User login
Is your patient on target? Optimizing diabetes management
› Aim for a glycated hemoglobin of <7% for most nonpregnant patients with type 2 diabetes, with a less stringent target for those with severe hypoglycemia, limited life expectancy, advanced micro- or macrovascular complications, and/or extensive comorbidities. B
› Attempt to treat patients with diabetes and hypertension to a target blood pressure<140/90mm Hg. B
› Prescribe statin therapy regardless of baseline lipid levels for all patients
who have diabetes and are between the ages of 40 and 75years. A
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
CASE › Dennis D, age 63, was recently diagnosed with diabetes. his glycated hemoglobin (HbA1c) is 7.8%, his blood pressure (BP) is mildly elevated (145/95 mm Hg), and his body mass index (BMI) is 28.5, but his low-density lipoprotein (LDL) cholesterol is 100 mg/dl, his high-density lipoprotein (HDL) cholesterol is 52 mg/dL, and he has no history of cardiovascular disease (CVD). After an unsuccessful attempt to treat him with lifestyle modification, it is time to initiate diabetes therapy.
Other than an alpha-blocker for benign prostatic hyperplasia and a prostaglandin for glaucoma, Mr. D takes no other medications. You prescribe metformin 500 mg twice daily and consider what else to add to keep his diabetes well controlled. Should you prescribe an antihypertensive? And, despite the patient’s normal lipid levels, should he begin taking a statin?
Type 2 diabetes has been extensively studied in rigorous randomized controlled trials (RCTs). While studies have provided ample evidence in support of optimal treatment, differing interpretations of the findings are reflected in consensus guidelines developed by expert panels that don’t always see eye to eye on what diabetes treatment targets should be and how best to prevent micro- and macrovascular complications.
What’s more, recommendations continue to be updated as new data emerge. In February 2014, the Joint Committee on Prevention, Evaluation, and Treatment of High Blood Pressure (JCN 8) revised its target for patients with diabetes to <140/90 mm Hg (from <130/80 mm Hg).1 This is likely to lead to revisions in other leading consensus guidelines, as well.
Thus, primary care physicians managing the care of patients with diabetes face the challenge of using the latest recommendations in a manner that addresses the entire clinical picture, considering each patient’s age and overall health status, priorities, and preferences. We developed this evidence-based review and guide- line summary with that in mind.
HbA1c target: How low should you go?
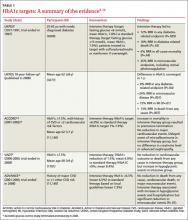
The Diabetes Control and Complications Trial (DCCT), published nearly 20 years ago, studied patients with type 1 diabetes, and found that intensive insulin therapy (HbA1c ≤6%) delayed the onset of retinopathy, nephropathy, and neuropathy.2 However, there was an important adverse effect of such intensive therapy: Patients in this group suffered from severe hypoglycemic episodes 3 times more frequently than those in the usual care group. Nonetheless, the microvascular benefits of intensive control observed in those with type 1 diabetes were thought to be similar for patients with type 2 diabetes.
The United Kingdom Prospective Diabetes Study (UKPDS), published in 1999, was the first major study to investigate targets for glucose control in patients with type 2 diabetes.3 Participants treated intensively (mean HbA1c goal, 7%) had a 25% reduction in microvascular complications, including the need for retinal photocoagulation, com- pared with those on standard control (mean HbA1c, 7.9%). There was also a nonsignificant trend toward a reduction in macrovascular complications in the intensive therapy group, but no difference in overall mortality rate.3
A 10-year follow-up of the UKPDS showed that while baseline differences in HbA1c between the 2 groups were lost by one year, reductions in microvascular complications continued to occur in the intensive treatment group.4 Reductions in myocardial infarction (MI) and death emerged over time, a possible legacy effect (ie, the result of intense treatment early in the course of the disease).
The Action to Control Cardiovascular Risk in Diabetes (ACCORD) trial, published in 2008, studied patients at risk for CVD, defined by either a prior history of CVD or ≥2 other cardiovascular risk factors.5 Participants, all of whom had poorly controlled type 2 diabetes (mean HbA1c, 8.1%), were randomized to either intensive treatment (HbA1c goal, <6%) or standard therapy (HbA1c goal, 7%-7.9%). The study was discontinued after a mean follow-up of 3.5 years, when those in the intensive therapy group were found to have a higher mortality rate.5
The rate of nonfatal MI reported by the ACCORD trial was lower in the intensive therapy group, however, and participants in this group also had delayed onset of microalbuminuria.6 No differences were seen in serum creatinine concentrations, advanced nephropathy, diabetic eye complications, or nonfatal stroke. Five-year follow up confirmed an increased mortality rate in the intensive therapy group,7 the result of severe hypoglycemia.8
The Veterans Affairs Diabetes Trial (VADT) randomized patients with poorly controlled type 2 diabetes to intensive or standard therapy.9 At 6 months, the intensive therapy group’s HbA1c averaged 6.9%, compared with 8.4% for the standard therapy group. Except for a delay in the progression of albuminuria, no significant effects of intensive therapy were found: Rates of other microvascular complications, major cardiovascular events, and death were similar.9 It should be noted that the VADT involved fewer participants and shorter follow-up than the other trials cited (TABLE 1),3-10 which may have affected its findings.
The Action in Diabetes and Vascular Disease (ADVANCE) trial, which included participants with either a history of major CVD or ≥1 other CVD risk factors, compared an intensive control group (mean HbA1c, 6.5%) with a standard care group (mean HbA1c, 7.3%)—with mixed results.10 Microalbuminuria occurred less frequently in the intensive therapy group, but hypoglycemia and hospitalization increased. No reduction in death from any cause, in cardiovascular death, or in major macrovascular events was found.
How to proceed? What the experts recommend
In updated standards for the medical care of diabetes released in January 2013,11 the American Diabetes Association (ADA) calls for an HbA1c goal <7% for most nonpregnant adults with type 2 diabetes. This is in line with the 2012 International Diabetes Federation (IDF) guideline.12
The 2011 guideline from the American Association of Clinical Endocrinologists (AACE),13 however, recommends tighter control—an HbA1c of ≤6.5% for most patients. For patients with diabetes of short duration, a long life expectancy, and no significant history of CVD, the AACE believes that this more aggressive goal has the potential to further reduce the risk of microvascular complications.
A less stringent target (eg, <8%) may be more appropriate for patients with a higher risk of adverse effects. That would apply to those with a history of severe hypoglycemia, a limited life expectancy, advanced micro- or macrovascular complications, or extensive comorbid conditions, as well as to any patient for whom stricter control is difficult to attain even with intensive therapy.13
Setting a BP target
In 2003, the 7th report of the Joint Committee on Prevention, Evaluation, and Treatment of High Blood Pressure (JCN 7) recommended a target BP <130/80 mm Hg for diabetes patients.14 Most major diabetes guidelines, including those of the AACE13 and IDF,12 echoed this recommendation. As noted earlier, JNC 8, published earlier this year, loosened the recommendation to <140/90 mm Hg.1 Although evidence has shown that treatment to a systolic BP <150 mm Hg improves cardiovascular and cerebrovascular outcomes for patients with diabetes,15 no RCTs have addressed whether more intensive treatment to achieve a systolic BP <140 mm Hg provides further benefit.
The BP of participants in the UKPDS has been examined, with patients with tighter control (<150/85 mm Hg) compared with those with less stringent control (<180/105 mm Hg). The tight control group showed a significant reduction in both death and complications related to diabetes, progression of diabetic retinopathy, and deterioration in visual acuity.15 Further investigation found that each 10 mm Hg reduction in systolic pressure was associated with a risk reduction of 15% for death related to diabetes, 12% for diabetes-related complications, 11% for MI, and 13% for microvascular complications.16
The ACCORD trial randomized participants to more intensive control (systolic BP <120 mm Hg, with a mean of 119.3) or standard therapy (systolic BP <140 mm Hg, mean 133.).17 After 4.7 years, no difference was found in the rates of MI, stroke, or death. However, a significant increase in the rate of serious adverse effects from antihypertensive treatment (including hypotension, syncope, bradycardia, hypokalemia, angioedema, and renal failure) occurred in the intensive control group.17
A subgroup analysis of patients with type 2 diabetes enrolled in the International Verapamil SR-Trandolapril Study (INVEST) evaluated systolic BP control and cardiovascular outcomes in those with preexisting coronary artery disease.18 Participants were categorized as having tight control if their systolic BP <130 mm Hg; usual control, if systolic pressure was between 130 and <140 mm Hg; and uncontrolled, if systolic BP ≥140 mm Hg. Those in the usual control group had lower risks of death, nonfatal MI, and stroke compared with those in the uncontrolled group, but little difference was found between patients in the usual control and tight control groups. The studies are summarized in TABLE 2.15-18
Interpreting the results: The experts disagree
The ADA recommends that patients with diabetes and hypertension be treated to a goal <140 mm Hg systolic and <80 mm Hg diastolic pressure11—more lenient than the recommendations of either the AACE or the IDF. It is not clear whether these recommendations will change, however, given the recent JNC 8 report.1 A lower systolic target may be appropriate for certain patients, if it can be achieved without undue adverse effects from antihypertensive medication. Older patients in particular may be at risk for orthostasis or falls as a result of more aggressive treatment.
CASE › Mr. D’s most recent BP is 145/95. Given that his goal is <140/90, you elect to start lisinopril 10 mg daily, advise him to monitor his BP at home, and refer him to a dietician to discuss the Dietary Approaches to Stop Hypertension diet.
Lipid levels: When to add statin therapy
Like glucose and BP control, lipid control and, concomitantly, the benefit of statin therapy for patients with type 2 diabetes has been studied extensively (TABLE 3).19-24
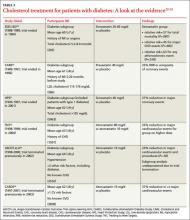
The Scandinavian Simvastatin Survival Study (4S) recruited participants with a history of MI or angina, and included a small diabetes subgroup.19 Participants were randomized to simvastatin 20 mg daily, with blinded titration up to 40 mg/d, or placebo. Among those with diabetes, patients on simvastatin had a 55% reduction in risk for major coronary heart disease events and a 43% reduction in total mortality. The risk reduction did not depend on baseline levels of total cholesterol, LDL cholesterol, HDL cholesterol, or triglycerides.
Cholesterol and Recurrent Events (CARE), which studied participants with a history of MI 3 to 20 months prior to the start of the study and also included a diabetes subgroup, had a similar outcome.20 Compared with placebo, treatment with pravastatin 40 mg/d reduced the risk of both coronary events and revascularization procedures by 25%.
The Heart Protection Study randomized patients with either diabetes or a history of occlusive arterial disease to receive simvastatin 40 mg daily or placebo.21 In the treatment group, the risk of major vascular events was reduced in patients with diabetes by 27%. Improvements were seen in patients with LDL cholesterol levels both above and below 116 mg/dL.
Multiple studies have evaluated the benefits of atorvastatin for patients with diabetes. All have demonstrated a significant reduction in the risk of MI and death in those on statin therapy. The Treating to New Targets study showed a 25% reduction in major cardiovascular events in those treated with 80 mg atorvastatin daily (mean LDL, 77 mg/dL) vs those treated with 10 mg of the drug (mean LDL, 86 mg/dL).22 The Anglo-Scandinavian Cardiac Outcomes Trial–Lipid-Lowering Arm (ASCOT-LLA)23 and the Collaborative Atorvastatin Diabetes Study (CARDS)24 were both terminated early due to the magnitude of benefit seen with statin therapy. In contrast to LDL, evidence for non-LDL treatment goals is lacking in the diabetes literature. Also, there is little evidence to support nonstatin cholesterol-lowering therapy for the management of diabetes patients.
Statin use is widely recommended
In 2008, the ADA and the American College of Cardiology Foundation (ACCF) produced a joint consensus statement regarding lipoprotein management for patients with diabetes and multiple CVD risk factors.25 Target LDL was recommended at <100 mg/dL for moderately high-risk primary prevention patients, including those with diabetes. For patients with diabetes and ≥1 other risk factors, the ADA/ACCF recommended an LDL goal <70 mg/dL. The 2011 AACE guideline has the same treatment goals,13 while the 2012 IDF guidelines are more aggressive.12 For primary prevention, the AACE endorses an LDL goal <80 mg/dL, and <70 mg/dL for those with known CVD.13
The updated standards released by the ADA in January 2013 recommend statin therapy regardless of LDL level for patients who have diabetes and known CVD, as well as for those ages 40 years and older who do not have CVD but have ≥1 other risk factors. Specific risk factors include hypertension, dyslipidemia, albuminuria, and a family history of CVD.11
The latest statin guideline. In November 2013, the American College of Cardiology and American Heart Association (ACC/AHA) published a new guideline for the treatment of cholesterol to reduce cardiovascular risk,26 but said nothing for or against specific LDL or non-HDL cholesterol targets. The ACC/AHA recommends that all patients who have diabetes and are between the ages of 40 and 75 years be treated with a moderate dose of a statin—a target supported with strong (strength of recommendation: A) evidence.
Patients with diabetes and an estimated 10-year risk of CVD >7.5% should be considered for high-intensity statin therapy, according to the ACC/AHA.26 For patients younger than 40 or older than 75, the decision to initiate statin therapy should be made by weighing the potential cardiovascular benefits, the risk of adverse effects, and the potential for drug-drug-interactions, as well as patient preference.
CASE › You discuss the need for moderate-dose statin therapy with Mr. D. He is hesitant at first, referring to a coworker who had “leg cramps” when he was taking a statin. You emphasize the importance of prevention in the care of his diabetes and convince the patient to begin a trial of atorvastatin 40 mg daily.
You warn Mr. D of the possibility of an allergic reaction, rash, or cough from lisinopril and loose stools from metformin, and advise him to call if he develops muscle cramps that could be associated with the statin. Finally, you stress the importance of lifestyle modification, including diet and weight loss, and schedule a follow-up visit in 3 months.
At Mr. D’s next visit, you will check his HbA1c and BP. If his HbA1c is still >7.0%, you may increase the dose of metformin or add a sulfonylurea. The dose of lisinopril could be increased if the patient’s BP continues to be elevated. There will be no need to recheck Mr. D’s cholesterol levels, however, because the purpose of the statin therapy is to improve overall outcomes, rather than to achieve a target goal.
CORRESPONDENCE
Kathryn M. Harmes, MD, Department of Family Medicine, University of Michigan Medical School, 1150 West Medical Center Drive, M7300 Med Sci I, SPC 5625, Ann Arbor, MI 48109-5625; [email protected]
1. James PA, Oparil S, Carter BL, et al. 2014 evidence-based guideline for the management of high blood pressure in adults: report from the panel members appointed to the Eighth Joint National Committee (JNC 8). JAMA. 2014;311:507-520.
2. The Diabetes Control and Complications Trial Research Group. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. The Diabetes Control and Complications Trial Research Group. N Engl J Med. 1993;329:977-986.
3. King P, Peacock I, Donnelly R. The UK Prospective Diabetes Study (UKPDS): clinical and therapeutic implications for type 2 diabetes. Br J Clin Pharmacol. 1999;48:643-648.
4. Holman RR, Paul SK, Bethel MA, et al. 10-year follow-up of intensive glucose control in type 2 diabetes. N Engl J Med. 2008;359:1577-1589.
5. Gerstein HC, Miller ME, Byington RP, et al; Action to Con- trol Cardiovascular Risk in Diabetes Study Group. Effects of intensive glucose lowering in type 2 diabetes. N Engl J Med. 2008;358:2545-2559.
6. Ismail-Beigi F, Craven T, Banerji MA, et al; ACCORD Trial Group. Effect of intensive treatment of hyperglycaemia on microvascular outcomes in type 2 diabetes: an analysis of the ACCORD randomised trial. Lancet. 2010;376:419-430.
7. Gerstein HC, Miller ME, Genuth S, et al; ACCORD Study Group. Long-term effects of intensive glucose lowering on cardiovascular outcomes. N Engl J Med. 2011;364:818-828.
8. Bonds DE, Miller ME, Bergenstal RM, et al. The association between symptomatic, severe hypoglycaemia and mortality in type 2 diabetes: retrospective epidemiological analysis of the ACCORD study. BMJ. 2010;340:b4909.
9. Duckworth W, Abraira C, Moritz T, et al; VADT Investigators. Glucose control and vascular complications in veterans with type 2 diabetes. N Engl J Med. 2009;360:129-139.
10. Patel A, MacMahon S, Chalmers J, et al; ADVANCE Collab- orative Group. Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes. N Engl J Med. 2008;358:2560-2572.
11. American Diabetes Association. Standards of medical care in diabetes—2013. Diabetes Care. 2013;36(suppl 1):S11-S66.
12. International Diabetes Foundation Clinical Guidelines Task Force. Global Guideline for Type 2 Diabetes. International Diabetes Federation. Brussels, Belgium; 2012.
13. Handelsman Y, Mechanick JI, Blonde L, et al; AACE Task Force for Developing Diabetes Comprehensive Care Plan. Ameri- can Association of Clinical Endocrinologists Medical Guidelines for clinical practice for developing a diabetes mellitus comprehensive care plan: executive summary. Endocr Pract. 2011;17(suppl 2):S1-S53
14. Chobanian AV, Bakris GL, Black HR, et al; National Heart, Lung, and Blood Institute Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure; National High Blood Pressure Education Program Coordinating Committee. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC 7 report. JAMA. 2003;289:2560-2572.
15. UK Prospective Diabetes Study Group. Tight blood pressure control and risk of macrovascular and microvascular complications in type 2 diabetes: UKPDS 38. UK Prospective Diabetes Study Group. BMJ. 1998;317:703-713.
16. Adler AI, Stratton IM, Neil HA, et al. Association of systolic blood pressure with macrovascular and microvascular complications of type 2 diabetes (UKPDS 36): prospective observational study. BMJ. 2000;321:412-419.
17. Cushman WC, Evans GW, Byington RP, et al; ACCORD Study Group. Effects of intensive blood-pressure control in type 2 diabetes mellitus. N Engl J Med. 2010;362:1575-1585.
18. Cooper-DeHoff RM, Gong Y, Handberg EM, et al. Tight blood pressure control and cardiovascular outcomes among hypertensive patients with diabetes and coronary artery disease. JAMA. 2010;304:61-68.
19. Pyörälä K, Pedersen TR, Kjekshus J, et al. Cholesterol lowering with simvastatin improves prognosis of diabetic patients with coronary heart disease. A subgroup analysis of the Scandinavian Simvastatin Survival Study (4S). Diabetes Care. 1997;20:614-620.
20. Goldberg RB, Mellies MJ, Sacks FM, et al; The Care Investigators. Cardiovascular events and their reduction with pravastatin in diabetic and glucose-intolerant myocardial infarction survivors with average cholesterol levels: subgroup analyses in the cholesterol and recurrent events (CARE) trial. The Care Investigators. Circulation. 1998;98:2513-2519.
21. Collins R, Armitage J, Parish S, et al; Heart Protection Study Collaborative Group. MRC/BHF Heart Protection Study of cholesterol-lowering with simvastatin in 5963 people with diabetes: a randomised placebo-controlled trial. Lancet. 2003;361:2005-2016.
22. Shepherd J, Barter P, Carmena R, et al. Effect of lowering LDL cholesterol substantially below currently recommended levels in patients with coronary heart disease and diabetes: the Treating to New Targets (TNT) study. Diabetes Care. 2006;29:1220-1226.
23. Sever PS, Poulter NR, Dahlöf B, et al. Reduction in cardiovascular events with atorvastatin in 2,532 patients with type 2 diabetes: Anglo-Scandinavian Cardiac Outcomes Trial—lipid-lowering arm (ASCOT-LLA). Diabetes Care. 2005;28:1151-1157.
24. Colhoun HM, Betteridge DJ, Durrington PN, et al; CARDS investigators. Primary prevention of cardiovascular disease with atorvastatin in type 2 diabetes in the Collaborative Atorvastatin Diabetes Study (CARDS): multicentre randomized placebo-controlled trial. Lancet. 2004;364:685-696.
25. Brunzell JD, Davidson M, Furberg CD, et al; American Diabetes Association; American College of Cardiology Foundation. Lipoprotein management in patients with cardiometabolic risk: consensus statement from the American Diabetes Association and the American College of Cardiology Foundation. Diabetes Care. 2008;31:811-822.
26. Stone NJ, Robinson JG, Lichtenstein AH, et al. 2013 ACC/AHA Guideline on the Treatment of Blood Cholesterol to Reduce Atherosclerotic Cardiovascular Risk in Adults: A Report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2014;63(25 pt B):2889-2934.
› Aim for a glycated hemoglobin of <7% for most nonpregnant patients with type 2 diabetes, with a less stringent target for those with severe hypoglycemia, limited life expectancy, advanced micro- or macrovascular complications, and/or extensive comorbidities. B
› Attempt to treat patients with diabetes and hypertension to a target blood pressure<140/90mm Hg. B
› Prescribe statin therapy regardless of baseline lipid levels for all patients
who have diabetes and are between the ages of 40 and 75years. A
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
CASE › Dennis D, age 63, was recently diagnosed with diabetes. his glycated hemoglobin (HbA1c) is 7.8%, his blood pressure (BP) is mildly elevated (145/95 mm Hg), and his body mass index (BMI) is 28.5, but his low-density lipoprotein (LDL) cholesterol is 100 mg/dl, his high-density lipoprotein (HDL) cholesterol is 52 mg/dL, and he has no history of cardiovascular disease (CVD). After an unsuccessful attempt to treat him with lifestyle modification, it is time to initiate diabetes therapy.
Other than an alpha-blocker for benign prostatic hyperplasia and a prostaglandin for glaucoma, Mr. D takes no other medications. You prescribe metformin 500 mg twice daily and consider what else to add to keep his diabetes well controlled. Should you prescribe an antihypertensive? And, despite the patient’s normal lipid levels, should he begin taking a statin?
Type 2 diabetes has been extensively studied in rigorous randomized controlled trials (RCTs). While studies have provided ample evidence in support of optimal treatment, differing interpretations of the findings are reflected in consensus guidelines developed by expert panels that don’t always see eye to eye on what diabetes treatment targets should be and how best to prevent micro- and macrovascular complications.
What’s more, recommendations continue to be updated as new data emerge. In February 2014, the Joint Committee on Prevention, Evaluation, and Treatment of High Blood Pressure (JCN 8) revised its target for patients with diabetes to <140/90 mm Hg (from <130/80 mm Hg).1 This is likely to lead to revisions in other leading consensus guidelines, as well.
Thus, primary care physicians managing the care of patients with diabetes face the challenge of using the latest recommendations in a manner that addresses the entire clinical picture, considering each patient’s age and overall health status, priorities, and preferences. We developed this evidence-based review and guide- line summary with that in mind.
HbA1c target: How low should you go?
The Diabetes Control and Complications Trial (DCCT), published nearly 20 years ago, studied patients with type 1 diabetes, and found that intensive insulin therapy (HbA1c ≤6%) delayed the onset of retinopathy, nephropathy, and neuropathy.2 However, there was an important adverse effect of such intensive therapy: Patients in this group suffered from severe hypoglycemic episodes 3 times more frequently than those in the usual care group. Nonetheless, the microvascular benefits of intensive control observed in those with type 1 diabetes were thought to be similar for patients with type 2 diabetes.
The United Kingdom Prospective Diabetes Study (UKPDS), published in 1999, was the first major study to investigate targets for glucose control in patients with type 2 diabetes.3 Participants treated intensively (mean HbA1c goal, 7%) had a 25% reduction in microvascular complications, including the need for retinal photocoagulation, com- pared with those on standard control (mean HbA1c, 7.9%). There was also a nonsignificant trend toward a reduction in macrovascular complications in the intensive therapy group, but no difference in overall mortality rate.3
A 10-year follow-up of the UKPDS showed that while baseline differences in HbA1c between the 2 groups were lost by one year, reductions in microvascular complications continued to occur in the intensive treatment group.4 Reductions in myocardial infarction (MI) and death emerged over time, a possible legacy effect (ie, the result of intense treatment early in the course of the disease).
The Action to Control Cardiovascular Risk in Diabetes (ACCORD) trial, published in 2008, studied patients at risk for CVD, defined by either a prior history of CVD or ≥2 other cardiovascular risk factors.5 Participants, all of whom had poorly controlled type 2 diabetes (mean HbA1c, 8.1%), were randomized to either intensive treatment (HbA1c goal, <6%) or standard therapy (HbA1c goal, 7%-7.9%). The study was discontinued after a mean follow-up of 3.5 years, when those in the intensive therapy group were found to have a higher mortality rate.5
The rate of nonfatal MI reported by the ACCORD trial was lower in the intensive therapy group, however, and participants in this group also had delayed onset of microalbuminuria.6 No differences were seen in serum creatinine concentrations, advanced nephropathy, diabetic eye complications, or nonfatal stroke. Five-year follow up confirmed an increased mortality rate in the intensive therapy group,7 the result of severe hypoglycemia.8
The Veterans Affairs Diabetes Trial (VADT) randomized patients with poorly controlled type 2 diabetes to intensive or standard therapy.9 At 6 months, the intensive therapy group’s HbA1c averaged 6.9%, compared with 8.4% for the standard therapy group. Except for a delay in the progression of albuminuria, no significant effects of intensive therapy were found: Rates of other microvascular complications, major cardiovascular events, and death were similar.9 It should be noted that the VADT involved fewer participants and shorter follow-up than the other trials cited (TABLE 1),3-10 which may have affected its findings.
The Action in Diabetes and Vascular Disease (ADVANCE) trial, which included participants with either a history of major CVD or ≥1 other CVD risk factors, compared an intensive control group (mean HbA1c, 6.5%) with a standard care group (mean HbA1c, 7.3%)—with mixed results.10 Microalbuminuria occurred less frequently in the intensive therapy group, but hypoglycemia and hospitalization increased. No reduction in death from any cause, in cardiovascular death, or in major macrovascular events was found.
How to proceed? What the experts recommend
In updated standards for the medical care of diabetes released in January 2013,11 the American Diabetes Association (ADA) calls for an HbA1c goal <7% for most nonpregnant adults with type 2 diabetes. This is in line with the 2012 International Diabetes Federation (IDF) guideline.12
The 2011 guideline from the American Association of Clinical Endocrinologists (AACE),13 however, recommends tighter control—an HbA1c of ≤6.5% for most patients. For patients with diabetes of short duration, a long life expectancy, and no significant history of CVD, the AACE believes that this more aggressive goal has the potential to further reduce the risk of microvascular complications.
A less stringent target (eg, <8%) may be more appropriate for patients with a higher risk of adverse effects. That would apply to those with a history of severe hypoglycemia, a limited life expectancy, advanced micro- or macrovascular complications, or extensive comorbid conditions, as well as to any patient for whom stricter control is difficult to attain even with intensive therapy.13
Setting a BP target
In 2003, the 7th report of the Joint Committee on Prevention, Evaluation, and Treatment of High Blood Pressure (JCN 7) recommended a target BP <130/80 mm Hg for diabetes patients.14 Most major diabetes guidelines, including those of the AACE13 and IDF,12 echoed this recommendation. As noted earlier, JNC 8, published earlier this year, loosened the recommendation to <140/90 mm Hg.1 Although evidence has shown that treatment to a systolic BP <150 mm Hg improves cardiovascular and cerebrovascular outcomes for patients with diabetes,15 no RCTs have addressed whether more intensive treatment to achieve a systolic BP <140 mm Hg provides further benefit.
The BP of participants in the UKPDS has been examined, with patients with tighter control (<150/85 mm Hg) compared with those with less stringent control (<180/105 mm Hg). The tight control group showed a significant reduction in both death and complications related to diabetes, progression of diabetic retinopathy, and deterioration in visual acuity.15 Further investigation found that each 10 mm Hg reduction in systolic pressure was associated with a risk reduction of 15% for death related to diabetes, 12% for diabetes-related complications, 11% for MI, and 13% for microvascular complications.16
The ACCORD trial randomized participants to more intensive control (systolic BP <120 mm Hg, with a mean of 119.3) or standard therapy (systolic BP <140 mm Hg, mean 133.).17 After 4.7 years, no difference was found in the rates of MI, stroke, or death. However, a significant increase in the rate of serious adverse effects from antihypertensive treatment (including hypotension, syncope, bradycardia, hypokalemia, angioedema, and renal failure) occurred in the intensive control group.17
A subgroup analysis of patients with type 2 diabetes enrolled in the International Verapamil SR-Trandolapril Study (INVEST) evaluated systolic BP control and cardiovascular outcomes in those with preexisting coronary artery disease.18 Participants were categorized as having tight control if their systolic BP <130 mm Hg; usual control, if systolic pressure was between 130 and <140 mm Hg; and uncontrolled, if systolic BP ≥140 mm Hg. Those in the usual control group had lower risks of death, nonfatal MI, and stroke compared with those in the uncontrolled group, but little difference was found between patients in the usual control and tight control groups. The studies are summarized in TABLE 2.15-18
Interpreting the results: The experts disagree
The ADA recommends that patients with diabetes and hypertension be treated to a goal <140 mm Hg systolic and <80 mm Hg diastolic pressure11—more lenient than the recommendations of either the AACE or the IDF. It is not clear whether these recommendations will change, however, given the recent JNC 8 report.1 A lower systolic target may be appropriate for certain patients, if it can be achieved without undue adverse effects from antihypertensive medication. Older patients in particular may be at risk for orthostasis or falls as a result of more aggressive treatment.
CASE › Mr. D’s most recent BP is 145/95. Given that his goal is <140/90, you elect to start lisinopril 10 mg daily, advise him to monitor his BP at home, and refer him to a dietician to discuss the Dietary Approaches to Stop Hypertension diet.
Lipid levels: When to add statin therapy
Like glucose and BP control, lipid control and, concomitantly, the benefit of statin therapy for patients with type 2 diabetes has been studied extensively (TABLE 3).19-24
The Scandinavian Simvastatin Survival Study (4S) recruited participants with a history of MI or angina, and included a small diabetes subgroup.19 Participants were randomized to simvastatin 20 mg daily, with blinded titration up to 40 mg/d, or placebo. Among those with diabetes, patients on simvastatin had a 55% reduction in risk for major coronary heart disease events and a 43% reduction in total mortality. The risk reduction did not depend on baseline levels of total cholesterol, LDL cholesterol, HDL cholesterol, or triglycerides.
Cholesterol and Recurrent Events (CARE), which studied participants with a history of MI 3 to 20 months prior to the start of the study and also included a diabetes subgroup, had a similar outcome.20 Compared with placebo, treatment with pravastatin 40 mg/d reduced the risk of both coronary events and revascularization procedures by 25%.
The Heart Protection Study randomized patients with either diabetes or a history of occlusive arterial disease to receive simvastatin 40 mg daily or placebo.21 In the treatment group, the risk of major vascular events was reduced in patients with diabetes by 27%. Improvements were seen in patients with LDL cholesterol levels both above and below 116 mg/dL.
Multiple studies have evaluated the benefits of atorvastatin for patients with diabetes. All have demonstrated a significant reduction in the risk of MI and death in those on statin therapy. The Treating to New Targets study showed a 25% reduction in major cardiovascular events in those treated with 80 mg atorvastatin daily (mean LDL, 77 mg/dL) vs those treated with 10 mg of the drug (mean LDL, 86 mg/dL).22 The Anglo-Scandinavian Cardiac Outcomes Trial–Lipid-Lowering Arm (ASCOT-LLA)23 and the Collaborative Atorvastatin Diabetes Study (CARDS)24 were both terminated early due to the magnitude of benefit seen with statin therapy. In contrast to LDL, evidence for non-LDL treatment goals is lacking in the diabetes literature. Also, there is little evidence to support nonstatin cholesterol-lowering therapy for the management of diabetes patients.
Statin use is widely recommended
In 2008, the ADA and the American College of Cardiology Foundation (ACCF) produced a joint consensus statement regarding lipoprotein management for patients with diabetes and multiple CVD risk factors.25 Target LDL was recommended at <100 mg/dL for moderately high-risk primary prevention patients, including those with diabetes. For patients with diabetes and ≥1 other risk factors, the ADA/ACCF recommended an LDL goal <70 mg/dL. The 2011 AACE guideline has the same treatment goals,13 while the 2012 IDF guidelines are more aggressive.12 For primary prevention, the AACE endorses an LDL goal <80 mg/dL, and <70 mg/dL for those with known CVD.13
The updated standards released by the ADA in January 2013 recommend statin therapy regardless of LDL level for patients who have diabetes and known CVD, as well as for those ages 40 years and older who do not have CVD but have ≥1 other risk factors. Specific risk factors include hypertension, dyslipidemia, albuminuria, and a family history of CVD.11
The latest statin guideline. In November 2013, the American College of Cardiology and American Heart Association (ACC/AHA) published a new guideline for the treatment of cholesterol to reduce cardiovascular risk,26 but said nothing for or against specific LDL or non-HDL cholesterol targets. The ACC/AHA recommends that all patients who have diabetes and are between the ages of 40 and 75 years be treated with a moderate dose of a statin—a target supported with strong (strength of recommendation: A) evidence.
Patients with diabetes and an estimated 10-year risk of CVD >7.5% should be considered for high-intensity statin therapy, according to the ACC/AHA.26 For patients younger than 40 or older than 75, the decision to initiate statin therapy should be made by weighing the potential cardiovascular benefits, the risk of adverse effects, and the potential for drug-drug-interactions, as well as patient preference.
CASE › You discuss the need for moderate-dose statin therapy with Mr. D. He is hesitant at first, referring to a coworker who had “leg cramps” when he was taking a statin. You emphasize the importance of prevention in the care of his diabetes and convince the patient to begin a trial of atorvastatin 40 mg daily.
You warn Mr. D of the possibility of an allergic reaction, rash, or cough from lisinopril and loose stools from metformin, and advise him to call if he develops muscle cramps that could be associated with the statin. Finally, you stress the importance of lifestyle modification, including diet and weight loss, and schedule a follow-up visit in 3 months.
At Mr. D’s next visit, you will check his HbA1c and BP. If his HbA1c is still >7.0%, you may increase the dose of metformin or add a sulfonylurea. The dose of lisinopril could be increased if the patient’s BP continues to be elevated. There will be no need to recheck Mr. D’s cholesterol levels, however, because the purpose of the statin therapy is to improve overall outcomes, rather than to achieve a target goal.
CORRESPONDENCE
Kathryn M. Harmes, MD, Department of Family Medicine, University of Michigan Medical School, 1150 West Medical Center Drive, M7300 Med Sci I, SPC 5625, Ann Arbor, MI 48109-5625; [email protected]
› Aim for a glycated hemoglobin of <7% for most nonpregnant patients with type 2 diabetes, with a less stringent target for those with severe hypoglycemia, limited life expectancy, advanced micro- or macrovascular complications, and/or extensive comorbidities. B
› Attempt to treat patients with diabetes and hypertension to a target blood pressure<140/90mm Hg. B
› Prescribe statin therapy regardless of baseline lipid levels for all patients
who have diabetes and are between the ages of 40 and 75years. A
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
CASE › Dennis D, age 63, was recently diagnosed with diabetes. his glycated hemoglobin (HbA1c) is 7.8%, his blood pressure (BP) is mildly elevated (145/95 mm Hg), and his body mass index (BMI) is 28.5, but his low-density lipoprotein (LDL) cholesterol is 100 mg/dl, his high-density lipoprotein (HDL) cholesterol is 52 mg/dL, and he has no history of cardiovascular disease (CVD). After an unsuccessful attempt to treat him with lifestyle modification, it is time to initiate diabetes therapy.
Other than an alpha-blocker for benign prostatic hyperplasia and a prostaglandin for glaucoma, Mr. D takes no other medications. You prescribe metformin 500 mg twice daily and consider what else to add to keep his diabetes well controlled. Should you prescribe an antihypertensive? And, despite the patient’s normal lipid levels, should he begin taking a statin?
Type 2 diabetes has been extensively studied in rigorous randomized controlled trials (RCTs). While studies have provided ample evidence in support of optimal treatment, differing interpretations of the findings are reflected in consensus guidelines developed by expert panels that don’t always see eye to eye on what diabetes treatment targets should be and how best to prevent micro- and macrovascular complications.
What’s more, recommendations continue to be updated as new data emerge. In February 2014, the Joint Committee on Prevention, Evaluation, and Treatment of High Blood Pressure (JCN 8) revised its target for patients with diabetes to <140/90 mm Hg (from <130/80 mm Hg).1 This is likely to lead to revisions in other leading consensus guidelines, as well.
Thus, primary care physicians managing the care of patients with diabetes face the challenge of using the latest recommendations in a manner that addresses the entire clinical picture, considering each patient’s age and overall health status, priorities, and preferences. We developed this evidence-based review and guide- line summary with that in mind.
HbA1c target: How low should you go?
The Diabetes Control and Complications Trial (DCCT), published nearly 20 years ago, studied patients with type 1 diabetes, and found that intensive insulin therapy (HbA1c ≤6%) delayed the onset of retinopathy, nephropathy, and neuropathy.2 However, there was an important adverse effect of such intensive therapy: Patients in this group suffered from severe hypoglycemic episodes 3 times more frequently than those in the usual care group. Nonetheless, the microvascular benefits of intensive control observed in those with type 1 diabetes were thought to be similar for patients with type 2 diabetes.
The United Kingdom Prospective Diabetes Study (UKPDS), published in 1999, was the first major study to investigate targets for glucose control in patients with type 2 diabetes.3 Participants treated intensively (mean HbA1c goal, 7%) had a 25% reduction in microvascular complications, including the need for retinal photocoagulation, com- pared with those on standard control (mean HbA1c, 7.9%). There was also a nonsignificant trend toward a reduction in macrovascular complications in the intensive therapy group, but no difference in overall mortality rate.3
A 10-year follow-up of the UKPDS showed that while baseline differences in HbA1c between the 2 groups were lost by one year, reductions in microvascular complications continued to occur in the intensive treatment group.4 Reductions in myocardial infarction (MI) and death emerged over time, a possible legacy effect (ie, the result of intense treatment early in the course of the disease).
The Action to Control Cardiovascular Risk in Diabetes (ACCORD) trial, published in 2008, studied patients at risk for CVD, defined by either a prior history of CVD or ≥2 other cardiovascular risk factors.5 Participants, all of whom had poorly controlled type 2 diabetes (mean HbA1c, 8.1%), were randomized to either intensive treatment (HbA1c goal, <6%) or standard therapy (HbA1c goal, 7%-7.9%). The study was discontinued after a mean follow-up of 3.5 years, when those in the intensive therapy group were found to have a higher mortality rate.5
The rate of nonfatal MI reported by the ACCORD trial was lower in the intensive therapy group, however, and participants in this group also had delayed onset of microalbuminuria.6 No differences were seen in serum creatinine concentrations, advanced nephropathy, diabetic eye complications, or nonfatal stroke. Five-year follow up confirmed an increased mortality rate in the intensive therapy group,7 the result of severe hypoglycemia.8
The Veterans Affairs Diabetes Trial (VADT) randomized patients with poorly controlled type 2 diabetes to intensive or standard therapy.9 At 6 months, the intensive therapy group’s HbA1c averaged 6.9%, compared with 8.4% for the standard therapy group. Except for a delay in the progression of albuminuria, no significant effects of intensive therapy were found: Rates of other microvascular complications, major cardiovascular events, and death were similar.9 It should be noted that the VADT involved fewer participants and shorter follow-up than the other trials cited (TABLE 1),3-10 which may have affected its findings.
The Action in Diabetes and Vascular Disease (ADVANCE) trial, which included participants with either a history of major CVD or ≥1 other CVD risk factors, compared an intensive control group (mean HbA1c, 6.5%) with a standard care group (mean HbA1c, 7.3%)—with mixed results.10 Microalbuminuria occurred less frequently in the intensive therapy group, but hypoglycemia and hospitalization increased. No reduction in death from any cause, in cardiovascular death, or in major macrovascular events was found.
How to proceed? What the experts recommend
In updated standards for the medical care of diabetes released in January 2013,11 the American Diabetes Association (ADA) calls for an HbA1c goal <7% for most nonpregnant adults with type 2 diabetes. This is in line with the 2012 International Diabetes Federation (IDF) guideline.12
The 2011 guideline from the American Association of Clinical Endocrinologists (AACE),13 however, recommends tighter control—an HbA1c of ≤6.5% for most patients. For patients with diabetes of short duration, a long life expectancy, and no significant history of CVD, the AACE believes that this more aggressive goal has the potential to further reduce the risk of microvascular complications.
A less stringent target (eg, <8%) may be more appropriate for patients with a higher risk of adverse effects. That would apply to those with a history of severe hypoglycemia, a limited life expectancy, advanced micro- or macrovascular complications, or extensive comorbid conditions, as well as to any patient for whom stricter control is difficult to attain even with intensive therapy.13
Setting a BP target
In 2003, the 7th report of the Joint Committee on Prevention, Evaluation, and Treatment of High Blood Pressure (JCN 7) recommended a target BP <130/80 mm Hg for diabetes patients.14 Most major diabetes guidelines, including those of the AACE13 and IDF,12 echoed this recommendation. As noted earlier, JNC 8, published earlier this year, loosened the recommendation to <140/90 mm Hg.1 Although evidence has shown that treatment to a systolic BP <150 mm Hg improves cardiovascular and cerebrovascular outcomes for patients with diabetes,15 no RCTs have addressed whether more intensive treatment to achieve a systolic BP <140 mm Hg provides further benefit.
The BP of participants in the UKPDS has been examined, with patients with tighter control (<150/85 mm Hg) compared with those with less stringent control (<180/105 mm Hg). The tight control group showed a significant reduction in both death and complications related to diabetes, progression of diabetic retinopathy, and deterioration in visual acuity.15 Further investigation found that each 10 mm Hg reduction in systolic pressure was associated with a risk reduction of 15% for death related to diabetes, 12% for diabetes-related complications, 11% for MI, and 13% for microvascular complications.16
The ACCORD trial randomized participants to more intensive control (systolic BP <120 mm Hg, with a mean of 119.3) or standard therapy (systolic BP <140 mm Hg, mean 133.).17 After 4.7 years, no difference was found in the rates of MI, stroke, or death. However, a significant increase in the rate of serious adverse effects from antihypertensive treatment (including hypotension, syncope, bradycardia, hypokalemia, angioedema, and renal failure) occurred in the intensive control group.17
A subgroup analysis of patients with type 2 diabetes enrolled in the International Verapamil SR-Trandolapril Study (INVEST) evaluated systolic BP control and cardiovascular outcomes in those with preexisting coronary artery disease.18 Participants were categorized as having tight control if their systolic BP <130 mm Hg; usual control, if systolic pressure was between 130 and <140 mm Hg; and uncontrolled, if systolic BP ≥140 mm Hg. Those in the usual control group had lower risks of death, nonfatal MI, and stroke compared with those in the uncontrolled group, but little difference was found between patients in the usual control and tight control groups. The studies are summarized in TABLE 2.15-18
Interpreting the results: The experts disagree
The ADA recommends that patients with diabetes and hypertension be treated to a goal <140 mm Hg systolic and <80 mm Hg diastolic pressure11—more lenient than the recommendations of either the AACE or the IDF. It is not clear whether these recommendations will change, however, given the recent JNC 8 report.1 A lower systolic target may be appropriate for certain patients, if it can be achieved without undue adverse effects from antihypertensive medication. Older patients in particular may be at risk for orthostasis or falls as a result of more aggressive treatment.
CASE › Mr. D’s most recent BP is 145/95. Given that his goal is <140/90, you elect to start lisinopril 10 mg daily, advise him to monitor his BP at home, and refer him to a dietician to discuss the Dietary Approaches to Stop Hypertension diet.
Lipid levels: When to add statin therapy
Like glucose and BP control, lipid control and, concomitantly, the benefit of statin therapy for patients with type 2 diabetes has been studied extensively (TABLE 3).19-24
The Scandinavian Simvastatin Survival Study (4S) recruited participants with a history of MI or angina, and included a small diabetes subgroup.19 Participants were randomized to simvastatin 20 mg daily, with blinded titration up to 40 mg/d, or placebo. Among those with diabetes, patients on simvastatin had a 55% reduction in risk for major coronary heart disease events and a 43% reduction in total mortality. The risk reduction did not depend on baseline levels of total cholesterol, LDL cholesterol, HDL cholesterol, or triglycerides.
Cholesterol and Recurrent Events (CARE), which studied participants with a history of MI 3 to 20 months prior to the start of the study and also included a diabetes subgroup, had a similar outcome.20 Compared with placebo, treatment with pravastatin 40 mg/d reduced the risk of both coronary events and revascularization procedures by 25%.
The Heart Protection Study randomized patients with either diabetes or a history of occlusive arterial disease to receive simvastatin 40 mg daily or placebo.21 In the treatment group, the risk of major vascular events was reduced in patients with diabetes by 27%. Improvements were seen in patients with LDL cholesterol levels both above and below 116 mg/dL.
Multiple studies have evaluated the benefits of atorvastatin for patients with diabetes. All have demonstrated a significant reduction in the risk of MI and death in those on statin therapy. The Treating to New Targets study showed a 25% reduction in major cardiovascular events in those treated with 80 mg atorvastatin daily (mean LDL, 77 mg/dL) vs those treated with 10 mg of the drug (mean LDL, 86 mg/dL).22 The Anglo-Scandinavian Cardiac Outcomes Trial–Lipid-Lowering Arm (ASCOT-LLA)23 and the Collaborative Atorvastatin Diabetes Study (CARDS)24 were both terminated early due to the magnitude of benefit seen with statin therapy. In contrast to LDL, evidence for non-LDL treatment goals is lacking in the diabetes literature. Also, there is little evidence to support nonstatin cholesterol-lowering therapy for the management of diabetes patients.
Statin use is widely recommended
In 2008, the ADA and the American College of Cardiology Foundation (ACCF) produced a joint consensus statement regarding lipoprotein management for patients with diabetes and multiple CVD risk factors.25 Target LDL was recommended at <100 mg/dL for moderately high-risk primary prevention patients, including those with diabetes. For patients with diabetes and ≥1 other risk factors, the ADA/ACCF recommended an LDL goal <70 mg/dL. The 2011 AACE guideline has the same treatment goals,13 while the 2012 IDF guidelines are more aggressive.12 For primary prevention, the AACE endorses an LDL goal <80 mg/dL, and <70 mg/dL for those with known CVD.13
The updated standards released by the ADA in January 2013 recommend statin therapy regardless of LDL level for patients who have diabetes and known CVD, as well as for those ages 40 years and older who do not have CVD but have ≥1 other risk factors. Specific risk factors include hypertension, dyslipidemia, albuminuria, and a family history of CVD.11
The latest statin guideline. In November 2013, the American College of Cardiology and American Heart Association (ACC/AHA) published a new guideline for the treatment of cholesterol to reduce cardiovascular risk,26 but said nothing for or against specific LDL or non-HDL cholesterol targets. The ACC/AHA recommends that all patients who have diabetes and are between the ages of 40 and 75 years be treated with a moderate dose of a statin—a target supported with strong (strength of recommendation: A) evidence.
Patients with diabetes and an estimated 10-year risk of CVD >7.5% should be considered for high-intensity statin therapy, according to the ACC/AHA.26 For patients younger than 40 or older than 75, the decision to initiate statin therapy should be made by weighing the potential cardiovascular benefits, the risk of adverse effects, and the potential for drug-drug-interactions, as well as patient preference.
CASE › You discuss the need for moderate-dose statin therapy with Mr. D. He is hesitant at first, referring to a coworker who had “leg cramps” when he was taking a statin. You emphasize the importance of prevention in the care of his diabetes and convince the patient to begin a trial of atorvastatin 40 mg daily.
You warn Mr. D of the possibility of an allergic reaction, rash, or cough from lisinopril and loose stools from metformin, and advise him to call if he develops muscle cramps that could be associated with the statin. Finally, you stress the importance of lifestyle modification, including diet and weight loss, and schedule a follow-up visit in 3 months.
At Mr. D’s next visit, you will check his HbA1c and BP. If his HbA1c is still >7.0%, you may increase the dose of metformin or add a sulfonylurea. The dose of lisinopril could be increased if the patient’s BP continues to be elevated. There will be no need to recheck Mr. D’s cholesterol levels, however, because the purpose of the statin therapy is to improve overall outcomes, rather than to achieve a target goal.
CORRESPONDENCE
Kathryn M. Harmes, MD, Department of Family Medicine, University of Michigan Medical School, 1150 West Medical Center Drive, M7300 Med Sci I, SPC 5625, Ann Arbor, MI 48109-5625; [email protected]
1. James PA, Oparil S, Carter BL, et al. 2014 evidence-based guideline for the management of high blood pressure in adults: report from the panel members appointed to the Eighth Joint National Committee (JNC 8). JAMA. 2014;311:507-520.
2. The Diabetes Control and Complications Trial Research Group. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. The Diabetes Control and Complications Trial Research Group. N Engl J Med. 1993;329:977-986.
3. King P, Peacock I, Donnelly R. The UK Prospective Diabetes Study (UKPDS): clinical and therapeutic implications for type 2 diabetes. Br J Clin Pharmacol. 1999;48:643-648.
4. Holman RR, Paul SK, Bethel MA, et al. 10-year follow-up of intensive glucose control in type 2 diabetes. N Engl J Med. 2008;359:1577-1589.
5. Gerstein HC, Miller ME, Byington RP, et al; Action to Con- trol Cardiovascular Risk in Diabetes Study Group. Effects of intensive glucose lowering in type 2 diabetes. N Engl J Med. 2008;358:2545-2559.
6. Ismail-Beigi F, Craven T, Banerji MA, et al; ACCORD Trial Group. Effect of intensive treatment of hyperglycaemia on microvascular outcomes in type 2 diabetes: an analysis of the ACCORD randomised trial. Lancet. 2010;376:419-430.
7. Gerstein HC, Miller ME, Genuth S, et al; ACCORD Study Group. Long-term effects of intensive glucose lowering on cardiovascular outcomes. N Engl J Med. 2011;364:818-828.
8. Bonds DE, Miller ME, Bergenstal RM, et al. The association between symptomatic, severe hypoglycaemia and mortality in type 2 diabetes: retrospective epidemiological analysis of the ACCORD study. BMJ. 2010;340:b4909.
9. Duckworth W, Abraira C, Moritz T, et al; VADT Investigators. Glucose control and vascular complications in veterans with type 2 diabetes. N Engl J Med. 2009;360:129-139.
10. Patel A, MacMahon S, Chalmers J, et al; ADVANCE Collab- orative Group. Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes. N Engl J Med. 2008;358:2560-2572.
11. American Diabetes Association. Standards of medical care in diabetes—2013. Diabetes Care. 2013;36(suppl 1):S11-S66.
12. International Diabetes Foundation Clinical Guidelines Task Force. Global Guideline for Type 2 Diabetes. International Diabetes Federation. Brussels, Belgium; 2012.
13. Handelsman Y, Mechanick JI, Blonde L, et al; AACE Task Force for Developing Diabetes Comprehensive Care Plan. Ameri- can Association of Clinical Endocrinologists Medical Guidelines for clinical practice for developing a diabetes mellitus comprehensive care plan: executive summary. Endocr Pract. 2011;17(suppl 2):S1-S53
14. Chobanian AV, Bakris GL, Black HR, et al; National Heart, Lung, and Blood Institute Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure; National High Blood Pressure Education Program Coordinating Committee. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC 7 report. JAMA. 2003;289:2560-2572.
15. UK Prospective Diabetes Study Group. Tight blood pressure control and risk of macrovascular and microvascular complications in type 2 diabetes: UKPDS 38. UK Prospective Diabetes Study Group. BMJ. 1998;317:703-713.
16. Adler AI, Stratton IM, Neil HA, et al. Association of systolic blood pressure with macrovascular and microvascular complications of type 2 diabetes (UKPDS 36): prospective observational study. BMJ. 2000;321:412-419.
17. Cushman WC, Evans GW, Byington RP, et al; ACCORD Study Group. Effects of intensive blood-pressure control in type 2 diabetes mellitus. N Engl J Med. 2010;362:1575-1585.
18. Cooper-DeHoff RM, Gong Y, Handberg EM, et al. Tight blood pressure control and cardiovascular outcomes among hypertensive patients with diabetes and coronary artery disease. JAMA. 2010;304:61-68.
19. Pyörälä K, Pedersen TR, Kjekshus J, et al. Cholesterol lowering with simvastatin improves prognosis of diabetic patients with coronary heart disease. A subgroup analysis of the Scandinavian Simvastatin Survival Study (4S). Diabetes Care. 1997;20:614-620.
20. Goldberg RB, Mellies MJ, Sacks FM, et al; The Care Investigators. Cardiovascular events and their reduction with pravastatin in diabetic and glucose-intolerant myocardial infarction survivors with average cholesterol levels: subgroup analyses in the cholesterol and recurrent events (CARE) trial. The Care Investigators. Circulation. 1998;98:2513-2519.
21. Collins R, Armitage J, Parish S, et al; Heart Protection Study Collaborative Group. MRC/BHF Heart Protection Study of cholesterol-lowering with simvastatin in 5963 people with diabetes: a randomised placebo-controlled trial. Lancet. 2003;361:2005-2016.
22. Shepherd J, Barter P, Carmena R, et al. Effect of lowering LDL cholesterol substantially below currently recommended levels in patients with coronary heart disease and diabetes: the Treating to New Targets (TNT) study. Diabetes Care. 2006;29:1220-1226.
23. Sever PS, Poulter NR, Dahlöf B, et al. Reduction in cardiovascular events with atorvastatin in 2,532 patients with type 2 diabetes: Anglo-Scandinavian Cardiac Outcomes Trial—lipid-lowering arm (ASCOT-LLA). Diabetes Care. 2005;28:1151-1157.
24. Colhoun HM, Betteridge DJ, Durrington PN, et al; CARDS investigators. Primary prevention of cardiovascular disease with atorvastatin in type 2 diabetes in the Collaborative Atorvastatin Diabetes Study (CARDS): multicentre randomized placebo-controlled trial. Lancet. 2004;364:685-696.
25. Brunzell JD, Davidson M, Furberg CD, et al; American Diabetes Association; American College of Cardiology Foundation. Lipoprotein management in patients with cardiometabolic risk: consensus statement from the American Diabetes Association and the American College of Cardiology Foundation. Diabetes Care. 2008;31:811-822.
26. Stone NJ, Robinson JG, Lichtenstein AH, et al. 2013 ACC/AHA Guideline on the Treatment of Blood Cholesterol to Reduce Atherosclerotic Cardiovascular Risk in Adults: A Report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2014;63(25 pt B):2889-2934.
1. James PA, Oparil S, Carter BL, et al. 2014 evidence-based guideline for the management of high blood pressure in adults: report from the panel members appointed to the Eighth Joint National Committee (JNC 8). JAMA. 2014;311:507-520.
2. The Diabetes Control and Complications Trial Research Group. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. The Diabetes Control and Complications Trial Research Group. N Engl J Med. 1993;329:977-986.
3. King P, Peacock I, Donnelly R. The UK Prospective Diabetes Study (UKPDS): clinical and therapeutic implications for type 2 diabetes. Br J Clin Pharmacol. 1999;48:643-648.
4. Holman RR, Paul SK, Bethel MA, et al. 10-year follow-up of intensive glucose control in type 2 diabetes. N Engl J Med. 2008;359:1577-1589.
5. Gerstein HC, Miller ME, Byington RP, et al; Action to Con- trol Cardiovascular Risk in Diabetes Study Group. Effects of intensive glucose lowering in type 2 diabetes. N Engl J Med. 2008;358:2545-2559.
6. Ismail-Beigi F, Craven T, Banerji MA, et al; ACCORD Trial Group. Effect of intensive treatment of hyperglycaemia on microvascular outcomes in type 2 diabetes: an analysis of the ACCORD randomised trial. Lancet. 2010;376:419-430.
7. Gerstein HC, Miller ME, Genuth S, et al; ACCORD Study Group. Long-term effects of intensive glucose lowering on cardiovascular outcomes. N Engl J Med. 2011;364:818-828.
8. Bonds DE, Miller ME, Bergenstal RM, et al. The association between symptomatic, severe hypoglycaemia and mortality in type 2 diabetes: retrospective epidemiological analysis of the ACCORD study. BMJ. 2010;340:b4909.
9. Duckworth W, Abraira C, Moritz T, et al; VADT Investigators. Glucose control and vascular complications in veterans with type 2 diabetes. N Engl J Med. 2009;360:129-139.
10. Patel A, MacMahon S, Chalmers J, et al; ADVANCE Collab- orative Group. Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes. N Engl J Med. 2008;358:2560-2572.
11. American Diabetes Association. Standards of medical care in diabetes—2013. Diabetes Care. 2013;36(suppl 1):S11-S66.
12. International Diabetes Foundation Clinical Guidelines Task Force. Global Guideline for Type 2 Diabetes. International Diabetes Federation. Brussels, Belgium; 2012.
13. Handelsman Y, Mechanick JI, Blonde L, et al; AACE Task Force for Developing Diabetes Comprehensive Care Plan. Ameri- can Association of Clinical Endocrinologists Medical Guidelines for clinical practice for developing a diabetes mellitus comprehensive care plan: executive summary. Endocr Pract. 2011;17(suppl 2):S1-S53
14. Chobanian AV, Bakris GL, Black HR, et al; National Heart, Lung, and Blood Institute Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure; National High Blood Pressure Education Program Coordinating Committee. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC 7 report. JAMA. 2003;289:2560-2572.
15. UK Prospective Diabetes Study Group. Tight blood pressure control and risk of macrovascular and microvascular complications in type 2 diabetes: UKPDS 38. UK Prospective Diabetes Study Group. BMJ. 1998;317:703-713.
16. Adler AI, Stratton IM, Neil HA, et al. Association of systolic blood pressure with macrovascular and microvascular complications of type 2 diabetes (UKPDS 36): prospective observational study. BMJ. 2000;321:412-419.
17. Cushman WC, Evans GW, Byington RP, et al; ACCORD Study Group. Effects of intensive blood-pressure control in type 2 diabetes mellitus. N Engl J Med. 2010;362:1575-1585.
18. Cooper-DeHoff RM, Gong Y, Handberg EM, et al. Tight blood pressure control and cardiovascular outcomes among hypertensive patients with diabetes and coronary artery disease. JAMA. 2010;304:61-68.
19. Pyörälä K, Pedersen TR, Kjekshus J, et al. Cholesterol lowering with simvastatin improves prognosis of diabetic patients with coronary heart disease. A subgroup analysis of the Scandinavian Simvastatin Survival Study (4S). Diabetes Care. 1997;20:614-620.
20. Goldberg RB, Mellies MJ, Sacks FM, et al; The Care Investigators. Cardiovascular events and their reduction with pravastatin in diabetic and glucose-intolerant myocardial infarction survivors with average cholesterol levels: subgroup analyses in the cholesterol and recurrent events (CARE) trial. The Care Investigators. Circulation. 1998;98:2513-2519.
21. Collins R, Armitage J, Parish S, et al; Heart Protection Study Collaborative Group. MRC/BHF Heart Protection Study of cholesterol-lowering with simvastatin in 5963 people with diabetes: a randomised placebo-controlled trial. Lancet. 2003;361:2005-2016.
22. Shepherd J, Barter P, Carmena R, et al. Effect of lowering LDL cholesterol substantially below currently recommended levels in patients with coronary heart disease and diabetes: the Treating to New Targets (TNT) study. Diabetes Care. 2006;29:1220-1226.
23. Sever PS, Poulter NR, Dahlöf B, et al. Reduction in cardiovascular events with atorvastatin in 2,532 patients with type 2 diabetes: Anglo-Scandinavian Cardiac Outcomes Trial—lipid-lowering arm (ASCOT-LLA). Diabetes Care. 2005;28:1151-1157.
24. Colhoun HM, Betteridge DJ, Durrington PN, et al; CARDS investigators. Primary prevention of cardiovascular disease with atorvastatin in type 2 diabetes in the Collaborative Atorvastatin Diabetes Study (CARDS): multicentre randomized placebo-controlled trial. Lancet. 2004;364:685-696.
25. Brunzell JD, Davidson M, Furberg CD, et al; American Diabetes Association; American College of Cardiology Foundation. Lipoprotein management in patients with cardiometabolic risk: consensus statement from the American Diabetes Association and the American College of Cardiology Foundation. Diabetes Care. 2008;31:811-822.
26. Stone NJ, Robinson JG, Lichtenstein AH, et al. 2013 ACC/AHA Guideline on the Treatment of Blood Cholesterol to Reduce Atherosclerotic Cardiovascular Risk in Adults: A Report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2014;63(25 pt B):2889-2934.
Strategies to help reduce hospital readmissions
› Use risk stratification methods such as the Probability of Repeated Admission (Pra) or the LACE index to identify patients at high risk for readmission. B
› Take steps to ensure that follow-up appointments are made within the first one to 2 weeks of discharge, depending on the patient’s risk of readmission. C
› Reconcile preadmission and postdischarge medications to identify discrepancies and possible interactions. B
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
CASE › Charles T, age 74, has a 3-year history of myocardial infarction (MI) and congestive heart failure (CHF) and a 10-year his-tory of type 2 diabetes with retinopathy. You have cared for him in the outpatient setting for 8 years. You are notified that he is in the emergency department (ED) and being admitted to the hospital, again. This is his third ED visit in the past 3 months; he was hospitalized for 6 days during his last admission 3 weeks ago.
What should you do with this information? How can you best communicate with the admitting team?
Hospital readmissions are widespread, costly, and often avoidable. Nearly 20% of Medicare beneficiaries discharged from hospitals are rehospitalized within 30 days, and 34% are rehospitalized within 90 days.1 For patients with conditions like CHF, the rate of readmission within 30 days approaches 25%.2 The estimated cost to Medicare for unplanned rehospitalizations in 2004 was $17.4 billion.1 The Centers for Medicare and Medicaid Services penalizes hospitals for high rates of readmission within 30 days of discharge for patients with CHF, MI, and pneumonia.
“Avoidable” hospitalizations are those that may be prevented by effective outpatient management and improved care coordination. Although efforts to reduce readmissions have focused on improving the discharge process, family physicians (FPs) can play a central role in reducing readmissions. This article describes key approaches that FPs can take to address this important issue. Because patients ages ≥65 years consistently have the highest rate of hospital readmissions,1 we will focus on this population.
Multiple complex factors are associated with hospital readmissions
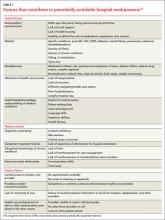
Characteristics of the patient, physician, and health care setting contribute to potentially avoidable readmissions (TABLE 1).3,4
Medical conditions and comorbidities associated with high rates of rehospitalization include CHF, acute MI, pneumonia, diabetes, and chronic obstructive pulmonary disease. However, a recent study found that a diverse range of conditions, frequently differing from the index cause of hospitalization, were responsible for 30-day readmissions of Medicare patients.5
Identifying those at high risk: Why and how
Determining which patients are at highest risk for readmission enables health care teams to match the intensity of interventions to the individual’s likelihood of readmission. However, current readmission risk prediction models remain a work in progress6 and few models have been tested in the outpatient setting. Despite numerous limitations, it’s still important to focus resources more efficiently. Thus, we recommend using risk stratification tools to identify patients at high risk for readmission.
Many risk stratification methods use data from electronic medical records (EMRs) and administrative databases or self-reported data from patients.7 Risk prediction tools that are relatively simple and easy to administer or generate through EMRs—such as the Probability of Repeated Admission (Pra),8 the LACE (Length of stay, acuity of the admission, comorbidities, ED visits in the previous 6 months) index,9 or the Community Assessment Risk Screen (CARS)10—may be best for use in the primary care setting. These tools generally identify key risk factors, such as prior health care utilization, presence of specific conditions such as heart disease or cognitive impairment, self-reported health status, absence of a caregiver, and/or need for assistance with daily routines.
Many of these tools have been used to identify high-risk older adults and may not be appropriate for patients who are likely to be readmitted for different reasons, such as mental illness, substance abuse, or chronic pain. Therefore, it is important to use a risk stratification method that captures the issues most likely to cause readmissions in your patient population, or to consider using a variety of methods.
The American Academy of Family Physicians (AAFP) offers resources to help FPs design methods for determining a patient’s health risk status and linking higher levels of risk to increasing care management at http://www.aafp.org/practice-management/pcmh/initiatives/cpci/rscm.html.
CASE › Mr. T has been admitted to the hospital 3 times in the past 3 months, so you use the lace index to evaluate his risk. You determine that Mr. T’s score is 15, which means his expected risk of death or unplanned readmission is 26.6% (TABLE 2).8,11 What are your next steps?
Foster communication between the hospital and outpatient office
Patients are particularly vulnerable during the transition from hospital to home. Delayed or inaccurate information adversely affects continuity of care, patient safety and satisfaction, and efficient use of resources.12 Discharge summaries are the main method of communication between providers, but their content, timeliness, availability, and quality frequently are lacking.13 Discharge summaries are available at only 12% to 34% of first postdischarge visits, and these summaries often lack important information such as diagnostic test results (33%-63%) or discharge medications (2%-40%).12 Although researchers have not consistently found that transferring a discharge summary to an outpatient physician reduces readmission rates, it is likely that direct communication can improve the handoff process independent of its effects on readmissions.12,14
Timely follow-up appointments are essential
Many factors influence the need for rapid follow-up, including disease severity, management complexity, ability of the patient to provide sufficient self-care, and adequacy of social supports.15,16 Studies have found that discharged patients who receive timely outpatient follow-up are less likely to be readmitted.1,17 While the optimal time interval between discharge and the first follow-up appointment is unknown, some literature supports follow-up within 4 weeks.15,18 However, because readmissions often cluster in the first several days or week following discharge,18 follow-up within the first 2 weeks (and within the first week for higher-risk patients) may be appropriate.19 Ideally, follow-up appointments should be scheduled before the patient is discharged. Patients who schedule a follow-up appointment before they are discharged are more likely to make their follow-up visit than those who are asked to call after discharge and schedule their own appointment.12
Employ outpatient follow-up alternatives
Follow-up telephone calls to patients after discharge help patients understand and adhere to discharge instructions and troubleshoot problems. Clinicians who use scripted telephone calls can evaluate symptoms related to the index hospitalization, provide patient education, schedule relevant appointments or testing, and, most importantly, initiate medication reconciliation, which is described at right.20 The FIGURE includes the script we use at our practice.
Home visits may be appropriate for certain patients, including the frail elderly. Home visits allow clinicians to evaluate the patient’s environmental safety, social sup port, and medication adherence.12 Preventive home visits generally have not been found to reduce hospital readmissions, but do enhance patient satisfaction with care.21
Bundled interventions, such as alternating home visits and follow-up telephone calls, may be more effective than individual interventions in reducing readmission.22
Reconciling medications may have far-reaching benefits
Medication discrepancies are observed in up to 70% of all patients at admission or discharge and are associated with adverse drug events (ADEs).23 To prevent ADEs and possibly readmission, take the following steps to reconcile a patient’s medications23:
Obtain a complete list of current medications. Information on all of the patient’s prescription and nonprescription medications should be collected from the patient/caregiver, the discharge summary, prescription bottles, home visits, and pharmacies.12,24
Reconcile preadmission and postdischarge medications. Clarify any discrepancies, review all medications for safety and appropriateness, and, when appropriate, resume any held medications and/or discontinue unnecessary ones.
Research shows that patients who received a phone call from a pharmacist within 3 to 7 days of discharge had lower readmission rates.Enlist pharmacy support. Pharmacists are uniquely positioned to review indications as well as potential duplication and interactions of a patient’s medications. Inpatient studies have demonstrated that partnering with pharmacists results in fewer ADEs.12,25 One study showed that patients at high risk for readmission who received a phone call from a pharmacist 3 to 7 days after discharge had lower readmission rates.26 The pharmacist reconciled the patients’ medications and ensured that patients had a clear understanding of each medication, its common safety concerns, and how often they were supposed to take it.26
Make medication adherence as easy as possible
As many as half of all patients don’t take their medications as prescribed.27 There is limited data on health outcomes associated with medication nonadherence, and existing data frequently are contradictory—some studies have found that as many as 11% of hospital admissions are attributed to nonadherence, while others show no association.28
Factors that affect adherence include psychiatric or cognitive impairment, limited insight into disease process or lack of belief in benefit of treatment, medication cost or adverse effect profile, poor provider-patient relationship, limited access to care or medication, or complexity of treatment.29 To promote medication adherence, consider the following educational and behavioral strategies30:
Identify patients at risk for nonadherence. This includes those with complex regimens and/or uncontrolled disease states or symptoms.
Increase patient communication and counseling. Patient education, particularly on the importance of adherence, is one of the few solo interventions that can improve compliance.31 Involving caregivers and using both verbal and written materials provides additional benefit.31,32
Simplify dosing schedules. Simple, convenient medication regimens may im- prove adherence. For example, adjusting dosing from 3 times a day to once a day can increase adherence from 59% to 83%.33 Aids such as pillboxes to organize medications may be of benefit.29,32
Ensure consistent follow-up. Patients who miss appointments are more likely to be nonadherent. They may benefit from easy access, help with scheduling, and frequent visits.32
Be mindful of patients’ out-of-pocket expenses. Reducing copayments improves adherence rates.30
Minimize polypharmacy. Polypharmacy has been independently associated with nonadherence and increased risk for ADEs.34
Identify patients who have limited health literacy. Limited health literacy may be linked to increased medication errors and nonadherence.12,35 Patients with low health literacy may be unable to identify medications recorded in their medical record. TABLE W336-41 outlines strategies for identifying patients with low health literacy and improving communication with them.
