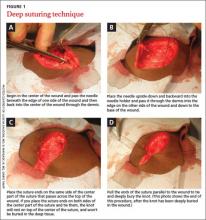
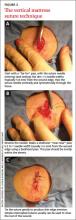
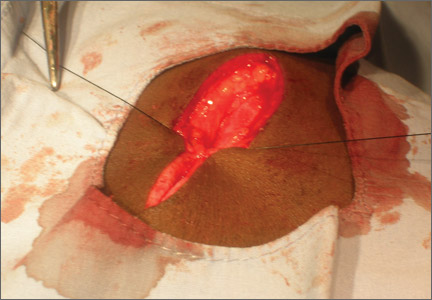
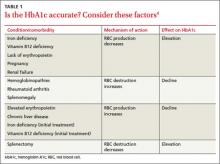
User login
Chest pain—tools to improve your in-office evaluation
› Seek immediate emergency care for patients with chest pain that is exertional, radiating to one or both arms, similar to or worse than prior cardiac chest pain, or associated with nausea, vomiting, or diaphoresis. A
› Be aware that patients with chest pain that is stabbing, pleuritic, positional, or reproducible with palpation are at very low risk for acute coronary syndrome and most likely have chest wall pain. A
› Consider a 2-week course of high-dose proton-pump inhibitor therapy to help identify patients whose chest pain may be from undiagnosed gastroesophageal reflux disease. A
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
CASE › Your patient, Amy Z, age 58, was given a diagnosis of hypertension 10 years ago and since then has been maintained on hydrochlorothiazide 50 mg/d and lisinopril 10 mg/d. In the office today, she reports intermittent chest tightness and heaviness. She has no history of coronary artery disease (CAD), cerebrovascular disease, or peripheral vascular disease. She attributes her chest discomfort to emotional stress. She recently started a job after having been unemployed, but still has no health insurance and is concerned about losing her house.
She denies orthopnea and resting or exertional dyspnea, and says she never gets chest pain while climbing stairs. Her blood pressure is elevated at 180/110 mm Hg, but her other vital signs are normal (pulse, 70 beats per minute; respiratory rate, 18 breaths per minute). On physical examination, she has no venous distension in her neck and her lungs are clear. A cardiac exam reveals a regular rate and rhythm, with a normally split S1 and S2 and no murmurs, rubs, or gallops. Palpation of the chest does not reproduce her chest pain.
You are concerned that your patient’s chest pain could be from heart disease, but she wants to defer additional testing because of the cost, stating, “It’s all due to my stress.”
How would you proceed?
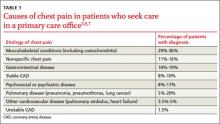
Musculoskeletal chest wall pain is the most common cause of chest pain in patients who seek treatment in the office, followed by GI disease and stable heart disease.
Whether they go to the emergency department (ED) or to their family physician’s office, most patients who seek treatment for chest pain don’t have life-threatening cardiac illness. Of the 8 million patients who visit an ED for chest pain each year, only 13% are diagnosed with acute coronary syndrome (ACS).1,2 Among those seen for chest pain in a primary care office, only a minority (approximately 1.5%) have unstable heart disease.3-5 Cross-sectional studies indicate that musculoskeletal chest wall pain (or “chest wall syndrome [CWS]”) is the most common cause of chest pain in patients who seek treatment in the office, followed by gastrointestinal (GI) disease, stable heart disease, psychosocial or psychiatric conditions, pulmonary disease, and other cardiovascular conditions (TABLE 1).3,6,7
When evaluating patients with chest pain in the office, the challenge is to appropriately evaluate and manage those who are at low risk of ACS, while at the same time identifying and arranging prompt transfer or referral for the minority of patients who are at high cardiac risk. This article describes how to determine which patients require emergency treatment, which tools to use to screen for ACS and other potential causes of chest pain, and how to proceed when initial evaluation and testing do not point to a clear diagnosis.
Start with the ABCs
When a patient presents in primary care with a chief complaint of chest pain, it’s of course critical that you quickly determine if he or she is stable by evaluating the “ABCs” (airway, breathing, and circulation). Any potentially unstable patient should be immediately transferred for emergency care.8 A patient who shows no signs of respiratory distress and whose vital signs are within a normal range is unlikely to be acutely unstable, and can be further evaluated in the office.
If the patient is stable, obtain a history of the onset and evolution of the chest pain, especially its location, quality, duration, and aggravating or alleviating factors. Also ask about a personal or family history of heart disease, hypertension, diabetes, or hypercholesterolemia, and about tobacco use. While the presence of any of these cardiac risk factors may increase suspicion for a cardiac cause for chest pain, the absence of such factors does not eliminate the need for a careful diagnostic evaluation.
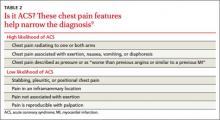
Patients with “typical” chest pain have a higher risk of ACS. In a 2005 review of observational prospective and retrospective studies and systematic reviews, Swap et al9 corroborated the description of “typical” anginal chest pain, indicating that patients whose chest pain is exertional, radiating to one or both arms, similar to or worse than prior cardiac chest pain, or associated with nausea, vomiting, or diaphoresis are at high risk for ACS (TABLE 2).9 These researchers also found that chest pain that is stabbing, pleuritic, positional, or reproducible with palpation suggests that a patient is at low risk for ACS. Pain that is not exertional or that is in a small inframammary area of the chest also suggests a low risk for ACS.9
Marburg Heart Score and other tests can help rule out ACS
As part of your initial physical examination, assess the patient’s overall condition and stability. Be aware, however, that an older literature review found that a physical exam is only minimally helpful in assessing ACS risk in a patient with chest pain. Findings that may increase the risk of ACS are a third heart sound (positive likelihood ratio [LR+] = 3.2; 95% confidence interval [CI], 1.6-6.5), systolic blood pressure <80 mm/Hg (LR+ = 3.1; 95% CI, 1.8-5.2), and pulmonary crackles on auscultation (LR+ = 2.1; 95% CI, 1.4–3.1); however, the absence of these findings does not exclude ACS.10 The most helpful sign or symptom in evaluating a patient with chest pain is chest wall tenderness on palpation, which largely rules out ACS in low-prevalence settings, such as a primary care office.11
Bösner et al12 developed the Marburg Heart Score (MHS) to help primary care physicians evaluate the risk of CAD in patients with chest pain (TABLE 3).12,13 A subsequent validation study found that an MHS ≥3 had a sensitivity of 89.1% (95% CI, 81.1%-94%) and a specificity of 63.5% (95% CI, 60%-66.9%) for CAD.13 The test’s negative predictive value (NPV) of 97.9% (95% CI, 96.2%-98.9%) means that patients with an MHS ≤2 are very unlikely to have CAD; however, the low positive predictive value (PPV) of only 23.3% (95% CI, 19.2%-28.0%) means an MHS ≥3 is not particularly helpful in diagnosing CAD.12,13
Unless it is clear that your patient’s chest pain is unlikely to have a cardiac cause (eg, pain is reproducible on palpation, or an MHS ≤2), order an electrocardiogram (EKG). If the EKG shows ST-segment elevation in 2 or more contiguous leads, presumed new left bundle branch block, ischemic ST-segment depression >.5 mm (.05 mV), or dynamic T-wave inversion with pain or discomfort, the patient needs urgent referral for emergency care.8 If the EKG is nondiagnostic but the chest pain is suspicious for CAD, then further testing with cardiac biomarkers (eg, troponin I or T) is recommended to evaluate for non-ST elevation myocardial infarction. Consider chest radiography if there is evidence of respiratory disease (cough, dyspnea, or a history of pulmonary disease).
Don’t overlook chest wall syndrome, GERD, or panic disorder
There are several conditions to consider in the differential diagnosis of patients whose chest pain does not appear to have a cardiac cause:
CWS is the most common cause of chest pain in primary care patients.14,15 While there are several specific types of chest wall pain—including musculoskeletal pain, parietal or intercostal pain, Tietze’s syndrome, and costochondral pain—all are manifestations of a musculoskeletal disorder and associated with tenderness of the chest wall. CWS is not life threatening, but one study found high rates of anxiety (54%-93%) among patients with moderate to severe CWS.14,15
Few trials have evaluated treatments for chest wall pain or costochondritis, though typical recommendations include nonsteroidal anti-inflammatory medications, use of heat or cold, physical therapy, or injection of local anesthetic.16 One study found that stretching exercises might benefit patients with costochondritis.17
GI disorders. Patients with esophagitis or gastroesophageal reflux disease (GERD) often report heartburn, chronic cough, chronic laryngitis, and asthma.18 However, the sensitivity and specificity of these symptoms are too low to allow diagnosis or exclusion of GERD based on history alone.18
Acid suppression therapy can be used to test for GERD. A 2005 meta-analysis of 6 studies found the sensitivity and specificity of a proton-pump inhibitor (PPI) acid suppression test for the diagnosis of GERD in patients with noncardiac chest pain were 80% (95% CI, 71%-87%) and 74% (95% CI, 64%-83%), respectively.19 One study demonstrated that relief of chest pain after a 14-day course of omeprazole 40 mg/d was more sensitive than endoscopy, manometry, or 24-hour esophageal pH monitoring in diagnosing GERD.20 Another study found that in patients with noncardiac chest pain and normal upper endoscopy, symptomatic relief with lansoprazole 30 mg/d for 4 weeks can be used to diagnose endoscopy-negative GERD.21
It is appropriate to try a high-dose course of a PPI (ie, omeprazole 40 mg twice daily, lansoprazole 30 mg/d, or esomeprazole 40 mg twice daily) to evaluate for GERD as the cause of chest pain in patients who:20-22
• do not initially describe typical reflux symptoms (eg, heartburn, chronic regurgitation, chronic cough, or a sore or burning throat)
• have no history of surgery in the upper GI tract, esophagus, or thorax, and
• have no signs or symptoms that indicate they have a serious or malignant disease (eg, weight loss, anemia, or dysphagia).
Panic disorder. Several tools have been proposed for screening for panic disorder (PD),23,24 but none have been tested in patients with chest pain. Dammen et al25 developed a 3-item questionnaire to assess for PD among patients with chest pain who were referred for cardiac evaluation (TABLE 4).25 A score ≥5 on the Dammen questionnaire had 55% sensitivity and 86% specificity for PD, with a PPV of 71% and an NPV of 76%.25 Although this instrument has not been subjected to validation studies, using it may help clarify whether further investigation for PD is warranted.
Psychotherapeutic interventions may be effective for patients whose chest pain is caused by PD. A Cochrane review of 15 randomized controlled trials of psychological interventions for chest pain in patients with normal coronary anatomy found that cognitive-behavior therapy, and possibly hypnotherapy, reduced patient reports of chest pain, reduced chest pain frequency, and increased the number of chest pain-free days, at least for 3 months.26
What to do when the diagnosis remains unclear
When your initial evaluation and diagnostic testing yield no clear diagnosis, appropriate follow-up is vital because in the year after primary care patients first develop chest pain, they are 1.5 to 3 times more likely than the general population to be diagnosed with musculoskeletal, GI, psychological, or respiratory problems, nearly 5 times as likely to be diagnosed with heart failure, and nearly 15 times as likely to be diagnosed with coronary heart disease.27,28
Consider ordering exercise or chemical stress testing within 3 to 7 days for a patient with chest pain that suggests ACS but who has normal results on EKG and biomarker testing.8 Interestingly, though, in a study of 4181 patients in an ED chest pain unit who had 2 sets of normal serum troponins during a 6-hour period followed by exercise or chemical stress testing, only 470 patients (11%) had abnormal stress test results and only 37 (.9%) had obstructive CAD that would have potentially benefited from revascularization.29 Thus, testing troponin levels twice over 6 hours is a reasonable alternative to stress testing for a primary care patient with chest pain; stress testing would be unnecessary if both troponin values were normal.
CASE › Based on her current chest pain symptoms, Ms. Z’s MHS is a reassuringly low 1, so CAD is unlikely. However, she scores 5 on the Dammen panic disorder screen. Due to her financial concerns, you decide to forgo stress testing and instead draw a serum troponin now, with plans to repeat later in the afternoon at your clinic lab if the initial result is normal. You encourage her to try a high-dose PPI for 2 weeks to determine whether GERD may be contributing to her symptoms, and offer to help her explore counseling options to address her emotional stressors.
CORRESPONDENCE
William E. Cayley Jr, MD, MDiv, University of Wisconsin, UW Health Augusta Family Medicine Clinic, 207 West Lincoln, Augusta, WI 54722; [email protected]
1. Amsterdam EA, Kirk JD, Bluemke DA, et al; American Heart Association Exercise, Cardiac Rehabilitation, and Prevention Committee of the Council on Clinical Cardiology, Council on Cardiovascular Nursing, and Interdisciplinary Council on Quality of Care and Outcomes Research. Testing of low-risk patients presenting to the emergency department with chest pain: a scientific statement from the American Heart Association. Circulation. 2010;122:1756-7176.
2. Bhuiya FA, Pitts SR, McCaig LF. Emergency department visits for chest pain and abdominal pain: United States, 1999-2008. NCHS Data Brief. 2010;43:1-8.
3. Klinkman MS, Stevens D, Gorenflo DW. Episodes of care for chest pain: a preliminary report from MIRNET. Michigan Research Network. J Fam Pract. 1994;38:345-352.
4. McConaghy JR, Oza RS. Outpatient diagnosis of acute chest pain in adults. Am Fam Physician. 2013;87:177-82.
5. Nilsson S, Scheike M, Engblom D, et al. Chest pain and ischaemic heart disease in primary care. Br J Gen Pract. 2003;53:378-382.
6. Buntinx F, Knockaert D, Bruyninckx R, et al. Chest pain in general practice or in the hospital emergency department: is it the same? Fam Pract. 2001;18:586-589.
7. Jonsbu E, Dammen T, Morken G, et al. Cardiac and psychiatric diagnoses among patients referred for chest pain and palpitations. Scand Cardiovasc J. 2009;43:256-259.
8. O’Connor RE, Brady W, Brooks SC, et al. Part 10: acute coronary syndromes: 2010 American Heart Association Guidelines for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care. Circulation. 2010;122(18 suppl 3):S787-S817.
9. Swap CJ, Nagurney JT. Value and limitations of chest pain history in the evaluation of patients with suspected acute coronary syndromes. JAMA. 2005;294:2623-2629.
10. Panju AA, Hemmelgarn BR, Guyatt GH, et al. The rational clinical examination. Is this patient having a myocardial infarction? JAMA. 1998;280:1256-1263.
11. Bruyninckx R, Aertgeerts B, Bruyninckx P, et al. Signs and symptoms in diagnosing acute myocardial infarction and acute coronary syndrome: a diagnostic meta-analysis. Br J Gen Pract. 2008;58:105-111.
12. Bösner S, Haasenritter J, Becker A, et al. Ruling out coronary artery disease in primary care: development and validation of a simple prediction rule. CMAJ. 2010;182:1295-1300.
13. Haasenritter J, Bösner S, Vaucher P, et al. Ruling out coronary heart disease in primary care: external validation of a clinical prediction rule. Br J Gen Pract. 2012;62:e415-e421.
14. Bösner S, Becker A, Hani MA, et al. Chest wall syndrome in primary care patients with chest pain: presentation, associated features and diagnosis. Fam Pract. 2010;27:363-369.
15. Verdon F, Burnand B, Herzig L, et al. Chest wall syndrome among primary care patients: a cohort study. BMC Fam Pract. 2007;8:51.
16. Proulx AM, Zryd TW. Costochondritis: diagnosis and treatment. Am Fam Physician. 2009;80:617-620.
17. Rovetta G, Sessarego P, Monteforte P. Stretching exercises for costochondritis pain. G Ital Med Lav Ergon. 2009;31:169-171.
18. Lacy BE, Weiser K, Chertoff J, et al. The diagnosis of gastroesophageal reflux disease. Am J Med. 2010;123: 583-592.
19. Wang WH, Huang JQ, Zheng GF, et al. Is proton pump inhibitor testing an effective approach to diagnose gastroesophageal reflux disease in patients with noncardiac chest pain?: a metaanalysis. Arch Intern Med. 2005;165:1222-1228.
20. Pandak WM, Arezo S, Everett S, et al. Short course of omeprazole: a better first diagnostic approach to noncardiac chest pain than endoscopy, manometry, or 24-hour esophageal pH monitoring. J Clin Gastroenterol. 2002;35:307-314.
21. Xia HH, Lai KC, Lam SK, et al. Symptomatic response to lansoprazole predicts abnormal acid reflux in endoscopy-negative patients with non-cardiac chest pain. Aliment Pharmacol Ther. 2003;17:369-377.
22. Flook NW, Moayyedi P, Dent J, et al. Acid-suppressive therapy with esomeprazole for relief of unexplained chest pain in primary care: a randomized, double-blind, placebo-controlled trial. Am J Gastroenterol. 2013;108:56-64.
23. Stein MB, Roy-Byrne PP, McQuaid JR, et al. Development of a brief diagnostic screen for panic disorder in primary care. Psychosom Med. 1999;61:359-364.
24. Ballenger JC. Treatment of panic disorder in the general medical setting. J Psychosom Res. 1998;44:5-15.
25. Dammen T, Ekeberg O, Arnesen H, et al. The detection of panic disorder in chest pain patients. Gen Hosp Psychiatry. 1999;21:323-332.
26. Kisely SR, Campbell LA, Yelland MJ, et al. Psychological interventions for symptomatic management of non-specific chest pain in patients with normal coronary anatomy. Cochrane Database Syst Rev. 2012;6:CD004101.
27. Ruigómez A, Rodríguez LA, Wallander MA, et al. Chest pain in general practice: incidence, comorbidity and mortality. Fam Pract. 2006;23:167-174.
28. Ruigómez A, Massó-González EL, Johansson S, et al. Chest pain without established ischaemic heart disease in primary care patients: associated comorbidities and mortality. Br J Gen Pract. 2009;59:e78-e86.
29. Hermann LK, Newman DH, Pleasant WA, et al. Yield of routine provocative cardiac testing among patients in an emergency department-based chest pain unit. JAMA Intern Med. 2013;173:1128-1133.
› Seek immediate emergency care for patients with chest pain that is exertional, radiating to one or both arms, similar to or worse than prior cardiac chest pain, or associated with nausea, vomiting, or diaphoresis. A
› Be aware that patients with chest pain that is stabbing, pleuritic, positional, or reproducible with palpation are at very low risk for acute coronary syndrome and most likely have chest wall pain. A
› Consider a 2-week course of high-dose proton-pump inhibitor therapy to help identify patients whose chest pain may be from undiagnosed gastroesophageal reflux disease. A
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
CASE › Your patient, Amy Z, age 58, was given a diagnosis of hypertension 10 years ago and since then has been maintained on hydrochlorothiazide 50 mg/d and lisinopril 10 mg/d. In the office today, she reports intermittent chest tightness and heaviness. She has no history of coronary artery disease (CAD), cerebrovascular disease, or peripheral vascular disease. She attributes her chest discomfort to emotional stress. She recently started a job after having been unemployed, but still has no health insurance and is concerned about losing her house.
She denies orthopnea and resting or exertional dyspnea, and says she never gets chest pain while climbing stairs. Her blood pressure is elevated at 180/110 mm Hg, but her other vital signs are normal (pulse, 70 beats per minute; respiratory rate, 18 breaths per minute). On physical examination, she has no venous distension in her neck and her lungs are clear. A cardiac exam reveals a regular rate and rhythm, with a normally split S1 and S2 and no murmurs, rubs, or gallops. Palpation of the chest does not reproduce her chest pain.
You are concerned that your patient’s chest pain could be from heart disease, but she wants to defer additional testing because of the cost, stating, “It’s all due to my stress.”
How would you proceed?
Musculoskeletal chest wall pain is the most common cause of chest pain in patients who seek treatment in the office, followed by GI disease and stable heart disease.
Whether they go to the emergency department (ED) or to their family physician’s office, most patients who seek treatment for chest pain don’t have life-threatening cardiac illness. Of the 8 million patients who visit an ED for chest pain each year, only 13% are diagnosed with acute coronary syndrome (ACS).1,2 Among those seen for chest pain in a primary care office, only a minority (approximately 1.5%) have unstable heart disease.3-5 Cross-sectional studies indicate that musculoskeletal chest wall pain (or “chest wall syndrome [CWS]”) is the most common cause of chest pain in patients who seek treatment in the office, followed by gastrointestinal (GI) disease, stable heart disease, psychosocial or psychiatric conditions, pulmonary disease, and other cardiovascular conditions (TABLE 1).3,6,7
When evaluating patients with chest pain in the office, the challenge is to appropriately evaluate and manage those who are at low risk of ACS, while at the same time identifying and arranging prompt transfer or referral for the minority of patients who are at high cardiac risk. This article describes how to determine which patients require emergency treatment, which tools to use to screen for ACS and other potential causes of chest pain, and how to proceed when initial evaluation and testing do not point to a clear diagnosis.
Start with the ABCs
When a patient presents in primary care with a chief complaint of chest pain, it’s of course critical that you quickly determine if he or she is stable by evaluating the “ABCs” (airway, breathing, and circulation). Any potentially unstable patient should be immediately transferred for emergency care.8 A patient who shows no signs of respiratory distress and whose vital signs are within a normal range is unlikely to be acutely unstable, and can be further evaluated in the office.
If the patient is stable, obtain a history of the onset and evolution of the chest pain, especially its location, quality, duration, and aggravating or alleviating factors. Also ask about a personal or family history of heart disease, hypertension, diabetes, or hypercholesterolemia, and about tobacco use. While the presence of any of these cardiac risk factors may increase suspicion for a cardiac cause for chest pain, the absence of such factors does not eliminate the need for a careful diagnostic evaluation.
Patients with “typical” chest pain have a higher risk of ACS. In a 2005 review of observational prospective and retrospective studies and systematic reviews, Swap et al9 corroborated the description of “typical” anginal chest pain, indicating that patients whose chest pain is exertional, radiating to one or both arms, similar to or worse than prior cardiac chest pain, or associated with nausea, vomiting, or diaphoresis are at high risk for ACS (TABLE 2).9 These researchers also found that chest pain that is stabbing, pleuritic, positional, or reproducible with palpation suggests that a patient is at low risk for ACS. Pain that is not exertional or that is in a small inframammary area of the chest also suggests a low risk for ACS.9
Marburg Heart Score and other tests can help rule out ACS
As part of your initial physical examination, assess the patient’s overall condition and stability. Be aware, however, that an older literature review found that a physical exam is only minimally helpful in assessing ACS risk in a patient with chest pain. Findings that may increase the risk of ACS are a third heart sound (positive likelihood ratio [LR+] = 3.2; 95% confidence interval [CI], 1.6-6.5), systolic blood pressure <80 mm/Hg (LR+ = 3.1; 95% CI, 1.8-5.2), and pulmonary crackles on auscultation (LR+ = 2.1; 95% CI, 1.4–3.1); however, the absence of these findings does not exclude ACS.10 The most helpful sign or symptom in evaluating a patient with chest pain is chest wall tenderness on palpation, which largely rules out ACS in low-prevalence settings, such as a primary care office.11
Bösner et al12 developed the Marburg Heart Score (MHS) to help primary care physicians evaluate the risk of CAD in patients with chest pain (TABLE 3).12,13 A subsequent validation study found that an MHS ≥3 had a sensitivity of 89.1% (95% CI, 81.1%-94%) and a specificity of 63.5% (95% CI, 60%-66.9%) for CAD.13 The test’s negative predictive value (NPV) of 97.9% (95% CI, 96.2%-98.9%) means that patients with an MHS ≤2 are very unlikely to have CAD; however, the low positive predictive value (PPV) of only 23.3% (95% CI, 19.2%-28.0%) means an MHS ≥3 is not particularly helpful in diagnosing CAD.12,13
Unless it is clear that your patient’s chest pain is unlikely to have a cardiac cause (eg, pain is reproducible on palpation, or an MHS ≤2), order an electrocardiogram (EKG). If the EKG shows ST-segment elevation in 2 or more contiguous leads, presumed new left bundle branch block, ischemic ST-segment depression >.5 mm (.05 mV), or dynamic T-wave inversion with pain or discomfort, the patient needs urgent referral for emergency care.8 If the EKG is nondiagnostic but the chest pain is suspicious for CAD, then further testing with cardiac biomarkers (eg, troponin I or T) is recommended to evaluate for non-ST elevation myocardial infarction. Consider chest radiography if there is evidence of respiratory disease (cough, dyspnea, or a history of pulmonary disease).
Don’t overlook chest wall syndrome, GERD, or panic disorder
There are several conditions to consider in the differential diagnosis of patients whose chest pain does not appear to have a cardiac cause:
CWS is the most common cause of chest pain in primary care patients.14,15 While there are several specific types of chest wall pain—including musculoskeletal pain, parietal or intercostal pain, Tietze’s syndrome, and costochondral pain—all are manifestations of a musculoskeletal disorder and associated with tenderness of the chest wall. CWS is not life threatening, but one study found high rates of anxiety (54%-93%) among patients with moderate to severe CWS.14,15
Few trials have evaluated treatments for chest wall pain or costochondritis, though typical recommendations include nonsteroidal anti-inflammatory medications, use of heat or cold, physical therapy, or injection of local anesthetic.16 One study found that stretching exercises might benefit patients with costochondritis.17
GI disorders. Patients with esophagitis or gastroesophageal reflux disease (GERD) often report heartburn, chronic cough, chronic laryngitis, and asthma.18 However, the sensitivity and specificity of these symptoms are too low to allow diagnosis or exclusion of GERD based on history alone.18
Acid suppression therapy can be used to test for GERD. A 2005 meta-analysis of 6 studies found the sensitivity and specificity of a proton-pump inhibitor (PPI) acid suppression test for the diagnosis of GERD in patients with noncardiac chest pain were 80% (95% CI, 71%-87%) and 74% (95% CI, 64%-83%), respectively.19 One study demonstrated that relief of chest pain after a 14-day course of omeprazole 40 mg/d was more sensitive than endoscopy, manometry, or 24-hour esophageal pH monitoring in diagnosing GERD.20 Another study found that in patients with noncardiac chest pain and normal upper endoscopy, symptomatic relief with lansoprazole 30 mg/d for 4 weeks can be used to diagnose endoscopy-negative GERD.21
It is appropriate to try a high-dose course of a PPI (ie, omeprazole 40 mg twice daily, lansoprazole 30 mg/d, or esomeprazole 40 mg twice daily) to evaluate for GERD as the cause of chest pain in patients who:20-22
• do not initially describe typical reflux symptoms (eg, heartburn, chronic regurgitation, chronic cough, or a sore or burning throat)
• have no history of surgery in the upper GI tract, esophagus, or thorax, and
• have no signs or symptoms that indicate they have a serious or malignant disease (eg, weight loss, anemia, or dysphagia).
Panic disorder. Several tools have been proposed for screening for panic disorder (PD),23,24 but none have been tested in patients with chest pain. Dammen et al25 developed a 3-item questionnaire to assess for PD among patients with chest pain who were referred for cardiac evaluation (TABLE 4).25 A score ≥5 on the Dammen questionnaire had 55% sensitivity and 86% specificity for PD, with a PPV of 71% and an NPV of 76%.25 Although this instrument has not been subjected to validation studies, using it may help clarify whether further investigation for PD is warranted.
Psychotherapeutic interventions may be effective for patients whose chest pain is caused by PD. A Cochrane review of 15 randomized controlled trials of psychological interventions for chest pain in patients with normal coronary anatomy found that cognitive-behavior therapy, and possibly hypnotherapy, reduced patient reports of chest pain, reduced chest pain frequency, and increased the number of chest pain-free days, at least for 3 months.26
What to do when the diagnosis remains unclear
When your initial evaluation and diagnostic testing yield no clear diagnosis, appropriate follow-up is vital because in the year after primary care patients first develop chest pain, they are 1.5 to 3 times more likely than the general population to be diagnosed with musculoskeletal, GI, psychological, or respiratory problems, nearly 5 times as likely to be diagnosed with heart failure, and nearly 15 times as likely to be diagnosed with coronary heart disease.27,28
Consider ordering exercise or chemical stress testing within 3 to 7 days for a patient with chest pain that suggests ACS but who has normal results on EKG and biomarker testing.8 Interestingly, though, in a study of 4181 patients in an ED chest pain unit who had 2 sets of normal serum troponins during a 6-hour period followed by exercise or chemical stress testing, only 470 patients (11%) had abnormal stress test results and only 37 (.9%) had obstructive CAD that would have potentially benefited from revascularization.29 Thus, testing troponin levels twice over 6 hours is a reasonable alternative to stress testing for a primary care patient with chest pain; stress testing would be unnecessary if both troponin values were normal.
CASE › Based on her current chest pain symptoms, Ms. Z’s MHS is a reassuringly low 1, so CAD is unlikely. However, she scores 5 on the Dammen panic disorder screen. Due to her financial concerns, you decide to forgo stress testing and instead draw a serum troponin now, with plans to repeat later in the afternoon at your clinic lab if the initial result is normal. You encourage her to try a high-dose PPI for 2 weeks to determine whether GERD may be contributing to her symptoms, and offer to help her explore counseling options to address her emotional stressors.
CORRESPONDENCE
William E. Cayley Jr, MD, MDiv, University of Wisconsin, UW Health Augusta Family Medicine Clinic, 207 West Lincoln, Augusta, WI 54722; [email protected]
› Seek immediate emergency care for patients with chest pain that is exertional, radiating to one or both arms, similar to or worse than prior cardiac chest pain, or associated with nausea, vomiting, or diaphoresis. A
› Be aware that patients with chest pain that is stabbing, pleuritic, positional, or reproducible with palpation are at very low risk for acute coronary syndrome and most likely have chest wall pain. A
› Consider a 2-week course of high-dose proton-pump inhibitor therapy to help identify patients whose chest pain may be from undiagnosed gastroesophageal reflux disease. A
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
CASE › Your patient, Amy Z, age 58, was given a diagnosis of hypertension 10 years ago and since then has been maintained on hydrochlorothiazide 50 mg/d and lisinopril 10 mg/d. In the office today, she reports intermittent chest tightness and heaviness. She has no history of coronary artery disease (CAD), cerebrovascular disease, or peripheral vascular disease. She attributes her chest discomfort to emotional stress. She recently started a job after having been unemployed, but still has no health insurance and is concerned about losing her house.
She denies orthopnea and resting or exertional dyspnea, and says she never gets chest pain while climbing stairs. Her blood pressure is elevated at 180/110 mm Hg, but her other vital signs are normal (pulse, 70 beats per minute; respiratory rate, 18 breaths per minute). On physical examination, she has no venous distension in her neck and her lungs are clear. A cardiac exam reveals a regular rate and rhythm, with a normally split S1 and S2 and no murmurs, rubs, or gallops. Palpation of the chest does not reproduce her chest pain.
You are concerned that your patient’s chest pain could be from heart disease, but she wants to defer additional testing because of the cost, stating, “It’s all due to my stress.”
How would you proceed?
Musculoskeletal chest wall pain is the most common cause of chest pain in patients who seek treatment in the office, followed by GI disease and stable heart disease.
Whether they go to the emergency department (ED) or to their family physician’s office, most patients who seek treatment for chest pain don’t have life-threatening cardiac illness. Of the 8 million patients who visit an ED for chest pain each year, only 13% are diagnosed with acute coronary syndrome (ACS).1,2 Among those seen for chest pain in a primary care office, only a minority (approximately 1.5%) have unstable heart disease.3-5 Cross-sectional studies indicate that musculoskeletal chest wall pain (or “chest wall syndrome [CWS]”) is the most common cause of chest pain in patients who seek treatment in the office, followed by gastrointestinal (GI) disease, stable heart disease, psychosocial or psychiatric conditions, pulmonary disease, and other cardiovascular conditions (TABLE 1).3,6,7
When evaluating patients with chest pain in the office, the challenge is to appropriately evaluate and manage those who are at low risk of ACS, while at the same time identifying and arranging prompt transfer or referral for the minority of patients who are at high cardiac risk. This article describes how to determine which patients require emergency treatment, which tools to use to screen for ACS and other potential causes of chest pain, and how to proceed when initial evaluation and testing do not point to a clear diagnosis.
Start with the ABCs
When a patient presents in primary care with a chief complaint of chest pain, it’s of course critical that you quickly determine if he or she is stable by evaluating the “ABCs” (airway, breathing, and circulation). Any potentially unstable patient should be immediately transferred for emergency care.8 A patient who shows no signs of respiratory distress and whose vital signs are within a normal range is unlikely to be acutely unstable, and can be further evaluated in the office.
If the patient is stable, obtain a history of the onset and evolution of the chest pain, especially its location, quality, duration, and aggravating or alleviating factors. Also ask about a personal or family history of heart disease, hypertension, diabetes, or hypercholesterolemia, and about tobacco use. While the presence of any of these cardiac risk factors may increase suspicion for a cardiac cause for chest pain, the absence of such factors does not eliminate the need for a careful diagnostic evaluation.
Patients with “typical” chest pain have a higher risk of ACS. In a 2005 review of observational prospective and retrospective studies and systematic reviews, Swap et al9 corroborated the description of “typical” anginal chest pain, indicating that patients whose chest pain is exertional, radiating to one or both arms, similar to or worse than prior cardiac chest pain, or associated with nausea, vomiting, or diaphoresis are at high risk for ACS (TABLE 2).9 These researchers also found that chest pain that is stabbing, pleuritic, positional, or reproducible with palpation suggests that a patient is at low risk for ACS. Pain that is not exertional or that is in a small inframammary area of the chest also suggests a low risk for ACS.9
Marburg Heart Score and other tests can help rule out ACS
As part of your initial physical examination, assess the patient’s overall condition and stability. Be aware, however, that an older literature review found that a physical exam is only minimally helpful in assessing ACS risk in a patient with chest pain. Findings that may increase the risk of ACS are a third heart sound (positive likelihood ratio [LR+] = 3.2; 95% confidence interval [CI], 1.6-6.5), systolic blood pressure <80 mm/Hg (LR+ = 3.1; 95% CI, 1.8-5.2), and pulmonary crackles on auscultation (LR+ = 2.1; 95% CI, 1.4–3.1); however, the absence of these findings does not exclude ACS.10 The most helpful sign or symptom in evaluating a patient with chest pain is chest wall tenderness on palpation, which largely rules out ACS in low-prevalence settings, such as a primary care office.11
Bösner et al12 developed the Marburg Heart Score (MHS) to help primary care physicians evaluate the risk of CAD in patients with chest pain (TABLE 3).12,13 A subsequent validation study found that an MHS ≥3 had a sensitivity of 89.1% (95% CI, 81.1%-94%) and a specificity of 63.5% (95% CI, 60%-66.9%) for CAD.13 The test’s negative predictive value (NPV) of 97.9% (95% CI, 96.2%-98.9%) means that patients with an MHS ≤2 are very unlikely to have CAD; however, the low positive predictive value (PPV) of only 23.3% (95% CI, 19.2%-28.0%) means an MHS ≥3 is not particularly helpful in diagnosing CAD.12,13
Unless it is clear that your patient’s chest pain is unlikely to have a cardiac cause (eg, pain is reproducible on palpation, or an MHS ≤2), order an electrocardiogram (EKG). If the EKG shows ST-segment elevation in 2 or more contiguous leads, presumed new left bundle branch block, ischemic ST-segment depression >.5 mm (.05 mV), or dynamic T-wave inversion with pain or discomfort, the patient needs urgent referral for emergency care.8 If the EKG is nondiagnostic but the chest pain is suspicious for CAD, then further testing with cardiac biomarkers (eg, troponin I or T) is recommended to evaluate for non-ST elevation myocardial infarction. Consider chest radiography if there is evidence of respiratory disease (cough, dyspnea, or a history of pulmonary disease).
Don’t overlook chest wall syndrome, GERD, or panic disorder
There are several conditions to consider in the differential diagnosis of patients whose chest pain does not appear to have a cardiac cause:
CWS is the most common cause of chest pain in primary care patients.14,15 While there are several specific types of chest wall pain—including musculoskeletal pain, parietal or intercostal pain, Tietze’s syndrome, and costochondral pain—all are manifestations of a musculoskeletal disorder and associated with tenderness of the chest wall. CWS is not life threatening, but one study found high rates of anxiety (54%-93%) among patients with moderate to severe CWS.14,15
Few trials have evaluated treatments for chest wall pain or costochondritis, though typical recommendations include nonsteroidal anti-inflammatory medications, use of heat or cold, physical therapy, or injection of local anesthetic.16 One study found that stretching exercises might benefit patients with costochondritis.17
GI disorders. Patients with esophagitis or gastroesophageal reflux disease (GERD) often report heartburn, chronic cough, chronic laryngitis, and asthma.18 However, the sensitivity and specificity of these symptoms are too low to allow diagnosis or exclusion of GERD based on history alone.18
Acid suppression therapy can be used to test for GERD. A 2005 meta-analysis of 6 studies found the sensitivity and specificity of a proton-pump inhibitor (PPI) acid suppression test for the diagnosis of GERD in patients with noncardiac chest pain were 80% (95% CI, 71%-87%) and 74% (95% CI, 64%-83%), respectively.19 One study demonstrated that relief of chest pain after a 14-day course of omeprazole 40 mg/d was more sensitive than endoscopy, manometry, or 24-hour esophageal pH monitoring in diagnosing GERD.20 Another study found that in patients with noncardiac chest pain and normal upper endoscopy, symptomatic relief with lansoprazole 30 mg/d for 4 weeks can be used to diagnose endoscopy-negative GERD.21
It is appropriate to try a high-dose course of a PPI (ie, omeprazole 40 mg twice daily, lansoprazole 30 mg/d, or esomeprazole 40 mg twice daily) to evaluate for GERD as the cause of chest pain in patients who:20-22
• do not initially describe typical reflux symptoms (eg, heartburn, chronic regurgitation, chronic cough, or a sore or burning throat)
• have no history of surgery in the upper GI tract, esophagus, or thorax, and
• have no signs or symptoms that indicate they have a serious or malignant disease (eg, weight loss, anemia, or dysphagia).
Panic disorder. Several tools have been proposed for screening for panic disorder (PD),23,24 but none have been tested in patients with chest pain. Dammen et al25 developed a 3-item questionnaire to assess for PD among patients with chest pain who were referred for cardiac evaluation (TABLE 4).25 A score ≥5 on the Dammen questionnaire had 55% sensitivity and 86% specificity for PD, with a PPV of 71% and an NPV of 76%.25 Although this instrument has not been subjected to validation studies, using it may help clarify whether further investigation for PD is warranted.
Psychotherapeutic interventions may be effective for patients whose chest pain is caused by PD. A Cochrane review of 15 randomized controlled trials of psychological interventions for chest pain in patients with normal coronary anatomy found that cognitive-behavior therapy, and possibly hypnotherapy, reduced patient reports of chest pain, reduced chest pain frequency, and increased the number of chest pain-free days, at least for 3 months.26
What to do when the diagnosis remains unclear
When your initial evaluation and diagnostic testing yield no clear diagnosis, appropriate follow-up is vital because in the year after primary care patients first develop chest pain, they are 1.5 to 3 times more likely than the general population to be diagnosed with musculoskeletal, GI, psychological, or respiratory problems, nearly 5 times as likely to be diagnosed with heart failure, and nearly 15 times as likely to be diagnosed with coronary heart disease.27,28
Consider ordering exercise or chemical stress testing within 3 to 7 days for a patient with chest pain that suggests ACS but who has normal results on EKG and biomarker testing.8 Interestingly, though, in a study of 4181 patients in an ED chest pain unit who had 2 sets of normal serum troponins during a 6-hour period followed by exercise or chemical stress testing, only 470 patients (11%) had abnormal stress test results and only 37 (.9%) had obstructive CAD that would have potentially benefited from revascularization.29 Thus, testing troponin levels twice over 6 hours is a reasonable alternative to stress testing for a primary care patient with chest pain; stress testing would be unnecessary if both troponin values were normal.
CASE › Based on her current chest pain symptoms, Ms. Z’s MHS is a reassuringly low 1, so CAD is unlikely. However, she scores 5 on the Dammen panic disorder screen. Due to her financial concerns, you decide to forgo stress testing and instead draw a serum troponin now, with plans to repeat later in the afternoon at your clinic lab if the initial result is normal. You encourage her to try a high-dose PPI for 2 weeks to determine whether GERD may be contributing to her symptoms, and offer to help her explore counseling options to address her emotional stressors.
CORRESPONDENCE
William E. Cayley Jr, MD, MDiv, University of Wisconsin, UW Health Augusta Family Medicine Clinic, 207 West Lincoln, Augusta, WI 54722; [email protected]
1. Amsterdam EA, Kirk JD, Bluemke DA, et al; American Heart Association Exercise, Cardiac Rehabilitation, and Prevention Committee of the Council on Clinical Cardiology, Council on Cardiovascular Nursing, and Interdisciplinary Council on Quality of Care and Outcomes Research. Testing of low-risk patients presenting to the emergency department with chest pain: a scientific statement from the American Heart Association. Circulation. 2010;122:1756-7176.
2. Bhuiya FA, Pitts SR, McCaig LF. Emergency department visits for chest pain and abdominal pain: United States, 1999-2008. NCHS Data Brief. 2010;43:1-8.
3. Klinkman MS, Stevens D, Gorenflo DW. Episodes of care for chest pain: a preliminary report from MIRNET. Michigan Research Network. J Fam Pract. 1994;38:345-352.
4. McConaghy JR, Oza RS. Outpatient diagnosis of acute chest pain in adults. Am Fam Physician. 2013;87:177-82.
5. Nilsson S, Scheike M, Engblom D, et al. Chest pain and ischaemic heart disease in primary care. Br J Gen Pract. 2003;53:378-382.
6. Buntinx F, Knockaert D, Bruyninckx R, et al. Chest pain in general practice or in the hospital emergency department: is it the same? Fam Pract. 2001;18:586-589.
7. Jonsbu E, Dammen T, Morken G, et al. Cardiac and psychiatric diagnoses among patients referred for chest pain and palpitations. Scand Cardiovasc J. 2009;43:256-259.
8. O’Connor RE, Brady W, Brooks SC, et al. Part 10: acute coronary syndromes: 2010 American Heart Association Guidelines for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care. Circulation. 2010;122(18 suppl 3):S787-S817.
9. Swap CJ, Nagurney JT. Value and limitations of chest pain history in the evaluation of patients with suspected acute coronary syndromes. JAMA. 2005;294:2623-2629.
10. Panju AA, Hemmelgarn BR, Guyatt GH, et al. The rational clinical examination. Is this patient having a myocardial infarction? JAMA. 1998;280:1256-1263.
11. Bruyninckx R, Aertgeerts B, Bruyninckx P, et al. Signs and symptoms in diagnosing acute myocardial infarction and acute coronary syndrome: a diagnostic meta-analysis. Br J Gen Pract. 2008;58:105-111.
12. Bösner S, Haasenritter J, Becker A, et al. Ruling out coronary artery disease in primary care: development and validation of a simple prediction rule. CMAJ. 2010;182:1295-1300.
13. Haasenritter J, Bösner S, Vaucher P, et al. Ruling out coronary heart disease in primary care: external validation of a clinical prediction rule. Br J Gen Pract. 2012;62:e415-e421.
14. Bösner S, Becker A, Hani MA, et al. Chest wall syndrome in primary care patients with chest pain: presentation, associated features and diagnosis. Fam Pract. 2010;27:363-369.
15. Verdon F, Burnand B, Herzig L, et al. Chest wall syndrome among primary care patients: a cohort study. BMC Fam Pract. 2007;8:51.
16. Proulx AM, Zryd TW. Costochondritis: diagnosis and treatment. Am Fam Physician. 2009;80:617-620.
17. Rovetta G, Sessarego P, Monteforte P. Stretching exercises for costochondritis pain. G Ital Med Lav Ergon. 2009;31:169-171.
18. Lacy BE, Weiser K, Chertoff J, et al. The diagnosis of gastroesophageal reflux disease. Am J Med. 2010;123: 583-592.
19. Wang WH, Huang JQ, Zheng GF, et al. Is proton pump inhibitor testing an effective approach to diagnose gastroesophageal reflux disease in patients with noncardiac chest pain?: a metaanalysis. Arch Intern Med. 2005;165:1222-1228.
20. Pandak WM, Arezo S, Everett S, et al. Short course of omeprazole: a better first diagnostic approach to noncardiac chest pain than endoscopy, manometry, or 24-hour esophageal pH monitoring. J Clin Gastroenterol. 2002;35:307-314.
21. Xia HH, Lai KC, Lam SK, et al. Symptomatic response to lansoprazole predicts abnormal acid reflux in endoscopy-negative patients with non-cardiac chest pain. Aliment Pharmacol Ther. 2003;17:369-377.
22. Flook NW, Moayyedi P, Dent J, et al. Acid-suppressive therapy with esomeprazole for relief of unexplained chest pain in primary care: a randomized, double-blind, placebo-controlled trial. Am J Gastroenterol. 2013;108:56-64.
23. Stein MB, Roy-Byrne PP, McQuaid JR, et al. Development of a brief diagnostic screen for panic disorder in primary care. Psychosom Med. 1999;61:359-364.
24. Ballenger JC. Treatment of panic disorder in the general medical setting. J Psychosom Res. 1998;44:5-15.
25. Dammen T, Ekeberg O, Arnesen H, et al. The detection of panic disorder in chest pain patients. Gen Hosp Psychiatry. 1999;21:323-332.
26. Kisely SR, Campbell LA, Yelland MJ, et al. Psychological interventions for symptomatic management of non-specific chest pain in patients with normal coronary anatomy. Cochrane Database Syst Rev. 2012;6:CD004101.
27. Ruigómez A, Rodríguez LA, Wallander MA, et al. Chest pain in general practice: incidence, comorbidity and mortality. Fam Pract. 2006;23:167-174.
28. Ruigómez A, Massó-González EL, Johansson S, et al. Chest pain without established ischaemic heart disease in primary care patients: associated comorbidities and mortality. Br J Gen Pract. 2009;59:e78-e86.
29. Hermann LK, Newman DH, Pleasant WA, et al. Yield of routine provocative cardiac testing among patients in an emergency department-based chest pain unit. JAMA Intern Med. 2013;173:1128-1133.
1. Amsterdam EA, Kirk JD, Bluemke DA, et al; American Heart Association Exercise, Cardiac Rehabilitation, and Prevention Committee of the Council on Clinical Cardiology, Council on Cardiovascular Nursing, and Interdisciplinary Council on Quality of Care and Outcomes Research. Testing of low-risk patients presenting to the emergency department with chest pain: a scientific statement from the American Heart Association. Circulation. 2010;122:1756-7176.
2. Bhuiya FA, Pitts SR, McCaig LF. Emergency department visits for chest pain and abdominal pain: United States, 1999-2008. NCHS Data Brief. 2010;43:1-8.
3. Klinkman MS, Stevens D, Gorenflo DW. Episodes of care for chest pain: a preliminary report from MIRNET. Michigan Research Network. J Fam Pract. 1994;38:345-352.
4. McConaghy JR, Oza RS. Outpatient diagnosis of acute chest pain in adults. Am Fam Physician. 2013;87:177-82.
5. Nilsson S, Scheike M, Engblom D, et al. Chest pain and ischaemic heart disease in primary care. Br J Gen Pract. 2003;53:378-382.
6. Buntinx F, Knockaert D, Bruyninckx R, et al. Chest pain in general practice or in the hospital emergency department: is it the same? Fam Pract. 2001;18:586-589.
7. Jonsbu E, Dammen T, Morken G, et al. Cardiac and psychiatric diagnoses among patients referred for chest pain and palpitations. Scand Cardiovasc J. 2009;43:256-259.
8. O’Connor RE, Brady W, Brooks SC, et al. Part 10: acute coronary syndromes: 2010 American Heart Association Guidelines for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care. Circulation. 2010;122(18 suppl 3):S787-S817.
9. Swap CJ, Nagurney JT. Value and limitations of chest pain history in the evaluation of patients with suspected acute coronary syndromes. JAMA. 2005;294:2623-2629.
10. Panju AA, Hemmelgarn BR, Guyatt GH, et al. The rational clinical examination. Is this patient having a myocardial infarction? JAMA. 1998;280:1256-1263.
11. Bruyninckx R, Aertgeerts B, Bruyninckx P, et al. Signs and symptoms in diagnosing acute myocardial infarction and acute coronary syndrome: a diagnostic meta-analysis. Br J Gen Pract. 2008;58:105-111.
12. Bösner S, Haasenritter J, Becker A, et al. Ruling out coronary artery disease in primary care: development and validation of a simple prediction rule. CMAJ. 2010;182:1295-1300.
13. Haasenritter J, Bösner S, Vaucher P, et al. Ruling out coronary heart disease in primary care: external validation of a clinical prediction rule. Br J Gen Pract. 2012;62:e415-e421.
14. Bösner S, Becker A, Hani MA, et al. Chest wall syndrome in primary care patients with chest pain: presentation, associated features and diagnosis. Fam Pract. 2010;27:363-369.
15. Verdon F, Burnand B, Herzig L, et al. Chest wall syndrome among primary care patients: a cohort study. BMC Fam Pract. 2007;8:51.
16. Proulx AM, Zryd TW. Costochondritis: diagnosis and treatment. Am Fam Physician. 2009;80:617-620.
17. Rovetta G, Sessarego P, Monteforte P. Stretching exercises for costochondritis pain. G Ital Med Lav Ergon. 2009;31:169-171.
18. Lacy BE, Weiser K, Chertoff J, et al. The diagnosis of gastroesophageal reflux disease. Am J Med. 2010;123: 583-592.
19. Wang WH, Huang JQ, Zheng GF, et al. Is proton pump inhibitor testing an effective approach to diagnose gastroesophageal reflux disease in patients with noncardiac chest pain?: a metaanalysis. Arch Intern Med. 2005;165:1222-1228.
20. Pandak WM, Arezo S, Everett S, et al. Short course of omeprazole: a better first diagnostic approach to noncardiac chest pain than endoscopy, manometry, or 24-hour esophageal pH monitoring. J Clin Gastroenterol. 2002;35:307-314.
21. Xia HH, Lai KC, Lam SK, et al. Symptomatic response to lansoprazole predicts abnormal acid reflux in endoscopy-negative patients with non-cardiac chest pain. Aliment Pharmacol Ther. 2003;17:369-377.
22. Flook NW, Moayyedi P, Dent J, et al. Acid-suppressive therapy with esomeprazole for relief of unexplained chest pain in primary care: a randomized, double-blind, placebo-controlled trial. Am J Gastroenterol. 2013;108:56-64.
23. Stein MB, Roy-Byrne PP, McQuaid JR, et al. Development of a brief diagnostic screen for panic disorder in primary care. Psychosom Med. 1999;61:359-364.
24. Ballenger JC. Treatment of panic disorder in the general medical setting. J Psychosom Res. 1998;44:5-15.
25. Dammen T, Ekeberg O, Arnesen H, et al. The detection of panic disorder in chest pain patients. Gen Hosp Psychiatry. 1999;21:323-332.
26. Kisely SR, Campbell LA, Yelland MJ, et al. Psychological interventions for symptomatic management of non-specific chest pain in patients with normal coronary anatomy. Cochrane Database Syst Rev. 2012;6:CD004101.
27. Ruigómez A, Rodríguez LA, Wallander MA, et al. Chest pain in general practice: incidence, comorbidity and mortality. Fam Pract. 2006;23:167-174.
28. Ruigómez A, Massó-González EL, Johansson S, et al. Chest pain without established ischaemic heart disease in primary care patients: associated comorbidities and mortality. Br J Gen Pract. 2009;59:e78-e86.
29. Hermann LK, Newman DH, Pleasant WA, et al. Yield of routine provocative cardiac testing among patients in an emergency department-based chest pain unit. JAMA Intern Med. 2013;173:1128-1133.
Beyond chronic pain: How best to treat psychological comorbidities
› To achieve optimal outcomes for patients with chronic pain, treat the constellation of symptoms that often accompany it—eg, disordered sleep, depression or anxiety, and/or substance abuse—as well as the pain. A
› Individualize drug therapy for patients with chronic pain (eg, specific comorbidities and symptoms) while considering drug-based factors, including adverse effect profiles and the potential for interaction with other agents. A
› Consider using a tricyclic antidepressant, a serotonergic/noradrenergic antidepressant, gabapentin, or pregabalin for patients who have chronic pain and depression or anxiety. A
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Primary care physicians often have the lead role in caring for patients with chronic pain from a myriad of causes, including arthritis, low back injury, migraine, neuropathic pain, and more.1 To ensure optimal outcomes for such patients, understanding chronic pain syndromes and their negative effect on sleep, mood, and daily functioning is key.
Studies of the interaction of chronic pain, insomnia, and psychiatric disorders are increasing awareness of the way patients with this constellation of comorbidities respond to treatment. What they show is that optimal outcomes are possible only if we treat these co-occurring disorders simultaneously.
Pain affects multiple functions
While pain is thought to originate from a primary dysfunction in the nervous system, the mind and body are involved in the constellation of pain, sleep disturbances, depression, anxiety disorders, and substance abuse/dependence. Patients with chronic pain typically report a higher degree of impairment in all dimensions of quality of life and sleep, and have higher scores on anxiety or depression screens than those without chronic pain.2
Sleep. About two-thirds (65%) of patients with chronic pain and the vast majority (96%) of those with fibromyalgia report sleep disturbances, with difficulty falling asleep, staying asleep, or both.3,4 Sleep deprivation has a hyperalgesic effect, which leads to decreased pain tolerance and greater severity and pain-related disability.5,6 While there does not appear to be a causal link between poor sleep and the onset of new pain symptoms, treatment directed toward improving sleep may help to reduce pain severity.
Depression. In primary care settings, more than 27% of patients with chronic pain meet diagnostic criteria for comorbid depression.7 The relationship between pain and depression is bidirectional, whereby chronic pain predicts the onset of new depressive episodes and depression predicts the onset of chronic pain.8 Having both conditions is associated with greater pain intensity, greater interference with usual activities, and a lower likelihood of responding to treatment.8 That finding highlights the importance of screening for depression in patients who present with somatic complaints, such as fatigue and headache, and in treating both depression and the pain simultaneously.
Anxiety. The relationship between pain and anxiety also appears to be bidirectional. The prevalence of anxiety disorders—including generalized anxiety disorder (GAD), panic disorder, and social phobia—is about twice as high among patients with chronic pain than in the general population.9
In primary care settings, anxiety disorders often are unrecognized and untreated. What’s more, anxiety can cause or exacerbate pain symptoms10; higher prevalence rates for arthritis, migraines, and back pain have been found in patients with a GAD diagnosis than in those without it.9 In older adults, pain conditions such as arthritis and migraines are associated with significantly higher rates of anxiety.11
Substance-related disorders. Substance abuse and dependence are an increasing problem worldwide, especially in developed countries. In North America, according to a 2012 report from the International Narcotics Control Board, approximately one in every 20 deaths of individuals ages 15 to 64 years is related to substance abuse.12 Canada has been found to have the world’s highest per capita consumption of high-potency opioids.13 In the United States, prescription drug abuse has been targeted as a public health epidemic.14 Also of note: Chronic pain affects 24% to 67% of patients with substance use disorders.15
Because of their analgesic effect, opioids often are given to patients with chronic noncancer pain, but substance misuse is common. Patients with a history of substance abuse or dependence are 4 times more likely to receive a prescription for opioids than those without such a history, and often are given higher potency opioids at higher doses.16 What’s more, individuals with chronic pain and a history of substance abuse/dependence generally have poorer outcomes, typically because they require more intensive treatment but rarely get it.17 These findings highlight the need to develop strategies to manage the symptoms of chronic pain in individuals who have a history of substance abuse or dependence—and to prevent addiction in patients without such a history.
Take aim at most—or all—of the patient's symptoms
In treating a patient with multiple comorbidities, it is best to initiate treatment with an agent that will address most—or all—of his or her symptoms. Using one drug whenever possible will reduce costs, prevent drug-drug interactions, and limit the likelihood of adverse effects. The American Psychiatric Association recommends the use of tricyclic antidepressants (TCAs) and serotonin-norepinephrine reuptake inhibitors (SNRIs) for treating chronic pain and comorbid depression.18 There is evidence of the effectiveness of “unconventional” analgesics, including anticonvulsants and antidepressants, for the treatment of chronic pain, as well. Opioids are, of course, an option too. In addition, nonpharmacologic treatments, such as cognitive-behavioral therapy (CBT), are recommended.19
Start with an anticonvulsant?
The anticonvulsants gabapentin and pregabalin have been shown to be effective in reducing certain types of neuropathic pain and alleviating insomnia.20,21 In studies investigating the use of these drugs in patients with GAD, both gabapentin and pregabalin led to improvement in anxiety symptoms, as well as in pain and sleep.20,21 At a dose of about 600 mg/d, pregabalin has been shown to significantly reduce pain levels in patients diagnosed with diabetic peripheral pain syndrome22; it has also been found to help prevent relapse23 and reduce sleep disturbances associated with GAD.24
The most common adverse effects of pregabalin are mild-to-moderate somnolence, dry mouth, headache, dizziness, and peripheral edema20,22; dizziness, somnolence, peripheral edema, and gait disturbance are most commonly associated with gabapentin treatment.25 These tend to stabilize over time, but occasionally the dose must be lowered or the drug discontinued.
Lamotrigine has been shown to reduce pain in patients with diabetic and sensory neuropathy compared with placebo,26 but was not effective in treating patients with pain due to spinal cord injury. The drug should be initiated at a low dose and slowly titrated to minimize the risk of serious adverse effects such as Stevens-Johnson syndrome.26
Try a tricyclic or an SNRI
TCAs, including amitriptyline, nortriptyline, desipramine, and imipramine, are recommended by the Canadian Pain Society as first-line therapy for chronic pain and often have benefit in the treatment of comorbid mood or anxiety disorders.27 Noradrenergic antidepressants—including TCAs—appear to have particular efficacy in treating moderate to severe neuropathic pain in patients with a comorbid substance disorder who take the drugs regularly, while undergoing frequent assessments.15
Overdose is a risk associated with TCAs, which have higher toxicity than other classes of antidepressants.28 Thus, it is essential to avoid prescribing TCAs for depressed patients until you carefully assess their risk of overdose. TCAs should not be prescribed for any patient at increased risk for cardiac arrhythmias.29
If you do prescribe a TCA… The doses of TCAs used to treat mood and anxiety symptoms often are much higher than doses needed for pain relief. As a result patients are often at risk of experiencing side effects.
What about an SNRI? In general, SNRIs, which target both serotonin and norepinephrine, have a greater analgesic effect than antidepressants targeting either neurotransmitter alone.30 Duloxetine, an SNRI, has been shown to effectively reduce symptoms in patients with pain disorders and comorbid depression.31 Other SNRIs studied in the treatment of pain and associated symptoms include venlafaxine, which has been effective in treating patients in a primary care setting who had both pain and depression,32 and milnacipran, which has been used successfully to treat pain associated with fibromyalgia.33
SNRIs may interfere with sleep. SNRIs have been associated with an increase in arousal and in rapid eye movement sleep suppression.34 Thus, another type of medication may be preferable for patients with pain and a sleep disturbance or, if an SNRI is prescribed, it may be necessary to lower the dose or add a sleep aid.
The role of SSRIs
Despite the recognized utility and widespread use of selective serotonin reuptake inhibitors (SSRIs) in the treatment of depressive and anxiety disorders, their role in managing neuropathic pain is less clear. Although some agents, such as escitalopram, have demonstrated mild pain-relieving effects in patients with painful polyneuropathy, the magnitude of the effect was clinically relevant at best for only a small number of patients.35 The effectiveness of other SSRIs in painful diabetic neuropathy has been shown to be less than that of TCAs.36 SSRIs generally are not recommended for the treatment of chronic neuropathic pain, even when it is associated with mood and anxiety symptoms.27
Opioids for which patients?
Chronic pain often is treated with opioids. Particular caution is required, however, when treating patients with pain and substance abuse or dependence.15,37 In order to prevent relapse in such individuals when they’re suffering from chronic pain, opioids should be used only if:15,38
• the pain is moderate to severe and has a significant impact on the patient’s functioning and overall quality of life;
• nonopioid medications have been tried but were unsuccessful; and
• the patient agrees to be closely monitored while taking opioids.
The opioids tramadol and methadone are recommended as third-line therapy, along with nonopioid medications such as cannabinoids, lamotrigine, topiramate, and valproic acid.27
Use a comprehensive pain scale, such as the Brief Pain Inventory, to assess the pain of any patient with a history of a substance-related disorder rather than asking him or her to rate the pain level on a general Likert-type scale.15
Long-acting opioids, such as sustained-release morphine, oxycodone, or fentanyl patch, are preferable to short-acting immediate-release opioids, which have a higher addictive profile because of their fast onset of action.15 Keep in mind, however, that long-acting opioids also have the potential for abuse, and patients taking them must be carefully monitored, as well.
Nonpharmacologic therapy often helps, too
Evidence suggests that even the most potent drugs significantly decrease pain in only about half of those taking them.39 And whether or not adequate pain relief is achieved, patients with the constellation of pain and sleep, mood, anxiety, and/or substance disorders can benefit from nonpharmacologic interventions, as well. Let patients know that CBT, in particular, has been shown to have a positive effect on psychological function and comorbid psychological disorders, particularly when it is combined with pharmacologic therapy.40 In addition, other nonpharmacologic treatments, including biofeedback41-44 and meditation,45-47 have shown preliminary value in managing pain.
