User login
Fifth and sixth diseases: More than a fever and a rash
› Reserve serologic testing for parvovirus B19 for pregnant women with known exposure to the virus, immunocompromised individuals, or patients with chronic hemolytic conditions or severe or persistent arthropathy. B
› Keep in mind that
up to 15% of children infected with human herpes virus 6 can experience febrile seizures. Treat with an antiepileptic drug, as you would for any febrile seizure that lasts >5 minutes. C
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Fifth and sixth diseases are frequently encountered viral exanthems in family medicine. This article delineates the unique clinical characteristics of these disorders, describes rare but serious sequelae of each, and offers recommendations to guide your practice.
Fifth disease
Parvovirus B19, an infectious agent found worldwide, is the cause of fifth disease, also known as slapped cheek syndrome or erythema infectiosum. It is transmitted via respiratory droplets, most commonly in late winter and early spring. The peak incidence of parvovirus B19 infection is in children ages 5 to 15 years.1 Approximately 20% of parvovirus B19 infections remain subclinical.1,2 An observational study of children in the United Kingdom who were 6 months to 16 years of age and had been immunized for measles and rubella revealed that parvovirus B19 was the number one identifiable cause of febrile rash, responsible for 17% of cases.3 Seroprevalence increases with age, and 40% to 60% of adults test positive for prior infection.1
Clinical presentation: Not necessarily limited to fever and rash
Associated arthritis. Parvovirus B19 may also cause a symmetric polyarthritis of the hands, wrists, knees, or ankles, particularly in adult females. The course of arthritis usually lasts 1 to 3 weeks, but up to 20% may evolve into a chronic arthritis.6 In addition, numerous case studies suggest that parvovirus B19 may, in rare cases, cause a viral myocarditis in infants and children.7
Hemolytic complications. The target of parvovirus B19 is the erythroid blood cell line.1 Consequently, immunocompromised patients and those with chronic hemolytic conditions (eg, sickle cell disease, thalassemia, spherocytosis, or pyruvate kinase deficiency) may develop hematologic complications such as aplastic crisis, chronic anemia, thrombocytopenia, neutropenia, or pancytopenia. Patients with hemolytic complications can be quite ill, presenting with fever, malaise, tachycardia, tachypnea, and profound anemia.
Perinatal perils. Approximately one-third of pregnant mothers are at risk for parvovirus B19 infection, and having children at home, a severe medical condition, or stressful employment have been shown to increase their risk of active infection.8 The annual incidence of symptomatic parvovirus B19 during pregnancy is 1.5%, increasing to 13% during epidemics.9 Such infection can cause significant morbidity and mortality for the fetus. Mothers newly infected during the first trimester have experienced a 71% increased risk of intrauterine fetal demise (fetal loss <20 weeks gestation) when compared with baseline risk of fetal loss.9 In one prospective observational study, fetal death was only observed when mothers were infected prior to 20 weeks of gestation.10 Intrauterine B19 infection during any trimester carries a 4% overall risk of hydrops fetalis, thought to be due to high output cardiac failure secondary to severe anemia.10
Rely on clinical findings to diagnose; restrict serologic testing
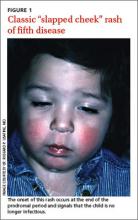
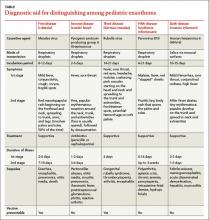
The characteristic “slapped cheek” rash usually distinguishes fifth disease from other causes of febrile rash. Differential diagnosis includes measles, scarlet fever, roseola infantum, enterovirus, and adenovirus. A diagnostic tool (TABLE) can help differentiate fifth disease from other viral exanthems.
In most cases of suspected parvovirus B19 infection, serologic testing is not indicated. However, consider serologic testing for pregnant women with known exposure to the virus, immunocompromised patients, patients with chronic hemolytic conditions, or patients with severe or persistent arthropathy. Serum immunoglobulin M can usually be detected 10 days after infection and can persist for 3 months, while serum immunoglobulin G is produced 2 weeks after inoculation and presumably lasts for life.11
Treat supportively
No specific treatment exists for parvovirus B19 infection. Management is supportive and the infection is usually mild and self-limiting. A nonsteroidal anti-inflammatory agent may be sufficient for associated arthritis; if needed, a low-dose oral corticosteroid can be used without prolonging the viral illness.6 Refer for hematologic consultation any immunocompromised patient with confirmed parvovirus who develops a hematologic complication, which may require intravenous immunoglobulin treatment or, in severe cases, bone marrow transplantation.
Clinical recommendations
Parvovirus B19 is communicable only during the nonspecific prodromal period—the 4 to 21 days of incubation in which the patient seems to have a common cold, with coryza, sore throat, and headache. With the appearance of the “slapped cheek” rash (an immune-mediated, postinfectious sequela), a child with erythema infectiosum is no longer infectious. At this stage, exclusion from school or child care is unnecessary.1
Perform serologic testing to determine immunity for all pregnant women with documented exposure to parvovirus B19.12 Retest women who are initially nonimmune after 3 to 4 weeks. Patients who seroconvert should undergo serial ultrasounds for 10 weeks to evaluate for hydrops fetalis or growth restriction. Repeat testing is unwarranted for those who do not seroconvert. There is no evidence to suggest that seronegative pregnant women should avoid work environments during endemic periods of infection.13
Sixth disease
Human herpesvirus 6 (HHV-6) causes sixth disease, also known as roseola infantum or exanthem subitum. Ninety percent of children have been infected by 2 years of age, with peak incidence occurring between 9 and 21 months of age.14 HHV-6 is most likely transmitted via the saliva of healthy individuals and enters the body via a mucosal surface. One percent of HHV-6 infection is acquired congenitally without known sequelae, similar to the transmission rate of cytomegalovirus.15
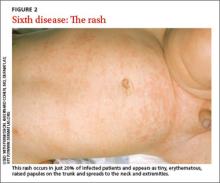
Clinical presentation: Only 20% may exhibit a rash
Complications. Fifteen percent of infected children have febrile seizures.1 Based on several case reports, HHV-6 infection has been associated with meningoencephalitis, acute disseminated demyelination, hepatitis, and myocarditis.17 It is unknown whether seizures increase the risk of these complications. Long-term sequelae from these manifestations of HHV-6 infection include developmental disorders and autism-spectrum disorders.18,19
Treat supportively
Patients with primary HHV-6 infection usually require antipyretics and frequent hydration. Reserve antivirals such as ganciclovir, foscarnet, and cidofovir for immunocompromised patients or those with HHV-6 encephalitis.20
Clinical recommendations
Treat seizures associated with HHV-6 infection as you would any other febrile seizure, giving an antiepileptic (diazepam, lorazepam, or midazolam) if the seizure lasts >5 minutes. Risk of seizure recurrence with HHV-6 is equivalent to that seen with other causes of febrile seizure.1
Because of the ubiquitous prevalence of HHV-6 infection, there are no effective preventive measures. Little is known about the effect of HHV-6 exposure during pregnancy because most pregnant mothers are immune to the virus.21 Exclusion from school or child care is not recommended because of the prolonged shedding of the virus.16,22
CORRESPONDENCE
Jason S. O’Grady, MD, Department of Family Medicine, Mayo Clinic, 200 First Street SW, Rochester, MN 55905; [email protected]
ACKNOWLEDGEMENT
The author thanks Anne Mounsey, MD, Department of Family Medicine, University of North Carolina at Chapel Hill, for her invaluable assistance in editing this manuscript.
1. Kliegman RM, Stanton BMD, St. Geme J, et al. Nelson Textbook of Pediatrics. 19th ed. Philadelphia, PA: Elsevier/Saunders; 2011.
2. Tuckerman JG, Brown T, Cohen BJ. Erythema infectiosum in a village primary school: clinical and virological studies. J R Coll Gen Pract. 1986;36:267-270.
3. Ramsay M, Reacher M, O’Flynn C, et al. Causes of morbilliform rash in a highly immunised English population. Arch Dis Child. 2002;87:202-206.
4. Anderson LJ. Role of parvovirus B19 in human disease. Pediatr Infect Dis J. 1987;6:711-718.
5. Smith PT, Landry ML, Carey H, et al. Papular-purpuric gloves and socks syndrome associated with acute parvovirus B19 infection: case report and review. Clin Infect Dis. 1998;27:164-168.
6. Tello-Winniczuk N, Diaz-Jouanen E, Diaz-Borjón A. Parvovirus B19-associated arthritis: report on a community outbreak. J Clin Rheumatol. 2011;17:449-450.
7. Molina KM, Garcia X, Denfield SW, et al. Parvovirus B19 myocarditis causes significant morbidity and mortality in children. Pediatr Cardiol. 2013;34:390-397.
8. Jensen IP, Thorsen P, Jeune B, et al. An epidemic of parvovirus B19 in a population of 3,596 pregnant women: a study of sociodemographic and medical risk factors. BJOG. 2000;107:637-643.
9. Lassen J, Jensen AK, Bager P, et al. Parvovirus B19 infection in the first trimester of pregnancy and risk of fetal loss: a population-based case-control study. Am J Epidemiol. 2012;176:803-807.
10. Enders M, Weidner A, Zoellner I, et al. Fetal morbidity and mortality after acute human parvovirus B19 infection in pregnancy: prospective evaluation of 1018 cases. Prenat Diagn. 2004;24:513-518.
11. Heegaard ED, Brown KE. Human parvovirus B19. Clin Microbiol Rev. 2002;15:485-505.
12. American College of Obstetrics and Gynecologists. ACOG practice bulletin. Perinatal viral and parasitic infections. Number 20, September 2000. (Replaces educational bulletin number 177, February 1993). Int J Gynaecol Obstet. 2002;76:95-107.
13. Harger JH, Adler SP, Koch WC, et al. Prospective evaluation of 618 pregnant women exposed to parvovirus B19: risks and symptoms. Obstet Gynecol. 1998;91:413-420.
14. Zerr DM, Meier AS, Selke SS, et al. A population-based study of primary human herpesvirus 6 infection. N Engl J Med. 2005;352:768-776.
15. Hall CB, Caserta MT, Schnabel KC, et al. Congenital infections with human herpesvirus 6 (HHV6) and human herpesvirus 7 (HHV7). J Pediatr. 2004;145:472-477.
16. Richardson M, Elliman D, Maguire H, et al. Evidence base of incubation periods, periods of infectiousness and exclusion policies for the control of communicable diseases in schools and pre-schools. Pediatr Infect Dis J. 2001;20:380-391.
17. Gewurz BE, Marty FM, Baden LR, et al. Human herpesvirus 6 encephalitis. Curr Infect Dis Rep. 2008;10:292-299.
18. Howell KB, Tiedemann K, Haeusler G, et al. Symptomatic generalized epilepsy after HHV6 posttransplant acute limbic encephalitis in children. Epilepsia. 2012;53:e122-e126.
19. Nicolson GL, Gan R, Nicolson NL, et al. Evidence for Mycoplasma ssp., Chlamydia pneunomiae, and human herpes virus-6 coinfections in the blood of patients with autistic spectrum disorders. J Neurosci Res. 2007;85:1143-1148.
20. De Bolle L, Naesens L, De Clercq E. Update on human herpesvirus 6 biology, clinical features, and therapy. Clin Microbiol Rev. 2005;18:217-245.
21. Baillargeon J, Piper J, Leach CT. Epidemiology of human herpesvirus 6 (HHV-6) infection in pregnant and nonpregnant women. J Clin Virol. 2000;16:149-157.
22. Levy JA, Ferro F, Greenspan D, et al. Frequent isolation of HHV-6 from saliva and high seroprevalence of the virus in the population. Lancet. 1990;335:1047-1050.
› Reserve serologic testing for parvovirus B19 for pregnant women with known exposure to the virus, immunocompromised individuals, or patients with chronic hemolytic conditions or severe or persistent arthropathy. B
› Keep in mind that
up to 15% of children infected with human herpes virus 6 can experience febrile seizures. Treat with an antiepileptic drug, as you would for any febrile seizure that lasts >5 minutes. C
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Fifth and sixth diseases are frequently encountered viral exanthems in family medicine. This article delineates the unique clinical characteristics of these disorders, describes rare but serious sequelae of each, and offers recommendations to guide your practice.
Fifth disease
Parvovirus B19, an infectious agent found worldwide, is the cause of fifth disease, also known as slapped cheek syndrome or erythema infectiosum. It is transmitted via respiratory droplets, most commonly in late winter and early spring. The peak incidence of parvovirus B19 infection is in children ages 5 to 15 years.1 Approximately 20% of parvovirus B19 infections remain subclinical.1,2 An observational study of children in the United Kingdom who were 6 months to 16 years of age and had been immunized for measles and rubella revealed that parvovirus B19 was the number one identifiable cause of febrile rash, responsible for 17% of cases.3 Seroprevalence increases with age, and 40% to 60% of adults test positive for prior infection.1
Clinical presentation: Not necessarily limited to fever and rash
Associated arthritis. Parvovirus B19 may also cause a symmetric polyarthritis of the hands, wrists, knees, or ankles, particularly in adult females. The course of arthritis usually lasts 1 to 3 weeks, but up to 20% may evolve into a chronic arthritis.6 In addition, numerous case studies suggest that parvovirus B19 may, in rare cases, cause a viral myocarditis in infants and children.7
Hemolytic complications. The target of parvovirus B19 is the erythroid blood cell line.1 Consequently, immunocompromised patients and those with chronic hemolytic conditions (eg, sickle cell disease, thalassemia, spherocytosis, or pyruvate kinase deficiency) may develop hematologic complications such as aplastic crisis, chronic anemia, thrombocytopenia, neutropenia, or pancytopenia. Patients with hemolytic complications can be quite ill, presenting with fever, malaise, tachycardia, tachypnea, and profound anemia.
Perinatal perils. Approximately one-third of pregnant mothers are at risk for parvovirus B19 infection, and having children at home, a severe medical condition, or stressful employment have been shown to increase their risk of active infection.8 The annual incidence of symptomatic parvovirus B19 during pregnancy is 1.5%, increasing to 13% during epidemics.9 Such infection can cause significant morbidity and mortality for the fetus. Mothers newly infected during the first trimester have experienced a 71% increased risk of intrauterine fetal demise (fetal loss <20 weeks gestation) when compared with baseline risk of fetal loss.9 In one prospective observational study, fetal death was only observed when mothers were infected prior to 20 weeks of gestation.10 Intrauterine B19 infection during any trimester carries a 4% overall risk of hydrops fetalis, thought to be due to high output cardiac failure secondary to severe anemia.10
Rely on clinical findings to diagnose; restrict serologic testing
The characteristic “slapped cheek” rash usually distinguishes fifth disease from other causes of febrile rash. Differential diagnosis includes measles, scarlet fever, roseola infantum, enterovirus, and adenovirus. A diagnostic tool (TABLE) can help differentiate fifth disease from other viral exanthems.
In most cases of suspected parvovirus B19 infection, serologic testing is not indicated. However, consider serologic testing for pregnant women with known exposure to the virus, immunocompromised patients, patients with chronic hemolytic conditions, or patients with severe or persistent arthropathy. Serum immunoglobulin M can usually be detected 10 days after infection and can persist for 3 months, while serum immunoglobulin G is produced 2 weeks after inoculation and presumably lasts for life.11
Treat supportively
No specific treatment exists for parvovirus B19 infection. Management is supportive and the infection is usually mild and self-limiting. A nonsteroidal anti-inflammatory agent may be sufficient for associated arthritis; if needed, a low-dose oral corticosteroid can be used without prolonging the viral illness.6 Refer for hematologic consultation any immunocompromised patient with confirmed parvovirus who develops a hematologic complication, which may require intravenous immunoglobulin treatment or, in severe cases, bone marrow transplantation.
Clinical recommendations
Parvovirus B19 is communicable only during the nonspecific prodromal period—the 4 to 21 days of incubation in which the patient seems to have a common cold, with coryza, sore throat, and headache. With the appearance of the “slapped cheek” rash (an immune-mediated, postinfectious sequela), a child with erythema infectiosum is no longer infectious. At this stage, exclusion from school or child care is unnecessary.1
Perform serologic testing to determine immunity for all pregnant women with documented exposure to parvovirus B19.12 Retest women who are initially nonimmune after 3 to 4 weeks. Patients who seroconvert should undergo serial ultrasounds for 10 weeks to evaluate for hydrops fetalis or growth restriction. Repeat testing is unwarranted for those who do not seroconvert. There is no evidence to suggest that seronegative pregnant women should avoid work environments during endemic periods of infection.13
Sixth disease
Human herpesvirus 6 (HHV-6) causes sixth disease, also known as roseola infantum or exanthem subitum. Ninety percent of children have been infected by 2 years of age, with peak incidence occurring between 9 and 21 months of age.14 HHV-6 is most likely transmitted via the saliva of healthy individuals and enters the body via a mucosal surface. One percent of HHV-6 infection is acquired congenitally without known sequelae, similar to the transmission rate of cytomegalovirus.15
Clinical presentation: Only 20% may exhibit a rash
Complications. Fifteen percent of infected children have febrile seizures.1 Based on several case reports, HHV-6 infection has been associated with meningoencephalitis, acute disseminated demyelination, hepatitis, and myocarditis.17 It is unknown whether seizures increase the risk of these complications. Long-term sequelae from these manifestations of HHV-6 infection include developmental disorders and autism-spectrum disorders.18,19
Treat supportively
Patients with primary HHV-6 infection usually require antipyretics and frequent hydration. Reserve antivirals such as ganciclovir, foscarnet, and cidofovir for immunocompromised patients or those with HHV-6 encephalitis.20
Clinical recommendations
Treat seizures associated with HHV-6 infection as you would any other febrile seizure, giving an antiepileptic (diazepam, lorazepam, or midazolam) if the seizure lasts >5 minutes. Risk of seizure recurrence with HHV-6 is equivalent to that seen with other causes of febrile seizure.1
Because of the ubiquitous prevalence of HHV-6 infection, there are no effective preventive measures. Little is known about the effect of HHV-6 exposure during pregnancy because most pregnant mothers are immune to the virus.21 Exclusion from school or child care is not recommended because of the prolonged shedding of the virus.16,22
CORRESPONDENCE
Jason S. O’Grady, MD, Department of Family Medicine, Mayo Clinic, 200 First Street SW, Rochester, MN 55905; [email protected]
ACKNOWLEDGEMENT
The author thanks Anne Mounsey, MD, Department of Family Medicine, University of North Carolina at Chapel Hill, for her invaluable assistance in editing this manuscript.
› Reserve serologic testing for parvovirus B19 for pregnant women with known exposure to the virus, immunocompromised individuals, or patients with chronic hemolytic conditions or severe or persistent arthropathy. B
› Keep in mind that
up to 15% of children infected with human herpes virus 6 can experience febrile seizures. Treat with an antiepileptic drug, as you would for any febrile seizure that lasts >5 minutes. C
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Fifth and sixth diseases are frequently encountered viral exanthems in family medicine. This article delineates the unique clinical characteristics of these disorders, describes rare but serious sequelae of each, and offers recommendations to guide your practice.
Fifth disease
Parvovirus B19, an infectious agent found worldwide, is the cause of fifth disease, also known as slapped cheek syndrome or erythema infectiosum. It is transmitted via respiratory droplets, most commonly in late winter and early spring. The peak incidence of parvovirus B19 infection is in children ages 5 to 15 years.1 Approximately 20% of parvovirus B19 infections remain subclinical.1,2 An observational study of children in the United Kingdom who were 6 months to 16 years of age and had been immunized for measles and rubella revealed that parvovirus B19 was the number one identifiable cause of febrile rash, responsible for 17% of cases.3 Seroprevalence increases with age, and 40% to 60% of adults test positive for prior infection.1
Clinical presentation: Not necessarily limited to fever and rash
Associated arthritis. Parvovirus B19 may also cause a symmetric polyarthritis of the hands, wrists, knees, or ankles, particularly in adult females. The course of arthritis usually lasts 1 to 3 weeks, but up to 20% may evolve into a chronic arthritis.6 In addition, numerous case studies suggest that parvovirus B19 may, in rare cases, cause a viral myocarditis in infants and children.7
Hemolytic complications. The target of parvovirus B19 is the erythroid blood cell line.1 Consequently, immunocompromised patients and those with chronic hemolytic conditions (eg, sickle cell disease, thalassemia, spherocytosis, or pyruvate kinase deficiency) may develop hematologic complications such as aplastic crisis, chronic anemia, thrombocytopenia, neutropenia, or pancytopenia. Patients with hemolytic complications can be quite ill, presenting with fever, malaise, tachycardia, tachypnea, and profound anemia.
Perinatal perils. Approximately one-third of pregnant mothers are at risk for parvovirus B19 infection, and having children at home, a severe medical condition, or stressful employment have been shown to increase their risk of active infection.8 The annual incidence of symptomatic parvovirus B19 during pregnancy is 1.5%, increasing to 13% during epidemics.9 Such infection can cause significant morbidity and mortality for the fetus. Mothers newly infected during the first trimester have experienced a 71% increased risk of intrauterine fetal demise (fetal loss <20 weeks gestation) when compared with baseline risk of fetal loss.9 In one prospective observational study, fetal death was only observed when mothers were infected prior to 20 weeks of gestation.10 Intrauterine B19 infection during any trimester carries a 4% overall risk of hydrops fetalis, thought to be due to high output cardiac failure secondary to severe anemia.10
Rely on clinical findings to diagnose; restrict serologic testing
The characteristic “slapped cheek” rash usually distinguishes fifth disease from other causes of febrile rash. Differential diagnosis includes measles, scarlet fever, roseola infantum, enterovirus, and adenovirus. A diagnostic tool (TABLE) can help differentiate fifth disease from other viral exanthems.
In most cases of suspected parvovirus B19 infection, serologic testing is not indicated. However, consider serologic testing for pregnant women with known exposure to the virus, immunocompromised patients, patients with chronic hemolytic conditions, or patients with severe or persistent arthropathy. Serum immunoglobulin M can usually be detected 10 days after infection and can persist for 3 months, while serum immunoglobulin G is produced 2 weeks after inoculation and presumably lasts for life.11
Treat supportively
No specific treatment exists for parvovirus B19 infection. Management is supportive and the infection is usually mild and self-limiting. A nonsteroidal anti-inflammatory agent may be sufficient for associated arthritis; if needed, a low-dose oral corticosteroid can be used without prolonging the viral illness.6 Refer for hematologic consultation any immunocompromised patient with confirmed parvovirus who develops a hematologic complication, which may require intravenous immunoglobulin treatment or, in severe cases, bone marrow transplantation.
Clinical recommendations
Parvovirus B19 is communicable only during the nonspecific prodromal period—the 4 to 21 days of incubation in which the patient seems to have a common cold, with coryza, sore throat, and headache. With the appearance of the “slapped cheek” rash (an immune-mediated, postinfectious sequela), a child with erythema infectiosum is no longer infectious. At this stage, exclusion from school or child care is unnecessary.1
Perform serologic testing to determine immunity for all pregnant women with documented exposure to parvovirus B19.12 Retest women who are initially nonimmune after 3 to 4 weeks. Patients who seroconvert should undergo serial ultrasounds for 10 weeks to evaluate for hydrops fetalis or growth restriction. Repeat testing is unwarranted for those who do not seroconvert. There is no evidence to suggest that seronegative pregnant women should avoid work environments during endemic periods of infection.13
Sixth disease
Human herpesvirus 6 (HHV-6) causes sixth disease, also known as roseola infantum or exanthem subitum. Ninety percent of children have been infected by 2 years of age, with peak incidence occurring between 9 and 21 months of age.14 HHV-6 is most likely transmitted via the saliva of healthy individuals and enters the body via a mucosal surface. One percent of HHV-6 infection is acquired congenitally without known sequelae, similar to the transmission rate of cytomegalovirus.15
Clinical presentation: Only 20% may exhibit a rash
Complications. Fifteen percent of infected children have febrile seizures.1 Based on several case reports, HHV-6 infection has been associated with meningoencephalitis, acute disseminated demyelination, hepatitis, and myocarditis.17 It is unknown whether seizures increase the risk of these complications. Long-term sequelae from these manifestations of HHV-6 infection include developmental disorders and autism-spectrum disorders.18,19
Treat supportively
Patients with primary HHV-6 infection usually require antipyretics and frequent hydration. Reserve antivirals such as ganciclovir, foscarnet, and cidofovir for immunocompromised patients or those with HHV-6 encephalitis.20
Clinical recommendations
Treat seizures associated with HHV-6 infection as you would any other febrile seizure, giving an antiepileptic (diazepam, lorazepam, or midazolam) if the seizure lasts >5 minutes. Risk of seizure recurrence with HHV-6 is equivalent to that seen with other causes of febrile seizure.1
Because of the ubiquitous prevalence of HHV-6 infection, there are no effective preventive measures. Little is known about the effect of HHV-6 exposure during pregnancy because most pregnant mothers are immune to the virus.21 Exclusion from school or child care is not recommended because of the prolonged shedding of the virus.16,22
CORRESPONDENCE
Jason S. O’Grady, MD, Department of Family Medicine, Mayo Clinic, 200 First Street SW, Rochester, MN 55905; [email protected]
ACKNOWLEDGEMENT
The author thanks Anne Mounsey, MD, Department of Family Medicine, University of North Carolina at Chapel Hill, for her invaluable assistance in editing this manuscript.
1. Kliegman RM, Stanton BMD, St. Geme J, et al. Nelson Textbook of Pediatrics. 19th ed. Philadelphia, PA: Elsevier/Saunders; 2011.
2. Tuckerman JG, Brown T, Cohen BJ. Erythema infectiosum in a village primary school: clinical and virological studies. J R Coll Gen Pract. 1986;36:267-270.
3. Ramsay M, Reacher M, O’Flynn C, et al. Causes of morbilliform rash in a highly immunised English population. Arch Dis Child. 2002;87:202-206.
4. Anderson LJ. Role of parvovirus B19 in human disease. Pediatr Infect Dis J. 1987;6:711-718.
5. Smith PT, Landry ML, Carey H, et al. Papular-purpuric gloves and socks syndrome associated with acute parvovirus B19 infection: case report and review. Clin Infect Dis. 1998;27:164-168.
6. Tello-Winniczuk N, Diaz-Jouanen E, Diaz-Borjón A. Parvovirus B19-associated arthritis: report on a community outbreak. J Clin Rheumatol. 2011;17:449-450.
7. Molina KM, Garcia X, Denfield SW, et al. Parvovirus B19 myocarditis causes significant morbidity and mortality in children. Pediatr Cardiol. 2013;34:390-397.
8. Jensen IP, Thorsen P, Jeune B, et al. An epidemic of parvovirus B19 in a population of 3,596 pregnant women: a study of sociodemographic and medical risk factors. BJOG. 2000;107:637-643.
9. Lassen J, Jensen AK, Bager P, et al. Parvovirus B19 infection in the first trimester of pregnancy and risk of fetal loss: a population-based case-control study. Am J Epidemiol. 2012;176:803-807.
10. Enders M, Weidner A, Zoellner I, et al. Fetal morbidity and mortality after acute human parvovirus B19 infection in pregnancy: prospective evaluation of 1018 cases. Prenat Diagn. 2004;24:513-518.
11. Heegaard ED, Brown KE. Human parvovirus B19. Clin Microbiol Rev. 2002;15:485-505.
12. American College of Obstetrics and Gynecologists. ACOG practice bulletin. Perinatal viral and parasitic infections. Number 20, September 2000. (Replaces educational bulletin number 177, February 1993). Int J Gynaecol Obstet. 2002;76:95-107.
13. Harger JH, Adler SP, Koch WC, et al. Prospective evaluation of 618 pregnant women exposed to parvovirus B19: risks and symptoms. Obstet Gynecol. 1998;91:413-420.
14. Zerr DM, Meier AS, Selke SS, et al. A population-based study of primary human herpesvirus 6 infection. N Engl J Med. 2005;352:768-776.
15. Hall CB, Caserta MT, Schnabel KC, et al. Congenital infections with human herpesvirus 6 (HHV6) and human herpesvirus 7 (HHV7). J Pediatr. 2004;145:472-477.
16. Richardson M, Elliman D, Maguire H, et al. Evidence base of incubation periods, periods of infectiousness and exclusion policies for the control of communicable diseases in schools and pre-schools. Pediatr Infect Dis J. 2001;20:380-391.
17. Gewurz BE, Marty FM, Baden LR, et al. Human herpesvirus 6 encephalitis. Curr Infect Dis Rep. 2008;10:292-299.
18. Howell KB, Tiedemann K, Haeusler G, et al. Symptomatic generalized epilepsy after HHV6 posttransplant acute limbic encephalitis in children. Epilepsia. 2012;53:e122-e126.
19. Nicolson GL, Gan R, Nicolson NL, et al. Evidence for Mycoplasma ssp., Chlamydia pneunomiae, and human herpes virus-6 coinfections in the blood of patients with autistic spectrum disorders. J Neurosci Res. 2007;85:1143-1148.
20. De Bolle L, Naesens L, De Clercq E. Update on human herpesvirus 6 biology, clinical features, and therapy. Clin Microbiol Rev. 2005;18:217-245.
21. Baillargeon J, Piper J, Leach CT. Epidemiology of human herpesvirus 6 (HHV-6) infection in pregnant and nonpregnant women. J Clin Virol. 2000;16:149-157.
22. Levy JA, Ferro F, Greenspan D, et al. Frequent isolation of HHV-6 from saliva and high seroprevalence of the virus in the population. Lancet. 1990;335:1047-1050.
1. Kliegman RM, Stanton BMD, St. Geme J, et al. Nelson Textbook of Pediatrics. 19th ed. Philadelphia, PA: Elsevier/Saunders; 2011.
2. Tuckerman JG, Brown T, Cohen BJ. Erythema infectiosum in a village primary school: clinical and virological studies. J R Coll Gen Pract. 1986;36:267-270.
3. Ramsay M, Reacher M, O’Flynn C, et al. Causes of morbilliform rash in a highly immunised English population. Arch Dis Child. 2002;87:202-206.
4. Anderson LJ. Role of parvovirus B19 in human disease. Pediatr Infect Dis J. 1987;6:711-718.
5. Smith PT, Landry ML, Carey H, et al. Papular-purpuric gloves and socks syndrome associated with acute parvovirus B19 infection: case report and review. Clin Infect Dis. 1998;27:164-168.
6. Tello-Winniczuk N, Diaz-Jouanen E, Diaz-Borjón A. Parvovirus B19-associated arthritis: report on a community outbreak. J Clin Rheumatol. 2011;17:449-450.
7. Molina KM, Garcia X, Denfield SW, et al. Parvovirus B19 myocarditis causes significant morbidity and mortality in children. Pediatr Cardiol. 2013;34:390-397.
8. Jensen IP, Thorsen P, Jeune B, et al. An epidemic of parvovirus B19 in a population of 3,596 pregnant women: a study of sociodemographic and medical risk factors. BJOG. 2000;107:637-643.
9. Lassen J, Jensen AK, Bager P, et al. Parvovirus B19 infection in the first trimester of pregnancy and risk of fetal loss: a population-based case-control study. Am J Epidemiol. 2012;176:803-807.
10. Enders M, Weidner A, Zoellner I, et al. Fetal morbidity and mortality after acute human parvovirus B19 infection in pregnancy: prospective evaluation of 1018 cases. Prenat Diagn. 2004;24:513-518.
11. Heegaard ED, Brown KE. Human parvovirus B19. Clin Microbiol Rev. 2002;15:485-505.
12. American College of Obstetrics and Gynecologists. ACOG practice bulletin. Perinatal viral and parasitic infections. Number 20, September 2000. (Replaces educational bulletin number 177, February 1993). Int J Gynaecol Obstet. 2002;76:95-107.
13. Harger JH, Adler SP, Koch WC, et al. Prospective evaluation of 618 pregnant women exposed to parvovirus B19: risks and symptoms. Obstet Gynecol. 1998;91:413-420.
14. Zerr DM, Meier AS, Selke SS, et al. A population-based study of primary human herpesvirus 6 infection. N Engl J Med. 2005;352:768-776.
15. Hall CB, Caserta MT, Schnabel KC, et al. Congenital infections with human herpesvirus 6 (HHV6) and human herpesvirus 7 (HHV7). J Pediatr. 2004;145:472-477.
16. Richardson M, Elliman D, Maguire H, et al. Evidence base of incubation periods, periods of infectiousness and exclusion policies for the control of communicable diseases in schools and pre-schools. Pediatr Infect Dis J. 2001;20:380-391.
17. Gewurz BE, Marty FM, Baden LR, et al. Human herpesvirus 6 encephalitis. Curr Infect Dis Rep. 2008;10:292-299.
18. Howell KB, Tiedemann K, Haeusler G, et al. Symptomatic generalized epilepsy after HHV6 posttransplant acute limbic encephalitis in children. Epilepsia. 2012;53:e122-e126.
19. Nicolson GL, Gan R, Nicolson NL, et al. Evidence for Mycoplasma ssp., Chlamydia pneunomiae, and human herpes virus-6 coinfections in the blood of patients with autistic spectrum disorders. J Neurosci Res. 2007;85:1143-1148.
20. De Bolle L, Naesens L, De Clercq E. Update on human herpesvirus 6 biology, clinical features, and therapy. Clin Microbiol Rev. 2005;18:217-245.
21. Baillargeon J, Piper J, Leach CT. Epidemiology of human herpesvirus 6 (HHV-6) infection in pregnant and nonpregnant women. J Clin Virol. 2000;16:149-157.
22. Levy JA, Ferro F, Greenspan D, et al. Frequent isolation of HHV-6 from saliva and high seroprevalence of the virus in the population. Lancet. 1990;335:1047-1050.
9 tips to help prevent derm biopsy mistakes
› Use an excisional biopsy for a melanocytic neoplasm. C
› Choose a punch biopsy over a shave biopsy for rashes. B
› Properly photograph and document the location of all lesions before biopsy. A
› Provide the pathologist with a sufficient history, including the distribution and appearance of the lesion, and how long the patient has had it. A
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Most physicians do a satisfactory job in choosing when and how to do a skin biopsy, but there is always room for improvement. The 9 pointers we provide here are based on standard of care practices and literature when available, and also on our collective experiences as a pathologist/dermatologist (JM), dermatopathologist (DZ), primary care physician (BR), and dermatologist/Mohs surgeon (EB).
1. Choose your biopsy type wisely.
Using the appropriate type of biopsy can have the greatest effect on a proper diagnosis. The decision of which biopsy type to use is not always easy. The most common biopsy types are shave, punch, excisional, and curettage. Several reference articles detail each type of biopsy commonly used in primary care and how to perform them.1,2 (For a series of how-to videos that illustrate how to perform some of these biopsies, visit The Journal of Family Practice Multimedia Library at http://www.jfponline.com/multimedia/video.html.)
Each type of biopsy has inherent advantages and disadvantages. In general, the shave biopsy is most commonly used for lesions that are solitary, elevated, and give the impression that a sufficient amount of tissue can be sampled using this technique. The punch biopsy is the biopsy of choice for most “rashes” (inflammatory skin disorders).2 Excisional biopsy is used to remove melanocytic neoplasms or larger lesions. And curettage, while still used by some clinicians for melanocytic lesions because of its speed and simplicity, should almost never be used for diagnostic purposes. Each of these techniques is described in greater detail in the tips that follow.
2. When performing a shave biopsy, avoid obtaining a sample that's too superficial.
The advantage of the shave biopsy is that it is minimally invasive and quick to perform. If kept small while not compromising the amount of sample retrieved, the scars left by shave biopsies have the potential to blend well. The major disadvantage of the shave biopsy is that occasionally, if the shave is not deep enough, an insufficient amount of tissue is obtained, which can make it challenging to establish an accurate diagnosis.
Balancing the need to obtain adequate tissue while minimizing scarring takes skill and experience. Taking a biopsy that is inadequate is a common occurrence. At times, the physician’s clinical impression may be that a biopsy has obtained adequate tissue, but histologically only the superficial part of the skin surface has been sampled. This often is because of thickening of the superficial skin, whether as a manifestation of the anatomic site (eg, acral skin) or the disease process itself.
Unfortunately, this superficial skin often is nondiagnostic when unaccompanied by underlying epidermis and dermis. It is important to keep this in mind when obtaining a skin biopsy, especially when dealing with lesions that are very scaly or keratinized. An equivocal biopsy wastes time, energy, and money, and can negatively impact patient care.3 It can be difficult to balance practical aspects of the biopsy (ie, optimizing cosmetic outcomes, minimizing scarring and wound size) with the need to obtain sufficient tissue sampling (FIGURE 1).
3. Choose punch over shave biopsy for rashes.
In a punch biopsy, a disposable metal cylinder with a sharpened edge is used to “punch” out a piece of skin that can be examined under the microscope. Punch biopsy is the preferred technique for almost all inflammatory skin conditions (rashes) because the pathologist is able to examine both the superficial and deep portions of the dermis4 (FIGURE 2).
Pathologists use the pattern of inflammation, in conjunction with epidermal changes, to distinguish different types of inflammatory processes. For example, lichen planus is typically associated with superficial inflammation, while lupus is known to have prominent superficial and deep inflammation.
An inadequate punch biopsy sample can hinder histological assessment of inflammatory skin disorders that involve both the superficial and deep portions of the dermis and can make arriving at a definitive diagnosis more challenging. The diameter of a punch cylinder ranges from 1 to 8 mm. Smaller punch biopsies often create diagnostic challenges because they provide so little sample. A punch biopsy size of 4 mm is commonly used for rashes.
An advantage of the punch biopsy is that patients are left with linear scars rather than round, potentially dyspigmented (darker or lighter) scars that are often associated with shave biopsy. A well-sutured punch biopsy can be cosmetically elegant, particularly if closure is oriented along relaxed skin tension lines. For this reason, punch biopsies are well suited for cosmetically sensitive locations such as the face, although shave biopsies are also often performed on the face.
4. Choose an excisional biopsy for a melanocytic neoplasm, when possible.
The purpose of an excisional biopsy (which typically includes a 1 to 3 mm rim of normal skin around the lesion) is to completely remove a lesion. The excisional biopsy generally is the preferred technique for clinically atypical melanocytic neoplasms (lesions that are not definitively benign).4-8
When suspicion for melanoma is high, excisional biopsies should be performed with minimal undermining to preserve the accuracy of any future sentinel lymph node biopsy surgeries. Excisional biopsy is the most involved type of biopsy and has the largest potential for cosmetic disfigurement if not properly planned and performed. While guidelines from the American Academy of Dermatology state that “narrow excisional biopsy that encompasses [the] entire breadth of lesion with clinically negative margins to ensure that the lesion is not transected” is preferred, they also acknowledge that partial sampling (incisional biopsy) is acceptable in select clinical circumstances,9 such as when a lesion is large or on a cosmetically sensitive site such as the face.10
While a larger punch biopsy (6 or 8 mm) or even deep shave/saucerization may function as an excisional biopsy for very small lesions, this approach can be problematic. For one thing, these biopsies are more likely than an excisional biopsy to leave a portion of the lesion in situ. Another concern is that a shave biopsy of a melanocytic lesion can lead to error or difficulty in obtaining the correct diagnosis on later biopsy.11 For pathologists, smaller or incomplete samples make it challenging to establish an accurate diagnosis.12 Among melanomas seen at a tertiary referral center, histopathological misdiagnosis was more common with a punch or shave biopsy than with an excisional biopsy.9
It has been shown that partial biopsy for melanoma results in more residual disease at wide local excision and makes it more challenging to properly stage the lesion.13,14 If a shave biopsy is used to sample a suspected melanocytic neoplasm, it is imperative to document the specific site of the biopsy, indicate the size of the melanocytic lesion on the pathology requisition form, and ensure that all (or nearly all) of the clinically evident lesion is sampled. Detailing the location of the lesion in the chart is not only essential in evaluating the present lesion, but it will serve you well in the future. Without knowing the patient’s clinical history, benign nevi that recur after a prior biopsy can be difficult to histologically distinguish from melanoma (FIGURE 3). For more on this, see tip #7.
5. Be careful with curettage.
6. Remember the importance of proper fixation and processing.
As obvious as it may sound, it is important to remember to promptly place sampled tissue in an adequate amount of formalin so that the tissue is submersed in it in the container.15 Failure to do so can result in improper fixation and will make it difficult to render an appropriate diagnosis. Conventionally, a 10:1 formalin volume to tissue volume ratio is recommended. If the “cold time”—the amount of time a tissue sample is out of formalin—is long enough (greater than a few hours), an appropriate assessment can be impossible.
Appropriate fixation and fixation times are important because molecular testing is being increasingly used to make pathological diagnoses.16 Additionally, aggressively manipulating a biopsy sample while extracting it or placing it in formalin can cause “crush” artifact, which can limit interpretability (FIGURE 5).
7. Properly photograph and document the biopsy location.
When performing a biopsy of a suspicious neoplasm, physicians often remove all of the lesion’s superficial components, which means that at the patient’s follow-up appointment and subsequent treatments, only a well-healed biopsy site will remain. The biopsy site may be so well healed that it blends seamlessly into the surrounding skin and is nearly impossible for the physician to identify. This problem is seen most often when patients present for surgical excision or Mohs micrographic surgery.17
To properly record the site of a biopsy for future dermatologic exams, take pictures of the lesion at the time of biopsy. The photographs should clearly document the lesion in question, and should be taken far enough from the site that surrounding lesions and/ or other anatomic landmarks are also visible. Biangulation or triangulation (taking a series of 2 or 3 measurements, respectively, from the site of the lesion to nearby anatomic landmarks) can be used in conjunction with photographs.
When using measurements, be as specific and accurate as possible with anatomic terms. For example, measuring the distance from the “ear” is not helpful. It would be more helpful to measure the distance from the “tragus” or the “root of the helix.” Without a properly photographed and documented biopsy site, surgical treatment may need to be delayed until the location can be confirmed.
8. Give the pathologist a pertinent history.
Providing the pathologist with a sufficient history, including the distribution and appearance of the lesion, and how long the patient has had it, can be essential in narrowing the diagnosis or making the differential diagnoses. Things like medication use or new exposures to perfumes, lotions, or plants can be especially helpful and are often overlooked when filling out the pathology requisition form.
When warranted, phone calls are helpful. You might, for example, call the pathologist and give him or her a more detailed physical examination description or additional pertinent history that was discovered after the requisition was filled out. Providing a good history can make the difference between a specific diagnosis and a broad differential.
9. Know when to refer.
There is no shame in asking for a second opinion when it comes to evaluating a skin issue, especially in regards to melanocytic neoplasms, where the stakes can be high, or skin eruptions that do not respond to conventional therapy. Remember, many cases are difficult, even for experts, and require a careful balance of clinical and histopathological judgment.18
CORRESPONDENCE
Jayson Miedema, MD, Department of Dermatology, University of North Carolina at Chapel Hill, 410 Market Street, Suite 400, Chapel Hill, NC 27516; [email protected]
1. Pickett H. Shave and punch biopsy for skin lesions. Am Fam Physician. 2011;84:995-1002.
2. Alguire PC, Mathes BM. Skin biopsy techniques for the internist. J Gen Intern Med. 1998;13:46-54.
3. Fernandez EM, Helm T, Ioffreda M, et al. The vanishing biopsy: the trend toward smaller specimens. Cutis. 2005;76:335-339.
4. Hieken TJ, Hernández-Irizarry R, Boll JM, et al. Accuracy of diagnostic biopsy for cutaneous melanoma: implications for surgical oncologists. Int J Surg Oncol. 2013;2013:196493.
5. Scolyer RA, Thompson JF, McCarthy SW, et al. Incomplete biopsy of melanocytic lesions can impair the accuracy of pathological diagnosis. Australas J Dermatol. 2006;47:71-75.
6. McCarthy SW, Scolyer RA. Pitfalls and important issues in the pathologic diagnosis of melanocytic tumors. Ochsner J. 2010;10:66-74.
7. Swanson NA, Lee KK, Gorman A, et al. Biopsy techniques. Diagnosis of melanoma. Dermatol Clin. 2002;20:677-680.
8. Chang TT, Somach SC, Wagamon K, et al. The inadequacy of punch-excised melanocytic lesions: sampling through the block for the determination of “margins”. J Am Acad Dermatol. 2009;60: 990-993.
9. Bichakjian CK, Halpern AC, Johnson TM, et al; American Academy of Dermatology. Guidelines of care for the management of primary cutaneous melanoma. American Academy of Dermatology. J Am Acad Dermatol. 2011;65:1032-1047.
10. Pardasani AG, Leshin B, Hallman JR, et al. Fusiform incisional biopsy for pigmented skin lesions. Dermatol Surg. 2000;26:622-624.
11. King R, Hayzen BA, Page RN, et al. Recurrent nevus phenomenon: a clinicopathologic study of 357 cases and histologic comparison with melanoma with regression. Mod Pathol. 2009;22:611-617.
12. Mills JK, White I, Diggs B, et al. Effect of biopsy type on outcomes in the treatment of primary cutaneous melanoma. Am J Surg. 2013;205:585-590.
13. Stell VH, Norton HJ, Smith KS, et al. Method of biopsy and incidence of positive margins in primary melanoma. Ann Surg Oncol. 2007;14:893-898.
14. Egnatios GL, Dueck AC, Macdonald JB, et al. The impact of biopsy technique on upstaging, residual disease, and outcome in cutaneous melanoma. Am J Surg. 2011;202:771-778.
15. Ackerman AB, Boer A, Bennin B, et al. Histologic Diagnosis of Inflammatory Skin Disease: An Algorithmic Method Based on Pattern Analysis. New York, NY: Ardor Scribendi; 2005.
16. Hewitt SM, Lewis FA, Cao Y, et al. Tissue handling and specimen preparation in surgical pathology: issues concerning the recovery of nucleic acids from formalin-fixed, paraffin-embedded tissue. Arch Pathol Lab Med. 2008;132:1929-1935.
17. Nemeth SA, Lawrence N. Site identification challenges in dermatologic surgery: a physician survey. J Am Acad Dermatol. 2012;67: 262-268.
18. Federman DG, Concato J, Kirsner RS. Comparison of dermatologic diagnoses by primary care practitioners and dermatologists. A review of the literature. Arch Fam Med. 1999;8:170-172.
› Use an excisional biopsy for a melanocytic neoplasm. C
› Choose a punch biopsy over a shave biopsy for rashes. B
› Properly photograph and document the location of all lesions before biopsy. A
› Provide the pathologist with a sufficient history, including the distribution and appearance of the lesion, and how long the patient has had it. A
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Most physicians do a satisfactory job in choosing when and how to do a skin biopsy, but there is always room for improvement. The 9 pointers we provide here are based on standard of care practices and literature when available, and also on our collective experiences as a pathologist/dermatologist (JM), dermatopathologist (DZ), primary care physician (BR), and dermatologist/Mohs surgeon (EB).
1. Choose your biopsy type wisely.
Using the appropriate type of biopsy can have the greatest effect on a proper diagnosis. The decision of which biopsy type to use is not always easy. The most common biopsy types are shave, punch, excisional, and curettage. Several reference articles detail each type of biopsy commonly used in primary care and how to perform them.1,2 (For a series of how-to videos that illustrate how to perform some of these biopsies, visit The Journal of Family Practice Multimedia Library at http://www.jfponline.com/multimedia/video.html.)
Each type of biopsy has inherent advantages and disadvantages. In general, the shave biopsy is most commonly used for lesions that are solitary, elevated, and give the impression that a sufficient amount of tissue can be sampled using this technique. The punch biopsy is the biopsy of choice for most “rashes” (inflammatory skin disorders).2 Excisional biopsy is used to remove melanocytic neoplasms or larger lesions. And curettage, while still used by some clinicians for melanocytic lesions because of its speed and simplicity, should almost never be used for diagnostic purposes. Each of these techniques is described in greater detail in the tips that follow.
2. When performing a shave biopsy, avoid obtaining a sample that's too superficial.
The advantage of the shave biopsy is that it is minimally invasive and quick to perform. If kept small while not compromising the amount of sample retrieved, the scars left by shave biopsies have the potential to blend well. The major disadvantage of the shave biopsy is that occasionally, if the shave is not deep enough, an insufficient amount of tissue is obtained, which can make it challenging to establish an accurate diagnosis.
Balancing the need to obtain adequate tissue while minimizing scarring takes skill and experience. Taking a biopsy that is inadequate is a common occurrence. At times, the physician’s clinical impression may be that a biopsy has obtained adequate tissue, but histologically only the superficial part of the skin surface has been sampled. This often is because of thickening of the superficial skin, whether as a manifestation of the anatomic site (eg, acral skin) or the disease process itself.
Unfortunately, this superficial skin often is nondiagnostic when unaccompanied by underlying epidermis and dermis. It is important to keep this in mind when obtaining a skin biopsy, especially when dealing with lesions that are very scaly or keratinized. An equivocal biopsy wastes time, energy, and money, and can negatively impact patient care.3 It can be difficult to balance practical aspects of the biopsy (ie, optimizing cosmetic outcomes, minimizing scarring and wound size) with the need to obtain sufficient tissue sampling (FIGURE 1).
3. Choose punch over shave biopsy for rashes.
In a punch biopsy, a disposable metal cylinder with a sharpened edge is used to “punch” out a piece of skin that can be examined under the microscope. Punch biopsy is the preferred technique for almost all inflammatory skin conditions (rashes) because the pathologist is able to examine both the superficial and deep portions of the dermis4 (FIGURE 2).
Pathologists use the pattern of inflammation, in conjunction with epidermal changes, to distinguish different types of inflammatory processes. For example, lichen planus is typically associated with superficial inflammation, while lupus is known to have prominent superficial and deep inflammation.
An inadequate punch biopsy sample can hinder histological assessment of inflammatory skin disorders that involve both the superficial and deep portions of the dermis and can make arriving at a definitive diagnosis more challenging. The diameter of a punch cylinder ranges from 1 to 8 mm. Smaller punch biopsies often create diagnostic challenges because they provide so little sample. A punch biopsy size of 4 mm is commonly used for rashes.
An advantage of the punch biopsy is that patients are left with linear scars rather than round, potentially dyspigmented (darker or lighter) scars that are often associated with shave biopsy. A well-sutured punch biopsy can be cosmetically elegant, particularly if closure is oriented along relaxed skin tension lines. For this reason, punch biopsies are well suited for cosmetically sensitive locations such as the face, although shave biopsies are also often performed on the face.
4. Choose an excisional biopsy for a melanocytic neoplasm, when possible.
The purpose of an excisional biopsy (which typically includes a 1 to 3 mm rim of normal skin around the lesion) is to completely remove a lesion. The excisional biopsy generally is the preferred technique for clinically atypical melanocytic neoplasms (lesions that are not definitively benign).4-8
When suspicion for melanoma is high, excisional biopsies should be performed with minimal undermining to preserve the accuracy of any future sentinel lymph node biopsy surgeries. Excisional biopsy is the most involved type of biopsy and has the largest potential for cosmetic disfigurement if not properly planned and performed. While guidelines from the American Academy of Dermatology state that “narrow excisional biopsy that encompasses [the] entire breadth of lesion with clinically negative margins to ensure that the lesion is not transected” is preferred, they also acknowledge that partial sampling (incisional biopsy) is acceptable in select clinical circumstances,9 such as when a lesion is large or on a cosmetically sensitive site such as the face.10
While a larger punch biopsy (6 or 8 mm) or even deep shave/saucerization may function as an excisional biopsy for very small lesions, this approach can be problematic. For one thing, these biopsies are more likely than an excisional biopsy to leave a portion of the lesion in situ. Another concern is that a shave biopsy of a melanocytic lesion can lead to error or difficulty in obtaining the correct diagnosis on later biopsy.11 For pathologists, smaller or incomplete samples make it challenging to establish an accurate diagnosis.12 Among melanomas seen at a tertiary referral center, histopathological misdiagnosis was more common with a punch or shave biopsy than with an excisional biopsy.9
It has been shown that partial biopsy for melanoma results in more residual disease at wide local excision and makes it more challenging to properly stage the lesion.13,14 If a shave biopsy is used to sample a suspected melanocytic neoplasm, it is imperative to document the specific site of the biopsy, indicate the size of the melanocytic lesion on the pathology requisition form, and ensure that all (or nearly all) of the clinically evident lesion is sampled. Detailing the location of the lesion in the chart is not only essential in evaluating the present lesion, but it will serve you well in the future. Without knowing the patient’s clinical history, benign nevi that recur after a prior biopsy can be difficult to histologically distinguish from melanoma (FIGURE 3). For more on this, see tip #7.
5. Be careful with curettage.
6. Remember the importance of proper fixation and processing.
As obvious as it may sound, it is important to remember to promptly place sampled tissue in an adequate amount of formalin so that the tissue is submersed in it in the container.15 Failure to do so can result in improper fixation and will make it difficult to render an appropriate diagnosis. Conventionally, a 10:1 formalin volume to tissue volume ratio is recommended. If the “cold time”—the amount of time a tissue sample is out of formalin—is long enough (greater than a few hours), an appropriate assessment can be impossible.
Appropriate fixation and fixation times are important because molecular testing is being increasingly used to make pathological diagnoses.16 Additionally, aggressively manipulating a biopsy sample while extracting it or placing it in formalin can cause “crush” artifact, which can limit interpretability (FIGURE 5).
7. Properly photograph and document the biopsy location.
When performing a biopsy of a suspicious neoplasm, physicians often remove all of the lesion’s superficial components, which means that at the patient’s follow-up appointment and subsequent treatments, only a well-healed biopsy site will remain. The biopsy site may be so well healed that it blends seamlessly into the surrounding skin and is nearly impossible for the physician to identify. This problem is seen most often when patients present for surgical excision or Mohs micrographic surgery.17
To properly record the site of a biopsy for future dermatologic exams, take pictures of the lesion at the time of biopsy. The photographs should clearly document the lesion in question, and should be taken far enough from the site that surrounding lesions and/ or other anatomic landmarks are also visible. Biangulation or triangulation (taking a series of 2 or 3 measurements, respectively, from the site of the lesion to nearby anatomic landmarks) can be used in conjunction with photographs.
When using measurements, be as specific and accurate as possible with anatomic terms. For example, measuring the distance from the “ear” is not helpful. It would be more helpful to measure the distance from the “tragus” or the “root of the helix.” Without a properly photographed and documented biopsy site, surgical treatment may need to be delayed until the location can be confirmed.
8. Give the pathologist a pertinent history.
Providing the pathologist with a sufficient history, including the distribution and appearance of the lesion, and how long the patient has had it, can be essential in narrowing the diagnosis or making the differential diagnoses. Things like medication use or new exposures to perfumes, lotions, or plants can be especially helpful and are often overlooked when filling out the pathology requisition form.
When warranted, phone calls are helpful. You might, for example, call the pathologist and give him or her a more detailed physical examination description or additional pertinent history that was discovered after the requisition was filled out. Providing a good history can make the difference between a specific diagnosis and a broad differential.
9. Know when to refer.
There is no shame in asking for a second opinion when it comes to evaluating a skin issue, especially in regards to melanocytic neoplasms, where the stakes can be high, or skin eruptions that do not respond to conventional therapy. Remember, many cases are difficult, even for experts, and require a careful balance of clinical and histopathological judgment.18
CORRESPONDENCE
Jayson Miedema, MD, Department of Dermatology, University of North Carolina at Chapel Hill, 410 Market Street, Suite 400, Chapel Hill, NC 27516; [email protected]
› Use an excisional biopsy for a melanocytic neoplasm. C
› Choose a punch biopsy over a shave biopsy for rashes. B
› Properly photograph and document the location of all lesions before biopsy. A
› Provide the pathologist with a sufficient history, including the distribution and appearance of the lesion, and how long the patient has had it. A
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Most physicians do a satisfactory job in choosing when and how to do a skin biopsy, but there is always room for improvement. The 9 pointers we provide here are based on standard of care practices and literature when available, and also on our collective experiences as a pathologist/dermatologist (JM), dermatopathologist (DZ), primary care physician (BR), and dermatologist/Mohs surgeon (EB).
1. Choose your biopsy type wisely.
Using the appropriate type of biopsy can have the greatest effect on a proper diagnosis. The decision of which biopsy type to use is not always easy. The most common biopsy types are shave, punch, excisional, and curettage. Several reference articles detail each type of biopsy commonly used in primary care and how to perform them.1,2 (For a series of how-to videos that illustrate how to perform some of these biopsies, visit The Journal of Family Practice Multimedia Library at http://www.jfponline.com/multimedia/video.html.)
Each type of biopsy has inherent advantages and disadvantages. In general, the shave biopsy is most commonly used for lesions that are solitary, elevated, and give the impression that a sufficient amount of tissue can be sampled using this technique. The punch biopsy is the biopsy of choice for most “rashes” (inflammatory skin disorders).2 Excisional biopsy is used to remove melanocytic neoplasms or larger lesions. And curettage, while still used by some clinicians for melanocytic lesions because of its speed and simplicity, should almost never be used for diagnostic purposes. Each of these techniques is described in greater detail in the tips that follow.
2. When performing a shave biopsy, avoid obtaining a sample that's too superficial.
The advantage of the shave biopsy is that it is minimally invasive and quick to perform. If kept small while not compromising the amount of sample retrieved, the scars left by shave biopsies have the potential to blend well. The major disadvantage of the shave biopsy is that occasionally, if the shave is not deep enough, an insufficient amount of tissue is obtained, which can make it challenging to establish an accurate diagnosis.
Balancing the need to obtain adequate tissue while minimizing scarring takes skill and experience. Taking a biopsy that is inadequate is a common occurrence. At times, the physician’s clinical impression may be that a biopsy has obtained adequate tissue, but histologically only the superficial part of the skin surface has been sampled. This often is because of thickening of the superficial skin, whether as a manifestation of the anatomic site (eg, acral skin) or the disease process itself.
Unfortunately, this superficial skin often is nondiagnostic when unaccompanied by underlying epidermis and dermis. It is important to keep this in mind when obtaining a skin biopsy, especially when dealing with lesions that are very scaly or keratinized. An equivocal biopsy wastes time, energy, and money, and can negatively impact patient care.3 It can be difficult to balance practical aspects of the biopsy (ie, optimizing cosmetic outcomes, minimizing scarring and wound size) with the need to obtain sufficient tissue sampling (FIGURE 1).
3. Choose punch over shave biopsy for rashes.
In a punch biopsy, a disposable metal cylinder with a sharpened edge is used to “punch” out a piece of skin that can be examined under the microscope. Punch biopsy is the preferred technique for almost all inflammatory skin conditions (rashes) because the pathologist is able to examine both the superficial and deep portions of the dermis4 (FIGURE 2).
Pathologists use the pattern of inflammation, in conjunction with epidermal changes, to distinguish different types of inflammatory processes. For example, lichen planus is typically associated with superficial inflammation, while lupus is known to have prominent superficial and deep inflammation.
An inadequate punch biopsy sample can hinder histological assessment of inflammatory skin disorders that involve both the superficial and deep portions of the dermis and can make arriving at a definitive diagnosis more challenging. The diameter of a punch cylinder ranges from 1 to 8 mm. Smaller punch biopsies often create diagnostic challenges because they provide so little sample. A punch biopsy size of 4 mm is commonly used for rashes.
An advantage of the punch biopsy is that patients are left with linear scars rather than round, potentially dyspigmented (darker or lighter) scars that are often associated with shave biopsy. A well-sutured punch biopsy can be cosmetically elegant, particularly if closure is oriented along relaxed skin tension lines. For this reason, punch biopsies are well suited for cosmetically sensitive locations such as the face, although shave biopsies are also often performed on the face.
4. Choose an excisional biopsy for a melanocytic neoplasm, when possible.
The purpose of an excisional biopsy (which typically includes a 1 to 3 mm rim of normal skin around the lesion) is to completely remove a lesion. The excisional biopsy generally is the preferred technique for clinically atypical melanocytic neoplasms (lesions that are not definitively benign).4-8
When suspicion for melanoma is high, excisional biopsies should be performed with minimal undermining to preserve the accuracy of any future sentinel lymph node biopsy surgeries. Excisional biopsy is the most involved type of biopsy and has the largest potential for cosmetic disfigurement if not properly planned and performed. While guidelines from the American Academy of Dermatology state that “narrow excisional biopsy that encompasses [the] entire breadth of lesion with clinically negative margins to ensure that the lesion is not transected” is preferred, they also acknowledge that partial sampling (incisional biopsy) is acceptable in select clinical circumstances,9 such as when a lesion is large or on a cosmetically sensitive site such as the face.10
While a larger punch biopsy (6 or 8 mm) or even deep shave/saucerization may function as an excisional biopsy for very small lesions, this approach can be problematic. For one thing, these biopsies are more likely than an excisional biopsy to leave a portion of the lesion in situ. Another concern is that a shave biopsy of a melanocytic lesion can lead to error or difficulty in obtaining the correct diagnosis on later biopsy.11 For pathologists, smaller or incomplete samples make it challenging to establish an accurate diagnosis.12 Among melanomas seen at a tertiary referral center, histopathological misdiagnosis was more common with a punch or shave biopsy than with an excisional biopsy.9
It has been shown that partial biopsy for melanoma results in more residual disease at wide local excision and makes it more challenging to properly stage the lesion.13,14 If a shave biopsy is used to sample a suspected melanocytic neoplasm, it is imperative to document the specific site of the biopsy, indicate the size of the melanocytic lesion on the pathology requisition form, and ensure that all (or nearly all) of the clinically evident lesion is sampled. Detailing the location of the lesion in the chart is not only essential in evaluating the present lesion, but it will serve you well in the future. Without knowing the patient’s clinical history, benign nevi that recur after a prior biopsy can be difficult to histologically distinguish from melanoma (FIGURE 3). For more on this, see tip #7.
5. Be careful with curettage.
6. Remember the importance of proper fixation and processing.
As obvious as it may sound, it is important to remember to promptly place sampled tissue in an adequate amount of formalin so that the tissue is submersed in it in the container.15 Failure to do so can result in improper fixation and will make it difficult to render an appropriate diagnosis. Conventionally, a 10:1 formalin volume to tissue volume ratio is recommended. If the “cold time”—the amount of time a tissue sample is out of formalin—is long enough (greater than a few hours), an appropriate assessment can be impossible.
Appropriate fixation and fixation times are important because molecular testing is being increasingly used to make pathological diagnoses.16 Additionally, aggressively manipulating a biopsy sample while extracting it or placing it in formalin can cause “crush” artifact, which can limit interpretability (FIGURE 5).
7. Properly photograph and document the biopsy location.
When performing a biopsy of a suspicious neoplasm, physicians often remove all of the lesion’s superficial components, which means that at the patient’s follow-up appointment and subsequent treatments, only a well-healed biopsy site will remain. The biopsy site may be so well healed that it blends seamlessly into the surrounding skin and is nearly impossible for the physician to identify. This problem is seen most often when patients present for surgical excision or Mohs micrographic surgery.17
To properly record the site of a biopsy for future dermatologic exams, take pictures of the lesion at the time of biopsy. The photographs should clearly document the lesion in question, and should be taken far enough from the site that surrounding lesions and/ or other anatomic landmarks are also visible. Biangulation or triangulation (taking a series of 2 or 3 measurements, respectively, from the site of the lesion to nearby anatomic landmarks) can be used in conjunction with photographs.
When using measurements, be as specific and accurate as possible with anatomic terms. For example, measuring the distance from the “ear” is not helpful. It would be more helpful to measure the distance from the “tragus” or the “root of the helix.” Without a properly photographed and documented biopsy site, surgical treatment may need to be delayed until the location can be confirmed.
8. Give the pathologist a pertinent history.
Providing the pathologist with a sufficient history, including the distribution and appearance of the lesion, and how long the patient has had it, can be essential in narrowing the diagnosis or making the differential diagnoses. Things like medication use or new exposures to perfumes, lotions, or plants can be especially helpful and are often overlooked when filling out the pathology requisition form.
When warranted, phone calls are helpful. You might, for example, call the pathologist and give him or her a more detailed physical examination description or additional pertinent history that was discovered after the requisition was filled out. Providing a good history can make the difference between a specific diagnosis and a broad differential.
9. Know when to refer.
There is no shame in asking for a second opinion when it comes to evaluating a skin issue, especially in regards to melanocytic neoplasms, where the stakes can be high, or skin eruptions that do not respond to conventional therapy. Remember, many cases are difficult, even for experts, and require a careful balance of clinical and histopathological judgment.18
CORRESPONDENCE
Jayson Miedema, MD, Department of Dermatology, University of North Carolina at Chapel Hill, 410 Market Street, Suite 400, Chapel Hill, NC 27516; [email protected]
1. Pickett H. Shave and punch biopsy for skin lesions. Am Fam Physician. 2011;84:995-1002.
2. Alguire PC, Mathes BM. Skin biopsy techniques for the internist. J Gen Intern Med. 1998;13:46-54.
3. Fernandez EM, Helm T, Ioffreda M, et al. The vanishing biopsy: the trend toward smaller specimens. Cutis. 2005;76:335-339.
4. Hieken TJ, Hernández-Irizarry R, Boll JM, et al. Accuracy of diagnostic biopsy for cutaneous melanoma: implications for surgical oncologists. Int J Surg Oncol. 2013;2013:196493.
5. Scolyer RA, Thompson JF, McCarthy SW, et al. Incomplete biopsy of melanocytic lesions can impair the accuracy of pathological diagnosis. Australas J Dermatol. 2006;47:71-75.
6. McCarthy SW, Scolyer RA. Pitfalls and important issues in the pathologic diagnosis of melanocytic tumors. Ochsner J. 2010;10:66-74.
7. Swanson NA, Lee KK, Gorman A, et al. Biopsy techniques. Diagnosis of melanoma. Dermatol Clin. 2002;20:677-680.
8. Chang TT, Somach SC, Wagamon K, et al. The inadequacy of punch-excised melanocytic lesions: sampling through the block for the determination of “margins”. J Am Acad Dermatol. 2009;60: 990-993.
9. Bichakjian CK, Halpern AC, Johnson TM, et al; American Academy of Dermatology. Guidelines of care for the management of primary cutaneous melanoma. American Academy of Dermatology. J Am Acad Dermatol. 2011;65:1032-1047.
10. Pardasani AG, Leshin B, Hallman JR, et al. Fusiform incisional biopsy for pigmented skin lesions. Dermatol Surg. 2000;26:622-624.
11. King R, Hayzen BA, Page RN, et al. Recurrent nevus phenomenon: a clinicopathologic study of 357 cases and histologic comparison with melanoma with regression. Mod Pathol. 2009;22:611-617.
12. Mills JK, White I, Diggs B, et al. Effect of biopsy type on outcomes in the treatment of primary cutaneous melanoma. Am J Surg. 2013;205:585-590.
13. Stell VH, Norton HJ, Smith KS, et al. Method of biopsy and incidence of positive margins in primary melanoma. Ann Surg Oncol. 2007;14:893-898.
