User login
Medication-Nonadherent Hypothyroidism Requiring Frequent Primary Care Visits to Achieve Euthyroidism
Nonadherence to medications is an issue across health care. In endocrinology, hypothyroidism, a deficiency of thyroid hormones, is most often treated with levothyroxine and if left untreated can lead to myxedema coma, which can lead to death due to multiorgan dysfunction.1 Therefore, adherence to levothyroxine is very important in preventing fatal complications.
We present the case of a patient with persistent primary hypothyroidism who was suspected to be nonadherent to levothyroxine, although the patient consistently claimed adherence. The patient’s plasma thyrotropin (TSH) level improved to reference range after 6 weeks of weekly primary care clinic visits. After stopping the visits, his plasma TSH level increased again, so 9 more weeks of visits resumed, which again helped bring down his plasma TSH levels.
Case Presentation
A male patient aged 67 years presented to the Dayton Veterans Affairs Medical Center (VAMC) endocrinology clinic for evaluation of thyroid nodules. The patient reported no history of neck irradiation and a physical examination was unremarkable. At that time, laboratory results showed a slightly elevated plasma TSH level of 4.35 uIU/mL (reference range, 0.35-4.00 uIU/mL) and normal free thyroxine (T4) of 1.00 ng/dL (reference range, 0.74-1.46 ng/dL). Later that year, the patient underwent a total thyroidectomy at the Cincinnati VAMC for Hurthle cell variant papillary thyroid carcinoma that was noted on biopsy at the Dayton VAMC. After surgical pathology results were available, the patient started levothyroxine 200 mcg daily, although 224 mcg would have been more appropriate based on his 142 kg weight. Due to a history of arrhythmia, the goal plasma TSH level was 0.10 to 0.50 uIU/mL. The patient subsequently underwent radioactive iodine ablation. After levothyroxine dose adjustments, the patient’s plasma TSH level was noted to be within his target range at 0.28 uIU/mL 3 months postablation.
Over the next 5 years the patient had regular laboratory tests during which his plasma TSH level rose and were typically high despite adjusting levothyroxine doses between 200 mcg and 325 mcg. The patient received counseling on taking the medication in the morning on an empty stomach and waiting at least 1 hour before consuming anything, and he went to many follow-up visits at the Dayton VAMC endocrinology clinic. He reported no vomiting or diarrhea but endorsed weight gain once. The patient also had high free T4 at times and did not take extra levothyroxine before undergoing laboratory tests.
Nonadherence to levothyroxine was suspected, but the patient insisted he was adherent. He received the medication in the mail regularly, generally had 90-day refills unless a dose change was made, used a pill box, and had social support from his son, but he did not use a phone alarm to remind him to take it. A home care nurse made weekly visits to make sure the remaining levothyroxine pill counts were correct; however, the patient continued to have difficulty maintaining daily adherence at home as indicated by the nurse’s pill counts not aligning with the number of pills which should have been left if the patient was talking the pills daily.
The patient was asked to visit a local community-based outpatient clinic (CBOC) weekly (to avoid patient travel time to Dayton VAMC > 1 hour) to check pill counts and assess adherence. The patient went to the CBOC clinic for these visits, during which pill counts indicated much better but not 100% adherence. After 6 weeks of clinic visits, his plasma TSH decreased to 1.01 uIU/mL, which was within the reference range, and the patient stopped coming to the weekly clinic visits (Table). Four months later, the patient's plasma TSH levels increased to 80.72 uIU/mL. Nonadherence to levothyroxine was suspected again. He was asked to resume weekly clinic visits, and the life-threatening effects of hypothyroidism and not taking levothyroxine were discussed with the patient and his son. The patient made CBOC clinic visits for 9 weeks, after which his plasma TSH level was low at 0.23 uIU/mL.
Discussion
There are multiple important causes to consider in patients with persistent hypothyroidism. One is medication nonadherence, which was most likely seen in the patient in this case. Missing even 1 day of levothyroxine can affect TSH and thyroid hormone levels for several days due to the long half-life of the medication.2 Hepp and colleagues found that patients with hypothyroidism were significantly more likely to be nonadherent to levothyroxine if they had comorbid conditions such as type 2 diabetes or were obese.3 Another study of levothyroxine adherence found that the most common reason for missing doses was forgetfulness.4 However, memory and cognition impairments can also be symptoms of hypothyroidism itself; Haskard-Zolnierek and colleagues found a significant association between nonadherence to levothyroxine and self-reported brain fog in patients with hypothyroidism.5
Another cause of persistent hypothyroidism is malabsorption. Absorption of levothyroxine can be affected by intestinal malabsorption due to inflammatory bowel disease, lactose intolerance, or gastrointestinal infection, as well as several foods, drinks (eg, coffee), medications, vitamins, and supplements (eg, proton-pump inhibitors and calcium).2,6 Levothyroxine is absorbed mainly at the jejunum and upper ileum, so any pathologies or ingested items that would directly or indirectly affect absorption at those sites can affect levothyroxine absorption.2
A liquid levothyroxine formulation can help with malabsorption.2 Alternatively, weight gain may lead to a need for increasing the dosage of levothyroxine.2,6 Other factors that can affect TSH levels include Addison disease, dysregulation of the hypothalamic-pituitary-thyroid axis, and TSH heterophile antibodies.2
Research describes methods that have effectively treated hypothyroidism in patients struggling with levothyroxine adherence. Two case reports describe weekly visits for levothyroxine administration successfully treating uncontrolled hypothyroidism.7,8 A meta-analysis found that while weekly levothyroxine tablets led to a higher mean TSH level than daily use, weekly use still led to reference-range TSH levels, suggesting that weekly levothyroxine may be a helpful alternative for nonadherent patients.9 Alternatively, patients taking levothyroxine tablets have been shown to forget to take their medication more frequently compared to those taking the liquid formulation.10,11 Additionally, a study by El Helou and colleagues found that adherence to levothyroxine was significantly improved when patients had endocrinology visits once a month and when the endocrinologist provided information about hypothyroidism.12
Another method that may improve adherence to levothyroxine is telehealth visits. This would be especially helpful for patients who live far from the clinic or do not have the time, transportation, or financial means to visit the clinic for weekly visits to assess medication adherence. Additionally, patients may be afraid of admitting to a health care professional that they are nonadherent. Clinicians must be tactful when asking about adherence to make the patient feel comfortable with admitting to nonadherence if their cognition is not impaired. Then, a patient-led conversation can occur regarding realistic ways the patient feels they can work toward adherence.
To our knowledge, the patient in this case report had no symptoms of intestinal malabsorption, and weight gain was not thought to be the issue, as levothyroxine dosage was adjusted multiple times. His plasma TSH levels returned to reference range after weekly pill count visits for 6 weeks and after weekly pill count visits for 9 weeks. Therefore, nonadherence to levothyroxine was suspected to be the cause of frequently elevated plasma TSH levels despite the patient’s insistence on adherence. While the patient did not report memory issues, cognitive impairments due to hypothyroidism may have been contributing to his probable nonadherence. Additionally, he had comorbidities, such as type 2 diabetes mellitus and obesity, which may have made adherence more difficult.
Levothyroxine was also only prescribed in daily tablet form, so the frequency and formulation may have also contributed to nonadherence. While the home nurse was originally sent to assess the patient’s adherence, the care team could have had the nurse start giving the patient weekly levothyroxine once nonadherence was determined to be a likely issue. The patient’s adherence only improved when he went to the clinic for pill counts but not when the home nurse came to his house weekly; this could be because the patient knew he had to invest the time to physically go to clinic visits for pill checks, motivating him to increase adherence.
Conclusions
This case reports a patient with frequently high plasma TSH levels achieving normalization of plasma TSH levels after weekly medication adherence checks at a primary care clinic. Weekly visits to a clinic seem impractical compared to weekly dosing with a visiting nurse; however, after review of the literature, this may be an approach to consider in the future. This strategy may especially help in cases of persistent abnormal plasma TSH levels in which no etiology can be found other than suspected medication nonadherence. Knowing their medication use will be checked at weekly clinic visits may motivate patients to be adherent.
1. Chaker L, Bianco AC, Jonklaas J, Peeters RP. Hypothyroidism. Lancet. 2017;390(10101):1550-1562. doi:10.1016/S0140-6736(17)30703-1
2. Centanni M, Benvenga S, Sachmechi I. Diagnosis and management of treatment-refractory hypothyroidism: an expert consensus report. J Endocrinol Invest. 2017;40(12):1289-1301. doi:10.1007/s40618-017-0706-y
3. Hepp Z, Lage MJ, Espaillat R, Gossain VV. The association between adherence to levothyroxine and economic and clinical outcomes in patients with hypothyroidism in the US. J Med Econ. 2018;21(9):912-919. doi:10.1080/13696998.2018.1484749
4. Shakya Shrestha S, Risal K, Shrestha R, Bhatta RD. Medication Adherence to Levothyroxine Therapy among Hypothyroid Patients and their Clinical Outcomes with Special Reference to Thyroid Function Parameters. Kathmandu Univ Med J (KUMJ). 2018;16(62):129-137.
5. Haskard-Zolnierek K, Wilson C, Pruin J, Deason R, Howard K. The Relationship Between Brain Fog and Medication Adherence for Individuals With Hypothyroidism. Clin Nurs Res. 2022;31(3):445-452. doi:10.1177/10547738211038127
6. McNally LJ, Ofiaeli CI, Oyibo SO. Treatment-refractory hypothyroidism. BMJ. 2019;364:l579. Published 2019 Feb 25. doi:10.1136/bmj.l579
7. Nakano Y, Hashimoto K, Ohkiba N, et al. A Case of Refractory Hypothyroidism due to Poor Compliance Treated with the Weekly Intravenous and Oral Levothyroxine Administration. Case Rep Endocrinol. 2019;2019:5986014. Published 2019 Feb 5. doi:10.1155/2019/5986014
8. Kiran Z, Shaikh KS, Fatima N, Tariq N, Baloch AA. Levothyroxine absorption test followed by directly observed treatment on an outpatient basis to address long-term high TSH levels in a hypothyroid patient: a case report. J Med Case Rep. 2023;17(1):24. Published 2023 Jan 25. doi:10.1186/s13256-023-03760-0
9. Chiu HH, Larrazabal R Jr, Uy AB, Jimeno C. Weekly Versus Daily Levothyroxine Tablet Replacement in Adults with Hypothyroidism: A Meta-Analysis. J ASEAN Fed Endocr Soc. 2021;36(2):156-160. doi:10.15605/jafes.036.02.07
10. Cappelli C, Castello R, Marini F, et al. Adherence to Levothyroxine Treatment Among Patients With Hypothyroidism: A Northeastern Italian Survey. Front Endocrinol (Lausanne). 2018;9:699. Published 2018 Nov 23. doi:10.3389/fendo.2018.00699
11. Bocale R, Desideri G, Barini A, et al. Long-Term Adherence to Levothyroxine Replacement Therapy in Thyroidectomized Patients. J Clin Med. 2022;11(15):4296. Published 2022 Jul 24. doi:10.3390/jcm11154296
12. El Helou S, Hallit S, Awada S, et al. Adherence to levothyroxine among patients with hypothyroidism in Lebanon. East Mediterr Health J. 2019;25(3):149-159. Published 2019 Apr 25. doi:10.26719/emhj.18.022
Nonadherence to medications is an issue across health care. In endocrinology, hypothyroidism, a deficiency of thyroid hormones, is most often treated with levothyroxine and if left untreated can lead to myxedema coma, which can lead to death due to multiorgan dysfunction.1 Therefore, adherence to levothyroxine is very important in preventing fatal complications.
We present the case of a patient with persistent primary hypothyroidism who was suspected to be nonadherent to levothyroxine, although the patient consistently claimed adherence. The patient’s plasma thyrotropin (TSH) level improved to reference range after 6 weeks of weekly primary care clinic visits. After stopping the visits, his plasma TSH level increased again, so 9 more weeks of visits resumed, which again helped bring down his plasma TSH levels.
Case Presentation
A male patient aged 67 years presented to the Dayton Veterans Affairs Medical Center (VAMC) endocrinology clinic for evaluation of thyroid nodules. The patient reported no history of neck irradiation and a physical examination was unremarkable. At that time, laboratory results showed a slightly elevated plasma TSH level of 4.35 uIU/mL (reference range, 0.35-4.00 uIU/mL) and normal free thyroxine (T4) of 1.00 ng/dL (reference range, 0.74-1.46 ng/dL). Later that year, the patient underwent a total thyroidectomy at the Cincinnati VAMC for Hurthle cell variant papillary thyroid carcinoma that was noted on biopsy at the Dayton VAMC. After surgical pathology results were available, the patient started levothyroxine 200 mcg daily, although 224 mcg would have been more appropriate based on his 142 kg weight. Due to a history of arrhythmia, the goal plasma TSH level was 0.10 to 0.50 uIU/mL. The patient subsequently underwent radioactive iodine ablation. After levothyroxine dose adjustments, the patient’s plasma TSH level was noted to be within his target range at 0.28 uIU/mL 3 months postablation.
Over the next 5 years the patient had regular laboratory tests during which his plasma TSH level rose and were typically high despite adjusting levothyroxine doses between 200 mcg and 325 mcg. The patient received counseling on taking the medication in the morning on an empty stomach and waiting at least 1 hour before consuming anything, and he went to many follow-up visits at the Dayton VAMC endocrinology clinic. He reported no vomiting or diarrhea but endorsed weight gain once. The patient also had high free T4 at times and did not take extra levothyroxine before undergoing laboratory tests.
Nonadherence to levothyroxine was suspected, but the patient insisted he was adherent. He received the medication in the mail regularly, generally had 90-day refills unless a dose change was made, used a pill box, and had social support from his son, but he did not use a phone alarm to remind him to take it. A home care nurse made weekly visits to make sure the remaining levothyroxine pill counts were correct; however, the patient continued to have difficulty maintaining daily adherence at home as indicated by the nurse’s pill counts not aligning with the number of pills which should have been left if the patient was talking the pills daily.