Further research is needed to understand the effectiveness of CBT in the management of chronic pain; however, it appears that CBT may have a positive effect on psychological functioning and comorbid psychological disorders.
The bottom line: Don’t overlook mood, anxiety symptoms in pain patients
In epidemiological studies of chronic pain, it is apparent that sleep, depressive, substance abuse/dependence, and anxiety syndromes often occur together, which supports the necessity of considering psychosocial dynamics to understand pain. Although there has been some inconsistency observed across findings (eg, Romano and Turner),48 clarifying the relationships amongst these disorders may be an avenue for future research. The literature to date suggests that mood- and anxiety-related symptoms should not be overlooked in pain patients, as there is a negative effect on prognosis when these disorders co-occur.6
Treatment should be based on individual patient factors, such as presenting symptoms and potential for drug side effects. Some pharmacologic agents have been shown to be effective in treating several symptoms of this pyramid. Such drugs offer the best success and relapse rates, and reduce the likelihood of drug interactions. CBT appears to offer added benefits, especially if combined with pharmacology. However, few controlled trials have been conducted in this area and further research is required to appropriately guide clinical management.
CORRESPONDENCE
Martin A. Katzman, MD, FRCPC, START Clinic for Mood and Anxiety Disorders, 32 Park Road, Toronto, Ontario, Canada M4W 2N4; [email protected]
1. Boulanger A, Clark AJ, Squire P, et al. Chronic pain in Canada: have we improved our management of chronic noncancer pain? Pain Res Manag. 2007;12:39-47.
2. Attal N, Lanteri-Minet M, Laurent B, et al. The specific disease burden of neuropathic pain: results of a French nationwide survey. Pain. 2011;152:2836-2843.
3. Bigatti SM, Hernandez AM, Cronan TA, et al. Sleep disturbances in fibromyalgia syndrome: relationship to pain and depression. Arthritis Rheum. 2008;59:961-967.
4. Morin CM, Gibson D, Wade J. Self-reported sleep and mood disturbance in chronic pain patients. Clin J Pain. 1998;14:311-314.
5. Onen SH, Alloui A, Gross A, et al. The effects of total sleep deprivation, selective sleep interruption and sleep recovery on pain tolerance thresholds in healthy subjects. J Sleep Res. 2001;10:35-42.
6. Naughton F, Ashworth P, Skevington SM. Does sleep quality predict pain-related disability in chronic pain patients? The mediating roles of depression and pain severity. Pain. 2007;127:243-252.
7. Bair MJ, Robinson RL, Katon W, et al. Depression and pain comorbidity: a literature review. Arch Intern Med. 2003;163:2433-2445.
8. Gureje O, Simon GE, Von Korff M. A cross-national study of the course of persistent pain in primary care. Pain. 2001;92:195-200.
9. McWilliams LA, Goodwin RD, Cox BJ. Depression and anxiety associated with three pain conditions: results from a nationally representative sample. Pain. 2004;111:77-83.
10. Jordan KD, Okifuji A. Anxiety disorders: differential diagnosis and their relationship to chronic pain. J Pain Palliat Care Pharmacother. 2011;25:231-245.
11. El-Gabalawy R, Mackenzie CS, Shooshtari S, et al. Comorbid physical health conditions and anxiety disorders: a population-based exploration of prevalence and health outcomes among older adults. Gen Hosp Psychiatry. 2011;33:556-564.
12. International Narcotics Control Board Web site. Report of the International Narcotics Control Board for 2012. Available at: http://www.incb.org/documents/Publications/AnnualReports/AR2012/AR_2012_E.pdf. Published January 2013. Accessed November 9, 2013.
13. Currie CL, Schopflocher DP, Wild TC. Prevalence and correlates of 12-month prescription drug misuse in Alberta. Can J Psychiatry. 2011;56:27-34.
14. Trust for America’s Health Web site. Prescription drug abuse: Strategies to stop the epidemic 2013. Available at: http://healthyamericans.org/assets/files/TFAH2013RxDrugAbuseRptFINAL.pdf. Published October 2013. Accessed November 14, 2013.
15. Olsen Y, Alford DP. Chronic pain management in patients with substance use disorders. Johns Hopkins Adv Stud Med. 2006;6:111-123.
16. Alford DP. Opioids for chronic pain in patients with substance abuse: too much, too little or just right? Pain. 2009;145:267-268.
17. Krashin D, Murinova N, Ballantyne J. Management of pain with comorbid substance abuse. Curr Psychiatry Rep. 2012;14:462-468.
18. Gelenberg AJ, Freeman MP, Markowitz JC, et al. Practice guideline for the treatment of patients with major depressive disorder. Third Edition. Available at: http://psychiatryonline.org/pdfaccess.ashx?ResourceID=243261&PDFSource=6. Published October 2010. Accessed April 22, 2014.
19. McQuay HJ, Moore RA. Antidepressants and chronic pain. BMJ. 1997;314:763-764.
20. Dworkin RH, Corbin AE, Young JP Jr, et al. Pregabalin for the treatment of postherpetic neuralgia: a randomized, placebocontrolled trial. Neurology. 2003;60:1274-1283.
21. Serpell MG; Neuropathic pain study group. Gabapentin in neuropathic pain syndromes: a randomised, double-blind, placebo-controlled trial. Pain. 2002;99:557-566.
22. Richter RW, Portenoy R, Sharma U, et al. Relief of painful diabetic peripheral neuropathy with pregabalin: a randomized, placebo-controlled trial. J Pain. 2005;6:253-260.
23. Feltner D, Wittchen HU, Kavoussi R, et al. Long-term efficacy of pregabalin in generalized anxiety disorder. Int Clin Psychopharmacol. 2008;23:18-28.
24. Bollu V, Bushmakin AG, Cappelleri JC, et al. Pregabalin reduces sleep disturbance in patients with generalized anxiety disorder via both direct and indirect mechanisms. Eur J Psychiat. 2010;24:18-27.
25. Moore RA, Wiffen PJ, Derry S, et al. Gabapentin for chronic neuropathic pain and fibromyalgia in adults. Cochrane Database Syst Rev. 2011;(3):CD007938.
26. Chandramouli, J. Newer anticonvulsant drugs in neuropathic pain and bipolar disorder. J Pain Palliat Care Pharmacother. 2002;16:19-37.
27. Moulin DE, Clark AJ, Gilron I, et al; Canadian Pain Society. Pharmacological management of chronic neuropathic pain: consensus statement and guidelines from the Canadian Pain Society. J Pain Res Manag. 2007;12:13-21.
28. Whyte IM, Dawson AH, Buckley NA. Relative toxicity of venlafaxine and selective serotonin reuptake inhibitors in overdose compared to tricyclic antidepressants. QJM. 2003;96:369-374.
29. Bosch TM, van der Werf TS, Uges DR, et al. Antidepressants selfpoisoning and ICU admissions in a university hospital in The Netherlands. Pharm World Sci. 2000;22:92-95.
30. Arnold LM, Palmer RH, Gendreau RM, et al. Relationships among pain, depressed mood, and global status in fibromyalgia patients: post hoc analyses of randomized, placebo-controlled trial of milnacipran. Psychosomatics. 2012;53:371-379.
31. Marangell LB, Clauw DJ, Choy E, et al. Comparative pain and mood effects in patients with comorbid fibromyalgia and major depressive disorder: secondary analyses of four pooled randomized controlled trials of duloxetine. Pain. 2011;152:31-37.
32. Begré S, Traber M, Gerber M, et al. Change in pain severity with open label venlafaxine use in patients with a depressive symptomatology: an observational study in primary care. Eur Psychiatry. 2008;23:178-186.
33. Branco JC, Zachrisson O, Perrot S, et al; Multinational Coordinator Study Group. A European multicenter randomized double-blind placebo-controlled monotherapy clinical trial of milnacipran in the treatment of fibromyalgia. J Rheumatol. 2010;37:851-859.
34. Salín-Pascual RJ, Galicia-Polo L, Drucker-Colin R. Sleep changes after 4 consecutive days of venlafaxine administration in normal volunteers. J Clin Psychiatry. 1997;58:348-350.
35. Otto M, Bach FW, Jensen TS, et al. Escitalopram in painful polyneuropathy: a randomized, placebo-controlled, cross-over trial. Pain. 2008;139:275-283.
36. Max MB, Lynch SA, Muir J, et al. Effects of desipramine, amitriptyline, and fluoxetine on pain in diabetic neuropathy. N Engl J Med. 1992;326:1250-1256.
37. Sellek S. Opioid abuse puts physicians between a rock and a hard place but resources can help them lead the debate on how to rein it in. Mich Med. 2013;112:10-13.
38. Nielsen S, Bruno R, Degenhardt L, et al. The sources of pharmaceuticals for problematic users of benzodiazepines and prescription opioids. Med J Aust. 2013;199:696-699.
39. Turk DC, Swanson KS, Tunks ER. Psychological approaches in the treatment of chronic pain patients–when pills, scalpels, and needles are not enough. Can J Psychiatry. 2008;53:213-223.
40. Basler HD, Jäkle C, Kröner-Herwig B. Incorporation of cognitive-behavioral treatment into the medical care of chronic low back patients: a controlled randomized study in German pain treatment centers. Patient Educ Couns. 1997;31:113-124.
41. Middaugh S, Thomas KJ, Smith AR, et al. EMG biofeedback and exercise for treatment of cervical and shoulder pain in individuals with a spinal cord injury: a pilot study. Top Spinal Cord Inj Rehabil. 2013;19:311-323.
42. Oravitan M, Avram C. The effectiveness of electromyographic biofeedback as part of a meniscal repair rehabilitation programme. J Sports Sci Med. 2013;12:526-532.eCollection 2013.
43. Glombiewski JA, Bernardy K, Häuser W. Efficacy of EMG- and EEG-biofeedback in fibromyalgia syndrome: A meta-analysis and a systematic review of randomized controlled trials. Evid Based Complement Alternat Med. 2013;2013:962741.
44. Kubik A, Biedroń A. Neurofeedback therapy in patients with acute and chronic pain syndromes—literature review and own experience. Przegl Lek. 2013;70:440-442.
45. Overcash J, Will KM, Lipetz DW. The benefits of medical qigong in patients with cancer: a descriptive pilot study. Clin J Oncol Nurs. 2013;17:654-658.
46. Jastrowski Mano KE, Salamon KS, Hainsworth KR, et al. A randomized, controlled pilot study of mindfulness-based stress reduction for pediatric chronic pain. Altern Ther Health Med. 2013;19:8-14.
47. Ahmed M, Modak S, Sequeira S. Acute pain relief after Mantram meditation in children with neuroblastoma undergoing anti-GD2 monoclonal antibody therapy. J Pediatr Hematol Oncol. 2014;36:152-155.
48. Romano JM, Turner JA. Chronic pain and depression: does the evidence support a relationship? Psychol Bull. 1985;97:18-34.
› To achieve optimal outcomes for patients with chronic pain, treat the constellation of symptoms that often accompany it—eg, disordered sleep, depression or anxiety, and/or substance abuse—as well as the pain. A
› Individualize drug therapy for patients with chronic pain (eg, specific comorbidities and symptoms) while considering drug-based factors, including adverse effect profiles and the potential for interaction with other agents. A
› Consider using a tricyclic antidepressant, a serotonergic/noradrenergic antidepressant, gabapentin, or pregabalin for patients who have chronic pain and depression or anxiety. A
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Primary care physicians often have the lead role in caring for patients with chronic pain from a myriad of causes, including arthritis, low back injury, migraine, neuropathic pain, and more.1 To ensure optimal outcomes for such patients, understanding chronic pain syndromes and their negative effect on sleep, mood, and daily functioning is key.
Studies of the interaction of chronic pain, insomnia, and psychiatric disorders are increasing awareness of the way patients with this constellation of comorbidities respond to treatment. What they show is that optimal outcomes are possible only if we treat these co-occurring disorders simultaneously.
Pain affects multiple functions
While pain is thought to originate from a primary dysfunction in the nervous system, the mind and body are involved in the constellation of pain, sleep disturbances, depression, anxiety disorders, and substance abuse/dependence. Patients with chronic pain typically report a higher degree of impairment in all dimensions of quality of life and sleep, and have higher scores on anxiety or depression screens than those without chronic pain.2
Sleep. About two-thirds (65%) of patients with chronic pain and the vast majority (96%) of those with fibromyalgia report sleep disturbances, with difficulty falling asleep, staying asleep, or both.3,4 Sleep deprivation has a hyperalgesic effect, which leads to decreased pain tolerance and greater severity and pain-related disability.5,6 While there does not appear to be a causal link between poor sleep and the onset of new pain symptoms, treatment directed toward improving sleep may help to reduce pain severity.
Depression. In primary care settings, more than 27% of patients with chronic pain meet diagnostic criteria for comorbid depression.7 The relationship between pain and depression is bidirectional, whereby chronic pain predicts the onset of new depressive episodes and depression predicts the onset of chronic pain.8 Having both conditions is associated with greater pain intensity, greater interference with usual activities, and a lower likelihood of responding to treatment.8 That finding highlights the importance of screening for depression in patients who present with somatic complaints, such as fatigue and headache, and in treating both depression and the pain simultaneously.
Anxiety. The relationship between pain and anxiety also appears to be bidirectional. The prevalence of anxiety disorders—including generalized anxiety disorder (GAD), panic disorder, and social phobia—is about twice as high among patients with chronic pain than in the general population.9
In primary care settings, anxiety disorders often are unrecognized and untreated. What’s more, anxiety can cause or exacerbate pain symptoms10; higher prevalence rates for arthritis, migraines, and back pain have been found in patients with a GAD diagnosis than in those without it.9 In older adults, pain conditions such as arthritis and migraines are associated with significantly higher rates of anxiety.11
Substance-related disorders. Substance abuse and dependence are an increasing problem worldwide, especially in developed countries. In North America, according to a 2012 report from the International Narcotics Control Board, approximately one in every 20 deaths of individuals ages 15 to 64 years is related to substance abuse.12 Canada has been found to have the world’s highest per capita consumption of high-potency opioids.13 In the United States, prescription drug abuse has been targeted as a public health epidemic.14 Also of note: Chronic pain affects 24% to 67% of patients with substance use disorders.15
Because of their analgesic effect, opioids often are given to patients with chronic noncancer pain, but substance misuse is common. Patients with a history of substance abuse or dependence are 4 times more likely to receive a prescription for opioids than those without such a history, and often are given higher potency opioids at higher doses.16 What’s more, individuals with chronic pain and a history of substance abuse/dependence generally have poorer outcomes, typically because they require more intensive treatment but rarely get it.17 These findings highlight the need to develop strategies to manage the symptoms of chronic pain in individuals who have a history of substance abuse or dependence—and to prevent addiction in patients without such a history.
Take aim at most—or all—of the patient's symptoms
In treating a patient with multiple comorbidities, it is best to initiate treatment with an agent that will address most—or all—of his or her symptoms. Using one drug whenever possible will reduce costs, prevent drug-drug interactions, and limit the likelihood of adverse effects. The American Psychiatric Association recommends the use of tricyclic antidepressants (TCAs) and serotonin-norepinephrine reuptake inhibitors (SNRIs) for treating chronic pain and comorbid depression.18 There is evidence of the effectiveness of “unconventional” analgesics, including anticonvulsants and antidepressants, for the treatment of chronic pain, as well. Opioids are, of course, an option too. In addition, nonpharmacologic treatments, such as cognitive-behavioral therapy (CBT), are recommended.19
Start with an anticonvulsant?
The anticonvulsants gabapentin and pregabalin have been shown to be effective in reducing certain types of neuropathic pain and alleviating insomnia.20,21 In studies investigating the use of these drugs in patients with GAD, both gabapentin and pregabalin led to improvement in anxiety symptoms, as well as in pain and sleep.20,21 At a dose of about 600 mg/d, pregabalin has been shown to significantly reduce pain levels in patients diagnosed with diabetic peripheral pain syndrome22; it has also been found to help prevent relapse23 and reduce sleep disturbances associated with GAD.24
The most common adverse effects of pregabalin are mild-to-moderate somnolence, dry mouth, headache, dizziness, and peripheral edema20,22; dizziness, somnolence, peripheral edema, and gait disturbance are most commonly associated with gabapentin treatment.25 These tend to stabilize over time, but occasionally the dose must be lowered or the drug discontinued.
Lamotrigine has been shown to reduce pain in patients with diabetic and sensory neuropathy compared with placebo,26 but was not effective in treating patients with pain due to spinal cord injury. The drug should be initiated at a low dose and slowly titrated to minimize the risk of serious adverse effects such as Stevens-Johnson syndrome.26
Try a tricyclic or an SNRI
TCAs, including amitriptyline, nortriptyline, desipramine, and imipramine, are recommended by the Canadian Pain Society as first-line therapy for chronic pain and often have benefit in the treatment of comorbid mood or anxiety disorders.27 Noradrenergic antidepressants—including TCAs—appear to have particular efficacy in treating moderate to severe neuropathic pain in patients with a comorbid substance disorder who take the drugs regularly, while undergoing frequent assessments.15
Overdose is a risk associated with TCAs, which have higher toxicity than other classes of antidepressants.28 Thus, it is essential to avoid prescribing TCAs for depressed patients until you carefully assess their risk of overdose. TCAs should not be prescribed for any patient at increased risk for cardiac arrhythmias.29
If you do prescribe a TCA… The doses of TCAs used to treat mood and anxiety symptoms often are much higher than doses needed for pain relief. As a result patients are often at risk of experiencing side effects.
What about an SNRI? In general, SNRIs, which target both serotonin and norepinephrine, have a greater analgesic effect than antidepressants targeting either neurotransmitter alone.30 Duloxetine, an SNRI, has been shown to effectively reduce symptoms in patients with pain disorders and comorbid depression.31 Other SNRIs studied in the treatment of pain and associated symptoms include venlafaxine, which has been effective in treating patients in a primary care setting who had both pain and depression,32 and milnacipran, which has been used successfully to treat pain associated with fibromyalgia.33
SNRIs may interfere with sleep. SNRIs have been associated with an increase in arousal and in rapid eye movement sleep suppression.34 Thus, another type of medication may be preferable for patients with pain and a sleep disturbance or, if an SNRI is prescribed, it may be necessary to lower the dose or add a sleep aid.
The role of SSRIs
Despite the recognized utility and widespread use of selective serotonin reuptake inhibitors (SSRIs) in the treatment of depressive and anxiety disorders, their role in managing neuropathic pain is less clear. Although some agents, such as escitalopram, have demonstrated mild pain-relieving effects in patients with painful polyneuropathy, the magnitude of the effect was clinically relevant at best for only a small number of patients.35 The effectiveness of other SSRIs in painful diabetic neuropathy has been shown to be less than that of TCAs.36 SSRIs generally are not recommended for the treatment of chronic neuropathic pain, even when it is associated with mood and anxiety symptoms.27
Opioids for which patients?
Chronic pain often is treated with opioids. Particular caution is required, however, when treating patients with pain and substance abuse or dependence.15,37 In order to prevent relapse in such individuals when they’re suffering from chronic pain, opioids should be used only if:15,38
• the pain is moderate to severe and has a significant impact on the patient’s functioning and overall quality of life;
• nonopioid medications have been tried but were unsuccessful; and
• the patient agrees to be closely monitored while taking opioids.
The opioids tramadol and methadone are recommended as third-line therapy, along with nonopioid medications such as cannabinoids, lamotrigine, topiramate, and valproic acid.27
Use a comprehensive pain scale, such as the Brief Pain Inventory, to assess the pain of any patient with a history of a substance-related disorder rather than asking him or her to rate the pain level on a general Likert-type scale.15
Long-acting opioids, such as sustained-release morphine, oxycodone, or fentanyl patch, are preferable to short-acting immediate-release opioids, which have a higher addictive profile because of their fast onset of action.15 Keep in mind, however, that long-acting opioids also have the potential for abuse, and patients taking them must be carefully monitored, as well.
Nonpharmacologic therapy often helps, too
Evidence suggests that even the most potent drugs significantly decrease pain in only about half of those taking them.39 And whether or not adequate pain relief is achieved, patients with the constellation of pain and sleep, mood, anxiety, and/or substance disorders can benefit from nonpharmacologic interventions, as well. Let patients know that CBT, in particular, has been shown to have a positive effect on psychological function and comorbid psychological disorders, particularly when it is combined with pharmacologic therapy.40 In addition, other nonpharmacologic treatments, including biofeedback41-44 and meditation,45-47 have shown preliminary value in managing pain.
Further research is needed to understand the effectiveness of CBT in the management of chronic pain; however, it appears that CBT may have a positive effect on psychological functioning and comorbid psychological disorders.
The bottom line: Don’t overlook mood, anxiety symptoms in pain patients
In epidemiological studies of chronic pain, it is apparent that sleep, depressive, substance abuse/dependence, and anxiety syndromes often occur together, which supports the necessity of considering psychosocial dynamics to understand pain. Although there has been some inconsistency observed across findings (eg, Romano and Turner),48 clarifying the relationships amongst these disorders may be an avenue for future research. The literature to date suggests that mood- and anxiety-related symptoms should not be overlooked in pain patients, as there is a negative effect on prognosis when these disorders co-occur.6
Treatment should be based on individual patient factors, such as presenting symptoms and potential for drug side effects. Some pharmacologic agents have been shown to be effective in treating several symptoms of this pyramid. Such drugs offer the best success and relapse rates, and reduce the likelihood of drug interactions. CBT appears to offer added benefits, especially if combined with pharmacology. However, few controlled trials have been conducted in this area and further research is required to appropriately guide clinical management.
CORRESPONDENCE
Martin A. Katzman, MD, FRCPC, START Clinic for Mood and Anxiety Disorders, 32 Park Road, Toronto, Ontario, Canada M4W 2N4; [email protected]
› To achieve optimal outcomes for patients with chronic pain, treat the constellation of symptoms that often accompany it—eg, disordered sleep, depression or anxiety, and/or substance abuse—as well as the pain. A
› Individualize drug therapy for patients with chronic pain (eg, specific comorbidities and symptoms) while considering drug-based factors, including adverse effect profiles and the potential for interaction with other agents. A
› Consider using a tricyclic antidepressant, a serotonergic/noradrenergic antidepressant, gabapentin, or pregabalin for patients who have chronic pain and depression or anxiety. A
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Primary care physicians often have the lead role in caring for patients with chronic pain from a myriad of causes, including arthritis, low back injury, migraine, neuropathic pain, and more.1 To ensure optimal outcomes for such patients, understanding chronic pain syndromes and their negative effect on sleep, mood, and daily functioning is key.
Studies of the interaction of chronic pain, insomnia, and psychiatric disorders are increasing awareness of the way patients with this constellation of comorbidities respond to treatment. What they show is that optimal outcomes are possible only if we treat these co-occurring disorders simultaneously.
Pain affects multiple functions
While pain is thought to originate from a primary dysfunction in the nervous system, the mind and body are involved in the constellation of pain, sleep disturbances, depression, anxiety disorders, and substance abuse/dependence. Patients with chronic pain typically report a higher degree of impairment in all dimensions of quality of life and sleep, and have higher scores on anxiety or depression screens than those without chronic pain.2
Sleep. About two-thirds (65%) of patients with chronic pain and the vast majority (96%) of those with fibromyalgia report sleep disturbances, with difficulty falling asleep, staying asleep, or both.3,4 Sleep deprivation has a hyperalgesic effect, which leads to decreased pain tolerance and greater severity and pain-related disability.5,6 While there does not appear to be a causal link between poor sleep and the onset of new pain symptoms, treatment directed toward improving sleep may help to reduce pain severity.
Depression. In primary care settings, more than 27% of patients with chronic pain meet diagnostic criteria for comorbid depression.7 The relationship between pain and depression is bidirectional, whereby chronic pain predicts the onset of new depressive episodes and depression predicts the onset of chronic pain.8 Having both conditions is associated with greater pain intensity, greater interference with usual activities, and a lower likelihood of responding to treatment.8 That finding highlights the importance of screening for depression in patients who present with somatic complaints, such as fatigue and headache, and in treating both depression and the pain simultaneously.
Anxiety. The relationship between pain and anxiety also appears to be bidirectional. The prevalence of anxiety disorders—including generalized anxiety disorder (GAD), panic disorder, and social phobia—is about twice as high among patients with chronic pain than in the general population.9
In primary care settings, anxiety disorders often are unrecognized and untreated. What’s more, anxiety can cause or exacerbate pain symptoms10; higher prevalence rates for arthritis, migraines, and back pain have been found in patients with a GAD diagnosis than in those without it.9 In older adults, pain conditions such as arthritis and migraines are associated with significantly higher rates of anxiety.11
Substance-related disorders. Substance abuse and dependence are an increasing problem worldwide, especially in developed countries. In North America, according to a 2012 report from the International Narcotics Control Board, approximately one in every 20 deaths of individuals ages 15 to 64 years is related to substance abuse.12 Canada has been found to have the world’s highest per capita consumption of high-potency opioids.13 In the United States, prescription drug abuse has been targeted as a public health epidemic.14 Also of note: Chronic pain affects 24% to 67% of patients with substance use disorders.15
Because of their analgesic effect, opioids often are given to patients with chronic noncancer pain, but substance misuse is common. Patients with a history of substance abuse or dependence are 4 times more likely to receive a prescription for opioids than those without such a history, and often are given higher potency opioids at higher doses.16 What’s more, individuals with chronic pain and a history of substance abuse/dependence generally have poorer outcomes, typically because they require more intensive treatment but rarely get it.17 These findings highlight the need to develop strategies to manage the symptoms of chronic pain in individuals who have a history of substance abuse or dependence—and to prevent addiction in patients without such a history.
Take aim at most—or all—of the patient's symptoms
In treating a patient with multiple comorbidities, it is best to initiate treatment with an agent that will address most—or all—of his or her symptoms. Using one drug whenever possible will reduce costs, prevent drug-drug interactions, and limit the likelihood of adverse effects. The American Psychiatric Association recommends the use of tricyclic antidepressants (TCAs) and serotonin-norepinephrine reuptake inhibitors (SNRIs) for treating chronic pain and comorbid depression.18 There is evidence of the effectiveness of “unconventional” analgesics, including anticonvulsants and antidepressants, for the treatment of chronic pain, as well. Opioids are, of course, an option too. In addition, nonpharmacologic treatments, such as cognitive-behavioral therapy (CBT), are recommended.19
Start with an anticonvulsant?
The anticonvulsants gabapentin and pregabalin have been shown to be effective in reducing certain types of neuropathic pain and alleviating insomnia.20,21 In studies investigating the use of these drugs in patients with GAD, both gabapentin and pregabalin led to improvement in anxiety symptoms, as well as in pain and sleep.20,21 At a dose of about 600 mg/d, pregabalin has been shown to significantly reduce pain levels in patients diagnosed with diabetic peripheral pain syndrome22; it has also been found to help prevent relapse23 and reduce sleep disturbances associated with GAD.24
The most common adverse effects of pregabalin are mild-to-moderate somnolence, dry mouth, headache, dizziness, and peripheral edema20,22; dizziness, somnolence, peripheral edema, and gait disturbance are most commonly associated with gabapentin treatment.25 These tend to stabilize over time, but occasionally the dose must be lowered or the drug discontinued.
Lamotrigine has been shown to reduce pain in patients with diabetic and sensory neuropathy compared with placebo,26 but was not effective in treating patients with pain due to spinal cord injury. The drug should be initiated at a low dose and slowly titrated to minimize the risk of serious adverse effects such as Stevens-Johnson syndrome.26
Try a tricyclic or an SNRI
TCAs, including amitriptyline, nortriptyline, desipramine, and imipramine, are recommended by the Canadian Pain Society as first-line therapy for chronic pain and often have benefit in the treatment of comorbid mood or anxiety disorders.27 Noradrenergic antidepressants—including TCAs—appear to have particular efficacy in treating moderate to severe neuropathic pain in patients with a comorbid substance disorder who take the drugs regularly, while undergoing frequent assessments.15
Overdose is a risk associated with TCAs, which have higher toxicity than other classes of antidepressants.28 Thus, it is essential to avoid prescribing TCAs for depressed patients until you carefully assess their risk of overdose. TCAs should not be prescribed for any patient at increased risk for cardiac arrhythmias.29
If you do prescribe a TCA… The doses of TCAs used to treat mood and anxiety symptoms often are much higher than doses needed for pain relief. As a result patients are often at risk of experiencing side effects.
What about an SNRI? In general, SNRIs, which target both serotonin and norepinephrine, have a greater analgesic effect than antidepressants targeting either neurotransmitter alone.30 Duloxetine, an SNRI, has been shown to effectively reduce symptoms in patients with pain disorders and comorbid depression.31 Other SNRIs studied in the treatment of pain and associated symptoms include venlafaxine, which has been effective in treating patients in a primary care setting who had both pain and depression,32 and milnacipran, which has been used successfully to treat pain associated with fibromyalgia.33
SNRIs may interfere with sleep. SNRIs have been associated with an increase in arousal and in rapid eye movement sleep suppression.34 Thus, another type of medication may be preferable for patients with pain and a sleep disturbance or, if an SNRI is prescribed, it may be necessary to lower the dose or add a sleep aid.
The role of SSRIs
Despite the recognized utility and widespread use of selective serotonin reuptake inhibitors (SSRIs) in the treatment of depressive and anxiety disorders, their role in managing neuropathic pain is less clear. Although some agents, such as escitalopram, have demonstrated mild pain-relieving effects in patients with painful polyneuropathy, the magnitude of the effect was clinically relevant at best for only a small number of patients.35 The effectiveness of other SSRIs in painful diabetic neuropathy has been shown to be less than that of TCAs.36 SSRIs generally are not recommended for the treatment of chronic neuropathic pain, even when it is associated with mood and anxiety symptoms.27
Opioids for which patients?
Chronic pain often is treated with opioids. Particular caution is required, however, when treating patients with pain and substance abuse or dependence.15,37 In order to prevent relapse in such individuals when they’re suffering from chronic pain, opioids should be used only if:15,38
• the pain is moderate to severe and has a significant impact on the patient’s functioning and overall quality of life;
• nonopioid medications have been tried but were unsuccessful; and
• the patient agrees to be closely monitored while taking opioids.
The opioids tramadol and methadone are recommended as third-line therapy, along with nonopioid medications such as cannabinoids, lamotrigine, topiramate, and valproic acid.27
Use a comprehensive pain scale, such as the Brief Pain Inventory, to assess the pain of any patient with a history of a substance-related disorder rather than asking him or her to rate the pain level on a general Likert-type scale.15
Long-acting opioids, such as sustained-release morphine, oxycodone, or fentanyl patch, are preferable to short-acting immediate-release opioids, which have a higher addictive profile because of their fast onset of action.15 Keep in mind, however, that long-acting opioids also have the potential for abuse, and patients taking them must be carefully monitored, as well.
Nonpharmacologic therapy often helps, too
Evidence suggests that even the most potent drugs significantly decrease pain in only about half of those taking them.39 And whether or not adequate pain relief is achieved, patients with the constellation of pain and sleep, mood, anxiety, and/or substance disorders can benefit from nonpharmacologic interventions, as well. Let patients know that CBT, in particular, has been shown to have a positive effect on psychological function and comorbid psychological disorders, particularly when it is combined with pharmacologic therapy.40 In addition, other nonpharmacologic treatments, including biofeedback41-44 and meditation,45-47 have shown preliminary value in managing pain.
Further research is needed to understand the effectiveness of CBT in the management of chronic pain; however, it appears that CBT may have a positive effect on psychological functioning and comorbid psychological disorders.
The bottom line: Don’t overlook mood, anxiety symptoms in pain patients
In epidemiological studies of chronic pain, it is apparent that sleep, depressive, substance abuse/dependence, and anxiety syndromes often occur together, which supports the necessity of considering psychosocial dynamics to understand pain. Although there has been some inconsistency observed across findings (eg, Romano and Turner),48 clarifying the relationships amongst these disorders may be an avenue for future research. The literature to date suggests that mood- and anxiety-related symptoms should not be overlooked in pain patients, as there is a negative effect on prognosis when these disorders co-occur.6
Treatment should be based on individual patient factors, such as presenting symptoms and potential for drug side effects. Some pharmacologic agents have been shown to be effective in treating several symptoms of this pyramid. Such drugs offer the best success and relapse rates, and reduce the likelihood of drug interactions. CBT appears to offer added benefits, especially if combined with pharmacology. However, few controlled trials have been conducted in this area and further research is required to appropriately guide clinical management.
CORRESPONDENCE
Martin A. Katzman, MD, FRCPC, START Clinic for Mood and Anxiety Disorders, 32 Park Road, Toronto, Ontario, Canada M4W 2N4; [email protected]
1. Boulanger A, Clark AJ, Squire P, et al. Chronic pain in Canada: have we improved our management of chronic noncancer pain? Pain Res Manag. 2007;12:39-47.
2. Attal N, Lanteri-Minet M, Laurent B, et al. The specific disease burden of neuropathic pain: results of a French nationwide survey. Pain. 2011;152:2836-2843.
3. Bigatti SM, Hernandez AM, Cronan TA, et al. Sleep disturbances in fibromyalgia syndrome: relationship to pain and depression. Arthritis Rheum. 2008;59:961-967.
4. Morin CM, Gibson D, Wade J. Self-reported sleep and mood disturbance in chronic pain patients. Clin J Pain. 1998;14:311-314.
5. Onen SH, Alloui A, Gross A, et al. The effects of total sleep deprivation, selective sleep interruption and sleep recovery on pain tolerance thresholds in healthy subjects. J Sleep Res. 2001;10:35-42.
6. Naughton F, Ashworth P, Skevington SM. Does sleep quality predict pain-related disability in chronic pain patients? The mediating roles of depression and pain severity. Pain. 2007;127:243-252.
7. Bair MJ, Robinson RL, Katon W, et al. Depression and pain comorbidity: a literature review. Arch Intern Med. 2003;163:2433-2445.
8. Gureje O, Simon GE, Von Korff M. A cross-national study of the course of persistent pain in primary care. Pain. 2001;92:195-200.
9. McWilliams LA, Goodwin RD, Cox BJ. Depression and anxiety associated with three pain conditions: results from a nationally representative sample. Pain. 2004;111:77-83.
10. Jordan KD, Okifuji A. Anxiety disorders: differential diagnosis and their relationship to chronic pain. J Pain Palliat Care Pharmacother. 2011;25:231-245.
11. El-Gabalawy R, Mackenzie CS, Shooshtari S, et al. Comorbid physical health conditions and anxiety disorders: a population-based exploration of prevalence and health outcomes among older adults. Gen Hosp Psychiatry. 2011;33:556-564.
12. International Narcotics Control Board Web site. Report of the International Narcotics Control Board for 2012. Available at: http://www.incb.org/documents/Publications/AnnualReports/AR2012/AR_2012_E.pdf. Published January 2013. Accessed November 9, 2013.
13. Currie CL, Schopflocher DP, Wild TC. Prevalence and correlates of 12-month prescription drug misuse in Alberta. Can J Psychiatry. 2011;56:27-34.
14. Trust for America’s Health Web site. Prescription drug abuse: Strategies to stop the epidemic 2013. Available at: http://healthyamericans.org/assets/files/TFAH2013RxDrugAbuseRptFINAL.pdf. Published October 2013. Accessed November 14, 2013.
15. Olsen Y, Alford DP. Chronic pain management in patients with substance use disorders. Johns Hopkins Adv Stud Med. 2006;6:111-123.
16. Alford DP. Opioids for chronic pain in patients with substance abuse: too much, too little or just right? Pain. 2009;145:267-268.
17. Krashin D, Murinova N, Ballantyne J. Management of pain with comorbid substance abuse. Curr Psychiatry Rep. 2012;14:462-468.
18. Gelenberg AJ, Freeman MP, Markowitz JC, et al. Practice guideline for the treatment of patients with major depressive disorder. Third Edition. Available at: http://psychiatryonline.org/pdfaccess.ashx?ResourceID=243261&PDFSource=6. Published October 2010. Accessed April 22, 2014.
19. McQuay HJ, Moore RA. Antidepressants and chronic pain. BMJ. 1997;314:763-764.
20. Dworkin RH, Corbin AE, Young JP Jr, et al. Pregabalin for the treatment of postherpetic neuralgia: a randomized, placebocontrolled trial. Neurology. 2003;60:1274-1283.
21. Serpell MG; Neuropathic pain study group. Gabapentin in neuropathic pain syndromes: a randomised, double-blind, placebo-controlled trial. Pain. 2002;99:557-566.
22. Richter RW, Portenoy R, Sharma U, et al. Relief of painful diabetic peripheral neuropathy with pregabalin: a randomized, placebo-controlled trial. J Pain. 2005;6:253-260.
23. Feltner D, Wittchen HU, Kavoussi R, et al. Long-term efficacy of pregabalin in generalized anxiety disorder. Int Clin Psychopharmacol. 2008;23:18-28.
24. Bollu V, Bushmakin AG, Cappelleri JC, et al. Pregabalin reduces sleep disturbance in patients with generalized anxiety disorder via both direct and indirect mechanisms. Eur J Psychiat. 2010;24:18-27.
25. Moore RA, Wiffen PJ, Derry S, et al. Gabapentin for chronic neuropathic pain and fibromyalgia in adults. Cochrane Database Syst Rev. 2011;(3):CD007938.
26. Chandramouli, J. Newer anticonvulsant drugs in neuropathic pain and bipolar disorder. J Pain Palliat Care Pharmacother. 2002;16:19-37.
27. Moulin DE, Clark AJ, Gilron I, et al; Canadian Pain Society. Pharmacological management of chronic neuropathic pain: consensus statement and guidelines from the Canadian Pain Society. J Pain Res Manag. 2007;12:13-21.
28. Whyte IM, Dawson AH, Buckley NA. Relative toxicity of venlafaxine and selective serotonin reuptake inhibitors in overdose compared to tricyclic antidepressants. QJM. 2003;96:369-374.
29. Bosch TM, van der Werf TS, Uges DR, et al. Antidepressants selfpoisoning and ICU admissions in a university hospital in The Netherlands. Pharm World Sci. 2000;22:92-95.
30. Arnold LM, Palmer RH, Gendreau RM, et al. Relationships among pain, depressed mood, and global status in fibromyalgia patients: post hoc analyses of randomized, placebo-controlled trial of milnacipran. Psychosomatics. 2012;53:371-379.
31. Marangell LB, Clauw DJ, Choy E, et al. Comparative pain and mood effects in patients with comorbid fibromyalgia and major depressive disorder: secondary analyses of four pooled randomized controlled trials of duloxetine. Pain. 2011;152:31-37.
32. Begré S, Traber M, Gerber M, et al. Change in pain severity with open label venlafaxine use in patients with a depressive symptomatology: an observational study in primary care. Eur Psychiatry. 2008;23:178-186.
33. Branco JC, Zachrisson O, Perrot S, et al; Multinational Coordinator Study Group. A European multicenter randomized double-blind placebo-controlled monotherapy clinical trial of milnacipran in the treatment of fibromyalgia. J Rheumatol. 2010;37:851-859.
34. Salín-Pascual RJ, Galicia-Polo L, Drucker-Colin R. Sleep changes after 4 consecutive days of venlafaxine administration in normal volunteers. J Clin Psychiatry. 1997;58:348-350.
35. Otto M, Bach FW, Jensen TS, et al. Escitalopram in painful polyneuropathy: a randomized, placebo-controlled, cross-over trial. Pain. 2008;139:275-283.
36. Max MB, Lynch SA, Muir J, et al. Effects of desipramine, amitriptyline, and fluoxetine on pain in diabetic neuropathy. N Engl J Med. 1992;326:1250-1256.
37. Sellek S. Opioid abuse puts physicians between a rock and a hard place but resources can help them lead the debate on how to rein it in. Mich Med. 2013;112:10-13.
38. Nielsen S, Bruno R, Degenhardt L, et al. The sources of pharmaceuticals for problematic users of benzodiazepines and prescription opioids. Med J Aust. 2013;199:696-699.
39. Turk DC, Swanson KS, Tunks ER. Psychological approaches in the treatment of chronic pain patients–when pills, scalpels, and needles are not enough. Can J Psychiatry. 2008;53:213-223.
40. Basler HD, Jäkle C, Kröner-Herwig B. Incorporation of cognitive-behavioral treatment into the medical care of chronic low back patients: a controlled randomized study in German pain treatment centers. Patient Educ Couns. 1997;31:113-124.
41. Middaugh S, Thomas KJ, Smith AR, et al. EMG biofeedback and exercise for treatment of cervical and shoulder pain in individuals with a spinal cord injury: a pilot study. Top Spinal Cord Inj Rehabil. 2013;19:311-323.
42. Oravitan M, Avram C. The effectiveness of electromyographic biofeedback as part of a meniscal repair rehabilitation programme. J Sports Sci Med. 2013;12:526-532.eCollection 2013.
43. Glombiewski JA, Bernardy K, Häuser W. Efficacy of EMG- and EEG-biofeedback in fibromyalgia syndrome: A meta-analysis and a systematic review of randomized controlled trials. Evid Based Complement Alternat Med. 2013;2013:962741.
44. Kubik A, Biedroń A. Neurofeedback therapy in patients with acute and chronic pain syndromes—literature review and own experience. Przegl Lek. 2013;70:440-442.
45. Overcash J, Will KM, Lipetz DW. The benefits of medical qigong in patients with cancer: a descriptive pilot study. Clin J Oncol Nurs. 2013;17:654-658.
46. Jastrowski Mano KE, Salamon KS, Hainsworth KR, et al. A randomized, controlled pilot study of mindfulness-based stress reduction for pediatric chronic pain. Altern Ther Health Med. 2013;19:8-14.
47. Ahmed M, Modak S, Sequeira S. Acute pain relief after Mantram meditation in children with neuroblastoma undergoing anti-GD2 monoclonal antibody therapy. J Pediatr Hematol Oncol. 2014;36:152-155.
48. Romano JM, Turner JA. Chronic pain and depression: does the evidence support a relationship? Psychol Bull. 1985;97:18-34.
1. Boulanger A, Clark AJ, Squire P, et al. Chronic pain in Canada: have we improved our management of chronic noncancer pain? Pain Res Manag. 2007;12:39-47.
2. Attal N, Lanteri-Minet M, Laurent B, et al. The specific disease burden of neuropathic pain: results of a French nationwide survey. Pain. 2011;152:2836-2843.
3. Bigatti SM, Hernandez AM, Cronan TA, et al. Sleep disturbances in fibromyalgia syndrome: relationship to pain and depression. Arthritis Rheum. 2008;59:961-967.
4. Morin CM, Gibson D, Wade J. Self-reported sleep and mood disturbance in chronic pain patients. Clin J Pain. 1998;14:311-314.
5. Onen SH, Alloui A, Gross A, et al. The effects of total sleep deprivation, selective sleep interruption and sleep recovery on pain tolerance thresholds in healthy subjects. J Sleep Res. 2001;10:35-42.
6. Naughton F, Ashworth P, Skevington SM. Does sleep quality predict pain-related disability in chronic pain patients? The mediating roles of depression and pain severity. Pain. 2007;127:243-252.
7. Bair MJ, Robinson RL, Katon W, et al. Depression and pain comorbidity: a literature review. Arch Intern Med. 2003;163:2433-2445.
8. Gureje O, Simon GE, Von Korff M. A cross-national study of the course of persistent pain in primary care. Pain. 2001;92:195-200.
9. McWilliams LA, Goodwin RD, Cox BJ. Depression and anxiety associated with three pain conditions: results from a nationally representative sample. Pain. 2004;111:77-83.
10. Jordan KD, Okifuji A. Anxiety disorders: differential diagnosis and their relationship to chronic pain. J Pain Palliat Care Pharmacother. 2011;25:231-245.
11. El-Gabalawy R, Mackenzie CS, Shooshtari S, et al. Comorbid physical health conditions and anxiety disorders: a population-based exploration of prevalence and health outcomes among older adults. Gen Hosp Psychiatry. 2011;33:556-564.
12. International Narcotics Control Board Web site. Report of the International Narcotics Control Board for 2012. Available at: http://www.incb.org/documents/Publications/AnnualReports/AR2012/AR_2012_E.pdf. Published January 2013. Accessed November 9, 2013.
13. Currie CL, Schopflocher DP, Wild TC. Prevalence and correlates of 12-month prescription drug misuse in Alberta. Can J Psychiatry. 2011;56:27-34.
14. Trust for America’s Health Web site. Prescription drug abuse: Strategies to stop the epidemic 2013. Available at: http://healthyamericans.org/assets/files/TFAH2013RxDrugAbuseRptFINAL.pdf. Published October 2013. Accessed November 14, 2013.
15. Olsen Y, Alford DP. Chronic pain management in patients with substance use disorders. Johns Hopkins Adv Stud Med. 2006;6:111-123.
16. Alford DP. Opioids for chronic pain in patients with substance abuse: too much, too little or just right? Pain. 2009;145:267-268.
17. Krashin D, Murinova N, Ballantyne J. Management of pain with comorbid substance abuse. Curr Psychiatry Rep. 2012;14:462-468.
18. Gelenberg AJ, Freeman MP, Markowitz JC, et al. Practice guideline for the treatment of patients with major depressive disorder. Third Edition. Available at: http://psychiatryonline.org/pdfaccess.ashx?ResourceID=243261&PDFSource=6. Published October 2010. Accessed April 22, 2014.
19. McQuay HJ, Moore RA. Antidepressants and chronic pain. BMJ. 1997;314:763-764.
20. Dworkin RH, Corbin AE, Young JP Jr, et al. Pregabalin for the treatment of postherpetic neuralgia: a randomized, placebocontrolled trial. Neurology. 2003;60:1274-1283.
21. Serpell MG; Neuropathic pain study group. Gabapentin in neuropathic pain syndromes: a randomised, double-blind, placebo-controlled trial. Pain. 2002;99:557-566.
22. Richter RW, Portenoy R, Sharma U, et al. Relief of painful diabetic peripheral neuropathy with pregabalin: a randomized, placebo-controlled trial. J Pain. 2005;6:253-260.
23. Feltner D, Wittchen HU, Kavoussi R, et al. Long-term efficacy of pregabalin in generalized anxiety disorder. Int Clin Psychopharmacol. 2008;23:18-28.
24. Bollu V, Bushmakin AG, Cappelleri JC, et al. Pregabalin reduces sleep disturbance in patients with generalized anxiety disorder via both direct and indirect mechanisms. Eur J Psychiat. 2010;24:18-27.
25. Moore RA, Wiffen PJ, Derry S, et al. Gabapentin for chronic neuropathic pain and fibromyalgia in adults. Cochrane Database Syst Rev. 2011;(3):CD007938.
26. Chandramouli, J. Newer anticonvulsant drugs in neuropathic pain and bipolar disorder. J Pain Palliat Care Pharmacother. 2002;16:19-37.
27. Moulin DE, Clark AJ, Gilron I, et al; Canadian Pain Society. Pharmacological management of chronic neuropathic pain: consensus statement and guidelines from the Canadian Pain Society. J Pain Res Manag. 2007;12:13-21.
28. Whyte IM, Dawson AH, Buckley NA. Relative toxicity of venlafaxine and selective serotonin reuptake inhibitors in overdose compared to tricyclic antidepressants. QJM. 2003;96:369-374.
29. Bosch TM, van der Werf TS, Uges DR, et al. Antidepressants selfpoisoning and ICU admissions in a university hospital in The Netherlands. Pharm World Sci. 2000;22:92-95.
30. Arnold LM, Palmer RH, Gendreau RM, et al. Relationships among pain, depressed mood, and global status in fibromyalgia patients: post hoc analyses of randomized, placebo-controlled trial of milnacipran. Psychosomatics. 2012;53:371-379.
31. Marangell LB, Clauw DJ, Choy E, et al. Comparative pain and mood effects in patients with comorbid fibromyalgia and major depressive disorder: secondary analyses of four pooled randomized controlled trials of duloxetine. Pain. 2011;152:31-37.
32. Begré S, Traber M, Gerber M, et al. Change in pain severity with open label venlafaxine use in patients with a depressive symptomatology: an observational study in primary care. Eur Psychiatry. 2008;23:178-186.
33. Branco JC, Zachrisson O, Perrot S, et al; Multinational Coordinator Study Group. A European multicenter randomized double-blind placebo-controlled monotherapy clinical trial of milnacipran in the treatment of fibromyalgia. J Rheumatol. 2010;37:851-859.
34. Salín-Pascual RJ, Galicia-Polo L, Drucker-Colin R. Sleep changes after 4 consecutive days of venlafaxine administration in normal volunteers. J Clin Psychiatry. 1997;58:348-350.
35. Otto M, Bach FW, Jensen TS, et al. Escitalopram in painful polyneuropathy: a randomized, placebo-controlled, cross-over trial. Pain. 2008;139:275-283.
36. Max MB, Lynch SA, Muir J, et al. Effects of desipramine, amitriptyline, and fluoxetine on pain in diabetic neuropathy. N Engl J Med. 1992;326:1250-1256.
37. Sellek S. Opioid abuse puts physicians between a rock and a hard place but resources can help them lead the debate on how to rein it in. Mich Med. 2013;112:10-13.
38. Nielsen S, Bruno R, Degenhardt L, et al. The sources of pharmaceuticals for problematic users of benzodiazepines and prescription opioids. Med J Aust. 2013;199:696-699.
39. Turk DC, Swanson KS, Tunks ER. Psychological approaches in the treatment of chronic pain patients–when pills, scalpels, and needles are not enough. Can J Psychiatry. 2008;53:213-223.
40. Basler HD, Jäkle C, Kröner-Herwig B. Incorporation of cognitive-behavioral treatment into the medical care of chronic low back patients: a controlled randomized study in German pain treatment centers. Patient Educ Couns. 1997;31:113-124.
41. Middaugh S, Thomas KJ, Smith AR, et al. EMG biofeedback and exercise for treatment of cervical and shoulder pain in individuals with a spinal cord injury: a pilot study. Top Spinal Cord Inj Rehabil. 2013;19:311-323.
42. Oravitan M, Avram C. The effectiveness of electromyographic biofeedback as part of a meniscal repair rehabilitation programme. J Sports Sci Med. 2013;12:526-532.eCollection 2013.
43. Glombiewski JA, Bernardy K, Häuser W. Efficacy of EMG- and EEG-biofeedback in fibromyalgia syndrome: A meta-analysis and a systematic review of randomized controlled trials. Evid Based Complement Alternat Med. 2013;2013:962741.
44. Kubik A, Biedroń A. Neurofeedback therapy in patients with acute and chronic pain syndromes—literature review and own experience. Przegl Lek. 2013;70:440-442.
45. Overcash J, Will KM, Lipetz DW. The benefits of medical qigong in patients with cancer: a descriptive pilot study. Clin J Oncol Nurs. 2013;17:654-658.
46. Jastrowski Mano KE, Salamon KS, Hainsworth KR, et al. A randomized, controlled pilot study of mindfulness-based stress reduction for pediatric chronic pain. Altern Ther Health Med. 2013;19:8-14.
47. Ahmed M, Modak S, Sequeira S. Acute pain relief after Mantram meditation in children with neuroblastoma undergoing anti-GD2 monoclonal antibody therapy. J Pediatr Hematol Oncol. 2014;36:152-155.
48. Romano JM, Turner JA. Chronic pain and depression: does the evidence support a relationship? Psychol Bull. 1985;97:18-34.
Should you suspect the female athlete triad?
› Screen all adolescent female athletes for components of the female athlete triad at the preparticipation examination or whenever they present with any of the triad’s symptoms. C
› Order a dual-energy x-ray absorptiometry scan to measure bone mineral density on all female athletes with a history of stress fracture—not just those who also have amenorrhea, oligomenorrhea, or disordered eating. C
› Prescribe oral contraceptives to regulate an athlete’s menstrual period only as a last measure for those who, despite following recommendations, do not have a normal return to menses after 6 months. B
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
CASE › Cassidy, age 14, comes to you for a physical in preparation for track and field tryouts. If she makes the team, she will practice 90 minutes every afternoon with optional practices 2 mornings a week.
She says that her period has been irregular since it started a year ago, and she complains of knee and shin pain that her mother attributes to “growing pains.” She says she usually skips breakfast due to a lack of time in the morning, but eats the school lunches. She is considering becoming a vegetarian. You suspect that the female athlete triad is at work here. How would you proceed?
The female athlete triad (“the triad”) is considered a spectrum of 3 interrelated disorders: low energy availability, menstrual dysfunction, and altered bone mineral density.1 Low energy availability—total dietary energy in (calories in) minus total exercise energy expended (calories out)—is considered the key cause. Previously, the triad was described as disordered eating, amenorrhea (having no menstruation for >3 sequential months), and osteoporosis.2 However, this definition has been expanded to encourage detection before clinical problems progress. In most instances, an athlete will develop only one or 2 of the 3 components of the triad.3,4 This article describes the clinical manifestations of the triad, how to screen patients for it, and indications for referring affected athletes.
How common is the triad?
The prevalence of the triad is difficult to determine because published studies often feature poor standardization of definitions and scales, small sample sizes, and no control groups. In limited studies, the estimated prevalence of female athletes with the complete triad ranges from 1.3% to 4.3%.3,4 Many studies, however, focus on just one of the following 3 components:
Low energy availability. Few studies have specifically evaluated the prevalence of low energy availability among female athletes. The prevalence of disordered eating among females ranges from 25% to 31% of those in “thin build” sports (eg, running, gymnastics, and figure skating) vs 5% to 9% of nonathletes.5,6
Menstrual dysfunction. The prevalence of menstrual dysfunction in female athletes is reported to be as high as 79%.1 Primary amenorrhea (a delay in the age of menarche past age 15) has been reported in 22% of gymnasts, cheerleaders, and divers vs <1% of the general population.7 Subclinical menstrual dysfunction is highly prevalent. For example, one study found 78% of normally menstruating recreational runners had luteal deficiency or anovulation in one-third of their cycles.8
Altered bone mineral density (BMD). BMD is increased in most athletes compared to sedentary controls, but low BMD often is seen in amenorrheic athletes. One review found 22% to 50% of amenorrheic athletes had osteopenia vs 12% of controls.9 The prevalence of osteoporosis in this group was as high as 13% vs 2.3% of controls.9
Three interrelated problems
As noted earlier, low energy availability is believed to be the key underlying etiology of the triad. Energy availability is the dietary energy left in the body after exercise is completed, or total dietary energy in (calories in) minus total exercise energy expended (calories out).10,11
Low energy availability is not synonymous with disordered eating. Low energy availability may be the result of either decreased caloric intake or increased output. For example, athletes who increase their training requirements (increased output) need to increase their caloric intake or they will suffer an energy imbalance. Disordered eating also can result in decreased caloric intake. Disordered eating ranges from poor eating habits such as skipping meals to psychiatric conditions such as anorexia nervosa, bulimia, or binge eating disorder. Behaviors may include restricting calories, purging, or using diet pills, diuretics, or laxatives. For a summary of the most recent changes to eating disorder diagnoses, go to http://www.dsm5.org/documents/eating%20disorders%20fact%20sheet.pdf.
Low energy availability leads to hormonal abnormalities that may exacerbate other triad symptoms. When energy is restricted, the body conserves energy by altering metabolism by several methods, including suppressing usual hormonal cycling. For example, inadequate energy availability results in suppression of gonadotropin-releasing hormone pulsatility and disruption of the number of luteinizing hormone (LH) and follicle-stimulating hormone (FSH) pulses, which results in decreased estrogen levels.12
When an athlete has decreased body fat as a result of low energy availability, she may have decreased adipokines, particularly leptin, which is believed to play a role in the ovulatory (normal) menstrual cycle. Abnormal eating also can suppress ghrelin, a short-term hormonal regulator of eating cycles/food intake and a long-term regulator of energy balance. Thus, short-term ghrelin dysregulation can self-perpetuate and have long-term consequences.
In anorexia nervosa, the intestinally derived anorexigen peptide YY is elevated,13 which may contribute to bone loss. Patients with anorexia nervosa also have androgen deficiency and elevated cortisol, both of which are factors in energy metabolism and maintenance of bone density.14
Menstrual dysfunction. Female athletes may experience amenorrhea, oligomenorrhea (menstrual periods with intervals >35 days), or subclinical menstrual dysfunction. Amenorrhea that occurs after menarche is considered secondary amenorrhea. Brancaccio et al15 found the incidence of secondary amenorrhea was significantly higher in high school athletes (30%) than in controls (15%). The researchers noted that “strenuous training alone has not been shown to alter menstrual cycles; it is necessary for dietary restriction to occur.”15
Altered BMD. Osteoporosis is characterized by compromised bone strength that increases the risk of fracture,16 and BMD is an aspect of bone strength. Osteoporosis may be caused by inadequate accumulation of optimal BMD during childhood and adolescence. A diagnosis of osteoporosis in patients ages 5 to 19 requires the presence of low bone mass and a clinically significant fracture history.17
BMD is assessed by dual-energy x-ray absorptiometry (DXA). DXA results are reported as a T-score, in which a patient’s bone density is compared with that of a healthy 30-year-old woman, or Z-score, which compares patients’ BMD with age- and sex-matched controls. The International Society of Clinical Densitometry recommends using Z-scores instead of T-scores when screening for osteoporosis in premenopausal women and in children.18
Z-scores are expressed as the number of standard deviations above or below the average value of the reference group. For every reduction by one standard deviation in BMD, the patient’s fracture risk doubles. The American College of Sports Medicine defines “low BMD” as a Z-score between -1 and -2.0 and osteoporosis as a Z-score ≤-2.0.1 For both definitions, the patient must have at least one secondary clinical risk factor for fracture, such as low estrogen, history of stress fracture, or nutritional deficiencies.
Screening for the triad: What to ask, what to look for
Screen all adolescent female athletes at preparticipation physicals or whenever they present with any of the triad’s signs and symptoms. Look for risk factors such as calorie restriction practices, vegetarianism, a history of injuries, extended exercise periods, or increased training, particularly sport-specific training.1 Also consider social, genetic, and psychiatric issues, such as abuse and family dysfunction.
If you believe a patient is at risk, ask her about her exercise and eating habits, menstrual cycles, and fracture history (TABLE 1).19 During the physical exam, look for signs that suggest the triad, including lanugo, enlarged parotid glands, and bradycardia (TABLE 2).1 Be sure to calculate the patient’s body mass index or body fat percentage.
Based on the history and physical findings, consider laboratory testing for a complete blood count with differential, ferritin, serum iron, B12, folate, comprehensive metabolic panel, thyroid function tests, erythrocyte sedimentation rate, and a urinalysis.20 For an athlete in whom you highly suspect the triad, order urine electrolytes, salivary amylase, and stool guaiac tests, as well as an electrocardiogram.19
If your patient is amenorrheic, be aware that functional amenorrhea is a diagnosis of exclusion. Other diagnoses to consider include pregnancy, polycystic ovary syndrome, prolactinoma, anatomic defect, and ovarian failure. A pregnancy test, FSH and LH levels, prolactin, and thyroid-stimulating hormone testing should all be considered during evaluation for amenorrhea.
All patients with a history of stress fracture should undergo a DXA scan, whether or not they have comorbid amenorrhea, oligomenorrhea, or disordered eating.20 In patients with amenorrhea, the DXA may need to be repeated in one year if menses does not resume.
For patients who screen positive for disordered eating, amenorrhea, or decreased BMD, the International Olympic Committee (IOC) guidelines are an excellent starting point for further evaluation.19 The IOC has “decision trees” for female athletes with disordered eating, amenorrhea, and osteoporosis that are available at http://www.olympic.org/Documents/Reports/EN/en_report_917.pdf.19
In addition to screening female athletes for the triad, consider addressing eating attitudes, menses, and fracture history in routine office physicals for all female patients. Also, be aware that triad symptoms are not limited to female athletes; male athletes, particularly those in sports that focus on leanness, physique, or weight classes (eg, wrestling) also are at risk for low energy availability, disordered eating, and low BMD.
Tx: First, restore sufficient nutrition
When treating a patient with the triad, consider consulting with a sports medicine specialist because these physicians typically are trained in diagnosing and treating this condition. Because low energy availability is the cornerstone of the triad, the priority in treating an affected athlete is to restore sufficient nutrition for caloric needs. Referral to a registered dietitian for full nutritional assessment and meal planning is recommended. If your athlete is unwilling or unable to follow dietary recommendations, refer her to an eating disorder specialist team. Ideally, this specialist team would consist of a registered sport nutritionist, a physician, and a psychologist or psychiatrist who specializes in eating disorders.
Drugs that can augment your efforts
Although they play a small role in treating the triad, pharmacologic therapies may be used to augment nutrition counseling. The selective serotonin reuptake inhibitor fluoxetine is the only medication approved by the US Food and Drug Administration for treating patients with bulimia; it is not approved for those with anorexia nervosa.21
Oral contraceptives may help women return to monthly menses, but they do not normalize the metabolic factors that impair bone formation and bone health. They can be used as a last measure in athletes who will not follow dietary or exercise recommendations, or those who, despite following recommendations, do not have a return to normal menses after 6 months.
Nasal calcitonin may be used to treat low BMD; order a follow-up DXA scan in 12 months to monitor improvement. However, prolonged use of nasal calcitonin may increase the risk for cancer, and in October 2013 nasal calcitonin was withdrawn from the Canadian market.22 For amenorrheic athletes, recommend oral calcium, 1000 to 1300 mg/d, and vitamin D, 400 to 800 IU/d. Ideally, patients should receive these levels of nutrients via dietary intake, but if that is not realistic, supplements may be considered.1,23 Bisphosphonates and selective estrogen receptor modulators are contraindicated for premenopausal athletes.19
Can the patient return to play?