14. Egnatios GL, Dueck AC, Macdonald JB, et al. The impact of biopsy technique on upstaging, residual disease, and outcome in cutaneous melanoma. Am J Surg. 2011;202:771-778.
15. Ackerman AB, Boer A, Bennin B, et al. Histologic Diagnosis of Inflammatory Skin Disease: An Algorithmic Method Based on Pattern Analysis. New York, NY: Ardor Scribendi; 2005.
16. Hewitt SM, Lewis FA, Cao Y, et al. Tissue handling and specimen preparation in surgical pathology: issues concerning the recovery of nucleic acids from formalin-fixed, paraffin-embedded tissue. Arch Pathol Lab Med. 2008;132:1929-1935.
17. Nemeth SA, Lawrence N. Site identification challenges in dermatologic surgery: a physician survey. J Am Acad Dermatol. 2012;67: 262-268.
18. Federman DG, Concato J, Kirsner RS. Comparison of dermatologic diagnoses by primary care practitioners and dermatologists. A review of the literature. Arch Fam Med. 1999;8:170-172.
1. Pickett H. Shave and punch biopsy for skin lesions. Am Fam Physician. 2011;84:995-1002.
2. Alguire PC, Mathes BM. Skin biopsy techniques for the internist. J Gen Intern Med. 1998;13:46-54.
3. Fernandez EM, Helm T, Ioffreda M, et al. The vanishing biopsy: the trend toward smaller specimens. Cutis. 2005;76:335-339.
4. Hieken TJ, Hernández-Irizarry R, Boll JM, et al. Accuracy of diagnostic biopsy for cutaneous melanoma: implications for surgical oncologists. Int J Surg Oncol. 2013;2013:196493.
5. Scolyer RA, Thompson JF, McCarthy SW, et al. Incomplete biopsy of melanocytic lesions can impair the accuracy of pathological diagnosis. Australas J Dermatol. 2006;47:71-75.
6. McCarthy SW, Scolyer RA. Pitfalls and important issues in the pathologic diagnosis of melanocytic tumors. Ochsner J. 2010;10:66-74.
7. Swanson NA, Lee KK, Gorman A, et al. Biopsy techniques. Diagnosis of melanoma. Dermatol Clin. 2002;20:677-680.
8. Chang TT, Somach SC, Wagamon K, et al. The inadequacy of punch-excised melanocytic lesions: sampling through the block for the determination of “margins”. J Am Acad Dermatol. 2009;60: 990-993.
9. Bichakjian CK, Halpern AC, Johnson TM, et al; American Academy of Dermatology. Guidelines of care for the management of primary cutaneous melanoma. American Academy of Dermatology. J Am Acad Dermatol. 2011;65:1032-1047.
10. Pardasani AG, Leshin B, Hallman JR, et al. Fusiform incisional biopsy for pigmented skin lesions. Dermatol Surg. 2000;26:622-624.
11. King R, Hayzen BA, Page RN, et al. Recurrent nevus phenomenon: a clinicopathologic study of 357 cases and histologic comparison with melanoma with regression. Mod Pathol. 2009;22:611-617.
12. Mills JK, White I, Diggs B, et al. Effect of biopsy type on outcomes in the treatment of primary cutaneous melanoma. Am J Surg. 2013;205:585-590.
13. Stell VH, Norton HJ, Smith KS, et al. Method of biopsy and incidence of positive margins in primary melanoma. Ann Surg Oncol. 2007;14:893-898.
14. Egnatios GL, Dueck AC, Macdonald JB, et al. The impact of biopsy technique on upstaging, residual disease, and outcome in cutaneous melanoma. Am J Surg. 2011;202:771-778.
15. Ackerman AB, Boer A, Bennin B, et al. Histologic Diagnosis of Inflammatory Skin Disease: An Algorithmic Method Based on Pattern Analysis. New York, NY: Ardor Scribendi; 2005.
16. Hewitt SM, Lewis FA, Cao Y, et al. Tissue handling and specimen preparation in surgical pathology: issues concerning the recovery of nucleic acids from formalin-fixed, paraffin-embedded tissue. Arch Pathol Lab Med. 2008;132:1929-1935.
17. Nemeth SA, Lawrence N. Site identification challenges in dermatologic surgery: a physician survey. J Am Acad Dermatol. 2012;67: 262-268.
18. Federman DG, Concato J, Kirsner RS. Comparison of dermatologic diagnoses by primary care practitioners and dermatologists. A review of the literature. Arch Fam Med. 1999;8:170-172.
Which CAM modalities are worth considering?
› Consider referring your patients for guided imagery to reduce anxiety or pain. A
› Recommend a trial of glucosamine sulfate 1500 mg/d for 3 months for patients with osteoarthritis. B
› Consider acupuncture as a treatment option for patients with chronic pain. B
› Use probiotics to prevent antibiotic-associated diarrhea in pediatric patients, except for those who are immunocompromised or have an indwelling medical device. B
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
CASE › Bob F, age 54, seeks care for chronic low back pain. The conservative treatments you have prescribed, including physical therapy, regular exercise, and an over-the-counter nonsteroidal anti-inflammatory drug, have provided minimal pain relief. Mr. F is reluctant to take a prescription pain medication and has expressed interest in trying a complementary and alternative medicine (CAM) therapy, such as acupuncture or yoga. What should you tell him?
Almost 40% of Americans use CAM modalities to treat specific conditions or for overall well-being,1 and these practices are increasingly becoming a part of our approach to health care, as evidenced by the nearly 50 of facilities across the country that boast integrative health care programs, which combine CAM modalities with conventional medicine.2 Emerging evidence suggests several integrative practices may offer health benefits, and primary care physicians must become well-versed in these modalities to effectively communicate potential benefits and harms to patients. In this article, we present evidence from Cochrane reviews and other studies of 8 commonly used CAM therapies, including dietary interventions, a psychotherapeutic modality, and other treatments (TABLE).1,3-30 And while motivational interviewing technically is not a form of CAM, we also review this modality, which has proven useful in the treatment of patients for substance use. (See “Motivational interviewing for substance abuse.”)
Fish oil for hypertriglyceridemia
High triglyceride levels are a risk factor for cardiovascular disease and a component of metabolic syndrome.8 A 2008 review of 47 randomized controlled trials (RCTs) that included 16,511 participants found that omega-3 fatty acid (fish oil) supplements significantly reduced triglyceride levels compared to placebo.7 The American Heart Association recommends 2 to 4 g/d of eicosapentaenoic acid (EPA) plus docosahexaenoic acid (DHA) to lower triglyceride levels.8
Most studies have found that fish oil supplements are associated with few adverse effects; gastrointestinal (GI) complaints are most common. However, these supplements should be discontinued following an acute bleeding event, such as hemorrhagic stroke, due to their anticoagulant properties.9 Some evidence suggests that the risk for prostate cancer is increased in men with high blood levels of omega-3 fatty acids.10
Glucosamine for osteoarthritis
Glucosamine is an amino sugar that is a building block of cartilage proteoglycans. Although it occurs naturally in the body, the glucosamine used in supplements is typically harvested from seashells. Glucosamine stimulates the metabolism of synovial cells and chondrocytes in articular cartilage and may delay joint degeneration.31,32
Glucosamine is widely used in the United States as a dietary supplement, most often as glucosamine sulfate but also as n-acetyl glucosamine and glucosamine hydrochloride, although there is limited evidence of effectiveness for the latter formulations.33
Most studies have examined the effects of oral glucosamine sulfate, 500 mg taken 3 times a day for 30 to 90 days. Once-a-day dosing as high as 1500 mg also has been used.
A Cochrane review of 25 studies with 4963 patients concluded that oral glucosamine sulfate may reduce osteoarthritis (OA) pain and improve functionality, without many adverse effects.11 A 2-year double-blind RCT compared the effects of glucosamine hydrochloride 500 mg tid, chondroitin sulfate 400 mg tid, glucosamine plus chondroitin, celecoxib 200 mg/d, or placebo in 662 patients with knee OA.12 While all groups experienced early and sustained symptomatic relief, the odds of achieving a 20% reduction in pain and improved functioning were highest with celecoxib and glucosamine.
Oral glucosamine sulfate can cause mild GI effects, but drowsiness, skin reactions, and headache also have been reported. Shellfish allergy also is a concern; however, shellfish allergies occur due to the proteins in the meat, and not from the shell from which glucosamine is derived. Glucosamine may increase glucose levels and the anticoagulant effects of warfarin.13
Probiotics to prevent antibiotic-associated diarrhea
Antibiotic-associated diarrhea (AAD) is a common problem.21 Probiotics—microorganisms found in oral supplements, yogurt, and other food—are commonly used to help maintain the balance of intestinal flora.34 A recent Cochrane review of 16 RCTs that included approximately 3400 patients found evidence that probiotics can prevent AAD.22 A 2012 systematic review and meta-analysis of 63 RCTs with more than 11,000 participants concluded that probiotics lowered the relative risk of developing diarrhea compared to control groups.35 The American Academy of Pediatrics supports the use of probiotics, citing results from a meta-analysis that found probiotics reduced the risk of developing diarrhea from 28.5% to 11.9% compared to placebo.36
Exact dosages for probiotics have not been established, and recommendations range from 5 billion to 40 billion colony-forming units/d.22 The most commonly used probiotics are from the Lactobacillus and Saccharomyces genera; relatively little evidence supports other genera.21,22,35,36
Probiotics are considered relatively safe, but are not recommended for patients who are immunocompromised or have an indwelling medical device.23 Adverse effects are rare, but may include flatulence, vomiting, rash, chest pain, and increased phlegm.21
For a review of the latest evidence on using probiotics to reduce crying in infants with colic, see "Probiotics for colic? A PURL update."
Soy for hyperlipidemia
Soybeans are a species of legume that contain significant amounts of protein, fiber, potassium, and iron. Although soy has been used to prevent or treat cancer, osteoporosis, and menopausal symptoms, current evidence is unfavorable or inconclusive for such conditions. Some RCTs have found soy has small, favorable effects on serum levels of low-density lipoprotein and total cholesterol,24 while others have shown modest improvements in triglyceride levels without significant improvements in other lipid levels.25
A 2011 meta-analysis of 10 RCTs that included 268 participants found that a diet high in non-soy legume products, such as alfalfa, lentils, and other beans, also improved lipid levels.37 A review of 136 studies that described 22 dietary interventions concluded that among other helpful dietary approaches to controlling hyperlipidemia, dietary soy—which contains fiber and polyunsaturated fats—is favored over supplementation of soy protein alone.38
Use caution when recommending soy for patients with thyroid dysfunction or hormone-sensitive cancers because some evidence suggests soy may interfere with absorption of levothyroxine and increase the risk of developing clinical symptoms of hypothyroidism.39
Soy also contains phytoestrogens, and prolonged use of soy supplements may increase the risk of endometrial hyperplasia.24 This risk has been documented only in the use of soy supplements, and not from dietary soy. GI disturbances and rare allergic reactions also have been reported.24
St. John’s wort for depression
Hypericum perforatum (St. John’s wort), a perennial herb, has been used to treat mood disorders and other ailments for more than 2000 years.40,41 Commercial preparations typically are alcohol extracts with an herb-to-extract ratio of 4:1 to 8:1.26 The normal dose ranges from 900 to 1500 mg/d in 2 to 3 divided doses of the alcohol extract standardized to 0.3% hypericin and/or 3% to 5% hyperforin.
St. John’s wort has been studied extensively as a treatment for depressive disorders. A 2001 double-blind RCT conducted in 11 US academic medical centers and community clinics between 1998 and 2000 that included 200 patients found that St. John’s wort was not effective for moderately severe major depression; a trend toward a positive effect was noted in both the placebo and St. John’s wort groups.26
However, a 2009 Cochrane review of 29 international studies (5489 patients) concluded that St. John’s wort may be better than placebo and as effective as antidepressants for mild to moderate major depression,27 and appeared to have fewer side effects than antidepressants. This review, conducted in German-speaking countries where medical professionals have long prescribed St. John’s wort, reported more positive results than those conducted in other countries.
St. John’s wort interacts with many medications, including antidepressants, oral contraceptives, cyclosporine, digoxin, indinavir, phenytoin, phenobarbital, warfarin, and others. It induces cytochrome P450 (CYP450) enzymes, and therefore can potentially reduce the efficacy of any medication that is metabolized by a CYP450 enzyme. When used in high doses in combination with antidepressants, St. John’s wort may cause serotonin syndrome. Other side effects include photosensitivity, GI complaints, fatigue, and increased risk of cataracts. Due to a lack of clinical data, St. John’s wort is contraindicated in women who are pregnant or breastfeeding.42
Motivational interviewing (MI) is an alternative approach to traditional provider-patient communication that entails using open-ended questions, reflective listening, affirmation, and assessing readiness to change.1 MI facilitators aim to elicit change and assist patients in forming a self-management plan with specific, measurable, achievable, realistic, and timely (SMART) goals.1-3
MI can be efficiently implemented in diverse settings and by a variety of trained facilitators.3-5 For example, the Brief Negotiation Interview requires only 7 minutes per emergency department patient and effectively improves long-term outcomes for substance abusers.4 A randomized controlled trial that included 135 patients admitted to a psychiatric emergency inpatient unit for substance abuse found that those who received 2 sessions of MI reported significantly less substance use than controls 2 years after the intervention.3
Training for providers to ensure proper implementation of MI techniques is essential because poor use of MI can be counter-therapeutic.5 Tools such as the Motivational Interviewing Treatment Integrity Scale and the Client Evaluation of Motivational Interviewing can be used to ensure providers are competent.4,6
References
1. Miller WR, Rollnick S. Motivational Interviewing: Preparing People to Change Addictive Behavior. New York, NY: Guilford Press; 1991.
2. Levensky ER, Forcehimes A, O’Donohue WT, et al. Motivational interviewing: an evidence-based approach to counseling helps patients follow treatment recommendations. Am J Nurse. 2007;107:50-59.
3. Bagøien G, Bjørngaard JH, Østensen C, et al. The effects of motivational interviewing on patients with comorbid substance use admitted to a psychiatric emergency unit - a randomized controlled trial with two year follow-up. BMC Psychiatry. 2013;13:93.
4. D’Onofrio G, Fiellin DA, Pantalon MV, et al. A brief intervention reduces hazardous and harmful drinking in emergency department patients. Ann Emerg Med. 2012;60:181-192.
5. Tollison SJ, Mastroleo NR, Mallett KA, et al. The relationship between baseline drinking status, peer motivational interviewing microskills, and drinking outcomes in a brief alcohol intervention for matriculating college students: a replication. Behav Ther. 2013;44:137-151.
6. Madson MB, Mohn RS, Zuckoff A, et al. Measuring client perceptions of motivational interviewing: factor analysis of the Client Evaluation of Motivational Interviewing scale. J Subst Abuse Treat. 2013;44:330-335.
Guided imagery for anxiety and pain
Guided imagery is a relaxation technique that involves visualizing positive outcomes to reduce one’s reaction to anxiety-provoking or painful experiences.43 It can be practiced independently or under the direction of an instructor. One RCT of 96 women with newly diagnosed breast cancer found that adding relaxation and guided imagery to standard breast cancer treatment protocols positively affected mood and quality of life.14 While this study saw no change in pathologic responses to chemotherapy,14 a more recent RCT concluded that such biochemical advantages may be possible.44 Guided imagery has been linked to decreased anxiety in diverse studies of students, women in labor, individuals suffering from nightmares, and in occupational settings such as training for pilots and surgeons.15-18
A review of 9 RCTs of guided imagery for decreasing musculoskeletal pain involving 201 patients found 8 studies reported positive results, though the methodological quality of the studies was low.19 Of 6 high-quality studies included in a 2012 systematic review, 5 supported the use of guided imagery for postoperative, abdominal, and other nonmusculoskeletal pain.20 This initial evidence is promising, but additional research of high methodological quality is needed to validate the use of guided imagery for anxiety and pain.
Acupuncture for pain
From 2002 to 2007, the use of acupuncture significantly increased in the United States, primarily for the treatment of pain.1 A 2012 meta-analysis of 29 RCTs that included almost 18,000 participants evaluated the clinical usefulness of acupuncture for back, neck, and shoulder pain, OA, and headache.3 Compared to no treatment, both acupuncture and sham acupuncture significantly improved pain scores. The authors of this meta-analysis found that acupuncture offered a small but significant advantage over sham acupuncture, and concluded that the benefits of acupuncture were not due to a placebo effect.
In 2007, the American College of Physicians (ACP) and the American Pain Society (APS) issued a joint statement indicating that acupuncture should be considered for patients with chronic low back pain who do not respond to conventional therapies.4 The North American Spine Society also supports acupuncture, stating that it provides “...better short-term pain relief and functional improvement than no treatment and the addition of acupuncture to other treatment modalities provides a greater benefit than those treatments alone.”5 Additional evidence found acupuncture for chronic low back pain improves function and serves as an adjunct therapy.6
Reported adverse effects of acupuncture include—but may not be limited to—infection, skin irritation, hematoma, pneumothorax, and spontaneous needle migration.1,3-6
Yoga for low back pain
Back pain is the most common reason patients use CAM therapies.1 A systematic review of 10 RCTs that included 967 participants with chronic low back pain found strong evidence for the short-term effectiveness and moderate evidence for the long-term effectiveness of yoga.28 A review of 17 studies that included 1626 patients concluded that yoga improves both pain and functionality; this review did not recommend a specific type of yoga practice.29 In a recent study of 95 minority adults with moderate-to-severe chronic low back pain, once-weekly and twice-weekly yoga for 12 weeks were similarly effective for reducing pain and improving functionality.30
Guidelines from the ACP and the APS recommend yoga as part of an intensive interdisciplinary rehabilitation program for patients with chronic or subacute low back pain who do not improve using other self-care options.4 This recommendation is specifically for Viniyoga, a practice in which the instructor recommends modifications to body positioning for each individual based on past injuries and overall physical condition. (For more information on therapeutic uses of yoga, see “Yoga as therapy: When is it helpful?”)
CORRESPONDENCE
Roger Zoorob, MD, MPH, FAAFP; Department of Family and Community Medicine, Baylor College of Medicine, 3701 Kirby Drive, Suite 600, Houston, TX 77098; [email protected]
1. Barnes PM, Bloom B, Nahin RL. Complementary and alternative medicine use among adults and children: United States, 2007. Natl Health Stat Report. 2008;10:1-23.
2. American Holistic Medicine Association. Integrative medicine centers. American Holistic Medicine Association Web site. Available at: http://www.holisticmedicine.org/content. asp?pl=30&sl=2&contentid=74. Accessed September 8, 2014.
3. Vickers AJ, Cronin AM, Maschino AC, et al; Acupuncture Trialists’ Collaboration. Acupuncture for chronic pain: individual patient data meta-analysis. Arch Intern Med. 2012;172:1444-1453.
4. Chou R, Qaseem A, Snow V, et al; Clinical Efficacy Assessment Subcommittee of the American College of Physicians; American College of Physicians; American Pain Society Low Back Pain Guidelines Panel. Diagnosis and treatment of low back pain: a joint clinical practice guideline from the American College of Physicians and the American Pain Society. Ann Intern Med. 2007;147:478-491.
5. Berman BM, Langevin HM, Witt CM, et al. Acupuncture for chronic low back pain. N Engl J Med. 2010;363:454-461.
6. Last AR, Hulbert K. Chronic low back pain: evaluation and management. Am Fam Physician. 2009;79:1067-1074.
7. Eslik GD, Howe PR, Smith C, et al. Benefits of fish oil supplementation in hyperlipidemia: a systematic review and meta-analysis. Int J Cardiol. 2009;136:4-16.
8. Miller M, Stone NJ, Ballantyne C, et al; American Heart Association Clinical Lipidology, Thrombosis, and Prevention Committeeof the Council on Nutrition, Physical Activity, and Metabolism; Council on Arteriosclerosis, Thrombosis and Vascular Biology; Council on Cardiovascular Nursing; Council on the Kidney in Cardiovascular Disease. Triglyceride and cardiovascular disease: a scientific statement from the American Heart Association. Circulation. 2011;123:2292-2333.
9. Bays HE. Safety considerations with omega-3 fatty acid therapy. Am J Cardiol. 2007;99:35C-43C.
10. Brasky TM, Darke AK, Song X, et al. Plasma phospholipid fatty acids and prostate cancer risk in the SELECT trial. J Natl Cancer Inst. 2013;105:1132-1141.
11. Towheed TE, Maxwell L, Anastassiades TP, et al. Glucosamine therapy for treating osteoarthritis. Cochrane Database Syst Rev. 2005;(2):CD002946.
12. Sawitzke AD, Shi H, Finco MF, et al. Clinical efficacy and safety of glucosamine, chondroitin sulphate, their combination, celecoxib or placebo taken to treat osteoarthritis of the knee: 2-year results from GAIT. Ann Rheum Dis. 2010;69:1459-1464.
13. Dostrovsky NR, Towheed TE, Hudson RW, et al. The effect of glucosamine on glucose metabolism in humans: a systematic review of the literature. Osteoarthritis Cartilage. 2011;19:375-380.
14. Walker LG, Walker MB, Ogston K, et al. Psychological, clinical and pathological effects of relaxation training and guided imagery during primary chemotherapy. Br J Cancer. 1999;80:262-268.
15. Thünker J, Pietrowsky R. Effectiveness of a manualized imagery rehearsal therapy for patients suffering from nightmare disorders with and without a comorbidity of depression or PTSD. Behav Res Ther. 2012;50:558-564.
16. Marc I, Toureche N, Ernst E, et al. Mind-body interventions during pregnancy for preventing or treating women’s anxiety. Cochrane Database Syst Rev. 2011;(7):CD007559.
17. Jing X, Wu P, Liu F, et al. Guided imagery, anxiety, heart rate, and heart rate variability during centrifuge training. Aviat Space Environ Med. 2011;82:92-96.
18. Arora S, Aggarwal R, Moran A, et al. Mental practice: effective stress management training for novice surgeons. J Am Coll Surg. 2011;212:225-233.
19. Posadzki P, Ernst E. Guided imagery for musculoskeletal pain: a systematic review. Clin J Pain. 2011;27:648-653.
20. Posadzki P, Lewandowski W, Terry R, et al. Guided imagery for non-musculoskeletal pain: a systematic review of randomized clinical trials. J Pain Symptom Manage. 2012;44:95-104.
21. Arvola T, Laiho K, Torkkeli S, et al. Prophylactic Lactobacillus GG reduces antibiotic-associated diarrhea in children with respiratory infections: a randomized study. Pediatrics. 1999;104:e64.
22. Johnston BC, Goldenberg JZ, Vandvik PO, et al. Probiotics for the prevention of pediatric antibiotic-associated diarrhea. Cochrane Database Syst Rev. 2011;(11):CD004827.
23. Williams NT. Probiotics. Am J Health-Syst Pharm. 2010;67:449-458.
24. Sacks FM, Lichtenstein A, Van Horn L, et al; American Heart Association Nutrition Committee. Soy protein, isoflavones, and cardiovascular health: an American Heart Association Science Advisory for professionals from the Nutrition Committee. Circulation. 2006;113:1034-1044.
25. Qin Y, Niu K, Zeng Y, et al. Isoflavones for hypercholesterolaemia in adults. Cochrane Database Syst Rev. 2013;6:CD009518.
26. Shelton RC, Keller MB, Gelenberg A, et al. Effectiveness of St John’s wort in major depression: a randomized controlled trial. JAMA. 2001;285:1978-1986.
27. Linde K, Berner MM, Kriston L. St John’s wort for major depression. Cochrane Database Syst Rev. 2008;(4):CD000448.
28. Cramer H, Lauche R, Haller H, et al. A systematic review and meta-analysis of yoga for low back pain. Clin J Pain. 2013;29: 450-460.
29. Ward L, Stebbings S, Cherkin D, et al. Yoga for functional ability, pain and psychosocial outcomes in musculoskeletal conditions: a systematic review and meta-analysis. Musculoskeletal Care. 2013;11:203-217.
30. Saper RB, Boah AR, Keosaian J, et al. Comparing once- versus twice-weekly yoga classes for chronic low back pain in predominantly low income minorities: a randomized dosing trial. Evid Based Complement Alternat Med. 2013;2013:658030.
31. Uitterlinden EJ, Koevoet JLM, Verkoelen CF, et al. Glucosamine increases hyaluronic acid production in human osteoarthritic synovium explants. BMC Musculoskelet Disord. 2008;9:120.
32. Calamia V, Ruiz-Romero C, Rocha B, et al. Pharmacoproteomic study of the effects of chondroitin and glucosamine sulfate on human articular chondrocytes. Arthritis Res Ther. 2010;12:R138.
33. Rovati LC, Girolami F, Persiani S. Crystalline glucosamine sulfate in the management of knee osteoarthritis: efficacy, safety, and pharmacokinetic properties. Ther Adv Musculoskelet Dis. 2012;4:167-180.
34. National Center for Complementary and Alternative Medicine. Oral probiotics: An introduction. National Center for Complementary and Alternative Medicine Web site. Available at: http:// nccam.nih.gov/health/probiotics/introduction.htm. Updated December 2012. Accessed August 29, 2013.
35. Hempel S, Newberry SJ, Maher AR, et al. Probiotics for the prevention and treatment of antibiotic-associated diarrhea: a systematic review and meta-analysis. JAMA. 2012;307:1959-1969.
36. Thomas DW, Greer FR; American Academy of Pediatrics Committee on Nutrition; American Academy of Pediatrics Section on Gastroenterology, Hepatology, and Nutrition. Probiotics and prebiotics in pediatrics. Pediatrics. 2010;126:1217-1231.
37. Bazzano LA, Thompson AM, Tees MT, et al. Non-soy legume consumption lowers cholesterol levels: a meta-analysis of randomized controlled trials. Nutr Metab Cardiovasc Dis. 2011;21:94-103.
38. Huang J, Frohlich J, Ignaszewski AP. The impact of dietary changes and dietary supplements on lipid profile. Can J Cardiol. 2011;27:488-505.
39. Messina M, Redmond G. Effects of soy protein and soybean isoflavones on thyroid function in healthy adults and hypothyroid patients: a review of the relevant literature. Thyroid. 2006;16: 249-258.
40. National Center for Complementary and Alternative Medicine. St. John’s Wort. National Center for Complementary and Alternative Medicine Web site. Available at: http://nccam.nih.gov/ health/stjohnswort. Accessed October 14, 2013.
41. Schulz V, Hänsel R, Tyler VE. Rational Phytotherapy: A Physicians’ Guide to Herbal Medicine. Berlin: Springer; 2001:62-81.
42. Mischoulon D. Update and critique of natural remedies as antidepressant treatments. Obstet Gynecol Clin North Am. 2009;36: 789-807.
43. Hanssen MM, Peters ML, Vlaeyen JW, et al. Optimism lowers pain: evidence of the causal status and underlying mechanisms. Pain. 2013;154:53-58.
44. Eremin O, Walker MB, Simpson E, et al. Immuno-modulatory effects of relaxation training and guided imagery in women with locally advanced breast cancer undergoing multimodality therapy: a randomised controlled trial. Breast. 2009;18:17-25.
› Consider referring your patients for guided imagery to reduce anxiety or pain. A
› Recommend a trial of glucosamine sulfate 1500 mg/d for 3 months for patients with osteoarthritis. B
› Consider acupuncture as a treatment option for patients with chronic pain. B
› Use probiotics to prevent antibiotic-associated diarrhea in pediatric patients, except for those who are immunocompromised or have an indwelling medical device. B
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
CASE › Bob F, age 54, seeks care for chronic low back pain. The conservative treatments you have prescribed, including physical therapy, regular exercise, and an over-the-counter nonsteroidal anti-inflammatory drug, have provided minimal pain relief. Mr. F is reluctant to take a prescription pain medication and has expressed interest in trying a complementary and alternative medicine (CAM) therapy, such as acupuncture or yoga. What should you tell him?
Almost 40% of Americans use CAM modalities to treat specific conditions or for overall well-being,1 and these practices are increasingly becoming a part of our approach to health care, as evidenced by the nearly 50 of facilities across the country that boast integrative health care programs, which combine CAM modalities with conventional medicine.2 Emerging evidence suggests several integrative practices may offer health benefits, and primary care physicians must become well-versed in these modalities to effectively communicate potential benefits and harms to patients. In this article, we present evidence from Cochrane reviews and other studies of 8 commonly used CAM therapies, including dietary interventions, a psychotherapeutic modality, and other treatments (TABLE).1,3-30 And while motivational interviewing technically is not a form of CAM, we also review this modality, which has proven useful in the treatment of patients for substance use. (See “Motivational interviewing for substance abuse.”)
Fish oil for hypertriglyceridemia
High triglyceride levels are a risk factor for cardiovascular disease and a component of metabolic syndrome.8 A 2008 review of 47 randomized controlled trials (RCTs) that included 16,511 participants found that omega-3 fatty acid (fish oil) supplements significantly reduced triglyceride levels compared to placebo.7 The American Heart Association recommends 2 to 4 g/d of eicosapentaenoic acid (EPA) plus docosahexaenoic acid (DHA) to lower triglyceride levels.8
Most studies have found that fish oil supplements are associated with few adverse effects; gastrointestinal (GI) complaints are most common. However, these supplements should be discontinued following an acute bleeding event, such as hemorrhagic stroke, due to their anticoagulant properties.9 Some evidence suggests that the risk for prostate cancer is increased in men with high blood levels of omega-3 fatty acids.10
Glucosamine for osteoarthritis
Glucosamine is an amino sugar that is a building block of cartilage proteoglycans. Although it occurs naturally in the body, the glucosamine used in supplements is typically harvested from seashells. Glucosamine stimulates the metabolism of synovial cells and chondrocytes in articular cartilage and may delay joint degeneration.31,32
Glucosamine is widely used in the United States as a dietary supplement, most often as glucosamine sulfate but also as n-acetyl glucosamine and glucosamine hydrochloride, although there is limited evidence of effectiveness for the latter formulations.33
Most studies have examined the effects of oral glucosamine sulfate, 500 mg taken 3 times a day for 30 to 90 days. Once-a-day dosing as high as 1500 mg also has been used.
A Cochrane review of 25 studies with 4963 patients concluded that oral glucosamine sulfate may reduce osteoarthritis (OA) pain and improve functionality, without many adverse effects.11 A 2-year double-blind RCT compared the effects of glucosamine hydrochloride 500 mg tid, chondroitin sulfate 400 mg tid, glucosamine plus chondroitin, celecoxib 200 mg/d, or placebo in 662 patients with knee OA.12 While all groups experienced early and sustained symptomatic relief, the odds of achieving a 20% reduction in pain and improved functioning were highest with celecoxib and glucosamine.
Oral glucosamine sulfate can cause mild GI effects, but drowsiness, skin reactions, and headache also have been reported. Shellfish allergy also is a concern; however, shellfish allergies occur due to the proteins in the meat, and not from the shell from which glucosamine is derived. Glucosamine may increase glucose levels and the anticoagulant effects of warfarin.13
Probiotics to prevent antibiotic-associated diarrhea
Antibiotic-associated diarrhea (AAD) is a common problem.21 Probiotics—microorganisms found in oral supplements, yogurt, and other food—are commonly used to help maintain the balance of intestinal flora.34 A recent Cochrane review of 16 RCTs that included approximately 3400 patients found evidence that probiotics can prevent AAD.22 A 2012 systematic review and meta-analysis of 63 RCTs with more than 11,000 participants concluded that probiotics lowered the relative risk of developing diarrhea compared to control groups.35 The American Academy of Pediatrics supports the use of probiotics, citing results from a meta-analysis that found probiotics reduced the risk of developing diarrhea from 28.5% to 11.9% compared to placebo.36
Exact dosages for probiotics have not been established, and recommendations range from 5 billion to 40 billion colony-forming units/d.22 The most commonly used probiotics are from the Lactobacillus and Saccharomyces genera; relatively little evidence supports other genera.21,22,35,36
Probiotics are considered relatively safe, but are not recommended for patients who are immunocompromised or have an indwelling medical device.23 Adverse effects are rare, but may include flatulence, vomiting, rash, chest pain, and increased phlegm.21
For a review of the latest evidence on using probiotics to reduce crying in infants with colic, see "Probiotics for colic? A PURL update."
Soy for hyperlipidemia
Soybeans are a species of legume that contain significant amounts of protein, fiber, potassium, and iron. Although soy has been used to prevent or treat cancer, osteoporosis, and menopausal symptoms, current evidence is unfavorable or inconclusive for such conditions. Some RCTs have found soy has small, favorable effects on serum levels of low-density lipoprotein and total cholesterol,24 while others have shown modest improvements in triglyceride levels without significant improvements in other lipid levels.25
A 2011 meta-analysis of 10 RCTs that included 268 participants found that a diet high in non-soy legume products, such as alfalfa, lentils, and other beans, also improved lipid levels.37 A review of 136 studies that described 22 dietary interventions concluded that among other helpful dietary approaches to controlling hyperlipidemia, dietary soy—which contains fiber and polyunsaturated fats—is favored over supplementation of soy protein alone.38
Use caution when recommending soy for patients with thyroid dysfunction or hormone-sensitive cancers because some evidence suggests soy may interfere with absorption of levothyroxine and increase the risk of developing clinical symptoms of hypothyroidism.39
Soy also contains phytoestrogens, and prolonged use of soy supplements may increase the risk of endometrial hyperplasia.24 This risk has been documented only in the use of soy supplements, and not from dietary soy. GI disturbances and rare allergic reactions also have been reported.24
St. John’s wort for depression
Hypericum perforatum (St. John’s wort), a perennial herb, has been used to treat mood disorders and other ailments for more than 2000 years.40,41 Commercial preparations typically are alcohol extracts with an herb-to-extract ratio of 4:1 to 8:1.26 The normal dose ranges from 900 to 1500 mg/d in 2 to 3 divided doses of the alcohol extract standardized to 0.3% hypericin and/or 3% to 5% hyperforin.
St. John’s wort has been studied extensively as a treatment for depressive disorders. A 2001 double-blind RCT conducted in 11 US academic medical centers and community clinics between 1998 and 2000 that included 200 patients found that St. John’s wort was not effective for moderately severe major depression; a trend toward a positive effect was noted in both the placebo and St. John’s wort groups.26
However, a 2009 Cochrane review of 29 international studies (5489 patients) concluded that St. John’s wort may be better than placebo and as effective as antidepressants for mild to moderate major depression,27 and appeared to have fewer side effects than antidepressants. This review, conducted in German-speaking countries where medical professionals have long prescribed St. John’s wort, reported more positive results than those conducted in other countries.
St. John’s wort interacts with many medications, including antidepressants, oral contraceptives, cyclosporine, digoxin, indinavir, phenytoin, phenobarbital, warfarin, and others. It induces cytochrome P450 (CYP450) enzymes, and therefore can potentially reduce the efficacy of any medication that is metabolized by a CYP450 enzyme. When used in high doses in combination with antidepressants, St. John’s wort may cause serotonin syndrome. Other side effects include photosensitivity, GI complaints, fatigue, and increased risk of cataracts. Due to a lack of clinical data, St. John’s wort is contraindicated in women who are pregnant or breastfeeding.42
Motivational interviewing (MI) is an alternative approach to traditional provider-patient communication that entails using open-ended questions, reflective listening, affirmation, and assessing readiness to change.1 MI facilitators aim to elicit change and assist patients in forming a self-management plan with specific, measurable, achievable, realistic, and timely (SMART) goals.1-3
MI can be efficiently implemented in diverse settings and by a variety of trained facilitators.3-5 For example, the Brief Negotiation Interview requires only 7 minutes per emergency department patient and effectively improves long-term outcomes for substance abusers.4 A randomized controlled trial that included 135 patients admitted to a psychiatric emergency inpatient unit for substance abuse found that those who received 2 sessions of MI reported significantly less substance use than controls 2 years after the intervention.3
Training for providers to ensure proper implementation of MI techniques is essential because poor use of MI can be counter-therapeutic.5 Tools such as the Motivational Interviewing Treatment Integrity Scale and the Client Evaluation of Motivational Interviewing can be used to ensure providers are competent.4,6
References
1. Miller WR, Rollnick S. Motivational Interviewing: Preparing People to Change Addictive Behavior. New York, NY: Guilford Press; 1991.
2. Levensky ER, Forcehimes A, O’Donohue WT, et al. Motivational interviewing: an evidence-based approach to counseling helps patients follow treatment recommendations. Am J Nurse. 2007;107:50-59.
3. Bagøien G, Bjørngaard JH, Østensen C, et al. The effects of motivational interviewing on patients with comorbid substance use admitted to a psychiatric emergency unit - a randomized controlled trial with two year follow-up. BMC Psychiatry. 2013;13:93.
4. D’Onofrio G, Fiellin DA, Pantalon MV, et al. A brief intervention reduces hazardous and harmful drinking in emergency department patients. Ann Emerg Med. 2012;60:181-192.
5. Tollison SJ, Mastroleo NR, Mallett KA, et al. The relationship between baseline drinking status, peer motivational interviewing microskills, and drinking outcomes in a brief alcohol intervention for matriculating college students: a replication. Behav Ther. 2013;44:137-151.
6. Madson MB, Mohn RS, Zuckoff A, et al. Measuring client perceptions of motivational interviewing: factor analysis of the Client Evaluation of Motivational Interviewing scale. J Subst Abuse Treat. 2013;44:330-335.
Guided imagery for anxiety and pain
Guided imagery is a relaxation technique that involves visualizing positive outcomes to reduce one’s reaction to anxiety-provoking or painful experiences.43 It can be practiced independently or under the direction of an instructor. One RCT of 96 women with newly diagnosed breast cancer found that adding relaxation and guided imagery to standard breast cancer treatment protocols positively affected mood and quality of life.14 While this study saw no change in pathologic responses to chemotherapy,14 a more recent RCT concluded that such biochemical advantages may be possible.44 Guided imagery has been linked to decreased anxiety in diverse studies of students, women in labor, individuals suffering from nightmares, and in occupational settings such as training for pilots and surgeons.15-18
A review of 9 RCTs of guided imagery for decreasing musculoskeletal pain involving 201 patients found 8 studies reported positive results, though the methodological quality of the studies was low.19 Of 6 high-quality studies included in a 2012 systematic review, 5 supported the use of guided imagery for postoperative, abdominal, and other nonmusculoskeletal pain.20 This initial evidence is promising, but additional research of high methodological quality is needed to validate the use of guided imagery for anxiety and pain.
Acupuncture for pain
From 2002 to 2007, the use of acupuncture significantly increased in the United States, primarily for the treatment of pain.1 A 2012 meta-analysis of 29 RCTs that included almost 18,000 participants evaluated the clinical usefulness of acupuncture for back, neck, and shoulder pain, OA, and headache.3 Compared to no treatment, both acupuncture and sham acupuncture significantly improved pain scores. The authors of this meta-analysis found that acupuncture offered a small but significant advantage over sham acupuncture, and concluded that the benefits of acupuncture were not due to a placebo effect.
In 2007, the American College of Physicians (ACP) and the American Pain Society (APS) issued a joint statement indicating that acupuncture should be considered for patients with chronic low back pain who do not respond to conventional therapies.4 The North American Spine Society also supports acupuncture, stating that it provides “...better short-term pain relief and functional improvement than no treatment and the addition of acupuncture to other treatment modalities provides a greater benefit than those treatments alone.”5 Additional evidence found acupuncture for chronic low back pain improves function and serves as an adjunct therapy.6
Reported adverse effects of acupuncture include—but may not be limited to—infection, skin irritation, hematoma, pneumothorax, and spontaneous needle migration.1,3-6
Yoga for low back pain
Back pain is the most common reason patients use CAM therapies.1 A systematic review of 10 RCTs that included 967 participants with chronic low back pain found strong evidence for the short-term effectiveness and moderate evidence for the long-term effectiveness of yoga.28 A review of 17 studies that included 1626 patients concluded that yoga improves both pain and functionality; this review did not recommend a specific type of yoga practice.29 In a recent study of 95 minority adults with moderate-to-severe chronic low back pain, once-weekly and twice-weekly yoga for 12 weeks were similarly effective for reducing pain and improving functionality.30
Guidelines from the ACP and the APS recommend yoga as part of an intensive interdisciplinary rehabilitation program for patients with chronic or subacute low back pain who do not improve using other self-care options.4 This recommendation is specifically for Viniyoga, a practice in which the instructor recommends modifications to body positioning for each individual based on past injuries and overall physical condition. (For more information on therapeutic uses of yoga, see “Yoga as therapy: When is it helpful?”)
CORRESPONDENCE
Roger Zoorob, MD, MPH, FAAFP; Department of Family and Community Medicine, Baylor College of Medicine, 3701 Kirby Drive, Suite 600, Houston, TX 77098; [email protected]
› Consider referring your patients for guided imagery to reduce anxiety or pain. A
› Recommend a trial of glucosamine sulfate 1500 mg/d for 3 months for patients with osteoarthritis. B
› Consider acupuncture as a treatment option for patients with chronic pain. B
› Use probiotics to prevent antibiotic-associated diarrhea in pediatric patients, except for those who are immunocompromised or have an indwelling medical device. B
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
CASE › Bob F, age 54, seeks care for chronic low back pain. The conservative treatments you have prescribed, including physical therapy, regular exercise, and an over-the-counter nonsteroidal anti-inflammatory drug, have provided minimal pain relief. Mr. F is reluctant to take a prescription pain medication and has expressed interest in trying a complementary and alternative medicine (CAM) therapy, such as acupuncture or yoga. What should you tell him?
Almost 40% of Americans use CAM modalities to treat specific conditions or for overall well-being,1 and these practices are increasingly becoming a part of our approach to health care, as evidenced by the nearly 50 of facilities across the country that boast integrative health care programs, which combine CAM modalities with conventional medicine.2 Emerging evidence suggests several integrative practices may offer health benefits, and primary care physicians must become well-versed in these modalities to effectively communicate potential benefits and harms to patients. In this article, we present evidence from Cochrane reviews and other studies of 8 commonly used CAM therapies, including dietary interventions, a psychotherapeutic modality, and other treatments (TABLE).1,3-30 And while motivational interviewing technically is not a form of CAM, we also review this modality, which has proven useful in the treatment of patients for substance use. (See “Motivational interviewing for substance abuse.”)
Fish oil for hypertriglyceridemia
High triglyceride levels are a risk factor for cardiovascular disease and a component of metabolic syndrome.8 A 2008 review of 47 randomized controlled trials (RCTs) that included 16,511 participants found that omega-3 fatty acid (fish oil) supplements significantly reduced triglyceride levels compared to placebo.7 The American Heart Association recommends 2 to 4 g/d of eicosapentaenoic acid (EPA) plus docosahexaenoic acid (DHA) to lower triglyceride levels.8
Most studies have found that fish oil supplements are associated with few adverse effects; gastrointestinal (GI) complaints are most common. However, these supplements should be discontinued following an acute bleeding event, such as hemorrhagic stroke, due to their anticoagulant properties.9 Some evidence suggests that the risk for prostate cancer is increased in men with high blood levels of omega-3 fatty acids.10
Glucosamine for osteoarthritis
Glucosamine is an amino sugar that is a building block of cartilage proteoglycans. Although it occurs naturally in the body, the glucosamine used in supplements is typically harvested from seashells. Glucosamine stimulates the metabolism of synovial cells and chondrocytes in articular cartilage and may delay joint degeneration.31,32
Glucosamine is widely used in the United States as a dietary supplement, most often as glucosamine sulfate but also as n-acetyl glucosamine and glucosamine hydrochloride, although there is limited evidence of effectiveness for the latter formulations.33
Most studies have examined the effects of oral glucosamine sulfate, 500 mg taken 3 times a day for 30 to 90 days. Once-a-day dosing as high as 1500 mg also has been used.
A Cochrane review of 25 studies with 4963 patients concluded that oral glucosamine sulfate may reduce osteoarthritis (OA) pain and improve functionality, without many adverse effects.11 A 2-year double-blind RCT compared the effects of glucosamine hydrochloride 500 mg tid, chondroitin sulfate 400 mg tid, glucosamine plus chondroitin, celecoxib 200 mg/d, or placebo in 662 patients with knee OA.12 While all groups experienced early and sustained symptomatic relief, the odds of achieving a 20% reduction in pain and improved functioning were highest with celecoxib and glucosamine.
Oral glucosamine sulfate can cause mild GI effects, but drowsiness, skin reactions, and headache also have been reported. Shellfish allergy also is a concern; however, shellfish allergies occur due to the proteins in the meat, and not from the shell from which glucosamine is derived. Glucosamine may increase glucose levels and the anticoagulant effects of warfarin.13
Probiotics to prevent antibiotic-associated diarrhea
Antibiotic-associated diarrhea (AAD) is a common problem.21 Probiotics—microorganisms found in oral supplements, yogurt, and other food—are commonly used to help maintain the balance of intestinal flora.34 A recent Cochrane review of 16 RCTs that included approximately 3400 patients found evidence that probiotics can prevent AAD.22 A 2012 systematic review and meta-analysis of 63 RCTs with more than 11,000 participants concluded that probiotics lowered the relative risk of developing diarrhea compared to control groups.35 The American Academy of Pediatrics supports the use of probiotics, citing results from a meta-analysis that found probiotics reduced the risk of developing diarrhea from 28.5% to 11.9% compared to placebo.36
Exact dosages for probiotics have not been established, and recommendations range from 5 billion to 40 billion colony-forming units/d.22 The most commonly used probiotics are from the Lactobacillus and Saccharomyces genera; relatively little evidence supports other genera.21,22,35,36
Probiotics are considered relatively safe, but are not recommended for patients who are immunocompromised or have an indwelling medical device.23 Adverse effects are rare, but may include flatulence, vomiting, rash, chest pain, and increased phlegm.21
For a review of the latest evidence on using probiotics to reduce crying in infants with colic, see "Probiotics for colic? A PURL update."
Soy for hyperlipidemia
Soybeans are a species of legume that contain significant amounts of protein, fiber, potassium, and iron. Although soy has been used to prevent or treat cancer, osteoporosis, and menopausal symptoms, current evidence is unfavorable or inconclusive for such conditions. Some RCTs have found soy has small, favorable effects on serum levels of low-density lipoprotein and total cholesterol,24 while others have shown modest improvements in triglyceride levels without significant improvements in other lipid levels.25
A 2011 meta-analysis of 10 RCTs that included 268 participants found that a diet high in non-soy legume products, such as alfalfa, lentils, and other beans, also improved lipid levels.37 A review of 136 studies that described 22 dietary interventions concluded that among other helpful dietary approaches to controlling hyperlipidemia, dietary soy—which contains fiber and polyunsaturated fats—is favored over supplementation of soy protein alone.38
Use caution when recommending soy for patients with thyroid dysfunction or hormone-sensitive cancers because some evidence suggests soy may interfere with absorption of levothyroxine and increase the risk of developing clinical symptoms of hypothyroidism.39
Soy also contains phytoestrogens, and prolonged use of soy supplements may increase the risk of endometrial hyperplasia.24 This risk has been documented only in the use of soy supplements, and not from dietary soy. GI disturbances and rare allergic reactions also have been reported.24
St. John’s wort for depression
Hypericum perforatum (St. John’s wort), a perennial herb, has been used to treat mood disorders and other ailments for more than 2000 years.40,41 Commercial preparations typically are alcohol extracts with an herb-to-extract ratio of 4:1 to 8:1.26 The normal dose ranges from 900 to 1500 mg/d in 2 to 3 divided doses of the alcohol extract standardized to 0.3% hypericin and/or 3% to 5% hyperforin.
St. John’s wort has been studied extensively as a treatment for depressive disorders. A 2001 double-blind RCT conducted in 11 US academic medical centers and community clinics between 1998 and 2000 that included 200 patients found that St. John’s wort was not effective for moderately severe major depression; a trend toward a positive effect was noted in both the placebo and St. John’s wort groups.26
However, a 2009 Cochrane review of 29 international studies (5489 patients) concluded that St. John’s wort may be better than placebo and as effective as antidepressants for mild to moderate major depression,27 and appeared to have fewer side effects than antidepressants. This review, conducted in German-speaking countries where medical professionals have long prescribed St. John’s wort, reported more positive results than those conducted in other countries.
St. John’s wort interacts with many medications, including antidepressants, oral contraceptives, cyclosporine, digoxin, indinavir, phenytoin, phenobarbital, warfarin, and others. It induces cytochrome P450 (CYP450) enzymes, and therefore can potentially reduce the efficacy of any medication that is metabolized by a CYP450 enzyme. When used in high doses in combination with antidepressants, St. John’s wort may cause serotonin syndrome. Other side effects include photosensitivity, GI complaints, fatigue, and increased risk of cataracts. Due to a lack of clinical data, St. John’s wort is contraindicated in women who are pregnant or breastfeeding.42
Motivational interviewing (MI) is an alternative approach to traditional provider-patient communication that entails using open-ended questions, reflective listening, affirmation, and assessing readiness to change.1 MI facilitators aim to elicit change and assist patients in forming a self-management plan with specific, measurable, achievable, realistic, and timely (SMART) goals.1-3
MI can be efficiently implemented in diverse settings and by a variety of trained facilitators.3-5 For example, the Brief Negotiation Interview requires only 7 minutes per emergency department patient and effectively improves long-term outcomes for substance abusers.4 A randomized controlled trial that included 135 patients admitted to a psychiatric emergency inpatient unit for substance abuse found that those who received 2 sessions of MI reported significantly less substance use than controls 2 years after the intervention.3
Training for providers to ensure proper implementation of MI techniques is essential because poor use of MI can be counter-therapeutic.5 Tools such as the Motivational Interviewing Treatment Integrity Scale and the Client Evaluation of Motivational Interviewing can be used to ensure providers are competent.4,6
References
1. Miller WR, Rollnick S. Motivational Interviewing: Preparing People to Change Addictive Behavior. New York, NY: Guilford Press; 1991.
2. Levensky ER, Forcehimes A, O’Donohue WT, et al. Motivational interviewing: an evidence-based approach to counseling helps patients follow treatment recommendations. Am J Nurse. 2007;107:50-59.
3. Bagøien G, Bjørngaard JH, Østensen C, et al. The effects of motivational interviewing on patients with comorbid substance use admitted to a psychiatric emergency unit - a randomized controlled trial with two year follow-up. BMC Psychiatry. 2013;13:93.
4. D’Onofrio G, Fiellin DA, Pantalon MV, et al. A brief intervention reduces hazardous and harmful drinking in emergency department patients. Ann Emerg Med. 2012;60:181-192.
5. Tollison SJ, Mastroleo NR, Mallett KA, et al. The relationship between baseline drinking status, peer motivational interviewing microskills, and drinking outcomes in a brief alcohol intervention for matriculating college students: a replication. Behav Ther. 2013;44:137-151.
6. Madson MB, Mohn RS, Zuckoff A, et al. Measuring client perceptions of motivational interviewing: factor analysis of the Client Evaluation of Motivational Interviewing scale. J Subst Abuse Treat. 2013;44:330-335.
Guided imagery for anxiety and pain
Guided imagery is a relaxation technique that involves visualizing positive outcomes to reduce one’s reaction to anxiety-provoking or painful experiences.43 It can be practiced independently or under the direction of an instructor. One RCT of 96 women with newly diagnosed breast cancer found that adding relaxation and guided imagery to standard breast cancer treatment protocols positively affected mood and quality of life.14 While this study saw no change in pathologic responses to chemotherapy,14 a more recent RCT concluded that such biochemical advantages may be possible.44 Guided imagery has been linked to decreased anxiety in diverse studies of students, women in labor, individuals suffering from nightmares, and in occupational settings such as training for pilots and surgeons.15-18
A review of 9 RCTs of guided imagery for decreasing musculoskeletal pain involving 201 patients found 8 studies reported positive results, though the methodological quality of the studies was low.19 Of 6 high-quality studies included in a 2012 systematic review, 5 supported the use of guided imagery for postoperative, abdominal, and other nonmusculoskeletal pain.20 This initial evidence is promising, but additional research of high methodological quality is needed to validate the use of guided imagery for anxiety and pain.
Acupuncture for pain
From 2002 to 2007, the use of acupuncture significantly increased in the United States, primarily for the treatment of pain.1 A 2012 meta-analysis of 29 RCTs that included almost 18,000 participants evaluated the clinical usefulness of acupuncture for back, neck, and shoulder pain, OA, and headache.3 Compared to no treatment, both acupuncture and sham acupuncture significantly improved pain scores. The authors of this meta-analysis found that acupuncture offered a small but significant advantage over sham acupuncture, and concluded that the benefits of acupuncture were not due to a placebo effect.
In 2007, the American College of Physicians (ACP) and the American Pain Society (APS) issued a joint statement indicating that acupuncture should be considered for patients with chronic low back pain who do not respond to conventional therapies.4 The North American Spine Society also supports acupuncture, stating that it provides “...better short-term pain relief and functional improvement than no treatment and the addition of acupuncture to other treatment modalities provides a greater benefit than those treatments alone.”5 Additional evidence found acupuncture for chronic low back pain improves function and serves as an adjunct therapy.6
Reported adverse effects of acupuncture include—but may not be limited to—infection, skin irritation, hematoma, pneumothorax, and spontaneous needle migration.1,3-6
Yoga for low back pain
Back pain is the most common reason patients use CAM therapies.1 A systematic review of 10 RCTs that included 967 participants with chronic low back pain found strong evidence for the short-term effectiveness and moderate evidence for the long-term effectiveness of yoga.28 A review of 17 studies that included 1626 patients concluded that yoga improves both pain and functionality; this review did not recommend a specific type of yoga practice.29 In a recent study of 95 minority adults with moderate-to-severe chronic low back pain, once-weekly and twice-weekly yoga for 12 weeks were similarly effective for reducing pain and improving functionality.30
Guidelines from the ACP and the APS recommend yoga as part of an intensive interdisciplinary rehabilitation program for patients with chronic or subacute low back pain who do not improve using other self-care options.4 This recommendation is specifically for Viniyoga, a practice in which the instructor recommends modifications to body positioning for each individual based on past injuries and overall physical condition. (For more information on therapeutic uses of yoga, see “Yoga as therapy: When is it helpful?”)
CORRESPONDENCE
Roger Zoorob, MD, MPH, FAAFP; Department of Family and Community Medicine, Baylor College of Medicine, 3701 Kirby Drive, Suite 600, Houston, TX 77098; [email protected]
1. Barnes PM, Bloom B, Nahin RL. Complementary and alternative medicine use among adults and children: United States, 2007. Natl Health Stat Report. 2008;10:1-23.
2. American Holistic Medicine Association. Integrative medicine centers. American Holistic Medicine Association Web site. Available at: http://www.holisticmedicine.org/content. asp?pl=30&sl=2&contentid=74. Accessed September 8, 2014.
3. Vickers AJ, Cronin AM, Maschino AC, et al; Acupuncture Trialists’ Collaboration. Acupuncture for chronic pain: individual patient data meta-analysis. Arch Intern Med. 2012;172:1444-1453.
4. Chou R, Qaseem A, Snow V, et al; Clinical Efficacy Assessment Subcommittee of the American College of Physicians; American College of Physicians; American Pain Society Low Back Pain Guidelines Panel. Diagnosis and treatment of low back pain: a joint clinical practice guideline from the American College of Physicians and the American Pain Society. Ann Intern Med. 2007;147:478-491.
5. Berman BM, Langevin HM, Witt CM, et al. Acupuncture for chronic low back pain. N Engl J Med. 2010;363:454-461.
6. Last AR, Hulbert K. Chronic low back pain: evaluation and management. Am Fam Physician. 2009;79:1067-1074.
7. Eslik GD, Howe PR, Smith C, et al. Benefits of fish oil supplementation in hyperlipidemia: a systematic review and meta-analysis. Int J Cardiol. 2009;136:4-16.
8. Miller M, Stone NJ, Ballantyne C, et al; American Heart Association Clinical Lipidology, Thrombosis, and Prevention Committeeof the Council on Nutrition, Physical Activity, and Metabolism; Council on Arteriosclerosis, Thrombosis and Vascular Biology; Council on Cardiovascular Nursing; Council on the Kidney in Cardiovascular Disease. Triglyceride and cardiovascular disease: a scientific statement from the American Heart Association. Circulation. 2011;123:2292-2333.
9. Bays HE. Safety considerations with omega-3 fatty acid therapy. Am J Cardiol. 2007;99:35C-43C.
10. Brasky TM, Darke AK, Song X, et al. Plasma phospholipid fatty acids and prostate cancer risk in the SELECT trial. J Natl Cancer Inst. 2013;105:1132-1141.
11. Towheed TE, Maxwell L, Anastassiades TP, et al. Glucosamine therapy for treating osteoarthritis. Cochrane Database Syst Rev. 2005;(2):CD002946.
12. Sawitzke AD, Shi H, Finco MF, et al. Clinical efficacy and safety of glucosamine, chondroitin sulphate, their combination, celecoxib or placebo taken to treat osteoarthritis of the knee: 2-year results from GAIT. Ann Rheum Dis. 2010;69:1459-1464.
13. Dostrovsky NR, Towheed TE, Hudson RW, et al. The effect of glucosamine on glucose metabolism in humans: a systematic review of the literature. Osteoarthritis Cartilage. 2011;19:375-380.
14. Walker LG, Walker MB, Ogston K, et al. Psychological, clinical and pathological effects of relaxation training and guided imagery during primary chemotherapy. Br J Cancer. 1999;80:262-268.
15. Thünker J, Pietrowsky R. Effectiveness of a manualized imagery rehearsal therapy for patients suffering from nightmare disorders with and without a comorbidity of depression or PTSD. Behav Res Ther. 2012;50:558-564.
16. Marc I, Toureche N, Ernst E, et al. Mind-body interventions during pregnancy for preventing or treating women’s anxiety. Cochrane Database Syst Rev. 2011;(7):CD007559.
17. Jing X, Wu P, Liu F, et al. Guided imagery, anxiety, heart rate, and heart rate variability during centrifuge training. Aviat Space Environ Med. 2011;82:92-96.
18. Arora S, Aggarwal R, Moran A, et al. Mental practice: effective stress management training for novice surgeons. J Am Coll Surg. 2011;212:225-233.
19. Posadzki P, Ernst E. Guided imagery for musculoskeletal pain: a systematic review. Clin J Pain. 2011;27:648-653.
20. Posadzki P, Lewandowski W, Terry R, et al. Guided imagery for non-musculoskeletal pain: a systematic review of randomized clinical trials. J Pain Symptom Manage. 2012;44:95-104.
21. Arvola T, Laiho K, Torkkeli S, et al. Prophylactic Lactobacillus GG reduces antibiotic-associated diarrhea in children with respiratory infections: a randomized study. Pediatrics. 1999;104:e64.
22. Johnston BC, Goldenberg JZ, Vandvik PO, et al. Probiotics for the prevention of pediatric antibiotic-associated diarrhea. Cochrane Database Syst Rev. 2011;(11):CD004827.
23. Williams NT. Probiotics. Am J Health-Syst Pharm. 2010;67:449-458.
24. Sacks FM, Lichtenstein A, Van Horn L, et al; American Heart Association Nutrition Committee. Soy protein, isoflavones, and cardiovascular health: an American Heart Association Science Advisory for professionals from the Nutrition Committee. Circulation. 2006;113:1034-1044.
25. Qin Y, Niu K, Zeng Y, et al. Isoflavones for hypercholesterolaemia in adults. Cochrane Database Syst Rev. 2013;6:CD009518.
26. Shelton RC, Keller MB, Gelenberg A, et al. Effectiveness of St John’s wort in major depression: a randomized controlled trial. JAMA. 2001;285:1978-1986.
27. Linde K, Berner MM, Kriston L. St John’s wort for major depression. Cochrane Database Syst Rev. 2008;(4):CD000448.
28. Cramer H, Lauche R, Haller H, et al. A systematic review and meta-analysis of yoga for low back pain. Clin J Pain. 2013;29: 450-460.
29. Ward L, Stebbings S, Cherkin D, et al. Yoga for functional ability, pain and psychosocial outcomes in musculoskeletal conditions: a systematic review and meta-analysis. Musculoskeletal Care. 2013;11:203-217.
30. Saper RB, Boah AR, Keosaian J, et al. Comparing once- versus twice-weekly yoga classes for chronic low back pain in predominantly low income minorities: a randomized dosing trial. Evid Based Complement Alternat Med. 2013;2013:658030.
31. Uitterlinden EJ, Koevoet JLM, Verkoelen CF, et al. Glucosamine increases hyaluronic acid production in human osteoarthritic synovium explants. BMC Musculoskelet Disord. 2008;9:120.
32. Calamia V, Ruiz-Romero C, Rocha B, et al. Pharmacoproteomic study of the effects of chondroitin and glucosamine sulfate on human articular chondrocytes. Arthritis Res Ther. 2010;12:R138.
33. Rovati LC, Girolami F, Persiani S. Crystalline glucosamine sulfate in the management of knee osteoarthritis: efficacy, safety, and pharmacokinetic properties. Ther Adv Musculoskelet Dis. 2012;4:167-180.
34. National Center for Complementary and Alternative Medicine. Oral probiotics: An introduction. National Center for Complementary and Alternative Medicine Web site. Available at: http:// nccam.nih.gov/health/probiotics/introduction.htm. Updated December 2012. Accessed August 29, 2013.
35. Hempel S, Newberry SJ, Maher AR, et al. Probiotics for the prevention and treatment of antibiotic-associated diarrhea: a systematic review and meta-analysis. JAMA. 2012;307:1959-1969.
36. Thomas DW, Greer FR; American Academy of Pediatrics Committee on Nutrition; American Academy of Pediatrics Section on Gastroenterology, Hepatology, and Nutrition. Probiotics and prebiotics in pediatrics. Pediatrics. 2010;126:1217-1231.
37. Bazzano LA, Thompson AM, Tees MT, et al. Non-soy legume consumption lowers cholesterol levels: a meta-analysis of randomized controlled trials. Nutr Metab Cardiovasc Dis. 2011;21:94-103.
38. Huang J, Frohlich J, Ignaszewski AP. The impact of dietary changes and dietary supplements on lipid profile. Can J Cardiol. 2011;27:488-505.
39. Messina M, Redmond G. Effects of soy protein and soybean isoflavones on thyroid function in healthy adults and hypothyroid patients: a review of the relevant literature. Thyroid. 2006;16: 249-258.
40. National Center for Complementary and Alternative Medicine. St. John’s Wort. National Center for Complementary and Alternative Medicine Web site. Available at: http://nccam.nih.gov/ health/stjohnswort. Accessed October 14, 2013.
41. Schulz V, Hänsel R, Tyler VE. Rational Phytotherapy: A Physicians’ Guide to Herbal Medicine. Berlin: Springer; 2001:62-81.
42. Mischoulon D. Update and critique of natural remedies as antidepressant treatments. Obstet Gynecol Clin North Am. 2009;36: 789-807.
43. Hanssen MM, Peters ML, Vlaeyen JW, et al. Optimism lowers pain: evidence of the causal status and underlying mechanisms. Pain. 2013;154:53-58.
44. Eremin O, Walker MB, Simpson E, et al. Immuno-modulatory effects of relaxation training and guided imagery in women with locally advanced breast cancer undergoing multimodality therapy: a randomised controlled trial. Breast. 2009;18:17-25.
1. Barnes PM, Bloom B, Nahin RL. Complementary and alternative medicine use among adults and children: United States, 2007. Natl Health Stat Report. 2008;10:1-23.
2. American Holistic Medicine Association. Integrative medicine centers. American Holistic Medicine Association Web site. Available at: http://www.holisticmedicine.org/content. asp?pl=30&sl=2&contentid=74. Accessed September 8, 2014.
3. Vickers AJ, Cronin AM, Maschino AC, et al; Acupuncture Trialists’ Collaboration. Acupuncture for chronic pain: individual patient data meta-analysis. Arch Intern Med. 2012;172:1444-1453.
4. Chou R, Qaseem A, Snow V, et al; Clinical Efficacy Assessment Subcommittee of the American College of Physicians; American College of Physicians; American Pain Society Low Back Pain Guidelines Panel. Diagnosis and treatment of low back pain: a joint clinical practice guideline from the American College of Physicians and the American Pain Society. Ann Intern Med. 2007;147:478-491.
5. Berman BM, Langevin HM, Witt CM, et al. Acupuncture for chronic low back pain. N Engl J Med. 2010;363:454-461.
6. Last AR, Hulbert K. Chronic low back pain: evaluation and management. Am Fam Physician. 2009;79:1067-1074.
7. Eslik GD, Howe PR, Smith C, et al. Benefits of fish oil supplementation in hyperlipidemia: a systematic review and meta-analysis. Int J Cardiol. 2009;136:4-16.
8. Miller M, Stone NJ, Ballantyne C, et al; American Heart Association Clinical Lipidology, Thrombosis, and Prevention Committeeof the Council on Nutrition, Physical Activity, and Metabolism; Council on Arteriosclerosis, Thrombosis and Vascular Biology; Council on Cardiovascular Nursing; Council on the Kidney in Cardiovascular Disease. Triglyceride and cardiovascular disease: a scientific statement from the American Heart Association. Circulation. 2011;123:2292-2333.
9. Bays HE. Safety considerations with omega-3 fatty acid therapy. Am J Cardiol. 2007;99:35C-43C.
10. Brasky TM, Darke AK, Song X, et al. Plasma phospholipid fatty acids and prostate cancer risk in the SELECT trial. J Natl Cancer Inst. 2013;105:1132-1141.
11. Towheed TE, Maxwell L, Anastassiades TP, et al. Glucosamine therapy for treating osteoarthritis. Cochrane Database Syst Rev. 2005;(2):CD002946.
12. Sawitzke AD, Shi H, Finco MF, et al. Clinical efficacy and safety of glucosamine, chondroitin sulphate, their combination, celecoxib or placebo taken to treat osteoarthritis of the knee: 2-year results from GAIT. Ann Rheum Dis. 2010;69:1459-1464.
13. Dostrovsky NR, Towheed TE, Hudson RW, et al. The effect of glucosamine on glucose metabolism in humans: a systematic review of the literature. Osteoarthritis Cartilage. 2011;19:375-380.
14. Walker LG, Walker MB, Ogston K, et al. Psychological, clinical and pathological effects of relaxation training and guided imagery during primary chemotherapy. Br J Cancer. 1999;80:262-268.
15. Thünker J, Pietrowsky R. Effectiveness of a manualized imagery rehearsal therapy for patients suffering from nightmare disorders with and without a comorbidity of depression or PTSD. Behav Res Ther. 2012;50:558-564.
16. Marc I, Toureche N, Ernst E, et al. Mind-body interventions during pregnancy for preventing or treating women’s anxiety. Cochrane Database Syst Rev. 2011;(7):CD007559.
