User login
Gone but Not Forgotten: Acute Appendicitis Postappendectomy
Acute appendicitis is a common condition emergency physicians (EPs) encounter in the ED, and it is also one of the most common general surgeries.1Although stump appendicitis is a rare, long-term complication of appendectomy, it should always be included in the differential diagnosis of patients presenting with right-sided abdominal pain and a history of appendectomy. Delays in diagnosing stump appendicitis can lead to perforation, gangrene, and sepsis.2
Case
A 33-year-old previously healthy man, whose medical history was significant for an appendectomy 6 months earlier, presented to the ED with progressive and worsening right lower quadrant abdominal pain that radiated to his right testicle. The patient stated that the pain started 3 days prior while he was lifting a bale of hay. He further noted having a fever of 102oF, nausea, and vomiting hours prior to his arrival at the ED.
Upon presentation, the patient’s vital signs were: heart rate, 89 beats/min; respiratory rate, 17 breaths/min; blood pressure, 132/84 mm Hg; and temperature, 98.9°F. Oxygen saturation was 98% on room air. Physical examination revealed exquisite tenderness in the right lower quadrant and suprapubic region. The testicular examination and the remainder of the physical examination were normal. Laboratory evaluation included a complete blood count and urinalysis, the results of which were significant for an elevated white blood cell count of 17 x 109/Lmicroscopic hematuria, trace leukocyte esterase, and ketones.
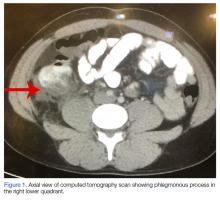
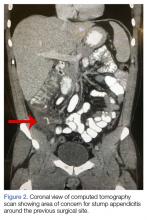
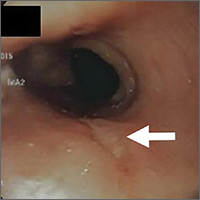
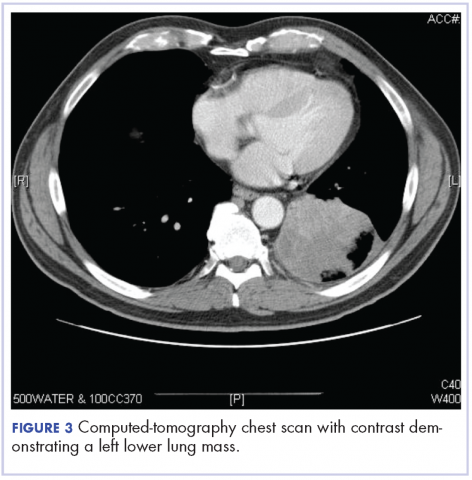
A computed tomography (CT) scan of the abdomen and pelvis with intravenous (IV) and oral contrast demonstrated a phlegmonous process surrounding the surgical site, which was concerning for stump appendicitis. The terminal ilium and colon were noted to be normal (Figures 1 and 2).
The patient was started on IV fluids and IV antibiotics, and received Zosyn in the ED. Surgical service was consulted, and the patient was admitted to the hospital where he continued nonoperative treatment with IV ciprofloxacin and metronidazole. The patient was discharged home on hospital day 3 without further complication. A repeat CT scan was taken of the abdomen and pelvis 3 weeks after discharge, and demonstrated complete resolution of the inflammatory process at the appendiceal stump with chronic scarring.
Discussion
Approximately 7% of patients who present to the ED with abdominal pain are diagnosed with appendicitis.3 Although appendectomy is one of the most common surgical procedures, stump appendicitis is a rare postsurgical complication, with a reported incidence of 1 in 50,000 cases.4,5
Stump appendicitis is an acute inflammation of the residual appendicular stump; the incidence of stump perforation is approximately 60% to 70%.4,6 Thus, stump appendicitis has a high morbidity and complication rate. Unfortunately, though stump appendicitis is a condition in which timely diagnosis and intervention are essential to prevent morbidity, due to its rarity and low occurrence, there is often a delay in diagnosis. It is therefore important that EPs include stump appendicitis in the differential diagnosis of patients presenting with right-sided abdominal pain and a history of appendectomy.
Stump appendicitis was initially described by Rose et al in 1945.2 This condition is underreported, and the exact causes are still unclear.Of the reported cases of stump appendicitis, approximately 66% developed following an open surgical appendectomy;5 therefore, complicated surgery or difficult dissection of the appendix is considered a risk factor for stump appendicitis. Conversely, adequate visualization of the appendiceal base during appendectomy and a stump measuring less than 3 to 5 mm1,4 are associated with a lower risk for stump appendicitis.
Stump appendicitis can develop as early as a few days postappendectomy or as late as 50 years postappendectomy. Patients with stump appendicitis present with signs and symptoms similar to that of acute appendicitis.2,4,7 Diagnosis can be made through ultrasound or CT studies, though CT is the preferred modality due to its higher specificity and ability to exclude other causes of right-sided abdominal pain.4
Management
Surgical intervention to remove the appendiceal stump is typically the preferred treatment. However, as with our patient, cases of successful and uncomplicated medical management have been reported.1,2,4
Conclusion
While stump appendicitis is rare, there has been a rise in the number of reported cases over the past few years due to the increasing use and availability of CT.4 The diagnosis of stump appendicitis is time-critical to prevent associated complications of stump perforation, gangrene, and sepsis. It is therefore imperative that EPs consider this
1. Shah T, Gupta RK, Karkee RJ, Agarwal CS. Recurrent pain abdomen following appendectomy: stump appendicitis, a surgeon’s dilemma. Clin Case Rep. 2017;5(3):215-217. doi:10.1002/ccr3.781.
2. Giwa A, Reyes M. Three times a charm…a case of repeat appendicitis status post two prior appendectomies. Am J Emerg Med. 2018;36(3):528.e1-528.e2. doi:10.1016/j.ajem.2017.12.024.
3. Addiss DG, Shaffer N, Fowler BS, Tauxe RV. The epidemiology of appendicitis and appendectomy in the United States. Am J Epidemiol. 1990;132(5):910-925.
4. Hendahewa R, Shekhar A, Ratnayake S. The dilemma of stump appendicitis—a case report and literature review. Int J Surg. Case Rep. 2015;14:101-103. doi:10.1016/j.ijscr.2015.07.017.
5. Liang MK, Lo HG, Marks JL. Stump appendicitis: a comprehensive review of literature. Am Surg. 2006;72(2):162-166.
6. Parthsarathi R, Jankar SV, Chittawadgi B, et al. Laraposcopic management of symptomatic residual appendicular tip: a rare case report. J Minim Access Surg. 2017;13(2):154-156. doi:10.4103/0972-9941.199610.
7. Kanona H, Al Samaraee A, Nice C, Bhattacharya V. Stump appendicitis: a review. Int J Surg. 2012;10(9):425-428. doi:10.1016/j.ijsu.2012.07.007.
Acute appendicitis is a common condition emergency physicians (EPs) encounter in the ED, and it is also one of the most common general surgeries.1Although stump appendicitis is a rare, long-term complication of appendectomy, it should always be included in the differential diagnosis of patients presenting with right-sided abdominal pain and a history of appendectomy. Delays in diagnosing stump appendicitis can lead to perforation, gangrene, and sepsis.2
Case
A 33-year-old previously healthy man, whose medical history was significant for an appendectomy 6 months earlier, presented to the ED with progressive and worsening right lower quadrant abdominal pain that radiated to his right testicle. The patient stated that the pain started 3 days prior while he was lifting a bale of hay. He further noted having a fever of 102oF, nausea, and vomiting hours prior to his arrival at the ED.
Upon presentation, the patient’s vital signs were: heart rate, 89 beats/min; respiratory rate, 17 breaths/min; blood pressure, 132/84 mm Hg; and temperature, 98.9°F. Oxygen saturation was 98% on room air. Physical examination revealed exquisite tenderness in the right lower quadrant and suprapubic region. The testicular examination and the remainder of the physical examination were normal. Laboratory evaluation included a complete blood count and urinalysis, the results of which were significant for an elevated white blood cell count of 17 x 109/Lmicroscopic hematuria, trace leukocyte esterase, and ketones.
A computed tomography (CT) scan of the abdomen and pelvis with intravenous (IV) and oral contrast demonstrated a phlegmonous process surrounding the surgical site, which was concerning for stump appendicitis. The terminal ilium and colon were noted to be normal (Figures 1 and 2).
The patient was started on IV fluids and IV antibiotics, and received Zosyn in the ED. Surgical service was consulted, and the patient was admitted to the hospital where he continued nonoperative treatment with IV ciprofloxacin and metronidazole. The patient was discharged home on hospital day 3 without further complication. A repeat CT scan was taken of the abdomen and pelvis 3 weeks after discharge, and demonstrated complete resolution of the inflammatory process at the appendiceal stump with chronic scarring.
Discussion
Approximately 7% of patients who present to the ED with abdominal pain are diagnosed with appendicitis.3 Although appendectomy is one of the most common surgical procedures, stump appendicitis is a rare postsurgical complication, with a reported incidence of 1 in 50,000 cases.4,5
Stump appendicitis is an acute inflammation of the residual appendicular stump; the incidence of stump perforation is approximately 60% to 70%.4,6 Thus, stump appendicitis has a high morbidity and complication rate. Unfortunately, though stump appendicitis is a condition in which timely diagnosis and intervention are essential to prevent morbidity, due to its rarity and low occurrence, there is often a delay in diagnosis. It is therefore important that EPs include stump appendicitis in the differential diagnosis of patients presenting with right-sided abdominal pain and a history of appendectomy.
Stump appendicitis was initially described by Rose et al in 1945.2 This condition is underreported, and the exact causes are still unclear.Of the reported cases of stump appendicitis, approximately 66% developed following an open surgical appendectomy;5 therefore, complicated surgery or difficult dissection of the appendix is considered a risk factor for stump appendicitis. Conversely, adequate visualization of the appendiceal base during appendectomy and a stump measuring less than 3 to 5 mm1,4 are associated with a lower risk for stump appendicitis.
Stump appendicitis can develop as early as a few days postappendectomy or as late as 50 years postappendectomy. Patients with stump appendicitis present with signs and symptoms similar to that of acute appendicitis.2,4,7 Diagnosis can be made through ultrasound or CT studies, though CT is the preferred modality due to its higher specificity and ability to exclude other causes of right-sided abdominal pain.4
Management
Surgical intervention to remove the appendiceal stump is typically the preferred treatment. However, as with our patient, cases of successful and uncomplicated medical management have been reported.1,2,4
Conclusion
While stump appendicitis is rare, there has been a rise in the number of reported cases over the past few years due to the increasing use and availability of CT.4 The diagnosis of stump appendicitis is time-critical to prevent associated complications of stump perforation, gangrene, and sepsis. It is therefore imperative that EPs consider this
Acute appendicitis is a common condition emergency physicians (EPs) encounter in the ED, and it is also one of the most common general surgeries.1Although stump appendicitis is a rare, long-term complication of appendectomy, it should always be included in the differential diagnosis of patients presenting with right-sided abdominal pain and a history of appendectomy. Delays in diagnosing stump appendicitis can lead to perforation, gangrene, and sepsis.2
Case
A 33-year-old previously healthy man, whose medical history was significant for an appendectomy 6 months earlier, presented to the ED with progressive and worsening right lower quadrant abdominal pain that radiated to his right testicle. The patient stated that the pain started 3 days prior while he was lifting a bale of hay. He further noted having a fever of 102oF, nausea, and vomiting hours prior to his arrival at the ED.
Upon presentation, the patient’s vital signs were: heart rate, 89 beats/min; respiratory rate, 17 breaths/min; blood pressure, 132/84 mm Hg; and temperature, 98.9°F. Oxygen saturation was 98% on room air. Physical examination revealed exquisite tenderness in the right lower quadrant and suprapubic region. The testicular examination and the remainder of the physical examination were normal. Laboratory evaluation included a complete blood count and urinalysis, the results of which were significant for an elevated white blood cell count of 17 x 109/Lmicroscopic hematuria, trace leukocyte esterase, and ketones.
A computed tomography (CT) scan of the abdomen and pelvis with intravenous (IV) and oral contrast demonstrated a phlegmonous process surrounding the surgical site, which was concerning for stump appendicitis. The terminal ilium and colon were noted to be normal (Figures 1 and 2).
The patient was started on IV fluids and IV antibiotics, and received Zosyn in the ED. Surgical service was consulted, and the patient was admitted to the hospital where he continued nonoperative treatment with IV ciprofloxacin and metronidazole. The patient was discharged home on hospital day 3 without further complication. A repeat CT scan was taken of the abdomen and pelvis 3 weeks after discharge, and demonstrated complete resolution of the inflammatory process at the appendiceal stump with chronic scarring.
Discussion
Approximately 7% of patients who present to the ED with abdominal pain are diagnosed with appendicitis.3 Although appendectomy is one of the most common surgical procedures, stump appendicitis is a rare postsurgical complication, with a reported incidence of 1 in 50,000 cases.4,5
Stump appendicitis is an acute inflammation of the residual appendicular stump; the incidence of stump perforation is approximately 60% to 70%.4,6 Thus, stump appendicitis has a high morbidity and complication rate. Unfortunately, though stump appendicitis is a condition in which timely diagnosis and intervention are essential to prevent morbidity, due to its rarity and low occurrence, there is often a delay in diagnosis. It is therefore important that EPs include stump appendicitis in the differential diagnosis of patients presenting with right-sided abdominal pain and a history of appendectomy.
Stump appendicitis was initially described by Rose et al in 1945.2 This condition is underreported, and the exact causes are still unclear.Of the reported cases of stump appendicitis, approximately 66% developed following an open surgical appendectomy;5 therefore, complicated surgery or difficult dissection of the appendix is considered a risk factor for stump appendicitis. Conversely, adequate visualization of the appendiceal base during appendectomy and a stump measuring less than 3 to 5 mm1,4 are associated with a lower risk for stump appendicitis.
Stump appendicitis can develop as early as a few days postappendectomy or as late as 50 years postappendectomy. Patients with stump appendicitis present with signs and symptoms similar to that of acute appendicitis.2,4,7 Diagnosis can be made through ultrasound or CT studies, though CT is the preferred modality due to its higher specificity and ability to exclude other causes of right-sided abdominal pain.4
Management
Surgical intervention to remove the appendiceal stump is typically the preferred treatment. However, as with our patient, cases of successful and uncomplicated medical management have been reported.1,2,4
Conclusion
While stump appendicitis is rare, there has been a rise in the number of reported cases over the past few years due to the increasing use and availability of CT.4 The diagnosis of stump appendicitis is time-critical to prevent associated complications of stump perforation, gangrene, and sepsis. It is therefore imperative that EPs consider this
1. Shah T, Gupta RK, Karkee RJ, Agarwal CS. Recurrent pain abdomen following appendectomy: stump appendicitis, a surgeon’s dilemma. Clin Case Rep. 2017;5(3):215-217. doi:10.1002/ccr3.781.
2. Giwa A, Reyes M. Three times a charm…a case of repeat appendicitis status post two prior appendectomies. Am J Emerg Med. 2018;36(3):528.e1-528.e2. doi:10.1016/j.ajem.2017.12.024.
3. Addiss DG, Shaffer N, Fowler BS, Tauxe RV. The epidemiology of appendicitis and appendectomy in the United States. Am J Epidemiol. 1990;132(5):910-925.
4. Hendahewa R, Shekhar A, Ratnayake S. The dilemma of stump appendicitis—a case report and literature review. Int J Surg. Case Rep. 2015;14:101-103. doi:10.1016/j.ijscr.2015.07.017.
5. Liang MK, Lo HG, Marks JL. Stump appendicitis: a comprehensive review of literature. Am Surg. 2006;72(2):162-166.
6. Parthsarathi R, Jankar SV, Chittawadgi B, et al. Laraposcopic management of symptomatic residual appendicular tip: a rare case report. J Minim Access Surg. 2017;13(2):154-156. doi:10.4103/0972-9941.199610.
7. Kanona H, Al Samaraee A, Nice C, Bhattacharya V. Stump appendicitis: a review. Int J Surg. 2012;10(9):425-428. doi:10.1016/j.ijsu.2012.07.007.
1. Shah T, Gupta RK, Karkee RJ, Agarwal CS. Recurrent pain abdomen following appendectomy: stump appendicitis, a surgeon’s dilemma. Clin Case Rep. 2017;5(3):215-217. doi:10.1002/ccr3.781.
2. Giwa A, Reyes M. Three times a charm…a case of repeat appendicitis status post two prior appendectomies. Am J Emerg Med. 2018;36(3):528.e1-528.e2. doi:10.1016/j.ajem.2017.12.024.
3. Addiss DG, Shaffer N, Fowler BS, Tauxe RV. The epidemiology of appendicitis and appendectomy in the United States. Am J Epidemiol. 1990;132(5):910-925.
4. Hendahewa R, Shekhar A, Ratnayake S. The dilemma of stump appendicitis—a case report and literature review. Int J Surg. Case Rep. 2015;14:101-103. doi:10.1016/j.ijscr.2015.07.017.
5. Liang MK, Lo HG, Marks JL. Stump appendicitis: a comprehensive review of literature. Am Surg. 2006;72(2):162-166.
6. Parthsarathi R, Jankar SV, Chittawadgi B, et al. Laraposcopic management of symptomatic residual appendicular tip: a rare case report. J Minim Access Surg. 2017;13(2):154-156. doi:10.4103/0972-9941.199610.
7. Kanona H, Al Samaraee A, Nice C, Bhattacharya V. Stump appendicitis: a review. Int J Surg. 2012;10(9):425-428. doi:10.1016/j.ijsu.2012.07.007.
The Clinical Pathophysiology of Chronic Systemic Sclerosis
Systemic sclerosis (SSc), also called scleroderma, is a rare but serious autoimmune connective tissue disease that has multiple fluctuating pathologic manifestations throughout its temporal course. Estimates have shown that the incidence is 10 to 20 cases per 1 million, and the prevalence is 4 to 253 cases per 1 million.1,2 Given the rarity of this incurable condition, it is vital that primary care providers (PCPs) are able to recognize its unique features early to limit and prevent acute and chronic complications. This case report discusses a patient’s journey with late-diagnosed scleroderma in order to convey these broad manifestations and what providers can do to manage it with their patients.
Case Presentation
Mr. P is a 60-year-old African American male with a history of hypertension, recurrent digital ulcers, pulmonary hypertension (PH), interstitial lung disease (ILD), kidney involvement, congestive heart failure (CHF), and gastroesophageal reflux disease (GERD). Mr. P’s workup began in his late 40s with resistant hypertension, resistant GERD, and multiple hospitalizations for hypertensive urgency. It was not until he was 54 years old that he was diagnosed with mixed connective tissue disorder with sclerodermatous predominance.
Review of systems throughout his medical examinations in his 50s were notable for skin tightening over his hands and shoulders, skin hypopigmentation over his scalp and face, and hair loss. Mr. P was found to have Raynaud phenomenon beginning with his original presentation and digital ulceration without complications of gangrene or autoamputation. Aggregate physical examinations were notable for digital ulceration, skin tightening/sclerodactyly, and telangiectasia. Serologic markers were notable for the following:
- Positive ANA (antinuclear antibody) with titer of 1:1,280/homogenous pattern;
- Positive anti-RNP (antiribonucleoprotein) with titer of 171.2;
- Positive anti-Scl-70 (antitopoisomerase I) with titer of 108.1;
- Positive anti-SM (anti-Smith antibody) with titer of 30.2;
- Positive anti-Ro (SSA) with titer of 107.6;
- Negative anti-La (SSB) with titer of 1.3;
- Negative anti-dsDNA (anti-double stranded DNA) with titer of 9; and
- Negative ACA (anticentromere antibody).
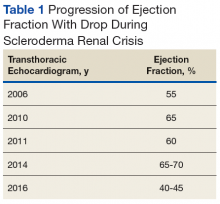
Early transthoracic echocardiograms revealed an ejection fraction (EF) initially at 55% with evidence of left ventricular hypertrophy. Following treatment with phosphodiesterase-5 inhibitors (PDE-5 inhibitors) and endothelin-1 antagonists for pulmonary hypertension, serial transthoracic echocardiograms showed improvement in his EF.
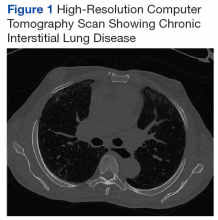
A chest X-ray did not show signs of ILD, but a subsequent high-resolution computed tomography (HRCT) scan was consistent with chronic ILD with a main pulmonary artery diameter of 3 cm (Figure 1).
In subsequent years, Mr. P was hospitalized several times, secondary to digit pain, ulcerations, and osteomyelitis. His first episode was 1 year after his scleroderma diagnosis, when he was hospitalized for 6 days for complications of SSc and finger pain. The following year, he had a 3-day hospitalization for hypertensive urgency and right third-digit osteomyelitis, treated initially with IV fluids, levofloxacin, and vancomycin, and then ceftaroline for 1 month. Throughout the next 6 years, Mr. P presented multiple times with fingertip ulcerations and was followed in the Infectious Disease clinic for recurrent osteomyelitis. He found some relief with systemic antibiotics, including augmentin, minocycline, moxifloxacin, and doxycycline.
At age 59, he was hospitalized for scleroderma renal crisis (SRC). Early in his disease, his kidney function was normal, but the SRC was discovered after an abrupt rise in his blood pressure (BP) and an increase in serum creatinine (SCr) from 1.2 mg/dL at baseline to 3.06 mg/dL. The presence of brown granular cast in his urine prompted a renal biopsy that showed thrombotic microangiopathy with schistocytes. Mr. P was started on captopril and remained stable with outpatient follow-up for this renal complication.
Discussion
Initial presentation of SSc can occur along a spectrum of its pathophysiology. A more severe presentation, like the one seen in Mr. P, seems to occur more frequently in African American patients relative to white patients.3 Differentiating between the 2 types—diffuse vs limited SSc—is vital to managing patients and disease progression. Limited-type SSc is more common (60%) and less severe with slower progression than is diffuse SSc.
Diffuse-type SSc (35%) includes features such as skin thickening and tightening, ILD, SRC, tendon friction rubs (palpable crepitus over tendons), and skin pigment changes.4 The specific involvement of the renal and cardiopulmonary systems accounts for the higher mortality rates in the diffuse-type.5
Many patients with SSc require periodic hospitalizations throughout their life for the acute complications of the disease. Hospitalized patients often range from age 45 to 64 years and are more often female. However, of hospitalized patients with SSc, in-hospital death rates are higher among men.3,6 Although these rates have decreased as the pathogenesis of SSc has become better understood, it is important to note that in-hospital mortality in 1995 for all patients with SSc was 7.1% and mean length of stay was 7.5 days, and in 2002 to 2003, 6.3% and 6.6 days, respectively.3 Though the burden of this disease has decreased, mortality and hospitalizations continue to persist at high rates. Understanding the pathogenesis, progression, and treatments of SSc are essential to aiding patients with this diagnosis.
Skin Involvement
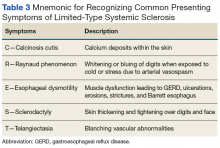
A common finding and presentation for patients with SSc is related to skin involvement. Common patient complaints and exam findings include calcinosis along extensor tendons and digits, Raynaud phenomenon (seen in more than 95% of patients), sclerodactyly, telangiectasias, hyper/hypopigmentation, and pruritus.4 These findings are useful in diagnosing and monitoring patients for disease progression.
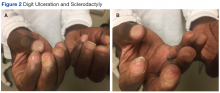
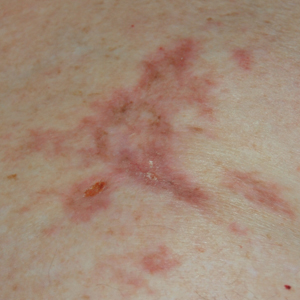
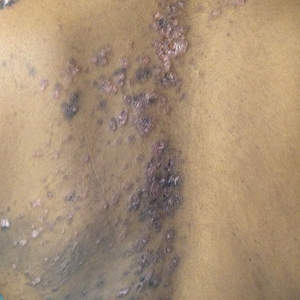
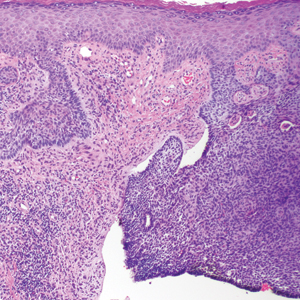
Many of the listed skin manifestations affect patients’ quality of life (QOL) but are not directly associated with mortality. However, a common and feared complication includes skin ulcers and osteomyelitis, seen in 48% and 7.7% of patients, respectively.7 Digit ulcers, areas with loss of dermis and epidermis distal to the proximal interphalangeal joints (Figures 2A and 2B), are significant because they parallel a more rapid progression of internal organ involvement.8
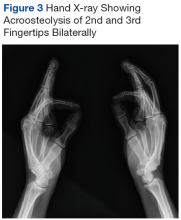
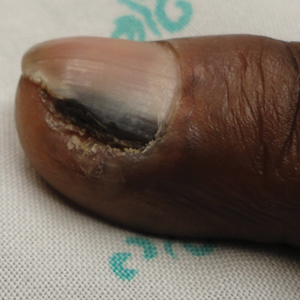
Mr. P required multiple hospitalizations and antibiotic regimens for painful digit ulcers complicated by osteomyelitis (Figure 3).
Treatment usually is aimed at infections that complicate these skin ulcers and are based on site-specific cultures. Preventive measures are aimed at the risk factors associated with digit ulcers, including decreased whole-body warmth, direct trauma to digits, smoking, and vasoconstrictors (eg, cocaine, sympathomimetics).8 Some patients may prevent ulcers by using D-penicillamine, mycophenolate mofetil, and cyclophosphamide, although definitive treatment has not been found.4 Calcium channel blockers, PDE-5 inhibitors, endothelin receptor antagonists, and prostacyclin analogues also have been used to reduce the severity of Raynaud phenomenon attacks and to decrease the number of digital ulcers (in addition to their beneficial effects on pulmonary involvement).8 Additional pharmacologic agents that have been linked to an improvement in Raynaud phenomenon and digital ulcers include statins, angiotensin-converting enzyme (ACE) inhibitors, angiotensin receptor blockers, intravenous N-acetylcysteine, vitamin E gel, and surgical options (eg, revascularization and sympathectomy).8
Pulmonary Involvement
Systemic sclerosis can lead to 2 complications in the lungs, both present in Mr. P: PAH (mean pulmonary arterial pressure > 25 mm Hg) and ILD, which together make lung involvement the leading cause of death for these patients. Limited-type SSc usually is restricted to PAH, whereas diffuse-type SSc usually leads to ILD.4
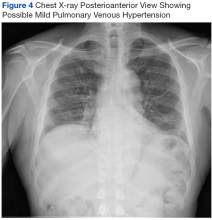
On diagnosis of SSc, an HRCT is indicated to assess the degree of lung involvement. Mr. P’s HRCT showed a 3-cm main pulmonary artery, suggestive of PAH, in addition to evidence of ILD seen on the chest X-ray (Figure 4).
Pulmonary arterial hypertension is a common finding in patients with SSc and carries a severe prognosis. Risk factors for the development of PAH, when not present on initial diagnosis, include limited-type SSc; late age of onset; Raynaud phenomenon; decreased DLCO, FVC/DLCO < 1.6; increased N-terminal pro-B-type natriuretic peptide (NT-proBNP) serum levels; and the presence of antibodies.9 Patients with both SSc and PAH have a 50% to 87% 1-year survival, whereas patients with idiopathic PAH without connective tissue disease have an 88% 1-year survival.9
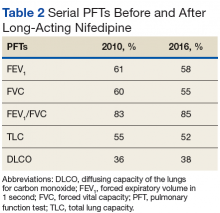
Many patients with lung involvement are asymptomatic, but some have findings of crackles and interstitial thickening on chest X-ray that can progress to cyanosis and right heart failure (cor pulmonale).10 On discovery of lung involvement (Figure 5), it is important to follow up with a PFT, primarily because the outcomes and prognosis for patients with SSc are correlated with the presenting severity of ILD and the subsequent progression of their DLCO.4,10
Treatment is directed at PAH and ILD separately. For PAH, a PDE-5 inhibitor, such as tadalafil or sildenafil, and an endothelin-1 receptor antagonist, such as ambrisentan or bosentan, are indicated. Other PAH treatments include diuretics and prostacyclin analogues (eg, epoprostenol, treprostinil, or iloprost) in addition to warfarin if patients have a history of thrombotic events.4 Mr. P’s pulmonologists deferred treatment with prostacyclin analogues given the potential for adverse effects with endothelin-1 receptor inhibitors, PDE-5 inhibitors, and calcium-channel blockers, although combination studies with bosentan and inhaled iloprost have shown promise.11
Like the skin manifestations of SSc, ILD lacks definitive treatment to prevent disease progression. However, some patients benefit from cyclophosphamide, followed by mycophenolate mofetil—which is usually better tolerated—azathioprine, haematopoietic stem cell transplant as rescue therapy, and lung transplant for life-saving treatment.10
Renal Involvement
Renal involvement in patients with SSc can have profound effects on QOL. Even without clinical renal involvement, glomerular filtration rate (GFR) usually decreases with the progression of vascular damage correlated with age and disease duration.12 Patients with a history of digital ulcers, like Mr. P, usually have a lower GFR than that of patients without digital ulcers.12 Monitoring renal function is vital in caring for these patients, because SRC occurs in 2% to 15% of all patients with SSc.13,14
Scleroderma renal crisis is generally defined as an abruptly elevated BP (> 140/90 mm Hgor > 30 mm Hg rise from baseline) with acute renal failure (elevated SCr) and decreased urine output.14 Given the rarity of SSc in the population, diagnosis of SRC requires high clinical suspicion. In the case of Mr. P, a workup involving serum analysis, urinalysis, and renal biopsy allowed for a definitive diagnosis. A renal biopsy can show microangiopathic hemolytic anemia and confirm SRC, although it may not be necessary in patients with known SSc presenting with new hypertension, rising creatinine, and unremarkable urine sediment on microscopy.14,15
Although an acute SRC can be difficult to predict, monitoring renal function and attention to key factors can assist in discovering this SSc complication. Scleroderma renal crisis usually occurs within the first 4 years of SSc diagnosis, often paralleling rapid progression of skin thickening and tightening with higher rates in both African Americans and males.13,14 Additional predictive factors include diffuse skin involvement, rapid progression of skin involvement, positive anti-RNA polymerase III antibodies, new anemia, new cardiac events (eg, pericardial effusion, pericarditis, left ventricular insufficiency), CHF, tendon friction rubs, arthritis, and recent (within 3 months) high-dose glucocorticoid use.4,13-15
The presentation of SRC can be nonspecific, often resembling findings related to acute kidney injury. Patients may report malaise, fatigue, fever, headache, seizure, blurred vision, or dyspnea.13 Clinical parametersto examine include systolic BP > 140 mm Hgand/or diastolic BP > 90 mm Hg (or an abrupt rise of > 30 or > 20, respectively), SCr increase by 50%+ or > 120% of the upper normal limit, proteinuria at 2+, high protein:creatinine ratio, hematuria > 2+ or > 10 red blood cells (RBCs), platelets < 100,000/mm3, and hypertensive encephalopathy.13 Mr. P presented with fatigue, dyspnea, an abrupt rise in BP (156/96 mm Hg), foamy urine, bilateral lower extremity edema (3.9 g/dL albumin), 2+ RBCs, and a SCr of 2.46 mg/dL on admission (a 205% increase from his last baseline of 1.2 mg/dL).
Treatments have had a large impact on the mortality rates of SRC. Following the introduction of ACE inhibitors, mortality from SRC has decreased from 76% to < 10% over recent decades.13 In addition to improving survival, these medications also have improved hospitalization outcomes for these patients.3 Captopril is often the medication of choice given its rapid onset and short duration of action relative to other medications in the same class, such as benazepril, enalapril, fosinopril, lisinopril, quinapril, and ramipril.4,13 Current clinical trials are assessing the specific renal benefits of endothelin-1 receptor antagonists, which often are used for pulmonary and skin involvement.14 For patients presenting with acute SRC and uremia, dialysis may be necessary (typically predicted by elevated NT-proBNP serum levels), while the decision for transplantation may be indicated at least 2 years after SRC onset and resolution.4,13-15
Once the acute crisis resolves, it is important to discuss prognosis with patients. The 1-, 2-, 3-, 5-, and 10-year survival rates are 82%, 74%, 71%, 59%, and 47%, respectively; however, when looking at only male patients at 10 years, the survival rate is 17%.13 Prevention of SRC can be addressed with daily BP checks and advising patients to seek medical care if they notice a consecutive 2-day abrupt rise.13
Cardiac Involvement
Systemic sclerosis has significant effects on patients’ heart function. The introduction of ACE inhibitors has shifted mortality in SSc patients from predominantly SRC to cardiac causes.6 Cardiac involvement can occur through a range of processes, including abnormal cardiac conduction, CHF, diastolic dysfunction, mitral valve nodular thickening, and pericardial effusion.3 Even though patients often present with skin findings, an initial cardiac workup is crucial to understanding disease progression and patient prognosis. The severity of the cutaneous manifestations often predicts the degree of diastolic dysfunction.16
Clinical evidence of cardiac involvement is seen in 20% to 25% of patients with SSc and is associated with a 70% mortality at 5 years when symptoms are evident.16 Additionally, right ventricular dysfunction at presentation is the strongest marker for all-mortality prognosis, representing the degree of pulmonary involvement, and includes findings such as progressive shortness of breath and systemic edema.9
Given the increasing survival of patients with SSc, cardiac involvement is becoming more evident and prominent. Direct treatments for cardiac manifestations are based on the causative feature, namely, focusing on pulmonary and renal involvement, which can be assessed with periodic echocardiograms evaluating left ventricular EF.
Gastrointestinal Involvement
Along with skin manifestations, the gastrointestinal (GI) involvement of SSc can have a significant impact on patients’ QOL without direct contribution to mortality. One of Mr. P’s earliest symptoms that led to a diagnosis of SSc was GERD, which caused a chronic cough, dental erosions, esophageal erosions, duodenal ulcers, dysphagia, abdominal pain, halitosis, pharyngitis, and weight loss. Esophageal involvement occurs in up to 96% of patients with SSc and can include motility abnormalities (eg, strictures and/or muscle dysfunction), lower esophageal sphincter abnormalities, and Barrett esophagus.17
Additional symptoms of the GI system linked with SSc can occur anywhere along the GI tract and include gastric antral vascular ectasia, causing GI bleeds and pernicious anemia, gastroparesis, bacterial overgrowth, intestinal malabsorption, pseudo-obstruction due to hypomotility, fecal incontinence due to anorectal involvement, and rarely, primary biliary cirrhosis.4,17 Decreased mobility of the oral aperture secondary to skin thickening and tightening also can contribute to malnutrition by decreasing oral intake.
Treatments are supportive and target symptom relief. Chronic treatment of GERD is often necessary and includes antacids, histamine-2 receptor blockers, and proton pump inhibitors.4 Other medications that can help with symptom relief include motility agents (such as metoclopramide, domperidone, prucalopride, tegaserod, and macrolides), osmotic laxatives, and ursodeoxycholic acid for primary biliary cirrhosis.17 Surgical intervention should be considered depending on the severity and progression of involvement within the GI tract. Behavioral changes that can improve patient symptoms include facial grimacing and other mouth-stretching exercises, frequent smaller meals followed by maintaining a vertical posture, and high fiber diets.17
Conclusion
Systemic sclerosis is an autoimmune and connective tissue disease with a pathophysiology that can manifest throughout the body. The organ systems that impact patient outcomes include skin, pulmonary, renal, cardiac, and GI. Primary care providers caring for patients diagnosed with SSc should monitor acute management and disease progression in all these systems. Important acute events that can impact morbidity, mortality, and/or QOL include Raynaud phenomenon, SRC, and pericardial effusion. Chronic manifestations that may be present on diagnosis of SSc or may develop while a patient is under a provider’s care include sclerodactyly, tendon calcinosis, PAH, ILD, chronic kidney injury, chronic cardiac damage, GERD, and esophageal dysmotility. While this discussion serves as a pertinent overview of patients with SSc, it is summative, and providers are encouraged to seek a stronger understanding of both the common and rarer manifestations within each of their patients.
1. Lawrence RC, Helmick CG, Arnett FC, et al. Estimates of the prevalence of arthritis and selected musculoskeletal disorders in the United States. Arthritis Rheum. 1998;41(5):778-799.
2. Silman AG. Scleroderma. In: Epidemiology of the Rheumatic Diseases. 2nd ed. Silman AJ, Hochberg MC, eds. Oxford, UK: Oxford University Press. 1993:chap 8.
3. Chung L, Krishnan E, Chakravarty EF. Hospitalizations and mortality in systemic sclerosis: results from the Nationwide Inpatient Sample. Rheumatology (Oxford). 2007;46(12):1808-1813.
4. Hinchcliff M, Varga J. Systemic sclerosis/scleroderma: a treatable multisystem disease. Am Fam Physician. 2008;78(8):961-968.
5. Tyndall AJ, Bannert B, Vonk M, et al. Causes and risk factors for death in systemic sclerosis: a study from the EULAR Scleroderma Trials and Research (EUSTAR) database. Ann Rheum Dis. 2010;69(10):1809-1815.
6. Piga M, Casula L, Sanna S, et al. Population-based analysis of hospitalizations for patients with systemic sclerosis in a West-European region over the period 2001-2012. Rheumatol Int. 2016;36(1):73-81.
7. Giuggioli D, Manfredi A, Colaci M, Lumetti F, Ferri C. Osteomyelitis complicating scleroderma digital ulcers. Clin Rheumatol. 2013;32(5):623-627.
8. Schiopu E, Impens AJ, Phillips K. Digital ischemia in scleroderma spectrum of diseases. Int J Rheumatol. 2010;2010:923743.
9. Hassoun PM. Therapies for scleroderma-related pulmonary arterial hypertension. Expert Rev Respir Med. 2009;3(2):187-196.
10. Giacomelli R, Liakouli V, Berardicurti O, et al. Interstitial lung disease in systemic sclerosis: current and future treatment. Rheumatol Int. 2017;37(6):853-863.
11. McLaughlin V, Humbert M, Coghlan G, Nash P, Steen V. Pulmonary arterial hypertension: the most devastating vascular complication of systemic sclerosis. Rheumatology (Oxford). 2009; (suppl 3):iii25-iii31.
12. Gigante A, Barbano B, Granata G, et al. Evaluation of estimated glomerular filtration rate and clinical variables in systemic sclerosis patients. Clin Nephrol. 2016;85(6):326-331.
13. Bose N, Chiesa-Vottero A, Chatterjee S. Scleroderma renal crisis. Semin Arthritis Rheum. 2015;44(6):687-694.
14. Woodworth TG, Suliman YA, Furst DE, Clements P. Scleroderma renal crisis and renal involvement in systemic sclerosis. Nat Rev Nephrol. 2016;12(11):678-691.
15. Mouthon L, Bérezné A, Bussone G, Noël LH, Villiger PM, Guillevin L. Scleroderma renal crisis: a rare but severe complication of systemic sclerosis. Clin Rev Allergy Immunol. 2011;40(2):84-91.
16. Champion HC. The heart in scleroderma. Rheum Dis Clin North Am. 2008;34(1):181-90.
17. Tian XP, Zhang X. Gastrointestinal complications of systemic sclerosis. World J Gastroenterol. 2013;19(41):7062-7068.
Systemic sclerosis (SSc), also called scleroderma, is a rare but serious autoimmune connective tissue disease that has multiple fluctuating pathologic manifestations throughout its temporal course. Estimates have shown that the incidence is 10 to 20 cases per 1 million, and the prevalence is 4 to 253 cases per 1 million.1,2 Given the rarity of this incurable condition, it is vital that primary care providers (PCPs) are able to recognize its unique features early to limit and prevent acute and chronic complications. This case report discusses a patient’s journey with late-diagnosed scleroderma in order to convey these broad manifestations and what providers can do to manage it with their patients.
Case Presentation
Mr. P is a 60-year-old African American male with a history of hypertension, recurrent digital ulcers, pulmonary hypertension (PH), interstitial lung disease (ILD), kidney involvement, congestive heart failure (CHF), and gastroesophageal reflux disease (GERD). Mr. P’s workup began in his late 40s with resistant hypertension, resistant GERD, and multiple hospitalizations for hypertensive urgency. It was not until he was 54 years old that he was diagnosed with mixed connective tissue disorder with sclerodermatous predominance.
Review of systems throughout his medical examinations in his 50s were notable for skin tightening over his hands and shoulders, skin hypopigmentation over his scalp and face, and hair loss. Mr. P was found to have Raynaud phenomenon beginning with his original presentation and digital ulceration without complications of gangrene or autoamputation. Aggregate physical examinations were notable for digital ulceration, skin tightening/sclerodactyly, and telangiectasia. Serologic markers were notable for the following:
- Positive ANA (antinuclear antibody) with titer of 1:1,280/homogenous pattern;
- Positive anti-RNP (antiribonucleoprotein) with titer of 171.2;
- Positive anti-Scl-70 (antitopoisomerase I) with titer of 108.1;
- Positive anti-SM (anti-Smith antibody) with titer of 30.2;
- Positive anti-Ro (SSA) with titer of 107.6;
- Negative anti-La (SSB) with titer of 1.3;
- Negative anti-dsDNA (anti-double stranded DNA) with titer of 9; and
- Negative ACA (anticentromere antibody).
Early transthoracic echocardiograms revealed an ejection fraction (EF) initially at 55% with evidence of left ventricular hypertrophy. Following treatment with phosphodiesterase-5 inhibitors (PDE-5 inhibitors) and endothelin-1 antagonists for pulmonary hypertension, serial transthoracic echocardiograms showed improvement in his EF.
A chest X-ray did not show signs of ILD, but a subsequent high-resolution computed tomography (HRCT) scan was consistent with chronic ILD with a main pulmonary artery diameter of 3 cm (Figure 1).
In subsequent years, Mr. P was hospitalized several times, secondary to digit pain, ulcerations, and osteomyelitis. His first episode was 1 year after his scleroderma diagnosis, when he was hospitalized for 6 days for complications of SSc and finger pain. The following year, he had a 3-day hospitalization for hypertensive urgency and right third-digit osteomyelitis, treated initially with IV fluids, levofloxacin, and vancomycin, and then ceftaroline for 1 month. Throughout the next 6 years, Mr. P presented multiple times with fingertip ulcerations and was followed in the Infectious Disease clinic for recurrent osteomyelitis. He found some relief with systemic antibiotics, including augmentin, minocycline, moxifloxacin, and doxycycline.
At age 59, he was hospitalized for scleroderma renal crisis (SRC). Early in his disease, his kidney function was normal, but the SRC was discovered after an abrupt rise in his blood pressure (BP) and an increase in serum creatinine (SCr) from 1.2 mg/dL at baseline to 3.06 mg/dL. The presence of brown granular cast in his urine prompted a renal biopsy that showed thrombotic microangiopathy with schistocytes. Mr. P was started on captopril and remained stable with outpatient follow-up for this renal complication.
Discussion
Initial presentation of SSc can occur along a spectrum of its pathophysiology. A more severe presentation, like the one seen in Mr. P, seems to occur more frequently in African American patients relative to white patients.3 Differentiating between the 2 types—diffuse vs limited SSc—is vital to managing patients and disease progression. Limited-type SSc is more common (60%) and less severe with slower progression than is diffuse SSc.
Diffuse-type SSc (35%) includes features such as skin thickening and tightening, ILD, SRC, tendon friction rubs (palpable crepitus over tendons), and skin pigment changes.4 The specific involvement of the renal and cardiopulmonary systems accounts for the higher mortality rates in the diffuse-type.5
Many patients with SSc require periodic hospitalizations throughout their life for the acute complications of the disease. Hospitalized patients often range from age 45 to 64 years and are more often female. However, of hospitalized patients with SSc, in-hospital death rates are higher among men.3,6 Although these rates have decreased as the pathogenesis of SSc has become better understood, it is important to note that in-hospital mortality in 1995 for all patients with SSc was 7.1% and mean length of stay was 7.5 days, and in 2002 to 2003, 6.3% and 6.6 days, respectively.3 Though the burden of this disease has decreased, mortality and hospitalizations continue to persist at high rates. Understanding the pathogenesis, progression, and treatments of SSc are essential to aiding patients with this diagnosis.
Skin Involvement
A common finding and presentation for patients with SSc is related to skin involvement. Common patient complaints and exam findings include calcinosis along extensor tendons and digits, Raynaud phenomenon (seen in more than 95% of patients), sclerodactyly, telangiectasias, hyper/hypopigmentation, and pruritus.4 These findings are useful in diagnosing and monitoring patients for disease progression.
Many of the listed skin manifestations affect patients’ quality of life (QOL) but are not directly associated with mortality. However, a common and feared complication includes skin ulcers and osteomyelitis, seen in 48% and 7.7% of patients, respectively.7 Digit ulcers, areas with loss of dermis and epidermis distal to the proximal interphalangeal joints (Figures 2A and 2B), are significant because they parallel a more rapid progression of internal organ involvement.8
Mr. P required multiple hospitalizations and antibiotic regimens for painful digit ulcers complicated by osteomyelitis (Figure 3).
Treatment usually is aimed at infections that complicate these skin ulcers and are based on site-specific cultures. Preventive measures are aimed at the risk factors associated with digit ulcers, including decreased whole-body warmth, direct trauma to digits, smoking, and vasoconstrictors (eg, cocaine, sympathomimetics).8 Some patients may prevent ulcers by using D-penicillamine, mycophenolate mofetil, and cyclophosphamide, although definitive treatment has not been found.4 Calcium channel blockers, PDE-5 inhibitors, endothelin receptor antagonists, and prostacyclin analogues also have been used to reduce the severity of Raynaud phenomenon attacks and to decrease the number of digital ulcers (in addition to their beneficial effects on pulmonary involvement).8 Additional pharmacologic agents that have been linked to an improvement in Raynaud phenomenon and digital ulcers include statins, angiotensin-converting enzyme (ACE) inhibitors, angiotensin receptor blockers, intravenous N-acetylcysteine, vitamin E gel, and surgical options (eg, revascularization and sympathectomy).8
Pulmonary Involvement
Systemic sclerosis can lead to 2 complications in the lungs, both present in Mr. P: PAH (mean pulmonary arterial pressure > 25 mm Hg) and ILD, which together make lung involvement the leading cause of death for these patients. Limited-type SSc usually is restricted to PAH, whereas diffuse-type SSc usually leads to ILD.4
