User login
Intralymphatic Histiocytosis Treated With Intralesional Triamcinolone Acetonide and Pressure Bandage
Intralymphatic histiocytosis was first described in 1994.1 To date, at least 70 cases have been reported in the English-language literature, the majority being associated with systemic or local inflammatory conditions such as rheumatoid arthritis (RA), malignancy, and metal prostheses. The remaining cases arose independent of any detectable disease process.2 The clinical lesion localizes to areas around surgical scars or inflamed joints and generally presents with erythematous livedoid papules and plaques. Because of its rarity, pathologists and clinicians may be unfamiliar with this entity, leading to delayed or missed diagnoses.
Although the pathogenesis of intralymphatic histiocytosis remains unclear, it may be related to dysregulated immune signaling. The condition follows a chronic, relapsing-remitting course that has shown variable response to topical and systemic treatments. We present a rare case of intralymphatic histiocytosis associated with joint replacement/metal prosthesis3-14 that was responsive to a novel treatment with intralesional steroid injection and pressure bandage.
Case Report
An 89-year-old woman presented with a relapsing and remitting rash on the right calf and popliteal fossa of 11 months’ duration. It was becoming more painful over time and recently began to hurt when walking. Her medical history was remarkable for deep vein thromboses of the bilateral legs, Factor V Leiden deficiency, osteoarthritis, and a popliteal (Baker) cyst on the right leg that ruptured 22 months prior to presentation. Her surgical history included bilateral knee replacements (10 years and 2 years prior to the current presentation for the right and left knees, respectively). Her international normalized ratio (2.0) was therapeutic on warfarin.
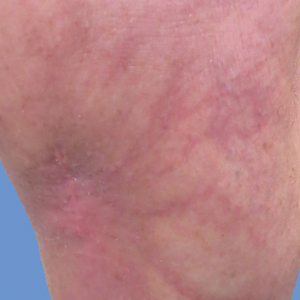
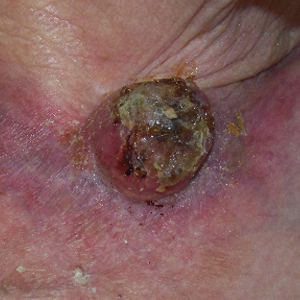
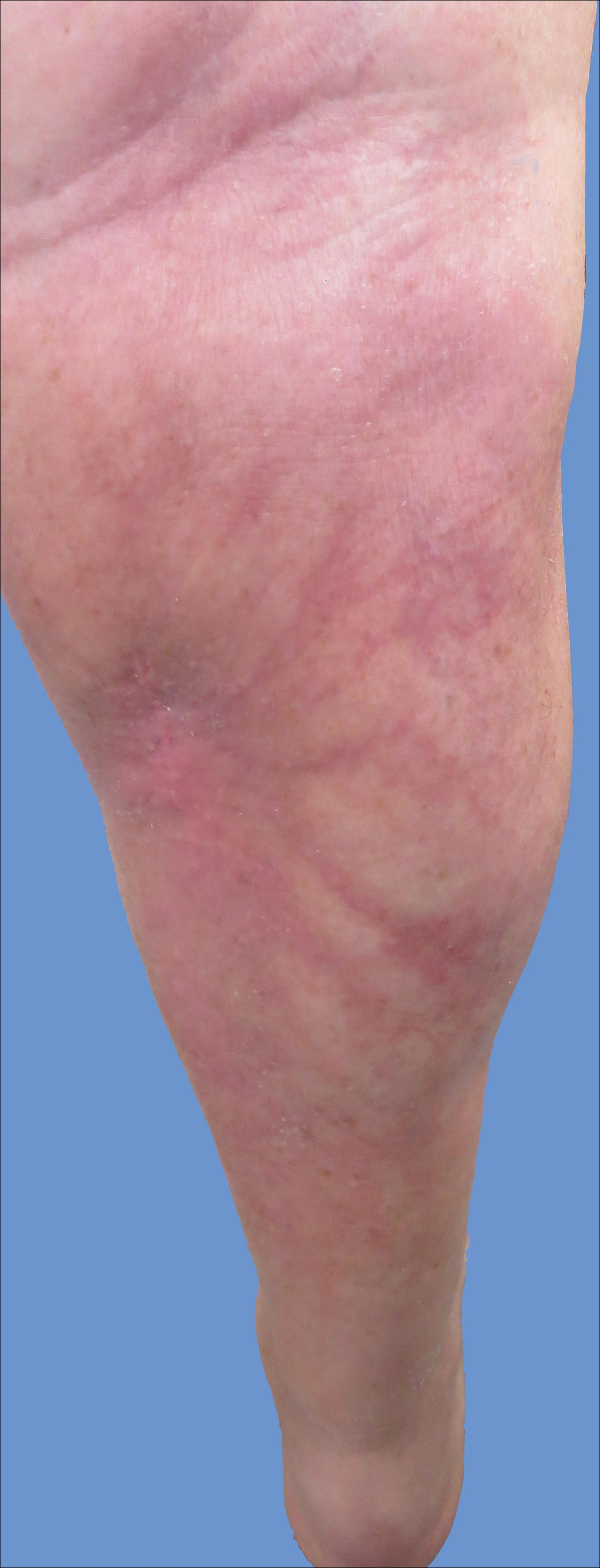
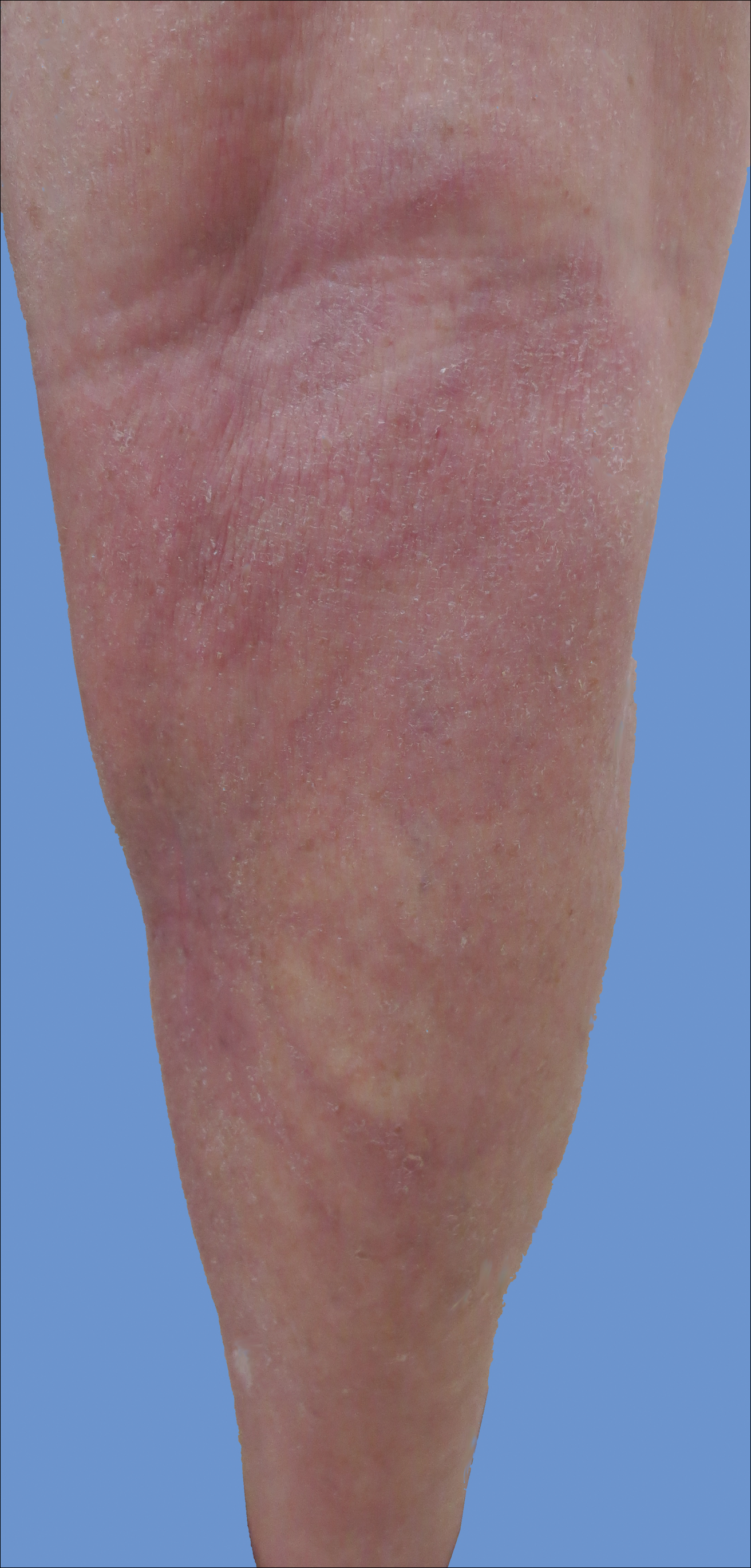
Initially, swelling, pain, and redness developed in the right calf, and recurrent right-leg deep venous thrombosis was ruled out by Doppler ultrasound. The findings were considered to be secondary to inflammation from a popliteal cyst. Symptoms persisted despite application of warm compresses, leg elevation, and compression stockings. Treatment with doxycycline prescribed by the patient’s primary care physician 9 months prior for presumed cellulitis produced little improvement. Physical examination revealed a well-healed vertical scar on the right calf from an incisional biopsy within an 8-cm, tender, erythematous, indurated, sclerotic plaque with erythematous streaks radiating from the center of the plaque (Figure 1). There also was red-brown, indurated discoloration on the right shin.
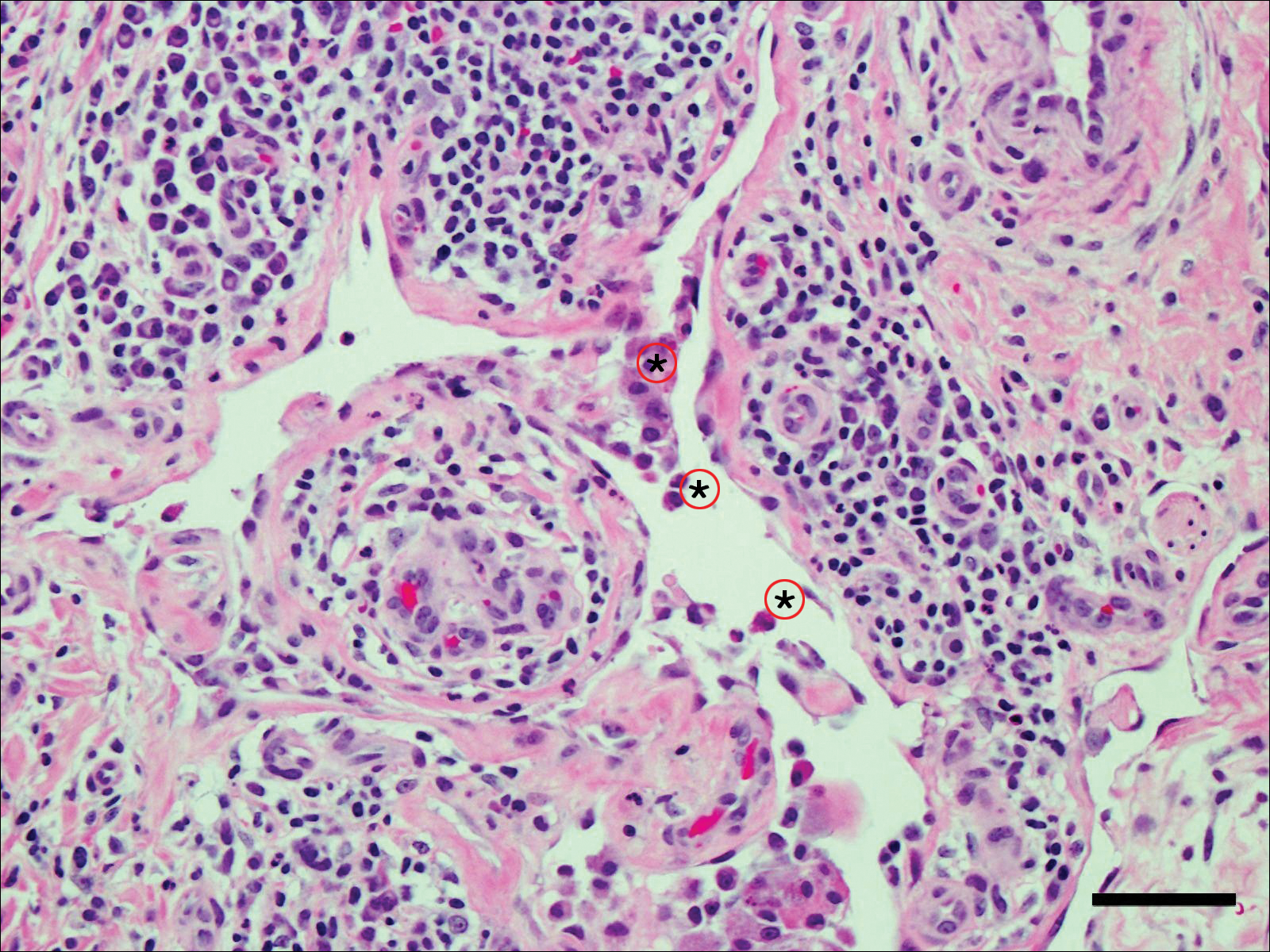
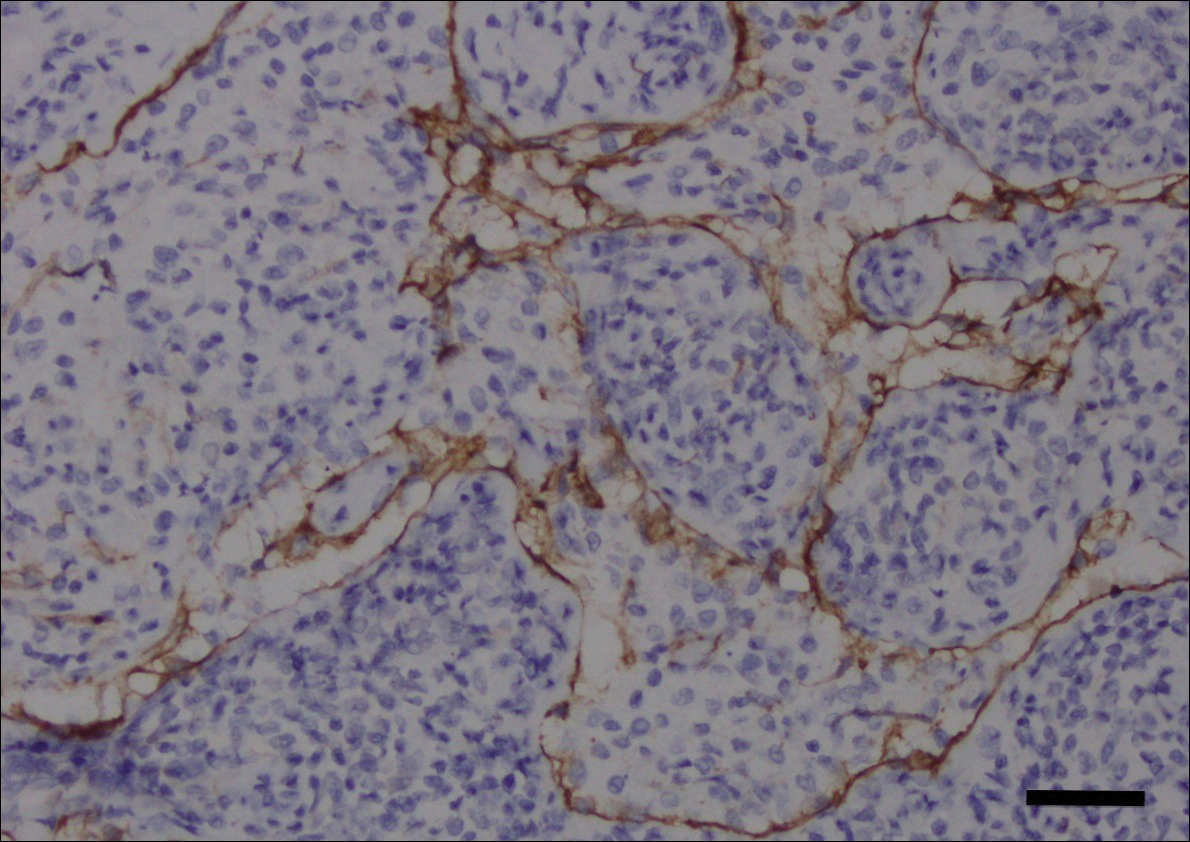
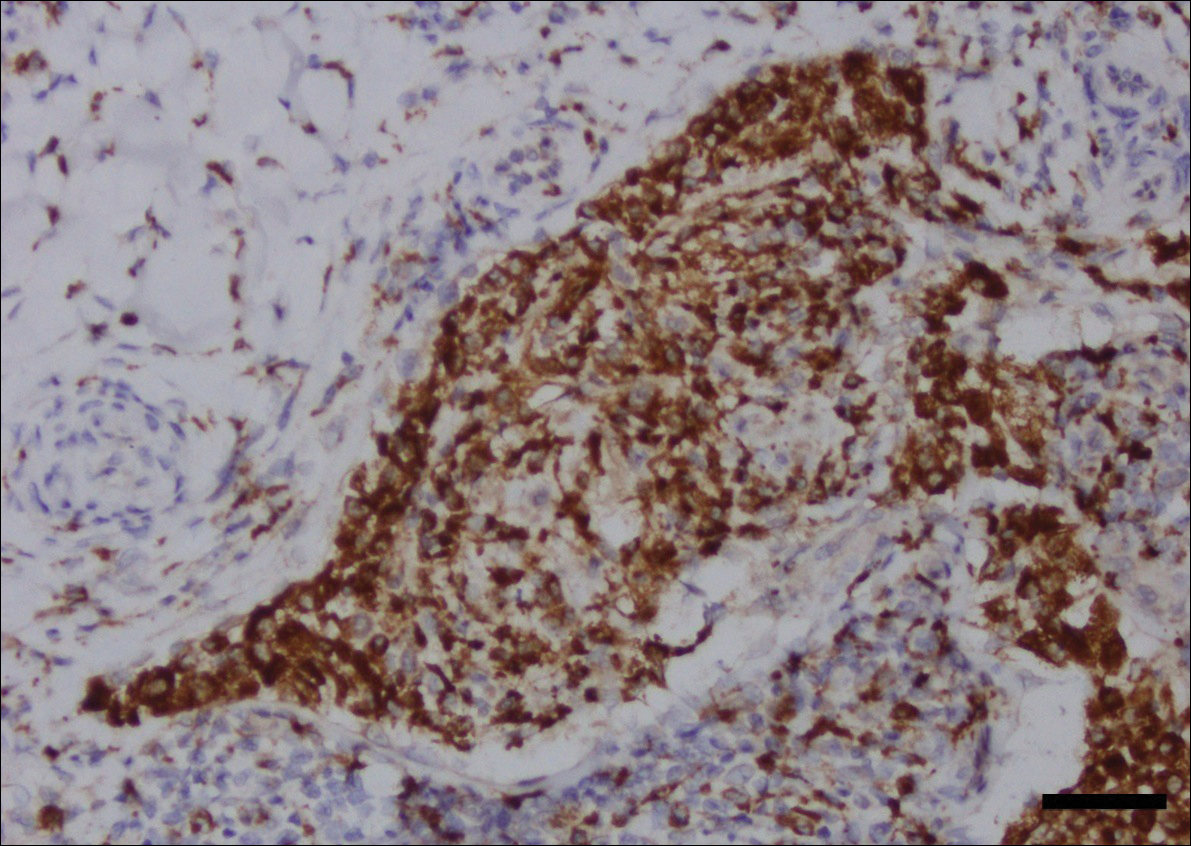
Fine-needle aspiration of the lesion revealed red blood cells and histiocytes. Laboratory studies showed an elevated erythrocyte sedimentation rate of 74 mm/h (reference range, 0–30 mm/h) and a C-reactive protein level of 39 mg/L (reference range, 0–10 mg/L). An incisional biopsy including the muscular fascia showed dense dermal fibrosis with chronic inflammation and scarring. A dermatopathologist (G. A. S.) reviewed the case and confirmed variable fibrosis and chronic inflammation associated with edema in the dermis and epidermal acanthosis. Inspection of vessels in the mid to upper dermis in one area revealed stellate, thin-walled, vascular structures that contained bland epithelioid cells lining the lumen as well as packed within the vessels. The epithelioid cells did not show atypia or mitotic figures, and they did not show intracytoplasmic vacuoles (Figure 2). Immunocytochemical staining for D2-40 was strongly positive in cells lining the vessels, consistent with lymphatics (Figure 3). CD68 immunohistochemistry for histiocytes stained the cells within the lymphatics (Figure 4). A diagnosis of intralymphatic histiocytosis was made.
Intralesional triamcinolone acetonide 10 mg/cc×1.6 cc was injected into the plaque once monthly for 2 consecutive months, and daily compression with a pressure bandage of the right lower leg was initiated. Four months after the first treatment with this regimen, the plaque was smaller and no longer sclerotic or painful, and the erythema was markedly reduced (Figure 5). Clinical and symptomatic improvement continued at 1-year follow-up.
Comment
Intralymphatic histiocytosis is a rare cutaneous disorder defined histologically by histiocytes within the lumina of lymphatics. In addition to the current case, our review of PubMed articles indexed for MEDLINE using the search term intralymphatic histiocytosis yielded more than 70 total cases. The condition has a slight female predominance and typically is seen in individuals over the age of 60 years (age range, 16–89 years).12 Many cases are associated with RA/elevated rheumatoid factor.2,4,8,15-30 At least 9 cases of intralymphatic histiocytosis were associated with premalignant or malignant conditions (ie, adenocarcinoma of the breasts, lungs, and colon; Merkel cell carcinoma; melanoma; melanoma in situ; Mullerian carcinoma, gammopathy).4,15,31-34 Primary disease, defined as occurring in patients who are otherwise healthy, was noted in at least 10 cases.1,2,4,12,35,36 Finally, intralymphatic histiocytosis was identified in areas adjacent to metal implants and joint replacements or exploration in approximately 15 cases (including the current case).3-14,29,37
The condition presents with papules, plaques, and nodules in the setting of characteristic livedoid discoloration; however, some patients present with nonspecific nodules or plaques. Lesions may be symptomatic (eg, pruritic, tender) or asymptomatic. The histologic features of intralymphatic histiocytosis are distinctive but may be focal, as in our case, and the diagnosis is easily missed. The histologic differential diagnosis includes diseases in which intravascular accumulations of cells may be seen, including intravascular B-cell lymphoma, which can be excluded with stains that detect B cells (CD20/CD79a), and reactive angioendotheliomatosis, a benign proliferation of endothelial cells, which may be excluded with stains against endothelial markers (CD31/CD34). The course typically is chronic, and treatment with topical steroids,3,9,15,22,26 cyclophosphamide,15 local radiation,1 thalidomide,35 pentoxifylline,7 and RA medications (eg, prednisolone, methotrexate, nonsteroidal anti-inflammatory drugs, hydroxychloroquine) generally are ineffective.2,16,20,25 Symptoms may improve with joint replacement,4 excision of the involved lesion, treatment of an associated malignancy/infection,33,36,38,39 nonsteroidal anti-inflammatory drugs, intra-articular steroid injection,18 amoxicillin and aspirin,19 infliximab,25 pressure bandage application,26 steroid-containing adhesive application,18 arthrocentesis,3,27 oral pentoxifylline,21 tacrolimus,29 CO2 laser,40 prednisolone,41 and tocilizumab.28 Treatment of associated RA is beneficial in rare cases.2,15,20,25,26
The pathogenesis of intralymphatic histiocytosis has not been elucidated with certainty but may represent an abnormal proliferative response of histiocytes and vessels in response to chronic systemic or local inflammation. Lymphangiectasis caused by lymphatic obstruction secondary to trauma, surgical manipulation, or chronic inflammation can promote lymphostasis and slowed clearance of antigens producing an accumulation of histiocytes and subsequent local immunologic reactions, thus an “immunocompromised district” is formed.42 It also is thought that rheumatic or prosthetic joints produce inflammatory mediator–rich (namely tumor necrosis factor α) synovial fluid that drains and collects within the dilated lymphatics, creating a nidus for histiocytes.1,5 In one case, treatment with an anti–tumor necrosis factor antibody (infliximab) improved the skin presentation and rheumatoid joint pain.25 Bakr et al2 noted an association with increased intralymphatic macrophage HLA-DR expression. This T-cell surface receptor typically is upregulated in cases of chronic antigen stimulation and autoimmune conditions.
Conclusion
Our patient had a history of a joint prosthesis and a popliteal cyst, which could have altered lymphatic drainage promoting abnormal immune cell trafficking contributing to the development of intralymphatic histiocytosis. The response to intralesional steroids supports this pathogenic hypothesis. Specifically, direct injection of the area suppressed the immune dysregulation, while compression lessened the degree of lymphostasis. In light of previously reported cases of intralymphatic histiocytosis in association with metal implants,3-9 we suggest that the condition should be considered in patients with chronic painful livedoid nodules or plaques around an affected joint, even in the absence of RA. The dermatopathologist should be warned to search carefully for the subtle but distinctive histologic features of the disease that confirm the diagnosis. Treatment with intralesional triamcinolone acetonide with an overlying pressure wrap has minimal side effects and can work quickly with sustained benefits.
- O’Grady JT, Shahidullah H, Doherty VR, et al. Intravascular histiocytosis. Histopathology. 1994;24:265-268.
- Bakr F, Webber N, Fassihi H, et al. Primary and secondary intralymphatic histiocytosis [published online January 17, 2014]. J Am Acad Dermatol. 2014;70:927-933.
- Watanabe T, Yamada N, Yoshida Y, et al. Intralymphatic histiocytosis with granuloma formation associated with orthopaedic metal implants [published online November 10, 2007]. Br J Dermatol. 2008;158:402-404.
- Requena L, El-Shabrawi-Caelen L, Walsh SN, et al. Intralymphatic histiocytosis. a clinicopathologic study of 16 cases. Am J Dermatopathol. 2009;31:140-151.
- Grekin S, Mesfin M, Kang S, et al. Intralymphatic histiocytosis following placement of a metal implant. J Cutan Pathol. 2011;38:351-353.
- Rossari S, Scatena C, Gori A, et al. Intralymphatic histiocytosis: cutaneous nodules and metal implants [published online March 6, 2011]. J Cutan Pathol. 2011;38:534-535.
- de Unamuno Bustos B, García Rabasco A, Ballester Sánchez R, et al. Erythematous indurated plaque on the right upper limb. intralymphatic histiocytosis (IH) associated with orthopedic metal implant. Int J Dermatol. 2013;52:547-549.
- Chiu YE, Maloney JE, Bengana C. Erythematous patch overlying a swollen knee—quiz case. intralymphatic histiocytosis. Arch Dermatol. 2010;146:1037-1042.
- Saggar S, Lee B, Krivo J, et al. Intralymphatic histiocytosis associated with orthopedic implants. J Drugs Dermatol. 2011;10:1208-1209.
- Bidier M, Hamsch C, Kutzner H, et al. Two cases of intralymphatic histiocytosis following hip replacement [published online June 9, 2015]. J Dtsch Dermatol Ges. 2015;13:700-702.
- Darling MD, Akin R, Tarbox MB, et al. Intralymphatic histiocytosis overlying hip implantation treated with pentoxifilline. J Biol Regul Homeost Agents. 2015;29(1 suppl):117-121.
- Demirkesen C, Kran T, Leblebici C, et al. Intravascular/intralymphatic histiocytosis: a report of 3 cases. Am J Dermatopathol. 2015;37:783-789.
- Gómez-Sánchez ME, Azaña-Defez JM, Martínez-Martínez ML, et al. Intralymphatic histiocytosis: a report of 2 cases. Actas Dermosifiliogr. 2018;109:E1-E5.
- Haitz KA, Chapman MS, Seidel GD. Intralymphatic histiocytosis associated with an orthopedic metal implant. Cutis. 2016;97:E12-E14.
- Rieger E, Soyer HP, Leboit PE, et al. Reactive angioendotheliomatosis or intravascular histiocytosis? an immunohistochemical and ultrastructural study in two cases of intravascular histiocytic cell proliferation. Br J Dermatol. 1999;140:497-504.
- Pruim B, Strutton G, Congdon S, et al. Cutaneous histiocytic lymphangitis: an unusual manifestation of rheumatoid arthritis. Australas J Dermatol. 2000;41:101-105.
- Magro CM, Crowson AN. The spectrum of cutaneous lesions in rheumatoid arthritis: a clinical and pathological study of 43 patients. J Cutan Pathol. 2003;30:1-10.
- Takiwaki H, Adachi A, Kohno H, et al. Intravascular or intralymphatic histiocytosis associated with rheumatoid arthritis: a report of 4 cases.J Am Acad Dermatol. 2004;50:585-590.
- Mensing CH, Krengel S, Tronnier M, et al. Reactive angioendotheliomatosis: is it “intravascular histiocytosis”? J Eur Acad Dermatol Venereol. 2005;19:216-219.
- Okazaki A, Asada H, Niizeki H, et al. Intravascular histiocytosis associated with rheumatoid arthritis: report of a case with lymphatic endothelial proliferation. Br J Dermatol. 2005;152:1385-1387.
- Catalina-Fernández I, Alvárez AC, Martin FC, et al. Cutaneous intralymphatic histiocytosis associated with rheumatoid arthritis: report of a case and review of the literature. Am J Dermatopathol. 2007;29:165-168.
- Nishie W, Sawamura D, Iitoyo M, et al. Intravascular histiocytosis associated with rheumatoid arthritis. Dermatology. 2008;217:144-145.
- Okamoto N, Tanioka M, Yamamoto T, et al. Intralymphatic histiocytosis associated with rheumatoid arthritis. Clin Exp Dermatol. 2008;33:516-518.
- Huang H-Y, Liang C-W, Hu S-L, et al. Cutaneous intravascular histiocytosis associated with rheumatoid arthritis: a case report and review of the literature. Clin Exp Dermatol. 2009;34:E302-E303.
- Sakaguchi M, Nagai H, Tsuji G, et al. Effectiveness of infliximab for intralymphatic histiocytosis with rheumatoid arthritis. Arch Dermatol. 2011;147:131-133.
- Washio K, Nakata K, Nakamura A, et al. Pressure bandage as an effective treatment for intralymphatic histiocytosis associated with rheumatoid arthritis. Dermatology. 2011;223:20-24.
- Kaneko T, Takeuchi S, Nakano H, et al. Intralymphatic histiocytosis with rheumatoid arthritis: possible association with the joint involvement. Case Reports Clin Med. 2014;3:149-152.
- Nakajima T, Kawabata D, Nakabo S, et al. Successful treatment with tocilizumab in a case of intralymphatic histiocytosis associated with rheumatoid arthritis. Intern Med. 2014;53:2255-2258.
- Tsujiwaki M, Hata H, Miyauchi T, et al. Warty intralymphatic histiocytosis successfully treated with topical tacrolimus. J Eur Acad Dermatol Venereol. 2015;29:2267-2269.
- Tanaka M, Funasaka Y, Tsuruta K, et al. Intralymphatic histiocytosis with massive interstitial granulomatous foci in a patient with rheumatoid arthritis. Ann Dermatol. 2017;29:237-238.
- Cornejo KM, Cosar EF, O’Donnell P. Cutaneous intralymphatic histiocytosis associated with lung adenocarcinoma. Am J Dermatopathol. 2016;38:568-570.
- Tran TAN, Tran Q, Carlson JA. Intralymphatic histiocytosis of the appendix and fallopian tube associated with primary peritoneal high-grade, poorly differentiated adenocarcinoma of Müllerian origin. Int J Surg Pathol. 2017;25:357-364.
- Echeverría-García B, Botella-Estrada R, Requena C, et al. Intralymphatic histiocytosis and cancer of the colon [in Spanish]. Actas Dermosifiliogr. 2010;101:257-262.
- Ergen EN, Zwerner JP. Cover image: intralymphatic histiocytosis with giant blanching violaceous plaques. Br J Dermatol. 2017;177:325-326.
- Wang Y, Yang H, Tu P. Upper facial swelling: an uncommon manifestation of intralymphatic histiocytosis. Eur J Dermatol. 2012;22:814-815.
- Rhee D-Y, Lee D-W, Chang S-E, et al. Intravascular histiocytosis without rheumatoid arthritis. J Dermatol. 2008;35:691-693.
- Gilchrest BA, Eller MS, Geller AC, et al. The pathogenesis of melanoma induced by ultraviolet radiation. N Engl J Med. 1999;340:1341-1348.
- Asagoe K, Torigoe R, Ofuji R, et al. Reactive intravascular histiocytosis associated with tonsillitis. Br J Dermatol. 2006;154:560-563.
- Pouryazdanparast P, Yu L, Dalton VK, et al. Intravascular histiocytosis presenting with extensive vulvar necrosis. J Cutan Pathol. 2009;(36 suppl 1):1-7.
- Reznitsky M, Daugaard S, Charabi BW. Two rare cases of laryngeal intralymphatic histiocytosis. Eur Arch Otorhinolaryngol. 2016;273:783-788.
- Fujimoto N, Nakanishi G, Manabe T, et al. Intralymphatic histiocytosis comprises M2 macrophages in superficial dermal lymphatics with or without smooth muscles. J Cutan Pathol. 2016;43:898-902.
- Piccolo V, Ruocco E, Russo T, et al. A possible relationship between metal implant-induced intralymphatic histiocytosis and the concept of the immunocompromised district. Int J Dermatol. 2014;53:E365.
Intralymphatic histiocytosis was first described in 1994.1 To date, at least 70 cases have been reported in the English-language literature, the majority being associated with systemic or local inflammatory conditions such as rheumatoid arthritis (RA), malignancy, and metal prostheses. The remaining cases arose independent of any detectable disease process.2 The clinical lesion localizes to areas around surgical scars or inflamed joints and generally presents with erythematous livedoid papules and plaques. Because of its rarity, pathologists and clinicians may be unfamiliar with this entity, leading to delayed or missed diagnoses.
Although the pathogenesis of intralymphatic histiocytosis remains unclear, it may be related to dysregulated immune signaling. The condition follows a chronic, relapsing-remitting course that has shown variable response to topical and systemic treatments. We present a rare case of intralymphatic histiocytosis associated with joint replacement/metal prosthesis3-14 that was responsive to a novel treatment with intralesional steroid injection and pressure bandage.
Case Report
An 89-year-old woman presented with a relapsing and remitting rash on the right calf and popliteal fossa of 11 months’ duration. It was becoming more painful over time and recently began to hurt when walking. Her medical history was remarkable for deep vein thromboses of the bilateral legs, Factor V Leiden deficiency, osteoarthritis, and a popliteal (Baker) cyst on the right leg that ruptured 22 months prior to presentation. Her surgical history included bilateral knee replacements (10 years and 2 years prior to the current presentation for the right and left knees, respectively). Her international normalized ratio (2.0) was therapeutic on warfarin.
Initially, swelling, pain, and redness developed in the right calf, and recurrent right-leg deep venous thrombosis was ruled out by Doppler ultrasound. The findings were considered to be secondary to inflammation from a popliteal cyst. Symptoms persisted despite application of warm compresses, leg elevation, and compression stockings. Treatment with doxycycline prescribed by the patient’s primary care physician 9 months prior for presumed cellulitis produced little improvement. Physical examination revealed a well-healed vertical scar on the right calf from an incisional biopsy within an 8-cm, tender, erythematous, indurated, sclerotic plaque with erythematous streaks radiating from the center of the plaque (Figure 1). There also was red-brown, indurated discoloration on the right shin.

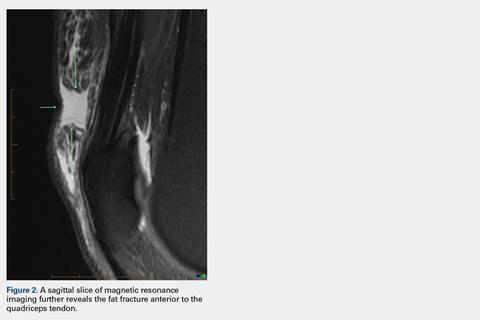
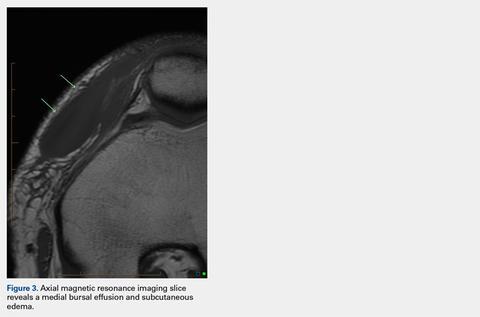
Fine-needle aspiration of the lesion revealed red blood cells and histiocytes. Laboratory studies showed an elevated erythrocyte sedimentation rate of 74 mm/h (reference range, 0–30 mm/h) and a C-reactive protein level of 39 mg/L (reference range, 0–10 mg/L). An incisional biopsy including the muscular fascia showed dense dermal fibrosis with chronic inflammation and scarring. A dermatopathologist (G. A. S.) reviewed the case and confirmed variable fibrosis and chronic inflammation associated with edema in the dermis and epidermal acanthosis. Inspection of vessels in the mid to upper dermis in one area revealed stellate, thin-walled, vascular structures that contained bland epithelioid cells lining the lumen as well as packed within the vessels. The epithelioid cells did not show atypia or mitotic figures, and they did not show intracytoplasmic vacuoles (Figure 2). Immunocytochemical staining for D2-40 was strongly positive in cells lining the vessels, consistent with lymphatics (Figure 3). CD68 immunohistochemistry for histiocytes stained the cells within the lymphatics (Figure 4). A diagnosis of intralymphatic histiocytosis was made.



Intralesional triamcinolone acetonide 10 mg/cc×1.6 cc was injected into the plaque once monthly for 2 consecutive months, and daily compression with a pressure bandage of the right lower leg was initiated. Four months after the first treatment with this regimen, the plaque was smaller and no longer sclerotic or painful, and the erythema was markedly reduced (Figure 5). Clinical and symptomatic improvement continued at 1-year follow-up.

