User login
Evaluation of shoulder pain
- Shoulder pain is a common complaint seen in primary care.
- Subacromial impingement syndrome and rotator cuff tears are the most common disorders encountered.
- The history and physical examination are keys to most shoulder pain diagnoses, particularly when used in combination.
- Imaging studies are indicated for failed conservative therapy, severe shoulder pathology, or unclear diagnosis.
Shoulder pain is a common problem that can pose difficult diagnostic and therapeutic challenges for the family physician. It is the third most common musculoskeletal complaint in the general population, and accounts for 5% of all general practitioner musculoskeletal consults.1,2 The incidence of shoulder pain is 6.6 to 25 cases per 1000 patients, with a peak incidence in the fourth through sixth decades.3-6 Shoulder pain is second only to knee pain for referrals to orthopedic surgery or primary care sports medicine clinics.7,8 Furthermore, 8% to 13% of athletic injuries involve the shoulder and account for up to 3.9% of new emergency department visits.9,10
Differential diagnosis
The challenge for the physician evaluating shoulder pain is the myriad of etiologies and the potential for multiple disorders. Compounding the challenge is a lack of uniformity in the literature regarding diagnostic classification.11 As Table 1 shows, the age of the patient will help focus the differential diagnosis. Patients younger than 30 years old tend to have biomechanical or mild inflammatory etiologies for their pain such as atraumatic instability, tendinosis, and arthropathies. Less than 1% of shoulder injuries in persons younger than 30 years are complete rotator cuff tears, which occur in 35% of patients older than 45 years with shoulder pain.12,13
The rotator cuff is the most commonly affected structure in the shoulder, and subacromial impingement syndrome is the leading cause of rotator cuff injury.4,12,14-16 Neer14 described 3 stages of shoulder impingement that he estimated lead to 95% of rotator cuff tears. Impingement can be caused by repetitive overhead activities, acute trauma, or subtle instability (atraumatic instability). The current theory is that inflammation of the rotator cuff tendons and/or bursa, caused by irritation against the coracoacromial arch, can progress to a complete rotator cuff tear over time.
Referred sources of shoulder pain should be included in the differential diagnosis of shoulder pain. Potential sources include cervical spondylolysis, cervical arthritis, cervical disc disease, myocardial ischemia, reflex sympathetic dystrophy, diaphragmatic irritation, thoracic outlet syndrome, and gallbladder disease.
TABLE 1
Differential diagnosis of shoulder pain
| Diagnosis | Primary care setting4-15(%) | Age (y) of presentation, Mean (SD)14 |
|---|---|---|
| Subacromial impingement syndrome | 48–72 | |
| Stage I (edema and hemorrhage) | 16 | 23 (7) |
| Stage II (cuff fibrosis and partial tear) | 42 | 41 (11) |
| Stage III (full-thickness tear) | 15 | 62 (12) |
| Adhesive capsulitis | 16–22 | 53 (10) |
| Acute bursitis | 17 | |
| Calcific tendonitis | 6 | |
| Myofascial pain syndrome | 5 | |
| Glenohumeral joint arthrosis | 2.5 | 64 (10) |
| Thoracic outlet syndrome | 2 | |
| Biceps tendonitis | 0.8 |
Using the history and physical examination
As noted above, the likelihood of specific conditions such as a complete rotator cuff tear varies with the setting, age of the patient, and specialty of the physi-cian.4,13,17,18 It is important to keep this pretest probability in mind while interpreting the history and physical examination. For example, a positive empty can test in a 50-year-old patient almost certainly represents a rotator cuff tear, whereas many younger patients with this finding will not have a tear. Moreover, certain components of the history and physical examination are more indicative of disorders while others are better at ruling them out. This concept is represented by the positive and negative likelihood ratios listed in Table 2.
The clinical evaluation begins with identification of the chief complaint and a thorough history. Common complaints include pain, weakness, stiffness, instability, locking, catching, and deformity.26 Determining the duration of symptoms and mechanism of injury will narrow the differential diagnosis. If trauma occurred, the mechanism can determine radiological needs. Aggravating and alleviating factors should be reviewed, including work, recreation, sports, or hobbies. Night pain when lying on the affected side and a history of trauma in a patient older than 65 years both suggest a rotator cuff tear, but no individual symptom is definitive for the diagnosis (Table 2).19 Pain with overhead work may indicate impingement syndrome, especially if the patient is symptomatic through the arc of 60 to 120 degrees.
The physical examination should include observation, palpation, range of motion (ROM), and provocative testing. Observation requires adequate exposure of the shoulders bilaterally to identify any gross deformities or abnormalities, including muscle atrophy, acromioclavicular joint disparity, or evidence of trauma. Muscle atrophy of either the supraspinatus or infraspinatus muscles is moderately predictive of rotator cuff tears in the elderly population, with a positive predictive value of 81%. However, this sign is not useful if absent, with a negative predictive value of only 43%.19 No studies have assessed the role of palpation in the evaluation of shoulder pain. Nevertheless, the role of palpation in discerning acromioclavicular joint pathology from shoulder and neck makes it a useful part of the examination.
The shoulder’s ROM should be evaluated both actively and passively. The shoulder is a mobile joint with a complexity of movements. These include flexion to 180 degrees, extension to 40 degrees, abduction to 120 degrees with palms down and 180 degrees with palms up, internal rotation to 55 degrees, and external rotation to 45 degrees with arms at the side. Although determining abduction ROM is consistent among examiners,27 interrater reliability is poor for assessment of external rotation ROM. Lack of full ROM that is equally limited with both passive and active examination is found in arthropathies and adhesive capsulitis.
Pain between 60 and 120 degrees of abduction “the painful arc”) is associated with subacromial impingement, whereas pain after 120 degrees is an indication of acromioclavicular joint origin. However, Calis and coworkers17 found that the presence of subacromial impingement has a positive likelihood ratio of only 1.7.
After assessing the ROM, the next steps are to evaluate the rotator cuff and biceps tendon, perform impingement testing, check for instability, and finally assess the acromioclavicular joint. The tests are listed in Table 2 in our preferred order of examination and represent the tests best supported by the evidence; the results are based on a literature search of Medline, PubMed, DARE, and Sports Discuss. The technique of each examination maneuver has been published elsewhere and is not described in detail here. Figure 1 through 4 illustrate several common examination maneuvers described below. A Web site that demonstrates the physical examination more thoroughly can be found at http://www.nismat.org/orthocor/exam/shoulder.html#Evaluation.
TABLE 2
Use of history and physical examination to diagnose shoulder pain
| History or maneuver | Study quality (1A–5)* | Sensitivity | Specificity | LR+ | LR− | PV+ | PV− |
|---|---|---|---|---|---|---|---|
| Rotator cuff tear | |||||||
| History of trauma19 | 2B | 36 | 73 | 1.3 | 0.88 | 72 | 37 |
| Night pain19 | 2B | 88 | 20 | 1.1 | 0.6 | 70 | 43 |
| Painful arc17 | 2B | 33 | 81 | 1.7 | 0.83 | 81 | 33 |
| Empty can test18,20,21 | 1B | 84–89 | 50–58 | 1.7–2 | 0.22–0.28 | 36–98 | 22–93 |
| Drop sign21 | 1B | 21 | 100 | >25 | 0.79 | 100 | 32 |
| Lift off test (for subscapularis tears)21 | 1B | 62 | 100 | >25 | 0.38 | 100 | 69 |
| Impingement | |||||||
| Hawkin’s test20,22 | 1B | 87–89 | 60 | 2.2 | 0.18 | 71 | 83 |
| Instability | |||||||
| Relocation test23 | 2B | 57 | 100 | >25 | 0.43 | 100 | 73 |
| Augmented apprehension23 | 2B | 68 | 100 | >25 | 0.32 | 100 | 78 |
| Labral tear | |||||||
| Crank test24 | 2B | 91 | 93 | 13 | 0.10 | 94 | 90 |
| Active compression test25 | 1B | 100 | 99 | >25 | 0.01 | 95 | 100 |
| Acromioclavicular joint | |||||||
| Active compression test25 | 1B | 100 | 97 | >25 | 0.01 | 89 | 100 |
| *Based on the guidelines for evidence quality outlined by the Center for Evidence-Based Medicine (http://163.1.96.10/docs/levels.html). | |||||||
| LR+ = positive likelihood ratio; LR− = negative likelihood ratio; PV+ = positive predictive value; PV− = negative predictive value. | |||||||
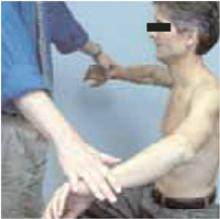
Figure 1
The empty can test
Rotator cuff tests
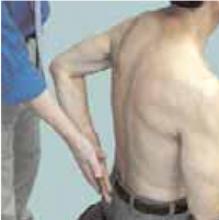
The drop arm test assesses the integrity of the rotator cuff, predominantly the supraspinatus muscle. The empty can test (Figure 1) isolates the supraspinatus against resistance. The lift off test (Figure 2) assesses the subscapularis integrity.
Figure 2
The lift off test
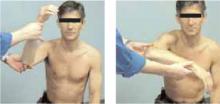
Impingement syndrome
Hawkin’s sign (Figure 3) is a test for evidence of impingement by re-creation of its symptoms.
Figures 3A & 3B
Hawkin’s sign
Glenohumeral joint stability
The augmented anterior apprehension test evaluates anterior shoulder instability. The relocation test, which helps confirm anterior instability, is carried out immediately after a positive anterior apprehension test.
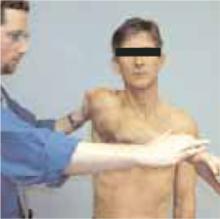
Labral tears
The crank test is used to identify chronic labral injury, whereas the active compression test25 (Figure 4) indicates labral injury if pain is deep in the shoulder.
Figure 4
The active compression test
Acromioclavicular joint
The active compression test25 (Figure 4) indicates acromioclavicular joint inflammation, arthritis, or injury if pain is localized to the top of the shoulder.
Diagnostic tests
Imaging studies used in the evaluation of shoulder pain include plain radiographs, arthrography, computed tomography (CT), ultrasound (US), and magnetic resonance imaging (MRI). Often no imaging is required, or plain radiographs are the sole imaging study needed. Soft tissue injuries are best identified by MRI or US, whereas bony pathology is seen best with plain radiographs or CT. Indications for imaging include severe injury, uncontrolled pain, failure of conservative therapy, return to play considerations, and examiner discretion. Table 3 outlines the accuracy of imaging modalities organized by diagnosis.
TABLE 3
Imaging tests to diagnose shoulder pain
| Diagnostic test | Study quality (1A–5)* | Sensitivity | Specificity | LR+ | LR− | PV+ | PV− |
|---|---|---|---|---|---|---|---|
| MRI | |||||||
| Rotator cuff tears | |||||||
| Partial28 | 2B | 82 | 85 | 5.5 | 0.21 | 82 | 85 |
| Complete15 | 1B | 81 | 78 | 3.7 | 0.24 | — | — |
| Overall16,29,30 | 2B | 89–96 | 49–100 | 1.9 to >25 | 0.08 | 58 | 94 |
| Impingement28 | 2B | 93 | 87 | 7.2 | 0.08 | 93 | 87 |
| Labral tears31-32 | 1B | 75–89 | 97–100 | >25 | 0.11–0.25 | 100 | 41 |
| Plain arthrogram | |||||||
| Rotator cuff tears | |||||||
| Partial33 | 1B | 70 | — | — | — | — | — |
| Complete15 | 1A | 50 | 96 | 13 | 0.52 | — | — |
| CT arthrogram | |||||||
| Rotator cuff tears | |||||||
| Partial33 | 1B | 70 | — | — | — | — | — |
| Complete33 | 1B | 95 | — | — | — | — | — |
| Overall33 | 1B | 86 | 98 | >25 | 0.14 | 96 | 93 |
| Ultrasound | |||||||
| Rotator cuff tears | |||||||
| Partial33 | 1B | 80 | — | — | — | — | — |
| Complete33 | 1B | 90 | — | — | — | — | — |
| Overall33,34 | 1B | 86 | 91 | 9.6 | 0.15 | 96 | 73 |
| *Based on the guidelines for evidence quality outlined by the Center for Evidence-Based Medicine (http://163.1.96.10/docs/levels.html). | |||||||
| CT, computed tomography; LR+ = positive likelihood ratio; LR− = negative likelihood ratio; MRI, magnetic resonance imaging; PV+ = positive predictive value; PV− = negative predictive value. | |||||||
Plain radiographs
Plain radiographs are the first step in diagnostic imaging. They can reveal fractures, dislocation, subluxation, bony lesions, outlet obstruction, acromioclavicular joint pathology, and arthritic changes. No definitive clinical studies on the needs of radiographs have been done. Plain radiographs should be taken when ROM is lost, especially when there is abduction of less than 90 degrees, severe pain, and after trauma. Our preferred x-rays include a glenohumeral anteroposterior (AP) view, a supraspinatus outlet view, and an axillary view. Anteroposterior views with internal and external rotation are added in cases of trauma to help rule out fracture. Positive acromioclavicular joint tests (crossover or palpation) should be followed by acromioclavicular joint radiographs because a shoulder series does not give a clear view of this joint. Additional views of the neck as well as a chest x-ray or abdominal imaging should be considered if a referred source of shoulder pain remains a possibility.
Arthrography
Arthrography was the diagnostic test of choice before MRI. It is specific for rotator cuff tears but lacks sensitivity15 because it cannot detect partial-thickness or associated soft tissue injuries of the shoulder. Arthrography still has a role in evaluating adhesive capsulitis by demonstrating decreased intracapsular volume.26 The test can be therapeutic if the capsule is dilated during the procedure. Additionally, patients with claustrophobia may be good candidates for arthrography if a full-thickness tear is suspected and MRI is not possible.
Computed tomography
Computed tomography may be used to evaluate bony lesions, including glenoid rim fractures, humoral fractures, and acromioclavicular joint disease. Computed tomography arthrograms may have a role in assessing labral tears and full-thickness rotator cuff tears.35 The use of CT arthrography has fallen into disfavor compared with MRI because of the risks associated with contrast exposure and poor sensitivity for partial-thickness rotator cuff tears or associated soft tissue injury.
Ultrasound
Ultrasound has been used in the evaluation of rotator cuff tears with varying degrees of sensitivity and specificity.12,29,34 This inconsistency may be related to variation in operator skill. Advantages of US include relatively low cost, speed, and noninvasiveness.
Magnetic resonance imaging
Magnetic resonance imaging has become the gold standard for diagnostic imaging of the shoulder related to soft tissue injury. The advantages include its noninvasive nature, lack of contrast exposure, nonionizing radiation, high degree of resolution, and the ability to evaluate multiple potential pathologic processes.36 Magnetic resonance imaging is the preferred test for evaluating impingement syndrome and rotator cuff pathology. A normal MRI greatly reduces the chances of a rotator cuff tear, with a negative likelihood ratio of 0.08.16,29,30 Magnetic resonance imaging is also useful in the evaluation of avascular necrosis, biceps tendon disorders, inflammatory processes, and tumors.13 The diagnosis of labral lesions can be challenging given the relatively low sensitivity and negative predictive value noted in several trials.16,28,31 Finally, it is important to note that up to one third of all asymptomatic patients and more than half of those older than 60 years demonstrate asymptomatic rotator cuff tears on MRI.37
Approach to the patient
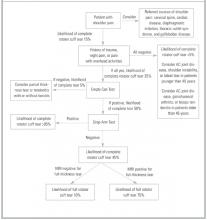
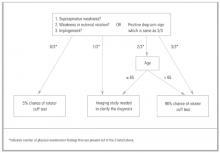
A general approach to the patient with shoulder pain is summarized in Figure 5. Pre- and posttest probabilities are included to give an understanding of how tests may help diagnose or rule out a complete rotator cuff tear. A recent prospective study combining multiple examination maneuvers demonstrated that a combination of 3 physical examination findings (supraspinatus weakness, weakness in external rotation, and impingement) along with the patient’s age can often diagnose or rule out a rotator cuff tear.38 This group of tests did not distinguish full versus partial thickness tears. This approach is summarized in Figure 6.
Figure 5
Basic approach to assess for complete rotator cuff tear
Figure 6
Alternative approach to a suspected rotator cuff tear
1. Urwin M, Symmons D, Allison T, et al. Estimating the burden of musculoskeletal disorders in the community: the comparative prevalence of symptoms at different anatomical sites, and the relation to social deprivation. Ann Rheum Dis 1998;57:649-55.
2. Peters D, Davies P, Pietroni P. Musculoskeletal clinic in general practice: study of one year’s referrals. Br J Gen Pract 1994;44:25-9.
3. Croft P. Soft-tissue rheumatism. In: Sillman AJ, Hochberg MC, Eds. Epidemiology of the Rheumatic Disease. Oxford, England: Oxford University Press; 1993;375-421.
4. Van der Windt DA, Koes BW, De Jong BA, Bouter LM. Shoulder disorders in general practice: incidence, patient characteristics, and management. Ann Rheum Dis 1995;54:959-64.
5. Bjelle A. Epidemiology of shoulder problems. Baillieres Clin Rheumatol 1989;3:437-51.
6. Lamberts H, Brouwer HJ, Mohrs J. Reason for Encounter-, Episode-and Process-Oriented Standard Output From Transition Project. Part I. Amsterdam: Department of General Practice/Family Medicine. University of Amsterdam; 1991.
7. Glockner SM. Shoulder pain: a diagnostic dilemma. Am Fam Physician 1995;51:1677-87-1690-2.
8. Butcher JD. Patient profile, referral sources, and consultant utilization in a primary care sports medicine clinic. J Fam Pract 1996;43:556-60.
9. Hill JA. Epidemiological perspective on shoulder injuries. Clin Sports Med 1983;2:241-6.
10. Watters DA, Brooks S, Elton RA, Little K. Sports injuries in an accident and emergency department. Arch Emerg Med 1984;1:105-11.
11. Croft P. Measuring up to shoulder pain. Ann Rheum Dis 1998;57:65-6.
12. Teefey SA, Hasan SA, Middleton WD, Patel M, Wright RW, Yamaguchi K. Ultrasonography of the rotator cuff. A comparison of ultrasonographic and arthroscopic findings in one hundred consecutive cases. J Bone Joint Surg Am 2000;82:498-504.
13. Matsen FA, Lippitt SB, Sidles JA, Harryman DT. Practical Evaluation and Management of the Shoulder. Philadelphia: W.B. Saunders; 1994.
14. Neer CS. Anterior acromioplasty for chronic impingement syndrome of shoulder. J Bone Joint Surg 1972;54A:41-50.
15. Blanchard TK, Bearcroft PW, Constant CR, Griffin DR, Dixon AK. Diagnostic and therapeutic impact of MRI and arthrography in the investigation of full-thickness rotator cuff tears. Eur Radiol 1999;9:638-42.
16. Torstensen ET, Hollinshead RM. Comparison of magnetic resonance imaging and arthroscopy in the evaluation of shoulder pathology. J Shoulder Elbow Surg 1999;8:42-5.
17. Calis M, Akgun K, Birtane M, Karacan I, Calis H, Tuzun F. Diagnostic values of clinical diagnostic tests in subacromial impingement syndrome. Ann Rheum Dis 2000;59:44-7.
18. Itoi E, Kido T, Samo A, Urayama M, Sato K. Which is more useful, the “full can test” or the “empty can test,” in detecting the torn supraspinatus tendon? Am J Sports Med 1999;27:65-8.
19. Litaker D, Pioro M, El Bilbeisi H, Brems J. Returning to the bedside: using the history and physical exam to identify rotator cuff tears. J Am Geriatr Soc 2000;48:1633-7.
20. Leroux JL, Thomas E, Bonnel F, Blotman F. Diagnostic value of clinical tests for shoulder impingement syndrome. Rev Rhum Engl Ed 1995;62:423-8.
21. Hertel R, Ballmer RT, Lombert SM, Gerber C. Lag signs in the diagnosis of rotator cuff rupture. J Shoulder Elbow Surg 1996;5:307-13.
22. MacDonald PB, Clark P, Sutherland K. An analysis of the diagnostic accuracy of the Hawkins and Neer subacromial impingement signs. J Shoulder Elbow Surg 2000;9:299-301.
23. Speer KP, Hannafin JA, Altchek DW, Warren RF. An evaluation of shoulder relocation test. Am J Sports Med 1994;22:177-83.
24. Liu SH, Henry MH, Nuccion SL. A prospective evaluation of a new physical examination in predicting glenoid labral tears. Am J Sports Med 1996;24:721-5.
25. O’Brien SJ, Pagnani MJ, Fealy S, McGlynn SR, Wilson JB. The active compression test: a new and effective test for diagnosing labral tears and acromioclavicular joint abnormality. Am J Sports Med 1998;26:610-3.
26. Howard T, O’Connor FG. The injured shoulder: primary care assessment. Arch Fam Med 1997;6:376-84.
27. Croft P, Pope D, Boswell R, Rigby A, Silman A. Observer variability in measuring elevation and external rotation of the shoulder. Primary Care Rheumatology Society Shoulder Study Group. Br J Rheumatol 1994;3 3:942-6.
28. Iannotti JP, Zlatkin MB, Esterhai JL, Kressel HY, Dalinka MK, Spindler KP. Magnetic resonance imaging of the shoulder. Sensitivity, specificity, and predictive value. J Bone Joint Surg 1991;73:17-29.
29. Burk DL, Jr, Karasick D, Kurtz AB, et al. Rotator cuff tears: prospective comparison of MR imaging with arthrography, sonography, and surgery. AJR Am J Roentgenol 1989;153:87-92.
30. Yeu K, Jiang CC, Shih TT. Correlation between MRI and operative findings of the rotator cuff tear. J Formos Med Assoc 1994;93:134-9.
31. Green MR, Christensen KP. Magnetic resonance imaging of the glenoid labrum in anterior shoulder instability. Am J Sports Med 1994;22:493-8.
32. Gusmer PB, Potter HG, Schaltz JA, et al. Labral injuries: accuracy of detection with unenhanced MR imaging of the shoulder. Radiology 1996;200:519-24.
33. Farin PU, Kaukanen E, Jaroma H, Vaatainen U, Miettinen H, Soimakallio S. Site and size of rotator-cuff tear. Findings at ultrasound, double-contrast arthrography, and computed tomography arthrography with surgical correlation. Invest Radiol 1996;31:387-94.
34. Van Moppes FI, Veldkam O, Roorda J. Role of shoulder ultrasonography in the evaluation of the painful shoulder. Eur J Radiol 1995;19:142-6.
35. Wilson AJ, Totty WG, Murphy WA, Hardy DC. Shoulder joint: arthrographic CT and long term follow-up, with surgical correlation. Radiology 1989;173:329-33.
36. Meyer SJ, Dalinka MK. Magnetic resonance imaging of the shoulder. Orthop Clin North Am 1990;21:497-513.
37. Sher JS, Uribe JW, Posada A, Murphy B, Zlatkin MB. Abnormal findings on magnetic resonance images of asymptomatic shoulders. J Bone Joint Surg Am 1995;77-A:10-5.
38. Murrell G, Walton J. Diagnosis of rotator cuff tears. Lancet 2001;357:769-70.
- Shoulder pain is a common complaint seen in primary care.
- Subacromial impingement syndrome and rotator cuff tears are the most common disorders encountered.
- The history and physical examination are keys to most shoulder pain diagnoses, particularly when used in combination.
- Imaging studies are indicated for failed conservative therapy, severe shoulder pathology, or unclear diagnosis.
Shoulder pain is a common problem that can pose difficult diagnostic and therapeutic challenges for the family physician. It is the third most common musculoskeletal complaint in the general population, and accounts for 5% of all general practitioner musculoskeletal consults.1,2 The incidence of shoulder pain is 6.6 to 25 cases per 1000 patients, with a peak incidence in the fourth through sixth decades.3-6 Shoulder pain is second only to knee pain for referrals to orthopedic surgery or primary care sports medicine clinics.7,8 Furthermore, 8% to 13% of athletic injuries involve the shoulder and account for up to 3.9% of new emergency department visits.9,10
Differential diagnosis
The challenge for the physician evaluating shoulder pain is the myriad of etiologies and the potential for multiple disorders. Compounding the challenge is a lack of uniformity in the literature regarding diagnostic classification.11 As Table 1 shows, the age of the patient will help focus the differential diagnosis. Patients younger than 30 years old tend to have biomechanical or mild inflammatory etiologies for their pain such as atraumatic instability, tendinosis, and arthropathies. Less than 1% of shoulder injuries in persons younger than 30 years are complete rotator cuff tears, which occur in 35% of patients older than 45 years with shoulder pain.12,13
The rotator cuff is the most commonly affected structure in the shoulder, and subacromial impingement syndrome is the leading cause of rotator cuff injury.4,12,14-16 Neer14 described 3 stages of shoulder impingement that he estimated lead to 95% of rotator cuff tears. Impingement can be caused by repetitive overhead activities, acute trauma, or subtle instability (atraumatic instability). The current theory is that inflammation of the rotator cuff tendons and/or bursa, caused by irritation against the coracoacromial arch, can progress to a complete rotator cuff tear over time.
Referred sources of shoulder pain should be included in the differential diagnosis of shoulder pain. Potential sources include cervical spondylolysis, cervical arthritis, cervical disc disease, myocardial ischemia, reflex sympathetic dystrophy, diaphragmatic irritation, thoracic outlet syndrome, and gallbladder disease.
TABLE 1
Differential diagnosis of shoulder pain
| Diagnosis | Primary care setting4-15(%) | Age (y) of presentation, Mean (SD)14 |
|---|---|---|
| Subacromial impingement syndrome | 48–72 | |
| Stage I (edema and hemorrhage) | 16 | 23 (7) |
| Stage II (cuff fibrosis and partial tear) | 42 | 41 (11) |
| Stage III (full-thickness tear) | 15 | 62 (12) |
| Adhesive capsulitis | 16–22 | 53 (10) |
| Acute bursitis | 17 | |
| Calcific tendonitis | 6 | |
| Myofascial pain syndrome | 5 | |
| Glenohumeral joint arthrosis | 2.5 | 64 (10) |
| Thoracic outlet syndrome | 2 | |
| Biceps tendonitis | 0.8 |
Using the history and physical examination
As noted above, the likelihood of specific conditions such as a complete rotator cuff tear varies with the setting, age of the patient, and specialty of the physi-cian.4,13,17,18 It is important to keep this pretest probability in mind while interpreting the history and physical examination. For example, a positive empty can test in a 50-year-old patient almost certainly represents a rotator cuff tear, whereas many younger patients with this finding will not have a tear. Moreover, certain components of the history and physical examination are more indicative of disorders while others are better at ruling them out. This concept is represented by the positive and negative likelihood ratios listed in Table 2.
The clinical evaluation begins with identification of the chief complaint and a thorough history. Common complaints include pain, weakness, stiffness, instability, locking, catching, and deformity.26 Determining the duration of symptoms and mechanism of injury will narrow the differential diagnosis. If trauma occurred, the mechanism can determine radiological needs. Aggravating and alleviating factors should be reviewed, including work, recreation, sports, or hobbies. Night pain when lying on the affected side and a history of trauma in a patient older than 65 years both suggest a rotator cuff tear, but no individual symptom is definitive for the diagnosis (Table 2).19 Pain with overhead work may indicate impingement syndrome, especially if the patient is symptomatic through the arc of 60 to 120 degrees.
The physical examination should include observation, palpation, range of motion (ROM), and provocative testing. Observation requires adequate exposure of the shoulders bilaterally to identify any gross deformities or abnormalities, including muscle atrophy, acromioclavicular joint disparity, or evidence of trauma. Muscle atrophy of either the supraspinatus or infraspinatus muscles is moderately predictive of rotator cuff tears in the elderly population, with a positive predictive value of 81%. However, this sign is not useful if absent, with a negative predictive value of only 43%.19 No studies have assessed the role of palpation in the evaluation of shoulder pain. Nevertheless, the role of palpation in discerning acromioclavicular joint pathology from shoulder and neck makes it a useful part of the examination.
The shoulder’s ROM should be evaluated both actively and passively. The shoulder is a mobile joint with a complexity of movements. These include flexion to 180 degrees, extension to 40 degrees, abduction to 120 degrees with palms down and 180 degrees with palms up, internal rotation to 55 degrees, and external rotation to 45 degrees with arms at the side. Although determining abduction ROM is consistent among examiners,27 interrater reliability is poor for assessment of external rotation ROM. Lack of full ROM that is equally limited with both passive and active examination is found in arthropathies and adhesive capsulitis.
Pain between 60 and 120 degrees of abduction “the painful arc”) is associated with subacromial impingement, whereas pain after 120 degrees is an indication of acromioclavicular joint origin. However, Calis and coworkers17 found that the presence of subacromial impingement has a positive likelihood ratio of only 1.7.
After assessing the ROM, the next steps are to evaluate the rotator cuff and biceps tendon, perform impingement testing, check for instability, and finally assess the acromioclavicular joint. The tests are listed in Table 2 in our preferred order of examination and represent the tests best supported by the evidence; the results are based on a literature search of Medline, PubMed, DARE, and Sports Discuss. The technique of each examination maneuver has been published elsewhere and is not described in detail here. Figure 1 through 4 illustrate several common examination maneuvers described below. A Web site that demonstrates the physical examination more thoroughly can be found at http://www.nismat.org/orthocor/exam/shoulder.html#Evaluation.
TABLE 2
Use of history and physical examination to diagnose shoulder pain
| History or maneuver | Study quality (1A–5)* | Sensitivity | Specificity | LR+ | LR− | PV+ | PV− |
|---|---|---|---|---|---|---|---|
| Rotator cuff tear | |||||||
| History of trauma19 | 2B | 36 | 73 | 1.3 | 0.88 | 72 | 37 |
| Night pain19 | 2B | 88 | 20 | 1.1 | 0.6 | 70 | 43 |
| Painful arc17 | 2B | 33 | 81 | 1.7 | 0.83 | 81 | 33 |
| Empty can test18,20,21 | 1B | 84–89 | 50–58 | 1.7–2 | 0.22–0.28 | 36–98 | 22–93 |
| Drop sign21 | 1B | 21 | 100 | >25 | 0.79 | 100 | 32 |
| Lift off test (for subscapularis tears)21 | 1B | 62 | 100 | >25 | 0.38 | 100 | 69 |
| Impingement | |||||||
| Hawkin’s test20,22 | 1B | 87–89 | 60 | 2.2 | 0.18 | 71 | 83 |
| Instability | |||||||
| Relocation test23 | 2B | 57 | 100 | >25 | 0.43 | 100 | 73 |
| Augmented apprehension23 | 2B | 68 | 100 | >25 | 0.32 | 100 | 78 |
| Labral tear | |||||||
| Crank test24 | 2B | 91 | 93 | 13 | 0.10 | 94 | 90 |
| Active compression test25 | 1B | 100 | 99 | >25 | 0.01 | 95 | 100 |
| Acromioclavicular joint | |||||||
| Active compression test25 | 1B | 100 | 97 | >25 | 0.01 | 89 | 100 |
| *Based on the guidelines for evidence quality outlined by the Center for Evidence-Based Medicine (http://163.1.96.10/docs/levels.html). | |||||||
| LR+ = positive likelihood ratio; LR− = negative likelihood ratio; PV+ = positive predictive value; PV− = negative predictive value. | |||||||
Figure 1
The empty can test
Rotator cuff tests
The drop arm test assesses the integrity of the rotator cuff, predominantly the supraspinatus muscle. The empty can test (Figure 1) isolates the supraspinatus against resistance. The lift off test (Figure 2) assesses the subscapularis integrity.
Figure 2
The lift off test
Impingement syndrome
Hawkin’s sign (Figure 3) is a test for evidence of impingement by re-creation of its symptoms.
Figures 3A & 3B
Hawkin’s sign
Glenohumeral joint stability
The augmented anterior apprehension test evaluates anterior shoulder instability. The relocation test, which helps confirm anterior instability, is carried out immediately after a positive anterior apprehension test.
Labral tears
The crank test is used to identify chronic labral injury, whereas the active compression test25 (Figure 4) indicates labral injury if pain is deep in the shoulder.
Figure 4
The active compression test
Acromioclavicular joint
The active compression test25 (Figure 4) indicates acromioclavicular joint inflammation, arthritis, or injury if pain is localized to the top of the shoulder.
Diagnostic tests
Imaging studies used in the evaluation of shoulder pain include plain radiographs, arthrography, computed tomography (CT), ultrasound (US), and magnetic resonance imaging (MRI). Often no imaging is required, or plain radiographs are the sole imaging study needed. Soft tissue injuries are best identified by MRI or US, whereas bony pathology is seen best with plain radiographs or CT. Indications for imaging include severe injury, uncontrolled pain, failure of conservative therapy, return to play considerations, and examiner discretion. Table 3 outlines the accuracy of imaging modalities organized by diagnosis.
TABLE 3
Imaging tests to diagnose shoulder pain
| Diagnostic test | Study quality (1A–5)* | Sensitivity | Specificity | LR+ | LR− | PV+ | PV− |
|---|---|---|---|---|---|---|---|
| MRI | |||||||
| Rotator cuff tears | |||||||
| Partial28 | 2B | 82 | 85 | 5.5 | 0.21 | 82 | 85 |
| Complete15 | 1B | 81 | 78 | 3.7 | 0.24 | — | — |
| Overall16,29,30 | 2B | 89–96 | 49–100 | 1.9 to >25 | 0.08 | 58 | 94 |
| Impingement28 | 2B | 93 | 87 | 7.2 | 0.08 | 93 | 87 |
| Labral tears31-32 | 1B | 75–89 | 97–100 | >25 | 0.11–0.25 | 100 | 41 |
| Plain arthrogram | |||||||
| Rotator cuff tears | |||||||
| Partial33 | 1B | 70 | — | — | — | — | — |
| Complete15 | 1A | 50 | 96 | 13 | 0.52 | — | — |
| CT arthrogram | |||||||
| Rotator cuff tears | |||||||
| Partial33 | 1B | 70 | — | — | — | — | — |
| Complete33 | 1B | 95 | — | — | — | — | — |
| Overall33 | 1B | 86 | 98 | >25 | 0.14 | 96 | 93 |
| Ultrasound | |||||||
| Rotator cuff tears | |||||||
| Partial33 | 1B | 80 | — | — | — | — | — |
| Complete33 | 1B | 90 | — | — | — | — | — |
| Overall33,34 | 1B | 86 | 91 | 9.6 | 0.15 | 96 | 73 |
| *Based on the guidelines for evidence quality outlined by the Center for Evidence-Based Medicine (http://163.1.96.10/docs/levels.html). | |||||||
| CT, computed tomography; LR+ = positive likelihood ratio; LR− = negative likelihood ratio; MRI, magnetic resonance imaging; PV+ = positive predictive value; PV− = negative predictive value. | |||||||
Plain radiographs
Plain radiographs are the first step in diagnostic imaging. They can reveal fractures, dislocation, subluxation, bony lesions, outlet obstruction, acromioclavicular joint pathology, and arthritic changes. No definitive clinical studies on the needs of radiographs have been done. Plain radiographs should be taken when ROM is lost, especially when there is abduction of less than 90 degrees, severe pain, and after trauma. Our preferred x-rays include a glenohumeral anteroposterior (AP) view, a supraspinatus outlet view, and an axillary view. Anteroposterior views with internal and external rotation are added in cases of trauma to help rule out fracture. Positive acromioclavicular joint tests (crossover or palpation) should be followed by acromioclavicular joint radiographs because a shoulder series does not give a clear view of this joint. Additional views of the neck as well as a chest x-ray or abdominal imaging should be considered if a referred source of shoulder pain remains a possibility.
Arthrography
Arthrography was the diagnostic test of choice before MRI. It is specific for rotator cuff tears but lacks sensitivity15 because it cannot detect partial-thickness or associated soft tissue injuries of the shoulder. Arthrography still has a role in evaluating adhesive capsulitis by demonstrating decreased intracapsular volume.26 The test can be therapeutic if the capsule is dilated during the procedure. Additionally, patients with claustrophobia may be good candidates for arthrography if a full-thickness tear is suspected and MRI is not possible.
Computed tomography
Computed tomography may be used to evaluate bony lesions, including glenoid rim fractures, humoral fractures, and acromioclavicular joint disease. Computed tomography arthrograms may have a role in assessing labral tears and full-thickness rotator cuff tears.35 The use of CT arthrography has fallen into disfavor compared with MRI because of the risks associated with contrast exposure and poor sensitivity for partial-thickness rotator cuff tears or associated soft tissue injury.
Ultrasound
Ultrasound has been used in the evaluation of rotator cuff tears with varying degrees of sensitivity and specificity.12,29,34 This inconsistency may be related to variation in operator skill. Advantages of US include relatively low cost, speed, and noninvasiveness.
Magnetic resonance imaging
Magnetic resonance imaging has become the gold standard for diagnostic imaging of the shoulder related to soft tissue injury. The advantages include its noninvasive nature, lack of contrast exposure, nonionizing radiation, high degree of resolution, and the ability to evaluate multiple potential pathologic processes.36 Magnetic resonance imaging is the preferred test for evaluating impingement syndrome and rotator cuff pathology. A normal MRI greatly reduces the chances of a rotator cuff tear, with a negative likelihood ratio of 0.08.16,29,30 Magnetic resonance imaging is also useful in the evaluation of avascular necrosis, biceps tendon disorders, inflammatory processes, and tumors.13 The diagnosis of labral lesions can be challenging given the relatively low sensitivity and negative predictive value noted in several trials.16,28,31 Finally, it is important to note that up to one third of all asymptomatic patients and more than half of those older than 60 years demonstrate asymptomatic rotator cuff tears on MRI.37
Approach to the patient
A general approach to the patient with shoulder pain is summarized in Figure 5. Pre- and posttest probabilities are included to give an understanding of how tests may help diagnose or rule out a complete rotator cuff tear. A recent prospective study combining multiple examination maneuvers demonstrated that a combination of 3 physical examination findings (supraspinatus weakness, weakness in external rotation, and impingement) along with the patient’s age can often diagnose or rule out a rotator cuff tear.38 This group of tests did not distinguish full versus partial thickness tears. This approach is summarized in Figure 6.
Figure 5
Basic approach to assess for complete rotator cuff tear
Figure 6
Alternative approach to a suspected rotator cuff tear
- Shoulder pain is a common complaint seen in primary care.
- Subacromial impingement syndrome and rotator cuff tears are the most common disorders encountered.
- The history and physical examination are keys to most shoulder pain diagnoses, particularly when used in combination.
- Imaging studies are indicated for failed conservative therapy, severe shoulder pathology, or unclear diagnosis.
Shoulder pain is a common problem that can pose difficult diagnostic and therapeutic challenges for the family physician. It is the third most common musculoskeletal complaint in the general population, and accounts for 5% of all general practitioner musculoskeletal consults.1,2 The incidence of shoulder pain is 6.6 to 25 cases per 1000 patients, with a peak incidence in the fourth through sixth decades.3-6 Shoulder pain is second only to knee pain for referrals to orthopedic surgery or primary care sports medicine clinics.7,8 Furthermore, 8% to 13% of athletic injuries involve the shoulder and account for up to 3.9% of new emergency department visits.9,10
Differential diagnosis
The challenge for the physician evaluating shoulder pain is the myriad of etiologies and the potential for multiple disorders. Compounding the challenge is a lack of uniformity in the literature regarding diagnostic classification.11 As Table 1 shows, the age of the patient will help focus the differential diagnosis. Patients younger than 30 years old tend to have biomechanical or mild inflammatory etiologies for their pain such as atraumatic instability, tendinosis, and arthropathies. Less than 1% of shoulder injuries in persons younger than 30 years are complete rotator cuff tears, which occur in 35% of patients older than 45 years with shoulder pain.12,13
The rotator cuff is the most commonly affected structure in the shoulder, and subacromial impingement syndrome is the leading cause of rotator cuff injury.4,12,14-16 Neer14 described 3 stages of shoulder impingement that he estimated lead to 95% of rotator cuff tears. Impingement can be caused by repetitive overhead activities, acute trauma, or subtle instability (atraumatic instability). The current theory is that inflammation of the rotator cuff tendons and/or bursa, caused by irritation against the coracoacromial arch, can progress to a complete rotator cuff tear over time.
Referred sources of shoulder pain should be included in the differential diagnosis of shoulder pain. Potential sources include cervical spondylolysis, cervical arthritis, cervical disc disease, myocardial ischemia, reflex sympathetic dystrophy, diaphragmatic irritation, thoracic outlet syndrome, and gallbladder disease.
TABLE 1
Differential diagnosis of shoulder pain
| Diagnosis | Primary care setting4-15(%) | Age (y) of presentation, Mean (SD)14 |
|---|---|---|
| Subacromial impingement syndrome | 48–72 | |
| Stage I (edema and hemorrhage) | 16 | 23 (7) |
| Stage II (cuff fibrosis and partial tear) | 42 | 41 (11) |
| Stage III (full-thickness tear) | 15 | 62 (12) |
| Adhesive capsulitis | 16–22 | 53 (10) |
| Acute bursitis | 17 | |
| Calcific tendonitis | 6 | |
| Myofascial pain syndrome | 5 | |
| Glenohumeral joint arthrosis | 2.5 | 64 (10) |
| Thoracic outlet syndrome | 2 | |
| Biceps tendonitis | 0.8 |
Using the history and physical examination
As noted above, the likelihood of specific conditions such as a complete rotator cuff tear varies with the setting, age of the patient, and specialty of the physi-cian.4,13,17,18 It is important to keep this pretest probability in mind while interpreting the history and physical examination. For example, a positive empty can test in a 50-year-old patient almost certainly represents a rotator cuff tear, whereas many younger patients with this finding will not have a tear. Moreover, certain components of the history and physical examination are more indicative of disorders while others are better at ruling them out. This concept is represented by the positive and negative likelihood ratios listed in Table 2.
The clinical evaluation begins with identification of the chief complaint and a thorough history. Common complaints include pain, weakness, stiffness, instability, locking, catching, and deformity.26 Determining the duration of symptoms and mechanism of injury will narrow the differential diagnosis. If trauma occurred, the mechanism can determine radiological needs. Aggravating and alleviating factors should be reviewed, including work, recreation, sports, or hobbies. Night pain when lying on the affected side and a history of trauma in a patient older than 65 years both suggest a rotator cuff tear, but no individual symptom is definitive for the diagnosis (Table 2).19 Pain with overhead work may indicate impingement syndrome, especially if the patient is symptomatic through the arc of 60 to 120 degrees.
The physical examination should include observation, palpation, range of motion (ROM), and provocative testing. Observation requires adequate exposure of the shoulders bilaterally to identify any gross deformities or abnormalities, including muscle atrophy, acromioclavicular joint disparity, or evidence of trauma. Muscle atrophy of either the supraspinatus or infraspinatus muscles is moderately predictive of rotator cuff tears in the elderly population, with a positive predictive value of 81%. However, this sign is not useful if absent, with a negative predictive value of only 43%.19 No studies have assessed the role of palpation in the evaluation of shoulder pain. Nevertheless, the role of palpation in discerning acromioclavicular joint pathology from shoulder and neck makes it a useful part of the examination.
The shoulder’s ROM should be evaluated both actively and passively. The shoulder is a mobile joint with a complexity of movements. These include flexion to 180 degrees, extension to 40 degrees, abduction to 120 degrees with palms down and 180 degrees with palms up, internal rotation to 55 degrees, and external rotation to 45 degrees with arms at the side. Although determining abduction ROM is consistent among examiners,27 interrater reliability is poor for assessment of external rotation ROM. Lack of full ROM that is equally limited with both passive and active examination is found in arthropathies and adhesive capsulitis.
Pain between 60 and 120 degrees of abduction “the painful arc”) is associated with subacromial impingement, whereas pain after 120 degrees is an indication of acromioclavicular joint origin. However, Calis and coworkers17 found that the presence of subacromial impingement has a positive likelihood ratio of only 1.7.
After assessing the ROM, the next steps are to evaluate the rotator cuff and biceps tendon, perform impingement testing, check for instability, and finally assess the acromioclavicular joint. The tests are listed in Table 2 in our preferred order of examination and represent the tests best supported by the evidence; the results are based on a literature search of Medline, PubMed, DARE, and Sports Discuss. The technique of each examination maneuver has been published elsewhere and is not described in detail here. Figure 1 through 4 illustrate several common examination maneuvers described below. A Web site that demonstrates the physical examination more thoroughly can be found at http://www.nismat.org/orthocor/exam/shoulder.html#Evaluation.
TABLE 2
Use of history and physical examination to diagnose shoulder pain
| History or maneuver | Study quality (1A–5)* | Sensitivity | Specificity | LR+ | LR− | PV+ | PV− |
|---|---|---|---|---|---|---|---|
| Rotator cuff tear | |||||||
| History of trauma19 | 2B | 36 | 73 | 1.3 | 0.88 | 72 | 37 |
| Night pain19 | 2B | 88 | 20 | 1.1 | 0.6 | 70 | 43 |
| Painful arc17 | 2B | 33 | 81 | 1.7 | 0.83 | 81 | 33 |
| Empty can test18,20,21 | 1B | 84–89 | 50–58 | 1.7–2 | 0.22–0.28 | 36–98 | 22–93 |
| Drop sign21 | 1B | 21 | 100 | >25 | 0.79 | 100 | 32 |
| Lift off test (for subscapularis tears)21 | 1B | 62 | 100 | >25 | 0.38 | 100 | 69 |
| Impingement | |||||||
| Hawkin’s test20,22 | 1B | 87–89 | 60 | 2.2 | 0.18 | 71 | 83 |
| Instability | |||||||
| Relocation test23 | 2B | 57 | 100 | >25 | 0.43 | 100 | 73 |
| Augmented apprehension23 | 2B | 68 | 100 | >25 | 0.32 | 100 | 78 |
| Labral tear | |||||||
| Crank test24 | 2B | 91 | 93 | 13 | 0.10 | 94 | 90 |
| Active compression test25 | 1B | 100 | 99 | >25 | 0.01 | 95 | 100 |
| Acromioclavicular joint | |||||||
| Active compression test25 | 1B | 100 | 97 | >25 | 0.01 | 89 | 100 |
| *Based on the guidelines for evidence quality outlined by the Center for Evidence-Based Medicine (http://163.1.96.10/docs/levels.html). | |||||||
| LR+ = positive likelihood ratio; LR− = negative likelihood ratio; PV+ = positive predictive value; PV− = negative predictive value. | |||||||
Figure 1
The empty can test
Rotator cuff tests
The drop arm test assesses the integrity of the rotator cuff, predominantly the supraspinatus muscle. The empty can test (Figure 1) isolates the supraspinatus against resistance. The lift off test (Figure 2) assesses the subscapularis integrity.
Figure 2
The lift off test
Impingement syndrome
Hawkin’s sign (Figure 3) is a test for evidence of impingement by re-creation of its symptoms.
Figures 3A & 3B
Hawkin’s sign
Glenohumeral joint stability
The augmented anterior apprehension test evaluates anterior shoulder instability. The relocation test, which helps confirm anterior instability, is carried out immediately after a positive anterior apprehension test.
Labral tears
The crank test is used to identify chronic labral injury, whereas the active compression test25 (Figure 4) indicates labral injury if pain is deep in the shoulder.
Figure 4
The active compression test
Acromioclavicular joint
The active compression test25 (Figure 4) indicates acromioclavicular joint inflammation, arthritis, or injury if pain is localized to the top of the shoulder.
Diagnostic tests
Imaging studies used in the evaluation of shoulder pain include plain radiographs, arthrography, computed tomography (CT), ultrasound (US), and magnetic resonance imaging (MRI). Often no imaging is required, or plain radiographs are the sole imaging study needed. Soft tissue injuries are best identified by MRI or US, whereas bony pathology is seen best with plain radiographs or CT. Indications for imaging include severe injury, uncontrolled pain, failure of conservative therapy, return to play considerations, and examiner discretion. Table 3 outlines the accuracy of imaging modalities organized by diagnosis.
TABLE 3
Imaging tests to diagnose shoulder pain
| Diagnostic test | Study quality (1A–5)* | Sensitivity | Specificity | LR+ | LR− | PV+ | PV− |
|---|---|---|---|---|---|---|---|
| MRI | |||||||
| Rotator cuff tears | |||||||
| Partial28 | 2B | 82 | 85 | 5.5 | 0.21 | 82 | 85 |
| Complete15 | 1B | 81 | 78 | 3.7 | 0.24 | — | — |
| Overall16,29,30 | 2B | 89–96 | 49–100 | 1.9 to >25 | 0.08 | 58 | 94 |
| Impingement28 | 2B | 93 | 87 | 7.2 | 0.08 | 93 | 87 |
| Labral tears31-32 | 1B | 75–89 | 97–100 | >25 | 0.11–0.25 | 100 | 41 |
| Plain arthrogram | |||||||
| Rotator cuff tears | |||||||
| Partial33 | 1B | 70 | — | — | — | — | — |
| Complete15 | 1A | 50 | 96 | 13 | 0.52 | — | — |
| CT arthrogram | |||||||
| Rotator cuff tears | |||||||
| Partial33 | 1B | 70 | — | — | — | — | — |
| Complete33 | 1B | 95 | — | — | — | — | — |
| Overall33 | 1B | 86 | 98 | >25 | 0.14 | 96 | 93 |
| Ultrasound | |||||||
| Rotator cuff tears | |||||||
| Partial33 | 1B | 80 | — | — | — | — | — |
| Complete33 | 1B | 90 | — | — | — | — | — |
| Overall33,34 | 1B | 86 | 91 | 9.6 | 0.15 | 96 | 73 |
| *Based on the guidelines for evidence quality outlined by the Center for Evidence-Based Medicine (http://163.1.96.10/docs/levels.html). | |||||||
| CT, computed tomography; LR+ = positive likelihood ratio; LR− = negative likelihood ratio; MRI, magnetic resonance imaging; PV+ = positive predictive value; PV− = negative predictive value. | |||||||
Plain radiographs
Plain radiographs are the first step in diagnostic imaging. They can reveal fractures, dislocation, subluxation, bony lesions, outlet obstruction, acromioclavicular joint pathology, and arthritic changes. No definitive clinical studies on the needs of radiographs have been done. Plain radiographs should be taken when ROM is lost, especially when there is abduction of less than 90 degrees, severe pain, and after trauma. Our preferred x-rays include a glenohumeral anteroposterior (AP) view, a supraspinatus outlet view, and an axillary view. Anteroposterior views with internal and external rotation are added in cases of trauma to help rule out fracture. Positive acromioclavicular joint tests (crossover or palpation) should be followed by acromioclavicular joint radiographs because a shoulder series does not give a clear view of this joint. Additional views of the neck as well as a chest x-ray or abdominal imaging should be considered if a referred source of shoulder pain remains a possibility.
Arthrography
Arthrography was the diagnostic test of choice before MRI. It is specific for rotator cuff tears but lacks sensitivity15 because it cannot detect partial-thickness or associated soft tissue injuries of the shoulder. Arthrography still has a role in evaluating adhesive capsulitis by demonstrating decreased intracapsular volume.26 The test can be therapeutic if the capsule is dilated during the procedure. Additionally, patients with claustrophobia may be good candidates for arthrography if a full-thickness tear is suspected and MRI is not possible.
Computed tomography
Computed tomography may be used to evaluate bony lesions, including glenoid rim fractures, humoral fractures, and acromioclavicular joint disease. Computed tomography arthrograms may have a role in assessing labral tears and full-thickness rotator cuff tears.35 The use of CT arthrography has fallen into disfavor compared with MRI because of the risks associated with contrast exposure and poor sensitivity for partial-thickness rotator cuff tears or associated soft tissue injury.
Ultrasound
Ultrasound has been used in the evaluation of rotator cuff tears with varying degrees of sensitivity and specificity.12,29,34 This inconsistency may be related to variation in operator skill. Advantages of US include relatively low cost, speed, and noninvasiveness.
Magnetic resonance imaging
Magnetic resonance imaging has become the gold standard for diagnostic imaging of the shoulder related to soft tissue injury. The advantages include its noninvasive nature, lack of contrast exposure, nonionizing radiation, high degree of resolution, and the ability to evaluate multiple potential pathologic processes.36 Magnetic resonance imaging is the preferred test for evaluating impingement syndrome and rotator cuff pathology. A normal MRI greatly reduces the chances of a rotator cuff tear, with a negative likelihood ratio of 0.08.16,29,30 Magnetic resonance imaging is also useful in the evaluation of avascular necrosis, biceps tendon disorders, inflammatory processes, and tumors.13 The diagnosis of labral lesions can be challenging given the relatively low sensitivity and negative predictive value noted in several trials.16,28,31 Finally, it is important to note that up to one third of all asymptomatic patients and more than half of those older than 60 years demonstrate asymptomatic rotator cuff tears on MRI.37
Approach to the patient
A general approach to the patient with shoulder pain is summarized in Figure 5. Pre- and posttest probabilities are included to give an understanding of how tests may help diagnose or rule out a complete rotator cuff tear. A recent prospective study combining multiple examination maneuvers demonstrated that a combination of 3 physical examination findings (supraspinatus weakness, weakness in external rotation, and impingement) along with the patient’s age can often diagnose or rule out a rotator cuff tear.38 This group of tests did not distinguish full versus partial thickness tears. This approach is summarized in Figure 6.
Figure 5
Basic approach to assess for complete rotator cuff tear
Figure 6
Alternative approach to a suspected rotator cuff tear
1. Urwin M, Symmons D, Allison T, et al. Estimating the burden of musculoskeletal disorders in the community: the comparative prevalence of symptoms at different anatomical sites, and the relation to social deprivation. Ann Rheum Dis 1998;57:649-55.
2. Peters D, Davies P, Pietroni P. Musculoskeletal clinic in general practice: study of one year’s referrals. Br J Gen Pract 1994;44:25-9.
3. Croft P. Soft-tissue rheumatism. In: Sillman AJ, Hochberg MC, Eds. Epidemiology of the Rheumatic Disease. Oxford, England: Oxford University Press; 1993;375-421.
4. Van der Windt DA, Koes BW, De Jong BA, Bouter LM. Shoulder disorders in general practice: incidence, patient characteristics, and management. Ann Rheum Dis 1995;54:959-64.
5. Bjelle A. Epidemiology of shoulder problems. Baillieres Clin Rheumatol 1989;3:437-51.
6. Lamberts H, Brouwer HJ, Mohrs J. Reason for Encounter-, Episode-and Process-Oriented Standard Output From Transition Project. Part I. Amsterdam: Department of General Practice/Family Medicine. University of Amsterdam; 1991.
7. Glockner SM. Shoulder pain: a diagnostic dilemma. Am Fam Physician 1995;51:1677-87-1690-2.
8. Butcher JD. Patient profile, referral sources, and consultant utilization in a primary care sports medicine clinic. J Fam Pract 1996;43:556-60.
9. Hill JA. Epidemiological perspective on shoulder injuries. Clin Sports Med 1983;2:241-6.
10. Watters DA, Brooks S, Elton RA, Little K. Sports injuries in an accident and emergency department. Arch Emerg Med 1984;1:105-11.
11. Croft P. Measuring up to shoulder pain. Ann Rheum Dis 1998;57:65-6.
12. Teefey SA, Hasan SA, Middleton WD, Patel M, Wright RW, Yamaguchi K. Ultrasonography of the rotator cuff. A comparison of ultrasonographic and arthroscopic findings in one hundred consecutive cases. J Bone Joint Surg Am 2000;82:498-504.
13. Matsen FA, Lippitt SB, Sidles JA, Harryman DT. Practical Evaluation and Management of the Shoulder. Philadelphia: W.B. Saunders; 1994.
14. Neer CS. Anterior acromioplasty for chronic impingement syndrome of shoulder. J Bone Joint Surg 1972;54A:41-50.
15. Blanchard TK, Bearcroft PW, Constant CR, Griffin DR, Dixon AK. Diagnostic and therapeutic impact of MRI and arthrography in the investigation of full-thickness rotator cuff tears. Eur Radiol 1999;9:638-42.
16. Torstensen ET, Hollinshead RM. Comparison of magnetic resonance imaging and arthroscopy in the evaluation of shoulder pathology. J Shoulder Elbow Surg 1999;8:42-5.
17. Calis M, Akgun K, Birtane M, Karacan I, Calis H, Tuzun F. Diagnostic values of clinical diagnostic tests in subacromial impingement syndrome. Ann Rheum Dis 2000;59:44-7.
18. Itoi E, Kido T, Samo A, Urayama M, Sato K. Which is more useful, the “full can test” or the “empty can test,” in detecting the torn supraspinatus tendon? Am J Sports Med 1999;27:65-8.
19. Litaker D, Pioro M, El Bilbeisi H, Brems J. Returning to the bedside: using the history and physical exam to identify rotator cuff tears. J Am Geriatr Soc 2000;48:1633-7.
20. Leroux JL, Thomas E, Bonnel F, Blotman F. Diagnostic value of clinical tests for shoulder impingement syndrome. Rev Rhum Engl Ed 1995;62:423-8.
21. Hertel R, Ballmer RT, Lombert SM, Gerber C. Lag signs in the diagnosis of rotator cuff rupture. J Shoulder Elbow Surg 1996;5:307-13.
22. MacDonald PB, Clark P, Sutherland K. An analysis of the diagnostic accuracy of the Hawkins and Neer subacromial impingement signs. J Shoulder Elbow Surg 2000;9:299-301.
23. Speer KP, Hannafin JA, Altchek DW, Warren RF. An evaluation of shoulder relocation test. Am J Sports Med 1994;22:177-83.
24. Liu SH, Henry MH, Nuccion SL. A prospective evaluation of a new physical examination in predicting glenoid labral tears. Am J Sports Med 1996;24:721-5.
25. O’Brien SJ, Pagnani MJ, Fealy S, McGlynn SR, Wilson JB. The active compression test: a new and effective test for diagnosing labral tears and acromioclavicular joint abnormality. Am J Sports Med 1998;26:610-3.
26. Howard T, O’Connor FG. The injured shoulder: primary care assessment. Arch Fam Med 1997;6:376-84.
27. Croft P, Pope D, Boswell R, Rigby A, Silman A. Observer variability in measuring elevation and external rotation of the shoulder. Primary Care Rheumatology Society Shoulder Study Group. Br J Rheumatol 1994;3 3:942-6.
28. Iannotti JP, Zlatkin MB, Esterhai JL, Kressel HY, Dalinka MK, Spindler KP. Magnetic resonance imaging of the shoulder. Sensitivity, specificity, and predictive value. J Bone Joint Surg 1991;73:17-29.
29. Burk DL, Jr, Karasick D, Kurtz AB, et al. Rotator cuff tears: prospective comparison of MR imaging with arthrography, sonography, and surgery. AJR Am J Roentgenol 1989;153:87-92.
30. Yeu K, Jiang CC, Shih TT. Correlation between MRI and operative findings of the rotator cuff tear. J Formos Med Assoc 1994;93:134-9.
31. Green MR, Christensen KP. Magnetic resonance imaging of the glenoid labrum in anterior shoulder instability. Am J Sports Med 1994;22:493-8.
32. Gusmer PB, Potter HG, Schaltz JA, et al. Labral injuries: accuracy of detection with unenhanced MR imaging of the shoulder. Radiology 1996;200:519-24.
33. Farin PU, Kaukanen E, Jaroma H, Vaatainen U, Miettinen H, Soimakallio S. Site and size of rotator-cuff tear. Findings at ultrasound, double-contrast arthrography, and computed tomography arthrography with surgical correlation. Invest Radiol 1996;31:387-94.
34. Van Moppes FI, Veldkam O, Roorda J. Role of shoulder ultrasonography in the evaluation of the painful shoulder. Eur J Radiol 1995;19:142-6.
35. Wilson AJ, Totty WG, Murphy WA, Hardy DC. Shoulder joint: arthrographic CT and long term follow-up, with surgical correlation. Radiology 1989;173:329-33.
36. Meyer SJ, Dalinka MK. Magnetic resonance imaging of the shoulder. Orthop Clin North Am 1990;21:497-513.
37. Sher JS, Uribe JW, Posada A, Murphy B, Zlatkin MB. Abnormal findings on magnetic resonance images of asymptomatic shoulders. J Bone Joint Surg Am 1995;77-A:10-5.
38. Murrell G, Walton J. Diagnosis of rotator cuff tears. Lancet 2001;357:769-70.
1. Urwin M, Symmons D, Allison T, et al. Estimating the burden of musculoskeletal disorders in the community: the comparative prevalence of symptoms at different anatomical sites, and the relation to social deprivation. Ann Rheum Dis 1998;57:649-55.
2. Peters D, Davies P, Pietroni P. Musculoskeletal clinic in general practice: study of one year’s referrals. Br J Gen Pract 1994;44:25-9.
3. Croft P. Soft-tissue rheumatism. In: Sillman AJ, Hochberg MC, Eds. Epidemiology of the Rheumatic Disease. Oxford, England: Oxford University Press; 1993;375-421.
4. Van der Windt DA, Koes BW, De Jong BA, Bouter LM. Shoulder disorders in general practice: incidence, patient characteristics, and management. Ann Rheum Dis 1995;54:959-64.
5. Bjelle A. Epidemiology of shoulder problems. Baillieres Clin Rheumatol 1989;3:437-51.
6. Lamberts H, Brouwer HJ, Mohrs J. Reason for Encounter-, Episode-and Process-Oriented Standard Output From Transition Project. Part I. Amsterdam: Department of General Practice/Family Medicine. University of Amsterdam; 1991.
7. Glockner SM. Shoulder pain: a diagnostic dilemma. Am Fam Physician 1995;51:1677-87-1690-2.
8. Butcher JD. Patient profile, referral sources, and consultant utilization in a primary care sports medicine clinic. J Fam Pract 1996;43:556-60.
9. Hill JA. Epidemiological perspective on shoulder injuries. Clin Sports Med 1983;2:241-6.
10. Watters DA, Brooks S, Elton RA, Little K. Sports injuries in an accident and emergency department. Arch Emerg Med 1984;1:105-11.
11. Croft P. Measuring up to shoulder pain. Ann Rheum Dis 1998;57:65-6.
12. Teefey SA, Hasan SA, Middleton WD, Patel M, Wright RW, Yamaguchi K. Ultrasonography of the rotator cuff. A comparison of ultrasonographic and arthroscopic findings in one hundred consecutive cases. J Bone Joint Surg Am 2000;82:498-504.
13. Matsen FA, Lippitt SB, Sidles JA, Harryman DT. Practical Evaluation and Management of the Shoulder. Philadelphia: W.B. Saunders; 1994.
14. Neer CS. Anterior acromioplasty for chronic impingement syndrome of shoulder. J Bone Joint Surg 1972;54A:41-50.
15. Blanchard TK, Bearcroft PW, Constant CR, Griffin DR, Dixon AK. Diagnostic and therapeutic impact of MRI and arthrography in the investigation of full-thickness rotator cuff tears. Eur Radiol 1999;9:638-42.
16. Torstensen ET, Hollinshead RM. Comparison of magnetic resonance imaging and arthroscopy in the evaluation of shoulder pathology. J Shoulder Elbow Surg 1999;8:42-5.
17. Calis M, Akgun K, Birtane M, Karacan I, Calis H, Tuzun F. Diagnostic values of clinical diagnostic tests in subacromial impingement syndrome. Ann Rheum Dis 2000;59:44-7.
18. Itoi E, Kido T, Samo A, Urayama M, Sato K. Which is more useful, the “full can test” or the “empty can test,” in detecting the torn supraspinatus tendon? Am J Sports Med 1999;27:65-8.
19. Litaker D, Pioro M, El Bilbeisi H, Brems J. Returning to the bedside: using the history and physical exam to identify rotator cuff tears. J Am Geriatr Soc 2000;48:1633-7.
20. Leroux JL, Thomas E, Bonnel F, Blotman F. Diagnostic value of clinical tests for shoulder impingement syndrome. Rev Rhum Engl Ed 1995;62:423-8.
21. Hertel R, Ballmer RT, Lombert SM, Gerber C. Lag signs in the diagnosis of rotator cuff rupture. J Shoulder Elbow Surg 1996;5:307-13.
22. MacDonald PB, Clark P, Sutherland K. An analysis of the diagnostic accuracy of the Hawkins and Neer subacromial impingement signs. J Shoulder Elbow Surg 2000;9:299-301.
23. Speer KP, Hannafin JA, Altchek DW, Warren RF. An evaluation of shoulder relocation test. Am J Sports Med 1994;22:177-83.
24. Liu SH, Henry MH, Nuccion SL. A prospective evaluation of a new physical examination in predicting glenoid labral tears. Am J Sports Med 1996;24:721-5.
25. O’Brien SJ, Pagnani MJ, Fealy S, McGlynn SR, Wilson JB. The active compression test: a new and effective test for diagnosing labral tears and acromioclavicular joint abnormality. Am J Sports Med 1998;26:610-3.
26. Howard T, O’Connor FG. The injured shoulder: primary care assessment. Arch Fam Med 1997;6:376-84.
27. Croft P, Pope D, Boswell R, Rigby A, Silman A. Observer variability in measuring elevation and external rotation of the shoulder. Primary Care Rheumatology Society Shoulder Study Group. Br J Rheumatol 1994;3 3:942-6.
28. Iannotti JP, Zlatkin MB, Esterhai JL, Kressel HY, Dalinka MK, Spindler KP. Magnetic resonance imaging of the shoulder. Sensitivity, specificity, and predictive value. J Bone Joint Surg 1991;73:17-29.
29. Burk DL, Jr, Karasick D, Kurtz AB, et al. Rotator cuff tears: prospective comparison of MR imaging with arthrography, sonography, and surgery. AJR Am J Roentgenol 1989;153:87-92.
30. Yeu K, Jiang CC, Shih TT. Correlation between MRI and operative findings of the rotator cuff tear. J Formos Med Assoc 1994;93:134-9.
31. Green MR, Christensen KP. Magnetic resonance imaging of the glenoid labrum in anterior shoulder instability. Am J Sports Med 1994;22:493-8.
32. Gusmer PB, Potter HG, Schaltz JA, et al. Labral injuries: accuracy of detection with unenhanced MR imaging of the shoulder. Radiology 1996;200:519-24.
33. Farin PU, Kaukanen E, Jaroma H, Vaatainen U, Miettinen H, Soimakallio S. Site and size of rotator-cuff tear. Findings at ultrasound, double-contrast arthrography, and computed tomography arthrography with surgical correlation. Invest Radiol 1996;31:387-94.
34. Van Moppes FI, Veldkam O, Roorda J. Role of shoulder ultrasonography in the evaluation of the painful shoulder. Eur J Radiol 1995;19:142-6.
35. Wilson AJ, Totty WG, Murphy WA, Hardy DC. Shoulder joint: arthrographic CT and long term follow-up, with surgical correlation. Radiology 1989;173:329-33.
36. Meyer SJ, Dalinka MK. Magnetic resonance imaging of the shoulder. Orthop Clin North Am 1990;21:497-513.
37. Sher JS, Uribe JW, Posada A, Murphy B, Zlatkin MB. Abnormal findings on magnetic resonance images of asymptomatic shoulders. J Bone Joint Surg Am 1995;77-A:10-5.
38. Murrell G, Walton J. Diagnosis of rotator cuff tears. Lancet 2001;357:769-70.
Outpatient treatment of heart failure
- Control the risks for the development and progression of heart failure (HF) by controlling hypertension, diabetes, myocardial ischemia, and tobacco and alcohol use.
- Treat HF with angiotensin-converting enzyme inhibitors, angiotensin receptor blockers, or beta-blockers, used alone or in combination; add spironolactone and carvedilol (or change current beta-blocker to carvedilol) in severe HF; institute aerobic exercise program.
- Control symptoms with diuretics, restricted dietary sodium intake, and digoxin.
- Provide close follow-up that is comprehensive and multidisciplinary, including intensive patient education; self-monitoring of weight, symptoms, and blood pressure; and periodic telephone or in-home follow-up between scheduled office visits.
Heart failure (HF) affects more than 2 million adults in the United States.1 This common, costly, and disabling disorder mainly affects the elderly, with prevalence rates of up to 10% in patients older than 65 years.2,3 The management of HF is responsible for millions of outpatient visits per year,4 is the most common discharge diagnosis for Medicare beneficiaries,5 and accounts for more than 5% of total health care dollars spent.6
Treatment
Major advances in the pharmacologic treatment of heart failure (HF) have emerged in recent years. An approach to the diagnosis and evaluation of HF is described elsewhere.7 This article summarizes the evidence for outpatient treatment of HF. Current intervention trials do not distinguish between systolic and diastolic heart failure; it is therefore unknown whether or how drug therapy should be tailored according to the type of HF. The treatment of cardiac dysrhythmias in the setting of HF is beyond the scope of this article and is presented elsewhere.8Table 1 compares the available outpatient treatments of HF and includes the levels of evidence, numbers needed to treat, and appropriate situations for use. In the remainder of this article, we will discuss pharmacologic and nonpharmacologic management, including identification of ineffective treatments.
TABLE 1
Treatment options in heart failure
| Strength of recommendation (level of evidence)* | Treatment | NNT (Time)† | Use in NYHA class | Comments |
|---|---|---|---|---|
| A (1a) | Angiotensin-converting9-14 enzyme (ACE) inhibitors | 24 (90 days to 2 years) | I–IV | Even moderate doses (equivalent to 10 to 20 mg enalapril per day) provide benefit |
| A (1b) | Angiotensin-receptor blockers (ARBs)15,16 | Similar to ACE inhibitors | I–IV | Useful in patients who do not tolerate ACE inhibitors; may be combined with ACE inhibitors or beta-blockers, but not both |
| A (1a) | Beta-blockers (metoprolol, bisoprolol, carvedilol)17-20 | 24 (1 to 2 years) | I–IV | Usually added to ACE inhibitors or ARBs. May also be useful if concomitant tachydysrhythmias are present and in the post-MI period |
| A (1b) | Carvedilol21 | 18 (10 months) | III–IV | Add carvedilol if not already taking beta-blocker or change current beta-blocker to carvedilol |
| A (1b) | Spironolactone23 | 9 (2 years) | III–IV | NNT = 4 (2 years) to prevent hospitalization for HF. Severe hyperkalemia important safety concern (NNH = 195 over 2 years) |
| A (1b) | Hydralazine + isosorbide dinitrate (ISDN)24,25 | 19 (6 years) | I–IV | Use limited by poor tolerability |
| B (1a) | Digoxin26-28 | N/A | I–IV | No mortality benefit. NNT = 22 to prevent 1 hospitalization over 3 years. Increased risk of hospitalization for digoxin toxicity (NNH = 94 over 3 years) |
| B (2b) | Diuretics (furosemide, bumetanide, torsemide)29-32 | N/A | I–IV | Used for fluid, sodium, and symptom control. No data on mortality benefit |
| A (1b) | Aerobic exercise38-40 | 4 (14 months) | I–IV | Decreases hospitalization for HF (NNT = 5). Even brief symptom-limited exercise in severe HF has benefit in improving quality of life |
| A (1b) | Comprehensive, multi-disciplinary outpatient visits | N/A | I–IV | No mortality benefit. NNT = 5 for 3 months to prevent repeat hospitalization. Includes some combination of intensive education, medication monitoring, individualized diet modification, telephone/home visit follow-up between scheduled outpatient visits |
| B (5) | Dietary sodium restriction8,36,37 | N/A | I–IV | Recommended as standard practice, but no morbidity or mortality data from RCTs |
| C (2a) | Antiplatelet therapy and anticoagulation in HF with sinus rhythm33,35,58 | N/A | N/A | Antiplatelet therapy not useful. No data to support routine anticoagulation, although may be useful in severe HF. Patients with concomitant atrial fibrillation should be anticoagulated if no contraindications |
| D (1b) | Calcium channel blockers (CCBs)46-50 | N/A | N/A | Short-acting CCBs worsen HF. Newer, long-acting CCBs do not worsen HF, but there is no evidence of morbidity or mortality benefit |
| D (1b) | Intermittent positive inotrope (oral or intravenous)(dobutamine, milrinone)51-53 | N/A | N/A | Increased mortality (NNH = 17 over 5 months), increased hospitalizations for worsening HF (NNH = 20), and serious adverse reactions (NNH = 25) |
| *Based on the guidelines for evidence quality outlined by the Center for Evidence-Based Medicine. Available at http://cebm.jr2.ox.ac.uk/docs/levels.html. | ||||
| †NNT = number needed to treat to prevent 1 death over specified time period unless otherwise noted. | ||||
| HF, heart failure; MI, myocardial infarction; NNH, number needed to harm; NNT, number needed to treat; NYHA, New York Heart Association classification; RCT, randomized controlled trial. | ||||
Pharmacologic treatment
Angiotensin-converting enzyme inhibitors. A systematic review9 of 32 trials with a total of 7105 patients demonstrated that mortality rates were lower in patients taking an angiotensin-converting enzyme (ACE) inhibitor than in those not taking one (number needed to treat [NNT] = 24 for > 90 days, meaning that 1 fewer death occurs for every 24 patients who take an ACE inhibitor for more than 90 days). In addition, there is a reduction in the combined endpoints of death and hospitalization because of HF (NNT = 11). Although most of this benefit was realized in the first 90 days of therapy, benefits lasted for 4 to 5 years and were more pronounced in patients categorized in more severe New York Heart Association (NYHA) HF classes10 (class I: no limitation of activities; class II: slight limitation of activity; class III: marked limitation of activity and comfortable only at rest; class IV: symptoms at rest).
Dosage comparison studies demonstrate that HF patients can benefit from even moderate doses of ACE inhibitors. A recent multicenter trial comparing moderate dose enalapril (10 mg twice a day) with a higher dose (30 mg twice a day) in patients with a left ventricular ejection fraction (LVEF) of less than 20% found no differences in mortality at 1 year between the 2 groups.11 In addition, both groups achieved similar increases in functional status and LVEF.
Several trials have demonstrated good tolerability of ACE inhibitors.12-14 Dropout rates of 15% to 30% were similar between patients in the ACE inhibitor and placebo groups, mainly because of side effects, including dizziness, altered taste, hypotension, hyperkalemia, and cough.
Angiotensin-receptor blockers. Angiotensin-receptor blockers (ARBs) reduce all-cause mortality and HF-related hospitalizations in patients with NYHA class II and III HF at rates comparable with those of ACE inhibitors.15,16 Cough is not a side effect of ARBs. Although they are more expensive, ARBs offer a reasonable alternative for patients who do not tolerate ACE inhibitors.
Beta-blockers. The beta-blockers carvedilol, metoprolol, and bisoprolol have a proven mortality benefit for patients with HF.17-19 Pooled results of 6 randomized controlled trials (RCTs), including more than 9000 patients already taking ACE inhibitors, showed a significant reduction in total mortality (NNT = 24 over 1–2 years) and sudden death (NNT = 35), regardless of NYHA classification.20 The average dropout rate of 16% was similar in the betablocker and placebo groups.
Early beta-blocker studies included few NYHA class IV patients until a recent study of the use of carvedilol in severe chronic HF.21 In this study, all patients were taking diuretics plus either an ACE inhibitor or ARB and were permitted to take digoxin, nitrates, hydralazine, spironolactone, or amiodarone. Carvedilol at an average dose of 37 mg per day decreased mortality (NNT = 18 for 10 months) and lowered combined mortality and hospitalization for worsening HF (NNT = 13). Study patients taking carvedilol withdrew from the study at a lower rate (approximately 15%) than placebo.
Because the pharmacologic properties of betablockers vary, clinicians have wondered which are most beneficial. The investigators in a study comparing metoprolol (a beta-1 antagonist) with carvedilol (a beta-1, beta-2, and alpha-1 antagonist) in NYHA class II or III patients found no differences in quality-of-life measures or changes in NYHA classification.22
Spironolactone. The addition of spironolactone to standard care can help patients with severe HF.23 In NYHA class III and IV HF patients, spironolactone at doses ranging from 25 mg every other day to 50 mg per day reduces mortality (NNT = 9 for 2 years), reduces hospitalization from all cardiac causes (NNT = 4), and reduces hospitalization for worsening HF (NNT = 3). The most common serious adverse event in the spironolactone group was severe hyperkalemia (number needed to harm [NNH] = 195). Ten percent of men taking spironolactone experienced breast pain and gynecomastia.
Hydralazine and isosorbide dinitrate. The combination of hydralazine and isosorbide dinitrate (ISDN) reduces mortality in HF patients, but tolerability is an issue. In earlier trials, men with HF symptoms that were optimally controlled with digoxin and diuretics and treated with hydralazine (average dose = 270 mg/day) plus ISDN (average dose = 136 mg/day) had a decrease in all-cause mortality of 28% (NNT = 19 for 6 years).24 A more recent trial comparing hydralazine plus ISDN with enalapril25 (average daily doses of hydralazine = 300 mg/day; ISDN = 160 mg/day; enalapril = 20 mg/day) in NYHA class II–III patients showed no differences in mortality between the 2 groups over 3 years. Tolerability was a problem in these trials; more than 30% of patients stopped taking hydralazine, nitrate, or both.
Digoxin. Digoxin is effective for treating the symptoms of HF in the absence of dysrhythmias but there are no data demonstrating a mortality benefit. Digoxin increases functional capacity in NYHA class II–III patients and heart failure symptoms worsen if digoxin is withdrawn.26 Although there are no differences in all-cause mortality with the use of digoxin, there are fewer hospitalizations due to worsening HF (NNT = 27–114 over 3 years) and a lower rate of clinical deterioration (NNT = 4–75).27 In a randomized trial comparing digoxin and placebo, patients taking digoxin were twice as likely to be hospitalized for suspected digoxin toxicity (2.0% vs 0.9%; P < .001; NNH = 52).28
Diuretics. Diuretics are a mainstay of the symptomatic treatment of heart failure. Short-term studies have shown that diuretics improve the symptoms of sodium and fluid retention and increase exercise tolerance and cardiac function regardless of NYHA classification.29-32 No studies that examine their effects on morbidity and mortality are available.
Antiplatelet therapy and anticoagulation. Patients with HF have an increased risk for thromboembolic events of 1.6% to 3.2% per year.33 One systematic review concluded that antiplatelet therapy is not useful in preventing thromboembolism in patients with HF in sinus rhythm and may even be harmful.34 Another systematic review also concluded that the data do not support the routine use of anticoagulants (eg, warfarin) in patients with HF and sinus rhythm.35 Anticoagulation may be beneficial, however, if there is echocardiographic visualization of a left ventricular thrombus or in cases of “severe” HF or concomitant atrial fibrillation.35
Nonpharmacologic management
Dietary sodium restriction. There is consensus that dietary sodium restriction is important in the treatment of HF36 and is recommended in published guidelines.8,37 Sodium restriction assists with fluid volume control and minimizes the dosages of HF drugs used. These recommendations are based on the retention of sodium and water in symptomatic HF. No studies, however, have examined the effect of dietary sodium restriction on morbidity or mortality, either alone or in combination with pharmacologic treatments.
Exercise training. Moderate exercise training improves quality of life and decreases mortality in patients with stable chronic HF. A recent RCT demonstrated a decrease in mortality (NNT = 4 for 14 months) and hospital readmission for HF (NNT = 5) with only moderate exercise on a stationary bicycle (60% of maximum exercise capacity) for 2 to 3 hours per week.38 Other studies have demonstrated improvements in physiologic markers39 and in quality-of-life ratings with short-term, symptom-limited exercise.40
Multidisciplinary or case-management approach. A case-based or disease-management approach to patients with HF decreases the frequency of unplanned and repeat hospitalizations, increases functional status, and increases quality of life.41 Even a single in-home visit by a clinical pharmacist and a nurse results in fewer unplanned readmissions and fewer days of hospitalization up to 18 months after discharge.42,43 A small study of 27 patients in a Veterans Affairs hospital demonstrated that patient instruction in the self-monitoring of weight and blood pressure, combined with frequent telephone follow-up from a nurse, lowered repeat hospitalizations over 1 year, with the effect more pronounced in patients with more severe NYHA classifications.44 A large RCT demonstrated that a multidisciplinary management approach (intensive patient education about HF and its treatment, dietary assessment and instruction, medication analysis and elimination of unnecessary medications, and telephone and home visit follow-up) results in fewer hospitalizations (NNT = 5 for 3 months) and reduced costs of care.45
Treatments that have no benefit or are harmful
Calcium-channel blockers. Although some of the newer, longer-acting calcium-channel blockers (CCBs) appear to be safe in the treatment of heart failure,46-49 no trials are available demonstrating that they lower mortality, decrease hospitalizations, or improve quality of life in patients with a failing heart. Older, short-acting CCBs can worsen HF.50
Positive inotropic therapy. Intermittent positive inotropic therapy, either orally (milrinone) or intravenously (dobutamine), should be avoided. Although short-term studies have shown some increase in cardiac function and symptoms,51 long-term studies demonstrate no mortality benefit.52 One RCT of milrinone demonstrated an increase in mortality (NNH = 17 for 5 months), an increased rate of hospitalization for worsening HF (NNH = 20), and more serious side effects (NNH = 25).53
Prognosis
Despite the increased longevity in Western developed nations and increased survival from coronary artery disease over recent decades, the overall prognosis of HF has improved very little.6,54 Mortality data derived from several different sources, the largest being the Framingham Heart Study,2,55 have shown that HF remains highly lethal, with a 5-year survival rate of 25% in men and 38% in women with NYHA II–IV heart failure. Mortality data from the placebo arms of intervention trials show an average 1-year mortality of 18%.9,17,19,20,56 A recent population-based study of patients with a new diagnosis of HF showed survival rates of only 62% at 12 months and 57% at 18 months.57 Despite these dismal population-based data, predicting the likelihood of survival in individuals with HF is largely unreliable.8 Estimating individual prognosis is only somewhat useful in making end-of-life care and hospice decisions for patients with very advanced HF. Table 2 summarizes specific prognostic factors for patients with HF.
TABLE 2
Factors that affect prognosis in patients with heart failure (HF)
| Factor | Result | Comment |
|---|---|---|
| Age1,2,6,59 | Increasing age and age older than 55 years decreases survival | Framingham data: survival rates of older women are twice as long as those of older men despite significant age difference (women: 72 years; men: 68 years). |
| Sex56,60-62 | Mortality higher in men | Women are underrepresented in HF trials and frequently have HF associated with diastolic dysfunction. Women rate their quality of inpatient care lower than men do. |
| Race63-65 | African Americans have higher mortality rates and higher rates of recurrent hospitalization | HF affects approximately 3% of all African Americans. They develop symptoms at an earlier tage. The disease progresses more rapidly than in whites. African Americans are underrepresented in HF trials. |
| Attending physician specialty66-68 | No difference in 6-month cardiac and all-cause mortality between family physician or generalist and cardiologist care | Family physician or generalist: Twofold increased risk of readmission in 6 months; tend to overestimate risks of ACE inhibitors and therefore under-prescribe them. |
| Cardiologist (as attending or consultant): Increased testing, hospital lengths of stay, and hospital charges, but better patient-perceived quality of life. |
Suggested management of patients with heart failure
Although the optimal sequence of pharmacologic interventions for treating HF has not been examined in RCTs, recommendations can be made on the basis of existing evidence in HF management (Figure). This approach can be divided into 4 steps performed simultaneously: (1) control risks for the development and progression of HF (treat concomitant diseases); (2) HF treatment; (3) symptom control; (4) close follow-up.
Control risks. Risks for the development and worsening of HF should be addressed as described else-where.8 Steps include longitudinal surveillance; identification and treatment of hypertension, diabetes and thyroid diseases; management of atherosclerotic and coronary artery disease and myocardial ischemia; and the elimination of alcohol and tobacco use.
Heart failure treatment. All patients with HF should take a drug or a combination of drugs that affects the disease process. Drugs shown by the preponderance of evidence to decrease morbidity and mortality include ACE inhibitors, beta-blockers, and ARBs. For most HF patients, regardless of NYHA class, ACE inhibitors should be the initial baseline treatment because of their proven track record and the observation that most recent HF trials include patients who are already taking these medications. ARBs are similar in efficacy to ACE inhibitors and, therefore, are an adequate alternative when ACE inhibitors are not tolerated. Beta-blockers (metoprolol and bisoprolol) added to ACE inhibitors are also useful as a baseline treatment in most HF patients and may be especially useful in the case of tachydysrhythmias and in the postmyocardial infarction period.
For severe HF (NYHA III–IV), spironolactone and carvedilol are useful additions to baseline drug therapy. Carvedilol may be added if a beta-blocker is not currently used. If the patient is currently taking a beta-blocker, the drug should be discontinued before the patient is switched to carvedilol.
The hydralazine–nitrate combination has been proved effective, but tolerability and ease-of-use issues limit its usefulness. No data are available to support the use of nitrates other than isosorbide dinitrate. Nitrates may be useful, however, for concomitant chronic myocardial ischemia.
Patients with stable HF should be encouraged to begin and maintain a regular aerobic exercise program. The level of exercise can range from brief, symptom-limited exercise to moderate exercise (60% capacity) for 3 or more hours per week.
The use of antiplatelet therapy or the routine use of anticoagulation in patients with HF who are in sinus rhythm provides no benefit. Anticoagulation may be useful if the patient has severe HF or has a known mural thrombus. HF patients with atrial fibrillation should be considered for antiplatelet or anticoagulation therapy as described elsewhere.58
Short-acting CCBs may worsen HF. No data support the use of any CCB in the primary treatment of HF. Similarly, intermittent use of milrinone or dobutamine is not indicated.
Symptom control. The symptomatic treatment of HF includes the use of diuretics and dietary sodium restriction to control sodium levels and volume status. Symptom control should be accomplished along with the pharmacologic disease management outlined above.
The role of digoxin in the failing heart without dysrhythmias is unclear. Digoxin may be most useful in symptom control, as it reduces hospitalizations attributed to worsening HF. This benefit must be balanced against an increased risk of hospitalization caused by digoxin toxicity. Patients who are already taking digoxin should probably continue to do so. The role of digoxin in newly diagnosed HF patients is unknown.
Close follow-up. Comprehensive follow-up, with the patient as a more active participant and in which care is extended beyond the hospital or office to the home, is a key strategy in the long-term care of HF patients. This aspect of HF management should include educating patients about their disease process and their dietary and pharmacologic treatments; teaching them how to monitor their weight, symptoms, and blood pressure and to understand when to seek care; and following up periodically by telephone between scheduled office visits.
FIGURE
Management of adults with heart failure
1. Schocken DD, Arrieta MI, Leaverton PE, Ross EA. Prevalence and mortality rate of congestive heart failure in the United States. J Am Coll Cardiol 1992;20:301-6.
2. McKee PA, Castelli WP, McNamara PM, Kannel WB. The natural history of congestive heart failure. The Framingham study. N Engl J Med 1971;285:1442-6.
3. Kannel WB, Plehn JF, Cupples LA. Cardiac failure and sudden death in the Framingham Study. Am Heart J 1988;115:869-75.
4. Schappert SM. Ambulatory care visits to physician offices, hospital outpatient departments, and emergency departments: United States, 1997. Vital Health Stat 13. 1999;No. 143:i-iv,1-39.
5. Krumholz HM, Parent EM, Tu N, et al. Readmission after hospitalization for congestive heart failure among Medicare beneficiaries. Arch Intern Med 1997;157:99-104.
6. McMurray JJ, Stewart S. Heart failure. Epidemiology, etiology, and prognosis of heart failure. Heart 2000;83:596-602.
7. Mair FS, Lloyd-Williams F. Evaluation of suspected left ventricular systolic dysfunction. J Fam Pract 2002;51:466-471.
8. Hunt SA, Baker DW, Chin MH, et al. ACC/AHA guidelines for the evaluation and management of chronic heart failure in the adult: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee to Revise the 1995 Guidelines for the Evaluation and Management of Heart Failure). 2001. American College of Cardiology Web site. Available at: http://www.acc.org/clinical/guidelines/failure/hf_index.htm.
9. Garg R, Yusuf S. Overview of randomized trials of angiotensin-converting enzyme inhibitors on mortality and morbidity in patients with heart failure. JAMA 1995;273:1450-6.
10. The Criteria Committee of the New York Heart Association. Diseases of the Heart and Blood Vessels: Nomenclature and Criteria for Diagnosis. 6th ed. Boston, MA: Little, Brown; 1964.
11. Nanas JN, Alexopoulus G, Anastasiou-Nana MI, et al. Outcome of patients with congestive heart failure treated with standard versus high doses of enalapril: a multicenter study. J Am Coll Cardiol 2000;36:2090-5.
12. CONSENSUS Trial Study Group. Effects of enalapril on mortality in severe congestive heart failure. N Engl J Med 1987;316:1429-35.
13. SOLVD Investigators. Effect of enalapril on survival in patients with reduced left ventricular ejection fractions and congestive heart failure. N Engl J Med 1991;325:293-302.
14. Pfeffer MA, Braunwald E, Moye LA, et al. Effect of captopril on mortality and morbidity in patients with left ventricular dysfunction after myocardial infarction. Results of the survival and ventricular enlargement (SAVE) trial. N Engl J Med 1992;327:659-77.
15. Pitt B, Poole-Wilson PA, Segal R, et al. Effect of losartan compared with captopril on mortality in patients with symptomatic heart failure: a randomized trial. The losartan heart failure survival study (ELITE II). Lancet 2000;355:1582-7.
16. Cohn JN, Tognoni G. for the Valsartan Heart Failure Trial Investigators. A randomized trial of the angiotensin-receptor blocker valsartan in chronic heart failure. N Engl J Med 2001;345:1667-75.
17. Packer M, Bristow MR, Cohn JN, et al. The effect of carvedilol on morbidity and mortality in patients with chronic heart failure. N Engl J Med 1996;334:1349-55.
18. MERIT-HF Study Group. Effect of metoprolol CR/XL in chronic heart failure: metoprolol CR/XL randomised intervention trial in congestive heart failure (MERIT-HF). Lancet 1999;353:2001-7.
19. CIBIS-II Investigators. The cardiac insufficiency bisoprolol study II (CIBIS-II): a randomized trial. Lancet 1999;353:9-13.
20. Lee S, Spencer A. Beta-blockers to reduce mortality in patients with systolic dysfunction. J Fam Pract 2001;6:499-504.
21. Packer M, Coats AJS, Fowler MB, et al. Effect of carvedilol on survival in severe chronic heart failure. N Engl J Med 2001;344:1651-8.
22. Metra M, Giubbini R, Nodari S, et al. Differential effects of betablockers in patients with heart failure: a prospective, randomized, double-blind comparison of the long-term effects of metoprolol versus carvedilol. Circulation 2000;102:546-51.
23. Pitt B, Zannad F, Remm WJ, et al. The effect of spironolactone on morbidity and mortality in patients with severe heart failure. N Engl J Med 1999;341:707-17.
24. Cohn J, Archibald DG, Ziesche S, et al. Effect of vasodilator therapy on mortality in chronic congestive heart failure. N Engl J Med 1986;314:1547-52.
25. Cohn JN, Johnson G, Ziesche S, et al. Comparison of enalapril with hydralazine–isosorbide dinitrate in the treatment of chronic congestive heart failure. N Engl J Med 1991;325:303-10.
26. Packer M, Gheorghiade M, Young JB, et al. Withdrawal of digoxin from patients with chronic heart failure treated with angiotensin-converting enzyme inhibitors. N Engl J Med 1993;329:1-7.
27. Hood WB, Jr, Dans A, Guyatt GH, Jaescke R, McMurray JV. Digitalis for treatment of congestive heart failure in patients in sinus rhythm. In: The Cochrane Library, Issue 4, 2001. Oxford, England: Update Software.
28. The Digitalis Investigation Group. The effect of digoxin on mortality and morbidity in patients with heart failure. N Engl J Med 1997;336:525-33.
29. Brater DC. Diuretic therapy. N Engl J Med 1998;339:387-95.
30. Cody RJ, Kubo SH, Pickworth KK. Diuretic treatment for the sodium retention of congestive heart failure. Arch Intern Med 1994;154:1905-14.
31. Wilson JR, Reichek N, Dunkman WB, Goldberg S. Effect of diuresis on the performance of the failing left ventricle in man. Am J Med 1981;70:234-9.
32. Richardson A, Bayliss J, Scriven AJ, Parameshwar J, Poole-Wilson PA, Sutton GC. Double-blind comparison of captopril alone against furosemide plus amiloride in mild heart failure. Lancet 1987;2:709-11.