17. Jing X, Wu P, Liu F, et al. Guided imagery, anxiety, heart rate, and heart rate variability during centrifuge training. Aviat Space Environ Med. 2011;82:92-96.
18. Arora S, Aggarwal R, Moran A, et al. Mental practice: effective stress management training for novice surgeons. J Am Coll Surg. 2011;212:225-233.
19. Posadzki P, Ernst E. Guided imagery for musculoskeletal pain: a systematic review. Clin J Pain. 2011;27:648-653.
20. Posadzki P, Lewandowski W, Terry R, et al. Guided imagery for non-musculoskeletal pain: a systematic review of randomized clinical trials. J Pain Symptom Manage. 2012;44:95-104.
21. Arvola T, Laiho K, Torkkeli S, et al. Prophylactic Lactobacillus GG reduces antibiotic-associated diarrhea in children with respiratory infections: a randomized study. Pediatrics. 1999;104:e64.
22. Johnston BC, Goldenberg JZ, Vandvik PO, et al. Probiotics for the prevention of pediatric antibiotic-associated diarrhea. Cochrane Database Syst Rev. 2011;(11):CD004827.
23. Williams NT. Probiotics. Am J Health-Syst Pharm. 2010;67:449-458.
24. Sacks FM, Lichtenstein A, Van Horn L, et al; American Heart Association Nutrition Committee. Soy protein, isoflavones, and cardiovascular health: an American Heart Association Science Advisory for professionals from the Nutrition Committee. Circulation. 2006;113:1034-1044.
25. Qin Y, Niu K, Zeng Y, et al. Isoflavones for hypercholesterolaemia in adults. Cochrane Database Syst Rev. 2013;6:CD009518.
26. Shelton RC, Keller MB, Gelenberg A, et al. Effectiveness of St John’s wort in major depression: a randomized controlled trial. JAMA. 2001;285:1978-1986.
27. Linde K, Berner MM, Kriston L. St John’s wort for major depression. Cochrane Database Syst Rev. 2008;(4):CD000448.
28. Cramer H, Lauche R, Haller H, et al. A systematic review and meta-analysis of yoga for low back pain. Clin J Pain. 2013;29: 450-460.
29. Ward L, Stebbings S, Cherkin D, et al. Yoga for functional ability, pain and psychosocial outcomes in musculoskeletal conditions: a systematic review and meta-analysis. Musculoskeletal Care. 2013;11:203-217.
30. Saper RB, Boah AR, Keosaian J, et al. Comparing once- versus twice-weekly yoga classes for chronic low back pain in predominantly low income minorities: a randomized dosing trial. Evid Based Complement Alternat Med. 2013;2013:658030.
31. Uitterlinden EJ, Koevoet JLM, Verkoelen CF, et al. Glucosamine increases hyaluronic acid production in human osteoarthritic synovium explants. BMC Musculoskelet Disord. 2008;9:120.
32. Calamia V, Ruiz-Romero C, Rocha B, et al. Pharmacoproteomic study of the effects of chondroitin and glucosamine sulfate on human articular chondrocytes. Arthritis Res Ther. 2010;12:R138.
33. Rovati LC, Girolami F, Persiani S. Crystalline glucosamine sulfate in the management of knee osteoarthritis: efficacy, safety, and pharmacokinetic properties. Ther Adv Musculoskelet Dis. 2012;4:167-180.
34. National Center for Complementary and Alternative Medicine. Oral probiotics: An introduction. National Center for Complementary and Alternative Medicine Web site. Available at: http:// nccam.nih.gov/health/probiotics/introduction.htm. Updated December 2012. Accessed August 29, 2013.
35. Hempel S, Newberry SJ, Maher AR, et al. Probiotics for the prevention and treatment of antibiotic-associated diarrhea: a systematic review and meta-analysis. JAMA. 2012;307:1959-1969.
36. Thomas DW, Greer FR; American Academy of Pediatrics Committee on Nutrition; American Academy of Pediatrics Section on Gastroenterology, Hepatology, and Nutrition. Probiotics and prebiotics in pediatrics. Pediatrics. 2010;126:1217-1231.
37. Bazzano LA, Thompson AM, Tees MT, et al. Non-soy legume consumption lowers cholesterol levels: a meta-analysis of randomized controlled trials. Nutr Metab Cardiovasc Dis. 2011;21:94-103.
38. Huang J, Frohlich J, Ignaszewski AP. The impact of dietary changes and dietary supplements on lipid profile. Can J Cardiol. 2011;27:488-505.
39. Messina M, Redmond G. Effects of soy protein and soybean isoflavones on thyroid function in healthy adults and hypothyroid patients: a review of the relevant literature. Thyroid. 2006;16: 249-258.
40. National Center for Complementary and Alternative Medicine. St. John’s Wort. National Center for Complementary and Alternative Medicine Web site. Available at: http://nccam.nih.gov/ health/stjohnswort. Accessed October 14, 2013.
41. Schulz V, Hänsel R, Tyler VE. Rational Phytotherapy: A Physicians’ Guide to Herbal Medicine. Berlin: Springer; 2001:62-81.
42. Mischoulon D. Update and critique of natural remedies as antidepressant treatments. Obstet Gynecol Clin North Am. 2009;36: 789-807.
43. Hanssen MM, Peters ML, Vlaeyen JW, et al. Optimism lowers pain: evidence of the causal status and underlying mechanisms. Pain. 2013;154:53-58.
44. Eremin O, Walker MB, Simpson E, et al. Immuno-modulatory effects of relaxation training and guided imagery in women with locally advanced breast cancer undergoing multimodality therapy: a randomised controlled trial. Breast. 2009;18:17-25.
JNC 8: What's covered, what's not, and what else to consider
› Initiate pharmacologic treatment for patients
60 years or older with systolic blood pressure (BP) ≥150 mm Hg and/or diastolic BP ≥90 mm Hg. A
› Start antihypertensive treatment for systolic BP ≥140 mm Hg and/or diastolic BP ≥90 mm Hg in patients who are younger than 60 or have chronic kidney disease or diabetes. C
› Select either a thiazide diuretic or a calcium channel blocker as first-line therapy for African Americans, whether or not they have diabetes. C
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
CASE › Carla S is a 64-year old African American whom you’re seeing for the first time. Her health has been excellent over the last 10 years, she reports, with one caveat: She has “borderline hypertension,” but has never been treated for it and denies any symptoms. Her blood pressure (BP) today is 154/82 mm Hg. A physical exam is unremarkable. Blood tests reveal a normal blood count and normal renal function, and a nonfasting glucose level of 145 mg/dL. You ask Ms. S to return in a week for a repeat BP and fasting lab work.
Hypertension is the most common condition seen by physicians in primary care,1 and a major risk factor for cardiovascular disease (CVD) and the morbidity and mortality associated with it. US treatment costs are an estimated $131 billion per year.2-4 With this in mind, the Joint National Committee on Hypertension (JNC) released its eighth report (JNC 8) in December 20131—the first update in a decade.
In many ways, JNC 8 guidelines are simpler than those of JNC 7,2 with more evidence-based recommendations and less reliance on expert opinion. The JNC has eliminated definitions such as stage 1, 2, and 3 hypertension, and focuses on outcomes instead. At the heart of the recommendations are 3 key questions:
1. At what BP should treatment be initiated to improve outcomes?
2. What should the target BP be for those undergoing treatment?
3. Which medications are best?
Answers to the first 2 questions, of course, go hand in hand. In other words, if the threshold for treatment is a systolic BP ≥140 mm Hg (more on that in a moment), then the target of treatment is a systolic BP of <140 mm Hg. In answer to the third question, JNC 8 offers guidance but gives physicians greater discretion in determining which type of drug to use when initiating treatment.1
In the text, algorithm, and table that follow, we present an overview of JNC 8. We also discuss the optimal treatment of hypertension in patients with heart failure (HF) and coronary artery disease (CAD)—populations JNC 8 does not address.
Age-based recommendations are a bit less stringent
60 years and older. Unlike JNC 7, which recommended initiating treatment for otherwise healthy patients of all ages with a BP ≥140/90 mm Hg,2 JNC 8 clearly delineates its recommendations by age. It calls for treating patients ages 60 or older with systolic BP ≥150 mm Hg and/or diastolic BP ≥90.1
The change is evidence-based: Moderate- to high-quality randomized controlled trials (RCTs) have found a reduced incidence of stroke, HF, and coronary heart disease when BP was treated to <150/90, but no additional benefit from a systolic BP target of <140 mm Hg for patients in this age group.5,6 Notably, JNC 8 does not recommend a change in medication for patients 60 years or older for whom the more stringent target is being maintained without adverse effects.1
18 to 59 years. For adults younger than 60, JNC 8 recommends treating systolic BP ≥140 and diastolic BP ≥90 mm Hg.1 The systolic BP guideline is based on expert opinion, however, as there is no high-quality evidence for a systolic threshold in this age group. This is largely because most patients younger than 60 who have systolic BP ≥140 also have diastolic BP ≥90, making it difficult to study the treatment of systolic BP alone. High-quality trials have shown improved health outcomes when patients ages 30 to 59 years were treated for diastolic BP ≥90, however.7-12 For patients younger than 30, the recommendation for treatment of diastolic pressure is based on expert consensus, as no sufficiently high-quality evidence exists.
Targets for patients with CKD and diabetes
Chronic kidney disease (CKD). JNC 8 recommends treating patients ages 18 to 69 years who have CKD and BP ≥140/90 mm Hg. JNC 7’s more stringent recommendation—treating such patients with BP ≥130/80 mm Hg2—was relaxed because there is little evidence of a lower mortality rate or cardiovascular or cerebrovascular benefits as a result of tighter control. In patients younger than 70, CKD is defined as an estimated (or measured) glomerular filtration rate (GFR) <60 mL/min/1.73 m2 or albuminuria (>30 mg of albumin per g of creatinine).1
It is important to note that this goal does not apply to individuals who have CKD and are 70 years or older. This is due to insufficient evidence, as well as uncertainty about the accuracy of an estimated GFR in this patient population. JNC 8 recommends that treatment of BP in patients 70 or older be based on comorbidities, including albuminuria, among other patient-specific considerations.1
Diabetes. JNC 8 recommends treating patients age 18 years or older who have diabetes and BP ≥140/90 mm Hg, as JNC 7 did.2 This is based largely on expert opinion.
Studies suggest that adults with both hypertension and diabetes have a reduction in mortality and improved cardiovascular and cerebrovascular outcomes when systolic BP is <150 mm Hg,13-15 but no strong data support a goal of <140/90 mm Hg. The Action to Control Cardiovascular Risk in Diabetes (ACCORD) BP trial, for example, showed comparable outcomes in patients with systolic BP of 150 or 140 mm Hg.16 The use of expert opinion vs well-designed studies in this instance seems at odds with JNC 8’s general policy of placing greater emphasis on evidence.
CASE › On her second visit, Ms. S’s BP is 144/82 mm Hg and her cholesterol levels are within the normal range. Her fasting glucose level is 104 mg/dL and glycated hemoglobin (HbA1c) is 6%. At a repeat visit one month later, her BP is 146/76 mm Hg. Given these 2 acceptable readings (<150/90 mm Hg for individuals age 60 and older who do not have diabetes), you do not initiate antihypertensive treatment.
However, you explain to the patient that her fasting glucose and HbA1c are evidence of insulin resistance. Although a diagnosis of diabetes is not warranted, you arrange for Ms. S to meet with a diabetes nurse educator for help in improving her diet and following an exercise regimen.
Pharmacotherapy: JNC 8 offers wider latitude
Like its predecessor, JNC 8 stresses the importance of diet and exercise. (See “Controlling hypertension starts with lifestyle modification”17 in this article.) It diverges from JNC 7, however, in its recommendations for initiating treatment (ALGORITHM).1 The earlier version recommended thiazide diuretics as first-line therapy but included multiple indications for initiating therapy with other drug classes. JNC 8 guidelines are less specific.
Starting therapy with a thiazide diuretic, angiotensin-converting enzyme inhibitor (ACEI), angiotensin receptor blocker (ARB), or calcium channel blocker (CCB)—all of which have high-quality evidence of improved outcomes18-20—is recommended for most patients, including those with diabetes. (Blacks and patients with CKD are exceptions.) The recommended doses of these medications, summarized in the TABLE,1,21 are similar to those used in RCTs. Other types of drugs are not recommended, either because they were shown to be inferior to another class of antihypertensive or because there is insufficient evidence of their efficacy.
For most blacks... JNC 8 recommends thiazide diuretics and CCBs as first-line therapy—a recommendation that is evidence-based. The Antihypertensive and Lipid Lowering Treatment to Prevent Heart Attack Trial (ALLHAT)22 revealed that black patients taking thiazide diuretics had fewer cerebrovascular and cardiovascular events and a lower rate of HF compared with those taking ACEIs, whether or not they had diabetes. Diuretics were more effective than CCBs in preventing HF, but no difference in rates of cerebrovascular and cardiovascular events, kidney disease, or overall mortality was found.22
For patients with CKD and proteinuria, regardless of race, JNC 8 calls for either an ACEI or an ARB as first-line agent to prevent progression to end-stage renal disease. This recommendation is based on expert consensus, and intended to prevent progression to end-stage renal disease.1,23
The optimal first-line agent for patients who have CKD without proteinuria is less clear. For such patients, JNC 8 notes, any of the 4 recommended drug classes can be used for initial therapy.1
Guidance on starting—and titrating—therapy
JNC 7 guidelines featured a complex means of diagnosing and monitoring hypertension.2 JNC 8 has simplified the recommendations, which call for patients to be reassessed within a month of initiating therapy.
The new guidelines include 3 distinct methods of dosing antihypertensive medications, none of which has demonstrated better outcomes than any other. All call for replacing one type of drug with another if the first trial is ineffective or results in adverse effects. And all stress the importance of avoiding ACEI and ARB combinations due to increases in serum creatinine and hyperkalemia and the need for monitoring. Note, however, that Method 3 is recommended for patients with more severe hypertension.1
Method 1. Initiate one medication from any of the 4 classes of antihypertensives recommended for initial treatment, and titrate to the maximum effective dose. If the BP goal is not achieved at maximum dose, add a medication from a second class and titrate that drug to the maximum effective dose, as well. If the goal is still not reached, add a medication from a third class and titrate up as needed.
Method 2. Initiate one medication, then add a second agent from a different drug class, if necessary, and titrate until both are at the maximum effective dose. If the goal still has not been reached, add a third agent and titrate that until BP is well controlled.
Method 3. Initiate 2 medications from 2 different classes of drugs simultaneously. If BP is not at goal after a reasonable trial, add a third agent and titrate to maximum effective dose. (Use this approach for patients who have systolic BP >160 mm Hg and/or diastolic BP >100 mm Hg or systolic BP >20 mm Hg above goal and/or diastolic BP >10 mm Hg above goal.)
As a general rule, a trial with monotherapy should be considered if BP is ≤160/100; a 2-agent combination is recommended as first-line therapy for pressure that exceeds that threshold. If a patient’s BP target is not reached even with the above strategies, a consultation with a hypertension specialist may be needed.
Treating patients with cardiovascular comorbidities
As noted earlier, JNC 8 offers no guidance in treating patients with HF or CAD and multiple comorbidities. In such cases, we turn to the American College of Cardiology (ACC) and American Heart Association (AHA).24
Recent ACC/AHA guidelines recommend a beta-blocker and ACEI for patients with a history of symptomatic stable HF and a left ventricular ejection fraction (EF) ≤40%, unless contraindications exist.24 Beta-blockers and an ACEI or an ARB should be used to prevent HF in patients with a history of myocardial infarction (MI) or acute coronary syndrome and a reduced EF. Beta-blockers with evidence to support their use in such cases include carvedilol, bisoprolol, and sustained-release metoprolol succinate.24
For symptomatic patients with dyspnea or other mild fluid retention, a loop diuretic or a thiazide diuretic can be used. Nondihydropyridine CCBs should be avoided in post-MI patients with low left ventricular EF due to the medication’s negative inotropic effects.24 The optimal drug regimen for secondary stroke prevention is not clear due to a lack of studies comparing drug regimens, but data suggest that a diuretic or a diuretic-ACEI combination is beneficial.25
Evaluating treatment-resistant hypertension
When a patient presents with treatment-resistant hypertension—elevated BP that is not controlled with a 3-drug regimen, all at maximum doses—start by asking several questions.26 Is the patient:
- having difficulty following a drug regimen that calls for multiple daily doses?
- drinking excessive amounts of alcohol?
- failing to adhere to a low-salt dietary regimen?
- taking any other medications or supplements that might elevate BP (eg, nonsteroidal anti-inflammatory agents, pseudoephedrine, ephedra, or licorice)?
- unable to afford all the drugs prescribed?
If no such issues are identified, consider a referral to a specialist for further evaluation and to rule out disorders associated with treatment-resistant hypertension, including CKD, renal artery stenosis, hyperaldosteronemia, sleep apnea, and coarctation of the aorta.26
For most people, cardiovascular health is dependent on exercise and weight control. That’s particularly true for those with hypertension, for whom limiting alcohol and salt consumption is crucial, as well.
JNC 8 calls for lifestyle management,1 but specific recommendations come from the American College of Cardiology (ACC)/American Heart Association (AHA)’s 2013 Lifestyle Work Group.17 The guidelines call for patients with elevated blood pressure (BP) to follow a diet rich in vegetables, fruits, and whole grains, including low-fat dairy, poultry, fish, legumes, nuts, and nontropical vegetable oils, such as the DASH (Dietary Approaches to Stop Hypertension) or AHA diet. Salt consumption should not exceed 2400 mg/d—and, ideally, be limited to 1500 mg/d or reflect a reduction of at least 1000 mg/d.17
Stress the importance of regular physical activity in controlling BP, as well. The ACC/AHA call for adults to engage in moderate to vigorous aerobic activity 3 to 4 times a week, averaging about 40 minutes per session.17
CASE › When Ms. S returns 3 months later, her BP is 140/70 mm Hg, her fasting glucose is 94 mg/dL, and her HbA1c is 5.7%. You encourage her to continue her new dietary and exercise regimen and schedule a follow-up visit in 6 months.
CORRESPONDENCE
Tracy D. Mahvan, PharmD, University of Wyoming, School of Pharmacy, Health Sciences Center, Room 292, 1000 East University Avenue, Department 3375, Laramie, WY 82071; [email protected]
1. James PA, Oparil S, Carter BL, et al. 2014 evidence-based guideline for the management of high blood pressure in adults: report from the panel members appointed to the Eighth Joint National Committee (JNC 8). JAMA. 2014;311:507-520.
2. Chobanian AV, Bakris GL, Black HR, et al; Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. National Heart, Lung, and Blood Institute; National High Blood Pressure Education Program Coordinating Committee. Seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. Hypertension. 2003;42:1206-1252.
3. Roger VL, Go AS, Lloyd-Jones DM, et al; American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics—2012 update: a report from the American Heart Association. Circulation. 2012;125:e2-e220.
4. Heidenreich PA, Trogdon JG, Khavjou OA, et al; American Heart Association Advocacy Coordinating Committee; Stroke Council; Council on Cardiovascular Radiology and Intervention; Council on Clinical Cardiology; Council on Epidemiology and Prevention; Council on Arteriosclerosis; Thrombosis and Vascular Biology; Council on Cardiopulmonary; Critical Care; Perioperative and Resuscitation; Council on Cardiovascular Nursing; Council on the Kidney in Cardiovascular Disease; Council on Cardiovascular Surgery and Anesthesia, and Interdisciplinary Council on Quality of Care and Outcomes Research. Forecasting the future of cardiovascular disease in the United States: a policy statement from the American Heart Association. Circulation. 2011;123:933-944.
5. JATOS Study Group. Principal results of the Japanese trial to assess optimal systolic blood pressure in elderly hypertensive patients (JATOS). Hypertens Res. 2008;31:2115-2127.
6. Ogihara T, Saruta T, Rakugi H, et al; Valsartan in Elderly Isolated Systolic Hypertension Study Group. Target blood pressure for treatment of isolated systolic hypertension in the elderly: valsartan in elderly isolated systolic hypertension study. Hypertension. 2010;56:196-202.
7. Hypertension Detection and Follow-up Program Cooperative Group. Five-year findings of the hypertension detection and follow-up program. I. Reduction in mortality of persons with high blood pressure, including mild hypertension. JAMA. 1979;242:2562-2571.
8. Hypertension Detection and Follow-up Program Cooperative Group. Five-year findings of the hypertension detection and follow-up program. III. Reduction in stroke incidence among persons with high blood pressure. JAMA. 1982;247:633-638.
9. Hypertension-Stroke Cooperative Study Group. Effect of antihypertensive treatment on stroke recurrence. JAMA. 1974;229:409-418.
10. Medical Research Council Working Party. MRC trial of treatment of mild hypertension: principal results. Br Med J (Clin Res Ed). 1985;291:97-104.
11. The Australian therapeutic trial in mild hypertension. Report by the Management Committee. Lancet. 1980;1:1261-1267.
12. Effects of treatment on morbidity in hypertension. II. Results in patients with diastolic blood pressure averaging 90 through 114 mm Hg. JAMA. 1970;213:1143-1152.
13. Curb JD, Pressel SL, Cutler JA, et al; Systolic Hypertension in the Elderly Program Cooperative Research Group. Effect of diuretic-based antihypertensive treatment on cardiovascular disease risk in older diabetic patients with isolated systolic hypertension. JAMA. 1996;276:1886-1892.
14. Tuomilehto J, Rastenyte D, Birkenhäger WH, et al; Systolic Hypertension in Europe Trial Investigators. Effects of calcium-channel blockade in older patients with diabetes and systolic hypertension. N Engl J Med. 1999;340:677-684.
15. UK Prospective Diabetes Study Group. Tight blood pressure control and risk of macrovascular and microvascular complications in type 2 diabetes: UKPDS 38. BMJ. 1998;317:703-713.
16. Cushman WC, Evans GW, Byington RP, et al; ACCORD Study Group. Effects of intensive blood-pressure control in type 2 diabetes mellitus. N Engl J Med. 2010;362:1575-1585.
17. Eckel RH, Jakicic JM, Ard JD, et al. 2013 AHA/ACC guideline on lifestyle management to reduce cardiovascular risk: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014;129(25 suppl 2):S76-S99.
18. SHEP Cooperative Research Group. Prevention of stroke by antihypertensive drug treatment in older persons with isolated systolic hypertension. Final results of the Systolic Hypertension in the Elderly Program (SHEP). JAMA. 1991;265:3255-3264.
19. Hypertension Detection and Follow-up Program Cooperative Group. Five-year findings of the hypertension detection and follow-up program. I. Reduction in mortality of persons with high blood pressure, including mild hypertension. JAMA. 1979;242:2562-2571.
20. Effects of treatment on morbidity in hypertension. II. Results in patients with diastolic blood pressure averaging 90 through 114 mm Hg. JAMA. 1970;213:1143-1152.
21. Mann JFE. Choice of drug therapy in primary (essential) hypertension: recommendations. UpToDate Web site. Available at: http://www.uptodate.com/contents/choice-of-drug-therapy-in-primary-essential-hypertension-recommendations. Accessed March 3, 2014.
22. ALLHAT Officers and Coordinators for the ALLHAT Collaborative Research Group. The Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial. Major outcomes in high-risk hypertensive patients randomized to angiotensin-converting enzyme inhibitor or calcium channel blocker vs diuretic: The Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial (ALLHAT). JAMA. 2002;288:2981-2997.
23. Wright JT Jr, Bakris G, Greene T, et al; African American Study of Kidney Disease and Hypertension Study Group. Effect of blood pressure lowering and antihypertensive drug class on progression of hypertensive kidney disease: results from the AASK trial. JAMA. 2002;288:2421-2431.
24. Yancy CW, Jessup M, Bozkurt B, et al; American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation. 2013;128:e240-e319.
25. Furie KL, Kasner SE, Adams RJ, et al; American Heart Assocaition Stroke Council, Council on Cardiovascular Nursing, Council on Clinical Cardiology, and Interdisciplinary Council on Quality of Care and Outcomes Research. Guidelines for the prevention of stroke in patients with stroke or transient ischemic attack: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. American Heart Association Stroke Council, Council on Cardiovascular Nursing, Council on Clinical Cardiology, and Interdisciplinary Council on Quality of Care and Outcomes Research. Stroke. 2011;42:227-276.
26. Weber MA, Schiffrin EL, White WB, et al. Clinical practice guidelines for the management of hypertension in the community: a statement by the American Society of Hypertension and the International Society of Hypertension. J Clin Hypertens (Greenwich). 2014;16:14-26.
› Initiate pharmacologic treatment for patients
60 years or older with systolic blood pressure (BP) ≥150 mm Hg and/or diastolic BP ≥90 mm Hg. A
› Start antihypertensive treatment for systolic BP ≥140 mm Hg and/or diastolic BP ≥90 mm Hg in patients who are younger than 60 or have chronic kidney disease or diabetes. C
› Select either a thiazide diuretic or a calcium channel blocker as first-line therapy for African Americans, whether or not they have diabetes. C
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
CASE › Carla S is a 64-year old African American whom you’re seeing for the first time. Her health has been excellent over the last 10 years, she reports, with one caveat: She has “borderline hypertension,” but has never been treated for it and denies any symptoms. Her blood pressure (BP) today is 154/82 mm Hg. A physical exam is unremarkable. Blood tests reveal a normal blood count and normal renal function, and a nonfasting glucose level of 145 mg/dL. You ask Ms. S to return in a week for a repeat BP and fasting lab work.
Hypertension is the most common condition seen by physicians in primary care,1 and a major risk factor for cardiovascular disease (CVD) and the morbidity and mortality associated with it. US treatment costs are an estimated $131 billion per year.2-4 With this in mind, the Joint National Committee on Hypertension (JNC) released its eighth report (JNC 8) in December 20131—the first update in a decade.
In many ways, JNC 8 guidelines are simpler than those of JNC 7,2 with more evidence-based recommendations and less reliance on expert opinion. The JNC has eliminated definitions such as stage 1, 2, and 3 hypertension, and focuses on outcomes instead. At the heart of the recommendations are 3 key questions:
1. At what BP should treatment be initiated to improve outcomes?
2. What should the target BP be for those undergoing treatment?
3. Which medications are best?
Answers to the first 2 questions, of course, go hand in hand. In other words, if the threshold for treatment is a systolic BP ≥140 mm Hg (more on that in a moment), then the target of treatment is a systolic BP of <140 mm Hg. In answer to the third question, JNC 8 offers guidance but gives physicians greater discretion in determining which type of drug to use when initiating treatment.1
In the text, algorithm, and table that follow, we present an overview of JNC 8. We also discuss the optimal treatment of hypertension in patients with heart failure (HF) and coronary artery disease (CAD)—populations JNC 8 does not address.
Age-based recommendations are a bit less stringent
60 years and older. Unlike JNC 7, which recommended initiating treatment for otherwise healthy patients of all ages with a BP ≥140/90 mm Hg,2 JNC 8 clearly delineates its recommendations by age. It calls for treating patients ages 60 or older with systolic BP ≥150 mm Hg and/or diastolic BP ≥90.1
The change is evidence-based: Moderate- to high-quality randomized controlled trials (RCTs) have found a reduced incidence of stroke, HF, and coronary heart disease when BP was treated to <150/90, but no additional benefit from a systolic BP target of <140 mm Hg for patients in this age group.5,6 Notably, JNC 8 does not recommend a change in medication for patients 60 years or older for whom the more stringent target is being maintained without adverse effects.1
18 to 59 years. For adults younger than 60, JNC 8 recommends treating systolic BP ≥140 and diastolic BP ≥90 mm Hg.1 The systolic BP guideline is based on expert opinion, however, as there is no high-quality evidence for a systolic threshold in this age group. This is largely because most patients younger than 60 who have systolic BP ≥140 also have diastolic BP ≥90, making it difficult to study the treatment of systolic BP alone. High-quality trials have shown improved health outcomes when patients ages 30 to 59 years were treated for diastolic BP ≥90, however.7-12 For patients younger than 30, the recommendation for treatment of diastolic pressure is based on expert consensus, as no sufficiently high-quality evidence exists.
Targets for patients with CKD and diabetes
Chronic kidney disease (CKD). JNC 8 recommends treating patients ages 18 to 69 years who have CKD and BP ≥140/90 mm Hg. JNC 7’s more stringent recommendation—treating such patients with BP ≥130/80 mm Hg2—was relaxed because there is little evidence of a lower mortality rate or cardiovascular or cerebrovascular benefits as a result of tighter control. In patients younger than 70, CKD is defined as an estimated (or measured) glomerular filtration rate (GFR) <60 mL/min/1.73 m2 or albuminuria (>30 mg of albumin per g of creatinine).1
It is important to note that this goal does not apply to individuals who have CKD and are 70 years or older. This is due to insufficient evidence, as well as uncertainty about the accuracy of an estimated GFR in this patient population. JNC 8 recommends that treatment of BP in patients 70 or older be based on comorbidities, including albuminuria, among other patient-specific considerations.1
Diabetes. JNC 8 recommends treating patients age 18 years or older who have diabetes and BP ≥140/90 mm Hg, as JNC 7 did.2 This is based largely on expert opinion.
Studies suggest that adults with both hypertension and diabetes have a reduction in mortality and improved cardiovascular and cerebrovascular outcomes when systolic BP is <150 mm Hg,13-15 but no strong data support a goal of <140/90 mm Hg. The Action to Control Cardiovascular Risk in Diabetes (ACCORD) BP trial, for example, showed comparable outcomes in patients with systolic BP of 150 or 140 mm Hg.16 The use of expert opinion vs well-designed studies in this instance seems at odds with JNC 8’s general policy of placing greater emphasis on evidence.
CASE › On her second visit, Ms. S’s BP is 144/82 mm Hg and her cholesterol levels are within the normal range. Her fasting glucose level is 104 mg/dL and glycated hemoglobin (HbA1c) is 6%. At a repeat visit one month later, her BP is 146/76 mm Hg. Given these 2 acceptable readings (<150/90 mm Hg for individuals age 60 and older who do not have diabetes), you do not initiate antihypertensive treatment.
However, you explain to the patient that her fasting glucose and HbA1c are evidence of insulin resistance. Although a diagnosis of diabetes is not warranted, you arrange for Ms. S to meet with a diabetes nurse educator for help in improving her diet and following an exercise regimen.
Pharmacotherapy: JNC 8 offers wider latitude
Like its predecessor, JNC 8 stresses the importance of diet and exercise. (See “Controlling hypertension starts with lifestyle modification”17 in this article.) It diverges from JNC 7, however, in its recommendations for initiating treatment (ALGORITHM).1 The earlier version recommended thiazide diuretics as first-line therapy but included multiple indications for initiating therapy with other drug classes. JNC 8 guidelines are less specific.
Starting therapy with a thiazide diuretic, angiotensin-converting enzyme inhibitor (ACEI), angiotensin receptor blocker (ARB), or calcium channel blocker (CCB)—all of which have high-quality evidence of improved outcomes18-20—is recommended for most patients, including those with diabetes. (Blacks and patients with CKD are exceptions.) The recommended doses of these medications, summarized in the TABLE,1,21 are similar to those used in RCTs. Other types of drugs are not recommended, either because they were shown to be inferior to another class of antihypertensive or because there is insufficient evidence of their efficacy.
For most blacks... JNC 8 recommends thiazide diuretics and CCBs as first-line therapy—a recommendation that is evidence-based. The Antihypertensive and Lipid Lowering Treatment to Prevent Heart Attack Trial (ALLHAT)22 revealed that black patients taking thiazide diuretics had fewer cerebrovascular and cardiovascular events and a lower rate of HF compared with those taking ACEIs, whether or not they had diabetes. Diuretics were more effective than CCBs in preventing HF, but no difference in rates of cerebrovascular and cardiovascular events, kidney disease, or overall mortality was found.22
For patients with CKD and proteinuria, regardless of race, JNC 8 calls for either an ACEI or an ARB as first-line agent to prevent progression to end-stage renal disease. This recommendation is based on expert consensus, and intended to prevent progression to end-stage renal disease.1,23
The optimal first-line agent for patients who have CKD without proteinuria is less clear. For such patients, JNC 8 notes, any of the 4 recommended drug classes can be used for initial therapy.1
Guidance on starting—and titrating—therapy
JNC 7 guidelines featured a complex means of diagnosing and monitoring hypertension.2 JNC 8 has simplified the recommendations, which call for patients to be reassessed within a month of initiating therapy.
The new guidelines include 3 distinct methods of dosing antihypertensive medications, none of which has demonstrated better outcomes than any other. All call for replacing one type of drug with another if the first trial is ineffective or results in adverse effects. And all stress the importance of avoiding ACEI and ARB combinations due to increases in serum creatinine and hyperkalemia and the need for monitoring. Note, however, that Method 3 is recommended for patients with more severe hypertension.1
Method 1. Initiate one medication from any of the 4 classes of antihypertensives recommended for initial treatment, and titrate to the maximum effective dose. If the BP goal is not achieved at maximum dose, add a medication from a second class and titrate that drug to the maximum effective dose, as well. If the goal is still not reached, add a medication from a third class and titrate up as needed.
Method 2. Initiate one medication, then add a second agent from a different drug class, if necessary, and titrate until both are at the maximum effective dose. If the goal still has not been reached, add a third agent and titrate that until BP is well controlled.
Method 3. Initiate 2 medications from 2 different classes of drugs simultaneously. If BP is not at goal after a reasonable trial, add a third agent and titrate to maximum effective dose. (Use this approach for patients who have systolic BP >160 mm Hg and/or diastolic BP >100 mm Hg or systolic BP >20 mm Hg above goal and/or diastolic BP >10 mm Hg above goal.)
As a general rule, a trial with monotherapy should be considered if BP is ≤160/100; a 2-agent combination is recommended as first-line therapy for pressure that exceeds that threshold. If a patient’s BP target is not reached even with the above strategies, a consultation with a hypertension specialist may be needed.
Treating patients with cardiovascular comorbidities
As noted earlier, JNC 8 offers no guidance in treating patients with HF or CAD and multiple comorbidities. In such cases, we turn to the American College of Cardiology (ACC) and American Heart Association (AHA).24
Recent ACC/AHA guidelines recommend a beta-blocker and ACEI for patients with a history of symptomatic stable HF and a left ventricular ejection fraction (EF) ≤40%, unless contraindications exist.24 Beta-blockers and an ACEI or an ARB should be used to prevent HF in patients with a history of myocardial infarction (MI) or acute coronary syndrome and a reduced EF. Beta-blockers with evidence to support their use in such cases include carvedilol, bisoprolol, and sustained-release metoprolol succinate.24
For symptomatic patients with dyspnea or other mild fluid retention, a loop diuretic or a thiazide diuretic can be used. Nondihydropyridine CCBs should be avoided in post-MI patients with low left ventricular EF due to the medication’s negative inotropic effects.24 The optimal drug regimen for secondary stroke prevention is not clear due to a lack of studies comparing drug regimens, but data suggest that a diuretic or a diuretic-ACEI combination is beneficial.25
Evaluating treatment-resistant hypertension
When a patient presents with treatment-resistant hypertension—elevated BP that is not controlled with a 3-drug regimen, all at maximum doses—start by asking several questions.26 Is the patient:
- having difficulty following a drug regimen that calls for multiple daily doses?
- drinking excessive amounts of alcohol?
- failing to adhere to a low-salt dietary regimen?
- taking any other medications or supplements that might elevate BP (eg, nonsteroidal anti-inflammatory agents, pseudoephedrine, ephedra, or licorice)?
- unable to afford all the drugs prescribed?
If no such issues are identified, consider a referral to a specialist for further evaluation and to rule out disorders associated with treatment-resistant hypertension, including CKD, renal artery stenosis, hyperaldosteronemia, sleep apnea, and coarctation of the aorta.26
For most people, cardiovascular health is dependent on exercise and weight control. That’s particularly true for those with hypertension, for whom limiting alcohol and salt consumption is crucial, as well.
JNC 8 calls for lifestyle management,1 but specific recommendations come from the American College of Cardiology (ACC)/American Heart Association (AHA)’s 2013 Lifestyle Work Group.17 The guidelines call for patients with elevated blood pressure (BP) to follow a diet rich in vegetables, fruits, and whole grains, including low-fat dairy, poultry, fish, legumes, nuts, and nontropical vegetable oils, such as the DASH (Dietary Approaches to Stop Hypertension) or AHA diet. Salt consumption should not exceed 2400 mg/d—and, ideally, be limited to 1500 mg/d or reflect a reduction of at least 1000 mg/d.17
Stress the importance of regular physical activity in controlling BP, as well. The ACC/AHA call for adults to engage in moderate to vigorous aerobic activity 3 to 4 times a week, averaging about 40 minutes per session.17
CASE › When Ms. S returns 3 months later, her BP is 140/70 mm Hg, her fasting glucose is 94 mg/dL, and her HbA1c is 5.7%. You encourage her to continue her new dietary and exercise regimen and schedule a follow-up visit in 6 months.
CORRESPONDENCE
Tracy D. Mahvan, PharmD, University of Wyoming, School of Pharmacy, Health Sciences Center, Room 292, 1000 East University Avenue, Department 3375, Laramie, WY 82071; [email protected]
› Initiate pharmacologic treatment for patients
60 years or older with systolic blood pressure (BP) ≥150 mm Hg and/or diastolic BP ≥90 mm Hg. A
› Start antihypertensive treatment for systolic BP ≥140 mm Hg and/or diastolic BP ≥90 mm Hg in patients who are younger than 60 or have chronic kidney disease or diabetes. C
› Select either a thiazide diuretic or a calcium channel blocker as first-line therapy for African Americans, whether or not they have diabetes. C
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
CASE › Carla S is a 64-year old African American whom you’re seeing for the first time. Her health has been excellent over the last 10 years, she reports, with one caveat: She has “borderline hypertension,” but has never been treated for it and denies any symptoms. Her blood pressure (BP) today is 154/82 mm Hg. A physical exam is unremarkable. Blood tests reveal a normal blood count and normal renal function, and a nonfasting glucose level of 145 mg/dL. You ask Ms. S to return in a week for a repeat BP and fasting lab work.
Hypertension is the most common condition seen by physicians in primary care,1 and a major risk factor for cardiovascular disease (CVD) and the morbidity and mortality associated with it. US treatment costs are an estimated $131 billion per year.2-4 With this in mind, the Joint National Committee on Hypertension (JNC) released its eighth report (JNC 8) in December 20131—the first update in a decade.
In many ways, JNC 8 guidelines are simpler than those of JNC 7,2 with more evidence-based recommendations and less reliance on expert opinion. The JNC has eliminated definitions such as stage 1, 2, and 3 hypertension, and focuses on outcomes instead. At the heart of the recommendations are 3 key questions:
1. At what BP should treatment be initiated to improve outcomes?
2. What should the target BP be for those undergoing treatment?
3. Which medications are best?
Answers to the first 2 questions, of course, go hand in hand. In other words, if the threshold for treatment is a systolic BP ≥140 mm Hg (more on that in a moment), then the target of treatment is a systolic BP of <140 mm Hg. In answer to the third question, JNC 8 offers guidance but gives physicians greater discretion in determining which type of drug to use when initiating treatment.1
In the text, algorithm, and table that follow, we present an overview of JNC 8. We also discuss the optimal treatment of hypertension in patients with heart failure (HF) and coronary artery disease (CAD)—populations JNC 8 does not address.
Age-based recommendations are a bit less stringent
60 years and older. Unlike JNC 7, which recommended initiating treatment for otherwise healthy patients of all ages with a BP ≥140/90 mm Hg,2 JNC 8 clearly delineates its recommendations by age. It calls for treating patients ages 60 or older with systolic BP ≥150 mm Hg and/or diastolic BP ≥90.1
The change is evidence-based: Moderate- to high-quality randomized controlled trials (RCTs) have found a reduced incidence of stroke, HF, and coronary heart disease when BP was treated to <150/90, but no additional benefit from a systolic BP target of <140 mm Hg for patients in this age group.5,6 Notably, JNC 8 does not recommend a change in medication for patients 60 years or older for whom the more stringent target is being maintained without adverse effects.1
18 to 59 years. For adults younger than 60, JNC 8 recommends treating systolic BP ≥140 and diastolic BP ≥90 mm Hg.1 The systolic BP guideline is based on expert opinion, however, as there is no high-quality evidence for a systolic threshold in this age group. This is largely because most patients younger than 60 who have systolic BP ≥140 also have diastolic BP ≥90, making it difficult to study the treatment of systolic BP alone. High-quality trials have shown improved health outcomes when patients ages 30 to 59 years were treated for diastolic BP ≥90, however.7-12 For patients younger than 30, the recommendation for treatment of diastolic pressure is based on expert consensus, as no sufficiently high-quality evidence exists.
Targets for patients with CKD and diabetes
Chronic kidney disease (CKD). JNC 8 recommends treating patients ages 18 to 69 years who have CKD and BP ≥140/90 mm Hg. JNC 7’s more stringent recommendation—treating such patients with BP ≥130/80 mm Hg2—was relaxed because there is little evidence of a lower mortality rate or cardiovascular or cerebrovascular benefits as a result of tighter control. In patients younger than 70, CKD is defined as an estimated (or measured) glomerular filtration rate (GFR) <60 mL/min/1.73 m2 or albuminuria (>30 mg of albumin per g of creatinine).1
It is important to note that this goal does not apply to individuals who have CKD and are 70 years or older. This is due to insufficient evidence, as well as uncertainty about the accuracy of an estimated GFR in this patient population. JNC 8 recommends that treatment of BP in patients 70 or older be based on comorbidities, including albuminuria, among other patient-specific considerations.1
Diabetes. JNC 8 recommends treating patients age 18 years or older who have diabetes and BP ≥140/90 mm Hg, as JNC 7 did.2 This is based largely on expert opinion.
Studies suggest that adults with both hypertension and diabetes have a reduction in mortality and improved cardiovascular and cerebrovascular outcomes when systolic BP is <150 mm Hg,13-15 but no strong data support a goal of <140/90 mm Hg. The Action to Control Cardiovascular Risk in Diabetes (ACCORD) BP trial, for example, showed comparable outcomes in patients with systolic BP of 150 or 140 mm Hg.16 The use of expert opinion vs well-designed studies in this instance seems at odds with JNC 8’s general policy of placing greater emphasis on evidence.
CASE › On her second visit, Ms. S’s BP is 144/82 mm Hg and her cholesterol levels are within the normal range. Her fasting glucose level is 104 mg/dL and glycated hemoglobin (HbA1c) is 6%. At a repeat visit one month later, her BP is 146/76 mm Hg. Given these 2 acceptable readings (<150/90 mm Hg for individuals age 60 and older who do not have diabetes), you do not initiate antihypertensive treatment.
However, you explain to the patient that her fasting glucose and HbA1c are evidence of insulin resistance. Although a diagnosis of diabetes is not warranted, you arrange for Ms. S to meet with a diabetes nurse educator for help in improving her diet and following an exercise regimen.
Pharmacotherapy: JNC 8 offers wider latitude
Like its predecessor, JNC 8 stresses the importance of diet and exercise. (See “Controlling hypertension starts with lifestyle modification”17 in this article.) It diverges from JNC 7, however, in its recommendations for initiating treatment (ALGORITHM).1 The earlier version recommended thiazide diuretics as first-line therapy but included multiple indications for initiating therapy with other drug classes. JNC 8 guidelines are less specific.
Starting therapy with a thiazide diuretic, angiotensin-converting enzyme inhibitor (ACEI), angiotensin receptor blocker (ARB), or calcium channel blocker (CCB)—all of which have high-quality evidence of improved outcomes18-20—is recommended for most patients, including those with diabetes. (Blacks and patients with CKD are exceptions.) The recommended doses of these medications, summarized in the TABLE,1,21 are similar to those used in RCTs. Other types of drugs are not recommended, either because they were shown to be inferior to another class of antihypertensive or because there is insufficient evidence of their efficacy.
For most blacks... JNC 8 recommends thiazide diuretics and CCBs as first-line therapy—a recommendation that is evidence-based. The Antihypertensive and Lipid Lowering Treatment to Prevent Heart Attack Trial (ALLHAT)22 revealed that black patients taking thiazide diuretics had fewer cerebrovascular and cardiovascular events and a lower rate of HF compared with those taking ACEIs, whether or not they had diabetes. Diuretics were more effective than CCBs in preventing HF, but no difference in rates of cerebrovascular and cardiovascular events, kidney disease, or overall mortality was found.22
For patients with CKD and proteinuria, regardless of race, JNC 8 calls for either an ACEI or an ARB as first-line agent to prevent progression to end-stage renal disease. This recommendation is based on expert consensus, and intended to prevent progression to end-stage renal disease.1,23
The optimal first-line agent for patients who have CKD without proteinuria is less clear. For such patients, JNC 8 notes, any of the 4 recommended drug classes can be used for initial therapy.1
Guidance on starting—and titrating—therapy
JNC 7 guidelines featured a complex means of diagnosing and monitoring hypertension.2 JNC 8 has simplified the recommendations, which call for patients to be reassessed within a month of initiating therapy.
The new guidelines include 3 distinct methods of dosing antihypertensive medications, none of which has demonstrated better outcomes than any other. All call for replacing one type of drug with another if the first trial is ineffective or results in adverse effects. And all stress the importance of avoiding ACEI and ARB combinations due to increases in serum creatinine and hyperkalemia and the need for monitoring. Note, however, that Method 3 is recommended for patients with more severe hypertension.1
Method 1. Initiate one medication from any of the 4 classes of antihypertensives recommended for initial treatment, and titrate to the maximum effective dose. If the BP goal is not achieved at maximum dose, add a medication from a second class and titrate that drug to the maximum effective dose, as well. If the goal is still not reached, add a medication from a third class and titrate up as needed.
Method 2. Initiate one medication, then add a second agent from a different drug class, if necessary, and titrate until both are at the maximum effective dose. If the goal still has not been reached, add a third agent and titrate that until BP is well controlled.
Method 3. Initiate 2 medications from 2 different classes of drugs simultaneously. If BP is not at goal after a reasonable trial, add a third agent and titrate to maximum effective dose. (Use this approach for patients who have systolic BP >160 mm Hg and/or diastolic BP >100 mm Hg or systolic BP >20 mm Hg above goal and/or diastolic BP >10 mm Hg above goal.)
As a general rule, a trial with monotherapy should be considered if BP is ≤160/100; a 2-agent combination is recommended as first-line therapy for pressure that exceeds that threshold. If a patient’s BP target is not reached even with the above strategies, a consultation with a hypertension specialist may be needed.
Treating patients with cardiovascular comorbidities
As noted earlier, JNC 8 offers no guidance in treating patients with HF or CAD and multiple comorbidities. In such cases, we turn to the American College of Cardiology (ACC) and American Heart Association (AHA).24
Recent ACC/AHA guidelines recommend a beta-blocker and ACEI for patients with a history of symptomatic stable HF and a left ventricular ejection fraction (EF) ≤40%, unless contraindications exist.24 Beta-blockers and an ACEI or an ARB should be used to prevent HF in patients with a history of myocardial infarction (MI) or acute coronary syndrome and a reduced EF. Beta-blockers with evidence to support their use in such cases include carvedilol, bisoprolol, and sustained-release metoprolol succinate.24
For symptomatic patients with dyspnea or other mild fluid retention, a loop diuretic or a thiazide diuretic can be used. Nondihydropyridine CCBs should be avoided in post-MI patients with low left ventricular EF due to the medication’s negative inotropic effects.24 The optimal drug regimen for secondary stroke prevention is not clear due to a lack of studies comparing drug regimens, but data suggest that a diuretic or a diuretic-ACEI combination is beneficial.25
Evaluating treatment-resistant hypertension
When a patient presents with treatment-resistant hypertension—elevated BP that is not controlled with a 3-drug regimen, all at maximum doses—start by asking several questions.26 Is the patient:
- having difficulty following a drug regimen that calls for multiple daily doses?
- drinking excessive amounts of alcohol?
- failing to adhere to a low-salt dietary regimen?
- taking any other medications or supplements that might elevate BP (eg, nonsteroidal anti-inflammatory agents, pseudoephedrine, ephedra, or licorice)?
- unable to afford all the drugs prescribed?
If no such issues are identified, consider a referral to a specialist for further evaluation and to rule out disorders associated with treatment-resistant hypertension, including CKD, renal artery stenosis, hyperaldosteronemia, sleep apnea, and coarctation of the aorta.26
For most people, cardiovascular health is dependent on exercise and weight control. That’s particularly true for those with hypertension, for whom limiting alcohol and salt consumption is crucial, as well.
JNC 8 calls for lifestyle management,1 but specific recommendations come from the American College of Cardiology (ACC)/American Heart Association (AHA)’s 2013 Lifestyle Work Group.17 The guidelines call for patients with elevated blood pressure (BP) to follow a diet rich in vegetables, fruits, and whole grains, including low-fat dairy, poultry, fish, legumes, nuts, and nontropical vegetable oils, such as the DASH (Dietary Approaches to Stop Hypertension) or AHA diet. Salt consumption should not exceed 2400 mg/d—and, ideally, be limited to 1500 mg/d or reflect a reduction of at least 1000 mg/d.17
Stress the importance of regular physical activity in controlling BP, as well. The ACC/AHA call for adults to engage in moderate to vigorous aerobic activity 3 to 4 times a week, averaging about 40 minutes per session.17
CASE › When Ms. S returns 3 months later, her BP is 140/70 mm Hg, her fasting glucose is 94 mg/dL, and her HbA1c is 5.7%. You encourage her to continue her new dietary and exercise regimen and schedule a follow-up visit in 6 months.
CORRESPONDENCE
Tracy D. Mahvan, PharmD, University of Wyoming, School of Pharmacy, Health Sciences Center, Room 292, 1000 East University Avenue, Department 3375, Laramie, WY 82071; [email protected]
1. James PA, Oparil S, Carter BL, et al. 2014 evidence-based guideline for the management of high blood pressure in adults: report from the panel members appointed to the Eighth Joint National Committee (JNC 8). JAMA. 2014;311:507-520.
2. Chobanian AV, Bakris GL, Black HR, et al; Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. National Heart, Lung, and Blood Institute; National High Blood Pressure Education Program Coordinating Committee. Seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. Hypertension. 2003;42:1206-1252.
3. Roger VL, Go AS, Lloyd-Jones DM, et al; American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics—2012 update: a report from the American Heart Association. Circulation. 2012;125:e2-e220.
4. Heidenreich PA, Trogdon JG, Khavjou OA, et al; American Heart Association Advocacy Coordinating Committee; Stroke Council; Council on Cardiovascular Radiology and Intervention; Council on Clinical Cardiology; Council on Epidemiology and Prevention; Council on Arteriosclerosis; Thrombosis and Vascular Biology; Council on Cardiopulmonary; Critical Care; Perioperative and Resuscitation; Council on Cardiovascular Nursing; Council on the Kidney in Cardiovascular Disease; Council on Cardiovascular Surgery and Anesthesia, and Interdisciplinary Council on Quality of Care and Outcomes Research. Forecasting the future of cardiovascular disease in the United States: a policy statement from the American Heart Association. Circulation. 2011;123:933-944.
5. JATOS Study Group. Principal results of the Japanese trial to assess optimal systolic blood pressure in elderly hypertensive patients (JATOS). Hypertens Res. 2008;31:2115-2127.
6. Ogihara T, Saruta T, Rakugi H, et al; Valsartan in Elderly Isolated Systolic Hypertension Study Group. Target blood pressure for treatment of isolated systolic hypertension in the elderly: valsartan in elderly isolated systolic hypertension study. Hypertension. 2010;56:196-202.
7. Hypertension Detection and Follow-up Program Cooperative Group. Five-year findings of the hypertension detection and follow-up program. I. Reduction in mortality of persons with high blood pressure, including mild hypertension. JAMA. 1979;242:2562-2571.
8. Hypertension Detection and Follow-up Program Cooperative Group. Five-year findings of the hypertension detection and follow-up program. III. Reduction in stroke incidence among persons with high blood pressure. JAMA. 1982;247:633-638.
9. Hypertension-Stroke Cooperative Study Group. Effect of antihypertensive treatment on stroke recurrence. JAMA. 1974;229:409-418.
10. Medical Research Council Working Party. MRC trial of treatment of mild hypertension: principal results. Br Med J (Clin Res Ed). 1985;291:97-104.
11. The Australian therapeutic trial in mild hypertension. Report by the Management Committee. Lancet. 1980;1:1261-1267.
12. Effects of treatment on morbidity in hypertension. II. Results in patients with diastolic blood pressure averaging 90 through 114 mm Hg. JAMA. 1970;213:1143-1152.
13. Curb JD, Pressel SL, Cutler JA, et al; Systolic Hypertension in the Elderly Program Cooperative Research Group. Effect of diuretic-based antihypertensive treatment on cardiovascular disease risk in older diabetic patients with isolated systolic hypertension. JAMA. 1996;276:1886-1892.
14. Tuomilehto J, Rastenyte D, Birkenhäger WH, et al; Systolic Hypertension in Europe Trial Investigators. Effects of calcium-channel blockade in older patients with diabetes and systolic hypertension. N Engl J Med. 1999;340:677-684.
15. UK Prospective Diabetes Study Group. Tight blood pressure control and risk of macrovascular and microvascular complications in type 2 diabetes: UKPDS 38. BMJ. 1998;317:703-713.
16. Cushman WC, Evans GW, Byington RP, et al; ACCORD Study Group. Effects of intensive blood-pressure control in type 2 diabetes mellitus. N Engl J Med. 2010;362:1575-1585.
17. Eckel RH, Jakicic JM, Ard JD, et al. 2013 AHA/ACC guideline on lifestyle management to reduce cardiovascular risk: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014;129(25 suppl 2):S76-S99.
18. SHEP Cooperative Research Group. Prevention of stroke by antihypertensive drug treatment in older persons with isolated systolic hypertension. Final results of the Systolic Hypertension in the Elderly Program (SHEP). JAMA. 1991;265:3255-3264.
19. Hypertension Detection and Follow-up Program Cooperative Group. Five-year findings of the hypertension detection and follow-up program. I. Reduction in mortality of persons with high blood pressure, including mild hypertension. JAMA. 1979;242:2562-2571.
20. Effects of treatment on morbidity in hypertension. II. Results in patients with diastolic blood pressure averaging 90 through 114 mm Hg. JAMA. 1970;213:1143-1152.
21. Mann JFE. Choice of drug therapy in primary (essential) hypertension: recommendations. UpToDate Web site. Available at: http://www.uptodate.com/contents/choice-of-drug-therapy-in-primary-essential-hypertension-recommendations. Accessed March 3, 2014.
22. ALLHAT Officers and Coordinators for the ALLHAT Collaborative Research Group. The Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial. Major outcomes in high-risk hypertensive patients randomized to angiotensin-converting enzyme inhibitor or calcium channel blocker vs diuretic: The Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial (ALLHAT). JAMA. 2002;288:2981-2997.
23. Wright JT Jr, Bakris G, Greene T, et al; African American Study of Kidney Disease and Hypertension Study Group. Effect of blood pressure lowering and antihypertensive drug class on progression of hypertensive kidney disease: results from the AASK trial. JAMA. 2002;288:2421-2431.
24. Yancy CW, Jessup M, Bozkurt B, et al; American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation. 2013;128:e240-e319.
25. Furie KL, Kasner SE, Adams RJ, et al; American Heart Assocaition Stroke Council, Council on Cardiovascular Nursing, Council on Clinical Cardiology, and Interdisciplinary Council on Quality of Care and Outcomes Research. Guidelines for the prevention of stroke in patients with stroke or transient ischemic attack: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. American Heart Association Stroke Council, Council on Cardiovascular Nursing, Council on Clinical Cardiology, and Interdisciplinary Council on Quality of Care and Outcomes Research. Stroke. 2011;42:227-276.
26. Weber MA, Schiffrin EL, White WB, et al. Clinical practice guidelines for the management of hypertension in the community: a statement by the American Society of Hypertension and the International Society of Hypertension. J Clin Hypertens (Greenwich). 2014;16:14-26.
1. James PA, Oparil S, Carter BL, et al. 2014 evidence-based guideline for the management of high blood pressure in adults: report from the panel members appointed to the Eighth Joint National Committee (JNC 8). JAMA. 2014;311:507-520.
2. Chobanian AV, Bakris GL, Black HR, et al; Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. National Heart, Lung, and Blood Institute; National High Blood Pressure Education Program Coordinating Committee. Seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. Hypertension. 2003;42:1206-1252.
3. Roger VL, Go AS, Lloyd-Jones DM, et al; American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics—2012 update: a report from the American Heart Association. Circulation. 2012;125:e2-e220.
4. Heidenreich PA, Trogdon JG, Khavjou OA, et al; American Heart Association Advocacy Coordinating Committee; Stroke Council; Council on Cardiovascular Radiology and Intervention; Council on Clinical Cardiology; Council on Epidemiology and Prevention; Council on Arteriosclerosis; Thrombosis and Vascular Biology; Council on Cardiopulmonary; Critical Care; Perioperative and Resuscitation; Council on Cardiovascular Nursing; Council on the Kidney in Cardiovascular Disease; Council on Cardiovascular Surgery and Anesthesia, and Interdisciplinary Council on Quality of Care and Outcomes Research. Forecasting the future of cardiovascular disease in the United States: a policy statement from the American Heart Association. Circulation. 2011;123:933-944.
5. JATOS Study Group. Principal results of the Japanese trial to assess optimal systolic blood pressure in elderly hypertensive patients (JATOS). Hypertens Res. 2008;31:2115-2127.
6. Ogihara T, Saruta T, Rakugi H, et al; Valsartan in Elderly Isolated Systolic Hypertension Study Group. Target blood pressure for treatment of isolated systolic hypertension in the elderly: valsartan in elderly isolated systolic hypertension study. Hypertension. 2010;56:196-202.
7. Hypertension Detection and Follow-up Program Cooperative Group. Five-year findings of the hypertension detection and follow-up program. I. Reduction in mortality of persons with high blood pressure, including mild hypertension. JAMA. 1979;242:2562-2571.
8. Hypertension Detection and Follow-up Program Cooperative Group. Five-year findings of the hypertension detection and follow-up program. III. Reduction in stroke incidence among persons with high blood pressure. JAMA. 1982;247:633-638.
9. Hypertension-Stroke Cooperative Study Group. Effect of antihypertensive treatment on stroke recurrence. JAMA. 1974;229:409-418.
10. Medical Research Council Working Party. MRC trial of treatment of mild hypertension: principal results. Br Med J (Clin Res Ed). 1985;291:97-104.
11. The Australian therapeutic trial in mild hypertension. Report by the Management Committee. Lancet. 1980;1:1261-1267.
12. Effects of treatment on morbidity in hypertension. II. Results in patients with diastolic blood pressure averaging 90 through 114 mm Hg. JAMA. 1970;213:1143-1152.
13. Curb JD, Pressel SL, Cutler JA, et al; Systolic Hypertension in the Elderly Program Cooperative Research Group. Effect of diuretic-based antihypertensive treatment on cardiovascular disease risk in older diabetic patients with isolated systolic hypertension. JAMA. 1996;276:1886-1892.
14. Tuomilehto J, Rastenyte D, Birkenhäger WH, et al; Systolic Hypertension in Europe Trial Investigators. Effects of calcium-channel blockade in older patients with diabetes and systolic hypertension. N Engl J Med. 1999;340:677-684.
15. UK Prospective Diabetes Study Group. Tight blood pressure control and risk of macrovascular and microvascular complications in type 2 diabetes: UKPDS 38. BMJ. 1998;317:703-713.
16. Cushman WC, Evans GW, Byington RP, et al; ACCORD Study Group. Effects of intensive blood-pressure control in type 2 diabetes mellitus. N Engl J Med. 2010;362:1575-1585.
17. Eckel RH, Jakicic JM, Ard JD, et al. 2013 AHA/ACC guideline on lifestyle management to reduce cardiovascular risk: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014;129(25 suppl 2):S76-S99.
18. SHEP Cooperative Research Group. Prevention of stroke by antihypertensive drug treatment in older persons with isolated systolic hypertension. Final results of the Systolic Hypertension in the Elderly Program (SHEP). JAMA. 1991;265:3255-3264.
19. Hypertension Detection and Follow-up Program Cooperative Group. Five-year findings of the hypertension detection and follow-up program. I. Reduction in mortality of persons with high blood pressure, including mild hypertension. JAMA. 1979;242:2562-2571.
20. Effects of treatment on morbidity in hypertension. II. Results in patients with diastolic blood pressure averaging 90 through 114 mm Hg. JAMA. 1970;213:1143-1152.
21. Mann JFE. Choice of drug therapy in primary (essential) hypertension: recommendations. UpToDate Web site. Available at: http://www.uptodate.com/contents/choice-of-drug-therapy-in-primary-essential-hypertension-recommendations. Accessed March 3, 2014.
22. ALLHAT Officers and Coordinators for the ALLHAT Collaborative Research Group. The Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial. Major outcomes in high-risk hypertensive patients randomized to angiotensin-converting enzyme inhibitor or calcium channel blocker vs diuretic: The Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial (ALLHAT). JAMA. 2002;288:2981-2997.
23. Wright JT Jr, Bakris G, Greene T, et al; African American Study of Kidney Disease and Hypertension Study Group. Effect of blood pressure lowering and antihypertensive drug class on progression of hypertensive kidney disease: results from the AASK trial. JAMA. 2002;288:2421-2431.
24. Yancy CW, Jessup M, Bozkurt B, et al; American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation. 2013;128:e240-e319.
25. Furie KL, Kasner SE, Adams RJ, et al; American Heart Assocaition Stroke Council, Council on Cardiovascular Nursing, Council on Clinical Cardiology, and Interdisciplinary Council on Quality of Care and Outcomes Research. Guidelines for the prevention of stroke in patients with stroke or transient ischemic attack: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. American Heart Association Stroke Council, Council on Cardiovascular Nursing, Council on Clinical Cardiology, and Interdisciplinary Council on Quality of Care and Outcomes Research. Stroke. 2011;42:227-276.
26. Weber MA, Schiffrin EL, White WB, et al. Clinical practice guidelines for the management of hypertension in the community: a statement by the American Society of Hypertension and the International Society of Hypertension. J Clin Hypertens (Greenwich). 2014;16:14-26.
Statin adverse effects: Sorting out the evidence
› Advise patients starting statin therapy to stop taking the medication and call your office immediately if they develop severe muscle pain or weakness, as statins are associated with a small increased risk of rhabdomyolysis. B
› Obtain a baseline creatine kinase level for patients with an increased risk of musculoskeletal disorders; routine monitoring is needed only for those who experience muscle pain or weakness while on statin therapy. C
› Prescribe statins for patients with chronic kidney or liver disease when indicated; statin therapy is not associated with an increased risk of renal or hepatic failure. B
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
CASE › Carl L, a 57-year-old obese patient (body mass index [BMI] >40) who had not been to a doctor in a decade, comes to see you after a health fair screening revealed dyslipidemia (low-density lipoprotein [LDL] cholesterol, 188 mg/dL; high-density lipoprotein cholesterol [HDL], 32 mg/dL; total cholesterol, 240 mg/dL; triglycerides, 100 mg/dL). His blood pressure (BP) is 146/90 mm Hg and his fasting glucose is 101 mg/dL. Labs drawn that day reveal a glycated hemoglobin (HbA1c) of 5.9%, alanine aminotransferase (ALT) of 45 U/L, and aspartate aminotransferase (AST) of 62 U/L. In taking his history, you discover that Mr. L also has a prominent family history of heart disease.
Mr. L agrees to take a low-dose statin, and you prescribe atorvastatin 10 mg and a thiazide diuretic. You advise the patient to contact you immediately if he develops significant myalgia, jaundice, dark urine, or symptoms of hyperglycemia such as excessive thirst or urination, and schedule a follow-up visit in 8 weeks.
Long recognized as the bedrock of hyperlipidemia therapy, statins achieved even greater prominence when the American College of Cardiology/American Heart Association (ACC/AHA) issued a new cholesterol guideline1 late last year. The ACC and AHA now recommend statins for a wider range of patients, often at a higher starting dose. (To read about the controversy the recommendations generated, see “The new cholesterol guideline: Beyond the headlines,” J Fam Pract. 2013;62:730.)
Based on the new recommendations, the use of statins is likely to rise.2 (A statin [rosuvastatin] is already the nation’s most widely prescribed medication.2) Thus, it is more important than ever for physicians to be knowledgeable about the risks associated with statins and able to assess the benefits of therapy for individual patients.
A 2013 retrospective cohort study of >100,000 patients on statins found that 17% developed adverse effects (AEs). Therapy was withheld, at least temporarily, for 10% of study participants (60% of those experiencing AEs).3 At the same time, the authors of a large meta-analysis (135 randomized controlled trials [RCTs] and >240,000 patients) reported that AEs associated with statins as a class were uncommon. The meta-analysis also found that the overall discontinuation rate for statin users—5.7%—was not significantly different from that of patients on placebo.4
Such discrepancies regarding particular risks as well as the overall incidence of AEs and discontinuation rates make the evidence difficult to sort out. We created this update with that in mind.
Musculoskeletal symptoms are most common
Skeletal muscle symptoms are the most common AEs reported by patients taking statins.5 These range from muscle weakness, fatigue, and pain to (rarely) rhabdomyolysis—a life-threatening condition characterized by severe muscle pain, muscle weakness, a 10-fold increase in creatine kinase (CK), and increased serum creatinine, often with myoglobinuria.5
Patients with myopathy—an umbrella term for any muscle disease—may report stiffness, weakness, tenderness, soreness, cramping, or heaviness. Symptoms usually are symmetrical and often involve the proximal limbs and trunk.6 Studies indicate that exercise increases the risk of statin-induced myalgia—muscle pain or weakness without an increase in CK—and that patients taking statins are more prone to exercise-related injury.7,8
A baseline CK is recommended for patients with an increased risk of muscular disorders.1 Risk factors include a personal or family history of statin intolerance or muscle disease, age >75 years, low levels of vitamin D, and concomitant use of medications that may increase the risk of myopathy (TABLE 1).1 Routine monitoring of CK is not recommended, but CK levels should be obtained for those who exhibit muscle symptoms while on statin therapy.1
What the studies show
The incidence of myalgia reported in clinical studies is highly variable, ranging from <1% to 20%.1,9,10 The ACC/AHA guideline reports only one additional case of myopathy per 10,000 statin users compared with those on placebo and cites a rhabdomyolysis occurrence rate of <.06% over 5 years.1
A 2006 systematic review estimated the absolute risk of rhabdomyolysis to be 3.4 per 100,000 person-years, but the incidence was 10 times higher for patients taking both a statin and gemfibrozil.11 (See TABLE 212,13 for more on drug-drug interactions.) But both the meta-analysis cited earlier4 and an earlier systematic review14 (35 RCTs and >74,000 patients) found that statins as a class do not increase the incidence of myalgia or rhabdomyolysis.