Comment
Intralymphatic histiocytosis is a rare cutaneous disorder defined histologically by histiocytes within the lumina of lymphatics. In addition to the current case, our review of PubMed articles indexed for MEDLINE using the search term intralymphatic histiocytosis yielded more than 70 total cases. The condition has a slight female predominance and typically is seen in individuals over the age of 60 years (age range, 16–89 years).12 Many cases are associated with RA/elevated rheumatoid factor.2,4,8,15-30 At least 9 cases of intralymphatic histiocytosis were associated with premalignant or malignant conditions (ie, adenocarcinoma of the breasts, lungs, and colon; Merkel cell carcinoma; melanoma; melanoma in situ; Mullerian carcinoma, gammopathy).4,15,31-34 Primary disease, defined as occurring in patients who are otherwise healthy, was noted in at least 10 cases.1,2,4,12,35,36 Finally, intralymphatic histiocytosis was identified in areas adjacent to metal implants and joint replacements or exploration in approximately 15 cases (including the current case).3-14,29,37
The condition presents with papules, plaques, and nodules in the setting of characteristic livedoid discoloration; however, some patients present with nonspecific nodules or plaques. Lesions may be symptomatic (eg, pruritic, tender) or asymptomatic. The histologic features of intralymphatic histiocytosis are distinctive but may be focal, as in our case, and the diagnosis is easily missed. The histologic differential diagnosis includes diseases in which intravascular accumulations of cells may be seen, including intravascular B-cell lymphoma, which can be excluded with stains that detect B cells (CD20/CD79a), and reactive angioendotheliomatosis, a benign proliferation of endothelial cells, which may be excluded with stains against endothelial markers (CD31/CD34). The course typically is chronic, and treatment with topical steroids,3,9,15,22,26 cyclophosphamide,15 local radiation,1 thalidomide,35 pentoxifylline,7 and RA medications (eg, prednisolone, methotrexate, nonsteroidal anti-inflammatory drugs, hydroxychloroquine) generally are ineffective.2,16,20,25 Symptoms may improve with joint replacement,4 excision of the involved lesion, treatment of an associated malignancy/infection,33,36,38,39 nonsteroidal anti-inflammatory drugs, intra-articular steroid injection,18 amoxicillin and aspirin,19 infliximab,25 pressure bandage application,26 steroid-containing adhesive application,18 arthrocentesis,3,27 oral pentoxifylline,21 tacrolimus,29 CO2 laser,40 prednisolone,41 and tocilizumab.28 Treatment of associated RA is beneficial in rare cases.2,15,20,25,26
The pathogenesis of intralymphatic histiocytosis has not been elucidated with certainty but may represent an abnormal proliferative response of histiocytes and vessels in response to chronic systemic or local inflammation. Lymphangiectasis caused by lymphatic obstruction secondary to trauma, surgical manipulation, or chronic inflammation can promote lymphostasis and slowed clearance of antigens producing an accumulation of histiocytes and subsequent local immunologic reactions, thus an “immunocompromised district” is formed.42 It also is thought that rheumatic or prosthetic joints produce inflammatory mediator–rich (namely tumor necrosis factor α) synovial fluid that drains and collects within the dilated lymphatics, creating a nidus for histiocytes.1,5 In one case, treatment with an anti–tumor necrosis factor antibody (infliximab) improved the skin presentation and rheumatoid joint pain.25 Bakr et al2 noted an association with increased intralymphatic macrophage HLA-DR expression. This T-cell surface receptor typically is upregulated in cases of chronic antigen stimulation and autoimmune conditions.
Conclusion
Our patient had a history of a joint prosthesis and a popliteal cyst, which could have altered lymphatic drainage promoting abnormal immune cell trafficking contributing to the development of intralymphatic histiocytosis. The response to intralesional steroids supports this pathogenic hypothesis. Specifically, direct injection of the area suppressed the immune dysregulation, while compression lessened the degree of lymphostasis. In light of previously reported cases of intralymphatic histiocytosis in association with metal implants,3-9 we suggest that the condition should be considered in patients with chronic painful livedoid nodules or plaques around an affected joint, even in the absence of RA. The dermatopathologist should be warned to search carefully for the subtle but distinctive histologic features of the disease that confirm the diagnosis. Treatment with intralesional triamcinolone acetonide with an overlying pressure wrap has minimal side effects and can work quickly with sustained benefits.
Intralymphatic histiocytosis was first described in 1994.1 To date, at least 70 cases have been reported in the English-language literature, the majority being associated with systemic or local inflammatory conditions such as rheumatoid arthritis (RA), malignancy, and metal prostheses. The remaining cases arose independent of any detectable disease process.2 The clinical lesion localizes to areas around surgical scars or inflamed joints and generally presents with erythematous livedoid papules and plaques. Because of its rarity, pathologists and clinicians may be unfamiliar with this entity, leading to delayed or missed diagnoses.
Although the pathogenesis of intralymphatic histiocytosis remains unclear, it may be related to dysregulated immune signaling. The condition follows a chronic, relapsing-remitting course that has shown variable response to topical and systemic treatments. We present a rare case of intralymphatic histiocytosis associated with joint replacement/metal prosthesis3-14 that was responsive to a novel treatment with intralesional steroid injection and pressure bandage.
Case Report
An 89-year-old woman presented with a relapsing and remitting rash on the right calf and popliteal fossa of 11 months’ duration. It was becoming more painful over time and recently began to hurt when walking. Her medical history was remarkable for deep vein thromboses of the bilateral legs, Factor V Leiden deficiency, osteoarthritis, and a popliteal (Baker) cyst on the right leg that ruptured 22 months prior to presentation. Her surgical history included bilateral knee replacements (10 years and 2 years prior to the current presentation for the right and left knees, respectively). Her international normalized ratio (2.0) was therapeutic on warfarin.
Initially, swelling, pain, and redness developed in the right calf, and recurrent right-leg deep venous thrombosis was ruled out by Doppler ultrasound. The findings were considered to be secondary to inflammation from a popliteal cyst. Symptoms persisted despite application of warm compresses, leg elevation, and compression stockings. Treatment with doxycycline prescribed by the patient’s primary care physician 9 months prior for presumed cellulitis produced little improvement. Physical examination revealed a well-healed vertical scar on the right calf from an incisional biopsy within an 8-cm, tender, erythematous, indurated, sclerotic plaque with erythematous streaks radiating from the center of the plaque (Figure 1). There also was red-brown, indurated discoloration on the right shin.

Fine-needle aspiration of the lesion revealed red blood cells and histiocytes. Laboratory studies showed an elevated erythrocyte sedimentation rate of 74 mm/h (reference range, 0–30 mm/h) and a C-reactive protein level of 39 mg/L (reference range, 0–10 mg/L). An incisional biopsy including the muscular fascia showed dense dermal fibrosis with chronic inflammation and scarring. A dermatopathologist (G. A. S.) reviewed the case and confirmed variable fibrosis and chronic inflammation associated with edema in the dermis and epidermal acanthosis. Inspection of vessels in the mid to upper dermis in one area revealed stellate, thin-walled, vascular structures that contained bland epithelioid cells lining the lumen as well as packed within the vessels. The epithelioid cells did not show atypia or mitotic figures, and they did not show intracytoplasmic vacuoles (Figure 2). Immunocytochemical staining for D2-40 was strongly positive in cells lining the vessels, consistent with lymphatics (Figure 3). CD68 immunohistochemistry for histiocytes stained the cells within the lymphatics (Figure 4). A diagnosis of intralymphatic histiocytosis was made.



Intralesional triamcinolone acetonide 10 mg/cc×1.6 cc was injected into the plaque once monthly for 2 consecutive months, and daily compression with a pressure bandage of the right lower leg was initiated. Four months after the first treatment with this regimen, the plaque was smaller and no longer sclerotic or painful, and the erythema was markedly reduced (Figure 5). Clinical and symptomatic improvement continued at 1-year follow-up.