The patient was asked to visit a local community-based outpatient clinic (CBOC) weekly (to avoid patient travel time to Dayton VAMC > 1 hour) to check pill counts and assess adherence. The patient went to the CBOC clinic for these visits, during which pill counts indicated much better but not 100% adherence. After 6 weeks of clinic visits, his plasma TSH decreased to 1.01 uIU/mL, which was within the reference range, and the patient stopped coming to the weekly clinic visits (Table). Four months later, the patient's plasma TSH levels increased to 80.72 uIU/mL. Nonadherence to levothyroxine was suspected again. He was asked to resume weekly clinic visits, and the life-threatening effects of hypothyroidism and not taking levothyroxine were discussed with the patient and his son. The patient made CBOC clinic visits for 9 weeks, after which his plasma TSH level was low at 0.23 uIU/mL.
Discussion
There are multiple important causes to consider in patients with persistent hypothyroidism. One is medication nonadherence, which was most likely seen in the patient in this case. Missing even 1 day of levothyroxine can affect TSH and thyroid hormone levels for several days due to the long half-life of the medication.2 Hepp and colleagues found that patients with hypothyroidism were significantly more likely to be nonadherent to levothyroxine if they had comorbid conditions such as type 2 diabetes or were obese.3 Another study of levothyroxine adherence found that the most common reason for missing doses was forgetfulness.4 However, memory and cognition impairments can also be symptoms of hypothyroidism itself; Haskard-Zolnierek and colleagues found a significant association between nonadherence to levothyroxine and self-reported brain fog in patients with hypothyroidism.5
Another cause of persistent hypothyroidism is malabsorption. Absorption of levothyroxine can be affected by intestinal malabsorption due to inflammatory bowel disease, lactose intolerance, or gastrointestinal infection, as well as several foods, drinks (eg, coffee), medications, vitamins, and supplements (eg, proton-pump inhibitors and calcium).2,6 Levothyroxine is absorbed mainly at the jejunum and upper ileum, so any pathologies or ingested items that would directly or indirectly affect absorption at those sites can affect levothyroxine absorption.2
A liquid levothyroxine formulation can help with malabsorption.2 Alternatively, weight gain may lead to a need for increasing the dosage of levothyroxine.2,6 Other factors that can affect TSH levels include Addison disease, dysregulation of the hypothalamic-pituitary-thyroid axis, and TSH heterophile antibodies.2
Research describes methods that have effectively treated hypothyroidism in patients struggling with levothyroxine adherence. Two case reports describe weekly visits for levothyroxine administration successfully treating uncontrolled hypothyroidism.7,8 A meta-analysis found that while weekly levothyroxine tablets led to a higher mean TSH level than daily use, weekly use still led to reference-range TSH levels, suggesting that weekly levothyroxine may be a helpful alternative for nonadherent patients.9 Alternatively, patients taking levothyroxine tablets have been shown to forget to take their medication more frequently compared to those taking the liquid formulation.10,11 Additionally, a study by El Helou and colleagues found that adherence to levothyroxine was significantly improved when patients had endocrinology visits once a month and when the endocrinologist provided information about hypothyroidism.12
Another method that may improve adherence to levothyroxine is telehealth visits. This would be especially helpful for patients who live far from the clinic or do not have the time, transportation, or financial means to visit the clinic for weekly visits to assess medication adherence. Additionally, patients may be afraid of admitting to a health care professional that they are nonadherent. Clinicians must be tactful when asking about adherence to make the patient feel comfortable with admitting to nonadherence if their cognition is not impaired. Then, a patient-led conversation can occur regarding realistic ways the patient feels they can work toward adherence.
To our knowledge, the patient in this case report had no symptoms of intestinal malabsorption, and weight gain was not thought to be the issue, as levothyroxine dosage was adjusted multiple times. His plasma TSH levels returned to reference range after weekly pill count visits for 6 weeks and after weekly pill count visits for 9 weeks. Therefore, nonadherence to levothyroxine was suspected to be the cause of frequently elevated plasma TSH levels despite the patient’s insistence on adherence. While the patient did not report memory issues, cognitive impairments due to hypothyroidism may have been contributing to his probable nonadherence. Additionally, he had comorbidities, such as type 2 diabetes mellitus and obesity, which may have made adherence more difficult.
Levothyroxine was also only prescribed in daily tablet form, so the frequency and formulation may have also contributed to nonadherence. While the home nurse was originally sent to assess the patient’s adherence, the care team could have had the nurse start giving the patient weekly levothyroxine once nonadherence was determined to be a likely issue. The patient’s adherence only improved when he went to the clinic for pill counts but not when the home nurse came to his house weekly; this could be because the patient knew he had to invest the time to physically go to clinic visits for pill checks, motivating him to increase adherence.
Conclusions
This case reports a patient with frequently high plasma TSH levels achieving normalization of plasma TSH levels after weekly medication adherence checks at a primary care clinic. Weekly visits to a clinic seem impractical compared to weekly dosing with a visiting nurse; however, after review of the literature, this may be an approach to consider in the future. This strategy may especially help in cases of persistent abnormal plasma TSH levels in which no etiology can be found other than suspected medication nonadherence. Knowing their medication use will be checked at weekly clinic visits may motivate patients to be adherent.
Nonadherence to medications is an issue across health care. In endocrinology, hypothyroidism, a deficiency of thyroid hormones, is most often treated with levothyroxine and if left untreated can lead to myxedema coma, which can lead to death due to multiorgan dysfunction.1 Therefore, adherence to levothyroxine is very important in preventing fatal complications.
We present the case of a patient with persistent primary hypothyroidism who was suspected to be nonadherent to levothyroxine, although the patient consistently claimed adherence. The patient’s plasma thyrotropin (TSH) level improved to reference range after 6 weeks of weekly primary care clinic visits. After stopping the visits, his plasma TSH level increased again, so 9 more weeks of visits resumed, which again helped bring down his plasma TSH levels.
Case Presentation
A male patient aged 67 years presented to the Dayton Veterans Affairs Medical Center (VAMC) endocrinology clinic for evaluation of thyroid nodules. The patient reported no history of neck irradiation and a physical examination was unremarkable. At that time, laboratory results showed a slightly elevated plasma TSH level of 4.35 uIU/mL (reference range, 0.35-4.00 uIU/mL) and normal free thyroxine (T4) of 1.00 ng/dL (reference range, 0.74-1.46 ng/dL). Later that year, the patient underwent a total thyroidectomy at the Cincinnati VAMC for Hurthle cell variant papillary thyroid carcinoma that was noted on biopsy at the Dayton VAMC. After surgical pathology results were available, the patient started levothyroxine 200 mcg daily, although 224 mcg would have been more appropriate based on his 142 kg weight. Due to a history of arrhythmia, the goal plasma TSH level was 0.10 to 0.50 uIU/mL. The patient subsequently underwent radioactive iodine ablation. After levothyroxine dose adjustments, the patient’s plasma TSH level was noted to be within his target range at 0.28 uIU/mL 3 months postablation.
Over the next 5 years the patient had regular laboratory tests during which his plasma TSH level rose and were typically high despite adjusting levothyroxine doses between 200 mcg and 325 mcg. The patient received counseling on taking the medication in the morning on an empty stomach and waiting at least 1 hour before consuming anything, and he went to many follow-up visits at the Dayton VAMC endocrinology clinic. He reported no vomiting or diarrhea but endorsed weight gain once. The patient also had high free T4 at times and did not take extra levothyroxine before undergoing laboratory tests.
Nonadherence to levothyroxine was suspected, but the patient insisted he was adherent. He received the medication in the mail regularly, generally had 90-day refills unless a dose change was made, used a pill box, and had social support from his son, but he did not use a phone alarm to remind him to take it. A home care nurse made weekly visits to make sure the remaining levothyroxine pill counts were correct; however, the patient continued to have difficulty maintaining daily adherence at home as indicated by the nurse’s pill counts not aligning with the number of pills which should have been left if the patient was talking the pills daily.
The patient was asked to visit a local community-based outpatient clinic (CBOC) weekly (to avoid patient travel time to Dayton VAMC > 1 hour) to check pill counts and assess adherence. The patient went to the CBOC clinic for these visits, during which pill counts indicated much better but not 100% adherence. After 6 weeks of clinic visits, his plasma TSH decreased to 1.01 uIU/mL, which was within the reference range, and the patient stopped coming to the weekly clinic visits (Table). Four months later, the patient's plasma TSH levels increased to 80.72 uIU/mL. Nonadherence to levothyroxine was suspected again. He was asked to resume weekly clinic visits, and the life-threatening effects of hypothyroidism and not taking levothyroxine were discussed with the patient and his son. The patient made CBOC clinic visits for 9 weeks, after which his plasma TSH level was low at 0.23 uIU/mL.
Discussion
There are multiple important causes to consider in patients with persistent hypothyroidism. One is medication nonadherence, which was most likely seen in the patient in this case. Missing even 1 day of levothyroxine can affect TSH and thyroid hormone levels for several days due to the long half-life of the medication.2 Hepp and colleagues found that patients with hypothyroidism were significantly more likely to be nonadherent to levothyroxine if they had comorbid conditions such as type 2 diabetes or were obese.3 Another study of levothyroxine adherence found that the most common reason for missing doses was forgetfulness.4 However, memory and cognition impairments can also be symptoms of hypothyroidism itself; Haskard-Zolnierek and colleagues found a significant association between nonadherence to levothyroxine and self-reported brain fog in patients with hypothyroidism.5
Another cause of persistent hypothyroidism is malabsorption. Absorption of levothyroxine can be affected by intestinal malabsorption due to inflammatory bowel disease, lactose intolerance, or gastrointestinal infection, as well as several foods, drinks (eg, coffee), medications, vitamins, and supplements (eg, proton-pump inhibitors and calcium).2,6 Levothyroxine is absorbed mainly at the jejunum and upper ileum, so any pathologies or ingested items that would directly or indirectly affect absorption at those sites can affect levothyroxine absorption.2
A liquid levothyroxine formulation can help with malabsorption.2 Alternatively, weight gain may lead to a need for increasing the dosage of levothyroxine.2,6 Other factors that can affect TSH levels include Addison disease, dysregulation of the hypothalamic-pituitary-thyroid axis, and TSH heterophile antibodies.2
Research describes methods that have effectively treated hypothyroidism in patients struggling with levothyroxine adherence. Two case reports describe weekly visits for levothyroxine administration successfully treating uncontrolled hypothyroidism.7,8 A meta-analysis found that while weekly levothyroxine tablets led to a higher mean TSH level than daily use, weekly use still led to reference-range TSH levels, suggesting that weekly levothyroxine may be a helpful alternative for nonadherent patients.9 Alternatively, patients taking levothyroxine tablets have been shown to forget to take their medication more frequently compared to those taking the liquid formulation.10,11 Additionally, a study by El Helou and colleagues found that adherence to levothyroxine was significantly improved when patients had endocrinology visits once a month and when the endocrinologist provided information about hypothyroidism.12
Another method that may improve adherence to levothyroxine is telehealth visits. This would be especially helpful for patients who live far from the clinic or do not have the time, transportation, or financial means to visit the clinic for weekly visits to assess medication adherence. Additionally, patients may be afraid of admitting to a health care professional that they are nonadherent. Clinicians must be tactful when asking about adherence to make the patient feel comfortable with admitting to nonadherence if their cognition is not impaired. Then, a patient-led conversation can occur regarding realistic ways the patient feels they can work toward adherence.
To our knowledge, the patient in this case report had no symptoms of intestinal malabsorption, and weight gain was not thought to be the issue, as levothyroxine dosage was adjusted multiple times. His plasma TSH levels returned to reference range after weekly pill count visits for 6 weeks and after weekly pill count visits for 9 weeks. Therefore, nonadherence to levothyroxine was suspected to be the cause of frequently elevated plasma TSH levels despite the patient’s insistence on adherence. While the patient did not report memory issues, cognitive impairments due to hypothyroidism may have been contributing to his probable nonadherence. Additionally, he had comorbidities, such as type 2 diabetes mellitus and obesity, which may have made adherence more difficult.
Levothyroxine was also only prescribed in daily tablet form, so the frequency and formulation may have also contributed to nonadherence. While the home nurse was originally sent to assess the patient’s adherence, the care team could have had the nurse start giving the patient weekly levothyroxine once nonadherence was determined to be a likely issue. The patient’s adherence only improved when he went to the clinic for pill counts but not when the home nurse came to his house weekly; this could be because the patient knew he had to invest the time to physically go to clinic visits for pill checks, motivating him to increase adherence.
Conclusions
This case reports a patient with frequently high plasma TSH levels achieving normalization of plasma TSH levels after weekly medication adherence checks at a primary care clinic. Weekly visits to a clinic seem impractical compared to weekly dosing with a visiting nurse; however, after review of the literature, this may be an approach to consider in the future. This strategy may especially help in cases of persistent abnormal plasma TSH levels in which no etiology can be found other than suspected medication nonadherence. Knowing their medication use will be checked at weekly clinic visits may motivate patients to be adherent.
1. Chaker L, Bianco AC, Jonklaas J, Peeters RP. Hypothyroidism. Lancet. 2017;390(10101):1550-1562. doi:10.1016/S0140-6736(17)30703-1
2. Centanni M, Benvenga S, Sachmechi I. Diagnosis and management of treatment-refractory hypothyroidism: an expert consensus report. J Endocrinol Invest. 2017;40(12):1289-1301. doi:10.1007/s40618-017-0706-y
3. Hepp Z, Lage MJ, Espaillat R, Gossain VV. The association between adherence to levothyroxine and economic and clinical outcomes in patients with hypothyroidism in the US. J Med Econ. 2018;21(9):912-919. doi:10.1080/13696998.2018.1484749
4. Shakya Shrestha S, Risal K, Shrestha R, Bhatta RD. Medication Adherence to Levothyroxine Therapy among Hypothyroid Patients and their Clinical Outcomes with Special Reference to Thyroid Function Parameters. Kathmandu Univ Med J (KUMJ). 2018;16(62):129-137.