CASE › By speaking with hospital staff before Mr. T is discharged, you are able to confirm that he has scheduled a follow-up visit with you for one week after discharge, and that a discharge summary will be available for him to bring to that visit. Mr. T brings his discharge summary with him to your office, and you reconcile his medication list. Because he is your last patient of the day, you have some time to sit with him and his wife to explore his goals of care.
Improve care—and possibly reduce readmissions—through goal setting
Goal setting is an important element of postdischarge follow-up, particularly for elderly patients and those with progressive or end-stage diseases. Goal setting can improve patient care by linking care plans with desired outcomes and keeping diagnostic and therapeutic interventions relevant to the patient.42 A patient who understands the purpose of a recommendation—especially when directly linked to a patient-derived goal—may be more likely to adhere to the plan of care.
Asking patients to articulate their goals of care using “Ask-Tell-Ask” framework described in TABLE W336-41 will allow you to deliver the prognosis, reinforce treatment options to achieve patient-specific goals, empower patients to assert their preferences, and develop a follow-up plan to see if treatment is successful.
Empowering patients
Consider using both verbal and written approaches when educating patients about self-care behaviors such as monitoring symptoms and adhering to dietary/behavior restrictions and medication instructions. One study showed that a brief one-on-one patient education session decreased readmissions in patients with heart failure,43 although another study found that patient education alone yielded a nonsignificant decrease.44
Providing caregivers with education and support is a critical and perhaps overlooked opportunity to reduce readmissions.45 Involving key family members in discharge planning, preparation, follow-up, and ongoing management is essential in caring for patients with functional deficits and/or complex care needs. Educating caregivers can help them feel more prepared and effective in their roles.
Establish an “action plan.” For patients with chronic, periodically symptomatic diseases such as asthma and heart failure, action planning can be useful. Action plans should include information that reinforces patients’ daily self-care behaviors and instructions for what to do if symptoms get worse. Action planning also might include simple if-then plans (“if x happens, then I will do y”), which can help with problem solving for common scenarios. Action plans have been shown to reduce admissions for children with asthma46 and adults with heart failure when coupled with home monitoring or telephone support from a registered nurse.16,47
Generate an individualized care plan for each patient, taking into account your patient’s health literacy, goals of care, and level of social support. This care plan may include educational and behavioral interventions, action planning, and follow-up plans. Most successful approaches to reducing readmissions have included both system-level and patient-level interventions that use an interdisciplinary team of providers.48
Make the most of follow-up visits. The traditional 15-minute FP visit can make it challenging to provide the level of care necessary for recently discharged patients. Multiple models of team-based care have been proposed to improve this situation, including using the “teamlet” model, which may include a clinician and one or 2 health coaches.49 During each visit, the health coaches—often medical assistants trained in chronic disease self-management skills—see patients before and after the physician. They also contact patients be- tween visits to facilitate action planning and to promote self-management.
Palliative care programs: A resource for FPs
The growth of palliative care programs in US hospitals has helped increase the emphasis on establishing goals of care. Inpatient-based palliative care consultation programs work with patients and families to establish goals. However, after discharge, many of these goals and plans begin to unravel due to gaps in the current health care model, including lack of follow-up and support.50 Outpatient palliative care programs have begun to address these gaps in care.50 Comprehensive palliative care programs are quickly becoming an important resource for FPs to help address transitional care issues.
CASE › When you ask Mr. and Mrs. T about his goals for treatment, they say are getting tired of the “back and forth” to the hospital. After discussing his lengthy history of worsening CHF and diabetes, you raise the idea of palliative care, including hospice, with the couple. They acknowledge that they have had family members get hospice care, and they are open to it—just not yet. The 3 of you craft an “if-then” plan of care to use at home. You schedule a 2-week follow-up visit and remind Mr. T and his wife of your office’s 24-hour on-call service.
CORRESPONDENCE
Danielle Snyderman, MD, Department of Family and Community Medicine, Jefferson University, 1015 Walnut Street, Suite 401, Philadelphia, Pa 19107; [email protected]
1. Jencks SF, Williams MV, Coleman EA. Rehospitalizations among patients in the Medicare fee-for-service program. N Engl J Med. 2009;360:1418-1428
2. O’Connor CM, Miller AB, Blair JE, et al; Efficacy of Vasopressin Antagonism in heart Failure Outcome Study with Tolvaptan (EVEREST) investigators. Causes of death and rehospitalization in patients hospitalized with worsening heart failure and reduce left ventricular ejection fraction; results from EVEREST program. Am Heart J. 2010;159:841-849.e1.
3. Garrison GM, Mansukhani MP, Bohn B. Predictors of thirty-day readmission among hospitalized family medicine patients. J Am Board Fam Med. 2013;26:71-77.
4. Boult C, Dowd B, McCaffrey D, et al. Screening elders for risk of hospital admission. J Am Geriatr Soc. 1993;41:811-817.
5. Dharmarajan K, Hsieh AF, Lin Z, et al. Diagnoses and timing of 30-day readmissions after hospitalization for heart failure, acute myocardial infarction, or pneumonia. JAMA. 2013;309:355-363.
6. Kansagara D, Englander H, Salanitro A, et al. Risk prediction models for hospital readmission: a systematic review. JAMA. 2011;306:1688-1698.
7. Haas LR, Takahashi PY, Shah ND, et al. Risk-stratification methods for identifying patients for care coordination. Am J Manag Care. 2013;19:725-732.
8. Wallace E, Hinchey T, Dimitrov BD, et al. A systematic review of the probability of repeated admission score in community-dwelling adults. J Am Geriatr Soc. 2013;61:357-364.
9. Cotter PE, Bhalla VK, Wallis SJ, et al. Predicting readmissions: poor performance of the LACE index in an older UK population. Age Ageing. 2012;41:784-789.
10. Shelton P, Sager MA, Schraeder C. The community assessment risk screen (CARS): identifying elderly persons at risk for hospitalization or emergency department visit. Am J Manag Care. 2000;6:925-933.
11. Charlson ME, Pompei P, Ales KL, et al. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chron Dis. 1987;40:373-383.
12. Kripalani S, Jackson AT, Schnipper JL, et al. Promoting effective transitions of care at hospital discharge: a review of key issues for hospitalists. J Hosp Med. 2007;2:314-323.
13. Kim CS, Flanders SA. In the clinic. Transitions of care. Ann Intern Med. 2013;158(5 pt 1):ITC3-1.
14. Hansen LO, Strater A, Smith L, et al. Hospital discharge documentation and risk of rehospitalisation. BMJ Qual Saf. 2011;20:773-778.
15. Vaduganathan M, Bonow RO, Gheorghiade M. Thirty-day readmissions: the clock is ticking. JAMA. 2013;309:345-346.
16. Hansen LO, Young RS, Hinami K, et al. Interventions to reduce 30-day rehospitalization: a systematic review. Ann Intern Med. 2011;155:520-528.
17. Misky GJ, Wald HL, Coleman EA. Post-hospitalization transitions: Examining the effects of timing of primary care provider follow-up. J Hosp Med. 2010;5:392-397.
18. van Walraven C, Jennings A, Taljaard M, et al. Incidence of potentially avoidable urgent readmissions and their relation to all-cause urgent readmissions. CMAJ. 2011;183:E1067-E1072.
19. Tang, N. A primary care physician’s ideal transitions of care—where’s the evidence? J Hosp Med. 2013;8:472-477.
20. Crocker JB, Crocker JT, Greenwald JL. Telephone follow-up as a primary care intervention for postdischarge outcomes improvement: a systematic review. Am J Med. 2012;125:915-921.
21. Wong FK, Chow S, Chung L, et al. Can home visits help reduce hospital readmissions? Randomized controlled trial. J Adv Nurs. 2008;62:585-595.
22. Wong FK, Chow SK, Chan TM, et al. Comparison of effects between home visits with telephone calls and telephone calls only for transitional discharge support: a randomised controlled trial. Age Ageing. 2014;43:91-97.
23. Mueller SK, Sponsler KC, Kripalani S, et al. Hospital-based medication reconciliation practices: a systematic review. Arch Intern Med. 2012;172:1057-1069.
24. Glintborg B, Andersen SE, Dalhoff K. Insufficient communication about medication use at the interface between hospital and primary care. Qual Saf Health Care. 2007;16:34-39.
25. Schnipper JL, Kirwin JL, Cotugno MC, et al. Role of pharmacist counseling in preventing adverse drug events after hospitalization. Arch Intern Med. 2006;166:565-571.
26. Kilcup M, Schultz D, Carlson J, et al. Postdischarge pharmacist medication reconciliation: impact on readmission rates and financial savings. J Am Pharm Assoc (2003). 2013;53:78-84.
27. Vermeire E, Hearnshaw H, Van Royen P, et al. Patient adherence to treatment: three decades of research. A comprehensive review. J Clin Pharm Ther. 2001;26:331-342.
28. Vik SA, Maxwell CJ, Hogan DB. Measurement, correlates, and health outcomes of medication adherence among seniors. Ann Pharmacother. 2004;38:303-312.
29. Osterberg L, Blaschke T. Adherence to medication. N Engl J Med. 2005;353:487-497.
30. Viswanathan M, Golin CE, Jones CD, et al. Interventions to improve adherence to self-administered medications for chronic diseases in the United States: a systematic review. Ann Intern Med. 2012;157:785-795.
31. McDonald HP, Garg AX, Haynes RB. Interventions to enhance patient adherence to medication prescriptions: scientific review. JAMA. 2002;288:2868-2879.
32. Kripalani S, Yao X, Haynes RB. Interventions to enhance medication adherence in chronic medical conditions: a systematic review. Arch Intern Med. 2007;167:540-550.
33. Eisen SA, Miller DK, Woodward RS, et al. The effect of prescribed daily dose frequency on patient medication compliance. Arch Intern Med. 1990;150:1881-1884.
34. Field TS, Gurwitz JH, Avorn J, et al. Risk factors for adverse drug events among nursing home residents. Arch Intern Med. 2001;161:1629-1634.
35. Persell SD, Osborn CY, Richard R, et al. Limited health literacy is a barrier to medication reconciliation in ambulatory care. J Gen Intern Med. 2007;22:1523-1526.
36. Weiss BD. Health Literacy and Patient Safety: Help Patients Understand. Manual for Clinicians. Chicago, IL: American Medical Association Foundation; 2007.
37. Chew LD, Bradley KA, Bokyo EJ. Brief questions to identify patients with inadequate health literacy. Fam Med. 2004;36:588-594.
38. Wallace LS, Rogers ES, Roskos SE, et al. Brief report: screening items to identify patients with limited health literacy skills. J Gen Intern Med. 2006;21:874-877.
39. Doak CC, Doak LG, Root JH. Teaching Patients with Low Literacy Skills. 2nd ed. Philadelphia, PA: JB Lippincott Company; 1996.
40. Back AL, Arnold RM, Baile WF, et al. Approaching difficult communication tasks in oncology. CA Cancer J Clin. 2005;55: 164-177.
41. Doak LG, Doak CC, eds. Pfizer Principles for Clear Health Communication: A Handbook for Creating Patient Education Materials that Enhance Understanding and Promote Health Outcomes. 2nd ed. New York, NY: Pfizer; 2004.
42. Bradley EH, Bogardus ST Jr, Tinetti M, et al. Goal-setting in clinical medicine. Soc Sci Med. 1999;49:267-278.
43. Koelling TM, Johnson ML, Cody RJ, et al. Discharge education improves clinical outcomes in patients with chronic heart failure. Circulation. 2005;111:179-185.
44. Krumholz HM, Amatruda J, Smith GL, et al. Randomized trial of an education and support intervention to prevent readmission of patients with heart failure. J Am Coll Cardiol. 2002;39:83-89.
45. Burke RE, Coleman EA. Interventions to decrease hospital readmissions: keys for cost-effectiveness. JAMA Intern Med. 2013;173:695-698.
46. Kessler KR. Relationship between the use of asthma action plans and asthma exacerbations in children with asthma: A systematic review. J Asthma Allergy Educators. 2011;2:11-21.
47. Maric B, Kaan A, Ignaszewski A, et al. A systematic review of telemonitoring technologies in heart failure. Eur J Heart Fail. 2009;11:506-517.
48. Boutwell A, Hwu S. Effective Interventions to Reduce Rehospitalizations: A Survey of the Published Evidence. Cambridge, MA: Institute for Healthcare Improvement; 2009.
49. Bodenheimer T, Laing BY. The teamlet model of primary care. Ann Fam Med. 2007;5:457-461.
50. Meier D, Beresford L. Outpatient clinics are a new frontier for palliative care. J Pall Med. 2008;11:823-828.
› Use risk stratification methods such as the Probability of Repeated Admission (Pra) or the LACE index to identify patients at high risk for readmission. B
› Take steps to ensure that follow-up appointments are made within the first one to 2 weeks of discharge, depending on the patient’s risk of readmission. C
› Reconcile preadmission and postdischarge medications to identify discrepancies and possible interactions. B
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
CASE › Charles T, age 74, has a 3-year history of myocardial infarction (MI) and congestive heart failure (CHF) and a 10-year his-tory of type 2 diabetes with retinopathy. You have cared for him in the outpatient setting for 8 years. You are notified that he is in the emergency department (ED) and being admitted to the hospital, again. This is his third ED visit in the past 3 months; he was hospitalized for 6 days during his last admission 3 weeks ago.
What should you do with this information? How can you best communicate with the admitting team?
Hospital readmissions are widespread, costly, and often avoidable. Nearly 20% of Medicare beneficiaries discharged from hospitals are rehospitalized within 30 days, and 34% are rehospitalized within 90 days.1 For patients with conditions like CHF, the rate of readmission within 30 days approaches 25%.2 The estimated cost to Medicare for unplanned rehospitalizations in 2004 was $17.4 billion.1 The Centers for Medicare and Medicaid Services penalizes hospitals for high rates of readmission within 30 days of discharge for patients with CHF, MI, and pneumonia.
“Avoidable” hospitalizations are those that may be prevented by effective outpatient management and improved care coordination. Although efforts to reduce readmissions have focused on improving the discharge process, family physicians (FPs) can play a central role in reducing readmissions. This article describes key approaches that FPs can take to address this important issue. Because patients ages ≥65 years consistently have the highest rate of hospital readmissions,1 we will focus on this population.
Multiple complex factors are associated with hospital readmissions
Characteristics of the patient, physician, and health care setting contribute to potentially avoidable readmissions (TABLE 1).3,4
Medical conditions and comorbidities associated with high rates of rehospitalization include CHF, acute MI, pneumonia, diabetes, and chronic obstructive pulmonary disease. However, a recent study found that a diverse range of conditions, frequently differing from the index cause of hospitalization, were responsible for 30-day readmissions of Medicare patients.5
Identifying those at high risk: Why and how
Determining which patients are at highest risk for readmission enables health care teams to match the intensity of interventions to the individual’s likelihood of readmission. However, current readmission risk prediction models remain a work in progress6 and few models have been tested in the outpatient setting. Despite numerous limitations, it’s still important to focus resources more efficiently. Thus, we recommend using risk stratification tools to identify patients at high risk for readmission.
Many risk stratification methods use data from electronic medical records (EMRs) and administrative databases or self-reported data from patients.7 Risk prediction tools that are relatively simple and easy to administer or generate through EMRs—such as the Probability of Repeated Admission (Pra),8 the LACE (Length of stay, acuity of the admission, comorbidities, ED visits in the previous 6 months) index,9 or the Community Assessment Risk Screen (CARS)10—may be best for use in the primary care setting. These tools generally identify key risk factors, such as prior health care utilization, presence of specific conditions such as heart disease or cognitive impairment, self-reported health status, absence of a caregiver, and/or need for assistance with daily routines.
Many of these tools have been used to identify high-risk older adults and may not be appropriate for patients who are likely to be readmitted for different reasons, such as mental illness, substance abuse, or chronic pain. Therefore, it is important to use a risk stratification method that captures the issues most likely to cause readmissions in your patient population, or to consider using a variety of methods.
The American Academy of Family Physicians (AAFP) offers resources to help FPs design methods for determining a patient’s health risk status and linking higher levels of risk to increasing care management at http://www.aafp.org/practice-management/pcmh/initiatives/cpci/rscm.html.
CASE › Mr. T has been admitted to the hospital 3 times in the past 3 months, so you use the lace index to evaluate his risk. You determine that Mr. T’s score is 15, which means his expected risk of death or unplanned readmission is 26.6% (TABLE 2).8,11 What are your next steps?
Foster communication between the hospital and outpatient office
Patients are particularly vulnerable during the transition from hospital to home. Delayed or inaccurate information adversely affects continuity of care, patient safety and satisfaction, and efficient use of resources.12 Discharge summaries are the main method of communication between providers, but their content, timeliness, availability, and quality frequently are lacking.13 Discharge summaries are available at only 12% to 34% of first postdischarge visits, and these summaries often lack important information such as diagnostic test results (33%-63%) or discharge medications (2%-40%).12 Although researchers have not consistently found that transferring a discharge summary to an outpatient physician reduces readmission rates, it is likely that direct communication can improve the handoff process independent of its effects on readmissions.12,14
Timely follow-up appointments are essential
Many factors influence the need for rapid follow-up, including disease severity, management complexity, ability of the patient to provide sufficient self-care, and adequacy of social supports.15,16 Studies have found that discharged patients who receive timely outpatient follow-up are less likely to be readmitted.1,17 While the optimal time interval between discharge and the first follow-up appointment is unknown, some literature supports follow-up within 4 weeks.15,18 However, because readmissions often cluster in the first several days or week following discharge,18 follow-up within the first 2 weeks (and within the first week for higher-risk patients) may be appropriate.19 Ideally, follow-up appointments should be scheduled before the patient is discharged. Patients who schedule a follow-up appointment before they are discharged are more likely to make their follow-up visit than those who are asked to call after discharge and schedule their own appointment.12
Employ outpatient follow-up alternatives
Follow-up telephone calls to patients after discharge help patients understand and adhere to discharge instructions and troubleshoot problems. Clinicians who use scripted telephone calls can evaluate symptoms related to the index hospitalization, provide patient education, schedule relevant appointments or testing, and, most importantly, initiate medication reconciliation, which is described at right.20 The FIGURE includes the script we use at our practice.
Home visits may be appropriate for certain patients, including the frail elderly. Home visits allow clinicians to evaluate the patient’s environmental safety, social sup port, and medication adherence.12 Preventive home visits generally have not been found to reduce hospital readmissions, but do enhance patient satisfaction with care.21
Bundled interventions, such as alternating home visits and follow-up telephone calls, may be more effective than individual interventions in reducing readmission.22
Reconciling medications may have far-reaching benefits
Medication discrepancies are observed in up to 70% of all patients at admission or discharge and are associated with adverse drug events (ADEs).23 To prevent ADEs and possibly readmission, take the following steps to reconcile a patient’s medications23:
Obtain a complete list of current medications. Information on all of the patient’s prescription and nonprescription medications should be collected from the patient/caregiver, the discharge summary, prescription bottles, home visits, and pharmacies.12,24
Reconcile preadmission and postdischarge medications. Clarify any discrepancies, review all medications for safety and appropriateness, and, when appropriate, resume any held medications and/or discontinue unnecessary ones.
Research shows that patients who received a phone call from a pharmacist within 3 to 7 days of discharge had lower readmission rates.Enlist pharmacy support. Pharmacists are uniquely positioned to review indications as well as potential duplication and interactions of a patient’s medications. Inpatient studies have demonstrated that partnering with pharmacists results in fewer ADEs.12,25 One study showed that patients at high risk for readmission who received a phone call from a pharmacist 3 to 7 days after discharge had lower readmission rates.26 The pharmacist reconciled the patients’ medications and ensured that patients had a clear understanding of each medication, its common safety concerns, and how often they were supposed to take it.26
Make medication adherence as easy as possible
As many as half of all patients don’t take their medications as prescribed.27 There is limited data on health outcomes associated with medication nonadherence, and existing data frequently are contradictory—some studies have found that as many as 11% of hospital admissions are attributed to nonadherence, while others show no association.28
Factors that affect adherence include psychiatric or cognitive impairment, limited insight into disease process or lack of belief in benefit of treatment, medication cost or adverse effect profile, poor provider-patient relationship, limited access to care or medication, or complexity of treatment.29 To promote medication adherence, consider the following educational and behavioral strategies30:
Identify patients at risk for nonadherence. This includes those with complex regimens and/or uncontrolled disease states or symptoms.
Increase patient communication and counseling. Patient education, particularly on the importance of adherence, is one of the few solo interventions that can improve compliance.31 Involving caregivers and using both verbal and written materials provides additional benefit.31,32
Simplify dosing schedules. Simple, convenient medication regimens may im- prove adherence. For example, adjusting dosing from 3 times a day to once a day can increase adherence from 59% to 83%.33 Aids such as pillboxes to organize medications may be of benefit.29,32
Ensure consistent follow-up. Patients who miss appointments are more likely to be nonadherent. They may benefit from easy access, help with scheduling, and frequent visits.32
Be mindful of patients’ out-of-pocket expenses. Reducing copayments improves adherence rates.30
Minimize polypharmacy. Polypharmacy has been independently associated with nonadherence and increased risk for ADEs.34
Identify patients who have limited health literacy. Limited health literacy may be linked to increased medication errors and nonadherence.12,35 Patients with low health literacy may be unable to identify medications recorded in their medical record. TABLE W336-41 outlines strategies for identifying patients with low health literacy and improving communication with them.
CASE › By speaking with hospital staff before Mr. T is discharged, you are able to confirm that he has scheduled a follow-up visit with you for one week after discharge, and that a discharge summary will be available for him to bring to that visit. Mr. T brings his discharge summary with him to your office, and you reconcile his medication list. Because he is your last patient of the day, you have some time to sit with him and his wife to explore his goals of care.
Improve care—and possibly reduce readmissions—through goal setting
Goal setting is an important element of postdischarge follow-up, particularly for elderly patients and those with progressive or end-stage diseases. Goal setting can improve patient care by linking care plans with desired outcomes and keeping diagnostic and therapeutic interventions relevant to the patient.42 A patient who understands the purpose of a recommendation—especially when directly linked to a patient-derived goal—may be more likely to adhere to the plan of care.
Asking patients to articulate their goals of care using “Ask-Tell-Ask” framework described in TABLE W336-41 will allow you to deliver the prognosis, reinforce treatment options to achieve patient-specific goals, empower patients to assert their preferences, and develop a follow-up plan to see if treatment is successful.
Empowering patients
Consider using both verbal and written approaches when educating patients about self-care behaviors such as monitoring symptoms and adhering to dietary/behavior restrictions and medication instructions. One study showed that a brief one-on-one patient education session decreased readmissions in patients with heart failure,43 although another study found that patient education alone yielded a nonsignificant decrease.44
Providing caregivers with education and support is a critical and perhaps overlooked opportunity to reduce readmissions.45 Involving key family members in discharge planning, preparation, follow-up, and ongoing management is essential in caring for patients with functional deficits and/or complex care needs. Educating caregivers can help them feel more prepared and effective in their roles.
Establish an “action plan.” For patients with chronic, periodically symptomatic diseases such as asthma and heart failure, action planning can be useful. Action plans should include information that reinforces patients’ daily self-care behaviors and instructions for what to do if symptoms get worse. Action planning also might include simple if-then plans (“if x happens, then I will do y”), which can help with problem solving for common scenarios. Action plans have been shown to reduce admissions for children with asthma46 and adults with heart failure when coupled with home monitoring or telephone support from a registered nurse.16,47
Generate an individualized care plan for each patient, taking into account your patient’s health literacy, goals of care, and level of social support. This care plan may include educational and behavioral interventions, action planning, and follow-up plans. Most successful approaches to reducing readmissions have included both system-level and patient-level interventions that use an interdisciplinary team of providers.48
Make the most of follow-up visits. The traditional 15-minute FP visit can make it challenging to provide the level of care necessary for recently discharged patients. Multiple models of team-based care have been proposed to improve this situation, including using the “teamlet” model, which may include a clinician and one or 2 health coaches.49 During each visit, the health coaches—often medical assistants trained in chronic disease self-management skills—see patients before and after the physician. They also contact patients be- tween visits to facilitate action planning and to promote self-management.
Palliative care programs: A resource for FPs
The growth of palliative care programs in US hospitals has helped increase the emphasis on establishing goals of care. Inpatient-based palliative care consultation programs work with patients and families to establish goals. However, after discharge, many of these goals and plans begin to unravel due to gaps in the current health care model, including lack of follow-up and support.50 Outpatient palliative care programs have begun to address these gaps in care.50 Comprehensive palliative care programs are quickly becoming an important resource for FPs to help address transitional care issues.
CASE › When you ask Mr. and Mrs. T about his goals for treatment, they say are getting tired of the “back and forth” to the hospital. After discussing his lengthy history of worsening CHF and diabetes, you raise the idea of palliative care, including hospice, with the couple. They acknowledge that they have had family members get hospice care, and they are open to it—just not yet. The 3 of you craft an “if-then” plan of care to use at home. You schedule a 2-week follow-up visit and remind Mr. T and his wife of your office’s 24-hour on-call service.
CORRESPONDENCE
Danielle Snyderman, MD, Department of Family and Community Medicine, Jefferson University, 1015 Walnut Street, Suite 401, Philadelphia, Pa 19107; [email protected]
› Use risk stratification methods such as the Probability of Repeated Admission (Pra) or the LACE index to identify patients at high risk for readmission. B
› Take steps to ensure that follow-up appointments are made within the first one to 2 weeks of discharge, depending on the patient’s risk of readmission. C
› Reconcile preadmission and postdischarge medications to identify discrepancies and possible interactions. B
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
CASE › Charles T, age 74, has a 3-year history of myocardial infarction (MI) and congestive heart failure (CHF) and a 10-year his-tory of type 2 diabetes with retinopathy. You have cared for him in the outpatient setting for 8 years. You are notified that he is in the emergency department (ED) and being admitted to the hospital, again. This is his third ED visit in the past 3 months; he was hospitalized for 6 days during his last admission 3 weeks ago.
What should you do with this information? How can you best communicate with the admitting team?
Hospital readmissions are widespread, costly, and often avoidable. Nearly 20% of Medicare beneficiaries discharged from hospitals are rehospitalized within 30 days, and 34% are rehospitalized within 90 days.1 For patients with conditions like CHF, the rate of readmission within 30 days approaches 25%.2 The estimated cost to Medicare for unplanned rehospitalizations in 2004 was $17.4 billion.1 The Centers for Medicare and Medicaid Services penalizes hospitals for high rates of readmission within 30 days of discharge for patients with CHF, MI, and pneumonia.
“Avoidable” hospitalizations are those that may be prevented by effective outpatient management and improved care coordination. Although efforts to reduce readmissions have focused on improving the discharge process, family physicians (FPs) can play a central role in reducing readmissions. This article describes key approaches that FPs can take to address this important issue. Because patients ages ≥65 years consistently have the highest rate of hospital readmissions,1 we will focus on this population.
Multiple complex factors are associated with hospital readmissions
Characteristics of the patient, physician, and health care setting contribute to potentially avoidable readmissions (TABLE 1).3,4
Medical conditions and comorbidities associated with high rates of rehospitalization include CHF, acute MI, pneumonia, diabetes, and chronic obstructive pulmonary disease. However, a recent study found that a diverse range of conditions, frequently differing from the index cause of hospitalization, were responsible for 30-day readmissions of Medicare patients.5
Identifying those at high risk: Why and how
Determining which patients are at highest risk for readmission enables health care teams to match the intensity of interventions to the individual’s likelihood of readmission. However, current readmission risk prediction models remain a work in progress6 and few models have been tested in the outpatient setting. Despite numerous limitations, it’s still important to focus resources more efficiently. Thus, we recommend using risk stratification tools to identify patients at high risk for readmission.
Many risk stratification methods use data from electronic medical records (EMRs) and administrative databases or self-reported data from patients.7 Risk prediction tools that are relatively simple and easy to administer or generate through EMRs—such as the Probability of Repeated Admission (Pra),8 the LACE (Length of stay, acuity of the admission, comorbidities, ED visits in the previous 6 months) index,9 or the Community Assessment Risk Screen (CARS)10—may be best for use in the primary care setting. These tools generally identify key risk factors, such as prior health care utilization, presence of specific conditions such as heart disease or cognitive impairment, self-reported health status, absence of a caregiver, and/or need for assistance with daily routines.
Many of these tools have been used to identify high-risk older adults and may not be appropriate for patients who are likely to be readmitted for different reasons, such as mental illness, substance abuse, or chronic pain. Therefore, it is important to use a risk stratification method that captures the issues most likely to cause readmissions in your patient population, or to consider using a variety of methods.
The American Academy of Family Physicians (AAFP) offers resources to help FPs design methods for determining a patient’s health risk status and linking higher levels of risk to increasing care management at http://www.aafp.org/practice-management/pcmh/initiatives/cpci/rscm.html.
CASE › Mr. T has been admitted to the hospital 3 times in the past 3 months, so you use the lace index to evaluate his risk. You determine that Mr. T’s score is 15, which means his expected risk of death or unplanned readmission is 26.6% (TABLE 2).8,11 What are your next steps?
Foster communication between the hospital and outpatient office
Patients are particularly vulnerable during the transition from hospital to home. Delayed or inaccurate information adversely affects continuity of care, patient safety and satisfaction, and efficient use of resources.12 Discharge summaries are the main method of communication between providers, but their content, timeliness, availability, and quality frequently are lacking.13 Discharge summaries are available at only 12% to 34% of first postdischarge visits, and these summaries often lack important information such as diagnostic test results (33%-63%) or discharge medications (2%-40%).12 Although researchers have not consistently found that transferring a discharge summary to an outpatient physician reduces readmission rates, it is likely that direct communication can improve the handoff process independent of its effects on readmissions.12,14
Timely follow-up appointments are essential
Many factors influence the need for rapid follow-up, including disease severity, management complexity, ability of the patient to provide sufficient self-care, and adequacy of social supports.15,16 Studies have found that discharged patients who receive timely outpatient follow-up are less likely to be readmitted.1,17 While the optimal time interval between discharge and the first follow-up appointment is unknown, some literature supports follow-up within 4 weeks.15,18 However, because readmissions often cluster in the first several days or week following discharge,18 follow-up within the first 2 weeks (and within the first week for higher-risk patients) may be appropriate.19 Ideally, follow-up appointments should be scheduled before the patient is discharged. Patients who schedule a follow-up appointment before they are discharged are more likely to make their follow-up visit than those who are asked to call after discharge and schedule their own appointment.12
Employ outpatient follow-up alternatives
Follow-up telephone calls to patients after discharge help patients understand and adhere to discharge instructions and troubleshoot problems. Clinicians who use scripted telephone calls can evaluate symptoms related to the index hospitalization, provide patient education, schedule relevant appointments or testing, and, most importantly, initiate medication reconciliation, which is described at right.20 The FIGURE includes the script we use at our practice.
Home visits may be appropriate for certain patients, including the frail elderly. Home visits allow clinicians to evaluate the patient’s environmental safety, social sup port, and medication adherence.12 Preventive home visits generally have not been found to reduce hospital readmissions, but do enhance patient satisfaction with care.21
Bundled interventions, such as alternating home visits and follow-up telephone calls, may be more effective than individual interventions in reducing readmission.22
Reconciling medications may have far-reaching benefits
Medication discrepancies are observed in up to 70% of all patients at admission or discharge and are associated with adverse drug events (ADEs).23 To prevent ADEs and possibly readmission, take the following steps to reconcile a patient’s medications23:
Obtain a complete list of current medications. Information on all of the patient’s prescription and nonprescription medications should be collected from the patient/caregiver, the discharge summary, prescription bottles, home visits, and pharmacies.12,24
Reconcile preadmission and postdischarge medications. Clarify any discrepancies, review all medications for safety and appropriateness, and, when appropriate, resume any held medications and/or discontinue unnecessary ones.
Research shows that patients who received a phone call from a pharmacist within 3 to 7 days of discharge had lower readmission rates.Enlist pharmacy support. Pharmacists are uniquely positioned to review indications as well as potential duplication and interactions of a patient’s medications. Inpatient studies have demonstrated that partnering with pharmacists results in fewer ADEs.12,25 One study showed that patients at high risk for readmission who received a phone call from a pharmacist 3 to 7 days after discharge had lower readmission rates.26 The pharmacist reconciled the patients’ medications and ensured that patients had a clear understanding of each medication, its common safety concerns, and how often they were supposed to take it.26
Make medication adherence as easy as possible
As many as half of all patients don’t take their medications as prescribed.27 There is limited data on health outcomes associated with medication nonadherence, and existing data frequently are contradictory—some studies have found that as many as 11% of hospital admissions are attributed to nonadherence, while others show no association.28
Factors that affect adherence include psychiatric or cognitive impairment, limited insight into disease process or lack of belief in benefit of treatment, medication cost or adverse effect profile, poor provider-patient relationship, limited access to care or medication, or complexity of treatment.29 To promote medication adherence, consider the following educational and behavioral strategies30:
Identify patients at risk for nonadherence. This includes those with complex regimens and/or uncontrolled disease states or symptoms.
Increase patient communication and counseling. Patient education, particularly on the importance of adherence, is one of the few solo interventions that can improve compliance.31 Involving caregivers and using both verbal and written materials provides additional benefit.31,32
Simplify dosing schedules. Simple, convenient medication regimens may im- prove adherence. For example, adjusting dosing from 3 times a day to once a day can increase adherence from 59% to 83%.33 Aids such as pillboxes to organize medications may be of benefit.29,32
Ensure consistent follow-up. Patients who miss appointments are more likely to be nonadherent. They may benefit from easy access, help with scheduling, and frequent visits.32
Be mindful of patients’ out-of-pocket expenses. Reducing copayments improves adherence rates.30
Minimize polypharmacy. Polypharmacy has been independently associated with nonadherence and increased risk for ADEs.34
Identify patients who have limited health literacy. Limited health literacy may be linked to increased medication errors and nonadherence.12,35 Patients with low health literacy may be unable to identify medications recorded in their medical record. TABLE W336-41 outlines strategies for identifying patients with low health literacy and improving communication with them.
CASE › By speaking with hospital staff before Mr. T is discharged, you are able to confirm that he has scheduled a follow-up visit with you for one week after discharge, and that a discharge summary will be available for him to bring to that visit. Mr. T brings his discharge summary with him to your office, and you reconcile his medication list. Because he is your last patient of the day, you have some time to sit with him and his wife to explore his goals of care.
Improve care—and possibly reduce readmissions—through goal setting
Goal setting is an important element of postdischarge follow-up, particularly for elderly patients and those with progressive or end-stage diseases. Goal setting can improve patient care by linking care plans with desired outcomes and keeping diagnostic and therapeutic interventions relevant to the patient.42 A patient who understands the purpose of a recommendation—especially when directly linked to a patient-derived goal—may be more likely to adhere to the plan of care.
Asking patients to articulate their goals of care using “Ask-Tell-Ask” framework described in TABLE W336-41 will allow you to deliver the prognosis, reinforce treatment options to achieve patient-specific goals, empower patients to assert their preferences, and develop a follow-up plan to see if treatment is successful.
Empowering patients
Consider using both verbal and written approaches when educating patients about self-care behaviors such as monitoring symptoms and adhering to dietary/behavior restrictions and medication instructions. One study showed that a brief one-on-one patient education session decreased readmissions in patients with heart failure,43 although another study found that patient education alone yielded a nonsignificant decrease.44
Providing caregivers with education and support is a critical and perhaps overlooked opportunity to reduce readmissions.45 Involving key family members in discharge planning, preparation, follow-up, and ongoing management is essential in caring for patients with functional deficits and/or complex care needs. Educating caregivers can help them feel more prepared and effective in their roles.
Establish an “action plan.” For patients with chronic, periodically symptomatic diseases such as asthma and heart failure, action planning can be useful. Action plans should include information that reinforces patients’ daily self-care behaviors and instructions for what to do if symptoms get worse. Action planning also might include simple if-then plans (“if x happens, then I will do y”), which can help with problem solving for common scenarios. Action plans have been shown to reduce admissions for children with asthma46 and adults with heart failure when coupled with home monitoring or telephone support from a registered nurse.16,47
Generate an individualized care plan for each patient, taking into account your patient’s health literacy, goals of care, and level of social support. This care plan may include educational and behavioral interventions, action planning, and follow-up plans. Most successful approaches to reducing readmissions have included both system-level and patient-level interventions that use an interdisciplinary team of providers.48
Make the most of follow-up visits. The traditional 15-minute FP visit can make it challenging to provide the level of care necessary for recently discharged patients. Multiple models of team-based care have been proposed to improve this situation, including using the “teamlet” model, which may include a clinician and one or 2 health coaches.49 During each visit, the health coaches—often medical assistants trained in chronic disease self-management skills—see patients before and after the physician. They also contact patients be- tween visits to facilitate action planning and to promote self-management.
Palliative care programs: A resource for FPs
The growth of palliative care programs in US hospitals has helped increase the emphasis on establishing goals of care. Inpatient-based palliative care consultation programs work with patients and families to establish goals. However, after discharge, many of these goals and plans begin to unravel due to gaps in the current health care model, including lack of follow-up and support.50 Outpatient palliative care programs have begun to address these gaps in care.50 Comprehensive palliative care programs are quickly becoming an important resource for FPs to help address transitional care issues.
CASE › When you ask Mr. and Mrs. T about his goals for treatment, they say are getting tired of the “back and forth” to the hospital. After discussing his lengthy history of worsening CHF and diabetes, you raise the idea of palliative care, including hospice, with the couple. They acknowledge that they have had family members get hospice care, and they are open to it—just not yet. The 3 of you craft an “if-then” plan of care to use at home. You schedule a 2-week follow-up visit and remind Mr. T and his wife of your office’s 24-hour on-call service.
CORRESPONDENCE
Danielle Snyderman, MD, Department of Family and Community Medicine, Jefferson University, 1015 Walnut Street, Suite 401, Philadelphia, Pa 19107; [email protected]
1. Jencks SF, Williams MV, Coleman EA. Rehospitalizations among patients in the Medicare fee-for-service program. N Engl J Med. 2009;360:1418-1428
2. O’Connor CM, Miller AB, Blair JE, et al; Efficacy of Vasopressin Antagonism in heart Failure Outcome Study with Tolvaptan (EVEREST) investigators. Causes of death and rehospitalization in patients hospitalized with worsening heart failure and reduce left ventricular ejection fraction; results from EVEREST program. Am Heart J. 2010;159:841-849.e1.
3. Garrison GM, Mansukhani MP, Bohn B. Predictors of thirty-day readmission among hospitalized family medicine patients. J Am Board Fam Med. 2013;26:71-77.
4. Boult C, Dowd B, McCaffrey D, et al. Screening elders for risk of hospital admission. J Am Geriatr Soc. 1993;41:811-817.
5. Dharmarajan K, Hsieh AF, Lin Z, et al. Diagnoses and timing of 30-day readmissions after hospitalization for heart failure, acute myocardial infarction, or pneumonia. JAMA. 2013;309:355-363.
6. Kansagara D, Englander H, Salanitro A, et al. Risk prediction models for hospital readmission: a systematic review. JAMA. 2011;306:1688-1698.
7. Haas LR, Takahashi PY, Shah ND, et al. Risk-stratification methods for identifying patients for care coordination. Am J Manag Care. 2013;19:725-732.
8. Wallace E, Hinchey T, Dimitrov BD, et al. A systematic review of the probability of repeated admission score in community-dwelling adults. J Am Geriatr Soc. 2013;61:357-364.
9. Cotter PE, Bhalla VK, Wallis SJ, et al. Predicting readmissions: poor performance of the LACE index in an older UK population. Age Ageing. 2012;41:784-789.
10. Shelton P, Sager MA, Schraeder C. The community assessment risk screen (CARS): identifying elderly persons at risk for hospitalization or emergency department visit. Am J Manag Care. 2000;6:925-933.
11. Charlson ME, Pompei P, Ales KL, et al. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chron Dis. 1987;40:373-383.
12. Kripalani S, Jackson AT, Schnipper JL, et al. Promoting effective transitions of care at hospital discharge: a review of key issues for hospitalists. J Hosp Med. 2007;2:314-323.
13. Kim CS, Flanders SA. In the clinic. Transitions of care. Ann Intern Med. 2013;158(5 pt 1):ITC3-1.
14. Hansen LO, Strater A, Smith L, et al. Hospital discharge documentation and risk of rehospitalisation. BMJ Qual Saf. 2011;20:773-778.
15. Vaduganathan M, Bonow RO, Gheorghiade M. Thirty-day readmissions: the clock is ticking. JAMA. 2013;309:345-346.
16. Hansen LO, Young RS, Hinami K, et al. Interventions to reduce 30-day rehospitalization: a systematic review. Ann Intern Med. 2011;155:520-528.
17. Misky GJ, Wald HL, Coleman EA. Post-hospitalization transitions: Examining the effects of timing of primary care provider follow-up. J Hosp Med. 2010;5:392-397.
18. van Walraven C, Jennings A, Taljaard M, et al. Incidence of potentially avoidable urgent readmissions and their relation to all-cause urgent readmissions. CMAJ. 2011;183:E1067-E1072.
19. Tang, N. A primary care physician’s ideal transitions of care—where’s the evidence? J Hosp Med. 2013;8:472-477.
20. Crocker JB, Crocker JT, Greenwald JL. Telephone follow-up as a primary care intervention for postdischarge outcomes improvement: a systematic review. Am J Med. 2012;125:915-921.
21. Wong FK, Chow S, Chung L, et al. Can home visits help reduce hospital readmissions? Randomized controlled trial. J Adv Nurs. 2008;62:585-595.
22. Wong FK, Chow SK, Chan TM, et al. Comparison of effects between home visits with telephone calls and telephone calls only for transitional discharge support: a randomised controlled trial. Age Ageing. 2014;43:91-97.
23. Mueller SK, Sponsler KC, Kripalani S, et al. Hospital-based medication reconciliation practices: a systematic review. Arch Intern Med. 2012;172:1057-1069.
24. Glintborg B, Andersen SE, Dalhoff K. Insufficient communication about medication use at the interface between hospital and primary care. Qual Saf Health Care. 2007;16:34-39.
25. Schnipper JL, Kirwin JL, Cotugno MC, et al. Role of pharmacist counseling in preventing adverse drug events after hospitalization. Arch Intern Med. 2006;166:565-571.
26. Kilcup M, Schultz D, Carlson J, et al. Postdischarge pharmacist medication reconciliation: impact on readmission rates and financial savings. J Am Pharm Assoc (2003). 2013;53:78-84.
27. Vermeire E, Hearnshaw H, Van Royen P, et al. Patient adherence to treatment: three decades of research. A comprehensive review. J Clin Pharm Ther. 2001;26:331-342.
28. Vik SA, Maxwell CJ, Hogan DB. Measurement, correlates, and health outcomes of medication adherence among seniors. Ann Pharmacother. 2004;38:303-312.
29. Osterberg L, Blaschke T. Adherence to medication. N Engl J Med. 2005;353:487-497.
30. Viswanathan M, Golin CE, Jones CD, et al. Interventions to improve adherence to self-administered medications for chronic diseases in the United States: a systematic review. Ann Intern Med. 2012;157:785-795.
31. McDonald HP, Garg AX, Haynes RB. Interventions to enhance patient adherence to medication prescriptions: scientific review. JAMA. 2002;288:2868-2879.
32. Kripalani S, Yao X, Haynes RB. Interventions to enhance medication adherence in chronic medical conditions: a systematic review. Arch Intern Med. 2007;167:540-550.
33. Eisen SA, Miller DK, Woodward RS, et al. The effect of prescribed daily dose frequency on patient medication compliance. Arch Intern Med. 1990;150:1881-1884.
34. Field TS, Gurwitz JH, Avorn J, et al. Risk factors for adverse drug events among nursing home residents. Arch Intern Med. 2001;161:1629-1634.
35. Persell SD, Osborn CY, Richard R, et al. Limited health literacy is a barrier to medication reconciliation in ambulatory care. J Gen Intern Med. 2007;22:1523-1526.
36. Weiss BD. Health Literacy and Patient Safety: Help Patients Understand. Manual for Clinicians. Chicago, IL: American Medical Association Foundation; 2007.
37. Chew LD, Bradley KA, Bokyo EJ. Brief questions to identify patients with inadequate health literacy. Fam Med. 2004;36:588-594.
38. Wallace LS, Rogers ES, Roskos SE, et al. Brief report: screening items to identify patients with limited health literacy skills. J Gen Intern Med. 2006;21:874-877.
39. Doak CC, Doak LG, Root JH. Teaching Patients with Low Literacy Skills. 2nd ed. Philadelphia, PA: JB Lippincott Company; 1996.
40. Back AL, Arnold RM, Baile WF, et al. Approaching difficult communication tasks in oncology. CA Cancer J Clin. 2005;55: 164-177.
41. Doak LG, Doak CC, eds. Pfizer Principles for Clear Health Communication: A Handbook for Creating Patient Education Materials that Enhance Understanding and Promote Health Outcomes. 2nd ed. New York, NY: Pfizer; 2004.
42. Bradley EH, Bogardus ST Jr, Tinetti M, et al. Goal-setting in clinical medicine. Soc Sci Med. 1999;49:267-278.
43. Koelling TM, Johnson ML, Cody RJ, et al. Discharge education improves clinical outcomes in patients with chronic heart failure. Circulation. 2005;111:179-185.
44. Krumholz HM, Amatruda J, Smith GL, et al. Randomized trial of an education and support intervention to prevent readmission of patients with heart failure. J Am Coll Cardiol. 2002;39:83-89.
45. Burke RE, Coleman EA. Interventions to decrease hospital readmissions: keys for cost-effectiveness. JAMA Intern Med. 2013;173:695-698.
46. Kessler KR. Relationship between the use of asthma action plans and asthma exacerbations in children with asthma: A systematic review. J Asthma Allergy Educators. 2011;2:11-21.
47. Maric B, Kaan A, Ignaszewski A, et al. A systematic review of telemonitoring technologies in heart failure. Eur J Heart Fail. 2009;11:506-517.
48. Boutwell A, Hwu S. Effective Interventions to Reduce Rehospitalizations: A Survey of the Published Evidence. Cambridge, MA: Institute for Healthcare Improvement; 2009.
49. Bodenheimer T, Laing BY. The teamlet model of primary care. Ann Fam Med. 2007;5:457-461.
50. Meier D, Beresford L. Outpatient clinics are a new frontier for palliative care. J Pall Med. 2008;11:823-828.
1. Jencks SF, Williams MV, Coleman EA. Rehospitalizations among patients in the Medicare fee-for-service program. N Engl J Med. 2009;360:1418-1428
2. O’Connor CM, Miller AB, Blair JE, et al; Efficacy of Vasopressin Antagonism in heart Failure Outcome Study with Tolvaptan (EVEREST) investigators. Causes of death and rehospitalization in patients hospitalized with worsening heart failure and reduce left ventricular ejection fraction; results from EVEREST program. Am Heart J. 2010;159:841-849.e1.
3. Garrison GM, Mansukhani MP, Bohn B. Predictors of thirty-day readmission among hospitalized family medicine patients. J Am Board Fam Med. 2013;26:71-77.
4. Boult C, Dowd B, McCaffrey D, et al. Screening elders for risk of hospital admission. J Am Geriatr Soc. 1993;41:811-817.
5. Dharmarajan K, Hsieh AF, Lin Z, et al. Diagnoses and timing of 30-day readmissions after hospitalization for heart failure, acute myocardial infarction, or pneumonia. JAMA. 2013;309:355-363.
6. Kansagara D, Englander H, Salanitro A, et al. Risk prediction models for hospital readmission: a systematic review. JAMA. 2011;306:1688-1698.
7. Haas LR, Takahashi PY, Shah ND, et al. Risk-stratification methods for identifying patients for care coordination. Am J Manag Care. 2013;19:725-732.
8. Wallace E, Hinchey T, Dimitrov BD, et al. A systematic review of the probability of repeated admission score in community-dwelling adults. J Am Geriatr Soc. 2013;61:357-364.
9. Cotter PE, Bhalla VK, Wallis SJ, et al. Predicting readmissions: poor performance of the LACE index in an older UK population. Age Ageing. 2012;41:784-789.
10. Shelton P, Sager MA, Schraeder C. The community assessment risk screen (CARS): identifying elderly persons at risk for hospitalization or emergency department visit. Am J Manag Care. 2000;6:925-933.
11. Charlson ME, Pompei P, Ales KL, et al. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chron Dis. 1987;40:373-383.
12. Kripalani S, Jackson AT, Schnipper JL, et al. Promoting effective transitions of care at hospital discharge: a review of key issues for hospitalists. J Hosp Med. 2007;2:314-323.
13. Kim CS, Flanders SA. In the clinic. Transitions of care. Ann Intern Med. 2013;158(5 pt 1):ITC3-1.
14. Hansen LO, Strater A, Smith L, et al. Hospital discharge documentation and risk of rehospitalisation. BMJ Qual Saf. 2011;20:773-778.
15. Vaduganathan M, Bonow RO, Gheorghiade M. Thirty-day readmissions: the clock is ticking. JAMA. 2013;309:345-346.
16. Hansen LO, Young RS, Hinami K, et al. Interventions to reduce 30-day rehospitalization: a systematic review. Ann Intern Med. 2011;155:520-528.
17. Misky GJ, Wald HL, Coleman EA. Post-hospitalization transitions: Examining the effects of timing of primary care provider follow-up. J Hosp Med. 2010;5:392-397.
18. van Walraven C, Jennings A, Taljaard M, et al. Incidence of potentially avoidable urgent readmissions and their relation to all-cause urgent readmissions. CMAJ. 2011;183:E1067-E1072.
19. Tang, N. A primary care physician’s ideal transitions of care—where’s the evidence? J Hosp Med. 2013;8:472-477.
20. Crocker JB, Crocker JT, Greenwald JL. Telephone follow-up as a primary care intervention for postdischarge outcomes improvement: a systematic review. Am J Med. 2012;125:915-921.
21. Wong FK, Chow S, Chung L, et al. Can home visits help reduce hospital readmissions? Randomized controlled trial. J Adv Nurs. 2008;62:585-595.
22. Wong FK, Chow SK, Chan TM, et al. Comparison of effects between home visits with telephone calls and telephone calls only for transitional discharge support: a randomised controlled trial. Age Ageing. 2014;43:91-97.
23. Mueller SK, Sponsler KC, Kripalani S, et al. Hospital-based medication reconciliation practices: a systematic review. Arch Intern Med. 2012;172:1057-1069.
24. Glintborg B, Andersen SE, Dalhoff K. Insufficient communication about medication use at the interface between hospital and primary care. Qual Saf Health Care. 2007;16:34-39.
25. Schnipper JL, Kirwin JL, Cotugno MC, et al. Role of pharmacist counseling in preventing adverse drug events after hospitalization. Arch Intern Med. 2006;166:565-571.
26. Kilcup M, Schultz D, Carlson J, et al. Postdischarge pharmacist medication reconciliation: impact on readmission rates and financial savings. J Am Pharm Assoc (2003). 2013;53:78-84.
27. Vermeire E, Hearnshaw H, Van Royen P, et al. Patient adherence to treatment: three decades of research. A comprehensive review. J Clin Pharm Ther. 2001;26:331-342.
28. Vik SA, Maxwell CJ, Hogan DB. Measurement, correlates, and health outcomes of medication adherence among seniors. Ann Pharmacother. 2004;38:303-312.
29. Osterberg L, Blaschke T. Adherence to medication. N Engl J Med. 2005;353:487-497.
30. Viswanathan M, Golin CE, Jones CD, et al. Interventions to improve adherence to self-administered medications for chronic diseases in the United States: a systematic review. Ann Intern Med. 2012;157:785-795.
31. McDonald HP, Garg AX, Haynes RB. Interventions to enhance patient adherence to medication prescriptions: scientific review. JAMA. 2002;288:2868-2879.
32. Kripalani S, Yao X, Haynes RB. Interventions to enhance medication adherence in chronic medical conditions: a systematic review. Arch Intern Med. 2007;167:540-550.
33. Eisen SA, Miller DK, Woodward RS, et al. The effect of prescribed daily dose frequency on patient medication compliance. Arch Intern Med. 1990;150:1881-1884.
34. Field TS, Gurwitz JH, Avorn J, et al. Risk factors for adverse drug events among nursing home residents. Arch Intern Med. 2001;161:1629-1634.
35. Persell SD, Osborn CY, Richard R, et al. Limited health literacy is a barrier to medication reconciliation in ambulatory care. J Gen Intern Med. 2007;22:1523-1526.
36. Weiss BD. Health Literacy and Patient Safety: Help Patients Understand. Manual for Clinicians. Chicago, IL: American Medical Association Foundation; 2007.
37. Chew LD, Bradley KA, Bokyo EJ. Brief questions to identify patients with inadequate health literacy. Fam Med. 2004;36:588-594.
38. Wallace LS, Rogers ES, Roskos SE, et al. Brief report: screening items to identify patients with limited health literacy skills. J Gen Intern Med. 2006;21:874-877.
39. Doak CC, Doak LG, Root JH. Teaching Patients with Low Literacy Skills. 2nd ed. Philadelphia, PA: JB Lippincott Company; 1996.
40. Back AL, Arnold RM, Baile WF, et al. Approaching difficult communication tasks in oncology. CA Cancer J Clin. 2005;55: 164-177.
41. Doak LG, Doak CC, eds. Pfizer Principles for Clear Health Communication: A Handbook for Creating Patient Education Materials that Enhance Understanding and Promote Health Outcomes. 2nd ed. New York, NY: Pfizer; 2004.
42. Bradley EH, Bogardus ST Jr, Tinetti M, et al. Goal-setting in clinical medicine. Soc Sci Med. 1999;49:267-278.
43. Koelling TM, Johnson ML, Cody RJ, et al. Discharge education improves clinical outcomes in patients with chronic heart failure. Circulation. 2005;111:179-185.
44. Krumholz HM, Amatruda J, Smith GL, et al. Randomized trial of an education and support intervention to prevent readmission of patients with heart failure. J Am Coll Cardiol. 2002;39:83-89.
45. Burke RE, Coleman EA. Interventions to decrease hospital readmissions: keys for cost-effectiveness. JAMA Intern Med. 2013;173:695-698.
46. Kessler KR. Relationship between the use of asthma action plans and asthma exacerbations in children with asthma: A systematic review. J Asthma Allergy Educators. 2011;2:11-21.
47. Maric B, Kaan A, Ignaszewski A, et al. A systematic review of telemonitoring technologies in heart failure. Eur J Heart Fail. 2009;11:506-517.
48. Boutwell A, Hwu S. Effective Interventions to Reduce Rehospitalizations: A Survey of the Published Evidence. Cambridge, MA: Institute for Healthcare Improvement; 2009.
49. Bodenheimer T, Laing BY. The teamlet model of primary care. Ann Fam Med. 2007;5:457-461.
50. Meier D, Beresford L. Outpatient clinics are a new frontier for palliative care. J Pall Med. 2008;11:823-828.
HIV screening: How we can do better
› Screen all adolescents and adults ages 15 to 65 years for human immunodeficiency virus (HIV) infection. A
› Screen younger adolescents and older adults who are at increased risk for HIV infection on an annual basis. A
› Screen all pregnant women for HIV infection, including those who are in labor and who are untested or whose HIV status is unknown. A
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
For the first 15 years of the epidemic, human immunodeficiency virus and acquired immunodeficiency syndrome (HIV/AIDs) was uniformly fatal. Between 1981 and 1996, approximately 362,000 people in the United States succumbed to the disease.1 That began to change in the mid 1990s, though, when highly active antiretroviral therapy (HAART) came into routine use. From that point forward, HIV became a chronic, manageable disease for most patients; an estimated 1.2 million people in the United States are now living with HIV infection.2
Unfortunately, the number of new infections continues to grow. There are more than 50,000 new infections in the United States each year,2 and an estimated approximately 200,000 people have it but are undiagnosed, leading to further spread of the disease.3 The Office of National AIDS Policy has issued a National HIV/AIDS Strategy that seeks to reduce new infections by 25% in 2015, in part by identifying people with the disease who do not know their HIV status.4
But screening still has not gotten the uptake by clinicians that health officials would like.
Lack of awareness by physicians? Or an unwillingness of patients?
In 2006, the Centers for Disease Control and Prevention (CDC) began recommending routine HIV screening for individuals between the ages of 13 and 64, with patients given the ability to opt out of such testing.5 That same year, the CDC also removed some prior barriers to testing, such as requiring written consent and pretest counseling. But as of 2009, fewer than 50% of US adults had ever been tested for HIV6—possibly the result of physicians being unaware of the guidelines, patients being unwilling to be tested, and/or reimbursement issues.
Conflicting recommendations may have played a role. When the CDC released its 2006 recommendations, the United States Preventive Services Task Force (USPSTF) felt there was insufficient evidence to support routine HIV screening and issued a grade C recommendation. At that time, the USPSTF recommended that only high-risk individuals and pregnant women be tested (A recommendation, meaning there was high certainty that the net benefit was substantial).
However, in April 2013, based on new evidence regarding the clinical and public health benefits of early identification of HIV infection and subsequent treatment, the USPSTF updated its recommendations. The USPSTF now encourages clinicians to screen all adolescents and adults age 15 to 65 years for HIV (A recommendation).7 Shortly thereafter, the American Academy of Family Physicians (AAFP) also endorsed routine HIV screening, although the AAFP calls for such screening to begin at age 18.8
Insurance now covers it… A USPSTF A recommendation carries significant health policy implications because the Affordable Care Act requires private and public health insurance plans to cover preventive services recommended by USPSTF.9
Integrating screening into your practice
Serologic tests have come a long way. The first HIV antibody test was an enzyme immunoassay (EIA) that was introduced in 1985 and used mainly to screen the blood supply. This first-generation EIA identified only immunoglobulin G (IgG) antibodies to HIV type 1 (HIV-1). More sensitive and specific second- and third-generation EIAs have since been developed to detect both IgG and IgM antibodies, as well as antibodies to HIV-2. The third-generation assays also can detect antibodies as soon as 3 weeks after infection.
The fourth-generation EIAs were approved by the US Food and Drug Administration (FDA) in 201010 and are the first step in the CDC’s current HIV diagnostic testing algorithm. These tests can detect HIV-1/HIV-2 IgG and IgM antibodies and also p24 antigen, which is present within 7 days of the appearance of HIV RNA.11 The fourth-generation assay allows for reliable detection within about 2 weeks of infection (FIGURE 1).10
Rapid HIV tests are also an option.12 These tests can detect IgG and IgM antibodies in samples of saliva, whole blood, serum, and plasma. Results of rapid tests usually are available in 20 to 30 minutes and allow physicians to give patients the results while they are still in the office. In 2013 the FDA approved a combination p24 antigen/antibody rapid HIV assay that according to the manufacturer can detect infection earlier than other currently available rapid tests.13
When rapid tests are most useful. Rapid tests can be particularly useful for testing women presenting in labor who have not been screened for HIV as part of prenatal care. They also can be used to determine the need for postexposure prophylaxis in the event of a needlestick injury. According to manufacturer’s data, the sensitivity of rapid tests ranges from 99.3% to 100% and specificity from 99.7% to 99.9%.12 However, in real-world experience these numbers have been slightly lower.12 By comparison, the sensitivity and specificity of the fourth-generation EIAs are 99.4% and 99.5%, respectively.14
The downside... A disadvantage of rapid HIV testing is that under current FDA-approval status and CDC guidance, tests performed on oral fluid must have serologic confirmation. In addition, patients tested during the “window period” of seroconversion (after infection occurs but before antibodies are detectable) will test negative with rapid HIV tests and must be reminded that repeat testing should be done within 4 to 6 weeks of their last potential exposure to the virus. In high-prevalence settings such as urban emergency departments (EDs), rapid HIV tests have detected a significant number of new infections.15 However, ED physicians and urgent care providers have been reluctant to perform HIV tests due to the lack of follow-up for most patients treated in these settings.
Over-the-counter (OTC) tests. Approved by the FDA in 2012, the OraQuick In-Home HIV Test is the only available OTC test for use at home. Patients can go to the company’s Web site at www.oraquick.com to learn more about HIV and testing, and the company offers 24-hour phone support. It’s not clear how many patients are taking advantage of this home testing option. The test costs approximately $40 and several studies suggest that this price may deter patients from using it.16 In addition, it is not clear how patients who test positive using an OTC test will access medical care or get appropriate medical follow-up.
New testing algorithm eliminates Western blot
Historically, a patient with a reactive (positive) EIA result would undergo the Western blot assay as a confirmatory test. Although the Western blot for HIV is highly specific (99.7%), it tests only for the IgG antibody. This could lead to a false negative test in a patient in whom IgG seroconversion has not yet occurred. Additionally, the time for HIV confirmation with the Western blot often is one week or longer.
Recently, the CDC has made available for public comment a diagnostic algorithm that removes the Western blot as a recommended test (FIGURE 2).17 This algorithm replaces the Western blot with an assay to differentiate HIV-1 and HIV-2 antibodies. Patients for whom this test is negative should undergo additional testing for HIV RNA to determine if HIV-1 is present. Positive HIV RNA would indicate acute or more recent infection. Studies suggest that this new algorithm is better than the existing algorithm at detecting HIV infections, and many reference labs have already adapted it.17,18
Choosing your words carefully when giving patients their results
Patients can be given the results of a rapid HIV test during their visit, but a positive result on a rapid test should be confirmed by serologic testing. When speaking with a patient who tests positive on a rapid test, consider using the phrase “preliminary positive” results. This allows the patient to more easily process the results, knowing that a confirmatory blood draw will be done. State laws vary regarding how patients can receive HIV test results. Most states allow negative serologic test results to be given over the telephone (or electronically). For positive tests, it is preferable to give these results at a face-to-face consultation so that you can ensure the patient will have access to medical care. For more on HIV testing and lab reporting laws by state, see http://www.cdc.gov/hiv/policies/law/states/index.html.
CORRESPONDENCE
Jeffrey T. Kirchner, DO, FAAFP, AAHIVS, Family and Community Medicine, Lancaster General Hospital, 555 N. Duke Street #3555, Lancaster, PA 17602; [email protected]
1. amFAR. Thirty years of HIV/AIDS: Snapshots of an epidemic. amfAR, The Foundation for AIDS Research Web site. Available at: http://www.amfar.org/thirty-years-of-hiv/aids-snapshots-of-anepidemic. Accessed May 9, 2014.
2. Centers for Disease Control and Prevention. HIV Surveillance Report, 2011. Vol. 23. Centers for Disease Control and Prevention Web site. Available at: http://www.cdc.gov/hiv/pdf/statistics_2011_HIV_Surveillance_Report_vol_23.pdf. Published February 2013. Accessed October 19, 2013.
3. Centers for Disease Control and Prevention. HIV in the United States: At a glance. Centers for Disease Control and Prevention Web site. Available at: http://www.cdc.gov/hiv/statistics/basics/ataglance.html. Accessed May 9, 2014.
4. The White House Office of National AIDS Policy. National HIV/ AIDS Strategy for the United States. AIDS.gov Web site. Available at: http://aids.gov/federal-resources/national-hiv-aids-strategy/nhas.pdf. Accessed October 25, 2013.
5. Branson BM, Handsfield HH, Lampe MA, et al; Centers for Disease Control and Prevention (CDC). Revised recommendations for HIV testing of adults, adolescents, and pregnant women in health-care settings. MMWR Recomm Rep. 2006;55(RR-14):1-17;quiz CE1-CE4.
6. Centers for Disease Control and Prevention (CDC). Vital signs: HIV testing and diagnosis among adults-- United States, 2001-2009. MMWR Morb Mortal Wkly Rep. 2010;59:1550-1555.
7. Moyer VA; U.S. Preventive Services Task Force. Screening for HIV: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2013;159:51-60.
8. Brown M. AAFP, USPSTF recommend routine HIV screening but differ on age to begin. Available at: http://www.aafp.org/news/health-of-the-public/20130429hivscreenrecs.html. Accessed August 17, 2013.
9. Bayer R, Oppenheimer GM. Routine HIV testing, public health, and the USPSTF--an end to the debate. N Engl J Med. 2013;368:881-884
10. Cornett JK, Kirn TJ. Laboratory diagnosis of HIV in adults: a review of current methods. Clin Infect Dis. 2013;57:712-718.
11. Baron EJ, Miller MJ, Weinstein MP, et al. A guide to the utilization of the microbiology laboratory for diagnosis of infectious diseases: 2013 recommendations by the Infectious Diseases Society of America (IDSA) and the American Society for Microbiology (ASM)(a). Clin Infect Dis. 2013;57:e22-e121.
12. Delaney KP, Branson BM, Uniyal A, et al. Evaluation of the performance characteristics of 6 rapid HIV antibody tests. Clin Infect Dis. 2011;52:257-263.
13. FDA. FDA approves first rapid diagnostic test to detect both HIV-1 antigen and HIV-1/2 antibodies [press release]. US Food and Drug Administration Web site. Available at: http://www.fda.gov/newsevents/newsroom/pressannouncements/ucm364480.htm. Silver Spring, MD: US Food and Drug Administration; August 8, 2013. Accessed October 26, 2013.
14. Chavez P, Wesolowski L, Patel P, et al. Evaluation of the performance of the Abbott ARCHITECT HIV Ag/Ab Combo Assay. J Clin Virol. 2011;52:S51-S55.
15. Centers for Disease Control and Prevention (CDC). Rapid HIV testing in emergency departments—three U.S. sites, January 2005-March 2006. MMWR Morb Mortal Wkly Rep. 2007;56:597-601.
16. Napierala Mavedzenge S, Baggaley R, Corbett EL. A review of self-testing for HIV: research and policy priorities in a new era of HIV prevention. Clin Infect Dis. 2013;57:126-138.
17. Centers for Disease Control and Prevention (CDC). Detection of acute HIV infection in two evaluations of a new HIV diagnostic testing algorithm - United States, 2011-2013. MMWR Morb Mortal Wkly Rep. 2013;62:489-494.
18. Nasrullah M, Wesolowski LG, Meyer WA 3rd, et al. Performance of a fourth-generation HIV screening assay and an alternative HIV diagnostic testing algorithm. AIDS. 2013;27:731-737.
› Screen all adolescents and adults ages 15 to 65 years for human immunodeficiency virus (HIV) infection. A
› Screen younger adolescents and older adults who are at increased risk for HIV infection on an annual basis. A
› Screen all pregnant women for HIV infection, including those who are in labor and who are untested or whose HIV status is unknown. A
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
For the first 15 years of the epidemic, human immunodeficiency virus and acquired immunodeficiency syndrome (HIV/AIDs) was uniformly fatal. Between 1981 and 1996, approximately 362,000 people in the United States succumbed to the disease.1 That began to change in the mid 1990s, though, when highly active antiretroviral therapy (HAART) came into routine use. From that point forward, HIV became a chronic, manageable disease for most patients; an estimated 1.2 million people in the United States are now living with HIV infection.2
Unfortunately, the number of new infections continues to grow. There are more than 50,000 new infections in the United States each year,2 and an estimated approximately 200,000 people have it but are undiagnosed, leading to further spread of the disease.3 The Office of National AIDS Policy has issued a National HIV/AIDS Strategy that seeks to reduce new infections by 25% in 2015, in part by identifying people with the disease who do not know their HIV status.4
But screening still has not gotten the uptake by clinicians that health officials would like.
Lack of awareness by physicians? Or an unwillingness of patients?
In 2006, the Centers for Disease Control and Prevention (CDC) began recommending routine HIV screening for individuals between the ages of 13 and 64, with patients given the ability to opt out of such testing.5 That same year, the CDC also removed some prior barriers to testing, such as requiring written consent and pretest counseling. But as of 2009, fewer than 50% of US adults had ever been tested for HIV6—possibly the result of physicians being unaware of the guidelines, patients being unwilling to be tested, and/or reimbursement issues.
Conflicting recommendations may have played a role. When the CDC released its 2006 recommendations, the United States Preventive Services Task Force (USPSTF) felt there was insufficient evidence to support routine HIV screening and issued a grade C recommendation. At that time, the USPSTF recommended that only high-risk individuals and pregnant women be tested (A recommendation, meaning there was high certainty that the net benefit was substantial).
However, in April 2013, based on new evidence regarding the clinical and public health benefits of early identification of HIV infection and subsequent treatment, the USPSTF updated its recommendations. The USPSTF now encourages clinicians to screen all adolescents and adults age 15 to 65 years for HIV (A recommendation).7 Shortly thereafter, the American Academy of Family Physicians (AAFP) also endorsed routine HIV screening, although the AAFP calls for such screening to begin at age 18.8
Insurance now covers it… A USPSTF A recommendation carries significant health policy implications because the Affordable Care Act requires private and public health insurance plans to cover preventive services recommended by USPSTF.9
Integrating screening into your practice
Serologic tests have come a long way. The first HIV antibody test was an enzyme immunoassay (EIA) that was introduced in 1985 and used mainly to screen the blood supply. This first-generation EIA identified only immunoglobulin G (IgG) antibodies to HIV type 1 (HIV-1). More sensitive and specific second- and third-generation EIAs have since been developed to detect both IgG and IgM antibodies, as well as antibodies to HIV-2. The third-generation assays also can detect antibodies as soon as 3 weeks after infection.