Comment
Intralymphatic histiocytosis is a rare cutaneous disorder defined histologically by histiocytes within the lumina of lymphatics. In addition to the current case, our review of PubMed articles indexed for MEDLINE using the search term intralymphatic histiocytosis yielded more than 70 total cases. The condition has a slight female predominance and typically is seen in individuals over the age of 60 years (age range, 16–89 years).12 Many cases are associated with RA/elevated rheumatoid factor.2,4,8,15-30 At least 9 cases of intralymphatic histiocytosis were associated with premalignant or malignant conditions (ie, adenocarcinoma of the breasts, lungs, and colon; Merkel cell carcinoma; melanoma; melanoma in situ; Mullerian carcinoma, gammopathy).4,15,31-34 Primary disease, defined as occurring in patients who are otherwise healthy, was noted in at least 10 cases.1,2,4,12,35,36 Finally, intralymphatic histiocytosis was identified in areas adjacent to metal implants and joint replacements or exploration in approximately 15 cases (including the current case).3-14,29,37
The condition presents with papules, plaques, and nodules in the setting of characteristic livedoid discoloration; however, some patients present with nonspecific nodules or plaques. Lesions may be symptomatic (eg, pruritic, tender) or asymptomatic. The histologic features of intralymphatic histiocytosis are distinctive but may be focal, as in our case, and the diagnosis is easily missed. The histologic differential diagnosis includes diseases in which intravascular accumulations of cells may be seen, including intravascular B-cell lymphoma, which can be excluded with stains that detect B cells (CD20/CD79a), and reactive angioendotheliomatosis, a benign proliferation of endothelial cells, which may be excluded with stains against endothelial markers (CD31/CD34). The course typically is chronic, and treatment with topical steroids,3,9,15,22,26 cyclophosphamide,15 local radiation,1 thalidomide,35 pentoxifylline,7 and RA medications (eg, prednisolone, methotrexate, nonsteroidal anti-inflammatory drugs, hydroxychloroquine) generally are ineffective.2,16,20,25 Symptoms may improve with joint replacement,4 excision of the involved lesion, treatment of an associated malignancy/infection,33,36,38,39 nonsteroidal anti-inflammatory drugs, intra-articular steroid injection,18 amoxicillin and aspirin,19 infliximab,25 pressure bandage application,26 steroid-containing adhesive application,18 arthrocentesis,3,27 oral pentoxifylline,21 tacrolimus,29 CO2 laser,40 prednisolone,41 and tocilizumab.28 Treatment of associated RA is beneficial in rare cases.2,15,20,25,26
The pathogenesis of intralymphatic histiocytosis has not been elucidated with certainty but may represent an abnormal proliferative response of histiocytes and vessels in response to chronic systemic or local inflammation. Lymphangiectasis caused by lymphatic obstruction secondary to trauma, surgical manipulation, or chronic inflammation can promote lymphostasis and slowed clearance of antigens producing an accumulation of histiocytes and subsequent local immunologic reactions, thus an “immunocompromised district” is formed.42 It also is thought that rheumatic or prosthetic joints produce inflammatory mediator–rich (namely tumor necrosis factor α) synovial fluid that drains and collects within the dilated lymphatics, creating a nidus for histiocytes.1,5 In one case, treatment with an anti–tumor necrosis factor antibody (infliximab) improved the skin presentation and rheumatoid joint pain.25 Bakr et al2 noted an association with increased intralymphatic macrophage HLA-DR expression. This T-cell surface receptor typically is upregulated in cases of chronic antigen stimulation and autoimmune conditions.
Conclusion
Our patient had a history of a joint prosthesis and a popliteal cyst, which could have altered lymphatic drainage promoting abnormal immune cell trafficking contributing to the development of intralymphatic histiocytosis. The response to intralesional steroids supports this pathogenic hypothesis. Specifically, direct injection of the area suppressed the immune dysregulation, while compression lessened the degree of lymphostasis. In light of previously reported cases of intralymphatic histiocytosis in association with metal implants,3-9 we suggest that the condition should be considered in patients with chronic painful livedoid nodules or plaques around an affected joint, even in the absence of RA. The dermatopathologist should be warned to search carefully for the subtle but distinctive histologic features of the disease that confirm the diagnosis. Treatment with intralesional triamcinolone acetonide with an overlying pressure wrap has minimal side effects and can work quickly with sustained benefits.
- O’Grady JT, Shahidullah H, Doherty VR, et al. Intravascular histiocytosis. Histopathology. 1994;24:265-268.
- Bakr F, Webber N, Fassihi H, et al. Primary and secondary intralymphatic histiocytosis [published online January 17, 2014]. J Am Acad Dermatol. 2014;70:927-933.
- Watanabe T, Yamada N, Yoshida Y, et al. Intralymphatic histiocytosis with granuloma formation associated with orthopaedic metal implants [published online November 10, 2007]. Br J Dermatol. 2008;158:402-404.
- Requena L, El-Shabrawi-Caelen L, Walsh SN, et al. Intralymphatic histiocytosis. a clinicopathologic study of 16 cases. Am J Dermatopathol. 2009;31:140-151.
- Grekin S, Mesfin M, Kang S, et al. Intralymphatic histiocytosis following placement of a metal implant. J Cutan Pathol. 2011;38:351-353.
- Rossari S, Scatena C, Gori A, et al. Intralymphatic histiocytosis: cutaneous nodules and metal implants [published online March 6, 2011]. J Cutan Pathol. 2011;38:534-535.
- de Unamuno Bustos B, García Rabasco A, Ballester Sánchez R, et al. Erythematous indurated plaque on the right upper limb. intralymphatic histiocytosis (IH) associated with orthopedic metal implant. Int J Dermatol. 2013;52:547-549.
- Chiu YE, Maloney JE, Bengana C. Erythematous patch overlying a swollen knee—quiz case. intralymphatic histiocytosis. Arch Dermatol. 2010;146:1037-1042.
- Saggar S, Lee B, Krivo J, et al. Intralymphatic histiocytosis associated with orthopedic implants. J Drugs Dermatol. 2011;10:1208-1209.
- Bidier M, Hamsch C, Kutzner H, et al. Two cases of intralymphatic histiocytosis following hip replacement [published online June 9, 2015]. J Dtsch Dermatol Ges. 2015;13:700-702.
- Darling MD, Akin R, Tarbox MB, et al. Intralymphatic histiocytosis overlying hip implantation treated with pentoxifilline. J Biol Regul Homeost Agents. 2015;29(1 suppl):117-121.
- Demirkesen C, Kran T, Leblebici C, et al. Intravascular/intralymphatic histiocytosis: a report of 3 cases. Am J Dermatopathol. 2015;37:783-789.
- Gómez-Sánchez ME, Azaña-Defez JM, Martínez-Martínez ML, et al. Intralymphatic histiocytosis: a report of 2 cases. Actas Dermosifiliogr. 2018;109:E1-E5.
- Haitz KA, Chapman MS, Seidel GD. Intralymphatic histiocytosis associated with an orthopedic metal implant. Cutis. 2016;97:E12-E14.
- Rieger E, Soyer HP, Leboit PE, et al. Reactive angioendotheliomatosis or intravascular histiocytosis? an immunohistochemical and ultrastructural study in two cases of intravascular histiocytic cell proliferation. Br J Dermatol. 1999;140:497-504.
- Pruim B, Strutton G, Congdon S, et al. Cutaneous histiocytic lymphangitis: an unusual manifestation of rheumatoid arthritis. Australas J Dermatol. 2000;41:101-105.
- Magro CM, Crowson AN. The spectrum of cutaneous lesions in rheumatoid arthritis: a clinical and pathological study of 43 patients. J Cutan Pathol. 2003;30:1-10.
- Takiwaki H, Adachi A, Kohno H, et al. Intravascular or intralymphatic histiocytosis associated with rheumatoid arthritis: a report of 4 cases.J Am Acad Dermatol. 2004;50:585-590.
- Mensing CH, Krengel S, Tronnier M, et al. Reactive angioendotheliomatosis: is it “intravascular histiocytosis”? J Eur Acad Dermatol Venereol. 2005;19:216-219.
- Okazaki A, Asada H, Niizeki H, et al. Intravascular histiocytosis associated with rheumatoid arthritis: report of a case with lymphatic endothelial proliferation. Br J Dermatol. 2005;152:1385-1387.
- Catalina-Fernández I, Alvárez AC, Martin FC, et al. Cutaneous intralymphatic histiocytosis associated with rheumatoid arthritis: report of a case and review of the literature. Am J Dermatopathol. 2007;29:165-168.
- Nishie W, Sawamura D, Iitoyo M, et al. Intravascular histiocytosis associated with rheumatoid arthritis. Dermatology. 2008;217:144-145.
- Okamoto N, Tanioka M, Yamamoto T, et al. Intralymphatic histiocytosis associated with rheumatoid arthritis. Clin Exp Dermatol. 2008;33:516-518.
- Huang H-Y, Liang C-W, Hu S-L, et al. Cutaneous intravascular histiocytosis associated with rheumatoid arthritis: a case report and review of the literature. Clin Exp Dermatol. 2009;34:E302-E303.
- Sakaguchi M, Nagai H, Tsuji G, et al. Effectiveness of infliximab for intralymphatic histiocytosis with rheumatoid arthritis. Arch Dermatol. 2011;147:131-133.
- Washio K, Nakata K, Nakamura A, et al. Pressure bandage as an effective treatment for intralymphatic histiocytosis associated with rheumatoid arthritis. Dermatology. 2011;223:20-24.
- Kaneko T, Takeuchi S, Nakano H, et al. Intralymphatic histiocytosis with rheumatoid arthritis: possible association with the joint involvement. Case Reports Clin Med. 2014;3:149-152.
- Nakajima T, Kawabata D, Nakabo S, et al. Successful treatment with tocilizumab in a case of intralymphatic histiocytosis associated with rheumatoid arthritis. Intern Med. 2014;53:2255-2258.
- Tsujiwaki M, Hata H, Miyauchi T, et al. Warty intralymphatic histiocytosis successfully treated with topical tacrolimus. J Eur Acad Dermatol Venereol. 2015;29:2267-2269.
- Tanaka M, Funasaka Y, Tsuruta K, et al. Intralymphatic histiocytosis with massive interstitial granulomatous foci in a patient with rheumatoid arthritis. Ann Dermatol. 2017;29:237-238.
- Cornejo KM, Cosar EF, O’Donnell P. Cutaneous intralymphatic histiocytosis associated with lung adenocarcinoma. Am J Dermatopathol. 2016;38:568-570.
- Tran TAN, Tran Q, Carlson JA. Intralymphatic histiocytosis of the appendix and fallopian tube associated with primary peritoneal high-grade, poorly differentiated adenocarcinoma of Müllerian origin. Int J Surg Pathol. 2017;25:357-364.
- Echeverría-García B, Botella-Estrada R, Requena C, et al. Intralymphatic histiocytosis and cancer of the colon [in Spanish]. Actas Dermosifiliogr. 2010;101:257-262.
- Ergen EN, Zwerner JP. Cover image: intralymphatic histiocytosis with giant blanching violaceous plaques. Br J Dermatol. 2017;177:325-326.
- Wang Y, Yang H, Tu P. Upper facial swelling: an uncommon manifestation of intralymphatic histiocytosis. Eur J Dermatol. 2012;22:814-815.
- Rhee D-Y, Lee D-W, Chang S-E, et al. Intravascular histiocytosis without rheumatoid arthritis. J Dermatol. 2008;35:691-693.
- Gilchrest BA, Eller MS, Geller AC, et al. The pathogenesis of melanoma induced by ultraviolet radiation. N Engl J Med. 1999;340:1341-1348.
- Asagoe K, Torigoe R, Ofuji R, et al. Reactive intravascular histiocytosis associated with tonsillitis. Br J Dermatol. 2006;154:560-563.
- Pouryazdanparast P, Yu L, Dalton VK, et al. Intravascular histiocytosis presenting with extensive vulvar necrosis. J Cutan Pathol. 2009;(36 suppl 1):1-7.
- Reznitsky M, Daugaard S, Charabi BW. Two rare cases of laryngeal intralymphatic histiocytosis. Eur Arch Otorhinolaryngol. 2016;273:783-788.
- Fujimoto N, Nakanishi G, Manabe T, et al. Intralymphatic histiocytosis comprises M2 macrophages in superficial dermal lymphatics with or without smooth muscles. J Cutan Pathol. 2016;43:898-902.
- Piccolo V, Ruocco E, Russo T, et al. A possible relationship between metal implant-induced intralymphatic histiocytosis and the concept of the immunocompromised district. Int J Dermatol. 2014;53:E365.
- O’Grady JT, Shahidullah H, Doherty VR, et al. Intravascular histiocytosis. Histopathology. 1994;24:265-268.
- Bakr F, Webber N, Fassihi H, et al. Primary and secondary intralymphatic histiocytosis [published online January 17, 2014]. J Am Acad Dermatol. 2014;70:927-933.
- Watanabe T, Yamada N, Yoshida Y, et al. Intralymphatic histiocytosis with granuloma formation associated with orthopaedic metal implants [published online November 10, 2007]. Br J Dermatol. 2008;158:402-404.
- Requena L, El-Shabrawi-Caelen L, Walsh SN, et al. Intralymphatic histiocytosis. a clinicopathologic study of 16 cases. Am J Dermatopathol. 2009;31:140-151.
- Grekin S, Mesfin M, Kang S, et al. Intralymphatic histiocytosis following placement of a metal implant. J Cutan Pathol. 2011;38:351-353.
- Rossari S, Scatena C, Gori A, et al. Intralymphatic histiocytosis: cutaneous nodules and metal implants [published online March 6, 2011]. J Cutan Pathol. 2011;38:534-535.
- de Unamuno Bustos B, García Rabasco A, Ballester Sánchez R, et al. Erythematous indurated plaque on the right upper limb. intralymphatic histiocytosis (IH) associated with orthopedic metal implant. Int J Dermatol. 2013;52:547-549.
- Chiu YE, Maloney JE, Bengana C. Erythematous patch overlying a swollen knee—quiz case. intralymphatic histiocytosis. Arch Dermatol. 2010;146:1037-1042.
- Saggar S, Lee B, Krivo J, et al. Intralymphatic histiocytosis associated with orthopedic implants. J Drugs Dermatol. 2011;10:1208-1209.
- Bidier M, Hamsch C, Kutzner H, et al. Two cases of intralymphatic histiocytosis following hip replacement [published online June 9, 2015]. J Dtsch Dermatol Ges. 2015;13:700-702.
- Darling MD, Akin R, Tarbox MB, et al. Intralymphatic histiocytosis overlying hip implantation treated with pentoxifilline. J Biol Regul Homeost Agents. 2015;29(1 suppl):117-121.
- Demirkesen C, Kran T, Leblebici C, et al. Intravascular/intralymphatic histiocytosis: a report of 3 cases. Am J Dermatopathol. 2015;37:783-789.
- Gómez-Sánchez ME, Azaña-Defez JM, Martínez-Martínez ML, et al. Intralymphatic histiocytosis: a report of 2 cases. Actas Dermosifiliogr. 2018;109:E1-E5.
- Haitz KA, Chapman MS, Seidel GD. Intralymphatic histiocytosis associated with an orthopedic metal implant. Cutis. 2016;97:E12-E14.
- Rieger E, Soyer HP, Leboit PE, et al. Reactive angioendotheliomatosis or intravascular histiocytosis? an immunohistochemical and ultrastructural study in two cases of intravascular histiocytic cell proliferation. Br J Dermatol. 1999;140:497-504.
- Pruim B, Strutton G, Congdon S, et al. Cutaneous histiocytic lymphangitis: an unusual manifestation of rheumatoid arthritis. Australas J Dermatol. 2000;41:101-105.
- Magro CM, Crowson AN. The spectrum of cutaneous lesions in rheumatoid arthritis: a clinical and pathological study of 43 patients. J Cutan Pathol. 2003;30:1-10.
- Takiwaki H, Adachi A, Kohno H, et al. Intravascular or intralymphatic histiocytosis associated with rheumatoid arthritis: a report of 4 cases.J Am Acad Dermatol. 2004;50:585-590.
- Mensing CH, Krengel S, Tronnier M, et al. Reactive angioendotheliomatosis: is it “intravascular histiocytosis”? J Eur Acad Dermatol Venereol. 2005;19:216-219.
- Okazaki A, Asada H, Niizeki H, et al. Intravascular histiocytosis associated with rheumatoid arthritis: report of a case with lymphatic endothelial proliferation. Br J Dermatol. 2005;152:1385-1387.
- Catalina-Fernández I, Alvárez AC, Martin FC, et al. Cutaneous intralymphatic histiocytosis associated with rheumatoid arthritis: report of a case and review of the literature. Am J Dermatopathol. 2007;29:165-168.
- Nishie W, Sawamura D, Iitoyo M, et al. Intravascular histiocytosis associated with rheumatoid arthritis. Dermatology. 2008;217:144-145.
- Okamoto N, Tanioka M, Yamamoto T, et al. Intralymphatic histiocytosis associated with rheumatoid arthritis. Clin Exp Dermatol. 2008;33:516-518.
- Huang H-Y, Liang C-W, Hu S-L, et al. Cutaneous intravascular histiocytosis associated with rheumatoid arthritis: a case report and review of the literature. Clin Exp Dermatol. 2009;34:E302-E303.
- Sakaguchi M, Nagai H, Tsuji G, et al. Effectiveness of infliximab for intralymphatic histiocytosis with rheumatoid arthritis. Arch Dermatol. 2011;147:131-133.
- Washio K, Nakata K, Nakamura A, et al. Pressure bandage as an effective treatment for intralymphatic histiocytosis associated with rheumatoid arthritis. Dermatology. 2011;223:20-24.
- Kaneko T, Takeuchi S, Nakano H, et al. Intralymphatic histiocytosis with rheumatoid arthritis: possible association with the joint involvement. Case Reports Clin Med. 2014;3:149-152.
- Nakajima T, Kawabata D, Nakabo S, et al. Successful treatment with tocilizumab in a case of intralymphatic histiocytosis associated with rheumatoid arthritis. Intern Med. 2014;53:2255-2258.
- Tsujiwaki M, Hata H, Miyauchi T, et al. Warty intralymphatic histiocytosis successfully treated with topical tacrolimus. J Eur Acad Dermatol Venereol. 2015;29:2267-2269.
- Tanaka M, Funasaka Y, Tsuruta K, et al. Intralymphatic histiocytosis with massive interstitial granulomatous foci in a patient with rheumatoid arthritis. Ann Dermatol. 2017;29:237-238.
- Cornejo KM, Cosar EF, O’Donnell P. Cutaneous intralymphatic histiocytosis associated with lung adenocarcinoma. Am J Dermatopathol. 2016;38:568-570.
- Tran TAN, Tran Q, Carlson JA. Intralymphatic histiocytosis of the appendix and fallopian tube associated with primary peritoneal high-grade, poorly differentiated adenocarcinoma of Müllerian origin. Int J Surg Pathol. 2017;25:357-364.
- Echeverría-García B, Botella-Estrada R, Requena C, et al. Intralymphatic histiocytosis and cancer of the colon [in Spanish]. Actas Dermosifiliogr. 2010;101:257-262.
- Ergen EN, Zwerner JP. Cover image: intralymphatic histiocytosis with giant blanching violaceous plaques. Br J Dermatol. 2017;177:325-326.
- Wang Y, Yang H, Tu P. Upper facial swelling: an uncommon manifestation of intralymphatic histiocytosis. Eur J Dermatol. 2012;22:814-815.
- Rhee D-Y, Lee D-W, Chang S-E, et al. Intravascular histiocytosis without rheumatoid arthritis. J Dermatol. 2008;35:691-693.
- Gilchrest BA, Eller MS, Geller AC, et al. The pathogenesis of melanoma induced by ultraviolet radiation. N Engl J Med. 1999;340:1341-1348.
- Asagoe K, Torigoe R, Ofuji R, et al. Reactive intravascular histiocytosis associated with tonsillitis. Br J Dermatol. 2006;154:560-563.
- Pouryazdanparast P, Yu L, Dalton VK, et al. Intravascular histiocytosis presenting with extensive vulvar necrosis. J Cutan Pathol. 2009;(36 suppl 1):1-7.
- Reznitsky M, Daugaard S, Charabi BW. Two rare cases of laryngeal intralymphatic histiocytosis. Eur Arch Otorhinolaryngol. 2016;273:783-788.
- Fujimoto N, Nakanishi G, Manabe T, et al. Intralymphatic histiocytosis comprises M2 macrophages in superficial dermal lymphatics with or without smooth muscles. J Cutan Pathol. 2016;43:898-902.
- Piccolo V, Ruocco E, Russo T, et al. A possible relationship between metal implant-induced intralymphatic histiocytosis and the concept of the immunocompromised district. Int J Dermatol. 2014;53:E365.
Practice Points
- Intralymphatic histiocytosis is a rare disorder often associated with rheumatic arthritis and joint prostheses.
- The diagnosis is made by histopathology as well as D2-40 and CD68 immunostaining.
- While there is no gold standard of treatment for intralymphatic histiocytosis, intralesional triamcinolone proved efficacious in this case with prolonged results.
Synovial Chondromatosis: An Unusual Case of Knee Pain and Swelling
Joint mice or loose/rice bodies are infrequently encountered within joints. Usually, they are either fibrin or cartilaginous. The fibrin type, typically results from bleeding within a joint from synovitis, rheumatoid arthritis (RA), or tuberculosis, and the cartilaginous/osteocartilaginous type develop from trauma or osteoarthritis.1 A rare cause of osteocartilaginous joint mice is synovial chondromatosis(SC), which can produce multiple loose bodies that originate from the synovial membranes of joints, bursae, and tendon sheaths of large joints; the knee being the most common (50%-65 % of cases).1,2
A case of a male who had multiple years of left knee pain and swelling without a documented traumatic cause is presented
Case Presentation
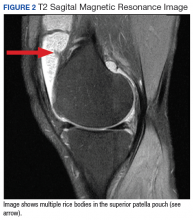
A 34-year-old male veteran was evaluated and treated in a VA orthopedic outpatient clinic by a physician assistant for anterior left knee pain and swelling of insidious onset that had persisted for 1.5 years. The patient reported experiencing no trauma. His primary care provider already was treating the patient with nonsteroidal anti-inflammatory medications (NSAIDs), icing, and bracing. He had full motion in his knee with extension/flexion 0° to 130°. Collateral and cruciate ligaments were stable. He had a positive McMurray test. The X-rays showed no pathology. Due to the positive meniscal tear signs, a magnetic resonance image (MRI) was ordered.
The patient was intermittently nonadherent with follow-up care. The MRI results were available at a subsequent appointment 3 months after the index evaluation, which revealed a large joint effusion with rice bodies, small erosion of the posterior tibialplateau, and synovial proliferation of the anterior knee joint. A steroid injection to his affected knee was given. Concerns for possible RA led to a workup. The laboratory results included rheumatoid factor (weakly positive), antinuclear-antibodies (negative), human immunodeficiency virus (positivewith western blot negative), C-reactive protein (< 0.02 mg/L), erythrocyte sedimentation rate (5 mm/h), white blood cell count (4.6 µL), hepatitis B surface antigen (reactive), hepatitis A antibody (IgG reactive), synovial fluid cultures, and Gram stain (negative).
The patient saw a rheumatologist 7 months after a RA referral was processed. The consulting rheumatologist was unconcerned by a weakly positive rheumatoid factor, which was later repeated and was negative. The rheumatologist excluded the possibility of RA, and the patient was diagnosed with oligoarthritis. The treatment rendered was to continue NSAIDs and to return to the orthopedic clinic for continued care.
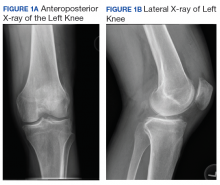
The patient had irregular follow-up visits where he received multiple methylprednisolone acetate intra-articular injections. His motion regressed until extension/flexion had decreased to 5°/85°. At this point the patient was forwarded to an operative orthopedic surgeon for evaluation for surgical intervention. Recent anteroposterior and lateral left knee X-rays showed faint intra-articular calcification, joint effusion, with mild arthritic changes of the patellofemoral joint (Figures 1A and 1B).
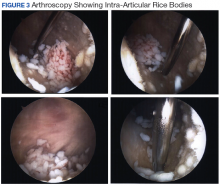
At surgery, on placing the infralateral portal, clear straw-colored fluid exited the cannula followed by copious small white rice bodies, which were sent to pathology for evaluation. The knee was surgically evaluated, and extensive rice bodies were encountered (Figure 3). These were extracted with a full radius shaver. The chondral surfaces were inspected. There were no arthritic changes, but the synovial lining of the joint was hypertrophied and reactive (Milgram phase 2). After all loose bodies were extracted, the patient’s incisions were closed with nylon suture, and he was placed in sterile dressings with a postoperative range of motion brace.
The patient presented for his routine postoperative visit 14 days after surgery. Pathology results showed synociocytes, and inflammatory cells were negative for malignancy. The patient was forwarded to a local hospital for further evaluation and treatment by an orthopedic oncologist due to a reported 5% chance of malignant transformation.1-3
Discussion
Synovial chondromatosis or osteochondromatosis is a rare, benign, metaplastic, typically monoarticular disorder of the synovial lining of joints, bursae, and synovial sheaths, usually affecting large joints.1-5 Although any joint can be involved, such as metacarpalphalangeal joints, temporomandibular joints, distal radio-ulnar joints, and the hips, the knee is the most common with an occurrence rate 50% to 65%.3-5 Extra-articular proliferation can be seen in cases of osteochondromatosis.2 It is characterized by the formation of intra-articular nodules of the synovium that can detach and become loose bodies, which can secondarily become calcified/ossified.4,6 The differential diagnosis associated with SC should include synovial hemangioma, pigmented villonodular synovitis, synovial cyst, lipoma arborescence, and malignancies, such as synovial chondrosarcoma or synovial sarcoma.3
Men are affected twice as much as are women, usually in the fourth through sixth decades of life, and a mean age of 47.7 years.1,3-5,7,8 The SC occurrence rate in adults is 1:100,000.2 Patients typically present with insidious gradual mechanical symptoms, such as pain (> 85% of cases), swelling (42%-58%), and decreased motion (38%-55%) in the affected joint.2,3,6 Often there is crepitus with motion, diffuse tenderness, effusion, and occasionally nodules can be palpated.2,3 Histologically, the synovium exhibits condrocytic metaplasia of fibroblasts with influence from transforming growth factor-β and bone morphogenic proteins.1,4
Synovial chondromatosis can mimic osteoarthritis or meniscal pathology.3 Because of a chance of malignant transformation, any patient with rapid late deterioration of clinical features should be evaluated for chondrosarcoma or synovial sarcoma.1-4 Plain radiographs may help differentiate the cause showing calcific joint mice and peri-articular erosions. However, the intra-articular loose bodies are frequently radiolucent, and a MRI may be warranted to definitively differentiate the diagnosis.2,7,8
Loose bodies tend to exhibit a low signal on T1-weighted images and a high signal on T2-weighted images, although there may a be low signal on all images where there is extensive calcification of the loose bodies.2 Ultrasound also is a useful diagnostic tool that can show numerous echogenic bodies, effusion, and synovial hypertrophy.2
A classic article by Milgram discussed the phases of the proliferative changes associated with SC, where phase 1 shows active intrasynovial disease with no loose bodies.9 Phase 2 has transitional lesions with osteochondral nodules within the synovial membrane and free bodies within the joint cavity. Last, in phase 3, there are multiple osteochondral free bodies but quiescent intrasynovial disease. The patient in this case study exhibited intra-articular activity mimicking phase 2 with extensive intra-articular loose bodies and reactive synovial lining.3,9
In the early phase of the disease, conservative management may be trialed with NSAIDs, bracing, and injections, but typically surgical intervention is warranted after free bodies are found present, because they limit motion and cause recalcitrant swelling.2,8 There is a controversy whether arthroscopic removal of loose bodies or excision with synovectomy is the treatment of choice.6 Ogilvie-Harris and colleagues reviewed the results of both procedures and found that although removal of loose bodies alone may be sufficient, there is the potential for recurrence.9,10 In order to reduce potential recurrence, removal of loose bodies with anterior and posterior synovectomy is the treatment of choice.9
If arthroscopic removal of loose bodies without synovectomy is performed, then the patient should be followed closely for recurrence, which Jesalpura and colleagues reported to occur for 11.5% of patients.9,11 If there is a reappearance, then a synovectomy should be performed.10 A recommended treatment option for recalcitrant SC is radiation, but this carries the added risk of perpetuating malignant transformation.1,7
Unfortunately, osteoarthritis can be a significant long-term postoperative adverse effect.3,6-8 This typically is related to the amount of articular damage that is present at surgery. Many times, the arthritis becomes significant enough to require total joint arthroplasty.4 Close long-term follow-up is recommended, because although rare, there is a chance of malignant change.1-4
Conclusion
Synovial chondromatosis is an uncommon cause of knee pain and swelling and should be included in the differential diagnosis when evaluating any adult aged 30 years to 50 years with knee pain of insidious onset. Appropriate workup, intervention, and treatment will allow final diagnosis and correlating care to be administered to the patient.
1. Libbey NP, Mirrer F. Synovial chondromatosis. Med Health R I. 2011;94(9):274-275.
2. Giancane G, Tanturri de Horatio L, Buonuomo PS, Barbuti D, Lais G, Cortis E. Swollen knee due to primary synovial chondromatosis in pediatrics: a rare and possibly misdiagnosed condition. Rheumatol Int. 2013;33(8):2183-2185.
3. Serbest S, Tiftikçi U, Karaaslan F, Tosun HB, Sevinç HF, Balci M. A neglected case of giant synovial chondromatosis in knee joint. Pan Afr Med J. 2015;22:5.
4. Hallam P, Ashwood N, Cobb J, Fazal A, Heatley W. Malignant transformation in synovial chondromatosis of the knee? Knee. 2001;8(3):239-242.
5. Pimentel Cde Q, Hoff LS, de Sousa LF, Cordeiro RA, Pereira RM. Primary synovial osteochondromatosis of the knee. Rheumatol (Oxford). 2015;54(10):1815.
6. Damron TA, Sim FH. Soft-tissue tumors about the knee. J Am Acad Orthop Surg. 1997;5(3):141-152.
7. Krych A, Odland A, Rose P, et al. Onconlogic conditions that simulate common sports injuries. J Am Acad Orthop Surg. 2014;22(4):223-234.
8. Adelani MA, Wupperman RM, Holt GE. Benign synovial disorders. J Am Acad Orthop Surg. 2008;16(5):268-275.
9. Migram JW. Synovial osteochondromatosis: a histopathological study of thirty cases. J Bone Joint Surg Am. 1977;59(6):792-801.
10. Ogilvie-Harris DJ, Saleh K. Generalized synovial chondromatosis of the knee: a comparison of removal of the loose bodies alone with arthroscopic synovectomy. Arthroscopy.1994;10(2):166-170.
11. Jesalpura JP, Chung HW, Patnaik S, Choi HW, Kim JI, Nha KW. Athroscopic treatment of localized synovial chondromatosis of the posterior knee joint. Orthopedics. 2010;33(1):49
Joint mice or loose/rice bodies are infrequently encountered within joints. Usually, they are either fibrin or cartilaginous. The fibrin type, typically results from bleeding within a joint from synovitis, rheumatoid arthritis (RA), or tuberculosis, and the cartilaginous/osteocartilaginous type develop from trauma or osteoarthritis.1 A rare cause of osteocartilaginous joint mice is synovial chondromatosis(SC), which can produce multiple loose bodies that originate from the synovial membranes of joints, bursae, and tendon sheaths of large joints; the knee being the most common (50%-65 % of cases).1,2
A case of a male who had multiple years of left knee pain and swelling without a documented traumatic cause is presented
Case Presentation
A 34-year-old male veteran was evaluated and treated in a VA orthopedic outpatient clinic by a physician assistant for anterior left knee pain and swelling of insidious onset that had persisted for 1.5 years. The patient reported experiencing no trauma. His primary care provider already was treating the patient with nonsteroidal anti-inflammatory medications (NSAIDs), icing, and bracing. He had full motion in his knee with extension/flexion 0° to 130°. Collateral and cruciate ligaments were stable. He had a positive McMurray test. The X-rays showed no pathology. Due to the positive meniscal tear signs, a magnetic resonance image (MRI) was ordered.
The patient was intermittently nonadherent with follow-up care. The MRI results were available at a subsequent appointment 3 months after the index evaluation, which revealed a large joint effusion with rice bodies, small erosion of the posterior tibialplateau, and synovial proliferation of the anterior knee joint. A steroid injection to his affected knee was given. Concerns for possible RA led to a workup. The laboratory results included rheumatoid factor (weakly positive), antinuclear-antibodies (negative), human immunodeficiency virus (positivewith western blot negative), C-reactive protein (< 0.02 mg/L), erythrocyte sedimentation rate (5 mm/h), white blood cell count (4.6 µL), hepatitis B surface antigen (reactive), hepatitis A antibody (IgG reactive), synovial fluid cultures, and Gram stain (negative).
The patient saw a rheumatologist 7 months after a RA referral was processed. The consulting rheumatologist was unconcerned by a weakly positive rheumatoid factor, which was later repeated and was negative. The rheumatologist excluded the possibility of RA, and the patient was diagnosed with oligoarthritis. The treatment rendered was to continue NSAIDs and to return to the orthopedic clinic for continued care.
The patient had irregular follow-up visits where he received multiple methylprednisolone acetate intra-articular injections. His motion regressed until extension/flexion had decreased to 5°/85°. At this point the patient was forwarded to an operative orthopedic surgeon for evaluation for surgical intervention. Recent anteroposterior and lateral left knee X-rays showed faint intra-articular calcification, joint effusion, with mild arthritic changes of the patellofemoral joint (Figures 1A and 1B).
At surgery, on placing the infralateral portal, clear straw-colored fluid exited the cannula followed by copious small white rice bodies, which were sent to pathology for evaluation. The knee was surgically evaluated, and extensive rice bodies were encountered (Figure 3). These were extracted with a full radius shaver. The chondral surfaces were inspected. There were no arthritic changes, but the synovial lining of the joint was hypertrophied and reactive (Milgram phase 2). After all loose bodies were extracted, the patient’s incisions were closed with nylon suture, and he was placed in sterile dressings with a postoperative range of motion brace.
The patient presented for his routine postoperative visit 14 days after surgery. Pathology results showed synociocytes, and inflammatory cells were negative for malignancy. The patient was forwarded to a local hospital for further evaluation and treatment by an orthopedic oncologist due to a reported 5% chance of malignant transformation.1-3
Discussion
Synovial chondromatosis or osteochondromatosis is a rare, benign, metaplastic, typically monoarticular disorder of the synovial lining of joints, bursae, and synovial sheaths, usually affecting large joints.1-5 Although any joint can be involved, such as metacarpalphalangeal joints, temporomandibular joints, distal radio-ulnar joints, and the hips, the knee is the most common with an occurrence rate 50% to 65%.3-5 Extra-articular proliferation can be seen in cases of osteochondromatosis.2 It is characterized by the formation of intra-articular nodules of the synovium that can detach and become loose bodies, which can secondarily become calcified/ossified.4,6 The differential diagnosis associated with SC should include synovial hemangioma, pigmented villonodular synovitis, synovial cyst, lipoma arborescence, and malignancies, such as synovial chondrosarcoma or synovial sarcoma.3
Men are affected twice as much as are women, usually in the fourth through sixth decades of life, and a mean age of 47.7 years.1,3-5,7,8 The SC occurrence rate in adults is 1:100,000.2 Patients typically present with insidious gradual mechanical symptoms, such as pain (> 85% of cases), swelling (42%-58%), and decreased motion (38%-55%) in the affected joint.2,3,6 Often there is crepitus with motion, diffuse tenderness, effusion, and occasionally nodules can be palpated.2,3 Histologically, the synovium exhibits condrocytic metaplasia of fibroblasts with influence from transforming growth factor-β and bone morphogenic proteins.1,4
Synovial chondromatosis can mimic osteoarthritis or meniscal pathology.3 Because of a chance of malignant transformation, any patient with rapid late deterioration of clinical features should be evaluated for chondrosarcoma or synovial sarcoma.1-4 Plain radiographs may help differentiate the cause showing calcific joint mice and peri-articular erosions. However, the intra-articular loose bodies are frequently radiolucent, and a MRI may be warranted to definitively differentiate the diagnosis.2,7,8
Loose bodies tend to exhibit a low signal on T1-weighted images and a high signal on T2-weighted images, although there may a be low signal on all images where there is extensive calcification of the loose bodies.2 Ultrasound also is a useful diagnostic tool that can show numerous echogenic bodies, effusion, and synovial hypertrophy.2
A classic article by Milgram discussed the phases of the proliferative changes associated with SC, where phase 1 shows active intrasynovial disease with no loose bodies.9 Phase 2 has transitional lesions with osteochondral nodules within the synovial membrane and free bodies within the joint cavity. Last, in phase 3, there are multiple osteochondral free bodies but quiescent intrasynovial disease. The patient in this case study exhibited intra-articular activity mimicking phase 2 with extensive intra-articular loose bodies and reactive synovial lining.3,9
In the early phase of the disease, conservative management may be trialed with NSAIDs, bracing, and injections, but typically surgical intervention is warranted after free bodies are found present, because they limit motion and cause recalcitrant swelling.2,8 There is a controversy whether arthroscopic removal of loose bodies or excision with synovectomy is the treatment of choice.6 Ogilvie-Harris and colleagues reviewed the results of both procedures and found that although removal of loose bodies alone may be sufficient, there is the potential for recurrence.9,10 In order to reduce potential recurrence, removal of loose bodies with anterior and posterior synovectomy is the treatment of choice.9
If arthroscopic removal of loose bodies without synovectomy is performed, then the patient should be followed closely for recurrence, which Jesalpura and colleagues reported to occur for 11.5% of patients.9,11 If there is a reappearance, then a synovectomy should be performed.10 A recommended treatment option for recalcitrant SC is radiation, but this carries the added risk of perpetuating malignant transformation.1,7
Unfortunately, osteoarthritis can be a significant long-term postoperative adverse effect.3,6-8 This typically is related to the amount of articular damage that is present at surgery. Many times, the arthritis becomes significant enough to require total joint arthroplasty.4 Close long-term follow-up is recommended, because although rare, there is a chance of malignant change.1-4
Conclusion
Synovial chondromatosis is an uncommon cause of knee pain and swelling and should be included in the differential diagnosis when evaluating any adult aged 30 years to 50 years with knee pain of insidious onset. Appropriate workup, intervention, and treatment will allow final diagnosis and correlating care to be administered to the patient.
Joint mice or loose/rice bodies are infrequently encountered within joints. Usually, they are either fibrin or cartilaginous. The fibrin type, typically results from bleeding within a joint from synovitis, rheumatoid arthritis (RA), or tuberculosis, and the cartilaginous/osteocartilaginous type develop from trauma or osteoarthritis.1 A rare cause of osteocartilaginous joint mice is synovial chondromatosis(SC), which can produce multiple loose bodies that originate from the synovial membranes of joints, bursae, and tendon sheaths of large joints; the knee being the most common (50%-65 % of cases).1,2
A case of a male who had multiple years of left knee pain and swelling without a documented traumatic cause is presented
Case Presentation
A 34-year-old male veteran was evaluated and treated in a VA orthopedic outpatient clinic by a physician assistant for anterior left knee pain and swelling of insidious onset that had persisted for 1.5 years. The patient reported experiencing no trauma. His primary care provider already was treating the patient with nonsteroidal anti-inflammatory medications (NSAIDs), icing, and bracing. He had full motion in his knee with extension/flexion 0° to 130°. Collateral and cruciate ligaments were stable. He had a positive McMurray test. The X-rays showed no pathology. Due to the positive meniscal tear signs, a magnetic resonance image (MRI) was ordered.
The patient was intermittently nonadherent with follow-up care. The MRI results were available at a subsequent appointment 3 months after the index evaluation, which revealed a large joint effusion with rice bodies, small erosion of the posterior tibialplateau, and synovial proliferation of the anterior knee joint. A steroid injection to his affected knee was given. Concerns for possible RA led to a workup. The laboratory results included rheumatoid factor (weakly positive), antinuclear-antibodies (negative), human immunodeficiency virus (positivewith western blot negative), C-reactive protein (< 0.02 mg/L), erythrocyte sedimentation rate (5 mm/h), white blood cell count (4.6 µL), hepatitis B surface antigen (reactive), hepatitis A antibody (IgG reactive), synovial fluid cultures, and Gram stain (negative).
The patient saw a rheumatologist 7 months after a RA referral was processed. The consulting rheumatologist was unconcerned by a weakly positive rheumatoid factor, which was later repeated and was negative. The rheumatologist excluded the possibility of RA, and the patient was diagnosed with oligoarthritis. The treatment rendered was to continue NSAIDs and to return to the orthopedic clinic for continued care.
The patient had irregular follow-up visits where he received multiple methylprednisolone acetate intra-articular injections. His motion regressed until extension/flexion had decreased to 5°/85°. At this point the patient was forwarded to an operative orthopedic surgeon for evaluation for surgical intervention. Recent anteroposterior and lateral left knee X-rays showed faint intra-articular calcification, joint effusion, with mild arthritic changes of the patellofemoral joint (Figures 1A and 1B).
At surgery, on placing the infralateral portal, clear straw-colored fluid exited the cannula followed by copious small white rice bodies, which were sent to pathology for evaluation. The knee was surgically evaluated, and extensive rice bodies were encountered (Figure 3). These were extracted with a full radius shaver. The chondral surfaces were inspected. There were no arthritic changes, but the synovial lining of the joint was hypertrophied and reactive (Milgram phase 2). After all loose bodies were extracted, the patient’s incisions were closed with nylon suture, and he was placed in sterile dressings with a postoperative range of motion brace.
The patient presented for his routine postoperative visit 14 days after surgery. Pathology results showed synociocytes, and inflammatory cells were negative for malignancy. The patient was forwarded to a local hospital for further evaluation and treatment by an orthopedic oncologist due to a reported 5% chance of malignant transformation.1-3
Discussion
Synovial chondromatosis or osteochondromatosis is a rare, benign, metaplastic, typically monoarticular disorder of the synovial lining of joints, bursae, and synovial sheaths, usually affecting large joints.1-5 Although any joint can be involved, such as metacarpalphalangeal joints, temporomandibular joints, distal radio-ulnar joints, and the hips, the knee is the most common with an occurrence rate 50% to 65%.3-5 Extra-articular proliferation can be seen in cases of osteochondromatosis.2 It is characterized by the formation of intra-articular nodules of the synovium that can detach and become loose bodies, which can secondarily become calcified/ossified.4,6 The differential diagnosis associated with SC should include synovial hemangioma, pigmented villonodular synovitis, synovial cyst, lipoma arborescence, and malignancies, such as synovial chondrosarcoma or synovial sarcoma.3
Men are affected twice as much as are women, usually in the fourth through sixth decades of life, and a mean age of 47.7 years.1,3-5,7,8 The SC occurrence rate in adults is 1:100,000.2 Patients typically present with insidious gradual mechanical symptoms, such as pain (> 85% of cases), swelling (42%-58%), and decreased motion (38%-55%) in the affected joint.2,3,6 Often there is crepitus with motion, diffuse tenderness, effusion, and occasionally nodules can be palpated.2,3 Histologically, the synovium exhibits condrocytic metaplasia of fibroblasts with influence from transforming growth factor-β and bone morphogenic proteins.1,4
Synovial chondromatosis can mimic osteoarthritis or meniscal pathology.3 Because of a chance of malignant transformation, any patient with rapid late deterioration of clinical features should be evaluated for chondrosarcoma or synovial sarcoma.1-4 Plain radiographs may help differentiate the cause showing calcific joint mice and peri-articular erosions. However, the intra-articular loose bodies are frequently radiolucent, and a MRI may be warranted to definitively differentiate the diagnosis.2,7,8
Loose bodies tend to exhibit a low signal on T1-weighted images and a high signal on T2-weighted images, although there may a be low signal on all images where there is extensive calcification of the loose bodies.2 Ultrasound also is a useful diagnostic tool that can show numerous echogenic bodies, effusion, and synovial hypertrophy.2
A classic article by Milgram discussed the phases of the proliferative changes associated with SC, where phase 1 shows active intrasynovial disease with no loose bodies.9 Phase 2 has transitional lesions with osteochondral nodules within the synovial membrane and free bodies within the joint cavity. Last, in phase 3, there are multiple osteochondral free bodies but quiescent intrasynovial disease. The patient in this case study exhibited intra-articular activity mimicking phase 2 with extensive intra-articular loose bodies and reactive synovial lining.3,9
In the early phase of the disease, conservative management may be trialed with NSAIDs, bracing, and injections, but typically surgical intervention is warranted after free bodies are found present, because they limit motion and cause recalcitrant swelling.2,8 There is a controversy whether arthroscopic removal of loose bodies or excision with synovectomy is the treatment of choice.6 Ogilvie-Harris and colleagues reviewed the results of both procedures and found that although removal of loose bodies alone may be sufficient, there is the potential for recurrence.9,10 In order to reduce potential recurrence, removal of loose bodies with anterior and posterior synovectomy is the treatment of choice.9
If arthroscopic removal of loose bodies without synovectomy is performed, then the patient should be followed closely for recurrence, which Jesalpura and colleagues reported to occur for 11.5% of patients.9,11 If there is a reappearance, then a synovectomy should be performed.10 A recommended treatment option for recalcitrant SC is radiation, but this carries the added risk of perpetuating malignant transformation.1,7
Unfortunately, osteoarthritis can be a significant long-term postoperative adverse effect.3,6-8 This typically is related to the amount of articular damage that is present at surgery. Many times, the arthritis becomes significant enough to require total joint arthroplasty.4 Close long-term follow-up is recommended, because although rare, there is a chance of malignant change.1-4
Conclusion
Synovial chondromatosis is an uncommon cause of knee pain and swelling and should be included in the differential diagnosis when evaluating any adult aged 30 years to 50 years with knee pain of insidious onset. Appropriate workup, intervention, and treatment will allow final diagnosis and correlating care to be administered to the patient.
1. Libbey NP, Mirrer F. Synovial chondromatosis. Med Health R I. 2011;94(9):274-275.
2. Giancane G, Tanturri de Horatio L, Buonuomo PS, Barbuti D, Lais G, Cortis E. Swollen knee due to primary synovial chondromatosis in pediatrics: a rare and possibly misdiagnosed condition. Rheumatol Int. 2013;33(8):2183-2185.
3. Serbest S, Tiftikçi U, Karaaslan F, Tosun HB, Sevinç HF, Balci M. A neglected case of giant synovial chondromatosis in knee joint. Pan Afr Med J. 2015;22:5.
4. Hallam P, Ashwood N, Cobb J, Fazal A, Heatley W. Malignant transformation in synovial chondromatosis of the knee? Knee. 2001;8(3):239-242.
5. Pimentel Cde Q, Hoff LS, de Sousa LF, Cordeiro RA, Pereira RM. Primary synovial osteochondromatosis of the knee. Rheumatol (Oxford). 2015;54(10):1815.
6. Damron TA, Sim FH. Soft-tissue tumors about the knee. J Am Acad Orthop Surg. 1997;5(3):141-152.
7. Krych A, Odland A, Rose P, et al. Onconlogic conditions that simulate common sports injuries. J Am Acad Orthop Surg. 2014;22(4):223-234.
8. Adelani MA, Wupperman RM, Holt GE. Benign synovial disorders. J Am Acad Orthop Surg. 2008;16(5):268-275.
9. Migram JW. Synovial osteochondromatosis: a histopathological study of thirty cases. J Bone Joint Surg Am. 1977;59(6):792-801.
10. Ogilvie-Harris DJ, Saleh K. Generalized synovial chondromatosis of the knee: a comparison of removal of the loose bodies alone with arthroscopic synovectomy. Arthroscopy.1994;10(2):166-170.
11. Jesalpura JP, Chung HW, Patnaik S, Choi HW, Kim JI, Nha KW. Athroscopic treatment of localized synovial chondromatosis of the posterior knee joint. Orthopedics. 2010;33(1):49
1. Libbey NP, Mirrer F. Synovial chondromatosis. Med Health R I. 2011;94(9):274-275.
2. Giancane G, Tanturri de Horatio L, Buonuomo PS, Barbuti D, Lais G, Cortis E. Swollen knee due to primary synovial chondromatosis in pediatrics: a rare and possibly misdiagnosed condition. Rheumatol Int. 2013;33(8):2183-2185.
3. Serbest S, Tiftikçi U, Karaaslan F, Tosun HB, Sevinç HF, Balci M. A neglected case of giant synovial chondromatosis in knee joint. Pan Afr Med J. 2015;22:5.
4. Hallam P, Ashwood N, Cobb J, Fazal A, Heatley W. Malignant transformation in synovial chondromatosis of the knee? Knee. 2001;8(3):239-242.
5. Pimentel Cde Q, Hoff LS, de Sousa LF, Cordeiro RA, Pereira RM. Primary synovial osteochondromatosis of the knee. Rheumatol (Oxford). 2015;54(10):1815.
6. Damron TA, Sim FH. Soft-tissue tumors about the knee. J Am Acad Orthop Surg. 1997;5(3):141-152.
7. Krych A, Odland A, Rose P, et al. Onconlogic conditions that simulate common sports injuries. J Am Acad Orthop Surg. 2014;22(4):223-234.
8. Adelani MA, Wupperman RM, Holt GE. Benign synovial disorders. J Am Acad Orthop Surg. 2008;16(5):268-275.
9. Migram JW. Synovial osteochondromatosis: a histopathological study of thirty cases. J Bone Joint Surg Am. 1977;59(6):792-801.
10. Ogilvie-Harris DJ, Saleh K. Generalized synovial chondromatosis of the knee: a comparison of removal of the loose bodies alone with arthroscopic synovectomy. Arthroscopy.1994;10(2):166-170.
11. Jesalpura JP, Chung HW, Patnaik S, Choi HW, Kim JI, Nha KW. Athroscopic treatment of localized synovial chondromatosis of the posterior knee joint. Orthopedics. 2010;33(1):49
Acute Exertional Upper-Extremity Rhabdomyolysis in 3 Female Trainees
Acute exertional rhabdomyolysis (AER) is the breakdown/destruction of muscle tissue from extreme physical exertion. Risks that lead to AER include exercise in hot and humid conditions, improper hydration, inadequate recovery between bouts of exercise, intense physical training, and inadequate fitness levels. Other risk factors include sickle cell trait, ingestion of performance enhancing agents, anabolic steroids, and previous history of AER. This article, describes 3 cases of AER after a vigorous, upper body, organized, and supervised training session.
Rhabdomyolysis is not uncommon in competitive athletics,1-3 military training,4-8 and individual training.9-12 It is more common in the lower extremities after intense training or marathons. Creatine kinase (CK) levels rise within 12 hours of muscle injury, peak in 24 to 36 hours, and decrease at a rate of 30% to 40% per day.13 The serum half-life of CK is about 36 hours. The CK levels decline 3 to 5 days after resolution of muscle injury.14 Failure of CK levels to decrease suggests ongoing muscle injury or development of a compartment syndrome. The peak CK level, especially when it is > 15,000 U/L, may be predictive of renal failure.15
Total CK elevation is a sensitive but nonspecific marker for rhabdomyolysis. A CK level that is 1.5 × above the reference range suggests rhabdomyolysis, although CK levels in rhabdomyolysis often are as high as 100 times the reference range or more.12 Health care providers (HCPs) should suspect early rhabdomyolysis and initiate a full laboratory workup for patients with serum CK levels > 2 × the reference range and risk factors for rhabdomyolysis. Because the total CK may increase from the initial values, draw it is important to repeat total CK levels every 6 to 12 hours until a peak level is established.
Case Presentations
An upper body physical training session was conducted with a group of trainees (men and women, aged 24-35 years) 12 weeks into a 21-week program. The training session consisted of 125 push-ups and 85 pull-ups (assisted) performed within a 12-minute period in an indoor, climate-controlled facility. Liberal hydration with water or sports drinks was not allowed. On day 3 following the training session, 1 woman, and on day 4, 2 additional women presented to the clinic with extreme bilateral upper extremity muscle weakness, pain, and marked swelling of their upper-extremities from the shoulder to the forearm. None had firm compartments of the arm or forearm. Further, none had signs of compartment syndrome. There were trace blood and protein in their urine, and their serum CK ranged from 10,000 to 78,000 U/L.
Case 1
The first case to present to the clinic was a 26-year-old white female without any underlying disease. She was taking oral contraceptive medication. She usually exercised by running 10 to 12 miles/week with upper body workouts as required by the training program and was in excellent physical health at the halfway point in the training program.
Following the workout of 125 push-ups and 85 pull-ups, the patient experienced muscle soreness and fatigue and took ibuprofen 800 mg that evening. Over the next 2 days, she experienced continued muscle soreness/pain, increasing weakness, and marked swelling of her arms. On day 3 after the workout, she presented to the clinic in moderate distress, unable to raise her arms above chest level. Her urine showed trace blood and protein, and her serum CK level was 73,044 U/L (Table 1).
The patient was diagnosed with rhabdomyolysis and admitted to the hospital. She received IV therapy, improved, and was discharged after 8 days, and had no sequelae.
Case 2
The second case involved a 27-year-old white female without any underlying disease who was not taking any medications and was in excellent physical health. Following the workout of 125 push-ups and 85 pull-ups, she experienced muscle soreness and fatigue. Over the next 3 days, she experienced continued muscle soreness/pain, increasing weakness, and marked swelling of her arms. On day 4 postworkout, she presented to the clinic in moderate distress. Her urine showed trace blood and protein, and her serum CK level was > 8,000 U/L (the hospital stopped dilutions when the CK level exceeded 8,000 U/L) (Table 2).
The patient was diagnosed with rhabdomyolysis and admitted to the hospital. She received IV therapy, improved, and was discharged after 7 days, and had no sequelae.
Case 3
The third case involved a 33-year-old white female without any underlying disease who was taking oral contraceptive medication and was in excellent physical health. Following the 125 push-ups and 85 pull-ups workout, she experienced muscle soreness and fatigue. Over the next 3 days, she experienced continued muscle soreness/pain, increasing weakness, and marked swelling of her arms. On day 4 postworkout, she presented to the clinic in moderate distress. Her urine showed trace blood and protein, and her serum CK level was 10,971 U/L (Table 3).
The patient was diagnosed with rhabdomyolysis and admitted to the hospital. She received IV therapy, improved, and was discharged after 7 days, and had no sequelae.
Clinical Observation
The importance of brown urine is stressed as a key factor in the diagnosis of AER in much of the literature on the condition.1-10 It is suggested that brown urine is pathognomonic for this condition. However, the urine for the individuals presented here with significant AER was not brown or even tinged. In a field evaluation, an inexperienced HCP might miss an AER diagnosis in the absence of urine findings. In addition, given that AER can occur in stages, it is essential that the HCP have a situational awareness of this condition in today’s culture of fitness and exercise.
Other Causes
Cocaine is a common cause of rhabdomyolysis, namely, in urban patient populations. Prolonged vasoconstriction of intramuscular arteries may result in muscle ischemia and acute rhabdomyolysis, but there also is a direct toxic effect that can produce acute skeletal myofibrillar degeneration.
A number of prescription drugs have been implicated in cases of rhabdomyolysis, including colchicine, zidovudine, isoniazid, benzodiazepines, opiates, corticosteroids, statins, and fibric acid derivatives. One particular interaction that is clinically significant is the interaction between statins and fibrates.16
The pathogenesis of rhabdomyolysis precipitated by infections (whether bacterial, viral, or fungal) is thought to be the result of direct cell invasion of striated muscle and cellular degeneration by the pathogen. Substantial morbidity (57% of cases with acute renal failure) and mortality (death in almost 40% of cases) are linked to bacterial causes of rhabdomyolysis.
In adult patients, Legionella species most often are associated with rhabdomyolysis. Other bacteria linked to rhabdomyolysis include group A β-hemolytic streptococci, Salmonella species, Francisella tularensis and Escherichia coli. Viruses, such as influenza, parainfluenza, coxsackievirus, Epstein-Barr virus, adenovirus, HIV, and cytomegalovirus also have been associated with this condition.
Rhabdomyolysis also can be observed in septic patients without direct muscle infection when the damage is caused by a toxin, associated fever, dehydration, and rigors. Electrolyte disorders, such as hyponatremia or hypernatremia, hypokalemia, and hypophosphatemia can result in rhabdomyolysis, distorting the permeability and the functions of sarcolemma in the muscles. Some endocrine disorders (ie, pheochromocytoma and thyrotoxicosis) also are able to potentiate rhabdomyolysis due to hypermetabolism.
Prevention
A workout program should progress gradually according to the individual’s current level of fitness, whether it’s cardiovascular, circuit training, or weight training. Fluid intake should be monitored, particularly when the workout is long, intense, or hot and especially when the workout meets all 3 conditions. Fluid replacement is important, and for strenuous and longer training evolutions, electrolyte replacement should be considered. Hard exercise while maintaining a low-calorie diet or after long fasting periods should be avoided. Sufficient caloric and fluid intake to allow muscles to work efficiently during strenuous workout period is needed. Recreational drugs, including alcohol should be limited before exercise, and illicit recreational or performance-enhancing drugs should be avoided.
Conclusion
Acute exertional rhabdomyolysis is more common in the lower extremities and in males. Extremely rigorous upper-extremity training can result in AER. The presentation usually is clear with an inciting event and muscle pain in the extremity. Besides identifying associated risk factors and performing a thorough examination, the patient should be examined for compartment syndrome. Early diagnosis and comprehensive management are crucial to ensure full recovery and avoidance of complications, such as acute tubular necrosis, renal failure, cardiac arrhythmias from hyperkalemia, and death. As in most cases with early diagnosis and aggressive management, these patients fully recovered and experienced no sequelae at 8 weeks postevent.
1. Galvez R, Stacy J, Howley A. Exertional rhabdomyolysis in seven division-1 swimming athletes. Clin J Sport Med. 2008;18(4):366-368.
2. Smoot MK, Amendola A, Cramer E, et al. A cluster of exertional rhabdomyolysis affecting a division 1 football team. Clin J Sport Med. 2013;23(5):365-372.
3. Kahanov L, Eberman LE, Wasik M, Alvey T. Exertional rhabdomyolysis in a collegiate American football player after preventive cold-water immersion: a case report. J Athl Train. 2012;47(2):228-232.
4. Aizawa H, Morita K, Minami H, Sasaki N, Tobise K. Exertional rhabdomyolysis as a result of strenuous military training. J Neurol Sci. 1995;132(2):239-240.
5. Tietjen DP, Guzzi LM. Exertional rhabdomyolysis and acute renal failure following the Army Physical Fitness Test. Mil Med. 154(1):23-25.
6. Gardner JW, Kark JA. Fatal rhabdomyolysis presenting as mild heat illness in military training. Mil Med. 159(2):160-163.
7. Armed Forces Health Surveillance Center (AFHSC). Exertional rhabdomyolysis among U.S. military members, 2004-2007. MSMR. 2008;15(2):8-11.
8. Gitin EL, Demos MA. Acute exertional rhabdomyolysis: a syndrome of increasing importance to the military physician. Mil Med.1974;139(1):33-36.
9. Springer BL, Clarkson PM. Two cases of exertional rhabdomyolysis precipitated by personal trainers. Med Sci Sports Exerc. 2003;35(9):1499-1502.
10. Lin A, Lin C, Wang T, Leu J. Rhabdomyolysis in 119 students after repetitive exercise. Br J Sports Med. 2005;39(1):e3.
11. Hamer R. When exercise goes awry: exertional rhabdomyolysis. South Med J. 1997;90(5):548-551.
12. Soni SN, McDonald E, Marino C. Rhabdomyolysis after exercise. Postgrad Med. 1993;94(6):128-132.
13. Lappalainen H, Tiula E, Uotila L, Mänttäri M. Elimination kinetics of myoglobin and creatine kinase in rhabdomyolysis: implications for follow-up. Crit Care Med. 2002;30(10):2212-2215.
14. Huerta-Alardín AL, Varon J, Marik PE. Bench-to-bedside review: rhabdomyolysis—an overview for clinicians. Crit Care. 2005;9(2):158-169.
15. Minnema BJ, Neligan PC, Quraishi NA, Fehlings MG, Prakash S. A case of occult compartment syndrome and nonresolving rhabdomyolysis. J Gen Intern Med. 2008;23(6):871-874.
16. Efstratiadis G, Voulgaridou A, Nikiforou D, Kyventidis A, Kourkouni E, Vergoulas G. Rhabdomyolysis updated. Hippokratia. 2007;11(3):129-137.
Acute exertional rhabdomyolysis (AER) is the breakdown/destruction of muscle tissue from extreme physical exertion. Risks that lead to AER include exercise in hot and humid conditions, improper hydration, inadequate recovery between bouts of exercise, intense physical training, and inadequate fitness levels. Other risk factors include sickle cell trait, ingestion of performance enhancing agents, anabolic steroids, and previous history of AER. This article, describes 3 cases of AER after a vigorous, upper body, organized, and supervised training session.
Rhabdomyolysis is not uncommon in competitive athletics,1-3 military training,4-8 and individual training.9-12 It is more common in the lower extremities after intense training or marathons. Creatine kinase (CK) levels rise within 12 hours of muscle injury, peak in 24 to 36 hours, and decrease at a rate of 30% to 40% per day.13 The serum half-life of CK is about 36 hours. The CK levels decline 3 to 5 days after resolution of muscle injury.14 Failure of CK levels to decrease suggests ongoing muscle injury or development of a compartment syndrome. The peak CK level, especially when it is > 15,000 U/L, may be predictive of renal failure.15
Total CK elevation is a sensitive but nonspecific marker for rhabdomyolysis. A CK level that is 1.5 × above the reference range suggests rhabdomyolysis, although CK levels in rhabdomyolysis often are as high as 100 times the reference range or more.12 Health care providers (HCPs) should suspect early rhabdomyolysis and initiate a full laboratory workup for patients with serum CK levels > 2 × the reference range and risk factors for rhabdomyolysis. Because the total CK may increase from the initial values, draw it is important to repeat total CK levels every 6 to 12 hours until a peak level is established.
Case Presentations
An upper body physical training session was conducted with a group of trainees (men and women, aged 24-35 years) 12 weeks into a 21-week program. The training session consisted of 125 push-ups and 85 pull-ups (assisted) performed within a 12-minute period in an indoor, climate-controlled facility. Liberal hydration with water or sports drinks was not allowed. On day 3 following the training session, 1 woman, and on day 4, 2 additional women presented to the clinic with extreme bilateral upper extremity muscle weakness, pain, and marked swelling of their upper-extremities from the shoulder to the forearm. None had firm compartments of the arm or forearm. Further, none had signs of compartment syndrome. There were trace blood and protein in their urine, and their serum CK ranged from 10,000 to 78,000 U/L.
Case 1
The first case to present to the clinic was a 26-year-old white female without any underlying disease. She was taking oral contraceptive medication. She usually exercised by running 10 to 12 miles/week with upper body workouts as required by the training program and was in excellent physical health at the halfway point in the training program.
Following the workout of 125 push-ups and 85 pull-ups, the patient experienced muscle soreness and fatigue and took ibuprofen 800 mg that evening. Over the next 2 days, she experienced continued muscle soreness/pain, increasing weakness, and marked swelling of her arms. On day 3 after the workout, she presented to the clinic in moderate distress, unable to raise her arms above chest level. Her urine showed trace blood and protein, and her serum CK level was 73,044 U/L (Table 1).
The patient was diagnosed with rhabdomyolysis and admitted to the hospital. She received IV therapy, improved, and was discharged after 8 days, and had no sequelae.
Case 2
The second case involved a 27-year-old white female without any underlying disease who was not taking any medications and was in excellent physical health. Following the workout of 125 push-ups and 85 pull-ups, she experienced muscle soreness and fatigue. Over the next 3 days, she experienced continued muscle soreness/pain, increasing weakness, and marked swelling of her arms. On day 4 postworkout, she presented to the clinic in moderate distress. Her urine showed trace blood and protein, and her serum CK level was > 8,000 U/L (the hospital stopped dilutions when the CK level exceeded 8,000 U/L) (Table 2).
The patient was diagnosed with rhabdomyolysis and admitted to the hospital. She received IV therapy, improved, and was discharged after 7 days, and had no sequelae.
Case 3
The third case involved a 33-year-old white female without any underlying disease who was taking oral contraceptive medication and was in excellent physical health. Following the 125 push-ups and 85 pull-ups workout, she experienced muscle soreness and fatigue. Over the next 3 days, she experienced continued muscle soreness/pain, increasing weakness, and marked swelling of her arms. On day 4 postworkout, she presented to the clinic in moderate distress. Her urine showed trace blood and protein, and her serum CK level was 10,971 U/L (Table 3).
The patient was diagnosed with rhabdomyolysis and admitted to the hospital. She received IV therapy, improved, and was discharged after 7 days, and had no sequelae.
Clinical Observation
The importance of brown urine is stressed as a key factor in the diagnosis of AER in much of the literature on the condition.1-10 It is suggested that brown urine is pathognomonic for this condition. However, the urine for the individuals presented here with significant AER was not brown or even tinged. In a field evaluation, an inexperienced HCP might miss an AER diagnosis in the absence of urine findings. In addition, given that AER can occur in stages, it is essential that the HCP have a situational awareness of this condition in today’s culture of fitness and exercise.
Other Causes
Cocaine is a common cause of rhabdomyolysis, namely, in urban patient populations. Prolonged vasoconstriction of intramuscular arteries may result in muscle ischemia and acute rhabdomyolysis, but there also is a direct toxic effect that can produce acute skeletal myofibrillar degeneration.
A number of prescription drugs have been implicated in cases of rhabdomyolysis, including colchicine, zidovudine, isoniazid, benzodiazepines, opiates, corticosteroids, statins, and fibric acid derivatives. One particular interaction that is clinically significant is the interaction between statins and fibrates.16
The pathogenesis of rhabdomyolysis precipitated by infections (whether bacterial, viral, or fungal) is thought to be the result of direct cell invasion of striated muscle and cellular degeneration by the pathogen. Substantial morbidity (57% of cases with acute renal failure) and mortality (death in almost 40% of cases) are linked to bacterial causes of rhabdomyolysis.
In adult patients, Legionella species most often are associated with rhabdomyolysis. Other bacteria linked to rhabdomyolysis include group A β-hemolytic streptococci, Salmonella species, Francisella tularensis and Escherichia coli. Viruses, such as influenza, parainfluenza, coxsackievirus, Epstein-Barr virus, adenovirus, HIV, and cytomegalovirus also have been associated with this condition.
Rhabdomyolysis also can be observed in septic patients without direct muscle infection when the damage is caused by a toxin, associated fever, dehydration, and rigors. Electrolyte disorders, such as hyponatremia or hypernatremia, hypokalemia, and hypophosphatemia can result in rhabdomyolysis, distorting the permeability and the functions of sarcolemma in the muscles. Some endocrine disorders (ie, pheochromocytoma and thyrotoxicosis) also are able to potentiate rhabdomyolysis due to hypermetabolism.
Prevention
A workout program should progress gradually according to the individual’s current level of fitness, whether it’s cardiovascular, circuit training, or weight training. Fluid intake should be monitored, particularly when the workout is long, intense, or hot and especially when the workout meets all 3 conditions. Fluid replacement is important, and for strenuous and longer training evolutions, electrolyte replacement should be considered. Hard exercise while maintaining a low-calorie diet or after long fasting periods should be avoided. Sufficient caloric and fluid intake to allow muscles to work efficiently during strenuous workout period is needed. Recreational drugs, including alcohol should be limited before exercise, and illicit recreational or performance-enhancing drugs should be avoided.
Conclusion
Acute exertional rhabdomyolysis is more common in the lower extremities and in males. Extremely rigorous upper-extremity training can result in AER. The presentation usually is clear with an inciting event and muscle pain in the extremity. Besides identifying associated risk factors and performing a thorough examination, the patient should be examined for compartment syndrome. Early diagnosis and comprehensive management are crucial to ensure full recovery and avoidance of complications, such as acute tubular necrosis, renal failure, cardiac arrhythmias from hyperkalemia, and death. As in most cases with early diagnosis and aggressive management, these patients fully recovered and experienced no sequelae at 8 weeks postevent.
Acute exertional rhabdomyolysis (AER) is the breakdown/destruction of muscle tissue from extreme physical exertion. Risks that lead to AER include exercise in hot and humid conditions, improper hydration, inadequate recovery between bouts of exercise, intense physical training, and inadequate fitness levels. Other risk factors include sickle cell trait, ingestion of performance enhancing agents, anabolic steroids, and previous history of AER. This article, describes 3 cases of AER after a vigorous, upper body, organized, and supervised training session.
Rhabdomyolysis is not uncommon in competitive athletics,1-3 military training,4-8 and individual training.9-12 It is more common in the lower extremities after intense training or marathons. Creatine kinase (CK) levels rise within 12 hours of muscle injury, peak in 24 to 36 hours, and decrease at a rate of 30% to 40% per day.13 The serum half-life of CK is about 36 hours. The CK levels decline 3 to 5 days after resolution of muscle injury.14 Failure of CK levels to decrease suggests ongoing muscle injury or development of a compartment syndrome. The peak CK level, especially when it is > 15,000 U/L, may be predictive of renal failure.15
Total CK elevation is a sensitive but nonspecific marker for rhabdomyolysis. A CK level that is 1.5 × above the reference range suggests rhabdomyolysis, although CK levels in rhabdomyolysis often are as high as 100 times the reference range or more.12 Health care providers (HCPs) should suspect early rhabdomyolysis and initiate a full laboratory workup for patients with serum CK levels > 2 × the reference range and risk factors for rhabdomyolysis. Because the total CK may increase from the initial values, draw it is important to repeat total CK levels every 6 to 12 hours until a peak level is established.
Case Presentations
An upper body physical training session was conducted with a group of trainees (men and women, aged 24-35 years) 12 weeks into a 21-week program. The training session consisted of 125 push-ups and 85 pull-ups (assisted) performed within a 12-minute period in an indoor, climate-controlled facility. Liberal hydration with water or sports drinks was not allowed. On day 3 following the training session, 1 woman, and on day 4, 2 additional women presented to the clinic with extreme bilateral upper extremity muscle weakness, pain, and marked swelling of their upper-extremities from the shoulder to the forearm. None had firm compartments of the arm or forearm. Further, none had signs of compartment syndrome. There were trace blood and protein in their urine, and their serum CK ranged from 10,000 to 78,000 U/L.
Case 1
The first case to present to the clinic was a 26-year-old white female without any underlying disease. She was taking oral contraceptive medication. She usually exercised by running 10 to 12 miles/week with upper body workouts as required by the training program and was in excellent physical health at the halfway point in the training program.
Following the workout of 125 push-ups and 85 pull-ups, the patient experienced muscle soreness and fatigue and took ibuprofen 800 mg that evening. Over the next 2 days, she experienced continued muscle soreness/pain, increasing weakness, and marked swelling of her arms. On day 3 after the workout, she presented to the clinic in moderate distress, unable to raise her arms above chest level. Her urine showed trace blood and protein, and her serum CK level was 73,044 U/L (Table 1).
The patient was diagnosed with rhabdomyolysis and admitted to the hospital. She received IV therapy, improved, and was discharged after 8 days, and had no sequelae.
Case 2
The second case involved a 27-year-old white female without any underlying disease who was not taking any medications and was in excellent physical health. Following the workout of 125 push-ups and 85 pull-ups, she experienced muscle soreness and fatigue. Over the next 3 days, she experienced continued muscle soreness/pain, increasing weakness, and marked swelling of her arms. On day 4 postworkout, she presented to the clinic in moderate distress. Her urine showed trace blood and protein, and her serum CK level was > 8,000 U/L (the hospital stopped dilutions when the CK level exceeded 8,000 U/L) (Table 2).
The patient was diagnosed with rhabdomyolysis and admitted to the hospital. She received IV therapy, improved, and was discharged after 7 days, and had no sequelae.
Case 3
The third case involved a 33-year-old white female without any underlying disease who was taking oral contraceptive medication and was in excellent physical health. Following the 125 push-ups and 85 pull-ups workout, she experienced muscle soreness and fatigue. Over the next 3 days, she experienced continued muscle soreness/pain, increasing weakness, and marked swelling of her arms. On day 4 postworkout, she presented to the clinic in moderate distress. Her urine showed trace blood and protein, and her serum CK level was 10,971 U/L (Table 3).
The patient was diagnosed with rhabdomyolysis and admitted to the hospital. She received IV therapy, improved, and was discharged after 7 days, and had no sequelae.
Clinical Observation
The importance of brown urine is stressed as a key factor in the diagnosis of AER in much of the literature on the condition.1-10 It is suggested that brown urine is pathognomonic for this condition. However, the urine for the individuals presented here with significant AER was not brown or even tinged. In a field evaluation, an inexperienced HCP might miss an AER diagnosis in the absence of urine findings. In addition, given that AER can occur in stages, it is essential that the HCP have a situational awareness of this condition in today’s culture of fitness and exercise.
Other Causes
Cocaine is a common cause of rhabdomyolysis, namely, in urban patient populations. Prolonged vasoconstriction of intramuscular arteries may result in muscle ischemia and acute rhabdomyolysis, but there also is a direct toxic effect that can produce acute skeletal myofibrillar degeneration.
A number of prescription drugs have been implicated in cases of rhabdomyolysis, including colchicine, zidovudine, isoniazid, benzodiazepines, opiates, corticosteroids, statins, and fibric acid derivatives. One particular interaction that is clinically significant is the interaction between statins and fibrates.16
The pathogenesis of rhabdomyolysis precipitated by infections (whether bacterial, viral, or fungal) is thought to be the result of direct cell invasion of striated muscle and cellular degeneration by the pathogen. Substantial morbidity (57% of cases with acute renal failure) and mortality (death in almost 40% of cases) are linked to bacterial causes of rhabdomyolysis.
In adult patients, Legionella species most often are associated with rhabdomyolysis. Other bacteria linked to rhabdomyolysis include group A β-hemolytic streptococci, Salmonella species, Francisella tularensis and Escherichia coli. Viruses, such as influenza, parainfluenza, coxsackievirus, Epstein-Barr virus, adenovirus, HIV, and cytomegalovirus also have been associated with this condition.
Rhabdomyolysis also can be observed in septic patients without direct muscle infection when the damage is caused by a toxin, associated fever, dehydration, and rigors. Electrolyte disorders, such as hyponatremia or hypernatremia, hypokalemia, and hypophosphatemia can result in rhabdomyolysis, distorting the permeability and the functions of sarcolemma in the muscles. Some endocrine disorders (ie, pheochromocytoma and thyrotoxicosis) also are able to potentiate rhabdomyolysis due to hypermetabolism.
Prevention
A workout program should progress gradually according to the individual’s current level of fitness, whether it’s cardiovascular, circuit training, or weight training. Fluid intake should be monitored, particularly when the workout is long, intense, or hot and especially when the workout meets all 3 conditions. Fluid replacement is important, and for strenuous and longer training evolutions, electrolyte replacement should be considered. Hard exercise while maintaining a low-calorie diet or after long fasting periods should be avoided. Sufficient caloric and fluid intake to allow muscles to work efficiently during strenuous workout period is needed. Recreational drugs, including alcohol should be limited before exercise, and illicit recreational or performance-enhancing drugs should be avoided.
Conclusion
Acute exertional rhabdomyolysis is more common in the lower extremities and in males. Extremely rigorous upper-extremity training can result in AER. The presentation usually is clear with an inciting event and muscle pain in the extremity. Besides identifying associated risk factors and performing a thorough examination, the patient should be examined for compartment syndrome. Early diagnosis and comprehensive management are crucial to ensure full recovery and avoidance of complications, such as acute tubular necrosis, renal failure, cardiac arrhythmias from hyperkalemia, and death. As in most cases with early diagnosis and aggressive management, these patients fully recovered and experienced no sequelae at 8 weeks postevent.
1. Galvez R, Stacy J, Howley A. Exertional rhabdomyolysis in seven division-1 swimming athletes. Clin J Sport Med. 2008;18(4):366-368.
2. Smoot MK, Amendola A, Cramer E, et al. A cluster of exertional rhabdomyolysis affecting a division 1 football team. Clin J Sport Med. 2013;23(5):365-372.
3. Kahanov L, Eberman LE, Wasik M, Alvey T. Exertional rhabdomyolysis in a collegiate American football player after preventive cold-water immersion: a case report. J Athl Train. 2012;47(2):228-232.
4. Aizawa H, Morita K, Minami H, Sasaki N, Tobise K. Exertional rhabdomyolysis as a result of strenuous military training. J Neurol Sci. 1995;132(2):239-240.
5. Tietjen DP, Guzzi LM. Exertional rhabdomyolysis and acute renal failure following the Army Physical Fitness Test. Mil Med. 154(1):23-25.
6. Gardner JW, Kark JA. Fatal rhabdomyolysis presenting as mild heat illness in military training. Mil Med. 159(2):160-163.
7. Armed Forces Health Surveillance Center (AFHSC). Exertional rhabdomyolysis among U.S. military members, 2004-2007. MSMR. 2008;15(2):8-11.
8. Gitin EL, Demos MA. Acute exertional rhabdomyolysis: a syndrome of increasing importance to the military physician. Mil Med.1974;139(1):33-36.
9. Springer BL, Clarkson PM. Two cases of exertional rhabdomyolysis precipitated by personal trainers. Med Sci Sports Exerc. 2003;35(9):1499-1502.
10. Lin A, Lin C, Wang T, Leu J. Rhabdomyolysis in 119 students after repetitive exercise. Br J Sports Med. 2005;39(1):e3.
11. Hamer R. When exercise goes awry: exertional rhabdomyolysis. South Med J. 1997;90(5):548-551.
12. Soni SN, McDonald E, Marino C. Rhabdomyolysis after exercise. Postgrad Med. 1993;94(6):128-132.
13. Lappalainen H, Tiula E, Uotila L, Mänttäri M. Elimination kinetics of myoglobin and creatine kinase in rhabdomyolysis: implications for follow-up. Crit Care Med. 2002;30(10):2212-2215.
14. Huerta-Alardín AL, Varon J, Marik PE. Bench-to-bedside review: rhabdomyolysis—an overview for clinicians. Crit Care. 2005;9(2):158-169.
15. Minnema BJ, Neligan PC, Quraishi NA, Fehlings MG, Prakash S. A case of occult compartment syndrome and nonresolving rhabdomyolysis. J Gen Intern Med. 2008;23(6):871-874.
16. Efstratiadis G, Voulgaridou A, Nikiforou D, Kyventidis A, Kourkouni E, Vergoulas G. Rhabdomyolysis updated. Hippokratia. 2007;11(3):129-137.
1. Galvez R, Stacy J, Howley A. Exertional rhabdomyolysis in seven division-1 swimming athletes. Clin J Sport Med. 2008;18(4):366-368.
2. Smoot MK, Amendola A, Cramer E, et al. A cluster of exertional rhabdomyolysis affecting a division 1 football team. Clin J Sport Med. 2013;23(5):365-372.
3. Kahanov L, Eberman LE, Wasik M, Alvey T. Exertional rhabdomyolysis in a collegiate American football player after preventive cold-water immersion: a case report. J Athl Train. 2012;47(2):228-232.
4. Aizawa H, Morita K, Minami H, Sasaki N, Tobise K. Exertional rhabdomyolysis as a result of strenuous military training. J Neurol Sci. 1995;132(2):239-240.
5. Tietjen DP, Guzzi LM. Exertional rhabdomyolysis and acute renal failure following the Army Physical Fitness Test. Mil Med. 154(1):23-25.
6. Gardner JW, Kark JA. Fatal rhabdomyolysis presenting as mild heat illness in military training. Mil Med. 159(2):160-163.
7. Armed Forces Health Surveillance Center (AFHSC). Exertional rhabdomyolysis among U.S. military members, 2004-2007. MSMR. 2008;15(2):8-11.
8. Gitin EL, Demos MA. Acute exertional rhabdomyolysis: a syndrome of increasing importance to the military physician. Mil Med.1974;139(1):33-36.
9. Springer BL, Clarkson PM. Two cases of exertional rhabdomyolysis precipitated by personal trainers. Med Sci Sports Exerc. 2003;35(9):1499-1502.
10. Lin A, Lin C, Wang T, Leu J. Rhabdomyolysis in 119 students after repetitive exercise. Br J Sports Med. 2005;39(1):e3.
11. Hamer R. When exercise goes awry: exertional rhabdomyolysis. South Med J. 1997;90(5):548-551.
12. Soni SN, McDonald E, Marino C. Rhabdomyolysis after exercise. Postgrad Med. 1993;94(6):128-132.
13. Lappalainen H, Tiula E, Uotila L, Mänttäri M. Elimination kinetics of myoglobin and creatine kinase in rhabdomyolysis: implications for follow-up. Crit Care Med. 2002;30(10):2212-2215.
14. Huerta-Alardín AL, Varon J, Marik PE. Bench-to-bedside review: rhabdomyolysis—an overview for clinicians. Crit Care. 2005;9(2):158-169.
15. Minnema BJ, Neligan PC, Quraishi NA, Fehlings MG, Prakash S. A case of occult compartment syndrome and nonresolving rhabdomyolysis. J Gen Intern Med. 2008;23(6):871-874.
16. Efstratiadis G, Voulgaridou A, Nikiforou D, Kyventidis A, Kourkouni E, Vergoulas G. Rhabdomyolysis updated. Hippokratia. 2007;11(3):129-137.
Fat Fracture: A Rare Cause of Anterior and Medial Knee Pain in a Professional Baseball Player
ABSTRACT
Blunt trauma to the anterior knee typically results in a contusion or fracture of the patella. Additionally, injury to the extensor mechanism may come from a partial or full disruption of the patellar or quadriceps tendon. A professional baseball player suffered an injury to his knee after he collided with an outfield wall. Acute swelling in the suprapatellar soft tissues concealed a palpable defect, which initially was suspected to be an injury to the quadriceps tendon. Magnetic resonance imaging of the knee revealed an intact extensor mechanism; moreover, a fracture of the subcutaneous fat anterior to the quadriceps tendon was evident and diagnosed as a fat fracture.
Fat fracture is a rare diagnosis, and to the best of our knowledge, this is the first reported diagnosis in a professional athlete. Conservative management including, but not limited to, range of motion exercises, hydrotherapy, and iontophoresis effectively treated the athlete’s injury.
Blunt trauma to the anterior knee can result in a contusion or fracture of the patella, subluxation of the patella, and injury to the quadriceps or patellar tendon. Typically, a contusion or non-displaced fracture of the patella clinically presents with a direct anterior effusion and point tenderness. A displaced fracture or tendon deficit typically has an extensor lag or weakness in extension. Fat fracture or traumatic lipomata has been previously described in 1 case of anterior knee pain after blunt injury.1
In this article, we present the case of a 32-year-old professional baseball player who suffered a blunt injury to his left knee after collision with the outfield wall and experienced both anterior and medial knee pain. The patient provided written informed consent for print and electronic publication of this case report.
CASE
A 32-year-old outfielder for a professional baseball team was attempting a catch in the outfield when his left knee collided with the padded outfield wall in a semiflexed position. The player was able to walk off the field in the middle of the inning; however, he then experienced increasing pain and was unable to return to play. He had no prior history of significant knee pain or injury. He complained only of pain, with no instability or sensation of catching or locking.
Continue to: Physical examination of the patient...
Physical examination of the patient revealed a grade 1+ swelling over the anterior aspect of the superior pole of the patella in the prepatellar region, as well as medially over the medial femoral condyle. However, there was no joint effusion. Palpation of the superomedial aspect of the patella elicited pain, but no medial joint line tenderness was elicited. Percussion testing to the patella was negative. There were no gross palpable defects in the extensor mechanism, and the patient was able to perform a straight leg raise against resistance with pain.
Mild coronal laxity of the patella was noted compared with that of the contralateral knee. Hip range of motion (ROM) was intact, but knee ROM was limited to 110° of flexion, with the complaint of anterior tightness at this position. He was able to fully extend his knee without symptoms. The knee was stable to varus and valgus stress at both 0° and 30° of flexion. Lachman and anterior and posterior drawer tests were negative and symmetric to the contralateral knee. The McMurray test for meniscal pathology also was negative. Radiographs of the left knee were completed and were negative for fracture.
OUTCOMES
The initial clinical diagnosis was a patellar contusion and sprain of the medial retinaculum, and the athlete was treated with multiple modalities available in the athletic training room. Rehabilitation included activity modifications, passive and active ROM activities, quadriceps isometric exercises, and neuromuscular control activities. Adjunctive modalities included cryotherapy, hydrotherapy, topical hematoma cream, and iontophoresis.2 This aggressive treatment was continued for 3 days with decreased but persistent pain with running drills and limited knee flexion. Repeat clinical examination revealed a decreased swelling, but there was evidence of a clinically palpable defect anteriorly proximal to the patella. Although the patient could perform a straight leg raise, a partial injury to the quadriceps became plausible. Magnetic resonance imaging (MRI) of the left knee was performed, owing to the persistent pain and limited flexion despite aggressive conservative management, as well as the palpable soft-tissue defect.
MRI was performed using a 3T (Tesla) system (GE Healthcare) with a GE Healthcare Precision 8-channel knee coil. Routine knee protocol imaging was performed to include the distal quadriceps tendon due to clinical concern for a quadriceps tear. Sagittal proton density and proton-density fat-saturated (PD FS), coronal T1 and PD FS, and axial T1 and PD FS sequences were acquired.
An acutely marginated, 1.5 cm × 3 cm, longitudinal and transverse fluid defect “crevasse” was identified at the midline in the prepatellar subcutaneous fat overlying the distal quadriceps tendon and corresponded to a clinically palpable abnormality (Figures 1, 2).
Continue to: These findings explained...
These findings explained the delayed course in resolution of symptoms. Over the next 48 hours, continued conservative management, as outlined above, led to the resolution of symptoms, and the athlete returned to play. At a 2-month follow-up, the athlete described normal function in his knee without any residual symptoms. He returned to play without any symptoms. At 6 months, the athlete underwent MRI of the same knee for an unrelated reason. MRI revealed a healed fat fracture with resolution of the fluid defect in the subcutaneous fat (Figures 4A, 4B).
DISCUSSION
A fat fracture was first described in 1972 in 12 cases of buttock fat fractures after blunt trauma.3 The authors explained that fat lobules are typically arranged in layers and supported by horizontal and vertical fibrous septa. Typical loads flatten the lobules and disperse the forces throughout the layer. However, abnormal loads to a local area disrupt the fat lobules and shear the septa, resulting in decreased integrity of the interface between the epidermis and the fascia.
However, the extremities typically have less adipose tissue than in the buttocks, and the anterior knee is prone to blunt trauma. A previous description of a fat fracture in the knee noted a palpable defect in the quadriceps tendon and an inability to perform a straight leg raise. Our case initially presented with swelling, which concealed any soft-tissue defect. Furthermore, a straight leg raise was always intact despite the fat fracture defect surfacing after anterior swelling subsided. However, the disparity in these 2 cases highlights the spectrum of injury that is possible, as well as the difficulty in diagnosing a fat fracture. The previous report used ultrasound to confirm the diagnosis and assess the integrity of adjacent musculotendinous structures. An ultrasound may be readily available in athletic training rooms.1 Of note, to the best of our knowledge, this is the first case in the literature to report a fat fracture in a professional athlete and in baseball players. Furthermore, this case report describes an athlete who presented with anterior and medial knee pain. The edema from the fat fracture dispersed into the medial prepatellar bursa, which could be confused with edema from an injury to the medial-sided soft tissues.
Although these injuries do not require operative management, conservative measures may not be as effective as those in a patellar contusion or ligamentous sprain, and prolonged treatment may be necessary. Additionally, healthcare providers should be aware of this possible source of injury and counsel on an appropriate recovery time. Ideally, further recognition of such injuries can facilitate improved management and a faster return to activity.
1. Thomas RH, Holt MD, James SH, White PG. 'Fat fracture'—a physical sign mimicking tendon rupture. J Bone Joint Surg Br. 2001;83(2):204-205.
2. Antich T, Randall CC, Westbrook RA, Morrissey MC, Brewster CE. Physical therapy treatment of knee extensor mechanism disorders: comparison of four treatment modalities*. J Orthop Sports Phys Ther. 1986;8(5):255-259.
3. Meggitt BF, Wilson JN. The battered buttock syndrome—fat fractures. A report on a group of traumatic lipomata. Br J Surg. 1972;59(3):165-169.
ABSTRACT
Blunt trauma to the anterior knee typically results in a contusion or fracture of the patella. Additionally, injury to the extensor mechanism may come from a partial or full disruption of the patellar or quadriceps tendon. A professional baseball player suffered an injury to his knee after he collided with an outfield wall. Acute swelling in the suprapatellar soft tissues concealed a palpable defect, which initially was suspected to be an injury to the quadriceps tendon. Magnetic resonance imaging of the knee revealed an intact extensor mechanism; moreover, a fracture of the subcutaneous fat anterior to the quadriceps tendon was evident and diagnosed as a fat fracture.
Fat fracture is a rare diagnosis, and to the best of our knowledge, this is the first reported diagnosis in a professional athlete. Conservative management including, but not limited to, range of motion exercises, hydrotherapy, and iontophoresis effectively treated the athlete’s injury.
Blunt trauma to the anterior knee can result in a contusion or fracture of the patella, subluxation of the patella, and injury to the quadriceps or patellar tendon. Typically, a contusion or non-displaced fracture of the patella clinically presents with a direct anterior effusion and point tenderness. A displaced fracture or tendon deficit typically has an extensor lag or weakness in extension. Fat fracture or traumatic lipomata has been previously described in 1 case of anterior knee pain after blunt injury.1
In this article, we present the case of a 32-year-old professional baseball player who suffered a blunt injury to his left knee after collision with the outfield wall and experienced both anterior and medial knee pain. The patient provided written informed consent for print and electronic publication of this case report.
CASE
A 32-year-old outfielder for a professional baseball team was attempting a catch in the outfield when his left knee collided with the padded outfield wall in a semiflexed position. The player was able to walk off the field in the middle of the inning; however, he then experienced increasing pain and was unable to return to play. He had no prior history of significant knee pain or injury. He complained only of pain, with no instability or sensation of catching or locking.
Continue to: Physical examination of the patient...
Physical examination of the patient revealed a grade 1+ swelling over the anterior aspect of the superior pole of the patella in the prepatellar region, as well as medially over the medial femoral condyle. However, there was no joint effusion. Palpation of the superomedial aspect of the patella elicited pain, but no medial joint line tenderness was elicited. Percussion testing to the patella was negative. There were no gross palpable defects in the extensor mechanism, and the patient was able to perform a straight leg raise against resistance with pain.
Mild coronal laxity of the patella was noted compared with that of the contralateral knee. Hip range of motion (ROM) was intact, but knee ROM was limited to 110° of flexion, with the complaint of anterior tightness at this position. He was able to fully extend his knee without symptoms. The knee was stable to varus and valgus stress at both 0° and 30° of flexion. Lachman and anterior and posterior drawer tests were negative and symmetric to the contralateral knee. The McMurray test for meniscal pathology also was negative. Radiographs of the left knee were completed and were negative for fracture.
OUTCOMES
The initial clinical diagnosis was a patellar contusion and sprain of the medial retinaculum, and the athlete was treated with multiple modalities available in the athletic training room. Rehabilitation included activity modifications, passive and active ROM activities, quadriceps isometric exercises, and neuromuscular control activities. Adjunctive modalities included cryotherapy, hydrotherapy, topical hematoma cream, and iontophoresis.2 This aggressive treatment was continued for 3 days with decreased but persistent pain with running drills and limited knee flexion. Repeat clinical examination revealed a decreased swelling, but there was evidence of a clinically palpable defect anteriorly proximal to the patella. Although the patient could perform a straight leg raise, a partial injury to the quadriceps became plausible. Magnetic resonance imaging (MRI) of the left knee was performed, owing to the persistent pain and limited flexion despite aggressive conservative management, as well as the palpable soft-tissue defect.
MRI was performed using a 3T (Tesla) system (GE Healthcare) with a GE Healthcare Precision 8-channel knee coil. Routine knee protocol imaging was performed to include the distal quadriceps tendon due to clinical concern for a quadriceps tear. Sagittal proton density and proton-density fat-saturated (PD FS), coronal T1 and PD FS, and axial T1 and PD FS sequences were acquired.
An acutely marginated, 1.5 cm × 3 cm, longitudinal and transverse fluid defect “crevasse” was identified at the midline in the prepatellar subcutaneous fat overlying the distal quadriceps tendon and corresponded to a clinically palpable abnormality (Figures 1, 2).
Continue to: These findings explained...
These findings explained the delayed course in resolution of symptoms. Over the next 48 hours, continued conservative management, as outlined above, led to the resolution of symptoms, and the athlete returned to play. At a 2-month follow-up, the athlete described normal function in his knee without any residual symptoms. He returned to play without any symptoms. At 6 months, the athlete underwent MRI of the same knee for an unrelated reason. MRI revealed a healed fat fracture with resolution of the fluid defect in the subcutaneous fat (Figures 4A, 4B).
DISCUSSION
A fat fracture was first described in 1972 in 12 cases of buttock fat fractures after blunt trauma.3 The authors explained that fat lobules are typically arranged in layers and supported by horizontal and vertical fibrous septa. Typical loads flatten the lobules and disperse the forces throughout the layer. However, abnormal loads to a local area disrupt the fat lobules and shear the septa, resulting in decreased integrity of the interface between the epidermis and the fascia.
However, the extremities typically have less adipose tissue than in the buttocks, and the anterior knee is prone to blunt trauma. A previous description of a fat fracture in the knee noted a palpable defect in the quadriceps tendon and an inability to perform a straight leg raise. Our case initially presented with swelling, which concealed any soft-tissue defect. Furthermore, a straight leg raise was always intact despite the fat fracture defect surfacing after anterior swelling subsided. However, the disparity in these 2 cases highlights the spectrum of injury that is possible, as well as the difficulty in diagnosing a fat fracture. The previous report used ultrasound to confirm the diagnosis and assess the integrity of adjacent musculotendinous structures. An ultrasound may be readily available in athletic training rooms.1 Of note, to the best of our knowledge, this is the first case in the literature to report a fat fracture in a professional athlete and in baseball players. Furthermore, this case report describes an athlete who presented with anterior and medial knee pain. The edema from the fat fracture dispersed into the medial prepatellar bursa, which could be confused with edema from an injury to the medial-sided soft tissues.
Although these injuries do not require operative management, conservative measures may not be as effective as those in a patellar contusion or ligamentous sprain, and prolonged treatment may be necessary. Additionally, healthcare providers should be aware of this possible source of injury and counsel on an appropriate recovery time. Ideally, further recognition of such injuries can facilitate improved management and a faster return to activity.
ABSTRACT
Blunt trauma to the anterior knee typically results in a contusion or fracture of the patella. Additionally, injury to the extensor mechanism may come from a partial or full disruption of the patellar or quadriceps tendon. A professional baseball player suffered an injury to his knee after he collided with an outfield wall. Acute swelling in the suprapatellar soft tissues concealed a palpable defect, which initially was suspected to be an injury to the quadriceps tendon. Magnetic resonance imaging of the knee revealed an intact extensor mechanism; moreover, a fracture of the subcutaneous fat anterior to the quadriceps tendon was evident and diagnosed as a fat fracture.
Fat fracture is a rare diagnosis, and to the best of our knowledge, this is the first reported diagnosis in a professional athlete. Conservative management including, but not limited to, range of motion exercises, hydrotherapy, and iontophoresis effectively treated the athlete’s injury.
Blunt trauma to the anterior knee can result in a contusion or fracture of the patella, subluxation of the patella, and injury to the quadriceps or patellar tendon. Typically, a contusion or non-displaced fracture of the patella clinically presents with a direct anterior effusion and point tenderness. A displaced fracture or tendon deficit typically has an extensor lag or weakness in extension. Fat fracture or traumatic lipomata has been previously described in 1 case of anterior knee pain after blunt injury.1
In this article, we present the case of a 32-year-old professional baseball player who suffered a blunt injury to his left knee after collision with the outfield wall and experienced both anterior and medial knee pain. The patient provided written informed consent for print and electronic publication of this case report.
CASE
A 32-year-old outfielder for a professional baseball team was attempting a catch in the outfield when his left knee collided with the padded outfield wall in a semiflexed position. The player was able to walk off the field in the middle of the inning; however, he then experienced increasing pain and was unable to return to play. He had no prior history of significant knee pain or injury. He complained only of pain, with no instability or sensation of catching or locking.
Continue to: Physical examination of the patient...
Physical examination of the patient revealed a grade 1+ swelling over the anterior aspect of the superior pole of the patella in the prepatellar region, as well as medially over the medial femoral condyle. However, there was no joint effusion. Palpation of the superomedial aspect of the patella elicited pain, but no medial joint line tenderness was elicited. Percussion testing to the patella was negative. There were no gross palpable defects in the extensor mechanism, and the patient was able to perform a straight leg raise against resistance with pain.
Mild coronal laxity of the patella was noted compared with that of the contralateral knee. Hip range of motion (ROM) was intact, but knee ROM was limited to 110° of flexion, with the complaint of anterior tightness at this position. He was able to fully extend his knee without symptoms. The knee was stable to varus and valgus stress at both 0° and 30° of flexion. Lachman and anterior and posterior drawer tests were negative and symmetric to the contralateral knee. The McMurray test for meniscal pathology also was negative. Radiographs of the left knee were completed and were negative for fracture.
OUTCOMES
The initial clinical diagnosis was a patellar contusion and sprain of the medial retinaculum, and the athlete was treated with multiple modalities available in the athletic training room. Rehabilitation included activity modifications, passive and active ROM activities, quadriceps isometric exercises, and neuromuscular control activities. Adjunctive modalities included cryotherapy, hydrotherapy, topical hematoma cream, and iontophoresis.2 This aggressive treatment was continued for 3 days with decreased but persistent pain with running drills and limited knee flexion. Repeat clinical examination revealed a decreased swelling, but there was evidence of a clinically palpable defect anteriorly proximal to the patella. Although the patient could perform a straight leg raise, a partial injury to the quadriceps became plausible. Magnetic resonance imaging (MRI) of the left knee was performed, owing to the persistent pain and limited flexion despite aggressive conservative management, as well as the palpable soft-tissue defect.
MRI was performed using a 3T (Tesla) system (GE Healthcare) with a GE Healthcare Precision 8-channel knee coil. Routine knee protocol imaging was performed to include the distal quadriceps tendon due to clinical concern for a quadriceps tear. Sagittal proton density and proton-density fat-saturated (PD FS), coronal T1 and PD FS, and axial T1 and PD FS sequences were acquired.
An acutely marginated, 1.5 cm × 3 cm, longitudinal and transverse fluid defect “crevasse” was identified at the midline in the prepatellar subcutaneous fat overlying the distal quadriceps tendon and corresponded to a clinically palpable abnormality (Figures 1, 2).
Continue to: These findings explained...
These findings explained the delayed course in resolution of symptoms. Over the next 48 hours, continued conservative management, as outlined above, led to the resolution of symptoms, and the athlete returned to play. At a 2-month follow-up, the athlete described normal function in his knee without any residual symptoms. He returned to play without any symptoms. At 6 months, the athlete underwent MRI of the same knee for an unrelated reason. MRI revealed a healed fat fracture with resolution of the fluid defect in the subcutaneous fat (Figures 4A, 4B).
DISCUSSION
A fat fracture was first described in 1972 in 12 cases of buttock fat fractures after blunt trauma.3 The authors explained that fat lobules are typically arranged in layers and supported by horizontal and vertical fibrous septa. Typical loads flatten the lobules and disperse the forces throughout the layer. However, abnormal loads to a local area disrupt the fat lobules and shear the septa, resulting in decreased integrity of the interface between the epidermis and the fascia.
However, the extremities typically have less adipose tissue than in the buttocks, and the anterior knee is prone to blunt trauma. A previous description of a fat fracture in the knee noted a palpable defect in the quadriceps tendon and an inability to perform a straight leg raise. Our case initially presented with swelling, which concealed any soft-tissue defect. Furthermore, a straight leg raise was always intact despite the fat fracture defect surfacing after anterior swelling subsided. However, the disparity in these 2 cases highlights the spectrum of injury that is possible, as well as the difficulty in diagnosing a fat fracture. The previous report used ultrasound to confirm the diagnosis and assess the integrity of adjacent musculotendinous structures. An ultrasound may be readily available in athletic training rooms.1 Of note, to the best of our knowledge, this is the first case in the literature to report a fat fracture in a professional athlete and in baseball players. Furthermore, this case report describes an athlete who presented with anterior and medial knee pain. The edema from the fat fracture dispersed into the medial prepatellar bursa, which could be confused with edema from an injury to the medial-sided soft tissues.
Although these injuries do not require operative management, conservative measures may not be as effective as those in a patellar contusion or ligamentous sprain, and prolonged treatment may be necessary. Additionally, healthcare providers should be aware of this possible source of injury and counsel on an appropriate recovery time. Ideally, further recognition of such injuries can facilitate improved management and a faster return to activity.
1. Thomas RH, Holt MD, James SH, White PG. 'Fat fracture'—a physical sign mimicking tendon rupture. J Bone Joint Surg Br. 2001;83(2):204-205.
2. Antich T, Randall CC, Westbrook RA, Morrissey MC, Brewster CE. Physical therapy treatment of knee extensor mechanism disorders: comparison of four treatment modalities*. J Orthop Sports Phys Ther. 1986;8(5):255-259.
3. Meggitt BF, Wilson JN. The battered buttock syndrome—fat fractures. A report on a group of traumatic lipomata. Br J Surg. 1972;59(3):165-169.
1. Thomas RH, Holt MD, James SH, White PG. 'Fat fracture'—a physical sign mimicking tendon rupture. J Bone Joint Surg Br. 2001;83(2):204-205.
2. Antich T, Randall CC, Westbrook RA, Morrissey MC, Brewster CE. Physical therapy treatment of knee extensor mechanism disorders: comparison of four treatment modalities*. J Orthop Sports Phys Ther. 1986;8(5):255-259.
3. Meggitt BF, Wilson JN. The battered buttock syndrome—fat fractures. A report on a group of traumatic lipomata. Br J Surg. 1972;59(3):165-169.
TAKE-HOME POINTS
- A fat fracture should be considered in the setting of a blunt injury to the anterior knee when a palpable soft-tissue defect is observed and the extensor mechanism is clinically intact.
- An ultrasound or MRI can assist in making the diagnosis, which can aid in guiding the patient with management and in determining the expected duration of symptoms.
- Injuries to the anterior knee that may present as contusions but have a prolonged course of symptoms should not be overlooked.
Spontaneous Regression of Merkel Cell Carcinoma
Merkel cell carcinoma (MCC) is a rare, rapidly growing, aggressive neoplasm with a generally poor prognosis. The cells of origin are highly anaplastic and share structural and immunohistochemical features with various neuroectodermally derived cells. Although Merkel cells, which are slow-acting cutaneous mechanoreceptors located in the basal layer of the epidermis, and MCC share immunohistochemical and ultrastructural features, there is limited evidence of a direct histogenetic relationship between the two.1,2 Additionally, some extracutaneous neuroendocrine tumors have features similar to MCC; therefore, although it may be more accurate and perhaps more practical to describe these lesions as primary neuroendocrine carcinomas of the skin, the term MCC is more commonly used both in the literature and in clinical practice.1,2
Merkel cell carcinoma typically presents in the head and neck region in white patients older than 70 years of age and in the immunocompromised population.3-6 The mean age of diagnosis is 76 years for women and 74 years for men.7 The incidence of MCC in the United States tripled over a 15-year period, and there are approximately 1500 new cases of MCC diagnosed each year, making it about 40 times less common than melanoma.8 The 5-year survival rate for patients without lymph node involvement is 75%, whereas the 5-year survival rate for patients with distant metastases is 25%.9
Merkel cell carcinoma is thought to develop through 1 of 2 distinct pathways. In a virally mediated pathway, which represents at least 80% of cases, the Merkel cell polyomavirus (MCV) monoclonally integrates into the host genome and promotes oncogenesis via altered p53 and retinoblastoma protein expression.10-12 The remainder of cases are believed to develop via a nonvirally mediated pathway in which genetic anomalies, immune status, and environmental factors influence oncogenesis.10-13
Due to the similarity between MCC and metastatic neuroendocrine neoplasms, especially small-cell lung carcinomas, immunohistochemistry is important in making the diagnosis. Cytokeratin 20 and neuron-specific enolase positivity and thyroid transcription factor 1 negativity are the most useful markers in identifying MCC.
Regression of MCC is a very rare and poorly understood event. A 2010 review of the literature described 22 cases of spontaneous regression.14 We report a rare case of rapid and complete regression of MCC following punch biopsy in a 96-year-old woman.
Case Report