5. Haskard-Zolnierek K, Wilson C, Pruin J, Deason R, Howard K. The Relationship Between Brain Fog and Medication Adherence for Individuals With Hypothyroidism. Clin Nurs Res. 2022;31(3):445-452. doi:10.1177/10547738211038127
6. McNally LJ, Ofiaeli CI, Oyibo SO. Treatment-refractory hypothyroidism. BMJ. 2019;364:l579. Published 2019 Feb 25. doi:10.1136/bmj.l579
7. Nakano Y, Hashimoto K, Ohkiba N, et al. A Case of Refractory Hypothyroidism due to Poor Compliance Treated with the Weekly Intravenous and Oral Levothyroxine Administration. Case Rep Endocrinol. 2019;2019:5986014. Published 2019 Feb 5. doi:10.1155/2019/5986014
8. Kiran Z, Shaikh KS, Fatima N, Tariq N, Baloch AA. Levothyroxine absorption test followed by directly observed treatment on an outpatient basis to address long-term high TSH levels in a hypothyroid patient: a case report. J Med Case Rep. 2023;17(1):24. Published 2023 Jan 25. doi:10.1186/s13256-023-03760-0
9. Chiu HH, Larrazabal R Jr, Uy AB, Jimeno C. Weekly Versus Daily Levothyroxine Tablet Replacement in Adults with Hypothyroidism: A Meta-Analysis. J ASEAN Fed Endocr Soc. 2021;36(2):156-160. doi:10.15605/jafes.036.02.07
10. Cappelli C, Castello R, Marini F, et al. Adherence to Levothyroxine Treatment Among Patients With Hypothyroidism: A Northeastern Italian Survey. Front Endocrinol (Lausanne). 2018;9:699. Published 2018 Nov 23. doi:10.3389/fendo.2018.00699
11. Bocale R, Desideri G, Barini A, et al. Long-Term Adherence to Levothyroxine Replacement Therapy in Thyroidectomized Patients. J Clin Med. 2022;11(15):4296. Published 2022 Jul 24. doi:10.3390/jcm11154296
12. El Helou S, Hallit S, Awada S, et al. Adherence to levothyroxine among patients with hypothyroidism in Lebanon. East Mediterr Health J. 2019;25(3):149-159. Published 2019 Apr 25. doi:10.26719/emhj.18.022
1. Chaker L, Bianco AC, Jonklaas J, Peeters RP. Hypothyroidism. Lancet. 2017;390(10101):1550-1562. doi:10.1016/S0140-6736(17)30703-1
2. Centanni M, Benvenga S, Sachmechi I. Diagnosis and management of treatment-refractory hypothyroidism: an expert consensus report. J Endocrinol Invest. 2017;40(12):1289-1301. doi:10.1007/s40618-017-0706-y
3. Hepp Z, Lage MJ, Espaillat R, Gossain VV. The association between adherence to levothyroxine and economic and clinical outcomes in patients with hypothyroidism in the US. J Med Econ. 2018;21(9):912-919. doi:10.1080/13696998.2018.1484749
4. Shakya Shrestha S, Risal K, Shrestha R, Bhatta RD. Medication Adherence to Levothyroxine Therapy among Hypothyroid Patients and their Clinical Outcomes with Special Reference to Thyroid Function Parameters. Kathmandu Univ Med J (KUMJ). 2018;16(62):129-137.
5. Haskard-Zolnierek K, Wilson C, Pruin J, Deason R, Howard K. The Relationship Between Brain Fog and Medication Adherence for Individuals With Hypothyroidism. Clin Nurs Res. 2022;31(3):445-452. doi:10.1177/10547738211038127
6. McNally LJ, Ofiaeli CI, Oyibo SO. Treatment-refractory hypothyroidism. BMJ. 2019;364:l579. Published 2019 Feb 25. doi:10.1136/bmj.l579
7. Nakano Y, Hashimoto K, Ohkiba N, et al. A Case of Refractory Hypothyroidism due to Poor Compliance Treated with the Weekly Intravenous and Oral Levothyroxine Administration. Case Rep Endocrinol. 2019;2019:5986014. Published 2019 Feb 5. doi:10.1155/2019/5986014
8. Kiran Z, Shaikh KS, Fatima N, Tariq N, Baloch AA. Levothyroxine absorption test followed by directly observed treatment on an outpatient basis to address long-term high TSH levels in a hypothyroid patient: a case report. J Med Case Rep. 2023;17(1):24. Published 2023 Jan 25. doi:10.1186/s13256-023-03760-0
9. Chiu HH, Larrazabal R Jr, Uy AB, Jimeno C. Weekly Versus Daily Levothyroxine Tablet Replacement in Adults with Hypothyroidism: A Meta-Analysis. J ASEAN Fed Endocr Soc. 2021;36(2):156-160. doi:10.15605/jafes.036.02.07
10. Cappelli C, Castello R, Marini F, et al. Adherence to Levothyroxine Treatment Among Patients With Hypothyroidism: A Northeastern Italian Survey. Front Endocrinol (Lausanne). 2018;9:699. Published 2018 Nov 23. doi:10.3389/fendo.2018.00699
11. Bocale R, Desideri G, Barini A, et al. Long-Term Adherence to Levothyroxine Replacement Therapy in Thyroidectomized Patients. J Clin Med. 2022;11(15):4296. Published 2022 Jul 24. doi:10.3390/jcm11154296
12. El Helou S, Hallit S, Awada S, et al. Adherence to levothyroxine among patients with hypothyroidism in Lebanon. East Mediterr Health J. 2019;25(3):149-159. Published 2019 Apr 25. doi:10.26719/emhj.18.022
Dermatologic Reactions Following COVID-19 Vaccination: A Case Series
Cutaneous reactions associated with the Pfizer-BioNTech COVID-19 vaccine have been reported worldwide since December 2020. Local injection site reactions (<1%) such as erythema, swelling, delayed local reactions (1%–10%), morbilliform rash, urticarial reactions, pityriasis rosea, Rowell syndrome, and lichen planus have been reported following the Pfizer-BioNTech COVID-19 vaccine.1 Cutaneous reactions reported in association with the Sinovac-Coronavac COVID-19 vaccine include swelling, redness, itching, discoloration, induration (1%–10%), urticaria, petechial rash, and exacerbation of psoriasis at the local injection site (<1%).2
We describe 7 patients from Turkey who presented with various dermatologic problems 5 to 28 days after COVID-19 vaccination, highlighting the possibility of early and late cutaneous reactions related to the vaccine (Table).
Case Reports
Patient 1—A 44-year-old woman was admitted to the dermatology clinic with painful lesions on the trunk of 3 days’ duration. Dermatologic examination revealed grouped erythematous vesicles showing dermatomal spread in the right thoracolumbar (dermatome T10) region. The patient reported that she had received 2 doses of the Sinovac-Coronavac vaccine (doses 1 and 2) and 2 doses of the BioNTech COVID-19 vaccine (doses 3 and 4); the rash had developed 28 days after she received the 4th dose. Her medical history was unremarkable. The lesions regressed after 1 week of treatment with oral valacyclovir 1000 mg 3 times daily, but she developed postherpetic neuralgia 1 week after starting treatment, which resolved after 8 weeks.
Patient 2—A 68-year-old woman presented to the dermatology clinic for evaluation of painful sores on the upper lip of 1 day’s duration. She had a history of rheumatoid arthritis, hypertension, and atopy and was currently taking prednisone and etanercept. Dermatologic examination revealed grouped vesicles on an erythematous base on the upper lip. A diagnosis of herpes labialis was made. The patient reported that she had received a third dose of the Sinovac-Coronavac vaccine 10 days prior to the appearance of the lesions. Her symptoms resolved completely within 2 weeks of treatment with topical acyclovir.
Patient 3—A 64-year-old woman was admitted to the hospital with pain, redness, and watery sores on and around the left eyelid of 2 days’ duration. Dermatologic evaluation revealed the erythematous surface of the left eyelid and periorbital area showed partial crusts, clustered vesicles, erythema, and edema. Additionally, the conjunctiva was purulent and erythematous. The patient’s medical history was notable for allergic asthma, hypertension, anxiety, and depression. For this reason, the patient was prescribed an angiotensin receptor blocker and a selective serotonin reuptake inhibitor. She noted that a similar rash had developed around the left eye 6 years prior that was diagnosed as herpes zoster (HZ). She also reported that she had received 2 doses of the Sinovac-Coronavac COVID-19 vaccine followed by 1 dose of the BioNTech COVID-19 vaccine, which she had received 2 weeks before the rash developed. The patient was treated at the eye clinic and was found to have ocular involvement. Ophthalmology was consulted and a diagnosis of herpes zoster ophthalmicus (HZO) was made. Systemic valacyclovir treatment was initiated, resulting in clinical improvement within 3 weeks.
Patient 4—A 75-year-old man was admitted to the hospital with chest and back pain and widespread muscle pain of several days’ duration. His medical history was remarkable for diabetes mellitus, hypertension, depression, and coronary artery bypass surgery. A medication history revealed treatment with a β-blocker, acetylsalicylic acid, a calcium channel blocker, a dipeptidyl peptidase 4 inhibitor, and a selective serotonin reuptake inhibitor. Dermatologic examination revealed grouped vesicles on an erythematous background in dermatome T5 on the right chest and back. A diagnosis of HZ was made. The patient reported that he had received 2 doses of the Sinovac-Coronavac vaccine followed by 1 dose of the Pfizer-BioNTech vaccine 2 weeks prior to the current presentation. He was treated with valacyclovir for 1 week, and his symptoms resolved entirely within 3 weeks.
Patient 5—A 50-year-old woman presented to the hospital for evaluation of painful sores on the back, chest, groin, and abdomen of 10 days’ duration. The lesions initially had developed 7 days after receiving the BioNTech COVID-19 vaccine; she previously had received 2 doses of the Sinovac-Coronavac vaccine. The patient had a history of untreated psoriasis. Dermatologic examination revealed grouped vesicles on an erythematous background in the T2–L2 dermatomes on the left side of the trunk. A diagnosis of HZ was made. The lesions resolved after 1 week of treatment with systemic valacyclovir; however, she subsequently developed postherpetic neuralgia, hypoesthesia, and postinflammatory hyperpigmentation in the affected regions.
Patient 6—A 37-year-old woman presented to the hospital with redness, swelling, and itching all over the body of 3 days’ duration. The patient noted that the rash would subside and reappear throughout the day. Her medical history was unremarkable, except for COVID-19 infection 6 months prior. She had received a second dose of the BioNTech vaccine 20 days prior to development of symptoms. Dermatologic examination revealed widespread erythematous urticarial plaques. A diagnosis of acute urticaria was made. The patient recovered completely after 1 week of treatment with a systemic steroid and 3 weeks of antihistamine treatment.
Patient 7—A 63-year-old woman presented to the hospital with widespread itching and rash that appeared 5 days after the first dose of the BioNTech COVID-19 vaccine. The patient reported that the rash resolved spontaneously within a few hours but then reappeared. Her medical history revealed that she was taking tamoxifen for breast cancer and that she previously had received 2 doses of the Sinovac-Coronavac vaccine. Dermatologic examination revealed erythematous urticarial plaques on the trunk and arms. A diagnosis of urticaria was made, and her symptoms resolved after 6 weeks of antihistamine treatment.
Comment
Skin lesions associated with COVID-19 infection have been reported worldwide3,4 as well as dermatologic reactions following COVID-19 vaccination. In one case from Turkey, HZ infection was reported in a 68-year-old man 5 days after he received a second dose of the COVID-19 vaccine.5 In another case, HZ infection developed in a 78-year-old man 5 days after COVID-19 vaccination.6 Numerous cases of HZ infection developing within 1 to 26 days of COVID-19 vaccination have been reported worldwide.7-9
In a study conducted in the United States, 40 skin reactions associated with the COVID-19 vaccine were investigated; of these cases, 87.5% (35/40) were reported as varicella-zoster virus, and 12.5% (5/40) were reported as herpes simplex reactivation; 54% (19/35) and 80% (4/5) of these cases, respectively, were associated with the Pfizer-BioNTech vaccine.10 The average age of patients who developed a skin reaction was 46 years, and 70% (28/40) were women. The time to onset of the reaction was 2 to 13 days after vaccination, and symptoms were reported to improve within 7 days on average.10
Another study from Spain examined 405 vaccine-related skin reactions, 40.2% of which were related to the Pfizer-BioNTech vaccine. Among them, 80.2% occurred in women; 13.8% of cases were diagnosed as varicella-zoster virus or HZ virus reactivation, and 14.6% were urticaria. Eighty reactions (21%) were classified as severe/very severe and 81% required treatment.11 One study reported 414 skin reactions from the COVID-19 vaccine from December 2020 to February 2021; of these cases, 83% occurred after the Moderna vaccine, which is not available in Turkey, and 17% occurred after the Pfizer-BioNTech vaccine.12A systematic review of 91 patients who developed HZ infection after COVID-19 vaccination reported that 10% (9/91) of cases were receiving immunosuppressive therapy and 13% (12/91) had an autoimmune disease.7 In our case series, it is known that at least 2 of the patients (patients 2 and 5), including 1 patient with rheumatoid arthritis (patient 2) who was on immunosuppressive treatment, had autoimmune disorders. However, reports in the literature indicate that most patients with autoimmune inflammatory rheumatic diseases remain stable after vaccination.13
Herpes zoster ophthalmicus is a rare form of HZ caused by involvement of the ophthalmic branch of the trigeminal nerve that manifests as vesicular lesions and retinitis, uveitis, keratitis, conjunctivitis, and pain on an erythematous background. Two cases of women who developed HZO infection after Pfizer-BioNTech vaccination were reported in the literature.14 Although patient 3 in our case series had a history of HZO 6 years prior, the possibility of the Pfizer-BioNTech vaccine triggering HZO should be taken into consideration.