On diagnosis of SSc, an HRCT is indicated to assess the degree of lung involvement. Mr. P’s HRCT showed a 3-cm main pulmonary artery, suggestive of PAH, in addition to evidence of ILD seen on the chest X-ray (Figure 4).
Pulmonary arterial hypertension is a common finding in patients with SSc and carries a severe prognosis. Risk factors for the development of PAH, when not present on initial diagnosis, include limited-type SSc; late age of onset; Raynaud phenomenon; decreased DLCO, FVC/DLCO < 1.6; increased N-terminal pro-B-type natriuretic peptide (NT-proBNP) serum levels; and the presence of antibodies.9 Patients with both SSc and PAH have a 50% to 87% 1-year survival, whereas patients with idiopathic PAH without connective tissue disease have an 88% 1-year survival.9
Many patients with lung involvement are asymptomatic, but some have findings of crackles and interstitial thickening on chest X-ray that can progress to cyanosis and right heart failure (cor pulmonale).10 On discovery of lung involvement (Figure 5), it is important to follow up with a PFT, primarily because the outcomes and prognosis for patients with SSc are correlated with the presenting severity of ILD and the subsequent progression of their DLCO.4,10
Treatment is directed at PAH and ILD separately. For PAH, a PDE-5 inhibitor, such as tadalafil or sildenafil, and an endothelin-1 receptor antagonist, such as ambrisentan or bosentan, are indicated. Other PAH treatments include diuretics and prostacyclin analogues (eg, epoprostenol, treprostinil, or iloprost) in addition to warfarin if patients have a history of thrombotic events.4 Mr. P’s pulmonologists deferred treatment with prostacyclin analogues given the potential for adverse effects with endothelin-1 receptor inhibitors, PDE-5 inhibitors, and calcium-channel blockers, although combination studies with bosentan and inhaled iloprost have shown promise.11
Like the skin manifestations of SSc, ILD lacks definitive treatment to prevent disease progression. However, some patients benefit from cyclophosphamide, followed by mycophenolate mofetil—which is usually better tolerated—azathioprine, haematopoietic stem cell transplant as rescue therapy, and lung transplant for life-saving treatment.10
Renal Involvement
Renal involvement in patients with SSc can have profound effects on QOL. Even without clinical renal involvement, glomerular filtration rate (GFR) usually decreases with the progression of vascular damage correlated with age and disease duration.12 Patients with a history of digital ulcers, like Mr. P, usually have a lower GFR than that of patients without digital ulcers.12 Monitoring renal function is vital in caring for these patients, because SRC occurs in 2% to 15% of all patients with SSc.13,14
Scleroderma renal crisis is generally defined as an abruptly elevated BP (> 140/90 mm Hgor > 30 mm Hg rise from baseline) with acute renal failure (elevated SCr) and decreased urine output.14 Given the rarity of SSc in the population, diagnosis of SRC requires high clinical suspicion. In the case of Mr. P, a workup involving serum analysis, urinalysis, and renal biopsy allowed for a definitive diagnosis. A renal biopsy can show microangiopathic hemolytic anemia and confirm SRC, although it may not be necessary in patients with known SSc presenting with new hypertension, rising creatinine, and unremarkable urine sediment on microscopy.14,15
Although an acute SRC can be difficult to predict, monitoring renal function and attention to key factors can assist in discovering this SSc complication. Scleroderma renal crisis usually occurs within the first 4 years of SSc diagnosis, often paralleling rapid progression of skin thickening and tightening with higher rates in both African Americans and males.13,14 Additional predictive factors include diffuse skin involvement, rapid progression of skin involvement, positive anti-RNA polymerase III antibodies, new anemia, new cardiac events (eg, pericardial effusion, pericarditis, left ventricular insufficiency), CHF, tendon friction rubs, arthritis, and recent (within 3 months) high-dose glucocorticoid use.4,13-15
The presentation of SRC can be nonspecific, often resembling findings related to acute kidney injury. Patients may report malaise, fatigue, fever, headache, seizure, blurred vision, or dyspnea.13 Clinical parametersto examine include systolic BP > 140 mm Hgand/or diastolic BP > 90 mm Hg (or an abrupt rise of > 30 or > 20, respectively), SCr increase by 50%+ or > 120% of the upper normal limit, proteinuria at 2+, high protein:creatinine ratio, hematuria > 2+ or > 10 red blood cells (RBCs), platelets < 100,000/mm3, and hypertensive encephalopathy.13 Mr. P presented with fatigue, dyspnea, an abrupt rise in BP (156/96 mm Hg), foamy urine, bilateral lower extremity edema (3.9 g/dL albumin), 2+ RBCs, and a SCr of 2.46 mg/dL on admission (a 205% increase from his last baseline of 1.2 mg/dL).
Treatments have had a large impact on the mortality rates of SRC. Following the introduction of ACE inhibitors, mortality from SRC has decreased from 76% to < 10% over recent decades.13 In addition to improving survival, these medications also have improved hospitalization outcomes for these patients.3 Captopril is often the medication of choice given its rapid onset and short duration of action relative to other medications in the same class, such as benazepril, enalapril, fosinopril, lisinopril, quinapril, and ramipril.4,13 Current clinical trials are assessing the specific renal benefits of endothelin-1 receptor antagonists, which often are used for pulmonary and skin involvement.14 For patients presenting with acute SRC and uremia, dialysis may be necessary (typically predicted by elevated NT-proBNP serum levels), while the decision for transplantation may be indicated at least 2 years after SRC onset and resolution.4,13-15
Once the acute crisis resolves, it is important to discuss prognosis with patients. The 1-, 2-, 3-, 5-, and 10-year survival rates are 82%, 74%, 71%, 59%, and 47%, respectively; however, when looking at only male patients at 10 years, the survival rate is 17%.13 Prevention of SRC can be addressed with daily BP checks and advising patients to seek medical care if they notice a consecutive 2-day abrupt rise.13
Cardiac Involvement
Systemic sclerosis has significant effects on patients’ heart function. The introduction of ACE inhibitors has shifted mortality in SSc patients from predominantly SRC to cardiac causes.6 Cardiac involvement can occur through a range of processes, including abnormal cardiac conduction, CHF, diastolic dysfunction, mitral valve nodular thickening, and pericardial effusion.3 Even though patients often present with skin findings, an initial cardiac workup is crucial to understanding disease progression and patient prognosis. The severity of the cutaneous manifestations often predicts the degree of diastolic dysfunction.16
Clinical evidence of cardiac involvement is seen in 20% to 25% of patients with SSc and is associated with a 70% mortality at 5 years when symptoms are evident.16 Additionally, right ventricular dysfunction at presentation is the strongest marker for all-mortality prognosis, representing the degree of pulmonary involvement, and includes findings such as progressive shortness of breath and systemic edema.9
Given the increasing survival of patients with SSc, cardiac involvement is becoming more evident and prominent. Direct treatments for cardiac manifestations are based on the causative feature, namely, focusing on pulmonary and renal involvement, which can be assessed with periodic echocardiograms evaluating left ventricular EF.
Gastrointestinal Involvement
Along with skin manifestations, the gastrointestinal (GI) involvement of SSc can have a significant impact on patients’ QOL without direct contribution to mortality. One of Mr. P’s earliest symptoms that led to a diagnosis of SSc was GERD, which caused a chronic cough, dental erosions, esophageal erosions, duodenal ulcers, dysphagia, abdominal pain, halitosis, pharyngitis, and weight loss. Esophageal involvement occurs in up to 96% of patients with SSc and can include motility abnormalities (eg, strictures and/or muscle dysfunction), lower esophageal sphincter abnormalities, and Barrett esophagus.17
Additional symptoms of the GI system linked with SSc can occur anywhere along the GI tract and include gastric antral vascular ectasia, causing GI bleeds and pernicious anemia, gastroparesis, bacterial overgrowth, intestinal malabsorption, pseudo-obstruction due to hypomotility, fecal incontinence due to anorectal involvement, and rarely, primary biliary cirrhosis.4,17 Decreased mobility of the oral aperture secondary to skin thickening and tightening also can contribute to malnutrition by decreasing oral intake.
Treatments are supportive and target symptom relief. Chronic treatment of GERD is often necessary and includes antacids, histamine-2 receptor blockers, and proton pump inhibitors.4 Other medications that can help with symptom relief include motility agents (such as metoclopramide, domperidone, prucalopride, tegaserod, and macrolides), osmotic laxatives, and ursodeoxycholic acid for primary biliary cirrhosis.17 Surgical intervention should be considered depending on the severity and progression of involvement within the GI tract. Behavioral changes that can improve patient symptoms include facial grimacing and other mouth-stretching exercises, frequent smaller meals followed by maintaining a vertical posture, and high fiber diets.17
Conclusion
Systemic sclerosis is an autoimmune and connective tissue disease with a pathophysiology that can manifest throughout the body. The organ systems that impact patient outcomes include skin, pulmonary, renal, cardiac, and GI. Primary care providers caring for patients diagnosed with SSc should monitor acute management and disease progression in all these systems. Important acute events that can impact morbidity, mortality, and/or QOL include Raynaud phenomenon, SRC, and pericardial effusion. Chronic manifestations that may be present on diagnosis of SSc or may develop while a patient is under a provider’s care include sclerodactyly, tendon calcinosis, PAH, ILD, chronic kidney injury, chronic cardiac damage, GERD, and esophageal dysmotility. While this discussion serves as a pertinent overview of patients with SSc, it is summative, and providers are encouraged to seek a stronger understanding of both the common and rarer manifestations within each of their patients.
Systemic sclerosis (SSc), also called scleroderma, is a rare but serious autoimmune connective tissue disease that has multiple fluctuating pathologic manifestations throughout its temporal course. Estimates have shown that the incidence is 10 to 20 cases per 1 million, and the prevalence is 4 to 253 cases per 1 million.1,2 Given the rarity of this incurable condition, it is vital that primary care providers (PCPs) are able to recognize its unique features early to limit and prevent acute and chronic complications. This case report discusses a patient’s journey with late-diagnosed scleroderma in order to convey these broad manifestations and what providers can do to manage it with their patients.
Case Presentation
Mr. P is a 60-year-old African American male with a history of hypertension, recurrent digital ulcers, pulmonary hypertension (PH), interstitial lung disease (ILD), kidney involvement, congestive heart failure (CHF), and gastroesophageal reflux disease (GERD). Mr. P’s workup began in his late 40s with resistant hypertension, resistant GERD, and multiple hospitalizations for hypertensive urgency. It was not until he was 54 years old that he was diagnosed with mixed connective tissue disorder with sclerodermatous predominance.
Review of systems throughout his medical examinations in his 50s were notable for skin tightening over his hands and shoulders, skin hypopigmentation over his scalp and face, and hair loss. Mr. P was found to have Raynaud phenomenon beginning with his original presentation and digital ulceration without complications of gangrene or autoamputation. Aggregate physical examinations were notable for digital ulceration, skin tightening/sclerodactyly, and telangiectasia. Serologic markers were notable for the following:
- Positive ANA (antinuclear antibody) with titer of 1:1,280/homogenous pattern;
- Positive anti-RNP (antiribonucleoprotein) with titer of 171.2;
- Positive anti-Scl-70 (antitopoisomerase I) with titer of 108.1;
- Positive anti-SM (anti-Smith antibody) with titer of 30.2;
- Positive anti-Ro (SSA) with titer of 107.6;
- Negative anti-La (SSB) with titer of 1.3;
- Negative anti-dsDNA (anti-double stranded DNA) with titer of 9; and
- Negative ACA (anticentromere antibody).
Early transthoracic echocardiograms revealed an ejection fraction (EF) initially at 55% with evidence of left ventricular hypertrophy. Following treatment with phosphodiesterase-5 inhibitors (PDE-5 inhibitors) and endothelin-1 antagonists for pulmonary hypertension, serial transthoracic echocardiograms showed improvement in his EF.
A chest X-ray did not show signs of ILD, but a subsequent high-resolution computed tomography (HRCT) scan was consistent with chronic ILD with a main pulmonary artery diameter of 3 cm (Figure 1).
In subsequent years, Mr. P was hospitalized several times, secondary to digit pain, ulcerations, and osteomyelitis. His first episode was 1 year after his scleroderma diagnosis, when he was hospitalized for 6 days for complications of SSc and finger pain. The following year, he had a 3-day hospitalization for hypertensive urgency and right third-digit osteomyelitis, treated initially with IV fluids, levofloxacin, and vancomycin, and then ceftaroline for 1 month. Throughout the next 6 years, Mr. P presented multiple times with fingertip ulcerations and was followed in the Infectious Disease clinic for recurrent osteomyelitis. He found some relief with systemic antibiotics, including augmentin, minocycline, moxifloxacin, and doxycycline.
At age 59, he was hospitalized for scleroderma renal crisis (SRC). Early in his disease, his kidney function was normal, but the SRC was discovered after an abrupt rise in his blood pressure (BP) and an increase in serum creatinine (SCr) from 1.2 mg/dL at baseline to 3.06 mg/dL. The presence of brown granular cast in his urine prompted a renal biopsy that showed thrombotic microangiopathy with schistocytes. Mr. P was started on captopril and remained stable with outpatient follow-up for this renal complication.
Discussion
Initial presentation of SSc can occur along a spectrum of its pathophysiology. A more severe presentation, like the one seen in Mr. P, seems to occur more frequently in African American patients relative to white patients.3 Differentiating between the 2 types—diffuse vs limited SSc—is vital to managing patients and disease progression. Limited-type SSc is more common (60%) and less severe with slower progression than is diffuse SSc.
Diffuse-type SSc (35%) includes features such as skin thickening and tightening, ILD, SRC, tendon friction rubs (palpable crepitus over tendons), and skin pigment changes.4 The specific involvement of the renal and cardiopulmonary systems accounts for the higher mortality rates in the diffuse-type.5
Many patients with SSc require periodic hospitalizations throughout their life for the acute complications of the disease. Hospitalized patients often range from age 45 to 64 years and are more often female. However, of hospitalized patients with SSc, in-hospital death rates are higher among men.3,6 Although these rates have decreased as the pathogenesis of SSc has become better understood, it is important to note that in-hospital mortality in 1995 for all patients with SSc was 7.1% and mean length of stay was 7.5 days, and in 2002 to 2003, 6.3% and 6.6 days, respectively.3 Though the burden of this disease has decreased, mortality and hospitalizations continue to persist at high rates. Understanding the pathogenesis, progression, and treatments of SSc are essential to aiding patients with this diagnosis.
Skin Involvement
A common finding and presentation for patients with SSc is related to skin involvement. Common patient complaints and exam findings include calcinosis along extensor tendons and digits, Raynaud phenomenon (seen in more than 95% of patients), sclerodactyly, telangiectasias, hyper/hypopigmentation, and pruritus.4 These findings are useful in diagnosing and monitoring patients for disease progression.
Many of the listed skin manifestations affect patients’ quality of life (QOL) but are not directly associated with mortality. However, a common and feared complication includes skin ulcers and osteomyelitis, seen in 48% and 7.7% of patients, respectively.7 Digit ulcers, areas with loss of dermis and epidermis distal to the proximal interphalangeal joints (Figures 2A and 2B), are significant because they parallel a more rapid progression of internal organ involvement.8
Mr. P required multiple hospitalizations and antibiotic regimens for painful digit ulcers complicated by osteomyelitis (Figure 3).
Treatment usually is aimed at infections that complicate these skin ulcers and are based on site-specific cultures. Preventive measures are aimed at the risk factors associated with digit ulcers, including decreased whole-body warmth, direct trauma to digits, smoking, and vasoconstrictors (eg, cocaine, sympathomimetics).8 Some patients may prevent ulcers by using D-penicillamine, mycophenolate mofetil, and cyclophosphamide, although definitive treatment has not been found.4 Calcium channel blockers, PDE-5 inhibitors, endothelin receptor antagonists, and prostacyclin analogues also have been used to reduce the severity of Raynaud phenomenon attacks and to decrease the number of digital ulcers (in addition to their beneficial effects on pulmonary involvement).8 Additional pharmacologic agents that have been linked to an improvement in Raynaud phenomenon and digital ulcers include statins, angiotensin-converting enzyme (ACE) inhibitors, angiotensin receptor blockers, intravenous N-acetylcysteine, vitamin E gel, and surgical options (eg, revascularization and sympathectomy).8
Pulmonary Involvement
Systemic sclerosis can lead to 2 complications in the lungs, both present in Mr. P: PAH (mean pulmonary arterial pressure > 25 mm Hg) and ILD, which together make lung involvement the leading cause of death for these patients. Limited-type SSc usually is restricted to PAH, whereas diffuse-type SSc usually leads to ILD.4
On diagnosis of SSc, an HRCT is indicated to assess the degree of lung involvement. Mr. P’s HRCT showed a 3-cm main pulmonary artery, suggestive of PAH, in addition to evidence of ILD seen on the chest X-ray (Figure 4).
Pulmonary arterial hypertension is a common finding in patients with SSc and carries a severe prognosis. Risk factors for the development of PAH, when not present on initial diagnosis, include limited-type SSc; late age of onset; Raynaud phenomenon; decreased DLCO, FVC/DLCO < 1.6; increased N-terminal pro-B-type natriuretic peptide (NT-proBNP) serum levels; and the presence of antibodies.9 Patients with both SSc and PAH have a 50% to 87% 1-year survival, whereas patients with idiopathic PAH without connective tissue disease have an 88% 1-year survival.9
Many patients with lung involvement are asymptomatic, but some have findings of crackles and interstitial thickening on chest X-ray that can progress to cyanosis and right heart failure (cor pulmonale).10 On discovery of lung involvement (Figure 5), it is important to follow up with a PFT, primarily because the outcomes and prognosis for patients with SSc are correlated with the presenting severity of ILD and the subsequent progression of their DLCO.4,10
Treatment is directed at PAH and ILD separately. For PAH, a PDE-5 inhibitor, such as tadalafil or sildenafil, and an endothelin-1 receptor antagonist, such as ambrisentan or bosentan, are indicated. Other PAH treatments include diuretics and prostacyclin analogues (eg, epoprostenol, treprostinil, or iloprost) in addition to warfarin if patients have a history of thrombotic events.4 Mr. P’s pulmonologists deferred treatment with prostacyclin analogues given the potential for adverse effects with endothelin-1 receptor inhibitors, PDE-5 inhibitors, and calcium-channel blockers, although combination studies with bosentan and inhaled iloprost have shown promise.11
Like the skin manifestations of SSc, ILD lacks definitive treatment to prevent disease progression. However, some patients benefit from cyclophosphamide, followed by mycophenolate mofetil—which is usually better tolerated—azathioprine, haematopoietic stem cell transplant as rescue therapy, and lung transplant for life-saving treatment.10
Renal Involvement
Renal involvement in patients with SSc can have profound effects on QOL. Even without clinical renal involvement, glomerular filtration rate (GFR) usually decreases with the progression of vascular damage correlated with age and disease duration.12 Patients with a history of digital ulcers, like Mr. P, usually have a lower GFR than that of patients without digital ulcers.12 Monitoring renal function is vital in caring for these patients, because SRC occurs in 2% to 15% of all patients with SSc.13,14
Scleroderma renal crisis is generally defined as an abruptly elevated BP (> 140/90 mm Hgor > 30 mm Hg rise from baseline) with acute renal failure (elevated SCr) and decreased urine output.14 Given the rarity of SSc in the population, diagnosis of SRC requires high clinical suspicion. In the case of Mr. P, a workup involving serum analysis, urinalysis, and renal biopsy allowed for a definitive diagnosis. A renal biopsy can show microangiopathic hemolytic anemia and confirm SRC, although it may not be necessary in patients with known SSc presenting with new hypertension, rising creatinine, and unremarkable urine sediment on microscopy.14,15
Although an acute SRC can be difficult to predict, monitoring renal function and attention to key factors can assist in discovering this SSc complication. Scleroderma renal crisis usually occurs within the first 4 years of SSc diagnosis, often paralleling rapid progression of skin thickening and tightening with higher rates in both African Americans and males.13,14 Additional predictive factors include diffuse skin involvement, rapid progression of skin involvement, positive anti-RNA polymerase III antibodies, new anemia, new cardiac events (eg, pericardial effusion, pericarditis, left ventricular insufficiency), CHF, tendon friction rubs, arthritis, and recent (within 3 months) high-dose glucocorticoid use.4,13-15
The presentation of SRC can be nonspecific, often resembling findings related to acute kidney injury. Patients may report malaise, fatigue, fever, headache, seizure, blurred vision, or dyspnea.13 Clinical parametersto examine include systolic BP > 140 mm Hgand/or diastolic BP > 90 mm Hg (or an abrupt rise of > 30 or > 20, respectively), SCr increase by 50%+ or > 120% of the upper normal limit, proteinuria at 2+, high protein:creatinine ratio, hematuria > 2+ or > 10 red blood cells (RBCs), platelets < 100,000/mm3, and hypertensive encephalopathy.13 Mr. P presented with fatigue, dyspnea, an abrupt rise in BP (156/96 mm Hg), foamy urine, bilateral lower extremity edema (3.9 g/dL albumin), 2+ RBCs, and a SCr of 2.46 mg/dL on admission (a 205% increase from his last baseline of 1.2 mg/dL).
Treatments have had a large impact on the mortality rates of SRC. Following the introduction of ACE inhibitors, mortality from SRC has decreased from 76% to < 10% over recent decades.13 In addition to improving survival, these medications also have improved hospitalization outcomes for these patients.3 Captopril is often the medication of choice given its rapid onset and short duration of action relative to other medications in the same class, such as benazepril, enalapril, fosinopril, lisinopril, quinapril, and ramipril.4,13 Current clinical trials are assessing the specific renal benefits of endothelin-1 receptor antagonists, which often are used for pulmonary and skin involvement.14 For patients presenting with acute SRC and uremia, dialysis may be necessary (typically predicted by elevated NT-proBNP serum levels), while the decision for transplantation may be indicated at least 2 years after SRC onset and resolution.4,13-15
Once the acute crisis resolves, it is important to discuss prognosis with patients. The 1-, 2-, 3-, 5-, and 10-year survival rates are 82%, 74%, 71%, 59%, and 47%, respectively; however, when looking at only male patients at 10 years, the survival rate is 17%.13 Prevention of SRC can be addressed with daily BP checks and advising patients to seek medical care if they notice a consecutive 2-day abrupt rise.13
Cardiac Involvement
Systemic sclerosis has significant effects on patients’ heart function. The introduction of ACE inhibitors has shifted mortality in SSc patients from predominantly SRC to cardiac causes.6 Cardiac involvement can occur through a range of processes, including abnormal cardiac conduction, CHF, diastolic dysfunction, mitral valve nodular thickening, and pericardial effusion.3 Even though patients often present with skin findings, an initial cardiac workup is crucial to understanding disease progression and patient prognosis. The severity of the cutaneous manifestations often predicts the degree of diastolic dysfunction.16
Clinical evidence of cardiac involvement is seen in 20% to 25% of patients with SSc and is associated with a 70% mortality at 5 years when symptoms are evident.16 Additionally, right ventricular dysfunction at presentation is the strongest marker for all-mortality prognosis, representing the degree of pulmonary involvement, and includes findings such as progressive shortness of breath and systemic edema.9
Given the increasing survival of patients with SSc, cardiac involvement is becoming more evident and prominent. Direct treatments for cardiac manifestations are based on the causative feature, namely, focusing on pulmonary and renal involvement, which can be assessed with periodic echocardiograms evaluating left ventricular EF.
Gastrointestinal Involvement
Along with skin manifestations, the gastrointestinal (GI) involvement of SSc can have a significant impact on patients’ QOL without direct contribution to mortality. One of Mr. P’s earliest symptoms that led to a diagnosis of SSc was GERD, which caused a chronic cough, dental erosions, esophageal erosions, duodenal ulcers, dysphagia, abdominal pain, halitosis, pharyngitis, and weight loss. Esophageal involvement occurs in up to 96% of patients with SSc and can include motility abnormalities (eg, strictures and/or muscle dysfunction), lower esophageal sphincter abnormalities, and Barrett esophagus.17
Additional symptoms of the GI system linked with SSc can occur anywhere along the GI tract and include gastric antral vascular ectasia, causing GI bleeds and pernicious anemia, gastroparesis, bacterial overgrowth, intestinal malabsorption, pseudo-obstruction due to hypomotility, fecal incontinence due to anorectal involvement, and rarely, primary biliary cirrhosis.4,17 Decreased mobility of the oral aperture secondary to skin thickening and tightening also can contribute to malnutrition by decreasing oral intake.
Treatments are supportive and target symptom relief. Chronic treatment of GERD is often necessary and includes antacids, histamine-2 receptor blockers, and proton pump inhibitors.4 Other medications that can help with symptom relief include motility agents (such as metoclopramide, domperidone, prucalopride, tegaserod, and macrolides), osmotic laxatives, and ursodeoxycholic acid for primary biliary cirrhosis.17 Surgical intervention should be considered depending on the severity and progression of involvement within the GI tract. Behavioral changes that can improve patient symptoms include facial grimacing and other mouth-stretching exercises, frequent smaller meals followed by maintaining a vertical posture, and high fiber diets.17
Conclusion
Systemic sclerosis is an autoimmune and connective tissue disease with a pathophysiology that can manifest throughout the body. The organ systems that impact patient outcomes include skin, pulmonary, renal, cardiac, and GI. Primary care providers caring for patients diagnosed with SSc should monitor acute management and disease progression in all these systems. Important acute events that can impact morbidity, mortality, and/or QOL include Raynaud phenomenon, SRC, and pericardial effusion. Chronic manifestations that may be present on diagnosis of SSc or may develop while a patient is under a provider’s care include sclerodactyly, tendon calcinosis, PAH, ILD, chronic kidney injury, chronic cardiac damage, GERD, and esophageal dysmotility. While this discussion serves as a pertinent overview of patients with SSc, it is summative, and providers are encouraged to seek a stronger understanding of both the common and rarer manifestations within each of their patients.
1. Lawrence RC, Helmick CG, Arnett FC, et al. Estimates of the prevalence of arthritis and selected musculoskeletal disorders in the United States. Arthritis Rheum. 1998;41(5):778-799.
2. Silman AG. Scleroderma. In: Epidemiology of the Rheumatic Diseases. 2nd ed. Silman AJ, Hochberg MC, eds. Oxford, UK: Oxford University Press. 1993:chap 8.
3. Chung L, Krishnan E, Chakravarty EF. Hospitalizations and mortality in systemic sclerosis: results from the Nationwide Inpatient Sample. Rheumatology (Oxford). 2007;46(12):1808-1813.
4. Hinchcliff M, Varga J. Systemic sclerosis/scleroderma: a treatable multisystem disease. Am Fam Physician. 2008;78(8):961-968.
5. Tyndall AJ, Bannert B, Vonk M, et al. Causes and risk factors for death in systemic sclerosis: a study from the EULAR Scleroderma Trials and Research (EUSTAR) database. Ann Rheum Dis. 2010;69(10):1809-1815.
6. Piga M, Casula L, Sanna S, et al. Population-based analysis of hospitalizations for patients with systemic sclerosis in a West-European region over the period 2001-2012. Rheumatol Int. 2016;36(1):73-81.
7. Giuggioli D, Manfredi A, Colaci M, Lumetti F, Ferri C. Osteomyelitis complicating scleroderma digital ulcers. Clin Rheumatol. 2013;32(5):623-627.
8. Schiopu E, Impens AJ, Phillips K. Digital ischemia in scleroderma spectrum of diseases. Int J Rheumatol. 2010;2010:923743.
9. Hassoun PM. Therapies for scleroderma-related pulmonary arterial hypertension. Expert Rev Respir Med. 2009;3(2):187-196.
10. Giacomelli R, Liakouli V, Berardicurti O, et al. Interstitial lung disease in systemic sclerosis: current and future treatment. Rheumatol Int. 2017;37(6):853-863.
11. McLaughlin V, Humbert M, Coghlan G, Nash P, Steen V. Pulmonary arterial hypertension: the most devastating vascular complication of systemic sclerosis. Rheumatology (Oxford). 2009; (suppl 3):iii25-iii31.
12. Gigante A, Barbano B, Granata G, et al. Evaluation of estimated glomerular filtration rate and clinical variables in systemic sclerosis patients. Clin Nephrol. 2016;85(6):326-331.
13. Bose N, Chiesa-Vottero A, Chatterjee S. Scleroderma renal crisis. Semin Arthritis Rheum. 2015;44(6):687-694.
14. Woodworth TG, Suliman YA, Furst DE, Clements P. Scleroderma renal crisis and renal involvement in systemic sclerosis. Nat Rev Nephrol. 2016;12(11):678-691.
15. Mouthon L, Bérezné A, Bussone G, Noël LH, Villiger PM, Guillevin L. Scleroderma renal crisis: a rare but severe complication of systemic sclerosis. Clin Rev Allergy Immunol. 2011;40(2):84-91.
16. Champion HC. The heart in scleroderma. Rheum Dis Clin North Am. 2008;34(1):181-90.
17. Tian XP, Zhang X. Gastrointestinal complications of systemic sclerosis. World J Gastroenterol. 2013;19(41):7062-7068.
1. Lawrence RC, Helmick CG, Arnett FC, et al. Estimates of the prevalence of arthritis and selected musculoskeletal disorders in the United States. Arthritis Rheum. 1998;41(5):778-799.
2. Silman AG. Scleroderma. In: Epidemiology of the Rheumatic Diseases. 2nd ed. Silman AJ, Hochberg MC, eds. Oxford, UK: Oxford University Press. 1993:chap 8.
3. Chung L, Krishnan E, Chakravarty EF. Hospitalizations and mortality in systemic sclerosis: results from the Nationwide Inpatient Sample. Rheumatology (Oxford). 2007;46(12):1808-1813.
4. Hinchcliff M, Varga J. Systemic sclerosis/scleroderma: a treatable multisystem disease. Am Fam Physician. 2008;78(8):961-968.
5. Tyndall AJ, Bannert B, Vonk M, et al. Causes and risk factors for death in systemic sclerosis: a study from the EULAR Scleroderma Trials and Research (EUSTAR) database. Ann Rheum Dis. 2010;69(10):1809-1815.
6. Piga M, Casula L, Sanna S, et al. Population-based analysis of hospitalizations for patients with systemic sclerosis in a West-European region over the period 2001-2012. Rheumatol Int. 2016;36(1):73-81.
7. Giuggioli D, Manfredi A, Colaci M, Lumetti F, Ferri C. Osteomyelitis complicating scleroderma digital ulcers. Clin Rheumatol. 2013;32(5):623-627.
8. Schiopu E, Impens AJ, Phillips K. Digital ischemia in scleroderma spectrum of diseases. Int J Rheumatol. 2010;2010:923743.
9. Hassoun PM. Therapies for scleroderma-related pulmonary arterial hypertension. Expert Rev Respir Med. 2009;3(2):187-196.
10. Giacomelli R, Liakouli V, Berardicurti O, et al. Interstitial lung disease in systemic sclerosis: current and future treatment. Rheumatol Int. 2017;37(6):853-863.
11. McLaughlin V, Humbert M, Coghlan G, Nash P, Steen V. Pulmonary arterial hypertension: the most devastating vascular complication of systemic sclerosis. Rheumatology (Oxford). 2009; (suppl 3):iii25-iii31.
12. Gigante A, Barbano B, Granata G, et al. Evaluation of estimated glomerular filtration rate and clinical variables in systemic sclerosis patients. Clin Nephrol. 2016;85(6):326-331.
13. Bose N, Chiesa-Vottero A, Chatterjee S. Scleroderma renal crisis. Semin Arthritis Rheum. 2015;44(6):687-694.
14. Woodworth TG, Suliman YA, Furst DE, Clements P. Scleroderma renal crisis and renal involvement in systemic sclerosis. Nat Rev Nephrol. 2016;12(11):678-691.
15. Mouthon L, Bérezné A, Bussone G, Noël LH, Villiger PM, Guillevin L. Scleroderma renal crisis: a rare but severe complication of systemic sclerosis. Clin Rev Allergy Immunol. 2011;40(2):84-91.
16. Champion HC. The heart in scleroderma. Rheum Dis Clin North Am. 2008;34(1):181-90.
17. Tian XP, Zhang X. Gastrointestinal complications of systemic sclerosis. World J Gastroenterol. 2013;19(41):7062-7068.
Metastatic Meningioma of the Scalp
Meningiomas generally present as slow-growing, expanding intracranial lesions and are the most common benign intracranial tumor in adults.1 Rarely, meningioma exhibits malignant potential and presents as an extracranial soft-tissue mass through extension or as a primary extracranial cutaneous neoplasm. The differential diagnosis of scalp neoplasms must be broadened to include uncommon tumors such as meningioma. We present a rare case of a 68-year-old woman with scalp metastasis of meningioma 11 years after initial resection of the primary tumor.
Case Report
A 68-year-old woman presented for evaluation of an asymptomatic nodule on the left parietal scalp of 2 years’ duration. She denied any headaches, difficulty with balance, vision changes, or changes in mentation. Her medical history was remarkable for a benign meningioma removed from the right parietal scalp 11 years prior without radiation therapy, as well as type 2 diabetes mellitus and arthritis. The patient’s son died from a brain tumor, but the exact tumor type and age at the time of death were unknown. Her current medications included metformin, insulin glargine, aspirin, and a daily multivitamin. She denied any allergies or history of smoking.
Physical examination of the scalp revealed 4 fixed, nontender, flesh-colored nodules: 2 on the left parietal scalp measuring 3.0 cm and 0.8 cm, respectively (Figure 1A); a 0.4-cm nodule on the right posterior occipital scalp; and a 1.6-cm sausage-shaped nodule on the right temple (Figure 1B). No positive lymph nodes were appreciated, and no additional lesions were noted. No additional atypical lesions were noted on full cutaneous examination.
A diagnostic 6-mm punch biopsy of the largest nodule was performed. Intraoperatively, there was no apparent cyst wall, but coiled, loose, stringlike, pink-yellow tissue was removed from the base of the wound before closing with sutures.
The primary histologic finding was cells within fibrous tissue containing delicate round-oval nuclei, inconspicuous nucleoli, and lightly eosinophilic cytoplasm with an indistinct border (Figure 2). Immunohistochemical studies for S100 protein were focal and limited to the cytoplasm of a subset of neoplastic cells (Figure 3). Tumor cells stained positive for epithelial membrane antigen (EMA) and were focally positive for progesterone receptor (Figure 4). Tumor cells were negative for CD31 and CD34. Based on the clinical and histologic findings, a diagnosis of metastatic meningioma of the scalp was made.
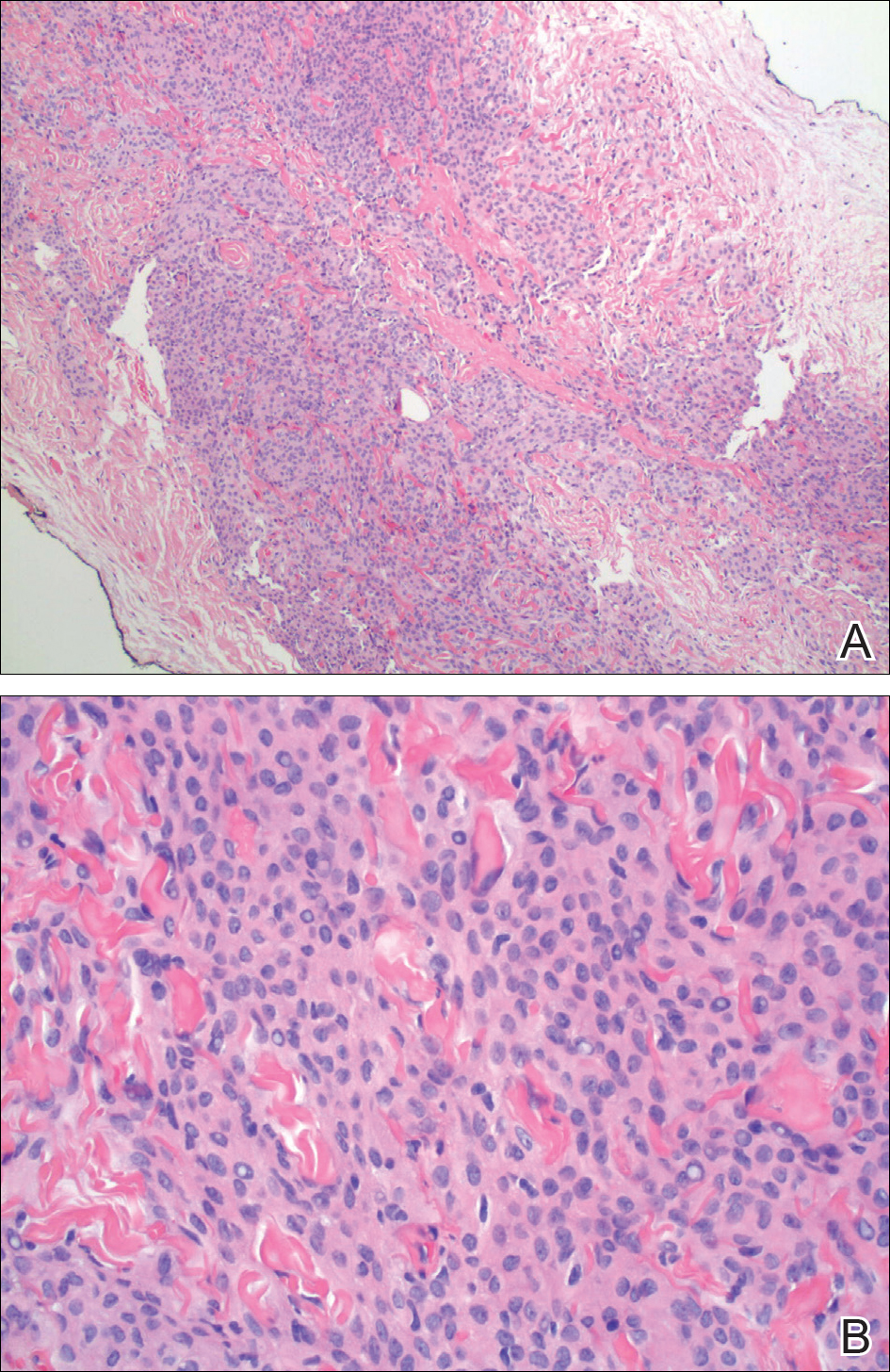
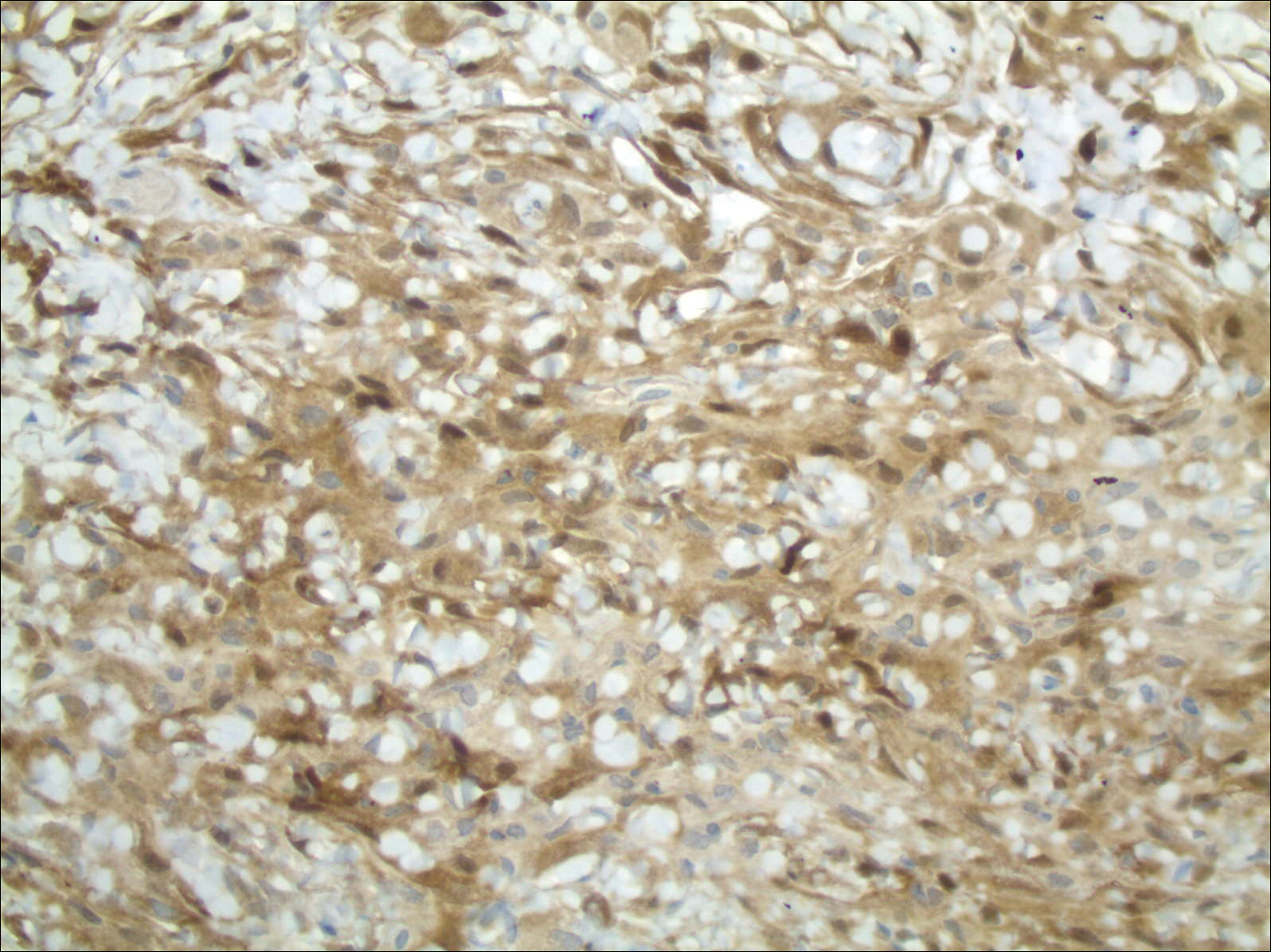
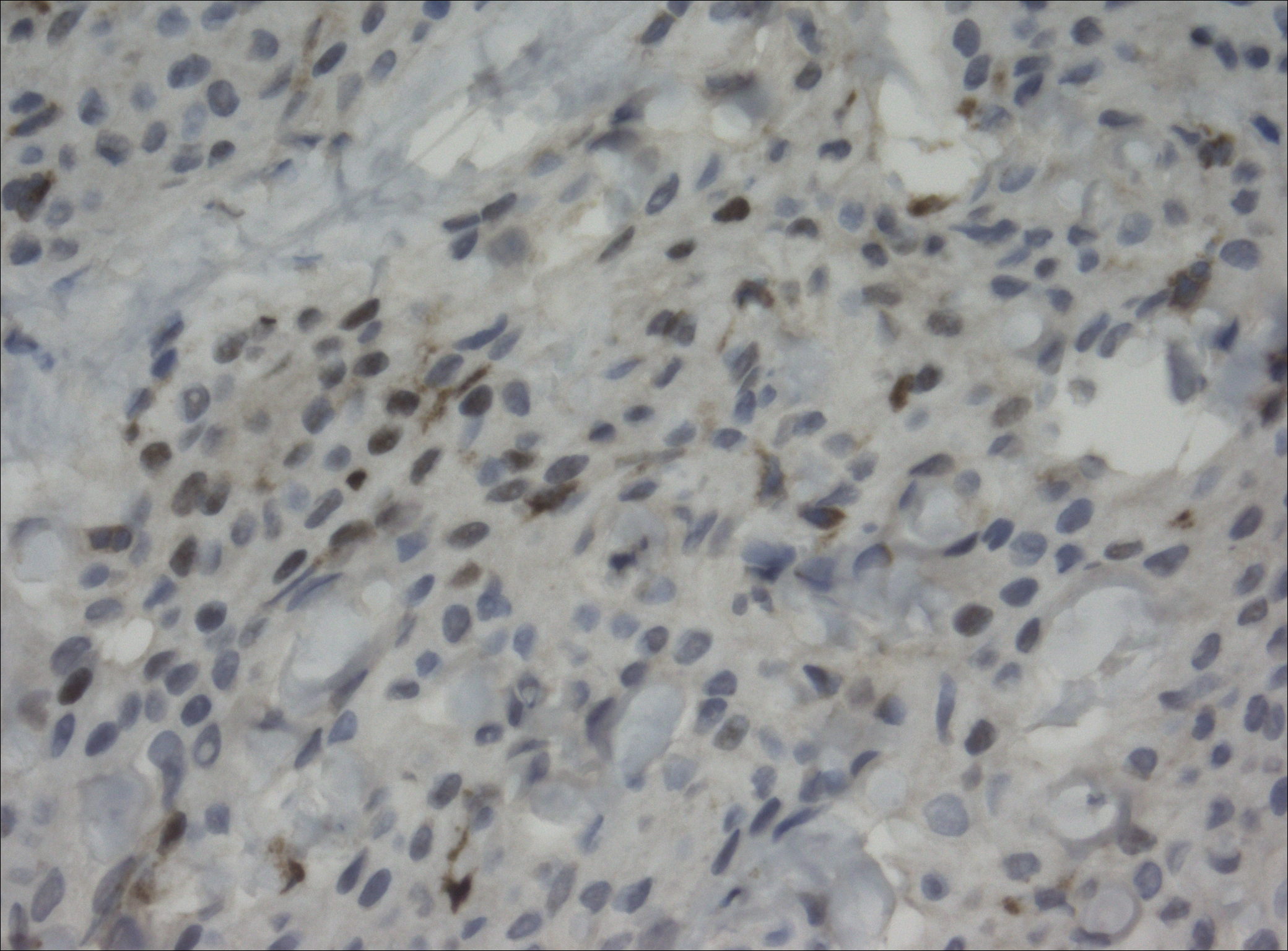
Magnetic resonance imaging and positron emission tomography of the head, neck, and chest demonstrated 3 residual subcutaneous nodules on the scalp and an indeterminate subcentimeter nodule in the right lung. The 0.4-cm nodule on the right posterior occipital scalp was removed without complication, and no radiation therapy was administered. The rest of the lesions were monitored. She remained under the close observation of a neurosurgeon and underwent repeat imaging of the scalp nodules and lungs, initially at 3 months and then routinely at the patient’s comfort. The patient currently denies any neurologic symptoms.
Comment
Meningiomas are derived from meningothelial cells found in the leptomeninges and in the choroid plexus of the ventricles of the brain.2 They are common intracranial neoplasms that generally are associated with a benign course and present during the fourth to sixth decades of life. Meningiomas constitute 13% to 30% of intracranial neoplasms and usually are female predominant (3:1).3,4 Rarely, malignant transformation can lead to local and distant metastasis to the lungs,5,6 liver,7 and skeletal system.8 In cases of metastatic spread, there is an increased incidence in males versus females.9-11
Risk Factors
Although many meningiomas are sporadic, numerous risk factors have been associated with the disease development. One study showed a link between exposure to ionizing radiation and subsequent development of meningioma.12 Another study found a population link between a higher incidence of meningioma and nuclear exposure in Hiroshima, Japan, after the atomic bomb blast in 1980.13 There is an increased incidence of meningioma in patients exposed to radiography from frequent dental imaging, particularly when older machines with higher levels of radiation exposure are used.14Another study demonstrated a correlation between meningioma and hormonal factors (eg, estrogen for hormone therapy) and exacerbation of symptoms during pregnancy.15 There also is an increased incidence of meningioma in breast cancer patients.4 Genetic alterations also have been implicated in the development of meningioma. It was found that 50% of patients with a mutation in the neurofibromatosis 2 gene (which codes for the merlin protein) had associated meningiomas.16,17 Scalp nodules in patients with neurofibromatosis type 2 increases suspicion of a scalp meningioma and necessitates biopsy.
Clinical Presentation
Cutaneous meningiomas typically present as firm, subcutaneous nodules. Scalp nodules ranging from alopecia18,19 to hypertrichosis20 have been reported. These neoplasms can be painless or painful, depending on mass effect and location.
Classification
The primary clinical classification system of metastatic meningioma was first described in 1974.21 Type 1 meningioma refers to congenital lesions that tend to cluster closer to the midline. Type 2 refers to ectopic soft-tissue lesions that extend to the skin from likely remnants of arachnoid cells. These lesions are more likely to be found around the eyes, ears, nose, and mouth. Type 3 meningiomas extend from intracranial tumors that secondarily involve the skin through proliferation through bone or anatomic defects. Type 3 is the result of direct extension and the location of the cutaneous presentation depends on the location of the intracranial lesion.4,22,23
Pathology
Meningiomas exhibit a range of morphologic appearances on histopathology. In almost all meningiomas, tumor cells are concentrically wrapped in tight whorls with round-oval nuclei and delicate chromatin, central clearing, and pale pseudonuclear inclusions. Lamellate calcifications known as psammoma bodies are a common finding. Immunohistochemical studies show that most meningiomas are positive for EMA, vimentin, and progesterone receptor. S100 protein expression, if present, usually is focal.
Differential Diagnosis
Asymptomatic nodules on the scalp may present a diagnostic challenge to physicians. Most common scalp lesions tend to be cystic or lipomatous. In children, a broad differential diagnosis should be considered, including dermoid and epidermoid tumors, dermal sinus tumors, hemangiomas, metastasis of another tumor, aplasia cutis congenita, pilomatricoma, and lipoma. In adults, the differential should focus on epidermoid cysts, lipomas, metastasis of other tumors, osteomas, arteriovenous fistulae, and heterotopic brain tissue. Often, microscopic examination is necessary, along with additional immunohistochemical staining (eg, EMA, vimentin).
Treatment
Treatment options for meningioma include observation, surgical resection, radiotherapy, and systemic therapy, as well as a combination of these modalities. The choice of therapy depends on such variables as patient age; performance status; comorbidities; presence or absence of symptoms (including focal neurologic deficits); and tumor location, size, and grade. It is important to note that there is limited knowledge looking at the results of various treatment modalities, and no consensus approach has been established.
Conclusion
Our patient’s medical history was remarkable for an intracranial meningioma 11 years prior to the current presentation, and she was found to have biopsy-proven metastatic meningioma without recurrence of the initial tumor. Patients presenting with a scalp nodule warrant a thorough medical history and consideration beyond common cysts and lipomas.
- Mackay B, Bruner JM, Luna MA. Malignant meningioma of the scalp. Ultrastruc Pathol. 1994;18:235-240.
- Whittle IR, Smith C, Navoo P, et al. Meningiomas. Lancet. 2004;363:1535-1543.
- Bauman G, Fisher B, Schild S, et al. Meningioma, ependymoma, and other adult brain tumors. In: Gunderson LL, Tepper JE, eds. Clinical Radiation Oncology. Philadelphia, PA: Elsevier Churchill Livingstone; 2007:539-566.
- Claus EB, Bondy ML, Schildkraut JM, et al. Epidemiology of intracranial meningioma. Neurosurgery. 2005;57:1088-1095.
- Tworek JA, Mikhail AA, Blaivas M. Meningioma: local recurrence and pulmonary metastasis diagnosed by fine needle aspiration. Acta Cytol. 1997;41:946-947.
- Shin MS, Holman WL, Herrera GA, et al. Extensive pulmonary metastasis of an intracranial meningioma with repeated recurrence: radiographic and pathologic features. South Med J. 1996;89:313-318.
- Ferguson JM, Flinn J. Intracranial meningioma with hepatic metastases and hypoglycaemia treated by selective hepatic arterial chemo-embolization. Australas Radiol.1995;39:97-99.
- Palmer JD, Cook PL, Ellison DW. Extracranial osseous metastases from intracranial meningioma. Br J Neurosurg. 1994;8:215-218.
- Glasauer FE, Yuan RH. Intracranial tumours with extracranial metastases. case report and review of the literature. J Neurosurg. 1963;20:474-493.
- Shuangshoti S, Hongsaprabhas C, Netsky MG. Metastasizing meningioma. Cancer. 1970;26:832-841.
- Ohta M, Iwaki T, Kitamoto T, et al. MIB-1 staining index and scoring of histological features in meningioma. Cancer. 1994;74:3176-3189.
- Wrensch M, Minn Y, Chew T, et al. Epidemiology of primary brain tumors: current concepts and review of the literature. Neuro Oncol. 2002;4:278-299.
- Shintani T, Hayakawa N, Hoshi M, et al. High incidence of meningioma among Hiroshima atomic bomb survivors. J Rad Res. 1999;40:49-57.
- Claus EB, Calvocoressi L, Bondy ML, et al. Dental x-rays and risk of meningioma. Cancer. 2012;118:4530-4537.
- Blitshteyn S, Crook JE, Jaeckle KA. Is there an association between meningioma and hormone replacement therapy? J Clin Oncol. 2008;26:279-282.
- Fontaine B, Rouleau GA, Seizinger BR, et al. Molecular genetics of neurofibromatosis 2 and related tumors (acoustic neuromas and meningioma). Ann N Y Acad Sci. 1991;615:338-343.
- Rabin BM, Meyer JR, Berlin JW, et al. Radiation-induced changes of the central nervous system and head and neck. Radiographics. 1996;16:1055-1072.
- Tanaka S, Okazaki M, Egusa G, et al. A case of pheochromocytoma associated with meningioma. J Intern Med. 1991;229:371-373.
- Zeikus P, Robinson-Bostom L, Stopa E. Primary cutaneous meningioma in association with a sinus pericranii. J Am Acad Dermatol. 2006;54(2 suppl):S49-S50.
- Junaid TA, Nkposong EO, Kolawole TM. Cutaneous meningiomas and an ovarian fibroma in a three-year-old girl. J Pathol. 1972;108:165-167.
- Lopez DA, Silvers DN, Helwig EB. Cutaneous meningioma—a clinicopathologic study. Cancer. 1974;34:728-744.
- Shuangshoti S, Boonjunwetwat D, Kaoroptham S. Association of primary intraspinal meningiomas and subcutaneous meningioma of the cervical region: case report and review of literature. Surg Neurol. 1992;38:129-134.
- Miedema JR, Zedek D. Cutaneous meningioma. Arch Pathol. 2012;136:208-211.
Meningiomas generally present as slow-growing, expanding intracranial lesions and are the most common benign intracranial tumor in adults.1 Rarely, meningioma exhibits malignant potential and presents as an extracranial soft-tissue mass through extension or as a primary extracranial cutaneous neoplasm. The differential diagnosis of scalp neoplasms must be broadened to include uncommon tumors such as meningioma. We present a rare case of a 68-year-old woman with scalp metastasis of meningioma 11 years after initial resection of the primary tumor.
Case Report
A 68-year-old woman presented for evaluation of an asymptomatic nodule on the left parietal scalp of 2 years’ duration. She denied any headaches, difficulty with balance, vision changes, or changes in mentation. Her medical history was remarkable for a benign meningioma removed from the right parietal scalp 11 years prior without radiation therapy, as well as type 2 diabetes mellitus and arthritis. The patient’s son died from a brain tumor, but the exact tumor type and age at the time of death were unknown. Her current medications included metformin, insulin glargine, aspirin, and a daily multivitamin. She denied any allergies or history of smoking.
Physical examination of the scalp revealed 4 fixed, nontender, flesh-colored nodules: 2 on the left parietal scalp measuring 3.0 cm and 0.8 cm, respectively (Figure 1A); a 0.4-cm nodule on the right posterior occipital scalp; and a 1.6-cm sausage-shaped nodule on the right temple (Figure 1B). No positive lymph nodes were appreciated, and no additional lesions were noted. No additional atypical lesions were noted on full cutaneous examination.