The athlete will need to be medically and psychologically cleared before being allowed to return to play (RTP). If she has menstrual dysfunction or low BMD, these conditions should be addressed as a prerequisite for RTP. If the treating physician, nutritionist, and/or eating disorder specialist team recommends specific treatments or other interventions, the athlete should agree to the treatment plan in order to RTP. The physician or assessment team should determine the time frame for RTP on an individual basis. Athletes who do not comply with treatment regimens should, for their health and safety, be prohibited from return to sports participation.
Focus on prevention
Primary prevention should focus on educating female (and male) athletes about regarding food as fuel, discouraging unhealthy weight loss, and enlisting the support of coaches and governing bodies. An athlete’s coaches may be the first to notice symptoms of the triad as changes in performance or behavior, but coaches should not encourage athletes to lose weight or be involved in determining an athlete’s weight.19
CORRESPONDENCE
Jennifer Payne, MD, Family Medicine Residency Program, Lancaster General Hospital, 555 N Duke Street, Lancaster, PA 17604; [email protected]
1. Nattiv A, Loucks AB, Manore MM, et al; American College of Sports Medicine. American College of Sports Medicine position stand on the female athlete triad. Med Sci Sports Exerc. 2007;39:1867-1882.
2. Nattiv A, Agostini R, Drinkwater B, et al. The female athlete triad. The inter-relatedness of disordered eating, amenorrhea, and osteoporosis. Clin Sports Med. 1994;13:405-418.
3. Nichols JF, Rauh MJ, Lawson MJ, et al. Prevalence of the female athlete triad syndrome among high school athletes. Arch Pediatr Adolesc Med. 2006;160:137-142.
4. Torstveit MK, Sungot-Borgen J. The female athlete triad exists in both athletes and controls. Med Sci Sports Exerc. 2005; 37:1449-1459.
5. Byrne S, McLean N. Elite athletes: effects of the pressure to be thin. J Sci Med Sport. 2002;5:80-94.
6. Sundgot-Borgen J, Torstveit MK. Prevalence of eating disorders in elite athletes is higher than in the general population. Clin J Sport Med. 2004;14:25-32.
7. Beals KA, Manore MM. Disorders of the female athlete triad among collegiate athletes. Int J Sport Nutr Exerc Metab. 2002;12:281-293.
8. De Souza MJ, Miller BE, Loucks AB, et al. High frequency of luteal phase deficiency and anovulation in recreational women runners: blunted elevation in follicle-stimulating hormone observed during luteal-follicular transition. J Clin Endocrinol Metab. 1998;83:4220-4232.
9. Khan KM, Liu-Ambrose T, Sran MM, et al. New criteria for the female athlete triad syndrome? As osteoporosis is rare, should osteopenia be among the criteria for defining the female athlete triad syndrome? Br J Sports Med. 2002;36:10-13.
10. Loucks AB. Effects of exercise training on the menstrual cycle: existence and mechanisms. Med Sci Sports Exerc. 1990;22:275-280.
11. Loucks AB, Verdun M, Heath EM. Low energy availability, not the stress of exercise, alters LH pulsatility in exercising women. J Appl Physiol (1985). 1998;84:37-46.
12. Mendelsohn FA, Warren MP. Anorexia, bulimia, and the female athlete triad: evaluation and management. Endocrinol Metab Clin N Am. 2010;39:155-167.
13. Misra M, Miller KK, Tsai P, et al. Elevated peptide YY levels in adolescent girls with anorexia nervosa. J Clin Endocrinol Metab. 2006;91:1027-1033.
14. Lawson EA, Klibanski A. Endocrine abnormalities in anorexia nervosa. Nat Clin Pract Endocrinol Metab. 2008;4:407-414.
15. Brancaccio P, Maffulli N, Buonauro R, et al. Serum enzyme monitoring in sports medicine. Clin Sports Med. 2008;27:1-18, vii.
16. NIH Consensus Development Panel on Osteoporosis Prevention, Diagnosis, and Therapy. Osteoporosis prevention, diagnosis, and therapy. JAMA. 2001;285:785-795.
17. 2007 ISCD Official Positions–Pediatric. The International Society for Clinical Densitometry Web site. Available at: http://www.iscd.org/official-positions/2007-iscd-official-positions-pediatric. Accessed February 26, 2014.
18. 2013 ISCD Official Positions–Adult. The International Society for Clinical Densitometry Web site. Available at: http://www.iscd.org/official-positions/2013-iscd-official-positions-adult. Accessed February 26, 2014.
19. Position stand on the female athlete triad. The Internal Olympic Committee Web site. Available at: http://www.olympic.org/Documents/Reports/EN/en_report_917.pdf. Accessed February 3, 2014.
20. The Female Athlete Triad Coalition Web site. Available at: http://www.femaleathletetriad.org. Accessed February 3, 2014.
21. Eating disorders. National Institute of Mental Health Web site. Available at: http://www.nimh.nih.gov/health/publications/eating-disorders/index.shtml. Accessed February 26, 2014.
22. Synthetic Calcitonin (Salmon) nasal spray (ns)—market withdrawal of all products, effective October 1st, 2013—for health professionals. Government of Canada Health Canadians Web site. Available at: http://healthycanadians.gc.ca/recall-alertrappel-avis/hc-sc/2013/34783a-eng.php. Accessed February 13, 2014.
23. Greer FR, Krebs NF; American Academy of Pediatrics Committee on Nutrition. Optimizing bone health and calcium intakes of infants, children, and adolescents. Pediatrics. 2006;117:578-585.
› Screen all adolescent female athletes for components of the female athlete triad at the preparticipation examination or whenever they present with any of the triad’s symptoms. C
› Order a dual-energy x-ray absorptiometry scan to measure bone mineral density on all female athletes with a history of stress fracture—not just those who also have amenorrhea, oligomenorrhea, or disordered eating. C
› Prescribe oral contraceptives to regulate an athlete’s menstrual period only as a last measure for those who, despite following recommendations, do not have a normal return to menses after 6 months. B
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
CASE › Cassidy, age 14, comes to you for a physical in preparation for track and field tryouts. If she makes the team, she will practice 90 minutes every afternoon with optional practices 2 mornings a week.
She says that her period has been irregular since it started a year ago, and she complains of knee and shin pain that her mother attributes to “growing pains.” She says she usually skips breakfast due to a lack of time in the morning, but eats the school lunches. She is considering becoming a vegetarian. You suspect that the female athlete triad is at work here. How would you proceed?
The female athlete triad (“the triad”) is considered a spectrum of 3 interrelated disorders: low energy availability, menstrual dysfunction, and altered bone mineral density.1 Low energy availability—total dietary energy in (calories in) minus total exercise energy expended (calories out)—is considered the key cause. Previously, the triad was described as disordered eating, amenorrhea (having no menstruation for >3 sequential months), and osteoporosis.2 However, this definition has been expanded to encourage detection before clinical problems progress. In most instances, an athlete will develop only one or 2 of the 3 components of the triad.3,4 This article describes the clinical manifestations of the triad, how to screen patients for it, and indications for referring affected athletes.
How common is the triad?
The prevalence of the triad is difficult to determine because published studies often feature poor standardization of definitions and scales, small sample sizes, and no control groups. In limited studies, the estimated prevalence of female athletes with the complete triad ranges from 1.3% to 4.3%.3,4 Many studies, however, focus on just one of the following 3 components:
Low energy availability. Few studies have specifically evaluated the prevalence of low energy availability among female athletes. The prevalence of disordered eating among females ranges from 25% to 31% of those in “thin build” sports (eg, running, gymnastics, and figure skating) vs 5% to 9% of nonathletes.5,6
Menstrual dysfunction. The prevalence of menstrual dysfunction in female athletes is reported to be as high as 79%.1 Primary amenorrhea (a delay in the age of menarche past age 15) has been reported in 22% of gymnasts, cheerleaders, and divers vs <1% of the general population.7 Subclinical menstrual dysfunction is highly prevalent. For example, one study found 78% of normally menstruating recreational runners had luteal deficiency or anovulation in one-third of their cycles.8
Altered bone mineral density (BMD). BMD is increased in most athletes compared to sedentary controls, but low BMD often is seen in amenorrheic athletes. One review found 22% to 50% of amenorrheic athletes had osteopenia vs 12% of controls.9 The prevalence of osteoporosis in this group was as high as 13% vs 2.3% of controls.9
Three interrelated problems
As noted earlier, low energy availability is believed to be the key underlying etiology of the triad. Energy availability is the dietary energy left in the body after exercise is completed, or total dietary energy in (calories in) minus total exercise energy expended (calories out).10,11
Low energy availability is not synonymous with disordered eating. Low energy availability may be the result of either decreased caloric intake or increased output. For example, athletes who increase their training requirements (increased output) need to increase their caloric intake or they will suffer an energy imbalance. Disordered eating also can result in decreased caloric intake. Disordered eating ranges from poor eating habits such as skipping meals to psychiatric conditions such as anorexia nervosa, bulimia, or binge eating disorder. Behaviors may include restricting calories, purging, or using diet pills, diuretics, or laxatives. For a summary of the most recent changes to eating disorder diagnoses, go to http://www.dsm5.org/documents/eating%20disorders%20fact%20sheet.pdf.
Low energy availability leads to hormonal abnormalities that may exacerbate other triad symptoms. When energy is restricted, the body conserves energy by altering metabolism by several methods, including suppressing usual hormonal cycling. For example, inadequate energy availability results in suppression of gonadotropin-releasing hormone pulsatility and disruption of the number of luteinizing hormone (LH) and follicle-stimulating hormone (FSH) pulses, which results in decreased estrogen levels.12
When an athlete has decreased body fat as a result of low energy availability, she may have decreased adipokines, particularly leptin, which is believed to play a role in the ovulatory (normal) menstrual cycle. Abnormal eating also can suppress ghrelin, a short-term hormonal regulator of eating cycles/food intake and a long-term regulator of energy balance. Thus, short-term ghrelin dysregulation can self-perpetuate and have long-term consequences.
In anorexia nervosa, the intestinally derived anorexigen peptide YY is elevated,13 which may contribute to bone loss. Patients with anorexia nervosa also have androgen deficiency and elevated cortisol, both of which are factors in energy metabolism and maintenance of bone density.14
Menstrual dysfunction. Female athletes may experience amenorrhea, oligomenorrhea (menstrual periods with intervals >35 days), or subclinical menstrual dysfunction. Amenorrhea that occurs after menarche is considered secondary amenorrhea. Brancaccio et al15 found the incidence of secondary amenorrhea was significantly higher in high school athletes (30%) than in controls (15%). The researchers noted that “strenuous training alone has not been shown to alter menstrual cycles; it is necessary for dietary restriction to occur.”15
Altered BMD. Osteoporosis is characterized by compromised bone strength that increases the risk of fracture,16 and BMD is an aspect of bone strength. Osteoporosis may be caused by inadequate accumulation of optimal BMD during childhood and adolescence. A diagnosis of osteoporosis in patients ages 5 to 19 requires the presence of low bone mass and a clinically significant fracture history.17
BMD is assessed by dual-energy x-ray absorptiometry (DXA). DXA results are reported as a T-score, in which a patient’s bone density is compared with that of a healthy 30-year-old woman, or Z-score, which compares patients’ BMD with age- and sex-matched controls. The International Society of Clinical Densitometry recommends using Z-scores instead of T-scores when screening for osteoporosis in premenopausal women and in children.18
Z-scores are expressed as the number of standard deviations above or below the average value of the reference group. For every reduction by one standard deviation in BMD, the patient’s fracture risk doubles. The American College of Sports Medicine defines “low BMD” as a Z-score between -1 and -2.0 and osteoporosis as a Z-score ≤-2.0.1 For both definitions, the patient must have at least one secondary clinical risk factor for fracture, such as low estrogen, history of stress fracture, or nutritional deficiencies.
Screening for the triad: What to ask, what to look for
Screen all adolescent female athletes at preparticipation physicals or whenever they present with any of the triad’s signs and symptoms. Look for risk factors such as calorie restriction practices, vegetarianism, a history of injuries, extended exercise periods, or increased training, particularly sport-specific training.1 Also consider social, genetic, and psychiatric issues, such as abuse and family dysfunction.
If you believe a patient is at risk, ask her about her exercise and eating habits, menstrual cycles, and fracture history (TABLE 1).19 During the physical exam, look for signs that suggest the triad, including lanugo, enlarged parotid glands, and bradycardia (TABLE 2).1 Be sure to calculate the patient’s body mass index or body fat percentage.
Based on the history and physical findings, consider laboratory testing for a complete blood count with differential, ferritin, serum iron, B12, folate, comprehensive metabolic panel, thyroid function tests, erythrocyte sedimentation rate, and a urinalysis.20 For an athlete in whom you highly suspect the triad, order urine electrolytes, salivary amylase, and stool guaiac tests, as well as an electrocardiogram.19
If your patient is amenorrheic, be aware that functional amenorrhea is a diagnosis of exclusion. Other diagnoses to consider include pregnancy, polycystic ovary syndrome, prolactinoma, anatomic defect, and ovarian failure. A pregnancy test, FSH and LH levels, prolactin, and thyroid-stimulating hormone testing should all be considered during evaluation for amenorrhea.
All patients with a history of stress fracture should undergo a DXA scan, whether or not they have comorbid amenorrhea, oligomenorrhea, or disordered eating.20 In patients with amenorrhea, the DXA may need to be repeated in one year if menses does not resume.
For patients who screen positive for disordered eating, amenorrhea, or decreased BMD, the International Olympic Committee (IOC) guidelines are an excellent starting point for further evaluation.19 The IOC has “decision trees” for female athletes with disordered eating, amenorrhea, and osteoporosis that are available at http://www.olympic.org/Documents/Reports/EN/en_report_917.pdf.19
In addition to screening female athletes for the triad, consider addressing eating attitudes, menses, and fracture history in routine office physicals for all female patients. Also, be aware that triad symptoms are not limited to female athletes; male athletes, particularly those in sports that focus on leanness, physique, or weight classes (eg, wrestling) also are at risk for low energy availability, disordered eating, and low BMD.
Tx: First, restore sufficient nutrition
When treating a patient with the triad, consider consulting with a sports medicine specialist because these physicians typically are trained in diagnosing and treating this condition. Because low energy availability is the cornerstone of the triad, the priority in treating an affected athlete is to restore sufficient nutrition for caloric needs. Referral to a registered dietitian for full nutritional assessment and meal planning is recommended. If your athlete is unwilling or unable to follow dietary recommendations, refer her to an eating disorder specialist team. Ideally, this specialist team would consist of a registered sport nutritionist, a physician, and a psychologist or psychiatrist who specializes in eating disorders.
Drugs that can augment your efforts
Although they play a small role in treating the triad, pharmacologic therapies may be used to augment nutrition counseling. The selective serotonin reuptake inhibitor fluoxetine is the only medication approved by the US Food and Drug Administration for treating patients with bulimia; it is not approved for those with anorexia nervosa.21
Oral contraceptives may help women return to monthly menses, but they do not normalize the metabolic factors that impair bone formation and bone health. They can be used as a last measure in athletes who will not follow dietary or exercise recommendations, or those who, despite following recommendations, do not have a return to normal menses after 6 months.
Nasal calcitonin may be used to treat low BMD; order a follow-up DXA scan in 12 months to monitor improvement. However, prolonged use of nasal calcitonin may increase the risk for cancer, and in October 2013 nasal calcitonin was withdrawn from the Canadian market.22 For amenorrheic athletes, recommend oral calcium, 1000 to 1300 mg/d, and vitamin D, 400 to 800 IU/d. Ideally, patients should receive these levels of nutrients via dietary intake, but if that is not realistic, supplements may be considered.1,23 Bisphosphonates and selective estrogen receptor modulators are contraindicated for premenopausal athletes.19
Can the patient return to play?
The athlete will need to be medically and psychologically cleared before being allowed to return to play (RTP). If she has menstrual dysfunction or low BMD, these conditions should be addressed as a prerequisite for RTP. If the treating physician, nutritionist, and/or eating disorder specialist team recommends specific treatments or other interventions, the athlete should agree to the treatment plan in order to RTP. The physician or assessment team should determine the time frame for RTP on an individual basis. Athletes who do not comply with treatment regimens should, for their health and safety, be prohibited from return to sports participation.
Focus on prevention
Primary prevention should focus on educating female (and male) athletes about regarding food as fuel, discouraging unhealthy weight loss, and enlisting the support of coaches and governing bodies. An athlete’s coaches may be the first to notice symptoms of the triad as changes in performance or behavior, but coaches should not encourage athletes to lose weight or be involved in determining an athlete’s weight.19
CORRESPONDENCE
Jennifer Payne, MD, Family Medicine Residency Program, Lancaster General Hospital, 555 N Duke Street, Lancaster, PA 17604; [email protected]
› Screen all adolescent female athletes for components of the female athlete triad at the preparticipation examination or whenever they present with any of the triad’s symptoms. C
› Order a dual-energy x-ray absorptiometry scan to measure bone mineral density on all female athletes with a history of stress fracture—not just those who also have amenorrhea, oligomenorrhea, or disordered eating. C
› Prescribe oral contraceptives to regulate an athlete’s menstrual period only as a last measure for those who, despite following recommendations, do not have a normal return to menses after 6 months. B
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
CASE › Cassidy, age 14, comes to you for a physical in preparation for track and field tryouts. If she makes the team, she will practice 90 minutes every afternoon with optional practices 2 mornings a week.
She says that her period has been irregular since it started a year ago, and she complains of knee and shin pain that her mother attributes to “growing pains.” She says she usually skips breakfast due to a lack of time in the morning, but eats the school lunches. She is considering becoming a vegetarian. You suspect that the female athlete triad is at work here. How would you proceed?
The female athlete triad (“the triad”) is considered a spectrum of 3 interrelated disorders: low energy availability, menstrual dysfunction, and altered bone mineral density.1 Low energy availability—total dietary energy in (calories in) minus total exercise energy expended (calories out)—is considered the key cause. Previously, the triad was described as disordered eating, amenorrhea (having no menstruation for >3 sequential months), and osteoporosis.2 However, this definition has been expanded to encourage detection before clinical problems progress. In most instances, an athlete will develop only one or 2 of the 3 components of the triad.3,4 This article describes the clinical manifestations of the triad, how to screen patients for it, and indications for referring affected athletes.
How common is the triad?
The prevalence of the triad is difficult to determine because published studies often feature poor standardization of definitions and scales, small sample sizes, and no control groups. In limited studies, the estimated prevalence of female athletes with the complete triad ranges from 1.3% to 4.3%.3,4 Many studies, however, focus on just one of the following 3 components:
Low energy availability. Few studies have specifically evaluated the prevalence of low energy availability among female athletes. The prevalence of disordered eating among females ranges from 25% to 31% of those in “thin build” sports (eg, running, gymnastics, and figure skating) vs 5% to 9% of nonathletes.5,6
Menstrual dysfunction. The prevalence of menstrual dysfunction in female athletes is reported to be as high as 79%.1 Primary amenorrhea (a delay in the age of menarche past age 15) has been reported in 22% of gymnasts, cheerleaders, and divers vs <1% of the general population.7 Subclinical menstrual dysfunction is highly prevalent. For example, one study found 78% of normally menstruating recreational runners had luteal deficiency or anovulation in one-third of their cycles.8
Altered bone mineral density (BMD). BMD is increased in most athletes compared to sedentary controls, but low BMD often is seen in amenorrheic athletes. One review found 22% to 50% of amenorrheic athletes had osteopenia vs 12% of controls.9 The prevalence of osteoporosis in this group was as high as 13% vs 2.3% of controls.9
Three interrelated problems
As noted earlier, low energy availability is believed to be the key underlying etiology of the triad. Energy availability is the dietary energy left in the body after exercise is completed, or total dietary energy in (calories in) minus total exercise energy expended (calories out).10,11
Low energy availability is not synonymous with disordered eating. Low energy availability may be the result of either decreased caloric intake or increased output. For example, athletes who increase their training requirements (increased output) need to increase their caloric intake or they will suffer an energy imbalance. Disordered eating also can result in decreased caloric intake. Disordered eating ranges from poor eating habits such as skipping meals to psychiatric conditions such as anorexia nervosa, bulimia, or binge eating disorder. Behaviors may include restricting calories, purging, or using diet pills, diuretics, or laxatives. For a summary of the most recent changes to eating disorder diagnoses, go to http://www.dsm5.org/documents/eating%20disorders%20fact%20sheet.pdf.
Low energy availability leads to hormonal abnormalities that may exacerbate other triad symptoms. When energy is restricted, the body conserves energy by altering metabolism by several methods, including suppressing usual hormonal cycling. For example, inadequate energy availability results in suppression of gonadotropin-releasing hormone pulsatility and disruption of the number of luteinizing hormone (LH) and follicle-stimulating hormone (FSH) pulses, which results in decreased estrogen levels.12
When an athlete has decreased body fat as a result of low energy availability, she may have decreased adipokines, particularly leptin, which is believed to play a role in the ovulatory (normal) menstrual cycle. Abnormal eating also can suppress ghrelin, a short-term hormonal regulator of eating cycles/food intake and a long-term regulator of energy balance. Thus, short-term ghrelin dysregulation can self-perpetuate and have long-term consequences.
In anorexia nervosa, the intestinally derived anorexigen peptide YY is elevated,13 which may contribute to bone loss. Patients with anorexia nervosa also have androgen deficiency and elevated cortisol, both of which are factors in energy metabolism and maintenance of bone density.14
Menstrual dysfunction. Female athletes may experience amenorrhea, oligomenorrhea (menstrual periods with intervals >35 days), or subclinical menstrual dysfunction. Amenorrhea that occurs after menarche is considered secondary amenorrhea. Brancaccio et al15 found the incidence of secondary amenorrhea was significantly higher in high school athletes (30%) than in controls (15%). The researchers noted that “strenuous training alone has not been shown to alter menstrual cycles; it is necessary for dietary restriction to occur.”15
Altered BMD. Osteoporosis is characterized by compromised bone strength that increases the risk of fracture,16 and BMD is an aspect of bone strength. Osteoporosis may be caused by inadequate accumulation of optimal BMD during childhood and adolescence. A diagnosis of osteoporosis in patients ages 5 to 19 requires the presence of low bone mass and a clinically significant fracture history.17
BMD is assessed by dual-energy x-ray absorptiometry (DXA). DXA results are reported as a T-score, in which a patient’s bone density is compared with that of a healthy 30-year-old woman, or Z-score, which compares patients’ BMD with age- and sex-matched controls. The International Society of Clinical Densitometry recommends using Z-scores instead of T-scores when screening for osteoporosis in premenopausal women and in children.18
Z-scores are expressed as the number of standard deviations above or below the average value of the reference group. For every reduction by one standard deviation in BMD, the patient’s fracture risk doubles. The American College of Sports Medicine defines “low BMD” as a Z-score between -1 and -2.0 and osteoporosis as a Z-score ≤-2.0.1 For both definitions, the patient must have at least one secondary clinical risk factor for fracture, such as low estrogen, history of stress fracture, or nutritional deficiencies.
Screening for the triad: What to ask, what to look for
Screen all adolescent female athletes at preparticipation physicals or whenever they present with any of the triad’s signs and symptoms. Look for risk factors such as calorie restriction practices, vegetarianism, a history of injuries, extended exercise periods, or increased training, particularly sport-specific training.1 Also consider social, genetic, and psychiatric issues, such as abuse and family dysfunction.
If you believe a patient is at risk, ask her about her exercise and eating habits, menstrual cycles, and fracture history (TABLE 1).19 During the physical exam, look for signs that suggest the triad, including lanugo, enlarged parotid glands, and bradycardia (TABLE 2).1 Be sure to calculate the patient’s body mass index or body fat percentage.
Based on the history and physical findings, consider laboratory testing for a complete blood count with differential, ferritin, serum iron, B12, folate, comprehensive metabolic panel, thyroid function tests, erythrocyte sedimentation rate, and a urinalysis.20 For an athlete in whom you highly suspect the triad, order urine electrolytes, salivary amylase, and stool guaiac tests, as well as an electrocardiogram.19
If your patient is amenorrheic, be aware that functional amenorrhea is a diagnosis of exclusion. Other diagnoses to consider include pregnancy, polycystic ovary syndrome, prolactinoma, anatomic defect, and ovarian failure. A pregnancy test, FSH and LH levels, prolactin, and thyroid-stimulating hormone testing should all be considered during evaluation for amenorrhea.
All patients with a history of stress fracture should undergo a DXA scan, whether or not they have comorbid amenorrhea, oligomenorrhea, or disordered eating.20 In patients with amenorrhea, the DXA may need to be repeated in one year if menses does not resume.
For patients who screen positive for disordered eating, amenorrhea, or decreased BMD, the International Olympic Committee (IOC) guidelines are an excellent starting point for further evaluation.19 The IOC has “decision trees” for female athletes with disordered eating, amenorrhea, and osteoporosis that are available at http://www.olympic.org/Documents/Reports/EN/en_report_917.pdf.19
In addition to screening female athletes for the triad, consider addressing eating attitudes, menses, and fracture history in routine office physicals for all female patients. Also, be aware that triad symptoms are not limited to female athletes; male athletes, particularly those in sports that focus on leanness, physique, or weight classes (eg, wrestling) also are at risk for low energy availability, disordered eating, and low BMD.
Tx: First, restore sufficient nutrition
When treating a patient with the triad, consider consulting with a sports medicine specialist because these physicians typically are trained in diagnosing and treating this condition. Because low energy availability is the cornerstone of the triad, the priority in treating an affected athlete is to restore sufficient nutrition for caloric needs. Referral to a registered dietitian for full nutritional assessment and meal planning is recommended. If your athlete is unwilling or unable to follow dietary recommendations, refer her to an eating disorder specialist team. Ideally, this specialist team would consist of a registered sport nutritionist, a physician, and a psychologist or psychiatrist who specializes in eating disorders.
Drugs that can augment your efforts
Although they play a small role in treating the triad, pharmacologic therapies may be used to augment nutrition counseling. The selective serotonin reuptake inhibitor fluoxetine is the only medication approved by the US Food and Drug Administration for treating patients with bulimia; it is not approved for those with anorexia nervosa.21
Oral contraceptives may help women return to monthly menses, but they do not normalize the metabolic factors that impair bone formation and bone health. They can be used as a last measure in athletes who will not follow dietary or exercise recommendations, or those who, despite following recommendations, do not have a return to normal menses after 6 months.
Nasal calcitonin may be used to treat low BMD; order a follow-up DXA scan in 12 months to monitor improvement. However, prolonged use of nasal calcitonin may increase the risk for cancer, and in October 2013 nasal calcitonin was withdrawn from the Canadian market.22 For amenorrheic athletes, recommend oral calcium, 1000 to 1300 mg/d, and vitamin D, 400 to 800 IU/d. Ideally, patients should receive these levels of nutrients via dietary intake, but if that is not realistic, supplements may be considered.1,23 Bisphosphonates and selective estrogen receptor modulators are contraindicated for premenopausal athletes.19
Can the patient return to play?
The athlete will need to be medically and psychologically cleared before being allowed to return to play (RTP). If she has menstrual dysfunction or low BMD, these conditions should be addressed as a prerequisite for RTP. If the treating physician, nutritionist, and/or eating disorder specialist team recommends specific treatments or other interventions, the athlete should agree to the treatment plan in order to RTP. The physician or assessment team should determine the time frame for RTP on an individual basis. Athletes who do not comply with treatment regimens should, for their health and safety, be prohibited from return to sports participation.
Focus on prevention
Primary prevention should focus on educating female (and male) athletes about regarding food as fuel, discouraging unhealthy weight loss, and enlisting the support of coaches and governing bodies. An athlete’s coaches may be the first to notice symptoms of the triad as changes in performance or behavior, but coaches should not encourage athletes to lose weight or be involved in determining an athlete’s weight.19
CORRESPONDENCE
Jennifer Payne, MD, Family Medicine Residency Program, Lancaster General Hospital, 555 N Duke Street, Lancaster, PA 17604; [email protected]
1. Nattiv A, Loucks AB, Manore MM, et al; American College of Sports Medicine. American College of Sports Medicine position stand on the female athlete triad. Med Sci Sports Exerc. 2007;39:1867-1882.
2. Nattiv A, Agostini R, Drinkwater B, et al. The female athlete triad. The inter-relatedness of disordered eating, amenorrhea, and osteoporosis. Clin Sports Med. 1994;13:405-418.
3. Nichols JF, Rauh MJ, Lawson MJ, et al. Prevalence of the female athlete triad syndrome among high school athletes. Arch Pediatr Adolesc Med. 2006;160:137-142.
4. Torstveit MK, Sungot-Borgen J. The female athlete triad exists in both athletes and controls. Med Sci Sports Exerc. 2005; 37:1449-1459.
5. Byrne S, McLean N. Elite athletes: effects of the pressure to be thin. J Sci Med Sport. 2002;5:80-94.
6. Sundgot-Borgen J, Torstveit MK. Prevalence of eating disorders in elite athletes is higher than in the general population. Clin J Sport Med. 2004;14:25-32.
7. Beals KA, Manore MM. Disorders of the female athlete triad among collegiate athletes. Int J Sport Nutr Exerc Metab. 2002;12:281-293.
8. De Souza MJ, Miller BE, Loucks AB, et al. High frequency of luteal phase deficiency and anovulation in recreational women runners: blunted elevation in follicle-stimulating hormone observed during luteal-follicular transition. J Clin Endocrinol Metab. 1998;83:4220-4232.
9. Khan KM, Liu-Ambrose T, Sran MM, et al. New criteria for the female athlete triad syndrome? As osteoporosis is rare, should osteopenia be among the criteria for defining the female athlete triad syndrome? Br J Sports Med. 2002;36:10-13.
10. Loucks AB. Effects of exercise training on the menstrual cycle: existence and mechanisms. Med Sci Sports Exerc. 1990;22:275-280.
11. Loucks AB, Verdun M, Heath EM. Low energy availability, not the stress of exercise, alters LH pulsatility in exercising women. J Appl Physiol (1985). 1998;84:37-46.
12. Mendelsohn FA, Warren MP. Anorexia, bulimia, and the female athlete triad: evaluation and management. Endocrinol Metab Clin N Am. 2010;39:155-167.
13. Misra M, Miller KK, Tsai P, et al. Elevated peptide YY levels in adolescent girls with anorexia nervosa. J Clin Endocrinol Metab. 2006;91:1027-1033.
14. Lawson EA, Klibanski A. Endocrine abnormalities in anorexia nervosa. Nat Clin Pract Endocrinol Metab. 2008;4:407-414.
15. Brancaccio P, Maffulli N, Buonauro R, et al. Serum enzyme monitoring in sports medicine. Clin Sports Med. 2008;27:1-18, vii.
16. NIH Consensus Development Panel on Osteoporosis Prevention, Diagnosis, and Therapy. Osteoporosis prevention, diagnosis, and therapy. JAMA. 2001;285:785-795.
17. 2007 ISCD Official Positions–Pediatric. The International Society for Clinical Densitometry Web site. Available at: http://www.iscd.org/official-positions/2007-iscd-official-positions-pediatric. Accessed February 26, 2014.
18. 2013 ISCD Official Positions–Adult. The International Society for Clinical Densitometry Web site. Available at: http://www.iscd.org/official-positions/2013-iscd-official-positions-adult. Accessed February 26, 2014.
19. Position stand on the female athlete triad. The Internal Olympic Committee Web site. Available at: http://www.olympic.org/Documents/Reports/EN/en_report_917.pdf. Accessed February 3, 2014.
20. The Female Athlete Triad Coalition Web site. Available at: http://www.femaleathletetriad.org. Accessed February 3, 2014.
21. Eating disorders. National Institute of Mental Health Web site. Available at: http://www.nimh.nih.gov/health/publications/eating-disorders/index.shtml. Accessed February 26, 2014.
22. Synthetic Calcitonin (Salmon) nasal spray (ns)—market withdrawal of all products, effective October 1st, 2013—for health professionals. Government of Canada Health Canadians Web site. Available at: http://healthycanadians.gc.ca/recall-alertrappel-avis/hc-sc/2013/34783a-eng.php. Accessed February 13, 2014.
23. Greer FR, Krebs NF; American Academy of Pediatrics Committee on Nutrition. Optimizing bone health and calcium intakes of infants, children, and adolescents. Pediatrics. 2006;117:578-585.
1. Nattiv A, Loucks AB, Manore MM, et al; American College of Sports Medicine. American College of Sports Medicine position stand on the female athlete triad. Med Sci Sports Exerc. 2007;39:1867-1882.
2. Nattiv A, Agostini R, Drinkwater B, et al. The female athlete triad. The inter-relatedness of disordered eating, amenorrhea, and osteoporosis. Clin Sports Med. 1994;13:405-418.
3. Nichols JF, Rauh MJ, Lawson MJ, et al. Prevalence of the female athlete triad syndrome among high school athletes. Arch Pediatr Adolesc Med. 2006;160:137-142.
4. Torstveit MK, Sungot-Borgen J. The female athlete triad exists in both athletes and controls. Med Sci Sports Exerc. 2005; 37:1449-1459.
5. Byrne S, McLean N. Elite athletes: effects of the pressure to be thin. J Sci Med Sport. 2002;5:80-94.
6. Sundgot-Borgen J, Torstveit MK. Prevalence of eating disorders in elite athletes is higher than in the general population. Clin J Sport Med. 2004;14:25-32.
7. Beals KA, Manore MM. Disorders of the female athlete triad among collegiate athletes. Int J Sport Nutr Exerc Metab. 2002;12:281-293.
8. De Souza MJ, Miller BE, Loucks AB, et al. High frequency of luteal phase deficiency and anovulation in recreational women runners: blunted elevation in follicle-stimulating hormone observed during luteal-follicular transition. J Clin Endocrinol Metab. 1998;83:4220-4232.
9. Khan KM, Liu-Ambrose T, Sran MM, et al. New criteria for the female athlete triad syndrome? As osteoporosis is rare, should osteopenia be among the criteria for defining the female athlete triad syndrome? Br J Sports Med. 2002;36:10-13.
10. Loucks AB. Effects of exercise training on the menstrual cycle: existence and mechanisms. Med Sci Sports Exerc. 1990;22:275-280.
11. Loucks AB, Verdun M, Heath EM. Low energy availability, not the stress of exercise, alters LH pulsatility in exercising women. J Appl Physiol (1985). 1998;84:37-46.
12. Mendelsohn FA, Warren MP. Anorexia, bulimia, and the female athlete triad: evaluation and management. Endocrinol Metab Clin N Am. 2010;39:155-167.
13. Misra M, Miller KK, Tsai P, et al. Elevated peptide YY levels in adolescent girls with anorexia nervosa. J Clin Endocrinol Metab. 2006;91:1027-1033.
14. Lawson EA, Klibanski A. Endocrine abnormalities in anorexia nervosa. Nat Clin Pract Endocrinol Metab. 2008;4:407-414.
15. Brancaccio P, Maffulli N, Buonauro R, et al. Serum enzyme monitoring in sports medicine. Clin Sports Med. 2008;27:1-18, vii.
16. NIH Consensus Development Panel on Osteoporosis Prevention, Diagnosis, and Therapy. Osteoporosis prevention, diagnosis, and therapy. JAMA. 2001;285:785-795.
17. 2007 ISCD Official Positions–Pediatric. The International Society for Clinical Densitometry Web site. Available at: http://www.iscd.org/official-positions/2007-iscd-official-positions-pediatric. Accessed February 26, 2014.
18. 2013 ISCD Official Positions–Adult. The International Society for Clinical Densitometry Web site. Available at: http://www.iscd.org/official-positions/2013-iscd-official-positions-adult. Accessed February 26, 2014.
19. Position stand on the female athlete triad. The Internal Olympic Committee Web site. Available at: http://www.olympic.org/Documents/Reports/EN/en_report_917.pdf. Accessed February 3, 2014.
20. The Female Athlete Triad Coalition Web site. Available at: http://www.femaleathletetriad.org. Accessed February 3, 2014.
21. Eating disorders. National Institute of Mental Health Web site. Available at: http://www.nimh.nih.gov/health/publications/eating-disorders/index.shtml. Accessed February 26, 2014.
22. Synthetic Calcitonin (Salmon) nasal spray (ns)—market withdrawal of all products, effective October 1st, 2013—for health professionals. Government of Canada Health Canadians Web site. Available at: http://healthycanadians.gc.ca/recall-alertrappel-avis/hc-sc/2013/34783a-eng.php. Accessed February 13, 2014.
23. Greer FR, Krebs NF; American Academy of Pediatrics Committee on Nutrition. Optimizing bone health and calcium intakes of infants, children, and adolescents. Pediatrics. 2006;117:578-585.
A guide to better wound closures
› If the skin edges of a wound require pressure to approximate, consider undermining the edges. C
› When removing a cutaneous neoplasm, avoid resecting excessive tissue. A
› For deep lacerations with potential “dead space,” use vertical mattress sutures to approximate the wound edges. C
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Skin procedures such as closing wounds and removing neoplasms are an integral part of most family medicine practices. While closure of simple lacerations and small surgical procedures are relatively straightforward, some lesions require extra techniques and attention to achieve the best outcomes.
This article describes some steps you can take to achieve the best outcomes for wound healing and cosmesis. Although suturing basics are important for successful wound closure, that information is not covered in this article. Forsch,1 however, provides an excellent discussion of basic wound skin preparation and suturing techniques.
Before beginning, visualize the ending
Before beginning any skin repair—whether it is a planned surgical procedure or a more urgent wound closure—review the patient’s medical record for conditions or medications that may adversely affect wound healing. Key in on patients with poorly controlled diabetes or those taking anticoagulants or antiplatelet drugs. Good diabetes control before surgery improves healing2 and drugs that promote bleeding sometimes may be temporarily discontinued before a procedure. If a patient’s diabetes is poorly controlled, an elective procedure can be postponed. If a patient is taking an anticoagulant and the international normalized ratio is within the therapeutic window, the procedure can be performed, but the physician must be ready to address bleeding by having electrical or chemical cautery available.
During your initial evaluation of the patient, assess and document the condition of the joints, muscles, tendons, and ligaments in the area in question before the procedure. Being aware of the local anatomy will help you avoid inadvertently damaging nerves, tendons, vessels, and other vital structures. Also, any time a procedure is beyond your comfort level, ask for help or refer the patient to a subspecialist. For example, if a lesion is on a patient’s neck near the carotid artery, the procedure is better left to an ear, nose, and throat specialist.
On the day of the procedure, plan how you will close the wound before you make the first cut, and include this information in your informed consent. Mentally review the anatomy of the area before starting a procedure to anticipate obstacles or structures that may be inadvertently injured. Visualize how the skin will fit together and how it will heal before making an incision. Reassure the patient that you will use techniques to minimize pain and scarring. Consider taking photos both before and after the procedure for documentation.
The best cosmetic results usually are achieved when the final closure line (and therefore the scar) lies parallel to the lines of least skin tension. When doing an excision, start choosing possible closures by considering layouts that produce this result. Eliminate from consideration any closures that would pull skin tension from areas that are immobile or that would cause cosmetic problems (such as a closure on or near the eyebrows or mouth, since this would change the contours of these structures). For such closures, a skin rearrangement flap may offer a cosmetically better outcome, especially on the face.
Tips for irrigating the wound and prepping the skin
If you are repairing a wound that is the result of an injury, you will need to clean the laceration gently but thoroughly. Avoid using products on open wounds that can damage regenerative tissue, such as organified iodines or hydrogen peroxide.3,4 Before irrigating a wound with normal saline, thoroughly examine it for foreign bodies, which may promote infection and impede healing. Consider giving local anesthesia before irrigation. Typically, irrigation pressure of approximately 8 pounds per square inch (psi) is considered ideal.5 Using irrigation pressures of <4 psi only moistens the wound, and >15 psi can damage the wound and drive bacteria deeper into tissue.6 Pressures of 8 to 11 psi can be achieved using a 18- to 19-gauge needle or angiocatheter with a 30 cc to 35 cc syringe.
Consider snipping off the tip of the cap and leaving it snugly on the needle. This will help prevent needle sticks and allow for thorough and safer irrigation. If commercial irrigation equipment is available, use it as directed by the manufacturer. Always be aware of potential splashback while irrigating and use appropriate personal protective equipment.
For wounds that result from a surgical procedure, employ appropriate site preparation and sterile technique. Iodophors provide broad-spectrum coverage, are not associated with microbial resistance, and provide a bacteriostatic effect as long as they remain on the skin. They require at least 2 minutes of contact to release free iodine, which exerts antibacterial activity.
Chlorhexidine gluconate offers broad-spectrum coverage against bacteria, yeast, and molds. It appears to provide greater reduction in skin microflora than povidone-iodine, and remains active for hours after application.7 Use it with caution around the eyes because of the risk for conjunctival irritation, keratitis, or corneal ulceration.8 Make sure to apply the prep solution to an area larger than the area exposed by the fenestrated drape because the drape may move around during the procedure, inadvertently contaminating the surgical field.
When excising a neoplasm, consider the margin
When performing a surgical procedure such as removing a cutaneous neoplasm, don’t resect excessive tissue, but do excise adequate margins. (For adequate margins of various cutaneous neoplasms, see TABLE 1.9-11) Mark the outlines of the visible tumor and the excision margin and show them to the patient, because some patients mistakenly assume that only the visible tumor will be removed.
Keep in mind when treating basal cell carcinomas (BCC) or squamous cell carcinomas (SCC) that the presence of high-risk characteristics may require larger surgical margins. The cure rates are lower for higher-risk BCCs, such as lesions that have morpheaform morphology or aggressive histologic features, or those that are ≥2 cm, recurrent, or located on the lips or paranasal or periocular regions.10-12
Cure rates for these higher-risk BCCs can be improved substantially by using intraoperative margin evaluation (frozen sections) or Mohs micrographic surgery. (For more on Mohs surgery, see “When to consider Mohs surgery,” J Fam Pract. 2013;62:558.)
Primary SCCs that are ≥2 cm in diameter, poorly differentiated on histology, in high-risk sites, or invading subcutaneous tissues require larger margins.13 The approach to a suspicious pigmented cutaneous neoplasm consists of an initial biopsy or excision with 1 to 2 mm margins and pathologic evaluation.14
Because hematoma formation inhibits wound healing, pay special attention to hemostasis when excising a neoplasm. Use pressure for minor bleeding. Use mosquito forceps to clamp a bleeder followed by ligation with an absorbable suture for larger bleeders. Other techniques for hemostasis include electrical or chemical cauterization and hemostatic solutions.
Positioning of edges is key to minimize scarring
When suturing a wound—whether it is from an injury or a procedure—take care to evert the skin edges; the underlying dermis from both edges should touch. This is to compensate for future contracture of the wound and thus, to produce a flat scar. It is difficult to evert the edges when they are too far apart or under tension, and excess skin tends to invert the skin edges, which is undesirable. If the pressure generated by the suture is greater than the closing pressure of the skin capillaries, the result will be local necrosis.
Before placing the suture, gently pinch the skin edges together. If the skin edges require pressure to approximate, consider undermining the skin edges, that is, cutting the fibrous septae that connect the skin to the underlying fascia so you can more easily pull the wound edges together. Undermining can be performed with a scalpel blade, scissors, or by bluntly using a hemostat. Use skin hooks or forceps to lift the wound edge. The safest level of undermining is in the fat, just below the dermal-fat junction. It usually takes 2 to 3 cm of undermining to free up 1 cm of tissue. Periodically check to see how much tissue has been released and undermine the minimum amount necessary.
Use the recommended size suture for the area of the body and remove sutures at recommended times (TABLE 2).7 In general, use the thinnest suture for the least amount of time possible. When giving the patient wound care instructions, emphasize the importance of having the sutures removed on time. Delay may cause local irritation and increased scarring. To prevent suture marks, consider earlier removal of a single suture that causes extra tension (such as a vertical mattress suture, which is described on page 186) within a line of simple sutures. Infection or patient factors such as age, presence of vascular or chronic disease, and nutritional status may influence healing times and suture removal times, so carefully assess wound healing; it may be necessary to remove sutures earlier or later than the recommended time.
Avoid dog ears. Dog ears (bunching of skin at one end of a wound closure) usually are the result of unequal amounts of opposing tissue. Causes range from ragged lacerations and flap procedures to uneven apportion of tissue when suturing.
If the difference in the lengths of the 2 sides of a wound is ≤15%, the halving technique (placing the first suture in the center of the wound, the next suture in the center of each remaining segment, and so on) works well to avoid dog ears. Otherwise, a Burow triangle repair—a procedure that slightly extends the wound but removes the excess tissue and results in a better cosmetic outcome—may be required.
If while in the process of repairing a relatively small wound you notice that dog ears occur as the result of poor technique, consider removing and redoing the sutures.
Deep suturing reduces tension, improves outcomes
To achieve the best possible cosmetic outcomes when closing a particularly deep wound, consider placing deep sutures. Because scars remodel for about a year post-repair, tension across the wound area may produce a wider and more unsightly scar as time goes by. Deep closure of a wound with dissolvable sutures can:15
• reduce or eliminate wound tension when suturing the epidermis
• close potential space below the skin
• stop subcutaneous bleeding
• reduce hematoma and seroma formation.
The technique for deep suture placement is shown in FIGURES 1A to 1D. The decreased tension in the healing scar that results from placing deep-buried sutures will reduce the final width of the scar. Buried dermal sutures do not increase the risk of infection in clean, uncontaminated lacerations. However, animal studies suggest that deep sutures should be avoided in highly contaminated wounds.16
For deep lacerations with potential space remaining, use vertical mattress sutures to approximate the wound edges.15 The vertical mattress suture technique is shown in FIGURES 2A to 2C. This technique incorporates a large amount of tissue within the passage of the suture loops and provides good tensile strength in closing wound edges over a distance or under tension. It also is used for wounds in locations where wound edges tend to invert, such as on the posterior neck, behind the ear, in the groin, in the inframammary crease, or on concave body surfaces. Early removal (half of the generally recommended days) of vertical mattress sutures can help prevent suture marks, especially if nearby simple interrupted sutures can remain in place for the recommended duration.
When to consider tape, staples, or adhesive
Don’t overlook wound closure tapes for superficial wounds. Even if tape is a poor candidate for the primary wound closure, it still may be used during the early stages of wound healing to support other closures by spreading tension over a larger area than just the suture area. Apply one strip at a time on one side of the wound, then pull the tape across, everting the edges of the wound. This can help eliminate the “railroad track” scars sometimes caused by tight sutures.
Often, the tip of a triangular flap will refuse to nudge up to the corner, even with a well-placed corner suture. Tape applied and then pulled over the tip can help approximate the tissue for this type of wound. An alternative use of tape is to strengthen fragile skin to allow for suturing. The tape can be applied to either side of a wound and the suture needle can be driven though the tape and skin so you can close as usual. To improve adhesion, use tincture of benzoin over the areas on which you want to apply tape.
When using staples for closure, be certain to approximate the tissue carefully. When possible, have an assistant approximate the edges. Hold the stapler with the center mark at the middle of the skin edges. Squeeze the handle completely while keeping the stapler still during application. Properly applied staples are much less painful to remove, which your patients will appreciate.
Using tissue adhesives can save time because the procedure is quick and does not require suture or staple removal. Clean, low-tension wounds on the face tend to do well with adhesives. When on the fence about whether to suture or use adhesive, choose suture. Any wound for which you would consider using adhesive should be small enough to suture in just a few minutes.
CORRESPONDENCE
Luke M. Baudoin, MD, Louisiana State University Health Sciences Center, 1501 Kings Highway, Shreveport, LA 71130; [email protected]
1. Forsch RT. Essentials of skin laceration repair. Am Fam Physician. 2008;78(8):945-951.
2. Ekmektzoglou KA, Zografos GC. A concomitant review of the effects of diabetes mellitus and hypothyroidism in wound healing. World J Gastroenterol. 2006;12:2721-2729.
3. Balin AK, Pratt L. Dilute povidone-iodine solutions inhibit human skin fibroblast growth. Dermatol Surg. 2002;28:210-214.
4. Lineaweaver W, McMorris S, Soucy D, et al. Cellular and bacterial toxicities of topical antimicrobials. Plast Reconstr Surg. 1985;75:394-396.
5. Fletcher J. Wound cleansing. Professional Nurse. 1997;12:793-797.
6. Krasner D. The 12 commandments of wound care. Nursing. 1992;22:34-41.
7. Mayeaux Jr EJ. The Essential Guide to Primary Care Procedures. Philadelphia, PA: Lippincott Williams & Wilkins; 2009.
8. Darouiche RO, Wall MJ Jr, Itani KM, et al. Chlorhexidine-alcohol versus povidone-iodine for surgical-site antisepsis. N Engl J Med. 2010;362:18-26.
9. Usatine RP, Smith MA, Mayeaux Jr EJ, et al, eds. The Color Atlas of Family Medicine. 2nd ed. New York, NY: McGraw-Hill Education; 2013.
10. Wolf DJ, Zitelli JA. Surgical margins for basal cell carcinoma. Arch Dermatol. 1987;123:340-344.
11. Gulleth Y, Goldberg N, Silverman RP, et al. What is the best surgical margin for a Basal cell carcinoma: a meta-analysis of the literature. Plast Reconstr Surg. 2010;126:1222-1231.
12. Thomas DJ, King AR, Peat BG. Excision margins for nonmelanotic skin cancer. Plast Reconstr Surg. 2003;112:57-63.
13. Huang CC, Boyce SM. Surgical margins of excision for basal cell carcinoma and squamous cell carcinoma. Semin Cutan Med Surg. 2004;23:167-173.
14. Bichakjian CK, Halpern AC, Johnson TM, et al; American Academy of Dermatology. Guidelines of care for the management of primary cutaneous melanoma. J Am Acad Dermatol. 2011;65:1032-1047.
15. Zuber TJ. The mattress sutures: vertical, horizontal, and corner stitch. Am Fam Physician. 2002;66:2231-2236.
16. Mehta, PH, Dunn, KA, Bradfield, JF, et al. Contaminated wounds: infection rates with subcutaneous sutures. Ann Emerg Med. 1996;27:43-48.
› If the skin edges of a wound require pressure to approximate, consider undermining the edges. C
› When removing a cutaneous neoplasm, avoid resecting excessive tissue. A
› For deep lacerations with potential “dead space,” use vertical mattress sutures to approximate the wound edges. C
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Skin procedures such as closing wounds and removing neoplasms are an integral part of most family medicine practices. While closure of simple lacerations and small surgical procedures are relatively straightforward, some lesions require extra techniques and attention to achieve the best outcomes.
This article describes some steps you can take to achieve the best outcomes for wound healing and cosmesis. Although suturing basics are important for successful wound closure, that information is not covered in this article. Forsch,1 however, provides an excellent discussion of basic wound skin preparation and suturing techniques.
Before beginning, visualize the ending
Before beginning any skin repair—whether it is a planned surgical procedure or a more urgent wound closure—review the patient’s medical record for conditions or medications that may adversely affect wound healing. Key in on patients with poorly controlled diabetes or those taking anticoagulants or antiplatelet drugs. Good diabetes control before surgery improves healing2 and drugs that promote bleeding sometimes may be temporarily discontinued before a procedure. If a patient’s diabetes is poorly controlled, an elective procedure can be postponed. If a patient is taking an anticoagulant and the international normalized ratio is within the therapeutic window, the procedure can be performed, but the physician must be ready to address bleeding by having electrical or chemical cautery available.
During your initial evaluation of the patient, assess and document the condition of the joints, muscles, tendons, and ligaments in the area in question before the procedure. Being aware of the local anatomy will help you avoid inadvertently damaging nerves, tendons, vessels, and other vital structures. Also, any time a procedure is beyond your comfort level, ask for help or refer the patient to a subspecialist. For example, if a lesion is on a patient’s neck near the carotid artery, the procedure is better left to an ear, nose, and throat specialist.
On the day of the procedure, plan how you will close the wound before you make the first cut, and include this information in your informed consent. Mentally review the anatomy of the area before starting a procedure to anticipate obstacles or structures that may be inadvertently injured. Visualize how the skin will fit together and how it will heal before making an incision. Reassure the patient that you will use techniques to minimize pain and scarring. Consider taking photos both before and after the procedure for documentation.
The best cosmetic results usually are achieved when the final closure line (and therefore the scar) lies parallel to the lines of least skin tension. When doing an excision, start choosing possible closures by considering layouts that produce this result. Eliminate from consideration any closures that would pull skin tension from areas that are immobile or that would cause cosmetic problems (such as a closure on or near the eyebrows or mouth, since this would change the contours of these structures). For such closures, a skin rearrangement flap may offer a cosmetically better outcome, especially on the face.
Tips for irrigating the wound and prepping the skin
If you are repairing a wound that is the result of an injury, you will need to clean the laceration gently but thoroughly. Avoid using products on open wounds that can damage regenerative tissue, such as organified iodines or hydrogen peroxide.3,4 Before irrigating a wound with normal saline, thoroughly examine it for foreign bodies, which may promote infection and impede healing. Consider giving local anesthesia before irrigation. Typically, irrigation pressure of approximately 8 pounds per square inch (psi) is considered ideal.5 Using irrigation pressures of <4 psi only moistens the wound, and >15 psi can damage the wound and drive bacteria deeper into tissue.6 Pressures of 8 to 11 psi can be achieved using a 18- to 19-gauge needle or angiocatheter with a 30 cc to 35 cc syringe.
Consider snipping off the tip of the cap and leaving it snugly on the needle. This will help prevent needle sticks and allow for thorough and safer irrigation. If commercial irrigation equipment is available, use it as directed by the manufacturer. Always be aware of potential splashback while irrigating and use appropriate personal protective equipment.
For wounds that result from a surgical procedure, employ appropriate site preparation and sterile technique. Iodophors provide broad-spectrum coverage, are not associated with microbial resistance, and provide a bacteriostatic effect as long as they remain on the skin. They require at least 2 minutes of contact to release free iodine, which exerts antibacterial activity.
Chlorhexidine gluconate offers broad-spectrum coverage against bacteria, yeast, and molds. It appears to provide greater reduction in skin microflora than povidone-iodine, and remains active for hours after application.7 Use it with caution around the eyes because of the risk for conjunctival irritation, keratitis, or corneal ulceration.8 Make sure to apply the prep solution to an area larger than the area exposed by the fenestrated drape because the drape may move around during the procedure, inadvertently contaminating the surgical field.
When excising a neoplasm, consider the margin
When performing a surgical procedure such as removing a cutaneous neoplasm, don’t resect excessive tissue, but do excise adequate margins. (For adequate margins of various cutaneous neoplasms, see TABLE 1.9-11) Mark the outlines of the visible tumor and the excision margin and show them to the patient, because some patients mistakenly assume that only the visible tumor will be removed.
Keep in mind when treating basal cell carcinomas (BCC) or squamous cell carcinomas (SCC) that the presence of high-risk characteristics may require larger surgical margins. The cure rates are lower for higher-risk BCCs, such as lesions that have morpheaform morphology or aggressive histologic features, or those that are ≥2 cm, recurrent, or located on the lips or paranasal or periocular regions.10-12
Cure rates for these higher-risk BCCs can be improved substantially by using intraoperative margin evaluation (frozen sections) or Mohs micrographic surgery. (For more on Mohs surgery, see “When to consider Mohs surgery,” J Fam Pract. 2013;62:558.)
Primary SCCs that are ≥2 cm in diameter, poorly differentiated on histology, in high-risk sites, or invading subcutaneous tissues require larger margins.13 The approach to a suspicious pigmented cutaneous neoplasm consists of an initial biopsy or excision with 1 to 2 mm margins and pathologic evaluation.14
Because hematoma formation inhibits wound healing, pay special attention to hemostasis when excising a neoplasm. Use pressure for minor bleeding. Use mosquito forceps to clamp a bleeder followed by ligation with an absorbable suture for larger bleeders. Other techniques for hemostasis include electrical or chemical cauterization and hemostatic solutions.
Positioning of edges is key to minimize scarring
When suturing a wound—whether it is from an injury or a procedure—take care to evert the skin edges; the underlying dermis from both edges should touch. This is to compensate for future contracture of the wound and thus, to produce a flat scar. It is difficult to evert the edges when they are too far apart or under tension, and excess skin tends to invert the skin edges, which is undesirable. If the pressure generated by the suture is greater than the closing pressure of the skin capillaries, the result will be local necrosis.
Before placing the suture, gently pinch the skin edges together. If the skin edges require pressure to approximate, consider undermining the skin edges, that is, cutting the fibrous septae that connect the skin to the underlying fascia so you can more easily pull the wound edges together. Undermining can be performed with a scalpel blade, scissors, or by bluntly using a hemostat. Use skin hooks or forceps to lift the wound edge. The safest level of undermining is in the fat, just below the dermal-fat junction. It usually takes 2 to 3 cm of undermining to free up 1 cm of tissue. Periodically check to see how much tissue has been released and undermine the minimum amount necessary.
Use the recommended size suture for the area of the body and remove sutures at recommended times (TABLE 2).7 In general, use the thinnest suture for the least amount of time possible. When giving the patient wound care instructions, emphasize the importance of having the sutures removed on time. Delay may cause local irritation and increased scarring. To prevent suture marks, consider earlier removal of a single suture that causes extra tension (such as a vertical mattress suture, which is described on page 186) within a line of simple sutures. Infection or patient factors such as age, presence of vascular or chronic disease, and nutritional status may influence healing times and suture removal times, so carefully assess wound healing; it may be necessary to remove sutures earlier or later than the recommended time.
Avoid dog ears. Dog ears (bunching of skin at one end of a wound closure) usually are the result of unequal amounts of opposing tissue. Causes range from ragged lacerations and flap procedures to uneven apportion of tissue when suturing.
If the difference in the lengths of the 2 sides of a wound is ≤15%, the halving technique (placing the first suture in the center of the wound, the next suture in the center of each remaining segment, and so on) works well to avoid dog ears. Otherwise, a Burow triangle repair—a procedure that slightly extends the wound but removes the excess tissue and results in a better cosmetic outcome—may be required.
If while in the process of repairing a relatively small wound you notice that dog ears occur as the result of poor technique, consider removing and redoing the sutures.
Deep suturing reduces tension, improves outcomes
To achieve the best possible cosmetic outcomes when closing a particularly deep wound, consider placing deep sutures. Because scars remodel for about a year post-repair, tension across the wound area may produce a wider and more unsightly scar as time goes by. Deep closure of a wound with dissolvable sutures can:15
• reduce or eliminate wound tension when suturing the epidermis
• close potential space below the skin
• stop subcutaneous bleeding
• reduce hematoma and seroma formation.
The technique for deep suture placement is shown in FIGURES 1A to 1D. The decreased tension in the healing scar that results from placing deep-buried sutures will reduce the final width of the scar. Buried dermal sutures do not increase the risk of infection in clean, uncontaminated lacerations. However, animal studies suggest that deep sutures should be avoided in highly contaminated wounds.16
For deep lacerations with potential space remaining, use vertical mattress sutures to approximate the wound edges.15 The vertical mattress suture technique is shown in FIGURES 2A to 2C. This technique incorporates a large amount of tissue within the passage of the suture loops and provides good tensile strength in closing wound edges over a distance or under tension. It also is used for wounds in locations where wound edges tend to invert, such as on the posterior neck, behind the ear, in the groin, in the inframammary crease, or on concave body surfaces. Early removal (half of the generally recommended days) of vertical mattress sutures can help prevent suture marks, especially if nearby simple interrupted sutures can remain in place for the recommended duration.
When to consider tape, staples, or adhesive
Don’t overlook wound closure tapes for superficial wounds. Even if tape is a poor candidate for the primary wound closure, it still may be used during the early stages of wound healing to support other closures by spreading tension over a larger area than just the suture area. Apply one strip at a time on one side of the wound, then pull the tape across, everting the edges of the wound. This can help eliminate the “railroad track” scars sometimes caused by tight sutures.
Often, the tip of a triangular flap will refuse to nudge up to the corner, even with a well-placed corner suture. Tape applied and then pulled over the tip can help approximate the tissue for this type of wound. An alternative use of tape is to strengthen fragile skin to allow for suturing. The tape can be applied to either side of a wound and the suture needle can be driven though the tape and skin so you can close as usual. To improve adhesion, use tincture of benzoin over the areas on which you want to apply tape.
When using staples for closure, be certain to approximate the tissue carefully. When possible, have an assistant approximate the edges. Hold the stapler with the center mark at the middle of the skin edges. Squeeze the handle completely while keeping the stapler still during application. Properly applied staples are much less painful to remove, which your patients will appreciate.
Using tissue adhesives can save time because the procedure is quick and does not require suture or staple removal. Clean, low-tension wounds on the face tend to do well with adhesives. When on the fence about whether to suture or use adhesive, choose suture. Any wound for which you would consider using adhesive should be small enough to suture in just a few minutes.
CORRESPONDENCE
Luke M. Baudoin, MD, Louisiana State University Health Sciences Center, 1501 Kings Highway, Shreveport, LA 71130; [email protected]
› If the skin edges of a wound require pressure to approximate, consider undermining the edges. C
› When removing a cutaneous neoplasm, avoid resecting excessive tissue. A
› For deep lacerations with potential “dead space,” use vertical mattress sutures to approximate the wound edges. C
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Skin procedures such as closing wounds and removing neoplasms are an integral part of most family medicine practices. While closure of simple lacerations and small surgical procedures are relatively straightforward, some lesions require extra techniques and attention to achieve the best outcomes.