Although cutaneous reactions after the Sinovac-Coronavac vaccine were observed in only 1 of 7 patients in our case series, skin reactions after Sinovac-Coronavac (an inactivated viral vaccine) have been reported in the literature. In one study, after a total of 35,229 injections, the incidence of cutaneous adverse events due to Sinovac-Coronavac was reported to be 0.94% and 0.70% after the first and second doses, respectively.15 Therefore, further study results are needed to directly attribute the reactions to COVID-19 vaccination.
Conclusion
Our case series highlights that clinicians should be vigilant in diagnosing cutaneous reactions following COVID-19 vaccination early to prevent potential complications. Early recognition of reactions is crucial, and the prognosis can be improved with appropriate treatment. Despite the potential dermatologic adverse effects of the COVID-19 vaccine, the most effective way to protect against serious COVID-19 infection is to continue to be vaccinated.
- Polack FP, Thomas SJ, Kitchin N, et al. Safety and efficacy of the BNT162b2 mRNA COVID-19 vaccine. N Engl J Med. 2020;383:2603-2615.
- Zhang Y, Zeng G, Pan H, et al. Safety, tolerability, and immunogenicity of an inactivated SARS-CoV-2 vaccine in healthy adults aged 18–59 years: a randomised, double-blind, placebo-controlled, phase 1/2 clinical trial. Lancet Infect Dis. 2021;21:181-192.
- Tan SW, Tam YC, Oh CC. Skin manifestations of COVID-19: a worldwide review. JAAD Int. 2021;2:119-133.
- Singh H, Kaur H, Singh K, et al. Cutaneous manifestations of COVID-19: a systematic review. advances in wound care. 2021;10:51-80.
- Aksu SB, Öztürk GZ. A rare case of shingles after COVID-19 vaccine: is it a possible adverse effect? clinical and experimental vaccine research. 2021;10:198-201.
- Bostan E, Yalici-Armagan B. Herpes zoster following inactivated COVID-19 vaccine: a coexistence or coincidence? J Cosmet Dermatol. 2021;20:1566-1567.
- Katsikas Triantafyllidis K, Giannos P, Mian IT, et al. Varicella zoster virus reactivation following COVID-19 vaccination: a systematic review of case reports. Vaccines (Basel). 2021;9:1013. doi:10.3390/vaccines9091013
- Rodríguez-Jiménez P, Chicharro P, Cabrera LM, et al. Varicella-zoster virus reactivation after SARS-CoV-2 BNT162b2 mRNA vaccination: report of 5 cases. JAAD Case Rep. 2021;12:58-59. doi:10.1016/j.jdcr.2021.04.014
- Lee C, Cotter D, Basa J, et al. 20 Post-COVID-19 vaccine-related shingles cases seen at the Las Vegas Dermatology clinic and sent to us via social media. J Cosmet Dermatol. 2021;20:1960-1964.
- Fathy RA, McMahon DE, Lee C, et al. Varicella-zoster and herpes simplex virus reactivation post-COVID-19 vaccination: a review of 40 cases in an International Dermatology Registry. J Eur Acad Dermatol Venerol. 2022;36:E6-E9.
- Català A, Muñoz-Santos C, Galván-Casas C, et al. Cutaneous reactions after SARS-CoV-2 vaccination: a cross-sectional Spanish nationwide study of 405 cases. Br J Dermatol. 2022;186:142-152.
- McMahon DE, Amerson E, Rosenbach M, et al. Cutaneous reactions reported after Moderna and Pfizer COVID-19 vaccination: a registry-based study of 414 cases. J Am Acad Dermatol. 2021;85:46-55.
- Furer V, Eviatar T, Zisman D, et al. Immunogenicity and safety of the BNT162b2 mRNA COVID-19 vaccine in adult patients with autoimmune inflammatory rheumatic diseases and in the general population: a multicentre study. Ann Rheum Dis. 2021;80:1330-1338.
- Bernardini N, Skroza N, Mambrin A, et al. Herpes zoster ophthalmicus in two women after Pfizer-BioNTech (BNT162b2) vaccine. J Med Virol. 2022;94:817-818.
- Rerknimitr P, Puaratanaarunkon T, Wongtada C, et al. Cutaneous adverse reactions from 35,229 doses of Sinovac and AstraZeneca COVID-19 vaccination: a prospective cohort study in healthcare workers. J Eur Acad Dermatol Venereol. 2022;36:E158-E161.
Cutaneous reactions associated with the Pfizer-BioNTech COVID-19 vaccine have been reported worldwide since December 2020. Local injection site reactions (<1%) such as erythema, swelling, delayed local reactions (1%–10%), morbilliform rash, urticarial reactions, pityriasis rosea, Rowell syndrome, and lichen planus have been reported following the Pfizer-BioNTech COVID-19 vaccine.1 Cutaneous reactions reported in association with the Sinovac-Coronavac COVID-19 vaccine include swelling, redness, itching, discoloration, induration (1%–10%), urticaria, petechial rash, and exacerbation of psoriasis at the local injection site (<1%).2
We describe 7 patients from Turkey who presented with various dermatologic problems 5 to 28 days after COVID-19 vaccination, highlighting the possibility of early and late cutaneous reactions related to the vaccine (Table).

Case Reports
Patient 1—A 44-year-old woman was admitted to the dermatology clinic with painful lesions on the trunk of 3 days’ duration. Dermatologic examination revealed grouped erythematous vesicles showing dermatomal spread in the right thoracolumbar (dermatome T10) region. The patient reported that she had received 2 doses of the Sinovac-Coronavac vaccine (doses 1 and 2) and 2 doses of the BioNTech COVID-19 vaccine (doses 3 and 4); the rash had developed 28 days after she received the 4th dose. Her medical history was unremarkable. The lesions regressed after 1 week of treatment with oral valacyclovir 1000 mg 3 times daily, but she developed postherpetic neuralgia 1 week after starting treatment, which resolved after 8 weeks.
Patient 2—A 68-year-old woman presented to the dermatology clinic for evaluation of painful sores on the upper lip of 1 day’s duration. She had a history of rheumatoid arthritis, hypertension, and atopy and was currently taking prednisone and etanercept. Dermatologic examination revealed grouped vesicles on an erythematous base on the upper lip. A diagnosis of herpes labialis was made. The patient reported that she had received a third dose of the Sinovac-Coronavac vaccine 10 days prior to the appearance of the lesions. Her symptoms resolved completely within 2 weeks of treatment with topical acyclovir.
Patient 3—A 64-year-old woman was admitted to the hospital with pain, redness, and watery sores on and around the left eyelid of 2 days’ duration. Dermatologic evaluation revealed the erythematous surface of the left eyelid and periorbital area showed partial crusts, clustered vesicles, erythema, and edema. Additionally, the conjunctiva was purulent and erythematous. The patient’s medical history was notable for allergic asthma, hypertension, anxiety, and depression. For this reason, the patient was prescribed an angiotensin receptor blocker and a selective serotonin reuptake inhibitor. She noted that a similar rash had developed around the left eye 6 years prior that was diagnosed as herpes zoster (HZ). She also reported that she had received 2 doses of the Sinovac-Coronavac COVID-19 vaccine followed by 1 dose of the BioNTech COVID-19 vaccine, which she had received 2 weeks before the rash developed. The patient was treated at the eye clinic and was found to have ocular involvement. Ophthalmology was consulted and a diagnosis of herpes zoster ophthalmicus (HZO) was made. Systemic valacyclovir treatment was initiated, resulting in clinical improvement within 3 weeks.
Patient 4—A 75-year-old man was admitted to the hospital with chest and back pain and widespread muscle pain of several days’ duration. His medical history was remarkable for diabetes mellitus, hypertension, depression, and coronary artery bypass surgery. A medication history revealed treatment with a β-blocker, acetylsalicylic acid, a calcium channel blocker, a dipeptidyl peptidase 4 inhibitor, and a selective serotonin reuptake inhibitor. Dermatologic examination revealed grouped vesicles on an erythematous background in dermatome T5 on the right chest and back. A diagnosis of HZ was made. The patient reported that he had received 2 doses of the Sinovac-Coronavac vaccine followed by 1 dose of the Pfizer-BioNTech vaccine 2 weeks prior to the current presentation. He was treated with valacyclovir for 1 week, and his symptoms resolved entirely within 3 weeks.
Patient 5—A 50-year-old woman presented to the hospital for evaluation of painful sores on the back, chest, groin, and abdomen of 10 days’ duration. The lesions initially had developed 7 days after receiving the BioNTech COVID-19 vaccine; she previously had received 2 doses of the Sinovac-Coronavac vaccine. The patient had a history of untreated psoriasis. Dermatologic examination revealed grouped vesicles on an erythematous background in the T2–L2 dermatomes on the left side of the trunk. A diagnosis of HZ was made. The lesions resolved after 1 week of treatment with systemic valacyclovir; however, she subsequently developed postherpetic neuralgia, hypoesthesia, and postinflammatory hyperpigmentation in the affected regions.
Patient 6—A 37-year-old woman presented to the hospital with redness, swelling, and itching all over the body of 3 days’ duration. The patient noted that the rash would subside and reappear throughout the day. Her medical history was unremarkable, except for COVID-19 infection 6 months prior. She had received a second dose of the BioNTech vaccine 20 days prior to development of symptoms. Dermatologic examination revealed widespread erythematous urticarial plaques. A diagnosis of acute urticaria was made. The patient recovered completely after 1 week of treatment with a systemic steroid and 3 weeks of antihistamine treatment.
Patient 7—A 63-year-old woman presented to the hospital with widespread itching and rash that appeared 5 days after the first dose of the BioNTech COVID-19 vaccine. The patient reported that the rash resolved spontaneously within a few hours but then reappeared. Her medical history revealed that she was taking tamoxifen for breast cancer and that she previously had received 2 doses of the Sinovac-Coronavac vaccine. Dermatologic examination revealed erythematous urticarial plaques on the trunk and arms. A diagnosis of urticaria was made, and her symptoms resolved after 6 weeks of antihistamine treatment.
Comment
Skin lesions associated with COVID-19 infection have been reported worldwide3,4 as well as dermatologic reactions following COVID-19 vaccination. In one case from Turkey, HZ infection was reported in a 68-year-old man 5 days after he received a second dose of the COVID-19 vaccine.5 In another case, HZ infection developed in a 78-year-old man 5 days after COVID-19 vaccination.6 Numerous cases of HZ infection developing within 1 to 26 days of COVID-19 vaccination have been reported worldwide.7-9
In a study conducted in the United States, 40 skin reactions associated with the COVID-19 vaccine were investigated; of these cases, 87.5% (35/40) were reported as varicella-zoster virus, and 12.5% (5/40) were reported as herpes simplex reactivation; 54% (19/35) and 80% (4/5) of these cases, respectively, were associated with the Pfizer-BioNTech vaccine.10 The average age of patients who developed a skin reaction was 46 years, and 70% (28/40) were women. The time to onset of the reaction was 2 to 13 days after vaccination, and symptoms were reported to improve within 7 days on average.10
Another study from Spain examined 405 vaccine-related skin reactions, 40.2% of which were related to the Pfizer-BioNTech vaccine. Among them, 80.2% occurred in women; 13.8% of cases were diagnosed as varicella-zoster virus or HZ virus reactivation, and 14.6% were urticaria. Eighty reactions (21%) were classified as severe/very severe and 81% required treatment.11 One study reported 414 skin reactions from the COVID-19 vaccine from December 2020 to February 2021; of these cases, 83% occurred after the Moderna vaccine, which is not available in Turkey, and 17% occurred after the Pfizer-BioNTech vaccine.12A systematic review of 91 patients who developed HZ infection after COVID-19 vaccination reported that 10% (9/91) of cases were receiving immunosuppressive therapy and 13% (12/91) had an autoimmune disease.7 In our case series, it is known that at least 2 of the patients (patients 2 and 5), including 1 patient with rheumatoid arthritis (patient 2) who was on immunosuppressive treatment, had autoimmune disorders. However, reports in the literature indicate that most patients with autoimmune inflammatory rheumatic diseases remain stable after vaccination.13
Herpes zoster ophthalmicus is a rare form of HZ caused by involvement of the ophthalmic branch of the trigeminal nerve that manifests as vesicular lesions and retinitis, uveitis, keratitis, conjunctivitis, and pain on an erythematous background. Two cases of women who developed HZO infection after Pfizer-BioNTech vaccination were reported in the literature.14 Although patient 3 in our case series had a history of HZO 6 years prior, the possibility of the Pfizer-BioNTech vaccine triggering HZO should be taken into consideration.
Although cutaneous reactions after the Sinovac-Coronavac vaccine were observed in only 1 of 7 patients in our case series, skin reactions after Sinovac-Coronavac (an inactivated viral vaccine) have been reported in the literature. In one study, after a total of 35,229 injections, the incidence of cutaneous adverse events due to Sinovac-Coronavac was reported to be 0.94% and 0.70% after the first and second doses, respectively.15 Therefore, further study results are needed to directly attribute the reactions to COVID-19 vaccination.
Conclusion
Our case series highlights that clinicians should be vigilant in diagnosing cutaneous reactions following COVID-19 vaccination early to prevent potential complications. Early recognition of reactions is crucial, and the prognosis can be improved with appropriate treatment. Despite the potential dermatologic adverse effects of the COVID-19 vaccine, the most effective way to protect against serious COVID-19 infection is to continue to be vaccinated.