A diagnostic 6-mm punch biopsy of the largest nodule was performed. Intraoperatively, there was no apparent cyst wall, but coiled, loose, stringlike, pink-yellow tissue was removed from the base of the wound before closing with sutures.
The primary histologic finding was cells within fibrous tissue containing delicate round-oval nuclei, inconspicuous nucleoli, and lightly eosinophilic cytoplasm with an indistinct border (Figure 2). Immunohistochemical studies for S100 protein were focal and limited to the cytoplasm of a subset of neoplastic cells (Figure 3). Tumor cells stained positive for epithelial membrane antigen (EMA) and were focally positive for progesterone receptor (Figure 4). Tumor cells were negative for CD31 and CD34. Based on the clinical and histologic findings, a diagnosis of metastatic meningioma of the scalp was made.



Magnetic resonance imaging and positron emission tomography of the head, neck, and chest demonstrated 3 residual subcutaneous nodules on the scalp and an indeterminate subcentimeter nodule in the right lung. The 0.4-cm nodule on the right posterior occipital scalp was removed without complication, and no radiation therapy was administered. The rest of the lesions were monitored. She remained under the close observation of a neurosurgeon and underwent repeat imaging of the scalp nodules and lungs, initially at 3 months and then routinely at the patient’s comfort. The patient currently denies any neurologic symptoms.
Comment
Meningiomas are derived from meningothelial cells found in the leptomeninges and in the choroid plexus of the ventricles of the brain.2 They are common intracranial neoplasms that generally are associated with a benign course and present during the fourth to sixth decades of life. Meningiomas constitute 13% to 30% of intracranial neoplasms and usually are female predominant (3:1).3,4 Rarely, malignant transformation can lead to local and distant metastasis to the lungs,5,6 liver,7 and skeletal system.8 In cases of metastatic spread, there is an increased incidence in males versus females.9-11
Risk Factors
Although many meningiomas are sporadic, numerous risk factors have been associated with the disease development. One study showed a link between exposure to ionizing radiation and subsequent development of meningioma.12 Another study found a population link between a higher incidence of meningioma and nuclear exposure in Hiroshima, Japan, after the atomic bomb blast in 1980.13 There is an increased incidence of meningioma in patients exposed to radiography from frequent dental imaging, particularly when older machines with higher levels of radiation exposure are used.14Another study demonstrated a correlation between meningioma and hormonal factors (eg, estrogen for hormone therapy) and exacerbation of symptoms during pregnancy.15 There also is an increased incidence of meningioma in breast cancer patients.4 Genetic alterations also have been implicated in the development of meningioma. It was found that 50% of patients with a mutation in the neurofibromatosis 2 gene (which codes for the merlin protein) had associated meningiomas.16,17 Scalp nodules in patients with neurofibromatosis type 2 increases suspicion of a scalp meningioma and necessitates biopsy.
Clinical Presentation
Cutaneous meningiomas typically present as firm, subcutaneous nodules. Scalp nodules ranging from alopecia18,19 to hypertrichosis20 have been reported. These neoplasms can be painless or painful, depending on mass effect and location.
Classification
The primary clinical classification system of metastatic meningioma was first described in 1974.21 Type 1 meningioma refers to congenital lesions that tend to cluster closer to the midline. Type 2 refers to ectopic soft-tissue lesions that extend to the skin from likely remnants of arachnoid cells. These lesions are more likely to be found around the eyes, ears, nose, and mouth. Type 3 meningiomas extend from intracranial tumors that secondarily involve the skin through proliferation through bone or anatomic defects. Type 3 is the result of direct extension and the location of the cutaneous presentation depends on the location of the intracranial lesion.4,22,23
Pathology
Meningiomas exhibit a range of morphologic appearances on histopathology. In almost all meningiomas, tumor cells are concentrically wrapped in tight whorls with round-oval nuclei and delicate chromatin, central clearing, and pale pseudonuclear inclusions. Lamellate calcifications known as psammoma bodies are a common finding. Immunohistochemical studies show that most meningiomas are positive for EMA, vimentin, and progesterone receptor. S100 protein expression, if present, usually is focal.
Differential Diagnosis
Asymptomatic nodules on the scalp may present a diagnostic challenge to physicians. Most common scalp lesions tend to be cystic or lipomatous. In children, a broad differential diagnosis should be considered, including dermoid and epidermoid tumors, dermal sinus tumors, hemangiomas, metastasis of another tumor, aplasia cutis congenita, pilomatricoma, and lipoma. In adults, the differential should focus on epidermoid cysts, lipomas, metastasis of other tumors, osteomas, arteriovenous fistulae, and heterotopic brain tissue. Often, microscopic examination is necessary, along with additional immunohistochemical staining (eg, EMA, vimentin).
Treatment
Treatment options for meningioma include observation, surgical resection, radiotherapy, and systemic therapy, as well as a combination of these modalities. The choice of therapy depends on such variables as patient age; performance status; comorbidities; presence or absence of symptoms (including focal neurologic deficits); and tumor location, size, and grade. It is important to note that there is limited knowledge looking at the results of various treatment modalities, and no consensus approach has been established.
Conclusion
Our patient’s medical history was remarkable for an intracranial meningioma 11 years prior to the current presentation, and she was found to have biopsy-proven metastatic meningioma without recurrence of the initial tumor. Patients presenting with a scalp nodule warrant a thorough medical history and consideration beyond common cysts and lipomas.
Meningiomas generally present as slow-growing, expanding intracranial lesions and are the most common benign intracranial tumor in adults.1 Rarely, meningioma exhibits malignant potential and presents as an extracranial soft-tissue mass through extension or as a primary extracranial cutaneous neoplasm. The differential diagnosis of scalp neoplasms must be broadened to include uncommon tumors such as meningioma. We present a rare case of a 68-year-old woman with scalp metastasis of meningioma 11 years after initial resection of the primary tumor.
Case Report
A 68-year-old woman presented for evaluation of an asymptomatic nodule on the left parietal scalp of 2 years’ duration. She denied any headaches, difficulty with balance, vision changes, or changes in mentation. Her medical history was remarkable for a benign meningioma removed from the right parietal scalp 11 years prior without radiation therapy, as well as type 2 diabetes mellitus and arthritis. The patient’s son died from a brain tumor, but the exact tumor type and age at the time of death were unknown. Her current medications included metformin, insulin glargine, aspirin, and a daily multivitamin. She denied any allergies or history of smoking.
Physical examination of the scalp revealed 4 fixed, nontender, flesh-colored nodules: 2 on the left parietal scalp measuring 3.0 cm and 0.8 cm, respectively (Figure 1A); a 0.4-cm nodule on the right posterior occipital scalp; and a 1.6-cm sausage-shaped nodule on the right temple (Figure 1B). No positive lymph nodes were appreciated, and no additional lesions were noted. No additional atypical lesions were noted on full cutaneous examination.

A diagnostic 6-mm punch biopsy of the largest nodule was performed. Intraoperatively, there was no apparent cyst wall, but coiled, loose, stringlike, pink-yellow tissue was removed from the base of the wound before closing with sutures.
The primary histologic finding was cells within fibrous tissue containing delicate round-oval nuclei, inconspicuous nucleoli, and lightly eosinophilic cytoplasm with an indistinct border (Figure 2). Immunohistochemical studies for S100 protein were focal and limited to the cytoplasm of a subset of neoplastic cells (Figure 3). Tumor cells stained positive for epithelial membrane antigen (EMA) and were focally positive for progesterone receptor (Figure 4). Tumor cells were negative for CD31 and CD34. Based on the clinical and histologic findings, a diagnosis of metastatic meningioma of the scalp was made.