33. Dunkman WB. Thromboembolism and antithrombotic therapy in congestive heart failure. J Cardiovasc Risk 1995;2:107-17.
34. Lip GYH, Gibbs CR. Antiplatelet agents versus control or anticoagulation for heart failure in sinus rhythm. In: The Cochrane Library, Issue 4, 2001. Oxford, England: Update Software.
35. Lip GYH, Gibbs CR. Anticoagulation for heart failure in sinus rhythm. In: The Cochrane Library, Issue 4, 2001. Oxford, England: Update Software.
36. Lenihan DJ, Uretsky BF. Nonpharmacologic treatment of heart failure in the elderly. Clin Geriatr Med 2000;16:477-87.
37. Williams JF, Bristow MR, Fowler MB, et al. Guidelines for the evaluation and management of chronic heart failure. Report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee on Evaluation and Management of Heart Failure). Circulation 1995;92:2764-84.
38. Belardinelli R, Georgiou D, Ciance G, Purcaro A. Randomized controlled trial of long-term moderate exercise training in chronic heart failure. Effects on functional capacity, quality of life, and clinical outcome. Circulation 1999;99:1173-82.
39. Sullivan MJ, Higginbotham MB, Cobb FR. Exercise training in patients with severe left ventricular dysfunction. Hemodynamic and metabolic effects. Circulation 1988;78:506-15.
40. Tokmakova M, Dobreva B, Kostianev S. Effects of short-term exercise training in patients with heart failure. Folia Medica (Plovdiv) 1999;41:68-71. Abstract.
41. Rich MW. Heart failure disease management: a critical review. J Card Fail 1999;5:64-75.
42. Stewart S, Pearson S, Horowitz JD. Effects of a home-based intervention among patients with congestive heart failure discharged from acute hospital care. Arch Intern Med 1998;158:1067-72.
43. Stewart S, Vandenbroek AJ, Pearson S, Horowitz JD. Prolonged beneficial effects of a home-based intervention on unplanned readmissions and mortality among patients with congestive heart failure. Arch Intern Med 1999;159:257-61.
44. Shah NB, Der E, Ruggerio C, Heidenreich PA, Massie BM. Prevention of hospitalizations for heart failure with an interactive home monitoring program. Am Heart J 1998;135:373-8.
45. Rich MW, Beckham V, Wittenberg C, Leven CL, Freedland KE, Carney RM. A multidisciplinary intervention to prevent the readmission of elderly patients with congestive heart failure. N Engl J Med 1995;333:1190-5.
46. Levine TN, Bernink PJ, Caspi A, et al. Effect of mibefradil, a T-type calcium channel blocker, on morbidity and mortality in moderate to severe congestive heart failure: the MACH-1 study. Mortality Assessment in Congestive Heart Failure Trial. Circulation 2000;101:758-64.
47. Mahon N, McKenna WJ. Calcium-channel blockers in cardiac failure. Prog Card Dis 1998;41:191-206.
48. deVries RJ, van Veldhuisen DJ, Dunselman PH. Efficacy and safety of calcium channel blockers in heart failure: focus on recent trials with second-generation dihydropyridines. Am Heart J 2000;139(2 pt 1):185-94.
49. O’Connor CM, Carson PE, Miller AB, et al. Effect of amlodipine on mode of death among patients with advanced heart failure in the PRAISE trial. Prospective randomized amlodipine survival evaluation. Am J Cardiol 1998;82:881-7.
50. Packer M, Kessler PD, Lee WH. Calcium-channel blockade in the management of severe chronic congestive heart failure: a bridge too far. Circulation 1987;75:V56-64.
51. Anderson JL. Hemodynamic and clinical benefits with intravenous milrinone in severe chronic heart failure: results of a multicenter study in the United States. Am Heart J 1991;121:1956-64.
52. Elis A, Bental T, Kimchi O, Ravid M, Lishner M. Intermittent dobutamine treatment in patients with chronic refractory congestive heart failure: a randomized, double-blind, placebo-controlled study. Clin Pharmacol Ther 1998;63:682-5.
53. Packer M, Carver JR, Rodeheffer RJ, et al. Effect of oral milrinone on mortality in severe chronic heart failure. The PROMISE Study Research Group. N Engl J Med 1991;325:1468-75.
54. Cleland JGF, Clark A. Has the survival of the heart failure population changed? Lessons from trials. Am J Cardiol 1999;83:112D-19D.
55. Ho KKL, Pinsky JL, Kannel WB, Levy D. The epidemiology of heart failure: the Framingham study. J Am Coll Cardiol 1993;22(suppl A):6A-13A.
56. Clinical Quality Improvement Network Investigators. Mortality risk and patterns of practice in 4606 acute care patients with congestive heart failure. The relative importance of age, sex, and medical therapy. Arch Intern Med 1996;156:1669-73.
57. Cowie MR, Wood DA, Coats AJ, et al. Survival of patients with a new diagnosis of heart failure: a population-based study. Heart 2000;83:505-10.
58. Albers GW, Dalen JE, Laupacis A, Manning WJ, Petersen P, Singer DE. Antithrombotic therapy in atrial fibrillation. Chest 2001;119:194S-206S.
59. Doba N, Tomiyama H, Nakayama T. Drugs, heart failure and quality of life: what are we achieving? What should we be trying to achieve? Drugs Aging 1999;14:153-63.
60. Petrie MC, Dawson NF, Murdoch DR, Davie AP, McMurray JJV. Failure of women’s hearts. Circulation 1999;99:2334-41.
61. Burns RB, McCarthy EP, Moskowitz MA, Ash A, Kane RL, Finch M. Outcomes for older men and women with congestive heart failure. J Am Geriatr Soc 1997;45:276-80.
62. Chin MH, Goldman L. Gender differences in 1-year survival and quality of life among patients admitted with congestive heart failure. Med Care 1998;36:1033-46.
63. Dries DL, Exner DV, Gersh BJ, Cooper HA, Carson PE, Domanski MJ. Racial differences in the outcome of left ventricular dysfunction [published erratum appears in N Engl J Med 1999; 341:298]. N Engl J Med 1999;340:609-16.
64. Alexander M, Grumbach K, Remy L, Rowell R, Massie BM. Congestive heart failure hospitalizations and survival in California: patterns according to race/ethnicity. Am Heart J 1999;137:919-27.
65. Philbin EF, DiSalvo TG. Influence of race and gender on care process, resource use, and hospital-based outcomes in congestive heart failure. Am J Cardiol 1998;82:76-81.
66. Reis SE, Holubkov R, Edmundowicz D, et al. Treatment of patients admitted to the hospital with congestive heart failure: specialty-related disparities in practice patterns and outcomes. J Am Coll Cardiol 1997;30:733-8.
67. Philbin EF, Weil HFC, Erb TA, Jenkins PL. Cardiology or primary care for heart failure in the community setting. Process of care and clinical outcomes. Chest 1999;116:346-54.
68. Baker DW, Hayes RP, Massie BM, Craig CA. Variations in family physicians’ and cardiologists’ care for patients with heart failure. Am Heart J 1999;138:826-34.
- Control the risks for the development and progression of heart failure (HF) by controlling hypertension, diabetes, myocardial ischemia, and tobacco and alcohol use.
- Treat HF with angiotensin-converting enzyme inhibitors, angiotensin receptor blockers, or beta-blockers, used alone or in combination; add spironolactone and carvedilol (or change current beta-blocker to carvedilol) in severe HF; institute aerobic exercise program.
- Control symptoms with diuretics, restricted dietary sodium intake, and digoxin.
- Provide close follow-up that is comprehensive and multidisciplinary, including intensive patient education; self-monitoring of weight, symptoms, and blood pressure; and periodic telephone or in-home follow-up between scheduled office visits.
Heart failure (HF) affects more than 2 million adults in the United States.1 This common, costly, and disabling disorder mainly affects the elderly, with prevalence rates of up to 10% in patients older than 65 years.2,3 The management of HF is responsible for millions of outpatient visits per year,4 is the most common discharge diagnosis for Medicare beneficiaries,5 and accounts for more than 5% of total health care dollars spent.6
Treatment
Major advances in the pharmacologic treatment of heart failure (HF) have emerged in recent years. An approach to the diagnosis and evaluation of HF is described elsewhere.7 This article summarizes the evidence for outpatient treatment of HF. Current intervention trials do not distinguish between systolic and diastolic heart failure; it is therefore unknown whether or how drug therapy should be tailored according to the type of HF. The treatment of cardiac dysrhythmias in the setting of HF is beyond the scope of this article and is presented elsewhere.8Table 1 compares the available outpatient treatments of HF and includes the levels of evidence, numbers needed to treat, and appropriate situations for use. In the remainder of this article, we will discuss pharmacologic and nonpharmacologic management, including identification of ineffective treatments.
TABLE 1
Treatment options in heart failure
| Strength of recommendation (level of evidence)* | Treatment | NNT (Time)† | Use in NYHA class | Comments |
|---|---|---|---|---|
| A (1a) | Angiotensin-converting9-14 enzyme (ACE) inhibitors | 24 (90 days to 2 years) | I–IV | Even moderate doses (equivalent to 10 to 20 mg enalapril per day) provide benefit |
| A (1b) | Angiotensin-receptor blockers (ARBs)15,16 | Similar to ACE inhibitors | I–IV | Useful in patients who do not tolerate ACE inhibitors; may be combined with ACE inhibitors or beta-blockers, but not both |
| A (1a) | Beta-blockers (metoprolol, bisoprolol, carvedilol)17-20 | 24 (1 to 2 years) | I–IV | Usually added to ACE inhibitors or ARBs. May also be useful if concomitant tachydysrhythmias are present and in the post-MI period |
| A (1b) | Carvedilol21 | 18 (10 months) | III–IV | Add carvedilol if not already taking beta-blocker or change current beta-blocker to carvedilol |
| A (1b) | Spironolactone23 | 9 (2 years) | III–IV | NNT = 4 (2 years) to prevent hospitalization for HF. Severe hyperkalemia important safety concern (NNH = 195 over 2 years) |
| A (1b) | Hydralazine + isosorbide dinitrate (ISDN)24,25 | 19 (6 years) | I–IV | Use limited by poor tolerability |
| B (1a) | Digoxin26-28 | N/A | I–IV | No mortality benefit. NNT = 22 to prevent 1 hospitalization over 3 years. Increased risk of hospitalization for digoxin toxicity (NNH = 94 over 3 years) |
| B (2b) | Diuretics (furosemide, bumetanide, torsemide)29-32 | N/A | I–IV | Used for fluid, sodium, and symptom control. No data on mortality benefit |
| A (1b) | Aerobic exercise38-40 | 4 (14 months) | I–IV | Decreases hospitalization for HF (NNT = 5). Even brief symptom-limited exercise in severe HF has benefit in improving quality of life |
| A (1b) | Comprehensive, multi-disciplinary outpatient visits | N/A | I–IV | No mortality benefit. NNT = 5 for 3 months to prevent repeat hospitalization. Includes some combination of intensive education, medication monitoring, individualized diet modification, telephone/home visit follow-up between scheduled outpatient visits |
| B (5) | Dietary sodium restriction8,36,37 | N/A | I–IV | Recommended as standard practice, but no morbidity or mortality data from RCTs |
| C (2a) | Antiplatelet therapy and anticoagulation in HF with sinus rhythm33,35,58 | N/A | N/A | Antiplatelet therapy not useful. No data to support routine anticoagulation, although may be useful in severe HF. Patients with concomitant atrial fibrillation should be anticoagulated if no contraindications |
| D (1b) | Calcium channel blockers (CCBs)46-50 | N/A | N/A | Short-acting CCBs worsen HF. Newer, long-acting CCBs do not worsen HF, but there is no evidence of morbidity or mortality benefit |
| D (1b) | Intermittent positive inotrope (oral or intravenous)(dobutamine, milrinone)51-53 | N/A | N/A | Increased mortality (NNH = 17 over 5 months), increased hospitalizations for worsening HF (NNH = 20), and serious adverse reactions (NNH = 25) |
| *Based on the guidelines for evidence quality outlined by the Center for Evidence-Based Medicine. Available at http://cebm.jr2.ox.ac.uk/docs/levels.html. | ||||
| †NNT = number needed to treat to prevent 1 death over specified time period unless otherwise noted. | ||||
| HF, heart failure; MI, myocardial infarction; NNH, number needed to harm; NNT, number needed to treat; NYHA, New York Heart Association classification; RCT, randomized controlled trial. | ||||
Pharmacologic treatment
Angiotensin-converting enzyme inhibitors. A systematic review9 of 32 trials with a total of 7105 patients demonstrated that mortality rates were lower in patients taking an angiotensin-converting enzyme (ACE) inhibitor than in those not taking one (number needed to treat [NNT] = 24 for > 90 days, meaning that 1 fewer death occurs for every 24 patients who take an ACE inhibitor for more than 90 days). In addition, there is a reduction in the combined endpoints of death and hospitalization because of HF (NNT = 11). Although most of this benefit was realized in the first 90 days of therapy, benefits lasted for 4 to 5 years and were more pronounced in patients categorized in more severe New York Heart Association (NYHA) HF classes10 (class I: no limitation of activities; class II: slight limitation of activity; class III: marked limitation of activity and comfortable only at rest; class IV: symptoms at rest).
Dosage comparison studies demonstrate that HF patients can benefit from even moderate doses of ACE inhibitors. A recent multicenter trial comparing moderate dose enalapril (10 mg twice a day) with a higher dose (30 mg twice a day) in patients with a left ventricular ejection fraction (LVEF) of less than 20% found no differences in mortality at 1 year between the 2 groups.11 In addition, both groups achieved similar increases in functional status and LVEF.
Several trials have demonstrated good tolerability of ACE inhibitors.12-14 Dropout rates of 15% to 30% were similar between patients in the ACE inhibitor and placebo groups, mainly because of side effects, including dizziness, altered taste, hypotension, hyperkalemia, and cough.
Angiotensin-receptor blockers. Angiotensin-receptor blockers (ARBs) reduce all-cause mortality and HF-related hospitalizations in patients with NYHA class II and III HF at rates comparable with those of ACE inhibitors.15,16 Cough is not a side effect of ARBs. Although they are more expensive, ARBs offer a reasonable alternative for patients who do not tolerate ACE inhibitors.
Beta-blockers. The beta-blockers carvedilol, metoprolol, and bisoprolol have a proven mortality benefit for patients with HF.17-19 Pooled results of 6 randomized controlled trials (RCTs), including more than 9000 patients already taking ACE inhibitors, showed a significant reduction in total mortality (NNT = 24 over 1–2 years) and sudden death (NNT = 35), regardless of NYHA classification.20 The average dropout rate of 16% was similar in the betablocker and placebo groups.
Early beta-blocker studies included few NYHA class IV patients until a recent study of the use of carvedilol in severe chronic HF.21 In this study, all patients were taking diuretics plus either an ACE inhibitor or ARB and were permitted to take digoxin, nitrates, hydralazine, spironolactone, or amiodarone. Carvedilol at an average dose of 37 mg per day decreased mortality (NNT = 18 for 10 months) and lowered combined mortality and hospitalization for worsening HF (NNT = 13). Study patients taking carvedilol withdrew from the study at a lower rate (approximately 15%) than placebo.
Because the pharmacologic properties of betablockers vary, clinicians have wondered which are most beneficial. The investigators in a study comparing metoprolol (a beta-1 antagonist) with carvedilol (a beta-1, beta-2, and alpha-1 antagonist) in NYHA class II or III patients found no differences in quality-of-life measures or changes in NYHA classification.22
Spironolactone. The addition of spironolactone to standard care can help patients with severe HF.23 In NYHA class III and IV HF patients, spironolactone at doses ranging from 25 mg every other day to 50 mg per day reduces mortality (NNT = 9 for 2 years), reduces hospitalization from all cardiac causes (NNT = 4), and reduces hospitalization for worsening HF (NNT = 3). The most common serious adverse event in the spironolactone group was severe hyperkalemia (number needed to harm [NNH] = 195). Ten percent of men taking spironolactone experienced breast pain and gynecomastia.
Hydralazine and isosorbide dinitrate. The combination of hydralazine and isosorbide dinitrate (ISDN) reduces mortality in HF patients, but tolerability is an issue. In earlier trials, men with HF symptoms that were optimally controlled with digoxin and diuretics and treated with hydralazine (average dose = 270 mg/day) plus ISDN (average dose = 136 mg/day) had a decrease in all-cause mortality of 28% (NNT = 19 for 6 years).24 A more recent trial comparing hydralazine plus ISDN with enalapril25 (average daily doses of hydralazine = 300 mg/day; ISDN = 160 mg/day; enalapril = 20 mg/day) in NYHA class II–III patients showed no differences in mortality between the 2 groups over 3 years. Tolerability was a problem in these trials; more than 30% of patients stopped taking hydralazine, nitrate, or both.
Digoxin. Digoxin is effective for treating the symptoms of HF in the absence of dysrhythmias but there are no data demonstrating a mortality benefit. Digoxin increases functional capacity in NYHA class II–III patients and heart failure symptoms worsen if digoxin is withdrawn.26 Although there are no differences in all-cause mortality with the use of digoxin, there are fewer hospitalizations due to worsening HF (NNT = 27–114 over 3 years) and a lower rate of clinical deterioration (NNT = 4–75).27 In a randomized trial comparing digoxin and placebo, patients taking digoxin were twice as likely to be hospitalized for suspected digoxin toxicity (2.0% vs 0.9%; P < .001; NNH = 52).28
Diuretics. Diuretics are a mainstay of the symptomatic treatment of heart failure. Short-term studies have shown that diuretics improve the symptoms of sodium and fluid retention and increase exercise tolerance and cardiac function regardless of NYHA classification.29-32 No studies that examine their effects on morbidity and mortality are available.
Antiplatelet therapy and anticoagulation. Patients with HF have an increased risk for thromboembolic events of 1.6% to 3.2% per year.33 One systematic review concluded that antiplatelet therapy is not useful in preventing thromboembolism in patients with HF in sinus rhythm and may even be harmful.34 Another systematic review also concluded that the data do not support the routine use of anticoagulants (eg, warfarin) in patients with HF and sinus rhythm.35 Anticoagulation may be beneficial, however, if there is echocardiographic visualization of a left ventricular thrombus or in cases of “severe” HF or concomitant atrial fibrillation.35
Nonpharmacologic management
Dietary sodium restriction. There is consensus that dietary sodium restriction is important in the treatment of HF36 and is recommended in published guidelines.8,37 Sodium restriction assists with fluid volume control and minimizes the dosages of HF drugs used. These recommendations are based on the retention of sodium and water in symptomatic HF. No studies, however, have examined the effect of dietary sodium restriction on morbidity or mortality, either alone or in combination with pharmacologic treatments.
Exercise training. Moderate exercise training improves quality of life and decreases mortality in patients with stable chronic HF. A recent RCT demonstrated a decrease in mortality (NNT = 4 for 14 months) and hospital readmission for HF (NNT = 5) with only moderate exercise on a stationary bicycle (60% of maximum exercise capacity) for 2 to 3 hours per week.38 Other studies have demonstrated improvements in physiologic markers39 and in quality-of-life ratings with short-term, symptom-limited exercise.40
Multidisciplinary or case-management approach. A case-based or disease-management approach to patients with HF decreases the frequency of unplanned and repeat hospitalizations, increases functional status, and increases quality of life.41 Even a single in-home visit by a clinical pharmacist and a nurse results in fewer unplanned readmissions and fewer days of hospitalization up to 18 months after discharge.42,43 A small study of 27 patients in a Veterans Affairs hospital demonstrated that patient instruction in the self-monitoring of weight and blood pressure, combined with frequent telephone follow-up from a nurse, lowered repeat hospitalizations over 1 year, with the effect more pronounced in patients with more severe NYHA classifications.44 A large RCT demonstrated that a multidisciplinary management approach (intensive patient education about HF and its treatment, dietary assessment and instruction, medication analysis and elimination of unnecessary medications, and telephone and home visit follow-up) results in fewer hospitalizations (NNT = 5 for 3 months) and reduced costs of care.45
Treatments that have no benefit or are harmful
Calcium-channel blockers. Although some of the newer, longer-acting calcium-channel blockers (CCBs) appear to be safe in the treatment of heart failure,46-49 no trials are available demonstrating that they lower mortality, decrease hospitalizations, or improve quality of life in patients with a failing heart. Older, short-acting CCBs can worsen HF.50
Positive inotropic therapy. Intermittent positive inotropic therapy, either orally (milrinone) or intravenously (dobutamine), should be avoided. Although short-term studies have shown some increase in cardiac function and symptoms,51 long-term studies demonstrate no mortality benefit.52 One RCT of milrinone demonstrated an increase in mortality (NNH = 17 for 5 months), an increased rate of hospitalization for worsening HF (NNH = 20), and more serious side effects (NNH = 25).53
Prognosis
Despite the increased longevity in Western developed nations and increased survival from coronary artery disease over recent decades, the overall prognosis of HF has improved very little.6,54 Mortality data derived from several different sources, the largest being the Framingham Heart Study,2,55 have shown that HF remains highly lethal, with a 5-year survival rate of 25% in men and 38% in women with NYHA II–IV heart failure. Mortality data from the placebo arms of intervention trials show an average 1-year mortality of 18%.9,17,19,20,56 A recent population-based study of patients with a new diagnosis of HF showed survival rates of only 62% at 12 months and 57% at 18 months.57 Despite these dismal population-based data, predicting the likelihood of survival in individuals with HF is largely unreliable.8 Estimating individual prognosis is only somewhat useful in making end-of-life care and hospice decisions for patients with very advanced HF. Table 2 summarizes specific prognostic factors for patients with HF.
TABLE 2
Factors that affect prognosis in patients with heart failure (HF)
| Factor | Result | Comment |
|---|---|---|
| Age1,2,6,59 | Increasing age and age older than 55 years decreases survival | Framingham data: survival rates of older women are twice as long as those of older men despite significant age difference (women: 72 years; men: 68 years). |
| Sex56,60-62 | Mortality higher in men | Women are underrepresented in HF trials and frequently have HF associated with diastolic dysfunction. Women rate their quality of inpatient care lower than men do. |
| Race63-65 | African Americans have higher mortality rates and higher rates of recurrent hospitalization | HF affects approximately 3% of all African Americans. They develop symptoms at an earlier tage. The disease progresses more rapidly than in whites. African Americans are underrepresented in HF trials. |
| Attending physician specialty66-68 | No difference in 6-month cardiac and all-cause mortality between family physician or generalist and cardiologist care | Family physician or generalist: Twofold increased risk of readmission in 6 months; tend to overestimate risks of ACE inhibitors and therefore under-prescribe them. |
| Cardiologist (as attending or consultant): Increased testing, hospital lengths of stay, and hospital charges, but better patient-perceived quality of life. |
Suggested management of patients with heart failure
Although the optimal sequence of pharmacologic interventions for treating HF has not been examined in RCTs, recommendations can be made on the basis of existing evidence in HF management (Figure). This approach can be divided into 4 steps performed simultaneously: (1) control risks for the development and progression of HF (treat concomitant diseases); (2) HF treatment; (3) symptom control; (4) close follow-up.
Control risks. Risks for the development and worsening of HF should be addressed as described else-where.8 Steps include longitudinal surveillance; identification and treatment of hypertension, diabetes and thyroid diseases; management of atherosclerotic and coronary artery disease and myocardial ischemia; and the elimination of alcohol and tobacco use.
Heart failure treatment. All patients with HF should take a drug or a combination of drugs that affects the disease process. Drugs shown by the preponderance of evidence to decrease morbidity and mortality include ACE inhibitors, beta-blockers, and ARBs. For most HF patients, regardless of NYHA class, ACE inhibitors should be the initial baseline treatment because of their proven track record and the observation that most recent HF trials include patients who are already taking these medications. ARBs are similar in efficacy to ACE inhibitors and, therefore, are an adequate alternative when ACE inhibitors are not tolerated. Beta-blockers (metoprolol and bisoprolol) added to ACE inhibitors are also useful as a baseline treatment in most HF patients and may be especially useful in the case of tachydysrhythmias and in the postmyocardial infarction period.
For severe HF (NYHA III–IV), spironolactone and carvedilol are useful additions to baseline drug therapy. Carvedilol may be added if a beta-blocker is not currently used. If the patient is currently taking a beta-blocker, the drug should be discontinued before the patient is switched to carvedilol.
The hydralazine–nitrate combination has been proved effective, but tolerability and ease-of-use issues limit its usefulness. No data are available to support the use of nitrates other than isosorbide dinitrate. Nitrates may be useful, however, for concomitant chronic myocardial ischemia.
Patients with stable HF should be encouraged to begin and maintain a regular aerobic exercise program. The level of exercise can range from brief, symptom-limited exercise to moderate exercise (60% capacity) for 3 or more hours per week.
The use of antiplatelet therapy or the routine use of anticoagulation in patients with HF who are in sinus rhythm provides no benefit. Anticoagulation may be useful if the patient has severe HF or has a known mural thrombus. HF patients with atrial fibrillation should be considered for antiplatelet or anticoagulation therapy as described elsewhere.58
Short-acting CCBs may worsen HF. No data support the use of any CCB in the primary treatment of HF. Similarly, intermittent use of milrinone or dobutamine is not indicated.
Symptom control. The symptomatic treatment of HF includes the use of diuretics and dietary sodium restriction to control sodium levels and volume status. Symptom control should be accomplished along with the pharmacologic disease management outlined above.
The role of digoxin in the failing heart without dysrhythmias is unclear. Digoxin may be most useful in symptom control, as it reduces hospitalizations attributed to worsening HF. This benefit must be balanced against an increased risk of hospitalization caused by digoxin toxicity. Patients who are already taking digoxin should probably continue to do so. The role of digoxin in newly diagnosed HF patients is unknown.
Close follow-up. Comprehensive follow-up, with the patient as a more active participant and in which care is extended beyond the hospital or office to the home, is a key strategy in the long-term care of HF patients. This aspect of HF management should include educating patients about their disease process and their dietary and pharmacologic treatments; teaching them how to monitor their weight, symptoms, and blood pressure and to understand when to seek care; and following up periodically by telephone between scheduled office visits.
FIGURE
Management of adults with heart failure
- Control the risks for the development and progression of heart failure (HF) by controlling hypertension, diabetes, myocardial ischemia, and tobacco and alcohol use.
- Treat HF with angiotensin-converting enzyme inhibitors, angiotensin receptor blockers, or beta-blockers, used alone or in combination; add spironolactone and carvedilol (or change current beta-blocker to carvedilol) in severe HF; institute aerobic exercise program.
- Control symptoms with diuretics, restricted dietary sodium intake, and digoxin.
- Provide close follow-up that is comprehensive and multidisciplinary, including intensive patient education; self-monitoring of weight, symptoms, and blood pressure; and periodic telephone or in-home follow-up between scheduled office visits.
Heart failure (HF) affects more than 2 million adults in the United States.1 This common, costly, and disabling disorder mainly affects the elderly, with prevalence rates of up to 10% in patients older than 65 years.2,3 The management of HF is responsible for millions of outpatient visits per year,4 is the most common discharge diagnosis for Medicare beneficiaries,5 and accounts for more than 5% of total health care dollars spent.6
Treatment
Major advances in the pharmacologic treatment of heart failure (HF) have emerged in recent years. An approach to the diagnosis and evaluation of HF is described elsewhere.7 This article summarizes the evidence for outpatient treatment of HF. Current intervention trials do not distinguish between systolic and diastolic heart failure; it is therefore unknown whether or how drug therapy should be tailored according to the type of HF. The treatment of cardiac dysrhythmias in the setting of HF is beyond the scope of this article and is presented elsewhere.8Table 1 compares the available outpatient treatments of HF and includes the levels of evidence, numbers needed to treat, and appropriate situations for use. In the remainder of this article, we will discuss pharmacologic and nonpharmacologic management, including identification of ineffective treatments.
TABLE 1
Treatment options in heart failure
| Strength of recommendation (level of evidence)* | Treatment | NNT (Time)† | Use in NYHA class | Comments |
|---|---|---|---|---|
| A (1a) | Angiotensin-converting9-14 enzyme (ACE) inhibitors | 24 (90 days to 2 years) | I–IV | Even moderate doses (equivalent to 10 to 20 mg enalapril per day) provide benefit |
| A (1b) | Angiotensin-receptor blockers (ARBs)15,16 | Similar to ACE inhibitors | I–IV | Useful in patients who do not tolerate ACE inhibitors; may be combined with ACE inhibitors or beta-blockers, but not both |
| A (1a) | Beta-blockers (metoprolol, bisoprolol, carvedilol)17-20 | 24 (1 to 2 years) | I–IV | Usually added to ACE inhibitors or ARBs. May also be useful if concomitant tachydysrhythmias are present and in the post-MI period |
| A (1b) | Carvedilol21 | 18 (10 months) | III–IV | Add carvedilol if not already taking beta-blocker or change current beta-blocker to carvedilol |
| A (1b) | Spironolactone23 | 9 (2 years) | III–IV | NNT = 4 (2 years) to prevent hospitalization for HF. Severe hyperkalemia important safety concern (NNH = 195 over 2 years) |
| A (1b) | Hydralazine + isosorbide dinitrate (ISDN)24,25 | 19 (6 years) | I–IV | Use limited by poor tolerability |
| B (1a) | Digoxin26-28 | N/A | I–IV | No mortality benefit. NNT = 22 to prevent 1 hospitalization over 3 years. Increased risk of hospitalization for digoxin toxicity (NNH = 94 over 3 years) |
| B (2b) | Diuretics (furosemide, bumetanide, torsemide)29-32 | N/A | I–IV | Used for fluid, sodium, and symptom control. No data on mortality benefit |
| A (1b) | Aerobic exercise38-40 | 4 (14 months) | I–IV | Decreases hospitalization for HF (NNT = 5). Even brief symptom-limited exercise in severe HF has benefit in improving quality of life |
| A (1b) | Comprehensive, multi-disciplinary outpatient visits | N/A | I–IV | No mortality benefit. NNT = 5 for 3 months to prevent repeat hospitalization. Includes some combination of intensive education, medication monitoring, individualized diet modification, telephone/home visit follow-up between scheduled outpatient visits |
| B (5) | Dietary sodium restriction8,36,37 | N/A | I–IV | Recommended as standard practice, but no morbidity or mortality data from RCTs |
| C (2a) | Antiplatelet therapy and anticoagulation in HF with sinus rhythm33,35,58 | N/A | N/A | Antiplatelet therapy not useful. No data to support routine anticoagulation, although may be useful in severe HF. Patients with concomitant atrial fibrillation should be anticoagulated if no contraindications |
| D (1b) | Calcium channel blockers (CCBs)46-50 | N/A | N/A | Short-acting CCBs worsen HF. Newer, long-acting CCBs do not worsen HF, but there is no evidence of morbidity or mortality benefit |
| D (1b) | Intermittent positive inotrope (oral or intravenous)(dobutamine, milrinone)51-53 | N/A | N/A | Increased mortality (NNH = 17 over 5 months), increased hospitalizations for worsening HF (NNH = 20), and serious adverse reactions (NNH = 25) |
| *Based on the guidelines for evidence quality outlined by the Center for Evidence-Based Medicine. Available at http://cebm.jr2.ox.ac.uk/docs/levels.html. | ||||
| †NNT = number needed to treat to prevent 1 death over specified time period unless otherwise noted. | ||||
| HF, heart failure; MI, myocardial infarction; NNH, number needed to harm; NNT, number needed to treat; NYHA, New York Heart Association classification; RCT, randomized controlled trial. | ||||
Pharmacologic treatment
Angiotensin-converting enzyme inhibitors. A systematic review9 of 32 trials with a total of 7105 patients demonstrated that mortality rates were lower in patients taking an angiotensin-converting enzyme (ACE) inhibitor than in those not taking one (number needed to treat [NNT] = 24 for > 90 days, meaning that 1 fewer death occurs for every 24 patients who take an ACE inhibitor for more than 90 days). In addition, there is a reduction in the combined endpoints of death and hospitalization because of HF (NNT = 11). Although most of this benefit was realized in the first 90 days of therapy, benefits lasted for 4 to 5 years and were more pronounced in patients categorized in more severe New York Heart Association (NYHA) HF classes10 (class I: no limitation of activities; class II: slight limitation of activity; class III: marked limitation of activity and comfortable only at rest; class IV: symptoms at rest).
Dosage comparison studies demonstrate that HF patients can benefit from even moderate doses of ACE inhibitors. A recent multicenter trial comparing moderate dose enalapril (10 mg twice a day) with a higher dose (30 mg twice a day) in patients with a left ventricular ejection fraction (LVEF) of less than 20% found no differences in mortality at 1 year between the 2 groups.11 In addition, both groups achieved similar increases in functional status and LVEF.
Several trials have demonstrated good tolerability of ACE inhibitors.12-14 Dropout rates of 15% to 30% were similar between patients in the ACE inhibitor and placebo groups, mainly because of side effects, including dizziness, altered taste, hypotension, hyperkalemia, and cough.
Angiotensin-receptor blockers. Angiotensin-receptor blockers (ARBs) reduce all-cause mortality and HF-related hospitalizations in patients with NYHA class II and III HF at rates comparable with those of ACE inhibitors.15,16 Cough is not a side effect of ARBs. Although they are more expensive, ARBs offer a reasonable alternative for patients who do not tolerate ACE inhibitors.
Beta-blockers. The beta-blockers carvedilol, metoprolol, and bisoprolol have a proven mortality benefit for patients with HF.17-19 Pooled results of 6 randomized controlled trials (RCTs), including more than 9000 patients already taking ACE inhibitors, showed a significant reduction in total mortality (NNT = 24 over 1–2 years) and sudden death (NNT = 35), regardless of NYHA classification.20 The average dropout rate of 16% was similar in the betablocker and placebo groups.
Early beta-blocker studies included few NYHA class IV patients until a recent study of the use of carvedilol in severe chronic HF.21 In this study, all patients were taking diuretics plus either an ACE inhibitor or ARB and were permitted to take digoxin, nitrates, hydralazine, spironolactone, or amiodarone. Carvedilol at an average dose of 37 mg per day decreased mortality (NNT = 18 for 10 months) and lowered combined mortality and hospitalization for worsening HF (NNT = 13). Study patients taking carvedilol withdrew from the study at a lower rate (approximately 15%) than placebo.
Because the pharmacologic properties of betablockers vary, clinicians have wondered which are most beneficial. The investigators in a study comparing metoprolol (a beta-1 antagonist) with carvedilol (a beta-1, beta-2, and alpha-1 antagonist) in NYHA class II or III patients found no differences in quality-of-life measures or changes in NYHA classification.22
Spironolactone. The addition of spironolactone to standard care can help patients with severe HF.23 In NYHA class III and IV HF patients, spironolactone at doses ranging from 25 mg every other day to 50 mg per day reduces mortality (NNT = 9 for 2 years), reduces hospitalization from all cardiac causes (NNT = 4), and reduces hospitalization for worsening HF (NNT = 3). The most common serious adverse event in the spironolactone group was severe hyperkalemia (number needed to harm [NNH] = 195). Ten percent of men taking spironolactone experienced breast pain and gynecomastia.
Hydralazine and isosorbide dinitrate. The combination of hydralazine and isosorbide dinitrate (ISDN) reduces mortality in HF patients, but tolerability is an issue. In earlier trials, men with HF symptoms that were optimally controlled with digoxin and diuretics and treated with hydralazine (average dose = 270 mg/day) plus ISDN (average dose = 136 mg/day) had a decrease in all-cause mortality of 28% (NNT = 19 for 6 years).24 A more recent trial comparing hydralazine plus ISDN with enalapril25 (average daily doses of hydralazine = 300 mg/day; ISDN = 160 mg/day; enalapril = 20 mg/day) in NYHA class II–III patients showed no differences in mortality between the 2 groups over 3 years. Tolerability was a problem in these trials; more than 30% of patients stopped taking hydralazine, nitrate, or both.
Digoxin. Digoxin is effective for treating the symptoms of HF in the absence of dysrhythmias but there are no data demonstrating a mortality benefit. Digoxin increases functional capacity in NYHA class II–III patients and heart failure symptoms worsen if digoxin is withdrawn.26 Although there are no differences in all-cause mortality with the use of digoxin, there are fewer hospitalizations due to worsening HF (NNT = 27–114 over 3 years) and a lower rate of clinical deterioration (NNT = 4–75).27 In a randomized trial comparing digoxin and placebo, patients taking digoxin were twice as likely to be hospitalized for suspected digoxin toxicity (2.0% vs 0.9%; P < .001; NNH = 52).28
Diuretics. Diuretics are a mainstay of the symptomatic treatment of heart failure. Short-term studies have shown that diuretics improve the symptoms of sodium and fluid retention and increase exercise tolerance and cardiac function regardless of NYHA classification.29-32 No studies that examine their effects on morbidity and mortality are available.
Antiplatelet therapy and anticoagulation. Patients with HF have an increased risk for thromboembolic events of 1.6% to 3.2% per year.33 One systematic review concluded that antiplatelet therapy is not useful in preventing thromboembolism in patients with HF in sinus rhythm and may even be harmful.34 Another systematic review also concluded that the data do not support the routine use of anticoagulants (eg, warfarin) in patients with HF and sinus rhythm.35 Anticoagulation may be beneficial, however, if there is echocardiographic visualization of a left ventricular thrombus or in cases of “severe” HF or concomitant atrial fibrillation.35
Nonpharmacologic management
Dietary sodium restriction. There is consensus that dietary sodium restriction is important in the treatment of HF36 and is recommended in published guidelines.8,37 Sodium restriction assists with fluid volume control and minimizes the dosages of HF drugs used. These recommendations are based on the retention of sodium and water in symptomatic HF. No studies, however, have examined the effect of dietary sodium restriction on morbidity or mortality, either alone or in combination with pharmacologic treatments.
Exercise training. Moderate exercise training improves quality of life and decreases mortality in patients with stable chronic HF. A recent RCT demonstrated a decrease in mortality (NNT = 4 for 14 months) and hospital readmission for HF (NNT = 5) with only moderate exercise on a stationary bicycle (60% of maximum exercise capacity) for 2 to 3 hours per week.38 Other studies have demonstrated improvements in physiologic markers39 and in quality-of-life ratings with short-term, symptom-limited exercise.40
Multidisciplinary or case-management approach. A case-based or disease-management approach to patients with HF decreases the frequency of unplanned and repeat hospitalizations, increases functional status, and increases quality of life.41 Even a single in-home visit by a clinical pharmacist and a nurse results in fewer unplanned readmissions and fewer days of hospitalization up to 18 months after discharge.42,43 A small study of 27 patients in a Veterans Affairs hospital demonstrated that patient instruction in the self-monitoring of weight and blood pressure, combined with frequent telephone follow-up from a nurse, lowered repeat hospitalizations over 1 year, with the effect more pronounced in patients with more severe NYHA classifications.44 A large RCT demonstrated that a multidisciplinary management approach (intensive patient education about HF and its treatment, dietary assessment and instruction, medication analysis and elimination of unnecessary medications, and telephone and home visit follow-up) results in fewer hospitalizations (NNT = 5 for 3 months) and reduced costs of care.45
Treatments that have no benefit or are harmful
Calcium-channel blockers. Although some of the newer, longer-acting calcium-channel blockers (CCBs) appear to be safe in the treatment of heart failure,46-49 no trials are available demonstrating that they lower mortality, decrease hospitalizations, or improve quality of life in patients with a failing heart. Older, short-acting CCBs can worsen HF.50
Positive inotropic therapy. Intermittent positive inotropic therapy, either orally (milrinone) or intravenously (dobutamine), should be avoided. Although short-term studies have shown some increase in cardiac function and symptoms,51 long-term studies demonstrate no mortality benefit.52 One RCT of milrinone demonstrated an increase in mortality (NNH = 17 for 5 months), an increased rate of hospitalization for worsening HF (NNH = 20), and more serious side effects (NNH = 25).53
Prognosis
Despite the increased longevity in Western developed nations and increased survival from coronary artery disease over recent decades, the overall prognosis of HF has improved very little.6,54 Mortality data derived from several different sources, the largest being the Framingham Heart Study,2,55 have shown that HF remains highly lethal, with a 5-year survival rate of 25% in men and 38% in women with NYHA II–IV heart failure. Mortality data from the placebo arms of intervention trials show an average 1-year mortality of 18%.9,17,19,20,56 A recent population-based study of patients with a new diagnosis of HF showed survival rates of only 62% at 12 months and 57% at 18 months.57 Despite these dismal population-based data, predicting the likelihood of survival in individuals with HF is largely unreliable.8 Estimating individual prognosis is only somewhat useful in making end-of-life care and hospice decisions for patients with very advanced HF. Table 2 summarizes specific prognostic factors for patients with HF.
TABLE 2
Factors that affect prognosis in patients with heart failure (HF)
| Factor | Result | Comment |
|---|---|---|
| Age1,2,6,59 | Increasing age and age older than 55 years decreases survival | Framingham data: survival rates of older women are twice as long as those of older men despite significant age difference (women: 72 years; men: 68 years). |
| Sex56,60-62 | Mortality higher in men | Women are underrepresented in HF trials and frequently have HF associated with diastolic dysfunction. Women rate their quality of inpatient care lower than men do. |
| Race63-65 | African Americans have higher mortality rates and higher rates of recurrent hospitalization | HF affects approximately 3% of all African Americans. They develop symptoms at an earlier tage. The disease progresses more rapidly than in whites. African Americans are underrepresented in HF trials. |
| Attending physician specialty66-68 | No difference in 6-month cardiac and all-cause mortality between family physician or generalist and cardiologist care | Family physician or generalist: Twofold increased risk of readmission in 6 months; tend to overestimate risks of ACE inhibitors and therefore under-prescribe them. |
| Cardiologist (as attending or consultant): Increased testing, hospital lengths of stay, and hospital charges, but better patient-perceived quality of life. |
Suggested management of patients with heart failure
Although the optimal sequence of pharmacologic interventions for treating HF has not been examined in RCTs, recommendations can be made on the basis of existing evidence in HF management (Figure). This approach can be divided into 4 steps performed simultaneously: (1) control risks for the development and progression of HF (treat concomitant diseases); (2) HF treatment; (3) symptom control; (4) close follow-up.
Control risks. Risks for the development and worsening of HF should be addressed as described else-where.8 Steps include longitudinal surveillance; identification and treatment of hypertension, diabetes and thyroid diseases; management of atherosclerotic and coronary artery disease and myocardial ischemia; and the elimination of alcohol and tobacco use.
Heart failure treatment. All patients with HF should take a drug or a combination of drugs that affects the disease process. Drugs shown by the preponderance of evidence to decrease morbidity and mortality include ACE inhibitors, beta-blockers, and ARBs. For most HF patients, regardless of NYHA class, ACE inhibitors should be the initial baseline treatment because of their proven track record and the observation that most recent HF trials include patients who are already taking these medications. ARBs are similar in efficacy to ACE inhibitors and, therefore, are an adequate alternative when ACE inhibitors are not tolerated. Beta-blockers (metoprolol and bisoprolol) added to ACE inhibitors are also useful as a baseline treatment in most HF patients and may be especially useful in the case of tachydysrhythmias and in the postmyocardial infarction period.
For severe HF (NYHA III–IV), spironolactone and carvedilol are useful additions to baseline drug therapy. Carvedilol may be added if a beta-blocker is not currently used. If the patient is currently taking a beta-blocker, the drug should be discontinued before the patient is switched to carvedilol.
The hydralazine–nitrate combination has been proved effective, but tolerability and ease-of-use issues limit its usefulness. No data are available to support the use of nitrates other than isosorbide dinitrate. Nitrates may be useful, however, for concomitant chronic myocardial ischemia.
Patients with stable HF should be encouraged to begin and maintain a regular aerobic exercise program. The level of exercise can range from brief, symptom-limited exercise to moderate exercise (60% capacity) for 3 or more hours per week.
The use of antiplatelet therapy or the routine use of anticoagulation in patients with HF who are in sinus rhythm provides no benefit. Anticoagulation may be useful if the patient has severe HF or has a known mural thrombus. HF patients with atrial fibrillation should be considered for antiplatelet or anticoagulation therapy as described elsewhere.58
Short-acting CCBs may worsen HF. No data support the use of any CCB in the primary treatment of HF. Similarly, intermittent use of milrinone or dobutamine is not indicated.
Symptom control. The symptomatic treatment of HF includes the use of diuretics and dietary sodium restriction to control sodium levels and volume status. Symptom control should be accomplished along with the pharmacologic disease management outlined above.
The role of digoxin in the failing heart without dysrhythmias is unclear. Digoxin may be most useful in symptom control, as it reduces hospitalizations attributed to worsening HF. This benefit must be balanced against an increased risk of hospitalization caused by digoxin toxicity. Patients who are already taking digoxin should probably continue to do so. The role of digoxin in newly diagnosed HF patients is unknown.
Close follow-up. Comprehensive follow-up, with the patient as a more active participant and in which care is extended beyond the hospital or office to the home, is a key strategy in the long-term care of HF patients. This aspect of HF management should include educating patients about their disease process and their dietary and pharmacologic treatments; teaching them how to monitor their weight, symptoms, and blood pressure and to understand when to seek care; and following up periodically by telephone between scheduled office visits.
FIGURE
Management of adults with heart failure
1. Schocken DD, Arrieta MI, Leaverton PE, Ross EA. Prevalence and mortality rate of congestive heart failure in the United States. J Am Coll Cardiol 1992;20:301-6.
2. McKee PA, Castelli WP, McNamara PM, Kannel WB. The natural history of congestive heart failure. The Framingham study. N Engl J Med 1971;285:1442-6.
3. Kannel WB, Plehn JF, Cupples LA. Cardiac failure and sudden death in the Framingham Study. Am Heart J 1988;115:869-75.
4. Schappert SM. Ambulatory care visits to physician offices, hospital outpatient departments, and emergency departments: United States, 1997. Vital Health Stat 13. 1999;No. 143:i-iv,1-39.
5. Krumholz HM, Parent EM, Tu N, et al. Readmission after hospitalization for congestive heart failure among Medicare beneficiaries. Arch Intern Med 1997;157:99-104.
6. McMurray JJ, Stewart S. Heart failure. Epidemiology, etiology, and prognosis of heart failure. Heart 2000;83:596-602.
7. Mair FS, Lloyd-Williams F. Evaluation of suspected left ventricular systolic dysfunction. J Fam Pract 2002;51:466-471.
8. Hunt SA, Baker DW, Chin MH, et al. ACC/AHA guidelines for the evaluation and management of chronic heart failure in the adult: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee to Revise the 1995 Guidelines for the Evaluation and Management of Heart Failure). 2001. American College of Cardiology Web site. Available at: http://www.acc.org/clinical/guidelines/failure/hf_index.htm.
9. Garg R, Yusuf S. Overview of randomized trials of angiotensin-converting enzyme inhibitors on mortality and morbidity in patients with heart failure. JAMA 1995;273:1450-6.
10. The Criteria Committee of the New York Heart Association. Diseases of the Heart and Blood Vessels: Nomenclature and Criteria for Diagnosis. 6th ed. Boston, MA: Little, Brown; 1964.
11. Nanas JN, Alexopoulus G, Anastasiou-Nana MI, et al. Outcome of patients with congestive heart failure treated with standard versus high doses of enalapril: a multicenter study. J Am Coll Cardiol 2000;36:2090-5.
12. CONSENSUS Trial Study Group. Effects of enalapril on mortality in severe congestive heart failure. N Engl J Med 1987;316:1429-35.
13. SOLVD Investigators. Effect of enalapril on survival in patients with reduced left ventricular ejection fractions and congestive heart failure. N Engl J Med 1991;325:293-302.
14. Pfeffer MA, Braunwald E, Moye LA, et al. Effect of captopril on mortality and morbidity in patients with left ventricular dysfunction after myocardial infarction. Results of the survival and ventricular enlargement (SAVE) trial. N Engl J Med 1992;327:659-77.
15. Pitt B, Poole-Wilson PA, Segal R, et al. Effect of losartan compared with captopril on mortality in patients with symptomatic heart failure: a randomized trial. The losartan heart failure survival study (ELITE II). Lancet 2000;355:1582-7.
16. Cohn JN, Tognoni G. for the Valsartan Heart Failure Trial Investigators. A randomized trial of the angiotensin-receptor blocker valsartan in chronic heart failure. N Engl J Med 2001;345:1667-75.
17. Packer M, Bristow MR, Cohn JN, et al. The effect of carvedilol on morbidity and mortality in patients with chronic heart failure. N Engl J Med 1996;334:1349-55.
18. MERIT-HF Study Group. Effect of metoprolol CR/XL in chronic heart failure: metoprolol CR/XL randomised intervention trial in congestive heart failure (MERIT-HF). Lancet 1999;353:2001-7.
19. CIBIS-II Investigators. The cardiac insufficiency bisoprolol study II (CIBIS-II): a randomized trial. Lancet 1999;353:9-13.
20. Lee S, Spencer A. Beta-blockers to reduce mortality in patients with systolic dysfunction. J Fam Pract 2001;6:499-504.
21. Packer M, Coats AJS, Fowler MB, et al. Effect of carvedilol on survival in severe chronic heart failure. N Engl J Med 2001;344:1651-8.
22. Metra M, Giubbini R, Nodari S, et al. Differential effects of betablockers in patients with heart failure: a prospective, randomized, double-blind comparison of the long-term effects of metoprolol versus carvedilol. Circulation 2000;102:546-51.
23. Pitt B, Zannad F, Remm WJ, et al. The effect of spironolactone on morbidity and mortality in patients with severe heart failure. N Engl J Med 1999;341:707-17.
24. Cohn J, Archibald DG, Ziesche S, et al. Effect of vasodilator therapy on mortality in chronic congestive heart failure. N Engl J Med 1986;314:1547-52.
25. Cohn JN, Johnson G, Ziesche S, et al. Comparison of enalapril with hydralazine–isosorbide dinitrate in the treatment of chronic congestive heart failure. N Engl J Med 1991;325:303-10.
26. Packer M, Gheorghiade M, Young JB, et al. Withdrawal of digoxin from patients with chronic heart failure treated with angiotensin-converting enzyme inhibitors. N Engl J Med 1993;329:1-7.
27. Hood WB, Jr, Dans A, Guyatt GH, Jaescke R, McMurray JV. Digitalis for treatment of congestive heart failure in patients in sinus rhythm. In: The Cochrane Library, Issue 4, 2001. Oxford, England: Update Software.
28. The Digitalis Investigation Group. The effect of digoxin on mortality and morbidity in patients with heart failure. N Engl J Med 1997;336:525-33.
29. Brater DC. Diuretic therapy. N Engl J Med 1998;339:387-95.
30. Cody RJ, Kubo SH, Pickworth KK. Diuretic treatment for the sodium retention of congestive heart failure. Arch Intern Med 1994;154:1905-14.
31. Wilson JR, Reichek N, Dunkman WB, Goldberg S. Effect of diuresis on the performance of the failing left ventricle in man. Am J Med 1981;70:234-9.
32. Richardson A, Bayliss J, Scriven AJ, Parameshwar J, Poole-Wilson PA, Sutton GC. Double-blind comparison of captopril alone against furosemide plus amiloride in mild heart failure. Lancet 1987;2:709-11.
33. Dunkman WB. Thromboembolism and antithrombotic therapy in congestive heart failure. J Cardiovasc Risk 1995;2:107-17.
34. Lip GYH, Gibbs CR. Antiplatelet agents versus control or anticoagulation for heart failure in sinus rhythm. In: The Cochrane Library, Issue 4, 2001. Oxford, England: Update Software.
35. Lip GYH, Gibbs CR. Anticoagulation for heart failure in sinus rhythm. In: The Cochrane Library, Issue 4, 2001. Oxford, England: Update Software.
36. Lenihan DJ, Uretsky BF. Nonpharmacologic treatment of heart failure in the elderly. Clin Geriatr Med 2000;16:477-87.
37. Williams JF, Bristow MR, Fowler MB, et al. Guidelines for the evaluation and management of chronic heart failure. Report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee on Evaluation and Management of Heart Failure). Circulation 1995;92:2764-84.
38. Belardinelli R, Georgiou D, Ciance G, Purcaro A. Randomized controlled trial of long-term moderate exercise training in chronic heart failure. Effects on functional capacity, quality of life, and clinical outcome. Circulation 1999;99:1173-82.
39. Sullivan MJ, Higginbotham MB, Cobb FR. Exercise training in patients with severe left ventricular dysfunction. Hemodynamic and metabolic effects. Circulation 1988;78:506-15.
40. Tokmakova M, Dobreva B, Kostianev S. Effects of short-term exercise training in patients with heart failure. Folia Medica (Plovdiv) 1999;41:68-71. Abstract.
41. Rich MW. Heart failure disease management: a critical review. J Card Fail 1999;5:64-75.
42. Stewart S, Pearson S, Horowitz JD. Effects of a home-based intervention among patients with congestive heart failure discharged from acute hospital care. Arch Intern Med 1998;158:1067-72.
43. Stewart S, Vandenbroek AJ, Pearson S, Horowitz JD. Prolonged beneficial effects of a home-based intervention on unplanned readmissions and mortality among patients with congestive heart failure. Arch Intern Med 1999;159:257-61.
44. Shah NB, Der E, Ruggerio C, Heidenreich PA, Massie BM. Prevention of hospitalizations for heart failure with an interactive home monitoring program. Am Heart J 1998;135:373-8.
45. Rich MW, Beckham V, Wittenberg C, Leven CL, Freedland KE, Carney RM. A multidisciplinary intervention to prevent the readmission of elderly patients with congestive heart failure. N Engl J Med 1995;333:1190-5.
46. Levine TN, Bernink PJ, Caspi A, et al. Effect of mibefradil, a T-type calcium channel blocker, on morbidity and mortality in moderate to severe congestive heart failure: the MACH-1 study. Mortality Assessment in Congestive Heart Failure Trial. Circulation 2000;101:758-64.
47. Mahon N, McKenna WJ. Calcium-channel blockers in cardiac failure. Prog Card Dis 1998;41:191-206.
48. deVries RJ, van Veldhuisen DJ, Dunselman PH. Efficacy and safety of calcium channel blockers in heart failure: focus on recent trials with second-generation dihydropyridines. Am Heart J 2000;139(2 pt 1):185-94.
49. O’Connor CM, Carson PE, Miller AB, et al. Effect of amlodipine on mode of death among patients with advanced heart failure in the PRAISE trial. Prospective randomized amlodipine survival evaluation. Am J Cardiol 1998;82:881-7.
50. Packer M, Kessler PD, Lee WH. Calcium-channel blockade in the management of severe chronic congestive heart failure: a bridge too far. Circulation 1987;75:V56-64.
51. Anderson JL. Hemodynamic and clinical benefits with intravenous milrinone in severe chronic heart failure: results of a multicenter study in the United States. Am Heart J 1991;121:1956-64.
52. Elis A, Bental T, Kimchi O, Ravid M, Lishner M. Intermittent dobutamine treatment in patients with chronic refractory congestive heart failure: a randomized, double-blind, placebo-controlled study. Clin Pharmacol Ther 1998;63:682-5.
53. Packer M, Carver JR, Rodeheffer RJ, et al. Effect of oral milrinone on mortality in severe chronic heart failure. The PROMISE Study Research Group. N Engl J Med 1991;325:1468-75.
54. Cleland JGF, Clark A. Has the survival of the heart failure population changed? Lessons from trials. Am J Cardiol 1999;83:112D-19D.
55. Ho KKL, Pinsky JL, Kannel WB, Levy D. The epidemiology of heart failure: the Framingham study. J Am Coll Cardiol 1993;22(suppl A):6A-13A.
56. Clinical Quality Improvement Network Investigators. Mortality risk and patterns of practice in 4606 acute care patients with congestive heart failure. The relative importance of age, sex, and medical therapy. Arch Intern Med 1996;156:1669-73.
57. Cowie MR, Wood DA, Coats AJ, et al. Survival of patients with a new diagnosis of heart failure: a population-based study. Heart 2000;83:505-10.
58. Albers GW, Dalen JE, Laupacis A, Manning WJ, Petersen P, Singer DE. Antithrombotic therapy in atrial fibrillation. Chest 2001;119:194S-206S.
59. Doba N, Tomiyama H, Nakayama T. Drugs, heart failure and quality of life: what are we achieving? What should we be trying to achieve? Drugs Aging 1999;14:153-63.
60. Petrie MC, Dawson NF, Murdoch DR, Davie AP, McMurray JJV. Failure of women’s hearts. Circulation 1999;99:2334-41.
61. Burns RB, McCarthy EP, Moskowitz MA, Ash A, Kane RL, Finch M. Outcomes for older men and women with congestive heart failure. J Am Geriatr Soc 1997;45:276-80.
62. Chin MH, Goldman L. Gender differences in 1-year survival and quality of life among patients admitted with congestive heart failure. Med Care 1998;36:1033-46.
63. Dries DL, Exner DV, Gersh BJ, Cooper HA, Carson PE, Domanski MJ. Racial differences in the outcome of left ventricular dysfunction [published erratum appears in N Engl J Med 1999; 341:298]. N Engl J Med 1999;340:609-16.
64. Alexander M, Grumbach K, Remy L, Rowell R, Massie BM. Congestive heart failure hospitalizations and survival in California: patterns according to race/ethnicity. Am Heart J 1999;137:919-27.
65. Philbin EF, DiSalvo TG. Influence of race and gender on care process, resource use, and hospital-based outcomes in congestive heart failure. Am J Cardiol 1998;82:76-81.
66. Reis SE, Holubkov R, Edmundowicz D, et al. Treatment of patients admitted to the hospital with congestive heart failure: specialty-related disparities in practice patterns and outcomes. J Am Coll Cardiol 1997;30:733-8.
67. Philbin EF, Weil HFC, Erb TA, Jenkins PL. Cardiology or primary care for heart failure in the community setting. Process of care and clinical outcomes. Chest 1999;116:346-54.
68. Baker DW, Hayes RP, Massie BM, Craig CA. Variations in family physicians’ and cardiologists’ care for patients with heart failure. Am Heart J 1999;138:826-34.
1. Schocken DD, Arrieta MI, Leaverton PE, Ross EA. Prevalence and mortality rate of congestive heart failure in the United States. J Am Coll Cardiol 1992;20:301-6.
2. McKee PA, Castelli WP, McNamara PM, Kannel WB. The natural history of congestive heart failure. The Framingham study. N Engl J Med 1971;285:1442-6.
3. Kannel WB, Plehn JF, Cupples LA. Cardiac failure and sudden death in the Framingham Study. Am Heart J 1988;115:869-75.
4. Schappert SM. Ambulatory care visits to physician offices, hospital outpatient departments, and emergency departments: United States, 1997. Vital Health Stat 13. 1999;No. 143:i-iv,1-39.
5. Krumholz HM, Parent EM, Tu N, et al. Readmission after hospitalization for congestive heart failure among Medicare beneficiaries. Arch Intern Med 1997;157:99-104.
6. McMurray JJ, Stewart S. Heart failure. Epidemiology, etiology, and prognosis of heart failure. Heart 2000;83:596-602.
7. Mair FS, Lloyd-Williams F. Evaluation of suspected left ventricular systolic dysfunction. J Fam Pract 2002;51:466-471.
8. Hunt SA, Baker DW, Chin MH, et al. ACC/AHA guidelines for the evaluation and management of chronic heart failure in the adult: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee to Revise the 1995 Guidelines for the Evaluation and Management of Heart Failure). 2001. American College of Cardiology Web site. Available at: http://www.acc.org/clinical/guidelines/failure/hf_index.htm.
9. Garg R, Yusuf S. Overview of randomized trials of angiotensin-converting enzyme inhibitors on mortality and morbidity in patients with heart failure. JAMA 1995;273:1450-6.
10. The Criteria Committee of the New York Heart Association. Diseases of the Heart and Blood Vessels: Nomenclature and Criteria for Diagnosis. 6th ed. Boston, MA: Little, Brown; 1964.
11. Nanas JN, Alexopoulus G, Anastasiou-Nana MI, et al. Outcome of patients with congestive heart failure treated with standard versus high doses of enalapril: a multicenter study. J Am Coll Cardiol 2000;36:2090-5.
12. CONSENSUS Trial Study Group. Effects of enalapril on mortality in severe congestive heart failure. N Engl J Med 1987;316:1429-35.
13. SOLVD Investigators. Effect of enalapril on survival in patients with reduced left ventricular ejection fractions and congestive heart failure. N Engl J Med 1991;325:293-302.
14. Pfeffer MA, Braunwald E, Moye LA, et al. Effect of captopril on mortality and morbidity in patients with left ventricular dysfunction after myocardial infarction. Results of the survival and ventricular enlargement (SAVE) trial. N Engl J Med 1992;327:659-77.
15. Pitt B, Poole-Wilson PA, Segal R, et al. Effect of losartan compared with captopril on mortality in patients with symptomatic heart failure: a randomized trial. The losartan heart failure survival study (ELITE II). Lancet 2000;355:1582-7.
16. Cohn JN, Tognoni G. for the Valsartan Heart Failure Trial Investigators. A randomized trial of the angiotensin-receptor blocker valsartan in chronic heart failure. N Engl J Med 2001;345:1667-75.
17. Packer M, Bristow MR, Cohn JN, et al. The effect of carvedilol on morbidity and mortality in patients with chronic heart failure. N Engl J Med 1996;334:1349-55.
18. MERIT-HF Study Group. Effect of metoprolol CR/XL in chronic heart failure: metoprolol CR/XL randomised intervention trial in congestive heart failure (MERIT-HF). Lancet 1999;353:2001-7.
19. CIBIS-II Investigators. The cardiac insufficiency bisoprolol study II (CIBIS-II): a randomized trial. Lancet 1999;353:9-13.
20. Lee S, Spencer A. Beta-blockers to reduce mortality in patients with systolic dysfunction. J Fam Pract 2001;6:499-504.
21. Packer M, Coats AJS, Fowler MB, et al. Effect of carvedilol on survival in severe chronic heart failure. N Engl J Med 2001;344:1651-8.
22. Metra M, Giubbini R, Nodari S, et al. Differential effects of betablockers in patients with heart failure: a prospective, randomized, double-blind comparison of the long-term effects of metoprolol versus carvedilol. Circulation 2000;102:546-51.
23. Pitt B, Zannad F, Remm WJ, et al. The effect of spironolactone on morbidity and mortality in patients with severe heart failure. N Engl J Med 1999;341:707-17.
24. Cohn J, Archibald DG, Ziesche S, et al. Effect of vasodilator therapy on mortality in chronic congestive heart failure. N Engl J Med 1986;314:1547-52.
25. Cohn JN, Johnson G, Ziesche S, et al. Comparison of enalapril with hydralazine–isosorbide dinitrate in the treatment of chronic congestive heart failure. N Engl J Med 1991;325:303-10.
26. Packer M, Gheorghiade M, Young JB, et al. Withdrawal of digoxin from patients with chronic heart failure treated with angiotensin-converting enzyme inhibitors. N Engl J Med 1993;329:1-7.
27. Hood WB, Jr, Dans A, Guyatt GH, Jaescke R, McMurray JV. Digitalis for treatment of congestive heart failure in patients in sinus rhythm. In: The Cochrane Library, Issue 4, 2001. Oxford, England: Update Software.
28. The Digitalis Investigation Group. The effect of digoxin on mortality and morbidity in patients with heart failure. N Engl J Med 1997;336:525-33.
29. Brater DC. Diuretic therapy. N Engl J Med 1998;339:387-95.
30. Cody RJ, Kubo SH, Pickworth KK. Diuretic treatment for the sodium retention of congestive heart failure. Arch Intern Med 1994;154:1905-14.
31. Wilson JR, Reichek N, Dunkman WB, Goldberg S. Effect of diuresis on the performance of the failing left ventricle in man. Am J Med 1981;70:234-9.
32. Richardson A, Bayliss J, Scriven AJ, Parameshwar J, Poole-Wilson PA, Sutton GC. Double-blind comparison of captopril alone against furosemide plus amiloride in mild heart failure. Lancet 1987;2:709-11.
33. Dunkman WB. Thromboembolism and antithrombotic therapy in congestive heart failure. J Cardiovasc Risk 1995;2:107-17.
34. Lip GYH, Gibbs CR. Antiplatelet agents versus control or anticoagulation for heart failure in sinus rhythm. In: The Cochrane Library, Issue 4, 2001. Oxford, England: Update Software.
35. Lip GYH, Gibbs CR. Anticoagulation for heart failure in sinus rhythm. In: The Cochrane Library, Issue 4, 2001. Oxford, England: Update Software.
36. Lenihan DJ, Uretsky BF. Nonpharmacologic treatment of heart failure in the elderly. Clin Geriatr Med 2000;16:477-87.
37. Williams JF, Bristow MR, Fowler MB, et al. Guidelines for the evaluation and management of chronic heart failure. Report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee on Evaluation and Management of Heart Failure). Circulation 1995;92:2764-84.
38. Belardinelli R, Georgiou D, Ciance G, Purcaro A. Randomized controlled trial of long-term moderate exercise training in chronic heart failure. Effects on functional capacity, quality of life, and clinical outcome. Circulation 1999;99:1173-82.
39. Sullivan MJ, Higginbotham MB, Cobb FR. Exercise training in patients with severe left ventricular dysfunction. Hemodynamic and metabolic effects. Circulation 1988;78:506-15.
40. Tokmakova M, Dobreva B, Kostianev S. Effects of short-term exercise training in patients with heart failure. Folia Medica (Plovdiv) 1999;41:68-71. Abstract.
41. Rich MW. Heart failure disease management: a critical review. J Card Fail 1999;5:64-75.
42. Stewart S, Pearson S, Horowitz JD. Effects of a home-based intervention among patients with congestive heart failure discharged from acute hospital care. Arch Intern Med 1998;158:1067-72.
43. Stewart S, Vandenbroek AJ, Pearson S, Horowitz JD. Prolonged beneficial effects of a home-based intervention on unplanned readmissions and mortality among patients with congestive heart failure. Arch Intern Med 1999;159:257-61.
44. Shah NB, Der E, Ruggerio C, Heidenreich PA, Massie BM. Prevention of hospitalizations for heart failure with an interactive home monitoring program. Am Heart J 1998;135:373-8.
45. Rich MW, Beckham V, Wittenberg C, Leven CL, Freedland KE, Carney RM. A multidisciplinary intervention to prevent the readmission of elderly patients with congestive heart failure. N Engl J Med 1995;333:1190-5.
46. Levine TN, Bernink PJ, Caspi A, et al. Effect of mibefradil, a T-type calcium channel blocker, on morbidity and mortality in moderate to severe congestive heart failure: the MACH-1 study. Mortality Assessment in Congestive Heart Failure Trial. Circulation 2000;101:758-64.
47. Mahon N, McKenna WJ. Calcium-channel blockers in cardiac failure. Prog Card Dis 1998;41:191-206.
48. deVries RJ, van Veldhuisen DJ, Dunselman PH. Efficacy and safety of calcium channel blockers in heart failure: focus on recent trials with second-generation dihydropyridines. Am Heart J 2000;139(2 pt 1):185-94.
49. O’Connor CM, Carson PE, Miller AB, et al. Effect of amlodipine on mode of death among patients with advanced heart failure in the PRAISE trial. Prospective randomized amlodipine survival evaluation. Am J Cardiol 1998;82:881-7.
50. Packer M, Kessler PD, Lee WH. Calcium-channel blockade in the management of severe chronic congestive heart failure: a bridge too far. Circulation 1987;75:V56-64.
51. Anderson JL. Hemodynamic and clinical benefits with intravenous milrinone in severe chronic heart failure: results of a multicenter study in the United States. Am Heart J 1991;121:1956-64.
52. Elis A, Bental T, Kimchi O, Ravid M, Lishner M. Intermittent dobutamine treatment in patients with chronic refractory congestive heart failure: a randomized, double-blind, placebo-controlled study. Clin Pharmacol Ther 1998;63:682-5.
53. Packer M, Carver JR, Rodeheffer RJ, et al. Effect of oral milrinone on mortality in severe chronic heart failure. The PROMISE Study Research Group. N Engl J Med 1991;325:1468-75.
54. Cleland JGF, Clark A. Has the survival of the heart failure population changed? Lessons from trials. Am J Cardiol 1999;83:112D-19D.
55. Ho KKL, Pinsky JL, Kannel WB, Levy D. The epidemiology of heart failure: the Framingham study. J Am Coll Cardiol 1993;22(suppl A):6A-13A.
56. Clinical Quality Improvement Network Investigators. Mortality risk and patterns of practice in 4606 acute care patients with congestive heart failure. The relative importance of age, sex, and medical therapy. Arch Intern Med 1996;156:1669-73.
57. Cowie MR, Wood DA, Coats AJ, et al. Survival of patients with a new diagnosis of heart failure: a population-based study. Heart 2000;83:505-10.
58. Albers GW, Dalen JE, Laupacis A, Manning WJ, Petersen P, Singer DE. Antithrombotic therapy in atrial fibrillation. Chest 2001;119:194S-206S.
59. Doba N, Tomiyama H, Nakayama T. Drugs, heart failure and quality of life: what are we achieving? What should we be trying to achieve? Drugs Aging 1999;14:153-63.
60. Petrie MC, Dawson NF, Murdoch DR, Davie AP, McMurray JJV. Failure of women’s hearts. Circulation 1999;99:2334-41.
61. Burns RB, McCarthy EP, Moskowitz MA, Ash A, Kane RL, Finch M. Outcomes for older men and women with congestive heart failure. J Am Geriatr Soc 1997;45:276-80.
62. Chin MH, Goldman L. Gender differences in 1-year survival and quality of life among patients admitted with congestive heart failure. Med Care 1998;36:1033-46.
63. Dries DL, Exner DV, Gersh BJ, Cooper HA, Carson PE, Domanski MJ. Racial differences in the outcome of left ventricular dysfunction [published erratum appears in N Engl J Med 1999; 341:298]. N Engl J Med 1999;340:609-16.
64. Alexander M, Grumbach K, Remy L, Rowell R, Massie BM. Congestive heart failure hospitalizations and survival in California: patterns according to race/ethnicity. Am Heart J 1999;137:919-27.
65. Philbin EF, DiSalvo TG. Influence of race and gender on care process, resource use, and hospital-based outcomes in congestive heart failure. Am J Cardiol 1998;82:76-81.
66. Reis SE, Holubkov R, Edmundowicz D, et al. Treatment of patients admitted to the hospital with congestive heart failure: specialty-related disparities in practice patterns and outcomes. J Am Coll Cardiol 1997;30:733-8.
67. Philbin EF, Weil HFC, Erb TA, Jenkins PL. Cardiology or primary care for heart failure in the community setting. Process of care and clinical outcomes. Chest 1999;116:346-54.
68. Baker DW, Hayes RP, Massie BM, Craig CA. Variations in family physicians’ and cardiologists’ care for patients with heart failure. Am Heart J 1999;138:826-34.
Evaluation of suspected left ventricular systolic dysfunction
- Heart failure is an increasingly common problem in primary care, with a mortality rate higher than that of most cancers.
- The absence of dyspnea on exertion or a normal electrocardiogram (ECG) result indicates that heart failure is unlikely; a gallop rhythm or laterally displaced apical rhythm is strong evidence in favor of heart failure.
- The history and physical examination and ECG alone are usually inadequate to confirm diagnosis of left ventricular systolic dysfunction, and echocardiography remains the gold standard to confirm the diagnosis.
Heart failure is increasing in incidence and prevalence; it currently affects 0.4% to 2% of the general population and 8% to 10% of the elderly.1,2 In the United States, heart failure is the second most common cardiovascular reason for an outpatient visit in the ambulatory care setting and remains the most common cause for hospitalization among patients older than 65 years.3 The total cost for heart failure management in 1999 was estimated to approach $56 billion.4 Those suffering with this illness experience high levels of morbidity and mortality5 that are reflected in the workloads of both primary and secondary care. Heart failure admission rates are rising, and the prognosis of heart failure has been compared with that of malignancy, with a 6-year mortality rate of 84% in men and 77% in women.6,7
A number of heart failure guidelines8-14 provide direction regarding “best practice” with regard to diagnosis and management. These guidelines have all been produced by expert panels and base their evidence on systematic critical reviews of the literature, plus expert consensus opinion. The evidence underlying the development of these guidelines ranges from well-conducted randomized controlled trials to expert opinion. These guidelines all emphasize the ways in which approaches to the diagnosis and management of heart failure have altered substantially in recent years and are continuing to change rapidly. The need to detect heart failure at an early stage to slow the progression of left ventricular systolic dysfunction (LVSD) is now well accepted.15
The following provides an overview of the current recommended approaches to diagnosis, focusing specifically on LVSD, the most common type of heart failure and also the usual focus of most guidelines. Accurate diagnosis of LVSD is the single most important step in management.16 An adequate diagnosis should establish the existence of heart failure, differentiate systolic from diastolic dysfunction, and identify the main underlying cause and any subsidiary diagnoses that may exacerbate heart failure. The etiology of heart failure and the presence of exacerbating factors or other diseases need to be carefully considered in all patients. Coronary artery disease remains the most common potentially reversible etiologic factor in heart failure.8
Using the history and physical examination
The major symptoms of heart failure are fatigue, exercise intolerance, exertional dyspnea, orthopnea, paroxysmal nocturnal dyspnea, and dependent edema. However, such symptoms are similar to those of many other diseases, particularly pulmonary diseases. For example, exertional dypsnea is a common symptom in heart failure but can be due to a wide range of other causes, such as chronic obstructive pulmonary disease, interstitial lung disease, asthma, respiratory infection, deconditioning, or obesity. Many patients with impaired left ventricular function may have no obvious symptoms.17 This highlights the importance of exploring past medical and medication history as these contribute to the overall clinical assessment.
Physical findings that may support a diagnosis of heart failure include raised jugular venous pressure, peripheral edema not due to venous insufficiency, presence of a third heart sound, gallop rhythm, laterally displaced apical impulse, tachycardia, and pulmonary rales that do not clear with coughing. Although clinical findings are particularly useful in acute severe heart failure at the time of hospitalization,18 it is difficult to accurately diagnose mild heart failure in the community on the basis of clinical grounds alone.2,9 The value of different symptoms and aspects of the medical history and use of medications in the evaluation of potential heart failure patients have been examined by researchers.18, 20-23 Similarly, the utility of physical examination has also undergone investigation.18,20,22-28Table 1 summarizes the study findings with regard to clinical symptoms and signs.
Davie and colleagues20 assessed the value of symptoms, past history, medications, and signs in the evaluation of patients who may have LVSD. No one clinical feature predicted LVSD, as assessed by echocardiography with sensitivity, specificity, and a high positive and negative predictive value. Absence of dyspnea on exertion essentially ruled out heart failure (negative likelihood ratio [LR-] = 0.06), while gallop rhythm (positive likelihood ratio [LR+] = 24.0), laterally displaced apical impulse (LR+ 16.4), and elevated jugular venous pulsation (LR+ = 8.9) are strong evidence in favor of the diagnosis. Furthermore, the combination of history of myocardial infarction and displaced apex on physical examination, although not particularly sensitive (39% sensitivity) was very specific (99% specificity) with high positive (89%) and negative (89%) predictive values. The authors also suggest that a breathless patient with a past history of myocardial infarction and a displaced apex beat on physical examination will almost certainly have heart failure and, if resources are limited, may not need echocardiography to confirm the diagnosis. However, less than 50% of breathless patients will have this combination, and the other half would therefore need echocardiography as the gold standard diagnostic tool for LVSD.
Morgan and coworkers28 assessed the prevalence and clinical characteristics of LVSD among elderly patients (those aged 70 years to 84 years) in a primary care setting by echocardiographic assessment of ventricular function. They found that no single clinical symptom or sign was both sensitive and specific, and concluded that diagnosis should not be based on clinical history and examination alone. They found that a substantial number of elderly individuals had asymptomatic or misdiagnosed LVSD, and suggested this might be due to the extremely limited sensitivity and specificity of clinical history taking and examination. For example, only 11% of patients with LVSD had a raised jugular venous pressure, and bilateral ankle edema was common but nonspecific. Researchers have therefore concluded that although these clinical findings are useful in acute severe heart failure, they have only a small role in detecting LVSD in the community.18
TABLE 1
The use of clinical symptoms and signs to diagnose heart failure, by study
| Sign or symptom | N | Setting* | Study quality (1a-5)† | Sensitivity (%) | Specificity (%) | LR+ | LR- | PV+(%) | PV-(%) |
|---|---|---|---|---|---|---|---|---|---|
| Previous myocardial infarction | |||||||||
| Davie, 199720 | 259 | R | 2b | 59 | 86 | 4.1 | 0.48 | 44 | 92 |
| Morgan, 199928 | 817 | P | 2b | 39 | 91 | 4.3 | 0.67 | ||
| Dyspnea on exertion | |||||||||
| Davie, 199720 | 259 | R | 2b | 100 | 17 | 1.20 | 0.06 | 18 | 100 |
| Morgan, 199928 | 817 | P | 2b | 15 | 97 | 5.4 | 0.88 | ||
| Orthopnea | |||||||||
| Davie, 199720 | 259 | R | 2b | 22 | 74 | 0.85 | 1.05 | 14 | 83 |
| Paroxysmal nocturnal dyspnea | |||||||||
| Davie, 199720 | 259 | R | 2b | 39 | 80 | 1.95 | 0.76 | 27 | 87 |
| History of peripheral edema | |||||||||
| Davie, 199720 | 259 | R | 2b | 49 | 47 | 0.92 | 1.09 | 15 | 83 |
| Tachycardia | |||||||||
| Davie, 199720 | 259 | R | 2b | 22 | 92 | 2.75 | 0.85 | 33 | 86 |
| Elevated JVP | |||||||||
| Davie, 199720 | 259 | R | 2b | 17 | 98 | 8.95 | 0.84 | 64 | 86 |
| Morgan, 199928 | 817 | R | 2b | 11 | 97 | 3.6 | 0.92 | ||
| Gallop rhythm | |||||||||
| Davie, 199720 | 259 | P | 2b | 24 | 99 | 24.0 | 0.77 | 77 | 87 |
| 3rd heart sound | |||||||||
| Rihal, 199524 | 554 | H | 2b | 9 | 97 | 3.00 | 0.94 | 54 | 78 |
| Laterally displaced apical impulse | |||||||||
| Davie, 199720 | 259 | R | 2b | 66 | 96 | 16.4 | 0.35 | 75 | 94 |
| Pulmonary rales | |||||||||
| Davie, 199720 | 259 | R | 2b | 29 | 77 | 1.26 | 0.92 | 19 | 85 |
| Morgan, 199928 | 817 | P | 2b | 44 | 82 | 2.4 | 0.68 | ||
| Peripheral edema on examination | |||||||||
| Davie, 199720 | 259 | R | 2b | 20 | 86 | 1.43 | 0.93 | 21 | 85 |
| Morgan, 199928 | 817 | P | 2b | 18 | 91 | 2.0 | 0.90 | ||
| NOTE: Pretest probability = 50%. | |||||||||
| *P denotes cross-sectional primary care population; R, primary care patients referred for suspected heart failure; H, hospitalized patients undergoing angiography. | |||||||||
| † Level 1a is the most rigorous; level 5 is the least rigorous. | |||||||||
| LR+ denotes positive likelihood ratio; LR-, negative likelihood ratio; PV+, positive predictive value; PV-, negative predictive value. | |||||||||
Laboratory and imaging evaluation
Although an important and valuable part of the evaluation, the history and physical examination alone are insufficient to confirm a diagnosis in most cases. Recommended initial tests for patients with signs or symptoms of heart failure include complete blood count (CBC), serum electrolytes, serum creatinine, serum albumin, liver function tests, urinalysis, electrocardiogram, and chest x-ray (Figure).
Blood tests. For those older than 65 years or with atrial fibrillation or evidence of thyroid disease, thyroid function tests should also be performed because heart failure due to thyrotoxicosis is frequently associated with rapid atrial fibrillation and hypothyroidism may also present as heart failure.8,10 The other routine blood tests are important as a way to exclude alternative diagnoses; they also help with the search for predisposing or exacerbating causes of the heart failure. These baseline tests also help guide future therapeutic decision making. For example, electrolyte and renal function results are pertinent when initiating angiotensin-converting enzyme (ACE) inhibitors. Anemia can exacerbate pre-existing heart failure, and measurement of renal function is essential to distinguish fluid overload due to heart failure from renal failure. Liver enzymes may be affected by hepatic congestion. Urinalysis is valuable in the detection of underlying renal disease or diabetes.8
Electrocardiography. An electrocardiogram (ECG) is another recommended part of the evaluation of the suspected heart failure patient.8-14 Considerable attention has been paid to examining the value of this test in the diagnosis of LVSD.29-32 Davie and colleagues29 assessed the value of the ECG in identifying patients with possible heart failure by examining referrals for echocardiography by primary care practitioners. A total of 534 patients were referred for echocardiography for possible heart failure, of whom 18% (n = 96) had LVSD. They showed that LVSD was extremely unlikely if the ECG result was normal, but that 1 in 3 patients with an abnormal result had significant LVSD. Thus, a normal ECG result virtually excludes chronic heart failure due to LVSD. However, the ECG is not a substitute for echocardiography, as an abnormal result does not accurately predict the presence of LVSD (Table 2).
Others have confirmed these findings.30,32 Talreja and colleagues30 found that of 330 consecutive in-patients referred for echocardiographic assessment of left ventricular function, 124 (41%) had LVSD. Only 2 of 124 patients with LVSD had a normal electrocardiogram result. When the ECG result is normal, the authors suggest that echocardiography is not needed. However, they concede that physicians are unlikely to adhere to this because many may not be as sophisticated in interpreting the ECG and may feel it important to get an accurate measure of ejection fraction. Guidelines published by the European Society of Cardiology10 state that a normal ECG result in patients with suspected heart failure should lead us to doubt the accuracy of the diagnosis.
Chest x-ray. The chest x-ray is most valuable as a test to exclude pulmonary causes. However, the existing evidence suggests it is not a reliable way to exclude LVSD.24,26,33,34 (Table 2) provides information about the value of radiography in predicting LVSD. A systematic review of the literature concluded that redistribution and cardiomegaly were the best chest radiographic findings for diagnosing increased preload and reduced ejection fraction, respectively.34 However, neither finding alone could adequately exclude or confirm LVSD. Studies published since that review have confirmed this finding.33 Although part of the evaluation of the heart failure patient, radiography is only one part of the diagnostic process and cannot be used to provide definitive diagnostic information.
Echocardiography. The most important step in the evaluation of the heart failure patient is the assessment of left ventricular systolic function. Both echocardiography and radionuclide ventriculography have been advocated.8-14 However, echocardiography is preferred as it is widely available, simple, noninvasive, safe, usually less expensive, and provides more information about valve function and left ventricular hypertrophy. Table 2 demonstrates the high sensitivity and specificity of echocardiography.35 In view of this, it is recommended as a standard adjunct to the clinical diagnosis of patients with dyspnea on exertion and suspected heart failure. Between 8% and 18% of patients will have inadequate echocardiograms, in which case radionuclide ventriculography is advocated.8
Neurohormonal markers. In recent years there has been increasing interest in the potential role of neurohormonal markers, such as B-type natriuretic peptide (BNP), atrial natriuretic peptide (ANP), N-terminal pro-ANP (N-ANP), and N-terminal pro-BNP (N-BNP) as indices of LVSD.36-42 Most of the data relating to these markers are relatively recent; therefore their use is not addressed in any detail in any of the aforementioned guidelines.
Some studies41,42 suggest that BNP and N-BNP are useful for diagnosing LVSD even when the positive predictive values are low, because of their high negative predictive values. One of the most recent studies44 examined the utility of BNP in an urgent care setting and suggested that BNP was an extremely reliable indicator of LVSD. In this population of patients with acute dyspnea where 39% had a final diagnosis of heart failure, 90% with a positive BNP had heart failure and 98% of those with a negative BNP did not. Although there appears to be a growing body of evidence supporting the role of these neurohormonal markers in the evaluation of the patient with LVSD, Table 2 illustrates that there have also been conflicting findings. This is partly because of differences in study design, study populations, cut-off points for ANP and BNP, and the definition of LVSD. Most studies agree that assessment of BNP, in particular, may be a cost-effective method for initial screening for LVSD, but should still be followed by an echocardiogram to confirm the diagnosis.
Management follows diagnosis. Making the correct diagnosis is the crucial first step in the management of chronic heart failure. Figure 1 summarizes the steps currently recommended for the evaluation of the patient with LVSD. A confirmation of a diagnosis of LVSD, however, is not the end of the story. Management will then need to include initiation of appropriate therapies and consideration of treatable and reversible etiologies, a subject to be addressed in the June 2002 issue of this journal.
TABLE 2
Key investigations used for the diagnosis of left ventricular systolic dysfunction
| Test | N | Setting* | Study quality † | Sensitivity (%) | Specificity(%) | LR+ | LR- | PV+ (%) | PV- (%) |
|---|---|---|---|---|---|---|---|---|---|
| Electrocardiogram | |||||||||
| Davie, 199729 | 534 | R | 1c | 94 | 61 | 2.43 | 0.10 | 35 | 98 |
| Lindsay, 200043 | 416 | R | 1c | 90 | 65 | 2.59 | 2.76 | 43 | 90 |
| Mosterd, 199732 | 1980 | R | 1c | 54 | 79 | 2.55 | 0.58 | 7 | 98 |
| Electrocardiogram (patient older than 70 years) | |||||||||
| Mosterd, 199732 | 1980 | R | 1c | 67 | 64 | 1.88 | 0.52 | 7 | 98 |
| Talreja, 200030 | 330 | H | 1c | 65 | 98 | 38.2 | 0.36 | 98 | 64 |
| Chest x-ray | |||||||||
| Badgett, 199634 | 29 studies | 2a | 51 | 79 | 2.43 | 0.62 | 71 | 62 | |
| Rihal, 199524 | 554 | H | 2b | 20 | 89 | 1.82 | 0.90 | 34 | 79 |
| Echocardiogram | |||||||||
| Erbel, 198435 | 110 | H | 1c | 80 | 100 | 80.0 | 0.20 | 100 | 85 |
| N-terminal ANP > 4.4 ng/mL | |||||||||
| McClure, 199840 | 134 | M | 2b | — | — | 1.08 | 0.96 | 52 | 51 |
| N-terminal pro-BNP > 275 fmol/mL | |||||||||
| Talwar, 199942 | 249 | R | 2b | 94 | 55 | 2.09 | 011 | 58 | 93 |
| BNP > 75 pg/mL | |||||||||
| Maisel, 200137 | 200 | R | 1c | 86 | 98 | 43.0 | 0.14 | 98 | 89 |
| Dao, 200144 | 250 | U | 1b | 98 | 92 | 12.2 | 0.02 | 92 | 98 |
| BNP > 46 pg/mL | |||||||||
| McClure, 199840 | 134 | M | 2b | — | — | 2.25 | 0.83 | 69 | 55 |
| BNP > 17.9 pg/mL | |||||||||
| McDonagh, 199841 | 1653 | P | 2b | 76 | 87 | 5.85 | 0.28 | 16 | 97 |
| NOTE: Pretest probability = 50%. | |||||||||
| *P denotes cross-sectional primary care population; R, primary care patients referred for suspected heart failure; H, hospitalized patients undergoing angiography; U, urgent care center; M, long-term myocardial infarction survivors recalled by their family physician. | |||||||||
| † Level 1a is the most rigorous; level 5 is the least rigorous. | |||||||||
| ANP, atrial natriuretic peptide; BNP, B-type natriuretic peptide; LR+, positive likelihood ratio; LR-, negative likelihood ratio; PV+, positive predictive value; PV-, negative predictive value. | |||||||||
Figure
Steps in the assessment of the patient with suspected heart failure
ACKNOWLEDGMENTS
We wish to thank Mr Chris Shiels at the Department of Primary Care, University of Liverpool, for his valuable advice regarding this manuscript.
1. Mair FS, Crowley TS, Bundred PE. Prevalence, aetiology and management of heart failure in general practice. Br J Gen Pract 1996;46:77-9.
2. Wheeldon NM, MacDonald TM, Flucker CJ, McDermitt DG, Struthers AD. An electrocardiographic study of chronic heart failure in the community. QJM 1993;86:17-23.
3. O’Connell JB, Bristow MR. Economic impact of heart failure in the United States: time for a different approach. J Heart Lung Transplan 1994;13:S107-112.
4. O’Connell JB. The economic burden of heart failure. Clin Cardiol 2000;23 (3 Suppl):III6-10.
5. English MA, Mastrean MB. Congestive heart failure: public and private burden. Crit Care Nurs Q 1995;18:1-6.
6. Croft JB, Giles WH, Pollard RA, Keenan NL, Casper ML, Anda RF. Heart failure survival among older adults in the United States: a poor prognosis for an emerging epidemic in the Medicare population. Arch Intern Med 1999;159:505-10.
7. Stewart S, MacIntyre K, Hole DJ, Capewell S, McMurray JJV. More ‘malignant’ than cancer? Five-year survival following a first admission for heart failure. Eur J Heart Fail 2001;3:315-22.
8. Konstam M, Dracup K, Baker D, et al. Heart failure: evaluation and care of patients with left-ventricular systolic dysfunction. Clinical Practice Guideline No. 11. AHCPR Publication No. 94-0612. Agency for Health Care Policy and Research, Public Health Service, US Department of Health and Human Services, Rockville, Md.
9. Guidelines for the evaluation and management of heart failure. Report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee on Evaluation and Management of Heart Failure). Circulation 1995;92:2764-84.
10. The Task Force on Heart Failure of the European Society of Cardiology. Guidelines for the diagnosis of heart failure. Eur Heart J 1995;16:741-51.
11. Heart failure —systolic dysfunction. University of Michigan Health System. August 1999. (Available at http://cme.med.umich.edu/pdf/guideline/heart.pdf)
12. The Task Force of the Working Group on Heart Failure of the European Society of Cardiolog. The treatment of heart failure. Eur Heart J 1997;18:736-53.
13. Diagnosis and treatment of heart failure due to left ventricular systolic dysfunction. A national clinical guideline. Scottish Intercollegiate Guidelines Network (SIGN); SIGN publication; no. 35. Feb 1999; 68.
14. Anonymous. Heart Failure Society of America (HFSA) practice guidelines. HFSA guidelines for management of patients with heart failure caused by left ventricular systolic dysfunction—pharmacological approaches [published erratum appears in J Card Fail 2000 Mar; 6:74]. J Card Fail 5:357-82.
15. McMurray JJV, Dargie HJ. Diagnosis and management of heart failure. BMJ 1994;308:321-8.
16. Mair FS. Management of heart failure. Am Fam Phys 1996;54:245-54.
17. Marantz PR, Tobin JN, Wassertheil-Smoller S, et al. The relationship between left ventricular systolic function and congestive heart failure diagnosed by clinical criteria. Circulation 1988;77:607-12.
18. Gillespie ND, McNeill G, Pringle T, Ogston S, Struthers AD, Pringle SD. Cross sectional study of contribution of clinical assessment and simple cardiac investigations to diagnosis of left ventricular systolic dysfunction in patients admitted with acute dyspnoea. BMJ 1997;314:936-40.
19. Remes J, Miettinen H, Reunanen A, Pyorala K. Validity of clinical diagnosis of heart failure in primary health care. Eur Heart J 1991;12:315-21.
20. Davie AP, Francis CM, Caruana L, Sutherland GR, McMurray JJV. Assessing diagnosis in heart failure: which features are of any use? QJM 1997;90:335-9.
21. Gadsboll N, Hoilund-Carlsen PF, Nielsen GG, et al. Symptoms and signs of heart failure in patients with myocardial infarction: reproducibility and relationship to chest X-ray, radionuclide ventriculog-raphy, and right heart catheterisation. Eur Heart J 1989;10:1017-28.
22. Stevenson LW, Perloff JK. The limited reliability of physical signs for estimating hemodynamics in chronic heart failure. JAMA 1989;261:884-8.
23. Ishmail AA, Wing S, Ferguson J, Hutchinson TA, Magder S, Flegel KM. Interobserver agreement by auscultation in the presence of a third heart sound in patients with congestive heart failure. Chest 1987;91:870-3.
24. Rihal CS, Davis KB, Kennedy W, Gersh BJ. The utility of clinical, electrocardiographic and roentgenographic variables in the prediction of left ventricular function. Am J Cardiol 1995;752:220-3.
25. Harlan WR, Oberman A, Grimm R, Rosati RA. Chronic congestive heart failure in coronary artery disease: clinical criteria. Ann Intern Med 1977;86:133-8.