The fourth-generation EIAs were approved by the US Food and Drug Administration (FDA) in 201010 and are the first step in the CDC’s current HIV diagnostic testing algorithm. These tests can detect HIV-1/HIV-2 IgG and IgM antibodies and also p24 antigen, which is present within 7 days of the appearance of HIV RNA.11 The fourth-generation assay allows for reliable detection within about 2 weeks of infection (FIGURE 1).10
Rapid HIV tests are also an option.12 These tests can detect IgG and IgM antibodies in samples of saliva, whole blood, serum, and plasma. Results of rapid tests usually are available in 20 to 30 minutes and allow physicians to give patients the results while they are still in the office. In 2013 the FDA approved a combination p24 antigen/antibody rapid HIV assay that according to the manufacturer can detect infection earlier than other currently available rapid tests.13
When rapid tests are most useful. Rapid tests can be particularly useful for testing women presenting in labor who have not been screened for HIV as part of prenatal care. They also can be used to determine the need for postexposure prophylaxis in the event of a needlestick injury. According to manufacturer’s data, the sensitivity of rapid tests ranges from 99.3% to 100% and specificity from 99.7% to 99.9%.12 However, in real-world experience these numbers have been slightly lower.12 By comparison, the sensitivity and specificity of the fourth-generation EIAs are 99.4% and 99.5%, respectively.14
The downside... A disadvantage of rapid HIV testing is that under current FDA-approval status and CDC guidance, tests performed on oral fluid must have serologic confirmation. In addition, patients tested during the “window period” of seroconversion (after infection occurs but before antibodies are detectable) will test negative with rapid HIV tests and must be reminded that repeat testing should be done within 4 to 6 weeks of their last potential exposure to the virus. In high-prevalence settings such as urban emergency departments (EDs), rapid HIV tests have detected a significant number of new infections.15 However, ED physicians and urgent care providers have been reluctant to perform HIV tests due to the lack of follow-up for most patients treated in these settings.
Over-the-counter (OTC) tests. Approved by the FDA in 2012, the OraQuick In-Home HIV Test is the only available OTC test for use at home. Patients can go to the company’s Web site at www.oraquick.com to learn more about HIV and testing, and the company offers 24-hour phone support. It’s not clear how many patients are taking advantage of this home testing option. The test costs approximately $40 and several studies suggest that this price may deter patients from using it.16 In addition, it is not clear how patients who test positive using an OTC test will access medical care or get appropriate medical follow-up.
New testing algorithm eliminates Western blot
Historically, a patient with a reactive (positive) EIA result would undergo the Western blot assay as a confirmatory test. Although the Western blot for HIV is highly specific (99.7%), it tests only for the IgG antibody. This could lead to a false negative test in a patient in whom IgG seroconversion has not yet occurred. Additionally, the time for HIV confirmation with the Western blot often is one week or longer.
Recently, the CDC has made available for public comment a diagnostic algorithm that removes the Western blot as a recommended test (FIGURE 2).17 This algorithm replaces the Western blot with an assay to differentiate HIV-1 and HIV-2 antibodies. Patients for whom this test is negative should undergo additional testing for HIV RNA to determine if HIV-1 is present. Positive HIV RNA would indicate acute or more recent infection. Studies suggest that this new algorithm is better than the existing algorithm at detecting HIV infections, and many reference labs have already adapted it.17,18
Choosing your words carefully when giving patients their results
Patients can be given the results of a rapid HIV test during their visit, but a positive result on a rapid test should be confirmed by serologic testing. When speaking with a patient who tests positive on a rapid test, consider using the phrase “preliminary positive” results. This allows the patient to more easily process the results, knowing that a confirmatory blood draw will be done. State laws vary regarding how patients can receive HIV test results. Most states allow negative serologic test results to be given over the telephone (or electronically). For positive tests, it is preferable to give these results at a face-to-face consultation so that you can ensure the patient will have access to medical care. For more on HIV testing and lab reporting laws by state, see http://www.cdc.gov/hiv/policies/law/states/index.html.
CORRESPONDENCE
Jeffrey T. Kirchner, DO, FAAFP, AAHIVS, Family and Community Medicine, Lancaster General Hospital, 555 N. Duke Street #3555, Lancaster, PA 17602; [email protected]
› Screen all adolescents and adults ages 15 to 65 years for human immunodeficiency virus (HIV) infection. A
› Screen younger adolescents and older adults who are at increased risk for HIV infection on an annual basis. A
› Screen all pregnant women for HIV infection, including those who are in labor and who are untested or whose HIV status is unknown. A
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
For the first 15 years of the epidemic, human immunodeficiency virus and acquired immunodeficiency syndrome (HIV/AIDs) was uniformly fatal. Between 1981 and 1996, approximately 362,000 people in the United States succumbed to the disease.1 That began to change in the mid 1990s, though, when highly active antiretroviral therapy (HAART) came into routine use. From that point forward, HIV became a chronic, manageable disease for most patients; an estimated 1.2 million people in the United States are now living with HIV infection.2
Unfortunately, the number of new infections continues to grow. There are more than 50,000 new infections in the United States each year,2 and an estimated approximately 200,000 people have it but are undiagnosed, leading to further spread of the disease.3 The Office of National AIDS Policy has issued a National HIV/AIDS Strategy that seeks to reduce new infections by 25% in 2015, in part by identifying people with the disease who do not know their HIV status.4
But screening still has not gotten the uptake by clinicians that health officials would like.
Lack of awareness by physicians? Or an unwillingness of patients?
In 2006, the Centers for Disease Control and Prevention (CDC) began recommending routine HIV screening for individuals between the ages of 13 and 64, with patients given the ability to opt out of such testing.5 That same year, the CDC also removed some prior barriers to testing, such as requiring written consent and pretest counseling. But as of 2009, fewer than 50% of US adults had ever been tested for HIV6—possibly the result of physicians being unaware of the guidelines, patients being unwilling to be tested, and/or reimbursement issues.
Conflicting recommendations may have played a role. When the CDC released its 2006 recommendations, the United States Preventive Services Task Force (USPSTF) felt there was insufficient evidence to support routine HIV screening and issued a grade C recommendation. At that time, the USPSTF recommended that only high-risk individuals and pregnant women be tested (A recommendation, meaning there was high certainty that the net benefit was substantial).
However, in April 2013, based on new evidence regarding the clinical and public health benefits of early identification of HIV infection and subsequent treatment, the USPSTF updated its recommendations. The USPSTF now encourages clinicians to screen all adolescents and adults age 15 to 65 years for HIV (A recommendation).7 Shortly thereafter, the American Academy of Family Physicians (AAFP) also endorsed routine HIV screening, although the AAFP calls for such screening to begin at age 18.8
Insurance now covers it… A USPSTF A recommendation carries significant health policy implications because the Affordable Care Act requires private and public health insurance plans to cover preventive services recommended by USPSTF.9
Integrating screening into your practice
Serologic tests have come a long way. The first HIV antibody test was an enzyme immunoassay (EIA) that was introduced in 1985 and used mainly to screen the blood supply. This first-generation EIA identified only immunoglobulin G (IgG) antibodies to HIV type 1 (HIV-1). More sensitive and specific second- and third-generation EIAs have since been developed to detect both IgG and IgM antibodies, as well as antibodies to HIV-2. The third-generation assays also can detect antibodies as soon as 3 weeks after infection.
The fourth-generation EIAs were approved by the US Food and Drug Administration (FDA) in 201010 and are the first step in the CDC’s current HIV diagnostic testing algorithm. These tests can detect HIV-1/HIV-2 IgG and IgM antibodies and also p24 antigen, which is present within 7 days of the appearance of HIV RNA.11 The fourth-generation assay allows for reliable detection within about 2 weeks of infection (FIGURE 1).10
Rapid HIV tests are also an option.12 These tests can detect IgG and IgM antibodies in samples of saliva, whole blood, serum, and plasma. Results of rapid tests usually are available in 20 to 30 minutes and allow physicians to give patients the results while they are still in the office. In 2013 the FDA approved a combination p24 antigen/antibody rapid HIV assay that according to the manufacturer can detect infection earlier than other currently available rapid tests.13
When rapid tests are most useful. Rapid tests can be particularly useful for testing women presenting in labor who have not been screened for HIV as part of prenatal care. They also can be used to determine the need for postexposure prophylaxis in the event of a needlestick injury. According to manufacturer’s data, the sensitivity of rapid tests ranges from 99.3% to 100% and specificity from 99.7% to 99.9%.12 However, in real-world experience these numbers have been slightly lower.12 By comparison, the sensitivity and specificity of the fourth-generation EIAs are 99.4% and 99.5%, respectively.14
The downside... A disadvantage of rapid HIV testing is that under current FDA-approval status and CDC guidance, tests performed on oral fluid must have serologic confirmation. In addition, patients tested during the “window period” of seroconversion (after infection occurs but before antibodies are detectable) will test negative with rapid HIV tests and must be reminded that repeat testing should be done within 4 to 6 weeks of their last potential exposure to the virus. In high-prevalence settings such as urban emergency departments (EDs), rapid HIV tests have detected a significant number of new infections.15 However, ED physicians and urgent care providers have been reluctant to perform HIV tests due to the lack of follow-up for most patients treated in these settings.
Over-the-counter (OTC) tests. Approved by the FDA in 2012, the OraQuick In-Home HIV Test is the only available OTC test for use at home. Patients can go to the company’s Web site at www.oraquick.com to learn more about HIV and testing, and the company offers 24-hour phone support. It’s not clear how many patients are taking advantage of this home testing option. The test costs approximately $40 and several studies suggest that this price may deter patients from using it.16 In addition, it is not clear how patients who test positive using an OTC test will access medical care or get appropriate medical follow-up.
New testing algorithm eliminates Western blot
Historically, a patient with a reactive (positive) EIA result would undergo the Western blot assay as a confirmatory test. Although the Western blot for HIV is highly specific (99.7%), it tests only for the IgG antibody. This could lead to a false negative test in a patient in whom IgG seroconversion has not yet occurred. Additionally, the time for HIV confirmation with the Western blot often is one week or longer.
Recently, the CDC has made available for public comment a diagnostic algorithm that removes the Western blot as a recommended test (FIGURE 2).17 This algorithm replaces the Western blot with an assay to differentiate HIV-1 and HIV-2 antibodies. Patients for whom this test is negative should undergo additional testing for HIV RNA to determine if HIV-1 is present. Positive HIV RNA would indicate acute or more recent infection. Studies suggest that this new algorithm is better than the existing algorithm at detecting HIV infections, and many reference labs have already adapted it.17,18
Choosing your words carefully when giving patients their results
Patients can be given the results of a rapid HIV test during their visit, but a positive result on a rapid test should be confirmed by serologic testing. When speaking with a patient who tests positive on a rapid test, consider using the phrase “preliminary positive” results. This allows the patient to more easily process the results, knowing that a confirmatory blood draw will be done. State laws vary regarding how patients can receive HIV test results. Most states allow negative serologic test results to be given over the telephone (or electronically). For positive tests, it is preferable to give these results at a face-to-face consultation so that you can ensure the patient will have access to medical care. For more on HIV testing and lab reporting laws by state, see http://www.cdc.gov/hiv/policies/law/states/index.html.
CORRESPONDENCE
Jeffrey T. Kirchner, DO, FAAFP, AAHIVS, Family and Community Medicine, Lancaster General Hospital, 555 N. Duke Street #3555, Lancaster, PA 17602; [email protected]
1. amFAR. Thirty years of HIV/AIDS: Snapshots of an epidemic. amfAR, The Foundation for AIDS Research Web site. Available at: http://www.amfar.org/thirty-years-of-hiv/aids-snapshots-of-anepidemic. Accessed May 9, 2014.
2. Centers for Disease Control and Prevention. HIV Surveillance Report, 2011. Vol. 23. Centers for Disease Control and Prevention Web site. Available at: http://www.cdc.gov/hiv/pdf/statistics_2011_HIV_Surveillance_Report_vol_23.pdf. Published February 2013. Accessed October 19, 2013.
3. Centers for Disease Control and Prevention. HIV in the United States: At a glance. Centers for Disease Control and Prevention Web site. Available at: http://www.cdc.gov/hiv/statistics/basics/ataglance.html. Accessed May 9, 2014.
4. The White House Office of National AIDS Policy. National HIV/ AIDS Strategy for the United States. AIDS.gov Web site. Available at: http://aids.gov/federal-resources/national-hiv-aids-strategy/nhas.pdf. Accessed October 25, 2013.
5. Branson BM, Handsfield HH, Lampe MA, et al; Centers for Disease Control and Prevention (CDC). Revised recommendations for HIV testing of adults, adolescents, and pregnant women in health-care settings. MMWR Recomm Rep. 2006;55(RR-14):1-17;quiz CE1-CE4.
6. Centers for Disease Control and Prevention (CDC). Vital signs: HIV testing and diagnosis among adults-- United States, 2001-2009. MMWR Morb Mortal Wkly Rep. 2010;59:1550-1555.
7. Moyer VA; U.S. Preventive Services Task Force. Screening for HIV: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2013;159:51-60.
8. Brown M. AAFP, USPSTF recommend routine HIV screening but differ on age to begin. Available at: http://www.aafp.org/news/health-of-the-public/20130429hivscreenrecs.html. Accessed August 17, 2013.
9. Bayer R, Oppenheimer GM. Routine HIV testing, public health, and the USPSTF--an end to the debate. N Engl J Med. 2013;368:881-884
10. Cornett JK, Kirn TJ. Laboratory diagnosis of HIV in adults: a review of current methods. Clin Infect Dis. 2013;57:712-718.
11. Baron EJ, Miller MJ, Weinstein MP, et al. A guide to the utilization of the microbiology laboratory for diagnosis of infectious diseases: 2013 recommendations by the Infectious Diseases Society of America (IDSA) and the American Society for Microbiology (ASM)(a). Clin Infect Dis. 2013;57:e22-e121.
12. Delaney KP, Branson BM, Uniyal A, et al. Evaluation of the performance characteristics of 6 rapid HIV antibody tests. Clin Infect Dis. 2011;52:257-263.
13. FDA. FDA approves first rapid diagnostic test to detect both HIV-1 antigen and HIV-1/2 antibodies [press release]. US Food and Drug Administration Web site. Available at: http://www.fda.gov/newsevents/newsroom/pressannouncements/ucm364480.htm. Silver Spring, MD: US Food and Drug Administration; August 8, 2013. Accessed October 26, 2013.
14. Chavez P, Wesolowski L, Patel P, et al. Evaluation of the performance of the Abbott ARCHITECT HIV Ag/Ab Combo Assay. J Clin Virol. 2011;52:S51-S55.
15. Centers for Disease Control and Prevention (CDC). Rapid HIV testing in emergency departments—three U.S. sites, January 2005-March 2006. MMWR Morb Mortal Wkly Rep. 2007;56:597-601.
16. Napierala Mavedzenge S, Baggaley R, Corbett EL. A review of self-testing for HIV: research and policy priorities in a new era of HIV prevention. Clin Infect Dis. 2013;57:126-138.
17. Centers for Disease Control and Prevention (CDC). Detection of acute HIV infection in two evaluations of a new HIV diagnostic testing algorithm - United States, 2011-2013. MMWR Morb Mortal Wkly Rep. 2013;62:489-494.
18. Nasrullah M, Wesolowski LG, Meyer WA 3rd, et al. Performance of a fourth-generation HIV screening assay and an alternative HIV diagnostic testing algorithm. AIDS. 2013;27:731-737.
1. amFAR. Thirty years of HIV/AIDS: Snapshots of an epidemic. amfAR, The Foundation for AIDS Research Web site. Available at: http://www.amfar.org/thirty-years-of-hiv/aids-snapshots-of-anepidemic. Accessed May 9, 2014.
2. Centers for Disease Control and Prevention. HIV Surveillance Report, 2011. Vol. 23. Centers for Disease Control and Prevention Web site. Available at: http://www.cdc.gov/hiv/pdf/statistics_2011_HIV_Surveillance_Report_vol_23.pdf. Published February 2013. Accessed October 19, 2013.
3. Centers for Disease Control and Prevention. HIV in the United States: At a glance. Centers for Disease Control and Prevention Web site. Available at: http://www.cdc.gov/hiv/statistics/basics/ataglance.html. Accessed May 9, 2014.
4. The White House Office of National AIDS Policy. National HIV/ AIDS Strategy for the United States. AIDS.gov Web site. Available at: http://aids.gov/federal-resources/national-hiv-aids-strategy/nhas.pdf. Accessed October 25, 2013.
5. Branson BM, Handsfield HH, Lampe MA, et al; Centers for Disease Control and Prevention (CDC). Revised recommendations for HIV testing of adults, adolescents, and pregnant women in health-care settings. MMWR Recomm Rep. 2006;55(RR-14):1-17;quiz CE1-CE4.
6. Centers for Disease Control and Prevention (CDC). Vital signs: HIV testing and diagnosis among adults-- United States, 2001-2009. MMWR Morb Mortal Wkly Rep. 2010;59:1550-1555.
7. Moyer VA; U.S. Preventive Services Task Force. Screening for HIV: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2013;159:51-60.
8. Brown M. AAFP, USPSTF recommend routine HIV screening but differ on age to begin. Available at: http://www.aafp.org/news/health-of-the-public/20130429hivscreenrecs.html. Accessed August 17, 2013.
9. Bayer R, Oppenheimer GM. Routine HIV testing, public health, and the USPSTF--an end to the debate. N Engl J Med. 2013;368:881-884
10. Cornett JK, Kirn TJ. Laboratory diagnosis of HIV in adults: a review of current methods. Clin Infect Dis. 2013;57:712-718.
11. Baron EJ, Miller MJ, Weinstein MP, et al. A guide to the utilization of the microbiology laboratory for diagnosis of infectious diseases: 2013 recommendations by the Infectious Diseases Society of America (IDSA) and the American Society for Microbiology (ASM)(a). Clin Infect Dis. 2013;57:e22-e121.
12. Delaney KP, Branson BM, Uniyal A, et al. Evaluation of the performance characteristics of 6 rapid HIV antibody tests. Clin Infect Dis. 2011;52:257-263.
13. FDA. FDA approves first rapid diagnostic test to detect both HIV-1 antigen and HIV-1/2 antibodies [press release]. US Food and Drug Administration Web site. Available at: http://www.fda.gov/newsevents/newsroom/pressannouncements/ucm364480.htm. Silver Spring, MD: US Food and Drug Administration; August 8, 2013. Accessed October 26, 2013.
14. Chavez P, Wesolowski L, Patel P, et al. Evaluation of the performance of the Abbott ARCHITECT HIV Ag/Ab Combo Assay. J Clin Virol. 2011;52:S51-S55.
15. Centers for Disease Control and Prevention (CDC). Rapid HIV testing in emergency departments—three U.S. sites, January 2005-March 2006. MMWR Morb Mortal Wkly Rep. 2007;56:597-601.
16. Napierala Mavedzenge S, Baggaley R, Corbett EL. A review of self-testing for HIV: research and policy priorities in a new era of HIV prevention. Clin Infect Dis. 2013;57:126-138.
17. Centers for Disease Control and Prevention (CDC). Detection of acute HIV infection in two evaluations of a new HIV diagnostic testing algorithm - United States, 2011-2013. MMWR Morb Mortal Wkly Rep. 2013;62:489-494.
18. Nasrullah M, Wesolowski LG, Meyer WA 3rd, et al. Performance of a fourth-generation HIV screening assay and an alternative HIV diagnostic testing algorithm. AIDS. 2013;27:731-737.
4 EKG abnormalities: What are the lifesaving diagnoses?
When evaluating a patient with a history of chest pain, palpitations, syncope, and/or new-onset seizures, an electrocardiogram (EKG) may be the key to identifying a potentially life-threatening condition. Here we present 4 cases in which EKG findings were the clue to underlying medical conditions that, if left untreated, could be fatal. Because each of these conditions may not have associated findings on a physical exam, early recognition of these EKG findings can be lifesaving.
CASE 1 › A 15-year-old boy suddenly collapses while walking, and bystanders report seizure-like activity. The patient doesn’t remember the event. Vital signs and physical exam are normal, and his blood glucose level is 86 mg/dL (normal: 70-100 mg/dL). He doesn’t take any medications and denies illicit drug use or recent illness.
What EKG abnormality (FIGURE 1) likely explains the cause of the patient’s collapse?
The EKG abnormality and diagnosis. The patient’s EKG revealed a prolonged QT interval (FIGURE 1, BRACKETS). His QTc (QT interval corrected for heart rate) was .470 seconds, which is at the high end of the normal range for his age and gender.1 The patient had no family history of syncope, sudden cardiac death (SCD), or seizure disorder. Evaluation uncovered a calcium level of 4.4 mEq/L (normal: 4.5-5.5 mEq/L) and a phosphate level of 7.8 mg/dL (normal: 2.4-4.1 mg/dL).
This patient had a low parathyroid hormone from primary hypoparathyroidism. His conduction abnormality was treated with both oral calcium and vitamin D supplements.
Etiology and epidemiology. A prolonged QT interval may be the result of a primary long QT syndrome (LQTS) or an acquired condition from electrolyte imbalance, medication effect, or toxin exposure.
In the United States, the incidence of a genetic mutation that causes LQTS is 1 in 2500 people.2 Patients with LQTS usually remain asymptomatic unless the QT interval is further prolonged by a condition or medication. There are several hundred congenital LQTS subtypes based on specific ion channel defects; the most common is LQTS1, with an inherited defect in the KCNQ1 gene, which regulates the slow potassium ion channel.
Acquired LQTS is much more common than congenital LQTS.3 Many drugs have been linked to an increased risk of LQTS, including certain antiarrhythmics, antibiotics, and antipsychotics (TABLE 1).4 In addition, electrolyte disturbances such as hypokalemia, hypocalcemia, and hypomagnesemia can be etiologic factors.
Be aware that an acquired LQTS may mask an underlying congenital LQTS. Therefore, patients in whom the offending agent or condition is corrected should still have a follow-up EKG. Screening family members for LQTS is worthwhile, even in those without symptoms.
Clinical features. Patients with symptomatic LQTS may have dizziness, palpitations, and syncope. SCD also is possible. These signs and symptoms may be triggered by strong emotions (in LQTS2) or physical activity (in LQTS1). They likely are caused by torsades de pointes and ventricular fibrillation. A brief aura may precede these arrhythmias, and patients may experience urinary or fecal incontinence.5
The key to making a diagnosis of LQTS is correctly measuring the QT interval. The QT interval is measured from the beginning of the Q-wave to the end of the T-wave as measured from the intersection of a line tangent to the downslope of the T-wave and the isoelectric line. This can be difficult to determine in EKGs showing bundle branch block or an irregular rhythm, such as atrial fibrillation (AF).6,7 A common error in measuring the QT interval occurs when clinicians inadvertently include a U-wave in the measurement.1 Some EKG machines may provide QT interval and QTc measurements. Normal QT intervals are ≤.450 seconds for men and ≤.470 seconds for women.8
It is essential to confirm the QT interval by using the Bazett formula (QTc equals the QT in seconds divided by the square root of the RR interval in seconds) for all patients with a history that suggests a possible arrhythmia.
Our patient had hypocalcemia, which on an EKG can cause T-wave widening with a normal ST segment, rather than a normal T-wave with a long ST segment, as is typically seen in LQTS. This distinction may be difficult to discern and should not preclude the search for either an acquired prolonged QTc or an underlying LQTS.9
Treatment. First rule out or treat any causes of acquired LQTS by taking a careful medication history and evaluating the patient’s electrolytes. Once these have been addressed, a beta-blocker is first-line therapy for symptomatic patients.5
Unfortunately, up to 20% of individuals treated with beta-blockers may continue to have syncope.5 For these patients, options include a left cardiac sympathetic denervation (LCSD) or placement of an automatic implantable cardioverter-defibrillator (AICD). An LCSD involves removal of the left-sided stellate and/or thoracic ganglia. This procedure can be used instead of, or in addition to, beta-blockers. If the patient’s syncope persists, AICDs are an option. AICDs can be lifesaving, but patients run the risk of adverse effects that include inappropriate shocks and infection.10 As the result of these therapies, mortality associated with LQTS has dropped to approximately 1%.11
CASE 2 › A 14-year-old boy has a syncopal episode while at rest. A similar event occurred 3 years earlier; at that time, an echocardiogram and EKG were normal. For 2 days, he’s had a cough and low-grade fever. His temperature is 102ºF and he has a productive cough. Based on this EKG (FIGURE 2), what is the likely diagnosis? What is the significance of his fever?
The EKG abnormality and diagnosis. This patient’s EKG showed a type 1 Brugada pattern (FIGURE 2, ARROWS), which strongly supported the diagnosis of Brugada syndrome (BS). BS is an inherited condition caused by a genetic defect in cardiac ion channel function that leads to characteristic EKG changes and a predisposition to ventricular fibrillation.12 In this case, the fever likely unmasked these EKG findings.
The patient was transferred to a local hospital for treatment of community-acquired pneumonia, and ultimately received an AICD.
Etiology and epidemiology. BS was first described in 199213 and is a major cause of SCD, responsible for up to 4% of all cases of SCD, and 20% of cases of patients without structural heart disease.14 BS is more common in men, and the mean age of diagnosis is 40 to 45.15-18
Mutations in at least 17 cardiac ion channel genes have been linked to BS.19 The SCN5A gene—a cardiac sodium channel—is the most commonly implicated, but accounts for only 11% to 24% of all BS cases.15
Clinical features. Patients with BS may present with syncope, nocturnal agonal respirations, or ventricular arrhythmias.12 EKG findings include partial or complete right bundle branch block (RBBB) and ST segment elevation in right precordial leads V1 to V3.12 There are 2 Brugada EKG patterns, a type 1 cloved pattern as seen in our patient’s EKG and a type 2 saddleback pattern.20 EKG findings are dynamic over time and may alternate between normal, type 1, and type 2.20 Factors that modulate EKG appearance include fever, intoxication, vagal tone, electrolyte imbalance, and sodium channel blockade.12,20
Diagnosis requires a type 1 Brugada pattern on EKG plus a family history of BS, documented ventricular arrhythmia, or arrhythmia-related symptoms such as syncope.12 Patients with a type 2 Brugada pattern may undergo electrophysiology (EP) testing with Class I antiarrhythmic drugs to induce a diagnostic type 1 Brugada pattern.12,21 Patients who have Brugada EKG findings but none of the other diagnostic criteria are considered to have a Brugada pattern (rather than Brugada syndrome).12
The most concerning outcome of BS is ventricular fibrillation. The estimated annual rate of cardiac events is 7.7% among patients who have experienced an aborted SCD, 1.9% among those who have experienced syncope, and 0.5% in asymptomatic patients.18
Treatment. The only effective treatment for BS is placement of an AICD; however, complications of AICD placement cause significant morbidity.6 Ten years after AICD placement, 37% of patients experienced inappropriate shocks and 29% experienced lead failure.22 Recent modifications in device programming and the addition of remote monitoring have decreased complication rates.12,22
The decision to place an AICD is based on the patient’s prior symptoms, EKG findings, and other factors. Recent guidelines recommend an AICD for all patients with a type 1 Brugada pattern (spontaneous or induced) who also have had an aborted SCD, syncope, or documented ventricular arrhythmia.12
Management of asymptomatic patients with type 1 Brugada pattern remains controversial because the rate of cardiac events is low, although such events can be fatal. Asymptomatic patients with type 1 Brugada findings should undergo further EP testing, and should receive AICD only upon demonstration of inducible ventricular arrhythmia.12
TABLE 2
Arrhythmias associated with Wolff-Parkinson-White syndrome23
| Arrhythmia | EKG findings | Treatment—unstable patients | Treatment—stable patients (in preferred treatment order) |
| PSVT, orthodromic | Narrow QRS, loss of delta wave, rate 160-260 beats/min | Synchronized cardioversion | Vagal maneuvers, adenosine, calcium channel blockers, beta-blockers, digoxin, procainamide |
| PSVT, antidromic | Wide complex tachycardia | Synchronized cardioversion | Procainamide |
| Atrial fibrillation | Irregularly irregular (RR interval variable with no pattern), ventricular rates that can exceed 300 beats/min | Synchronized cardioversion | Synchronized cardioversion, procainamide |
| Atrial flutter | Flutter waves, rate normal to tachycardic depending on conduction rate | Synchronized cardioversion | Synchronized cardioversion, procainamide |
| Ventricular fibrillation | Rapid, erratic electrical impulses | Defibrillation | N/A |
EKG, electrocardiogram; N/A, not applicable; PSVT, paroxysmal supraventricular tachycardia.
CASE 3 › A 21-year-old man with no medical history presents with sudden onset of lightheadedness followed by syncope. He denies any chest pain or other associated symptoms. At the time of evaluation, he is asymptomatic. His EKG (FIGURE 3) is diagnostic of what syndrome?
The EKG abnormality and diagnosis. The patient had a classic presentation for Wolff-Parkinson-White (WPW) syndrome, a common congenital disorder that alters normal cardiac conduction. He described 2 past instances of unexplained light-headedness and palpitations. Subsequent EP studies demonstrated that the patient had an accessory atrioventricular (AV) tract, causing electrical activity in the heart to bypass the AV node, resulting in a delta wave on EKG (FIGURE 3, GREEN ARROWS).
The patient opted for ablation therapy, which successfully eliminated the delta wave on EKG. Five years later he has had no recurrences.
Epidemiology. The prevalence of WPW syndrome is .1% to 3%.23 Accessory AV tracts are found in men twice as often as in women. Only half of individuals with confirmed tracts develop a tachyarrhythmia. The estimated risk of sudden death due to WPW syndrome is .5% to 4%.24
Pathophysiology. Normally cardiac conduction originates from the sinus node and travels to the AV node, where conduction is slowed, and then proceeds to the His-Purkinje system, and finally to the rest of the ventricular myocardium. In WPW syndrome, ventricular depolarization occurs first by an accessory AV tract called the bundle of Kent, followed shortly thereafter by the His-Purkinje system. This sequence of depolarization is what leads to the EKG findings characteristic of WPW syndrome: a PR interval <.12 seconds, presence of a delta wave, widened QRS complex (>.12 seconds), and repolarization changes seen as ST segment and T-wave changes discordant to (opposite direction) the delta wave and QRS complex (FIGURE 3, RED ARROW).
Factors that influence electrical conduction through the bundle of Kent include cardioactive medications, physiological stress, circulating catecholamines, coronary ischemia, and aging. The end result is a propensity for the heart to convert to one of 4 arrhythmias: paroxysmal supraventricular tachycardia (PSVT), AF, atrial flutter, or ventricular fibrillation (TABLE 2).23
The most common arrhythmia in WPW syndrome is PSVT.23 This rhythm is induced by the formation of a reentry circuit—a pattern in which the heart’s electrical signal loops back on itself—involving the normal conduction pathway and the bundle of Kent. Reentry progressing down the His-Purkinje system and traveling up the bundle of Kent is referred to as orthodromic (anterograde) PSVT. Antidromic (retrograde) PSVT is due to a reentry circuit conducting from the bundle of Kent to the ventricles, and then retrograde through the His-Purkinje system and AV node to the atria.
Clinical features. Under normal circumstances, patients with WPW syndrome are asymptomatic. As was the case with our patient, individuals who develop one of the 4 characteristic arrhythmias can experience light-headedness and syncope.
Treatment. An unstable patient who is experiencing PSVT, AF, or atrial flutter should receive synchronized cardioversion; those experiencing ventricular fibrillation should receive defibrillation (TABLE 2).23 For stable patients, therapy is tailored to the type of arrhythmia. Calcium channel blockers, beta-blockers, and adenosine might be appropriate for patients with orthodromic PSVT but should be avoided in patients with antidromic PSVT, AF, or atrial flutter because these medications block AV node conduction and thus facilitate conduction down the bundle of Kent, which can result in potentially unstable arrhythmias. In general, the longer an arrhythmia has been present, the less effective the pharmacologic intervention because of the increasing sympathetic tone.
Preventive long-term therapies for WPW patients who have experienced arrhythmia include antiarrhythmic medications or ablative procedures. Long-term antiarrhythmic therapy often is reserved for older, more sedentary individuals with less frequent arrhythmias that are not life-threatening. Radiofrequency ablation is a popular option, with long-term success rates as high as 95% and complication rates <1%.23 Patients in whom a WPW pattern is identified incidentally on EKG should be referred to cardiology for EP studies and risk stratification.25
CASE 4 › A 61-year-old woman has an episode of substernal exertional chest pressure that lasted approximately 2 hours but resolved before she arrived at her physician’s office. She also experienced mild nausea. She has no history of coronary artery disease but says that she has experienced similar episodes of chest pressure. What abnormality is seen on her EKG (FIGURE 4)? What is the most likely cause of her symptoms?
The EKG abnormality and diagnosis. Although classically associated with syncope, hypertrophic cardiomyopathy (HCM) often presents similarly to acute coronary syndromes, with chest pain and dyspnea on exertion.26 This patient had no history of cardiac disease or family history of SCD or cardiomyopathy; however, her EKG showed changes indicating left ventricular hypertrophy (LVH), which is consistent with HCM (FIGURE 4, ARROWS). Echocardiography identified myocardial hypertrophy, normal left ventricular ejection fraction, but severe left ventricular outflow obstruction and mild diastolic dysfunction. She was treated with metoprolol and verapamil.
Etiology and epidemiology. Hypertrophic cardiomyopathy is an autosomal dominant intrinsic myocardial disorder resulting in LVH that is commonly associated with SCD during extreme physical activity.26,27 The prevalence of HCM is approximately 1 in 500.26 Although it can present at any age, it is the most common cause of SCD in young people (under age 30), responsible for 33% of deaths during athletic events.28
TABLE 3
4 diagnoses and what you'll see on EKG
| Diagnosis | EKG finding |
| Prolonged QT interval | QTc interval >.450 sec (men) or >.470 sec (women) |
| Brugada syndrome | Partial or complete RBBB, and ST segment elevation in right precordial leads V1-V3 |
| Wolff-Parkinson-White syndrome | Delta wave, widened QRS, short PR interval, ST segment and T-wave changes |
| Hypertrophic cardiomyopathy | No definitive finding; may have left ventricular hypertrophy or abnormal Q-waves |
EKG, electrocardiogram; RBBB, right bundle branch block.
Clinical features. The severity of HCM ranges from asymptomatic to fatal. Symptoms of HCM include chest pain, dyspnea, and syncope. The disorder causes morbidity and mortality in at least one of 3 ways: ventricular tachyarrhythmias (often in younger patients), heart failure (from left ventricular outflow obstruction), and/or thromboembolism.27
Although echocardiography typically is used to make the diagnosis,27 an EKG often is the initial screening tool. EKG changes are seen in 75% to 95% of affected patients and include the presence of Q-waves and increased voltages related to LVH.27,29 Infarct-like patterns may be present before wall thickening on echocardiogram. Abnormal Q-waves are found in 20% to 50% of HCM patients, and are more common in younger patients. Konno et al30 have shown that Q-waves >3 mm in depth and/or >.040 seconds in duration in at least 2 leads other than aVR is specific (90%) in identifying carriers of HCM genes before they develop clinical symptoms.
Ambulatory monitoring may be useful for risk stratifying HCM patients; those with nonsustained ventricular tachycardia (NSVT) are at higher risk of SCD. Holter monitoring is recommended in initial evaluation because evidence of ventricular tachyarrhythmias may warrant AICD placement.26
Treatment. The risk of SCD in HCM is approximately 1%, but higher in those with a family history of SCD, syncope, NSVT, hypotension during exercise, or severe LVH (left ventricle thickness >30 mm).26 AICDs are recommended for HCM patients with prior cardiac arrest, patients with ≥2 of these risk factors, or patients with one risk factor who have experienced syncope related to arrhythmia.26
For patients who are symptomatic but have <2 risk factors, beta-blockers are firstline therapy.26 Verapamil is used as a second line treatment. Both beta-blockers and calcium channel blockers reduce dyspnea, palpitations, and chest pain.27
For patients who don’t respond to medical therapy, septal reduction therapy may be performed, either by septal myectomy or alcohol septal ablation.27 It is also important to consider genetic screening and counseling for the family.
A summary of all 4 diagnoses described in this article, their associated EKG findings, and their pathophysiology appears in TABLE 3.
CORRESPONDENCE
Samir Haydar, DO, MPH, FACEP, Tufts University School of Medicine, Maine Medical Center, Department of Emergency Medicine, 47 Bramhall St., Portland, ME 04103; [email protected]
1. Taggart NW, Haglund CM, Tester DJ, et al. Diagnostic miscues in congenital long-QT syndrome. Circulation. 2007;115:2613-2620.
2. Schwartz PJ, Stramba-Badiale M, Crotti L, et al. Prevalance of the congenital long QT syndrome. Circulation. 2009;120:1761-1767.
3. van Noord C, Eijgelsheim M, Stricker BH. Drug- and nondrug-associated QT interval prolongation. Br J Clin Pharmacol. 2010;70:16-23.
4. Credible Meds Web site. Available at: http://crediblemeds.org. Accessed April 8, 2014.
5. Schwartz PJ, Priori SG, Spazzolini C, et al. Genotype-phenotype correlation in the long QT syndrome: gene specific triggers for life threatening arrhythmias. Circulation. 2001;103:89.
6. Chiladakis JK, Kalogeropoulos A, Koutsogiannis N, et al. Optimal QT/JT interval assessment in patients with complete bundle branch block. Ann Noninvasive Electrocardiol. 2012;17:268-276.
7. Ercan S, Altunbas G, Oylumlu M, et al. Congenital long QT syndrome masked by atrial fibrillation and unmasked by hypokalemia. Am J Emerg Med. 2013;31:451.e3-451.e6.
8. Goldenberg I, Moss AJ, Zareba W. QT interval: how to measure it and what is “normal.” J Cardiovasc Electrophysiol. 2006;17:333-336.
9. Podrid PJ. ECG Response: August 20, 2013. Circulation. 2013;128:869.
10. Olde Nordkamp LR, Wilde AA, Tijssen JG, et al. The ICD for primary prevention in patients with inherited cardiac diseases: indications, use, and outcome: a comparison with secondary prevention. Circ Arrhythm Electrophysiol. 2013;6:91-100.
11. Schwartz PJ. Pharmacological and non-pharmacological management of the congenital long QT syndrome: the rationale. Pharmacol Ther. 2011;131:171-177.
12. Berne P, Brugada J. Brugada syndrome 2012. Circ J. 2012;76:1563-1571.
13. Brugada P, Brugada J. Right bundle branch block, persistent ST segment elevation and sudden cardiac death: a distinct clinical and electrocardiographic syndrome. A multicenter report. J Am Coll Cardiol. 1992;20:1391-1396.
14. Antzelevitch C, Brugada P, Borggrefe M, et al. Brugada syndrome: report of the second consensus conference: endorsed by the Heart Rhythm Society and the European Heart Rhythm Association. Circulation. 2005;111:659-670.
15. Brugada J, Brugada R, Antzelevitch C, et al. Long-term followup of individuals with the electrocardiographic pattern of right bundle-branch block and ST-segment elevation in precordial leads V1 to V3. Circulation. 2002;105:73-78.
16. Eckardt L, Probst V, Smits JP, et al. Long-term prognosis of individuals with right precordial ST-segment-elevation Brugada syndrome. Circulation. 2005;111:257-263.
17. Giustetto C, Drago S, Demarchi PG, et al; Italian Association of Arrhythmology and Cardiostimulation (AIAC)-Piedmont Section. Risk stratification of the patients with Brugada type electrocardiogram: a community-based prospective study. Europace. 2009;11:507-513.
18. Probst V, Veltmann C, Eckardt L, et al. Long-term prognosis of patients diagnosed with Brugada syndrome: Results from the FINGER Brugada Syndrome Registry. Circulation. 2010;121:635-643.
19. Nielsen MW, Holst AG, Olesen SP, et al. The genetic component of Brugada syndrome. Front Physiol. 2013;4:179.
20. Bayés de Luna A, Brugada J, Baranchuk A, et al. Current electrocardiographic criteria for diagnosis of Brugada pattern: a consensus report. J Electrocardiol. 2012;45:433-442.
21. Veltmann C, Schimpf R, Echternach C, et al. A prospective study on spontaneous fluctuations between diagnostic and non-diagnostic ECGs in Brugada syndrome: implications for correct phenotyping and risk stratification. Eur Heart J. 2006;27:2544-2552.
22. Sacher F, Probst V, Maury P, et al. Outcome after implantation of a cardioverter-defibrillator in patients with Brugada syndrome: a multicenter study-part 2. Circulation. 2013;128:1739-1747.
23. Rosner MH, Brady WJ Jr, Kefer MP, et al. Electrocardiography in the patient with the Wolff-Parkinson-White syndrome: diagnostic and initial therapeutic issues. Am J Emerg Med. 1999;17:705-714.
24. Keating L, Morris FP, Brady WJ. Electrocardiographic features of Wolff-Parkinson-White syndrome. Emerg Med J. 2003;20:491-493.
25. Blomström-Lundqvist C, Scheinman MM, Aliot EM, et al; European Society of Cardiology Committee, NASPE-Heart Rhythm Society. ACC/AHA/ESC guidelines for the management of patients with supraventricular arrhythmias—executive summary. A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the European Society of Cardiology Committee for Practice Guidelines (writing committee to develop guidelines for the management of patients with supraventricular arrhythmias) developed in collaboration with NASPE-Heart Rhythm Society. J Am Coll Cardiol. 2003;42:1493-1531.
26. Ho CY. Hypertrophic cardiomyopathy in 2012. Circulation. 2012;125:1432-1438.
27. Gersh BJ, Maron BJ, Bonow RO, et al; American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines; American Association for Thoracic Surgery; American Society of Echocardiography; American Society of Nuclear Cardiology; Heart Failure Society of America; Heart Rhythm Society; Society for Cardiovascular Angiography and Interventions; Society of Thoracic Surgeons. 2011 ACCF/AHA guideline for the diagnosis and treatment of hypertrophic cardiomyopathy: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation. 2011;124:e783-e831.
28. Paterick TE, Jan MF, Paterick ZR, et al. Cardiac evaluation of collegiate student athletes: a medical and legal perspective. Am J Med. 2012;125:742-752.
29. Maron BJ. Hypertrophic cardiomyopathy. In: Bonow RO, Mann DL, Zipes DP, et al (eds). Braunwald’s Heart Disease: A Textbook of Cardiovascular Medicine. 9th ed. Philadelphia, PA: Saunders; 2011:1582-1594.
30. Konno T, Shimizu M, Ino H, et al. Diagnostic value of abnormal Q waves for identification of preclinical carriers of hypertrophic cardiomyopathy based on molecular genetic diagnosis. Eur Heart J. 2004;25:246-251.
When evaluating a patient with a history of chest pain, palpitations, syncope, and/or new-onset seizures, an electrocardiogram (EKG) may be the key to identifying a potentially life-threatening condition. Here we present 4 cases in which EKG findings were the clue to underlying medical conditions that, if left untreated, could be fatal. Because each of these conditions may not have associated findings on a physical exam, early recognition of these EKG findings can be lifesaving.
CASE 1 › A 15-year-old boy suddenly collapses while walking, and bystanders report seizure-like activity. The patient doesn’t remember the event. Vital signs and physical exam are normal, and his blood glucose level is 86 mg/dL (normal: 70-100 mg/dL). He doesn’t take any medications and denies illicit drug use or recent illness.
What EKG abnormality (FIGURE 1) likely explains the cause of the patient’s collapse?
The EKG abnormality and diagnosis. The patient’s EKG revealed a prolonged QT interval (FIGURE 1, BRACKETS). His QTc (QT interval corrected for heart rate) was .470 seconds, which is at the high end of the normal range for his age and gender.1 The patient had no family history of syncope, sudden cardiac death (SCD), or seizure disorder. Evaluation uncovered a calcium level of 4.4 mEq/L (normal: 4.5-5.5 mEq/L) and a phosphate level of 7.8 mg/dL (normal: 2.4-4.1 mg/dL).
This patient had a low parathyroid hormone from primary hypoparathyroidism. His conduction abnormality was treated with both oral calcium and vitamin D supplements.
Etiology and epidemiology. A prolonged QT interval may be the result of a primary long QT syndrome (LQTS) or an acquired condition from electrolyte imbalance, medication effect, or toxin exposure.
In the United States, the incidence of a genetic mutation that causes LQTS is 1 in 2500 people.2 Patients with LQTS usually remain asymptomatic unless the QT interval is further prolonged by a condition or medication. There are several hundred congenital LQTS subtypes based on specific ion channel defects; the most common is LQTS1, with an inherited defect in the KCNQ1 gene, which regulates the slow potassium ion channel.
Acquired LQTS is much more common than congenital LQTS.3 Many drugs have been linked to an increased risk of LQTS, including certain antiarrhythmics, antibiotics, and antipsychotics (TABLE 1).4 In addition, electrolyte disturbances such as hypokalemia, hypocalcemia, and hypomagnesemia can be etiologic factors.
Be aware that an acquired LQTS may mask an underlying congenital LQTS. Therefore, patients in whom the offending agent or condition is corrected should still have a follow-up EKG. Screening family members for LQTS is worthwhile, even in those without symptoms.
Clinical features. Patients with symptomatic LQTS may have dizziness, palpitations, and syncope. SCD also is possible. These signs and symptoms may be triggered by strong emotions (in LQTS2) or physical activity (in LQTS1). They likely are caused by torsades de pointes and ventricular fibrillation. A brief aura may precede these arrhythmias, and patients may experience urinary or fecal incontinence.5
The key to making a diagnosis of LQTS is correctly measuring the QT interval. The QT interval is measured from the beginning of the Q-wave to the end of the T-wave as measured from the intersection of a line tangent to the downslope of the T-wave and the isoelectric line. This can be difficult to determine in EKGs showing bundle branch block or an irregular rhythm, such as atrial fibrillation (AF).6,7 A common error in measuring the QT interval occurs when clinicians inadvertently include a U-wave in the measurement.1 Some EKG machines may provide QT interval and QTc measurements. Normal QT intervals are ≤.450 seconds for men and ≤.470 seconds for women.8
It is essential to confirm the QT interval by using the Bazett formula (QTc equals the QT in seconds divided by the square root of the RR interval in seconds) for all patients with a history that suggests a possible arrhythmia.
Our patient had hypocalcemia, which on an EKG can cause T-wave widening with a normal ST segment, rather than a normal T-wave with a long ST segment, as is typically seen in LQTS. This distinction may be difficult to discern and should not preclude the search for either an acquired prolonged QTc or an underlying LQTS.9
Treatment. First rule out or treat any causes of acquired LQTS by taking a careful medication history and evaluating the patient’s electrolytes. Once these have been addressed, a beta-blocker is first-line therapy for symptomatic patients.5
Unfortunately, up to 20% of individuals treated with beta-blockers may continue to have syncope.5 For these patients, options include a left cardiac sympathetic denervation (LCSD) or placement of an automatic implantable cardioverter-defibrillator (AICD). An LCSD involves removal of the left-sided stellate and/or thoracic ganglia. This procedure can be used instead of, or in addition to, beta-blockers. If the patient’s syncope persists, AICDs are an option. AICDs can be lifesaving, but patients run the risk of adverse effects that include inappropriate shocks and infection.10 As the result of these therapies, mortality associated with LQTS has dropped to approximately 1%.11
CASE 2 › A 14-year-old boy has a syncopal episode while at rest. A similar event occurred 3 years earlier; at that time, an echocardiogram and EKG were normal. For 2 days, he’s had a cough and low-grade fever. His temperature is 102ºF and he has a productive cough. Based on this EKG (FIGURE 2), what is the likely diagnosis? What is the significance of his fever?
The EKG abnormality and diagnosis. This patient’s EKG showed a type 1 Brugada pattern (FIGURE 2, ARROWS), which strongly supported the diagnosis of Brugada syndrome (BS). BS is an inherited condition caused by a genetic defect in cardiac ion channel function that leads to characteristic EKG changes and a predisposition to ventricular fibrillation.12 In this case, the fever likely unmasked these EKG findings.
The patient was transferred to a local hospital for treatment of community-acquired pneumonia, and ultimately received an AICD.
Etiology and epidemiology. BS was first described in 199213 and is a major cause of SCD, responsible for up to 4% of all cases of SCD, and 20% of cases of patients without structural heart disease.14 BS is more common in men, and the mean age of diagnosis is 40 to 45.15-18
Mutations in at least 17 cardiac ion channel genes have been linked to BS.19 The SCN5A gene—a cardiac sodium channel—is the most commonly implicated, but accounts for only 11% to 24% of all BS cases.15
Clinical features. Patients with BS may present with syncope, nocturnal agonal respirations, or ventricular arrhythmias.12 EKG findings include partial or complete right bundle branch block (RBBB) and ST segment elevation in right precordial leads V1 to V3.12 There are 2 Brugada EKG patterns, a type 1 cloved pattern as seen in our patient’s EKG and a type 2 saddleback pattern.20 EKG findings are dynamic over time and may alternate between normal, type 1, and type 2.20 Factors that modulate EKG appearance include fever, intoxication, vagal tone, electrolyte imbalance, and sodium channel blockade.12,20
Diagnosis requires a type 1 Brugada pattern on EKG plus a family history of BS, documented ventricular arrhythmia, or arrhythmia-related symptoms such as syncope.12 Patients with a type 2 Brugada pattern may undergo electrophysiology (EP) testing with Class I antiarrhythmic drugs to induce a diagnostic type 1 Brugada pattern.12,21 Patients who have Brugada EKG findings but none of the other diagnostic criteria are considered to have a Brugada pattern (rather than Brugada syndrome).12
The most concerning outcome of BS is ventricular fibrillation. The estimated annual rate of cardiac events is 7.7% among patients who have experienced an aborted SCD, 1.9% among those who have experienced syncope, and 0.5% in asymptomatic patients.18
Treatment. The only effective treatment for BS is placement of an AICD; however, complications of AICD placement cause significant morbidity.6 Ten years after AICD placement, 37% of patients experienced inappropriate shocks and 29% experienced lead failure.22 Recent modifications in device programming and the addition of remote monitoring have decreased complication rates.12,22
The decision to place an AICD is based on the patient’s prior symptoms, EKG findings, and other factors. Recent guidelines recommend an AICD for all patients with a type 1 Brugada pattern (spontaneous or induced) who also have had an aborted SCD, syncope, or documented ventricular arrhythmia.12
Management of asymptomatic patients with type 1 Brugada pattern remains controversial because the rate of cardiac events is low, although such events can be fatal. Asymptomatic patients with type 1 Brugada findings should undergo further EP testing, and should receive AICD only upon demonstration of inducible ventricular arrhythmia.12
TABLE 2
Arrhythmias associated with Wolff-Parkinson-White syndrome23
| Arrhythmia | EKG findings | Treatment—unstable patients | Treatment—stable patients (in preferred treatment order) |
| PSVT, orthodromic | Narrow QRS, loss of delta wave, rate 160-260 beats/min | Synchronized cardioversion | Vagal maneuvers, adenosine, calcium channel blockers, beta-blockers, digoxin, procainamide |
| PSVT, antidromic | Wide complex tachycardia | Synchronized cardioversion | Procainamide |
| Atrial fibrillation | Irregularly irregular (RR interval variable with no pattern), ventricular rates that can exceed 300 beats/min | Synchronized cardioversion | Synchronized cardioversion, procainamide |
| Atrial flutter | Flutter waves, rate normal to tachycardic depending on conduction rate | Synchronized cardioversion | Synchronized cardioversion, procainamide |
| Ventricular fibrillation | Rapid, erratic electrical impulses | Defibrillation | N/A |
EKG, electrocardiogram; N/A, not applicable; PSVT, paroxysmal supraventricular tachycardia.
CASE 3 › A 21-year-old man with no medical history presents with sudden onset of lightheadedness followed by syncope. He denies any chest pain or other associated symptoms. At the time of evaluation, he is asymptomatic. His EKG (FIGURE 3) is diagnostic of what syndrome?
The EKG abnormality and diagnosis. The patient had a classic presentation for Wolff-Parkinson-White (WPW) syndrome, a common congenital disorder that alters normal cardiac conduction. He described 2 past instances of unexplained light-headedness and palpitations. Subsequent EP studies demonstrated that the patient had an accessory atrioventricular (AV) tract, causing electrical activity in the heart to bypass the AV node, resulting in a delta wave on EKG (FIGURE 3, GREEN ARROWS).
The patient opted for ablation therapy, which successfully eliminated the delta wave on EKG. Five years later he has had no recurrences.
Epidemiology. The prevalence of WPW syndrome is .1% to 3%.23 Accessory AV tracts are found in men twice as often as in women. Only half of individuals with confirmed tracts develop a tachyarrhythmia. The estimated risk of sudden death due to WPW syndrome is .5% to 4%.24
Pathophysiology. Normally cardiac conduction originates from the sinus node and travels to the AV node, where conduction is slowed, and then proceeds to the His-Purkinje system, and finally to the rest of the ventricular myocardium. In WPW syndrome, ventricular depolarization occurs first by an accessory AV tract called the bundle of Kent, followed shortly thereafter by the His-Purkinje system. This sequence of depolarization is what leads to the EKG findings characteristic of WPW syndrome: a PR interval <.12 seconds, presence of a delta wave, widened QRS complex (>.12 seconds), and repolarization changes seen as ST segment and T-wave changes discordant to (opposite direction) the delta wave and QRS complex (FIGURE 3, RED ARROW).
Factors that influence electrical conduction through the bundle of Kent include cardioactive medications, physiological stress, circulating catecholamines, coronary ischemia, and aging. The end result is a propensity for the heart to convert to one of 4 arrhythmias: paroxysmal supraventricular tachycardia (PSVT), AF, atrial flutter, or ventricular fibrillation (TABLE 2).23
The most common arrhythmia in WPW syndrome is PSVT.23 This rhythm is induced by the formation of a reentry circuit—a pattern in which the heart’s electrical signal loops back on itself—involving the normal conduction pathway and the bundle of Kent. Reentry progressing down the His-Purkinje system and traveling up the bundle of Kent is referred to as orthodromic (anterograde) PSVT. Antidromic (retrograde) PSVT is due to a reentry circuit conducting from the bundle of Kent to the ventricles, and then retrograde through the His-Purkinje system and AV node to the atria.
Clinical features. Under normal circumstances, patients with WPW syndrome are asymptomatic. As was the case with our patient, individuals who develop one of the 4 characteristic arrhythmias can experience light-headedness and syncope.
Treatment. An unstable patient who is experiencing PSVT, AF, or atrial flutter should receive synchronized cardioversion; those experiencing ventricular fibrillation should receive defibrillation (TABLE 2).23 For stable patients, therapy is tailored to the type of arrhythmia. Calcium channel blockers, beta-blockers, and adenosine might be appropriate for patients with orthodromic PSVT but should be avoided in patients with antidromic PSVT, AF, or atrial flutter because these medications block AV node conduction and thus facilitate conduction down the bundle of Kent, which can result in potentially unstable arrhythmias. In general, the longer an arrhythmia has been present, the less effective the pharmacologic intervention because of the increasing sympathetic tone.
Preventive long-term therapies for WPW patients who have experienced arrhythmia include antiarrhythmic medications or ablative procedures. Long-term antiarrhythmic therapy often is reserved for older, more sedentary individuals with less frequent arrhythmias that are not life-threatening. Radiofrequency ablation is a popular option, with long-term success rates as high as 95% and complication rates <1%.23 Patients in whom a WPW pattern is identified incidentally on EKG should be referred to cardiology for EP studies and risk stratification.25
CASE 4 › A 61-year-old woman has an episode of substernal exertional chest pressure that lasted approximately 2 hours but resolved before she arrived at her physician’s office. She also experienced mild nausea. She has no history of coronary artery disease but says that she has experienced similar episodes of chest pressure. What abnormality is seen on her EKG (FIGURE 4)? What is the most likely cause of her symptoms?
The EKG abnormality and diagnosis. Although classically associated with syncope, hypertrophic cardiomyopathy (HCM) often presents similarly to acute coronary syndromes, with chest pain and dyspnea on exertion.26 This patient had no history of cardiac disease or family history of SCD or cardiomyopathy; however, her EKG showed changes indicating left ventricular hypertrophy (LVH), which is consistent with HCM (FIGURE 4, ARROWS). Echocardiography identified myocardial hypertrophy, normal left ventricular ejection fraction, but severe left ventricular outflow obstruction and mild diastolic dysfunction. She was treated with metoprolol and verapamil.
Etiology and epidemiology. Hypertrophic cardiomyopathy is an autosomal dominant intrinsic myocardial disorder resulting in LVH that is commonly associated with SCD during extreme physical activity.26,27 The prevalence of HCM is approximately 1 in 500.26 Although it can present at any age, it is the most common cause of SCD in young people (under age 30), responsible for 33% of deaths during athletic events.28
TABLE 3
4 diagnoses and what you'll see on EKG
| Diagnosis | EKG finding |
| Prolonged QT interval | QTc interval >.450 sec (men) or >.470 sec (women) |
| Brugada syndrome | Partial or complete RBBB, and ST segment elevation in right precordial leads V1-V3 |
| Wolff-Parkinson-White syndrome | Delta wave, widened QRS, short PR interval, ST segment and T-wave changes |
| Hypertrophic cardiomyopathy | No definitive finding; may have left ventricular hypertrophy or abnormal Q-waves |
EKG, electrocardiogram; RBBB, right bundle branch block.
Clinical features. The severity of HCM ranges from asymptomatic to fatal. Symptoms of HCM include chest pain, dyspnea, and syncope. The disorder causes morbidity and mortality in at least one of 3 ways: ventricular tachyarrhythmias (often in younger patients), heart failure (from left ventricular outflow obstruction), and/or thromboembolism.27
Although echocardiography typically is used to make the diagnosis,27 an EKG often is the initial screening tool. EKG changes are seen in 75% to 95% of affected patients and include the presence of Q-waves and increased voltages related to LVH.27,29 Infarct-like patterns may be present before wall thickening on echocardiogram. Abnormal Q-waves are found in 20% to 50% of HCM patients, and are more common in younger patients. Konno et al30 have shown that Q-waves >3 mm in depth and/or >.040 seconds in duration in at least 2 leads other than aVR is specific (90%) in identifying carriers of HCM genes before they develop clinical symptoms.
Ambulatory monitoring may be useful for risk stratifying HCM patients; those with nonsustained ventricular tachycardia (NSVT) are at higher risk of SCD. Holter monitoring is recommended in initial evaluation because evidence of ventricular tachyarrhythmias may warrant AICD placement.26
Treatment. The risk of SCD in HCM is approximately 1%, but higher in those with a family history of SCD, syncope, NSVT, hypotension during exercise, or severe LVH (left ventricle thickness >30 mm).26 AICDs are recommended for HCM patients with prior cardiac arrest, patients with ≥2 of these risk factors, or patients with one risk factor who have experienced syncope related to arrhythmia.26
For patients who are symptomatic but have <2 risk factors, beta-blockers are firstline therapy.26 Verapamil is used as a second line treatment. Both beta-blockers and calcium channel blockers reduce dyspnea, palpitations, and chest pain.27
For patients who don’t respond to medical therapy, septal reduction therapy may be performed, either by septal myectomy or alcohol septal ablation.27 It is also important to consider genetic screening and counseling for the family.
A summary of all 4 diagnoses described in this article, their associated EKG findings, and their pathophysiology appears in TABLE 3.
CORRESPONDENCE
Samir Haydar, DO, MPH, FACEP, Tufts University School of Medicine, Maine Medical Center, Department of Emergency Medicine, 47 Bramhall St., Portland, ME 04103; [email protected]
When evaluating a patient with a history of chest pain, palpitations, syncope, and/or new-onset seizures, an electrocardiogram (EKG) may be the key to identifying a potentially life-threatening condition. Here we present 4 cases in which EKG findings were the clue to underlying medical conditions that, if left untreated, could be fatal. Because each of these conditions may not have associated findings on a physical exam, early recognition of these EKG findings can be lifesaving.
CASE 1 › A 15-year-old boy suddenly collapses while walking, and bystanders report seizure-like activity. The patient doesn’t remember the event. Vital signs and physical exam are normal, and his blood glucose level is 86 mg/dL (normal: 70-100 mg/dL). He doesn’t take any medications and denies illicit drug use or recent illness.
What EKG abnormality (FIGURE 1) likely explains the cause of the patient’s collapse?
The EKG abnormality and diagnosis. The patient’s EKG revealed a prolonged QT interval (FIGURE 1, BRACKETS). His QTc (QT interval corrected for heart rate) was .470 seconds, which is at the high end of the normal range for his age and gender.1 The patient had no family history of syncope, sudden cardiac death (SCD), or seizure disorder. Evaluation uncovered a calcium level of 4.4 mEq/L (normal: 4.5-5.5 mEq/L) and a phosphate level of 7.8 mg/dL (normal: 2.4-4.1 mg/dL).
This patient had a low parathyroid hormone from primary hypoparathyroidism. His conduction abnormality was treated with both oral calcium and vitamin D supplements.
Etiology and epidemiology. A prolonged QT interval may be the result of a primary long QT syndrome (LQTS) or an acquired condition from electrolyte imbalance, medication effect, or toxin exposure.
In the United States, the incidence of a genetic mutation that causes LQTS is 1 in 2500 people.2 Patients with LQTS usually remain asymptomatic unless the QT interval is further prolonged by a condition or medication. There are several hundred congenital LQTS subtypes based on specific ion channel defects; the most common is LQTS1, with an inherited defect in the KCNQ1 gene, which regulates the slow potassium ion channel.
Acquired LQTS is much more common than congenital LQTS.3 Many drugs have been linked to an increased risk of LQTS, including certain antiarrhythmics, antibiotics, and antipsychotics (TABLE 1).4 In addition, electrolyte disturbances such as hypokalemia, hypocalcemia, and hypomagnesemia can be etiologic factors.
Be aware that an acquired LQTS may mask an underlying congenital LQTS. Therefore, patients in whom the offending agent or condition is corrected should still have a follow-up EKG. Screening family members for LQTS is worthwhile, even in those without symptoms.
Clinical features. Patients with symptomatic LQTS may have dizziness, palpitations, and syncope. SCD also is possible. These signs and symptoms may be triggered by strong emotions (in LQTS2) or physical activity (in LQTS1). They likely are caused by torsades de pointes and ventricular fibrillation. A brief aura may precede these arrhythmias, and patients may experience urinary or fecal incontinence.5
The key to making a diagnosis of LQTS is correctly measuring the QT interval. The QT interval is measured from the beginning of the Q-wave to the end of the T-wave as measured from the intersection of a line tangent to the downslope of the T-wave and the isoelectric line. This can be difficult to determine in EKGs showing bundle branch block or an irregular rhythm, such as atrial fibrillation (AF).6,7 A common error in measuring the QT interval occurs when clinicians inadvertently include a U-wave in the measurement.1 Some EKG machines may provide QT interval and QTc measurements. Normal QT intervals are ≤.450 seconds for men and ≤.470 seconds for women.8
It is essential to confirm the QT interval by using the Bazett formula (QTc equals the QT in seconds divided by the square root of the RR interval in seconds) for all patients with a history that suggests a possible arrhythmia.
Our patient had hypocalcemia, which on an EKG can cause T-wave widening with a normal ST segment, rather than a normal T-wave with a long ST segment, as is typically seen in LQTS. This distinction may be difficult to discern and should not preclude the search for either an acquired prolonged QTc or an underlying LQTS.9
Treatment. First rule out or treat any causes of acquired LQTS by taking a careful medication history and evaluating the patient’s electrolytes. Once these have been addressed, a beta-blocker is first-line therapy for symptomatic patients.5
Unfortunately, up to 20% of individuals treated with beta-blockers may continue to have syncope.5 For these patients, options include a left cardiac sympathetic denervation (LCSD) or placement of an automatic implantable cardioverter-defibrillator (AICD). An LCSD involves removal of the left-sided stellate and/or thoracic ganglia. This procedure can be used instead of, or in addition to, beta-blockers. If the patient’s syncope persists, AICDs are an option. AICDs can be lifesaving, but patients run the risk of adverse effects that include inappropriate shocks and infection.10 As the result of these therapies, mortality associated with LQTS has dropped to approximately 1%.11
CASE 2 › A 14-year-old boy has a syncopal episode while at rest. A similar event occurred 3 years earlier; at that time, an echocardiogram and EKG were normal. For 2 days, he’s had a cough and low-grade fever. His temperature is 102ºF and he has a productive cough. Based on this EKG (FIGURE 2), what is the likely diagnosis? What is the significance of his fever?
The EKG abnormality and diagnosis. This patient’s EKG showed a type 1 Brugada pattern (FIGURE 2, ARROWS), which strongly supported the diagnosis of Brugada syndrome (BS). BS is an inherited condition caused by a genetic defect in cardiac ion channel function that leads to characteristic EKG changes and a predisposition to ventricular fibrillation.12 In this case, the fever likely unmasked these EKG findings.
The patient was transferred to a local hospital for treatment of community-acquired pneumonia, and ultimately received an AICD.
Etiology and epidemiology. BS was first described in 199213 and is a major cause of SCD, responsible for up to 4% of all cases of SCD, and 20% of cases of patients without structural heart disease.14 BS is more common in men, and the mean age of diagnosis is 40 to 45.15-18
Mutations in at least 17 cardiac ion channel genes have been linked to BS.19 The SCN5A gene—a cardiac sodium channel—is the most commonly implicated, but accounts for only 11% to 24% of all BS cases.15
Clinical features. Patients with BS may present with syncope, nocturnal agonal respirations, or ventricular arrhythmias.12 EKG findings include partial or complete right bundle branch block (RBBB) and ST segment elevation in right precordial leads V1 to V3.12 There are 2 Brugada EKG patterns, a type 1 cloved pattern as seen in our patient’s EKG and a type 2 saddleback pattern.20 EKG findings are dynamic over time and may alternate between normal, type 1, and type 2.20 Factors that modulate EKG appearance include fever, intoxication, vagal tone, electrolyte imbalance, and sodium channel blockade.12,20
Diagnosis requires a type 1 Brugada pattern on EKG plus a family history of BS, documented ventricular arrhythmia, or arrhythmia-related symptoms such as syncope.12 Patients with a type 2 Brugada pattern may undergo electrophysiology (EP) testing with Class I antiarrhythmic drugs to induce a diagnostic type 1 Brugada pattern.12,21 Patients who have Brugada EKG findings but none of the other diagnostic criteria are considered to have a Brugada pattern (rather than Brugada syndrome).12
The most concerning outcome of BS is ventricular fibrillation. The estimated annual rate of cardiac events is 7.7% among patients who have experienced an aborted SCD, 1.9% among those who have experienced syncope, and 0.5% in asymptomatic patients.18
Treatment. The only effective treatment for BS is placement of an AICD; however, complications of AICD placement cause significant morbidity.6 Ten years after AICD placement, 37% of patients experienced inappropriate shocks and 29% experienced lead failure.22 Recent modifications in device programming and the addition of remote monitoring have decreased complication rates.12,22
The decision to place an AICD is based on the patient’s prior symptoms, EKG findings, and other factors. Recent guidelines recommend an AICD for all patients with a type 1 Brugada pattern (spontaneous or induced) who also have had an aborted SCD, syncope, or documented ventricular arrhythmia.12
Management of asymptomatic patients with type 1 Brugada pattern remains controversial because the rate of cardiac events is low, although such events can be fatal. Asymptomatic patients with type 1 Brugada findings should undergo further EP testing, and should receive AICD only upon demonstration of inducible ventricular arrhythmia.12
TABLE 2
Arrhythmias associated with Wolff-Parkinson-White syndrome23
| Arrhythmia | EKG findings | Treatment—unstable patients | Treatment—stable patients (in preferred treatment order) |
| PSVT, orthodromic | Narrow QRS, loss of delta wave, rate 160-260 beats/min | Synchronized cardioversion | Vagal maneuvers, adenosine, calcium channel blockers, beta-blockers, digoxin, procainamide |
| PSVT, antidromic | Wide complex tachycardia | Synchronized cardioversion | Procainamide |
| Atrial fibrillation | Irregularly irregular (RR interval variable with no pattern), ventricular rates that can exceed 300 beats/min | Synchronized cardioversion | Synchronized cardioversion, procainamide |
| Atrial flutter | Flutter waves, rate normal to tachycardic depending on conduction rate | Synchronized cardioversion | Synchronized cardioversion, procainamide |
| Ventricular fibrillation | Rapid, erratic electrical impulses | Defibrillation | N/A |
EKG, electrocardiogram; N/A, not applicable; PSVT, paroxysmal supraventricular tachycardia.
CASE 3 › A 21-year-old man with no medical history presents with sudden onset of lightheadedness followed by syncope. He denies any chest pain or other associated symptoms. At the time of evaluation, he is asymptomatic. His EKG (FIGURE 3) is diagnostic of what syndrome?
The EKG abnormality and diagnosis. The patient had a classic presentation for Wolff-Parkinson-White (WPW) syndrome, a common congenital disorder that alters normal cardiac conduction. He described 2 past instances of unexplained light-headedness and palpitations. Subsequent EP studies demonstrated that the patient had an accessory atrioventricular (AV) tract, causing electrical activity in the heart to bypass the AV node, resulting in a delta wave on EKG (FIGURE 3, GREEN ARROWS).
The patient opted for ablation therapy, which successfully eliminated the delta wave on EKG. Five years later he has had no recurrences.