A 4-mm punch biopsy was obtained at a follow-up visit 4 weeks later (12 weeks after the reported onset of the lesion). Hematoxylin and eosin staining showed a small-cell neoplasm with stippled nuclei and scant cytoplasm forming a nested and somewhat trabecular pattern. Mitotic activity, apoptosis, and nuclear molding also were present (Figure 2). The tumor cells were positive for cytokeratin 20 with a dotlike, paranuclear pattern (Figure 3). Staining for CAM 5.2 also was positive. Cytokeratin 5/6, human melanoma black 45, and leukocyte common antigen were negative. The immunophenotyping of the lymphocytic response to the tumor showed that the majority of intratumoral lymphocytes were CD8 positive (Figure 4). CD4-positive lymphocytes were predominantly seen at the periphery of the tumor nests without tumor infiltration (Figure 5). Based on these findings, a diagnosis of MCC was made. The patient’s family declined treatment based on her advanced age and current health status, which included advanced dementia.
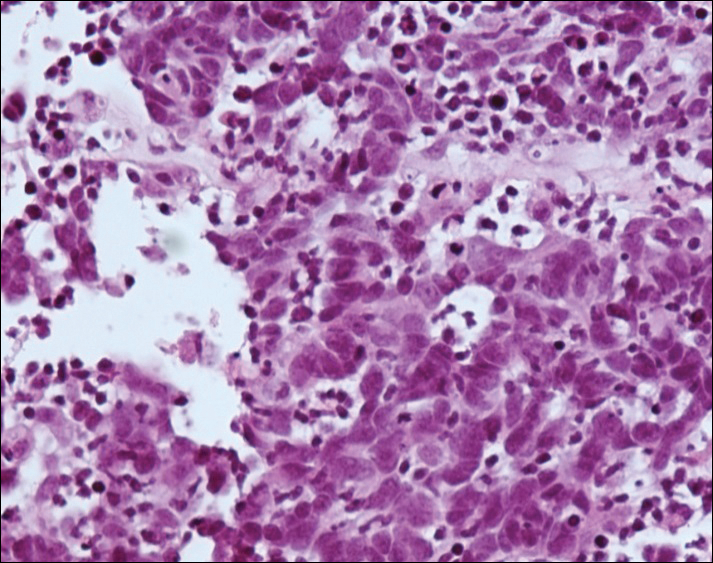

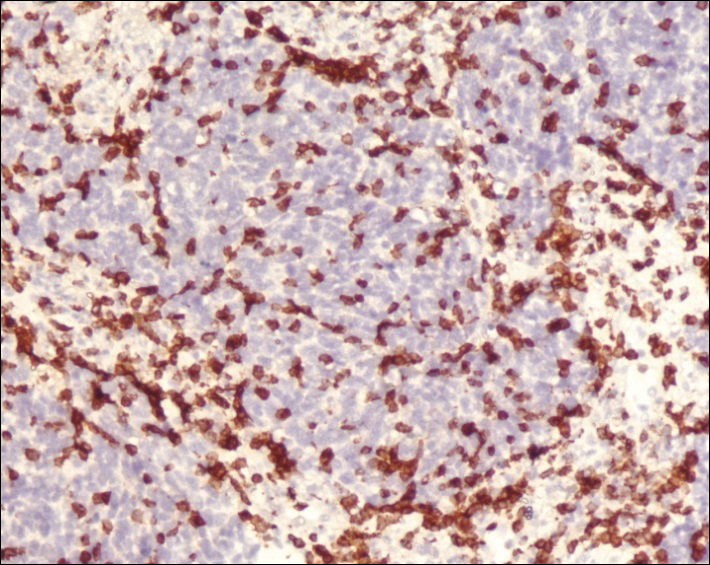
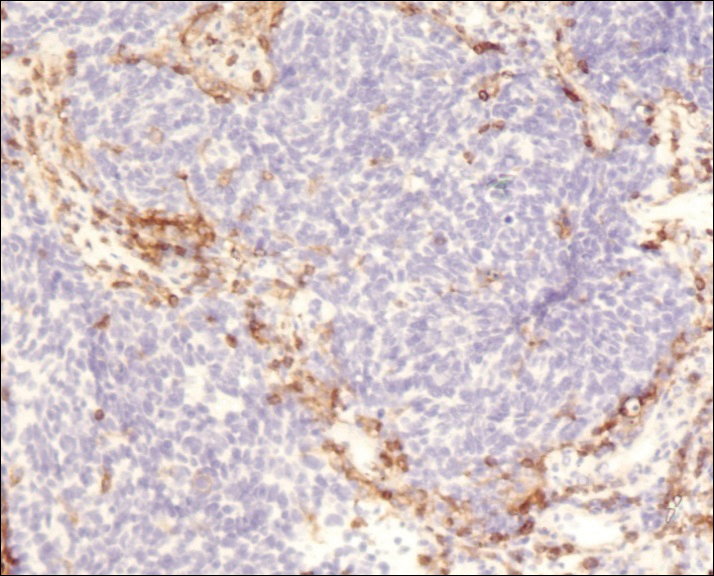
Two weeks after the punch biopsy, the lesion had noticeably decreased in size and lost its dome-shaped appearance. Within 8 weeks after biopsy (20 weeks since the lesion first appeared), the lesion had completely resolved (Figure 6). The patient was lost to follow-up months later, but no recurrence of the lesion was reported.