This article describes some steps you can take to achieve the best outcomes for wound healing and cosmesis. Although suturing basics are important for successful wound closure, that information is not covered in this article. Forsch,1 however, provides an excellent discussion of basic wound skin preparation and suturing techniques.
Before beginning, visualize the ending
Before beginning any skin repair—whether it is a planned surgical procedure or a more urgent wound closure—review the patient’s medical record for conditions or medications that may adversely affect wound healing. Key in on patients with poorly controlled diabetes or those taking anticoagulants or antiplatelet drugs. Good diabetes control before surgery improves healing2 and drugs that promote bleeding sometimes may be temporarily discontinued before a procedure. If a patient’s diabetes is poorly controlled, an elective procedure can be postponed. If a patient is taking an anticoagulant and the international normalized ratio is within the therapeutic window, the procedure can be performed, but the physician must be ready to address bleeding by having electrical or chemical cautery available.
During your initial evaluation of the patient, assess and document the condition of the joints, muscles, tendons, and ligaments in the area in question before the procedure. Being aware of the local anatomy will help you avoid inadvertently damaging nerves, tendons, vessels, and other vital structures. Also, any time a procedure is beyond your comfort level, ask for help or refer the patient to a subspecialist. For example, if a lesion is on a patient’s neck near the carotid artery, the procedure is better left to an ear, nose, and throat specialist.
On the day of the procedure, plan how you will close the wound before you make the first cut, and include this information in your informed consent. Mentally review the anatomy of the area before starting a procedure to anticipate obstacles or structures that may be inadvertently injured. Visualize how the skin will fit together and how it will heal before making an incision. Reassure the patient that you will use techniques to minimize pain and scarring. Consider taking photos both before and after the procedure for documentation.
The best cosmetic results usually are achieved when the final closure line (and therefore the scar) lies parallel to the lines of least skin tension. When doing an excision, start choosing possible closures by considering layouts that produce this result. Eliminate from consideration any closures that would pull skin tension from areas that are immobile or that would cause cosmetic problems (such as a closure on or near the eyebrows or mouth, since this would change the contours of these structures). For such closures, a skin rearrangement flap may offer a cosmetically better outcome, especially on the face.
Tips for irrigating the wound and prepping the skin
If you are repairing a wound that is the result of an injury, you will need to clean the laceration gently but thoroughly. Avoid using products on open wounds that can damage regenerative tissue, such as organified iodines or hydrogen peroxide.3,4 Before irrigating a wound with normal saline, thoroughly examine it for foreign bodies, which may promote infection and impede healing. Consider giving local anesthesia before irrigation. Typically, irrigation pressure of approximately 8 pounds per square inch (psi) is considered ideal.5 Using irrigation pressures of <4 psi only moistens the wound, and >15 psi can damage the wound and drive bacteria deeper into tissue.6 Pressures of 8 to 11 psi can be achieved using a 18- to 19-gauge needle or angiocatheter with a 30 cc to 35 cc syringe.
Consider snipping off the tip of the cap and leaving it snugly on the needle. This will help prevent needle sticks and allow for thorough and safer irrigation. If commercial irrigation equipment is available, use it as directed by the manufacturer. Always be aware of potential splashback while irrigating and use appropriate personal protective equipment.
For wounds that result from a surgical procedure, employ appropriate site preparation and sterile technique. Iodophors provide broad-spectrum coverage, are not associated with microbial resistance, and provide a bacteriostatic effect as long as they remain on the skin. They require at least 2 minutes of contact to release free iodine, which exerts antibacterial activity.
Chlorhexidine gluconate offers broad-spectrum coverage against bacteria, yeast, and molds. It appears to provide greater reduction in skin microflora than povidone-iodine, and remains active for hours after application.7 Use it with caution around the eyes because of the risk for conjunctival irritation, keratitis, or corneal ulceration.8 Make sure to apply the prep solution to an area larger than the area exposed by the fenestrated drape because the drape may move around during the procedure, inadvertently contaminating the surgical field.
When excising a neoplasm, consider the margin
When performing a surgical procedure such as removing a cutaneous neoplasm, don’t resect excessive tissue, but do excise adequate margins. (For adequate margins of various cutaneous neoplasms, see TABLE 1.9-11) Mark the outlines of the visible tumor and the excision margin and show them to the patient, because some patients mistakenly assume that only the visible tumor will be removed.
Keep in mind when treating basal cell carcinomas (BCC) or squamous cell carcinomas (SCC) that the presence of high-risk characteristics may require larger surgical margins. The cure rates are lower for higher-risk BCCs, such as lesions that have morpheaform morphology or aggressive histologic features, or those that are ≥2 cm, recurrent, or located on the lips or paranasal or periocular regions.10-12
Cure rates for these higher-risk BCCs can be improved substantially by using intraoperative margin evaluation (frozen sections) or Mohs micrographic surgery. (For more on Mohs surgery, see “When to consider Mohs surgery,” J Fam Pract. 2013;62:558.)
Primary SCCs that are ≥2 cm in diameter, poorly differentiated on histology, in high-risk sites, or invading subcutaneous tissues require larger margins.13 The approach to a suspicious pigmented cutaneous neoplasm consists of an initial biopsy or excision with 1 to 2 mm margins and pathologic evaluation.14
Because hematoma formation inhibits wound healing, pay special attention to hemostasis when excising a neoplasm. Use pressure for minor bleeding. Use mosquito forceps to clamp a bleeder followed by ligation with an absorbable suture for larger bleeders. Other techniques for hemostasis include electrical or chemical cauterization and hemostatic solutions.
Positioning of edges is key to minimize scarring
When suturing a wound—whether it is from an injury or a procedure—take care to evert the skin edges; the underlying dermis from both edges should touch. This is to compensate for future contracture of the wound and thus, to produce a flat scar. It is difficult to evert the edges when they are too far apart or under tension, and excess skin tends to invert the skin edges, which is undesirable. If the pressure generated by the suture is greater than the closing pressure of the skin capillaries, the result will be local necrosis.
Before placing the suture, gently pinch the skin edges together. If the skin edges require pressure to approximate, consider undermining the skin edges, that is, cutting the fibrous septae that connect the skin to the underlying fascia so you can more easily pull the wound edges together. Undermining can be performed with a scalpel blade, scissors, or by bluntly using a hemostat. Use skin hooks or forceps to lift the wound edge. The safest level of undermining is in the fat, just below the dermal-fat junction. It usually takes 2 to 3 cm of undermining to free up 1 cm of tissue. Periodically check to see how much tissue has been released and undermine the minimum amount necessary.
Use the recommended size suture for the area of the body and remove sutures at recommended times (TABLE 2).7 In general, use the thinnest suture for the least amount of time possible. When giving the patient wound care instructions, emphasize the importance of having the sutures removed on time. Delay may cause local irritation and increased scarring. To prevent suture marks, consider earlier removal of a single suture that causes extra tension (such as a vertical mattress suture, which is described on page 186) within a line of simple sutures. Infection or patient factors such as age, presence of vascular or chronic disease, and nutritional status may influence healing times and suture removal times, so carefully assess wound healing; it may be necessary to remove sutures earlier or later than the recommended time.
Avoid dog ears. Dog ears (bunching of skin at one end of a wound closure) usually are the result of unequal amounts of opposing tissue. Causes range from ragged lacerations and flap procedures to uneven apportion of tissue when suturing.
If the difference in the lengths of the 2 sides of a wound is ≤15%, the halving technique (placing the first suture in the center of the wound, the next suture in the center of each remaining segment, and so on) works well to avoid dog ears. Otherwise, a Burow triangle repair—a procedure that slightly extends the wound but removes the excess tissue and results in a better cosmetic outcome—may be required.
If while in the process of repairing a relatively small wound you notice that dog ears occur as the result of poor technique, consider removing and redoing the sutures.
Deep suturing reduces tension, improves outcomes
To achieve the best possible cosmetic outcomes when closing a particularly deep wound, consider placing deep sutures. Because scars remodel for about a year post-repair, tension across the wound area may produce a wider and more unsightly scar as time goes by. Deep closure of a wound with dissolvable sutures can:15
• reduce or eliminate wound tension when suturing the epidermis
• close potential space below the skin
• stop subcutaneous bleeding
• reduce hematoma and seroma formation.
The technique for deep suture placement is shown in FIGURES 1A to 1D. The decreased tension in the healing scar that results from placing deep-buried sutures will reduce the final width of the scar. Buried dermal sutures do not increase the risk of infection in clean, uncontaminated lacerations. However, animal studies suggest that deep sutures should be avoided in highly contaminated wounds.16
For deep lacerations with potential space remaining, use vertical mattress sutures to approximate the wound edges.15 The vertical mattress suture technique is shown in FIGURES 2A to 2C. This technique incorporates a large amount of tissue within the passage of the suture loops and provides good tensile strength in closing wound edges over a distance or under tension. It also is used for wounds in locations where wound edges tend to invert, such as on the posterior neck, behind the ear, in the groin, in the inframammary crease, or on concave body surfaces. Early removal (half of the generally recommended days) of vertical mattress sutures can help prevent suture marks, especially if nearby simple interrupted sutures can remain in place for the recommended duration.
When to consider tape, staples, or adhesive
Don’t overlook wound closure tapes for superficial wounds. Even if tape is a poor candidate for the primary wound closure, it still may be used during the early stages of wound healing to support other closures by spreading tension over a larger area than just the suture area. Apply one strip at a time on one side of the wound, then pull the tape across, everting the edges of the wound. This can help eliminate the “railroad track” scars sometimes caused by tight sutures.
Often, the tip of a triangular flap will refuse to nudge up to the corner, even with a well-placed corner suture. Tape applied and then pulled over the tip can help approximate the tissue for this type of wound. An alternative use of tape is to strengthen fragile skin to allow for suturing. The tape can be applied to either side of a wound and the suture needle can be driven though the tape and skin so you can close as usual. To improve adhesion, use tincture of benzoin over the areas on which you want to apply tape.
When using staples for closure, be certain to approximate the tissue carefully. When possible, have an assistant approximate the edges. Hold the stapler with the center mark at the middle of the skin edges. Squeeze the handle completely while keeping the stapler still during application. Properly applied staples are much less painful to remove, which your patients will appreciate.
Using tissue adhesives can save time because the procedure is quick and does not require suture or staple removal. Clean, low-tension wounds on the face tend to do well with adhesives. When on the fence about whether to suture or use adhesive, choose suture. Any wound for which you would consider using adhesive should be small enough to suture in just a few minutes.
CORRESPONDENCE
Luke M. Baudoin, MD, Louisiana State University Health Sciences Center, 1501 Kings Highway, Shreveport, LA 71130; [email protected]
1. Forsch RT. Essentials of skin laceration repair. Am Fam Physician. 2008;78(8):945-951.
2. Ekmektzoglou KA, Zografos GC. A concomitant review of the effects of diabetes mellitus and hypothyroidism in wound healing. World J Gastroenterol. 2006;12:2721-2729.
3. Balin AK, Pratt L. Dilute povidone-iodine solutions inhibit human skin fibroblast growth. Dermatol Surg. 2002;28:210-214.
4. Lineaweaver W, McMorris S, Soucy D, et al. Cellular and bacterial toxicities of topical antimicrobials. Plast Reconstr Surg. 1985;75:394-396.
5. Fletcher J. Wound cleansing. Professional Nurse. 1997;12:793-797.
6. Krasner D. The 12 commandments of wound care. Nursing. 1992;22:34-41.
7. Mayeaux Jr EJ. The Essential Guide to Primary Care Procedures. Philadelphia, PA: Lippincott Williams & Wilkins; 2009.
8. Darouiche RO, Wall MJ Jr, Itani KM, et al. Chlorhexidine-alcohol versus povidone-iodine for surgical-site antisepsis. N Engl J Med. 2010;362:18-26.
9. Usatine RP, Smith MA, Mayeaux Jr EJ, et al, eds. The Color Atlas of Family Medicine. 2nd ed. New York, NY: McGraw-Hill Education; 2013.
10. Wolf DJ, Zitelli JA. Surgical margins for basal cell carcinoma. Arch Dermatol. 1987;123:340-344.
11. Gulleth Y, Goldberg N, Silverman RP, et al. What is the best surgical margin for a Basal cell carcinoma: a meta-analysis of the literature. Plast Reconstr Surg. 2010;126:1222-1231.
12. Thomas DJ, King AR, Peat BG. Excision margins for nonmelanotic skin cancer. Plast Reconstr Surg. 2003;112:57-63.
13. Huang CC, Boyce SM. Surgical margins of excision for basal cell carcinoma and squamous cell carcinoma. Semin Cutan Med Surg. 2004;23:167-173.
14. Bichakjian CK, Halpern AC, Johnson TM, et al; American Academy of Dermatology. Guidelines of care for the management of primary cutaneous melanoma. J Am Acad Dermatol. 2011;65:1032-1047.
15. Zuber TJ. The mattress sutures: vertical, horizontal, and corner stitch. Am Fam Physician. 2002;66:2231-2236.
16. Mehta, PH, Dunn, KA, Bradfield, JF, et al. Contaminated wounds: infection rates with subcutaneous sutures. Ann Emerg Med. 1996;27:43-48.
1. Forsch RT. Essentials of skin laceration repair. Am Fam Physician. 2008;78(8):945-951.
2. Ekmektzoglou KA, Zografos GC. A concomitant review of the effects of diabetes mellitus and hypothyroidism in wound healing. World J Gastroenterol. 2006;12:2721-2729.
3. Balin AK, Pratt L. Dilute povidone-iodine solutions inhibit human skin fibroblast growth. Dermatol Surg. 2002;28:210-214.
4. Lineaweaver W, McMorris S, Soucy D, et al. Cellular and bacterial toxicities of topical antimicrobials. Plast Reconstr Surg. 1985;75:394-396.
5. Fletcher J. Wound cleansing. Professional Nurse. 1997;12:793-797.
6. Krasner D. The 12 commandments of wound care. Nursing. 1992;22:34-41.
7. Mayeaux Jr EJ. The Essential Guide to Primary Care Procedures. Philadelphia, PA: Lippincott Williams & Wilkins; 2009.
8. Darouiche RO, Wall MJ Jr, Itani KM, et al. Chlorhexidine-alcohol versus povidone-iodine for surgical-site antisepsis. N Engl J Med. 2010;362:18-26.
9. Usatine RP, Smith MA, Mayeaux Jr EJ, et al, eds. The Color Atlas of Family Medicine. 2nd ed. New York, NY: McGraw-Hill Education; 2013.
10. Wolf DJ, Zitelli JA. Surgical margins for basal cell carcinoma. Arch Dermatol. 1987;123:340-344.
11. Gulleth Y, Goldberg N, Silverman RP, et al. What is the best surgical margin for a Basal cell carcinoma: a meta-analysis of the literature. Plast Reconstr Surg. 2010;126:1222-1231.
12. Thomas DJ, King AR, Peat BG. Excision margins for nonmelanotic skin cancer. Plast Reconstr Surg. 2003;112:57-63.
13. Huang CC, Boyce SM. Surgical margins of excision for basal cell carcinoma and squamous cell carcinoma. Semin Cutan Med Surg. 2004;23:167-173.
14. Bichakjian CK, Halpern AC, Johnson TM, et al; American Academy of Dermatology. Guidelines of care for the management of primary cutaneous melanoma. J Am Acad Dermatol. 2011;65:1032-1047.
15. Zuber TJ. The mattress sutures: vertical, horizontal, and corner stitch. Am Fam Physician. 2002;66:2231-2236.
16. Mehta, PH, Dunn, KA, Bradfield, JF, et al. Contaminated wounds: infection rates with subcutaneous sutures. Ann Emerg Med. 1996;27:43-48.
How to discuss sex with elderly patients
› Keep in mind that elderly patients may want to discuss matters of sexuality but can also be embarrassed, fearful, or reluctant to do so with a younger caregiver. C
› Consider making a patient’s sexual history part of your general health screening, perhaps using the PLISSIT model for facilitating discussion. C
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Sexuality is a central aspect of being human. It encompasses sex, gender identities and roles, sexual orientation, pleasure, eroticism, and intimacy, and is a major contributor to an individual’s quality of life and sense of wellbeing.1,2 Positive sexual relationships and behaviors are integral to maintaining good health and general well-being later in life, as well.2,3 Cynthia Graber, a reporter with Scientific American, reported that sex is a key reason retirees have a happy life.4
While there is a decline in sexual activity with age, a great number of men and women continue to engage in vaginal or anal intercourse, oral sex, and masturbation into the eighth and ninth decades of life.2,5 In a survey conducted among married men and women, about 90% of respondents between the ages of 60 and 64 and almost 30% of those older than age 80 said they were still sexually active.2 Another study reported that 62% of men and 30% of women 80 to 102 years of age were still sexually active.6 However, sexuality is rarely discussed with the elderly, and most physicians are unsure about how to handle such conversations.7
The baby boomer population is aging in the United States and elsewhere. By 2030, 20% of the US population will be ≥65 years old, and 4% (3 million) will be lesbian, gay, bisexual, transgender, and queer (LGBTQ) elderly adults.3,8 Given the impact of sex on maintaining quality of life, it is important for health care providers to be comfortable discussing sexuality with the elderly.9
Barriers to discussing sexuality
Physician barriers
Primary care physicians typically are the first point of contact for elderly adults experiencing health problems, including sexual dysfunction. According to the American Psychological Association, sex is not discussed enough with the elderly. Most physicians do not address sexual health proactively, and rarely do they include a sexual history as part of general health screening in the elderly.2,10,11 Inadequate training of physicians in sexual health is likely a contributing factor.5 Physicians also often feel discomfort when discussing such matters with patients of the opposite sex.12 (For a suggested approach to these conversations, see “Discussing sexuality with elderly patients: Getting beyond ‘don’t ask, don’t tell,” below.) With the increasing number of LGBTQ elderly adults, physicians should not assume their patients have any particular sexual behavior or orientation. This will help elderly LGBTQ patients feel more comfortable discussing their sexual health needs.8
The PLISSIT model, developed in 1976 by clinical psychologist Dr. Jack Annon, can facilitate a discussion of sexuality with elderly patients.11,13 First, the healthcare provider seeks permission (P) to discuss sexuality with the patient. After permission is given, the provider can share limited information (LI) about sexual issues that affect the older adult. Next, the provider may offer specific suggestions (SS) to improve sexual health or resolve problems. Finally, referral for intensive therapy (IT) may be needed for someone whose sexual dysfunction goes beyond the scope of the health care provider’s expertise. In 2000, open-ended questions were added to the PLISSIT model to more effectively guide an assessment of sexuality in older adults13,14:
• Can you tell me how you express your sexuality?
• What concerns or questions do you have about fulfilling your continuing sexual needs?
• In what ways has your sexual relationship with your partner changed as you have aged?
Many physicians have only a vague understanding of the sexual needs of the elderly, and some may even consider sexuality among elderly people a taboo.5 The reality is that elderly adults need to be touched, held, and feel loved, and this does not diminish with age.15-17 Unfortunately, many healthcare professionals have a mindset of, “I don’t want to think about my parents having sex, let alone my grandparents.” It is critical that physicians address intimacy needs as part of a medical assessment of the elderly.
Loss of physical and emotional intimacy is profound and often ignored as a source of suffering for the elderly. Most elderly patients want to discuss sexual issues with their physician, according to the Global Study of Sexual Attitudes among men and women ages 40 to 80 years.18 Surprisingly, even geriatricians often fail to take a sexual history of their patients. In one study, only 57% of 120 geriatricians surveyed routinely took a sexual history, even though 97% of them believed that patients with sexual problems should be managed further.1
Patient barriers
Even given a desire to discuss sexual concerns with their health care provider, elderly patients can be reluctant due to embarrassment or a fear of sexuality. Others may hesitate because their caregiver is younger than they or is of the opposite sex.19,20 The attitude of a medical professional has a powerful impact on the sexual attitudes and behaviors of elderly patients, and on their level of comfort in discussing sexual issues.21 Elderly patients do not usually complain to their physicians about sexual dysfunctions; 92% of men and 96% of women who reported at least one sexual problem in a survey had not sought help at all.18
Addressing issues in sexual dysfunction
Though sexual desires and needs may not decline with age, sexual function might, for any number of reasons.1,2,7 Many chronic diseases are known to interfere with sexual function (TABLE).2 Polypharmacy can lead to physical challenges, cognitive changes, and impaired sexual arousal, especially in men.3 However, the reason cited most often for absence of sexual activity is lack of a partner or a willing partner.2 Unfortunately as one ages, the chance of finding a partner diminishes. Hence the need to discuss alternative expressions of sexuality that may not require a partner.3 Many elderly individuals enjoy masturbation as a form of sexual expression.
Men and women have different sexual problems, but they are all treatable. For instance, with normal aging, levels of testosterone in men and estrogen in women decrease.5,15 Despite the number of sexual health dysfunctions, only 14% of men and 1% of women use medications to treat them.2,5 With men who have erectile dysfunction, discuss possible testosterone replacement or medication. For women with postmenopausal (atrophic) vaginitis, estrogen therapy or a lubricant (for those with contraindication to estrogen therapy) can improve sexual function. Anorgasmia and low libido are other concerns for postmenopausal women, and may warrant gynecologic referral.
For elderly adults moving into assisted living or a nursing home, the transition can signal the end of a sexual life.16,22 There is limited opportunity for men and women in residential settings to engage in sexual activity, in part due to a lack of privacy.23 The nursing home is still a home, and facility staff should provide opportunities for privacy and intimacy. In a study conducted in a residential setting, more than 25% of those ages 65 to 85 reported an active sex life, while 90% of those surveyed had sexual thoughts and fantasies.22 Of course, many elderly adults enter residential settings without a partner. They should be allowed to engage in sexual activities if they can understand, consent to, and form a relationship. Sexual needs remain even in those with dementia. But cognitive impairment frequently manifests as inappropriate sexual behavior. A study of cognitively impaired older adults revealed that 1.8% had displayed sexually inappropriate verbal or physical behavior.24 In these situations, a behavior medicine specialist can be of great help.
Health risks of sexual activity in the elderly
In 2011, the Centers for Disease Control and Prevention reported that 5% of new human immunodeficiency virus (HIV) cases occurred in those ≥55 years, and almost 2% of new diagnoses were in the those ≥65 years.25 Sexually active elderly individuals are at risk for acquiring HIV, in part because they do not consider themselves to be at risk for sexually transmitted diseases (STDs).26 They also might not have received education about the importance of condom use.11,26 In addition, prescribing erectile dysfunction medications for men and hormone replacement therapy for women might have played a part in increasing STDs among the elderly, particularly Chlamydia and HIV.27 The long-term effects of STDs left untreated can easily be mistaken for other symptoms or diseases of aging, which further underscores the importance of discussing sexuality with elderly patients.
CORRESPONDENCE
Folashade Omole, MD, FAAFP, 1513 East Cleveland Avenue, Building 100, Suite 300-A, East Point, GA 30344; [email protected]
1. Balami JS. Are geriatricians guilty of failure to take a sexual history? J Clin Gerontol Geriatr. 2011;2:17-20.
2. Lindau ST, Schumm LP, Laumann EO, et al. A study of sexuality and health among older adults in the United States. N Engl J Med. 2007;357:762-774.
3. Bradford A, Meston CM. Senior sexual health: The effects of aging on sexuality. In: VandeCreek L, Petersen FL, Bley JW, eds. Innovations in Clinical Practice: Focus on Sexual Health. Sarasota, FL: Professional Resource Press; 2007:35-45.
4. Graber C. Sex keeps elderly happier in marriage. Scientific American.
Available at: http://www.scientificamerican.com/podcast/episode/sex-keeps-elderly-happier-in-marria-11-11-29. Accessed March 26, 2014.
5. Hinchliff S, Gott M. Seeking medical help for sexual concerns in mid- and later life: a review of the literature. J Sex Res. 2011;48:106-117.
6. Tobin JM, Harindra V. Attendance by older patients at a genitourinary medicine clinic. Sex Transm Infect. 2001;77:289-291.
7. Bauer M, McAuliffe L, Nay R. Sexuality, health care and the older person: an overview of the literature. Int J Older People Nurs. 2007;2:63-68.
8. Wallace SP, Cochran SD, Durazo EM, et al. The health of aging lesbian, gay and bisexual adults in California. Policy Brief UCLA Cent Health Policy Res. 2011;(PB2011-2):1-8.
9. Henry J, McNab W. Forever young: a health promotion focus on sexuality and aging. Gerontol Geriatr Education. 2003;23:57-74.
10. Gott M, Hinchliff S, Galena E. General practitioner attitudes to discussing sexual health issues with older people. Soc Sci Med. 2004;58:2093-2103.
11. Nusbaum MR, Hamilton CD. The proactive sexual health history. Am Fam Physician. 2002;66:1705-1712.
12. Burd ID, Nevadunsky N, Bachmann G. Impact of physician gender on sexual history taking in a multispecialty practice. J Sex Med. 2006;3:194-200.
13. Kazer MW. Sexuality Assessment for Older Adults. Hartford Institute for Geriatric Nursing Web site. Available at: http://consultgerirn.org/uploads/File/trythis/try_this_10.pdf. Updated 2012. Accessed March 14, 2014.
14. Wallace MA. Assessment of sexual health in older adults. Am J Nursing. 2012;108:52-60.
15. Sexuality in later life. National Institute on Aging Web site. Available at: http://www.nia.nih.gov/health/publication/sexualitylater-life. Updated March 11, 2014. Accessed March 21, 2014.
16. Hajjar RR, Kamel HK. Sexuality in the nursing home, part 1: attitudes and barriers to sexual expression. J Am Med Dir Assoc. 2004;5(2 suppl):S42-S47.
17. Bildtgård T. The sexuality of elderly people on film—visual limitations. J Aging Identity. 2000;5:169-183.
18. Moreira ED Jr, Brock G, Glasser DB, et al; GSSAB Investigators’ Group. Help-seeking behaviour for sexual problems: the global study of sexual attitudes and behaviors. Int J Clin Pract. 2005;59:6-16.
19. Gott M, Hinchliff S. Barriers to seeking treatment for sexual problems in primary care: a qualitative study with older people. Fam Pract. 2003;20:690-695.
20. Politi MC, Clark MA, Armstrong G, et al. Patient-provider communication about sexual health among unmarried middle-aged and older women. J Gen Intern Med. 2009;24:511-516.
21. Bouman W, Arcelus J, Benbow S. Nottingham study of sexuality & ageing (NoSSA I). Attitudes regarding sexuality and older people: a review of the literature. Sex Relationship Ther. 2006;21:149-161.
22. Low LPL, Lui MHL, Lee DTF, et al. Promoting awareness of sexuality of older people in residential care. Electronic J Human Sexuality. 2005;8:8-16.
23. Rheaume C, Mitty E. Sexuality and intimacy in older adults. Geriatr Nurs. 2008;29:342-349.
24. Nagaratnam N, Gayagay G Jr. Hypersexuality in nursing care facilities—a descriptive study. Arch Gerontol Geriatr. 2002;35:195-203.
25. HIV among older Americans. Centers for Disease Control and Prevention Web site. Available at: http://www.cdc.gov/hiv/risk/age/olderamericans/. Updated December 23, 2013. Accessed February 28, 2014.
26. Nguyen N, Holodniy M. HIV infection in the elderly. Clin Interv Aging. 2008;3:453-472.
27. Jena AB, Goldman DP, Kamdar A, et al. Sexually transmitted diseases among users of erectile dysfunction drugs: analysis of claims data. Ann Intern Med. 2010;153:1-7.
› Keep in mind that elderly patients may want to discuss matters of sexuality but can also be embarrassed, fearful, or reluctant to do so with a younger caregiver. C
› Consider making a patient’s sexual history part of your general health screening, perhaps using the PLISSIT model for facilitating discussion. C
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Sexuality is a central aspect of being human. It encompasses sex, gender identities and roles, sexual orientation, pleasure, eroticism, and intimacy, and is a major contributor to an individual’s quality of life and sense of wellbeing.1,2 Positive sexual relationships and behaviors are integral to maintaining good health and general well-being later in life, as well.2,3 Cynthia Graber, a reporter with Scientific American, reported that sex is a key reason retirees have a happy life.4
While there is a decline in sexual activity with age, a great number of men and women continue to engage in vaginal or anal intercourse, oral sex, and masturbation into the eighth and ninth decades of life.2,5 In a survey conducted among married men and women, about 90% of respondents between the ages of 60 and 64 and almost 30% of those older than age 80 said they were still sexually active.2 Another study reported that 62% of men and 30% of women 80 to 102 years of age were still sexually active.6 However, sexuality is rarely discussed with the elderly, and most physicians are unsure about how to handle such conversations.7
The baby boomer population is aging in the United States and elsewhere. By 2030, 20% of the US population will be ≥65 years old, and 4% (3 million) will be lesbian, gay, bisexual, transgender, and queer (LGBTQ) elderly adults.3,8 Given the impact of sex on maintaining quality of life, it is important for health care providers to be comfortable discussing sexuality with the elderly.9
Barriers to discussing sexuality
Physician barriers
Primary care physicians typically are the first point of contact for elderly adults experiencing health problems, including sexual dysfunction. According to the American Psychological Association, sex is not discussed enough with the elderly. Most physicians do not address sexual health proactively, and rarely do they include a sexual history as part of general health screening in the elderly.2,10,11 Inadequate training of physicians in sexual health is likely a contributing factor.5 Physicians also often feel discomfort when discussing such matters with patients of the opposite sex.12 (For a suggested approach to these conversations, see “Discussing sexuality with elderly patients: Getting beyond ‘don’t ask, don’t tell,” below.) With the increasing number of LGBTQ elderly adults, physicians should not assume their patients have any particular sexual behavior or orientation. This will help elderly LGBTQ patients feel more comfortable discussing their sexual health needs.8
The PLISSIT model, developed in 1976 by clinical psychologist Dr. Jack Annon, can facilitate a discussion of sexuality with elderly patients.11,13 First, the healthcare provider seeks permission (P) to discuss sexuality with the patient. After permission is given, the provider can share limited information (LI) about sexual issues that affect the older adult. Next, the provider may offer specific suggestions (SS) to improve sexual health or resolve problems. Finally, referral for intensive therapy (IT) may be needed for someone whose sexual dysfunction goes beyond the scope of the health care provider’s expertise. In 2000, open-ended questions were added to the PLISSIT model to more effectively guide an assessment of sexuality in older adults13,14:
• Can you tell me how you express your sexuality?
• What concerns or questions do you have about fulfilling your continuing sexual needs?
• In what ways has your sexual relationship with your partner changed as you have aged?
Many physicians have only a vague understanding of the sexual needs of the elderly, and some may even consider sexuality among elderly people a taboo.5 The reality is that elderly adults need to be touched, held, and feel loved, and this does not diminish with age.15-17 Unfortunately, many healthcare professionals have a mindset of, “I don’t want to think about my parents having sex, let alone my grandparents.” It is critical that physicians address intimacy needs as part of a medical assessment of the elderly.
Loss of physical and emotional intimacy is profound and often ignored as a source of suffering for the elderly. Most elderly patients want to discuss sexual issues with their physician, according to the Global Study of Sexual Attitudes among men and women ages 40 to 80 years.18 Surprisingly, even geriatricians often fail to take a sexual history of their patients. In one study, only 57% of 120 geriatricians surveyed routinely took a sexual history, even though 97% of them believed that patients with sexual problems should be managed further.1
Patient barriers
Even given a desire to discuss sexual concerns with their health care provider, elderly patients can be reluctant due to embarrassment or a fear of sexuality. Others may hesitate because their caregiver is younger than they or is of the opposite sex.19,20 The attitude of a medical professional has a powerful impact on the sexual attitudes and behaviors of elderly patients, and on their level of comfort in discussing sexual issues.21 Elderly patients do not usually complain to their physicians about sexual dysfunctions; 92% of men and 96% of women who reported at least one sexual problem in a survey had not sought help at all.18
Addressing issues in sexual dysfunction
Though sexual desires and needs may not decline with age, sexual function might, for any number of reasons.1,2,7 Many chronic diseases are known to interfere with sexual function (TABLE).2 Polypharmacy can lead to physical challenges, cognitive changes, and impaired sexual arousal, especially in men.3 However, the reason cited most often for absence of sexual activity is lack of a partner or a willing partner.2 Unfortunately as one ages, the chance of finding a partner diminishes. Hence the need to discuss alternative expressions of sexuality that may not require a partner.3 Many elderly individuals enjoy masturbation as a form of sexual expression.
Men and women have different sexual problems, but they are all treatable. For instance, with normal aging, levels of testosterone in men and estrogen in women decrease.5,15 Despite the number of sexual health dysfunctions, only 14% of men and 1% of women use medications to treat them.2,5 With men who have erectile dysfunction, discuss possible testosterone replacement or medication. For women with postmenopausal (atrophic) vaginitis, estrogen therapy or a lubricant (for those with contraindication to estrogen therapy) can improve sexual function. Anorgasmia and low libido are other concerns for postmenopausal women, and may warrant gynecologic referral.
For elderly adults moving into assisted living or a nursing home, the transition can signal the end of a sexual life.16,22 There is limited opportunity for men and women in residential settings to engage in sexual activity, in part due to a lack of privacy.23 The nursing home is still a home, and facility staff should provide opportunities for privacy and intimacy. In a study conducted in a residential setting, more than 25% of those ages 65 to 85 reported an active sex life, while 90% of those surveyed had sexual thoughts and fantasies.22 Of course, many elderly adults enter residential settings without a partner. They should be allowed to engage in sexual activities if they can understand, consent to, and form a relationship. Sexual needs remain even in those with dementia. But cognitive impairment frequently manifests as inappropriate sexual behavior. A study of cognitively impaired older adults revealed that 1.8% had displayed sexually inappropriate verbal or physical behavior.24 In these situations, a behavior medicine specialist can be of great help.
Health risks of sexual activity in the elderly
In 2011, the Centers for Disease Control and Prevention reported that 5% of new human immunodeficiency virus (HIV) cases occurred in those ≥55 years, and almost 2% of new diagnoses were in the those ≥65 years.25 Sexually active elderly individuals are at risk for acquiring HIV, in part because they do not consider themselves to be at risk for sexually transmitted diseases (STDs).26 They also might not have received education about the importance of condom use.11,26 In addition, prescribing erectile dysfunction medications for men and hormone replacement therapy for women might have played a part in increasing STDs among the elderly, particularly Chlamydia and HIV.27 The long-term effects of STDs left untreated can easily be mistaken for other symptoms or diseases of aging, which further underscores the importance of discussing sexuality with elderly patients.
CORRESPONDENCE
Folashade Omole, MD, FAAFP, 1513 East Cleveland Avenue, Building 100, Suite 300-A, East Point, GA 30344; [email protected]
› Keep in mind that elderly patients may want to discuss matters of sexuality but can also be embarrassed, fearful, or reluctant to do so with a younger caregiver. C
› Consider making a patient’s sexual history part of your general health screening, perhaps using the PLISSIT model for facilitating discussion. C
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Sexuality is a central aspect of being human. It encompasses sex, gender identities and roles, sexual orientation, pleasure, eroticism, and intimacy, and is a major contributor to an individual’s quality of life and sense of wellbeing.1,2 Positive sexual relationships and behaviors are integral to maintaining good health and general well-being later in life, as well.2,3 Cynthia Graber, a reporter with Scientific American, reported that sex is a key reason retirees have a happy life.4
While there is a decline in sexual activity with age, a great number of men and women continue to engage in vaginal or anal intercourse, oral sex, and masturbation into the eighth and ninth decades of life.2,5 In a survey conducted among married men and women, about 90% of respondents between the ages of 60 and 64 and almost 30% of those older than age 80 said they were still sexually active.2 Another study reported that 62% of men and 30% of women 80 to 102 years of age were still sexually active.6 However, sexuality is rarely discussed with the elderly, and most physicians are unsure about how to handle such conversations.7
The baby boomer population is aging in the United States and elsewhere. By 2030, 20% of the US population will be ≥65 years old, and 4% (3 million) will be lesbian, gay, bisexual, transgender, and queer (LGBTQ) elderly adults.3,8 Given the impact of sex on maintaining quality of life, it is important for health care providers to be comfortable discussing sexuality with the elderly.9
Barriers to discussing sexuality
Physician barriers
Primary care physicians typically are the first point of contact for elderly adults experiencing health problems, including sexual dysfunction. According to the American Psychological Association, sex is not discussed enough with the elderly. Most physicians do not address sexual health proactively, and rarely do they include a sexual history as part of general health screening in the elderly.2,10,11 Inadequate training of physicians in sexual health is likely a contributing factor.5 Physicians also often feel discomfort when discussing such matters with patients of the opposite sex.12 (For a suggested approach to these conversations, see “Discussing sexuality with elderly patients: Getting beyond ‘don’t ask, don’t tell,” below.) With the increasing number of LGBTQ elderly adults, physicians should not assume their patients have any particular sexual behavior or orientation. This will help elderly LGBTQ patients feel more comfortable discussing their sexual health needs.8
The PLISSIT model, developed in 1976 by clinical psychologist Dr. Jack Annon, can facilitate a discussion of sexuality with elderly patients.11,13 First, the healthcare provider seeks permission (P) to discuss sexuality with the patient. After permission is given, the provider can share limited information (LI) about sexual issues that affect the older adult. Next, the provider may offer specific suggestions (SS) to improve sexual health or resolve problems. Finally, referral for intensive therapy (IT) may be needed for someone whose sexual dysfunction goes beyond the scope of the health care provider’s expertise. In 2000, open-ended questions were added to the PLISSIT model to more effectively guide an assessment of sexuality in older adults13,14:
• Can you tell me how you express your sexuality?
• What concerns or questions do you have about fulfilling your continuing sexual needs?
• In what ways has your sexual relationship with your partner changed as you have aged?
Many physicians have only a vague understanding of the sexual needs of the elderly, and some may even consider sexuality among elderly people a taboo.5 The reality is that elderly adults need to be touched, held, and feel loved, and this does not diminish with age.15-17 Unfortunately, many healthcare professionals have a mindset of, “I don’t want to think about my parents having sex, let alone my grandparents.” It is critical that physicians address intimacy needs as part of a medical assessment of the elderly.
Loss of physical and emotional intimacy is profound and often ignored as a source of suffering for the elderly. Most elderly patients want to discuss sexual issues with their physician, according to the Global Study of Sexual Attitudes among men and women ages 40 to 80 years.18 Surprisingly, even geriatricians often fail to take a sexual history of their patients. In one study, only 57% of 120 geriatricians surveyed routinely took a sexual history, even though 97% of them believed that patients with sexual problems should be managed further.1
Patient barriers
Even given a desire to discuss sexual concerns with their health care provider, elderly patients can be reluctant due to embarrassment or a fear of sexuality. Others may hesitate because their caregiver is younger than they or is of the opposite sex.19,20 The attitude of a medical professional has a powerful impact on the sexual attitudes and behaviors of elderly patients, and on their level of comfort in discussing sexual issues.21 Elderly patients do not usually complain to their physicians about sexual dysfunctions; 92% of men and 96% of women who reported at least one sexual problem in a survey had not sought help at all.18
Addressing issues in sexual dysfunction
Though sexual desires and needs may not decline with age, sexual function might, for any number of reasons.1,2,7 Many chronic diseases are known to interfere with sexual function (TABLE).2 Polypharmacy can lead to physical challenges, cognitive changes, and impaired sexual arousal, especially in men.3 However, the reason cited most often for absence of sexual activity is lack of a partner or a willing partner.2 Unfortunately as one ages, the chance of finding a partner diminishes. Hence the need to discuss alternative expressions of sexuality that may not require a partner.3 Many elderly individuals enjoy masturbation as a form of sexual expression.
Men and women have different sexual problems, but they are all treatable. For instance, with normal aging, levels of testosterone in men and estrogen in women decrease.5,15 Despite the number of sexual health dysfunctions, only 14% of men and 1% of women use medications to treat them.2,5 With men who have erectile dysfunction, discuss possible testosterone replacement or medication. For women with postmenopausal (atrophic) vaginitis, estrogen therapy or a lubricant (for those with contraindication to estrogen therapy) can improve sexual function. Anorgasmia and low libido are other concerns for postmenopausal women, and may warrant gynecologic referral.
For elderly adults moving into assisted living or a nursing home, the transition can signal the end of a sexual life.16,22 There is limited opportunity for men and women in residential settings to engage in sexual activity, in part due to a lack of privacy.23 The nursing home is still a home, and facility staff should provide opportunities for privacy and intimacy. In a study conducted in a residential setting, more than 25% of those ages 65 to 85 reported an active sex life, while 90% of those surveyed had sexual thoughts and fantasies.22 Of course, many elderly adults enter residential settings without a partner. They should be allowed to engage in sexual activities if they can understand, consent to, and form a relationship. Sexual needs remain even in those with dementia. But cognitive impairment frequently manifests as inappropriate sexual behavior. A study of cognitively impaired older adults revealed that 1.8% had displayed sexually inappropriate verbal or physical behavior.24 In these situations, a behavior medicine specialist can be of great help.
Health risks of sexual activity in the elderly
In 2011, the Centers for Disease Control and Prevention reported that 5% of new human immunodeficiency virus (HIV) cases occurred in those ≥55 years, and almost 2% of new diagnoses were in the those ≥65 years.25 Sexually active elderly individuals are at risk for acquiring HIV, in part because they do not consider themselves to be at risk for sexually transmitted diseases (STDs).26 They also might not have received education about the importance of condom use.11,26 In addition, prescribing erectile dysfunction medications for men and hormone replacement therapy for women might have played a part in increasing STDs among the elderly, particularly Chlamydia and HIV.27 The long-term effects of STDs left untreated can easily be mistaken for other symptoms or diseases of aging, which further underscores the importance of discussing sexuality with elderly patients.
CORRESPONDENCE
Folashade Omole, MD, FAAFP, 1513 East Cleveland Avenue, Building 100, Suite 300-A, East Point, GA 30344; [email protected]
1. Balami JS. Are geriatricians guilty of failure to take a sexual history? J Clin Gerontol Geriatr. 2011;2:17-20.
2. Lindau ST, Schumm LP, Laumann EO, et al. A study of sexuality and health among older adults in the United States. N Engl J Med. 2007;357:762-774.
3. Bradford A, Meston CM. Senior sexual health: The effects of aging on sexuality. In: VandeCreek L, Petersen FL, Bley JW, eds. Innovations in Clinical Practice: Focus on Sexual Health. Sarasota, FL: Professional Resource Press; 2007:35-45.
4. Graber C. Sex keeps elderly happier in marriage. Scientific American.
Available at: http://www.scientificamerican.com/podcast/episode/sex-keeps-elderly-happier-in-marria-11-11-29. Accessed March 26, 2014.
5. Hinchliff S, Gott M. Seeking medical help for sexual concerns in mid- and later life: a review of the literature. J Sex Res. 2011;48:106-117.
6. Tobin JM, Harindra V. Attendance by older patients at a genitourinary medicine clinic. Sex Transm Infect. 2001;77:289-291.
7. Bauer M, McAuliffe L, Nay R. Sexuality, health care and the older person: an overview of the literature. Int J Older People Nurs. 2007;2:63-68.
8. Wallace SP, Cochran SD, Durazo EM, et al. The health of aging lesbian, gay and bisexual adults in California. Policy Brief UCLA Cent Health Policy Res. 2011;(PB2011-2):1-8.
9. Henry J, McNab W. Forever young: a health promotion focus on sexuality and aging. Gerontol Geriatr Education. 2003;23:57-74.
10. Gott M, Hinchliff S, Galena E. General practitioner attitudes to discussing sexual health issues with older people. Soc Sci Med. 2004;58:2093-2103.
11. Nusbaum MR, Hamilton CD. The proactive sexual health history. Am Fam Physician. 2002;66:1705-1712.
12. Burd ID, Nevadunsky N, Bachmann G. Impact of physician gender on sexual history taking in a multispecialty practice. J Sex Med. 2006;3:194-200.
13. Kazer MW. Sexuality Assessment for Older Adults. Hartford Institute for Geriatric Nursing Web site. Available at: http://consultgerirn.org/uploads/File/trythis/try_this_10.pdf. Updated 2012. Accessed March 14, 2014.
14. Wallace MA. Assessment of sexual health in older adults. Am J Nursing. 2012;108:52-60.
15. Sexuality in later life. National Institute on Aging Web site. Available at: http://www.nia.nih.gov/health/publication/sexualitylater-life. Updated March 11, 2014. Accessed March 21, 2014.
16. Hajjar RR, Kamel HK. Sexuality in the nursing home, part 1: attitudes and barriers to sexual expression. J Am Med Dir Assoc. 2004;5(2 suppl):S42-S47.
17. Bildtgård T. The sexuality of elderly people on film—visual limitations. J Aging Identity. 2000;5:169-183.
18. Moreira ED Jr, Brock G, Glasser DB, et al; GSSAB Investigators’ Group. Help-seeking behaviour for sexual problems: the global study of sexual attitudes and behaviors. Int J Clin Pract. 2005;59:6-16.
19. Gott M, Hinchliff S. Barriers to seeking treatment for sexual problems in primary care: a qualitative study with older people. Fam Pract. 2003;20:690-695.
20. Politi MC, Clark MA, Armstrong G, et al. Patient-provider communication about sexual health among unmarried middle-aged and older women. J Gen Intern Med. 2009;24:511-516.
21. Bouman W, Arcelus J, Benbow S. Nottingham study of sexuality & ageing (NoSSA I). Attitudes regarding sexuality and older people: a review of the literature. Sex Relationship Ther. 2006;21:149-161.
22. Low LPL, Lui MHL, Lee DTF, et al. Promoting awareness of sexuality of older people in residential care. Electronic J Human Sexuality. 2005;8:8-16.
23. Rheaume C, Mitty E. Sexuality and intimacy in older adults. Geriatr Nurs. 2008;29:342-349.
24. Nagaratnam N, Gayagay G Jr. Hypersexuality in nursing care facilities—a descriptive study. Arch Gerontol Geriatr. 2002;35:195-203.
25. HIV among older Americans. Centers for Disease Control and Prevention Web site. Available at: http://www.cdc.gov/hiv/risk/age/olderamericans/. Updated December 23, 2013. Accessed February 28, 2014.
26. Nguyen N, Holodniy M. HIV infection in the elderly. Clin Interv Aging. 2008;3:453-472.
27. Jena AB, Goldman DP, Kamdar A, et al. Sexually transmitted diseases among users of erectile dysfunction drugs: analysis of claims data. Ann Intern Med. 2010;153:1-7.
1. Balami JS. Are geriatricians guilty of failure to take a sexual history? J Clin Gerontol Geriatr. 2011;2:17-20.
2. Lindau ST, Schumm LP, Laumann EO, et al. A study of sexuality and health among older adults in the United States. N Engl J Med. 2007;357:762-774.
3. Bradford A, Meston CM. Senior sexual health: The effects of aging on sexuality. In: VandeCreek L, Petersen FL, Bley JW, eds. Innovations in Clinical Practice: Focus on Sexual Health. Sarasota, FL: Professional Resource Press; 2007:35-45.
4. Graber C. Sex keeps elderly happier in marriage. Scientific American.
Available at: http://www.scientificamerican.com/podcast/episode/sex-keeps-elderly-happier-in-marria-11-11-29. Accessed March 26, 2014.
5. Hinchliff S, Gott M. Seeking medical help for sexual concerns in mid- and later life: a review of the literature. J Sex Res. 2011;48:106-117.
6. Tobin JM, Harindra V. Attendance by older patients at a genitourinary medicine clinic. Sex Transm Infect. 2001;77:289-291.
7. Bauer M, McAuliffe L, Nay R. Sexuality, health care and the older person: an overview of the literature. Int J Older People Nurs. 2007;2:63-68.
8. Wallace SP, Cochran SD, Durazo EM, et al. The health of aging lesbian, gay and bisexual adults in California. Policy Brief UCLA Cent Health Policy Res. 2011;(PB2011-2):1-8.
9. Henry J, McNab W. Forever young: a health promotion focus on sexuality and aging. Gerontol Geriatr Education. 2003;23:57-74.
10. Gott M, Hinchliff S, Galena E. General practitioner attitudes to discussing sexual health issues with older people. Soc Sci Med. 2004;58:2093-2103.
11. Nusbaum MR, Hamilton CD. The proactive sexual health history. Am Fam Physician. 2002;66:1705-1712.
12. Burd ID, Nevadunsky N, Bachmann G. Impact of physician gender on sexual history taking in a multispecialty practice. J Sex Med. 2006;3:194-200.
13. Kazer MW. Sexuality Assessment for Older Adults. Hartford Institute for Geriatric Nursing Web site. Available at: http://consultgerirn.org/uploads/File/trythis/try_this_10.pdf. Updated 2012. Accessed March 14, 2014.
14. Wallace MA. Assessment of sexual health in older adults. Am J Nursing. 2012;108:52-60.
15. Sexuality in later life. National Institute on Aging Web site. Available at: http://www.nia.nih.gov/health/publication/sexualitylater-life. Updated March 11, 2014. Accessed March 21, 2014.
16. Hajjar RR, Kamel HK. Sexuality in the nursing home, part 1: attitudes and barriers to sexual expression. J Am Med Dir Assoc. 2004;5(2 suppl):S42-S47.
17. Bildtgård T. The sexuality of elderly people on film—visual limitations. J Aging Identity. 2000;5:169-183.
18. Moreira ED Jr, Brock G, Glasser DB, et al; GSSAB Investigators’ Group. Help-seeking behaviour for sexual problems: the global study of sexual attitudes and behaviors. Int J Clin Pract. 2005;59:6-16.
19. Gott M, Hinchliff S. Barriers to seeking treatment for sexual problems in primary care: a qualitative study with older people. Fam Pract. 2003;20:690-695.
20. Politi MC, Clark MA, Armstrong G, et al. Patient-provider communication about sexual health among unmarried middle-aged and older women. J Gen Intern Med. 2009;24:511-516.
21. Bouman W, Arcelus J, Benbow S. Nottingham study of sexuality & ageing (NoSSA I). Attitudes regarding sexuality and older people: a review of the literature. Sex Relationship Ther. 2006;21:149-161.
22. Low LPL, Lui MHL, Lee DTF, et al. Promoting awareness of sexuality of older people in residential care. Electronic J Human Sexuality. 2005;8:8-16.
23. Rheaume C, Mitty E. Sexuality and intimacy in older adults. Geriatr Nurs. 2008;29:342-349.
24. Nagaratnam N, Gayagay G Jr. Hypersexuality in nursing care facilities—a descriptive study. Arch Gerontol Geriatr. 2002;35:195-203.
25. HIV among older Americans. Centers for Disease Control and Prevention Web site. Available at: http://www.cdc.gov/hiv/risk/age/olderamericans/. Updated December 23, 2013. Accessed February 28, 2014.
26. Nguyen N, Holodniy M. HIV infection in the elderly. Clin Interv Aging. 2008;3:453-472.
27. Jena AB, Goldman DP, Kamdar A, et al. Sexually transmitted diseases among users of erectile dysfunction drugs: analysis of claims data. Ann Intern Med. 2010;153:1-7.
Pitfalls & pearls for 8 common lab tests
› When interpreting hemoglobin A1c (HbA1c) levels, assess for anemia and other comorbidities that can significantly affect the lifespan of red blood cells and skew HbA1c test results. B
› Order nonfasting lipid panels for patients for whom fasting laboratory tests are difficult to obtain, as they have good clinical utility in screening and initial treatment. A
› Avoid routine thyroid-stimulating hormone (TSH) testing in asymptomatic adults; when testing is indicated, start with TSH. A
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Laboratory mistakes are not defined as diagnostic errors, but they contribute significantly to the thousands of medical errors that occur every year.1 Part of the problem: While accurate interpretation of lab tests often depends on the use of statistical concepts we all learned in medical training, it is difficult to find the time to incorporate these principles into a busy practice.
Overuse of lab tests presents problems, as well. Because “normal ranges” for test results are based on statistical analysis, as many as 5% of patients in a standard distribution fall outside of the range.2 It is important to order only the tests you really need, as extra testing automatically means more false positive results.
This article was written with such pitfalls in mind. In the pages that follow, we focus on 8 types of tests family physicians rely on regularly—all cases in which test results are reliable only if comorbidities, pre- and post-test probabilities, and clinical context are carefully considered. To help you put these lab tests into the proper context, we’ve addressed a key question—and highlighted both pitfalls and pearls—about each.
1. Hemoglobin A1c: How does anemia affect it?
Hemoglobin A1c (HbA1c) can be measured in many ways, including high-performance liquid chromatography, boronate affinity, capillary electrophoresis, and immunoassay, all of which can provide equivalent values without significant variability.3,4 In interpreting these tests, however, it is important to understand the effect that anemia has on HbA1c.
It's important to order only the tests you really need, as extra testing automatically means more false positive results.
Two primary variables influencing HbA1c are the average glucose level and the average lifespan of red blood cells (RBCs). Normally, there is a direct correlation between average serum glucose and HbA1c.4 In patients with anemia, however, this relationship is less clear, and may be affected by erythropoiesis and RBC destruction.5 In iron deficiency anemia (IDA),6,7 hemoglobin production falls secondary to iron stores, resulting in microcytic cells with a longer lifespan and elevated HbA1c. In at least one study,5,7 HbA1c approached levels associated with diabetes (with increases as high as 1.5%) in nondiabetic patients, but resolved with treatment of IDA.
Increased destruction as well as increased production of RBCs lowers their lifespan, and in turn decreases HbA1c levels (TABLE 1).4 This can be seen in conditions such as splenomegaly and hemoglobinopathies. In patients with hemoglobinopathies, the percentage of hemoglobin A is significantly decreased, often to undetectable levels—thereby making HbA1c tests inaccurate. Hemoglobin electrophoresis and determination of glycation by capillary electrophoresis or high-performance liquid chromatography can be used instead, but neither is practical because of cost and limited availability.4,8,9
THE TAKEAWAY: When you evaluate HbA1c test results, it is crucial to assess the patient for anemia and other conditions or comorbidities that can significantly affect RBC lifespan and skew test results.2,4-6
2. D-dimer: When should you use it?
D-dimer is a fibrin degradation product that is increased when active clotting is present,10 and its assay—which has high sensitivity and low specificity—is widely used to screen for pulmonary embolism (PE) and deep vein thrombosis (DVT). While the minimal number of false negatives makes the D-dimer a good screening test, the higher rate of false positives makes it difficult to arrive at a definitive diagnosis. Appropriate use of the D-dimer assay is crucial to minimize the potential for adverse consequences, such as bleeding in patients who are subjected to unnecessary anticoagulation because of false positive results.
Further testing typically follows. A positive D-dimer test is commonly followed by a computed tomography (CT) scan of the chest or a ventilation/perfusion scan to establish a PE or DVT diagnosis. But this subsequent testing increases both the cost of health care and the patient’s radiation exposure. Use of these subsequent scans can be reduced by first considering the patient’s pretest probability for PE or DVT. The Wells’ Criteria (available at www.mdcalc.com/wells-criteriafor-pulmonary-embolism-pe/) and Geneva Score (Revised) (www.mdcalc.com/genevascore-revised-for-pulmonary-embolism/) can both be used for this purpose.10,11
Patients with high pretest probability should undergo immediate scanning, foregoing the D-dimer—which should be reserved for patients who have a low or moderate pretest probability but sufficient reason to suspect PE or DVT.10-12
The low specificity of the D-dimer assay poses another challenge to its effective use. There are many things that can increase D-dimer levels, such as age, cancer, prolonged immobility, autoimmune disease, inflammation, sickle cell disease, pregnancy, trauma, and surgery.13-15 All these factors must be taken into consideration prior to ordering this test.
In fact, one recent study found that using an age-adjusted D-dimer cutoff (patient’s age in years x 10 mcg/L)—rather than a conventional cutoff of 500 mcg/L—for patients older than 50 years reduces false positives without substantially increasing false negatives.16
Also of note: An anticoagulant can decrease D-dimer levels in plasma, so the test should not be used to rule out PE or DVT in patients who are undergoing anticoagulation.13,15
THE TAKEAWAY: In evaluating patients for PE or DVT, use the Wells’ Criteria or Geneva Score (Revised) to determine a patient’s pretest probability of disease. Use the D-dimer assay to safely rule out these conditions in patients with a low or intermediate pretest probability, but go directly to scans for those with a high pretest probability.
3. Lipid panels: How important is fasting?
Patients are often instructed to report for fasting lab studies, specifically for lipid profiles. Traditionally, this had been defined as an 8- to 12-hour period without food.17 In clinical practice, however, this is often misinterpreted by patients, who may be confused about the duration of the fast or unsure about whether to eat or drink immediately before the test.
Studies investigating the effect of meals on lab values have found that triglycerides are consistently elevated postprandially, to a maximum of 12 hours.18-21 The effect of the fasting state on total cholesterol, low-density lipoprotein (LDL) cholesterol, and high-density lipoprotein (HDL) cholesterol is more controversial; while some postprandial differences have been detected, the clinical relevance is equivocal.18-21
Nonfasting lipid values can offer useful information, particularly in patients who are unwilling or unable to return for fasting labs. The US Preventive Services Task Force (USPSTF) supports this practice.22 Because guidelines for evaluation and treatment are based on fasting lipids, however, fasting lab work should be used, whenever possible, for initiating treatment and monitoring patients with abnormal values. If nonfasting lipids are used, it is crucial to factor in the postprandial effects on triglycerides and the subsequent difficulty of assessing LDL cholesterol levels.
THE TAKEAWAY: The clinical relevance of postprandial vs fasting lipid levels is equivocal. Nonfasting lipid panels have reasonable clinical utility in screening and initial treatment, particularly in cases in which obtaining fasting lab values may be problematic.18,19
4. Mononucleoosis spot test: When should you use it?
The monospot test is a latex assay that causes hemagglutination of horse RBCs in the presence of heterophile antibodies characteristic of infectious mononucleosis.23 The antibodies develop within the first 7 days of onset of symptoms, but do not peak for 2 to 5 weeks.24 As a result, monospot testing yields a high incidence of false negatives during the first 2 weeks of active infection.25 False negatives are also common in patients younger than 14 years. Heterophile antibodies may be present for up to a year after active infection.24
Patients at increased risk for splenic rupture, such as athletes, pose considerable diagnostic difficulty.26 When there is strong clinical suspicion of mononucleosis despite a negative monospot test in such high-risk individuals, follow-up testing is recommended to differentiate it from other mononucleosis-like illnesses (TABLE 2).27 The optimal combination of Epstein-Barr virus (EBV) serologic testing consists of the antibody titration of 4 markers: immunoglobulins M (IgM) and G (IgG) to the viral capsid antigen, IgM to the early antigen, and antibody to Epstein-Barr nuclear antigen (EBNA).28 Acute phase reactants in the setting of an antibody to EBNA could indicate reactivation. A positive test does not exclude other medical causes, however, because up to 20% of patients have acute phase antibodies that persist for years.29
Appropriate diagnosis is important because of the significant morbidity associated with EBV. Risk of splenic injury is greatest between 4 and 21 days after onset of symptoms but persists at 7 weeks,26 so conservative therapy followed by monospot retesting one week later is a reasonable approach.
Mononucleosis or routine tonsillitis? It is important to note that there is no evidence that a positive monospot test will affect the management or outcome of routine tonsillitis, raising questions of the utility of the test in such cases. A better approach: Reserve testing for patients with additional findings—ie, splenomegaly—or whose symptoms have persisted ≥ 2 weeks.
THE TAKEAWAY: Wait at least 2 weeks to conduct monospot testing in patients with routine tonsillitis. If strong clinical suspicion exists, proceed with specific IgM and IgG serologic testing.24,25,27,28
5. Evaluating prescription drug levels: Which factors interfere?
Correct interpretation of lab tests conducted to measure prescription drug levels has major implications with regard to patient safety, particularly for medications with a narrow therapeutic index.
Most drug level tests measure the total concentration, which includes both bound and unbound (free) forms. The unbound forms are the active components of the drug; thus, for an accurate evaluation, it is important to be aware of factors that increase free drug concentration. Chief among them is low protein levels, or hypoalbuminemia.30
Risk factors for hypoalbuminemia include significant burns, advanced age, pregnancy, malnutrition, and human immunodeficiency virus/acquired immune deficiency syndrome (HIV/AIDS).30 HIV/AIDS is a particularly high risk because certain protease inhibitors are highly protein bound.
Drug protein binding is classified as low, moderate, or high. The main proteins involved in the process are albumin, alpha-1-acid glycoprotein, and lipoprotein. Medications that are highly protein bound (>80%) are the most affected by low protein levels: Problems can arise when drugs completely bind to all the available proteins and excess drug availability increases free drug levels.