Epidemiology. The prevalence of WPW syndrome is .1% to 3%.23 Accessory AV tracts are found in men twice as often as in women. Only half of individuals with confirmed tracts develop a tachyarrhythmia. The estimated risk of sudden death due to WPW syndrome is .5% to 4%.24
Pathophysiology. Normally cardiac conduction originates from the sinus node and travels to the AV node, where conduction is slowed, and then proceeds to the His-Purkinje system, and finally to the rest of the ventricular myocardium. In WPW syndrome, ventricular depolarization occurs first by an accessory AV tract called the bundle of Kent, followed shortly thereafter by the His-Purkinje system. This sequence of depolarization is what leads to the EKG findings characteristic of WPW syndrome: a PR interval <.12 seconds, presence of a delta wave, widened QRS complex (>.12 seconds), and repolarization changes seen as ST segment and T-wave changes discordant to (opposite direction) the delta wave and QRS complex (FIGURE 3, RED ARROW).
Factors that influence electrical conduction through the bundle of Kent include cardioactive medications, physiological stress, circulating catecholamines, coronary ischemia, and aging. The end result is a propensity for the heart to convert to one of 4 arrhythmias: paroxysmal supraventricular tachycardia (PSVT), AF, atrial flutter, or ventricular fibrillation (TABLE 2).23
The most common arrhythmia in WPW syndrome is PSVT.23 This rhythm is induced by the formation of a reentry circuit—a pattern in which the heart’s electrical signal loops back on itself—involving the normal conduction pathway and the bundle of Kent. Reentry progressing down the His-Purkinje system and traveling up the bundle of Kent is referred to as orthodromic (anterograde) PSVT. Antidromic (retrograde) PSVT is due to a reentry circuit conducting from the bundle of Kent to the ventricles, and then retrograde through the His-Purkinje system and AV node to the atria.
Clinical features. Under normal circumstances, patients with WPW syndrome are asymptomatic. As was the case with our patient, individuals who develop one of the 4 characteristic arrhythmias can experience light-headedness and syncope.
Treatment. An unstable patient who is experiencing PSVT, AF, or atrial flutter should receive synchronized cardioversion; those experiencing ventricular fibrillation should receive defibrillation (TABLE 2).23 For stable patients, therapy is tailored to the type of arrhythmia. Calcium channel blockers, beta-blockers, and adenosine might be appropriate for patients with orthodromic PSVT but should be avoided in patients with antidromic PSVT, AF, or atrial flutter because these medications block AV node conduction and thus facilitate conduction down the bundle of Kent, which can result in potentially unstable arrhythmias. In general, the longer an arrhythmia has been present, the less effective the pharmacologic intervention because of the increasing sympathetic tone.
Preventive long-term therapies for WPW patients who have experienced arrhythmia include antiarrhythmic medications or ablative procedures. Long-term antiarrhythmic therapy often is reserved for older, more sedentary individuals with less frequent arrhythmias that are not life-threatening. Radiofrequency ablation is a popular option, with long-term success rates as high as 95% and complication rates <1%.23 Patients in whom a WPW pattern is identified incidentally on EKG should be referred to cardiology for EP studies and risk stratification.25
CASE 4 › A 61-year-old woman has an episode of substernal exertional chest pressure that lasted approximately 2 hours but resolved before she arrived at her physician’s office. She also experienced mild nausea. She has no history of coronary artery disease but says that she has experienced similar episodes of chest pressure. What abnormality is seen on her EKG (FIGURE 4)? What is the most likely cause of her symptoms?
The EKG abnormality and diagnosis. Although classically associated with syncope, hypertrophic cardiomyopathy (HCM) often presents similarly to acute coronary syndromes, with chest pain and dyspnea on exertion.26 This patient had no history of cardiac disease or family history of SCD or cardiomyopathy; however, her EKG showed changes indicating left ventricular hypertrophy (LVH), which is consistent with HCM (FIGURE 4, ARROWS). Echocardiography identified myocardial hypertrophy, normal left ventricular ejection fraction, but severe left ventricular outflow obstruction and mild diastolic dysfunction. She was treated with metoprolol and verapamil.
Etiology and epidemiology. Hypertrophic cardiomyopathy is an autosomal dominant intrinsic myocardial disorder resulting in LVH that is commonly associated with SCD during extreme physical activity.26,27 The prevalence of HCM is approximately 1 in 500.26 Although it can present at any age, it is the most common cause of SCD in young people (under age 30), responsible for 33% of deaths during athletic events.28
TABLE 3
4 diagnoses and what you'll see on EKG
| Diagnosis | EKG finding |
| Prolonged QT interval | QTc interval >.450 sec (men) or >.470 sec (women) |
| Brugada syndrome | Partial or complete RBBB, and ST segment elevation in right precordial leads V1-V3 |
| Wolff-Parkinson-White syndrome | Delta wave, widened QRS, short PR interval, ST segment and T-wave changes |
| Hypertrophic cardiomyopathy | No definitive finding; may have left ventricular hypertrophy or abnormal Q-waves |
EKG, electrocardiogram; RBBB, right bundle branch block.
Clinical features. The severity of HCM ranges from asymptomatic to fatal. Symptoms of HCM include chest pain, dyspnea, and syncope. The disorder causes morbidity and mortality in at least one of 3 ways: ventricular tachyarrhythmias (often in younger patients), heart failure (from left ventricular outflow obstruction), and/or thromboembolism.27
Although echocardiography typically is used to make the diagnosis,27 an EKG often is the initial screening tool. EKG changes are seen in 75% to 95% of affected patients and include the presence of Q-waves and increased voltages related to LVH.27,29 Infarct-like patterns may be present before wall thickening on echocardiogram. Abnormal Q-waves are found in 20% to 50% of HCM patients, and are more common in younger patients. Konno et al30 have shown that Q-waves >3 mm in depth and/or >.040 seconds in duration in at least 2 leads other than aVR is specific (90%) in identifying carriers of HCM genes before they develop clinical symptoms.
Ambulatory monitoring may be useful for risk stratifying HCM patients; those with nonsustained ventricular tachycardia (NSVT) are at higher risk of SCD. Holter monitoring is recommended in initial evaluation because evidence of ventricular tachyarrhythmias may warrant AICD placement.26
Treatment. The risk of SCD in HCM is approximately 1%, but higher in those with a family history of SCD, syncope, NSVT, hypotension during exercise, or severe LVH (left ventricle thickness >30 mm).26 AICDs are recommended for HCM patients with prior cardiac arrest, patients with ≥2 of these risk factors, or patients with one risk factor who have experienced syncope related to arrhythmia.26
For patients who are symptomatic but have <2 risk factors, beta-blockers are firstline therapy.26 Verapamil is used as a second line treatment. Both beta-blockers and calcium channel blockers reduce dyspnea, palpitations, and chest pain.27
For patients who don’t respond to medical therapy, septal reduction therapy may be performed, either by septal myectomy or alcohol septal ablation.27 It is also important to consider genetic screening and counseling for the family.
A summary of all 4 diagnoses described in this article, their associated EKG findings, and their pathophysiology appears in TABLE 3.
CORRESPONDENCE
Samir Haydar, DO, MPH, FACEP, Tufts University School of Medicine, Maine Medical Center, Department of Emergency Medicine, 47 Bramhall St., Portland, ME 04103; [email protected]
1. Taggart NW, Haglund CM, Tester DJ, et al. Diagnostic miscues in congenital long-QT syndrome. Circulation. 2007;115:2613-2620.
2. Schwartz PJ, Stramba-Badiale M, Crotti L, et al. Prevalance of the congenital long QT syndrome. Circulation. 2009;120:1761-1767.
3. van Noord C, Eijgelsheim M, Stricker BH. Drug- and nondrug-associated QT interval prolongation. Br J Clin Pharmacol. 2010;70:16-23.
4. Credible Meds Web site. Available at: http://crediblemeds.org. Accessed April 8, 2014.
5. Schwartz PJ, Priori SG, Spazzolini C, et al. Genotype-phenotype correlation in the long QT syndrome: gene specific triggers for life threatening arrhythmias. Circulation. 2001;103:89.
6. Chiladakis JK, Kalogeropoulos A, Koutsogiannis N, et al. Optimal QT/JT interval assessment in patients with complete bundle branch block. Ann Noninvasive Electrocardiol. 2012;17:268-276.
7. Ercan S, Altunbas G, Oylumlu M, et al. Congenital long QT syndrome masked by atrial fibrillation and unmasked by hypokalemia. Am J Emerg Med. 2013;31:451.e3-451.e6.
8. Goldenberg I, Moss AJ, Zareba W. QT interval: how to measure it and what is “normal.” J Cardiovasc Electrophysiol. 2006;17:333-336.
9. Podrid PJ. ECG Response: August 20, 2013. Circulation. 2013;128:869.
10. Olde Nordkamp LR, Wilde AA, Tijssen JG, et al. The ICD for primary prevention in patients with inherited cardiac diseases: indications, use, and outcome: a comparison with secondary prevention. Circ Arrhythm Electrophysiol. 2013;6:91-100.
11. Schwartz PJ. Pharmacological and non-pharmacological management of the congenital long QT syndrome: the rationale. Pharmacol Ther. 2011;131:171-177.
12. Berne P, Brugada J. Brugada syndrome 2012. Circ J. 2012;76:1563-1571.
13. Brugada P, Brugada J. Right bundle branch block, persistent ST segment elevation and sudden cardiac death: a distinct clinical and electrocardiographic syndrome. A multicenter report. J Am Coll Cardiol. 1992;20:1391-1396.
14. Antzelevitch C, Brugada P, Borggrefe M, et al. Brugada syndrome: report of the second consensus conference: endorsed by the Heart Rhythm Society and the European Heart Rhythm Association. Circulation. 2005;111:659-670.
15. Brugada J, Brugada R, Antzelevitch C, et al. Long-term followup of individuals with the electrocardiographic pattern of right bundle-branch block and ST-segment elevation in precordial leads V1 to V3. Circulation. 2002;105:73-78.
16. Eckardt L, Probst V, Smits JP, et al. Long-term prognosis of individuals with right precordial ST-segment-elevation Brugada syndrome. Circulation. 2005;111:257-263.
17. Giustetto C, Drago S, Demarchi PG, et al; Italian Association of Arrhythmology and Cardiostimulation (AIAC)-Piedmont Section. Risk stratification of the patients with Brugada type electrocardiogram: a community-based prospective study. Europace. 2009;11:507-513.
18. Probst V, Veltmann C, Eckardt L, et al. Long-term prognosis of patients diagnosed with Brugada syndrome: Results from the FINGER Brugada Syndrome Registry. Circulation. 2010;121:635-643.
19. Nielsen MW, Holst AG, Olesen SP, et al. The genetic component of Brugada syndrome. Front Physiol. 2013;4:179.
20. Bayés de Luna A, Brugada J, Baranchuk A, et al. Current electrocardiographic criteria for diagnosis of Brugada pattern: a consensus report. J Electrocardiol. 2012;45:433-442.
21. Veltmann C, Schimpf R, Echternach C, et al. A prospective study on spontaneous fluctuations between diagnostic and non-diagnostic ECGs in Brugada syndrome: implications for correct phenotyping and risk stratification. Eur Heart J. 2006;27:2544-2552.
22. Sacher F, Probst V, Maury P, et al. Outcome after implantation of a cardioverter-defibrillator in patients with Brugada syndrome: a multicenter study-part 2. Circulation. 2013;128:1739-1747.
23. Rosner MH, Brady WJ Jr, Kefer MP, et al. Electrocardiography in the patient with the Wolff-Parkinson-White syndrome: diagnostic and initial therapeutic issues. Am J Emerg Med. 1999;17:705-714.
24. Keating L, Morris FP, Brady WJ. Electrocardiographic features of Wolff-Parkinson-White syndrome. Emerg Med J. 2003;20:491-493.
25. Blomström-Lundqvist C, Scheinman MM, Aliot EM, et al; European Society of Cardiology Committee, NASPE-Heart Rhythm Society. ACC/AHA/ESC guidelines for the management of patients with supraventricular arrhythmias—executive summary. A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the European Society of Cardiology Committee for Practice Guidelines (writing committee to develop guidelines for the management of patients with supraventricular arrhythmias) developed in collaboration with NASPE-Heart Rhythm Society. J Am Coll Cardiol. 2003;42:1493-1531.
26. Ho CY. Hypertrophic cardiomyopathy in 2012. Circulation. 2012;125:1432-1438.
27. Gersh BJ, Maron BJ, Bonow RO, et al; American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines; American Association for Thoracic Surgery; American Society of Echocardiography; American Society of Nuclear Cardiology; Heart Failure Society of America; Heart Rhythm Society; Society for Cardiovascular Angiography and Interventions; Society of Thoracic Surgeons. 2011 ACCF/AHA guideline for the diagnosis and treatment of hypertrophic cardiomyopathy: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation. 2011;124:e783-e831.
28. Paterick TE, Jan MF, Paterick ZR, et al. Cardiac evaluation of collegiate student athletes: a medical and legal perspective. Am J Med. 2012;125:742-752.
29. Maron BJ. Hypertrophic cardiomyopathy. In: Bonow RO, Mann DL, Zipes DP, et al (eds). Braunwald’s Heart Disease: A Textbook of Cardiovascular Medicine. 9th ed. Philadelphia, PA: Saunders; 2011:1582-1594.
30. Konno T, Shimizu M, Ino H, et al. Diagnostic value of abnormal Q waves for identification of preclinical carriers of hypertrophic cardiomyopathy based on molecular genetic diagnosis. Eur Heart J. 2004;25:246-251.
1. Taggart NW, Haglund CM, Tester DJ, et al. Diagnostic miscues in congenital long-QT syndrome. Circulation. 2007;115:2613-2620.
2. Schwartz PJ, Stramba-Badiale M, Crotti L, et al. Prevalance of the congenital long QT syndrome. Circulation. 2009;120:1761-1767.
3. van Noord C, Eijgelsheim M, Stricker BH. Drug- and nondrug-associated QT interval prolongation. Br J Clin Pharmacol. 2010;70:16-23.
4. Credible Meds Web site. Available at: http://crediblemeds.org. Accessed April 8, 2014.
5. Schwartz PJ, Priori SG, Spazzolini C, et al. Genotype-phenotype correlation in the long QT syndrome: gene specific triggers for life threatening arrhythmias. Circulation. 2001;103:89.
6. Chiladakis JK, Kalogeropoulos A, Koutsogiannis N, et al. Optimal QT/JT interval assessment in patients with complete bundle branch block. Ann Noninvasive Electrocardiol. 2012;17:268-276.
7. Ercan S, Altunbas G, Oylumlu M, et al. Congenital long QT syndrome masked by atrial fibrillation and unmasked by hypokalemia. Am J Emerg Med. 2013;31:451.e3-451.e6.
8. Goldenberg I, Moss AJ, Zareba W. QT interval: how to measure it and what is “normal.” J Cardiovasc Electrophysiol. 2006;17:333-336.
9. Podrid PJ. ECG Response: August 20, 2013. Circulation. 2013;128:869.
10. Olde Nordkamp LR, Wilde AA, Tijssen JG, et al. The ICD for primary prevention in patients with inherited cardiac diseases: indications, use, and outcome: a comparison with secondary prevention. Circ Arrhythm Electrophysiol. 2013;6:91-100.
11. Schwartz PJ. Pharmacological and non-pharmacological management of the congenital long QT syndrome: the rationale. Pharmacol Ther. 2011;131:171-177.
12. Berne P, Brugada J. Brugada syndrome 2012. Circ J. 2012;76:1563-1571.
13. Brugada P, Brugada J. Right bundle branch block, persistent ST segment elevation and sudden cardiac death: a distinct clinical and electrocardiographic syndrome. A multicenter report. J Am Coll Cardiol. 1992;20:1391-1396.
14. Antzelevitch C, Brugada P, Borggrefe M, et al. Brugada syndrome: report of the second consensus conference: endorsed by the Heart Rhythm Society and the European Heart Rhythm Association. Circulation. 2005;111:659-670.
15. Brugada J, Brugada R, Antzelevitch C, et al. Long-term followup of individuals with the electrocardiographic pattern of right bundle-branch block and ST-segment elevation in precordial leads V1 to V3. Circulation. 2002;105:73-78.
16. Eckardt L, Probst V, Smits JP, et al. Long-term prognosis of individuals with right precordial ST-segment-elevation Brugada syndrome. Circulation. 2005;111:257-263.
17. Giustetto C, Drago S, Demarchi PG, et al; Italian Association of Arrhythmology and Cardiostimulation (AIAC)-Piedmont Section. Risk stratification of the patients with Brugada type electrocardiogram: a community-based prospective study. Europace. 2009;11:507-513.
18. Probst V, Veltmann C, Eckardt L, et al. Long-term prognosis of patients diagnosed with Brugada syndrome: Results from the FINGER Brugada Syndrome Registry. Circulation. 2010;121:635-643.
19. Nielsen MW, Holst AG, Olesen SP, et al. The genetic component of Brugada syndrome. Front Physiol. 2013;4:179.
20. Bayés de Luna A, Brugada J, Baranchuk A, et al. Current electrocardiographic criteria for diagnosis of Brugada pattern: a consensus report. J Electrocardiol. 2012;45:433-442.
21. Veltmann C, Schimpf R, Echternach C, et al. A prospective study on spontaneous fluctuations between diagnostic and non-diagnostic ECGs in Brugada syndrome: implications for correct phenotyping and risk stratification. Eur Heart J. 2006;27:2544-2552.
22. Sacher F, Probst V, Maury P, et al. Outcome after implantation of a cardioverter-defibrillator in patients with Brugada syndrome: a multicenter study-part 2. Circulation. 2013;128:1739-1747.
23. Rosner MH, Brady WJ Jr, Kefer MP, et al. Electrocardiography in the patient with the Wolff-Parkinson-White syndrome: diagnostic and initial therapeutic issues. Am J Emerg Med. 1999;17:705-714.
24. Keating L, Morris FP, Brady WJ. Electrocardiographic features of Wolff-Parkinson-White syndrome. Emerg Med J. 2003;20:491-493.
25. Blomström-Lundqvist C, Scheinman MM, Aliot EM, et al; European Society of Cardiology Committee, NASPE-Heart Rhythm Society. ACC/AHA/ESC guidelines for the management of patients with supraventricular arrhythmias—executive summary. A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the European Society of Cardiology Committee for Practice Guidelines (writing committee to develop guidelines for the management of patients with supraventricular arrhythmias) developed in collaboration with NASPE-Heart Rhythm Society. J Am Coll Cardiol. 2003;42:1493-1531.
26. Ho CY. Hypertrophic cardiomyopathy in 2012. Circulation. 2012;125:1432-1438.
27. Gersh BJ, Maron BJ, Bonow RO, et al; American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines; American Association for Thoracic Surgery; American Society of Echocardiography; American Society of Nuclear Cardiology; Heart Failure Society of America; Heart Rhythm Society; Society for Cardiovascular Angiography and Interventions; Society of Thoracic Surgeons. 2011 ACCF/AHA guideline for the diagnosis and treatment of hypertrophic cardiomyopathy: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation. 2011;124:e783-e831.
28. Paterick TE, Jan MF, Paterick ZR, et al. Cardiac evaluation of collegiate student athletes: a medical and legal perspective. Am J Med. 2012;125:742-752.
29. Maron BJ. Hypertrophic cardiomyopathy. In: Bonow RO, Mann DL, Zipes DP, et al (eds). Braunwald’s Heart Disease: A Textbook of Cardiovascular Medicine. 9th ed. Philadelphia, PA: Saunders; 2011:1582-1594.
30. Konno T, Shimizu M, Ino H, et al. Diagnostic value of abnormal Q waves for identification of preclinical carriers of hypertrophic cardiomyopathy based on molecular genetic diagnosis. Eur Heart J. 2004;25:246-251.
Pica: An age-old eating disorder that’s often missed
› Ask about pica behavior or unusual cravings in certain high-risk groups: pregnant women, immigrants or refugees, and children and adults with autism or other developmental disabilities. C
› Obtain serum hemoglobin and hematocrit levels along with iron levels, if necessary, in patients who report cravings for unusual substances. B
› Check serum lead levels and consider testing for ova and parasites in patients who eat dirt. C
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
CASE › A 6-year-old African girl, developing and growing appropriately for age, was brought to our clinic by her father with the chief complaint of “eating the textbooks at school.” The child had eaten paper for years, the father said; he never thought it unusual until her teacher brought it to his attention. The father reported that his daughter had met all developmental milestones and was up to date with her immunizations. When asked why she ate paper, the child responded, “I don’t know.”
The child was diagnosed with pica and, because we were concerned that she was eating other nonnutritive foods, we ordered hematologic studies. Her lead level (2 mcg/dL) was within the normal range; her hemoglobin/hematocrit was 10.4 g/dL/32.3%. Iron therapy was started. At follow-up 4 weeks later, the child’s paper-eating behavior had resolved.
The word pica comes from the Latin word for magpie, a bird with a reputation for eating practically anything. The Diagnostic and Statistical Manual of Mental Disorders, 5th edition, defines pica as persistent eating of nonnutritive substances for at least 1 month that is inappropriate to developmental level and not part of a culturally supported or socially normative practice.1
Case reports on paper pica are few, but numerous reports describe other forms of the behavior, including eating ice; dirt, soil, and clay; starch; burnt matches; cardboard; hair; laundry detergent; chalk; soap; firecrackers; and metal artifacts such as coins.2-16
Pica has been described in the literature as “underreported” and “unrecognized.” Its true prevalence is difficult to assess because most people don’t report it and the methodology of data collection varies among populations, as does the definition of pica. According to some estimates, more than 50% of children ages 18 to 36 months seek and ingest nonfood items. The practice reportedly decreases as a child ages, but an estimated 10% of children older than 12 years may engage in it.17
Pica has been reported since antiquity. Many medical and anthropological studies refer to the practice of geophagia, or dirt eating, which is prevalent in Africa and among small children and women, particularly women who are native to the southern United States, African-American, or pregnant.5-10,18,19
Pica often occurs in people with developmental disabilities such as autism and is considered a psychiatric condition in that context.3,11,15,20-31 However, because many forms of pica, especially geophagia, aren’t associated with mental health issues, researchers disagree about whether to consider it an abnormal behavior. A 2000 workshop on pica organized by the Agency for Toxic Substances and Disease Registry concluded that geophagia is not an abnormal behavior.17 One of the most compelling arguments for this view is that dirt eating is far too common around the world to be considered abnormal, and dirt is held in some cultures to have therapeutic powers.7,13,24
Adverse outcomes linked to pica
Pica is associated with adverse outcomes, however. A study by the Agency for Healthcare Research and Quality found that despite an overall decline in hospitalizations for eating disorders, hospitalizations for pica have risen.25 From 1999 to 2009, pica-related hospitalizations jumped 93%, although the overall number of patients hospitalized for the condition remains small (964 in 1999 to 2000, 1862 in 2008-2009).
Documented adverse effects of pica include potassium abnormalities and gastrointestinal conditions ranging from irritation and abdominal pain to perforation, blockage, and colon ischemia.3,11,26-29 Reported bidirectional effects (which both result from and contribute to pica) include iron deficiency, parasitic infections, and heavy metal exposure—particularly lead, mercury, and arsenic.4,6,9,20,30-38
Diagnosis: Focus on history and selective testing
Pica is a clinical diagnosis, confirmed by the patient’s history, not any single laboratory test. Providers should ask about pica behavior or unusual cravings in certain high-risk groups: pregnant women, particularly women from the southern United States, immigrants or refugees, and children and adults with autism or other developmental disabilities.18,22
Testing should be based on the type of pica behavior. Because various forms of pica are commonly associated with iron-deficiency anemia, obtain serum hemoglobin and hematocrit levels along with iron levels if necessary in patients who report cravings for unusual substances. Pica in pregnancy is a sign of iron deficiency, but it also may signal iron deficiency in patients who aren’t pregnant. In one study of 262 nonpregnant adults with iron-deficiency anemia, 45% reported pica behaviors; of these, 87.3% reported eating ice.34
Check serum lead levels in children who engage in geophagia since dirt may contain lead. Because ingestion of soil or clay is associated with soil-borne parasitic infections, also consider testing for ova and parasites if clinically indicated. Patients who eat paper may be exposed to mercury poisoning, so a serum mercury level is advisable.
Management: Prevention and behavior modification are key
Treatment for pica varies by patient and the specific behavior. Management approaches are primarily preventive, educational, and directed toward behavior modification.
Prevention. Residential facilities and primary care offices that care for people with developmental disabilities may screen for pica by means of prevalence surveys, direct observation, stool checks, review of medical history records, and interviews with caregivers.
Residential facilities can create a pica-safe environment by training staff in pica prevention, instituting regular on-site monitoring to ensure that no dangerous objects are available, and developing procedures to guide staff behavior, such as safe disposal of rubber gloves.22 Parents and caregivers of young children or children with developmental disabilities who don’t live in residential facilities should be aware of pica and monitor what their children are ingesting.
Behavior modification. Behavior-based approaches have proved effective for treating pica in developmentally disabled patients. Applied behavioral analysis “was found to have the most robust empirical support to treat this behavior.”39 Patients found to have pica may be referred for further assessment to a behavior specialist or a psychologist with experience in treating the condition.22,39
A review of 26 studies found that, in 25 studies, behavioral therapy reduced pica behavior by 80% or more.23 Behavioral treatments included reinforcement procedures alone, response reduction procedures alone, and combined reinforcement and response reduction procedures. Reinforcement shapes behavior by controlling the consequences of the behavior using a combination of rewards and punishments.23 Response reduction, or blocking, involves obstructing every attempt to eat inedible items.22
Treatments that combined reinforcement and response reduction showed good efficacy.23 An example of the combined approach would be to stop the patient from eating nonnutritive items while redirecting him to eat food instead.22
Supplementation. Iron supplementation has decreased or even reversed pica in patients whose clinical symptoms and behavior were associated with iron deficiency.35,40
Medications. Successful treatment with selective serotonin reuptake inhibitors (escitalopram), atypical neuroleptics (olanzapine), and attention-deficit/hyperactivity disorder medications (methylphenidate) has been reported in some patients, but case reports are few, and the evidence for the drugs’ efficacy is limited.41-43
Be alert for pica. Primary care physicians need to be aware of pica and proactively seek information about cravings or behaviors suggesting the condition from patients in high-risk populations—pregnant women, children, immigrants and refugees, people with developmental disabilities—or their caregivers. Once pica is identified, clinicians should undertake appropriate laboratory investigation and behavior modification attempts.
CORRESPONDENCE
Ranit Mishori, MD, MHS, Department of Family Medicine, Georgetown University School of Medicine, Pre-Clinical Building, GB-01D, 3900 Reservoir Road, NW, Washington, DC 20007; [email protected]
1. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th ed. Arlington, VA: American Psychiatric Association; 2013.
2. Yalug I, Kirmizi-Alsan E, Tufan AE. Adult-onset paper pica in the context of anorexia nervosa with major depressive disorder and a history of childhood geophagia: a case report. Prog Neuropsychopharmacol Biol Psychiatry. 2007;31:1341-1342.
3. Spaniolas K, Ou S, Findeis-Hosey J, et al. Paper pica: an unusual cause of colonic ischemia. J Gastrointest Surg. 2010;14:1065-1066.
4. Olynyk F, Sharpe DH. Mercury poisoning in paper pica. N Engl J Med. 1982;306:1056-1057.
5. Guney M, Zagury GJ, Dogan N, et al. Exposure assessment and risk characterization from trace elements following soil ingestion by children exposed to playgrounds, parks and picnic areas. J Hazard Mater. 2010;182:656-664.
6. Kawai K, Saathoff E, Antelman G, et al. Geophagy (soil-eating) in relation to anemia and helminth infection among HIV-infected pregnant women in Tanzania. Am J Trop Med Hyg. 2009;80:36-43.
7. Woywodt A, Kiss A. Geophagia: the history of earth-eating. J R Soc Med. 2002;95:143-146.
8. Stokes T. The earth-eaters. Nature. 2006;444:543-544.
9. Kutalek R, Wewalka G, Gundacker C, et al. Geophagy and potential health implications: geohelminths, microbes and heavy metals. Trans R Soc Trop Med Hyg. 2010;104:787-795.
10. Keith D, Keith L, Berger GS, et al. Amylophagia during pregnancy: some maternal and perinatal correlations. Mt Sinai J Med. 1975;42:410-414.
11. Abu-Hamdan DK, Sondheimer JH, Mahajan SK. Cautopyreiophagia. Cause of life-threatening hyperkalemia in a patient undergoing hemodialysis. Am J Med. 1985;79:517-519.
12. Ewert P, Keim L, Schulte-Markwort M. Trichobezoar. A rare cause of recurrent upper abdominal pain [in German]. Monatsschr Kinderheilkd. 1992;140:811-813.
13. Grigsby RK, Thyer BA, Waller RJ, et al. Chalk eating in middle Georgia: a culture-bound syndrome of pica? South Med J. 1999;92:190-192.
14. Ahishali E, Boynueğrı B, Dabak R, et al. A case of severe acute hepatitis due to oral intake of firecrackers. Turk J Gastroenterol. 2010;21:325-326.
15. Rashid F, Davies L, Iftikhar SY. Magnetised intragastric foreign body collection and autism: An advice for careers and literature review. Autism. 2010;14:139-145.
16. Martindale JL, Bunker CJ, Noble VE. Ingested foreign bodies in a patient with pica. Gastroenterol Hepatol (N Y). 2010;6:582-584.
17. Agency for Toxic Substances and Disease Registry. Summary Report for the ATSDR Soil-Pica Workshop June 2000, Atlanta, Georgia. Agency for Toxic Substances and Disease Registry Web site. Available at: www.atsdr.cdc.gov/child/soilpica.html. Accessed June 2, 2012.
18. Njiru H, Elchalal U, Paltiel O. Geophagy during pregnancy in Africa: a literature review. Obstet Gynecol Surv. 2011;66:452-459.
19. Young SL. Pica in pregnancy: new ideas about an old condition. Annu Rev Nutr. 2010;30:403-422.
20. Clark B, Vandermeer B, Simonetti A, et al. Is lead a concern in Canadian autistic children? Paediatr Child Health. 2010;15:17-22.
21. Matson JL, Sipes M, Fodstad JC, et al. Issues in the management of challenging behaviours of adults with autism spectrum disorder. CNS Drugs. 2011;25:597-606.
22. Williams DE, McAdam D. Assessment, behavioral treatment, and prevention of pica: clinical guidelines and recommendations for practitioners. Res Dev Disabil. 2012;33:2050-2057.
23. Hagopian LP, Rooker GW, Rolider NU. Identifying empirically supported treatments for pica in individuals with intellectual disabilities. Res Dev Disabil. 2011;32:2114-2120.
24. Engberg DE. Geophagy: adaptive or aberrant behavior. Nebraska Anthropologist. 1995;12:57-68.
25. Agency for Healthcare Research and Quality. Hospitalizations for eating disorder decline, but big increase seen in pica disorder. Agency for Healthcare Research and Quality Web site. Available at: www.ahrq.gov/news/nn/nn090811.htm. Accessed June 2, 2014.
26. Stroman D, Young C, Rubano AR, et al. Adult-onset pica leading to acute intestinal obstruction. Psychosomatics. 2011;52:393-394.
27. Young SL, Khalfan SS, Farag TH, et al. Association of pica with anemia and gastrointestinal distress among pregnant women in Zanzibar, Tanzania. Am J Trop Med Hyg. 2010;83:144-151.
28. Altepeter T, Annes J, Meller J. Foam bezoar: resection of perforated terminal ileum in a 17-year-old with sickle b+thalassemia and pica. J Pediatr Surg. 2011;46:E31-E32.
29. Chatzimavroudis G, Christopoulos P, Atmatzidis S, et al. Pica: an uncommon cause of acute abdominal pain in children. Indian J Pediatr. 2011;78:886-887.
30. Rector WG Jr. Pica: its frequency and significance in patients with iron-deficiency anemia due to chronic gastrointestinal blood loss. J Gen Intern Med. 1989;4:512-513.
31. Sontag C, Kettaneh A, Fain O, et al. Rapid regression of prolonged pagophagia after treatment of iron deficiency [in French]. Presse Med. 2001;30:321-323.
32. Sharma TR, Kavuru B, Aly M. Coprophagia and pica in individuals with mild to moderate dementia and mixed (iron deficiency and macrocytic) anemia. J Am Geriatr Soc. 2011;59:2375-2377.
33. Kushner RF, Shanta Retelny V. Emergence of pica (ingestion of non-food substances) accompanying iron deficiency anemia after gastric bypass surgery. Obes Surg. 2005;15:1491-1495.
34. Barton JC, Barton JC, Bertoli LF. Pica associated with iron deficiency or depletion: clinical and laboratory correlates in 262 nonpregnant adult outpatients. BMC Blood Disord. 2010;10:9.
35. Khan Y, Tisman G. Pica in iron deficiency: a case series. J Med Case Rep. 2010;4:86.
36. Bakhireva LN, Rowland AS, Young BN, et al. Sources of potential lead exposure among pregnant women in New Mexico. Matern Child Health J. 2013;17:172-179.
37. Thihalolipavan S, Candalla BM, Ehrlich J. Examining pica in NYC pregnant women with elevated blood lead levels. Matern Child Health J. 2013;17:49-55.
38. Al-Rmalli SW, Jenkins RO, Watts MJ, et al. Risk of human exposure to arsenic and other toxic elements from geophagy: trace element analysis of baked clay using inductively coupled plasma mass spectrometry. Environ Health. 2010;9:79.
39. Matson JL, Hattier MA, Belva B, et al. Pica in persons with developmental disabilities: approaches to treatment. Res Dev Disabil. 2013;34:2564-2571.
40. Bryant BJ1, Yau YY, Arceo SM, et al. Ascertainment of iron deficiency and depletion in blood donors through screening questions for pica and restless legs syndrome. Transfusion. 2013;53:1637-1644.
41. Lerner AJ. Treatment of pica behavior with olanzapine. CNS Spectr. 2008;13:19.
42. Hergüner S, Hergüner AS. Pica in a child with attention deficit hyperactivity disorder and successful treatment with methylphenidate. Prog Neuropsychopharmacol Biol Psychiatry. 2010;34:1155-1156.
43. Bhatia MS, Gupta R. Pica responding to SSRI: an OCD Spectrum Disorder? World J Biol Psychiatry. 2009;10(4 pt 3):936-938.
› Ask about pica behavior or unusual cravings in certain high-risk groups: pregnant women, immigrants or refugees, and children and adults with autism or other developmental disabilities. C
› Obtain serum hemoglobin and hematocrit levels along with iron levels, if necessary, in patients who report cravings for unusual substances. B
› Check serum lead levels and consider testing for ova and parasites in patients who eat dirt. C
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
CASE › A 6-year-old African girl, developing and growing appropriately for age, was brought to our clinic by her father with the chief complaint of “eating the textbooks at school.” The child had eaten paper for years, the father said; he never thought it unusual until her teacher brought it to his attention. The father reported that his daughter had met all developmental milestones and was up to date with her immunizations. When asked why she ate paper, the child responded, “I don’t know.”
The child was diagnosed with pica and, because we were concerned that she was eating other nonnutritive foods, we ordered hematologic studies. Her lead level (2 mcg/dL) was within the normal range; her hemoglobin/hematocrit was 10.4 g/dL/32.3%. Iron therapy was started. At follow-up 4 weeks later, the child’s paper-eating behavior had resolved.
The word pica comes from the Latin word for magpie, a bird with a reputation for eating practically anything. The Diagnostic and Statistical Manual of Mental Disorders, 5th edition, defines pica as persistent eating of nonnutritive substances for at least 1 month that is inappropriate to developmental level and not part of a culturally supported or socially normative practice.1
Case reports on paper pica are few, but numerous reports describe other forms of the behavior, including eating ice; dirt, soil, and clay; starch; burnt matches; cardboard; hair; laundry detergent; chalk; soap; firecrackers; and metal artifacts such as coins.2-16
Pica has been described in the literature as “underreported” and “unrecognized.” Its true prevalence is difficult to assess because most people don’t report it and the methodology of data collection varies among populations, as does the definition of pica. According to some estimates, more than 50% of children ages 18 to 36 months seek and ingest nonfood items. The practice reportedly decreases as a child ages, but an estimated 10% of children older than 12 years may engage in it.17
Pica has been reported since antiquity. Many medical and anthropological studies refer to the practice of geophagia, or dirt eating, which is prevalent in Africa and among small children and women, particularly women who are native to the southern United States, African-American, or pregnant.5-10,18,19
Pica often occurs in people with developmental disabilities such as autism and is considered a psychiatric condition in that context.3,11,15,20-31 However, because many forms of pica, especially geophagia, aren’t associated with mental health issues, researchers disagree about whether to consider it an abnormal behavior. A 2000 workshop on pica organized by the Agency for Toxic Substances and Disease Registry concluded that geophagia is not an abnormal behavior.17 One of the most compelling arguments for this view is that dirt eating is far too common around the world to be considered abnormal, and dirt is held in some cultures to have therapeutic powers.7,13,24
Adverse outcomes linked to pica
Pica is associated with adverse outcomes, however. A study by the Agency for Healthcare Research and Quality found that despite an overall decline in hospitalizations for eating disorders, hospitalizations for pica have risen.25 From 1999 to 2009, pica-related hospitalizations jumped 93%, although the overall number of patients hospitalized for the condition remains small (964 in 1999 to 2000, 1862 in 2008-2009).
Documented adverse effects of pica include potassium abnormalities and gastrointestinal conditions ranging from irritation and abdominal pain to perforation, blockage, and colon ischemia.3,11,26-29 Reported bidirectional effects (which both result from and contribute to pica) include iron deficiency, parasitic infections, and heavy metal exposure—particularly lead, mercury, and arsenic.4,6,9,20,30-38
Diagnosis: Focus on history and selective testing
Pica is a clinical diagnosis, confirmed by the patient’s history, not any single laboratory test. Providers should ask about pica behavior or unusual cravings in certain high-risk groups: pregnant women, particularly women from the southern United States, immigrants or refugees, and children and adults with autism or other developmental disabilities.18,22
Testing should be based on the type of pica behavior. Because various forms of pica are commonly associated with iron-deficiency anemia, obtain serum hemoglobin and hematocrit levels along with iron levels if necessary in patients who report cravings for unusual substances. Pica in pregnancy is a sign of iron deficiency, but it also may signal iron deficiency in patients who aren’t pregnant. In one study of 262 nonpregnant adults with iron-deficiency anemia, 45% reported pica behaviors; of these, 87.3% reported eating ice.34
Check serum lead levels in children who engage in geophagia since dirt may contain lead. Because ingestion of soil or clay is associated with soil-borne parasitic infections, also consider testing for ova and parasites if clinically indicated. Patients who eat paper may be exposed to mercury poisoning, so a serum mercury level is advisable.
Management: Prevention and behavior modification are key
Treatment for pica varies by patient and the specific behavior. Management approaches are primarily preventive, educational, and directed toward behavior modification.
Prevention. Residential facilities and primary care offices that care for people with developmental disabilities may screen for pica by means of prevalence surveys, direct observation, stool checks, review of medical history records, and interviews with caregivers.
Residential facilities can create a pica-safe environment by training staff in pica prevention, instituting regular on-site monitoring to ensure that no dangerous objects are available, and developing procedures to guide staff behavior, such as safe disposal of rubber gloves.22 Parents and caregivers of young children or children with developmental disabilities who don’t live in residential facilities should be aware of pica and monitor what their children are ingesting.
Behavior modification. Behavior-based approaches have proved effective for treating pica in developmentally disabled patients. Applied behavioral analysis “was found to have the most robust empirical support to treat this behavior.”39 Patients found to have pica may be referred for further assessment to a behavior specialist or a psychologist with experience in treating the condition.22,39
A review of 26 studies found that, in 25 studies, behavioral therapy reduced pica behavior by 80% or more.23 Behavioral treatments included reinforcement procedures alone, response reduction procedures alone, and combined reinforcement and response reduction procedures. Reinforcement shapes behavior by controlling the consequences of the behavior using a combination of rewards and punishments.23 Response reduction, or blocking, involves obstructing every attempt to eat inedible items.22
Treatments that combined reinforcement and response reduction showed good efficacy.23 An example of the combined approach would be to stop the patient from eating nonnutritive items while redirecting him to eat food instead.22
Supplementation. Iron supplementation has decreased or even reversed pica in patients whose clinical symptoms and behavior were associated with iron deficiency.35,40
Medications. Successful treatment with selective serotonin reuptake inhibitors (escitalopram), atypical neuroleptics (olanzapine), and attention-deficit/hyperactivity disorder medications (methylphenidate) has been reported in some patients, but case reports are few, and the evidence for the drugs’ efficacy is limited.41-43
Be alert for pica. Primary care physicians need to be aware of pica and proactively seek information about cravings or behaviors suggesting the condition from patients in high-risk populations—pregnant women, children, immigrants and refugees, people with developmental disabilities—or their caregivers. Once pica is identified, clinicians should undertake appropriate laboratory investigation and behavior modification attempts.
CORRESPONDENCE
Ranit Mishori, MD, MHS, Department of Family Medicine, Georgetown University School of Medicine, Pre-Clinical Building, GB-01D, 3900 Reservoir Road, NW, Washington, DC 20007; [email protected]
› Ask about pica behavior or unusual cravings in certain high-risk groups: pregnant women, immigrants or refugees, and children and adults with autism or other developmental disabilities. C
› Obtain serum hemoglobin and hematocrit levels along with iron levels, if necessary, in patients who report cravings for unusual substances. B
› Check serum lead levels and consider testing for ova and parasites in patients who eat dirt. C
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
CASE › A 6-year-old African girl, developing and growing appropriately for age, was brought to our clinic by her father with the chief complaint of “eating the textbooks at school.” The child had eaten paper for years, the father said; he never thought it unusual until her teacher brought it to his attention. The father reported that his daughter had met all developmental milestones and was up to date with her immunizations. When asked why she ate paper, the child responded, “I don’t know.”
The child was diagnosed with pica and, because we were concerned that she was eating other nonnutritive foods, we ordered hematologic studies. Her lead level (2 mcg/dL) was within the normal range; her hemoglobin/hematocrit was 10.4 g/dL/32.3%. Iron therapy was started. At follow-up 4 weeks later, the child’s paper-eating behavior had resolved.
The word pica comes from the Latin word for magpie, a bird with a reputation for eating practically anything. The Diagnostic and Statistical Manual of Mental Disorders, 5th edition, defines pica as persistent eating of nonnutritive substances for at least 1 month that is inappropriate to developmental level and not part of a culturally supported or socially normative practice.1
Case reports on paper pica are few, but numerous reports describe other forms of the behavior, including eating ice; dirt, soil, and clay; starch; burnt matches; cardboard; hair; laundry detergent; chalk; soap; firecrackers; and metal artifacts such as coins.2-16
Pica has been described in the literature as “underreported” and “unrecognized.” Its true prevalence is difficult to assess because most people don’t report it and the methodology of data collection varies among populations, as does the definition of pica. According to some estimates, more than 50% of children ages 18 to 36 months seek and ingest nonfood items. The practice reportedly decreases as a child ages, but an estimated 10% of children older than 12 years may engage in it.17
Pica has been reported since antiquity. Many medical and anthropological studies refer to the practice of geophagia, or dirt eating, which is prevalent in Africa and among small children and women, particularly women who are native to the southern United States, African-American, or pregnant.5-10,18,19
Pica often occurs in people with developmental disabilities such as autism and is considered a psychiatric condition in that context.3,11,15,20-31 However, because many forms of pica, especially geophagia, aren’t associated with mental health issues, researchers disagree about whether to consider it an abnormal behavior. A 2000 workshop on pica organized by the Agency for Toxic Substances and Disease Registry concluded that geophagia is not an abnormal behavior.17 One of the most compelling arguments for this view is that dirt eating is far too common around the world to be considered abnormal, and dirt is held in some cultures to have therapeutic powers.7,13,24
Adverse outcomes linked to pica
Pica is associated with adverse outcomes, however. A study by the Agency for Healthcare Research and Quality found that despite an overall decline in hospitalizations for eating disorders, hospitalizations for pica have risen.25 From 1999 to 2009, pica-related hospitalizations jumped 93%, although the overall number of patients hospitalized for the condition remains small (964 in 1999 to 2000, 1862 in 2008-2009).
Documented adverse effects of pica include potassium abnormalities and gastrointestinal conditions ranging from irritation and abdominal pain to perforation, blockage, and colon ischemia.3,11,26-29 Reported bidirectional effects (which both result from and contribute to pica) include iron deficiency, parasitic infections, and heavy metal exposure—particularly lead, mercury, and arsenic.4,6,9,20,30-38
Diagnosis: Focus on history and selective testing
Pica is a clinical diagnosis, confirmed by the patient’s history, not any single laboratory test. Providers should ask about pica behavior or unusual cravings in certain high-risk groups: pregnant women, particularly women from the southern United States, immigrants or refugees, and children and adults with autism or other developmental disabilities.18,22
Testing should be based on the type of pica behavior. Because various forms of pica are commonly associated with iron-deficiency anemia, obtain serum hemoglobin and hematocrit levels along with iron levels if necessary in patients who report cravings for unusual substances. Pica in pregnancy is a sign of iron deficiency, but it also may signal iron deficiency in patients who aren’t pregnant. In one study of 262 nonpregnant adults with iron-deficiency anemia, 45% reported pica behaviors; of these, 87.3% reported eating ice.34
Check serum lead levels in children who engage in geophagia since dirt may contain lead. Because ingestion of soil or clay is associated with soil-borne parasitic infections, also consider testing for ova and parasites if clinically indicated. Patients who eat paper may be exposed to mercury poisoning, so a serum mercury level is advisable.
Management: Prevention and behavior modification are key
Treatment for pica varies by patient and the specific behavior. Management approaches are primarily preventive, educational, and directed toward behavior modification.
Prevention. Residential facilities and primary care offices that care for people with developmental disabilities may screen for pica by means of prevalence surveys, direct observation, stool checks, review of medical history records, and interviews with caregivers.
Residential facilities can create a pica-safe environment by training staff in pica prevention, instituting regular on-site monitoring to ensure that no dangerous objects are available, and developing procedures to guide staff behavior, such as safe disposal of rubber gloves.22 Parents and caregivers of young children or children with developmental disabilities who don’t live in residential facilities should be aware of pica and monitor what their children are ingesting.
Behavior modification. Behavior-based approaches have proved effective for treating pica in developmentally disabled patients. Applied behavioral analysis “was found to have the most robust empirical support to treat this behavior.”39 Patients found to have pica may be referred for further assessment to a behavior specialist or a psychologist with experience in treating the condition.22,39
A review of 26 studies found that, in 25 studies, behavioral therapy reduced pica behavior by 80% or more.23 Behavioral treatments included reinforcement procedures alone, response reduction procedures alone, and combined reinforcement and response reduction procedures. Reinforcement shapes behavior by controlling the consequences of the behavior using a combination of rewards and punishments.23 Response reduction, or blocking, involves obstructing every attempt to eat inedible items.22
Treatments that combined reinforcement and response reduction showed good efficacy.23 An example of the combined approach would be to stop the patient from eating nonnutritive items while redirecting him to eat food instead.22
Supplementation. Iron supplementation has decreased or even reversed pica in patients whose clinical symptoms and behavior were associated with iron deficiency.35,40
Medications. Successful treatment with selective serotonin reuptake inhibitors (escitalopram), atypical neuroleptics (olanzapine), and attention-deficit/hyperactivity disorder medications (methylphenidate) has been reported in some patients, but case reports are few, and the evidence for the drugs’ efficacy is limited.41-43
Be alert for pica. Primary care physicians need to be aware of pica and proactively seek information about cravings or behaviors suggesting the condition from patients in high-risk populations—pregnant women, children, immigrants and refugees, people with developmental disabilities—or their caregivers. Once pica is identified, clinicians should undertake appropriate laboratory investigation and behavior modification attempts.
CORRESPONDENCE
Ranit Mishori, MD, MHS, Department of Family Medicine, Georgetown University School of Medicine, Pre-Clinical Building, GB-01D, 3900 Reservoir Road, NW, Washington, DC 20007; [email protected]
1. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th ed. Arlington, VA: American Psychiatric Association; 2013.
2. Yalug I, Kirmizi-Alsan E, Tufan AE. Adult-onset paper pica in the context of anorexia nervosa with major depressive disorder and a history of childhood geophagia: a case report. Prog Neuropsychopharmacol Biol Psychiatry. 2007;31:1341-1342.
3. Spaniolas K, Ou S, Findeis-Hosey J, et al. Paper pica: an unusual cause of colonic ischemia. J Gastrointest Surg. 2010;14:1065-1066.
4. Olynyk F, Sharpe DH. Mercury poisoning in paper pica. N Engl J Med. 1982;306:1056-1057.
5. Guney M, Zagury GJ, Dogan N, et al. Exposure assessment and risk characterization from trace elements following soil ingestion by children exposed to playgrounds, parks and picnic areas. J Hazard Mater. 2010;182:656-664.
6. Kawai K, Saathoff E, Antelman G, et al. Geophagy (soil-eating) in relation to anemia and helminth infection among HIV-infected pregnant women in Tanzania. Am J Trop Med Hyg. 2009;80:36-43.
7. Woywodt A, Kiss A. Geophagia: the history of earth-eating. J R Soc Med. 2002;95:143-146.
8. Stokes T. The earth-eaters. Nature. 2006;444:543-544.
9. Kutalek R, Wewalka G, Gundacker C, et al. Geophagy and potential health implications: geohelminths, microbes and heavy metals. Trans R Soc Trop Med Hyg. 2010;104:787-795.
10. Keith D, Keith L, Berger GS, et al. Amylophagia during pregnancy: some maternal and perinatal correlations. Mt Sinai J Med. 1975;42:410-414.
11. Abu-Hamdan DK, Sondheimer JH, Mahajan SK. Cautopyreiophagia. Cause of life-threatening hyperkalemia in a patient undergoing hemodialysis. Am J Med. 1985;79:517-519.
12. Ewert P, Keim L, Schulte-Markwort M. Trichobezoar. A rare cause of recurrent upper abdominal pain [in German]. Monatsschr Kinderheilkd. 1992;140:811-813.
13. Grigsby RK, Thyer BA, Waller RJ, et al. Chalk eating in middle Georgia: a culture-bound syndrome of pica? South Med J. 1999;92:190-192.
14. Ahishali E, Boynueğrı B, Dabak R, et al. A case of severe acute hepatitis due to oral intake of firecrackers. Turk J Gastroenterol. 2010;21:325-326.
15. Rashid F, Davies L, Iftikhar SY. Magnetised intragastric foreign body collection and autism: An advice for careers and literature review. Autism. 2010;14:139-145.
16. Martindale JL, Bunker CJ, Noble VE. Ingested foreign bodies in a patient with pica. Gastroenterol Hepatol (N Y). 2010;6:582-584.
17. Agency for Toxic Substances and Disease Registry. Summary Report for the ATSDR Soil-Pica Workshop June 2000, Atlanta, Georgia. Agency for Toxic Substances and Disease Registry Web site. Available at: www.atsdr.cdc.gov/child/soilpica.html. Accessed June 2, 2012.
18. Njiru H, Elchalal U, Paltiel O. Geophagy during pregnancy in Africa: a literature review. Obstet Gynecol Surv. 2011;66:452-459.
19. Young SL. Pica in pregnancy: new ideas about an old condition. Annu Rev Nutr. 2010;30:403-422.
20. Clark B, Vandermeer B, Simonetti A, et al. Is lead a concern in Canadian autistic children? Paediatr Child Health. 2010;15:17-22.
21. Matson JL, Sipes M, Fodstad JC, et al. Issues in the management of challenging behaviours of adults with autism spectrum disorder. CNS Drugs. 2011;25:597-606.
22. Williams DE, McAdam D. Assessment, behavioral treatment, and prevention of pica: clinical guidelines and recommendations for practitioners. Res Dev Disabil. 2012;33:2050-2057.
23. Hagopian LP, Rooker GW, Rolider NU. Identifying empirically supported treatments for pica in individuals with intellectual disabilities. Res Dev Disabil. 2011;32:2114-2120.
24. Engberg DE. Geophagy: adaptive or aberrant behavior. Nebraska Anthropologist. 1995;12:57-68.
25. Agency for Healthcare Research and Quality. Hospitalizations for eating disorder decline, but big increase seen in pica disorder. Agency for Healthcare Research and Quality Web site. Available at: www.ahrq.gov/news/nn/nn090811.htm. Accessed June 2, 2014.
26. Stroman D, Young C, Rubano AR, et al. Adult-onset pica leading to acute intestinal obstruction. Psychosomatics. 2011;52:393-394.
27. Young SL, Khalfan SS, Farag TH, et al. Association of pica with anemia and gastrointestinal distress among pregnant women in Zanzibar, Tanzania. Am J Trop Med Hyg. 2010;83:144-151.
28. Altepeter T, Annes J, Meller J. Foam bezoar: resection of perforated terminal ileum in a 17-year-old with sickle b+thalassemia and pica. J Pediatr Surg. 2011;46:E31-E32.
29. Chatzimavroudis G, Christopoulos P, Atmatzidis S, et al. Pica: an uncommon cause of acute abdominal pain in children. Indian J Pediatr. 2011;78:886-887.
30. Rector WG Jr. Pica: its frequency and significance in patients with iron-deficiency anemia due to chronic gastrointestinal blood loss. J Gen Intern Med. 1989;4:512-513.
31. Sontag C, Kettaneh A, Fain O, et al. Rapid regression of prolonged pagophagia after treatment of iron deficiency [in French]. Presse Med. 2001;30:321-323.
32. Sharma TR, Kavuru B, Aly M. Coprophagia and pica in individuals with mild to moderate dementia and mixed (iron deficiency and macrocytic) anemia. J Am Geriatr Soc. 2011;59:2375-2377.
33. Kushner RF, Shanta Retelny V. Emergence of pica (ingestion of non-food substances) accompanying iron deficiency anemia after gastric bypass surgery. Obes Surg. 2005;15:1491-1495.
34. Barton JC, Barton JC, Bertoli LF. Pica associated with iron deficiency or depletion: clinical and laboratory correlates in 262 nonpregnant adult outpatients. BMC Blood Disord. 2010;10:9.
35. Khan Y, Tisman G. Pica in iron deficiency: a case series. J Med Case Rep. 2010;4:86.
36. Bakhireva LN, Rowland AS, Young BN, et al. Sources of potential lead exposure among pregnant women in New Mexico. Matern Child Health J. 2013;17:172-179.
37. Thihalolipavan S, Candalla BM, Ehrlich J. Examining pica in NYC pregnant women with elevated blood lead levels. Matern Child Health J. 2013;17:49-55.
38. Al-Rmalli SW, Jenkins RO, Watts MJ, et al. Risk of human exposure to arsenic and other toxic elements from geophagy: trace element analysis of baked clay using inductively coupled plasma mass spectrometry. Environ Health. 2010;9:79.
39. Matson JL, Hattier MA, Belva B, et al. Pica in persons with developmental disabilities: approaches to treatment. Res Dev Disabil. 2013;34:2564-2571.
40. Bryant BJ1, Yau YY, Arceo SM, et al. Ascertainment of iron deficiency and depletion in blood donors through screening questions for pica and restless legs syndrome. Transfusion. 2013;53:1637-1644.
41. Lerner AJ. Treatment of pica behavior with olanzapine. CNS Spectr. 2008;13:19.
42. Hergüner S, Hergüner AS. Pica in a child with attention deficit hyperactivity disorder and successful treatment with methylphenidate. Prog Neuropsychopharmacol Biol Psychiatry. 2010;34:1155-1156.
43. Bhatia MS, Gupta R. Pica responding to SSRI: an OCD Spectrum Disorder? World J Biol Psychiatry. 2009;10(4 pt 3):936-938.
1. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th ed. Arlington, VA: American Psychiatric Association; 2013.
2. Yalug I, Kirmizi-Alsan E, Tufan AE. Adult-onset paper pica in the context of anorexia nervosa with major depressive disorder and a history of childhood geophagia: a case report. Prog Neuropsychopharmacol Biol Psychiatry. 2007;31:1341-1342.
3. Spaniolas K, Ou S, Findeis-Hosey J, et al. Paper pica: an unusual cause of colonic ischemia. J Gastrointest Surg. 2010;14:1065-1066.
4. Olynyk F, Sharpe DH. Mercury poisoning in paper pica. N Engl J Med. 1982;306:1056-1057.
5. Guney M, Zagury GJ, Dogan N, et al. Exposure assessment and risk characterization from trace elements following soil ingestion by children exposed to playgrounds, parks and picnic areas. J Hazard Mater. 2010;182:656-664.
6. Kawai K, Saathoff E, Antelman G, et al. Geophagy (soil-eating) in relation to anemia and helminth infection among HIV-infected pregnant women in Tanzania. Am J Trop Med Hyg. 2009;80:36-43.
7. Woywodt A, Kiss A. Geophagia: the history of earth-eating. J R Soc Med. 2002;95:143-146.
8. Stokes T. The earth-eaters. Nature. 2006;444:543-544.
9. Kutalek R, Wewalka G, Gundacker C, et al. Geophagy and potential health implications: geohelminths, microbes and heavy metals. Trans R Soc Trop Med Hyg. 2010;104:787-795.
10. Keith D, Keith L, Berger GS, et al. Amylophagia during pregnancy: some maternal and perinatal correlations. Mt Sinai J Med. 1975;42:410-414.
11. Abu-Hamdan DK, Sondheimer JH, Mahajan SK. Cautopyreiophagia. Cause of life-threatening hyperkalemia in a patient undergoing hemodialysis. Am J Med. 1985;79:517-519.
12. Ewert P, Keim L, Schulte-Markwort M. Trichobezoar. A rare cause of recurrent upper abdominal pain [in German]. Monatsschr Kinderheilkd. 1992;140:811-813.
13. Grigsby RK, Thyer BA, Waller RJ, et al. Chalk eating in middle Georgia: a culture-bound syndrome of pica? South Med J. 1999;92:190-192.
14. Ahishali E, Boynueğrı B, Dabak R, et al. A case of severe acute hepatitis due to oral intake of firecrackers. Turk J Gastroenterol. 2010;21:325-326.
15. Rashid F, Davies L, Iftikhar SY. Magnetised intragastric foreign body collection and autism: An advice for careers and literature review. Autism. 2010;14:139-145.
16. Martindale JL, Bunker CJ, Noble VE. Ingested foreign bodies in a patient with pica. Gastroenterol Hepatol (N Y). 2010;6:582-584.
17. Agency for Toxic Substances and Disease Registry. Summary Report for the ATSDR Soil-Pica Workshop June 2000, Atlanta, Georgia. Agency for Toxic Substances and Disease Registry Web site. Available at: www.atsdr.cdc.gov/child/soilpica.html. Accessed June 2, 2012.
18. Njiru H, Elchalal U, Paltiel O. Geophagy during pregnancy in Africa: a literature review. Obstet Gynecol Surv. 2011;66:452-459.
19. Young SL. Pica in pregnancy: new ideas about an old condition. Annu Rev Nutr. 2010;30:403-422.
20. Clark B, Vandermeer B, Simonetti A, et al. Is lead a concern in Canadian autistic children? Paediatr Child Health. 2010;15:17-22.
21. Matson JL, Sipes M, Fodstad JC, et al. Issues in the management of challenging behaviours of adults with autism spectrum disorder. CNS Drugs. 2011;25:597-606.
22. Williams DE, McAdam D. Assessment, behavioral treatment, and prevention of pica: clinical guidelines and recommendations for practitioners. Res Dev Disabil. 2012;33:2050-2057.
23. Hagopian LP, Rooker GW, Rolider NU. Identifying empirically supported treatments for pica in individuals with intellectual disabilities. Res Dev Disabil. 2011;32:2114-2120.
24. Engberg DE. Geophagy: adaptive or aberrant behavior. Nebraska Anthropologist. 1995;12:57-68.
25. Agency for Healthcare Research and Quality. Hospitalizations for eating disorder decline, but big increase seen in pica disorder. Agency for Healthcare Research and Quality Web site. Available at: www.ahrq.gov/news/nn/nn090811.htm. Accessed June 2, 2014.
26. Stroman D, Young C, Rubano AR, et al. Adult-onset pica leading to acute intestinal obstruction. Psychosomatics. 2011;52:393-394.
27. Young SL, Khalfan SS, Farag TH, et al. Association of pica with anemia and gastrointestinal distress among pregnant women in Zanzibar, Tanzania. Am J Trop Med Hyg. 2010;83:144-151.
28. Altepeter T, Annes J, Meller J. Foam bezoar: resection of perforated terminal ileum in a 17-year-old with sickle b+thalassemia and pica. J Pediatr Surg. 2011;46:E31-E32.
29. Chatzimavroudis G, Christopoulos P, Atmatzidis S, et al. Pica: an uncommon cause of acute abdominal pain in children. Indian J Pediatr. 2011;78:886-887.
30. Rector WG Jr. Pica: its frequency and significance in patients with iron-deficiency anemia due to chronic gastrointestinal blood loss. J Gen Intern Med. 1989;4:512-513.
31. Sontag C, Kettaneh A, Fain O, et al. Rapid regression of prolonged pagophagia after treatment of iron deficiency [in French]. Presse Med. 2001;30:321-323.
32. Sharma TR, Kavuru B, Aly M. Coprophagia and pica in individuals with mild to moderate dementia and mixed (iron deficiency and macrocytic) anemia. J Am Geriatr Soc. 2011;59:2375-2377.
33. Kushner RF, Shanta Retelny V. Emergence of pica (ingestion of non-food substances) accompanying iron deficiency anemia after gastric bypass surgery. Obes Surg. 2005;15:1491-1495.
34. Barton JC, Barton JC, Bertoli LF. Pica associated with iron deficiency or depletion: clinical and laboratory correlates in 262 nonpregnant adult outpatients. BMC Blood Disord. 2010;10:9.
35. Khan Y, Tisman G. Pica in iron deficiency: a case series. J Med Case Rep. 2010;4:86.
36. Bakhireva LN, Rowland AS, Young BN, et al. Sources of potential lead exposure among pregnant women in New Mexico. Matern Child Health J. 2013;17:172-179.
37. Thihalolipavan S, Candalla BM, Ehrlich J. Examining pica in NYC pregnant women with elevated blood lead levels. Matern Child Health J. 2013;17:49-55.
38. Al-Rmalli SW, Jenkins RO, Watts MJ, et al. Risk of human exposure to arsenic and other toxic elements from geophagy: trace element analysis of baked clay using inductively coupled plasma mass spectrometry. Environ Health. 2010;9:79.
39. Matson JL, Hattier MA, Belva B, et al. Pica in persons with developmental disabilities: approaches to treatment. Res Dev Disabil. 2013;34:2564-2571.
40. Bryant BJ1, Yau YY, Arceo SM, et al. Ascertainment of iron deficiency and depletion in blood donors through screening questions for pica and restless legs syndrome. Transfusion. 2013;53:1637-1644.
41. Lerner AJ. Treatment of pica behavior with olanzapine. CNS Spectr. 2008;13:19.
42. Hergüner S, Hergüner AS. Pica in a child with attention deficit hyperactivity disorder and successful treatment with methylphenidate. Prog Neuropsychopharmacol Biol Psychiatry. 2010;34:1155-1156.
43. Bhatia MS, Gupta R. Pica responding to SSRI: an OCD Spectrum Disorder? World J Biol Psychiatry. 2009;10(4 pt 3):936-938.
Autism: Why the rise in rates?
For many years, articles about autism cited prevalence rates of approximately 7 in 10,000.1 Over the past few years, however, there appears to have been an explosion in the rate at which autism is diagnosed: More recent estimates range from about 30 in 10,0002 to one in 68.3 References to an autism epidemic appear to have originated in a 2002 California legislative report suggesting a 273% increase in autism from 1987 to 1998.4
Concerns about rising rates of autism, however, are not new. In 1943, Leo Kanner, MD, a psychiatrist and pioneer in the study of autism, published a paper titled, “Autistic disturbances of affective contact.”5 The result? “Almost overnight, the country seemed to be populated by a multitude of autistic children,” he later observed.6
To what should we attribute the current rise in reported autism rates? Even a casual review of the autism literature suggests a number of potential causes that may account for at least a portion of the recent increase.
We know more about the disorder
In his paper, Kanner described the “peculiarities” of 11 children whom he had cared for.5 While several had been diagnosed with mental retardation, childhood schizophrenia, or both, what stood out to Kanner was an “autistic aloneness” evident from the beginning of life. This was in contrast to childhood schizophrenia, in which a child experienced a departure from previously normal interrelations. His insight contributed to our understanding of what is now recognized as autism spectrum disorder (ASD).
Also in 1943, Hans Asperger, MD, was studying families with children exhibiting behaviors similar to those described by Kanner. The following year, Asperger published an article (in German) describing these children. Unfortunately, this paper—titled “Autistic psychopathy in childhood”7—was not translated into English until the early 1990s.8
Since Kanner and Asperger first called attention to the disorder, there have been numerous changes in societal and medical understanding of autism. Bruno Bettelheim, PhD, an Austrian-born child psychologist with a particular interest in emotionally disturbed children, theorized that poor maternal bonding and lack of maternal affection were responsible for autistic characteristics.9 Bernard Rimland, PhD, a psychologist and the father of an autistic child, argued for a biological basis of the disorder.10 Rimland’s theory of autism as a neurodevelopmental disorder with an unidentified organic etiology is most consistent with current medical opinion.
Definitions and diagnostic criteria have evolved
The first edition of the Diagnostic and Statistical Manual of Mental Disorders (DSM) was published in 1952.11 Although it was nearly a decade after Dr. Kanner clearly described autism as an entity separate from childhood schizophrenia, the word autism was used in this edition only once—to describe psychotic reactions associated with schizophrenia. Similarly, autism was referred to only in relation to childhood schizophrenia in the DSMII,12 published in 1968.
DSM-III adds diagnostic criteria. In DSM-III (1980),13 specific diagnostic criteria for infantile autism and pervasive developmental disorder (PDD) appeared for the first time. Both diagnoses were clearly contrasted with a diagnosis of schizophrenia, and were used to identify children who exhibited a pervasive lack of responsiveness to others.
The DSM-III-Revised (R) (1987)14 added a classification scheme more consistent with the current standard. It included: 1) qualitative impairment in reciprocal social interaction; 2) qualitative impairment in communication and imaginative activity; and 3) restricted activity/interests.
PDD Not Otherwise Specified was also included, and served to identify those who had qualitative impairments in social interaction and communication skills but did not meet the full criteria for autism disorder or PDD.
The publication of DSM-IV (1994)15 brought another change: Specific criteria were outlined for the diagnoses of Asperger syndrome, Rett syndrome, and childhood disintegrative disorders; in the DSM-IV-Text Revision (TR) (2000),16 this structure remained relatively stable.
DSM-5 (2013) took another step, consolidating these various disorders into a single, unifying diagnosis of ASD.17 Significant controversy surrounded this change, with some viewing it as an oversimplification that does not accurately reflect important distinctions among divergent disorders18 and others arguing that it will result in unrecognized cases and exclusion of affected individuals.19 Notably, a recent study using concurrent DSM-IV and DSM-5 criteria to diagnose autism and PDD in more than 4000 children documented a high level of agreement between them.20
Diagnostic tools have improved
As the number of children diagnosed with autism has increased, so have efforts to more accurately diagnose autism as a distinct disorder.
In the 1960s, guidelines for diagnosing autism focused primarily on Kanner’s original descriptive criteria. Even in the 1980s, after DSM-III criteria identified autism as a distinct disorder, children were being evaluated with generalized developmental screening tools focused on behaviors characteristic of a severe mental handicap, without differentiating between autistic and nonautistic children.
Early autism-specific observational and structured interview tools (eg, Childhood Autism Rating Scale,21 Autism Diagnostic Interview [ADI],22 and Autistic Diagnostic Observation Schedule)23 emerged from a need for standardized diagnostic instruments that were comparable and reproducible.24 But because these tools were highly specific and initially studied in research settings with high-risk populations, they lacked the sensitivity to identify children at risk in the general population, particularly those with milder symptoms.
As the diagnosis of autism became more standardized following publication of the DSM III-R in 1987, developmental specialists were able to construct increasingly sensitive evaluation tools. The ADI was revised25 to facilitate earlier and more efficient diagnosis, allowing for assessment of children as young as 19 months of age. The Checklist for Autism in Toddlers (CHAT)26 and subsequent modification (M-CHAT)27 were among the earliest and most effective screening tools, appropriate for use in children as young as 16 months old.
Tools that followed the original CHAT (eg, the Autism Spectrum Screening Questionnaire [ASSQ]28) were adapted to better identify high-functioning children with Asperger syndrome, as well as those with autism.
Another revision of the M-CHAT—the M-CHAT-R/F (Revised with Follow-up) was validated earlier this year. In a study involving 16,000 children, 95% of those who had positive tests were found to have some form of developmental delay and almost half (47%) received an ASD diagnosis.29
Other diagnostic aids are being explored as a means of promoting earlier identification of ASD. For example, a blood test to identify differences in gene expression between children with and without ASD30 has shown initial promise, particularly in males. This test is licensed by SynapDx (Lexington, Mass) and a clinical trial to evaluate it has begun.
Results of another study demonstrating normalization of brain activity in autistic children after they’ve undergone intensive treatment31 raise the possibility of using cortical activation as measured by electroencephalography as an early biomarker for autism.