Comment
Spontaneous regression is not unique to MCC, as this phenomenon also has been reported in keratoacanthoma, lymphoma, basal cell carcinoma, and melanoma.15 Complete spontaneous regression is defined as occurring in the absence of therapy that is intended to have a treatment effect.15,16 Spontaneous regression is estimated to occur in malignant neoplasms at a rate of 1 case per 60,000 to 100,000 (approximately 0.0013% of all malignant neoplasms).17 Considering the reported prevalence of MCC and the number of cases that have been known to regress, the estimated incidence of complete spontaneous regression may be as high as 1.5%.14 Though spontaneous regression of MCC is more prevalent than expected, it still is considered a rare phenomenon. A 2010 review of the literature yielded 22 cases of complete spontaneous regression of MCC.14 No recurrences have been observed; however, follow-up was relatively short in some cases.
In a unique report by Bertolotti et al,18 a patient with MCC on the nasal tip presented 4 weeks after biopsy with complete spontaneous regression of the tumor, which was associated with bilateral cervical lymph node involvement as noted by hypermetabolic uptake on positron emission tomography scanning. The patient underwent radiation therapy and was disease free at 12 months’ follow-up.18
Complete spontaneous regression has been described in MCC patients with local disease, regional recurrences, and metastatic disease.19 In
The histopathologic features observed in our case, specifically intratumoral CD8-positive cytotoxic lymphocytes and peritumoral CD4-positive cells, were similar to the findings in other reported cases. In one series of 2 cases, the one case showed scar tissue with a moderate, predominantly T-lymphocytic infiltrate and no tumor cells, and the second showed cellular proliferation in the deep dermis with dense lymphocytic infiltrates primarily composed of CD3-positive T cells.14 Other studies of regression of both localized and metastatic MCC demonstrated infiltration by CD4-positive, CD8-positive, and CD3-positive lymphocytes and foamy macrophages.21-23
The discovery of the MCV was one of the most important advances in elucidating the pathogenesis of MCC.10,24-26 Merkel cell polyomavirus DNA has been detected in a majority of MCC cases.25,27 Viral integration has been shown to take place early, prior to tumor clonal expansion.10 Importantly, not all cases of MCC show MCV infection, and MCV infection is not exclusive to MCC.28 Merkel cell polyomavirus is considered to be part of the normal human flora, and asymptomatic infection is quite common.29 It has been identified in 80% of adults older than 50 years of age and, interestingly, in 35% of children by 13 years of age or younger.30,31 It remains unclear what role the presence of MCV plays in determining MCC prognosis. Several reports have demonstrated lower disease-specific mortality associated with MCV-positive MCC.32-35 In contrast, Schrama et al36 correlated the MCV status of 174 MCC tumors and found no difference in clinical behavior or prognosis between MCV-positive and MCV-negative MCCs.
Immunosuppression also may play a role in the development of MCC.5,25 There is increased prevalence of MCC in the human immunodeficiency virus–positive population, as well as in organ-transplant recipients and patients with leukemia. Chronic lymphocytic leukemia seems to be the most frequent neoplasia associated with development of MCC.37
The mechanism of MCC regression remains unclear, but many investigators emphasize the importance of T-cell–mediated immunity.16,21-23,38,39 Apoptosis also has been shown to play an important role.40 Our case showed tumor-infiltrating CD8-positive lymphocytes and CD4-positive lymphocytes present predominantly at the periphery of the tumor, with close proximity to the tumor nests but with no tumor infiltration (Figure 3). This distribution was consistently present in multiple sections of the tumor. These findings are consistent with prior reports of both CD4-positive and CD8-positive T lymphocytes associated with MCC regression. Our findings confirm that immune response may play an important role in spontaneous regression of MCC.
There is much speculation regarding the initial biopsy of an MCC lesion (or other traumatic event) and its role in tumor regression. Koba et al41 examined the effect of biopsy on CD8-positive lymphocytic infiltration of MCC tumor cells and found that biopsy does not commonly alter intratumoral CD8-positive infiltration. These findings suggest trauma does not directly induce immunologic recognition of this cancer.
Conclusion
We report a case of complete spontaneous regression of a localized MCC following a punch biopsy. The histopathology showed a brisk T-lymphocyte response with intratumoral CD8-positive cytotoxic lymphocytes and peritumoral CD4-positive cells. The age and clinical profile of our patient as well as the clinicopathologic characteristics of the tumor regression are similar to other reported cases. Further research is needed to elucidate the mechanism of MCC regression, and a better understanding of this fascinating phenomenon could help in development of new immunotherapeutic approaches.
- Sibley RK, Dehner LP, Rosai J. Primary neuroendocrine (Merkel cell?) carcinoma of the skin. I. a clinicopathologic and ultrastructural study of 43 cases. Am J Surg Pathol. 1985;9:95-108.
- Sibley RK, Dahl D. Primary neuroendocrine (Merkel cell?) carcinoma of the skin. II. an immunocytochemical study of 21 cases. Am J Surg Pathol. 1985;9:109-116.
- Heath M, Jaimes N, Lemos B, et al. Clinical characteristics of Merkel cell carcinoma at diagnosis in 195 patients: the AEIOU features. J Am Acad Dermatol. 2008;58:375-381.
- Penn I, First MR. Merkel’s cell carcinoma in organ recipients: report of 41 cases. Transplantation. 1999;68:1717-1721.
- Gooptu C, Woolloons A, Ross J, et al. Merkel cell carcinoma arising after therapeutic immunosuppression. Br J Dermatol. 1997;137:637-641.
- Plunkett TA, Harris AJ, Ogg CS, et al. The treatment of Merkel cell carcinoma and its association with immunosuppression. Br J Dermatol. 1998;139:345-346.
- Calder KB, Smoller BR. New insights into Merkel cell carcinoma. Adv Anat Pathol. 2010;17:155-161.
- Hodgson NC. Merkel cell carcinoma: changing incidence trends. J Surg Oncol. 2005;89:1-4.
- Agelli M, Clegg LX. Epidemiology of primary Merkel cell carcinoma in the United States. J Am Acad Dermatol. 2003;49:832-841.
- Feng H, Shuda M, Chang Y, et al. Clonal integration of a polyomavirus in human Merkel cell carcinoma. Science. 2008;319:1096-1100.
- Amber K, McLeod MP, Nouri K. The Merkel cell polyomavirus and its involvement in Merkel cell carcinoma. Dermatol Surg. 2013;39:232-238.
- Decaprio JA. Does detection of Merkel cell polyomavirus in Merkel cell carcinoma provide prognostic information? J Natl Cancer Inst. 2009;101:905-907.
- Popp S, Waltering S, Herbst C, et al. UV-B-type mutations and chromosomal imbalances indicate common pathways for the development of Merkel and skin squamous cell carcinomas. Int J Cancer. 2002;99:352-360.
- Ciudad C, Avilés JA, Alfageme F, et al. Spontaneous regression in Merkel cell carcinoma: report of two cases with description of dermoscopic features and review of literature. Dermatol Surg. 2010;36:687-693.
- O’Rourke MGE, Bell JR. Merkel cell tumor with spontaneous regression. J Dermatol Surg Oncol. 1986;12:994-997.
- Connelly TJ, Cribier B, Brown TJ, et al. Complete spontaneous regression of Merkel cell carcinoma: a review of 10 reported cases. Dermatol Surg. 2000;26:853-856.
- Cole WH. Efforts to explain spontaneous regression of cancer. J Surg Oncol. 1981;17:201-209.
- Bertolotti A, Conte H, Francois L, et al. Merkel cell carcinoma: complete clinical remission associated with disease progression. JAMA Dermatol. 2013;149:501-502.
- Pang C, Sharma D, Sankar T. Spontaneous regression of Merkel cell carcinoma: a case report and review of the literature [published online November 13, 2014]. Int J Surg Case Rep. 2015;7C:104-108.
- Richetta AG, Mancini M, Torroni A, et al. Total spontaneous regression of advanced Merkel cell carcinoma after biopsy: review and a new case. Dermatol Surg. 2008;34:815-822.
- Vesely MJ, Murray DJ, Neligan PC, et al. Complete spontaneous regression in Merkel cell carcinoma. J Plast Reconstr Aesthet Surg. 2008;61:165-171.
- Kayashima K, Ono T, Johno M, et al. Spontaneous regression in Merkel cell (neuroendocrine) carcinoma of the skin. Arch Dermatol. 1991;127:550-553.
- Maruo K, Kayashima KI, Ono T. Regressing Merkel cell carcinoma-a case showing replacement of tumour cells by foamy cells. Br J Dermatol. 2000;142:1184-1189.
- Duncavage E, Zehnbauer B, Pfeifer J. Prevalence of Merkel cell polyomavirus in Merkel cell carcinoma. Mod Pathol. 2009;22:516-521.
- Kassem A, Schopflin A, Diaz C, et al. Frequent detection of Merkel cell polyomavirus in human Merkel cell carcinomas and identification of unique deletion in the VP1 gene. Cancer Res. 2008;68:5009-5013.
- Becker J, Schrama D, Houben R. Merkel cell carcinoma. Cell Mol Life Sci. 2009;66:1-8.
- Haitz KA, Rady PL, Nguyen HP, et al. Merkel cell polyomavirus DNA detection in a patient with Merkel cell carcinoma and multiple other skin cancers. Int J Dermatol. 2012;51:442-444.
- Andres C, Puchta U, Sander CA, et al. Prevalence of Merkel cell polyomavirus DNA in cutaneous lymphomas, pseudolymphomas, and inflammatory skin diseases. Am J Dermatopathol. 2010;32:593-598.
- Showalter RM, Pastrana DV, Pumphrey KA, et al. Merkel cell polyomavirus and two previously unknown polyomaviruses are chronically shed from human skin. Cell Host Microbe. 2010;7:509-515.
- Tolstov YL, Pastrana DV, Feng H, et al. Human Merkel cell polyomavirus infection II. MCV is a common human infection that can be detected by conformational capsid epitope immunoassays. Int J Cancer. 2009;125:1250-1256.
- Chen T, Hedman L, Mattila PS, et al. Serological evidence of Merkel cell polyomavirus primary infections in childhood. J Clin Virol. 2011;50:125-129.
- Laude HC, Jonchère B, Maubec E, et al. Distinct Merkel cell polyomavirus molecular features in tumour and non tumour specimens from patients with Merkel cell carcinoma. PLoS Pathog. 2010;6:e1001076.
- Waltari M, Sihto H, Kukko H, et al. Association of Merkel cell polyomavirus infection with tumor p53, KIT, stem cell factor, PDGFR-alpha and survival in Merkel cell carcinoma. Int J Cancer. 2011;129:619-628.
- Sihto H, Kukko H, Koljonen V, et al. Clinical factors associated with Merkel cell polyomavirus infection in Merkel cell carcinoma. J Natl Cancer Inst. 2009;101:938-945.
- Paulson KG, Lemos BD, Feng B, et al. Array-CGH reveals recurrent genomic changes in Merkel cell carcinoma including amplification of L-Myc. J Invest Dermatol. 2009;129:1547-1555.
- Schrama D, Peitsch WK, Zapatka M, et al. Merkel cell polyomavirus status is not associated with clinical course of Merkel cell carcinoma. J Invest Dermatol. 2011;131:1631-1638.
- Tadmor T, Aviv A, Polliack A. Merkel cell carcinoma, chronic lymphocytic leukemia and other lymphoproliferative disorders: an old bond with possible new viral ties. Ann Oncol. 2011;22:250-256.
- Wooff J, Trites JR, Walsh NM, et al. Complete spontaneous regression of metastatic Merkel cell carcinoma: a case report and review of the literature. Am J Dermatopathol. 2010;32:614-617.
- Turk TO, Smoljan I, Nacinovic A, et al. Spontaneous regression of Merkel cell carcinoma in a patient with chronic lymphocytic leukemia: a case report. J Med Case Rep. 2009;3:7270.
- Mori Y, Tanaka K, Cui CY, et al. A study of apoptosis in Merkel cell carcinoma. an immunohistochemical, ultrasctructural, DNA ladder and TUNEL labeling study. Am J Dermatopathol. 2001;23:16-23.
- Koba S, Paulson KG, Nagase K, et al. Diagnostic biopsy does not commonly induce intratumoral CD8 T cell infiltration in Merkel cell carcinoma. PLoS ONE. 2012;7:e41465.
Merkel cell carcinoma (MCC) is a rare, rapidly growing, aggressive neoplasm with a generally poor prognosis. The cells of origin are highly anaplastic and share structural and immunohistochemical features with various neuroectodermally derived cells. Although Merkel cells, which are slow-acting cutaneous mechanoreceptors located in the basal layer of the epidermis, and MCC share immunohistochemical and ultrastructural features, there is limited evidence of a direct histogenetic relationship between the two.1,2 Additionally, some extracutaneous neuroendocrine tumors have features similar to MCC; therefore, although it may be more accurate and perhaps more practical to describe these lesions as primary neuroendocrine carcinomas of the skin, the term MCC is more commonly used both in the literature and in clinical practice.1,2
Merkel cell carcinoma typically presents in the head and neck region in white patients older than 70 years of age and in the immunocompromised population.3-6 The mean age of diagnosis is 76 years for women and 74 years for men.7 The incidence of MCC in the United States tripled over a 15-year period, and there are approximately 1500 new cases of MCC diagnosed each year, making it about 40 times less common than melanoma.8 The 5-year survival rate for patients without lymph node involvement is 75%, whereas the 5-year survival rate for patients with distant metastases is 25%.9
Merkel cell carcinoma is thought to develop through 1 of 2 distinct pathways. In a virally mediated pathway, which represents at least 80% of cases, the Merkel cell polyomavirus (MCV) monoclonally integrates into the host genome and promotes oncogenesis via altered p53 and retinoblastoma protein expression.10-12 The remainder of cases are believed to develop via a nonvirally mediated pathway in which genetic anomalies, immune status, and environmental factors influence oncogenesis.10-13
Due to the similarity between MCC and metastatic neuroendocrine neoplasms, especially small-cell lung carcinomas, immunohistochemistry is important in making the diagnosis. Cytokeratin 20 and neuron-specific enolase positivity and thyroid transcription factor 1 negativity are the most useful markers in identifying MCC.
Regression of MCC is a very rare and poorly understood event. A 2010 review of the literature described 22 cases of spontaneous regression.14 We report a rare case of rapid and complete regression of MCC following punch biopsy in a 96-year-old woman.
Case Report

A 4-mm punch biopsy was obtained at a follow-up visit 4 weeks later (12 weeks after the reported onset of the lesion). Hematoxylin and eosin staining showed a small-cell neoplasm with stippled nuclei and scant cytoplasm forming a nested and somewhat trabecular pattern. Mitotic activity, apoptosis, and nuclear molding also were present (Figure 2). The tumor cells were positive for cytokeratin 20 with a dotlike, paranuclear pattern (Figure 3). Staining for CAM 5.2 also was positive. Cytokeratin 5/6, human melanoma black 45, and leukocyte common antigen were negative. The immunophenotyping of the lymphocytic response to the tumor showed that the majority of intratumoral lymphocytes were CD8 positive (Figure 4). CD4-positive lymphocytes were predominantly seen at the periphery of the tumor nests without tumor infiltration (Figure 5). Based on these findings, a diagnosis of MCC was made. The patient’s family declined treatment based on her advanced age and current health status, which included advanced dementia.




Two weeks after the punch biopsy, the lesion had noticeably decreased in size and lost its dome-shaped appearance. Within 8 weeks after biopsy (20 weeks since the lesion first appeared), the lesion had completely resolved (Figure 6). The patient was lost to follow-up months later, but no recurrence of the lesion was reported.

Comment
Spontaneous regression is not unique to MCC, as this phenomenon also has been reported in keratoacanthoma, lymphoma, basal cell carcinoma, and melanoma.15 Complete spontaneous regression is defined as occurring in the absence of therapy that is intended to have a treatment effect.15,16 Spontaneous regression is estimated to occur in malignant neoplasms at a rate of 1 case per 60,000 to 100,000 (approximately 0.0013% of all malignant neoplasms).17 Considering the reported prevalence of MCC and the number of cases that have been known to regress, the estimated incidence of complete spontaneous regression may be as high as 1.5%.14 Though spontaneous regression of MCC is more prevalent than expected, it still is considered a rare phenomenon. A 2010 review of the literature yielded 22 cases of complete spontaneous regression of MCC.14 No recurrences have been observed; however, follow-up was relatively short in some cases.
In a unique report by Bertolotti et al,18 a patient with MCC on the nasal tip presented 4 weeks after biopsy with complete spontaneous regression of the tumor, which was associated with bilateral cervical lymph node involvement as noted by hypermetabolic uptake on positron emission tomography scanning. The patient underwent radiation therapy and was disease free at 12 months’ follow-up.18
Complete spontaneous regression has been described in MCC patients with local disease, regional recurrences, and metastatic disease.19 In
The histopathologic features observed in our case, specifically intratumoral CD8-positive cytotoxic lymphocytes and peritumoral CD4-positive cells, were similar to the findings in other reported cases. In one series of 2 cases, the one case showed scar tissue with a moderate, predominantly T-lymphocytic infiltrate and no tumor cells, and the second showed cellular proliferation in the deep dermis with dense lymphocytic infiltrates primarily composed of CD3-positive T cells.14 Other studies of regression of both localized and metastatic MCC demonstrated infiltration by CD4-positive, CD8-positive, and CD3-positive lymphocytes and foamy macrophages.21-23
The discovery of the MCV was one of the most important advances in elucidating the pathogenesis of MCC.10,24-26 Merkel cell polyomavirus DNA has been detected in a majority of MCC cases.25,27 Viral integration has been shown to take place early, prior to tumor clonal expansion.10 Importantly, not all cases of MCC show MCV infection, and MCV infection is not exclusive to MCC.28 Merkel cell polyomavirus is considered to be part of the normal human flora, and asymptomatic infection is quite common.29 It has been identified in 80% of adults older than 50 years of age and, interestingly, in 35% of children by 13 years of age or younger.30,31 It remains unclear what role the presence of MCV plays in determining MCC prognosis. Several reports have demonstrated lower disease-specific mortality associated with MCV-positive MCC.32-35 In contrast, Schrama et al36 correlated the MCV status of 174 MCC tumors and found no difference in clinical behavior or prognosis between MCV-positive and MCV-negative MCCs.
Immunosuppression also may play a role in the development of MCC.5,25 There is increased prevalence of MCC in the human immunodeficiency virus–positive population, as well as in organ-transplant recipients and patients with leukemia. Chronic lymphocytic leukemia seems to be the most frequent neoplasia associated with development of MCC.37
The mechanism of MCC regression remains unclear, but many investigators emphasize the importance of T-cell–mediated immunity.16,21-23,38,39 Apoptosis also has been shown to play an important role.40 Our case showed tumor-infiltrating CD8-positive lymphocytes and CD4-positive lymphocytes present predominantly at the periphery of the tumor, with close proximity to the tumor nests but with no tumor infiltration (Figure 3). This distribution was consistently present in multiple sections of the tumor. These findings are consistent with prior reports of both CD4-positive and CD8-positive T lymphocytes associated with MCC regression. Our findings confirm that immune response may play an important role in spontaneous regression of MCC.
There is much speculation regarding the initial biopsy of an MCC lesion (or other traumatic event) and its role in tumor regression. Koba et al41 examined the effect of biopsy on CD8-positive lymphocytic infiltration of MCC tumor cells and found that biopsy does not commonly alter intratumoral CD8-positive infiltration. These findings suggest trauma does not directly induce immunologic recognition of this cancer.
Conclusion
We report a case of complete spontaneous regression of a localized MCC following a punch biopsy. The histopathology showed a brisk T-lymphocyte response with intratumoral CD8-positive cytotoxic lymphocytes and peritumoral CD4-positive cells. The age and clinical profile of our patient as well as the clinicopathologic characteristics of the tumor regression are similar to other reported cases. Further research is needed to elucidate the mechanism of MCC regression, and a better understanding of this fascinating phenomenon could help in development of new immunotherapeutic approaches.
Merkel cell carcinoma (MCC) is a rare, rapidly growing, aggressive neoplasm with a generally poor prognosis. The cells of origin are highly anaplastic and share structural and immunohistochemical features with various neuroectodermally derived cells. Although Merkel cells, which are slow-acting cutaneous mechanoreceptors located in the basal layer of the epidermis, and MCC share immunohistochemical and ultrastructural features, there is limited evidence of a direct histogenetic relationship between the two.1,2 Additionally, some extracutaneous neuroendocrine tumors have features similar to MCC; therefore, although it may be more accurate and perhaps more practical to describe these lesions as primary neuroendocrine carcinomas of the skin, the term MCC is more commonly used both in the literature and in clinical practice.1,2
Merkel cell carcinoma typically presents in the head and neck region in white patients older than 70 years of age and in the immunocompromised population.3-6 The mean age of diagnosis is 76 years for women and 74 years for men.7 The incidence of MCC in the United States tripled over a 15-year period, and there are approximately 1500 new cases of MCC diagnosed each year, making it about 40 times less common than melanoma.8 The 5-year survival rate for patients without lymph node involvement is 75%, whereas the 5-year survival rate for patients with distant metastases is 25%.9
Merkel cell carcinoma is thought to develop through 1 of 2 distinct pathways. In a virally mediated pathway, which represents at least 80% of cases, the Merkel cell polyomavirus (MCV) monoclonally integrates into the host genome and promotes oncogenesis via altered p53 and retinoblastoma protein expression.10-12 The remainder of cases are believed to develop via a nonvirally mediated pathway in which genetic anomalies, immune status, and environmental factors influence oncogenesis.10-13
Due to the similarity between MCC and metastatic neuroendocrine neoplasms, especially small-cell lung carcinomas, immunohistochemistry is important in making the diagnosis. Cytokeratin 20 and neuron-specific enolase positivity and thyroid transcription factor 1 negativity are the most useful markers in identifying MCC.
Regression of MCC is a very rare and poorly understood event. A 2010 review of the literature described 22 cases of spontaneous regression.14 We report a rare case of rapid and complete regression of MCC following punch biopsy in a 96-year-old woman.
Case Report

A 4-mm punch biopsy was obtained at a follow-up visit 4 weeks later (12 weeks after the reported onset of the lesion). Hematoxylin and eosin staining showed a small-cell neoplasm with stippled nuclei and scant cytoplasm forming a nested and somewhat trabecular pattern. Mitotic activity, apoptosis, and nuclear molding also were present (Figure 2). The tumor cells were positive for cytokeratin 20 with a dotlike, paranuclear pattern (Figure 3). Staining for CAM 5.2 also was positive. Cytokeratin 5/6, human melanoma black 45, and leukocyte common antigen were negative. The immunophenotyping of the lymphocytic response to the tumor showed that the majority of intratumoral lymphocytes were CD8 positive (Figure 4). CD4-positive lymphocytes were predominantly seen at the periphery of the tumor nests without tumor infiltration (Figure 5). Based on these findings, a diagnosis of MCC was made. The patient’s family declined treatment based on her advanced age and current health status, which included advanced dementia.




Two weeks after the punch biopsy, the lesion had noticeably decreased in size and lost its dome-shaped appearance. Within 8 weeks after biopsy (20 weeks since the lesion first appeared), the lesion had completely resolved (Figure 6). The patient was lost to follow-up months later, but no recurrence of the lesion was reported.

Comment
Spontaneous regression is not unique to MCC, as this phenomenon also has been reported in keratoacanthoma, lymphoma, basal cell carcinoma, and melanoma.15 Complete spontaneous regression is defined as occurring in the absence of therapy that is intended to have a treatment effect.15,16 Spontaneous regression is estimated to occur in malignant neoplasms at a rate of 1 case per 60,000 to 100,000 (approximately 0.0013% of all malignant neoplasms).17 Considering the reported prevalence of MCC and the number of cases that have been known to regress, the estimated incidence of complete spontaneous regression may be as high as 1.5%.14 Though spontaneous regression of MCC is more prevalent than expected, it still is considered a rare phenomenon. A 2010 review of the literature yielded 22 cases of complete spontaneous regression of MCC.14 No recurrences have been observed; however, follow-up was relatively short in some cases.
In a unique report by Bertolotti et al,18 a patient with MCC on the nasal tip presented 4 weeks after biopsy with complete spontaneous regression of the tumor, which was associated with bilateral cervical lymph node involvement as noted by hypermetabolic uptake on positron emission tomography scanning. The patient underwent radiation therapy and was disease free at 12 months’ follow-up.18
Complete spontaneous regression has been described in MCC patients with local disease, regional recurrences, and metastatic disease.19 In
The histopathologic features observed in our case, specifically intratumoral CD8-positive cytotoxic lymphocytes and peritumoral CD4-positive cells, were similar to the findings in other reported cases. In one series of 2 cases, the one case showed scar tissue with a moderate, predominantly T-lymphocytic infiltrate and no tumor cells, and the second showed cellular proliferation in the deep dermis with dense lymphocytic infiltrates primarily composed of CD3-positive T cells.14 Other studies of regression of both localized and metastatic MCC demonstrated infiltration by CD4-positive, CD8-positive, and CD3-positive lymphocytes and foamy macrophages.21-23
The discovery of the MCV was one of the most important advances in elucidating the pathogenesis of MCC.10,24-26 Merkel cell polyomavirus DNA has been detected in a majority of MCC cases.25,27 Viral integration has been shown to take place early, prior to tumor clonal expansion.10 Importantly, not all cases of MCC show MCV infection, and MCV infection is not exclusive to MCC.28 Merkel cell polyomavirus is considered to be part of the normal human flora, and asymptomatic infection is quite common.29 It has been identified in 80% of adults older than 50 years of age and, interestingly, in 35% of children by 13 years of age or younger.30,31 It remains unclear what role the presence of MCV plays in determining MCC prognosis. Several reports have demonstrated lower disease-specific mortality associated with MCV-positive MCC.32-35 In contrast, Schrama et al36 correlated the MCV status of 174 MCC tumors and found no difference in clinical behavior or prognosis between MCV-positive and MCV-negative MCCs.
Immunosuppression also may play a role in the development of MCC.5,25 There is increased prevalence of MCC in the human immunodeficiency virus–positive population, as well as in organ-transplant recipients and patients with leukemia. Chronic lymphocytic leukemia seems to be the most frequent neoplasia associated with development of MCC.37
The mechanism of MCC regression remains unclear, but many investigators emphasize the importance of T-cell–mediated immunity.16,21-23,38,39 Apoptosis also has been shown to play an important role.40 Our case showed tumor-infiltrating CD8-positive lymphocytes and CD4-positive lymphocytes present predominantly at the periphery of the tumor, with close proximity to the tumor nests but with no tumor infiltration (Figure 3). This distribution was consistently present in multiple sections of the tumor. These findings are consistent with prior reports of both CD4-positive and CD8-positive T lymphocytes associated with MCC regression. Our findings confirm that immune response may play an important role in spontaneous regression of MCC.
There is much speculation regarding the initial biopsy of an MCC lesion (or other traumatic event) and its role in tumor regression. Koba et al41 examined the effect of biopsy on CD8-positive lymphocytic infiltration of MCC tumor cells and found that biopsy does not commonly alter intratumoral CD8-positive infiltration. These findings suggest trauma does not directly induce immunologic recognition of this cancer.
Conclusion
We report a case of complete spontaneous regression of a localized MCC following a punch biopsy. The histopathology showed a brisk T-lymphocyte response with intratumoral CD8-positive cytotoxic lymphocytes and peritumoral CD4-positive cells. The age and clinical profile of our patient as well as the clinicopathologic characteristics of the tumor regression are similar to other reported cases. Further research is needed to elucidate the mechanism of MCC regression, and a better understanding of this fascinating phenomenon could help in development of new immunotherapeutic approaches.
- Sibley RK, Dehner LP, Rosai J. Primary neuroendocrine (Merkel cell?) carcinoma of the skin. I. a clinicopathologic and ultrastructural study of 43 cases. Am J Surg Pathol. 1985;9:95-108.
- Sibley RK, Dahl D. Primary neuroendocrine (Merkel cell?) carcinoma of the skin. II. an immunocytochemical study of 21 cases. Am J Surg Pathol. 1985;9:109-116.
- Heath M, Jaimes N, Lemos B, et al. Clinical characteristics of Merkel cell carcinoma at diagnosis in 195 patients: the AEIOU features. J Am Acad Dermatol. 2008;58:375-381.
- Penn I, First MR. Merkel’s cell carcinoma in organ recipients: report of 41 cases. Transplantation. 1999;68:1717-1721.
- Gooptu C, Woolloons A, Ross J, et al. Merkel cell carcinoma arising after therapeutic immunosuppression. Br J Dermatol. 1997;137:637-641.
- Plunkett TA, Harris AJ, Ogg CS, et al. The treatment of Merkel cell carcinoma and its association with immunosuppression. Br J Dermatol. 1998;139:345-346.
- Calder KB, Smoller BR. New insights into Merkel cell carcinoma. Adv Anat Pathol. 2010;17:155-161.
- Hodgson NC. Merkel cell carcinoma: changing incidence trends. J Surg Oncol. 2005;89:1-4.
- Agelli M, Clegg LX. Epidemiology of primary Merkel cell carcinoma in the United States. J Am Acad Dermatol. 2003;49:832-841.
- Feng H, Shuda M, Chang Y, et al. Clonal integration of a polyomavirus in human Merkel cell carcinoma. Science. 2008;319:1096-1100.
- Amber K, McLeod MP, Nouri K. The Merkel cell polyomavirus and its involvement in Merkel cell carcinoma. Dermatol Surg. 2013;39:232-238.
- Decaprio JA. Does detection of Merkel cell polyomavirus in Merkel cell carcinoma provide prognostic information? J Natl Cancer Inst. 2009;101:905-907.
- Popp S, Waltering S, Herbst C, et al. UV-B-type mutations and chromosomal imbalances indicate common pathways for the development of Merkel and skin squamous cell carcinomas. Int J Cancer. 2002;99:352-360.
- Ciudad C, Avilés JA, Alfageme F, et al. Spontaneous regression in Merkel cell carcinoma: report of two cases with description of dermoscopic features and review of literature. Dermatol Surg. 2010;36:687-693.
- O’Rourke MGE, Bell JR. Merkel cell tumor with spontaneous regression. J Dermatol Surg Oncol. 1986;12:994-997.
- Connelly TJ, Cribier B, Brown TJ, et al. Complete spontaneous regression of Merkel cell carcinoma: a review of 10 reported cases. Dermatol Surg. 2000;26:853-856.
- Cole WH. Efforts to explain spontaneous regression of cancer. J Surg Oncol. 1981;17:201-209.
- Bertolotti A, Conte H, Francois L, et al. Merkel cell carcinoma: complete clinical remission associated with disease progression. JAMA Dermatol. 2013;149:501-502.
- Pang C, Sharma D, Sankar T. Spontaneous regression of Merkel cell carcinoma: a case report and review of the literature [published online November 13, 2014]. Int J Surg Case Rep. 2015;7C:104-108.
- Richetta AG, Mancini M, Torroni A, et al. Total spontaneous regression of advanced Merkel cell carcinoma after biopsy: review and a new case. Dermatol Surg. 2008;34:815-822.
- Vesely MJ, Murray DJ, Neligan PC, et al. Complete spontaneous regression in Merkel cell carcinoma. J Plast Reconstr Aesthet Surg. 2008;61:165-171.
- Kayashima K, Ono T, Johno M, et al. Spontaneous regression in Merkel cell (neuroendocrine) carcinoma of the skin. Arch Dermatol. 1991;127:550-553.
- Maruo K, Kayashima KI, Ono T. Regressing Merkel cell carcinoma-a case showing replacement of tumour cells by foamy cells. Br J Dermatol. 2000;142:1184-1189.
- Duncavage E, Zehnbauer B, Pfeifer J. Prevalence of Merkel cell polyomavirus in Merkel cell carcinoma. Mod Pathol. 2009;22:516-521.
- Kassem A, Schopflin A, Diaz C, et al. Frequent detection of Merkel cell polyomavirus in human Merkel cell carcinomas and identification of unique deletion in the VP1 gene. Cancer Res. 2008;68:5009-5013.
- Becker J, Schrama D, Houben R. Merkel cell carcinoma. Cell Mol Life Sci. 2009;66:1-8.
- Haitz KA, Rady PL, Nguyen HP, et al. Merkel cell polyomavirus DNA detection in a patient with Merkel cell carcinoma and multiple other skin cancers. Int J Dermatol. 2012;51:442-444.
- Andres C, Puchta U, Sander CA, et al. Prevalence of Merkel cell polyomavirus DNA in cutaneous lymphomas, pseudolymphomas, and inflammatory skin diseases. Am J Dermatopathol. 2010;32:593-598.
- Showalter RM, Pastrana DV, Pumphrey KA, et al. Merkel cell polyomavirus and two previously unknown polyomaviruses are chronically shed from human skin. Cell Host Microbe. 2010;7:509-515.
- Tolstov YL, Pastrana DV, Feng H, et al. Human Merkel cell polyomavirus infection II. MCV is a common human infection that can be detected by conformational capsid epitope immunoassays. Int J Cancer. 2009;125:1250-1256.
- Chen T, Hedman L, Mattila PS, et al. Serological evidence of Merkel cell polyomavirus primary infections in childhood. J Clin Virol. 2011;50:125-129.
- Laude HC, Jonchère B, Maubec E, et al. Distinct Merkel cell polyomavirus molecular features in tumour and non tumour specimens from patients with Merkel cell carcinoma. PLoS Pathog. 2010;6:e1001076.
- Waltari M, Sihto H, Kukko H, et al. Association of Merkel cell polyomavirus infection with tumor p53, KIT, stem cell factor, PDGFR-alpha and survival in Merkel cell carcinoma. Int J Cancer. 2011;129:619-628.
- Sihto H, Kukko H, Koljonen V, et al. Clinical factors associated with Merkel cell polyomavirus infection in Merkel cell carcinoma. J Natl Cancer Inst. 2009;101:938-945.
- Paulson KG, Lemos BD, Feng B, et al. Array-CGH reveals recurrent genomic changes in Merkel cell carcinoma including amplification of L-Myc. J Invest Dermatol. 2009;129:1547-1555.
- Schrama D, Peitsch WK, Zapatka M, et al. Merkel cell polyomavirus status is not associated with clinical course of Merkel cell carcinoma. J Invest Dermatol. 2011;131:1631-1638.
- Tadmor T, Aviv A, Polliack A. Merkel cell carcinoma, chronic lymphocytic leukemia and other lymphoproliferative disorders: an old bond with possible new viral ties. Ann Oncol. 2011;22:250-256.
- Wooff J, Trites JR, Walsh NM, et al. Complete spontaneous regression of metastatic Merkel cell carcinoma: a case report and review of the literature. Am J Dermatopathol. 2010;32:614-617.
- Turk TO, Smoljan I, Nacinovic A, et al. Spontaneous regression of Merkel cell carcinoma in a patient with chronic lymphocytic leukemia: a case report. J Med Case Rep. 2009;3:7270.
- Mori Y, Tanaka K, Cui CY, et al. A study of apoptosis in Merkel cell carcinoma. an immunohistochemical, ultrasctructural, DNA ladder and TUNEL labeling study. Am J Dermatopathol. 2001;23:16-23.
- Koba S, Paulson KG, Nagase K, et al. Diagnostic biopsy does not commonly induce intratumoral CD8 T cell infiltration in Merkel cell carcinoma. PLoS ONE. 2012;7:e41465.
- Sibley RK, Dehner LP, Rosai J. Primary neuroendocrine (Merkel cell?) carcinoma of the skin. I. a clinicopathologic and ultrastructural study of 43 cases. Am J Surg Pathol. 1985;9:95-108.
- Sibley RK, Dahl D. Primary neuroendocrine (Merkel cell?) carcinoma of the skin. II. an immunocytochemical study of 21 cases. Am J Surg Pathol. 1985;9:109-116.
- Heath M, Jaimes N, Lemos B, et al. Clinical characteristics of Merkel cell carcinoma at diagnosis in 195 patients: the AEIOU features. J Am Acad Dermatol. 2008;58:375-381.
- Penn I, First MR. Merkel’s cell carcinoma in organ recipients: report of 41 cases. Transplantation. 1999;68:1717-1721.
- Gooptu C, Woolloons A, Ross J, et al. Merkel cell carcinoma arising after therapeutic immunosuppression. Br J Dermatol. 1997;137:637-641.
- Plunkett TA, Harris AJ, Ogg CS, et al. The treatment of Merkel cell carcinoma and its association with immunosuppression. Br J Dermatol. 1998;139:345-346.
- Calder KB, Smoller BR. New insights into Merkel cell carcinoma. Adv Anat Pathol. 2010;17:155-161.
- Hodgson NC. Merkel cell carcinoma: changing incidence trends. J Surg Oncol. 2005;89:1-4.
- Agelli M, Clegg LX. Epidemiology of primary Merkel cell carcinoma in the United States. J Am Acad Dermatol. 2003;49:832-841.
- Feng H, Shuda M, Chang Y, et al. Clonal integration of a polyomavirus in human Merkel cell carcinoma. Science. 2008;319:1096-1100.
- Amber K, McLeod MP, Nouri K. The Merkel cell polyomavirus and its involvement in Merkel cell carcinoma. Dermatol Surg. 2013;39:232-238.
- Decaprio JA. Does detection of Merkel cell polyomavirus in Merkel cell carcinoma provide prognostic information? J Natl Cancer Inst. 2009;101:905-907.
- Popp S, Waltering S, Herbst C, et al. UV-B-type mutations and chromosomal imbalances indicate common pathways for the development of Merkel and skin squamous cell carcinomas. Int J Cancer. 2002;99:352-360.
- Ciudad C, Avilés JA, Alfageme F, et al. Spontaneous regression in Merkel cell carcinoma: report of two cases with description of dermoscopic features and review of literature. Dermatol Surg. 2010;36:687-693.
- O’Rourke MGE, Bell JR. Merkel cell tumor with spontaneous regression. J Dermatol Surg Oncol. 1986;12:994-997.
- Connelly TJ, Cribier B, Brown TJ, et al. Complete spontaneous regression of Merkel cell carcinoma: a review of 10 reported cases. Dermatol Surg. 2000;26:853-856.
- Cole WH. Efforts to explain spontaneous regression of cancer. J Surg Oncol. 1981;17:201-209.
- Bertolotti A, Conte H, Francois L, et al. Merkel cell carcinoma: complete clinical remission associated with disease progression. JAMA Dermatol. 2013;149:501-502.
- Pang C, Sharma D, Sankar T. Spontaneous regression of Merkel cell carcinoma: a case report and review of the literature [published online November 13, 2014]. Int J Surg Case Rep. 2015;7C:104-108.
- Richetta AG, Mancini M, Torroni A, et al. Total spontaneous regression of advanced Merkel cell carcinoma after biopsy: review and a new case. Dermatol Surg. 2008;34:815-822.
- Vesely MJ, Murray DJ, Neligan PC, et al. Complete spontaneous regression in Merkel cell carcinoma. J Plast Reconstr Aesthet Surg. 2008;61:165-171.
- Kayashima K, Ono T, Johno M, et al. Spontaneous regression in Merkel cell (neuroendocrine) carcinoma of the skin. Arch Dermatol. 1991;127:550-553.
- Maruo K, Kayashima KI, Ono T. Regressing Merkel cell carcinoma-a case showing replacement of tumour cells by foamy cells. Br J Dermatol. 2000;142:1184-1189.
- Duncavage E, Zehnbauer B, Pfeifer J. Prevalence of Merkel cell polyomavirus in Merkel cell carcinoma. Mod Pathol. 2009;22:516-521.
- Kassem A, Schopflin A, Diaz C, et al. Frequent detection of Merkel cell polyomavirus in human Merkel cell carcinomas and identification of unique deletion in the VP1 gene. Cancer Res. 2008;68:5009-5013.
- Becker J, Schrama D, Houben R. Merkel cell carcinoma. Cell Mol Life Sci. 2009;66:1-8.
- Haitz KA, Rady PL, Nguyen HP, et al. Merkel cell polyomavirus DNA detection in a patient with Merkel cell carcinoma and multiple other skin cancers. Int J Dermatol. 2012;51:442-444.
- Andres C, Puchta U, Sander CA, et al. Prevalence of Merkel cell polyomavirus DNA in cutaneous lymphomas, pseudolymphomas, and inflammatory skin diseases. Am J Dermatopathol. 2010;32:593-598.
- Showalter RM, Pastrana DV, Pumphrey KA, et al. Merkel cell polyomavirus and two previously unknown polyomaviruses are chronically shed from human skin. Cell Host Microbe. 2010;7:509-515.
- Tolstov YL, Pastrana DV, Feng H, et al. Human Merkel cell polyomavirus infection II. MCV is a common human infection that can be detected by conformational capsid epitope immunoassays. Int J Cancer. 2009;125:1250-1256.
- Chen T, Hedman L, Mattila PS, et al. Serological evidence of Merkel cell polyomavirus primary infections in childhood. J Clin Virol. 2011;50:125-129.
- Laude HC, Jonchère B, Maubec E, et al. Distinct Merkel cell polyomavirus molecular features in tumour and non tumour specimens from patients with Merkel cell carcinoma. PLoS Pathog. 2010;6:e1001076.
- Waltari M, Sihto H, Kukko H, et al. Association of Merkel cell polyomavirus infection with tumor p53, KIT, stem cell factor, PDGFR-alpha and survival in Merkel cell carcinoma. Int J Cancer. 2011;129:619-628.
- Sihto H, Kukko H, Koljonen V, et al. Clinical factors associated with Merkel cell polyomavirus infection in Merkel cell carcinoma. J Natl Cancer Inst. 2009;101:938-945.
- Paulson KG, Lemos BD, Feng B, et al. Array-CGH reveals recurrent genomic changes in Merkel cell carcinoma including amplification of L-Myc. J Invest Dermatol. 2009;129:1547-1555.
- Schrama D, Peitsch WK, Zapatka M, et al. Merkel cell polyomavirus status is not associated with clinical course of Merkel cell carcinoma. J Invest Dermatol. 2011;131:1631-1638.
- Tadmor T, Aviv A, Polliack A. Merkel cell carcinoma, chronic lymphocytic leukemia and other lymphoproliferative disorders: an old bond with possible new viral ties. Ann Oncol. 2011;22:250-256.
- Wooff J, Trites JR, Walsh NM, et al. Complete spontaneous regression of metastatic Merkel cell carcinoma: a case report and review of the literature. Am J Dermatopathol. 2010;32:614-617.
- Turk TO, Smoljan I, Nacinovic A, et al. Spontaneous regression of Merkel cell carcinoma in a patient with chronic lymphocytic leukemia: a case report. J Med Case Rep. 2009;3:7270.
- Mori Y, Tanaka K, Cui CY, et al. A study of apoptosis in Merkel cell carcinoma. an immunohistochemical, ultrasctructural, DNA ladder and TUNEL labeling study. Am J Dermatopathol. 2001;23:16-23.
- Koba S, Paulson KG, Nagase K, et al. Diagnostic biopsy does not commonly induce intratumoral CD8 T cell infiltration in Merkel cell carcinoma. PLoS ONE. 2012;7:e41465.
Practice Points
- Merkel cell carcinoma (MCC) is a rare malignancy with a high rate of metastasis and poor prognosis.
- T-cell mediated immunity appears to play an important role in tumor regression in MCC.
- Merkel cell polyomavirus appears to play a role in the pathogenesis of MCC and may be associated with a better prognosis.
- A better understanding of spontaneous regression of MCC could help in the development of new immunotherapeutic approaches to this malignancy.
Facial Involvement in Progressive Macular Hypomelanosis
Progressive macular hypomelanosis (PMH) is a noninflammatory skin disorder characterized by ill-defined, nummular, hypopigmented, and nonscaly macules. Historically, various names have been used to describe this entity. Several of these terms, including cutis trunci variata and nummular and confluent hypomelanosis of the trunk, reflected its predominantly truncal distribution.1,2 Less frequently, involvement on the neck, buttocks, and arms and legs has been noted.1,2 A lack of facial involvement previously has been highlighted as a key clinical feature of PMH.3
Progressive macular hypomelanosis is a diagnosis of exclusion. Hypopigmented diseases commonly considered in the differential include those caused by fungi and yeasts (eg, tinea versicolor, seborrheic dermatitis), inflammatory skin disorders (eg, pityriasis alba, postinflammatory dyschromia), and mycosis fungoides (MF) as well as leprosy.
The hypopigmented macules of PMH have nonspecific histopathologic findings; lesional skin often shows minimal alterations as compared to normal skin. A sparse perivascular lymphocytic infiltrate often is observed,4,5 and at times, a decrease in epidermal melanin content can be detected.1-3,6,7
We report 4 cases with considerable facial involvement of hypopigmented macules that were determined to be consistent with PMH. We propose that characteristic macules that are not clinically or histopathologically consistent with other disease entities are compatible with a diagnosis of PMH, regardless of the distribution. A diagnosis of PMH should be considered in the differential when there are suggestive facial lesions in addition to truncal lesions.
Case Reports
Patient 1
A 40-year-old man presented with hypopigmented macules on the face (Figure 1), trunk, chest, arms, and legs of 2 years’ duration. The lesions were asymptomatic and had started on the forehead as hypopigmented macules, then progressed to the trunk, arms, and legs. The patient denied any prior rash, injury, or hyperpigmentation associated with the distribution of the lesions.
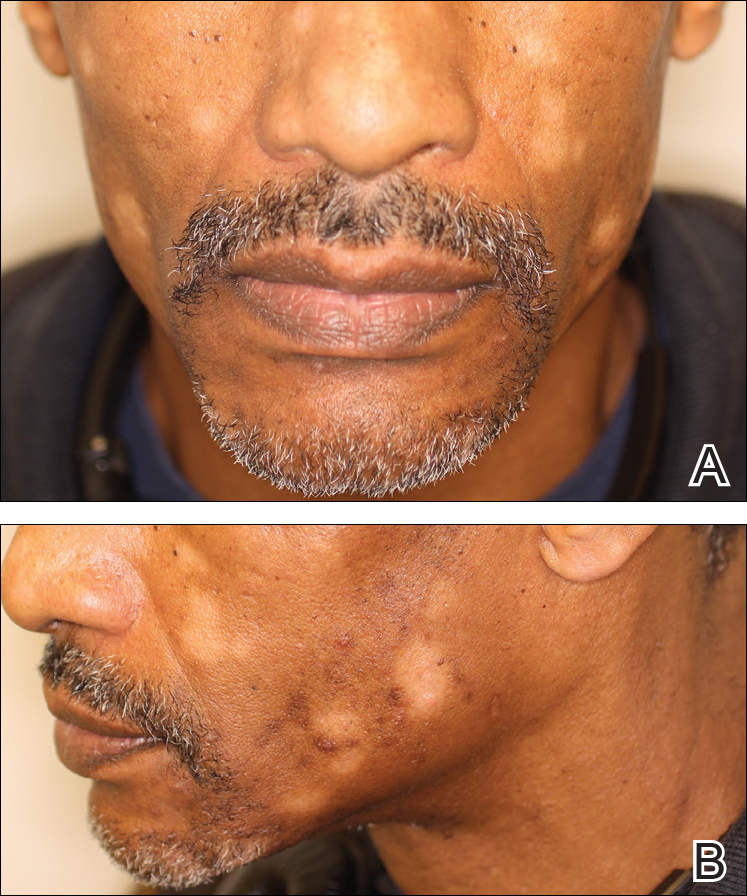
A rapid plasma reagin (RPR) test was conducted to rule out secondary syphilis and was nonreactive. During a series of clinical encounters over several months, a total of 5 biopsies of lesions on the face and back were performed. All specimens contained mild mononuclear perivascular inflammation (Figure 2). In some foci, staining for Melan-A revealed a decrease in epidermal melanocytes (Figure 3). Periodic acid–Schiff staining performed on one section revealed a few pityriasis spores but no hyphal elements, suggesting colonization rather than infection.