Cutaneous reactions associated with the Pfizer-BioNTech COVID-19 vaccine have been reported worldwide since December 2020. Local injection site reactions (<1%) such as erythema, swelling, delayed local reactions (1%–10%), morbilliform rash, urticarial reactions, pityriasis rosea, Rowell syndrome, and lichen planus have been reported following the Pfizer-BioNTech COVID-19 vaccine.1 Cutaneous reactions reported in association with the Sinovac-Coronavac COVID-19 vaccine include swelling, redness, itching, discoloration, induration (1%–10%), urticaria, petechial rash, and exacerbation of psoriasis at the local injection site (<1%).2
We describe 7 patients from Turkey who presented with various dermatologic problems 5 to 28 days after COVID-19 vaccination, highlighting the possibility of early and late cutaneous reactions related to the vaccine (Table).

Case Reports
Patient 1—A 44-year-old woman was admitted to the dermatology clinic with painful lesions on the trunk of 3 days’ duration. Dermatologic examination revealed grouped erythematous vesicles showing dermatomal spread in the right thoracolumbar (dermatome T10) region. The patient reported that she had received 2 doses of the Sinovac-Coronavac vaccine (doses 1 and 2) and 2 doses of the BioNTech COVID-19 vaccine (doses 3 and 4); the rash had developed 28 days after she received the 4th dose. Her medical history was unremarkable. The lesions regressed after 1 week of treatment with oral valacyclovir 1000 mg 3 times daily, but she developed postherpetic neuralgia 1 week after starting treatment, which resolved after 8 weeks.
Patient 2—A 68-year-old woman presented to the dermatology clinic for evaluation of painful sores on the upper lip of 1 day’s duration. She had a history of rheumatoid arthritis, hypertension, and atopy and was currently taking prednisone and etanercept. Dermatologic examination revealed grouped vesicles on an erythematous base on the upper lip. A diagnosis of herpes labialis was made. The patient reported that she had received a third dose of the Sinovac-Coronavac vaccine 10 days prior to the appearance of the lesions. Her symptoms resolved completely within 2 weeks of treatment with topical acyclovir.
Patient 3—A 64-year-old woman was admitted to the hospital with pain, redness, and watery sores on and around the left eyelid of 2 days’ duration. Dermatologic evaluation revealed the erythematous surface of the left eyelid and periorbital area showed partial crusts, clustered vesicles, erythema, and edema. Additionally, the conjunctiva was purulent and erythematous. The patient’s medical history was notable for allergic asthma, hypertension, anxiety, and depression. For this reason, the patient was prescribed an angiotensin receptor blocker and a selective serotonin reuptake inhibitor. She noted that a similar rash had developed around the left eye 6 years prior that was diagnosed as herpes zoster (HZ). She also reported that she had received 2 doses of the Sinovac-Coronavac COVID-19 vaccine followed by 1 dose of the BioNTech COVID-19 vaccine, which she had received 2 weeks before the rash developed. The patient was treated at the eye clinic and was found to have ocular involvement. Ophthalmology was consulted and a diagnosis of herpes zoster ophthalmicus (HZO) was made. Systemic valacyclovir treatment was initiated, resulting in clinical improvement within 3 weeks.
Patient 4—A 75-year-old man was admitted to the hospital with chest and back pain and widespread muscle pain of several days’ duration. His medical history was remarkable for diabetes mellitus, hypertension, depression, and coronary artery bypass surgery. A medication history revealed treatment with a β-blocker, acetylsalicylic acid, a calcium channel blocker, a dipeptidyl peptidase 4 inhibitor, and a selective serotonin reuptake inhibitor. Dermatologic examination revealed grouped vesicles on an erythematous background in dermatome T5 on the right chest and back. A diagnosis of HZ was made. The patient reported that he had received 2 doses of the Sinovac-Coronavac vaccine followed by 1 dose of the Pfizer-BioNTech vaccine 2 weeks prior to the current presentation. He was treated with valacyclovir for 1 week, and his symptoms resolved entirely within 3 weeks.
Patient 5—A 50-year-old woman presented to the hospital for evaluation of painful sores on the back, chest, groin, and abdomen of 10 days’ duration. The lesions initially had developed 7 days after receiving the BioNTech COVID-19 vaccine; she previously had received 2 doses of the Sinovac-Coronavac vaccine. The patient had a history of untreated psoriasis. Dermatologic examination revealed grouped vesicles on an erythematous background in the T2–L2 dermatomes on the left side of the trunk. A diagnosis of HZ was made. The lesions resolved after 1 week of treatment with systemic valacyclovir; however, she subsequently developed postherpetic neuralgia, hypoesthesia, and postinflammatory hyperpigmentation in the affected regions.
Patient 6—A 37-year-old woman presented to the hospital with redness, swelling, and itching all over the body of 3 days’ duration. The patient noted that the rash would subside and reappear throughout the day. Her medical history was unremarkable, except for COVID-19 infection 6 months prior. She had received a second dose of the BioNTech vaccine 20 days prior to development of symptoms. Dermatologic examination revealed widespread erythematous urticarial plaques. A diagnosis of acute urticaria was made. The patient recovered completely after 1 week of treatment with a systemic steroid and 3 weeks of antihistamine treatment.
Patient 7—A 63-year-old woman presented to the hospital with widespread itching and rash that appeared 5 days after the first dose of the BioNTech COVID-19 vaccine. The patient reported that the rash resolved spontaneously within a few hours but then reappeared. Her medical history revealed that she was taking tamoxifen for breast cancer and that she previously had received 2 doses of the Sinovac-Coronavac vaccine. Dermatologic examination revealed erythematous urticarial plaques on the trunk and arms. A diagnosis of urticaria was made, and her symptoms resolved after 6 weeks of antihistamine treatment.
Comment
Skin lesions associated with COVID-19 infection have been reported worldwide3,4 as well as dermatologic reactions following COVID-19 vaccination. In one case from Turkey, HZ infection was reported in a 68-year-old man 5 days after he received a second dose of the COVID-19 vaccine.5 In another case, HZ infection developed in a 78-year-old man 5 days after COVID-19 vaccination.6 Numerous cases of HZ infection developing within 1 to 26 days of COVID-19 vaccination have been reported worldwide.7-9
In a study conducted in the United States, 40 skin reactions associated with the COVID-19 vaccine were investigated; of these cases, 87.5% (35/40) were reported as varicella-zoster virus, and 12.5% (5/40) were reported as herpes simplex reactivation; 54% (19/35) and 80% (4/5) of these cases, respectively, were associated with the Pfizer-BioNTech vaccine.10 The average age of patients who developed a skin reaction was 46 years, and 70% (28/40) were women. The time to onset of the reaction was 2 to 13 days after vaccination, and symptoms were reported to improve within 7 days on average.10
Another study from Spain examined 405 vaccine-related skin reactions, 40.2% of which were related to the Pfizer-BioNTech vaccine. Among them, 80.2% occurred in women; 13.8% of cases were diagnosed as varicella-zoster virus or HZ virus reactivation, and 14.6% were urticaria. Eighty reactions (21%) were classified as severe/very severe and 81% required treatment.11 One study reported 414 skin reactions from the COVID-19 vaccine from December 2020 to February 2021; of these cases, 83% occurred after the Moderna vaccine, which is not available in Turkey, and 17% occurred after the Pfizer-BioNTech vaccine.12A systematic review of 91 patients who developed HZ infection after COVID-19 vaccination reported that 10% (9/91) of cases were receiving immunosuppressive therapy and 13% (12/91) had an autoimmune disease.7 In our case series, it is known that at least 2 of the patients (patients 2 and 5), including 1 patient with rheumatoid arthritis (patient 2) who was on immunosuppressive treatment, had autoimmune disorders. However, reports in the literature indicate that most patients with autoimmune inflammatory rheumatic diseases remain stable after vaccination.13
Herpes zoster ophthalmicus is a rare form of HZ caused by involvement of the ophthalmic branch of the trigeminal nerve that manifests as vesicular lesions and retinitis, uveitis, keratitis, conjunctivitis, and pain on an erythematous background. Two cases of women who developed HZO infection after Pfizer-BioNTech vaccination were reported in the literature.14 Although patient 3 in our case series had a history of HZO 6 years prior, the possibility of the Pfizer-BioNTech vaccine triggering HZO should be taken into consideration.
Although cutaneous reactions after the Sinovac-Coronavac vaccine were observed in only 1 of 7 patients in our case series, skin reactions after Sinovac-Coronavac (an inactivated viral vaccine) have been reported in the literature. In one study, after a total of 35,229 injections, the incidence of cutaneous adverse events due to Sinovac-Coronavac was reported to be 0.94% and 0.70% after the first and second doses, respectively.15 Therefore, further study results are needed to directly attribute the reactions to COVID-19 vaccination.
Conclusion
Our case series highlights that clinicians should be vigilant in diagnosing cutaneous reactions following COVID-19 vaccination early to prevent potential complications. Early recognition of reactions is crucial, and the prognosis can be improved with appropriate treatment. Despite the potential dermatologic adverse effects of the COVID-19 vaccine, the most effective way to protect against serious COVID-19 infection is to continue to be vaccinated.
- Polack FP, Thomas SJ, Kitchin N, et al. Safety and efficacy of the BNT162b2 mRNA COVID-19 vaccine. N Engl J Med. 2020;383:2603-2615.
- Zhang Y, Zeng G, Pan H, et al. Safety, tolerability, and immunogenicity of an inactivated SARS-CoV-2 vaccine in healthy adults aged 18–59 years: a randomised, double-blind, placebo-controlled, phase 1/2 clinical trial. Lancet Infect Dis. 2021;21:181-192.
- Tan SW, Tam YC, Oh CC. Skin manifestations of COVID-19: a worldwide review. JAAD Int. 2021;2:119-133.
- Singh H, Kaur H, Singh K, et al. Cutaneous manifestations of COVID-19: a systematic review. advances in wound care. 2021;10:51-80.
- Aksu SB, Öztürk GZ. A rare case of shingles after COVID-19 vaccine: is it a possible adverse effect? clinical and experimental vaccine research. 2021;10:198-201.
- Bostan E, Yalici-Armagan B. Herpes zoster following inactivated COVID-19 vaccine: a coexistence or coincidence? J Cosmet Dermatol. 2021;20:1566-1567.
- Katsikas Triantafyllidis K, Giannos P, Mian IT, et al. Varicella zoster virus reactivation following COVID-19 vaccination: a systematic review of case reports. Vaccines (Basel). 2021;9:1013. doi:10.3390/vaccines9091013
- Rodríguez-Jiménez P, Chicharro P, Cabrera LM, et al. Varicella-zoster virus reactivation after SARS-CoV-2 BNT162b2 mRNA vaccination: report of 5 cases. JAAD Case Rep. 2021;12:58-59. doi:10.1016/j.jdcr.2021.04.014
- Lee C, Cotter D, Basa J, et al. 20 Post-COVID-19 vaccine-related shingles cases seen at the Las Vegas Dermatology clinic and sent to us via social media. J Cosmet Dermatol. 2021;20:1960-1964.
- Fathy RA, McMahon DE, Lee C, et al. Varicella-zoster and herpes simplex virus reactivation post-COVID-19 vaccination: a review of 40 cases in an International Dermatology Registry. J Eur Acad Dermatol Venerol. 2022;36:E6-E9.
- Català A, Muñoz-Santos C, Galván-Casas C, et al. Cutaneous reactions after SARS-CoV-2 vaccination: a cross-sectional Spanish nationwide study of 405 cases. Br J Dermatol. 2022;186:142-152.
- McMahon DE, Amerson E, Rosenbach M, et al. Cutaneous reactions reported after Moderna and Pfizer COVID-19 vaccination: a registry-based study of 414 cases. J Am Acad Dermatol. 2021;85:46-55.
- Furer V, Eviatar T, Zisman D, et al. Immunogenicity and safety of the BNT162b2 mRNA COVID-19 vaccine in adult patients with autoimmune inflammatory rheumatic diseases and in the general population: a multicentre study. Ann Rheum Dis. 2021;80:1330-1338.
- Bernardini N, Skroza N, Mambrin A, et al. Herpes zoster ophthalmicus in two women after Pfizer-BioNTech (BNT162b2) vaccine. J Med Virol. 2022;94:817-818.
- Rerknimitr P, Puaratanaarunkon T, Wongtada C, et al. Cutaneous adverse reactions from 35,229 doses of Sinovac and AstraZeneca COVID-19 vaccination: a prospective cohort study in healthcare workers. J Eur Acad Dermatol Venereol. 2022;36:E158-E161.
- Polack FP, Thomas SJ, Kitchin N, et al. Safety and efficacy of the BNT162b2 mRNA COVID-19 vaccine. N Engl J Med. 2020;383:2603-2615.
- Zhang Y, Zeng G, Pan H, et al. Safety, tolerability, and immunogenicity of an inactivated SARS-CoV-2 vaccine in healthy adults aged 18–59 years: a randomised, double-blind, placebo-controlled, phase 1/2 clinical trial. Lancet Infect Dis. 2021;21:181-192.
- Tan SW, Tam YC, Oh CC. Skin manifestations of COVID-19: a worldwide review. JAAD Int. 2021;2:119-133.
- Singh H, Kaur H, Singh K, et al. Cutaneous manifestations of COVID-19: a systematic review. advances in wound care. 2021;10:51-80.
- Aksu SB, Öztürk GZ. A rare case of shingles after COVID-19 vaccine: is it a possible adverse effect? clinical and experimental vaccine research. 2021;10:198-201.
- Bostan E, Yalici-Armagan B. Herpes zoster following inactivated COVID-19 vaccine: a coexistence or coincidence? J Cosmet Dermatol. 2021;20:1566-1567.