Magnetic resonance imaging and positron emission tomography of the head, neck, and chest demonstrated 3 residual subcutaneous nodules on the scalp and an indeterminate subcentimeter nodule in the right lung. The 0.4-cm nodule on the right posterior occipital scalp was removed without complication, and no radiation therapy was administered. The rest of the lesions were monitored. She remained under the close observation of a neurosurgeon and underwent repeat imaging of the scalp nodules and lungs, initially at 3 months and then routinely at the patient’s comfort. The patient currently denies any neurologic symptoms.
Comment
Meningiomas are derived from meningothelial cells found in the leptomeninges and in the choroid plexus of the ventricles of the brain.2 They are common intracranial neoplasms that generally are associated with a benign course and present during the fourth to sixth decades of life. Meningiomas constitute 13% to 30% of intracranial neoplasms and usually are female predominant (3:1).3,4 Rarely, malignant transformation can lead to local and distant metastasis to the lungs,5,6 liver,7 and skeletal system.8 In cases of metastatic spread, there is an increased incidence in males versus females.9-11
Risk Factors
Although many meningiomas are sporadic, numerous risk factors have been associated with the disease development. One study showed a link between exposure to ionizing radiation and subsequent development of meningioma.12 Another study found a population link between a higher incidence of meningioma and nuclear exposure in Hiroshima, Japan, after the atomic bomb blast in 1980.13 There is an increased incidence of meningioma in patients exposed to radiography from frequent dental imaging, particularly when older machines with higher levels of radiation exposure are used.14Another study demonstrated a correlation between meningioma and hormonal factors (eg, estrogen for hormone therapy) and exacerbation of symptoms during pregnancy.15 There also is an increased incidence of meningioma in breast cancer patients.4 Genetic alterations also have been implicated in the development of meningioma. It was found that 50% of patients with a mutation in the neurofibromatosis 2 gene (which codes for the merlin protein) had associated meningiomas.16,17 Scalp nodules in patients with neurofibromatosis type 2 increases suspicion of a scalp meningioma and necessitates biopsy.
Clinical Presentation
Cutaneous meningiomas typically present as firm, subcutaneous nodules. Scalp nodules ranging from alopecia18,19 to hypertrichosis20 have been reported. These neoplasms can be painless or painful, depending on mass effect and location.
Classification
The primary clinical classification system of metastatic meningioma was first described in 1974.21 Type 1 meningioma refers to congenital lesions that tend to cluster closer to the midline. Type 2 refers to ectopic soft-tissue lesions that extend to the skin from likely remnants of arachnoid cells. These lesions are more likely to be found around the eyes, ears, nose, and mouth. Type 3 meningiomas extend from intracranial tumors that secondarily involve the skin through proliferation through bone or anatomic defects. Type 3 is the result of direct extension and the location of the cutaneous presentation depends on the location of the intracranial lesion.4,22,23
Pathology
Meningiomas exhibit a range of morphologic appearances on histopathology. In almost all meningiomas, tumor cells are concentrically wrapped in tight whorls with round-oval nuclei and delicate chromatin, central clearing, and pale pseudonuclear inclusions. Lamellate calcifications known as psammoma bodies are a common finding. Immunohistochemical studies show that most meningiomas are positive for EMA, vimentin, and progesterone receptor. S100 protein expression, if present, usually is focal.
Differential Diagnosis
Asymptomatic nodules on the scalp may present a diagnostic challenge to physicians. Most common scalp lesions tend to be cystic or lipomatous. In children, a broad differential diagnosis should be considered, including dermoid and epidermoid tumors, dermal sinus tumors, hemangiomas, metastasis of another tumor, aplasia cutis congenita, pilomatricoma, and lipoma. In adults, the differential should focus on epidermoid cysts, lipomas, metastasis of other tumors, osteomas, arteriovenous fistulae, and heterotopic brain tissue. Often, microscopic examination is necessary, along with additional immunohistochemical staining (eg, EMA, vimentin).
Treatment
Treatment options for meningioma include observation, surgical resection, radiotherapy, and systemic therapy, as well as a combination of these modalities. The choice of therapy depends on such variables as patient age; performance status; comorbidities; presence or absence of symptoms (including focal neurologic deficits); and tumor location, size, and grade. It is important to note that there is limited knowledge looking at the results of various treatment modalities, and no consensus approach has been established.
Conclusion
Our patient’s medical history was remarkable for an intracranial meningioma 11 years prior to the current presentation, and she was found to have biopsy-proven metastatic meningioma without recurrence of the initial tumor. Patients presenting with a scalp nodule warrant a thorough medical history and consideration beyond common cysts and lipomas.
- Mackay B, Bruner JM, Luna MA. Malignant meningioma of the scalp. Ultrastruc Pathol. 1994;18:235-240.
- Whittle IR, Smith C, Navoo P, et al. Meningiomas. Lancet. 2004;363:1535-1543.
- Bauman G, Fisher B, Schild S, et al. Meningioma, ependymoma, and other adult brain tumors. In: Gunderson LL, Tepper JE, eds. Clinical Radiation Oncology. Philadelphia, PA: Elsevier Churchill Livingstone; 2007:539-566.
- Claus EB, Bondy ML, Schildkraut JM, et al. Epidemiology of intracranial meningioma. Neurosurgery. 2005;57:1088-1095.
- Tworek JA, Mikhail AA, Blaivas M. Meningioma: local recurrence and pulmonary metastasis diagnosed by fine needle aspiration. Acta Cytol. 1997;41:946-947.
- Shin MS, Holman WL, Herrera GA, et al. Extensive pulmonary metastasis of an intracranial meningioma with repeated recurrence: radiographic and pathologic features. South Med J. 1996;89:313-318.
- Ferguson JM, Flinn J. Intracranial meningioma with hepatic metastases and hypoglycaemia treated by selective hepatic arterial chemo-embolization. Australas Radiol.1995;39:97-99.
- Palmer JD, Cook PL, Ellison DW. Extracranial osseous metastases from intracranial meningioma. Br J Neurosurg. 1994;8:215-218.
- Glasauer FE, Yuan RH. Intracranial tumours with extracranial metastases. case report and review of the literature. J Neurosurg. 1963;20:474-493.
- Shuangshoti S, Hongsaprabhas C, Netsky MG. Metastasizing meningioma. Cancer. 1970;26:832-841.
- Ohta M, Iwaki T, Kitamoto T, et al. MIB-1 staining index and scoring of histological features in meningioma. Cancer. 1994;74:3176-3189.
- Wrensch M, Minn Y, Chew T, et al. Epidemiology of primary brain tumors: current concepts and review of the literature. Neuro Oncol. 2002;4:278-299.
- Shintani T, Hayakawa N, Hoshi M, et al. High incidence of meningioma among Hiroshima atomic bomb survivors. J Rad Res. 1999;40:49-57.
- Claus EB, Calvocoressi L, Bondy ML, et al. Dental x-rays and risk of meningioma. Cancer. 2012;118:4530-4537.
- Blitshteyn S, Crook JE, Jaeckle KA. Is there an association between meningioma and hormone replacement therapy? J Clin Oncol. 2008;26:279-282.
- Fontaine B, Rouleau GA, Seizinger BR, et al. Molecular genetics of neurofibromatosis 2 and related tumors (acoustic neuromas and meningioma). Ann N Y Acad Sci. 1991;615:338-343.
- Rabin BM, Meyer JR, Berlin JW, et al. Radiation-induced changes of the central nervous system and head and neck. Radiographics. 1996;16:1055-1072.
- Tanaka S, Okazaki M, Egusa G, et al. A case of pheochromocytoma associated with meningioma. J Intern Med. 1991;229:371-373.
- Zeikus P, Robinson-Bostom L, Stopa E. Primary cutaneous meningioma in association with a sinus pericranii. J Am Acad Dermatol. 2006;54(2 suppl):S49-S50.
- Junaid TA, Nkposong EO, Kolawole TM. Cutaneous meningiomas and an ovarian fibroma in a three-year-old girl. J Pathol. 1972;108:165-167.
- Lopez DA, Silvers DN, Helwig EB. Cutaneous meningioma—a clinicopathologic study. Cancer. 1974;34:728-744.
- Shuangshoti S, Boonjunwetwat D, Kaoroptham S. Association of primary intraspinal meningiomas and subcutaneous meningioma of the cervical region: case report and review of literature. Surg Neurol. 1992;38:129-134.
- Miedema JR, Zedek D. Cutaneous meningioma. Arch Pathol. 2012;136:208-211.
- Mackay B, Bruner JM, Luna MA. Malignant meningioma of the scalp. Ultrastruc Pathol. 1994;18:235-240.
- Whittle IR, Smith C, Navoo P, et al. Meningiomas. Lancet. 2004;363:1535-1543.
- Bauman G, Fisher B, Schild S, et al. Meningioma, ependymoma, and other adult brain tumors. In: Gunderson LL, Tepper JE, eds. Clinical Radiation Oncology. Philadelphia, PA: Elsevier Churchill Livingstone; 2007:539-566.
- Claus EB, Bondy ML, Schildkraut JM, et al. Epidemiology of intracranial meningioma. Neurosurgery. 2005;57:1088-1095.
- Tworek JA, Mikhail AA, Blaivas M. Meningioma: local recurrence and pulmonary metastasis diagnosed by fine needle aspiration. Acta Cytol. 1997;41:946-947.
- Shin MS, Holman WL, Herrera GA, et al. Extensive pulmonary metastasis of an intracranial meningioma with repeated recurrence: radiographic and pathologic features. South Med J. 1996;89:313-318.
- Ferguson JM, Flinn J. Intracranial meningioma with hepatic metastases and hypoglycaemia treated by selective hepatic arterial chemo-embolization. Australas Radiol.1995;39:97-99.
- Palmer JD, Cook PL, Ellison DW. Extracranial osseous metastases from intracranial meningioma. Br J Neurosurg. 1994;8:215-218.
- Glasauer FE, Yuan RH. Intracranial tumours with extracranial metastases. case report and review of the literature. J Neurosurg. 1963;20:474-493.
- Shuangshoti S, Hongsaprabhas C, Netsky MG. Metastasizing meningioma. Cancer. 1970;26:832-841.
- Ohta M, Iwaki T, Kitamoto T, et al. MIB-1 staining index and scoring of histological features in meningioma. Cancer. 1994;74:3176-3189.
- Wrensch M, Minn Y, Chew T, et al. Epidemiology of primary brain tumors: current concepts and review of the literature. Neuro Oncol. 2002;4:278-299.
- Shintani T, Hayakawa N, Hoshi M, et al. High incidence of meningioma among Hiroshima atomic bomb survivors. J Rad Res. 1999;40:49-57.
- Claus EB, Calvocoressi L, Bondy ML, et al. Dental x-rays and risk of meningioma. Cancer. 2012;118:4530-4537.
- Blitshteyn S, Crook JE, Jaeckle KA. Is there an association between meningioma and hormone replacement therapy? J Clin Oncol. 2008;26:279-282.
- Fontaine B, Rouleau GA, Seizinger BR, et al. Molecular genetics of neurofibromatosis 2 and related tumors (acoustic neuromas and meningioma). Ann N Y Acad Sci. 1991;615:338-343.
- Rabin BM, Meyer JR, Berlin JW, et al. Radiation-induced changes of the central nervous system and head and neck. Radiographics. 1996;16:1055-1072.
- Tanaka S, Okazaki M, Egusa G, et al. A case of pheochromocytoma associated with meningioma. J Intern Med. 1991;229:371-373.
- Zeikus P, Robinson-Bostom L, Stopa E. Primary cutaneous meningioma in association with a sinus pericranii. J Am Acad Dermatol. 2006;54(2 suppl):S49-S50.
- Junaid TA, Nkposong EO, Kolawole TM. Cutaneous meningiomas and an ovarian fibroma in a three-year-old girl. J Pathol. 1972;108:165-167.
- Lopez DA, Silvers DN, Helwig EB. Cutaneous meningioma—a clinicopathologic study. Cancer. 1974;34:728-744.
- Shuangshoti S, Boonjunwetwat D, Kaoroptham S. Association of primary intraspinal meningiomas and subcutaneous meningioma of the cervical region: case report and review of literature. Surg Neurol. 1992;38:129-134.
- Miedema JR, Zedek D. Cutaneous meningioma. Arch Pathol. 2012;136:208-211.
Squamoid Eccrine Ductal Carcinoma
Eccrine carcinomas are uncommon cutaneous neoplasms demonstrating nonuniform histologic features, behavior, and nomenclature. Given the rarity of these tumors, no known criteria by which to diagnose the tumor or guidelines for treatment have been proposed. We report a rare case of an immunocompromised patient with a primary squamoid eccrine ductal carcinoma (SEDC) who was subsequently treated with radical resection and axillary dissection. It was later determined that the patient had distant metastasis of SEDC. A review of the literature on the diagnosis, treatment, and surveillance of SEDC also is provided.
Case Report
A 77-year-old man whose medical history was remarkable for chronic lymphocytic leukemia (CLL) and numerous previous basal cell carcinomas and squamous cell carcinomas (SCCs) presented with a 5-cm, stellate, sclerotic plaque on the left chest of approximately 2 years’ duration (Figure 1) and a 3-mm pink papule on the right nasal sidewall of 2 months’ duration. Initial histology of both lesions revealed carcinoma with squamous and ductal differentiation extending from the undersurface of the epidermis, favoring a diagnosis of SEDC (Figure 2). At the time of initial presentation, the patient also had a 6-mm pink papule on the right chest of several months duration that was consistent with a well-differentiated sebaceous carcinoma on histology.
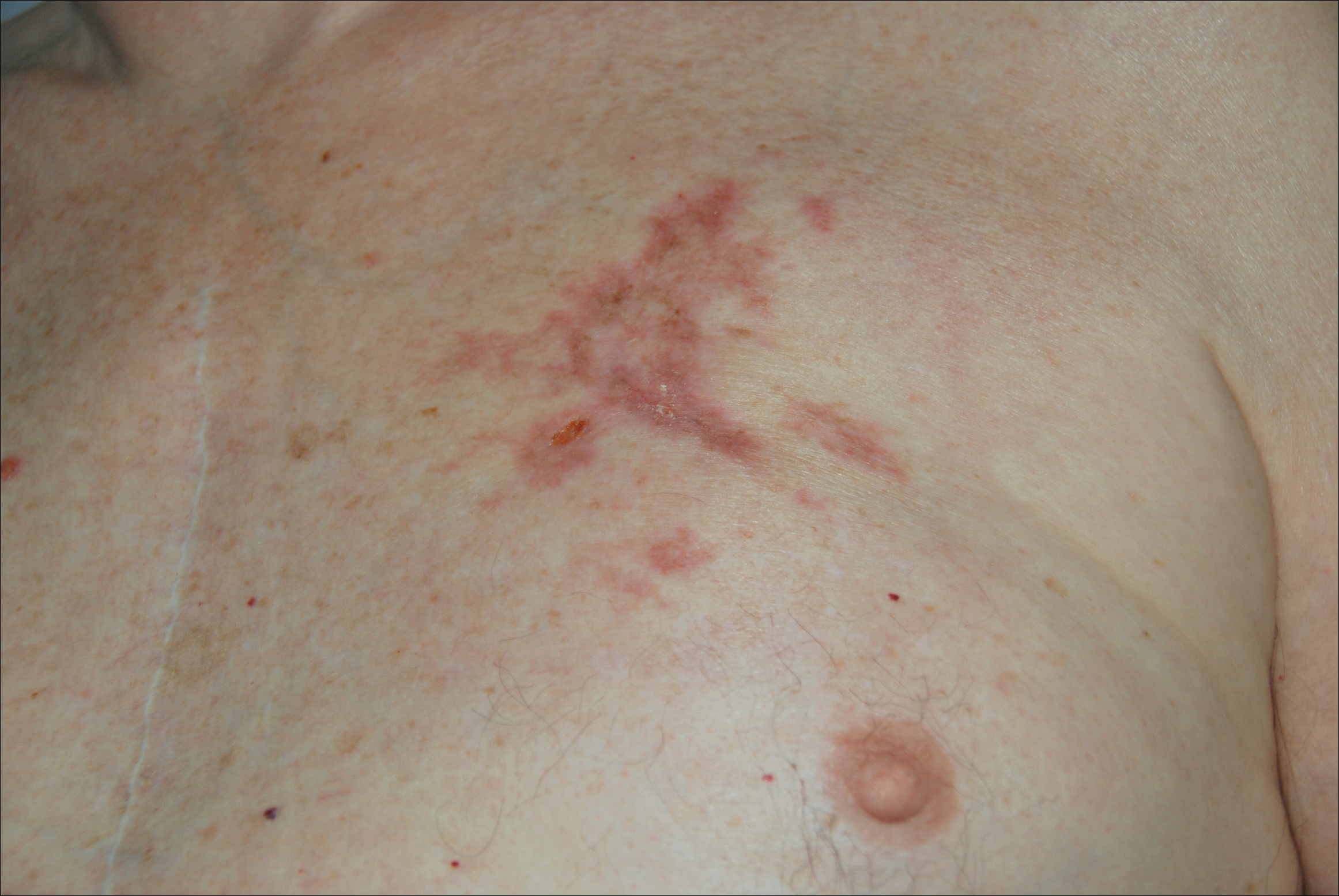
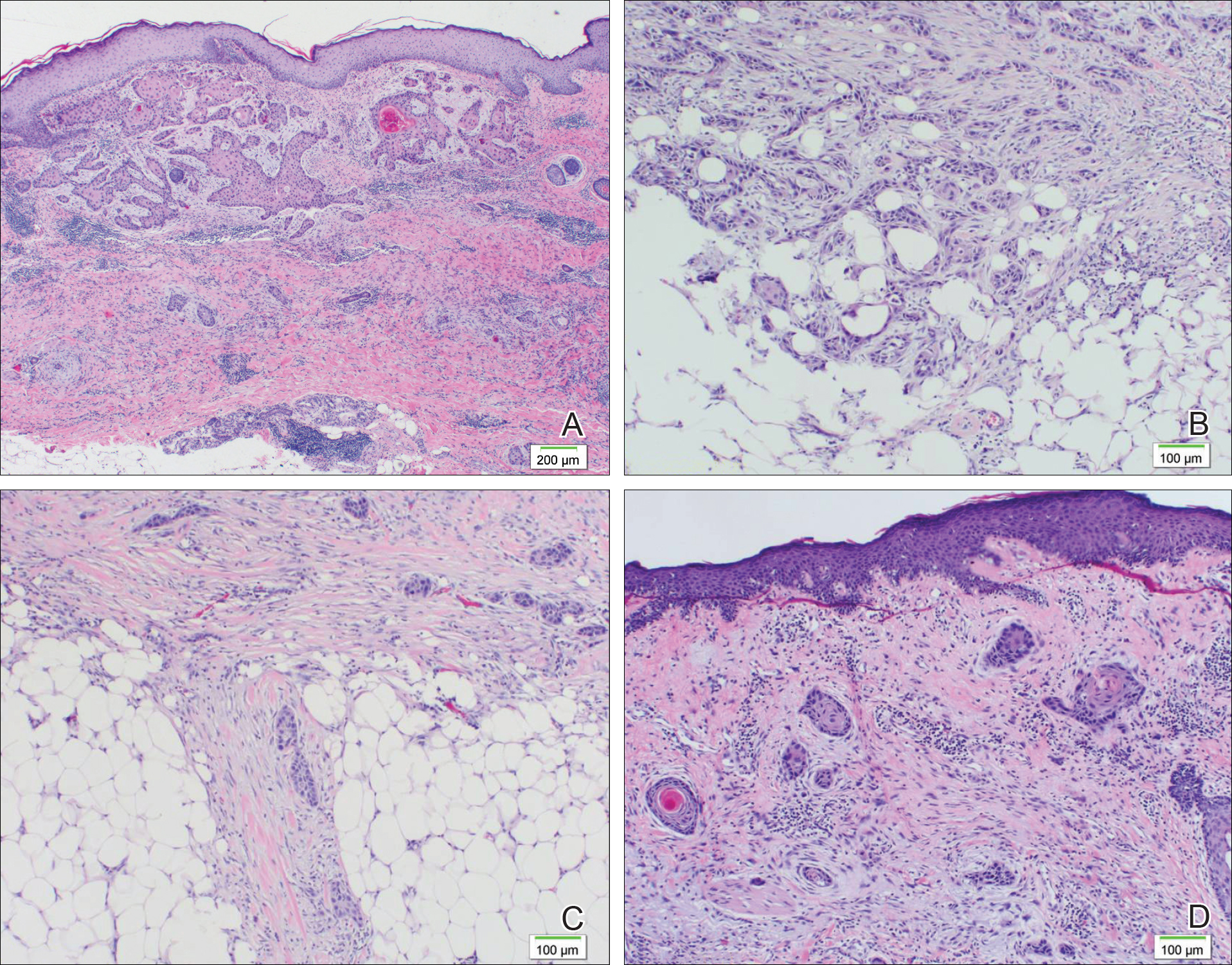
Further analysis of the lesion on the left chest revealed positive staining for cytokeratin (CK) 5/14 and p63, suggestive of a cutaneous malignancy. Staining for S100 protein highlighted rare cells in the basal layer of tumor aggregates. The immunohistochemical profile showed negative staining for CK7, CK5D3, epithelial membrane antigen (EMA), estrogen receptor, progesterone receptor, and human epidermal growth factor 2.
Diagnosis of SEDC of the chest and nasal lesions was based on the morphologic architecture, which included ductal formation noted within the tumor. The chest lesion also had prominent squamoid differentiation. Another histologic feature consistent with SEDC was poorly demarcated, infiltrative neoplastic cells extending into the dermis and subcutis. Although there was some positive focal staining for carcinoembryonic antigen (CEA), variegation within the tumor and the prominent squamoid component might have contributed to this unexpected staining pattern.
The patient was admitted to the hospital for excision of the lesion on the chest wall. Initial workup revealed macrocytic anemia, which required transfusion, and an incidental finding of non–small-cell lung cancer. The chest lesion was unrelated to the non–small-cell lung cancer based on the staining profile. Material from the lung stained positive for thyroid transcription factor 1 (TTF-1) and exhibited rare staining for p63; however, the chest lesion did not stain positive for TTF-1 and had strong staining affinity for p63, indicative of a cutaneous malignancy.
The lesion on the chest wall was definitively excised. Pathologic analysis revealed a dermal-based infiltrative tumor of irregular nests and cords of squamoid cells with focal ductal formation in a fibromyxoid background stroma, suggestive of an adnexal carcinoma with a considerable degree of squamous differentiation and favoring a diagnosis of SEDC. Focal perineural invasion was noted, but no lymphovascular spread was identified; however, metastasis was identified in 1 of 26 axillary lymph nodes. The patient underwent 9 sessions of radiation therapy for the lung cancer and also was given cetuximab.
Three months later, the nasal tumor was subsequently excised in an outpatient procedure, and the final biopsy report indicated a diagnosis of basal cell carcinoma. One-and-a-half years later, in follow-up with surgery after removal of the chest lesion, a 2×3-cm mass was excised from the left neck that demonstrated lymph nodes consistent with metastatic SEDC. Careful evaluation of this patient, including family history and genetic screening, was considered. Our patient continues to follow-up with the dermatology department every 3 months. He has been doing well and has had multiple additional primary SCCs in the subsequent 5 years of follow-up.
Comment
Eccrine carcinoma is the most common subtype of adnexal carcinoma, representing 0.01% of all cutaneous tumors.1 S
Eccrine carcinoma is observed clinically as a slow-growing, nodular plaque on the scalp, arms, legs, or trunk in middle-aged and elderly individuals.1 Squamoid eccrine ductal carcinoma also has been reported in a young woman.5 Another immunocompromised patient was identified in the literature with a great toe lesion that showed follicular differentiation along with the usual SEDC features of squamoid and ductal differentiation.6 The etiology of SEDC is controversial but is thought to be an SCC arising from eccrine glands, a subtype of eccrine carcinoma with extensive squamoid differentiation, or a biphenotypic carcinoma.1,7
Histologically, SEDC is poorly circumscribed with an infiltrative growth pattern and deep extension into the dermis and subcutaneous tissue. The lesion is characterized by prominent squamous epithelial proliferation superficially with cellular atypia, keratinous cyst formation, squamous eddies, and eccrine ductal differentiation.1
The differential diagnosis of SEDC includes SCC; metastatic carcinoma with squamoid features; and eccrine tumors, including eccrine poroma, microcystic adnexal carcinoma, and porocarcinoma with squamous differentiation.1
Immunohistochemistry has a role in the diagnosis of SEDC. Findings include positive staining for S100 protein, EMA, CKs, and CEA. Glandular tissue stains positive for EMA and CEA, supporting an adnexal origin.1 Positivity for p63 and CK5/6 supports the conclusion that this is a primary cutaneous malignancy, not a metastatic disease.1
Squamoid eccrine ductal carcinoma has an indeterminate malignant potential. There is a disparity of clinical behavior between SCC and eccrine cancers; however, because squamous differentiation sometimes dominates the histological picture, eccrine carcinomas can be misdiagnosed as SCC.1,8 Eccrine adnexal tumors are characterized by multiple local recurrences (70%–80% of cases); perineural invasion; and metastasis (50% of cases) to regional lymph nodes and viscera, including the lungs, liver, bones, and brain.1 Squamous cell carcinoma, however, has a markedly lower recurrence rate (3.1%–18.7% of cases) and rate of metastasis (5.2%–37.8%).1
Squamoid eccrine ductal carcinoma is classified as one of the less aggressive eccrine tumors, although the low number of cases makes it a controversial conclusion.1 To our knowledge, no cases of SEDC metastasis have been reported with SEDC. Recurrence of SEDC has been reported locally, and perineural or perivascular invasion (or both) has been demonstrated in 3 cases.1
Since SEDC has invasive and metastatic potential, as demonstrated in our case, along with elevated local recurrence rates, physicians must be able to properly diagnose this rare entity and recommend an appropriate surgical modality. Due to the low incidence of SEDC, there are no known randomized studies comparing treatment modalities.1 O
Surgical extirpation with complete margin examination is recommended, as SEDC tends to be underestimated in size, is aggressive in its infiltration, and is predisposed to perineural and perivascular invasion. T
Along with the rarity of SEDC in our patient, the simultaneous occurrence of 3 primary malignancies also is unusual. Patients with CLL have progressive defects of cell- and humoral-mediated immunity, causing immunosuppression. In a retrospective study, Tsimberidou et al9 reviewed the records of 2028 untreated CLL patients and determined that 27% had another primary malignancy, including skin (30%) and lung cancers (6%), which were two of the malignancies seen in our patient. The investigators concluded that patients with CLL have more than twice the risk of developing a second primary malignancy and an increased frequency of certain cancer types.9 Furthermore, treatment regimens for CLL have been considered to increase cell- and humoral-mediated immune defects at specific cancer sites,10 although the exact mechanism of this action is unknown. Development of a second primary malignancy (or even a third) in patients with SEDC is increasingly being reported in CLL patients.9,10
A high index of suspicion with SEDC in the differential diagnosis should be maintained in elderly men with slow-growing, solitary, nodular lesions of the scalp, nose, arms, legs, or trunk.
- Clark S, Young A, Piatigorsky E, et al. Mohs micrographic surgery in the setting of squamoid eccrine ductal carcinoma: addressing a diagnostic and therapeutic challenge. Clin Aesthet Dermatol. 2013;6:33-36.
- Saraiva MI, Vieira MA, Portocarrero LK, et al. Squamoid eccrine ductal carcinoma. An Bras Dermatol. 2016;916:799-802.
- van der Horst MP, Garcia-Herrera A, Markiewicz D, et al. Squamoid eccrine ductal carcinoma: a clinicopathologic study of 30 cases. Am J Surg Pathol. 2016;40:755-760.
- Frouin E, Vignon-Pennamen MD, Balme B, et al. Anatomoclinical study of 30 cases of sclerosing sweat duct carcinomas (microcystic adnexal carcinoma, syringomatous carcinoma and squamoid eccrine ductal carcinoma)[published online April 15, 2015]. J Eur Acad Dermatol Venereol. 2015;29:1978-1994.
- Kim YJ, Kim AR, Yu DS. Mohs micrographic surgery for squamoid eccrine ductal carcinoma. Dermatol Surg. 2005;31:1462-1464.
- Kavand S, Cassarino DS. Squamoid eccrine ductal carcinoma: an unusual low-grade case with follicular differentiation. are these tumors squamoid variants of microcystic adnexal carcinoma? Am J Dermatopathol. 2009;31:849-852.
- Terushkin E, Leffell DJ, Futoryan T, et al. Squamoid eccrine ductal carcinoma: a case report and review of the literature. Am J Dermatopathol. 2010;32:287-292.
- Chhibber V, Lyle S, Mahalingam M. Ductal eccrine carcinoma with squamous differentiation: apropos a case. J Cutan Pathol. 2007;34:503-507.
- Tsimberidou AM, Wen S, McLaughlin P, et al. Other malignancies in chronic lymphocytic leukemia/small lymphocytic lymphoma. J Clin Oncol. 2009;27:904-910.
- Dasanu CA, Alexandrescu DT. Risk for second nonlymphoid neoplasms in chronic lymphocytic leukemia. Med Gen Med. 2007;9:35.
Eccrine carcinomas are uncommon cutaneous neoplasms demonstrating nonuniform histologic features, behavior, and nomenclature. Given the rarity of these tumors, no known criteria by which to diagnose the tumor or guidelines for treatment have been proposed. We report a rare case of an immunocompromised patient with a primary squamoid eccrine ductal carcinoma (SEDC) who was subsequently treated with radical resection and axillary dissection. It was later determined that the patient had distant metastasis of SEDC. A review of the literature on the diagnosis, treatment, and surveillance of SEDC also is provided.
Case Report
A 77-year-old man whose medical history was remarkable for chronic lymphocytic leukemia (CLL) and numerous previous basal cell carcinomas and squamous cell carcinomas (SCCs) presented with a 5-cm, stellate, sclerotic plaque on the left chest of approximately 2 years’ duration (Figure 1) and a 3-mm pink papule on the right nasal sidewall of 2 months’ duration. Initial histology of both lesions revealed carcinoma with squamous and ductal differentiation extending from the undersurface of the epidermis, favoring a diagnosis of SEDC (Figure 2). At the time of initial presentation, the patient also had a 6-mm pink papule on the right chest of several months duration that was consistent with a well-differentiated sebaceous carcinoma on histology.


Further analysis of the lesion on the left chest revealed positive staining for cytokeratin (CK) 5/14 and p63, suggestive of a cutaneous malignancy. Staining for S100 protein highlighted rare cells in the basal layer of tumor aggregates. The immunohistochemical profile showed negative staining for CK7, CK5D3, epithelial membrane antigen (EMA), estrogen receptor, progesterone receptor, and human epidermal growth factor 2.
Diagnosis of SEDC of the chest and nasal lesions was based on the morphologic architecture, which included ductal formation noted within the tumor. The chest lesion also had prominent squamoid differentiation. Another histologic feature consistent with SEDC was poorly demarcated, infiltrative neoplastic cells extending into the dermis and subcutis. Although there was some positive focal staining for carcinoembryonic antigen (CEA), variegation within the tumor and the prominent squamoid component might have contributed to this unexpected staining pattern.
The patient was admitted to the hospital for excision of the lesion on the chest wall. Initial workup revealed macrocytic anemia, which required transfusion, and an incidental finding of non–small-cell lung cancer. The chest lesion was unrelated to the non–small-cell lung cancer based on the staining profile. Material from the lung stained positive for thyroid transcription factor 1 (TTF-1) and exhibited rare staining for p63; however, the chest lesion did not stain positive for TTF-1 and had strong staining affinity for p63, indicative of a cutaneous malignancy.
The lesion on the chest wall was definitively excised. Pathologic analysis revealed a dermal-based infiltrative tumor of irregular nests and cords of squamoid cells with focal ductal formation in a fibromyxoid background stroma, suggestive of an adnexal carcinoma with a considerable degree of squamous differentiation and favoring a diagnosis of SEDC. Focal perineural invasion was noted, but no lymphovascular spread was identified; however, metastasis was identified in 1 of 26 axillary lymph nodes. The patient underwent 9 sessions of radiation therapy for the lung cancer and also was given cetuximab.
Three months later, the nasal tumor was subsequently excised in an outpatient procedure, and the final biopsy report indicated a diagnosis of basal cell carcinoma. One-and-a-half years later, in follow-up with surgery after removal of the chest lesion, a 2×3-cm mass was excised from the left neck that demonstrated lymph nodes consistent with metastatic SEDC. Careful evaluation of this patient, including family history and genetic screening, was considered. Our patient continues to follow-up with the dermatology department every 3 months. He has been doing well and has had multiple additional primary SCCs in the subsequent 5 years of follow-up.
Comment
Eccrine carcinoma is the most common subtype of adnexal carcinoma, representing 0.01% of all cutaneous tumors.1 S
Eccrine carcinoma is observed clinically as a slow-growing, nodular plaque on the scalp, arms, legs, or trunk in middle-aged and elderly individuals.1 Squamoid eccrine ductal carcinoma also has been reported in a young woman.5 Another immunocompromised patient was identified in the literature with a great toe lesion that showed follicular differentiation along with the usual SEDC features of squamoid and ductal differentiation.6 The etiology of SEDC is controversial but is thought to be an SCC arising from eccrine glands, a subtype of eccrine carcinoma with extensive squamoid differentiation, or a biphenotypic carcinoma.1,7
Histologically, SEDC is poorly circumscribed with an infiltrative growth pattern and deep extension into the dermis and subcutaneous tissue. The lesion is characterized by prominent squamous epithelial proliferation superficially with cellular atypia, keratinous cyst formation, squamous eddies, and eccrine ductal differentiation.1
The differential diagnosis of SEDC includes SCC; metastatic carcinoma with squamoid features; and eccrine tumors, including eccrine poroma, microcystic adnexal carcinoma, and porocarcinoma with squamous differentiation.1
Immunohistochemistry has a role in the diagnosis of SEDC. Findings include positive staining for S100 protein, EMA, CKs, and CEA. Glandular tissue stains positive for EMA and CEA, supporting an adnexal origin.1 Positivity for p63 and CK5/6 supports the conclusion that this is a primary cutaneous malignancy, not a metastatic disease.1
Squamoid eccrine ductal carcinoma has an indeterminate malignant potential. There is a disparity of clinical behavior between SCC and eccrine cancers; however, because squamous differentiation sometimes dominates the histological picture, eccrine carcinomas can be misdiagnosed as SCC.1,8 Eccrine adnexal tumors are characterized by multiple local recurrences (70%–80% of cases); perineural invasion; and metastasis (50% of cases) to regional lymph nodes and viscera, including the lungs, liver, bones, and brain.1 Squamous cell carcinoma, however, has a markedly lower recurrence rate (3.1%–18.7% of cases) and rate of metastasis (5.2%–37.8%).1
Squamoid eccrine ductal carcinoma is classified as one of the less aggressive eccrine tumors, although the low number of cases makes it a controversial conclusion.1 To our knowledge, no cases of SEDC metastasis have been reported with SEDC. Recurrence of SEDC has been reported locally, and perineural or perivascular invasion (or both) has been demonstrated in 3 cases.1
Since SEDC has invasive and metastatic potential, as demonstrated in our case, along with elevated local recurrence rates, physicians must be able to properly diagnose this rare entity and recommend an appropriate surgical modality. Due to the low incidence of SEDC, there are no known randomized studies comparing treatment modalities.1 O
Surgical extirpation with complete margin examination is recommended, as SEDC tends to be underestimated in size, is aggressive in its infiltration, and is predisposed to perineural and perivascular invasion. T
Along with the rarity of SEDC in our patient, the simultaneous occurrence of 3 primary malignancies also is unusual. Patients with CLL have progressive defects of cell- and humoral-mediated immunity, causing immunosuppression. In a retrospective study, Tsimberidou et al9 reviewed the records of 2028 untreated CLL patients and determined that 27% had another primary malignancy, including skin (30%) and lung cancers (6%), which were two of the malignancies seen in our patient. The investigators concluded that patients with CLL have more than twice the risk of developing a second primary malignancy and an increased frequency of certain cancer types.9 Furthermore, treatment regimens for CLL have been considered to increase cell- and humoral-mediated immune defects at specific cancer sites,10 although the exact mechanism of this action is unknown. Development of a second primary malignancy (or even a third) in patients with SEDC is increasingly being reported in CLL patients.9,10
A high index of suspicion with SEDC in the differential diagnosis should be maintained in elderly men with slow-growing, solitary, nodular lesions of the scalp, nose, arms, legs, or trunk.
Eccrine carcinomas are uncommon cutaneous neoplasms demonstrating nonuniform histologic features, behavior, and nomenclature. Given the rarity of these tumors, no known criteria by which to diagnose the tumor or guidelines for treatment have been proposed. We report a rare case of an immunocompromised patient with a primary squamoid eccrine ductal carcinoma (SEDC) who was subsequently treated with radical resection and axillary dissection. It was later determined that the patient had distant metastasis of SEDC. A review of the literature on the diagnosis, treatment, and surveillance of SEDC also is provided.
Case Report
A 77-year-old man whose medical history was remarkable for chronic lymphocytic leukemia (CLL) and numerous previous basal cell carcinomas and squamous cell carcinomas (SCCs) presented with a 5-cm, stellate, sclerotic plaque on the left chest of approximately 2 years’ duration (Figure 1) and a 3-mm pink papule on the right nasal sidewall of 2 months’ duration. Initial histology of both lesions revealed carcinoma with squamous and ductal differentiation extending from the undersurface of the epidermis, favoring a diagnosis of SEDC (Figure 2). At the time of initial presentation, the patient also had a 6-mm pink papule on the right chest of several months duration that was consistent with a well-differentiated sebaceous carcinoma on histology.


Further analysis of the lesion on the left chest revealed positive staining for cytokeratin (CK) 5/14 and p63, suggestive of a cutaneous malignancy. Staining for S100 protein highlighted rare cells in the basal layer of tumor aggregates. The immunohistochemical profile showed negative staining for CK7, CK5D3, epithelial membrane antigen (EMA), estrogen receptor, progesterone receptor, and human epidermal growth factor 2.
Diagnosis of SEDC of the chest and nasal lesions was based on the morphologic architecture, which included ductal formation noted within the tumor. The chest lesion also had prominent squamoid differentiation. Another histologic feature consistent with SEDC was poorly demarcated, infiltrative neoplastic cells extending into the dermis and subcutis. Although there was some positive focal staining for carcinoembryonic antigen (CEA), variegation within the tumor and the prominent squamoid component might have contributed to this unexpected staining pattern.
The patient was admitted to the hospital for excision of the lesion on the chest wall. Initial workup revealed macrocytic anemia, which required transfusion, and an incidental finding of non–small-cell lung cancer. The chest lesion was unrelated to the non–small-cell lung cancer based on the staining profile. Material from the lung stained positive for thyroid transcription factor 1 (TTF-1) and exhibited rare staining for p63; however, the chest lesion did not stain positive for TTF-1 and had strong staining affinity for p63, indicative of a cutaneous malignancy.
The lesion on the chest wall was definitively excised. Pathologic analysis revealed a dermal-based infiltrative tumor of irregular nests and cords of squamoid cells with focal ductal formation in a fibromyxoid background stroma, suggestive of an adnexal carcinoma with a considerable degree of squamous differentiation and favoring a diagnosis of SEDC. Focal perineural invasion was noted, but no lymphovascular spread was identified; however, metastasis was identified in 1 of 26 axillary lymph nodes. The patient underwent 9 sessions of radiation therapy for the lung cancer and also was given cetuximab.
Three months later, the nasal tumor was subsequently excised in an outpatient procedure, and the final biopsy report indicated a diagnosis of basal cell carcinoma. One-and-a-half years later, in follow-up with surgery after removal of the chest lesion, a 2×3-cm mass was excised from the left neck that demonstrated lymph nodes consistent with metastatic SEDC. Careful evaluation of this patient, including family history and genetic screening, was considered. Our patient continues to follow-up with the dermatology department every 3 months. He has been doing well and has had multiple additional primary SCCs in the subsequent 5 years of follow-up.
Comment
Eccrine carcinoma is the most common subtype of adnexal carcinoma, representing 0.01% of all cutaneous tumors.1 S
Eccrine carcinoma is observed clinically as a slow-growing, nodular plaque on the scalp, arms, legs, or trunk in middle-aged and elderly individuals.1 Squamoid eccrine ductal carcinoma also has been reported in a young woman.5 Another immunocompromised patient was identified in the literature with a great toe lesion that showed follicular differentiation along with the usual SEDC features of squamoid and ductal differentiation.6 The etiology of SEDC is controversial but is thought to be an SCC arising from eccrine glands, a subtype of eccrine carcinoma with extensive squamoid differentiation, or a biphenotypic carcinoma.1,7
Histologically, SEDC is poorly circumscribed with an infiltrative growth pattern and deep extension into the dermis and subcutaneous tissue. The lesion is characterized by prominent squamous epithelial proliferation superficially with cellular atypia, keratinous cyst formation, squamous eddies, and eccrine ductal differentiation.1
The differential diagnosis of SEDC includes SCC; metastatic carcinoma with squamoid features; and eccrine tumors, including eccrine poroma, microcystic adnexal carcinoma, and porocarcinoma with squamous differentiation.1
Immunohistochemistry has a role in the diagnosis of SEDC. Findings include positive staining for S100 protein, EMA, CKs, and CEA. Glandular tissue stains positive for EMA and CEA, supporting an adnexal origin.1 Positivity for p63 and CK5/6 supports the conclusion that this is a primary cutaneous malignancy, not a metastatic disease.1
Squamoid eccrine ductal carcinoma has an indeterminate malignant potential. There is a disparity of clinical behavior between SCC and eccrine cancers; however, because squamous differentiation sometimes dominates the histological picture, eccrine carcinomas can be misdiagnosed as SCC.1,8 Eccrine adnexal tumors are characterized by multiple local recurrences (70%–80% of cases); perineural invasion; and metastasis (50% of cases) to regional lymph nodes and viscera, including the lungs, liver, bones, and brain.1 Squamous cell carcinoma, however, has a markedly lower recurrence rate (3.1%–18.7% of cases) and rate of metastasis (5.2%–37.8%).1
Squamoid eccrine ductal carcinoma is classified as one of the less aggressive eccrine tumors, although the low number of cases makes it a controversial conclusion.1 To our knowledge, no cases of SEDC metastasis have been reported with SEDC. Recurrence of SEDC has been reported locally, and perineural or perivascular invasion (or both) has been demonstrated in 3 cases.1
Since SEDC has invasive and metastatic potential, as demonstrated in our case, along with elevated local recurrence rates, physicians must be able to properly diagnose this rare entity and recommend an appropriate surgical modality. Due to the low incidence of SEDC, there are no known randomized studies comparing treatment modalities.1 O
Surgical extirpation with complete margin examination is recommended, as SEDC tends to be underestimated in size, is aggressive in its infiltration, and is predisposed to perineural and perivascular invasion. T
Along with the rarity of SEDC in our patient, the simultaneous occurrence of 3 primary malignancies also is unusual. Patients with CLL have progressive defects of cell- and humoral-mediated immunity, causing immunosuppression. In a retrospective study, Tsimberidou et al9 reviewed the records of 2028 untreated CLL patients and determined that 27% had another primary malignancy, including skin (30%) and lung cancers (6%), which were two of the malignancies seen in our patient. The investigators concluded that patients with CLL have more than twice the risk of developing a second primary malignancy and an increased frequency of certain cancer types.9 Furthermore, treatment regimens for CLL have been considered to increase cell- and humoral-mediated immune defects at specific cancer sites,10 although the exact mechanism of this action is unknown. Development of a second primary malignancy (or even a third) in patients with SEDC is increasingly being reported in CLL patients.9,10
A high index of suspicion with SEDC in the differential diagnosis should be maintained in elderly men with slow-growing, solitary, nodular lesions of the scalp, nose, arms, legs, or trunk.
- Clark S, Young A, Piatigorsky E, et al. Mohs micrographic surgery in the setting of squamoid eccrine ductal carcinoma: addressing a diagnostic and therapeutic challenge. Clin Aesthet Dermatol. 2013;6:33-36.
- Saraiva MI, Vieira MA, Portocarrero LK, et al. Squamoid eccrine ductal carcinoma. An Bras Dermatol. 2016;916:799-802.
- van der Horst MP, Garcia-Herrera A, Markiewicz D, et al. Squamoid eccrine ductal carcinoma: a clinicopathologic study of 30 cases. Am J Surg Pathol. 2016;40:755-760.
- Frouin E, Vignon-Pennamen MD, Balme B, et al. Anatomoclinical study of 30 cases of sclerosing sweat duct carcinomas (microcystic adnexal carcinoma, syringomatous carcinoma and squamoid eccrine ductal carcinoma)[published online April 15, 2015]. J Eur Acad Dermatol Venereol. 2015;29:1978-1994.
- Kim YJ, Kim AR, Yu DS. Mohs micrographic surgery for squamoid eccrine ductal carcinoma. Dermatol Surg. 2005;31:1462-1464.
- Kavand S, Cassarino DS. Squamoid eccrine ductal carcinoma: an unusual low-grade case with follicular differentiation. are these tumors squamoid variants of microcystic adnexal carcinoma? Am J Dermatopathol. 2009;31:849-852.
- Terushkin E, Leffell DJ, Futoryan T, et al. Squamoid eccrine ductal carcinoma: a case report and review of the literature. Am J Dermatopathol. 2010;32:287-292.
- Chhibber V, Lyle S, Mahalingam M. Ductal eccrine carcinoma with squamous differentiation: apropos a case. J Cutan Pathol. 2007;34:503-507.
- Tsimberidou AM, Wen S, McLaughlin P, et al. Other malignancies in chronic lymphocytic leukemia/small lymphocytic lymphoma. J Clin Oncol. 2009;27:904-910.
- Dasanu CA, Alexandrescu DT. Risk for second nonlymphoid neoplasms in chronic lymphocytic leukemia. Med Gen Med. 2007;9:35.
- Clark S, Young A, Piatigorsky E, et al. Mohs micrographic surgery in the setting of squamoid eccrine ductal carcinoma: addressing a diagnostic and therapeutic challenge. Clin Aesthet Dermatol. 2013;6:33-36.
- Saraiva MI, Vieira MA, Portocarrero LK, et al. Squamoid eccrine ductal carcinoma. An Bras Dermatol. 2016;916:799-802.
- van der Horst MP, Garcia-Herrera A, Markiewicz D, et al. Squamoid eccrine ductal carcinoma: a clinicopathologic study of 30 cases. Am J Surg Pathol. 2016;40:755-760.
- Frouin E, Vignon-Pennamen MD, Balme B, et al. Anatomoclinical study of 30 cases of sclerosing sweat duct carcinomas (microcystic adnexal carcinoma, syringomatous carcinoma and squamoid eccrine ductal carcinoma)[published online April 15, 2015]. J Eur Acad Dermatol Venereol. 2015;29:1978-1994.
- Kim YJ, Kim AR, Yu DS. Mohs micrographic surgery for squamoid eccrine ductal carcinoma. Dermatol Surg. 2005;31:1462-1464.
- Kavand S, Cassarino DS. Squamoid eccrine ductal carcinoma: an unusual low-grade case with follicular differentiation. are these tumors squamoid variants of microcystic adnexal carcinoma? Am J Dermatopathol. 2009;31:849-852.
- Terushkin E, Leffell DJ, Futoryan T, et al. Squamoid eccrine ductal carcinoma: a case report and review of the literature. Am J Dermatopathol. 2010;32:287-292.
- Chhibber V, Lyle S, Mahalingam M. Ductal eccrine carcinoma with squamous differentiation: apropos a case. J Cutan Pathol. 2007;34:503-507.
- Tsimberidou AM, Wen S, McLaughlin P, et al. Other malignancies in chronic lymphocytic leukemia/small lymphocytic lymphoma. J Clin Oncol. 2009;27:904-910.
- Dasanu CA, Alexandrescu DT. Risk for second nonlymphoid neoplasms in chronic lymphocytic leukemia. Med Gen Med. 2007;9:35.
Practice Points
- Squamoid eccrine ductal carcinoma (SEDC) is an extremely rare cutaneous tumor of unknown etiology.
- A high index of suspicion with SEDC in the differential diagnosis should be maintained in elderly men with slow-growing, solitary, nodular lesions of the scalp, nose, arms, legs, or trunk.
- Development of a second or even a third primary malignancy in patients with SEDC is increasingly being reported in CLL patients.
Pigmented Squamous Cell Carcinoma Presenting as Longitudinal Melanonychia in a Transplant Recipient
Case Report
A 62-year-old black man presented for examination of a dark longitudinal streak located adjacent to the lateral nail fold on the third finger of the left hand. The lesion had been present for several months, during which time it had slowly expanded in size. The fingertip had recently become tender, which interfered with the patient’s ability to work. His past medical history was remarkable for end-stage renal disease secondary to glomerulonephritis with nephrotic syndrome of unclear etiology. He initially was treated by an outside physician using peritoneal dialysis for 3 years until he underwent renal transplantation in 2004 with a cadaveric organ. Other remarkable medical conditions included posttransplantation diabetes, hyperlipidemia, and gout. His multidrug regimen included 2 immunosuppressive medications: oral cyclosporine 125 mg twice daily and oral mycophenolate mofetil 250 mg twice daily.
A broad, irregular, black, pigmented, subungual band was noted on the left third finger. The lesion appeared to emanate from below the nail cuticle and traveled along the nail longitudinally toward the distal tip. The band appeared darker at the edge adjacent to the lateral nail fold and grew lighter near the middle of the nail where its free edge was noted to be irregular. A slightly thickened lateral nail fold with an irregular, small, sawtoothlike hyperkeratosis and hyperpigmentation also was noted (Figure 1).
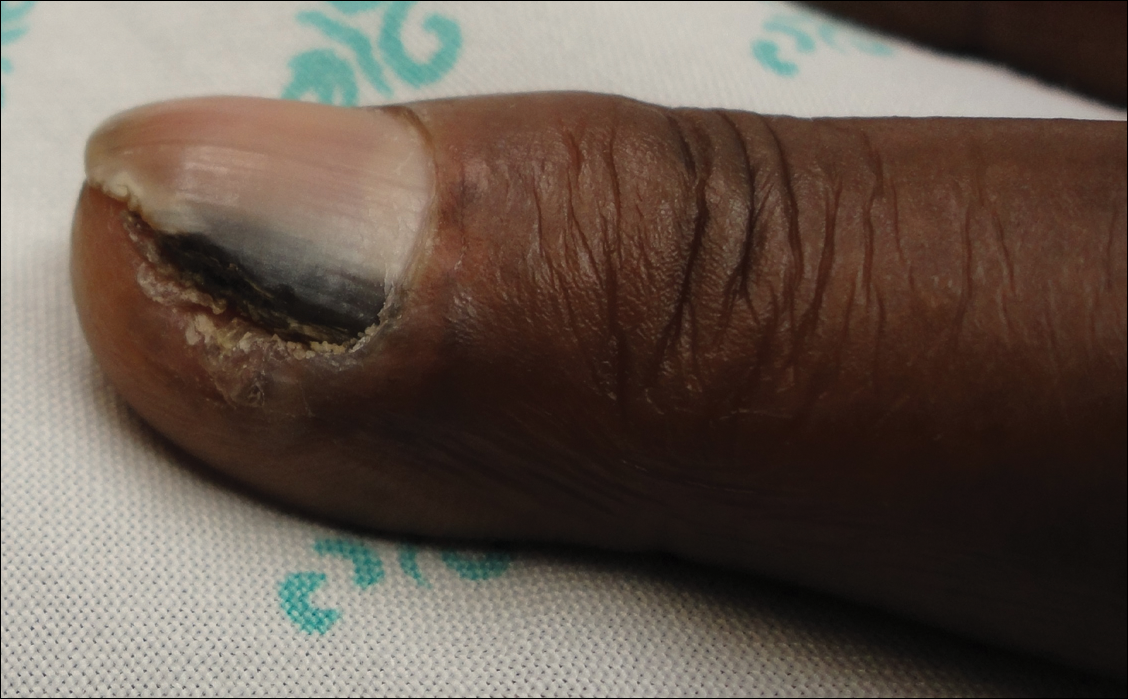
Subungual melanoma, onychomycosis, squamous cell carcinoma (SCC), and a verruca copresenting with onychomycosis were considered in the differential diagnosis. The patient underwent nail avulsion and biopsy of the nail bed as well as the nail matrix. Histopathology was notable for malignant dyskeratosis with a lack of nuclear maturation, occasional mitoses, multinucleation, and individual cell keratinization (Figure 2). Immunostaining for S100 was negative, while staining for cytokeratins AE1/AE3 was positive. Deposition of melanin pigment in the malignant dyskeratotic cells was noted. Periodic acid–Schiff staining identified pseudohyphae without invasion of the nail plate. A diagnosis of pigmented SCC (pSCC) was made. The patient’s nail also was sent for fungal cultures that later grew Candida glabrata and Candida parapsilosis.
The patient underwent Mohs micrographic surgery for removal of the pSCC, which was found to be more extensive than originally suspected and required en bloc excision of the nail repaired with a full-thickness skin graft from the left forearm. The area healed well with some hyperpigmentation (Figure 3).
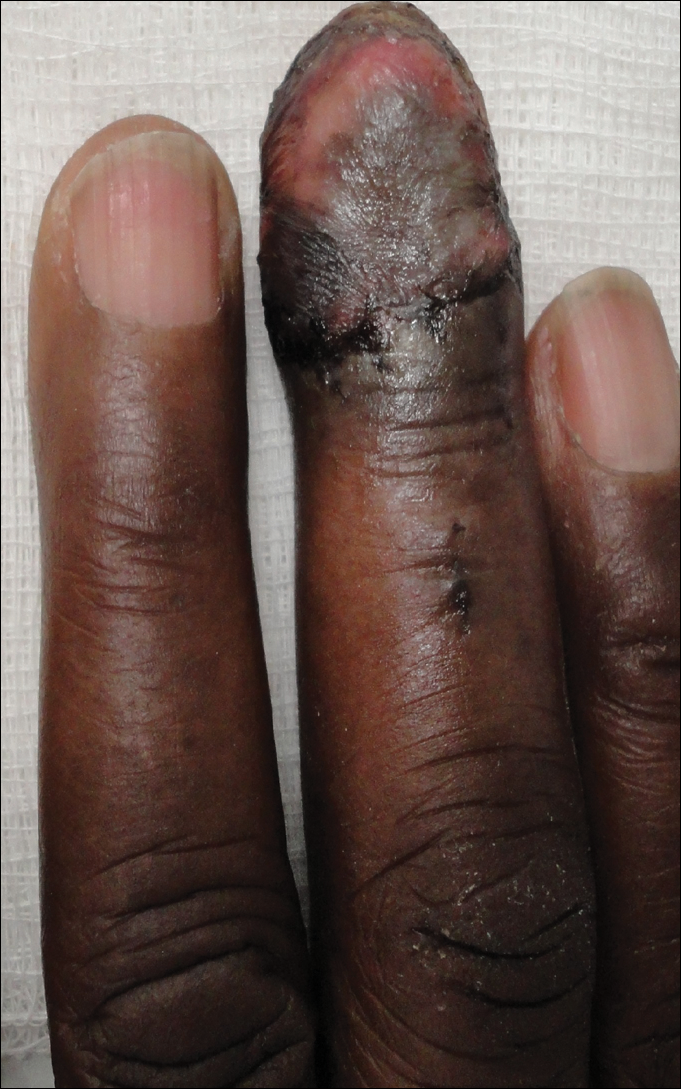
Comment
Among the various types of skin cancer, an estimated 700,000 patients are diagnosed with SCC annually, making it the second most common form of skin cancer in the United States.1 Basal cell carcinoma (BCC) is the most common skin cancer among whites in the United States, while in contrast SCC is the most common skin cancer in patients with skin of color.2 Only an estimated 2% to 5% of all SCCs are pigmented, and this variant is more commonly seen in patients with skin of color.3-5 One analysis of 52 cases of pSCC showed that common features included a flat or slightly raised appearance and hyperpigmentation with varying levels of scaling.6 Studies have shown an altered presentation of pSCC in black skin with increased melanin production and thickness of the stratum corneum in contrast with cases seen in white patients.7 Other potential features include scaling, erosive changes, and sharply demarcated borders. Squamous cell carcinoma typically occurs in sun-exposed areas, reflecting its association with UV light damage; however, SCC in skin of color patients has been noted to occur in sun-protected areas and in areas of chronic scarring.8 Pigmented SCC also appears to follow this distribution, as affected areas are not necessarily in direct exposure to the sun. Pigmented SCCs have been associated with pruritus and/or burning pain, which also was seen in our case when our patient complained of tenderness at the site.
We describe the case of a subungual pSCC clinically presenting as longitudinal melanonychia. Pigmented SCC presenting as longitudinal melanonychia was first described by Baran and Simon in 1988.9 Since that time, it has been reported that approximately 10% of subungual pSCCs clinically present as longitudinal melanonychia.10,11 A retrospective study reviewing 35 cases of SCC of the nail apparatus found that 5 (14.3%) cases presented as longitudinal melanonychia.10 Another retrospective study found that 6 of 51 (11.8%) cases of SCCs affecting the nail unit presented as the warty type of SCC in association with longitudinal melanonychia.12 Cases of pSCC in situ appearing as longitudinal melanonychia also have been reported.13,14
Risk factors for the development of pSCC include advanced age, male sex, presence of human papilloma virus, and use of immunosuppressants.15 Male predominance and advanced age at the time of diagnosis (mean age, 67 years) have been observed in pSCC cases.16 It is now well established that renal transplant recipients have an increased risk of SCC, with a reported incidence rate of 5% to 6%.16 When these patients develop an SCC, they typically follow a more aggressive course. Renal transplantation has a higher ratio than cardiac transplantation for SCC development (2.37:1), whereas cardiac transplantation is associated with a higher risk of BCC development.17 A study of 384 transplant recipients found that 96 (25.0%) had a postsurgical nonmelanoma skin cancer (NMSC), with a ratio of SCC to BCC of 1.2:1.16 The calculated incidence of NMSC at 10 and 20 years posttransplantation was 24.2% and 54.4%, respectively. Another study also determined that SCC rates (50.0%) in postrenal transplant recipients were approximately twice that of BCC (27.0%).18
A daily regimen of immunosuppressive medications such as cyclosporine and mycophenolate mofetil showed an increased risk for development of NMSC.15 Immunosuppressive medications play an important role in the pathogenesis of SCC due to a direct oncogenic effect as well as impairment of the immune system’s ability to fight precancerous developments.15 A 4-year study of 100 renal transplant recipients using mycophenolate mofetil as part of an immunosuppressive regimen reported 22% NMSC findings among 9 patients.19 On average, patients developed an NMSC approximately 61 months posttransplantation, with a wide range from 2 to 120 months.
Advanced age was another important risk factor, with each decade of life producing a 60% increase in instantaneous risk of SCC development for transplant recipients.15 A steady increase in risk was related to the length of time adhering to an immunosuppressive regimen, especially from 2 to 6 years, and then remaining constant in subsequent years. For older patients on immunosuppressant regimens for more than 8 years, the calculated relative risk was noted to be over 200 times greater than the normal population’s development of skin cancers.18
Conclusion
Although cases of pSCC presenting as longitudinal melanonychia have previously been reported,9-14,20 our case is unique in that it describes pSCC in a renal transplant recipient. Our patient had many of the known risk factors for the development of pSCC including male sex, advanced age, skin of color, history of renal transplantation, and immunosuppressive therapy. Although regular full-body skin examinations are an accepted part of renal transplantation follow-up due to SCC risk, our case emphasizes the need to remain vigilant due to possible atypical presentations among the immunosuppressed. The nail unit should not be overlooked during the clinical examination of renal transplant recipients as demonstrated by our patient’s rare presentation of pSCC in the nail.
- Karia PS, Han J, Schmults CD. Cutaneous squamous cell carcinoma: estimated incidence of disease, nodal metastasis, and deaths from disease in the United States, 2012 [published online February 1, 2013]. J Am Acad Dermatol. 2013;68:957-966.
- Tan KB, Tan SH, Aw DC, et al. Simulators of squamous cell carcinoma of the skin: diagnostic challenges on small biopsies and clinicopathological correlation [published online June 25, 2013]. J Skin Cancer. 2013;2013:752864.
- McCall CO, Chen SC. Squamous cell carcinoma of the legs in African Americans. J Am Acad Dermatol. 2002;47:524-529.
- Krishna R, Lewis A, Orengo IF, et al. Pigmented Bowen’s disease (squamous cell carcinoma in situ): a mimic of malignant melanoma. Dermatol Surg. 2001;27:673-674.
- Brinca A, Teixeira V, Goncalo M, et al. A large pigmented lesion mimicking malignant melanoma. Clin Exp Dermatol. 2012;37:817-818.
- Cameron A, Rosendahl C, Tschandl P, et al. Dermatoscopy of pigmented Bowen’s disease. J Am Acad Dermatol. 2010;62:597-604.
- Singh B, Bhaya M, Shaha A, et al. Presentation, course, and outcome of head and neck cancers in African Americans: a case-control study. Laryngoscope. 1998;108(8 pt 1):1159-1163.
- Cancer Facts and Figures 2006. Atlanta, GA: American Cancer Society; 2006.
- Baran R, Simon C. Longitudinal melanonychia: a symptom of Bowen’s disease. J Am Acad Dermatol. 1988;18:1359-1360.
- Dalle S, Depape L, Phan A, et al. Squamous cell carcinoma of the nail apparatus: clinicopathological study of 35 cases. Br J Dermatol. 2007;156:871-874.
- Ishida M, Iwai M, Yoshida K, et al. Subungual pigmented squamous cell carcinoma presenting as longitudinal melanonychia: a case report with review of the literature. Int J Clin Exp Pathol. 2014;7:844-847.
- Lecerf P, Richert B, Theunis A, et al. A retrospective study of squamous cell carcinoma of the nail unit diagnosed in a Belgian general hospital over a 15-year period. J Am Acad Dermatol. 2013;69:253-261.
- Saito T, Uchi H, Moroi Y, et al. Subungual Bowen disease revealed by longitudinal melanonychia. J Am Acad Dermatol. 2012;67:E240-E241.
- Saxena A, Kasper DA, Campanelli CD, et al. Pigmented Bowen’s disease clinically mimicking melanoma on the nail. Dermatol Surg. 2006;32:1522-1525.
- Mackenzie KA, Wells JE, Lynn KL, et al. First and subsequent nonmelanoma skin cancers: incidence and predictors in a population of New Zealand renal transplant recipients. Nephrol Dial Transplant. 2010;25:300-306.
- Gutiérrez-Mendoza D, Narro-Llorente R, Karam-Orantes M, et al. Dermoscopy clues in pigmented Bowen’s disease [published online ahead of print September 16, 2010]. Dermatol Res Pract. 2010;2010.
- Euvards S, Kanitakis J, Pouteil-Noble C, et al. Comparative epidemiologic study of premalignant and malignant epithelial cutaneous lesions developing after kidney and heart transplantation. J Am Acad Dermatol. 1995;33(2 pt 1):222-229.
- Moloney FJ, Comber H, O’Lorcain P, et al. A population-based study of skin cancer incidence and prevalence in renal transplant patients. Br J Dermatol. 2006;154:498-504.
- Formicone F, Fargnoli MC, Pisani F, et al. Cutaneous manifestations in Italian kidney transplant recipients. Transplant Proc. 2005;37:2527-2528.
- Fernandes Massa A, Debarbieux S, Depaepe L, et al. Pigmented squamous cell carcinoma of the nail bed presenting as a melanonychia striata: diagnosis by perioperative reflectance confocal microscopy. Br J Dermatol. 2013;169:198-199.
Case Report
A 62-year-old black man presented for examination of a dark longitudinal streak located adjacent to the lateral nail fold on the third finger of the left hand. The lesion had been present for several months, during which time it had slowly expanded in size. The fingertip had recently become tender, which interfered with the patient’s ability to work. His past medical history was remarkable for end-stage renal disease secondary to glomerulonephritis with nephrotic syndrome of unclear etiology. He initially was treated by an outside physician using peritoneal dialysis for 3 years until he underwent renal transplantation in 2004 with a cadaveric organ. Other remarkable medical conditions included posttransplantation diabetes, hyperlipidemia, and gout. His multidrug regimen included 2 immunosuppressive medications: oral cyclosporine 125 mg twice daily and oral mycophenolate mofetil 250 mg twice daily.
A broad, irregular, black, pigmented, subungual band was noted on the left third finger. The lesion appeared to emanate from below the nail cuticle and traveled along the nail longitudinally toward the distal tip. The band appeared darker at the edge adjacent to the lateral nail fold and grew lighter near the middle of the nail where its free edge was noted to be irregular. A slightly thickened lateral nail fold with an irregular, small, sawtoothlike hyperkeratosis and hyperpigmentation also was noted (Figure 1).