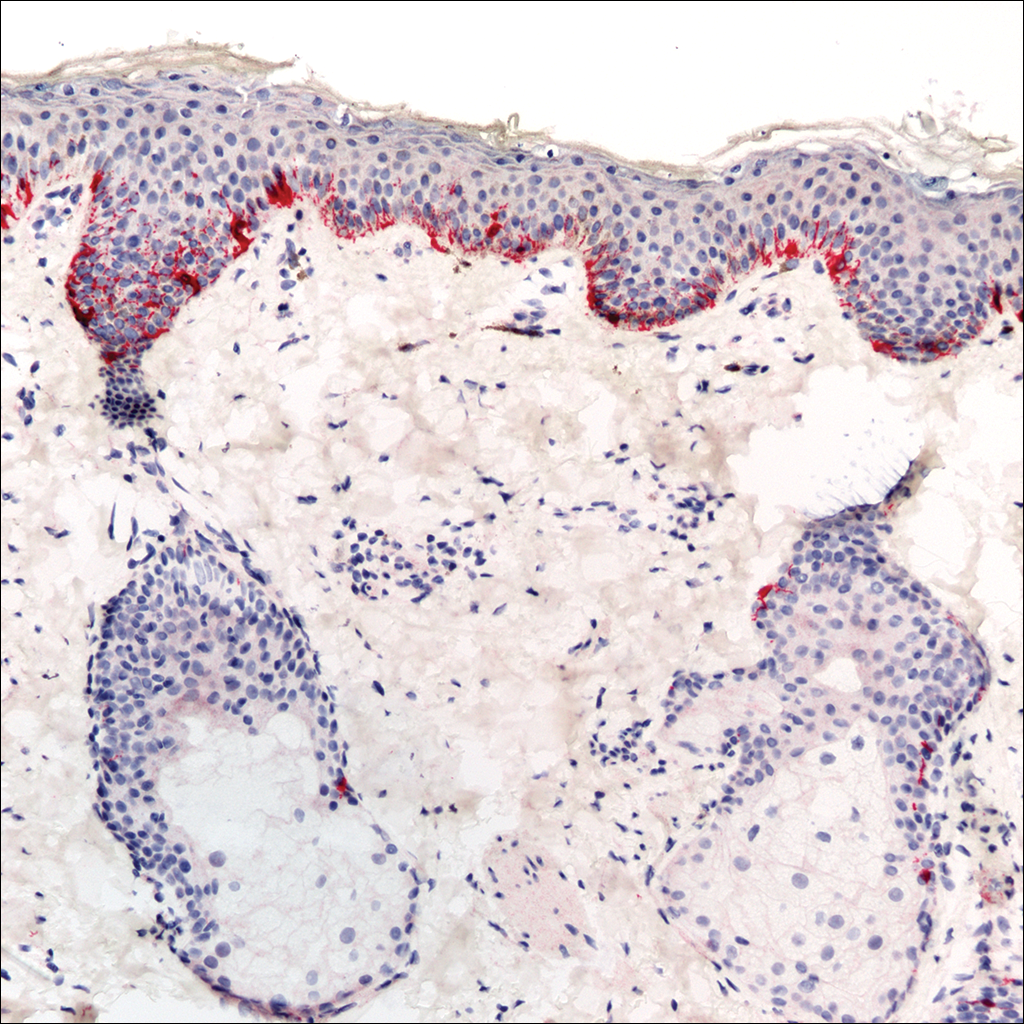
The patient initially was started on tacrolimus ointment 0.1% once daily and narrowband UVB phototherapy twice weekly for 3 months without benefit. A diagnosis of tinea versicolor was revisited and the patient was switched to ketoconazole shampoo 1% two to 3 times weekly on the face, trunk, arms, and legs for 10 to 15 minutes prior to rinsing, and ketoconazole cream 2% was applied twice daily to the affected areas for 2 months without notable improvement. Once-weekly 150-mg pulse doses of oral fluconazole for 8 weeks were started but proved equally ineffective. Antibiotic therapy aimed at eradicating Propionibacterium acnes was considered following a provisional diagnosis of PMH after the patient failed 5 months of therapy for tinea versicolor.
Patient 2
A 54-year-old man presented with hypopigmented to depigmented nonscaly macules on the face, trunk, chest, and arms of several months’ duration. The patient initially noted hypopigmentation on the face that gradually spread to the rest of the body. The patient denied any prior rash or hyperpigmentation in the affected areas. At the initial visit to our clinic, a potassium hydroxide (KOH) preparation of the face and back was positive for tinea versicolor. The patient was treated with ketoconazole shampoo 1% two to 3 times weekly for several weeks on the scalp, face, trunk, arms, and legs for 10 to 15 minutes prior to rinsing and 2 total doses of oral fluconazole 150 mg taken 1 week apart.
Three months later the patient returned with no improvement of the existing lesions and with progression of the disease to previously uninvolved areas of the trunk, arms, and legs. Biopsy of a facial lesion was performed, and laboratory studies including RPR, thyroid-stimulating hormone, and antinuclear antibody tests were conducted to screen for possible systemic disease. Microscopic analysis of the biopsied facial lesion revealed a sparse perivascular infiltrate of lymphocytes and plasma cells but no evidence of yeast or hyphal elements. Melan-A staining did not reveal a decreased number of epidermal melanocytes. All laboratory studies were negative or within normal limits. Desonide ointment 0.05% was prescribed to relieve the patient’s occasional pruritus. Although the patient’s symptoms resolved, the hypopigmented macules continued to progress, making a diagnosis of PMH more likely given the lack of improvement on treatment for tinea versicolor. Pimecrolimus cream 1% was started with discontinuation of desonide for steroid-sparing therapy.
Patient 3
A 63-year-old man presented with progressive nonscaly and asymptomatic hypopigmented macules on the face, trunk, abdomen, and back of 5 years’ duration. He first noted lesions on the abdomen and they subsequently spread to the rest of the body. The patient denied any prior rash, hyperpigmentation, or other lesions in the involved areas.
One year prior to the current presentation, KOH scrapings from the lesions performed by an outside physician were negative. During his initial visit to our clinic, an abdominal biopsy was performed, and histopathologic analysis showed postinflammatory pigmentary alteration; however, the patient denied any prior history of rash or injury in the distribution of the lesions that would correlate with the histopathologic findings of postinflammatory pigmentation. Because the histopathologic findings showed postinflammatory pigmentary alteration, additional stains including Melan-A were not performed.
The patient was provisionally treated with ketoconazole shampoo 1% two to 3 times weekly on the face, trunk, arms, and legs for 10 to 15 minutes prior to rinsing and ketoconazole cream 2% twice daily to the affected areas. After several months on this regimen, the patient did not report any improvement. An abdominal skin biopsy was again performed and revealed similar histopathology. Periodic acid–Schiff staining was negative for fungus. A diagnosis of PMH was made, and the patient was started on benzoyl peroxide wash 5% and clindamycin lotion.
Patient 4
A 45-year-old woman presented with hypopigmented, nonscaly macules on the face, neck, chest, trunk, and back. She first noted the lesions on the face and trunk more than 8 years prior, and they subsequently progressed. Potassium hydroxide scrapings performed on the lesions at the current presentation were negative, and a skin biopsy from the neck revealed postinflammatory pigmentary alteration, although the patient had no history of rash or injury in the areas in which the lesions were distributed.
Fontana-Masson and Melan-A staining of the skin biopsy of the neck revealed a normal distribution of melanocytes and pigment at the dermoepidermal junction. An RPR test was nonreactive. A diagnosis of PMH was made, and the patient was started on benzoyl peroxide wash 5% and clindamycin phosphate lotion 1%.
Comment
The 4 cases of PMH reported here showed extensive facial involvement in addition to the characteristic hypopigmented lesions on the trunk, arms, and legs. It is unclear why the lesions in these patients had a predominantly facial distribution. Involvement of the face in PMH has not been commonly reported in the literature. Martínez-Martínez et al3 reported 12 PMH patients with lesions only presenting in lumbar and abdominal distributions. Kim et al8 presented a series of 23 PMH patients treated with narrowband UVB in whom 56% (9/16) saw repigmentation in 90% of the lesions following treatment. The most commonly affected area was the lower back, followed by the abdomen, upper back, chest, sacral region, flank, and shoulders, respectively.8 In a review by Relyveld et al,1 PMH is described as a predominantly truncal disease that can occasionally extend to the neck, face, and proximal arms and legs; however, no specific cases were reported.
Previous case series have reported PMH primarily in adolescents and young adults, with mean ages ranging from 26 to 30 years.1,3 The 4 patients reported here were older, ranging in age from 40 to 65 years. This discrepancy in age may contribute to the facial distribution encountered in this patient population; however, given the small number of patients in our case series, such extrapolation is premature. Most recently, Westerhof et al6 demonstrated a relationship between the presence of P acnes, a common skin commensal of the face, and the hypopigmented macules of PMH. The investigators suggested that some strains of P acnes produce a factor that is yet to be identified that interferes with melanogenesis. The response of PMH lesions to topical treatments such as benzoyl peroxide, clindamycin, and phototherapy has lent credence to the potential etiologic role of P acnes in this condition.9,10 The interplay between age, PMH distribution, and P acnes requires further investigation.
The biopsies in our 4 patients were consistent with the nonspecific histopathologic characteristics of PMH lesions. Biopsies in all 4 patients revealed a sparse perivascular lymphocytic infiltrate, and in 2 of the cases, postinflammatory pigmentary alteration was noted. Such changes often are described in PMH lesions.4,5 In other cases detailed in the literature, lesional and nonlesional skin often are indistinguishable on hematoxylin and eosin staining.11 In the 3 patients for whom we performed additional immunohistochemical studies, results were mixed: Melan-A staining revealed a decreased number of melanocytes in Patient 1 but not in Patients 2 or 4. Many reported cases in the literature have not demonstrated a decrease in melanocyte density but instead show a decrease in melanin content in lesional skin.1-3,6,7 Although additional stains performed in Patient 4 revealed neither a decrease in the number of melanocytes nor a decrease in the melanin content, such histopathologic findings of PMH often are subtle. Additional stains were not performed in Patient 3. More studies are needed to characterize the immunohistochemical staining patterns of lesional skin in patients with PMH.
Tinea versicolor, pityriasis alba, mycosis fungoides, sarcoidosis, leprosy, and syphilis typically are included in the differential diagnosis for PMH. Tinea versicolor traditionally is diagnosed based on the combination of irregular hypopigmented or hyperpigmented scaly macules and a KOH preparation that is positive for hyphae and spores. Similar to PMH, tinea versicolor is most often found on the trunk, but unusual cases have been reported involving the face.12
Patient 2 reflected how it can be difficult diagnostically to distinguish between tinea versicolor and PMH. Although this patient initially had a KOH scraping suggestive for tinea versicolor, adequate treatment with oral fluconazole and ketoconazole shampoo did not result in improvement. The hypopigmented lesions in this patient continued to progress despite therapy. Additionally, his hypopigmented to depigmented nonscaly macules were more clinically consistent with the characteristic description of lesion configuration in PMH than with the irregular, more sharply defined, asymmetric, and scaly spots of tinea versicolor. Furthermore, the inflammatory findings on biopsy favored a diagnosis of PMH.
Pityriasis alba, most frequently presents on the face in the form of hypopigmented, sometimes slightly scaly macules but also can occur on the body. It usually occurs in younger patients who often have an atopic diathesis. Histologic findings generally are nonspecific, but discrete eczematous changes can sometimes be appreciated in the epidermis and dermis. None of our patients had histories suggestive of an atopic diathesis or lesion distributions typical of pityriasis alba. Histologic findings also were more consistent with PMH than pityriasis alba.
A diagnosis of patch-stage hypopigmented MF should also be entertained in patients with hypopigmented macules, as it can appear similar to the lesions of PMH. Hypopigmented MF often is associated with subtle atrophy, scaling, poikiloderma, and erythema. These features were not present in the 4 cases presented here. Histologically, atypical lymphocytes with prominent epidermotropism and tagging of the epidermis by large lymphocytic infiltrates are seen in cases of hypopigmented MF. These findings were not present in biopsies from our patients.
Hypopigmented sarcoidosis, leprosy, and syphilis are other systemic diseases associated with hypopigmented lesions. Histologically, noncaseasting granulomas in the dermis or subcutaneous tissue would favor a diagnosis of sarcoidosis over PMH. In patients who live in endemic areas, a diagnosis of leprosy for an anesthetic hypopigmented lesion would be higher in the differential. Finally, it is important to rule out secondary syphilis when diagnosing PMH. Known as the great imitator, secondary syphilis may present in a patient in the form of hypopigmented macules. Patients 1, 2, and 4 had nonreactive RPR tests; unfortunately, RPR was not checked in Patient 3. He denied all risk factors for syphilis.
Various topical and oral treatments were prescribed for each patient, but so far none have been unequivocally effective. In the literature, there are reports supporting the efficacy of topical antimicrobial agents targeting P acnes.9,10 One case report noted improvement in a patient with PMH after isotretinoin use.13 Phototherapy also has been reported to improve PMH in several case reports4-8; however, consistent response to these therapies has not been documented. Unfortunately for patients with a diagnosis of PMH, a lack of effective treatment options often exists.
This series of 4 cases highlights the importance of considering PMH in the differential of hypopigmented macules, even when they appear predominantly on the face.
- Relyveld G, Menke H, Westerhof W. Progressive macular hypomelanosis: an overview. Am J Clin Dermatol. 2007;8:13-19.
- Hwang SW, Hong SK, Kim SH, et al. Progressive macular hypomelanosis in Korean patients: a clinicopathologic study. Ann Dermatol. 2009;21:261-267.
- Martinéz-Martinéz ML, Azaña-Defez JM, Rodríguez-Vázquez M, et al. Progressive macular hypomelanosis. Pediatr Dermatol. 2012;29:460-462.
- Montero LC, Belinchonón I, Toledo F, et al. Progressive macular hypomelanosis, excellent response with narrow-band ultraviolet B phototherapy. Photodermatol Photoimmunol Photomed. 2011;27:162-163.
- Choi YJ, Hann SK. Two cases of progressive macular hypomelanosis of the trunk. Korean J Dermatol. 2000;38:655-658.
- Westerhof W, Rlyveld G, Kingswijk M, et al. Propionibacterium acnes and the pathogenesis of progressive macular hypomelanosis. Arch Dermatol. 2004;140:210-214.
- Wu SG, Xu AE, Song XZ, et al. Clinical, pathologic, and ultrastructural studies of progressive macular hypomelanosis. Int J Dermatol. 2010;29:1127-1132.
- Kim MB, Kim GW, Cho HH, et al. Narrowband UVB treatment of progressive macular hypomelanosis. J Am Acad Dermatol. 2012;66:598-605.
- Revlyveld GN, Menkie HE, Westerhof W. Benzoyl peroxide/clindamycin/UVA is more effective than fluticasone/UVA in progressive macular hypomelanosis: a randomized study. Am J Clin Dermatol. 2006;55:836-843.
- Santos JB, Almeida OL, Silva LM, et al. Efficacy of topical combination of benzoyl peroxide 5% and clindamcyin 1% for the treatment of progressive macular hypomelanosis: a randomized, doubleblind, placebo-controlled trial [in Portuguese]. An Bras Dermatol. 2011;86:50-54.
- Kumarasinghe SP, Tan SH, Thng S, et al. Progressive macular hypomelanosis in Singapore: a clinico-pathological study. Int J Dermatol. 2006;45:737-742.
- Terragni L, Lasagni A, Oriani A. Pityriasis versicolor of the face. Mycoses. 1991;34:345-347.
- Kim YK, Lee DY, Lee, JY, et al. Progressive macular hypomelanosis showing excellent response to oral isotretinoin [published online June 23, 2012]. J Dermatol. 2012;39:937-938.
Progressive macular hypomelanosis (PMH) is a noninflammatory skin disorder characterized by ill-defined, nummular, hypopigmented, and nonscaly macules. Historically, various names have been used to describe this entity. Several of these terms, including cutis trunci variata and nummular and confluent hypomelanosis of the trunk, reflected its predominantly truncal distribution.1,2 Less frequently, involvement on the neck, buttocks, and arms and legs has been noted.1,2 A lack of facial involvement previously has been highlighted as a key clinical feature of PMH.3
Progressive macular hypomelanosis is a diagnosis of exclusion. Hypopigmented diseases commonly considered in the differential include those caused by fungi and yeasts (eg, tinea versicolor, seborrheic dermatitis), inflammatory skin disorders (eg, pityriasis alba, postinflammatory dyschromia), and mycosis fungoides (MF) as well as leprosy.
The hypopigmented macules of PMH have nonspecific histopathologic findings; lesional skin often shows minimal alterations as compared to normal skin. A sparse perivascular lymphocytic infiltrate often is observed,4,5 and at times, a decrease in epidermal melanin content can be detected.1-3,6,7
We report 4 cases with considerable facial involvement of hypopigmented macules that were determined to be consistent with PMH. We propose that characteristic macules that are not clinically or histopathologically consistent with other disease entities are compatible with a diagnosis of PMH, regardless of the distribution. A diagnosis of PMH should be considered in the differential when there are suggestive facial lesions in addition to truncal lesions.
Case Reports
Patient 1
A 40-year-old man presented with hypopigmented macules on the face (Figure 1), trunk, chest, arms, and legs of 2 years’ duration. The lesions were asymptomatic and had started on the forehead as hypopigmented macules, then progressed to the trunk, arms, and legs. The patient denied any prior rash, injury, or hyperpigmentation associated with the distribution of the lesions.

A rapid plasma reagin (RPR) test was conducted to rule out secondary syphilis and was nonreactive. During a series of clinical encounters over several months, a total of 5 biopsies of lesions on the face and back were performed. All specimens contained mild mononuclear perivascular inflammation (Figure 2). In some foci, staining for Melan-A revealed a decrease in epidermal melanocytes (Figure 3). Periodic acid–Schiff staining performed on one section revealed a few pityriasis spores but no hyphal elements, suggesting colonization rather than infection.


The patient initially was started on tacrolimus ointment 0.1% once daily and narrowband UVB phototherapy twice weekly for 3 months without benefit. A diagnosis of tinea versicolor was revisited and the patient was switched to ketoconazole shampoo 1% two to 3 times weekly on the face, trunk, arms, and legs for 10 to 15 minutes prior to rinsing, and ketoconazole cream 2% was applied twice daily to the affected areas for 2 months without notable improvement. Once-weekly 150-mg pulse doses of oral fluconazole for 8 weeks were started but proved equally ineffective. Antibiotic therapy aimed at eradicating Propionibacterium acnes was considered following a provisional diagnosis of PMH after the patient failed 5 months of therapy for tinea versicolor.
Patient 2
A 54-year-old man presented with hypopigmented to depigmented nonscaly macules on the face, trunk, chest, and arms of several months’ duration. The patient initially noted hypopigmentation on the face that gradually spread to the rest of the body. The patient denied any prior rash or hyperpigmentation in the affected areas. At the initial visit to our clinic, a potassium hydroxide (KOH) preparation of the face and back was positive for tinea versicolor. The patient was treated with ketoconazole shampoo 1% two to 3 times weekly for several weeks on the scalp, face, trunk, arms, and legs for 10 to 15 minutes prior to rinsing and 2 total doses of oral fluconazole 150 mg taken 1 week apart.
Three months later the patient returned with no improvement of the existing lesions and with progression of the disease to previously uninvolved areas of the trunk, arms, and legs. Biopsy of a facial lesion was performed, and laboratory studies including RPR, thyroid-stimulating hormone, and antinuclear antibody tests were conducted to screen for possible systemic disease. Microscopic analysis of the biopsied facial lesion revealed a sparse perivascular infiltrate of lymphocytes and plasma cells but no evidence of yeast or hyphal elements. Melan-A staining did not reveal a decreased number of epidermal melanocytes. All laboratory studies were negative or within normal limits. Desonide ointment 0.05% was prescribed to relieve the patient’s occasional pruritus. Although the patient’s symptoms resolved, the hypopigmented macules continued to progress, making a diagnosis of PMH more likely given the lack of improvement on treatment for tinea versicolor. Pimecrolimus cream 1% was started with discontinuation of desonide for steroid-sparing therapy.
Patient 3
A 63-year-old man presented with progressive nonscaly and asymptomatic hypopigmented macules on the face, trunk, abdomen, and back of 5 years’ duration. He first noted lesions on the abdomen and they subsequently spread to the rest of the body. The patient denied any prior rash, hyperpigmentation, or other lesions in the involved areas.
One year prior to the current presentation, KOH scrapings from the lesions performed by an outside physician were negative. During his initial visit to our clinic, an abdominal biopsy was performed, and histopathologic analysis showed postinflammatory pigmentary alteration; however, the patient denied any prior history of rash or injury in the distribution of the lesions that would correlate with the histopathologic findings of postinflammatory pigmentation. Because the histopathologic findings showed postinflammatory pigmentary alteration, additional stains including Melan-A were not performed.
The patient was provisionally treated with ketoconazole shampoo 1% two to 3 times weekly on the face, trunk, arms, and legs for 10 to 15 minutes prior to rinsing and ketoconazole cream 2% twice daily to the affected areas. After several months on this regimen, the patient did not report any improvement. An abdominal skin biopsy was again performed and revealed similar histopathology. Periodic acid–Schiff staining was negative for fungus. A diagnosis of PMH was made, and the patient was started on benzoyl peroxide wash 5% and clindamycin lotion.
Patient 4
A 45-year-old woman presented with hypopigmented, nonscaly macules on the face, neck, chest, trunk, and back. She first noted the lesions on the face and trunk more than 8 years prior, and they subsequently progressed. Potassium hydroxide scrapings performed on the lesions at the current presentation were negative, and a skin biopsy from the neck revealed postinflammatory pigmentary alteration, although the patient had no history of rash or injury in the areas in which the lesions were distributed.
Fontana-Masson and Melan-A staining of the skin biopsy of the neck revealed a normal distribution of melanocytes and pigment at the dermoepidermal junction. An RPR test was nonreactive. A diagnosis of PMH was made, and the patient was started on benzoyl peroxide wash 5% and clindamycin phosphate lotion 1%.
Comment
The 4 cases of PMH reported here showed extensive facial involvement in addition to the characteristic hypopigmented lesions on the trunk, arms, and legs. It is unclear why the lesions in these patients had a predominantly facial distribution. Involvement of the face in PMH has not been commonly reported in the literature. Martínez-Martínez et al3 reported 12 PMH patients with lesions only presenting in lumbar and abdominal distributions. Kim et al8 presented a series of 23 PMH patients treated with narrowband UVB in whom 56% (9/16) saw repigmentation in 90% of the lesions following treatment. The most commonly affected area was the lower back, followed by the abdomen, upper back, chest, sacral region, flank, and shoulders, respectively.8 In a review by Relyveld et al,1 PMH is described as a predominantly truncal disease that can occasionally extend to the neck, face, and proximal arms and legs; however, no specific cases were reported.
Previous case series have reported PMH primarily in adolescents and young adults, with mean ages ranging from 26 to 30 years.1,3 The 4 patients reported here were older, ranging in age from 40 to 65 years. This discrepancy in age may contribute to the facial distribution encountered in this patient population; however, given the small number of patients in our case series, such extrapolation is premature. Most recently, Westerhof et al6 demonstrated a relationship between the presence of P acnes, a common skin commensal of the face, and the hypopigmented macules of PMH. The investigators suggested that some strains of P acnes produce a factor that is yet to be identified that interferes with melanogenesis. The response of PMH lesions to topical treatments such as benzoyl peroxide, clindamycin, and phototherapy has lent credence to the potential etiologic role of P acnes in this condition.9,10 The interplay between age, PMH distribution, and P acnes requires further investigation.
The biopsies in our 4 patients were consistent with the nonspecific histopathologic characteristics of PMH lesions. Biopsies in all 4 patients revealed a sparse perivascular lymphocytic infiltrate, and in 2 of the cases, postinflammatory pigmentary alteration was noted. Such changes often are described in PMH lesions.4,5 In other cases detailed in the literature, lesional and nonlesional skin often are indistinguishable on hematoxylin and eosin staining.11 In the 3 patients for whom we performed additional immunohistochemical studies, results were mixed: Melan-A staining revealed a decreased number of melanocytes in Patient 1 but not in Patients 2 or 4. Many reported cases in the literature have not demonstrated a decrease in melanocyte density but instead show a decrease in melanin content in lesional skin.1-3,6,7 Although additional stains performed in Patient 4 revealed neither a decrease in the number of melanocytes nor a decrease in the melanin content, such histopathologic findings of PMH often are subtle. Additional stains were not performed in Patient 3. More studies are needed to characterize the immunohistochemical staining patterns of lesional skin in patients with PMH.
Tinea versicolor, pityriasis alba, mycosis fungoides, sarcoidosis, leprosy, and syphilis typically are included in the differential diagnosis for PMH. Tinea versicolor traditionally is diagnosed based on the combination of irregular hypopigmented or hyperpigmented scaly macules and a KOH preparation that is positive for hyphae and spores. Similar to PMH, tinea versicolor is most often found on the trunk, but unusual cases have been reported involving the face.12
Patient 2 reflected how it can be difficult diagnostically to distinguish between tinea versicolor and PMH. Although this patient initially had a KOH scraping suggestive for tinea versicolor, adequate treatment with oral fluconazole and ketoconazole shampoo did not result in improvement. The hypopigmented lesions in this patient continued to progress despite therapy. Additionally, his hypopigmented to depigmented nonscaly macules were more clinically consistent with the characteristic description of lesion configuration in PMH than with the irregular, more sharply defined, asymmetric, and scaly spots of tinea versicolor. Furthermore, the inflammatory findings on biopsy favored a diagnosis of PMH.
Pityriasis alba, most frequently presents on the face in the form of hypopigmented, sometimes slightly scaly macules but also can occur on the body. It usually occurs in younger patients who often have an atopic diathesis. Histologic findings generally are nonspecific, but discrete eczematous changes can sometimes be appreciated in the epidermis and dermis. None of our patients had histories suggestive of an atopic diathesis or lesion distributions typical of pityriasis alba. Histologic findings also were more consistent with PMH than pityriasis alba.
A diagnosis of patch-stage hypopigmented MF should also be entertained in patients with hypopigmented macules, as it can appear similar to the lesions of PMH. Hypopigmented MF often is associated with subtle atrophy, scaling, poikiloderma, and erythema. These features were not present in the 4 cases presented here. Histologically, atypical lymphocytes with prominent epidermotropism and tagging of the epidermis by large lymphocytic infiltrates are seen in cases of hypopigmented MF. These findings were not present in biopsies from our patients.
Hypopigmented sarcoidosis, leprosy, and syphilis are other systemic diseases associated with hypopigmented lesions. Histologically, noncaseasting granulomas in the dermis or subcutaneous tissue would favor a diagnosis of sarcoidosis over PMH. In patients who live in endemic areas, a diagnosis of leprosy for an anesthetic hypopigmented lesion would be higher in the differential. Finally, it is important to rule out secondary syphilis when diagnosing PMH. Known as the great imitator, secondary syphilis may present in a patient in the form of hypopigmented macules. Patients 1, 2, and 4 had nonreactive RPR tests; unfortunately, RPR was not checked in Patient 3. He denied all risk factors for syphilis.
Various topical and oral treatments were prescribed for each patient, but so far none have been unequivocally effective. In the literature, there are reports supporting the efficacy of topical antimicrobial agents targeting P acnes.9,10 One case report noted improvement in a patient with PMH after isotretinoin use.13 Phototherapy also has been reported to improve PMH in several case reports4-8; however, consistent response to these therapies has not been documented. Unfortunately for patients with a diagnosis of PMH, a lack of effective treatment options often exists.
This series of 4 cases highlights the importance of considering PMH in the differential of hypopigmented macules, even when they appear predominantly on the face.
Progressive macular hypomelanosis (PMH) is a noninflammatory skin disorder characterized by ill-defined, nummular, hypopigmented, and nonscaly macules. Historically, various names have been used to describe this entity. Several of these terms, including cutis trunci variata and nummular and confluent hypomelanosis of the trunk, reflected its predominantly truncal distribution.1,2 Less frequently, involvement on the neck, buttocks, and arms and legs has been noted.1,2 A lack of facial involvement previously has been highlighted as a key clinical feature of PMH.3
Progressive macular hypomelanosis is a diagnosis of exclusion. Hypopigmented diseases commonly considered in the differential include those caused by fungi and yeasts (eg, tinea versicolor, seborrheic dermatitis), inflammatory skin disorders (eg, pityriasis alba, postinflammatory dyschromia), and mycosis fungoides (MF) as well as leprosy.
The hypopigmented macules of PMH have nonspecific histopathologic findings; lesional skin often shows minimal alterations as compared to normal skin. A sparse perivascular lymphocytic infiltrate often is observed,4,5 and at times, a decrease in epidermal melanin content can be detected.1-3,6,7
We report 4 cases with considerable facial involvement of hypopigmented macules that were determined to be consistent with PMH. We propose that characteristic macules that are not clinically or histopathologically consistent with other disease entities are compatible with a diagnosis of PMH, regardless of the distribution. A diagnosis of PMH should be considered in the differential when there are suggestive facial lesions in addition to truncal lesions.
Case Reports
Patient 1
A 40-year-old man presented with hypopigmented macules on the face (Figure 1), trunk, chest, arms, and legs of 2 years’ duration. The lesions were asymptomatic and had started on the forehead as hypopigmented macules, then progressed to the trunk, arms, and legs. The patient denied any prior rash, injury, or hyperpigmentation associated with the distribution of the lesions.

A rapid plasma reagin (RPR) test was conducted to rule out secondary syphilis and was nonreactive. During a series of clinical encounters over several months, a total of 5 biopsies of lesions on the face and back were performed. All specimens contained mild mononuclear perivascular inflammation (Figure 2). In some foci, staining for Melan-A revealed a decrease in epidermal melanocytes (Figure 3). Periodic acid–Schiff staining performed on one section revealed a few pityriasis spores but no hyphal elements, suggesting colonization rather than infection.