26. Chakko S, Woska D, Martinez H, et al. Clinical, radiographic, and hemodynamic correlations in chronic congestive heart failure: conflicting results may lead to inappropriate care. Am J Med 1991;90:353-9.
27. Mattleman SJ, Hakki A, Iskandrian AS, Segal BL, Kane SA. Reliability of bedside evaluation in determining left ventricular function: correlation with left ventricular ejection fraction determined by radionuclide ventriculography. J Am Coll Cardio 1983;1:417-20.
28. Morgan S, Smith H, Simpson I, et al. Prevalence and clinical characteristics of left ventricular dysfunction among elderly patients in general practice setting: cross sectional survey. BMJ 1999;318:368-72.
29. Davie AP, Francis CM, Love MP, et al. Value of electrocardiogram in identifying heart failure due to left ventricular systolic dysfunction. BMJ 1996;312:222-3.
30. Talreja D, Gruver C, Sklenar J, Dent J, Kaul S. Efficient utilization of echocardiography for the assessment of left ventricular systolic function. Am Heart J 2000;139:394-8.
31. Murkofsky RL, Dangas G, Diamond JA, et al. A prolonged QRS duration on surface electrocardiogram is a specific indicator of left ventricular dysfunction. J Am Coll Cardiol 1998;32:476-82.
32. Mosterd A, de Bruijne MC, Hoes AW, et al. Usefulness of echocardiography in detecting left ventricular dysfunction in population-based studies (The Rotterdam Study). Am J Cardiol 1997;79:103-4.
33. Clark AL, Coats ALS. Unreliability of cardiothoracic ratio as a marker of left ventricular impairment: comparison with radionuclide ventriculography and echocardiography. Postgrad Med J 2000;76:289-91.
34. Badgett RG, Mulrow CD, Otto PM, Ramirez G. How well can the chest radiograph diagnose left ventricular dysfunction? J Gen Intern Med 1996;11:625-34.
35. Erbel R, Schweizer P, Drebs W, Meyer J, Effert S. Sensitivity and specificity of two-dimensional echocardiography in detection of impaired left ventricular function. Eur Heart J 1984;5:477-89.
36. Davis M, Espiner E, Richards G, et al. Plasma brain natriuretic peptide in assessment of acute dyspnoea. Lancet 1994;343:440-4.
37. Maisel AS, Koon J, Krishnaswamy P, et al. Utility of B-natriuretic peptide as a rapid, point-of-care test for screening patients undergoing echocardiography to determine left ventricular dysfunction. Am Heart J 2001;141:367-74.
38. Schirmer H, Omland T. Circulating N-terminal pro-atrial natriuretic peptide is an independent predictor of left ventricular hypertrophy in the general population. The Tromsø Study. Eur Heart J 1999;20:755-63.
39. Lerman A, Gibbons RJ, Rodeheffer RJ, et al. Circulating N-terminal atrial natriuretic peptide as a marker for symptomless left-ventricular dysfunction. Lancet 1993;341:1105-9.
40. McClure SJ, Davie AP, Goldthorp S, et al. Cohort study of plasma natriuretic peptides for identifying left ventricular systolic dysfunction in primary care. BMJ 1998;317:516-9.
41. McDonagh TA, Robb SD, Murdoch DR, et al. Biochemical detection of left-ventricular systolic dysfunction. Lancet 1998;351:9-13,
42. Talwar S, Squire IB, Davies JE, et al. Plasma N-terminal pro-brain natriuretic peptide and the ECG in the assessment of left-ventricular systolic dysfunction in a high risk population. Eur Heart J 1999;20:1736-1744.
43. Lindsay MM, Goodfield NER, Hogg KJ, Dunn EG. Optimising direct access echo referral in suspected heart failure. Scot Med J 2000;45:043-044.
44. Dao Q, Krishnaswamy P, Kazanegra R, et al. Utility of B-Type natriuretic peptide in the diagnosis of congestive heart failure in an urgent care setting. J Am Coll Cardiol 2001;37:379-85.
- Heart failure is an increasingly common problem in primary care, with a mortality rate higher than that of most cancers.
- The absence of dyspnea on exertion or a normal electrocardiogram (ECG) result indicates that heart failure is unlikely; a gallop rhythm or laterally displaced apical rhythm is strong evidence in favor of heart failure.
- The history and physical examination and ECG alone are usually inadequate to confirm diagnosis of left ventricular systolic dysfunction, and echocardiography remains the gold standard to confirm the diagnosis.
Heart failure is increasing in incidence and prevalence; it currently affects 0.4% to 2% of the general population and 8% to 10% of the elderly.1,2 In the United States, heart failure is the second most common cardiovascular reason for an outpatient visit in the ambulatory care setting and remains the most common cause for hospitalization among patients older than 65 years.3 The total cost for heart failure management in 1999 was estimated to approach $56 billion.4 Those suffering with this illness experience high levels of morbidity and mortality5 that are reflected in the workloads of both primary and secondary care. Heart failure admission rates are rising, and the prognosis of heart failure has been compared with that of malignancy, with a 6-year mortality rate of 84% in men and 77% in women.6,7
A number of heart failure guidelines8-14 provide direction regarding “best practice” with regard to diagnosis and management. These guidelines have all been produced by expert panels and base their evidence on systematic critical reviews of the literature, plus expert consensus opinion. The evidence underlying the development of these guidelines ranges from well-conducted randomized controlled trials to expert opinion. These guidelines all emphasize the ways in which approaches to the diagnosis and management of heart failure have altered substantially in recent years and are continuing to change rapidly. The need to detect heart failure at an early stage to slow the progression of left ventricular systolic dysfunction (LVSD) is now well accepted.15
The following provides an overview of the current recommended approaches to diagnosis, focusing specifically on LVSD, the most common type of heart failure and also the usual focus of most guidelines. Accurate diagnosis of LVSD is the single most important step in management.16 An adequate diagnosis should establish the existence of heart failure, differentiate systolic from diastolic dysfunction, and identify the main underlying cause and any subsidiary diagnoses that may exacerbate heart failure. The etiology of heart failure and the presence of exacerbating factors or other diseases need to be carefully considered in all patients. Coronary artery disease remains the most common potentially reversible etiologic factor in heart failure.8
Using the history and physical examination
The major symptoms of heart failure are fatigue, exercise intolerance, exertional dyspnea, orthopnea, paroxysmal nocturnal dyspnea, and dependent edema. However, such symptoms are similar to those of many other diseases, particularly pulmonary diseases. For example, exertional dypsnea is a common symptom in heart failure but can be due to a wide range of other causes, such as chronic obstructive pulmonary disease, interstitial lung disease, asthma, respiratory infection, deconditioning, or obesity. Many patients with impaired left ventricular function may have no obvious symptoms.17 This highlights the importance of exploring past medical and medication history as these contribute to the overall clinical assessment.
Physical findings that may support a diagnosis of heart failure include raised jugular venous pressure, peripheral edema not due to venous insufficiency, presence of a third heart sound, gallop rhythm, laterally displaced apical impulse, tachycardia, and pulmonary rales that do not clear with coughing. Although clinical findings are particularly useful in acute severe heart failure at the time of hospitalization,18 it is difficult to accurately diagnose mild heart failure in the community on the basis of clinical grounds alone.2,9 The value of different symptoms and aspects of the medical history and use of medications in the evaluation of potential heart failure patients have been examined by researchers.18, 20-23 Similarly, the utility of physical examination has also undergone investigation.18,20,22-28Table 1 summarizes the study findings with regard to clinical symptoms and signs.
Davie and colleagues20 assessed the value of symptoms, past history, medications, and signs in the evaluation of patients who may have LVSD. No one clinical feature predicted LVSD, as assessed by echocardiography with sensitivity, specificity, and a high positive and negative predictive value. Absence of dyspnea on exertion essentially ruled out heart failure (negative likelihood ratio [LR-] = 0.06), while gallop rhythm (positive likelihood ratio [LR+] = 24.0), laterally displaced apical impulse (LR+ 16.4), and elevated jugular venous pulsation (LR+ = 8.9) are strong evidence in favor of the diagnosis. Furthermore, the combination of history of myocardial infarction and displaced apex on physical examination, although not particularly sensitive (39% sensitivity) was very specific (99% specificity) with high positive (89%) and negative (89%) predictive values. The authors also suggest that a breathless patient with a past history of myocardial infarction and a displaced apex beat on physical examination will almost certainly have heart failure and, if resources are limited, may not need echocardiography to confirm the diagnosis. However, less than 50% of breathless patients will have this combination, and the other half would therefore need echocardiography as the gold standard diagnostic tool for LVSD.
Morgan and coworkers28 assessed the prevalence and clinical characteristics of LVSD among elderly patients (those aged 70 years to 84 years) in a primary care setting by echocardiographic assessment of ventricular function. They found that no single clinical symptom or sign was both sensitive and specific, and concluded that diagnosis should not be based on clinical history and examination alone. They found that a substantial number of elderly individuals had asymptomatic or misdiagnosed LVSD, and suggested this might be due to the extremely limited sensitivity and specificity of clinical history taking and examination. For example, only 11% of patients with LVSD had a raised jugular venous pressure, and bilateral ankle edema was common but nonspecific. Researchers have therefore concluded that although these clinical findings are useful in acute severe heart failure, they have only a small role in detecting LVSD in the community.18
TABLE 1
The use of clinical symptoms and signs to diagnose heart failure, by study
| Sign or symptom | N | Setting* | Study quality (1a-5)† | Sensitivity (%) | Specificity (%) | LR+ | LR- | PV+(%) | PV-(%) |
|---|---|---|---|---|---|---|---|---|---|
| Previous myocardial infarction | |||||||||
| Davie, 199720 | 259 | R | 2b | 59 | 86 | 4.1 | 0.48 | 44 | 92 |
| Morgan, 199928 | 817 | P | 2b | 39 | 91 | 4.3 | 0.67 | ||
| Dyspnea on exertion | |||||||||
| Davie, 199720 | 259 | R | 2b | 100 | 17 | 1.20 | 0.06 | 18 | 100 |
| Morgan, 199928 | 817 | P | 2b | 15 | 97 | 5.4 | 0.88 | ||
| Orthopnea | |||||||||
| Davie, 199720 | 259 | R | 2b | 22 | 74 | 0.85 | 1.05 | 14 | 83 |
| Paroxysmal nocturnal dyspnea | |||||||||
| Davie, 199720 | 259 | R | 2b | 39 | 80 | 1.95 | 0.76 | 27 | 87 |
| History of peripheral edema | |||||||||
| Davie, 199720 | 259 | R | 2b | 49 | 47 | 0.92 | 1.09 | 15 | 83 |
| Tachycardia | |||||||||
| Davie, 199720 | 259 | R | 2b | 22 | 92 | 2.75 | 0.85 | 33 | 86 |
| Elevated JVP | |||||||||
| Davie, 199720 | 259 | R | 2b | 17 | 98 | 8.95 | 0.84 | 64 | 86 |
| Morgan, 199928 | 817 | R | 2b | 11 | 97 | 3.6 | 0.92 | ||
| Gallop rhythm | |||||||||
| Davie, 199720 | 259 | P | 2b | 24 | 99 | 24.0 | 0.77 | 77 | 87 |
| 3rd heart sound | |||||||||
| Rihal, 199524 | 554 | H | 2b | 9 | 97 | 3.00 | 0.94 | 54 | 78 |
| Laterally displaced apical impulse | |||||||||
| Davie, 199720 | 259 | R | 2b | 66 | 96 | 16.4 | 0.35 | 75 | 94 |
| Pulmonary rales | |||||||||
| Davie, 199720 | 259 | R | 2b | 29 | 77 | 1.26 | 0.92 | 19 | 85 |
| Morgan, 199928 | 817 | P | 2b | 44 | 82 | 2.4 | 0.68 | ||
| Peripheral edema on examination | |||||||||
| Davie, 199720 | 259 | R | 2b | 20 | 86 | 1.43 | 0.93 | 21 | 85 |
| Morgan, 199928 | 817 | P | 2b | 18 | 91 | 2.0 | 0.90 | ||
| NOTE: Pretest probability = 50%. | |||||||||
| *P denotes cross-sectional primary care population; R, primary care patients referred for suspected heart failure; H, hospitalized patients undergoing angiography. | |||||||||
| † Level 1a is the most rigorous; level 5 is the least rigorous. | |||||||||
| LR+ denotes positive likelihood ratio; LR-, negative likelihood ratio; PV+, positive predictive value; PV-, negative predictive value. | |||||||||
Laboratory and imaging evaluation
Although an important and valuable part of the evaluation, the history and physical examination alone are insufficient to confirm a diagnosis in most cases. Recommended initial tests for patients with signs or symptoms of heart failure include complete blood count (CBC), serum electrolytes, serum creatinine, serum albumin, liver function tests, urinalysis, electrocardiogram, and chest x-ray (Figure).
Blood tests. For those older than 65 years or with atrial fibrillation or evidence of thyroid disease, thyroid function tests should also be performed because heart failure due to thyrotoxicosis is frequently associated with rapid atrial fibrillation and hypothyroidism may also present as heart failure.8,10 The other routine blood tests are important as a way to exclude alternative diagnoses; they also help with the search for predisposing or exacerbating causes of the heart failure. These baseline tests also help guide future therapeutic decision making. For example, electrolyte and renal function results are pertinent when initiating angiotensin-converting enzyme (ACE) inhibitors. Anemia can exacerbate pre-existing heart failure, and measurement of renal function is essential to distinguish fluid overload due to heart failure from renal failure. Liver enzymes may be affected by hepatic congestion. Urinalysis is valuable in the detection of underlying renal disease or diabetes.8
Electrocardiography. An electrocardiogram (ECG) is another recommended part of the evaluation of the suspected heart failure patient.8-14 Considerable attention has been paid to examining the value of this test in the diagnosis of LVSD.29-32 Davie and colleagues29 assessed the value of the ECG in identifying patients with possible heart failure by examining referrals for echocardiography by primary care practitioners. A total of 534 patients were referred for echocardiography for possible heart failure, of whom 18% (n = 96) had LVSD. They showed that LVSD was extremely unlikely if the ECG result was normal, but that 1 in 3 patients with an abnormal result had significant LVSD. Thus, a normal ECG result virtually excludes chronic heart failure due to LVSD. However, the ECG is not a substitute for echocardiography, as an abnormal result does not accurately predict the presence of LVSD (Table 2).
Others have confirmed these findings.30,32 Talreja and colleagues30 found that of 330 consecutive in-patients referred for echocardiographic assessment of left ventricular function, 124 (41%) had LVSD. Only 2 of 124 patients with LVSD had a normal electrocardiogram result. When the ECG result is normal, the authors suggest that echocardiography is not needed. However, they concede that physicians are unlikely to adhere to this because many may not be as sophisticated in interpreting the ECG and may feel it important to get an accurate measure of ejection fraction. Guidelines published by the European Society of Cardiology10 state that a normal ECG result in patients with suspected heart failure should lead us to doubt the accuracy of the diagnosis.
Chest x-ray. The chest x-ray is most valuable as a test to exclude pulmonary causes. However, the existing evidence suggests it is not a reliable way to exclude LVSD.24,26,33,34 (Table 2) provides information about the value of radiography in predicting LVSD. A systematic review of the literature concluded that redistribution and cardiomegaly were the best chest radiographic findings for diagnosing increased preload and reduced ejection fraction, respectively.34 However, neither finding alone could adequately exclude or confirm LVSD. Studies published since that review have confirmed this finding.33 Although part of the evaluation of the heart failure patient, radiography is only one part of the diagnostic process and cannot be used to provide definitive diagnostic information.
Echocardiography. The most important step in the evaluation of the heart failure patient is the assessment of left ventricular systolic function. Both echocardiography and radionuclide ventriculography have been advocated.8-14 However, echocardiography is preferred as it is widely available, simple, noninvasive, safe, usually less expensive, and provides more information about valve function and left ventricular hypertrophy. Table 2 demonstrates the high sensitivity and specificity of echocardiography.35 In view of this, it is recommended as a standard adjunct to the clinical diagnosis of patients with dyspnea on exertion and suspected heart failure. Between 8% and 18% of patients will have inadequate echocardiograms, in which case radionuclide ventriculography is advocated.8
Neurohormonal markers. In recent years there has been increasing interest in the potential role of neurohormonal markers, such as B-type natriuretic peptide (BNP), atrial natriuretic peptide (ANP), N-terminal pro-ANP (N-ANP), and N-terminal pro-BNP (N-BNP) as indices of LVSD.36-42 Most of the data relating to these markers are relatively recent; therefore their use is not addressed in any detail in any of the aforementioned guidelines.
Some studies41,42 suggest that BNP and N-BNP are useful for diagnosing LVSD even when the positive predictive values are low, because of their high negative predictive values. One of the most recent studies44 examined the utility of BNP in an urgent care setting and suggested that BNP was an extremely reliable indicator of LVSD. In this population of patients with acute dyspnea where 39% had a final diagnosis of heart failure, 90% with a positive BNP had heart failure and 98% of those with a negative BNP did not. Although there appears to be a growing body of evidence supporting the role of these neurohormonal markers in the evaluation of the patient with LVSD, Table 2 illustrates that there have also been conflicting findings. This is partly because of differences in study design, study populations, cut-off points for ANP and BNP, and the definition of LVSD. Most studies agree that assessment of BNP, in particular, may be a cost-effective method for initial screening for LVSD, but should still be followed by an echocardiogram to confirm the diagnosis.
Management follows diagnosis. Making the correct diagnosis is the crucial first step in the management of chronic heart failure. Figure 1 summarizes the steps currently recommended for the evaluation of the patient with LVSD. A confirmation of a diagnosis of LVSD, however, is not the end of the story. Management will then need to include initiation of appropriate therapies and consideration of treatable and reversible etiologies, a subject to be addressed in the June 2002 issue of this journal.
TABLE 2
Key investigations used for the diagnosis of left ventricular systolic dysfunction
| Test | N | Setting* | Study quality † | Sensitivity (%) | Specificity(%) | LR+ | LR- | PV+ (%) | PV- (%) |
|---|---|---|---|---|---|---|---|---|---|
| Electrocardiogram | |||||||||
| Davie, 199729 | 534 | R | 1c | 94 | 61 | 2.43 | 0.10 | 35 | 98 |
| Lindsay, 200043 | 416 | R | 1c | 90 | 65 | 2.59 | 2.76 | 43 | 90 |
| Mosterd, 199732 | 1980 | R | 1c | 54 | 79 | 2.55 | 0.58 | 7 | 98 |
| Electrocardiogram (patient older than 70 years) | |||||||||
| Mosterd, 199732 | 1980 | R | 1c | 67 | 64 | 1.88 | 0.52 | 7 | 98 |
| Talreja, 200030 | 330 | H | 1c | 65 | 98 | 38.2 | 0.36 | 98 | 64 |
| Chest x-ray | |||||||||
| Badgett, 199634 | 29 studies | 2a | 51 | 79 | 2.43 | 0.62 | 71 | 62 | |
| Rihal, 199524 | 554 | H | 2b | 20 | 89 | 1.82 | 0.90 | 34 | 79 |
| Echocardiogram | |||||||||
| Erbel, 198435 | 110 | H | 1c | 80 | 100 | 80.0 | 0.20 | 100 | 85 |
| N-terminal ANP > 4.4 ng/mL | |||||||||
| McClure, 199840 | 134 | M | 2b | — | — | 1.08 | 0.96 | 52 | 51 |
| N-terminal pro-BNP > 275 fmol/mL | |||||||||
| Talwar, 199942 | 249 | R | 2b | 94 | 55 | 2.09 | 011 | 58 | 93 |
| BNP > 75 pg/mL | |||||||||
| Maisel, 200137 | 200 | R | 1c | 86 | 98 | 43.0 | 0.14 | 98 | 89 |
| Dao, 200144 | 250 | U | 1b | 98 | 92 | 12.2 | 0.02 | 92 | 98 |
| BNP > 46 pg/mL | |||||||||
| McClure, 199840 | 134 | M | 2b | — | — | 2.25 | 0.83 | 69 | 55 |
| BNP > 17.9 pg/mL | |||||||||
| McDonagh, 199841 | 1653 | P | 2b | 76 | 87 | 5.85 | 0.28 | 16 | 97 |
| NOTE: Pretest probability = 50%. | |||||||||
| *P denotes cross-sectional primary care population; R, primary care patients referred for suspected heart failure; H, hospitalized patients undergoing angiography; U, urgent care center; M, long-term myocardial infarction survivors recalled by their family physician. | |||||||||
| † Level 1a is the most rigorous; level 5 is the least rigorous. | |||||||||
| ANP, atrial natriuretic peptide; BNP, B-type natriuretic peptide; LR+, positive likelihood ratio; LR-, negative likelihood ratio; PV+, positive predictive value; PV-, negative predictive value. | |||||||||
Figure
Steps in the assessment of the patient with suspected heart failure
ACKNOWLEDGMENTS
We wish to thank Mr Chris Shiels at the Department of Primary Care, University of Liverpool, for his valuable advice regarding this manuscript.
- Heart failure is an increasingly common problem in primary care, with a mortality rate higher than that of most cancers.
- The absence of dyspnea on exertion or a normal electrocardiogram (ECG) result indicates that heart failure is unlikely; a gallop rhythm or laterally displaced apical rhythm is strong evidence in favor of heart failure.
- The history and physical examination and ECG alone are usually inadequate to confirm diagnosis of left ventricular systolic dysfunction, and echocardiography remains the gold standard to confirm the diagnosis.
Heart failure is increasing in incidence and prevalence; it currently affects 0.4% to 2% of the general population and 8% to 10% of the elderly.1,2 In the United States, heart failure is the second most common cardiovascular reason for an outpatient visit in the ambulatory care setting and remains the most common cause for hospitalization among patients older than 65 years.3 The total cost for heart failure management in 1999 was estimated to approach $56 billion.4 Those suffering with this illness experience high levels of morbidity and mortality5 that are reflected in the workloads of both primary and secondary care. Heart failure admission rates are rising, and the prognosis of heart failure has been compared with that of malignancy, with a 6-year mortality rate of 84% in men and 77% in women.6,7
A number of heart failure guidelines8-14 provide direction regarding “best practice” with regard to diagnosis and management. These guidelines have all been produced by expert panels and base their evidence on systematic critical reviews of the literature, plus expert consensus opinion. The evidence underlying the development of these guidelines ranges from well-conducted randomized controlled trials to expert opinion. These guidelines all emphasize the ways in which approaches to the diagnosis and management of heart failure have altered substantially in recent years and are continuing to change rapidly. The need to detect heart failure at an early stage to slow the progression of left ventricular systolic dysfunction (LVSD) is now well accepted.15
The following provides an overview of the current recommended approaches to diagnosis, focusing specifically on LVSD, the most common type of heart failure and also the usual focus of most guidelines. Accurate diagnosis of LVSD is the single most important step in management.16 An adequate diagnosis should establish the existence of heart failure, differentiate systolic from diastolic dysfunction, and identify the main underlying cause and any subsidiary diagnoses that may exacerbate heart failure. The etiology of heart failure and the presence of exacerbating factors or other diseases need to be carefully considered in all patients. Coronary artery disease remains the most common potentially reversible etiologic factor in heart failure.8
Using the history and physical examination
The major symptoms of heart failure are fatigue, exercise intolerance, exertional dyspnea, orthopnea, paroxysmal nocturnal dyspnea, and dependent edema. However, such symptoms are similar to those of many other diseases, particularly pulmonary diseases. For example, exertional dypsnea is a common symptom in heart failure but can be due to a wide range of other causes, such as chronic obstructive pulmonary disease, interstitial lung disease, asthma, respiratory infection, deconditioning, or obesity. Many patients with impaired left ventricular function may have no obvious symptoms.17 This highlights the importance of exploring past medical and medication history as these contribute to the overall clinical assessment.
Physical findings that may support a diagnosis of heart failure include raised jugular venous pressure, peripheral edema not due to venous insufficiency, presence of a third heart sound, gallop rhythm, laterally displaced apical impulse, tachycardia, and pulmonary rales that do not clear with coughing. Although clinical findings are particularly useful in acute severe heart failure at the time of hospitalization,18 it is difficult to accurately diagnose mild heart failure in the community on the basis of clinical grounds alone.2,9 The value of different symptoms and aspects of the medical history and use of medications in the evaluation of potential heart failure patients have been examined by researchers.18, 20-23 Similarly, the utility of physical examination has also undergone investigation.18,20,22-28Table 1 summarizes the study findings with regard to clinical symptoms and signs.
Davie and colleagues20 assessed the value of symptoms, past history, medications, and signs in the evaluation of patients who may have LVSD. No one clinical feature predicted LVSD, as assessed by echocardiography with sensitivity, specificity, and a high positive and negative predictive value. Absence of dyspnea on exertion essentially ruled out heart failure (negative likelihood ratio [LR-] = 0.06), while gallop rhythm (positive likelihood ratio [LR+] = 24.0), laterally displaced apical impulse (LR+ 16.4), and elevated jugular venous pulsation (LR+ = 8.9) are strong evidence in favor of the diagnosis. Furthermore, the combination of history of myocardial infarction and displaced apex on physical examination, although not particularly sensitive (39% sensitivity) was very specific (99% specificity) with high positive (89%) and negative (89%) predictive values. The authors also suggest that a breathless patient with a past history of myocardial infarction and a displaced apex beat on physical examination will almost certainly have heart failure and, if resources are limited, may not need echocardiography to confirm the diagnosis. However, less than 50% of breathless patients will have this combination, and the other half would therefore need echocardiography as the gold standard diagnostic tool for LVSD.
Morgan and coworkers28 assessed the prevalence and clinical characteristics of LVSD among elderly patients (those aged 70 years to 84 years) in a primary care setting by echocardiographic assessment of ventricular function. They found that no single clinical symptom or sign was both sensitive and specific, and concluded that diagnosis should not be based on clinical history and examination alone. They found that a substantial number of elderly individuals had asymptomatic or misdiagnosed LVSD, and suggested this might be due to the extremely limited sensitivity and specificity of clinical history taking and examination. For example, only 11% of patients with LVSD had a raised jugular venous pressure, and bilateral ankle edema was common but nonspecific. Researchers have therefore concluded that although these clinical findings are useful in acute severe heart failure, they have only a small role in detecting LVSD in the community.18
TABLE 1
The use of clinical symptoms and signs to diagnose heart failure, by study
| Sign or symptom | N | Setting* | Study quality (1a-5)† | Sensitivity (%) | Specificity (%) | LR+ | LR- | PV+(%) | PV-(%) |
|---|---|---|---|---|---|---|---|---|---|
| Previous myocardial infarction | |||||||||
| Davie, 199720 | 259 | R | 2b | 59 | 86 | 4.1 | 0.48 | 44 | 92 |
| Morgan, 199928 | 817 | P | 2b | 39 | 91 | 4.3 | 0.67 | ||
| Dyspnea on exertion | |||||||||
| Davie, 199720 | 259 | R | 2b | 100 | 17 | 1.20 | 0.06 | 18 | 100 |
| Morgan, 199928 | 817 | P | 2b | 15 | 97 | 5.4 | 0.88 | ||
| Orthopnea | |||||||||
| Davie, 199720 | 259 | R | 2b | 22 | 74 | 0.85 | 1.05 | 14 | 83 |
| Paroxysmal nocturnal dyspnea | |||||||||
| Davie, 199720 | 259 | R | 2b | 39 | 80 | 1.95 | 0.76 | 27 | 87 |
| History of peripheral edema | |||||||||
| Davie, 199720 | 259 | R | 2b | 49 | 47 | 0.92 | 1.09 | 15 | 83 |
| Tachycardia | |||||||||
| Davie, 199720 | 259 | R | 2b | 22 | 92 | 2.75 | 0.85 | 33 | 86 |
| Elevated JVP | |||||||||
| Davie, 199720 | 259 | R | 2b | 17 | 98 | 8.95 | 0.84 | 64 | 86 |
| Morgan, 199928 | 817 | R | 2b | 11 | 97 | 3.6 | 0.92 | ||
| Gallop rhythm | |||||||||
| Davie, 199720 | 259 | P | 2b | 24 | 99 | 24.0 | 0.77 | 77 | 87 |
| 3rd heart sound | |||||||||
| Rihal, 199524 | 554 | H | 2b | 9 | 97 | 3.00 | 0.94 | 54 | 78 |
| Laterally displaced apical impulse | |||||||||
| Davie, 199720 | 259 | R | 2b | 66 | 96 | 16.4 | 0.35 | 75 | 94 |
| Pulmonary rales | |||||||||
| Davie, 199720 | 259 | R | 2b | 29 | 77 | 1.26 | 0.92 | 19 | 85 |
| Morgan, 199928 | 817 | P | 2b | 44 | 82 | 2.4 | 0.68 | ||
| Peripheral edema on examination | |||||||||
| Davie, 199720 | 259 | R | 2b | 20 | 86 | 1.43 | 0.93 | 21 | 85 |
| Morgan, 199928 | 817 | P | 2b | 18 | 91 | 2.0 | 0.90 | ||
| NOTE: Pretest probability = 50%. | |||||||||
| *P denotes cross-sectional primary care population; R, primary care patients referred for suspected heart failure; H, hospitalized patients undergoing angiography. | |||||||||
| † Level 1a is the most rigorous; level 5 is the least rigorous. | |||||||||
| LR+ denotes positive likelihood ratio; LR-, negative likelihood ratio; PV+, positive predictive value; PV-, negative predictive value. | |||||||||
Laboratory and imaging evaluation
Although an important and valuable part of the evaluation, the history and physical examination alone are insufficient to confirm a diagnosis in most cases. Recommended initial tests for patients with signs or symptoms of heart failure include complete blood count (CBC), serum electrolytes, serum creatinine, serum albumin, liver function tests, urinalysis, electrocardiogram, and chest x-ray (Figure).
Blood tests. For those older than 65 years or with atrial fibrillation or evidence of thyroid disease, thyroid function tests should also be performed because heart failure due to thyrotoxicosis is frequently associated with rapid atrial fibrillation and hypothyroidism may also present as heart failure.8,10 The other routine blood tests are important as a way to exclude alternative diagnoses; they also help with the search for predisposing or exacerbating causes of the heart failure. These baseline tests also help guide future therapeutic decision making. For example, electrolyte and renal function results are pertinent when initiating angiotensin-converting enzyme (ACE) inhibitors. Anemia can exacerbate pre-existing heart failure, and measurement of renal function is essential to distinguish fluid overload due to heart failure from renal failure. Liver enzymes may be affected by hepatic congestion. Urinalysis is valuable in the detection of underlying renal disease or diabetes.8
Electrocardiography. An electrocardiogram (ECG) is another recommended part of the evaluation of the suspected heart failure patient.8-14 Considerable attention has been paid to examining the value of this test in the diagnosis of LVSD.29-32 Davie and colleagues29 assessed the value of the ECG in identifying patients with possible heart failure by examining referrals for echocardiography by primary care practitioners. A total of 534 patients were referred for echocardiography for possible heart failure, of whom 18% (n = 96) had LVSD. They showed that LVSD was extremely unlikely if the ECG result was normal, but that 1 in 3 patients with an abnormal result had significant LVSD. Thus, a normal ECG result virtually excludes chronic heart failure due to LVSD. However, the ECG is not a substitute for echocardiography, as an abnormal result does not accurately predict the presence of LVSD (Table 2).
Others have confirmed these findings.30,32 Talreja and colleagues30 found that of 330 consecutive in-patients referred for echocardiographic assessment of left ventricular function, 124 (41%) had LVSD. Only 2 of 124 patients with LVSD had a normal electrocardiogram result. When the ECG result is normal, the authors suggest that echocardiography is not needed. However, they concede that physicians are unlikely to adhere to this because many may not be as sophisticated in interpreting the ECG and may feel it important to get an accurate measure of ejection fraction. Guidelines published by the European Society of Cardiology10 state that a normal ECG result in patients with suspected heart failure should lead us to doubt the accuracy of the diagnosis.
Chest x-ray. The chest x-ray is most valuable as a test to exclude pulmonary causes. However, the existing evidence suggests it is not a reliable way to exclude LVSD.24,26,33,34 (Table 2) provides information about the value of radiography in predicting LVSD. A systematic review of the literature concluded that redistribution and cardiomegaly were the best chest radiographic findings for diagnosing increased preload and reduced ejection fraction, respectively.34 However, neither finding alone could adequately exclude or confirm LVSD. Studies published since that review have confirmed this finding.33 Although part of the evaluation of the heart failure patient, radiography is only one part of the diagnostic process and cannot be used to provide definitive diagnostic information.
Echocardiography. The most important step in the evaluation of the heart failure patient is the assessment of left ventricular systolic function. Both echocardiography and radionuclide ventriculography have been advocated.8-14 However, echocardiography is preferred as it is widely available, simple, noninvasive, safe, usually less expensive, and provides more information about valve function and left ventricular hypertrophy. Table 2 demonstrates the high sensitivity and specificity of echocardiography.35 In view of this, it is recommended as a standard adjunct to the clinical diagnosis of patients with dyspnea on exertion and suspected heart failure. Between 8% and 18% of patients will have inadequate echocardiograms, in which case radionuclide ventriculography is advocated.8
Neurohormonal markers. In recent years there has been increasing interest in the potential role of neurohormonal markers, such as B-type natriuretic peptide (BNP), atrial natriuretic peptide (ANP), N-terminal pro-ANP (N-ANP), and N-terminal pro-BNP (N-BNP) as indices of LVSD.36-42 Most of the data relating to these markers are relatively recent; therefore their use is not addressed in any detail in any of the aforementioned guidelines.
Some studies41,42 suggest that BNP and N-BNP are useful for diagnosing LVSD even when the positive predictive values are low, because of their high negative predictive values. One of the most recent studies44 examined the utility of BNP in an urgent care setting and suggested that BNP was an extremely reliable indicator of LVSD. In this population of patients with acute dyspnea where 39% had a final diagnosis of heart failure, 90% with a positive BNP had heart failure and 98% of those with a negative BNP did not. Although there appears to be a growing body of evidence supporting the role of these neurohormonal markers in the evaluation of the patient with LVSD, Table 2 illustrates that there have also been conflicting findings. This is partly because of differences in study design, study populations, cut-off points for ANP and BNP, and the definition of LVSD. Most studies agree that assessment of BNP, in particular, may be a cost-effective method for initial screening for LVSD, but should still be followed by an echocardiogram to confirm the diagnosis.
Management follows diagnosis. Making the correct diagnosis is the crucial first step in the management of chronic heart failure. Figure 1 summarizes the steps currently recommended for the evaluation of the patient with LVSD. A confirmation of a diagnosis of LVSD, however, is not the end of the story. Management will then need to include initiation of appropriate therapies and consideration of treatable and reversible etiologies, a subject to be addressed in the June 2002 issue of this journal.
TABLE 2
Key investigations used for the diagnosis of left ventricular systolic dysfunction
| Test | N | Setting* | Study quality † | Sensitivity (%) | Specificity(%) | LR+ | LR- | PV+ (%) | PV- (%) |
|---|---|---|---|---|---|---|---|---|---|
| Electrocardiogram | |||||||||
| Davie, 199729 | 534 | R | 1c | 94 | 61 | 2.43 | 0.10 | 35 | 98 |
| Lindsay, 200043 | 416 | R | 1c | 90 | 65 | 2.59 | 2.76 | 43 | 90 |
| Mosterd, 199732 | 1980 | R | 1c | 54 | 79 | 2.55 | 0.58 | 7 | 98 |
| Electrocardiogram (patient older than 70 years) | |||||||||
| Mosterd, 199732 | 1980 | R | 1c | 67 | 64 | 1.88 | 0.52 | 7 | 98 |
| Talreja, 200030 | 330 | H | 1c | 65 | 98 | 38.2 | 0.36 | 98 | 64 |
| Chest x-ray | |||||||||
| Badgett, 199634 | 29 studies | 2a | 51 | 79 | 2.43 | 0.62 | 71 | 62 | |
| Rihal, 199524 | 554 | H | 2b | 20 | 89 | 1.82 | 0.90 | 34 | 79 |
| Echocardiogram | |||||||||
| Erbel, 198435 | 110 | H | 1c | 80 | 100 | 80.0 | 0.20 | 100 | 85 |
| N-terminal ANP > 4.4 ng/mL | |||||||||
| McClure, 199840 | 134 | M | 2b | — | — | 1.08 | 0.96 | 52 | 51 |
| N-terminal pro-BNP > 275 fmol/mL | |||||||||
| Talwar, 199942 | 249 | R | 2b | 94 | 55 | 2.09 | 011 | 58 | 93 |
| BNP > 75 pg/mL | |||||||||
| Maisel, 200137 | 200 | R | 1c | 86 | 98 | 43.0 | 0.14 | 98 | 89 |
| Dao, 200144 | 250 | U | 1b | 98 | 92 | 12.2 | 0.02 | 92 | 98 |
| BNP > 46 pg/mL | |||||||||
| McClure, 199840 | 134 | M | 2b | — | — | 2.25 | 0.83 | 69 | 55 |
| BNP > 17.9 pg/mL | |||||||||
| McDonagh, 199841 | 1653 | P | 2b | 76 | 87 | 5.85 | 0.28 | 16 | 97 |
| NOTE: Pretest probability = 50%. | |||||||||
| *P denotes cross-sectional primary care population; R, primary care patients referred for suspected heart failure; H, hospitalized patients undergoing angiography; U, urgent care center; M, long-term myocardial infarction survivors recalled by their family physician. | |||||||||
| † Level 1a is the most rigorous; level 5 is the least rigorous. | |||||||||
| ANP, atrial natriuretic peptide; BNP, B-type natriuretic peptide; LR+, positive likelihood ratio; LR-, negative likelihood ratio; PV+, positive predictive value; PV-, negative predictive value. | |||||||||
Figure
Steps in the assessment of the patient with suspected heart failure
ACKNOWLEDGMENTS
We wish to thank Mr Chris Shiels at the Department of Primary Care, University of Liverpool, for his valuable advice regarding this manuscript.
1. Mair FS, Crowley TS, Bundred PE. Prevalence, aetiology and management of heart failure in general practice. Br J Gen Pract 1996;46:77-9.
2. Wheeldon NM, MacDonald TM, Flucker CJ, McDermitt DG, Struthers AD. An electrocardiographic study of chronic heart failure in the community. QJM 1993;86:17-23.
3. O’Connell JB, Bristow MR. Economic impact of heart failure in the United States: time for a different approach. J Heart Lung Transplan 1994;13:S107-112.
4. O’Connell JB. The economic burden of heart failure. Clin Cardiol 2000;23 (3 Suppl):III6-10.
5. English MA, Mastrean MB. Congestive heart failure: public and private burden. Crit Care Nurs Q 1995;18:1-6.
6. Croft JB, Giles WH, Pollard RA, Keenan NL, Casper ML, Anda RF. Heart failure survival among older adults in the United States: a poor prognosis for an emerging epidemic in the Medicare population. Arch Intern Med 1999;159:505-10.
7. Stewart S, MacIntyre K, Hole DJ, Capewell S, McMurray JJV. More ‘malignant’ than cancer? Five-year survival following a first admission for heart failure. Eur J Heart Fail 2001;3:315-22.
8. Konstam M, Dracup K, Baker D, et al. Heart failure: evaluation and care of patients with left-ventricular systolic dysfunction. Clinical Practice Guideline No. 11. AHCPR Publication No. 94-0612. Agency for Health Care Policy and Research, Public Health Service, US Department of Health and Human Services, Rockville, Md.
9. Guidelines for the evaluation and management of heart failure. Report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee on Evaluation and Management of Heart Failure). Circulation 1995;92:2764-84.
10. The Task Force on Heart Failure of the European Society of Cardiology. Guidelines for the diagnosis of heart failure. Eur Heart J 1995;16:741-51.
11. Heart failure —systolic dysfunction. University of Michigan Health System. August 1999. (Available at http://cme.med.umich.edu/pdf/guideline/heart.pdf)
12. The Task Force of the Working Group on Heart Failure of the European Society of Cardiolog. The treatment of heart failure. Eur Heart J 1997;18:736-53.
13. Diagnosis and treatment of heart failure due to left ventricular systolic dysfunction. A national clinical guideline. Scottish Intercollegiate Guidelines Network (SIGN); SIGN publication; no. 35. Feb 1999; 68.
14. Anonymous. Heart Failure Society of America (HFSA) practice guidelines. HFSA guidelines for management of patients with heart failure caused by left ventricular systolic dysfunction—pharmacological approaches [published erratum appears in J Card Fail 2000 Mar; 6:74]. J Card Fail 5:357-82.
15. McMurray JJV, Dargie HJ. Diagnosis and management of heart failure. BMJ 1994;308:321-8.
16. Mair FS. Management of heart failure. Am Fam Phys 1996;54:245-54.
17. Marantz PR, Tobin JN, Wassertheil-Smoller S, et al. The relationship between left ventricular systolic function and congestive heart failure diagnosed by clinical criteria. Circulation 1988;77:607-12.
18. Gillespie ND, McNeill G, Pringle T, Ogston S, Struthers AD, Pringle SD. Cross sectional study of contribution of clinical assessment and simple cardiac investigations to diagnosis of left ventricular systolic dysfunction in patients admitted with acute dyspnoea. BMJ 1997;314:936-40.
19. Remes J, Miettinen H, Reunanen A, Pyorala K. Validity of clinical diagnosis of heart failure in primary health care. Eur Heart J 1991;12:315-21.
20. Davie AP, Francis CM, Caruana L, Sutherland GR, McMurray JJV. Assessing diagnosis in heart failure: which features are of any use? QJM 1997;90:335-9.
21. Gadsboll N, Hoilund-Carlsen PF, Nielsen GG, et al. Symptoms and signs of heart failure in patients with myocardial infarction: reproducibility and relationship to chest X-ray, radionuclide ventriculog-raphy, and right heart catheterisation. Eur Heart J 1989;10:1017-28.
22. Stevenson LW, Perloff JK. The limited reliability of physical signs for estimating hemodynamics in chronic heart failure. JAMA 1989;261:884-8.
23. Ishmail AA, Wing S, Ferguson J, Hutchinson TA, Magder S, Flegel KM. Interobserver agreement by auscultation in the presence of a third heart sound in patients with congestive heart failure. Chest 1987;91:870-3.
24. Rihal CS, Davis KB, Kennedy W, Gersh BJ. The utility of clinical, electrocardiographic and roentgenographic variables in the prediction of left ventricular function. Am J Cardiol 1995;752:220-3.
25. Harlan WR, Oberman A, Grimm R, Rosati RA. Chronic congestive heart failure in coronary artery disease: clinical criteria. Ann Intern Med 1977;86:133-8.
26. Chakko S, Woska D, Martinez H, et al. Clinical, radiographic, and hemodynamic correlations in chronic congestive heart failure: conflicting results may lead to inappropriate care. Am J Med 1991;90:353-9.
27. Mattleman SJ, Hakki A, Iskandrian AS, Segal BL, Kane SA. Reliability of bedside evaluation in determining left ventricular function: correlation with left ventricular ejection fraction determined by radionuclide ventriculography. J Am Coll Cardio 1983;1:417-20.
28. Morgan S, Smith H, Simpson I, et al. Prevalence and clinical characteristics of left ventricular dysfunction among elderly patients in general practice setting: cross sectional survey. BMJ 1999;318:368-72.
29. Davie AP, Francis CM, Love MP, et al. Value of electrocardiogram in identifying heart failure due to left ventricular systolic dysfunction. BMJ 1996;312:222-3.
30. Talreja D, Gruver C, Sklenar J, Dent J, Kaul S. Efficient utilization of echocardiography for the assessment of left ventricular systolic function. Am Heart J 2000;139:394-8.
31. Murkofsky RL, Dangas G, Diamond JA, et al. A prolonged QRS duration on surface electrocardiogram is a specific indicator of left ventricular dysfunction. J Am Coll Cardiol 1998;32:476-82.
32. Mosterd A, de Bruijne MC, Hoes AW, et al. Usefulness of echocardiography in detecting left ventricular dysfunction in population-based studies (The Rotterdam Study). Am J Cardiol 1997;79:103-4.
33. Clark AL, Coats ALS. Unreliability of cardiothoracic ratio as a marker of left ventricular impairment: comparison with radionuclide ventriculography and echocardiography. Postgrad Med J 2000;76:289-91.
34. Badgett RG, Mulrow CD, Otto PM, Ramirez G. How well can the chest radiograph diagnose left ventricular dysfunction? J Gen Intern Med 1996;11:625-34.
35. Erbel R, Schweizer P, Drebs W, Meyer J, Effert S. Sensitivity and specificity of two-dimensional echocardiography in detection of impaired left ventricular function. Eur Heart J 1984;5:477-89.
36. Davis M, Espiner E, Richards G, et al. Plasma brain natriuretic peptide in assessment of acute dyspnoea. Lancet 1994;343:440-4.
37. Maisel AS, Koon J, Krishnaswamy P, et al. Utility of B-natriuretic peptide as a rapid, point-of-care test for screening patients undergoing echocardiography to determine left ventricular dysfunction. Am Heart J 2001;141:367-74.
38. Schirmer H, Omland T. Circulating N-terminal pro-atrial natriuretic peptide is an independent predictor of left ventricular hypertrophy in the general population. The Tromsø Study. Eur Heart J 1999;20:755-63.
39. Lerman A, Gibbons RJ, Rodeheffer RJ, et al. Circulating N-terminal atrial natriuretic peptide as a marker for symptomless left-ventricular dysfunction. Lancet 1993;341:1105-9.
40. McClure SJ, Davie AP, Goldthorp S, et al. Cohort study of plasma natriuretic peptides for identifying left ventricular systolic dysfunction in primary care. BMJ 1998;317:516-9.
41. McDonagh TA, Robb SD, Murdoch DR, et al. Biochemical detection of left-ventricular systolic dysfunction. Lancet 1998;351:9-13,
42. Talwar S, Squire IB, Davies JE, et al. Plasma N-terminal pro-brain natriuretic peptide and the ECG in the assessment of left-ventricular systolic dysfunction in a high risk population. Eur Heart J 1999;20:1736-1744.
43. Lindsay MM, Goodfield NER, Hogg KJ, Dunn EG. Optimising direct access echo referral in suspected heart failure. Scot Med J 2000;45:043-044.
44. Dao Q, Krishnaswamy P, Kazanegra R, et al. Utility of B-Type natriuretic peptide in the diagnosis of congestive heart failure in an urgent care setting. J Am Coll Cardiol 2001;37:379-85.
1. Mair FS, Crowley TS, Bundred PE. Prevalence, aetiology and management of heart failure in general practice. Br J Gen Pract 1996;46:77-9.
2. Wheeldon NM, MacDonald TM, Flucker CJ, McDermitt DG, Struthers AD. An electrocardiographic study of chronic heart failure in the community. QJM 1993;86:17-23.
3. O’Connell JB, Bristow MR. Economic impact of heart failure in the United States: time for a different approach. J Heart Lung Transplan 1994;13:S107-112.
4. O’Connell JB. The economic burden of heart failure. Clin Cardiol 2000;23 (3 Suppl):III6-10.
5. English MA, Mastrean MB. Congestive heart failure: public and private burden. Crit Care Nurs Q 1995;18:1-6.
6. Croft JB, Giles WH, Pollard RA, Keenan NL, Casper ML, Anda RF. Heart failure survival among older adults in the United States: a poor prognosis for an emerging epidemic in the Medicare population. Arch Intern Med 1999;159:505-10.
7. Stewart S, MacIntyre K, Hole DJ, Capewell S, McMurray JJV. More ‘malignant’ than cancer? Five-year survival following a first admission for heart failure. Eur J Heart Fail 2001;3:315-22.
8. Konstam M, Dracup K, Baker D, et al. Heart failure: evaluation and care of patients with left-ventricular systolic dysfunction. Clinical Practice Guideline No. 11. AHCPR Publication No. 94-0612. Agency for Health Care Policy and Research, Public Health Service, US Department of Health and Human Services, Rockville, Md.
9. Guidelines for the evaluation and management of heart failure. Report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee on Evaluation and Management of Heart Failure). Circulation 1995;92:2764-84.
10. The Task Force on Heart Failure of the European Society of Cardiology. Guidelines for the diagnosis of heart failure. Eur Heart J 1995;16:741-51.
11. Heart failure —systolic dysfunction. University of Michigan Health System. August 1999. (Available at http://cme.med.umich.edu/pdf/guideline/heart.pdf)
12. The Task Force of the Working Group on Heart Failure of the European Society of Cardiolog. The treatment of heart failure. Eur Heart J 1997;18:736-53.
13. Diagnosis and treatment of heart failure due to left ventricular systolic dysfunction. A national clinical guideline. Scottish Intercollegiate Guidelines Network (SIGN); SIGN publication; no. 35. Feb 1999; 68.
14. Anonymous. Heart Failure Society of America (HFSA) practice guidelines. HFSA guidelines for management of patients with heart failure caused by left ventricular systolic dysfunction—pharmacological approaches [published erratum appears in J Card Fail 2000 Mar; 6:74]. J Card Fail 5:357-82.
15. McMurray JJV, Dargie HJ. Diagnosis and management of heart failure. BMJ 1994;308:321-8.
16. Mair FS. Management of heart failure. Am Fam Phys 1996;54:245-54.
17. Marantz PR, Tobin JN, Wassertheil-Smoller S, et al. The relationship between left ventricular systolic function and congestive heart failure diagnosed by clinical criteria. Circulation 1988;77:607-12.
18. Gillespie ND, McNeill G, Pringle T, Ogston S, Struthers AD, Pringle SD. Cross sectional study of contribution of clinical assessment and simple cardiac investigations to diagnosis of left ventricular systolic dysfunction in patients admitted with acute dyspnoea. BMJ 1997;314:936-40.
19. Remes J, Miettinen H, Reunanen A, Pyorala K. Validity of clinical diagnosis of heart failure in primary health care. Eur Heart J 1991;12:315-21.
20. Davie AP, Francis CM, Caruana L, Sutherland GR, McMurray JJV. Assessing diagnosis in heart failure: which features are of any use? QJM 1997;90:335-9.
21. Gadsboll N, Hoilund-Carlsen PF, Nielsen GG, et al. Symptoms and signs of heart failure in patients with myocardial infarction: reproducibility and relationship to chest X-ray, radionuclide ventriculog-raphy, and right heart catheterisation. Eur Heart J 1989;10:1017-28.
22. Stevenson LW, Perloff JK. The limited reliability of physical signs for estimating hemodynamics in chronic heart failure. JAMA 1989;261:884-8.
23. Ishmail AA, Wing S, Ferguson J, Hutchinson TA, Magder S, Flegel KM. Interobserver agreement by auscultation in the presence of a third heart sound in patients with congestive heart failure. Chest 1987;91:870-3.
24. Rihal CS, Davis KB, Kennedy W, Gersh BJ. The utility of clinical, electrocardiographic and roentgenographic variables in the prediction of left ventricular function. Am J Cardiol 1995;752:220-3.
25. Harlan WR, Oberman A, Grimm R, Rosati RA. Chronic congestive heart failure in coronary artery disease: clinical criteria. Ann Intern Med 1977;86:133-8.
26. Chakko S, Woska D, Martinez H, et al. Clinical, radiographic, and hemodynamic correlations in chronic congestive heart failure: conflicting results may lead to inappropriate care. Am J Med 1991;90:353-9.
27. Mattleman SJ, Hakki A, Iskandrian AS, Segal BL, Kane SA. Reliability of bedside evaluation in determining left ventricular function: correlation with left ventricular ejection fraction determined by radionuclide ventriculography. J Am Coll Cardio 1983;1:417-20.
28. Morgan S, Smith H, Simpson I, et al. Prevalence and clinical characteristics of left ventricular dysfunction among elderly patients in general practice setting: cross sectional survey. BMJ 1999;318:368-72.
29. Davie AP, Francis CM, Love MP, et al. Value of electrocardiogram in identifying heart failure due to left ventricular systolic dysfunction. BMJ 1996;312:222-3.
30. Talreja D, Gruver C, Sklenar J, Dent J, Kaul S. Efficient utilization of echocardiography for the assessment of left ventricular systolic function. Am Heart J 2000;139:394-8.
31. Murkofsky RL, Dangas G, Diamond JA, et al. A prolonged QRS duration on surface electrocardiogram is a specific indicator of left ventricular dysfunction. J Am Coll Cardiol 1998;32:476-82.
32. Mosterd A, de Bruijne MC, Hoes AW, et al. Usefulness of echocardiography in detecting left ventricular dysfunction in population-based studies (The Rotterdam Study). Am J Cardiol 1997;79:103-4.
33. Clark AL, Coats ALS. Unreliability of cardiothoracic ratio as a marker of left ventricular impairment: comparison with radionuclide ventriculography and echocardiography. Postgrad Med J 2000;76:289-91.
34. Badgett RG, Mulrow CD, Otto PM, Ramirez G. How well can the chest radiograph diagnose left ventricular dysfunction? J Gen Intern Med 1996;11:625-34.
35. Erbel R, Schweizer P, Drebs W, Meyer J, Effert S. Sensitivity and specificity of two-dimensional echocardiography in detection of impaired left ventricular function. Eur Heart J 1984;5:477-89.
36. Davis M, Espiner E, Richards G, et al. Plasma brain natriuretic peptide in assessment of acute dyspnoea. Lancet 1994;343:440-4.
37. Maisel AS, Koon J, Krishnaswamy P, et al. Utility of B-natriuretic peptide as a rapid, point-of-care test for screening patients undergoing echocardiography to determine left ventricular dysfunction. Am Heart J 2001;141:367-74.
38. Schirmer H, Omland T. Circulating N-terminal pro-atrial natriuretic peptide is an independent predictor of left ventricular hypertrophy in the general population. The Tromsø Study. Eur Heart J 1999;20:755-63.
39. Lerman A, Gibbons RJ, Rodeheffer RJ, et al. Circulating N-terminal atrial natriuretic peptide as a marker for symptomless left-ventricular dysfunction. Lancet 1993;341:1105-9.
40. McClure SJ, Davie AP, Goldthorp S, et al. Cohort study of plasma natriuretic peptides for identifying left ventricular systolic dysfunction in primary care. BMJ 1998;317:516-9.
41. McDonagh TA, Robb SD, Murdoch DR, et al. Biochemical detection of left-ventricular systolic dysfunction. Lancet 1998;351:9-13,
42. Talwar S, Squire IB, Davies JE, et al. Plasma N-terminal pro-brain natriuretic peptide and the ECG in the assessment of left-ventricular systolic dysfunction in a high risk population. Eur Heart J 1999;20:1736-1744.
43. Lindsay MM, Goodfield NER, Hogg KJ, Dunn EG. Optimising direct access echo referral in suspected heart failure. Scot Med J 2000;45:043-044.
44. Dao Q, Krishnaswamy P, Kazanegra R, et al. Utility of B-Type natriuretic peptide in the diagnosis of congestive heart failure in an urgent care setting. J Am Coll Cardiol 2001;37:379-85.
Is lansoprazole (Prevacid) or omeprazole (Prilosec) more effective in treating erosive esophagitis?
ABSTRACT
BACKGROUND: While the superiority of proton pump inhibitors (PPIs) over histamine-2 receptor antagonists in symptom control of gastroesophageal reflux disease (GERD) has been well established, limited work has been done comparing the efficacy of different PPIs. Theoretically, differences in pharmacokinetic properties, such as increased bioavailability of lansoprazole, could play a role in efficacy of symptom control. The purpose of this study was to demonstrate a difference between PPIs in GERD symptom control.
POPULATION STUDIED: The patient population for this study consisted of 3510 individuals over age 18 years with endoscopically confirmed erosive esophagitis of grade 2 severity or higher who were gathered through a large multicenter clinical trial. To enter the study, patients had to have experienced at least 1 episode of moderate to very severe heartburn within 3 days before their screening visit. Comparison of treatment groups showed the only significant demographic difference was increased reported tobacco use in the omeprazole group (28%) versus the lansoprazole group (25%).
STUDY DESIGN AND VALIDITY: This study was a double-blind multicenter clinical trial in which participants were randomized to receive either 30 mg lansoprazole or 20 mg omeprazole once daily for 8 weeks. Allocation concealment was not mentioned. Follow-up visits were conducted at the end of weeks 1, 2, and 8 of treatment. Analysis was by intention to treat.
OUTCOMES MEASURED: This study looked primarily at onset and duration of symptom relief and severity as recorded by patients in a diary. Specifically, daytime and nighttime heartburn symptoms were analyzed with regard to percentage of complete heartburn relief as well as average heartburn severity at days 1 to 3 and the end of weeks 1, 2, and 8 of treatment.
RESULTS: The group treated with lansoprazole showed a statistically significant advantage in symptom relief throughout the treatment period. On day 1 of treatment, the lansoprazole group was found to be 33% heartburn free as compared with 25% in the omeprazole group (P < .0001). The number needed to treat (NNT) to see this statistically significant difference was 12.5. Patients receiving lansoprazole versus omeprazole had small but statistically significant decreases in numbers of heartburn-free days (56% vs 49% in first 3 days of treatment, NNT = 14) and nights (NNT = 14) as well as daytime heartburn severity and nighttime severity. The lansoprazoletreated group also showed increased sustained resolution of symptoms over the omeprazole-treated group during the 8-week study period. Overall, however, these differences were extremely small and narrowed as the study progressed to 8 weeks.
Lansoprazole provided a small but sustained advantage over omeprazole in the treatment of heartburn. However, although statistically significant, these differences in efficacy are minor and diminished over the 8-week course of treatment. In deciding to use one PPI over another, clinicians should consider other factors, primarily cost or availability.
ABSTRACT
BACKGROUND: While the superiority of proton pump inhibitors (PPIs) over histamine-2 receptor antagonists in symptom control of gastroesophageal reflux disease (GERD) has been well established, limited work has been done comparing the efficacy of different PPIs. Theoretically, differences in pharmacokinetic properties, such as increased bioavailability of lansoprazole, could play a role in efficacy of symptom control. The purpose of this study was to demonstrate a difference between PPIs in GERD symptom control.
POPULATION STUDIED: The patient population for this study consisted of 3510 individuals over age 18 years with endoscopically confirmed erosive esophagitis of grade 2 severity or higher who were gathered through a large multicenter clinical trial. To enter the study, patients had to have experienced at least 1 episode of moderate to very severe heartburn within 3 days before their screening visit. Comparison of treatment groups showed the only significant demographic difference was increased reported tobacco use in the omeprazole group (28%) versus the lansoprazole group (25%).
STUDY DESIGN AND VALIDITY: This study was a double-blind multicenter clinical trial in which participants were randomized to receive either 30 mg lansoprazole or 20 mg omeprazole once daily for 8 weeks. Allocation concealment was not mentioned. Follow-up visits were conducted at the end of weeks 1, 2, and 8 of treatment. Analysis was by intention to treat.
OUTCOMES MEASURED: This study looked primarily at onset and duration of symptom relief and severity as recorded by patients in a diary. Specifically, daytime and nighttime heartburn symptoms were analyzed with regard to percentage of complete heartburn relief as well as average heartburn severity at days 1 to 3 and the end of weeks 1, 2, and 8 of treatment.
RESULTS: The group treated with lansoprazole showed a statistically significant advantage in symptom relief throughout the treatment period. On day 1 of treatment, the lansoprazole group was found to be 33% heartburn free as compared with 25% in the omeprazole group (P < .0001). The number needed to treat (NNT) to see this statistically significant difference was 12.5. Patients receiving lansoprazole versus omeprazole had small but statistically significant decreases in numbers of heartburn-free days (56% vs 49% in first 3 days of treatment, NNT = 14) and nights (NNT = 14) as well as daytime heartburn severity and nighttime severity. The lansoprazoletreated group also showed increased sustained resolution of symptoms over the omeprazole-treated group during the 8-week study period. Overall, however, these differences were extremely small and narrowed as the study progressed to 8 weeks.
Lansoprazole provided a small but sustained advantage over omeprazole in the treatment of heartburn. However, although statistically significant, these differences in efficacy are minor and diminished over the 8-week course of treatment. In deciding to use one PPI over another, clinicians should consider other factors, primarily cost or availability.
ABSTRACT
BACKGROUND: While the superiority of proton pump inhibitors (PPIs) over histamine-2 receptor antagonists in symptom control of gastroesophageal reflux disease (GERD) has been well established, limited work has been done comparing the efficacy of different PPIs. Theoretically, differences in pharmacokinetic properties, such as increased bioavailability of lansoprazole, could play a role in efficacy of symptom control. The purpose of this study was to demonstrate a difference between PPIs in GERD symptom control.
POPULATION STUDIED: The patient population for this study consisted of 3510 individuals over age 18 years with endoscopically confirmed erosive esophagitis of grade 2 severity or higher who were gathered through a large multicenter clinical trial. To enter the study, patients had to have experienced at least 1 episode of moderate to very severe heartburn within 3 days before their screening visit. Comparison of treatment groups showed the only significant demographic difference was increased reported tobacco use in the omeprazole group (28%) versus the lansoprazole group (25%).
STUDY DESIGN AND VALIDITY: This study was a double-blind multicenter clinical trial in which participants were randomized to receive either 30 mg lansoprazole or 20 mg omeprazole once daily for 8 weeks. Allocation concealment was not mentioned. Follow-up visits were conducted at the end of weeks 1, 2, and 8 of treatment. Analysis was by intention to treat.
OUTCOMES MEASURED: This study looked primarily at onset and duration of symptom relief and severity as recorded by patients in a diary. Specifically, daytime and nighttime heartburn symptoms were analyzed with regard to percentage of complete heartburn relief as well as average heartburn severity at days 1 to 3 and the end of weeks 1, 2, and 8 of treatment.
RESULTS: The group treated with lansoprazole showed a statistically significant advantage in symptom relief throughout the treatment period. On day 1 of treatment, the lansoprazole group was found to be 33% heartburn free as compared with 25% in the omeprazole group (P < .0001). The number needed to treat (NNT) to see this statistically significant difference was 12.5. Patients receiving lansoprazole versus omeprazole had small but statistically significant decreases in numbers of heartburn-free days (56% vs 49% in first 3 days of treatment, NNT = 14) and nights (NNT = 14) as well as daytime heartburn severity and nighttime severity. The lansoprazoletreated group also showed increased sustained resolution of symptoms over the omeprazole-treated group during the 8-week study period. Overall, however, these differences were extremely small and narrowed as the study progressed to 8 weeks.
Lansoprazole provided a small but sustained advantage over omeprazole in the treatment of heartburn. However, although statistically significant, these differences in efficacy are minor and diminished over the 8-week course of treatment. In deciding to use one PPI over another, clinicians should consider other factors, primarily cost or availability.
Treatment of Hyperlipidemia
- The new NCEP III provides revised guidelines for the treatment of hyperlipidemia.
- Combining traditional risk factor assessment with the calculated 10-year risk of coronary artery disease allows for optimal patient-centered counseling.
- Statins are normally the first-line therapy for hyperlipidemia.
In 1995 and 1996, US adults made more than 18 million office visits for the evaluation and treatment of hyperlipidemia, including 3.4% of all visits to family physicians. Among visits to family physicians, 4.1% included measurement of cholesterol levels.1 Overall, mean cholesterol levels decreased from 220 in 1960–1962 to 203 in 1988–1994. During the same time period, the proportion of adults with elevated total cholesterol levels (> 240) decreased from 32% to 19%.2 Despite this progress, the availability of more effective drugs, guidelines advocating increasingly aggressive treatment, and populationwide goals established in Healthy People 2010 will continue to increase the number of patients seen by family physicians for screening, diagnosis, and treatment of hyperlipidemia.3
When to treat
The National Cholesterol Education Program (NCEP), a program within the National Institute of Health’s Heart, Lung, and Blood Institute, published a guideline in 1993 for screening and treating hyperlipidemia. Physicians have since become familiar with the NCEP concept of basing treatment decisions on assessment of patient risk factors (smoking, age, diabetes, hypertension, family history of early coronary artery disease [CAD]) and application of algorithms linked to desired low-density lipoprotein (LDL) cholesterol levels. The advantage of this strategy is its simplicity. Physicians assess whether the NCEP risk factors are present and then work with their patients to achieve the desired LDL level through lifestyle modification, drug therapy, or both.
Unfortunately, the NCEP guideline did not assess the individual’s actual risk of CAD. In its recently released Third Report, the NCEP has recognized the value of this strategy by incorporating the Framingham tables to calculate the 10-year risk of developing clinical CAD based on a patient’s individual risk factors, including cholesterol levels (Table 1).4 This new NCEP III guideline recommends traditional risk factor counting coupled, in certain situations, with the 10-year risk derived from the Framingham scoring system.
TABLE 1
FRAMINGHAM TABLES FOR CALCULATING CORONARY ARTERY DISEASE RISK
Therapy is based on the individual patient’s risk category and LDL levels (Figure). Patients whose 10-year risk is greater than 20% or those who have CAD-equivalent conditions (ie, diabetes, peripheral arterial disease, abdominal aortic aneurysm, symptomatic carotid artery disease) are considered to have a risk equivalent to that of patients with known CAD; all have an LDL goal of 100 or less.
For those with a 10-year CAD risk less than 20%, the number of positive risk factors determines the LDL goal. This new method allows physicians to communicate with their patients more clearly about individual risk and enhances shared decision making. While the NCEP III report is based on extensive literature review, the recommendations of its expert panel are not characterized according to the strength of the supporting evidence, as is done by the US Preventive Services Task Force.
Figure
TREATMENT STRATEGY BASED ON LDL LEVEL AND RISK CATEGORY
Explaining treatment benefits
The NCEP III report does not make explicit the effect of the treatment on the patient; that is, how much the proposed treatment will reduce the risk of CAD. This determination depends in part on whether the patient being treated has known CAD or a CAD-equivalent condition (secondary prevention) versus no known CAD (primary prevention). The benefits of treatment have been most clearly quantified for drug treatment and are most easily evaluated using the number needed to treat (NNT). The NNT refers to the number of patients who would have to be treated for 5 years to prevent 1 CAD event. Physicians may use the NNTs to assist patients in determining their preferences for treatment, bearing in mind that the NNT refers to an outcome for a population, such as men with high cholesterol levels. For a given individual, their risk of an adverse outcome is all or none. Nonetheless, patients may find the NNT a useful way to assess their personal values in making treatment decisions.
Treatment
Lifestyle modification
Diet modification is the cornerstone of therapy for mild to moderate hyperlipidemia. Modifying the diet is also recommended along with pharmacologic therapy in people at higher risk of CAD. NCEP III recommends a diet for “therapeutic lifestyle changes” that includes < 200 mg cholesterol per day, < 7% saturated fat, 25% to 35% total fat, 50% to 60% carbohydrates, and 15% protein of total calories.4
Although diet therapy has shown a modest redution in total cholesterol in clinical trials, no clear evidence shows that a diet low in saturated fat and cholesterol will reduce cardiovascular morbidity and mortality.5,6 Many people find it difficult to change their dietary habits and to maintain healthier ones. Systematic reviews of observational studies have found that increased consumption of fruits and vegetables is associated with lower incidence of heart attack and stroke. However, the potential for bias and confounding factors in such studies makes them less convincing than randomized controlled trials (RCTs).5
The Ornish program, in which CAD or CAD-equivalent patients pursue intense lifestyle modification for up to 3 years, has shown that revascularization procedures can be avoided. Treatment groups that ate a very-low-fat diet, received intervention on stress management, and followed a prescribed exercise program showed similar improvement in angina symptoms versus the revascularization group. Another trial showed regression of atherosclerotic plaque on angiograms.10-12
Other nonpharmacologic options include plant stanols (2 grams/day) and soluble fiber (10 to 25 grams/day) to reduce LDL-cholesterol. Plant stanols have a structure similar to that of cholesterol and interfere with cholesterol absorption when eaten along with a typical diet, resulting in reduction of blood cholesterol levels. Plant stanols and sterols can be found in certain margarines and salad oils and can be taken with each meal as substitutes for other sources of dietary fat.7-9 No RCTs have shown that these substances reduce cardiovascular events or overall mortality.
Herbal products and dietary supplements
A survey found that as many as 50% of respondents with elevated cholesterol levels would prefer an over-the-counter product such as garlic, yeast, or soy products.13 Studies of products promoted for lipid-lowering effects were found to have a modest effect on lipid levels13-18 (Table 2); however, no RCTs were found that assessed patient-oriented outcomes. Because herbal products and supplements have modest effects on lipid levels and because long-term safety data are lacking, such products should be used with caution for treatment of hyperlipidemia.
TABLE 2
PHARMACOLOGIC AND NONPHARMACOLOGIC INTERVENTIONS
| Strength of Recommendation* | Treatment | Type of Benefit | Cost Per Month ($) | Comments |
|---|---|---|---|---|
| A | Statins | OM, CVM | 40–110 | Well tolerated |
| B | Fibric acids | CVE | 60–70 | All male subjects in both primary and secondary trials |
| B | Niacin | CVE | 10–80 | Watch for adverse reactions (flushing, elevated glucose, liver function tests) |
| B | Bile acid resin | CVE | 40–60 | Ideal agent for patients with severe liver disease; watch for drug interactions |
| B | Lifestyle modification | Lipid | Varies | No strong evidence from randomized clinical trials on primary prevention of major coronary events or mortality |
| B | Soy products | Lipid | 20 | FDA has approved labeling soy products for cholesterol reduction |
| B | Red yeast | Lipid | 20–30 | Active ingredient is lovastatin; should be treated as lovastatin |
| B | Plant stanols | Lipid | 20–30 | Substitute for other source of fat calories; must be taken with each meal |
| C | Fish oils | Lipid | 5–10 | Use with caution because of high caloric value and cholesterol content in products; may increase cholesterol level with long-term use |
| C | Garlic | Lipid | 10–20 | Conflicting results with clinical trials |
| C | Green tea | Lipid | 15 | Epidemiologic study data |
| * Criteria correspond to US Preventive Services Task Force categories (A = strong evidence to support recommendation, B = fair evidence to support recommendation, C = insufficient evidence to recommend for or against). CVE denotes reduction in cardiovascular events; CVM, reduction in cardiovascular mortality; lipid, reduction in lipid levels only; OM, reduction in overall mortality. | ||||
Pharmacologic treatment
Clinical trials of hyperlipidemia therapy should address outcomes that matter most to patients, such as morbidity, mortality, quality of life, and cost, rather than stressing disease-oriented evidence, such as the ability to reduce cholesterol levels. For this review we identified major long-term RCTs that included significant coronary events or mortality as the primary outcomes. Table 3 summarizes the results of primary and secondary prevention studies.
TABLE 3
PHARMACOLOGIC INTERVENTION
| Reduction in Risk | ||||
|---|---|---|---|---|
| Intervention | Major Coronary Events | All-Cause Mortality | Comments | |
| ARR (%) | NNT | NNT | ||
| Primary Prevention | ||||
| Statins | 2.0–2.3 | 44–49 | NS | Studies on normal and hypercholesterolemic patients. Mean age was 47–58 years; all patients were men except for 1 statin study that included a small number of women |
| Gemfibrozil | 1.4 | 71 | NS | |
| Cholestyramine | 1.7 | 59 | NS | |
| Secondary Prevention | ||||
| Statins | 3–3.6 | 28–33 | 24–28 | Mean age was 55–64 years. Participants were male except for the 3 statin studies and the benzafibrate study that enrolled a small number of women. Cholesterol eligibility criteria varied among the studies and included patients with normal or elevated total and LDL levels or low HDL levels |
| Gemfibrozil | 4.4 | 23 | NS | |
| Benzafibrate | 1.4 | 71 | NS | |
| Niacin | 6.2 | 17 | NS | |
| ARR denotes absolute risk reduction in percent; NNT, number of needed to treat for 5 years to prevent 1 adverse outcome; NS, not significant. | ||||
Primary prevention
Primary prevention studies have investigated the treatment of middle-aged men with hyperlipidemia and of men and women with average cholesterol levels.19-23 Results showed similar positive outcomes on reducing coronary events in all groups (Table 3). A systematic review and a meta-analysis of primary prevention studies also demonstrated that drug therapy reduced cholesterol levels and resulted in statistically significant lowering of cardiovascular events in the treated group compared with placebo without any significant reduction in overall mortality.5,24 Absolute risk reductions ranged from 1.4% to 2.3%. In other words, the number of patients that would have to be treated for 5 years to prevent a single major coronary event was 44 to 49 for the statins, 71 for gemfibrozil, and 59 for cholestyramine.
Secondary prevention
In secondary prevention trials, RCTs have demonstrated a strong, consistent relationship between cholesterol lowering and the reduction of risk for a coronary event Table 3.25-30 Patients with preexisting CAD and elevated or average lipid levels benefit from medical therapy. The relative risk of cardiovascular events was reduced by an average of 30% in the active treatment groups.
In these trials, the NNT for 5 years to prevent 1 coronary heart event or nonfatal myocardial infarction (MI) was 28 to 33 for statins, 23 for gemfibrozil, 71 for bezafibrate, and 17 for niacin. There was also a significant risk reduction for all-cause mortality in the statin trials.27,28 These data support the recommendations from NCEP III to treat patients with preexisting CAD aggressively. People with diabetes should receive similar treatment because they are more prone to the development of new CAD within 10 years.4 In addition, subgroup analyses of diabetics treated with statins in primary prevention trials demonstrated a decreased risk of cardiovascular events.26,29
While cholesterol-modifying agents include 4 different classes—statins, fibric acid derivatives, bile acid resins, and nicotinic acid—studies cited in this paper predominantly involved statins and fibric acids. In systematic reviews of both primary and secondary prevention trials, statins were the most effective agents for both cholesterol lowering and cardiovascular risk reduction.5 We found no RCTs that directly compared outcomes between cholesterol-lowering medications. Although women represented a small number of participants in these trials, a meta-analysis showed that statin therapy decreased their risk of heart disease, with an NNT of 31 for reduction of major coronary events.31 No evidence was found to support the effectiveness of hyperlipidemia therapy for people aged more than 75 years. For people aged 65 to 75 years, there is evidence to support drug therapy for secondary prevention but not for primary prevention.
Statins are well tolerated; the most common adverse reactions are gastrointestinal related and occur in approximately 3% of patients. The more serious but uncommon events associated with the use of statins are hepatitis and myopathy. Asymptomatic increases in hepatic transaminases to more than 3 times the upper normal limit occur in approximately 1% of patients.32 Therapy can be discontinued for 1 to 2 weeks; enzyme levels should return to normal if the elevations are medication related. It is not necessary to stop therapy when enzymes are elevated at less than 3 times the upper normal limit.
General guidelines on liver monitoring call for performing a baseline liver function test and repeating it 6 weeks later.33 Once a stable dose has been established, the manufacturer recommends periodic testing; however, no clear evidence supports a specific interval. Clinicians may choose to individualize decisions on testing frequency based on factors such as potential drug interactions (statins with fibric acids or niacin) or the presence of conditions that increase the risk of liver disease.34
Myopathy, defined as generalized muscle aches and pain with a serum creatine kinase level greater than 1000 U/L, occurs rarely (< 0.1%) but may be more likely to occur when statins are used concomitantly with medications such as fibric acid, antifungals, erythromycin, and cyclosporine.31,35 The best preventive strategy is to educate patients about early recognition of the signs and symptoms of myopathy. Because most statins are metabolized by the cytochrome P450-3A4, any medications that inhibit this enzyme can increase statin serum levels and increase the risk of hepatotoxicity and myopathy.
The NCEP III recommends the use of statins as firstline therapy. A standard dose of a statin decreases LDL levels by 20% to 50%, increases HDL levels by 5% to 10%, and reduces triglyceride levels by 10% to 20%. Atorvastatin and simvastatin can produce the highest reductions in LDL levels: up to 50%. Only pravastatin, simvastatin, and lovastatin have been involved in longterm RCTs of primary and secondary prevention. Atorvastatin had positive benefits in a short-term secondary prevention trial.37 Unfortunately, the only head-to-head comparisons of statins have looked at disease-oriented outcomes such as lipid levels.37 Statins are patient friendly. They require a daily evening dose because cholesterol synthesis is more active during the night. Atorvastatin can be given at any time of day because of its long half-life.
Gemfibrozil, a fibric acid, is often used to treat hypertriglyceridemia and as an adjunctive agent to statin therapy. It decreases triglycerides by 40% to 50% but has minimal effects on the rest of the lipid panel. Adverse effects are generally mild. Liver function monitoring is recommended. The usual dosage regimen for fibric acids is 2 times a day and should be adjusted for renal function.
Niacin can increase HDL by 30% and decrease triglycerides by 30% and LDL by 20%. Major adverse reactions include flushing, gastrointestinal symptoms, elevation of liver function tests, uric acid, and serum glucose levels. The new longer-acting formulation has been associated with less flushing. Another class, the bile acid resins, including cholestyramine and colestipol, may play an adjunctive role in therapy. Their effect on the lipid panel is mild compared with those of the other class and they can increase triglyceride levels. Many patients find the gritty taste of the granular formulation unpalatable. The bile acid resins have a favorable safety profile. Most adverse events occur locally in the gut.
Conclusions
The emergence of statins as a safe and effective, although costly, therapy for hyperlipidemia and the development of clinical guidelines advocating their increased use will place family physicians under added pressure to screen for and treat hyperlipidemia. While the general value of lifestyle changes is recognized in national recommendations, more effective ways for physicians to implement them successfully in ambulatory settings are needed.
An optimal evidence-based approach to hyperlipidemia uses the new NCEP III guideline, which combines traditional risk factor assessment with assessment for CAD using the Framingham tables to determine LDL goals and appropriate treatment modalities. Statins are first-line agents for patients who are candidates for drug therapy. Discussions between clinicians and patients of the NNTs for primary and secondary prevention will help foster patient-centered discussions on the role of medical, economic, and quality-of-life issues in the decision-making process.
1. Schappert SM, Nelson C. National Ambulatory Medical Care Survey: 1995–1996 summary. National Center for Health Statistics. Vital Health Stat 1999;13(142).-
2. National Center for Health Statistics. Health, United States, 1999, with health and aging chartbook. Hyattsville, Md: 1999.
3. US Department of Health and Human Services. Tracking Healthy People 2010. Washington, DC: US Government Printing Office. November 2000. Available at: http://www.cdc.gov/hchs/hphome.htm.
4. Third report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III). Executive Summary. Available at: http://www.nhlbi.nih.gov/guidelines/cholesterol/atp3xsum.pdf Accessed April 16, 2001.
5. Clinical evidence London, England: BMJ Publishing Group; June 2001. Available at: www.clinicalevidence.org.
6. Henkin Y, Shai I, Zuk R, et al. Dietary treatment of hyperlipidemia: Do dietitians do it better? A randomized, controlled trial. Am J Med 2000;109:549-55.
7. Ornish D. Avoiding revascularization with lifestyle changes: the multicenter lifestyle demonstration project. Am J Cardiol 1998;82:72T-76T.
8. Ornish D, Scherwitz LW, Billings JH, et al. Intensive lifestyle changes for reversal of coronary heart disease. JAMA 1998;280:2001-7.
9. Gould KL, Ornish D, Scherwitz L, et al. Changes in myocardial perfusion abnormalities by positron emission tomography after long-term, intense risk factor modification. JAMA 1995;274:894-901.
10. Mensink RP, Plat J. Efficacy of dietary plant stanols. In: New developments in the dietary management of high cholesterol. New York: McGraw-Hill; 1998;27-31.
11. Blair SN, Capuzzi DM, Gottlieb SO, et al. Incremental reduction of serum total cholesterol and LDL with the addition of plant stanol ester-containing spread to statin therapy. Am J Cardiol 2000;86:46-52.
12. Miettinen TA, Puska P, Gylling H, et al. Reduction of serum cholesterol with sitostanol-ester margarine in a mildly hypercholesteremic population. N Engl J Med 1995;333:1308-12.
13. Caron MF, White CM. Evaluation of the antihyperlipidemic properties of dietary supplements. Pharmacotherapy 2001;21:481-7.
14. Harris WS. Nonpharmacologic treatment of hypertriglyceridemia: focus on fish oils. Clin Cardiol 1999;22(suppl 2):II40-3.
15. Stevinson C, Pittler MH, Ernst E. Garlic for treating hyperlipidemia. Ann Intern Med 2000;133:420-9.
16. EBM Reviews. Database of abstracts of reviews of effectiveness [database online]. Psyllium-enriched cereals lower blood total cholesterol and LDL cholesterol, but not HDL cholesterol, in hypercholesterolemic adults: results of a meta-analysis. July 2001; v1, accession no. 00125498-100000000-00737. Available at: http://www.ovid.com/products/databases. Accessed Oct. 29, 2001.
17. EBM Reviews. ACP Journal Club [database online]. Soy protein intake decreases total and LDL cholesterol and triglyceride levels. March/April 1996; 124:41, accession no. 00021607-199603000-00013. Available at: http://www.ovid.com/products/databases. Accessed April 4, 2001.
18. Jellin JM, Batz F, Hitchens K. Natural Medicines Comprehensive Database, 3rd ed. Stockton, Calif: Therapeutic Research Faculty; 2000.
19. Frick MH, Elo O, Haapa K, et al. Helsinki heart study: primary-prevention trial with gemfibrozil in middle-aged men with dyslipidemia. N Engl J Med 1987;317:1237-45.
20. The lipid research clinics coronary primary prevention trial results I. Reduction in incidence of coronary heart disease. JAMA 1984;251:351-64.
21. The lipid research clinics coronary primary prevention trial results II. The relationship of reduction in incidence of coronary heart disease to cholesterol lowering. JAMA 1984;251:365-74.
22. Shepherd J, Cobbe SM, Ford I, et al. Prevention of coronary heart disease with pravastatin in men with hyperlipidemia. N Engl J Med 1995;333:1301-6.
23. Downs JR, Clearfield M, Weis S, et al. Primary prevention of acute coronary events with lovastatin in men and women with average cholesterol levels. JAMA 1998;279:1615-22.
24. Pignone M, Phillips C, Mulrow C. Use of lipid lowering drugs for primary prevention of coronary heart disease: meta-analysis of randomized trials. BMJ 2000;321:983-5.
25. Secondary prevention by raising HDL cholesterol and reducing triglycerides in patients with coronary artery disease: the Bezafibrate Infarction Prevention (BIP) Study. Circulation 2000;102:21-7.
26. Rubins HB, Robins SJ, Collins D, et al. Gemfibrozil for the secondary prevention of coronary heart disease in men with low levels of HDL-cholesterol. N Engl J Med 1999;341:410-8.
27. Randomised trial of cholesterol lowering in 4444 patients with coronary heart disease: the Scandinavian simvastatin survival study (4S). Lancet 1994;344:1383-9.
28. Preventio of cardiovascular events and death with pravastatin in patients with coronary heart disease and a broad range of initial cholesterol levels. The Long-Term Intervention with Pravastatin in Ischaemic Disease. N Engl J Med 1998;339:1349-57.
29. Sacks FM, Pfeffer MA, Moye LA, et al. The effect of pravastatin on coronary events after myocardial infarction in patients with average cholesterol levels. N Engl J Med 1996;335:1001-9.
30. Canner PL, Berge KG, Wenger NK, et al. Fifteen year mortality in coronary drug project patients: long-term benefit with niacin. J Am Coll Cardiol 1986;8:1245-55.
31. LaRosa JC, He J, Vupputuri S. Effect of statins on risk of coronary disease; a meta-analysis of randomized controlled trials. JAMA 1999;24:2340-6.
32. Hsu I, Spinler SA, Johnson N. Comparative evaluation of the safety and efficacy of HMG-CoA reductase inhibitor monotherapy in the treatment of primary hyperlipidemia. Ann Pharmacother 1995;29:743-59.
33. Tice SA, Parry D. Medications that require hepatic monitoring. Hosp Pharm 2001;36:456-64.
34. Weismantel D. What lab monitoring is appropriate to detect adverse drug reactions in patients on cholesterol-lowering agents? J Fam Pract 2001;50:927.-
35. American College of Clinical Pharmacy. PSAP: pharmacotherapy self-assessment program, 4th ed. Kansas City, Mo: ACCP; 2001;66-7.
36. Schwartz GG, Olsson AG, Ezekowitz MD, et al. Effects of atorvastatin on early recurrent ischemic events in acute coronary syndromes. JAMA 2001;285:1711-8.
37. Jones P, Kafonek S, Laurora I, et al. Comparative dose efficacy study of atorvastatin vs. simvastatin, pravastatin, lovastatin, and fluvastatin in patients with hyperlipidemia (the CURVES study). Am J Cardiol 1998;81:582-7.
- The new NCEP III provides revised guidelines for the treatment of hyperlipidemia.
- Combining traditional risk factor assessment with the calculated 10-year risk of coronary artery disease allows for optimal patient-centered counseling.
- Statins are normally the first-line therapy for hyperlipidemia.
In 1995 and 1996, US adults made more than 18 million office visits for the evaluation and treatment of hyperlipidemia, including 3.4% of all visits to family physicians. Among visits to family physicians, 4.1% included measurement of cholesterol levels.1 Overall, mean cholesterol levels decreased from 220 in 1960–1962 to 203 in 1988–1994. During the same time period, the proportion of adults with elevated total cholesterol levels (> 240) decreased from 32% to 19%.2 Despite this progress, the availability of more effective drugs, guidelines advocating increasingly aggressive treatment, and populationwide goals established in Healthy People 2010 will continue to increase the number of patients seen by family physicians for screening, diagnosis, and treatment of hyperlipidemia.3
When to treat
The National Cholesterol Education Program (NCEP), a program within the National Institute of Health’s Heart, Lung, and Blood Institute, published a guideline in 1993 for screening and treating hyperlipidemia. Physicians have since become familiar with the NCEP concept of basing treatment decisions on assessment of patient risk factors (smoking, age, diabetes, hypertension, family history of early coronary artery disease [CAD]) and application of algorithms linked to desired low-density lipoprotein (LDL) cholesterol levels. The advantage of this strategy is its simplicity. Physicians assess whether the NCEP risk factors are present and then work with their patients to achieve the desired LDL level through lifestyle modification, drug therapy, or both.
Unfortunately, the NCEP guideline did not assess the individual’s actual risk of CAD. In its recently released Third Report, the NCEP has recognized the value of this strategy by incorporating the Framingham tables to calculate the 10-year risk of developing clinical CAD based on a patient’s individual risk factors, including cholesterol levels (Table 1).4 This new NCEP III guideline recommends traditional risk factor counting coupled, in certain situations, with the 10-year risk derived from the Framingham scoring system.
TABLE 1
FRAMINGHAM TABLES FOR CALCULATING CORONARY ARTERY DISEASE RISK
Therapy is based on the individual patient’s risk category and LDL levels (Figure). Patients whose 10-year risk is greater than 20% or those who have CAD-equivalent conditions (ie, diabetes, peripheral arterial disease, abdominal aortic aneurysm, symptomatic carotid artery disease) are considered to have a risk equivalent to that of patients with known CAD; all have an LDL goal of 100 or less.
For those with a 10-year CAD risk less than 20%, the number of positive risk factors determines the LDL goal. This new method allows physicians to communicate with their patients more clearly about individual risk and enhances shared decision making. While the NCEP III report is based on extensive literature review, the recommendations of its expert panel are not characterized according to the strength of the supporting evidence, as is done by the US Preventive Services Task Force.
Figure
TREATMENT STRATEGY BASED ON LDL LEVEL AND RISK CATEGORY
Explaining treatment benefits
The NCEP III report does not make explicit the effect of the treatment on the patient; that is, how much the proposed treatment will reduce the risk of CAD. This determination depends in part on whether the patient being treated has known CAD or a CAD-equivalent condition (secondary prevention) versus no known CAD (primary prevention). The benefits of treatment have been most clearly quantified for drug treatment and are most easily evaluated using the number needed to treat (NNT). The NNT refers to the number of patients who would have to be treated for 5 years to prevent 1 CAD event. Physicians may use the NNTs to assist patients in determining their preferences for treatment, bearing in mind that the NNT refers to an outcome for a population, such as men with high cholesterol levels. For a given individual, their risk of an adverse outcome is all or none. Nonetheless, patients may find the NNT a useful way to assess their personal values in making treatment decisions.
Treatment
Lifestyle modification
Diet modification is the cornerstone of therapy for mild to moderate hyperlipidemia. Modifying the diet is also recommended along with pharmacologic therapy in people at higher risk of CAD. NCEP III recommends a diet for “therapeutic lifestyle changes” that includes < 200 mg cholesterol per day, < 7% saturated fat, 25% to 35% total fat, 50% to 60% carbohydrates, and 15% protein of total calories.4
Although diet therapy has shown a modest redution in total cholesterol in clinical trials, no clear evidence shows that a diet low in saturated fat and cholesterol will reduce cardiovascular morbidity and mortality.5,6 Many people find it difficult to change their dietary habits and to maintain healthier ones. Systematic reviews of observational studies have found that increased consumption of fruits and vegetables is associated with lower incidence of heart attack and stroke. However, the potential for bias and confounding factors in such studies makes them less convincing than randomized controlled trials (RCTs).5
The Ornish program, in which CAD or CAD-equivalent patients pursue intense lifestyle modification for up to 3 years, has shown that revascularization procedures can be avoided. Treatment groups that ate a very-low-fat diet, received intervention on stress management, and followed a prescribed exercise program showed similar improvement in angina symptoms versus the revascularization group. Another trial showed regression of atherosclerotic plaque on angiograms.10-12
Other nonpharmacologic options include plant stanols (2 grams/day) and soluble fiber (10 to 25 grams/day) to reduce LDL-cholesterol. Plant stanols have a structure similar to that of cholesterol and interfere with cholesterol absorption when eaten along with a typical diet, resulting in reduction of blood cholesterol levels. Plant stanols and sterols can be found in certain margarines and salad oils and can be taken with each meal as substitutes for other sources of dietary fat.7-9 No RCTs have shown that these substances reduce cardiovascular events or overall mortality.
Herbal products and dietary supplements
A survey found that as many as 50% of respondents with elevated cholesterol levels would prefer an over-the-counter product such as garlic, yeast, or soy products.13 Studies of products promoted for lipid-lowering effects were found to have a modest effect on lipid levels13-18 (Table 2); however, no RCTs were found that assessed patient-oriented outcomes. Because herbal products and supplements have modest effects on lipid levels and because long-term safety data are lacking, such products should be used with caution for treatment of hyperlipidemia.
TABLE 2
PHARMACOLOGIC AND NONPHARMACOLOGIC INTERVENTIONS
| Strength of Recommendation* | Treatment | Type of Benefit | Cost Per Month ($) | Comments |
|---|---|---|---|---|
| A | Statins | OM, CVM | 40–110 | Well tolerated |
| B | Fibric acids | CVE | 60–70 | All male subjects in both primary and secondary trials |
| B | Niacin | CVE | 10–80 | Watch for adverse reactions (flushing, elevated glucose, liver function tests) |
| B | Bile acid resin | CVE | 40–60 | Ideal agent for patients with severe liver disease; watch for drug interactions |
| B | Lifestyle modification | Lipid | Varies | No strong evidence from randomized clinical trials on primary prevention of major coronary events or mortality |
| B | Soy products | Lipid | 20 | FDA has approved labeling soy products for cholesterol reduction |
| B | Red yeast | Lipid | 20–30 | Active ingredient is lovastatin; should be treated as lovastatin |
| B | Plant stanols | Lipid | 20–30 | Substitute for other source of fat calories; must be taken with each meal |
| C | Fish oils | Lipid | 5–10 | Use with caution because of high caloric value and cholesterol content in products; may increase cholesterol level with long-term use |
| C | Garlic | Lipid | 10–20 | Conflicting results with clinical trials |
| C | Green tea | Lipid | 15 | Epidemiologic study data |
| * Criteria correspond to US Preventive Services Task Force categories (A = strong evidence to support recommendation, B = fair evidence to support recommendation, C = insufficient evidence to recommend for or against). CVE denotes reduction in cardiovascular events; CVM, reduction in cardiovascular mortality; lipid, reduction in lipid levels only; OM, reduction in overall mortality. | ||||
Pharmacologic treatment
Clinical trials of hyperlipidemia therapy should address outcomes that matter most to patients, such as morbidity, mortality, quality of life, and cost, rather than stressing disease-oriented evidence, such as the ability to reduce cholesterol levels. For this review we identified major long-term RCTs that included significant coronary events or mortality as the primary outcomes. Table 3 summarizes the results of primary and secondary prevention studies.