Subungual melanoma, onychomycosis, squamous cell carcinoma (SCC), and a verruca copresenting with onychomycosis were considered in the differential diagnosis. The patient underwent nail avulsion and biopsy of the nail bed as well as the nail matrix. Histopathology was notable for malignant dyskeratosis with a lack of nuclear maturation, occasional mitoses, multinucleation, and individual cell keratinization (Figure 2). Immunostaining for S100 was negative, while staining for cytokeratins AE1/AE3 was positive. Deposition of melanin pigment in the malignant dyskeratotic cells was noted. Periodic acid–Schiff staining identified pseudohyphae without invasion of the nail plate. A diagnosis of pigmented SCC (pSCC) was made. The patient’s nail also was sent for fungal cultures that later grew Candida glabrata and Candida parapsilosis.
The patient underwent Mohs micrographic surgery for removal of the pSCC, which was found to be more extensive than originally suspected and required en bloc excision of the nail repaired with a full-thickness skin graft from the left forearm. The area healed well with some hyperpigmentation (Figure 3).


Comment
Among the various types of skin cancer, an estimated 700,000 patients are diagnosed with SCC annually, making it the second most common form of skin cancer in the United States.1 Basal cell carcinoma (BCC) is the most common skin cancer among whites in the United States, while in contrast SCC is the most common skin cancer in patients with skin of color.2 Only an estimated 2% to 5% of all SCCs are pigmented, and this variant is more commonly seen in patients with skin of color.3-5 One analysis of 52 cases of pSCC showed that common features included a flat or slightly raised appearance and hyperpigmentation with varying levels of scaling.6 Studies have shown an altered presentation of pSCC in black skin with increased melanin production and thickness of the stratum corneum in contrast with cases seen in white patients.7 Other potential features include scaling, erosive changes, and sharply demarcated borders. Squamous cell carcinoma typically occurs in sun-exposed areas, reflecting its association with UV light damage; however, SCC in skin of color patients has been noted to occur in sun-protected areas and in areas of chronic scarring.8 Pigmented SCC also appears to follow this distribution, as affected areas are not necessarily in direct exposure to the sun. Pigmented SCCs have been associated with pruritus and/or burning pain, which also was seen in our case when our patient complained of tenderness at the site.
We describe the case of a subungual pSCC clinically presenting as longitudinal melanonychia. Pigmented SCC presenting as longitudinal melanonychia was first described by Baran and Simon in 1988.9 Since that time, it has been reported that approximately 10% of subungual pSCCs clinically present as longitudinal melanonychia.10,11 A retrospective study reviewing 35 cases of SCC of the nail apparatus found that 5 (14.3%) cases presented as longitudinal melanonychia.10 Another retrospective study found that 6 of 51 (11.8%) cases of SCCs affecting the nail unit presented as the warty type of SCC in association with longitudinal melanonychia.12 Cases of pSCC in situ appearing as longitudinal melanonychia also have been reported.13,14
Risk factors for the development of pSCC include advanced age, male sex, presence of human papilloma virus, and use of immunosuppressants.15 Male predominance and advanced age at the time of diagnosis (mean age, 67 years) have been observed in pSCC cases.16 It is now well established that renal transplant recipients have an increased risk of SCC, with a reported incidence rate of 5% to 6%.16 When these patients develop an SCC, they typically follow a more aggressive course. Renal transplantation has a higher ratio than cardiac transplantation for SCC development (2.37:1), whereas cardiac transplantation is associated with a higher risk of BCC development.17 A study of 384 transplant recipients found that 96 (25.0%) had a postsurgical nonmelanoma skin cancer (NMSC), with a ratio of SCC to BCC of 1.2:1.16 The calculated incidence of NMSC at 10 and 20 years posttransplantation was 24.2% and 54.4%, respectively. Another study also determined that SCC rates (50.0%) in postrenal transplant recipients were approximately twice that of BCC (27.0%).18
A daily regimen of immunosuppressive medications such as cyclosporine and mycophenolate mofetil showed an increased risk for development of NMSC.15 Immunosuppressive medications play an important role in the pathogenesis of SCC due to a direct oncogenic effect as well as impairment of the immune system’s ability to fight precancerous developments.15 A 4-year study of 100 renal transplant recipients using mycophenolate mofetil as part of an immunosuppressive regimen reported 22% NMSC findings among 9 patients.19 On average, patients developed an NMSC approximately 61 months posttransplantation, with a wide range from 2 to 120 months.
Advanced age was another important risk factor, with each decade of life producing a 60% increase in instantaneous risk of SCC development for transplant recipients.15 A steady increase in risk was related to the length of time adhering to an immunosuppressive regimen, especially from 2 to 6 years, and then remaining constant in subsequent years. For older patients on immunosuppressant regimens for more than 8 years, the calculated relative risk was noted to be over 200 times greater than the normal population’s development of skin cancers.18
Conclusion
Although cases of pSCC presenting as longitudinal melanonychia have previously been reported,9-14,20 our case is unique in that it describes pSCC in a renal transplant recipient. Our patient had many of the known risk factors for the development of pSCC including male sex, advanced age, skin of color, history of renal transplantation, and immunosuppressive therapy. Although regular full-body skin examinations are an accepted part of renal transplantation follow-up due to SCC risk, our case emphasizes the need to remain vigilant due to possible atypical presentations among the immunosuppressed. The nail unit should not be overlooked during the clinical examination of renal transplant recipients as demonstrated by our patient’s rare presentation of pSCC in the nail.
Case Report
A 62-year-old black man presented for examination of a dark longitudinal streak located adjacent to the lateral nail fold on the third finger of the left hand. The lesion had been present for several months, during which time it had slowly expanded in size. The fingertip had recently become tender, which interfered with the patient’s ability to work. His past medical history was remarkable for end-stage renal disease secondary to glomerulonephritis with nephrotic syndrome of unclear etiology. He initially was treated by an outside physician using peritoneal dialysis for 3 years until he underwent renal transplantation in 2004 with a cadaveric organ. Other remarkable medical conditions included posttransplantation diabetes, hyperlipidemia, and gout. His multidrug regimen included 2 immunosuppressive medications: oral cyclosporine 125 mg twice daily and oral mycophenolate mofetil 250 mg twice daily.
A broad, irregular, black, pigmented, subungual band was noted on the left third finger. The lesion appeared to emanate from below the nail cuticle and traveled along the nail longitudinally toward the distal tip. The band appeared darker at the edge adjacent to the lateral nail fold and grew lighter near the middle of the nail where its free edge was noted to be irregular. A slightly thickened lateral nail fold with an irregular, small, sawtoothlike hyperkeratosis and hyperpigmentation also was noted (Figure 1).

Subungual melanoma, onychomycosis, squamous cell carcinoma (SCC), and a verruca copresenting with onychomycosis were considered in the differential diagnosis. The patient underwent nail avulsion and biopsy of the nail bed as well as the nail matrix. Histopathology was notable for malignant dyskeratosis with a lack of nuclear maturation, occasional mitoses, multinucleation, and individual cell keratinization (Figure 2). Immunostaining for S100 was negative, while staining for cytokeratins AE1/AE3 was positive. Deposition of melanin pigment in the malignant dyskeratotic cells was noted. Periodic acid–Schiff staining identified pseudohyphae without invasion of the nail plate. A diagnosis of pigmented SCC (pSCC) was made. The patient’s nail also was sent for fungal cultures that later grew Candida glabrata and Candida parapsilosis.
The patient underwent Mohs micrographic surgery for removal of the pSCC, which was found to be more extensive than originally suspected and required en bloc excision of the nail repaired with a full-thickness skin graft from the left forearm. The area healed well with some hyperpigmentation (Figure 3).


Comment
Among the various types of skin cancer, an estimated 700,000 patients are diagnosed with SCC annually, making it the second most common form of skin cancer in the United States.1 Basal cell carcinoma (BCC) is the most common skin cancer among whites in the United States, while in contrast SCC is the most common skin cancer in patients with skin of color.2 Only an estimated 2% to 5% of all SCCs are pigmented, and this variant is more commonly seen in patients with skin of color.3-5 One analysis of 52 cases of pSCC showed that common features included a flat or slightly raised appearance and hyperpigmentation with varying levels of scaling.6 Studies have shown an altered presentation of pSCC in black skin with increased melanin production and thickness of the stratum corneum in contrast with cases seen in white patients.7 Other potential features include scaling, erosive changes, and sharply demarcated borders. Squamous cell carcinoma typically occurs in sun-exposed areas, reflecting its association with UV light damage; however, SCC in skin of color patients has been noted to occur in sun-protected areas and in areas of chronic scarring.8 Pigmented SCC also appears to follow this distribution, as affected areas are not necessarily in direct exposure to the sun. Pigmented SCCs have been associated with pruritus and/or burning pain, which also was seen in our case when our patient complained of tenderness at the site.
We describe the case of a subungual pSCC clinically presenting as longitudinal melanonychia. Pigmented SCC presenting as longitudinal melanonychia was first described by Baran and Simon in 1988.9 Since that time, it has been reported that approximately 10% of subungual pSCCs clinically present as longitudinal melanonychia.10,11 A retrospective study reviewing 35 cases of SCC of the nail apparatus found that 5 (14.3%) cases presented as longitudinal melanonychia.10 Another retrospective study found that 6 of 51 (11.8%) cases of SCCs affecting the nail unit presented as the warty type of SCC in association with longitudinal melanonychia.12 Cases of pSCC in situ appearing as longitudinal melanonychia also have been reported.13,14
Risk factors for the development of pSCC include advanced age, male sex, presence of human papilloma virus, and use of immunosuppressants.15 Male predominance and advanced age at the time of diagnosis (mean age, 67 years) have been observed in pSCC cases.16 It is now well established that renal transplant recipients have an increased risk of SCC, with a reported incidence rate of 5% to 6%.16 When these patients develop an SCC, they typically follow a more aggressive course. Renal transplantation has a higher ratio than cardiac transplantation for SCC development (2.37:1), whereas cardiac transplantation is associated with a higher risk of BCC development.17 A study of 384 transplant recipients found that 96 (25.0%) had a postsurgical nonmelanoma skin cancer (NMSC), with a ratio of SCC to BCC of 1.2:1.16 The calculated incidence of NMSC at 10 and 20 years posttransplantation was 24.2% and 54.4%, respectively. Another study also determined that SCC rates (50.0%) in postrenal transplant recipients were approximately twice that of BCC (27.0%).18
A daily regimen of immunosuppressive medications such as cyclosporine and mycophenolate mofetil showed an increased risk for development of NMSC.15 Immunosuppressive medications play an important role in the pathogenesis of SCC due to a direct oncogenic effect as well as impairment of the immune system’s ability to fight precancerous developments.15 A 4-year study of 100 renal transplant recipients using mycophenolate mofetil as part of an immunosuppressive regimen reported 22% NMSC findings among 9 patients.19 On average, patients developed an NMSC approximately 61 months posttransplantation, with a wide range from 2 to 120 months.
Advanced age was another important risk factor, with each decade of life producing a 60% increase in instantaneous risk of SCC development for transplant recipients.15 A steady increase in risk was related to the length of time adhering to an immunosuppressive regimen, especially from 2 to 6 years, and then remaining constant in subsequent years. For older patients on immunosuppressant regimens for more than 8 years, the calculated relative risk was noted to be over 200 times greater than the normal population’s development of skin cancers.18
Conclusion
Although cases of pSCC presenting as longitudinal melanonychia have previously been reported,9-14,20 our case is unique in that it describes pSCC in a renal transplant recipient. Our patient had many of the known risk factors for the development of pSCC including male sex, advanced age, skin of color, history of renal transplantation, and immunosuppressive therapy. Although regular full-body skin examinations are an accepted part of renal transplantation follow-up due to SCC risk, our case emphasizes the need to remain vigilant due to possible atypical presentations among the immunosuppressed. The nail unit should not be overlooked during the clinical examination of renal transplant recipients as demonstrated by our patient’s rare presentation of pSCC in the nail.
- Karia PS, Han J, Schmults CD. Cutaneous squamous cell carcinoma: estimated incidence of disease, nodal metastasis, and deaths from disease in the United States, 2012 [published online February 1, 2013]. J Am Acad Dermatol. 2013;68:957-966.
- Tan KB, Tan SH, Aw DC, et al. Simulators of squamous cell carcinoma of the skin: diagnostic challenges on small biopsies and clinicopathological correlation [published online June 25, 2013]. J Skin Cancer. 2013;2013:752864.
- McCall CO, Chen SC. Squamous cell carcinoma of the legs in African Americans. J Am Acad Dermatol. 2002;47:524-529.
- Krishna R, Lewis A, Orengo IF, et al. Pigmented Bowen’s disease (squamous cell carcinoma in situ): a mimic of malignant melanoma. Dermatol Surg. 2001;27:673-674.
- Brinca A, Teixeira V, Goncalo M, et al. A large pigmented lesion mimicking malignant melanoma. Clin Exp Dermatol. 2012;37:817-818.
- Cameron A, Rosendahl C, Tschandl P, et al. Dermatoscopy of pigmented Bowen’s disease. J Am Acad Dermatol. 2010;62:597-604.
- Singh B, Bhaya M, Shaha A, et al. Presentation, course, and outcome of head and neck cancers in African Americans: a case-control study. Laryngoscope. 1998;108(8 pt 1):1159-1163.
- Cancer Facts and Figures 2006. Atlanta, GA: American Cancer Society; 2006.
- Baran R, Simon C. Longitudinal melanonychia: a symptom of Bowen’s disease. J Am Acad Dermatol. 1988;18:1359-1360.
- Dalle S, Depape L, Phan A, et al. Squamous cell carcinoma of the nail apparatus: clinicopathological study of 35 cases. Br J Dermatol. 2007;156:871-874.
- Ishida M, Iwai M, Yoshida K, et al. Subungual pigmented squamous cell carcinoma presenting as longitudinal melanonychia: a case report with review of the literature. Int J Clin Exp Pathol. 2014;7:844-847.
- Lecerf P, Richert B, Theunis A, et al. A retrospective study of squamous cell carcinoma of the nail unit diagnosed in a Belgian general hospital over a 15-year period. J Am Acad Dermatol. 2013;69:253-261.
- Saito T, Uchi H, Moroi Y, et al. Subungual Bowen disease revealed by longitudinal melanonychia. J Am Acad Dermatol. 2012;67:E240-E241.
- Saxena A, Kasper DA, Campanelli CD, et al. Pigmented Bowen’s disease clinically mimicking melanoma on the nail. Dermatol Surg. 2006;32:1522-1525.
- Mackenzie KA, Wells JE, Lynn KL, et al. First and subsequent nonmelanoma skin cancers: incidence and predictors in a population of New Zealand renal transplant recipients. Nephrol Dial Transplant. 2010;25:300-306.
- Gutiérrez-Mendoza D, Narro-Llorente R, Karam-Orantes M, et al. Dermoscopy clues in pigmented Bowen’s disease [published online ahead of print September 16, 2010]. Dermatol Res Pract. 2010;2010.
- Euvards S, Kanitakis J, Pouteil-Noble C, et al. Comparative epidemiologic study of premalignant and malignant epithelial cutaneous lesions developing after kidney and heart transplantation. J Am Acad Dermatol. 1995;33(2 pt 1):222-229.
- Moloney FJ, Comber H, O’Lorcain P, et al. A population-based study of skin cancer incidence and prevalence in renal transplant patients. Br J Dermatol. 2006;154:498-504.
- Formicone F, Fargnoli MC, Pisani F, et al. Cutaneous manifestations in Italian kidney transplant recipients. Transplant Proc. 2005;37:2527-2528.
- Fernandes Massa A, Debarbieux S, Depaepe L, et al. Pigmented squamous cell carcinoma of the nail bed presenting as a melanonychia striata: diagnosis by perioperative reflectance confocal microscopy. Br J Dermatol. 2013;169:198-199.
- Karia PS, Han J, Schmults CD. Cutaneous squamous cell carcinoma: estimated incidence of disease, nodal metastasis, and deaths from disease in the United States, 2012 [published online February 1, 2013]. J Am Acad Dermatol. 2013;68:957-966.
- Tan KB, Tan SH, Aw DC, et al. Simulators of squamous cell carcinoma of the skin: diagnostic challenges on small biopsies and clinicopathological correlation [published online June 25, 2013]. J Skin Cancer. 2013;2013:752864.
- McCall CO, Chen SC. Squamous cell carcinoma of the legs in African Americans. J Am Acad Dermatol. 2002;47:524-529.
- Krishna R, Lewis A, Orengo IF, et al. Pigmented Bowen’s disease (squamous cell carcinoma in situ): a mimic of malignant melanoma. Dermatol Surg. 2001;27:673-674.
- Brinca A, Teixeira V, Goncalo M, et al. A large pigmented lesion mimicking malignant melanoma. Clin Exp Dermatol. 2012;37:817-818.
- Cameron A, Rosendahl C, Tschandl P, et al. Dermatoscopy of pigmented Bowen’s disease. J Am Acad Dermatol. 2010;62:597-604.
- Singh B, Bhaya M, Shaha A, et al. Presentation, course, and outcome of head and neck cancers in African Americans: a case-control study. Laryngoscope. 1998;108(8 pt 1):1159-1163.
- Cancer Facts and Figures 2006. Atlanta, GA: American Cancer Society; 2006.
- Baran R, Simon C. Longitudinal melanonychia: a symptom of Bowen’s disease. J Am Acad Dermatol. 1988;18:1359-1360.
- Dalle S, Depape L, Phan A, et al. Squamous cell carcinoma of the nail apparatus: clinicopathological study of 35 cases. Br J Dermatol. 2007;156:871-874.
- Ishida M, Iwai M, Yoshida K, et al. Subungual pigmented squamous cell carcinoma presenting as longitudinal melanonychia: a case report with review of the literature. Int J Clin Exp Pathol. 2014;7:844-847.
- Lecerf P, Richert B, Theunis A, et al. A retrospective study of squamous cell carcinoma of the nail unit diagnosed in a Belgian general hospital over a 15-year period. J Am Acad Dermatol. 2013;69:253-261.
- Saito T, Uchi H, Moroi Y, et al. Subungual Bowen disease revealed by longitudinal melanonychia. J Am Acad Dermatol. 2012;67:E240-E241.
- Saxena A, Kasper DA, Campanelli CD, et al. Pigmented Bowen’s disease clinically mimicking melanoma on the nail. Dermatol Surg. 2006;32:1522-1525.
- Mackenzie KA, Wells JE, Lynn KL, et al. First and subsequent nonmelanoma skin cancers: incidence and predictors in a population of New Zealand renal transplant recipients. Nephrol Dial Transplant. 2010;25:300-306.
- Gutiérrez-Mendoza D, Narro-Llorente R, Karam-Orantes M, et al. Dermoscopy clues in pigmented Bowen’s disease [published online ahead of print September 16, 2010]. Dermatol Res Pract. 2010;2010.
- Euvards S, Kanitakis J, Pouteil-Noble C, et al. Comparative epidemiologic study of premalignant and malignant epithelial cutaneous lesions developing after kidney and heart transplantation. J Am Acad Dermatol. 1995;33(2 pt 1):222-229.
- Moloney FJ, Comber H, O’Lorcain P, et al. A population-based study of skin cancer incidence and prevalence in renal transplant patients. Br J Dermatol. 2006;154:498-504.
- Formicone F, Fargnoli MC, Pisani F, et al. Cutaneous manifestations in Italian kidney transplant recipients. Transplant Proc. 2005;37:2527-2528.
- Fernandes Massa A, Debarbieux S, Depaepe L, et al. Pigmented squamous cell carcinoma of the nail bed presenting as a melanonychia striata: diagnosis by perioperative reflectance confocal microscopy. Br J Dermatol. 2013;169:198-199.
Practice Points
- Risk factors for the development of pigmented squamous cell carcinoma (pSCC) include older age, male sex, and use of immunosuppressant medications.
- Subungual pSCC can present as longitudinal melanonychia and should be considered in the differential diagnosis for melanonychia in patients with skin of color or those who are immunosuppressed.
Discoid Lupus Erythematosus Following Herpes Zoster
Cutaneous manifestations of systemic lupus erythematosus (SLE) can be classified as lupus-specific or lupus-nonspecific skin lesions. Lupus-specific lesions commonly are photodistributed, with involvement of the malar region, arms, and trunk. The development of discoid lupus erythematosus (DLE) in areas of trauma, including sun-exposed skin, is not uncommon and may be associated with an isomorphic response. We present a rare case of an isomorphic response following herpes zoster (HZ) in a young woman undergoing treatment with immunosuppressive agents for SLE and DLE. Potential prophylactic therapy also is discussed.
Case Report
A 19-year-old woman initially presented to an outside dermatologist for evaluation of new-onset scarring alopecia, crusted erythematous plaques on the face and arms, and arthralgia. A punch biopsy of a lesion on the left arm demonstrated a lichenoid and perivascular lymphocytic infiltrate with scattered necrotic keratinocytes, perifollicular inflammation, and focally thickened basement membrane at the dermoepidermal junction consistent with discoid lupus erythematosus (DLE). A laboratory workup for SLE revealed 1:1280 antinuclear antibodies (reference range, negative <1:80) with elevated titers of double-stranded DNA, Smith, ribonucleoprotein, Sjögren syndrome A, and Sjögren syndrome B autoantibodies with low complement levels. Based on these findings, a diagnosis of SLE and DLE was made.
At that time, the patient was started on hydroxychloroquine 200 mg twice daily for SLE. Four days later she developed swelling in both hands and feet, and hydroxychloroquine was stopped due to a presumed adverse reaction; however, her symptoms subsequently were determined to be polyarthritis secondary to a lupus flare. Prednisone 10 mg once daily was then initiated. The patient was encouraged to restart hydroxychloroquine, but she declined.
Over the next 13 months, the patient developed severe photosensitivity, oral ulcers, Raynaud phenomenon, anemia, and nephrotic-range proteinuria. She ultimately was diagnosed by the nephrology department at our institution with mixed diffuse proliferative and membranous glomerulonephritis. Induction therapy with oral mycophenolate mofetil 1000 mg twice daily and prednisone 60 mg once daily was started, followed by the addition of tacrolimus 1 mg twice daily. Despite immunosuppressive therapy, she continued to develop new discoid lesions on the face, chest, and arms. Th
After 4 weeks of treatment with mycophenolate mofetil, prednisone, and tacrolimus, the patient developed a painful vesicular rash on the left breast with extension over the left axilla and scapula in a T3 to T4 dermatomal distribution. A clinical diagnosis of HZ was made, and she was started on intravenous acyclovir 10 mg/kg in dextrose 5% every 8 hours for 4 days followed by oral valacyclovir 1000 mg every 8 hours for 14 days, which led to resolution of the eruption.
Over the next 4 months, the patient continued to experience pain confined to the same dermatomal area as the HZ, which was consistent with postherpetic neuralgia. Mycophenolate mofetil was discontinued after she developed acute liver toxicity attributed to the drug. Upon discontinuation, the patient developed a new pruritic rash on both arms and the back. Physical examination by the dermatology department at our institution revealed diffuse, scaly, hyperpigmented papules and annular plaques with central pink hypopigmentation on the face, ears, anterior chest, arms, hands, and back. On the left anterior chest and back, the distribution was strikingly unilateral and multidermatomal (Figure 1). Upon further questioning, the patient confirmed that the areas of the new rash coincided with areas previously affected by HZ. Histologic examination of a representative lesion from the left lateral breast revealed hyperkeratosis, follicular plugging, a patchy lichenoid and perivascular mononuclear cell infiltrate, and pigment incontinence (Figure 2A). These histologic features were subtle and were not diagnostic for lupus; however, direct immunofluorescence demonstrated a continuous granular band of IgG and C3 along the dermoepidermal junction, confirming the diagnosis of DLE (Figure 2B). The histologic findings and clinical presentation were consistent with the development of DLE in areas of previous trauma from HZ. The patient continues to follow-up with the rheumatology and nephrology departments but was lost to dermatology follow-up.
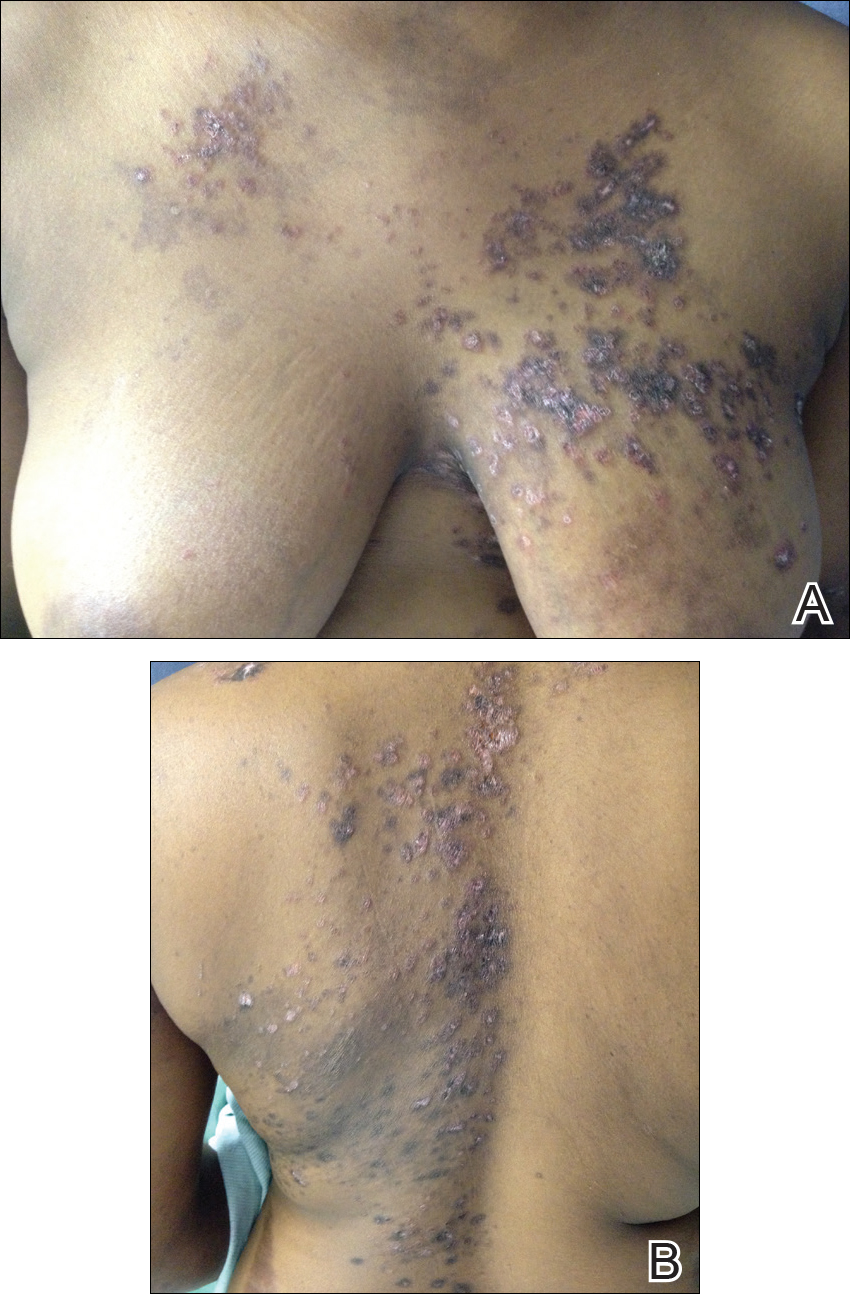
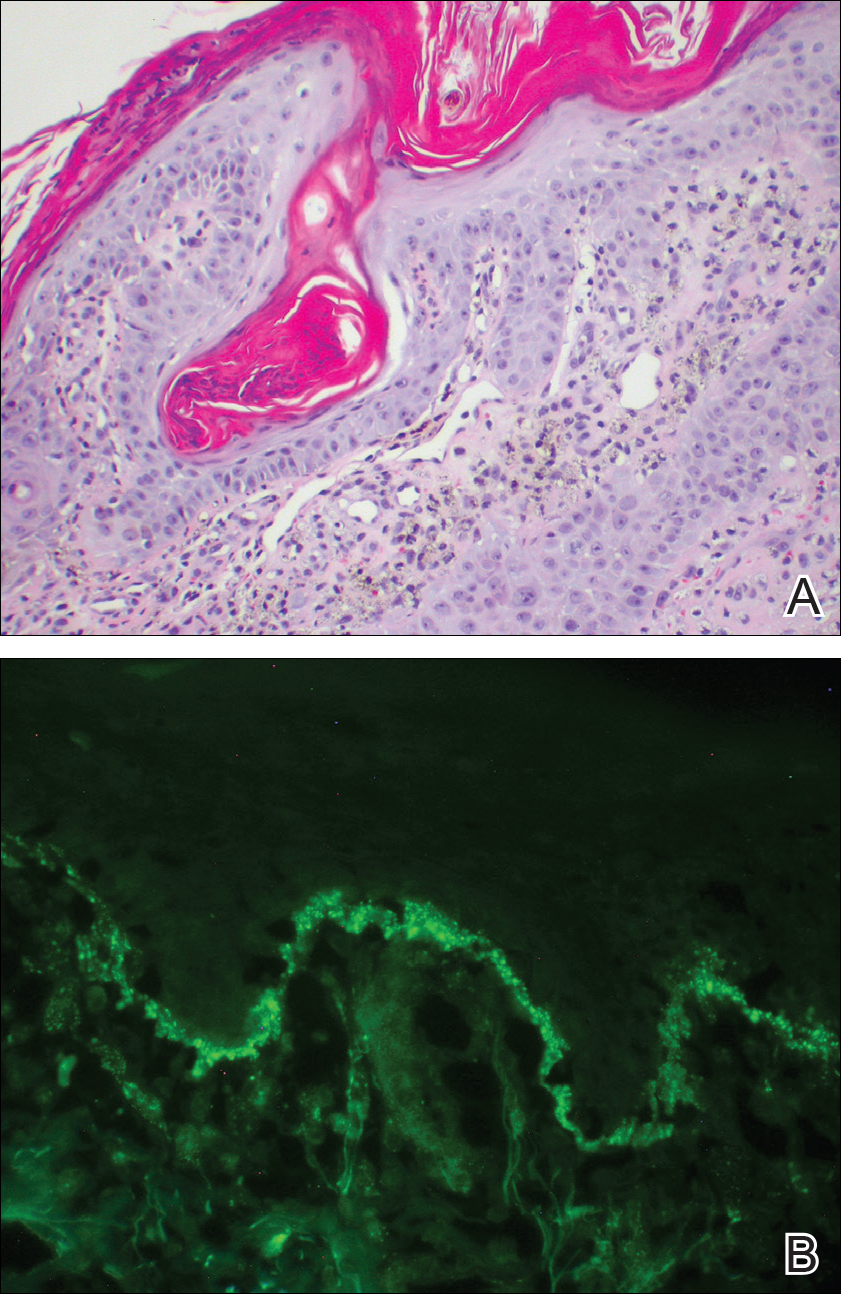
Comment
The pathogenesis of DLE is poorly understood but is thought to be multifactorial, involving genetics, sun exposure, and immune dysregulation.1 Development of DLE lesions in skin traumatized by tattoos, scratches, scars, and prolonged heat exposure has been reported.2 Clarification of the mechanism(s) underlying these traumatized areas may provide insight into the pathophysiology of DLE.
The isomorphic response, also known as the Köbner phenomenon, is the development of a preexisting skin condition at a site of trauma. This phenomenon has been observed in several dermatologic conditions including psoriasis, lichen planus, systemic sclerosis, dermatomyositis, sarcoidosis, vitiligo, and DLE.3 Koebnerization may result from trauma to the skin caused by scratches, sun exposure, radiography, prolonged heat and cold exposure, pressure, tattoos, scars, and inflammatory dermatoses.2,4 Ueki4 suggested that localized trauma to the skin stimulates an immune response that makes the traumatized site a target for a preexisting skin condition. Inflammatory mediators such as IL-1, tumor necrosis factor α, IL-6, and interferon γ have been implicated in the pathophysiology of the isomorphic response.4
Wolf isotopic response is a similar entity that refers to the development of a novel skin condition at the site of a distinct, previously resolved skin disorder. This phenomenon was described by Wolf et al5 in 1995, and since then over 170 cases have been reported.5-7 In most cases the initial skin condition is HZ, although herpes simplex virus has also been implicated. The common resulting skin conditions include granulomatous reactions, malignant tumors, lichen planus, morphea, and infections. The notion that the antecedent skin disease alters the affected site and causes it to be more susceptible to autoimmunity has been proposed as a mechanism for the isotopic response.7,8 While one might consider our presentation of DLE following HZ to be an isotopic response, we believe this case is best classified as an isomorphic response, as the patient already had an established diagnosis of DLE.
The development of DLE at the site of a previous HZ eruption has been described in 2 other cases of young women with SLE.9,10 Unique to our case is the development of a multidermatomal eruption, which may be an indication of her degree of immunosuppression, as immunosuppressed patients are more likely to present with multidermatomal reactivation of varicella zoster virus and postherpetic neuralgia.11 The similarities between our case and the 2 prior reports—including the patients’ age, sex, history of SLE, and degree of immunosuppression—are noteworthy in that they may represent a subset of SLE patients who are predisposed to developing koebnerization following HZ. Physicians should be aware of this phenomenon and consider being proactive in preventing long-term damage.
When feasible, physicians should consider administering the HZ vaccine to reduce the course and severity of HZ before prescribing immunosuppressive agents. When HZ presents in young, immunosuppressed women with a history of SLE, we suggest monitoring the affected sites closely for any evidence of DLE. Topical corticosteroids should be applied to involved areas of the face or body at the earliest appearance of such lesions, which may prevent the isomorphic response and its potentially scarring DLE lesions. This will be our therapeutic approach if we encounter a similar clinical situation in the future. Fur
Acknowledgment
We thank Carolyn E. Grotkowski, MD, from the Department of Pathology, Cooper Medical School of Rowan University, Camden, New Jersey, for her assistance in photographing the pathology slides.
- Lin JH, Dutz JP, Sontheimer RD, et al. Pathophysiology of cutaneous lupus erythematosus. Clinic Rev Allerg Immunol. 2007;33:85-106.
- Ueki H. Köbner phenomenon in lupus erythematosus [in German]. Hautarzt. 1994;45:154-160.
- Boyd AS, Neldner KH. The isomorphic response of Koebner. Int J Dermatol. 1990;29:401-410.
- Ueki H. Koebner phenomenon in lupus erythematosus with special consideration of clinical findings. Autoimmun Rev. 2005;4:219-223.
- Wolf R, Brenner S, Ruocco V, et al. Isotopic response. Int J Dermatol. 1995;34:341-348.
- Wolf R, Wolf D, Ruocco E, et al. Wolf’s isotopic response. Clin Dermatol. 2011;29:237-240.
- Ruocco V, Brunetti G, Puca RV, et al. The immunocompromised district: a unifying concept for lymphoedematous, herpes-infected and otherwise damaged sites. J Eur Acad Dermatol Venereol. 2009;23:1364-1373.
- Martires KJ, Baird K, Citrin DE, et al. Localization of sclerotic-type chronic graft-vs-host disease to sites of skin injury. Arch Dermatol. 2011;147:1081-1086.
- Lee NY, Daniel AS, Dasher DA, et al. Cutaneous lupus after herpes zoster: isomorphic, isotopic, or both [published online May 29, 2012]? Pediatr Dermatol. 2013;30:e110-e113.
- Longhi BS, Centeville M, Marini R, et al. Koebner’s phenomenon in systemic lupus erythematosus. Rheumatol Int. 2012;32:1403-1405.
- Failla V, Jacques J, Castronovo C, et al. Herpes zoster in patients treated with biologicals. Dermatology. 2012;224:251-256.
Cutaneous manifestations of systemic lupus erythematosus (SLE) can be classified as lupus-specific or lupus-nonspecific skin lesions. Lupus-specific lesions commonly are photodistributed, with involvement of the malar region, arms, and trunk. The development of discoid lupus erythematosus (DLE) in areas of trauma, including sun-exposed skin, is not uncommon and may be associated with an isomorphic response. We present a rare case of an isomorphic response following herpes zoster (HZ) in a young woman undergoing treatment with immunosuppressive agents for SLE and DLE. Potential prophylactic therapy also is discussed.
Case Report
A 19-year-old woman initially presented to an outside dermatologist for evaluation of new-onset scarring alopecia, crusted erythematous plaques on the face and arms, and arthralgia. A punch biopsy of a lesion on the left arm demonstrated a lichenoid and perivascular lymphocytic infiltrate with scattered necrotic keratinocytes, perifollicular inflammation, and focally thickened basement membrane at the dermoepidermal junction consistent with discoid lupus erythematosus (DLE). A laboratory workup for SLE revealed 1:1280 antinuclear antibodies (reference range, negative <1:80) with elevated titers of double-stranded DNA, Smith, ribonucleoprotein, Sjögren syndrome A, and Sjögren syndrome B autoantibodies with low complement levels. Based on these findings, a diagnosis of SLE and DLE was made.
At that time, the patient was started on hydroxychloroquine 200 mg twice daily for SLE. Four days later she developed swelling in both hands and feet, and hydroxychloroquine was stopped due to a presumed adverse reaction; however, her symptoms subsequently were determined to be polyarthritis secondary to a lupus flare. Prednisone 10 mg once daily was then initiated. The patient was encouraged to restart hydroxychloroquine, but she declined.
Over the next 13 months, the patient developed severe photosensitivity, oral ulcers, Raynaud phenomenon, anemia, and nephrotic-range proteinuria. She ultimately was diagnosed by the nephrology department at our institution with mixed diffuse proliferative and membranous glomerulonephritis. Induction therapy with oral mycophenolate mofetil 1000 mg twice daily and prednisone 60 mg once daily was started, followed by the addition of tacrolimus 1 mg twice daily. Despite immunosuppressive therapy, she continued to develop new discoid lesions on the face, chest, and arms. Th
After 4 weeks of treatment with mycophenolate mofetil, prednisone, and tacrolimus, the patient developed a painful vesicular rash on the left breast with extension over the left axilla and scapula in a T3 to T4 dermatomal distribution. A clinical diagnosis of HZ was made, and she was started on intravenous acyclovir 10 mg/kg in dextrose 5% every 8 hours for 4 days followed by oral valacyclovir 1000 mg every 8 hours for 14 days, which led to resolution of the eruption.
Over the next 4 months, the patient continued to experience pain confined to the same dermatomal area as the HZ, which was consistent with postherpetic neuralgia. Mycophenolate mofetil was discontinued after she developed acute liver toxicity attributed to the drug. Upon discontinuation, the patient developed a new pruritic rash on both arms and the back. Physical examination by the dermatology department at our institution revealed diffuse, scaly, hyperpigmented papules and annular plaques with central pink hypopigmentation on the face, ears, anterior chest, arms, hands, and back. On the left anterior chest and back, the distribution was strikingly unilateral and multidermatomal (Figure 1). Upon further questioning, the patient confirmed that the areas of the new rash coincided with areas previously affected by HZ. Histologic examination of a representative lesion from the left lateral breast revealed hyperkeratosis, follicular plugging, a patchy lichenoid and perivascular mononuclear cell infiltrate, and pigment incontinence (Figure 2A). These histologic features were subtle and were not diagnostic for lupus; however, direct immunofluorescence demonstrated a continuous granular band of IgG and C3 along the dermoepidermal junction, confirming the diagnosis of DLE (Figure 2B). The histologic findings and clinical presentation were consistent with the development of DLE in areas of previous trauma from HZ. The patient continues to follow-up with the rheumatology and nephrology departments but was lost to dermatology follow-up.