Differences in the way muscular disorders are defined has been suggested as one reason for the discrepancies.10 In addition, many clinical trials exclude patients at higher risk of statin-associated AEs, such as those with renal or hepatic insufficiency, prior muscular complaints, poorly controlled diabetes, or potential drug-drug interactions.1
An FDA advisory. In a safety communication last updated in February 2012, the US Food and Drug Administration (FDA) cautioned against starting patients on the highest dose of simvastatin (80 mg).15 The warning is based on a large study (N=12,064) that found an increased risk of myopathy (0.9%) and rhabdomyolysis (0.2%) in patients on the 80-mg dose vs those taking 20 mg (0.02% and 0%, respectively).16
With the ACC/AHA now recommending intensive therapy (atorvastatin 40-80 mg or rosuvastatin 20-40 mg) to achieve an LDL reduction >50% for many patients,1 it is important to be aware that this risk is specific to simvastatin. A recent meta-analysis of studies directly comparing patients receiving intensive statin therapy with those on low to moderate doses did not find any increased risk in rhabdomyolysis associated with more intensive therapy when those taking 80 mg simvastatin were excluded.17
THE BOTTOM LINE: Although rhabdomyolysis is rare, its severity—a fatality rate of 10%11—makes it critical to educate patients about the disorder and instruct them to stop taking the statin and call the office immediately if they develop severe muscle pain or weakness.
Recommend CoQ10 for statin-induced myopathy
Although the exact mechanism of statin-induced myopathy is unknown, the most likely explanation is a depletion of coenzyme Q10 (CoQ10), which has negative effects on mitochondrial energy production.18 While studies using CoQ10 to treat this AE have been small and had mixed results, the overall evidence suggests that it decreases the development and/or severity of symptoms.18-20
In fact, CoQ10 supplementation is the only treatment that has shown promise in treating statin-induced muscle symptoms.18-20 Doses of about 100 mg bid have been found to be beneficial and safe; no clinically relevant AEs have been seen with doses <300 mg/d.18,20,21 A large placebo-controlled study is currently evaluating a 600 mg/d dose of CoQ10 in patients with statin-induced myopathy.19
CASE › On his next visit, Mr. L reports a new ache in his left shoulder and upper back, which he describes as mild, but annoying. He also tells you his memory seems to be getting worse and that he has developed an odd tingling in his hands. These symptoms began about a month after he started the medications, Mr. L says. He also began a new exercise program, but his BMI is unchanged.
On examination, you find the affected shoulder and upper back modestly and diffusely tender to palpation, but with no decline in strength. Mr. L’s BP has fallen to 134/84 mm Hg and his fasting glucose is 105 mg/dL. Lab tests reveal an LDL of 144 mg/dL and HDL of 36 mg/dL, HbA1c of 6.1%, ALT of 105 U/L, AST of 61 U/L, and a normal CK.
You recommend 100 mg CoQ10 bid. Because it is available only over the counter, you advise the patient to look for a product whose purity and potency have been verified by an external source, such as the US Pharmacopeial Convention. You also prescribe metformin 500 mg bid for insulin resistance, refer the patient to a nutritionist and diabetes specialist, and order tests to evaluate his other symptoms.
Hepatic effects are rare
Historically, statins have been linked to potential hepatotoxicity, with case reports of serum transaminase elevation, cholestasis, hepatitis, and acute liver failure. It is now recognized that hepatic AEs are rare and that statins are not associated with a risk of acute or chronic liver failure.1,11 In patients with coronary heart disease, the incidence of hepatotoxicity with statin use is reported to be <1.5% over the course of 5 years, and appears to be dose-dependent.1
In 2012, the FDA revised the labeling for most statins, relaxing its earlier recommendations for monitoring of liver function, clarifying the risk of myopathy, and providing additional information about drug-drug interactions.13
Checking transaminase levels before initiating therapy is recommended by both the ACC/AHA and FDA.1,13 Routine monitoring is not necessary, the ACC/AHA guideline states, because RCTs have found little evidence of ALT/AST elevation.1 But here, too, evidence varies. An older meta-analysis (13 trials and nearly 50,000 participants) concluded that as a class, statins have no greater risk of transaminase elevations than placebo.22 But the 135-RCT meta-analysis4 found otherwise: Statins did increase the risk of transaminase elevation (odds ratio [OR], 1.51; 95% confidence interval [CI], 1.24-1.84) compared with placebo, with differences associated with particular drugs and higher doses associated with more clinically significant elevations.4 It is important to note, however, that there was significant heterogeneity among the studies and no consistent definition of clinical significance.
THE BOTTOM LINE: Statins have been shown in multiple prospective studies to be safe for patients with chronic liver disease.22,23
Statin use and diabetes: Is there a link?
Recent studies have found an increased risk of new-onset type 2 diabetes in statin users, with a greater risk associated with higher potency statins, including rosuvastatin and atorvastatin.4,24 Although the exact mechanism is not known, statins may modify insulin signaling in peripheral tissues or directly impair insulin secretion.
The ACC/AHA guideline reports an excess rate of diabetes of one per 1000 patient-years for moderate-intensity therapy and 3 per 1000 patient years for high-intensity therapy.1 The 2013 meta-analysis found that the elevated risk of diabetes was relatively small (OR=1.09; 95% CI, 1.02-1.16).4 No difference among various statins was found.
In another meta-analysis—this one encompassing 17 RCTs and >110,000 patients—no statistically significant difference in the incidence of new-onset diabetes was seen based on either the specific statin being taken or the intensity of therapy (high vs moderate).24
THE BOTTOM LINE: Physicians should monitor patients taking statins for signs and symptoms of hyperglycemia.
Statins may be renoprotective
Statin use has been found to be associated with an increased risk of tubular proteinuria—an effect that is both dose- and potency-dependent.25 Nonetheless, it has been suggested that statins may be a rare example of a drug class that is renoprotective in the long term, despite having an increased rate of proteinuria in the short term.25
The evidence? In prospective studies, statin therapy has been shown to slow the progression of kidney disease in diverse patient populations, including renal transplant recipients and those with chronic kidney disease (CKD).26,27
The Kidney Expert Panel of the National Lipid Association (NLA) has concluded that statins do not appear to cause significant proteinuria or acute kidney injury. The panel does not recommend routine monitoring for proteinuria or kidney function in statin users unless otherwise indicated, but does recommend a lower dose for patients with CKD.28
THE BOTTOM LINE: Kidney Disease Improving Global Outcomes guidelines recommend that patients who have CKD, but are not on dialysis, be treated with statin therapy. Statins are contraindicated for patients on dialysis, as clinical trials have failed to show significant cardiovascular benefit.29
Intracerebral hemorrhage: Statins increase recurrence risk
In recent years there has been considerable concern about a statin-induced increased risk for intracerebral hemorrhage (ICH). In a major prospective study in which patients were put on high-dose statin therapy or placebo after an acute ischemic or hemorrhagic stroke, the overall incidence of a recurrent stroke was significantly lower in the statin group.30 Among those who’d had an ICH, however, the recurrence rate was 73% higher for patients taking statins.
A subanalysis that looked only at patients who’d had a hemorrhagic stroke as their initial event (n=93) found that the absolute risk of recurrent ICH was 15.6% for patients randomized to atorvastatin vs 4.2% for those on placebo.31 Despite being based on a small subset of the original study group, the increased risk was statistically significant in multivariate analysis (hazard ratio [HR]=1.69; 95% CI, 1.1-2.6).
A subsequent decision analysis study based on these results proposed that patients with a history of spontaneous deep ICH would need an exceedingly high 10-year cardiovascular event risk (>40%) for the benefits of statin therapy to outweigh the risk.32 The risk is particularly high for those with a history of lobar ICH, which has an extremely high recurrence rate. However, subsequent retrospective and observational studies have found that patients who were already on statins when the ICH occurred had less severe strokes and more favorable outcomes, with a lower mortality rate at 90 days post-ICH.33-35
A 2010 ICH guideline from the AHA/American Stroke Association states that there is “insufficient data to recommend restrictions on use of statin agents” for patients who have had an ICH.36
THE BOTTOM LINE: Physicians should carefully evaluate the anticipated cardiovascular risk for patients who have had a hemorrhagic stroke to determine whether statin therapy would be beneficial.
Other serious adverse effects: Which reports are accurate?
Statin use has been associated with a number of other serious AEs. Some reports appear to be accurate; others do not hold up after a close look at the evidence.
Malignancy. A potential link between statins and an increased risk of malignancy has been considered for years. A large trial (N=5804) from 2002 found a correlation between pravastatin and an increased risk of new cancer diagnoses compared with placebo (HR=1.25; 95% CI, 1.04-1.51; P=.02).37 But a 10-year follow-up did not substantiate this finding, and it is now believed that the original result may have been due to chance.38 Numerous other meta-analyses and systematic reviews have found no link between statin use and malignancy.39-41
Cataracts. Potential ocular effects have been widely studied and debated in recent years. Observational studies reporting an association between statin use and cataracts have had conflicting results, with some showing statins as protective42-45 and others finding an increased risk.46,47 However, a recent propensity-score matched analysis found that statin users do indeed have an increased risk of developing cataracts.48 The authors concluded that for primary prevention, the risk-benefit equation for statin use should include this added risk.48
In addition, a review of the databases of the National Registry of Drug-Induced Ocular Side Effects, the World Health Organization, and the FDA from 1987 to 2008 indicates that statin therapy may also cause diplopia, ptosis, and ophthalmoplegia.49
Peripheral neuropathy. Despite case reports of statin-induced peripheral neuropathy, the NLA’s Neurology Expert Panel states that statins do not appear to cause this condition. If a patient receiving statin therapy develops peripheral neuropathy, a full work-up for other causes should be initiated before considering a modification of statin therapy, the panel advises.28
Statins have also been linked to headache and dizziness, respiratory symptoms, gastrointestinal problems, and rash, among other AEs (TABLE 3).50
Which drug? Potential differences in statins
The meta-analysis with >240,000 participants evaluated patients taking 7 different statins (atorvastatin, fluvastatin, lovastatin, pravastatin, pitavastatin, rosuvastatin, and simvastatin), looking at AEs of the drugs both collectively and individually.4 As noted earlier, the overall discontinuation rate due to AEs for all statins was 5.7%. Discontinuation rates for each agent were not reported.4
The researchers did report, however, that atorvastatin and rosuvastatin had the highest discontinuation rates; atorvastatin and fluvastatin had the highest incidence of transaminase elevations (OR, 2.6 and 5.2, respectively); and pravastatin and simvastatin appeared to be the best tolerated and safest statins, with the lowest discontinuation rates. However, higher doses of simvastatin (>40 mg/d) significantly increased the risk of CK and transaminase elevations (OR, 4.1 and 2.8, respectively),4 as well as the risk of rhabdomyolysis at the highest dose.15,16
Are statins safe for these patients?
When considering statin therapy, there are some patient populations that warrant particular concern:
Women of childbearing age. Statins are contraindicated in women who are pregnant or breastfeeding,1 and should not be initiated in women who are trying to conceive.
Children and adolescents (ages 8-18 years). Statins have been shown to be safe and effective for children and adolescents with familial hyperlipidemia. No effect on growth or maturation has been seen.51 As with adults, however, higher statin doses and the use of concomitant interacting drugs increase the risk of AEs.
Asians. The new ACC/AHA guideline suggests taking Asian ancestry into consideration when prescribing statins because Asians may be more sensitive to medications metabolized by the CYP450 system.1 However, there are no reports of an increased risk of AEs in Asian patients on statins.52 (To read more about statin use in particular patient populations, see “Statin therapy: When to think twice,” J Fam Pract. 2013;62:726-732.)
Patient factors that increase risk
Risk factors for statin-induced AEs include:1
- multiple and/or serious comorbidities (eg, hypothyroidism, impaired renal or hepatic function, rheumatic disorders)
- unexplained ALT elevation >3 times the upper limit of normal
- history of prior statin intolerance or concomitant use of drugs that affect statin metabolism
- age >75 years
- preexisting muscle disorders
- low vitamin D levels.
If a patient who would clearly benefit from statin therapy develops an AE requiring discontinuation, a retrial—with the same drug or a different statin—is generally recommended once the symptoms resolve.1
CASE › The risk of elevated serum transaminases, insulin resistance, cognitive impairment, and neuropathy associated with statin use is minimal, and further evaluation revealed that Mr. L’s recent symptoms had other causes. The elevated transaminases were due to fatty liver disease, the cognitive impairment was secondary to sleep apnea (both linked to his obesity), and the tingling in his hands was the result of carpal tunnel syndrome caused by his exercise regimen.
When he returns in 6 months, Mr. L reports that he has been working with both a nutritionist and an athletic trainer. He has sustained a 15-lb weight loss. He is still taking atorvastatin 10 mg; after he began taking CoQ10, his muscle pain resolved. The patient’s cholesterol and transaminase levels are normal, and the cognitive impairment and peripheral neuropathy he reported at his last visit have improved significantly.
CORRESPONDENCE
Tracy D. Mahvan, PharmD, University of Wyoming, School of Pharmacy Health Sciences Center, Room 292, 1000 E. University Avenue, Laramie, WY 82071; [email protected]
1. Stone NJ, Robinson JG, Lichtenstein AH, et al. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association task force on practice guidelines. Circulation. 2014;129:S1-S45.
2. Lowes R. Top 100 selling drugs through September reported. Medscape Med News. WebMD, LLC. 2013. Medscape Web site. Available at: http://www.medscape.com/viewarticle/813571#3. Accessed December 11, 2013.
3. Zhang H, Plutzky J, Skentzos S, et al. Discontinuation of statins in routine care settings: a cohort study. Ann Intern Med. 2013;158:526-534.
4. Naci H, Brugts J, Ades T. Comparative tolerability and harms of individual statins: a study-level network meta-analysis of 246,955 participants from 135 randomized, controlled trials. Circ Cardiovasc Qual Outcomes. 2013;6:390-399.
5. Pasternak RC, Smith SC Jr, Bairey-Merz CN, et al; American College of Cardiology; American Heart Association; National Heart, Lung and Blood Institute. ACC/AHA/NHLBI clinical advisory on the use and safety of statins. Circulation. 2002;106:1024-1028.
6. Eckel RH. Approach to the patient who is intolerant of statin therapy. J Clin Endocrinol Metab. 2010;95:2015-2022.
7. Parker BA, Thompson PD. Effect of statins on skeletal muscle: exercise, myopathy, and muscle outcomes. Exerc Sport Sci Rev. 2012;40:188-194.
8. Mansi I, Frei CR, Pugh MJ, et al. Statins and musculoskeletal conditions, arthropathies, and injuries. JAMA Intern Med. 2013;173: 1-10.
9. Bruckert E, Hayem G, Dejager S, et al. Mild to moderate muscular symptoms with high-dosage statin therapy in hyperlipidemic patients—the PRIMO study. Cardiovasc Drugs Ther. 2005;19: 403-414.
10. Fernandez G, Spatz ES, Jablecki C, et al. Statin myopathy: a common dilemma not reflected in clinical trials. Cleve Clin J Med. 2011;78:393-403.
11. Law M, Rudnicka AR. Statin safety: a systematic review. Am J Cardiol. 2006;97:52C-60C.
12. Elsevier/Gold Standard. Gold Standard Drug Database. Elsevier/Gold Standard Web site. Available at: http://www.goldstandard.com/product/gold-standard-drug-database/. Accessed December 4, 2013.
13. US Food and Drug Administration. FDA drug safety communication: Important safety label changes to cholesterol-lowering statin drugs. US Food and Drug Administration Web site. Available at: http://www.fda.gov/drugs/drugsafety/ucm293101.htm. Accessed July 23, 2014.
14. Kashani A, Phillips CO, Foody JM, et al. Risks associated with statin therapy: a systematic overview of randomized clinical trials. Circulation. 2006;114:2788-2797.
15. US Food and Drug Administration. FDA drug safety communication: Ongoing safety review of high-dose Zocor (simvastatin) and increased risk of muscle injury. US Food and Drug Administration Web site. Available at: http://www.fda.gov/Drugs/DrugSafety/PostmarketDrugSafetyInformationforPatientsandProviders/ucm204882.htm. Updated February 15, 2012. Accessed December 9, 2013.
16. Bowman L, Armitage J, Bulbulia R, et al; SEARCH Study Collaborative Group. Study of the effectiveness of additional reductions in cholesterol and homocysteine (SEARCH): characteristics of a randomized trial among 12064 myocardial infarction survivors. Am J Heart. 2007;154:815-823.
17. Mills EJ, O’Regan C, Eyawo O, et al. Intensive statin therapy compared with moderate dosing for prevention of cardiovascular events: a meta-analysis of >40,000 patients. Euro Heart J. 2011;32:1409-1415.
18. Bookstaver DA, Burkhalter NA, Hatzigeorgiou C. Effect of coenzyme Q10 supplementation on statin-induced myalgias. Am J Cardiol. 2012;110:526-529.
19. Parker BA, Gregory SM, Lorson L, et al. A randomized trial of coenzyme Q10 in patients with statin myopathy: rationale and study design. J Clin Lipidol. 2013;7:187-193.
20. Fedacko J, Pella D, Fedackova P, et al. Coenzyme Q(10) and selenium in statin-associated myopathy treatment. Can J Physiol Pharmacol. 2013;91:165-170.
21. Jellin JM, Gregory PJ, et al. Natural Medicines Comprehensive Database. University of Wyoming Libraries Web site. Available at: http://www.naturaldatabase.com.libproxy.uwyo.edu. Accessed December 4, 2013.
22. de Denus S, Spinler SA, Miller K, et al. Statins and liver toxicity: a meta-analysis. Pharmacotherapy. 2004;24:584-591.
23. Lewis JH. Clinical perspective: statins and the liver—harmful or helpful? Dig Dis Sci. 2012;57:1754-1763.
24. Navarese EP, Buffon A, Andreotti F, et al. Meta-analysis of impact of different types and doses of statins on new-onset diabetes mellitus. Am J Cardiol. 2013;111:1123-1130.
25. Agarwal R. Effects of statins on renal function. Am J Cardiol. 2006;97:748-755.
26. Fried LF, Orchard TJ, Lasiske BL. Effect of lipid reduction on the progression of renal disease: a meta-analysis. Kidney Int. 2001;59:260-269.
27. Fellström B, Holdaas H, Jardine AG, et al; Assessment of Lescol in Renal Transportation Study Investigators. Effect of fluvastatin on renal end points in the Assessment of Lescol in Renal Transplant (ALERT) Trial. Kidney Int. 2004;66:1549-1555.
28. McKenney JM, Davidson MH, Jacobson TA, et al; National Lipid Association Statin Safety Assessment Task Force. Final conclusions and recommendations of the National Lipid Association Statin Safety Assessment Task Force. Am J Cardiol. 2006;97:89C-94C.
29. KDIGO Clinical Practice Guideline for Lipid Management in Chronic Kidney Disease. Kidney Int. 2013;3(suppl):S259-S305.
30. Goldstein LB, Amarenco P, Szarek M, et al; SPARCL Investigators. Hemorrhagic stroke in the Stroke Prevention by Aggressive Reduction in Cholesterol Levels study. Neurology. 2008;70(24 pt 2):2364-2370.
31. Goldstein LB, Amarenco P, Lamonte M, et al; SPARCL investigators. Relative effects of statin therapy on stroke and cardiovascular events in men and women: secondary analysis of the Stroke Prevention by Aggressive Reduction in Cholesterol Levels (SPARCL) Study. Stroke. 2008;39:2444-2448.
32. Westover MB, Bianchi MT, Eckman MH, et al. Statin use following intracerebral hemorrhage: a decision analysis. Arch Neurol. 2011;68:573-579.
33. Biffi A, Devan WJ, Anderson CD, et al. Statin use and outcome after intracerebral hemorrhage: case-control study and meta-analysis. Neurology. 2011;76:1581-1588.
34. Dowlatshahi D, Demchuck AM, Fang J, et al; Registry of the Canadian Stroke Network. Association of statins and statin discontinuation with poor outcome and survival after intracerebral hemorrhage. Stroke. 2012;43:1518-1523.
35. Bustamante A, Montaner J. Statin therapy should not be discontinued in patients with intracerebral hemorrhage. Stroke. 2013;44:2060-2061.
36. Morgenstern LB, Hemphill JC 3rd, Anderson C, et al; American Heart Association Stroke Council and Council on Cardiovascular Nursing. Guidelines for the management of spontaneous intracerebral hemorrhage: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2010;41:2108-2129.
37. Shepherd J, Blauw GJ, Murphy MB, et al; PROSPER study group. PROspective Study of Pravastatin in the Elderly at Risk. Pravastatin in elderly individuals at risk of vascular disease (PROSPER): a randomised controlled trial. Lancet. 2002;360:1623-1630.
38. Jukema JW, Cannon CP, de Craen AJ, et al. The controversies of statin therapy: weighing the evidence. J Am Coll Cardiol. 2012;60:875-881.
39. Alberton M, Wu P, Druyts E, et al. Adverse events associated with individual statin treatments for cardiovascular disease: an indirect comparison meta-analysis. QJM. 2012;105:145-157.
40. Baigent C, Blackwell L, Emberson J, et al; Cholesterol Treatment Trialists’ (CTT) Collaboration. Efficacy and safety of more intensive lowering of LDL cholesterol: a meta-analysis of data from 170,000 participants in 26 randomised trials. Lancet. 2010;376:1670-1681.
41. Emberson JR, Kearney PM, Blackwell L, et al; Cholesterol Treatment Trialists’ (CTT) Collaboration. Lack of effect of lowering LDL cholesterol on cancer: meta-analysis of individual data from 175,000 people in 27 randomised trials of statin therapy. PLoS One. 2012;7:e29849.
42. Klein BE, Klein R, Lee KE, et al. Statin use and incident nuclear cataract. JAMA. 2006;295:2752-2758.
43. Fong DS, Poon KY. Recent statin use and cataract surgery. Am J Ophthalmol. 2012;153:222-228.e1.
44. Chodick G, Heymann AD, Flash S, et al. Persistence with statins and incident cataract: a population-based historical cohort study. Ann Epidemiol. 2010;20:136-142.
45. Tan JS, Mitchell P, Rochtchina E, et al. Statin use and the long-term risk of incident cataract: the Blue Mountains Eye Study. Am J Ophthalmol. 2007;143:687-689.
46. Machan CM, Hrynchak PK, Irving EL. Age-related cataract is associated with type 2 diabetes and statin use. Optom Vis Sci. 2012;89:1165-1171.
47. Hippisley-Cox J, Coupland C. Unintended effects of statins in men and women in England and Wales: population based cohort study using the QResearch database. BMJ. 2010;340:c2197.
48. Leuschen J, Mortensen EM, Frei CR, et al. Association of statin use with cataracts: a propensity score-matched analysis. JAMA Ophthalmol. 2013;131:1427-1434.
49. Fraunfelder FW, Richards AB. Diplopia, blepharoptosis, and ophthalmoplegia and 3-hydroxy-3-methyl-glutaryl-CoA reductase inhibitor use. Ophthalmology. 2008;115:2282-2285.
50. AHFS Drug Information 2013. Bethesda, MD: American Society of Health-System Pharmacists; 2013.
51. Expert Panel on Integrated Guidelines for Cardiovascular Health and Risk Reduction in Children and Adolescents; National Heart, Lung, and Blood Institute. Expert panel on integrated guidelines for cardiovascular health and risk reduction in children and adolescents: summary report. Pediatrics. 2011;128(suppl 5): S213-S256.
52. Liao JK. Safety and efficacy of statins in Asians. Am J Cardiol. 2007;99:410-414.
› Advise patients starting statin therapy to stop taking the medication and call your office immediately if they develop severe muscle pain or weakness, as statins are associated with a small increased risk of rhabdomyolysis. B
› Obtain a baseline creatine kinase level for patients with an increased risk of musculoskeletal disorders; routine monitoring is needed only for those who experience muscle pain or weakness while on statin therapy. C
› Prescribe statins for patients with chronic kidney or liver disease when indicated; statin therapy is not associated with an increased risk of renal or hepatic failure. B
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
CASE › Carl L, a 57-year-old obese patient (body mass index [BMI] >40) who had not been to a doctor in a decade, comes to see you after a health fair screening revealed dyslipidemia (low-density lipoprotein [LDL] cholesterol, 188 mg/dL; high-density lipoprotein cholesterol [HDL], 32 mg/dL; total cholesterol, 240 mg/dL; triglycerides, 100 mg/dL). His blood pressure (BP) is 146/90 mm Hg and his fasting glucose is 101 mg/dL. Labs drawn that day reveal a glycated hemoglobin (HbA1c) of 5.9%, alanine aminotransferase (ALT) of 45 U/L, and aspartate aminotransferase (AST) of 62 U/L. In taking his history, you discover that Mr. L also has a prominent family history of heart disease.
Mr. L agrees to take a low-dose statin, and you prescribe atorvastatin 10 mg and a thiazide diuretic. You advise the patient to contact you immediately if he develops significant myalgia, jaundice, dark urine, or symptoms of hyperglycemia such as excessive thirst or urination, and schedule a follow-up visit in 8 weeks.
Long recognized as the bedrock of hyperlipidemia therapy, statins achieved even greater prominence when the American College of Cardiology/American Heart Association (ACC/AHA) issued a new cholesterol guideline1 late last year. The ACC and AHA now recommend statins for a wider range of patients, often at a higher starting dose. (To read about the controversy the recommendations generated, see “The new cholesterol guideline: Beyond the headlines,” J Fam Pract. 2013;62:730.)
Based on the new recommendations, the use of statins is likely to rise.2 (A statin [rosuvastatin] is already the nation’s most widely prescribed medication.2) Thus, it is more important than ever for physicians to be knowledgeable about the risks associated with statins and able to assess the benefits of therapy for individual patients.
A 2013 retrospective cohort study of >100,000 patients on statins found that 17% developed adverse effects (AEs). Therapy was withheld, at least temporarily, for 10% of study participants (60% of those experiencing AEs).3 At the same time, the authors of a large meta-analysis (135 randomized controlled trials [RCTs] and >240,000 patients) reported that AEs associated with statins as a class were uncommon. The meta-analysis also found that the overall discontinuation rate for statin users—5.7%—was not significantly different from that of patients on placebo.4
Such discrepancies regarding particular risks as well as the overall incidence of AEs and discontinuation rates make the evidence difficult to sort out. We created this update with that in mind.
Musculoskeletal symptoms are most common
Skeletal muscle symptoms are the most common AEs reported by patients taking statins.5 These range from muscle weakness, fatigue, and pain to (rarely) rhabdomyolysis—a life-threatening condition characterized by severe muscle pain, muscle weakness, a 10-fold increase in creatine kinase (CK), and increased serum creatinine, often with myoglobinuria.5
Patients with myopathy—an umbrella term for any muscle disease—may report stiffness, weakness, tenderness, soreness, cramping, or heaviness. Symptoms usually are symmetrical and often involve the proximal limbs and trunk.6 Studies indicate that exercise increases the risk of statin-induced myalgia—muscle pain or weakness without an increase in CK—and that patients taking statins are more prone to exercise-related injury.7,8
A baseline CK is recommended for patients with an increased risk of muscular disorders.1 Risk factors include a personal or family history of statin intolerance or muscle disease, age >75 years, low levels of vitamin D, and concomitant use of medications that may increase the risk of myopathy (TABLE 1).1 Routine monitoring of CK is not recommended, but CK levels should be obtained for those who exhibit muscle symptoms while on statin therapy.1
What the studies show
The incidence of myalgia reported in clinical studies is highly variable, ranging from <1% to 20%.1,9,10 The ACC/AHA guideline reports only one additional case of myopathy per 10,000 statin users compared with those on placebo and cites a rhabdomyolysis occurrence rate of <.06% over 5 years.1
A 2006 systematic review estimated the absolute risk of rhabdomyolysis to be 3.4 per 100,000 person-years, but the incidence was 10 times higher for patients taking both a statin and gemfibrozil.11 (See TABLE 212,13 for more on drug-drug interactions.) But both the meta-analysis cited earlier4 and an earlier systematic review14 (35 RCTs and >74,000 patients) found that statins as a class do not increase the incidence of myalgia or rhabdomyolysis.
Differences in the way muscular disorders are defined has been suggested as one reason for the discrepancies.10 In addition, many clinical trials exclude patients at higher risk of statin-associated AEs, such as those with renal or hepatic insufficiency, prior muscular complaints, poorly controlled diabetes, or potential drug-drug interactions.1
An FDA advisory. In a safety communication last updated in February 2012, the US Food and Drug Administration (FDA) cautioned against starting patients on the highest dose of simvastatin (80 mg).15 The warning is based on a large study (N=12,064) that found an increased risk of myopathy (0.9%) and rhabdomyolysis (0.2%) in patients on the 80-mg dose vs those taking 20 mg (0.02% and 0%, respectively).16
With the ACC/AHA now recommending intensive therapy (atorvastatin 40-80 mg or rosuvastatin 20-40 mg) to achieve an LDL reduction >50% for many patients,1 it is important to be aware that this risk is specific to simvastatin. A recent meta-analysis of studies directly comparing patients receiving intensive statin therapy with those on low to moderate doses did not find any increased risk in rhabdomyolysis associated with more intensive therapy when those taking 80 mg simvastatin were excluded.17
THE BOTTOM LINE: Although rhabdomyolysis is rare, its severity—a fatality rate of 10%11—makes it critical to educate patients about the disorder and instruct them to stop taking the statin and call the office immediately if they develop severe muscle pain or weakness.
Recommend CoQ10 for statin-induced myopathy
Although the exact mechanism of statin-induced myopathy is unknown, the most likely explanation is a depletion of coenzyme Q10 (CoQ10), which has negative effects on mitochondrial energy production.18 While studies using CoQ10 to treat this AE have been small and had mixed results, the overall evidence suggests that it decreases the development and/or severity of symptoms.18-20
In fact, CoQ10 supplementation is the only treatment that has shown promise in treating statin-induced muscle symptoms.18-20 Doses of about 100 mg bid have been found to be beneficial and safe; no clinically relevant AEs have been seen with doses <300 mg/d.18,20,21 A large placebo-controlled study is currently evaluating a 600 mg/d dose of CoQ10 in patients with statin-induced myopathy.19
CASE › On his next visit, Mr. L reports a new ache in his left shoulder and upper back, which he describes as mild, but annoying. He also tells you his memory seems to be getting worse and that he has developed an odd tingling in his hands. These symptoms began about a month after he started the medications, Mr. L says. He also began a new exercise program, but his BMI is unchanged.
On examination, you find the affected shoulder and upper back modestly and diffusely tender to palpation, but with no decline in strength. Mr. L’s BP has fallen to 134/84 mm Hg and his fasting glucose is 105 mg/dL. Lab tests reveal an LDL of 144 mg/dL and HDL of 36 mg/dL, HbA1c of 6.1%, ALT of 105 U/L, AST of 61 U/L, and a normal CK.
You recommend 100 mg CoQ10 bid. Because it is available only over the counter, you advise the patient to look for a product whose purity and potency have been verified by an external source, such as the US Pharmacopeial Convention. You also prescribe metformin 500 mg bid for insulin resistance, refer the patient to a nutritionist and diabetes specialist, and order tests to evaluate his other symptoms.
Hepatic effects are rare
Historically, statins have been linked to potential hepatotoxicity, with case reports of serum transaminase elevation, cholestasis, hepatitis, and acute liver failure. It is now recognized that hepatic AEs are rare and that statins are not associated with a risk of acute or chronic liver failure.1,11 In patients with coronary heart disease, the incidence of hepatotoxicity with statin use is reported to be <1.5% over the course of 5 years, and appears to be dose-dependent.1
In 2012, the FDA revised the labeling for most statins, relaxing its earlier recommendations for monitoring of liver function, clarifying the risk of myopathy, and providing additional information about drug-drug interactions.13
Checking transaminase levels before initiating therapy is recommended by both the ACC/AHA and FDA.1,13 Routine monitoring is not necessary, the ACC/AHA guideline states, because RCTs have found little evidence of ALT/AST elevation.1 But here, too, evidence varies. An older meta-analysis (13 trials and nearly 50,000 participants) concluded that as a class, statins have no greater risk of transaminase elevations than placebo.22 But the 135-RCT meta-analysis4 found otherwise: Statins did increase the risk of transaminase elevation (odds ratio [OR], 1.51; 95% confidence interval [CI], 1.24-1.84) compared with placebo, with differences associated with particular drugs and higher doses associated with more clinically significant elevations.4 It is important to note, however, that there was significant heterogeneity among the studies and no consistent definition of clinical significance.
THE BOTTOM LINE: Statins have been shown in multiple prospective studies to be safe for patients with chronic liver disease.22,23
Statin use and diabetes: Is there a link?
Recent studies have found an increased risk of new-onset type 2 diabetes in statin users, with a greater risk associated with higher potency statins, including rosuvastatin and atorvastatin.4,24 Although the exact mechanism is not known, statins may modify insulin signaling in peripheral tissues or directly impair insulin secretion.
The ACC/AHA guideline reports an excess rate of diabetes of one per 1000 patient-years for moderate-intensity therapy and 3 per 1000 patient years for high-intensity therapy.1 The 2013 meta-analysis found that the elevated risk of diabetes was relatively small (OR=1.09; 95% CI, 1.02-1.16).4 No difference among various statins was found.
In another meta-analysis—this one encompassing 17 RCTs and >110,000 patients—no statistically significant difference in the incidence of new-onset diabetes was seen based on either the specific statin being taken or the intensity of therapy (high vs moderate).24
THE BOTTOM LINE: Physicians should monitor patients taking statins for signs and symptoms of hyperglycemia.
Statins may be renoprotective
Statin use has been found to be associated with an increased risk of tubular proteinuria—an effect that is both dose- and potency-dependent.25 Nonetheless, it has been suggested that statins may be a rare example of a drug class that is renoprotective in the long term, despite having an increased rate of proteinuria in the short term.25
The evidence? In prospective studies, statin therapy has been shown to slow the progression of kidney disease in diverse patient populations, including renal transplant recipients and those with chronic kidney disease (CKD).26,27
The Kidney Expert Panel of the National Lipid Association (NLA) has concluded that statins do not appear to cause significant proteinuria or acute kidney injury. The panel does not recommend routine monitoring for proteinuria or kidney function in statin users unless otherwise indicated, but does recommend a lower dose for patients with CKD.28
THE BOTTOM LINE: Kidney Disease Improving Global Outcomes guidelines recommend that patients who have CKD, but are not on dialysis, be treated with statin therapy. Statins are contraindicated for patients on dialysis, as clinical trials have failed to show significant cardiovascular benefit.29
Intracerebral hemorrhage: Statins increase recurrence risk
In recent years there has been considerable concern about a statin-induced increased risk for intracerebral hemorrhage (ICH). In a major prospective study in which patients were put on high-dose statin therapy or placebo after an acute ischemic or hemorrhagic stroke, the overall incidence of a recurrent stroke was significantly lower in the statin group.30 Among those who’d had an ICH, however, the recurrence rate was 73% higher for patients taking statins.
A subanalysis that looked only at patients who’d had a hemorrhagic stroke as their initial event (n=93) found that the absolute risk of recurrent ICH was 15.6% for patients randomized to atorvastatin vs 4.2% for those on placebo.31 Despite being based on a small subset of the original study group, the increased risk was statistically significant in multivariate analysis (hazard ratio [HR]=1.69; 95% CI, 1.1-2.6).
A subsequent decision analysis study based on these results proposed that patients with a history of spontaneous deep ICH would need an exceedingly high 10-year cardiovascular event risk (>40%) for the benefits of statin therapy to outweigh the risk.32 The risk is particularly high for those with a history of lobar ICH, which has an extremely high recurrence rate. However, subsequent retrospective and observational studies have found that patients who were already on statins when the ICH occurred had less severe strokes and more favorable outcomes, with a lower mortality rate at 90 days post-ICH.33-35
A 2010 ICH guideline from the AHA/American Stroke Association states that there is “insufficient data to recommend restrictions on use of statin agents” for patients who have had an ICH.36
THE BOTTOM LINE: Physicians should carefully evaluate the anticipated cardiovascular risk for patients who have had a hemorrhagic stroke to determine whether statin therapy would be beneficial.
Other serious adverse effects: Which reports are accurate?
Statin use has been associated with a number of other serious AEs. Some reports appear to be accurate; others do not hold up after a close look at the evidence.
Malignancy. A potential link between statins and an increased risk of malignancy has been considered for years. A large trial (N=5804) from 2002 found a correlation between pravastatin and an increased risk of new cancer diagnoses compared with placebo (HR=1.25; 95% CI, 1.04-1.51; P=.02).37 But a 10-year follow-up did not substantiate this finding, and it is now believed that the original result may have been due to chance.38 Numerous other meta-analyses and systematic reviews have found no link between statin use and malignancy.39-41
Cataracts. Potential ocular effects have been widely studied and debated in recent years. Observational studies reporting an association between statin use and cataracts have had conflicting results, with some showing statins as protective42-45 and others finding an increased risk.46,47 However, a recent propensity-score matched analysis found that statin users do indeed have an increased risk of developing cataracts.48 The authors concluded that for primary prevention, the risk-benefit equation for statin use should include this added risk.48
In addition, a review of the databases of the National Registry of Drug-Induced Ocular Side Effects, the World Health Organization, and the FDA from 1987 to 2008 indicates that statin therapy may also cause diplopia, ptosis, and ophthalmoplegia.49
Peripheral neuropathy. Despite case reports of statin-induced peripheral neuropathy, the NLA’s Neurology Expert Panel states that statins do not appear to cause this condition. If a patient receiving statin therapy develops peripheral neuropathy, a full work-up for other causes should be initiated before considering a modification of statin therapy, the panel advises.28
Statins have also been linked to headache and dizziness, respiratory symptoms, gastrointestinal problems, and rash, among other AEs (TABLE 3).50
Which drug? Potential differences in statins
The meta-analysis with >240,000 participants evaluated patients taking 7 different statins (atorvastatin, fluvastatin, lovastatin, pravastatin, pitavastatin, rosuvastatin, and simvastatin), looking at AEs of the drugs both collectively and individually.4 As noted earlier, the overall discontinuation rate due to AEs for all statins was 5.7%. Discontinuation rates for each agent were not reported.4
The researchers did report, however, that atorvastatin and rosuvastatin had the highest discontinuation rates; atorvastatin and fluvastatin had the highest incidence of transaminase elevations (OR, 2.6 and 5.2, respectively); and pravastatin and simvastatin appeared to be the best tolerated and safest statins, with the lowest discontinuation rates. However, higher doses of simvastatin (>40 mg/d) significantly increased the risk of CK and transaminase elevations (OR, 4.1 and 2.8, respectively),4 as well as the risk of rhabdomyolysis at the highest dose.15,16
Are statins safe for these patients?
When considering statin therapy, there are some patient populations that warrant particular concern:
Women of childbearing age. Statins are contraindicated in women who are pregnant or breastfeeding,1 and should not be initiated in women who are trying to conceive.
Children and adolescents (ages 8-18 years). Statins have been shown to be safe and effective for children and adolescents with familial hyperlipidemia. No effect on growth or maturation has been seen.51 As with adults, however, higher statin doses and the use of concomitant interacting drugs increase the risk of AEs.
Asians. The new ACC/AHA guideline suggests taking Asian ancestry into consideration when prescribing statins because Asians may be more sensitive to medications metabolized by the CYP450 system.1 However, there are no reports of an increased risk of AEs in Asian patients on statins.52 (To read more about statin use in particular patient populations, see “Statin therapy: When to think twice,” J Fam Pract. 2013;62:726-732.)
Patient factors that increase risk
Risk factors for statin-induced AEs include:1
- multiple and/or serious comorbidities (eg, hypothyroidism, impaired renal or hepatic function, rheumatic disorders)
- unexplained ALT elevation >3 times the upper limit of normal
- history of prior statin intolerance or concomitant use of drugs that affect statin metabolism
- age >75 years
- preexisting muscle disorders
- low vitamin D levels.
If a patient who would clearly benefit from statin therapy develops an AE requiring discontinuation, a retrial—with the same drug or a different statin—is generally recommended once the symptoms resolve.1
CASE › The risk of elevated serum transaminases, insulin resistance, cognitive impairment, and neuropathy associated with statin use is minimal, and further evaluation revealed that Mr. L’s recent symptoms had other causes. The elevated transaminases were due to fatty liver disease, the cognitive impairment was secondary to sleep apnea (both linked to his obesity), and the tingling in his hands was the result of carpal tunnel syndrome caused by his exercise regimen.
When he returns in 6 months, Mr. L reports that he has been working with both a nutritionist and an athletic trainer. He has sustained a 15-lb weight loss. He is still taking atorvastatin 10 mg; after he began taking CoQ10, his muscle pain resolved. The patient’s cholesterol and transaminase levels are normal, and the cognitive impairment and peripheral neuropathy he reported at his last visit have improved significantly.
CORRESPONDENCE
Tracy D. Mahvan, PharmD, University of Wyoming, School of Pharmacy Health Sciences Center, Room 292, 1000 E. University Avenue, Laramie, WY 82071; [email protected]
› Advise patients starting statin therapy to stop taking the medication and call your office immediately if they develop severe muscle pain or weakness, as statins are associated with a small increased risk of rhabdomyolysis. B
› Obtain a baseline creatine kinase level for patients with an increased risk of musculoskeletal disorders; routine monitoring is needed only for those who experience muscle pain or weakness while on statin therapy. C
› Prescribe statins for patients with chronic kidney or liver disease when indicated; statin therapy is not associated with an increased risk of renal or hepatic failure. B
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
CASE › Carl L, a 57-year-old obese patient (body mass index [BMI] >40) who had not been to a doctor in a decade, comes to see you after a health fair screening revealed dyslipidemia (low-density lipoprotein [LDL] cholesterol, 188 mg/dL; high-density lipoprotein cholesterol [HDL], 32 mg/dL; total cholesterol, 240 mg/dL; triglycerides, 100 mg/dL). His blood pressure (BP) is 146/90 mm Hg and his fasting glucose is 101 mg/dL. Labs drawn that day reveal a glycated hemoglobin (HbA1c) of 5.9%, alanine aminotransferase (ALT) of 45 U/L, and aspartate aminotransferase (AST) of 62 U/L. In taking his history, you discover that Mr. L also has a prominent family history of heart disease.
Mr. L agrees to take a low-dose statin, and you prescribe atorvastatin 10 mg and a thiazide diuretic. You advise the patient to contact you immediately if he develops significant myalgia, jaundice, dark urine, or symptoms of hyperglycemia such as excessive thirst or urination, and schedule a follow-up visit in 8 weeks.
Long recognized as the bedrock of hyperlipidemia therapy, statins achieved even greater prominence when the American College of Cardiology/American Heart Association (ACC/AHA) issued a new cholesterol guideline1 late last year. The ACC and AHA now recommend statins for a wider range of patients, often at a higher starting dose. (To read about the controversy the recommendations generated, see “The new cholesterol guideline: Beyond the headlines,” J Fam Pract. 2013;62:730.)
Based on the new recommendations, the use of statins is likely to rise.2 (A statin [rosuvastatin] is already the nation’s most widely prescribed medication.2) Thus, it is more important than ever for physicians to be knowledgeable about the risks associated with statins and able to assess the benefits of therapy for individual patients.
A 2013 retrospective cohort study of >100,000 patients on statins found that 17% developed adverse effects (AEs). Therapy was withheld, at least temporarily, for 10% of study participants (60% of those experiencing AEs).3 At the same time, the authors of a large meta-analysis (135 randomized controlled trials [RCTs] and >240,000 patients) reported that AEs associated with statins as a class were uncommon. The meta-analysis also found that the overall discontinuation rate for statin users—5.7%—was not significantly different from that of patients on placebo.4
Such discrepancies regarding particular risks as well as the overall incidence of AEs and discontinuation rates make the evidence difficult to sort out. We created this update with that in mind.
Musculoskeletal symptoms are most common
Skeletal muscle symptoms are the most common AEs reported by patients taking statins.5 These range from muscle weakness, fatigue, and pain to (rarely) rhabdomyolysis—a life-threatening condition characterized by severe muscle pain, muscle weakness, a 10-fold increase in creatine kinase (CK), and increased serum creatinine, often with myoglobinuria.5
Patients with myopathy—an umbrella term for any muscle disease—may report stiffness, weakness, tenderness, soreness, cramping, or heaviness. Symptoms usually are symmetrical and often involve the proximal limbs and trunk.6 Studies indicate that exercise increases the risk of statin-induced myalgia—muscle pain or weakness without an increase in CK—and that patients taking statins are more prone to exercise-related injury.7,8
A baseline CK is recommended for patients with an increased risk of muscular disorders.1 Risk factors include a personal or family history of statin intolerance or muscle disease, age >75 years, low levels of vitamin D, and concomitant use of medications that may increase the risk of myopathy (TABLE 1).1 Routine monitoring of CK is not recommended, but CK levels should be obtained for those who exhibit muscle symptoms while on statin therapy.1
What the studies show
The incidence of myalgia reported in clinical studies is highly variable, ranging from <1% to 20%.1,9,10 The ACC/AHA guideline reports only one additional case of myopathy per 10,000 statin users compared with those on placebo and cites a rhabdomyolysis occurrence rate of <.06% over 5 years.1
A 2006 systematic review estimated the absolute risk of rhabdomyolysis to be 3.4 per 100,000 person-years, but the incidence was 10 times higher for patients taking both a statin and gemfibrozil.11 (See TABLE 212,13 for more on drug-drug interactions.) But both the meta-analysis cited earlier4 and an earlier systematic review14 (35 RCTs and >74,000 patients) found that statins as a class do not increase the incidence of myalgia or rhabdomyolysis.
Differences in the way muscular disorders are defined has been suggested as one reason for the discrepancies.10 In addition, many clinical trials exclude patients at higher risk of statin-associated AEs, such as those with renal or hepatic insufficiency, prior muscular complaints, poorly controlled diabetes, or potential drug-drug interactions.1
An FDA advisory. In a safety communication last updated in February 2012, the US Food and Drug Administration (FDA) cautioned against starting patients on the highest dose of simvastatin (80 mg).15 The warning is based on a large study (N=12,064) that found an increased risk of myopathy (0.9%) and rhabdomyolysis (0.2%) in patients on the 80-mg dose vs those taking 20 mg (0.02% and 0%, respectively).16
With the ACC/AHA now recommending intensive therapy (atorvastatin 40-80 mg or rosuvastatin 20-40 mg) to achieve an LDL reduction >50% for many patients,1 it is important to be aware that this risk is specific to simvastatin. A recent meta-analysis of studies directly comparing patients receiving intensive statin therapy with those on low to moderate doses did not find any increased risk in rhabdomyolysis associated with more intensive therapy when those taking 80 mg simvastatin were excluded.17
THE BOTTOM LINE: Although rhabdomyolysis is rare, its severity—a fatality rate of 10%11—makes it critical to educate patients about the disorder and instruct them to stop taking the statin and call the office immediately if they develop severe muscle pain or weakness.
Recommend CoQ10 for statin-induced myopathy
Although the exact mechanism of statin-induced myopathy is unknown, the most likely explanation is a depletion of coenzyme Q10 (CoQ10), which has negative effects on mitochondrial energy production.18 While studies using CoQ10 to treat this AE have been small and had mixed results, the overall evidence suggests that it decreases the development and/or severity of symptoms.18-20
In fact, CoQ10 supplementation is the only treatment that has shown promise in treating statin-induced muscle symptoms.18-20 Doses of about 100 mg bid have been found to be beneficial and safe; no clinically relevant AEs have been seen with doses <300 mg/d.18,20,21 A large placebo-controlled study is currently evaluating a 600 mg/d dose of CoQ10 in patients with statin-induced myopathy.19
CASE › On his next visit, Mr. L reports a new ache in his left shoulder and upper back, which he describes as mild, but annoying. He also tells you his memory seems to be getting worse and that he has developed an odd tingling in his hands. These symptoms began about a month after he started the medications, Mr. L says. He also began a new exercise program, but his BMI is unchanged.
On examination, you find the affected shoulder and upper back modestly and diffusely tender to palpation, but with no decline in strength. Mr. L’s BP has fallen to 134/84 mm Hg and his fasting glucose is 105 mg/dL. Lab tests reveal an LDL of 144 mg/dL and HDL of 36 mg/dL, HbA1c of 6.1%, ALT of 105 U/L, AST of 61 U/L, and a normal CK.
You recommend 100 mg CoQ10 bid. Because it is available only over the counter, you advise the patient to look for a product whose purity and potency have been verified by an external source, such as the US Pharmacopeial Convention. You also prescribe metformin 500 mg bid for insulin resistance, refer the patient to a nutritionist and diabetes specialist, and order tests to evaluate his other symptoms.
Hepatic effects are rare
Historically, statins have been linked to potential hepatotoxicity, with case reports of serum transaminase elevation, cholestasis, hepatitis, and acute liver failure. It is now recognized that hepatic AEs are rare and that statins are not associated with a risk of acute or chronic liver failure.1,11 In patients with coronary heart disease, the incidence of hepatotoxicity with statin use is reported to be <1.5% over the course of 5 years, and appears to be dose-dependent.1
In 2012, the FDA revised the labeling for most statins, relaxing its earlier recommendations for monitoring of liver function, clarifying the risk of myopathy, and providing additional information about drug-drug interactions.13
Checking transaminase levels before initiating therapy is recommended by both the ACC/AHA and FDA.1,13 Routine monitoring is not necessary, the ACC/AHA guideline states, because RCTs have found little evidence of ALT/AST elevation.1 But here, too, evidence varies. An older meta-analysis (13 trials and nearly 50,000 participants) concluded that as a class, statins have no greater risk of transaminase elevations than placebo.22 But the 135-RCT meta-analysis4 found otherwise: Statins did increase the risk of transaminase elevation (odds ratio [OR], 1.51; 95% confidence interval [CI], 1.24-1.84) compared with placebo, with differences associated with particular drugs and higher doses associated with more clinically significant elevations.4 It is important to note, however, that there was significant heterogeneity among the studies and no consistent definition of clinical significance.
THE BOTTOM LINE: Statins have been shown in multiple prospective studies to be safe for patients with chronic liver disease.22,23
Statin use and diabetes: Is there a link?
Recent studies have found an increased risk of new-onset type 2 diabetes in statin users, with a greater risk associated with higher potency statins, including rosuvastatin and atorvastatin.4,24 Although the exact mechanism is not known, statins may modify insulin signaling in peripheral tissues or directly impair insulin secretion.
The ACC/AHA guideline reports an excess rate of diabetes of one per 1000 patient-years for moderate-intensity therapy and 3 per 1000 patient years for high-intensity therapy.1 The 2013 meta-analysis found that the elevated risk of diabetes was relatively small (OR=1.09; 95% CI, 1.02-1.16).4 No difference among various statins was found.
In another meta-analysis—this one encompassing 17 RCTs and >110,000 patients—no statistically significant difference in the incidence of new-onset diabetes was seen based on either the specific statin being taken or the intensity of therapy (high vs moderate).24
THE BOTTOM LINE: Physicians should monitor patients taking statins for signs and symptoms of hyperglycemia.
Statins may be renoprotective
Statin use has been found to be associated with an increased risk of tubular proteinuria—an effect that is both dose- and potency-dependent.25 Nonetheless, it has been suggested that statins may be a rare example of a drug class that is renoprotective in the long term, despite having an increased rate of proteinuria in the short term.25
The evidence? In prospective studies, statin therapy has been shown to slow the progression of kidney disease in diverse patient populations, including renal transplant recipients and those with chronic kidney disease (CKD).26,27
The Kidney Expert Panel of the National Lipid Association (NLA) has concluded that statins do not appear to cause significant proteinuria or acute kidney injury. The panel does not recommend routine monitoring for proteinuria or kidney function in statin users unless otherwise indicated, but does recommend a lower dose for patients with CKD.28
THE BOTTOM LINE: Kidney Disease Improving Global Outcomes guidelines recommend that patients who have CKD, but are not on dialysis, be treated with statin therapy. Statins are contraindicated for patients on dialysis, as clinical trials have failed to show significant cardiovascular benefit.29
Intracerebral hemorrhage: Statins increase recurrence risk
In recent years there has been considerable concern about a statin-induced increased risk for intracerebral hemorrhage (ICH). In a major prospective study in which patients were put on high-dose statin therapy or placebo after an acute ischemic or hemorrhagic stroke, the overall incidence of a recurrent stroke was significantly lower in the statin group.30 Among those who’d had an ICH, however, the recurrence rate was 73% higher for patients taking statins.
A subanalysis that looked only at patients who’d had a hemorrhagic stroke as their initial event (n=93) found that the absolute risk of recurrent ICH was 15.6% for patients randomized to atorvastatin vs 4.2% for those on placebo.31 Despite being based on a small subset of the original study group, the increased risk was statistically significant in multivariate analysis (hazard ratio [HR]=1.69; 95% CI, 1.1-2.6).
A subsequent decision analysis study based on these results proposed that patients with a history of spontaneous deep ICH would need an exceedingly high 10-year cardiovascular event risk (>40%) for the benefits of statin therapy to outweigh the risk.32 The risk is particularly high for those with a history of lobar ICH, which has an extremely high recurrence rate. However, subsequent retrospective and observational studies have found that patients who were already on statins when the ICH occurred had less severe strokes and more favorable outcomes, with a lower mortality rate at 90 days post-ICH.33-35
A 2010 ICH guideline from the AHA/American Stroke Association states that there is “insufficient data to recommend restrictions on use of statin agents” for patients who have had an ICH.36
THE BOTTOM LINE: Physicians should carefully evaluate the anticipated cardiovascular risk for patients who have had a hemorrhagic stroke to determine whether statin therapy would be beneficial.
Other serious adverse effects: Which reports are accurate?
Statin use has been associated with a number of other serious AEs. Some reports appear to be accurate; others do not hold up after a close look at the evidence.
Malignancy. A potential link between statins and an increased risk of malignancy has been considered for years. A large trial (N=5804) from 2002 found a correlation between pravastatin and an increased risk of new cancer diagnoses compared with placebo (HR=1.25; 95% CI, 1.04-1.51; P=.02).37 But a 10-year follow-up did not substantiate this finding, and it is now believed that the original result may have been due to chance.38 Numerous other meta-analyses and systematic reviews have found no link between statin use and malignancy.39-41
Cataracts. Potential ocular effects have been widely studied and debated in recent years. Observational studies reporting an association between statin use and cataracts have had conflicting results, with some showing statins as protective42-45 and others finding an increased risk.46,47 However, a recent propensity-score matched analysis found that statin users do indeed have an increased risk of developing cataracts.48 The authors concluded that for primary prevention, the risk-benefit equation for statin use should include this added risk.48
In addition, a review of the databases of the National Registry of Drug-Induced Ocular Side Effects, the World Health Organization, and the FDA from 1987 to 2008 indicates that statin therapy may also cause diplopia, ptosis, and ophthalmoplegia.49
Peripheral neuropathy. Despite case reports of statin-induced peripheral neuropathy, the NLA’s Neurology Expert Panel states that statins do not appear to cause this condition. If a patient receiving statin therapy develops peripheral neuropathy, a full work-up for other causes should be initiated before considering a modification of statin therapy, the panel advises.28
Statins have also been linked to headache and dizziness, respiratory symptoms, gastrointestinal problems, and rash, among other AEs (TABLE 3).50
Which drug? Potential differences in statins
The meta-analysis with >240,000 participants evaluated patients taking 7 different statins (atorvastatin, fluvastatin, lovastatin, pravastatin, pitavastatin, rosuvastatin, and simvastatin), looking at AEs of the drugs both collectively and individually.4 As noted earlier, the overall discontinuation rate due to AEs for all statins was 5.7%. Discontinuation rates for each agent were not reported.4
The researchers did report, however, that atorvastatin and rosuvastatin had the highest discontinuation rates; atorvastatin and fluvastatin had the highest incidence of transaminase elevations (OR, 2.6 and 5.2, respectively); and pravastatin and simvastatin appeared to be the best tolerated and safest statins, with the lowest discontinuation rates. However, higher doses of simvastatin (>40 mg/d) significantly increased the risk of CK and transaminase elevations (OR, 4.1 and 2.8, respectively),4 as well as the risk of rhabdomyolysis at the highest dose.15,16
Are statins safe for these patients?
When considering statin therapy, there are some patient populations that warrant particular concern:
Women of childbearing age. Statins are contraindicated in women who are pregnant or breastfeeding,1 and should not be initiated in women who are trying to conceive.
Children and adolescents (ages 8-18 years). Statins have been shown to be safe and effective for children and adolescents with familial hyperlipidemia. No effect on growth or maturation has been seen.51 As with adults, however, higher statin doses and the use of concomitant interacting drugs increase the risk of AEs.
Asians. The new ACC/AHA guideline suggests taking Asian ancestry into consideration when prescribing statins because Asians may be more sensitive to medications metabolized by the CYP450 system.1 However, there are no reports of an increased risk of AEs in Asian patients on statins.52 (To read more about statin use in particular patient populations, see “Statin therapy: When to think twice,” J Fam Pract. 2013;62:726-732.)
Patient factors that increase risk
Risk factors for statin-induced AEs include:1
- multiple and/or serious comorbidities (eg, hypothyroidism, impaired renal or hepatic function, rheumatic disorders)
- unexplained ALT elevation >3 times the upper limit of normal
- history of prior statin intolerance or concomitant use of drugs that affect statin metabolism
- age >75 years
- preexisting muscle disorders
- low vitamin D levels.
If a patient who would clearly benefit from statin therapy develops an AE requiring discontinuation, a retrial—with the same drug or a different statin—is generally recommended once the symptoms resolve.1
CASE › The risk of elevated serum transaminases, insulin resistance, cognitive impairment, and neuropathy associated with statin use is minimal, and further evaluation revealed that Mr. L’s recent symptoms had other causes. The elevated transaminases were due to fatty liver disease, the cognitive impairment was secondary to sleep apnea (both linked to his obesity), and the tingling in his hands was the result of carpal tunnel syndrome caused by his exercise regimen.
When he returns in 6 months, Mr. L reports that he has been working with both a nutritionist and an athletic trainer. He has sustained a 15-lb weight loss. He is still taking atorvastatin 10 mg; after he began taking CoQ10, his muscle pain resolved. The patient’s cholesterol and transaminase levels are normal, and the cognitive impairment and peripheral neuropathy he reported at his last visit have improved significantly.
CORRESPONDENCE
Tracy D. Mahvan, PharmD, University of Wyoming, School of Pharmacy Health Sciences Center, Room 292, 1000 E. University Avenue, Laramie, WY 82071; [email protected]
1. Stone NJ, Robinson JG, Lichtenstein AH, et al. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association task force on practice guidelines. Circulation. 2014;129:S1-S45.
2. Lowes R. Top 100 selling drugs through September reported. Medscape Med News. WebMD, LLC. 2013. Medscape Web site. Available at: http://www.medscape.com/viewarticle/813571#3. Accessed December 11, 2013.
3. Zhang H, Plutzky J, Skentzos S, et al. Discontinuation of statins in routine care settings: a cohort study. Ann Intern Med. 2013;158:526-534.
4. Naci H, Brugts J, Ades T. Comparative tolerability and harms of individual statins: a study-level network meta-analysis of 246,955 participants from 135 randomized, controlled trials. Circ Cardiovasc Qual Outcomes. 2013;6:390-399.
5. Pasternak RC, Smith SC Jr, Bairey-Merz CN, et al; American College of Cardiology; American Heart Association; National Heart, Lung and Blood Institute. ACC/AHA/NHLBI clinical advisory on the use and safety of statins. Circulation. 2002;106:1024-1028.
6. Eckel RH. Approach to the patient who is intolerant of statin therapy. J Clin Endocrinol Metab. 2010;95:2015-2022.
7. Parker BA, Thompson PD. Effect of statins on skeletal muscle: exercise, myopathy, and muscle outcomes. Exerc Sport Sci Rev. 2012;40:188-194.
8. Mansi I, Frei CR, Pugh MJ, et al. Statins and musculoskeletal conditions, arthropathies, and injuries. JAMA Intern Med. 2013;173: 1-10.
9. Bruckert E, Hayem G, Dejager S, et al. Mild to moderate muscular symptoms with high-dosage statin therapy in hyperlipidemic patients—the PRIMO study. Cardiovasc Drugs Ther. 2005;19: 403-414.
10. Fernandez G, Spatz ES, Jablecki C, et al. Statin myopathy: a common dilemma not reflected in clinical trials. Cleve Clin J Med. 2011;78:393-403.
11. Law M, Rudnicka AR. Statin safety: a systematic review. Am J Cardiol. 2006;97:52C-60C.
12. Elsevier/Gold Standard. Gold Standard Drug Database. Elsevier/Gold Standard Web site. Available at: http://www.goldstandard.com/product/gold-standard-drug-database/. Accessed December 4, 2013.
13. US Food and Drug Administration. FDA drug safety communication: Important safety label changes to cholesterol-lowering statin drugs. US Food and Drug Administration Web site. Available at: http://www.fda.gov/drugs/drugsafety/ucm293101.htm. Accessed July 23, 2014.
14. Kashani A, Phillips CO, Foody JM, et al. Risks associated with statin therapy: a systematic overview of randomized clinical trials. Circulation. 2006;114:2788-2797.
15. US Food and Drug Administration. FDA drug safety communication: Ongoing safety review of high-dose Zocor (simvastatin) and increased risk of muscle injury. US Food and Drug Administration Web site. Available at: http://www.fda.gov/Drugs/DrugSafety/PostmarketDrugSafetyInformationforPatientsandProviders/ucm204882.htm. Updated February 15, 2012. Accessed December 9, 2013.
16. Bowman L, Armitage J, Bulbulia R, et al; SEARCH Study Collaborative Group. Study of the effectiveness of additional reductions in cholesterol and homocysteine (SEARCH): characteristics of a randomized trial among 12064 myocardial infarction survivors. Am J Heart. 2007;154:815-823.
17. Mills EJ, O’Regan C, Eyawo O, et al. Intensive statin therapy compared with moderate dosing for prevention of cardiovascular events: a meta-analysis of >40,000 patients. Euro Heart J. 2011;32:1409-1415.
18. Bookstaver DA, Burkhalter NA, Hatzigeorgiou C. Effect of coenzyme Q10 supplementation on statin-induced myalgias. Am J Cardiol. 2012;110:526-529.
19. Parker BA, Gregory SM, Lorson L, et al. A randomized trial of coenzyme Q10 in patients with statin myopathy: rationale and study design. J Clin Lipidol. 2013;7:187-193.
20. Fedacko J, Pella D, Fedackova P, et al. Coenzyme Q(10) and selenium in statin-associated myopathy treatment. Can J Physiol Pharmacol. 2013;91:165-170.
21. Jellin JM, Gregory PJ, et al. Natural Medicines Comprehensive Database. University of Wyoming Libraries Web site. Available at: http://www.naturaldatabase.com.libproxy.uwyo.edu. Accessed December 4, 2013.
22. de Denus S, Spinler SA, Miller K, et al. Statins and liver toxicity: a meta-analysis. Pharmacotherapy. 2004;24:584-591.
23. Lewis JH. Clinical perspective: statins and the liver—harmful or helpful? Dig Dis Sci. 2012;57:1754-1763.
24. Navarese EP, Buffon A, Andreotti F, et al. Meta-analysis of impact of different types and doses of statins on new-onset diabetes mellitus. Am J Cardiol. 2013;111:1123-1130.
25. Agarwal R. Effects of statins on renal function. Am J Cardiol. 2006;97:748-755.
26. Fried LF, Orchard TJ, Lasiske BL. Effect of lipid reduction on the progression of renal disease: a meta-analysis. Kidney Int. 2001;59:260-269.
27. Fellström B, Holdaas H, Jardine AG, et al; Assessment of Lescol in Renal Transportation Study Investigators. Effect of fluvastatin on renal end points in the Assessment of Lescol in Renal Transplant (ALERT) Trial. Kidney Int. 2004;66:1549-1555.
28. McKenney JM, Davidson MH, Jacobson TA, et al; National Lipid Association Statin Safety Assessment Task Force. Final conclusions and recommendations of the National Lipid Association Statin Safety Assessment Task Force. Am J Cardiol. 2006;97:89C-94C.
29. KDIGO Clinical Practice Guideline for Lipid Management in Chronic Kidney Disease. Kidney Int. 2013;3(suppl):S259-S305.
30. Goldstein LB, Amarenco P, Szarek M, et al; SPARCL Investigators. Hemorrhagic stroke in the Stroke Prevention by Aggressive Reduction in Cholesterol Levels study. Neurology. 2008;70(24 pt 2):2364-2370.
31. Goldstein LB, Amarenco P, Lamonte M, et al; SPARCL investigators. Relative effects of statin therapy on stroke and cardiovascular events in men and women: secondary analysis of the Stroke Prevention by Aggressive Reduction in Cholesterol Levels (SPARCL) Study. Stroke. 2008;39:2444-2448.
32. Westover MB, Bianchi MT, Eckman MH, et al. Statin use following intracerebral hemorrhage: a decision analysis. Arch Neurol. 2011;68:573-579.
33. Biffi A, Devan WJ, Anderson CD, et al. Statin use and outcome after intracerebral hemorrhage: case-control study and meta-analysis. Neurology. 2011;76:1581-1588.
34. Dowlatshahi D, Demchuck AM, Fang J, et al; Registry of the Canadian Stroke Network. Association of statins and statin discontinuation with poor outcome and survival after intracerebral hemorrhage. Stroke. 2012;43:1518-1523.
35. Bustamante A, Montaner J. Statin therapy should not be discontinued in patients with intracerebral hemorrhage. Stroke. 2013;44:2060-2061.
36. Morgenstern LB, Hemphill JC 3rd, Anderson C, et al; American Heart Association Stroke Council and Council on Cardiovascular Nursing. Guidelines for the management of spontaneous intracerebral hemorrhage: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2010;41:2108-2129.
37. Shepherd J, Blauw GJ, Murphy MB, et al; PROSPER study group. PROspective Study of Pravastatin in the Elderly at Risk. Pravastatin in elderly individuals at risk of vascular disease (PROSPER): a randomised controlled trial. Lancet. 2002;360:1623-1630.
38. Jukema JW, Cannon CP, de Craen AJ, et al. The controversies of statin therapy: weighing the evidence. J Am Coll Cardiol. 2012;60:875-881.
39. Alberton M, Wu P, Druyts E, et al. Adverse events associated with individual statin treatments for cardiovascular disease: an indirect comparison meta-analysis. QJM. 2012;105:145-157.