TABLE 3
PHARMACOLOGIC INTERVENTION
| Reduction in Risk | ||||
|---|---|---|---|---|
| Intervention | Major Coronary Events | All-Cause Mortality | Comments | |
| ARR (%) | NNT | NNT | ||
| Primary Prevention | ||||
| Statins | 2.0–2.3 | 44–49 | NS | Studies on normal and hypercholesterolemic patients. Mean age was 47–58 years; all patients were men except for 1 statin study that included a small number of women |
| Gemfibrozil | 1.4 | 71 | NS | |
| Cholestyramine | 1.7 | 59 | NS | |
| Secondary Prevention | ||||
| Statins | 3–3.6 | 28–33 | 24–28 | Mean age was 55–64 years. Participants were male except for the 3 statin studies and the benzafibrate study that enrolled a small number of women. Cholesterol eligibility criteria varied among the studies and included patients with normal or elevated total and LDL levels or low HDL levels |
| Gemfibrozil | 4.4 | 23 | NS | |
| Benzafibrate | 1.4 | 71 | NS | |
| Niacin | 6.2 | 17 | NS | |
| ARR denotes absolute risk reduction in percent; NNT, number of needed to treat for 5 years to prevent 1 adverse outcome; NS, not significant. | ||||
Primary prevention
Primary prevention studies have investigated the treatment of middle-aged men with hyperlipidemia and of men and women with average cholesterol levels.19-23 Results showed similar positive outcomes on reducing coronary events in all groups (Table 3). A systematic review and a meta-analysis of primary prevention studies also demonstrated that drug therapy reduced cholesterol levels and resulted in statistically significant lowering of cardiovascular events in the treated group compared with placebo without any significant reduction in overall mortality.5,24 Absolute risk reductions ranged from 1.4% to 2.3%. In other words, the number of patients that would have to be treated for 5 years to prevent a single major coronary event was 44 to 49 for the statins, 71 for gemfibrozil, and 59 for cholestyramine.
Secondary prevention
In secondary prevention trials, RCTs have demonstrated a strong, consistent relationship between cholesterol lowering and the reduction of risk for a coronary event Table 3.25-30 Patients with preexisting CAD and elevated or average lipid levels benefit from medical therapy. The relative risk of cardiovascular events was reduced by an average of 30% in the active treatment groups.
In these trials, the NNT for 5 years to prevent 1 coronary heart event or nonfatal myocardial infarction (MI) was 28 to 33 for statins, 23 for gemfibrozil, 71 for bezafibrate, and 17 for niacin. There was also a significant risk reduction for all-cause mortality in the statin trials.27,28 These data support the recommendations from NCEP III to treat patients with preexisting CAD aggressively. People with diabetes should receive similar treatment because they are more prone to the development of new CAD within 10 years.4 In addition, subgroup analyses of diabetics treated with statins in primary prevention trials demonstrated a decreased risk of cardiovascular events.26,29
While cholesterol-modifying agents include 4 different classes—statins, fibric acid derivatives, bile acid resins, and nicotinic acid—studies cited in this paper predominantly involved statins and fibric acids. In systematic reviews of both primary and secondary prevention trials, statins were the most effective agents for both cholesterol lowering and cardiovascular risk reduction.5 We found no RCTs that directly compared outcomes between cholesterol-lowering medications. Although women represented a small number of participants in these trials, a meta-analysis showed that statin therapy decreased their risk of heart disease, with an NNT of 31 for reduction of major coronary events.31 No evidence was found to support the effectiveness of hyperlipidemia therapy for people aged more than 75 years. For people aged 65 to 75 years, there is evidence to support drug therapy for secondary prevention but not for primary prevention.
Statins are well tolerated; the most common adverse reactions are gastrointestinal related and occur in approximately 3% of patients. The more serious but uncommon events associated with the use of statins are hepatitis and myopathy. Asymptomatic increases in hepatic transaminases to more than 3 times the upper normal limit occur in approximately 1% of patients.32 Therapy can be discontinued for 1 to 2 weeks; enzyme levels should return to normal if the elevations are medication related. It is not necessary to stop therapy when enzymes are elevated at less than 3 times the upper normal limit.
General guidelines on liver monitoring call for performing a baseline liver function test and repeating it 6 weeks later.33 Once a stable dose has been established, the manufacturer recommends periodic testing; however, no clear evidence supports a specific interval. Clinicians may choose to individualize decisions on testing frequency based on factors such as potential drug interactions (statins with fibric acids or niacin) or the presence of conditions that increase the risk of liver disease.34
Myopathy, defined as generalized muscle aches and pain with a serum creatine kinase level greater than 1000 U/L, occurs rarely (< 0.1%) but may be more likely to occur when statins are used concomitantly with medications such as fibric acid, antifungals, erythromycin, and cyclosporine.31,35 The best preventive strategy is to educate patients about early recognition of the signs and symptoms of myopathy. Because most statins are metabolized by the cytochrome P450-3A4, any medications that inhibit this enzyme can increase statin serum levels and increase the risk of hepatotoxicity and myopathy.
The NCEP III recommends the use of statins as firstline therapy. A standard dose of a statin decreases LDL levels by 20% to 50%, increases HDL levels by 5% to 10%, and reduces triglyceride levels by 10% to 20%. Atorvastatin and simvastatin can produce the highest reductions in LDL levels: up to 50%. Only pravastatin, simvastatin, and lovastatin have been involved in longterm RCTs of primary and secondary prevention. Atorvastatin had positive benefits in a short-term secondary prevention trial.37 Unfortunately, the only head-to-head comparisons of statins have looked at disease-oriented outcomes such as lipid levels.37 Statins are patient friendly. They require a daily evening dose because cholesterol synthesis is more active during the night. Atorvastatin can be given at any time of day because of its long half-life.
Gemfibrozil, a fibric acid, is often used to treat hypertriglyceridemia and as an adjunctive agent to statin therapy. It decreases triglycerides by 40% to 50% but has minimal effects on the rest of the lipid panel. Adverse effects are generally mild. Liver function monitoring is recommended. The usual dosage regimen for fibric acids is 2 times a day and should be adjusted for renal function.
Niacin can increase HDL by 30% and decrease triglycerides by 30% and LDL by 20%. Major adverse reactions include flushing, gastrointestinal symptoms, elevation of liver function tests, uric acid, and serum glucose levels. The new longer-acting formulation has been associated with less flushing. Another class, the bile acid resins, including cholestyramine and colestipol, may play an adjunctive role in therapy. Their effect on the lipid panel is mild compared with those of the other class and they can increase triglyceride levels. Many patients find the gritty taste of the granular formulation unpalatable. The bile acid resins have a favorable safety profile. Most adverse events occur locally in the gut.
Conclusions
The emergence of statins as a safe and effective, although costly, therapy for hyperlipidemia and the development of clinical guidelines advocating their increased use will place family physicians under added pressure to screen for and treat hyperlipidemia. While the general value of lifestyle changes is recognized in national recommendations, more effective ways for physicians to implement them successfully in ambulatory settings are needed.
An optimal evidence-based approach to hyperlipidemia uses the new NCEP III guideline, which combines traditional risk factor assessment with assessment for CAD using the Framingham tables to determine LDL goals and appropriate treatment modalities. Statins are first-line agents for patients who are candidates for drug therapy. Discussions between clinicians and patients of the NNTs for primary and secondary prevention will help foster patient-centered discussions on the role of medical, economic, and quality-of-life issues in the decision-making process.
- The new NCEP III provides revised guidelines for the treatment of hyperlipidemia.
- Combining traditional risk factor assessment with the calculated 10-year risk of coronary artery disease allows for optimal patient-centered counseling.
- Statins are normally the first-line therapy for hyperlipidemia.
In 1995 and 1996, US adults made more than 18 million office visits for the evaluation and treatment of hyperlipidemia, including 3.4% of all visits to family physicians. Among visits to family physicians, 4.1% included measurement of cholesterol levels.1 Overall, mean cholesterol levels decreased from 220 in 1960–1962 to 203 in 1988–1994. During the same time period, the proportion of adults with elevated total cholesterol levels (> 240) decreased from 32% to 19%.2 Despite this progress, the availability of more effective drugs, guidelines advocating increasingly aggressive treatment, and populationwide goals established in Healthy People 2010 will continue to increase the number of patients seen by family physicians for screening, diagnosis, and treatment of hyperlipidemia.3
When to treat
The National Cholesterol Education Program (NCEP), a program within the National Institute of Health’s Heart, Lung, and Blood Institute, published a guideline in 1993 for screening and treating hyperlipidemia. Physicians have since become familiar with the NCEP concept of basing treatment decisions on assessment of patient risk factors (smoking, age, diabetes, hypertension, family history of early coronary artery disease [CAD]) and application of algorithms linked to desired low-density lipoprotein (LDL) cholesterol levels. The advantage of this strategy is its simplicity. Physicians assess whether the NCEP risk factors are present and then work with their patients to achieve the desired LDL level through lifestyle modification, drug therapy, or both.
Unfortunately, the NCEP guideline did not assess the individual’s actual risk of CAD. In its recently released Third Report, the NCEP has recognized the value of this strategy by incorporating the Framingham tables to calculate the 10-year risk of developing clinical CAD based on a patient’s individual risk factors, including cholesterol levels (Table 1).4 This new NCEP III guideline recommends traditional risk factor counting coupled, in certain situations, with the 10-year risk derived from the Framingham scoring system.
TABLE 1
FRAMINGHAM TABLES FOR CALCULATING CORONARY ARTERY DISEASE RISK
Therapy is based on the individual patient’s risk category and LDL levels (Figure). Patients whose 10-year risk is greater than 20% or those who have CAD-equivalent conditions (ie, diabetes, peripheral arterial disease, abdominal aortic aneurysm, symptomatic carotid artery disease) are considered to have a risk equivalent to that of patients with known CAD; all have an LDL goal of 100 or less.
For those with a 10-year CAD risk less than 20%, the number of positive risk factors determines the LDL goal. This new method allows physicians to communicate with their patients more clearly about individual risk and enhances shared decision making. While the NCEP III report is based on extensive literature review, the recommendations of its expert panel are not characterized according to the strength of the supporting evidence, as is done by the US Preventive Services Task Force.
Figure
TREATMENT STRATEGY BASED ON LDL LEVEL AND RISK CATEGORY
Explaining treatment benefits
The NCEP III report does not make explicit the effect of the treatment on the patient; that is, how much the proposed treatment will reduce the risk of CAD. This determination depends in part on whether the patient being treated has known CAD or a CAD-equivalent condition (secondary prevention) versus no known CAD (primary prevention). The benefits of treatment have been most clearly quantified for drug treatment and are most easily evaluated using the number needed to treat (NNT). The NNT refers to the number of patients who would have to be treated for 5 years to prevent 1 CAD event. Physicians may use the NNTs to assist patients in determining their preferences for treatment, bearing in mind that the NNT refers to an outcome for a population, such as men with high cholesterol levels. For a given individual, their risk of an adverse outcome is all or none. Nonetheless, patients may find the NNT a useful way to assess their personal values in making treatment decisions.
Treatment
Lifestyle modification
Diet modification is the cornerstone of therapy for mild to moderate hyperlipidemia. Modifying the diet is also recommended along with pharmacologic therapy in people at higher risk of CAD. NCEP III recommends a diet for “therapeutic lifestyle changes” that includes < 200 mg cholesterol per day, < 7% saturated fat, 25% to 35% total fat, 50% to 60% carbohydrates, and 15% protein of total calories.4
Although diet therapy has shown a modest redution in total cholesterol in clinical trials, no clear evidence shows that a diet low in saturated fat and cholesterol will reduce cardiovascular morbidity and mortality.5,6 Many people find it difficult to change their dietary habits and to maintain healthier ones. Systematic reviews of observational studies have found that increased consumption of fruits and vegetables is associated with lower incidence of heart attack and stroke. However, the potential for bias and confounding factors in such studies makes them less convincing than randomized controlled trials (RCTs).5
The Ornish program, in which CAD or CAD-equivalent patients pursue intense lifestyle modification for up to 3 years, has shown that revascularization procedures can be avoided. Treatment groups that ate a very-low-fat diet, received intervention on stress management, and followed a prescribed exercise program showed similar improvement in angina symptoms versus the revascularization group. Another trial showed regression of atherosclerotic plaque on angiograms.10-12
Other nonpharmacologic options include plant stanols (2 grams/day) and soluble fiber (10 to 25 grams/day) to reduce LDL-cholesterol. Plant stanols have a structure similar to that of cholesterol and interfere with cholesterol absorption when eaten along with a typical diet, resulting in reduction of blood cholesterol levels. Plant stanols and sterols can be found in certain margarines and salad oils and can be taken with each meal as substitutes for other sources of dietary fat.7-9 No RCTs have shown that these substances reduce cardiovascular events or overall mortality.
Herbal products and dietary supplements
A survey found that as many as 50% of respondents with elevated cholesterol levels would prefer an over-the-counter product such as garlic, yeast, or soy products.13 Studies of products promoted for lipid-lowering effects were found to have a modest effect on lipid levels13-18 (Table 2); however, no RCTs were found that assessed patient-oriented outcomes. Because herbal products and supplements have modest effects on lipid levels and because long-term safety data are lacking, such products should be used with caution for treatment of hyperlipidemia.
TABLE 2
PHARMACOLOGIC AND NONPHARMACOLOGIC INTERVENTIONS
| Strength of Recommendation* | Treatment | Type of Benefit | Cost Per Month ($) | Comments |
|---|---|---|---|---|
| A | Statins | OM, CVM | 40–110 | Well tolerated |
| B | Fibric acids | CVE | 60–70 | All male subjects in both primary and secondary trials |
| B | Niacin | CVE | 10–80 | Watch for adverse reactions (flushing, elevated glucose, liver function tests) |
| B | Bile acid resin | CVE | 40–60 | Ideal agent for patients with severe liver disease; watch for drug interactions |
| B | Lifestyle modification | Lipid | Varies | No strong evidence from randomized clinical trials on primary prevention of major coronary events or mortality |
| B | Soy products | Lipid | 20 | FDA has approved labeling soy products for cholesterol reduction |
| B | Red yeast | Lipid | 20–30 | Active ingredient is lovastatin; should be treated as lovastatin |
| B | Plant stanols | Lipid | 20–30 | Substitute for other source of fat calories; must be taken with each meal |
| C | Fish oils | Lipid | 5–10 | Use with caution because of high caloric value and cholesterol content in products; may increase cholesterol level with long-term use |
| C | Garlic | Lipid | 10–20 | Conflicting results with clinical trials |
| C | Green tea | Lipid | 15 | Epidemiologic study data |
| * Criteria correspond to US Preventive Services Task Force categories (A = strong evidence to support recommendation, B = fair evidence to support recommendation, C = insufficient evidence to recommend for or against). CVE denotes reduction in cardiovascular events; CVM, reduction in cardiovascular mortality; lipid, reduction in lipid levels only; OM, reduction in overall mortality. | ||||
Pharmacologic treatment
Clinical trials of hyperlipidemia therapy should address outcomes that matter most to patients, such as morbidity, mortality, quality of life, and cost, rather than stressing disease-oriented evidence, such as the ability to reduce cholesterol levels. For this review we identified major long-term RCTs that included significant coronary events or mortality as the primary outcomes. Table 3 summarizes the results of primary and secondary prevention studies.
TABLE 3
PHARMACOLOGIC INTERVENTION
| Reduction in Risk | ||||
|---|---|---|---|---|
| Intervention | Major Coronary Events | All-Cause Mortality | Comments | |
| ARR (%) | NNT | NNT | ||
| Primary Prevention | ||||
| Statins | 2.0–2.3 | 44–49 | NS | Studies on normal and hypercholesterolemic patients. Mean age was 47–58 years; all patients were men except for 1 statin study that included a small number of women |
| Gemfibrozil | 1.4 | 71 | NS | |
| Cholestyramine | 1.7 | 59 | NS | |
| Secondary Prevention | ||||
| Statins | 3–3.6 | 28–33 | 24–28 | Mean age was 55–64 years. Participants were male except for the 3 statin studies and the benzafibrate study that enrolled a small number of women. Cholesterol eligibility criteria varied among the studies and included patients with normal or elevated total and LDL levels or low HDL levels |
| Gemfibrozil | 4.4 | 23 | NS | |
| Benzafibrate | 1.4 | 71 | NS | |
| Niacin | 6.2 | 17 | NS | |
| ARR denotes absolute risk reduction in percent; NNT, number of needed to treat for 5 years to prevent 1 adverse outcome; NS, not significant. | ||||
Primary prevention
Primary prevention studies have investigated the treatment of middle-aged men with hyperlipidemia and of men and women with average cholesterol levels.19-23 Results showed similar positive outcomes on reducing coronary events in all groups (Table 3). A systematic review and a meta-analysis of primary prevention studies also demonstrated that drug therapy reduced cholesterol levels and resulted in statistically significant lowering of cardiovascular events in the treated group compared with placebo without any significant reduction in overall mortality.5,24 Absolute risk reductions ranged from 1.4% to 2.3%. In other words, the number of patients that would have to be treated for 5 years to prevent a single major coronary event was 44 to 49 for the statins, 71 for gemfibrozil, and 59 for cholestyramine.
Secondary prevention
In secondary prevention trials, RCTs have demonstrated a strong, consistent relationship between cholesterol lowering and the reduction of risk for a coronary event Table 3.25-30 Patients with preexisting CAD and elevated or average lipid levels benefit from medical therapy. The relative risk of cardiovascular events was reduced by an average of 30% in the active treatment groups.
In these trials, the NNT for 5 years to prevent 1 coronary heart event or nonfatal myocardial infarction (MI) was 28 to 33 for statins, 23 for gemfibrozil, 71 for bezafibrate, and 17 for niacin. There was also a significant risk reduction for all-cause mortality in the statin trials.27,28 These data support the recommendations from NCEP III to treat patients with preexisting CAD aggressively. People with diabetes should receive similar treatment because they are more prone to the development of new CAD within 10 years.4 In addition, subgroup analyses of diabetics treated with statins in primary prevention trials demonstrated a decreased risk of cardiovascular events.26,29
While cholesterol-modifying agents include 4 different classes—statins, fibric acid derivatives, bile acid resins, and nicotinic acid—studies cited in this paper predominantly involved statins and fibric acids. In systematic reviews of both primary and secondary prevention trials, statins were the most effective agents for both cholesterol lowering and cardiovascular risk reduction.5 We found no RCTs that directly compared outcomes between cholesterol-lowering medications. Although women represented a small number of participants in these trials, a meta-analysis showed that statin therapy decreased their risk of heart disease, with an NNT of 31 for reduction of major coronary events.31 No evidence was found to support the effectiveness of hyperlipidemia therapy for people aged more than 75 years. For people aged 65 to 75 years, there is evidence to support drug therapy for secondary prevention but not for primary prevention.
Statins are well tolerated; the most common adverse reactions are gastrointestinal related and occur in approximately 3% of patients. The more serious but uncommon events associated with the use of statins are hepatitis and myopathy. Asymptomatic increases in hepatic transaminases to more than 3 times the upper normal limit occur in approximately 1% of patients.32 Therapy can be discontinued for 1 to 2 weeks; enzyme levels should return to normal if the elevations are medication related. It is not necessary to stop therapy when enzymes are elevated at less than 3 times the upper normal limit.
General guidelines on liver monitoring call for performing a baseline liver function test and repeating it 6 weeks later.33 Once a stable dose has been established, the manufacturer recommends periodic testing; however, no clear evidence supports a specific interval. Clinicians may choose to individualize decisions on testing frequency based on factors such as potential drug interactions (statins with fibric acids or niacin) or the presence of conditions that increase the risk of liver disease.34
Myopathy, defined as generalized muscle aches and pain with a serum creatine kinase level greater than 1000 U/L, occurs rarely (< 0.1%) but may be more likely to occur when statins are used concomitantly with medications such as fibric acid, antifungals, erythromycin, and cyclosporine.31,35 The best preventive strategy is to educate patients about early recognition of the signs and symptoms of myopathy. Because most statins are metabolized by the cytochrome P450-3A4, any medications that inhibit this enzyme can increase statin serum levels and increase the risk of hepatotoxicity and myopathy.
The NCEP III recommends the use of statins as firstline therapy. A standard dose of a statin decreases LDL levels by 20% to 50%, increases HDL levels by 5% to 10%, and reduces triglyceride levels by 10% to 20%. Atorvastatin and simvastatin can produce the highest reductions in LDL levels: up to 50%. Only pravastatin, simvastatin, and lovastatin have been involved in longterm RCTs of primary and secondary prevention. Atorvastatin had positive benefits in a short-term secondary prevention trial.37 Unfortunately, the only head-to-head comparisons of statins have looked at disease-oriented outcomes such as lipid levels.37 Statins are patient friendly. They require a daily evening dose because cholesterol synthesis is more active during the night. Atorvastatin can be given at any time of day because of its long half-life.
Gemfibrozil, a fibric acid, is often used to treat hypertriglyceridemia and as an adjunctive agent to statin therapy. It decreases triglycerides by 40% to 50% but has minimal effects on the rest of the lipid panel. Adverse effects are generally mild. Liver function monitoring is recommended. The usual dosage regimen for fibric acids is 2 times a day and should be adjusted for renal function.
Niacin can increase HDL by 30% and decrease triglycerides by 30% and LDL by 20%. Major adverse reactions include flushing, gastrointestinal symptoms, elevation of liver function tests, uric acid, and serum glucose levels. The new longer-acting formulation has been associated with less flushing. Another class, the bile acid resins, including cholestyramine and colestipol, may play an adjunctive role in therapy. Their effect on the lipid panel is mild compared with those of the other class and they can increase triglyceride levels. Many patients find the gritty taste of the granular formulation unpalatable. The bile acid resins have a favorable safety profile. Most adverse events occur locally in the gut.
Conclusions
The emergence of statins as a safe and effective, although costly, therapy for hyperlipidemia and the development of clinical guidelines advocating their increased use will place family physicians under added pressure to screen for and treat hyperlipidemia. While the general value of lifestyle changes is recognized in national recommendations, more effective ways for physicians to implement them successfully in ambulatory settings are needed.
An optimal evidence-based approach to hyperlipidemia uses the new NCEP III guideline, which combines traditional risk factor assessment with assessment for CAD using the Framingham tables to determine LDL goals and appropriate treatment modalities. Statins are first-line agents for patients who are candidates for drug therapy. Discussions between clinicians and patients of the NNTs for primary and secondary prevention will help foster patient-centered discussions on the role of medical, economic, and quality-of-life issues in the decision-making process.
1. Schappert SM, Nelson C. National Ambulatory Medical Care Survey: 1995–1996 summary. National Center for Health Statistics. Vital Health Stat 1999;13(142).-
2. National Center for Health Statistics. Health, United States, 1999, with health and aging chartbook. Hyattsville, Md: 1999.
3. US Department of Health and Human Services. Tracking Healthy People 2010. Washington, DC: US Government Printing Office. November 2000. Available at: http://www.cdc.gov/hchs/hphome.htm.
4. Third report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III). Executive Summary. Available at: http://www.nhlbi.nih.gov/guidelines/cholesterol/atp3xsum.pdf Accessed April 16, 2001.
5. Clinical evidence London, England: BMJ Publishing Group; June 2001. Available at: www.clinicalevidence.org.
6. Henkin Y, Shai I, Zuk R, et al. Dietary treatment of hyperlipidemia: Do dietitians do it better? A randomized, controlled trial. Am J Med 2000;109:549-55.
7. Ornish D. Avoiding revascularization with lifestyle changes: the multicenter lifestyle demonstration project. Am J Cardiol 1998;82:72T-76T.
8. Ornish D, Scherwitz LW, Billings JH, et al. Intensive lifestyle changes for reversal of coronary heart disease. JAMA 1998;280:2001-7.
9. Gould KL, Ornish D, Scherwitz L, et al. Changes in myocardial perfusion abnormalities by positron emission tomography after long-term, intense risk factor modification. JAMA 1995;274:894-901.
10. Mensink RP, Plat J. Efficacy of dietary plant stanols. In: New developments in the dietary management of high cholesterol. New York: McGraw-Hill; 1998;27-31.
11. Blair SN, Capuzzi DM, Gottlieb SO, et al. Incremental reduction of serum total cholesterol and LDL with the addition of plant stanol ester-containing spread to statin therapy. Am J Cardiol 2000;86:46-52.
12. Miettinen TA, Puska P, Gylling H, et al. Reduction of serum cholesterol with sitostanol-ester margarine in a mildly hypercholesteremic population. N Engl J Med 1995;333:1308-12.
13. Caron MF, White CM. Evaluation of the antihyperlipidemic properties of dietary supplements. Pharmacotherapy 2001;21:481-7.
14. Harris WS. Nonpharmacologic treatment of hypertriglyceridemia: focus on fish oils. Clin Cardiol 1999;22(suppl 2):II40-3.
15. Stevinson C, Pittler MH, Ernst E. Garlic for treating hyperlipidemia. Ann Intern Med 2000;133:420-9.
16. EBM Reviews. Database of abstracts of reviews of effectiveness [database online]. Psyllium-enriched cereals lower blood total cholesterol and LDL cholesterol, but not HDL cholesterol, in hypercholesterolemic adults: results of a meta-analysis. July 2001; v1, accession no. 00125498-100000000-00737. Available at: http://www.ovid.com/products/databases. Accessed Oct. 29, 2001.
17. EBM Reviews. ACP Journal Club [database online]. Soy protein intake decreases total and LDL cholesterol and triglyceride levels. March/April 1996; 124:41, accession no. 00021607-199603000-00013. Available at: http://www.ovid.com/products/databases. Accessed April 4, 2001.
18. Jellin JM, Batz F, Hitchens K. Natural Medicines Comprehensive Database, 3rd ed. Stockton, Calif: Therapeutic Research Faculty; 2000.
19. Frick MH, Elo O, Haapa K, et al. Helsinki heart study: primary-prevention trial with gemfibrozil in middle-aged men with dyslipidemia. N Engl J Med 1987;317:1237-45.
20. The lipid research clinics coronary primary prevention trial results I. Reduction in incidence of coronary heart disease. JAMA 1984;251:351-64.
21. The lipid research clinics coronary primary prevention trial results II. The relationship of reduction in incidence of coronary heart disease to cholesterol lowering. JAMA 1984;251:365-74.
22. Shepherd J, Cobbe SM, Ford I, et al. Prevention of coronary heart disease with pravastatin in men with hyperlipidemia. N Engl J Med 1995;333:1301-6.
23. Downs JR, Clearfield M, Weis S, et al. Primary prevention of acute coronary events with lovastatin in men and women with average cholesterol levels. JAMA 1998;279:1615-22.
24. Pignone M, Phillips C, Mulrow C. Use of lipid lowering drugs for primary prevention of coronary heart disease: meta-analysis of randomized trials. BMJ 2000;321:983-5.
25. Secondary prevention by raising HDL cholesterol and reducing triglycerides in patients with coronary artery disease: the Bezafibrate Infarction Prevention (BIP) Study. Circulation 2000;102:21-7.
26. Rubins HB, Robins SJ, Collins D, et al. Gemfibrozil for the secondary prevention of coronary heart disease in men with low levels of HDL-cholesterol. N Engl J Med 1999;341:410-8.
27. Randomised trial of cholesterol lowering in 4444 patients with coronary heart disease: the Scandinavian simvastatin survival study (4S). Lancet 1994;344:1383-9.
28. Preventio of cardiovascular events and death with pravastatin in patients with coronary heart disease and a broad range of initial cholesterol levels. The Long-Term Intervention with Pravastatin in Ischaemic Disease. N Engl J Med 1998;339:1349-57.
29. Sacks FM, Pfeffer MA, Moye LA, et al. The effect of pravastatin on coronary events after myocardial infarction in patients with average cholesterol levels. N Engl J Med 1996;335:1001-9.
30. Canner PL, Berge KG, Wenger NK, et al. Fifteen year mortality in coronary drug project patients: long-term benefit with niacin. J Am Coll Cardiol 1986;8:1245-55.
31. LaRosa JC, He J, Vupputuri S. Effect of statins on risk of coronary disease; a meta-analysis of randomized controlled trials. JAMA 1999;24:2340-6.
32. Hsu I, Spinler SA, Johnson N. Comparative evaluation of the safety and efficacy of HMG-CoA reductase inhibitor monotherapy in the treatment of primary hyperlipidemia. Ann Pharmacother 1995;29:743-59.
33. Tice SA, Parry D. Medications that require hepatic monitoring. Hosp Pharm 2001;36:456-64.
34. Weismantel D. What lab monitoring is appropriate to detect adverse drug reactions in patients on cholesterol-lowering agents? J Fam Pract 2001;50:927.-
35. American College of Clinical Pharmacy. PSAP: pharmacotherapy self-assessment program, 4th ed. Kansas City, Mo: ACCP; 2001;66-7.
36. Schwartz GG, Olsson AG, Ezekowitz MD, et al. Effects of atorvastatin on early recurrent ischemic events in acute coronary syndromes. JAMA 2001;285:1711-8.
37. Jones P, Kafonek S, Laurora I, et al. Comparative dose efficacy study of atorvastatin vs. simvastatin, pravastatin, lovastatin, and fluvastatin in patients with hyperlipidemia (the CURVES study). Am J Cardiol 1998;81:582-7.
1. Schappert SM, Nelson C. National Ambulatory Medical Care Survey: 1995–1996 summary. National Center for Health Statistics. Vital Health Stat 1999;13(142).-
2. National Center for Health Statistics. Health, United States, 1999, with health and aging chartbook. Hyattsville, Md: 1999.
3. US Department of Health and Human Services. Tracking Healthy People 2010. Washington, DC: US Government Printing Office. November 2000. Available at: http://www.cdc.gov/hchs/hphome.htm.
4. Third report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III). Executive Summary. Available at: http://www.nhlbi.nih.gov/guidelines/cholesterol/atp3xsum.pdf Accessed April 16, 2001.
5. Clinical evidence London, England: BMJ Publishing Group; June 2001. Available at: www.clinicalevidence.org.
6. Henkin Y, Shai I, Zuk R, et al. Dietary treatment of hyperlipidemia: Do dietitians do it better? A randomized, controlled trial. Am J Med 2000;109:549-55.
7. Ornish D. Avoiding revascularization with lifestyle changes: the multicenter lifestyle demonstration project. Am J Cardiol 1998;82:72T-76T.
8. Ornish D, Scherwitz LW, Billings JH, et al. Intensive lifestyle changes for reversal of coronary heart disease. JAMA 1998;280:2001-7.
9. Gould KL, Ornish D, Scherwitz L, et al. Changes in myocardial perfusion abnormalities by positron emission tomography after long-term, intense risk factor modification. JAMA 1995;274:894-901.
10. Mensink RP, Plat J. Efficacy of dietary plant stanols. In: New developments in the dietary management of high cholesterol. New York: McGraw-Hill; 1998;27-31.
11. Blair SN, Capuzzi DM, Gottlieb SO, et al. Incremental reduction of serum total cholesterol and LDL with the addition of plant stanol ester-containing spread to statin therapy. Am J Cardiol 2000;86:46-52.
12. Miettinen TA, Puska P, Gylling H, et al. Reduction of serum cholesterol with sitostanol-ester margarine in a mildly hypercholesteremic population. N Engl J Med 1995;333:1308-12.
13. Caron MF, White CM. Evaluation of the antihyperlipidemic properties of dietary supplements. Pharmacotherapy 2001;21:481-7.
14. Harris WS. Nonpharmacologic treatment of hypertriglyceridemia: focus on fish oils. Clin Cardiol 1999;22(suppl 2):II40-3.
15. Stevinson C, Pittler MH, Ernst E. Garlic for treating hyperlipidemia. Ann Intern Med 2000;133:420-9.
16. EBM Reviews. Database of abstracts of reviews of effectiveness [database online]. Psyllium-enriched cereals lower blood total cholesterol and LDL cholesterol, but not HDL cholesterol, in hypercholesterolemic adults: results of a meta-analysis. July 2001; v1, accession no. 00125498-100000000-00737. Available at: http://www.ovid.com/products/databases. Accessed Oct. 29, 2001.
17. EBM Reviews. ACP Journal Club [database online]. Soy protein intake decreases total and LDL cholesterol and triglyceride levels. March/April 1996; 124:41, accession no. 00021607-199603000-00013. Available at: http://www.ovid.com/products/databases. Accessed April 4, 2001.
18. Jellin JM, Batz F, Hitchens K. Natural Medicines Comprehensive Database, 3rd ed. Stockton, Calif: Therapeutic Research Faculty; 2000.
19. Frick MH, Elo O, Haapa K, et al. Helsinki heart study: primary-prevention trial with gemfibrozil in middle-aged men with dyslipidemia. N Engl J Med 1987;317:1237-45.
20. The lipid research clinics coronary primary prevention trial results I. Reduction in incidence of coronary heart disease. JAMA 1984;251:351-64.
21. The lipid research clinics coronary primary prevention trial results II. The relationship of reduction in incidence of coronary heart disease to cholesterol lowering. JAMA 1984;251:365-74.
22. Shepherd J, Cobbe SM, Ford I, et al. Prevention of coronary heart disease with pravastatin in men with hyperlipidemia. N Engl J Med 1995;333:1301-6.
23. Downs JR, Clearfield M, Weis S, et al. Primary prevention of acute coronary events with lovastatin in men and women with average cholesterol levels. JAMA 1998;279:1615-22.
24. Pignone M, Phillips C, Mulrow C. Use of lipid lowering drugs for primary prevention of coronary heart disease: meta-analysis of randomized trials. BMJ 2000;321:983-5.
25. Secondary prevention by raising HDL cholesterol and reducing triglycerides in patients with coronary artery disease: the Bezafibrate Infarction Prevention (BIP) Study. Circulation 2000;102:21-7.
26. Rubins HB, Robins SJ, Collins D, et al. Gemfibrozil for the secondary prevention of coronary heart disease in men with low levels of HDL-cholesterol. N Engl J Med 1999;341:410-8.
27. Randomised trial of cholesterol lowering in 4444 patients with coronary heart disease: the Scandinavian simvastatin survival study (4S). Lancet 1994;344:1383-9.
28. Preventio of cardiovascular events and death with pravastatin in patients with coronary heart disease and a broad range of initial cholesterol levels. The Long-Term Intervention with Pravastatin in Ischaemic Disease. N Engl J Med 1998;339:1349-57.
29. Sacks FM, Pfeffer MA, Moye LA, et al. The effect of pravastatin on coronary events after myocardial infarction in patients with average cholesterol levels. N Engl J Med 1996;335:1001-9.
30. Canner PL, Berge KG, Wenger NK, et al. Fifteen year mortality in coronary drug project patients: long-term benefit with niacin. J Am Coll Cardiol 1986;8:1245-55.
31. LaRosa JC, He J, Vupputuri S. Effect of statins on risk of coronary disease; a meta-analysis of randomized controlled trials. JAMA 1999;24:2340-6.
32. Hsu I, Spinler SA, Johnson N. Comparative evaluation of the safety and efficacy of HMG-CoA reductase inhibitor monotherapy in the treatment of primary hyperlipidemia. Ann Pharmacother 1995;29:743-59.
33. Tice SA, Parry D. Medications that require hepatic monitoring. Hosp Pharm 2001;36:456-64.
34. Weismantel D. What lab monitoring is appropriate to detect adverse drug reactions in patients on cholesterol-lowering agents? J Fam Pract 2001;50:927.-
35. American College of Clinical Pharmacy. PSAP: pharmacotherapy self-assessment program, 4th ed. Kansas City, Mo: ACCP; 2001;66-7.
36. Schwartz GG, Olsson AG, Ezekowitz MD, et al. Effects of atorvastatin on early recurrent ischemic events in acute coronary syndromes. JAMA 2001;285:1711-8.
37. Jones P, Kafonek S, Laurora I, et al. Comparative dose efficacy study of atorvastatin vs. simvastatin, pravastatin, lovastatin, and fluvastatin in patients with hyperlipidemia (the CURVES study). Am J Cardiol 1998;81:582-7.
Approach to the Perimenopausal Patient
- Laboratory testing is not indicated to initiate treatment of perimenopausal symptoms.
- While estrogens are the best established of the options to treat vasomotor symptoms at perimenopause, they are not a proven treatment for major depression or poor libido.
- Little evidence exists regarding the benefits and risks of androgens for perimenopausal women, suggesting a cautious approach to their use.
- Routine use of hormone replacement therapy, especially beyond 5 years’ duration, is not recommended because of uncertainties regarding risks and benefits.
Menopause has been successfully promoted as an estrogen-deficient state. Prescriptions in the United States for noncontraceptive estrogen formulations increased from 16 million to 39 million between 1982 and 1992; progestin sales reached 4.7 million by 1992 after their introduction in 1986.1 A condition for which half of the population becomes eligible for pharmacologic treatment for 30 years or more of their life spans is worthy of family physicians’ attention. Counseling of women regarding menopause has also been incorporated into the Health Employer Data Information Set (HEDIS) for measuring the quality of care provided by health care plans.
The women of the generation born from 1946 to 1965 are now 36 to 55 years old. About half will at some time seek medical attention for relief of symptoms believed to be related to the menopausal transition.2 The clinical picture, however, can be confusing: women at midlife are susceptible to diseases that may affect or be affected by the menopausal transition. Life cycle changes can also provoke dysphoric symptoms similar to those of menopause or aggravate symptoms that already exist.
Natural history
A woman’s hormonal rhythm changes gradually, usually in the early to middle forties. Ovarian mass decreases progressively; production of ovarian hormones decreases as well. The menstrual cycles tend to be somewhat shorter. Follicle-stimulating hormone (FSH) and estrogen levels fluctuate. Estrogen levels may be transiently higher than in former years in response to higher FSH levels, recruiting more ovarian follicles. Anovulatory cycles are more frequent. Perimenopausal menstrual irregularity typically lasts for approximately 4 years; the large majority of women experience such irregularity for 1 to 7 years.2 For 10% of women, menses simply cease without prior menstrual irregularity.
The best estimate of mean age at menopause in the United States, based on a cohort of primarily Caucasian women, is 51.3 years.2 Smokers experience menopause 1.8 years earlier than nonsmokers (50.2 versus 52.0 years). Less than 10% of women reach menopause before age 46, while approximately 30% do so before age 50.2 A recent review3 concluded that the lifetime number of ovulatory cycles is predictive of age at menopause: earlier for women with shorter cycles and nulliparous women, later for multigravid women and those with a history of oral contraceptive use. A familial tendency toward similarity in age at menopause has been noted.
Premature menopause or premature ovarian failure is defined as cessation of menstrual periods before 40 years of age. The prevalence of premature ovarian failure is approximately 1% by age 40 and 0.1% by 30 years of age.4 Premature ovarian failure is frequently an autoimmune disorder.5
Diagnosis of menopause
The gold standard for diagnosing menopause is to do so retrospectively, 1 year after the last menstrual period. In general, a diagnosis of menopause based on menstrual history or hormone levels is not considered necessary to begin treatment for perimenopausal symptoms, which often begin several years before the onset of menopause.
Laboratory diagnosis
The extent to which FSH or other serologic markers can be used to diagnose menopause is controversial. The most important clinical reason to do so is to discontinue contraceptive methods safely. Some consider an FSH level greater than 40 mIU/mL to be diagnostic. This value was chosen because it is about 2 standard deviations above the periovulatory peak in FSH levels in regularly cycling women. However, longitudinal studies6,7 during the perimenopausal years have demonstrated that hormonal patterns that include FSH values greater than 40 mIU/mL often abruptly revert to premenopausal patterns and are accompanied by ovulatory cycles. For the individual patient, hormone levels do not appear to rule out fertility reliably.8 Studies defining test characteristics (sensitivity, specificity, likelihood ratios) of hormone assays for the diagnosis of menopause are needed.
History and physical examination
A large population-based survey of Swedish women9 found that the most common climacteric symptoms are, in order of frequency, vasomotor symptoms (hot flashes), mood disturbances, sleep disturbances, decreased libido, and vaginal dryness. Several observational studies10-13 have shown that vasomotor symptoms have the clearest temporal association with the menstrual cycle changes of the climacteric. These symptoms result from a sudden change in the hypothalamic control of temperature regulation,14 although the precise triggers have not been elucidated. Hot flashes occur commonly among women in their late thirties and forties who have regular menstrual cycles.15 Several studies2,10,13,16 have shown that the prevalence of hot flashes peaks in the year immediately following the final menstrual period. A typical pattern prevalence of hot flashes is 25% in premenopausal women, 69% in perimenopausal women, and 39% in late-postmenopausal women (more than 4.5 years).17 Fifteen years after menopause, 10% of women may continue to have moderate to severe hot flashes,18 which can be lifelong.
Irritability and mood swings are common climacteric complaints. Women often compare them with their earlier premenstrual symptoms. Studies of depressive symptoms in menopausal women indicate that menopause is not associated with increased rates of major depression.19 Stressful life context and poor health status appear to be more important risk factors for depression than symptoms of menopause in climacteric women.20
Many perimenopausal women complain of poor sleep, often attributed to nocturnal hot flashes. Subjective impairment of sleep quality that is associated with climacteric vasomotor symptoms does not manifest as abnormalities in polysomnographic sleep recordings.21 It does not appear to be related to sleep apnea.
Sexual dysfunction is common in women at midlife and beyond. Dyspareunia, associated with vaginal dryness, increases in frequency with increasing time after menopause.9 The other complaint is decreased libido. Multiple factors may contribute to lack of sexual interest. Both aging and the menopause are independently associated with decreases in sexual responsiveness.22 The roles of declining endogenous sex steroid hormones in this process have not been elucidated.
Treatment
Vasomotor symptoms
Table 1 summarizes treatment options for vasomotor symptoms. Numerous well-designed clinical trials have demonstrated the effectiveness of oral or transdermal estrogen replacement therapy (ERT) for hot flashes.18,23-25 Low-dose oral contraceptive formulations are approved until 50 years of age for nonsmoking women.26 In a well-designed randomized controlled trial (RCT) of 93 women, low-dose estrogen (0.625 mg conjugated equine estrogens daily) plus 1.25 mg methyltestosterone daily was shown to be more effective than low-dose estrogen only and as effective as high-dose estrogen (1.25 mg conjugated estrogens daily).27
Phytoestrogens may be helpful, but have not yet been studied extensively. One RCT28 of 104 postmenopausal women comparing ingestion of 60 g soy protein daily with that of 60 g casein (placebo) daily showed a 45% relative reduction of hot flashes at 12 weeks in the group taking soy versus the control group. A second RCT29 of 51 women comparing soy protein with carbohydrate placebo showed a decrease in severity, but not frequency, of hot flashes. Another well-designed RCT30 including 69 women treated with 40 g soy daily versus whey protein for 24 weeks showed no difference between treatment groups and improvement in symptom scores over time in both groups. It is difficult to include a 40-g to 60-g protein supplement in the daily diet because of the accompanying caloric intake required. Recent reports of randomized placebo-controlled trials of black cohosh31 and dong quai32 and a systematic review33 of controlled trials of red clover have found no benefit.
Alternatives to estrogen for treatment of hot flashes include methyldopa, clonidine, transdermal progesterone, and megestrol acetate. Megestrol, which reduces symptoms by 70%, appears to be the most effective of these.34 Although long-term use of megestrol acetate by cancer survivors for the treatment of hot flashes has been demonstrated to be effective and well tolerated,35 it is not customarily used at menopause. A 20% reduction in hot flashes can be expected with clonidine at a dose of 0.1 to 0.2 mg daily,34,36 although this regimen may cause an increase in difficulty sleeping37 as well as dry mouth, constipation, and low blood pressure. Transdermal progesterone cream alone has been shown to improve vasomotor symptoms, although without protective effect regarding bone loss.38 One small study39 of behavioral approaches showed symptom reduction with deep-breathing relaxation techniques. Pilot studies of sertraline,40 venlafaxine,41 and paroxetine42 show promise in the treatment of hot flashes.
The remainder of this article focuses on hormonal treatment effects and risks for menopausal women. A summary appears in Table 2.
TABLE 1
TREATMENT OF VASOMOTOR SYMPTOMS
| Strength of Recommendation | Treatment | Comment |
|---|---|---|
| A | Estrogens | Many preparations with both oral and transdermal delivery have been studied |
| A | Estrogen + MPA | Other progestins not well studied |
| B | Transdermal progesterone | One RCT38 |
| B | Estrogen + testosterone | One RCT27; long-term safety is a theoretical concern |
| B | Megasterol | Cohort35; long experience with cancer patients gives some assurance of safety |
| C | Behavioral approaches | One small RCT39; deep breathing was beneficial |
| C | Clonidine | Small RCTs36,37 with important loss of subjects because of side effects |
| D | Antidepressants | Pilot studies of sertraline,40 venlafaxine,41 and paroxetine42 |
| D | Phytoestrogens | Conflicting RCT results |
| D | Exercise | Weak observational studies suggest benefit74,76 |
| No benefit seen | ||
| B | Black cohosh | No benefit seen in one RCT31 |
| B | Dong quai | No benefit seen in one RCT32 |
| B | Red clover | No benefit seen in systematic review33 |
| Grades of recommendation are based on Oxford Centre for Evidence-Based Medicine guidelines. | ||
| MPA denotes medroxyprogesterone; RCT, randomized clinical trial. | ||
TABLE 2
SUMMARY OF RISKS AND BENEFITS OF TREATMENTS FOR PERIMENOPAUSAL SYMPTOMS
| Treatment | VMS | Mood | Libido | Bone | CAD | Breast CA |
|---|---|---|---|---|---|---|
| Estrogens | Benefit | Benefit | No benefit | Benefit | Uncertain | Risk |
| Estrogen + MPA | Benefit | ? benefit | No benefit | Benefit | Uncertain* | Risk† |
| Progesterone | Benefit | Risk | No benefit | NS | Uncertain | NS |
| Testosterone | Benefit | NS | ? benefit | Benefit | NS | NS |
| Phytoestrogens | ? benefit | NS | NS | No benefit | NS | NS |
| DHEA | NS | ? benefit | ? benefit | NS | NS | NS |
| * Not beneficial for secondary prevention. † Increased risk over estrogen alone. | ||||||
| CA denotes cancer; CAD, coronary artery disease; DHEA, dihydroepiandrosterone; MPA, medroxyprogesterone acetate; NS, not studied; VMS, vasomotor symptoms. | ||||||
Mood disorders
In a meta-analysis43 including 26 RCTs of the effects of hormone replacement therapy (HRT) on depressed mood, estrogen showed limited effectiveness in improving mood. The addition of synthetic progestins reduced the estrogen effect. More recent short trials of unopposed transdermal estrogen showed benefit.44,45 Other reviews46,47 have concluded that ERT or HRT has little effect in the treatment of psychological symptoms, including anxiety, cognitive, and affective symptoms. As an adjuvant to psychotropic therapy, it may have limited effect. There is insufficient evidence to support prophylactic ERT or HRT to prevent depression in women whose medical history includes prior postpartum depression.47 Estrogens do not affect the ability of a woman with moderate to severe vasomotor symptoms to cope with stress.48 Clinical trials reporting the effects of testosterone treatment on mood in women were not identified.
Women with mild psychological and predominantly vasomotor symptoms may benefit from a trial of HRT before psychotropic medication. For women who meet criteria for a diagnosis of major depression, initial treatment with an antidepressant alone or concurrent with HRT is advisable.
Sleep disturbance
In a survey of more than 6000 women aged 40 to 64 years, 30% of HRT users reported sleep improvement that they attributed to therapy.49 Other standard approaches to insomnia, such as sleep hygiene measures and progressive relaxation techniques, can also be used. If sleep apnea is suspected, a sleep study may be indicated.
Sexual dysfunction
In a systematic review50 of HRT for climacteric sexual dysfunction, vaginal dryness improved with ERT in 7 of 8 studies. Dyspareunia improved in only 1 of 6 studies using transdermal 17-beta-estradiol. Orgasm increased in only 1 of 5 trials using ethinyl estradiol. Sexual interest increased in none of 7 studies that used conjugated estrogens. However, taking testosterone appeared to increase sexual interest. The evidence regarding the safety and efficacy of androgens (testosterone and dehydroepiandrosterone [DHEA]) for the treatment of sexual dysfunction in perimenopause is incomplete; therefore, these drugs should not routinely be prescribed.51
Bone
Although HRT prevents the rapid bone loss observed in the early menopausal period, this effect is lost when treatment is stopped. The positive effect of estrogen alone on bone mineral density was not diminished by medroxyprogesterone acetate (MPA) or micronized progesterone over a 3-year follow-up period.52 The long-term effects of MPA on fracture risk in postmenopausal women have not been reported. Use of transdermal progesterone alone does not prevent bone loss.38
Cancer
Estrogen alone for women with an intact uterus is currently considered unacceptable because adding the hormone poses endometrial cancer risk. An exception is low-dose estrogen administered intravaginally; this method does not alter the endometrium.53
Estrogen alone or in combination with progestins has been associated with an increased risk of breast cancer in many observational studies and meta-analyses. A comprehensive reanalysis54 of 51 mostly observational studies, including 52,705 cases of breast cancer and more than 100,000 controls, examined the association of breast cancer with HRT, predominantly unopposed estrogen. These authors concluded that there is an increase in incidence of breast cancer of 0.2%, 0.6%. and 1.2% with 5, 10, and 15 years of use, respectively. Thus, 1 additional case of breast cancer occurs for every 167 women treated for 10 years (number needed to harm [NNH] = 167). Two recent observational studies have documented up to a fourfold increase in breast cancer with estrogen plus progesterone over estrogen alone.55,56
Cardiovascular disease
HRT has been widely advocated for prevention of coronary artery disease (CAD), based on many observational studies. A meta-analysis57 of 25 studies published through 1997 gave a relative risk (RR) of 0.7 (CI 0.65-0.75) for coronary events in women using HRT. However, a consistent bias in these studies of selecting healthy, compliant women for inclusion may explain the observed benefit.
A meta-analysis58 of 22 trials of 4124 women comparing HRT with placebo, no therapy, or vitamins, in which cardiovascular events were secondary endpoints, revealed that there was no benefit regarding cardiac events and there were small increases in absolute risk of stroke and venous thromboembolism (VET). In the Heart and Estrogen/Progestin Replacement (HERS) study,59 conjugated equine estrogens (CEE) plus MPA, administered to women with established CAD for a mean of 4.1 years, did not reduce risk of cardiovascular events. An increase in events, particularly VET and stroke,60 occurred in the first year of use. Small increases in the absolute risks of stroke61,62 and VTE63,64 have also been described in observational studies.
Randomized trial evidence is currently lacking for a role of HRT in the primary prevention of cardiovascular disease. A large study of low-risk postmenopausal women, the Women’s Health Initiative,65 is currently under way. Its objective is to investigate strategies for the prevention and control of some of the most common causes of morbidity and mortality in postmenopausal women. The study includes 27,000 women randomized to CEE plus MPA or placebo. Results are expected in 2007. The American Heart Association now recommends against estrogen therapy with or without progestin solely for the prevention of heart disease.66 Long-term effects of androgens on cardiovascular risk have not been studied; concerns exist about their use.51
Other effects
A meta-analysis67 of trials of HRT for urinary incontinence showed no benefit. The HERS study68 showed an increase in urinary incontinence episodes with combined HRT for women with incontinence at baseline (NNH = 8). HRT also increases the risk of gallbladder disease69 and may worsen cognitive function for women with mild to moderate dementia.70
Prognosis
The symptoms of perimenopause are not life threatening and are usually limited in time. Climacteric symptoms are generally more severe and difficult to treat in women who have undergone bilateral oophorectomy before experiencing natural menopause.71 Women with multiple chronic medical conditions,13,72,73 psychiatric illnesses,15,74 or a history of premenstrual syndrome11,12,75 are also likely to experience more difficulty with symptoms attributed to the menopausal transition. Table 3 provides a list of resources for patient education regarding menopause.
TABLE 3
RESOURCES FOR PATIENT EDUCATION ABOUT MENOPAUSE
| Organization | Contact Information | Description |
|---|---|---|
| Ottawa Health Decision Centre | [email protected] 613-798-5555 | Making Choices: Hormones After Menopause (audiotape and workbook) |
| American Academy of Family Physicians | www.aafp.org 1-800-274-2237 | Brochures: “Menopause: What to Expect When Your Body Is Changing”; “Osteoporosis: Keeping Your Bones Healthy and Strong” |
| American College of Obstetricians and Gynecologists | www.acog.org 1-800-410-ACOG | Brochures: “Midlife Transitions: A Guide to the Menopause Years”; “Hormone Replacement Therapy” |
1. Wysowski D, Golden L, Burke L. Use of menopausal estrogens and medroxyprogesterone in the United States, 1982-1992. Obstet Gynecol 1995;85:6-10.
2. McKinlay S, Brambilla PJ, Posnere JG. The normal menopause transition. Maturitas 1992;14:13-5.
3. Harlow B, Signorello LB. Factors associated with early menopause. Maturitas 2000;35:3-9.
4. Coulam C, Adamson SC, Annegers JF. Incidence of premature ovarian failure. Obstet Gynecol 1986;67:604-6.
5. Kalantaridou S, Nelson LM. Premature ovarian failure is not premature menopause. Ann NY Acad Sci 2000;900:393-402.
6. Hee J, MacNaughton J, Bangah M, Burger HG. Perimenopausal patterns of gonadotropins, immunoreactive inhibin, oestradiol and progesterone. Maturitas 1993;18:9-20.
7. Rannevik G, Jeppsson S, Johnell O, Bjerre B, Laurell-Borulf Y, Svanberg L. A longitudinal study of the perimenopausal transition: altered profiles of steroid and pituitary hormones, SHBG and bone moneral density. Maturitas 1995;21:103-13.
8. Burger H. Diagnostic role of follicle-stimulating hormone (FSH) measurements during the menopausal transition—an analysis of FSH, oestradiol and inhibin. Eur J Endocrinol 1994;130:38-42.
9. Stadberg E, Mattson LA, Milsom I. The prevalence and severity of climacteric symptoms and the use of different treatment regimens in a Swedish population. Acta Obstet Gynecol Scand 1997;76:442-8.
10. Holte A, Mikkelsen A. The menopausal syndrome: a factor analytic replication. Maturitas 1991;13:193-203.
11. Hunter M. The South-East England Longitudinal Study of the climacteric and postmenopause. Maturitas 1992;14:217-28.
12. Collins A, Landgren BM. Reproductive health, use of estrogen and experience of symptoms in perimenopausal women: a population-based study. Maturitas 1995;20:101-11.
13. Dennerstein L, Smith AMA, Morse C, Burger H, Green A, Hopper J, et al. Menopausal symptoms in Australian women. Med J Aust 1993;159:232-6.
14. Mashchak C, Kletsky QA, Artel R, et al. The relation of physiological changes to subjective symptoms in postmenopausal women with and without hot flushes. Maturitas 1985;6:301-8.
15. Grisso J, Freeman EW, Maurin E, Garcia-Espans B, Berlin JA. Racial differences in menopause information and the experience of hot flashes. J Gen Intern Med 1999;14:98-103.
16. Oldenhave A, Jaszmann LJB, Haspels AA, et al. Impact of climacteric on well-being. Am J Obstet Gynecol 1993;168:772-80.
17. Barentsen R, Groeneveld FP, Bareman FP, et al. Women’s opinion on withdrawal bleeding with hormone replacement therapy. Eur J Obstet Gynecol Reprod Biol 1993;51:203-7.
18. Bachman G. Vasomotor flushes in menopausal women. Am J Obstet Gynecol 1999;180:S312-6.
19. Pearlstein T, Rosen K, Stone AB. Mood disorders and menopause. Endocr Metab Clin North Am 1997;26:279-94.
20. Woods N, Mitchell ES. Pathways to depressed mood for midlife women: observations from the Seattle midlife women’s health study. Res Nurs Health 1997;20:119-29.
21. Polo-Kantola P, Erkkola R. Climacteric symptoms and sleep quality. Obstet Gynecol 1999;94:219-24.
22. Dennerstein L, Dudley E, Burger H. Are changes in sexual functioning during midlife due to aging or menopause? Fertil Steril 2001;76:456-60.
23. Notelovitz M, Cassel D, Hille D, et al. Efficacy of continuous sequential transdermal estradiol and norethindrone acetate in relieving vasomotor symptoms associated with menopause. Am J Obstet Gynecol 2000;182:7-12.
24. Utian W, Burry KA, Archer DF, et al. Efficacy and safety of low, standard, and high dosages of an estradiol transdermal system (Esclim) compared with placebo on vasomotor symptoms in highly symptomatic menopausal patients. Am J Obstet Gynecol 1999;181:71-9.
25. Greendale G, Reboussin BA, Hogan P, et al. Symptom relief and side effects of postmenopausal hormones: results from the postmenopausal estrogen/progestin interventions trial. Obstet Gynecol 1998;92:982-8.
26. World Health Organization. Improving access to quality care in family planning:medical eligibility criteria for contraceptive use. Geneva, Switzerland; 1996.
27. Simon J, Kaiber E, Wiita B, Bowen YHA. Differential effects of estrogen-androgen and estrogen-only therapy on vasomotor symptoms, gonadotropin secretion, and endogenous androgen bioavailability in postmenopausal women. Menopause 1999;6:138-46.
28. Albertazzi P, Pansini F, Bonaccorsi G, Zanotti L, Forini E, de Aloysio D. The effect of dietary soy supplementation on hot flushes. Obstet Gynecol 1998;91:6-11.
29. Washburn S, Burke GL, Morgan T, Anthony M. Effect of soy protein on serum lipoproteins, blood pressure, and menopausal symptoms in perimenopausal women. Menopause 1999;6:7-13.
30. St Germaine A, Peterson CT, Robinson JG. Isoflavone-rich or isoflavone-poor soy protein does not reduce menopausal symptoms during 24 weeks of treatment. Menopause 2001;8:17-26.
31. Jacobson J, Troxel AB, Evans J. Randomized trial of black cohosh for the treatment of hot flashes among women with a history of breast cancer. J Clin Oncol 2001;19:2739-45.
32. Hirata J, Swiersz LM, Zell B, Small R, Ettinger B. Does dong quai have estrogenic effects in postmenopausal women? A double-blind, placebo-controlled trial. Fertil Steril 1997;68:981-6.
33. Fugh-Berman A, Kronenberg F. Red clover (trifolium pratense) for menopausal women: current state of knowledge. Menopause 2001;8:333-7.
34. Greendale G, Lee NP, Arriola ER. The menopause. Lancet 1999;353:571-80.
35. Quella S, Loprinzi CL, Sloan JA, et al. Long term use of megestrol acetate by cancer survivors for the treatment of hot flashes. Cancer 1998;82:1784-8.
36. Laufer L, Erlik Y, Meldrum DR, Judd HL. Effect of clonidine on hot flashes in postmenopausal women. Obstet Gynecol 1982;60:583-6.
37. Pandya K, Raubertas RF, Flynn PJ, et al. Oral clonidine in postmenopausal patients with breast cancer experiencing tamoxifen-induced hot flashes: a University of Rochester Cancer Center Community Clinical Oncology Program study. Ann Int Med 2000;132:788-93.
38. Leonetti H, Longo S, Anasti JN. Transdermal progresterone cream for vasomotor symptoms and postmenopausal bone loss. Obstet Gynecol 1999;94:225-8.
39. Freedman R, Woodward S. Behavioral treatment of menopausal hot flushes: evaluation by ambulatory monitoring. Am J Obstet Gynecol 1992;167436-9.
40. Roth A, Sacher HI. Sertraline relieves hot flashes secondary to medical castration as treatment of advanced prostate cancer. Psychooncology 1998;7:129-32.
41. Loprinzi C, Pisansky TM, Fonseca R, et al. Pilot evaluation of venlafaxine hydrochloride for the therapy of hot flashes in cancer survivors. J Clin Oncol 1998;16:2377-81.
42. Stearns V, Isaacs C, Rowland J, et al. A pilot trial of paroxetine hydrochloride in controlling hot flashes in breast cancer survivors. Ann Oncol 2000;11:17-22.
43. Zweifel J, O’Brien WH. Meta-analysis of the effect of hormone replacement therapy on depressed mood. Psychoneuroendocrinology 1997;22:189-212.
44. Soares C, Almeida OP, Joffe H, Cohen LS. Efficacy of estradiol for the treatment of depressive disorders in perimenopausal women: a double-blind, randomized, placebo-controlled trial. Arch Gen Psychiatry 2001;58:529-34.
45. Schmidt P, Neiman L, Danaceau MA, et al. Estrogen replacement in perimenopause-related depression: a preliminary report. Am J Obstet Gynecol 2000;183:414-20.
46. Bech P, Munk-Jensen N, Obel EB, et al. Combined versus sequential hormonal replacement therapy: a double-blind, placebo-controlled study on quality of life-relationed outcome measures. Psychother Psychosom 1998;7:259-65.
47. Joffe H, Cohen LS. Estrogen, serotonin and mood disturbance: where is the therapeutic bridge? Biol Psychiatry 1998;44:798-811.
48. Nedstrand E, Wijma K, Lindgren M, Hammar M. The relationship between stress-coping and vasomotor symptoms in postmenopausal women. Maturitas 1998;31:29-34.
49. Asplund R, Aberg HE. Body mass index and sleep in women aged 40-64 years. Maturitas 1995;22:1-8.
50. McCoy N. Sexual issues for postmenopausal women. Top Geriatr Rehab 1997;12:28-39.
51. American College of Obstetricians and Gynecologists Committee on Gyecologic Practice. Androgen treatment of decreased libido. Obstet Gynecol 2000;96:244-5.
52. Writing Group for the PEPI Trial. Effects of hormone therapy on bone mineral density. JAMA 1996;276:1389.-
53. Botsis D, Kassanos D, Kalogiro D, et al. Vaginal ultrasound of the endometrium in postmenopausal women with symptoms of uro genital atrophy on low-dose estrogen or tibolone treatment: a comparison. Maturitas 1997;26:57-62.
54. Collaborative Group on Hormonal Factors in Breast Cancer. Breast cancer and hormone replacement therapy: collaborative reanalysis of data from 51 epidemiological studies of 52,705 women with breast cancer and 108,411 women without breast cancer. Lancet 1997;350:1047-59.
55. Ross R, Paganini-Hill A, Wan PC, et al. Effect of hormone replacement therapy on breast cancer risk: estrogen versus estrogen plus progestin. J Natl Cancer Inst 2000;92:328-32.
56. Schairer C, Lubin J, Triosi R, et al. Menopausal estrogen and estrogen-progestin replacement therapy and breast cancer risk. JAMA 2000;283:485-91.
57. Barrett-Connor E, Grady D. Hormone replacement therapy, heart disease, and other conditions. Ann Rev Public Health 1998;19:55-72.
58. Hemminki E, McPherson K. Impact of postmenopausal therapy on cardiovascular events and cancer: pooled data from clinical trials. BMJ 1997;315:149-53.
59. Hulley S, Grady D, Bush T, et al. Randomized trial of estrogen plus progestin for secondary prevention of coronary heart disease in postmenopausal women. Heart and Estrogen/Progestin Replacement Study (HERS) Research Group. JAMA 1998;280:605-13.
60. Simon J, Hsia J, Cauley JA, et al. Postmenopausal hormone therapy and risk of stroke: The Heart and Estrogen/Progestin Replacement Study (HERS). Circulation 2001;103:638-42.
61. Wilson P, Garrison RJ, Castelli WP. Postmenopausal estrogen use, cigarette smoking, and cardiovascular morbidity in women over 50. N Engl J Med 1985;313:1038-45.
62. Oger E, Scarabin PY. Risk of stroke among users of hormone replacement therapy. Ann d’Endocrinol 1999;60:232-41.
63. Gutthann S, Rodriguez LA, Castellsague J, Oliart AD. Hormone replacement therapy and risk of thromboembolism: population based case-control study. BMJ 1997;314:796-800.
64. Daly E, Vessey MP, Hawkins MM, et al. Risk of venous thromboembolism in users of hormone replacement therapy. Lancet 1996;348:977-80.
65. Women’s Health Initiative Study Group. Design of the Women’s Health Initiative clinical trial and observational study. Control Clin Trials 1998;19:61-109.
66. Mosca L, Grundy SM, Judelson D, King K, Limacher M, Oparil S. AHA/ACC scientific statement: consensus panel statement. Guide to preventive cardiology for women. J Am Coll Cardiol 1999;33:1751-5.
67. Fantl J, Cardozo L, McClish DK. Estrogen therapy in the management of urinary incontinence in postmenopausal women: a meta-analysis. Obstet Gynecol 1994;83:12-8.
68. Grady D, Brown JS, Vittinghoff E, Applegate W, Warner E, Snyder T. Postmenopausal hormones and incontinence: the Heart and Estrogen/Progestin Replacement Study. Obstet Gynecol 2001;97:116-20.
69. Uhler M, Marks JW, Judd HL. Estrogen replacement therapy and gallbladder disease in postmenopausal women. Menopause 2000;7:162-7.
70. Mulnard R, Cotman CW, Kawas C, et al. Estrogen replacement therapy for mild to moderate Alzheimer’s disease: a randomized controlled trial. JAMA 2000;283:1007-15.
71. Langenberg P, Kjerulff KH, Stolley PD. Hormone replacement and menopausal symptoms following hysterectomy. Am J Epidemiol 1997;146:870-80.
72. Kuh D, Wadsworth M, Hardy R. Women’s health in midlife: the influence of the menopause, social factors and health in earlier life. Br J Obstet Gynaecol 1997;104:923-33.
73. Kirchengast S. Relations between anthropometric characteristics and degree of severity of the climacteric syndrome in Austrian women. Maturitas 1993;17:167-80.
74. Stadberg E, Mattson LA, Milsom I. Factors associated with climacteric symptoms and the use of hormone replacement therapy. Acta Obstet Gynecol Scand 2000;79:286-92.
75. Guthrie J, Dennerstein L, Hopper JL, Burger HG. Hot flushes, menstrual status, and hormone levels in a population-based sample of midlife women. Obstet Gynecol 1996;88:437-42.
76. Hammar M, Berg G, Lindgren R. Does physical exercise influence the frequency of postmenopausal hot flushes? Acta Obstet Gynecol Scand 1990;69:409-12.
- Laboratory testing is not indicated to initiate treatment of perimenopausal symptoms.
- While estrogens are the best established of the options to treat vasomotor symptoms at perimenopause, they are not a proven treatment for major depression or poor libido.
- Little evidence exists regarding the benefits and risks of androgens for perimenopausal women, suggesting a cautious approach to their use.
- Routine use of hormone replacement therapy, especially beyond 5 years’ duration, is not recommended because of uncertainties regarding risks and benefits.
Menopause has been successfully promoted as an estrogen-deficient state. Prescriptions in the United States for noncontraceptive estrogen formulations increased from 16 million to 39 million between 1982 and 1992; progestin sales reached 4.7 million by 1992 after their introduction in 1986.1 A condition for which half of the population becomes eligible for pharmacologic treatment for 30 years or more of their life spans is worthy of family physicians’ attention. Counseling of women regarding menopause has also been incorporated into the Health Employer Data Information Set (HEDIS) for measuring the quality of care provided by health care plans.
The women of the generation born from 1946 to 1965 are now 36 to 55 years old. About half will at some time seek medical attention for relief of symptoms believed to be related to the menopausal transition.2 The clinical picture, however, can be confusing: women at midlife are susceptible to diseases that may affect or be affected by the menopausal transition. Life cycle changes can also provoke dysphoric symptoms similar to those of menopause or aggravate symptoms that already exist.
Natural history
A woman’s hormonal rhythm changes gradually, usually in the early to middle forties. Ovarian mass decreases progressively; production of ovarian hormones decreases as well. The menstrual cycles tend to be somewhat shorter. Follicle-stimulating hormone (FSH) and estrogen levels fluctuate. Estrogen levels may be transiently higher than in former years in response to higher FSH levels, recruiting more ovarian follicles. Anovulatory cycles are more frequent. Perimenopausal menstrual irregularity typically lasts for approximately 4 years; the large majority of women experience such irregularity for 1 to 7 years.2 For 10% of women, menses simply cease without prior menstrual irregularity.
The best estimate of mean age at menopause in the United States, based on a cohort of primarily Caucasian women, is 51.3 years.2 Smokers experience menopause 1.8 years earlier than nonsmokers (50.2 versus 52.0 years). Less than 10% of women reach menopause before age 46, while approximately 30% do so before age 50.2 A recent review3 concluded that the lifetime number of ovulatory cycles is predictive of age at menopause: earlier for women with shorter cycles and nulliparous women, later for multigravid women and those with a history of oral contraceptive use. A familial tendency toward similarity in age at menopause has been noted.
Premature menopause or premature ovarian failure is defined as cessation of menstrual periods before 40 years of age. The prevalence of premature ovarian failure is approximately 1% by age 40 and 0.1% by 30 years of age.4 Premature ovarian failure is frequently an autoimmune disorder.5
Diagnosis of menopause
The gold standard for diagnosing menopause is to do so retrospectively, 1 year after the last menstrual period. In general, a diagnosis of menopause based on menstrual history or hormone levels is not considered necessary to begin treatment for perimenopausal symptoms, which often begin several years before the onset of menopause.
Laboratory diagnosis
The extent to which FSH or other serologic markers can be used to diagnose menopause is controversial. The most important clinical reason to do so is to discontinue contraceptive methods safely. Some consider an FSH level greater than 40 mIU/mL to be diagnostic. This value was chosen because it is about 2 standard deviations above the periovulatory peak in FSH levels in regularly cycling women. However, longitudinal studies6,7 during the perimenopausal years have demonstrated that hormonal patterns that include FSH values greater than 40 mIU/mL often abruptly revert to premenopausal patterns and are accompanied by ovulatory cycles. For the individual patient, hormone levels do not appear to rule out fertility reliably.8 Studies defining test characteristics (sensitivity, specificity, likelihood ratios) of hormone assays for the diagnosis of menopause are needed.
History and physical examination
A large population-based survey of Swedish women9 found that the most common climacteric symptoms are, in order of frequency, vasomotor symptoms (hot flashes), mood disturbances, sleep disturbances, decreased libido, and vaginal dryness. Several observational studies10-13 have shown that vasomotor symptoms have the clearest temporal association with the menstrual cycle changes of the climacteric. These symptoms result from a sudden change in the hypothalamic control of temperature regulation,14 although the precise triggers have not been elucidated. Hot flashes occur commonly among women in their late thirties and forties who have regular menstrual cycles.15 Several studies2,10,13,16 have shown that the prevalence of hot flashes peaks in the year immediately following the final menstrual period. A typical pattern prevalence of hot flashes is 25% in premenopausal women, 69% in perimenopausal women, and 39% in late-postmenopausal women (more than 4.5 years).17 Fifteen years after menopause, 10% of women may continue to have moderate to severe hot flashes,18 which can be lifelong.
Irritability and mood swings are common climacteric complaints. Women often compare them with their earlier premenstrual symptoms. Studies of depressive symptoms in menopausal women indicate that menopause is not associated with increased rates of major depression.19 Stressful life context and poor health status appear to be more important risk factors for depression than symptoms of menopause in climacteric women.20
Many perimenopausal women complain of poor sleep, often attributed to nocturnal hot flashes. Subjective impairment of sleep quality that is associated with climacteric vasomotor symptoms does not manifest as abnormalities in polysomnographic sleep recordings.21 It does not appear to be related to sleep apnea.
Sexual dysfunction is common in women at midlife and beyond. Dyspareunia, associated with vaginal dryness, increases in frequency with increasing time after menopause.9 The other complaint is decreased libido. Multiple factors may contribute to lack of sexual interest. Both aging and the menopause are independently associated with decreases in sexual responsiveness.22 The roles of declining endogenous sex steroid hormones in this process have not been elucidated.
Treatment
Vasomotor symptoms
Table 1 summarizes treatment options for vasomotor symptoms. Numerous well-designed clinical trials have demonstrated the effectiveness of oral or transdermal estrogen replacement therapy (ERT) for hot flashes.18,23-25 Low-dose oral contraceptive formulations are approved until 50 years of age for nonsmoking women.26 In a well-designed randomized controlled trial (RCT) of 93 women, low-dose estrogen (0.625 mg conjugated equine estrogens daily) plus 1.25 mg methyltestosterone daily was shown to be more effective than low-dose estrogen only and as effective as high-dose estrogen (1.25 mg conjugated estrogens daily).27
Phytoestrogens may be helpful, but have not yet been studied extensively. One RCT28 of 104 postmenopausal women comparing ingestion of 60 g soy protein daily with that of 60 g casein (placebo) daily showed a 45% relative reduction of hot flashes at 12 weeks in the group taking soy versus the control group. A second RCT29 of 51 women comparing soy protein with carbohydrate placebo showed a decrease in severity, but not frequency, of hot flashes. Another well-designed RCT30 including 69 women treated with 40 g soy daily versus whey protein for 24 weeks showed no difference between treatment groups and improvement in symptom scores over time in both groups. It is difficult to include a 40-g to 60-g protein supplement in the daily diet because of the accompanying caloric intake required. Recent reports of randomized placebo-controlled trials of black cohosh31 and dong quai32 and a systematic review33 of controlled trials of red clover have found no benefit.
Alternatives to estrogen for treatment of hot flashes include methyldopa, clonidine, transdermal progesterone, and megestrol acetate. Megestrol, which reduces symptoms by 70%, appears to be the most effective of these.34 Although long-term use of megestrol acetate by cancer survivors for the treatment of hot flashes has been demonstrated to be effective and well tolerated,35 it is not customarily used at menopause. A 20% reduction in hot flashes can be expected with clonidine at a dose of 0.1 to 0.2 mg daily,34,36 although this regimen may cause an increase in difficulty sleeping37 as well as dry mouth, constipation, and low blood pressure. Transdermal progesterone cream alone has been shown to improve vasomotor symptoms, although without protective effect regarding bone loss.38 One small study39 of behavioral approaches showed symptom reduction with deep-breathing relaxation techniques. Pilot studies of sertraline,40 venlafaxine,41 and paroxetine42 show promise in the treatment of hot flashes.
The remainder of this article focuses on hormonal treatment effects and risks for menopausal women. A summary appears in Table 2.
TABLE 1
TREATMENT OF VASOMOTOR SYMPTOMS
| Strength of Recommendation | Treatment | Comment |
|---|---|---|
| A | Estrogens | Many preparations with both oral and transdermal delivery have been studied |
| A | Estrogen + MPA | Other progestins not well studied |
| B | Transdermal progesterone | One RCT38 |
| B | Estrogen + testosterone | One RCT27; long-term safety is a theoretical concern |
| B | Megasterol | Cohort35; long experience with cancer patients gives some assurance of safety |
| C | Behavioral approaches | One small RCT39; deep breathing was beneficial |
| C | Clonidine | Small RCTs36,37 with important loss of subjects because of side effects |
| D | Antidepressants | Pilot studies of sertraline,40 venlafaxine,41 and paroxetine42 |
| D | Phytoestrogens | Conflicting RCT results |
| D | Exercise | Weak observational studies suggest benefit74,76 |
| No benefit seen | ||
| B | Black cohosh | No benefit seen in one RCT31 |
| B | Dong quai | No benefit seen in one RCT32 |
| B | Red clover | No benefit seen in systematic review33 |
| Grades of recommendation are based on Oxford Centre for Evidence-Based Medicine guidelines. | ||
| MPA denotes medroxyprogesterone; RCT, randomized clinical trial. | ||
TABLE 2
SUMMARY OF RISKS AND BENEFITS OF TREATMENTS FOR PERIMENOPAUSAL SYMPTOMS
| Treatment | VMS | Mood | Libido | Bone | CAD | Breast CA |
|---|---|---|---|---|---|---|
| Estrogens | Benefit | Benefit | No benefit | Benefit | Uncertain | Risk |
| Estrogen + MPA | Benefit | ? benefit | No benefit | Benefit | Uncertain* | Risk† |
| Progesterone | Benefit | Risk | No benefit | NS | Uncertain | NS |
| Testosterone | Benefit | NS | ? benefit | Benefit | NS | NS |
| Phytoestrogens | ? benefit | NS | NS | No benefit | NS | NS |
| DHEA | NS | ? benefit | ? benefit | NS | NS | NS |
| * Not beneficial for secondary prevention. † Increased risk over estrogen alone. | ||||||
| CA denotes cancer; CAD, coronary artery disease; DHEA, dihydroepiandrosterone; MPA, medroxyprogesterone acetate; NS, not studied; VMS, vasomotor symptoms. | ||||||
Mood disorders
In a meta-analysis43 including 26 RCTs of the effects of hormone replacement therapy (HRT) on depressed mood, estrogen showed limited effectiveness in improving mood. The addition of synthetic progestins reduced the estrogen effect. More recent short trials of unopposed transdermal estrogen showed benefit.44,45 Other reviews46,47 have concluded that ERT or HRT has little effect in the treatment of psychological symptoms, including anxiety, cognitive, and affective symptoms. As an adjuvant to psychotropic therapy, it may have limited effect. There is insufficient evidence to support prophylactic ERT or HRT to prevent depression in women whose medical history includes prior postpartum depression.47 Estrogens do not affect the ability of a woman with moderate to severe vasomotor symptoms to cope with stress.48 Clinical trials reporting the effects of testosterone treatment on mood in women were not identified.
Women with mild psychological and predominantly vasomotor symptoms may benefit from a trial of HRT before psychotropic medication. For women who meet criteria for a diagnosis of major depression, initial treatment with an antidepressant alone or concurrent with HRT is advisable.
Sleep disturbance
In a survey of more than 6000 women aged 40 to 64 years, 30% of HRT users reported sleep improvement that they attributed to therapy.49 Other standard approaches to insomnia, such as sleep hygiene measures and progressive relaxation techniques, can also be used. If sleep apnea is suspected, a sleep study may be indicated.
Sexual dysfunction
In a systematic review50 of HRT for climacteric sexual dysfunction, vaginal dryness improved with ERT in 7 of 8 studies. Dyspareunia improved in only 1 of 6 studies using transdermal 17-beta-estradiol. Orgasm increased in only 1 of 5 trials using ethinyl estradiol. Sexual interest increased in none of 7 studies that used conjugated estrogens. However, taking testosterone appeared to increase sexual interest. The evidence regarding the safety and efficacy of androgens (testosterone and dehydroepiandrosterone [DHEA]) for the treatment of sexual dysfunction in perimenopause is incomplete; therefore, these drugs should not routinely be prescribed.51
Bone
Although HRT prevents the rapid bone loss observed in the early menopausal period, this effect is lost when treatment is stopped. The positive effect of estrogen alone on bone mineral density was not diminished by medroxyprogesterone acetate (MPA) or micronized progesterone over a 3-year follow-up period.52 The long-term effects of MPA on fracture risk in postmenopausal women have not been reported. Use of transdermal progesterone alone does not prevent bone loss.38
Cancer
Estrogen alone for women with an intact uterus is currently considered unacceptable because adding the hormone poses endometrial cancer risk. An exception is low-dose estrogen administered intravaginally; this method does not alter the endometrium.53
Estrogen alone or in combination with progestins has been associated with an increased risk of breast cancer in many observational studies and meta-analyses. A comprehensive reanalysis54 of 51 mostly observational studies, including 52,705 cases of breast cancer and more than 100,000 controls, examined the association of breast cancer with HRT, predominantly unopposed estrogen. These authors concluded that there is an increase in incidence of breast cancer of 0.2%, 0.6%. and 1.2% with 5, 10, and 15 years of use, respectively. Thus, 1 additional case of breast cancer occurs for every 167 women treated for 10 years (number needed to harm [NNH] = 167). Two recent observational studies have documented up to a fourfold increase in breast cancer with estrogen plus progesterone over estrogen alone.55,56
Cardiovascular disease
HRT has been widely advocated for prevention of coronary artery disease (CAD), based on many observational studies. A meta-analysis57 of 25 studies published through 1997 gave a relative risk (RR) of 0.7 (CI 0.65-0.75) for coronary events in women using HRT. However, a consistent bias in these studies of selecting healthy, compliant women for inclusion may explain the observed benefit.
A meta-analysis58 of 22 trials of 4124 women comparing HRT with placebo, no therapy, or vitamins, in which cardiovascular events were secondary endpoints, revealed that there was no benefit regarding cardiac events and there were small increases in absolute risk of stroke and venous thromboembolism (VET). In the Heart and Estrogen/Progestin Replacement (HERS) study,59 conjugated equine estrogens (CEE) plus MPA, administered to women with established CAD for a mean of 4.1 years, did not reduce risk of cardiovascular events. An increase in events, particularly VET and stroke,60 occurred in the first year of use. Small increases in the absolute risks of stroke61,62 and VTE63,64 have also been described in observational studies.
Randomized trial evidence is currently lacking for a role of HRT in the primary prevention of cardiovascular disease. A large study of low-risk postmenopausal women, the Women’s Health Initiative,65 is currently under way. Its objective is to investigate strategies for the prevention and control of some of the most common causes of morbidity and mortality in postmenopausal women. The study includes 27,000 women randomized to CEE plus MPA or placebo. Results are expected in 2007. The American Heart Association now recommends against estrogen therapy with or without progestin solely for the prevention of heart disease.66 Long-term effects of androgens on cardiovascular risk have not been studied; concerns exist about their use.51
Other effects
A meta-analysis67 of trials of HRT for urinary incontinence showed no benefit. The HERS study68 showed an increase in urinary incontinence episodes with combined HRT for women with incontinence at baseline (NNH = 8). HRT also increases the risk of gallbladder disease69 and may worsen cognitive function for women with mild to moderate dementia.70
Prognosis
The symptoms of perimenopause are not life threatening and are usually limited in time. Climacteric symptoms are generally more severe and difficult to treat in women who have undergone bilateral oophorectomy before experiencing natural menopause.71 Women with multiple chronic medical conditions,13,72,73 psychiatric illnesses,15,74 or a history of premenstrual syndrome11,12,75 are also likely to experience more difficulty with symptoms attributed to the menopausal transition. Table 3 provides a list of resources for patient education regarding menopause.
TABLE 3
RESOURCES FOR PATIENT EDUCATION ABOUT MENOPAUSE
| Organization | Contact Information | Description |
|---|---|---|
| Ottawa Health Decision Centre | [email protected] 613-798-5555 | Making Choices: Hormones After Menopause (audiotape and workbook) |
| American Academy of Family Physicians | www.aafp.org 1-800-274-2237 | Brochures: “Menopause: What to Expect When Your Body Is Changing”; “Osteoporosis: Keeping Your Bones Healthy and Strong” |
| American College of Obstetricians and Gynecologists | www.acog.org 1-800-410-ACOG | Brochures: “Midlife Transitions: A Guide to the Menopause Years”; “Hormone Replacement Therapy” |
- Laboratory testing is not indicated to initiate treatment of perimenopausal symptoms.
- While estrogens are the best established of the options to treat vasomotor symptoms at perimenopause, they are not a proven treatment for major depression or poor libido.
- Little evidence exists regarding the benefits and risks of androgens for perimenopausal women, suggesting a cautious approach to their use.
- Routine use of hormone replacement therapy, especially beyond 5 years’ duration, is not recommended because of uncertainties regarding risks and benefits.
Menopause has been successfully promoted as an estrogen-deficient state. Prescriptions in the United States for noncontraceptive estrogen formulations increased from 16 million to 39 million between 1982 and 1992; progestin sales reached 4.7 million by 1992 after their introduction in 1986.1 A condition for which half of the population becomes eligible for pharmacologic treatment for 30 years or more of their life spans is worthy of family physicians’ attention. Counseling of women regarding menopause has also been incorporated into the Health Employer Data Information Set (HEDIS) for measuring the quality of care provided by health care plans.
The women of the generation born from 1946 to 1965 are now 36 to 55 years old. About half will at some time seek medical attention for relief of symptoms believed to be related to the menopausal transition.2 The clinical picture, however, can be confusing: women at midlife are susceptible to diseases that may affect or be affected by the menopausal transition. Life cycle changes can also provoke dysphoric symptoms similar to those of menopause or aggravate symptoms that already exist.
Natural history
A woman’s hormonal rhythm changes gradually, usually in the early to middle forties. Ovarian mass decreases progressively; production of ovarian hormones decreases as well. The menstrual cycles tend to be somewhat shorter. Follicle-stimulating hormone (FSH) and estrogen levels fluctuate. Estrogen levels may be transiently higher than in former years in response to higher FSH levels, recruiting more ovarian follicles. Anovulatory cycles are more frequent. Perimenopausal menstrual irregularity typically lasts for approximately 4 years; the large majority of women experience such irregularity for 1 to 7 years.2 For 10% of women, menses simply cease without prior menstrual irregularity.
The best estimate of mean age at menopause in the United States, based on a cohort of primarily Caucasian women, is 51.3 years.2 Smokers experience menopause 1.8 years earlier than nonsmokers (50.2 versus 52.0 years). Less than 10% of women reach menopause before age 46, while approximately 30% do so before age 50.2 A recent review3 concluded that the lifetime number of ovulatory cycles is predictive of age at menopause: earlier for women with shorter cycles and nulliparous women, later for multigravid women and those with a history of oral contraceptive use. A familial tendency toward similarity in age at menopause has been noted.
Premature menopause or premature ovarian failure is defined as cessation of menstrual periods before 40 years of age. The prevalence of premature ovarian failure is approximately 1% by age 40 and 0.1% by 30 years of age.4 Premature ovarian failure is frequently an autoimmune disorder.5
Diagnosis of menopause
The gold standard for diagnosing menopause is to do so retrospectively, 1 year after the last menstrual period. In general, a diagnosis of menopause based on menstrual history or hormone levels is not considered necessary to begin treatment for perimenopausal symptoms, which often begin several years before the onset of menopause.
Laboratory diagnosis
The extent to which FSH or other serologic markers can be used to diagnose menopause is controversial. The most important clinical reason to do so is to discontinue contraceptive methods safely. Some consider an FSH level greater than 40 mIU/mL to be diagnostic. This value was chosen because it is about 2 standard deviations above the periovulatory peak in FSH levels in regularly cycling women. However, longitudinal studies6,7 during the perimenopausal years have demonstrated that hormonal patterns that include FSH values greater than 40 mIU/mL often abruptly revert to premenopausal patterns and are accompanied by ovulatory cycles. For the individual patient, hormone levels do not appear to rule out fertility reliably.8 Studies defining test characteristics (sensitivity, specificity, likelihood ratios) of hormone assays for the diagnosis of menopause are needed.
History and physical examination
A large population-based survey of Swedish women9 found that the most common climacteric symptoms are, in order of frequency, vasomotor symptoms (hot flashes), mood disturbances, sleep disturbances, decreased libido, and vaginal dryness. Several observational studies10-13 have shown that vasomotor symptoms have the clearest temporal association with the menstrual cycle changes of the climacteric. These symptoms result from a sudden change in the hypothalamic control of temperature regulation,14 although the precise triggers have not been elucidated. Hot flashes occur commonly among women in their late thirties and forties who have regular menstrual cycles.15 Several studies2,10,13,16 have shown that the prevalence of hot flashes peaks in the year immediately following the final menstrual period. A typical pattern prevalence of hot flashes is 25% in premenopausal women, 69% in perimenopausal women, and 39% in late-postmenopausal women (more than 4.5 years).17 Fifteen years after menopause, 10% of women may continue to have moderate to severe hot flashes,18 which can be lifelong.
Irritability and mood swings are common climacteric complaints. Women often compare them with their earlier premenstrual symptoms. Studies of depressive symptoms in menopausal women indicate that menopause is not associated with increased rates of major depression.19 Stressful life context and poor health status appear to be more important risk factors for depression than symptoms of menopause in climacteric women.20
Many perimenopausal women complain of poor sleep, often attributed to nocturnal hot flashes. Subjective impairment of sleep quality that is associated with climacteric vasomotor symptoms does not manifest as abnormalities in polysomnographic sleep recordings.21 It does not appear to be related to sleep apnea.
Sexual dysfunction is common in women at midlife and beyond. Dyspareunia, associated with vaginal dryness, increases in frequency with increasing time after menopause.9 The other complaint is decreased libido. Multiple factors may contribute to lack of sexual interest. Both aging and the menopause are independently associated with decreases in sexual responsiveness.22 The roles of declining endogenous sex steroid hormones in this process have not been elucidated.
Treatment
Vasomotor symptoms
Table 1 summarizes treatment options for vasomotor symptoms. Numerous well-designed clinical trials have demonstrated the effectiveness of oral or transdermal estrogen replacement therapy (ERT) for hot flashes.18,23-25 Low-dose oral contraceptive formulations are approved until 50 years of age for nonsmoking women.26 In a well-designed randomized controlled trial (RCT) of 93 women, low-dose estrogen (0.625 mg conjugated equine estrogens daily) plus 1.25 mg methyltestosterone daily was shown to be more effective than low-dose estrogen only and as effective as high-dose estrogen (1.25 mg conjugated estrogens daily).27
Phytoestrogens may be helpful, but have not yet been studied extensively. One RCT28 of 104 postmenopausal women comparing ingestion of 60 g soy protein daily with that of 60 g casein (placebo) daily showed a 45% relative reduction of hot flashes at 12 weeks in the group taking soy versus the control group. A second RCT29 of 51 women comparing soy protein with carbohydrate placebo showed a decrease in severity, but not frequency, of hot flashes. Another well-designed RCT30 including 69 women treated with 40 g soy daily versus whey protein for 24 weeks showed no difference between treatment groups and improvement in symptom scores over time in both groups. It is difficult to include a 40-g to 60-g protein supplement in the daily diet because of the accompanying caloric intake required. Recent reports of randomized placebo-controlled trials of black cohosh31 and dong quai32 and a systematic review33 of controlled trials of red clover have found no benefit.
Alternatives to estrogen for treatment of hot flashes include methyldopa, clonidine, transdermal progesterone, and megestrol acetate. Megestrol, which reduces symptoms by 70%, appears to be the most effective of these.34 Although long-term use of megestrol acetate by cancer survivors for the treatment of hot flashes has been demonstrated to be effective and well tolerated,35 it is not customarily used at menopause. A 20% reduction in hot flashes can be expected with clonidine at a dose of 0.1 to 0.2 mg daily,34,36 although this regimen may cause an increase in difficulty sleeping37 as well as dry mouth, constipation, and low blood pressure. Transdermal progesterone cream alone has been shown to improve vasomotor symptoms, although without protective effect regarding bone loss.38 One small study39 of behavioral approaches showed symptom reduction with deep-breathing relaxation techniques. Pilot studies of sertraline,40 venlafaxine,41 and paroxetine42 show promise in the treatment of hot flashes.
The remainder of this article focuses on hormonal treatment effects and risks for menopausal women. A summary appears in Table 2.
TABLE 1
TREATMENT OF VASOMOTOR SYMPTOMS
| Strength of Recommendation | Treatment | Comment |
|---|---|---|
| A | Estrogens | Many preparations with both oral and transdermal delivery have been studied |
| A | Estrogen + MPA | Other progestins not well studied |
| B | Transdermal progesterone | One RCT38 |
| B | Estrogen + testosterone | One RCT27; long-term safety is a theoretical concern |
| B | Megasterol | Cohort35; long experience with cancer patients gives some assurance of safety |
| C | Behavioral approaches | One small RCT39; deep breathing was beneficial |
| C | Clonidine | Small RCTs36,37 with important loss of subjects because of side effects |
| D | Antidepressants | Pilot studies of sertraline,40 venlafaxine,41 and paroxetine42 |
| D | Phytoestrogens | Conflicting RCT results |
| D | Exercise | Weak observational studies suggest benefit74,76 |
| No benefit seen | ||
| B | Black cohosh | No benefit seen in one RCT31 |
| B | Dong quai | No benefit seen in one RCT32 |
| B | Red clover | No benefit seen in systematic review33 |
| Grades of recommendation are based on Oxford Centre for Evidence-Based Medicine guidelines. | ||
| MPA denotes medroxyprogesterone; RCT, randomized clinical trial. | ||
TABLE 2
SUMMARY OF RISKS AND BENEFITS OF TREATMENTS FOR PERIMENOPAUSAL SYMPTOMS
| Treatment | VMS | Mood | Libido | Bone | CAD | Breast CA |
|---|---|---|---|---|---|---|
| Estrogens | Benefit | Benefit | No benefit | Benefit | Uncertain | Risk |
| Estrogen + MPA | Benefit | ? benefit | No benefit | Benefit | Uncertain* | Risk† |
| Progesterone | Benefit | Risk | No benefit | NS | Uncertain | NS |
| Testosterone | Benefit | NS | ? benefit | Benefit | NS | NS |
| Phytoestrogens | ? benefit | NS | NS | No benefit | NS | NS |
| DHEA | NS | ? benefit | ? benefit | NS | NS | NS |
| * Not beneficial for secondary prevention. † Increased risk over estrogen alone. | ||||||
| CA denotes cancer; CAD, coronary artery disease; DHEA, dihydroepiandrosterone; MPA, medroxyprogesterone acetate; NS, not studied; VMS, vasomotor symptoms. | ||||||
Mood disorders
In a meta-analysis43 including 26 RCTs of the effects of hormone replacement therapy (HRT) on depressed mood, estrogen showed limited effectiveness in improving mood. The addition of synthetic progestins reduced the estrogen effect. More recent short trials of unopposed transdermal estrogen showed benefit.44,45 Other reviews46,47 have concluded that ERT or HRT has little effect in the treatment of psychological symptoms, including anxiety, cognitive, and affective symptoms. As an adjuvant to psychotropic therapy, it may have limited effect. There is insufficient evidence to support prophylactic ERT or HRT to prevent depression in women whose medical history includes prior postpartum depression.47 Estrogens do not affect the ability of a woman with moderate to severe vasomotor symptoms to cope with stress.48 Clinical trials reporting the effects of testosterone treatment on mood in women were not identified.