Medications that are most likely to be affected by a high degree of protein binding include carbamazepine, cyclosporine, mycophenolic acid, phenytoin, protease inhibitors (with the exception of indinavir), tacrolimus, and valproic acid. It is important to consider free levels when you order medication assays for these drugs to avoid misinterpreting the serum levels as being too low-a scenario that raises the risk of drug toxicity and adverse outcomes.30,31
A study of 119 phenytoin samples from 70 patients found significantly higher free phenytoin levels in patients with lower albumin levels.32 Higher free phenytoin levels were also seen in older patients and in those with diminished renal function (creatinine clearance <25 mL/min).32 The degree of protein binding is affected by both the serum drug concentration and the albumin level, with saturable protein binding occurring at higher drug levels.33
Calculate phenytoin levels with this equation. To calculate corrected phenytoin levels in patients with low albumin levels, use the following formula, known as the Sheiner-Tozer equation:34
Concentration adjusted=concentration reported/([adjustment x serum albumin] + 0.1); adjustment=0.2 for creatinine clearance ≥20 or 0.1 for creatinine clearance <20.
Additional causes of misinterpreted drug levels. While hypoalbuminemia plays a major role in the misinterpretation of drug levels, other factors affect serum drug concentration, as well. These include drug-drug interactions, which can significantly increase the concentration of the medications involved, and the timing of the test with regard to medication administration. Digoxin levels, in particular, need to be drawn at least 6 to 8 hours after the last dose is taken to allow for appropriate drug distribution.35
THE TAKEAWAY: It is essential to consider free drug level monitoring in patients who either have hypoalbuminemia or have one or more risk factors for hypoalbuminemia to avoid falsely low estimation of drug levels.36,37
6 Liver function tests: Necessary for patients on statin therapy?
Since statins gained US Food and Drug Administration (FDA) approval, the drugs have been associated with increased liver function tests (LFTs). Indeed, there had been a long-standing belief, based on clinical trials, that by monitoring alanine aminotransferase (ALT) and maintaining it at <3 times the upper limit normal (ULN), hepatotoxicity could be avoided.38 In clinical practice, however, further ALT elevation is frequently allowed based on patient tolerability.
In February 2012, the FDA revised its safety data to reflect this practice.39 The FDA update confirmed that routine LFT monitoring is unnecessary for patients on statins—and that it is not very effective in identifying or preventing liver damage.
Overall, serious hepatotoxicity is very rare, with an incidence ≤2 per 1 million patient-years.39 The National Lipid Association Statin Safety Assessment Task Force recommends repeating LFTs that are 3 to 5 times the ULN within 6 months and continuing with the statin dose if the patient is asymptomatic.38
THE TAKEAWAY: Routine liver function monitoring is not necessary for patients on statins. A better approach: Obtain baseline ALT levels, and repeat the testing only as clinically indicated thereafter.38,39
7. Urine drug screens: Which factors affect their accuracy?
The gold standard for testing for drugs of abuse, urine drug screens (UDS) have good sensitivity and specificity, easy administration, and reasonable cost.40 UDS can detect various narcotics, such as morphine, oxycodone, ,and methadone, and identify other illicit drugs, although which drugs and metabolites are tested for is laboratory- and test-specific.
Cross-reactivity. There are 6 currently available immunoassays, all of which use competitive binding between the sample drug and a drug chemically labeled with an enzyme, radioisotope, or fluorophore. The sample drug and labeled drug compete for substrate binding sites on drug-specific antibodies.41,42 Similar to competitive binding for enzymatic reactions in the body, the substrate binding site can experience cross-reactivity—causing substances other than the drug in question to bind to the immunoglobulin, leading to a false positive result (TABLE 3).43 Other factors that can alter the results include the cutoff value of the test and the absorption, distribution, metabolism, and excretion of the drug.42 Thus, a confirmatory test of gas chromatography-mass spectrometry is recommended before making decisions based on the results of UDS.43-45
Routine screens for patients on chronic opioid therapy. Routine use of UDS in emergency departments is no longer recommended, based on evidence that the results are unlikely to have a significant effect on patient management.46 For patients on chronic opioid therapy, however, routine screening has proven helpful in detecting prescription opioid abuse, illicit drug use, and diversion. Up to 34% of patients on prescription opioids have been found to be using illicit drugs, as well.42
THE TAKEAWAY: Use UDS as a tool in managing patients on chronic opioid therapy, but before acting on results, assess for factors, such as the use of oral or topical medications and the cutoff value of the test, that may be associated with false positive or false negative results.43-45
8. Thyroid function testing: When should you test?
Thyroid-stimulating hormone (TSH) is the first-line test when investigating presumed hyper- or hypothyroidism.47,48 Third-generation chemiluminometric assays can reliably measure TSH concentrations <0.01 mU/L by using multiple antibodies to produce a sandwich-type effect on the molecule in question.49
TSH levels exhibit diurnal variation, however, and are affected by other medications, including steroids, opiates, and some antihistamines, among others, as well as comorbidities.47,48 Chronic and acute conditions unrelated to thyroid disease can cause transient changes in TSH concentrations, and have the potential to modify the binding capacity of plasma thyroid hormone binding proteins.48 Thus, TSH should be ordered for hospitalized patients only when clinical suspicion of a thyroid problem exists.48 The USPSTF recommends against routine TSH screening for asymptomatic adults.46
How to respond to abnormal results. For patients found to have abnormal TSH levels, free T4 (fT4) is the next test to order.47,49 An fT4 assay is a superior indicator of thyroid status because it is not affected by changes in iodothyronine-binding proteins, which influence total hormone measurements.49 The results will be elevated in hyperthyroidism and reduced in hypothyroidism.47
Triiodothyronine (T3) measures can be useful in diagnosing Graves’ disease, in which T3 toxicosis may be the initial symptom—or an indication of a relapse. Because T3 is often a peripheral product, however, nonthyroid illnesses and medications can cause artifactually abnormal results.49
Other thyroid-specific labs include thyroid ,antibodies such as antithyroid peroxidase, antithyroglobulin, and TSH receptor, both blocking and stimulating.49 Thyroglobulin is a precursor form of thyroid hormone and should be measured when factitious hyperthyroidism is suspected. Management of hyper- and hypothyroidism often is independent of etiology. Retesting TSH to assess treatment response should be postponed until ≥2 months after any change in medication or dosing.50
Thyroid studies can be very difficult to interpret. TSH should be the first test ordered. However, if TSH values do not match the clinical picture, fT4, T3, and other thyroid tests that are less affected by outside factors can be useful in identifying the cause.
THE TAKEAWAY: Routine TSH testing is not indicated for asymptomatic adults. When evaluating thyroid function is clinically indicated, TSH is the initial test of choice.47,48,51
CORRESPONDENCE
Joshua Tessier, DO, Iowa Lutheran Family Medicine Residency, 840 East University Avenue, Des Moines, IA 50316; [email protected]
1. Ottomano C. Errors in medicine and errors in laboratory medicine: what is the difference? Blood Transfus. 2010;8;79-81.
2. Wallach JB. Introduction to normal values (reference ranges). Interpretation of Diagnostic Tests. 8th ed. Philadelphia, PA: Lippincott Williams & Wilkins; 2007:3-7.
3. Halwachs-Baumann G, Katzensteiner S, Schnedl W, et al. Comparative evaluation of three assay systems for automated determination of hemoglobin A1c. Clin Chem. 1997;43:511-517.
4. Gallagher EJ, Le Roith D, Bloomgarden Z. Review of hemoglobin A(1c) in the management of diabetes. J Diabetes. 2009;1:9-17.
5. Franco RS. The measurement and importance of red cell survival. Am J Hematol. 2009;84:109-114.
6. Tarim O, Küçükerdog˘an A, Gunay U, et al. Effects of iron deficiency anemia on hemoglobin A1c in type 1 diabetes mellitus. Pediatr Int. 1999;41:357-362.
7. Kim C, Bullard KM, Herman WH, et al. Association between iron deficiency and A1C levels among adults without diabetes in the National Health and Nutrition Examination Survey, 1999-2006. Diabetes Care. 2010;33:780-785.
8. Higgins T, Stewart D, Boehr E. Challenges in HbA1c analysis and reporting: an interesting case illustrating the many pitfalls. Clin Biochem. 2008;41:1104-1106.
9. Mongia SK, Little RR, Rohlfing CL, et al. Effects of hemoglobin C and S traits on the results of 14 commercial glycated hemoglobin assays. Am J Clin Pathol. 2008;130:136-140.
10. Brown MD, Rowe BH, Reeves MJ, et al. The accuracy of the enzyme-linked immunosorbent assay D-dimer test in the diagnosis of pulmonary embolism: a meta-analysis. Ann Emerg Med. 2002;40:133-144.
11. Squizzato A, Ageno W. What is the next step in D-dimer research? Education of physicians. Intern Emerg Med. 2006;1:165.
12. Kabrhel C, Mark Courtney D, Camargo CA Jr, et al. Potential impact of adjusting the threshold of the quantitative D-dimer based on pretest probability of acute pulmonary embolism. Acad Emerg Med. 2009;16:325-332.
13. Kabrhel C, Mark Courtney MD, Camargo CA Jr, et al. Factors associated with positive D-dimer results in patients evaluated for pulmonary embolism. Acad Emerg Med. 2010;17:589-597.
14. Berman AR. Pulmonary embolism in the elderly. Clin Geriatr Med. 2001;17:107-130.
15. Bruinstroop E, van de Ree MA, Huisman MV. The use of Ddimer in specific clinical conditions: a narrative review. Eur J Intern Med. 2009;20:441-446.
16. Schouten HJ, Geersing GI, Koek HL, et al. Diagnostic accuracy ,of conventional or age adjusted D-dimer cut-off values in older patients with suspected venous thromboembolism: systematic review and meta-analysis. BMJ. 2013;346:f2492.
17. Turgeon ML. Linne & Ringsrud’s Clinical Laboratory Science. 5th ed. Saint Louis, MO: Mosby; 2007:50.
18. Cohn JS,McNamara JR, Schaefer EJ. Lipoprotein cholesterol concentrations in the plasma of human subjects as measured in the fed and fasted states. Clin Chem. 1988;34:2456-2459.
19. Watts GF, Cohn JS. Whither the lipid profile: feast, famine, or no free lunch? Clin Chem. 2011;57:363-365.
20. Mora S, Rifai N, Buring JE, et al. Fasting compared with nonfasting lipids and apolipoproteins for predicting incident cardiovascular events. Circulation. 2008;118:993-1001.
21. Nordestgaard BG, Langsted A, Freiberg JJ. Nonfasting hyperlipidemia and cardiovascular disease. Curr Drug Targets. 2009;10:328-335.
22. Screening for lipid disorders in adults: US Preventive Services Task Force Recommendation statement. US Preventive Services Task Force Web site. Available at: http://www.uspreventiveservicestaskforce.org/uspstf08/lipid/lipidrs.htm. Accessed March 13, 2014.
23. Wolf DM, Friedrichs I, Toma AG. Lymphocyte-white blood cell count ratio: a quickly available screening tool to differentiate acute purulent tonsillitis from glandular fever. Arch Otolaryngol Head Neck Surg. 2007;133:61-64.
24. McCormack R, O’Shea T. The uptake and use of the Monospot test in patients with tonsillitis. Ir Med J. 2009;102:226-228.
25. Ebell MH. Epstein-Barr virus infectious mononucleosis. Am Fam Physician. 2004;70:1279-1287.
26. Waninger KN, Harcke HT. Determination of safe return to play for athletes recovering from infectious mononucleosis: a review of the literature. Clin J Sport Med. 2005;15:410-416.
27. Hurt C, Tammaro D. Diagnostic evaluation of mononucleosislike illnesses. Am J Med. 2007;120:911.e1-911.e8.
28. Vouloumanou EK, Rafailidis PI, Falagas ME. Current diagnosis and management of infectious mononucleosis. Curr Opin Hematol. 2012;19:14-20.
29. Epstein-Barr virus and infectious mononucleosis. Centers for Disease Control and Prevention Web site. Available at: http://www.cdc.gov/epstein-barr/laboratory-testing.html. Updated January 7, 2014. Accessed March 12, 2014.
30. Dasgupta A. Clinical utility of free drug monitoring. Clin Chem Lab Med. 2002;40:986-993.
31. Dasgupta A. Usefulness of monitoring free (unbound) concentrations of therapeutic drugs in patient management. Clin Chim Acta. 2007;377:1-13.
32. Iwamoto T, Kagawa Y, Naito Y, et al. Clinical evaluation of plasma free phenytoin measurement and factors influencing its protein binding. Biopharm Drug Dispos. 2006;27:77-84.
33. Hermida J, Tutor JC. A theoretical method for normalizing total serum valproic acid concentration in hypoalbuminemic patients. J Pharmacol Sci. 2005;97:489-493.
34. Wolf GK, McClain CD, Zurakowski D, et al. Total phenytoin concentrations do not accurately predict free phenytoin concentrations in critically ill children. Pediatr Crit Care Med. 2006;7:434-439; quiz 440.
35. Lanoxin (digoxin) [package insert]. Research Triangle Park, NC: GlaxoSmithKline; 2011.
36. National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III). Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) final report. Circulation. 2002;106:3143-3421.
37. De Backer G, Ambrosini E, Borch-Johnsen K, et al; Third Joint Force of European and other Societies on Cardiovascular Disease and Prevention in Clinical Practice. European guidelines on cardiovascular disease and prevention in clinical practice. Atherosclerosis. 2003;171:145-155.
38. McKenney JM, Davidson MH, Jacobson TA, et al. Final conclusions and recommendations of the National Lipid Association Statin Safety Assessment Task Force. Am J Cardiol. 2006;97:89C-94C.
39. FDA drug safety communication: important safety label changes to cholesterol-lowering statin drugs. US Food and Drug Administration Web site. Available at: http://www.fda.gov/Drugs/DrugSafety/ucm293101.htm. Updated July 3, 2012. Accessed May 17, 2013.
40. Eskridge KD, Guthrie SK. Clinical issues associated with urine testing of substances of abuse. Pharmacotherapy. 1997;17:497-510.
41. Herring C, Muzyk AJ, Johnston C. Interferences with urine drug screens. J Pharm Pract. 2001;24:102-108.
42. Jaffee WB, Trucco E, Teter C, et al. Focus on alcohol & drug abuse: ensuring validity in urine drug testing. Psychiatr Serv. 2008;59:140-142.
43. Brahm NC, Yeager LL, Fox MD, et al. Commonly prescribed medications and potential false-positive urine drug screens. Am J Health Syst Pharm. 2010;67:1344-1350.
44. Moeller KE, Lee KC, Kissack JC. Urine drug screening: Practical guide for clinicians. Mayo Clin Proc. 2008;83:66-76.
45. Christo PJ, Manchikanti L, Ruan X, et al. Urine drug testing in chronic pain. Pain Physician. 2011;14:123-143.
46. Tenenbein M. Do you really need that emergency drug screen? Clin Toxicol (Phila). 2009;47:286-291.
47. Gaitonde DY, Rowley KD, Sweeney LB. Hypothyroidism: an update. Am Fam Physician. 2012;86:244-251.
48. UK guidelines for the use of thyroid function tests. British Thyroid Association Web site. Available at: http://www.british-thyroid-association.org/info-for-patients/Docs/TFT_guideline_final_version_July_2006.pdf. Accessed March 11, 2014.
49. Volpé, R. Rational use of thyroid function tests. Crit Rev Clin Lab Sci. 1997;34:405-438.
50. Graber JR, Cobin RH, Gharib H, et al; American Association of Clinical Endocrinologists and American Thyroid Association Taskforce on Hypothyroidism in Adults. Clinical practice guidelines for hypothyroidism in adults: cosponsored by the American Association of Clinical Endocrinologists and the American Thyroid Association. Endocr Pract. 2012;18:988-1028.
51. Helfand M; US Preventive Services Task Force. Screening for subclinical thyroid dysfunction in nonpregnant adults: a summary of the evidence for the US Preventive Services Task Force. Ann Intern Med. 2004;140:128-141.
› When interpreting hemoglobin A1c (HbA1c) levels, assess for anemia and other comorbidities that can significantly affect the lifespan of red blood cells and skew HbA1c test results. B
› Order nonfasting lipid panels for patients for whom fasting laboratory tests are difficult to obtain, as they have good clinical utility in screening and initial treatment. A
› Avoid routine thyroid-stimulating hormone (TSH) testing in asymptomatic adults; when testing is indicated, start with TSH. A
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Laboratory mistakes are not defined as diagnostic errors, but they contribute significantly to the thousands of medical errors that occur every year.1 Part of the problem: While accurate interpretation of lab tests often depends on the use of statistical concepts we all learned in medical training, it is difficult to find the time to incorporate these principles into a busy practice.
Overuse of lab tests presents problems, as well. Because “normal ranges” for test results are based on statistical analysis, as many as 5% of patients in a standard distribution fall outside of the range.2 It is important to order only the tests you really need, as extra testing automatically means more false positive results.
This article was written with such pitfalls in mind. In the pages that follow, we focus on 8 types of tests family physicians rely on regularly—all cases in which test results are reliable only if comorbidities, pre- and post-test probabilities, and clinical context are carefully considered. To help you put these lab tests into the proper context, we’ve addressed a key question—and highlighted both pitfalls and pearls—about each.
1. Hemoglobin A1c: How does anemia affect it?
Hemoglobin A1c (HbA1c) can be measured in many ways, including high-performance liquid chromatography, boronate affinity, capillary electrophoresis, and immunoassay, all of which can provide equivalent values without significant variability.3,4 In interpreting these tests, however, it is important to understand the effect that anemia has on HbA1c.
It's important to order only the tests you really need, as extra testing automatically means more false positive results.
Two primary variables influencing HbA1c are the average glucose level and the average lifespan of red blood cells (RBCs). Normally, there is a direct correlation between average serum glucose and HbA1c.4 In patients with anemia, however, this relationship is less clear, and may be affected by erythropoiesis and RBC destruction.5 In iron deficiency anemia (IDA),6,7 hemoglobin production falls secondary to iron stores, resulting in microcytic cells with a longer lifespan and elevated HbA1c. In at least one study,5,7 HbA1c approached levels associated with diabetes (with increases as high as 1.5%) in nondiabetic patients, but resolved with treatment of IDA.
Increased destruction as well as increased production of RBCs lowers their lifespan, and in turn decreases HbA1c levels (TABLE 1).4 This can be seen in conditions such as splenomegaly and hemoglobinopathies. In patients with hemoglobinopathies, the percentage of hemoglobin A is significantly decreased, often to undetectable levels—thereby making HbA1c tests inaccurate. Hemoglobin electrophoresis and determination of glycation by capillary electrophoresis or high-performance liquid chromatography can be used instead, but neither is practical because of cost and limited availability.4,8,9
THE TAKEAWAY: When you evaluate HbA1c test results, it is crucial to assess the patient for anemia and other conditions or comorbidities that can significantly affect RBC lifespan and skew test results.2,4-6
2. D-dimer: When should you use it?
D-dimer is a fibrin degradation product that is increased when active clotting is present,10 and its assay—which has high sensitivity and low specificity—is widely used to screen for pulmonary embolism (PE) and deep vein thrombosis (DVT). While the minimal number of false negatives makes the D-dimer a good screening test, the higher rate of false positives makes it difficult to arrive at a definitive diagnosis. Appropriate use of the D-dimer assay is crucial to minimize the potential for adverse consequences, such as bleeding in patients who are subjected to unnecessary anticoagulation because of false positive results.
Further testing typically follows. A positive D-dimer test is commonly followed by a computed tomography (CT) scan of the chest or a ventilation/perfusion scan to establish a PE or DVT diagnosis. But this subsequent testing increases both the cost of health care and the patient’s radiation exposure. Use of these subsequent scans can be reduced by first considering the patient’s pretest probability for PE or DVT. The Wells’ Criteria (available at www.mdcalc.com/wells-criteriafor-pulmonary-embolism-pe/) and Geneva Score (Revised) (www.mdcalc.com/genevascore-revised-for-pulmonary-embolism/) can both be used for this purpose.10,11
Patients with high pretest probability should undergo immediate scanning, foregoing the D-dimer—which should be reserved for patients who have a low or moderate pretest probability but sufficient reason to suspect PE or DVT.10-12
The low specificity of the D-dimer assay poses another challenge to its effective use. There are many things that can increase D-dimer levels, such as age, cancer, prolonged immobility, autoimmune disease, inflammation, sickle cell disease, pregnancy, trauma, and surgery.13-15 All these factors must be taken into consideration prior to ordering this test.
In fact, one recent study found that using an age-adjusted D-dimer cutoff (patient’s age in years x 10 mcg/L)—rather than a conventional cutoff of 500 mcg/L—for patients older than 50 years reduces false positives without substantially increasing false negatives.16
Also of note: An anticoagulant can decrease D-dimer levels in plasma, so the test should not be used to rule out PE or DVT in patients who are undergoing anticoagulation.13,15
THE TAKEAWAY: In evaluating patients for PE or DVT, use the Wells’ Criteria or Geneva Score (Revised) to determine a patient’s pretest probability of disease. Use the D-dimer assay to safely rule out these conditions in patients with a low or intermediate pretest probability, but go directly to scans for those with a high pretest probability.
3. Lipid panels: How important is fasting?
Patients are often instructed to report for fasting lab studies, specifically for lipid profiles. Traditionally, this had been defined as an 8- to 12-hour period without food.17 In clinical practice, however, this is often misinterpreted by patients, who may be confused about the duration of the fast or unsure about whether to eat or drink immediately before the test.
Studies investigating the effect of meals on lab values have found that triglycerides are consistently elevated postprandially, to a maximum of 12 hours.18-21 The effect of the fasting state on total cholesterol, low-density lipoprotein (LDL) cholesterol, and high-density lipoprotein (HDL) cholesterol is more controversial; while some postprandial differences have been detected, the clinical relevance is equivocal.18-21
Nonfasting lipid values can offer useful information, particularly in patients who are unwilling or unable to return for fasting labs. The US Preventive Services Task Force (USPSTF) supports this practice.22 Because guidelines for evaluation and treatment are based on fasting lipids, however, fasting lab work should be used, whenever possible, for initiating treatment and monitoring patients with abnormal values. If nonfasting lipids are used, it is crucial to factor in the postprandial effects on triglycerides and the subsequent difficulty of assessing LDL cholesterol levels.
THE TAKEAWAY: The clinical relevance of postprandial vs fasting lipid levels is equivocal. Nonfasting lipid panels have reasonable clinical utility in screening and initial treatment, particularly in cases in which obtaining fasting lab values may be problematic.18,19
4. Mononucleoosis spot test: When should you use it?
The monospot test is a latex assay that causes hemagglutination of horse RBCs in the presence of heterophile antibodies characteristic of infectious mononucleosis.23 The antibodies develop within the first 7 days of onset of symptoms, but do not peak for 2 to 5 weeks.24 As a result, monospot testing yields a high incidence of false negatives during the first 2 weeks of active infection.25 False negatives are also common in patients younger than 14 years. Heterophile antibodies may be present for up to a year after active infection.24
Patients at increased risk for splenic rupture, such as athletes, pose considerable diagnostic difficulty.26 When there is strong clinical suspicion of mononucleosis despite a negative monospot test in such high-risk individuals, follow-up testing is recommended to differentiate it from other mononucleosis-like illnesses (TABLE 2).27 The optimal combination of Epstein-Barr virus (EBV) serologic testing consists of the antibody titration of 4 markers: immunoglobulins M (IgM) and G (IgG) to the viral capsid antigen, IgM to the early antigen, and antibody to Epstein-Barr nuclear antigen (EBNA).28 Acute phase reactants in the setting of an antibody to EBNA could indicate reactivation. A positive test does not exclude other medical causes, however, because up to 20% of patients have acute phase antibodies that persist for years.29
Appropriate diagnosis is important because of the significant morbidity associated with EBV. Risk of splenic injury is greatest between 4 and 21 days after onset of symptoms but persists at 7 weeks,26 so conservative therapy followed by monospot retesting one week later is a reasonable approach.
Mononucleosis or routine tonsillitis? It is important to note that there is no evidence that a positive monospot test will affect the management or outcome of routine tonsillitis, raising questions of the utility of the test in such cases. A better approach: Reserve testing for patients with additional findings—ie, splenomegaly—or whose symptoms have persisted ≥ 2 weeks.
THE TAKEAWAY: Wait at least 2 weeks to conduct monospot testing in patients with routine tonsillitis. If strong clinical suspicion exists, proceed with specific IgM and IgG serologic testing.24,25,27,28
5. Evaluating prescription drug levels: Which factors interfere?
Correct interpretation of lab tests conducted to measure prescription drug levels has major implications with regard to patient safety, particularly for medications with a narrow therapeutic index.
Most drug level tests measure the total concentration, which includes both bound and unbound (free) forms. The unbound forms are the active components of the drug; thus, for an accurate evaluation, it is important to be aware of factors that increase free drug concentration. Chief among them is low protein levels, or hypoalbuminemia.30
Risk factors for hypoalbuminemia include significant burns, advanced age, pregnancy, malnutrition, and human immunodeficiency virus/acquired immune deficiency syndrome (HIV/AIDS).30 HIV/AIDS is a particularly high risk because certain protease inhibitors are highly protein bound.
Drug protein binding is classified as low, moderate, or high. The main proteins involved in the process are albumin, alpha-1-acid glycoprotein, and lipoprotein. Medications that are highly protein bound (>80%) are the most affected by low protein levels: Problems can arise when drugs completely bind to all the available proteins and excess drug availability increases free drug levels.
Medications that are most likely to be affected by a high degree of protein binding include carbamazepine, cyclosporine, mycophenolic acid, phenytoin, protease inhibitors (with the exception of indinavir), tacrolimus, and valproic acid. It is important to consider free levels when you order medication assays for these drugs to avoid misinterpreting the serum levels as being too low-a scenario that raises the risk of drug toxicity and adverse outcomes.30,31
A study of 119 phenytoin samples from 70 patients found significantly higher free phenytoin levels in patients with lower albumin levels.32 Higher free phenytoin levels were also seen in older patients and in those with diminished renal function (creatinine clearance <25 mL/min).32 The degree of protein binding is affected by both the serum drug concentration and the albumin level, with saturable protein binding occurring at higher drug levels.33
Calculate phenytoin levels with this equation. To calculate corrected phenytoin levels in patients with low albumin levels, use the following formula, known as the Sheiner-Tozer equation:34
Concentration adjusted=concentration reported/([adjustment x serum albumin] + 0.1); adjustment=0.2 for creatinine clearance ≥20 or 0.1 for creatinine clearance <20.
Additional causes of misinterpreted drug levels. While hypoalbuminemia plays a major role in the misinterpretation of drug levels, other factors affect serum drug concentration, as well. These include drug-drug interactions, which can significantly increase the concentration of the medications involved, and the timing of the test with regard to medication administration. Digoxin levels, in particular, need to be drawn at least 6 to 8 hours after the last dose is taken to allow for appropriate drug distribution.35
THE TAKEAWAY: It is essential to consider free drug level monitoring in patients who either have hypoalbuminemia or have one or more risk factors for hypoalbuminemia to avoid falsely low estimation of drug levels.36,37
6 Liver function tests: Necessary for patients on statin therapy?
Since statins gained US Food and Drug Administration (FDA) approval, the drugs have been associated with increased liver function tests (LFTs). Indeed, there had been a long-standing belief, based on clinical trials, that by monitoring alanine aminotransferase (ALT) and maintaining it at <3 times the upper limit normal (ULN), hepatotoxicity could be avoided.38 In clinical practice, however, further ALT elevation is frequently allowed based on patient tolerability.
In February 2012, the FDA revised its safety data to reflect this practice.39 The FDA update confirmed that routine LFT monitoring is unnecessary for patients on statins—and that it is not very effective in identifying or preventing liver damage.
Overall, serious hepatotoxicity is very rare, with an incidence ≤2 per 1 million patient-years.39 The National Lipid Association Statin Safety Assessment Task Force recommends repeating LFTs that are 3 to 5 times the ULN within 6 months and continuing with the statin dose if the patient is asymptomatic.38
THE TAKEAWAY: Routine liver function monitoring is not necessary for patients on statins. A better approach: Obtain baseline ALT levels, and repeat the testing only as clinically indicated thereafter.38,39
7. Urine drug screens: Which factors affect their accuracy?
The gold standard for testing for drugs of abuse, urine drug screens (UDS) have good sensitivity and specificity, easy administration, and reasonable cost.40 UDS can detect various narcotics, such as morphine, oxycodone, ,and methadone, and identify other illicit drugs, although which drugs and metabolites are tested for is laboratory- and test-specific.
Cross-reactivity. There are 6 currently available immunoassays, all of which use competitive binding between the sample drug and a drug chemically labeled with an enzyme, radioisotope, or fluorophore. The sample drug and labeled drug compete for substrate binding sites on drug-specific antibodies.41,42 Similar to competitive binding for enzymatic reactions in the body, the substrate binding site can experience cross-reactivity—causing substances other than the drug in question to bind to the immunoglobulin, leading to a false positive result (TABLE 3).43 Other factors that can alter the results include the cutoff value of the test and the absorption, distribution, metabolism, and excretion of the drug.42 Thus, a confirmatory test of gas chromatography-mass spectrometry is recommended before making decisions based on the results of UDS.43-45
Routine screens for patients on chronic opioid therapy. Routine use of UDS in emergency departments is no longer recommended, based on evidence that the results are unlikely to have a significant effect on patient management.46 For patients on chronic opioid therapy, however, routine screening has proven helpful in detecting prescription opioid abuse, illicit drug use, and diversion. Up to 34% of patients on prescription opioids have been found to be using illicit drugs, as well.42
THE TAKEAWAY: Use UDS as a tool in managing patients on chronic opioid therapy, but before acting on results, assess for factors, such as the use of oral or topical medications and the cutoff value of the test, that may be associated with false positive or false negative results.43-45
8. Thyroid function testing: When should you test?
Thyroid-stimulating hormone (TSH) is the first-line test when investigating presumed hyper- or hypothyroidism.47,48 Third-generation chemiluminometric assays can reliably measure TSH concentrations <0.01 mU/L by using multiple antibodies to produce a sandwich-type effect on the molecule in question.49
TSH levels exhibit diurnal variation, however, and are affected by other medications, including steroids, opiates, and some antihistamines, among others, as well as comorbidities.47,48 Chronic and acute conditions unrelated to thyroid disease can cause transient changes in TSH concentrations, and have the potential to modify the binding capacity of plasma thyroid hormone binding proteins.48 Thus, TSH should be ordered for hospitalized patients only when clinical suspicion of a thyroid problem exists.48 The USPSTF recommends against routine TSH screening for asymptomatic adults.46
How to respond to abnormal results. For patients found to have abnormal TSH levels, free T4 (fT4) is the next test to order.47,49 An fT4 assay is a superior indicator of thyroid status because it is not affected by changes in iodothyronine-binding proteins, which influence total hormone measurements.49 The results will be elevated in hyperthyroidism and reduced in hypothyroidism.47
Triiodothyronine (T3) measures can be useful in diagnosing Graves’ disease, in which T3 toxicosis may be the initial symptom—or an indication of a relapse. Because T3 is often a peripheral product, however, nonthyroid illnesses and medications can cause artifactually abnormal results.49
Other thyroid-specific labs include thyroid ,antibodies such as antithyroid peroxidase, antithyroglobulin, and TSH receptor, both blocking and stimulating.49 Thyroglobulin is a precursor form of thyroid hormone and should be measured when factitious hyperthyroidism is suspected. Management of hyper- and hypothyroidism often is independent of etiology. Retesting TSH to assess treatment response should be postponed until ≥2 months after any change in medication or dosing.50
Thyroid studies can be very difficult to interpret. TSH should be the first test ordered. However, if TSH values do not match the clinical picture, fT4, T3, and other thyroid tests that are less affected by outside factors can be useful in identifying the cause.
THE TAKEAWAY: Routine TSH testing is not indicated for asymptomatic adults. When evaluating thyroid function is clinically indicated, TSH is the initial test of choice.47,48,51
CORRESPONDENCE
Joshua Tessier, DO, Iowa Lutheran Family Medicine Residency, 840 East University Avenue, Des Moines, IA 50316; [email protected]
› When interpreting hemoglobin A1c (HbA1c) levels, assess for anemia and other comorbidities that can significantly affect the lifespan of red blood cells and skew HbA1c test results. B
› Order nonfasting lipid panels for patients for whom fasting laboratory tests are difficult to obtain, as they have good clinical utility in screening and initial treatment. A
› Avoid routine thyroid-stimulating hormone (TSH) testing in asymptomatic adults; when testing is indicated, start with TSH. A
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Laboratory mistakes are not defined as diagnostic errors, but they contribute significantly to the thousands of medical errors that occur every year.1 Part of the problem: While accurate interpretation of lab tests often depends on the use of statistical concepts we all learned in medical training, it is difficult to find the time to incorporate these principles into a busy practice.
Overuse of lab tests presents problems, as well. Because “normal ranges” for test results are based on statistical analysis, as many as 5% of patients in a standard distribution fall outside of the range.2 It is important to order only the tests you really need, as extra testing automatically means more false positive results.
This article was written with such pitfalls in mind. In the pages that follow, we focus on 8 types of tests family physicians rely on regularly—all cases in which test results are reliable only if comorbidities, pre- and post-test probabilities, and clinical context are carefully considered. To help you put these lab tests into the proper context, we’ve addressed a key question—and highlighted both pitfalls and pearls—about each.
1. Hemoglobin A1c: How does anemia affect it?
Hemoglobin A1c (HbA1c) can be measured in many ways, including high-performance liquid chromatography, boronate affinity, capillary electrophoresis, and immunoassay, all of which can provide equivalent values without significant variability.3,4 In interpreting these tests, however, it is important to understand the effect that anemia has on HbA1c.
It's important to order only the tests you really need, as extra testing automatically means more false positive results.
Two primary variables influencing HbA1c are the average glucose level and the average lifespan of red blood cells (RBCs). Normally, there is a direct correlation between average serum glucose and HbA1c.4 In patients with anemia, however, this relationship is less clear, and may be affected by erythropoiesis and RBC destruction.5 In iron deficiency anemia (IDA),6,7 hemoglobin production falls secondary to iron stores, resulting in microcytic cells with a longer lifespan and elevated HbA1c. In at least one study,5,7 HbA1c approached levels associated with diabetes (with increases as high as 1.5%) in nondiabetic patients, but resolved with treatment of IDA.
Increased destruction as well as increased production of RBCs lowers their lifespan, and in turn decreases HbA1c levels (TABLE 1).4 This can be seen in conditions such as splenomegaly and hemoglobinopathies. In patients with hemoglobinopathies, the percentage of hemoglobin A is significantly decreased, often to undetectable levels—thereby making HbA1c tests inaccurate. Hemoglobin electrophoresis and determination of glycation by capillary electrophoresis or high-performance liquid chromatography can be used instead, but neither is practical because of cost and limited availability.4,8,9
THE TAKEAWAY: When you evaluate HbA1c test results, it is crucial to assess the patient for anemia and other conditions or comorbidities that can significantly affect RBC lifespan and skew test results.2,4-6
2. D-dimer: When should you use it?
D-dimer is a fibrin degradation product that is increased when active clotting is present,10 and its assay—which has high sensitivity and low specificity—is widely used to screen for pulmonary embolism (PE) and deep vein thrombosis (DVT). While the minimal number of false negatives makes the D-dimer a good screening test, the higher rate of false positives makes it difficult to arrive at a definitive diagnosis. Appropriate use of the D-dimer assay is crucial to minimize the potential for adverse consequences, such as bleeding in patients who are subjected to unnecessary anticoagulation because of false positive results.
Further testing typically follows. A positive D-dimer test is commonly followed by a computed tomography (CT) scan of the chest or a ventilation/perfusion scan to establish a PE or DVT diagnosis. But this subsequent testing increases both the cost of health care and the patient’s radiation exposure. Use of these subsequent scans can be reduced by first considering the patient’s pretest probability for PE or DVT. The Wells’ Criteria (available at www.mdcalc.com/wells-criteriafor-pulmonary-embolism-pe/) and Geneva Score (Revised) (www.mdcalc.com/genevascore-revised-for-pulmonary-embolism/) can both be used for this purpose.10,11
Patients with high pretest probability should undergo immediate scanning, foregoing the D-dimer—which should be reserved for patients who have a low or moderate pretest probability but sufficient reason to suspect PE or DVT.10-12
The low specificity of the D-dimer assay poses another challenge to its effective use. There are many things that can increase D-dimer levels, such as age, cancer, prolonged immobility, autoimmune disease, inflammation, sickle cell disease, pregnancy, trauma, and surgery.13-15 All these factors must be taken into consideration prior to ordering this test.
In fact, one recent study found that using an age-adjusted D-dimer cutoff (patient’s age in years x 10 mcg/L)—rather than a conventional cutoff of 500 mcg/L—for patients older than 50 years reduces false positives without substantially increasing false negatives.16
Also of note: An anticoagulant can decrease D-dimer levels in plasma, so the test should not be used to rule out PE or DVT in patients who are undergoing anticoagulation.13,15
THE TAKEAWAY: In evaluating patients for PE or DVT, use the Wells’ Criteria or Geneva Score (Revised) to determine a patient’s pretest probability of disease. Use the D-dimer assay to safely rule out these conditions in patients with a low or intermediate pretest probability, but go directly to scans for those with a high pretest probability.
3. Lipid panels: How important is fasting?
Patients are often instructed to report for fasting lab studies, specifically for lipid profiles. Traditionally, this had been defined as an 8- to 12-hour period without food.17 In clinical practice, however, this is often misinterpreted by patients, who may be confused about the duration of the fast or unsure about whether to eat or drink immediately before the test.
Studies investigating the effect of meals on lab values have found that triglycerides are consistently elevated postprandially, to a maximum of 12 hours.18-21 The effect of the fasting state on total cholesterol, low-density lipoprotein (LDL) cholesterol, and high-density lipoprotein (HDL) cholesterol is more controversial; while some postprandial differences have been detected, the clinical relevance is equivocal.18-21
Nonfasting lipid values can offer useful information, particularly in patients who are unwilling or unable to return for fasting labs. The US Preventive Services Task Force (USPSTF) supports this practice.22 Because guidelines for evaluation and treatment are based on fasting lipids, however, fasting lab work should be used, whenever possible, for initiating treatment and monitoring patients with abnormal values. If nonfasting lipids are used, it is crucial to factor in the postprandial effects on triglycerides and the subsequent difficulty of assessing LDL cholesterol levels.
THE TAKEAWAY: The clinical relevance of postprandial vs fasting lipid levels is equivocal. Nonfasting lipid panels have reasonable clinical utility in screening and initial treatment, particularly in cases in which obtaining fasting lab values may be problematic.18,19
4. Mononucleoosis spot test: When should you use it?
The monospot test is a latex assay that causes hemagglutination of horse RBCs in the presence of heterophile antibodies characteristic of infectious mononucleosis.23 The antibodies develop within the first 7 days of onset of symptoms, but do not peak for 2 to 5 weeks.24 As a result, monospot testing yields a high incidence of false negatives during the first 2 weeks of active infection.25 False negatives are also common in patients younger than 14 years. Heterophile antibodies may be present for up to a year after active infection.24
Patients at increased risk for splenic rupture, such as athletes, pose considerable diagnostic difficulty.26 When there is strong clinical suspicion of mononucleosis despite a negative monospot test in such high-risk individuals, follow-up testing is recommended to differentiate it from other mononucleosis-like illnesses (TABLE 2).27 The optimal combination of Epstein-Barr virus (EBV) serologic testing consists of the antibody titration of 4 markers: immunoglobulins M (IgM) and G (IgG) to the viral capsid antigen, IgM to the early antigen, and antibody to Epstein-Barr nuclear antigen (EBNA).28 Acute phase reactants in the setting of an antibody to EBNA could indicate reactivation. A positive test does not exclude other medical causes, however, because up to 20% of patients have acute phase antibodies that persist for years.29
Appropriate diagnosis is important because of the significant morbidity associated with EBV. Risk of splenic injury is greatest between 4 and 21 days after onset of symptoms but persists at 7 weeks,26 so conservative therapy followed by monospot retesting one week later is a reasonable approach.
Mononucleosis or routine tonsillitis? It is important to note that there is no evidence that a positive monospot test will affect the management or outcome of routine tonsillitis, raising questions of the utility of the test in such cases. A better approach: Reserve testing for patients with additional findings—ie, splenomegaly—or whose symptoms have persisted ≥ 2 weeks.
THE TAKEAWAY: Wait at least 2 weeks to conduct monospot testing in patients with routine tonsillitis. If strong clinical suspicion exists, proceed with specific IgM and IgG serologic testing.24,25,27,28
5. Evaluating prescription drug levels: Which factors interfere?
Correct interpretation of lab tests conducted to measure prescription drug levels has major implications with regard to patient safety, particularly for medications with a narrow therapeutic index.
Most drug level tests measure the total concentration, which includes both bound and unbound (free) forms. The unbound forms are the active components of the drug; thus, for an accurate evaluation, it is important to be aware of factors that increase free drug concentration. Chief among them is low protein levels, or hypoalbuminemia.30
Risk factors for hypoalbuminemia include significant burns, advanced age, pregnancy, malnutrition, and human immunodeficiency virus/acquired immune deficiency syndrome (HIV/AIDS).30 HIV/AIDS is a particularly high risk because certain protease inhibitors are highly protein bound.
Drug protein binding is classified as low, moderate, or high. The main proteins involved in the process are albumin, alpha-1-acid glycoprotein, and lipoprotein. Medications that are highly protein bound (>80%) are the most affected by low protein levels: Problems can arise when drugs completely bind to all the available proteins and excess drug availability increases free drug levels.
Medications that are most likely to be affected by a high degree of protein binding include carbamazepine, cyclosporine, mycophenolic acid, phenytoin, protease inhibitors (with the exception of indinavir), tacrolimus, and valproic acid. It is important to consider free levels when you order medication assays for these drugs to avoid misinterpreting the serum levels as being too low-a scenario that raises the risk of drug toxicity and adverse outcomes.30,31
A study of 119 phenytoin samples from 70 patients found significantly higher free phenytoin levels in patients with lower albumin levels.32 Higher free phenytoin levels were also seen in older patients and in those with diminished renal function (creatinine clearance <25 mL/min).32 The degree of protein binding is affected by both the serum drug concentration and the albumin level, with saturable protein binding occurring at higher drug levels.33
Calculate phenytoin levels with this equation. To calculate corrected phenytoin levels in patients with low albumin levels, use the following formula, known as the Sheiner-Tozer equation:34
Concentration adjusted=concentration reported/([adjustment x serum albumin] + 0.1); adjustment=0.2 for creatinine clearance ≥20 or 0.1 for creatinine clearance <20.
Additional causes of misinterpreted drug levels. While hypoalbuminemia plays a major role in the misinterpretation of drug levels, other factors affect serum drug concentration, as well. These include drug-drug interactions, which can significantly increase the concentration of the medications involved, and the timing of the test with regard to medication administration. Digoxin levels, in particular, need to be drawn at least 6 to 8 hours after the last dose is taken to allow for appropriate drug distribution.35
THE TAKEAWAY: It is essential to consider free drug level monitoring in patients who either have hypoalbuminemia or have one or more risk factors for hypoalbuminemia to avoid falsely low estimation of drug levels.36,37
6 Liver function tests: Necessary for patients on statin therapy?
Since statins gained US Food and Drug Administration (FDA) approval, the drugs have been associated with increased liver function tests (LFTs). Indeed, there had been a long-standing belief, based on clinical trials, that by monitoring alanine aminotransferase (ALT) and maintaining it at <3 times the upper limit normal (ULN), hepatotoxicity could be avoided.38 In clinical practice, however, further ALT elevation is frequently allowed based on patient tolerability.
In February 2012, the FDA revised its safety data to reflect this practice.39 The FDA update confirmed that routine LFT monitoring is unnecessary for patients on statins—and that it is not very effective in identifying or preventing liver damage.
Overall, serious hepatotoxicity is very rare, with an incidence ≤2 per 1 million patient-years.39 The National Lipid Association Statin Safety Assessment Task Force recommends repeating LFTs that are 3 to 5 times the ULN within 6 months and continuing with the statin dose if the patient is asymptomatic.38
THE TAKEAWAY: Routine liver function monitoring is not necessary for patients on statins. A better approach: Obtain baseline ALT levels, and repeat the testing only as clinically indicated thereafter.38,39
7. Urine drug screens: Which factors affect their accuracy?
The gold standard for testing for drugs of abuse, urine drug screens (UDS) have good sensitivity and specificity, easy administration, and reasonable cost.40 UDS can detect various narcotics, such as morphine, oxycodone, ,and methadone, and identify other illicit drugs, although which drugs and metabolites are tested for is laboratory- and test-specific.
Cross-reactivity. There are 6 currently available immunoassays, all of which use competitive binding between the sample drug and a drug chemically labeled with an enzyme, radioisotope, or fluorophore. The sample drug and labeled drug compete for substrate binding sites on drug-specific antibodies.41,42 Similar to competitive binding for enzymatic reactions in the body, the substrate binding site can experience cross-reactivity—causing substances other than the drug in question to bind to the immunoglobulin, leading to a false positive result (TABLE 3).43 Other factors that can alter the results include the cutoff value of the test and the absorption, distribution, metabolism, and excretion of the drug.42 Thus, a confirmatory test of gas chromatography-mass spectrometry is recommended before making decisions based on the results of UDS.43-45
Routine screens for patients on chronic opioid therapy. Routine use of UDS in emergency departments is no longer recommended, based on evidence that the results are unlikely to have a significant effect on patient management.46 For patients on chronic opioid therapy, however, routine screening has proven helpful in detecting prescription opioid abuse, illicit drug use, and diversion. Up to 34% of patients on prescription opioids have been found to be using illicit drugs, as well.42
THE TAKEAWAY: Use UDS as a tool in managing patients on chronic opioid therapy, but before acting on results, assess for factors, such as the use of oral or topical medications and the cutoff value of the test, that may be associated with false positive or false negative results.43-45
8. Thyroid function testing: When should you test?
Thyroid-stimulating hormone (TSH) is the first-line test when investigating presumed hyper- or hypothyroidism.47,48 Third-generation chemiluminometric assays can reliably measure TSH concentrations <0.01 mU/L by using multiple antibodies to produce a sandwich-type effect on the molecule in question.49
TSH levels exhibit diurnal variation, however, and are affected by other medications, including steroids, opiates, and some antihistamines, among others, as well as comorbidities.47,48 Chronic and acute conditions unrelated to thyroid disease can cause transient changes in TSH concentrations, and have the potential to modify the binding capacity of plasma thyroid hormone binding proteins.48 Thus, TSH should be ordered for hospitalized patients only when clinical suspicion of a thyroid problem exists.48 The USPSTF recommends against routine TSH screening for asymptomatic adults.46
How to respond to abnormal results. For patients found to have abnormal TSH levels, free T4 (fT4) is the next test to order.47,49 An fT4 assay is a superior indicator of thyroid status because it is not affected by changes in iodothyronine-binding proteins, which influence total hormone measurements.49 The results will be elevated in hyperthyroidism and reduced in hypothyroidism.47
Triiodothyronine (T3) measures can be useful in diagnosing Graves’ disease, in which T3 toxicosis may be the initial symptom—or an indication of a relapse. Because T3 is often a peripheral product, however, nonthyroid illnesses and medications can cause artifactually abnormal results.49
Other thyroid-specific labs include thyroid ,antibodies such as antithyroid peroxidase, antithyroglobulin, and TSH receptor, both blocking and stimulating.49 Thyroglobulin is a precursor form of thyroid hormone and should be measured when factitious hyperthyroidism is suspected. Management of hyper- and hypothyroidism often is independent of etiology. Retesting TSH to assess treatment response should be postponed until ≥2 months after any change in medication or dosing.50
Thyroid studies can be very difficult to interpret. TSH should be the first test ordered. However, if TSH values do not match the clinical picture, fT4, T3, and other thyroid tests that are less affected by outside factors can be useful in identifying the cause.
THE TAKEAWAY: Routine TSH testing is not indicated for asymptomatic adults. When evaluating thyroid function is clinically indicated, TSH is the initial test of choice.47,48,51
CORRESPONDENCE
Joshua Tessier, DO, Iowa Lutheran Family Medicine Residency, 840 East University Avenue, Des Moines, IA 50316; [email protected]
1. Ottomano C. Errors in medicine and errors in laboratory medicine: what is the difference? Blood Transfus. 2010;8;79-81.
2. Wallach JB. Introduction to normal values (reference ranges). Interpretation of Diagnostic Tests. 8th ed. Philadelphia, PA: Lippincott Williams & Wilkins; 2007:3-7.
3. Halwachs-Baumann G, Katzensteiner S, Schnedl W, et al. Comparative evaluation of three assay systems for automated determination of hemoglobin A1c. Clin Chem. 1997;43:511-517.
4. Gallagher EJ, Le Roith D, Bloomgarden Z. Review of hemoglobin A(1c) in the management of diabetes. J Diabetes. 2009;1:9-17.
5. Franco RS. The measurement and importance of red cell survival. Am J Hematol. 2009;84:109-114.
6. Tarim O, Küçükerdog˘an A, Gunay U, et al. Effects of iron deficiency anemia on hemoglobin A1c in type 1 diabetes mellitus. Pediatr Int. 1999;41:357-362.
7. Kim C, Bullard KM, Herman WH, et al. Association between iron deficiency and A1C levels among adults without diabetes in the National Health and Nutrition Examination Survey, 1999-2006. Diabetes Care. 2010;33:780-785.
8. Higgins T, Stewart D, Boehr E. Challenges in HbA1c analysis and reporting: an interesting case illustrating the many pitfalls. Clin Biochem. 2008;41:1104-1106.
9. Mongia SK, Little RR, Rohlfing CL, et al. Effects of hemoglobin C and S traits on the results of 14 commercial glycated hemoglobin assays. Am J Clin Pathol. 2008;130:136-140.
10. Brown MD, Rowe BH, Reeves MJ, et al. The accuracy of the enzyme-linked immunosorbent assay D-dimer test in the diagnosis of pulmonary embolism: a meta-analysis. Ann Emerg Med. 2002;40:133-144.
11. Squizzato A, Ageno W. What is the next step in D-dimer research? Education of physicians. Intern Emerg Med. 2006;1:165.
12. Kabrhel C, Mark Courtney D, Camargo CA Jr, et al. Potential impact of adjusting the threshold of the quantitative D-dimer based on pretest probability of acute pulmonary embolism. Acad Emerg Med. 2009;16:325-332.
13. Kabrhel C, Mark Courtney MD, Camargo CA Jr, et al. Factors associated with positive D-dimer results in patients evaluated for pulmonary embolism. Acad Emerg Med. 2010;17:589-597.
14. Berman AR. Pulmonary embolism in the elderly. Clin Geriatr Med. 2001;17:107-130.
15. Bruinstroop E, van de Ree MA, Huisman MV. The use of Ddimer in specific clinical conditions: a narrative review. Eur J Intern Med. 2009;20:441-446.
16. Schouten HJ, Geersing GI, Koek HL, et al. Diagnostic accuracy ,of conventional or age adjusted D-dimer cut-off values in older patients with suspected venous thromboembolism: systematic review and meta-analysis. BMJ. 2013;346:f2492.
17. Turgeon ML. Linne & Ringsrud’s Clinical Laboratory Science. 5th ed. Saint Louis, MO: Mosby; 2007:50.
18. Cohn JS,McNamara JR, Schaefer EJ. Lipoprotein cholesterol concentrations in the plasma of human subjects as measured in the fed and fasted states. Clin Chem. 1988;34:2456-2459.
19. Watts GF, Cohn JS. Whither the lipid profile: feast, famine, or no free lunch? Clin Chem. 2011;57:363-365.
20. Mora S, Rifai N, Buring JE, et al. Fasting compared with nonfasting lipids and apolipoproteins for predicting incident cardiovascular events. Circulation. 2008;118:993-1001.
21. Nordestgaard BG, Langsted A, Freiberg JJ. Nonfasting hyperlipidemia and cardiovascular disease. Curr Drug Targets. 2009;10:328-335.
22. Screening for lipid disorders in adults: US Preventive Services Task Force Recommendation statement. US Preventive Services Task Force Web site. Available at: http://www.uspreventiveservicestaskforce.org/uspstf08/lipid/lipidrs.htm. Accessed March 13, 2014.
23. Wolf DM, Friedrichs I, Toma AG. Lymphocyte-white blood cell count ratio: a quickly available screening tool to differentiate acute purulent tonsillitis from glandular fever. Arch Otolaryngol Head Neck Surg. 2007;133:61-64.
24. McCormack R, O’Shea T. The uptake and use of the Monospot test in patients with tonsillitis. Ir Med J. 2009;102:226-228.
25. Ebell MH. Epstein-Barr virus infectious mononucleosis. Am Fam Physician. 2004;70:1279-1287.
26. Waninger KN, Harcke HT. Determination of safe return to play for athletes recovering from infectious mononucleosis: a review of the literature. Clin J Sport Med. 2005;15:410-416.
27. Hurt C, Tammaro D. Diagnostic evaluation of mononucleosislike illnesses. Am J Med. 2007;120:911.e1-911.e8.
28. Vouloumanou EK, Rafailidis PI, Falagas ME. Current diagnosis and management of infectious mononucleosis. Curr Opin Hematol. 2012;19:14-20.
29. Epstein-Barr virus and infectious mononucleosis. Centers for Disease Control and Prevention Web site. Available at: http://www.cdc.gov/epstein-barr/laboratory-testing.html. Updated January 7, 2014. Accessed March 12, 2014.
30. Dasgupta A. Clinical utility of free drug monitoring. Clin Chem Lab Med. 2002;40:986-993.
31. Dasgupta A. Usefulness of monitoring free (unbound) concentrations of therapeutic drugs in patient management. Clin Chim Acta. 2007;377:1-13.
32. Iwamoto T, Kagawa Y, Naito Y, et al. Clinical evaluation of plasma free phenytoin measurement and factors influencing its protein binding. Biopharm Drug Dispos. 2006;27:77-84.
33. Hermida J, Tutor JC. A theoretical method for normalizing total serum valproic acid concentration in hypoalbuminemic patients. J Pharmacol Sci. 2005;97:489-493.
34. Wolf GK, McClain CD, Zurakowski D, et al. Total phenytoin concentrations do not accurately predict free phenytoin concentrations in critically ill children. Pediatr Crit Care Med. 2006;7:434-439; quiz 440.
35. Lanoxin (digoxin) [package insert]. Research Triangle Park, NC: GlaxoSmithKline; 2011.
36. National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III). Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) final report. Circulation. 2002;106:3143-3421.
37. De Backer G, Ambrosini E, Borch-Johnsen K, et al; Third Joint Force of European and other Societies on Cardiovascular Disease and Prevention in Clinical Practice. European guidelines on cardiovascular disease and prevention in clinical practice. Atherosclerosis. 2003;171:145-155.
38. McKenney JM, Davidson MH, Jacobson TA, et al. Final conclusions and recommendations of the National Lipid Association Statin Safety Assessment Task Force. Am J Cardiol. 2006;97:89C-94C.
39. FDA drug safety communication: important safety label changes to cholesterol-lowering statin drugs. US Food and Drug Administration Web site. Available at: http://www.fda.gov/Drugs/DrugSafety/ucm293101.htm. Updated July 3, 2012. Accessed May 17, 2013.
40. Eskridge KD, Guthrie SK. Clinical issues associated with urine testing of substances of abuse. Pharmacotherapy. 1997;17:497-510.
41. Herring C, Muzyk AJ, Johnston C. Interferences with urine drug screens. J Pharm Pract. 2001;24:102-108.
42. Jaffee WB, Trucco E, Teter C, et al. Focus on alcohol & drug abuse: ensuring validity in urine drug testing. Psychiatr Serv. 2008;59:140-142.
43. Brahm NC, Yeager LL, Fox MD, et al. Commonly prescribed medications and potential false-positive urine drug screens. Am J Health Syst Pharm. 2010;67:1344-1350.
44. Moeller KE, Lee KC, Kissack JC. Urine drug screening: Practical guide for clinicians. Mayo Clin Proc. 2008;83:66-76.
45. Christo PJ, Manchikanti L, Ruan X, et al. Urine drug testing in chronic pain. Pain Physician. 2011;14:123-143.
46. Tenenbein M. Do you really need that emergency drug screen? Clin Toxicol (Phila). 2009;47:286-291.
47. Gaitonde DY, Rowley KD, Sweeney LB. Hypothyroidism: an update. Am Fam Physician. 2012;86:244-251.
48. UK guidelines for the use of thyroid function tests. British Thyroid Association Web site. Available at: http://www.british-thyroid-association.org/info-for-patients/Docs/TFT_guideline_final_version_July_2006.pdf. Accessed March 11, 2014.
49. Volpé, R. Rational use of thyroid function tests. Crit Rev Clin Lab Sci. 1997;34:405-438.
50. Graber JR, Cobin RH, Gharib H, et al; American Association of Clinical Endocrinologists and American Thyroid Association Taskforce on Hypothyroidism in Adults. Clinical practice guidelines for hypothyroidism in adults: cosponsored by the American Association of Clinical Endocrinologists and the American Thyroid Association. Endocr Pract. 2012;18:988-1028.
51. Helfand M; US Preventive Services Task Force. Screening for subclinical thyroid dysfunction in nonpregnant adults: a summary of the evidence for the US Preventive Services Task Force. Ann Intern Med. 2004;140:128-141.
1. Ottomano C. Errors in medicine and errors in laboratory medicine: what is the difference? Blood Transfus. 2010;8;79-81.
2. Wallach JB. Introduction to normal values (reference ranges). Interpretation of Diagnostic Tests. 8th ed. Philadelphia, PA: Lippincott Williams & Wilkins; 2007:3-7.
3. Halwachs-Baumann G, Katzensteiner S, Schnedl W, et al. Comparative evaluation of three assay systems for automated determination of hemoglobin A1c. Clin Chem. 1997;43:511-517.
4. Gallagher EJ, Le Roith D, Bloomgarden Z. Review of hemoglobin A(1c) in the management of diabetes. J Diabetes. 2009;1:9-17.
5. Franco RS. The measurement and importance of red cell survival. Am J Hematol. 2009;84:109-114.
6. Tarim O, Küçükerdog˘an A, Gunay U, et al. Effects of iron deficiency anemia on hemoglobin A1c in type 1 diabetes mellitus. Pediatr Int. 1999;41:357-362.
7. Kim C, Bullard KM, Herman WH, et al. Association between iron deficiency and A1C levels among adults without diabetes in the National Health and Nutrition Examination Survey, 1999-2006. Diabetes Care. 2010;33:780-785.
8. Higgins T, Stewart D, Boehr E. Challenges in HbA1c analysis and reporting: an interesting case illustrating the many pitfalls. Clin Biochem. 2008;41:1104-1106.
9. Mongia SK, Little RR, Rohlfing CL, et al. Effects of hemoglobin C and S traits on the results of 14 commercial glycated hemoglobin assays. Am J Clin Pathol. 2008;130:136-140.
10. Brown MD, Rowe BH, Reeves MJ, et al. The accuracy of the enzyme-linked immunosorbent assay D-dimer test in the diagnosis of pulmonary embolism: a meta-analysis. Ann Emerg Med. 2002;40:133-144.
11. Squizzato A, Ageno W. What is the next step in D-dimer research? Education of physicians. Intern Emerg Med. 2006;1:165.
12. Kabrhel C, Mark Courtney D, Camargo CA Jr, et al. Potential impact of adjusting the threshold of the quantitative D-dimer based on pretest probability of acute pulmonary embolism. Acad Emerg Med. 2009;16:325-332.
13. Kabrhel C, Mark Courtney MD, Camargo CA Jr, et al. Factors associated with positive D-dimer results in patients evaluated for pulmonary embolism. Acad Emerg Med. 2010;17:589-597.
14. Berman AR. Pulmonary embolism in the elderly. Clin Geriatr Med. 2001;17:107-130.
15. Bruinstroop E, van de Ree MA, Huisman MV. The use of Ddimer in specific clinical conditions: a narrative review. Eur J Intern Med. 2009;20:441-446.
16. Schouten HJ, Geersing GI, Koek HL, et al. Diagnostic accuracy ,of conventional or age adjusted D-dimer cut-off values in older patients with suspected venous thromboembolism: systematic review and meta-analysis. BMJ. 2013;346:f2492.
17. Turgeon ML. Linne & Ringsrud’s Clinical Laboratory Science. 5th ed. Saint Louis, MO: Mosby; 2007:50.
18. Cohn JS,McNamara JR, Schaefer EJ. Lipoprotein cholesterol concentrations in the plasma of human subjects as measured in the fed and fasted states. Clin Chem. 1988;34:2456-2459.
19. Watts GF, Cohn JS. Whither the lipid profile: feast, famine, or no free lunch? Clin Chem. 2011;57:363-365.
20. Mora S, Rifai N, Buring JE, et al. Fasting compared with nonfasting lipids and apolipoproteins for predicting incident cardiovascular events. Circulation. 2008;118:993-1001.
21. Nordestgaard BG, Langsted A, Freiberg JJ. Nonfasting hyperlipidemia and cardiovascular disease. Curr Drug Targets. 2009;10:328-335.
22. Screening for lipid disorders in adults: US Preventive Services Task Force Recommendation statement. US Preventive Services Task Force Web site. Available at: http://www.uspreventiveservicestaskforce.org/uspstf08/lipid/lipidrs.htm. Accessed March 13, 2014.
23. Wolf DM, Friedrichs I, Toma AG. Lymphocyte-white blood cell count ratio: a quickly available screening tool to differentiate acute purulent tonsillitis from glandular fever. Arch Otolaryngol Head Neck Surg. 2007;133:61-64.