40. Baigent C, Blackwell L, Emberson J, et al; Cholesterol Treatment Trialists’ (CTT) Collaboration. Efficacy and safety of more intensive lowering of LDL cholesterol: a meta-analysis of data from 170,000 participants in 26 randomised trials. Lancet. 2010;376:1670-1681.
41. Emberson JR, Kearney PM, Blackwell L, et al; Cholesterol Treatment Trialists’ (CTT) Collaboration. Lack of effect of lowering LDL cholesterol on cancer: meta-analysis of individual data from 175,000 people in 27 randomised trials of statin therapy. PLoS One. 2012;7:e29849.
42. Klein BE, Klein R, Lee KE, et al. Statin use and incident nuclear cataract. JAMA. 2006;295:2752-2758.
43. Fong DS, Poon KY. Recent statin use and cataract surgery. Am J Ophthalmol. 2012;153:222-228.e1.
44. Chodick G, Heymann AD, Flash S, et al. Persistence with statins and incident cataract: a population-based historical cohort study. Ann Epidemiol. 2010;20:136-142.
45. Tan JS, Mitchell P, Rochtchina E, et al. Statin use and the long-term risk of incident cataract: the Blue Mountains Eye Study. Am J Ophthalmol. 2007;143:687-689.
46. Machan CM, Hrynchak PK, Irving EL. Age-related cataract is associated with type 2 diabetes and statin use. Optom Vis Sci. 2012;89:1165-1171.
47. Hippisley-Cox J, Coupland C. Unintended effects of statins in men and women in England and Wales: population based cohort study using the QResearch database. BMJ. 2010;340:c2197.
48. Leuschen J, Mortensen EM, Frei CR, et al. Association of statin use with cataracts: a propensity score-matched analysis. JAMA Ophthalmol. 2013;131:1427-1434.
49. Fraunfelder FW, Richards AB. Diplopia, blepharoptosis, and ophthalmoplegia and 3-hydroxy-3-methyl-glutaryl-CoA reductase inhibitor use. Ophthalmology. 2008;115:2282-2285.
50. AHFS Drug Information 2013. Bethesda, MD: American Society of Health-System Pharmacists; 2013.
51. Expert Panel on Integrated Guidelines for Cardiovascular Health and Risk Reduction in Children and Adolescents; National Heart, Lung, and Blood Institute. Expert panel on integrated guidelines for cardiovascular health and risk reduction in children and adolescents: summary report. Pediatrics. 2011;128(suppl 5): S213-S256.
52. Liao JK. Safety and efficacy of statins in Asians. Am J Cardiol. 2007;99:410-414.
1. Stone NJ, Robinson JG, Lichtenstein AH, et al. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association task force on practice guidelines. Circulation. 2014;129:S1-S45.
2. Lowes R. Top 100 selling drugs through September reported. Medscape Med News. WebMD, LLC. 2013. Medscape Web site. Available at: http://www.medscape.com/viewarticle/813571#3. Accessed December 11, 2013.
3. Zhang H, Plutzky J, Skentzos S, et al. Discontinuation of statins in routine care settings: a cohort study. Ann Intern Med. 2013;158:526-534.
4. Naci H, Brugts J, Ades T. Comparative tolerability and harms of individual statins: a study-level network meta-analysis of 246,955 participants from 135 randomized, controlled trials. Circ Cardiovasc Qual Outcomes. 2013;6:390-399.
5. Pasternak RC, Smith SC Jr, Bairey-Merz CN, et al; American College of Cardiology; American Heart Association; National Heart, Lung and Blood Institute. ACC/AHA/NHLBI clinical advisory on the use and safety of statins. Circulation. 2002;106:1024-1028.
6. Eckel RH. Approach to the patient who is intolerant of statin therapy. J Clin Endocrinol Metab. 2010;95:2015-2022.
7. Parker BA, Thompson PD. Effect of statins on skeletal muscle: exercise, myopathy, and muscle outcomes. Exerc Sport Sci Rev. 2012;40:188-194.
8. Mansi I, Frei CR, Pugh MJ, et al. Statins and musculoskeletal conditions, arthropathies, and injuries. JAMA Intern Med. 2013;173: 1-10.
9. Bruckert E, Hayem G, Dejager S, et al. Mild to moderate muscular symptoms with high-dosage statin therapy in hyperlipidemic patients—the PRIMO study. Cardiovasc Drugs Ther. 2005;19: 403-414.
10. Fernandez G, Spatz ES, Jablecki C, et al. Statin myopathy: a common dilemma not reflected in clinical trials. Cleve Clin J Med. 2011;78:393-403.
11. Law M, Rudnicka AR. Statin safety: a systematic review. Am J Cardiol. 2006;97:52C-60C.
12. Elsevier/Gold Standard. Gold Standard Drug Database. Elsevier/Gold Standard Web site. Available at: http://www.goldstandard.com/product/gold-standard-drug-database/. Accessed December 4, 2013.
13. US Food and Drug Administration. FDA drug safety communication: Important safety label changes to cholesterol-lowering statin drugs. US Food and Drug Administration Web site. Available at: http://www.fda.gov/drugs/drugsafety/ucm293101.htm. Accessed July 23, 2014.
14. Kashani A, Phillips CO, Foody JM, et al. Risks associated with statin therapy: a systematic overview of randomized clinical trials. Circulation. 2006;114:2788-2797.
15. US Food and Drug Administration. FDA drug safety communication: Ongoing safety review of high-dose Zocor (simvastatin) and increased risk of muscle injury. US Food and Drug Administration Web site. Available at: http://www.fda.gov/Drugs/DrugSafety/PostmarketDrugSafetyInformationforPatientsandProviders/ucm204882.htm. Updated February 15, 2012. Accessed December 9, 2013.
16. Bowman L, Armitage J, Bulbulia R, et al; SEARCH Study Collaborative Group. Study of the effectiveness of additional reductions in cholesterol and homocysteine (SEARCH): characteristics of a randomized trial among 12064 myocardial infarction survivors. Am J Heart. 2007;154:815-823.
17. Mills EJ, O’Regan C, Eyawo O, et al. Intensive statin therapy compared with moderate dosing for prevention of cardiovascular events: a meta-analysis of >40,000 patients. Euro Heart J. 2011;32:1409-1415.
18. Bookstaver DA, Burkhalter NA, Hatzigeorgiou C. Effect of coenzyme Q10 supplementation on statin-induced myalgias. Am J Cardiol. 2012;110:526-529.
19. Parker BA, Gregory SM, Lorson L, et al. A randomized trial of coenzyme Q10 in patients with statin myopathy: rationale and study design. J Clin Lipidol. 2013;7:187-193.
20. Fedacko J, Pella D, Fedackova P, et al. Coenzyme Q(10) and selenium in statin-associated myopathy treatment. Can J Physiol Pharmacol. 2013;91:165-170.
21. Jellin JM, Gregory PJ, et al. Natural Medicines Comprehensive Database. University of Wyoming Libraries Web site. Available at: http://www.naturaldatabase.com.libproxy.uwyo.edu. Accessed December 4, 2013.
22. de Denus S, Spinler SA, Miller K, et al. Statins and liver toxicity: a meta-analysis. Pharmacotherapy. 2004;24:584-591.
23. Lewis JH. Clinical perspective: statins and the liver—harmful or helpful? Dig Dis Sci. 2012;57:1754-1763.
24. Navarese EP, Buffon A, Andreotti F, et al. Meta-analysis of impact of different types and doses of statins on new-onset diabetes mellitus. Am J Cardiol. 2013;111:1123-1130.
25. Agarwal R. Effects of statins on renal function. Am J Cardiol. 2006;97:748-755.
26. Fried LF, Orchard TJ, Lasiske BL. Effect of lipid reduction on the progression of renal disease: a meta-analysis. Kidney Int. 2001;59:260-269.
27. Fellström B, Holdaas H, Jardine AG, et al; Assessment of Lescol in Renal Transportation Study Investigators. Effect of fluvastatin on renal end points in the Assessment of Lescol in Renal Transplant (ALERT) Trial. Kidney Int. 2004;66:1549-1555.
28. McKenney JM, Davidson MH, Jacobson TA, et al; National Lipid Association Statin Safety Assessment Task Force. Final conclusions and recommendations of the National Lipid Association Statin Safety Assessment Task Force. Am J Cardiol. 2006;97:89C-94C.
29. KDIGO Clinical Practice Guideline for Lipid Management in Chronic Kidney Disease. Kidney Int. 2013;3(suppl):S259-S305.
30. Goldstein LB, Amarenco P, Szarek M, et al; SPARCL Investigators. Hemorrhagic stroke in the Stroke Prevention by Aggressive Reduction in Cholesterol Levels study. Neurology. 2008;70(24 pt 2):2364-2370.
31. Goldstein LB, Amarenco P, Lamonte M, et al; SPARCL investigators. Relative effects of statin therapy on stroke and cardiovascular events in men and women: secondary analysis of the Stroke Prevention by Aggressive Reduction in Cholesterol Levels (SPARCL) Study. Stroke. 2008;39:2444-2448.
32. Westover MB, Bianchi MT, Eckman MH, et al. Statin use following intracerebral hemorrhage: a decision analysis. Arch Neurol. 2011;68:573-579.
33. Biffi A, Devan WJ, Anderson CD, et al. Statin use and outcome after intracerebral hemorrhage: case-control study and meta-analysis. Neurology. 2011;76:1581-1588.
34. Dowlatshahi D, Demchuck AM, Fang J, et al; Registry of the Canadian Stroke Network. Association of statins and statin discontinuation with poor outcome and survival after intracerebral hemorrhage. Stroke. 2012;43:1518-1523.
35. Bustamante A, Montaner J. Statin therapy should not be discontinued in patients with intracerebral hemorrhage. Stroke. 2013;44:2060-2061.
36. Morgenstern LB, Hemphill JC 3rd, Anderson C, et al; American Heart Association Stroke Council and Council on Cardiovascular Nursing. Guidelines for the management of spontaneous intracerebral hemorrhage: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2010;41:2108-2129.
37. Shepherd J, Blauw GJ, Murphy MB, et al; PROSPER study group. PROspective Study of Pravastatin in the Elderly at Risk. Pravastatin in elderly individuals at risk of vascular disease (PROSPER): a randomised controlled trial. Lancet. 2002;360:1623-1630.
38. Jukema JW, Cannon CP, de Craen AJ, et al. The controversies of statin therapy: weighing the evidence. J Am Coll Cardiol. 2012;60:875-881.
39. Alberton M, Wu P, Druyts E, et al. Adverse events associated with individual statin treatments for cardiovascular disease: an indirect comparison meta-analysis. QJM. 2012;105:145-157.
40. Baigent C, Blackwell L, Emberson J, et al; Cholesterol Treatment Trialists’ (CTT) Collaboration. Efficacy and safety of more intensive lowering of LDL cholesterol: a meta-analysis of data from 170,000 participants in 26 randomised trials. Lancet. 2010;376:1670-1681.
41. Emberson JR, Kearney PM, Blackwell L, et al; Cholesterol Treatment Trialists’ (CTT) Collaboration. Lack of effect of lowering LDL cholesterol on cancer: meta-analysis of individual data from 175,000 people in 27 randomised trials of statin therapy. PLoS One. 2012;7:e29849.
42. Klein BE, Klein R, Lee KE, et al. Statin use and incident nuclear cataract. JAMA. 2006;295:2752-2758.
43. Fong DS, Poon KY. Recent statin use and cataract surgery. Am J Ophthalmol. 2012;153:222-228.e1.
44. Chodick G, Heymann AD, Flash S, et al. Persistence with statins and incident cataract: a population-based historical cohort study. Ann Epidemiol. 2010;20:136-142.
45. Tan JS, Mitchell P, Rochtchina E, et al. Statin use and the long-term risk of incident cataract: the Blue Mountains Eye Study. Am J Ophthalmol. 2007;143:687-689.
46. Machan CM, Hrynchak PK, Irving EL. Age-related cataract is associated with type 2 diabetes and statin use. Optom Vis Sci. 2012;89:1165-1171.
47. Hippisley-Cox J, Coupland C. Unintended effects of statins in men and women in England and Wales: population based cohort study using the QResearch database. BMJ. 2010;340:c2197.
48. Leuschen J, Mortensen EM, Frei CR, et al. Association of statin use with cataracts: a propensity score-matched analysis. JAMA Ophthalmol. 2013;131:1427-1434.
49. Fraunfelder FW, Richards AB. Diplopia, blepharoptosis, and ophthalmoplegia and 3-hydroxy-3-methyl-glutaryl-CoA reductase inhibitor use. Ophthalmology. 2008;115:2282-2285.
50. AHFS Drug Information 2013. Bethesda, MD: American Society of Health-System Pharmacists; 2013.
51. Expert Panel on Integrated Guidelines for Cardiovascular Health and Risk Reduction in Children and Adolescents; National Heart, Lung, and Blood Institute. Expert panel on integrated guidelines for cardiovascular health and risk reduction in children and adolescents: summary report. Pediatrics. 2011;128(suppl 5): S213-S256.
52. Liao JK. Safety and efficacy of statins in Asians. Am J Cardiol. 2007;99:410-414.
Yoga as therapy: When is it helpful?
› Consider recommending Iyengar yoga or Viniyoga for the treatment of chronic low back pain in patients who express an interest in this modality. B
› Consider recommending yoga for the treatment of depression and anxiety symptoms in patients who are interested in exploring this approach. B
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Yoga is practiced by 15.8 million Americans,1 and is often recommended as therapy for a variety of medical conditions. However, the scientific literature on yoga is limited in scope and quality. This article presents good evidence for yoga as treatment for chronic back pain, depression, and anxiety, and fair evidence for treating asthma, symptoms of menopause, hypertension, and mobility issues in the elderly.
Yoga’s rising popularity as therapy
Yoga is a system of movement and breathing exercises meant to foster mind-body connection. Its roots are in ancient Indian practices codified by the writer Patanjali in the first or second century BCE.2
The practice of yoga was introduced to the Western world by a series of popular gurus from the 1930s to 1970s and consists primarily of asanas, or postures, and breathing exercises known as pranayama. Since then, yoga has been further subdivided into different schools and brands (TABLE1,2), some of which are extremely taxing and vigorous and should be performed only by fit and healthy individuals, while others are gentle and accessible to anyone. Yoga has steadily gained in popularity, and nearly half of those who practice it say they do so to improve their health.1
How useful is the research on yoga therapy?
Yoga has been a subject of Western scientific inquiry for more than 100 years. It has been deemed effective for treating conditions from hypertension to epilepsy,3 but many claims are poorly substantiated. Most studies report on a single case or series. The few investigational studies are mainly very small, of short duration, and lacking in appropriate blinding.
Moreover, yoga practices used in the interventions vary markedly, making comparison of results difficult. Interventions range from a single 1-hour session to weekly sessions over several months to inpatient treatment that includes many lifestyle modifications. Some studies required subjects to practice physically demanding asanas, while others focused on pranayama or practices similar to guided relaxation.
Helping patients navigate the yoga domain
The variability in practices described as “yoga” and the lack of a standardized credentialing for yoga teachers make it challenging for patients to find a source suitable for their particular needs. Although choosing a style of yoga appropriate to one’s fitness level and finding an experienced instructor are not straightforward undertakings, physicians familiar with the styles, risks, and benefits of yoga can help direct patients seeking this type of therapy.
The Yoga Alliance is the best-known credentialing organization; it offers a 200-hour and 500-hour curriculum covering anatomy, yoga philosophy, and hands-on practice, and grants credit for years of experience in teaching.4 However, the Yoga Alliance began its current credentialing project just 7 years ago, and it is far from ubiquitous in the industry. Some types of yoga, such as Iyengar and Bikram, have their own certification systems that teachers may preferentially use.
Therapy credentialing. The International Association of Yoga Therapists (IAYT) was founded in 1989 to define yoga therapy and to organize practitioners attempting to use yoga to treat health conditions. As of July 2012, it had published suggested curricula for yoga therapists requiring 800 hours of study.4 Clearly, it will take time for these standards to become disseminated through the industry. At this point, IAYT membership does not require any certification or credentials.4 Moreover, the broad and decentralized nature of yoga practice means that any type of teacher and therapist credentialing or licensure will be controversial and not universally accepted among practitioners. Because of the relative newness of teacher and therapist licensing programs, many experienced and well-respected instructors may lack formal credentials or certifications.
Patients should do extensive research before choosing a type of yoga and an instructor (see “Finding a yoga instructor”). They should choose a type of yoga suited to their fitness level and general health (TABLE1,2) to avoid serious injury, which can include fractures, neuralgia, and arterial dissection.2
Two organizations may be useful in helping your patient locate a yoga instructor or therapist in your area. The International Association of Yoga Therapists (IAYT) and the Yoga Alliance both offer online search tools: http://iayt.site-ym.com/search/custom.asp?id=1156 IA (IAYT) and https://www.yogaalliance.org/yogaregistry (Yoga Alliance). Important areas of questioning for potential therapists include length of teaching experience, training programs completed, and the amount of experience the instructor or therapist has had in working with individuals with a specific medical condition. It may be prudent in certain situations to refer patients to a physical therapist for evaluation before beginning yoga study.
The evidence for yoga’s benefits for specific conditions
The promotion of yoga as medical treatment is rife with dubious claims, but there is solid evidence for its benefits in some common conditions. The evidence summaries that follow reflect searches on Medline, via PubMed, and the Cochrane Database using the phrase “yoga review.”
Back pain
Often a stress-related musculoskeletal problem, back pain seems an appropriate indication for treatment with yoga, and there is a large body of literature on the subject.5 In a systematic review, Chou and Huffman6 found only 3 studies meeting inclusion criteria on yoga’s effectiveness for subacute or chronic low back pain. One large study found 6 weeks of Viniyoga was superior to conventional exercise programs and a self-care booklet in reducing pain and “bothersomeness” scores, as well as reducing the need for analgesic medication.7 Physician visits for back pain were not reduced in the treatment group, however.7 Also included in the systematic review were 2 smaller studies of Iyengar yoga on low back pain; results did not rise to statistical significance.6
A review by Posadzki and Ernst8 included 4 randomized controlled trials (RCTs) not included in Chou and Huffman, although only one of these had >50 subjects. Yoga practices for the treatment groups were mostly Iyengar and Viniyoga and lasted for 12 to 24 weeks, although one study used a 7-day intensive inpatient treatment program. Yoga practitioners had lower pain scores and lower Roland Morris Disability scores.8 A 2004 Clinical Inquiry in The Journal of Family Practice found limited evidence to suggest yoga may speed healing for patients with chronic back pain.9
Most recently, Cramer et al10 found 12 studies meeting inclusion criteria that reported on Viniyoga, Iyengar, and Hatha yoga interventions. Ten of these studies were included in the meta-analysis, which strongly favored yoga over control interventions for reducing pain and disability scores.10
Depression and anxiety
Yoga therapy for depression and anxiety has been commonly studied, given that aspects of mindfulness and relaxation are thought to be important parts of treatment. Moreover, patients uncomfortable with pharmacologic therapy for their disorders may be amenable to yoga treatment. In a recent Clinical Inquiry, Skowronek et al11 found evidence (strength of recommendation [SOR] B) for yoga to treat depression and anxiety symptoms based on 3 recently published review articles that commented on a total of 23 RCTs.
A handful of additional review papers on this subject have selected slightly different groups of studies to include in their analyses, but all have found generally positive results.12-14 Inclusion criteria varied: one review omitted breathing-only modalities such as Sudarshan Kriya yoga, while another included them.12,14 One omitted Mindfulness-Based Stress Reduction (MBSR), which is a program developed in the United States based on several Eastern and Western methodologies including yoga.12 MBSR already has a large body of literature supporting its use for anxiety and depression.12
One of these reviews,12 which involved a meta-analysis of 9 studies regarding depression, also included a meta-analysis of 5 studies on yoga for anxiety. Pooled results for depression showed significant benefit for yoga over usual care, and smaller but still significant benefit for yoga over aerobic exercise or other relaxation techniques. For anxiety, pooled analysis showed yoga to be equal to usual care but superior to other relaxation modalities.12 As with earlier reviews, study groups were heterogeneous and included young and older adults, caregivers for dementia patients, and those receiving inpatient treatment for alcohol dependency; symptoms of depression ranged from mild to severe.12
In a review focusing on anxiety disorders, Kirkwood et al15 located 8 trials, 6 of which were randomized. Many of these were published in the 1970s and 80s. The yoga interventions varied and included weekly Kundalini sessions, pranayama techniques, and savasana (a pose in which practitioners lie supine while focusing on breathing and muscle relaxation). These practices were compared with anxiolytic medication, progressive muscular relaxation, placebo capsule, and no treatment. All found a statistically significant reduction in anxiety indices in the yoga treatment groups, and the authors noted that the positive effects of yoga for those suffering from obsessive-compulsive disorders are particularly well documented.15 More recently, Li and Goldsmith16 reviewed 6 interventional studies that included some trials without randomization, blinding, or a control group. Subjects of the studies included cancer patients, postmenopausal women, pregnant women, and firefighters. Six of 9 trials showed improvement in externally validated anxiety indices such as the State-Trait Anxiety Inventory or Perceived Stress Scale.
Asthma
With its focus on awareness of breath and the mechanics of breathing, yoga would seem a natural adjunct to conventional asthma therapy. One systematic review found 4 trials (3 RCTs) that showed statistically significant improvements in spirometric measurements in patients with asthma who practiced yoga techniques.17 An additional 3 RCTs showed no improvements with yoga over conventional treatments.17 Overall, the reviewers noted that study quality was poor, although they said several studies were appropriately designed. Again, the interventions described as “yoga” varied considerably, from Iyengar-type classes to meditation-focused techniques to pranayama exercises. Follow-up ranged from 6 weeks to 6 months.17
A more recent and thorough review found 14 RCTs using yoga to treat asthma symptoms.18 The investigators performed pooled analysis despite significant heterogeneity in the studies. The analysis showed some improvement in the yoga group compared with usual therapy, but no difference in comparison with sham yoga or non-yoga breathing exercises.18
Symptoms of menopause
Studies have focused on alternative or adjunctive therapies for menopause symptoms, primarily hot flashes, since hormone replacement therapy and other conventional medical therapies have been found to have a high incidence of adverse effects. However, evidence that yoga can reduce hot flashes is sparse.
A Cochrane review examined the effects of exercise on hot flashes and found 2 RCTs using yoga as a treatment modality. Neither one found statistically significant differences between the yoga groups and conventional exercise groups.19 The authors concluded there was insufficient evidence to show yoga was more effective than other forms of exercise on vasomotor symptoms of menopause. However, a large RCT included in the Cochrane review did show lower stress levels and decreased overall symptoms in the yoga arm.20
The yoga intervention in this study consisted of pranayama, sun salutation (a repetitive sequence of 12 yoga postures), and cyclic meditation.20 Lee et al21 reviewed the 2 studies used in the Cochrane paper as well as 5 other studies. Two were RCTs showing that yoga intervention was not superior to a no-treatment control. Four studies showed favorable results for yoga interventions; however, one was a nonrandomized controlled trial and 3 lacked control groups.
Cramer et al22 attempted pooled analysis of 5 studies, including those in the Cochrane paper, with similar results: Yoga interventions were not efficacious for somatic, vasomotor, or urogenital symptoms of menopause. Yoga was somewhat efficacious for psychological symptoms associated with menopause.22 More recently, an RCT (N=249) found that yoga reduces vasomotor symptoms no more frequently than non-yoga exercise.23
Hypertension
Yoga is often said to reduce blood pressure (BP), which would make sense given the emphasis put on relaxation by many schools of yoga. In the past 2 years, 3 review articles have been published, as well as 2 relevant RCTs not included in those reviews.
Hagins et al24 found 17 RCTs using yoga to treat adults with hypertension and prehypertension. These included both blinded and unblinded studies, and yoga interventions were compared with usual treatment, education, or non-yoga exercise. The authors included only studies of asanas intervention, and excluded interventions using only breathing or relaxation techniques.24 In meta-analysis, pooled data showed the yoga treatment decreased both diastolic BP (DBP) and systolic BP (SBP) by 3 to 4 mm Hg compared with usual treatment, but not when compared with other exercise therapies.24 Reviewers concluded that yoga was likely as effective for lowering BP as other types of physical activity.24
In a review without meta-analysis, Posadzki et al25 also found 17 blinded RCTs using yoga to treat hypertension or prehypertension in adults. Eleven of the 17 studies favored yoga, with 8 showing a decrease in SBP and 5 in DBP.25 All but 2 studies were found to be of poor quality, especially with regard to blinding.25 The authors noted that studies using subjects with prehypertension or hypertension with comorbidities were more likely to show significant results, speculating that yoga may be more effective for these populations.25
In an ambitious review article on yoga as treatment for a variety of risk factors for cardiovascular disease, Cramer et al26 located 28 RCTs that addressed effects of yoga on BP. Seven of the studies in the Posadzki review25 were included. Meta-analysis showed a statistically significant decrease in SBP of 5.85 mm Hg and in DBP of 4.12 mm Hg.26 Although wide in scope, this meta-analysis included many studies of healthy patients without hypertension who could conceivably have differing neuroendocrine responses to yoga practice.
In a pilot RCT, Cohen et al27 found a significant decrease in BP among subjects randomized into Iyengar yoga classes for 24 weeks compared with a control group educated about lifestyle modification. A larger study with 102 subjects is currently underway.28 These studies were unique in that no subjects were currently being treated with antihypertensive medications27,28; most other trials on this subject enrolled participants on antihypertensive medications if their regimens had been stable for some time.
In an RCT published recently by Hagins et al,29 68 subjects with pre- or stage I hypertension were randomized into Ashtanga yoga classes or non-aerobic exercise classes formulated to burn equivalent METs. After 12 weeks of treatment, the yoga subjects’ BP had significantly decreased from starting values, but was not improved compared with the exercise subjects.29 This further supports the assertion that yoga is equivalent to other forms of physical activity in decreasing BP among hypertensive subjects.
Balance and stability in the elderly
With its emphasis on strength, balance, and body awareness, yoga would seem a helpful intervention for older patients at risk of injury from falls. Unfortunately this area of research lacks significant numbers of controlled trails. In a Cochrane review of exercise interventions for improving balance in the elderly, the reviewers were unable find any studies specifically using yoga that met their criteria.30 Jeter et al31 attempted a review more recently, and found 15 studies meeting inclusion criteria, 5 of which were RCTs. Overall, however, the poor quality of the studies and variation in both the type of yoga used as intervention and measurements of balance precluded pooled analysis, although some studies did have positive results.
A small but well-designed pilot RCT was recently published showing that an Iyengar yoga intervention significantly improved timed one-leg balancing among community dwelling older adults.32 However, this study did not show a significant difference in a standardized fall risk survey after the intervention.32
Cautioning against yoga in this context are several articles chronicling increased risks of some yoga exercises, especially for those with osteoporosis or other risks for fractures.33 At this point, the well-documented risks of yoga practice in this group probably outweigh the unsubstantiated rewards.
CORRESPONDENCE
Genevieve Verrastro, MD, MAHEC Family Health Center at Biltmore, 123 Hendersonville Road, Asheville, NC 28803; [email protected]
1. Yoga Journal. Yoga in America study 2012 [press release]. Santa Cruz, CA: Santa Cruz Bay Publishing; 2008. Available at: http://www.yogajournal.com/press/yoga_in_america. Accessed August 19, 2014.
2. Broad WJ. The Science of Yoga: The Risks and Rewards. New York, New York: Simon & Schuster; 2012.
3. Lamb T. Health Benefits of Yoga. International Association of Yoga Therapists Web site. Available at: http://www.iayt.org/?page=HealthBenefitsofYoga. Accessed August 21, 2014.
4. Yoga Alliance. 200-hour standards for yoga teacher trainings. Yoga Alliance Web site. Available at: http://yogaalliance.org/content/200-hour-standards. Accessed August 19, 2014.
5. Wren AA, Wright MA, Carson JW, et al. Yoga for persistent pain: New findings and directions for an ancient practice. Pain. 2011;152:477-480.
6. Chou R, Huffman LH; American Pain Society; American College of Physicians. Nonpharmacologic therapies for acute and chronic low back pain: a review of the evidence for an American Pain Society/American College of Physicians clinical practice guideline. Ann Intern Med. 2007;147:492-504.
7. Sherman KJ, Cherkin DC, Erro J, et al. Comparing yoga, exercise, and a self-care book for chronic low back pain: a randomized, controlled trial. Ann Intern Med. 2005;143:849-856.
8. Posadzki P, Ernst E. Yoga for low back pain: a systematic review of randomized clinical trials. Clin Rheumatol. 2011;30:1257-1262.
9. Graves N, Krepcho M, Mayo HG, et al. Does yoga speed healing for patients with low back pain? J Fam Pract. 2004;53:661-662.
10. Cramer H, Lauche R, Haller H, et al. A systematic review and meta-analysis of yoga for low back pain. Clin J Pain. 2013;29:450-460.
11. Skowronek IB, Mounsey A, Handler L. Can yoga reduce symptoms of anxiety and depression? J Fam Pract. 2013;63:398-399,407.
12. Cramer H, Lauche R, Langhorst J, et al. Yoga for depression: a systematic review and meta-analysis. Depress Anxiety. 2013;30: 1068-1083.
13. D’Silva S, Poscablo C, Habousha R, et al. Mind-body medicine therapies for a range of depression severity: a systematic review. Psychosomatics. 2012;53:407-423.
14. Balasubramaniam M, Telles S, Doraiswamy PM. Yoga on our minds: a systematic review of yoga for neuropsychiatric disorders. Front Psychiatry. 2013;3:117.
15. Kirkwood G, Rampes H, Tuffrey V, et al. Yoga for anxiety: a systematic review of the research evidence. Br J Sports Med. 2005;39: 884-891.
16. Li AW, Goldsmith CA. The effects of yoga on anxiety and stress. Altern Med Rev. 2012;17:21-35.
17. Posadzki P, Ernst E. Yoga for asthma? A systematic review of randomized clinical trials. J Asthma. 2011;48:632-639.
18. Cramer H, Posadzki P, Dobos G, et al. Yoga for asthma: a systematic review and meta-analysis. Ann Allergy Asthma Immunol. 2014;112:503-510.e5.
19. Daley A, Stokes-Lampard H, Macarthur C. Exercise for vasomotor menopausal symptoms. Cochrane Database Syst Rev. 2011;(5):CD006108.
20. Chattha R, Nagarathna R, Padmalatha V, et al. Effect of yoga on cognitive functions in climacteric syndrome: a randomised control study. BJOG. 2008;115:991-1000.
21. Lee MS, Kim JI, Ha JY, et al. Yoga for menopausal symptoms: a systematic review. Menopause. 2009;16:602-608.
22. Cramer H, Lauche R, Langhorst J, et al. Effectiveness of yoga for menopausal symptoms: a systematic review and meta-analysis of randomized controlled trials. Evid Based Complement Alternat Med. 2012;2012:863905.
23. Newton KM, Reed SD, Guthrie KA, et al. Efficacy of yoga for vasomotor symptoms: a randomized controlled trial. Menopause. 2014;21:339-346.
24. Hagins M, States R, Selfe T, et al. Effectiveness of yoga for hypertension: systematic review and meta-analysis. Evid Based Complement Alternat Med. 2013;2013:649836.
25. Posadzki P, Cramer H, Kuzdzal A, et al. Yoga for hypertension: a systematic review of randomized clinical trials. Complement Ther Med. 2014;22:511-522.
26. Cramer H, Lauche R, Haller H, et al. Effects of yoga on cardiovascular disease risk factors: a systematic review and meta-analysis. Int J Cardiol. 2014;173:170-183.
27. Cohen DL, Bloedon LT, Rothman RL, et al. Iyengar yoga versus enhanced usual care on blood pressure in patients with prehypertension to stage I hypertension: a randomized controlled trial. Evid Based Complement Alternat Med. 2011;2011:546428.
28. Cohen DL, Bowler A, Fisher SA, et al. Lifestyle Modification in Blood Pressure Study II (LIMBS): study protocol of a randomized controlled trial assessing the efficacy of a 24 week structured yoga program versus lifestyle modification on blood pressure reduction. Contemp Clin Trials. 2013;36:32-40.
29. Hagins M, Rundle A, Consedine N, et al. A randomized controlled trial comparing the effects of yoga with an active control on ambulatory blood pressure in individuals with pre- and stage 1 hypertension. J Clin Hypertens (Greenwich). 2014;16:54-62.
30. Howe TE, Rochester L, Neil F, et al. Exercise for improving balance in older people. Cochrane Database Syst Rev. 2011;(11):CD004963.
31. Jeter PE, Nkodo AF, Moonaz SH, et al. A systematic review of yoga for balance in a healthy population. J Altern Complement Med. 2014;20:221-232.
32. Tiedemann A, O’Rourke S, Sesto R, et al. A 12-week Iyengar yoga program improved balance and mobility in older community-dwelling people: a pilot randomized controlled trial. J Gerontol A Biol Sci Med Sci. 2013;68:1068-1075.
33. Sinaki M. Yoga spinal flexion positions and vertebral compression fracture in osteopenia or osteoporosis of spine: case series. Pain Pract. 2013;13:68-75.
› Consider recommending Iyengar yoga or Viniyoga for the treatment of chronic low back pain in patients who express an interest in this modality. B
› Consider recommending yoga for the treatment of depression and anxiety symptoms in patients who are interested in exploring this approach. B
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Yoga is practiced by 15.8 million Americans,1 and is often recommended as therapy for a variety of medical conditions. However, the scientific literature on yoga is limited in scope and quality. This article presents good evidence for yoga as treatment for chronic back pain, depression, and anxiety, and fair evidence for treating asthma, symptoms of menopause, hypertension, and mobility issues in the elderly.
Yoga’s rising popularity as therapy
Yoga is a system of movement and breathing exercises meant to foster mind-body connection. Its roots are in ancient Indian practices codified by the writer Patanjali in the first or second century BCE.2
The practice of yoga was introduced to the Western world by a series of popular gurus from the 1930s to 1970s and consists primarily of asanas, or postures, and breathing exercises known as pranayama. Since then, yoga has been further subdivided into different schools and brands (TABLE1,2), some of which are extremely taxing and vigorous and should be performed only by fit and healthy individuals, while others are gentle and accessible to anyone. Yoga has steadily gained in popularity, and nearly half of those who practice it say they do so to improve their health.1
How useful is the research on yoga therapy?
Yoga has been a subject of Western scientific inquiry for more than 100 years. It has been deemed effective for treating conditions from hypertension to epilepsy,3 but many claims are poorly substantiated. Most studies report on a single case or series. The few investigational studies are mainly very small, of short duration, and lacking in appropriate blinding.
Moreover, yoga practices used in the interventions vary markedly, making comparison of results difficult. Interventions range from a single 1-hour session to weekly sessions over several months to inpatient treatment that includes many lifestyle modifications. Some studies required subjects to practice physically demanding asanas, while others focused on pranayama or practices similar to guided relaxation.
Helping patients navigate the yoga domain
The variability in practices described as “yoga” and the lack of a standardized credentialing for yoga teachers make it challenging for patients to find a source suitable for their particular needs. Although choosing a style of yoga appropriate to one’s fitness level and finding an experienced instructor are not straightforward undertakings, physicians familiar with the styles, risks, and benefits of yoga can help direct patients seeking this type of therapy.
The Yoga Alliance is the best-known credentialing organization; it offers a 200-hour and 500-hour curriculum covering anatomy, yoga philosophy, and hands-on practice, and grants credit for years of experience in teaching.4 However, the Yoga Alliance began its current credentialing project just 7 years ago, and it is far from ubiquitous in the industry. Some types of yoga, such as Iyengar and Bikram, have their own certification systems that teachers may preferentially use.
Therapy credentialing. The International Association of Yoga Therapists (IAYT) was founded in 1989 to define yoga therapy and to organize practitioners attempting to use yoga to treat health conditions. As of July 2012, it had published suggested curricula for yoga therapists requiring 800 hours of study.4 Clearly, it will take time for these standards to become disseminated through the industry. At this point, IAYT membership does not require any certification or credentials.4 Moreover, the broad and decentralized nature of yoga practice means that any type of teacher and therapist credentialing or licensure will be controversial and not universally accepted among practitioners. Because of the relative newness of teacher and therapist licensing programs, many experienced and well-respected instructors may lack formal credentials or certifications.
Patients should do extensive research before choosing a type of yoga and an instructor (see “Finding a yoga instructor”). They should choose a type of yoga suited to their fitness level and general health (TABLE1,2) to avoid serious injury, which can include fractures, neuralgia, and arterial dissection.2
Two organizations may be useful in helping your patient locate a yoga instructor or therapist in your area. The International Association of Yoga Therapists (IAYT) and the Yoga Alliance both offer online search tools: http://iayt.site-ym.com/search/custom.asp?id=1156 IA (IAYT) and https://www.yogaalliance.org/yogaregistry (Yoga Alliance). Important areas of questioning for potential therapists include length of teaching experience, training programs completed, and the amount of experience the instructor or therapist has had in working with individuals with a specific medical condition. It may be prudent in certain situations to refer patients to a physical therapist for evaluation before beginning yoga study.
The evidence for yoga’s benefits for specific conditions
The promotion of yoga as medical treatment is rife with dubious claims, but there is solid evidence for its benefits in some common conditions. The evidence summaries that follow reflect searches on Medline, via PubMed, and the Cochrane Database using the phrase “yoga review.”
Back pain
Often a stress-related musculoskeletal problem, back pain seems an appropriate indication for treatment with yoga, and there is a large body of literature on the subject.5 In a systematic review, Chou and Huffman6 found only 3 studies meeting inclusion criteria on yoga’s effectiveness for subacute or chronic low back pain. One large study found 6 weeks of Viniyoga was superior to conventional exercise programs and a self-care booklet in reducing pain and “bothersomeness” scores, as well as reducing the need for analgesic medication.7 Physician visits for back pain were not reduced in the treatment group, however.7 Also included in the systematic review were 2 smaller studies of Iyengar yoga on low back pain; results did not rise to statistical significance.6
A review by Posadzki and Ernst8 included 4 randomized controlled trials (RCTs) not included in Chou and Huffman, although only one of these had >50 subjects. Yoga practices for the treatment groups were mostly Iyengar and Viniyoga and lasted for 12 to 24 weeks, although one study used a 7-day intensive inpatient treatment program. Yoga practitioners had lower pain scores and lower Roland Morris Disability scores.8 A 2004 Clinical Inquiry in The Journal of Family Practice found limited evidence to suggest yoga may speed healing for patients with chronic back pain.9
Most recently, Cramer et al10 found 12 studies meeting inclusion criteria that reported on Viniyoga, Iyengar, and Hatha yoga interventions. Ten of these studies were included in the meta-analysis, which strongly favored yoga over control interventions for reducing pain and disability scores.10
Depression and anxiety
Yoga therapy for depression and anxiety has been commonly studied, given that aspects of mindfulness and relaxation are thought to be important parts of treatment. Moreover, patients uncomfortable with pharmacologic therapy for their disorders may be amenable to yoga treatment. In a recent Clinical Inquiry, Skowronek et al11 found evidence (strength of recommendation [SOR] B) for yoga to treat depression and anxiety symptoms based on 3 recently published review articles that commented on a total of 23 RCTs.
A handful of additional review papers on this subject have selected slightly different groups of studies to include in their analyses, but all have found generally positive results.12-14 Inclusion criteria varied: one review omitted breathing-only modalities such as Sudarshan Kriya yoga, while another included them.12,14 One omitted Mindfulness-Based Stress Reduction (MBSR), which is a program developed in the United States based on several Eastern and Western methodologies including yoga.12 MBSR already has a large body of literature supporting its use for anxiety and depression.12
One of these reviews,12 which involved a meta-analysis of 9 studies regarding depression, also included a meta-analysis of 5 studies on yoga for anxiety. Pooled results for depression showed significant benefit for yoga over usual care, and smaller but still significant benefit for yoga over aerobic exercise or other relaxation techniques. For anxiety, pooled analysis showed yoga to be equal to usual care but superior to other relaxation modalities.12 As with earlier reviews, study groups were heterogeneous and included young and older adults, caregivers for dementia patients, and those receiving inpatient treatment for alcohol dependency; symptoms of depression ranged from mild to severe.12
In a review focusing on anxiety disorders, Kirkwood et al15 located 8 trials, 6 of which were randomized. Many of these were published in the 1970s and 80s. The yoga interventions varied and included weekly Kundalini sessions, pranayama techniques, and savasana (a pose in which practitioners lie supine while focusing on breathing and muscle relaxation). These practices were compared with anxiolytic medication, progressive muscular relaxation, placebo capsule, and no treatment. All found a statistically significant reduction in anxiety indices in the yoga treatment groups, and the authors noted that the positive effects of yoga for those suffering from obsessive-compulsive disorders are particularly well documented.15 More recently, Li and Goldsmith16 reviewed 6 interventional studies that included some trials without randomization, blinding, or a control group. Subjects of the studies included cancer patients, postmenopausal women, pregnant women, and firefighters. Six of 9 trials showed improvement in externally validated anxiety indices such as the State-Trait Anxiety Inventory or Perceived Stress Scale.
Asthma
With its focus on awareness of breath and the mechanics of breathing, yoga would seem a natural adjunct to conventional asthma therapy. One systematic review found 4 trials (3 RCTs) that showed statistically significant improvements in spirometric measurements in patients with asthma who practiced yoga techniques.17 An additional 3 RCTs showed no improvements with yoga over conventional treatments.17 Overall, the reviewers noted that study quality was poor, although they said several studies were appropriately designed. Again, the interventions described as “yoga” varied considerably, from Iyengar-type classes to meditation-focused techniques to pranayama exercises. Follow-up ranged from 6 weeks to 6 months.17
A more recent and thorough review found 14 RCTs using yoga to treat asthma symptoms.18 The investigators performed pooled analysis despite significant heterogeneity in the studies. The analysis showed some improvement in the yoga group compared with usual therapy, but no difference in comparison with sham yoga or non-yoga breathing exercises.18
Symptoms of menopause
Studies have focused on alternative or adjunctive therapies for menopause symptoms, primarily hot flashes, since hormone replacement therapy and other conventional medical therapies have been found to have a high incidence of adverse effects. However, evidence that yoga can reduce hot flashes is sparse.
A Cochrane review examined the effects of exercise on hot flashes and found 2 RCTs using yoga as a treatment modality. Neither one found statistically significant differences between the yoga groups and conventional exercise groups.19 The authors concluded there was insufficient evidence to show yoga was more effective than other forms of exercise on vasomotor symptoms of menopause. However, a large RCT included in the Cochrane review did show lower stress levels and decreased overall symptoms in the yoga arm.20
The yoga intervention in this study consisted of pranayama, sun salutation (a repetitive sequence of 12 yoga postures), and cyclic meditation.20 Lee et al21 reviewed the 2 studies used in the Cochrane paper as well as 5 other studies. Two were RCTs showing that yoga intervention was not superior to a no-treatment control. Four studies showed favorable results for yoga interventions; however, one was a nonrandomized controlled trial and 3 lacked control groups.
Cramer et al22 attempted pooled analysis of 5 studies, including those in the Cochrane paper, with similar results: Yoga interventions were not efficacious for somatic, vasomotor, or urogenital symptoms of menopause. Yoga was somewhat efficacious for psychological symptoms associated with menopause.22 More recently, an RCT (N=249) found that yoga reduces vasomotor symptoms no more frequently than non-yoga exercise.23
Hypertension
Yoga is often said to reduce blood pressure (BP), which would make sense given the emphasis put on relaxation by many schools of yoga. In the past 2 years, 3 review articles have been published, as well as 2 relevant RCTs not included in those reviews.
Hagins et al24 found 17 RCTs using yoga to treat adults with hypertension and prehypertension. These included both blinded and unblinded studies, and yoga interventions were compared with usual treatment, education, or non-yoga exercise. The authors included only studies of asanas intervention, and excluded interventions using only breathing or relaxation techniques.24 In meta-analysis, pooled data showed the yoga treatment decreased both diastolic BP (DBP) and systolic BP (SBP) by 3 to 4 mm Hg compared with usual treatment, but not when compared with other exercise therapies.24 Reviewers concluded that yoga was likely as effective for lowering BP as other types of physical activity.24
In a review without meta-analysis, Posadzki et al25 also found 17 blinded RCTs using yoga to treat hypertension or prehypertension in adults. Eleven of the 17 studies favored yoga, with 8 showing a decrease in SBP and 5 in DBP.25 All but 2 studies were found to be of poor quality, especially with regard to blinding.25 The authors noted that studies using subjects with prehypertension or hypertension with comorbidities were more likely to show significant results, speculating that yoga may be more effective for these populations.25
In an ambitious review article on yoga as treatment for a variety of risk factors for cardiovascular disease, Cramer et al26 located 28 RCTs that addressed effects of yoga on BP. Seven of the studies in the Posadzki review25 were included. Meta-analysis showed a statistically significant decrease in SBP of 5.85 mm Hg and in DBP of 4.12 mm Hg.26 Although wide in scope, this meta-analysis included many studies of healthy patients without hypertension who could conceivably have differing neuroendocrine responses to yoga practice.
In a pilot RCT, Cohen et al27 found a significant decrease in BP among subjects randomized into Iyengar yoga classes for 24 weeks compared with a control group educated about lifestyle modification. A larger study with 102 subjects is currently underway.28 These studies were unique in that no subjects were currently being treated with antihypertensive medications27,28; most other trials on this subject enrolled participants on antihypertensive medications if their regimens had been stable for some time.
In an RCT published recently by Hagins et al,29 68 subjects with pre- or stage I hypertension were randomized into Ashtanga yoga classes or non-aerobic exercise classes formulated to burn equivalent METs. After 12 weeks of treatment, the yoga subjects’ BP had significantly decreased from starting values, but was not improved compared with the exercise subjects.29 This further supports the assertion that yoga is equivalent to other forms of physical activity in decreasing BP among hypertensive subjects.
Balance and stability in the elderly
With its emphasis on strength, balance, and body awareness, yoga would seem a helpful intervention for older patients at risk of injury from falls. Unfortunately this area of research lacks significant numbers of controlled trails. In a Cochrane review of exercise interventions for improving balance in the elderly, the reviewers were unable find any studies specifically using yoga that met their criteria.30 Jeter et al31 attempted a review more recently, and found 15 studies meeting inclusion criteria, 5 of which were RCTs. Overall, however, the poor quality of the studies and variation in both the type of yoga used as intervention and measurements of balance precluded pooled analysis, although some studies did have positive results.
A small but well-designed pilot RCT was recently published showing that an Iyengar yoga intervention significantly improved timed one-leg balancing among community dwelling older adults.32 However, this study did not show a significant difference in a standardized fall risk survey after the intervention.32
Cautioning against yoga in this context are several articles chronicling increased risks of some yoga exercises, especially for those with osteoporosis or other risks for fractures.33 At this point, the well-documented risks of yoga practice in this group probably outweigh the unsubstantiated rewards.
CORRESPONDENCE
Genevieve Verrastro, MD, MAHEC Family Health Center at Biltmore, 123 Hendersonville Road, Asheville, NC 28803; [email protected]
› Consider recommending Iyengar yoga or Viniyoga for the treatment of chronic low back pain in patients who express an interest in this modality. B
› Consider recommending yoga for the treatment of depression and anxiety symptoms in patients who are interested in exploring this approach. B
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Yoga is practiced by 15.8 million Americans,1 and is often recommended as therapy for a variety of medical conditions. However, the scientific literature on yoga is limited in scope and quality. This article presents good evidence for yoga as treatment for chronic back pain, depression, and anxiety, and fair evidence for treating asthma, symptoms of menopause, hypertension, and mobility issues in the elderly.
Yoga’s rising popularity as therapy
Yoga is a system of movement and breathing exercises meant to foster mind-body connection. Its roots are in ancient Indian practices codified by the writer Patanjali in the first or second century BCE.2
The practice of yoga was introduced to the Western world by a series of popular gurus from the 1930s to 1970s and consists primarily of asanas, or postures, and breathing exercises known as pranayama. Since then, yoga has been further subdivided into different schools and brands (TABLE1,2), some of which are extremely taxing and vigorous and should be performed only by fit and healthy individuals, while others are gentle and accessible to anyone. Yoga has steadily gained in popularity, and nearly half of those who practice it say they do so to improve their health.1
How useful is the research on yoga therapy?
Yoga has been a subject of Western scientific inquiry for more than 100 years. It has been deemed effective for treating conditions from hypertension to epilepsy,3 but many claims are poorly substantiated. Most studies report on a single case or series. The few investigational studies are mainly very small, of short duration, and lacking in appropriate blinding.
Moreover, yoga practices used in the interventions vary markedly, making comparison of results difficult. Interventions range from a single 1-hour session to weekly sessions over several months to inpatient treatment that includes many lifestyle modifications. Some studies required subjects to practice physically demanding asanas, while others focused on pranayama or practices similar to guided relaxation.
Helping patients navigate the yoga domain
The variability in practices described as “yoga” and the lack of a standardized credentialing for yoga teachers make it challenging for patients to find a source suitable for their particular needs. Although choosing a style of yoga appropriate to one’s fitness level and finding an experienced instructor are not straightforward undertakings, physicians familiar with the styles, risks, and benefits of yoga can help direct patients seeking this type of therapy.
The Yoga Alliance is the best-known credentialing organization; it offers a 200-hour and 500-hour curriculum covering anatomy, yoga philosophy, and hands-on practice, and grants credit for years of experience in teaching.4 However, the Yoga Alliance began its current credentialing project just 7 years ago, and it is far from ubiquitous in the industry. Some types of yoga, such as Iyengar and Bikram, have their own certification systems that teachers may preferentially use.
Therapy credentialing. The International Association of Yoga Therapists (IAYT) was founded in 1989 to define yoga therapy and to organize practitioners attempting to use yoga to treat health conditions. As of July 2012, it had published suggested curricula for yoga therapists requiring 800 hours of study.4 Clearly, it will take time for these standards to become disseminated through the industry. At this point, IAYT membership does not require any certification or credentials.4 Moreover, the broad and decentralized nature of yoga practice means that any type of teacher and therapist credentialing or licensure will be controversial and not universally accepted among practitioners. Because of the relative newness of teacher and therapist licensing programs, many experienced and well-respected instructors may lack formal credentials or certifications.
Patients should do extensive research before choosing a type of yoga and an instructor (see “Finding a yoga instructor”). They should choose a type of yoga suited to their fitness level and general health (TABLE1,2) to avoid serious injury, which can include fractures, neuralgia, and arterial dissection.2
Two organizations may be useful in helping your patient locate a yoga instructor or therapist in your area. The International Association of Yoga Therapists (IAYT) and the Yoga Alliance both offer online search tools: http://iayt.site-ym.com/search/custom.asp?id=1156 IA (IAYT) and https://www.yogaalliance.org/yogaregistry (Yoga Alliance). Important areas of questioning for potential therapists include length of teaching experience, training programs completed, and the amount of experience the instructor or therapist has had in working with individuals with a specific medical condition. It may be prudent in certain situations to refer patients to a physical therapist for evaluation before beginning yoga study.
The evidence for yoga’s benefits for specific conditions
The promotion of yoga as medical treatment is rife with dubious claims, but there is solid evidence for its benefits in some common conditions. The evidence summaries that follow reflect searches on Medline, via PubMed, and the Cochrane Database using the phrase “yoga review.”
Back pain
Often a stress-related musculoskeletal problem, back pain seems an appropriate indication for treatment with yoga, and there is a large body of literature on the subject.5 In a systematic review, Chou and Huffman6 found only 3 studies meeting inclusion criteria on yoga’s effectiveness for subacute or chronic low back pain. One large study found 6 weeks of Viniyoga was superior to conventional exercise programs and a self-care booklet in reducing pain and “bothersomeness” scores, as well as reducing the need for analgesic medication.7 Physician visits for back pain were not reduced in the treatment group, however.7 Also included in the systematic review were 2 smaller studies of Iyengar yoga on low back pain; results did not rise to statistical significance.6
A review by Posadzki and Ernst8 included 4 randomized controlled trials (RCTs) not included in Chou and Huffman, although only one of these had >50 subjects. Yoga practices for the treatment groups were mostly Iyengar and Viniyoga and lasted for 12 to 24 weeks, although one study used a 7-day intensive inpatient treatment program. Yoga practitioners had lower pain scores and lower Roland Morris Disability scores.8 A 2004 Clinical Inquiry in The Journal of Family Practice found limited evidence to suggest yoga may speed healing for patients with chronic back pain.9
Most recently, Cramer et al10 found 12 studies meeting inclusion criteria that reported on Viniyoga, Iyengar, and Hatha yoga interventions. Ten of these studies were included in the meta-analysis, which strongly favored yoga over control interventions for reducing pain and disability scores.10
Depression and anxiety
Yoga therapy for depression and anxiety has been commonly studied, given that aspects of mindfulness and relaxation are thought to be important parts of treatment. Moreover, patients uncomfortable with pharmacologic therapy for their disorders may be amenable to yoga treatment. In a recent Clinical Inquiry, Skowronek et al11 found evidence (strength of recommendation [SOR] B) for yoga to treat depression and anxiety symptoms based on 3 recently published review articles that commented on a total of 23 RCTs.
A handful of additional review papers on this subject have selected slightly different groups of studies to include in their analyses, but all have found generally positive results.12-14 Inclusion criteria varied: one review omitted breathing-only modalities such as Sudarshan Kriya yoga, while another included them.12,14 One omitted Mindfulness-Based Stress Reduction (MBSR), which is a program developed in the United States based on several Eastern and Western methodologies including yoga.12 MBSR already has a large body of literature supporting its use for anxiety and depression.12
One of these reviews,12 which involved a meta-analysis of 9 studies regarding depression, also included a meta-analysis of 5 studies on yoga for anxiety. Pooled results for depression showed significant benefit for yoga over usual care, and smaller but still significant benefit for yoga over aerobic exercise or other relaxation techniques. For anxiety, pooled analysis showed yoga to be equal to usual care but superior to other relaxation modalities.12 As with earlier reviews, study groups were heterogeneous and included young and older adults, caregivers for dementia patients, and those receiving inpatient treatment for alcohol dependency; symptoms of depression ranged from mild to severe.12
In a review focusing on anxiety disorders, Kirkwood et al15 located 8 trials, 6 of which were randomized. Many of these were published in the 1970s and 80s. The yoga interventions varied and included weekly Kundalini sessions, pranayama techniques, and savasana (a pose in which practitioners lie supine while focusing on breathing and muscle relaxation). These practices were compared with anxiolytic medication, progressive muscular relaxation, placebo capsule, and no treatment. All found a statistically significant reduction in anxiety indices in the yoga treatment groups, and the authors noted that the positive effects of yoga for those suffering from obsessive-compulsive disorders are particularly well documented.15 More recently, Li and Goldsmith16 reviewed 6 interventional studies that included some trials without randomization, blinding, or a control group. Subjects of the studies included cancer patients, postmenopausal women, pregnant women, and firefighters. Six of 9 trials showed improvement in externally validated anxiety indices such as the State-Trait Anxiety Inventory or Perceived Stress Scale.
Asthma
With its focus on awareness of breath and the mechanics of breathing, yoga would seem a natural adjunct to conventional asthma therapy. One systematic review found 4 trials (3 RCTs) that showed statistically significant improvements in spirometric measurements in patients with asthma who practiced yoga techniques.17 An additional 3 RCTs showed no improvements with yoga over conventional treatments.17 Overall, the reviewers noted that study quality was poor, although they said several studies were appropriately designed. Again, the interventions described as “yoga” varied considerably, from Iyengar-type classes to meditation-focused techniques to pranayama exercises. Follow-up ranged from 6 weeks to 6 months.17
A more recent and thorough review found 14 RCTs using yoga to treat asthma symptoms.18 The investigators performed pooled analysis despite significant heterogeneity in the studies. The analysis showed some improvement in the yoga group compared with usual therapy, but no difference in comparison with sham yoga or non-yoga breathing exercises.18
Symptoms of menopause
Studies have focused on alternative or adjunctive therapies for menopause symptoms, primarily hot flashes, since hormone replacement therapy and other conventional medical therapies have been found to have a high incidence of adverse effects. However, evidence that yoga can reduce hot flashes is sparse.
A Cochrane review examined the effects of exercise on hot flashes and found 2 RCTs using yoga as a treatment modality. Neither one found statistically significant differences between the yoga groups and conventional exercise groups.19 The authors concluded there was insufficient evidence to show yoga was more effective than other forms of exercise on vasomotor symptoms of menopause. However, a large RCT included in the Cochrane review did show lower stress levels and decreased overall symptoms in the yoga arm.20
The yoga intervention in this study consisted of pranayama, sun salutation (a repetitive sequence of 12 yoga postures), and cyclic meditation.20 Lee et al21 reviewed the 2 studies used in the Cochrane paper as well as 5 other studies. Two were RCTs showing that yoga intervention was not superior to a no-treatment control. Four studies showed favorable results for yoga interventions; however, one was a nonrandomized controlled trial and 3 lacked control groups.
Cramer et al22 attempted pooled analysis of 5 studies, including those in the Cochrane paper, with similar results: Yoga interventions were not efficacious for somatic, vasomotor, or urogenital symptoms of menopause. Yoga was somewhat efficacious for psychological symptoms associated with menopause.22 More recently, an RCT (N=249) found that yoga reduces vasomotor symptoms no more frequently than non-yoga exercise.23
Hypertension
Yoga is often said to reduce blood pressure (BP), which would make sense given the emphasis put on relaxation by many schools of yoga. In the past 2 years, 3 review articles have been published, as well as 2 relevant RCTs not included in those reviews.
Hagins et al24 found 17 RCTs using yoga to treat adults with hypertension and prehypertension. These included both blinded and unblinded studies, and yoga interventions were compared with usual treatment, education, or non-yoga exercise. The authors included only studies of asanas intervention, and excluded interventions using only breathing or relaxation techniques.24 In meta-analysis, pooled data showed the yoga treatment decreased both diastolic BP (DBP) and systolic BP (SBP) by 3 to 4 mm Hg compared with usual treatment, but not when compared with other exercise therapies.24 Reviewers concluded that yoga was likely as effective for lowering BP as other types of physical activity.24
In a review without meta-analysis, Posadzki et al25 also found 17 blinded RCTs using yoga to treat hypertension or prehypertension in adults. Eleven of the 17 studies favored yoga, with 8 showing a decrease in SBP and 5 in DBP.25 All but 2 studies were found to be of poor quality, especially with regard to blinding.25 The authors noted that studies using subjects with prehypertension or hypertension with comorbidities were more likely to show significant results, speculating that yoga may be more effective for these populations.25
In an ambitious review article on yoga as treatment for a variety of risk factors for cardiovascular disease, Cramer et al26 located 28 RCTs that addressed effects of yoga on BP. Seven of the studies in the Posadzki review25 were included. Meta-analysis showed a statistically significant decrease in SBP of 5.85 mm Hg and in DBP of 4.12 mm Hg.26 Although wide in scope, this meta-analysis included many studies of healthy patients without hypertension who could conceivably have differing neuroendocrine responses to yoga practice.
In a pilot RCT, Cohen et al27 found a significant decrease in BP among subjects randomized into Iyengar yoga classes for 24 weeks compared with a control group educated about lifestyle modification. A larger study with 102 subjects is currently underway.28 These studies were unique in that no subjects were currently being treated with antihypertensive medications27,28; most other trials on this subject enrolled participants on antihypertensive medications if their regimens had been stable for some time.
In an RCT published recently by Hagins et al,29 68 subjects with pre- or stage I hypertension were randomized into Ashtanga yoga classes or non-aerobic exercise classes formulated to burn equivalent METs. After 12 weeks of treatment, the yoga subjects’ BP had significantly decreased from starting values, but was not improved compared with the exercise subjects.29 This further supports the assertion that yoga is equivalent to other forms of physical activity in decreasing BP among hypertensive subjects.
Balance and stability in the elderly
With its emphasis on strength, balance, and body awareness, yoga would seem a helpful intervention for older patients at risk of injury from falls. Unfortunately this area of research lacks significant numbers of controlled trails. In a Cochrane review of exercise interventions for improving balance in the elderly, the reviewers were unable find any studies specifically using yoga that met their criteria.30 Jeter et al31 attempted a review more recently, and found 15 studies meeting inclusion criteria, 5 of which were RCTs. Overall, however, the poor quality of the studies and variation in both the type of yoga used as intervention and measurements of balance precluded pooled analysis, although some studies did have positive results.
A small but well-designed pilot RCT was recently published showing that an Iyengar yoga intervention significantly improved timed one-leg balancing among community dwelling older adults.32 However, this study did not show a significant difference in a standardized fall risk survey after the intervention.32
Cautioning against yoga in this context are several articles chronicling increased risks of some yoga exercises, especially for those with osteoporosis or other risks for fractures.33 At this point, the well-documented risks of yoga practice in this group probably outweigh the unsubstantiated rewards.
CORRESPONDENCE
Genevieve Verrastro, MD, MAHEC Family Health Center at Biltmore, 123 Hendersonville Road, Asheville, NC 28803; [email protected]
1. Yoga Journal. Yoga in America study 2012 [press release]. Santa Cruz, CA: Santa Cruz Bay Publishing; 2008. Available at: http://www.yogajournal.com/press/yoga_in_america. Accessed August 19, 2014.
2. Broad WJ. The Science of Yoga: The Risks and Rewards. New York, New York: Simon & Schuster; 2012.
3. Lamb T. Health Benefits of Yoga. International Association of Yoga Therapists Web site. Available at: http://www.iayt.org/?page=HealthBenefitsofYoga. Accessed August 21, 2014.
4. Yoga Alliance. 200-hour standards for yoga teacher trainings. Yoga Alliance Web site. Available at: http://yogaalliance.org/content/200-hour-standards. Accessed August 19, 2014.
5. Wren AA, Wright MA, Carson JW, et al. Yoga for persistent pain: New findings and directions for an ancient practice. Pain. 2011;152:477-480.
6. Chou R, Huffman LH; American Pain Society; American College of Physicians. Nonpharmacologic therapies for acute and chronic low back pain: a review of the evidence for an American Pain Society/American College of Physicians clinical practice guideline. Ann Intern Med. 2007;147:492-504.
7. Sherman KJ, Cherkin DC, Erro J, et al. Comparing yoga, exercise, and a self-care book for chronic low back pain: a randomized, controlled trial. Ann Intern Med. 2005;143:849-856.
8. Posadzki P, Ernst E. Yoga for low back pain: a systematic review of randomized clinical trials. Clin Rheumatol. 2011;30:1257-1262.
9. Graves N, Krepcho M, Mayo HG, et al. Does yoga speed healing for patients with low back pain? J Fam Pract. 2004;53:661-662.
10. Cramer H, Lauche R, Haller H, et al. A systematic review and meta-analysis of yoga for low back pain. Clin J Pain. 2013;29:450-460.
11. Skowronek IB, Mounsey A, Handler L. Can yoga reduce symptoms of anxiety and depression? J Fam Pract. 2013;63:398-399,407.
12. Cramer H, Lauche R, Langhorst J, et al. Yoga for depression: a systematic review and meta-analysis. Depress Anxiety. 2013;30: 1068-1083.
13. D’Silva S, Poscablo C, Habousha R, et al. Mind-body medicine therapies for a range of depression severity: a systematic review. Psychosomatics. 2012;53:407-423.
14. Balasubramaniam M, Telles S, Doraiswamy PM. Yoga on our minds: a systematic review of yoga for neuropsychiatric disorders. Front Psychiatry. 2013;3:117.
15. Kirkwood G, Rampes H, Tuffrey V, et al. Yoga for anxiety: a systematic review of the research evidence. Br J Sports Med. 2005;39: 884-891.
16. Li AW, Goldsmith CA. The effects of yoga on anxiety and stress. Altern Med Rev. 2012;17:21-35.
17. Posadzki P, Ernst E. Yoga for asthma? A systematic review of randomized clinical trials. J Asthma. 2011;48:632-639.
18. Cramer H, Posadzki P, Dobos G, et al. Yoga for asthma: a systematic review and meta-analysis. Ann Allergy Asthma Immunol. 2014;112:503-510.e5.
19. Daley A, Stokes-Lampard H, Macarthur C. Exercise for vasomotor menopausal symptoms. Cochrane Database Syst Rev. 2011;(5):CD006108.
20. Chattha R, Nagarathna R, Padmalatha V, et al. Effect of yoga on cognitive functions in climacteric syndrome: a randomised control study. BJOG. 2008;115:991-1000.
21. Lee MS, Kim JI, Ha JY, et al. Yoga for menopausal symptoms: a systematic review. Menopause. 2009;16:602-608.
22. Cramer H, Lauche R, Langhorst J, et al. Effectiveness of yoga for menopausal symptoms: a systematic review and meta-analysis of randomized controlled trials. Evid Based Complement Alternat Med. 2012;2012:863905.
23. Newton KM, Reed SD, Guthrie KA, et al. Efficacy of yoga for vasomotor symptoms: a randomized controlled trial. Menopause. 2014;21:339-346.
24. Hagins M, States R, Selfe T, et al. Effectiveness of yoga for hypertension: systematic review and meta-analysis. Evid Based Complement Alternat Med. 2013;2013:649836.
25. Posadzki P, Cramer H, Kuzdzal A, et al. Yoga for hypertension: a systematic review of randomized clinical trials. Complement Ther Med. 2014;22:511-522.
26. Cramer H, Lauche R, Haller H, et al. Effects of yoga on cardiovascular disease risk factors: a systematic review and meta-analysis. Int J Cardiol. 2014;173:170-183.
27. Cohen DL, Bloedon LT, Rothman RL, et al. Iyengar yoga versus enhanced usual care on blood pressure in patients with prehypertension to stage I hypertension: a randomized controlled trial. Evid Based Complement Alternat Med. 2011;2011:546428.
28. Cohen DL, Bowler A, Fisher SA, et al. Lifestyle Modification in Blood Pressure Study II (LIMBS): study protocol of a randomized controlled trial assessing the efficacy of a 24 week structured yoga program versus lifestyle modification on blood pressure reduction. Contemp Clin Trials. 2013;36:32-40.
29. Hagins M, Rundle A, Consedine N, et al. A randomized controlled trial comparing the effects of yoga with an active control on ambulatory blood pressure in individuals with pre- and stage 1 hypertension. J Clin Hypertens (Greenwich). 2014;16:54-62.
30. Howe TE, Rochester L, Neil F, et al. Exercise for improving balance in older people. Cochrane Database Syst Rev. 2011;(11):CD004963.
31. Jeter PE, Nkodo AF, Moonaz SH, et al. A systematic review of yoga for balance in a healthy population. J Altern Complement Med. 2014;20:221-232.
32. Tiedemann A, O’Rourke S, Sesto R, et al. A 12-week Iyengar yoga program improved balance and mobility in older community-dwelling people: a pilot randomized controlled trial. J Gerontol A Biol Sci Med Sci. 2013;68:1068-1075.