Comment
The pathogenesis of DLE is poorly understood but is thought to be multifactorial, involving genetics, sun exposure, and immune dysregulation.1 Development of DLE lesions in skin traumatized by tattoos, scratches, scars, and prolonged heat exposure has been reported.2 Clarification of the mechanism(s) underlying these traumatized areas may provide insight into the pathophysiology of DLE.
The isomorphic response, also known as the Köbner phenomenon, is the development of a preexisting skin condition at a site of trauma. This phenomenon has been observed in several dermatologic conditions including psoriasis, lichen planus, systemic sclerosis, dermatomyositis, sarcoidosis, vitiligo, and DLE.3 Koebnerization may result from trauma to the skin caused by scratches, sun exposure, radiography, prolonged heat and cold exposure, pressure, tattoos, scars, and inflammatory dermatoses.2,4 Ueki4 suggested that localized trauma to the skin stimulates an immune response that makes the traumatized site a target for a preexisting skin condition. Inflammatory mediators such as IL-1, tumor necrosis factor α, IL-6, and interferon γ have been implicated in the pathophysiology of the isomorphic response.4
Wolf isotopic response is a similar entity that refers to the development of a novel skin condition at the site of a distinct, previously resolved skin disorder. This phenomenon was described by Wolf et al5 in 1995, and since then over 170 cases have been reported.5-7 In most cases the initial skin condition is HZ, although herpes simplex virus has also been implicated. The common resulting skin conditions include granulomatous reactions, malignant tumors, lichen planus, morphea, and infections. The notion that the antecedent skin disease alters the affected site and causes it to be more susceptible to autoimmunity has been proposed as a mechanism for the isotopic response.7,8 While one might consider our presentation of DLE following HZ to be an isotopic response, we believe this case is best classified as an isomorphic response, as the patient already had an established diagnosis of DLE.
The development of DLE at the site of a previous HZ eruption has been described in 2 other cases of young women with SLE.9,10 Unique to our case is the development of a multidermatomal eruption, which may be an indication of her degree of immunosuppression, as immunosuppressed patients are more likely to present with multidermatomal reactivation of varicella zoster virus and postherpetic neuralgia.11 The similarities between our case and the 2 prior reports—including the patients’ age, sex, history of SLE, and degree of immunosuppression—are noteworthy in that they may represent a subset of SLE patients who are predisposed to developing koebnerization following HZ. Physicians should be aware of this phenomenon and consider being proactive in preventing long-term damage.
When feasible, physicians should consider administering the HZ vaccine to reduce the course and severity of HZ before prescribing immunosuppressive agents. When HZ presents in young, immunosuppressed women with a history of SLE, we suggest monitoring the affected sites closely for any evidence of DLE. Topical corticosteroids should be applied to involved areas of the face or body at the earliest appearance of such lesions, which may prevent the isomorphic response and its potentially scarring DLE lesions. This will be our therapeutic approach if we encounter a similar clinical situation in the future. Fur
Acknowledgment
We thank Carolyn E. Grotkowski, MD, from the Department of Pathology, Cooper Medical School of Rowan University, Camden, New Jersey, for her assistance in photographing the pathology slides.
Cutaneous manifestations of systemic lupus erythematosus (SLE) can be classified as lupus-specific or lupus-nonspecific skin lesions. Lupus-specific lesions commonly are photodistributed, with involvement of the malar region, arms, and trunk. The development of discoid lupus erythematosus (DLE) in areas of trauma, including sun-exposed skin, is not uncommon and may be associated with an isomorphic response. We present a rare case of an isomorphic response following herpes zoster (HZ) in a young woman undergoing treatment with immunosuppressive agents for SLE and DLE. Potential prophylactic therapy also is discussed.
Case Report
A 19-year-old woman initially presented to an outside dermatologist for evaluation of new-onset scarring alopecia, crusted erythematous plaques on the face and arms, and arthralgia. A punch biopsy of a lesion on the left arm demonstrated a lichenoid and perivascular lymphocytic infiltrate with scattered necrotic keratinocytes, perifollicular inflammation, and focally thickened basement membrane at the dermoepidermal junction consistent with discoid lupus erythematosus (DLE). A laboratory workup for SLE revealed 1:1280 antinuclear antibodies (reference range, negative <1:80) with elevated titers of double-stranded DNA, Smith, ribonucleoprotein, Sjögren syndrome A, and Sjögren syndrome B autoantibodies with low complement levels. Based on these findings, a diagnosis of SLE and DLE was made.
At that time, the patient was started on hydroxychloroquine 200 mg twice daily for SLE. Four days later she developed swelling in both hands and feet, and hydroxychloroquine was stopped due to a presumed adverse reaction; however, her symptoms subsequently were determined to be polyarthritis secondary to a lupus flare. Prednisone 10 mg once daily was then initiated. The patient was encouraged to restart hydroxychloroquine, but she declined.
Over the next 13 months, the patient developed severe photosensitivity, oral ulcers, Raynaud phenomenon, anemia, and nephrotic-range proteinuria. She ultimately was diagnosed by the nephrology department at our institution with mixed diffuse proliferative and membranous glomerulonephritis. Induction therapy with oral mycophenolate mofetil 1000 mg twice daily and prednisone 60 mg once daily was started, followed by the addition of tacrolimus 1 mg twice daily. Despite immunosuppressive therapy, she continued to develop new discoid lesions on the face, chest, and arms. Th
After 4 weeks of treatment with mycophenolate mofetil, prednisone, and tacrolimus, the patient developed a painful vesicular rash on the left breast with extension over the left axilla and scapula in a T3 to T4 dermatomal distribution. A clinical diagnosis of HZ was made, and she was started on intravenous acyclovir 10 mg/kg in dextrose 5% every 8 hours for 4 days followed by oral valacyclovir 1000 mg every 8 hours for 14 days, which led to resolution of the eruption.
Over the next 4 months, the patient continued to experience pain confined to the same dermatomal area as the HZ, which was consistent with postherpetic neuralgia. Mycophenolate mofetil was discontinued after she developed acute liver toxicity attributed to the drug. Upon discontinuation, the patient developed a new pruritic rash on both arms and the back. Physical examination by the dermatology department at our institution revealed diffuse, scaly, hyperpigmented papules and annular plaques with central pink hypopigmentation on the face, ears, anterior chest, arms, hands, and back. On the left anterior chest and back, the distribution was strikingly unilateral and multidermatomal (Figure 1). Upon further questioning, the patient confirmed that the areas of the new rash coincided with areas previously affected by HZ. Histologic examination of a representative lesion from the left lateral breast revealed hyperkeratosis, follicular plugging, a patchy lichenoid and perivascular mononuclear cell infiltrate, and pigment incontinence (Figure 2A). These histologic features were subtle and were not diagnostic for lupus; however, direct immunofluorescence demonstrated a continuous granular band of IgG and C3 along the dermoepidermal junction, confirming the diagnosis of DLE (Figure 2B). The histologic findings and clinical presentation were consistent with the development of DLE in areas of previous trauma from HZ. The patient continues to follow-up with the rheumatology and nephrology departments but was lost to dermatology follow-up.


Comment
The pathogenesis of DLE is poorly understood but is thought to be multifactorial, involving genetics, sun exposure, and immune dysregulation.1 Development of DLE lesions in skin traumatized by tattoos, scratches, scars, and prolonged heat exposure has been reported.2 Clarification of the mechanism(s) underlying these traumatized areas may provide insight into the pathophysiology of DLE.
The isomorphic response, also known as the Köbner phenomenon, is the development of a preexisting skin condition at a site of trauma. This phenomenon has been observed in several dermatologic conditions including psoriasis, lichen planus, systemic sclerosis, dermatomyositis, sarcoidosis, vitiligo, and DLE.3 Koebnerization may result from trauma to the skin caused by scratches, sun exposure, radiography, prolonged heat and cold exposure, pressure, tattoos, scars, and inflammatory dermatoses.2,4 Ueki4 suggested that localized trauma to the skin stimulates an immune response that makes the traumatized site a target for a preexisting skin condition. Inflammatory mediators such as IL-1, tumor necrosis factor α, IL-6, and interferon γ have been implicated in the pathophysiology of the isomorphic response.4
Wolf isotopic response is a similar entity that refers to the development of a novel skin condition at the site of a distinct, previously resolved skin disorder. This phenomenon was described by Wolf et al5 in 1995, and since then over 170 cases have been reported.5-7 In most cases the initial skin condition is HZ, although herpes simplex virus has also been implicated. The common resulting skin conditions include granulomatous reactions, malignant tumors, lichen planus, morphea, and infections. The notion that the antecedent skin disease alters the affected site and causes it to be more susceptible to autoimmunity has been proposed as a mechanism for the isotopic response.7,8 While one might consider our presentation of DLE following HZ to be an isotopic response, we believe this case is best classified as an isomorphic response, as the patient already had an established diagnosis of DLE.
The development of DLE at the site of a previous HZ eruption has been described in 2 other cases of young women with SLE.9,10 Unique to our case is the development of a multidermatomal eruption, which may be an indication of her degree of immunosuppression, as immunosuppressed patients are more likely to present with multidermatomal reactivation of varicella zoster virus and postherpetic neuralgia.11 The similarities between our case and the 2 prior reports—including the patients’ age, sex, history of SLE, and degree of immunosuppression—are noteworthy in that they may represent a subset of SLE patients who are predisposed to developing koebnerization following HZ. Physicians should be aware of this phenomenon and consider being proactive in preventing long-term damage.
When feasible, physicians should consider administering the HZ vaccine to reduce the course and severity of HZ before prescribing immunosuppressive agents. When HZ presents in young, immunosuppressed women with a history of SLE, we suggest monitoring the affected sites closely for any evidence of DLE. Topical corticosteroids should be applied to involved areas of the face or body at the earliest appearance of such lesions, which may prevent the isomorphic response and its potentially scarring DLE lesions. This will be our therapeutic approach if we encounter a similar clinical situation in the future. Fur
Acknowledgment
We thank Carolyn E. Grotkowski, MD, from the Department of Pathology, Cooper Medical School of Rowan University, Camden, New Jersey, for her assistance in photographing the pathology slides.
- Lin JH, Dutz JP, Sontheimer RD, et al. Pathophysiology of cutaneous lupus erythematosus. Clinic Rev Allerg Immunol. 2007;33:85-106.
- Ueki H. Köbner phenomenon in lupus erythematosus [in German]. Hautarzt. 1994;45:154-160.
- Boyd AS, Neldner KH. The isomorphic response of Koebner. Int J Dermatol. 1990;29:401-410.
- Ueki H. Koebner phenomenon in lupus erythematosus with special consideration of clinical findings. Autoimmun Rev. 2005;4:219-223.
- Wolf R, Brenner S, Ruocco V, et al. Isotopic response. Int J Dermatol. 1995;34:341-348.
- Wolf R, Wolf D, Ruocco E, et al. Wolf’s isotopic response. Clin Dermatol. 2011;29:237-240.
- Ruocco V, Brunetti G, Puca RV, et al. The immunocompromised district: a unifying concept for lymphoedematous, herpes-infected and otherwise damaged sites. J Eur Acad Dermatol Venereol. 2009;23:1364-1373.
- Martires KJ, Baird K, Citrin DE, et al. Localization of sclerotic-type chronic graft-vs-host disease to sites of skin injury. Arch Dermatol. 2011;147:1081-1086.
- Lee NY, Daniel AS, Dasher DA, et al. Cutaneous lupus after herpes zoster: isomorphic, isotopic, or both [published online May 29, 2012]? Pediatr Dermatol. 2013;30:e110-e113.
- Longhi BS, Centeville M, Marini R, et al. Koebner’s phenomenon in systemic lupus erythematosus. Rheumatol Int. 2012;32:1403-1405.
- Failla V, Jacques J, Castronovo C, et al. Herpes zoster in patients treated with biologicals. Dermatology. 2012;224:251-256.
- Lin JH, Dutz JP, Sontheimer RD, et al. Pathophysiology of cutaneous lupus erythematosus. Clinic Rev Allerg Immunol. 2007;33:85-106.
- Ueki H. Köbner phenomenon in lupus erythematosus [in German]. Hautarzt. 1994;45:154-160.
- Boyd AS, Neldner KH. The isomorphic response of Koebner. Int J Dermatol. 1990;29:401-410.
- Ueki H. Koebner phenomenon in lupus erythematosus with special consideration of clinical findings. Autoimmun Rev. 2005;4:219-223.
- Wolf R, Brenner S, Ruocco V, et al. Isotopic response. Int J Dermatol. 1995;34:341-348.
- Wolf R, Wolf D, Ruocco E, et al. Wolf’s isotopic response. Clin Dermatol. 2011;29:237-240.
- Ruocco V, Brunetti G, Puca RV, et al. The immunocompromised district: a unifying concept for lymphoedematous, herpes-infected and otherwise damaged sites. J Eur Acad Dermatol Venereol. 2009;23:1364-1373.
- Martires KJ, Baird K, Citrin DE, et al. Localization of sclerotic-type chronic graft-vs-host disease to sites of skin injury. Arch Dermatol. 2011;147:1081-1086.
- Lee NY, Daniel AS, Dasher DA, et al. Cutaneous lupus after herpes zoster: isomorphic, isotopic, or both [published online May 29, 2012]? Pediatr Dermatol. 2013;30:e110-e113.
- Longhi BS, Centeville M, Marini R, et al. Koebner’s phenomenon in systemic lupus erythematosus. Rheumatol Int. 2012;32:1403-1405.
- Failla V, Jacques J, Castronovo C, et al. Herpes zoster in patients treated with biologicals. Dermatology. 2012;224:251-256.
Practice Points
- Discoid lupus erythematosus (DLE) most commonly presents as scaling and crusted plaques in sun-exposed areas of the face and arms. It also may present in skin traumatized by tattoos, scratches, scars, prolonged heat exposure, andherpes zoster (HZ).
- Patients with a history of DLE who subsequently develop HZ should be followed closely for the development of DLE in HZ-affected dermatomes.
- Following resolution of HZ, topical corticosteroids may have a role in prevention of DLE in HZ-affected dermatomes.
Secukinumab Emerges as a Rapidly Effective Therapy for Pityriasis Rubra Pilaris
Although there currently are no formal guidelines for the treatment of refractory pityriasis rubra pilaris (PRP), successful off-label treatment of the condition with multiple biologics approved for psoriasis has been reported.1,2 Secukinumab, an IL-17A antagonist, has shown particularly striking results in the treatment of PRP in 2 recent case reports.3,4 We report 2 additional cases of severe refractory PRP that responded rapidly to treatment with secukinumab. In both cases, the patients’ erythematous plaques resolved or had nearly resolved by week 4 of treatment. Our findings suggest that IL-17 plays an important role in PRP pathogenesis and support future clinical trials of anti–IL-17 agents for treatment of this entity.
Case Reports
Patient 1
A 60-year-old man with a history of biopsy-proven PRP presented with persistent generalized erythema, scattered patches of normal skin, and hyperkeratotic plaques on the bilateral palms of 1 year’s duration. Previous therapies included topical steroids, topical calcipotriene, adalimumab 40 mg once every other week, infliximab 5 mg/kg once every 8 weeks, ustekinumab 90 mg once every 12 weeks, acitretin 25 mg once daily, and most recently cyclosporine 200 mg twice daily. Of these treatments, infliximab was the
At 4 weeks’ follow-up, there was a marked decrease in erythema and scaling. The body surface area affected had decreased to 5%, and improvement of palmar keratoderma was noted. The patient continued with maintenance dosing of secukinumab 300 mg once every 4 weeks. By week 8, the erythema had fully resolved (Figure 1B), and he remained clear at week 24. No adverse events were noted since initiation of therapy.
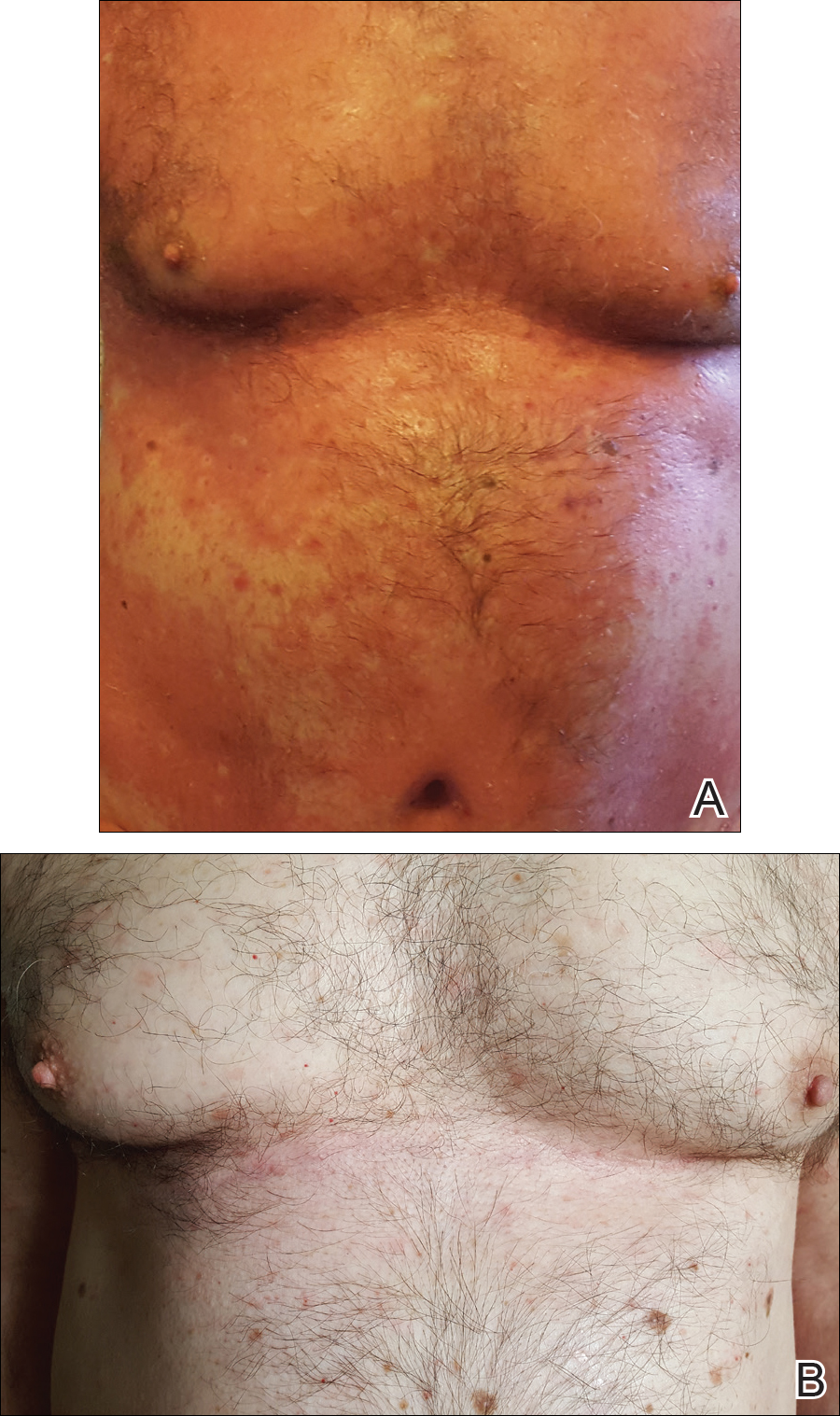
Patient 2
A 74-year-old woman with a history of PRP that had previously been misdiagnosed as psoriasis by an outside physician presented for evaluation of palmoplantar keratoderma (Figure 2A), follicular hyperkeratosis, and erythematous plaques on the trunk and arms of 5 years’ duration. Previous therapies included topical steroids, topical urea, methotrexate 20 mg once weekly, adalimumab 40 mg once every other week, infliximab 10 mg/kg once every 4 weeks, ustekinumab 90 mg once every 12 weeks, and most recently acitretin 50 mg once daily.
The patient had been maintained on ustekinumab and acitretin for 2 years with only mild improvement. Ustekinumab was then discontinued, and after 3 months treatment with secukinumab was added to the once-daily acitretin. Similar to Patient 1, loading doses of secukinumab 300 mg were administered once weekly for 5 weeks. The plaques on the trunk and arms had resolved by week 4, but the palmoplantar keratoderma persisted. The patient continued with the maintenance dose of secukinumab 300 mg once every 4 weeks and reported an increase in peeling of the palms and soles at week 8.
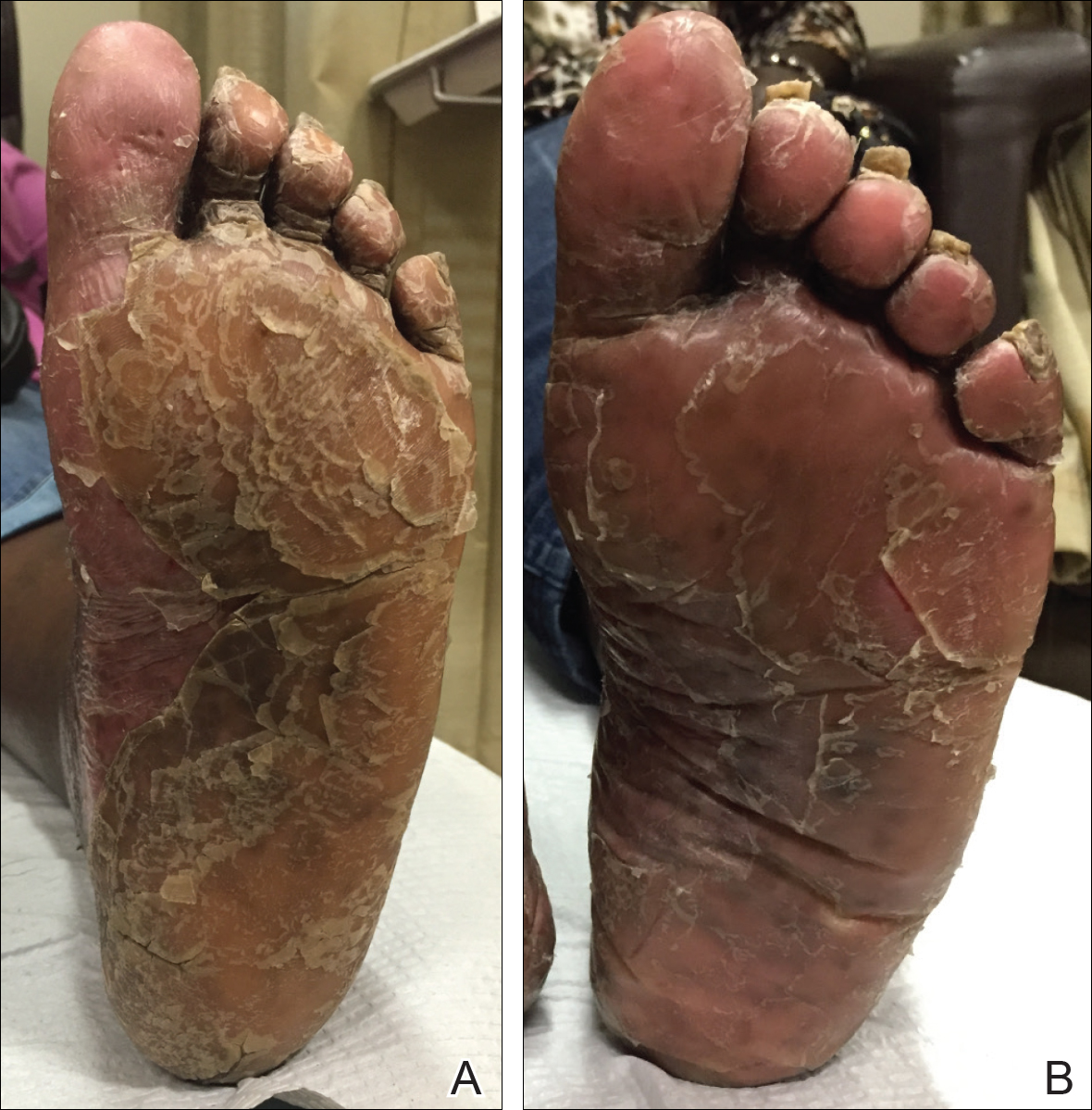
By week 12 of treatment, the palmar keratoderma had resolved, and debridement of the soles revealed patches of normal skin (Figure 2B). By week 52, no adverse events had been noted. The patient continued to experience mild keratoderma of the soles, making us reluctant to discontinue acitretin; however, she has maintained her maximal response, and her quality of life has significantly improved. The patient was continued on acitretin and secukinumab, and her condition remained stable.
Comment
Because there are no formal treatment guidelines for refractory PRP, case reports play an important role in clinical decision-making. When a patient is unresponsive to topical medications and first-line traditional systemic therapies (eg, methotrexate, cyclosporine, acitretin), biologic drugs effective in the treatment of psoriasis are widely accepted as the next therapeutic step.1 The biologic medications that are most often reported in the treatment of PRP are the TNF-α antagonists, as they have been available the longest.1-2 In a systematic review of 15 patients with PRP who were treated with TNF-α antagonists,2 80% of patients achieved complete response (mean time to maximal response, 5 months). There also are a number of reports of successful treatment of PRP with the IL-12/23 antagonist ustekinumab, which has been commercially available since 2009.5-9 Although improvement was noted in most of these patients at the time of the second injection (week 4 of therapy), maximal response with ustekinumab typically occurs between weeks 12 and 28.10
In our cases of PRP treated with secukinumab as well as 2 others that were recently reported in the literature, resolution of erythema and plaques was rapid. This superiority of the response rate parallels the performance of secukinumab relative to ustekinumab in patients with psoriasis11 In one case of a 67-year-old man with PRP treated with secukinumab, scaling and pruritus were reduced by week 3 of treatment and erythema had cleared by week 8.3 In another case of a 33-year-old woman with PRP, pruritus resolved after 1 week of treatment and erythematous plaques and palmoplantar keratoderma improved by week 2.4 In both of our cases, plaques had resolved or nearly resolved by week 4 of follow-up. Patient 1 achieved complete response at week 8 of therapy. Patient 2 never attained complete response, but by week 12 she achieved maximal response, which still resulted in markedly increased quality of life. We do not intend to make additions to her treatment plan because she is currently the clearest she has been since onset of symptoms and is happy with her present condition.
Although it is difficult to predict the long-term prognosis in our 2 patients, we will continue their current regimens indefinitely—as long as the response persists and no adverse events are experienced. This approach is consistent with guidelines for management of plaque psoriasis with secukinumab.12
This accumulation of evidence suggests the importance of the role of IL-17 in the pathogenesis of PRP. The serum level of IL-17 was not evaluated in our patients, but elevation of IL-17 has been reported in a case of PRP.13 Further studies are needed to clarify the role of IL-17 in this disease entity.
Conclusion
Given the refractory nature of PRP and the relative safety of targeted immunotherapy, trials of new biologics and potent small molecules approved for psoriasis treatment are worth exploring for PRP. In light of our reports and those in the literature and given the relative safety of anti–IL-17 agents, it may be reasonable to consider such agents as a first-line therapy for this predictably refractory disease.
- Klein A, Landthaler M, Karrer S. Pityriasis rubra pilaris. Am J Clin Dermatol. 2010;11:157-170.
- Petrof G, Almaani N, Archer CB, et al. A systematic review of the literature on the treatment of pityriasis rubra pilaris type 1 with TNF-antagonists. J Eur Acad Dermatol Venereol. 2013;27:E131-E135.
- Schuster D, Pfister-Wartha A, Bruckner-Tuderman L, et al. Successful treatment of refractory pityriasis rubra pilaris with secukinumab. JAMA Dermatol. 2016;152:1278-1280.
- Gauci ML, Jachiet M, Gottlieb J, et al. Successful treatment of type II pityriasis rubra pilaris with secukinumab. JAAD Case Rep. 2016;2:462-264.
- Chowdhary M, Davila U, Cohen DJ. Ustekinumab as an alternative treatment option for chronic pityriasis rubra pilaris. Case Rep Dermatol. 2015;7:46-50.
- Wohlrab J, Kreft B. Treatment of pityriasis rubra pilaris with ustekinumab. Br J Dermatol. 2010;163:655-656.
- Villaverde RR, Cano DS. Successful treatment of type 1 pityriasis rubra pilaris with ustekinumab therapy. Eur J Dermatol. 2010;20:630-631.
- Di Stefani A, Galluzzo M, Talamonti M, et al. Long-term ustekinumab treatment for refractory type I pityriasis rubra pilaris. J Dermatol Case Rep. 2013;7:5-9.
- Eytan O, Sarig O, Sprecher E, et al. Clinical response to ustekinumab in familial pityriasis rubra pilaris caused by a novel mutation in CARD14. Br J Dermatol. 2014;171:420-422.
- Papp KA, Langley RG, Lebwohl M, et al. Efficacy and safety of ustekinumab, a human interleukin-12/23 monoclonal antibody, in patients with psoriasis: 52-week results from a randomised, double-blind, placebo-controlled trial (PHOENIX 2). Lancet. 2008;371:1675-1684.
- Thaçi D, Blauvelt A, Reich K, et al. Secukinumab is superior to ustekinumab in clearing skin of subjects with moderate to severe plaque psoriasis: CLEAR, a randomized controlled trial. J Am Acad Dermatol. 2015;73:400-409.
- van de Kerkhof PC, Griffiths CE, Reich K, et al. Secukinumab long-term safety experience: a pooled analysis of 10 phase II and III clinical studies in patients with moderate to severe plaque psoriasis. J Am Acad Dermatol. 2016;75:83-98.
- Adnot-Desanlis L, Antonicelli F, Tabary T, et al. Effectiveness of infliximab in pityriasis rubra pilaris is associated with pro-inflammatory cytokine inhibition. Dermatology. 2013;226:41-46.
Although there currently are no formal guidelines for the treatment of refractory pityriasis rubra pilaris (PRP), successful off-label treatment of the condition with multiple biologics approved for psoriasis has been reported.1,2 Secukinumab, an IL-17A antagonist, has shown particularly striking results in the treatment of PRP in 2 recent case reports.3,4 We report 2 additional cases of severe refractory PRP that responded rapidly to treatment with secukinumab. In both cases, the patients’ erythematous plaques resolved or had nearly resolved by week 4 of treatment. Our findings suggest that IL-17 plays an important role in PRP pathogenesis and support future clinical trials of anti–IL-17 agents for treatment of this entity.
Case Reports
Patient 1
A 60-year-old man with a history of biopsy-proven PRP presented with persistent generalized erythema, scattered patches of normal skin, and hyperkeratotic plaques on the bilateral palms of 1 year’s duration. Previous therapies included topical steroids, topical calcipotriene, adalimumab 40 mg once every other week, infliximab 5 mg/kg once every 8 weeks, ustekinumab 90 mg once every 12 weeks, acitretin 25 mg once daily, and most recently cyclosporine 200 mg twice daily. Of these treatments, infliximab was the
At 4 weeks’ follow-up, there was a marked decrease in erythema and scaling. The body surface area affected had decreased to 5%, and improvement of palmar keratoderma was noted. The patient continued with maintenance dosing of secukinumab 300 mg once every 4 weeks. By week 8, the erythema had fully resolved (Figure 1B), and he remained clear at week 24. No adverse events were noted since initiation of therapy.