The patient initially was started on tacrolimus ointment 0.1% once daily and narrowband UVB phototherapy twice weekly for 3 months without benefit. A diagnosis of tinea versicolor was revisited and the patient was switched to ketoconazole shampoo 1% two to 3 times weekly on the face, trunk, arms, and legs for 10 to 15 minutes prior to rinsing, and ketoconazole cream 2% was applied twice daily to the affected areas for 2 months without notable improvement. Once-weekly 150-mg pulse doses of oral fluconazole for 8 weeks were started but proved equally ineffective. Antibiotic therapy aimed at eradicating Propionibacterium acnes was considered following a provisional diagnosis of PMH after the patient failed 5 months of therapy for tinea versicolor.
Patient 2
A 54-year-old man presented with hypopigmented to depigmented nonscaly macules on the face, trunk, chest, and arms of several months’ duration. The patient initially noted hypopigmentation on the face that gradually spread to the rest of the body. The patient denied any prior rash or hyperpigmentation in the affected areas. At the initial visit to our clinic, a potassium hydroxide (KOH) preparation of the face and back was positive for tinea versicolor. The patient was treated with ketoconazole shampoo 1% two to 3 times weekly for several weeks on the scalp, face, trunk, arms, and legs for 10 to 15 minutes prior to rinsing and 2 total doses of oral fluconazole 150 mg taken 1 week apart.
Three months later the patient returned with no improvement of the existing lesions and with progression of the disease to previously uninvolved areas of the trunk, arms, and legs. Biopsy of a facial lesion was performed, and laboratory studies including RPR, thyroid-stimulating hormone, and antinuclear antibody tests were conducted to screen for possible systemic disease. Microscopic analysis of the biopsied facial lesion revealed a sparse perivascular infiltrate of lymphocytes and plasma cells but no evidence of yeast or hyphal elements. Melan-A staining did not reveal a decreased number of epidermal melanocytes. All laboratory studies were negative or within normal limits. Desonide ointment 0.05% was prescribed to relieve the patient’s occasional pruritus. Although the patient’s symptoms resolved, the hypopigmented macules continued to progress, making a diagnosis of PMH more likely given the lack of improvement on treatment for tinea versicolor. Pimecrolimus cream 1% was started with discontinuation of desonide for steroid-sparing therapy.
Patient 3
A 63-year-old man presented with progressive nonscaly and asymptomatic hypopigmented macules on the face, trunk, abdomen, and back of 5 years’ duration. He first noted lesions on the abdomen and they subsequently spread to the rest of the body. The patient denied any prior rash, hyperpigmentation, or other lesions in the involved areas.
One year prior to the current presentation, KOH scrapings from the lesions performed by an outside physician were negative. During his initial visit to our clinic, an abdominal biopsy was performed, and histopathologic analysis showed postinflammatory pigmentary alteration; however, the patient denied any prior history of rash or injury in the distribution of the lesions that would correlate with the histopathologic findings of postinflammatory pigmentation. Because the histopathologic findings showed postinflammatory pigmentary alteration, additional stains including Melan-A were not performed.
The patient was provisionally treated with ketoconazole shampoo 1% two to 3 times weekly on the face, trunk, arms, and legs for 10 to 15 minutes prior to rinsing and ketoconazole cream 2% twice daily to the affected areas. After several months on this regimen, the patient did not report any improvement. An abdominal skin biopsy was again performed and revealed similar histopathology. Periodic acid–Schiff staining was negative for fungus. A diagnosis of PMH was made, and the patient was started on benzoyl peroxide wash 5% and clindamycin lotion.
Patient 4
A 45-year-old woman presented with hypopigmented, nonscaly macules on the face, neck, chest, trunk, and back. She first noted the lesions on the face and trunk more than 8 years prior, and they subsequently progressed. Potassium hydroxide scrapings performed on the lesions at the current presentation were negative, and a skin biopsy from the neck revealed postinflammatory pigmentary alteration, although the patient had no history of rash or injury in the areas in which the lesions were distributed.
Fontana-Masson and Melan-A staining of the skin biopsy of the neck revealed a normal distribution of melanocytes and pigment at the dermoepidermal junction. An RPR test was nonreactive. A diagnosis of PMH was made, and the patient was started on benzoyl peroxide wash 5% and clindamycin phosphate lotion 1%.
Comment
The 4 cases of PMH reported here showed extensive facial involvement in addition to the characteristic hypopigmented lesions on the trunk, arms, and legs. It is unclear why the lesions in these patients had a predominantly facial distribution. Involvement of the face in PMH has not been commonly reported in the literature. Martínez-Martínez et al3 reported 12 PMH patients with lesions only presenting in lumbar and abdominal distributions. Kim et al8 presented a series of 23 PMH patients treated with narrowband UVB in whom 56% (9/16) saw repigmentation in 90% of the lesions following treatment. The most commonly affected area was the lower back, followed by the abdomen, upper back, chest, sacral region, flank, and shoulders, respectively.8 In a review by Relyveld et al,1 PMH is described as a predominantly truncal disease that can occasionally extend to the neck, face, and proximal arms and legs; however, no specific cases were reported.
Previous case series have reported PMH primarily in adolescents and young adults, with mean ages ranging from 26 to 30 years.1,3 The 4 patients reported here were older, ranging in age from 40 to 65 years. This discrepancy in age may contribute to the facial distribution encountered in this patient population; however, given the small number of patients in our case series, such extrapolation is premature. Most recently, Westerhof et al6 demonstrated a relationship between the presence of P acnes, a common skin commensal of the face, and the hypopigmented macules of PMH. The investigators suggested that some strains of P acnes produce a factor that is yet to be identified that interferes with melanogenesis. The response of PMH lesions to topical treatments such as benzoyl peroxide, clindamycin, and phototherapy has lent credence to the potential etiologic role of P acnes in this condition.9,10 The interplay between age, PMH distribution, and P acnes requires further investigation.
The biopsies in our 4 patients were consistent with the nonspecific histopathologic characteristics of PMH lesions. Biopsies in all 4 patients revealed a sparse perivascular lymphocytic infiltrate, and in 2 of the cases, postinflammatory pigmentary alteration was noted. Such changes often are described in PMH lesions.4,5 In other cases detailed in the literature, lesional and nonlesional skin often are indistinguishable on hematoxylin and eosin staining.11 In the 3 patients for whom we performed additional immunohistochemical studies, results were mixed: Melan-A staining revealed a decreased number of melanocytes in Patient 1 but not in Patients 2 or 4. Many reported cases in the literature have not demonstrated a decrease in melanocyte density but instead show a decrease in melanin content in lesional skin.1-3,6,7 Although additional stains performed in Patient 4 revealed neither a decrease in the number of melanocytes nor a decrease in the melanin content, such histopathologic findings of PMH often are subtle. Additional stains were not performed in Patient 3. More studies are needed to characterize the immunohistochemical staining patterns of lesional skin in patients with PMH.
Tinea versicolor, pityriasis alba, mycosis fungoides, sarcoidosis, leprosy, and syphilis typically are included in the differential diagnosis for PMH. Tinea versicolor traditionally is diagnosed based on the combination of irregular hypopigmented or hyperpigmented scaly macules and a KOH preparation that is positive for hyphae and spores. Similar to PMH, tinea versicolor is most often found on the trunk, but unusual cases have been reported involving the face.12
Patient 2 reflected how it can be difficult diagnostically to distinguish between tinea versicolor and PMH. Although this patient initially had a KOH scraping suggestive for tinea versicolor, adequate treatment with oral fluconazole and ketoconazole shampoo did not result in improvement. The hypopigmented lesions in this patient continued to progress despite therapy. Additionally, his hypopigmented to depigmented nonscaly macules were more clinically consistent with the characteristic description of lesion configuration in PMH than with the irregular, more sharply defined, asymmetric, and scaly spots of tinea versicolor. Furthermore, the inflammatory findings on biopsy favored a diagnosis of PMH.
Pityriasis alba, most frequently presents on the face in the form of hypopigmented, sometimes slightly scaly macules but also can occur on the body. It usually occurs in younger patients who often have an atopic diathesis. Histologic findings generally are nonspecific, but discrete eczematous changes can sometimes be appreciated in the epidermis and dermis. None of our patients had histories suggestive of an atopic diathesis or lesion distributions typical of pityriasis alba. Histologic findings also were more consistent with PMH than pityriasis alba.
A diagnosis of patch-stage hypopigmented MF should also be entertained in patients with hypopigmented macules, as it can appear similar to the lesions of PMH. Hypopigmented MF often is associated with subtle atrophy, scaling, poikiloderma, and erythema. These features were not present in the 4 cases presented here. Histologically, atypical lymphocytes with prominent epidermotropism and tagging of the epidermis by large lymphocytic infiltrates are seen in cases of hypopigmented MF. These findings were not present in biopsies from our patients.
Hypopigmented sarcoidosis, leprosy, and syphilis are other systemic diseases associated with hypopigmented lesions. Histologically, noncaseasting granulomas in the dermis or subcutaneous tissue would favor a diagnosis of sarcoidosis over PMH. In patients who live in endemic areas, a diagnosis of leprosy for an anesthetic hypopigmented lesion would be higher in the differential. Finally, it is important to rule out secondary syphilis when diagnosing PMH. Known as the great imitator, secondary syphilis may present in a patient in the form of hypopigmented macules. Patients 1, 2, and 4 had nonreactive RPR tests; unfortunately, RPR was not checked in Patient 3. He denied all risk factors for syphilis.
Various topical and oral treatments were prescribed for each patient, but so far none have been unequivocally effective. In the literature, there are reports supporting the efficacy of topical antimicrobial agents targeting P acnes.9,10 One case report noted improvement in a patient with PMH after isotretinoin use.13 Phototherapy also has been reported to improve PMH in several case reports4-8; however, consistent response to these therapies has not been documented. Unfortunately for patients with a diagnosis of PMH, a lack of effective treatment options often exists.
This series of 4 cases highlights the importance of considering PMH in the differential of hypopigmented macules, even when they appear predominantly on the face.
- Relyveld G, Menke H, Westerhof W. Progressive macular hypomelanosis: an overview. Am J Clin Dermatol. 2007;8:13-19.
- Hwang SW, Hong SK, Kim SH, et al. Progressive macular hypomelanosis in Korean patients: a clinicopathologic study. Ann Dermatol. 2009;21:261-267.
- Martinéz-Martinéz ML, Azaña-Defez JM, Rodríguez-Vázquez M, et al. Progressive macular hypomelanosis. Pediatr Dermatol. 2012;29:460-462.
- Montero LC, Belinchonón I, Toledo F, et al. Progressive macular hypomelanosis, excellent response with narrow-band ultraviolet B phototherapy. Photodermatol Photoimmunol Photomed. 2011;27:162-163.
- Choi YJ, Hann SK. Two cases of progressive macular hypomelanosis of the trunk. Korean J Dermatol. 2000;38:655-658.
- Westerhof W, Rlyveld G, Kingswijk M, et al. Propionibacterium acnes and the pathogenesis of progressive macular hypomelanosis. Arch Dermatol. 2004;140:210-214.
- Wu SG, Xu AE, Song XZ, et al. Clinical, pathologic, and ultrastructural studies of progressive macular hypomelanosis. Int J Dermatol. 2010;29:1127-1132.
- Kim MB, Kim GW, Cho HH, et al. Narrowband UVB treatment of progressive macular hypomelanosis. J Am Acad Dermatol. 2012;66:598-605.
- Revlyveld GN, Menkie HE, Westerhof W. Benzoyl peroxide/clindamycin/UVA is more effective than fluticasone/UVA in progressive macular hypomelanosis: a randomized study. Am J Clin Dermatol. 2006;55:836-843.
- Santos JB, Almeida OL, Silva LM, et al. Efficacy of topical combination of benzoyl peroxide 5% and clindamcyin 1% for the treatment of progressive macular hypomelanosis: a randomized, doubleblind, placebo-controlled trial [in Portuguese]. An Bras Dermatol. 2011;86:50-54.
- Kumarasinghe SP, Tan SH, Thng S, et al. Progressive macular hypomelanosis in Singapore: a clinico-pathological study. Int J Dermatol. 2006;45:737-742.
- Terragni L, Lasagni A, Oriani A. Pityriasis versicolor of the face. Mycoses. 1991;34:345-347.
- Kim YK, Lee DY, Lee, JY, et al. Progressive macular hypomelanosis showing excellent response to oral isotretinoin [published online June 23, 2012]. J Dermatol. 2012;39:937-938.
- Relyveld G, Menke H, Westerhof W. Progressive macular hypomelanosis: an overview. Am J Clin Dermatol. 2007;8:13-19.
- Hwang SW, Hong SK, Kim SH, et al. Progressive macular hypomelanosis in Korean patients: a clinicopathologic study. Ann Dermatol. 2009;21:261-267.
- Martinéz-Martinéz ML, Azaña-Defez JM, Rodríguez-Vázquez M, et al. Progressive macular hypomelanosis. Pediatr Dermatol. 2012;29:460-462.
- Montero LC, Belinchonón I, Toledo F, et al. Progressive macular hypomelanosis, excellent response with narrow-band ultraviolet B phototherapy. Photodermatol Photoimmunol Photomed. 2011;27:162-163.
- Choi YJ, Hann SK. Two cases of progressive macular hypomelanosis of the trunk. Korean J Dermatol. 2000;38:655-658.
- Westerhof W, Rlyveld G, Kingswijk M, et al. Propionibacterium acnes and the pathogenesis of progressive macular hypomelanosis. Arch Dermatol. 2004;140:210-214.
- Wu SG, Xu AE, Song XZ, et al. Clinical, pathologic, and ultrastructural studies of progressive macular hypomelanosis. Int J Dermatol. 2010;29:1127-1132.
- Kim MB, Kim GW, Cho HH, et al. Narrowband UVB treatment of progressive macular hypomelanosis. J Am Acad Dermatol. 2012;66:598-605.
- Revlyveld GN, Menkie HE, Westerhof W. Benzoyl peroxide/clindamycin/UVA is more effective than fluticasone/UVA in progressive macular hypomelanosis: a randomized study. Am J Clin Dermatol. 2006;55:836-843.
- Santos JB, Almeida OL, Silva LM, et al. Efficacy of topical combination of benzoyl peroxide 5% and clindamcyin 1% for the treatment of progressive macular hypomelanosis: a randomized, doubleblind, placebo-controlled trial [in Portuguese]. An Bras Dermatol. 2011;86:50-54.
- Kumarasinghe SP, Tan SH, Thng S, et al. Progressive macular hypomelanosis in Singapore: a clinico-pathological study. Int J Dermatol. 2006;45:737-742.
- Terragni L, Lasagni A, Oriani A. Pityriasis versicolor of the face. Mycoses. 1991;34:345-347.
- Kim YK, Lee DY, Lee, JY, et al. Progressive macular hypomelanosis showing excellent response to oral isotretinoin [published online June 23, 2012]. J Dermatol. 2012;39:937-938.
Practice Points
- Progressive macular hypomelanosis should be considered in the differential diagnosis for hypopigmented facial lesions.
- Progressive macular hypomelanosis proves to be a diagnosis of exclusion.
Drug-induced Linear IgA Bullous Dermatosis in a Patient With a Vancomycin-impregnated Cement Spacer
Case Report
A 77-year-old man was admitted to the general medicine service at our institution for treatment of a diffuse macular eruption and hemorrhagic bullae 12 days after undergoing left-knee revision arthroplasty during which a cement spacer impregnated with vancomycin and tobramycin was placed. At the time of the surgery, the patient also received intravenous (IV) vancomycin and oral ciprofloxacin, which were continued postoperatively until his hospital presentation. The patient was recovering well until postoperative day 7, when he developed painful swelling and erythema surrounding the surgical wound on the left knee. Concerned that his symptoms indicated a flare of gout, he restarted a former allopurinol prescription from an outside physician after 2 years of nonuse. The skin changes progressed distally on the left leg over the next 48 hours. By postoperative day 10, he had developed serosanguinous blisters on the left knee (Figure 1A) and oral mucosa (Figure 1B), as well as erythematous nodules on the bilateral palms. He presented to our institution for emergent care on postoperative day 12 following progression of the eruption to the inguinal region (Figure 2A), buttocks (Figure 2B), and abdominal region.


Due to concerns about a potential drug reaction, the IV vancomycin, oral ciprofloxacin, and oral allopurinol were discontinued on hospital admission.

Oral prednisone 60 mg once daily and oral dapsone 25 mg once daily were initiated on hospital days 4 and 6 (postoperative days 15 and 17), respectively. A 6-week course of oral ciprofloxacin 750 mg twice daily and daptomycin 8 mg/kg once daily was initiated for bacterial coverage on hospital day 5 (postoperative day 16). Topical triamcinolone and an anesthetic mouthwash also were used to treat the mucosal involvement. The lesions stabilized on the third day of steroid therapy, and the patient was discharged 7 days after hospital admission (postoperative day 18). Dapsone was rapidly increased to 100 mg once daily over the next week for Pneumocystis jirovecii pneumonia prophylaxis. An increase in prednisone to 80 mg once daily was required 3 days after the patient was discharged due to worsening oral lesions. Five days after discharge, the patient was readmitted to the hospital for 3 days due to acute kidney injury (AKI) in which his baseline creatinine level tripled. The cause of renal impairment was unknown, resulting in empiric discontinuation of dapsone on postoperative day 27. Prophylaxis for P jirovecii pneumonia was replaced with once-monthly inhaled pentamidine. Prednisone was tapered 20 days after the original presentation (postoperative day 32) following gradual improvement of both the skin and oral lesions. At dermatology follow-up 2 weeks later, doxycycline 100 mg twice daily was added for residual inflammation of the left leg. A deep vein thrombosis was discovered in the left leg 10 days later, and 3 months of anticoagulation therapy was initiated with discontinuation of the doxycycline. The patient continued to have renal insufficiency several weeks after dapsone discontinuation and developed prominent peripheral motor neuropathy with bilateral thenar atrophy. He did not experience any skin eruptions or relapses in the weeks following prednisone cessation and underwent successful removal of the cement spacer with full left-knee reconstruction 4 months after his initial presentation to our institution. At 9-month dermatology follow-up, the LABD remained in remission.
Comment
Linear IgA bullous dermatosis is a well-documented autoimmune mucocutaneous disorder characterized by linear IgA deposits at the dermoepidermal junction. The development of autoantibodies to antigens within the basement membrane zone leads to both cellular and humoral immune responses that facilitate the subepidermal blistering rash in LABD.2,3 Linear IgA bullous dermatosis affects all ages and races with a bimodal epidemiology. The adult form typically appears after 60 years of age, whereas the childhood form (chronic bullous disease of childhood) appears between 6 months and 6 years of age.3 Medications—particularly vancomycin—are responsible for a substantial portion of cases.1-4 In one review, vancomycin was implicated in almost half (22/52 [42.3%]) of drug-related cases of LABD.4 Other associated medications include captopril, trimethoprim-sulfamethoxazole, phenytoin, and diclo-fenac.3,4 Vancomycin-associated LABD has a substantially shorter time to onset of symptoms, with a mean of 8.6 days compared to 63.8 days for other causative agents.4
The initial treatment of drug-induced LABD is immediate discontinuation of the suspected agent(s) and supportive care.9 Although future avoidance of vancomycin is recommended in patients with a history of LABD, there are reported cases of successful rechallenges.4,10 The early removal of our patient’s cement spacer was discouraged by both the orthopedics and infectious disease consultation services due to potential complications as well as the patient’s gradual improvement during his hospital course.
Dapsone is considered the standard systemic treatment for LABD. Sulfapyridine is an alternative to dapsone, or a combination of these 2 drugs may be used. Corticosteroids can be added to each of these regimens to achieve remission, as in our case.2 Although dapsone was discontinued in the setting of the patient’s AKI, the vancomycin in the dual-eluting spacer was more likely the culprit. A review of 544 postoperative outcomes following the use of an antibiotic-impregnated cement spacer (AICS) during 2-stage arthroplasty displayed an 8- to 10-fold increase in the development of AKIs compared to the rate of AKIs following primary joint arthroplasty.10 While our patient’s AKI was not attributed to dapsone, his prominent peripheral motor neuropathy with resultant bilateral thenar atrophy was a rare complication of dapsone use. While dapsone-associated neuropathy has been reported in daily dosages of as low as 75 mg, it typically is seen in doses of at least 300 mg per day and in larger cumulative dosages.11
Despite having a well-characterized vancomycin-induced LABD in the setting of known vancomycin exposure, our patient’s case was particularly challenging given the continued presence of the vancomycin-impregnated cement spacer (VICS) in the left knee, resulting in vancomycin levels at admission and during subsequent measurements over 2 weeks that were all several-fold higher than the renal clearance predicted.
Vancomycin-associated LABD does not appear to be dose dependent and has been reported at both subtherapeutic1-3 and supratherapeutic levels,5-9 whereas toxicity reactions are more common at supratherapeutic levels.9 The literature on AICS use suggests that drug elution occurs at relatively unpredictable rates based on a variety of factors, including the type of cement used and the initial antibiotic concentration.12,13 Furthermore, the addition of tobramycin to VICSs has been found to increase the rate of vancomycin delivery through a phenomenon known as passive opportunism.14
As AICS devices allow for the delivery of higher concentrations of antibiotics to a localized area, systemic complications are considered rare but have been reported.13 Our report describes a rare case of LABD in the setting of a VICS. One clinical aspect of our case that supports the implication of VICS as the cause of the patient’s LABD is the concentration of bullae overlying the incision site on the left knee. A case of a desquamating rash in a patient with an implanted VICS has been documented in which the early lesions were localized to the surgical leg, as in our case.15 Unlike our case, there was a history of Stevens-Johnson syndrome following previous vancomycin exposure. A case of a gentamicin-impregnated cement spacer causing allergic dermatitis that was most prominent in the surgical leg also has been reported.16 An isomorphic phenomenon (Köbner phenomenon) has been suggested in the setting of
- Plunkett RW, Chiarello SE, Beutner EH. Linear IgA bullous dermatosis in one of two piroxicam-induced eruptions: a distinct direct immunofluorescence trend revealed by the literature. J Am Acad Dermatol. 2001;45:691-696.
- Guide SV, Marinkovich MP. Linear IgA bullous dermatosis. Clin Dermatol. 2001;19:719-727.
- Fortuna G, Marinkovich MP. Linear immunoglobulin A bullous dermatosis. Clin Dermatol. 2012;30:38-50.
- Fortuna G, Salas-Alanis JC, Guidetti E, et al. A critical reappraisal of the current data on drug-induced linear immunoglobulin A bullous dermatosis: a real and separate nosological entity? J Am Acad Dermatol. 2012;66:988-994.
- Kuechle MK, Stegemeir E, Maynard B, et al. Drug-induced linear IgA bullous dermatosis: report of six cases and review of the literature. J Am Acad Dermatol. 1994;30(2, pt 1):187-192.
- Neughebauer BI, Negron G, Pelton S, et al. Bullous skin disease: an unusual allergic reaction to vancomycin. Am J Med Sci. 2002;323:273-278.
- Naranjo CA, Busto U, Sellers EM, et al. A method for estimating the probability of adverse drug reactions. Clin Pharmacol Ther. 1981;30:239-245.
- Wiadrowski TP, Reid CM. Drug-induced linear IgA bullous disease following antibiotics. Australas J Dermatol. 2001;42:196-199.
- Dang LV, Byrom L, Muir J, et al. Vancomycin-induced linear IgA with mucosal and ocular involvement: a case report. Infect Dis Clin Pract. 2014;22:e119-e121.
- Luu A, Syed F, Raman G, et al. Two-stage arthroplasty for prosthetic joint infection: a systematic review of acute kidney injury, systemic toxicity and infection control [published online April 8, 2013]. J Arthroplasty. 2013;28:1490.e1-1498.e1.
- Daneshmend TK. The neurotoxicity of dapsone. Adverse Drug React Acute Poisoning Rev. 1984;3:43-58.
- Jacobs C, Christensen CP, Berend ME. Static and mobile antibiotic-impregnated cement spacers for the management of prosthetic joint infection. J Am Acad Orthop Surg. 2009;17:356-368.
- Springer BD, Lee GC, Osmon D, et al. Systemic safety of high-dose antibiotic-loaded cement spacers after resection of an infected total knee arthroplasty. Clin Orthop Relat Res. 2004;427:47-51.
- Penner MJ, Masri BA, Duncan CP. Elution characteristics of vancomycin and tobramycin combined in acrylic bone-cement. J Arthroplasty. 1996;11:939-944.
- Williams B, Hanson A, Sha B. Diffuse desquamating rash following exposure to vancomycin-impregnated bone cement. Ann Pharmacother. 2014;48:1061-1065.
- Haeberle M, Wittner B. Is gentamicin-loaded bone cement a risk for developing systemic allergic dermatitis? Contact Dermatitis. 2009;60:176-177.
- McDonald HC, York NR, Pandya AG. Drug-induced linear IgA bullous dermatosis demonstrating the isomorphic phenomenon. J Am Acad Dermatol. 2010;62:897-898.
Case Report
A 77-year-old man was admitted to the general medicine service at our institution for treatment of a diffuse macular eruption and hemorrhagic bullae 12 days after undergoing left-knee revision arthroplasty during which a cement spacer impregnated with vancomycin and tobramycin was placed. At the time of the surgery, the patient also received intravenous (IV) vancomycin and oral ciprofloxacin, which were continued postoperatively until his hospital presentation. The patient was recovering well until postoperative day 7, when he developed painful swelling and erythema surrounding the surgical wound on the left knee. Concerned that his symptoms indicated a flare of gout, he restarted a former allopurinol prescription from an outside physician after 2 years of nonuse. The skin changes progressed distally on the left leg over the next 48 hours. By postoperative day 10, he had developed serosanguinous blisters on the left knee (Figure 1A) and oral mucosa (Figure 1B), as well as erythematous nodules on the bilateral palms. He presented to our institution for emergent care on postoperative day 12 following progression of the eruption to the inguinal region (Figure 2A), buttocks (Figure 2B), and abdominal region.


Due to concerns about a potential drug reaction, the IV vancomycin, oral ciprofloxacin, and oral allopurinol were discontinued on hospital admission.

Oral prednisone 60 mg once daily and oral dapsone 25 mg once daily were initiated on hospital days 4 and 6 (postoperative days 15 and 17), respectively. A 6-week course of oral ciprofloxacin 750 mg twice daily and daptomycin 8 mg/kg once daily was initiated for bacterial coverage on hospital day 5 (postoperative day 16). Topical triamcinolone and an anesthetic mouthwash also were used to treat the mucosal involvement. The lesions stabilized on the third day of steroid therapy, and the patient was discharged 7 days after hospital admission (postoperative day 18). Dapsone was rapidly increased to 100 mg once daily over the next week for Pneumocystis jirovecii pneumonia prophylaxis. An increase in prednisone to 80 mg once daily was required 3 days after the patient was discharged due to worsening oral lesions. Five days after discharge, the patient was readmitted to the hospital for 3 days due to acute kidney injury (AKI) in which his baseline creatinine level tripled. The cause of renal impairment was unknown, resulting in empiric discontinuation of dapsone on postoperative day 27. Prophylaxis for P jirovecii pneumonia was replaced with once-monthly inhaled pentamidine. Prednisone was tapered 20 days after the original presentation (postoperative day 32) following gradual improvement of both the skin and oral lesions. At dermatology follow-up 2 weeks later, doxycycline 100 mg twice daily was added for residual inflammation of the left leg. A deep vein thrombosis was discovered in the left leg 10 days later, and 3 months of anticoagulation therapy was initiated with discontinuation of the doxycycline. The patient continued to have renal insufficiency several weeks after dapsone discontinuation and developed prominent peripheral motor neuropathy with bilateral thenar atrophy. He did not experience any skin eruptions or relapses in the weeks following prednisone cessation and underwent successful removal of the cement spacer with full left-knee reconstruction 4 months after his initial presentation to our institution. At 9-month dermatology follow-up, the LABD remained in remission.
Comment
Linear IgA bullous dermatosis is a well-documented autoimmune mucocutaneous disorder characterized by linear IgA deposits at the dermoepidermal junction. The development of autoantibodies to antigens within the basement membrane zone leads to both cellular and humoral immune responses that facilitate the subepidermal blistering rash in LABD.2,3 Linear IgA bullous dermatosis affects all ages and races with a bimodal epidemiology. The adult form typically appears after 60 years of age, whereas the childhood form (chronic bullous disease of childhood) appears between 6 months and 6 years of age.3 Medications—particularly vancomycin—are responsible for a substantial portion of cases.1-4 In one review, vancomycin was implicated in almost half (22/52 [42.3%]) of drug-related cases of LABD.4 Other associated medications include captopril, trimethoprim-sulfamethoxazole, phenytoin, and diclo-fenac.3,4 Vancomycin-associated LABD has a substantially shorter time to onset of symptoms, with a mean of 8.6 days compared to 63.8 days for other causative agents.4
The initial treatment of drug-induced LABD is immediate discontinuation of the suspected agent(s) and supportive care.9 Although future avoidance of vancomycin is recommended in patients with a history of LABD, there are reported cases of successful rechallenges.4,10 The early removal of our patient’s cement spacer was discouraged by both the orthopedics and infectious disease consultation services due to potential complications as well as the patient’s gradual improvement during his hospital course.
Dapsone is considered the standard systemic treatment for LABD. Sulfapyridine is an alternative to dapsone, or a combination of these 2 drugs may be used. Corticosteroids can be added to each of these regimens to achieve remission, as in our case.2 Although dapsone was discontinued in the setting of the patient’s AKI, the vancomycin in the dual-eluting spacer was more likely the culprit. A review of 544 postoperative outcomes following the use of an antibiotic-impregnated cement spacer (AICS) during 2-stage arthroplasty displayed an 8- to 10-fold increase in the development of AKIs compared to the rate of AKIs following primary joint arthroplasty.10 While our patient’s AKI was not attributed to dapsone, his prominent peripheral motor neuropathy with resultant bilateral thenar atrophy was a rare complication of dapsone use. While dapsone-associated neuropathy has been reported in daily dosages of as low as 75 mg, it typically is seen in doses of at least 300 mg per day and in larger cumulative dosages.11
Despite having a well-characterized vancomycin-induced LABD in the setting of known vancomycin exposure, our patient’s case was particularly challenging given the continued presence of the vancomycin-impregnated cement spacer (VICS) in the left knee, resulting in vancomycin levels at admission and during subsequent measurements over 2 weeks that were all several-fold higher than the renal clearance predicted.
Vancomycin-associated LABD does not appear to be dose dependent and has been reported at both subtherapeutic1-3 and supratherapeutic levels,5-9 whereas toxicity reactions are more common at supratherapeutic levels.9 The literature on AICS use suggests that drug elution occurs at relatively unpredictable rates based on a variety of factors, including the type of cement used and the initial antibiotic concentration.12,13 Furthermore, the addition of tobramycin to VICSs has been found to increase the rate of vancomycin delivery through a phenomenon known as passive opportunism.14
As AICS devices allow for the delivery of higher concentrations of antibiotics to a localized area, systemic complications are considered rare but have been reported.13 Our report describes a rare case of LABD in the setting of a VICS. One clinical aspect of our case that supports the implication of VICS as the cause of the patient’s LABD is the concentration of bullae overlying the incision site on the left knee. A case of a desquamating rash in a patient with an implanted VICS has been documented in which the early lesions were localized to the surgical leg, as in our case.15 Unlike our case, there was a history of Stevens-Johnson syndrome following previous vancomycin exposure. A case of a gentamicin-impregnated cement spacer causing allergic dermatitis that was most prominent in the surgical leg also has been reported.16 An isomorphic phenomenon (Köbner phenomenon) has been suggested in the setting of
Case Report
A 77-year-old man was admitted to the general medicine service at our institution for treatment of a diffuse macular eruption and hemorrhagic bullae 12 days after undergoing left-knee revision arthroplasty during which a cement spacer impregnated with vancomycin and tobramycin was placed. At the time of the surgery, the patient also received intravenous (IV) vancomycin and oral ciprofloxacin, which were continued postoperatively until his hospital presentation. The patient was recovering well until postoperative day 7, when he developed painful swelling and erythema surrounding the surgical wound on the left knee. Concerned that his symptoms indicated a flare of gout, he restarted a former allopurinol prescription from an outside physician after 2 years of nonuse. The skin changes progressed distally on the left leg over the next 48 hours. By postoperative day 10, he had developed serosanguinous blisters on the left knee (Figure 1A) and oral mucosa (Figure 1B), as well as erythematous nodules on the bilateral palms. He presented to our institution for emergent care on postoperative day 12 following progression of the eruption to the inguinal region (Figure 2A), buttocks (Figure 2B), and abdominal region.


Due to concerns about a potential drug reaction, the IV vancomycin, oral ciprofloxacin, and oral allopurinol were discontinued on hospital admission.