Women with mild psychological and predominantly vasomotor symptoms may benefit from a trial of HRT before psychotropic medication. For women who meet criteria for a diagnosis of major depression, initial treatment with an antidepressant alone or concurrent with HRT is advisable.
Sleep disturbance
In a survey of more than 6000 women aged 40 to 64 years, 30% of HRT users reported sleep improvement that they attributed to therapy.49 Other standard approaches to insomnia, such as sleep hygiene measures and progressive relaxation techniques, can also be used. If sleep apnea is suspected, a sleep study may be indicated.
Sexual dysfunction
In a systematic review50 of HRT for climacteric sexual dysfunction, vaginal dryness improved with ERT in 7 of 8 studies. Dyspareunia improved in only 1 of 6 studies using transdermal 17-beta-estradiol. Orgasm increased in only 1 of 5 trials using ethinyl estradiol. Sexual interest increased in none of 7 studies that used conjugated estrogens. However, taking testosterone appeared to increase sexual interest. The evidence regarding the safety and efficacy of androgens (testosterone and dehydroepiandrosterone [DHEA]) for the treatment of sexual dysfunction in perimenopause is incomplete; therefore, these drugs should not routinely be prescribed.51
Bone
Although HRT prevents the rapid bone loss observed in the early menopausal period, this effect is lost when treatment is stopped. The positive effect of estrogen alone on bone mineral density was not diminished by medroxyprogesterone acetate (MPA) or micronized progesterone over a 3-year follow-up period.52 The long-term effects of MPA on fracture risk in postmenopausal women have not been reported. Use of transdermal progesterone alone does not prevent bone loss.38
Cancer
Estrogen alone for women with an intact uterus is currently considered unacceptable because adding the hormone poses endometrial cancer risk. An exception is low-dose estrogen administered intravaginally; this method does not alter the endometrium.53
Estrogen alone or in combination with progestins has been associated with an increased risk of breast cancer in many observational studies and meta-analyses. A comprehensive reanalysis54 of 51 mostly observational studies, including 52,705 cases of breast cancer and more than 100,000 controls, examined the association of breast cancer with HRT, predominantly unopposed estrogen. These authors concluded that there is an increase in incidence of breast cancer of 0.2%, 0.6%. and 1.2% with 5, 10, and 15 years of use, respectively. Thus, 1 additional case of breast cancer occurs for every 167 women treated for 10 years (number needed to harm [NNH] = 167). Two recent observational studies have documented up to a fourfold increase in breast cancer with estrogen plus progesterone over estrogen alone.55,56
Cardiovascular disease
HRT has been widely advocated for prevention of coronary artery disease (CAD), based on many observational studies. A meta-analysis57 of 25 studies published through 1997 gave a relative risk (RR) of 0.7 (CI 0.65-0.75) for coronary events in women using HRT. However, a consistent bias in these studies of selecting healthy, compliant women for inclusion may explain the observed benefit.
A meta-analysis58 of 22 trials of 4124 women comparing HRT with placebo, no therapy, or vitamins, in which cardiovascular events were secondary endpoints, revealed that there was no benefit regarding cardiac events and there were small increases in absolute risk of stroke and venous thromboembolism (VET). In the Heart and Estrogen/Progestin Replacement (HERS) study,59 conjugated equine estrogens (CEE) plus MPA, administered to women with established CAD for a mean of 4.1 years, did not reduce risk of cardiovascular events. An increase in events, particularly VET and stroke,60 occurred in the first year of use. Small increases in the absolute risks of stroke61,62 and VTE63,64 have also been described in observational studies.
Randomized trial evidence is currently lacking for a role of HRT in the primary prevention of cardiovascular disease. A large study of low-risk postmenopausal women, the Women’s Health Initiative,65 is currently under way. Its objective is to investigate strategies for the prevention and control of some of the most common causes of morbidity and mortality in postmenopausal women. The study includes 27,000 women randomized to CEE plus MPA or placebo. Results are expected in 2007. The American Heart Association now recommends against estrogen therapy with or without progestin solely for the prevention of heart disease.66 Long-term effects of androgens on cardiovascular risk have not been studied; concerns exist about their use.51
Other effects
A meta-analysis67 of trials of HRT for urinary incontinence showed no benefit. The HERS study68 showed an increase in urinary incontinence episodes with combined HRT for women with incontinence at baseline (NNH = 8). HRT also increases the risk of gallbladder disease69 and may worsen cognitive function for women with mild to moderate dementia.70
Prognosis
The symptoms of perimenopause are not life threatening and are usually limited in time. Climacteric symptoms are generally more severe and difficult to treat in women who have undergone bilateral oophorectomy before experiencing natural menopause.71 Women with multiple chronic medical conditions,13,72,73 psychiatric illnesses,15,74 or a history of premenstrual syndrome11,12,75 are also likely to experience more difficulty with symptoms attributed to the menopausal transition. Table 3 provides a list of resources for patient education regarding menopause.
TABLE 3
RESOURCES FOR PATIENT EDUCATION ABOUT MENOPAUSE
| Organization | Contact Information | Description |
|---|---|---|
| Ottawa Health Decision Centre | [email protected] 613-798-5555 | Making Choices: Hormones After Menopause (audiotape and workbook) |
| American Academy of Family Physicians | www.aafp.org 1-800-274-2237 | Brochures: “Menopause: What to Expect When Your Body Is Changing”; “Osteoporosis: Keeping Your Bones Healthy and Strong” |
| American College of Obstetricians and Gynecologists | www.acog.org 1-800-410-ACOG | Brochures: “Midlife Transitions: A Guide to the Menopause Years”; “Hormone Replacement Therapy” |
1. Wysowski D, Golden L, Burke L. Use of menopausal estrogens and medroxyprogesterone in the United States, 1982-1992. Obstet Gynecol 1995;85:6-10.
2. McKinlay S, Brambilla PJ, Posnere JG. The normal menopause transition. Maturitas 1992;14:13-5.
3. Harlow B, Signorello LB. Factors associated with early menopause. Maturitas 2000;35:3-9.
4. Coulam C, Adamson SC, Annegers JF. Incidence of premature ovarian failure. Obstet Gynecol 1986;67:604-6.
5. Kalantaridou S, Nelson LM. Premature ovarian failure is not premature menopause. Ann NY Acad Sci 2000;900:393-402.
6. Hee J, MacNaughton J, Bangah M, Burger HG. Perimenopausal patterns of gonadotropins, immunoreactive inhibin, oestradiol and progesterone. Maturitas 1993;18:9-20.
7. Rannevik G, Jeppsson S, Johnell O, Bjerre B, Laurell-Borulf Y, Svanberg L. A longitudinal study of the perimenopausal transition: altered profiles of steroid and pituitary hormones, SHBG and bone moneral density. Maturitas 1995;21:103-13.
8. Burger H. Diagnostic role of follicle-stimulating hormone (FSH) measurements during the menopausal transition—an analysis of FSH, oestradiol and inhibin. Eur J Endocrinol 1994;130:38-42.
9. Stadberg E, Mattson LA, Milsom I. The prevalence and severity of climacteric symptoms and the use of different treatment regimens in a Swedish population. Acta Obstet Gynecol Scand 1997;76:442-8.
10. Holte A, Mikkelsen A. The menopausal syndrome: a factor analytic replication. Maturitas 1991;13:193-203.
11. Hunter M. The South-East England Longitudinal Study of the climacteric and postmenopause. Maturitas 1992;14:217-28.
12. Collins A, Landgren BM. Reproductive health, use of estrogen and experience of symptoms in perimenopausal women: a population-based study. Maturitas 1995;20:101-11.
13. Dennerstein L, Smith AMA, Morse C, Burger H, Green A, Hopper J, et al. Menopausal symptoms in Australian women. Med J Aust 1993;159:232-6.
14. Mashchak C, Kletsky QA, Artel R, et al. The relation of physiological changes to subjective symptoms in postmenopausal women with and without hot flushes. Maturitas 1985;6:301-8.
15. Grisso J, Freeman EW, Maurin E, Garcia-Espans B, Berlin JA. Racial differences in menopause information and the experience of hot flashes. J Gen Intern Med 1999;14:98-103.
16. Oldenhave A, Jaszmann LJB, Haspels AA, et al. Impact of climacteric on well-being. Am J Obstet Gynecol 1993;168:772-80.
17. Barentsen R, Groeneveld FP, Bareman FP, et al. Women’s opinion on withdrawal bleeding with hormone replacement therapy. Eur J Obstet Gynecol Reprod Biol 1993;51:203-7.
18. Bachman G. Vasomotor flushes in menopausal women. Am J Obstet Gynecol 1999;180:S312-6.
19. Pearlstein T, Rosen K, Stone AB. Mood disorders and menopause. Endocr Metab Clin North Am 1997;26:279-94.
20. Woods N, Mitchell ES. Pathways to depressed mood for midlife women: observations from the Seattle midlife women’s health study. Res Nurs Health 1997;20:119-29.
21. Polo-Kantola P, Erkkola R. Climacteric symptoms and sleep quality. Obstet Gynecol 1999;94:219-24.
22. Dennerstein L, Dudley E, Burger H. Are changes in sexual functioning during midlife due to aging or menopause? Fertil Steril 2001;76:456-60.
23. Notelovitz M, Cassel D, Hille D, et al. Efficacy of continuous sequential transdermal estradiol and norethindrone acetate in relieving vasomotor symptoms associated with menopause. Am J Obstet Gynecol 2000;182:7-12.
24. Utian W, Burry KA, Archer DF, et al. Efficacy and safety of low, standard, and high dosages of an estradiol transdermal system (Esclim) compared with placebo on vasomotor symptoms in highly symptomatic menopausal patients. Am J Obstet Gynecol 1999;181:71-9.
25. Greendale G, Reboussin BA, Hogan P, et al. Symptom relief and side effects of postmenopausal hormones: results from the postmenopausal estrogen/progestin interventions trial. Obstet Gynecol 1998;92:982-8.
26. World Health Organization. Improving access to quality care in family planning:medical eligibility criteria for contraceptive use. Geneva, Switzerland; 1996.
27. Simon J, Kaiber E, Wiita B, Bowen YHA. Differential effects of estrogen-androgen and estrogen-only therapy on vasomotor symptoms, gonadotropin secretion, and endogenous androgen bioavailability in postmenopausal women. Menopause 1999;6:138-46.
28. Albertazzi P, Pansini F, Bonaccorsi G, Zanotti L, Forini E, de Aloysio D. The effect of dietary soy supplementation on hot flushes. Obstet Gynecol 1998;91:6-11.
29. Washburn S, Burke GL, Morgan T, Anthony M. Effect of soy protein on serum lipoproteins, blood pressure, and menopausal symptoms in perimenopausal women. Menopause 1999;6:7-13.
30. St Germaine A, Peterson CT, Robinson JG. Isoflavone-rich or isoflavone-poor soy protein does not reduce menopausal symptoms during 24 weeks of treatment. Menopause 2001;8:17-26.
31. Jacobson J, Troxel AB, Evans J. Randomized trial of black cohosh for the treatment of hot flashes among women with a history of breast cancer. J Clin Oncol 2001;19:2739-45.
32. Hirata J, Swiersz LM, Zell B, Small R, Ettinger B. Does dong quai have estrogenic effects in postmenopausal women? A double-blind, placebo-controlled trial. Fertil Steril 1997;68:981-6.
33. Fugh-Berman A, Kronenberg F. Red clover (trifolium pratense) for menopausal women: current state of knowledge. Menopause 2001;8:333-7.
34. Greendale G, Lee NP, Arriola ER. The menopause. Lancet 1999;353:571-80.
35. Quella S, Loprinzi CL, Sloan JA, et al. Long term use of megestrol acetate by cancer survivors for the treatment of hot flashes. Cancer 1998;82:1784-8.
36. Laufer L, Erlik Y, Meldrum DR, Judd HL. Effect of clonidine on hot flashes in postmenopausal women. Obstet Gynecol 1982;60:583-6.
37. Pandya K, Raubertas RF, Flynn PJ, et al. Oral clonidine in postmenopausal patients with breast cancer experiencing tamoxifen-induced hot flashes: a University of Rochester Cancer Center Community Clinical Oncology Program study. Ann Int Med 2000;132:788-93.
38. Leonetti H, Longo S, Anasti JN. Transdermal progresterone cream for vasomotor symptoms and postmenopausal bone loss. Obstet Gynecol 1999;94:225-8.
39. Freedman R, Woodward S. Behavioral treatment of menopausal hot flushes: evaluation by ambulatory monitoring. Am J Obstet Gynecol 1992;167436-9.
40. Roth A, Sacher HI. Sertraline relieves hot flashes secondary to medical castration as treatment of advanced prostate cancer. Psychooncology 1998;7:129-32.
41. Loprinzi C, Pisansky TM, Fonseca R, et al. Pilot evaluation of venlafaxine hydrochloride for the therapy of hot flashes in cancer survivors. J Clin Oncol 1998;16:2377-81.
42. Stearns V, Isaacs C, Rowland J, et al. A pilot trial of paroxetine hydrochloride in controlling hot flashes in breast cancer survivors. Ann Oncol 2000;11:17-22.
43. Zweifel J, O’Brien WH. Meta-analysis of the effect of hormone replacement therapy on depressed mood. Psychoneuroendocrinology 1997;22:189-212.
44. Soares C, Almeida OP, Joffe H, Cohen LS. Efficacy of estradiol for the treatment of depressive disorders in perimenopausal women: a double-blind, randomized, placebo-controlled trial. Arch Gen Psychiatry 2001;58:529-34.
45. Schmidt P, Neiman L, Danaceau MA, et al. Estrogen replacement in perimenopause-related depression: a preliminary report. Am J Obstet Gynecol 2000;183:414-20.
46. Bech P, Munk-Jensen N, Obel EB, et al. Combined versus sequential hormonal replacement therapy: a double-blind, placebo-controlled study on quality of life-relationed outcome measures. Psychother Psychosom 1998;7:259-65.
47. Joffe H, Cohen LS. Estrogen, serotonin and mood disturbance: where is the therapeutic bridge? Biol Psychiatry 1998;44:798-811.
48. Nedstrand E, Wijma K, Lindgren M, Hammar M. The relationship between stress-coping and vasomotor symptoms in postmenopausal women. Maturitas 1998;31:29-34.
49. Asplund R, Aberg HE. Body mass index and sleep in women aged 40-64 years. Maturitas 1995;22:1-8.
50. McCoy N. Sexual issues for postmenopausal women. Top Geriatr Rehab 1997;12:28-39.
51. American College of Obstetricians and Gynecologists Committee on Gyecologic Practice. Androgen treatment of decreased libido. Obstet Gynecol 2000;96:244-5.
52. Writing Group for the PEPI Trial. Effects of hormone therapy on bone mineral density. JAMA 1996;276:1389.-
53. Botsis D, Kassanos D, Kalogiro D, et al. Vaginal ultrasound of the endometrium in postmenopausal women with symptoms of uro genital atrophy on low-dose estrogen or tibolone treatment: a comparison. Maturitas 1997;26:57-62.
54. Collaborative Group on Hormonal Factors in Breast Cancer. Breast cancer and hormone replacement therapy: collaborative reanalysis of data from 51 epidemiological studies of 52,705 women with breast cancer and 108,411 women without breast cancer. Lancet 1997;350:1047-59.
55. Ross R, Paganini-Hill A, Wan PC, et al. Effect of hormone replacement therapy on breast cancer risk: estrogen versus estrogen plus progestin. J Natl Cancer Inst 2000;92:328-32.
56. Schairer C, Lubin J, Triosi R, et al. Menopausal estrogen and estrogen-progestin replacement therapy and breast cancer risk. JAMA 2000;283:485-91.
57. Barrett-Connor E, Grady D. Hormone replacement therapy, heart disease, and other conditions. Ann Rev Public Health 1998;19:55-72.
58. Hemminki E, McPherson K. Impact of postmenopausal therapy on cardiovascular events and cancer: pooled data from clinical trials. BMJ 1997;315:149-53.
59. Hulley S, Grady D, Bush T, et al. Randomized trial of estrogen plus progestin for secondary prevention of coronary heart disease in postmenopausal women. Heart and Estrogen/Progestin Replacement Study (HERS) Research Group. JAMA 1998;280:605-13.
60. Simon J, Hsia J, Cauley JA, et al. Postmenopausal hormone therapy and risk of stroke: The Heart and Estrogen/Progestin Replacement Study (HERS). Circulation 2001;103:638-42.
61. Wilson P, Garrison RJ, Castelli WP. Postmenopausal estrogen use, cigarette smoking, and cardiovascular morbidity in women over 50. N Engl J Med 1985;313:1038-45.
62. Oger E, Scarabin PY. Risk of stroke among users of hormone replacement therapy. Ann d’Endocrinol 1999;60:232-41.
63. Gutthann S, Rodriguez LA, Castellsague J, Oliart AD. Hormone replacement therapy and risk of thromboembolism: population based case-control study. BMJ 1997;314:796-800.
64. Daly E, Vessey MP, Hawkins MM, et al. Risk of venous thromboembolism in users of hormone replacement therapy. Lancet 1996;348:977-80.
65. Women’s Health Initiative Study Group. Design of the Women’s Health Initiative clinical trial and observational study. Control Clin Trials 1998;19:61-109.
66. Mosca L, Grundy SM, Judelson D, King K, Limacher M, Oparil S. AHA/ACC scientific statement: consensus panel statement. Guide to preventive cardiology for women. J Am Coll Cardiol 1999;33:1751-5.
67. Fantl J, Cardozo L, McClish DK. Estrogen therapy in the management of urinary incontinence in postmenopausal women: a meta-analysis. Obstet Gynecol 1994;83:12-8.
68. Grady D, Brown JS, Vittinghoff E, Applegate W, Warner E, Snyder T. Postmenopausal hormones and incontinence: the Heart and Estrogen/Progestin Replacement Study. Obstet Gynecol 2001;97:116-20.
69. Uhler M, Marks JW, Judd HL. Estrogen replacement therapy and gallbladder disease in postmenopausal women. Menopause 2000;7:162-7.
70. Mulnard R, Cotman CW, Kawas C, et al. Estrogen replacement therapy for mild to moderate Alzheimer’s disease: a randomized controlled trial. JAMA 2000;283:1007-15.
71. Langenberg P, Kjerulff KH, Stolley PD. Hormone replacement and menopausal symptoms following hysterectomy. Am J Epidemiol 1997;146:870-80.
72. Kuh D, Wadsworth M, Hardy R. Women’s health in midlife: the influence of the menopause, social factors and health in earlier life. Br J Obstet Gynaecol 1997;104:923-33.
73. Kirchengast S. Relations between anthropometric characteristics and degree of severity of the climacteric syndrome in Austrian women. Maturitas 1993;17:167-80.
74. Stadberg E, Mattson LA, Milsom I. Factors associated with climacteric symptoms and the use of hormone replacement therapy. Acta Obstet Gynecol Scand 2000;79:286-92.
75. Guthrie J, Dennerstein L, Hopper JL, Burger HG. Hot flushes, menstrual status, and hormone levels in a population-based sample of midlife women. Obstet Gynecol 1996;88:437-42.
76. Hammar M, Berg G, Lindgren R. Does physical exercise influence the frequency of postmenopausal hot flushes? Acta Obstet Gynecol Scand 1990;69:409-12.
1. Wysowski D, Golden L, Burke L. Use of menopausal estrogens and medroxyprogesterone in the United States, 1982-1992. Obstet Gynecol 1995;85:6-10.
2. McKinlay S, Brambilla PJ, Posnere JG. The normal menopause transition. Maturitas 1992;14:13-5.
3. Harlow B, Signorello LB. Factors associated with early menopause. Maturitas 2000;35:3-9.
4. Coulam C, Adamson SC, Annegers JF. Incidence of premature ovarian failure. Obstet Gynecol 1986;67:604-6.
5. Kalantaridou S, Nelson LM. Premature ovarian failure is not premature menopause. Ann NY Acad Sci 2000;900:393-402.
6. Hee J, MacNaughton J, Bangah M, Burger HG. Perimenopausal patterns of gonadotropins, immunoreactive inhibin, oestradiol and progesterone. Maturitas 1993;18:9-20.
7. Rannevik G, Jeppsson S, Johnell O, Bjerre B, Laurell-Borulf Y, Svanberg L. A longitudinal study of the perimenopausal transition: altered profiles of steroid and pituitary hormones, SHBG and bone moneral density. Maturitas 1995;21:103-13.
8. Burger H. Diagnostic role of follicle-stimulating hormone (FSH) measurements during the menopausal transition—an analysis of FSH, oestradiol and inhibin. Eur J Endocrinol 1994;130:38-42.
9. Stadberg E, Mattson LA, Milsom I. The prevalence and severity of climacteric symptoms and the use of different treatment regimens in a Swedish population. Acta Obstet Gynecol Scand 1997;76:442-8.
10. Holte A, Mikkelsen A. The menopausal syndrome: a factor analytic replication. Maturitas 1991;13:193-203.
11. Hunter M. The South-East England Longitudinal Study of the climacteric and postmenopause. Maturitas 1992;14:217-28.
12. Collins A, Landgren BM. Reproductive health, use of estrogen and experience of symptoms in perimenopausal women: a population-based study. Maturitas 1995;20:101-11.
13. Dennerstein L, Smith AMA, Morse C, Burger H, Green A, Hopper J, et al. Menopausal symptoms in Australian women. Med J Aust 1993;159:232-6.
14. Mashchak C, Kletsky QA, Artel R, et al. The relation of physiological changes to subjective symptoms in postmenopausal women with and without hot flushes. Maturitas 1985;6:301-8.
15. Grisso J, Freeman EW, Maurin E, Garcia-Espans B, Berlin JA. Racial differences in menopause information and the experience of hot flashes. J Gen Intern Med 1999;14:98-103.
16. Oldenhave A, Jaszmann LJB, Haspels AA, et al. Impact of climacteric on well-being. Am J Obstet Gynecol 1993;168:772-80.
17. Barentsen R, Groeneveld FP, Bareman FP, et al. Women’s opinion on withdrawal bleeding with hormone replacement therapy. Eur J Obstet Gynecol Reprod Biol 1993;51:203-7.
18. Bachman G. Vasomotor flushes in menopausal women. Am J Obstet Gynecol 1999;180:S312-6.
19. Pearlstein T, Rosen K, Stone AB. Mood disorders and menopause. Endocr Metab Clin North Am 1997;26:279-94.
20. Woods N, Mitchell ES. Pathways to depressed mood for midlife women: observations from the Seattle midlife women’s health study. Res Nurs Health 1997;20:119-29.
21. Polo-Kantola P, Erkkola R. Climacteric symptoms and sleep quality. Obstet Gynecol 1999;94:219-24.
22. Dennerstein L, Dudley E, Burger H. Are changes in sexual functioning during midlife due to aging or menopause? Fertil Steril 2001;76:456-60.
23. Notelovitz M, Cassel D, Hille D, et al. Efficacy of continuous sequential transdermal estradiol and norethindrone acetate in relieving vasomotor symptoms associated with menopause. Am J Obstet Gynecol 2000;182:7-12.
24. Utian W, Burry KA, Archer DF, et al. Efficacy and safety of low, standard, and high dosages of an estradiol transdermal system (Esclim) compared with placebo on vasomotor symptoms in highly symptomatic menopausal patients. Am J Obstet Gynecol 1999;181:71-9.
25. Greendale G, Reboussin BA, Hogan P, et al. Symptom relief and side effects of postmenopausal hormones: results from the postmenopausal estrogen/progestin interventions trial. Obstet Gynecol 1998;92:982-8.
26. World Health Organization. Improving access to quality care in family planning:medical eligibility criteria for contraceptive use. Geneva, Switzerland; 1996.
27. Simon J, Kaiber E, Wiita B, Bowen YHA. Differential effects of estrogen-androgen and estrogen-only therapy on vasomotor symptoms, gonadotropin secretion, and endogenous androgen bioavailability in postmenopausal women. Menopause 1999;6:138-46.
28. Albertazzi P, Pansini F, Bonaccorsi G, Zanotti L, Forini E, de Aloysio D. The effect of dietary soy supplementation on hot flushes. Obstet Gynecol 1998;91:6-11.
29. Washburn S, Burke GL, Morgan T, Anthony M. Effect of soy protein on serum lipoproteins, blood pressure, and menopausal symptoms in perimenopausal women. Menopause 1999;6:7-13.
30. St Germaine A, Peterson CT, Robinson JG. Isoflavone-rich or isoflavone-poor soy protein does not reduce menopausal symptoms during 24 weeks of treatment. Menopause 2001;8:17-26.
31. Jacobson J, Troxel AB, Evans J. Randomized trial of black cohosh for the treatment of hot flashes among women with a history of breast cancer. J Clin Oncol 2001;19:2739-45.
32. Hirata J, Swiersz LM, Zell B, Small R, Ettinger B. Does dong quai have estrogenic effects in postmenopausal women? A double-blind, placebo-controlled trial. Fertil Steril 1997;68:981-6.
33. Fugh-Berman A, Kronenberg F. Red clover (trifolium pratense) for menopausal women: current state of knowledge. Menopause 2001;8:333-7.
34. Greendale G, Lee NP, Arriola ER. The menopause. Lancet 1999;353:571-80.
35. Quella S, Loprinzi CL, Sloan JA, et al. Long term use of megestrol acetate by cancer survivors for the treatment of hot flashes. Cancer 1998;82:1784-8.
36. Laufer L, Erlik Y, Meldrum DR, Judd HL. Effect of clonidine on hot flashes in postmenopausal women. Obstet Gynecol 1982;60:583-6.
37. Pandya K, Raubertas RF, Flynn PJ, et al. Oral clonidine in postmenopausal patients with breast cancer experiencing tamoxifen-induced hot flashes: a University of Rochester Cancer Center Community Clinical Oncology Program study. Ann Int Med 2000;132:788-93.
38. Leonetti H, Longo S, Anasti JN. Transdermal progresterone cream for vasomotor symptoms and postmenopausal bone loss. Obstet Gynecol 1999;94:225-8.
39. Freedman R, Woodward S. Behavioral treatment of menopausal hot flushes: evaluation by ambulatory monitoring. Am J Obstet Gynecol 1992;167436-9.
40. Roth A, Sacher HI. Sertraline relieves hot flashes secondary to medical castration as treatment of advanced prostate cancer. Psychooncology 1998;7:129-32.
41. Loprinzi C, Pisansky TM, Fonseca R, et al. Pilot evaluation of venlafaxine hydrochloride for the therapy of hot flashes in cancer survivors. J Clin Oncol 1998;16:2377-81.
42. Stearns V, Isaacs C, Rowland J, et al. A pilot trial of paroxetine hydrochloride in controlling hot flashes in breast cancer survivors. Ann Oncol 2000;11:17-22.
43. Zweifel J, O’Brien WH. Meta-analysis of the effect of hormone replacement therapy on depressed mood. Psychoneuroendocrinology 1997;22:189-212.
44. Soares C, Almeida OP, Joffe H, Cohen LS. Efficacy of estradiol for the treatment of depressive disorders in perimenopausal women: a double-blind, randomized, placebo-controlled trial. Arch Gen Psychiatry 2001;58:529-34.
45. Schmidt P, Neiman L, Danaceau MA, et al. Estrogen replacement in perimenopause-related depression: a preliminary report. Am J Obstet Gynecol 2000;183:414-20.
46. Bech P, Munk-Jensen N, Obel EB, et al. Combined versus sequential hormonal replacement therapy: a double-blind, placebo-controlled study on quality of life-relationed outcome measures. Psychother Psychosom 1998;7:259-65.
47. Joffe H, Cohen LS. Estrogen, serotonin and mood disturbance: where is the therapeutic bridge? Biol Psychiatry 1998;44:798-811.
48. Nedstrand E, Wijma K, Lindgren M, Hammar M. The relationship between stress-coping and vasomotor symptoms in postmenopausal women. Maturitas 1998;31:29-34.
49. Asplund R, Aberg HE. Body mass index and sleep in women aged 40-64 years. Maturitas 1995;22:1-8.
50. McCoy N. Sexual issues for postmenopausal women. Top Geriatr Rehab 1997;12:28-39.
51. American College of Obstetricians and Gynecologists Committee on Gyecologic Practice. Androgen treatment of decreased libido. Obstet Gynecol 2000;96:244-5.
52. Writing Group for the PEPI Trial. Effects of hormone therapy on bone mineral density. JAMA 1996;276:1389.-
53. Botsis D, Kassanos D, Kalogiro D, et al. Vaginal ultrasound of the endometrium in postmenopausal women with symptoms of uro genital atrophy on low-dose estrogen or tibolone treatment: a comparison. Maturitas 1997;26:57-62.
54. Collaborative Group on Hormonal Factors in Breast Cancer. Breast cancer and hormone replacement therapy: collaborative reanalysis of data from 51 epidemiological studies of 52,705 women with breast cancer and 108,411 women without breast cancer. Lancet 1997;350:1047-59.
55. Ross R, Paganini-Hill A, Wan PC, et al. Effect of hormone replacement therapy on breast cancer risk: estrogen versus estrogen plus progestin. J Natl Cancer Inst 2000;92:328-32.
56. Schairer C, Lubin J, Triosi R, et al. Menopausal estrogen and estrogen-progestin replacement therapy and breast cancer risk. JAMA 2000;283:485-91.
57. Barrett-Connor E, Grady D. Hormone replacement therapy, heart disease, and other conditions. Ann Rev Public Health 1998;19:55-72.
58. Hemminki E, McPherson K. Impact of postmenopausal therapy on cardiovascular events and cancer: pooled data from clinical trials. BMJ 1997;315:149-53.
59. Hulley S, Grady D, Bush T, et al. Randomized trial of estrogen plus progestin for secondary prevention of coronary heart disease in postmenopausal women. Heart and Estrogen/Progestin Replacement Study (HERS) Research Group. JAMA 1998;280:605-13.
60. Simon J, Hsia J, Cauley JA, et al. Postmenopausal hormone therapy and risk of stroke: The Heart and Estrogen/Progestin Replacement Study (HERS). Circulation 2001;103:638-42.
61. Wilson P, Garrison RJ, Castelli WP. Postmenopausal estrogen use, cigarette smoking, and cardiovascular morbidity in women over 50. N Engl J Med 1985;313:1038-45.
62. Oger E, Scarabin PY. Risk of stroke among users of hormone replacement therapy. Ann d’Endocrinol 1999;60:232-41.
63. Gutthann S, Rodriguez LA, Castellsague J, Oliart AD. Hormone replacement therapy and risk of thromboembolism: population based case-control study. BMJ 1997;314:796-800.
64. Daly E, Vessey MP, Hawkins MM, et al. Risk of venous thromboembolism in users of hormone replacement therapy. Lancet 1996;348:977-80.
65. Women’s Health Initiative Study Group. Design of the Women’s Health Initiative clinical trial and observational study. Control Clin Trials 1998;19:61-109.
66. Mosca L, Grundy SM, Judelson D, King K, Limacher M, Oparil S. AHA/ACC scientific statement: consensus panel statement. Guide to preventive cardiology for women. J Am Coll Cardiol 1999;33:1751-5.
67. Fantl J, Cardozo L, McClish DK. Estrogen therapy in the management of urinary incontinence in postmenopausal women: a meta-analysis. Obstet Gynecol 1994;83:12-8.
68. Grady D, Brown JS, Vittinghoff E, Applegate W, Warner E, Snyder T. Postmenopausal hormones and incontinence: the Heart and Estrogen/Progestin Replacement Study. Obstet Gynecol 2001;97:116-20.
69. Uhler M, Marks JW, Judd HL. Estrogen replacement therapy and gallbladder disease in postmenopausal women. Menopause 2000;7:162-7.
70. Mulnard R, Cotman CW, Kawas C, et al. Estrogen replacement therapy for mild to moderate Alzheimer’s disease: a randomized controlled trial. JAMA 2000;283:1007-15.
71. Langenberg P, Kjerulff KH, Stolley PD. Hormone replacement and menopausal symptoms following hysterectomy. Am J Epidemiol 1997;146:870-80.
72. Kuh D, Wadsworth M, Hardy R. Women’s health in midlife: the influence of the menopause, social factors and health in earlier life. Br J Obstet Gynaecol 1997;104:923-33.
73. Kirchengast S. Relations between anthropometric characteristics and degree of severity of the climacteric syndrome in Austrian women. Maturitas 1993;17:167-80.
74. Stadberg E, Mattson LA, Milsom I. Factors associated with climacteric symptoms and the use of hormone replacement therapy. Acta Obstet Gynecol Scand 2000;79:286-92.
75. Guthrie J, Dennerstein L, Hopper JL, Burger HG. Hot flushes, menstrual status, and hormone levels in a population-based sample of midlife women. Obstet Gynecol 1996;88:437-42.
76. Hammar M, Berg G, Lindgren R. Does physical exercise influence the frequency of postmenopausal hot flushes? Acta Obstet Gynecol Scand 1990;69:409-12.
Evaluation and Treatment of the Adult Patient With Migraine
- The International Headache Society criteria provide a useful standardized way to diagnose migraine clinically.
- Neuroimaging is not necessary for patients who clearly meet clinical criteria for migraine and whose neurologic examination results are normal.
- Over-the-counter drugs (including aspirin, ibuprofen, and the combination of aspirin, acetaminophen, and caffeine) work well and are first-line treatments for mild migraine.
- Migraine-specific medications (including intranasal dihydroergotamine [DHE] and the triptans) are recommended for more severe migraine; little evidence exists to suggest one drug over another.
- Prophylaxis is recommended if patients find the severity or frequency of headaches bothersome enough to warrant preventive measures; amitriptyline, divalproex sodium, and propranolol are effective prophylaxes.
Migraine is a common and disabling condition. Among adults in the United States, approximately 18% of women and 6% of men report symptoms consistent with migraine1; less than half have been diagnosed by a physician or received prescription treatment from a physician.2 Migraine accounts for more than 2.8 million visits per year to US physicians and is the reason for encounter in about 1 visit per week to the typical family physician.3 Migraine is estimated to cost US employers more than $13 billion each year; direct medical costs exceed $1 billion annually.4
Pathophysiology
The exact pathophysiology of migraine is unknown. The prevailing theory is that a trigger (such as fatigue, stress, or certain foods) sets off a wave of brief neuronal activation, followed by a more sustained neuronal inhibition known as cortical spreading depression (CSD). At some point the trigeminovascular system is activated (possibly by CSD), releasing vasoactive neuropeptides that cause a painful inflammatory response in the meninges. Stimulation of presynaptic serotonin receptors inhibits release of the inflammatory neuropeptides; this is one possible explanation for the effectiveness of the triptans.5
Diagnosis
Migraine is a syndrome diagnosed by a certain combination of signs and symptoms. The International Headache Society (IHS) diagnostic criteria (Table 1) are widely accepted as the reference standard for the diagnosis of migraine, as well as that of other types of headache.6 Although they were originally intended to assist in the standardization of research subjects, the criteria for the most common headache disorders can be adapted for diagnosis in the clinical setting. Migraine diagnosis is based almost entirely on the history; the main role of the physical examination is to screen for life-threatening conditions, such as intracranial hemorrhage or tumors.
In some patients, migraine is difficult to distinguish from other primary or secondary headaches, especially tension-type headache. A recent meta-analysis demonstrated that the features most helpful to rule in migraine (compared with tension-type headache) are nausea (positive likelihood ratio [LR+] = 19.2), photophobia (LR+ = 5.8), and phonophobia (LR+ = 5.2). Table 2 provides additional information regarding these and other significant findings, including the post-test probabilities of migraine given the reported prevalence among adult men and women in the United States. The likelihood ratios are probably somewhat inflated, since many of these symptoms are also part of the criteria for the reference standard (“incorporation bias”). The IHS criteria for migraine without aura require nausea or the combination of photophobia and phonophobia to make the diagnosis; it is therefore not surprising that these findings would be the most specific.
While the presence of any single feature may not be sufficient to clinch the diagnosis, sequentially combining the post-test probabilities can prove useful in cases that are not straightforward. For example, a woman with a family history of migraine who complains of a unilateral headache accompanied by photophobia but no nausea has an approximately 80% post-test probability of migraine. This conclusion assumes statistical independence of these symptoms and thus may overestimate the probability somewhat.8
Migraine has no specific diagnostic findings on computed tomography (CT) or magnetic resonance imaging (MRI). The best evidence addressing the use of neuroimaging studies in migraine, as well as most other diagnostic and management issues, comes from the United States Headache Consortium (USHC), a panel of experts from several specialty societies and professional organizations, including the American Academy of Family Physicians. In April 2000, the USHC issued diagnosis and treatment guidelines based on rigorous evidence-based reviews of the medical literature.9 A USHC meta-analysis showed that the prevalence of significant abnormalities on head CT or MRI for migraine patients with a normal neurologic examination ranged from 0% to 3.1% with an overall prevalence of 0.18% (1 in 555).10 Therefore, the USHC does not recommend neuroimaging for patients with a normal neurologic examination who meet the IHS diagnostic criteria for migraine (level of evidence [LOE]: B, using the Centre for Evidence-Based Medicine classification scheme). Neuroimaging should be considered for patients for whom a diagnosis is less clear cut (LOE: C).
Further support for this recommendation is found in a well-designed retrospective study demonstrating that the rate of significant intracranial pathology (mass lesion or hemorrhage) in patients presenting to primary care practices with a new headache and no neurologic findings was only 0.35%.11 Some patients and physicians will find even this low risk unacceptable and will obtain neuroimaging studies for reasons (such as litigation fears, risk perception, and so forth) that likely are not amenable to statistical argument.
TABLE 1
DIAGNOSTIC CRITERIA FOR MIGRAINE
Migraine Without Aura
|
Migraine With Aura
|
| In both cases, the diagnosis of migraine cannot be made if the history or physical examination suggests another disorder unless that disorder has been ruled out by appropriate testing or the migraine attacks do not occur for the first time in close temporal relation to the disorder. |
| Adapted, with permission, from Headache Classification Committee of the International Headache Society. Diagnostic Criteria. Available at: http://www.i-h-s.org/ihsnew/guidelines/pdfs/diagnost.pdf. Accessed May 28, 2001. |
TABLE 2
DIAGNOSTIC FEATURES IN MIGRAINE
| Finding | Sensitivity (%) | Specificity (%) | LR+ | LR- | PV+% (M/F) | PV-% (M/F) |
|---|---|---|---|---|---|---|
| Versus Patients With Tension-Type Headache | ||||||
| Nausea | 81 | 96 | 19.2 | 0.20 | 56/82 | 1.2/4.2 |
| Photophobia | 79 | 86 | 5.8 | 0.25 | 26/55 | 1.5/5.1 |
| Phonophobia | 67 | 87 | 5.2 | 0.38 | 25/53 | 2.4/7.7 |
| Exacerbation by physical activity | 81 | 78 | 3.7 | 0.24 | 19/45 | 1.5/5.1 |
| Unilateral location | 65 | 82 | 3.7 | 0.43 | 19/44 | 2.7/8.6 |
| Throbbing or pulsating quality | 73 | 75 | 2.9 | 0.36 | 16/39 | 2.2/7.3 |
| Precipitated by chocolate | 33 | 95 | 7.1 | 0.70 | 30/59 | 4.3/13 |
| Versus Patients Without History of Headache | ||||||
| Family history of migraine | 58 | 88 | 5.0 | 0.47 | 24/51 | 3.0/10 |
| LR+ denotes positive likelihood ratio; LR-, negative likelihood ratio; PV+, probability of migraine given a positive finding; PV-, probability of migraine given a negative finding. | ||||||
| Prevalence of migraine in the US population is 6% for men (M) and 18% for women (F).1 | ||||||
| Adapted, with permission, from Smetana GW. The diagnostic value of historical features in primary headache syndromes: a comprehensive review. Arch Intern Med 2000; 160:2729-37. ©2000 American Medical Association. | ||||||
Treatment
General principles
Although the diagnostic criteria for migraine are relatively straightforward, the expression of these symptoms can be highly variable, both between patients and in any given patient between attacks. In addition, patients with migraine often experience intercurrent tension or other primary headaches, complicating both the diagnosis and the interpretation of response to a therapeutic trial. Consequently, finding the right medication for an individual is often challenging. The choice of treatment may be suggested or limited by coexisting conditions. The presence of severe nausea or vomiting during a migraine may require use of a medication that can be dosed other than by mouth.
Patient education and involvement in the development and evaluation of a migraine treatment plan is essential. Just as migraine sufferers differ in the type, frequency, and severity of their symptoms, they also differ in their treatment preferences and goals. Some are unable to tolerate certain side effects; others are more interested in rapid relief of pain. Discussions regarding expected responses to treatment can prevent patients’ disappointment and losing patients to follow-up. For example, a reduction in the frequency of headaches over the course of several months is a more realistic goal than immediate prevention of all headaches. A patient’s headache diary can aid the patient in identifying and possibly eliminating migraine triggers and greatly assist the physician in adjusting and refining a treatment plan.
Various treatments, both pharmacologic and nonpharmacologic, have been used to treat patients with migraine. This article examines treatments in 3 categories: abortive medications, prophylactic medications, and nonpharmacologic treatments.
Abortive medications
Table 3 lists over-the-counter (OTC) and prescription medications for acute migraine attacks. Until recently, little if any high-quality evidence existed to guide the physician in selecting the appropriate medication for a specific patient. The USHC issued a consensus recommendation that nonsteroidal antiinflammatory drugs (NSAIDs) and over-the-counter analgesics be considered first-line treatments, especially for mild migraine headaches, and that migraine-specific agents be used for patients with more severe episodes.12
Further support for this stratified-care approach to migraine treatment has since been provided by the Disability in Strategies of Care (DISC) Study. DISC demonstrated that patients whose treatment was chosen according to their Migraine Disability Assessment Scale (MIDAS) score (those with a score of I or II were treated with aspirin and metoclopramide; those with a score of III or IV were treated with zolmitriptan) had less disability and a significantly greater headache response at 2 hours than patients who were given zolmitriptan if their headaches failed to respond to aspirin and metoclopramide.13 The study supports the expert consensus that patients with a history of mild disability associated with migraine can be treated effectively with simple OTC analgesics, whereas patients with significant migraine-associated disability will have better outcomes if treated with migraine-specific medications (LOE: B).
Mild to moderate migraine can be treated effectively with an oral combination of aspirin, acetaminophen, and caffeine (Excedrin or generic substitutes) or aspirin plus metoclopramide (LOE: A). Patients who cannot take aspirin may respond to 1000 mg acetaminophen alone (LOE: B).
Triptans (5-hydroxytryptamine1B/1D receptor agonists) are the drugs of choice for the acute treatment of moderate to severe migraine (except hemiplegic or basilar migraine) (LOE: A). Contraindications include coronary artery disease, uncontrolled hypertension, pregnancy, and recent monoamine oxidase inhibitor or ergot alkaloid use. Little evidence exists to recommend one triptan over another. A few studies suggest that the newer oral triptans may be slightly more efficacious than oral sumatriptan, although the differences do not appear overwhelming.14-16
There have been no recent studies on isometheptene-containing compounds such as Midrin. Three randomized placebo-controlled trials in the mid-1970s found a modest but statistically significant effect on migraine pain.17-19 However, the lack of standardized inclusion criteria and outcome measures makes it difficult to draw firm, valid conclusions about the efficacy of isometheptene.20 These drugs should be considered second line in the acute treatment of migraine (LOE: B).
A number of randomized controlled studies have demonstrated the efficacy of acetaminophen–codeine combinations in the acute treatment of migraine.21-23 Some of these trials have used combinations that included other medications in addition to acetaminophen and codeine; no study has been done on the dose most readily available in the United States (ie, 300 mg acetaminophen plus 30 mg codeine). Concerns about abuse, tolerance, and rebound headache appropriately limit their use. In addition, there is no evidence that they are more effective than other abortive treatments; one study showed no difference between the acute migraine relief provided by 1000 mg plain aspirin versus 400 mg acetaminophen and 25 mg codeine.21 While acetaminophen plus codeine combinations probably are effective in migraine, they are second-line drugs (LOE: B).
No randomized, placebo-controlled trials have evaluated the efficacy of butalbital-containing agents for migraine. Because of concerns relating to dependence, withdrawal, and rebound headache, the USHC recommends that use of these agents “should be limited and carefully monitored” (LOE: D).12
In the emergency department setting, prochlorperazine (10 mg given intravenously [IV]) is a safe and effective treatment for migraine (LOE: A).24 Dihydroergotamine (DHE) given IV or intramuscularly (IM) in combination with antiemetics is at least as good as meperidine (IV or IM) in relieving the pain of migraine (LOE: A).25,26 Despite the widespread use of parenteral meperidine in this setting, there are no placebo-controlled studies documenting its effectiveness in the treatment of migraine headache.
TABLE 3
SELF-ADMINISTERED ACUTE TREATMENT OPTIONS IN MIGRAINE
| Strength of Recommendation | Treatment (Route of Administration) | Comments |
|---|---|---|
| A | Acetaminophen +aspirin + caffeine (PO) | NNT* 3.9 (3.2 to 4.9)51 |
| A | Aspirin (PO) | NNT range from 3.5 to 5.552 |
| A | Aspirin + metoclopramide (PO) | NNT 3.2 (2.6 to 4.0)53 |
| A | Butorphanol (IN) | Abuse/dependence and rebound headache potential |
| A | DHE (IN) | NNT 2.5 (1.9 to 3.7)54 |
| A | NSAIDs (PO) | NNT 7.5 (4.5 to 22) (for ibuprofen)55 |
| A | Triptans (PO) | NNT range from 2.7 to 5.456 |
| A | Sumatriptan (IN) | NNT 3.4 (2.9 to 4.1)56 |
| A | Sumatriptan (SC) | NNT 2.0 (1.8 to 2.2)56 |
| B | Acetaminophen (PO) | NNT 5.2 (3.3 to 13)57 |
| B | Acetaminophen + codeine (PO) | Abuse/dependence and rebound headache potential |
| B | Isometheptene compounds (PO) | Limited clinical trial data. |
| D | Butalbital compounds (PO) | No clinical trials; risk of rebound headache |
| D | Ergotamine (PO) | Conflicting evidence; increased risk of adverse effects |
| * Numbers needed to treat (NNT; 95% confidence interval) in this column are for headache response (reduction in headache severity from “severe” or “moderate” to “mild” or “none”) at 2 hours; included when available data permit. | ||
| IN denotes intranasal; PO, by mouth; SC, subcutaneous. | ||
Prophylactic medications
The USHC recommends that preventive treatment be considered for patients with migraine who desire a reduction in the frequency or severity of their headaches for any reason, including but not limited to frequent headaches that significantly interfere with daily activities despite acute treatment, unpleasant side effects associated with abortive medications, or the cost of abortive medications (LOE: D).27Table 4 lists medications available in the United States that are used in the prophylaxis of migraine.
Beta blockers, particularly propranolol, are commonly prescribed and are very effective in reducing the frequency of migraine (LOE: A).27-30 Most authorities consider them the migraine prophylactic of choice in patients with no contraindications (eg, asthma, congestive heart failure, or heart block).
Amitriptyline is the only antidepressant to demonstrate consistent efficacy in migraine prophylaxis.27,31,32 This medication may be especially useful in patients who suffer from both migraine and tension headaches.33 Divalproex sodium is another drug clearly shown effective against migraine prophylactically.27,30,34 The risk of significant hematologic and hepatic side effects requires laboratory monitoring and may limit its use in many patients.
Calcium channel blockers (CCBs), particularly verapamil, are widely used by both primary care physicians and neurologists for the prevention of migraine,35 and yet only 3 controlled trials of verapamil are reported in the English language literature. Two methodologically weak studies showed a small but significant effect from verapamil36,37; the third demonstrated no advantage over placebo.38 The only CCB consistently shown effective for migraine prophylaxis is flunarizine.27 Unfortunately, it is not available in the United States.
Besides use for acute treatment, NSAIDs are occasionally prescribed to prevent migraine. Naproxen sodium, the most frequently studied NSAID, shows a small but significant effect in overall improvement compared with placebo in several trials.39-41 Two of these studies showed a reduction in the number of severe headaches per week but no significant change in the total number of headaches per week.40,41
Some recent studies support the use of novel migraine prophylactics. One study of riboflavin (400 mg daily) showed a moderate reduction in migraine frequency.42 Achieving maximal therapeutic effect required 3 months of use. Another study found that 10 mg lisinopril daily can significantly reduce migraine frequency and severity when compared with placebo.43
TABLE 4
PROPHYLACTIC TREATMENT OPTIONS IN MIGRAINE
| Strength of Recommendation | Treatment | Comments |
|---|---|---|
| A | Amitriptyline | Evidence for no significant difference versus propranolol32, 33 |
| A | Divalproex sodium | NNT* range from 2.1 to 2.930, 34 |
| A | Propranolol | NNT range from 2.3 to 528-30 |
| B | Lisinopril | Based on 1 study (level 1b)43 |
| B | Naproxen sodium | Risk of rebound headache |
| B | Riboflavin | NNT 2.842 based on 1 study (level 1b) |
| D | Verapamil | Considered effective by many experts; limited, poor-quality clinical trials (see text) |
| * Numbers needed to treat (NNT) in this column are for a 50% reduction in headache frequency compared with baseline; reported when available data permit. | ||
Nonpharmacologic treatments
Although the data for nonpharmacologic migraine treatment are neither so extensive nor so rigorous as those for medications, some evidence is available. The Duke Center for Clinical Health Policy Research performed a comprehensive systematic review and meta-analysis of behavioral and physical treatments for migraine for the Agency for Healthcare Research and Quality.44 This review forms the evidence base for the USHC guideline in this area.45 The authors note that most studies were conducted on patients recruited at specialized headache centers; thus, caution should be exercised in generalizing the results to a primary care population.
The meta-analysis showed that cognitive–behavioral (including stress management) therapy, electromyelogram biofeedback, relaxation training, and thermal biofeedback combined with relaxation training are effective in migraine prophylaxis (LOE: B).44 An earlier meta-analysis concluded that the prophylactic benefit of combined relaxation and thermal biofeedback training was equivalent to the benefit obtained from propranolol.46 Because of limited or mixed evidence, no clear recommendations can be made with regard to acupuncture, cervical manipulation, hyperbaric oxygen, hypnosis, occlusal adjustment, or transcutaneous electronic nerve stimulation.45
Prognosis
Little evidence is available concerning the long-term prognosis of migraine, either with or without treatment. For many patients, migraine persists, but slowly decreases in frequency over a lifetime.47,48 For patients who respond well to prophylaxis, no data are available to help the clinician decide how long to continue using it. One small case series showed that while a few patients had a lasting reduction in the frequency of their migraines after stopping effective prophylactic medication, most experienced relapse.49
A subset of patients with migraine develops headaches of increasing frequency, often resulting in daily or continuous headaches. This syndrome has been known as transformed or malignant migraine. Many such patients use migraine medications on a daily basis. Although no controlled trials have been reported, the daily or near-daily use of most acute migraine medications (including acetaminophen, aspirin, dihydroergotamine, ergotamine, NSAIDs, opioids, and triptans) is believed capable of provoking medication-overuse headaches.50 Some of these patients can reduce the frequency of their headaches if they can break the cycle of medication use.47
Conclusions
Migraine headache is a common and disabling condition. The diagnosis often can be made on the basis of key findings in the patient’s history. A classic history, in combination with a normal neurologic examination, obviates head imaging. Available evidence clearly shows that effective methods for both acute and prophylactic treatment of migraine exist. The Figure contains an algorithm summarizing such treatment. Wider implementation of the USHC evidence-based guidelines by primary care physicians treating those with migraine should result in decreased pain and increased productivity for many patients.
FIGURE
ALGORITHM FOR TREATMENT OF MIGRAINE
Acknowledgments
The author would like to thank John R. Holman, MD, MPH, for reviewing the manuscript and Anne J. O’Connor for her help in obtaining the references.
1. Stewart WF, Lipton RB, Celentano DD, Reed ML. Prevalence of migraine headache in the United States: relation to age, income, race and other sociodemographic factors. JAMA 1992;267:64-9.
2. Stewart WF, Lipton RB. Migraine headache: epidemiology and health care utilization. Cephalalgin 1993;13(suppl 12):41-6.
3. Schappert SM, Nelson C. National Ambulatory Medical Care Survey, 1995-1996 Summary. National Center for Health Statistics. Vital Health Stat 13(142). 1999.
4. Hu XH, Markson LE, Lipton RB, Stewart WF, Berger ML. Burden of migraine in the United States: disability and economic costs. Arch Intern Med 1999;159:813-8.
5. Goadsby PJ. Current concepts of the pathophysiology of migraine. Neurol Clin 1997;15:27-42.
6. Headache Classification Committee of the International Headache Society. Diagnostic criteria. Available at: www.i-h-s.org/ihsnew/guidelines/pdfs/diagnost.pdf/. Accessed May 28, 2001.
7. Smetana GW. The diagnostic value of historical features in primary headache syndromes: a comprehensive review. Arch Intern Med 2000;160:2729-37.
8. Diagnosing migraine. Available at: http://www.jr2.ox.ac.uk/bandolier/booth/Migraine/Diagmig.html/. Accessed November 6, 2001.
9. McCrory DC, Matchar DB, Rosenberg JH, Silberstein SD. Evidenced-based guidelines for migraine headache: overview of program description and methodology. Available at: www.aan.com/public/practiceguidelines/01.pdf/. Accessed November 29, 2001.
10. Frishberg BM, Rosenberg JH, Matchar DB, McCrory DC, Rozen TD, Silberstein SD. Evidence-based guidelines in the primary care setting: neuroimaging in patients with nonacute headache. Available at: www.aan.com/public/practiceguidelines/02.pdf/. Accessed November 29, 2001.
11. Becker LA, Green LA, Beaufait D, Kirk J, Froom J, Freeman WL. Detection of intracranial tumors, subarachnoid hemorrhages, and subdural hematomas in primary care patients: a report from ASPN, Part 2. J Fam Pract 1993;37:135-41.
12. Matchar DB, Young WB, Rosenberg JH, et al. Evidence-based guidelines for migraine headache in the primary care setting: pharmacological management of acute attacks. Available at: www.aan.com/public/practiceguidelines/03.pdf/. Accessed November 29, 2001.
13. Lipton RB, Stewart WF, Stone AM, Lainez MJA, Sawyer JPC. Stratified care vs step care strategies for migraine. The disability in strategies of care (DISC) study: a randomized trial. JAMA 2000;284:2599-605.
14. Tfelt-Hansen P, Teall J, Rodriguez F, et al. Oral rizatriptan versus oral sumatriptan: a direct comparative study in the acute treatment of migraine. Headache 1998;38:748-55.
15. Gallagher R, Dennish G, Spierings EL, Chitra R. A comparative trial of zolmitriptan and sumatriptan for the acute oral treatment of migraine. Headache 2000;40:119-28.
16. Gobel H, Winter P, Boswell D, Crisp A, Becker W, Hauge T, et al. Comparison of naratriptan and sumatriptan in recurrence-prone migraine patients. Clin Ther 2000;22:981-9.
17. Ryan RE. A study of Midrin in the symptomatic relief of migraine headache. Headache 1974;14:33-42.
18. Diamond S, Medina JL. Isometheptene-a non-ergot drug in the treatment of migraine. Headache 1975;15:211-3.
19. Diamond S. Treatment of migraine with isometheptene, acetaminophen, and dichloralphenazone combination: a double-blind, crossover trial. Headache 1976;15:282-7.
20. Isometheptene for acute migraine. Available at: http://www.jr2.ox.ac.uk/bandolier/booth/Migraine/IsomORacu.html/. Accessed November 9, 2001.
21. Boureau F, Joubert JM, Lasserre V, Prum B, Delecoeuillerie G. Double-blind comparison of an acetaminophen 400 mg-codeine 25 mg combination versus aspirin 1000 mg and placebo in acute migraine attack. Cephalalgia 1994;14:156-61.
22. Adam EI. A treatment for the acute migraine attack. J Int Med Res 1987;15:71-5.
23. Carasso RL, Yehuda S. The prevention and treatment of migraine with an analgesic combination. Br J Clin Pract 1984;38:25-7.
24. Coppola M, Yealy DM, Leibold RA. Randomized, placebo-controlled evaluation of prochlorperazine versus metoclopramide for emergency department treatment of migraine headache. Ann Emerg Med 1995;26:541-6.
25. Klapper JA, Stanton J. Current emergency treatment of severe migraine headaches. Headache 1993;33:560-2.
26. Carleton SC, Shesser RF, Pietrzak MP, et al. Double-blind, multicenter trial to compare the efficacy of intramuscular dihydroergotamine plus hydroxyzine versus intramuscular meperidine plus hydroxyzine for the emergency department treatment of acute migraine headache. Ann Emerg Med 1998;32:129-38.
27. Ramadan NM, Silberstein SD, Freitag FG, Gilbert TT, Frishberg BM. Evidence-based guidelines for migraine headache in the primary care setting: pharmacological management for the prevention of migraine. Available at: www.aan.com/public/practiceguidelines/05.pdf/. Accessed November 29, 2001.
28. Borgesen SE, Nielsen JL, Moller CE. Prophylactic treatment of migraine with propranolol: a clinical trial. Acta Neurol Scand 1974;50:651-6.
29. Tfelt-Hansen P, Standnes B, Kangasniemi P, Hakkarainen H, Olesen J. Timolol vs. propranolol vs. placebo in common migraine prophylaxis: a double-blind multicenter trial. Acta Neurol Scand 1984;69:1-8.
30. Kaniecki RG. A comparison of divalproex with propranolol and placebo for the prophylaxis of migraine without aura. Arch Neurol 1997;54:1141-5.
31. Couch JR, Hassanein RS. Amitriptyline in migraine prophylaxis. Arch Neurol 1979;36:695-9.
32. Ziegler DK, Hurwitz A, Hassanein RS, Kodanaz HA, Preskorn SH, Mason J. Migraine prophylaxis: a comparison of propranolol and amitriptyline. Arch Neurol 1987;44:486-9.
33. Mathew NT. Prophylaxis of migraine and mixed headache. A randomized controlled study. Headache 1981;21:105-9.
34. Mathew NT, Saper JR, Silberstein SD, et al. Migraine prophylaxis with divalproex. Arch Neurol 1995;52:281-6.
35. Ramadan NM, Schultz LL, Gilkey SJ. Migraine prophylactic drugs: proof of efficacy, utilization and cost. Cephalalgia 1997;17:73-80.
36. Solomon GD, Steel JG, Spaccavento LJ. Verapamil prophylaxis of migraine: a double-blind, placebo-controlled study. JAMA 1983;250:2500-2.
37. Markley HG, Cheronis JC, Piepho RW. Verapamil in prophylactic therapy of migraine. Neurology 1984;34:973-6.
38. Solomon GD. Verapamil and propranolol in migraine prophylaxis; a double-blind, cross-over study. Headache 1986;26:325.-
39. Ziegler DK, Ellis DJ. Naproxen in prophylaxis of migraine. Arch Neurol 1985;42:582-4.
40. Sargent J, Solbach P, Damasio H, et al. A comparison of naproxen sodium to propranolol hydrochloride and a placebo control for the prophylaxis of migraine headache. Headache 1985;25:320-4.
41. Welch KMA, Ellis DJ, Keenan PA. Successful migraine prophylaxis with naproxen sodium. Neurology 1985;35:1304-10.
42. Schoenen J, Jacquy J, Lenaerts M. Effectiveness of high-dose riboflavin in migraine prophylaxis: a randomized controlled trial. Neurology 1998;50:466-70.
43. Schrader H, Stoner LJ, Helde G, Sand T, Bovim G. Prophylactic treatment of migraine with angiotensin converting enzyme inhibitor (lisinopril): randomised, placebo controlled, crossover study. BMJ 2001;322:1-5.
44. Goslin RE, Gray RN, McCrory DC, Penzien D, Rains J, Hasselblad V. Behavioral and physical treatments for migraine headache. Technical review 2.2. February 1999. Prepared for the Agency for Health Care Policy and Research under Contract No. 290-94-2025. Available at: http://www.clinpol.mc.duke.edu./Pubs/Publications/Behavioral_Manuscript.pdf/. Accessed November 27, 2001.
45. Campbell JK, Penzien DB, Wall EM. Evidence-based guidelines for migraine headache: behavioral and physical treatments. Available at: www.aan.com/public/practiceguidelines/04.pdf/. Accessed November 29, 2001.
46. Holroyd KA, Penzien DB. Pharmacological versus non-pharmacological prophylaxis of recurrent migraine headache: a meta-analytic review of clinical trials. Pain 1990;42:1-13.
47. Silberstein SD, Lipton RB. Headache epidemiology. Emphasis on migraine. Neurol Clin 1996;14:421-34.
48. Silberstein SD, Young WB. Headache and facial pain. In: Goetz CG, Pappert EJ, eds. Textbook of clinical neurology. 1st ed. Philadelphia, Pa: Saunders; 1999:1095.
49. Wober C, Wober-Bingol C, Kock G, Wessely P. Long-term results of migraine prophylaxis with flunarazine and beta-blockers. Cephalalgia. 1991;11:251-6.
50. Diener HC, Dahlof CGH. Headache associated with chronic use of substances. In: Olesen J, Tfelt-Hansen P, Welch KMA, eds. The headaches. 2nd ed. Philadelphia, Pa: Lippincott, Williams & Wilkins; 2000:871-7.
51. Paracetamol, aspirin caffeine incombination (Excedrin) for acute migraine. Available at: http://www.jr2.ox.ac.uk/bandolier/booth/Migraine/Excedr.html/. Accessed November 9, 2001.
52. Aspirin for acute migraine. Available at: http://www.jr2.ox.ac.uk/bandolier/booth/Migraine/Aspacute.html/. Accessed November 9, 2001.
53. Aspirin plus metoclopramide for acute migraine. Available at: http://www.jr2.ox.ac.uk/bandolier/booth/Migraine/AsMcOacu.html/. Accessed November 9, 2001.
54. Intranasal dihydroergotamine for acute migraine Available at: http://www.jr2.ox.ac.uk/bandolier/booth/Migraine/DHEINacu.html/. Accessed November 9, 2001.
55. Ibuprofen for acute migraine (new RCT). Available at: http://www.jr2.ox.ac.uk/bandolier/booth/Migraine/ibu2.html/. Accessed November 9, 2001.
56. Migraine league table: acute treatments and two hour headache response. Available at: http://www.jr2.ox.ac.uk/bandolier/booth/Migraine/League.html/. Accessed November 9, 2001.
57. Lipton RB, Baggish JS, Stewart WF, Codispoti JR, Fu M. Efficacy and safety of acetaminophen in the treatment of migraine: results of a randomized, double-blind, placebo-controlled, population-based study. Arch Intern Med 2000;160:3486-92
- The International Headache Society criteria provide a useful standardized way to diagnose migraine clinically.
- Neuroimaging is not necessary for patients who clearly meet clinical criteria for migraine and whose neurologic examination results are normal.
- Over-the-counter drugs (including aspirin, ibuprofen, and the combination of aspirin, acetaminophen, and caffeine) work well and are first-line treatments for mild migraine.
- Migraine-specific medications (including intranasal dihydroergotamine [DHE] and the triptans) are recommended for more severe migraine; little evidence exists to suggest one drug over another.
- Prophylaxis is recommended if patients find the severity or frequency of headaches bothersome enough to warrant preventive measures; amitriptyline, divalproex sodium, and propranolol are effective prophylaxes.
Migraine is a common and disabling condition. Among adults in the United States, approximately 18% of women and 6% of men report symptoms consistent with migraine1; less than half have been diagnosed by a physician or received prescription treatment from a physician.2 Migraine accounts for more than 2.8 million visits per year to US physicians and is the reason for encounter in about 1 visit per week to the typical family physician.3 Migraine is estimated to cost US employers more than $13 billion each year; direct medical costs exceed $1 billion annually.4
Pathophysiology
The exact pathophysiology of migraine is unknown. The prevailing theory is that a trigger (such as fatigue, stress, or certain foods) sets off a wave of brief neuronal activation, followed by a more sustained neuronal inhibition known as cortical spreading depression (CSD). At some point the trigeminovascular system is activated (possibly by CSD), releasing vasoactive neuropeptides that cause a painful inflammatory response in the meninges. Stimulation of presynaptic serotonin receptors inhibits release of the inflammatory neuropeptides; this is one possible explanation for the effectiveness of the triptans.5
Diagnosis
Migraine is a syndrome diagnosed by a certain combination of signs and symptoms. The International Headache Society (IHS) diagnostic criteria (Table 1) are widely accepted as the reference standard for the diagnosis of migraine, as well as that of other types of headache.6 Although they were originally intended to assist in the standardization of research subjects, the criteria for the most common headache disorders can be adapted for diagnosis in the clinical setting. Migraine diagnosis is based almost entirely on the history; the main role of the physical examination is to screen for life-threatening conditions, such as intracranial hemorrhage or tumors.
In some patients, migraine is difficult to distinguish from other primary or secondary headaches, especially tension-type headache. A recent meta-analysis demonstrated that the features most helpful to rule in migraine (compared with tension-type headache) are nausea (positive likelihood ratio [LR+] = 19.2), photophobia (LR+ = 5.8), and phonophobia (LR+ = 5.2). Table 2 provides additional information regarding these and other significant findings, including the post-test probabilities of migraine given the reported prevalence among adult men and women in the United States. The likelihood ratios are probably somewhat inflated, since many of these symptoms are also part of the criteria for the reference standard (“incorporation bias”). The IHS criteria for migraine without aura require nausea or the combination of photophobia and phonophobia to make the diagnosis; it is therefore not surprising that these findings would be the most specific.
While the presence of any single feature may not be sufficient to clinch the diagnosis, sequentially combining the post-test probabilities can prove useful in cases that are not straightforward. For example, a woman with a family history of migraine who complains of a unilateral headache accompanied by photophobia but no nausea has an approximately 80% post-test probability of migraine. This conclusion assumes statistical independence of these symptoms and thus may overestimate the probability somewhat.8
Migraine has no specific diagnostic findings on computed tomography (CT) or magnetic resonance imaging (MRI). The best evidence addressing the use of neuroimaging studies in migraine, as well as most other diagnostic and management issues, comes from the United States Headache Consortium (USHC), a panel of experts from several specialty societies and professional organizations, including the American Academy of Family Physicians. In April 2000, the USHC issued diagnosis and treatment guidelines based on rigorous evidence-based reviews of the medical literature.9 A USHC meta-analysis showed that the prevalence of significant abnormalities on head CT or MRI for migraine patients with a normal neurologic examination ranged from 0% to 3.1% with an overall prevalence of 0.18% (1 in 555).10 Therefore, the USHC does not recommend neuroimaging for patients with a normal neurologic examination who meet the IHS diagnostic criteria for migraine (level of evidence [LOE]: B, using the Centre for Evidence-Based Medicine classification scheme). Neuroimaging should be considered for patients for whom a diagnosis is less clear cut (LOE: C).
Further support for this recommendation is found in a well-designed retrospective study demonstrating that the rate of significant intracranial pathology (mass lesion or hemorrhage) in patients presenting to primary care practices with a new headache and no neurologic findings was only 0.35%.11 Some patients and physicians will find even this low risk unacceptable and will obtain neuroimaging studies for reasons (such as litigation fears, risk perception, and so forth) that likely are not amenable to statistical argument.
TABLE 1
DIAGNOSTIC CRITERIA FOR MIGRAINE
Migraine Without Aura
|
Migraine With Aura
|
| In both cases, the diagnosis of migraine cannot be made if the history or physical examination suggests another disorder unless that disorder has been ruled out by appropriate testing or the migraine attacks do not occur for the first time in close temporal relation to the disorder. |
| Adapted, with permission, from Headache Classification Committee of the International Headache Society. Diagnostic Criteria. Available at: http://www.i-h-s.org/ihsnew/guidelines/pdfs/diagnost.pdf. Accessed May 28, 2001. |
TABLE 2
DIAGNOSTIC FEATURES IN MIGRAINE
| Finding | Sensitivity (%) | Specificity (%) | LR+ | LR- | PV+% (M/F) | PV-% (M/F) |
|---|---|---|---|---|---|---|
| Versus Patients With Tension-Type Headache | ||||||
| Nausea | 81 | 96 | 19.2 | 0.20 | 56/82 | 1.2/4.2 |
| Photophobia | 79 | 86 | 5.8 | 0.25 | 26/55 | 1.5/5.1 |
| Phonophobia | 67 | 87 | 5.2 | 0.38 | 25/53 | 2.4/7.7 |
| Exacerbation by physical activity | 81 | 78 | 3.7 | 0.24 | 19/45 | 1.5/5.1 |
| Unilateral location | 65 | 82 | 3.7 | 0.43 | 19/44 | 2.7/8.6 |
| Throbbing or pulsating quality | 73 | 75 | 2.9 | 0.36 | 16/39 | 2.2/7.3 |
| Precipitated by chocolate | 33 | 95 | 7.1 | 0.70 | 30/59 | 4.3/13 |
| Versus Patients Without History of Headache | ||||||
| Family history of migraine | 58 | 88 | 5.0 | 0.47 | 24/51 | 3.0/10 |
| LR+ denotes positive likelihood ratio; LR-, negative likelihood ratio; PV+, probability of migraine given a positive finding; PV-, probability of migraine given a negative finding. | ||||||
| Prevalence of migraine in the US population is 6% for men (M) and 18% for women (F).1 | ||||||
| Adapted, with permission, from Smetana GW. The diagnostic value of historical features in primary headache syndromes: a comprehensive review. Arch Intern Med 2000; 160:2729-37. ©2000 American Medical Association. | ||||||
Treatment
General principles
Although the diagnostic criteria for migraine are relatively straightforward, the expression of these symptoms can be highly variable, both between patients and in any given patient between attacks. In addition, patients with migraine often experience intercurrent tension or other primary headaches, complicating both the diagnosis and the interpretation of response to a therapeutic trial. Consequently, finding the right medication for an individual is often challenging. The choice of treatment may be suggested or limited by coexisting conditions. The presence of severe nausea or vomiting during a migraine may require use of a medication that can be dosed other than by mouth.
Patient education and involvement in the development and evaluation of a migraine treatment plan is essential. Just as migraine sufferers differ in the type, frequency, and severity of their symptoms, they also differ in their treatment preferences and goals. Some are unable to tolerate certain side effects; others are more interested in rapid relief of pain. Discussions regarding expected responses to treatment can prevent patients’ disappointment and losing patients to follow-up. For example, a reduction in the frequency of headaches over the course of several months is a more realistic goal than immediate prevention of all headaches. A patient’s headache diary can aid the patient in identifying and possibly eliminating migraine triggers and greatly assist the physician in adjusting and refining a treatment plan.
Various treatments, both pharmacologic and nonpharmacologic, have been used to treat patients with migraine. This article examines treatments in 3 categories: abortive medications, prophylactic medications, and nonpharmacologic treatments.
Abortive medications
Table 3 lists over-the-counter (OTC) and prescription medications for acute migraine attacks. Until recently, little if any high-quality evidence existed to guide the physician in selecting the appropriate medication for a specific patient. The USHC issued a consensus recommendation that nonsteroidal antiinflammatory drugs (NSAIDs) and over-the-counter analgesics be considered first-line treatments, especially for mild migraine headaches, and that migraine-specific agents be used for patients with more severe episodes.12
Further support for this stratified-care approach to migraine treatment has since been provided by the Disability in Strategies of Care (DISC) Study. DISC demonstrated that patients whose treatment was chosen according to their Migraine Disability Assessment Scale (MIDAS) score (those with a score of I or II were treated with aspirin and metoclopramide; those with a score of III or IV were treated with zolmitriptan) had less disability and a significantly greater headache response at 2 hours than patients who were given zolmitriptan if their headaches failed to respond to aspirin and metoclopramide.13 The study supports the expert consensus that patients with a history of mild disability associated with migraine can be treated effectively with simple OTC analgesics, whereas patients with significant migraine-associated disability will have better outcomes if treated with migraine-specific medications (LOE: B).
Mild to moderate migraine can be treated effectively with an oral combination of aspirin, acetaminophen, and caffeine (Excedrin or generic substitutes) or aspirin plus metoclopramide (LOE: A). Patients who cannot take aspirin may respond to 1000 mg acetaminophen alone (LOE: B).
Triptans (5-hydroxytryptamine1B/1D receptor agonists) are the drugs of choice for the acute treatment of moderate to severe migraine (except hemiplegic or basilar migraine) (LOE: A). Contraindications include coronary artery disease, uncontrolled hypertension, pregnancy, and recent monoamine oxidase inhibitor or ergot alkaloid use. Little evidence exists to recommend one triptan over another. A few studies suggest that the newer oral triptans may be slightly more efficacious than oral sumatriptan, although the differences do not appear overwhelming.14-16
There have been no recent studies on isometheptene-containing compounds such as Midrin. Three randomized placebo-controlled trials in the mid-1970s found a modest but statistically significant effect on migraine pain.17-19 However, the lack of standardized inclusion criteria and outcome measures makes it difficult to draw firm, valid conclusions about the efficacy of isometheptene.20 These drugs should be considered second line in the acute treatment of migraine (LOE: B).
A number of randomized controlled studies have demonstrated the efficacy of acetaminophen–codeine combinations in the acute treatment of migraine.21-23 Some of these trials have used combinations that included other medications in addition to acetaminophen and codeine; no study has been done on the dose most readily available in the United States (ie, 300 mg acetaminophen plus 30 mg codeine). Concerns about abuse, tolerance, and rebound headache appropriately limit their use. In addition, there is no evidence that they are more effective than other abortive treatments; one study showed no difference between the acute migraine relief provided by 1000 mg plain aspirin versus 400 mg acetaminophen and 25 mg codeine.21 While acetaminophen plus codeine combinations probably are effective in migraine, they are second-line drugs (LOE: B).
No randomized, placebo-controlled trials have evaluated the efficacy of butalbital-containing agents for migraine. Because of concerns relating to dependence, withdrawal, and rebound headache, the USHC recommends that use of these agents “should be limited and carefully monitored” (LOE: D).12
In the emergency department setting, prochlorperazine (10 mg given intravenously [IV]) is a safe and effective treatment for migraine (LOE: A).24 Dihydroergotamine (DHE) given IV or intramuscularly (IM) in combination with antiemetics is at least as good as meperidine (IV or IM) in relieving the pain of migraine (LOE: A).25,26 Despite the widespread use of parenteral meperidine in this setting, there are no placebo-controlled studies documenting its effectiveness in the treatment of migraine headache.
TABLE 3
SELF-ADMINISTERED ACUTE TREATMENT OPTIONS IN MIGRAINE
| Strength of Recommendation | Treatment (Route of Administration) | Comments |
|---|---|---|
| A | Acetaminophen +aspirin + caffeine (PO) | NNT* 3.9 (3.2 to 4.9)51 |
| A | Aspirin (PO) | NNT range from 3.5 to 5.552 |
| A | Aspirin + metoclopramide (PO) | NNT 3.2 (2.6 to 4.0)53 |
| A | Butorphanol (IN) | Abuse/dependence and rebound headache potential |
| A | DHE (IN) | NNT 2.5 (1.9 to 3.7)54 |
| A | NSAIDs (PO) | NNT 7.5 (4.5 to 22) (for ibuprofen)55 |
| A | Triptans (PO) | NNT range from 2.7 to 5.456 |
| A | Sumatriptan (IN) | NNT 3.4 (2.9 to 4.1)56 |
| A | Sumatriptan (SC) | NNT 2.0 (1.8 to 2.2)56 |
| B | Acetaminophen (PO) | NNT 5.2 (3.3 to 13)57 |
| B | Acetaminophen + codeine (PO) | Abuse/dependence and rebound headache potential |
| B | Isometheptene compounds (PO) | Limited clinical trial data. |
| D | Butalbital compounds (PO) | No clinical trials; risk of rebound headache |
| D | Ergotamine (PO) | Conflicting evidence; increased risk of adverse effects |
| * Numbers needed to treat (NNT; 95% confidence interval) in this column are for headache response (reduction in headache severity from “severe” or “moderate” to “mild” or “none”) at 2 hours; included when available data permit. | ||
| IN denotes intranasal; PO, by mouth; SC, subcutaneous. | ||
Prophylactic medications
The USHC recommends that preventive treatment be considered for patients with migraine who desire a reduction in the frequency or severity of their headaches for any reason, including but not limited to frequent headaches that significantly interfere with daily activities despite acute treatment, unpleasant side effects associated with abortive medications, or the cost of abortive medications (LOE: D).27Table 4 lists medications available in the United States that are used in the prophylaxis of migraine.
Beta blockers, particularly propranolol, are commonly prescribed and are very effective in reducing the frequency of migraine (LOE: A).27-30 Most authorities consider them the migraine prophylactic of choice in patients with no contraindications (eg, asthma, congestive heart failure, or heart block).
Amitriptyline is the only antidepressant to demonstrate consistent efficacy in migraine prophylaxis.27,31,32 This medication may be especially useful in patients who suffer from both migraine and tension headaches.33 Divalproex sodium is another drug clearly shown effective against migraine prophylactically.27,30,34 The risk of significant hematologic and hepatic side effects requires laboratory monitoring and may limit its use in many patients.
Calcium channel blockers (CCBs), particularly verapamil, are widely used by both primary care physicians and neurologists for the prevention of migraine,35 and yet only 3 controlled trials of verapamil are reported in the English language literature. Two methodologically weak studies showed a small but significant effect from verapamil36,37; the third demonstrated no advantage over placebo.38 The only CCB consistently shown effective for migraine prophylaxis is flunarizine.27 Unfortunately, it is not available in the United States.
Besides use for acute treatment, NSAIDs are occasionally prescribed to prevent migraine. Naproxen sodium, the most frequently studied NSAID, shows a small but significant effect in overall improvement compared with placebo in several trials.39-41 Two of these studies showed a reduction in the number of severe headaches per week but no significant change in the total number of headaches per week.40,41
Some recent studies support the use of novel migraine prophylactics. One study of riboflavin (400 mg daily) showed a moderate reduction in migraine frequency.42 Achieving maximal therapeutic effect required 3 months of use. Another study found that 10 mg lisinopril daily can significantly reduce migraine frequency and severity when compared with placebo.43
TABLE 4
PROPHYLACTIC TREATMENT OPTIONS IN MIGRAINE
| Strength of Recommendation | Treatment | Comments |
|---|---|---|
| A | Amitriptyline | Evidence for no significant difference versus propranolol32, 33 |
| A | Divalproex sodium | NNT* range from 2.1 to 2.930, 34 |
| A | Propranolol | NNT range from 2.3 to 528-30 |
| B | Lisinopril | Based on 1 study (level 1b)43 |
| B | Naproxen sodium | Risk of rebound headache |
| B | Riboflavin | NNT 2.842 based on 1 study (level 1b) |
| D | Verapamil | Considered effective by many experts; limited, poor-quality clinical trials (see text) |
| * Numbers needed to treat (NNT) in this column are for a 50% reduction in headache frequency compared with baseline; reported when available data permit. | ||
Nonpharmacologic treatments
Although the data for nonpharmacologic migraine treatment are neither so extensive nor so rigorous as those for medications, some evidence is available. The Duke Center for Clinical Health Policy Research performed a comprehensive systematic review and meta-analysis of behavioral and physical treatments for migraine for the Agency for Healthcare Research and Quality.44 This review forms the evidence base for the USHC guideline in this area.45 The authors note that most studies were conducted on patients recruited at specialized headache centers; thus, caution should be exercised in generalizing the results to a primary care population.
The meta-analysis showed that cognitive–behavioral (including stress management) therapy, electromyelogram biofeedback, relaxation training, and thermal biofeedback combined with relaxation training are effective in migraine prophylaxis (LOE: B).44 An earlier meta-analysis concluded that the prophylactic benefit of combined relaxation and thermal biofeedback training was equivalent to the benefit obtained from propranolol.46 Because of limited or mixed evidence, no clear recommendations can be made with regard to acupuncture, cervical manipulation, hyperbaric oxygen, hypnosis, occlusal adjustment, or transcutaneous electronic nerve stimulation.45
Prognosis
Little evidence is available concerning the long-term prognosis of migraine, either with or without treatment. For many patients, migraine persists, but slowly decreases in frequency over a lifetime.47,48 For patients who respond well to prophylaxis, no data are available to help the clinician decide how long to continue using it. One small case series showed that while a few patients had a lasting reduction in the frequency of their migraines after stopping effective prophylactic medication, most experienced relapse.49
A subset of patients with migraine develops headaches of increasing frequency, often resulting in daily or continuous headaches. This syndrome has been known as transformed or malignant migraine. Many such patients use migraine medications on a daily basis. Although no controlled trials have been reported, the daily or near-daily use of most acute migraine medications (including acetaminophen, aspirin, dihydroergotamine, ergotamine, NSAIDs, opioids, and triptans) is believed capable of provoking medication-overuse headaches.50 Some of these patients can reduce the frequency of their headaches if they can break the cycle of medication use.47
Conclusions
Migraine headache is a common and disabling condition. The diagnosis often can be made on the basis of key findings in the patient’s history. A classic history, in combination with a normal neurologic examination, obviates head imaging. Available evidence clearly shows that effective methods for both acute and prophylactic treatment of migraine exist. The Figure contains an algorithm summarizing such treatment. Wider implementation of the USHC evidence-based guidelines by primary care physicians treating those with migraine should result in decreased pain and increased productivity for many patients.
FIGURE
ALGORITHM FOR TREATMENT OF MIGRAINE
Acknowledgments
The author would like to thank John R. Holman, MD, MPH, for reviewing the manuscript and Anne J. O’Connor for her help in obtaining the references.
- The International Headache Society criteria provide a useful standardized way to diagnose migraine clinically.
- Neuroimaging is not necessary for patients who clearly meet clinical criteria for migraine and whose neurologic examination results are normal.
- Over-the-counter drugs (including aspirin, ibuprofen, and the combination of aspirin, acetaminophen, and caffeine) work well and are first-line treatments for mild migraine.
- Migraine-specific medications (including intranasal dihydroergotamine [DHE] and the triptans) are recommended for more severe migraine; little evidence exists to suggest one drug over another.
- Prophylaxis is recommended if patients find the severity or frequency of headaches bothersome enough to warrant preventive measures; amitriptyline, divalproex sodium, and propranolol are effective prophylaxes.
Migraine is a common and disabling condition. Among adults in the United States, approximately 18% of women and 6% of men report symptoms consistent with migraine1; less than half have been diagnosed by a physician or received prescription treatment from a physician.2 Migraine accounts for more than 2.8 million visits per year to US physicians and is the reason for encounter in about 1 visit per week to the typical family physician.3 Migraine is estimated to cost US employers more than $13 billion each year; direct medical costs exceed $1 billion annually.4
Pathophysiology
The exact pathophysiology of migraine is unknown. The prevailing theory is that a trigger (such as fatigue, stress, or certain foods) sets off a wave of brief neuronal activation, followed by a more sustained neuronal inhibition known as cortical spreading depression (CSD). At some point the trigeminovascular system is activated (possibly by CSD), releasing vasoactive neuropeptides that cause a painful inflammatory response in the meninges. Stimulation of presynaptic serotonin receptors inhibits release of the inflammatory neuropeptides; this is one possible explanation for the effectiveness of the triptans.5
Diagnosis
Migraine is a syndrome diagnosed by a certain combination of signs and symptoms. The International Headache Society (IHS) diagnostic criteria (Table 1) are widely accepted as the reference standard for the diagnosis of migraine, as well as that of other types of headache.6 Although they were originally intended to assist in the standardization of research subjects, the criteria for the most common headache disorders can be adapted for diagnosis in the clinical setting. Migraine diagnosis is based almost entirely on the history; the main role of the physical examination is to screen for life-threatening conditions, such as intracranial hemorrhage or tumors.
In some patients, migraine is difficult to distinguish from other primary or secondary headaches, especially tension-type headache. A recent meta-analysis demonstrated that the features most helpful to rule in migraine (compared with tension-type headache) are nausea (positive likelihood ratio [LR+] = 19.2), photophobia (LR+ = 5.8), and phonophobia (LR+ = 5.2). Table 2 provides additional information regarding these and other significant findings, including the post-test probabilities of migraine given the reported prevalence among adult men and women in the United States. The likelihood ratios are probably somewhat inflated, since many of these symptoms are also part of the criteria for the reference standard (“incorporation bias”). The IHS criteria for migraine without aura require nausea or the combination of photophobia and phonophobia to make the diagnosis; it is therefore not surprising that these findings would be the most specific.
While the presence of any single feature may not be sufficient to clinch the diagnosis, sequentially combining the post-test probabilities can prove useful in cases that are not straightforward. For example, a woman with a family history of migraine who complains of a unilateral headache accompanied by photophobia but no nausea has an approximately 80% post-test probability of migraine. This conclusion assumes statistical independence of these symptoms and thus may overestimate the probability somewhat.8
Migraine has no specific diagnostic findings on computed tomography (CT) or magnetic resonance imaging (MRI). The best evidence addressing the use of neuroimaging studies in migraine, as well as most other diagnostic and management issues, comes from the United States Headache Consortium (USHC), a panel of experts from several specialty societies and professional organizations, including the American Academy of Family Physicians. In April 2000, the USHC issued diagnosis and treatment guidelines based on rigorous evidence-based reviews of the medical literature.9 A USHC meta-analysis showed that the prevalence of significant abnormalities on head CT or MRI for migraine patients with a normal neurologic examination ranged from 0% to 3.1% with an overall prevalence of 0.18% (1 in 555).10 Therefore, the USHC does not recommend neuroimaging for patients with a normal neurologic examination who meet the IHS diagnostic criteria for migraine (level of evidence [LOE]: B, using the Centre for Evidence-Based Medicine classification scheme). Neuroimaging should be considered for patients for whom a diagnosis is less clear cut (LOE: C).
Further support for this recommendation is found in a well-designed retrospective study demonstrating that the rate of significant intracranial pathology (mass lesion or hemorrhage) in patients presenting to primary care practices with a new headache and no neurologic findings was only 0.35%.11 Some patients and physicians will find even this low risk unacceptable and will obtain neuroimaging studies for reasons (such as litigation fears, risk perception, and so forth) that likely are not amenable to statistical argument.
TABLE 1
DIAGNOSTIC CRITERIA FOR MIGRAINE
Migraine Without Aura
|
Migraine With Aura
|
| In both cases, the diagnosis of migraine cannot be made if the history or physical examination suggests another disorder unless that disorder has been ruled out by appropriate testing or the migraine attacks do not occur for the first time in close temporal relation to the disorder. |
| Adapted, with permission, from Headache Classification Committee of the International Headache Society. Diagnostic Criteria. Available at: http://www.i-h-s.org/ihsnew/guidelines/pdfs/diagnost.pdf. Accessed May 28, 2001. |
TABLE 2
DIAGNOSTIC FEATURES IN MIGRAINE
| Finding | Sensitivity (%) | Specificity (%) | LR+ | LR- | PV+% (M/F) | PV-% (M/F) |
|---|---|---|---|---|---|---|
| Versus Patients With Tension-Type Headache | ||||||
| Nausea | 81 | 96 | 19.2 | 0.20 | 56/82 | 1.2/4.2 |
| Photophobia | 79 | 86 | 5.8 | 0.25 | 26/55 | 1.5/5.1 |
| Phonophobia | 67 | 87 | 5.2 | 0.38 | 25/53 | 2.4/7.7 |
| Exacerbation by physical activity | 81 | 78 | 3.7 | 0.24 | 19/45 | 1.5/5.1 |
| Unilateral location | 65 | 82 | 3.7 | 0.43 | 19/44 | 2.7/8.6 |
| Throbbing or pulsating quality | 73 | 75 | 2.9 | 0.36 | 16/39 | 2.2/7.3 |
| Precipitated by chocolate | 33 | 95 | 7.1 | 0.70 | 30/59 | 4.3/13 |
| Versus Patients Without History of Headache | ||||||
| Family history of migraine | 58 | 88 | 5.0 | 0.47 | 24/51 | 3.0/10 |
| LR+ denotes positive likelihood ratio; LR-, negative likelihood ratio; PV+, probability of migraine given a positive finding; PV-, probability of migraine given a negative finding. | ||||||
| Prevalence of migraine in the US population is 6% for men (M) and 18% for women (F).1 | ||||||
| Adapted, with permission, from Smetana GW. The diagnostic value of historical features in primary headache syndromes: a comprehensive review. Arch Intern Med 2000; 160:2729-37. ©2000 American Medical Association. | ||||||
Treatment
General principles
Although the diagnostic criteria for migraine are relatively straightforward, the expression of these symptoms can be highly variable, both between patients and in any given patient between attacks. In addition, patients with migraine often experience intercurrent tension or other primary headaches, complicating both the diagnosis and the interpretation of response to a therapeutic trial. Consequently, finding the right medication for an individual is often challenging. The choice of treatment may be suggested or limited by coexisting conditions. The presence of severe nausea or vomiting during a migraine may require use of a medication that can be dosed other than by mouth.
Patient education and involvement in the development and evaluation of a migraine treatment plan is essential. Just as migraine sufferers differ in the type, frequency, and severity of their symptoms, they also differ in their treatment preferences and goals. Some are unable to tolerate certain side effects; others are more interested in rapid relief of pain. Discussions regarding expected responses to treatment can prevent patients’ disappointment and losing patients to follow-up. For example, a reduction in the frequency of headaches over the course of several months is a more realistic goal than immediate prevention of all headaches. A patient’s headache diary can aid the patient in identifying and possibly eliminating migraine triggers and greatly assist the physician in adjusting and refining a treatment plan.
Various treatments, both pharmacologic and nonpharmacologic, have been used to treat patients with migraine. This article examines treatments in 3 categories: abortive medications, prophylactic medications, and nonpharmacologic treatments.
Abortive medications
Table 3 lists over-the-counter (OTC) and prescription medications for acute migraine attacks. Until recently, little if any high-quality evidence existed to guide the physician in selecting the appropriate medication for a specific patient. The USHC issued a consensus recommendation that nonsteroidal antiinflammatory drugs (NSAIDs) and over-the-counter analgesics be considered first-line treatments, especially for mild migraine headaches, and that migraine-specific agents be used for patients with more severe episodes.12
Further support for this stratified-care approach to migraine treatment has since been provided by the Disability in Strategies of Care (DISC) Study. DISC demonstrated that patients whose treatment was chosen according to their Migraine Disability Assessment Scale (MIDAS) score (those with a score of I or II were treated with aspirin and metoclopramide; those with a score of III or IV were treated with zolmitriptan) had less disability and a significantly greater headache response at 2 hours than patients who were given zolmitriptan if their headaches failed to respond to aspirin and metoclopramide.13 The study supports the expert consensus that patients with a history of mild disability associated with migraine can be treated effectively with simple OTC analgesics, whereas patients with significant migraine-associated disability will have better outcomes if treated with migraine-specific medications (LOE: B).
Mild to moderate migraine can be treated effectively with an oral combination of aspirin, acetaminophen, and caffeine (Excedrin or generic substitutes) or aspirin plus metoclopramide (LOE: A). Patients who cannot take aspirin may respond to 1000 mg acetaminophen alone (LOE: B).