Patient 2
A 74-year-old woman with a history of PRP that had previously been misdiagnosed as psoriasis by an outside physician presented for evaluation of palmoplantar keratoderma (Figure 2A), follicular hyperkeratosis, and erythematous plaques on the trunk and arms of 5 years’ duration. Previous therapies included topical steroids, topical urea, methotrexate 20 mg once weekly, adalimumab 40 mg once every other week, infliximab 10 mg/kg once every 4 weeks, ustekinumab 90 mg once every 12 weeks, and most recently acitretin 50 mg once daily.
The patient had been maintained on ustekinumab and acitretin for 2 years with only mild improvement. Ustekinumab was then discontinued, and after 3 months treatment with secukinumab was added to the once-daily acitretin. Similar to Patient 1, loading doses of secukinumab 300 mg were administered once weekly for 5 weeks. The plaques on the trunk and arms had resolved by week 4, but the palmoplantar keratoderma persisted. The patient continued with the maintenance dose of secukinumab 300 mg once every 4 weeks and reported an increase in peeling of the palms and soles at week 8.

By week 12 of treatment, the palmar keratoderma had resolved, and debridement of the soles revealed patches of normal skin (Figure 2B). By week 52, no adverse events had been noted. The patient continued to experience mild keratoderma of the soles, making us reluctant to discontinue acitretin; however, she has maintained her maximal response, and her quality of life has significantly improved. The patient was continued on acitretin and secukinumab, and her condition remained stable.
Comment
Because there are no formal treatment guidelines for refractory PRP, case reports play an important role in clinical decision-making. When a patient is unresponsive to topical medications and first-line traditional systemic therapies (eg, methotrexate, cyclosporine, acitretin), biologic drugs effective in the treatment of psoriasis are widely accepted as the next therapeutic step.1 The biologic medications that are most often reported in the treatment of PRP are the TNF-α antagonists, as they have been available the longest.1-2 In a systematic review of 15 patients with PRP who were treated with TNF-α antagonists,2 80% of patients achieved complete response (mean time to maximal response, 5 months). There also are a number of reports of successful treatment of PRP with the IL-12/23 antagonist ustekinumab, which has been commercially available since 2009.5-9 Although improvement was noted in most of these patients at the time of the second injection (week 4 of therapy), maximal response with ustekinumab typically occurs between weeks 12 and 28.10
In our cases of PRP treated with secukinumab as well as 2 others that were recently reported in the literature, resolution of erythema and plaques was rapid. This superiority of the response rate parallels the performance of secukinumab relative to ustekinumab in patients with psoriasis11 In one case of a 67-year-old man with PRP treated with secukinumab, scaling and pruritus were reduced by week 3 of treatment and erythema had cleared by week 8.3 In another case of a 33-year-old woman with PRP, pruritus resolved after 1 week of treatment and erythematous plaques and palmoplantar keratoderma improved by week 2.4 In both of our cases, plaques had resolved or nearly resolved by week 4 of follow-up. Patient 1 achieved complete response at week 8 of therapy. Patient 2 never attained complete response, but by week 12 she achieved maximal response, which still resulted in markedly increased quality of life. We do not intend to make additions to her treatment plan because she is currently the clearest she has been since onset of symptoms and is happy with her present condition.
Although it is difficult to predict the long-term prognosis in our 2 patients, we will continue their current regimens indefinitely—as long as the response persists and no adverse events are experienced. This approach is consistent with guidelines for management of plaque psoriasis with secukinumab.12
This accumulation of evidence suggests the importance of the role of IL-17 in the pathogenesis of PRP. The serum level of IL-17 was not evaluated in our patients, but elevation of IL-17 has been reported in a case of PRP.13 Further studies are needed to clarify the role of IL-17 in this disease entity.
Conclusion
Given the refractory nature of PRP and the relative safety of targeted immunotherapy, trials of new biologics and potent small molecules approved for psoriasis treatment are worth exploring for PRP. In light of our reports and those in the literature and given the relative safety of anti–IL-17 agents, it may be reasonable to consider such agents as a first-line therapy for this predictably refractory disease.
Although there currently are no formal guidelines for the treatment of refractory pityriasis rubra pilaris (PRP), successful off-label treatment of the condition with multiple biologics approved for psoriasis has been reported.1,2 Secukinumab, an IL-17A antagonist, has shown particularly striking results in the treatment of PRP in 2 recent case reports.3,4 We report 2 additional cases of severe refractory PRP that responded rapidly to treatment with secukinumab. In both cases, the patients’ erythematous plaques resolved or had nearly resolved by week 4 of treatment. Our findings suggest that IL-17 plays an important role in PRP pathogenesis and support future clinical trials of anti–IL-17 agents for treatment of this entity.
Case Reports
Patient 1
A 60-year-old man with a history of biopsy-proven PRP presented with persistent generalized erythema, scattered patches of normal skin, and hyperkeratotic plaques on the bilateral palms of 1 year’s duration. Previous therapies included topical steroids, topical calcipotriene, adalimumab 40 mg once every other week, infliximab 5 mg/kg once every 8 weeks, ustekinumab 90 mg once every 12 weeks, acitretin 25 mg once daily, and most recently cyclosporine 200 mg twice daily. Of these treatments, infliximab was the
At 4 weeks’ follow-up, there was a marked decrease in erythema and scaling. The body surface area affected had decreased to 5%, and improvement of palmar keratoderma was noted. The patient continued with maintenance dosing of secukinumab 300 mg once every 4 weeks. By week 8, the erythema had fully resolved (Figure 1B), and he remained clear at week 24. No adverse events were noted since initiation of therapy.

Patient 2
A 74-year-old woman with a history of PRP that had previously been misdiagnosed as psoriasis by an outside physician presented for evaluation of palmoplantar keratoderma (Figure 2A), follicular hyperkeratosis, and erythematous plaques on the trunk and arms of 5 years’ duration. Previous therapies included topical steroids, topical urea, methotrexate 20 mg once weekly, adalimumab 40 mg once every other week, infliximab 10 mg/kg once every 4 weeks, ustekinumab 90 mg once every 12 weeks, and most recently acitretin 50 mg once daily.
The patient had been maintained on ustekinumab and acitretin for 2 years with only mild improvement. Ustekinumab was then discontinued, and after 3 months treatment with secukinumab was added to the once-daily acitretin. Similar to Patient 1, loading doses of secukinumab 300 mg were administered once weekly for 5 weeks. The plaques on the trunk and arms had resolved by week 4, but the palmoplantar keratoderma persisted. The patient continued with the maintenance dose of secukinumab 300 mg once every 4 weeks and reported an increase in peeling of the palms and soles at week 8.

By week 12 of treatment, the palmar keratoderma had resolved, and debridement of the soles revealed patches of normal skin (Figure 2B). By week 52, no adverse events had been noted. The patient continued to experience mild keratoderma of the soles, making us reluctant to discontinue acitretin; however, she has maintained her maximal response, and her quality of life has significantly improved. The patient was continued on acitretin and secukinumab, and her condition remained stable.
Comment
Because there are no formal treatment guidelines for refractory PRP, case reports play an important role in clinical decision-making. When a patient is unresponsive to topical medications and first-line traditional systemic therapies (eg, methotrexate, cyclosporine, acitretin), biologic drugs effective in the treatment of psoriasis are widely accepted as the next therapeutic step.1 The biologic medications that are most often reported in the treatment of PRP are the TNF-α antagonists, as they have been available the longest.1-2 In a systematic review of 15 patients with PRP who were treated with TNF-α antagonists,2 80% of patients achieved complete response (mean time to maximal response, 5 months). There also are a number of reports of successful treatment of PRP with the IL-12/23 antagonist ustekinumab, which has been commercially available since 2009.5-9 Although improvement was noted in most of these patients at the time of the second injection (week 4 of therapy), maximal response with ustekinumab typically occurs between weeks 12 and 28.10
In our cases of PRP treated with secukinumab as well as 2 others that were recently reported in the literature, resolution of erythema and plaques was rapid. This superiority of the response rate parallels the performance of secukinumab relative to ustekinumab in patients with psoriasis11 In one case of a 67-year-old man with PRP treated with secukinumab, scaling and pruritus were reduced by week 3 of treatment and erythema had cleared by week 8.3 In another case of a 33-year-old woman with PRP, pruritus resolved after 1 week of treatment and erythematous plaques and palmoplantar keratoderma improved by week 2.4 In both of our cases, plaques had resolved or nearly resolved by week 4 of follow-up. Patient 1 achieved complete response at week 8 of therapy. Patient 2 never attained complete response, but by week 12 she achieved maximal response, which still resulted in markedly increased quality of life. We do not intend to make additions to her treatment plan because she is currently the clearest she has been since onset of symptoms and is happy with her present condition.
Although it is difficult to predict the long-term prognosis in our 2 patients, we will continue their current regimens indefinitely—as long as the response persists and no adverse events are experienced. This approach is consistent with guidelines for management of plaque psoriasis with secukinumab.12
This accumulation of evidence suggests the importance of the role of IL-17 in the pathogenesis of PRP. The serum level of IL-17 was not evaluated in our patients, but elevation of IL-17 has been reported in a case of PRP.13 Further studies are needed to clarify the role of IL-17 in this disease entity.
Conclusion
Given the refractory nature of PRP and the relative safety of targeted immunotherapy, trials of new biologics and potent small molecules approved for psoriasis treatment are worth exploring for PRP. In light of our reports and those in the literature and given the relative safety of anti–IL-17 agents, it may be reasonable to consider such agents as a first-line therapy for this predictably refractory disease.
- Klein A, Landthaler M, Karrer S. Pityriasis rubra pilaris. Am J Clin Dermatol. 2010;11:157-170.
- Petrof G, Almaani N, Archer CB, et al. A systematic review of the literature on the treatment of pityriasis rubra pilaris type 1 with TNF-antagonists. J Eur Acad Dermatol Venereol. 2013;27:E131-E135.
- Schuster D, Pfister-Wartha A, Bruckner-Tuderman L, et al. Successful treatment of refractory pityriasis rubra pilaris with secukinumab. JAMA Dermatol. 2016;152:1278-1280.
- Gauci ML, Jachiet M, Gottlieb J, et al. Successful treatment of type II pityriasis rubra pilaris with secukinumab. JAAD Case Rep. 2016;2:462-264.
- Chowdhary M, Davila U, Cohen DJ. Ustekinumab as an alternative treatment option for chronic pityriasis rubra pilaris. Case Rep Dermatol. 2015;7:46-50.
- Wohlrab J, Kreft B. Treatment of pityriasis rubra pilaris with ustekinumab. Br J Dermatol. 2010;163:655-656.
- Villaverde RR, Cano DS. Successful treatment of type 1 pityriasis rubra pilaris with ustekinumab therapy. Eur J Dermatol. 2010;20:630-631.
- Di Stefani A, Galluzzo M, Talamonti M, et al. Long-term ustekinumab treatment for refractory type I pityriasis rubra pilaris. J Dermatol Case Rep. 2013;7:5-9.
- Eytan O, Sarig O, Sprecher E, et al. Clinical response to ustekinumab in familial pityriasis rubra pilaris caused by a novel mutation in CARD14. Br J Dermatol. 2014;171:420-422.
- Papp KA, Langley RG, Lebwohl M, et al. Efficacy and safety of ustekinumab, a human interleukin-12/23 monoclonal antibody, in patients with psoriasis: 52-week results from a randomised, double-blind, placebo-controlled trial (PHOENIX 2). Lancet. 2008;371:1675-1684.
- Thaçi D, Blauvelt A, Reich K, et al. Secukinumab is superior to ustekinumab in clearing skin of subjects with moderate to severe plaque psoriasis: CLEAR, a randomized controlled trial. J Am Acad Dermatol. 2015;73:400-409.
- van de Kerkhof PC, Griffiths CE, Reich K, et al. Secukinumab long-term safety experience: a pooled analysis of 10 phase II and III clinical studies in patients with moderate to severe plaque psoriasis. J Am Acad Dermatol. 2016;75:83-98.
- Adnot-Desanlis L, Antonicelli F, Tabary T, et al. Effectiveness of infliximab in pityriasis rubra pilaris is associated with pro-inflammatory cytokine inhibition. Dermatology. 2013;226:41-46.
- Klein A, Landthaler M, Karrer S. Pityriasis rubra pilaris. Am J Clin Dermatol. 2010;11:157-170.
- Petrof G, Almaani N, Archer CB, et al. A systematic review of the literature on the treatment of pityriasis rubra pilaris type 1 with TNF-antagonists. J Eur Acad Dermatol Venereol. 2013;27:E131-E135.
- Schuster D, Pfister-Wartha A, Bruckner-Tuderman L, et al. Successful treatment of refractory pityriasis rubra pilaris with secukinumab. JAMA Dermatol. 2016;152:1278-1280.
- Gauci ML, Jachiet M, Gottlieb J, et al. Successful treatment of type II pityriasis rubra pilaris with secukinumab. JAAD Case Rep. 2016;2:462-264.
- Chowdhary M, Davila U, Cohen DJ. Ustekinumab as an alternative treatment option for chronic pityriasis rubra pilaris. Case Rep Dermatol. 2015;7:46-50.
- Wohlrab J, Kreft B. Treatment of pityriasis rubra pilaris with ustekinumab. Br J Dermatol. 2010;163:655-656.
- Villaverde RR, Cano DS. Successful treatment of type 1 pityriasis rubra pilaris with ustekinumab therapy. Eur J Dermatol. 2010;20:630-631.
- Di Stefani A, Galluzzo M, Talamonti M, et al. Long-term ustekinumab treatment for refractory type I pityriasis rubra pilaris. J Dermatol Case Rep. 2013;7:5-9.
- Eytan O, Sarig O, Sprecher E, et al. Clinical response to ustekinumab in familial pityriasis rubra pilaris caused by a novel mutation in CARD14. Br J Dermatol. 2014;171:420-422.
- Papp KA, Langley RG, Lebwohl M, et al. Efficacy and safety of ustekinumab, a human interleukin-12/23 monoclonal antibody, in patients with psoriasis: 52-week results from a randomised, double-blind, placebo-controlled trial (PHOENIX 2). Lancet. 2008;371:1675-1684.
- Thaçi D, Blauvelt A, Reich K, et al. Secukinumab is superior to ustekinumab in clearing skin of subjects with moderate to severe plaque psoriasis: CLEAR, a randomized controlled trial. J Am Acad Dermatol. 2015;73:400-409.
- van de Kerkhof PC, Griffiths CE, Reich K, et al. Secukinumab long-term safety experience: a pooled analysis of 10 phase II and III clinical studies in patients with moderate to severe plaque psoriasis. J Am Acad Dermatol. 2016;75:83-98.
- Adnot-Desanlis L, Antonicelli F, Tabary T, et al. Effectiveness of infliximab in pityriasis rubra pilaris is associated with pro-inflammatory cytokine inhibition. Dermatology. 2013;226:41-46.
Practice Points
- In patients with pityriasis rubra pilaris (PRP) who have not responded to topical treatments, off-label treatment with systemic therapies approved for plaque psoriasis can be considered.
- Secukinumab, an IL-17A antagonist, has shown particularly striking results in the treatment of PRP.
Perianal Basal Cell Carcinoma Treated With Mohs Micrographic Surgery
Basal cell carcinoma (BCC) is the most common skin cancer in the United States1 and most commonly occurs in sun-exposed areas. Although BCCs can and do develop on other non–sun-exposed areas of the body, BCCs of the perianal or genital regions are very rare (0.27% of cases). It is estimated that perianal BCCs account for less than 0.08% of all BCCs.2
We present a case of a superficial nodular perianal BCC that was discovered following an annual total-body skin examination and was treated with Mohs micrographic surgery (MMS).
Case Report
A 76-year-old man presented to the dermatology clinic for an annual total-body skin examination as well as evaluation of a new submental skin lesion. The patient’s medical history included successfully treated malignant melanoma in situ, multiple actinic keratoses, and an eccrine carcinoma. His family history was noncontributory. Inspection of the submental lesion revealed a pearly, 1.8-cm, telangiectatic, nodular plaque that was highly suspected to be a BCC. During the examination, a 1-cm pinkish-red plaque was found on the skin in the left perianal region (Figure 1). The patient was unaware of the lesion and did not report any symptoms upon questioning.
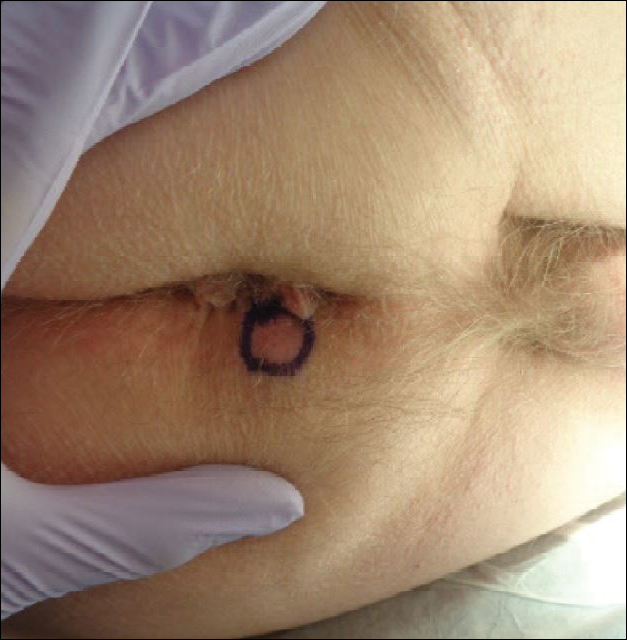
A shave biopsy of the submental lesion confirmed a diagnosis of micronodular BCC, and the patient was referred for MMS. It was decided to reevaluate the perianal lesion clinically at a follow-up appointment 2 months later and biopsy if it had not resolved. However, the patient did not attend the 2-month follow-up visit as scheduled, and it was not until the following year at his next annual total-body skin examination that the perianal lesion was rechecked. The lesion was unchanged at the time and was similar to the previous findings in both appearance and size. A punch biopsy was performed, and the pathology showed a superficial nodular perianal BCC (Figure 2). The perianal BCC was excised during a 2-stage MMS procedure with no recurrence at 6-month follow-up (Figure 3).

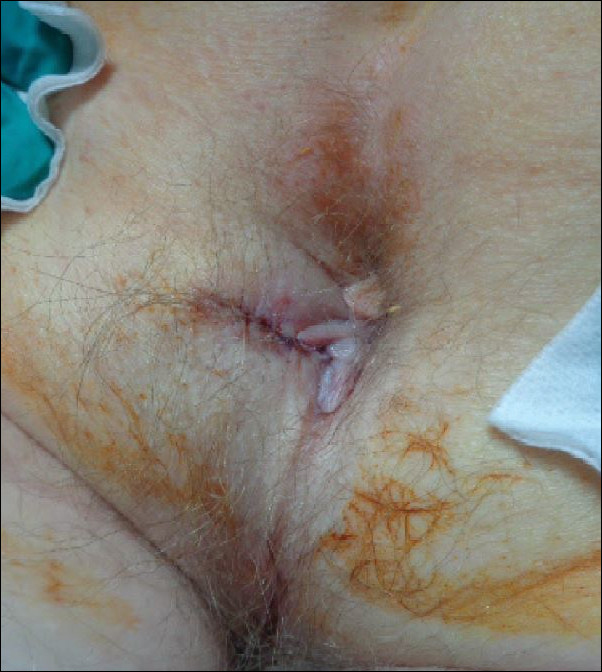
Comment
At the time of the patient’s initial visit, the differential diagnosis for this perianal lesion included an inflammatory or infectious dermatosis. Its asymptomatic nature made it difficult to determine how long it had been present. The lack of resolution on reevaluation of the lesion 1 year later raised the possibilities of amelanotic melanoma, squamous cell carcinoma, and lichen planus. Basal cell carcinoma was much lower in the differential diagnosis, as BCCs rarely are found in this area of the body; in fact, BCCs account for 0.2% of all anorectal neoplasms,3 and less than 0.08% of BCCs will occur in the perianal region.2
This challenging presentation is common for BCCs found in the perianal and perineal regions, as they are difficult to diagnose and often are overlooked as inflammatory dermatoses.4,5 The infrequency of perianal BCC reported in the literature as well as the predominance of BCC in sun-exposed areas makes it difficult for dermatologists to diagnose perianal BCC without biopsy. Another feature indicative of this diagnostic difficulty is that the average size of perianal and perineal BCCs has been found to be 1.95 cm.2 Without thorough and routine total-body skin examinations, there is no reliable way to catch asymptomatic BCCs in the perianal region until they have progressed far enough to become symptomatic. When possible, we recommend that dermatologists check the genital and anal regions during skin examinations and biopsy any suspicious lesions.
This case also highlights the challenge of missed appointments, which dermatologists also consistently face. Nonattendance rates in US dermatology clinics have been estimated at 17%,6 18.6%,7 19.4%,8 and 23.9%9 and present a challenge for even the best-run practices. Among patients with missed appointments, the most frequently stated reason in one survey was forgetting, and 24% of those contacted reported that they had not been reminded of their appointment.8 Many of the patients surveyed also expressed that they had preferred methods of receiving reminders such as e-mail or text message, which fell outside of traditional contact methods (eg, phone calls, voicemails). Confirming appointments ahead of time can reduce the number of missed appointments due to patient forgetfulness, and incorporating multiple communication modalities may lead to more effective appointment reminders.
Conclusion
Perianal BCC is challenging to diagnose and easy to overlook. Basal cell carcinoma is rarely found in the perianal regions and accounts for a fraction of all anorectal neoplasms. We recommend thorough total-body skin examinations that include the genital region and gluteal cleft when possible and encourage physicians to biopsy suspicious lesions in these regions. Routine, thorough total-body skin examinations can reveal neoplasms when they are smaller and asymptomatic. When surgical excision is indicated, MMS is an effective way to preserve as much tissue as possible and minimize recurrence.
- Rogers HW, Weinstock MA, Feldman SR, et al. Incidence estimate of nonmelanoma skin cancer (keratinocyte carcinomas) in the US population, 2012. JAMA Dermatology. 2015;151:1081-1086.
- Gibson GE, Ahmed I. Perianal and genital basal cell carcinoma: a clinicopathologic review of 51 cases. J Am Acad Dermatol. 2001;45:68-71.
- Leonard D, Beddy D, Dozois EJ. Neoplasms of anal canal and perianal skin. Clin Colon Rectal Surg. 2011;24:54-63.
- Bulur I, Boyuk E, Saracoglu ZN, et al. Perianal basal cell carcinoma. Case Rep Dermatol. 2015;7:25-28.
- Collins PS, Farber GA, Hegre AM. Basal-cell carcinoma of the vulva. J Dermatol Surg Oncol. 1981;7:711-714.
- Penneys NS, Glaser DA. The incidence of cancellation and nonattendance at a dermatology clinic. J Am Acad Dermatol. 1990;40:714-718.
- Cronin P, DeCoste L, Kimball A. A multivariate analysis of dermatology missed appointment predictors. JAMA Dermatology. 2013;149:1435-1437.
- Moustafa FA, Ramsey L, Huang KE, et al. Factors associated with missed dermatology appointments. Cutis. 2015;96:E20-E23.
- Canizares MJ, Penneys NS. The incidence of nonattendance at an urgent care dermatology clinic. J Am Acad Dermatol. 2002;46:457-459.
Basal cell carcinoma (BCC) is the most common skin cancer in the United States1 and most commonly occurs in sun-exposed areas. Although BCCs can and do develop on other non–sun-exposed areas of the body, BCCs of the perianal or genital regions are very rare (0.27% of cases). It is estimated that perianal BCCs account for less than 0.08% of all BCCs.2
We present a case of a superficial nodular perianal BCC that was discovered following an annual total-body skin examination and was treated with Mohs micrographic surgery (MMS).
Case Report
A 76-year-old man presented to the dermatology clinic for an annual total-body skin examination as well as evaluation of a new submental skin lesion. The patient’s medical history included successfully treated malignant melanoma in situ, multiple actinic keratoses, and an eccrine carcinoma. His family history was noncontributory. Inspection of the submental lesion revealed a pearly, 1.8-cm, telangiectatic, nodular plaque that was highly suspected to be a BCC. During the examination, a 1-cm pinkish-red plaque was found on the skin in the left perianal region (Figure 1). The patient was unaware of the lesion and did not report any symptoms upon questioning.

A shave biopsy of the submental lesion confirmed a diagnosis of micronodular BCC, and the patient was referred for MMS. It was decided to reevaluate the perianal lesion clinically at a follow-up appointment 2 months later and biopsy if it had not resolved. However, the patient did not attend the 2-month follow-up visit as scheduled, and it was not until the following year at his next annual total-body skin examination that the perianal lesion was rechecked. The lesion was unchanged at the time and was similar to the previous findings in both appearance and size. A punch biopsy was performed, and the pathology showed a superficial nodular perianal BCC (Figure 2). The perianal BCC was excised during a 2-stage MMS procedure with no recurrence at 6-month follow-up (Figure 3).


Comment
At the time of the patient’s initial visit, the differential diagnosis for this perianal lesion included an inflammatory or infectious dermatosis. Its asymptomatic nature made it difficult to determine how long it had been present. The lack of resolution on reevaluation of the lesion 1 year later raised the possibilities of amelanotic melanoma, squamous cell carcinoma, and lichen planus. Basal cell carcinoma was much lower in the differential diagnosis, as BCCs rarely are found in this area of the body; in fact, BCCs account for 0.2% of all anorectal neoplasms,3 and less than 0.08% of BCCs will occur in the perianal region.2
This challenging presentation is common for BCCs found in the perianal and perineal regions, as they are difficult to diagnose and often are overlooked as inflammatory dermatoses.4,5 The infrequency of perianal BCC reported in the literature as well as the predominance of BCC in sun-exposed areas makes it difficult for dermatologists to diagnose perianal BCC without biopsy. Another feature indicative of this diagnostic difficulty is that the average size of perianal and perineal BCCs has been found to be 1.95 cm.2 Without thorough and routine total-body skin examinations, there is no reliable way to catch asymptomatic BCCs in the perianal region until they have progressed far enough to become symptomatic. When possible, we recommend that dermatologists check the genital and anal regions during skin examinations and biopsy any suspicious lesions.
This case also highlights the challenge of missed appointments, which dermatologists also consistently face. Nonattendance rates in US dermatology clinics have been estimated at 17%,6 18.6%,7 19.4%,8 and 23.9%9 and present a challenge for even the best-run practices. Among patients with missed appointments, the most frequently stated reason in one survey was forgetting, and 24% of those contacted reported that they had not been reminded of their appointment.8 Many of the patients surveyed also expressed that they had preferred methods of receiving reminders such as e-mail or text message, which fell outside of traditional contact methods (eg, phone calls, voicemails). Confirming appointments ahead of time can reduce the number of missed appointments due to patient forgetfulness, and incorporating multiple communication modalities may lead to more effective appointment reminders.
Conclusion
Perianal BCC is challenging to diagnose and easy to overlook. Basal cell carcinoma is rarely found in the perianal regions and accounts for a fraction of all anorectal neoplasms. We recommend thorough total-body skin examinations that include the genital region and gluteal cleft when possible and encourage physicians to biopsy suspicious lesions in these regions. Routine, thorough total-body skin examinations can reveal neoplasms when they are smaller and asymptomatic. When surgical excision is indicated, MMS is an effective way to preserve as much tissue as possible and minimize recurrence.
Basal cell carcinoma (BCC) is the most common skin cancer in the United States1 and most commonly occurs in sun-exposed areas. Although BCCs can and do develop on other non–sun-exposed areas of the body, BCCs of the perianal or genital regions are very rare (0.27% of cases). It is estimated that perianal BCCs account for less than 0.08% of all BCCs.2
We present a case of a superficial nodular perianal BCC that was discovered following an annual total-body skin examination and was treated with Mohs micrographic surgery (MMS).
Case Report
A 76-year-old man presented to the dermatology clinic for an annual total-body skin examination as well as evaluation of a new submental skin lesion. The patient’s medical history included successfully treated malignant melanoma in situ, multiple actinic keratoses, and an eccrine carcinoma. His family history was noncontributory. Inspection of the submental lesion revealed a pearly, 1.8-cm, telangiectatic, nodular plaque that was highly suspected to be a BCC. During the examination, a 1-cm pinkish-red plaque was found on the skin in the left perianal region (Figure 1). The patient was unaware of the lesion and did not report any symptoms upon questioning.

A shave biopsy of the submental lesion confirmed a diagnosis of micronodular BCC, and the patient was referred for MMS. It was decided to reevaluate the perianal lesion clinically at a follow-up appointment 2 months later and biopsy if it had not resolved. However, the patient did not attend the 2-month follow-up visit as scheduled, and it was not until the following year at his next annual total-body skin examination that the perianal lesion was rechecked. The lesion was unchanged at the time and was similar to the previous findings in both appearance and size. A punch biopsy was performed, and the pathology showed a superficial nodular perianal BCC (Figure 2). The perianal BCC was excised during a 2-stage MMS procedure with no recurrence at 6-month follow-up (Figure 3).


Comment
At the time of the patient’s initial visit, the differential diagnosis for this perianal lesion included an inflammatory or infectious dermatosis. Its asymptomatic nature made it difficult to determine how long it had been present. The lack of resolution on reevaluation of the lesion 1 year later raised the possibilities of amelanotic melanoma, squamous cell carcinoma, and lichen planus. Basal cell carcinoma was much lower in the differential diagnosis, as BCCs rarely are found in this area of the body; in fact, BCCs account for 0.2% of all anorectal neoplasms,3 and less than 0.08% of BCCs will occur in the perianal region.2
This challenging presentation is common for BCCs found in the perianal and perineal regions, as they are difficult to diagnose and often are overlooked as inflammatory dermatoses.4,5 The infrequency of perianal BCC reported in the literature as well as the predominance of BCC in sun-exposed areas makes it difficult for dermatologists to diagnose perianal BCC without biopsy. Another feature indicative of this diagnostic difficulty is that the average size of perianal and perineal BCCs has been found to be 1.95 cm.2 Without thorough and routine total-body skin examinations, there is no reliable way to catch asymptomatic BCCs in the perianal region until they have progressed far enough to become symptomatic. When possible, we recommend that dermatologists check the genital and anal regions during skin examinations and biopsy any suspicious lesions.
This case also highlights the challenge of missed appointments, which dermatologists also consistently face. Nonattendance rates in US dermatology clinics have been estimated at 17%,6 18.6%,7 19.4%,8 and 23.9%9 and present a challenge for even the best-run practices. Among patients with missed appointments, the most frequently stated reason in one survey was forgetting, and 24% of those contacted reported that they had not been reminded of their appointment.8 Many of the patients surveyed also expressed that they had preferred methods of receiving reminders such as e-mail or text message, which fell outside of traditional contact methods (eg, phone calls, voicemails). Confirming appointments ahead of time can reduce the number of missed appointments due to patient forgetfulness, and incorporating multiple communication modalities may lead to more effective appointment reminders.
Conclusion
Perianal BCC is challenging to diagnose and easy to overlook. Basal cell carcinoma is rarely found in the perianal regions and accounts for a fraction of all anorectal neoplasms. We recommend thorough total-body skin examinations that include the genital region and gluteal cleft when possible and encourage physicians to biopsy suspicious lesions in these regions. Routine, thorough total-body skin examinations can reveal neoplasms when they are smaller and asymptomatic. When surgical excision is indicated, MMS is an effective way to preserve as much tissue as possible and minimize recurrence.
- Rogers HW, Weinstock MA, Feldman SR, et al. Incidence estimate of nonmelanoma skin cancer (keratinocyte carcinomas) in the US population, 2012. JAMA Dermatology. 2015;151:1081-1086.
- Gibson GE, Ahmed I. Perianal and genital basal cell carcinoma: a clinicopathologic review of 51 cases. J Am Acad Dermatol. 2001;45:68-71.
- Leonard D, Beddy D, Dozois EJ. Neoplasms of anal canal and perianal skin. Clin Colon Rectal Surg. 2011;24:54-63.
- Bulur I, Boyuk E, Saracoglu ZN, et al. Perianal basal cell carcinoma. Case Rep Dermatol. 2015;7:25-28.
- Collins PS, Farber GA, Hegre AM. Basal-cell carcinoma of the vulva. J Dermatol Surg Oncol. 1981;7:711-714.
- Penneys NS, Glaser DA. The incidence of cancellation and nonattendance at a dermatology clinic. J Am Acad Dermatol. 1990;40:714-718.
- Cronin P, DeCoste L, Kimball A. A multivariate analysis of dermatology missed appointment predictors. JAMA Dermatology. 2013;149:1435-1437.
- Moustafa FA, Ramsey L, Huang KE, et al. Factors associated with missed dermatology appointments. Cutis. 2015;96:E20-E23.
- Canizares MJ, Penneys NS. The incidence of nonattendance at an urgent care dermatology clinic. J Am Acad Dermatol. 2002;46:457-459.
- Rogers HW, Weinstock MA, Feldman SR, et al. Incidence estimate of nonmelanoma skin cancer (keratinocyte carcinomas) in the US population, 2012. JAMA Dermatology. 2015;151:1081-1086.
- Gibson GE, Ahmed I. Perianal and genital basal cell carcinoma: a clinicopathologic review of 51 cases. J Am Acad Dermatol. 2001;45:68-71.
- Leonard D, Beddy D, Dozois EJ. Neoplasms of anal canal and perianal skin. Clin Colon Rectal Surg. 2011;24:54-63.
- Bulur I, Boyuk E, Saracoglu ZN, et al. Perianal basal cell carcinoma. Case Rep Dermatol. 2015;7:25-28.
- Collins PS, Farber GA, Hegre AM. Basal-cell carcinoma of the vulva. J Dermatol Surg Oncol. 1981;7:711-714.
- Penneys NS, Glaser DA. The incidence of cancellation and nonattendance at a dermatology clinic. J Am Acad Dermatol. 1990;40:714-718.
- Cronin P, DeCoste L, Kimball A. A multivariate analysis of dermatology missed appointment predictors. JAMA Dermatology. 2013;149:1435-1437.
- Moustafa FA, Ramsey L, Huang KE, et al. Factors associated with missed dermatology appointments. Cutis. 2015;96:E20-E23.
- Canizares MJ, Penneys NS. The incidence of nonattendance at an urgent care dermatology clinic. J Am Acad Dermatol. 2002;46:457-459.
Practice Points
- Basal cell carcinoma is less common in non–sun-exposed areas of the body and is exceptionally rare in the perineal and perianal regions.
- Thorough total-body skin examinations may lead to early detection of asymptomatic skin lesions, allowing for earlier and less invasive treatment.
- Appointment attendance and patient compliance are common challenges that dermatologists face. Patient reminders via their preferred method of communication may help reduce missed dermatology appointments.
Critical anemia • light-headedness • bilateral leg swelling • Dx?
THE CASE
A 40-year-old man was referred to the emergency department (ED) with critical anemia after routine blood work at an outside clinic showed a hemoglobin level of 3.5 g/dL. On presentation, he reported symptoms of fatigue, shortness of breath, bilateral leg swelling, dizziness (characterized as light-headedness), and frequent heartburn. He said that the symptoms began 5 weeks earlier, after he was exposed to a relative with hand, foot, and mouth disease.
Additionally, the patient reported an intentional 14-lb weight loss over the 6 months prior to presentation. He denied fever, rash, chest pain, loss of consciousness, headache, abdominal pain, hematemesis, melena, and hematochezia. His medical history was significant for peptic ulcer disease (diagnosed and treated at age 8). He did not recall the specifics, and he denied any related chronic symptoms or complications. His family history (paternal) was significant for colon cancer.
The physical exam revealed conjunctival pallor, skin pallor, jaundice, +1 bilateral lower extremity edema, tachycardia, and tachypnea. Stool Hemoccult was negative. On repeat complete blood count (performed in the ED), hemoglobin was found to be 3.1 g/dL with a mean corpuscular volume of 47 fL.
THE DIAGNOSIS
The patient was admitted to the family medicine service and received 4 units of packed red blood cells, which increased his hemoglobin to the target goal of >7 g/dL. A colonoscopy and an esophagogastroduodenoscopy (EGD) were performed (FIGURE 1A-1C), with results suggestive of diverticulosis, probable Barrett’s mucosa, esophageal ulcer, huge hiatal hernia (at least one-half of the stomach was in the chest), and Cameron ulcers. Esophageal biopsies showed cardiac mucosa with chronic inflammation. Esophageal ulcer biopsies revealed Barrett’s esophagus without dysplasia. Duodenal biopsies displayed normal mucosa.
DISCUSSION
Although rare, Cameron ulcers must be considered in the differential diagnosis of patients with chronic anemia of unknown origin. The potential for these lesions to result in chronic blood loss, which could over time manifest as severe anemia or hypovolemic shock, makes proper diagnosis and prompt treatment especially important.4,5
In our patient’s case, his severe anemia was likely the result of a combination of the esophageal and Cameron ulcers evidenced on EGD, rather than any single ulcer. In our review of the literature, we found no reports of any patients with anemia and Cameron ulcers who presented with hemoglobin levels as low as our patient had.
Treat with a PPI and iron supplementation
Multiple EGDs may be needed to properly diagnose Cameron ulcers, as they can be difficult to identify. Once a patient receives the diagnosis, he or she will typically be put on a daily proton pump inhibitor (PPI) regimen, such as omeprazole 20 mg bid. However, since many patients with Cameron ulcers also have acid-related problems (as was true in this case), a multifactorial acid suppression approach may be warranted.1 This may include recommending lifestyle modifications (eg, eating small meals, avoiding foods that provoke symptoms, or losing weight) and prescribing medications in addition to a PPI, such as an H2 blocker (eg, 300 mg qid, before meals and at bedtime).
In addition, iron sulfate (325 mg/d, in this case) and blood transfusions may be required to treat the anemia. In refractory cases, endoscopic or surgical interventions, such as hemoclipping, Nissen fundoplication, or laparoscopic gastropexy, may need to be performed.2
Our patient was given a prescription for ferrous sulfate 325 mg/d and omeprazole 20 mg bid. His symptoms improved with treatment, and he was discharged on Day 5; his hemoglobin remained >7 g/dL.
THE TAKEAWAY
The association between chronic iron deficiency anemia and Cameron ulcers has been established but is commonly overlooked in patients presenting with unexplained anemia or an undiagnosed hiatal hernia. This is likely due to their rarity as a cause of anemia, in general.
Furthermore, the lesions can be missed on EGD; multiple EGDs may be needed to make the diagnosis. Once diagnosed, Cameron ulcers typically respond well to twice daily PPI treatment. Patients with refractory, recurrent, or severe lesions, or large, symptomatic hiatal hernias should be referred for surgical assessment.
CORRESPONDENCE
Megan Yee, 801 Broadward Avenue NW, Grand Rapids, MI 49504; [email protected].
1. Maganty K, Smith RL. Cameron lesions: unusual cause of gastrointestinal bleeding and anemia. Digestion. 2008;77:214-217.
2. Camus M, Jensen DM, Ohning GV, et al. Severe upper gastrointestinal hemorrhage from linear gastric ulcers in large hiatal hernias: a large prospective case series of Cameron ulcers. Endoscopy. 2013;45:397-400.
3. Kimer N, Schmidt PN, Krag, A. Cameron lesions: an often overlooked cause of iron deficiency anaemia in patients with large hiatal hernias. BMJ Case Rep. 2010;2010.
4. Kapadia S, Jagroop S, Kumar, A. Cameron ulcers: an atypical source for a massive upper gastrointestinal bleed. World J Gastroenterol. 2012;18:4959-4961.
5. Gupta P, Suryadevara M, Das A, et al. Cameron ulcer causing severe anemia in a patient with diaphragmatic hernia. Am J Case Rep. 2015;16:733-736.
THE CASE
A 40-year-old man was referred to the emergency department (ED) with critical anemia after routine blood work at an outside clinic showed a hemoglobin level of 3.5 g/dL. On presentation, he reported symptoms of fatigue, shortness of breath, bilateral leg swelling, dizziness (characterized as light-headedness), and frequent heartburn. He said that the symptoms began 5 weeks earlier, after he was exposed to a relative with hand, foot, and mouth disease.
Additionally, the patient reported an intentional 14-lb weight loss over the 6 months prior to presentation. He denied fever, rash, chest pain, loss of consciousness, headache, abdominal pain, hematemesis, melena, and hematochezia. His medical history was significant for peptic ulcer disease (diagnosed and treated at age 8). He did not recall the specifics, and he denied any related chronic symptoms or complications. His family history (paternal) was significant for colon cancer.
The physical exam revealed conjunctival pallor, skin pallor, jaundice, +1 bilateral lower extremity edema, tachycardia, and tachypnea. Stool Hemoccult was negative. On repeat complete blood count (performed in the ED), hemoglobin was found to be 3.1 g/dL with a mean corpuscular volume of 47 fL.
THE DIAGNOSIS
The patient was admitted to the family medicine service and received 4 units of packed red blood cells, which increased his hemoglobin to the target goal of >7 g/dL. A colonoscopy and an esophagogastroduodenoscopy (EGD) were performed (FIGURE 1A-1C), with results suggestive of diverticulosis, probable Barrett’s mucosa, esophageal ulcer, huge hiatal hernia (at least one-half of the stomach was in the chest), and Cameron ulcers. Esophageal biopsies showed cardiac mucosa with chronic inflammation. Esophageal ulcer biopsies revealed Barrett’s esophagus without dysplasia. Duodenal biopsies displayed normal mucosa.