24. McCormack R, O’Shea T. The uptake and use of the Monospot test in patients with tonsillitis. Ir Med J. 2009;102:226-228.
25. Ebell MH. Epstein-Barr virus infectious mononucleosis. Am Fam Physician. 2004;70:1279-1287.
26. Waninger KN, Harcke HT. Determination of safe return to play for athletes recovering from infectious mononucleosis: a review of the literature. Clin J Sport Med. 2005;15:410-416.
27. Hurt C, Tammaro D. Diagnostic evaluation of mononucleosislike illnesses. Am J Med. 2007;120:911.e1-911.e8.
28. Vouloumanou EK, Rafailidis PI, Falagas ME. Current diagnosis and management of infectious mononucleosis. Curr Opin Hematol. 2012;19:14-20.
29. Epstein-Barr virus and infectious mononucleosis. Centers for Disease Control and Prevention Web site. Available at: http://www.cdc.gov/epstein-barr/laboratory-testing.html. Updated January 7, 2014. Accessed March 12, 2014.
30. Dasgupta A. Clinical utility of free drug monitoring. Clin Chem Lab Med. 2002;40:986-993.
31. Dasgupta A. Usefulness of monitoring free (unbound) concentrations of therapeutic drugs in patient management. Clin Chim Acta. 2007;377:1-13.
32. Iwamoto T, Kagawa Y, Naito Y, et al. Clinical evaluation of plasma free phenytoin measurement and factors influencing its protein binding. Biopharm Drug Dispos. 2006;27:77-84.
33. Hermida J, Tutor JC. A theoretical method for normalizing total serum valproic acid concentration in hypoalbuminemic patients. J Pharmacol Sci. 2005;97:489-493.
34. Wolf GK, McClain CD, Zurakowski D, et al. Total phenytoin concentrations do not accurately predict free phenytoin concentrations in critically ill children. Pediatr Crit Care Med. 2006;7:434-439; quiz 440.
35. Lanoxin (digoxin) [package insert]. Research Triangle Park, NC: GlaxoSmithKline; 2011.
36. National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III). Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) final report. Circulation. 2002;106:3143-3421.
37. De Backer G, Ambrosini E, Borch-Johnsen K, et al; Third Joint Force of European and other Societies on Cardiovascular Disease and Prevention in Clinical Practice. European guidelines on cardiovascular disease and prevention in clinical practice. Atherosclerosis. 2003;171:145-155.
38. McKenney JM, Davidson MH, Jacobson TA, et al. Final conclusions and recommendations of the National Lipid Association Statin Safety Assessment Task Force. Am J Cardiol. 2006;97:89C-94C.
39. FDA drug safety communication: important safety label changes to cholesterol-lowering statin drugs. US Food and Drug Administration Web site. Available at: http://www.fda.gov/Drugs/DrugSafety/ucm293101.htm. Updated July 3, 2012. Accessed May 17, 2013.
40. Eskridge KD, Guthrie SK. Clinical issues associated with urine testing of substances of abuse. Pharmacotherapy. 1997;17:497-510.
41. Herring C, Muzyk AJ, Johnston C. Interferences with urine drug screens. J Pharm Pract. 2001;24:102-108.
42. Jaffee WB, Trucco E, Teter C, et al. Focus on alcohol & drug abuse: ensuring validity in urine drug testing. Psychiatr Serv. 2008;59:140-142.
43. Brahm NC, Yeager LL, Fox MD, et al. Commonly prescribed medications and potential false-positive urine drug screens. Am J Health Syst Pharm. 2010;67:1344-1350.
44. Moeller KE, Lee KC, Kissack JC. Urine drug screening: Practical guide for clinicians. Mayo Clin Proc. 2008;83:66-76.
45. Christo PJ, Manchikanti L, Ruan X, et al. Urine drug testing in chronic pain. Pain Physician. 2011;14:123-143.
46. Tenenbein M. Do you really need that emergency drug screen? Clin Toxicol (Phila). 2009;47:286-291.
47. Gaitonde DY, Rowley KD, Sweeney LB. Hypothyroidism: an update. Am Fam Physician. 2012;86:244-251.
48. UK guidelines for the use of thyroid function tests. British Thyroid Association Web site. Available at: http://www.british-thyroid-association.org/info-for-patients/Docs/TFT_guideline_final_version_July_2006.pdf. Accessed March 11, 2014.
49. Volpé, R. Rational use of thyroid function tests. Crit Rev Clin Lab Sci. 1997;34:405-438.
50. Graber JR, Cobin RH, Gharib H, et al; American Association of Clinical Endocrinologists and American Thyroid Association Taskforce on Hypothyroidism in Adults. Clinical practice guidelines for hypothyroidism in adults: cosponsored by the American Association of Clinical Endocrinologists and the American Thyroid Association. Endocr Pract. 2012;18:988-1028.
51. Helfand M; US Preventive Services Task Force. Screening for subclinical thyroid dysfunction in nonpregnant adults: a summary of the evidence for the US Preventive Services Task Force. Ann Intern Med. 2004;140:128-141.
What caused elevated liver enzymes in this postpartum patient?
CASE › 38-year-old, previously healthy G2 P2 woman arrives at your office with sudden-onset epigastric pain, chills, and nausea, but no vomiting. She has had no fever, shortness of breath, or pruritis. Her appetite is good and her weight is stable. Three days earlier, she gave birth to a healthy baby. The course of pregnancy had been uncomplicated, and delivery was vaginal at 35 weeks gestation without any complications. Her blood pressure (BP) was normal throughout pregnancy, and she had no signs of preeclampsia.
She does not smoke. Although she usually drinks 1 beer daily, she avoided alcohol during her pregnancy. She does not use illicit drugs. She has received no blood transfusions and has no history of viral hepatitis.
On examination she is alert and oriented. She is afebrile and anicteric. Her vital signs are normal with a BP of 116/80 mm Hg and a pulse rate of 86/min. Respiratory rate is 20/min, and oxygen saturation is 98% while breathing ambient air. On palpation, her abdomen is soft and nontender without organomegaly. There is no ascites and bowel sounds are audible.
Initial laboratory investigation yields the following results:
• alkaline phosphatase 436 U/L (normal, 40-135)
• alanine aminotransferase 685 U/L (4-55)
• total bilirubin 27 mcmol/L (2-20)
• serum albumin 25 g/L (35-55)
• international normalized ratio 0.9 (0.9-1.3)
• amylase 47 U/L (20-110)
• hemoglobin 146 g/L (140-180)
• platelets 296 3 109/L (150-400)
• white blood cell count 9.7 3 109/L (4.0-10.0)
• urinalysis reveals no proteins
• transferrin 4.58 g/L (1.32-3.02)
• iron saturation 29% (15-50)
• ferritin 70 mcg/L (10-200)
• serum copper 43.9 mcmol/L (9.0-27.0)
• ceruloplasmin 594 mg/L (200-600)
• Alpha-1-antitrypsin levels 2.05 g/L (1.06-1.58).
What is the differential diagnosis of abnormally elevated liver enzymes in the peripartum period?
Possible underlying causes of the patient’s findings include pregnancy-related liver diseases such as hyperemesis gravidarum (HG), intrahepatic cholestasis of pregnancy, preeclampsia, eclampsia, HELLP syndrome (hemolysis, elevated liver enzymes, and low platelets), and acute fatty liver of pregnancy (AFLP); or liver diseases unrelated to pregnancy such as viral hepatitis, autoimmune liver disease, Wilson’s disease, Budd-Chiari syndrome, cholecystitis, and drug-induced hepatotoxicity.
Narrowing the field. HG usually presents between 4 to 13 weeks of the start of pregnancy and is characterized by severe nausea, vomiting, weight loss, and electrolyte disturbances, none of which are present in this patient. The patient does not have neuropsychiatric symptoms and signs typical of Wilson’s disease, and the high-normal ceruloplasmin level despite above-normal serum copper also weighs against this diagnosis.
Our patient was not taking any hepatotoxic drugs or over-the-counter medications that cause liver damage.
With intrahepatic cholestasis of pregnancy, aminotransferase levels can be as high as 20 times the upper limit of normal. However, with this disorder, elevated serum bile acids during the second half of pregnancy cause pruritis. Absence of pruritis, jaundice, and features of obstructive jaundice, including pale stools and dark urine, makes intrahepatic cholestasis of pregnancy unlikely. Moreover, patients with this disorder do not have constitutional symptoms.1
Preeclampsia is characterized by hypertension and proteinuria after 20 weeks of gestation or within 48 hours of delivery. Absence of seizures differentiates it from eclampsia. Right upper quadrant pain, nausea, and vomiting may be the presenting features. Aminotransferase levels can be up to 10 times the upper limit of normal. Bilirubin concentrations are usually normal. These abnormalities typically resolve within 2 weeks of delivery. Though atypical clinical presentations have been known with preeclampsia—particularly as extremes of maternal childbearing age have been associated with preeclampsia2—the patient’s normal BP and an absence of proteinuria make both preeclampsia and eclampsia unlikely.
HELLP syndrome usually arises in the second or third trimester of pregnancy but also can develop after delivery. Right upper quadrant and epigastric pain, nausea, and vomiting are usual presenting symptoms. Hypertension and proteinuria are found in 85% of cases.3 Absence of hypertension and proteinuria and normal microangiopathic blood smear and platelet count make HELLP syndrome unlikely in the patient.
AFLP usually presents in the third trimester of pregnancy with nausea, abdominal pain, jaundice, and hepatic encephalopathy. Hypoglycemia, lactic acidosis, hyperammonemia, and disseminated intravascular coagulation may complicate the clinical picture. Leukocytosis occurs in 98% of patients.4 Elevated concentrations of bilirubin, aminotransferases, and uric acid are commonly found. The biochemical picture in our patient does not match that of AFLP and makes this diagnosis unlikely.
Remaining potential diagnoses. Hepatitis B and C are possibilities and must be excluded by appropriate serologic tests. Hepatitis E viral infection usually follows a more severe course in pregnancy. Pregnant women are more likely to acquire hepatitis E in the second or third trimester. Also, though it is rare for autoimmune hepatitis to first appear during pregnancy, it too, must be ruled out.
Pregnancy is a prothrombotic state, so you must exclude Budd-Chiari syndrome. Up to 20% of cases of Budd-Chiari syndrome occur in women who are on oral contraceptives or are pregnant or 2 months postpartum.5 Right upper quadrant pain, jaundice, and ascites are the common clinical features.
Gallstones are strongly associated with higher parity in women. Pre-pregnancy obesity and high serum leptin levels are strong risk factors for pregnancy-associated gallbladder disease. Gallbladder sludge and stones are common in pregnancy and the postpartum period, and cholecystectomy is frequently done within the first year postpartum.6 Serum alkaline phosphatase is less helpful in diagnosing cholecystitis in pregnancy because of elevated levels from the placenta.
With hepatitis, Budd-Chiari syndrome, and gallstones remaining in the differential, what other investigations would you pursue to narrow the differential?
A test for hepatitis A virus immunoglobulin M (IgM) proves negative. Hepatitis B surface antigen is negative, and hepatitis B surface antibody is 11.5 mIU/L, suggesting borderline protective level of antibody. Hepatitis C virus antibody also is negative. Hepatitis E occurs in the Indian subcontinent, Africa, and the Middle East, and is therefore unlikely in this patient. Serologies for cytomegalovirus IgG and Epstein-Barr virus IgM are negative. Herpes simplex type-1 specific IgG antibody is present. These serologic results exclude viral causes of hepatitis.
Antinuclear (ANA) and antimitochondrial antibodies are negative. Antismooth muscle antibody (ASMA) is positive at a titer of 1:20. Quantitative IgG is 7.23 g/L (normal, 5.52-17.24), IgA is 1.36 g/L (0.87-3.94), and IgM is 1.19 g/L (0.44-2.47). Negative ANA, weakly positive ASMA, and normal levels of immunoglobulins in our patient do not support a diagnosis of autoimmune liver disease.
Imaging is the next step for this patient. Even during pregnancy, ultrasound and magnetic resonance imaging are safe and readily available. The diagnostic accuracy of ultrasound for detecting gallstones is 95%. When ultrasound findings are equivocal in a pregnant patient, magnetic resonance cholangiopancreatography provides an accurate evaluation of the biliary system and can substitute for endoscopic retrograde cholangiopancreatography (ERCP).7
An ultrasound examination of the patient shows a normal liver with no significant fatty infiltration. The gallbladder, however, is packed with calculi. The common hepatic duct measures 4.6 mm and the common bile duct measures 8.5 mm. The intrahepatic ducts are not dilated. Doppler ultrasound of the hepatic and portal veins demonstrates normal flow without evidence of thromboses. Absence of jaundice, ascites, and hepatic vein thrombosis on ultrasound excludes Budd-Chiari syndrome.
The diagnosis
History of sudden-onset epigastric pain, chills, and nausea in the postpartum period, no history of liver disease, and an uneventful pregnancy makes cholecystitis the most likely diagnosis for the patient.
Gallstones are common in pregnancy and more than 4% of pregnant women have incident gallbladder sludge or stones persisting to the early postpartum. Cholesterol secretion is increased in the second and third trimester of pregnancy, thus increasing the lithogenicity of the bile.8
The outcome
ERCP showed several stones in the common bile duct. We performed a papillotomy and removed 15 pale, almost white-faced, stones. Subsequent laparoscopic cholecystectomy removed a large gallbladder with multiple remaining stones. Microscopic examination of the gallbladder wall showed thickened muscularis propria and fibrosis of the subserosa, findings consistent with chronic cholecystitis.
CORRESPONDENCE
H.U. Rehman, MBBS, Clinical Associate Professor, Department of Medicine, Regina General Hospital, 1440 14th Avenue, Regina, SK, S4P 0W5, Canada; [email protected]
1. Knox TA, Olans LB. Liver disease in pregnancy. N Engl J Med. 1996;335:569-576.
2. Joshi D, James A, Quaglia A, et al. Liver disease in pregnancy. Lancet. 2010;375:594-605.
3. Martin JN Jr, Rinehart BK, May WL, et al. The spectrum of severe preeclampsia: comparative analysis by HELLP (hemolysis, elevated liver enzyme levels, and low platelet count) syndrome classification. Am J Obstet Gynecol. 1999;180(6 pt 1):1373-1384.
4. Knight M, Nelson-Piercy C, Kurinczuk JJ, et al; UK Obstetric Surveillance System. A prospective national study of acute fatty liver of pregnancy in the UK. Gut. 2008;57:951–956.
5. Khuroo MS, Datta DV. Budd-Chiari syndrome following pregnancy. Report of 16 cases, with roentgenologic, hemodynamic and histologic studies of the hepatic outflow tract. Am J Med. 1980;68:113-121.
6. Ko CW, Beresford SA, Schulte SJ, et al. Incidence, natural history, and risk factors for biliary sludge and stones during pregnancy. Hepatology. 2005;41:359-365.
7. Oto A, Ernst R, Ghulmiyyah L, et al. The role of MR cholangiopancreatography in the evaluation of pregnant patients with acute pancreaticobiliary disease. Br J Radiol. 2009;82:279-285.
8. Donovan JM. Physical and metabolic factors in gallstone pathogenesis. Gastroenterol Clin North Am. 1999;28:75-97.
CASE › 38-year-old, previously healthy G2 P2 woman arrives at your office with sudden-onset epigastric pain, chills, and nausea, but no vomiting. She has had no fever, shortness of breath, or pruritis. Her appetite is good and her weight is stable. Three days earlier, she gave birth to a healthy baby. The course of pregnancy had been uncomplicated, and delivery was vaginal at 35 weeks gestation without any complications. Her blood pressure (BP) was normal throughout pregnancy, and she had no signs of preeclampsia.
She does not smoke. Although she usually drinks 1 beer daily, she avoided alcohol during her pregnancy. She does not use illicit drugs. She has received no blood transfusions and has no history of viral hepatitis.
On examination she is alert and oriented. She is afebrile and anicteric. Her vital signs are normal with a BP of 116/80 mm Hg and a pulse rate of 86/min. Respiratory rate is 20/min, and oxygen saturation is 98% while breathing ambient air. On palpation, her abdomen is soft and nontender without organomegaly. There is no ascites and bowel sounds are audible.
Initial laboratory investigation yields the following results:
• alkaline phosphatase 436 U/L (normal, 40-135)
• alanine aminotransferase 685 U/L (4-55)
• total bilirubin 27 mcmol/L (2-20)
• serum albumin 25 g/L (35-55)
• international normalized ratio 0.9 (0.9-1.3)
• amylase 47 U/L (20-110)
• hemoglobin 146 g/L (140-180)
• platelets 296 3 109/L (150-400)
• white blood cell count 9.7 3 109/L (4.0-10.0)
• urinalysis reveals no proteins
• transferrin 4.58 g/L (1.32-3.02)
• iron saturation 29% (15-50)
• ferritin 70 mcg/L (10-200)
• serum copper 43.9 mcmol/L (9.0-27.0)
• ceruloplasmin 594 mg/L (200-600)
• Alpha-1-antitrypsin levels 2.05 g/L (1.06-1.58).
What is the differential diagnosis of abnormally elevated liver enzymes in the peripartum period?
Possible underlying causes of the patient’s findings include pregnancy-related liver diseases such as hyperemesis gravidarum (HG), intrahepatic cholestasis of pregnancy, preeclampsia, eclampsia, HELLP syndrome (hemolysis, elevated liver enzymes, and low platelets), and acute fatty liver of pregnancy (AFLP); or liver diseases unrelated to pregnancy such as viral hepatitis, autoimmune liver disease, Wilson’s disease, Budd-Chiari syndrome, cholecystitis, and drug-induced hepatotoxicity.
Narrowing the field. HG usually presents between 4 to 13 weeks of the start of pregnancy and is characterized by severe nausea, vomiting, weight loss, and electrolyte disturbances, none of which are present in this patient. The patient does not have neuropsychiatric symptoms and signs typical of Wilson’s disease, and the high-normal ceruloplasmin level despite above-normal serum copper also weighs against this diagnosis.
Our patient was not taking any hepatotoxic drugs or over-the-counter medications that cause liver damage.
With intrahepatic cholestasis of pregnancy, aminotransferase levels can be as high as 20 times the upper limit of normal. However, with this disorder, elevated serum bile acids during the second half of pregnancy cause pruritis. Absence of pruritis, jaundice, and features of obstructive jaundice, including pale stools and dark urine, makes intrahepatic cholestasis of pregnancy unlikely. Moreover, patients with this disorder do not have constitutional symptoms.1
Preeclampsia is characterized by hypertension and proteinuria after 20 weeks of gestation or within 48 hours of delivery. Absence of seizures differentiates it from eclampsia. Right upper quadrant pain, nausea, and vomiting may be the presenting features. Aminotransferase levels can be up to 10 times the upper limit of normal. Bilirubin concentrations are usually normal. These abnormalities typically resolve within 2 weeks of delivery. Though atypical clinical presentations have been known with preeclampsia—particularly as extremes of maternal childbearing age have been associated with preeclampsia2—the patient’s normal BP and an absence of proteinuria make both preeclampsia and eclampsia unlikely.
HELLP syndrome usually arises in the second or third trimester of pregnancy but also can develop after delivery. Right upper quadrant and epigastric pain, nausea, and vomiting are usual presenting symptoms. Hypertension and proteinuria are found in 85% of cases.3 Absence of hypertension and proteinuria and normal microangiopathic blood smear and platelet count make HELLP syndrome unlikely in the patient.
AFLP usually presents in the third trimester of pregnancy with nausea, abdominal pain, jaundice, and hepatic encephalopathy. Hypoglycemia, lactic acidosis, hyperammonemia, and disseminated intravascular coagulation may complicate the clinical picture. Leukocytosis occurs in 98% of patients.4 Elevated concentrations of bilirubin, aminotransferases, and uric acid are commonly found. The biochemical picture in our patient does not match that of AFLP and makes this diagnosis unlikely.
Remaining potential diagnoses. Hepatitis B and C are possibilities and must be excluded by appropriate serologic tests. Hepatitis E viral infection usually follows a more severe course in pregnancy. Pregnant women are more likely to acquire hepatitis E in the second or third trimester. Also, though it is rare for autoimmune hepatitis to first appear during pregnancy, it too, must be ruled out.
Pregnancy is a prothrombotic state, so you must exclude Budd-Chiari syndrome. Up to 20% of cases of Budd-Chiari syndrome occur in women who are on oral contraceptives or are pregnant or 2 months postpartum.5 Right upper quadrant pain, jaundice, and ascites are the common clinical features.
Gallstones are strongly associated with higher parity in women. Pre-pregnancy obesity and high serum leptin levels are strong risk factors for pregnancy-associated gallbladder disease. Gallbladder sludge and stones are common in pregnancy and the postpartum period, and cholecystectomy is frequently done within the first year postpartum.6 Serum alkaline phosphatase is less helpful in diagnosing cholecystitis in pregnancy because of elevated levels from the placenta.
With hepatitis, Budd-Chiari syndrome, and gallstones remaining in the differential, what other investigations would you pursue to narrow the differential?
A test for hepatitis A virus immunoglobulin M (IgM) proves negative. Hepatitis B surface antigen is negative, and hepatitis B surface antibody is 11.5 mIU/L, suggesting borderline protective level of antibody. Hepatitis C virus antibody also is negative. Hepatitis E occurs in the Indian subcontinent, Africa, and the Middle East, and is therefore unlikely in this patient. Serologies for cytomegalovirus IgG and Epstein-Barr virus IgM are negative. Herpes simplex type-1 specific IgG antibody is present. These serologic results exclude viral causes of hepatitis.
Antinuclear (ANA) and antimitochondrial antibodies are negative. Antismooth muscle antibody (ASMA) is positive at a titer of 1:20. Quantitative IgG is 7.23 g/L (normal, 5.52-17.24), IgA is 1.36 g/L (0.87-3.94), and IgM is 1.19 g/L (0.44-2.47). Negative ANA, weakly positive ASMA, and normal levels of immunoglobulins in our patient do not support a diagnosis of autoimmune liver disease.
Imaging is the next step for this patient. Even during pregnancy, ultrasound and magnetic resonance imaging are safe and readily available. The diagnostic accuracy of ultrasound for detecting gallstones is 95%. When ultrasound findings are equivocal in a pregnant patient, magnetic resonance cholangiopancreatography provides an accurate evaluation of the biliary system and can substitute for endoscopic retrograde cholangiopancreatography (ERCP).7
An ultrasound examination of the patient shows a normal liver with no significant fatty infiltration. The gallbladder, however, is packed with calculi. The common hepatic duct measures 4.6 mm and the common bile duct measures 8.5 mm. The intrahepatic ducts are not dilated. Doppler ultrasound of the hepatic and portal veins demonstrates normal flow without evidence of thromboses. Absence of jaundice, ascites, and hepatic vein thrombosis on ultrasound excludes Budd-Chiari syndrome.
The diagnosis
History of sudden-onset epigastric pain, chills, and nausea in the postpartum period, no history of liver disease, and an uneventful pregnancy makes cholecystitis the most likely diagnosis for the patient.
Gallstones are common in pregnancy and more than 4% of pregnant women have incident gallbladder sludge or stones persisting to the early postpartum. Cholesterol secretion is increased in the second and third trimester of pregnancy, thus increasing the lithogenicity of the bile.8
The outcome
ERCP showed several stones in the common bile duct. We performed a papillotomy and removed 15 pale, almost white-faced, stones. Subsequent laparoscopic cholecystectomy removed a large gallbladder with multiple remaining stones. Microscopic examination of the gallbladder wall showed thickened muscularis propria and fibrosis of the subserosa, findings consistent with chronic cholecystitis.
CORRESPONDENCE
H.U. Rehman, MBBS, Clinical Associate Professor, Department of Medicine, Regina General Hospital, 1440 14th Avenue, Regina, SK, S4P 0W5, Canada; [email protected]
CASE › 38-year-old, previously healthy G2 P2 woman arrives at your office with sudden-onset epigastric pain, chills, and nausea, but no vomiting. She has had no fever, shortness of breath, or pruritis. Her appetite is good and her weight is stable. Three days earlier, she gave birth to a healthy baby. The course of pregnancy had been uncomplicated, and delivery was vaginal at 35 weeks gestation without any complications. Her blood pressure (BP) was normal throughout pregnancy, and she had no signs of preeclampsia.
She does not smoke. Although she usually drinks 1 beer daily, she avoided alcohol during her pregnancy. She does not use illicit drugs. She has received no blood transfusions and has no history of viral hepatitis.
On examination she is alert and oriented. She is afebrile and anicteric. Her vital signs are normal with a BP of 116/80 mm Hg and a pulse rate of 86/min. Respiratory rate is 20/min, and oxygen saturation is 98% while breathing ambient air. On palpation, her abdomen is soft and nontender without organomegaly. There is no ascites and bowel sounds are audible.
Initial laboratory investigation yields the following results:
• alkaline phosphatase 436 U/L (normal, 40-135)
• alanine aminotransferase 685 U/L (4-55)
• total bilirubin 27 mcmol/L (2-20)
• serum albumin 25 g/L (35-55)
• international normalized ratio 0.9 (0.9-1.3)
• amylase 47 U/L (20-110)
• hemoglobin 146 g/L (140-180)
• platelets 296 3 109/L (150-400)
• white blood cell count 9.7 3 109/L (4.0-10.0)
• urinalysis reveals no proteins
• transferrin 4.58 g/L (1.32-3.02)
• iron saturation 29% (15-50)
• ferritin 70 mcg/L (10-200)
• serum copper 43.9 mcmol/L (9.0-27.0)
• ceruloplasmin 594 mg/L (200-600)
• Alpha-1-antitrypsin levels 2.05 g/L (1.06-1.58).
What is the differential diagnosis of abnormally elevated liver enzymes in the peripartum period?
Possible underlying causes of the patient’s findings include pregnancy-related liver diseases such as hyperemesis gravidarum (HG), intrahepatic cholestasis of pregnancy, preeclampsia, eclampsia, HELLP syndrome (hemolysis, elevated liver enzymes, and low platelets), and acute fatty liver of pregnancy (AFLP); or liver diseases unrelated to pregnancy such as viral hepatitis, autoimmune liver disease, Wilson’s disease, Budd-Chiari syndrome, cholecystitis, and drug-induced hepatotoxicity.
Narrowing the field. HG usually presents between 4 to 13 weeks of the start of pregnancy and is characterized by severe nausea, vomiting, weight loss, and electrolyte disturbances, none of which are present in this patient. The patient does not have neuropsychiatric symptoms and signs typical of Wilson’s disease, and the high-normal ceruloplasmin level despite above-normal serum copper also weighs against this diagnosis.
Our patient was not taking any hepatotoxic drugs or over-the-counter medications that cause liver damage.
With intrahepatic cholestasis of pregnancy, aminotransferase levels can be as high as 20 times the upper limit of normal. However, with this disorder, elevated serum bile acids during the second half of pregnancy cause pruritis. Absence of pruritis, jaundice, and features of obstructive jaundice, including pale stools and dark urine, makes intrahepatic cholestasis of pregnancy unlikely. Moreover, patients with this disorder do not have constitutional symptoms.1
Preeclampsia is characterized by hypertension and proteinuria after 20 weeks of gestation or within 48 hours of delivery. Absence of seizures differentiates it from eclampsia. Right upper quadrant pain, nausea, and vomiting may be the presenting features. Aminotransferase levels can be up to 10 times the upper limit of normal. Bilirubin concentrations are usually normal. These abnormalities typically resolve within 2 weeks of delivery. Though atypical clinical presentations have been known with preeclampsia—particularly as extremes of maternal childbearing age have been associated with preeclampsia2—the patient’s normal BP and an absence of proteinuria make both preeclampsia and eclampsia unlikely.
HELLP syndrome usually arises in the second or third trimester of pregnancy but also can develop after delivery. Right upper quadrant and epigastric pain, nausea, and vomiting are usual presenting symptoms. Hypertension and proteinuria are found in 85% of cases.3 Absence of hypertension and proteinuria and normal microangiopathic blood smear and platelet count make HELLP syndrome unlikely in the patient.
AFLP usually presents in the third trimester of pregnancy with nausea, abdominal pain, jaundice, and hepatic encephalopathy. Hypoglycemia, lactic acidosis, hyperammonemia, and disseminated intravascular coagulation may complicate the clinical picture. Leukocytosis occurs in 98% of patients.4 Elevated concentrations of bilirubin, aminotransferases, and uric acid are commonly found. The biochemical picture in our patient does not match that of AFLP and makes this diagnosis unlikely.
Remaining potential diagnoses. Hepatitis B and C are possibilities and must be excluded by appropriate serologic tests. Hepatitis E viral infection usually follows a more severe course in pregnancy. Pregnant women are more likely to acquire hepatitis E in the second or third trimester. Also, though it is rare for autoimmune hepatitis to first appear during pregnancy, it too, must be ruled out.
Pregnancy is a prothrombotic state, so you must exclude Budd-Chiari syndrome. Up to 20% of cases of Budd-Chiari syndrome occur in women who are on oral contraceptives or are pregnant or 2 months postpartum.5 Right upper quadrant pain, jaundice, and ascites are the common clinical features.
Gallstones are strongly associated with higher parity in women. Pre-pregnancy obesity and high serum leptin levels are strong risk factors for pregnancy-associated gallbladder disease. Gallbladder sludge and stones are common in pregnancy and the postpartum period, and cholecystectomy is frequently done within the first year postpartum.6 Serum alkaline phosphatase is less helpful in diagnosing cholecystitis in pregnancy because of elevated levels from the placenta.
With hepatitis, Budd-Chiari syndrome, and gallstones remaining in the differential, what other investigations would you pursue to narrow the differential?
A test for hepatitis A virus immunoglobulin M (IgM) proves negative. Hepatitis B surface antigen is negative, and hepatitis B surface antibody is 11.5 mIU/L, suggesting borderline protective level of antibody. Hepatitis C virus antibody also is negative. Hepatitis E occurs in the Indian subcontinent, Africa, and the Middle East, and is therefore unlikely in this patient. Serologies for cytomegalovirus IgG and Epstein-Barr virus IgM are negative. Herpes simplex type-1 specific IgG antibody is present. These serologic results exclude viral causes of hepatitis.
Antinuclear (ANA) and antimitochondrial antibodies are negative. Antismooth muscle antibody (ASMA) is positive at a titer of 1:20. Quantitative IgG is 7.23 g/L (normal, 5.52-17.24), IgA is 1.36 g/L (0.87-3.94), and IgM is 1.19 g/L (0.44-2.47). Negative ANA, weakly positive ASMA, and normal levels of immunoglobulins in our patient do not support a diagnosis of autoimmune liver disease.
Imaging is the next step for this patient. Even during pregnancy, ultrasound and magnetic resonance imaging are safe and readily available. The diagnostic accuracy of ultrasound for detecting gallstones is 95%. When ultrasound findings are equivocal in a pregnant patient, magnetic resonance cholangiopancreatography provides an accurate evaluation of the biliary system and can substitute for endoscopic retrograde cholangiopancreatography (ERCP).7
An ultrasound examination of the patient shows a normal liver with no significant fatty infiltration. The gallbladder, however, is packed with calculi. The common hepatic duct measures 4.6 mm and the common bile duct measures 8.5 mm. The intrahepatic ducts are not dilated. Doppler ultrasound of the hepatic and portal veins demonstrates normal flow without evidence of thromboses. Absence of jaundice, ascites, and hepatic vein thrombosis on ultrasound excludes Budd-Chiari syndrome.
The diagnosis
History of sudden-onset epigastric pain, chills, and nausea in the postpartum period, no history of liver disease, and an uneventful pregnancy makes cholecystitis the most likely diagnosis for the patient.
Gallstones are common in pregnancy and more than 4% of pregnant women have incident gallbladder sludge or stones persisting to the early postpartum. Cholesterol secretion is increased in the second and third trimester of pregnancy, thus increasing the lithogenicity of the bile.8
The outcome
ERCP showed several stones in the common bile duct. We performed a papillotomy and removed 15 pale, almost white-faced, stones. Subsequent laparoscopic cholecystectomy removed a large gallbladder with multiple remaining stones. Microscopic examination of the gallbladder wall showed thickened muscularis propria and fibrosis of the subserosa, findings consistent with chronic cholecystitis.
CORRESPONDENCE
H.U. Rehman, MBBS, Clinical Associate Professor, Department of Medicine, Regina General Hospital, 1440 14th Avenue, Regina, SK, S4P 0W5, Canada; [email protected]
1. Knox TA, Olans LB. Liver disease in pregnancy. N Engl J Med. 1996;335:569-576.
2. Joshi D, James A, Quaglia A, et al. Liver disease in pregnancy. Lancet. 2010;375:594-605.
3. Martin JN Jr, Rinehart BK, May WL, et al. The spectrum of severe preeclampsia: comparative analysis by HELLP (hemolysis, elevated liver enzyme levels, and low platelet count) syndrome classification. Am J Obstet Gynecol. 1999;180(6 pt 1):1373-1384.
4. Knight M, Nelson-Piercy C, Kurinczuk JJ, et al; UK Obstetric Surveillance System. A prospective national study of acute fatty liver of pregnancy in the UK. Gut. 2008;57:951–956.
5. Khuroo MS, Datta DV. Budd-Chiari syndrome following pregnancy. Report of 16 cases, with roentgenologic, hemodynamic and histologic studies of the hepatic outflow tract. Am J Med. 1980;68:113-121.
6. Ko CW, Beresford SA, Schulte SJ, et al. Incidence, natural history, and risk factors for biliary sludge and stones during pregnancy. Hepatology. 2005;41:359-365.
7. Oto A, Ernst R, Ghulmiyyah L, et al. The role of MR cholangiopancreatography in the evaluation of pregnant patients with acute pancreaticobiliary disease. Br J Radiol. 2009;82:279-285.
8. Donovan JM. Physical and metabolic factors in gallstone pathogenesis. Gastroenterol Clin North Am. 1999;28:75-97.
1. Knox TA, Olans LB. Liver disease in pregnancy. N Engl J Med. 1996;335:569-576.
2. Joshi D, James A, Quaglia A, et al. Liver disease in pregnancy. Lancet. 2010;375:594-605.
3. Martin JN Jr, Rinehart BK, May WL, et al. The spectrum of severe preeclampsia: comparative analysis by HELLP (hemolysis, elevated liver enzyme levels, and low platelet count) syndrome classification. Am J Obstet Gynecol. 1999;180(6 pt 1):1373-1384.
4. Knight M, Nelson-Piercy C, Kurinczuk JJ, et al; UK Obstetric Surveillance System. A prospective national study of acute fatty liver of pregnancy in the UK. Gut. 2008;57:951–956.
5. Khuroo MS, Datta DV. Budd-Chiari syndrome following pregnancy. Report of 16 cases, with roentgenologic, hemodynamic and histologic studies of the hepatic outflow tract. Am J Med. 1980;68:113-121.
6. Ko CW, Beresford SA, Schulte SJ, et al. Incidence, natural history, and risk factors for biliary sludge and stones during pregnancy. Hepatology. 2005;41:359-365.
7. Oto A, Ernst R, Ghulmiyyah L, et al. The role of MR cholangiopancreatography in the evaluation of pregnant patients with acute pancreaticobiliary disease. Br J Radiol. 2009;82:279-285.
8. Donovan JM. Physical and metabolic factors in gallstone pathogenesis. Gastroenterol Clin North Am. 1999;28:75-97.
Managing patients on antipsychotics: Your domain, too
› Evaluate patients for movement disorders before initiating or adjusting antipsychotic therapy, then weekly until the dose is stabilized. A
› Use nonpharmacologic interventions—eg, positive reinforcement, music, light exercise—as first-line therapy for neuropsychiatric symptoms of dementia; consider antipsychotic therapy only if they fail. A
› Obtain a fasting glucose level before initiating or adjusting antipsychotic therapy, then at 12 weeks, and annually if the patient is taking a second-generation agent. B
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
CASE 1 › Steve B is a 43-year-old patient with bipolar disorder and a history of hypertension and high cholesterol. His body mass index (BMI) is 29. During a checkup, he tells you his psychiatrist recently started him on olanzapine. He reports that the medication is working, but he’s concerned about adverse effects, and asks whether he should be monitored for signs of diabetes.
CASE 2 › Mary F, an 83-year-old with Alzheimer’s disease and a history of stable coronary artery disease, is a resident in a long-term care facility, where staff members report that she is increasingly combative. The floor nurse says Ms. F has been striking out at the nurses’ aides who attempt to dress her and asks that you prescribe an antipsychotic to “calm her down.”
If Mr. B and Ms. F were your patients, what would you do?
In 1951, the chance discovery of an anesthetic’s calming properties was the first step in the development of the medications that came to be known as antipsychotics.1 In recent years, we have seen an expansion in both the number of antipsychotic agents on the market and the scope of their use, for conditions as varied as chronic pain, dementia, nausea and vomiting, and Tourette syndrome.
While antipsychotics often are prescribed by psychiatrists or other specialists, primary care physicians are increasingly likely to be involved in the management of patients who take them—and, at times, to prescribe antipsychotic agents themselves. We developed this guide to increase awareness of safe prescribing practices and principles guiding the initiation and management of antipsychotic agents. We start with a review of the mechanism of action of first- and second-generation antipsychotics (SGAs).
In the last decade, research has called into question whether second-generation antipsychotics really are more effective than first-generation agents
First- and second-generation agents: How they work and differ
Antipsychotics act at the level of the dopaminergic pathways in the central nervous system by blocking the D2 receptors. Action on the mesolimbic pathway is thought to be responsible for their effects on schizophrenia symptoms,2 while action at receptor sites in other dopaminergic pathways leads to common adverse effects, primarily the extrapyramidal symptoms (EPS) associated with first-generation antipsychotics (FGAs).
The distinction between first- and second-generation agents relates to SGAs’ blockage of serotonin receptors (thought to better relieve schizophrenia symptoms) and increased specificity for the mesolimbic pathway (which reduces the action on other dopamine pathways and is less likely to produce EPS).3 These differences largely accounted for the belief that SGAs were more effective and provided the rationale for their designation as atypical antipsychotics.
Are SGAs really better?
In the last decade, research has called such claims into question. Trials such as the Clinical Antipsychotic Trials of Intervention Effectiveness4 and Cost Utility of the Latest Antipsychotic Drugs in Schizophrenia study,5 as well as a meta-analysis,6 found that SGAs as a class are no more effective than FGAs. That said, 2 SGAs—clozapine and olanzapine—were found to be superior to FGAs for the treatment of schizophrenia. The studies also raised doubts about SGAs’ advantages regarding tolerability, as the time to discontinuation due to intolerable adverse effects was similar for first- and second-generation drugs.4-6
Approved and off-label indications: A look at the evidence
In addition to schizophrenia, many antipsychotics have US Food and Drug Administration (FDA) approval to treat various psychiatric and nonpsychiatric conditions (TABLE 1).7 Several are approved for use in bipolar disorder, 2 are approved as adjunctive treatment of major depressive disorder (MDD), and one is approved for the short-term treatment of generalized anxiety disorder (GAD). Porphyria, tetanus, and intractable hiccups are among the nonpsychiatric conditions for which some antipsychotics are approved.7
Evidence ranging from anecdotal to randomized controlled trials (RCTs) is steadily emerging about off-label uses of antipsychotics, with risperidone, quetiapine, and olanzapine foremost among them.8,9 Use of antipsychotics in the treatment of neuropsychiatric symptoms (NPS) of dementia has become particularly widespread, with off-label use of antipsychotics more prevalent in long-term care facilities than in outpatient settings.9
NPS. Antipsychotics’ efficacy in controlling dementia-related agitation, aggression, and psychosis has consistently shown a modest but statistically significant benefit. A Cochrane review found evidence that the use of risperidone and olanzapine resulted in improvements in agitation scale scores; risperidone was linked to improved scores on a psychosis scale, as well.10 A second meta-analysis showed small but statistically significant improvements in NPS with risperidone, olanzapine, and aripiprazole.8 Another study showed that nearly half (48%) of patients who had a positive response to risperidone relapsed when they stopped taking the drug.11
A rapidly aging population is expected to further increase the need for pharmacologic interventions to control NPS. Yet safety concerns about the use of antipsychotics in the elderly (more on this in a bit) call this practice into question.
Chronic pain. A 2008 Cochrane review analyzed the efficacy of antipsychotics for acute and chronic pain, pooling results of 11 studies of the treatment of conditions such as postherpetic neuralgia, tension headache, acute myocardial infarction (MI), and terminal cancer. Results from the pooled trials were described as mixed, although an overall statistically significant decrease in pain intensity was found.12
Polypharmacy. The simultaneous use of 2 or more antipsychotic agents is also increasingly prevalent,13 with levels exceeding 50% in one study of patients with schizophrenia.14 Because there is little data on the safety and efficacy of antipsychotic polypharmacy, this off-label approach should be considered only as a last resort.15
Off-label treatment of psychiatric conditions
GAD. In comparative effectiveness trials, quetiapine was found to be equal to both paroxetine and escitalopram for the treatment of GAD, with a favorable effect on symptoms 8 weeks after its initiation.8 Trials of other antipsychotics for the treatment of GAD have not demonstrated clear efficacy. Trifluoperazine is approved for GAD, as a short-term treatment.
MDD. Antipsychotics have been shown to be beneficial in the treatment of MDD, although only quetiapine and aripiprazole are approved (and only as adjunctive treatment). Evidence supports the use of both agents, as well as risperidone, as augmentation to selective serotonin reuptake inhibitors (SSRIs), and in pooled results from 5 placebo-controlled trials, quetiapine was found to be effective as monotherapy for MDD.9
Obsessive-compulsive disorder (OCD). Compared with placebo, risperidone showed a 4-fold increase in the likelihood of a favorable response (number needed to treat [NNT]=4) in patients with OCD,8 but the drug remains off-label for this purpose.
Posttraumatic stress disorder (PTSD). A meta-analysis of 7 studies demonstrated risperidone’s efficacy in the treatment of combat-related PTSD.9 In a large Veterans Administration study of patients with combat-related PTSD resistant to treatment with SSRIs, however, risperidone showed no benefit after 6 months of therapy.16 Antipsychotics have not been found beneficial for substance abuse, eating disorders, or insomnia.9
Identifying risk factos, monitoring for adverse effects
While FGAs carry an increased risk of EPS, SGAs increase the risk of obesity, hyperlipidemia, hypertension, and diabetes mellitus. The average life expectancy of patients with schizophrenia is 2 to 3 decades lower than that of age-matched controls,17 a finding largely attributed to the increased rate of cardiovascular disease. While this can be partly explained by differences in lifestyle and access to care, the metabolic effects of SGAs are a likely contributing factor.
Because of the adverse effects of FGAs and SGAs, the American Diabetes Association and American Psychiatric Association jointly issued guidelines addressing both the type and optimal frequency of monitoring for patients on antipsychotics (TABLE 2).18,19 Following them is critical, as both the initiation of an antipsychotic agent and any change in regimen can lead to the development—or exacerbation—of a number of diseases.
Before initiating antipsychotic therapy—or the first time you see a patient like Mr. B, whose care you will be monitoring—a thorough assessment of risk factors is needed. Foremost among them are overweight or obesity, insulin resistance or diabetes, a history of heart disease, and EPS.
In some cases, preexisting conditions and the potential harm of a specific drug must be weighed in determining which antipsychotic to prescribe. When adverse effects develop after drug therapy has been initiated, decisions about further actions should be based on both the degree of the unfavorable response and the availability of other treatments—and made, as appropriate, in consultation with the specialist who prescribed the drug.
CASE 1 › You tell Mr. B that metabolic side effects like weight gain, impaired glucose tolerance, and increased low-density lipoprotein cholesterol are common with SGAs like the one he is taking, and that you will monitor his fasting glucose levels to evaluate his risk for developing diabetes—starting with this visit. (Olanzapine, the drug he is taking, is 4 times more likely than an FGA to lead to diabetes.18)
You talk to him, too, about the importance of weight control and note that if his BMI increases by ≥1 point you will refer him to a nutritionist and recommend a structured exercise program. Finally, you schedule an appointment in 3 months.
Risks associated with older age and dementia
In 2010, there were 84,842 visits to US emergency departments (EDs) due to adverse drug events involving antipsychotic agents—a 110% increase since 2005. Nearly 30% of these ED visits involved patients 65 years or older.20
Among patients with dementia, use of antipsychotics has been found to dramatically increase the risk of stroke (rate ratio, 3.26 for FGAs and 5.86 for SGAs).21 The risk was greatest in the first 35 days of treatment, but persisted throughout the 175-day study period.
The rate of MI also was elevated in dementia patients (hazard ratio of 2.19 for the first 30 days of treatment, then falling to 1.15 for the first year).22 The risk of pulmonary embolism and deep vein thrombosis also rose for patients who had been on antipsychotics during the previous 24 months (odds ratio=1.32), with the highest risk within the first 90 days of treatment.23
Risk of death varies with agent and dose. Multiple studies have shown that the mortality risk associated with antipsychotics varies greatly among individual drugs, with haloperidol carrying the highest risk and quetiapine the lowest.24-26 The hazard ratio for death within the first 30 days was 3.2 for haloperidol, 1.6 for risperidone, and 1.5 for olanzapine; quetiapine had no statistically significant increase. The increased mortality risk was statistically significant only at higher doses.24
The FDA weighs in
Evidence of the elevated risk of death led the FDA to require black-box warnings on SGAs (in 2005)27 and FGAs (in 2008),28 stating that “antipsychotics are not indicated for the treatment of dementia-related psychosis.”28 More recently (in 2012), the American Geriatrics Society (AGS) published a guide on the management of NPS in patients with dementia.29 In it, the AGS acknowledges that despite FDA warnings, antipsychotics may be necessary for the treatment of NPS.
The AGS stresses the importance of nonpharmacologic interventions (eg, positive reinforcement, orientation to time and place, music, light exercise, pet therapy) as a first-line approach. If these measures fail and antipsychotics are necessary, the AGS calls for obtaining informed consent from a family member, using the lowest effective dose, and regularly attempting to wean the patient off the antipsychotic as the standard of care.28
CASE 2 › New or worsening aggressive behavior in an elderly patient with dementia requires a prompt assessment. You start with a complete medical evaluation of Ms. F, ruling out common causes of agitation such as infection, pain, constipation, and an adverse reaction to medication.
You also ask about the incidents of aggression: Does the same aide dress Ms. F daily? Does the aide introduce herself and explain what she’s about to do before attempting to dress the patient?
Next, you recommend nonpharmacologic therapies, such as calming music, participation in group activities, and pet therapy. You tell the floor nurse that if these measures fail and Ms. F’s threatening behavior continues, an antipsychotic may be considered.
Guard against abuse of antipsychotics
As antipsychotic use increases, so, too, does misuse and abuse, particularly of quetiapine. The drug has a reported street value of $3 to $8 for a 25- to 100-mg dose and is known as “quell,” “Susie-Q,” “and “baby heroin”; “Q-ball” is the name used for a combination of cocaine and quetiapine.30,31
The Drug Abuse Warning Network reported a 115% increase in ED visits related to the misuse or abuse of pharmaceuticals between 2004 and 2010.32 In 2010, 57,199 drug abuse cases—including 28,618 suicide attempts—were linked to antipsychotics.20
To optimize the benefit of antipsychotics and minimize the likelihood of abuse, ensure that every patient taking them has a clearly documented indication for an antipsychotic and a single responsible prescriber of the antipsychotic, often a psychiatrist. Your responsibilities: Schedule visits for monitoring, do a medication review to identify potential drug-drug interactions, and assess efficacy, all on a regular basis.
CASE 1 › At Mr. B’s next visit, you retest his fasting glucose (which is now 105 mg/dL) and recheck his BMI, which has climbed to 30. You tell him you will speak with his psychiatrist about his weight gain and your concern about the development of insulin resistance.
Meanwhile, you refer the patient to a nutritionist and encourage a healthy lifestyle. Because the medication has been effective, you schedule a follow-up visit in 6 weeks to see if the lifestyle interventions have been successful before consulting with the patient’s psychiatrist about a change in medication.
CASE 2 › When you return to the long-term care facility one week later, you find that Ms. F’s NPS have not abated. You realize an antipsychotic agent may be needed. Because she has a history of heart disease, however, she has a higher risk for cardiovascular events.
You meet with her son to review the benefits and risks of antipsychotic therapy, explaining that risperidone is a reasonable agent and that a low starting dose (0.25-0.5 mg) will reduce the risk. You obtain his informed consent, document your treatment goals—a decrease in threatening behavior and the ability of the staff to work with Ms. F to get her up and out of bed—and establish a plan to review in 2 weeks.
CORRESPONDENCE
Daniel DeJoseph, MD, Drexel Family Medicine, 3401 Market Street, Suite 105-B, Philadelphia, PA 19104; [email protected]
1. Shen WW. A history of antipsychotic drug development. Compr Psychiatry. 1999;40:407-414.
2. Miller R. Mechanism of action of antipsychotic drugs of different classes, refractoriness to therapeutic effects of classical neuroleptics, and individual variation in sensitivity to their actions. Curr Neuropharmacol. 2009;7:302-314.
3. Seeman P. Atypical antipsychotics: mechanism of action. Can J Psychiatry. 2002;47:27-38.
4. Lieberman JA, Stroup TS, McEvoy JP, et al; Clinical Antipsychotic Trials of Intervention Effectiveness (CATIE) Investigators. Effectiveness of antipsychotic drugs in patients with chronic schizophrenia. N Engl J Med. 2005;353:1209-1223.
5. Jones PB, Barnes TR, Davies L, et al. Randomized controlled trial of the effect on quality of life of second- vs first-generation antipsychotic drugs in schizophrenia: Cost Utility of the Latest Antipsychotic Drugs in Schizophrenia Study (CUtLASS 1). Arch Gen Psychiatry. 2006;63:1079-1087.
6. Leucht S, Corves C, Arbter D, et al. Second-generation versus first-generation antipsychotic drugs for schizophrenia: a meta-analysis. Lancet. 2009;373:31-41.
7. Christian R, Saavedra L, Gaynes BN, et al. Future research needs for first- and second-generation antipsychotics for children and young adults [Internet]. Agency for Healthcare Research and Quality. 2012:12-EHC042-EF.
8. Maher AR, Maglione M, Bagley S, et al. Efficacy and comparative effectiveness of atypical antipsychotic medications for off-label uses in adults: a systematic review and meta-analysis. JAMA. 2011;306:1359-1369.
9. Maglione M, Maher AR, Hu J, et al. Off-label use of atypical antipsychotics: An update [Internet]. Agency for Healthcare Research and Quality. 2011:11-EHC087-EF.
10. Ballard CG, Waite J. Atypical antipsychotics for aggression and psychosis in Alzheimer’s disease. Cochrane Database Syst Rev. 2006;(1):CD003476.
11. Devanand DP, Mintzer J, Schultz SK, et al. Relapse risk after discontinuation of risperidone in Alzheimer’s disease. N Engl J Med. 2012;367:1497-1507.
12. Seidel S, Aigner M, Ossege M, et al. Antipsychotics for acute and chronic pain in adults. Cochrane Database Syst Rev. 2008;(4):CD004844.
13. Mojtabai R, Olfson M. National trends in psychotropic medication polypharmacy in office-based psychiatry. Arch Gen Psychiatry. 2010;67:26-36.
14. Faries D, Ascher-Svanum H, Zhu B, et al. Antipsychotic monotherapy and polypharmacy in the naturalistic treatment of schizophrenia with atypical antipsychotics. BMC Psychiatry. 2005;5:26.
15. Ballon J, Stroup TS. Polypharmacy for schizophrenia. Curr Opin Psychiatry. 2013;26:208-213.
16. Krystal JH, Rosenheck RA, Cramer JA, et al; Veterans Affairs Cooperative Study No. 504 Group. Adjunctive risperidone treatment for antidepressant-resistant symptoms of chronic military service-related PTSD: a randomized trial. JAMA. 2011;306:493-502.
17. Institute of Medicine. Retooling for an Aging America: Building the Health Care Workforce. Washington, DC: National Academy of Sciences; 2008.
18. Marder SR, Essock SM, Miller AL, et al. Physical health monitoring of patients with schizophrenia. Am J Psychiatry. 2004;161:1334-1349.
19. Barrett E, Blonde L, Clement S, et al. Consensus development conference on antipsychotic drugs and obesity and diabetes. Diabetes Care. 2004;27:596-601.
20. Drug Abuse Warning Network, 2010: National Estimates of Drug-Related Emergency Department Visits. HHS Publication No. (SMA) 12-4733, DAWN Series D-38. Substance Abuse and Mental Health Services Administration Web site. Available at: http://www.samhsa.gov/data/2k13/DAWN2k10ED/DAWN2k10ED.htm. Accessed May 1, 2013.
21. Douglas IJ, Smeeth L. Exposure to antipsychotics and risk of stroke: self controlled case series study. BMJ. 2008;337:a1227.
22. Pariente A, Fourrier-Réglat A, Ducruet T, et al. Antipsychotic use and myocardial infarction in older patients with treated dementia. Arch Intern Med. 2012;172:648-653.
23. Parker C, Coupland C, Hippisley-Cox J. Antipsychotic drugs and risk of venous thromboembolism: nested case-control study. BMJ. 2010;341:c4245.
24. Rossom RC, Rector TS, Lederle FA, et al. Are all commonly prescribed antipsychotics associated with greater mortality in elderly male veterans with dementia? J Am Geriatr Soc. 2010;58:1027-1034.
25. Kales HC, Kim HM, Zivin K, et al. Risk of mortality among individual antipsychotics in patients with dementia. Am J Psychiatry. 2012;169:71-79.
26. Huybrechts KF, Gerhard T, Crystal S, et al. Differential risk of death in older residents in nursing homes prescribed specific antipsychotic drugs: population based cohort study. BMJ. 2012;344:e977.
27. US Food and Drug Public Health Advisory: Deaths with antipsychotics in elderly patients with behavioral disturbances. US Food and Drug Administration Web site Available at: http://1.usa.gov/1plsxPk. Accessed February 5, 2014.
28. Information for healthcare professionals: conventional antipsychotics. US Food and Drug Administration Web site. Available at: http://www.fda.gov/drugs/drugsafety/postmarketdrugsafetyinformationforpatientsandproviders/ucm124830.htm. Accessed February 5, 2014.
29. Guide to the management of psychotic disorders and neuropsychiatric symptoms of dementia in older adults. American Geriatric Society Web site. Available at: http://dementia.americangeriatrics.org/GeriPsych_index.php. Accessed April 15, 2013.
30. Bogart GT, Ott CA. Abuse of second-generation antipsychotics: What prescribers need to know. Curr Psychiatr. 2011;10:77-79.
31. Tarosoff G, Osti K. Black-market value of antipsychotics, antidepressants, and hypnotics in Las Vegas, Nevada. Am J Psychiatry. 2007;164:350.
32. Highlights of the 2010 Drug Abuse Warning Network (DAWN) findings on drug-related emergency department visits. Substance Abuse and Mental Health Services Administration, Center for Behavioral Health Statistics and Quality Web site. Available at: http://www.samhsa.gov/data/2k12/DAWN096/SR096EDHighlights2010.htm. Accessed May 1, 2013.
› Evaluate patients for movement disorders before initiating or adjusting antipsychotic therapy, then weekly until the dose is stabilized. A
› Use nonpharmacologic interventions—eg, positive reinforcement, music, light exercise—as first-line therapy for neuropsychiatric symptoms of dementia; consider antipsychotic therapy only if they fail. A
› Obtain a fasting glucose level before initiating or adjusting antipsychotic therapy, then at 12 weeks, and annually if the patient is taking a second-generation agent. B
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
CASE 1 › Steve B is a 43-year-old patient with bipolar disorder and a history of hypertension and high cholesterol. His body mass index (BMI) is 29. During a checkup, he tells you his psychiatrist recently started him on olanzapine. He reports that the medication is working, but he’s concerned about adverse effects, and asks whether he should be monitored for signs of diabetes.
CASE 2 › Mary F, an 83-year-old with Alzheimer’s disease and a history of stable coronary artery disease, is a resident in a long-term care facility, where staff members report that she is increasingly combative. The floor nurse says Ms. F has been striking out at the nurses’ aides who attempt to dress her and asks that you prescribe an antipsychotic to “calm her down.”
If Mr. B and Ms. F were your patients, what would you do?
In 1951, the chance discovery of an anesthetic’s calming properties was the first step in the development of the medications that came to be known as antipsychotics.1 In recent years, we have seen an expansion in both the number of antipsychotic agents on the market and the scope of their use, for conditions as varied as chronic pain, dementia, nausea and vomiting, and Tourette syndrome.
While antipsychotics often are prescribed by psychiatrists or other specialists, primary care physicians are increasingly likely to be involved in the management of patients who take them—and, at times, to prescribe antipsychotic agents themselves. We developed this guide to increase awareness of safe prescribing practices and principles guiding the initiation and management of antipsychotic agents. We start with a review of the mechanism of action of first- and second-generation antipsychotics (SGAs).
In the last decade, research has called into question whether second-generation antipsychotics really are more effective than first-generation agents
First- and second-generation agents: How they work and differ
Antipsychotics act at the level of the dopaminergic pathways in the central nervous system by blocking the D2 receptors. Action on the mesolimbic pathway is thought to be responsible for their effects on schizophrenia symptoms,2 while action at receptor sites in other dopaminergic pathways leads to common adverse effects, primarily the extrapyramidal symptoms (EPS) associated with first-generation antipsychotics (FGAs).
The distinction between first- and second-generation agents relates to SGAs’ blockage of serotonin receptors (thought to better relieve schizophrenia symptoms) and increased specificity for the mesolimbic pathway (which reduces the action on other dopamine pathways and is less likely to produce EPS).3 These differences largely accounted for the belief that SGAs were more effective and provided the rationale for their designation as atypical antipsychotics.
Are SGAs really better?
In the last decade, research has called such claims into question. Trials such as the Clinical Antipsychotic Trials of Intervention Effectiveness4 and Cost Utility of the Latest Antipsychotic Drugs in Schizophrenia study,5 as well as a meta-analysis,6 found that SGAs as a class are no more effective than FGAs. That said, 2 SGAs—clozapine and olanzapine—were found to be superior to FGAs for the treatment of schizophrenia. The studies also raised doubts about SGAs’ advantages regarding tolerability, as the time to discontinuation due to intolerable adverse effects was similar for first- and second-generation drugs.4-6
Approved and off-label indications: A look at the evidence
In addition to schizophrenia, many antipsychotics have US Food and Drug Administration (FDA) approval to treat various psychiatric and nonpsychiatric conditions (TABLE 1).7 Several are approved for use in bipolar disorder, 2 are approved as adjunctive treatment of major depressive disorder (MDD), and one is approved for the short-term treatment of generalized anxiety disorder (GAD). Porphyria, tetanus, and intractable hiccups are among the nonpsychiatric conditions for which some antipsychotics are approved.7
Evidence ranging from anecdotal to randomized controlled trials (RCTs) is steadily emerging about off-label uses of antipsychotics, with risperidone, quetiapine, and olanzapine foremost among them.8,9 Use of antipsychotics in the treatment of neuropsychiatric symptoms (NPS) of dementia has become particularly widespread, with off-label use of antipsychotics more prevalent in long-term care facilities than in outpatient settings.9
NPS. Antipsychotics’ efficacy in controlling dementia-related agitation, aggression, and psychosis has consistently shown a modest but statistically significant benefit. A Cochrane review found evidence that the use of risperidone and olanzapine resulted in improvements in agitation scale scores; risperidone was linked to improved scores on a psychosis scale, as well.10 A second meta-analysis showed small but statistically significant improvements in NPS with risperidone, olanzapine, and aripiprazole.8 Another study showed that nearly half (48%) of patients who had a positive response to risperidone relapsed when they stopped taking the drug.11
A rapidly aging population is expected to further increase the need for pharmacologic interventions to control NPS. Yet safety concerns about the use of antipsychotics in the elderly (more on this in a bit) call this practice into question.
Chronic pain. A 2008 Cochrane review analyzed the efficacy of antipsychotics for acute and chronic pain, pooling results of 11 studies of the treatment of conditions such as postherpetic neuralgia, tension headache, acute myocardial infarction (MI), and terminal cancer. Results from the pooled trials were described as mixed, although an overall statistically significant decrease in pain intensity was found.12
Polypharmacy. The simultaneous use of 2 or more antipsychotic agents is also increasingly prevalent,13 with levels exceeding 50% in one study of patients with schizophrenia.14 Because there is little data on the safety and efficacy of antipsychotic polypharmacy, this off-label approach should be considered only as a last resort.15
Off-label treatment of psychiatric conditions
GAD. In comparative effectiveness trials, quetiapine was found to be equal to both paroxetine and escitalopram for the treatment of GAD, with a favorable effect on symptoms 8 weeks after its initiation.8 Trials of other antipsychotics for the treatment of GAD have not demonstrated clear efficacy. Trifluoperazine is approved for GAD, as a short-term treatment.
MDD. Antipsychotics have been shown to be beneficial in the treatment of MDD, although only quetiapine and aripiprazole are approved (and only as adjunctive treatment). Evidence supports the use of both agents, as well as risperidone, as augmentation to selective serotonin reuptake inhibitors (SSRIs), and in pooled results from 5 placebo-controlled trials, quetiapine was found to be effective as monotherapy for MDD.9
Obsessive-compulsive disorder (OCD). Compared with placebo, risperidone showed a 4-fold increase in the likelihood of a favorable response (number needed to treat [NNT]=4) in patients with OCD,8 but the drug remains off-label for this purpose.