33. Sinaki M. Yoga spinal flexion positions and vertebral compression fracture in osteopenia or osteoporosis of spine: case series. Pain Pract. 2013;13:68-75.
1. Yoga Journal. Yoga in America study 2012 [press release]. Santa Cruz, CA: Santa Cruz Bay Publishing; 2008. Available at: http://www.yogajournal.com/press/yoga_in_america. Accessed August 19, 2014.
2. Broad WJ. The Science of Yoga: The Risks and Rewards. New York, New York: Simon & Schuster; 2012.
3. Lamb T. Health Benefits of Yoga. International Association of Yoga Therapists Web site. Available at: http://www.iayt.org/?page=HealthBenefitsofYoga. Accessed August 21, 2014.
4. Yoga Alliance. 200-hour standards for yoga teacher trainings. Yoga Alliance Web site. Available at: http://yogaalliance.org/content/200-hour-standards. Accessed August 19, 2014.
5. Wren AA, Wright MA, Carson JW, et al. Yoga for persistent pain: New findings and directions for an ancient practice. Pain. 2011;152:477-480.
6. Chou R, Huffman LH; American Pain Society; American College of Physicians. Nonpharmacologic therapies for acute and chronic low back pain: a review of the evidence for an American Pain Society/American College of Physicians clinical practice guideline. Ann Intern Med. 2007;147:492-504.
7. Sherman KJ, Cherkin DC, Erro J, et al. Comparing yoga, exercise, and a self-care book for chronic low back pain: a randomized, controlled trial. Ann Intern Med. 2005;143:849-856.
8. Posadzki P, Ernst E. Yoga for low back pain: a systematic review of randomized clinical trials. Clin Rheumatol. 2011;30:1257-1262.
9. Graves N, Krepcho M, Mayo HG, et al. Does yoga speed healing for patients with low back pain? J Fam Pract. 2004;53:661-662.
10. Cramer H, Lauche R, Haller H, et al. A systematic review and meta-analysis of yoga for low back pain. Clin J Pain. 2013;29:450-460.
11. Skowronek IB, Mounsey A, Handler L. Can yoga reduce symptoms of anxiety and depression? J Fam Pract. 2013;63:398-399,407.
12. Cramer H, Lauche R, Langhorst J, et al. Yoga for depression: a systematic review and meta-analysis. Depress Anxiety. 2013;30: 1068-1083.
13. D’Silva S, Poscablo C, Habousha R, et al. Mind-body medicine therapies for a range of depression severity: a systematic review. Psychosomatics. 2012;53:407-423.
14. Balasubramaniam M, Telles S, Doraiswamy PM. Yoga on our minds: a systematic review of yoga for neuropsychiatric disorders. Front Psychiatry. 2013;3:117.
15. Kirkwood G, Rampes H, Tuffrey V, et al. Yoga for anxiety: a systematic review of the research evidence. Br J Sports Med. 2005;39: 884-891.
16. Li AW, Goldsmith CA. The effects of yoga on anxiety and stress. Altern Med Rev. 2012;17:21-35.
17. Posadzki P, Ernst E. Yoga for asthma? A systematic review of randomized clinical trials. J Asthma. 2011;48:632-639.
18. Cramer H, Posadzki P, Dobos G, et al. Yoga for asthma: a systematic review and meta-analysis. Ann Allergy Asthma Immunol. 2014;112:503-510.e5.
19. Daley A, Stokes-Lampard H, Macarthur C. Exercise for vasomotor menopausal symptoms. Cochrane Database Syst Rev. 2011;(5):CD006108.
20. Chattha R, Nagarathna R, Padmalatha V, et al. Effect of yoga on cognitive functions in climacteric syndrome: a randomised control study. BJOG. 2008;115:991-1000.
21. Lee MS, Kim JI, Ha JY, et al. Yoga for menopausal symptoms: a systematic review. Menopause. 2009;16:602-608.
22. Cramer H, Lauche R, Langhorst J, et al. Effectiveness of yoga for menopausal symptoms: a systematic review and meta-analysis of randomized controlled trials. Evid Based Complement Alternat Med. 2012;2012:863905.
23. Newton KM, Reed SD, Guthrie KA, et al. Efficacy of yoga for vasomotor symptoms: a randomized controlled trial. Menopause. 2014;21:339-346.
24. Hagins M, States R, Selfe T, et al. Effectiveness of yoga for hypertension: systematic review and meta-analysis. Evid Based Complement Alternat Med. 2013;2013:649836.
25. Posadzki P, Cramer H, Kuzdzal A, et al. Yoga for hypertension: a systematic review of randomized clinical trials. Complement Ther Med. 2014;22:511-522.
26. Cramer H, Lauche R, Haller H, et al. Effects of yoga on cardiovascular disease risk factors: a systematic review and meta-analysis. Int J Cardiol. 2014;173:170-183.
27. Cohen DL, Bloedon LT, Rothman RL, et al. Iyengar yoga versus enhanced usual care on blood pressure in patients with prehypertension to stage I hypertension: a randomized controlled trial. Evid Based Complement Alternat Med. 2011;2011:546428.
28. Cohen DL, Bowler A, Fisher SA, et al. Lifestyle Modification in Blood Pressure Study II (LIMBS): study protocol of a randomized controlled trial assessing the efficacy of a 24 week structured yoga program versus lifestyle modification on blood pressure reduction. Contemp Clin Trials. 2013;36:32-40.
29. Hagins M, Rundle A, Consedine N, et al. A randomized controlled trial comparing the effects of yoga with an active control on ambulatory blood pressure in individuals with pre- and stage 1 hypertension. J Clin Hypertens (Greenwich). 2014;16:54-62.
30. Howe TE, Rochester L, Neil F, et al. Exercise for improving balance in older people. Cochrane Database Syst Rev. 2011;(11):CD004963.
31. Jeter PE, Nkodo AF, Moonaz SH, et al. A systematic review of yoga for balance in a healthy population. J Altern Complement Med. 2014;20:221-232.
32. Tiedemann A, O’Rourke S, Sesto R, et al. A 12-week Iyengar yoga program improved balance and mobility in older community-dwelling people: a pilot randomized controlled trial. J Gerontol A Biol Sci Med Sci. 2013;68:1068-1075.
33. Sinaki M. Yoga spinal flexion positions and vertebral compression fracture in osteopenia or osteoporosis of spine: case series. Pain Pract. 2013;13:68-75.
Unhealthy drug use: How to screen, when to intervene
› Implement screening and brief intervention (SBI) for unhealthy drug use among adults in primary care. C
› Consult the National Institute on Drug Abuse’s Screening for Drug Use in General Medical Settings Resource Guide for step-by-step recommendations for implementing a drug SBI. C
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
CASE › Joe M, age 54, comes to your office for his annual physical examination. As part of your routine screening, you ask him, “In the past year, how often have you used alcohol, tobacco, prescription drugs for nonmedical reasons, or illegal drugs?” Mr. M replies that he does not use tobacco and has not used prescription drugs for nonmedical reasons, but drinks alcohol weekly and uses cannabis and cocaine monthly.
If Mr. M were your patient, what would your next steps be?
One promising approach to alleviate substance use problems is screening and brief intervention (SBI), and—when appropriate—referral to an addiction treatment program. With strong evidence of efficacy, alcohol and tobacco SBIs have become recommended “usual” care for adults in primary care settings.1,2 Strategies for applying SBI to unhealthy drug use (“drug” SBI) in primary care have been a natural extension of the evidence that supports alcohol and tobacco SBIs.
Screening for unhealthy drug use consists of a quick risk appraisal, typically via a brief questionnaire.3-5 Patients with a positive screen then receive a more detailed assessment to estimate the extent of their substance use and severity of its consequences. If appropriate, this is followed with a brief intervention (BI), which is a time-limited, patient-centered counseling session designed to reduce substance use and/or related harm.4-6
So how can you make use of a drug SBI in your office setting?
Drug screening: What the evidence says
Currently, evidence on drug SBI is limited. The US Preventive Services Task Force (USPSTF) found insufficient evidence to recommend for or against universal drug SBI.4,7,8 The scarcity of validated screening and assessment tools that are brief enough to be used in primary care, patients’ use of multiple drugs, and confidentiality concerns likely contribute to the relative lack of research in this area.3,6,9
To our knowledge, results of only 5 randomized controlled trials (RCTs) of drug SBI that included universal screening have been published in English. Here is what these researchers found:
Bernstein et al10 investigated the efficacy of SBI for cocaine and heroin use among 23,699 adults in urgent care, women’s health, and homeless clinic settings. They randomized 1175 patients who screened positive on the Drug Abuse Screening Test11 to receive a single BI session or a handout. At 6 months, patients in the BI group were 1.5 times more likely than controls to be abstinent from cocaine (22% vs 17%; P=.045) and heroin (40% vs 31%; P=.050).
Zahradnik et al12 examined the efficacy of SBI in reducing the use of potentially addictive prescription drugs by hospitalized patients. After researchers screened 6000 inpatients, 126 patients who used, abused, or were dependent on prescription medications were randomized to receive 2 BI sessions or an information booklet. At 3 months, 52% of patients in the BI group had a ≥25% reduction in their daily doses of prescription drugs, compared to 30% in the control group (P=.017),12 However, this difference was not maintained at 12 months.13
Humeniuk et al14 evaluated the efficacy of SBI among primary care patients ages 16 to 62 years in Australia, Brazil, India, and the United States who used cannabis, cocaine, amphetamines, or opioids. Patients were screened and assessed using the World Health Organization Alcohol, Smoking, and Substance Involvement Screening Test (ASSIST).15 Patients whose scores indicated they had a moderate risk for problem use (N=731) were randomly assigned to receive a BI or usual care. At 3 months, patients in the BI group reported a reduction in total score for “illicit substance involvement” compared to controls (P<.001). However, country-specific analyses found that BI did not have a statistically significant effect on drug use by those in the United States (N=218), possibly due to protocol differences and a greater exposure to previous substance use treatment among US patients.14
Saitz et al16 investigated the efficacy of drug SBI among primary care patients (N=528) who had been screened using the ASSIST. The most commonly used drugs were marijuana (63% of patients), cocaine (19%), and opioids (17%). Patients were randomly assigned to a 10- to 15-minute BI, a 30- to 45-minute intervention, or no intervention. BI did not show efficacy for decreasing drug use at 6-month follow-up.
Roy-Byrne et al17 screened 10,337 primary care patients of “safety net” clinics serving low-income populations. Of 1621 patients who screened positive for problem drug use, 868 were enrolled and randomly assigned to either a BI group (one-time BI using motivational interviewing, a telephone booster session, and a handout, which included relevant drug-use related information and a list of substance abuse resources) or enhanced care as usual (usual care plus a handout). Over 12 months of follow-up, there were no differences between groups in drug use or related consequences. However, a subgroup analysis suggested that compared to enhanced usual care, BI may help reduce emergency department use and increase admissions to specialized drug treatment programs among those with severe drug problems.
In addition to these 5 RCTs, a large, prospective, uncontrolled trial looked at the efficacy of drug BI among 459,599 patients from various medical settings, including primary care.18 Twenty-three percent of patients screened positive for illicit drug use and were recommended BI (16%), brief treatment (3%) or specialty treatment (4%). At a 6-month follow-up, drug use among these patients decreased by 68% and heavy alcohol use decreased by 39% (P<.001). In addition, general health, mental health, employment, housing status, and criminal behavior improved among patients recommended for brief or specialty treatments (P<.001). Although this trial lent support for the efficacy of drug SBI in primary care, it was limited by the lack of a control group and low follow-up rates at some sites.
A step-by-step approach to drug screening
Although a variety of instruments can be used to screen and assess patients for unhealthy drug use, few have been validated in primary care (TABLE 1).11,15,19-27 Despite limited evidence, multiple professional organizations, including the American Academy of Family Physicians28 and the American Psychiatric Association,26 support routine implementation of drug SBI in primary care.
The National Institute on Drug Abuse (NIDA)’s Screening for Drug Use in General Medical Settings Resource Guide19 provides a step-by-step approach to drug SBI in primary care and other general medical settings. Primarily focused on drug SBI in adults, the NIDA guide details the use of the NIDA Quick Screen and the NIDA-Modified ASSIST (NM ASSIST). These tools are available as a PDF that you can print out and complete manually (http://www.drugabuse.gov/sites/default/ files/pdf/nmassist.pdf) or as a series of forms you can complete online (http://www.drugabuse.gov/nmassist). The NIDA guide also conveniently incorporates links to alcohol and tobacco SBI recommendations.
What to ask first. Following the NIDA algorithm, first screen patients with the Quick Screen, which consists of a single question about substance use: “In the past year, how often have you used alcohol, tobacco products, prescription drugs for nonmedical reasons, or illegal drugs?" (TABLE 2).19,29-32
A negative Quick Screen (a “never” response for all substances) completes the process. Patients with a negative screen should be praised and encouraged to continue their healthy lifestyle, then rescreened annually.
For patients who respond “Yes” to heavy drinking or any tobacco use, the NIDA guide recommends proceeding with an alcohol29 or tobacco30 SBI, respectively, and provides links to appropriate resources (TABLE 2).19,29-32 Those who screen positive for drugs (“Yes” to any drug use in the past year) should receive a detailed assessment using the NM ASSIST32 to determine their risk level for developing a substance use disorder. The NM ASSIST includes 8 questions about the patient’s desire for, use of, and problems related to the use of a wide range of drugs, including cannabis, cocaine, methamphetamine, hallucinogens, and other substances (eg, “In the past 3 months, how often have you used the following substances?” “How often have you had a strong desire or urge to use this substance?” “How often has your use of this substance led to health, social, legal or financial problems?”). The score on the NM ASSIST is used to calculate the patient’s risk level as low, moderate, or high.
For patients who use more than one drug, this risk level is scored separately for each drug and may differ from drug to drug. Multi-drug assessment can become time-consuming and may not be appropriate in some patients, especially if time is an issue (eg, the patient would like to focus on other concerns) or the patient is not interested in addressing certain drugs. In general, the decision about which substances to address should be clinically-driven, tailored to the needs of an individual patient. Focusing on the substance with the highest risk score or associated with the patient’s expressed greatest motivation to change may produce the best results.
CASE › Based on Mr. M’s response to your Quick Screen indicating he drinks alcohol and uses illicit drugs, you administer the NM ASSIST to perform a detailed assessment. His answer to a screening question for problematic alcohol use is negative (In the past year, he has not had >4 drinks in a day). Next, you calculate his NM ASSIST-based risk scores for cannabis and cocaine, and determine he is at moderate risk for developing problems due to cannabis use and at high risk for developing problems based on his use of cocaine. You also note Mr. M’s blood pressure (BP) is elevated (155/90 mm hg).
Conducting a brief intervention
Depending on the patient’s risk level for developing a substance use disorder, he or she should receive either brief advice (for those at low risk) or a BI (for those at moderate or high risk) and, if needed, a referral to treatment. Two popular approaches are FRAMES (Feedback, Responsibility, Advice, Menu of Strategies, Empathy, Self-efficacy) and the NIDA-recommended 5 As intervention. The latter approach entails Asking the patient about his drug use (via the Quick Screen); Advising the patient about his drug use by providing specific medical advice on why he should stop or cut down, and how; Assessing the patient’s readiness to quit or reduce use; Assisting the patient in making a change by creating a plan with specific goals; and Arranging a follow-up visit or specialty assessment and treatment by making referrals as appropriate.19
What about children and adolescents? Implementing a drug SBI in young patients often entails overcoming unique challenges and ethical dilemmas. Although the American Academy of Pediatrics recommends SBI for unhealthy drug and alcohol use among children and adolescents,33,34 the USPSTF did not find sufficient evidence to recommend the practice.1,8,35 Screening for drug use in minors often is complicated by questions about the age at which to start routine screening and issues related to confidentiality and parental involvement. The Center for Adolescent Health and the Law and the National Institute on Alcohol Abuse and Alcoholism provide useful resources related to youth SBI, including guidance on when to consider breeching a child’s confidentiality by engaging parents or guardians (TABLE 3).
TABLE 3
Resources
NIDA Resource Guide NIDA-Modified ASSIST Coding for SBI reimbursement SAMHSA’s Treatment Services Locator NIDA’s List of Community Treatment Programs SAMHSA Opioid Overdose Toolkit Buprenorphine training program Center for Adolescent Health and the Law NIAAA Alcohol Screening and Brief Intervention for Youth |
Help is available for securing treatment, reimbursement
In addition to NIDA, many other organizations offer resources to assist clinicians in using drug SBI and helping patients obtain treatment (TABLE 3). For reimbursement, the Centers for Medicare and Medicaid Services has adopted billing codes for SBI services.36,37 The Substance Abuse and Mental Health Services Administration (SAMHSA)’s Behavioral Health Treatment Services Locator and NIDA’s National Drug Abuse Treatment Clinical Trials Network List of Associated Community Treatment Programs can assist clinicians and patients in finding specialty treatment programs. Self-help groups such as Narcotics Anonymous, Alcoholic Anonymous, or Self-Managment and Recovery Training may help alleviate problems related to insurance coverage, location, and/or timing of services.
SAMHSA’s Opioid Overdose Toolkit provides guidance to clinicians and patients on ways to reduce the risk of overdose. Physicians also can complete a short training program in office-based buprenorphine maintenance therapy to provide evidence-based care to patients with opioid dependence; more details about this program are available from http://www.buppractice.com.
CASE › You decide to use the 5 as intervention with Mr. M. You explain to him that he is at high risk of developing a substance use disorder. You also discuss his elevated BP and the possible negative effects of drug use, especially cocaine, on BP. You advise him that medically it is in his best interest to stop using cocaine and stop or reduce using cannabis. When you ask Mr. M about his readiness to change his drug use, he expresses moderate interest in stopping cocaine but is not willing to reduce his cannabis use. At this time, he is not willing to discuss these issues further (“I’ll think about that”) or create a specific plan. You assure him of your ongoing support, provide him with resources on specialty treatment programs should he wish to consider those, and schedule a follow-up visit in 2 weeks to address BP and, if the patient agrees, drug use.
CORRESPONDENCE
Aleksandra Zgierska, MD, Phd, Department of Family Medicine, University of Wisconsin School of Medicine and Public Health, 1100 Delaplaine Court, Madison, WI 53715-1896; [email protected]
1. US Preventive Services Task Force. Screening and behavioral counseling interventions in primary care to reduce alcohol misuse. US Preventive Services Task Force Web site. Available at: http://www.uspreventiveservicestaskforce.org/uspstf/ uspsdrin.htm. Accessed March 4, 2013.
2. US Preventive Services Task Force. Counseling and interventions to prevent tobacco use and tobacco-caused disease in adults and pregnant women. US Preventive Services Task Force Web site. Available at: http://www.uspreventiveservicestaskforce.org/uspstf/uspstbac2.htm. Accessed March 4, 2014.
3. Saitz R, Alford DP, Bernstein J, et al. Screening and brief intervention for unhealthy drug use in primary care settings: randomized clinical trials are needed. J Addict Med. 2010;4: 123-130.
4. Pilowsky DJ, Wu LT. Screening for alcohol and drug use disorders among adults in primary care: a review. Subst Abuse Rehabil. 2012;3:25-34.
5. Substance Abuse and Mental Health Services Administration. Screening, Brief Intervention, and Referral to Treatment (SBIRT). Substance Abuse and Mental Health Services Administration Web site. Available at: http://www.samhsa.gov/ prevention/sbirt/. Accessed March 4, 2014.
6. Squires LE, Alford DP, Bernstein J, et al. Clinical case discussion: screening and brief intervention for drug use in primary care. J Addict Med. 2010;4:131-136.
7. Krupski A, Joesch JM, Dunn C, et al. Testing the effects of brief intervention in primary care for problem drug use in a randomized controlled trial: rationale, design, and methods. Addict Sci Clin Pract. 2012;7:27.
8. US Preventive Services Task Force. Screening for illicit drug use. US Preventive Services Task Force Web site. Available at: http://www.uspreventiveservicestaskforce.org/uspstf/uspsdrug.htm. Accessed March 4, 2014.
9. Lanier D, Ko S. Screening in Primary Care Settings for Illicit Drug Use: Assessment of Screening Instruments—A Supplemental Evidence Update for the U.S. Preventive Services Task Force. AHRQ Publication No. 08-05108-EF-2. Rockville, MD: Agency for Healthcare Research and Quality; 2008.
10. Bernstein J, Bernstein E, Tassiopoulos K, et al. Brief motivational intervention at a clinic visit reduces cocaine and heroin use. Drug Alcohol Depend. 2005;77:49-59.
11. Skinner HA. The drug abuse screening test. Addict Behav. 1982;7:363-371.
12. Zahradnik A, Otto C, Crackau B, et al. Randomized controlled trial of a brief intervention for problematic prescription drug use in non-treatment-seeking patients. Addiction. 2009;104:109-117.
13. Otto C, Crackau B, Löhrmann I, et al. Brief intervention in general hospital for problematic prescription drug use: 12-month outcome. Drug Alcohol Depend. 2009;105:221-226.
14. Humeniuk R, Ali R, Babor T, et al. A randomized controlled trial of a brief intervention for illicit drugs linked to the Alcohol, Smoking and Substance Involvement Screening Test (ASSIST) in clients recruited from primary health-care settings in four countries. Addiction. 2012;107:957-966.
15. WHO ASSIST Working Group. The Alcohol, Smoking and Substance Involvement Screening Test (ASSIST): development, reliability and feasibility. Addiction. 2002;97:1183-1194.
16. Saitz R, Palfai TP, Cheng DM, et al. Screening and brief intervention for drug use in primary care: the Assessing Screening Plus brief Intervention’s Resulting Efficacy to stop drug use (ASPIRE) randomized trial. Addict Sci Clin Pract. 2013;8(suppl 1):A61.
17. Roy-Byrne P, Bumgardner K, Krupski A, et al. Brief intervention for problem drug use in safety-net primary care settings: a randomized clinical trial. JAMA. 2014;312(5):492-501.
18. Madras BK, Compton WM, Avula D, et al. Screening, brief interventions, referral to treatment (SBIRT) for illicit drug and alcohol use at multiple healthcare sites: comparison at intake and 6 months later. Drug Alcohol Depend. 2009;99:280-295.
19. National Institute on Drug Abuse. Resource guide: Screening for drug use in general medical settings. National Institute on Drug Abuse Web site. Available at: http://www.drugabuse. gov/publications/resource-guide. Accessed March 8, 2014.
20. Saitz R, Cheng DM, Allensworth-Davies D, et al. The ability of single screening questions for unhealthy alcohol and other drug use to identify substance dependence in primary care. J Stud Alcohol Drugs. 2014;75:153-157.
21. Newcombe DA, Humeniuk RE, Ali R. Validation of the World Health Organization Alcohol, Smoking and Substance Involvement Screening Test (ASSIST): report of results from the Australian site. Drug Alcohol Rev. 2005;24:217-226.
22. Humeniuk R, Ali R, Babor TF, et al. Validation of the Alcohol, Smoking And Substance Involvement Screening Test (ASSIST). Addiction. 2008;103:1039-1047.
23. Mdege ND, Lang J. Screening instruments for detecting illicit drug use/abuse that could be useful in general hospital wards: a systematic review. Addict Behav. 2011;36:1111-1119.
24. Cassidy CM, Schmitz N, Malla A. Validation of the alcohol use disorders identification test and the drug abuse screening test in first episode psychosis. Can J Psychiatry. 2008;53:26-33.
25. Brown RL, Rounds LA. Conjoint screening questionnaires for alcohol and other drug abuse: criterion validity in a primary care practice. Wis Med J. 1995;94:135-140.
26. American Psychiatric Association. Position statement on substance use disorders. American Psychiatric Association Web site. Available at: http://www.psychiatry.org/File%20Library/Advocacy%20and%20Newsroom/Position%20Statements/ps2012_Substance.pdf. Accessed March 4, 2014.
27. Smith PC, Schmidt SM, Allensworth-Davies D, et al. A single-question screening test for drug use in primary care. Arch Intern Med. 2010;170:1155-1160.
28. American Academy of Family Physicians. Substance abuse and addiction. American Academy of Family Physicians Web site. Available at: http://www.aafp.org/about/policies/all/substance-abuse.html. Accessed March 4, 2014.
29. National Institute on Alcohol Abuse and Alcoholism. Helping patients who drink too much: A clinician’s guide. National Institute on Alcohol Abuse and Alcoholism Web site. Available at: http://pubs.niaaa.nih.gov/publications/Practitioner/CliniciansGuide2005/clinicians_guide.htm. Accessed March 4, 2014.
30. US Department of Health and Human Services Public Health Service. Helping smokers quit: A guide for clinicians. US Department of Health and Human Services Public Health Service Web site. Available at: http://www.ahrq.gov/professionals/clinicians-providers/guidelines-recommendations/tobacco/clinicians//clinhlpsmkqt/. Accessed March 4, 2014.
31. National Institute on Alcohol Abuse and Alcoholism. A Pocket Guide for Alcohol Screening and Brief Intervention. National Institute on Alcohol Abuse and Alcoholism Web site. Available at: http://pubs.niaaa.nih.gov/publications/Practitioner/pocketguide/pocket_guide.htm. Accessed July 30, 2014.
32. National Institute on Drug Abuse. NIDA-Quick Screen V1.0. National Institute on Drug Abuse Web site. Available at: http://www.drugabuse.gov/sites/default/files/pdf/nmassist.pdf. Accessed March 4, 2014.
33. Committee on Substance Abuse, Levy SJ, Kokotailo PK. Substance use screening, brief intervention, and referral to treatment for pediatricians. Pediatrics. 2011;128:e1330-e1340.
34. Kulig JW; American Academy of Pediatrics Committee on Substance Abuse. Tobacco, alcohol, and other drugs: the role of the pediatrician in prevention, identification, and management of substance abuse. Pediatrics. 2005;115:816-821.
35. US Preventive Services Task Force. Primary care behavioral interventions to reduce the nonmedical use of drugs in children and adolescents. US Preventive Services Task Force Web site. Available at: http://www.uspreventiveservicestaskforce.org/uspstf/uspsnonmed.htm. Accessed March 4, 2014.
36. Centers for Medicare & Medicaid Services. Screening, Brief Intervention, and Referral to Treatment (SBIRT) services. Centers for Medicare & Medicaid Services Web site. Available at: http://www.cms.gov/Outreach-and-Education/Medicare-Learning-Network-MLN/MLNProducts/downloads/sbirt_factsheet_icn904084.pdf. Accessed March 4, 2014.
37. Substance Abuse and Mental Health Services Administration. Coding for screening and brief intervention reimbursement. Substance Abuse and Mental Health Services Administration Web site. Available at: http://beta.samhsa.gov/sbirt/coding-reimbursement. Accessed August 4, 2014.
› Implement screening and brief intervention (SBI) for unhealthy drug use among adults in primary care. C
› Consult the National Institute on Drug Abuse’s Screening for Drug Use in General Medical Settings Resource Guide for step-by-step recommendations for implementing a drug SBI. C
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
CASE › Joe M, age 54, comes to your office for his annual physical examination. As part of your routine screening, you ask him, “In the past year, how often have you used alcohol, tobacco, prescription drugs for nonmedical reasons, or illegal drugs?” Mr. M replies that he does not use tobacco and has not used prescription drugs for nonmedical reasons, but drinks alcohol weekly and uses cannabis and cocaine monthly.
If Mr. M were your patient, what would your next steps be?
One promising approach to alleviate substance use problems is screening and brief intervention (SBI), and—when appropriate—referral to an addiction treatment program. With strong evidence of efficacy, alcohol and tobacco SBIs have become recommended “usual” care for adults in primary care settings.1,2 Strategies for applying SBI to unhealthy drug use (“drug” SBI) in primary care have been a natural extension of the evidence that supports alcohol and tobacco SBIs.
Screening for unhealthy drug use consists of a quick risk appraisal, typically via a brief questionnaire.3-5 Patients with a positive screen then receive a more detailed assessment to estimate the extent of their substance use and severity of its consequences. If appropriate, this is followed with a brief intervention (BI), which is a time-limited, patient-centered counseling session designed to reduce substance use and/or related harm.4-6
So how can you make use of a drug SBI in your office setting?
Drug screening: What the evidence says
Currently, evidence on drug SBI is limited. The US Preventive Services Task Force (USPSTF) found insufficient evidence to recommend for or against universal drug SBI.4,7,8 The scarcity of validated screening and assessment tools that are brief enough to be used in primary care, patients’ use of multiple drugs, and confidentiality concerns likely contribute to the relative lack of research in this area.3,6,9
To our knowledge, results of only 5 randomized controlled trials (RCTs) of drug SBI that included universal screening have been published in English. Here is what these researchers found:
Bernstein et al10 investigated the efficacy of SBI for cocaine and heroin use among 23,699 adults in urgent care, women’s health, and homeless clinic settings. They randomized 1175 patients who screened positive on the Drug Abuse Screening Test11 to receive a single BI session or a handout. At 6 months, patients in the BI group were 1.5 times more likely than controls to be abstinent from cocaine (22% vs 17%; P=.045) and heroin (40% vs 31%; P=.050).
Zahradnik et al12 examined the efficacy of SBI in reducing the use of potentially addictive prescription drugs by hospitalized patients. After researchers screened 6000 inpatients, 126 patients who used, abused, or were dependent on prescription medications were randomized to receive 2 BI sessions or an information booklet. At 3 months, 52% of patients in the BI group had a ≥25% reduction in their daily doses of prescription drugs, compared to 30% in the control group (P=.017),12 However, this difference was not maintained at 12 months.13
Humeniuk et al14 evaluated the efficacy of SBI among primary care patients ages 16 to 62 years in Australia, Brazil, India, and the United States who used cannabis, cocaine, amphetamines, or opioids. Patients were screened and assessed using the World Health Organization Alcohol, Smoking, and Substance Involvement Screening Test (ASSIST).15 Patients whose scores indicated they had a moderate risk for problem use (N=731) were randomly assigned to receive a BI or usual care. At 3 months, patients in the BI group reported a reduction in total score for “illicit substance involvement” compared to controls (P<.001). However, country-specific analyses found that BI did not have a statistically significant effect on drug use by those in the United States (N=218), possibly due to protocol differences and a greater exposure to previous substance use treatment among US patients.14
Saitz et al16 investigated the efficacy of drug SBI among primary care patients (N=528) who had been screened using the ASSIST. The most commonly used drugs were marijuana (63% of patients), cocaine (19%), and opioids (17%). Patients were randomly assigned to a 10- to 15-minute BI, a 30- to 45-minute intervention, or no intervention. BI did not show efficacy for decreasing drug use at 6-month follow-up.
Roy-Byrne et al17 screened 10,337 primary care patients of “safety net” clinics serving low-income populations. Of 1621 patients who screened positive for problem drug use, 868 were enrolled and randomly assigned to either a BI group (one-time BI using motivational interviewing, a telephone booster session, and a handout, which included relevant drug-use related information and a list of substance abuse resources) or enhanced care as usual (usual care plus a handout). Over 12 months of follow-up, there were no differences between groups in drug use or related consequences. However, a subgroup analysis suggested that compared to enhanced usual care, BI may help reduce emergency department use and increase admissions to specialized drug treatment programs among those with severe drug problems.
In addition to these 5 RCTs, a large, prospective, uncontrolled trial looked at the efficacy of drug BI among 459,599 patients from various medical settings, including primary care.18 Twenty-three percent of patients screened positive for illicit drug use and were recommended BI (16%), brief treatment (3%) or specialty treatment (4%). At a 6-month follow-up, drug use among these patients decreased by 68% and heavy alcohol use decreased by 39% (P<.001). In addition, general health, mental health, employment, housing status, and criminal behavior improved among patients recommended for brief or specialty treatments (P<.001). Although this trial lent support for the efficacy of drug SBI in primary care, it was limited by the lack of a control group and low follow-up rates at some sites.
A step-by-step approach to drug screening
Although a variety of instruments can be used to screen and assess patients for unhealthy drug use, few have been validated in primary care (TABLE 1).11,15,19-27 Despite limited evidence, multiple professional organizations, including the American Academy of Family Physicians28 and the American Psychiatric Association,26 support routine implementation of drug SBI in primary care.
The National Institute on Drug Abuse (NIDA)’s Screening for Drug Use in General Medical Settings Resource Guide19 provides a step-by-step approach to drug SBI in primary care and other general medical settings. Primarily focused on drug SBI in adults, the NIDA guide details the use of the NIDA Quick Screen and the NIDA-Modified ASSIST (NM ASSIST). These tools are available as a PDF that you can print out and complete manually (http://www.drugabuse.gov/sites/default/ files/pdf/nmassist.pdf) or as a series of forms you can complete online (http://www.drugabuse.gov/nmassist). The NIDA guide also conveniently incorporates links to alcohol and tobacco SBI recommendations.
What to ask first. Following the NIDA algorithm, first screen patients with the Quick Screen, which consists of a single question about substance use: “In the past year, how often have you used alcohol, tobacco products, prescription drugs for nonmedical reasons, or illegal drugs?" (TABLE 2).19,29-32
A negative Quick Screen (a “never” response for all substances) completes the process. Patients with a negative screen should be praised and encouraged to continue their healthy lifestyle, then rescreened annually.
For patients who respond “Yes” to heavy drinking or any tobacco use, the NIDA guide recommends proceeding with an alcohol29 or tobacco30 SBI, respectively, and provides links to appropriate resources (TABLE 2).19,29-32 Those who screen positive for drugs (“Yes” to any drug use in the past year) should receive a detailed assessment using the NM ASSIST32 to determine their risk level for developing a substance use disorder. The NM ASSIST includes 8 questions about the patient’s desire for, use of, and problems related to the use of a wide range of drugs, including cannabis, cocaine, methamphetamine, hallucinogens, and other substances (eg, “In the past 3 months, how often have you used the following substances?” “How often have you had a strong desire or urge to use this substance?” “How often has your use of this substance led to health, social, legal or financial problems?”). The score on the NM ASSIST is used to calculate the patient’s risk level as low, moderate, or high.
For patients who use more than one drug, this risk level is scored separately for each drug and may differ from drug to drug. Multi-drug assessment can become time-consuming and may not be appropriate in some patients, especially if time is an issue (eg, the patient would like to focus on other concerns) or the patient is not interested in addressing certain drugs. In general, the decision about which substances to address should be clinically-driven, tailored to the needs of an individual patient. Focusing on the substance with the highest risk score or associated with the patient’s expressed greatest motivation to change may produce the best results.
CASE › Based on Mr. M’s response to your Quick Screen indicating he drinks alcohol and uses illicit drugs, you administer the NM ASSIST to perform a detailed assessment. His answer to a screening question for problematic alcohol use is negative (In the past year, he has not had >4 drinks in a day). Next, you calculate his NM ASSIST-based risk scores for cannabis and cocaine, and determine he is at moderate risk for developing problems due to cannabis use and at high risk for developing problems based on his use of cocaine. You also note Mr. M’s blood pressure (BP) is elevated (155/90 mm hg).
Conducting a brief intervention
Depending on the patient’s risk level for developing a substance use disorder, he or she should receive either brief advice (for those at low risk) or a BI (for those at moderate or high risk) and, if needed, a referral to treatment. Two popular approaches are FRAMES (Feedback, Responsibility, Advice, Menu of Strategies, Empathy, Self-efficacy) and the NIDA-recommended 5 As intervention. The latter approach entails Asking the patient about his drug use (via the Quick Screen); Advising the patient about his drug use by providing specific medical advice on why he should stop or cut down, and how; Assessing the patient’s readiness to quit or reduce use; Assisting the patient in making a change by creating a plan with specific goals; and Arranging a follow-up visit or specialty assessment and treatment by making referrals as appropriate.19
What about children and adolescents? Implementing a drug SBI in young patients often entails overcoming unique challenges and ethical dilemmas. Although the American Academy of Pediatrics recommends SBI for unhealthy drug and alcohol use among children and adolescents,33,34 the USPSTF did not find sufficient evidence to recommend the practice.1,8,35 Screening for drug use in minors often is complicated by questions about the age at which to start routine screening and issues related to confidentiality and parental involvement. The Center for Adolescent Health and the Law and the National Institute on Alcohol Abuse and Alcoholism provide useful resources related to youth SBI, including guidance on when to consider breeching a child’s confidentiality by engaging parents or guardians (TABLE 3).
TABLE 3
Resources
NIDA Resource Guide NIDA-Modified ASSIST Coding for SBI reimbursement SAMHSA’s Treatment Services Locator NIDA’s List of Community Treatment Programs SAMHSA Opioid Overdose Toolkit Buprenorphine training program Center for Adolescent Health and the Law NIAAA Alcohol Screening and Brief Intervention for Youth |
Help is available for securing treatment, reimbursement
In addition to NIDA, many other organizations offer resources to assist clinicians in using drug SBI and helping patients obtain treatment (TABLE 3). For reimbursement, the Centers for Medicare and Medicaid Services has adopted billing codes for SBI services.36,37 The Substance Abuse and Mental Health Services Administration (SAMHSA)’s Behavioral Health Treatment Services Locator and NIDA’s National Drug Abuse Treatment Clinical Trials Network List of Associated Community Treatment Programs can assist clinicians and patients in finding specialty treatment programs. Self-help groups such as Narcotics Anonymous, Alcoholic Anonymous, or Self-Managment and Recovery Training may help alleviate problems related to insurance coverage, location, and/or timing of services.
SAMHSA’s Opioid Overdose Toolkit provides guidance to clinicians and patients on ways to reduce the risk of overdose. Physicians also can complete a short training program in office-based buprenorphine maintenance therapy to provide evidence-based care to patients with opioid dependence; more details about this program are available from http://www.buppractice.com.
CASE › You decide to use the 5 as intervention with Mr. M. You explain to him that he is at high risk of developing a substance use disorder. You also discuss his elevated BP and the possible negative effects of drug use, especially cocaine, on BP. You advise him that medically it is in his best interest to stop using cocaine and stop or reduce using cannabis. When you ask Mr. M about his readiness to change his drug use, he expresses moderate interest in stopping cocaine but is not willing to reduce his cannabis use. At this time, he is not willing to discuss these issues further (“I’ll think about that”) or create a specific plan. You assure him of your ongoing support, provide him with resources on specialty treatment programs should he wish to consider those, and schedule a follow-up visit in 2 weeks to address BP and, if the patient agrees, drug use.
CORRESPONDENCE
Aleksandra Zgierska, MD, Phd, Department of Family Medicine, University of Wisconsin School of Medicine and Public Health, 1100 Delaplaine Court, Madison, WI 53715-1896; [email protected]
› Implement screening and brief intervention (SBI) for unhealthy drug use among adults in primary care. C
› Consult the National Institute on Drug Abuse’s Screening for Drug Use in General Medical Settings Resource Guide for step-by-step recommendations for implementing a drug SBI. C
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
CASE › Joe M, age 54, comes to your office for his annual physical examination. As part of your routine screening, you ask him, “In the past year, how often have you used alcohol, tobacco, prescription drugs for nonmedical reasons, or illegal drugs?” Mr. M replies that he does not use tobacco and has not used prescription drugs for nonmedical reasons, but drinks alcohol weekly and uses cannabis and cocaine monthly.
If Mr. M were your patient, what would your next steps be?
One promising approach to alleviate substance use problems is screening and brief intervention (SBI), and—when appropriate—referral to an addiction treatment program. With strong evidence of efficacy, alcohol and tobacco SBIs have become recommended “usual” care for adults in primary care settings.1,2 Strategies for applying SBI to unhealthy drug use (“drug” SBI) in primary care have been a natural extension of the evidence that supports alcohol and tobacco SBIs.
Screening for unhealthy drug use consists of a quick risk appraisal, typically via a brief questionnaire.3-5 Patients with a positive screen then receive a more detailed assessment to estimate the extent of their substance use and severity of its consequences. If appropriate, this is followed with a brief intervention (BI), which is a time-limited, patient-centered counseling session designed to reduce substance use and/or related harm.4-6
So how can you make use of a drug SBI in your office setting?
Drug screening: What the evidence says
Currently, evidence on drug SBI is limited. The US Preventive Services Task Force (USPSTF) found insufficient evidence to recommend for or against universal drug SBI.4,7,8 The scarcity of validated screening and assessment tools that are brief enough to be used in primary care, patients’ use of multiple drugs, and confidentiality concerns likely contribute to the relative lack of research in this area.3,6,9
To our knowledge, results of only 5 randomized controlled trials (RCTs) of drug SBI that included universal screening have been published in English. Here is what these researchers found:
Bernstein et al10 investigated the efficacy of SBI for cocaine and heroin use among 23,699 adults in urgent care, women’s health, and homeless clinic settings. They randomized 1175 patients who screened positive on the Drug Abuse Screening Test11 to receive a single BI session or a handout. At 6 months, patients in the BI group were 1.5 times more likely than controls to be abstinent from cocaine (22% vs 17%; P=.045) and heroin (40% vs 31%; P=.050).
Zahradnik et al12 examined the efficacy of SBI in reducing the use of potentially addictive prescription drugs by hospitalized patients. After researchers screened 6000 inpatients, 126 patients who used, abused, or were dependent on prescription medications were randomized to receive 2 BI sessions or an information booklet. At 3 months, 52% of patients in the BI group had a ≥25% reduction in their daily doses of prescription drugs, compared to 30% in the control group (P=.017),12 However, this difference was not maintained at 12 months.13
Humeniuk et al14 evaluated the efficacy of SBI among primary care patients ages 16 to 62 years in Australia, Brazil, India, and the United States who used cannabis, cocaine, amphetamines, or opioids. Patients were screened and assessed using the World Health Organization Alcohol, Smoking, and Substance Involvement Screening Test (ASSIST).15 Patients whose scores indicated they had a moderate risk for problem use (N=731) were randomly assigned to receive a BI or usual care. At 3 months, patients in the BI group reported a reduction in total score for “illicit substance involvement” compared to controls (P<.001). However, country-specific analyses found that BI did not have a statistically significant effect on drug use by those in the United States (N=218), possibly due to protocol differences and a greater exposure to previous substance use treatment among US patients.14
Saitz et al16 investigated the efficacy of drug SBI among primary care patients (N=528) who had been screened using the ASSIST. The most commonly used drugs were marijuana (63% of patients), cocaine (19%), and opioids (17%). Patients were randomly assigned to a 10- to 15-minute BI, a 30- to 45-minute intervention, or no intervention. BI did not show efficacy for decreasing drug use at 6-month follow-up.
Roy-Byrne et al17 screened 10,337 primary care patients of “safety net” clinics serving low-income populations. Of 1621 patients who screened positive for problem drug use, 868 were enrolled and randomly assigned to either a BI group (one-time BI using motivational interviewing, a telephone booster session, and a handout, which included relevant drug-use related information and a list of substance abuse resources) or enhanced care as usual (usual care plus a handout). Over 12 months of follow-up, there were no differences between groups in drug use or related consequences. However, a subgroup analysis suggested that compared to enhanced usual care, BI may help reduce emergency department use and increase admissions to specialized drug treatment programs among those with severe drug problems.
In addition to these 5 RCTs, a large, prospective, uncontrolled trial looked at the efficacy of drug BI among 459,599 patients from various medical settings, including primary care.18 Twenty-three percent of patients screened positive for illicit drug use and were recommended BI (16%), brief treatment (3%) or specialty treatment (4%). At a 6-month follow-up, drug use among these patients decreased by 68% and heavy alcohol use decreased by 39% (P<.001). In addition, general health, mental health, employment, housing status, and criminal behavior improved among patients recommended for brief or specialty treatments (P<.001). Although this trial lent support for the efficacy of drug SBI in primary care, it was limited by the lack of a control group and low follow-up rates at some sites.
A step-by-step approach to drug screening
Although a variety of instruments can be used to screen and assess patients for unhealthy drug use, few have been validated in primary care (TABLE 1).11,15,19-27 Despite limited evidence, multiple professional organizations, including the American Academy of Family Physicians28 and the American Psychiatric Association,26 support routine implementation of drug SBI in primary care.
The National Institute on Drug Abuse (NIDA)’s Screening for Drug Use in General Medical Settings Resource Guide19 provides a step-by-step approach to drug SBI in primary care and other general medical settings. Primarily focused on drug SBI in adults, the NIDA guide details the use of the NIDA Quick Screen and the NIDA-Modified ASSIST (NM ASSIST). These tools are available as a PDF that you can print out and complete manually (http://www.drugabuse.gov/sites/default/ files/pdf/nmassist.pdf) or as a series of forms you can complete online (http://www.drugabuse.gov/nmassist). The NIDA guide also conveniently incorporates links to alcohol and tobacco SBI recommendations.
What to ask first. Following the NIDA algorithm, first screen patients with the Quick Screen, which consists of a single question about substance use: “In the past year, how often have you used alcohol, tobacco products, prescription drugs for nonmedical reasons, or illegal drugs?" (TABLE 2).19,29-32
A negative Quick Screen (a “never” response for all substances) completes the process. Patients with a negative screen should be praised and encouraged to continue their healthy lifestyle, then rescreened annually.
For patients who respond “Yes” to heavy drinking or any tobacco use, the NIDA guide recommends proceeding with an alcohol29 or tobacco30 SBI, respectively, and provides links to appropriate resources (TABLE 2).19,29-32 Those who screen positive for drugs (“Yes” to any drug use in the past year) should receive a detailed assessment using the NM ASSIST32 to determine their risk level for developing a substance use disorder. The NM ASSIST includes 8 questions about the patient’s desire for, use of, and problems related to the use of a wide range of drugs, including cannabis, cocaine, methamphetamine, hallucinogens, and other substances (eg, “In the past 3 months, how often have you used the following substances?” “How often have you had a strong desire or urge to use this substance?” “How often has your use of this substance led to health, social, legal or financial problems?”). The score on the NM ASSIST is used to calculate the patient’s risk level as low, moderate, or high.
For patients who use more than one drug, this risk level is scored separately for each drug and may differ from drug to drug. Multi-drug assessment can become time-consuming and may not be appropriate in some patients, especially if time is an issue (eg, the patient would like to focus on other concerns) or the patient is not interested in addressing certain drugs. In general, the decision about which substances to address should be clinically-driven, tailored to the needs of an individual patient. Focusing on the substance with the highest risk score or associated with the patient’s expressed greatest motivation to change may produce the best results.
CASE › Based on Mr. M’s response to your Quick Screen indicating he drinks alcohol and uses illicit drugs, you administer the NM ASSIST to perform a detailed assessment. His answer to a screening question for problematic alcohol use is negative (In the past year, he has not had >4 drinks in a day). Next, you calculate his NM ASSIST-based risk scores for cannabis and cocaine, and determine he is at moderate risk for developing problems due to cannabis use and at high risk for developing problems based on his use of cocaine. You also note Mr. M’s blood pressure (BP) is elevated (155/90 mm hg).
Conducting a brief intervention
Depending on the patient’s risk level for developing a substance use disorder, he or she should receive either brief advice (for those at low risk) or a BI (for those at moderate or high risk) and, if needed, a referral to treatment. Two popular approaches are FRAMES (Feedback, Responsibility, Advice, Menu of Strategies, Empathy, Self-efficacy) and the NIDA-recommended 5 As intervention. The latter approach entails Asking the patient about his drug use (via the Quick Screen); Advising the patient about his drug use by providing specific medical advice on why he should stop or cut down, and how; Assessing the patient’s readiness to quit or reduce use; Assisting the patient in making a change by creating a plan with specific goals; and Arranging a follow-up visit or specialty assessment and treatment by making referrals as appropriate.19
What about children and adolescents? Implementing a drug SBI in young patients often entails overcoming unique challenges and ethical dilemmas. Although the American Academy of Pediatrics recommends SBI for unhealthy drug and alcohol use among children and adolescents,33,34 the USPSTF did not find sufficient evidence to recommend the practice.1,8,35 Screening for drug use in minors often is complicated by questions about the age at which to start routine screening and issues related to confidentiality and parental involvement. The Center for Adolescent Health and the Law and the National Institute on Alcohol Abuse and Alcoholism provide useful resources related to youth SBI, including guidance on when to consider breeching a child’s confidentiality by engaging parents or guardians (TABLE 3).
TABLE 3
Resources
NIDA Resource Guide NIDA-Modified ASSIST Coding for SBI reimbursement SAMHSA’s Treatment Services Locator NIDA’s List of Community Treatment Programs SAMHSA Opioid Overdose Toolkit Buprenorphine training program Center for Adolescent Health and the Law NIAAA Alcohol Screening and Brief Intervention for Youth |
Help is available for securing treatment, reimbursement
In addition to NIDA, many other organizations offer resources to assist clinicians in using drug SBI and helping patients obtain treatment (TABLE 3). For reimbursement, the Centers for Medicare and Medicaid Services has adopted billing codes for SBI services.36,37 The Substance Abuse and Mental Health Services Administration (SAMHSA)’s Behavioral Health Treatment Services Locator and NIDA’s National Drug Abuse Treatment Clinical Trials Network List of Associated Community Treatment Programs can assist clinicians and patients in finding specialty treatment programs. Self-help groups such as Narcotics Anonymous, Alcoholic Anonymous, or Self-Managment and Recovery Training may help alleviate problems related to insurance coverage, location, and/or timing of services.
SAMHSA’s Opioid Overdose Toolkit provides guidance to clinicians and patients on ways to reduce the risk of overdose. Physicians also can complete a short training program in office-based buprenorphine maintenance therapy to provide evidence-based care to patients with opioid dependence; more details about this program are available from http://www.buppractice.com.
CASE › You decide to use the 5 as intervention with Mr. M. You explain to him that he is at high risk of developing a substance use disorder. You also discuss his elevated BP and the possible negative effects of drug use, especially cocaine, on BP. You advise him that medically it is in his best interest to stop using cocaine and stop or reduce using cannabis. When you ask Mr. M about his readiness to change his drug use, he expresses moderate interest in stopping cocaine but is not willing to reduce his cannabis use. At this time, he is not willing to discuss these issues further (“I’ll think about that”) or create a specific plan. You assure him of your ongoing support, provide him with resources on specialty treatment programs should he wish to consider those, and schedule a follow-up visit in 2 weeks to address BP and, if the patient agrees, drug use.
CORRESPONDENCE
Aleksandra Zgierska, MD, Phd, Department of Family Medicine, University of Wisconsin School of Medicine and Public Health, 1100 Delaplaine Court, Madison, WI 53715-1896; [email protected]
1. US Preventive Services Task Force. Screening and behavioral counseling interventions in primary care to reduce alcohol misuse. US Preventive Services Task Force Web site. Available at: http://www.uspreventiveservicestaskforce.org/uspstf/ uspsdrin.htm. Accessed March 4, 2013.
2. US Preventive Services Task Force. Counseling and interventions to prevent tobacco use and tobacco-caused disease in adults and pregnant women. US Preventive Services Task Force Web site. Available at: http://www.uspreventiveservicestaskforce.org/uspstf/uspstbac2.htm. Accessed March 4, 2014.
3. Saitz R, Alford DP, Bernstein J, et al. Screening and brief intervention for unhealthy drug use in primary care settings: randomized clinical trials are needed. J Addict Med. 2010;4: 123-130.
4. Pilowsky DJ, Wu LT. Screening for alcohol and drug use disorders among adults in primary care: a review. Subst Abuse Rehabil. 2012;3:25-34.
5. Substance Abuse and Mental Health Services Administration. Screening, Brief Intervention, and Referral to Treatment (SBIRT). Substance Abuse and Mental Health Services Administration Web site. Available at: http://www.samhsa.gov/ prevention/sbirt/. Accessed March 4, 2014.
6. Squires LE, Alford DP, Bernstein J, et al. Clinical case discussion: screening and brief intervention for drug use in primary care. J Addict Med. 2010;4:131-136.
7. Krupski A, Joesch JM, Dunn C, et al. Testing the effects of brief intervention in primary care for problem drug use in a randomized controlled trial: rationale, design, and methods. Addict Sci Clin Pract. 2012;7:27.
8. US Preventive Services Task Force. Screening for illicit drug use. US Preventive Services Task Force Web site. Available at: http://www.uspreventiveservicestaskforce.org/uspstf/uspsdrug.htm. Accessed March 4, 2014.
9. Lanier D, Ko S. Screening in Primary Care Settings for Illicit Drug Use: Assessment of Screening Instruments—A Supplemental Evidence Update for the U.S. Preventive Services Task Force. AHRQ Publication No. 08-05108-EF-2. Rockville, MD: Agency for Healthcare Research and Quality; 2008.
10. Bernstein J, Bernstein E, Tassiopoulos K, et al. Brief motivational intervention at a clinic visit reduces cocaine and heroin use. Drug Alcohol Depend. 2005;77:49-59.
11. Skinner HA. The drug abuse screening test. Addict Behav. 1982;7:363-371.
12. Zahradnik A, Otto C, Crackau B, et al. Randomized controlled trial of a brief intervention for problematic prescription drug use in non-treatment-seeking patients. Addiction. 2009;104:109-117.
13. Otto C, Crackau B, Löhrmann I, et al. Brief intervention in general hospital for problematic prescription drug use: 12-month outcome. Drug Alcohol Depend. 2009;105:221-226.
14. Humeniuk R, Ali R, Babor T, et al. A randomized controlled trial of a brief intervention for illicit drugs linked to the Alcohol, Smoking and Substance Involvement Screening Test (ASSIST) in clients recruited from primary health-care settings in four countries. Addiction. 2012;107:957-966.
15. WHO ASSIST Working Group. The Alcohol, Smoking and Substance Involvement Screening Test (ASSIST): development, reliability and feasibility. Addiction. 2002;97:1183-1194.
16. Saitz R, Palfai TP, Cheng DM, et al. Screening and brief intervention for drug use in primary care: the Assessing Screening Plus brief Intervention’s Resulting Efficacy to stop drug use (ASPIRE) randomized trial. Addict Sci Clin Pract. 2013;8(suppl 1):A61.
17. Roy-Byrne P, Bumgardner K, Krupski A, et al. Brief intervention for problem drug use in safety-net primary care settings: a randomized clinical trial. JAMA. 2014;312(5):492-501.
18. Madras BK, Compton WM, Avula D, et al. Screening, brief interventions, referral to treatment (SBIRT) for illicit drug and alcohol use at multiple healthcare sites: comparison at intake and 6 months later. Drug Alcohol Depend. 2009;99:280-295.
19. National Institute on Drug Abuse. Resource guide: Screening for drug use in general medical settings. National Institute on Drug Abuse Web site. Available at: http://www.drugabuse. gov/publications/resource-guide. Accessed March 8, 2014.
20. Saitz R, Cheng DM, Allensworth-Davies D, et al. The ability of single screening questions for unhealthy alcohol and other drug use to identify substance dependence in primary care. J Stud Alcohol Drugs. 2014;75:153-157.
21. Newcombe DA, Humeniuk RE, Ali R. Validation of the World Health Organization Alcohol, Smoking and Substance Involvement Screening Test (ASSIST): report of results from the Australian site. Drug Alcohol Rev. 2005;24:217-226.
22. Humeniuk R, Ali R, Babor TF, et al. Validation of the Alcohol, Smoking And Substance Involvement Screening Test (ASSIST). Addiction. 2008;103:1039-1047.
23. Mdege ND, Lang J. Screening instruments for detecting illicit drug use/abuse that could be useful in general hospital wards: a systematic review. Addict Behav. 2011;36:1111-1119.
24. Cassidy CM, Schmitz N, Malla A. Validation of the alcohol use disorders identification test and the drug abuse screening test in first episode psychosis. Can J Psychiatry. 2008;53:26-33.
25. Brown RL, Rounds LA. Conjoint screening questionnaires for alcohol and other drug abuse: criterion validity in a primary care practice. Wis Med J. 1995;94:135-140.
26. American Psychiatric Association. Position statement on substance use disorders. American Psychiatric Association Web site. Available at: http://www.psychiatry.org/File%20Library/Advocacy%20and%20Newsroom/Position%20Statements/ps2012_Substance.pdf. Accessed March 4, 2014.
27. Smith PC, Schmidt SM, Allensworth-Davies D, et al. A single-question screening test for drug use in primary care. Arch Intern Med. 2010;170:1155-1160.
28. American Academy of Family Physicians. Substance abuse and addiction. American Academy of Family Physicians Web site. Available at: http://www.aafp.org/about/policies/all/substance-abuse.html. Accessed March 4, 2014.
29. National Institute on Alcohol Abuse and Alcoholism. Helping patients who drink too much: A clinician’s guide. National Institute on Alcohol Abuse and Alcoholism Web site. Available at: http://pubs.niaaa.nih.gov/publications/Practitioner/CliniciansGuide2005/clinicians_guide.htm. Accessed March 4, 2014.
30. US Department of Health and Human Services Public Health Service. Helping smokers quit: A guide for clinicians. US Department of Health and Human Services Public Health Service Web site. Available at: http://www.ahrq.gov/professionals/clinicians-providers/guidelines-recommendations/tobacco/clinicians//clinhlpsmkqt/. Accessed March 4, 2014.
31. National Institute on Alcohol Abuse and Alcoholism. A Pocket Guide for Alcohol Screening and Brief Intervention. National Institute on Alcohol Abuse and Alcoholism Web site. Available at: http://pubs.niaaa.nih.gov/publications/Practitioner/pocketguide/pocket_guide.htm. Accessed July 30, 2014.
32. National Institute on Drug Abuse. NIDA-Quick Screen V1.0. National Institute on Drug Abuse Web site. Available at: http://www.drugabuse.gov/sites/default/files/pdf/nmassist.pdf. Accessed March 4, 2014.
33. Committee on Substance Abuse, Levy SJ, Kokotailo PK. Substance use screening, brief intervention, and referral to treatment for pediatricians. Pediatrics. 2011;128:e1330-e1340.
34. Kulig JW; American Academy of Pediatrics Committee on Substance Abuse. Tobacco, alcohol, and other drugs: the role of the pediatrician in prevention, identification, and management of substance abuse. Pediatrics. 2005;115:816-821.
35. US Preventive Services Task Force. Primary care behavioral interventions to reduce the nonmedical use of drugs in children and adolescents. US Preventive Services Task Force Web site. Available at: http://www.uspreventiveservicestaskforce.org/uspstf/uspsnonmed.htm. Accessed March 4, 2014.
36. Centers for Medicare & Medicaid Services. Screening, Brief Intervention, and Referral to Treatment (SBIRT) services. Centers for Medicare & Medicaid Services Web site. Available at: http://www.cms.gov/Outreach-and-Education/Medicare-Learning-Network-MLN/MLNProducts/downloads/sbirt_factsheet_icn904084.pdf. Accessed March 4, 2014.
37. Substance Abuse and Mental Health Services Administration. Coding for screening and brief intervention reimbursement. Substance Abuse and Mental Health Services Administration Web site. Available at: http://beta.samhsa.gov/sbirt/coding-reimbursement. Accessed August 4, 2014.
1. US Preventive Services Task Force. Screening and behavioral counseling interventions in primary care to reduce alcohol misuse. US Preventive Services Task Force Web site. Available at: http://www.uspreventiveservicestaskforce.org/uspstf/ uspsdrin.htm. Accessed March 4, 2013.
2. US Preventive Services Task Force. Counseling and interventions to prevent tobacco use and tobacco-caused disease in adults and pregnant women. US Preventive Services Task Force Web site. Available at: http://www.uspreventiveservicestaskforce.org/uspstf/uspstbac2.htm. Accessed March 4, 2014.
3. Saitz R, Alford DP, Bernstein J, et al. Screening and brief intervention for unhealthy drug use in primary care settings: randomized clinical trials are needed. J Addict Med. 2010;4: 123-130.
4. Pilowsky DJ, Wu LT. Screening for alcohol and drug use disorders among adults in primary care: a review. Subst Abuse Rehabil. 2012;3:25-34.
5. Substance Abuse and Mental Health Services Administration. Screening, Brief Intervention, and Referral to Treatment (SBIRT). Substance Abuse and Mental Health Services Administration Web site. Available at: http://www.samhsa.gov/ prevention/sbirt/. Accessed March 4, 2014.
6. Squires LE, Alford DP, Bernstein J, et al. Clinical case discussion: screening and brief intervention for drug use in primary care. J Addict Med. 2010;4:131-136.
7. Krupski A, Joesch JM, Dunn C, et al. Testing the effects of brief intervention in primary care for problem drug use in a randomized controlled trial: rationale, design, and methods. Addict Sci Clin Pract. 2012;7:27.
8. US Preventive Services Task Force. Screening for illicit drug use. US Preventive Services Task Force Web site. Available at: http://www.uspreventiveservicestaskforce.org/uspstf/uspsdrug.htm. Accessed March 4, 2014.
9. Lanier D, Ko S. Screening in Primary Care Settings for Illicit Drug Use: Assessment of Screening Instruments—A Supplemental Evidence Update for the U.S. Preventive Services Task Force. AHRQ Publication No. 08-05108-EF-2. Rockville, MD: Agency for Healthcare Research and Quality; 2008.
10. Bernstein J, Bernstein E, Tassiopoulos K, et al. Brief motivational intervention at a clinic visit reduces cocaine and heroin use. Drug Alcohol Depend. 2005;77:49-59.
11. Skinner HA. The drug abuse screening test. Addict Behav. 1982;7:363-371.
12. Zahradnik A, Otto C, Crackau B, et al. Randomized controlled trial of a brief intervention for problematic prescription drug use in non-treatment-seeking patients. Addiction. 2009;104:109-117.
13. Otto C, Crackau B, Löhrmann I, et al. Brief intervention in general hospital for problematic prescription drug use: 12-month outcome. Drug Alcohol Depend. 2009;105:221-226.
14. Humeniuk R, Ali R, Babor T, et al. A randomized controlled trial of a brief intervention for illicit drugs linked to the Alcohol, Smoking and Substance Involvement Screening Test (ASSIST) in clients recruited from primary health-care settings in four countries. Addiction. 2012;107:957-966.
15. WHO ASSIST Working Group. The Alcohol, Smoking and Substance Involvement Screening Test (ASSIST): development, reliability and feasibility. Addiction. 2002;97:1183-1194.
16. Saitz R, Palfai TP, Cheng DM, et al. Screening and brief intervention for drug use in primary care: the Assessing Screening Plus brief Intervention’s Resulting Efficacy to stop drug use (ASPIRE) randomized trial. Addict Sci Clin Pract. 2013;8(suppl 1):A61.
17. Roy-Byrne P, Bumgardner K, Krupski A, et al. Brief intervention for problem drug use in safety-net primary care settings: a randomized clinical trial. JAMA. 2014;312(5):492-501.
18. Madras BK, Compton WM, Avula D, et al. Screening, brief interventions, referral to treatment (SBIRT) for illicit drug and alcohol use at multiple healthcare sites: comparison at intake and 6 months later. Drug Alcohol Depend. 2009;99:280-295.
19. National Institute on Drug Abuse. Resource guide: Screening for drug use in general medical settings. National Institute on Drug Abuse Web site. Available at: http://www.drugabuse. gov/publications/resource-guide. Accessed March 8, 2014.
20. Saitz R, Cheng DM, Allensworth-Davies D, et al. The ability of single screening questions for unhealthy alcohol and other drug use to identify substance dependence in primary care. J Stud Alcohol Drugs. 2014;75:153-157.
21. Newcombe DA, Humeniuk RE, Ali R. Validation of the World Health Organization Alcohol, Smoking and Substance Involvement Screening Test (ASSIST): report of results from the Australian site. Drug Alcohol Rev. 2005;24:217-226.
22. Humeniuk R, Ali R, Babor TF, et al. Validation of the Alcohol, Smoking And Substance Involvement Screening Test (ASSIST). Addiction. 2008;103:1039-1047.
23. Mdege ND, Lang J. Screening instruments for detecting illicit drug use/abuse that could be useful in general hospital wards: a systematic review. Addict Behav. 2011;36:1111-1119.
24. Cassidy CM, Schmitz N, Malla A. Validation of the alcohol use disorders identification test and the drug abuse screening test in first episode psychosis. Can J Psychiatry. 2008;53:26-33.
25. Brown RL, Rounds LA. Conjoint screening questionnaires for alcohol and other drug abuse: criterion validity in a primary care practice. Wis Med J. 1995;94:135-140.
26. American Psychiatric Association. Position statement on substance use disorders. American Psychiatric Association Web site. Available at: http://www.psychiatry.org/File%20Library/Advocacy%20and%20Newsroom/Position%20Statements/ps2012_Substance.pdf. Accessed March 4, 2014.
27. Smith PC, Schmidt SM, Allensworth-Davies D, et al. A single-question screening test for drug use in primary care. Arch Intern Med. 2010;170:1155-1160.
28. American Academy of Family Physicians. Substance abuse and addiction. American Academy of Family Physicians Web site. Available at: http://www.aafp.org/about/policies/all/substance-abuse.html. Accessed March 4, 2014.
29. National Institute on Alcohol Abuse and Alcoholism. Helping patients who drink too much: A clinician’s guide. National Institute on Alcohol Abuse and Alcoholism Web site. Available at: http://pubs.niaaa.nih.gov/publications/Practitioner/CliniciansGuide2005/clinicians_guide.htm. Accessed March 4, 2014.
30. US Department of Health and Human Services Public Health Service. Helping smokers quit: A guide for clinicians. US Department of Health and Human Services Public Health Service Web site. Available at: http://www.ahrq.gov/professionals/clinicians-providers/guidelines-recommendations/tobacco/clinicians//clinhlpsmkqt/. Accessed March 4, 2014.
31. National Institute on Alcohol Abuse and Alcoholism. A Pocket Guide for Alcohol Screening and Brief Intervention. National Institute on Alcohol Abuse and Alcoholism Web site. Available at: http://pubs.niaaa.nih.gov/publications/Practitioner/pocketguide/pocket_guide.htm. Accessed July 30, 2014.
32. National Institute on Drug Abuse. NIDA-Quick Screen V1.0. National Institute on Drug Abuse Web site. Available at: http://www.drugabuse.gov/sites/default/files/pdf/nmassist.pdf. Accessed March 4, 2014.
33. Committee on Substance Abuse, Levy SJ, Kokotailo PK. Substance use screening, brief intervention, and referral to treatment for pediatricians. Pediatrics. 2011;128:e1330-e1340.
34. Kulig JW; American Academy of Pediatrics Committee on Substance Abuse. Tobacco, alcohol, and other drugs: the role of the pediatrician in prevention, identification, and management of substance abuse. Pediatrics. 2005;115:816-821.
35. US Preventive Services Task Force. Primary care behavioral interventions to reduce the nonmedical use of drugs in children and adolescents. US Preventive Services Task Force Web site. Available at: http://www.uspreventiveservicestaskforce.org/uspstf/uspsnonmed.htm. Accessed March 4, 2014.