Treatment options, advocacy affect rate of diagnosis
Improvements in diagnosis and targeted identification of potentially treatable symptoms32 led to the development of new treatment options. And greater use of day care and preschool programs prompted networking among parents, who touted the benefits of early evaluation, diagnosis, and treatment. Earlier screening, not surprisingly, led to an increase in the target population.
|
Local as well as national advocacy groups, led primarily by parents, have become powerful voices for improvements in services offered to children with autism. And research studies with varying degrees of sophistication continue to be published, further fueling the demand for school systems to provide supportive learning environments as required by the Individuals with Disabilities Education Act, initially enacted in 1975 and amended in 2004.33
Federal and state funds in the form of Medicaid waivers are available to provide long-term care services in home and community settings, while private insurers typically pay for associated treatment modalities for those with an autism diagnosis, including physical, occupational, and speech therapy, among other services.
Finally, politicians and celebrities with personal connections to autism have joined the effort to increase awareness and improve the quality and availability of services—further assuring that autism is recognized as a legitimate, definable, and treatable disorder.
As an autism diagnosis has become more socially acceptable, it has at times replaced diagnoses of learning disability and mental retardation, a trend known as “diagnostic substitution.”34 Indeed, having a child with an ASD diagnosis often makes it possible for parents to secure services that might otherwise be unavailable to them.
Is the incidence of autism linked to the environment?
Numerous environmental, nutritional, and pharmaceutical changes have been cited as reasons for what is perceived as an increasing incidence of autism in recent years. For example, some contend that greater use of food preservatives and greater exposure of young children to environmental toxins are contributing factors.35
Thimerosal. Perhaps most notable is the assertion—since disproven—that thimerosal, a substance previously used in the manufacture of several childhood vaccines, was a leading cause of autism.36,37 In fact, one study documented an increase in autism after thimerosal had been discontinued.38 (For more on the thimerosal controversy, see “Autism: 5 misconceptions that can complicate care”.)
Autism comorbidity. As autism is frequently comorbid with other developmental disabilities, advances in medical technology that have led to a decline in neonatal death and overall mortality among the disabled may mean more survivors are subsequently diagnosed with an autism comorbidity.
Biological factors. Recent studies suggest that advanced paternal age can increase the risk of autism.39 Twin studies suggest moderate genetic heritability, along with a substantial environmental contribution to the development of autism.40 And new research suggests that maternal stressors during pregnancy—eg, trauma, illness, or substance abuse—may increase a child’s risk of developing autism, among other psychiatric disorders.41
The important role you play in the diagnosis of autism
It is clear that autism is more common than previously thought3 and that various factors are at work. Ensuring that children are promptly and properly evaluated begins when primary care physicians take parents’ concerns seriously and keep an eye out for common symptoms and characteristic developmental delays that may be evident even in the first year of life.
If symptoms are not severe enough to be detected in the child’s first several years, the next likely presentation will be when a parent gets a call from school suggesting that their child be tested for autism. While this delayed presentation suggests a higher level of functioning, a full evaluation, including the use of questionnaires such as the Social Responsiveness Scale42 and ASSQ,28 and appropriate referrals are still vital.
Puberty is another time when characteristics suggestive of autism that escaped earlier detection may be noted, and vague behavioral issues or concerns about intellectual impairment may become sufficiently troublesome to prompt a thorough evaluation. While behavioral therapy can be very helpful in such cases, medical therapy directed at co-occurring conditions (ie, a mood or anxiety disorder, including obsessive-compulsive disorder) may also provide significant benefit.
Whatever the age of the child, his or her parents should be counseled as to the general nature of autism, reassured of the availability of treatment options and given the appropriate referrals, and encouraged to learn more by availing themselves of resources (TABLE) and support groups. A consumer update from the US Food and Drug Administration titled “Beware of false or misleading claims for treating autism” (http://www.fda.gov/ForConsumers/ConsumerUpdates/ucm394757.htm) will be helpful for many parents, as well.
CORRESPONDENCE
Robert G. Zylstra, EdD, LCSW, Department of Family Medicine, The University of Tennessee College of Medicine, 110 East Third Street, Chattanooga, TN 37403; [email protected]
1. Prater C, Zylstra R. Autism: a medical primer. Am Fam Physician. 2002;66:1667-1674.
2. Nassar N, Dixon G, Bourke J, et al. Autism spectrum disorders in young children: effect of changes in diagnostic practices. Int J Epidemiol. 2009;38:1245-1254.
3. Developmental Disabilities Monitoring Network Surveillance Year 2010 Principal Investigators; Centers for Disease Control and Prevention (CDC). Prevalence of autism spectrum disorder among children aged 8 years - Autism and Developmental Disabilities Monitoring Network, 11 sites, United States, 2010. MMWR Surveill Summ. 2014;63:1-21.
4. Report to the legislature on the principle findings from the epidemiology of autism in California: a comprehensive pilot study. State of California Department of Developmental Services Web site. Available at: http://www.dds.ca.gov/Autism/docs/study_final.pdf. Accessed May 1, 2014.
5. Kanner L. Autistic disturbances of affective contact. Nervous Child. 1943;2:217-250.
6. Kanner L. Infantile autism and the schizophrenias. Behav Sci. 1965;10:412-420.
7. Asperger H. Die ‘autistischen psychopathen’ im kindesalter. Archiv für Psychiatrie und Nervenkrankheiten. 1944;117:76-136.
8. Frith U. Autism and Asperger Syndrome. Cambridge, UK: Cambridge University Press; 1991.
9. Bettelheim B. The empty fortress: Infantile autism and the birth of the self. New York: Free Press; 1972.
10. Rimland B. Infantile autism: The syndrome and its implications for a neural theory of behavior. New York: Appleton-Century- Crofts; 1964.
11. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. Washington, DC: American Psychiatric Association; 1952.
12. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 2nd ed. Washington, DC: American Psychiatric Association;1968.
13. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 3rd ed. Washington, DC: American Psychiatric Association; 1980.
14. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 3rd ed, revised. Washington, DC: American Psychiatric Association; 1987.
15. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 4th ed. Washington, DC: American Psychiatric Association; 1994.
16. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 4th ed, text revision. Washington: DC: American Psychiatric Association; 2000.
17. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th ed. Arlington, VA: American Psychiatric Publishing; 2013.
18. Ghaziuddin M. Brief report: Should the DMS-V drop Asperger Syndrome? J Autism Dev Disord. 2010;40:1146-1148.
19. Wing L, Gould J, Gillberg C. Autism spectrum disorders in the DSM-V: better or worse than the DSM-IV? Res Dev Disabil. 2011;32:768-773.
20. Huerta M, Bishop S, Duncan A, et al. Application of DSM-5 criteria for Autism Spectrum Disorder to three samples of children with DSM-IV diagnoses of pervasive developmental disorders. Am J Psychiatry. 2012;169:1056-1064.
21. Schopler E, Reichler RJ, DeVellis RF. Toward objective classification of childhood autism: Childhood Autism Rating Scale (CARS). J Autism Dev Disord. 1980;10:91-103.
22. Le Couteur A, Rutter M, Lord C, et al. Autism diagnostic interview: a standardized investigator-based instrument. J Autism Dev Disord. 1989;19:363-387.
23. Lord C, Rutter M, Goode S, et al. Autism diagnostic observation schedule: a standardized observation of communicative and social behavior. J Autism Dev Disord. 1989;19:185-212.
24. Zwaigenbaum L. Advances in the early detection of autism. Curr Opin Neurol. 2010;23:97-102.
25. Lord C, Rutter M, Le Couteur A. Autism Diagnostic Interview-Revised: A revised version of a diagnostic interview for caregivers of individuals with possible pervasive developmental disorders. J Autism Dev Disord. 1994;24:659-685.
26. Baron-Cohen S, Allen J, Gillberg C. Can autism be detected at 18 months? The needle, the haystack, and the CHAT. Br J Psychiatry. 1992;161:839-843.
27. Robins DL, Fein D, Barton ML, et al. The Modified Checklist for Autism in Toddlers: an initial study investigating the early detection of autism and pervasive developmental disorders. J Autism Dev Disord. 2001;31:131-144.
28. Ehlers S, Gilberg C, Wing L. A screening questionnaire for Asperger syndrome and other high-functioning autism spectrum disorders in school age children. J Autism Dev Disord. 1999;29:129-141.
29. Robins DL, Casagrande K, Barton M, et al. Validation of the modified checklist for Autism in toddlers, revised with followup (M-CHAT-R/F). Pediatrics. 2014;133:37-45.
30. Kong SW, Collins CD, Shimizu-Motohashi Y, et al. Characteristics and predictive value of blood transcriptome signature in males with autism spectrum disorders. PLoS One. 2012;7:e49475.
31. Dawson G, Jones EJ, Merkle K, et al. Early behavioral intervention is associated with normalized brain activity in young children with autism. J Am Acad Child Adolesc Psychiatry. 2012;51:1150-1159.
32. Autism ALARM. National Center for Medical Home Implementation Web site. Available at: http://www.medicalhomeinfo.org/downloads/pdfs/AutismAlarm.pdf. Accessed March 4, 2013.
33. IDEA. Autism Community Web site. Available at: http://www.autism-community.com/education/idea/. Accessed April 25, 2014.
34. Shattuck P. The contribution of diagnostic substitution to the growing administrative prevalence of autism in US special education. Pediatrics. 2006;117:1028-1037.
35. Landrigan P. What causes autism? Exploring the environmental contribution. Curr Opin Pediatr. 2010;22:219-225.
36. The great thimerosal cover-up: Mercury, vaccines, autism and your child’s health. Natural Health News & Scientific Discoveries Web site. Available at: http://www.naturalnews.com/011764_thimerosal_mercury.html. Accessed March 4, 2013.
37. Price CS, Thompson WW, Goodson B, et al. Prenatal and infant exposure to thimerosal from vaccines and immunoglobulins and risk of autism. Pediatrics. 2010;126:656-664.
38. Fombonne E, Zakarian R, Bennett A, et al. Pervasive developmental disorders in Montreal, Quebec, Canada: prevalence and links with immunizations. Pediatrics. 2006;118:e139-e150.
39. Flatscher-Bader T, Foldi CJ, Chong S, et al. Increased de novo copy number variants in the offspring of older males. Transl Psychiatry. 2011;1:e34.
40. Hallmayer J, Cleveland S, Torres A, et al. Genetic heritability and shared environmental factors among twin pairs with autism. Arch Gen Psychiatry. 2011;68:1095-1102.
41. Fine R, Zhang J, Stevens HE. Prenatal stress and inhibitory neuron systems: implications for neuropsychiatric disorders Mol Psychiatry. 2014 April 22. [Epub ahead of print].
42. Joshi G, Petty DR, Fried R, et al. Discriminant and concurrent validity of a simplified DSM-based structured diagnostic instrument for the assessment of autism spectrum disorders in youth and young adults. BMC Psychiatry. 2011;11:204.
For many years, articles about autism cited prevalence rates of approximately 7 in 10,000.1 Over the past few years, however, there appears to have been an explosion in the rate at which autism is diagnosed: More recent estimates range from about 30 in 10,0002 to one in 68.3 References to an autism epidemic appear to have originated in a 2002 California legislative report suggesting a 273% increase in autism from 1987 to 1998.4
Concerns about rising rates of autism, however, are not new. In 1943, Leo Kanner, MD, a psychiatrist and pioneer in the study of autism, published a paper titled, “Autistic disturbances of affective contact.”5 The result? “Almost overnight, the country seemed to be populated by a multitude of autistic children,” he later observed.6
To what should we attribute the current rise in reported autism rates? Even a casual review of the autism literature suggests a number of potential causes that may account for at least a portion of the recent increase.
We know more about the disorder
In his paper, Kanner described the “peculiarities” of 11 children whom he had cared for.5 While several had been diagnosed with mental retardation, childhood schizophrenia, or both, what stood out to Kanner was an “autistic aloneness” evident from the beginning of life. This was in contrast to childhood schizophrenia, in which a child experienced a departure from previously normal interrelations. His insight contributed to our understanding of what is now recognized as autism spectrum disorder (ASD).
Also in 1943, Hans Asperger, MD, was studying families with children exhibiting behaviors similar to those described by Kanner. The following year, Asperger published an article (in German) describing these children. Unfortunately, this paper—titled “Autistic psychopathy in childhood”7—was not translated into English until the early 1990s.8
Since Kanner and Asperger first called attention to the disorder, there have been numerous changes in societal and medical understanding of autism. Bruno Bettelheim, PhD, an Austrian-born child psychologist with a particular interest in emotionally disturbed children, theorized that poor maternal bonding and lack of maternal affection were responsible for autistic characteristics.9 Bernard Rimland, PhD, a psychologist and the father of an autistic child, argued for a biological basis of the disorder.10 Rimland’s theory of autism as a neurodevelopmental disorder with an unidentified organic etiology is most consistent with current medical opinion.
Definitions and diagnostic criteria have evolved
The first edition of the Diagnostic and Statistical Manual of Mental Disorders (DSM) was published in 1952.11 Although it was nearly a decade after Dr. Kanner clearly described autism as an entity separate from childhood schizophrenia, the word autism was used in this edition only once—to describe psychotic reactions associated with schizophrenia. Similarly, autism was referred to only in relation to childhood schizophrenia in the DSMII,12 published in 1968.
DSM-III adds diagnostic criteria. In DSM-III (1980),13 specific diagnostic criteria for infantile autism and pervasive developmental disorder (PDD) appeared for the first time. Both diagnoses were clearly contrasted with a diagnosis of schizophrenia, and were used to identify children who exhibited a pervasive lack of responsiveness to others.
The DSM-III-Revised (R) (1987)14 added a classification scheme more consistent with the current standard. It included: 1) qualitative impairment in reciprocal social interaction; 2) qualitative impairment in communication and imaginative activity; and 3) restricted activity/interests.
PDD Not Otherwise Specified was also included, and served to identify those who had qualitative impairments in social interaction and communication skills but did not meet the full criteria for autism disorder or PDD.
The publication of DSM-IV (1994)15 brought another change: Specific criteria were outlined for the diagnoses of Asperger syndrome, Rett syndrome, and childhood disintegrative disorders; in the DSM-IV-Text Revision (TR) (2000),16 this structure remained relatively stable.
DSM-5 (2013) took another step, consolidating these various disorders into a single, unifying diagnosis of ASD.17 Significant controversy surrounded this change, with some viewing it as an oversimplification that does not accurately reflect important distinctions among divergent disorders18 and others arguing that it will result in unrecognized cases and exclusion of affected individuals.19 Notably, a recent study using concurrent DSM-IV and DSM-5 criteria to diagnose autism and PDD in more than 4000 children documented a high level of agreement between them.20
Diagnostic tools have improved
As the number of children diagnosed with autism has increased, so have efforts to more accurately diagnose autism as a distinct disorder.
In the 1960s, guidelines for diagnosing autism focused primarily on Kanner’s original descriptive criteria. Even in the 1980s, after DSM-III criteria identified autism as a distinct disorder, children were being evaluated with generalized developmental screening tools focused on behaviors characteristic of a severe mental handicap, without differentiating between autistic and nonautistic children.
Early autism-specific observational and structured interview tools (eg, Childhood Autism Rating Scale,21 Autism Diagnostic Interview [ADI],22 and Autistic Diagnostic Observation Schedule)23 emerged from a need for standardized diagnostic instruments that were comparable and reproducible.24 But because these tools were highly specific and initially studied in research settings with high-risk populations, they lacked the sensitivity to identify children at risk in the general population, particularly those with milder symptoms.
As the diagnosis of autism became more standardized following publication of the DSM III-R in 1987, developmental specialists were able to construct increasingly sensitive evaluation tools. The ADI was revised25 to facilitate earlier and more efficient diagnosis, allowing for assessment of children as young as 19 months of age. The Checklist for Autism in Toddlers (CHAT)26 and subsequent modification (M-CHAT)27 were among the earliest and most effective screening tools, appropriate for use in children as young as 16 months old.
Tools that followed the original CHAT (eg, the Autism Spectrum Screening Questionnaire [ASSQ]28) were adapted to better identify high-functioning children with Asperger syndrome, as well as those with autism.
Another revision of the M-CHAT—the M-CHAT-R/F (Revised with Follow-up) was validated earlier this year. In a study involving 16,000 children, 95% of those who had positive tests were found to have some form of developmental delay and almost half (47%) received an ASD diagnosis.29
Other diagnostic aids are being explored as a means of promoting earlier identification of ASD. For example, a blood test to identify differences in gene expression between children with and without ASD30 has shown initial promise, particularly in males. This test is licensed by SynapDx (Lexington, Mass) and a clinical trial to evaluate it has begun.
Results of another study demonstrating normalization of brain activity in autistic children after they’ve undergone intensive treatment31 raise the possibility of using cortical activation as measured by electroencephalography as an early biomarker for autism.
Treatment options, advocacy affect rate of diagnosis
Improvements in diagnosis and targeted identification of potentially treatable symptoms32 led to the development of new treatment options. And greater use of day care and preschool programs prompted networking among parents, who touted the benefits of early evaluation, diagnosis, and treatment. Earlier screening, not surprisingly, led to an increase in the target population.
|
Local as well as national advocacy groups, led primarily by parents, have become powerful voices for improvements in services offered to children with autism. And research studies with varying degrees of sophistication continue to be published, further fueling the demand for school systems to provide supportive learning environments as required by the Individuals with Disabilities Education Act, initially enacted in 1975 and amended in 2004.33
Federal and state funds in the form of Medicaid waivers are available to provide long-term care services in home and community settings, while private insurers typically pay for associated treatment modalities for those with an autism diagnosis, including physical, occupational, and speech therapy, among other services.
Finally, politicians and celebrities with personal connections to autism have joined the effort to increase awareness and improve the quality and availability of services—further assuring that autism is recognized as a legitimate, definable, and treatable disorder.
As an autism diagnosis has become more socially acceptable, it has at times replaced diagnoses of learning disability and mental retardation, a trend known as “diagnostic substitution.”34 Indeed, having a child with an ASD diagnosis often makes it possible for parents to secure services that might otherwise be unavailable to them.
Is the incidence of autism linked to the environment?
Numerous environmental, nutritional, and pharmaceutical changes have been cited as reasons for what is perceived as an increasing incidence of autism in recent years. For example, some contend that greater use of food preservatives and greater exposure of young children to environmental toxins are contributing factors.35
Thimerosal. Perhaps most notable is the assertion—since disproven—that thimerosal, a substance previously used in the manufacture of several childhood vaccines, was a leading cause of autism.36,37 In fact, one study documented an increase in autism after thimerosal had been discontinued.38 (For more on the thimerosal controversy, see “Autism: 5 misconceptions that can complicate care”.)
Autism comorbidity. As autism is frequently comorbid with other developmental disabilities, advances in medical technology that have led to a decline in neonatal death and overall mortality among the disabled may mean more survivors are subsequently diagnosed with an autism comorbidity.
Biological factors. Recent studies suggest that advanced paternal age can increase the risk of autism.39 Twin studies suggest moderate genetic heritability, along with a substantial environmental contribution to the development of autism.40 And new research suggests that maternal stressors during pregnancy—eg, trauma, illness, or substance abuse—may increase a child’s risk of developing autism, among other psychiatric disorders.41
The important role you play in the diagnosis of autism
It is clear that autism is more common than previously thought3 and that various factors are at work. Ensuring that children are promptly and properly evaluated begins when primary care physicians take parents’ concerns seriously and keep an eye out for common symptoms and characteristic developmental delays that may be evident even in the first year of life.
If symptoms are not severe enough to be detected in the child’s first several years, the next likely presentation will be when a parent gets a call from school suggesting that their child be tested for autism. While this delayed presentation suggests a higher level of functioning, a full evaluation, including the use of questionnaires such as the Social Responsiveness Scale42 and ASSQ,28 and appropriate referrals are still vital.
Puberty is another time when characteristics suggestive of autism that escaped earlier detection may be noted, and vague behavioral issues or concerns about intellectual impairment may become sufficiently troublesome to prompt a thorough evaluation. While behavioral therapy can be very helpful in such cases, medical therapy directed at co-occurring conditions (ie, a mood or anxiety disorder, including obsessive-compulsive disorder) may also provide significant benefit.
Whatever the age of the child, his or her parents should be counseled as to the general nature of autism, reassured of the availability of treatment options and given the appropriate referrals, and encouraged to learn more by availing themselves of resources (TABLE) and support groups. A consumer update from the US Food and Drug Administration titled “Beware of false or misleading claims for treating autism” (http://www.fda.gov/ForConsumers/ConsumerUpdates/ucm394757.htm) will be helpful for many parents, as well.
CORRESPONDENCE
Robert G. Zylstra, EdD, LCSW, Department of Family Medicine, The University of Tennessee College of Medicine, 110 East Third Street, Chattanooga, TN 37403; [email protected]
For many years, articles about autism cited prevalence rates of approximately 7 in 10,000.1 Over the past few years, however, there appears to have been an explosion in the rate at which autism is diagnosed: More recent estimates range from about 30 in 10,0002 to one in 68.3 References to an autism epidemic appear to have originated in a 2002 California legislative report suggesting a 273% increase in autism from 1987 to 1998.4
Concerns about rising rates of autism, however, are not new. In 1943, Leo Kanner, MD, a psychiatrist and pioneer in the study of autism, published a paper titled, “Autistic disturbances of affective contact.”5 The result? “Almost overnight, the country seemed to be populated by a multitude of autistic children,” he later observed.6
To what should we attribute the current rise in reported autism rates? Even a casual review of the autism literature suggests a number of potential causes that may account for at least a portion of the recent increase.
We know more about the disorder
In his paper, Kanner described the “peculiarities” of 11 children whom he had cared for.5 While several had been diagnosed with mental retardation, childhood schizophrenia, or both, what stood out to Kanner was an “autistic aloneness” evident from the beginning of life. This was in contrast to childhood schizophrenia, in which a child experienced a departure from previously normal interrelations. His insight contributed to our understanding of what is now recognized as autism spectrum disorder (ASD).
Also in 1943, Hans Asperger, MD, was studying families with children exhibiting behaviors similar to those described by Kanner. The following year, Asperger published an article (in German) describing these children. Unfortunately, this paper—titled “Autistic psychopathy in childhood”7—was not translated into English until the early 1990s.8
Since Kanner and Asperger first called attention to the disorder, there have been numerous changes in societal and medical understanding of autism. Bruno Bettelheim, PhD, an Austrian-born child psychologist with a particular interest in emotionally disturbed children, theorized that poor maternal bonding and lack of maternal affection were responsible for autistic characteristics.9 Bernard Rimland, PhD, a psychologist and the father of an autistic child, argued for a biological basis of the disorder.10 Rimland’s theory of autism as a neurodevelopmental disorder with an unidentified organic etiology is most consistent with current medical opinion.
Definitions and diagnostic criteria have evolved
The first edition of the Diagnostic and Statistical Manual of Mental Disorders (DSM) was published in 1952.11 Although it was nearly a decade after Dr. Kanner clearly described autism as an entity separate from childhood schizophrenia, the word autism was used in this edition only once—to describe psychotic reactions associated with schizophrenia. Similarly, autism was referred to only in relation to childhood schizophrenia in the DSMII,12 published in 1968.
DSM-III adds diagnostic criteria. In DSM-III (1980),13 specific diagnostic criteria for infantile autism and pervasive developmental disorder (PDD) appeared for the first time. Both diagnoses were clearly contrasted with a diagnosis of schizophrenia, and were used to identify children who exhibited a pervasive lack of responsiveness to others.
The DSM-III-Revised (R) (1987)14 added a classification scheme more consistent with the current standard. It included: 1) qualitative impairment in reciprocal social interaction; 2) qualitative impairment in communication and imaginative activity; and 3) restricted activity/interests.
PDD Not Otherwise Specified was also included, and served to identify those who had qualitative impairments in social interaction and communication skills but did not meet the full criteria for autism disorder or PDD.
The publication of DSM-IV (1994)15 brought another change: Specific criteria were outlined for the diagnoses of Asperger syndrome, Rett syndrome, and childhood disintegrative disorders; in the DSM-IV-Text Revision (TR) (2000),16 this structure remained relatively stable.
DSM-5 (2013) took another step, consolidating these various disorders into a single, unifying diagnosis of ASD.17 Significant controversy surrounded this change, with some viewing it as an oversimplification that does not accurately reflect important distinctions among divergent disorders18 and others arguing that it will result in unrecognized cases and exclusion of affected individuals.19 Notably, a recent study using concurrent DSM-IV and DSM-5 criteria to diagnose autism and PDD in more than 4000 children documented a high level of agreement between them.20
Diagnostic tools have improved
As the number of children diagnosed with autism has increased, so have efforts to more accurately diagnose autism as a distinct disorder.
In the 1960s, guidelines for diagnosing autism focused primarily on Kanner’s original descriptive criteria. Even in the 1980s, after DSM-III criteria identified autism as a distinct disorder, children were being evaluated with generalized developmental screening tools focused on behaviors characteristic of a severe mental handicap, without differentiating between autistic and nonautistic children.
Early autism-specific observational and structured interview tools (eg, Childhood Autism Rating Scale,21 Autism Diagnostic Interview [ADI],22 and Autistic Diagnostic Observation Schedule)23 emerged from a need for standardized diagnostic instruments that were comparable and reproducible.24 But because these tools were highly specific and initially studied in research settings with high-risk populations, they lacked the sensitivity to identify children at risk in the general population, particularly those with milder symptoms.
As the diagnosis of autism became more standardized following publication of the DSM III-R in 1987, developmental specialists were able to construct increasingly sensitive evaluation tools. The ADI was revised25 to facilitate earlier and more efficient diagnosis, allowing for assessment of children as young as 19 months of age. The Checklist for Autism in Toddlers (CHAT)26 and subsequent modification (M-CHAT)27 were among the earliest and most effective screening tools, appropriate for use in children as young as 16 months old.
Tools that followed the original CHAT (eg, the Autism Spectrum Screening Questionnaire [ASSQ]28) were adapted to better identify high-functioning children with Asperger syndrome, as well as those with autism.
Another revision of the M-CHAT—the M-CHAT-R/F (Revised with Follow-up) was validated earlier this year. In a study involving 16,000 children, 95% of those who had positive tests were found to have some form of developmental delay and almost half (47%) received an ASD diagnosis.29
Other diagnostic aids are being explored as a means of promoting earlier identification of ASD. For example, a blood test to identify differences in gene expression between children with and without ASD30 has shown initial promise, particularly in males. This test is licensed by SynapDx (Lexington, Mass) and a clinical trial to evaluate it has begun.
Results of another study demonstrating normalization of brain activity in autistic children after they’ve undergone intensive treatment31 raise the possibility of using cortical activation as measured by electroencephalography as an early biomarker for autism.
Treatment options, advocacy affect rate of diagnosis
Improvements in diagnosis and targeted identification of potentially treatable symptoms32 led to the development of new treatment options. And greater use of day care and preschool programs prompted networking among parents, who touted the benefits of early evaluation, diagnosis, and treatment. Earlier screening, not surprisingly, led to an increase in the target population.
|
Local as well as national advocacy groups, led primarily by parents, have become powerful voices for improvements in services offered to children with autism. And research studies with varying degrees of sophistication continue to be published, further fueling the demand for school systems to provide supportive learning environments as required by the Individuals with Disabilities Education Act, initially enacted in 1975 and amended in 2004.33
Federal and state funds in the form of Medicaid waivers are available to provide long-term care services in home and community settings, while private insurers typically pay for associated treatment modalities for those with an autism diagnosis, including physical, occupational, and speech therapy, among other services.
Finally, politicians and celebrities with personal connections to autism have joined the effort to increase awareness and improve the quality and availability of services—further assuring that autism is recognized as a legitimate, definable, and treatable disorder.
As an autism diagnosis has become more socially acceptable, it has at times replaced diagnoses of learning disability and mental retardation, a trend known as “diagnostic substitution.”34 Indeed, having a child with an ASD diagnosis often makes it possible for parents to secure services that might otherwise be unavailable to them.
Is the incidence of autism linked to the environment?
Numerous environmental, nutritional, and pharmaceutical changes have been cited as reasons for what is perceived as an increasing incidence of autism in recent years. For example, some contend that greater use of food preservatives and greater exposure of young children to environmental toxins are contributing factors.35
Thimerosal. Perhaps most notable is the assertion—since disproven—that thimerosal, a substance previously used in the manufacture of several childhood vaccines, was a leading cause of autism.36,37 In fact, one study documented an increase in autism after thimerosal had been discontinued.38 (For more on the thimerosal controversy, see “Autism: 5 misconceptions that can complicate care”.)
Autism comorbidity. As autism is frequently comorbid with other developmental disabilities, advances in medical technology that have led to a decline in neonatal death and overall mortality among the disabled may mean more survivors are subsequently diagnosed with an autism comorbidity.
Biological factors. Recent studies suggest that advanced paternal age can increase the risk of autism.39 Twin studies suggest moderate genetic heritability, along with a substantial environmental contribution to the development of autism.40 And new research suggests that maternal stressors during pregnancy—eg, trauma, illness, or substance abuse—may increase a child’s risk of developing autism, among other psychiatric disorders.41
The important role you play in the diagnosis of autism
It is clear that autism is more common than previously thought3 and that various factors are at work. Ensuring that children are promptly and properly evaluated begins when primary care physicians take parents’ concerns seriously and keep an eye out for common symptoms and characteristic developmental delays that may be evident even in the first year of life.
If symptoms are not severe enough to be detected in the child’s first several years, the next likely presentation will be when a parent gets a call from school suggesting that their child be tested for autism. While this delayed presentation suggests a higher level of functioning, a full evaluation, including the use of questionnaires such as the Social Responsiveness Scale42 and ASSQ,28 and appropriate referrals are still vital.
Puberty is another time when characteristics suggestive of autism that escaped earlier detection may be noted, and vague behavioral issues or concerns about intellectual impairment may become sufficiently troublesome to prompt a thorough evaluation. While behavioral therapy can be very helpful in such cases, medical therapy directed at co-occurring conditions (ie, a mood or anxiety disorder, including obsessive-compulsive disorder) may also provide significant benefit.
Whatever the age of the child, his or her parents should be counseled as to the general nature of autism, reassured of the availability of treatment options and given the appropriate referrals, and encouraged to learn more by availing themselves of resources (TABLE) and support groups. A consumer update from the US Food and Drug Administration titled “Beware of false or misleading claims for treating autism” (http://www.fda.gov/ForConsumers/ConsumerUpdates/ucm394757.htm) will be helpful for many parents, as well.
CORRESPONDENCE
Robert G. Zylstra, EdD, LCSW, Department of Family Medicine, The University of Tennessee College of Medicine, 110 East Third Street, Chattanooga, TN 37403; [email protected]
1. Prater C, Zylstra R. Autism: a medical primer. Am Fam Physician. 2002;66:1667-1674.
2. Nassar N, Dixon G, Bourke J, et al. Autism spectrum disorders in young children: effect of changes in diagnostic practices. Int J Epidemiol. 2009;38:1245-1254.
3. Developmental Disabilities Monitoring Network Surveillance Year 2010 Principal Investigators; Centers for Disease Control and Prevention (CDC). Prevalence of autism spectrum disorder among children aged 8 years - Autism and Developmental Disabilities Monitoring Network, 11 sites, United States, 2010. MMWR Surveill Summ. 2014;63:1-21.
4. Report to the legislature on the principle findings from the epidemiology of autism in California: a comprehensive pilot study. State of California Department of Developmental Services Web site. Available at: http://www.dds.ca.gov/Autism/docs/study_final.pdf. Accessed May 1, 2014.
5. Kanner L. Autistic disturbances of affective contact. Nervous Child. 1943;2:217-250.
6. Kanner L. Infantile autism and the schizophrenias. Behav Sci. 1965;10:412-420.
7. Asperger H. Die ‘autistischen psychopathen’ im kindesalter. Archiv für Psychiatrie und Nervenkrankheiten. 1944;117:76-136.
8. Frith U. Autism and Asperger Syndrome. Cambridge, UK: Cambridge University Press; 1991.
9. Bettelheim B. The empty fortress: Infantile autism and the birth of the self. New York: Free Press; 1972.
10. Rimland B. Infantile autism: The syndrome and its implications for a neural theory of behavior. New York: Appleton-Century- Crofts; 1964.
11. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. Washington, DC: American Psychiatric Association; 1952.
12. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 2nd ed. Washington, DC: American Psychiatric Association;1968.
13. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 3rd ed. Washington, DC: American Psychiatric Association; 1980.
14. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 3rd ed, revised. Washington, DC: American Psychiatric Association; 1987.
15. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 4th ed. Washington, DC: American Psychiatric Association; 1994.
16. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 4th ed, text revision. Washington: DC: American Psychiatric Association; 2000.
17. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th ed. Arlington, VA: American Psychiatric Publishing; 2013.
18. Ghaziuddin M. Brief report: Should the DMS-V drop Asperger Syndrome? J Autism Dev Disord. 2010;40:1146-1148.
19. Wing L, Gould J, Gillberg C. Autism spectrum disorders in the DSM-V: better or worse than the DSM-IV? Res Dev Disabil. 2011;32:768-773.
20. Huerta M, Bishop S, Duncan A, et al. Application of DSM-5 criteria for Autism Spectrum Disorder to three samples of children with DSM-IV diagnoses of pervasive developmental disorders. Am J Psychiatry. 2012;169:1056-1064.
21. Schopler E, Reichler RJ, DeVellis RF. Toward objective classification of childhood autism: Childhood Autism Rating Scale (CARS). J Autism Dev Disord. 1980;10:91-103.
22. Le Couteur A, Rutter M, Lord C, et al. Autism diagnostic interview: a standardized investigator-based instrument. J Autism Dev Disord. 1989;19:363-387.
23. Lord C, Rutter M, Goode S, et al. Autism diagnostic observation schedule: a standardized observation of communicative and social behavior. J Autism Dev Disord. 1989;19:185-212.
24. Zwaigenbaum L. Advances in the early detection of autism. Curr Opin Neurol. 2010;23:97-102.
25. Lord C, Rutter M, Le Couteur A. Autism Diagnostic Interview-Revised: A revised version of a diagnostic interview for caregivers of individuals with possible pervasive developmental disorders. J Autism Dev Disord. 1994;24:659-685.
26. Baron-Cohen S, Allen J, Gillberg C. Can autism be detected at 18 months? The needle, the haystack, and the CHAT. Br J Psychiatry. 1992;161:839-843.
27. Robins DL, Fein D, Barton ML, et al. The Modified Checklist for Autism in Toddlers: an initial study investigating the early detection of autism and pervasive developmental disorders. J Autism Dev Disord. 2001;31:131-144.
28. Ehlers S, Gilberg C, Wing L. A screening questionnaire for Asperger syndrome and other high-functioning autism spectrum disorders in school age children. J Autism Dev Disord. 1999;29:129-141.
29. Robins DL, Casagrande K, Barton M, et al. Validation of the modified checklist for Autism in toddlers, revised with followup (M-CHAT-R/F). Pediatrics. 2014;133:37-45.
30. Kong SW, Collins CD, Shimizu-Motohashi Y, et al. Characteristics and predictive value of blood transcriptome signature in males with autism spectrum disorders. PLoS One. 2012;7:e49475.
31. Dawson G, Jones EJ, Merkle K, et al. Early behavioral intervention is associated with normalized brain activity in young children with autism. J Am Acad Child Adolesc Psychiatry. 2012;51:1150-1159.
32. Autism ALARM. National Center for Medical Home Implementation Web site. Available at: http://www.medicalhomeinfo.org/downloads/pdfs/AutismAlarm.pdf. Accessed March 4, 2013.
33. IDEA. Autism Community Web site. Available at: http://www.autism-community.com/education/idea/. Accessed April 25, 2014.
34. Shattuck P. The contribution of diagnostic substitution to the growing administrative prevalence of autism in US special education. Pediatrics. 2006;117:1028-1037.
35. Landrigan P. What causes autism? Exploring the environmental contribution. Curr Opin Pediatr. 2010;22:219-225.
36. The great thimerosal cover-up: Mercury, vaccines, autism and your child’s health. Natural Health News & Scientific Discoveries Web site. Available at: http://www.naturalnews.com/011764_thimerosal_mercury.html. Accessed March 4, 2013.
37. Price CS, Thompson WW, Goodson B, et al. Prenatal and infant exposure to thimerosal from vaccines and immunoglobulins and risk of autism. Pediatrics. 2010;126:656-664.
38. Fombonne E, Zakarian R, Bennett A, et al. Pervasive developmental disorders in Montreal, Quebec, Canada: prevalence and links with immunizations. Pediatrics. 2006;118:e139-e150.
39. Flatscher-Bader T, Foldi CJ, Chong S, et al. Increased de novo copy number variants in the offspring of older males. Transl Psychiatry. 2011;1:e34.
40. Hallmayer J, Cleveland S, Torres A, et al. Genetic heritability and shared environmental factors among twin pairs with autism. Arch Gen Psychiatry. 2011;68:1095-1102.
41. Fine R, Zhang J, Stevens HE. Prenatal stress and inhibitory neuron systems: implications for neuropsychiatric disorders Mol Psychiatry. 2014 April 22. [Epub ahead of print].
42. Joshi G, Petty DR, Fried R, et al. Discriminant and concurrent validity of a simplified DSM-based structured diagnostic instrument for the assessment of autism spectrum disorders in youth and young adults. BMC Psychiatry. 2011;11:204.
1. Prater C, Zylstra R. Autism: a medical primer. Am Fam Physician. 2002;66:1667-1674.
2. Nassar N, Dixon G, Bourke J, et al. Autism spectrum disorders in young children: effect of changes in diagnostic practices. Int J Epidemiol. 2009;38:1245-1254.
3. Developmental Disabilities Monitoring Network Surveillance Year 2010 Principal Investigators; Centers for Disease Control and Prevention (CDC). Prevalence of autism spectrum disorder among children aged 8 years - Autism and Developmental Disabilities Monitoring Network, 11 sites, United States, 2010. MMWR Surveill Summ. 2014;63:1-21.
4. Report to the legislature on the principle findings from the epidemiology of autism in California: a comprehensive pilot study. State of California Department of Developmental Services Web site. Available at: http://www.dds.ca.gov/Autism/docs/study_final.pdf. Accessed May 1, 2014.
5. Kanner L. Autistic disturbances of affective contact. Nervous Child. 1943;2:217-250.
6. Kanner L. Infantile autism and the schizophrenias. Behav Sci. 1965;10:412-420.
7. Asperger H. Die ‘autistischen psychopathen’ im kindesalter. Archiv für Psychiatrie und Nervenkrankheiten. 1944;117:76-136.
8. Frith U. Autism and Asperger Syndrome. Cambridge, UK: Cambridge University Press; 1991.
9. Bettelheim B. The empty fortress: Infantile autism and the birth of the self. New York: Free Press; 1972.
10. Rimland B. Infantile autism: The syndrome and its implications for a neural theory of behavior. New York: Appleton-Century- Crofts; 1964.
11. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. Washington, DC: American Psychiatric Association; 1952.
12. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 2nd ed. Washington, DC: American Psychiatric Association;1968.
13. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 3rd ed. Washington, DC: American Psychiatric Association; 1980.
14. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 3rd ed, revised. Washington, DC: American Psychiatric Association; 1987.
15. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 4th ed. Washington, DC: American Psychiatric Association; 1994.
16. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 4th ed, text revision. Washington: DC: American Psychiatric Association; 2000.
17. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th ed. Arlington, VA: American Psychiatric Publishing; 2013.
18. Ghaziuddin M. Brief report: Should the DMS-V drop Asperger Syndrome? J Autism Dev Disord. 2010;40:1146-1148.
19. Wing L, Gould J, Gillberg C. Autism spectrum disorders in the DSM-V: better or worse than the DSM-IV? Res Dev Disabil. 2011;32:768-773.
20. Huerta M, Bishop S, Duncan A, et al. Application of DSM-5 criteria for Autism Spectrum Disorder to three samples of children with DSM-IV diagnoses of pervasive developmental disorders. Am J Psychiatry. 2012;169:1056-1064.
21. Schopler E, Reichler RJ, DeVellis RF. Toward objective classification of childhood autism: Childhood Autism Rating Scale (CARS). J Autism Dev Disord. 1980;10:91-103.
22. Le Couteur A, Rutter M, Lord C, et al. Autism diagnostic interview: a standardized investigator-based instrument. J Autism Dev Disord. 1989;19:363-387.
23. Lord C, Rutter M, Goode S, et al. Autism diagnostic observation schedule: a standardized observation of communicative and social behavior. J Autism Dev Disord. 1989;19:185-212.
24. Zwaigenbaum L. Advances in the early detection of autism. Curr Opin Neurol. 2010;23:97-102.
25. Lord C, Rutter M, Le Couteur A. Autism Diagnostic Interview-Revised: A revised version of a diagnostic interview for caregivers of individuals with possible pervasive developmental disorders. J Autism Dev Disord. 1994;24:659-685.
26. Baron-Cohen S, Allen J, Gillberg C. Can autism be detected at 18 months? The needle, the haystack, and the CHAT. Br J Psychiatry. 1992;161:839-843.
27. Robins DL, Fein D, Barton ML, et al. The Modified Checklist for Autism in Toddlers: an initial study investigating the early detection of autism and pervasive developmental disorders. J Autism Dev Disord. 2001;31:131-144.
28. Ehlers S, Gilberg C, Wing L. A screening questionnaire for Asperger syndrome and other high-functioning autism spectrum disorders in school age children. J Autism Dev Disord. 1999;29:129-141.
29. Robins DL, Casagrande K, Barton M, et al. Validation of the modified checklist for Autism in toddlers, revised with followup (M-CHAT-R/F). Pediatrics. 2014;133:37-45.
30. Kong SW, Collins CD, Shimizu-Motohashi Y, et al. Characteristics and predictive value of blood transcriptome signature in males with autism spectrum disorders. PLoS One. 2012;7:e49475.
31. Dawson G, Jones EJ, Merkle K, et al. Early behavioral intervention is associated with normalized brain activity in young children with autism. J Am Acad Child Adolesc Psychiatry. 2012;51:1150-1159.
32. Autism ALARM. National Center for Medical Home Implementation Web site. Available at: http://www.medicalhomeinfo.org/downloads/pdfs/AutismAlarm.pdf. Accessed March 4, 2013.
33. IDEA. Autism Community Web site. Available at: http://www.autism-community.com/education/idea/. Accessed April 25, 2014.
34. Shattuck P. The contribution of diagnostic substitution to the growing administrative prevalence of autism in US special education. Pediatrics. 2006;117:1028-1037.
35. Landrigan P. What causes autism? Exploring the environmental contribution. Curr Opin Pediatr. 2010;22:219-225.
36. The great thimerosal cover-up: Mercury, vaccines, autism and your child’s health. Natural Health News & Scientific Discoveries Web site. Available at: http://www.naturalnews.com/011764_thimerosal_mercury.html. Accessed March 4, 2013.
37. Price CS, Thompson WW, Goodson B, et al. Prenatal and infant exposure to thimerosal from vaccines and immunoglobulins and risk of autism. Pediatrics. 2010;126:656-664.
38. Fombonne E, Zakarian R, Bennett A, et al. Pervasive developmental disorders in Montreal, Quebec, Canada: prevalence and links with immunizations. Pediatrics. 2006;118:e139-e150.
39. Flatscher-Bader T, Foldi CJ, Chong S, et al. Increased de novo copy number variants in the offspring of older males. Transl Psychiatry. 2011;1:e34.
40. Hallmayer J, Cleveland S, Torres A, et al. Genetic heritability and shared environmental factors among twin pairs with autism. Arch Gen Psychiatry. 2011;68:1095-1102.
41. Fine R, Zhang J, Stevens HE. Prenatal stress and inhibitory neuron systems: implications for neuropsychiatric disorders Mol Psychiatry. 2014 April 22. [Epub ahead of print].
42. Joshi G, Petty DR, Fried R, et al. Discriminant and concurrent validity of a simplified DSM-based structured diagnostic instrument for the assessment of autism spectrum disorders in youth and young adults. BMC Psychiatry. 2011;11:204.
Autism: 5 misconceptions that can complicate care
› Screen children for developmental delays with a standardized screening tool at 9, 18, and 24 or 30 months of age, accompanied by surveillance at all well-child visits. C
› Use a parent-completed tool rather than a directly administered tool to screen for developmental delays. C
›Advise parents of a child diagnosed with autism spectrum disorder that early intensive behavioral therapy can improve cognitive, language, and adaptive skills. A
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Autism spectrum disorder (ASD) affects approximately one in 68 children in the United States, according to the Centers for Disease Control and Prevention (CDC).1 Growing public awareness of autism means that family physicians are increasingly likely to hear from anxious new (and expectant) parents. Unfortunately, misinformation about autism continues to be perpetuated, through word of mouth, the Internet, and misinformed advocacy groups. This article addresses 5 of the most common misconceptions, and can help you set the record straight and respond appropriately to parental concerns.
Misconception 1: Autism is a single condition
While autistic disorder was previously considered one of 5 pervasive developmental disorders, in 2013 the Diagnostic and Statistical Manual of Mental Disorder, 5th edition (DSM-5) redefined it. (To learn more about how shifts in our understanding of autism were reflected in each new edition of the DSM, see “Autism: Why the rise in rates?”)
ASD is now an umbrella term that encompasses autism, Asperger syndrome, pervasive developmental disorder not otherwise specified, childhood disintegrative disorder, and Rett syndrome.2 The new term is meant to highlight the continuum of symptoms and frequent variability of presentation among those affected, ranging from mild to more severe impairment. Anyone who was classified under DSM-IV criteria, of course, should continue to have an autism/ASD diagnosis.
As with previous definitions, ASD is characterized by communication deficits (eg, inappropriate responses in conversation, misinterpreted nonverbal interactions, and significant challenges in age-appropriate bonding/friendship development). While previous definitions were focused on identifying school-age deficits, the update requires early childhood symptoms—regardless of the age of formal diagnosis.2
Misconception 2: Only symptomatic children should be screened for ASD
Although the decision to screen all children remains a controversial one, at least one medical society—the American Academy of Pediatrics (AAP)—calls for universal screening.3 The American Academy of Family Physicians does not have or endorse a formal guideline about screening for ASD. The US Preventive Services Task Force has a guideline, but it is in the process of being revised.4
Given the advances in early childhood interventions, there is little doubt that early identification of those at risk for developmental delay is beneficial. But opponents of universal screening cite concerns about unnecessary testing, anxiety, and overdiagnosis due to false positives associated with traditional screening methods.
The AAP calls for screening and surveillance. Since 2006, the AAP has recommended surveillance at all well-child visits, combined with screening for developmental delays at 9, 18, and 24 or 30 months of age, using a standardized screening tool.3,5 A parent-completed tool (eg, the Modified Checklist for Autism in Toddlers [M-CHAT] or, most recently, the M-CHAT Revised with Follow-up6; Parents’ Evaluation of Developmental Status; or Ages and Stages Questionnaire, 3rd ed) should be used rather than a directly administered tool.3,5 An algorithm detailing the AAP’s approach is available at http://www.cdc.gov/ncbddd/actearly/autism/case-modules/pdf/diagnosis/AAP%20Screening%20Guidelines.pdf.
Physicians should also be prepared to evaluate any child whose parents raise concerns about his or her development during a routine visit. A “wait and see” approach is strongly discouraged. Parents of children with ASD often broach the subject by the baby’s first birthday.5 Common concerns include the child’s inability to babble, point or gesture meaningfully, or respond to his or her name; poor eye contact; failure to play with toys; and/or loss of (or failure to develop) language or social skills.5
An ASD mnemonic. The CDC, in collaboration with the AAP, the American Academy of Neurology, and the Child Neurology Society, has released a simplified guideline with the mnemonic ALARM to summarize recommendations for developmental screening, surveillance, diagnosis, and management of ASD.5 ALARM stands for:
• Autism is prevalent
• Listen to parents
• Act early
• Refer
• Monitor.
Misconception 3: Since ASD can't be cured, early intervention offers no benefit
While there is no cure for ASD and it is not considered reversible, there is an array of potential ASD therapies and proven benefits of early intervention. Therapies range from diet to medication and behavioral skills development, but only a few have ample evidence of efficacy (TABLE).7-17
Randomized controlled trials of early developmental and behavioral therapy have shown some promise in decreasing symptoms associated with ASD and improving parent-child communication and social engagement.18-21 A systematic review found that young children with ASD can improve cognitive performance, language skills, and adaptive behavioral skills through behavioral interventions or more comprehensive approaches using developmental and behavioral frameworks.22 In addition to increasing the likelihood of overall school success, early intervention programs that improve communication and social skills can have a significant impact on the individual’s eventual quality of life (QOL), employability, and independence.23-25
TABLE
Therapeutic options for ASD: What works?7-17
| Intervention | Effectiveness (SOR*) |
|
| Effective |
| Early intensive behavioral therapy7 | A |
| Melatonin (for sleep disturbance)8 | A |
| Parent-mediated early intervention9 | A |
| Risperidone (for behavioral issues)10 | A |
|
| Inadequate evidence to support |
| Acetylcholinesterase inhibitors14 | B |
| Acupuncture11 | B |
| Atypical antipsychotics12 | B |
| Auditory integration therapy13 | A |
| B6-magnesium supplementation14 | B |
| Gluten-free/casein-free diet15 | A |
| Music therapy13 | A |
| Naltrexone14 | B |
| Omega-3 fatty acids16 | A |
|
| Ineffective |
| Secretin IV17 | A |
ASD, autism spectrum disorder; IV, intravenous; SOR, strength of recommendation.
*SOR: A, Good-quality patient-oriented evidence; B, inconsistent or limited-quality patient-oriented evidence; C, consensus, usual practice, opinion, disease-oriented evidence, case series.
Misconception 4: Individuals with ASD are more intellectually disabled and can't function independently
As many as 96% of children with ASD have a coexisting developmental disability, such as generalized developmental delay (found in 80% of those with ASD), learning disabilities (affecting 60%), or attention-deficit/hyperactivity disorder (in 42%).26 Although many parents—and some physicians—assume that children with ASD are intellectually disabled, in fact, less than one in 5 (19%) has an intellectual disability.26
Individuals with ASD do, however, often have difficulty living independently. In one study of post high school living arrangements, those with ASD were less likely to have ever lived on their own than those with learning disabilities, intellectual disabilities, or emotional disturbances.23
Another analysis found that only about half (53.4%) of young adults with ASD had ever worked for pay outside the home since leaving high school—the lowest rate among disability groups.24 Of those who had worked outside the home, young adults with ASD earned an average of $8.10 per hour, significantly lower than the comparison groups. Not surprisingly, young adults with ASD who had better conversational and/or functional skills had a higher likelihood of ever having had worked for pay outside the home.24
Social participation also is considered an indicator of overall QOL and independent function. Young adults with ASD were found to be significantly more likely than those with other types of developmental delays to be socially isolated—never seeing friends, getting calls from friends, or being invited to activities. Lower communication and functional skills, as well as living with a parent, were predictors of less social participation in young adults with ASD.25
Misconception 5: Thimerosal vaccines cause ASD
The controversy and concern about a correlation between mercury and ASD began in the late 1990s, when a published study appeared to link thimerosal-containing vaccines to the increasing incidence of autism.27 This notion appeared to be further strengthened by a 2002 study,28 done by the same researchers and reaching similar conclusions. Since then, the correlation has been disproven by a number of studies and further review of the initial studies revealed them to be flawed.29
Despite the lack of evidence to support any long-term effects from the minimal exposure to mercury in the preservative, in July 1999, US Public Health Service agencies, the AAP, and vaccine manufacturers agreed that thimerosal should be reduced or eliminated in vaccines as a precautionary measure.30 With the exception of a limited number of multidose influenza vaccines, childhood vaccines are now thimerosal-free.
Fact: Early referral is key
When and where to refer parents for additional evaluation of a child with developmental delays depends on the resources in your community. Typically, a team of providers participates in the evaluation and management of a child with ASD, often including a developmental pediatrician, psychiatrist and other mental health professional, neurologist, speech pathologist, audiologist, physical therapist, and special education teacher.
Although every community may not have easy access to a pediatric subspecialist referral center, all 50 states and US territories are required by law to provide access to early intervention programs under Part C of the 2004 Individuals with Disabilities Education Act.31 (For a list of resources, see “Autism spectrum disorder: Where to learn more”.)
Help families prepare
Advise families whom you refer for evaluation that they are unlikely to have a definitive answer after the first visit. Explain that the evaluation is quite thorough and generally takes several visits. Typically, a child suspected of developmental delay will undergo a comprehensive evaluation, including history and physical exam, blood work (including lead testing and, in some locations, genetic testing), hearing and vision screening, speech and language evaluation, and sensorimotor and cognitive evaluation. Additional information may be requested from daycare providers, preschool teachers, or others who spend significant amounts of time with the child.
Once a diagnosis is made, the team will work with the parents to develop an individualized care plan for the child, which often includes a mix of cognitive, physical, and speech development services in addition to nutrition and support services. The primary care physician, of course, will continue to oversee, monitor, and coordinate care.
CORRESPONDENCE
Margot Savoy, MD, MPH, FAAF P, CPE, FA BC, Christiana Care Health System, Department of Family & Community Medicine, 1401 Foulk Road, Suite 100B, Wilmington, DE 19803; [email protected]
1. Developmental Disabilities Monitoring Network Surveillance Year 2010 Principal Investigators; Centers for Disease Control and Prevention (CDC). Prevalence of autism spectrum disorder among children aged 8 years - Autism and Developmental Disabilities Monitoring Network, 11 sites, United States, 2010. MMWR. 2014;63:1-21.
2. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th ed. Arlington, VA: American Psychiatric Publishing: 2013.
3. Council on Children With Disabilities; Section on Developmental Behavioral Pediatrics; Bright Futures Steering Committee; Medical Home Initiatives for Children With Special Needs Project Advisory Committee. Identifying infants and young children with developmental disorders in the medical home: an algorithm for developmental surveillance and screening. Pediatrics. 2006;118:405-420.
4. Screening for autism spectrum disorder in young children. US Preventive Services Task Force Web site. Available at: http://www.uspreventiveservicestaskforce.org/uspstf/uspsaut.htm. Accessed November 7, 2013.
5. Johnson CP, Myers SM; Council on Children With Disabilities. Identification and evaluation of children with autism spectrum disorders. Pediatrics. 2007;120:1183-1215.
6. Robins DL, Casagrande K, Barton M, et al. Validation of the modified checklist for Autism in toddlers, revised with followup (M-CHAT-R/F). Pediatrics. 2014;133:37-45.
7. Reichow B, Barton EE, Boyd BA, et al. Early intensive behavioral intervention (EIBI) for young children with autism spectrum disorders (ASD). Cochrane Database Syst Rev. 2012;10:CD009260.
8. Rossignol DA, Frye RE. Melatonin in autism spectrum disorders: a systematic review and meta-analysis. Dev Med Child Neurol. 2011;53:783-792.
9. Oono IP, Honey EJ, McConachie H. Parent-mediated early intervention for young children with autism spectrum disorders (ASD). Cochrane Database Syst Rev. 2013;4: CD009774.
10. Dove D, Warren Z, McPheeters ML, et al. Medications for adolescents and young adults with autism spectrum disorders: a systematic review. Pediatrics. 2012;130:717-726.
11. Cheuk DK, Wong V, Chen WX. Acupuncture for autism spectrum disorders (ASD). Cochrane Database Syst Rev. 2011;(9):CD007849.
12. Sochocky N, Millin R. Second generation antipsychotics in Asperger’s Disorder and high functioning autism: a systematic review of the literature and effectiveness of meta-analysis. Curr Clin Pharmacol. 2013;8:370-379.
13. Sinha Y, Silove N, Hayen A, et al. Auditory integration training and other sound therapies for autism spectrum disorders (ASD). Cochrane Database Syst Rev. 2011;(12):CD003681.
14. Rossignol DA. Novel and emerging treatments for autism spectrum disorders: a systematic review. Ann Clin Psychiatry. 2009;21:213-236.
15. Millward C, Ferriter M, Calver S, et al. Gluten- and casein-free diets for autistic spectrum disorder. Cochrane Database Syst Rev. 2008;(2):CD003498.
16. James S, Montgomery P, Williams K. Omega-3 fatty acids supplementation for autism spectrum disorders (ASD). Cochrane Database Syst Rev. 2011;(11):CD007992.
17. Williams K, Wray JA, Wheeler DM. Intravenous secretin for autism spectrum disorders (ASD). Cochrane Database Syst Rev. 2012;4:CD003495.
18. Green J, Charman T, McConachie H, et al; PACT Consortium. Parent-mediated communication-focused treatment in children with autism (PACT): a randomised controlled trial. Lancet. 2010;375:2152-2160.
19. Landa RJ, Holman KC, O’Neill AH, et al. Intervention targeting development of socially synchronous engagement in toddlers with autism spectrum disorder: a randomized controlled trial. J Child Psychol Psychiatry. 2011;52:13-21.
20. Kasari C, Gulsrud AC, Wong C, et al. Randomized controlled caregiver mediated joint engagement intervention for toddlers with autism. J Autism Dev Disord. 2010;40:1045-1056.
21. Dawson G, Rogers S, Munson J, et al. Randomized, controlled trial of an intervention for toddlers with autism: the Early Start Denver Model. Pediatrics. 2010;125:e17-e23.
22. Warren Z, McPheeters ML, Sathe N, et al. A systematic review of early intensive intervention for autism spectrum disorders. Pediatrics. 2011;127:e1303-e1311.
23. Anderson KA, Shattuck PT, Cooper BP, et al. Prevalence and correlates of postsecondary residential status among young adults with an autism spectrum disorder Autism. 2013 September 9. [Epub ahead of print].
24. Roux AM, Shattuck PT, Cooper BP, et al. Postsecondary employment experiences among young adults with an autism spectrum disorder. J Am Acad Child Adolesc Psychiatry. 2013;52:931-939.
25. Orsmond GI, Shattuck PT, Cooper BP, et al. Social participation among young adults with an autism spectrum disorder. J Autism Dev Disord. 2013;43:2710-2719.
26. Edelson MG. Are the majority of children with autism mentally retarded? A systematic evaluation of the data. Focus Autism Other Dev Disabl. 2006;21:66-83.
27. Wakefield AJ, Murch SH, Anthony A, et al. Ileal-lymphoidnodular hyperplasia, non-specific colitis, and pervasive developmental disorder in children. Lancet. 1998;351:637-641.
28. Uhlmann V, Martin CM, Sheils O, et al. Potential viral pathogenic mechanism for new variant inflammatory bowel disease. Mol Pathol. 2002;55:84-90.
29. Centers for Disease Control and Prevention: Immunization Safety and Autism. Centers for Disease Control and Prevention Web site. Available at: http://www.cdc.gov/vaccinesafety/Concerns/Autism/Index.html. Accessed May 20, 2014.
30. Centers for Disease Control and Prevention (CDC). Thimerosal in vaccines: a joint statement of the American Academy of Pediatrics and the Public Health Service. MMWR Morb Moral Wkly Rep. 1999;48:563-565.
31. IDEA. Autism Community Web site. Available at: http://www.autism-community.com/education/idea/. Accessed May 20, 2014.
› Screen children for developmental delays with a standardized screening tool at 9, 18, and 24 or 30 months of age, accompanied by surveillance at all well-child visits. C
› Use a parent-completed tool rather than a directly administered tool to screen for developmental delays. C
›Advise parents of a child diagnosed with autism spectrum disorder that early intensive behavioral therapy can improve cognitive, language, and adaptive skills. A
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Autism spectrum disorder (ASD) affects approximately one in 68 children in the United States, according to the Centers for Disease Control and Prevention (CDC).1 Growing public awareness of autism means that family physicians are increasingly likely to hear from anxious new (and expectant) parents. Unfortunately, misinformation about autism continues to be perpetuated, through word of mouth, the Internet, and misinformed advocacy groups. This article addresses 5 of the most common misconceptions, and can help you set the record straight and respond appropriately to parental concerns.
Misconception 1: Autism is a single condition
While autistic disorder was previously considered one of 5 pervasive developmental disorders, in 2013 the Diagnostic and Statistical Manual of Mental Disorder, 5th edition (DSM-5) redefined it. (To learn more about how shifts in our understanding of autism were reflected in each new edition of the DSM, see “Autism: Why the rise in rates?”)
ASD is now an umbrella term that encompasses autism, Asperger syndrome, pervasive developmental disorder not otherwise specified, childhood disintegrative disorder, and Rett syndrome.2 The new term is meant to highlight the continuum of symptoms and frequent variability of presentation among those affected, ranging from mild to more severe impairment. Anyone who was classified under DSM-IV criteria, of course, should continue to have an autism/ASD diagnosis.
As with previous definitions, ASD is characterized by communication deficits (eg, inappropriate responses in conversation, misinterpreted nonverbal interactions, and significant challenges in age-appropriate bonding/friendship development). While previous definitions were focused on identifying school-age deficits, the update requires early childhood symptoms—regardless of the age of formal diagnosis.2
Misconception 2: Only symptomatic children should be screened for ASD
Although the decision to screen all children remains a controversial one, at least one medical society—the American Academy of Pediatrics (AAP)—calls for universal screening.3 The American Academy of Family Physicians does not have or endorse a formal guideline about screening for ASD. The US Preventive Services Task Force has a guideline, but it is in the process of being revised.4
Given the advances in early childhood interventions, there is little doubt that early identification of those at risk for developmental delay is beneficial. But opponents of universal screening cite concerns about unnecessary testing, anxiety, and overdiagnosis due to false positives associated with traditional screening methods.
The AAP calls for screening and surveillance. Since 2006, the AAP has recommended surveillance at all well-child visits, combined with screening for developmental delays at 9, 18, and 24 or 30 months of age, using a standardized screening tool.3,5 A parent-completed tool (eg, the Modified Checklist for Autism in Toddlers [M-CHAT] or, most recently, the M-CHAT Revised with Follow-up6; Parents’ Evaluation of Developmental Status; or Ages and Stages Questionnaire, 3rd ed) should be used rather than a directly administered tool.3,5 An algorithm detailing the AAP’s approach is available at http://www.cdc.gov/ncbddd/actearly/autism/case-modules/pdf/diagnosis/AAP%20Screening%20Guidelines.pdf.
Physicians should also be prepared to evaluate any child whose parents raise concerns about his or her development during a routine visit. A “wait and see” approach is strongly discouraged. Parents of children with ASD often broach the subject by the baby’s first birthday.5 Common concerns include the child’s inability to babble, point or gesture meaningfully, or respond to his or her name; poor eye contact; failure to play with toys; and/or loss of (or failure to develop) language or social skills.5
An ASD mnemonic. The CDC, in collaboration with the AAP, the American Academy of Neurology, and the Child Neurology Society, has released a simplified guideline with the mnemonic ALARM to summarize recommendations for developmental screening, surveillance, diagnosis, and management of ASD.5 ALARM stands for:
• Autism is prevalent
• Listen to parents
• Act early
• Refer
• Monitor.
Misconception 3: Since ASD can't be cured, early intervention offers no benefit
While there is no cure for ASD and it is not considered reversible, there is an array of potential ASD therapies and proven benefits of early intervention. Therapies range from diet to medication and behavioral skills development, but only a few have ample evidence of efficacy (TABLE).7-17
Randomized controlled trials of early developmental and behavioral therapy have shown some promise in decreasing symptoms associated with ASD and improving parent-child communication and social engagement.18-21 A systematic review found that young children with ASD can improve cognitive performance, language skills, and adaptive behavioral skills through behavioral interventions or more comprehensive approaches using developmental and behavioral frameworks.22 In addition to increasing the likelihood of overall school success, early intervention programs that improve communication and social skills can have a significant impact on the individual’s eventual quality of life (QOL), employability, and independence.23-25
TABLE
Therapeutic options for ASD: What works?7-17
| Intervention | Effectiveness (SOR*) |
|
| Effective |
| Early intensive behavioral therapy7 | A |
| Melatonin (for sleep disturbance)8 | A |
| Parent-mediated early intervention9 | A |
| Risperidone (for behavioral issues)10 | A |
|
| Inadequate evidence to support |
| Acetylcholinesterase inhibitors14 | B |
| Acupuncture11 | B |
| Atypical antipsychotics12 | B |
| Auditory integration therapy13 | A |
| B6-magnesium supplementation14 | B |
| Gluten-free/casein-free diet15 | A |
| Music therapy13 | A |
| Naltrexone14 | B |
| Omega-3 fatty acids16 | A |
|
| Ineffective |
| Secretin IV17 | A |
ASD, autism spectrum disorder; IV, intravenous; SOR, strength of recommendation.
*SOR: A, Good-quality patient-oriented evidence; B, inconsistent or limited-quality patient-oriented evidence; C, consensus, usual practice, opinion, disease-oriented evidence, case series.
Misconception 4: Individuals with ASD are more intellectually disabled and can't function independently
As many as 96% of children with ASD have a coexisting developmental disability, such as generalized developmental delay (found in 80% of those with ASD), learning disabilities (affecting 60%), or attention-deficit/hyperactivity disorder (in 42%).26 Although many parents—and some physicians—assume that children with ASD are intellectually disabled, in fact, less than one in 5 (19%) has an intellectual disability.26
Individuals with ASD do, however, often have difficulty living independently. In one study of post high school living arrangements, those with ASD were less likely to have ever lived on their own than those with learning disabilities, intellectual disabilities, or emotional disturbances.23
Another analysis found that only about half (53.4%) of young adults with ASD had ever worked for pay outside the home since leaving high school—the lowest rate among disability groups.24 Of those who had worked outside the home, young adults with ASD earned an average of $8.10 per hour, significantly lower than the comparison groups. Not surprisingly, young adults with ASD who had better conversational and/or functional skills had a higher likelihood of ever having had worked for pay outside the home.24
Social participation also is considered an indicator of overall QOL and independent function. Young adults with ASD were found to be significantly more likely than those with other types of developmental delays to be socially isolated—never seeing friends, getting calls from friends, or being invited to activities. Lower communication and functional skills, as well as living with a parent, were predictors of less social participation in young adults with ASD.25
Misconception 5: Thimerosal vaccines cause ASD
The controversy and concern about a correlation between mercury and ASD began in the late 1990s, when a published study appeared to link thimerosal-containing vaccines to the increasing incidence of autism.27 This notion appeared to be further strengthened by a 2002 study,28 done by the same researchers and reaching similar conclusions. Since then, the correlation has been disproven by a number of studies and further review of the initial studies revealed them to be flawed.29
Despite the lack of evidence to support any long-term effects from the minimal exposure to mercury in the preservative, in July 1999, US Public Health Service agencies, the AAP, and vaccine manufacturers agreed that thimerosal should be reduced or eliminated in vaccines as a precautionary measure.30 With the exception of a limited number of multidose influenza vaccines, childhood vaccines are now thimerosal-free.
Fact: Early referral is key
When and where to refer parents for additional evaluation of a child with developmental delays depends on the resources in your community. Typically, a team of providers participates in the evaluation and management of a child with ASD, often including a developmental pediatrician, psychiatrist and other mental health professional, neurologist, speech pathologist, audiologist, physical therapist, and special education teacher.
Although every community may not have easy access to a pediatric subspecialist referral center, all 50 states and US territories are required by law to provide access to early intervention programs under Part C of the 2004 Individuals with Disabilities Education Act.31 (For a list of resources, see “Autism spectrum disorder: Where to learn more”.)
Help families prepare
Advise families whom you refer for evaluation that they are unlikely to have a definitive answer after the first visit. Explain that the evaluation is quite thorough and generally takes several visits. Typically, a child suspected of developmental delay will undergo a comprehensive evaluation, including history and physical exam, blood work (including lead testing and, in some locations, genetic testing), hearing and vision screening, speech and language evaluation, and sensorimotor and cognitive evaluation. Additional information may be requested from daycare providers, preschool teachers, or others who spend significant amounts of time with the child.
Once a diagnosis is made, the team will work with the parents to develop an individualized care plan for the child, which often includes a mix of cognitive, physical, and speech development services in addition to nutrition and support services. The primary care physician, of course, will continue to oversee, monitor, and coordinate care.
CORRESPONDENCE
Margot Savoy, MD, MPH, FAAF P, CPE, FA BC, Christiana Care Health System, Department of Family & Community Medicine, 1401 Foulk Road, Suite 100B, Wilmington, DE 19803; [email protected]
› Screen children for developmental delays with a standardized screening tool at 9, 18, and 24 or 30 months of age, accompanied by surveillance at all well-child visits. C
› Use a parent-completed tool rather than a directly administered tool to screen for developmental delays. C
›Advise parents of a child diagnosed with autism spectrum disorder that early intensive behavioral therapy can improve cognitive, language, and adaptive skills. A
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Autism spectrum disorder (ASD) affects approximately one in 68 children in the United States, according to the Centers for Disease Control and Prevention (CDC).1 Growing public awareness of autism means that family physicians are increasingly likely to hear from anxious new (and expectant) parents. Unfortunately, misinformation about autism continues to be perpetuated, through word of mouth, the Internet, and misinformed advocacy groups. This article addresses 5 of the most common misconceptions, and can help you set the record straight and respond appropriately to parental concerns.
Misconception 1: Autism is a single condition
While autistic disorder was previously considered one of 5 pervasive developmental disorders, in 2013 the Diagnostic and Statistical Manual of Mental Disorder, 5th edition (DSM-5) redefined it. (To learn more about how shifts in our understanding of autism were reflected in each new edition of the DSM, see “Autism: Why the rise in rates?”)