DISCUSSION
Although rare, Cameron ulcers must be considered in the differential diagnosis of patients with chronic anemia of unknown origin. The potential for these lesions to result in chronic blood loss, which could over time manifest as severe anemia or hypovolemic shock, makes proper diagnosis and prompt treatment especially important.4,5
In our patient’s case, his severe anemia was likely the result of a combination of the esophageal and Cameron ulcers evidenced on EGD, rather than any single ulcer. In our review of the literature, we found no reports of any patients with anemia and Cameron ulcers who presented with hemoglobin levels as low as our patient had.
Treat with a PPI and iron supplementation
Multiple EGDs may be needed to properly diagnose Cameron ulcers, as they can be difficult to identify. Once a patient receives the diagnosis, he or she will typically be put on a daily proton pump inhibitor (PPI) regimen, such as omeprazole 20 mg bid. However, since many patients with Cameron ulcers also have acid-related problems (as was true in this case), a multifactorial acid suppression approach may be warranted.1 This may include recommending lifestyle modifications (eg, eating small meals, avoiding foods that provoke symptoms, or losing weight) and prescribing medications in addition to a PPI, such as an H2 blocker (eg, 300 mg qid, before meals and at bedtime).
In addition, iron sulfate (325 mg/d, in this case) and blood transfusions may be required to treat the anemia. In refractory cases, endoscopic or surgical interventions, such as hemoclipping, Nissen fundoplication, or laparoscopic gastropexy, may need to be performed.2
Our patient was given a prescription for ferrous sulfate 325 mg/d and omeprazole 20 mg bid. His symptoms improved with treatment, and he was discharged on Day 5; his hemoglobin remained >7 g/dL.
THE TAKEAWAY
The association between chronic iron deficiency anemia and Cameron ulcers has been established but is commonly overlooked in patients presenting with unexplained anemia or an undiagnosed hiatal hernia. This is likely due to their rarity as a cause of anemia, in general.
Furthermore, the lesions can be missed on EGD; multiple EGDs may be needed to make the diagnosis. Once diagnosed, Cameron ulcers typically respond well to twice daily PPI treatment. Patients with refractory, recurrent, or severe lesions, or large, symptomatic hiatal hernias should be referred for surgical assessment.
CORRESPONDENCE
Megan Yee, 801 Broadward Avenue NW, Grand Rapids, MI 49504; [email protected].
THE CASE
A 40-year-old man was referred to the emergency department (ED) with critical anemia after routine blood work at an outside clinic showed a hemoglobin level of 3.5 g/dL. On presentation, he reported symptoms of fatigue, shortness of breath, bilateral leg swelling, dizziness (characterized as light-headedness), and frequent heartburn. He said that the symptoms began 5 weeks earlier, after he was exposed to a relative with hand, foot, and mouth disease.
Additionally, the patient reported an intentional 14-lb weight loss over the 6 months prior to presentation. He denied fever, rash, chest pain, loss of consciousness, headache, abdominal pain, hematemesis, melena, and hematochezia. His medical history was significant for peptic ulcer disease (diagnosed and treated at age 8). He did not recall the specifics, and he denied any related chronic symptoms or complications. His family history (paternal) was significant for colon cancer.
The physical exam revealed conjunctival pallor, skin pallor, jaundice, +1 bilateral lower extremity edema, tachycardia, and tachypnea. Stool Hemoccult was negative. On repeat complete blood count (performed in the ED), hemoglobin was found to be 3.1 g/dL with a mean corpuscular volume of 47 fL.
THE DIAGNOSIS
The patient was admitted to the family medicine service and received 4 units of packed red blood cells, which increased his hemoglobin to the target goal of >7 g/dL. A colonoscopy and an esophagogastroduodenoscopy (EGD) were performed (FIGURE 1A-1C), with results suggestive of diverticulosis, probable Barrett’s mucosa, esophageal ulcer, huge hiatal hernia (at least one-half of the stomach was in the chest), and Cameron ulcers. Esophageal biopsies showed cardiac mucosa with chronic inflammation. Esophageal ulcer biopsies revealed Barrett’s esophagus without dysplasia. Duodenal biopsies displayed normal mucosa.

DISCUSSION
Although rare, Cameron ulcers must be considered in the differential diagnosis of patients with chronic anemia of unknown origin. The potential for these lesions to result in chronic blood loss, which could over time manifest as severe anemia or hypovolemic shock, makes proper diagnosis and prompt treatment especially important.4,5
In our patient’s case, his severe anemia was likely the result of a combination of the esophageal and Cameron ulcers evidenced on EGD, rather than any single ulcer. In our review of the literature, we found no reports of any patients with anemia and Cameron ulcers who presented with hemoglobin levels as low as our patient had.
Treat with a PPI and iron supplementation
Multiple EGDs may be needed to properly diagnose Cameron ulcers, as they can be difficult to identify. Once a patient receives the diagnosis, he or she will typically be put on a daily proton pump inhibitor (PPI) regimen, such as omeprazole 20 mg bid. However, since many patients with Cameron ulcers also have acid-related problems (as was true in this case), a multifactorial acid suppression approach may be warranted.1 This may include recommending lifestyle modifications (eg, eating small meals, avoiding foods that provoke symptoms, or losing weight) and prescribing medications in addition to a PPI, such as an H2 blocker (eg, 300 mg qid, before meals and at bedtime).
In addition, iron sulfate (325 mg/d, in this case) and blood transfusions may be required to treat the anemia. In refractory cases, endoscopic or surgical interventions, such as hemoclipping, Nissen fundoplication, or laparoscopic gastropexy, may need to be performed.2
Our patient was given a prescription for ferrous sulfate 325 mg/d and omeprazole 20 mg bid. His symptoms improved with treatment, and he was discharged on Day 5; his hemoglobin remained >7 g/dL.
THE TAKEAWAY
The association between chronic iron deficiency anemia and Cameron ulcers has been established but is commonly overlooked in patients presenting with unexplained anemia or an undiagnosed hiatal hernia. This is likely due to their rarity as a cause of anemia, in general.
Furthermore, the lesions can be missed on EGD; multiple EGDs may be needed to make the diagnosis. Once diagnosed, Cameron ulcers typically respond well to twice daily PPI treatment. Patients with refractory, recurrent, or severe lesions, or large, symptomatic hiatal hernias should be referred for surgical assessment.
CORRESPONDENCE
Megan Yee, 801 Broadward Avenue NW, Grand Rapids, MI 49504; [email protected].
1. Maganty K, Smith RL. Cameron lesions: unusual cause of gastrointestinal bleeding and anemia. Digestion. 2008;77:214-217.
2. Camus M, Jensen DM, Ohning GV, et al. Severe upper gastrointestinal hemorrhage from linear gastric ulcers in large hiatal hernias: a large prospective case series of Cameron ulcers. Endoscopy. 2013;45:397-400.
3. Kimer N, Schmidt PN, Krag, A. Cameron lesions: an often overlooked cause of iron deficiency anaemia in patients with large hiatal hernias. BMJ Case Rep. 2010;2010.
4. Kapadia S, Jagroop S, Kumar, A. Cameron ulcers: an atypical source for a massive upper gastrointestinal bleed. World J Gastroenterol. 2012;18:4959-4961.
5. Gupta P, Suryadevara M, Das A, et al. Cameron ulcer causing severe anemia in a patient with diaphragmatic hernia. Am J Case Rep. 2015;16:733-736.
1. Maganty K, Smith RL. Cameron lesions: unusual cause of gastrointestinal bleeding and anemia. Digestion. 2008;77:214-217.
2. Camus M, Jensen DM, Ohning GV, et al. Severe upper gastrointestinal hemorrhage from linear gastric ulcers in large hiatal hernias: a large prospective case series of Cameron ulcers. Endoscopy. 2013;45:397-400.
3. Kimer N, Schmidt PN, Krag, A. Cameron lesions: an often overlooked cause of iron deficiency anaemia in patients with large hiatal hernias. BMJ Case Rep. 2010;2010.
4. Kapadia S, Jagroop S, Kumar, A. Cameron ulcers: an atypical source for a massive upper gastrointestinal bleed. World J Gastroenterol. 2012;18:4959-4961.
5. Gupta P, Suryadevara M, Das A, et al. Cameron ulcer causing severe anemia in a patient with diaphragmatic hernia. Am J Case Rep. 2015;16:733-736.
Isolated ocular metastases from lung cancer
Non–small cell lung cancer constitutes 80%-85% of lung cancers, and 40% of NSCLC are adenocarcinoma. It is rare to find intraocular metastasis from lung cancer. In this article, we present the case of a patient who presented with complaints of diminished vision redness of the eye and was found to have intra-ocular metastases from lung cancer.
Case presentation and summary
A 60-year-old man with a 40-pack per year history of smoking presented to multiple ophthalmologists with complaints of decreased vision and redness of the left eye. He was eventually evaluated by an ophthalmologist who performed a biopsy of the anterior chamber of the eye. Histologic findings were consistent with adenocarcinoma of lung primary (Figures 1 and 2).
After the diagnosis, a chest X-ray showed that the patient had a left lower lung mass. The results of his physical exam were all within normal limits, with the exception of decreased visual acuity in the left eye. The results of his laboratory studies, including complete blood count and serum chemistries, were also within normal limits. Imaging studies – including a computed-tomography (CT) scan of the chest, abdomen, and pelvis and a full-body positron-emission tomography–CT scan – showed a hypermetabolic left lower lobe mass 4.5 cm and right lower paratracheal lymph node metastasis 2 cm with a small focus of increased uptake alone the medial aspect of the left globe (Figures 3 and 4).
An MRI orbit was performed in an attempt to better characterize the left eye mass, but no optic lesion was identified. A biopsy of the left lower lung mass was consistent with non–small-cell lung cancer (NSCLC). Aside from the isolated left eye metastases, the patient did not have evidence of other distant metastatic involvement.
He was started on palliative chemotherapy on a clinical trial and received intravenous carboplatin AUC 6, pemetrexed 500 mg/m2, and bevacizumab 15 mg/kg every 3 weeks. He received 1 dose intraocular bevacizumab injection before initiation of systemic chemotherapy as he was symptomatic from the intraocular metastases. Within 2 weeks after intravitreal bevacizumab was administered, the patient had subjective improvement in vision. Mutational analysis to identify if the patient would benefit from targeted therapy showed no presence of EGFR mutation and ALK gene rearrangement, and that the patient was K-RAS mutant.
After treatment initiation, interval imaging studies (a computed-tomography scan of the chest, abdomen, pelvis; and magnetic-resonance imaging of the brain) after 3 cycles showed no evidence of disease progression, and after 4 cycles of chemotherapy with these drugs, the patient was started on maintenance chemotherapy with bevacizumab 15 mg/kg and pemetrexed 500 mg/m2.
Discussion
Choroidal metastasis is the most common site of intraocular tumor. In an autopsy study of 230 patients with carcinoma, 12% of cases demonstrated histologic evidence of ocular metastasis.1 A retrospective series of patients with malignant involvement of the eye, 66% of patients had a known history of primary cancer and in 34% of patients the ocular tumor was the first sign of cancer.2 The most common cancers that were found to have ocular metastasis were lung and breast cancer.2 Adenocarcinoma was the most common histologic type of lung cancer to result in ocular metastases and was seen in 41% of patients.3
Decreased or blurred vision with redness as the primary complaint of NSCLC is rare. Only a few case reports are available. Abundo and colleagues reported that 0.7%-12% of patients with lung cancer develop ocular metastases.4 Therefore, routine ophthalmologic screening for ocular metastases in patients with cancer has not been pursued in asymptomatic patients.5 Ophthalmological evaluation is recommended in symptomatic patients.
Metastatic involvement of two or more other organs was found to be a risk factor for development of choroidal metastasis in patients with lung cancer though in our patient no evidence of other organ involvement was found.5 The most common site of metastases in patients with NSCLC with ocular metastases was found to be the liver. Choroidal metastases was reported to be the sixth common site of metastases in patients with lung cancer.5
Treatment of ocular manifestations has been generally confined to surgical resection or radiation therapy, but advances in chemotherapy and development of novel targeted agents have shown promising results.7 Median life expectancy after a diagnosis of uveal metastases was reported to be 12 months in a retrospective study, which is similar to the reported median survival in metastatic NSCLC.8
Our patient was enrolled in a clinical trial and was treated with a regimen of carboplatin, paclitaxel, and bevacizumab. On presentation, he had significant impairment of vision with pain. He was treated with intravitreal bevacizumab yielding improvement in his visual symptoms. Bevacizumab is a vascular endothelial growth factor receptor monoclonal antibody approved for use in patients with metastatic lung cancer. Other pathways that have been reported in development of lung cancer involve the ALK gene translocation, and EGFR and K-RAS mutations, and targeted therapy has shown good results in cancer patients with these molecular defects. Randomized clinical trials in patients with advanced NSCLC and an EGFR mutation have shown significant improvement in overall survival with the use of erlotinib, a tyrosine kinase inhibitor targeting the epidermal growth factor receptor.9 Similarly, crizotinib has shown promising results in patients with metastatic NSCLC who have ELM-ALK rearrangement.10 As our patient’s tumor did not have either of these mutations, he was initiated on chemotherapy with bevacizumab. The presence of a K-RAS mutation in this patient further supported the use of front-line chemotherapy given that it may confer resistance against agents that target the EGFR pathway.
In our review of the literature, we found cases of patients with ocular metastases who responded well to therapy with targeted agents (Table).
Singh and colleagues did a systematic review of 55 cases of patients with lung cancer and choroidal metastases and found that the type of therapy depended on when the diagnosis had been made in relation to the advent of targeted therapy: cases diagnosed before targeted therapy had received radiation therapy or enucleation.6 As far as we could ascertain, there have been no randomized studies evaluating the impact of various targeted therapies or systemic chemotherapy on ocular metastases, although case reports have documented improvement in vision and regression of metastases with such therapy.
Conclusion
The goal of therapy in metastatic lung cancer is palliation of symptoms and improvement in patient quality of life with prolongation in overall survival. The newer targeted chemotherapeutic agents assist in achieving these goals and may decrease the morbidity associated from radiation or surgery with improvement in vision and regression of ocular metastatic lesions. Targeted therapies should be considered in the treatment of patients with ocular metastases from NSCLC.
1. Bloch RS, Gartner S. The incidence of ocular metastatic carcinoma. Arch Ophthalmol-Chic. 1971;85(6):673-675.
2. Shields CL, Shields JA, Gross NE, Schwartz GP, Lally SE. Survey of 520 eyes with uveal metastases. Ophthalmology. 1997;104(8):1265-1276.
3. Kreusel KM, Bechrakis NE, Wiegel T, Krause L, Foerster MH. Incidence and clinical characteristics of symptomatic choroidal metastasis from lung cancer. Acta Ophthalmol. 2008;86(5):515-519.
4. Abundo RE, Orenic CJ, Anderson SF, Townsend JC. Choroidal metastases resulting from carcinoma of the lung. J Am Optom Assoc. 1997;68(2):95-108.
5. Kreusel KM, Wiegel T, Stange M, Bornfeld N, Hinkelbein W, Foerster MH. Choroidal metastasis in disseminated lung cancer: frequency and risk factors. Am J Ophthalmol. 2002;134(3):445-447.
6. Singh N, Kulkarni P, Aggarwal AN, et al. Choroidal metastasis as a presenting manifestation of lung cancer: a report of 3 cases and systematic review of the literature. Medicine (Baltimore). 2012;91(4):179-194.
7. Chen CJ, McCoy AN, Brahmer J, Handa JT. Emerging treatments for choroidal metastases. Surv Ophthalmol. 2011;56(6):511-521.
8. Shah SU, Mashayekhi A, Shields CL, et al. Uveal metastasis from lung cancer: clinical features, treatment, and outcome in 194 patients. Ophthalmology. 2014;121(1):352-357.
9. Shepherd FA, Rodrigues Pereira J, Ciuleanu T, et al. Erlotinib in previously treated non-small-cell lung cancer. N Engl J Med. 2005;353(2):123-132.
10. Shaw AT, Kim DW, Nakagawa K, et al. Crizotinib versus chemotherapy in advanced ALK-positive lung cancer. N Engl J Med. 2013;368(25):2385-2394.
11. Kim SW, Kim MJ, Huh K, Oh J. Complete regression of choroidal metastasis secondary to non-small-cell lung cancer with intravitreal bevacizumab and oral erlotinib combination therapy. Ophthalmologica. 2009;223(6):411-413.
12. George B, Wirostko WJ, Connor TB, Choong NW. Complete and durable response of choroid metastasis from non-small cell lung cancer with systemic bevacizumab and chemotherapy. J Thorac Oncol. 2009;4(5):661-662.
13. Inoue M, Watanabe Y, Yamane S, et al. Choroidal metastasis with adenocarcinoma of the lung treated with gefitinib. Eur J Ophthalmol. 2010;20(5):963-965.
14. Shimomura I, Tada Y, Miura G, et al. Choroidal metastasis of non-small cell lung cancer that responded to gefitinib. https://www.hindawi.com/journals/criopm/2013/213124/. Published 2013. Accessed May 4, 2017.
15. Feng Y, Singh AD, Lanigan C, Tubbs RR, Ma PC. Choroidal metastases responsive to crizotinib therapy in a lung adenocarcinoma patient with ALK 2p23 fusion identified by ALK immunohistochemistry. J Thorac Oncol. 2013;8(12):e109-111.
Non–small cell lung cancer constitutes 80%-85% of lung cancers, and 40% of NSCLC are adenocarcinoma. It is rare to find intraocular metastasis from lung cancer. In this article, we present the case of a patient who presented with complaints of diminished vision redness of the eye and was found to have intra-ocular metastases from lung cancer.
Case presentation and summary
A 60-year-old man with a 40-pack per year history of smoking presented to multiple ophthalmologists with complaints of decreased vision and redness of the left eye. He was eventually evaluated by an ophthalmologist who performed a biopsy of the anterior chamber of the eye. Histologic findings were consistent with adenocarcinoma of lung primary (Figures 1 and 2).
After the diagnosis, a chest X-ray showed that the patient had a left lower lung mass. The results of his physical exam were all within normal limits, with the exception of decreased visual acuity in the left eye. The results of his laboratory studies, including complete blood count and serum chemistries, were also within normal limits. Imaging studies – including a computed-tomography (CT) scan of the chest, abdomen, and pelvis and a full-body positron-emission tomography–CT scan – showed a hypermetabolic left lower lobe mass 4.5 cm and right lower paratracheal lymph node metastasis 2 cm with a small focus of increased uptake alone the medial aspect of the left globe (Figures 3 and 4).
An MRI orbit was performed in an attempt to better characterize the left eye mass, but no optic lesion was identified. A biopsy of the left lower lung mass was consistent with non–small-cell lung cancer (NSCLC). Aside from the isolated left eye metastases, the patient did not have evidence of other distant metastatic involvement.
He was started on palliative chemotherapy on a clinical trial and received intravenous carboplatin AUC 6, pemetrexed 500 mg/m2, and bevacizumab 15 mg/kg every 3 weeks. He received 1 dose intraocular bevacizumab injection before initiation of systemic chemotherapy as he was symptomatic from the intraocular metastases. Within 2 weeks after intravitreal bevacizumab was administered, the patient had subjective improvement in vision. Mutational analysis to identify if the patient would benefit from targeted therapy showed no presence of EGFR mutation and ALK gene rearrangement, and that the patient was K-RAS mutant.
After treatment initiation, interval imaging studies (a computed-tomography scan of the chest, abdomen, pelvis; and magnetic-resonance imaging of the brain) after 3 cycles showed no evidence of disease progression, and after 4 cycles of chemotherapy with these drugs, the patient was started on maintenance chemotherapy with bevacizumab 15 mg/kg and pemetrexed 500 mg/m2.
Discussion
Choroidal metastasis is the most common site of intraocular tumor. In an autopsy study of 230 patients with carcinoma, 12% of cases demonstrated histologic evidence of ocular metastasis.1 A retrospective series of patients with malignant involvement of the eye, 66% of patients had a known history of primary cancer and in 34% of patients the ocular tumor was the first sign of cancer.2 The most common cancers that were found to have ocular metastasis were lung and breast cancer.2 Adenocarcinoma was the most common histologic type of lung cancer to result in ocular metastases and was seen in 41% of patients.3
Decreased or blurred vision with redness as the primary complaint of NSCLC is rare. Only a few case reports are available. Abundo and colleagues reported that 0.7%-12% of patients with lung cancer develop ocular metastases.4 Therefore, routine ophthalmologic screening for ocular metastases in patients with cancer has not been pursued in asymptomatic patients.5 Ophthalmological evaluation is recommended in symptomatic patients.
Metastatic involvement of two or more other organs was found to be a risk factor for development of choroidal metastasis in patients with lung cancer though in our patient no evidence of other organ involvement was found.5 The most common site of metastases in patients with NSCLC with ocular metastases was found to be the liver. Choroidal metastases was reported to be the sixth common site of metastases in patients with lung cancer.5
Treatment of ocular manifestations has been generally confined to surgical resection or radiation therapy, but advances in chemotherapy and development of novel targeted agents have shown promising results.7 Median life expectancy after a diagnosis of uveal metastases was reported to be 12 months in a retrospective study, which is similar to the reported median survival in metastatic NSCLC.8
Our patient was enrolled in a clinical trial and was treated with a regimen of carboplatin, paclitaxel, and bevacizumab. On presentation, he had significant impairment of vision with pain. He was treated with intravitreal bevacizumab yielding improvement in his visual symptoms. Bevacizumab is a vascular endothelial growth factor receptor monoclonal antibody approved for use in patients with metastatic lung cancer. Other pathways that have been reported in development of lung cancer involve the ALK gene translocation, and EGFR and K-RAS mutations, and targeted therapy has shown good results in cancer patients with these molecular defects. Randomized clinical trials in patients with advanced NSCLC and an EGFR mutation have shown significant improvement in overall survival with the use of erlotinib, a tyrosine kinase inhibitor targeting the epidermal growth factor receptor.9 Similarly, crizotinib has shown promising results in patients with metastatic NSCLC who have ELM-ALK rearrangement.10 As our patient’s tumor did not have either of these mutations, he was initiated on chemotherapy with bevacizumab. The presence of a K-RAS mutation in this patient further supported the use of front-line chemotherapy given that it may confer resistance against agents that target the EGFR pathway.
In our review of the literature, we found cases of patients with ocular metastases who responded well to therapy with targeted agents (Table).
Singh and colleagues did a systematic review of 55 cases of patients with lung cancer and choroidal metastases and found that the type of therapy depended on when the diagnosis had been made in relation to the advent of targeted therapy: cases diagnosed before targeted therapy had received radiation therapy or enucleation.6 As far as we could ascertain, there have been no randomized studies evaluating the impact of various targeted therapies or systemic chemotherapy on ocular metastases, although case reports have documented improvement in vision and regression of metastases with such therapy.
Conclusion
The goal of therapy in metastatic lung cancer is palliation of symptoms and improvement in patient quality of life with prolongation in overall survival. The newer targeted chemotherapeutic agents assist in achieving these goals and may decrease the morbidity associated from radiation or surgery with improvement in vision and regression of ocular metastatic lesions. Targeted therapies should be considered in the treatment of patients with ocular metastases from NSCLC.
Non–small cell lung cancer constitutes 80%-85% of lung cancers, and 40% of NSCLC are adenocarcinoma. It is rare to find intraocular metastasis from lung cancer. In this article, we present the case of a patient who presented with complaints of diminished vision redness of the eye and was found to have intra-ocular metastases from lung cancer.
Case presentation and summary
A 60-year-old man with a 40-pack per year history of smoking presented to multiple ophthalmologists with complaints of decreased vision and redness of the left eye. He was eventually evaluated by an ophthalmologist who performed a biopsy of the anterior chamber of the eye. Histologic findings were consistent with adenocarcinoma of lung primary (Figures 1 and 2).
After the diagnosis, a chest X-ray showed that the patient had a left lower lung mass. The results of his physical exam were all within normal limits, with the exception of decreased visual acuity in the left eye. The results of his laboratory studies, including complete blood count and serum chemistries, were also within normal limits. Imaging studies – including a computed-tomography (CT) scan of the chest, abdomen, and pelvis and a full-body positron-emission tomography–CT scan – showed a hypermetabolic left lower lobe mass 4.5 cm and right lower paratracheal lymph node metastasis 2 cm with a small focus of increased uptake alone the medial aspect of the left globe (Figures 3 and 4).
An MRI orbit was performed in an attempt to better characterize the left eye mass, but no optic lesion was identified. A biopsy of the left lower lung mass was consistent with non–small-cell lung cancer (NSCLC). Aside from the isolated left eye metastases, the patient did not have evidence of other distant metastatic involvement.
He was started on palliative chemotherapy on a clinical trial and received intravenous carboplatin AUC 6, pemetrexed 500 mg/m2, and bevacizumab 15 mg/kg every 3 weeks. He received 1 dose intraocular bevacizumab injection before initiation of systemic chemotherapy as he was symptomatic from the intraocular metastases. Within 2 weeks after intravitreal bevacizumab was administered, the patient had subjective improvement in vision. Mutational analysis to identify if the patient would benefit from targeted therapy showed no presence of EGFR mutation and ALK gene rearrangement, and that the patient was K-RAS mutant.
After treatment initiation, interval imaging studies (a computed-tomography scan of the chest, abdomen, pelvis; and magnetic-resonance imaging of the brain) after 3 cycles showed no evidence of disease progression, and after 4 cycles of chemotherapy with these drugs, the patient was started on maintenance chemotherapy with bevacizumab 15 mg/kg and pemetrexed 500 mg/m2.
Discussion
Choroidal metastasis is the most common site of intraocular tumor. In an autopsy study of 230 patients with carcinoma, 12% of cases demonstrated histologic evidence of ocular metastasis.1 A retrospective series of patients with malignant involvement of the eye, 66% of patients had a known history of primary cancer and in 34% of patients the ocular tumor was the first sign of cancer.2 The most common cancers that were found to have ocular metastasis were lung and breast cancer.2 Adenocarcinoma was the most common histologic type of lung cancer to result in ocular metastases and was seen in 41% of patients.3
Decreased or blurred vision with redness as the primary complaint of NSCLC is rare. Only a few case reports are available. Abundo and colleagues reported that 0.7%-12% of patients with lung cancer develop ocular metastases.4 Therefore, routine ophthalmologic screening for ocular metastases in patients with cancer has not been pursued in asymptomatic patients.5 Ophthalmological evaluation is recommended in symptomatic patients.
Metastatic involvement of two or more other organs was found to be a risk factor for development of choroidal metastasis in patients with lung cancer though in our patient no evidence of other organ involvement was found.5 The most common site of metastases in patients with NSCLC with ocular metastases was found to be the liver. Choroidal metastases was reported to be the sixth common site of metastases in patients with lung cancer.5
Treatment of ocular manifestations has been generally confined to surgical resection or radiation therapy, but advances in chemotherapy and development of novel targeted agents have shown promising results.7 Median life expectancy after a diagnosis of uveal metastases was reported to be 12 months in a retrospective study, which is similar to the reported median survival in metastatic NSCLC.8
Our patient was enrolled in a clinical trial and was treated with a regimen of carboplatin, paclitaxel, and bevacizumab. On presentation, he had significant impairment of vision with pain. He was treated with intravitreal bevacizumab yielding improvement in his visual symptoms. Bevacizumab is a vascular endothelial growth factor receptor monoclonal antibody approved for use in patients with metastatic lung cancer. Other pathways that have been reported in development of lung cancer involve the ALK gene translocation, and EGFR and K-RAS mutations, and targeted therapy has shown good results in cancer patients with these molecular defects. Randomized clinical trials in patients with advanced NSCLC and an EGFR mutation have shown significant improvement in overall survival with the use of erlotinib, a tyrosine kinase inhibitor targeting the epidermal growth factor receptor.9 Similarly, crizotinib has shown promising results in patients with metastatic NSCLC who have ELM-ALK rearrangement.10 As our patient’s tumor did not have either of these mutations, he was initiated on chemotherapy with bevacizumab. The presence of a K-RAS mutation in this patient further supported the use of front-line chemotherapy given that it may confer resistance against agents that target the EGFR pathway.
In our review of the literature, we found cases of patients with ocular metastases who responded well to therapy with targeted agents (Table).
Singh and colleagues did a systematic review of 55 cases of patients with lung cancer and choroidal metastases and found that the type of therapy depended on when the diagnosis had been made in relation to the advent of targeted therapy: cases diagnosed before targeted therapy had received radiation therapy or enucleation.6 As far as we could ascertain, there have been no randomized studies evaluating the impact of various targeted therapies or systemic chemotherapy on ocular metastases, although case reports have documented improvement in vision and regression of metastases with such therapy.
Conclusion
The goal of therapy in metastatic lung cancer is palliation of symptoms and improvement in patient quality of life with prolongation in overall survival. The newer targeted chemotherapeutic agents assist in achieving these goals and may decrease the morbidity associated from radiation or surgery with improvement in vision and regression of ocular metastatic lesions. Targeted therapies should be considered in the treatment of patients with ocular metastases from NSCLC.
1. Bloch RS, Gartner S. The incidence of ocular metastatic carcinoma. Arch Ophthalmol-Chic. 1971;85(6):673-675.
2. Shields CL, Shields JA, Gross NE, Schwartz GP, Lally SE. Survey of 520 eyes with uveal metastases. Ophthalmology. 1997;104(8):1265-1276.
3. Kreusel KM, Bechrakis NE, Wiegel T, Krause L, Foerster MH. Incidence and clinical characteristics of symptomatic choroidal metastasis from lung cancer. Acta Ophthalmol. 2008;86(5):515-519.
4. Abundo RE, Orenic CJ, Anderson SF, Townsend JC. Choroidal metastases resulting from carcinoma of the lung. J Am Optom Assoc. 1997;68(2):95-108.
5. Kreusel KM, Wiegel T, Stange M, Bornfeld N, Hinkelbein W, Foerster MH. Choroidal metastasis in disseminated lung cancer: frequency and risk factors. Am J Ophthalmol. 2002;134(3):445-447.
6. Singh N, Kulkarni P, Aggarwal AN, et al. Choroidal metastasis as a presenting manifestation of lung cancer: a report of 3 cases and systematic review of the literature. Medicine (Baltimore). 2012;91(4):179-194.
7. Chen CJ, McCoy AN, Brahmer J, Handa JT. Emerging treatments for choroidal metastases. Surv Ophthalmol. 2011;56(6):511-521.
8. Shah SU, Mashayekhi A, Shields CL, et al. Uveal metastasis from lung cancer: clinical features, treatment, and outcome in 194 patients. Ophthalmology. 2014;121(1):352-357.
9. Shepherd FA, Rodrigues Pereira J, Ciuleanu T, et al. Erlotinib in previously treated non-small-cell lung cancer. N Engl J Med. 2005;353(2):123-132.
10. Shaw AT, Kim DW, Nakagawa K, et al. Crizotinib versus chemotherapy in advanced ALK-positive lung cancer. N Engl J Med. 2013;368(25):2385-2394.
11. Kim SW, Kim MJ, Huh K, Oh J. Complete regression of choroidal metastasis secondary to non-small-cell lung cancer with intravitreal bevacizumab and oral erlotinib combination therapy. Ophthalmologica. 2009;223(6):411-413.
12. George B, Wirostko WJ, Connor TB, Choong NW. Complete and durable response of choroid metastasis from non-small cell lung cancer with systemic bevacizumab and chemotherapy. J Thorac Oncol. 2009;4(5):661-662.
13. Inoue M, Watanabe Y, Yamane S, et al. Choroidal metastasis with adenocarcinoma of the lung treated with gefitinib. Eur J Ophthalmol. 2010;20(5):963-965.
14. Shimomura I, Tada Y, Miura G, et al. Choroidal metastasis of non-small cell lung cancer that responded to gefitinib. https://www.hindawi.com/journals/criopm/2013/213124/. Published 2013. Accessed May 4, 2017.
15. Feng Y, Singh AD, Lanigan C, Tubbs RR, Ma PC. Choroidal metastases responsive to crizotinib therapy in a lung adenocarcinoma patient with ALK 2p23 fusion identified by ALK immunohistochemistry. J Thorac Oncol. 2013;8(12):e109-111.
1. Bloch RS, Gartner S. The incidence of ocular metastatic carcinoma. Arch Ophthalmol-Chic. 1971;85(6):673-675.
2. Shields CL, Shields JA, Gross NE, Schwartz GP, Lally SE. Survey of 520 eyes with uveal metastases. Ophthalmology. 1997;104(8):1265-1276.
3. Kreusel KM, Bechrakis NE, Wiegel T, Krause L, Foerster MH. Incidence and clinical characteristics of symptomatic choroidal metastasis from lung cancer. Acta Ophthalmol. 2008;86(5):515-519.
4. Abundo RE, Orenic CJ, Anderson SF, Townsend JC. Choroidal metastases resulting from carcinoma of the lung. J Am Optom Assoc. 1997;68(2):95-108.
5. Kreusel KM, Wiegel T, Stange M, Bornfeld N, Hinkelbein W, Foerster MH. Choroidal metastasis in disseminated lung cancer: frequency and risk factors. Am J Ophthalmol. 2002;134(3):445-447.
6. Singh N, Kulkarni P, Aggarwal AN, et al. Choroidal metastasis as a presenting manifestation of lung cancer: a report of 3 cases and systematic review of the literature. Medicine (Baltimore). 2012;91(4):179-194.
7. Chen CJ, McCoy AN, Brahmer J, Handa JT. Emerging treatments for choroidal metastases. Surv Ophthalmol. 2011;56(6):511-521.
8. Shah SU, Mashayekhi A, Shields CL, et al. Uveal metastasis from lung cancer: clinical features, treatment, and outcome in 194 patients. Ophthalmology. 2014;121(1):352-357.
9. Shepherd FA, Rodrigues Pereira J, Ciuleanu T, et al. Erlotinib in previously treated non-small-cell lung cancer. N Engl J Med. 2005;353(2):123-132.
10. Shaw AT, Kim DW, Nakagawa K, et al. Crizotinib versus chemotherapy in advanced ALK-positive lung cancer. N Engl J Med. 2013;368(25):2385-2394.
11. Kim SW, Kim MJ, Huh K, Oh J. Complete regression of choroidal metastasis secondary to non-small-cell lung cancer with intravitreal bevacizumab and oral erlotinib combination therapy. Ophthalmologica. 2009;223(6):411-413.
12. George B, Wirostko WJ, Connor TB, Choong NW. Complete and durable response of choroid metastasis from non-small cell lung cancer with systemic bevacizumab and chemotherapy. J Thorac Oncol. 2009;4(5):661-662.
13. Inoue M, Watanabe Y, Yamane S, et al. Choroidal metastasis with adenocarcinoma of the lung treated with gefitinib. Eur J Ophthalmol. 2010;20(5):963-965.
14. Shimomura I, Tada Y, Miura G, et al. Choroidal metastasis of non-small cell lung cancer that responded to gefitinib. https://www.hindawi.com/journals/criopm/2013/213124/. Published 2013. Accessed May 4, 2017.
15. Feng Y, Singh AD, Lanigan C, Tubbs RR, Ma PC. Choroidal metastases responsive to crizotinib therapy in a lung adenocarcinoma patient with ALK 2p23 fusion identified by ALK immunohistochemistry. J Thorac Oncol. 2013;8(12):e109-111.