Triptans (5-hydroxytryptamine1B/1D receptor agonists) are the drugs of choice for the acute treatment of moderate to severe migraine (except hemiplegic or basilar migraine) (LOE: A). Contraindications include coronary artery disease, uncontrolled hypertension, pregnancy, and recent monoamine oxidase inhibitor or ergot alkaloid use. Little evidence exists to recommend one triptan over another. A few studies suggest that the newer oral triptans may be slightly more efficacious than oral sumatriptan, although the differences do not appear overwhelming.14-16
There have been no recent studies on isometheptene-containing compounds such as Midrin. Three randomized placebo-controlled trials in the mid-1970s found a modest but statistically significant effect on migraine pain.17-19 However, the lack of standardized inclusion criteria and outcome measures makes it difficult to draw firm, valid conclusions about the efficacy of isometheptene.20 These drugs should be considered second line in the acute treatment of migraine (LOE: B).
A number of randomized controlled studies have demonstrated the efficacy of acetaminophen–codeine combinations in the acute treatment of migraine.21-23 Some of these trials have used combinations that included other medications in addition to acetaminophen and codeine; no study has been done on the dose most readily available in the United States (ie, 300 mg acetaminophen plus 30 mg codeine). Concerns about abuse, tolerance, and rebound headache appropriately limit their use. In addition, there is no evidence that they are more effective than other abortive treatments; one study showed no difference between the acute migraine relief provided by 1000 mg plain aspirin versus 400 mg acetaminophen and 25 mg codeine.21 While acetaminophen plus codeine combinations probably are effective in migraine, they are second-line drugs (LOE: B).
No randomized, placebo-controlled trials have evaluated the efficacy of butalbital-containing agents for migraine. Because of concerns relating to dependence, withdrawal, and rebound headache, the USHC recommends that use of these agents “should be limited and carefully monitored” (LOE: D).12
In the emergency department setting, prochlorperazine (10 mg given intravenously [IV]) is a safe and effective treatment for migraine (LOE: A).24 Dihydroergotamine (DHE) given IV or intramuscularly (IM) in combination with antiemetics is at least as good as meperidine (IV or IM) in relieving the pain of migraine (LOE: A).25,26 Despite the widespread use of parenteral meperidine in this setting, there are no placebo-controlled studies documenting its effectiveness in the treatment of migraine headache.
TABLE 3
SELF-ADMINISTERED ACUTE TREATMENT OPTIONS IN MIGRAINE
| Strength of Recommendation | Treatment (Route of Administration) | Comments |
|---|---|---|
| A | Acetaminophen +aspirin + caffeine (PO) | NNT* 3.9 (3.2 to 4.9)51 |
| A | Aspirin (PO) | NNT range from 3.5 to 5.552 |
| A | Aspirin + metoclopramide (PO) | NNT 3.2 (2.6 to 4.0)53 |
| A | Butorphanol (IN) | Abuse/dependence and rebound headache potential |
| A | DHE (IN) | NNT 2.5 (1.9 to 3.7)54 |
| A | NSAIDs (PO) | NNT 7.5 (4.5 to 22) (for ibuprofen)55 |
| A | Triptans (PO) | NNT range from 2.7 to 5.456 |
| A | Sumatriptan (IN) | NNT 3.4 (2.9 to 4.1)56 |
| A | Sumatriptan (SC) | NNT 2.0 (1.8 to 2.2)56 |
| B | Acetaminophen (PO) | NNT 5.2 (3.3 to 13)57 |
| B | Acetaminophen + codeine (PO) | Abuse/dependence and rebound headache potential |
| B | Isometheptene compounds (PO) | Limited clinical trial data. |
| D | Butalbital compounds (PO) | No clinical trials; risk of rebound headache |
| D | Ergotamine (PO) | Conflicting evidence; increased risk of adverse effects |
| * Numbers needed to treat (NNT; 95% confidence interval) in this column are for headache response (reduction in headache severity from “severe” or “moderate” to “mild” or “none”) at 2 hours; included when available data permit. | ||
| IN denotes intranasal; PO, by mouth; SC, subcutaneous. | ||
Prophylactic medications
The USHC recommends that preventive treatment be considered for patients with migraine who desire a reduction in the frequency or severity of their headaches for any reason, including but not limited to frequent headaches that significantly interfere with daily activities despite acute treatment, unpleasant side effects associated with abortive medications, or the cost of abortive medications (LOE: D).27Table 4 lists medications available in the United States that are used in the prophylaxis of migraine.
Beta blockers, particularly propranolol, are commonly prescribed and are very effective in reducing the frequency of migraine (LOE: A).27-30 Most authorities consider them the migraine prophylactic of choice in patients with no contraindications (eg, asthma, congestive heart failure, or heart block).
Amitriptyline is the only antidepressant to demonstrate consistent efficacy in migraine prophylaxis.27,31,32 This medication may be especially useful in patients who suffer from both migraine and tension headaches.33 Divalproex sodium is another drug clearly shown effective against migraine prophylactically.27,30,34 The risk of significant hematologic and hepatic side effects requires laboratory monitoring and may limit its use in many patients.
Calcium channel blockers (CCBs), particularly verapamil, are widely used by both primary care physicians and neurologists for the prevention of migraine,35 and yet only 3 controlled trials of verapamil are reported in the English language literature. Two methodologically weak studies showed a small but significant effect from verapamil36,37; the third demonstrated no advantage over placebo.38 The only CCB consistently shown effective for migraine prophylaxis is flunarizine.27 Unfortunately, it is not available in the United States.
Besides use for acute treatment, NSAIDs are occasionally prescribed to prevent migraine. Naproxen sodium, the most frequently studied NSAID, shows a small but significant effect in overall improvement compared with placebo in several trials.39-41 Two of these studies showed a reduction in the number of severe headaches per week but no significant change in the total number of headaches per week.40,41
Some recent studies support the use of novel migraine prophylactics. One study of riboflavin (400 mg daily) showed a moderate reduction in migraine frequency.42 Achieving maximal therapeutic effect required 3 months of use. Another study found that 10 mg lisinopril daily can significantly reduce migraine frequency and severity when compared with placebo.43
TABLE 4
PROPHYLACTIC TREATMENT OPTIONS IN MIGRAINE
| Strength of Recommendation | Treatment | Comments |
|---|---|---|
| A | Amitriptyline | Evidence for no significant difference versus propranolol32, 33 |
| A | Divalproex sodium | NNT* range from 2.1 to 2.930, 34 |
| A | Propranolol | NNT range from 2.3 to 528-30 |
| B | Lisinopril | Based on 1 study (level 1b)43 |
| B | Naproxen sodium | Risk of rebound headache |
| B | Riboflavin | NNT 2.842 based on 1 study (level 1b) |
| D | Verapamil | Considered effective by many experts; limited, poor-quality clinical trials (see text) |
| * Numbers needed to treat (NNT) in this column are for a 50% reduction in headache frequency compared with baseline; reported when available data permit. | ||
Nonpharmacologic treatments
Although the data for nonpharmacologic migraine treatment are neither so extensive nor so rigorous as those for medications, some evidence is available. The Duke Center for Clinical Health Policy Research performed a comprehensive systematic review and meta-analysis of behavioral and physical treatments for migraine for the Agency for Healthcare Research and Quality.44 This review forms the evidence base for the USHC guideline in this area.45 The authors note that most studies were conducted on patients recruited at specialized headache centers; thus, caution should be exercised in generalizing the results to a primary care population.
The meta-analysis showed that cognitive–behavioral (including stress management) therapy, electromyelogram biofeedback, relaxation training, and thermal biofeedback combined with relaxation training are effective in migraine prophylaxis (LOE: B).44 An earlier meta-analysis concluded that the prophylactic benefit of combined relaxation and thermal biofeedback training was equivalent to the benefit obtained from propranolol.46 Because of limited or mixed evidence, no clear recommendations can be made with regard to acupuncture, cervical manipulation, hyperbaric oxygen, hypnosis, occlusal adjustment, or transcutaneous electronic nerve stimulation.45
Prognosis
Little evidence is available concerning the long-term prognosis of migraine, either with or without treatment. For many patients, migraine persists, but slowly decreases in frequency over a lifetime.47,48 For patients who respond well to prophylaxis, no data are available to help the clinician decide how long to continue using it. One small case series showed that while a few patients had a lasting reduction in the frequency of their migraines after stopping effective prophylactic medication, most experienced relapse.49
A subset of patients with migraine develops headaches of increasing frequency, often resulting in daily or continuous headaches. This syndrome has been known as transformed or malignant migraine. Many such patients use migraine medications on a daily basis. Although no controlled trials have been reported, the daily or near-daily use of most acute migraine medications (including acetaminophen, aspirin, dihydroergotamine, ergotamine, NSAIDs, opioids, and triptans) is believed capable of provoking medication-overuse headaches.50 Some of these patients can reduce the frequency of their headaches if they can break the cycle of medication use.47
Conclusions
Migraine headache is a common and disabling condition. The diagnosis often can be made on the basis of key findings in the patient’s history. A classic history, in combination with a normal neurologic examination, obviates head imaging. Available evidence clearly shows that effective methods for both acute and prophylactic treatment of migraine exist. The Figure contains an algorithm summarizing such treatment. Wider implementation of the USHC evidence-based guidelines by primary care physicians treating those with migraine should result in decreased pain and increased productivity for many patients.
FIGURE
ALGORITHM FOR TREATMENT OF MIGRAINE
Acknowledgments
The author would like to thank John R. Holman, MD, MPH, for reviewing the manuscript and Anne J. O’Connor for her help in obtaining the references.
1. Stewart WF, Lipton RB, Celentano DD, Reed ML. Prevalence of migraine headache in the United States: relation to age, income, race and other sociodemographic factors. JAMA 1992;267:64-9.
2. Stewart WF, Lipton RB. Migraine headache: epidemiology and health care utilization. Cephalalgin 1993;13(suppl 12):41-6.
3. Schappert SM, Nelson C. National Ambulatory Medical Care Survey, 1995-1996 Summary. National Center for Health Statistics. Vital Health Stat 13(142). 1999.
4. Hu XH, Markson LE, Lipton RB, Stewart WF, Berger ML. Burden of migraine in the United States: disability and economic costs. Arch Intern Med 1999;159:813-8.
5. Goadsby PJ. Current concepts of the pathophysiology of migraine. Neurol Clin 1997;15:27-42.
6. Headache Classification Committee of the International Headache Society. Diagnostic criteria. Available at: www.i-h-s.org/ihsnew/guidelines/pdfs/diagnost.pdf/. Accessed May 28, 2001.
7. Smetana GW. The diagnostic value of historical features in primary headache syndromes: a comprehensive review. Arch Intern Med 2000;160:2729-37.
8. Diagnosing migraine. Available at: http://www.jr2.ox.ac.uk/bandolier/booth/Migraine/Diagmig.html/. Accessed November 6, 2001.
9. McCrory DC, Matchar DB, Rosenberg JH, Silberstein SD. Evidenced-based guidelines for migraine headache: overview of program description and methodology. Available at: www.aan.com/public/practiceguidelines/01.pdf/. Accessed November 29, 2001.
10. Frishberg BM, Rosenberg JH, Matchar DB, McCrory DC, Rozen TD, Silberstein SD. Evidence-based guidelines in the primary care setting: neuroimaging in patients with nonacute headache. Available at: www.aan.com/public/practiceguidelines/02.pdf/. Accessed November 29, 2001.
11. Becker LA, Green LA, Beaufait D, Kirk J, Froom J, Freeman WL. Detection of intracranial tumors, subarachnoid hemorrhages, and subdural hematomas in primary care patients: a report from ASPN, Part 2. J Fam Pract 1993;37:135-41.
12. Matchar DB, Young WB, Rosenberg JH, et al. Evidence-based guidelines for migraine headache in the primary care setting: pharmacological management of acute attacks. Available at: www.aan.com/public/practiceguidelines/03.pdf/. Accessed November 29, 2001.
13. Lipton RB, Stewart WF, Stone AM, Lainez MJA, Sawyer JPC. Stratified care vs step care strategies for migraine. The disability in strategies of care (DISC) study: a randomized trial. JAMA 2000;284:2599-605.
14. Tfelt-Hansen P, Teall J, Rodriguez F, et al. Oral rizatriptan versus oral sumatriptan: a direct comparative study in the acute treatment of migraine. Headache 1998;38:748-55.
15. Gallagher R, Dennish G, Spierings EL, Chitra R. A comparative trial of zolmitriptan and sumatriptan for the acute oral treatment of migraine. Headache 2000;40:119-28.
16. Gobel H, Winter P, Boswell D, Crisp A, Becker W, Hauge T, et al. Comparison of naratriptan and sumatriptan in recurrence-prone migraine patients. Clin Ther 2000;22:981-9.
17. Ryan RE. A study of Midrin in the symptomatic relief of migraine headache. Headache 1974;14:33-42.
18. Diamond S, Medina JL. Isometheptene-a non-ergot drug in the treatment of migraine. Headache 1975;15:211-3.
19. Diamond S. Treatment of migraine with isometheptene, acetaminophen, and dichloralphenazone combination: a double-blind, crossover trial. Headache 1976;15:282-7.
20. Isometheptene for acute migraine. Available at: http://www.jr2.ox.ac.uk/bandolier/booth/Migraine/IsomORacu.html/. Accessed November 9, 2001.
21. Boureau F, Joubert JM, Lasserre V, Prum B, Delecoeuillerie G. Double-blind comparison of an acetaminophen 400 mg-codeine 25 mg combination versus aspirin 1000 mg and placebo in acute migraine attack. Cephalalgia 1994;14:156-61.
22. Adam EI. A treatment for the acute migraine attack. J Int Med Res 1987;15:71-5.
23. Carasso RL, Yehuda S. The prevention and treatment of migraine with an analgesic combination. Br J Clin Pract 1984;38:25-7.
24. Coppola M, Yealy DM, Leibold RA. Randomized, placebo-controlled evaluation of prochlorperazine versus metoclopramide for emergency department treatment of migraine headache. Ann Emerg Med 1995;26:541-6.
25. Klapper JA, Stanton J. Current emergency treatment of severe migraine headaches. Headache 1993;33:560-2.
26. Carleton SC, Shesser RF, Pietrzak MP, et al. Double-blind, multicenter trial to compare the efficacy of intramuscular dihydroergotamine plus hydroxyzine versus intramuscular meperidine plus hydroxyzine for the emergency department treatment of acute migraine headache. Ann Emerg Med 1998;32:129-38.
27. Ramadan NM, Silberstein SD, Freitag FG, Gilbert TT, Frishberg BM. Evidence-based guidelines for migraine headache in the primary care setting: pharmacological management for the prevention of migraine. Available at: www.aan.com/public/practiceguidelines/05.pdf/. Accessed November 29, 2001.
28. Borgesen SE, Nielsen JL, Moller CE. Prophylactic treatment of migraine with propranolol: a clinical trial. Acta Neurol Scand 1974;50:651-6.
29. Tfelt-Hansen P, Standnes B, Kangasniemi P, Hakkarainen H, Olesen J. Timolol vs. propranolol vs. placebo in common migraine prophylaxis: a double-blind multicenter trial. Acta Neurol Scand 1984;69:1-8.
30. Kaniecki RG. A comparison of divalproex with propranolol and placebo for the prophylaxis of migraine without aura. Arch Neurol 1997;54:1141-5.
31. Couch JR, Hassanein RS. Amitriptyline in migraine prophylaxis. Arch Neurol 1979;36:695-9.
32. Ziegler DK, Hurwitz A, Hassanein RS, Kodanaz HA, Preskorn SH, Mason J. Migraine prophylaxis: a comparison of propranolol and amitriptyline. Arch Neurol 1987;44:486-9.
33. Mathew NT. Prophylaxis of migraine and mixed headache. A randomized controlled study. Headache 1981;21:105-9.
34. Mathew NT, Saper JR, Silberstein SD, et al. Migraine prophylaxis with divalproex. Arch Neurol 1995;52:281-6.
35. Ramadan NM, Schultz LL, Gilkey SJ. Migraine prophylactic drugs: proof of efficacy, utilization and cost. Cephalalgia 1997;17:73-80.
36. Solomon GD, Steel JG, Spaccavento LJ. Verapamil prophylaxis of migraine: a double-blind, placebo-controlled study. JAMA 1983;250:2500-2.
37. Markley HG, Cheronis JC, Piepho RW. Verapamil in prophylactic therapy of migraine. Neurology 1984;34:973-6.
38. Solomon GD. Verapamil and propranolol in migraine prophylaxis; a double-blind, cross-over study. Headache 1986;26:325.-
39. Ziegler DK, Ellis DJ. Naproxen in prophylaxis of migraine. Arch Neurol 1985;42:582-4.
40. Sargent J, Solbach P, Damasio H, et al. A comparison of naproxen sodium to propranolol hydrochloride and a placebo control for the prophylaxis of migraine headache. Headache 1985;25:320-4.
41. Welch KMA, Ellis DJ, Keenan PA. Successful migraine prophylaxis with naproxen sodium. Neurology 1985;35:1304-10.
42. Schoenen J, Jacquy J, Lenaerts M. Effectiveness of high-dose riboflavin in migraine prophylaxis: a randomized controlled trial. Neurology 1998;50:466-70.
43. Schrader H, Stoner LJ, Helde G, Sand T, Bovim G. Prophylactic treatment of migraine with angiotensin converting enzyme inhibitor (lisinopril): randomised, placebo controlled, crossover study. BMJ 2001;322:1-5.
44. Goslin RE, Gray RN, McCrory DC, Penzien D, Rains J, Hasselblad V. Behavioral and physical treatments for migraine headache. Technical review 2.2. February 1999. Prepared for the Agency for Health Care Policy and Research under Contract No. 290-94-2025. Available at: http://www.clinpol.mc.duke.edu./Pubs/Publications/Behavioral_Manuscript.pdf/. Accessed November 27, 2001.
45. Campbell JK, Penzien DB, Wall EM. Evidence-based guidelines for migraine headache: behavioral and physical treatments. Available at: www.aan.com/public/practiceguidelines/04.pdf/. Accessed November 29, 2001.
46. Holroyd KA, Penzien DB. Pharmacological versus non-pharmacological prophylaxis of recurrent migraine headache: a meta-analytic review of clinical trials. Pain 1990;42:1-13.
47. Silberstein SD, Lipton RB. Headache epidemiology. Emphasis on migraine. Neurol Clin 1996;14:421-34.
48. Silberstein SD, Young WB. Headache and facial pain. In: Goetz CG, Pappert EJ, eds. Textbook of clinical neurology. 1st ed. Philadelphia, Pa: Saunders; 1999:1095.
49. Wober C, Wober-Bingol C, Kock G, Wessely P. Long-term results of migraine prophylaxis with flunarazine and beta-blockers. Cephalalgia. 1991;11:251-6.
50. Diener HC, Dahlof CGH. Headache associated with chronic use of substances. In: Olesen J, Tfelt-Hansen P, Welch KMA, eds. The headaches. 2nd ed. Philadelphia, Pa: Lippincott, Williams & Wilkins; 2000:871-7.
51. Paracetamol, aspirin caffeine incombination (Excedrin) for acute migraine. Available at: http://www.jr2.ox.ac.uk/bandolier/booth/Migraine/Excedr.html/. Accessed November 9, 2001.
52. Aspirin for acute migraine. Available at: http://www.jr2.ox.ac.uk/bandolier/booth/Migraine/Aspacute.html/. Accessed November 9, 2001.
53. Aspirin plus metoclopramide for acute migraine. Available at: http://www.jr2.ox.ac.uk/bandolier/booth/Migraine/AsMcOacu.html/. Accessed November 9, 2001.
54. Intranasal dihydroergotamine for acute migraine Available at: http://www.jr2.ox.ac.uk/bandolier/booth/Migraine/DHEINacu.html/. Accessed November 9, 2001.
55. Ibuprofen for acute migraine (new RCT). Available at: http://www.jr2.ox.ac.uk/bandolier/booth/Migraine/ibu2.html/. Accessed November 9, 2001.
56. Migraine league table: acute treatments and two hour headache response. Available at: http://www.jr2.ox.ac.uk/bandolier/booth/Migraine/League.html/. Accessed November 9, 2001.
57. Lipton RB, Baggish JS, Stewart WF, Codispoti JR, Fu M. Efficacy and safety of acetaminophen in the treatment of migraine: results of a randomized, double-blind, placebo-controlled, population-based study. Arch Intern Med 2000;160:3486-92
1. Stewart WF, Lipton RB, Celentano DD, Reed ML. Prevalence of migraine headache in the United States: relation to age, income, race and other sociodemographic factors. JAMA 1992;267:64-9.
2. Stewart WF, Lipton RB. Migraine headache: epidemiology and health care utilization. Cephalalgin 1993;13(suppl 12):41-6.
3. Schappert SM, Nelson C. National Ambulatory Medical Care Survey, 1995-1996 Summary. National Center for Health Statistics. Vital Health Stat 13(142). 1999.
4. Hu XH, Markson LE, Lipton RB, Stewart WF, Berger ML. Burden of migraine in the United States: disability and economic costs. Arch Intern Med 1999;159:813-8.
5. Goadsby PJ. Current concepts of the pathophysiology of migraine. Neurol Clin 1997;15:27-42.
6. Headache Classification Committee of the International Headache Society. Diagnostic criteria. Available at: www.i-h-s.org/ihsnew/guidelines/pdfs/diagnost.pdf/. Accessed May 28, 2001.
7. Smetana GW. The diagnostic value of historical features in primary headache syndromes: a comprehensive review. Arch Intern Med 2000;160:2729-37.
8. Diagnosing migraine. Available at: http://www.jr2.ox.ac.uk/bandolier/booth/Migraine/Diagmig.html/. Accessed November 6, 2001.
9. McCrory DC, Matchar DB, Rosenberg JH, Silberstein SD. Evidenced-based guidelines for migraine headache: overview of program description and methodology. Available at: www.aan.com/public/practiceguidelines/01.pdf/. Accessed November 29, 2001.
10. Frishberg BM, Rosenberg JH, Matchar DB, McCrory DC, Rozen TD, Silberstein SD. Evidence-based guidelines in the primary care setting: neuroimaging in patients with nonacute headache. Available at: www.aan.com/public/practiceguidelines/02.pdf/. Accessed November 29, 2001.
11. Becker LA, Green LA, Beaufait D, Kirk J, Froom J, Freeman WL. Detection of intracranial tumors, subarachnoid hemorrhages, and subdural hematomas in primary care patients: a report from ASPN, Part 2. J Fam Pract 1993;37:135-41.
12. Matchar DB, Young WB, Rosenberg JH, et al. Evidence-based guidelines for migraine headache in the primary care setting: pharmacological management of acute attacks. Available at: www.aan.com/public/practiceguidelines/03.pdf/. Accessed November 29, 2001.
13. Lipton RB, Stewart WF, Stone AM, Lainez MJA, Sawyer JPC. Stratified care vs step care strategies for migraine. The disability in strategies of care (DISC) study: a randomized trial. JAMA 2000;284:2599-605.
14. Tfelt-Hansen P, Teall J, Rodriguez F, et al. Oral rizatriptan versus oral sumatriptan: a direct comparative study in the acute treatment of migraine. Headache 1998;38:748-55.
15. Gallagher R, Dennish G, Spierings EL, Chitra R. A comparative trial of zolmitriptan and sumatriptan for the acute oral treatment of migraine. Headache 2000;40:119-28.
16. Gobel H, Winter P, Boswell D, Crisp A, Becker W, Hauge T, et al. Comparison of naratriptan and sumatriptan in recurrence-prone migraine patients. Clin Ther 2000;22:981-9.
17. Ryan RE. A study of Midrin in the symptomatic relief of migraine headache. Headache 1974;14:33-42.
18. Diamond S, Medina JL. Isometheptene-a non-ergot drug in the treatment of migraine. Headache 1975;15:211-3.
19. Diamond S. Treatment of migraine with isometheptene, acetaminophen, and dichloralphenazone combination: a double-blind, crossover trial. Headache 1976;15:282-7.
20. Isometheptene for acute migraine. Available at: http://www.jr2.ox.ac.uk/bandolier/booth/Migraine/IsomORacu.html/. Accessed November 9, 2001.
21. Boureau F, Joubert JM, Lasserre V, Prum B, Delecoeuillerie G. Double-blind comparison of an acetaminophen 400 mg-codeine 25 mg combination versus aspirin 1000 mg and placebo in acute migraine attack. Cephalalgia 1994;14:156-61.
22. Adam EI. A treatment for the acute migraine attack. J Int Med Res 1987;15:71-5.
23. Carasso RL, Yehuda S. The prevention and treatment of migraine with an analgesic combination. Br J Clin Pract 1984;38:25-7.
24. Coppola M, Yealy DM, Leibold RA. Randomized, placebo-controlled evaluation of prochlorperazine versus metoclopramide for emergency department treatment of migraine headache. Ann Emerg Med 1995;26:541-6.
25. Klapper JA, Stanton J. Current emergency treatment of severe migraine headaches. Headache 1993;33:560-2.
26. Carleton SC, Shesser RF, Pietrzak MP, et al. Double-blind, multicenter trial to compare the efficacy of intramuscular dihydroergotamine plus hydroxyzine versus intramuscular meperidine plus hydroxyzine for the emergency department treatment of acute migraine headache. Ann Emerg Med 1998;32:129-38.
27. Ramadan NM, Silberstein SD, Freitag FG, Gilbert TT, Frishberg BM. Evidence-based guidelines for migraine headache in the primary care setting: pharmacological management for the prevention of migraine. Available at: www.aan.com/public/practiceguidelines/05.pdf/. Accessed November 29, 2001.
28. Borgesen SE, Nielsen JL, Moller CE. Prophylactic treatment of migraine with propranolol: a clinical trial. Acta Neurol Scand 1974;50:651-6.
29. Tfelt-Hansen P, Standnes B, Kangasniemi P, Hakkarainen H, Olesen J. Timolol vs. propranolol vs. placebo in common migraine prophylaxis: a double-blind multicenter trial. Acta Neurol Scand 1984;69:1-8.
30. Kaniecki RG. A comparison of divalproex with propranolol and placebo for the prophylaxis of migraine without aura. Arch Neurol 1997;54:1141-5.
31. Couch JR, Hassanein RS. Amitriptyline in migraine prophylaxis. Arch Neurol 1979;36:695-9.
32. Ziegler DK, Hurwitz A, Hassanein RS, Kodanaz HA, Preskorn SH, Mason J. Migraine prophylaxis: a comparison of propranolol and amitriptyline. Arch Neurol 1987;44:486-9.
33. Mathew NT. Prophylaxis of migraine and mixed headache. A randomized controlled study. Headache 1981;21:105-9.
34. Mathew NT, Saper JR, Silberstein SD, et al. Migraine prophylaxis with divalproex. Arch Neurol 1995;52:281-6.
35. Ramadan NM, Schultz LL, Gilkey SJ. Migraine prophylactic drugs: proof of efficacy, utilization and cost. Cephalalgia 1997;17:73-80.
36. Solomon GD, Steel JG, Spaccavento LJ. Verapamil prophylaxis of migraine: a double-blind, placebo-controlled study. JAMA 1983;250:2500-2.
37. Markley HG, Cheronis JC, Piepho RW. Verapamil in prophylactic therapy of migraine. Neurology 1984;34:973-6.
38. Solomon GD. Verapamil and propranolol in migraine prophylaxis; a double-blind, cross-over study. Headache 1986;26:325.-
39. Ziegler DK, Ellis DJ. Naproxen in prophylaxis of migraine. Arch Neurol 1985;42:582-4.
40. Sargent J, Solbach P, Damasio H, et al. A comparison of naproxen sodium to propranolol hydrochloride and a placebo control for the prophylaxis of migraine headache. Headache 1985;25:320-4.
41. Welch KMA, Ellis DJ, Keenan PA. Successful migraine prophylaxis with naproxen sodium. Neurology 1985;35:1304-10.
42. Schoenen J, Jacquy J, Lenaerts M. Effectiveness of high-dose riboflavin in migraine prophylaxis: a randomized controlled trial. Neurology 1998;50:466-70.
43. Schrader H, Stoner LJ, Helde G, Sand T, Bovim G. Prophylactic treatment of migraine with angiotensin converting enzyme inhibitor (lisinopril): randomised, placebo controlled, crossover study. BMJ 2001;322:1-5.
44. Goslin RE, Gray RN, McCrory DC, Penzien D, Rains J, Hasselblad V. Behavioral and physical treatments for migraine headache. Technical review 2.2. February 1999. Prepared for the Agency for Health Care Policy and Research under Contract No. 290-94-2025. Available at: http://www.clinpol.mc.duke.edu./Pubs/Publications/Behavioral_Manuscript.pdf/. Accessed November 27, 2001.
45. Campbell JK, Penzien DB, Wall EM. Evidence-based guidelines for migraine headache: behavioral and physical treatments. Available at: www.aan.com/public/practiceguidelines/04.pdf/. Accessed November 29, 2001.
46. Holroyd KA, Penzien DB. Pharmacological versus non-pharmacological prophylaxis of recurrent migraine headache: a meta-analytic review of clinical trials. Pain 1990;42:1-13.
47. Silberstein SD, Lipton RB. Headache epidemiology. Emphasis on migraine. Neurol Clin 1996;14:421-34.
48. Silberstein SD, Young WB. Headache and facial pain. In: Goetz CG, Pappert EJ, eds. Textbook of clinical neurology. 1st ed. Philadelphia, Pa: Saunders; 1999:1095.
49. Wober C, Wober-Bingol C, Kock G, Wessely P. Long-term results of migraine prophylaxis with flunarazine and beta-blockers. Cephalalgia. 1991;11:251-6.
50. Diener HC, Dahlof CGH. Headache associated with chronic use of substances. In: Olesen J, Tfelt-Hansen P, Welch KMA, eds. The headaches. 2nd ed. Philadelphia, Pa: Lippincott, Williams & Wilkins; 2000:871-7.
51. Paracetamol, aspirin caffeine incombination (Excedrin) for acute migraine. Available at: http://www.jr2.ox.ac.uk/bandolier/booth/Migraine/Excedr.html/. Accessed November 9, 2001.
52. Aspirin for acute migraine. Available at: http://www.jr2.ox.ac.uk/bandolier/booth/Migraine/Aspacute.html/. Accessed November 9, 2001.
53. Aspirin plus metoclopramide for acute migraine. Available at: http://www.jr2.ox.ac.uk/bandolier/booth/Migraine/AsMcOacu.html/. Accessed November 9, 2001.
54. Intranasal dihydroergotamine for acute migraine Available at: http://www.jr2.ox.ac.uk/bandolier/booth/Migraine/DHEINacu.html/. Accessed November 9, 2001.
55. Ibuprofen for acute migraine (new RCT). Available at: http://www.jr2.ox.ac.uk/bandolier/booth/Migraine/ibu2.html/. Accessed November 9, 2001.
56. Migraine league table: acute treatments and two hour headache response. Available at: http://www.jr2.ox.ac.uk/bandolier/booth/Migraine/League.html/. Accessed November 9, 2001.
57. Lipton RB, Baggish JS, Stewart WF, Codispoti JR, Fu M. Efficacy and safety of acetaminophen in the treatment of migraine: results of a randomized, double-blind, placebo-controlled, population-based study. Arch Intern Med 2000;160:3486-92
The Treatment of Adults with Essential Hypertension
- Only 53% of hypertensive patients are being treated, and only 24% have their hypertension under control.
- The first step in planning the treatment of a patient with essential hypertension is to categorize the patient’s risk status.
- The target blood pressure of patients who have diabetes or renal failure should be less than 130/85.
- Diuretics are safe, well tolerated, effective, relatively inexpensive, and convenient for initial drug treatment of hypertension in patients who do not have concomitant illness.
- Alpha-adrenergic blockers should be used with caution in the treatment of hypertension.
- Ambulatory blood pressure measurements predict cardiovascular events more closely than clinic blood pressure measurements.
Hypertension is arbitrarily defined as diastolic blood pressure (DBP) of 90 mm Hg or higher, systolic blood pressure (SBP) of 140 mm Hg or higher, or both, on 3 separate occasions. Essential hypertension is hypertension without an identifiable cause. Essential hypertension, also known as primary or idiopathic hypertension, accounts for at least 95% of all cases of hypertension.
According to the third National Health and Nutrition Examination Survey (NHANES III), approximately 60% of the 50 million Americans with hypertension are at increased risk for cardiovascular disease resulting from uncontrolled hypertension. This is because only 53% of hypertensive patients are being treated and only 24% have their hypertension under control.1 Physicians must play an active role in identifying and treating hypertension.
In an earlier Applied Evidence article2 an approach to the diagnosis of hypertension was presented. This article reviews the treatment of essential hypertension in adults and the prognosis of untreated hypertension. Risk stratification, alternative therapies, lifestyle modification, drug therapy, and prognosis will each be reviewed sequentially.
Risk stratification
The decision to treat hypertension and the choice of treatment is affected by the patient’s risk of morbidity and mortality if the blood pressure remains untreated or under-treated. According to the recommendations of the sixth report of the Joint National Committee on the Prevention, Diagnosis, Evaluation, and Treatment of High Blood Pressure (JNC-VI), the first step in planning treatment of a patient with essential hypertension is to categorize the patient’s risk status.3 The patient is placed in 1 of 9 treatment categories according to his or her blood pressure category, cardiovascular risk factors, and evidence of end-organ damage found during the initial evaluation (Table 1). Once the treatment category is identified, initial treatment should begin (Figure 1). Subsequent treatment depends on the patient’s response to initial treatment (Figure 2).
Patients should be monitored regularly to be sure they do not develop signs and symptoms that would place them in a different category and mandate more aggressive treatment. After a patient’s blood pressure has been controlled for 1 year, it may be possible to decrease the dose or the number of antihypertensive drugs—especially among patients who make significant lifestyle changes.4
The effectiveness of therapy varies depending on the patient’s cardiovascular risk. The New Zealand Guidelines Group has developed a helpful risk calculator based on the Framingham Heart Study for estimating a patient’s cardiovascular risk. This calculator incorporates sex, age, systolic blood pressure, smoking status, total cholesterol, high-density lipoprotein cholesterol, presence or absence of diabetes, and presence or absence of electrocardiogram evidence of left ventricular hypertrophy. This helpful risk calculator may be downloaded from the Web site of the New Zealand Guidelines Group at http://www.nzgg.org.nz/library/gl_complete/bloodpressure/appendix.cfm#app3. Alternatively, the University of Sheffield Medical School has developed tables to estimate an individual’s risk of heart disease based on cardiovascular risk factors including age, sex, cholesterol level, and presence or absence of smoking, hypertension, and diabetes—Sheffield tables.5 Software for handheld computers (Palm and PocketPC) that helps you estimate risk is available at www.jfponline.com.
Regardless of the method used, the benefit of treatment increases steadily as the patient’s current cardiovascular risk increases. With a 5-year cardiovascular risk of less than 2.5%, more than 120 patients have to be treated for 5 years to prevent 1 cardiovascular event; this number decreases to 25 patients with a risk of between 5% and 10%, and only 13 with a risk of between 20% and 24%.6 It is tempting to assume that the benefit of hypertension treatment is related to reduction in blood pressure whether achieved by drug therapy, lifestyle modification, or alternative therapy. However, this has not been established and it is important to consider the evidence supporting the benefit of each of these therapeutic options (Table 2).
TABLE 1
HYPERTENSION RISK STRATIFICATION AND TREATMENT CATEGORIES
| Blood Pressure Category | Risk Group A* | Risk Group B* | Risk Group C* |
|---|---|---|---|
| High-normal (130 – 139/85 – 89) | Lifestyle modification† | Lifestyle modification | Lifestyle modification and drug therapy |
| Stage 1 (140 – 159/0 – 99) | Lifestyle modification (12-month trial) | Lifestyle modification (6-month trial) | Lifestyle modification and drug therapy |
| Stage 2 or 3 (≥ 160 / ≤100) | Lifestyle modification and drug therapy | Lifestyle modification and drug therapy | Lifestyle modification and drug therapy |
| *Risk groups: A = no risk factors, end-organ damage, or clinical cardiovascular disease; B = 1 risk factor other than diabetes, no end-organ damage, and no clinical cardiovascular disease; C = Diabetes, end-organ damage, or clinic cardiovascular disease. | |||
| † Lifestyle modification should be included in the treatment plan of all patients receiving drug therapy. | |||
FIGURE 1
ALGORITHM FOR INITIAL MANAGEMENT OF HYPERTENSION
FIGURE 2
ALGORITHM FOR SUBSEQUENT MANAGEMENT OF HYPERTENSION
TABLE 2
NUMBER NEEDED TO TREAT (NNT) FOR SPECIFIC ANTIHYPERTENSIVE TREATMENTS
| Medication | Level of Evidence | NNT (95% CI)* | Comment |
|---|---|---|---|
| Low-Dose Thiazide | 1a | 18 (14-23) | Adults with systolic blood pressure |
| ≥ 160 or diastolic blood pressure | |||
| ≥90 regardless of age or comorbidities. | |||
| High-Dose Thiazide | 1a | 67 (48-111) | |
| Beta-Blocker | 1a | 142 (71-1000) | Drug vs no treatment comparison.14 |
| Calcium-Channel Blockers | 1b | 45 (30-102) | Isolated systolic hypertension in older patients, drug vs no treatment comparison.22 |
| ACE inhibitors | 1b | NS | Captopril versus diuretic or ß-blocker. |
| Alpha-agonists | 1b | NS | Doxazosin versus chlorthalidone, increased congestive heart in doxazosin group.7 |
| ARBs | NA | NA | Patient-oriented outcomes not available. |
| Sodium Restriction | 1a | NA | May reduce blood pressure but lacks evidence of reduced morbidity or mortality.27-35 |
| Weight Loss | 1a | NA | |
| Exercise | 1a | NA | |
| Low-Fat Diet | 1b | NA | |
| Limited Alcohol | 5 | NA | |
| Potassium Supplement | 1a | NA | |
| Fish Oil Supplement | 1a | NA | |
| Acupuncture | NA | NA | No evidence of blood pressure reduction or reduced morbidity or mortality.36-43 |
| Biofeedback | NA | NA | |
| Herbal Medicine | NA | NA | |
| Transcendental Meditation | NA | NA | |
| Yoga | NA | NA | |
| *For total cardiovascular events over 5 years. | |||
| NS denotes no significant difference from comparison drug; NA, not applicable; ACE, angiotensin-converting enzyme; ARBs, angiotensin-receptor blockers. | |||
Treatment
Drug Therapy
Patients who require drug treatment for hypertension should begin with a low dose of the initial medication, and that dose should be slowly titrated upward every 1 to 2 months (Figure 2). The JNC-VI recommends a diuretic or a ß-blocker with once daily dosing and 24-hour efficacy as the initial treatment for most hypertensive patients. However, the choice of initial medication will be affected by concomitant illnesses: (1) ß-blockers are recommended for the initial treatment of patients with hypertension and a history of coronary artery disease; (2) diuretics are suggested for the initial treatment of isolated systolic hypertension; (3) and angiotensin-converting enzyme (ACE) inhibitors are recommended for hypertensive patients who have systolic dysfunction after myocardial infarction, diabetic nephropathy, or congestive heart failure. Angiotensin II receptor blockers may be used in patients who cannot tolerate ACE inhibitors because of cough or rash. Alpha-adrenergic blockers should be used with caution in light of evidence that they may increase the risk of cardiovascular events (especially congestive heart failure).7
Among patients who do not have concomitant illness, the choice of drug therapy is controversial. A case-control study and a meta-analysis suggested that short-acting calcium channel blockers (CCBs) increase cardiovascular mortality.8,9 Unfortunately, these studies were not designed to establish a causal relationship. A recent nonsystematic review suggested that short-acting CCBs should be avoided and that conventional therapies were more effective than long-acting CCBs.10 An earlier non-systematic review suggested that short- and intermediate-acting CCBs were associated with increased cardiovascular mortality and morbidity. However, a well-designed cohort study of patients with coronary artery disease failed to reveal an increase in adverse effects among patients taking short-acting CCBs.11 Furthermore, randomized controlled trials suggest that diuretics, ß-blockers, and long-acting CCBs are equally effective in preventing cardiovascular mortality and morbidity.12,13 Physicians who treat hypertension must choose the best initial treatment for patients who do not have concomitant illness. Fortunately, safety, tolerability, efficacy, price, and simplicity can guide the physician to an ideal drug for most hypertensive patients.
Low-dose thiazide diuretics (the equivalent of 25 to 50 mg of hydrochlorothiazide) appear better tolerated than ß-blockers or CCBs.14 Treatment with ß-blockers, CCBs, and ACE inhibitors is also more expensive (75% to 85% more) than diuretic therapy.15 The cost savings offered by diuretics complement the fact that diuretics are safe, effective, and may be dosed once daily. In short, in addition to being the drug of choice for isolated systolic hypertension, low-dose thiazide diuretics are the ideal initial drug treatment of patients without concomitant illness. It should be noted that higher doses of thiazide diuretics offer proportionately less blood pressure reduction and greater risk of hypokalemia.16
Antihypertensive treatment reduces morbidity and mortality for all stages of hypertension, but people with the greatest baseline cardiovascular risk (eg, older patients and patients with higher levels of blood pressure) have the most to gain from treatment.17,18 There is no conclusive evidence to suggest that lowering blood pressure to below 140/80 reduces morbidity or mortality in most patients. However, patients who have diabetes or renal failure benefit from more aggressive management of blood pressure.19,20 Therefore, the JNC-VI recommends a target blood pressure of less than 130/85 for these patients.
The JNC-VI recommendation to start with a low-dose diuretic is supported by the evidence across a spectrum of patient-oriented outcomes. The effectiveness of diuretics and ß-blockers as first-line agents has been confirmed by long-term clinical trials.14,21 However, low-dose thiazides appear effective against a broader range of outcomes than high-dose thiazides and ß-blockers (Table 3). There is also evidence to suggest that CCBs and ACE inhibitors may be effective first-line agents, but fewer patients have been studied who take CCBs and ACE inhibitors than those who take diuretics and ß-blockers.22,23
Most patients with hypertension will respond to 1 (approximately 50%) or 2 (approximately 30%) antihypertensive medications.19,24,25 As noted earlier, failure to respond to treatment suggests an identifiable cause of hypertension. Among patients who do not have a secondary cause of hypertension, inadequate drug treatment (often failure to start a diuretic) and noncompliance are among the most common causes of resistant hypertension.26
When patients who are receiving drug therapy fail to reach the target blood pressure goal or fail to maintain the blood pressure goal, they should have the initial drug dose increased until the goal is reached (Figure 2). Those who fail initial drug therapy at full doses should have a second drug added and increased until the blood pressure goal is reached. Those who fail initial and second drug therapy at full doses should have a third drug added and increased until the pressure goal is reached. Patients who fail to reach the goal on maximal doses of 3 drugs have, by definition, resistant hypertension and will require evaluation by a physician with expertise in managing resistant hypertension. A cause should be sought each time a patient fails to respond to a drug or fails to maintain blood pressure control on a drug that had previously controlled the pressure (Table 3).
TABLE 3
PARTIAL LISTING OF CAUSES OF FAILURE TO REACH OR MAINTAIN TARGET BLOOD PRESSURE
| Volume overload: failure to start a diuretic |
| Nonadherence to therapy: dementia, side effects, complex regimen |
| Drug-induced: prescription, over-the-counter, herbal, or illicit drugs |
| Diet/stimulant induced: caffeine, licorice, salt, alcohol, nicotine |
| Associated conditions: obesity, sleep apnea, anxiety, chronic pain |
| Identifiable causes: chronic renal disease, renovascular disease, hyperaldosteronism, Cushing’s syndrome, pheochromocytoma |
| Pseudoresistance: wrong cuff size, white-coat hypertension |
Lifestyle modifications
Several lifestyle modifications are recommended in all treatment categories. Aerobic exercise (45 to 60 minutes at least 3 days per week), low-salt, low-fat, and high fruit and vegetable diet, limited alcohol consumption (less than 3 drinks per day), and modest weight loss (3% to 9% of total body weight) have been demonstrated to yield modest blood pressure reductions, but there is insufficient evidence to suggest that these measures alone reduce morbidity or mortality in hypertensive patients.27-33 A systematic review of randomized controlled trials found an average 4.4/2.5 mm Hg reduction in blood pressure with no evidence of harm (among patients who were not at risk for hyperkalemia) when diet was supplemented with about 2000 mg of potassium daily.34 A comparable reduction in blood pressure was seen with a daily supplement of more than 3 grams of fish oil.35 Research concerning the value of calcium and magnesium supplementation is conflicting and insufficient for supplementation to be considered standard therapy at this time.
Alternative therapy
The number and the quality of studies evaluating acupuncture, biofeedback, herbal medicine, transcendental meditation, and yoga are, for the most part, limited. They have focused on reduction in blood pressure, not patient-oriented outcomes, such as a reduction in morbidity and mortality. Acupuncture does not appear to have a significant effect on blood pressure levels.36,37 Biofeedback and other behavioral techniques have not been demonstrated to reduce blood pressure.38,39 The effect of garlic on blood pressure is unclear with mixed study results.40,41 Transcendental meditation and yoga may reduce blood pressure, but studies of these modalities are small and the experimental designs have a limited capacity to detect an independent treatment effect or a placebo effect.42,43
Therefore, physicians who include any of these modalities in their hypertension treatment plan should carefully monitor each patient for adequacy of blood pressure control, development of risk factors, and evidence of end-organ damage. At this time, alternative therapies should be considered experimental adjuncts to lifestyle modification and medical therapy that have not been shown to improve patient-oriented outcomes.
Follow-up of patients with hypertension
Follow-up visits should be designed to identify new risk factors, evidence of end-organ damage, and adequacy of blood pressure control. Follow-up visits may include an interval history, limited physical examination, radiologic evaluation, and laboratory testing. The frequency and nature of follow-up hypertension evaluations will vary according to the presence or absence of preexisting risk factors, evidence of end-organ damage, the nature of the treatment the patient is receiving, and the stability of blood pressure control. Unfortunately, there is little evidence to support specific recommendations for the frequency and nature of follow-up hypertension evaluations.
In the absence of evidence, several general principles may be suggested. Patients should be seen within 2 months of initiation of treatment. Follow-up history should focus on the cardiovascular and neurologic review of systems. The examination should include a focused cardiovascular work-up (eg, retinopathy, carotid bruits). Consideration should be given to periodic laboratory testing for diabetes, renal insufficiency, and hyperlipidemia. Periodic (but less frequent) chest x-rays and electrocardiograms may be helpful to detect cardiomegaly, but there is no evidence to support such testing in the absence of symptoms.
Follow-up visits should be more frequent among patients who have marginal blood pressure control, preexisting risk factors, or end-organ damage. Evaluations may be less frequent among those with good control and no preexisting risk factors or endorgan damage. Office visits and testing should be more frequent whenever changes are made in treatment. The frequency and nature of follow-up testing will also depend on the nature of treatment. Patients taking diuretics should have their potassium levels checked periodically. Renal function and potassium should be monitored in patients who are taking ACE inhibitors, especially during the first few weeks of therapy.
Patients willing to regularly monitor their blood pressure at home may require less frequent follow-up than those who leave it to the physician to check. Patients who monitor their blood pressure at home should have their sphygmomanometers validated initially and periodically.44 It is important to remember that home blood pressure measurements are consistently lower and more closely correlated with cardiovascular outcomes than are clinic blood pressure measurements.45-47
Prognosis
It is difficult to estimate the precise impact blood pressure control has on morbidity and mortality, but it is clear that high blood pressure, if unrecognized or untreated, substantially increases the morbidity and mortality associated with coronary disease, heart failure, renal failure, and stroke.17 In an early study of untreated hypertension there was a close relationship between blood pressure level and cardiovascular morbidity over 14 years of observation. This study revealed that hypertensive patients (those with a blood pressure of 160/95) had cardiovascular morbidity rates (coronary artery disease, claudication, stroke, and congestive heart failure) 2 to 3 times higher than normotensive patients.48 The impact of inadequately controlled blood pressure on morbidity and mortality among patients with diabetes is especially problematic.49 Over 9 years, when compared with diabetic patients with less tight control (< 180/105 mm Hg), those with tight blood pressure control (< 150/85 mm Hg) had a 24% reduction in sudden death, hyperglycemic or hypoglycemic death, fatal or nonfatal myocardial infarction, angina, heart failure, fatal or nonfatal stroke, renal failure, amputation, vitreous hemorrhage, and retinal hemorrhage.
Finally, renal function deteriorates more rapidly when blood pressure control is inadequate in patients with chronic renal disease of diverse causes.20 Over 2 years, when compared with patients with renal failure who had less tight control (mean arterial pressure 107 mm Hg), renal failure patients with tight control (mean arterial blood pressure 92 mm Hg) had significantly less proteinuria and lower rates of decline in renal function. Whether this translates into a significant improvement in the risk of end-stage renal disease is unknown.
1. Burt V, Whelton P, Rocella E, Brown C, Cutler J. Prevalence of hypertension in the US population: results from the third national health and nutrition examination survey, 1988-1991. Hypertension 1995;25:305-13.
2. Dosh S. The diagnosis of essential and secondary hypertension in adults. J Fam Pract 2001;50:707-12.
3. Joint National Committee on the Prevention Detection. Evaluation and Treatment of High Blood Pressure. the sixth report of the joint national committee on prevention, detection, evaluation, and treatment of high blood pressure (JNC VI). Arch Intern Med 1997;157:2413-46.
4. Whelton P, Appelgate W, Ettinger W, et al. Sodium reduction and weight loss in the treatment of hypertension in older persons: a randomized controlled trial of non-pharmacologic interventions in the elderly (TONE). JAMA 1996;279:839-46.
5. Wallis E, Ramsay L, Haq I, Ghahramani P, Jackson P. Coronary and cardiovascular risk estimation for the primary prevention: validation of a new Sheffield table in the 1995 Scottish health survey. Br Med J 2000;320:671-76.
6. Baker S, Priest P, Jackson R. Using thresholds based on risk of cardiovascular disease to target treatment for hypertension: modelling events averted and number treated. Br Med J 2000;320:680-85.
7. ALLHAT Collaborative Research Group. Major cardiovascular events in hypertensive patients randomized to doxazosin vs chlorthalidone. J Am Med Assoc 2000;283:1967-75.
8. Psaty B, Heckbert S, Koepsell T, et al. The risk of myocardial infarction associated with anti-hypertensive drug therapies. JAMA 1995;274:620-25.
9. Furberg C, Psaty B, Meyer J. Nifedipine: dose related increase in mortality in patients with coronary heart disease. Circulation 1995;92:1326-31.
10. Kizer J, Kimmel S. Epidemiologic review of the calcium channel blocker drugs. Arch Intern Med 2001;161:1145-58.
11. Braun S, Boyo V, Behar S, et al. Calcium antagonists did not increase mortality in patients with coronary artery disease. J Am Coll Cardiol 1996;28:7-11.
12. Hansson L, Hedner T, Lund-Johansen P, et al. Randomized trial of effects of calcium antagonists compared with diuretics and B-blockers on cardiovascular morbidity and mortality in hypertension: the Nordic Diltiazem (NORDIL) study. Lancet 2000;356:359-65.
13. Hansson L, Lindholm L, Ekbohm T, Dahlof B. Randomised trial of old and new antihypertensive drugs in elderly patients: cardiovascular mortality and morbidity. The Swedish trial in old patients with hypertension-2 study. Lancet 1999;354:1751-56.
14. Wright J, Lee C, Chambers G. Systematic review of antihypertensive therapies: does evidence assist in choosing a first-line drug? Can Med Assoc J 1999;161:25-32.
15. Ramsey S, Niel N, Sullivan S, Perfetto E. An economic evaluation of the JNC hypertension guidelines using data from a randomized controlled trial. J Am Board Fam Pract 1999;12:105-14.
16. Wright J. Choosing a first-line drug in the management of elevated blood pressure: What is the evidence?: 1 Thiazide diuretics. Can Med Assoc J 2000;163:57-60.
17. Gueyffier F, Froment A, Gouton M. New meta-analysis of treatment trials of hypertension: improving the estimate of therapeutic benefit. J Hum Hypertens 1996;10:1-8.
18. Mulrow C, Cornell J, Herrera C, Kadri A, Farnett L, Aguilar C. Hypertension in the elderly: implications and generalizability of randomized trials. JAMA 1994;272:1932-38.
19. Hansson L, Zanchetti A, Carruthers S, Dahlof B. Effects of intensive blood pressure lowering and low dose aspirin in patients with hypertension: principal results of the hypertension optimal treatment (HOT) randomised trial. Lancet 1998;351:1755-62.
20. Peterson J, Adler S, Burkart J, Greene J. Blood pressure control, proteinuria, and the progression of renal disease. Ann Intern Med 1995;123:754-62.
21. Psaty B, Smith N, Siscovick D, Koepsell T, Weiss N. Health outcomes associated with antihypertensive therapies used as first-line agents: A systematic review and meta-analysis. J Am Med Assoc 1197;277:739-45.
22. Staessen J, Fagard R, Celis H, Arabidze C, Birkenhager W. Randomised double-blind comparison of placebo and active treatment in older patients with isolated systolic hypertension. Lancet 1997;350:757-64.
23. Investigators THOPES. Effect of an angiotensin-converting-enzyme inhibitor, ramipril, on cardiovascular events in high-risk patients. New Eng J Med 2000;342:145-53.
24. Masterson B, Reda D, Preston R, et al. Response to a second single antihypertensive agent used as monotherapy for hypertension after failure of the initial drug. Arch Intern Med 1995;155:1757-62.
25. Tuomilehto J, Rastenyte D, Birkenhager W, et al. Systolic hypertension in Europe trial investigators. Effects of calcium-channel blockers in older patients with diabetes and systolic hypertension. New Eng J Med 1999;340:677-84.
26. Yakovlevitch M, Black HR. Resistant hypertension in a tertiary care clinic. Arch Intern Med 1991;151:1786-92.
27. Halbert J, Silagy C, Finucane P, Withers R. The effectiveness of exercise training in lowering blood pressure: a metaanalysis of randomized controlled trials of 4 weeks or longer. J Human Hypertension 1997;10:641-49.
28. Ebrahim S, Davey G. Lowering blood pressure: a systematic review of sustained effects of non-pharmacologic interventions. J Public Health Med 1998;4:441-48.
29. Appel L, Moore T, Obarzanek E, Vollmer W. A clinical trail of the effects of dietary patterns on blood pressure. New Eng J Med 1997;336:1117-24.
30. Appel L, Espeland M, Easter L, Wilson A, Folmar S, Lacy C. Effects of reduced sodium intake on hypertension control in older individuals. Results from the trial of nonpharmacologic interventions in the elderly (TONE). Arch Intern Med 2001;161:685-93.
31. Beilin L, Puddey I, Burke V. Alcohol and hypertension: kill or cure? J Human Hypertension 1996;10(Suppl 2):1-5.
32. Corrigan S, Raczynski J, Swencionis C, Jennings S. Weight reduction in the prevention and treatment of hypertension: a review of representative clinical trials. Am J Health Promo 1991;5:208-14.
33. Mulrow C, Chiquette E, Angel L, Cornell J. Dieting to reduce body weight for controlling hypertension in adults. The Cochrane Library 2001.
34. Whelton P, He J, Cutler J, Brancati F, Appel L. Effects of oral potassium on blood pressure: meta-analysis of randomized controlled trials. JAMA 1997;277:1624-32.
35. Morris M, Sacks F, Rosner B. Regulation on blood pressure: does fish oil lower blood pressure?: analysis of controlled trials. Circulation 1993;88:523-33.
36. Sugioka K, Woods M, Mueller R. An unsuccessful attempt to treat hypertension with acupuncture. Am J Chinese Med 1977;5:39-44.
37. Kraft K, Coulon S. Effect of a standardized acupuncture treatment on complaints, blood pressure, and serum lipids of hypertensive, postmenopausal women. A randomized controlled clinical study. Forschende Komplementarmedizin 1999;6:74-79.
38. Eisenberg D, Delblanco T, Berkey C, et al. Cognitive and behavioral techniques and hypertension: a meta-analysis. Ann Intern Med 1993;118:964-72.
39. Hunyor S, Henderson R, Saroj K, Carter N, et al. Placebo-controlled biofeedback blood pressure effect in hypertensive humans. Hypertension 1997;29:1225-31.
40. Auer W. Hypertension and hyperlipidemia: garlic helps in mild cases. Br J Clin Pract 1990;69(Suppl):3-6.
41. Ackerman R, Mulrow C, Ramirez G, Gardner C, Mobidoni L, Lawrence V. Garlic shows promise for improving some cardiovascular risk factors. Arch Intern Med 2001;161:813-24.
42. Patel C. Twelve-month follow-up of yoga and bio-feedback in the management of hypertension. Lancet 1975;1:62-64.
43. Sundar S, Agrawal S, Singh V, Bhattacharya S, et al. Role of yoga in management of essential hypertension. Acta Cardiologica 1984;39:203-08.
44. White W, Berson A, Robbins C, Jamieson M, Prisant L. National standard for measurement of resting and ambulatory blood pressure with automated sphygmomanometers. Hypertension 1993;21:504-09.
45. Staessen J, Byttebier G, Butinx F, Celis H, O’Brien E. Antihypertensive treatment based on conventional or ambulatory blood pressure measurement. J Am Med Assoc 1997;278:1065-72.
46. Ohkubo T, Imai Y, Tsuji I, Nagai K, Ito S. Reference values for 24-hour ambulatory blood pressure monitoring based on a prognostic criterion: the Ohasma study. Hypertension 1998;32:255-59.
47. Staessen J, Thijs L, Fagard R, O’Brien E, Eoin T. Predicting cardiovascular risk using conventional vs ambulatory blood pressure in older patients with systolic hypertension. JAMA 1999;282:539-46.
48. Kannel W, Wolf P, Verter J, McNamara P. Epidemiologic assessment of the role of blood pressure in stroke: the Framingham study. JAMA 1970;214:301-10.
49. Turner R, Holman R, Stratton I, Cull C. Tight blood pressure control and risk of macrovascular and microvascular complications in type 2 diabetes: UKPDS 38. Br Med J 1998;317:703-12.
- Only 53% of hypertensive patients are being treated, and only 24% have their hypertension under control.
- The first step in planning the treatment of a patient with essential hypertension is to categorize the patient’s risk status.
- The target blood pressure of patients who have diabetes or renal failure should be less than 130/85.
- Diuretics are safe, well tolerated, effective, relatively inexpensive, and convenient for initial drug treatment of hypertension in patients who do not have concomitant illness.
- Alpha-adrenergic blockers should be used with caution in the treatment of hypertension.
- Ambulatory blood pressure measurements predict cardiovascular events more closely than clinic blood pressure measurements.
Hypertension is arbitrarily defined as diastolic blood pressure (DBP) of 90 mm Hg or higher, systolic blood pressure (SBP) of 140 mm Hg or higher, or both, on 3 separate occasions. Essential hypertension is hypertension without an identifiable cause. Essential hypertension, also known as primary or idiopathic hypertension, accounts for at least 95% of all cases of hypertension.
According to the third National Health and Nutrition Examination Survey (NHANES III), approximately 60% of the 50 million Americans with hypertension are at increased risk for cardiovascular disease resulting from uncontrolled hypertension. This is because only 53% of hypertensive patients are being treated and only 24% have their hypertension under control.1 Physicians must play an active role in identifying and treating hypertension.
In an earlier Applied Evidence article2 an approach to the diagnosis of hypertension was presented. This article reviews the treatment of essential hypertension in adults and the prognosis of untreated hypertension. Risk stratification, alternative therapies, lifestyle modification, drug therapy, and prognosis will each be reviewed sequentially.
Risk stratification
The decision to treat hypertension and the choice of treatment is affected by the patient’s risk of morbidity and mortality if the blood pressure remains untreated or under-treated. According to the recommendations of the sixth report of the Joint National Committee on the Prevention, Diagnosis, Evaluation, and Treatment of High Blood Pressure (JNC-VI), the first step in planning treatment of a patient with essential hypertension is to categorize the patient’s risk status.3 The patient is placed in 1 of 9 treatment categories according to his or her blood pressure category, cardiovascular risk factors, and evidence of end-organ damage found during the initial evaluation (Table 1). Once the treatment category is identified, initial treatment should begin (Figure 1). Subsequent treatment depends on the patient’s response to initial treatment (Figure 2).
Patients should be monitored regularly to be sure they do not develop signs and symptoms that would place them in a different category and mandate more aggressive treatment. After a patient’s blood pressure has been controlled for 1 year, it may be possible to decrease the dose or the number of antihypertensive drugs—especially among patients who make significant lifestyle changes.4
The effectiveness of therapy varies depending on the patient’s cardiovascular risk. The New Zealand Guidelines Group has developed a helpful risk calculator based on the Framingham Heart Study for estimating a patient’s cardiovascular risk. This calculator incorporates sex, age, systolic blood pressure, smoking status, total cholesterol, high-density lipoprotein cholesterol, presence or absence of diabetes, and presence or absence of electrocardiogram evidence of left ventricular hypertrophy. This helpful risk calculator may be downloaded from the Web site of the New Zealand Guidelines Group at http://www.nzgg.org.nz/library/gl_complete/bloodpressure/appendix.cfm#app3. Alternatively, the University of Sheffield Medical School has developed tables to estimate an individual’s risk of heart disease based on cardiovascular risk factors including age, sex, cholesterol level, and presence or absence of smoking, hypertension, and diabetes—Sheffield tables.5 Software for handheld computers (Palm and PocketPC) that helps you estimate risk is available at www.jfponline.com.
Regardless of the method used, the benefit of treatment increases steadily as the patient’s current cardiovascular risk increases. With a 5-year cardiovascular risk of less than 2.5%, more than 120 patients have to be treated for 5 years to prevent 1 cardiovascular event; this number decreases to 25 patients with a risk of between 5% and 10%, and only 13 with a risk of between 20% and 24%.6 It is tempting to assume that the benefit of hypertension treatment is related to reduction in blood pressure whether achieved by drug therapy, lifestyle modification, or alternative therapy. However, this has not been established and it is important to consider the evidence supporting the benefit of each of these therapeutic options (Table 2).
TABLE 1
HYPERTENSION RISK STRATIFICATION AND TREATMENT CATEGORIES
| Blood Pressure Category | Risk Group A* | Risk Group B* | Risk Group C* |
|---|---|---|---|
| High-normal (130 – 139/85 – 89) | Lifestyle modification† | Lifestyle modification | Lifestyle modification and drug therapy |
| Stage 1 (140 – 159/0 – 99) | Lifestyle modification (12-month trial) | Lifestyle modification (6-month trial) | Lifestyle modification and drug therapy |
| Stage 2 or 3 (≥ 160 / ≤100) | Lifestyle modification and drug therapy | Lifestyle modification and drug therapy | Lifestyle modification and drug therapy |
| *Risk groups: A = no risk factors, end-organ damage, or clinical cardiovascular disease; B = 1 risk factor other than diabetes, no end-organ damage, and no clinical cardiovascular disease; C = Diabetes, end-organ damage, or clinic cardiovascular disease. | |||
| † Lifestyle modification should be included in the treatment plan of all patients receiving drug therapy. | |||
FIGURE 1
ALGORITHM FOR INITIAL MANAGEMENT OF HYPERTENSION
FIGURE 2
ALGORITHM FOR SUBSEQUENT MANAGEMENT OF HYPERTENSION
TABLE 2
NUMBER NEEDED TO TREAT (NNT) FOR SPECIFIC ANTIHYPERTENSIVE TREATMENTS
| Medication | Level of Evidence | NNT (95% CI)* | Comment |
|---|---|---|---|
| Low-Dose Thiazide | 1a | 18 (14-23) | Adults with systolic blood pressure |
| ≥ 160 or diastolic blood pressure | |||
| ≥90 regardless of age or comorbidities. | |||
| High-Dose Thiazide | 1a | 67 (48-111) | |
| Beta-Blocker | 1a | 142 (71-1000) | Drug vs no treatment comparison.14 |
| Calcium-Channel Blockers | 1b | 45 (30-102) | Isolated systolic hypertension in older patients, drug vs no treatment comparison.22 |
| ACE inhibitors | 1b | NS | Captopril versus diuretic or ß-blocker. |
| Alpha-agonists | 1b | NS | Doxazosin versus chlorthalidone, increased congestive heart in doxazosin group.7 |
| ARBs | NA | NA | Patient-oriented outcomes not available. |
| Sodium Restriction | 1a | NA | May reduce blood pressure but lacks evidence of reduced morbidity or mortality.27-35 |
| Weight Loss | 1a | NA | |
| Exercise | 1a | NA | |
| Low-Fat Diet | 1b | NA | |
| Limited Alcohol | 5 | NA | |
| Potassium Supplement | 1a | NA | |
| Fish Oil Supplement | 1a | NA | |
| Acupuncture | NA | NA | No evidence of blood pressure reduction or reduced morbidity or mortality.36-43 |
| Biofeedback | NA | NA | |
| Herbal Medicine | NA | NA | |
| Transcendental Meditation | NA | NA | |
| Yoga | NA | NA | |
| *For total cardiovascular events over 5 years. | |||
| NS denotes no significant difference from comparison drug; NA, not applicable; ACE, angiotensin-converting enzyme; ARBs, angiotensin-receptor blockers. | |||
Treatment
Drug Therapy
Patients who require drug treatment for hypertension should begin with a low dose of the initial medication, and that dose should be slowly titrated upward every 1 to 2 months (Figure 2). The JNC-VI recommends a diuretic or a ß-blocker with once daily dosing and 24-hour efficacy as the initial treatment for most hypertensive patients. However, the choice of initial medication will be affected by concomitant illnesses: (1) ß-blockers are recommended for the initial treatment of patients with hypertension and a history of coronary artery disease; (2) diuretics are suggested for the initial treatment of isolated systolic hypertension; (3) and angiotensin-converting enzyme (ACE) inhibitors are recommended for hypertensive patients who have systolic dysfunction after myocardial infarction, diabetic nephropathy, or congestive heart failure. Angiotensin II receptor blockers may be used in patients who cannot tolerate ACE inhibitors because of cough or rash. Alpha-adrenergic blockers should be used with caution in light of evidence that they may increase the risk of cardiovascular events (especially congestive heart failure).7
Among patients who do not have concomitant illness, the choice of drug therapy is controversial. A case-control study and a meta-analysis suggested that short-acting calcium channel blockers (CCBs) increase cardiovascular mortality.8,9 Unfortunately, these studies were not designed to establish a causal relationship. A recent nonsystematic review suggested that short-acting CCBs should be avoided and that conventional therapies were more effective than long-acting CCBs.10 An earlier non-systematic review suggested that short- and intermediate-acting CCBs were associated with increased cardiovascular mortality and morbidity. However, a well-designed cohort study of patients with coronary artery disease failed to reveal an increase in adverse effects among patients taking short-acting CCBs.11 Furthermore, randomized controlled trials suggest that diuretics, ß-blockers, and long-acting CCBs are equally effective in preventing cardiovascular mortality and morbidity.12,13 Physicians who treat hypertension must choose the best initial treatment for patients who do not have concomitant illness. Fortunately, safety, tolerability, efficacy, price, and simplicity can guide the physician to an ideal drug for most hypertensive patients.
Low-dose thiazide diuretics (the equivalent of 25 to 50 mg of hydrochlorothiazide) appear better tolerated than ß-blockers or CCBs.14 Treatment with ß-blockers, CCBs, and ACE inhibitors is also more expensive (75% to 85% more) than diuretic therapy.15 The cost savings offered by diuretics complement the fact that diuretics are safe, effective, and may be dosed once daily. In short, in addition to being the drug of choice for isolated systolic hypertension, low-dose thiazide diuretics are the ideal initial drug treatment of patients without concomitant illness. It should be noted that higher doses of thiazide diuretics offer proportionately less blood pressure reduction and greater risk of hypokalemia.16
Antihypertensive treatment reduces morbidity and mortality for all stages of hypertension, but people with the greatest baseline cardiovascular risk (eg, older patients and patients with higher levels of blood pressure) have the most to gain from treatment.17,18 There is no conclusive evidence to suggest that lowering blood pressure to below 140/80 reduces morbidity or mortality in most patients. However, patients who have diabetes or renal failure benefit from more aggressive management of blood pressure.19,20 Therefore, the JNC-VI recommends a target blood pressure of less than 130/85 for these patients.
The JNC-VI recommendation to start with a low-dose diuretic is supported by the evidence across a spectrum of patient-oriented outcomes. The effectiveness of diuretics and ß-blockers as first-line agents has been confirmed by long-term clinical trials.14,21 However, low-dose thiazides appear effective against a broader range of outcomes than high-dose thiazides and ß-blockers (Table 3). There is also evidence to suggest that CCBs and ACE inhibitors may be effective first-line agents, but fewer patients have been studied who take CCBs and ACE inhibitors than those who take diuretics and ß-blockers.22,23
Most patients with hypertension will respond to 1 (approximately 50%) or 2 (approximately 30%) antihypertensive medications.19,24,25 As noted earlier, failure to respond to treatment suggests an identifiable cause of hypertension. Among patients who do not have a secondary cause of hypertension, inadequate drug treatment (often failure to start a diuretic) and noncompliance are among the most common causes of resistant hypertension.26
When patients who are receiving drug therapy fail to reach the target blood pressure goal or fail to maintain the blood pressure goal, they should have the initial drug dose increased until the goal is reached (Figure 2). Those who fail initial drug therapy at full doses should have a second drug added and increased until the blood pressure goal is reached. Those who fail initial and second drug therapy at full doses should have a third drug added and increased until the pressure goal is reached. Patients who fail to reach the goal on maximal doses of 3 drugs have, by definition, resistant hypertension and will require evaluation by a physician with expertise in managing resistant hypertension. A cause should be sought each time a patient fails to respond to a drug or fails to maintain blood pressure control on a drug that had previously controlled the pressure (Table 3).
TABLE 3
PARTIAL LISTING OF CAUSES OF FAILURE TO REACH OR MAINTAIN TARGET BLOOD PRESSURE
| Volume overload: failure to start a diuretic |
| Nonadherence to therapy: dementia, side effects, complex regimen |
| Drug-induced: prescription, over-the-counter, herbal, or illicit drugs |
| Diet/stimulant induced: caffeine, licorice, salt, alcohol, nicotine |
| Associated conditions: obesity, sleep apnea, anxiety, chronic pain |
| Identifiable causes: chronic renal disease, renovascular disease, hyperaldosteronism, Cushing’s syndrome, pheochromocytoma |
| Pseudoresistance: wrong cuff size, white-coat hypertension |
Lifestyle modifications
Several lifestyle modifications are recommended in all treatment categories. Aerobic exercise (45 to 60 minutes at least 3 days per week), low-salt, low-fat, and high fruit and vegetable diet, limited alcohol consumption (less than 3 drinks per day), and modest weight loss (3% to 9% of total body weight) have been demonstrated to yield modest blood pressure reductions, but there is insufficient evidence to suggest that these measures alone reduce morbidity or mortality in hypertensive patients.27-33 A systematic review of randomized controlled trials found an average 4.4/2.5 mm Hg reduction in blood pressure with no evidence of harm (among patients who were not at risk for hyperkalemia) when diet was supplemented with about 2000 mg of potassium daily.34 A comparable reduction in blood pressure was seen with a daily supplement of more than 3 grams of fish oil.35 Research concerning the value of calcium and magnesium supplementation is conflicting and insufficient for supplementation to be considered standard therapy at this time.
Alternative therapy
The number and the quality of studies evaluating acupuncture, biofeedback, herbal medicine, transcendental meditation, and yoga are, for the most part, limited. They have focused on reduction in blood pressure, not patient-oriented outcomes, such as a reduction in morbidity and mortality. Acupuncture does not appear to have a significant effect on blood pressure levels.36,37 Biofeedback and other behavioral techniques have not been demonstrated to reduce blood pressure.38,39 The effect of garlic on blood pressure is unclear with mixed study results.40,41 Transcendental meditation and yoga may reduce blood pressure, but studies of these modalities are small and the experimental designs have a limited capacity to detect an independent treatment effect or a placebo effect.42,43
Therefore, physicians who include any of these modalities in their hypertension treatment plan should carefully monitor each patient for adequacy of blood pressure control, development of risk factors, and evidence of end-organ damage. At this time, alternative therapies should be considered experimental adjuncts to lifestyle modification and medical therapy that have not been shown to improve patient-oriented outcomes.
Follow-up of patients with hypertension
Follow-up visits should be designed to identify new risk factors, evidence of end-organ damage, and adequacy of blood pressure control. Follow-up visits may include an interval history, limited physical examination, radiologic evaluation, and laboratory testing. The frequency and nature of follow-up hypertension evaluations will vary according to the presence or absence of preexisting risk factors, evidence of end-organ damage, the nature of the treatment the patient is receiving, and the stability of blood pressure control. Unfortunately, there is little evidence to support specific recommendations for the frequency and nature of follow-up hypertension evaluations.
In the absence of evidence, several general principles may be suggested. Patients should be seen within 2 months of initiation of treatment. Follow-up history should focus on the cardiovascular and neurologic review of systems. The examination should include a focused cardiovascular work-up (eg, retinopathy, carotid bruits). Consideration should be given to periodic laboratory testing for diabetes, renal insufficiency, and hyperlipidemia. Periodic (but less frequent) chest x-rays and electrocardiograms may be helpful to detect cardiomegaly, but there is no evidence to support such testing in the absence of symptoms.
Follow-up visits should be more frequent among patients who have marginal blood pressure control, preexisting risk factors, or end-organ damage. Evaluations may be less frequent among those with good control and no preexisting risk factors or endorgan damage. Office visits and testing should be more frequent whenever changes are made in treatment. The frequency and nature of follow-up testing will also depend on the nature of treatment. Patients taking diuretics should have their potassium levels checked periodically. Renal function and potassium should be monitored in patients who are taking ACE inhibitors, especially during the first few weeks of therapy.
Patients willing to regularly monitor their blood pressure at home may require less frequent follow-up than those who leave it to the physician to check. Patients who monitor their blood pressure at home should have their sphygmomanometers validated initially and periodically.44 It is important to remember that home blood pressure measurements are consistently lower and more closely correlated with cardiovascular outcomes than are clinic blood pressure measurements.45-47
Prognosis
It is difficult to estimate the precise impact blood pressure control has on morbidity and mortality, but it is clear that high blood pressure, if unrecognized or untreated, substantially increases the morbidity and mortality associated with coronary disease, heart failure, renal failure, and stroke.17 In an early study of untreated hypertension there was a close relationship between blood pressure level and cardiovascular morbidity over 14 years of observation. This study revealed that hypertensive patients (those with a blood pressure of 160/95) had cardiovascular morbidity rates (coronary artery disease, claudication, stroke, and congestive heart failure) 2 to 3 times higher than normotensive patients.48 The impact of inadequately controlled blood pressure on morbidity and mortality among patients with diabetes is especially problematic.49 Over 9 years, when compared with diabetic patients with less tight control (< 180/105 mm Hg), those with tight blood pressure control (< 150/85 mm Hg) had a 24% reduction in sudden death, hyperglycemic or hypoglycemic death, fatal or nonfatal myocardial infarction, angina, heart failure, fatal or nonfatal stroke, renal failure, amputation, vitreous hemorrhage, and retinal hemorrhage.
Finally, renal function deteriorates more rapidly when blood pressure control is inadequate in patients with chronic renal disease of diverse causes.20 Over 2 years, when compared with patients with renal failure who had less tight control (mean arterial pressure 107 mm Hg), renal failure patients with tight control (mean arterial blood pressure 92 mm Hg) had significantly less proteinuria and lower rates of decline in renal function. Whether this translates into a significant improvement in the risk of end-stage renal disease is unknown.
- Only 53% of hypertensive patients are being treated, and only 24% have their hypertension under control.
- The first step in planning the treatment of a patient with essential hypertension is to categorize the patient’s risk status.
- The target blood pressure of patients who have diabetes or renal failure should be less than 130/85.
- Diuretics are safe, well tolerated, effective, relatively inexpensive, and convenient for initial drug treatment of hypertension in patients who do not have concomitant illness.
- Alpha-adrenergic blockers should be used with caution in the treatment of hypertension.
- Ambulatory blood pressure measurements predict cardiovascular events more closely than clinic blood pressure measurements.
Hypertension is arbitrarily defined as diastolic blood pressure (DBP) of 90 mm Hg or higher, systolic blood pressure (SBP) of 140 mm Hg or higher, or both, on 3 separate occasions. Essential hypertension is hypertension without an identifiable cause. Essential hypertension, also known as primary or idiopathic hypertension, accounts for at least 95% of all cases of hypertension.
According to the third National Health and Nutrition Examination Survey (NHANES III), approximately 60% of the 50 million Americans with hypertension are at increased risk for cardiovascular disease resulting from uncontrolled hypertension. This is because only 53% of hypertensive patients are being treated and only 24% have their hypertension under control.1 Physicians must play an active role in identifying and treating hypertension.
In an earlier Applied Evidence article2 an approach to the diagnosis of hypertension was presented. This article reviews the treatment of essential hypertension in adults and the prognosis of untreated hypertension. Risk stratification, alternative therapies, lifestyle modification, drug therapy, and prognosis will each be reviewed sequentially.
Risk stratification
The decision to treat hypertension and the choice of treatment is affected by the patient’s risk of morbidity and mortality if the blood pressure remains untreated or under-treated. According to the recommendations of the sixth report of the Joint National Committee on the Prevention, Diagnosis, Evaluation, and Treatment of High Blood Pressure (JNC-VI), the first step in planning treatment of a patient with essential hypertension is to categorize the patient’s risk status.3 The patient is placed in 1 of 9 treatment categories according to his or her blood pressure category, cardiovascular risk factors, and evidence of end-organ damage found during the initial evaluation (Table 1). Once the treatment category is identified, initial treatment should begin (Figure 1). Subsequent treatment depends on the patient’s response to initial treatment (Figure 2).
Patients should be monitored regularly to be sure they do not develop signs and symptoms that would place them in a different category and mandate more aggressive treatment. After a patient’s blood pressure has been controlled for 1 year, it may be possible to decrease the dose or the number of antihypertensive drugs—especially among patients who make significant lifestyle changes.4
The effectiveness of therapy varies depending on the patient’s cardiovascular risk. The New Zealand Guidelines Group has developed a helpful risk calculator based on the Framingham Heart Study for estimating a patient’s cardiovascular risk. This calculator incorporates sex, age, systolic blood pressure, smoking status, total cholesterol, high-density lipoprotein cholesterol, presence or absence of diabetes, and presence or absence of electrocardiogram evidence of left ventricular hypertrophy. This helpful risk calculator may be downloaded from the Web site of the New Zealand Guidelines Group at http://www.nzgg.org.nz/library/gl_complete/bloodpressure/appendix.cfm#app3. Alternatively, the University of Sheffield Medical School has developed tables to estimate an individual’s risk of heart disease based on cardiovascular risk factors including age, sex, cholesterol level, and presence or absence of smoking, hypertension, and diabetes—Sheffield tables.5 Software for handheld computers (Palm and PocketPC) that helps you estimate risk is available at www.jfponline.com.
Regardless of the method used, the benefit of treatment increases steadily as the patient’s current cardiovascular risk increases. With a 5-year cardiovascular risk of less than 2.5%, more than 120 patients have to be treated for 5 years to prevent 1 cardiovascular event; this number decreases to 25 patients with a risk of between 5% and 10%, and only 13 with a risk of between 20% and 24%.6 It is tempting to assume that the benefit of hypertension treatment is related to reduction in blood pressure whether achieved by drug therapy, lifestyle modification, or alternative therapy. However, this has not been established and it is important to consider the evidence supporting the benefit of each of these therapeutic options (Table 2).
TABLE 1
HYPERTENSION RISK STRATIFICATION AND TREATMENT CATEGORIES
| Blood Pressure Category | Risk Group A* | Risk Group B* | Risk Group C* |
|---|---|---|---|
| High-normal (130 – 139/85 – 89) | Lifestyle modification† | Lifestyle modification | Lifestyle modification and drug therapy |
| Stage 1 (140 – 159/0 – 99) | Lifestyle modification (12-month trial) | Lifestyle modification (6-month trial) | Lifestyle modification and drug therapy |
| Stage 2 or 3 (≥ 160 / ≤100) | Lifestyle modification and drug therapy | Lifestyle modification and drug therapy | Lifestyle modification and drug therapy |
| *Risk groups: A = no risk factors, end-organ damage, or clinical cardiovascular disease; B = 1 risk factor other than diabetes, no end-organ damage, and no clinical cardiovascular disease; C = Diabetes, end-organ damage, or clinic cardiovascular disease. | |||
| † Lifestyle modification should be included in the treatment plan of all patients receiving drug therapy. | |||
FIGURE 1
ALGORITHM FOR INITIAL MANAGEMENT OF HYPERTENSION
FIGURE 2
ALGORITHM FOR SUBSEQUENT MANAGEMENT OF HYPERTENSION
TABLE 2
NUMBER NEEDED TO TREAT (NNT) FOR SPECIFIC ANTIHYPERTENSIVE TREATMENTS
| Medication | Level of Evidence | NNT (95% CI)* | Comment |
|---|---|---|---|
| Low-Dose Thiazide | 1a | 18 (14-23) | Adults with systolic blood pressure |
| ≥ 160 or diastolic blood pressure | |||
| ≥90 regardless of age or comorbidities. | |||
| High-Dose Thiazide | 1a | 67 (48-111) | |
| Beta-Blocker | 1a | 142 (71-1000) | Drug vs no treatment comparison.14 |
| Calcium-Channel Blockers | 1b | 45 (30-102) | Isolated systolic hypertension in older patients, drug vs no treatment comparison.22 |
| ACE inhibitors | 1b | NS | Captopril versus diuretic or ß-blocker. |
| Alpha-agonists | 1b | NS | Doxazosin versus chlorthalidone, increased congestive heart in doxazosin group.7 |
| ARBs | NA | NA | Patient-oriented outcomes not available. |
| Sodium Restriction | 1a | NA | May reduce blood pressure but lacks evidence of reduced morbidity or mortality.27-35 |
| Weight Loss | 1a | NA | |
| Exercise | 1a | NA | |
| Low-Fat Diet | 1b | NA | |
| Limited Alcohol | 5 | NA | |
| Potassium Supplement | 1a | NA | |
| Fish Oil Supplement | 1a | NA | |
| Acupuncture | NA | NA | No evidence of blood pressure reduction or reduced morbidity or mortality.36-43 |
| Biofeedback | NA | NA | |
| Herbal Medicine | NA | NA | |
| Transcendental Meditation | NA | NA | |
| Yoga | NA | NA | |
| *For total cardiovascular events over 5 years. | |||
| NS denotes no significant difference from comparison drug; NA, not applicable; ACE, angiotensin-converting enzyme; ARBs, angiotensin-receptor blockers. | |||
Treatment
Drug Therapy
Patients who require drug treatment for hypertension should begin with a low dose of the initial medication, and that dose should be slowly titrated upward every 1 to 2 months (Figure 2). The JNC-VI recommends a diuretic or a ß-blocker with once daily dosing and 24-hour efficacy as the initial treatment for most hypertensive patients. However, the choice of initial medication will be affected by concomitant illnesses: (1) ß-blockers are recommended for the initial treatment of patients with hypertension and a history of coronary artery disease; (2) diuretics are suggested for the initial treatment of isolated systolic hypertension; (3) and angiotensin-converting enzyme (ACE) inhibitors are recommended for hypertensive patients who have systolic dysfunction after myocardial infarction, diabetic nephropathy, or congestive heart failure. Angiotensin II receptor blockers may be used in patients who cannot tolerate ACE inhibitors because of cough or rash. Alpha-adrenergic blockers should be used with caution in light of evidence that they may increase the risk of cardiovascular events (especially congestive heart failure).7
Among patients who do not have concomitant illness, the choice of drug therapy is controversial. A case-control study and a meta-analysis suggested that short-acting calcium channel blockers (CCBs) increase cardiovascular mortality.8,9 Unfortunately, these studies were not designed to establish a causal relationship. A recent nonsystematic review suggested that short-acting CCBs should be avoided and that conventional therapies were more effective than long-acting CCBs.10 An earlier non-systematic review suggested that short- and intermediate-acting CCBs were associated with increased cardiovascular mortality and morbidity. However, a well-designed cohort study of patients with coronary artery disease failed to reveal an increase in adverse effects among patients taking short-acting CCBs.11 Furthermore, randomized controlled trials suggest that diuretics, ß-blockers, and long-acting CCBs are equally effective in preventing cardiovascular mortality and morbidity.12,13 Physicians who treat hypertension must choose the best initial treatment for patients who do not have concomitant illness. Fortunately, safety, tolerability, efficacy, price, and simplicity can guide the physician to an ideal drug for most hypertensive patients.
Low-dose thiazide diuretics (the equivalent of 25 to 50 mg of hydrochlorothiazide) appear better tolerated than ß-blockers or CCBs.14 Treatment with ß-blockers, CCBs, and ACE inhibitors is also more expensive (75% to 85% more) than diuretic therapy.15 The cost savings offered by diuretics complement the fact that diuretics are safe, effective, and may be dosed once daily. In short, in addition to being the drug of choice for isolated systolic hypertension, low-dose thiazide diuretics are the ideal initial drug treatment of patients without concomitant illness. It should be noted that higher doses of thiazide diuretics offer proportionately less blood pressure reduction and greater risk of hypokalemia.16
Antihypertensive treatment reduces morbidity and mortality for all stages of hypertension, but people with the greatest baseline cardiovascular risk (eg, older patients and patients with higher levels of blood pressure) have the most to gain from treatment.17,18 There is no conclusive evidence to suggest that lowering blood pressure to below 140/80 reduces morbidity or mortality in most patients. However, patients who have diabetes or renal failure benefit from more aggressive management of blood pressure.19,20 Therefore, the JNC-VI recommends a target blood pressure of less than 130/85 for these patients.
The JNC-VI recommendation to start with a low-dose diuretic is supported by the evidence across a spectrum of patient-oriented outcomes. The effectiveness of diuretics and ß-blockers as first-line agents has been confirmed by long-term clinical trials.14,21 However, low-dose thiazides appear effective against a broader range of outcomes than high-dose thiazides and ß-blockers (Table 3). There is also evidence to suggest that CCBs and ACE inhibitors may be effective first-line agents, but fewer patients have been studied who take CCBs and ACE inhibitors than those who take diuretics and ß-blockers.22,23
Most patients with hypertension will respond to 1 (approximately 50%) or 2 (approximately 30%) antihypertensive medications.19,24,25 As noted earlier, failure to respond to treatment suggests an identifiable cause of hypertension. Among patients who do not have a secondary cause of hypertension, inadequate drug treatment (often failure to start a diuretic) and noncompliance are among the most common causes of resistant hypertension.26
When patients who are receiving drug therapy fail to reach the target blood pressure goal or fail to maintain the blood pressure goal, they should have the initial drug dose increased until the goal is reached (Figure 2). Those who fail initial drug therapy at full doses should have a second drug added and increased until the blood pressure goal is reached. Those who fail initial and second drug therapy at full doses should have a third drug added and increased until the pressure goal is reached. Patients who fail to reach the goal on maximal doses of 3 drugs have, by definition, resistant hypertension and will require evaluation by a physician with expertise in managing resistant hypertension. A cause should be sought each time a patient fails to respond to a drug or fails to maintain blood pressure control on a drug that had previously controlled the pressure (Table 3).
TABLE 3
PARTIAL LISTING OF CAUSES OF FAILURE TO REACH OR MAINTAIN TARGET BLOOD PRESSURE
| Volume overload: failure to start a diuretic |
| Nonadherence to therapy: dementia, side effects, complex regimen |
| Drug-induced: prescription, over-the-counter, herbal, or illicit drugs |
| Diet/stimulant induced: caffeine, licorice, salt, alcohol, nicotine |
| Associated conditions: obesity, sleep apnea, anxiety, chronic pain |
| Identifiable causes: chronic renal disease, renovascular disease, hyperaldosteronism, Cushing’s syndrome, pheochromocytoma |
| Pseudoresistance: wrong cuff size, white-coat hypertension |
Lifestyle modifications
Several lifestyle modifications are recommended in all treatment categories. Aerobic exercise (45 to 60 minutes at least 3 days per week), low-salt, low-fat, and high fruit and vegetable diet, limited alcohol consumption (less than 3 drinks per day), and modest weight loss (3% to 9% of total body weight) have been demonstrated to yield modest blood pressure reductions, but there is insufficient evidence to suggest that these measures alone reduce morbidity or mortality in hypertensive patients.27-33 A systematic review of randomized controlled trials found an average 4.4/2.5 mm Hg reduction in blood pressure with no evidence of harm (among patients who were not at risk for hyperkalemia) when diet was supplemented with about 2000 mg of potassium daily.34 A comparable reduction in blood pressure was seen with a daily supplement of more than 3 grams of fish oil.35 Research concerning the value of calcium and magnesium supplementation is conflicting and insufficient for supplementation to be considered standard therapy at this time.
Alternative therapy
The number and the quality of studies evaluating acupuncture, biofeedback, herbal medicine, transcendental meditation, and yoga are, for the most part, limited. They have focused on reduction in blood pressure, not patient-oriented outcomes, such as a reduction in morbidity and mortality. Acupuncture does not appear to have a significant effect on blood pressure levels.36,37 Biofeedback and other behavioral techniques have not been demonstrated to reduce blood pressure.38,39 The effect of garlic on blood pressure is unclear with mixed study results.40,41 Transcendental meditation and yoga may reduce blood pressure, but studies of these modalities are small and the experimental designs have a limited capacity to detect an independent treatment effect or a placebo effect.42,43
Therefore, physicians who include any of these modalities in their hypertension treatment plan should carefully monitor each patient for adequacy of blood pressure control, development of risk factors, and evidence of end-organ damage. At this time, alternative therapies should be considered experimental adjuncts to lifestyle modification and medical therapy that have not been shown to improve patient-oriented outcomes.
Follow-up of patients with hypertension
Follow-up visits should be designed to identify new risk factors, evidence of end-organ damage, and adequacy of blood pressure control. Follow-up visits may include an interval history, limited physical examination, radiologic evaluation, and laboratory testing. The frequency and nature of follow-up hypertension evaluations will vary according to the presence or absence of preexisting risk factors, evidence of end-organ damage, the nature of the treatment the patient is receiving, and the stability of blood pressure control. Unfortunately, there is little evidence to support specific recommendations for the frequency and nature of follow-up hypertension evaluations.
In the absence of evidence, several general principles may be suggested. Patients should be seen within 2 months of initiation of treatment. Follow-up history should focus on the cardiovascular and neurologic review of systems. The examination should include a focused cardiovascular work-up (eg, retinopathy, carotid bruits). Consideration should be given to periodic laboratory testing for diabetes, renal insufficiency, and hyperlipidemia. Periodic (but less frequent) chest x-rays and electrocardiograms may be helpful to detect cardiomegaly, but there is no evidence to support such testing in the absence of symptoms.
Follow-up visits should be more frequent among patients who have marginal blood pressure control, preexisting risk factors, or end-organ damage. Evaluations may be less frequent among those with good control and no preexisting risk factors or endorgan damage. Office visits and testing should be more frequent whenever changes are made in treatment. The frequency and nature of follow-up testing will also depend on the nature of treatment. Patients taking diuretics should have their potassium levels checked periodically. Renal function and potassium should be monitored in patients who are taking ACE inhibitors, especially during the first few weeks of therapy.
Patients willing to regularly monitor their blood pressure at home may require less frequent follow-up than those who leave it to the physician to check. Patients who monitor their blood pressure at home should have their sphygmomanometers validated initially and periodically.44 It is important to remember that home blood pressure measurements are consistently lower and more closely correlated with cardiovascular outcomes than are clinic blood pressure measurements.45-47
Prognosis
It is difficult to estimate the precise impact blood pressure control has on morbidity and mortality, but it is clear that high blood pressure, if unrecognized or untreated, substantially increases the morbidity and mortality associated with coronary disease, heart failure, renal failure, and stroke.17 In an early study of untreated hypertension there was a close relationship between blood pressure level and cardiovascular morbidity over 14 years of observation. This study revealed that hypertensive patients (those with a blood pressure of 160/95) had cardiovascular morbidity rates (coronary artery disease, claudication, stroke, and congestive heart failure) 2 to 3 times higher than normotensive patients.48 The impact of inadequately controlled blood pressure on morbidity and mortality among patients with diabetes is especially problematic.49 Over 9 years, when compared with diabetic patients with less tight control (< 180/105 mm Hg), those with tight blood pressure control (< 150/85 mm Hg) had a 24% reduction in sudden death, hyperglycemic or hypoglycemic death, fatal or nonfatal myocardial infarction, angina, heart failure, fatal or nonfatal stroke, renal failure, amputation, vitreous hemorrhage, and retinal hemorrhage.