ASD is now an umbrella term that encompasses autism, Asperger syndrome, pervasive developmental disorder not otherwise specified, childhood disintegrative disorder, and Rett syndrome.2 The new term is meant to highlight the continuum of symptoms and frequent variability of presentation among those affected, ranging from mild to more severe impairment. Anyone who was classified under DSM-IV criteria, of course, should continue to have an autism/ASD diagnosis.
As with previous definitions, ASD is characterized by communication deficits (eg, inappropriate responses in conversation, misinterpreted nonverbal interactions, and significant challenges in age-appropriate bonding/friendship development). While previous definitions were focused on identifying school-age deficits, the update requires early childhood symptoms—regardless of the age of formal diagnosis.2
Misconception 2: Only symptomatic children should be screened for ASD
Although the decision to screen all children remains a controversial one, at least one medical society—the American Academy of Pediatrics (AAP)—calls for universal screening.3 The American Academy of Family Physicians does not have or endorse a formal guideline about screening for ASD. The US Preventive Services Task Force has a guideline, but it is in the process of being revised.4
Given the advances in early childhood interventions, there is little doubt that early identification of those at risk for developmental delay is beneficial. But opponents of universal screening cite concerns about unnecessary testing, anxiety, and overdiagnosis due to false positives associated with traditional screening methods.
The AAP calls for screening and surveillance. Since 2006, the AAP has recommended surveillance at all well-child visits, combined with screening for developmental delays at 9, 18, and 24 or 30 months of age, using a standardized screening tool.3,5 A parent-completed tool (eg, the Modified Checklist for Autism in Toddlers [M-CHAT] or, most recently, the M-CHAT Revised with Follow-up6; Parents’ Evaluation of Developmental Status; or Ages and Stages Questionnaire, 3rd ed) should be used rather than a directly administered tool.3,5 An algorithm detailing the AAP’s approach is available at http://www.cdc.gov/ncbddd/actearly/autism/case-modules/pdf/diagnosis/AAP%20Screening%20Guidelines.pdf.
Physicians should also be prepared to evaluate any child whose parents raise concerns about his or her development during a routine visit. A “wait and see” approach is strongly discouraged. Parents of children with ASD often broach the subject by the baby’s first birthday.5 Common concerns include the child’s inability to babble, point or gesture meaningfully, or respond to his or her name; poor eye contact; failure to play with toys; and/or loss of (or failure to develop) language or social skills.5
An ASD mnemonic. The CDC, in collaboration with the AAP, the American Academy of Neurology, and the Child Neurology Society, has released a simplified guideline with the mnemonic ALARM to summarize recommendations for developmental screening, surveillance, diagnosis, and management of ASD.5 ALARM stands for:
• Autism is prevalent
• Listen to parents
• Act early
• Refer
• Monitor.
Misconception 3: Since ASD can't be cured, early intervention offers no benefit
While there is no cure for ASD and it is not considered reversible, there is an array of potential ASD therapies and proven benefits of early intervention. Therapies range from diet to medication and behavioral skills development, but only a few have ample evidence of efficacy (TABLE).7-17
Randomized controlled trials of early developmental and behavioral therapy have shown some promise in decreasing symptoms associated with ASD and improving parent-child communication and social engagement.18-21 A systematic review found that young children with ASD can improve cognitive performance, language skills, and adaptive behavioral skills through behavioral interventions or more comprehensive approaches using developmental and behavioral frameworks.22 In addition to increasing the likelihood of overall school success, early intervention programs that improve communication and social skills can have a significant impact on the individual’s eventual quality of life (QOL), employability, and independence.23-25
TABLE
Therapeutic options for ASD: What works?7-17
| Intervention | Effectiveness (SOR*) |
|
| Effective |
| Early intensive behavioral therapy7 | A |
| Melatonin (for sleep disturbance)8 | A |
| Parent-mediated early intervention9 | A |
| Risperidone (for behavioral issues)10 | A |
|
| Inadequate evidence to support |
| Acetylcholinesterase inhibitors14 | B |
| Acupuncture11 | B |
| Atypical antipsychotics12 | B |
| Auditory integration therapy13 | A |
| B6-magnesium supplementation14 | B |
| Gluten-free/casein-free diet15 | A |
| Music therapy13 | A |
| Naltrexone14 | B |
| Omega-3 fatty acids16 | A |
|
| Ineffective |
| Secretin IV17 | A |
ASD, autism spectrum disorder; IV, intravenous; SOR, strength of recommendation.
*SOR: A, Good-quality patient-oriented evidence; B, inconsistent or limited-quality patient-oriented evidence; C, consensus, usual practice, opinion, disease-oriented evidence, case series.
Misconception 4: Individuals with ASD are more intellectually disabled and can't function independently
As many as 96% of children with ASD have a coexisting developmental disability, such as generalized developmental delay (found in 80% of those with ASD), learning disabilities (affecting 60%), or attention-deficit/hyperactivity disorder (in 42%).26 Although many parents—and some physicians—assume that children with ASD are intellectually disabled, in fact, less than one in 5 (19%) has an intellectual disability.26
Individuals with ASD do, however, often have difficulty living independently. In one study of post high school living arrangements, those with ASD were less likely to have ever lived on their own than those with learning disabilities, intellectual disabilities, or emotional disturbances.23
Another analysis found that only about half (53.4%) of young adults with ASD had ever worked for pay outside the home since leaving high school—the lowest rate among disability groups.24 Of those who had worked outside the home, young adults with ASD earned an average of $8.10 per hour, significantly lower than the comparison groups. Not surprisingly, young adults with ASD who had better conversational and/or functional skills had a higher likelihood of ever having had worked for pay outside the home.24
Social participation also is considered an indicator of overall QOL and independent function. Young adults with ASD were found to be significantly more likely than those with other types of developmental delays to be socially isolated—never seeing friends, getting calls from friends, or being invited to activities. Lower communication and functional skills, as well as living with a parent, were predictors of less social participation in young adults with ASD.25
Misconception 5: Thimerosal vaccines cause ASD
The controversy and concern about a correlation between mercury and ASD began in the late 1990s, when a published study appeared to link thimerosal-containing vaccines to the increasing incidence of autism.27 This notion appeared to be further strengthened by a 2002 study,28 done by the same researchers and reaching similar conclusions. Since then, the correlation has been disproven by a number of studies and further review of the initial studies revealed them to be flawed.29
Despite the lack of evidence to support any long-term effects from the minimal exposure to mercury in the preservative, in July 1999, US Public Health Service agencies, the AAP, and vaccine manufacturers agreed that thimerosal should be reduced or eliminated in vaccines as a precautionary measure.30 With the exception of a limited number of multidose influenza vaccines, childhood vaccines are now thimerosal-free.
Fact: Early referral is key
When and where to refer parents for additional evaluation of a child with developmental delays depends on the resources in your community. Typically, a team of providers participates in the evaluation and management of a child with ASD, often including a developmental pediatrician, psychiatrist and other mental health professional, neurologist, speech pathologist, audiologist, physical therapist, and special education teacher.
Although every community may not have easy access to a pediatric subspecialist referral center, all 50 states and US territories are required by law to provide access to early intervention programs under Part C of the 2004 Individuals with Disabilities Education Act.31 (For a list of resources, see “Autism spectrum disorder: Where to learn more”.)
Help families prepare
Advise families whom you refer for evaluation that they are unlikely to have a definitive answer after the first visit. Explain that the evaluation is quite thorough and generally takes several visits. Typically, a child suspected of developmental delay will undergo a comprehensive evaluation, including history and physical exam, blood work (including lead testing and, in some locations, genetic testing), hearing and vision screening, speech and language evaluation, and sensorimotor and cognitive evaluation. Additional information may be requested from daycare providers, preschool teachers, or others who spend significant amounts of time with the child.
Once a diagnosis is made, the team will work with the parents to develop an individualized care plan for the child, which often includes a mix of cognitive, physical, and speech development services in addition to nutrition and support services. The primary care physician, of course, will continue to oversee, monitor, and coordinate care.
CORRESPONDENCE
Margot Savoy, MD, MPH, FAAF P, CPE, FA BC, Christiana Care Health System, Department of Family & Community Medicine, 1401 Foulk Road, Suite 100B, Wilmington, DE 19803; [email protected]
1. Developmental Disabilities Monitoring Network Surveillance Year 2010 Principal Investigators; Centers for Disease Control and Prevention (CDC). Prevalence of autism spectrum disorder among children aged 8 years - Autism and Developmental Disabilities Monitoring Network, 11 sites, United States, 2010. MMWR. 2014;63:1-21.
2. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th ed. Arlington, VA: American Psychiatric Publishing: 2013.
3. Council on Children With Disabilities; Section on Developmental Behavioral Pediatrics; Bright Futures Steering Committee; Medical Home Initiatives for Children With Special Needs Project Advisory Committee. Identifying infants and young children with developmental disorders in the medical home: an algorithm for developmental surveillance and screening. Pediatrics. 2006;118:405-420.
4. Screening for autism spectrum disorder in young children. US Preventive Services Task Force Web site. Available at: http://www.uspreventiveservicestaskforce.org/uspstf/uspsaut.htm. Accessed November 7, 2013.
5. Johnson CP, Myers SM; Council on Children With Disabilities. Identification and evaluation of children with autism spectrum disorders. Pediatrics. 2007;120:1183-1215.
6. Robins DL, Casagrande K, Barton M, et al. Validation of the modified checklist for Autism in toddlers, revised with followup (M-CHAT-R/F). Pediatrics. 2014;133:37-45.
7. Reichow B, Barton EE, Boyd BA, et al. Early intensive behavioral intervention (EIBI) for young children with autism spectrum disorders (ASD). Cochrane Database Syst Rev. 2012;10:CD009260.
8. Rossignol DA, Frye RE. Melatonin in autism spectrum disorders: a systematic review and meta-analysis. Dev Med Child Neurol. 2011;53:783-792.
9. Oono IP, Honey EJ, McConachie H. Parent-mediated early intervention for young children with autism spectrum disorders (ASD). Cochrane Database Syst Rev. 2013;4: CD009774.
10. Dove D, Warren Z, McPheeters ML, et al. Medications for adolescents and young adults with autism spectrum disorders: a systematic review. Pediatrics. 2012;130:717-726.
11. Cheuk DK, Wong V, Chen WX. Acupuncture for autism spectrum disorders (ASD). Cochrane Database Syst Rev. 2011;(9):CD007849.
12. Sochocky N, Millin R. Second generation antipsychotics in Asperger’s Disorder and high functioning autism: a systematic review of the literature and effectiveness of meta-analysis. Curr Clin Pharmacol. 2013;8:370-379.
13. Sinha Y, Silove N, Hayen A, et al. Auditory integration training and other sound therapies for autism spectrum disorders (ASD). Cochrane Database Syst Rev. 2011;(12):CD003681.
14. Rossignol DA. Novel and emerging treatments for autism spectrum disorders: a systematic review. Ann Clin Psychiatry. 2009;21:213-236.
15. Millward C, Ferriter M, Calver S, et al. Gluten- and casein-free diets for autistic spectrum disorder. Cochrane Database Syst Rev. 2008;(2):CD003498.
16. James S, Montgomery P, Williams K. Omega-3 fatty acids supplementation for autism spectrum disorders (ASD). Cochrane Database Syst Rev. 2011;(11):CD007992.
17. Williams K, Wray JA, Wheeler DM. Intravenous secretin for autism spectrum disorders (ASD). Cochrane Database Syst Rev. 2012;4:CD003495.
18. Green J, Charman T, McConachie H, et al; PACT Consortium. Parent-mediated communication-focused treatment in children with autism (PACT): a randomised controlled trial. Lancet. 2010;375:2152-2160.
19. Landa RJ, Holman KC, O’Neill AH, et al. Intervention targeting development of socially synchronous engagement in toddlers with autism spectrum disorder: a randomized controlled trial. J Child Psychol Psychiatry. 2011;52:13-21.
20. Kasari C, Gulsrud AC, Wong C, et al. Randomized controlled caregiver mediated joint engagement intervention for toddlers with autism. J Autism Dev Disord. 2010;40:1045-1056.
21. Dawson G, Rogers S, Munson J, et al. Randomized, controlled trial of an intervention for toddlers with autism: the Early Start Denver Model. Pediatrics. 2010;125:e17-e23.
22. Warren Z, McPheeters ML, Sathe N, et al. A systematic review of early intensive intervention for autism spectrum disorders. Pediatrics. 2011;127:e1303-e1311.
23. Anderson KA, Shattuck PT, Cooper BP, et al. Prevalence and correlates of postsecondary residential status among young adults with an autism spectrum disorder Autism. 2013 September 9. [Epub ahead of print].
24. Roux AM, Shattuck PT, Cooper BP, et al. Postsecondary employment experiences among young adults with an autism spectrum disorder. J Am Acad Child Adolesc Psychiatry. 2013;52:931-939.
25. Orsmond GI, Shattuck PT, Cooper BP, et al. Social participation among young adults with an autism spectrum disorder. J Autism Dev Disord. 2013;43:2710-2719.
26. Edelson MG. Are the majority of children with autism mentally retarded? A systematic evaluation of the data. Focus Autism Other Dev Disabl. 2006;21:66-83.
27. Wakefield AJ, Murch SH, Anthony A, et al. Ileal-lymphoidnodular hyperplasia, non-specific colitis, and pervasive developmental disorder in children. Lancet. 1998;351:637-641.
28. Uhlmann V, Martin CM, Sheils O, et al. Potential viral pathogenic mechanism for new variant inflammatory bowel disease. Mol Pathol. 2002;55:84-90.
29. Centers for Disease Control and Prevention: Immunization Safety and Autism. Centers for Disease Control and Prevention Web site. Available at: http://www.cdc.gov/vaccinesafety/Concerns/Autism/Index.html. Accessed May 20, 2014.
30. Centers for Disease Control and Prevention (CDC). Thimerosal in vaccines: a joint statement of the American Academy of Pediatrics and the Public Health Service. MMWR Morb Moral Wkly Rep. 1999;48:563-565.
31. IDEA. Autism Community Web site. Available at: http://www.autism-community.com/education/idea/. Accessed May 20, 2014.
1. Developmental Disabilities Monitoring Network Surveillance Year 2010 Principal Investigators; Centers for Disease Control and Prevention (CDC). Prevalence of autism spectrum disorder among children aged 8 years - Autism and Developmental Disabilities Monitoring Network, 11 sites, United States, 2010. MMWR. 2014;63:1-21.
2. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th ed. Arlington, VA: American Psychiatric Publishing: 2013.
3. Council on Children With Disabilities; Section on Developmental Behavioral Pediatrics; Bright Futures Steering Committee; Medical Home Initiatives for Children With Special Needs Project Advisory Committee. Identifying infants and young children with developmental disorders in the medical home: an algorithm for developmental surveillance and screening. Pediatrics. 2006;118:405-420.
4. Screening for autism spectrum disorder in young children. US Preventive Services Task Force Web site. Available at: http://www.uspreventiveservicestaskforce.org/uspstf/uspsaut.htm. Accessed November 7, 2013.
5. Johnson CP, Myers SM; Council on Children With Disabilities. Identification and evaluation of children with autism spectrum disorders. Pediatrics. 2007;120:1183-1215.
6. Robins DL, Casagrande K, Barton M, et al. Validation of the modified checklist for Autism in toddlers, revised with followup (M-CHAT-R/F). Pediatrics. 2014;133:37-45.
7. Reichow B, Barton EE, Boyd BA, et al. Early intensive behavioral intervention (EIBI) for young children with autism spectrum disorders (ASD). Cochrane Database Syst Rev. 2012;10:CD009260.
8. Rossignol DA, Frye RE. Melatonin in autism spectrum disorders: a systematic review and meta-analysis. Dev Med Child Neurol. 2011;53:783-792.
9. Oono IP, Honey EJ, McConachie H. Parent-mediated early intervention for young children with autism spectrum disorders (ASD). Cochrane Database Syst Rev. 2013;4: CD009774.
10. Dove D, Warren Z, McPheeters ML, et al. Medications for adolescents and young adults with autism spectrum disorders: a systematic review. Pediatrics. 2012;130:717-726.
11. Cheuk DK, Wong V, Chen WX. Acupuncture for autism spectrum disorders (ASD). Cochrane Database Syst Rev. 2011;(9):CD007849.
12. Sochocky N, Millin R. Second generation antipsychotics in Asperger’s Disorder and high functioning autism: a systematic review of the literature and effectiveness of meta-analysis. Curr Clin Pharmacol. 2013;8:370-379.
13. Sinha Y, Silove N, Hayen A, et al. Auditory integration training and other sound therapies for autism spectrum disorders (ASD). Cochrane Database Syst Rev. 2011;(12):CD003681.
14. Rossignol DA. Novel and emerging treatments for autism spectrum disorders: a systematic review. Ann Clin Psychiatry. 2009;21:213-236.
15. Millward C, Ferriter M, Calver S, et al. Gluten- and casein-free diets for autistic spectrum disorder. Cochrane Database Syst Rev. 2008;(2):CD003498.
16. James S, Montgomery P, Williams K. Omega-3 fatty acids supplementation for autism spectrum disorders (ASD). Cochrane Database Syst Rev. 2011;(11):CD007992.
17. Williams K, Wray JA, Wheeler DM. Intravenous secretin for autism spectrum disorders (ASD). Cochrane Database Syst Rev. 2012;4:CD003495.
18. Green J, Charman T, McConachie H, et al; PACT Consortium. Parent-mediated communication-focused treatment in children with autism (PACT): a randomised controlled trial. Lancet. 2010;375:2152-2160.
19. Landa RJ, Holman KC, O’Neill AH, et al. Intervention targeting development of socially synchronous engagement in toddlers with autism spectrum disorder: a randomized controlled trial. J Child Psychol Psychiatry. 2011;52:13-21.
20. Kasari C, Gulsrud AC, Wong C, et al. Randomized controlled caregiver mediated joint engagement intervention for toddlers with autism. J Autism Dev Disord. 2010;40:1045-1056.
21. Dawson G, Rogers S, Munson J, et al. Randomized, controlled trial of an intervention for toddlers with autism: the Early Start Denver Model. Pediatrics. 2010;125:e17-e23.
22. Warren Z, McPheeters ML, Sathe N, et al. A systematic review of early intensive intervention for autism spectrum disorders. Pediatrics. 2011;127:e1303-e1311.
23. Anderson KA, Shattuck PT, Cooper BP, et al. Prevalence and correlates of postsecondary residential status among young adults with an autism spectrum disorder Autism. 2013 September 9. [Epub ahead of print].
24. Roux AM, Shattuck PT, Cooper BP, et al. Postsecondary employment experiences among young adults with an autism spectrum disorder. J Am Acad Child Adolesc Psychiatry. 2013;52:931-939.
25. Orsmond GI, Shattuck PT, Cooper BP, et al. Social participation among young adults with an autism spectrum disorder. J Autism Dev Disord. 2013;43:2710-2719.
26. Edelson MG. Are the majority of children with autism mentally retarded? A systematic evaluation of the data. Focus Autism Other Dev Disabl. 2006;21:66-83.
27. Wakefield AJ, Murch SH, Anthony A, et al. Ileal-lymphoidnodular hyperplasia, non-specific colitis, and pervasive developmental disorder in children. Lancet. 1998;351:637-641.
28. Uhlmann V, Martin CM, Sheils O, et al. Potential viral pathogenic mechanism for new variant inflammatory bowel disease. Mol Pathol. 2002;55:84-90.
29. Centers for Disease Control and Prevention: Immunization Safety and Autism. Centers for Disease Control and Prevention Web site. Available at: http://www.cdc.gov/vaccinesafety/Concerns/Autism/Index.html. Accessed May 20, 2014.
30. Centers for Disease Control and Prevention (CDC). Thimerosal in vaccines: a joint statement of the American Academy of Pediatrics and the Public Health Service. MMWR Morb Moral Wkly Rep. 1999;48:563-565.
31. IDEA. Autism Community Web site. Available at: http://www.autism-community.com/education/idea/. Accessed May 20, 2014.
Health care reform: Possibilities & opportunities for primary care
Pressure to reform our health care system is at an all-time high, driven by relentlessly rising costs and fragmentation of care. These persistent problems have led to lower quality care and limited access to care for a large proportion of the US population—issues that accountable care organizations (ACOs), as well as other value-based models, are designed to address.
While the terms used to describe the means by which health care systems attempt to do more to meet the needs of those they serve may vary, the importance of reorganizing care delivery to better integrate services is gaining traction nationwide. As we move to new models, primary care takes center stage.
ACOs (or ACO-type arrangements) anchored by primary care networks can help meet the goals of health care reform by responding to changes in reimbursement, reducing fragmented care, and focusing on improving the quality of care for defined patient populations. In addition, these delivery models can take advantage of new health information technology (IT) and the move toward patient-centered medical homes (PCMHs).
In the pages that follow, we examine opportunities for new care delivery models to slow rising costs and improve population health in family medicine. The introduction of these models has important implications for patients, physicians, and provider organizations, and our aim is to ensure that family physicians are prepared to take these vital steps toward achieving health reform goals.
Shifting from volume-based to value-based reimbursement
According to the Congressional Budget Office, ACOs are expected to save $5 billion during their first 8 years of existence.1 After one year of ACO activity, the Centers for Medicare and Medicaid Services (CMS) reported savings of $30 million.2 The expected savings will be driven by the increased provider accountability associated with ACOs.
Various means of increasing provider accountability through changes in reimbursement strategies have been proposed; several are new, while others are improvements on—or variations of—methods that have been tried before. The most common approaches—shared savings, shared savings plus penalty, capitation, episodic payment, prospective payment, pay-for-performance, and hospital-physician bundling—are detailed in TABLE 1.3-7 Broad implementation of any of these reimbursement mechanisms within a new model of care would represent a shift away from the traditional volume-based provider payment model to a value-based system—a key step in slowing the rise in health care costs.
TABLE 1
Reimbursement strategies designed to promote physician accountability3-7
Model | Description | Implications for FPs |
Shared savings | FFS plus a portion of dollars saved relative to predicted costs if quality and patient satisfaction are enhanced3 | Focus on population health incentivizes well care and preventive services |
Shared savings plus penalty | Same as shared savings, plus a penalty if expenses exceed spending targets; bonus potential is increased to account for increased risk3 | Potential for care coordination payments in addition to shared savings |
Capitation | Flat payments plus bonuses and penalties; provider organization assumes full risk for a defined patient population3 | A better understanding of population management and IT now makes capitation a viable strategy in certain settings |
Episodic payments | Reimbursement is for defined episodes of care, which may extend from time of admission to days or weeks after discharge; may also include home health, extended care, or ancillary services4 | No incentive for prevention or PCMH coordination |
Prospective payment | Reimbursement for inpatient services based on a prepaid amount that covers a defined period of time; uses DRG system that bases payment on disease classification by CMS5 | It is important for FPs to partner with specialists willing to share reimbursement commensurate with the value of care provided |
Pay-for-performance | Reimbursement is tied to achievement of metrics (eg, number of patients immunized for a specific disease, desired clinical outcomes, high patient satisfaction scores) mutually agreed upon by ACO and payer6 | Be sure any agreed-upon “targets” are achievable and patient-focused |
Hospital-physician bundling | Reimbursement is based on the cost of a procedure or diagnosis that includes both hospital and physician components. One payment is made for the collective services associated with a hospitalization7 | Similar to prospective payment from FP perspective; it is important to work with those who value the care of FPs |
ACO, accountable care organization; CMS, Centers for Medicare and Medicaid Services; DRG, diagnosis-related group; FFS, fee-for-service; FPs, family physicians; IT, information technology; PCMH, patient-centered medical home.
Although it is too soon to be certain of the effect such changes will have on the earnings of family physicians, it is reasonable to think that new payment strategies—and a larger role for primary care providers—will improve their financial standing.
Moving toward population health management
New ACO-type models also make it easier to improve health care for specific populations, using strategies designed to organize, provide, and manage care for defined groups. In addition to controlling the cost of caring for specific groups, well-designed and implemented population health management strategies can increase continuity of care by ensuring oversight of patients across the spectrum of health care settings.
Five broad categories of population health management are most prominent: lifestyle management and demand management (both for relatively healthy people), disease management (for those with chronic conditions), catastrophic care management (for patients with rare or catastrophic illness or injury), and disability management (for groups of employees).8TABLE 28 describes the population targeted and activities associated with each. It is important to remember, however, that no single strategy is mutually exclusive for a particular group.
Comprehensive Primary Care (CPC) initiative. In a collaboration made possible by the Affordable Care Act, CMS initiated the CPC initiative in 2012.9 A 4-year project designed to test and further identify the benefits of population health management and strengthen coordination of care for Medicare patients within primary care settings, the CPC initiative involves 497 sites and 2347 providers caring for approximately 315,000 Medicare beneficiaries.9 More information is available at http://innovation.cms.gov/initiatives/comprehensive-primary-care-initiative/.
TABLE 2
Population health management strategies8
Strategy | Target/goal | Key elements | Evidence of effectiveness |
Lifestyle management | To help relatively healthy individuals make good choices about health behaviors and risks | • Prevention • Risk reduction • Self-care | • Adherence to guidelines for clinical screenings • Reduced costs resulting from prevention programs |
Demand management | To help relatively healthy individuals take an active role in decisions about health and medical care; aims to reduce inappropriate demand for services | • Telephone triage • Advice and referrals • Decision and behavioral support • Education to promote self-care | • Reduced variation in care unexplained by morbidity • Improved understanding of perceived need for care • Improved access, better outcomes, lower cost |
Disease management | To identify and target chronically ill patients (eg, those with diabetes, heart failure, or asthma) with specific interventions | • Clinical oversight/management of patients with chronic disease • Education and self-care • Coordination of care/providers | • Reduced costs for treatment of chronic diseases • Decreased complications associated with chronic illness |
Catastrophic care management | To identify those with rare or catastrophic illness or injury and provide services needed to improve outcomes | • Immediate referral to appropriate providers • Coordination of care • Medical/care management | • Reduced hospitalizations and total claims costs • Reduced morbidity; improved QOL • Realistic, patient-specific goals |
Disability management | To develop and deliver employer-driven initiatives for employees to reduce lost time from work, improve productivity, and optimize health and well-being | • Disability prevention programs • Return-to-work programs • Employer-based lifestyle management programs • Coordination of care/providers for employees with chronic disease, disability, and/or serious illness or injury • Absence management programs (ie, designed to control/limit unexplained, unscheduled, or excessive absenteeism) • Workplace rehabilitation | • Lower workers’ compensation/disability benefit costs • Reduced number of injuries • Reduced lost time from work • Increased productivity |
QOL, quality of life.
Building infrastructure and leveraging IT
ACOs and ACO-type models will take a variety of forms, depending in part on geographic need and local demographics. Yet, all share a common need for a strong infrastructure to support improved transitions, integration, and coordination of care. Incorporation of a strong health IT system is critical so that data regarding the process and cost of care, as well as outcomes, can be collected and put to optimal use.10-13
Across health care settings, health IT innovations are being successfully implemented in efforts to enhance physician decision support, improve patient safety, increase guideline adherence, and improve chronic disease treatment.11,13 In primary care, for example, an IT infrastructure can create disease registries so that data on patients with specific conditions can be tracked and acted upon—eg, a diabetes registry could be used to identify and contact patients who have an hemoglobin A1c >9 and have not been seen in 9 months or more.
Similarly, an IT system with the ability to identify patients at high risk for disease decompensation, hospitalization, and/or increased morbidity and mortality linked to progression of a chronic disease is needed. Identifying medical conditions associated with higher costs would make it possible to focus care coordination and chronic care management efforts on this targeted population.
As health IT continues to evolve, additional means of interacting with patients and improving patient care will be developed. Physicians and organizations that are ready to take advantage of these advances in technology will be well positioned to address the goals of health reform. (See “Health care reform: Recommendations for family physicians” below.3-7,14,15)
How the patient-centered medical home fits into the picture
The implementation of ACOs and other new models of care has promising implications for the establishment of PCMHs. Consistent with the goals of health reform, the PCMH movement focuses on a coordinated teamwork approach, anchored within a general practice or family medicine setting.
An evaluation of the PCMH National Demonstration Project funded by the American Academy of Family Physicians found that the adoption of more components of the PCMH at the practice level was associated with improvement in patient outcomes, as measured by the Ambulatory Care Quality Alliance starter set16 (a compilation of clinical performance measures developed by a broad coalition of providers, payers, consumers, and government agencies).
A recent look at the Southeastern Pennsylvania Chronic Care Initiative17 found less promising results. “A multipayer medical home pilot, in which participating practices adopted new structural capabilities and received NCQA [National Committee for Quality Assurance] certification, was associated with limited improvements in quality and was not associated with reductions in utilization of hospital, emergency department, or ambulatory care services or total costs over 3 years,” Friedberg et al17 concluded. The authors did note, however, that NCQA recognition was what the practices involved in this initiative were rewarded for—not PCMH activity.
It is also important to keep in mind that PCMH activity has been shown to improve care and reduce costs—not NCQA recognition in and of itself. In fact, a large body of evidence clearly demonstrates the positive patient care outcomes and reductions in overall cost associated with the PCMH. These findings were compiled by the Patient-Centered Primary Care Collaborative—which issues annual reports on the progress of the PCMH—in a January 2014 update.18
In a PCMH model, the focus shifts away from the procedure(s) or treatment to the whole person. However, all components of care (eg, primary and specialty care, hospital, ancillary services, laboratory, and radiology) are vital and need to be connected to increase efficiency and reduce cost—creating what is sometimes referred to as a “medical neighborhood.”19-21
What’s in the neighborhood?
In a medical neighborhood, such as an ACO, each patient is cared for by a team of providers at multiple locations. The PCMH serves as the base, ensuring that all providers work together toward a common goal. In addition to providing primary care, the PCMH coordinates each patient’s specialty and support services and communicates the care plan to all involved.
Successful implementation of a medical neighborhood requires a close working relationship among providers, payers, and community resources. For example, payers can provide real-time information about patients who have been admitted to the hospital or discharged from the emergency department, which enables close follow-up and coordination across multiple systems.
Given the emerging opportunities for new care delivery models to advance primary care, we urge family physicians to respond positively to these changes and challenges. Here’s what we recommend:
Carefully consider payment methodologies. Changes in the way physicians are paid will vary by payer source, as well as geographic market.3-7 Regardless of the reimbursement model you’re offered, however, do not agree to it until you have the opportunity to evaluate it, along with your particular circumstances, to ensure that you have the infrastructure to support whatever changes the new model will require.
Read the fine print. Look out for your own interests by carefully reading the terms you are presented with. Consider seeking advice from those who understand the particular nuances faced by family physicians under particular reimbursement strategies. Just because a payment method benefits a particular specialty does not mean it will be favorable to family physicians.
Before you join an ACO
Before joining an accountable care organization (ACO ) or a similar entity, find out whether it supports primary care principles and the patient-centered medical home (PCMH ). Some questions to ask:
• Does the ACO have the infrastructure necessary to be successful, including the requisite health information technology, administrative support, actuarial knowledge, and experience with population health management?
• Is the ACO founded on primary care principles? Find out, for example, whether primary care physicians are represented at all levels of the organization and provide appropriate input on all important issues.
If you practice in a rural area … The growth of ACO activity is expected to be slow in both rural and underserved metropolitan markets. To address this issue, the Centers for Medicare and Medicaid Services is allowing primary care physicians in such markets to participate in more than one Medicare ACO and providing financial incentives in the form of savings exemptions to smaller, rural ACOs.14,15 Another option for rural providers is the adoption of a “virtual ACO ”—a loosely organized group of providers, united in the effort to achieve high-quality care and reduced costs and willing to submit to computer analysis that will determine their relative contributions to efficiency and the distribution of savings.14
Get involved
It is important for all family physicians to engage in discussions about health care reform, and to represent both their patients and their specialty. Familiarity with what is happening is essential. One way to do that is to become an active member of your state or local American Academy of Family Physicians affiliate.
More information is available at:
• www.aafp.org
Practical information with regard to health reform, in addition to suggesting ways to get involved
• http://www.tafp.org/Media/Default/Downloads/practice%20resources/aco-guide.pdf
Information to consider before joining or forming an ACO
• http://www.ncqa.org/Programs/Recognition/PatientCenteredMedicalHomePCMH.aspx and http://www.transformed.com/
Resources for practitioners considering transformation to a PCMH
• http://innovation.cms.gov
Information, including webinars and forums, on innovative payment and service delivery models.
Nontraditional settings. Another facet of the medical neighborhood is the provision of health care services in nontraditional settings. For example, some grocery stores in our area employ nutritionists to whom we refer patients for nutritional counseling regarding their health in general or a disease process in particular.
Changes in reimbursement also will affect how care is delivered within the medical neighborhood. As we move away from fee-for-service (volume-based) to value-based payments, physicians who have made the transition from working individually with a panel of patients to providing team-based care within a PCMH will be better positioned to meet the goals of health care reform. (See “Team-based care is key inside the PCMH, too”22 below.) Nonetheless, the transition is a dynamic process. With changes in reimbursement and delivery models, physicians also will be expected to develop and implement continuous quality improvement measures so patient care can be continually evaluated and improved.
Now comes the hard part
While a PCMH requires primary care physicians to collaborate with other health professionals, it has the potential to lead to conflicts and debates about who is at the head of the health care team. This is particularly true within mental health services because, while primary care visits are frequently related to psychosocial issues, the mental health and general practice sectors have traditionally been distinct. In recent years, however, coordinated delivery models that integrate primary care and mental health services have been shown to increase access and reduce the stigma associated with mental health services—and to be cost-effective.23
In many ways, moving the primary care culture from the traditional focus on the physician as “captain of the ship” to a physician-led, team-based approach is one of the most difficult tasks for organizations attempting to transform their care delivery models.3,24 Physicians historically have been autonomous providers of medical care, relying on their own experience, expertise, and beliefs to guide decisions about patient care. Now they’re being asked to give up some of the direct control they may have had over patient care decisions and learn to work more collaboratively with other providers, as well as nonclinicians (eg, health coaches), to achieve desired outcomes.3 A successful transition depends on a reimbursement framework in which patient care goals are properly aligned with incentives for primary care physicians to work in a team-based environment.3,25-27
In addition to operating as a team with providers in other settings in the medical neighborhood, innovative primary care practices—typically those that have already achieved patient-centered medical home (PCMH ) status—have strong teams within their walls. In “In search of joy in practice: A report of 23 high-functioning primary care practices,” Sinsky et al22 highlight a number of ways in which the practices they studied are maximizing this approach.
Nonphysician care. A number of practices expanded the roles of medical assistants (MAs), nurses, and even nonclinician health coaches. In one case, MAs nearly tripled the time they spend with each patient, to enable them to do medication review, fill out forms, give immunizations, and book appointments for screening tests such as mammography. In another, registered nurses were given standing orders to treat routine problems such as ear infections and urinary tract infections; at a third, health coaches counsel patients with chronic conditions and MAs conduct depression screening, as needed.
Documentation and computerized order entry—which ties up many hours of physician time—is another area in which some practices have adopted a team approach. A number of practices use nurses or MAs as scribes, entering orders and preparing after-visit summaries, for example. Not only are the physicians more satisfied, but the MAs and nurses are happy to have more involvement in patient care, the researchers report.
Communication is crucial to a successful team approach. In some practices, this is accomplished with weekly physician-clinical staff meetings; in others, with brief group “huddles” or by an office design featuring “co-location.” In one example of the latter, MAs and physicians sit side-by-side, so they can easily talk to each other—the doctor could communicate key patient information that the MA would then follow up on, for example. Regular analysis of workflow to identify and address undue delays is an effective team function, as well.
Helping patients help themselves. Moving toward more patient-focused care will also require a concerted effort to increase patients’ engagement in their own health and medical care. In practice, very little of an individual’s time is spent in a physician’s office. Thus, optimal outcomes can be achieved only when patients are actively involved. Helping patients become proactive—ie, by arming them with the knowledge, skill, and confidence to do their part in staying healthy28—also represents a major shift in primary care culture, as patients become active participants in medical decision making rather than passive recipients of physicians’ advice.
Alternative approaches. To deliver the continuum of care that is central to new care delivery models and shift the culture of primary care toward a PCMH, physicians can implement a number of clinic-based engagement approaches—interacting with patients via e-visits such as e-mail through a secure portal, telemedicine, and group medical visits, for example. Physicians can encourage patient participation by starting patient interest groups and advisory panels29—recommended by the NCQA and the Agency for Healthcare Research and Quality and used at our institution—and conducting patient needs assessments on a regular basis. Opportunities for primary care practices to engage with the community include partnering with local health departments, churches, nonprofits, and advocacy organizations to conduct health promotion and educational activities.
CORRESPONDENCE
Randy Wexler, MD, MPH, Department of Family Medicine, College of Medicine, The Ohio State University, 2231 North High Street, Northwood and High Building, Columbus, OH 43201; [email protected]
1. Shortell SM, Casalino LP, Fisher ES. How the Center for Medicare and Medicaid Innovation should test accountable care organizations. Health Aff. 2010;29:1293-1298.
2. Medicare’s delivery system reform initiatives achieve significant savings and quality improvements - off to a strong start [press release]. Washington, DC: US Department of Health & Human Services; January 30, 2014. Available at: http://www.hhs.gov/news/press/2014pres/01/20140130a.html. Accessed March 28, 2014.
3. The family physician’s ACO blueprint for success: Preparing family medicine for the approaching accountable care era. Texas Academy of Family Physicians Web site. Available at: http://www.tafp.org/Media/Default/Downloads/practice%20resources/acoguide.pdf. Accessed March 28, 2014.
4. Mechanic RE. Opportunities and challenges for episode-based payment. N Engl J Med. 2011;365:777-779.
5. Medicare prospective payment systems (PPS): A summary. American Speech-Language-Hearing Association Web site. Available at: http://www.asha.org/practice/reimbursement/medicare/pps_sum.htm. Accessed March 28, 2014.
6. Pay for performance (P4P): AHRQ resources. Agency for Healthcare Research and Quality Web site. Available at: http://www.ahrq.gov/legacy/qual/pay4per.htm. Accessed March 29, 2014.
7. Komisar H, Feder J, Ginsburg PB. “Bundling” payment for episodes of hospital care: Issues and recommendations for the new pilot program in Medicare. Available at: http://www.americanprogress.org/issues/2011/07/pdf/medicare_bundling.pdf. Accessed May 20, 2014.
8. McAlearney AS. Population health management: Strategies to improve outcomes. Chicago, IL: Health Administration Press; 2003.
9. Centers for Medicare and Medicaid Services Web site. Comprehensive primary care initiative. Available at: http://innovation.cms.gov/initiatives/comprehensive-primary-care-initiative/. Accessed March 29, 2014.
10. Robertson DC, Lerner JC. Top technology issues for ambulatory care facilities this year and beyond. J Ambul Care Manag. 2009;32:303-319.
11. Linder JA, Ma J, Bates DW, et al. Electronic health record use and the quality of ambulatory care in the united states. Arch Intern Med. 2007;167:1400-1405.
12. Vest JR. Health information exchange and healthcare utilization. J Med Syst. 2009;33:223-231.
13. Parente ST, McCullough JS. Health information technology and patient safety: evidence from panel data. Health Affairs (Millwood). 2009;28:357-360.
14. FAQ on Accountable Care Organizations. American Academy of Family Physicians Web site. Available at: http://www.aafp.org/practice-management/payment/acos/faq.html. Accessed March 29, 2014.
15. Torrieri M. CMS appeals to rural practices with another ACO participation perk. Physicians Practice blog. June 1, 2011. Available at: http://www.physicianspractice.com/blog/cms-appealsrural-practices-another-aco-participation-perk. Accessed March 29, 2014.
16. Crabtree BF, Nutting PA, Miller WL, et al. Summary of the national demonstration project and recommendations for the patient-centered medical home. Ann Fam Med. 2010;8 suppl 1:S80-S92.
17. Friedberg MW, Schneider EC, Rosenthal MB, et al. Association between participation in a multipayer medical home intervention and changes in quality, utilization, and costs of care. JAMA. 2014;311:815-825.
18. Nielsen M, Olayiwola JN, Grundy P, et al. The patient-centered medical home’s impact on cost & quality: An annual update of the evidence, 2012-2013. Available at: http://www.pcpcc.org/resource/medical-homes-impact-cost-quality. Accessed May 20, 2014.
19. Laine C. Welcome to the patient-centered medical neighborhood. Ann Intern Med. 2011;154:60.
20. Pham HH. Good neighbors: how will the patient-centered medical home relate to the rest of the health-care delivery system? J Gen Intern Med. 2010;25:630-634.
21. Taylor EF, Lake T, Nysenbaum J, et al; Mathematica Policy Research. Coordinating care in the medical neighborhood: critical components and available mechanisms: White paper. Available at: http://pcmh.ahrq.gov/sites/default/files/attachments/Coordinating%20Care%20in%20the%20Medical%20Neighborhood.pdf. Accessed May 20, 2014.
22. Sinsky CA, Willard-Grace R, Schutzbank AM, et al. In search of joy in practice: a report of 23 high-functioning primary care practices. Ann Fam Med. 2013;11:272-278.
23. Collins C, Hewson DL, Munger R, et al. Evolving models of behavioral health integration in primary care. Available at: http://www.milbank.org/uploads/documents/10430EvolvingCare/EvolvingCare.pdf. Accessed April 10, 2014.
24. The cornerstones of accountable care. Health Leaders Media Web site. Available at: http://www.healthleadersmedia.com/content/256694.pdf. Accessed March 29, 2014.
25. AAFP statement: AAFP commends CMS for improving Medicare ACO final rule, announcing the advance payment model [press release]. Leawood, Kansas: American Academy of Family Physicians; October 21, 2011. Available at: http://www.aafp.org/media-center/releases-statements/all/2011/aco-final-rule.html. Accessed March 29, 2014.
26. Fisher ES, Staiger DO, Bynum JP, et al. Creating accountable care organizations: the extended hospital medical staff. Health Affairs (Millwood). 2007;26:w44-w57.
27. Shields MC, Patel PH, Manning M, et al. A model for integrating independent physicians into accountable care organizations. Health Affairs (Millwood). 2011;30:161-172.
28. Hibbard JH. Patient engagement in accountable care organizations. Webinar. 2008. https://acoregister.rti.org/display_docs7.cfm. Accessed April 11, 2014.
29. Developing a community-based patient safety advisory council. Agency for Healthcare Research and Quality Web site. Available at: http://www.ahrq.gov/professionals/quality-patient-safety/patient-safety-resources/resources/patient-safety-advisorycouncil/. Accessed March 31, 2014.
Pressure to reform our health care system is at an all-time high, driven by relentlessly rising costs and fragmentation of care. These persistent problems have led to lower quality care and limited access to care for a large proportion of the US population—issues that accountable care organizations (ACOs), as well as other value-based models, are designed to address.
While the terms used to describe the means by which health care systems attempt to do more to meet the needs of those they serve may vary, the importance of reorganizing care delivery to better integrate services is gaining traction nationwide. As we move to new models, primary care takes center stage.
ACOs (or ACO-type arrangements) anchored by primary care networks can help meet the goals of health care reform by responding to changes in reimbursement, reducing fragmented care, and focusing on improving the quality of care for defined patient populations. In addition, these delivery models can take advantage of new health information technology (IT) and the move toward patient-centered medical homes (PCMHs).
In the pages that follow, we examine opportunities for new care delivery models to slow rising costs and improve population health in family medicine. The introduction of these models has important implications for patients, physicians, and provider organizations, and our aim is to ensure that family physicians are prepared to take these vital steps toward achieving health reform goals.
Shifting from volume-based to value-based reimbursement
According to the Congressional Budget Office, ACOs are expected to save $5 billion during their first 8 years of existence.1 After one year of ACO activity, the Centers for Medicare and Medicaid Services (CMS) reported savings of $30 million.2 The expected savings will be driven by the increased provider accountability associated with ACOs.
Various means of increasing provider accountability through changes in reimbursement strategies have been proposed; several are new, while others are improvements on—or variations of—methods that have been tried before. The most common approaches—shared savings, shared savings plus penalty, capitation, episodic payment, prospective payment, pay-for-performance, and hospital-physician bundling—are detailed in TABLE 1.3-7 Broad implementation of any of these reimbursement mechanisms within a new model of care would represent a shift away from the traditional volume-based provider payment model to a value-based system—a key step in slowing the rise in health care costs.
TABLE 1
Reimbursement strategies designed to promote physician accountability3-7
Model | Description | Implications for FPs |
Shared savings | FFS plus a portion of dollars saved relative to predicted costs if quality and patient satisfaction are enhanced3 | Focus on population health incentivizes well care and preventive services |
Shared savings plus penalty | Same as shared savings, plus a penalty if expenses exceed spending targets; bonus potential is increased to account for increased risk3 | Potential for care coordination payments in addition to shared savings |
Capitation | Flat payments plus bonuses and penalties; provider organization assumes full risk for a defined patient population3 | A better understanding of population management and IT now makes capitation a viable strategy in certain settings |
Episodic payments | Reimbursement is for defined episodes of care, which may extend from time of admission to days or weeks after discharge; may also include home health, extended care, or ancillary services4 | No incentive for prevention or PCMH coordination |
Prospective payment | Reimbursement for inpatient services based on a prepaid amount that covers a defined period of time; uses DRG system that bases payment on disease classification by CMS5 | It is important for FPs to partner with specialists willing to share reimbursement commensurate with the value of care provided |
Pay-for-performance | Reimbursement is tied to achievement of metrics (eg, number of patients immunized for a specific disease, desired clinical outcomes, high patient satisfaction scores) mutually agreed upon by ACO and payer6 | Be sure any agreed-upon “targets” are achievable and patient-focused |
Hospital-physician bundling | Reimbursement is based on the cost of a procedure or diagnosis that includes both hospital and physician components. One payment is made for the collective services associated with a hospitalization7 | Similar to prospective payment from FP perspective; it is important to work with those who value the care of FPs |
ACO, accountable care organization; CMS, Centers for Medicare and Medicaid Services; DRG, diagnosis-related group; FFS, fee-for-service; FPs, family physicians; IT, information technology; PCMH, patient-centered medical home.
Although it is too soon to be certain of the effect such changes will have on the earnings of family physicians, it is reasonable to think that new payment strategies—and a larger role for primary care providers—will improve their financial standing.
Moving toward population health management
New ACO-type models also make it easier to improve health care for specific populations, using strategies designed to organize, provide, and manage care for defined groups. In addition to controlling the cost of caring for specific groups, well-designed and implemented population health management strategies can increase continuity of care by ensuring oversight of patients across the spectrum of health care settings.
Five broad categories of population health management are most prominent: lifestyle management and demand management (both for relatively healthy people), disease management (for those with chronic conditions), catastrophic care management (for patients with rare or catastrophic illness or injury), and disability management (for groups of employees).8TABLE 28 describes the population targeted and activities associated with each. It is important to remember, however, that no single strategy is mutually exclusive for a particular group.
Comprehensive Primary Care (CPC) initiative. In a collaboration made possible by the Affordable Care Act, CMS initiated the CPC initiative in 2012.9 A 4-year project designed to test and further identify the benefits of population health management and strengthen coordination of care for Medicare patients within primary care settings, the CPC initiative involves 497 sites and 2347 providers caring for approximately 315,000 Medicare beneficiaries.9 More information is available at http://innovation.cms.gov/initiatives/comprehensive-primary-care-initiative/.
TABLE 2
Population health management strategies8
Strategy | Target/goal | Key elements | Evidence of effectiveness |
Lifestyle management | To help relatively healthy individuals make good choices about health behaviors and risks | • Prevention • Risk reduction • Self-care | • Adherence to guidelines for clinical screenings • Reduced costs resulting from prevention programs |
Demand management | To help relatively healthy individuals take an active role in decisions about health and medical care; aims to reduce inappropriate demand for services | • Telephone triage • Advice and referrals • Decision and behavioral support • Education to promote self-care | • Reduced variation in care unexplained by morbidity • Improved understanding of perceived need for care • Improved access, better outcomes, lower cost |
Disease management | To identify and target chronically ill patients (eg, those with diabetes, heart failure, or asthma) with specific interventions | • Clinical oversight/management of patients with chronic disease • Education and self-care • Coordination of care/providers | • Reduced costs for treatment of chronic diseases • Decreased complications associated with chronic illness |
Catastrophic care management | To identify those with rare or catastrophic illness or injury and provide services needed to improve outcomes | • Immediate referral to appropriate providers • Coordination of care • Medical/care management | • Reduced hospitalizations and total claims costs • Reduced morbidity; improved QOL • Realistic, patient-specific goals |
Disability management | To develop and deliver employer-driven initiatives for employees to reduce lost time from work, improve productivity, and optimize health and well-being | • Disability prevention programs • Return-to-work programs • Employer-based lifestyle management programs • Coordination of care/providers for employees with chronic disease, disability, and/or serious illness or injury • Absence management programs (ie, designed to control/limit unexplained, unscheduled, or excessive absenteeism) • Workplace rehabilitation | • Lower workers’ compensation/disability benefit costs • Reduced number of injuries • Reduced lost time from work • Increased productivity |
QOL, quality of life.
Building infrastructure and leveraging IT
ACOs and ACO-type models will take a variety of forms, depending in part on geographic need and local demographics. Yet, all share a common need for a strong infrastructure to support improved transitions, integration, and coordination of care. Incorporation of a strong health IT system is critical so that data regarding the process and cost of care, as well as outcomes, can be collected and put to optimal use.10-13
Across health care settings, health IT innovations are being successfully implemented in efforts to enhance physician decision support, improve patient safety, increase guideline adherence, and improve chronic disease treatment.11,13 In primary care, for example, an IT infrastructure can create disease registries so that data on patients with specific conditions can be tracked and acted upon—eg, a diabetes registry could be used to identify and contact patients who have an hemoglobin A1c >9 and have not been seen in 9 months or more.
Similarly, an IT system with the ability to identify patients at high risk for disease decompensation, hospitalization, and/or increased morbidity and mortality linked to progression of a chronic disease is needed. Identifying medical conditions associated with higher costs would make it possible to focus care coordination and chronic care management efforts on this targeted population.
As health IT continues to evolve, additional means of interacting with patients and improving patient care will be developed. Physicians and organizations that are ready to take advantage of these advances in technology will be well positioned to address the goals of health reform. (See “Health care reform: Recommendations for family physicians” below.3-7,14,15)
How the patient-centered medical home fits into the picture
The implementation of ACOs and other new models of care has promising implications for the establishment of PCMHs. Consistent with the goals of health reform, the PCMH movement focuses on a coordinated teamwork approach, anchored within a general practice or family medicine setting.
An evaluation of the PCMH National Demonstration Project funded by the American Academy of Family Physicians found that the adoption of more components of the PCMH at the practice level was associated with improvement in patient outcomes, as measured by the Ambulatory Care Quality Alliance starter set16 (a compilation of clinical performance measures developed by a broad coalition of providers, payers, consumers, and government agencies).
A recent look at the Southeastern Pennsylvania Chronic Care Initiative17 found less promising results. “A multipayer medical home pilot, in which participating practices adopted new structural capabilities and received NCQA [National Committee for Quality Assurance] certification, was associated with limited improvements in quality and was not associated with reductions in utilization of hospital, emergency department, or ambulatory care services or total costs over 3 years,” Friedberg et al17 concluded. The authors did note, however, that NCQA recognition was what the practices involved in this initiative were rewarded for—not PCMH activity.
It is also important to keep in mind that PCMH activity has been shown to improve care and reduce costs—not NCQA recognition in and of itself. In fact, a large body of evidence clearly demonstrates the positive patient care outcomes and reductions in overall cost associated with the PCMH. These findings were compiled by the Patient-Centered Primary Care Collaborative—which issues annual reports on the progress of the PCMH—in a January 2014 update.18
In a PCMH model, the focus shifts away from the procedure(s) or treatment to the whole person. However, all components of care (eg, primary and specialty care, hospital, ancillary services, laboratory, and radiology) are vital and need to be connected to increase efficiency and reduce cost—creating what is sometimes referred to as a “medical neighborhood.”19-21
What’s in the neighborhood?
In a medical neighborhood, such as an ACO, each patient is cared for by a team of providers at multiple locations. The PCMH serves as the base, ensuring that all providers work together toward a common goal. In addition to providing primary care, the PCMH coordinates each patient’s specialty and support services and communicates the care plan to all involved.
Successful implementation of a medical neighborhood requires a close working relationship among providers, payers, and community resources. For example, payers can provide real-time information about patients who have been admitted to the hospital or discharged from the emergency department, which enables close follow-up and coordination across multiple systems.
Given the emerging opportunities for new care delivery models to advance primary care, we urge family physicians to respond positively to these changes and challenges. Here’s what we recommend:
Carefully consider payment methodologies. Changes in the way physicians are paid will vary by payer source, as well as geographic market.3-7 Regardless of the reimbursement model you’re offered, however, do not agree to it until you have the opportunity to evaluate it, along with your particular circumstances, to ensure that you have the infrastructure to support whatever changes the new model will require.
Read the fine print. Look out for your own interests by carefully reading the terms you are presented with. Consider seeking advice from those who understand the particular nuances faced by family physicians under particular reimbursement strategies. Just because a payment method benefits a particular specialty does not mean it will be favorable to family physicians.
Before you join an ACO
Before joining an accountable care organization (ACO ) or a similar entity, find out whether it supports primary care principles and the patient-centered medical home (PCMH ). Some questions to ask:
• Does the ACO have the infrastructure necessary to be successful, including the requisite health information technology, administrative support, actuarial knowledge, and experience with population health management?
• Is the ACO founded on primary care principles? Find out, for example, whether primary care physicians are represented at all levels of the organization and provide appropriate input on all important issues.
If you practice in a rural area … The growth of ACO activity is expected to be slow in both rural and underserved metropolitan markets. To address this issue, the Centers for Medicare and Medicaid Services is allowing primary care physicians in such markets to participate in more than one Medicare ACO and providing financial incentives in the form of savings exemptions to smaller, rural ACOs.14,15 Another option for rural providers is the adoption of a “virtual ACO ”—a loosely organized group of providers, united in the effort to achieve high-quality care and reduced costs and willing to submit to computer analysis that will determine their relative contributions to efficiency and the distribution of savings.14
Get involved
It is important for all family physicians to engage in discussions about health care reform, and to represent both their patients and their specialty. Familiarity with what is happening is essential. One way to do that is to become an active member of your state or local American Academy of Family Physicians affiliate.
More information is available at:
• www.aafp.org
Practical information with regard to health reform, in addition to suggesting ways to get involved
• http://www.tafp.org/Media/Default/Downloads/practice%20resources/aco-guide.pdf
Information to consider before joining or forming an ACO
• http://www.ncqa.org/Programs/Recognition/PatientCenteredMedicalHomePCMH.aspx and http://www.transformed.com/
Resources for practitioners considering transformation to a PCMH
• http://innovation.cms.gov
Information, including webinars and forums, on innovative payment and service delivery models.
Nontraditional settings. Another facet of the medical neighborhood is the provision of health care services in nontraditional settings. For example, some grocery stores in our area employ nutritionists to whom we refer patients for nutritional counseling regarding their health in general or a disease process in particular.
Changes in reimbursement also will affect how care is delivered within the medical neighborhood. As we move away from fee-for-service (volume-based) to value-based payments, physicians who have made the transition from working individually with a panel of patients to providing team-based care within a PCMH will be better positioned to meet the goals of health care reform. (See “Team-based care is key inside the PCMH, too”22 below.) Nonetheless, the transition is a dynamic process. With changes in reimbursement and delivery models, physicians also will be expected to develop and implement continuous quality improvement measures so patient care can be continually evaluated and improved.
Now comes the hard part
While a PCMH requires primary care physicians to collaborate with other health professionals, it has the potential to lead to conflicts and debates about who is at the head of the health care team. This is particularly true within mental health services because, while primary care visits are frequently related to psychosocial issues, the mental health and general practice sectors have traditionally been distinct. In recent years, however, coordinated delivery models that integrate primary care and mental health services have been shown to increase access and reduce the stigma associated with mental health services—and to be cost-effective.23
In many ways, moving the primary care culture from the traditional focus on the physician as “captain of the ship” to a physician-led, team-based approach is one of the most difficult tasks for organizations attempting to transform their care delivery models.3,24 Physicians historically have been autonomous providers of medical care, relying on their own experience, expertise, and beliefs to guide decisions about patient care. Now they’re being asked to give up some of the direct control they may have had over patient care decisions and learn to work more collaboratively with other providers, as well as nonclinicians (eg, health coaches), to achieve desired outcomes.3 A successful transition depends on a reimbursement framework in which patient care goals are properly aligned with incentives for primary care physicians to work in a team-based environment.3,25-27
In addition to operating as a team with providers in other settings in the medical neighborhood, innovative primary care practices—typically those that have already achieved patient-centered medical home (PCMH ) status—have strong teams within their walls. In “In search of joy in practice: A report of 23 high-functioning primary care practices,” Sinsky et al22 highlight a number of ways in which the practices they studied are maximizing this approach.
Nonphysician care. A number of practices expanded the roles of medical assistants (MAs), nurses, and even nonclinician health coaches. In one case, MAs nearly tripled the time they spend with each patient, to enable them to do medication review, fill out forms, give immunizations, and book appointments for screening tests such as mammography. In another, registered nurses were given standing orders to treat routine problems such as ear infections and urinary tract infections; at a third, health coaches counsel patients with chronic conditions and MAs conduct depression screening, as needed.
Documentation and computerized order entry—which ties up many hours of physician time—is another area in which some practices have adopted a team approach. A number of practices use nurses or MAs as scribes, entering orders and preparing after-visit summaries, for example. Not only are the physicians more satisfied, but the MAs and nurses are happy to have more involvement in patient care, the researchers report.
Communication is crucial to a successful team approach. In some practices, this is accomplished with weekly physician-clinical staff meetings; in others, with brief group “huddles” or by an office design featuring “co-location.” In one example of the latter, MAs and physicians sit side-by-side, so they can easily talk to each other—the doctor could communicate key patient information that the MA would then follow up on, for example. Regular analysis of workflow to identify and address undue delays is an effective team function, as well.
Helping patients help themselves. Moving toward more patient-focused care will also require a concerted effort to increase patients’ engagement in their own health and medical care. In practice, very little of an individual’s time is spent in a physician’s office. Thus, optimal outcomes can be achieved only when patients are actively involved. Helping patients become proactive—ie, by arming them with the knowledge, skill, and confidence to do their part in staying healthy28—also represents a major shift in primary care culture, as patients become active participants in medical decision making rather than passive recipients of physicians’ advice.
Alternative approaches. To deliver the continuum of care that is central to new care delivery models and shift the culture of primary care toward a PCMH, physicians can implement a number of clinic-based engagement approaches—interacting with patients via e-visits such as e-mail through a secure portal, telemedicine, and group medical visits, for example. Physicians can encourage patient participation by starting patient interest groups and advisory panels29—recommended by the NCQA and the Agency for Healthcare Research and Quality and used at our institution—and conducting patient needs assessments on a regular basis. Opportunities for primary care practices to engage with the community include partnering with local health departments, churches, nonprofits, and advocacy organizations to conduct health promotion and educational activities.
CORRESPONDENCE
Randy Wexler, MD, MPH, Department of Family Medicine, College of Medicine, The Ohio State University, 2231 North High Street, Northwood and High Building, Columbus, OH 43201; [email protected]
Pressure to reform our health care system is at an all-time high, driven by relentlessly rising costs and fragmentation of care. These persistent problems have led to lower quality care and limited access to care for a large proportion of the US population—issues that accountable care organizations (ACOs), as well as other value-based models, are designed to address.
While the terms used to describe the means by which health care systems attempt to do more to meet the needs of those they serve may vary, the importance of reorganizing care delivery to better integrate services is gaining traction nationwide. As we move to new models, primary care takes center stage.
ACOs (or ACO-type arrangements) anchored by primary care networks can help meet the goals of health care reform by responding to changes in reimbursement, reducing fragmented care, and focusing on improving the quality of care for defined patient populations. In addition, these delivery models can take advantage of new health information technology (IT) and the move toward patient-centered medical homes (PCMHs).
In the pages that follow, we examine opportunities for new care delivery models to slow rising costs and improve population health in family medicine. The introduction of these models has important implications for patients, physicians, and provider organizations, and our aim is to ensure that family physicians are prepared to take these vital steps toward achieving health reform goals.
Shifting from volume-based to value-based reimbursement
According to the Congressional Budget Office, ACOs are expected to save $5 billion during their first 8 years of existence.1 After one year of ACO activity, the Centers for Medicare and Medicaid Services (CMS) reported savings of $30 million.2 The expected savings will be driven by the increased provider accountability associated with ACOs.
Various means of increasing provider accountability through changes in reimbursement strategies have been proposed; several are new, while others are improvements on—or variations of—methods that have been tried before. The most common approaches—shared savings, shared savings plus penalty, capitation, episodic payment, prospective payment, pay-for-performance, and hospital-physician bundling—are detailed in TABLE 1.3-7 Broad implementation of any of these reimbursement mechanisms within a new model of care would represent a shift away from the traditional volume-based provider payment model to a value-based system—a key step in slowing the rise in health care costs.
TABLE 1
Reimbursement strategies designed to promote physician accountability3-7
Model | Description | Implications for FPs |
Shared savings | FFS plus a portion of dollars saved relative to predicted costs if quality and patient satisfaction are enhanced3 | Focus on population health incentivizes well care and preventive services |
Shared savings plus penalty | Same as shared savings, plus a penalty if expenses exceed spending targets; bonus potential is increased to account for increased risk3 | Potential for care coordination payments in addition to shared savings |
Capitation | Flat payments plus bonuses and penalties; provider organization assumes full risk for a defined patient population3 | A better understanding of population management and IT now makes capitation a viable strategy in certain settings |
Episodic payments | Reimbursement is for defined episodes of care, which may extend from time of admission to days or weeks after discharge; may also include home health, extended care, or ancillary services4 | No incentive for prevention or PCMH coordination |
Prospective payment | Reimbursement for inpatient services based on a prepaid amount that covers a defined period of time; uses DRG system that bases payment on disease classification by CMS5 | It is important for FPs to partner with specialists willing to share reimbursement commensurate with the value of care provided |
Pay-for-performance | Reimbursement is tied to achievement of metrics (eg, number of patients immunized for a specific disease, desired clinical outcomes, high patient satisfaction scores) mutually agreed upon by ACO and payer6 | Be sure any agreed-upon “targets” are achievable and patient-focused |
Hospital-physician bundling | Reimbursement is based on the cost of a procedure or diagnosis that includes both hospital and physician components. One payment is made for the collective services associated with a hospitalization7 | Similar to prospective payment from FP perspective; it is important to work with those who value the care of FPs |
ACO, accountable care organization; CMS, Centers for Medicare and Medicaid Services; DRG, diagnosis-related group; FFS, fee-for-service; FPs, family physicians; IT, information technology; PCMH, patient-centered medical home.
Although it is too soon to be certain of the effect such changes will have on the earnings of family physicians, it is reasonable to think that new payment strategies—and a larger role for primary care providers—will improve their financial standing.
Moving toward population health management
New ACO-type models also make it easier to improve health care for specific populations, using strategies designed to organize, provide, and manage care for defined groups. In addition to controlling the cost of caring for specific groups, well-designed and implemented population health management strategies can increase continuity of care by ensuring oversight of patients across the spectrum of health care settings.
Five broad categories of population health management are most prominent: lifestyle management and demand management (both for relatively healthy people), disease management (for those with chronic conditions), catastrophic care management (for patients with rare or catastrophic illness or injury), and disability management (for groups of employees).8TABLE 28 describes the population targeted and activities associated with each. It is important to remember, however, that no single strategy is mutually exclusive for a particular group.
Comprehensive Primary Care (CPC) initiative. In a collaboration made possible by the Affordable Care Act, CMS initiated the CPC initiative in 2012.9 A 4-year project designed to test and further identify the benefits of population health management and strengthen coordination of care for Medicare patients within primary care settings, the CPC initiative involves 497 sites and 2347 providers caring for approximately 315,000 Medicare beneficiaries.9 More information is available at http://innovation.cms.gov/initiatives/comprehensive-primary-care-initiative/.
TABLE 2
Population health management strategies8
Strategy | Target/goal | Key elements | Evidence of effectiveness |
Lifestyle management | To help relatively healthy individuals make good choices about health behaviors and risks | • Prevention • Risk reduction • Self-care | • Adherence to guidelines for clinical screenings • Reduced costs resulting from prevention programs |
Demand management | To help relatively healthy individuals take an active role in decisions about health and medical care; aims to reduce inappropriate demand for services | • Telephone triage • Advice and referrals • Decision and behavioral support • Education to promote self-care | • Reduced variation in care unexplained by morbidity • Improved understanding of perceived need for care • Improved access, better outcomes, lower cost |
Disease management | To identify and target chronically ill patients (eg, those with diabetes, heart failure, or asthma) with specific interventions | • Clinical oversight/management of patients with chronic disease • Education and self-care • Coordination of care/providers | • Reduced costs for treatment of chronic diseases • Decreased complications associated with chronic illness |
Catastrophic care management | To identify those with rare or catastrophic illness or injury and provide services needed to improve outcomes | • Immediate referral to appropriate providers • Coordination of care • Medical/care management | • Reduced hospitalizations and total claims costs • Reduced morbidity; improved QOL • Realistic, patient-specific goals |
Disability management | To develop and deliver employer-driven initiatives for employees to reduce lost time from work, improve productivity, and optimize health and well-being | • Disability prevention programs • Return-to-work programs • Employer-based lifestyle management programs • Coordination of care/providers for employees with chronic disease, disability, and/or serious illness or injury • Absence management programs (ie, designed to control/limit unexplained, unscheduled, or excessive absenteeism) • Workplace rehabilitation | • Lower workers’ compensation/disability benefit costs • Reduced number of injuries • Reduced lost time from work • Increased productivity |
QOL, quality of life.
Building infrastructure and leveraging IT
ACOs and ACO-type models will take a variety of forms, depending in part on geographic need and local demographics. Yet, all share a common need for a strong infrastructure to support improved transitions, integration, and coordination of care. Incorporation of a strong health IT system is critical so that data regarding the process and cost of care, as well as outcomes, can be collected and put to optimal use.10-13
Across health care settings, health IT innovations are being successfully implemented in efforts to enhance physician decision support, improve patient safety, increase guideline adherence, and improve chronic disease treatment.11,13 In primary care, for example, an IT infrastructure can create disease registries so that data on patients with specific conditions can be tracked and acted upon—eg, a diabetes registry could be used to identify and contact patients who have an hemoglobin A1c >9 and have not been seen in 9 months or more.
Similarly, an IT system with the ability to identify patients at high risk for disease decompensation, hospitalization, and/or increased morbidity and mortality linked to progression of a chronic disease is needed. Identifying medical conditions associated with higher costs would make it possible to focus care coordination and chronic care management efforts on this targeted population.
As health IT continues to evolve, additional means of interacting with patients and improving patient care will be developed. Physicians and organizations that are ready to take advantage of these advances in technology will be well positioned to address the goals of health reform. (See “Health care reform: Recommendations for family physicians” below.3-7,14,15)
How the patient-centered medical home fits into the picture
The implementation of ACOs and other new models of care has promising implications for the establishment of PCMHs. Consistent with the goals of health reform, the PCMH movement focuses on a coordinated teamwork approach, anchored within a general practice or family medicine setting.
An evaluation of the PCMH National Demonstration Project funded by the American Academy of Family Physicians found that the adoption of more components of the PCMH at the practice level was associated with improvement in patient outcomes, as measured by the Ambulatory Care Quality Alliance starter set16 (a compilation of clinical performance measures developed by a broad coalition of providers, payers, consumers, and government agencies).
A recent look at the Southeastern Pennsylvania Chronic Care Initiative17 found less promising results. “A multipayer medical home pilot, in which participating practices adopted new structural capabilities and received NCQA [National Committee for Quality Assurance] certification, was associated with limited improvements in quality and was not associated with reductions in utilization of hospital, emergency department, or ambulatory care services or total costs over 3 years,” Friedberg et al17 concluded. The authors did note, however, that NCQA recognition was what the practices involved in this initiative were rewarded for—not PCMH activity.
It is also important to keep in mind that PCMH activity has been shown to improve care and reduce costs—not NCQA recognition in and of itself. In fact, a large body of evidence clearly demonstrates the positive patient care outcomes and reductions in overall cost associated with the PCMH. These findings were compiled by the Patient-Centered Primary Care Collaborative—which issues annual reports on the progress of the PCMH—in a January 2014 update.18
In a PCMH model, the focus shifts away from the procedure(s) or treatment to the whole person. However, all components of care (eg, primary and specialty care, hospital, ancillary services, laboratory, and radiology) are vital and need to be connected to increase efficiency and reduce cost—creating what is sometimes referred to as a “medical neighborhood.”19-21
What’s in the neighborhood?
In a medical neighborhood, such as an ACO, each patient is cared for by a team of providers at multiple locations. The PCMH serves as the base, ensuring that all providers work together toward a common goal. In addition to providing primary care, the PCMH coordinates each patient’s specialty and support services and communicates the care plan to all involved.
Successful implementation of a medical neighborhood requires a close working relationship among providers, payers, and community resources. For example, payers can provide real-time information about patients who have been admitted to the hospital or discharged from the emergency department, which enables close follow-up and coordination across multiple systems.