36. Centers for Medicare & Medicaid Services. Screening, Brief Intervention, and Referral to Treatment (SBIRT) services. Centers for Medicare & Medicaid Services Web site. Available at: http://www.cms.gov/Outreach-and-Education/Medicare-Learning-Network-MLN/MLNProducts/downloads/sbirt_factsheet_icn904084.pdf. Accessed March 4, 2014.
37. Substance Abuse and Mental Health Services Administration. Coding for screening and brief intervention reimbursement. Substance Abuse and Mental Health Services Administration Web site. Available at: http://beta.samhsa.gov/sbirt/coding-reimbursement. Accessed August 4, 2014.
Why celiac disease is so easy to miss
› Do not rely on symptoms or symptom response to
a gluten-free diet alone
to diagnose celiac disease (CD); this approach does not differentiate CD from non-celiac gluten sensitivity. B
› Use HLA-DQ2 and -DQ8 genotype testing to effectively rule out the disease in selected clinical situations. B
› Test for CD in any
patient who has unexplained elevated serum aminotransferase levels, even in the absence of CD symptoms. A
› Screen all first-degree relatives of patients with
CD by testing for immunoglobulin A (IgA) tissue transglutaminase antibodies and serum IgA levels. A
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
CASE › It was a clinical conundrum. A 2011 case study1 described a 33-year-old woman with a 10-year history of progressive, debilitating pain and weakness. The patient had not received a unifying diagnosis or effective treatment despite multiple diagnostic tests and different recommendations from multiple specialists. The diagnosis remained elusive until a rheumatologist agreed to reexamine the case.
While reviewing the woman’s thick chart, the rheumatologist noted a series of negative results from upper and lower endoscopies and abdominal scans. Further investigation revealed an almost obfuscated clue—blood tests performed 2 years earlier that were positive for celiac disease (CD). However, a small intestine biopsy, which normally is done to confirm the diagnosis, was never performed.
The rheumatologist made a tentative diagnosis of CD and referred her to a nutritionist, who recommended the patient adhere to a strict gluten-free diet. Within 3 months, the patient experienced marked improvement and returned to work.
CD is an often-missed diagnosis. According to a study based on National Health and Nutrition Examination Survey data, only 17% of patients with CD are aware they have the disease.2 As such, it is imperative that primary care physicians familiarize themselves with CD’s myriad clinical presentations, diagnosis, and treatment.3-6
Gluten triggers an immune response in genetically susceptible patients
CD initially was known as “celiac sprue” because it shares characteristics with tropical sprue—diarrhea, malabsorption, and emaciation. It is a unique T-cell autoimmune enteropathy that is precipitated in genetically susceptible individuals by the ingestion of gluten, the major storage protein of wheat, barley, and rye.3,7
Upon ingestion, gluten breaks down to gliadin, which provokes an immune response in the intestinal mucosa of patients with CD. This response results in an inflammatory reaction, primarily in the upper small intestine, that destroys the absorption surface and causes villous atrophy, leading to nutrient malabsorption and chronic diarrhea.8 CD is associated with significant morbidity due to an abnormal excretion of fat (steatorrhea) and varying degrees of malabsorption of vitamins A, D, and K, as well as B complex vitamins including B12 and folate; carbohydrates; protein; water; and minerals such as magnesium, calcium, and iron.9
CD develops only in individuals who possess alleles that encode for HLA-DQ2 or HLA-DQ8 proteins, products of 2 of the HLA genes. And while 30% of Caucasians carry the HLA-DQ2 allele and virtually 100% consume wheat, only 1 in 100 will develop CD.3,10,11 Although the genes are necessary, it is the interplay between genes (both HLA and non-HLA associated) and environment (ie, gluten) that leads to the intestinal mucosa damage typical of the disease. The HLA-DQ region also is associated with increased risk of type 1 diabetes, which might explain the correlation of CD to a host of other autoimmune disorders, including Graves’ disease and rheumatoid arthritis.8,10,11
Increased prevalence reflects better recognition of celiac disease
CD affects .6% to 1% of the population worldwide, with wide regional variation.3 Before the development of serologic assays in the 1970s, CD was a clinical diagnosis based on classic symptoms. With the advent of assays for immunoglobulin A (IgA) antibodies, the prevalence of CD has drastically increased to the current estimates of 1:250 to 1:500.4,5 The prevalence will continue to increase as clinicians become more aware of the different presentations of the disease, which are described below.
CD runs in families. Most patients with CD have a family history of the disease based on inheritance of the HLA alleles. A US study determined that the prevalence of CD was 1:22 in first-degree relatives and 1:39 in second-degree relatives of patients with biopsy-proven CD.12
Less than half of patients have GI symptoms
The classic presentation of CD involves a constellation of signs and symptoms of malabsorption: diarrhea, muscle wasting, and weight loss. Other typical gastrointestinal (GI) symptoms include bloating, flatulence, and abdominal pain.
Recognizing CD can be challenging, however, because <50% of patients diagnosed with CD present with these classic GI symptoms.3 About 50% of CD patients present with extra-intestinal symptoms, such as iron deficiency anemia, aphthous stomatitis, chronic fatigue, osteopenia, and dental enamel hypoplasia.3,8,13 Other possible non-GI symptoms include abnormal liver function test results and skin disorders such as dermatitis herpetiformis, a pruritic rash with cutaneous IgA deposits.3,8 In addition, many patients are asymptomatic.14 This highly variable clinical picture is due to the genetic and immunologic basis of the disease, extent of mucosal injury, and patients’ dietary habits, gender, and age of onset.15 A common clue that suggests a patient may have CD is unexplained iron deficiency anemia that does not improve with oral iron supplementation.4,13
Because symptoms may be intermittent, a patient may delay seeking care until he or she develops secondary manifestations, which often are debilitating and overshadow the GI complaints. Chronic complications of untreated CD include lymphoma and adenocarcinomas of the jejunum, recurrent miscarriages, neurologic disorders, osteoporosis, and hyposplenism.3,4,8
Since CD can manifest with widely varying symptoms, some researchers believe the disease should be classified into 3 categories based on presentation: classic CD, which presents with diarrhea, weight loss, malabsorption, and vitamin deficiency; atypical CD, which presents with minimal GI symptoms but can include anemia, neurologic symptoms, arthritis, or infertility; and asymptomatic CD, which typically displays no symptoms but usually is identified on incidental screening.3,8,16 Non-celiac gluten sensitivity is a distinct condition in which the body reacts adversely to gluten; it is not an autoimmune disease with an inflammatory response.
Order serologic testing for at-risk patients
Because CD remains underdiagnosed,16 taking a thorough family history and dietary history and making sure to at least consider CD as a part of a differential diagnosis is important. Although population-based screening has been proposed, its benefits and cost-effectiveness remain unproven. As a result, serologic testing of at-risk groups—individuals with conditions known to be associated with CD—remains the current standard.3 The TABLE lists groups for whom serologic testing for CD is indicated.16,17
In addition, the American College of Gastroenterology (ACG) and the North American Society for Pediatric Gastroenterology, Hepatology, and Nutrition (NASPGHAN) provide guidance on the diagnosis and treatment of adults and children with CD. (An ACG diagnostic algorithm is available at http://www.nature.com/ajg/journal/v108/n5/pdf/ajg201379a.pdf.)
Adults. For patients who are consuming a diet that includes gluten and have symptoms that suggest CD, the ACG guidelines recommend initial testing for IgA tissue transglutaminase (tTG) antibodies.16 The IgA tTG has a sensitivity and specificity >95%.16 An alternative test, the IgA endomysial (IgA EMA) test, has similar sensitivity but is time-consuming and its accuracy depends on the experience and skill of the laboratory technician. A negative result for either test has a high negative predictive value for CD.3,16
IgA deficiency is much more common in patients with CD than in the general population and can result in a false negative test for tTG and EMA. Therefore, consider taking a baseline IgA measurement first. If the patient has an IgA deficiency, the test you’ll use next will change: The preferred test for CD is either immunoglobulin G (IgG) tTG or IgG deamidated gliadin peptides (DGP).3,16
If a patient is already gluten-free... To rule out CD in patients who are already consuming a gluten-free diet, order HLA-DQ2 and HLA-DQ8 testing because these markers have a specificity >99%; if the HLA test is negative, the disease is excluded.8,16
Children. NASPGHAN recommends taking a baseline IgA measurement in children at risk for CD and then testing for IgA tTG antibodies, but not until patients are 3 years old and have been on a diet that includes gluten for at least 1 year.17 Repeat testing at a later date it is recommended for those with negative results because some evidence suggests that in certain patients, later serologic testing will be positive. Alternatively, you may offer HLA testing. If the HLA test is negative, CD can be excluded >99% of the time.
Diagnosis usually is confirmed by intestinal biopsy
Positive results on serologic testing should be confirmed with a biopsy of the small bowel; findings characteristic of CD include an increased number of intraepithelial lymphocytes (>25 per 100 enterocytes), elongation of the crypts, and partial to total villous atrophy.4 Final confirmation of CD is resolution of symptoms by consuming a gluten-free diet.3,8
Alternate approaches to confirming the diagnosis. Although intestinal biopsy has long been considered the gold standard for diagnosis of CD, this may change. In 2012, the European Society for Paediatric Gastroenterology, Hepatology, and Nutrition proposed that the biopsy may not be necessary in children with the following 3 characteristics: classic intestinal symptoms of CD, IgA tTG levels >10 times higher than normal, and a positive HLA-DQ2.18
Catassi and Fasano19 have proposed shifting from relying on algorithms and intestinal biopsy to a quantitative approach. They suggest using the “4 out of 5” rule, meaning the diagnosis of CD can be confirmed if at least 4 of the following 5 criteria are satisfied: typical CD symptoms, a positive IgA tTG, a positive HLA-DQ2 or -DQ8, celiac enteropathy on small bowel biopsy, and response to a gluten-free diet.19
The only proven treatment: A gluten-free diet
Lifelong adherence to a gluten-free diet is the only effective treatment for CD.14,16 Previously, patients with CD were advised to also avoid oats, but most evidence supports the safety of oats (<2 oz/d), provided there is no cross-contamination with gluten.14 Adhering to a strict gluten-free diet can be challenging because cereal flours are ubiquitous in western foods, and some foods may be cross-contaminated. The Celiac Disease Foundation (http://www.celiac.org) offers guidance on maintaining a gluten-free diet.
Because avoiding gluten has become popular even among people who don’t have CD, product labeling that includes information on gluten content has become pervasive. However, determining which items contain gluten depends on accurate labeling, a standard that often is not met in many countries; in the United States, such labeling began to be phased in starting in July 2014.20 As a result, CD patients may unwittingly be exposed to gluten over the long term, which can result in greater morbidity and mortality. Unless a food is labeled “gluten-free," it is best to check with the manufacturer.
Compliance with a gluten-free diet can be monitored by following IgA tTG titers every 1 to 2 years, as these values normalize after a patient has been adhering to the diet for 6 to 24 months.3,16
In addition to lifelong adherence to a gluten-free diet, a National Institutes of Health Consensus Development Conference recommended that management of patients with CD should include21:
- consultation with a skilled dietitian
- education about the disease
- continuous long-term follow-up by a multidisciplinary team
- identification and follow-up of abnormalities found at baseline, such as abnormal liver function test results
- treatment of nutritional deficiencies.
The ACG also recommends that CD patients receive a dual energy x-ray scan for follow-up of osteopenia and a pneumococcal vaccine because functional hyposplenism is associated with CD, and pneumonia is a common complication of hyposplenism.16,18
Compared to infants who are breastfed and don’t receive gluten until ages 4 to 6 months, infants who are fed gluten in their first 3 months have a significantly increased risk of developing antibodies that are associated with celiac disease (CD).22 Recent studies suggest that the effects of breast milk on the microbiota composition of the intestine may help explain this difference. Breast milk selectively stimulates the growth of specific bacteria, including bifidobacteria, which are relatively depleted in children with CD.23,24 Researchers believe breastfeeding and delaying introduction of gluten-containing foods until 4 to 6 months of age might protect against CD.25
Should you recommend a gluten-free diet for other patients? Because avoiding gluten is now popular and many gluten-free products are marketed as “health food,” physicians may be reluctant to recommend a gluten-free diet for patients who have vague abdominal symptoms but negative CD test results. Despite the current popularity of “going gluten-free,” the reality is that in addition to CD, many other diseases may be helped by a gluten-free diet, such as dermatitis herpetiformis, irritable bowel syndrome, and neurologic diseases such as gluten-sensitive ataxia.19 In the end, whether to adopt a gluten-free diet is a decision that you and your patient will need to make together.
Researchers are searching for additional treatments
Because many patients find it difficult to adhere to a gluten-free diet, researchers are investigating several alternative treatments, including a derivative from cholera toxin that inhibits the opening of intestinal epithelial junctions, thereby reducing the resultant inflammatory response, and a desensitizing vaccine.19,22,23 Another intriguing approach involves using the parasite Necator americanus to modulate the immune response to gluten.18 Finally, certain infant feeding practices, including breastfeeding and delaying introduction of gluten to the diet, may minimize the risk of developing CD. (See "A link between infant feeding practices and the risk of CD?" above.22-25)
CORRESPONDENCE
Patrick T. Dowling, MD, MPH, Department of Family Medicine, 50-078 Center for Health Sciences, David Geffen School of Medicine at UCLA, Los Angeles, CA 90095-1683; [email protected]
1. Sanders L. Hurt all over. New York Times Sunday Magazine. November 11, 2011:MM22.
2. Rubio-Tapia A, Ludvigsson JF, Brantner TL, et al. The prevalence of celiac disease in the United States. Am J Gastroenterol. 2012;107:1538-1544.
3. Fasano A, Catassi C. Clinical practice. Celiac disease. N Engl J Med. 2012;367:2419-2426.
4. Green PH, Cellier C. Celiac disease. N Engl J Med. 2007;357: 1731-1743.
5. Fasano A, Berti I, Gerarduzzi T, et al. Prevalence of celiac disease in at-risk and not-at-risk groups in the United States: a large multicenter study. Arch Intern Med. 2003;163:286-292.
6. Mustalahti K, Catassi C, Reunanen A, et al; Coeliac EU Cluster, Project Epidemiology. The prevalence of celiac disease in Europe: results of centralized, international mass screening project. Ann Med. 2010;42:587-595.
7. Farrel R, Kelly C. Celiac disease and refractory celiac disease. In: Feldman M, Friedman LS, Brandt LJ. Sleisenger and Fordtran’s Gastrointestinal and Liver Disease: Pathophysiology, Diagnosis, Management. 9th ed. Philadelphia, PA: Saunders; 2010: 1797-1820.
8. Gujral N, Freeman HJ, Thomson Ab. Celiac disease: prevalence, diagnosis, pathogenesis and treatment. World J Gastroenterol. 2012;18:6036-6059.
9. Sleisenger MH. Diseases of malabsorption. In: Beeson PB, McDermott W. Cecil-Loeb Textbook of Medicine. 13th ed. Philadelphia, PA: WB Saunders Company; 1971:1285-1291.
10. Reilly NR, Green PH. Epidemiology and clinical presentations of celiac disease. Sem Immunopathol. 2012;34:473-478.
11. Kagnoff MF. Celiac disease. A gastrointestinal disease with environmental, genetic, and immunologic components. Gastroenterol Clin North Am. 1992;21:405-425.
12. Riddle MS, Murray JA, Porter CK. The incidence and risk of celiac disease in a healthy US adult population. Am J Gastroenterol. 2012;107:1248-1255.
13. Rampertab SD, Pooran N, Brar P, et al. Trends in the presentation of celiac disease. Am J Med. 2006;119:355.e9-355.e14.
14. Pietzak M. Celiac disease, wheat allergy, and gluten sensitivity: when gluten free is not a fad. JPEN J Parenter Enteral Nutr. 2012;36(1 suppl):68S-75S.
15. Sapone A, Bai JC, Ciacci C, et al. Spectrum of gluten-related disorders: consensus on new nomenclature and classification. BMC Med. 2012;10:13.
16. Rubio-Tapia A, Hill ID, Kelly CP, et al; American College of Gastroenterology. ACG clinical guidelines: diagnosis and management celiac disease. Am J Gastroenterol. 2013;108:656-676.
17. Hill ID, Dirks MH, Liptak GS, et al; North American Society for Pediatric Gastroenterology, Hepatology and Nutrition. Guidelines for the diagnosis and treatment of celiac disease in children: recommendations of the North American Society for Pediatric Gastroenterology, Hepatology and Nutrition. J Pediatr Gastroentertol Nutr. 2005;40:1-19.
18. Husby S, Koletsko S, Korponay-Szabó IR, et al; ESPGHAN Working Group on Coeliac Disease Diagnosis; ESPGHAN Gastoenterology Committee; European Society for Pediatric Gastroenterology, Hepatology, and Nutrition. European Society for Pediatric Gastroenterology, Hepatology and Nutrition Guidelines for the diagnosis of coeliac disease. J Pediatr Gastroenterol Nutr. 2012;54:136-160.
19. Catassi C, Fasano A. Celiac disease diagnosis: simple rules are better than complicated algorithms. Am J Med. 2010;123:691-693.
20. US Food and Drug Administration. Foods labeled gluten-free must now meet FDA's definition. Available at: http://www.fda. gov/Food/NewsEvents/ConstituentUpdates/ucm407867.htm. Accessed August 13, 2014.
21. National Institutes of Health Consensus Development Conference on Celiac Disease. National Institutes of Health Consensus Development Conference Statement. Available at: http://consensus.nih.gov/2004/2004celiacdisease118html.htm. Accessed August 13, 2014.
22. Norris JM, Barriga K, Hoffenberg EJ, et al. Risk of celiac disease autoimmunity and timing of gluten introduction in the diet of infants at increased risk of disease. JAMA. 2005;293:2343-2351.
23. Pozo-Rubio T, Olivares M, Nova E, et al. Immune development and intestinal microbiota in celiac disease. Clin Dev Immunol. 2012;2012:654143
24. Ivarsson A, Myléus A, Norström F, et al. Prevalence of childhood celiac disease and changes in infant feeding. Pediatrics. 2013;131:687-694.
25. Cataldo F, Montalto G. Celiac disease in the developing countries: a new and challenging public health problem. World J Gastroenterol. 2007;13:2153-2159.
› Do not rely on symptoms or symptom response to
a gluten-free diet alone
to diagnose celiac disease (CD); this approach does not differentiate CD from non-celiac gluten sensitivity. B
› Use HLA-DQ2 and -DQ8 genotype testing to effectively rule out the disease in selected clinical situations. B
› Test for CD in any
patient who has unexplained elevated serum aminotransferase levels, even in the absence of CD symptoms. A
› Screen all first-degree relatives of patients with
CD by testing for immunoglobulin A (IgA) tissue transglutaminase antibodies and serum IgA levels. A
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
CASE › It was a clinical conundrum. A 2011 case study1 described a 33-year-old woman with a 10-year history of progressive, debilitating pain and weakness. The patient had not received a unifying diagnosis or effective treatment despite multiple diagnostic tests and different recommendations from multiple specialists. The diagnosis remained elusive until a rheumatologist agreed to reexamine the case.
While reviewing the woman’s thick chart, the rheumatologist noted a series of negative results from upper and lower endoscopies and abdominal scans. Further investigation revealed an almost obfuscated clue—blood tests performed 2 years earlier that were positive for celiac disease (CD). However, a small intestine biopsy, which normally is done to confirm the diagnosis, was never performed.
The rheumatologist made a tentative diagnosis of CD and referred her to a nutritionist, who recommended the patient adhere to a strict gluten-free diet. Within 3 months, the patient experienced marked improvement and returned to work.
CD is an often-missed diagnosis. According to a study based on National Health and Nutrition Examination Survey data, only 17% of patients with CD are aware they have the disease.2 As such, it is imperative that primary care physicians familiarize themselves with CD’s myriad clinical presentations, diagnosis, and treatment.3-6
Gluten triggers an immune response in genetically susceptible patients
CD initially was known as “celiac sprue” because it shares characteristics with tropical sprue—diarrhea, malabsorption, and emaciation. It is a unique T-cell autoimmune enteropathy that is precipitated in genetically susceptible individuals by the ingestion of gluten, the major storage protein of wheat, barley, and rye.3,7
Upon ingestion, gluten breaks down to gliadin, which provokes an immune response in the intestinal mucosa of patients with CD. This response results in an inflammatory reaction, primarily in the upper small intestine, that destroys the absorption surface and causes villous atrophy, leading to nutrient malabsorption and chronic diarrhea.8 CD is associated with significant morbidity due to an abnormal excretion of fat (steatorrhea) and varying degrees of malabsorption of vitamins A, D, and K, as well as B complex vitamins including B12 and folate; carbohydrates; protein; water; and minerals such as magnesium, calcium, and iron.9
CD develops only in individuals who possess alleles that encode for HLA-DQ2 or HLA-DQ8 proteins, products of 2 of the HLA genes. And while 30% of Caucasians carry the HLA-DQ2 allele and virtually 100% consume wheat, only 1 in 100 will develop CD.3,10,11 Although the genes are necessary, it is the interplay between genes (both HLA and non-HLA associated) and environment (ie, gluten) that leads to the intestinal mucosa damage typical of the disease. The HLA-DQ region also is associated with increased risk of type 1 diabetes, which might explain the correlation of CD to a host of other autoimmune disorders, including Graves’ disease and rheumatoid arthritis.8,10,11
Increased prevalence reflects better recognition of celiac disease
CD affects .6% to 1% of the population worldwide, with wide regional variation.3 Before the development of serologic assays in the 1970s, CD was a clinical diagnosis based on classic symptoms. With the advent of assays for immunoglobulin A (IgA) antibodies, the prevalence of CD has drastically increased to the current estimates of 1:250 to 1:500.4,5 The prevalence will continue to increase as clinicians become more aware of the different presentations of the disease, which are described below.
CD runs in families. Most patients with CD have a family history of the disease based on inheritance of the HLA alleles. A US study determined that the prevalence of CD was 1:22 in first-degree relatives and 1:39 in second-degree relatives of patients with biopsy-proven CD.12
Less than half of patients have GI symptoms
The classic presentation of CD involves a constellation of signs and symptoms of malabsorption: diarrhea, muscle wasting, and weight loss. Other typical gastrointestinal (GI) symptoms include bloating, flatulence, and abdominal pain.
Recognizing CD can be challenging, however, because <50% of patients diagnosed with CD present with these classic GI symptoms.3 About 50% of CD patients present with extra-intestinal symptoms, such as iron deficiency anemia, aphthous stomatitis, chronic fatigue, osteopenia, and dental enamel hypoplasia.3,8,13 Other possible non-GI symptoms include abnormal liver function test results and skin disorders such as dermatitis herpetiformis, a pruritic rash with cutaneous IgA deposits.3,8 In addition, many patients are asymptomatic.14 This highly variable clinical picture is due to the genetic and immunologic basis of the disease, extent of mucosal injury, and patients’ dietary habits, gender, and age of onset.15 A common clue that suggests a patient may have CD is unexplained iron deficiency anemia that does not improve with oral iron supplementation.4,13
Because symptoms may be intermittent, a patient may delay seeking care until he or she develops secondary manifestations, which often are debilitating and overshadow the GI complaints. Chronic complications of untreated CD include lymphoma and adenocarcinomas of the jejunum, recurrent miscarriages, neurologic disorders, osteoporosis, and hyposplenism.3,4,8
Since CD can manifest with widely varying symptoms, some researchers believe the disease should be classified into 3 categories based on presentation: classic CD, which presents with diarrhea, weight loss, malabsorption, and vitamin deficiency; atypical CD, which presents with minimal GI symptoms but can include anemia, neurologic symptoms, arthritis, or infertility; and asymptomatic CD, which typically displays no symptoms but usually is identified on incidental screening.3,8,16 Non-celiac gluten sensitivity is a distinct condition in which the body reacts adversely to gluten; it is not an autoimmune disease with an inflammatory response.
Order serologic testing for at-risk patients
Because CD remains underdiagnosed,16 taking a thorough family history and dietary history and making sure to at least consider CD as a part of a differential diagnosis is important. Although population-based screening has been proposed, its benefits and cost-effectiveness remain unproven. As a result, serologic testing of at-risk groups—individuals with conditions known to be associated with CD—remains the current standard.3 The TABLE lists groups for whom serologic testing for CD is indicated.16,17
In addition, the American College of Gastroenterology (ACG) and the North American Society for Pediatric Gastroenterology, Hepatology, and Nutrition (NASPGHAN) provide guidance on the diagnosis and treatment of adults and children with CD. (An ACG diagnostic algorithm is available at http://www.nature.com/ajg/journal/v108/n5/pdf/ajg201379a.pdf.)
Adults. For patients who are consuming a diet that includes gluten and have symptoms that suggest CD, the ACG guidelines recommend initial testing for IgA tissue transglutaminase (tTG) antibodies.16 The IgA tTG has a sensitivity and specificity >95%.16 An alternative test, the IgA endomysial (IgA EMA) test, has similar sensitivity but is time-consuming and its accuracy depends on the experience and skill of the laboratory technician. A negative result for either test has a high negative predictive value for CD.3,16
IgA deficiency is much more common in patients with CD than in the general population and can result in a false negative test for tTG and EMA. Therefore, consider taking a baseline IgA measurement first. If the patient has an IgA deficiency, the test you’ll use next will change: The preferred test for CD is either immunoglobulin G (IgG) tTG or IgG deamidated gliadin peptides (DGP).3,16
If a patient is already gluten-free... To rule out CD in patients who are already consuming a gluten-free diet, order HLA-DQ2 and HLA-DQ8 testing because these markers have a specificity >99%; if the HLA test is negative, the disease is excluded.8,16
Children. NASPGHAN recommends taking a baseline IgA measurement in children at risk for CD and then testing for IgA tTG antibodies, but not until patients are 3 years old and have been on a diet that includes gluten for at least 1 year.17 Repeat testing at a later date it is recommended for those with negative results because some evidence suggests that in certain patients, later serologic testing will be positive. Alternatively, you may offer HLA testing. If the HLA test is negative, CD can be excluded >99% of the time.
Diagnosis usually is confirmed by intestinal biopsy
Positive results on serologic testing should be confirmed with a biopsy of the small bowel; findings characteristic of CD include an increased number of intraepithelial lymphocytes (>25 per 100 enterocytes), elongation of the crypts, and partial to total villous atrophy.4 Final confirmation of CD is resolution of symptoms by consuming a gluten-free diet.3,8
Alternate approaches to confirming the diagnosis. Although intestinal biopsy has long been considered the gold standard for diagnosis of CD, this may change. In 2012, the European Society for Paediatric Gastroenterology, Hepatology, and Nutrition proposed that the biopsy may not be necessary in children with the following 3 characteristics: classic intestinal symptoms of CD, IgA tTG levels >10 times higher than normal, and a positive HLA-DQ2.18
Catassi and Fasano19 have proposed shifting from relying on algorithms and intestinal biopsy to a quantitative approach. They suggest using the “4 out of 5” rule, meaning the diagnosis of CD can be confirmed if at least 4 of the following 5 criteria are satisfied: typical CD symptoms, a positive IgA tTG, a positive HLA-DQ2 or -DQ8, celiac enteropathy on small bowel biopsy, and response to a gluten-free diet.19
The only proven treatment: A gluten-free diet
Lifelong adherence to a gluten-free diet is the only effective treatment for CD.14,16 Previously, patients with CD were advised to also avoid oats, but most evidence supports the safety of oats (<2 oz/d), provided there is no cross-contamination with gluten.14 Adhering to a strict gluten-free diet can be challenging because cereal flours are ubiquitous in western foods, and some foods may be cross-contaminated. The Celiac Disease Foundation (http://www.celiac.org) offers guidance on maintaining a gluten-free diet.
Because avoiding gluten has become popular even among people who don’t have CD, product labeling that includes information on gluten content has become pervasive. However, determining which items contain gluten depends on accurate labeling, a standard that often is not met in many countries; in the United States, such labeling began to be phased in starting in July 2014.20 As a result, CD patients may unwittingly be exposed to gluten over the long term, which can result in greater morbidity and mortality. Unless a food is labeled “gluten-free," it is best to check with the manufacturer.
Compliance with a gluten-free diet can be monitored by following IgA tTG titers every 1 to 2 years, as these values normalize after a patient has been adhering to the diet for 6 to 24 months.3,16
In addition to lifelong adherence to a gluten-free diet, a National Institutes of Health Consensus Development Conference recommended that management of patients with CD should include21:
- consultation with a skilled dietitian
- education about the disease
- continuous long-term follow-up by a multidisciplinary team
- identification and follow-up of abnormalities found at baseline, such as abnormal liver function test results
- treatment of nutritional deficiencies.
The ACG also recommends that CD patients receive a dual energy x-ray scan for follow-up of osteopenia and a pneumococcal vaccine because functional hyposplenism is associated with CD, and pneumonia is a common complication of hyposplenism.16,18
Compared to infants who are breastfed and don’t receive gluten until ages 4 to 6 months, infants who are fed gluten in their first 3 months have a significantly increased risk of developing antibodies that are associated with celiac disease (CD).22 Recent studies suggest that the effects of breast milk on the microbiota composition of the intestine may help explain this difference. Breast milk selectively stimulates the growth of specific bacteria, including bifidobacteria, which are relatively depleted in children with CD.23,24 Researchers believe breastfeeding and delaying introduction of gluten-containing foods until 4 to 6 months of age might protect against CD.25
Should you recommend a gluten-free diet for other patients? Because avoiding gluten is now popular and many gluten-free products are marketed as “health food,” physicians may be reluctant to recommend a gluten-free diet for patients who have vague abdominal symptoms but negative CD test results. Despite the current popularity of “going gluten-free,” the reality is that in addition to CD, many other diseases may be helped by a gluten-free diet, such as dermatitis herpetiformis, irritable bowel syndrome, and neurologic diseases such as gluten-sensitive ataxia.19 In the end, whether to adopt a gluten-free diet is a decision that you and your patient will need to make together.
Researchers are searching for additional treatments
Because many patients find it difficult to adhere to a gluten-free diet, researchers are investigating several alternative treatments, including a derivative from cholera toxin that inhibits the opening of intestinal epithelial junctions, thereby reducing the resultant inflammatory response, and a desensitizing vaccine.19,22,23 Another intriguing approach involves using the parasite Necator americanus to modulate the immune response to gluten.18 Finally, certain infant feeding practices, including breastfeeding and delaying introduction of gluten to the diet, may minimize the risk of developing CD. (See "A link between infant feeding practices and the risk of CD?" above.22-25)
CORRESPONDENCE
Patrick T. Dowling, MD, MPH, Department of Family Medicine, 50-078 Center for Health Sciences, David Geffen School of Medicine at UCLA, Los Angeles, CA 90095-1683; [email protected]
› Do not rely on symptoms or symptom response to
a gluten-free diet alone
to diagnose celiac disease (CD); this approach does not differentiate CD from non-celiac gluten sensitivity. B
› Use HLA-DQ2 and -DQ8 genotype testing to effectively rule out the disease in selected clinical situations. B
› Test for CD in any
patient who has unexplained elevated serum aminotransferase levels, even in the absence of CD symptoms. A
› Screen all first-degree relatives of patients with
CD by testing for immunoglobulin A (IgA) tissue transglutaminase antibodies and serum IgA levels. A
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
CASE › It was a clinical conundrum. A 2011 case study1 described a 33-year-old woman with a 10-year history of progressive, debilitating pain and weakness. The patient had not received a unifying diagnosis or effective treatment despite multiple diagnostic tests and different recommendations from multiple specialists. The diagnosis remained elusive until a rheumatologist agreed to reexamine the case.
While reviewing the woman’s thick chart, the rheumatologist noted a series of negative results from upper and lower endoscopies and abdominal scans. Further investigation revealed an almost obfuscated clue—blood tests performed 2 years earlier that were positive for celiac disease (CD). However, a small intestine biopsy, which normally is done to confirm the diagnosis, was never performed.
The rheumatologist made a tentative diagnosis of CD and referred her to a nutritionist, who recommended the patient adhere to a strict gluten-free diet. Within 3 months, the patient experienced marked improvement and returned to work.
CD is an often-missed diagnosis. According to a study based on National Health and Nutrition Examination Survey data, only 17% of patients with CD are aware they have the disease.2 As such, it is imperative that primary care physicians familiarize themselves with CD’s myriad clinical presentations, diagnosis, and treatment.3-6
Gluten triggers an immune response in genetically susceptible patients
CD initially was known as “celiac sprue” because it shares characteristics with tropical sprue—diarrhea, malabsorption, and emaciation. It is a unique T-cell autoimmune enteropathy that is precipitated in genetically susceptible individuals by the ingestion of gluten, the major storage protein of wheat, barley, and rye.3,7
Upon ingestion, gluten breaks down to gliadin, which provokes an immune response in the intestinal mucosa of patients with CD. This response results in an inflammatory reaction, primarily in the upper small intestine, that destroys the absorption surface and causes villous atrophy, leading to nutrient malabsorption and chronic diarrhea.8 CD is associated with significant morbidity due to an abnormal excretion of fat (steatorrhea) and varying degrees of malabsorption of vitamins A, D, and K, as well as B complex vitamins including B12 and folate; carbohydrates; protein; water; and minerals such as magnesium, calcium, and iron.9
CD develops only in individuals who possess alleles that encode for HLA-DQ2 or HLA-DQ8 proteins, products of 2 of the HLA genes. And while 30% of Caucasians carry the HLA-DQ2 allele and virtually 100% consume wheat, only 1 in 100 will develop CD.3,10,11 Although the genes are necessary, it is the interplay between genes (both HLA and non-HLA associated) and environment (ie, gluten) that leads to the intestinal mucosa damage typical of the disease. The HLA-DQ region also is associated with increased risk of type 1 diabetes, which might explain the correlation of CD to a host of other autoimmune disorders, including Graves’ disease and rheumatoid arthritis.8,10,11
Increased prevalence reflects better recognition of celiac disease
CD affects .6% to 1% of the population worldwide, with wide regional variation.3 Before the development of serologic assays in the 1970s, CD was a clinical diagnosis based on classic symptoms. With the advent of assays for immunoglobulin A (IgA) antibodies, the prevalence of CD has drastically increased to the current estimates of 1:250 to 1:500.4,5 The prevalence will continue to increase as clinicians become more aware of the different presentations of the disease, which are described below.
CD runs in families. Most patients with CD have a family history of the disease based on inheritance of the HLA alleles. A US study determined that the prevalence of CD was 1:22 in first-degree relatives and 1:39 in second-degree relatives of patients with biopsy-proven CD.12
Less than half of patients have GI symptoms
The classic presentation of CD involves a constellation of signs and symptoms of malabsorption: diarrhea, muscle wasting, and weight loss. Other typical gastrointestinal (GI) symptoms include bloating, flatulence, and abdominal pain.
Recognizing CD can be challenging, however, because <50% of patients diagnosed with CD present with these classic GI symptoms.3 About 50% of CD patients present with extra-intestinal symptoms, such as iron deficiency anemia, aphthous stomatitis, chronic fatigue, osteopenia, and dental enamel hypoplasia.3,8,13 Other possible non-GI symptoms include abnormal liver function test results and skin disorders such as dermatitis herpetiformis, a pruritic rash with cutaneous IgA deposits.3,8 In addition, many patients are asymptomatic.14 This highly variable clinical picture is due to the genetic and immunologic basis of the disease, extent of mucosal injury, and patients’ dietary habits, gender, and age of onset.15 A common clue that suggests a patient may have CD is unexplained iron deficiency anemia that does not improve with oral iron supplementation.4,13
Because symptoms may be intermittent, a patient may delay seeking care until he or she develops secondary manifestations, which often are debilitating and overshadow the GI complaints. Chronic complications of untreated CD include lymphoma and adenocarcinomas of the jejunum, recurrent miscarriages, neurologic disorders, osteoporosis, and hyposplenism.3,4,8
Since CD can manifest with widely varying symptoms, some researchers believe the disease should be classified into 3 categories based on presentation: classic CD, which presents with diarrhea, weight loss, malabsorption, and vitamin deficiency; atypical CD, which presents with minimal GI symptoms but can include anemia, neurologic symptoms, arthritis, or infertility; and asymptomatic CD, which typically displays no symptoms but usually is identified on incidental screening.3,8,16 Non-celiac gluten sensitivity is a distinct condition in which the body reacts adversely to gluten; it is not an autoimmune disease with an inflammatory response.
Order serologic testing for at-risk patients
Because CD remains underdiagnosed,16 taking a thorough family history and dietary history and making sure to at least consider CD as a part of a differential diagnosis is important. Although population-based screening has been proposed, its benefits and cost-effectiveness remain unproven. As a result, serologic testing of at-risk groups—individuals with conditions known to be associated with CD—remains the current standard.3 The TABLE lists groups for whom serologic testing for CD is indicated.16,17
In addition, the American College of Gastroenterology (ACG) and the North American Society for Pediatric Gastroenterology, Hepatology, and Nutrition (NASPGHAN) provide guidance on the diagnosis and treatment of adults and children with CD. (An ACG diagnostic algorithm is available at http://www.nature.com/ajg/journal/v108/n5/pdf/ajg201379a.pdf.)
Adults. For patients who are consuming a diet that includes gluten and have symptoms that suggest CD, the ACG guidelines recommend initial testing for IgA tissue transglutaminase (tTG) antibodies.16 The IgA tTG has a sensitivity and specificity >95%.16 An alternative test, the IgA endomysial (IgA EMA) test, has similar sensitivity but is time-consuming and its accuracy depends on the experience and skill of the laboratory technician. A negative result for either test has a high negative predictive value for CD.3,16
IgA deficiency is much more common in patients with CD than in the general population and can result in a false negative test for tTG and EMA. Therefore, consider taking a baseline IgA measurement first. If the patient has an IgA deficiency, the test you’ll use next will change: The preferred test for CD is either immunoglobulin G (IgG) tTG or IgG deamidated gliadin peptides (DGP).3,16
If a patient is already gluten-free... To rule out CD in patients who are already consuming a gluten-free diet, order HLA-DQ2 and HLA-DQ8 testing because these markers have a specificity >99%; if the HLA test is negative, the disease is excluded.8,16
Children. NASPGHAN recommends taking a baseline IgA measurement in children at risk for CD and then testing for IgA tTG antibodies, but not until patients are 3 years old and have been on a diet that includes gluten for at least 1 year.17 Repeat testing at a later date it is recommended for those with negative results because some evidence suggests that in certain patients, later serologic testing will be positive. Alternatively, you may offer HLA testing. If the HLA test is negative, CD can be excluded >99% of the time.
Diagnosis usually is confirmed by intestinal biopsy
Positive results on serologic testing should be confirmed with a biopsy of the small bowel; findings characteristic of CD include an increased number of intraepithelial lymphocytes (>25 per 100 enterocytes), elongation of the crypts, and partial to total villous atrophy.4 Final confirmation of CD is resolution of symptoms by consuming a gluten-free diet.3,8
Alternate approaches to confirming the diagnosis. Although intestinal biopsy has long been considered the gold standard for diagnosis of CD, this may change. In 2012, the European Society for Paediatric Gastroenterology, Hepatology, and Nutrition proposed that the biopsy may not be necessary in children with the following 3 characteristics: classic intestinal symptoms of CD, IgA tTG levels >10 times higher than normal, and a positive HLA-DQ2.18
Catassi and Fasano19 have proposed shifting from relying on algorithms and intestinal biopsy to a quantitative approach. They suggest using the “4 out of 5” rule, meaning the diagnosis of CD can be confirmed if at least 4 of the following 5 criteria are satisfied: typical CD symptoms, a positive IgA tTG, a positive HLA-DQ2 or -DQ8, celiac enteropathy on small bowel biopsy, and response to a gluten-free diet.19
The only proven treatment: A gluten-free diet
Lifelong adherence to a gluten-free diet is the only effective treatment for CD.14,16 Previously, patients with CD were advised to also avoid oats, but most evidence supports the safety of oats (<2 oz/d), provided there is no cross-contamination with gluten.14 Adhering to a strict gluten-free diet can be challenging because cereal flours are ubiquitous in western foods, and some foods may be cross-contaminated. The Celiac Disease Foundation (http://www.celiac.org) offers guidance on maintaining a gluten-free diet.
Because avoiding gluten has become popular even among people who don’t have CD, product labeling that includes information on gluten content has become pervasive. However, determining which items contain gluten depends on accurate labeling, a standard that often is not met in many countries; in the United States, such labeling began to be phased in starting in July 2014.20 As a result, CD patients may unwittingly be exposed to gluten over the long term, which can result in greater morbidity and mortality. Unless a food is labeled “gluten-free," it is best to check with the manufacturer.
Compliance with a gluten-free diet can be monitored by following IgA tTG titers every 1 to 2 years, as these values normalize after a patient has been adhering to the diet for 6 to 24 months.3,16
In addition to lifelong adherence to a gluten-free diet, a National Institutes of Health Consensus Development Conference recommended that management of patients with CD should include21:
- consultation with a skilled dietitian
- education about the disease
- continuous long-term follow-up by a multidisciplinary team
- identification and follow-up of abnormalities found at baseline, such as abnormal liver function test results
- treatment of nutritional deficiencies.
The ACG also recommends that CD patients receive a dual energy x-ray scan for follow-up of osteopenia and a pneumococcal vaccine because functional hyposplenism is associated with CD, and pneumonia is a common complication of hyposplenism.16,18
Compared to infants who are breastfed and don’t receive gluten until ages 4 to 6 months, infants who are fed gluten in their first 3 months have a significantly increased risk of developing antibodies that are associated with celiac disease (CD).22 Recent studies suggest that the effects of breast milk on the microbiota composition of the intestine may help explain this difference. Breast milk selectively stimulates the growth of specific bacteria, including bifidobacteria, which are relatively depleted in children with CD.23,24 Researchers believe breastfeeding and delaying introduction of gluten-containing foods until 4 to 6 months of age might protect against CD.25
Should you recommend a gluten-free diet for other patients? Because avoiding gluten is now popular and many gluten-free products are marketed as “health food,” physicians may be reluctant to recommend a gluten-free diet for patients who have vague abdominal symptoms but negative CD test results. Despite the current popularity of “going gluten-free,” the reality is that in addition to CD, many other diseases may be helped by a gluten-free diet, such as dermatitis herpetiformis, irritable bowel syndrome, and neurologic diseases such as gluten-sensitive ataxia.19 In the end, whether to adopt a gluten-free diet is a decision that you and your patient will need to make together.
Researchers are searching for additional treatments
Because many patients find it difficult to adhere to a gluten-free diet, researchers are investigating several alternative treatments, including a derivative from cholera toxin that inhibits the opening of intestinal epithelial junctions, thereby reducing the resultant inflammatory response, and a desensitizing vaccine.19,22,23 Another intriguing approach involves using the parasite Necator americanus to modulate the immune response to gluten.18 Finally, certain infant feeding practices, including breastfeeding and delaying introduction of gluten to the diet, may minimize the risk of developing CD. (See "A link between infant feeding practices and the risk of CD?" above.22-25)
CORRESPONDENCE
Patrick T. Dowling, MD, MPH, Department of Family Medicine, 50-078 Center for Health Sciences, David Geffen School of Medicine at UCLA, Los Angeles, CA 90095-1683; [email protected]
1. Sanders L. Hurt all over. New York Times Sunday Magazine. November 11, 2011:MM22.
2. Rubio-Tapia A, Ludvigsson JF, Brantner TL, et al. The prevalence of celiac disease in the United States. Am J Gastroenterol. 2012;107:1538-1544.
3. Fasano A, Catassi C. Clinical practice. Celiac disease. N Engl J Med. 2012;367:2419-2426.
4. Green PH, Cellier C. Celiac disease. N Engl J Med. 2007;357: 1731-1743.
5. Fasano A, Berti I, Gerarduzzi T, et al. Prevalence of celiac disease in at-risk and not-at-risk groups in the United States: a large multicenter study. Arch Intern Med. 2003;163:286-292.
6. Mustalahti K, Catassi C, Reunanen A, et al; Coeliac EU Cluster, Project Epidemiology. The prevalence of celiac disease in Europe: results of centralized, international mass screening project. Ann Med. 2010;42:587-595.
7. Farrel R, Kelly C. Celiac disease and refractory celiac disease. In: Feldman M, Friedman LS, Brandt LJ. Sleisenger and Fordtran’s Gastrointestinal and Liver Disease: Pathophysiology, Diagnosis, Management. 9th ed. Philadelphia, PA: Saunders; 2010: 1797-1820.
8. Gujral N, Freeman HJ, Thomson Ab. Celiac disease: prevalence, diagnosis, pathogenesis and treatment. World J Gastroenterol. 2012;18:6036-6059.
9. Sleisenger MH. Diseases of malabsorption. In: Beeson PB, McDermott W. Cecil-Loeb Textbook of Medicine. 13th ed. Philadelphia, PA: WB Saunders Company; 1971:1285-1291.
10. Reilly NR, Green PH. Epidemiology and clinical presentations of celiac disease. Sem Immunopathol. 2012;34:473-478.
11. Kagnoff MF. Celiac disease. A gastrointestinal disease with environmental, genetic, and immunologic components. Gastroenterol Clin North Am. 1992;21:405-425.
12. Riddle MS, Murray JA, Porter CK. The incidence and risk of celiac disease in a healthy US adult population. Am J Gastroenterol. 2012;107:1248-1255.
13. Rampertab SD, Pooran N, Brar P, et al. Trends in the presentation of celiac disease. Am J Med. 2006;119:355.e9-355.e14.
14. Pietzak M. Celiac disease, wheat allergy, and gluten sensitivity: when gluten free is not a fad. JPEN J Parenter Enteral Nutr. 2012;36(1 suppl):68S-75S.
15. Sapone A, Bai JC, Ciacci C, et al. Spectrum of gluten-related disorders: consensus on new nomenclature and classification. BMC Med. 2012;10:13.
16. Rubio-Tapia A, Hill ID, Kelly CP, et al; American College of Gastroenterology. ACG clinical guidelines: diagnosis and management celiac disease. Am J Gastroenterol. 2013;108:656-676.
17. Hill ID, Dirks MH, Liptak GS, et al; North American Society for Pediatric Gastroenterology, Hepatology and Nutrition. Guidelines for the diagnosis and treatment of celiac disease in children: recommendations of the North American Society for Pediatric Gastroenterology, Hepatology and Nutrition. J Pediatr Gastroentertol Nutr. 2005;40:1-19.
18. Husby S, Koletsko S, Korponay-Szabó IR, et al; ESPGHAN Working Group on Coeliac Disease Diagnosis; ESPGHAN Gastoenterology Committee; European Society for Pediatric Gastroenterology, Hepatology, and Nutrition. European Society for Pediatric Gastroenterology, Hepatology and Nutrition Guidelines for the diagnosis of coeliac disease. J Pediatr Gastroenterol Nutr. 2012;54:136-160.
19. Catassi C, Fasano A. Celiac disease diagnosis: simple rules are better than complicated algorithms. Am J Med. 2010;123:691-693.
20. US Food and Drug Administration. Foods labeled gluten-free must now meet FDA's definition. Available at: http://www.fda. gov/Food/NewsEvents/ConstituentUpdates/ucm407867.htm. Accessed August 13, 2014.
21. National Institutes of Health Consensus Development Conference on Celiac Disease. National Institutes of Health Consensus Development Conference Statement. Available at: http://consensus.nih.gov/2004/2004celiacdisease118html.htm. Accessed August 13, 2014.
22. Norris JM, Barriga K, Hoffenberg EJ, et al. Risk of celiac disease autoimmunity and timing of gluten introduction in the diet of infants at increased risk of disease. JAMA. 2005;293:2343-2351.
23. Pozo-Rubio T, Olivares M, Nova E, et al. Immune development and intestinal microbiota in celiac disease. Clin Dev Immunol. 2012;2012:654143
24. Ivarsson A, Myléus A, Norström F, et al. Prevalence of childhood celiac disease and changes in infant feeding. Pediatrics. 2013;131:687-694.
25. Cataldo F, Montalto G. Celiac disease in the developing countries: a new and challenging public health problem. World J Gastroenterol. 2007;13:2153-2159.
1. Sanders L. Hurt all over. New York Times Sunday Magazine. November 11, 2011:MM22.
2. Rubio-Tapia A, Ludvigsson JF, Brantner TL, et al. The prevalence of celiac disease in the United States. Am J Gastroenterol. 2012;107:1538-1544.
3. Fasano A, Catassi C. Clinical practice. Celiac disease. N Engl J Med. 2012;367:2419-2426.
4. Green PH, Cellier C. Celiac disease. N Engl J Med. 2007;357: 1731-1743.
5. Fasano A, Berti I, Gerarduzzi T, et al. Prevalence of celiac disease in at-risk and not-at-risk groups in the United States: a large multicenter study. Arch Intern Med. 2003;163:286-292.
6. Mustalahti K, Catassi C, Reunanen A, et al; Coeliac EU Cluster, Project Epidemiology. The prevalence of celiac disease in Europe: results of centralized, international mass screening project. Ann Med. 2010;42:587-595.
7. Farrel R, Kelly C. Celiac disease and refractory celiac disease. In: Feldman M, Friedman LS, Brandt LJ. Sleisenger and Fordtran’s Gastrointestinal and Liver Disease: Pathophysiology, Diagnosis, Management. 9th ed. Philadelphia, PA: Saunders; 2010: 1797-1820.
8. Gujral N, Freeman HJ, Thomson Ab. Celiac disease: prevalence, diagnosis, pathogenesis and treatment. World J Gastroenterol. 2012;18:6036-6059.
9. Sleisenger MH. Diseases of malabsorption. In: Beeson PB, McDermott W. Cecil-Loeb Textbook of Medicine. 13th ed. Philadelphia, PA: WB Saunders Company; 1971:1285-1291.
10. Reilly NR, Green PH. Epidemiology and clinical presentations of celiac disease. Sem Immunopathol. 2012;34:473-478.
11. Kagnoff MF. Celiac disease. A gastrointestinal disease with environmental, genetic, and immunologic components. Gastroenterol Clin North Am. 1992;21:405-425.
12. Riddle MS, Murray JA, Porter CK. The incidence and risk of celiac disease in a healthy US adult population. Am J Gastroenterol. 2012;107:1248-1255.
13. Rampertab SD, Pooran N, Brar P, et al. Trends in the presentation of celiac disease. Am J Med. 2006;119:355.e9-355.e14.
14. Pietzak M. Celiac disease, wheat allergy, and gluten sensitivity: when gluten free is not a fad. JPEN J Parenter Enteral Nutr. 2012;36(1 suppl):68S-75S.
15. Sapone A, Bai JC, Ciacci C, et al. Spectrum of gluten-related disorders: consensus on new nomenclature and classification. BMC Med. 2012;10:13.
16. Rubio-Tapia A, Hill ID, Kelly CP, et al; American College of Gastroenterology. ACG clinical guidelines: diagnosis and management celiac disease. Am J Gastroenterol. 2013;108:656-676.
17. Hill ID, Dirks MH, Liptak GS, et al; North American Society for Pediatric Gastroenterology, Hepatology and Nutrition. Guidelines for the diagnosis and treatment of celiac disease in children: recommendations of the North American Society for Pediatric Gastroenterology, Hepatology and Nutrition. J Pediatr Gastroentertol Nutr. 2005;40:1-19.
18. Husby S, Koletsko S, Korponay-Szabó IR, et al; ESPGHAN Working Group on Coeliac Disease Diagnosis; ESPGHAN Gastoenterology Committee; European Society for Pediatric Gastroenterology, Hepatology, and Nutrition. European Society for Pediatric Gastroenterology, Hepatology and Nutrition Guidelines for the diagnosis of coeliac disease. J Pediatr Gastroenterol Nutr. 2012;54:136-160.
19. Catassi C, Fasano A. Celiac disease diagnosis: simple rules are better than complicated algorithms. Am J Med. 2010;123:691-693.
20. US Food and Drug Administration. Foods labeled gluten-free must now meet FDA's definition. Available at: http://www.fda. gov/Food/NewsEvents/ConstituentUpdates/ucm407867.htm. Accessed August 13, 2014.
21. National Institutes of Health Consensus Development Conference on Celiac Disease. National Institutes of Health Consensus Development Conference Statement. Available at: http://consensus.nih.gov/2004/2004celiacdisease118html.htm. Accessed August 13, 2014.
22. Norris JM, Barriga K, Hoffenberg EJ, et al. Risk of celiac disease autoimmunity and timing of gluten introduction in the diet of infants at increased risk of disease. JAMA. 2005;293:2343-2351.
23. Pozo-Rubio T, Olivares M, Nova E, et al. Immune development and intestinal microbiota in celiac disease. Clin Dev Immunol. 2012;2012:654143
24. Ivarsson A, Myléus A, Norström F, et al. Prevalence of childhood celiac disease and changes in infant feeding. Pediatrics. 2013;131:687-694.
25. Cataldo F, Montalto G. Celiac disease in the developing countries: a new and challenging public health problem. World J Gastroenterol. 2007;13:2153-2159.
Biliary pain, no gallstones—remove the gallbladder, anyway?
CASE 1 › A 28-year-old woman (G0P0) came to our office with recurrent episodes of postprandial epigastric and right upper quadrant pain. Upper and lower endoscopy, sonography, body imaging, and laboratory tests were normal. A biliary nuclear scan showed an ejection fraction (EF) of 95%; normal is >35%. We made a diagnosis of biliary dyskinesia (BD) and recommended a laparoscopic cholecystectomy. The patient underwent this procedure and her pain was relieved. She has been much improved for 2 years, although she has since been diagnosed with an autoimmune disorder.
CASE 2 › A 21-year-old woman with right upper quadrant, postprandial, colicky pain presented to the emergency department. The episode lasted approximately 30 minutes and was followed by residual soreness. This episode was one of several that had been increasing in frequency and intensity. A sonogram showed a normal gallbladder and common duct. All laboratory tests were normal. She improved and was discharged. Outpatient evaluation included body imaging and endoscopy, which were negative. A hepatobiliary (HIDA) scan revealed an EF of 90%, and the scan reproduced her symptoms.
We diagnosed BD in this patient. After reviewing the risks and benefits of cholecystectomy, the patient consented to the procedure. She has been asymptomatic for 2 years.
Family physicians often are the first to evaluate patients with recurrent biliary colic. Biliary colic without gallstones—also known as BD or acalculous cholecystitis—is a functional disorder of the gallbladder or bile duct. Approximately 8% of men and 21% of women with biliary pain do not have gallstones.1-5
BD has been successfully treated with cholecystectomy. Physicians typically have viewed cholecystectomy as being effective primarily for patients with biliary pain who have a low EF (<35%).2-4 However, recent studies and our experience with cholecystectomy in these 2 patients with high EFs suggest that EF is only one of several factors to consider when deciding whether cholecystectomy might be appropriate for a given patient.
Which patients are most likely to benefit from cholecystectomy?
BD is a diagnosis of exclusion, considered when other upper abdominal disorders are eliminated. To receive a diagnosis of BD, patients must meet the Rome III criteria (TABLE).2
Before the advent of oral cholecystography in the 1920s, biliary disease was a clinical diagnosis confirmed by examination of the excised gallbladder.6 In 2 large studies conducted before cholecystography was in common use, researchers noted improvement in 75% to 85% of BD patients after cholecystectomy.7,8 Several years later, with the benefit, of cholecystography, Mackey9 reported similar improvement rates among patients with BD who underwent cholecystectomy.
Cholecystography has largely been replaced with HIDA scanning, which provides an objective measure of EF. Although some studies have suggested low EFs may predict which patients will benefit from cholecystectomy, others have suggested this value doesn’t tell the whole story.2,4,10,11 In some studies, patients who had biliary symptoms and a low EF (<35%) were found to be most likely to experience relief after cholecystectomy.2,4 More recently, in a chart review, DuCoin et al10 found that of 19 BD patients with an EF >35% who underwent cholecystectomy, 17 had complete symptom resolution, one had partial resolution, and one was unchanged. Only one abstract of a study of cholecystectomy for BD patients with a high EF (>80%) has been published.11 Of 28 patients who received cholecystectomy, 22 were asymptomatic after cholecystectomy and 5 others improved.11
Other tests to consider. A cholecystokinin infusion without a scan has been used to reproduce biliary colic; some physicians consider this to be diagnostic of BD and sufficient for cholecystectomy.12 Others have advocated endoscopic injection of botulinum into the sphincter of Oddi to differentiate pain arising from the sphincter of Oddi from pain in the gallbladder.5,13 If symptoms are relieved by this injection, an endoscopic biliary sphincterotomy—cutting of the biliary sphincter—is done. Cholecystectomy is reserved for patients whose pain is not relieved by botulinum. In an initial report, 25 BD patients received botulinum injections into the sphincter of Oddi; of the 11 whose pain was relieved by this injection, 10 underwent endoscopic biliary sphincterotomy, and pain resolved for all of these patients.13
Why we chose cholecystectomy for our patients
Despite a plethora of tests available to visualize and assess gallbladder and bile duct function, clinical assessment of BD by experienced physicians may be sufficient to determine which BD patients will benefit from cholecystectomy. In the cases we report on here, each patient had a high EF, but both met Rome III criteria and were experiencing clinically significant pain. Also, for both patients, a cholecystokinin infusion administered to calculate EF reproduced their pain. This clinical picture led us to recommend laparoscopic cholecystectomy, which ultimately relieved their symptoms.
CORRESPONDENCE
Mazen Iskandar, MD, The Pancreas and Biliary Center of New York, Beth Israel Medical Center, 350 East 17th Street, 16 Baird Hall, New York, NY 10010; [email protected]
1. Drossman DA, Dumitrascu DL. Rome III: new standard for functional gastrointestinal disorders. J Gastrointest Liver Dis. 2006;15:237-241.
2. Hansel SL, DiBaise JK. Functional gallbladder disorder: gallbladder dyskinesia. Gastroenterol Clin North Am. 2010;39:369-379.
3. Francis G, Baillie J. Gallbladder dyskinesia: fact or fiction? Curr Gastroenterol Rep. 2011;13:188-192.
4. Yap L, Wycherley AG, Morphett AD, et al. Acalculous biliary pain; cholecystectomy alleviates symptoms in patients with abnormal cholescintigraphy. Gastroenterology. 1991;101:786-793.
5. Behar J, Corazziari E, Guelrud M, et al. Functional gallbladder and sphincter of oddi disorders. Gastroenterology. 2006;130: 1498-1509.
6. Graham EA, Cole WH. Roentgenologic examination of the gallbladder: preliminary report of a new method utilizing the intravenous injection of tetrabromophenolphthalein. JAMA. 1924;82:613-614.
7. Blalock A. A study of eight hundred and eighty-eight cases of biliary tract disease. Johns Hopkins Hosp Bull. 1924;35:391-409.
8. Whipple AO. Surgical criteria for cholecystectomy. Bull N Y Acad Med. 1926;2:302-306.
9. Mackey WA. Cholecystitis without stone an investigation of 264 operated cases from the clinical, radiological, and pathological aspects. An attempt to determine the factors of service in estimating prognosis. Br J Surg. 1934;22:274-295.
10. DuCoin C, Faber R, Ilagan M, et al. Normokinetic biliary dyskinesia: a novel diagnosis. Surg Endosc. 2012;26:3088-3093.
11. Holes-Lewis KA, Hakim S, Rehman F, al. CCK-induced gall bladder hyperkinesia: An indication for cholecystectomy and brain-GI connectivity research. J Nucl Med. 2009;50(suppl 2):1312.
12. Carr JA, Walls J, Bryan LJ, et al. The treatment of gallbladder dyskinesia based upon symptoms: results of a 2-year, prospective, nonrandomized, concurrent cohort study. Surg Laparosc Endosc Percutan Tech. 2009;19:222-226.
13. Murray WR. Botulinum toxin-induced relaxation of the sphincter of Oddi may select patients with acalculous biliary pain who will benefit from cholecystectomy. Surg Endosc. 2011;25:813-816.
CASE 1 › A 28-year-old woman (G0P0) came to our office with recurrent episodes of postprandial epigastric and right upper quadrant pain. Upper and lower endoscopy, sonography, body imaging, and laboratory tests were normal. A biliary nuclear scan showed an ejection fraction (EF) of 95%; normal is >35%. We made a diagnosis of biliary dyskinesia (BD) and recommended a laparoscopic cholecystectomy. The patient underwent this procedure and her pain was relieved. She has been much improved for 2 years, although she has since been diagnosed with an autoimmune disorder.
CASE 2 › A 21-year-old woman with right upper quadrant, postprandial, colicky pain presented to the emergency department. The episode lasted approximately 30 minutes and was followed by residual soreness. This episode was one of several that had been increasing in frequency and intensity. A sonogram showed a normal gallbladder and common duct. All laboratory tests were normal. She improved and was discharged. Outpatient evaluation included body imaging and endoscopy, which were negative. A hepatobiliary (HIDA) scan revealed an EF of 90%, and the scan reproduced her symptoms.
We diagnosed BD in this patient. After reviewing the risks and benefits of cholecystectomy, the patient consented to the procedure. She has been asymptomatic for 2 years.
Family physicians often are the first to evaluate patients with recurrent biliary colic. Biliary colic without gallstones—also known as BD or acalculous cholecystitis—is a functional disorder of the gallbladder or bile duct. Approximately 8% of men and 21% of women with biliary pain do not have gallstones.1-5
BD has been successfully treated with cholecystectomy. Physicians typically have viewed cholecystectomy as being effective primarily for patients with biliary pain who have a low EF (<35%).2-4 However, recent studies and our experience with cholecystectomy in these 2 patients with high EFs suggest that EF is only one of several factors to consider when deciding whether cholecystectomy might be appropriate for a given patient.
Which patients are most likely to benefit from cholecystectomy?
BD is a diagnosis of exclusion, considered when other upper abdominal disorders are eliminated. To receive a diagnosis of BD, patients must meet the Rome III criteria (TABLE).2
Before the advent of oral cholecystography in the 1920s, biliary disease was a clinical diagnosis confirmed by examination of the excised gallbladder.6 In 2 large studies conducted before cholecystography was in common use, researchers noted improvement in 75% to 85% of BD patients after cholecystectomy.7,8 Several years later, with the benefit, of cholecystography, Mackey9 reported similar improvement rates among patients with BD who underwent cholecystectomy.
Cholecystography has largely been replaced with HIDA scanning, which provides an objective measure of EF. Although some studies have suggested low EFs may predict which patients will benefit from cholecystectomy, others have suggested this value doesn’t tell the whole story.2,4,10,11 In some studies, patients who had biliary symptoms and a low EF (<35%) were found to be most likely to experience relief after cholecystectomy.2,4 More recently, in a chart review, DuCoin et al10 found that of 19 BD patients with an EF >35% who underwent cholecystectomy, 17 had complete symptom resolution, one had partial resolution, and one was unchanged. Only one abstract of a study of cholecystectomy for BD patients with a high EF (>80%) has been published.11 Of 28 patients who received cholecystectomy, 22 were asymptomatic after cholecystectomy and 5 others improved.11
Other tests to consider. A cholecystokinin infusion without a scan has been used to reproduce biliary colic; some physicians consider this to be diagnostic of BD and sufficient for cholecystectomy.12 Others have advocated endoscopic injection of botulinum into the sphincter of Oddi to differentiate pain arising from the sphincter of Oddi from pain in the gallbladder.5,13 If symptoms are relieved by this injection, an endoscopic biliary sphincterotomy—cutting of the biliary sphincter—is done. Cholecystectomy is reserved for patients whose pain is not relieved by botulinum. In an initial report, 25 BD patients received botulinum injections into the sphincter of Oddi; of the 11 whose pain was relieved by this injection, 10 underwent endoscopic biliary sphincterotomy, and pain resolved for all of these patients.13
Why we chose cholecystectomy for our patients
Despite a plethora of tests available to visualize and assess gallbladder and bile duct function, clinical assessment of BD by experienced physicians may be sufficient to determine which BD patients will benefit from cholecystectomy. In the cases we report on here, each patient had a high EF, but both met Rome III criteria and were experiencing clinically significant pain. Also, for both patients, a cholecystokinin infusion administered to calculate EF reproduced their pain. This clinical picture led us to recommend laparoscopic cholecystectomy, which ultimately relieved their symptoms.
CORRESPONDENCE
Mazen Iskandar, MD, The Pancreas and Biliary Center of New York, Beth Israel Medical Center, 350 East 17th Street, 16 Baird Hall, New York, NY 10010; [email protected]
CASE 1 › A 28-year-old woman (G0P0) came to our office with recurrent episodes of postprandial epigastric and right upper quadrant pain. Upper and lower endoscopy, sonography, body imaging, and laboratory tests were normal. A biliary nuclear scan showed an ejection fraction (EF) of 95%; normal is >35%. We made a diagnosis of biliary dyskinesia (BD) and recommended a laparoscopic cholecystectomy. The patient underwent this procedure and her pain was relieved. She has been much improved for 2 years, although she has since been diagnosed with an autoimmune disorder.
CASE 2 › A 21-year-old woman with right upper quadrant, postprandial, colicky pain presented to the emergency department. The episode lasted approximately 30 minutes and was followed by residual soreness. This episode was one of several that had been increasing in frequency and intensity. A sonogram showed a normal gallbladder and common duct. All laboratory tests were normal. She improved and was discharged. Outpatient evaluation included body imaging and endoscopy, which were negative. A hepatobiliary (HIDA) scan revealed an EF of 90%, and the scan reproduced her symptoms.
We diagnosed BD in this patient. After reviewing the risks and benefits of cholecystectomy, the patient consented to the procedure. She has been asymptomatic for 2 years.
Family physicians often are the first to evaluate patients with recurrent biliary colic. Biliary colic without gallstones—also known as BD or acalculous cholecystitis—is a functional disorder of the gallbladder or bile duct. Approximately 8% of men and 21% of women with biliary pain do not have gallstones.1-5
BD has been successfully treated with cholecystectomy. Physicians typically have viewed cholecystectomy as being effective primarily for patients with biliary pain who have a low EF (<35%).2-4 However, recent studies and our experience with cholecystectomy in these 2 patients with high EFs suggest that EF is only one of several factors to consider when deciding whether cholecystectomy might be appropriate for a given patient.
Which patients are most likely to benefit from cholecystectomy?
BD is a diagnosis of exclusion, considered when other upper abdominal disorders are eliminated. To receive a diagnosis of BD, patients must meet the Rome III criteria (TABLE).2
Before the advent of oral cholecystography in the 1920s, biliary disease was a clinical diagnosis confirmed by examination of the excised gallbladder.6 In 2 large studies conducted before cholecystography was in common use, researchers noted improvement in 75% to 85% of BD patients after cholecystectomy.7,8 Several years later, with the benefit, of cholecystography, Mackey9 reported similar improvement rates among patients with BD who underwent cholecystectomy.