Finally, renal function deteriorates more rapidly when blood pressure control is inadequate in patients with chronic renal disease of diverse causes.20 Over 2 years, when compared with patients with renal failure who had less tight control (mean arterial pressure 107 mm Hg), renal failure patients with tight control (mean arterial blood pressure 92 mm Hg) had significantly less proteinuria and lower rates of decline in renal function. Whether this translates into a significant improvement in the risk of end-stage renal disease is unknown.
1. Burt V, Whelton P, Rocella E, Brown C, Cutler J. Prevalence of hypertension in the US population: results from the third national health and nutrition examination survey, 1988-1991. Hypertension 1995;25:305-13.
2. Dosh S. The diagnosis of essential and secondary hypertension in adults. J Fam Pract 2001;50:707-12.
3. Joint National Committee on the Prevention Detection. Evaluation and Treatment of High Blood Pressure. the sixth report of the joint national committee on prevention, detection, evaluation, and treatment of high blood pressure (JNC VI). Arch Intern Med 1997;157:2413-46.
4. Whelton P, Appelgate W, Ettinger W, et al. Sodium reduction and weight loss in the treatment of hypertension in older persons: a randomized controlled trial of non-pharmacologic interventions in the elderly (TONE). JAMA 1996;279:839-46.
5. Wallis E, Ramsay L, Haq I, Ghahramani P, Jackson P. Coronary and cardiovascular risk estimation for the primary prevention: validation of a new Sheffield table in the 1995 Scottish health survey. Br Med J 2000;320:671-76.
6. Baker S, Priest P, Jackson R. Using thresholds based on risk of cardiovascular disease to target treatment for hypertension: modelling events averted and number treated. Br Med J 2000;320:680-85.
7. ALLHAT Collaborative Research Group. Major cardiovascular events in hypertensive patients randomized to doxazosin vs chlorthalidone. J Am Med Assoc 2000;283:1967-75.
8. Psaty B, Heckbert S, Koepsell T, et al. The risk of myocardial infarction associated with anti-hypertensive drug therapies. JAMA 1995;274:620-25.
9. Furberg C, Psaty B, Meyer J. Nifedipine: dose related increase in mortality in patients with coronary heart disease. Circulation 1995;92:1326-31.
10. Kizer J, Kimmel S. Epidemiologic review of the calcium channel blocker drugs. Arch Intern Med 2001;161:1145-58.
11. Braun S, Boyo V, Behar S, et al. Calcium antagonists did not increase mortality in patients with coronary artery disease. J Am Coll Cardiol 1996;28:7-11.
12. Hansson L, Hedner T, Lund-Johansen P, et al. Randomized trial of effects of calcium antagonists compared with diuretics and B-blockers on cardiovascular morbidity and mortality in hypertension: the Nordic Diltiazem (NORDIL) study. Lancet 2000;356:359-65.
13. Hansson L, Lindholm L, Ekbohm T, Dahlof B. Randomised trial of old and new antihypertensive drugs in elderly patients: cardiovascular mortality and morbidity. The Swedish trial in old patients with hypertension-2 study. Lancet 1999;354:1751-56.
14. Wright J, Lee C, Chambers G. Systematic review of antihypertensive therapies: does evidence assist in choosing a first-line drug? Can Med Assoc J 1999;161:25-32.
15. Ramsey S, Niel N, Sullivan S, Perfetto E. An economic evaluation of the JNC hypertension guidelines using data from a randomized controlled trial. J Am Board Fam Pract 1999;12:105-14.
16. Wright J. Choosing a first-line drug in the management of elevated blood pressure: What is the evidence?: 1 Thiazide diuretics. Can Med Assoc J 2000;163:57-60.
17. Gueyffier F, Froment A, Gouton M. New meta-analysis of treatment trials of hypertension: improving the estimate of therapeutic benefit. J Hum Hypertens 1996;10:1-8.
18. Mulrow C, Cornell J, Herrera C, Kadri A, Farnett L, Aguilar C. Hypertension in the elderly: implications and generalizability of randomized trials. JAMA 1994;272:1932-38.
19. Hansson L, Zanchetti A, Carruthers S, Dahlof B. Effects of intensive blood pressure lowering and low dose aspirin in patients with hypertension: principal results of the hypertension optimal treatment (HOT) randomised trial. Lancet 1998;351:1755-62.
20. Peterson J, Adler S, Burkart J, Greene J. Blood pressure control, proteinuria, and the progression of renal disease. Ann Intern Med 1995;123:754-62.
21. Psaty B, Smith N, Siscovick D, Koepsell T, Weiss N. Health outcomes associated with antihypertensive therapies used as first-line agents: A systematic review and meta-analysis. J Am Med Assoc 1197;277:739-45.
22. Staessen J, Fagard R, Celis H, Arabidze C, Birkenhager W. Randomised double-blind comparison of placebo and active treatment in older patients with isolated systolic hypertension. Lancet 1997;350:757-64.
23. Investigators THOPES. Effect of an angiotensin-converting-enzyme inhibitor, ramipril, on cardiovascular events in high-risk patients. New Eng J Med 2000;342:145-53.
24. Masterson B, Reda D, Preston R, et al. Response to a second single antihypertensive agent used as monotherapy for hypertension after failure of the initial drug. Arch Intern Med 1995;155:1757-62.
25. Tuomilehto J, Rastenyte D, Birkenhager W, et al. Systolic hypertension in Europe trial investigators. Effects of calcium-channel blockers in older patients with diabetes and systolic hypertension. New Eng J Med 1999;340:677-84.
26. Yakovlevitch M, Black HR. Resistant hypertension in a tertiary care clinic. Arch Intern Med 1991;151:1786-92.
27. Halbert J, Silagy C, Finucane P, Withers R. The effectiveness of exercise training in lowering blood pressure: a metaanalysis of randomized controlled trials of 4 weeks or longer. J Human Hypertension 1997;10:641-49.
28. Ebrahim S, Davey G. Lowering blood pressure: a systematic review of sustained effects of non-pharmacologic interventions. J Public Health Med 1998;4:441-48.
29. Appel L, Moore T, Obarzanek E, Vollmer W. A clinical trail of the effects of dietary patterns on blood pressure. New Eng J Med 1997;336:1117-24.
30. Appel L, Espeland M, Easter L, Wilson A, Folmar S, Lacy C. Effects of reduced sodium intake on hypertension control in older individuals. Results from the trial of nonpharmacologic interventions in the elderly (TONE). Arch Intern Med 2001;161:685-93.
31. Beilin L, Puddey I, Burke V. Alcohol and hypertension: kill or cure? J Human Hypertension 1996;10(Suppl 2):1-5.
32. Corrigan S, Raczynski J, Swencionis C, Jennings S. Weight reduction in the prevention and treatment of hypertension: a review of representative clinical trials. Am J Health Promo 1991;5:208-14.
33. Mulrow C, Chiquette E, Angel L, Cornell J. Dieting to reduce body weight for controlling hypertension in adults. The Cochrane Library 2001.
34. Whelton P, He J, Cutler J, Brancati F, Appel L. Effects of oral potassium on blood pressure: meta-analysis of randomized controlled trials. JAMA 1997;277:1624-32.
35. Morris M, Sacks F, Rosner B. Regulation on blood pressure: does fish oil lower blood pressure?: analysis of controlled trials. Circulation 1993;88:523-33.
36. Sugioka K, Woods M, Mueller R. An unsuccessful attempt to treat hypertension with acupuncture. Am J Chinese Med 1977;5:39-44.
37. Kraft K, Coulon S. Effect of a standardized acupuncture treatment on complaints, blood pressure, and serum lipids of hypertensive, postmenopausal women. A randomized controlled clinical study. Forschende Komplementarmedizin 1999;6:74-79.
38. Eisenberg D, Delblanco T, Berkey C, et al. Cognitive and behavioral techniques and hypertension: a meta-analysis. Ann Intern Med 1993;118:964-72.
39. Hunyor S, Henderson R, Saroj K, Carter N, et al. Placebo-controlled biofeedback blood pressure effect in hypertensive humans. Hypertension 1997;29:1225-31.
40. Auer W. Hypertension and hyperlipidemia: garlic helps in mild cases. Br J Clin Pract 1990;69(Suppl):3-6.
41. Ackerman R, Mulrow C, Ramirez G, Gardner C, Mobidoni L, Lawrence V. Garlic shows promise for improving some cardiovascular risk factors. Arch Intern Med 2001;161:813-24.
42. Patel C. Twelve-month follow-up of yoga and bio-feedback in the management of hypertension. Lancet 1975;1:62-64.
43. Sundar S, Agrawal S, Singh V, Bhattacharya S, et al. Role of yoga in management of essential hypertension. Acta Cardiologica 1984;39:203-08.
44. White W, Berson A, Robbins C, Jamieson M, Prisant L. National standard for measurement of resting and ambulatory blood pressure with automated sphygmomanometers. Hypertension 1993;21:504-09.
45. Staessen J, Byttebier G, Butinx F, Celis H, O’Brien E. Antihypertensive treatment based on conventional or ambulatory blood pressure measurement. J Am Med Assoc 1997;278:1065-72.
46. Ohkubo T, Imai Y, Tsuji I, Nagai K, Ito S. Reference values for 24-hour ambulatory blood pressure monitoring based on a prognostic criterion: the Ohasma study. Hypertension 1998;32:255-59.
47. Staessen J, Thijs L, Fagard R, O’Brien E, Eoin T. Predicting cardiovascular risk using conventional vs ambulatory blood pressure in older patients with systolic hypertension. JAMA 1999;282:539-46.
48. Kannel W, Wolf P, Verter J, McNamara P. Epidemiologic assessment of the role of blood pressure in stroke: the Framingham study. JAMA 1970;214:301-10.
49. Turner R, Holman R, Stratton I, Cull C. Tight blood pressure control and risk of macrovascular and microvascular complications in type 2 diabetes: UKPDS 38. Br Med J 1998;317:703-12.
1. Burt V, Whelton P, Rocella E, Brown C, Cutler J. Prevalence of hypertension in the US population: results from the third national health and nutrition examination survey, 1988-1991. Hypertension 1995;25:305-13.
2. Dosh S. The diagnosis of essential and secondary hypertension in adults. J Fam Pract 2001;50:707-12.
3. Joint National Committee on the Prevention Detection. Evaluation and Treatment of High Blood Pressure. the sixth report of the joint national committee on prevention, detection, evaluation, and treatment of high blood pressure (JNC VI). Arch Intern Med 1997;157:2413-46.
4. Whelton P, Appelgate W, Ettinger W, et al. Sodium reduction and weight loss in the treatment of hypertension in older persons: a randomized controlled trial of non-pharmacologic interventions in the elderly (TONE). JAMA 1996;279:839-46.
5. Wallis E, Ramsay L, Haq I, Ghahramani P, Jackson P. Coronary and cardiovascular risk estimation for the primary prevention: validation of a new Sheffield table in the 1995 Scottish health survey. Br Med J 2000;320:671-76.
6. Baker S, Priest P, Jackson R. Using thresholds based on risk of cardiovascular disease to target treatment for hypertension: modelling events averted and number treated. Br Med J 2000;320:680-85.
7. ALLHAT Collaborative Research Group. Major cardiovascular events in hypertensive patients randomized to doxazosin vs chlorthalidone. J Am Med Assoc 2000;283:1967-75.
8. Psaty B, Heckbert S, Koepsell T, et al. The risk of myocardial infarction associated with anti-hypertensive drug therapies. JAMA 1995;274:620-25.
9. Furberg C, Psaty B, Meyer J. Nifedipine: dose related increase in mortality in patients with coronary heart disease. Circulation 1995;92:1326-31.
10. Kizer J, Kimmel S. Epidemiologic review of the calcium channel blocker drugs. Arch Intern Med 2001;161:1145-58.
11. Braun S, Boyo V, Behar S, et al. Calcium antagonists did not increase mortality in patients with coronary artery disease. J Am Coll Cardiol 1996;28:7-11.
12. Hansson L, Hedner T, Lund-Johansen P, et al. Randomized trial of effects of calcium antagonists compared with diuretics and B-blockers on cardiovascular morbidity and mortality in hypertension: the Nordic Diltiazem (NORDIL) study. Lancet 2000;356:359-65.
13. Hansson L, Lindholm L, Ekbohm T, Dahlof B. Randomised trial of old and new antihypertensive drugs in elderly patients: cardiovascular mortality and morbidity. The Swedish trial in old patients with hypertension-2 study. Lancet 1999;354:1751-56.
14. Wright J, Lee C, Chambers G. Systematic review of antihypertensive therapies: does evidence assist in choosing a first-line drug? Can Med Assoc J 1999;161:25-32.
15. Ramsey S, Niel N, Sullivan S, Perfetto E. An economic evaluation of the JNC hypertension guidelines using data from a randomized controlled trial. J Am Board Fam Pract 1999;12:105-14.
16. Wright J. Choosing a first-line drug in the management of elevated blood pressure: What is the evidence?: 1 Thiazide diuretics. Can Med Assoc J 2000;163:57-60.
17. Gueyffier F, Froment A, Gouton M. New meta-analysis of treatment trials of hypertension: improving the estimate of therapeutic benefit. J Hum Hypertens 1996;10:1-8.
18. Mulrow C, Cornell J, Herrera C, Kadri A, Farnett L, Aguilar C. Hypertension in the elderly: implications and generalizability of randomized trials. JAMA 1994;272:1932-38.
19. Hansson L, Zanchetti A, Carruthers S, Dahlof B. Effects of intensive blood pressure lowering and low dose aspirin in patients with hypertension: principal results of the hypertension optimal treatment (HOT) randomised trial. Lancet 1998;351:1755-62.
20. Peterson J, Adler S, Burkart J, Greene J. Blood pressure control, proteinuria, and the progression of renal disease. Ann Intern Med 1995;123:754-62.
21. Psaty B, Smith N, Siscovick D, Koepsell T, Weiss N. Health outcomes associated with antihypertensive therapies used as first-line agents: A systematic review and meta-analysis. J Am Med Assoc 1197;277:739-45.
22. Staessen J, Fagard R, Celis H, Arabidze C, Birkenhager W. Randomised double-blind comparison of placebo and active treatment in older patients with isolated systolic hypertension. Lancet 1997;350:757-64.
23. Investigators THOPES. Effect of an angiotensin-converting-enzyme inhibitor, ramipril, on cardiovascular events in high-risk patients. New Eng J Med 2000;342:145-53.
24. Masterson B, Reda D, Preston R, et al. Response to a second single antihypertensive agent used as monotherapy for hypertension after failure of the initial drug. Arch Intern Med 1995;155:1757-62.
25. Tuomilehto J, Rastenyte D, Birkenhager W, et al. Systolic hypertension in Europe trial investigators. Effects of calcium-channel blockers in older patients with diabetes and systolic hypertension. New Eng J Med 1999;340:677-84.
26. Yakovlevitch M, Black HR. Resistant hypertension in a tertiary care clinic. Arch Intern Med 1991;151:1786-92.
27. Halbert J, Silagy C, Finucane P, Withers R. The effectiveness of exercise training in lowering blood pressure: a metaanalysis of randomized controlled trials of 4 weeks or longer. J Human Hypertension 1997;10:641-49.
28. Ebrahim S, Davey G. Lowering blood pressure: a systematic review of sustained effects of non-pharmacologic interventions. J Public Health Med 1998;4:441-48.
29. Appel L, Moore T, Obarzanek E, Vollmer W. A clinical trail of the effects of dietary patterns on blood pressure. New Eng J Med 1997;336:1117-24.
30. Appel L, Espeland M, Easter L, Wilson A, Folmar S, Lacy C. Effects of reduced sodium intake on hypertension control in older individuals. Results from the trial of nonpharmacologic interventions in the elderly (TONE). Arch Intern Med 2001;161:685-93.
31. Beilin L, Puddey I, Burke V. Alcohol and hypertension: kill or cure? J Human Hypertension 1996;10(Suppl 2):1-5.
32. Corrigan S, Raczynski J, Swencionis C, Jennings S. Weight reduction in the prevention and treatment of hypertension: a review of representative clinical trials. Am J Health Promo 1991;5:208-14.
33. Mulrow C, Chiquette E, Angel L, Cornell J. Dieting to reduce body weight for controlling hypertension in adults. The Cochrane Library 2001.
34. Whelton P, He J, Cutler J, Brancati F, Appel L. Effects of oral potassium on blood pressure: meta-analysis of randomized controlled trials. JAMA 1997;277:1624-32.
35. Morris M, Sacks F, Rosner B. Regulation on blood pressure: does fish oil lower blood pressure?: analysis of controlled trials. Circulation 1993;88:523-33.
36. Sugioka K, Woods M, Mueller R. An unsuccessful attempt to treat hypertension with acupuncture. Am J Chinese Med 1977;5:39-44.
37. Kraft K, Coulon S. Effect of a standardized acupuncture treatment on complaints, blood pressure, and serum lipids of hypertensive, postmenopausal women. A randomized controlled clinical study. Forschende Komplementarmedizin 1999;6:74-79.
38. Eisenberg D, Delblanco T, Berkey C, et al. Cognitive and behavioral techniques and hypertension: a meta-analysis. Ann Intern Med 1993;118:964-72.
39. Hunyor S, Henderson R, Saroj K, Carter N, et al. Placebo-controlled biofeedback blood pressure effect in hypertensive humans. Hypertension 1997;29:1225-31.
40. Auer W. Hypertension and hyperlipidemia: garlic helps in mild cases. Br J Clin Pract 1990;69(Suppl):3-6.
41. Ackerman R, Mulrow C, Ramirez G, Gardner C, Mobidoni L, Lawrence V. Garlic shows promise for improving some cardiovascular risk factors. Arch Intern Med 2001;161:813-24.
42. Patel C. Twelve-month follow-up of yoga and bio-feedback in the management of hypertension. Lancet 1975;1:62-64.
43. Sundar S, Agrawal S, Singh V, Bhattacharya S, et al. Role of yoga in management of essential hypertension. Acta Cardiologica 1984;39:203-08.
44. White W, Berson A, Robbins C, Jamieson M, Prisant L. National standard for measurement of resting and ambulatory blood pressure with automated sphygmomanometers. Hypertension 1993;21:504-09.
45. Staessen J, Byttebier G, Butinx F, Celis H, O’Brien E. Antihypertensive treatment based on conventional or ambulatory blood pressure measurement. J Am Med Assoc 1997;278:1065-72.
46. Ohkubo T, Imai Y, Tsuji I, Nagai K, Ito S. Reference values for 24-hour ambulatory blood pressure monitoring based on a prognostic criterion: the Ohasma study. Hypertension 1998;32:255-59.
47. Staessen J, Thijs L, Fagard R, O’Brien E, Eoin T. Predicting cardiovascular risk using conventional vs ambulatory blood pressure in older patients with systolic hypertension. JAMA 1999;282:539-46.
48. Kannel W, Wolf P, Verter J, McNamara P. Epidemiologic assessment of the role of blood pressure in stroke: the Framingham study. JAMA 1970;214:301-10.
49. Turner R, Holman R, Stratton I, Cull C. Tight blood pressure control and risk of macrovascular and microvascular complications in type 2 diabetes: UKPDS 38. Br Med J 1998;317:703-12.
Evaluation and Treatment of the Patient with Acute Undifferentiated Respiratory Tract Infection
The term acute upper respiratory tract infection (ARTI) refers to an infection, almost always viral,1 predominantly involving the nasopharynx, sinuses, and large bronchi. It encompasses what is frequently referred to as the common cold, sinusitis, pharyngitis, bronchitis, and otitis media. This review will focus primarily on the common cold, or undifferentiated ARTI, but there will be considerable overlap with the other diagnoses. The rationale for grouping these traditionally separate diagnostic categories together is straightforward. ARTI almost always presents with some combination of nasal congestion, rhinnorrhea, sore throat, and cough. Sometimes one or another of these symptoms predominates, but there is good reason to suggest that the traditional diagnostic distinctions are arbitrary.2 The overlap between the clinical signs, symptoms, and x-ray findings is so extensive that these terms are not very useful diagnostically. This symptom complex is among the top 3 reasons for visits to primary care doctors3 and accounts for approximately 100 million office visits in the United States per year4 at an annual cost of considerably more than 1 billion dollars.5 Overuse of antibiotics adds more than $11 to the cost of each encounter for ARTI.5
This review will summarize the evidence that patients who present with undifferentiated ARTI usually have self-limited disease, that complications are rare, effective treatments for symptoms are available, and antibiotics are not often indicated. The evidence is based on only adults and children over age 2 who have normal immune systems and do not have chronic respiratory disease. (J Fam Pract; 50:1070-1077)
Pathophysiology
Patients with undifferentiated ARTI present with any or all of the following symptoms: rhinnorrhea (which may be either clear or colored), nasal congestion, cough, sore throat, facial pain, malaise, headache, or fever. The etiology is almost always viral, most commonly rhinovirus, but other viruses have been implicated as well, particularly corona-viruses, parainfluenza, and influenza.6 Bacterial infection is rare in undifferentiated ARTI, occurring in approximately 2% of patients.1 The most common bacteria implicated are group A Streptococcus, Streptococcus pneumoniae, Haemophilus influenzae and Moraxella catarrhalis.7 Chlamydia pnuemoniae and Mycoplasma pnuemoniae have rarely been identified as well.6 Bordatella pertussis can occasionally be the cause of persistent cough in children and adults.8, 9
Diagnosis
The major diagnostic consideration in a patient who presents with ARTI symptoms is to rule out a more serious illness which would require aggressive treatment. These include pneumonia, pharyngitis caused by group A streptococci, and bacterial sinusitis. The traditional symptoms and signs that physicians use to distinguish viral from bacterial infection have not been determined helpful in making this distinction. Both a history of colored nasal discharge and maxillary sinus tenderness to palpation have a positive likelihood ratio (LR+) near 1 in predicting computed tomography–based diagnosis of acute bacterial sinusitis (a likelihood ratio of 1 indicates that the result does not change the likelihood of disease).10 Purulent sputum does not distinguish between viral ARTI and bacterial pneumonia.11 Combinations of symptoms, however, in the form of clinical decision rules, can be useful at ruling out more serious conditions.
Nasal Discharge
In patients who present primarily with nasal discharge, a study that correlated computed tomography (CT) scans with direct sinus puncture demonstrated that 90% of primary care patients with CT findings of total opacification or air fluid level in the maxillary sinuses have a bacterial etiology.12 Unfortunately, there is no combination of clinical signs or symptoms that reliably predicts opacification or air fluid levels.12 There have been 3 decision rules published based on clinical findings that may be useful, particularly in helping clinicians identify the most important symptoms on which they should focus during their examination. However, 2 of the rules require a sedimentation rate or C-reactive protein value,10,12 not typically available in a routine office visit. The only other rule based on clinical findings included adult men and used x-ray as the reference standard, making it less useful.13
The suspicion of sinusitis by a generalist is actually quite accurate diagnostically, since about 40% of patients with suspected sinusitis have the diagnosis confirmed by aspiration or CT imaging.14 One must therefore be guided by overall clinical judgment in these patients, but a practical approach to empiric treatment is presented in the section on treatment.
Sore Throat
In patients who present primarily with sore throat, the important consideration is to rule out group A streptococcus as the etiology. The prevalence of streptococcal pharyngitis varies markedly with age, season of the year, and presence or absence of an outbreak in the community. For children prevalences have been reported ranging from 12% to 35%. Prevalence seems to peak in the 5 years to 9 years age range, and in the autumn. Reported prevalences for adults range from 5% to 15%.15-20
A recent systematic review noted nine published decision rules for diagnosis of streptococcal pharyngitis,21 but the only one that has been prospectively validated in a primary care population of both children and adults is by McIssac22,23Table 1. Patients with a low risk of GABHS require no further testing; those with an intermediate risk should undergo rapid strep antigen testing; and those with a high risk of GABHS should either under rapid strep antigen testing with culture follow-up or empiric therapy. Culture for group A streptococcus is the most reliable means of diagnosis, but requires withholding treatment for 48 hours while awaiting the result.24
Infectious mononucleosis can also cause exudative pharyngitis. Palatine petechiae, posterior auricular and posterior cervical adenopathy, marked axillary adenopathy, and inguinal adenopathy are all associated with infectious mononucleosis,25 and if any of these signs are present a heterophile antibody test may be indicated to confirm the diagnosis.
Cough
Many patients with ARTI present with cough as a primary symptom. While the differential diagnosis for undifferentiated cough is long, in the setting of ARTI the primary diagnostic consideration is to rule out pneumonia. Decision rules by Heckerling11 and Diehr26 have been well validated Table 2, Table 3. If the patient does not have temperature higher than 100 ÞF, pulse rate higher than 100, rales, decreased breath sounds, or absence of wheezing, then pneumonia is very unlikely (negative likelihood ratio = 0.06). As the number of signs increases, the likelihood ratio increases, but the decision rule does not have sufficient positive predictive value to be used as a basis for antibiotic treatment. Purulent sputum is not predictive of pneumonia (LR+=1.3).11,27 Empiric antibiotics for pneumonia should not be given without a confirmatory chest x-ray.1
Treatment
Undifferentiated ARTI
The level of evidence supporting different interventions is summarized in Table 4, and each is discussed in more detail below.
Antibiotics. There have been numerous double blind placebo controlled randomized trials of antibiotics in patients with undifferentiated ARTI symptoms. All of these trials have been evaluated in a recent Cochrane systematic review.28 The review concludes that there is no consistent evidence of benefit from any antibiotic treatment and that there is a significant increase in adverse effects associated with antibiotic use. Increased cost and increased bacterial resistance in the patient and community are additional concerns.
Education. One of the reasons for inappropriate antibiotic use in ARTI is patient pressure for antibiotics. This can be subtle as well as direct and is difficult for physicians to resist.29 Several studies have shown that patient expectation for antibiotics is related to having been given antibiotics for respiratory infections in the past.30-32 Furthermore, McFarlane has shown that simple patient education techniques reduce both visits for ARTI and antibiotic usage.33 An essential part of treatment of these infections, therefore, is educating patients about the viral nature of the illness, the usual course and duration of symptoms (10 days for most, up to 3 weeks for some), the ineffectiveness of antibiotics for treatment, and the harm antibiotics can cause both to the patient and the community. Patient satisfaction has been shown to increase after this sort of intervention, even when patients initially had an expectation for antibiotic prescription.30,34 Some have advocated avoiding terms such as sinusitis and bronchitis with patients, instead using the terms head cold and chest cold to emphasize the viral nature of these illnesses.
Naproxen Sodium. Naproxen sodium was evaluated in a well-designed randomized placebo controlled trial using experimentally induced rhinovirus infections in volunteers.35 A statistically significant 29% reduction in total 5-day symptom scores was found. Specific symptoms that improved included sneezing, rhinnorrhea, nasal obstruction, sore throat, cough, headache, malaise and chilliness.
Vitamin C. A Cochrane Library meta-analysis of all the trials for ascorbic acid showed a small but significant effect on decreasing duration (.55 days per episode) and a modest effect on severity of symptoms.36 There was no evidence for an effect when taken prophylactically.
Zinc. Randomized trials of zinc have shown marked heterogeneity, but this may have been due to use of different preparations with different bioavailability.37 A more recent randomized trial used zinc acetate lozenges.38 Increased plasma zinc levels were documented in the treatment group. The lozenges contained 42.96 mg of zinc acetate dihydrate, and were administered every 2 to 3 hours, beginning 24 hours or less after the onset of symptoms. The treatment group had a reduction from 8.1 to 4.5 days in total duration of symptoms (number needed to treat [NNT] =3 at 5 days) and a 50% reduction in symptom scores compared to the placebo group. Symptoms included in the score were sore throat, nasal discharge, nasal congestion, sneezing, cough, scratchy throat, hoarseness, muscle ache, fever, and headache.
Echinacea. Echinacea was evaluated in another Cochrane Library Systematic Review.39 Sixteen trials were evaluated, two thirds of which had insufficient quality of reporting. There were many different extracts used, which made comparability of the results a problem. Most trials showed positive results, suggesting that preparations containing extract of echinacea may have some beneficial effect on prevention and treatment.
Intranasal Fluticasone. Intranasal fluticasone was evaluated in a randomized trial of 200 young adults with common cold symptoms.40 No clinically significant differences were noted in either duration or severity of symptoms between treatment and control groups.
Nasal Discharge
Antibiotics. As noted above under undifferentiated ARTI, a Cochrane systematic review of randomized antibiotic trials found no evidence of benefit of antibiotic treatment for clear or purulent rhinnorrhea.28 Another Cochrane systematic review focused on randomized trials of antibiotic treatment for sinusitis.41 There was modest benefit from amoxicillin treatment in patients with acute maxillary sinusitis confirmed radiographically (NNT=4). There was no evidence, even from more recent studies, that other antibiotics were any more effective than amoxicillin. Lindbaek was able to demonstrate in a randomized controlled trial of amoxicillin that the only patients who benefited from treatment were those who had either complete opacification of a sinus, or an air fluid level.42 Patients who had mucosal thickening alone showed no difference from the placebo group. Of the patients in the placebo group who did have opacification or air fluid level, half were well or much better within 10 days. There is also some evidence that patients who present with moderate to severe unilateral facial pain benefit from antibiotics.43 In children, a recent antibiotic trial of patients who had a clinical diagnosis of sinusitis with symptoms for between 14 and 28 days showed no difference in any outcome measure between treatment and placebo groups.44
Based on Lindbaek’s data for adults, assuming that 42% of patients with suspected bacterial sinusitis actually have the disease, the number needed to treat to benefit one patient is 8. The number needed to harm by adverse effects from antibiotics is 4, so harm may outweigh any benefit. Frontal sinusitis seems to represent a different disease process because of the anatomy of drainage from the frontal sinuses. These patients usually present with high fever, severe pain, are quite ill, and may require hospitalization, parenteral antibiotics and surgery.1,45,46 A practical approach is to treat all patients with nasal discharge symptomatically, unless they have severe pain, or appear very ill, in which case sinus films should be considered to rule out frontal sinusitis, or opacification or air fluid level in any sinus. Also, adult patients who have had sinus symptoms for more than two weeks without improvement are more likely to benefit from antibiotic treatment.42
Ipratropium. Ipratopium nasal spray used in a randomized controlled trial, reduced rhinnorrhea and sneezing in patients with cold symptoms by 31% compared to placebo saline nasal spray (ARR=16% NNT=7), and by 78% when compared with untreated patients (ARR=63% NNT=2).47
Brompheniramine. Brompheniramine likewise has a temporary modest effect on rhinnorrhea in adults. Brompheniramine was evaluated in a randomized controlled trial in volunteers infected with rhinovirus.48 The treatment group had a 20% reduction in symptom score on day 1 and a 26% reduction on day 2. The main symptoms improved were sneeze frequency, sneeze severity, and cough count. Drowsiness was a troubling side effect. Neither decongestants nor antihistamines, nor combinations of the 2 have shown any effect in randomized trials in children.49
Nasal Decongestants. The efficacy of oral psuedoephedrine or metalazone nasal spray were evaluated in a Cochrane Library meta-analysis.50 There was a 13% reduction in symptom score (subjective rating of severity of nasal congestion) after a single use of either agent, but no difference in combined symptom scores after use for 5 days.
Heated Humidified Air. Published studies of effectiveness of breathing heated humidified air for cold symptoms were evaluated in a Cochrane Library Systematic Review.51 Six studies were evaluated and the results were quite heterogeneous. One study in Israel and two in the UK showed significant improvement, while three in the US showed no difference between treatment and placebo groups.
Sore Throat
Antibiotics. The data for sore throat should reassure physicians who worry about the consequences of missing streptococcal pharyngitis. A Cochrane meta-analysis showed that treatment of streptococcal pharyngitis with penicillin did reduce duration of illness, but only by approximately half a day.52 There was only a minimal effect on prevention of suppurative complications as well (NNT=30 for children and NNT=145 for adults to prevent one case of otitis media). The incidence of rheumatic fever is so low in the industrialized world (.5 per 100,000 in the pediatric population in the United States )53 that the number needed to treat to prevent 1 case of rheumatic fever is exceedingly high. In fact, Howie54 has estimated that for a general practitioner in Scotland the NNT exceeds the number of patients he or she would see in his or her lifetime. Furthermore, it is estimated than only approximately 15% of patients with streptococcal pharyngitis ever present for treatment, further reducing the opportunity to prevent complications.52 Most of the symptomatic improvement from antibiotics comes in the first 3 days of illness, so that if culture is used as a treatment criterion, most patients will already be better by the time the results are available.55
Analgesics. Acetominophen, aspirin, ibuprofen, flurbiprofen, and the combination of aspirin and caffeine have all been shown to be effective for sore throat pain in randomized trials.56-60 Ibuprofen was found to be superior to acetominophen60 and aspirin/caffeine was found to be superior to aspirin alone.57
Benzocaine. I could find only one study evaluating benzocaine containing lozenges for sore throat61 and it did not include a placebo group. Although one lozenge (Merocaine) produced significantly better pain relief than the other (Tyrozets), the lack of a placebo comparison makes interpretation of these results problematic.
Cough
Antibiotics. There is no evidence, in the absence of pneumonia or pertussis, that antibiotics are effective for the treatment of acute cough, including cough productive of colored sputum in otherwise healthy patients.62 This does not include patients with acute exacerbation of chronic pulmonary disease.
Codeine, Dextromethorphan, Guaifenesin. There are few studies evaluating the effectiveness of cough medicines that contain combinations of guaifenesin and codeine or dextromethorphan. One randomized trial of the effectiveness of codeine for cough related to ARTI showed no difference between experimental and placebo groups.63 Another study found no difference between cough medicines containing guaifenesin alone, guaifenesin and codeine, and guaifenesin and dextromethorphan.64 Therefore, although studies are few, there is no evidence to suggest that any of these agents is effective for treatment of cough associated with ARTI. It is interesting to note that all studies showed a marked placebo effect.
b2 -Agonists. Initial studies appeared to show some benefit for inhaled b2-agonists in the treatment of cough.65-67 A recent systematic review by Smucny, however, found that only patients with demonstrated hyperreactivity of airways benefited from this treatment.68
A flow diagram depicting an algorithm for when to use antibiotic in addition to symptomatic treatment for ARTI is shown in the Figure 1. This closely approximates the most recent CDC guidelines on the judicious use of antibiotics for respiratory infections in children and adults. Table 5 summarizes the best clinical trial evidence regarding the use of antibiotics in ARTI.
Prognosis
The mean duration of symptoms for untreated ARTI is approximately 10 days.38,42,69 It is not unusual for symptoms, particularly nasal congestion and cough, however, to last for up to 3 weeks. One study of lower respiratory tract infection in primary care patients found that 60% were still coughing after 10 days.70 Another study of ARTI in children in Norway found that 50% still had nasal discharge and cough after 3 weeks.71 Complications are rare in primary care patients. Less than 2% of patients with ARTI go on to develop secondary bacterial infection requiring antibiotic treatment.1 Although there are no data in adults which specifically address whether antibiotic treatment prevents bacterial infection,72 there are at least 2 studies that demonstrate that antibiotic treatment of children with undifferentiated ARTI does not prevent pneumonia or otitis media.73,74
1. Gonzales R, Bartlett JG, Besser RE, Richelle JC, Hickner J, Jerome R, Hoffman JR. Principles of appropriate antibiotic use for treatment of acute respiratory tract infections in adults. Ann Intern Med 2001;134.-
2. Hueston WJ, Mainous AG, 3rd, Dacus EN, Hopper JE. Does acute bronchitis really exist? A reconceptualization of acute viral respiratory infections. J Fam Pract 2000;49:401-6.
3. Woodwell DA. National Ambulatory Medical Care Survey: 1996 summary. Adv Data 1997;1-25.
4. Gonzales R, Steiner JF, Sande MA. Antibiotic prescribing for adults with colds, upper respiratory tract infections, and bronchitis by ambulatory care physicians. JAMA 1997;278:901-4.
5. Mainous AG, 3rd, Hueston WJ. The cost of antibiotics in treating upper respiratory tract infections in a Medicaid population . Arch Fam Med 1998;7:45-9.
6. Makela MJ, Puhakka T, Ruuskanen O, et al. Viruses and bacteria in the etiology of the common cold. J Clin Microbiol 1998;36:539-42.
7. Ruoff G. Upper respiratory tract infections in family practice. Pediatr Infect Dis J 1998;17:S73-8.
8. Dowell SF, Schwartz B, Phillips WR. Appropriate use of antibiotics for URIs in children: Part II. Cough, pharyngitis and the common cold. The Pediatric URI Consensus Team. Am Fam Physician 1998;58:1335-42, 1345.
9. Gonzales R, Bartlett JG, Besser RE, et al. Principles of appropriate antibiotic use for treatment of acute respiratory tract infections in adults: background, specific aims, and methods. Ann Intern Med 2001;134:479-86.
10. Lindbaek M, Hjortdahl P, Johnsen UL. Use of symptoms, signs, and blood tests to diagnose acute sinus infections in primary care: comparison with computed tomography. Fam Med 1996;28:183-8.
11. Heckerling PS, Tape TG, Wigton RS, et al. Clinical prediction rule for pulmonary infiltrates. Ann Intern Med 1990;113:664-70.
12. Hansen JG, Schmidt H, Rosborg J, Lund E. Predicting acute maxillary sinusitis in a general practice population. BMJ 1995;311:233-6.
13. Williams JW, Jr, Simel DL, Roberts L, Samsa GP. Clinical evaluation for sinusitis. Making the diagnosis by history and physical examination. Ann Intern Med 1992;117:705-10.
14. Hickner JM, Bartlett JG, Besser RE, Gonzales R, Hoffman JR, Sande MA. Principles of appropriate antibiotic use for acute rhinosinusitis in adults: background. Ann Intern Med 2001;134:498-505.
15. Gunnarsson RK, Holm SE, Soderstrom M. The prevalence of potential pathogenic bacteria in nasopharyngeal samples from individuals with a respiratory tract infection and a sore throat—implications for the diagnosis of pharyngotonsillitis. Fam Pract 2001;18:266-71.
16. Pokorski SJ, Vetter EA, Wollan PC, Cockerill FR, 3rd. Comparison of Gen-Probe Group A streptococcus Direct Test with culture for diagnosing streptococcal pharyngitis. J Clin Microbiol 1994;32:1440-3.
17. Dagnelie CF, Bartelink ML, van der Graaf Y, Goessens W, de Melker RA. Towards a better diagnosis of throat infections (with group A beta-haemolytic streptococcus) in general practice. Br J Gen Pract 1998;48:959-62.
18. Hart AP, Buck LL, Morgan S, Saverio S, McLaughlin JC. A comparison of the BioStar Strep A OIA rapid antigen assay, group A Selective Strep Agar (ssA), and Todd-Hewitt broth cultures for the detection of group A Streptococcus in an outpatient family practice setting. Diagn Microbiol Infect Dis 1997;29:139-45.
19. Smith TD, Wilkinson V, Kaplan EL. Group A streptococcus-associated upper respiratory tract infections in a day-care center. Pediatrics 1989;83:380-4.
20. Drulak M, Raybould TJ, Yong J, Hsiung D, Smith H, Winston S. Comparison of Visuwell enzyme immunoassay to culture for detection of group A Streptococcus in throat swab specimens. Diagn Microbiol Infect Dis 1988;11:181-7.
21. Ebell MH SM, Barry HC, Ives K, Carey BS. Does this patient have strep throat? JAMA 2000;284:2912-2918.
22. McIsaac WJ, Goel V, To T, Low DE. The validity of a sore throat score in family practice. CMAJ 2000;163:811-5.
23. McIsaac WJ, White D, Tannenbaum D, Low DE. A clinical score to reduce unnecessary antibiotic use in patients with sore throat. CMAJ 1998;158:75-83.
24. Reed BD, Huck W, French T. Diagnosis of group A beta-hemolytic Streptococcus using clinical scoring criteria, Directigen 1-2-3 group A streptococcal test, and culture. Arch Intern Med 1990;150:1727-32.
25. Aronson MD, Komaroff AL, Pass TM, Ervin CT, Branch WT. Heterophil antibody in adults with sore throat: frequency and clinical presentation. Ann Intern Med 1982;96:505-8.
26. Diehr P, Wood RW, Bushyhead J, Krueger L, Wolcott B, Tompkins RK. Prediction of pneumonia in outpatients with acute cough—a statistical approach. J Chronic Dis 1984;37:215-25.
27. Metlay JP, Kapoor WN, Fine MJ. Does this patient have community-acquired pneumonia? Diagnosing pneumonia by history and physical examination. JAMA 1997;278:1440-5.
28. Arroll B, Kenealy T. Antibiotics for the common cold. Cochrane Database Syst Rev 2000;CD000247.-
29. Scott J, Cohen D, Dicicco-Bloom B, Orzano A, Jaen C, Crabtree BF. Unnecessary antibiotic use in acute respiratory Infections. JAMA 2001; In press.
30. Hamm RM, Hicks RJ, Bemben DA. Antibiotics and respiratory infections: do antibiotic prescriptions improve outcomes? J Okla State Med Assoc 1996;89:267-74.
31. Mainous AG, 3rd, Zoorob RJ, Oler MJ, Haynes DM. Patient knowledge of upper respiratory infections: implications for antibiotic expectations and unnecessary utilization. J Fam Pract 1997;45:75-83.
32. Little P, Williamson I, Warner G, Gould C, Gantley M, Kinmonth AL. Open randomised trial of prescribing strategies in managing sore throat. BMJ 1997;314:722-7.
33. Macfarlane JT, Holmes WF, Macfarlane RM. Reducing reconsultations for acute lower respiratory tract illness with an information leaflet: a randomized controlled study of patients in primary care. Br J Gen Pract 1997;47:719-22.
34. Sanchez-Menegay C, Hudes ES, Cummings SR. Patient expectations and satisfaction with medical care for upper respiratory infections. J Gen Intern Med 1992;7:432-4.
35. Sperber SJ, Hendley JO, Hayden FG, Riker DK, Sorrentino JV, Gwaltney JM, Jr. Effects of naproxen on experimental rhinovirus colds. A randomized, double-blind, controlled trial. Ann Intern Med 1992;117:37-41.
36. Douglas RM, Chalker EB, Treacy B. Vitamin C for preventing and treating the common cold. Cochrane Database Syst Rev 2000;2.-
37. Marshall I. Zinc for the common cold. Cochrane Database Syst Rev 2000;2.-
38. Prasad AS, Fitzgerald JT, Bao B, Beck FW, Chandrasekar PH. Duration of symptoms and plasma cytokine levels in patients with the common cold treated with zinc acetate. A randomized, double-blind, placebo-controlled trial. Ann Intern Med 2000;133:245-52.
39. Melchart D, Linde K, Fischer P, Kaesmayr J. Echinacea for preventing and treating the common cold. Cochrane Database Syst Rev 2000;2.-
40. Puhakka T, Makela MJ, Malmstrom K, et al. The common cold: effects of intranasal fluticasone propionate treatment. J Allergy Clin Immunol 1998;101:726-31.
41. Williams JW, Jr, Aguilar C, Makela M, et al. Antibiotics for acute maxillary sinusitis. Cochrane Database Syst Rev 2000;CD000243.-
42. Lindbaek M, Hjortdahl P, Johnsen UL. Randomised, double blind, placebo controlled trial of penicillin V and amoxycillin in treatment of acute sinus infections in adults. BMJ 1996;313:325-9.
43. Hansen JG, Schmidt H, Grinsted P. Randomised, double blind, placebo controlled trial of penicillin V in the treatment of acute maxillary sinusitis in adults in general practice. Scand J Prim Health Care 2000;18:44-7.
44. Garbutt JMMF, Goldstein MDDS, Gellman EMD, Shannon WP, Littenberg BMD. A randomized, placebo-controlled trial of antimicrobial treatment for children with clinically diagnosed acute sinusitis. Pediatrics April 2001;107:619-625.
45. Altman KW, Austin MB, Tom LW, Knox GW. Complications of frontal sinusitis in adolescents: case presentations and treatment options. Int J Pediatr Otorhinolaryngol 1997;41:9-20.
46. Giannoni CM, Stewart MG, Alford EL. Intracranial complications of sinusitis. Laryngoscope 1997;107:863-7.
47. Hayden FG, Diamond L, Wood PB, Korts DC, Wecker MT. Effectiveness and safety of intranasal ipratropium bromide in common colds. A randomized, double-blind, placebo-controlled trial. Ann Intern Med 1996;125:89-97.
48. Gwaltney JM, Druce HM. Efficacy of brompheniramine maleate for the treatment of rhinovirus colds. Clin Infect Dis 1997;25:1188-94.
49. Clemens CJ, Taylor JA, Almquist JR, Quinn HC, Mehta A, Naylor GS. Is an antihistamine-decongestant combination effective in temporarily relieving symptoms of the common cold in preschool children? J Pediatr 1997;130:463-6.
50. Taverner D, Bickford L, Draper M. Nasal decongestants for the common cold. Cochrane Database Syst Rev 2000;2.-
51. Singh M. Heated, humidified air for the common cold. Cochrane Database Syst Rev;2000;CD001728.-
52. Del Mar CB, Glasziou PP, Spinks AB. Antibiotics for sore throat. Cochrane Database Syst Rev;2000;CD000023.-
53. Olivier C. Rheumatic fever—is it still a problem? J Antimicrob Chemother 2000;45 Suppl:13-21.
54. Howie JG, Foggo BA. Antibiotics, sore throats and rheumatic fever. J R Coll Gen Pract 1985;35:223-4.
55. Del Mar C, Glasziou P, Hayem M. Are antibiotics indicated as initial treatment for children with acute otitis media? A meta-analysis. BMJ 1997;314:1526-9.
56. Schachtel BP, Fillingim JM, Thoden WR, Lane AC, Baybutt RI. Sore throat pain in the evaluation of mild analgesics. Clin Pharmacol Ther 1988;44:704-11.
57. Schachtel BP, Fillingim JM, Lane AC, Thoden WR, Baybutt RI. Caffeine as an analgesic adjuvant. A double-blind study comparing aspirin with caffeine to aspirin and placebo in patients with sore throat. Arch Intern Med 1991;151:733-7.
58. Wethington JF. Double-blind study of benzydamine hydrochloride, a new treatment for sore throat. Clinical Therapeutics 1985;7:641-6.
59. Watson N, Nimmo WS, Christian J, Charlesworth A, Speight J, Miller K. Relief of sore throat with the anti-inflammatory throat lozenge flurbiprofen 8.75 mg: a randomised, double-blind, placebo-controlled study of efficacy and safety. Int J Clin Pract 2000;54:490-6.
60. Schachtel BP, Thoden WR. A placebo-controlled model for assaying systemic analgesics in children. Pract Clin Pharmacol Ther 1993;53:593-601.
61. Kagan G, Huddlestone L, Wolstencroft P. Two lozenges containing benzocaine assessed in the relief of sore throat. J Int Med Res 1982;10:443-6.
62. Gonzales R, Bartlett JG, Besser RE, et al. Principles of appropriate antibiotic use for treatment of uncomplicated acute bronchitis: background. Ann Intern Med 2001;134:521-9.
63. Eccles R, Morris S, Jawad M. Lack of effect of codeine in the treatment of cough associated with acute upper respiratory tract infection. J Clin Pharm Ther 1992;17:175-80.
64. Croughan-Minihane MS, Petitti DB, Rodnick JE, Eliaser G. Clinical trial examining effectiveness of three cough syrups. J Am Board Fam Pract 1993;6:109-15.
65. Chang AB, Phelan PD, Carlin JB, Sawyer SM, Robertson CF. A randomised, placebo controlled trial of inhaled salbutamol and beclomethasone for recurrent cough. Arch Dis Child 1998;79:6-11.
66. Hueston WJ. A comparison of albuterol and erythromycin for the treatment of acute bronchitis. J Fam Pract 1991;33:476-80.
67. Hueston WJ. Albuterol delivered by metered-dose inhaler to treat acute bronchitis. J Fam Pract 1994;39:437-40.
68. Smucny J, Flynn C, Becker L, Glazier R. Beta2-agonists for acute bronchitis [Systematic Review]. Cochrane Database of Systematic Reviews 2001;1.-
69. Gwaltney JM, Jr, Phillips CD, Miller RD, Riker DK. Computed tomographic study of the common cold. N Engl J Med 1994;330:25-30.
70. Holmes WF, Macfarlane JT, Macfarlane RM, Hubbard R. Symptoms, signs, and prescribing for acute lower respiratory tract illness. Br J Gen Pract 2001;51:177-81.
71. Gulbrandsen P, Fugelli P, Kvarstein G, Moland L. The duration of acute respiratory tract infections in children. Scand J Prim Health Care 1989;7:219-23.
72. Gonzales R, Bartlett JG, Besser RE, Hickner JM, Hoffman JR, Sande MA. Principles of appropriate antibiotic use for treatment of nonspecific upper respiratory tract infections in adults: background. Ann Intern Med 2001;134:490-4.
73. Gadomski AM. Potential interventions for preventing pneumonia among young children: lack of effect of antibiotic treatment for upper respiratory infections. Pediatr Infect Dis J 1993;12:115-20.
74. Heikkinen T, Ruuskanen O, Ziegler T, Waris M, Puhakka H. Short-term use of amoxicillin-clavulanate during upper respiratory tract infection for prevention of acute otitis media. J Pediatr 1995;126:313-6.
The term acute upper respiratory tract infection (ARTI) refers to an infection, almost always viral,1 predominantly involving the nasopharynx, sinuses, and large bronchi. It encompasses what is frequently referred to as the common cold, sinusitis, pharyngitis, bronchitis, and otitis media. This review will focus primarily on the common cold, or undifferentiated ARTI, but there will be considerable overlap with the other diagnoses. The rationale for grouping these traditionally separate diagnostic categories together is straightforward. ARTI almost always presents with some combination of nasal congestion, rhinnorrhea, sore throat, and cough. Sometimes one or another of these symptoms predominates, but there is good reason to suggest that the traditional diagnostic distinctions are arbitrary.2 The overlap between the clinical signs, symptoms, and x-ray findings is so extensive that these terms are not very useful diagnostically. This symptom complex is among the top 3 reasons for visits to primary care doctors3 and accounts for approximately 100 million office visits in the United States per year4 at an annual cost of considerably more than 1 billion dollars.5 Overuse of antibiotics adds more than $11 to the cost of each encounter for ARTI.5
This review will summarize the evidence that patients who present with undifferentiated ARTI usually have self-limited disease, that complications are rare, effective treatments for symptoms are available, and antibiotics are not often indicated. The evidence is based on only adults and children over age 2 who have normal immune systems and do not have chronic respiratory disease. (J Fam Pract; 50:1070-1077)
Pathophysiology
Patients with undifferentiated ARTI present with any or all of the following symptoms: rhinnorrhea (which may be either clear or colored), nasal congestion, cough, sore throat, facial pain, malaise, headache, or fever. The etiology is almost always viral, most commonly rhinovirus, but other viruses have been implicated as well, particularly corona-viruses, parainfluenza, and influenza.6 Bacterial infection is rare in undifferentiated ARTI, occurring in approximately 2% of patients.1 The most common bacteria implicated are group A Streptococcus, Streptococcus pneumoniae, Haemophilus influenzae and Moraxella catarrhalis.7 Chlamydia pnuemoniae and Mycoplasma pnuemoniae have rarely been identified as well.6 Bordatella pertussis can occasionally be the cause of persistent cough in children and adults.8, 9
Diagnosis
The major diagnostic consideration in a patient who presents with ARTI symptoms is to rule out a more serious illness which would require aggressive treatment. These include pneumonia, pharyngitis caused by group A streptococci, and bacterial sinusitis. The traditional symptoms and signs that physicians use to distinguish viral from bacterial infection have not been determined helpful in making this distinction. Both a history of colored nasal discharge and maxillary sinus tenderness to palpation have a positive likelihood ratio (LR+) near 1 in predicting computed tomography–based diagnosis of acute bacterial sinusitis (a likelihood ratio of 1 indicates that the result does not change the likelihood of disease).10 Purulent sputum does not distinguish between viral ARTI and bacterial pneumonia.11 Combinations of symptoms, however, in the form of clinical decision rules, can be useful at ruling out more serious conditions.
Nasal Discharge
In patients who present primarily with nasal discharge, a study that correlated computed tomography (CT) scans with direct sinus puncture demonstrated that 90% of primary care patients with CT findings of total opacification or air fluid level in the maxillary sinuses have a bacterial etiology.12 Unfortunately, there is no combination of clinical signs or symptoms that reliably predicts opacification or air fluid levels.12 There have been 3 decision rules published based on clinical findings that may be useful, particularly in helping clinicians identify the most important symptoms on which they should focus during their examination. However, 2 of the rules require a sedimentation rate or C-reactive protein value,10,12 not typically available in a routine office visit. The only other rule based on clinical findings included adult men and used x-ray as the reference standard, making it less useful.13
The suspicion of sinusitis by a generalist is actually quite accurate diagnostically, since about 40% of patients with suspected sinusitis have the diagnosis confirmed by aspiration or CT imaging.14 One must therefore be guided by overall clinical judgment in these patients, but a practical approach to empiric treatment is presented in the section on treatment.
Sore Throat
In patients who present primarily with sore throat, the important consideration is to rule out group A streptococcus as the etiology. The prevalence of streptococcal pharyngitis varies markedly with age, season of the year, and presence or absence of an outbreak in the community. For children prevalences have been reported ranging from 12% to 35%. Prevalence seems to peak in the 5 years to 9 years age range, and in the autumn. Reported prevalences for adults range from 5% to 15%.15-20
A recent systematic review noted nine published decision rules for diagnosis of streptococcal pharyngitis,21 but the only one that has been prospectively validated in a primary care population of both children and adults is by McIssac22,23Table 1. Patients with a low risk of GABHS require no further testing; those with an intermediate risk should undergo rapid strep antigen testing; and those with a high risk of GABHS should either under rapid strep antigen testing with culture follow-up or empiric therapy. Culture for group A streptococcus is the most reliable means of diagnosis, but requires withholding treatment for 48 hours while awaiting the result.24
Infectious mononucleosis can also cause exudative pharyngitis. Palatine petechiae, posterior auricular and posterior cervical adenopathy, marked axillary adenopathy, and inguinal adenopathy are all associated with infectious mononucleosis,25 and if any of these signs are present a heterophile antibody test may be indicated to confirm the diagnosis.
Cough
Many patients with ARTI present with cough as a primary symptom. While the differential diagnosis for undifferentiated cough is long, in the setting of ARTI the primary diagnostic consideration is to rule out pneumonia. Decision rules by Heckerling11 and Diehr26 have been well validated Table 2, Table 3. If the patient does not have temperature higher than 100 ÞF, pulse rate higher than 100, rales, decreased breath sounds, or absence of wheezing, then pneumonia is very unlikely (negative likelihood ratio = 0.06). As the number of signs increases, the likelihood ratio increases, but the decision rule does not have sufficient positive predictive value to be used as a basis for antibiotic treatment. Purulent sputum is not predictive of pneumonia (LR+=1.3).11,27 Empiric antibiotics for pneumonia should not be given without a confirmatory chest x-ray.1
Treatment
Undifferentiated ARTI
The level of evidence supporting different interventions is summarized in Table 4, and each is discussed in more detail below.
Antibiotics. There have been numerous double blind placebo controlled randomized trials of antibiotics in patients with undifferentiated ARTI symptoms. All of these trials have been evaluated in a recent Cochrane systematic review.28 The review concludes that there is no consistent evidence of benefit from any antibiotic treatment and that there is a significant increase in adverse effects associated with antibiotic use. Increased cost and increased bacterial resistance in the patient and community are additional concerns.
Education. One of the reasons for inappropriate antibiotic use in ARTI is patient pressure for antibiotics. This can be subtle as well as direct and is difficult for physicians to resist.29 Several studies have shown that patient expectation for antibiotics is related to having been given antibiotics for respiratory infections in the past.30-32 Furthermore, McFarlane has shown that simple patient education techniques reduce both visits for ARTI and antibiotic usage.33 An essential part of treatment of these infections, therefore, is educating patients about the viral nature of the illness, the usual course and duration of symptoms (10 days for most, up to 3 weeks for some), the ineffectiveness of antibiotics for treatment, and the harm antibiotics can cause both to the patient and the community. Patient satisfaction has been shown to increase after this sort of intervention, even when patients initially had an expectation for antibiotic prescription.30,34 Some have advocated avoiding terms such as sinusitis and bronchitis with patients, instead using the terms head cold and chest cold to emphasize the viral nature of these illnesses.
Naproxen Sodium. Naproxen sodium was evaluated in a well-designed randomized placebo controlled trial using experimentally induced rhinovirus infections in volunteers.35 A statistically significant 29% reduction in total 5-day symptom scores was found. Specific symptoms that improved included sneezing, rhinnorrhea, nasal obstruction, sore throat, cough, headache, malaise and chilliness.
Vitamin C. A Cochrane Library meta-analysis of all the trials for ascorbic acid showed a small but significant effect on decreasing duration (.55 days per episode) and a modest effect on severity of symptoms.36 There was no evidence for an effect when taken prophylactically.
Zinc. Randomized trials of zinc have shown marked heterogeneity, but this may have been due to use of different preparations with different bioavailability.37 A more recent randomized trial used zinc acetate lozenges.38 Increased plasma zinc levels were documented in the treatment group. The lozenges contained 42.96 mg of zinc acetate dihydrate, and were administered every 2 to 3 hours, beginning 24 hours or less after the onset of symptoms. The treatment group had a reduction from 8.1 to 4.5 days in total duration of symptoms (number needed to treat [NNT] =3 at 5 days) and a 50% reduction in symptom scores compared to the placebo group. Symptoms included in the score were sore throat, nasal discharge, nasal congestion, sneezing, cough, scratchy throat, hoarseness, muscle ache, fever, and headache.
Echinacea. Echinacea was evaluated in another Cochrane Library Systematic Review.39 Sixteen trials were evaluated, two thirds of which had insufficient quality of reporting. There were many different extracts used, which made comparability of the results a problem. Most trials showed positive results, suggesting that preparations containing extract of echinacea may have some beneficial effect on prevention and treatment.
Intranasal Fluticasone. Intranasal fluticasone was evaluated in a randomized trial of 200 young adults with common cold symptoms.40 No clinically significant differences were noted in either duration or severity of symptoms between treatment and control groups.
Nasal Discharge
Antibiotics. As noted above under undifferentiated ARTI, a Cochrane systematic review of randomized antibiotic trials found no evidence of benefit of antibiotic treatment for clear or purulent rhinnorrhea.28 Another Cochrane systematic review focused on randomized trials of antibiotic treatment for sinusitis.41 There was modest benefit from amoxicillin treatment in patients with acute maxillary sinusitis confirmed radiographically (NNT=4). There was no evidence, even from more recent studies, that other antibiotics were any more effective than amoxicillin. Lindbaek was able to demonstrate in a randomized controlled trial of amoxicillin that the only patients who benefited from treatment were those who had either complete opacification of a sinus, or an air fluid level.42 Patients who had mucosal thickening alone showed no difference from the placebo group. Of the patients in the placebo group who did have opacification or air fluid level, half were well or much better within 10 days. There is also some evidence that patients who present with moderate to severe unilateral facial pain benefit from antibiotics.43 In children, a recent antibiotic trial of patients who had a clinical diagnosis of sinusitis with symptoms for between 14 and 28 days showed no difference in any outcome measure between treatment and placebo groups.44
Based on Lindbaek’s data for adults, assuming that 42% of patients with suspected bacterial sinusitis actually have the disease, the number needed to treat to benefit one patient is 8. The number needed to harm by adverse effects from antibiotics is 4, so harm may outweigh any benefit. Frontal sinusitis seems to represent a different disease process because of the anatomy of drainage from the frontal sinuses. These patients usually present with high fever, severe pain, are quite ill, and may require hospitalization, parenteral antibiotics and surgery.1,45,46 A practical approach is to treat all patients with nasal discharge symptomatically, unless they have severe pain, or appear very ill, in which case sinus films should be considered to rule out frontal sinusitis, or opacification or air fluid level in any sinus. Also, adult patients who have had sinus symptoms for more than two weeks without improvement are more likely to benefit from antibiotic treatment.42
Ipratropium. Ipratopium nasal spray used in a randomized controlled trial, reduced rhinnorrhea and sneezing in patients with cold symptoms by 31% compared to placebo saline nasal spray (ARR=16% NNT=7), and by 78% when compared with untreated patients (ARR=63% NNT=2).47
Brompheniramine. Brompheniramine likewise has a temporary modest effect on rhinnorrhea in adults. Brompheniramine was evaluated in a randomized controlled trial in volunteers infected with rhinovirus.48 The treatment group had a 20% reduction in symptom score on day 1 and a 26% reduction on day 2. The main symptoms improved were sneeze frequency, sneeze severity, and cough count. Drowsiness was a troubling side effect. Neither decongestants nor antihistamines, nor combinations of the 2 have shown any effect in randomized trials in children.49
Nasal Decongestants. The efficacy of oral psuedoephedrine or metalazone nasal spray were evaluated in a Cochrane Library meta-analysis.50 There was a 13% reduction in symptom score (subjective rating of severity of nasal congestion) after a single use of either agent, but no difference in combined symptom scores after use for 5 days.
Heated Humidified Air. Published studies of effectiveness of breathing heated humidified air for cold symptoms were evaluated in a Cochrane Library Systematic Review.51 Six studies were evaluated and the results were quite heterogeneous. One study in Israel and two in the UK showed significant improvement, while three in the US showed no difference between treatment and placebo groups.
Sore Throat
Antibiotics. The data for sore throat should reassure physicians who worry about the consequences of missing streptococcal pharyngitis. A Cochrane meta-analysis showed that treatment of streptococcal pharyngitis with penicillin did reduce duration of illness, but only by approximately half a day.52 There was only a minimal effect on prevention of suppurative complications as well (NNT=30 for children and NNT=145 for adults to prevent one case of otitis media). The incidence of rheumatic fever is so low in the industrialized world (.5 per 100,000 in the pediatric population in the United States )53 that the number needed to treat to prevent 1 case of rheumatic fever is exceedingly high. In fact, Howie54 has estimated that for a general practitioner in Scotland the NNT exceeds the number of patients he or she would see in his or her lifetime. Furthermore, it is estimated than only approximately 15% of patients with streptococcal pharyngitis ever present for treatment, further reducing the opportunity to prevent complications.52 Most of the symptomatic improvement from antibiotics comes in the first 3 days of illness, so that if culture is used as a treatment criterion, most patients will already be better by the time the results are available.55
Analgesics. Acetominophen, aspirin, ibuprofen, flurbiprofen, and the combination of aspirin and caffeine have all been shown to be effective for sore throat pain in randomized trials.56-60 Ibuprofen was found to be superior to acetominophen60 and aspirin/caffeine was found to be superior to aspirin alone.57
Benzocaine. I could find only one study evaluating benzocaine containing lozenges for sore throat61 and it did not include a placebo group. Although one lozenge (Merocaine) produced significantly better pain relief than the other (Tyrozets), the lack of a placebo comparison makes interpretation of these results problematic.
Cough
Antibiotics. There is no evidence, in the absence of pneumonia or pertussis, that antibiotics are effective for the treatment of acute cough, including cough productive of colored sputum in otherwise healthy patients.62 This does not include patients with acute exacerbation of chronic pulmonary disease.
Codeine, Dextromethorphan, Guaifenesin. There are few studies evaluating the effectiveness of cough medicines that contain combinations of guaifenesin and codeine or dextromethorphan. One randomized trial of the effectiveness of codeine for cough related to ARTI showed no difference between experimental and placebo groups.63 Another study found no difference between cough medicines containing guaifenesin alone, guaifenesin and codeine, and guaifenesin and dextromethorphan.64 Therefore, although studies are few, there is no evidence to suggest that any of these agents is effective for treatment of cough associated with ARTI. It is interesting to note that all studies showed a marked placebo effect.
b2 -Agonists. Initial studies appeared to show some benefit for inhaled b2-agonists in the treatment of cough.65-67 A recent systematic review by Smucny, however, found that only patients with demonstrated hyperreactivity of airways benefited from this treatment.68
A flow diagram depicting an algorithm for when to use antibiotic in addition to symptomatic treatment for ARTI is shown in the Figure 1. This closely approximates the most recent CDC guidelines on the judicious use of antibiotics for respiratory infections in children and adults. Table 5 summarizes the best clinical trial evidence regarding the use of antibiotics in ARTI.
Prognosis
The mean duration of symptoms for untreated ARTI is approximately 10 days.38,42,69 It is not unusual for symptoms, particularly nasal congestion and cough, however, to last for up to 3 weeks. One study of lower respiratory tract infection in primary care patients found that 60% were still coughing after 10 days.70 Another study of ARTI in children in Norway found that 50% still had nasal discharge and cough after 3 weeks.71 Complications are rare in primary care patients. Less than 2% of patients with ARTI go on to develop secondary bacterial infection requiring antibiotic treatment.1 Although there are no data in adults which specifically address whether antibiotic treatment prevents bacterial infection,72 there are at least 2 studies that demonstrate that antibiotic treatment of children with undifferentiated ARTI does not prevent pneumonia or otitis media.73,74
The term acute upper respiratory tract infection (ARTI) refers to an infection, almost always viral,1 predominantly involving the nasopharynx, sinuses, and large bronchi. It encompasses what is frequently referred to as the common cold, sinusitis, pharyngitis, bronchitis, and otitis media. This review will focus primarily on the common cold, or undifferentiated ARTI, but there will be considerable overlap with the other diagnoses. The rationale for grouping these traditionally separate diagnostic categories together is straightforward. ARTI almost always presents with some combination of nasal congestion, rhinnorrhea, sore throat, and cough. Sometimes one or another of these symptoms predominates, but there is good reason to suggest that the traditional diagnostic distinctions are arbitrary.2 The overlap between the clinical signs, symptoms, and x-ray findings is so extensive that these terms are not very useful diagnostically. This symptom complex is among the top 3 reasons for visits to primary care doctors3 and accounts for approximately 100 million office visits in the United States per year4 at an annual cost of considerably more than 1 billion dollars.5 Overuse of antibiotics adds more than $11 to the cost of each encounter for ARTI.5
This review will summarize the evidence that patients who present with undifferentiated ARTI usually have self-limited disease, that complications are rare, effective treatments for symptoms are available, and antibiotics are not often indicated. The evidence is based on only adults and children over age 2 who have normal immune systems and do not have chronic respiratory disease. (J Fam Pract; 50:1070-1077)
Pathophysiology
Patients with undifferentiated ARTI present with any or all of the following symptoms: rhinnorrhea (which may be either clear or colored), nasal congestion, cough, sore throat, facial pain, malaise, headache, or fever. The etiology is almost always viral, most commonly rhinovirus, but other viruses have been implicated as well, particularly corona-viruses, parainfluenza, and influenza.6 Bacterial infection is rare in undifferentiated ARTI, occurring in approximately 2% of patients.1 The most common bacteria implicated are group A Streptococcus, Streptococcus pneumoniae, Haemophilus influenzae and Moraxella catarrhalis.7 Chlamydia pnuemoniae and Mycoplasma pnuemoniae have rarely been identified as well.6 Bordatella pertussis can occasionally be the cause of persistent cough in children and adults.8, 9
Diagnosis
The major diagnostic consideration in a patient who presents with ARTI symptoms is to rule out a more serious illness which would require aggressive treatment. These include pneumonia, pharyngitis caused by group A streptococci, and bacterial sinusitis. The traditional symptoms and signs that physicians use to distinguish viral from bacterial infection have not been determined helpful in making this distinction. Both a history of colored nasal discharge and maxillary sinus tenderness to palpation have a positive likelihood ratio (LR+) near 1 in predicting computed tomography–based diagnosis of acute bacterial sinusitis (a likelihood ratio of 1 indicates that the result does not change the likelihood of disease).10 Purulent sputum does not distinguish between viral ARTI and bacterial pneumonia.11 Combinations of symptoms, however, in the form of clinical decision rules, can be useful at ruling out more serious conditions.
Nasal Discharge
In patients who present primarily with nasal discharge, a study that correlated computed tomography (CT) scans with direct sinus puncture demonstrated that 90% of primary care patients with CT findings of total opacification or air fluid level in the maxillary sinuses have a bacterial etiology.12 Unfortunately, there is no combination of clinical signs or symptoms that reliably predicts opacification or air fluid levels.12 There have been 3 decision rules published based on clinical findings that may be useful, particularly in helping clinicians identify the most important symptoms on which they should focus during their examination. However, 2 of the rules require a sedimentation rate or C-reactive protein value,10,12 not typically available in a routine office visit. The only other rule based on clinical findings included adult men and used x-ray as the reference standard, making it less useful.13
The suspicion of sinusitis by a generalist is actually quite accurate diagnostically, since about 40% of patients with suspected sinusitis have the diagnosis confirmed by aspiration or CT imaging.14 One must therefore be guided by overall clinical judgment in these patients, but a practical approach to empiric treatment is presented in the section on treatment.
Sore Throat
In patients who present primarily with sore throat, the important consideration is to rule out group A streptococcus as the etiology. The prevalence of streptococcal pharyngitis varies markedly with age, season of the year, and presence or absence of an outbreak in the community. For children prevalences have been reported ranging from 12% to 35%. Prevalence seems to peak in the 5 years to 9 years age range, and in the autumn. Reported prevalences for adults range from 5% to 15%.15-20
A recent systematic review noted nine published decision rules for diagnosis of streptococcal pharyngitis,21 but the only one that has been prospectively validated in a primary care population of both children and adults is by McIssac22,23Table 1. Patients with a low risk of GABHS require no further testing; those with an intermediate risk should undergo rapid strep antigen testing; and those with a high risk of GABHS should either under rapid strep antigen testing with culture follow-up or empiric therapy. Culture for group A streptococcus is the most reliable means of diagnosis, but requires withholding treatment for 48 hours while awaiting the result.24
Infectious mononucleosis can also cause exudative pharyngitis. Palatine petechiae, posterior auricular and posterior cervical adenopathy, marked axillary adenopathy, and inguinal adenopathy are all associated with infectious mononucleosis,25 and if any of these signs are present a heterophile antibody test may be indicated to confirm the diagnosis.
Cough
Many patients with ARTI present with cough as a primary symptom. While the differential diagnosis for undifferentiated cough is long, in the setting of ARTI the primary diagnostic consideration is to rule out pneumonia. Decision rules by Heckerling11 and Diehr26 have been well validated Table 2, Table 3. If the patient does not have temperature higher than 100 ÞF, pulse rate higher than 100, rales, decreased breath sounds, or absence of wheezing, then pneumonia is very unlikely (negative likelihood ratio = 0.06). As the number of signs increases, the likelihood ratio increases, but the decision rule does not have sufficient positive predictive value to be used as a basis for antibiotic treatment. Purulent sputum is not predictive of pneumonia (LR+=1.3).11,27 Empiric antibiotics for pneumonia should not be given without a confirmatory chest x-ray.1
Treatment
Undifferentiated ARTI
The level of evidence supporting different interventions is summarized in Table 4, and each is discussed in more detail below.
Antibiotics. There have been numerous double blind placebo controlled randomized trials of antibiotics in patients with undifferentiated ARTI symptoms. All of these trials have been evaluated in a recent Cochrane systematic review.28 The review concludes that there is no consistent evidence of benefit from any antibiotic treatment and that there is a significant increase in adverse effects associated with antibiotic use. Increased cost and increased bacterial resistance in the patient and community are additional concerns.
Education. One of the reasons for inappropriate antibiotic use in ARTI is patient pressure for antibiotics. This can be subtle as well as direct and is difficult for physicians to resist.29 Several studies have shown that patient expectation for antibiotics is related to having been given antibiotics for respiratory infections in the past.30-32 Furthermore, McFarlane has shown that simple patient education techniques reduce both visits for ARTI and antibiotic usage.33 An essential part of treatment of these infections, therefore, is educating patients about the viral nature of the illness, the usual course and duration of symptoms (10 days for most, up to 3 weeks for some), the ineffectiveness of antibiotics for treatment, and the harm antibiotics can cause both to the patient and the community. Patient satisfaction has been shown to increase after this sort of intervention, even when patients initially had an expectation for antibiotic prescription.30,34 Some have advocated avoiding terms such as sinusitis and bronchitis with patients, instead using the terms head cold and chest cold to emphasize the viral nature of these illnesses.
Naproxen Sodium. Naproxen sodium was evaluated in a well-designed randomized placebo controlled trial using experimentally induced rhinovirus infections in volunteers.35 A statistically significant 29% reduction in total 5-day symptom scores was found. Specific symptoms that improved included sneezing, rhinnorrhea, nasal obstruction, sore throat, cough, headache, malaise and chilliness.
Vitamin C. A Cochrane Library meta-analysis of all the trials for ascorbic acid showed a small but significant effect on decreasing duration (.55 days per episode) and a modest effect on severity of symptoms.36 There was no evidence for an effect when taken prophylactically.
Zinc. Randomized trials of zinc have shown marked heterogeneity, but this may have been due to use of different preparations with different bioavailability.37 A more recent randomized trial used zinc acetate lozenges.38 Increased plasma zinc levels were documented in the treatment group. The lozenges contained 42.96 mg of zinc acetate dihydrate, and were administered every 2 to 3 hours, beginning 24 hours or less after the onset of symptoms. The treatment group had a reduction from 8.1 to 4.5 days in total duration of symptoms (number needed to treat [NNT] =3 at 5 days) and a 50% reduction in symptom scores compared to the placebo group. Symptoms included in the score were sore throat, nasal discharge, nasal congestion, sneezing, cough, scratchy throat, hoarseness, muscle ache, fever, and headache.
Echinacea. Echinacea was evaluated in another Cochrane Library Systematic Review.39 Sixteen trials were evaluated, two thirds of which had insufficient quality of reporting. There were many different extracts used, which made comparability of the results a problem. Most trials showed positive results, suggesting that preparations containing extract of echinacea may have some beneficial effect on prevention and treatment.
Intranasal Fluticasone. Intranasal fluticasone was evaluated in a randomized trial of 200 young adults with common cold symptoms.40 No clinically significant differences were noted in either duration or severity of symptoms between treatment and control groups.
Nasal Discharge
Antibiotics. As noted above under undifferentiated ARTI, a Cochrane systematic review of randomized antibiotic trials found no evidence of benefit of antibiotic treatment for clear or purulent rhinnorrhea.28 Another Cochrane systematic review focused on randomized trials of antibiotic treatment for sinusitis.41 There was modest benefit from amoxicillin treatment in patients with acute maxillary sinusitis confirmed radiographically (NNT=4). There was no evidence, even from more recent studies, that other antibiotics were any more effective than amoxicillin. Lindbaek was able to demonstrate in a randomized controlled trial of amoxicillin that the only patients who benefited from treatment were those who had either complete opacification of a sinus, or an air fluid level.42 Patients who had mucosal thickening alone showed no difference from the placebo group. Of the patients in the placebo group who did have opacification or air fluid level, half were well or much better within 10 days. There is also some evidence that patients who present with moderate to severe unilateral facial pain benefit from antibiotics.43 In children, a recent antibiotic trial of patients who had a clinical diagnosis of sinusitis with symptoms for between 14 and 28 days showed no difference in any outcome measure between treatment and placebo groups.44
Based on Lindbaek’s data for adults, assuming that 42% of patients with suspected bacterial sinusitis actually have the disease, the number needed to treat to benefit one patient is 8. The number needed to harm by adverse effects from antibiotics is 4, so harm may outweigh any benefit. Frontal sinusitis seems to represent a different disease process because of the anatomy of drainage from the frontal sinuses. These patients usually present with high fever, severe pain, are quite ill, and may require hospitalization, parenteral antibiotics and surgery.1,45,46 A practical approach is to treat all patients with nasal discharge symptomatically, unless they have severe pain, or appear very ill, in which case sinus films should be considered to rule out frontal sinusitis, or opacification or air fluid level in any sinus. Also, adult patients who have had sinus symptoms for more than two weeks without improvement are more likely to benefit from antibiotic treatment.42
Ipratropium. Ipratopium nasal spray used in a randomized controlled trial, reduced rhinnorrhea and sneezing in patients with cold symptoms by 31% compared to placebo saline nasal spray (ARR=16% NNT=7), and by 78% when compared with untreated patients (ARR=63% NNT=2).47
Brompheniramine. Brompheniramine likewise has a temporary modest effect on rhinnorrhea in adults. Brompheniramine was evaluated in a randomized controlled trial in volunteers infected with rhinovirus.48 The treatment group had a 20% reduction in symptom score on day 1 and a 26% reduction on day 2. The main symptoms improved were sneeze frequency, sneeze severity, and cough count. Drowsiness was a troubling side effect. Neither decongestants nor antihistamines, nor combinations of the 2 have shown any effect in randomized trials in children.49
Nasal Decongestants. The efficacy of oral psuedoephedrine or metalazone nasal spray were evaluated in a Cochrane Library meta-analysis.50 There was a 13% reduction in symptom score (subjective rating of severity of nasal congestion) after a single use of either agent, but no difference in combined symptom scores after use for 5 days.
Heated Humidified Air. Published studies of effectiveness of breathing heated humidified air for cold symptoms were evaluated in a Cochrane Library Systematic Review.51 Six studies were evaluated and the results were quite heterogeneous. One study in Israel and two in the UK showed significant improvement, while three in the US showed no difference between treatment and placebo groups.
Sore Throat
Antibiotics. The data for sore throat should reassure physicians who worry about the consequences of missing streptococcal pharyngitis. A Cochrane meta-analysis showed that treatment of streptococcal pharyngitis with penicillin did reduce duration of illness, but only by approximately half a day.52 There was only a minimal effect on prevention of suppurative complications as well (NNT=30 for children and NNT=145 for adults to prevent one case of otitis media). The incidence of rheumatic fever is so low in the industrialized world (.5 per 100,000 in the pediatric population in the United States )53 that the number needed to treat to prevent 1 case of rheumatic fever is exceedingly high. In fact, Howie54 has estimated that for a general practitioner in Scotland the NNT exceeds the number of patients he or she would see in his or her lifetime. Furthermore, it is estimated than only approximately 15% of patients with streptococcal pharyngitis ever present for treatment, further reducing the opportunity to prevent complications.52 Most of the symptomatic improvement from antibiotics comes in the first 3 days of illness, so that if culture is used as a treatment criterion, most patients will already be better by the time the results are available.55
Analgesics. Acetominophen, aspirin, ibuprofen, flurbiprofen, and the combination of aspirin and caffeine have all been shown to be effective for sore throat pain in randomized trials.56-60 Ibuprofen was found to be superior to acetominophen60 and aspirin/caffeine was found to be superior to aspirin alone.57
Benzocaine. I could find only one study evaluating benzocaine containing lozenges for sore throat61 and it did not include a placebo group. Although one lozenge (Merocaine) produced significantly better pain relief than the other (Tyrozets), the lack of a placebo comparison makes interpretation of these results problematic.
Cough
Antibiotics. There is no evidence, in the absence of pneumonia or pertussis, that antibiotics are effective for the treatment of acute cough, including cough productive of colored sputum in otherwise healthy patients.62 This does not include patients with acute exacerbation of chronic pulmonary disease.
Codeine, Dextromethorphan, Guaifenesin. There are few studies evaluating the effectiveness of cough medicines that contain combinations of guaifenesin and codeine or dextromethorphan. One randomized trial of the effectiveness of codeine for cough related to ARTI showed no difference between experimental and placebo groups.63 Another study found no difference between cough medicines containing guaifenesin alone, guaifenesin and codeine, and guaifenesin and dextromethorphan.64 Therefore, although studies are few, there is no evidence to suggest that any of these agents is effective for treatment of cough associated with ARTI. It is interesting to note that all studies showed a marked placebo effect.
b2 -Agonists. Initial studies appeared to show some benefit for inhaled b2-agonists in the treatment of cough.65-67 A recent systematic review by Smucny, however, found that only patients with demonstrated hyperreactivity of airways benefited from this treatment.68
A flow diagram depicting an algorithm for when to use antibiotic in addition to symptomatic treatment for ARTI is shown in the Figure 1. This closely approximates the most recent CDC guidelines on the judicious use of antibiotics for respiratory infections in children and adults. Table 5 summarizes the best clinical trial evidence regarding the use of antibiotics in ARTI.
Prognosis
The mean duration of symptoms for untreated ARTI is approximately 10 days.38,42,69 It is not unusual for symptoms, particularly nasal congestion and cough, however, to last for up to 3 weeks. One study of lower respiratory tract infection in primary care patients found that 60% were still coughing after 10 days.70 Another study of ARTI in children in Norway found that 50% still had nasal discharge and cough after 3 weeks.71 Complications are rare in primary care patients. Less than 2% of patients with ARTI go on to develop secondary bacterial infection requiring antibiotic treatment.1 Although there are no data in adults which specifically address whether antibiotic treatment prevents bacterial infection,72 there are at least 2 studies that demonstrate that antibiotic treatment of children with undifferentiated ARTI does not prevent pneumonia or otitis media.73,74
1. Gonzales R, Bartlett JG, Besser RE, Richelle JC, Hickner J, Jerome R, Hoffman JR. Principles of appropriate antibiotic use for treatment of acute respiratory tract infections in adults. Ann Intern Med 2001;134.-
2. Hueston WJ, Mainous AG, 3rd, Dacus EN, Hopper JE. Does acute bronchitis really exist? A reconceptualization of acute viral respiratory infections. J Fam Pract 2000;49:401-6.
3. Woodwell DA. National Ambulatory Medical Care Survey: 1996 summary. Adv Data 1997;1-25.
4. Gonzales R, Steiner JF, Sande MA. Antibiotic prescribing for adults with colds, upper respiratory tract infections, and bronchitis by ambulatory care physicians. JAMA 1997;278:901-4.
5. Mainous AG, 3rd, Hueston WJ. The cost of antibiotics in treating upper respiratory tract infections in a Medicaid population . Arch Fam Med 1998;7:45-9.
6. Makela MJ, Puhakka T, Ruuskanen O, et al. Viruses and bacteria in the etiology of the common cold. J Clin Microbiol 1998;36:539-42.
7. Ruoff G. Upper respiratory tract infections in family practice. Pediatr Infect Dis J 1998;17:S73-8.
8. Dowell SF, Schwartz B, Phillips WR. Appropriate use of antibiotics for URIs in children: Part II. Cough, pharyngitis and the common cold. The Pediatric URI Consensus Team. Am Fam Physician 1998;58:1335-42, 1345.
9. Gonzales R, Bartlett JG, Besser RE, et al. Principles of appropriate antibiotic use for treatment of acute respiratory tract infections in adults: background, specific aims, and methods. Ann Intern Med 2001;134:479-86.
10. Lindbaek M, Hjortdahl P, Johnsen UL. Use of symptoms, signs, and blood tests to diagnose acute sinus infections in primary care: comparison with computed tomography. Fam Med 1996;28:183-8.
11. Heckerling PS, Tape TG, Wigton RS, et al. Clinical prediction rule for pulmonary infiltrates. Ann Intern Med 1990;113:664-70.
12. Hansen JG, Schmidt H, Rosborg J, Lund E. Predicting acute maxillary sinusitis in a general practice population. BMJ 1995;311:233-6.
13. Williams JW, Jr, Simel DL, Roberts L, Samsa GP. Clinical evaluation for sinusitis. Making the diagnosis by history and physical examination. Ann Intern Med 1992;117:705-10.
14. Hickner JM, Bartlett JG, Besser RE, Gonzales R, Hoffman JR, Sande MA. Principles of appropriate antibiotic use for acute rhinosinusitis in adults: background. Ann Intern Med 2001;134:498-505.
15. Gunnarsson RK, Holm SE, Soderstrom M. The prevalence of potential pathogenic bacteria in nasopharyngeal samples from individuals with a respiratory tract infection and a sore throat—implications for the diagnosis of pharyngotonsillitis. Fam Pract 2001;18:266-71.
16. Pokorski SJ, Vetter EA, Wollan PC, Cockerill FR, 3rd. Comparison of Gen-Probe Group A streptococcus Direct Test with culture for diagnosing streptococcal pharyngitis. J Clin Microbiol 1994;32:1440-3.
17. Dagnelie CF, Bartelink ML, van der Graaf Y, Goessens W, de Melker RA. Towards a better diagnosis of throat infections (with group A beta-haemolytic streptococcus) in general practice. Br J Gen Pract 1998;48:959-62.
18. Hart AP, Buck LL, Morgan S, Saverio S, McLaughlin JC. A comparison of the BioStar Strep A OIA rapid antigen assay, group A Selective Strep Agar (ssA), and Todd-Hewitt broth cultures for the detection of group A Streptococcus in an outpatient family practice setting. Diagn Microbiol Infect Dis 1997;29:139-45.
19. Smith TD, Wilkinson V, Kaplan EL. Group A streptococcus-associated upper respiratory tract infections in a day-care center. Pediatrics 1989;83:380-4.
20. Drulak M, Raybould TJ, Yong J, Hsiung D, Smith H, Winston S. Comparison of Visuwell enzyme immunoassay to culture for detection of group A Streptococcus in throat swab specimens. Diagn Microbiol Infect Dis 1988;11:181-7.
21. Ebell MH SM, Barry HC, Ives K, Carey BS. Does this patient have strep throat? JAMA 2000;284:2912-2918.
22. McIsaac WJ, Goel V, To T, Low DE. The validity of a sore throat score in family practice. CMAJ 2000;163:811-5.
23. McIsaac WJ, White D, Tannenbaum D, Low DE. A clinical score to reduce unnecessary antibiotic use in patients with sore throat. CMAJ 1998;158:75-83.
24. Reed BD, Huck W, French T. Diagnosis of group A beta-hemolytic Streptococcus using clinical scoring criteria, Directigen 1-2-3 group A streptococcal test, and culture. Arch Intern Med 1990;150:1727-32.
25. Aronson MD, Komaroff AL, Pass TM, Ervin CT, Branch WT. Heterophil antibody in adults with sore throat: frequency and clinical presentation. Ann Intern Med 1982;96:505-8.
26. Diehr P, Wood RW, Bushyhead J, Krueger L, Wolcott B, Tompkins RK. Prediction of pneumonia in outpatients with acute cough—a statistical approach. J Chronic Dis 1984;37:215-25.
27. Metlay JP, Kapoor WN, Fine MJ. Does this patient have community-acquired pneumonia? Diagnosing pneumonia by history and physical examination. JAMA 1997;278:1440-5.
28. Arroll B, Kenealy T. Antibiotics for the common cold. Cochrane Database Syst Rev 2000;CD000247.-
29. Scott J, Cohen D, Dicicco-Bloom B, Orzano A, Jaen C, Crabtree BF. Unnecessary antibiotic use in acute respiratory Infections. JAMA 2001; In press.
30. Hamm RM, Hicks RJ, Bemben DA. Antibiotics and respiratory infections: do antibiotic prescriptions improve outcomes? J Okla State Med Assoc 1996;89:267-74.
31. Mainous AG, 3rd, Zoorob RJ, Oler MJ, Haynes DM. Patient knowledge of upper respiratory infections: implications for antibiotic expectations and unnecessary utilization. J Fam Pract 1997;45:75-83.
32. Little P, Williamson I, Warner G, Gould C, Gantley M, Kinmonth AL. Open randomised trial of prescribing strategies in managing sore throat. BMJ 1997;314:722-7.
33. Macfarlane JT, Holmes WF, Macfarlane RM. Reducing reconsultations for acute lower respiratory tract illness with an information leaflet: a randomized controlled study of patients in primary care. Br J Gen Pract 1997;47:719-22.
34. Sanchez-Menegay C, Hudes ES, Cummings SR. Patient expectations and satisfaction with medical care for upper respiratory infections. J Gen Intern Med 1992;7:432-4.
35. Sperber SJ, Hendley JO, Hayden FG, Riker DK, Sorrentino JV, Gwaltney JM, Jr. Effects of naproxen on experimental rhinovirus colds. A randomized, double-blind, controlled trial. Ann Intern Med 1992;117:37-41.
36. Douglas RM, Chalker EB, Treacy B. Vitamin C for preventing and treating the common cold. Cochrane Database Syst Rev 2000;2.-
37. Marshall I. Zinc for the common cold. Cochrane Database Syst Rev 2000;2.-
38. Prasad AS, Fitzgerald JT, Bao B, Beck FW, Chandrasekar PH. Duration of symptoms and plasma cytokine levels in patients with the common cold treated with zinc acetate. A randomized, double-blind, placebo-controlled trial. Ann Intern Med 2000;133:245-52.
39. Melchart D, Linde K, Fischer P, Kaesmayr J. Echinacea for preventing and treating the common cold. Cochrane Database Syst Rev 2000;2.-
40. Puhakka T, Makela MJ, Malmstrom K, et al. The common cold: effects of intranasal fluticasone propionate treatment. J Allergy Clin Immunol 1998;101:726-31.
41. Williams JW, Jr, Aguilar C, Makela M, et al. Antibiotics for acute maxillary sinusitis. Cochrane Database Syst Rev 2000;CD000243.-
42. Lindbaek M, Hjortdahl P, Johnsen UL. Randomised, double blind, placebo controlled trial of penicillin V and amoxycillin in treatment of acute sinus infections in adults. BMJ 1996;313:325-9.
43. Hansen JG, Schmidt H, Grinsted P. Randomised, double blind, placebo controlled trial of penicillin V in the treatment of acute maxillary sinusitis in adults in general practice. Scand J Prim Health Care 2000;18:44-7.
44. Garbutt JMMF, Goldstein MDDS, Gellman EMD, Shannon WP, Littenberg BMD. A randomized, placebo-controlled trial of antimicrobial treatment for children with clinically diagnosed acute sinusitis. Pediatrics April 2001;107:619-625.
45. Altman KW, Austin MB, Tom LW, Knox GW. Complications of frontal sinusitis in adolescents: case presentations and treatment options. Int J Pediatr Otorhinolaryngol 1997;41:9-20.
46. Giannoni CM, Stewart MG, Alford EL. Intracranial complications of sinusitis. Laryngoscope 1997;107:863-7.
47. Hayden FG, Diamond L, Wood PB, Korts DC, Wecker MT. Effectiveness and safety of intranasal ipratropium bromide in common colds. A randomized, double-blind, placebo-controlled trial. Ann Intern Med 1996;125:89-97.
48. Gwaltney JM, Druce HM. Efficacy of brompheniramine maleate for the treatment of rhinovirus colds. Clin Infect Dis 1997;25:1188-94.
49. Clemens CJ, Taylor JA, Almquist JR, Quinn HC, Mehta A, Naylor GS. Is an antihistamine-decongestant combination effective in temporarily relieving symptoms of the common cold in preschool children? J Pediatr 1997;130:463-6.
50. Taverner D, Bickford L, Draper M. Nasal decongestants for the common cold. Cochrane Database Syst Rev 2000;2.-
51. Singh M. Heated, humidified air for the common cold. Cochrane Database Syst Rev;2000;CD001728.-
52. Del Mar CB, Glasziou PP, Spinks AB. Antibiotics for sore throat. Cochrane Database Syst Rev;2000;CD000023.-
53. Olivier C. Rheumatic fever—is it still a problem? J Antimicrob Chemother 2000;45 Suppl:13-21.
54. Howie JG, Foggo BA. Antibiotics, sore throats and rheumatic fever. J R Coll Gen Pract 1985;35:223-4.
55. Del Mar C, Glasziou P, Hayem M. Are antibiotics indicated as initial treatment for children with acute otitis media? A meta-analysis. BMJ 1997;314:1526-9.
56. Schachtel BP, Fillingim JM, Thoden WR, Lane AC, Baybutt RI. Sore throat pain in the evaluation of mild analgesics. Clin Pharmacol Ther 1988;44:704-11.
57. Schachtel BP, Fillingim JM, Lane AC, Thoden WR, Baybutt RI. Caffeine as an analgesic adjuvant. A double-blind study comparing aspirin with caffeine to aspirin and placebo in patients with sore throat. Arch Intern Med 1991;151:733-7.
58. Wethington JF. Double-blind study of benzydamine hydrochloride, a new treatment for sore throat. Clinical Therapeutics 1985;7:641-6.