Given the emerging opportunities for new care delivery models to advance primary care, we urge family physicians to respond positively to these changes and challenges. Here’s what we recommend:
Carefully consider payment methodologies. Changes in the way physicians are paid will vary by payer source, as well as geographic market.3-7 Regardless of the reimbursement model you’re offered, however, do not agree to it until you have the opportunity to evaluate it, along with your particular circumstances, to ensure that you have the infrastructure to support whatever changes the new model will require.
Read the fine print. Look out for your own interests by carefully reading the terms you are presented with. Consider seeking advice from those who understand the particular nuances faced by family physicians under particular reimbursement strategies. Just because a payment method benefits a particular specialty does not mean it will be favorable to family physicians.
Before you join an ACO
Before joining an accountable care organization (ACO ) or a similar entity, find out whether it supports primary care principles and the patient-centered medical home (PCMH ). Some questions to ask:
• Does the ACO have the infrastructure necessary to be successful, including the requisite health information technology, administrative support, actuarial knowledge, and experience with population health management?
• Is the ACO founded on primary care principles? Find out, for example, whether primary care physicians are represented at all levels of the organization and provide appropriate input on all important issues.
If you practice in a rural area … The growth of ACO activity is expected to be slow in both rural and underserved metropolitan markets. To address this issue, the Centers for Medicare and Medicaid Services is allowing primary care physicians in such markets to participate in more than one Medicare ACO and providing financial incentives in the form of savings exemptions to smaller, rural ACOs.14,15 Another option for rural providers is the adoption of a “virtual ACO ”—a loosely organized group of providers, united in the effort to achieve high-quality care and reduced costs and willing to submit to computer analysis that will determine their relative contributions to efficiency and the distribution of savings.14
Get involved
It is important for all family physicians to engage in discussions about health care reform, and to represent both their patients and their specialty. Familiarity with what is happening is essential. One way to do that is to become an active member of your state or local American Academy of Family Physicians affiliate.
More information is available at:
• www.aafp.org
Practical information with regard to health reform, in addition to suggesting ways to get involved
• http://www.tafp.org/Media/Default/Downloads/practice%20resources/aco-guide.pdf
Information to consider before joining or forming an ACO
• http://www.ncqa.org/Programs/Recognition/PatientCenteredMedicalHomePCMH.aspx and http://www.transformed.com/
Resources for practitioners considering transformation to a PCMH
• http://innovation.cms.gov
Information, including webinars and forums, on innovative payment and service delivery models.
Nontraditional settings. Another facet of the medical neighborhood is the provision of health care services in nontraditional settings. For example, some grocery stores in our area employ nutritionists to whom we refer patients for nutritional counseling regarding their health in general or a disease process in particular.
Changes in reimbursement also will affect how care is delivered within the medical neighborhood. As we move away from fee-for-service (volume-based) to value-based payments, physicians who have made the transition from working individually with a panel of patients to providing team-based care within a PCMH will be better positioned to meet the goals of health care reform. (See “Team-based care is key inside the PCMH, too”22 below.) Nonetheless, the transition is a dynamic process. With changes in reimbursement and delivery models, physicians also will be expected to develop and implement continuous quality improvement measures so patient care can be continually evaluated and improved.
Now comes the hard part
While a PCMH requires primary care physicians to collaborate with other health professionals, it has the potential to lead to conflicts and debates about who is at the head of the health care team. This is particularly true within mental health services because, while primary care visits are frequently related to psychosocial issues, the mental health and general practice sectors have traditionally been distinct. In recent years, however, coordinated delivery models that integrate primary care and mental health services have been shown to increase access and reduce the stigma associated with mental health services—and to be cost-effective.23
In many ways, moving the primary care culture from the traditional focus on the physician as “captain of the ship” to a physician-led, team-based approach is one of the most difficult tasks for organizations attempting to transform their care delivery models.3,24 Physicians historically have been autonomous providers of medical care, relying on their own experience, expertise, and beliefs to guide decisions about patient care. Now they’re being asked to give up some of the direct control they may have had over patient care decisions and learn to work more collaboratively with other providers, as well as nonclinicians (eg, health coaches), to achieve desired outcomes.3 A successful transition depends on a reimbursement framework in which patient care goals are properly aligned with incentives for primary care physicians to work in a team-based environment.3,25-27
In addition to operating as a team with providers in other settings in the medical neighborhood, innovative primary care practices—typically those that have already achieved patient-centered medical home (PCMH ) status—have strong teams within their walls. In “In search of joy in practice: A report of 23 high-functioning primary care practices,” Sinsky et al22 highlight a number of ways in which the practices they studied are maximizing this approach.
Nonphysician care. A number of practices expanded the roles of medical assistants (MAs), nurses, and even nonclinician health coaches. In one case, MAs nearly tripled the time they spend with each patient, to enable them to do medication review, fill out forms, give immunizations, and book appointments for screening tests such as mammography. In another, registered nurses were given standing orders to treat routine problems such as ear infections and urinary tract infections; at a third, health coaches counsel patients with chronic conditions and MAs conduct depression screening, as needed.
Documentation and computerized order entry—which ties up many hours of physician time—is another area in which some practices have adopted a team approach. A number of practices use nurses or MAs as scribes, entering orders and preparing after-visit summaries, for example. Not only are the physicians more satisfied, but the MAs and nurses are happy to have more involvement in patient care, the researchers report.
Communication is crucial to a successful team approach. In some practices, this is accomplished with weekly physician-clinical staff meetings; in others, with brief group “huddles” or by an office design featuring “co-location.” In one example of the latter, MAs and physicians sit side-by-side, so they can easily talk to each other—the doctor could communicate key patient information that the MA would then follow up on, for example. Regular analysis of workflow to identify and address undue delays is an effective team function, as well.
Helping patients help themselves. Moving toward more patient-focused care will also require a concerted effort to increase patients’ engagement in their own health and medical care. In practice, very little of an individual’s time is spent in a physician’s office. Thus, optimal outcomes can be achieved only when patients are actively involved. Helping patients become proactive—ie, by arming them with the knowledge, skill, and confidence to do their part in staying healthy28—also represents a major shift in primary care culture, as patients become active participants in medical decision making rather than passive recipients of physicians’ advice.
Alternative approaches. To deliver the continuum of care that is central to new care delivery models and shift the culture of primary care toward a PCMH, physicians can implement a number of clinic-based engagement approaches—interacting with patients via e-visits such as e-mail through a secure portal, telemedicine, and group medical visits, for example. Physicians can encourage patient participation by starting patient interest groups and advisory panels29—recommended by the NCQA and the Agency for Healthcare Research and Quality and used at our institution—and conducting patient needs assessments on a regular basis. Opportunities for primary care practices to engage with the community include partnering with local health departments, churches, nonprofits, and advocacy organizations to conduct health promotion and educational activities.
CORRESPONDENCE
Randy Wexler, MD, MPH, Department of Family Medicine, College of Medicine, The Ohio State University, 2231 North High Street, Northwood and High Building, Columbus, OH 43201; [email protected]
1. Shortell SM, Casalino LP, Fisher ES. How the Center for Medicare and Medicaid Innovation should test accountable care organizations. Health Aff. 2010;29:1293-1298.
2. Medicare’s delivery system reform initiatives achieve significant savings and quality improvements - off to a strong start [press release]. Washington, DC: US Department of Health & Human Services; January 30, 2014. Available at: http://www.hhs.gov/news/press/2014pres/01/20140130a.html. Accessed March 28, 2014.
3. The family physician’s ACO blueprint for success: Preparing family medicine for the approaching accountable care era. Texas Academy of Family Physicians Web site. Available at: http://www.tafp.org/Media/Default/Downloads/practice%20resources/acoguide.pdf. Accessed March 28, 2014.
4. Mechanic RE. Opportunities and challenges for episode-based payment. N Engl J Med. 2011;365:777-779.
5. Medicare prospective payment systems (PPS): A summary. American Speech-Language-Hearing Association Web site. Available at: http://www.asha.org/practice/reimbursement/medicare/pps_sum.htm. Accessed March 28, 2014.
6. Pay for performance (P4P): AHRQ resources. Agency for Healthcare Research and Quality Web site. Available at: http://www.ahrq.gov/legacy/qual/pay4per.htm. Accessed March 29, 2014.
7. Komisar H, Feder J, Ginsburg PB. “Bundling” payment for episodes of hospital care: Issues and recommendations for the new pilot program in Medicare. Available at: http://www.americanprogress.org/issues/2011/07/pdf/medicare_bundling.pdf. Accessed May 20, 2014.
8. McAlearney AS. Population health management: Strategies to improve outcomes. Chicago, IL: Health Administration Press; 2003.
9. Centers for Medicare and Medicaid Services Web site. Comprehensive primary care initiative. Available at: http://innovation.cms.gov/initiatives/comprehensive-primary-care-initiative/. Accessed March 29, 2014.
10. Robertson DC, Lerner JC. Top technology issues for ambulatory care facilities this year and beyond. J Ambul Care Manag. 2009;32:303-319.
11. Linder JA, Ma J, Bates DW, et al. Electronic health record use and the quality of ambulatory care in the united states. Arch Intern Med. 2007;167:1400-1405.
12. Vest JR. Health information exchange and healthcare utilization. J Med Syst. 2009;33:223-231.
13. Parente ST, McCullough JS. Health information technology and patient safety: evidence from panel data. Health Affairs (Millwood). 2009;28:357-360.
14. FAQ on Accountable Care Organizations. American Academy of Family Physicians Web site. Available at: http://www.aafp.org/practice-management/payment/acos/faq.html. Accessed March 29, 2014.
15. Torrieri M. CMS appeals to rural practices with another ACO participation perk. Physicians Practice blog. June 1, 2011. Available at: http://www.physicianspractice.com/blog/cms-appealsrural-practices-another-aco-participation-perk. Accessed March 29, 2014.
16. Crabtree BF, Nutting PA, Miller WL, et al. Summary of the national demonstration project and recommendations for the patient-centered medical home. Ann Fam Med. 2010;8 suppl 1:S80-S92.
17. Friedberg MW, Schneider EC, Rosenthal MB, et al. Association between participation in a multipayer medical home intervention and changes in quality, utilization, and costs of care. JAMA. 2014;311:815-825.
18. Nielsen M, Olayiwola JN, Grundy P, et al. The patient-centered medical home’s impact on cost & quality: An annual update of the evidence, 2012-2013. Available at: http://www.pcpcc.org/resource/medical-homes-impact-cost-quality. Accessed May 20, 2014.
19. Laine C. Welcome to the patient-centered medical neighborhood. Ann Intern Med. 2011;154:60.
20. Pham HH. Good neighbors: how will the patient-centered medical home relate to the rest of the health-care delivery system? J Gen Intern Med. 2010;25:630-634.
21. Taylor EF, Lake T, Nysenbaum J, et al; Mathematica Policy Research. Coordinating care in the medical neighborhood: critical components and available mechanisms: White paper. Available at: http://pcmh.ahrq.gov/sites/default/files/attachments/Coordinating%20Care%20in%20the%20Medical%20Neighborhood.pdf. Accessed May 20, 2014.
22. Sinsky CA, Willard-Grace R, Schutzbank AM, et al. In search of joy in practice: a report of 23 high-functioning primary care practices. Ann Fam Med. 2013;11:272-278.
23. Collins C, Hewson DL, Munger R, et al. Evolving models of behavioral health integration in primary care. Available at: http://www.milbank.org/uploads/documents/10430EvolvingCare/EvolvingCare.pdf. Accessed April 10, 2014.
24. The cornerstones of accountable care. Health Leaders Media Web site. Available at: http://www.healthleadersmedia.com/content/256694.pdf. Accessed March 29, 2014.
25. AAFP statement: AAFP commends CMS for improving Medicare ACO final rule, announcing the advance payment model [press release]. Leawood, Kansas: American Academy of Family Physicians; October 21, 2011. Available at: http://www.aafp.org/media-center/releases-statements/all/2011/aco-final-rule.html. Accessed March 29, 2014.
26. Fisher ES, Staiger DO, Bynum JP, et al. Creating accountable care organizations: the extended hospital medical staff. Health Affairs (Millwood). 2007;26:w44-w57.
27. Shields MC, Patel PH, Manning M, et al. A model for integrating independent physicians into accountable care organizations. Health Affairs (Millwood). 2011;30:161-172.
28. Hibbard JH. Patient engagement in accountable care organizations. Webinar. 2008. https://acoregister.rti.org/display_docs7.cfm. Accessed April 11, 2014.
29. Developing a community-based patient safety advisory council. Agency for Healthcare Research and Quality Web site. Available at: http://www.ahrq.gov/professionals/quality-patient-safety/patient-safety-resources/resources/patient-safety-advisorycouncil/. Accessed March 31, 2014.
1. Shortell SM, Casalino LP, Fisher ES. How the Center for Medicare and Medicaid Innovation should test accountable care organizations. Health Aff. 2010;29:1293-1298.
2. Medicare’s delivery system reform initiatives achieve significant savings and quality improvements - off to a strong start [press release]. Washington, DC: US Department of Health & Human Services; January 30, 2014. Available at: http://www.hhs.gov/news/press/2014pres/01/20140130a.html. Accessed March 28, 2014.
3. The family physician’s ACO blueprint for success: Preparing family medicine for the approaching accountable care era. Texas Academy of Family Physicians Web site. Available at: http://www.tafp.org/Media/Default/Downloads/practice%20resources/acoguide.pdf. Accessed March 28, 2014.
4. Mechanic RE. Opportunities and challenges for episode-based payment. N Engl J Med. 2011;365:777-779.
5. Medicare prospective payment systems (PPS): A summary. American Speech-Language-Hearing Association Web site. Available at: http://www.asha.org/practice/reimbursement/medicare/pps_sum.htm. Accessed March 28, 2014.
6. Pay for performance (P4P): AHRQ resources. Agency for Healthcare Research and Quality Web site. Available at: http://www.ahrq.gov/legacy/qual/pay4per.htm. Accessed March 29, 2014.
7. Komisar H, Feder J, Ginsburg PB. “Bundling” payment for episodes of hospital care: Issues and recommendations for the new pilot program in Medicare. Available at: http://www.americanprogress.org/issues/2011/07/pdf/medicare_bundling.pdf. Accessed May 20, 2014.
8. McAlearney AS. Population health management: Strategies to improve outcomes. Chicago, IL: Health Administration Press; 2003.
9. Centers for Medicare and Medicaid Services Web site. Comprehensive primary care initiative. Available at: http://innovation.cms.gov/initiatives/comprehensive-primary-care-initiative/. Accessed March 29, 2014.
10. Robertson DC, Lerner JC. Top technology issues for ambulatory care facilities this year and beyond. J Ambul Care Manag. 2009;32:303-319.
11. Linder JA, Ma J, Bates DW, et al. Electronic health record use and the quality of ambulatory care in the united states. Arch Intern Med. 2007;167:1400-1405.
12. Vest JR. Health information exchange and healthcare utilization. J Med Syst. 2009;33:223-231.
13. Parente ST, McCullough JS. Health information technology and patient safety: evidence from panel data. Health Affairs (Millwood). 2009;28:357-360.
14. FAQ on Accountable Care Organizations. American Academy of Family Physicians Web site. Available at: http://www.aafp.org/practice-management/payment/acos/faq.html. Accessed March 29, 2014.
15. Torrieri M. CMS appeals to rural practices with another ACO participation perk. Physicians Practice blog. June 1, 2011. Available at: http://www.physicianspractice.com/blog/cms-appealsrural-practices-another-aco-participation-perk. Accessed March 29, 2014.
16. Crabtree BF, Nutting PA, Miller WL, et al. Summary of the national demonstration project and recommendations for the patient-centered medical home. Ann Fam Med. 2010;8 suppl 1:S80-S92.
17. Friedberg MW, Schneider EC, Rosenthal MB, et al. Association between participation in a multipayer medical home intervention and changes in quality, utilization, and costs of care. JAMA. 2014;311:815-825.
18. Nielsen M, Olayiwola JN, Grundy P, et al. The patient-centered medical home’s impact on cost & quality: An annual update of the evidence, 2012-2013. Available at: http://www.pcpcc.org/resource/medical-homes-impact-cost-quality. Accessed May 20, 2014.
19. Laine C. Welcome to the patient-centered medical neighborhood. Ann Intern Med. 2011;154:60.
20. Pham HH. Good neighbors: how will the patient-centered medical home relate to the rest of the health-care delivery system? J Gen Intern Med. 2010;25:630-634.
21. Taylor EF, Lake T, Nysenbaum J, et al; Mathematica Policy Research. Coordinating care in the medical neighborhood: critical components and available mechanisms: White paper. Available at: http://pcmh.ahrq.gov/sites/default/files/attachments/Coordinating%20Care%20in%20the%20Medical%20Neighborhood.pdf. Accessed May 20, 2014.
22. Sinsky CA, Willard-Grace R, Schutzbank AM, et al. In search of joy in practice: a report of 23 high-functioning primary care practices. Ann Fam Med. 2013;11:272-278.
23. Collins C, Hewson DL, Munger R, et al. Evolving models of behavioral health integration in primary care. Available at: http://www.milbank.org/uploads/documents/10430EvolvingCare/EvolvingCare.pdf. Accessed April 10, 2014.
24. The cornerstones of accountable care. Health Leaders Media Web site. Available at: http://www.healthleadersmedia.com/content/256694.pdf. Accessed March 29, 2014.
25. AAFP statement: AAFP commends CMS for improving Medicare ACO final rule, announcing the advance payment model [press release]. Leawood, Kansas: American Academy of Family Physicians; October 21, 2011. Available at: http://www.aafp.org/media-center/releases-statements/all/2011/aco-final-rule.html. Accessed March 29, 2014.
26. Fisher ES, Staiger DO, Bynum JP, et al. Creating accountable care organizations: the extended hospital medical staff. Health Affairs (Millwood). 2007;26:w44-w57.
27. Shields MC, Patel PH, Manning M, et al. A model for integrating independent physicians into accountable care organizations. Health Affairs (Millwood). 2011;30:161-172.
28. Hibbard JH. Patient engagement in accountable care organizations. Webinar. 2008. https://acoregister.rti.org/display_docs7.cfm. Accessed April 11, 2014.
29. Developing a community-based patient safety advisory council. Agency for Healthcare Research and Quality Web site. Available at: http://www.ahrq.gov/professionals/quality-patient-safety/patient-safety-resources/resources/patient-safety-advisorycouncil/. Accessed March 31, 2014.
Intrauterine fetal demise: Care in the aftermath, and beyond
› Consider vaginal misoprostol for achieving delivery following intrauterine fetal demise (IUFD); it is as effective as other vaginal prostaglandin preparations and more effective than oral misoprostol. A
› Include in your postdelivery evaluation of IUFD autopsy, placental gross and histologic examination, fetal karyotype, and exam for fetomaternal hemorrhage. B
›Offer grieving parents early emotional support and counseling; research indicates it shortens the bereavement process. A
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
CASE › Louise T, age 26, is pregnant with her first child. She attends all prenatal care visits with her health care team and appears to be doing well. However, at Ms. T’s 28-week visit, her physician is unable to detect a fetal heartbeat, or any movement of the fetus. He orders an ultrasound, which confirms his suspicions. Ms. T opts for immediate induction of labor. In his postdelivery evaluation, Ms. T’s physician does not determine a definitive cause for the intrauterine fetal demise.
Intrauterine fetal demise (IUFD) is fetal death that occurs after 20 weeks gestation but before birth.1 If the gestational age is unknown at the time of death, a fetus that weighs ≥350 g is considered an IUFD. In 2005, IUFD occurred at a rate of 6.22 per 1000 pregnancies, which amounted to 25,894 deaths.1
Family physicians who provide obstetric care are likely to care for women who have experienced an IUFD. This article describes what that care should include.
Keep these risk factors in mind
IUFD has been attributed to an extensive range of risk factors and possible causes, including various maternal medical conditions, obstetric complications, and pathologic fetal or placental conditions (TABLE 1).2-8 The 2 most common risk factors—obesity (body mass index [BMI] >30) and smoking—are modifiable and increase the odds of IUFD approximately three-fold.2 Though less common, 2 other notable risk factors are lupus and chronic renal disease; their impact on IUFD risk varies depending on the severity of the disease.2 However, keep in mind that these factors may not be causal and that most pregnant women with these conditions will deliver healthy infants.
Induce labor, or wait?
A woman experiencing an IUFD is likely to seek care when she notices that the fetus isn’t moving or when she experiences contractions, loss of fluid, or vaginal bleeding. Alternatively, she could be asymptomatic, and it may be the physician who suspects IUFD when he/she is unable to hear fetal heart tones. The diagnosis is confirmed by the absence of fetal cardiac activity on ultrasound; physicians may wish to obtain a second ultrasound for confirmation of the diagnosis.
Once IUFD is confirmed, most women choose to immediately undergo induction of labor. However, some elect to wait for spontaneous labor. Approximately 84% to 90% of women will go into spontaneous labor within 2 weeks of fetal death.9 Unless there is a compelling indication for immediate delivery (eg, coagulopathy, evidence of intrauterine infection, preeclampsia), expectant management may be permitted.
If a mother chooses expectant management, she should undergo periodic followup exams to assess for abdominal pain, fever, bleeding, bruising, labor, and emotional lability.10 Tell patients to seek immediate care if they develop a fever, abdominal pain, foul-smelling or purulent vaginal discharge, moderate bleeding, or bruising, or if they go into labor.
Vaginal prostaglandin effectively induces labor
Options for labor induction include oral or vaginal prostaglandins, continuous oxytocin infusion, or mechanical dilation (cervical placement of laminaria or a Foley bulb). Factors that affect which method to use include concomitant maternal illness, gestational age, Bishop score, or the presence of a uterine scar from a previous Cesarean section or other surgery. A Cochrane review found vaginal misoprostol was as effective as other vaginal prostaglandin preparations (E2 and F2-alpha) and more effective than oral misoprostol in achieving delivery for second- and third-trimester terminations and fetal deaths.11 Due to the risk of uterine rupture, most experts advise against use of misoprostol for a woman with a previously scarred uterus at >24 to 26 weeks gestation.10,12 In this circumstance, consider mechanical dilation followed by oxytocin infusion.
Beyond 28 weeks gestation, misoprostol can be used to induce labor by following the standard protocols utilized for term pregnancies (TABLE 2).10,12 Some patients may require additional doses of misoprostol to complete the third stage of labor. Pain can be managed via narcotic patient controlled analgesia, periodic use of intravenous narcotics, or continuous epidural.
A systematic approach to postdelivery evaluation
Although 25% to 60% of IUFDs are classified as “unexplained,” in up to 40% of cases the lack of explanation may be due to an incomplete evaluation.2-4 When performing an evaluation, it is important to be systematic and not confuse association with causality. Kortweg et al13 analyzed laboratory studies obtained in the evaluation of 1025 fetal deaths in the Netherlands from 2002 to 2008. The most useful tests were placental examination, fetal autopsy, and fetal karyotype, which aided in assigning the cause of death in 96%, 73%, and 29% of cases, respectively. Compared to fetal karyotyping from postpartum tissue sampling, samples obtained via amniocentesis or chorionic villus sampling before inducing labor are much more successful in identifying the cause of death (85% vs 28%).14
Testing for fetomaternal hemorrhage, which is the cause of IUFD about 12% of the time, needs to be performed when IUFD is diagnosed.13 Additional laboratory testing may be helpful depending on the mother’s history or symptoms at the time of IUFD. For example, a maternal history of drug use, thyroid disease, diabetes mellitus, hypertension, venous thromboembolism, or febrile illness should prompt further studies (TABLE 3).10,12-15
How to help grieving parents
Mothers may feel tremendous guilt upon suffering an IUFD. When addressing grief, mourning, and bereavement after an IUFD, the goal is to support the parents through the grieving process and properly identify when grief becomes pathologic. Grief is pathologic when there is a prolonged response—usually longer than 6 months—and when it interferes with daily activities.16 In a prospective study, patient characteristics that affected the intensity of grief were advanced gestational age at loss, lack of children in the home, relatively older age, pre-loss neurotic personality, and pre-loss psychiatric symptoms.17
Unlike the grief experienced by parents who lose an infant or child, the grief experienced by parents who experience an IUFD may not be socially validated. Therefore, it is important for physicians to acknowledge that patients’ feelings of loss are legitimate. Additionally, reassure mothers that there was very likely nothing that she could have done to change the outcome, unless there is compelling evidence to the contrary (eg, drug abuse causing abruption and fetal demise). Avoid using phrases such as “you can always try again.”
In a study of 769 women who experienced an IUFD, many reported receiving “support” or “great support” from family members (91.7%), nurses (90%), and physicians (53.4%).18 Adequate health care support is associated with lower levels of depression and anxiety, but family support appears to be the most important.18 Women who are single, divorced, or widowed experience higher levels of depression after IUFD than those who are married or cohabitating.18
In a different study of support for women who experienced IUFD or death of their child shortly after birth, mothers did not blame doctors or feel that the doctors should make them feel better.19 However, they did want an explanation in simple language of what had gone wrong and for physicians to listen and accept their distress.19 This study also found that early support and counseling shortened the bereavement process.19 Half of women had symptoms of depression and anxiety 6 months after the death of their fetus or child and 20% had these symptoms at 14 months. Fathers, however, recovered more quickly—14% were symptomatic at 6 months.19
Managing a subsequent therapy
A retrospective cohort study found that for women who had experienced an IUFD, the odds ratio of having a recurrent IUFD was 1.94 (99% confidence interval, 1.29-2.92) compared to women who had a previous live birth.20 The risk of recurrent IUFD in a specific patient is related to the underlying pathophysiologic cause of the initial IUFD, and how that cause was addressed. For example, a patient who experienced an IUFD believed to be the result of uncontrolled diabetes can expect to have an improved outcome if her diabetes is brought under control before conceiving and she maintains control throughout the pregnancy. On the contrary, a patient who experienced an IUFD that was believed to be secondary to a nonmodifiable risk factor, such as lupus-induced chronic kidney disease, will continue to have a significant risk of recurrence of IUFD in the subsequent pregnancy.
When managing a subsequent pregnancy of a woman who has experienced an IUFD, review all data from the prior pregnancy as well as the mother’s medical conditions. Order any studies in TABLE 3 that were not previously obtained and are clinically indicated. If applicable, encourage the woman to quite smoking and achieve a healthy BMI (<25).
In addition to routine obstetric care measures, these women should be offered antepartum fetal surveillance starting at 32 weeks gestation, or one to 2 weeks before the gestational age of the fetus at the time of the previous IUFD (whichever is earlier), as well as serial ultrasonography starting at 28 weeks to assess for fetal growth restriction.12,21 Most experts advise delivery at 39 weeks unless indicated earlier.12
Psychological risks. IUFD is associated with posttraumatic stress disorder (PTSD) and anxiety in a subsequent pregnancy.22,23 Approximately 21% of women in one study met criteria for PTSD in the third trimester of the first subsequent pregnancy; this decreased to 4% at one year postpartum.22 Risk factors for PTSD and anxiety were conceiving within one year of IUFD and a perceived lack of support at time of loss.22,23 Additionally, women who said they had poor partner support at the time of IUFD were more likely to have more severe PTSD symptoms, such as recurring, involuntary distressing memories of the IUFD, 6 to 8 years later.24 Because women who become pregnant after having an IUFD are likely to be anxious, physicians should be aware that there may be “false alarms” during the course of these pregnancies.
CASE › Two years after experiencing an IUFD , Ms. T becomes pregnant. Her physician carefully reviews her medical records and begins fetal surveillance at 26 weeks gestation, including serial ultrasounds. Ms. T’s pregnancy and labor proceed without complications, and at 38 weeks, she delivers a healthy 6.3-lb. boy.
CORRESPONDENCE
Richard Temple, MD, Naval Hospital Camp Lejeune Family Medicine Residency, 100 Brewster Blvd., Camp Lejeune, NC 28547; [email protected]
ACKNOWLEDGMENT
The authors thank Anthony Viera, MD for his assistance in the preparation of this manuscript.
1. MacDorman MF, Kirmeyer S. Fetal and perinatal mortality, United States, 2005. Natl Vital Stat Rep. 2009;57:1-19.
2. Goldstein DP, Johnson JP, Reid DE. Management of intrauterine fetal death. Obstet Gynecol. 1963;21:523-529.
3. Fretts RC. Etiology and prevention of stillbirth. Am J Obstet Gynecol. 2005;193:1923-1935.
4. Huang DY, Usher RH, Kramer MS, et al. Determinants of unexplained antepartum fetal deaths. Obstet Gynecol. 2000;95:215-221.
5. Dudley DJ, Goldenberg R, Conway D, et al; Stillbirth Research Collaborative Network. A new system for determining the causes of stillbirth. Obstet Gynecol. 2010;116(2 pt 1):254-260.
6. Sims MA, Collins KA. Fetal death. A 10-year retrospective study. Am J Forensic Med Pathol. 2001;22:261-265.
7. Walsh CA, McMenamin MB, Foley ME, et al. Trends in intrapartum fetal death, 1979-2003. Am J Obstet Gynecol. 2008;198:47.e1-47.e7.
8. Smulian JC, Ananth CV, Vintzileos AM, et al. Fetal deaths in the United States. Influence of high-risk conditions and implications for management. Obstet Gynecol. 2002;100:1183-1189.
9. Silver RM. Fetal death. Obstet Gynecol. 2007;109:153-167.
10. Dodd JM, Crowther CA. Misoprostol for induction of labour to terminate pregnancy in the second or third trimester for women with a fetal anomaly or after intrauterine fetal death. Cochrane Database Syst Rev. 2010;(4):CD004901.
11. ACOG Practice Bulletin No. 102: management of stillbirth. Obstet Gynecol. 2009;113:748-761.
12. FrØen JF, Arnestad M, Frey K, et al. Risk factors for sudden intrauterine unexplained death: epidemiologic characteristics of singleton cases in Oslo, Norway, 1986-1995. Am J Obstet Gynecol. 2001;184:694-702.
13. Korteweg FJ, Erwich JJ, Timmer A, et al. Evaluation of 1025 fetal deaths: proposed diagnostic workup. Am J Obstet Gynecol. 2012;206:53.e1-53.e12.
14. Korteweg FJ, Bouman K, Erwich JJ, et al. Cytogenetic analysis after evaluation of 750 fetal deaths: proposal for diagnostic workup. Obstet Gynecol. 2008;111:865-874.
15. Silver RM, Varner MW, Reddy U, et al. Work-up of stillbirth: a review of the literature. Am J Obstet Gynecol. 2007;196:433-444.
16. Badenhorst W, Hughes P. Psychological aspects of perinatal loss. Best Pract Res Clin Obstet Gynaecol. 2007;21:249-259.
17. Janssen HJ, Cuisinier MC, de Graauw KP, et al. A prospective study of risk factors predicting grief intensity following pregnancy loss. Arch Gen Psychiatry. 1997;54:56-61.
18. Cacciatore J, Schnebly S, Froen JF. The effects of social support on maternal anxiety and depression after stillbirth. Health Soc Care Community. 2009;17:167-176.
19. Forrest GC, Standish E, Baum JD. Support after perinatal death: a study of support and counselling after perinatal bereavement. Br Med J (Clin Res Ed). 1982;285:1475-1479.
20. Bhattacharya S, Prescott GJ, Black M, et al. Recurrence risk of stillbirth in a second pregnancy. BJOG. 2010;117:1243-1247.
21. Reddy UM. Management of pregnancy after stillbirth. Clin Obstet Gynecol. 2010;53:700-709.
22. Turton P, Hughes P, Evans CD, et al. Incidence, correlates and predictors of post-traumatic stress disorder in the pregnancy after stillbirth. Br J Psychiatry. 2001;178:556-560.
23. Hughes PM, Turton P, Evans CD. Stillbirth as risk factor for depression and anxiety in the subsequent pregnancy: cohort study. BMJ. 1999;318:1721-1724.
24. Turton P, Evans C, Hughes P. Long-term psychosocial sequelae of stillbirth: phase II of a nested case-control cohort study. Arch Womens Ment Health. 2009;12:35-41.
› Consider vaginal misoprostol for achieving delivery following intrauterine fetal demise (IUFD); it is as effective as other vaginal prostaglandin preparations and more effective than oral misoprostol. A
› Include in your postdelivery evaluation of IUFD autopsy, placental gross and histologic examination, fetal karyotype, and exam for fetomaternal hemorrhage. B
›Offer grieving parents early emotional support and counseling; research indicates it shortens the bereavement process. A
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
CASE › Louise T, age 26, is pregnant with her first child. She attends all prenatal care visits with her health care team and appears to be doing well. However, at Ms. T’s 28-week visit, her physician is unable to detect a fetal heartbeat, or any movement of the fetus. He orders an ultrasound, which confirms his suspicions. Ms. T opts for immediate induction of labor. In his postdelivery evaluation, Ms. T’s physician does not determine a definitive cause for the intrauterine fetal demise.
Intrauterine fetal demise (IUFD) is fetal death that occurs after 20 weeks gestation but before birth.1 If the gestational age is unknown at the time of death, a fetus that weighs ≥350 g is considered an IUFD. In 2005, IUFD occurred at a rate of 6.22 per 1000 pregnancies, which amounted to 25,894 deaths.1
Family physicians who provide obstetric care are likely to care for women who have experienced an IUFD. This article describes what that care should include.
Keep these risk factors in mind
IUFD has been attributed to an extensive range of risk factors and possible causes, including various maternal medical conditions, obstetric complications, and pathologic fetal or placental conditions (TABLE 1).2-8 The 2 most common risk factors—obesity (body mass index [BMI] >30) and smoking—are modifiable and increase the odds of IUFD approximately three-fold.2 Though less common, 2 other notable risk factors are lupus and chronic renal disease; their impact on IUFD risk varies depending on the severity of the disease.2 However, keep in mind that these factors may not be causal and that most pregnant women with these conditions will deliver healthy infants.
Induce labor, or wait?
A woman experiencing an IUFD is likely to seek care when she notices that the fetus isn’t moving or when she experiences contractions, loss of fluid, or vaginal bleeding. Alternatively, she could be asymptomatic, and it may be the physician who suspects IUFD when he/she is unable to hear fetal heart tones. The diagnosis is confirmed by the absence of fetal cardiac activity on ultrasound; physicians may wish to obtain a second ultrasound for confirmation of the diagnosis.
Once IUFD is confirmed, most women choose to immediately undergo induction of labor. However, some elect to wait for spontaneous labor. Approximately 84% to 90% of women will go into spontaneous labor within 2 weeks of fetal death.9 Unless there is a compelling indication for immediate delivery (eg, coagulopathy, evidence of intrauterine infection, preeclampsia), expectant management may be permitted.
If a mother chooses expectant management, she should undergo periodic followup exams to assess for abdominal pain, fever, bleeding, bruising, labor, and emotional lability.10 Tell patients to seek immediate care if they develop a fever, abdominal pain, foul-smelling or purulent vaginal discharge, moderate bleeding, or bruising, or if they go into labor.
Vaginal prostaglandin effectively induces labor
Options for labor induction include oral or vaginal prostaglandins, continuous oxytocin infusion, or mechanical dilation (cervical placement of laminaria or a Foley bulb). Factors that affect which method to use include concomitant maternal illness, gestational age, Bishop score, or the presence of a uterine scar from a previous Cesarean section or other surgery. A Cochrane review found vaginal misoprostol was as effective as other vaginal prostaglandin preparations (E2 and F2-alpha) and more effective than oral misoprostol in achieving delivery for second- and third-trimester terminations and fetal deaths.11 Due to the risk of uterine rupture, most experts advise against use of misoprostol for a woman with a previously scarred uterus at >24 to 26 weeks gestation.10,12 In this circumstance, consider mechanical dilation followed by oxytocin infusion.
Beyond 28 weeks gestation, misoprostol can be used to induce labor by following the standard protocols utilized for term pregnancies (TABLE 2).10,12 Some patients may require additional doses of misoprostol to complete the third stage of labor. Pain can be managed via narcotic patient controlled analgesia, periodic use of intravenous narcotics, or continuous epidural.
A systematic approach to postdelivery evaluation
Although 25% to 60% of IUFDs are classified as “unexplained,” in up to 40% of cases the lack of explanation may be due to an incomplete evaluation.2-4 When performing an evaluation, it is important to be systematic and not confuse association with causality. Kortweg et al13 analyzed laboratory studies obtained in the evaluation of 1025 fetal deaths in the Netherlands from 2002 to 2008. The most useful tests were placental examination, fetal autopsy, and fetal karyotype, which aided in assigning the cause of death in 96%, 73%, and 29% of cases, respectively. Compared to fetal karyotyping from postpartum tissue sampling, samples obtained via amniocentesis or chorionic villus sampling before inducing labor are much more successful in identifying the cause of death (85% vs 28%).14
Testing for fetomaternal hemorrhage, which is the cause of IUFD about 12% of the time, needs to be performed when IUFD is diagnosed.13 Additional laboratory testing may be helpful depending on the mother’s history or symptoms at the time of IUFD. For example, a maternal history of drug use, thyroid disease, diabetes mellitus, hypertension, venous thromboembolism, or febrile illness should prompt further studies (TABLE 3).10,12-15
How to help grieving parents
Mothers may feel tremendous guilt upon suffering an IUFD. When addressing grief, mourning, and bereavement after an IUFD, the goal is to support the parents through the grieving process and properly identify when grief becomes pathologic. Grief is pathologic when there is a prolonged response—usually longer than 6 months—and when it interferes with daily activities.16 In a prospective study, patient characteristics that affected the intensity of grief were advanced gestational age at loss, lack of children in the home, relatively older age, pre-loss neurotic personality, and pre-loss psychiatric symptoms.17
Unlike the grief experienced by parents who lose an infant or child, the grief experienced by parents who experience an IUFD may not be socially validated. Therefore, it is important for physicians to acknowledge that patients’ feelings of loss are legitimate. Additionally, reassure mothers that there was very likely nothing that she could have done to change the outcome, unless there is compelling evidence to the contrary (eg, drug abuse causing abruption and fetal demise). Avoid using phrases such as “you can always try again.”
In a study of 769 women who experienced an IUFD, many reported receiving “support” or “great support” from family members (91.7%), nurses (90%), and physicians (53.4%).18 Adequate health care support is associated with lower levels of depression and anxiety, but family support appears to be the most important.18 Women who are single, divorced, or widowed experience higher levels of depression after IUFD than those who are married or cohabitating.18
In a different study of support for women who experienced IUFD or death of their child shortly after birth, mothers did not blame doctors or feel that the doctors should make them feel better.19 However, they did want an explanation in simple language of what had gone wrong and for physicians to listen and accept their distress.19 This study also found that early support and counseling shortened the bereavement process.19 Half of women had symptoms of depression and anxiety 6 months after the death of their fetus or child and 20% had these symptoms at 14 months. Fathers, however, recovered more quickly—14% were symptomatic at 6 months.19
Managing a subsequent therapy
A retrospective cohort study found that for women who had experienced an IUFD, the odds ratio of having a recurrent IUFD was 1.94 (99% confidence interval, 1.29-2.92) compared to women who had a previous live birth.20 The risk of recurrent IUFD in a specific patient is related to the underlying pathophysiologic cause of the initial IUFD, and how that cause was addressed. For example, a patient who experienced an IUFD believed to be the result of uncontrolled diabetes can expect to have an improved outcome if her diabetes is brought under control before conceiving and she maintains control throughout the pregnancy. On the contrary, a patient who experienced an IUFD that was believed to be secondary to a nonmodifiable risk factor, such as lupus-induced chronic kidney disease, will continue to have a significant risk of recurrence of IUFD in the subsequent pregnancy.
When managing a subsequent pregnancy of a woman who has experienced an IUFD, review all data from the prior pregnancy as well as the mother’s medical conditions. Order any studies in TABLE 3 that were not previously obtained and are clinically indicated. If applicable, encourage the woman to quite smoking and achieve a healthy BMI (<25).
In addition to routine obstetric care measures, these women should be offered antepartum fetal surveillance starting at 32 weeks gestation, or one to 2 weeks before the gestational age of the fetus at the time of the previous IUFD (whichever is earlier), as well as serial ultrasonography starting at 28 weeks to assess for fetal growth restriction.12,21 Most experts advise delivery at 39 weeks unless indicated earlier.12
Psychological risks. IUFD is associated with posttraumatic stress disorder (PTSD) and anxiety in a subsequent pregnancy.22,23 Approximately 21% of women in one study met criteria for PTSD in the third trimester of the first subsequent pregnancy; this decreased to 4% at one year postpartum.22 Risk factors for PTSD and anxiety were conceiving within one year of IUFD and a perceived lack of support at time of loss.22,23 Additionally, women who said they had poor partner support at the time of IUFD were more likely to have more severe PTSD symptoms, such as recurring, involuntary distressing memories of the IUFD, 6 to 8 years later.24 Because women who become pregnant after having an IUFD are likely to be anxious, physicians should be aware that there may be “false alarms” during the course of these pregnancies.
CASE › Two years after experiencing an IUFD , Ms. T becomes pregnant. Her physician carefully reviews her medical records and begins fetal surveillance at 26 weeks gestation, including serial ultrasounds. Ms. T’s pregnancy and labor proceed without complications, and at 38 weeks, she delivers a healthy 6.3-lb. boy.
CORRESPONDENCE
Richard Temple, MD, Naval Hospital Camp Lejeune Family Medicine Residency, 100 Brewster Blvd., Camp Lejeune, NC 28547; [email protected]
ACKNOWLEDGMENT
The authors thank Anthony Viera, MD for his assistance in the preparation of this manuscript.
› Consider vaginal misoprostol for achieving delivery following intrauterine fetal demise (IUFD); it is as effective as other vaginal prostaglandin preparations and more effective than oral misoprostol. A
› Include in your postdelivery evaluation of IUFD autopsy, placental gross and histologic examination, fetal karyotype, and exam for fetomaternal hemorrhage. B
›Offer grieving parents early emotional support and counseling; research indicates it shortens the bereavement process. A
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
CASE › Louise T, age 26, is pregnant with her first child. She attends all prenatal care visits with her health care team and appears to be doing well. However, at Ms. T’s 28-week visit, her physician is unable to detect a fetal heartbeat, or any movement of the fetus. He orders an ultrasound, which confirms his suspicions. Ms. T opts for immediate induction of labor. In his postdelivery evaluation, Ms. T’s physician does not determine a definitive cause for the intrauterine fetal demise.
Intrauterine fetal demise (IUFD) is fetal death that occurs after 20 weeks gestation but before birth.1 If the gestational age is unknown at the time of death, a fetus that weighs ≥350 g is considered an IUFD. In 2005, IUFD occurred at a rate of 6.22 per 1000 pregnancies, which amounted to 25,894 deaths.1
Family physicians who provide obstetric care are likely to care for women who have experienced an IUFD. This article describes what that care should include.
Keep these risk factors in mind
IUFD has been attributed to an extensive range of risk factors and possible causes, including various maternal medical conditions, obstetric complications, and pathologic fetal or placental conditions (TABLE 1).2-8 The 2 most common risk factors—obesity (body mass index [BMI] >30) and smoking—are modifiable and increase the odds of IUFD approximately three-fold.2 Though less common, 2 other notable risk factors are lupus and chronic renal disease; their impact on IUFD risk varies depending on the severity of the disease.2 However, keep in mind that these factors may not be causal and that most pregnant women with these conditions will deliver healthy infants.
Induce labor, or wait?
A woman experiencing an IUFD is likely to seek care when she notices that the fetus isn’t moving or when she experiences contractions, loss of fluid, or vaginal bleeding. Alternatively, she could be asymptomatic, and it may be the physician who suspects IUFD when he/she is unable to hear fetal heart tones. The diagnosis is confirmed by the absence of fetal cardiac activity on ultrasound; physicians may wish to obtain a second ultrasound for confirmation of the diagnosis.
Once IUFD is confirmed, most women choose to immediately undergo induction of labor. However, some elect to wait for spontaneous labor. Approximately 84% to 90% of women will go into spontaneous labor within 2 weeks of fetal death.9 Unless there is a compelling indication for immediate delivery (eg, coagulopathy, evidence of intrauterine infection, preeclampsia), expectant management may be permitted.
If a mother chooses expectant management, she should undergo periodic followup exams to assess for abdominal pain, fever, bleeding, bruising, labor, and emotional lability.10 Tell patients to seek immediate care if they develop a fever, abdominal pain, foul-smelling or purulent vaginal discharge, moderate bleeding, or bruising, or if they go into labor.
Vaginal prostaglandin effectively induces labor
Options for labor induction include oral or vaginal prostaglandins, continuous oxytocin infusion, or mechanical dilation (cervical placement of laminaria or a Foley bulb). Factors that affect which method to use include concomitant maternal illness, gestational age, Bishop score, or the presence of a uterine scar from a previous Cesarean section or other surgery. A Cochrane review found vaginal misoprostol was as effective as other vaginal prostaglandin preparations (E2 and F2-alpha) and more effective than oral misoprostol in achieving delivery for second- and third-trimester terminations and fetal deaths.11 Due to the risk of uterine rupture, most experts advise against use of misoprostol for a woman with a previously scarred uterus at >24 to 26 weeks gestation.10,12 In this circumstance, consider mechanical dilation followed by oxytocin infusion.
Beyond 28 weeks gestation, misoprostol can be used to induce labor by following the standard protocols utilized for term pregnancies (TABLE 2).10,12 Some patients may require additional doses of misoprostol to complete the third stage of labor. Pain can be managed via narcotic patient controlled analgesia, periodic use of intravenous narcotics, or continuous epidural.
A systematic approach to postdelivery evaluation
Although 25% to 60% of IUFDs are classified as “unexplained,” in up to 40% of cases the lack of explanation may be due to an incomplete evaluation.2-4 When performing an evaluation, it is important to be systematic and not confuse association with causality. Kortweg et al13 analyzed laboratory studies obtained in the evaluation of 1025 fetal deaths in the Netherlands from 2002 to 2008. The most useful tests were placental examination, fetal autopsy, and fetal karyotype, which aided in assigning the cause of death in 96%, 73%, and 29% of cases, respectively. Compared to fetal karyotyping from postpartum tissue sampling, samples obtained via amniocentesis or chorionic villus sampling before inducing labor are much more successful in identifying the cause of death (85% vs 28%).14
Testing for fetomaternal hemorrhage, which is the cause of IUFD about 12% of the time, needs to be performed when IUFD is diagnosed.13 Additional laboratory testing may be helpful depending on the mother’s history or symptoms at the time of IUFD. For example, a maternal history of drug use, thyroid disease, diabetes mellitus, hypertension, venous thromboembolism, or febrile illness should prompt further studies (TABLE 3).10,12-15
How to help grieving parents
Mothers may feel tremendous guilt upon suffering an IUFD. When addressing grief, mourning, and bereavement after an IUFD, the goal is to support the parents through the grieving process and properly identify when grief becomes pathologic. Grief is pathologic when there is a prolonged response—usually longer than 6 months—and when it interferes with daily activities.16 In a prospective study, patient characteristics that affected the intensity of grief were advanced gestational age at loss, lack of children in the home, relatively older age, pre-loss neurotic personality, and pre-loss psychiatric symptoms.17
Unlike the grief experienced by parents who lose an infant or child, the grief experienced by parents who experience an IUFD may not be socially validated. Therefore, it is important for physicians to acknowledge that patients’ feelings of loss are legitimate. Additionally, reassure mothers that there was very likely nothing that she could have done to change the outcome, unless there is compelling evidence to the contrary (eg, drug abuse causing abruption and fetal demise). Avoid using phrases such as “you can always try again.”
In a study of 769 women who experienced an IUFD, many reported receiving “support” or “great support” from family members (91.7%), nurses (90%), and physicians (53.4%).18 Adequate health care support is associated with lower levels of depression and anxiety, but family support appears to be the most important.18 Women who are single, divorced, or widowed experience higher levels of depression after IUFD than those who are married or cohabitating.18
In a different study of support for women who experienced IUFD or death of their child shortly after birth, mothers did not blame doctors or feel that the doctors should make them feel better.19 However, they did want an explanation in simple language of what had gone wrong and for physicians to listen and accept their distress.19 This study also found that early support and counseling shortened the bereavement process.19 Half of women had symptoms of depression and anxiety 6 months after the death of their fetus or child and 20% had these symptoms at 14 months. Fathers, however, recovered more quickly—14% were symptomatic at 6 months.19
Managing a subsequent therapy
A retrospective cohort study found that for women who had experienced an IUFD, the odds ratio of having a recurrent IUFD was 1.94 (99% confidence interval, 1.29-2.92) compared to women who had a previous live birth.20 The risk of recurrent IUFD in a specific patient is related to the underlying pathophysiologic cause of the initial IUFD, and how that cause was addressed. For example, a patient who experienced an IUFD believed to be the result of uncontrolled diabetes can expect to have an improved outcome if her diabetes is brought under control before conceiving and she maintains control throughout the pregnancy. On the contrary, a patient who experienced an IUFD that was believed to be secondary to a nonmodifiable risk factor, such as lupus-induced chronic kidney disease, will continue to have a significant risk of recurrence of IUFD in the subsequent pregnancy.
When managing a subsequent pregnancy of a woman who has experienced an IUFD, review all data from the prior pregnancy as well as the mother’s medical conditions. Order any studies in TABLE 3 that were not previously obtained and are clinically indicated. If applicable, encourage the woman to quite smoking and achieve a healthy BMI (<25).
In addition to routine obstetric care measures, these women should be offered antepartum fetal surveillance starting at 32 weeks gestation, or one to 2 weeks before the gestational age of the fetus at the time of the previous IUFD (whichever is earlier), as well as serial ultrasonography starting at 28 weeks to assess for fetal growth restriction.12,21 Most experts advise delivery at 39 weeks unless indicated earlier.12
Psychological risks. IUFD is associated with posttraumatic stress disorder (PTSD) and anxiety in a subsequent pregnancy.22,23 Approximately 21% of women in one study met criteria for PTSD in the third trimester of the first subsequent pregnancy; this decreased to 4% at one year postpartum.22 Risk factors for PTSD and anxiety were conceiving within one year of IUFD and a perceived lack of support at time of loss.22,23 Additionally, women who said they had poor partner support at the time of IUFD were more likely to have more severe PTSD symptoms, such as recurring, involuntary distressing memories of the IUFD, 6 to 8 years later.24 Because women who become pregnant after having an IUFD are likely to be anxious, physicians should be aware that there may be “false alarms” during the course of these pregnancies.
CASE › Two years after experiencing an IUFD , Ms. T becomes pregnant. Her physician carefully reviews her medical records and begins fetal surveillance at 26 weeks gestation, including serial ultrasounds. Ms. T’s pregnancy and labor proceed without complications, and at 38 weeks, she delivers a healthy 6.3-lb. boy.
CORRESPONDENCE
Richard Temple, MD, Naval Hospital Camp Lejeune Family Medicine Residency, 100 Brewster Blvd., Camp Lejeune, NC 28547; [email protected]
ACKNOWLEDGMENT
The authors thank Anthony Viera, MD for his assistance in the preparation of this manuscript.
1. MacDorman MF, Kirmeyer S. Fetal and perinatal mortality, United States, 2005. Natl Vital Stat Rep. 2009;57:1-19.
2. Goldstein DP, Johnson JP, Reid DE. Management of intrauterine fetal death. Obstet Gynecol. 1963;21:523-529.
3. Fretts RC. Etiology and prevention of stillbirth. Am J Obstet Gynecol. 2005;193:1923-1935.
4. Huang DY, Usher RH, Kramer MS, et al. Determinants of unexplained antepartum fetal deaths. Obstet Gynecol. 2000;95:215-221.
5. Dudley DJ, Goldenberg R, Conway D, et al; Stillbirth Research Collaborative Network. A new system for determining the causes of stillbirth. Obstet Gynecol. 2010;116(2 pt 1):254-260.
6. Sims MA, Collins KA. Fetal death. A 10-year retrospective study. Am J Forensic Med Pathol. 2001;22:261-265.
7. Walsh CA, McMenamin MB, Foley ME, et al. Trends in intrapartum fetal death, 1979-2003. Am J Obstet Gynecol. 2008;198:47.e1-47.e7.
8. Smulian JC, Ananth CV, Vintzileos AM, et al. Fetal deaths in the United States. Influence of high-risk conditions and implications for management. Obstet Gynecol. 2002;100:1183-1189.
9. Silver RM. Fetal death. Obstet Gynecol. 2007;109:153-167.
10. Dodd JM, Crowther CA. Misoprostol for induction of labour to terminate pregnancy in the second or third trimester for women with a fetal anomaly or after intrauterine fetal death. Cochrane Database Syst Rev. 2010;(4):CD004901.
11. ACOG Practice Bulletin No. 102: management of stillbirth. Obstet Gynecol. 2009;113:748-761.
12. FrØen JF, Arnestad M, Frey K, et al. Risk factors for sudden intrauterine unexplained death: epidemiologic characteristics of singleton cases in Oslo, Norway, 1986-1995. Am J Obstet Gynecol. 2001;184:694-702.
13. Korteweg FJ, Erwich JJ, Timmer A, et al. Evaluation of 1025 fetal deaths: proposed diagnostic workup. Am J Obstet Gynecol. 2012;206:53.e1-53.e12.
14. Korteweg FJ, Bouman K, Erwich JJ, et al. Cytogenetic analysis after evaluation of 750 fetal deaths: proposal for diagnostic workup. Obstet Gynecol. 2008;111:865-874.
15. Silver RM, Varner MW, Reddy U, et al. Work-up of stillbirth: a review of the literature. Am J Obstet Gynecol. 2007;196:433-444.
16. Badenhorst W, Hughes P. Psychological aspects of perinatal loss. Best Pract Res Clin Obstet Gynaecol. 2007;21:249-259.
17. Janssen HJ, Cuisinier MC, de Graauw KP, et al. A prospective study of risk factors predicting grief intensity following pregnancy loss. Arch Gen Psychiatry. 1997;54:56-61.
18. Cacciatore J, Schnebly S, Froen JF. The effects of social support on maternal anxiety and depression after stillbirth. Health Soc Care Community. 2009;17:167-176.
19. Forrest GC, Standish E, Baum JD. Support after perinatal death: a study of support and counselling after perinatal bereavement. Br Med J (Clin Res Ed). 1982;285:1475-1479.
20. Bhattacharya S, Prescott GJ, Black M, et al. Recurrence risk of stillbirth in a second pregnancy. BJOG. 2010;117:1243-1247.
21. Reddy UM. Management of pregnancy after stillbirth. Clin Obstet Gynecol. 2010;53:700-709.
22. Turton P, Hughes P, Evans CD, et al. Incidence, correlates and predictors of post-traumatic stress disorder in the pregnancy after stillbirth. Br J Psychiatry. 2001;178:556-560.
23. Hughes PM, Turton P, Evans CD. Stillbirth as risk factor for depression and anxiety in the subsequent pregnancy: cohort study. BMJ. 1999;318:1721-1724.
24. Turton P, Evans C, Hughes P. Long-term psychosocial sequelae of stillbirth: phase II of a nested case-control cohort study. Arch Womens Ment Health. 2009;12:35-41.
1. MacDorman MF, Kirmeyer S. Fetal and perinatal mortality, United States, 2005. Natl Vital Stat Rep. 2009;57:1-19.
2. Goldstein DP, Johnson JP, Reid DE. Management of intrauterine fetal death. Obstet Gynecol. 1963;21:523-529.
3. Fretts RC. Etiology and prevention of stillbirth. Am J Obstet Gynecol. 2005;193:1923-1935.
4. Huang DY, Usher RH, Kramer MS, et al. Determinants of unexplained antepartum fetal deaths. Obstet Gynecol. 2000;95:215-221.
5. Dudley DJ, Goldenberg R, Conway D, et al; Stillbirth Research Collaborative Network. A new system for determining the causes of stillbirth. Obstet Gynecol. 2010;116(2 pt 1):254-260.
6. Sims MA, Collins KA. Fetal death. A 10-year retrospective study. Am J Forensic Med Pathol. 2001;22:261-265.
7. Walsh CA, McMenamin MB, Foley ME, et al. Trends in intrapartum fetal death, 1979-2003. Am J Obstet Gynecol. 2008;198:47.e1-47.e7.
8. Smulian JC, Ananth CV, Vintzileos AM, et al. Fetal deaths in the United States. Influence of high-risk conditions and implications for management. Obstet Gynecol. 2002;100:1183-1189.
9. Silver RM. Fetal death. Obstet Gynecol. 2007;109:153-167.
10. Dodd JM, Crowther CA. Misoprostol for induction of labour to terminate pregnancy in the second or third trimester for women with a fetal anomaly or after intrauterine fetal death. Cochrane Database Syst Rev. 2010;(4):CD004901.
11. ACOG Practice Bulletin No. 102: management of stillbirth. Obstet Gynecol. 2009;113:748-761.
12. FrØen JF, Arnestad M, Frey K, et al. Risk factors for sudden intrauterine unexplained death: epidemiologic characteristics of singleton cases in Oslo, Norway, 1986-1995. Am J Obstet Gynecol. 2001;184:694-702.
13. Korteweg FJ, Erwich JJ, Timmer A, et al. Evaluation of 1025 fetal deaths: proposed diagnostic workup. Am J Obstet Gynecol. 2012;206:53.e1-53.e12.
14. Korteweg FJ, Bouman K, Erwich JJ, et al. Cytogenetic analysis after evaluation of 750 fetal deaths: proposal for diagnostic workup. Obstet Gynecol. 2008;111:865-874.
15. Silver RM, Varner MW, Reddy U, et al. Work-up of stillbirth: a review of the literature. Am J Obstet Gynecol. 2007;196:433-444.
16. Badenhorst W, Hughes P. Psychological aspects of perinatal loss. Best Pract Res Clin Obstet Gynaecol. 2007;21:249-259.
17. Janssen HJ, Cuisinier MC, de Graauw KP, et al. A prospective study of risk factors predicting grief intensity following pregnancy loss. Arch Gen Psychiatry. 1997;54:56-61.
18. Cacciatore J, Schnebly S, Froen JF. The effects of social support on maternal anxiety and depression after stillbirth. Health Soc Care Community. 2009;17:167-176.
19. Forrest GC, Standish E, Baum JD. Support after perinatal death: a study of support and counselling after perinatal bereavement. Br Med J (Clin Res Ed). 1982;285:1475-1479.
20. Bhattacharya S, Prescott GJ, Black M, et al. Recurrence risk of stillbirth in a second pregnancy. BJOG. 2010;117:1243-1247.
21. Reddy UM. Management of pregnancy after stillbirth. Clin Obstet Gynecol. 2010;53:700-709.
22. Turton P, Hughes P, Evans CD, et al. Incidence, correlates and predictors of post-traumatic stress disorder in the pregnancy after stillbirth. Br J Psychiatry. 2001;178:556-560.
23. Hughes PM, Turton P, Evans CD. Stillbirth as risk factor for depression and anxiety in the subsequent pregnancy: cohort study. BMJ. 1999;318:1721-1724.
24. Turton P, Evans C, Hughes P. Long-term psychosocial sequelae of stillbirth: phase II of a nested case-control cohort study. Arch Womens Ment Health. 2009;12:35-41.
Addressing the unique issues of student athletes with ADHD
› Schedule twice-monthly visits when prescribing a psychostimulant to assess symptom control, review adverse effects, and record blood pressure, pulse, height, and weight in determining the optimal dose. C
› Keep in mind that using a psychostimulant can put endurance athletes at risk for heat-related injury. C
› Advise college-bound athletes that the NCAA requires a therapeutic use exemption for those who take psychostimulant medications. C
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
The symptoms typical of attention-deficit/hyperactivity disorder (ADHD)—inability to focus concentration and maintain attention span, and associated hyperactivity—impair normal daily functioning and cause distress for affected individuals.1 For the student athlete with ADHD, sports are a natural outlet, fulfilling the need to be active. In the case of a developing child with ADHD, involvement in sports often is a haven from negative feedback that can occur in the classroom and an environment in which to experience success.
Symptoms of ADHD also may offer an advantage in sports. Impulsivity, or the ability to act without reflection, enables quick decision-making and the spontaneity required of a quarterback or point guard.2 Well-known athletes with ADHD have said that while tasks requiring long stretches of concentration are difficult, aspects of their sport involving instantaneous reactions help them to succeed. Evidence also shows a statistically significant decrease in markers of anxiety and depression among ADHD subjects with higher levels of sports participation.3
Given the positive experience sports can provide, children and adolescents with ADHD are likely to continue participating and be as large a segment of youth athletes as they are of the general population.2,4 Primary care providers often treat student athletes, and in this article we discuss the need for accurate diagnosis through comprehensive clinical evaluation, proper use of psychostimulant medication and other available treatments, and special health concerns for athletes who have ADHD.
Diagnosis: The need for awareness and accurate evaluation
The worldwide prevalence of ADHD is 5.3%.5 In the United States, it is 8.7% among adolescents and 4.4% among adults.6,7 One study of NFL athletes found that 14 of 159 players studied had either ADHD or a learning disability for a combined prevalence of 8.8%.8 ADHD is diagnosed 3 times more often in males than females9; however, studies have shown no gender effect on ADHD, and referral patterns contribute to the higher prevalence pattern for males.10
ADHD usually is diagnosed in childhood, but increasingly, it is not established until adolescence or adulthood.2,9 Although there is no age limit for the diagnosis, the Diagnostic and Statistical Manual of Mental Disorders (DSM-5) calls for the presence of some symptoms before age 12, and symptoms must cause impairment of functioning in multiple settings.1 While hyperactivity symptoms may decrease over time, a significant number of children and adolescents will experience inattention symptoms into adulthood.11 In fact, the disorder may not become evident until college entry, when academic demands overwhelm an individual’s usual coping strategies.2
Multiple reasons for an accurate diagnosis. Initiate evaluation for ADHD for any child 4 to 18 years of age who exhibits symptoms of inattention, hyperactivity, or impulsivity to such a degree that it causes distress or impairment at home, at school, or on the sports field.12 Making an accurate diagnosis of ADHD is vital in student athletes given that treatment, or lack thereof, may put their health at risk and adversely impact their academic and athletic performances. Diagnostic accuracy also aids in distinguishing the student athlete with a legitimate need for treatment from one who is fine and merely looking for a performance enhancer.9 Moreover, having a comprehensive assessment with diagnostic confirmation already in place when an individual enters college greatly facilitates completion of National Collegiate Athletic Association (NCAA) medical exemption documentation.
Essential diagnostic steps. The core clinical evaluation should cover the following:
• Ensure that DSM-5 criteria are met.
• Obtain objective reports to confirm the presence of symptoms in multiple settings. Commonly applied symptom assessment scales include the Brown, Vanderbilt, and Connors questionnaires administered to parents, teachers, and adolescent patients mature enough to complete a self-evaluation.
• Determine whether comorbid conditions are present.
• Rule out medical conditions that can mimic ADHD (eg, lead toxicity or thyroid disorder).
No neurocognitive or laboratory test for ADHD has sufficient sensitivity and specificity to qualify as a standard diagnostic test.2,13 In the future, advanced neuroimaging may provide a means of diagnosing ADHD. Functional magnetic resonance imaging has shown characteristic patterns of reduced activation in the basal ganglia, frontal lobe, and parietal lobes in patients with ADHD.14
The differential diagnosis for symptoms of inattention and hyperactivity is large (TABLE 1).1,3,4,6 Once underlying medical conditions have been ruled out, screen the patient for mental disorders, including depression and mood disorders, anxiety, and conduct disorders, before concluding that symptoms are likely due to ADHD. When compared with mood disorders, a patient with ADHD will have a persistent course of symptoms rather than periods of recurring and remitting symptoms.2 ADHD is a chronic condition that raises special health care concerns for children and adolescents.12 As many as two-thirds of children with ADHD have at least one coexisting neuropsychiatric condition, and symptoms may overlap, making for a significant diagnostic and management challenge.9 Difficult cases may necessitate consulting a specialist (psychiatrist, neurologist, or neuropsychologist) for guidance. Additionally, in ADHD youth the overall risk of developing a substance use disorder is twice that of children who do not have ADHD.2,15
Treatment: More than medication
Effective treatment for ADHD improves quality of life, decreases the rate of substance abuse, reduces errors when driving vehicles, and decreases the prevalence of comorbid psychological disorders.16,17 Pharmacologic and nonpharmacologic options are available. With athletes, it’s important to be aware of and consider alternatives to medication, particularly given the rules restricting the use of stimulant medication by the NCAA, International Olympic Committee (IOC), and the World Anti-Doping Agency (WADA). The IOC and WADA prohibit any use of stimulant medications, and the NCAA requires a therapeutic-use exemption (TUE) for athletes who take psychostimulant medications (detailed below).3,16
Nonpharmacologic treatment
Published guidelines on managing ADHD show greater agreement on pharmacologic treatment than on psychosocial interventions, based on strength of evidence.18 One evidence-based psychosocial intervention that has shown benefit is behavior therapy, which includes a broad set of specific interventions that modify physical and social environments to change behavior.19 Behavioral training, which primary care providers can introduce to parents, teachers, and coaches, involves the simple principles of reinforcing desired behavior through reward and ignoring undesired behavior to reduce or eliminate it. Consistent application of rewards or unresponsiveness helps patients increase attention to instructions, comply with rules, improve productivity, and decrease disruptive behavior.20
The athlete with ADHD will benefit from a structured environment and, depending on age and level of maturity, can be educated by coaches on self-management strategies such as time management, effective planning and organization, and avoidance of distractions.20 Exercise may help relieve subjective symptoms of ADHD and comorbid mood disorders, but evidence is insufficient to determine its direct impact on ADHD.
Pharmacologic treatment
Of the many available medications used to treat ADHD (TABLE 2),9,12,16,18,20,21 psychostimulants are most effective for reducing core symptoms of the disorder.22 It is estimated that 56% of patients with ADHD receive drug therapy, and most of these drugs are psychostimulants.16 These agents increase dopamine and norepinephrine concentrations in the brainstem, midbrain, and frontal cortex, which likely is responsible for increasing attention span and concentration.23 As judged by increased attention or decreased hyperactivity in a recent cohort-based study, the positive response rate to psychostimulants was 73.1%.24
Atomoxetine, a selective norepinephrine reuptake inhibitor, is the primary US Food and Drug Administration (FDA)-approved nonstimulant medication for the treatment of ADHD. In double-blind randomized trials, atomoxetine was roughly equivalent to psychostimulants in reducing target symptoms.21,25 Typically more expensive than psychostimulants, atomoxetine is an acceptable alternative and the more appropriate agent for the ADHD patient with a history of illicit substance abuse or the athlete whose sport bans the use of stimulant medications.
Medication adverse effects. Adverse effects common to psychostimulants are generally mild and include decreased appetite and sleep disturbances. Less common are nervousness, irritability, headache, and increased heart rate and blood pressure (BP).22 Overdose can result in drug-induced psychosis or cardiac arrest.26 Most of these effects are reversible or preventable through dose reduction, increasing the dosing interval, or changing time of dosing during the day. Linear growth rate deceleration in both height and weight may occur in children and adolescents, but this effect is thought to be small and reversible upon discontinuation of medication.27,28
Contraindications to using psychostimulant medications include symptomatic cardiovascular disease, structural heart disease, uncontrolled hypertension, hyperthyroidism, glaucoma, stimulant hypersensitivity, psychosis, and a history of drug dependence.29 Psychostimulants are Schedule II drugs, which means they pose a high potential for abuse and risk for development of physical dependence. The nonstimulant medications listed in TABLE 2 are not Schedule II drugs and, though not as efficacious, generally are safer and lack the adverse effects typically seen with psychostimulants. Atomoxetine, however, carries a black-box warning regarding the risk of suicidality in children and adolescents during the first month of treatment, and patients should be counseled accordingly. Long-term effects of ADHD medications, either adverse or positive, remain unknown; few studies have been done over a period longer than 24 months.25
Medication management
Psychostimulant therapy for ADHD has 3 essential stages: initiation/titration, maintenance, and termination.
With initiation and titration, determining the optimal dose requires twice monthly follow-up visits. With each visit, assess symptom control, review adverse effects, and record BP, pulse, height, and weight. The optimal dose is one at which target outcomes are achieved with minimal adverse effects. Long-acting agents are preferred to enhance compliance, ensure dosing consistency, and reduce abuse potential. If the desired outcome is not being achieved at the highest feasible dose, an alternative psychostimulant may be tried. If a desired response is still not achieved, reevaluate the diagnosis or consider the possibility of comorbid conditions or that the patient has stopped taking the medication.
During the maintenance stage, it is prudent to have monthly contact with the student athlete before writing refill prescription for a Schedule II medication.
Determining when to terminate treatment is a highly individualized decision that entails ongoing analysis of risks vs benefit.9,12,16,26,29 A student athlete’s diagnosis of ADHD might have been based on a positive response to medication in lieu of a comprehensive evaluation, which is regrettable. Response to medication cannot be used to confirm or refute a diagnosis of ADHD because psychostimulant medication will improve behavior in conditions other than ADHD, including learning disability and depression.22
Misuse of psychostimulants among athletes. Some athletes will use a psychostimulant primarily as an appetite suppressant for weight control. However, perceived ergogenic effects are what make psychostimulants especially problematic,16 and are the main reason they are banned from competitive sports. Potential performance enhancements include improved concentration and attention to tasks, increased aggression, decreased pain perception, and euphoria.