Oral prednisone 60 mg once daily and oral dapsone 25 mg once daily were initiated on hospital days 4 and 6 (postoperative days 15 and 17), respectively. A 6-week course of oral ciprofloxacin 750 mg twice daily and daptomycin 8 mg/kg once daily was initiated for bacterial coverage on hospital day 5 (postoperative day 16). Topical triamcinolone and an anesthetic mouthwash also were used to treat the mucosal involvement. The lesions stabilized on the third day of steroid therapy, and the patient was discharged 7 days after hospital admission (postoperative day 18). Dapsone was rapidly increased to 100 mg once daily over the next week for Pneumocystis jirovecii pneumonia prophylaxis. An increase in prednisone to 80 mg once daily was required 3 days after the patient was discharged due to worsening oral lesions. Five days after discharge, the patient was readmitted to the hospital for 3 days due to acute kidney injury (AKI) in which his baseline creatinine level tripled. The cause of renal impairment was unknown, resulting in empiric discontinuation of dapsone on postoperative day 27. Prophylaxis for P jirovecii pneumonia was replaced with once-monthly inhaled pentamidine. Prednisone was tapered 20 days after the original presentation (postoperative day 32) following gradual improvement of both the skin and oral lesions. At dermatology follow-up 2 weeks later, doxycycline 100 mg twice daily was added for residual inflammation of the left leg. A deep vein thrombosis was discovered in the left leg 10 days later, and 3 months of anticoagulation therapy was initiated with discontinuation of the doxycycline. The patient continued to have renal insufficiency several weeks after dapsone discontinuation and developed prominent peripheral motor neuropathy with bilateral thenar atrophy. He did not experience any skin eruptions or relapses in the weeks following prednisone cessation and underwent successful removal of the cement spacer with full left-knee reconstruction 4 months after his initial presentation to our institution. At 9-month dermatology follow-up, the LABD remained in remission.
Comment
Linear IgA bullous dermatosis is a well-documented autoimmune mucocutaneous disorder characterized by linear IgA deposits at the dermoepidermal junction. The development of autoantibodies to antigens within the basement membrane zone leads to both cellular and humoral immune responses that facilitate the subepidermal blistering rash in LABD.2,3 Linear IgA bullous dermatosis affects all ages and races with a bimodal epidemiology. The adult form typically appears after 60 years of age, whereas the childhood form (chronic bullous disease of childhood) appears between 6 months and 6 years of age.3 Medications—particularly vancomycin—are responsible for a substantial portion of cases.1-4 In one review, vancomycin was implicated in almost half (22/52 [42.3%]) of drug-related cases of LABD.4 Other associated medications include captopril, trimethoprim-sulfamethoxazole, phenytoin, and diclo-fenac.3,4 Vancomycin-associated LABD has a substantially shorter time to onset of symptoms, with a mean of 8.6 days compared to 63.8 days for other causative agents.4
The initial treatment of drug-induced LABD is immediate discontinuation of the suspected agent(s) and supportive care.9 Although future avoidance of vancomycin is recommended in patients with a history of LABD, there are reported cases of successful rechallenges.4,10 The early removal of our patient’s cement spacer was discouraged by both the orthopedics and infectious disease consultation services due to potential complications as well as the patient’s gradual improvement during his hospital course.
Dapsone is considered the standard systemic treatment for LABD. Sulfapyridine is an alternative to dapsone, or a combination of these 2 drugs may be used. Corticosteroids can be added to each of these regimens to achieve remission, as in our case.2 Although dapsone was discontinued in the setting of the patient’s AKI, the vancomycin in the dual-eluting spacer was more likely the culprit. A review of 544 postoperative outcomes following the use of an antibiotic-impregnated cement spacer (AICS) during 2-stage arthroplasty displayed an 8- to 10-fold increase in the development of AKIs compared to the rate of AKIs following primary joint arthroplasty.10 While our patient’s AKI was not attributed to dapsone, his prominent peripheral motor neuropathy with resultant bilateral thenar atrophy was a rare complication of dapsone use. While dapsone-associated neuropathy has been reported in daily dosages of as low as 75 mg, it typically is seen in doses of at least 300 mg per day and in larger cumulative dosages.11
Despite having a well-characterized vancomycin-induced LABD in the setting of known vancomycin exposure, our patient’s case was particularly challenging given the continued presence of the vancomycin-impregnated cement spacer (VICS) in the left knee, resulting in vancomycin levels at admission and during subsequent measurements over 2 weeks that were all several-fold higher than the renal clearance predicted.
Vancomycin-associated LABD does not appear to be dose dependent and has been reported at both subtherapeutic1-3 and supratherapeutic levels,5-9 whereas toxicity reactions are more common at supratherapeutic levels.9 The literature on AICS use suggests that drug elution occurs at relatively unpredictable rates based on a variety of factors, including the type of cement used and the initial antibiotic concentration.12,13 Furthermore, the addition of tobramycin to VICSs has been found to increase the rate of vancomycin delivery through a phenomenon known as passive opportunism.14
As AICS devices allow for the delivery of higher concentrations of antibiotics to a localized area, systemic complications are considered rare but have been reported.13 Our report describes a rare case of LABD in the setting of a VICS. One clinical aspect of our case that supports the implication of VICS as the cause of the patient’s LABD is the concentration of bullae overlying the incision site on the left knee. A case of a desquamating rash in a patient with an implanted VICS has been documented in which the early lesions were localized to the surgical leg, as in our case.15 Unlike our case, there was a history of Stevens-Johnson syndrome following previous vancomycin exposure. A case of a gentamicin-impregnated cement spacer causing allergic dermatitis that was most prominent in the surgical leg also has been reported.16 An isomorphic phenomenon (Köbner phenomenon) has been suggested in the setting of
- Plunkett RW, Chiarello SE, Beutner EH. Linear IgA bullous dermatosis in one of two piroxicam-induced eruptions: a distinct direct immunofluorescence trend revealed by the literature. J Am Acad Dermatol. 2001;45:691-696.
- Guide SV, Marinkovich MP. Linear IgA bullous dermatosis. Clin Dermatol. 2001;19:719-727.
- Fortuna G, Marinkovich MP. Linear immunoglobulin A bullous dermatosis. Clin Dermatol. 2012;30:38-50.
- Fortuna G, Salas-Alanis JC, Guidetti E, et al. A critical reappraisal of the current data on drug-induced linear immunoglobulin A bullous dermatosis: a real and separate nosological entity? J Am Acad Dermatol. 2012;66:988-994.
- Kuechle MK, Stegemeir E, Maynard B, et al. Drug-induced linear IgA bullous dermatosis: report of six cases and review of the literature. J Am Acad Dermatol. 1994;30(2, pt 1):187-192.
- Neughebauer BI, Negron G, Pelton S, et al. Bullous skin disease: an unusual allergic reaction to vancomycin. Am J Med Sci. 2002;323:273-278.
- Naranjo CA, Busto U, Sellers EM, et al. A method for estimating the probability of adverse drug reactions. Clin Pharmacol Ther. 1981;30:239-245.
- Wiadrowski TP, Reid CM. Drug-induced linear IgA bullous disease following antibiotics. Australas J Dermatol. 2001;42:196-199.
- Dang LV, Byrom L, Muir J, et al. Vancomycin-induced linear IgA with mucosal and ocular involvement: a case report. Infect Dis Clin Pract. 2014;22:e119-e121.
- Luu A, Syed F, Raman G, et al. Two-stage arthroplasty for prosthetic joint infection: a systematic review of acute kidney injury, systemic toxicity and infection control [published online April 8, 2013]. J Arthroplasty. 2013;28:1490.e1-1498.e1.
- Daneshmend TK. The neurotoxicity of dapsone. Adverse Drug React Acute Poisoning Rev. 1984;3:43-58.
- Jacobs C, Christensen CP, Berend ME. Static and mobile antibiotic-impregnated cement spacers for the management of prosthetic joint infection. J Am Acad Orthop Surg. 2009;17:356-368.
- Springer BD, Lee GC, Osmon D, et al. Systemic safety of high-dose antibiotic-loaded cement spacers after resection of an infected total knee arthroplasty. Clin Orthop Relat Res. 2004;427:47-51.
- Penner MJ, Masri BA, Duncan CP. Elution characteristics of vancomycin and tobramycin combined in acrylic bone-cement. J Arthroplasty. 1996;11:939-944.
- Williams B, Hanson A, Sha B. Diffuse desquamating rash following exposure to vancomycin-impregnated bone cement. Ann Pharmacother. 2014;48:1061-1065.
- Haeberle M, Wittner B. Is gentamicin-loaded bone cement a risk for developing systemic allergic dermatitis? Contact Dermatitis. 2009;60:176-177.
- McDonald HC, York NR, Pandya AG. Drug-induced linear IgA bullous dermatosis demonstrating the isomorphic phenomenon. J Am Acad Dermatol. 2010;62:897-898.
- Plunkett RW, Chiarello SE, Beutner EH. Linear IgA bullous dermatosis in one of two piroxicam-induced eruptions: a distinct direct immunofluorescence trend revealed by the literature. J Am Acad Dermatol. 2001;45:691-696.
- Guide SV, Marinkovich MP. Linear IgA bullous dermatosis. Clin Dermatol. 2001;19:719-727.
- Fortuna G, Marinkovich MP. Linear immunoglobulin A bullous dermatosis. Clin Dermatol. 2012;30:38-50.
- Fortuna G, Salas-Alanis JC, Guidetti E, et al. A critical reappraisal of the current data on drug-induced linear immunoglobulin A bullous dermatosis: a real and separate nosological entity? J Am Acad Dermatol. 2012;66:988-994.
- Kuechle MK, Stegemeir E, Maynard B, et al. Drug-induced linear IgA bullous dermatosis: report of six cases and review of the literature. J Am Acad Dermatol. 1994;30(2, pt 1):187-192.
- Neughebauer BI, Negron G, Pelton S, et al. Bullous skin disease: an unusual allergic reaction to vancomycin. Am J Med Sci. 2002;323:273-278.
- Naranjo CA, Busto U, Sellers EM, et al. A method for estimating the probability of adverse drug reactions. Clin Pharmacol Ther. 1981;30:239-245.
- Wiadrowski TP, Reid CM. Drug-induced linear IgA bullous disease following antibiotics. Australas J Dermatol. 2001;42:196-199.
- Dang LV, Byrom L, Muir J, et al. Vancomycin-induced linear IgA with mucosal and ocular involvement: a case report. Infect Dis Clin Pract. 2014;22:e119-e121.
- Luu A, Syed F, Raman G, et al. Two-stage arthroplasty for prosthetic joint infection: a systematic review of acute kidney injury, systemic toxicity and infection control [published online April 8, 2013]. J Arthroplasty. 2013;28:1490.e1-1498.e1.
- Daneshmend TK. The neurotoxicity of dapsone. Adverse Drug React Acute Poisoning Rev. 1984;3:43-58.
- Jacobs C, Christensen CP, Berend ME. Static and mobile antibiotic-impregnated cement spacers for the management of prosthetic joint infection. J Am Acad Orthop Surg. 2009;17:356-368.
- Springer BD, Lee GC, Osmon D, et al. Systemic safety of high-dose antibiotic-loaded cement spacers after resection of an infected total knee arthroplasty. Clin Orthop Relat Res. 2004;427:47-51.
- Penner MJ, Masri BA, Duncan CP. Elution characteristics of vancomycin and tobramycin combined in acrylic bone-cement. J Arthroplasty. 1996;11:939-944.
- Williams B, Hanson A, Sha B. Diffuse desquamating rash following exposure to vancomycin-impregnated bone cement. Ann Pharmacother. 2014;48:1061-1065.
- Haeberle M, Wittner B. Is gentamicin-loaded bone cement a risk for developing systemic allergic dermatitis? Contact Dermatitis. 2009;60:176-177.
- McDonald HC, York NR, Pandya AG. Drug-induced linear IgA bullous dermatosis demonstrating the isomorphic phenomenon. J Am Acad Dermatol. 2010;62:897-898.
Practice Points
- Linear IgA bullous dermatosis (LABD) is an autoimmune mucocutaneous disorder characterized by linear IgA deposits at the dermoepidermal junction.
- A substantial number of cases of LABD are drug related, with vancomycin most commonly implicated.
- While antibiotic-impregnated cement spacers deliver high concentrations of local medications, systemic reactions are still possible.
- Dapsone is the first-line treatment for LABD.
A Case of Pustular Psoriasis of Pregnancy With Positive Maternal-Fetal Outcomes
Pustular psoriasis of pregnancy (PPP), also known as impetigo herpetiformis, is a relatively rare cutaneous disorder of pregnancy wherein lesions typically appear in the third trimester and resolve after delivery; however, lesions may persist through the postpartum period. Pustular psoriasis of pregnancy may be considered a fifth dermatosis of pregnancy, alongside the classic dermatoses of atopic eruption of pregnancy, intrahepatic cholestasis of pregnancy, pemphigoid gestationis, and pruritic urticarial papules and plaques of pregnancy.1
As PPP is a rare disease, its effects on maternal-fetal health outcomes and management remain to be elucidated. Though maternal mortality is rare in PPP, it is a unique dermatosis of pregnancy because it may be associated with severe systemic maternal symptoms.2 Fetal morbidity and mortality are less predictable in PPP, with reported cases of stillbirth, fetal anomalies, and neonatal death thought to be due largely to placental insufficiency, even with control of symptoms.1,3 Given the risk of serious harm to the fetus, reporting of cases and discussion of PPP management is critical.
Case Report
An otherwise healthy 29-year-old G2P1 woman at 32 weeks’ gestation presented to our emergency department with a 1-week history of a pruritic, burning rash that started on the thighs then spread diffusely. She denied any similar rash in her prior pregnancy. She was not currently taking any medications except for prenatal vitamins and denied any systemic symptoms. The patient’s obstetrician initiated treatment with methylprednisolone 50 mg once daily for the rash 3 days prior to the current presentation, which had not seemed to help. On physical examination, edematous pink plaques studded with 1- to 2-mm collarettes of scaling and sparse 1-mm pustules involving the arms, chest, abdomen, back, groin, buttocks, and legs were noted. The plaques on the back and inner thighs had a peripheral rim of desquamative scaling. There were pink macules on the palms, and superficial desquamation was noted on the lips. The oral mucosa was otherwise spared (Figure 1).
Biopsy specimens from the left arm revealed discrete subcorneal pustules with mild acanthosis of the epidermis with spongiosis (Figure 2). The papillary dermis showed a sparse infiltrate of neutrophils with many marginated neutrophils within vessels. Direct immunofluorescence was negative for human IgG, IgA, IgM, complement component 3, and fibrinogen. Laboratory workup revealed leukocytosis of 21.5×109/L (reference range, 4.5–11.0×109/L) with neutrophilic predominance of 73.6% (reference range, 56%), an elevated erythrocyte sedimentation rate (ESR) of 40 mm/h (reference range, 0–20 mm/h), and a mild hypocalcemia of 8.6 mg/dL (reference range, 8.2–10.2 mg/dL). The patient was started on methylprednisone 40 mg once daily with a plan to taper the dose by 8 mg every 5 days.
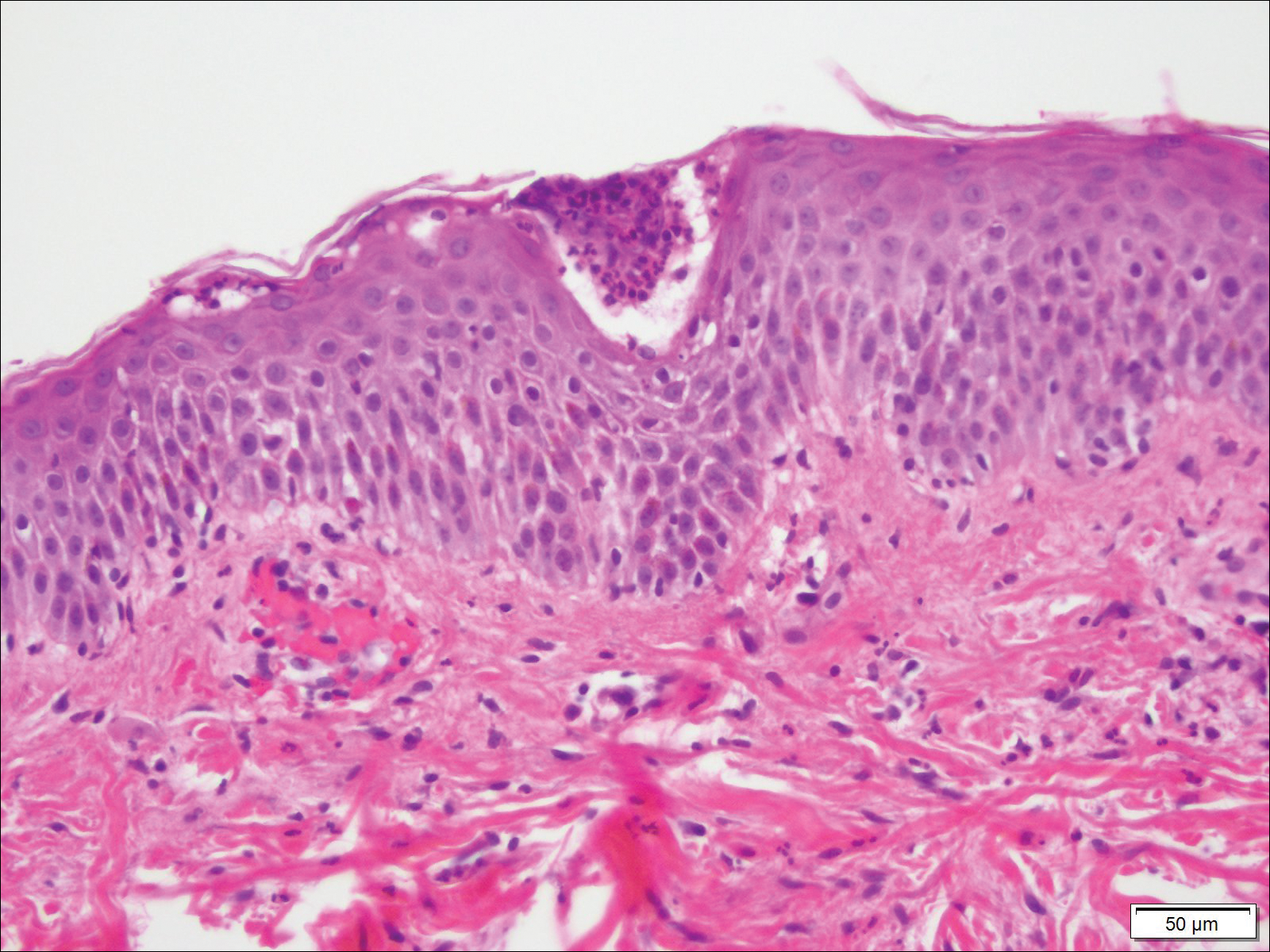
At 35 weeks’ gestation, the patient continued to report pruritus and burning in the areas where the rash had developed. The morphology of the rash had changed considerably, as she now had prominent, annular, pink plaques with central clearing, trailing scaling, and a border of subtle pustules on the legs. There also were rings of desquamative scaling on the palms. During follow-up at 37 weeks’ gestation, the back, chest, and abdomen were improved from the initial presentation, and annular pink plaques with central clearing were noted on the legs (Figure 3). Given the clinical and histopathologic findings, a diagnosis of PPP was made. It was recommended that she undergo increased fetal surveillance with close obstetric follow-up. Weekly office visits with obstetrics and twice-weekly Doppler ultrasounds and fetal nonstress tests were deemed appropriate management. The patient was scheduled for induction at 39 weeks’ gestation given the risk for potential harm to the fetus. She was maintained on low-dose methylprednisolone 4 mg once daily for the duration of the pregnancy. The patient continued to have gradual improvement of the rash at the low treatment dose.
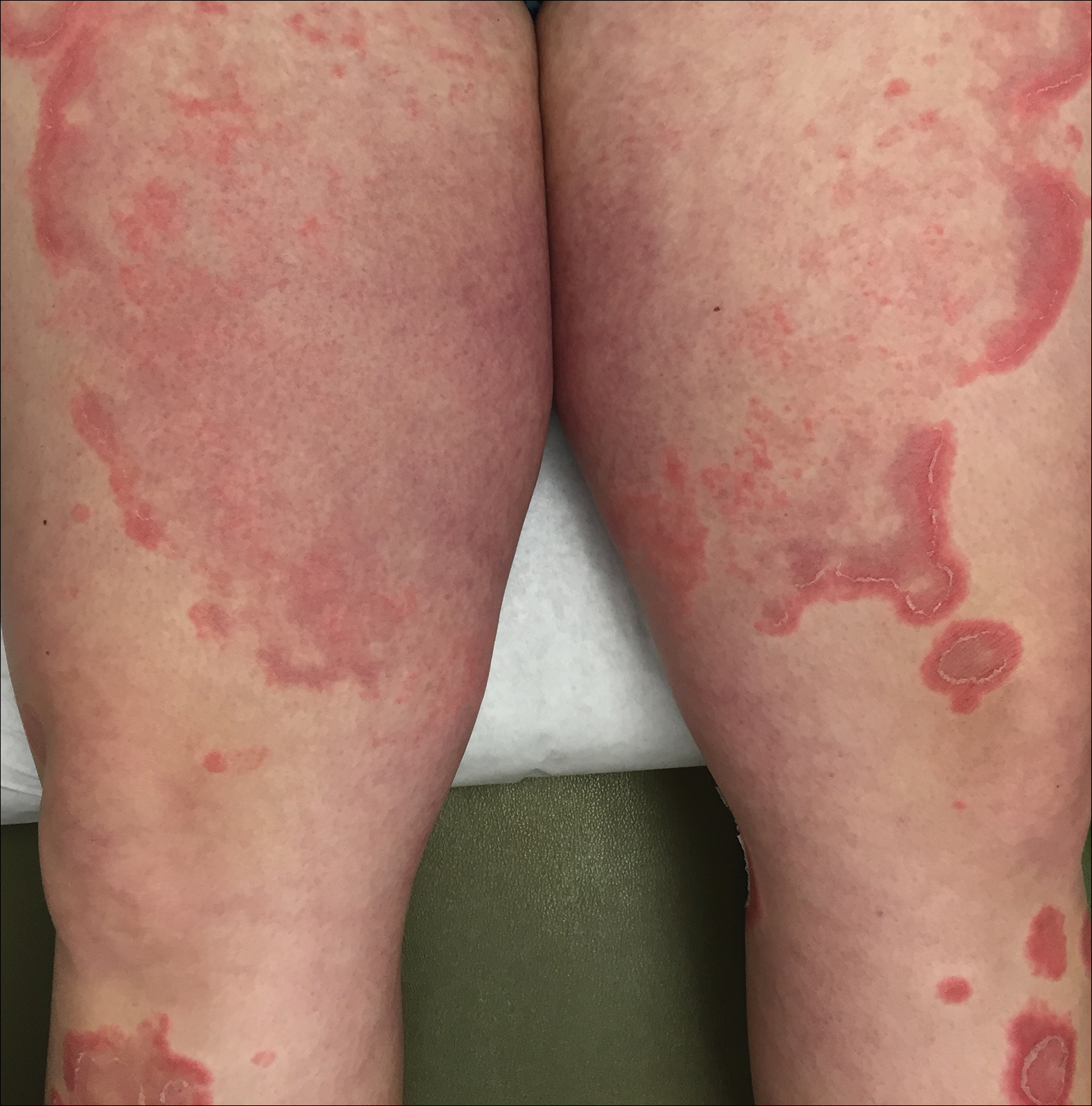
Following induction at 39 weeks’ gestation, the patient vaginally delivered a healthy, 6-lb male neonate at an outside hospital. She reported that the burning sensation improved within hours of delivery, and systemic steroids were stopped after delivery. At a follow-up visit 3 weeks postpartum, considerable improvement of the rash was noted with no evidence of pustules. Fading pink patches with a superficial scaling were noted on the back, chest, abdomen, arms, legs (Figure 4), and fingertips. The patient was counseled that PPP could recur in subsequent pregnancies and that she should be aware of the potential risks to the fetus.
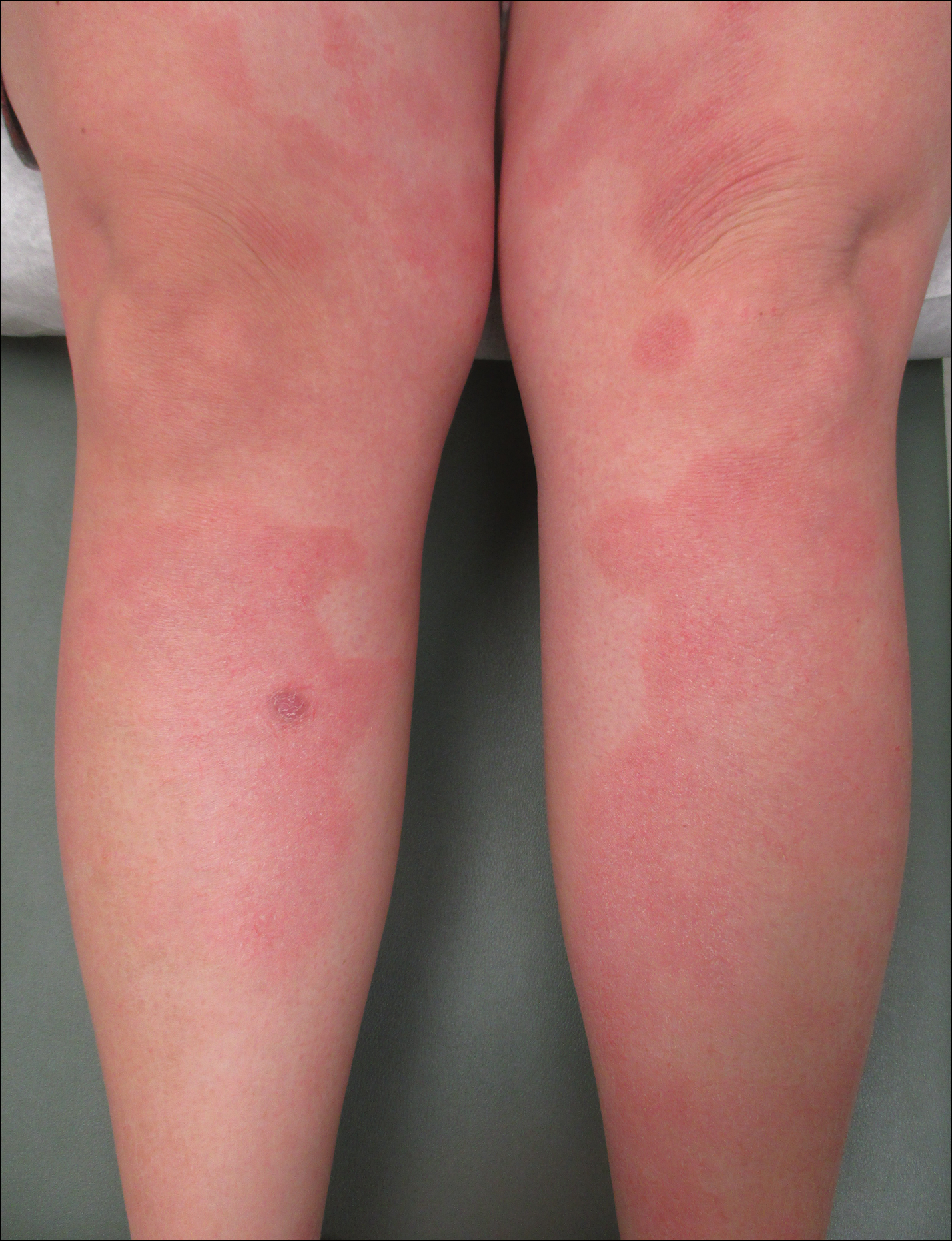
Comment
In our patient, the diagnosis of PPP was supported by the presence of erythematous, coalescent plaques with small pustules at the margins and central erosions as well as the histologic findings of subcorneal pustules with mild acanthosis of the epidermis with spongiosis and a sparse neutrophilic infiltrate into the dermis.
The typical presentation of PPP is characterized by lesions that initially develop in skin folds with centrifugal spread.3 The lesions usually begin as erythematous plaques with a pustular ring with a central erosion. The face, palms, and soles of the feet typically are spared with occasional involvement of oral and esophageal mucosae. Biopsy findings typically include spongiform pustules with neutrophil invasion into the epidermis. Typical laboratory findings include electrolyte derangements with elevated ESR and leukocytosis.1
Diagnosis of PPP is critical given the potential for associated fetal morbidity and mortality.4 Anticipatory guidance for the patient also is necessary, as PPP can recur with subsequent pregnancies or even use of oral contraceptive pills (OCPs). Notably, a patient with recurrences of PPP with each of 9 pregnancies also experienced a recurrence when taking a combination estrogen/progesterone OCP, but not with an estrogen-only diethylstilbestrol OCP.5 Although the pathophysiology is not entirely understood, the development of PPP is thought to be related to the hormonal changes that occur in the third trimester, most notably due to elevated progesterone levels.2 The presence of progesterone in OCPs and recurrences associated with their use supports this altered hormonal state, contributing to the underlying pathophysiology of PPP.
Pustular psoriasis of pregnancy can occur in women without any personal or family history of psoriasis, and as such, it is unclear whether PPP is a separate entity or a hormonally induced variation of generalized pustular psoriasis. Recent evidence included reports of women with PPP who had a mutation in the IL-36 receptor antagonist, leading to a relative abundance of IL-36 inflammatory cytokines.6
The mainstay of treatment for PPP is oral corticosteroids. Cases of PPP that are unresponsive to systemic steroids have been documented, requiring treatment with cyclosporine.9 Antitumor necrosis factors also have been used safely during pregnancy.10 Narrowband UVB phototherapy also has been proposed as a treatment alternative for patients who do not respond to oral corticosteroids.11
Conclusion
Pustular psoriasis of pregnancy is a rare dermatosis of pregnancy that, unlike most other common dermatoses of pregnancy, is associated with adverse fetal outcomes. Diagnosis and management of PPP are critical to ensure the best care and outcomes for the patient and fetus and for a successful delivery of a healthy neonate. Our patient with PPP presented with involvement of the body, palms, and oral mucosa in the absence of systemic symptoms. Close follow-up and comanagement with the patient’s obstetrician ensured safe outcomes for the patient and the neonate.
- Lehrhoff S, Pomeranz MK. Specific dermatoses of pregnancy and their treatment. Dermatol Ther. 2013;26:274-284.
- Kar S, Krishnan A, Shivkumar PV. Pregnancy and skin [published online August 28, 2012]. J Obstet Gynaecol India. 2012;62:268-275.
- Kondo RN, Araújo FM, Pereira AM, et al. Pustular psoriasis of pregnancy (impetigo herpetiformis)—case report. An Bras Dermatol. 2013;88(6 suppl 1):186-189.
- Oumeish OY, Parish JL. Impetigo herpetiformis. Clin Dermatol. 2006;24:101-104.
- Oumeish OY, Farraj SE, Bataineh AS. Some aspects of impetigo herpetiformis. Arch Dermatol. 1982;118:103-105.
- Sugiura K, Oiso N, Iinuma S, et al. IL36RN mutations underlie impetigo herpetiformis. J Invest Dermatol. 2014;134:2472-2474.
- Sugiura K. The genetic background of generalized pustular psoriasis: IL36RN mutations and CARD14 gain-of-function variants [published online March 5, 2014]. J Dermatol Sci. 2014;74:187-192.
- Li X, Chen M, Fu X, et al. Mutation analysis of the IL36RN gene in Chinese patients with generalized pustular psoriasis with/without psoriasis vulgaris. J Dermatol Sci. 2014;76:132-138.
- Hazarika D. Generalized pustular psoriasis of pregnancy successfully treated with cyclosporine. Indian J Dermatol Venereol Leprol. 2009;75:638.
- Puig L, Barco D, Alomar A. Treatment of psoriasis with anti-TNF drugs during pregnancy: case report and review of the literature. Dermatology. 2010;220:71-76.
- Bozdag K, Ozturk S, Ermete M. A case of recurrent impetigo herpetiformis treated with systemic corticosteroids and narrowband UVB [published online January 20, 2012]. Cutan Ocul Toxicol. 2012;31:67-69.
Pustular psoriasis of pregnancy (PPP), also known as impetigo herpetiformis, is a relatively rare cutaneous disorder of pregnancy wherein lesions typically appear in the third trimester and resolve after delivery; however, lesions may persist through the postpartum period. Pustular psoriasis of pregnancy may be considered a fifth dermatosis of pregnancy, alongside the classic dermatoses of atopic eruption of pregnancy, intrahepatic cholestasis of pregnancy, pemphigoid gestationis, and pruritic urticarial papules and plaques of pregnancy.1
As PPP is a rare disease, its effects on maternal-fetal health outcomes and management remain to be elucidated. Though maternal mortality is rare in PPP, it is a unique dermatosis of pregnancy because it may be associated with severe systemic maternal symptoms.2 Fetal morbidity and mortality are less predictable in PPP, with reported cases of stillbirth, fetal anomalies, and neonatal death thought to be due largely to placental insufficiency, even with control of symptoms.1,3 Given the risk of serious harm to the fetus, reporting of cases and discussion of PPP management is critical.
Case Report
An otherwise healthy 29-year-old G2P1 woman at 32 weeks’ gestation presented to our emergency department with a 1-week history of a pruritic, burning rash that started on the thighs then spread diffusely. She denied any similar rash in her prior pregnancy. She was not currently taking any medications except for prenatal vitamins and denied any systemic symptoms. The patient’s obstetrician initiated treatment with methylprednisolone 50 mg once daily for the rash 3 days prior to the current presentation, which had not seemed to help. On physical examination, edematous pink plaques studded with 1- to 2-mm collarettes of scaling and sparse 1-mm pustules involving the arms, chest, abdomen, back, groin, buttocks, and legs were noted. The plaques on the back and inner thighs had a peripheral rim of desquamative scaling. There were pink macules on the palms, and superficial desquamation was noted on the lips. The oral mucosa was otherwise spared (Figure 1).

Biopsy specimens from the left arm revealed discrete subcorneal pustules with mild acanthosis of the epidermis with spongiosis (Figure 2). The papillary dermis showed a sparse infiltrate of neutrophils with many marginated neutrophils within vessels. Direct immunofluorescence was negative for human IgG, IgA, IgM, complement component 3, and fibrinogen. Laboratory workup revealed leukocytosis of 21.5×109/L (reference range, 4.5–11.0×109/L) with neutrophilic predominance of 73.6% (reference range, 56%), an elevated erythrocyte sedimentation rate (ESR) of 40 mm/h (reference range, 0–20 mm/h), and a mild hypocalcemia of 8.6 mg/dL (reference range, 8.2–10.2 mg/dL). The patient was started on methylprednisone 40 mg once daily with a plan to taper the dose by 8 mg every 5 days.

At 35 weeks’ gestation, the patient continued to report pruritus and burning in the areas where the rash had developed. The morphology of the rash had changed considerably, as she now had prominent, annular, pink plaques with central clearing, trailing scaling, and a border of subtle pustules on the legs. There also were rings of desquamative scaling on the palms. During follow-up at 37 weeks’ gestation, the back, chest, and abdomen were improved from the initial presentation, and annular pink plaques with central clearing were noted on the legs (Figure 3). Given the clinical and histopathologic findings, a diagnosis of PPP was made. It was recommended that she undergo increased fetal surveillance with close obstetric follow-up. Weekly office visits with obstetrics and twice-weekly Doppler ultrasounds and fetal nonstress tests were deemed appropriate management. The patient was scheduled for induction at 39 weeks’ gestation given the risk for potential harm to the fetus. She was maintained on low-dose methylprednisolone 4 mg once daily for the duration of the pregnancy. The patient continued to have gradual improvement of the rash at the low treatment dose.

Following induction at 39 weeks’ gestation, the patient vaginally delivered a healthy, 6-lb male neonate at an outside hospital. She reported that the burning sensation improved within hours of delivery, and systemic steroids were stopped after delivery. At a follow-up visit 3 weeks postpartum, considerable improvement of the rash was noted with no evidence of pustules. Fading pink patches with a superficial scaling were noted on the back, chest, abdomen, arms, legs (Figure 4), and fingertips. The patient was counseled that PPP could recur in subsequent pregnancies and that she should be aware of the potential risks to the fetus.

Comment
In our patient, the diagnosis of PPP was supported by the presence of erythematous, coalescent plaques with small pustules at the margins and central erosions as well as the histologic findings of subcorneal pustules with mild acanthosis of the epidermis with spongiosis and a sparse neutrophilic infiltrate into the dermis.
The typical presentation of PPP is characterized by lesions that initially develop in skin folds with centrifugal spread.3 The lesions usually begin as erythematous plaques with a pustular ring with a central erosion. The face, palms, and soles of the feet typically are spared with occasional involvement of oral and esophageal mucosae. Biopsy findings typically include spongiform pustules with neutrophil invasion into the epidermis. Typical laboratory findings include electrolyte derangements with elevated ESR and leukocytosis.1
Diagnosis of PPP is critical given the potential for associated fetal morbidity and mortality.4 Anticipatory guidance for the patient also is necessary, as PPP can recur with subsequent pregnancies or even use of oral contraceptive pills (OCPs). Notably, a patient with recurrences of PPP with each of 9 pregnancies also experienced a recurrence when taking a combination estrogen/progesterone OCP, but not with an estrogen-only diethylstilbestrol OCP.5 Although the pathophysiology is not entirely understood, the development of PPP is thought to be related to the hormonal changes that occur in the third trimester, most notably due to elevated progesterone levels.2 The presence of progesterone in OCPs and recurrences associated with their use supports this altered hormonal state, contributing to the underlying pathophysiology of PPP.
Pustular psoriasis of pregnancy can occur in women without any personal or family history of psoriasis, and as such, it is unclear whether PPP is a separate entity or a hormonally induced variation of generalized pustular psoriasis. Recent evidence included reports of women with PPP who had a mutation in the IL-36 receptor antagonist, leading to a relative abundance of IL-36 inflammatory cytokines.6
The mainstay of treatment for PPP is oral corticosteroids. Cases of PPP that are unresponsive to systemic steroids have been documented, requiring treatment with cyclosporine.9 Antitumor necrosis factors also have been used safely during pregnancy.10 Narrowband UVB phototherapy also has been proposed as a treatment alternative for patients who do not respond to oral corticosteroids.11
Conclusion
Pustular psoriasis of pregnancy is a rare dermatosis of pregnancy that, unlike most other common dermatoses of pregnancy, is associated with adverse fetal outcomes. Diagnosis and management of PPP are critical to ensure the best care and outcomes for the patient and fetus and for a successful delivery of a healthy neonate. Our patient with PPP presented with involvement of the body, palms, and oral mucosa in the absence of systemic symptoms. Close follow-up and comanagement with the patient’s obstetrician ensured safe outcomes for the patient and the neonate.
Pustular psoriasis of pregnancy (PPP), also known as impetigo herpetiformis, is a relatively rare cutaneous disorder of pregnancy wherein lesions typically appear in the third trimester and resolve after delivery; however, lesions may persist through the postpartum period. Pustular psoriasis of pregnancy may be considered a fifth dermatosis of pregnancy, alongside the classic dermatoses of atopic eruption of pregnancy, intrahepatic cholestasis of pregnancy, pemphigoid gestationis, and pruritic urticarial papules and plaques of pregnancy.1
As PPP is a rare disease, its effects on maternal-fetal health outcomes and management remain to be elucidated. Though maternal mortality is rare in PPP, it is a unique dermatosis of pregnancy because it may be associated with severe systemic maternal symptoms.2 Fetal morbidity and mortality are less predictable in PPP, with reported cases of stillbirth, fetal anomalies, and neonatal death thought to be due largely to placental insufficiency, even with control of symptoms.1,3 Given the risk of serious harm to the fetus, reporting of cases and discussion of PPP management is critical.
Case Report
An otherwise healthy 29-year-old G2P1 woman at 32 weeks’ gestation presented to our emergency department with a 1-week history of a pruritic, burning rash that started on the thighs then spread diffusely. She denied any similar rash in her prior pregnancy. She was not currently taking any medications except for prenatal vitamins and denied any systemic symptoms. The patient’s obstetrician initiated treatment with methylprednisolone 50 mg once daily for the rash 3 days prior to the current presentation, which had not seemed to help. On physical examination, edematous pink plaques studded with 1- to 2-mm collarettes of scaling and sparse 1-mm pustules involving the arms, chest, abdomen, back, groin, buttocks, and legs were noted. The plaques on the back and inner thighs had a peripheral rim of desquamative scaling. There were pink macules on the palms, and superficial desquamation was noted on the lips. The oral mucosa was otherwise spared (Figure 1).

Biopsy specimens from the left arm revealed discrete subcorneal pustules with mild acanthosis of the epidermis with spongiosis (Figure 2). The papillary dermis showed a sparse infiltrate of neutrophils with many marginated neutrophils within vessels. Direct immunofluorescence was negative for human IgG, IgA, IgM, complement component 3, and fibrinogen. Laboratory workup revealed leukocytosis of 21.5×109/L (reference range, 4.5–11.0×109/L) with neutrophilic predominance of 73.6% (reference range, 56%), an elevated erythrocyte sedimentation rate (ESR) of 40 mm/h (reference range, 0–20 mm/h), and a mild hypocalcemia of 8.6 mg/dL (reference range, 8.2–10.2 mg/dL). The patient was started on methylprednisone 40 mg once daily with a plan to taper the dose by 8 mg every 5 days.

At 35 weeks’ gestation, the patient continued to report pruritus and burning in the areas where the rash had developed. The morphology of the rash had changed considerably, as she now had prominent, annular, pink plaques with central clearing, trailing scaling, and a border of subtle pustules on the legs. There also were rings of desquamative scaling on the palms. During follow-up at 37 weeks’ gestation, the back, chest, and abdomen were improved from the initial presentation, and annular pink plaques with central clearing were noted on the legs (Figure 3). Given the clinical and histopathologic findings, a diagnosis of PPP was made. It was recommended that she undergo increased fetal surveillance with close obstetric follow-up. Weekly office visits with obstetrics and twice-weekly Doppler ultrasounds and fetal nonstress tests were deemed appropriate management. The patient was scheduled for induction at 39 weeks’ gestation given the risk for potential harm to the fetus. She was maintained on low-dose methylprednisolone 4 mg once daily for the duration of the pregnancy. The patient continued to have gradual improvement of the rash at the low treatment dose.

Following induction at 39 weeks’ gestation, the patient vaginally delivered a healthy, 6-lb male neonate at an outside hospital. She reported that the burning sensation improved within hours of delivery, and systemic steroids were stopped after delivery. At a follow-up visit 3 weeks postpartum, considerable improvement of the rash was noted with no evidence of pustules. Fading pink patches with a superficial scaling were noted on the back, chest, abdomen, arms, legs (Figure 4), and fingertips. The patient was counseled that PPP could recur in subsequent pregnancies and that she should be aware of the potential risks to the fetus.

Comment
In our patient, the diagnosis of PPP was supported by the presence of erythematous, coalescent plaques with small pustules at the margins and central erosions as well as the histologic findings of subcorneal pustules with mild acanthosis of the epidermis with spongiosis and a sparse neutrophilic infiltrate into the dermis.
The typical presentation of PPP is characterized by lesions that initially develop in skin folds with centrifugal spread.3 The lesions usually begin as erythematous plaques with a pustular ring with a central erosion. The face, palms, and soles of the feet typically are spared with occasional involvement of oral and esophageal mucosae. Biopsy findings typically include spongiform pustules with neutrophil invasion into the epidermis. Typical laboratory findings include electrolyte derangements with elevated ESR and leukocytosis.1
Diagnosis of PPP is critical given the potential for associated fetal morbidity and mortality.4 Anticipatory guidance for the patient also is necessary, as PPP can recur with subsequent pregnancies or even use of oral contraceptive pills (OCPs). Notably, a patient with recurrences of PPP with each of 9 pregnancies also experienced a recurrence when taking a combination estrogen/progesterone OCP, but not with an estrogen-only diethylstilbestrol OCP.5 Although the pathophysiology is not entirely understood, the development of PPP is thought to be related to the hormonal changes that occur in the third trimester, most notably due to elevated progesterone levels.2 The presence of progesterone in OCPs and recurrences associated with their use supports this altered hormonal state, contributing to the underlying pathophysiology of PPP.
Pustular psoriasis of pregnancy can occur in women without any personal or family history of psoriasis, and as such, it is unclear whether PPP is a separate entity or a hormonally induced variation of generalized pustular psoriasis. Recent evidence included reports of women with PPP who had a mutation in the IL-36 receptor antagonist, leading to a relative abundance of IL-36 inflammatory cytokines.6
The mainstay of treatment for PPP is oral corticosteroids. Cases of PPP that are unresponsive to systemic steroids have been documented, requiring treatment with cyclosporine.9 Antitumor necrosis factors also have been used safely during pregnancy.10 Narrowband UVB phototherapy also has been proposed as a treatment alternative for patients who do not respond to oral corticosteroids.11
Conclusion
Pustular psoriasis of pregnancy is a rare dermatosis of pregnancy that, unlike most other common dermatoses of pregnancy, is associated with adverse fetal outcomes. Diagnosis and management of PPP are critical to ensure the best care and outcomes for the patient and fetus and for a successful delivery of a healthy neonate. Our patient with PPP presented with involvement of the body, palms, and oral mucosa in the absence of systemic symptoms. Close follow-up and comanagement with the patient’s obstetrician ensured safe outcomes for the patient and the neonate.
- Lehrhoff S, Pomeranz MK. Specific dermatoses of pregnancy and their treatment. Dermatol Ther. 2013;26:274-284.
- Kar S, Krishnan A, Shivkumar PV. Pregnancy and skin [published online August 28, 2012]. J Obstet Gynaecol India. 2012;62:268-275.
- Kondo RN, Araújo FM, Pereira AM, et al. Pustular psoriasis of pregnancy (impetigo herpetiformis)—case report. An Bras Dermatol. 2013;88(6 suppl 1):186-189.
- Oumeish OY, Parish JL. Impetigo herpetiformis. Clin Dermatol. 2006;24:101-104.
- Oumeish OY, Farraj SE, Bataineh AS. Some aspects of impetigo herpetiformis. Arch Dermatol. 1982;118:103-105.
- Sugiura K, Oiso N, Iinuma S, et al. IL36RN mutations underlie impetigo herpetiformis. J Invest Dermatol. 2014;134:2472-2474.
- Sugiura K. The genetic background of generalized pustular psoriasis: IL36RN mutations and CARD14 gain-of-function variants [published online March 5, 2014]. J Dermatol Sci. 2014;74:187-192.
- Li X, Chen M, Fu X, et al. Mutation analysis of the IL36RN gene in Chinese patients with generalized pustular psoriasis with/without psoriasis vulgaris. J Dermatol Sci. 2014;76:132-138.
- Hazarika D. Generalized pustular psoriasis of pregnancy successfully treated with cyclosporine. Indian J Dermatol Venereol Leprol. 2009;75:638.
- Puig L, Barco D, Alomar A. Treatment of psoriasis with anti-TNF drugs during pregnancy: case report and review of the literature. Dermatology. 2010;220:71-76.
- Bozdag K, Ozturk S, Ermete M. A case of recurrent impetigo herpetiformis treated with systemic corticosteroids and narrowband UVB [published online January 20, 2012]. Cutan Ocul Toxicol. 2012;31:67-69.
- Lehrhoff S, Pomeranz MK. Specific dermatoses of pregnancy and their treatment. Dermatol Ther. 2013;26:274-284.
- Kar S, Krishnan A, Shivkumar PV. Pregnancy and skin [published online August 28, 2012]. J Obstet Gynaecol India. 2012;62:268-275.
- Kondo RN, Araújo FM, Pereira AM, et al. Pustular psoriasis of pregnancy (impetigo herpetiformis)—case report. An Bras Dermatol. 2013;88(6 suppl 1):186-189.
- Oumeish OY, Parish JL. Impetigo herpetiformis. Clin Dermatol. 2006;24:101-104.
- Oumeish OY, Farraj SE, Bataineh AS. Some aspects of impetigo herpetiformis. Arch Dermatol. 1982;118:103-105.
- Sugiura K, Oiso N, Iinuma S, et al. IL36RN mutations underlie impetigo herpetiformis. J Invest Dermatol. 2014;134:2472-2474.
- Sugiura K. The genetic background of generalized pustular psoriasis: IL36RN mutations and CARD14 gain-of-function variants [published online March 5, 2014]. J Dermatol Sci. 2014;74:187-192.
- Li X, Chen M, Fu X, et al. Mutation analysis of the IL36RN gene in Chinese patients with generalized pustular psoriasis with/without psoriasis vulgaris. J Dermatol Sci. 2014;76:132-138.
- Hazarika D. Generalized pustular psoriasis of pregnancy successfully treated with cyclosporine. Indian J Dermatol Venereol Leprol. 2009;75:638.
- Puig L, Barco D, Alomar A. Treatment of psoriasis with anti-TNF drugs during pregnancy: case report and review of the literature. Dermatology. 2010;220:71-76.
- Bozdag K, Ozturk S, Ermete M. A case of recurrent impetigo herpetiformis treated with systemic corticosteroids and narrowband UVB [published online January 20, 2012]. Cutan Ocul Toxicol. 2012;31:67-69.
Practice Points
- Given its association with maternal and fetal morbidity/mortality, it is important for physicians to have a high suspicion for pustular psoriasis of pregnancy (PPP) in pregnant women with widespread cutaneous eruptions.
- Oral corticosteroids and close involvement of obstetric care is the mainstay of treatment for PPP.