59. Watson N, Nimmo WS, Christian J, Charlesworth A, Speight J, Miller K. Relief of sore throat with the anti-inflammatory throat lozenge flurbiprofen 8.75 mg: a randomised, double-blind, placebo-controlled study of efficacy and safety. Int J Clin Pract 2000;54:490-6.
60. Schachtel BP, Thoden WR. A placebo-controlled model for assaying systemic analgesics in children. Pract Clin Pharmacol Ther 1993;53:593-601.
61. Kagan G, Huddlestone L, Wolstencroft P. Two lozenges containing benzocaine assessed in the relief of sore throat. J Int Med Res 1982;10:443-6.
62. Gonzales R, Bartlett JG, Besser RE, et al. Principles of appropriate antibiotic use for treatment of uncomplicated acute bronchitis: background. Ann Intern Med 2001;134:521-9.
63. Eccles R, Morris S, Jawad M. Lack of effect of codeine in the treatment of cough associated with acute upper respiratory tract infection. J Clin Pharm Ther 1992;17:175-80.
64. Croughan-Minihane MS, Petitti DB, Rodnick JE, Eliaser G. Clinical trial examining effectiveness of three cough syrups. J Am Board Fam Pract 1993;6:109-15.
65. Chang AB, Phelan PD, Carlin JB, Sawyer SM, Robertson CF. A randomised, placebo controlled trial of inhaled salbutamol and beclomethasone for recurrent cough. Arch Dis Child 1998;79:6-11.
66. Hueston WJ. A comparison of albuterol and erythromycin for the treatment of acute bronchitis. J Fam Pract 1991;33:476-80.
67. Hueston WJ. Albuterol delivered by metered-dose inhaler to treat acute bronchitis. J Fam Pract 1994;39:437-40.
68. Smucny J, Flynn C, Becker L, Glazier R. Beta2-agonists for acute bronchitis [Systematic Review]. Cochrane Database of Systematic Reviews 2001;1.-
69. Gwaltney JM, Jr, Phillips CD, Miller RD, Riker DK. Computed tomographic study of the common cold. N Engl J Med 1994;330:25-30.
70. Holmes WF, Macfarlane JT, Macfarlane RM, Hubbard R. Symptoms, signs, and prescribing for acute lower respiratory tract illness. Br J Gen Pract 2001;51:177-81.
71. Gulbrandsen P, Fugelli P, Kvarstein G, Moland L. The duration of acute respiratory tract infections in children. Scand J Prim Health Care 1989;7:219-23.
72. Gonzales R, Bartlett JG, Besser RE, Hickner JM, Hoffman JR, Sande MA. Principles of appropriate antibiotic use for treatment of nonspecific upper respiratory tract infections in adults: background. Ann Intern Med 2001;134:490-4.
73. Gadomski AM. Potential interventions for preventing pneumonia among young children: lack of effect of antibiotic treatment for upper respiratory infections. Pediatr Infect Dis J 1993;12:115-20.
74. Heikkinen T, Ruuskanen O, Ziegler T, Waris M, Puhakka H. Short-term use of amoxicillin-clavulanate during upper respiratory tract infection for prevention of acute otitis media. J Pediatr 1995;126:313-6.
1. Gonzales R, Bartlett JG, Besser RE, Richelle JC, Hickner J, Jerome R, Hoffman JR. Principles of appropriate antibiotic use for treatment of acute respiratory tract infections in adults. Ann Intern Med 2001;134.-
2. Hueston WJ, Mainous AG, 3rd, Dacus EN, Hopper JE. Does acute bronchitis really exist? A reconceptualization of acute viral respiratory infections. J Fam Pract 2000;49:401-6.
3. Woodwell DA. National Ambulatory Medical Care Survey: 1996 summary. Adv Data 1997;1-25.
4. Gonzales R, Steiner JF, Sande MA. Antibiotic prescribing for adults with colds, upper respiratory tract infections, and bronchitis by ambulatory care physicians. JAMA 1997;278:901-4.
5. Mainous AG, 3rd, Hueston WJ. The cost of antibiotics in treating upper respiratory tract infections in a Medicaid population . Arch Fam Med 1998;7:45-9.
6. Makela MJ, Puhakka T, Ruuskanen O, et al. Viruses and bacteria in the etiology of the common cold. J Clin Microbiol 1998;36:539-42.
7. Ruoff G. Upper respiratory tract infections in family practice. Pediatr Infect Dis J 1998;17:S73-8.
8. Dowell SF, Schwartz B, Phillips WR. Appropriate use of antibiotics for URIs in children: Part II. Cough, pharyngitis and the common cold. The Pediatric URI Consensus Team. Am Fam Physician 1998;58:1335-42, 1345.
9. Gonzales R, Bartlett JG, Besser RE, et al. Principles of appropriate antibiotic use for treatment of acute respiratory tract infections in adults: background, specific aims, and methods. Ann Intern Med 2001;134:479-86.
10. Lindbaek M, Hjortdahl P, Johnsen UL. Use of symptoms, signs, and blood tests to diagnose acute sinus infections in primary care: comparison with computed tomography. Fam Med 1996;28:183-8.
11. Heckerling PS, Tape TG, Wigton RS, et al. Clinical prediction rule for pulmonary infiltrates. Ann Intern Med 1990;113:664-70.
12. Hansen JG, Schmidt H, Rosborg J, Lund E. Predicting acute maxillary sinusitis in a general practice population. BMJ 1995;311:233-6.
13. Williams JW, Jr, Simel DL, Roberts L, Samsa GP. Clinical evaluation for sinusitis. Making the diagnosis by history and physical examination. Ann Intern Med 1992;117:705-10.
14. Hickner JM, Bartlett JG, Besser RE, Gonzales R, Hoffman JR, Sande MA. Principles of appropriate antibiotic use for acute rhinosinusitis in adults: background. Ann Intern Med 2001;134:498-505.
15. Gunnarsson RK, Holm SE, Soderstrom M. The prevalence of potential pathogenic bacteria in nasopharyngeal samples from individuals with a respiratory tract infection and a sore throat—implications for the diagnosis of pharyngotonsillitis. Fam Pract 2001;18:266-71.
16. Pokorski SJ, Vetter EA, Wollan PC, Cockerill FR, 3rd. Comparison of Gen-Probe Group A streptococcus Direct Test with culture for diagnosing streptococcal pharyngitis. J Clin Microbiol 1994;32:1440-3.
17. Dagnelie CF, Bartelink ML, van der Graaf Y, Goessens W, de Melker RA. Towards a better diagnosis of throat infections (with group A beta-haemolytic streptococcus) in general practice. Br J Gen Pract 1998;48:959-62.
18. Hart AP, Buck LL, Morgan S, Saverio S, McLaughlin JC. A comparison of the BioStar Strep A OIA rapid antigen assay, group A Selective Strep Agar (ssA), and Todd-Hewitt broth cultures for the detection of group A Streptococcus in an outpatient family practice setting. Diagn Microbiol Infect Dis 1997;29:139-45.
19. Smith TD, Wilkinson V, Kaplan EL. Group A streptococcus-associated upper respiratory tract infections in a day-care center. Pediatrics 1989;83:380-4.
20. Drulak M, Raybould TJ, Yong J, Hsiung D, Smith H, Winston S. Comparison of Visuwell enzyme immunoassay to culture for detection of group A Streptococcus in throat swab specimens. Diagn Microbiol Infect Dis 1988;11:181-7.
21. Ebell MH SM, Barry HC, Ives K, Carey BS. Does this patient have strep throat? JAMA 2000;284:2912-2918.
22. McIsaac WJ, Goel V, To T, Low DE. The validity of a sore throat score in family practice. CMAJ 2000;163:811-5.
23. McIsaac WJ, White D, Tannenbaum D, Low DE. A clinical score to reduce unnecessary antibiotic use in patients with sore throat. CMAJ 1998;158:75-83.
24. Reed BD, Huck W, French T. Diagnosis of group A beta-hemolytic Streptococcus using clinical scoring criteria, Directigen 1-2-3 group A streptococcal test, and culture. Arch Intern Med 1990;150:1727-32.
25. Aronson MD, Komaroff AL, Pass TM, Ervin CT, Branch WT. Heterophil antibody in adults with sore throat: frequency and clinical presentation. Ann Intern Med 1982;96:505-8.
26. Diehr P, Wood RW, Bushyhead J, Krueger L, Wolcott B, Tompkins RK. Prediction of pneumonia in outpatients with acute cough—a statistical approach. J Chronic Dis 1984;37:215-25.
27. Metlay JP, Kapoor WN, Fine MJ. Does this patient have community-acquired pneumonia? Diagnosing pneumonia by history and physical examination. JAMA 1997;278:1440-5.
28. Arroll B, Kenealy T. Antibiotics for the common cold. Cochrane Database Syst Rev 2000;CD000247.-
29. Scott J, Cohen D, Dicicco-Bloom B, Orzano A, Jaen C, Crabtree BF. Unnecessary antibiotic use in acute respiratory Infections. JAMA 2001; In press.
30. Hamm RM, Hicks RJ, Bemben DA. Antibiotics and respiratory infections: do antibiotic prescriptions improve outcomes? J Okla State Med Assoc 1996;89:267-74.
31. Mainous AG, 3rd, Zoorob RJ, Oler MJ, Haynes DM. Patient knowledge of upper respiratory infections: implications for antibiotic expectations and unnecessary utilization. J Fam Pract 1997;45:75-83.
32. Little P, Williamson I, Warner G, Gould C, Gantley M, Kinmonth AL. Open randomised trial of prescribing strategies in managing sore throat. BMJ 1997;314:722-7.
33. Macfarlane JT, Holmes WF, Macfarlane RM. Reducing reconsultations for acute lower respiratory tract illness with an information leaflet: a randomized controlled study of patients in primary care. Br J Gen Pract 1997;47:719-22.
34. Sanchez-Menegay C, Hudes ES, Cummings SR. Patient expectations and satisfaction with medical care for upper respiratory infections. J Gen Intern Med 1992;7:432-4.
35. Sperber SJ, Hendley JO, Hayden FG, Riker DK, Sorrentino JV, Gwaltney JM, Jr. Effects of naproxen on experimental rhinovirus colds. A randomized, double-blind, controlled trial. Ann Intern Med 1992;117:37-41.
36. Douglas RM, Chalker EB, Treacy B. Vitamin C for preventing and treating the common cold. Cochrane Database Syst Rev 2000;2.-
37. Marshall I. Zinc for the common cold. Cochrane Database Syst Rev 2000;2.-
38. Prasad AS, Fitzgerald JT, Bao B, Beck FW, Chandrasekar PH. Duration of symptoms and plasma cytokine levels in patients with the common cold treated with zinc acetate. A randomized, double-blind, placebo-controlled trial. Ann Intern Med 2000;133:245-52.
39. Melchart D, Linde K, Fischer P, Kaesmayr J. Echinacea for preventing and treating the common cold. Cochrane Database Syst Rev 2000;2.-
40. Puhakka T, Makela MJ, Malmstrom K, et al. The common cold: effects of intranasal fluticasone propionate treatment. J Allergy Clin Immunol 1998;101:726-31.
41. Williams JW, Jr, Aguilar C, Makela M, et al. Antibiotics for acute maxillary sinusitis. Cochrane Database Syst Rev 2000;CD000243.-
42. Lindbaek M, Hjortdahl P, Johnsen UL. Randomised, double blind, placebo controlled trial of penicillin V and amoxycillin in treatment of acute sinus infections in adults. BMJ 1996;313:325-9.
43. Hansen JG, Schmidt H, Grinsted P. Randomised, double blind, placebo controlled trial of penicillin V in the treatment of acute maxillary sinusitis in adults in general practice. Scand J Prim Health Care 2000;18:44-7.
44. Garbutt JMMF, Goldstein MDDS, Gellman EMD, Shannon WP, Littenberg BMD. A randomized, placebo-controlled trial of antimicrobial treatment for children with clinically diagnosed acute sinusitis. Pediatrics April 2001;107:619-625.
45. Altman KW, Austin MB, Tom LW, Knox GW. Complications of frontal sinusitis in adolescents: case presentations and treatment options. Int J Pediatr Otorhinolaryngol 1997;41:9-20.
46. Giannoni CM, Stewart MG, Alford EL. Intracranial complications of sinusitis. Laryngoscope 1997;107:863-7.
47. Hayden FG, Diamond L, Wood PB, Korts DC, Wecker MT. Effectiveness and safety of intranasal ipratropium bromide in common colds. A randomized, double-blind, placebo-controlled trial. Ann Intern Med 1996;125:89-97.
48. Gwaltney JM, Druce HM. Efficacy of brompheniramine maleate for the treatment of rhinovirus colds. Clin Infect Dis 1997;25:1188-94.
49. Clemens CJ, Taylor JA, Almquist JR, Quinn HC, Mehta A, Naylor GS. Is an antihistamine-decongestant combination effective in temporarily relieving symptoms of the common cold in preschool children? J Pediatr 1997;130:463-6.
50. Taverner D, Bickford L, Draper M. Nasal decongestants for the common cold. Cochrane Database Syst Rev 2000;2.-
51. Singh M. Heated, humidified air for the common cold. Cochrane Database Syst Rev;2000;CD001728.-
52. Del Mar CB, Glasziou PP, Spinks AB. Antibiotics for sore throat. Cochrane Database Syst Rev;2000;CD000023.-
53. Olivier C. Rheumatic fever—is it still a problem? J Antimicrob Chemother 2000;45 Suppl:13-21.
54. Howie JG, Foggo BA. Antibiotics, sore throats and rheumatic fever. J R Coll Gen Pract 1985;35:223-4.
55. Del Mar C, Glasziou P, Hayem M. Are antibiotics indicated as initial treatment for children with acute otitis media? A meta-analysis. BMJ 1997;314:1526-9.
56. Schachtel BP, Fillingim JM, Thoden WR, Lane AC, Baybutt RI. Sore throat pain in the evaluation of mild analgesics. Clin Pharmacol Ther 1988;44:704-11.
57. Schachtel BP, Fillingim JM, Lane AC, Thoden WR, Baybutt RI. Caffeine as an analgesic adjuvant. A double-blind study comparing aspirin with caffeine to aspirin and placebo in patients with sore throat. Arch Intern Med 1991;151:733-7.
58. Wethington JF. Double-blind study of benzydamine hydrochloride, a new treatment for sore throat. Clinical Therapeutics 1985;7:641-6.
59. Watson N, Nimmo WS, Christian J, Charlesworth A, Speight J, Miller K. Relief of sore throat with the anti-inflammatory throat lozenge flurbiprofen 8.75 mg: a randomised, double-blind, placebo-controlled study of efficacy and safety. Int J Clin Pract 2000;54:490-6.
60. Schachtel BP, Thoden WR. A placebo-controlled model for assaying systemic analgesics in children. Pract Clin Pharmacol Ther 1993;53:593-601.
61. Kagan G, Huddlestone L, Wolstencroft P. Two lozenges containing benzocaine assessed in the relief of sore throat. J Int Med Res 1982;10:443-6.
62. Gonzales R, Bartlett JG, Besser RE, et al. Principles of appropriate antibiotic use for treatment of uncomplicated acute bronchitis: background. Ann Intern Med 2001;134:521-9.
63. Eccles R, Morris S, Jawad M. Lack of effect of codeine in the treatment of cough associated with acute upper respiratory tract infection. J Clin Pharm Ther 1992;17:175-80.
64. Croughan-Minihane MS, Petitti DB, Rodnick JE, Eliaser G. Clinical trial examining effectiveness of three cough syrups. J Am Board Fam Pract 1993;6:109-15.
65. Chang AB, Phelan PD, Carlin JB, Sawyer SM, Robertson CF. A randomised, placebo controlled trial of inhaled salbutamol and beclomethasone for recurrent cough. Arch Dis Child 1998;79:6-11.
66. Hueston WJ. A comparison of albuterol and erythromycin for the treatment of acute bronchitis. J Fam Pract 1991;33:476-80.
67. Hueston WJ. Albuterol delivered by metered-dose inhaler to treat acute bronchitis. J Fam Pract 1994;39:437-40.
68. Smucny J, Flynn C, Becker L, Glazier R. Beta2-agonists for acute bronchitis [Systematic Review]. Cochrane Database of Systematic Reviews 2001;1.-
69. Gwaltney JM, Jr, Phillips CD, Miller RD, Riker DK. Computed tomographic study of the common cold. N Engl J Med 1994;330:25-30.
70. Holmes WF, Macfarlane JT, Macfarlane RM, Hubbard R. Symptoms, signs, and prescribing for acute lower respiratory tract illness. Br J Gen Pract 2001;51:177-81.
71. Gulbrandsen P, Fugelli P, Kvarstein G, Moland L. The duration of acute respiratory tract infections in children. Scand J Prim Health Care 1989;7:219-23.
72. Gonzales R, Bartlett JG, Besser RE, Hickner JM, Hoffman JR, Sande MA. Principles of appropriate antibiotic use for treatment of nonspecific upper respiratory tract infections in adults: background. Ann Intern Med 2001;134:490-4.
73. Gadomski AM. Potential interventions for preventing pneumonia among young children: lack of effect of antibiotic treatment for upper respiratory infections. Pediatr Infect Dis J 1993;12:115-20.
74. Heikkinen T, Ruuskanen O, Ziegler T, Waris M, Puhakka H. Short-term use of amoxicillin-clavulanate during upper respiratory tract infection for prevention of acute otitis media. J Pediatr 1995;126:313-6.
The Evaluation and Treatment of Tobacco Use Disorder
Tobacco use is the leading cause of preventable diseases and deaths in the United States, accounting for approximately 435,000 deaths yearly.1 Smoking is responsible for an estimated $100 billion annually in direct medical and indirect nonmedical costs.2 Despite widespread efforts to educate the public on the risks of smoking, approximately 50 million American adults still smoke cigarettes.3 Cigarette smoking is an addiction, as powerful in many respects as cocaine or opiate dependence.4 Among those who have ever tried even one cigarette, almost one third develop nicotine dependence.5 Every year, primary care clinicians have access to 70% of smokers.6,7 One of the goals of Healthy People 20108 is to increase to 75% the proportion of primary care providers who routinely provide smoking cessation counseling.
Diagnosis
The Smoking Cessation Clinical Practice Guideline was originally published by the Agency for Healthcare Research and Quality (AHRQ) in 19969 and was updated in 2000 by AHRQ and a consortium of 7 government and nonprofit organizations.10 The 2000 guideline urged clinicians to treat tobacco use disorder as a chronic disease similar in many respects to other diseases like hypertension, diabetes, and hyperlipidemia and to provide patients with appropriate advice and pharmacotherapy. The updated guideline recommends a 5-step approach (the 5A’s: ask, advise, assess, assist, and arrange) to be used by primary care physicians. The first step (ask) is key in the management of tobacco use disorder. Tobacco use status should be asked and documented for all patients at every visit. The AHRQ recommends that tobacco use status be adopted as the “5th vital sign” along with blood pressure, temperature, pulse, and respiration. Data show that only approximately half of physicians in nonresearch settings consistently advise smokers to quit.11-13
Because the most common presentation of a smoker in the primary care setting is for general medical care not necessarily related to smoking, the recommendation to ask about tobacco use at every visit is a practical method to ensure early identification of smokers. Asking about tobacco use at every visit has been shown to result in better screening14-16 and increased cessation rates.17 Screening can be performed by the nurse or other trained member of the office staff who collects clinical information from the patient before being seen by the physician. Physicians should establish office-wide systems to enhance consistent identification and treatment of smokers in their practices. Organizational system approaches are cost-effective and have been shown to increase delivery of cessation interventions.18
Treatment
After identifying smokers during an office visit (ask), the next step is to strongly urge all smokers to quit (advise). Such initial advice should be given regardless of the patient’s state of readiness to quit. The transtheoretical model of stages of change (SOC)19 is useful for assessing the patient’s readiness to quit (assess). The SOC model identifies smoking behavior change as a process involving movement through a series of 5 motivational stages including precontemplation (not planning to quit within next 6 months), contemplation (planning to quit within next 6 months), preparation (planning to quit within next 30 days), action (has quit smoking for less than 6 months), and maintenance (has quit smoking for 6 months or longer). Interventions based on the SOC have been shown to enhance motivation20 and predict cessation.21 For patients unwilling to quit, physicians should identify reasons for resistance. For example, patients who are misinformed about the health risks of smoking should be provided with information relevant to their (or their family’s) health condition. Patients willing to make a quit attempt should be given specific advice about how to proceed, including setting a quit date and information on pharmacotherapy.
Behavioral interventions are beneficial to the long-term success of smoking cessation. Studies have shown that brief ( 5 minutes) advice on quitting given by physicians to smokers during an office visit have resulted in higher quit rates compared with no advice.22 A review of 20 studies conducted in primary care settings23 reported that 2% of all smokers who received brief physician advice quit smoking as a direct consequence, compared with less than 1% in smokers who received no advice. With additional encouragement and support (eg, follow-up letters, phone calls, demonstration of spirometry, and additional visits) quit rates increased to 5%.23,24 A more recent meta-analysis of 7 studies by the Clinical Practice Guideline Panel reported an abstinence rate of 8% when no cessation advice was given, compared with 10% with cessation advice.17 Although success rates are better with more intensive counseling, brief interventions appear to be more feasible in the primary care setting, given time constraints experienced by primary care physicians during office visits25 and the unwillingness of many patients to enter intensive programs.26
A recent Cochrane review27 found that group therapy is more effective than self-help materials but is not consistently better than personal contact. Although groups are theoretically more cost-effective, their usefulness may be limited by participant recruitment and retention problems.28,29 Current evidence does not support efficacy of acupuncture or hypnosis as treatment for smoking cessation.17,30
Pharmacotherapy
Because success rates associated with nonpharmacologic treatments are generally lower, pharmacotherapy should be offered to every smoker willing to make a quit attempt unless there is a medical contraindication.10 The 5 pharmacologic agents approved by the US Food and Drug Administration for treatment of tobacco use disorder include 4 nicotine replacement therapies (NRT)—gum, patch, spray, inhaler—and one non-nicotine therapy, bupropion. All 5 agents promote similar success rates in long-term smoking cessation if they are prescribed to meet the needs of the individual smoker.31
Nicotine gum. A meta-analysis of randomized controlled trials among specialized cessation clinics found higher success rates for patients treated with nicotine gum compared with use of placebo gum at 6 months (27% vs 18%).32 This is in contrast with studies conducted in general medical practices, where success rates (12%) with nicotine gum at 6 months were no different from placebo.33 Higher quit rates in specialized smoking cessation clinics may be a result of more in-depth counseling, better-trained counselors, and inclusion of smokers with a higher motivation to quit. The gum is available without prescription and comes in 2-mg and 4-mg doses. The 4-mg dose is more efficacious in more dependent smokers.34 Treatment is recommended for 8 weeks.
Nicotine patches. The patches have been shown to be effective under controlled as well as real-world settings.35-37 A meta-analysis of 17 randomized trials estimated the efficacy of the nicotine patch as 27% at end of treatment and 22% at 6 months compared with 13% and 9%, respectively, for placebo.38 Treatment beyond 8 weeks did not increase efficacy. The patches are available in 16-mg and 21-mg dosages (with 14-mg and 7-mg step-down doses). Although weaning is strongly encouraged by most marketers of nicotine patches, current data do not support added beneficial effect for this step-down approach.38 The highest dose should be used for those who smoke more than 10 cigarettes per day and reduced dosage forms for light smokers. The optimal dosage for light smokers is not known because of limited data in this group. The patches are contraindicated for patients with systemic eczema, unstable angina, and within 1 month of a myocardial infarction.
Nicotine nasal spray. Abstinence rate at 6 months from meta-analysis was 31% for the spray compared with 14% for placebo.17 A dose is one spray into each nostril; each spray delivers 0.5 mg of nicotine. Patients should use 1 to 2 doses every hour for 6 to 8 weeks. A drawback is that the spray seems to have the highest addictive potential of all NRTs.39,40 Patients who experience withdrawal symptoms with abrupt cessation of treatment should be considered for 4 to 6 weeks of tapering. Tapering could be achieved by reducing the dose by half every week. The most commonly reported side effects of the nicotine nasal spray include nasal irritation, runny nose, sneezing, throat irritation, coughing, and watery eyes. Patients usually develop tolerance to these effects within the first week.
Nicotine inhaler. A unique feature of the nicotine inhaler is that it mimics the hand-to-mouth routine similar to cigarette smoking and may therefore reduce fears associated with abrupt cessation of the hand-to-mouth ritual. The inhaler consists of a plastic mouthpiece to which a cartridge is attached. The cartridge contains 10 mg (but delivers only 4 mg) of nicotine plus 1 mg of menthol. The inhaler is different from typical inhalers in that patients puff on the mouthpiece, and nicotine is absorbed in the mouth rather than the lungs. Abstinence rates at 6 months were 23% for the inhaler and 11% for placebo.17 Recommended dosage is 6 to 16 cartridges per day for 8 weeks. Patients should self-titrate their dosing based on severity of withdrawal symptoms experienced. Adverse events are generally mild, consisting of throat irritation and cough.
Bupropion. This is an alternative for smokers who either cannot tolerate nicotine replacement therapy or prefer non-nicotine treatment. The efficacy of bupropion for smoking cessation has been demonstrated in 2 randomized controlled trials.41,42 Abstinence rates at 6 months were approximately 30% for bupropion versus 17% for placebo. Common adverse effects are generally mild, consisting of insomnia and dry mouth; headache and tremors are less common. This drug is contraindicated for patients with history of seizures, anorexia or bulimia, head trauma, or heavy alcohol use, and is category B for pregnancy.
Combination drug therapy. Combining the nicotine patch with a self-administered form of nicotine (eg, gum, spray, inhaler) is more efficacious than a single NRT.17 One randomized trial also showed that bupropion combined with the patch was more efficacious than the patch alone but not significantly better than bupropion alone. Combination treatments should be considered for smokers unable to quit because of significant craving or withdrawal despite adequate doses of single agents.
Other recommended pharmacotherapies. The 2000 clinical practice guidelines recommended the use of clonidine hydrochloride and nortriptyline hydrochloride as second-line agents. Controlled studies on both agents are limited,43-48 and neither agent is approved by the United States Food and Drug Administration for smoking cessation. Clonidine or nortriptyline should only be considered for patients who failed the first-line drugs or are unable to use them because of contraindications. Adverse events are generally more than for first-line agents.
Choice of Treatment
Few data exist on the comparative efficacy of the 5 approved pharmacotherapy aids Table 1. The STEPS (safety, tolerability, efficacy, price, simplicity) approach can be used to guide physicians in the choice of pharmacologic agents. All NRTs are considered generally safe, and adverse effects associated with their use are mild. The NRTs have similar cardiovascular precautions (ie, avoid use in unstable angina and within 1 month of a myocardial infarction), are pregnancy category D (there is evidence of human fetal risk, but use is acceptable if benefits outweigh risks and safer alternatives are unavailable or ineffective), except the gum, which is category C (animal studies have revealed adverse effects on the fetus, but there are no controlled studies in women) and should be used during pregnancy only if nonpharmacologic approaches are unsuccessful. Bupropion is also relatively safe with precaution as discussed earlier. Product-specific characteristics could make some NRTs less suitable for certain patients. For example, the gum is not appropriate for patients with dental or jaw problems and may be difficult to use correctly, since it requires special chewing techniques and high frequency of use. Very humid weather conditions may affect adhesiveness of the patch. The patch should also be avoided in patients with systemic eczema.
The only study that compared the efficacy of various NRTs reported similar results for all 4 NRTs. Although one study reported superior efficacy for bupropion over the patch,49 this finding has not been replicated. Bupropion costs slightly less than the NRTs Table 2. Of the NRTs, the patch appears to be the most convenient to use. In one randomized controlled trial,50 compliance was highest for the patch (82%) compared with the gum (38%), the spray (15%), and the inhaler (11%). A limitation common to all smoking cessation pharmacologic trials is that participants were volunteers with higher motivation to quit smoking and willing to comply with frequent follow-up contacts required in clinical trials. The effectiveness of these medications in real world settings may be lower than that reported in clinical trials. Also, the placebo arms in these trials typically receive substantially more counseling than what happens in real world settings. These factors combined produce higher quit rates in placebo patients than that found in typical unaided quit attempts. Physicians should consider using an algorithm Figure 1 to assist them in approach to and treatment of smokers.
Follow-up
Relapse is quite common among smokers trying to quit. On average, it takes 4 to 5 quit attempts before a smoker is successful.4 For this reason the last step of the AHRQ recommendations (arrange, ie, make arrangement for follow-up care for smoking cessation), is very important. The follow-up contact should occur within 1 week of patient’s quit date, because the risk of relapse back to smoking is highest during the first few days of abstinence.51,52 There are considerable data showing that additional follow-up contact beyond initial brief advice significantly increases quit rates.23,53,54
A variety of follow-up methods have been used in clinical trials, including face-to-face contact with a physician or other health care professional, letters, telephone, and self-help materials. Nurses and other office staff could be trained and designated to perform some or all the follow-up contact.18,55-57 In a recently published randomized trial,58 office nurses were trained to provide telephone follow-up contacts for low-income Medicaid managed care smokers. Abstinence rates at 3 months were 21% and 8% for telephone follow-up and usual care, respectively.
In addition to office-based telephone and printed self-help resources, physicians should be aware of a growing number of free telephone helplines and Internet-based resources Table 3 available for people trying to quit smoking. Patients without personal Internet access should be encouraged to make use of such services available at most public libraries.
Prognosis
Smoking cessation significantly reduces most of the increased morbidity and mortality from smoking.59 The degree of improvement, however, depends on the disease process involved, the amount of damage produced, and the reversibility of this damage at the time of cessation. Former smokers reduce their risk of developing coronary heart disease by 50% within 1 year of quitting.59 After 4 years, this risk becomes equal to that of people who have never smoked. Improvement in cancer risk varies with the type of cancer involved. The risk of lung cancer in former smokers, for example, always remains higher than that for those who have never smoked. However, this risk decreases progressively and considerably with the number of years of abstinence.59
In addition to reduction in morbidity and mortality, smoking cessation is among the most cost-effective measures in primary care. Studies have shown that the cost-effectiveness of physician smoking cessation counseling is similar to the treatment of mild to moderate hypertension or hypercholesterolemia.60 The estimated cost per year of life saved is $2000 for smoking cessation compared with $50,000 for screening mammography for breast cancer.61 Given the proven effectiveness of available smoking cessation interventions and the ready access primary care physicians have to smokers, effectively addressing tobacco use in primary care settings has a great potential of reducing tobacco-related morbidity and mortality.
Acknowledgments
Our work was supported by a grant from the Cancer Research Foundation of America (Dr Okuyemi) and a Robert Wood Johnson Foundation Generalist Physician Faculty Scholars Award (Dr Ahluwalia, #032586). Dr Wadland has received funding in the past from SmithKline-Beecham and Glaxo-Wellcome for nicotine replacement in community clinical trials. Dr Ahluwalia has received honoraria for presentations from Glaxo-Wellcome, Inc and Pharmacia Upjohn, Inc. Dr Okuyemi has received an honorarium for educational purposes from SmithKline Beecham.
1. Centers for Disease Control and Prevention. Smoking: attributable mortality and years of potential life lost—United States, 1984. MMWR 1997;46:444-51.
2. Centers for Disease Control and Prevention. Economic consequences of smoking: direct medical costs. MMWR 1994;43:469-72.
3. Center for Disease Control and Prevention. Cigarette smoking among adults. MMWR 1999;48:993-96.
4. US Department of Health and Human Services. The health consequences of smoking: nicotine addiction. A report of the Surgeon General. DHHS pub. no. (CDC) 88-8406. 1988.
5. Anthony J, Warner L, Kessler R. Comparative epidemiology of dependence on tobacco, alcohol, controlled substances, and inhalants: basic findings from the National Comorbidity Survey. Exp Clin Psychopharmacol 1994;2:244-68.
6. Centers for Disease Control and Prevention. Physicians and other health care professional counseling of smokers to quit—United States, 1991. MMWR 1993;42:854-57.
7. Ockene JK. Physician-delivered interventions for smoking cessation: strategies for increasing effectiveness. Prev Med 1987;16:723-37.
8. US Department of Health and Human Services. Healthy people 2010: objectives for improving health. Washington, DC: USDHHS; 2000.
9. US Department of Health and Human Services. Smoking cessation: clinical practice guideline. Rockville, Md: Public Health Service, Agency for Health Care Policy and Research; 1996.
10. US Department of Health and Human Services. The tobacco use and dependence clinical practice guideline panel staff and consortium representatives: a clinical practice guideline for treating tobacco use and dependence. JAMA 2000;283:3244-54.
11. Anda RF, Remington PL, Sienko DG, Davis RM. Are physicians advising smokers to quit? the patient’s perspective. JAMA 1987;257:1916-19.
12. Wells KB, Lewis CE, Leake B, Schleiter MK, Brook RH. The practices of general and subspecialty internists in counseling about smoking and exercise. Am J Public Health 1986;76:1009-13.
13. Doescher MP, Saver BG. Physicians’ advice to quit smoking: the glass remains half empty. J Fam Pract 2000;49:543-47.
14. Ahluwalia JS, Gibson CA, Kenney RE, Wallace DD, Resnicow K. Smoking status as a vital sign. J Gen Intern Med 1999;14:402-08.
15. Fiore MC, Jorenby DE, Schensky AE, Smith SS, Bauer RR, Baker TB. Smoking status as the new vital sign: effect on assessment and intervention in patients who smoke. Mayo Clin Proceed 1995;70:209-13.
16. Robinson MD, Laurent SL, Little JM. Including smoking status as a new vital sign: it works. J Fam Pract 1995;40:556-61.
17. Fiore M, Bailey W, Cohen S, et al. Treating tobacco use and dependence: clinical practice guideline. Rockville, Md: US Department of Health and Human Services, Public Health Service; 2000.
18. Solberg LI, Maxwell PL, Kottke TE, Gepner GJ, Brekke ML. A systemic primary care office-based smoking cessation program. J Fam Pract 1990;30:647-54.
19. Prochaska JO, DiClemente CC. Stages and processes of self-change of smoking: toward an integrative model of change. J Consult Clin Psychol 1983;51:390-95.
20. Goldberg DN, Hoffman AM, Farinha MF, et al. Physician delivery of smoking-cessation advice based on the stages-of-change model. Am J Prev Med 1994;10:267-74.
21. Farkas AJ, Pierce JP, Zhu SH, et al. Addiction versus stages of change models in predicting smoking cessation. Addiction 1996;91:1271-80; discussion 1281-92.
22. Slama K, Redman S, Perkins J, Reid A, Sanson-Fisher RW. The effectiveness of two smoking cessation programs for use in general practice: a randomized clinical trial. BMJ 1990;300:1707-09.
23. Law M, Tang JL. An analysis of the effectiveness of interventions intended to help people stop smoking. Arch Intern Med 1995;155:1933-41.
24. Risser NL, Belcher DW. Adding spirometry, carbon monoxide, and pulmonary symptom results to smoking cessation counseling: a randomized trial. J Gen Intern Med 1990;5:16-22.
25. Gilchrist V, Miller RS, Gillanders WR, et al. Does family practice at residency teaching sites reflect community practice? J Fam Pract 1993;37:555-63.
26. Lichtenstein E, Hollis J. Patient referral to a smoking cessation program: who follows through? J Fam Pract 1992;34:739-44.
27. Stead LF, Lancaster T. Group behaviour therapy programmes for smoking cessation. Cochrane Database Syst Rev 2000;2.-
28. Hollis JF, Lichtenstein E, Vogt TM, Stevens VJ, Biglan A. Nurse-assisted counseling for smokers in primary care. Ann Intern Med 1993;118:521-25.
29. Lancaster T, Stead L, Silagy C, Sowden A. Effectiveness of interventions to help people stop smoking: findings from the Cochrane library. BMJ 2000;321:355-58.
30. Abbot NC, Stead LF, White AR, Barnes J, Ernst E. Hypnotherapy for smoking cessation. Cochrane Database Syst Rev 2000;2.-
31. Hughes J, Goldstein MG, Hurt RD, Shiffman S. Recent advances in the pharmacotherapy of smoking. JAMA 1999;281:72-76.
32. Cepeda-Benito A. Meta-analytical review of the efficacy of nicotine chewing gum in smoking treatment programs. J Consult Clin Psychol 1993;61:822-30.
33. Lam W, Sacks HS, Sze PC, Chalmers TC. Meta analysis of randomised controlled trials of nicotine chewing gum. Lancet 1987;2:27-30.
34. Sachs DPL. Effectiveness of the 4-mg dose of nicotine polacrilex for the initial treatment of high-dependent smokers. Arch Intern Med 1995;155:1973-80.
35. Jolicoeur DG, Ahluwalia JS, Richter KP, et al. The use of nicotine patches with minimal intervention. Prevent Med 2000;30:504-12.
36. Orleans CT, Resch N, Noll E, et al. Use of transdermal nicotine in a state-level prescription plan for the elderly. JAMA 1994;271:601-07.
37. Silagy C, Mant D, Fowler G, Lodge M. Meta-analysis on efficacy of nicotine replacement therapies in smoking cessation. Lancet 1994;343:139-42.
38. Fiore MC, Smith SS, Jorenby DE, Baker TB. The effectiveness of the nicotine patch for smoking cessation: a meta-analysis. JAMA 1994;271:1940-47.
39. Sutherland G, Stapleton JA, Russell MAH, et al. Randomised controlled trial of nasal nicotine spray in smoking cessation. Lancet 1992;340:324-29.
40. Hughes JR, Gust SW, Keenan R, Fenwick JW, Skoog K, Higgins ST. Long-term use of nicotine vs placebo gum. Arch Intern Med 1991;151:1993-98.
41. Hurt RD, Sachs DPL, Glover ED, Offord KP, et al. A comparison of sustained-release bupropion and placebo for smoking cessation. N Engl J Medicine. 1997;337:1195-202.
42. Jorenby DE, Leischow SJ, Nides MA, et al. A controlled trial of sustained-release bupropion, a nicotine patch, or both for smoking cessation. N Engl J Med 1999;340:685-91.
43. Glassman AH, Stetner F, Walsh T, et al. Heavy smokers smoking cessation and clonidine. JAMA 1988;259:2863-66.
44. Glassman AH, Covey LS, Dalack GW, et al. Smoking cessation, clonidine, and vulnerability to nicotine among dependent smokers. Clin Pharmacol Ther 1993;54:670-79.
45. Hilleman DE, Mohiuddin SM, Delcore MG, Lucas BD, Jr. Randomized, controlled trial of transdermal clonidine for smoking cessation. Ann Pharmacother 1993;27:1025-28.
46. Hao W, Young D, Wei H. Effect of clonidine on cigarette cessation and in the alleviation of withdrawal symptoms. Br J Addict 1988;83:1221-26.
47. Hall SM, Reus VI, Munoz RF, et al. Nortriptyline and cognitive-behavioral therapy in the treatment of cigarette smoking. Arch Gen Psychiatry 1998;55:683-90.
48. Prochazka AV, Weaver MJ, Keller RT, Fryer GE, Licari PA, Lofaso D. A randomized trial of nortriptyline for smoking cessation. Arch Intern Med 1998;158:2035-39.
49. Jorenby D, Leischow S, Nides M, et al. Bupropion alone or with a nicotine patch increased smoking cessation rates. N Engl J Med 1999;340:685-91.
50. Hajek P, West R, Foulds J, Nilsson F, Burrows S, Meadow A. Randomized comparative trial of nicotine polacrilex, a transdermal patch, nasal spray, and an inhaler. Arch Intern Med 1999;159:2033-38.
51. Hughes JR, Gulliver SB, Fenwick JW, et al. Smoking cessation among self-quitters. Health Psychol 1992;11:331-34.
52. Kottke TE, Brekke ML, Solberg LI, Hughes JR. A randomized trial to increase smoking intervention by physicians (Doctors helping smokers, round I). JAMA 1989;261:2101-06.
53. Smoking cessation in patients: two further studies by the British Thoracic Society. Research Committee of the British Thoracic Society. Thorax 1990;45:835-40.
54. Richmond R, Webster I. Evaluation of general practitioners’ use of a smoking intervention programme. Int J Epidemiol 1985;14:396-401.
55. Duncan C, Stein MJ, Cummings SR. Staff involvement and special follow-up time increase physicians’ counseling about smoking cessation: a controlled trial. Am J Public Health 1991;81:899-901.
56. Pine D, David C, Sauser M, Sullivan S. Effects of a systemic approach to tobacco cessation in a community-based practice. Arch Fam Med 1997;6:363-67.
57. Wadland WC, Stoffelmayr B, Berger E, Crombach A, Ives K. Enhancing smoking cessation rates in primary care. J Fam Pract 1999;48:711-18.
58. Wadland WC, Soffelmayr B, Ives K. Enhancing smoking cessation of low-income smokers in managed care. J Fam Pract 2001;50:138-44.
59. US Department of Health and Human Services. The health benefits of smoking cessation. Public Health Service; CDC: Center for Chronic Disease Prevention and Health Promotion; OSH. DHHS pub. no. (CDC) 90-8416; 1990.
60. Cummings SR, Rubin SM, Oster G. The cost-effectiveness of counseling smokers to quit. JAMA 1989;261:75-79.
61. Marwick C. Intensive smoking cessation efforts cost-effective. JAMA 1996;276:1291.-
62. Ascher JA, Cole JO, Colin J-N, Feighner JP, et al. Bupropion: a review of its mechanism of antidepressant activity. J Clin Psychiatry 1995;56:395-401.
63. Benowitz NL. Pharmacologic aspects of cigarette smoking and nicotine addiction. N Engl J Med 1988;319:1318-30.
64. Kornitzer M, Boutsen M, Dramaix M, Thijs J, Gustavsson G. Combined use of nicotine patch and gum in smoking cessation: a placebo-controlled clinical trial. Prev Med 1995;24:41-47.
65. Blondal T, Gudmundsson LJ, Olafsdottir I, Gustavsson G, Westin A. Nicotine nasal spray with nicotine patch for smoking cessation: randomised trial with six year follow-up. BMJ 1999;318:285-89.
66. Bohadana A, Nilsson F, Rasmussen T, Martinet Y. Nicotine inhaler and nicotine patch as a combination therapy for smoking cessation: a randomized double blind placebo controlled trial. Arch Intern Med 2000;160:3128-34.
67. Okuyemi KS, Ahluwalia JS, Harris KJ. Pharmacotherapy of smoking cessation. Arch Fam Med 2000;9:270-81.
Tobacco use is the leading cause of preventable diseases and deaths in the United States, accounting for approximately 435,000 deaths yearly.1 Smoking is responsible for an estimated $100 billion annually in direct medical and indirect nonmedical costs.2 Despite widespread efforts to educate the public on the risks of smoking, approximately 50 million American adults still smoke cigarettes.3 Cigarette smoking is an addiction, as powerful in many respects as cocaine or opiate dependence.4 Among those who have ever tried even one cigarette, almost one third develop nicotine dependence.5 Every year, primary care clinicians have access to 70% of smokers.6,7 One of the goals of Healthy People 20108 is to increase to 75% the proportion of primary care providers who routinely provide smoking cessation counseling.
Diagnosis
The Smoking Cessation Clinical Practice Guideline was originally published by the Agency for Healthcare Research and Quality (AHRQ) in 19969 and was updated in 2000 by AHRQ and a consortium of 7 government and nonprofit organizations.10 The 2000 guideline urged clinicians to treat tobacco use disorder as a chronic disease similar in many respects to other diseases like hypertension, diabetes, and hyperlipidemia and to provide patients with appropriate advice and pharmacotherapy. The updated guideline recommends a 5-step approach (the 5A’s: ask, advise, assess, assist, and arrange) to be used by primary care physicians. The first step (ask) is key in the management of tobacco use disorder. Tobacco use status should be asked and documented for all patients at every visit. The AHRQ recommends that tobacco use status be adopted as the “5th vital sign” along with blood pressure, temperature, pulse, and respiration. Data show that only approximately half of physicians in nonresearch settings consistently advise smokers to quit.11-13
Because the most common presentation of a smoker in the primary care setting is for general medical care not necessarily related to smoking, the recommendation to ask about tobacco use at every visit is a practical method to ensure early identification of smokers. Asking about tobacco use at every visit has been shown to result in better screening14-16 and increased cessation rates.17 Screening can be performed by the nurse or other trained member of the office staff who collects clinical information from the patient before being seen by the physician. Physicians should establish office-wide systems to enhance consistent identification and treatment of smokers in their practices. Organizational system approaches are cost-effective and have been shown to increase delivery of cessation interventions.18
Treatment
After identifying smokers during an office visit (ask), the next step is to strongly urge all smokers to quit (advise). Such initial advice should be given regardless of the patient’s state of readiness to quit. The transtheoretical model of stages of change (SOC)19 is useful for assessing the patient’s readiness to quit (assess). The SOC model identifies smoking behavior change as a process involving movement through a series of 5 motivational stages including precontemplation (not planning to quit within next 6 months), contemplation (planning to quit within next 6 months), preparation (planning to quit within next 30 days), action (has quit smoking for less than 6 months), and maintenance (has quit smoking for 6 months or longer). Interventions based on the SOC have been shown to enhance motivation20 and predict cessation.21 For patients unwilling to quit, physicians should identify reasons for resistance. For example, patients who are misinformed about the health risks of smoking should be provided with information relevant to their (or their family’s) health condition. Patients willing to make a quit attempt should be given specific advice about how to proceed, including setting a quit date and information on pharmacotherapy.
Behavioral interventions are beneficial to the long-term success of smoking cessation. Studies have shown that brief ( 5 minutes) advice on quitting given by physicians to smokers during an office visit have resulted in higher quit rates compared with no advice.22 A review of 20 studies conducted in primary care settings23 reported that 2% of all smokers who received brief physician advice quit smoking as a direct consequence, compared with less than 1% in smokers who received no advice. With additional encouragement and support (eg, follow-up letters, phone calls, demonstration of spirometry, and additional visits) quit rates increased to 5%.23,24 A more recent meta-analysis of 7 studies by the Clinical Practice Guideline Panel reported an abstinence rate of 8% when no cessation advice was given, compared with 10% with cessation advice.17 Although success rates are better with more intensive counseling, brief interventions appear to be more feasible in the primary care setting, given time constraints experienced by primary care physicians during office visits25 and the unwillingness of many patients to enter intensive programs.26
A recent Cochrane review27 found that group therapy is more effective than self-help materials but is not consistently better than personal contact. Although groups are theoretically more cost-effective, their usefulness may be limited by participant recruitment and retention problems.28,29 Current evidence does not support efficacy of acupuncture or hypnosis as treatment for smoking cessation.17,30
Pharmacotherapy
Because success rates associated with nonpharmacologic treatments are generally lower, pharmacotherapy should be offered to every smoker willing to make a quit attempt unless there is a medical contraindication.10 The 5 pharmacologic agents approved by the US Food and Drug Administration for treatment of tobacco use disorder include 4 nicotine replacement therapies (NRT)—gum, patch, spray, inhaler—and one non-nicotine therapy, bupropion. All 5 agents promote similar success rates in long-term smoking cessation if they are prescribed to meet the needs of the individual smoker.31
Nicotine gum. A meta-analysis of randomized controlled trials among specialized cessation clinics found higher success rates for patients treated with nicotine gum compared with use of placebo gum at 6 months (27% vs 18%).32 This is in contrast with studies conducted in general medical practices, where success rates (12%) with nicotine gum at 6 months were no different from placebo.33 Higher quit rates in specialized smoking cessation clinics may be a result of more in-depth counseling, better-trained counselors, and inclusion of smokers with a higher motivation to quit. The gum is available without prescription and comes in 2-mg and 4-mg doses. The 4-mg dose is more efficacious in more dependent smokers.34 Treatment is recommended for 8 weeks.
Nicotine patches. The patches have been shown to be effective under controlled as well as real-world settings.35-37 A meta-analysis of 17 randomized trials estimated the efficacy of the nicotine patch as 27% at end of treatment and 22% at 6 months compared with 13% and 9%, respectively, for placebo.38 Treatment beyond 8 weeks did not increase efficacy. The patches are available in 16-mg and 21-mg dosages (with 14-mg and 7-mg step-down doses). Although weaning is strongly encouraged by most marketers of nicotine patches, current data do not support added beneficial effect for this step-down approach.38 The highest dose should be used for those who smoke more than 10 cigarettes per day and reduced dosage forms for light smokers. The optimal dosage for light smokers is not known because of limited data in this group. The patches are contraindicated for patients with systemic eczema, unstable angina, and within 1 month of a myocardial infarction.
Nicotine nasal spray. Abstinence rate at 6 months from meta-analysis was 31% for the spray compared with 14% for placebo.17 A dose is one spray into each nostril; each spray delivers 0.5 mg of nicotine. Patients should use 1 to 2 doses every hour for 6 to 8 weeks. A drawback is that the spray seems to have the highest addictive potential of all NRTs.39,40 Patients who experience withdrawal symptoms with abrupt cessation of treatment should be considered for 4 to 6 weeks of tapering. Tapering could be achieved by reducing the dose by half every week. The most commonly reported side effects of the nicotine nasal spray include nasal irritation, runny nose, sneezing, throat irritation, coughing, and watery eyes. Patients usually develop tolerance to these effects within the first week.
Nicotine inhaler. A unique feature of the nicotine inhaler is that it mimics the hand-to-mouth routine similar to cigarette smoking and may therefore reduce fears associated with abrupt cessation of the hand-to-mouth ritual. The inhaler consists of a plastic mouthpiece to which a cartridge is attached. The cartridge contains 10 mg (but delivers only 4 mg) of nicotine plus 1 mg of menthol. The inhaler is different from typical inhalers in that patients puff on the mouthpiece, and nicotine is absorbed in the mouth rather than the lungs. Abstinence rates at 6 months were 23% for the inhaler and 11% for placebo.17 Recommended dosage is 6 to 16 cartridges per day for 8 weeks. Patients should self-titrate their dosing based on severity of withdrawal symptoms experienced. Adverse events are generally mild, consisting of throat irritation and cough.
Bupropion. This is an alternative for smokers who either cannot tolerate nicotine replacement therapy or prefer non-nicotine treatment. The efficacy of bupropion for smoking cessation has been demonstrated in 2 randomized controlled trials.41,42 Abstinence rates at 6 months were approximately 30% for bupropion versus 17% for placebo. Common adverse effects are generally mild, consisting of insomnia and dry mouth; headache and tremors are less common. This drug is contraindicated for patients with history of seizures, anorexia or bulimia, head trauma, or heavy alcohol use, and is category B for pregnancy.
Combination drug therapy. Combining the nicotine patch with a self-administered form of nicotine (eg, gum, spray, inhaler) is more efficacious than a single NRT.17 One randomized trial also showed that bupropion combined with the patch was more efficacious than the patch alone but not significantly better than bupropion alone. Combination treatments should be considered for smokers unable to quit because of significant craving or withdrawal despite adequate doses of single agents.
Other recommended pharmacotherapies. The 2000 clinical practice guidelines recommended the use of clonidine hydrochloride and nortriptyline hydrochloride as second-line agents. Controlled studies on both agents are limited,43-48 and neither agent is approved by the United States Food and Drug Administration for smoking cessation. Clonidine or nortriptyline should only be considered for patients who failed the first-line drugs or are unable to use them because of contraindications. Adverse events are generally more than for first-line agents.
Choice of Treatment
Few data exist on the comparative efficacy of the 5 approved pharmacotherapy aids Table 1. The STEPS (safety, tolerability, efficacy, price, simplicity) approach can be used to guide physicians in the choice of pharmacologic agents. All NRTs are considered generally safe, and adverse effects associated with their use are mild. The NRTs have similar cardiovascular precautions (ie, avoid use in unstable angina and within 1 month of a myocardial infarction), are pregnancy category D (there is evidence of human fetal risk, but use is acceptable if benefits outweigh risks and safer alternatives are unavailable or ineffective), except the gum, which is category C (animal studies have revealed adverse effects on the fetus, but there are no controlled studies in women) and should be used during pregnancy only if nonpharmacologic approaches are unsuccessful. Bupropion is also relatively safe with precaution as discussed earlier. Product-specific characteristics could make some NRTs less suitable for certain patients. For example, the gum is not appropriate for patients with dental or jaw problems and may be difficult to use correctly, since it requires special chewing techniques and high frequency of use. Very humid weather conditions may affect adhesiveness of the patch. The patch should also be avoided in patients with systemic eczema.
The only study that compared the efficacy of various NRTs reported similar results for all 4 NRTs. Although one study reported superior efficacy for bupropion over the patch,49 this finding has not been replicated. Bupropion costs slightly less than the NRTs Table 2. Of the NRTs, the patch appears to be the most convenient to use. In one randomized controlled trial,50 compliance was highest for the patch (82%) compared with the gum (38%), the spray (15%), and the inhaler (11%). A limitation common to all smoking cessation pharmacologic trials is that participants were volunteers with higher motivation to quit smoking and willing to comply with frequent follow-up contacts required in clinical trials. The effectiveness of these medications in real world settings may be lower than that reported in clinical trials. Also, the placebo arms in these trials typically receive substantially more counseling than what happens in real world settings. These factors combined produce higher quit rates in placebo patients than that found in typical unaided quit attempts. Physicians should consider using an algorithm Figure 1 to assist them in approach to and treatment of smokers.
Follow-up
Relapse is quite common among smokers trying to quit. On average, it takes 4 to 5 quit attempts before a smoker is successful.4 For this reason the last step of the AHRQ recommendations (arrange, ie, make arrangement for follow-up care for smoking cessation), is very important. The follow-up contact should occur within 1 week of patient’s quit date, because the risk of relapse back to smoking is highest during the first few days of abstinence.51,52 There are considerable data showing that additional follow-up contact beyond initial brief advice significantly increases quit rates.23,53,54
A variety of follow-up methods have been used in clinical trials, including face-to-face contact with a physician or other health care professional, letters, telephone, and self-help materials. Nurses and other office staff could be trained and designated to perform some or all the follow-up contact.18,55-57 In a recently published randomized trial,58 office nurses were trained to provide telephone follow-up contacts for low-income Medicaid managed care smokers. Abstinence rates at 3 months were 21% and 8% for telephone follow-up and usual care, respectively.
In addition to office-based telephone and printed self-help resources, physicians should be aware of a growing number of free telephone helplines and Internet-based resources Table 3 available for people trying to quit smoking. Patients without personal Internet access should be encouraged to make use of such services available at most public libraries.
Prognosis
Smoking cessation significantly reduces most of the increased morbidity and mortality from smoking.59 The degree of improvement, however, depends on the disease process involved, the amount of damage produced, and the reversibility of this damage at the time of cessation. Former smokers reduce their risk of developing coronary heart disease by 50% within 1 year of quitting.59 After 4 years, this risk becomes equal to that of people who have never smoked. Improvement in cancer risk varies with the type of cancer involved. The risk of lung cancer in former smokers, for example, always remains higher than that for those who have never smoked. However, this risk decreases progressively and considerably with the number of years of abstinence.59
In addition to reduction in morbidity and mortality, smoking cessation is among the most cost-effective measures in primary care. Studies have shown that the cost-effectiveness of physician smoking cessation counseling is similar to the treatment of mild to moderate hypertension or hypercholesterolemia.60 The estimated cost per year of life saved is $2000 for smoking cessation compared with $50,000 for screening mammography for breast cancer.61 Given the proven effectiveness of available smoking cessation interventions and the ready access primary care physicians have to smokers, effectively addressing tobacco use in primary care settings has a great potential of reducing tobacco-related morbidity and mortality.
Acknowledgments
Our work was supported by a grant from the Cancer Research Foundation of America (Dr Okuyemi) and a Robert Wood Johnson Foundation Generalist Physician Faculty Scholars Award (Dr Ahluwalia, #032586). Dr Wadland has received funding in the past from SmithKline-Beecham and Glaxo-Wellcome for nicotine replacement in community clinical trials. Dr Ahluwalia has received honoraria for presentations from Glaxo-Wellcome, Inc and Pharmacia Upjohn, Inc. Dr Okuyemi has received an honorarium for educational purposes from SmithKline Beecham.
Tobacco use is the leading cause of preventable diseases and deaths in the United States, accounting for approximately 435,000 deaths yearly.1 Smoking is responsible for an estimated $100 billion annually in direct medical and indirect nonmedical costs.2 Despite widespread efforts to educate the public on the risks of smoking, approximately 50 million American adults still smoke cigarettes.3 Cigarette smoking is an addiction, as powerful in many respects as cocaine or opiate dependence.4 Among those who have ever tried even one cigarette, almost one third develop nicotine dependence.5 Every year, primary care clinicians have access to 70% of smokers.6,7 One of the goals of Healthy People 20108 is to increase to 75% the proportion of primary care providers who routinely provide smoking cessation counseling.
Diagnosis
The Smoking Cessation Clinical Practice Guideline was originally published by the Agency for Healthcare Research and Quality (AHRQ) in 19969 and was updated in 2000 by AHRQ and a consortium of 7 government and nonprofit organizations.10 The 2000 guideline urged clinicians to treat tobacco use disorder as a chronic disease similar in many respects to other diseases like hypertension, diabetes, and hyperlipidemia and to provide patients with appropriate advice and pharmacotherapy. The updated guideline recommends a 5-step approach (the 5A’s: ask, advise, assess, assist, and arrange) to be used by primary care physicians. The first step (ask) is key in the management of tobacco use disorder. Tobacco use status should be asked and documented for all patients at every visit. The AHRQ recommends that tobacco use status be adopted as the “5th vital sign” along with blood pressure, temperature, pulse, and respiration. Data show that only approximately half of physicians in nonresearch settings consistently advise smokers to quit.11-13
Because the most common presentation of a smoker in the primary care setting is for general medical care not necessarily related to smoking, the recommendation to ask about tobacco use at every visit is a practical method to ensure early identification of smokers. Asking about tobacco use at every visit has been shown to result in better screening14-16 and increased cessation rates.17 Screening can be performed by the nurse or other trained member of the office staff who collects clinical information from the patient before being seen by the physician. Physicians should establish office-wide systems to enhance consistent identification and treatment of smokers in their practices. Organizational system approaches are cost-effective and have been shown to increase delivery of cessation interventions.18
Treatment
After identifying smokers during an office visit (ask), the next step is to strongly urge all smokers to quit (advise). Such initial advice should be given regardless of the patient’s state of readiness to quit. The transtheoretical model of stages of change (SOC)19 is useful for assessing the patient’s readiness to quit (assess). The SOC model identifies smoking behavior change as a process involving movement through a series of 5 motivational stages including precontemplation (not planning to quit within next 6 months), contemplation (planning to quit within next 6 months), preparation (planning to quit within next 30 days), action (has quit smoking for less than 6 months), and maintenance (has quit smoking for 6 months or longer). Interventions based on the SOC have been shown to enhance motivation20 and predict cessation.21 For patients unwilling to quit, physicians should identify reasons for resistance. For example, patients who are misinformed about the health risks of smoking should be provided with information relevant to their (or their family’s) health condition. Patients willing to make a quit attempt should be given specific advice about how to proceed, including setting a quit date and information on pharmacotherapy.
Behavioral interventions are beneficial to the long-term success of smoking cessation. Studies have shown that brief ( 5 minutes) advice on quitting given by physicians to smokers during an office visit have resulted in higher quit rates compared with no advice.22 A review of 20 studies conducted in primary care settings23 reported that 2% of all smokers who received brief physician advice quit smoking as a direct consequence, compared with less than 1% in smokers who received no advice. With additional encouragement and support (eg, follow-up letters, phone calls, demonstration of spirometry, and additional visits) quit rates increased to 5%.23,24 A more recent meta-analysis of 7 studies by the Clinical Practice Guideline Panel reported an abstinence rate of 8% when no cessation advice was given, compared with 10% with cessation advice.17 Although success rates are better with more intensive counseling, brief interventions appear to be more feasible in the primary care setting, given time constraints experienced by primary care physicians during office visits25 and the unwillingness of many patients to enter intensive programs.26
A recent Cochrane review27 found that group therapy is more effective than self-help materials but is not consistently better than personal contact. Although groups are theoretically more cost-effective, their usefulness may be limited by participant recruitment and retention problems.28,29 Current evidence does not support efficacy of acupuncture or hypnosis as treatment for smoking cessation.17,30
Pharmacotherapy
Because success rates associated with nonpharmacologic treatments are generally lower, pharmacotherapy should be offered to every smoker willing to make a quit attempt unless there is a medical contraindication.10 The 5 pharmacologic agents approved by the US Food and Drug Administration for treatment of tobacco use disorder include 4 nicotine replacement therapies (NRT)—gum, patch, spray, inhaler—and one non-nicotine therapy, bupropion. All 5 agents promote similar success rates in long-term smoking cessation if they are prescribed to meet the needs of the individual smoker.31
Nicotine gum. A meta-analysis of randomized controlled trials among specialized cessation clinics found higher success rates for patients treated with nicotine gum compared with use of placebo gum at 6 months (27% vs 18%).32 This is in contrast with studies conducted in general medical practices, where success rates (12%) with nicotine gum at 6 months were no different from placebo.33 Higher quit rates in specialized smoking cessation clinics may be a result of more in-depth counseling, better-trained counselors, and inclusion of smokers with a higher motivation to quit. The gum is available without prescription and comes in 2-mg and 4-mg doses. The 4-mg dose is more efficacious in more dependent smokers.34 Treatment is recommended for 8 weeks.
Nicotine patches. The patches have been shown to be effective under controlled as well as real-world settings.35-37 A meta-analysis of 17 randomized trials estimated the efficacy of the nicotine patch as 27% at end of treatment and 22% at 6 months compared with 13% and 9%, respectively, for placebo.38 Treatment beyond 8 weeks did not increase efficacy. The patches are available in 16-mg and 21-mg dosages (with 14-mg and 7-mg step-down doses). Although weaning is strongly encouraged by most marketers of nicotine patches, current data do not support added beneficial effect for this step-down approach.38 The highest dose should be used for those who smoke more than 10 cigarettes per day and reduced dosage forms for light smokers. The optimal dosage for light smokers is not known because of limited data in this group. The patches are contraindicated for patients with systemic eczema, unstable angina, and within 1 month of a myocardial infarction.
Nicotine nasal spray. Abstinence rate at 6 months from meta-analysis was 31% for the spray compared with 14% for placebo.17 A dose is one spray into each nostril; each spray delivers 0.5 mg of nicotine. Patients should use 1 to 2 doses every hour for 6 to 8 weeks. A drawback is that the spray seems to have the highest addictive potential of all NRTs.39,40 Patients who experience withdrawal symptoms with abrupt cessation of treatment should be considered for 4 to 6 weeks of tapering. Tapering could be achieved by reducing the dose by half every week. The most commonly reported side effects of the nicotine nasal spray include nasal irritation, runny nose, sneezing, throat irritation, coughing, and watery eyes. Patients usually develop tolerance to these effects within the first week.
Nicotine inhaler. A unique feature of the nicotine inhaler is that it mimics the hand-to-mouth routine similar to cigarette smoking and may therefore reduce fears associated with abrupt cessation of the hand-to-mouth ritual. The inhaler consists of a plastic mouthpiece to which a cartridge is attached. The cartridge contains 10 mg (but delivers only 4 mg) of nicotine plus 1 mg of menthol. The inhaler is different from typical inhalers in that patients puff on the mouthpiece, and nicotine is absorbed in the mouth rather than the lungs. Abstinence rates at 6 months were 23% for the inhaler and 11% for placebo.17 Recommended dosage is 6 to 16 cartridges per day for 8 weeks. Patients should self-titrate their dosing based on severity of withdrawal symptoms experienced. Adverse events are generally mild, consisting of throat irritation and cough.
Bupropion. This is an alternative for smokers who either cannot tolerate nicotine replacement therapy or prefer non-nicotine treatment. The efficacy of bupropion for smoking cessation has been demonstrated in 2 randomized controlled trials.41,42 Abstinence rates at 6 months were approximately 30% for bupropion versus 17% for placebo. Common adverse effects are generally mild, consisting of insomnia and dry mouth; headache and tremors are less common. This drug is contraindicated for patients with history of seizures, anorexia or bulimia, head trauma, or heavy alcohol use, and is category B for pregnancy.
Combination drug therapy. Combining the nicotine patch with a self-administered form of nicotine (eg, gum, spray, inhaler) is more efficacious than a single NRT.17 One randomized trial also showed that bupropion combined with the patch was more efficacious than the patch alone but not significantly better than bupropion alone. Combination treatments should be considered for smokers unable to quit because of significant craving or withdrawal despite adequate doses of single agents.
Other recommended pharmacotherapies. The 2000 clinical practice guidelines recommended the use of clonidine hydrochloride and nortriptyline hydrochloride as second-line agents. Controlled studies on both agents are limited,43-48 and neither agent is approved by the United States Food and Drug Administration for smoking cessation. Clonidine or nortriptyline should only be considered for patients who failed the first-line drugs or are unable to use them because of contraindications. Adverse events are generally more than for first-line agents.
Choice of Treatment
Few data exist on the comparative efficacy of the 5 approved pharmacotherapy aids Table 1. The STEPS (safety, tolerability, efficacy, price, simplicity) approach can be used to guide physicians in the choice of pharmacologic agents. All NRTs are considered generally safe, and adverse effects associated with their use are mild. The NRTs have similar cardiovascular precautions (ie, avoid use in unstable angina and within 1 month of a myocardial infarction), are pregnancy category D (there is evidence of human fetal risk, but use is acceptable if benefits outweigh risks and safer alternatives are unavailable or ineffective), except the gum, which is category C (animal studies have revealed adverse effects on the fetus, but there are no controlled studies in women) and should be used during pregnancy only if nonpharmacologic approaches are unsuccessful. Bupropion is also relatively safe with precaution as discussed earlier. Product-specific characteristics could make some NRTs less suitable for certain patients. For example, the gum is not appropriate for patients with dental or jaw problems and may be difficult to use correctly, since it requires special chewing techniques and high frequency of use. Very humid weather conditions may affect adhesiveness of the patch. The patch should also be avoided in patients with systemic eczema.
The only study that compared the efficacy of various NRTs reported similar results for all 4 NRTs. Although one study reported superior efficacy for bupropion over the patch,49 this finding has not been replicated. Bupropion costs slightly less than the NRTs Table 2. Of the NRTs, the patch appears to be the most convenient to use. In one randomized controlled trial,50 compliance was highest for the patch (82%) compared with the gum (38%), the spray (15%), and the inhaler (11%). A limitation common to all smoking cessation pharmacologic trials is that participants were volunteers with higher motivation to quit smoking and willing to comply with frequent follow-up contacts required in clinical trials. The effectiveness of these medications in real world settings may be lower than that reported in clinical trials. Also, the placebo arms in these trials typically receive substantially more counseling than what happens in real world settings. These factors combined produce higher quit rates in placebo patients than that found in typical unaided quit attempts. Physicians should consider using an algorithm Figure 1 to assist them in approach to and treatment of smokers.
Follow-up
Relapse is quite common among smokers trying to quit. On average, it takes 4 to 5 quit attempts before a smoker is successful.4 For this reason the last step of the AHRQ recommendations (arrange, ie, make arrangement for follow-up care for smoking cessation), is very important. The follow-up contact should occur within 1 week of patient’s quit date, because the risk of relapse back to smoking is highest during the first few days of abstinence.51,52 There are considerable data showing that additional follow-up contact beyond initial brief advice significantly increases quit rates.23,53,54
A variety of follow-up methods have been used in clinical trials, including face-to-face contact with a physician or other health care professional, letters, telephone, and self-help materials. Nurses and other office staff could be trained and designated to perform some or all the follow-up contact.18,55-57 In a recently published randomized trial,58 office nurses were trained to provide telephone follow-up contacts for low-income Medicaid managed care smokers. Abstinence rates at 3 months were 21% and 8% for telephone follow-up and usual care, respectively.
In addition to office-based telephone and printed self-help resources, physicians should be aware of a growing number of free telephone helplines and Internet-based resources Table 3 available for people trying to quit smoking. Patients without personal Internet access should be encouraged to make use of such services available at most public libraries.
Prognosis
Smoking cessation significantly reduces most of the increased morbidity and mortality from smoking.59 The degree of improvement, however, depends on the disease process involved, the amount of damage produced, and the reversibility of this damage at the time of cessation. Former smokers reduce their risk of developing coronary heart disease by 50% within 1 year of quitting.59 After 4 years, this risk becomes equal to that of people who have never smoked. Improvement in cancer risk varies with the type of cancer involved. The risk of lung cancer in former smokers, for example, always remains higher than that for those who have never smoked. However, this risk decreases progressively and considerably with the number of years of abstinence.59
In addition to reduction in morbidity and mortality, smoking cessation is among the most cost-effective measures in primary care. Studies have shown that the cost-effectiveness of physician smoking cessation counseling is similar to the treatment of mild to moderate hypertension or hypercholesterolemia.60 The estimated cost per year of life saved is $2000 for smoking cessation compared with $50,000 for screening mammography for breast cancer.61 Given the proven effectiveness of available smoking cessation interventions and the ready access primary care physicians have to smokers, effectively addressing tobacco use in primary care settings has a great potential of reducing tobacco-related morbidity and mortality.
Acknowledgments
Our work was supported by a grant from the Cancer Research Foundation of America (Dr Okuyemi) and a Robert Wood Johnson Foundation Generalist Physician Faculty Scholars Award (Dr Ahluwalia, #032586). Dr Wadland has received funding in the past from SmithKline-Beecham and Glaxo-Wellcome for nicotine replacement in community clinical trials. Dr Ahluwalia has received honoraria for presentations from Glaxo-Wellcome, Inc and Pharmacia Upjohn, Inc. Dr Okuyemi has received an honorarium for educational purposes from SmithKline Beecham.
1. Centers for Disease Control and Prevention. Smoking: attributable mortality and years of potential life lost—United States, 1984. MMWR 1997;46:444-51.
2. Centers for Disease Control and Prevention. Economic consequences of smoking: direct medical costs. MMWR 1994;43:469-72.
3. Center for Disease Control and Prevention. Cigarette smoking among adults. MMWR 1999;48:993-96.
4. US Department of Health and Human Services. The health consequences of smoking: nicotine addiction. A report of the Surgeon General. DHHS pub. no. (CDC) 88-8406. 1988.
5. Anthony J, Warner L, Kessler R. Comparative epidemiology of dependence on tobacco, alcohol, controlled substances, and inhalants: basic findings from the National Comorbidity Survey. Exp Clin Psychopharmacol 1994;2:244-68.
6. Centers for Disease Control and Prevention. Physicians and other health care professional counseling of smokers to quit—United States, 1991. MMWR 1993;42:854-57.
7. Ockene JK. Physician-delivered interventions for smoking cessation: strategies for increasing effectiveness. Prev Med 1987;16:723-37.
8. US Department of Health and Human Services. Healthy people 2010: objectives for improving health. Washington, DC: USDHHS; 2000.
9. US Department of Health and Human Services. Smoking cessation: clinical practice guideline. Rockville, Md: Public Health Service, Agency for Health Care Policy and Research; 1996.
10. US Department of Health and Human Services. The tobacco use and dependence clinical practice guideline panel staff and consortium representatives: a clinical practice guideline for treating tobacco use and dependence. JAMA 2000;283:3244-54.
11. Anda RF, Remington PL, Sienko DG, Davis RM. Are physicians advising smokers to quit? the patient’s perspective. JAMA 1987;257:1916-19.
12. Wells KB, Lewis CE, Leake B, Schleiter MK, Brook RH. The practices of general and subspecialty internists in counseling about smoking and exercise. Am J Public Health 1986;76:1009-13.
13. Doescher MP, Saver BG. Physicians’ advice to quit smoking: the glass remains half empty. J Fam Pract 2000;49:543-47.
14. Ahluwalia JS, Gibson CA, Kenney RE, Wallace DD, Resnicow K. Smoking status as a vital sign. J Gen Intern Med 1999;14:402-08.
15. Fiore MC, Jorenby DE, Schensky AE, Smith SS, Bauer RR, Baker TB. Smoking status as the new vital sign: effect on assessment and intervention in patients who smoke. Mayo Clin Proceed 1995;70:209-13.
16. Robinson MD, Laurent SL, Little JM. Including smoking status as a new vital sign: it works. J Fam Pract 1995;40:556-61.
17. Fiore M, Bailey W, Cohen S, et al. Treating tobacco use and dependence: clinical practice guideline. Rockville, Md: US Department of Health and Human Services, Public Health Service; 2000.
18. Solberg LI, Maxwell PL, Kottke TE, Gepner GJ, Brekke ML. A systemic primary care office-based smoking cessation program. J Fam Pract 1990;30:647-54.
19. Prochaska JO, DiClemente CC. Stages and processes of self-change of smoking: toward an integrative model of change. J Consult Clin Psychol 1983;51:390-95.
20. Goldberg DN, Hoffman AM, Farinha MF, et al. Physician delivery of smoking-cessation advice based on the stages-of-change model. Am J Prev Med 1994;10:267-74.
21. Farkas AJ, Pierce JP, Zhu SH, et al. Addiction versus stages of change models in predicting smoking cessation. Addiction 1996;91:1271-80; discussion 1281-92.
22. Slama K, Redman S, Perkins J, Reid A, Sanson-Fisher RW. The effectiveness of two smoking cessation programs for use in general practice: a randomized clinical trial. BMJ 1990;300:1707-09.
23. Law M, Tang JL. An analysis of the effectiveness of interventions intended to help people stop smoking. Arch Intern Med 1995;155:1933-41.
24. Risser NL, Belcher DW. Adding spirometry, carbon monoxide, and pulmonary symptom results to smoking cessation counseling: a randomized trial. J Gen Intern Med 1990;5:16-22.
25. Gilchrist V, Miller RS, Gillanders WR, et al. Does family practice at residency teaching sites reflect community practice? J Fam Pract 1993;37:555-63.
26. Lichtenstein E, Hollis J. Patient referral to a smoking cessation program: who follows through? J Fam Pract 1992;34:739-44.
27. Stead LF, Lancaster T. Group behaviour therapy programmes for smoking cessation. Cochrane Database Syst Rev 2000;2.-
28. Hollis JF, Lichtenstein E, Vogt TM, Stevens VJ, Biglan A. Nurse-assisted counseling for smokers in primary care. Ann Intern Med 1993;118:521-25.
29. Lancaster T, Stead L, Silagy C, Sowden A. Effectiveness of interventions to help people stop smoking: findings from the Cochrane library. BMJ 2000;321:355-58.
30. Abbot NC, Stead LF, White AR, Barnes J, Ernst E. Hypnotherapy for smoking cessation. Cochrane Database Syst Rev 2000;2.-
31. Hughes J, Goldstein MG, Hurt RD, Shiffman S. Recent advances in the pharmacotherapy of smoking. JAMA 1999;281:72-76.
32. Cepeda-Benito A. Meta-analytical review of the efficacy of nicotine chewing gum in smoking treatment programs. J Consult Clin Psychol 1993;61:822-30.
33. Lam W, Sacks HS, Sze PC, Chalmers TC. Meta analysis of randomised controlled trials of nicotine chewing gum. Lancet 1987;2:27-30.
34. Sachs DPL. Effectiveness of the 4-mg dose of nicotine polacrilex for the initial treatment of high-dependent smokers. Arch Intern Med 1995;155:1973-80.
35. Jolicoeur DG, Ahluwalia JS, Richter KP, et al. The use of nicotine patches with minimal intervention. Prevent Med 2000;30:504-12.
36. Orleans CT, Resch N, Noll E, et al. Use of transdermal nicotine in a state-level prescription plan for the elderly. JAMA 1994;271:601-07.
37. Silagy C, Mant D, Fowler G, Lodge M. Meta-analysis on efficacy of nicotine replacement therapies in smoking cessation. Lancet 1994;343:139-42.
38. Fiore MC, Smith SS, Jorenby DE, Baker TB. The effectiveness of the nicotine patch for smoking cessation: a meta-analysis. JAMA 1994;271:1940-47.
39. Sutherland G, Stapleton JA, Russell MAH, et al. Randomised controlled trial of nasal nicotine spray in smoking cessation. Lancet 1992;340:324-29.
40. Hughes JR, Gust SW, Keenan R, Fenwick JW, Skoog K, Higgins ST. Long-term use of nicotine vs placebo gum. Arch Intern Med 1991;151:1993-98.
41. Hurt RD, Sachs DPL, Glover ED, Offord KP, et al. A comparison of sustained-release bupropion and placebo for smoking cessation. N Engl J Medicine. 1997;337:1195-202.
42. Jorenby DE, Leischow SJ, Nides MA, et al. A controlled trial of sustained-release bupropion, a nicotine patch, or both for smoking cessation. N Engl J Med 1999;340:685-91.
43. Glassman AH, Stetner F, Walsh T, et al. Heavy smokers smoking cessation and clonidine. JAMA 1988;259:2863-66.
44. Glassman AH, Covey LS, Dalack GW, et al. Smoking cessation, clonidine, and vulnerability to nicotine among dependent smokers. Clin Pharmacol Ther 1993;54:670-79.
45. Hilleman DE, Mohiuddin SM, Delcore MG, Lucas BD, Jr. Randomized, controlled trial of transdermal clonidine for smoking cessation. Ann Pharmacother 1993;27:1025-28.
46. Hao W, Young D, Wei H. Effect of clonidine on cigarette cessation and in the alleviation of withdrawal symptoms. Br J Addict 1988;83:1221-26.
47. Hall SM, Reus VI, Munoz RF, et al. Nortriptyline and cognitive-behavioral therapy in the treatment of cigarette smoking. Arch Gen Psychiatry 1998;55:683-90.
48. Prochazka AV, Weaver MJ, Keller RT, Fryer GE, Licari PA, Lofaso D. A randomized trial of nortriptyline for smoking cessation. Arch Intern Med 1998;158:2035-39.
49. Jorenby D, Leischow S, Nides M, et al. Bupropion alone or with a nicotine patch increased smoking cessation rates. N Engl J Med 1999;340:685-91.
50. Hajek P, West R, Foulds J, Nilsson F, Burrows S, Meadow A. Randomized comparative trial of nicotine polacrilex, a transdermal patch, nasal spray, and an inhaler. Arch Intern Med 1999;159:2033-38.
51. Hughes JR, Gulliver SB, Fenwick JW, et al. Smoking cessation among self-quitters. Health Psychol 1992;11:331-34.
52. Kottke TE, Brekke ML, Solberg LI, Hughes JR. A randomized trial to increase smoking intervention by physicians (Doctors helping smokers, round I). JAMA 1989;261:2101-06.
53. Smoking cessation in patients: two further studies by the British Thoracic Society. Research Committee of the British Thoracic Society. Thorax 1990;45:835-40.
54. Richmond R, Webster I. Evaluation of general practitioners’ use of a smoking intervention programme. Int J Epidemiol 1985;14:396-401.
55. Duncan C, Stein MJ, Cummings SR. Staff involvement and special follow-up time increase physicians’ counseling about smoking cessation: a controlled trial. Am J Public Health 1991;81:899-901.
56. Pine D, David C, Sauser M, Sullivan S. Effects of a systemic approach to tobacco cessation in a community-based practice. Arch Fam Med 1997;6:363-67.
57. Wadland WC, Stoffelmayr B, Berger E, Crombach A, Ives K. Enhancing smoking cessation rates in primary care. J Fam Pract 1999;48:711-18.
58. Wadland WC, Soffelmayr B, Ives K. Enhancing smoking cessation of low-income smokers in managed care. J Fam Pract 2001;50:138-44.
59. US Department of Health and Human Services. The health benefits of smoking cessation. Public Health Service; CDC: Center for Chronic Disease Prevention and Health Promotion; OSH. DHHS pub. no. (CDC) 90-8416; 1990.
60. Cummings SR, Rubin SM, Oster G. The cost-effectiveness of counseling smokers to quit. JAMA 1989;261:75-79.
61. Marwick C. Intensive smoking cessation efforts cost-effective. JAMA 1996;276:1291.-
62. Ascher JA, Cole JO, Colin J-N, Feighner JP, et al. Bupropion: a review of its mechanism of antidepressant activity. J Clin Psychiatry 1995;56:395-401.
63. Benowitz NL. Pharmacologic aspects of cigarette smoking and nicotine addiction. N Engl J Med 1988;319:1318-30.
64. Kornitzer M, Boutsen M, Dramaix M, Thijs J, Gustavsson G. Combined use of nicotine patch and gum in smoking cessation: a placebo-controlled clinical trial. Prev Med 1995;24:41-47.
65. Blondal T, Gudmundsson LJ, Olafsdottir I, Gustavsson G, Westin A. Nicotine nasal spray with nicotine patch for smoking cessation: randomised trial with six year follow-up. BMJ 1999;318:285-89.
66. Bohadana A, Nilsson F, Rasmussen T, Martinet Y. Nicotine inhaler and nicotine patch as a combination therapy for smoking cessation: a randomized double blind placebo controlled trial. Arch Intern Med 2000;160:3128-34.
67. Okuyemi KS, Ahluwalia JS, Harris KJ. Pharmacotherapy of smoking cessation. Arch Fam Med 2000;9:270-81.
1. Centers for Disease Control and Prevention. Smoking: attributable mortality and years of potential life lost—United States, 1984. MMWR 1997;46:444-51.
2. Centers for Disease Control and Prevention. Economic consequences of smoking: direct medical costs. MMWR 1994;43:469-72.
3. Center for Disease Control and Prevention. Cigarette smoking among adults. MMWR 1999;48:993-96.
4. US Department of Health and Human Services. The health consequences of smoking: nicotine addiction. A report of the Surgeon General. DHHS pub. no. (CDC) 88-8406. 1988.
5. Anthony J, Warner L, Kessler R. Comparative epidemiology of dependence on tobacco, alcohol, controlled substances, and inhalants: basic findings from the National Comorbidity Survey. Exp Clin Psychopharmacol 1994;2:244-68.
6. Centers for Disease Control and Prevention. Physicians and other health care professional counseling of smokers to quit—United States, 1991. MMWR 1993;42:854-57.
7. Ockene JK. Physician-delivered interventions for smoking cessation: strategies for increasing effectiveness. Prev Med 1987;16:723-37.
8. US Department of Health and Human Services. Healthy people 2010: objectives for improving health. Washington, DC: USDHHS; 2000.
9. US Department of Health and Human Services. Smoking cessation: clinical practice guideline. Rockville, Md: Public Health Service, Agency for Health Care Policy and Research; 1996.
10. US Department of Health and Human Services. The tobacco use and dependence clinical practice guideline panel staff and consortium representatives: a clinical practice guideline for treating tobacco use and dependence. JAMA 2000;283:3244-54.
11. Anda RF, Remington PL, Sienko DG, Davis RM. Are physicians advising smokers to quit? the patient’s perspective. JAMA 1987;257:1916-19.
12. Wells KB, Lewis CE, Leake B, Schleiter MK, Brook RH. The practices of general and subspecialty internists in counseling about smoking and exercise. Am J Public Health 1986;76:1009-13.
13. Doescher MP, Saver BG. Physicians’ advice to quit smoking: the glass remains half empty. J Fam Pract 2000;49:543-47.
14. Ahluwalia JS, Gibson CA, Kenney RE, Wallace DD, Resnicow K. Smoking status as a vital sign. J Gen Intern Med 1999;14:402-08.
15. Fiore MC, Jorenby DE, Schensky AE, Smith SS, Bauer RR, Baker TB. Smoking status as the new vital sign: effect on assessment and intervention in patients who smoke. Mayo Clin Proceed 1995;70:209-13.
16. Robinson MD, Laurent SL, Little JM. Including smoking status as a new vital sign: it works. J Fam Pract 1995;40:556-61.
17. Fiore M, Bailey W, Cohen S, et al. Treating tobacco use and dependence: clinical practice guideline. Rockville, Md: US Department of Health and Human Services, Public Health Service; 2000.
18. Solberg LI, Maxwell PL, Kottke TE, Gepner GJ, Brekke ML. A systemic primary care office-based smoking cessation program. J Fam Pract 1990;30:647-54.
19. Prochaska JO, DiClemente CC. Stages and processes of self-change of smoking: toward an integrative model of change. J Consult Clin Psychol 1983;51:390-95.
20. Goldberg DN, Hoffman AM, Farinha MF, et al. Physician delivery of smoking-cessation advice based on the stages-of-change model. Am J Prev Med 1994;10:267-74.
21. Farkas AJ, Pierce JP, Zhu SH, et al. Addiction versus stages of change models in predicting smoking cessation. Addiction 1996;91:1271-80; discussion 1281-92.
22. Slama K, Redman S, Perkins J, Reid A, Sanson-Fisher RW. The effectiveness of two smoking cessation programs for use in general practice: a randomized clinical trial. BMJ 1990;300:1707-09.
23. Law M, Tang JL. An analysis of the effectiveness of interventions intended to help people stop smoking. Arch Intern Med 1995;155:1933-41.
24. Risser NL, Belcher DW. Adding spirometry, carbon monoxide, and pulmonary symptom results to smoking cessation counseling: a randomized trial. J Gen Intern Med 1990;5:16-22.
25. Gilchrist V, Miller RS, Gillanders WR, et al. Does family practice at residency teaching sites reflect community practice? J Fam Pract 1993;37:555-63.
26. Lichtenstein E, Hollis J. Patient referral to a smoking cessation program: who follows through? J Fam Pract 1992;34:739-44.
27. Stead LF, Lancaster T. Group behaviour therapy programmes for smoking cessation. Cochrane Database Syst Rev 2000;2.-
28. Hollis JF, Lichtenstein E, Vogt TM, Stevens VJ, Biglan A. Nurse-assisted counseling for smokers in primary care. Ann Intern Med 1993;118:521-25.
29. Lancaster T, Stead L, Silagy C, Sowden A. Effectiveness of interventions to help people stop smoking: findings from the Cochrane library. BMJ 2000;321:355-58.
30. Abbot NC, Stead LF, White AR, Barnes J, Ernst E. Hypnotherapy for smoking cessation. Cochrane Database Syst Rev 2000;2.-
31. Hughes J, Goldstein MG, Hurt RD, Shiffman S. Recent advances in the pharmacotherapy of smoking. JAMA 1999;281:72-76.
32. Cepeda-Benito A. Meta-analytical review of the efficacy of nicotine chewing gum in smoking treatment programs. J Consult Clin Psychol 1993;61:822-30.
33. Lam W, Sacks HS, Sze PC, Chalmers TC. Meta analysis of randomised controlled trials of nicotine chewing gum. Lancet 1987;2:27-30.
34. Sachs DPL. Effectiveness of the 4-mg dose of nicotine polacrilex for the initial treatment of high-dependent smokers. Arch Intern Med 1995;155:1973-80.
35. Jolicoeur DG, Ahluwalia JS, Richter KP, et al. The use of nicotine patches with minimal intervention. Prevent Med 2000;30:504-12.
36. Orleans CT, Resch N, Noll E, et al. Use of transdermal nicotine in a state-level prescription plan for the elderly. JAMA 1994;271:601-07.
37. Silagy C, Mant D, Fowler G, Lodge M. Meta-analysis on efficacy of nicotine replacement therapies in smoking cessation. Lancet 1994;343:139-42.
38. Fiore MC, Smith SS, Jorenby DE, Baker TB. The effectiveness of the nicotine patch for smoking cessation: a meta-analysis. JAMA 1994;271:1940-47.
39. Sutherland G, Stapleton JA, Russell MAH, et al. Randomised controlled trial of nasal nicotine spray in smoking cessation. Lancet 1992;340:324-29.
40. Hughes JR, Gust SW, Keenan R, Fenwick JW, Skoog K, Higgins ST. Long-term use of nicotine vs placebo gum. Arch Intern Med 1991;151:1993-98.
41. Hurt RD, Sachs DPL, Glover ED, Offord KP, et al. A comparison of sustained-release bupropion and placebo for smoking cessation. N Engl J Medicine. 1997;337:1195-202.
42. Jorenby DE, Leischow SJ, Nides MA, et al. A controlled trial of sustained-release bupropion, a nicotine patch, or both for smoking cessation. N Engl J Med 1999;340:685-91.
43. Glassman AH, Stetner F, Walsh T, et al. Heavy smokers smoking cessation and clonidine. JAMA 1988;259:2863-66.
44. Glassman AH, Covey LS, Dalack GW, et al. Smoking cessation, clonidine, and vulnerability to nicotine among dependent smokers. Clin Pharmacol Ther 1993;54:670-79.
45. Hilleman DE, Mohiuddin SM, Delcore MG, Lucas BD, Jr. Randomized, controlled trial of transdermal clonidine for smoking cessation. Ann Pharmacother 1993;27:1025-28.
46. Hao W, Young D, Wei H. Effect of clonidine on cigarette cessation and in the alleviation of withdrawal symptoms. Br J Addict 1988;83:1221-26.
47. Hall SM, Reus VI, Munoz RF, et al. Nortriptyline and cognitive-behavioral therapy in the treatment of cigarette smoking. Arch Gen Psychiatry 1998;55:683-90.
48. Prochazka AV, Weaver MJ, Keller RT, Fryer GE, Licari PA, Lofaso D. A randomized trial of nortriptyline for smoking cessation. Arch Intern Med 1998;158:2035-39.
49. Jorenby D, Leischow S, Nides M, et al. Bupropion alone or with a nicotine patch increased smoking cessation rates. N Engl J Med 1999;340:685-91.
50. Hajek P, West R, Foulds J, Nilsson F, Burrows S, Meadow A. Randomized comparative trial of nicotine polacrilex, a transdermal patch, nasal spray, and an inhaler. Arch Intern Med 1999;159:2033-38.
51. Hughes JR, Gulliver SB, Fenwick JW, et al. Smoking cessation among self-quitters. Health Psychol 1992;11:331-34.
52. Kottke TE, Brekke ML, Solberg LI, Hughes JR. A randomized trial to increase smoking intervention by physicians (Doctors helping smokers, round I). JAMA 1989;261:2101-06.
53. Smoking cessation in patients: two further studies by the British Thoracic Society. Research Committee of the British Thoracic Society. Thorax 1990;45:835-40.
54. Richmond R, Webster I. Evaluation of general practitioners’ use of a smoking intervention programme. Int J Epidemiol 1985;14:396-401.
55. Duncan C, Stein MJ, Cummings SR. Staff involvement and special follow-up time increase physicians’ counseling about smoking cessation: a controlled trial. Am J Public Health 1991;81:899-901.
56. Pine D, David C, Sauser M, Sullivan S. Effects of a systemic approach to tobacco cessation in a community-based practice. Arch Fam Med 1997;6:363-67.
57. Wadland WC, Stoffelmayr B, Berger E, Crombach A, Ives K. Enhancing smoking cessation rates in primary care. J Fam Pract 1999;48:711-18.
58. Wadland WC, Soffelmayr B, Ives K. Enhancing smoking cessation of low-income smokers in managed care. J Fam Pract 2001;50:138-44.
59. US Department of Health and Human Services. The health benefits of smoking cessation. Public Health Service; CDC: Center for Chronic Disease Prevention and Health Promotion; OSH. DHHS pub. no. (CDC) 90-8416; 1990.
60. Cummings SR, Rubin SM, Oster G. The cost-effectiveness of counseling smokers to quit. JAMA 1989;261:75-79.
61. Marwick C. Intensive smoking cessation efforts cost-effective. JAMA 1996;276:1291.-
62. Ascher JA, Cole JO, Colin J-N, Feighner JP, et al. Bupropion: a review of its mechanism of antidepressant activity. J Clin Psychiatry 1995;56:395-401.
63. Benowitz NL. Pharmacologic aspects of cigarette smoking and nicotine addiction. N Engl J Med 1988;319:1318-30.
64. Kornitzer M, Boutsen M, Dramaix M, Thijs J, Gustavsson G. Combined use of nicotine patch and gum in smoking cessation: a placebo-controlled clinical trial. Prev Med 1995;24:41-47.
65. Blondal T, Gudmundsson LJ, Olafsdottir I, Gustavsson G, Westin A. Nicotine nasal spray with nicotine patch for smoking cessation: randomised trial with six year follow-up. BMJ 1999;318:285-89.
66. Bohadana A, Nilsson F, Rasmussen T, Martinet Y. Nicotine inhaler and nicotine patch as a combination therapy for smoking cessation: a randomized double blind placebo controlled trial. Arch Intern Med 2000;160:3128-34.
67. Okuyemi KS, Ahluwalia JS, Harris KJ. Pharmacotherapy of smoking cessation. Arch Fam Med 2000;9:270-81.