- Katsikas Triantafyllidis K, Giannos P, Mian IT, et al. Varicella zoster virus reactivation following COVID-19 vaccination: a systematic review of case reports. Vaccines (Basel). 2021;9:1013. doi:10.3390/vaccines9091013
- Rodríguez-Jiménez P, Chicharro P, Cabrera LM, et al. Varicella-zoster virus reactivation after SARS-CoV-2 BNT162b2 mRNA vaccination: report of 5 cases. JAAD Case Rep. 2021;12:58-59. doi:10.1016/j.jdcr.2021.04.014
- Lee C, Cotter D, Basa J, et al. 20 Post-COVID-19 vaccine-related shingles cases seen at the Las Vegas Dermatology clinic and sent to us via social media. J Cosmet Dermatol. 2021;20:1960-1964.
- Fathy RA, McMahon DE, Lee C, et al. Varicella-zoster and herpes simplex virus reactivation post-COVID-19 vaccination: a review of 40 cases in an International Dermatology Registry. J Eur Acad Dermatol Venerol. 2022;36:E6-E9.
- Català A, Muñoz-Santos C, Galván-Casas C, et al. Cutaneous reactions after SARS-CoV-2 vaccination: a cross-sectional Spanish nationwide study of 405 cases. Br J Dermatol. 2022;186:142-152.
- McMahon DE, Amerson E, Rosenbach M, et al. Cutaneous reactions reported after Moderna and Pfizer COVID-19 vaccination: a registry-based study of 414 cases. J Am Acad Dermatol. 2021;85:46-55.
- Furer V, Eviatar T, Zisman D, et al. Immunogenicity and safety of the BNT162b2 mRNA COVID-19 vaccine in adult patients with autoimmune inflammatory rheumatic diseases and in the general population: a multicentre study. Ann Rheum Dis. 2021;80:1330-1338.
- Bernardini N, Skroza N, Mambrin A, et al. Herpes zoster ophthalmicus in two women after Pfizer-BioNTech (BNT162b2) vaccine. J Med Virol. 2022;94:817-818.
- Rerknimitr P, Puaratanaarunkon T, Wongtada C, et al. Cutaneous adverse reactions from 35,229 doses of Sinovac and AstraZeneca COVID-19 vaccination: a prospective cohort study in healthcare workers. J Eur Acad Dermatol Venereol. 2022;36:E158-E161.
Practice Points
- Cutaneous reactions have been reported following COVID-19 vaccination.
- Herpes infections and urticarial reactions can be associated with COVID-19 vaccination, regardless of the delay in onset between the injection and symptom development.
My Kidney Is Fine, Can’t You Cystatin C?
Clinicians usually measure renal function by using surrogate markers because directly measuring glomerular filtration rate (GFR) is not routinely feasible in a clinical setting.1,2 Creatinine (Cr) and cystatin C (CysC) are the 2 main surrogate molecules used to estimate GFR.3
Creatine is a molecule nonenzymatically converted into Cr, weighing only 113 Da in skeletal muscles.4 It is then filtered at the glomeruli and secreted at the proximal tubules of the kidneys. However, serum Cr (sCr) levels are affected by several factors, including age, biological sex, liver function, diet, and muscle mass.5 Historically, sCr levels also are affected by race.5 In an early study of factors affecting accurate GFR, researchers reported that self-identified African American patients had a 16% higher GFR than those who did not when using Cr.6 Despite this, the inclusion of Cr on a basic metabolic panel has allowed automatic reporting of an estimated GFR using sCr (eGFRCr) to be readily available.7
In comparison to Cr, CysC is an endogenous protein weighing 13 kDa produced by all nucleated cells.8,9 CysC is filtered by the kidney at the glomeruli and completely reabsorbed and catabolized by epithelial cells at the proximal tubule.9 Since production is not dependent on skeletal muscle, there are fewer physiological impacts on serum concentration of CysC. Levels of CysC may be elevated by factors shown in the Table.
Estimating Glomerular Filtration Rates
Multiple equations were developed to mitigate the impact of extraneous factors on the accuracy of an eGFRCr. The first widely used equation that included a variable adjustment for race was the Modification of Diet in Renal Disease study, presented in 2006.10 The equation increased the accuracy of eGFRCr further by adjusting for sex and age. It was followed by the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation in 2009, which was more accurate at higher GFR levels.11
CysC was simultaneously studied as an alternative to Cr with multiple equation iterations shown to be viable in various populations as early as 2003.12-15 However, it was not until 2012 that an equation for the use of CysC was offered for widespread use as an alternative to Cr alongside further refinement of the CKD-EPI equation for Cr.16 A new formula was presented in 2021 to use both sCr and serum CysC levels to obtain a more accurate estimation of GFR.17 Research continues its effort to accurately estimate GFR for diagnosing kidney disease and assessing comorbidities relating to decreased kidney function.3
All historical equations attempted to mitigate the potential impact of race on sCr level when calculating eGFRCr by assigning a separate variable for African American patients. As an unintended adverse effect, these equations may have led to discrimination by having a different equation for African American patients.18 Moreover, these Cr-based equations remain less accurate in patients with varied muscle mass, such as older patients, bodybuilders, athletes, and individuals with varied extremes of daily protein intake.1,8,9,19 Several medications can also directly affect Cr clearance, reducing its ability to act as a surrogate for kidney function.1 In this case report, we discuss an African American patient with high muscle mass and protein intake who was initially diagnosed with kidney disease based on an elevated Cr and found to be misdiagnosed based on the use of CysC for a more accurate GFR estimation.
Case Presentation
A 35-year-old African American man serving in the military and recently diagnosed with HIV was referred to a nephrology clinic for further evaluation of an acute elevation in sCr. Before treatment for HIV, a brief record review showed a baseline Cr of about 1.3 mg/dL, with an eGFRCr of 75 mL/min/1.73 m2.20 In the same month, the patient was prescribed bictegravir/emtricitabine/tenofovir alafenamide, an HIV drug with nephrotoxic potential.21 The patient's total viral load remained low, and CD4 count remained > 500 after initiation of the HIV treatment. He was in his normal state of health and had no known contributory history before his HIV diagnosis. Cr readings peaked at 1.83 mg/dL after starting the HIV treatment and remained elevated to 1.73 mg/dL over the next few months, corresponding to CKD stage 3A. Because bictegravir/emtricitabine/tenofovir alafenamide is cleared by the kidneys and has a nephrotoxic profile, the clinical care team considered dosage adjustment or a medication switch given his observed elevated eGFRCr based on the CKD-EPI 2021 equation for Cr alone. It was also noted that the patient had a similar Cr spike to 1.83 mg/dL in 2018 without any identifiable renal insult or symptoms (Figure).
Diagnostic Evaluation
The primary care team ordered a renal ultrasound and referred the patient to the nephrology clinic. The nephrologist ordered the following laboratory studies: urine microalbumin to Cr ratio, basic metabolic panel (BMP), comprehensive metabolic panel (CMP), urinalysis, urine protein, urine Cr, parathyroid hormone level, hemoglobin A1c, complement component 3/4 panels, antinuclear and antineutrophil cytoplasmic antibodies titers, glomerular basement membrane antibody titer, urine light chains, serum protein electrophoresis, κ/λ ratio, viral hepatitis panel, and rapid plasma reagin testing. Much of this laboratory evaluation served to rule out any secondary causes of kidney disease, including autoimmune disease, monoclonal or polyclonal gammopathies, diabetic nephropathy or glomerulosclerosis, and nephrotic or nephritic syndromes.
All laboratory studies returned within normal limits; no proteinuria was discovered on urinalysis, and no abnormalities were visualized on renal ultrasound. Bictegravir/emtricitabine/tenofovir alafenamide nephrotoxicity was highest among the differential diagnoses due to the timing of Cr elevation coinciding with the initiation of the medications. The patient's CysC level was 0.85 mg/dL with a calculated eGFRCys of 125 mL/min/1.73 m2. The calculated sCR and serum cystatin C (eGFRCr-Cys) using the new 2021 equation and when adjusting for body surface area placed his eGFR at 92 mL/min/1.73 m2.20
The patient’s eGFRCys reassured the care team that the patient’s renal function was not acutely or chronically impacted by bictegravir/emtricitabine/tenofovir alafenamide, resulting in avoidance of unnecessary dosage adjustment or discontinuation of the HIV treatment. The patient reported a chronic habit of protein and creatine supplementation and bodybuilding, which likely further compounded the discrepancy between eGFRCr and eGFRCys and explained his previous elevation in Cr in 2018.
Follow-up
The patient underwent serial monitoring that revealed a stable Cr and unremarkable eGFR, ruling out CKD. There has been no evidence of worsening kidney disease to date, and the patient remained on his initial HIV regimen.
Discussion
This case shows the importance of using CysC as an alternative or confirmatory marker compared with sCr to estimate GFR in patients with high muscle mass and/or high creatine intake, such as many in the US Department of Defense (DoD) and US Department of Veterans Affairs (VA) patient populations. In the presented case, recorded Cr levels climbed from baseline Cr with the initiation of bictegravir/emtricitabine/tenofovir alafenamide. This raised the concern that HIV treatment was leading to the development of kidney damage.22
Diagnosis of kidney disease as opposed to the normal decline of eGFR with age in individuals without intrinsic CKD requires GFR ≥ 60 mL/min/1.73 m2 with kidney damage (proteinuria or radiological abnormalities, etc) or GFR < 135 to 140 mL/min/1.73 m2 minus the patient’s age in years.23 The patient’s Cr peak at 1.83 mg/dL in 2018 led to an inappropriate diagnosis of kidney disease stage 3a based on an eGFRCr (2021 equation) of 52 mL/min/1.73 m2 when not corrected for body surface area.20 However, using the new 2021 equation using both Cr and CysC, the patient’s eGFRCr-Cys was 92 mL/min/1.73 m2 after a correction for body surface area.
The 2009 CKD-EPI recommended the calculation of eGFR based on SCr concentration using age, sex, and race while the 2021 CKD-EPI recommended the exclusion of race.3 Both equations are less accurate in African American patients, individuals taking medications that interfere with Cr secretion and assay, and patients taking creatine supplements, high daily protein intake, or with high muscle mass.7 These settings result in a decreased eGFRCr without corresponding eGFRCys changes. Using SCr and CysC together, the eGFRCr-Cys yields improved concordance to measured GFR across race groups compared to GFR estimation based on Cr alone, which can avoid unnecessary expensive diagnostic workup, inappropriate kidney disease diagnosis, incorrect dosing of drugs, and accurately represent the military readiness of patients. Interestingly, in African American patients with recently diagnosed HIV, CKD-EPI using both Cr and CysC without race inclusion led to only a 2.9% overestimation of GFR and was the only equation with no statistically significant bias compared with measured GFR.24
A March 2023 case involving an otherwise healthy 26-year-old male active-duty US Navy member with a history of excessive protein supplement intake and intense exercise < 24 hours before laboratory work was diagnosed with CKD after a measured Cr of 16 mg/dL and an eGFRCr of 4 mL/min/1.73 m2 without any other evidence of kidney disease. His CysC remained within normal limits, resulting in a normal eGFRCys of 121 mL/min/1.73 m2, indicating no CKD. His Cr and eGFR recovered 10 days after his clinic visit and cessation of his supplement intake. These findings may not be uncommon given that 65% of active-duty military use protein supplements and 38% use other performance-enhancing supplements, such as creatine, according to a study.25
Unfortunately, the BMP/CMP traditionally used at VA centers use the eGFRCr equation, and it is unknown how many primary care practitioners recognize the limitations of these metabolic panels on accurate estimation of kidney function. However, in 2022 an expert panel including VA physicians recommended the immediate use of eGFRCr-Cys or eGFRCys for confirmatory testing and potentially screening of CKD.26 A small number of VAs have since adopted this recommendation, which should lead to fewer misdiagnoses among US military members as clinicians should now have access to more accurate measurements of GFR.
The VA spends about $18 billion (excluding dialysis) for care for 1.1 to 2.5 million VA patients with CKD.27 The majority of these diagnoses were undoubtedly made using the eGFRCr equation, raising the question of how many may be misdiagnosed. Assessment with CysC is currently relatively expensive, but it will likely become more affordable as the use of CysC as a confirmatory test increases.5 The cost of a sCr test is about $2.50, while CysC costs about $10.60, with variation from laboratory to laboratory.28 By comparison, a renal ultrasound costs $99 to $140 for uninsured patients.29 Furthermore, the cost of CysC testing is likely to trend downward as more facilities adopt the use of CysC measurements, which can be run on the same analytical equipment currently used for Cr measurements. Currently, most laboratories do not have established assays to use in-house and thus require CysC to be sent out to a laboratory, which increases result time and makes Cr a more attractive option. As more laboratories adopt assays for CysC, the cost of reagents will further decrease.
Given such considerations, confirmation testing of kidney function with CysC in specific patient populations with decreased eGFRCr without other features of CKD can offer great medical and financial benefits. A 2023 KDIGO report noted that many individuals may be mistakenly diagnosed with CKD when using eGFRCr.3 KDIGO noted that a 2013 meta-analysis of 90,000 individuals found that with a Cr-based eGFR of 45 to 59 mL/min/1.73 m2 (42%) had a CysC-based eGFR of ≥ 60 mL/min/1.73 m2. An eGFRCr of 45 to 59 represents 54% of all patients with CKD, amounting to millions of people (including current and former military personnel).3,29-31 Correcting a misdiagnosis of CKD would bring significant relief to patients and save millions in health care spending.
Conclusions
In patients who meet CKD criteria using eGFRCr but without other features of CKD, we recommend using confirmatory CysC levels and the eGFRCr-Cys equation. This will align care with the KDIGO guidelines and could be a cost-effective step toward improving military patient care. Further work in this area should focus on determining the knowledge gaps in primary care practitioners’ understanding of the limits of eGFRCr, the potential mitigation of concomitant CysC testing in equivocal CKD cases, and the cost-effectiveness and increased utilization of CysC.