Posttraumatic stress disorder (PTSD). A meta-analysis of 7 studies demonstrated risperidone’s efficacy in the treatment of combat-related PTSD.9 In a large Veterans Administration study of patients with combat-related PTSD resistant to treatment with SSRIs, however, risperidone showed no benefit after 6 months of therapy.16 Antipsychotics have not been found beneficial for substance abuse, eating disorders, or insomnia.9
Identifying risk factos, monitoring for adverse effects
While FGAs carry an increased risk of EPS, SGAs increase the risk of obesity, hyperlipidemia, hypertension, and diabetes mellitus. The average life expectancy of patients with schizophrenia is 2 to 3 decades lower than that of age-matched controls,17 a finding largely attributed to the increased rate of cardiovascular disease. While this can be partly explained by differences in lifestyle and access to care, the metabolic effects of SGAs are a likely contributing factor.
Because of the adverse effects of FGAs and SGAs, the American Diabetes Association and American Psychiatric Association jointly issued guidelines addressing both the type and optimal frequency of monitoring for patients on antipsychotics (TABLE 2).18,19 Following them is critical, as both the initiation of an antipsychotic agent and any change in regimen can lead to the development—or exacerbation—of a number of diseases.
Before initiating antipsychotic therapy—or the first time you see a patient like Mr. B, whose care you will be monitoring—a thorough assessment of risk factors is needed. Foremost among them are overweight or obesity, insulin resistance or diabetes, a history of heart disease, and EPS.
In some cases, preexisting conditions and the potential harm of a specific drug must be weighed in determining which antipsychotic to prescribe. When adverse effects develop after drug therapy has been initiated, decisions about further actions should be based on both the degree of the unfavorable response and the availability of other treatments—and made, as appropriate, in consultation with the specialist who prescribed the drug.
CASE 1 › You tell Mr. B that metabolic side effects like weight gain, impaired glucose tolerance, and increased low-density lipoprotein cholesterol are common with SGAs like the one he is taking, and that you will monitor his fasting glucose levels to evaluate his risk for developing diabetes—starting with this visit. (Olanzapine, the drug he is taking, is 4 times more likely than an FGA to lead to diabetes.18)
You talk to him, too, about the importance of weight control and note that if his BMI increases by ≥1 point you will refer him to a nutritionist and recommend a structured exercise program. Finally, you schedule an appointment in 3 months.
Risks associated with older age and dementia
In 2010, there were 84,842 visits to US emergency departments (EDs) due to adverse drug events involving antipsychotic agents—a 110% increase since 2005. Nearly 30% of these ED visits involved patients 65 years or older.20
Among patients with dementia, use of antipsychotics has been found to dramatically increase the risk of stroke (rate ratio, 3.26 for FGAs and 5.86 for SGAs).21 The risk was greatest in the first 35 days of treatment, but persisted throughout the 175-day study period.
The rate of MI also was elevated in dementia patients (hazard ratio of 2.19 for the first 30 days of treatment, then falling to 1.15 for the first year).22 The risk of pulmonary embolism and deep vein thrombosis also rose for patients who had been on antipsychotics during the previous 24 months (odds ratio=1.32), with the highest risk within the first 90 days of treatment.23
Risk of death varies with agent and dose. Multiple studies have shown that the mortality risk associated with antipsychotics varies greatly among individual drugs, with haloperidol carrying the highest risk and quetiapine the lowest.24-26 The hazard ratio for death within the first 30 days was 3.2 for haloperidol, 1.6 for risperidone, and 1.5 for olanzapine; quetiapine had no statistically significant increase. The increased mortality risk was statistically significant only at higher doses.24
The FDA weighs in
Evidence of the elevated risk of death led the FDA to require black-box warnings on SGAs (in 2005)27 and FGAs (in 2008),28 stating that “antipsychotics are not indicated for the treatment of dementia-related psychosis.”28 More recently (in 2012), the American Geriatrics Society (AGS) published a guide on the management of NPS in patients with dementia.29 In it, the AGS acknowledges that despite FDA warnings, antipsychotics may be necessary for the treatment of NPS.
The AGS stresses the importance of nonpharmacologic interventions (eg, positive reinforcement, orientation to time and place, music, light exercise, pet therapy) as a first-line approach. If these measures fail and antipsychotics are necessary, the AGS calls for obtaining informed consent from a family member, using the lowest effective dose, and regularly attempting to wean the patient off the antipsychotic as the standard of care.28
CASE 2 › New or worsening aggressive behavior in an elderly patient with dementia requires a prompt assessment. You start with a complete medical evaluation of Ms. F, ruling out common causes of agitation such as infection, pain, constipation, and an adverse reaction to medication.
You also ask about the incidents of aggression: Does the same aide dress Ms. F daily? Does the aide introduce herself and explain what she’s about to do before attempting to dress the patient?
Next, you recommend nonpharmacologic therapies, such as calming music, participation in group activities, and pet therapy. You tell the floor nurse that if these measures fail and Ms. F’s threatening behavior continues, an antipsychotic may be considered.
Guard against abuse of antipsychotics
As antipsychotic use increases, so, too, does misuse and abuse, particularly of quetiapine. The drug has a reported street value of $3 to $8 for a 25- to 100-mg dose and is known as “quell,” “Susie-Q,” “and “baby heroin”; “Q-ball” is the name used for a combination of cocaine and quetiapine.30,31
The Drug Abuse Warning Network reported a 115% increase in ED visits related to the misuse or abuse of pharmaceuticals between 2004 and 2010.32 In 2010, 57,199 drug abuse cases—including 28,618 suicide attempts—were linked to antipsychotics.20
To optimize the benefit of antipsychotics and minimize the likelihood of abuse, ensure that every patient taking them has a clearly documented indication for an antipsychotic and a single responsible prescriber of the antipsychotic, often a psychiatrist. Your responsibilities: Schedule visits for monitoring, do a medication review to identify potential drug-drug interactions, and assess efficacy, all on a regular basis.
CASE 1 › At Mr. B’s next visit, you retest his fasting glucose (which is now 105 mg/dL) and recheck his BMI, which has climbed to 30. You tell him you will speak with his psychiatrist about his weight gain and your concern about the development of insulin resistance.
Meanwhile, you refer the patient to a nutritionist and encourage a healthy lifestyle. Because the medication has been effective, you schedule a follow-up visit in 6 weeks to see if the lifestyle interventions have been successful before consulting with the patient’s psychiatrist about a change in medication.
CASE 2 › When you return to the long-term care facility one week later, you find that Ms. F’s NPS have not abated. You realize an antipsychotic agent may be needed. Because she has a history of heart disease, however, she has a higher risk for cardiovascular events.
You meet with her son to review the benefits and risks of antipsychotic therapy, explaining that risperidone is a reasonable agent and that a low starting dose (0.25-0.5 mg) will reduce the risk. You obtain his informed consent, document your treatment goals—a decrease in threatening behavior and the ability of the staff to work with Ms. F to get her up and out of bed—and establish a plan to review in 2 weeks.
CORRESPONDENCE
Daniel DeJoseph, MD, Drexel Family Medicine, 3401 Market Street, Suite 105-B, Philadelphia, PA 19104; [email protected]
› Evaluate patients for movement disorders before initiating or adjusting antipsychotic therapy, then weekly until the dose is stabilized. A
› Use nonpharmacologic interventions—eg, positive reinforcement, music, light exercise—as first-line therapy for neuropsychiatric symptoms of dementia; consider antipsychotic therapy only if they fail. A
› Obtain a fasting glucose level before initiating or adjusting antipsychotic therapy, then at 12 weeks, and annually if the patient is taking a second-generation agent. B
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
CASE 1 › Steve B is a 43-year-old patient with bipolar disorder and a history of hypertension and high cholesterol. His body mass index (BMI) is 29. During a checkup, he tells you his psychiatrist recently started him on olanzapine. He reports that the medication is working, but he’s concerned about adverse effects, and asks whether he should be monitored for signs of diabetes.
CASE 2 › Mary F, an 83-year-old with Alzheimer’s disease and a history of stable coronary artery disease, is a resident in a long-term care facility, where staff members report that she is increasingly combative. The floor nurse says Ms. F has been striking out at the nurses’ aides who attempt to dress her and asks that you prescribe an antipsychotic to “calm her down.”
If Mr. B and Ms. F were your patients, what would you do?
In 1951, the chance discovery of an anesthetic’s calming properties was the first step in the development of the medications that came to be known as antipsychotics.1 In recent years, we have seen an expansion in both the number of antipsychotic agents on the market and the scope of their use, for conditions as varied as chronic pain, dementia, nausea and vomiting, and Tourette syndrome.
While antipsychotics often are prescribed by psychiatrists or other specialists, primary care physicians are increasingly likely to be involved in the management of patients who take them—and, at times, to prescribe antipsychotic agents themselves. We developed this guide to increase awareness of safe prescribing practices and principles guiding the initiation and management of antipsychotic agents. We start with a review of the mechanism of action of first- and second-generation antipsychotics (SGAs).
In the last decade, research has called into question whether second-generation antipsychotics really are more effective than first-generation agents
First- and second-generation agents: How they work and differ
Antipsychotics act at the level of the dopaminergic pathways in the central nervous system by blocking the D2 receptors. Action on the mesolimbic pathway is thought to be responsible for their effects on schizophrenia symptoms,2 while action at receptor sites in other dopaminergic pathways leads to common adverse effects, primarily the extrapyramidal symptoms (EPS) associated with first-generation antipsychotics (FGAs).
The distinction between first- and second-generation agents relates to SGAs’ blockage of serotonin receptors (thought to better relieve schizophrenia symptoms) and increased specificity for the mesolimbic pathway (which reduces the action on other dopamine pathways and is less likely to produce EPS).3 These differences largely accounted for the belief that SGAs were more effective and provided the rationale for their designation as atypical antipsychotics.
Are SGAs really better?
In the last decade, research has called such claims into question. Trials such as the Clinical Antipsychotic Trials of Intervention Effectiveness4 and Cost Utility of the Latest Antipsychotic Drugs in Schizophrenia study,5 as well as a meta-analysis,6 found that SGAs as a class are no more effective than FGAs. That said, 2 SGAs—clozapine and olanzapine—were found to be superior to FGAs for the treatment of schizophrenia. The studies also raised doubts about SGAs’ advantages regarding tolerability, as the time to discontinuation due to intolerable adverse effects was similar for first- and second-generation drugs.4-6
Approved and off-label indications: A look at the evidence
In addition to schizophrenia, many antipsychotics have US Food and Drug Administration (FDA) approval to treat various psychiatric and nonpsychiatric conditions (TABLE 1).7 Several are approved for use in bipolar disorder, 2 are approved as adjunctive treatment of major depressive disorder (MDD), and one is approved for the short-term treatment of generalized anxiety disorder (GAD). Porphyria, tetanus, and intractable hiccups are among the nonpsychiatric conditions for which some antipsychotics are approved.7
Evidence ranging from anecdotal to randomized controlled trials (RCTs) is steadily emerging about off-label uses of antipsychotics, with risperidone, quetiapine, and olanzapine foremost among them.8,9 Use of antipsychotics in the treatment of neuropsychiatric symptoms (NPS) of dementia has become particularly widespread, with off-label use of antipsychotics more prevalent in long-term care facilities than in outpatient settings.9
NPS. Antipsychotics’ efficacy in controlling dementia-related agitation, aggression, and psychosis has consistently shown a modest but statistically significant benefit. A Cochrane review found evidence that the use of risperidone and olanzapine resulted in improvements in agitation scale scores; risperidone was linked to improved scores on a psychosis scale, as well.10 A second meta-analysis showed small but statistically significant improvements in NPS with risperidone, olanzapine, and aripiprazole.8 Another study showed that nearly half (48%) of patients who had a positive response to risperidone relapsed when they stopped taking the drug.11
A rapidly aging population is expected to further increase the need for pharmacologic interventions to control NPS. Yet safety concerns about the use of antipsychotics in the elderly (more on this in a bit) call this practice into question.
Chronic pain. A 2008 Cochrane review analyzed the efficacy of antipsychotics for acute and chronic pain, pooling results of 11 studies of the treatment of conditions such as postherpetic neuralgia, tension headache, acute myocardial infarction (MI), and terminal cancer. Results from the pooled trials were described as mixed, although an overall statistically significant decrease in pain intensity was found.12
Polypharmacy. The simultaneous use of 2 or more antipsychotic agents is also increasingly prevalent,13 with levels exceeding 50% in one study of patients with schizophrenia.14 Because there is little data on the safety and efficacy of antipsychotic polypharmacy, this off-label approach should be considered only as a last resort.15
Off-label treatment of psychiatric conditions
GAD. In comparative effectiveness trials, quetiapine was found to be equal to both paroxetine and escitalopram for the treatment of GAD, with a favorable effect on symptoms 8 weeks after its initiation.8 Trials of other antipsychotics for the treatment of GAD have not demonstrated clear efficacy. Trifluoperazine is approved for GAD, as a short-term treatment.
MDD. Antipsychotics have been shown to be beneficial in the treatment of MDD, although only quetiapine and aripiprazole are approved (and only as adjunctive treatment). Evidence supports the use of both agents, as well as risperidone, as augmentation to selective serotonin reuptake inhibitors (SSRIs), and in pooled results from 5 placebo-controlled trials, quetiapine was found to be effective as monotherapy for MDD.9
Obsessive-compulsive disorder (OCD). Compared with placebo, risperidone showed a 4-fold increase in the likelihood of a favorable response (number needed to treat [NNT]=4) in patients with OCD,8 but the drug remains off-label for this purpose.
Posttraumatic stress disorder (PTSD). A meta-analysis of 7 studies demonstrated risperidone’s efficacy in the treatment of combat-related PTSD.9 In a large Veterans Administration study of patients with combat-related PTSD resistant to treatment with SSRIs, however, risperidone showed no benefit after 6 months of therapy.16 Antipsychotics have not been found beneficial for substance abuse, eating disorders, or insomnia.9
Identifying risk factos, monitoring for adverse effects
While FGAs carry an increased risk of EPS, SGAs increase the risk of obesity, hyperlipidemia, hypertension, and diabetes mellitus. The average life expectancy of patients with schizophrenia is 2 to 3 decades lower than that of age-matched controls,17 a finding largely attributed to the increased rate of cardiovascular disease. While this can be partly explained by differences in lifestyle and access to care, the metabolic effects of SGAs are a likely contributing factor.
Because of the adverse effects of FGAs and SGAs, the American Diabetes Association and American Psychiatric Association jointly issued guidelines addressing both the type and optimal frequency of monitoring for patients on antipsychotics (TABLE 2).18,19 Following them is critical, as both the initiation of an antipsychotic agent and any change in regimen can lead to the development—or exacerbation—of a number of diseases.
Before initiating antipsychotic therapy—or the first time you see a patient like Mr. B, whose care you will be monitoring—a thorough assessment of risk factors is needed. Foremost among them are overweight or obesity, insulin resistance or diabetes, a history of heart disease, and EPS.
In some cases, preexisting conditions and the potential harm of a specific drug must be weighed in determining which antipsychotic to prescribe. When adverse effects develop after drug therapy has been initiated, decisions about further actions should be based on both the degree of the unfavorable response and the availability of other treatments—and made, as appropriate, in consultation with the specialist who prescribed the drug.
CASE 1 › You tell Mr. B that metabolic side effects like weight gain, impaired glucose tolerance, and increased low-density lipoprotein cholesterol are common with SGAs like the one he is taking, and that you will monitor his fasting glucose levels to evaluate his risk for developing diabetes—starting with this visit. (Olanzapine, the drug he is taking, is 4 times more likely than an FGA to lead to diabetes.18)
You talk to him, too, about the importance of weight control and note that if his BMI increases by ≥1 point you will refer him to a nutritionist and recommend a structured exercise program. Finally, you schedule an appointment in 3 months.
Risks associated with older age and dementia
In 2010, there were 84,842 visits to US emergency departments (EDs) due to adverse drug events involving antipsychotic agents—a 110% increase since 2005. Nearly 30% of these ED visits involved patients 65 years or older.20
Among patients with dementia, use of antipsychotics has been found to dramatically increase the risk of stroke (rate ratio, 3.26 for FGAs and 5.86 for SGAs).21 The risk was greatest in the first 35 days of treatment, but persisted throughout the 175-day study period.
The rate of MI also was elevated in dementia patients (hazard ratio of 2.19 for the first 30 days of treatment, then falling to 1.15 for the first year).22 The risk of pulmonary embolism and deep vein thrombosis also rose for patients who had been on antipsychotics during the previous 24 months (odds ratio=1.32), with the highest risk within the first 90 days of treatment.23
Risk of death varies with agent and dose. Multiple studies have shown that the mortality risk associated with antipsychotics varies greatly among individual drugs, with haloperidol carrying the highest risk and quetiapine the lowest.24-26 The hazard ratio for death within the first 30 days was 3.2 for haloperidol, 1.6 for risperidone, and 1.5 for olanzapine; quetiapine had no statistically significant increase. The increased mortality risk was statistically significant only at higher doses.24
The FDA weighs in
Evidence of the elevated risk of death led the FDA to require black-box warnings on SGAs (in 2005)27 and FGAs (in 2008),28 stating that “antipsychotics are not indicated for the treatment of dementia-related psychosis.”28 More recently (in 2012), the American Geriatrics Society (AGS) published a guide on the management of NPS in patients with dementia.29 In it, the AGS acknowledges that despite FDA warnings, antipsychotics may be necessary for the treatment of NPS.
The AGS stresses the importance of nonpharmacologic interventions (eg, positive reinforcement, orientation to time and place, music, light exercise, pet therapy) as a first-line approach. If these measures fail and antipsychotics are necessary, the AGS calls for obtaining informed consent from a family member, using the lowest effective dose, and regularly attempting to wean the patient off the antipsychotic as the standard of care.28
CASE 2 › New or worsening aggressive behavior in an elderly patient with dementia requires a prompt assessment. You start with a complete medical evaluation of Ms. F, ruling out common causes of agitation such as infection, pain, constipation, and an adverse reaction to medication.
You also ask about the incidents of aggression: Does the same aide dress Ms. F daily? Does the aide introduce herself and explain what she’s about to do before attempting to dress the patient?
Next, you recommend nonpharmacologic therapies, such as calming music, participation in group activities, and pet therapy. You tell the floor nurse that if these measures fail and Ms. F’s threatening behavior continues, an antipsychotic may be considered.
Guard against abuse of antipsychotics
As antipsychotic use increases, so, too, does misuse and abuse, particularly of quetiapine. The drug has a reported street value of $3 to $8 for a 25- to 100-mg dose and is known as “quell,” “Susie-Q,” “and “baby heroin”; “Q-ball” is the name used for a combination of cocaine and quetiapine.30,31
The Drug Abuse Warning Network reported a 115% increase in ED visits related to the misuse or abuse of pharmaceuticals between 2004 and 2010.32 In 2010, 57,199 drug abuse cases—including 28,618 suicide attempts—were linked to antipsychotics.20
To optimize the benefit of antipsychotics and minimize the likelihood of abuse, ensure that every patient taking them has a clearly documented indication for an antipsychotic and a single responsible prescriber of the antipsychotic, often a psychiatrist. Your responsibilities: Schedule visits for monitoring, do a medication review to identify potential drug-drug interactions, and assess efficacy, all on a regular basis.
CASE 1 › At Mr. B’s next visit, you retest his fasting glucose (which is now 105 mg/dL) and recheck his BMI, which has climbed to 30. You tell him you will speak with his psychiatrist about his weight gain and your concern about the development of insulin resistance.
Meanwhile, you refer the patient to a nutritionist and encourage a healthy lifestyle. Because the medication has been effective, you schedule a follow-up visit in 6 weeks to see if the lifestyle interventions have been successful before consulting with the patient’s psychiatrist about a change in medication.
CASE 2 › When you return to the long-term care facility one week later, you find that Ms. F’s NPS have not abated. You realize an antipsychotic agent may be needed. Because she has a history of heart disease, however, she has a higher risk for cardiovascular events.
You meet with her son to review the benefits and risks of antipsychotic therapy, explaining that risperidone is a reasonable agent and that a low starting dose (0.25-0.5 mg) will reduce the risk. You obtain his informed consent, document your treatment goals—a decrease in threatening behavior and the ability of the staff to work with Ms. F to get her up and out of bed—and establish a plan to review in 2 weeks.
CORRESPONDENCE
Daniel DeJoseph, MD, Drexel Family Medicine, 3401 Market Street, Suite 105-B, Philadelphia, PA 19104; [email protected]
1. Shen WW. A history of antipsychotic drug development. Compr Psychiatry. 1999;40:407-414.
2. Miller R. Mechanism of action of antipsychotic drugs of different classes, refractoriness to therapeutic effects of classical neuroleptics, and individual variation in sensitivity to their actions. Curr Neuropharmacol. 2009;7:302-314.
3. Seeman P. Atypical antipsychotics: mechanism of action. Can J Psychiatry. 2002;47:27-38.
4. Lieberman JA, Stroup TS, McEvoy JP, et al; Clinical Antipsychotic Trials of Intervention Effectiveness (CATIE) Investigators. Effectiveness of antipsychotic drugs in patients with chronic schizophrenia. N Engl J Med. 2005;353:1209-1223.
5. Jones PB, Barnes TR, Davies L, et al. Randomized controlled trial of the effect on quality of life of second- vs first-generation antipsychotic drugs in schizophrenia: Cost Utility of the Latest Antipsychotic Drugs in Schizophrenia Study (CUtLASS 1). Arch Gen Psychiatry. 2006;63:1079-1087.
6. Leucht S, Corves C, Arbter D, et al. Second-generation versus first-generation antipsychotic drugs for schizophrenia: a meta-analysis. Lancet. 2009;373:31-41.
7. Christian R, Saavedra L, Gaynes BN, et al. Future research needs for first- and second-generation antipsychotics for children and young adults [Internet]. Agency for Healthcare Research and Quality. 2012:12-EHC042-EF.
8. Maher AR, Maglione M, Bagley S, et al. Efficacy and comparative effectiveness of atypical antipsychotic medications for off-label uses in adults: a systematic review and meta-analysis. JAMA. 2011;306:1359-1369.
9. Maglione M, Maher AR, Hu J, et al. Off-label use of atypical antipsychotics: An update [Internet]. Agency for Healthcare Research and Quality. 2011:11-EHC087-EF.
10. Ballard CG, Waite J. Atypical antipsychotics for aggression and psychosis in Alzheimer’s disease. Cochrane Database Syst Rev. 2006;(1):CD003476.
11. Devanand DP, Mintzer J, Schultz SK, et al. Relapse risk after discontinuation of risperidone in Alzheimer’s disease. N Engl J Med. 2012;367:1497-1507.
12. Seidel S, Aigner M, Ossege M, et al. Antipsychotics for acute and chronic pain in adults. Cochrane Database Syst Rev. 2008;(4):CD004844.
13. Mojtabai R, Olfson M. National trends in psychotropic medication polypharmacy in office-based psychiatry. Arch Gen Psychiatry. 2010;67:26-36.
14. Faries D, Ascher-Svanum H, Zhu B, et al. Antipsychotic monotherapy and polypharmacy in the naturalistic treatment of schizophrenia with atypical antipsychotics. BMC Psychiatry. 2005;5:26.
15. Ballon J, Stroup TS. Polypharmacy for schizophrenia. Curr Opin Psychiatry. 2013;26:208-213.
16. Krystal JH, Rosenheck RA, Cramer JA, et al; Veterans Affairs Cooperative Study No. 504 Group. Adjunctive risperidone treatment for antidepressant-resistant symptoms of chronic military service-related PTSD: a randomized trial. JAMA. 2011;306:493-502.
17. Institute of Medicine. Retooling for an Aging America: Building the Health Care Workforce. Washington, DC: National Academy of Sciences; 2008.
18. Marder SR, Essock SM, Miller AL, et al. Physical health monitoring of patients with schizophrenia. Am J Psychiatry. 2004;161:1334-1349.
19. Barrett E, Blonde L, Clement S, et al. Consensus development conference on antipsychotic drugs and obesity and diabetes. Diabetes Care. 2004;27:596-601.
20. Drug Abuse Warning Network, 2010: National Estimates of Drug-Related Emergency Department Visits. HHS Publication No. (SMA) 12-4733, DAWN Series D-38. Substance Abuse and Mental Health Services Administration Web site. Available at: http://www.samhsa.gov/data/2k13/DAWN2k10ED/DAWN2k10ED.htm. Accessed May 1, 2013.
21. Douglas IJ, Smeeth L. Exposure to antipsychotics and risk of stroke: self controlled case series study. BMJ. 2008;337:a1227.
22. Pariente A, Fourrier-Réglat A, Ducruet T, et al. Antipsychotic use and myocardial infarction in older patients with treated dementia. Arch Intern Med. 2012;172:648-653.
23. Parker C, Coupland C, Hippisley-Cox J. Antipsychotic drugs and risk of venous thromboembolism: nested case-control study. BMJ. 2010;341:c4245.
24. Rossom RC, Rector TS, Lederle FA, et al. Are all commonly prescribed antipsychotics associated with greater mortality in elderly male veterans with dementia? J Am Geriatr Soc. 2010;58:1027-1034.
25. Kales HC, Kim HM, Zivin K, et al. Risk of mortality among individual antipsychotics in patients with dementia. Am J Psychiatry. 2012;169:71-79.
26. Huybrechts KF, Gerhard T, Crystal S, et al. Differential risk of death in older residents in nursing homes prescribed specific antipsychotic drugs: population based cohort study. BMJ. 2012;344:e977.
27. US Food and Drug Public Health Advisory: Deaths with antipsychotics in elderly patients with behavioral disturbances. US Food and Drug Administration Web site Available at: http://1.usa.gov/1plsxPk. Accessed February 5, 2014.
28. Information for healthcare professionals: conventional antipsychotics. US Food and Drug Administration Web site. Available at: http://www.fda.gov/drugs/drugsafety/postmarketdrugsafetyinformationforpatientsandproviders/ucm124830.htm. Accessed February 5, 2014.
29. Guide to the management of psychotic disorders and neuropsychiatric symptoms of dementia in older adults. American Geriatric Society Web site. Available at: http://dementia.americangeriatrics.org/GeriPsych_index.php. Accessed April 15, 2013.
30. Bogart GT, Ott CA. Abuse of second-generation antipsychotics: What prescribers need to know. Curr Psychiatr. 2011;10:77-79.
31. Tarosoff G, Osti K. Black-market value of antipsychotics, antidepressants, and hypnotics in Las Vegas, Nevada. Am J Psychiatry. 2007;164:350.
32. Highlights of the 2010 Drug Abuse Warning Network (DAWN) findings on drug-related emergency department visits. Substance Abuse and Mental Health Services Administration, Center for Behavioral Health Statistics and Quality Web site. Available at: http://www.samhsa.gov/data/2k12/DAWN096/SR096EDHighlights2010.htm. Accessed May 1, 2013.
1. Shen WW. A history of antipsychotic drug development. Compr Psychiatry. 1999;40:407-414.
2. Miller R. Mechanism of action of antipsychotic drugs of different classes, refractoriness to therapeutic effects of classical neuroleptics, and individual variation in sensitivity to their actions. Curr Neuropharmacol. 2009;7:302-314.
3. Seeman P. Atypical antipsychotics: mechanism of action. Can J Psychiatry. 2002;47:27-38.
4. Lieberman JA, Stroup TS, McEvoy JP, et al; Clinical Antipsychotic Trials of Intervention Effectiveness (CATIE) Investigators. Effectiveness of antipsychotic drugs in patients with chronic schizophrenia. N Engl J Med. 2005;353:1209-1223.
5. Jones PB, Barnes TR, Davies L, et al. Randomized controlled trial of the effect on quality of life of second- vs first-generation antipsychotic drugs in schizophrenia: Cost Utility of the Latest Antipsychotic Drugs in Schizophrenia Study (CUtLASS 1). Arch Gen Psychiatry. 2006;63:1079-1087.
6. Leucht S, Corves C, Arbter D, et al. Second-generation versus first-generation antipsychotic drugs for schizophrenia: a meta-analysis. Lancet. 2009;373:31-41.
7. Christian R, Saavedra L, Gaynes BN, et al. Future research needs for first- and second-generation antipsychotics for children and young adults [Internet]. Agency for Healthcare Research and Quality. 2012:12-EHC042-EF.
8. Maher AR, Maglione M, Bagley S, et al. Efficacy and comparative effectiveness of atypical antipsychotic medications for off-label uses in adults: a systematic review and meta-analysis. JAMA. 2011;306:1359-1369.
9. Maglione M, Maher AR, Hu J, et al. Off-label use of atypical antipsychotics: An update [Internet]. Agency for Healthcare Research and Quality. 2011:11-EHC087-EF.
10. Ballard CG, Waite J. Atypical antipsychotics for aggression and psychosis in Alzheimer’s disease. Cochrane Database Syst Rev. 2006;(1):CD003476.
11. Devanand DP, Mintzer J, Schultz SK, et al. Relapse risk after discontinuation of risperidone in Alzheimer’s disease. N Engl J Med. 2012;367:1497-1507.
12. Seidel S, Aigner M, Ossege M, et al. Antipsychotics for acute and chronic pain in adults. Cochrane Database Syst Rev. 2008;(4):CD004844.
13. Mojtabai R, Olfson M. National trends in psychotropic medication polypharmacy in office-based psychiatry. Arch Gen Psychiatry. 2010;67:26-36.
14. Faries D, Ascher-Svanum H, Zhu B, et al. Antipsychotic monotherapy and polypharmacy in the naturalistic treatment of schizophrenia with atypical antipsychotics. BMC Psychiatry. 2005;5:26.
15. Ballon J, Stroup TS. Polypharmacy for schizophrenia. Curr Opin Psychiatry. 2013;26:208-213.
16. Krystal JH, Rosenheck RA, Cramer JA, et al; Veterans Affairs Cooperative Study No. 504 Group. Adjunctive risperidone treatment for antidepressant-resistant symptoms of chronic military service-related PTSD: a randomized trial. JAMA. 2011;306:493-502.
17. Institute of Medicine. Retooling for an Aging America: Building the Health Care Workforce. Washington, DC: National Academy of Sciences; 2008.
18. Marder SR, Essock SM, Miller AL, et al. Physical health monitoring of patients with schizophrenia. Am J Psychiatry. 2004;161:1334-1349.
19. Barrett E, Blonde L, Clement S, et al. Consensus development conference on antipsychotic drugs and obesity and diabetes. Diabetes Care. 2004;27:596-601.
20. Drug Abuse Warning Network, 2010: National Estimates of Drug-Related Emergency Department Visits. HHS Publication No. (SMA) 12-4733, DAWN Series D-38. Substance Abuse and Mental Health Services Administration Web site. Available at: http://www.samhsa.gov/data/2k13/DAWN2k10ED/DAWN2k10ED.htm. Accessed May 1, 2013.
21. Douglas IJ, Smeeth L. Exposure to antipsychotics and risk of stroke: self controlled case series study. BMJ. 2008;337:a1227.
22. Pariente A, Fourrier-Réglat A, Ducruet T, et al. Antipsychotic use and myocardial infarction in older patients with treated dementia. Arch Intern Med. 2012;172:648-653.
23. Parker C, Coupland C, Hippisley-Cox J. Antipsychotic drugs and risk of venous thromboembolism: nested case-control study. BMJ. 2010;341:c4245.
24. Rossom RC, Rector TS, Lederle FA, et al. Are all commonly prescribed antipsychotics associated with greater mortality in elderly male veterans with dementia? J Am Geriatr Soc. 2010;58:1027-1034.
25. Kales HC, Kim HM, Zivin K, et al. Risk of mortality among individual antipsychotics in patients with dementia. Am J Psychiatry. 2012;169:71-79.
26. Huybrechts KF, Gerhard T, Crystal S, et al. Differential risk of death in older residents in nursing homes prescribed specific antipsychotic drugs: population based cohort study. BMJ. 2012;344:e977.
27. US Food and Drug Public Health Advisory: Deaths with antipsychotics in elderly patients with behavioral disturbances. US Food and Drug Administration Web site Available at: http://1.usa.gov/1plsxPk. Accessed February 5, 2014.
28. Information for healthcare professionals: conventional antipsychotics. US Food and Drug Administration Web site. Available at: http://www.fda.gov/drugs/drugsafety/postmarketdrugsafetyinformationforpatientsandproviders/ucm124830.htm. Accessed February 5, 2014.
29. Guide to the management of psychotic disorders and neuropsychiatric symptoms of dementia in older adults. American Geriatric Society Web site. Available at: http://dementia.americangeriatrics.org/GeriPsych_index.php. Accessed April 15, 2013.
30. Bogart GT, Ott CA. Abuse of second-generation antipsychotics: What prescribers need to know. Curr Psychiatr. 2011;10:77-79.
31. Tarosoff G, Osti K. Black-market value of antipsychotics, antidepressants, and hypnotics in Las Vegas, Nevada. Am J Psychiatry. 2007;164:350.
32. Highlights of the 2010 Drug Abuse Warning Network (DAWN) findings on drug-related emergency department visits. Substance Abuse and Mental Health Services Administration, Center for Behavioral Health Statistics and Quality Web site. Available at: http://www.samhsa.gov/data/2k12/DAWN096/SR096EDHighlights2010.htm. Accessed May 1, 2013.
Pediatric hypertension: Often missed and mismanaged
› Screen for hypertension in all children over the age of 3 at every visit. C
› Order laboratory evaluation, echocardiography, and renovascular imaging for all children given a diagnosis of hypertension. C
› Advise parents that children with prehypertension and stage 1 hypertension without target-organ damage are eligible to participate in competitive athletics, but those with stage 2 hypertension, target-organ damage, or symptomatic hypertension should not engage in high-static sports (eg, gymnastics, weightlifting, wrestling) until BP is well controlled. C
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Childhood hypertension is on the rise: Recent data from the National Health and Nutrition Survey suggest 10% of children and adolescents have prehypertension and 4% have hypertension.1-4 Unfortunately, the condition often is missed. In a study of 14,187 children and adolescents who had at least 3 well-child visits at an outpatient academic medical center, 507 patients met the criteria for hypertension, yet only 131 (26%) had this diagnosis documented in their electronic health record.5
In a survey of 89 pediatricians, >50% of respondents said they were not familiar with the most current published recommendations for diagnosing and treating pediatric hypertension.6 Respondents also indicated that the most common reason for not initiating pharmacotherapy for children with hypertension was a lack of familiarity with appropriate antihypertensive agents (54%), followed by concern for adverse medication effects. Delayed diagnosis, evaluation, and treatment of hypertension in young patients can increase the likelihood of serious consequences, including target-organ damage such as left ventricular hypertrophy (LVH). In this review, we’ll describe the factors that put children and adolescents at risk for hypertension, and offer an evidence-based approach to diagnosis and treatment.
Obesity is a key risk factor
An estimated 17% of children aged 2 to 19 are obese.7 Obesity increases a child’s risk for hypertension by approximately 3- to 5-fold, and body mass index (BMI) is greater in children with primary hypertension compared with those with secondary hypertension.8 Hypertension is more common among Hispanic and non-Hispanic black male children and adolescents compared with their white counterparts; these ethnic disparities are not found in females.9,10 Poor diets and physical inactivity further contribute to obesity and hypertension risk. Children who were born preterm or had a very low birth weight also are at increased risk.11
Unchecked hypertension can lead to cardiac, vascular damage
Some children and adolescents with undiagnosed and untreated hypertension have evidence of target-organ damage, including cardiac dysfunction and pathologic vascular abnormalities. LVH is present in 20% to 41% of children and adolescents with hypertension.12,13 Carotid intima-media thickness, an established surrogate marker for atherosclerosis, is abnormally increased in children with hypertension, even after adjusting for BMI.14 Other target organ effects include impaired cognitive function, reduced glomerular filtration rate, microalbuminuria, and retinal arteriolar narrowing.15-17
Pediatric hypertension may persist into adulthood. A meta-analysis of more than 50 studies found that elevated blood pressure (BP) in childhood increases the risk for hypertension as an adult.18
NHLBI recommendations call for a BP check at every visit
The National Heart, Lung, and Blood Institute (NHLBI) Fourth Report on the Diagnosis, Evaluation, and Treatment of High Blood Pressure in Children and Adolescents (“the 4th Report”) recommends measuring BP in all children over age 3 during every health care visit.12 Children under age 3 should have their BP checked in certain circumstances, including preterm delivery, congenital heart disease, recurrent urinary tract infections, renal/urologic disease, organ transplantation, malignancy, and systemic illnesses associated with hypertension.12 The 4th Report is endorsed by the American Academy of Pediatrics (AAP); however, the American Academy of Family Physicians and the US Preventive Services Task Force have concluded that the evidence is insufficient to recommend for or against routine screening for hypertension in children and adolescents to reduce the risk of cardiovascular disease (CVD).19,20
Does the child have hypertension? That depends on several factors
Determining whether a child has hypertension requires that you consult national BP standards to determine if he or she is within the normal range. Normal BP standards for children and adolescents are based on gender, age, and height percentile, and provide a precise classification based on body size.12 These tables are available from the NHLBI Web site at http://www.nhlbi.nih.gov/guidelines/hypertension/child_tbl.htm. Height percentiles in these tables correspond with the Centers for Disease Control and Prevention (CDC) growth charts published in 2000.21 The Baylor College of Medicine Children’s Nutrition Research Center has a web-based calculator to help physicians determine BP percentiles in children and adolescents; it is available at http://www.bcm.edu/bodycomplab/Flashapps/BPVAgeChartpage.html. The International Pediatric Hypertension Association also offers BP charts and calculators at http://www.iphapediatrichypertension.org.
The diagnostic parameters for pediatric hypertension are listed in TABLE 1.12 The higher systolic or diastolic BP percentile value is used to determine a child’s overall BP category. A child is considered normotensive if the BP is <90th percentile. Hypertension is an average systolic or diastolic BP that is ≥95th percentile on at least 3 separate occasions. Stage 1 hypertension is BP levels ranging from the 95th percentile to 5 mm Hg above the 99th percentile, and stage 2 hypertension is BP levels greater than 5 mm Hg above the 99th percentile.
For example, assume you are evaluating a 12-year-old boy who is 61 inches tall and has a BP of 129/87 mm Hg. According to the CDC growth charts, his height puts him in the 75th percentile for his age. Using the NHLBI chart, you determine that he falls in the 95th-99th percentile for BP, and thus, using the categories in TABLE 1, is given a diagnosis of Stage 1 hypertension.
Accurate BP measurement requires using an appropriate cuff size that covers 80% of the child’s upper arm. When the child is between cuff sizes, use the larger cuff because small cuffs overestimate BP readings. BP readings should be taken on the right arm with the arm supported at heart level after the child has been sitting quietly for at least 5 minutes.12 One study showed that the initial BP readings taken in the triage area were significantly higher—often by >10 mm Hg—compared with follow-up measurements in the examination room.22
The preferred method of BP measurement is auscultation; however, oscillometric devices also are acceptable. These devices are easier to use, help eliminate digit bias, and minimize observer variation, but they typically read approximately 6 to 9 mm Hg higher than auscultation.23 For any BP measurement obtained by oscillometry that is >90th percentile, repeat the measurement by auscultation at least twice during the same office visit, and use an average of the repeated measurements.12 Obtain measurements of a lower extremity when you suspect congenital heart disease (eg, aortic coarctation). For any patient in whom you confirm a BP measurement >95th percentile, repeat the measurement within 2 weeks; for BPs >99th percentile, reevaluation should occur within one week.
Ambulatory BP monitoring (ABPM). Because BP measurements have a circadian pattern (higher during the day and reduced by 10% during sleep24) an ABPM device that provides 50 to 60 readings over 24 hours can be useful when evaluating children and adolescents for white-coat hypertension (elevated clinic BP with normal ambulatory BP), masked hypertension (normal clinic BP with elevated ambulatory BP), prehypertension and secondary hypertension (BP generally does not follow circadian patterns).25 ABPM is more accurate than BP self-measurement, but usually is limited to children older than age 5
Steps to take for clinical evaluation
Start by conducting a thorough history and physical examination, looking for information that can help you select the most appropriate tests for the next phase of evaluation.8,12 Calculate the patient’s BMI to screen for obesity, ask about a family history of hypertension or CVD, and determine if the patient is taking any medications that might cause hypertension, such as amphetamines, corticosteroids, or cyclosporine.8 Assess for signs and symptoms that suggest an underlying disease, such as renal disease (hematuria, edema, fatigue) or heart disease (chest pain, exertional dyspnea, palpitations).12
All children diagnosed with hypertension should be screened for secondary causes (TABLE 2). The recommended evaluation is to obtain a renal function panel, electrolytes, urinalysis, urine culture, complete blood count, renovascular imaging, and echocardiogram.12 The most common etiologies for secondary hypertension are renal parenchymal disease (68%), renovascular abnormalities (10%), and endocrinopathies (10%).26 Other causes, such as aortic coarctation, obstructive sleep apnea, iatrogenic factors (eg, toxins, medications, drugs of abuse), and genitourinary abnormalities, account for only a small percentage of cases and should be investigated as clinically indicated.26
Renovascular assessment depends on facility expertise. Imaging options include renal ultrasound (with or without Doppler), computed tomography angiography, renal flow scan, and magnetic resonance angiography. These studies have similar sensitivities and specificities.27 For patients in whom you strongly suspect renovascular disease, renal arteriography (digital subtraction angiography) provides the best images, although it is the most invasive study.27
Refer children and adolescents who are found to have significant abnormalities during the initial evaluation to the appropriate specialist. BP measurements often improve when secondary causes are treated.
Which drugs for which patients?
Pharmacologic management is indicated for pediatric patients with stage 1 or stage 2 hypertension, secondary hypertension, and those with evidence of target-organ damage.12 The goal of therapy is to reduce BP to <95th percentile. In patients with target organ damage, renal disease, or diabetes mellitus, the goal is <90th percentile.12,15,28 Intensive management of BP (≤50th percentile) in children with chronic kidney disease has been shown to delay progression to renal failure,29 but it is uncertain if lower BP goals can slow or prevent additional subclinical target organ damage.
Pharmacotherapy for hypertensive children or adolescents can be challenging because recommendations of which medication to use are based upon expert opinion and extrapolation from randomized trials of adults. The length of therapy (often lifelong), potential adverse effects, and unproven direct mortality benefit complicate this decision. Medication choice usually is based on physician preference or experience.12 The most common antihypertensive drugs prescribed are angiotensin-converting enzyme (ACE) inhibitors (26%), followed by diuretics (20%), and beta-blockers (17%).30,31 The starting doses and other details of medications commonly used to treat pediatric hypertension are listed in TABLE 3.28,32-34
One approach to choosing an antihypertensive drug for children is to measure the patient’s ambulatory plasma renin activity (PRA) level before initiating therapy. Those with high PRA levels (>0.65 ng/mL/h), presumably due to peripheral vasoconstriction, may benefit more from ACE inhibitors, angiotensin receptor blockers (ARBs), or beta-adrenergic antagonists.35 Individuals with low PRA levels (<0.65 ng/mL/h) maintain higher volume/sodium excess and may benefit more from diuretics or calcium channel blockers.35
Ethnicity also may guide medication selection. African American adults do not respond well to ACE inhibitor monotherapy due to decreased PRA and increased salt hypersensitivity.36 One meta-analysis found that African American children and adolescents had inadequate BP response to 6 individual ACE inhibitors, even at higher doses compared with white children and those of other ethnicities, who showed significant improvement in BP.37 ARBs may be a more effective alternative for this population.
Most experts recommend initiating a single agent at a low dose.12 A systematic review found that except for African American children, pediatric patients experienced comparable reductions in BP with ACE inhibitors (10.7/8.1 mm Hg), ARBs (10.5/6.9 mm Hg), and calcium channel blockers (9.3/7.2 mm Hg).38 In addition, ACE inhibitors and ARBs significantly reduced proteinuria by 49% and 59%, respectively.38
Schedule follow-up visits for 2 to 4 weeks (or sooner for patients with stage 2 hypertension) after initiating pharmacotherapy. If BP response is suboptimal, consider increasing the dose before adding a second agent. If the patient experiences significant adverse effects or has an inadequate BP response, changing to a drug from a different class is recommended.39 Patients who do not adequately respond to these approaches may require combination therapy; in such cases, strongly consider consultation with pediatric nephrologist or cardiologist.39 Medication compliance should be verified (eg, by pill counting, parental supervision) in patients who do not respond to therapy. Once BP control has been achieved, visits every 3 to 4 months are appropriate, with periodic laboratory monitoring, especially for children taking diuretics, ACE inhibitors, or ARBs or who have underlying renal disease.
Recommend exercise, but carefully monitor athlete's BP
Encourage obese and overweight children and adolescents to lose weight to maintain a BMI <95th percentile. Current guidelines based on expert opinion recommend that children and adolescents should engage in 60 minutes of daily physical activity.12 A meta-analysis found physical activity led to a 1% and 3% reduction in systolic and diastolic BP, respectively, although these results were not statistically significant.40
Be aware, however, that children and adolescents with hypertension who engage in certain competitive sports can significantly increase their BP and may be at risk for complications.41 According to the AAP guidelines, patients with stage 2 hypertension should not engage in high-static sports (eg, gymnastics, weightlifting, wrestling, boxing, cycling, decathlon, triathlon) until BP is well controlled.41 Patients with target-organ damage, uncontrolled hypertension, or symptomatic hypertension should not participate until BP is well controlled. Patients with prehypertension and stage 1 hypertension without target-organ damage are eligible to participate in competitive athletics. Reassess BP every 6 months in patients who are prehypertensive and every one to 2 weeks for those with stage 1 hypertension. When the patient’s BP remains <90th percentile, routine surveillance every 3 to 6 months is recommended.
What about sodium? Encourage parents of pediatric patients with hypertension to limit their child’s salt intake to 1.2 g/d for those age 4 to 8 and 1.5 g/d for older children.42 A meta-analysis found salt reduction decreased systolic BP by 1.2 mm Hg and diastolic BP by 1.3 mm Hg.43 Though these benefits are small, reducing sodium intake can be one of several lifestyle modifications, such as increased activity and quitting smoking, that can reduce young patients’ risk of hypertension and related cardiovascular sequelae.
CORRESPONDENCE
Robert Gauer, MD, Womack Army Medical Center, 2817 Reilly Road, Fort Bragg NC 28310; [email protected]
1. McNiece KL, Poffenbarger TS, Tuner JL, et al. Prevalence of hypertension and pre-hypertension among adolescents. J Pediatr. 2007;150:640-644.e1.
2. Moore WE, Eichner JE, Cohn EM, et al. Blood pressure screening of school children in a multiracial school district: the Healthy Kids Project. Am J Hypertens. 2009;22:351-356.
3. Falkner B. What exactly do the trends mean? Circulation. 2007;116:1437-1439.
4. Feber J, Ahmed M. Hypertension in children: new trends and challenges. Clin Sci (Lond). 2010;119:151-161.
5. Hansen ML, Gunn PW, Kaelber DC. Under diagnosis of hypertension in children and adolescents. JAMA. 2007;298:874-879.
6. Boneparth A, Flynn JT. Evaluation and treatment of hypertension in general pediatric practice. Clin Pediatr (Phila). 2009;48:44-49.
7. Ogden CL, Carroll MD, Kit BK, et al. Prevalence of obesity and trends in body mass index among US children and adolescents, 1999-2010. JAMA. 2012;307:483-490.
8. Feld LG, Corey H. Hypertension in childhood. Pediatr Rev. 2007;28:283-298.
9. Rosner B, Cook N, Portman R, et al. Blood pressure differences by ethnic group among United States children and adolescents. Hypertension. 2009;54:502-508.
10. Din-Dzietham R, Liu Y, Bielo MV, et al. High blood pressure trends in children and adolescents in national surveys, 1963 to 2002. Circulation. 2007;116:1488-1496.
11. de Jong F, Monuteaux MC, van Elburg RM, et al. Systematic review and meta-analysis of preterm birth and later systolic blood pressure. Hypertension. 2012;59:226-234.
12. National High Blood Pressure Education Program Working Group on High Blood Pressure in Children and Adolescents. The fourth report on the diagnosis, evaluation, and treatment of high blood pressure in children and adolescents. Pediatrics. 2004;114(2 suppl 4th report):555-576.
13. Ramaswamy P, Lytrivi ID, Paul C, et al. Regression of left ventricular hypertrophy in children with antihypertensive therapy. Pediatr Nephrol. 2007;22:141-143.
14. Lande MB, Carson NL, Roy J, et al. Effects of childhood primary hypertension on carotid intima media thickness: a matched controlled study. Hypertension. 2006;48:40-44.
15. Flynn JT. Pediatric hypertension update. Curr Opin Nephrol Hypertens. 2010;19:292-297.
16. Mitchell P, Cheung N, de Haseth K, et al. Blood pressure and retinal arteriolar narrowing in children. Hypertension. 2007;49:1156-1162.
17. Kupferman JC, Lande MB, Adams HR, et al. Primary hypertension and neurocognitive and executive functioning in school-age children. Pediatr Nephrol. 2013;28:401-408.
18. Chen X, Wang Y. Tracking of blood pressure from childhood to adulthood: A systematic review of meta-regression analysis. Circulation. 2008;117:3171-3180.
19. Hypertension. American Academy of Family Physicians Web site. Available at: http://www.aafp.org/patient-care/clinical-recommendations/all/hypertension.html. Accessed February 7, 2014.
20. Screening for high blood pressure: reaffirmation recommendation statement. December 2007. AHRQ publication 08-05105-EF-2. US Preventive Services Task Force Web site. Available at: http://www.uspreventiveservicestaskforce.org/uspstf07/hbp/hbprs.htm. Accessed October 10, 2012.
21. Growth Charts. Centers for Disease Control and Prevention Web site. Available at: http://www.cdc.gov/growthcharts. Accessed July 31, 2012.
22. Podoll A, Grenier M, Croix B, et al. Inaccuracy in pediatric outpatient blood pressure measurement. Pediatrics. 2007;119:e538-e543.
23. Flynn JT, Pierce CB, Miller ER 3rd, et al; Chronic Kidney Disease in Children Study Group. Reliability of resting blood pressure measurement and classification using an oscillometric device in children with chronic kidney disease. J Pediatr. 2012;160:434-440.e.1.
24. Villar VA, Liu T, Jose PA. Recent trends in pediatric hypertension research. J Med Liban. 2010;58:179-184.
25. Swartz SJ, Srivaths PR, Croix B, et al. Cost-effectiveness of ambulatory blood pressure monitoring in the initial evaluation of hypertension in children. Pediatrics. 2008;122:1177-1181.
26. Brady TM, Feld LG. Pediatric approach to hypertension. Semin Nephrol. 2009;29:379-388.
27. Tullus K, Roebuck DJ, McLaren CA, et al. Imaging in the evaluation of renovascular disease. Pediatr Nephrol. 2010;25:1049-1056.
28. Flynn JT. Management of hypertension in the young: role of antihypertensive medications. J Cardiovasc Pharmacol. 2011;58:111-120.
29. ESCAPE Trial Group; Wühl E, Trivelli A, Picca S, et al. Strict blood pressure control and progression of renal failure in children. N Engl J Med. 2009;361:1639-1650.
30. Yoon EY, Cohn L, Rocchini A, et al. Antihypertensive prescribing patterns for adolescents with primary hypertension. Pediatrics. 2012;129:e1-e8.
31. Blowey DL. Update on the pharmacologic treatment of hypertension in pediatrics. J Clin Hypertens (Greenwich). 2012;14:383-387.
32. Welch WP, Yang W, Taylor-Zapata P, et al. Antihypertensive drug use by children: are the drugs labeled and indicated? J Clin Hypertens. 2012;14:388-395.
33. Lexicomp Pharmaceutical Reference, Version 1.8.3(155). Lexi-Comp Web site. Available at: http://online.lexi.com/crlsql/servlet/crlonline. Accessed July 31, 2012.
34. Robinson RF, Nahata MC, Batisky DL, et al. Pharmacologic treatment of chronic pediatric hypertension. Pediatr Drugs. 2005;7:27-40.
35. Hanevold CD. Concepts guiding therapy for hypertension in children. Expert Rev Cardiovasc Ther. 2009;7:647-657.
36. Brewster LM, van Montfrans GA, Kleijnen J. Systematic review: antihypertensive drug therapy in black patients. Ann Intern Med. 2004;141:614-627.
37. Li JS, Baker-Smith CM, Smith PB, et al. Racial differences in blood pressure response to angiotensin-converting enzyme inhibitors in children a meta-analysis. Clin Pharmacol Ther. 2008;84:315-319.
38. Simonetti GD, Rizzi M, Donadini R, et al. Effects of antihypertensive drugs on blood pressure and proteinuria in childhood. J Hypertens. 2007;25:2370-2376.
39. Lurbe E, Álvarez J, Redon J. Diagnosis and treatment of hypertension in children. Curr Hypertens Rep. 2010;12:480-486.
40. Kelley GA, Kelley KS, Tran ZV. The effects of exercise on resting blood pressure in children and adolescents: a meta-analysis of randomized controlled trials. Prev Cardiol. 2003;6:8-16.
41. McCambridge TM, Benjamin HJ, Breener JS, et al; Council on Sports Medicine and Fitness. Athletic participation by children and adolescents who have systemic hypertension. Pediatrics. 2010;125:1287-1294.
42. 2008 Physical Activity Guidelines for Americans. US Department of Health and Human Services Web site. Available at: http://www.health.gov/PAguidelines/guidelines/default.aspx. Updated March 11, 2013. Accessed February 7, 2014.
43. He FJ, MacGregor GA. Importance of salt in determining blood pressure in children: Meta-analysis of controlled trials. Hypertension. 2006;48:861-869.
› Screen for hypertension in all children over the age of 3 at every visit. C
› Order laboratory evaluation, echocardiography, and renovascular imaging for all children given a diagnosis of hypertension. C
› Advise parents that children with prehypertension and stage 1 hypertension without target-organ damage are eligible to participate in competitive athletics, but those with stage 2 hypertension, target-organ damage, or symptomatic hypertension should not engage in high-static sports (eg, gymnastics, weightlifting, wrestling) until BP is well controlled. C
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Childhood hypertension is on the rise: Recent data from the National Health and Nutrition Survey suggest 10% of children and adolescents have prehypertension and 4% have hypertension.1-4 Unfortunately, the condition often is missed. In a study of 14,187 children and adolescents who had at least 3 well-child visits at an outpatient academic medical center, 507 patients met the criteria for hypertension, yet only 131 (26%) had this diagnosis documented in their electronic health record.5
In a survey of 89 pediatricians, >50% of respondents said they were not familiar with the most current published recommendations for diagnosing and treating pediatric hypertension.6 Respondents also indicated that the most common reason for not initiating pharmacotherapy for children with hypertension was a lack of familiarity with appropriate antihypertensive agents (54%), followed by concern for adverse medication effects. Delayed diagnosis, evaluation, and treatment of hypertension in young patients can increase the likelihood of serious consequences, including target-organ damage such as left ventricular hypertrophy (LVH). In this review, we’ll describe the factors that put children and adolescents at risk for hypertension, and offer an evidence-based approach to diagnosis and treatment.
Obesity is a key risk factor
An estimated 17% of children aged 2 to 19 are obese.7 Obesity increases a child’s risk for hypertension by approximately 3- to 5-fold, and body mass index (BMI) is greater in children with primary hypertension compared with those with secondary hypertension.8 Hypertension is more common among Hispanic and non-Hispanic black male children and adolescents compared with their white counterparts; these ethnic disparities are not found in females.9,10 Poor diets and physical inactivity further contribute to obesity and hypertension risk. Children who were born preterm or had a very low birth weight also are at increased risk.11
Unchecked hypertension can lead to cardiac, vascular damage
Some children and adolescents with undiagnosed and untreated hypertension have evidence of target-organ damage, including cardiac dysfunction and pathologic vascular abnormalities. LVH is present in 20% to 41% of children and adolescents with hypertension.12,13 Carotid intima-media thickness, an established surrogate marker for atherosclerosis, is abnormally increased in children with hypertension, even after adjusting for BMI.14 Other target organ effects include impaired cognitive function, reduced glomerular filtration rate, microalbuminuria, and retinal arteriolar narrowing.15-17
Pediatric hypertension may persist into adulthood. A meta-analysis of more than 50 studies found that elevated blood pressure (BP) in childhood increases the risk for hypertension as an adult.18
NHLBI recommendations call for a BP check at every visit
The National Heart, Lung, and Blood Institute (NHLBI) Fourth Report on the Diagnosis, Evaluation, and Treatment of High Blood Pressure in Children and Adolescents (“the 4th Report”) recommends measuring BP in all children over age 3 during every health care visit.12 Children under age 3 should have their BP checked in certain circumstances, including preterm delivery, congenital heart disease, recurrent urinary tract infections, renal/urologic disease, organ transplantation, malignancy, and systemic illnesses associated with hypertension.12 The 4th Report is endorsed by the American Academy of Pediatrics (AAP); however, the American Academy of Family Physicians and the US Preventive Services Task Force have concluded that the evidence is insufficient to recommend for or against routine screening for hypertension in children and adolescents to reduce the risk of cardiovascular disease (CVD).19,20
Does the child have hypertension? That depends on several factors
Determining whether a child has hypertension requires that you consult national BP standards to determine if he or she is within the normal range. Normal BP standards for children and adolescents are based on gender, age, and height percentile, and provide a precise classification based on body size.12 These tables are available from the NHLBI Web site at http://www.nhlbi.nih.gov/guidelines/hypertension/child_tbl.htm. Height percentiles in these tables correspond with the Centers for Disease Control and Prevention (CDC) growth charts published in 2000.21 The Baylor College of Medicine Children’s Nutrition Research Center has a web-based calculator to help physicians determine BP percentiles in children and adolescents; it is available at http://www.bcm.edu/bodycomplab/Flashapps/BPVAgeChartpage.html. The International Pediatric Hypertension Association also offers BP charts and calculators at http://www.iphapediatrichypertension.org.
The diagnostic parameters for pediatric hypertension are listed in TABLE 1.12 The higher systolic or diastolic BP percentile value is used to determine a child’s overall BP category. A child is considered normotensive if the BP is <90th percentile. Hypertension is an average systolic or diastolic BP that is ≥95th percentile on at least 3 separate occasions. Stage 1 hypertension is BP levels ranging from the 95th percentile to 5 mm Hg above the 99th percentile, and stage 2 hypertension is BP levels greater than 5 mm Hg above the 99th percentile.
For example, assume you are evaluating a 12-year-old boy who is 61 inches tall and has a BP of 129/87 mm Hg. According to the CDC growth charts, his height puts him in the 75th percentile for his age. Using the NHLBI chart, you determine that he falls in the 95th-99th percentile for BP, and thus, using the categories in TABLE 1, is given a diagnosis of Stage 1 hypertension.
Accurate BP measurement requires using an appropriate cuff size that covers 80% of the child’s upper arm. When the child is between cuff sizes, use the larger cuff because small cuffs overestimate BP readings. BP readings should be taken on the right arm with the arm supported at heart level after the child has been sitting quietly for at least 5 minutes.12 One study showed that the initial BP readings taken in the triage area were significantly higher—often by >10 mm Hg—compared with follow-up measurements in the examination room.22
The preferred method of BP measurement is auscultation; however, oscillometric devices also are acceptable. These devices are easier to use, help eliminate digit bias, and minimize observer variation, but they typically read approximately 6 to 9 mm Hg higher than auscultation.23 For any BP measurement obtained by oscillometry that is >90th percentile, repeat the measurement by auscultation at least twice during the same office visit, and use an average of the repeated measurements.12 Obtain measurements of a lower extremity when you suspect congenital heart disease (eg, aortic coarctation). For any patient in whom you confirm a BP measurement >95th percentile, repeat the measurement within 2 weeks; for BPs >99th percentile, reevaluation should occur within one week.
Ambulatory BP monitoring (ABPM). Because BP measurements have a circadian pattern (higher during the day and reduced by 10% during sleep24) an ABPM device that provides 50 to 60 readings over 24 hours can be useful when evaluating children and adolescents for white-coat hypertension (elevated clinic BP with normal ambulatory BP), masked hypertension (normal clinic BP with elevated ambulatory BP), prehypertension and secondary hypertension (BP generally does not follow circadian patterns).25 ABPM is more accurate than BP self-measurement, but usually is limited to children older than age 5
Steps to take for clinical evaluation
Start by conducting a thorough history and physical examination, looking for information that can help you select the most appropriate tests for the next phase of evaluation.8,12 Calculate the patient’s BMI to screen for obesity, ask about a family history of hypertension or CVD, and determine if the patient is taking any medications that might cause hypertension, such as amphetamines, corticosteroids, or cyclosporine.8 Assess for signs and symptoms that suggest an underlying disease, such as renal disease (hematuria, edema, fatigue) or heart disease (chest pain, exertional dyspnea, palpitations).12
All children diagnosed with hypertension should be screened for secondary causes (TABLE 2). The recommended evaluation is to obtain a renal function panel, electrolytes, urinalysis, urine culture, complete blood count, renovascular imaging, and echocardiogram.12 The most common etiologies for secondary hypertension are renal parenchymal disease (68%), renovascular abnormalities (10%), and endocrinopathies (10%).26 Other causes, such as aortic coarctation, obstructive sleep apnea, iatrogenic factors (eg, toxins, medications, drugs of abuse), and genitourinary abnormalities, account for only a small percentage of cases and should be investigated as clinically indicated.26
Renovascular assessment depends on facility expertise. Imaging options include renal ultrasound (with or without Doppler), computed tomography angiography, renal flow scan, and magnetic resonance angiography. These studies have similar sensitivities and specificities.27 For patients in whom you strongly suspect renovascular disease, renal arteriography (digital subtraction angiography) provides the best images, although it is the most invasive study.27
Refer children and adolescents who are found to have significant abnormalities during the initial evaluation to the appropriate specialist. BP measurements often improve when secondary causes are treated.