A 2006 NCAA study of substance abuse habits of college student athletes (reflecting 2005-2006 data) demonstrated the following findings concerning ergogenic use of psychostimulants:30
• Psychostimulant use has continually increased since 1997 among all student athletes.
• Psychostimulant use has increased across all divisions, with highest use in Division III.
• Psychostimulant use increased in all men’s sports except basketball, football, and swimming.
• Psychostimulant use increased in all women’s sports except tennis, gymnastics, soccer, and volleyball.
• Respondents who used stimulants said they did so to get more energy or to treat ADHD.
• Respondents who didn’t use stimulants said they were concerned about the effect on health, side effects, and going against personal beliefs.30 (The latter issue regarding why student athletes do or do not use specific substances is a focus of the 2012-2013 NCAA National Study of Substance Use Habits of College Student-Athletes, currently underway.)
The rise in the nonprescription use of Adderall among National Football League (NFL) players has become a hot topic. Regarded by the league as a game-day performance enhancer, it has been banned since 2006. Muddying the waters on the true prevalence of Adderall use is the NFL’s policy of silence on identifying the specific performance-enhancing drug that triggered suspension. Only the player, if he so chooses, can disclose the substance in question. It has become convenient for players to name Adderall as the culprit, as it lacks the stigma attached to anabolic steroids and human growth hormone. Whether the drug is being used for ergogenic purposes or as an easy alibi, or both, remains unclear.31
Competition restrictions and therapeutic-use exemption
At the college level and beyond, psychostimulant use is highly regulated in competitive sports. Primary care providers can be supportive by being mindful of existing restrictions when making treatment decisions, and by keeping detailed documentation as stipulated in NCAA policy that became effective on August 1, 2009.30
The policy requires student athletes with ADHD who take psychostimulant medication to provide “evidence that the student athlete has undergone clinical assessment to diagnose the disorder, is being monitored routinely with use of psychostimulant medication and has a current prescription on file.” If the diagnosis of ADHD was made in childhood, policy requires the student athlete to provide their institution with a copy of the comprehensive assessment, including history of treatment. If such documents are not available, then a comprehensive assessment, must be performed to establish the diagnosis.
At minimum, documentation must include a description of the evaluation process and assessment tool(s) used; a statement of the diagnosis; a history of ADHD treatment, both previous and ongoing; a statement that a nonbanned alternative ADHD medication has been considered, if a psychostimulant is currently prescribed; and a statement reflecting evidence of ongoing follow-up/medication monitoring.
If a psychostimulant medication is prescribed, NCAA regulations require that a TUE be included in the documentation. The NCAA asks only that the prescribing physician consider nonstimulants first; they do not require an initial trial of a nonstimulant medication.2,9,16 Per NCAA regulation the student athlete must undergo, at minimum, an annual clinical evaluation by the team physician. The NCAA Committee on Safeguards and Medical Aspects of Sports has issued a new mandatory reporting form that contains criteria, including any known history of substance abuse, to help differentiate legitimate use worthy of medical exemption from use that is abusive.32
The student athlete participating in events sanctioned by WADA or IOC must be aware that use of psychostimulant medication is prohibited in competition. The only FDA-approved ADHD medication allowed for use in competition by all governing bodies is atomoxetine. Encourage student athletes to check governing organization Web sites to review current restrictions on use of psychostimulants in competition. Psychostimulants are banned in all professional sports, though many allow a TUE (except the National Hockey League). The process of obtaining a TUE is rigorous, and Major League Baseball requires a second opinion.2,9,16,33
Specific health concerns for student athletes treated for ADHD
Sudden cardiac death (SCD) is rare among athletes and most often associated with congenital abnormalities affecting heart structure and electrical conduction.16 Although there have been reports of cardiac arrhythmias related to the use of psychostimulants, no compelling clinical evidence has demonstrated a higher incidence of SCD in pediatric ADHD patients treated with psychostimulants compared with the general population.34
The American Academy of Pediatrics, in a policy statement subsequently endorsed by the American Medical Society for Sports Medicine, does not support the routine use of electrocardiograms before initiating psychostimulant therapy.16,34
In light of the cardiovascular side effects of psychostimulants, it remains prudent to obtain a thorough cardiovascular history before starting medication. If no preexisting cardiac disease is identified, psychostimulants can be safely prescribed for the ADHD athlete without worry about the risk of SCD.34
Psychostimulants can confer risk of heat injury
Endurance ADHD athletes on psychostimulants may be at increased risk of heat injury when exercising in warm conditions. Evidence suggests that psychostimulants can increase core temperature while also masking signs and symptoms of fatigue, allowing for a longer duration of exercise and delayed time to exhaustion in the presence of elevated core temperature and heart rate.35
In one placebo-controlled trial of exercise under warm conditions, core temperature measurements in athletes taking 20 mg of methylphenidate often exceeded 104˚F, and the athletes experienced no change in their perception of effort or thermal stress.36 These factors raise concerns for increased risk of heat-related injury in the ADHD athlete taking psychostimulant medication. Close monitoring is required.
Psychostimulant medication, with its direct actions and adverse effects, has great potential for misuse, and the past 10 years have seen a surge in nonprescription stimulant use among adolescents and young adults.26 The reason most commonly given for using a stimulant is to enhance academic performance through improved alertness and sharpened focus.
Adderall is the psychostimulant most in demand as a “study drug.” Among college students, evidence suggests the individual most likely to misuse Adderall is white, male, affiliated with a formal fraternity, and more likely to use other illicit substances.26 Adding to the perpetuation of this phenomenon is that it is relatively stigma-free: Public opinion does not consistently condemn the use of Adderall for academic means, effectively legitimizing nonprescription use.
Very few universities have an academic policy associating nonprescription use of psychostimulants with cheating. The result is an unprecedented demand for psychostimulant medications,37 which are increasingly obtained through diversion by profiteering peers or from clinicians under false pretenses.26
To help curb the problem of misuse, consider stigmatizing such behavior and stress that, in addition to significant health risks associated with inappropriate use, the vast majority of evidence shows no cognitive enhancement with stimulants when compared with placebo in healthy individuals. Given that psychostimulant misuse is more common with an immediate-release formulation, one means of prevention is to restrict legitimate prescriptions to long-acting formulation as much as possible.38-40
Your role as the primary care provider
An optimal treatment plan for the ADHD athlete, especially one using a psychostimulant medication, should always be individualized. Many factors come into play: the nature of impairing symptoms, presence of comorbidities, and prior response to medication.
How the psychostimulant is taken also can vary depending on an athlete’s preference and the nature of the sport. For example, some athletes will take the medication only for academic purposes (studying, testing). Other athletes feel their sport performance improves while on psychostimulants (eg, a baseball catcher who requires game-long concentration), while yet others prefer not to take it during an event so they can remain unfocused, move randomly, and maintain spontaneity (as with a basketball point guard).
If psychostimulants are to be used while playing, it is wise not to initiate therapy during a high-stress event, such as a championship game. In addition, it is important to know when to withhold medication, as in the case of an endurance athlete competing in hot weather.
Coordinating all aspects of care
In providing the best care for the ADHD athlete, the primary care physician must possess comprehensive knowledge of evidence-based best practices. Educate yourself about all available therapies, including behavioral management and use of psychostimulants. And become familiar with available resources and with the referral network (eg, neuropsychologist).
Acknowledgement of NCAA regulations/restrictions is vital to making treatment decisions. In light of the many regulations (both governmental and within the competitive sporting world), consider the use of nonbanned medications and behavioral therapies whenever possible. Throughout the treatment process, involve all stakeholders—parents, athletic trainers, coaches, teachers—to sustain a collaborative approach to care.
Be attentive to signs of inappropriate use of psychostimulant medication (See “Anticipating and addressing the misuse of psychostimulants” above6,37-40). However, fear of potential misuse is not justification for withholding medication, especially when a clear indication is evident. Failure to recognize ADHD as a legitimate problem puts both academic and social hurdles in the path of the student athlete. Evidence shows that adequately treating ADHD with indicated pharmacotherapy actually reduces subsequent substance abuse.41 Finally, education of every ADHD athlete on existing restrictions/regulations/requirements as posed by governing bodies (NCAA, US Anti-Doping Agency, WADA, and IOC) is imperative.
CORRESPONDENCE
Adam E. Perrin, MD, Family Medicine Center at Asylum Hill, University of Connecticut School of Medicine, 99 Woodland Street, Hartford, CT 06105-1207; [email protected]
1. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th ed. Washington, DC: American Psychiatric Association Press; 2013.
2. Parr JW. Attention-deficit hyperactivity disorder and the athlete: new advances and understanding. Clin Sports Med. 2011;30:591-610.
3. Kiluk BD, Weden S, Culotta VP. Sport participation and anxiety in children with ADHD. J Atten Disord. 2009;12:499-506.
4. Broshek DK, Freeman JR. Psychiatric and neuropsychological issues in sports medicine. Clin Sports Med. 2005;24:663-679,x.
5. Polanczyk G, de Lima MS, Horta BL, et al. The worldwide prevalence of ADHD: a systematic review and metaregression analysis. Am J Psychiatry. 2006;164:942-948.
6. Merikangas KR, He JP, Burstein M, et al. Lifetime prevalence of mental disorders in US adolescents: results from the National Comorbidity Stud—Adolescent Supplement (NCS-A). J Am Acad Child Adolesc Psychiatry. 2010;49:980-989.
7. Kessler RC, Adler L, Barkley R, et al. The prevalence and correlates of adult ADHD in the United States: results from the National Comorbidity Survey Replication. Am J Psychiatry. 2006;163:716-723.
8. Solomon GS, Haase RF. Biopsychosocial characteristics and neurocognitive test performance in National Football League players: an initial assessment. Arch Clinical Neuropsychol. 2008;23:563-577.
9. Kutcher JS. Treatment of attention-deficit hyperactivity disorder in athletes. Curr Sports Med Reports. 2011;10:32-36.
10 Biederman J, Kwon A, Aleardi M, et al. Absence of gender effects on attention deficit hyperactivity disorder: findings in nonreferred subjects. Am J Psychiatry. 2005;162:1083-1089.
11. Wilens TE, Faraone SV, Biederman J. Attention-deficit/hyperactivity disorder in adults. JAMA. 2004;292:619-623.
12. Subcommittee on Attention-Deficit/Hyperactivity Disorder; Steering Committee on Quality Improvement and Management; Wolraich M, Brown L, Brown RT, et al. ADHD: clinical practice guideline for diagnosis, evaluation, and treatment of attentiondeficit/hyperactivity disorder in children and adolescents. Pediatrics. 2011;128:1007-1022.
13. Boonstra AM, Osterlaan J, Sergeant JA, et al. Executive functioning in adult ADHD: a meta-analytic review. Psychol Med. 2005;35:1097-1108.
14. Silk T, Vance A, Rinehart N, et al. Fronto-parietal activation in attention-deficit/hyperactivity disorder, combined type: functional magnetic resonance imaging study. Br J Psychiatry. 2005;187:282-283.
15. Biederman J, Wilens TE, Mick E, et al. Does attention-deficit hyperactivity disorder impact the developmental course of drug and alcohol abuse and dependence? Biol Psychiatry. 1998;44:269-273.
16. Putukian M, Kreher JB, Coppel DB, et al. Attention deficit hyperactivity disorder and the athlete: an American Medical Society for Sports Medicine position statement. Clin J Sport Med. 2011;21:392-401.
17. Agarwal R, Goldenberg M, Perry R, et al. The quality of life of adults with attention deficit disorder: a systematic review. Innov Clin Neurosci. 2012;9:10-21.
18. Seixas M, Weiss M, Müller U. Systematic review of national and international guidelines on attention-deficit hyperactivity disorder. J Psychopharmacol. 2012;26:753-765.
19. Pelham WE Jr, Fabiano GA. Evidence-based psychosocial treatments for attention-deficit/hyperactivity disorder. J Clin Child Adolesc Psychol. 2008;37:184-214.
20. Searight HR, Burke JM, Rottneck F. Adult ADHD: evaluation and treatment in family medicine. Am Fam Physician. 2000;62:2077-2086,2091-2092.
21. Krull KR. Attention-deficit hyperactivity disorder in children and adolescents: Treatment with medications. Available at: http://www.uptodate.com/contents/attention-deficit-hyperactivitydisorder-in-children-and-adolescents-treatment-with-medications. Accessed March 17, 2014.
22. Conant-Norville DO, Tofler IR. Attention deficit/hyperactivity disorder and psychopharmacologic treatments in the athlete. Clin Sports Med. 2005;24:829-843,viii.
23. Vaidya CJ, Austin G, Kirkorian G, et al. Selective effects of methylphenidate in attention deficit hyperactivity disorder: a functional magnetic resonance study. Proc Natl Acad Sci USA. 1998;95:14494-14499.
24. Barbaresi WJ, Katusic SK, Colligan RC, et al. Long-term stimulant medication treatment of attention-deficit/hyperactivity disorder: results from a population-based study. J Dev Behav Pediatr. 2006;27:1-10.
25. Hanwella R, Senanayake M, de Silva V. Comparative efficacy and acceptability of methylphenidate and atomoxetine in treatment of attention deficit hyperactivity disorder in children and adolescents: a meta-analysis. BMC Psychiatry. 2011;11:176.
26. Lakhan SE, Kirchgessner A. Prescription stimulants in individuals with and without attention deficit hyperactivity disorder: misuse, cognitive impact, and adverse effects. Brain Behav. 2012:2:661-677.
27. Biederman J, Spencer TJ, Monuteaux MC, et al. A naturalistic 10-year prospective study of height and weight in children with attention-deficit hyperactivity disorder grown up: sex and treatment effects. J Pediatr. 2010;157:635-640.
28. Goldman RD. ADHD stimulants and their effect on height in children. Can Fam Physician. 2010;56:145-146.
29. Conant-Norville DO, Tofler IR. Attention deficit/hyperactivity disorder and psychopharmacologic treatments in the athlete. Clin Sports Med. 2005;24:829-843,viii.
30. National Collegiate Athletic Association Web site. 2012-13 NCAA Sports Medicine Handbook. Available at: http://www.ncaa.org/sites/default/files/MD12.pdf. Accessed March 17, 2014.
31. Battista J. Adderall, a drug of focus, is often blamed as NFL suspensions rise. New York Times. December 2, 2012. Available at: http://www.nytimes.com/2012/12/02/sports/football/adderall-a-drug-of-increased-focus-for-nfl-players.html?pagewanted=all&_r=0. Accessed March 17, 2014.
32. CBS Sports Web site. NCAA medical exception documentation reporting form to support the diagnosis of attention deficit hyperactivity disorder (ADHD) and treatment with banned stimulant medication. Available at: http://grfx.cstv.com/photos/schools/grva/genrel/auto_pdf/2012-13/misc_non_event/adhdreporting.pdf. Accessed March 17, 2014.
33. World Anti-Doping Agency Web site. The 2013 list of prohibited substances and methods. Available at: http://www.wada-ama.org/en/Science-Medicine/Prohibited-List/. Accessed March 17, 2014.
34. Perrin JM, Friedman RA, Knilans TK; Black Box Working Group; Section on Cardiology and Cardiac Surgery. Cardiovascular monitoring and stimulant drugs for attention-deficit/hyperactivity disorder. Pediatrics. 2008;122:451-453.
35. Watson P, Hasegawa H, Roelands B, et al. Acute dopamine/noradrenaline reuptake inhibition enhances human exercise performance in warm, but not temperate conditions. J Physiol. 2005;565(pt 3):873-883.
36. Roelands B, Hasegawa H, Watson P, et al. The effects of acute dopamine reuptake inhibition on performance. Med Sci Sports Exerc. 2008;40:879-885.
37. Sinclair L. ADHD drugs on critical list as medication shortages soar. Psychiatric News. Available at: http://psychnews.psychiatryonline.org/newsarticle.aspx?articleid=481189. Accessed March 17, 2014.
38. Forlini C, Gauthier S, Racine E. Should physicians prescribe cognitive enhancers to healthy individuals? CMAJ. 2013;185:1047-1050.
39. McDuff DR, Baron D. Substance abuse in athletes: a sports psychiatry perspective. Clin Sports Med. 2005;24:885-897,ix-x.
40. Desantis AD, Hane AC. “Adderall is definitely not a drug”: justifications for the illegal use of ADHD stimulants. Subst Use Misuse. 2010;45:31-46.
41. Wilens TE, Faraone SV, Biederman J, et al. Does stimulant therapy of attention-deficit/hyperactivity disorder beget later substance abuse? A meta-analytic review of the literature. Pediatrics. 2003;111:179-185.
› Schedule twice-monthly visits when prescribing a psychostimulant to assess symptom control, review adverse effects, and record blood pressure, pulse, height, and weight in determining the optimal dose. C
› Keep in mind that using a psychostimulant can put endurance athletes at risk for heat-related injury. C
› Advise college-bound athletes that the NCAA requires a therapeutic use exemption for those who take psychostimulant medications. C
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
The symptoms typical of attention-deficit/hyperactivity disorder (ADHD)—inability to focus concentration and maintain attention span, and associated hyperactivity—impair normal daily functioning and cause distress for affected individuals.1 For the student athlete with ADHD, sports are a natural outlet, fulfilling the need to be active. In the case of a developing child with ADHD, involvement in sports often is a haven from negative feedback that can occur in the classroom and an environment in which to experience success.
Symptoms of ADHD also may offer an advantage in sports. Impulsivity, or the ability to act without reflection, enables quick decision-making and the spontaneity required of a quarterback or point guard.2 Well-known athletes with ADHD have said that while tasks requiring long stretches of concentration are difficult, aspects of their sport involving instantaneous reactions help them to succeed. Evidence also shows a statistically significant decrease in markers of anxiety and depression among ADHD subjects with higher levels of sports participation.3
Given the positive experience sports can provide, children and adolescents with ADHD are likely to continue participating and be as large a segment of youth athletes as they are of the general population.2,4 Primary care providers often treat student athletes, and in this article we discuss the need for accurate diagnosis through comprehensive clinical evaluation, proper use of psychostimulant medication and other available treatments, and special health concerns for athletes who have ADHD.
Diagnosis: The need for awareness and accurate evaluation
The worldwide prevalence of ADHD is 5.3%.5 In the United States, it is 8.7% among adolescents and 4.4% among adults.6,7 One study of NFL athletes found that 14 of 159 players studied had either ADHD or a learning disability for a combined prevalence of 8.8%.8 ADHD is diagnosed 3 times more often in males than females9; however, studies have shown no gender effect on ADHD, and referral patterns contribute to the higher prevalence pattern for males.10
ADHD usually is diagnosed in childhood, but increasingly, it is not established until adolescence or adulthood.2,9 Although there is no age limit for the diagnosis, the Diagnostic and Statistical Manual of Mental Disorders (DSM-5) calls for the presence of some symptoms before age 12, and symptoms must cause impairment of functioning in multiple settings.1 While hyperactivity symptoms may decrease over time, a significant number of children and adolescents will experience inattention symptoms into adulthood.11 In fact, the disorder may not become evident until college entry, when academic demands overwhelm an individual’s usual coping strategies.2
Multiple reasons for an accurate diagnosis. Initiate evaluation for ADHD for any child 4 to 18 years of age who exhibits symptoms of inattention, hyperactivity, or impulsivity to such a degree that it causes distress or impairment at home, at school, or on the sports field.12 Making an accurate diagnosis of ADHD is vital in student athletes given that treatment, or lack thereof, may put their health at risk and adversely impact their academic and athletic performances. Diagnostic accuracy also aids in distinguishing the student athlete with a legitimate need for treatment from one who is fine and merely looking for a performance enhancer.9 Moreover, having a comprehensive assessment with diagnostic confirmation already in place when an individual enters college greatly facilitates completion of National Collegiate Athletic Association (NCAA) medical exemption documentation.
Essential diagnostic steps. The core clinical evaluation should cover the following:
• Ensure that DSM-5 criteria are met.
• Obtain objective reports to confirm the presence of symptoms in multiple settings. Commonly applied symptom assessment scales include the Brown, Vanderbilt, and Connors questionnaires administered to parents, teachers, and adolescent patients mature enough to complete a self-evaluation.
• Determine whether comorbid conditions are present.
• Rule out medical conditions that can mimic ADHD (eg, lead toxicity or thyroid disorder).
No neurocognitive or laboratory test for ADHD has sufficient sensitivity and specificity to qualify as a standard diagnostic test.2,13 In the future, advanced neuroimaging may provide a means of diagnosing ADHD. Functional magnetic resonance imaging has shown characteristic patterns of reduced activation in the basal ganglia, frontal lobe, and parietal lobes in patients with ADHD.14
The differential diagnosis for symptoms of inattention and hyperactivity is large (TABLE 1).1,3,4,6 Once underlying medical conditions have been ruled out, screen the patient for mental disorders, including depression and mood disorders, anxiety, and conduct disorders, before concluding that symptoms are likely due to ADHD. When compared with mood disorders, a patient with ADHD will have a persistent course of symptoms rather than periods of recurring and remitting symptoms.2 ADHD is a chronic condition that raises special health care concerns for children and adolescents.12 As many as two-thirds of children with ADHD have at least one coexisting neuropsychiatric condition, and symptoms may overlap, making for a significant diagnostic and management challenge.9 Difficult cases may necessitate consulting a specialist (psychiatrist, neurologist, or neuropsychologist) for guidance. Additionally, in ADHD youth the overall risk of developing a substance use disorder is twice that of children who do not have ADHD.2,15
Treatment: More than medication
Effective treatment for ADHD improves quality of life, decreases the rate of substance abuse, reduces errors when driving vehicles, and decreases the prevalence of comorbid psychological disorders.16,17 Pharmacologic and nonpharmacologic options are available. With athletes, it’s important to be aware of and consider alternatives to medication, particularly given the rules restricting the use of stimulant medication by the NCAA, International Olympic Committee (IOC), and the World Anti-Doping Agency (WADA). The IOC and WADA prohibit any use of stimulant medications, and the NCAA requires a therapeutic-use exemption (TUE) for athletes who take psychostimulant medications (detailed below).3,16
Nonpharmacologic treatment
Published guidelines on managing ADHD show greater agreement on pharmacologic treatment than on psychosocial interventions, based on strength of evidence.18 One evidence-based psychosocial intervention that has shown benefit is behavior therapy, which includes a broad set of specific interventions that modify physical and social environments to change behavior.19 Behavioral training, which primary care providers can introduce to parents, teachers, and coaches, involves the simple principles of reinforcing desired behavior through reward and ignoring undesired behavior to reduce or eliminate it. Consistent application of rewards or unresponsiveness helps patients increase attention to instructions, comply with rules, improve productivity, and decrease disruptive behavior.20
The athlete with ADHD will benefit from a structured environment and, depending on age and level of maturity, can be educated by coaches on self-management strategies such as time management, effective planning and organization, and avoidance of distractions.20 Exercise may help relieve subjective symptoms of ADHD and comorbid mood disorders, but evidence is insufficient to determine its direct impact on ADHD.
Pharmacologic treatment
Of the many available medications used to treat ADHD (TABLE 2),9,12,16,18,20,21 psychostimulants are most effective for reducing core symptoms of the disorder.22 It is estimated that 56% of patients with ADHD receive drug therapy, and most of these drugs are psychostimulants.16 These agents increase dopamine and norepinephrine concentrations in the brainstem, midbrain, and frontal cortex, which likely is responsible for increasing attention span and concentration.23 As judged by increased attention or decreased hyperactivity in a recent cohort-based study, the positive response rate to psychostimulants was 73.1%.24
Atomoxetine, a selective norepinephrine reuptake inhibitor, is the primary US Food and Drug Administration (FDA)-approved nonstimulant medication for the treatment of ADHD. In double-blind randomized trials, atomoxetine was roughly equivalent to psychostimulants in reducing target symptoms.21,25 Typically more expensive than psychostimulants, atomoxetine is an acceptable alternative and the more appropriate agent for the ADHD patient with a history of illicit substance abuse or the athlete whose sport bans the use of stimulant medications.
Medication adverse effects. Adverse effects common to psychostimulants are generally mild and include decreased appetite and sleep disturbances. Less common are nervousness, irritability, headache, and increased heart rate and blood pressure (BP).22 Overdose can result in drug-induced psychosis or cardiac arrest.26 Most of these effects are reversible or preventable through dose reduction, increasing the dosing interval, or changing time of dosing during the day. Linear growth rate deceleration in both height and weight may occur in children and adolescents, but this effect is thought to be small and reversible upon discontinuation of medication.27,28
Contraindications to using psychostimulant medications include symptomatic cardiovascular disease, structural heart disease, uncontrolled hypertension, hyperthyroidism, glaucoma, stimulant hypersensitivity, psychosis, and a history of drug dependence.29 Psychostimulants are Schedule II drugs, which means they pose a high potential for abuse and risk for development of physical dependence. The nonstimulant medications listed in TABLE 2 are not Schedule II drugs and, though not as efficacious, generally are safer and lack the adverse effects typically seen with psychostimulants. Atomoxetine, however, carries a black-box warning regarding the risk of suicidality in children and adolescents during the first month of treatment, and patients should be counseled accordingly. Long-term effects of ADHD medications, either adverse or positive, remain unknown; few studies have been done over a period longer than 24 months.25
Medication management
Psychostimulant therapy for ADHD has 3 essential stages: initiation/titration, maintenance, and termination.
With initiation and titration, determining the optimal dose requires twice monthly follow-up visits. With each visit, assess symptom control, review adverse effects, and record BP, pulse, height, and weight. The optimal dose is one at which target outcomes are achieved with minimal adverse effects. Long-acting agents are preferred to enhance compliance, ensure dosing consistency, and reduce abuse potential. If the desired outcome is not being achieved at the highest feasible dose, an alternative psychostimulant may be tried. If a desired response is still not achieved, reevaluate the diagnosis or consider the possibility of comorbid conditions or that the patient has stopped taking the medication.
During the maintenance stage, it is prudent to have monthly contact with the student athlete before writing refill prescription for a Schedule II medication.
Determining when to terminate treatment is a highly individualized decision that entails ongoing analysis of risks vs benefit.9,12,16,26,29 A student athlete’s diagnosis of ADHD might have been based on a positive response to medication in lieu of a comprehensive evaluation, which is regrettable. Response to medication cannot be used to confirm or refute a diagnosis of ADHD because psychostimulant medication will improve behavior in conditions other than ADHD, including learning disability and depression.22
Misuse of psychostimulants among athletes. Some athletes will use a psychostimulant primarily as an appetite suppressant for weight control. However, perceived ergogenic effects are what make psychostimulants especially problematic,16 and are the main reason they are banned from competitive sports. Potential performance enhancements include improved concentration and attention to tasks, increased aggression, decreased pain perception, and euphoria.
A 2006 NCAA study of substance abuse habits of college student athletes (reflecting 2005-2006 data) demonstrated the following findings concerning ergogenic use of psychostimulants:30
• Psychostimulant use has continually increased since 1997 among all student athletes.
• Psychostimulant use has increased across all divisions, with highest use in Division III.
• Psychostimulant use increased in all men’s sports except basketball, football, and swimming.
• Psychostimulant use increased in all women’s sports except tennis, gymnastics, soccer, and volleyball.
• Respondents who used stimulants said they did so to get more energy or to treat ADHD.
• Respondents who didn’t use stimulants said they were concerned about the effect on health, side effects, and going against personal beliefs.30 (The latter issue regarding why student athletes do or do not use specific substances is a focus of the 2012-2013 NCAA National Study of Substance Use Habits of College Student-Athletes, currently underway.)
The rise in the nonprescription use of Adderall among National Football League (NFL) players has become a hot topic. Regarded by the league as a game-day performance enhancer, it has been banned since 2006. Muddying the waters on the true prevalence of Adderall use is the NFL’s policy of silence on identifying the specific performance-enhancing drug that triggered suspension. Only the player, if he so chooses, can disclose the substance in question. It has become convenient for players to name Adderall as the culprit, as it lacks the stigma attached to anabolic steroids and human growth hormone. Whether the drug is being used for ergogenic purposes or as an easy alibi, or both, remains unclear.31
Competition restrictions and therapeutic-use exemption
At the college level and beyond, psychostimulant use is highly regulated in competitive sports. Primary care providers can be supportive by being mindful of existing restrictions when making treatment decisions, and by keeping detailed documentation as stipulated in NCAA policy that became effective on August 1, 2009.30
The policy requires student athletes with ADHD who take psychostimulant medication to provide “evidence that the student athlete has undergone clinical assessment to diagnose the disorder, is being monitored routinely with use of psychostimulant medication and has a current prescription on file.” If the diagnosis of ADHD was made in childhood, policy requires the student athlete to provide their institution with a copy of the comprehensive assessment, including history of treatment. If such documents are not available, then a comprehensive assessment, must be performed to establish the diagnosis.
At minimum, documentation must include a description of the evaluation process and assessment tool(s) used; a statement of the diagnosis; a history of ADHD treatment, both previous and ongoing; a statement that a nonbanned alternative ADHD medication has been considered, if a psychostimulant is currently prescribed; and a statement reflecting evidence of ongoing follow-up/medication monitoring.
If a psychostimulant medication is prescribed, NCAA regulations require that a TUE be included in the documentation. The NCAA asks only that the prescribing physician consider nonstimulants first; they do not require an initial trial of a nonstimulant medication.2,9,16 Per NCAA regulation the student athlete must undergo, at minimum, an annual clinical evaluation by the team physician. The NCAA Committee on Safeguards and Medical Aspects of Sports has issued a new mandatory reporting form that contains criteria, including any known history of substance abuse, to help differentiate legitimate use worthy of medical exemption from use that is abusive.32
The student athlete participating in events sanctioned by WADA or IOC must be aware that use of psychostimulant medication is prohibited in competition. The only FDA-approved ADHD medication allowed for use in competition by all governing bodies is atomoxetine. Encourage student athletes to check governing organization Web sites to review current restrictions on use of psychostimulants in competition. Psychostimulants are banned in all professional sports, though many allow a TUE (except the National Hockey League). The process of obtaining a TUE is rigorous, and Major League Baseball requires a second opinion.2,9,16,33
Specific health concerns for student athletes treated for ADHD
Sudden cardiac death (SCD) is rare among athletes and most often associated with congenital abnormalities affecting heart structure and electrical conduction.16 Although there have been reports of cardiac arrhythmias related to the use of psychostimulants, no compelling clinical evidence has demonstrated a higher incidence of SCD in pediatric ADHD patients treated with psychostimulants compared with the general population.34
The American Academy of Pediatrics, in a policy statement subsequently endorsed by the American Medical Society for Sports Medicine, does not support the routine use of electrocardiograms before initiating psychostimulant therapy.16,34
In light of the cardiovascular side effects of psychostimulants, it remains prudent to obtain a thorough cardiovascular history before starting medication. If no preexisting cardiac disease is identified, psychostimulants can be safely prescribed for the ADHD athlete without worry about the risk of SCD.34
Psychostimulants can confer risk of heat injury
Endurance ADHD athletes on psychostimulants may be at increased risk of heat injury when exercising in warm conditions. Evidence suggests that psychostimulants can increase core temperature while also masking signs and symptoms of fatigue, allowing for a longer duration of exercise and delayed time to exhaustion in the presence of elevated core temperature and heart rate.35
In one placebo-controlled trial of exercise under warm conditions, core temperature measurements in athletes taking 20 mg of methylphenidate often exceeded 104˚F, and the athletes experienced no change in their perception of effort or thermal stress.36 These factors raise concerns for increased risk of heat-related injury in the ADHD athlete taking psychostimulant medication. Close monitoring is required.
Psychostimulant medication, with its direct actions and adverse effects, has great potential for misuse, and the past 10 years have seen a surge in nonprescription stimulant use among adolescents and young adults.26 The reason most commonly given for using a stimulant is to enhance academic performance through improved alertness and sharpened focus.
Adderall is the psychostimulant most in demand as a “study drug.” Among college students, evidence suggests the individual most likely to misuse Adderall is white, male, affiliated with a formal fraternity, and more likely to use other illicit substances.26 Adding to the perpetuation of this phenomenon is that it is relatively stigma-free: Public opinion does not consistently condemn the use of Adderall for academic means, effectively legitimizing nonprescription use.
Very few universities have an academic policy associating nonprescription use of psychostimulants with cheating. The result is an unprecedented demand for psychostimulant medications,37 which are increasingly obtained through diversion by profiteering peers or from clinicians under false pretenses.26
To help curb the problem of misuse, consider stigmatizing such behavior and stress that, in addition to significant health risks associated with inappropriate use, the vast majority of evidence shows no cognitive enhancement with stimulants when compared with placebo in healthy individuals. Given that psychostimulant misuse is more common with an immediate-release formulation, one means of prevention is to restrict legitimate prescriptions to long-acting formulation as much as possible.38-40
Your role as the primary care provider
An optimal treatment plan for the ADHD athlete, especially one using a psychostimulant medication, should always be individualized. Many factors come into play: the nature of impairing symptoms, presence of comorbidities, and prior response to medication.
How the psychostimulant is taken also can vary depending on an athlete’s preference and the nature of the sport. For example, some athletes will take the medication only for academic purposes (studying, testing). Other athletes feel their sport performance improves while on psychostimulants (eg, a baseball catcher who requires game-long concentration), while yet others prefer not to take it during an event so they can remain unfocused, move randomly, and maintain spontaneity (as with a basketball point guard).
If psychostimulants are to be used while playing, it is wise not to initiate therapy during a high-stress event, such as a championship game. In addition, it is important to know when to withhold medication, as in the case of an endurance athlete competing in hot weather.
Coordinating all aspects of care
In providing the best care for the ADHD athlete, the primary care physician must possess comprehensive knowledge of evidence-based best practices. Educate yourself about all available therapies, including behavioral management and use of psychostimulants. And become familiar with available resources and with the referral network (eg, neuropsychologist).
Acknowledgement of NCAA regulations/restrictions is vital to making treatment decisions. In light of the many regulations (both governmental and within the competitive sporting world), consider the use of nonbanned medications and behavioral therapies whenever possible. Throughout the treatment process, involve all stakeholders—parents, athletic trainers, coaches, teachers—to sustain a collaborative approach to care.
Be attentive to signs of inappropriate use of psychostimulant medication (See “Anticipating and addressing the misuse of psychostimulants” above6,37-40). However, fear of potential misuse is not justification for withholding medication, especially when a clear indication is evident. Failure to recognize ADHD as a legitimate problem puts both academic and social hurdles in the path of the student athlete. Evidence shows that adequately treating ADHD with indicated pharmacotherapy actually reduces subsequent substance abuse.41 Finally, education of every ADHD athlete on existing restrictions/regulations/requirements as posed by governing bodies (NCAA, US Anti-Doping Agency, WADA, and IOC) is imperative.
CORRESPONDENCE
Adam E. Perrin, MD, Family Medicine Center at Asylum Hill, University of Connecticut School of Medicine, 99 Woodland Street, Hartford, CT 06105-1207; [email protected]
› Schedule twice-monthly visits when prescribing a psychostimulant to assess symptom control, review adverse effects, and record blood pressure, pulse, height, and weight in determining the optimal dose. C
› Keep in mind that using a psychostimulant can put endurance athletes at risk for heat-related injury. C
› Advise college-bound athletes that the NCAA requires a therapeutic use exemption for those who take psychostimulant medications. C
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
The symptoms typical of attention-deficit/hyperactivity disorder (ADHD)—inability to focus concentration and maintain attention span, and associated hyperactivity—impair normal daily functioning and cause distress for affected individuals.1 For the student athlete with ADHD, sports are a natural outlet, fulfilling the need to be active. In the case of a developing child with ADHD, involvement in sports often is a haven from negative feedback that can occur in the classroom and an environment in which to experience success.
Symptoms of ADHD also may offer an advantage in sports. Impulsivity, or the ability to act without reflection, enables quick decision-making and the spontaneity required of a quarterback or point guard.2 Well-known athletes with ADHD have said that while tasks requiring long stretches of concentration are difficult, aspects of their sport involving instantaneous reactions help them to succeed. Evidence also shows a statistically significant decrease in markers of anxiety and depression among ADHD subjects with higher levels of sports participation.3
Given the positive experience sports can provide, children and adolescents with ADHD are likely to continue participating and be as large a segment of youth athletes as they are of the general population.2,4 Primary care providers often treat student athletes, and in this article we discuss the need for accurate diagnosis through comprehensive clinical evaluation, proper use of psychostimulant medication and other available treatments, and special health concerns for athletes who have ADHD.
Diagnosis: The need for awareness and accurate evaluation
The worldwide prevalence of ADHD is 5.3%.5 In the United States, it is 8.7% among adolescents and 4.4% among adults.6,7 One study of NFL athletes found that 14 of 159 players studied had either ADHD or a learning disability for a combined prevalence of 8.8%.8 ADHD is diagnosed 3 times more often in males than females9; however, studies have shown no gender effect on ADHD, and referral patterns contribute to the higher prevalence pattern for males.10
ADHD usually is diagnosed in childhood, but increasingly, it is not established until adolescence or adulthood.2,9 Although there is no age limit for the diagnosis, the Diagnostic and Statistical Manual of Mental Disorders (DSM-5) calls for the presence of some symptoms before age 12, and symptoms must cause impairment of functioning in multiple settings.1 While hyperactivity symptoms may decrease over time, a significant number of children and adolescents will experience inattention symptoms into adulthood.11 In fact, the disorder may not become evident until college entry, when academic demands overwhelm an individual’s usual coping strategies.2
Multiple reasons for an accurate diagnosis. Initiate evaluation for ADHD for any child 4 to 18 years of age who exhibits symptoms of inattention, hyperactivity, or impulsivity to such a degree that it causes distress or impairment at home, at school, or on the sports field.12 Making an accurate diagnosis of ADHD is vital in student athletes given that treatment, or lack thereof, may put their health at risk and adversely impact their academic and athletic performances. Diagnostic accuracy also aids in distinguishing the student athlete with a legitimate need for treatment from one who is fine and merely looking for a performance enhancer.9 Moreover, having a comprehensive assessment with diagnostic confirmation already in place when an individual enters college greatly facilitates completion of National Collegiate Athletic Association (NCAA) medical exemption documentation.
Essential diagnostic steps. The core clinical evaluation should cover the following:
• Ensure that DSM-5 criteria are met.
• Obtain objective reports to confirm the presence of symptoms in multiple settings. Commonly applied symptom assessment scales include the Brown, Vanderbilt, and Connors questionnaires administered to parents, teachers, and adolescent patients mature enough to complete a self-evaluation.
• Determine whether comorbid conditions are present.
• Rule out medical conditions that can mimic ADHD (eg, lead toxicity or thyroid disorder).
No neurocognitive or laboratory test for ADHD has sufficient sensitivity and specificity to qualify as a standard diagnostic test.2,13 In the future, advanced neuroimaging may provide a means of diagnosing ADHD. Functional magnetic resonance imaging has shown characteristic patterns of reduced activation in the basal ganglia, frontal lobe, and parietal lobes in patients with ADHD.14
The differential diagnosis for symptoms of inattention and hyperactivity is large (TABLE 1).1,3,4,6 Once underlying medical conditions have been ruled out, screen the patient for mental disorders, including depression and mood disorders, anxiety, and conduct disorders, before concluding that symptoms are likely due to ADHD. When compared with mood disorders, a patient with ADHD will have a persistent course of symptoms rather than periods of recurring and remitting symptoms.2 ADHD is a chronic condition that raises special health care concerns for children and adolescents.12 As many as two-thirds of children with ADHD have at least one coexisting neuropsychiatric condition, and symptoms may overlap, making for a significant diagnostic and management challenge.9 Difficult cases may necessitate consulting a specialist (psychiatrist, neurologist, or neuropsychologist) for guidance. Additionally, in ADHD youth the overall risk of developing a substance use disorder is twice that of children who do not have ADHD.2,15
Treatment: More than medication
Effective treatment for ADHD improves quality of life, decreases the rate of substance abuse, reduces errors when driving vehicles, and decreases the prevalence of comorbid psychological disorders.16,17 Pharmacologic and nonpharmacologic options are available. With athletes, it’s important to be aware of and consider alternatives to medication, particularly given the rules restricting the use of stimulant medication by the NCAA, International Olympic Committee (IOC), and the World Anti-Doping Agency (WADA). The IOC and WADA prohibit any use of stimulant medications, and the NCAA requires a therapeutic-use exemption (TUE) for athletes who take psychostimulant medications (detailed below).3,16
Nonpharmacologic treatment
Published guidelines on managing ADHD show greater agreement on pharmacologic treatment than on psychosocial interventions, based on strength of evidence.18 One evidence-based psychosocial intervention that has shown benefit is behavior therapy, which includes a broad set of specific interventions that modify physical and social environments to change behavior.19 Behavioral training, which primary care providers can introduce to parents, teachers, and coaches, involves the simple principles of reinforcing desired behavior through reward and ignoring undesired behavior to reduce or eliminate it. Consistent application of rewards or unresponsiveness helps patients increase attention to instructions, comply with rules, improve productivity, and decrease disruptive behavior.20
The athlete with ADHD will benefit from a structured environment and, depending on age and level of maturity, can be educated by coaches on self-management strategies such as time management, effective planning and organization, and avoidance of distractions.20 Exercise may help relieve subjective symptoms of ADHD and comorbid mood disorders, but evidence is insufficient to determine its direct impact on ADHD.
Pharmacologic treatment
Of the many available medications used to treat ADHD (TABLE 2),9,12,16,18,20,21 psychostimulants are most effective for reducing core symptoms of the disorder.22 It is estimated that 56% of patients with ADHD receive drug therapy, and most of these drugs are psychostimulants.16 These agents increase dopamine and norepinephrine concentrations in the brainstem, midbrain, and frontal cortex, which likely is responsible for increasing attention span and concentration.23 As judged by increased attention or decreased hyperactivity in a recent cohort-based study, the positive response rate to psychostimulants was 73.1%.24
Atomoxetine, a selective norepinephrine reuptake inhibitor, is the primary US Food and Drug Administration (FDA)-approved nonstimulant medication for the treatment of ADHD. In double-blind randomized trials, atomoxetine was roughly equivalent to psychostimulants in reducing target symptoms.21,25 Typically more expensive than psychostimulants, atomoxetine is an acceptable alternative and the more appropriate agent for the ADHD patient with a history of illicit substance abuse or the athlete whose sport bans the use of stimulant medications.
Medication adverse effects. Adverse effects common to psychostimulants are generally mild and include decreased appetite and sleep disturbances. Less common are nervousness, irritability, headache, and increased heart rate and blood pressure (BP).22 Overdose can result in drug-induced psychosis or cardiac arrest.26 Most of these effects are reversible or preventable through dose reduction, increasing the dosing interval, or changing time of dosing during the day. Linear growth rate deceleration in both height and weight may occur in children and adolescents, but this effect is thought to be small and reversible upon discontinuation of medication.27,28
Contraindications to using psychostimulant medications include symptomatic cardiovascular disease, structural heart disease, uncontrolled hypertension, hyperthyroidism, glaucoma, stimulant hypersensitivity, psychosis, and a history of drug dependence.29 Psychostimulants are Schedule II drugs, which means they pose a high potential for abuse and risk for development of physical dependence. The nonstimulant medications listed in TABLE 2 are not Schedule II drugs and, though not as efficacious, generally are safer and lack the adverse effects typically seen with psychostimulants. Atomoxetine, however, carries a black-box warning regarding the risk of suicidality in children and adolescents during the first month of treatment, and patients should be counseled accordingly. Long-term effects of ADHD medications, either adverse or positive, remain unknown; few studies have been done over a period longer than 24 months.25
Medication management
Psychostimulant therapy for ADHD has 3 essential stages: initiation/titration, maintenance, and termination.
With initiation and titration, determining the optimal dose requires twice monthly follow-up visits. With each visit, assess symptom control, review adverse effects, and record BP, pulse, height, and weight. The optimal dose is one at which target outcomes are achieved with minimal adverse effects. Long-acting agents are preferred to enhance compliance, ensure dosing consistency, and reduce abuse potential. If the desired outcome is not being achieved at the highest feasible dose, an alternative psychostimulant may be tried. If a desired response is still not achieved, reevaluate the diagnosis or consider the possibility of comorbid conditions or that the patient has stopped taking the medication.
During the maintenance stage, it is prudent to have monthly contact with the student athlete before writing refill prescription for a Schedule II medication.
Determining when to terminate treatment is a highly individualized decision that entails ongoing analysis of risks vs benefit.9,12,16,26,29 A student athlete’s diagnosis of ADHD might have been based on a positive response to medication in lieu of a comprehensive evaluation, which is regrettable. Response to medication cannot be used to confirm or refute a diagnosis of ADHD because psychostimulant medication will improve behavior in conditions other than ADHD, including learning disability and depression.22
Misuse of psychostimulants among athletes. Some athletes will use a psychostimulant primarily as an appetite suppressant for weight control. However, perceived ergogenic effects are what make psychostimulants especially problematic,16 and are the main reason they are banned from competitive sports. Potential performance enhancements include improved concentration and attention to tasks, increased aggression, decreased pain perception, and euphoria.
A 2006 NCAA study of substance abuse habits of college student athletes (reflecting 2005-2006 data) demonstrated the following findings concerning ergogenic use of psychostimulants:30
• Psychostimulant use has continually increased since 1997 among all student athletes.
• Psychostimulant use has increased across all divisions, with highest use in Division III.
• Psychostimulant use increased in all men’s sports except basketball, football, and swimming.
• Psychostimulant use increased in all women’s sports except tennis, gymnastics, soccer, and volleyball.
• Respondents who used stimulants said they did so to get more energy or to treat ADHD.
• Respondents who didn’t use stimulants said they were concerned about the effect on health, side effects, and going against personal beliefs.30 (The latter issue regarding why student athletes do or do not use specific substances is a focus of the 2012-2013 NCAA National Study of Substance Use Habits of College Student-Athletes, currently underway.)
The rise in the nonprescription use of Adderall among National Football League (NFL) players has become a hot topic. Regarded by the league as a game-day performance enhancer, it has been banned since 2006. Muddying the waters on the true prevalence of Adderall use is the NFL’s policy of silence on identifying the specific performance-enhancing drug that triggered suspension. Only the player, if he so chooses, can disclose the substance in question. It has become convenient for players to name Adderall as the culprit, as it lacks the stigma attached to anabolic steroids and human growth hormone. Whether the drug is being used for ergogenic purposes or as an easy alibi, or both, remains unclear.31
Competition restrictions and therapeutic-use exemption
At the college level and beyond, psychostimulant use is highly regulated in competitive sports. Primary care providers can be supportive by being mindful of existing restrictions when making treatment decisions, and by keeping detailed documentation as stipulated in NCAA policy that became effective on August 1, 2009.30
The policy requires student athletes with ADHD who take psychostimulant medication to provide “evidence that the student athlete has undergone clinical assessment to diagnose the disorder, is being monitored routinely with use of psychostimulant medication and has a current prescription on file.” If the diagnosis of ADHD was made in childhood, policy requires the student athlete to provide their institution with a copy of the comprehensive assessment, including history of treatment. If such documents are not available, then a comprehensive assessment, must be performed to establish the diagnosis.
At minimum, documentation must include a description of the evaluation process and assessment tool(s) used; a statement of the diagnosis; a history of ADHD treatment, both previous and ongoing; a statement that a nonbanned alternative ADHD medication has been considered, if a psychostimulant is currently prescribed; and a statement reflecting evidence of ongoing follow-up/medication monitoring.
If a psychostimulant medication is prescribed, NCAA regulations require that a TUE be included in the documentation. The NCAA asks only that the prescribing physician consider nonstimulants first; they do not require an initial trial of a nonstimulant medication.2,9,16 Per NCAA regulation the student athlete must undergo, at minimum, an annual clinical evaluation by the team physician. The NCAA Committee on Safeguards and Medical Aspects of Sports has issued a new mandatory reporting form that contains criteria, including any known history of substance abuse, to help differentiate legitimate use worthy of medical exemption from use that is abusive.32
The student athlete participating in events sanctioned by WADA or IOC must be aware that use of psychostimulant medication is prohibited in competition. The only FDA-approved ADHD medication allowed for use in competition by all governing bodies is atomoxetine. Encourage student athletes to check governing organization Web sites to review current restrictions on use of psychostimulants in competition. Psychostimulants are banned in all professional sports, though many allow a TUE (except the National Hockey League). The process of obtaining a TUE is rigorous, and Major League Baseball requires a second opinion.2,9,16,33
Specific health concerns for student athletes treated for ADHD
Sudden cardiac death (SCD) is rare among athletes and most often associated with congenital abnormalities affecting heart structure and electrical conduction.16 Although there have been reports of cardiac arrhythmias related to the use of psychostimulants, no compelling clinical evidence has demonstrated a higher incidence of SCD in pediatric ADHD patients treated with psychostimulants compared with the general population.34
The American Academy of Pediatrics, in a policy statement subsequently endorsed by the American Medical Society for Sports Medicine, does not support the routine use of electrocardiograms before initiating psychostimulant therapy.16,34
In light of the cardiovascular side effects of psychostimulants, it remains prudent to obtain a thorough cardiovascular history before starting medication. If no preexisting cardiac disease is identified, psychostimulants can be safely prescribed for the ADHD athlete without worry about the risk of SCD.34
Psychostimulants can confer risk of heat injury
Endurance ADHD athletes on psychostimulants may be at increased risk of heat injury when exercising in warm conditions. Evidence suggests that psychostimulants can increase core temperature while also masking signs and symptoms of fatigue, allowing for a longer duration of exercise and delayed time to exhaustion in the presence of elevated core temperature and heart rate.35
In one placebo-controlled trial of exercise under warm conditions, core temperature measurements in athletes taking 20 mg of methylphenidate often exceeded 104˚F, and the athletes experienced no change in their perception of effort or thermal stress.36 These factors raise concerns for increased risk of heat-related injury in the ADHD athlete taking psychostimulant medication. Close monitoring is required.
Psychostimulant medication, with its direct actions and adverse effects, has great potential for misuse, and the past 10 years have seen a surge in nonprescription stimulant use among adolescents and young adults.26 The reason most commonly given for using a stimulant is to enhance academic performance through improved alertness and sharpened focus.
Adderall is the psychostimulant most in demand as a “study drug.” Among college students, evidence suggests the individual most likely to misuse Adderall is white, male, affiliated with a formal fraternity, and more likely to use other illicit substances.26 Adding to the perpetuation of this phenomenon is that it is relatively stigma-free: Public opinion does not consistently condemn the use of Adderall for academic means, effectively legitimizing nonprescription use.
Very few universities have an academic policy associating nonprescription use of psychostimulants with cheating. The result is an unprecedented demand for psychostimulant medications,37 which are increasingly obtained through diversion by profiteering peers or from clinicians under false pretenses.26
To help curb the problem of misuse, consider stigmatizing such behavior and stress that, in addition to significant health risks associated with inappropriate use, the vast majority of evidence shows no cognitive enhancement with stimulants when compared with placebo in healthy individuals. Given that psychostimulant misuse is more common with an immediate-release formulation, one means of prevention is to restrict legitimate prescriptions to long-acting formulation as much as possible.38-40
Your role as the primary care provider
An optimal treatment plan for the ADHD athlete, especially one using a psychostimulant medication, should always be individualized. Many factors come into play: the nature of impairing symptoms, presence of comorbidities, and prior response to medication.
How the psychostimulant is taken also can vary depending on an athlete’s preference and the nature of the sport. For example, some athletes will take the medication only for academic purposes (studying, testing). Other athletes feel their sport performance improves while on psychostimulants (eg, a baseball catcher who requires game-long concentration), while yet others prefer not to take it during an event so they can remain unfocused, move randomly, and maintain spontaneity (as with a basketball point guard).
If psychostimulants are to be used while playing, it is wise not to initiate therapy during a high-stress event, such as a championship game. In addition, it is important to know when to withhold medication, as in the case of an endurance athlete competing in hot weather.
Coordinating all aspects of care
In providing the best care for the ADHD athlete, the primary care physician must possess comprehensive knowledge of evidence-based best practices. Educate yourself about all available therapies, including behavioral management and use of psychostimulants. And become familiar with available resources and with the referral network (eg, neuropsychologist).
Acknowledgement of NCAA regulations/restrictions is vital to making treatment decisions. In light of the many regulations (both governmental and within the competitive sporting world), consider the use of nonbanned medications and behavioral therapies whenever possible. Throughout the treatment process, involve all stakeholders—parents, athletic trainers, coaches, teachers—to sustain a collaborative approach to care.
Be attentive to signs of inappropriate use of psychostimulant medication (See “Anticipating and addressing the misuse of psychostimulants” above6,37-40). However, fear of potential misuse is not justification for withholding medication, especially when a clear indication is evident. Failure to recognize ADHD as a legitimate problem puts both academic and social hurdles in the path of the student athlete. Evidence shows that adequately treating ADHD with indicated pharmacotherapy actually reduces subsequent substance abuse.41 Finally, education of every ADHD athlete on existing restrictions/regulations/requirements as posed by governing bodies (NCAA, US Anti-Doping Agency, WADA, and IOC) is imperative.
CORRESPONDENCE
Adam E. Perrin, MD, Family Medicine Center at Asylum Hill, University of Connecticut School of Medicine, 99 Woodland Street, Hartford, CT 06105-1207; [email protected]
1. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th ed. Washington, DC: American Psychiatric Association Press; 2013.
2. Parr JW. Attention-deficit hyperactivity disorder and the athlete: new advances and understanding. Clin Sports Med. 2011;30:591-610.
3. Kiluk BD, Weden S, Culotta VP. Sport participation and anxiety in children with ADHD. J Atten Disord. 2009;12:499-506.
4. Broshek DK, Freeman JR. Psychiatric and neuropsychological issues in sports medicine. Clin Sports Med. 2005;24:663-679,x.
5. Polanczyk G, de Lima MS, Horta BL, et al. The worldwide prevalence of ADHD: a systematic review and metaregression analysis. Am J Psychiatry. 2006;164:942-948.
6. Merikangas KR, He JP, Burstein M, et al. Lifetime prevalence of mental disorders in US adolescents: results from the National Comorbidity Stud—Adolescent Supplement (NCS-A). J Am Acad Child Adolesc Psychiatry. 2010;49:980-989.
7. Kessler RC, Adler L, Barkley R, et al. The prevalence and correlates of adult ADHD in the United States: results from the National Comorbidity Survey Replication. Am J Psychiatry. 2006;163:716-723.
8. Solomon GS, Haase RF. Biopsychosocial characteristics and neurocognitive test performance in National Football League players: an initial assessment. Arch Clinical Neuropsychol. 2008;23:563-577.
9. Kutcher JS. Treatment of attention-deficit hyperactivity disorder in athletes. Curr Sports Med Reports. 2011;10:32-36.
10 Biederman J, Kwon A, Aleardi M, et al. Absence of gender effects on attention deficit hyperactivity disorder: findings in nonreferred subjects. Am J Psychiatry. 2005;162:1083-1089.
11. Wilens TE, Faraone SV, Biederman J. Attention-deficit/hyperactivity disorder in adults. JAMA. 2004;292:619-623.
12. Subcommittee on Attention-Deficit/Hyperactivity Disorder; Steering Committee on Quality Improvement and Management; Wolraich M, Brown L, Brown RT, et al. ADHD: clinical practice guideline for diagnosis, evaluation, and treatment of attentiondeficit/hyperactivity disorder in children and adolescents. Pediatrics. 2011;128:1007-1022.
13. Boonstra AM, Osterlaan J, Sergeant JA, et al. Executive functioning in adult ADHD: a meta-analytic review. Psychol Med. 2005;35:1097-1108.
14. Silk T, Vance A, Rinehart N, et al. Fronto-parietal activation in attention-deficit/hyperactivity disorder, combined type: functional magnetic resonance imaging study. Br J Psychiatry. 2005;187:282-283.
15. Biederman J, Wilens TE, Mick E, et al. Does attention-deficit hyperactivity disorder impact the developmental course of drug and alcohol abuse and dependence? Biol Psychiatry. 1998;44:269-273.
16. Putukian M, Kreher JB, Coppel DB, et al. Attention deficit hyperactivity disorder and the athlete: an American Medical Society for Sports Medicine position statement. Clin J Sport Med. 2011;21:392-401.
17. Agarwal R, Goldenberg M, Perry R, et al. The quality of life of adults with attention deficit disorder: a systematic review. Innov Clin Neurosci. 2012;9:10-21.
18. Seixas M, Weiss M, Müller U. Systematic review of national and international guidelines on attention-deficit hyperactivity disorder. J Psychopharmacol. 2012;26:753-765.
19. Pelham WE Jr, Fabiano GA. Evidence-based psychosocial treatments for attention-deficit/hyperactivity disorder. J Clin Child Adolesc Psychol. 2008;37:184-214.
20. Searight HR, Burke JM, Rottneck F. Adult ADHD: evaluation and treatment in family medicine. Am Fam Physician. 2000;62:2077-2086,2091-2092.
21. Krull KR. Attention-deficit hyperactivity disorder in children and adolescents: Treatment with medications. Available at: http://www.uptodate.com/contents/attention-deficit-hyperactivitydisorder-in-children-and-adolescents-treatment-with-medications. Accessed March 17, 2014.
22. Conant-Norville DO, Tofler IR. Attention deficit/hyperactivity disorder and psychopharmacologic treatments in the athlete. Clin Sports Med. 2005;24:829-843,viii.
23. Vaidya CJ, Austin G, Kirkorian G, et al. Selective effects of methylphenidate in attention deficit hyperactivity disorder: a functional magnetic resonance study. Proc Natl Acad Sci USA. 1998;95:14494-14499.
24. Barbaresi WJ, Katusic SK, Colligan RC, et al. Long-term stimulant medication treatment of attention-deficit/hyperactivity disorder: results from a population-based study. J Dev Behav Pediatr. 2006;27:1-10.
25. Hanwella R, Senanayake M, de Silva V. Comparative efficacy and acceptability of methylphenidate and atomoxetine in treatment of attention deficit hyperactivity disorder in children and adolescents: a meta-analysis. BMC Psychiatry. 2011;11:176.
26. Lakhan SE, Kirchgessner A. Prescription stimulants in individuals with and without attention deficit hyperactivity disorder: misuse, cognitive impact, and adverse effects. Brain Behav. 2012:2:661-677.
27. Biederman J, Spencer TJ, Monuteaux MC, et al. A naturalistic 10-year prospective study of height and weight in children with attention-deficit hyperactivity disorder grown up: sex and treatment effects. J Pediatr. 2010;157:635-640.
28. Goldman RD. ADHD stimulants and their effect on height in children. Can Fam Physician. 2010;56:145-146.
29. Conant-Norville DO, Tofler IR. Attention deficit/hyperactivity disorder and psychopharmacologic treatments in the athlete. Clin Sports Med. 2005;24:829-843,viii.
30. National Collegiate Athletic Association Web site. 2012-13 NCAA Sports Medicine Handbook. Available at: http://www.ncaa.org/sites/default/files/MD12.pdf. Accessed March 17, 2014.
31. Battista J. Adderall, a drug of focus, is often blamed as NFL suspensions rise. New York Times. December 2, 2012. Available at: http://www.nytimes.com/2012/12/02/sports/football/adderall-a-drug-of-increased-focus-for-nfl-players.html?pagewanted=all&_r=0. Accessed March 17, 2014.
32. CBS Sports Web site. NCAA medical exception documentation reporting form to support the diagnosis of attention deficit hyperactivity disorder (ADHD) and treatment with banned stimulant medication. Available at: http://grfx.cstv.com/photos/schools/grva/genrel/auto_pdf/2012-13/misc_non_event/adhdreporting.pdf. Accessed March 17, 2014.
33. World Anti-Doping Agency Web site. The 2013 list of prohibited substances and methods. Available at: http://www.wada-ama.org/en/Science-Medicine/Prohibited-List/. Accessed March 17, 2014.
34. Perrin JM, Friedman RA, Knilans TK; Black Box Working Group; Section on Cardiology and Cardiac Surgery. Cardiovascular monitoring and stimulant drugs for attention-deficit/hyperactivity disorder. Pediatrics. 2008;122:451-453.
35. Watson P, Hasegawa H, Roelands B, et al. Acute dopamine/noradrenaline reuptake inhibition enhances human exercise performance in warm, but not temperate conditions. J Physiol. 2005;565(pt 3):873-883.
36. Roelands B, Hasegawa H, Watson P, et al. The effects of acute dopamine reuptake inhibition on performance. Med Sci Sports Exerc. 2008;40:879-885.
37. Sinclair L. ADHD drugs on critical list as medication shortages soar. Psychiatric News. Available at: http://psychnews.psychiatryonline.org/newsarticle.aspx?articleid=481189. Accessed March 17, 2014.
38. Forlini C, Gauthier S, Racine E. Should physicians prescribe cognitive enhancers to healthy individuals? CMAJ. 2013;185:1047-1050.
39. McDuff DR, Baron D. Substance abuse in athletes: a sports psychiatry perspective. Clin Sports Med. 2005;24:885-897,ix-x.
40. Desantis AD, Hane AC. “Adderall is definitely not a drug”: justifications for the illegal use of ADHD stimulants. Subst Use Misuse. 2010;45:31-46.
41. Wilens TE, Faraone SV, Biederman J, et al. Does stimulant therapy of attention-deficit/hyperactivity disorder beget later substance abuse? A meta-analytic review of the literature. Pediatrics. 2003;111:179-185.
1. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th ed. Washington, DC: American Psychiatric Association Press; 2013.
2. Parr JW. Attention-deficit hyperactivity disorder and the athlete: new advances and understanding. Clin Sports Med. 2011;30:591-610.
3. Kiluk BD, Weden S, Culotta VP. Sport participation and anxiety in children with ADHD. J Atten Disord. 2009;12:499-506.
4. Broshek DK, Freeman JR. Psychiatric and neuropsychological issues in sports medicine. Clin Sports Med. 2005;24:663-679,x.
5. Polanczyk G, de Lima MS, Horta BL, et al. The worldwide prevalence of ADHD: a systematic review and metaregression analysis. Am J Psychiatry. 2006;164:942-948.
6. Merikangas KR, He JP, Burstein M, et al. Lifetime prevalence of mental disorders in US adolescents: results from the National Comorbidity Stud—Adolescent Supplement (NCS-A). J Am Acad Child Adolesc Psychiatry. 2010;49:980-989.
7. Kessler RC, Adler L, Barkley R, et al. The prevalence and correlates of adult ADHD in the United States: results from the National Comorbidity Survey Replication. Am J Psychiatry. 2006;163:716-723.
8. Solomon GS, Haase RF. Biopsychosocial characteristics and neurocognitive test performance in National Football League players: an initial assessment. Arch Clinical Neuropsychol. 2008;23:563-577.
9. Kutcher JS. Treatment of attention-deficit hyperactivity disorder in athletes. Curr Sports Med Reports. 2011;10:32-36.
10 Biederman J, Kwon A, Aleardi M, et al. Absence of gender effects on attention deficit hyperactivity disorder: findings in nonreferred subjects. Am J Psychiatry. 2005;162:1083-1089.
11. Wilens TE, Faraone SV, Biederman J. Attention-deficit/hyperactivity disorder in adults. JAMA. 2004;292:619-623.
12. Subcommittee on Attention-Deficit/Hyperactivity Disorder; Steering Committee on Quality Improvement and Management; Wolraich M, Brown L, Brown RT, et al. ADHD: clinical practice guideline for diagnosis, evaluation, and treatment of attentiondeficit/hyperactivity disorder in children and adolescents. Pediatrics. 2011;128:1007-1022.
13. Boonstra AM, Osterlaan J, Sergeant JA, et al. Executive functioning in adult ADHD: a meta-analytic review. Psychol Med. 2005;35:1097-1108.
14. Silk T, Vance A, Rinehart N, et al. Fronto-parietal activation in attention-deficit/hyperactivity disorder, combined type: functional magnetic resonance imaging study. Br J Psychiatry. 2005;187:282-283.
15. Biederman J, Wilens TE, Mick E, et al. Does attention-deficit hyperactivity disorder impact the developmental course of drug and alcohol abuse and dependence? Biol Psychiatry. 1998;44:269-273.
16. Putukian M, Kreher JB, Coppel DB, et al. Attention deficit hyperactivity disorder and the athlete: an American Medical Society for Sports Medicine position statement. Clin J Sport Med. 2011;21:392-401.
17. Agarwal R, Goldenberg M, Perry R, et al. The quality of life of adults with attention deficit disorder: a systematic review. Innov Clin Neurosci. 2012;9:10-21.
18. Seixas M, Weiss M, Müller U. Systematic review of national and international guidelines on attention-deficit hyperactivity disorder. J Psychopharmacol. 2012;26:753-765.
19. Pelham WE Jr, Fabiano GA. Evidence-based psychosocial treatments for attention-deficit/hyperactivity disorder. J Clin Child Adolesc Psychol. 2008;37:184-214.
20. Searight HR, Burke JM, Rottneck F. Adult ADHD: evaluation and treatment in family medicine. Am Fam Physician. 2000;62:2077-2086,2091-2092.
21. Krull KR. Attention-deficit hyperactivity disorder in children and adolescents: Treatment with medications. Available at: http://www.uptodate.com/contents/attention-deficit-hyperactivitydisorder-in-children-and-adolescents-treatment-with-medications. Accessed March 17, 2014.
22. Conant-Norville DO, Tofler IR. Attention deficit/hyperactivity disorder and psychopharmacologic treatments in the athlete. Clin Sports Med. 2005;24:829-843,viii.
23. Vaidya CJ, Austin G, Kirkorian G, et al. Selective effects of methylphenidate in attention deficit hyperactivity disorder: a functional magnetic resonance study. Proc Natl Acad Sci USA. 1998;95:14494-14499.
24. Barbaresi WJ, Katusic SK, Colligan RC, et al. Long-term stimulant medication treatment of attention-deficit/hyperactivity disorder: results from a population-based study. J Dev Behav Pediatr. 2006;27:1-10.
25. Hanwella R, Senanayake M, de Silva V. Comparative efficacy and acceptability of methylphenidate and atomoxetine in treatment of attention deficit hyperactivity disorder in children and adolescents: a meta-analysis. BMC Psychiatry. 2011;11:176.
26. Lakhan SE, Kirchgessner A. Prescription stimulants in individuals with and without attention deficit hyperactivity disorder: misuse, cognitive impact, and adverse effects. Brain Behav. 2012:2:661-677.
27. Biederman J, Spencer TJ, Monuteaux MC, et al. A naturalistic 10-year prospective study of height and weight in children with attention-deficit hyperactivity disorder grown up: sex and treatment effects. J Pediatr. 2010;157:635-640.
28. Goldman RD. ADHD stimulants and their effect on height in children. Can Fam Physician. 2010;56:145-146.
29. Conant-Norville DO, Tofler IR. Attention deficit/hyperactivity disorder and psychopharmacologic treatments in the athlete. Clin Sports Med. 2005;24:829-843,viii.
30. National Collegiate Athletic Association Web site. 2012-13 NCAA Sports Medicine Handbook. Available at: http://www.ncaa.org/sites/default/files/MD12.pdf. Accessed March 17, 2014.
31. Battista J. Adderall, a drug of focus, is often blamed as NFL suspensions rise. New York Times. December 2, 2012. Available at: http://www.nytimes.com/2012/12/02/sports/football/adderall-a-drug-of-increased-focus-for-nfl-players.html?pagewanted=all&_r=0. Accessed March 17, 2014.
32. CBS Sports Web site. NCAA medical exception documentation reporting form to support the diagnosis of attention deficit hyperactivity disorder (ADHD) and treatment with banned stimulant medication. Available at: http://grfx.cstv.com/photos/schools/grva/genrel/auto_pdf/2012-13/misc_non_event/adhdreporting.pdf. Accessed March 17, 2014.
33. World Anti-Doping Agency Web site. The 2013 list of prohibited substances and methods. Available at: http://www.wada-ama.org/en/Science-Medicine/Prohibited-List/. Accessed March 17, 2014.
34. Perrin JM, Friedman RA, Knilans TK; Black Box Working Group; Section on Cardiology and Cardiac Surgery. Cardiovascular monitoring and stimulant drugs for attention-deficit/hyperactivity disorder. Pediatrics. 2008;122:451-453.
35. Watson P, Hasegawa H, Roelands B, et al. Acute dopamine/noradrenaline reuptake inhibition enhances human exercise performance in warm, but not temperate conditions. J Physiol. 2005;565(pt 3):873-883.
36. Roelands B, Hasegawa H, Watson P, et al. The effects of acute dopamine reuptake inhibition on performance. Med Sci Sports Exerc. 2008;40:879-885.
37. Sinclair L. ADHD drugs on critical list as medication shortages soar. Psychiatric News. Available at: http://psychnews.psychiatryonline.org/newsarticle.aspx?articleid=481189. Accessed March 17, 2014.
38. Forlini C, Gauthier S, Racine E. Should physicians prescribe cognitive enhancers to healthy individuals? CMAJ. 2013;185:1047-1050.
39. McDuff DR, Baron D. Substance abuse in athletes: a sports psychiatry perspective. Clin Sports Med. 2005;24:885-897,ix-x.
40. Desantis AD, Hane AC. “Adderall is definitely not a drug”: justifications for the illegal use of ADHD stimulants. Subst Use Misuse. 2010;45:31-46.
41. Wilens TE, Faraone SV, Biederman J, et al. Does stimulant therapy of attention-deficit/hyperactivity disorder beget later substance abuse? A meta-analytic review of the literature. Pediatrics. 2003;111:179-185.