1. Gabriel R. Time to scrap creatinine clearance? Br Med J (Clin Res Ed). 1986;293(6555):1119-1120. doi:10.1136/bmj.293.6555.1119
2. Swan SK. The search continues—an ideal marker of GFR. Clin Chem. 1997;43(6):913-914.doi:10.1093/clinchem/43.6.913 3. KDIGO 2012 clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Int Suppl. 2013;3(1).
4. Wyss M, Kaddurah-Daouk R. Creatine and creatinine metabolism. Physiol Rev. 2000;80(3):1107-1213. doi:10.1152/physrev.2000.80.3.1107
5. Ferguson TW, Komenda P, Tangri N. Cystatin C as a biomarker for estimating glomerular filtration rate. Curr Opin Nephrol Hypertens. 2015;24(3):295-300. doi:10.1097/mnh.0000000000000115
6. Levey AS, Titan SM, Powe NR, Coresh J, Inker LA. Kidney disease, race, and GFR estimation. Clin J Am Soc Nephrol. 2020;15(8):1203-1212. doi:10.2215/cjn.12791019
7. Shlipak MG, Tummalapalli SL, Boulware LE, et al; Conference Participants. The case for early identification and intervention of chronic kidney disease: conclusions from a Kidney Disease: Improving Global Outcomes (KDIGO) controversies conference. Kidney Int. 2021;99(1):34-47. doi:10.1016/j.kint.2020.10.012
8. O’Riordan SE, Webb MC, Stowe HJ, et al. Cystatin C improves the detection of mild renal dysfunction in older patients. Ann Clin Biochem. 2003;40(pt 6):648-655. doi:10.1258/000456303770367243
9. Stevens LA, Schmid CH, Greene T, et al. Factors other than glomerular filtration rate affect serum cystatin C levels. Kidney Int. 2009;75(6):652-660. doi:10.1038/ki.2008.638
10. Levey AS, Coresh J, Greene T, et al; Chronic Kidney Disease Epidemiology Collaboration. Using standardized serum creatinine values in the modification of diet in renal disease study equation for estimating glomerular filtration rate. Ann Intern Med. 2006;145(4):247-254. doi:10.7326/0003-4819-145-4-200608150-00004
11. Levey AS, Stevens LA, Schmid CH, et al; Chronic Kidney Disease Epidemiology Collaboration. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150(9):604-612. doi:10.7326/0003-4819-150-9-200905050-00006
12. Pöge U, Gerhardt T, Stoffel-Wagner B, Klehr HU, Sauerbruch T, Woitas RP. Calculation of glomerular filtration rate based on cystatin C in cirrhotic patients. Nephrol Dial Transplant. 2006;21(3):660-664. doi:10.1093/ndt/gfi305
13. Larsson A, Malm J, Grubb A, Hansson LO. Calculation of glomerular filtration rate expressed in mL/min from plasma cystatin C values in mg/L. Scand J Clin Lab Invest. 2004;64(1):25-30. doi:10.1080/00365510410003723.
14. Macisaac RJ, Tsalamandris C, Thomas MC, et al. Estimating glomerular filtration rate in diabetes: a comparison of cystatin-C- and creatinine-based methods. Diabetologia. 2006;49(7):1686-1689. doi:10.1007/s00125-006-0275-7
15. Rule AD, Bergstralh EJ, Slezak JM, Bergert J, Larson TS. Glomerular filtration rate estimated by cystatin C among different clinical presentations. Kidney Int. 2006;69(2):399-405. doi:10.1038/sj.ki.5000073
16. Inker LA, Schmid CH, Tighiouart H, et al; Chronic Kidney Disease Epidemiology Collaboration Investigators. Estimating glomerular filtration rate from serum creatinine and cystatin C. N Engl J Med. 2012;367(1):20-29. doi:10.1056/NEJMoa1114248
17. Shlipak MG, Matsushita K, Ärnlöv J, et al; CKD Prognosis Consortium. Cystatin C versus creatinine in determining risk based on kidney function. N Engl J Med. 2013;369(10):932-943. doi:10.1056/NEJMoa1214234
18. Inker LA, Eneanya ND, Coresh J, et al; Chronic Kidney Disease Epidemiology Collaboration. New creatinine- and cystatin C–Based equations to estimate GFR without race. N Engl J Med. 2021;385(19):1737-1749. doi:10.1056/NEJMoa2102953
19. Oterdoom LH, Gansevoort RT, Schouten JP, de Jong PE, Gans ROB, Bakker SJL. Urinary creatinine excretion, an indirect measure of muscle mass, is an independent predictor of cardiovascular disease and mortality in the general population. Atherosclerosis. 2009;207(2):534-540. doi.10.1016/j.atherosclerosis.2009.05.010
20. National Kidney Foundation Inc. eGFR calculator. Accessed October 20, 2023. https://www.kidney.org/professionals/kdoqi/gfr_calculator
21. Ueaphongsukkit T, Gatechompol S, Avihingsanon A, et al. Tenofovir alafenamide nephrotoxicity: a case report and literature review. AIDS Res Ther. 2021;18(1):53. doi:10.1186/s12981-021-00380-w
22. D’Agati V, Appel GB. Renal pathology of human immunodeficiency virus infection. Semin Nephrol. 1998;18(4):406-421.
23. Glassock RJ, Winearls C. Ageing and the glomerular filtration rate: truths and consequences. Trans Am Clin Climatol Assoc. 2009;120:419-428.
24. Seape T, Gounden V, van Deventer HE, Candy GP, George JA. Cystatin C- and creatinine-based equations in the assessment of renal function in HIV-positive patients prior to commencing highly active antiretroviral therapy. Ann Clin Biochem. 2016;53(pt 1):58-66. doi:10.1177/0004563215579695
25. Tobin TW, Thurlow JS, Yuan CM. A healthy active duty soldier with an elevated serum creatinine. Mil Med. 2023;188(3-4):e866-e869. doi:10.1093/milmed/usab163
26. Delgado C, Baweja M, Crews DC, et al. A unifying approach for GFR estimation: recommendations of the NKF-ASN Task Force on Reassessing the Inclusion of Race in Diagnosing Kidney Disease. Am J Kidney Dis. 2022;79(2):268-288.e1. doi:10.1053/j.ajkd.2021.08.003
27. Saran R, Pearson A, Tilea A, et al; VA-REINS Steering Committee; VA Advisory Board. Burden and cost of caring for us veterans with CKD: initial findings from the VA Renal Information System (VA-REINS). Am J Kidney Dis. 2021;77(3):397-405. doi:10.1053/j.ajkd.2020.07.013
28. Zoler ML. Nephrologists make the case for cystatin C-based eGFR. Accessed October 20, 2023. https://www.medscape.com/viewarticle/951335#vp_2
29. Versaw N. How much does an ultrasound cost? Updated February 2022. Accessed October 20, 2023. https://www.compare.com/health/healthcare-resources/how-much-does-an-ultrasound-cost
30. Levey AS, Coresh J. Chronic kidney disease. Lancet. 2012;379(9811):165-180. doi:10.1016/S0140-6736(11)60178-5
31. Shlipak MG, Matsushita K, Ärnlöv J, et al; CKD Prognosis Consortium. Cystatin C versus creatinine in determining risk based on kidney function. N Engl J Med. 2013;369(10):932-943. doi:10.1056/NEJMoa1214234
Clinicians usually measure renal function by using surrogate markers because directly measuring glomerular filtration rate (GFR) is not routinely feasible in a clinical setting.1,2 Creatinine (Cr) and cystatin C (CysC) are the 2 main surrogate molecules used to estimate GFR.3
Creatine is a molecule nonenzymatically converted into Cr, weighing only 113 Da in skeletal muscles.4 It is then filtered at the glomeruli and secreted at the proximal tubules of the kidneys. However, serum Cr (sCr) levels are affected by several factors, including age, biological sex, liver function, diet, and muscle mass.5 Historically, sCr levels also are affected by race.5 In an early study of factors affecting accurate GFR, researchers reported that self-identified African American patients had a 16% higher GFR than those who did not when using Cr.6 Despite this, the inclusion of Cr on a basic metabolic panel has allowed automatic reporting of an estimated GFR using sCr (eGFRCr) to be readily available.7
In comparison to Cr, CysC is an endogenous protein weighing 13 kDa produced by all nucleated cells.8,9 CysC is filtered by the kidney at the glomeruli and completely reabsorbed and catabolized by epithelial cells at the proximal tubule.9 Since production is not dependent on skeletal muscle, there are fewer physiological impacts on serum concentration of CysC. Levels of CysC may be elevated by factors shown in the Table.
Estimating Glomerular Filtration Rates
Multiple equations were developed to mitigate the impact of extraneous factors on the accuracy of an eGFRCr. The first widely used equation that included a variable adjustment for race was the Modification of Diet in Renal Disease study, presented in 2006.10 The equation increased the accuracy of eGFRCr further by adjusting for sex and age. It was followed by the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation in 2009, which was more accurate at higher GFR levels.11
CysC was simultaneously studied as an alternative to Cr with multiple equation iterations shown to be viable in various populations as early as 2003.12-15 However, it was not until 2012 that an equation for the use of CysC was offered for widespread use as an alternative to Cr alongside further refinement of the CKD-EPI equation for Cr.16 A new formula was presented in 2021 to use both sCr and serum CysC levels to obtain a more accurate estimation of GFR.17 Research continues its effort to accurately estimate GFR for diagnosing kidney disease and assessing comorbidities relating to decreased kidney function.3
All historical equations attempted to mitigate the potential impact of race on sCr level when calculating eGFRCr by assigning a separate variable for African American patients. As an unintended adverse effect, these equations may have led to discrimination by having a different equation for African American patients.18 Moreover, these Cr-based equations remain less accurate in patients with varied muscle mass, such as older patients, bodybuilders, athletes, and individuals with varied extremes of daily protein intake.1,8,9,19 Several medications can also directly affect Cr clearance, reducing its ability to act as a surrogate for kidney function.1 In this case report, we discuss an African American patient with high muscle mass and protein intake who was initially diagnosed with kidney disease based on an elevated Cr and found to be misdiagnosed based on the use of CysC for a more accurate GFR estimation.
Case Presentation
A 35-year-old African American man serving in the military and recently diagnosed with HIV was referred to a nephrology clinic for further evaluation of an acute elevation in sCr. Before treatment for HIV, a brief record review showed a baseline Cr of about 1.3 mg/dL, with an eGFRCr of 75 mL/min/1.73 m2.20 In the same month, the patient was prescribed bictegravir/emtricitabine/tenofovir alafenamide, an HIV drug with nephrotoxic potential.21 The patient's total viral load remained low, and CD4 count remained > 500 after initiation of the HIV treatment. He was in his normal state of health and had no known contributory history before his HIV diagnosis. Cr readings peaked at 1.83 mg/dL after starting the HIV treatment and remained elevated to 1.73 mg/dL over the next few months, corresponding to CKD stage 3A. Because bictegravir/emtricitabine/tenofovir alafenamide is cleared by the kidneys and has a nephrotoxic profile, the clinical care team considered dosage adjustment or a medication switch given his observed elevated eGFRCr based on the CKD-EPI 2021 equation for Cr alone. It was also noted that the patient had a similar Cr spike to 1.83 mg/dL in 2018 without any identifiable renal insult or symptoms (Figure).
Diagnostic Evaluation
The primary care team ordered a renal ultrasound and referred the patient to the nephrology clinic. The nephrologist ordered the following laboratory studies: urine microalbumin to Cr ratio, basic metabolic panel (BMP), comprehensive metabolic panel (CMP), urinalysis, urine protein, urine Cr, parathyroid hormone level, hemoglobin A1c, complement component 3/4 panels, antinuclear and antineutrophil cytoplasmic antibodies titers, glomerular basement membrane antibody titer, urine light chains, serum protein electrophoresis, κ/λ ratio, viral hepatitis panel, and rapid plasma reagin testing. Much of this laboratory evaluation served to rule out any secondary causes of kidney disease, including autoimmune disease, monoclonal or polyclonal gammopathies, diabetic nephropathy or glomerulosclerosis, and nephrotic or nephritic syndromes.
All laboratory studies returned within normal limits; no proteinuria was discovered on urinalysis, and no abnormalities were visualized on renal ultrasound. Bictegravir/emtricitabine/tenofovir alafenamide nephrotoxicity was highest among the differential diagnoses due to the timing of Cr elevation coinciding with the initiation of the medications. The patient's CysC level was 0.85 mg/dL with a calculated eGFRCys of 125 mL/min/1.73 m2. The calculated sCR and serum cystatin C (eGFRCr-Cys) using the new 2021 equation and when adjusting for body surface area placed his eGFR at 92 mL/min/1.73 m2.20
The patient’s eGFRCys reassured the care team that the patient’s renal function was not acutely or chronically impacted by bictegravir/emtricitabine/tenofovir alafenamide, resulting in avoidance of unnecessary dosage adjustment or discontinuation of the HIV treatment. The patient reported a chronic habit of protein and creatine supplementation and bodybuilding, which likely further compounded the discrepancy between eGFRCr and eGFRCys and explained his previous elevation in Cr in 2018.
Follow-up
The patient underwent serial monitoring that revealed a stable Cr and unremarkable eGFR, ruling out CKD. There has been no evidence of worsening kidney disease to date, and the patient remained on his initial HIV regimen.