Cholecystography has largely been replaced with HIDA scanning, which provides an objective measure of EF. Although some studies have suggested low EFs may predict which patients will benefit from cholecystectomy, others have suggested this value doesn’t tell the whole story.2,4,10,11 In some studies, patients who had biliary symptoms and a low EF (<35%) were found to be most likely to experience relief after cholecystectomy.2,4 More recently, in a chart review, DuCoin et al10 found that of 19 BD patients with an EF >35% who underwent cholecystectomy, 17 had complete symptom resolution, one had partial resolution, and one was unchanged. Only one abstract of a study of cholecystectomy for BD patients with a high EF (>80%) has been published.11 Of 28 patients who received cholecystectomy, 22 were asymptomatic after cholecystectomy and 5 others improved.11
Other tests to consider. A cholecystokinin infusion without a scan has been used to reproduce biliary colic; some physicians consider this to be diagnostic of BD and sufficient for cholecystectomy.12 Others have advocated endoscopic injection of botulinum into the sphincter of Oddi to differentiate pain arising from the sphincter of Oddi from pain in the gallbladder.5,13 If symptoms are relieved by this injection, an endoscopic biliary sphincterotomy—cutting of the biliary sphincter—is done. Cholecystectomy is reserved for patients whose pain is not relieved by botulinum. In an initial report, 25 BD patients received botulinum injections into the sphincter of Oddi; of the 11 whose pain was relieved by this injection, 10 underwent endoscopic biliary sphincterotomy, and pain resolved for all of these patients.13
Why we chose cholecystectomy for our patients
Despite a plethora of tests available to visualize and assess gallbladder and bile duct function, clinical assessment of BD by experienced physicians may be sufficient to determine which BD patients will benefit from cholecystectomy. In the cases we report on here, each patient had a high EF, but both met Rome III criteria and were experiencing clinically significant pain. Also, for both patients, a cholecystokinin infusion administered to calculate EF reproduced their pain. This clinical picture led us to recommend laparoscopic cholecystectomy, which ultimately relieved their symptoms.
CORRESPONDENCE
Mazen Iskandar, MD, The Pancreas and Biliary Center of New York, Beth Israel Medical Center, 350 East 17th Street, 16 Baird Hall, New York, NY 10010; [email protected]
1. Drossman DA, Dumitrascu DL. Rome III: new standard for functional gastrointestinal disorders. J Gastrointest Liver Dis. 2006;15:237-241.
2. Hansel SL, DiBaise JK. Functional gallbladder disorder: gallbladder dyskinesia. Gastroenterol Clin North Am. 2010;39:369-379.
3. Francis G, Baillie J. Gallbladder dyskinesia: fact or fiction? Curr Gastroenterol Rep. 2011;13:188-192.
4. Yap L, Wycherley AG, Morphett AD, et al. Acalculous biliary pain; cholecystectomy alleviates symptoms in patients with abnormal cholescintigraphy. Gastroenterology. 1991;101:786-793.
5. Behar J, Corazziari E, Guelrud M, et al. Functional gallbladder and sphincter of oddi disorders. Gastroenterology. 2006;130: 1498-1509.
6. Graham EA, Cole WH. Roentgenologic examination of the gallbladder: preliminary report of a new method utilizing the intravenous injection of tetrabromophenolphthalein. JAMA. 1924;82:613-614.
7. Blalock A. A study of eight hundred and eighty-eight cases of biliary tract disease. Johns Hopkins Hosp Bull. 1924;35:391-409.
8. Whipple AO. Surgical criteria for cholecystectomy. Bull N Y Acad Med. 1926;2:302-306.
9. Mackey WA. Cholecystitis without stone an investigation of 264 operated cases from the clinical, radiological, and pathological aspects. An attempt to determine the factors of service in estimating prognosis. Br J Surg. 1934;22:274-295.
10. DuCoin C, Faber R, Ilagan M, et al. Normokinetic biliary dyskinesia: a novel diagnosis. Surg Endosc. 2012;26:3088-3093.
11. Holes-Lewis KA, Hakim S, Rehman F, al. CCK-induced gall bladder hyperkinesia: An indication for cholecystectomy and brain-GI connectivity research. J Nucl Med. 2009;50(suppl 2):1312.
12. Carr JA, Walls J, Bryan LJ, et al. The treatment of gallbladder dyskinesia based upon symptoms: results of a 2-year, prospective, nonrandomized, concurrent cohort study. Surg Laparosc Endosc Percutan Tech. 2009;19:222-226.
13. Murray WR. Botulinum toxin-induced relaxation of the sphincter of Oddi may select patients with acalculous biliary pain who will benefit from cholecystectomy. Surg Endosc. 2011;25:813-816.
1. Drossman DA, Dumitrascu DL. Rome III: new standard for functional gastrointestinal disorders. J Gastrointest Liver Dis. 2006;15:237-241.
2. Hansel SL, DiBaise JK. Functional gallbladder disorder: gallbladder dyskinesia. Gastroenterol Clin North Am. 2010;39:369-379.
3. Francis G, Baillie J. Gallbladder dyskinesia: fact or fiction? Curr Gastroenterol Rep. 2011;13:188-192.
4. Yap L, Wycherley AG, Morphett AD, et al. Acalculous biliary pain; cholecystectomy alleviates symptoms in patients with abnormal cholescintigraphy. Gastroenterology. 1991;101:786-793.
5. Behar J, Corazziari E, Guelrud M, et al. Functional gallbladder and sphincter of oddi disorders. Gastroenterology. 2006;130: 1498-1509.
6. Graham EA, Cole WH. Roentgenologic examination of the gallbladder: preliminary report of a new method utilizing the intravenous injection of tetrabromophenolphthalein. JAMA. 1924;82:613-614.
7. Blalock A. A study of eight hundred and eighty-eight cases of biliary tract disease. Johns Hopkins Hosp Bull. 1924;35:391-409.
8. Whipple AO. Surgical criteria for cholecystectomy. Bull N Y Acad Med. 1926;2:302-306.
9. Mackey WA. Cholecystitis without stone an investigation of 264 operated cases from the clinical, radiological, and pathological aspects. An attempt to determine the factors of service in estimating prognosis. Br J Surg. 1934;22:274-295.
10. DuCoin C, Faber R, Ilagan M, et al. Normokinetic biliary dyskinesia: a novel diagnosis. Surg Endosc. 2012;26:3088-3093.
11. Holes-Lewis KA, Hakim S, Rehman F, al. CCK-induced gall bladder hyperkinesia: An indication for cholecystectomy and brain-GI connectivity research. J Nucl Med. 2009;50(suppl 2):1312.
12. Carr JA, Walls J, Bryan LJ, et al. The treatment of gallbladder dyskinesia based upon symptoms: results of a 2-year, prospective, nonrandomized, concurrent cohort study. Surg Laparosc Endosc Percutan Tech. 2009;19:222-226.
13. Murray WR. Botulinum toxin-induced relaxation of the sphincter of Oddi may select patients with acalculous biliary pain who will benefit from cholecystectomy. Surg Endosc. 2011;25:813-816.
How to avoid 3 common errors in dementia screening
› Use age- and education-corrected normative data when using dementia screening tools. C
› Use verbatim instructions and the same size stimuli and response pages provided in a test’s manual. C
› Ensure that norms used for comparisons are current. C
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Treatment options for dementia are expanding and improving, giving extra impetus to detecting this progressive disease as early as possible. For example, research on the cholinesterase inhibitor donepezil has shown it can delay cognitive decline by 6 months or more compared with controls1,2 and possibly postpone institutionalization. With the number of elderly individuals and cases of dementia projected to grow significantly over the next 20 years,3 primary care physicians will increasingly be screening for cognitive impairment. Given the time constraints and patient loads in today’s practices, it’s not surprising that physicians tend to use evaluation tools that are brief and simple to administer. However, there are also serious pitfalls in the use of these tools.
When to screen. Many health-related organizations address screening for dementia4,5 and offer screening criteria (eg, the Alzheimer’s Association,6 the US Preventive Services Task Force7). Our experience suggests that specific behavioral changes are reasonable indicators of suspected dementia that should prompt cognitive screening. Using the Kingston Standardized Behavioural Assessment,8 we demonstrated a consistent pattern of earliest behavior change in a community-dwelling group with dementia.9 Meaningful clues are a decreased ability to engage in specific functional activities (including participation in favorite pastimes, ability to eat properly if left to prepare one’s own food, handling of personal finances, word finding, and reading) and unsteadiness. These specific behavioral changes reported by family or a caregiver suggest the need for cognitive screening.
Pitfalls associated with common screening tools, if not taken into account, can seriously limit the usefulness of information gained during assessment and potentially lead to a wrong conclusion. Screening tools are just that: a means of detecting the possible existence of a condition. Results are based on probability and subject to error. Therefore, a single test score is insufficient to render a diagnosis of dementia, and is one variable in a set of diagnostic criteria.
The purpose of this article is to review some of the most commonly used tools and procedures for dementia screening, identify procedural or interpretive errors made in everyday clinical practice, and suggest practical yet simple strategies to address these problems and improve the accuracy of assessments. We illustrate key points with clinical examples and vignettes using the Mini-Mental State Examination (MMSE),10 an Animal Naming Task, and the Trail Making Test.11
Common error #1: Reliance on simple, single cutoff scores
There are a number of important considerations to keep in mind when trying to make sense of scores from the many available cognitive tests.
The range of normal test results is wide. The normal range for most physiologic measures, such as glucose levels or hemoglobin counts, is relatively narrow. However, human cognitive functions can naturally differ from person to person, and the range of normal can be extremely large.
A single, all-purpose cutoff score ignores critical factors. Very often, clinicians have dealt with the issue of wide variance in cognition scores by establishing a general cutoff point to serve as a pass-fail mark. But this practice can result in both under- and overidentification of dementia, and it ignores the 2 components that chiefly determine how individuals differ cognitively: age and intelligence.
Practical fix: Use age-, intelligence-corrected normative data
Level of cognitive performance can be revealing when adjustments are made for age and intelligence. Not taking these factors into account can lead to many errors in clinical decision making.
Age matters. Many cognitive capacities decline as part of normal aging even in otherwise healthy individuals (eg, reaction time, spatial abilities, flexibility in novel problem solving).12 With this in mind, psychologists often have made the distinction between “hold” tests (remaining stable or even improving with age) and “no-hold” tests (declining with age).13 Therefore it is critical to ask, “What is normal, given a particular patient’s age?” If normative data corrected for age are available for a given test, use them.
Intelligence is a factor, too. Intelligence, like most human qualities, is distributed along a bell-shaped curve of normal distribution, wherein most people fall somewhere in the middle and a smaller number will be at the lower and higher tails of the curve. Not all of us fall into the average range of intelligence; indeed, psychometrically, only half of us do. The other half are found somewhere in the more extreme ends. In evaluating a person for dementia, it is critical to compare test results with those found in the appropriate intellectual group. But how does the physician looking for a brief assessment strategy determine a patient’s premorbid level of intellectual functioning?
A widely used and accepted heuristic for gauging intelligence is “years of education.” Of course, education is not perfectly correlated with intelligence, particularly as those who are now elderly may have been denied the opportunity to attend school due to the Great Depression, war, or other life events. Nevertheless, with these limitations in mind, level of education is a reasonable approximation of intelligence. In practical application, premorbid intellectual level is determined by using education-corrected normative data.
Typically with cognitive tests, cutoff scores and score ranges are defined for general levels of education (eg, less than grade 12 or more than grade 12; elementary school, high school, post-secondary, etc). Adjusted norms for age and education are usually determined by taking large samples of subjects and stratifying the distribution by subgroups—eg, 5-year age groups; levels of education such as elementary school or high school—and then statistically analyzing each group and noting the relative differences between them.
Illustration: MMSE. Although not designed for the overall measurement of cognitive impairment in dementia, the MMSE10 has become widely used for that purpose. It is fairly insensitive to cognitive changes associated with earlier stages of dementia,14 and is intended only as a means of identifying patients in need of more comprehensive assessment. However, the MMSE is increasingly used to make a diagnosis of dementia.15 In some areas (eg, Ontario, Canada), it is used to justify paying for treatment with cognitive enhancers.
The universal cutoff score proves inadequate. Although several dementia cutoff scores for the MMSE have been proposed, it is common practice to use an MMSE score ≥24 to rule out dementia.16 In our clinical practice, however, many patients who ultimately are diagnosed with early dementia often perform well on the MMSE, although rather poorly on other dementia screens, such as the Kingston Standardized Cognitive Assessment-Revised (KSCAr)17 or the mini-KSCAr.18
Recently, we reviewed cases of >70 individuals from our outpatient clinic who were given the MMSE and were also diagnosed as having dementia by both DSM-IV (Diagnostic and Statistical Manual of Mental Disorders)19 and the National Institute of Neurological and Communicative Disorders and Stroke and the Alzheimer’s Disease and Related Disorders Association20 criteria. Over three-quarters (78%) of these cases had an MMSE score of ≥24. Based on MMSE scores alone, these individuals would have been declared “not demented.”17
Correcting for age and intelligence increases accuracy. Published age and education norms are available for the MMSE.21 In applying these norms to our sample described above, the number of misidentified patients drops to approximately one-third (35.7%). This means that instead of misidentifying 2 out of 3 cases, the age and education corrections reduced this to about one out of 3, thereby increasing sensitivity and specificity. While this is still an unacceptably high rate of false negatives, it shows the considerable value of using age and education corrections.
The challenge of optimizing sensitivity and specificity of dementia screening tools is ongoing. As a matter of interest, we include TABLE 1,4,18,22-24 which shows calculated sensitivities and specificities of some commonly used screening tests.
Another practical fix: Use distributions and percentile-based normative data
Instead of simple cutoff scores, test scores can be, and often are, translated into percentiles to provide a meaningful context for evaluation and to make it easier to compare scores between patients. Someone with a score at the 70th percentile has performed as well as or better than 70% of others in the group who have taken the test. Usually, the average range of a normal population is defined as being between the 25th to 75th percentiles, encompassing 50% of that population. In general, percentiles make interpreting performance easier. Percentile-based test norms can also help determine with increased accuracy if there has been a decline over time.
Illustration: Animal naming task. In a common version of this task, patients are asked to name as many animals as they can in 60 seconds. This task has its roots in neuro- psychological tests of verbal fluency, such as the Controlled Oral Word Association Task.25 Verbal fluency tasks such as naming animals tap verbal generativity/problem-solving and self-monitoring, but are also highly dependent on vocabulary (word knowledge), a cognitive ability that is quite stable and even improves as one ages until individuals are well into their 80s.26
It is common practice with this procedure to consider a cutoff score of 15 as a minimally acceptable level of performance.27 Here again, there are potentially great differences in expected performance based on age and intelligence. TABLE 2 shows the effect of age and education on verbal fluency, expressed as percentiles, using a raw score of 15.28 For an individual in their early 60s who has a university degree, naming just 15 animals puts their performance at the 12th percentile (below average). The same performance for someone in their 90s who has only 8 years of education puts them in the 79th percentile (above the average range of 25th-75th percentiles). This performance would indicate impairment for the 60-year-old university-educated individual, but strong cognitive function for the 90-year-old.
Common error #2: Deviating from standardized procedures
While clinicians specifically trained in cognitive measurement are familiar with the rigor by which tests are constructed, those with less training are often unaware that even seemingly minor deviations in procedure can contaminate results as surely as using nonsterile containers in biologic testing, leading to inaccurate interpretations of cognition.
Practical fix: Administer tests using verbatim instructions
Failing to follow instructions can significantly bias acquired data, particularly when using performance tests that are timed.
Illustration: Trail Making Test. Trail Making is an old 2-part test developed for the United States Army in the 1940s,11 and used in the Halstead-Reitan neuropsychological battery. Part A is a timed measure of an individual’s ability to join up a series of numbered circles in ascending order. Part B measures the ability to alternately switch between 2 related tasks: namely, alternately joining numbered and lettered circles, in ascending order. This is considered a measure of complex attention, which is often disrupted in early dementia.29
The test uses a specific standardized set of instructions, and Part B’s interpretation depends on having first administered Part A. Anecdotally, we have increasingly seen clinician reports using only Part B. Eliminating Part A removes a significant opportunity for patients to become familiar with the task’s demands, placing them at a considerable disadvantage on Part B and thereby invalidating the normative data.
In addition, follow the exact phrasing of the instructions and use stimuli and response pages that are the same size as those provided in the manual. If a patient errs at any point, it’s important that the test administrator reads, verbatim, the provided correction statements because these statements influence the amount of time spent correcting an error and therefore the final score.
Common error #3: Using outdated normative data
Neglecting to use updated norms that reflect current cohort differences can compromise screening accuracy.
Practical fix: Ensure current norms are used for comparisons
Societal influences—computers and other technologies, nutrition, etc—have led to steady improvements in cognitive and physical abilities. In basic psychology, this pattern of improving cognition, documented as an approximate increase of 3 IQ points per decade, is referred to as the Flynn effect.30 Therefore, not only do age and education need to be controlled for, but normative data must be current.
Cognitive screening tools are usually published with norms compiled at the time of the test’s development. However, scores are periodically “re-normed” to reflect current levels of ability. These updated norms are readily available in published journal articles or online. (Current norms for each of the tests used as examples in this article are provided in the references).21,28,31
Illustration: Trail Making Test. The normative data for this test are not only age- and education-sensitive, but are also highly sensitive to cohort effects. Early norms such as those of Davies,32 while often still quoted in literature and even in some training initiatives, are now seriously outdated and should not be used for interpretation. TABLE 3 shows how an average individual (ie, 50th percentile) in the 1960s, in one of 2 age groups, would compare in speed to an individual of similar age today.31 A time score that was at the 50th percentile in 1968 is now at or below the 1st percentile. More recent norms are also usually corrected for education, as are those provided by Tombaugh.31
In “A 'case' for using optimal procedures” (below), TABLE 4 shows the results of using outdated Trail Making norms vs current Trail Making norms.
George is a 77-year-old retired school teacher with >15 years of education who was referred to us for complaints of memory loss and suspicion of progressive cognitive deficits. On cognitive screening he scored 26/30 on the Mini-Mental State Examination, generated 16 animal names in 60 seconds, and completed Parts A and B of the Trail Making test in 80 seconds and 196 seconds, respectively. TABLE 4 summarizes test scores and interpretation with and without appropriate corrections.
George’s case dramatically illustrates the clinical impact of using (or not using) optimal interpretive procedures—ie, age and education corrections and current (not outdated) norms. Using the basic cutoff scores without corrections, George’s performance is within acceptable limits on all 3 screening tests, and he is sent home with the comforting news that his performance was within normal limits. However, by using appropriate comparative data, the same scores on all 3 screens indicate impairment. A likely next step would be referral for specialized testing. Monitoring for progressive deterioration is advisable, and perhaps initiation of medication.
TABLE 4
Trail Making: Outdated norms vs current norms
| Version 1 – No corrections for age or education for MMSE or COWAT; outdated Trail Making norms | |||
| Test | Score | Results | Suggests dementia |
| MMSE | 26 | ≥24 within normal limits10 | No |
| COWAT | 16 | >15 within normal limits25 | No |
| Trail Making A | 80 secs | 50th percentile32 | No |
| Trail Making B | 196 secs | 50th percentile32 | No |
| Decision: Negative for dementia | |||
|
|
|
|
|
| Version 2 – Applied age and education corrections for MMSE and COWAT; current Trail Making norms | |||
| Test | Score | Results | Suggests dementia |
| MMSE | 26 | Expected = 2822 | Yes |
| COWAT | 16 | 38th percentile28 | Yes |
| Trail Making A | 80 secs | <1st percentile31 | Yes |
| Trail Making B | 196 secs | <2nd percentile31 | Yes |
| Decision: Positive for dementia | |||
COWAT, Controlled Oral Word Association Task; MMSE, Mini-Mental State Examination.
Patients deserve an accurate assessment
A diagnosis of dementia profoundly affects patients and families. Progressive dementia such as Alzheimer’s disease means an individual will spend the rest of his or her life (usually 8-10 years) with decreasing cognitive capacity and quality of life.33-35 It also means families will spend years providing or arranging for care, and watching their family member deteriorate. Early detection can afford affected individuals and families the opportunity to make plans for fulfilling wishes and dreams before increased impairment makes such plans unattainable. The importance of rigor in assessment is therefore essential.
Optimizing accuracy in screening for dementia also can enable physicians to reasonably reassure patients that they likely do not suffer from a dementia at the present time, or to at least recommend that they be further assessed by a specialist. Without rigor, time and resources are wasted and the important question that triggered the referral is neither satisfactorily—nor accurately—addressed. Thus, there is a need to use not just simple cutoff scores but to apply the most current age and education normative data, and adhere to administrative instructions verbatim.
CORRESPONDENCE
Lindy A. Kilik, PhD, Geriatric Psychiatry Program, Providence Care Mental Health Services, PO Bag 603, Kingston, Ontario, Canada K7L 4X3; [email protected]
1. Loveman E, Green C, Kirby J, et al. The clinical and cost-effectiveness of donepezil, rivastigmine, galantamine and memantine for Alzheimer’s disease. Health Technol Assess. 2006;10:iii-iv,ix- xi,1-160.
2. Medical Care Corporation. Delaying the onset and progression of Alzheimer’s disease. Prevent AD Web site. Available at: http://www.preventad.com/pdf/support/article/DelayingADProgression.pdf. Accessed June 18, 2014.
3. Hopkins RW. Dementia projections for the counties, regional municipalities and districts of Ontario. Geriatric Psychiatry Unit Clinical/Research Bulletin, No. 16. Providence Care Web site. Available at: http://www.providencecare.ca/clinical-tools/Documents/Ontario-Dementia-Projections-2010.pdf. Accessed June 18, 2014.
4. Simmons BB, Hartmann B, Dejoseph D. Evaluation of suspected dementia. Am Fam Physician. 2011;84:895-902.
5. McCarten JR, Borson S. Should family physicians routinely screen patients for cognitive impairment? Yes: screening is the first step toward improving care. Am Fam Physician. 2014;89: 861-862.
6. Alzheimer’s Association. Health Care Professionals and Alzheimer’s. Alzheimer’s Association Web site. Available at: http://www.alz.org/health-care-professionals/cognitive-tests-patient-assessment.asp. Accessed June 18, 2014.
7. US Preventive Services Task Force. Screening for cognitive impairment in older adults. US Preventive Services Task Force Web site. Available at: http://www.uspreventiveservicestaskforce.org/uspstf/uspsdeme.htm. Accessed June 18, 2014.
8. Hopkins RW, Kilik LA, Day D, et al. Kingston Standardized Behavioural Assessment. Am J Alzheimers Dis Other Demen. 2006;21:339-346.
9. Kilik LA, Hopkins RW, Day D, et al. The progression of behaviour in dementia: an in-office guide for clinicians. Am J Alzheimers Dis Other Demen. 2008;23:242-249.
10. Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189-198.
11. Army Individual Test Battery. Manual of Directions and Scoring. Washington, DC: War Department, Adjutant General’s Office; 1944.
12. Wechsler D. The Measurement and Appraisal of Adult Intelligence. 4th ed. The Williams & Wilkins Company: Baltimore, MD; 1958.
13. Larrabee GJ, Largen JW, Levin HS. Sensitivity of age-decline resistant (“hold”) WAIS subtests to Alzheimer’s disease. J Clin Exp Neuropsychol. 1985;7:497-504.
14. Herndon RM. Assessment of the elderly with dementia. In: Handbook of Neurologic Rating Scales. 2nd ed. Demos Medical Publishing LLC: New York, NY; 2006:199.
15. Brugnolo A, Nobili F, Barbieri MP, et al. The factorial structure of the mini mental state examination (MMSE) in Alzheimer’s disease. Arch Gerontol Geriatr. 2009;49:180-185.
16. Folstein M, Anthony JC, Parhad I, et al. The meaning of cognitive impairment in the elderly. J Am Geriatr Soc. 1985;33:228-235.
17. Hopkins RW, Kilik LA, Day DJ, et al. The Revised Kingston Standardized Cognitive Assessment. Int J Geriatr Psychiatry. 2004;19:320-326.
18. Hopkins R, Kilik L. The mini-Kingston Standardized Cognitive Assessment. Kingston Scales Web site. Available at: http://www.kingstonscales.org. Accessed June 18, 2014.
19. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 4th ed. Washington, DC: American Psychiatric Association; 1994.
20. McKhann G, Drachman D, Folstein M, et al. Clinical diagnosis of Alzheimer’s disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer’s Disease. Neurology. 1984;34: 939-944.
21. Crum RM, Anthony JC, Bassett SS, et al. Population-based norms for the Mini-Mental State Examination by age and educational level. JAMA. 1993;269:2386-2391.
22. O’Bryant SE, Humphreys JD, Smith GE, et al. Detecting dementia with the mini-mental state examination in highly educated individuals. Arch Neurol. 2008;65:963-967.
23. O’Sullivan M, Morris RG, Markus HS. Brief cognitive assessment for patients with cerebral small vessel disease. J Neurol Neurosurg Psychiatry. 2005;76:1140-1145.
24. Nasreddine ZS, Phillips NA, Bédirian V, et al. The Montreal Cognitive Assessment (MoCA): a brief screening tool for mild cognitive impairment. J Am Geriatr Soc. 2005;53:695-699.
25. Benton AL, Hamsher K. Multilingual Aphasia Examination. 2nd ed. Iowa City, OA: AJA Associates, Inc; 1976.
26. Wechsler D. WAIS-III Administration and Scoring Manual. San Antonio, TX: The Psychological Corporation; 1997.
27. Morris JC, Heyman A, Mohs RC, et al. The Consortium to Establish a Registry for Alzheimer’s Disease (CERAD). Part I. Clinical and neuropsychological assesment of Alzheimer’s disease. Neurology. 1989;39:1159-1165.
28. Gladsjo JA, Miller SW, Heaton RK. Norms for Letter and Category Fluency: Demographic Corrections for Age, Education and Ethnicity. Odessa, FL: Psychological Assessment Resources; 1999.
29. Perry R, Hodges J. Attention and executive deficits in Alzheimer’s disease. A critical review. Brain. 1999;122(pt 3):383-404.
30. Flynn JR. The mean IQ of Americans: Massive gains 1932 to 1978. Psychol Bull. 1984;95:29-51.
31. Tombaugh TN. Trail Making Test A and B: normative data stratified by age and education. Arch Clin Neuropsychol. 2004;19: 203-214.
32. Davies A. The influence of age on trail making test performance. J Clin Psychol. 1968;24:96-98.
33. Bianchetti A, Trabucch M. Clinical aspects of Alzheimer’s disease. Aging (Milano). 2001;13:221-230.
34. Kay D, Forster DP, Newens AJ. Long-term survival, place of death, and death certification in clinically diagnosed pre-senile dementia in northern England. Follow-up after 8-12 years. Br J Psychiatry. 2000;177:156-162.
35. Chaussalet T, Thompson WA. Data requirements in a model of the natural history of Alzheimer’s disease. Health Care Manag Sci. 2001;4:13-19.
› Use age- and education-corrected normative data when using dementia screening tools. C
› Use verbatim instructions and the same size stimuli and response pages provided in a test’s manual. C
› Ensure that norms used for comparisons are current. C
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Treatment options for dementia are expanding and improving, giving extra impetus to detecting this progressive disease as early as possible. For example, research on the cholinesterase inhibitor donepezil has shown it can delay cognitive decline by 6 months or more compared with controls1,2 and possibly postpone institutionalization. With the number of elderly individuals and cases of dementia projected to grow significantly over the next 20 years,3 primary care physicians will increasingly be screening for cognitive impairment. Given the time constraints and patient loads in today’s practices, it’s not surprising that physicians tend to use evaluation tools that are brief and simple to administer. However, there are also serious pitfalls in the use of these tools.
When to screen. Many health-related organizations address screening for dementia4,5 and offer screening criteria (eg, the Alzheimer’s Association,6 the US Preventive Services Task Force7). Our experience suggests that specific behavioral changes are reasonable indicators of suspected dementia that should prompt cognitive screening. Using the Kingston Standardized Behavioural Assessment,8 we demonstrated a consistent pattern of earliest behavior change in a community-dwelling group with dementia.9 Meaningful clues are a decreased ability to engage in specific functional activities (including participation in favorite pastimes, ability to eat properly if left to prepare one’s own food, handling of personal finances, word finding, and reading) and unsteadiness. These specific behavioral changes reported by family or a caregiver suggest the need for cognitive screening.
Pitfalls associated with common screening tools, if not taken into account, can seriously limit the usefulness of information gained during assessment and potentially lead to a wrong conclusion. Screening tools are just that: a means of detecting the possible existence of a condition. Results are based on probability and subject to error. Therefore, a single test score is insufficient to render a diagnosis of dementia, and is one variable in a set of diagnostic criteria.
The purpose of this article is to review some of the most commonly used tools and procedures for dementia screening, identify procedural or interpretive errors made in everyday clinical practice, and suggest practical yet simple strategies to address these problems and improve the accuracy of assessments. We illustrate key points with clinical examples and vignettes using the Mini-Mental State Examination (MMSE),10 an Animal Naming Task, and the Trail Making Test.11
Common error #1: Reliance on simple, single cutoff scores
There are a number of important considerations to keep in mind when trying to make sense of scores from the many available cognitive tests.
The range of normal test results is wide. The normal range for most physiologic measures, such as glucose levels or hemoglobin counts, is relatively narrow. However, human cognitive functions can naturally differ from person to person, and the range of normal can be extremely large.
A single, all-purpose cutoff score ignores critical factors. Very often, clinicians have dealt with the issue of wide variance in cognition scores by establishing a general cutoff point to serve as a pass-fail mark. But this practice can result in both under- and overidentification of dementia, and it ignores the 2 components that chiefly determine how individuals differ cognitively: age and intelligence.
Practical fix: Use age-, intelligence-corrected normative data
Level of cognitive performance can be revealing when adjustments are made for age and intelligence. Not taking these factors into account can lead to many errors in clinical decision making.
Age matters. Many cognitive capacities decline as part of normal aging even in otherwise healthy individuals (eg, reaction time, spatial abilities, flexibility in novel problem solving).12 With this in mind, psychologists often have made the distinction between “hold” tests (remaining stable or even improving with age) and “no-hold” tests (declining with age).13 Therefore it is critical to ask, “What is normal, given a particular patient’s age?” If normative data corrected for age are available for a given test, use them.
Intelligence is a factor, too. Intelligence, like most human qualities, is distributed along a bell-shaped curve of normal distribution, wherein most people fall somewhere in the middle and a smaller number will be at the lower and higher tails of the curve. Not all of us fall into the average range of intelligence; indeed, psychometrically, only half of us do. The other half are found somewhere in the more extreme ends. In evaluating a person for dementia, it is critical to compare test results with those found in the appropriate intellectual group. But how does the physician looking for a brief assessment strategy determine a patient’s premorbid level of intellectual functioning?
A widely used and accepted heuristic for gauging intelligence is “years of education.” Of course, education is not perfectly correlated with intelligence, particularly as those who are now elderly may have been denied the opportunity to attend school due to the Great Depression, war, or other life events. Nevertheless, with these limitations in mind, level of education is a reasonable approximation of intelligence. In practical application, premorbid intellectual level is determined by using education-corrected normative data.
Typically with cognitive tests, cutoff scores and score ranges are defined for general levels of education (eg, less than grade 12 or more than grade 12; elementary school, high school, post-secondary, etc). Adjusted norms for age and education are usually determined by taking large samples of subjects and stratifying the distribution by subgroups—eg, 5-year age groups; levels of education such as elementary school or high school—and then statistically analyzing each group and noting the relative differences between them.
Illustration: MMSE. Although not designed for the overall measurement of cognitive impairment in dementia, the MMSE10 has become widely used for that purpose. It is fairly insensitive to cognitive changes associated with earlier stages of dementia,14 and is intended only as a means of identifying patients in need of more comprehensive assessment. However, the MMSE is increasingly used to make a diagnosis of dementia.15 In some areas (eg, Ontario, Canada), it is used to justify paying for treatment with cognitive enhancers.
The universal cutoff score proves inadequate. Although several dementia cutoff scores for the MMSE have been proposed, it is common practice to use an MMSE score ≥24 to rule out dementia.16 In our clinical practice, however, many patients who ultimately are diagnosed with early dementia often perform well on the MMSE, although rather poorly on other dementia screens, such as the Kingston Standardized Cognitive Assessment-Revised (KSCAr)17 or the mini-KSCAr.18
Recently, we reviewed cases of >70 individuals from our outpatient clinic who were given the MMSE and were also diagnosed as having dementia by both DSM-IV (Diagnostic and Statistical Manual of Mental Disorders)19 and the National Institute of Neurological and Communicative Disorders and Stroke and the Alzheimer’s Disease and Related Disorders Association20 criteria. Over three-quarters (78%) of these cases had an MMSE score of ≥24. Based on MMSE scores alone, these individuals would have been declared “not demented.”17
Correcting for age and intelligence increases accuracy. Published age and education norms are available for the MMSE.21 In applying these norms to our sample described above, the number of misidentified patients drops to approximately one-third (35.7%). This means that instead of misidentifying 2 out of 3 cases, the age and education corrections reduced this to about one out of 3, thereby increasing sensitivity and specificity. While this is still an unacceptably high rate of false negatives, it shows the considerable value of using age and education corrections.
The challenge of optimizing sensitivity and specificity of dementia screening tools is ongoing. As a matter of interest, we include TABLE 1,4,18,22-24 which shows calculated sensitivities and specificities of some commonly used screening tests.
Another practical fix: Use distributions and percentile-based normative data
Instead of simple cutoff scores, test scores can be, and often are, translated into percentiles to provide a meaningful context for evaluation and to make it easier to compare scores between patients. Someone with a score at the 70th percentile has performed as well as or better than 70% of others in the group who have taken the test. Usually, the average range of a normal population is defined as being between the 25th to 75th percentiles, encompassing 50% of that population. In general, percentiles make interpreting performance easier. Percentile-based test norms can also help determine with increased accuracy if there has been a decline over time.
Illustration: Animal naming task. In a common version of this task, patients are asked to name as many animals as they can in 60 seconds. This task has its roots in neuro- psychological tests of verbal fluency, such as the Controlled Oral Word Association Task.25 Verbal fluency tasks such as naming animals tap verbal generativity/problem-solving and self-monitoring, but are also highly dependent on vocabulary (word knowledge), a cognitive ability that is quite stable and even improves as one ages until individuals are well into their 80s.26
It is common practice with this procedure to consider a cutoff score of 15 as a minimally acceptable level of performance.27 Here again, there are potentially great differences in expected performance based on age and intelligence. TABLE 2 shows the effect of age and education on verbal fluency, expressed as percentiles, using a raw score of 15.28 For an individual in their early 60s who has a university degree, naming just 15 animals puts their performance at the 12th percentile (below average). The same performance for someone in their 90s who has only 8 years of education puts them in the 79th percentile (above the average range of 25th-75th percentiles). This performance would indicate impairment for the 60-year-old university-educated individual, but strong cognitive function for the 90-year-old.
Common error #2: Deviating from standardized procedures
While clinicians specifically trained in cognitive measurement are familiar with the rigor by which tests are constructed, those with less training are often unaware that even seemingly minor deviations in procedure can contaminate results as surely as using nonsterile containers in biologic testing, leading to inaccurate interpretations of cognition.
Practical fix: Administer tests using verbatim instructions
Failing to follow instructions can significantly bias acquired data, particularly when using performance tests that are timed.
Illustration: Trail Making Test. Trail Making is an old 2-part test developed for the United States Army in the 1940s,11 and used in the Halstead-Reitan neuropsychological battery. Part A is a timed measure of an individual’s ability to join up a series of numbered circles in ascending order. Part B measures the ability to alternately switch between 2 related tasks: namely, alternately joining numbered and lettered circles, in ascending order. This is considered a measure of complex attention, which is often disrupted in early dementia.29
The test uses a specific standardized set of instructions, and Part B’s interpretation depends on having first administered Part A. Anecdotally, we have increasingly seen clinician reports using only Part B. Eliminating Part A removes a significant opportunity for patients to become familiar with the task’s demands, placing them at a considerable disadvantage on Part B and thereby invalidating the normative data.
In addition, follow the exact phrasing of the instructions and use stimuli and response pages that are the same size as those provided in the manual. If a patient errs at any point, it’s important that the test administrator reads, verbatim, the provided correction statements because these statements influence the amount of time spent correcting an error and therefore the final score.
Common error #3: Using outdated normative data
Neglecting to use updated norms that reflect current cohort differences can compromise screening accuracy.
Practical fix: Ensure current norms are used for comparisons
Societal influences—computers and other technologies, nutrition, etc—have led to steady improvements in cognitive and physical abilities. In basic psychology, this pattern of improving cognition, documented as an approximate increase of 3 IQ points per decade, is referred to as the Flynn effect.30 Therefore, not only do age and education need to be controlled for, but normative data must be current.
Cognitive screening tools are usually published with norms compiled at the time of the test’s development. However, scores are periodically “re-normed” to reflect current levels of ability. These updated norms are readily available in published journal articles or online. (Current norms for each of the tests used as examples in this article are provided in the references).21,28,31
Illustration: Trail Making Test. The normative data for this test are not only age- and education-sensitive, but are also highly sensitive to cohort effects. Early norms such as those of Davies,32 while often still quoted in literature and even in some training initiatives, are now seriously outdated and should not be used for interpretation. TABLE 3 shows how an average individual (ie, 50th percentile) in the 1960s, in one of 2 age groups, would compare in speed to an individual of similar age today.31 A time score that was at the 50th percentile in 1968 is now at or below the 1st percentile. More recent norms are also usually corrected for education, as are those provided by Tombaugh.31
In “A 'case' for using optimal procedures” (below), TABLE 4 shows the results of using outdated Trail Making norms vs current Trail Making norms.
George is a 77-year-old retired school teacher with >15 years of education who was referred to us for complaints of memory loss and suspicion of progressive cognitive deficits. On cognitive screening he scored 26/30 on the Mini-Mental State Examination, generated 16 animal names in 60 seconds, and completed Parts A and B of the Trail Making test in 80 seconds and 196 seconds, respectively. TABLE 4 summarizes test scores and interpretation with and without appropriate corrections.
George’s case dramatically illustrates the clinical impact of using (or not using) optimal interpretive procedures—ie, age and education corrections and current (not outdated) norms. Using the basic cutoff scores without corrections, George’s performance is within acceptable limits on all 3 screening tests, and he is sent home with the comforting news that his performance was within normal limits. However, by using appropriate comparative data, the same scores on all 3 screens indicate impairment. A likely next step would be referral for specialized testing. Monitoring for progressive deterioration is advisable, and perhaps initiation of medication.
TABLE 4
Trail Making: Outdated norms vs current norms
| Version 1 – No corrections for age or education for MMSE or COWAT; outdated Trail Making norms | |||
| Test | Score | Results | Suggests dementia |
| MMSE | 26 | ≥24 within normal limits10 | No |
| COWAT | 16 | >15 within normal limits25 | No |
| Trail Making A | 80 secs | 50th percentile32 | No |
| Trail Making B | 196 secs | 50th percentile32 | No |
| Decision: Negative for dementia | |||
|
|
|
|
|
| Version 2 – Applied age and education corrections for MMSE and COWAT; current Trail Making norms | |||
| Test | Score | Results | Suggests dementia |
| MMSE | 26 | Expected = 2822 | Yes |
| COWAT | 16 | 38th percentile28 | Yes |
| Trail Making A | 80 secs | <1st percentile31 | Yes |
| Trail Making B | 196 secs | <2nd percentile31 | Yes |
| Decision: Positive for dementia | |||
COWAT, Controlled Oral Word Association Task; MMSE, Mini-Mental State Examination.
Patients deserve an accurate assessment
A diagnosis of dementia profoundly affects patients and families. Progressive dementia such as Alzheimer’s disease means an individual will spend the rest of his or her life (usually 8-10 years) with decreasing cognitive capacity and quality of life.33-35 It also means families will spend years providing or arranging for care, and watching their family member deteriorate. Early detection can afford affected individuals and families the opportunity to make plans for fulfilling wishes and dreams before increased impairment makes such plans unattainable. The importance of rigor in assessment is therefore essential.
Optimizing accuracy in screening for dementia also can enable physicians to reasonably reassure patients that they likely do not suffer from a dementia at the present time, or to at least recommend that they be further assessed by a specialist. Without rigor, time and resources are wasted and the important question that triggered the referral is neither satisfactorily—nor accurately—addressed. Thus, there is a need to use not just simple cutoff scores but to apply the most current age and education normative data, and adhere to administrative instructions verbatim.
CORRESPONDENCE
Lindy A. Kilik, PhD, Geriatric Psychiatry Program, Providence Care Mental Health Services, PO Bag 603, Kingston, Ontario, Canada K7L 4X3; [email protected]
› Use age- and education-corrected normative data when using dementia screening tools. C
› Use verbatim instructions and the same size stimuli and response pages provided in a test’s manual. C
› Ensure that norms used for comparisons are current. C
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Treatment options for dementia are expanding and improving, giving extra impetus to detecting this progressive disease as early as possible. For example, research on the cholinesterase inhibitor donepezil has shown it can delay cognitive decline by 6 months or more compared with controls1,2 and possibly postpone institutionalization. With the number of elderly individuals and cases of dementia projected to grow significantly over the next 20 years,3 primary care physicians will increasingly be screening for cognitive impairment. Given the time constraints and patient loads in today’s practices, it’s not surprising that physicians tend to use evaluation tools that are brief and simple to administer. However, there are also serious pitfalls in the use of these tools.
When to screen. Many health-related organizations address screening for dementia4,5 and offer screening criteria (eg, the Alzheimer’s Association,6 the US Preventive Services Task Force7). Our experience suggests that specific behavioral changes are reasonable indicators of suspected dementia that should prompt cognitive screening. Using the Kingston Standardized Behavioural Assessment,8 we demonstrated a consistent pattern of earliest behavior change in a community-dwelling group with dementia.9 Meaningful clues are a decreased ability to engage in specific functional activities (including participation in favorite pastimes, ability to eat properly if left to prepare one’s own food, handling of personal finances, word finding, and reading) and unsteadiness. These specific behavioral changes reported by family or a caregiver suggest the need for cognitive screening.
Pitfalls associated with common screening tools, if not taken into account, can seriously limit the usefulness of information gained during assessment and potentially lead to a wrong conclusion. Screening tools are just that: a means of detecting the possible existence of a condition. Results are based on probability and subject to error. Therefore, a single test score is insufficient to render a diagnosis of dementia, and is one variable in a set of diagnostic criteria.
The purpose of this article is to review some of the most commonly used tools and procedures for dementia screening, identify procedural or interpretive errors made in everyday clinical practice, and suggest practical yet simple strategies to address these problems and improve the accuracy of assessments. We illustrate key points with clinical examples and vignettes using the Mini-Mental State Examination (MMSE),10 an Animal Naming Task, and the Trail Making Test.11
Common error #1: Reliance on simple, single cutoff scores
There are a number of important considerations to keep in mind when trying to make sense of scores from the many available cognitive tests.
The range of normal test results is wide. The normal range for most physiologic measures, such as glucose levels or hemoglobin counts, is relatively narrow. However, human cognitive functions can naturally differ from person to person, and the range of normal can be extremely large.
A single, all-purpose cutoff score ignores critical factors. Very often, clinicians have dealt with the issue of wide variance in cognition scores by establishing a general cutoff point to serve as a pass-fail mark. But this practice can result in both under- and overidentification of dementia, and it ignores the 2 components that chiefly determine how individuals differ cognitively: age and intelligence.
Practical fix: Use age-, intelligence-corrected normative data
Level of cognitive performance can be revealing when adjustments are made for age and intelligence. Not taking these factors into account can lead to many errors in clinical decision making.
Age matters. Many cognitive capacities decline as part of normal aging even in otherwise healthy individuals (eg, reaction time, spatial abilities, flexibility in novel problem solving).12 With this in mind, psychologists often have made the distinction between “hold” tests (remaining stable or even improving with age) and “no-hold” tests (declining with age).13 Therefore it is critical to ask, “What is normal, given a particular patient’s age?” If normative data corrected for age are available for a given test, use them.
Intelligence is a factor, too. Intelligence, like most human qualities, is distributed along a bell-shaped curve of normal distribution, wherein most people fall somewhere in the middle and a smaller number will be at the lower and higher tails of the curve. Not all of us fall into the average range of intelligence; indeed, psychometrically, only half of us do. The other half are found somewhere in the more extreme ends. In evaluating a person for dementia, it is critical to compare test results with those found in the appropriate intellectual group. But how does the physician looking for a brief assessment strategy determine a patient’s premorbid level of intellectual functioning?
A widely used and accepted heuristic for gauging intelligence is “years of education.” Of course, education is not perfectly correlated with intelligence, particularly as those who are now elderly may have been denied the opportunity to attend school due to the Great Depression, war, or other life events. Nevertheless, with these limitations in mind, level of education is a reasonable approximation of intelligence. In practical application, premorbid intellectual level is determined by using education-corrected normative data.
Typically with cognitive tests, cutoff scores and score ranges are defined for general levels of education (eg, less than grade 12 or more than grade 12; elementary school, high school, post-secondary, etc). Adjusted norms for age and education are usually determined by taking large samples of subjects and stratifying the distribution by subgroups—eg, 5-year age groups; levels of education such as elementary school or high school—and then statistically analyzing each group and noting the relative differences between them.
Illustration: MMSE. Although not designed for the overall measurement of cognitive impairment in dementia, the MMSE10 has become widely used for that purpose. It is fairly insensitive to cognitive changes associated with earlier stages of dementia,14 and is intended only as a means of identifying patients in need of more comprehensive assessment. However, the MMSE is increasingly used to make a diagnosis of dementia.15 In some areas (eg, Ontario, Canada), it is used to justify paying for treatment with cognitive enhancers.
The universal cutoff score proves inadequate. Although several dementia cutoff scores for the MMSE have been proposed, it is common practice to use an MMSE score ≥24 to rule out dementia.16 In our clinical practice, however, many patients who ultimately are diagnosed with early dementia often perform well on the MMSE, although rather poorly on other dementia screens, such as the Kingston Standardized Cognitive Assessment-Revised (KSCAr)17 or the mini-KSCAr.18
Recently, we reviewed cases of >70 individuals from our outpatient clinic who were given the MMSE and were also diagnosed as having dementia by both DSM-IV (Diagnostic and Statistical Manual of Mental Disorders)19 and the National Institute of Neurological and Communicative Disorders and Stroke and the Alzheimer’s Disease and Related Disorders Association20 criteria. Over three-quarters (78%) of these cases had an MMSE score of ≥24. Based on MMSE scores alone, these individuals would have been declared “not demented.”17
Correcting for age and intelligence increases accuracy. Published age and education norms are available for the MMSE.21 In applying these norms to our sample described above, the number of misidentified patients drops to approximately one-third (35.7%). This means that instead of misidentifying 2 out of 3 cases, the age and education corrections reduced this to about one out of 3, thereby increasing sensitivity and specificity. While this is still an unacceptably high rate of false negatives, it shows the considerable value of using age and education corrections.
The challenge of optimizing sensitivity and specificity of dementia screening tools is ongoing. As a matter of interest, we include TABLE 1,4,18,22-24 which shows calculated sensitivities and specificities of some commonly used screening tests.
Another practical fix: Use distributions and percentile-based normative data
Instead of simple cutoff scores, test scores can be, and often are, translated into percentiles to provide a meaningful context for evaluation and to make it easier to compare scores between patients. Someone with a score at the 70th percentile has performed as well as or better than 70% of others in the group who have taken the test. Usually, the average range of a normal population is defined as being between the 25th to 75th percentiles, encompassing 50% of that population. In general, percentiles make interpreting performance easier. Percentile-based test norms can also help determine with increased accuracy if there has been a decline over time.
Illustration: Animal naming task. In a common version of this task, patients are asked to name as many animals as they can in 60 seconds. This task has its roots in neuro- psychological tests of verbal fluency, such as the Controlled Oral Word Association Task.25 Verbal fluency tasks such as naming animals tap verbal generativity/problem-solving and self-monitoring, but are also highly dependent on vocabulary (word knowledge), a cognitive ability that is quite stable and even improves as one ages until individuals are well into their 80s.26
It is common practice with this procedure to consider a cutoff score of 15 as a minimally acceptable level of performance.27 Here again, there are potentially great differences in expected performance based on age and intelligence. TABLE 2 shows the effect of age and education on verbal fluency, expressed as percentiles, using a raw score of 15.28 For an individual in their early 60s who has a university degree, naming just 15 animals puts their performance at the 12th percentile (below average). The same performance for someone in their 90s who has only 8 years of education puts them in the 79th percentile (above the average range of 25th-75th percentiles). This performance would indicate impairment for the 60-year-old university-educated individual, but strong cognitive function for the 90-year-old.
Common error #2: Deviating from standardized procedures
While clinicians specifically trained in cognitive measurement are familiar with the rigor by which tests are constructed, those with less training are often unaware that even seemingly minor deviations in procedure can contaminate results as surely as using nonsterile containers in biologic testing, leading to inaccurate interpretations of cognition.
Practical fix: Administer tests using verbatim instructions
Failing to follow instructions can significantly bias acquired data, particularly when using performance tests that are timed.
Illustration: Trail Making Test. Trail Making is an old 2-part test developed for the United States Army in the 1940s,11 and used in the Halstead-Reitan neuropsychological battery. Part A is a timed measure of an individual’s ability to join up a series of numbered circles in ascending order. Part B measures the ability to alternately switch between 2 related tasks: namely, alternately joining numbered and lettered circles, in ascending order. This is considered a measure of complex attention, which is often disrupted in early dementia.29
The test uses a specific standardized set of instructions, and Part B’s interpretation depends on having first administered Part A. Anecdotally, we have increasingly seen clinician reports using only Part B. Eliminating Part A removes a significant opportunity for patients to become familiar with the task’s demands, placing them at a considerable disadvantage on Part B and thereby invalidating the normative data.
In addition, follow the exact phrasing of the instructions and use stimuli and response pages that are the same size as those provided in the manual. If a patient errs at any point, it’s important that the test administrator reads, verbatim, the provided correction statements because these statements influence the amount of time spent correcting an error and therefore the final score.
Common error #3: Using outdated normative data
Neglecting to use updated norms that reflect current cohort differences can compromise screening accuracy.
Practical fix: Ensure current norms are used for comparisons
Societal influences—computers and other technologies, nutrition, etc—have led to steady improvements in cognitive and physical abilities. In basic psychology, this pattern of improving cognition, documented as an approximate increase of 3 IQ points per decade, is referred to as the Flynn effect.30 Therefore, not only do age and education need to be controlled for, but normative data must be current.
Cognitive screening tools are usually published with norms compiled at the time of the test’s development. However, scores are periodically “re-normed” to reflect current levels of ability. These updated norms are readily available in published journal articles or online. (Current norms for each of the tests used as examples in this article are provided in the references).21,28,31
Illustration: Trail Making Test. The normative data for this test are not only age- and education-sensitive, but are also highly sensitive to cohort effects. Early norms such as those of Davies,32 while often still quoted in literature and even in some training initiatives, are now seriously outdated and should not be used for interpretation. TABLE 3 shows how an average individual (ie, 50th percentile) in the 1960s, in one of 2 age groups, would compare in speed to an individual of similar age today.31 A time score that was at the 50th percentile in 1968 is now at or below the 1st percentile. More recent norms are also usually corrected for education, as are those provided by Tombaugh.31
In “A 'case' for using optimal procedures” (below), TABLE 4 shows the results of using outdated Trail Making norms vs current Trail Making norms.
George is a 77-year-old retired school teacher with >15 years of education who was referred to us for complaints of memory loss and suspicion of progressive cognitive deficits. On cognitive screening he scored 26/30 on the Mini-Mental State Examination, generated 16 animal names in 60 seconds, and completed Parts A and B of the Trail Making test in 80 seconds and 196 seconds, respectively. TABLE 4 summarizes test scores and interpretation with and without appropriate corrections.
George’s case dramatically illustrates the clinical impact of using (or not using) optimal interpretive procedures—ie, age and education corrections and current (not outdated) norms. Using the basic cutoff scores without corrections, George’s performance is within acceptable limits on all 3 screening tests, and he is sent home with the comforting news that his performance was within normal limits. However, by using appropriate comparative data, the same scores on all 3 screens indicate impairment. A likely next step would be referral for specialized testing. Monitoring for progressive deterioration is advisable, and perhaps initiation of medication.
TABLE 4
Trail Making: Outdated norms vs current norms
| Version 1 – No corrections for age or education for MMSE or COWAT; outdated Trail Making norms | |||
| Test | Score | Results | Suggests dementia |
| MMSE | 26 | ≥24 within normal limits10 | No |
| COWAT | 16 | >15 within normal limits25 | No |
| Trail Making A | 80 secs | 50th percentile32 | No |
| Trail Making B | 196 secs | 50th percentile32 | No |
| Decision: Negative for dementia | |||
|
|
|
|
|
| Version 2 – Applied age and education corrections for MMSE and COWAT; current Trail Making norms | |||
| Test | Score | Results | Suggests dementia |
| MMSE | 26 | Expected = 2822 | Yes |
| COWAT | 16 | 38th percentile28 | Yes |
| Trail Making A | 80 secs | <1st percentile31 | Yes |
| Trail Making B | 196 secs | <2nd percentile31 | Yes |
| Decision: Positive for dementia | |||
COWAT, Controlled Oral Word Association Task; MMSE, Mini-Mental State Examination.
Patients deserve an accurate assessment
A diagnosis of dementia profoundly affects patients and families. Progressive dementia such as Alzheimer’s disease means an individual will spend the rest of his or her life (usually 8-10 years) with decreasing cognitive capacity and quality of life.33-35 It also means families will spend years providing or arranging for care, and watching their family member deteriorate. Early detection can afford affected individuals and families the opportunity to make plans for fulfilling wishes and dreams before increased impairment makes such plans unattainable. The importance of rigor in assessment is therefore essential.
Optimizing accuracy in screening for dementia also can enable physicians to reasonably reassure patients that they likely do not suffer from a dementia at the present time, or to at least recommend that they be further assessed by a specialist. Without rigor, time and resources are wasted and the important question that triggered the referral is neither satisfactorily—nor accurately—addressed. Thus, there is a need to use not just simple cutoff scores but to apply the most current age and education normative data, and adhere to administrative instructions verbatim.
CORRESPONDENCE
Lindy A. Kilik, PhD, Geriatric Psychiatry Program, Providence Care Mental Health Services, PO Bag 603, Kingston, Ontario, Canada K7L 4X3; [email protected]
1. Loveman E, Green C, Kirby J, et al. The clinical and cost-effectiveness of donepezil, rivastigmine, galantamine and memantine for Alzheimer’s disease. Health Technol Assess. 2006;10:iii-iv,ix- xi,1-160.
2. Medical Care Corporation. Delaying the onset and progression of Alzheimer’s disease. Prevent AD Web site. Available at: http://www.preventad.com/pdf/support/article/DelayingADProgression.pdf. Accessed June 18, 2014.
3. Hopkins RW. Dementia projections for the counties, regional municipalities and districts of Ontario. Geriatric Psychiatry Unit Clinical/Research Bulletin, No. 16. Providence Care Web site. Available at: http://www.providencecare.ca/clinical-tools/Documents/Ontario-Dementia-Projections-2010.pdf. Accessed June 18, 2014.
4. Simmons BB, Hartmann B, Dejoseph D. Evaluation of suspected dementia. Am Fam Physician. 2011;84:895-902.
5. McCarten JR, Borson S. Should family physicians routinely screen patients for cognitive impairment? Yes: screening is the first step toward improving care. Am Fam Physician. 2014;89: 861-862.
6. Alzheimer’s Association. Health Care Professionals and Alzheimer’s. Alzheimer’s Association Web site. Available at: http://www.alz.org/health-care-professionals/cognitive-tests-patient-assessment.asp. Accessed June 18, 2014.
7. US Preventive Services Task Force. Screening for cognitive impairment in older adults. US Preventive Services Task Force Web site. Available at: http://www.uspreventiveservicestaskforce.org/uspstf/uspsdeme.htm. Accessed June 18, 2014.
8. Hopkins RW, Kilik LA, Day D, et al. Kingston Standardized Behavioural Assessment. Am J Alzheimers Dis Other Demen. 2006;21:339-346.
9. Kilik LA, Hopkins RW, Day D, et al. The progression of behaviour in dementia: an in-office guide for clinicians. Am J Alzheimers Dis Other Demen. 2008;23:242-249.
10. Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189-198.
11. Army Individual Test Battery. Manual of Directions and Scoring. Washington, DC: War Department, Adjutant General’s Office; 1944.
12. Wechsler D. The Measurement and Appraisal of Adult Intelligence. 4th ed. The Williams & Wilkins Company: Baltimore, MD; 1958.
13. Larrabee GJ, Largen JW, Levin HS. Sensitivity of age-decline resistant (“hold”) WAIS subtests to Alzheimer’s disease. J Clin Exp Neuropsychol. 1985;7:497-504.
14. Herndon RM. Assessment of the elderly with dementia. In: Handbook of Neurologic Rating Scales. 2nd ed. Demos Medical Publishing LLC: New York, NY; 2006:199.
15. Brugnolo A, Nobili F, Barbieri MP, et al. The factorial structure of the mini mental state examination (MMSE) in Alzheimer’s disease. Arch Gerontol Geriatr. 2009;49:180-185.
16. Folstein M, Anthony JC, Parhad I, et al. The meaning of cognitive impairment in the elderly. J Am Geriatr Soc. 1985;33:228-235.
17. Hopkins RW, Kilik LA, Day DJ, et al. The Revised Kingston Standardized Cognitive Assessment. Int J Geriatr Psychiatry. 2004;19:320-326.
18. Hopkins R, Kilik L. The mini-Kingston Standardized Cognitive Assessment. Kingston Scales Web site. Available at: http://www.kingstonscales.org. Accessed June 18, 2014.
19. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 4th ed. Washington, DC: American Psychiatric Association; 1994.
20. McKhann G, Drachman D, Folstein M, et al. Clinical diagnosis of Alzheimer’s disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer’s Disease. Neurology. 1984;34: 939-944.
21. Crum RM, Anthony JC, Bassett SS, et al. Population-based norms for the Mini-Mental State Examination by age and educational level. JAMA. 1993;269:2386-2391.
22. O’Bryant SE, Humphreys JD, Smith GE, et al. Detecting dementia with the mini-mental state examination in highly educated individuals. Arch Neurol. 2008;65:963-967.
23. O’Sullivan M, Morris RG, Markus HS. Brief cognitive assessment for patients with cerebral small vessel disease. J Neurol Neurosurg Psychiatry. 2005;76:1140-1145.
24. Nasreddine ZS, Phillips NA, Bédirian V, et al. The Montreal Cognitive Assessment (MoCA): a brief screening tool for mild cognitive impairment. J Am Geriatr Soc. 2005;53:695-699.
25. Benton AL, Hamsher K. Multilingual Aphasia Examination. 2nd ed. Iowa City, OA: AJA Associates, Inc; 1976.
26. Wechsler D. WAIS-III Administration and Scoring Manual. San Antonio, TX: The Psychological Corporation; 1997.
27. Morris JC, Heyman A, Mohs RC, et al. The Consortium to Establish a Registry for Alzheimer’s Disease (CERAD). Part I. Clinical and neuropsychological assesment of Alzheimer’s disease. Neurology. 1989;39:1159-1165.
28. Gladsjo JA, Miller SW, Heaton RK. Norms for Letter and Category Fluency: Demographic Corrections for Age, Education and Ethnicity. Odessa, FL: Psychological Assessment Resources; 1999.
29. Perry R, Hodges J. Attention and executive deficits in Alzheimer’s disease. A critical review. Brain. 1999;122(pt 3):383-404.
30. Flynn JR. The mean IQ of Americans: Massive gains 1932 to 1978. Psychol Bull. 1984;95:29-51.
31. Tombaugh TN. Trail Making Test A and B: normative data stratified by age and education. Arch Clin Neuropsychol. 2004;19: 203-214.
32. Davies A. The influence of age on trail making test performance. J Clin Psychol. 1968;24:96-98.
33. Bianchetti A, Trabucch M. Clinical aspects of Alzheimer’s disease. Aging (Milano). 2001;13:221-230.
34. Kay D, Forster DP, Newens AJ. Long-term survival, place of death, and death certification in clinically diagnosed pre-senile dementia in northern England. Follow-up after 8-12 years. Br J Psychiatry. 2000;177:156-162.
35. Chaussalet T, Thompson WA. Data requirements in a model of the natural history of Alzheimer’s disease. Health Care Manag Sci. 2001;4:13-19.
1. Loveman E, Green C, Kirby J, et al. The clinical and cost-effectiveness of donepezil, rivastigmine, galantamine and memantine for Alzheimer’s disease. Health Technol Assess. 2006;10:iii-iv,ix- xi,1-160.
2. Medical Care Corporation. Delaying the onset and progression of Alzheimer’s disease. Prevent AD Web site. Available at: http://www.preventad.com/pdf/support/article/DelayingADProgression.pdf. Accessed June 18, 2014.
3. Hopkins RW. Dementia projections for the counties, regional municipalities and districts of Ontario. Geriatric Psychiatry Unit Clinical/Research Bulletin, No. 16. Providence Care Web site. Available at: http://www.providencecare.ca/clinical-tools/Documents/Ontario-Dementia-Projections-2010.pdf. Accessed June 18, 2014.
4. Simmons BB, Hartmann B, Dejoseph D. Evaluation of suspected dementia. Am Fam Physician. 2011;84:895-902.
5. McCarten JR, Borson S. Should family physicians routinely screen patients for cognitive impairment? Yes: screening is the first step toward improving care. Am Fam Physician. 2014;89: 861-862.
6. Alzheimer’s Association. Health Care Professionals and Alzheimer’s. Alzheimer’s Association Web site. Available at: http://www.alz.org/health-care-professionals/cognitive-tests-patient-assessment.asp. Accessed June 18, 2014.
7. US Preventive Services Task Force. Screening for cognitive impairment in older adults. US Preventive Services Task Force Web site. Available at: http://www.uspreventiveservicestaskforce.org/uspstf/uspsdeme.htm. Accessed June 18, 2014.
8. Hopkins RW, Kilik LA, Day D, et al. Kingston Standardized Behavioural Assessment. Am J Alzheimers Dis Other Demen. 2006;21:339-346.
9. Kilik LA, Hopkins RW, Day D, et al. The progression of behaviour in dementia: an in-office guide for clinicians. Am J Alzheimers Dis Other Demen. 2008;23:242-249.
10. Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189-198.
11. Army Individual Test Battery. Manual of Directions and Scoring. Washington, DC: War Department, Adjutant General’s Office; 1944.
12. Wechsler D. The Measurement and Appraisal of Adult Intelligence. 4th ed. The Williams & Wilkins Company: Baltimore, MD; 1958.
13. Larrabee GJ, Largen JW, Levin HS. Sensitivity of age-decline resistant (“hold”) WAIS subtests to Alzheimer’s disease. J Clin Exp Neuropsychol. 1985;7:497-504.
14. Herndon RM. Assessment of the elderly with dementia. In: Handbook of Neurologic Rating Scales. 2nd ed. Demos Medical Publishing LLC: New York, NY; 2006:199.
15. Brugnolo A, Nobili F, Barbieri MP, et al. The factorial structure of the mini mental state examination (MMSE) in Alzheimer’s disease. Arch Gerontol Geriatr. 2009;49:180-185.
16. Folstein M, Anthony JC, Parhad I, et al. The meaning of cognitive impairment in the elderly. J Am Geriatr Soc. 1985;33:228-235.
17. Hopkins RW, Kilik LA, Day DJ, et al. The Revised Kingston Standardized Cognitive Assessment. Int J Geriatr Psychiatry. 2004;19:320-326.
18. Hopkins R, Kilik L. The mini-Kingston Standardized Cognitive Assessment. Kingston Scales Web site. Available at: http://www.kingstonscales.org. Accessed June 18, 2014.
19. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 4th ed. Washington, DC: American Psychiatric Association; 1994.
20. McKhann G, Drachman D, Folstein M, et al. Clinical diagnosis of Alzheimer’s disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer’s Disease. Neurology. 1984;34: 939-944.
21. Crum RM, Anthony JC, Bassett SS, et al. Population-based norms for the Mini-Mental State Examination by age and educational level. JAMA. 1993;269:2386-2391.
22. O’Bryant SE, Humphreys JD, Smith GE, et al. Detecting dementia with the mini-mental state examination in highly educated individuals. Arch Neurol. 2008;65:963-967.
23. O’Sullivan M, Morris RG, Markus HS. Brief cognitive assessment for patients with cerebral small vessel disease. J Neurol Neurosurg Psychiatry. 2005;76:1140-1145.
24. Nasreddine ZS, Phillips NA, Bédirian V, et al. The Montreal Cognitive Assessment (MoCA): a brief screening tool for mild cognitive impairment. J Am Geriatr Soc. 2005;53:695-699.
25. Benton AL, Hamsher K. Multilingual Aphasia Examination. 2nd ed. Iowa City, OA: AJA Associates, Inc; 1976.
26. Wechsler D. WAIS-III Administration and Scoring Manual. San Antonio, TX: The Psychological Corporation; 1997.
27. Morris JC, Heyman A, Mohs RC, et al. The Consortium to Establish a Registry for Alzheimer’s Disease (CERAD). Part I. Clinical and neuropsychological assesment of Alzheimer’s disease. Neurology. 1989;39:1159-1165.
28. Gladsjo JA, Miller SW, Heaton RK. Norms for Letter and Category Fluency: Demographic Corrections for Age, Education and Ethnicity. Odessa, FL: Psychological Assessment Resources; 1999.
29. Perry R, Hodges J. Attention and executive deficits in Alzheimer’s disease. A critical review. Brain. 1999;122(pt 3):383-404.
30. Flynn JR. The mean IQ of Americans: Massive gains 1932 to 1978. Psychol Bull. 1984;95:29-51.
31. Tombaugh TN. Trail Making Test A and B: normative data stratified by age and education. Arch Clin Neuropsychol. 2004;19: 203-214.
32. Davies A. The influence of age on trail making test performance. J Clin Psychol. 1968;24:96-98.
33. Bianchetti A, Trabucch M. Clinical aspects of Alzheimer’s disease. Aging (Milano). 2001;13:221-230.
34. Kay D, Forster DP, Newens AJ. Long-term survival, place of death, and death certification in clinically diagnosed pre-senile dementia in northern England. Follow-up after 8-12 years. Br J Psychiatry. 2000;177:156-162.
35. Chaussalet T, Thompson WA. Data requirements in a model of the natural history of Alzheimer’s disease. Health Care Manag Sci. 2001;4:13-19.