Which drugs for which patients?
Pharmacologic management is indicated for pediatric patients with stage 1 or stage 2 hypertension, secondary hypertension, and those with evidence of target-organ damage.12 The goal of therapy is to reduce BP to <95th percentile. In patients with target organ damage, renal disease, or diabetes mellitus, the goal is <90th percentile.12,15,28 Intensive management of BP (≤50th percentile) in children with chronic kidney disease has been shown to delay progression to renal failure,29 but it is uncertain if lower BP goals can slow or prevent additional subclinical target organ damage.
Pharmacotherapy for hypertensive children or adolescents can be challenging because recommendations of which medication to use are based upon expert opinion and extrapolation from randomized trials of adults. The length of therapy (often lifelong), potential adverse effects, and unproven direct mortality benefit complicate this decision. Medication choice usually is based on physician preference or experience.12 The most common antihypertensive drugs prescribed are angiotensin-converting enzyme (ACE) inhibitors (26%), followed by diuretics (20%), and beta-blockers (17%).30,31 The starting doses and other details of medications commonly used to treat pediatric hypertension are listed in TABLE 3.28,32-34
One approach to choosing an antihypertensive drug for children is to measure the patient’s ambulatory plasma renin activity (PRA) level before initiating therapy. Those with high PRA levels (>0.65 ng/mL/h), presumably due to peripheral vasoconstriction, may benefit more from ACE inhibitors, angiotensin receptor blockers (ARBs), or beta-adrenergic antagonists.35 Individuals with low PRA levels (<0.65 ng/mL/h) maintain higher volume/sodium excess and may benefit more from diuretics or calcium channel blockers.35
Ethnicity also may guide medication selection. African American adults do not respond well to ACE inhibitor monotherapy due to decreased PRA and increased salt hypersensitivity.36 One meta-analysis found that African American children and adolescents had inadequate BP response to 6 individual ACE inhibitors, even at higher doses compared with white children and those of other ethnicities, who showed significant improvement in BP.37 ARBs may be a more effective alternative for this population.
Most experts recommend initiating a single agent at a low dose.12 A systematic review found that except for African American children, pediatric patients experienced comparable reductions in BP with ACE inhibitors (10.7/8.1 mm Hg), ARBs (10.5/6.9 mm Hg), and calcium channel blockers (9.3/7.2 mm Hg).38 In addition, ACE inhibitors and ARBs significantly reduced proteinuria by 49% and 59%, respectively.38
Schedule follow-up visits for 2 to 4 weeks (or sooner for patients with stage 2 hypertension) after initiating pharmacotherapy. If BP response is suboptimal, consider increasing the dose before adding a second agent. If the patient experiences significant adverse effects or has an inadequate BP response, changing to a drug from a different class is recommended.39 Patients who do not adequately respond to these approaches may require combination therapy; in such cases, strongly consider consultation with pediatric nephrologist or cardiologist.39 Medication compliance should be verified (eg, by pill counting, parental supervision) in patients who do not respond to therapy. Once BP control has been achieved, visits every 3 to 4 months are appropriate, with periodic laboratory monitoring, especially for children taking diuretics, ACE inhibitors, or ARBs or who have underlying renal disease.
Recommend exercise, but carefully monitor athlete's BP
Encourage obese and overweight children and adolescents to lose weight to maintain a BMI <95th percentile. Current guidelines based on expert opinion recommend that children and adolescents should engage in 60 minutes of daily physical activity.12 A meta-analysis found physical activity led to a 1% and 3% reduction in systolic and diastolic BP, respectively, although these results were not statistically significant.40
Be aware, however, that children and adolescents with hypertension who engage in certain competitive sports can significantly increase their BP and may be at risk for complications.41 According to the AAP guidelines, patients with stage 2 hypertension should not engage in high-static sports (eg, gymnastics, weightlifting, wrestling, boxing, cycling, decathlon, triathlon) until BP is well controlled.41 Patients with target-organ damage, uncontrolled hypertension, or symptomatic hypertension should not participate until BP is well controlled. Patients with prehypertension and stage 1 hypertension without target-organ damage are eligible to participate in competitive athletics. Reassess BP every 6 months in patients who are prehypertensive and every one to 2 weeks for those with stage 1 hypertension. When the patient’s BP remains <90th percentile, routine surveillance every 3 to 6 months is recommended.
What about sodium? Encourage parents of pediatric patients with hypertension to limit their child’s salt intake to 1.2 g/d for those age 4 to 8 and 1.5 g/d for older children.42 A meta-analysis found salt reduction decreased systolic BP by 1.2 mm Hg and diastolic BP by 1.3 mm Hg.43 Though these benefits are small, reducing sodium intake can be one of several lifestyle modifications, such as increased activity and quitting smoking, that can reduce young patients’ risk of hypertension and related cardiovascular sequelae.
CORRESPONDENCE
Robert Gauer, MD, Womack Army Medical Center, 2817 Reilly Road, Fort Bragg NC 28310; [email protected]
› Screen for hypertension in all children over the age of 3 at every visit. C
› Order laboratory evaluation, echocardiography, and renovascular imaging for all children given a diagnosis of hypertension. C
› Advise parents that children with prehypertension and stage 1 hypertension without target-organ damage are eligible to participate in competitive athletics, but those with stage 2 hypertension, target-organ damage, or symptomatic hypertension should not engage in high-static sports (eg, gymnastics, weightlifting, wrestling) until BP is well controlled. C
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Childhood hypertension is on the rise: Recent data from the National Health and Nutrition Survey suggest 10% of children and adolescents have prehypertension and 4% have hypertension.1-4 Unfortunately, the condition often is missed. In a study of 14,187 children and adolescents who had at least 3 well-child visits at an outpatient academic medical center, 507 patients met the criteria for hypertension, yet only 131 (26%) had this diagnosis documented in their electronic health record.5
In a survey of 89 pediatricians, >50% of respondents said they were not familiar with the most current published recommendations for diagnosing and treating pediatric hypertension.6 Respondents also indicated that the most common reason for not initiating pharmacotherapy for children with hypertension was a lack of familiarity with appropriate antihypertensive agents (54%), followed by concern for adverse medication effects. Delayed diagnosis, evaluation, and treatment of hypertension in young patients can increase the likelihood of serious consequences, including target-organ damage such as left ventricular hypertrophy (LVH). In this review, we’ll describe the factors that put children and adolescents at risk for hypertension, and offer an evidence-based approach to diagnosis and treatment.
Obesity is a key risk factor
An estimated 17% of children aged 2 to 19 are obese.7 Obesity increases a child’s risk for hypertension by approximately 3- to 5-fold, and body mass index (BMI) is greater in children with primary hypertension compared with those with secondary hypertension.8 Hypertension is more common among Hispanic and non-Hispanic black male children and adolescents compared with their white counterparts; these ethnic disparities are not found in females.9,10 Poor diets and physical inactivity further contribute to obesity and hypertension risk. Children who were born preterm or had a very low birth weight also are at increased risk.11
Unchecked hypertension can lead to cardiac, vascular damage
Some children and adolescents with undiagnosed and untreated hypertension have evidence of target-organ damage, including cardiac dysfunction and pathologic vascular abnormalities. LVH is present in 20% to 41% of children and adolescents with hypertension.12,13 Carotid intima-media thickness, an established surrogate marker for atherosclerosis, is abnormally increased in children with hypertension, even after adjusting for BMI.14 Other target organ effects include impaired cognitive function, reduced glomerular filtration rate, microalbuminuria, and retinal arteriolar narrowing.15-17
Pediatric hypertension may persist into adulthood. A meta-analysis of more than 50 studies found that elevated blood pressure (BP) in childhood increases the risk for hypertension as an adult.18
NHLBI recommendations call for a BP check at every visit
The National Heart, Lung, and Blood Institute (NHLBI) Fourth Report on the Diagnosis, Evaluation, and Treatment of High Blood Pressure in Children and Adolescents (“the 4th Report”) recommends measuring BP in all children over age 3 during every health care visit.12 Children under age 3 should have their BP checked in certain circumstances, including preterm delivery, congenital heart disease, recurrent urinary tract infections, renal/urologic disease, organ transplantation, malignancy, and systemic illnesses associated with hypertension.12 The 4th Report is endorsed by the American Academy of Pediatrics (AAP); however, the American Academy of Family Physicians and the US Preventive Services Task Force have concluded that the evidence is insufficient to recommend for or against routine screening for hypertension in children and adolescents to reduce the risk of cardiovascular disease (CVD).19,20
Does the child have hypertension? That depends on several factors
Determining whether a child has hypertension requires that you consult national BP standards to determine if he or she is within the normal range. Normal BP standards for children and adolescents are based on gender, age, and height percentile, and provide a precise classification based on body size.12 These tables are available from the NHLBI Web site at http://www.nhlbi.nih.gov/guidelines/hypertension/child_tbl.htm. Height percentiles in these tables correspond with the Centers for Disease Control and Prevention (CDC) growth charts published in 2000.21 The Baylor College of Medicine Children’s Nutrition Research Center has a web-based calculator to help physicians determine BP percentiles in children and adolescents; it is available at http://www.bcm.edu/bodycomplab/Flashapps/BPVAgeChartpage.html. The International Pediatric Hypertension Association also offers BP charts and calculators at http://www.iphapediatrichypertension.org.
The diagnostic parameters for pediatric hypertension are listed in TABLE 1.12 The higher systolic or diastolic BP percentile value is used to determine a child’s overall BP category. A child is considered normotensive if the BP is <90th percentile. Hypertension is an average systolic or diastolic BP that is ≥95th percentile on at least 3 separate occasions. Stage 1 hypertension is BP levels ranging from the 95th percentile to 5 mm Hg above the 99th percentile, and stage 2 hypertension is BP levels greater than 5 mm Hg above the 99th percentile.
For example, assume you are evaluating a 12-year-old boy who is 61 inches tall and has a BP of 129/87 mm Hg. According to the CDC growth charts, his height puts him in the 75th percentile for his age. Using the NHLBI chart, you determine that he falls in the 95th-99th percentile for BP, and thus, using the categories in TABLE 1, is given a diagnosis of Stage 1 hypertension.
Accurate BP measurement requires using an appropriate cuff size that covers 80% of the child’s upper arm. When the child is between cuff sizes, use the larger cuff because small cuffs overestimate BP readings. BP readings should be taken on the right arm with the arm supported at heart level after the child has been sitting quietly for at least 5 minutes.12 One study showed that the initial BP readings taken in the triage area were significantly higher—often by >10 mm Hg—compared with follow-up measurements in the examination room.22
The preferred method of BP measurement is auscultation; however, oscillometric devices also are acceptable. These devices are easier to use, help eliminate digit bias, and minimize observer variation, but they typically read approximately 6 to 9 mm Hg higher than auscultation.23 For any BP measurement obtained by oscillometry that is >90th percentile, repeat the measurement by auscultation at least twice during the same office visit, and use an average of the repeated measurements.12 Obtain measurements of a lower extremity when you suspect congenital heart disease (eg, aortic coarctation). For any patient in whom you confirm a BP measurement >95th percentile, repeat the measurement within 2 weeks; for BPs >99th percentile, reevaluation should occur within one week.
Ambulatory BP monitoring (ABPM). Because BP measurements have a circadian pattern (higher during the day and reduced by 10% during sleep24) an ABPM device that provides 50 to 60 readings over 24 hours can be useful when evaluating children and adolescents for white-coat hypertension (elevated clinic BP with normal ambulatory BP), masked hypertension (normal clinic BP with elevated ambulatory BP), prehypertension and secondary hypertension (BP generally does not follow circadian patterns).25 ABPM is more accurate than BP self-measurement, but usually is limited to children older than age 5
Steps to take for clinical evaluation
Start by conducting a thorough history and physical examination, looking for information that can help you select the most appropriate tests for the next phase of evaluation.8,12 Calculate the patient’s BMI to screen for obesity, ask about a family history of hypertension or CVD, and determine if the patient is taking any medications that might cause hypertension, such as amphetamines, corticosteroids, or cyclosporine.8 Assess for signs and symptoms that suggest an underlying disease, such as renal disease (hematuria, edema, fatigue) or heart disease (chest pain, exertional dyspnea, palpitations).12
All children diagnosed with hypertension should be screened for secondary causes (TABLE 2). The recommended evaluation is to obtain a renal function panel, electrolytes, urinalysis, urine culture, complete blood count, renovascular imaging, and echocardiogram.12 The most common etiologies for secondary hypertension are renal parenchymal disease (68%), renovascular abnormalities (10%), and endocrinopathies (10%).26 Other causes, such as aortic coarctation, obstructive sleep apnea, iatrogenic factors (eg, toxins, medications, drugs of abuse), and genitourinary abnormalities, account for only a small percentage of cases and should be investigated as clinically indicated.26
Renovascular assessment depends on facility expertise. Imaging options include renal ultrasound (with or without Doppler), computed tomography angiography, renal flow scan, and magnetic resonance angiography. These studies have similar sensitivities and specificities.27 For patients in whom you strongly suspect renovascular disease, renal arteriography (digital subtraction angiography) provides the best images, although it is the most invasive study.27
Refer children and adolescents who are found to have significant abnormalities during the initial evaluation to the appropriate specialist. BP measurements often improve when secondary causes are treated.
Which drugs for which patients?
Pharmacologic management is indicated for pediatric patients with stage 1 or stage 2 hypertension, secondary hypertension, and those with evidence of target-organ damage.12 The goal of therapy is to reduce BP to <95th percentile. In patients with target organ damage, renal disease, or diabetes mellitus, the goal is <90th percentile.12,15,28 Intensive management of BP (≤50th percentile) in children with chronic kidney disease has been shown to delay progression to renal failure,29 but it is uncertain if lower BP goals can slow or prevent additional subclinical target organ damage.
Pharmacotherapy for hypertensive children or adolescents can be challenging because recommendations of which medication to use are based upon expert opinion and extrapolation from randomized trials of adults. The length of therapy (often lifelong), potential adverse effects, and unproven direct mortality benefit complicate this decision. Medication choice usually is based on physician preference or experience.12 The most common antihypertensive drugs prescribed are angiotensin-converting enzyme (ACE) inhibitors (26%), followed by diuretics (20%), and beta-blockers (17%).30,31 The starting doses and other details of medications commonly used to treat pediatric hypertension are listed in TABLE 3.28,32-34
One approach to choosing an antihypertensive drug for children is to measure the patient’s ambulatory plasma renin activity (PRA) level before initiating therapy. Those with high PRA levels (>0.65 ng/mL/h), presumably due to peripheral vasoconstriction, may benefit more from ACE inhibitors, angiotensin receptor blockers (ARBs), or beta-adrenergic antagonists.35 Individuals with low PRA levels (<0.65 ng/mL/h) maintain higher volume/sodium excess and may benefit more from diuretics or calcium channel blockers.35
Ethnicity also may guide medication selection. African American adults do not respond well to ACE inhibitor monotherapy due to decreased PRA and increased salt hypersensitivity.36 One meta-analysis found that African American children and adolescents had inadequate BP response to 6 individual ACE inhibitors, even at higher doses compared with white children and those of other ethnicities, who showed significant improvement in BP.37 ARBs may be a more effective alternative for this population.
Most experts recommend initiating a single agent at a low dose.12 A systematic review found that except for African American children, pediatric patients experienced comparable reductions in BP with ACE inhibitors (10.7/8.1 mm Hg), ARBs (10.5/6.9 mm Hg), and calcium channel blockers (9.3/7.2 mm Hg).38 In addition, ACE inhibitors and ARBs significantly reduced proteinuria by 49% and 59%, respectively.38
Schedule follow-up visits for 2 to 4 weeks (or sooner for patients with stage 2 hypertension) after initiating pharmacotherapy. If BP response is suboptimal, consider increasing the dose before adding a second agent. If the patient experiences significant adverse effects or has an inadequate BP response, changing to a drug from a different class is recommended.39 Patients who do not adequately respond to these approaches may require combination therapy; in such cases, strongly consider consultation with pediatric nephrologist or cardiologist.39 Medication compliance should be verified (eg, by pill counting, parental supervision) in patients who do not respond to therapy. Once BP control has been achieved, visits every 3 to 4 months are appropriate, with periodic laboratory monitoring, especially for children taking diuretics, ACE inhibitors, or ARBs or who have underlying renal disease.
Recommend exercise, but carefully monitor athlete's BP
Encourage obese and overweight children and adolescents to lose weight to maintain a BMI <95th percentile. Current guidelines based on expert opinion recommend that children and adolescents should engage in 60 minutes of daily physical activity.12 A meta-analysis found physical activity led to a 1% and 3% reduction in systolic and diastolic BP, respectively, although these results were not statistically significant.40
Be aware, however, that children and adolescents with hypertension who engage in certain competitive sports can significantly increase their BP and may be at risk for complications.41 According to the AAP guidelines, patients with stage 2 hypertension should not engage in high-static sports (eg, gymnastics, weightlifting, wrestling, boxing, cycling, decathlon, triathlon) until BP is well controlled.41 Patients with target-organ damage, uncontrolled hypertension, or symptomatic hypertension should not participate until BP is well controlled. Patients with prehypertension and stage 1 hypertension without target-organ damage are eligible to participate in competitive athletics. Reassess BP every 6 months in patients who are prehypertensive and every one to 2 weeks for those with stage 1 hypertension. When the patient’s BP remains <90th percentile, routine surveillance every 3 to 6 months is recommended.
What about sodium? Encourage parents of pediatric patients with hypertension to limit their child’s salt intake to 1.2 g/d for those age 4 to 8 and 1.5 g/d for older children.42 A meta-analysis found salt reduction decreased systolic BP by 1.2 mm Hg and diastolic BP by 1.3 mm Hg.43 Though these benefits are small, reducing sodium intake can be one of several lifestyle modifications, such as increased activity and quitting smoking, that can reduce young patients’ risk of hypertension and related cardiovascular sequelae.
CORRESPONDENCE
Robert Gauer, MD, Womack Army Medical Center, 2817 Reilly Road, Fort Bragg NC 28310; [email protected]
1. McNiece KL, Poffenbarger TS, Tuner JL, et al. Prevalence of hypertension and pre-hypertension among adolescents. J Pediatr. 2007;150:640-644.e1.
2. Moore WE, Eichner JE, Cohn EM, et al. Blood pressure screening of school children in a multiracial school district: the Healthy Kids Project. Am J Hypertens. 2009;22:351-356.
3. Falkner B. What exactly do the trends mean? Circulation. 2007;116:1437-1439.
4. Feber J, Ahmed M. Hypertension in children: new trends and challenges. Clin Sci (Lond). 2010;119:151-161.
5. Hansen ML, Gunn PW, Kaelber DC. Under diagnosis of hypertension in children and adolescents. JAMA. 2007;298:874-879.
6. Boneparth A, Flynn JT. Evaluation and treatment of hypertension in general pediatric practice. Clin Pediatr (Phila). 2009;48:44-49.
7. Ogden CL, Carroll MD, Kit BK, et al. Prevalence of obesity and trends in body mass index among US children and adolescents, 1999-2010. JAMA. 2012;307:483-490.
8. Feld LG, Corey H. Hypertension in childhood. Pediatr Rev. 2007;28:283-298.
9. Rosner B, Cook N, Portman R, et al. Blood pressure differences by ethnic group among United States children and adolescents. Hypertension. 2009;54:502-508.
10. Din-Dzietham R, Liu Y, Bielo MV, et al. High blood pressure trends in children and adolescents in national surveys, 1963 to 2002. Circulation. 2007;116:1488-1496.
11. de Jong F, Monuteaux MC, van Elburg RM, et al. Systematic review and meta-analysis of preterm birth and later systolic blood pressure. Hypertension. 2012;59:226-234.
12. National High Blood Pressure Education Program Working Group on High Blood Pressure in Children and Adolescents. The fourth report on the diagnosis, evaluation, and treatment of high blood pressure in children and adolescents. Pediatrics. 2004;114(2 suppl 4th report):555-576.
13. Ramaswamy P, Lytrivi ID, Paul C, et al. Regression of left ventricular hypertrophy in children with antihypertensive therapy. Pediatr Nephrol. 2007;22:141-143.
14. Lande MB, Carson NL, Roy J, et al. Effects of childhood primary hypertension on carotid intima media thickness: a matched controlled study. Hypertension. 2006;48:40-44.
15. Flynn JT. Pediatric hypertension update. Curr Opin Nephrol Hypertens. 2010;19:292-297.
16. Mitchell P, Cheung N, de Haseth K, et al. Blood pressure and retinal arteriolar narrowing in children. Hypertension. 2007;49:1156-1162.
17. Kupferman JC, Lande MB, Adams HR, et al. Primary hypertension and neurocognitive and executive functioning in school-age children. Pediatr Nephrol. 2013;28:401-408.
18. Chen X, Wang Y. Tracking of blood pressure from childhood to adulthood: A systematic review of meta-regression analysis. Circulation. 2008;117:3171-3180.
19. Hypertension. American Academy of Family Physicians Web site. Available at: http://www.aafp.org/patient-care/clinical-recommendations/all/hypertension.html. Accessed February 7, 2014.
20. Screening for high blood pressure: reaffirmation recommendation statement. December 2007. AHRQ publication 08-05105-EF-2. US Preventive Services Task Force Web site. Available at: http://www.uspreventiveservicestaskforce.org/uspstf07/hbp/hbprs.htm. Accessed October 10, 2012.
21. Growth Charts. Centers for Disease Control and Prevention Web site. Available at: http://www.cdc.gov/growthcharts. Accessed July 31, 2012.
22. Podoll A, Grenier M, Croix B, et al. Inaccuracy in pediatric outpatient blood pressure measurement. Pediatrics. 2007;119:e538-e543.
23. Flynn JT, Pierce CB, Miller ER 3rd, et al; Chronic Kidney Disease in Children Study Group. Reliability of resting blood pressure measurement and classification using an oscillometric device in children with chronic kidney disease. J Pediatr. 2012;160:434-440.e.1.
24. Villar VA, Liu T, Jose PA. Recent trends in pediatric hypertension research. J Med Liban. 2010;58:179-184.
25. Swartz SJ, Srivaths PR, Croix B, et al. Cost-effectiveness of ambulatory blood pressure monitoring in the initial evaluation of hypertension in children. Pediatrics. 2008;122:1177-1181.
26. Brady TM, Feld LG. Pediatric approach to hypertension. Semin Nephrol. 2009;29:379-388.
27. Tullus K, Roebuck DJ, McLaren CA, et al. Imaging in the evaluation of renovascular disease. Pediatr Nephrol. 2010;25:1049-1056.
28. Flynn JT. Management of hypertension in the young: role of antihypertensive medications. J Cardiovasc Pharmacol. 2011;58:111-120.
29. ESCAPE Trial Group; Wühl E, Trivelli A, Picca S, et al. Strict blood pressure control and progression of renal failure in children. N Engl J Med. 2009;361:1639-1650.
30. Yoon EY, Cohn L, Rocchini A, et al. Antihypertensive prescribing patterns for adolescents with primary hypertension. Pediatrics. 2012;129:e1-e8.
31. Blowey DL. Update on the pharmacologic treatment of hypertension in pediatrics. J Clin Hypertens (Greenwich). 2012;14:383-387.
32. Welch WP, Yang W, Taylor-Zapata P, et al. Antihypertensive drug use by children: are the drugs labeled and indicated? J Clin Hypertens. 2012;14:388-395.
33. Lexicomp Pharmaceutical Reference, Version 1.8.3(155). Lexi-Comp Web site. Available at: http://online.lexi.com/crlsql/servlet/crlonline. Accessed July 31, 2012.
34. Robinson RF, Nahata MC, Batisky DL, et al. Pharmacologic treatment of chronic pediatric hypertension. Pediatr Drugs. 2005;7:27-40.
35. Hanevold CD. Concepts guiding therapy for hypertension in children. Expert Rev Cardiovasc Ther. 2009;7:647-657.
36. Brewster LM, van Montfrans GA, Kleijnen J. Systematic review: antihypertensive drug therapy in black patients. Ann Intern Med. 2004;141:614-627.
37. Li JS, Baker-Smith CM, Smith PB, et al. Racial differences in blood pressure response to angiotensin-converting enzyme inhibitors in children a meta-analysis. Clin Pharmacol Ther. 2008;84:315-319.
38. Simonetti GD, Rizzi M, Donadini R, et al. Effects of antihypertensive drugs on blood pressure and proteinuria in childhood. J Hypertens. 2007;25:2370-2376.
39. Lurbe E, Álvarez J, Redon J. Diagnosis and treatment of hypertension in children. Curr Hypertens Rep. 2010;12:480-486.
40. Kelley GA, Kelley KS, Tran ZV. The effects of exercise on resting blood pressure in children and adolescents: a meta-analysis of randomized controlled trials. Prev Cardiol. 2003;6:8-16.
41. McCambridge TM, Benjamin HJ, Breener JS, et al; Council on Sports Medicine and Fitness. Athletic participation by children and adolescents who have systemic hypertension. Pediatrics. 2010;125:1287-1294.
42. 2008 Physical Activity Guidelines for Americans. US Department of Health and Human Services Web site. Available at: http://www.health.gov/PAguidelines/guidelines/default.aspx. Updated March 11, 2013. Accessed February 7, 2014.
43. He FJ, MacGregor GA. Importance of salt in determining blood pressure in children: Meta-analysis of controlled trials. Hypertension. 2006;48:861-869.
1. McNiece KL, Poffenbarger TS, Tuner JL, et al. Prevalence of hypertension and pre-hypertension among adolescents. J Pediatr. 2007;150:640-644.e1.
2. Moore WE, Eichner JE, Cohn EM, et al. Blood pressure screening of school children in a multiracial school district: the Healthy Kids Project. Am J Hypertens. 2009;22:351-356.
3. Falkner B. What exactly do the trends mean? Circulation. 2007;116:1437-1439.
4. Feber J, Ahmed M. Hypertension in children: new trends and challenges. Clin Sci (Lond). 2010;119:151-161.
5. Hansen ML, Gunn PW, Kaelber DC. Under diagnosis of hypertension in children and adolescents. JAMA. 2007;298:874-879.
6. Boneparth A, Flynn JT. Evaluation and treatment of hypertension in general pediatric practice. Clin Pediatr (Phila). 2009;48:44-49.
7. Ogden CL, Carroll MD, Kit BK, et al. Prevalence of obesity and trends in body mass index among US children and adolescents, 1999-2010. JAMA. 2012;307:483-490.
8. Feld LG, Corey H. Hypertension in childhood. Pediatr Rev. 2007;28:283-298.
9. Rosner B, Cook N, Portman R, et al. Blood pressure differences by ethnic group among United States children and adolescents. Hypertension. 2009;54:502-508.
10. Din-Dzietham R, Liu Y, Bielo MV, et al. High blood pressure trends in children and adolescents in national surveys, 1963 to 2002. Circulation. 2007;116:1488-1496.
11. de Jong F, Monuteaux MC, van Elburg RM, et al. Systematic review and meta-analysis of preterm birth and later systolic blood pressure. Hypertension. 2012;59:226-234.
12. National High Blood Pressure Education Program Working Group on High Blood Pressure in Children and Adolescents. The fourth report on the diagnosis, evaluation, and treatment of high blood pressure in children and adolescents. Pediatrics. 2004;114(2 suppl 4th report):555-576.
13. Ramaswamy P, Lytrivi ID, Paul C, et al. Regression of left ventricular hypertrophy in children with antihypertensive therapy. Pediatr Nephrol. 2007;22:141-143.
14. Lande MB, Carson NL, Roy J, et al. Effects of childhood primary hypertension on carotid intima media thickness: a matched controlled study. Hypertension. 2006;48:40-44.
15. Flynn JT. Pediatric hypertension update. Curr Opin Nephrol Hypertens. 2010;19:292-297.
16. Mitchell P, Cheung N, de Haseth K, et al. Blood pressure and retinal arteriolar narrowing in children. Hypertension. 2007;49:1156-1162.
17. Kupferman JC, Lande MB, Adams HR, et al. Primary hypertension and neurocognitive and executive functioning in school-age children. Pediatr Nephrol. 2013;28:401-408.
18. Chen X, Wang Y. Tracking of blood pressure from childhood to adulthood: A systematic review of meta-regression analysis. Circulation. 2008;117:3171-3180.
19. Hypertension. American Academy of Family Physicians Web site. Available at: http://www.aafp.org/patient-care/clinical-recommendations/all/hypertension.html. Accessed February 7, 2014.
20. Screening for high blood pressure: reaffirmation recommendation statement. December 2007. AHRQ publication 08-05105-EF-2. US Preventive Services Task Force Web site. Available at: http://www.uspreventiveservicestaskforce.org/uspstf07/hbp/hbprs.htm. Accessed October 10, 2012.
21. Growth Charts. Centers for Disease Control and Prevention Web site. Available at: http://www.cdc.gov/growthcharts. Accessed July 31, 2012.
22. Podoll A, Grenier M, Croix B, et al. Inaccuracy in pediatric outpatient blood pressure measurement. Pediatrics. 2007;119:e538-e543.
23. Flynn JT, Pierce CB, Miller ER 3rd, et al; Chronic Kidney Disease in Children Study Group. Reliability of resting blood pressure measurement and classification using an oscillometric device in children with chronic kidney disease. J Pediatr. 2012;160:434-440.e.1.
24. Villar VA, Liu T, Jose PA. Recent trends in pediatric hypertension research. J Med Liban. 2010;58:179-184.
25. Swartz SJ, Srivaths PR, Croix B, et al. Cost-effectiveness of ambulatory blood pressure monitoring in the initial evaluation of hypertension in children. Pediatrics. 2008;122:1177-1181.
26. Brady TM, Feld LG. Pediatric approach to hypertension. Semin Nephrol. 2009;29:379-388.
27. Tullus K, Roebuck DJ, McLaren CA, et al. Imaging in the evaluation of renovascular disease. Pediatr Nephrol. 2010;25:1049-1056.
28. Flynn JT. Management of hypertension in the young: role of antihypertensive medications. J Cardiovasc Pharmacol. 2011;58:111-120.
29. ESCAPE Trial Group; Wühl E, Trivelli A, Picca S, et al. Strict blood pressure control and progression of renal failure in children. N Engl J Med. 2009;361:1639-1650.
30. Yoon EY, Cohn L, Rocchini A, et al. Antihypertensive prescribing patterns for adolescents with primary hypertension. Pediatrics. 2012;129:e1-e8.
31. Blowey DL. Update on the pharmacologic treatment of hypertension in pediatrics. J Clin Hypertens (Greenwich). 2012;14:383-387.
32. Welch WP, Yang W, Taylor-Zapata P, et al. Antihypertensive drug use by children: are the drugs labeled and indicated? J Clin Hypertens. 2012;14:388-395.
33. Lexicomp Pharmaceutical Reference, Version 1.8.3(155). Lexi-Comp Web site. Available at: http://online.lexi.com/crlsql/servlet/crlonline. Accessed July 31, 2012.
34. Robinson RF, Nahata MC, Batisky DL, et al. Pharmacologic treatment of chronic pediatric hypertension. Pediatr Drugs. 2005;7:27-40.
35. Hanevold CD. Concepts guiding therapy for hypertension in children. Expert Rev Cardiovasc Ther. 2009;7:647-657.
36. Brewster LM, van Montfrans GA, Kleijnen J. Systematic review: antihypertensive drug therapy in black patients. Ann Intern Med. 2004;141:614-627.
37. Li JS, Baker-Smith CM, Smith PB, et al. Racial differences in blood pressure response to angiotensin-converting enzyme inhibitors in children a meta-analysis. Clin Pharmacol Ther. 2008;84:315-319.
38. Simonetti GD, Rizzi M, Donadini R, et al. Effects of antihypertensive drugs on blood pressure and proteinuria in childhood. J Hypertens. 2007;25:2370-2376.
39. Lurbe E, Álvarez J, Redon J. Diagnosis and treatment of hypertension in children. Curr Hypertens Rep. 2010;12:480-486.
40. Kelley GA, Kelley KS, Tran ZV. The effects of exercise on resting blood pressure in children and adolescents: a meta-analysis of randomized controlled trials. Prev Cardiol. 2003;6:8-16.
41. McCambridge TM, Benjamin HJ, Breener JS, et al; Council on Sports Medicine and Fitness. Athletic participation by children and adolescents who have systemic hypertension. Pediatrics. 2010;125:1287-1294.
42. 2008 Physical Activity Guidelines for Americans. US Department of Health and Human Services Web site. Available at: http://www.health.gov/PAguidelines/guidelines/default.aspx. Updated March 11, 2013. Accessed February 7, 2014.
43. He FJ, MacGregor GA. Importance of salt in determining blood pressure in children: Meta-analysis of controlled trials. Hypertension. 2006;48:861-869.
7 questions to ask when evaluating a noninferiority trial
The traditional clinical trial, designed to test whether a new treatment is better than a placebo or another active treatment, is known as a “superiority” trial—although rarely labeled as such. In contrast, the goal of a noninferiority trial is simply to demonstrate that a new treatment is not substantially less effective than the standard therapy.
Such trials are useful when a new therapy is thought to be safer, easier to administer, or less costly than the existing treatment, but not necessarily more effective. And, because it would be unethical to randomize patients with a serious condition for which there already is an effective treatment to placebo, a noninferiority trial is another means of determining if the new treatment is effective.
Noninferiority trials have unique design features and methodology and require a different analysis than traditional superiority trials. Yet many physicians know far less about them; many investigators appear to be less than proficient, as well. A review of 116 noninferiority trials and 46 equivalence trials found that only 20% fulfilled generally accepted quality criteria.1 To improve the quality of noninferiority trials, the CONSORT (Consolidated Standards of Reporting Trials) Group has published a checklist for trial design and reporting standards.2,3 Based on this checklist, we came up with 7 key questions to consider when evaluating a noninferiority trial. In the pages that follow, you’ll also find an at-a-glance guide (TABLE) and a methodology review using a hypothetical case (page E7).
1. Is a noninferiority trial appropriate?
The introduction to a noninferiority trial should provide the rationale for this design and the absence of a placebo control group. Look for a review of the evidence of the efficacy of the reference treatment that placebo-controlled trials have revealed, along with the effect size. The advantages of the new treatment over the standard treatment—eg, fewer adverse effects, easier administration, or lower cost—should be discussed, as well.
In the Randomized Evaluation of Long-term Anticoagulation Therapy (RE-LY)—a prominent noninferiority trial—investigators compared the standard anticoagulant (warfarin) for patients with atrial fibrillation (AF) at risk of stroke with a new agent, dabigatran.4 In the methods section of the abstract and the statistical analysis section of the main body, the authors clearly indicated that this was a noninferiority trial. They began by referring to the existing evidence of warfarin’s effectiveness, then detailed the qualities that make warfarin cumbersome to use, including the need for frequent laboratory monitoring. This was followed by evidence that many patients stop taking warfarin and that even for those who persist with treatment, adequate anticoagulation is difficult to maintain.
The authors went on to state that because dabigatran requires no long-term monitoring, it is easier to use. Therefore, if dabigatran could be shown to be no worse than warfarin in preventing strokes, it would be a reasonable alternative, leaving no doubt that this was an appropriate noninferiority trial.
2. Is the noninferiority margin based on clinical judgment and statistical reasoning?
The noninferiority margin should be based on clinical judgment as to how effective a new treatment must be in order to be declared not clinically inferior to the standard treatment. This can be based on several factors, including the severity of the outcome and the expected advantages of the new treatment. The margin should also take into account the size of the standard treatment’s effect vs placebo. In RELY, for example, the authors noted that the noninferiority margin was based on the desire to preserve at least 50% of the lower limit of the confidence interval (CI) of warfarin’s estimated effect; this was done using data from a previously published meta-analysis of 6 trials comparing warfarin with placebo for stroke prevention in patients with AF.4-6
3. Are the hypothesis and statistical analysis formulated correctly?
The clinical hypothesis in a noninferiority trial is that the new treatment is not worse than the standard treatment by a prespecified margin; therefore, the statistical null hypothesis to be tested is that the new treatment is worse than the reference treatment by more than that margin. Rejecting a true null hypothesis (for example, because the P value is <.05) is known as a type l error. In this setting, making a type I error would mean accepting a new treatment that is truly worse than the standard by at least the specified margin. Failure to reject a false null hypothesis is known as a type II error, which in this case would mean failing to identify a new treatment that is truly noninferior to the standard.7
In RE-LY, the authors stated that the upper limit of the one-sided 97.5% CI for the relative risk of a stroke with dabigatran vs warfarin had to fall below 1.46.4 (This is the same as testing the null hypothesis that the hazard ratio is ≥1.46.) Thus, the hypothesis was formulated correctly.
4. Is the sample size appropriate and justified?
The sample size in a noninferiority trial should provide high power to reject the null hypothesis that the difference (or relative risk) between groups is equal to or greater than the noninferiority margin under some clinically meaningful assumption about the true difference (or absolute risk reduction) between groups. A true difference of 0 (or a relative risk of 1) is typically assumed for sample size calculation. However, assuming that the new treatment is truly slightly better or slightly worse than the standard may be clinically appropriate in some cases. This would indicate a need for a smaller or larger sample size, respectively, than that required under the usual assumption of no difference.
When the justification for the sample size in a noninferiority trial is not provided or the number of participants is based on an inappropriate approach (eg, using superiority trial calculations for a noninferiority trial), questions about the quality of the trial arise. The primary concern is whether the noninferiority margin was actually selected before the trial began, as it should have been. And if the researchers used overly optimistic assumptions about the efficacy of the new treatment relative to the standard therapy, the failure to rule out the margin could be misleading. (As with superiority trials that fail to reject the null hypothesis, post hoc power calculations should be avoided.) After the study has ended, the resulting CIs should be used to evaluate whether the study was large enough to adequately assess the relative effectiveness of the treatments.
The RE-LY trial calculated the sample size that was expected to provide 84% power to rule out the prespecified hazard ratio of 1.46, assuming a true event rate of 1.6% per year (presumably for both groups), a recruitment period of 2 years, and at least one year of follow-up. The sample size was subsequently increased from 15,000 to 18,000 to maintain power in case of a low event rate.4,5
5. Is the noninferiority trial as similar as possible to the trial(s) comparing the standard treatment with placebo?
Characteristics of participants, setting, reference treatment, and outcomes used in a noninferiority trial should be as close as possible to those in the trial(s) comparing the treatment with placebo. This is known as the constancy assumption, and it is key to researchers’ ability to draw a conclusion about noninferiority.
The trials used to calculate the noninferiority margin and the RE-LY trial itself involved similar populations of patients with AF, and the outcome (stroke) was similar.
6. Is a per protocol analysis reported in the results?
In randomized controlled superiority trials, the participants should be analyzed in the groups to which they were originally allocated, regardless of whether they adhered to treatment during the entire follow-up period. Such intention-to-treat (ITT) analysis is important because it provides a more conservative estimate of treatment effect—taking into account that some people who are offered treatment will not accept it and others will discontinue treatment. An ITT analysis therefore tends to minimize treatment effects compared with a “per protocol” analysis, in which participants are analyzed according to the treatment they actually received and are often removed from the analysis if they discontinue or do not adhere to treatment.
In noninferiority trials, if patients in the intervention group cross over to the standard treatment group or those in the standard treatment group have poor adherence, an ITT analysis can increase the risk of wrongly claiming noninferiority.7 Therefore, a per protocol analysis should be included—and indeed may be preferable.
In RE-LY, ITT analyses were reported, and complete follow-up data were available for 99.9% of patients. However, the rates of treatment discontinuation at one year were about 15% for those on dabigatran and 10% for the warfarin group, and 21% and 17%, respectively, at 2 years.4,5 If the new treatment were truly less efficacious than the standard treatment, these moderate discontinuation rates could lead to more similar rates of stroke in the 2 groups than would be expected with higher continuation rates, biasing results towards the alternative of noninferiority. Although the original publication of trial results did not include a per protocol analysis, the RE-LY authors later reported that a per protocol analysis yielded similar results to the ITT analysis.
7. Are the overall design and execution of the trial high quality?
Because a poor quality noninferiority trial can appear to demonstrate noninferiority, looking at such studies critically is crucial. Appropriate randomization, concealed allocation, masking, and careful attention to participant flow must all be assessed.2,3
To continue with our example, the RE-LY trial was well conducted. Randomization was performed centrally via an automated telephone system and 2 doses of dabigatran were administered in a masked fashion, while warfarin was open-label. Remarkably, follow-up was achieved for 99.9% of participants over a median of 2 years, and independent adjudicators masked to treatment group assessed outcomes.4,5
CORRESPONDENCE
Anne Mounsey, MD, UNC Chapel Hill Department of Family Medicine, 590 Manning Drive, CB 7595, Chapel Hill, NC 27590; [email protected]
1. Le Henanff A, Giraudeau B, Baron G, et al. Quality of reporting of noninferiority and equivalence randomized trials. JAMA. 2006;295:1147-1151.
2. Piaggio G, Elbourne DR, Pocock SJ, et al; CONSORT Group. Reporting of noninferiority and equivalence randomized trials: extension of the CONSORT 2010 statement. JAMA. 2012;308:2594-2604.
3. Moher D, Schulz KF, Altman D; CONSORT Group (Consolidated Standards of Reporting Trials). The CONSORT statement: revised recommendations for improving the quality of reports of parallel-group randomized trials. JAMA. 2001;285:1987-1991.
4. Connolly SJ, Ezekowitz MD, Yusuf S, et al; RE-LY Steering Committee and Investigators. Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med. 2009;361:1139-1151.
5. Ezekowitz MD, Connolly S, Parekh A, et al. Rationale and design of RE-LY: randomized evaluation of long-term anticoagulant therapy, warfarin, compared with dabigatran. Am Heart J. 2009;157:805-810, 810.e1-2.
6. Hart RG, Benavente O, McBride R, et al. Antithrombotic therapy to prevent stroke in patients with atrial fibrillation: a meta-analysis. Ann Intern Med. 1999;131:492-501.
7. US Department of Health and Human Services. Guidance for industry non-inferiority clinical trials. US Food and Drug Administration Web site. March 2010. Available at: http://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/UCM202140.pdf. Accessed February 4, 2014.
The traditional clinical trial, designed to test whether a new treatment is better than a placebo or another active treatment, is known as a “superiority” trial—although rarely labeled as such. In contrast, the goal of a noninferiority trial is simply to demonstrate that a new treatment is not substantially less effective than the standard therapy.
Such trials are useful when a new therapy is thought to be safer, easier to administer, or less costly than the existing treatment, but not necessarily more effective. And, because it would be unethical to randomize patients with a serious condition for which there already is an effective treatment to placebo, a noninferiority trial is another means of determining if the new treatment is effective.
Noninferiority trials have unique design features and methodology and require a different analysis than traditional superiority trials. Yet many physicians know far less about them; many investigators appear to be less than proficient, as well. A review of 116 noninferiority trials and 46 equivalence trials found that only 20% fulfilled generally accepted quality criteria.1 To improve the quality of noninferiority trials, the CONSORT (Consolidated Standards of Reporting Trials) Group has published a checklist for trial design and reporting standards.2,3 Based on this checklist, we came up with 7 key questions to consider when evaluating a noninferiority trial. In the pages that follow, you’ll also find an at-a-glance guide (TABLE) and a methodology review using a hypothetical case (page E7).
1. Is a noninferiority trial appropriate?
The introduction to a noninferiority trial should provide the rationale for this design and the absence of a placebo control group. Look for a review of the evidence of the efficacy of the reference treatment that placebo-controlled trials have revealed, along with the effect size. The advantages of the new treatment over the standard treatment—eg, fewer adverse effects, easier administration, or lower cost—should be discussed, as well.
In the Randomized Evaluation of Long-term Anticoagulation Therapy (RE-LY)—a prominent noninferiority trial—investigators compared the standard anticoagulant (warfarin) for patients with atrial fibrillation (AF) at risk of stroke with a new agent, dabigatran.4 In the methods section of the abstract and the statistical analysis section of the main body, the authors clearly indicated that this was a noninferiority trial. They began by referring to the existing evidence of warfarin’s effectiveness, then detailed the qualities that make warfarin cumbersome to use, including the need for frequent laboratory monitoring. This was followed by evidence that many patients stop taking warfarin and that even for those who persist with treatment, adequate anticoagulation is difficult to maintain.
The authors went on to state that because dabigatran requires no long-term monitoring, it is easier to use. Therefore, if dabigatran could be shown to be no worse than warfarin in preventing strokes, it would be a reasonable alternative, leaving no doubt that this was an appropriate noninferiority trial.
2. Is the noninferiority margin based on clinical judgment and statistical reasoning?
The noninferiority margin should be based on clinical judgment as to how effective a new treatment must be in order to be declared not clinically inferior to the standard treatment. This can be based on several factors, including the severity of the outcome and the expected advantages of the new treatment. The margin should also take into account the size of the standard treatment’s effect vs placebo. In RELY, for example, the authors noted that the noninferiority margin was based on the desire to preserve at least 50% of the lower limit of the confidence interval (CI) of warfarin’s estimated effect; this was done using data from a previously published meta-analysis of 6 trials comparing warfarin with placebo for stroke prevention in patients with AF.4-6
3. Are the hypothesis and statistical analysis formulated correctly?
The clinical hypothesis in a noninferiority trial is that the new treatment is not worse than the standard treatment by a prespecified margin; therefore, the statistical null hypothesis to be tested is that the new treatment is worse than the reference treatment by more than that margin. Rejecting a true null hypothesis (for example, because the P value is <.05) is known as a type l error. In this setting, making a type I error would mean accepting a new treatment that is truly worse than the standard by at least the specified margin. Failure to reject a false null hypothesis is known as a type II error, which in this case would mean failing to identify a new treatment that is truly noninferior to the standard.7
In RE-LY, the authors stated that the upper limit of the one-sided 97.5% CI for the relative risk of a stroke with dabigatran vs warfarin had to fall below 1.46.4 (This is the same as testing the null hypothesis that the hazard ratio is ≥1.46.) Thus, the hypothesis was formulated correctly.
4. Is the sample size appropriate and justified?
The sample size in a noninferiority trial should provide high power to reject the null hypothesis that the difference (or relative risk) between groups is equal to or greater than the noninferiority margin under some clinically meaningful assumption about the true difference (or absolute risk reduction) between groups. A true difference of 0 (or a relative risk of 1) is typically assumed for sample size calculation. However, assuming that the new treatment is truly slightly better or slightly worse than the standard may be clinically appropriate in some cases. This would indicate a need for a smaller or larger sample size, respectively, than that required under the usual assumption of no difference.
When the justification for the sample size in a noninferiority trial is not provided or the number of participants is based on an inappropriate approach (eg, using superiority trial calculations for a noninferiority trial), questions about the quality of the trial arise. The primary concern is whether the noninferiority margin was actually selected before the trial began, as it should have been. And if the researchers used overly optimistic assumptions about the efficacy of the new treatment relative to the standard therapy, the failure to rule out the margin could be misleading. (As with superiority trials that fail to reject the null hypothesis, post hoc power calculations should be avoided.) After the study has ended, the resulting CIs should be used to evaluate whether the study was large enough to adequately assess the relative effectiveness of the treatments.
The RE-LY trial calculated the sample size that was expected to provide 84% power to rule out the prespecified hazard ratio of 1.46, assuming a true event rate of 1.6% per year (presumably for both groups), a recruitment period of 2 years, and at least one year of follow-up. The sample size was subsequently increased from 15,000 to 18,000 to maintain power in case of a low event rate.4,5
5. Is the noninferiority trial as similar as possible to the trial(s) comparing the standard treatment with placebo?
Characteristics of participants, setting, reference treatment, and outcomes used in a noninferiority trial should be as close as possible to those in the trial(s) comparing the treatment with placebo. This is known as the constancy assumption, and it is key to researchers’ ability to draw a conclusion about noninferiority.
The trials used to calculate the noninferiority margin and the RE-LY trial itself involved similar populations of patients with AF, and the outcome (stroke) was similar.
6. Is a per protocol analysis reported in the results?
In randomized controlled superiority trials, the participants should be analyzed in the groups to which they were originally allocated, regardless of whether they adhered to treatment during the entire follow-up period. Such intention-to-treat (ITT) analysis is important because it provides a more conservative estimate of treatment effect—taking into account that some people who are offered treatment will not accept it and others will discontinue treatment. An ITT analysis therefore tends to minimize treatment effects compared with a “per protocol” analysis, in which participants are analyzed according to the treatment they actually received and are often removed from the analysis if they discontinue or do not adhere to treatment.
In noninferiority trials, if patients in the intervention group cross over to the standard treatment group or those in the standard treatment group have poor adherence, an ITT analysis can increase the risk of wrongly claiming noninferiority.7 Therefore, a per protocol analysis should be included—and indeed may be preferable.
In RE-LY, ITT analyses were reported, and complete follow-up data were available for 99.9% of patients. However, the rates of treatment discontinuation at one year were about 15% for those on dabigatran and 10% for the warfarin group, and 21% and 17%, respectively, at 2 years.4,5 If the new treatment were truly less efficacious than the standard treatment, these moderate discontinuation rates could lead to more similar rates of stroke in the 2 groups than would be expected with higher continuation rates, biasing results towards the alternative of noninferiority. Although the original publication of trial results did not include a per protocol analysis, the RE-LY authors later reported that a per protocol analysis yielded similar results to the ITT analysis.
7. Are the overall design and execution of the trial high quality?
Because a poor quality noninferiority trial can appear to demonstrate noninferiority, looking at such studies critically is crucial. Appropriate randomization, concealed allocation, masking, and careful attention to participant flow must all be assessed.2,3
To continue with our example, the RE-LY trial was well conducted. Randomization was performed centrally via an automated telephone system and 2 doses of dabigatran were administered in a masked fashion, while warfarin was open-label. Remarkably, follow-up was achieved for 99.9% of participants over a median of 2 years, and independent adjudicators masked to treatment group assessed outcomes.4,5
CORRESPONDENCE
Anne Mounsey, MD, UNC Chapel Hill Department of Family Medicine, 590 Manning Drive, CB 7595, Chapel Hill, NC 27590; [email protected]
The traditional clinical trial, designed to test whether a new treatment is better than a placebo or another active treatment, is known as a “superiority” trial—although rarely labeled as such. In contrast, the goal of a noninferiority trial is simply to demonstrate that a new treatment is not substantially less effective than the standard therapy.
Such trials are useful when a new therapy is thought to be safer, easier to administer, or less costly than the existing treatment, but not necessarily more effective. And, because it would be unethical to randomize patients with a serious condition for which there already is an effective treatment to placebo, a noninferiority trial is another means of determining if the new treatment is effective.
Noninferiority trials have unique design features and methodology and require a different analysis than traditional superiority trials. Yet many physicians know far less about them; many investigators appear to be less than proficient, as well. A review of 116 noninferiority trials and 46 equivalence trials found that only 20% fulfilled generally accepted quality criteria.1 To improve the quality of noninferiority trials, the CONSORT (Consolidated Standards of Reporting Trials) Group has published a checklist for trial design and reporting standards.2,3 Based on this checklist, we came up with 7 key questions to consider when evaluating a noninferiority trial. In the pages that follow, you’ll also find an at-a-glance guide (TABLE) and a methodology review using a hypothetical case (page E7).
1. Is a noninferiority trial appropriate?
The introduction to a noninferiority trial should provide the rationale for this design and the absence of a placebo control group. Look for a review of the evidence of the efficacy of the reference treatment that placebo-controlled trials have revealed, along with the effect size. The advantages of the new treatment over the standard treatment—eg, fewer adverse effects, easier administration, or lower cost—should be discussed, as well.
In the Randomized Evaluation of Long-term Anticoagulation Therapy (RE-LY)—a prominent noninferiority trial—investigators compared the standard anticoagulant (warfarin) for patients with atrial fibrillation (AF) at risk of stroke with a new agent, dabigatran.4 In the methods section of the abstract and the statistical analysis section of the main body, the authors clearly indicated that this was a noninferiority trial. They began by referring to the existing evidence of warfarin’s effectiveness, then detailed the qualities that make warfarin cumbersome to use, including the need for frequent laboratory monitoring. This was followed by evidence that many patients stop taking warfarin and that even for those who persist with treatment, adequate anticoagulation is difficult to maintain.
The authors went on to state that because dabigatran requires no long-term monitoring, it is easier to use. Therefore, if dabigatran could be shown to be no worse than warfarin in preventing strokes, it would be a reasonable alternative, leaving no doubt that this was an appropriate noninferiority trial.
2. Is the noninferiority margin based on clinical judgment and statistical reasoning?
The noninferiority margin should be based on clinical judgment as to how effective a new treatment must be in order to be declared not clinically inferior to the standard treatment. This can be based on several factors, including the severity of the outcome and the expected advantages of the new treatment. The margin should also take into account the size of the standard treatment’s effect vs placebo. In RELY, for example, the authors noted that the noninferiority margin was based on the desire to preserve at least 50% of the lower limit of the confidence interval (CI) of warfarin’s estimated effect; this was done using data from a previously published meta-analysis of 6 trials comparing warfarin with placebo for stroke prevention in patients with AF.4-6
3. Are the hypothesis and statistical analysis formulated correctly?
The clinical hypothesis in a noninferiority trial is that the new treatment is not worse than the standard treatment by a prespecified margin; therefore, the statistical null hypothesis to be tested is that the new treatment is worse than the reference treatment by more than that margin. Rejecting a true null hypothesis (for example, because the P value is <.05) is known as a type l error. In this setting, making a type I error would mean accepting a new treatment that is truly worse than the standard by at least the specified margin. Failure to reject a false null hypothesis is known as a type II error, which in this case would mean failing to identify a new treatment that is truly noninferior to the standard.7
In RE-LY, the authors stated that the upper limit of the one-sided 97.5% CI for the relative risk of a stroke with dabigatran vs warfarin had to fall below 1.46.4 (This is the same as testing the null hypothesis that the hazard ratio is ≥1.46.) Thus, the hypothesis was formulated correctly.
4. Is the sample size appropriate and justified?
The sample size in a noninferiority trial should provide high power to reject the null hypothesis that the difference (or relative risk) between groups is equal to or greater than the noninferiority margin under some clinically meaningful assumption about the true difference (or absolute risk reduction) between groups. A true difference of 0 (or a relative risk of 1) is typically assumed for sample size calculation. However, assuming that the new treatment is truly slightly better or slightly worse than the standard may be clinically appropriate in some cases. This would indicate a need for a smaller or larger sample size, respectively, than that required under the usual assumption of no difference.
When the justification for the sample size in a noninferiority trial is not provided or the number of participants is based on an inappropriate approach (eg, using superiority trial calculations for a noninferiority trial), questions about the quality of the trial arise. The primary concern is whether the noninferiority margin was actually selected before the trial began, as it should have been. And if the researchers used overly optimistic assumptions about the efficacy of the new treatment relative to the standard therapy, the failure to rule out the margin could be misleading. (As with superiority trials that fail to reject the null hypothesis, post hoc power calculations should be avoided.) After the study has ended, the resulting CIs should be used to evaluate whether the study was large enough to adequately assess the relative effectiveness of the treatments.
The RE-LY trial calculated the sample size that was expected to provide 84% power to rule out the prespecified hazard ratio of 1.46, assuming a true event rate of 1.6% per year (presumably for both groups), a recruitment period of 2 years, and at least one year of follow-up. The sample size was subsequently increased from 15,000 to 18,000 to maintain power in case of a low event rate.4,5
5. Is the noninferiority trial as similar as possible to the trial(s) comparing the standard treatment with placebo?
Characteristics of participants, setting, reference treatment, and outcomes used in a noninferiority trial should be as close as possible to those in the trial(s) comparing the treatment with placebo. This is known as the constancy assumption, and it is key to researchers’ ability to draw a conclusion about noninferiority.
The trials used to calculate the noninferiority margin and the RE-LY trial itself involved similar populations of patients with AF, and the outcome (stroke) was similar.
6. Is a per protocol analysis reported in the results?
In randomized controlled superiority trials, the participants should be analyzed in the groups to which they were originally allocated, regardless of whether they adhered to treatment during the entire follow-up period. Such intention-to-treat (ITT) analysis is important because it provides a more conservative estimate of treatment effect—taking into account that some people who are offered treatment will not accept it and others will discontinue treatment. An ITT analysis therefore tends to minimize treatment effects compared with a “per protocol” analysis, in which participants are analyzed according to the treatment they actually received and are often removed from the analysis if they discontinue or do not adhere to treatment.
In noninferiority trials, if patients in the intervention group cross over to the standard treatment group or those in the standard treatment group have poor adherence, an ITT analysis can increase the risk of wrongly claiming noninferiority.7 Therefore, a per protocol analysis should be included—and indeed may be preferable.
In RE-LY, ITT analyses were reported, and complete follow-up data were available for 99.9% of patients. However, the rates of treatment discontinuation at one year were about 15% for those on dabigatran and 10% for the warfarin group, and 21% and 17%, respectively, at 2 years.4,5 If the new treatment were truly less efficacious than the standard treatment, these moderate discontinuation rates could lead to more similar rates of stroke in the 2 groups than would be expected with higher continuation rates, biasing results towards the alternative of noninferiority. Although the original publication of trial results did not include a per protocol analysis, the RE-LY authors later reported that a per protocol analysis yielded similar results to the ITT analysis.
7. Are the overall design and execution of the trial high quality?
Because a poor quality noninferiority trial can appear to demonstrate noninferiority, looking at such studies critically is crucial. Appropriate randomization, concealed allocation, masking, and careful attention to participant flow must all be assessed.2,3
To continue with our example, the RE-LY trial was well conducted. Randomization was performed centrally via an automated telephone system and 2 doses of dabigatran were administered in a masked fashion, while warfarin was open-label. Remarkably, follow-up was achieved for 99.9% of participants over a median of 2 years, and independent adjudicators masked to treatment group assessed outcomes.4,5
CORRESPONDENCE
Anne Mounsey, MD, UNC Chapel Hill Department of Family Medicine, 590 Manning Drive, CB 7595, Chapel Hill, NC 27590; [email protected]
1. Le Henanff A, Giraudeau B, Baron G, et al. Quality of reporting of noninferiority and equivalence randomized trials. JAMA. 2006;295:1147-1151.
2. Piaggio G, Elbourne DR, Pocock SJ, et al; CONSORT Group. Reporting of noninferiority and equivalence randomized trials: extension of the CONSORT 2010 statement. JAMA. 2012;308:2594-2604.
3. Moher D, Schulz KF, Altman D; CONSORT Group (Consolidated Standards of Reporting Trials). The CONSORT statement: revised recommendations for improving the quality of reports of parallel-group randomized trials. JAMA. 2001;285:1987-1991.
4. Connolly SJ, Ezekowitz MD, Yusuf S, et al; RE-LY Steering Committee and Investigators. Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med. 2009;361:1139-1151.
5. Ezekowitz MD, Connolly S, Parekh A, et al. Rationale and design of RE-LY: randomized evaluation of long-term anticoagulant therapy, warfarin, compared with dabigatran. Am Heart J. 2009;157:805-810, 810.e1-2.
6. Hart RG, Benavente O, McBride R, et al. Antithrombotic therapy to prevent stroke in patients with atrial fibrillation: a meta-analysis. Ann Intern Med. 1999;131:492-501.
7. US Department of Health and Human Services. Guidance for industry non-inferiority clinical trials. US Food and Drug Administration Web site. March 2010. Available at: http://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/UCM202140.pdf. Accessed February 4, 2014.
1. Le Henanff A, Giraudeau B, Baron G, et al. Quality of reporting of noninferiority and equivalence randomized trials. JAMA. 2006;295:1147-1151.
2. Piaggio G, Elbourne DR, Pocock SJ, et al; CONSORT Group. Reporting of noninferiority and equivalence randomized trials: extension of the CONSORT 2010 statement. JAMA. 2012;308:2594-2604.
3. Moher D, Schulz KF, Altman D; CONSORT Group (Consolidated Standards of Reporting Trials). The CONSORT statement: revised recommendations for improving the quality of reports of parallel-group randomized trials. JAMA. 2001;285:1987-1991.
4. Connolly SJ, Ezekowitz MD, Yusuf S, et al; RE-LY Steering Committee and Investigators. Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med. 2009;361:1139-1151.
5. Ezekowitz MD, Connolly S, Parekh A, et al. Rationale and design of RE-LY: randomized evaluation of long-term anticoagulant therapy, warfarin, compared with dabigatran. Am Heart J. 2009;157:805-810, 810.e1-2.
6. Hart RG, Benavente O, McBride R, et al. Antithrombotic therapy to prevent stroke in patients with atrial fibrillation: a meta-analysis. Ann Intern Med. 1999;131:492-501.
7. US Department of Health and Human Services. Guidance for industry non-inferiority clinical trials. US Food and Drug Administration Web site. March 2010. Available at: http://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/UCM202140.pdf. Accessed February 4, 2014.