Discussion
This case shows the importance of using CysC as an alternative or confirmatory marker compared with sCr to estimate GFR in patients with high muscle mass and/or high creatine intake, such as many in the US Department of Defense (DoD) and US Department of Veterans Affairs (VA) patient populations. In the presented case, recorded Cr levels climbed from baseline Cr with the initiation of bictegravir/emtricitabine/tenofovir alafenamide. This raised the concern that HIV treatment was leading to the development of kidney damage.22
Diagnosis of kidney disease as opposed to the normal decline of eGFR with age in individuals without intrinsic CKD requires GFR ≥ 60 mL/min/1.73 m2 with kidney damage (proteinuria or radiological abnormalities, etc) or GFR < 135 to 140 mL/min/1.73 m2 minus the patient’s age in years.23 The patient’s Cr peak at 1.83 mg/dL in 2018 led to an inappropriate diagnosis of kidney disease stage 3a based on an eGFRCr (2021 equation) of 52 mL/min/1.73 m2 when not corrected for body surface area.20 However, using the new 2021 equation using both Cr and CysC, the patient’s eGFRCr-Cys was 92 mL/min/1.73 m2 after a correction for body surface area.
The 2009 CKD-EPI recommended the calculation of eGFR based on SCr concentration using age, sex, and race while the 2021 CKD-EPI recommended the exclusion of race.3 Both equations are less accurate in African American patients, individuals taking medications that interfere with Cr secretion and assay, and patients taking creatine supplements, high daily protein intake, or with high muscle mass.7 These settings result in a decreased eGFRCr without corresponding eGFRCys changes. Using SCr and CysC together, the eGFRCr-Cys yields improved concordance to measured GFR across race groups compared to GFR estimation based on Cr alone, which can avoid unnecessary expensive diagnostic workup, inappropriate kidney disease diagnosis, incorrect dosing of drugs, and accurately represent the military readiness of patients. Interestingly, in African American patients with recently diagnosed HIV, CKD-EPI using both Cr and CysC without race inclusion led to only a 2.9% overestimation of GFR and was the only equation with no statistically significant bias compared with measured GFR.24
A March 2023 case involving an otherwise healthy 26-year-old male active-duty US Navy member with a history of excessive protein supplement intake and intense exercise < 24 hours before laboratory work was diagnosed with CKD after a measured Cr of 16 mg/dL and an eGFRCr of 4 mL/min/1.73 m2 without any other evidence of kidney disease. His CysC remained within normal limits, resulting in a normal eGFRCys of 121 mL/min/1.73 m2, indicating no CKD. His Cr and eGFR recovered 10 days after his clinic visit and cessation of his supplement intake. These findings may not be uncommon given that 65% of active-duty military use protein supplements and 38% use other performance-enhancing supplements, such as creatine, according to a study.25
Unfortunately, the BMP/CMP traditionally used at VA centers use the eGFRCr equation, and it is unknown how many primary care practitioners recognize the limitations of these metabolic panels on accurate estimation of kidney function. However, in 2022 an expert panel including VA physicians recommended the immediate use of eGFRCr-Cys or eGFRCys for confirmatory testing and potentially screening of CKD.26 A small number of VAs have since adopted this recommendation, which should lead to fewer misdiagnoses among US military members as clinicians should now have access to more accurate measurements of GFR.
The VA spends about $18 billion (excluding dialysis) for care for 1.1 to 2.5 million VA patients with CKD.27 The majority of these diagnoses were undoubtedly made using the eGFRCr equation, raising the question of how many may be misdiagnosed. Assessment with CysC is currently relatively expensive, but it will likely become more affordable as the use of CysC as a confirmatory test increases.5 The cost of a sCr test is about $2.50, while CysC costs about $10.60, with variation from laboratory to laboratory.28 By comparison, a renal ultrasound costs $99 to $140 for uninsured patients.29 Furthermore, the cost of CysC testing is likely to trend downward as more facilities adopt the use of CysC measurements, which can be run on the same analytical equipment currently used for Cr measurements. Currently, most laboratories do not have established assays to use in-house and thus require CysC to be sent out to a laboratory, which increases result time and makes Cr a more attractive option. As more laboratories adopt assays for CysC, the cost of reagents will further decrease.
Given such considerations, confirmation testing of kidney function with CysC in specific patient populations with decreased eGFRCr without other features of CKD can offer great medical and financial benefits. A 2023 KDIGO report noted that many individuals may be mistakenly diagnosed with CKD when using eGFRCr.3 KDIGO noted that a 2013 meta-analysis of 90,000 individuals found that with a Cr-based eGFR of 45 to 59 mL/min/1.73 m2 (42%) had a CysC-based eGFR of ≥ 60 mL/min/1.73 m2. An eGFRCr of 45 to 59 represents 54% of all patients with CKD, amounting to millions of people (including current and former military personnel).3,29-31 Correcting a misdiagnosis of CKD would bring significant relief to patients and save millions in health care spending.
Conclusions
In patients who meet CKD criteria using eGFRCr but without other features of CKD, we recommend using confirmatory CysC levels and the eGFRCr-Cys equation. This will align care with the KDIGO guidelines and could be a cost-effective step toward improving military patient care. Further work in this area should focus on determining the knowledge gaps in primary care practitioners’ understanding of the limits of eGFRCr, the potential mitigation of concomitant CysC testing in equivocal CKD cases, and the cost-effectiveness and increased utilization of CysC.
Clinicians usually measure renal function by using surrogate markers because directly measuring glomerular filtration rate (GFR) is not routinely feasible in a clinical setting.1,2 Creatinine (Cr) and cystatin C (CysC) are the 2 main surrogate molecules used to estimate GFR.3
Creatine is a molecule nonenzymatically converted into Cr, weighing only 113 Da in skeletal muscles.4 It is then filtered at the glomeruli and secreted at the proximal tubules of the kidneys. However, serum Cr (sCr) levels are affected by several factors, including age, biological sex, liver function, diet, and muscle mass.5 Historically, sCr levels also are affected by race.5 In an early study of factors affecting accurate GFR, researchers reported that self-identified African American patients had a 16% higher GFR than those who did not when using Cr.6 Despite this, the inclusion of Cr on a basic metabolic panel has allowed automatic reporting of an estimated GFR using sCr (eGFRCr) to be readily available.7
In comparison to Cr, CysC is an endogenous protein weighing 13 kDa produced by all nucleated cells.8,9 CysC is filtered by the kidney at the glomeruli and completely reabsorbed and catabolized by epithelial cells at the proximal tubule.9 Since production is not dependent on skeletal muscle, there are fewer physiological impacts on serum concentration of CysC. Levels of CysC may be elevated by factors shown in the Table.
Estimating Glomerular Filtration Rates
Multiple equations were developed to mitigate the impact of extraneous factors on the accuracy of an eGFRCr. The first widely used equation that included a variable adjustment for race was the Modification of Diet in Renal Disease study, presented in 2006.10 The equation increased the accuracy of eGFRCr further by adjusting for sex and age. It was followed by the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation in 2009, which was more accurate at higher GFR levels.11
CysC was simultaneously studied as an alternative to Cr with multiple equation iterations shown to be viable in various populations as early as 2003.12-15 However, it was not until 2012 that an equation for the use of CysC was offered for widespread use as an alternative to Cr alongside further refinement of the CKD-EPI equation for Cr.16 A new formula was presented in 2021 to use both sCr and serum CysC levels to obtain a more accurate estimation of GFR.17 Research continues its effort to accurately estimate GFR for diagnosing kidney disease and assessing comorbidities relating to decreased kidney function.3
All historical equations attempted to mitigate the potential impact of race on sCr level when calculating eGFRCr by assigning a separate variable for African American patients. As an unintended adverse effect, these equations may have led to discrimination by having a different equation for African American patients.18 Moreover, these Cr-based equations remain less accurate in patients with varied muscle mass, such as older patients, bodybuilders, athletes, and individuals with varied extremes of daily protein intake.1,8,9,19 Several medications can also directly affect Cr clearance, reducing its ability to act as a surrogate for kidney function.1 In this case report, we discuss an African American patient with high muscle mass and protein intake who was initially diagnosed with kidney disease based on an elevated Cr and found to be misdiagnosed based on the use of CysC for a more accurate GFR estimation.
Case Presentation
A 35-year-old African American man serving in the military and recently diagnosed with HIV was referred to a nephrology clinic for further evaluation of an acute elevation in sCr. Before treatment for HIV, a brief record review showed a baseline Cr of about 1.3 mg/dL, with an eGFRCr of 75 mL/min/1.73 m2.20 In the same month, the patient was prescribed bictegravir/emtricitabine/tenofovir alafenamide, an HIV drug with nephrotoxic potential.21 The patient's total viral load remained low, and CD4 count remained > 500 after initiation of the HIV treatment. He was in his normal state of health and had no known contributory history before his HIV diagnosis. Cr readings peaked at 1.83 mg/dL after starting the HIV treatment and remained elevated to 1.73 mg/dL over the next few months, corresponding to CKD stage 3A. Because bictegravir/emtricitabine/tenofovir alafenamide is cleared by the kidneys and has a nephrotoxic profile, the clinical care team considered dosage adjustment or a medication switch given his observed elevated eGFRCr based on the CKD-EPI 2021 equation for Cr alone. It was also noted that the patient had a similar Cr spike to 1.83 mg/dL in 2018 without any identifiable renal insult or symptoms (Figure).
Diagnostic Evaluation
The primary care team ordered a renal ultrasound and referred the patient to the nephrology clinic. The nephrologist ordered the following laboratory studies: urine microalbumin to Cr ratio, basic metabolic panel (BMP), comprehensive metabolic panel (CMP), urinalysis, urine protein, urine Cr, parathyroid hormone level, hemoglobin A1c, complement component 3/4 panels, antinuclear and antineutrophil cytoplasmic antibodies titers, glomerular basement membrane antibody titer, urine light chains, serum protein electrophoresis, κ/λ ratio, viral hepatitis panel, and rapid plasma reagin testing. Much of this laboratory evaluation served to rule out any secondary causes of kidney disease, including autoimmune disease, monoclonal or polyclonal gammopathies, diabetic nephropathy or glomerulosclerosis, and nephrotic or nephritic syndromes.
All laboratory studies returned within normal limits; no proteinuria was discovered on urinalysis, and no abnormalities were visualized on renal ultrasound. Bictegravir/emtricitabine/tenofovir alafenamide nephrotoxicity was highest among the differential diagnoses due to the timing of Cr elevation coinciding with the initiation of the medications. The patient's CysC level was 0.85 mg/dL with a calculated eGFRCys of 125 mL/min/1.73 m2. The calculated sCR and serum cystatin C (eGFRCr-Cys) using the new 2021 equation and when adjusting for body surface area placed his eGFR at 92 mL/min/1.73 m2.20
The patient’s eGFRCys reassured the care team that the patient’s renal function was not acutely or chronically impacted by bictegravir/emtricitabine/tenofovir alafenamide, resulting in avoidance of unnecessary dosage adjustment or discontinuation of the HIV treatment. The patient reported a chronic habit of protein and creatine supplementation and bodybuilding, which likely further compounded the discrepancy between eGFRCr and eGFRCys and explained his previous elevation in Cr in 2018.
Follow-up
The patient underwent serial monitoring that revealed a stable Cr and unremarkable eGFR, ruling out CKD. There has been no evidence of worsening kidney disease to date, and the patient remained on his initial HIV regimen.
Discussion
This case shows the importance of using CysC as an alternative or confirmatory marker compared with sCr to estimate GFR in patients with high muscle mass and/or high creatine intake, such as many in the US Department of Defense (DoD) and US Department of Veterans Affairs (VA) patient populations. In the presented case, recorded Cr levels climbed from baseline Cr with the initiation of bictegravir/emtricitabine/tenofovir alafenamide. This raised the concern that HIV treatment was leading to the development of kidney damage.22
Diagnosis of kidney disease as opposed to the normal decline of eGFR with age in individuals without intrinsic CKD requires GFR ≥ 60 mL/min/1.73 m2 with kidney damage (proteinuria or radiological abnormalities, etc) or GFR < 135 to 140 mL/min/1.73 m2 minus the patient’s age in years.23 The patient’s Cr peak at 1.83 mg/dL in 2018 led to an inappropriate diagnosis of kidney disease stage 3a based on an eGFRCr (2021 equation) of 52 mL/min/1.73 m2 when not corrected for body surface area.20 However, using the new 2021 equation using both Cr and CysC, the patient’s eGFRCr-Cys was 92 mL/min/1.73 m2 after a correction for body surface area.
The 2009 CKD-EPI recommended the calculation of eGFR based on SCr concentration using age, sex, and race while the 2021 CKD-EPI recommended the exclusion of race.3 Both equations are less accurate in African American patients, individuals taking medications that interfere with Cr secretion and assay, and patients taking creatine supplements, high daily protein intake, or with high muscle mass.7 These settings result in a decreased eGFRCr without corresponding eGFRCys changes. Using SCr and CysC together, the eGFRCr-Cys yields improved concordance to measured GFR across race groups compared to GFR estimation based on Cr alone, which can avoid unnecessary expensive diagnostic workup, inappropriate kidney disease diagnosis, incorrect dosing of drugs, and accurately represent the military readiness of patients. Interestingly, in African American patients with recently diagnosed HIV, CKD-EPI using both Cr and CysC without race inclusion led to only a 2.9% overestimation of GFR and was the only equation with no statistically significant bias compared with measured GFR.24
A March 2023 case involving an otherwise healthy 26-year-old male active-duty US Navy member with a history of excessive protein supplement intake and intense exercise < 24 hours before laboratory work was diagnosed with CKD after a measured Cr of 16 mg/dL and an eGFRCr of 4 mL/min/1.73 m2 without any other evidence of kidney disease. His CysC remained within normal limits, resulting in a normal eGFRCys of 121 mL/min/1.73 m2, indicating no CKD. His Cr and eGFR recovered 10 days after his clinic visit and cessation of his supplement intake. These findings may not be uncommon given that 65% of active-duty military use protein supplements and 38% use other performance-enhancing supplements, such as creatine, according to a study.25
Unfortunately, the BMP/CMP traditionally used at VA centers use the eGFRCr equation, and it is unknown how many primary care practitioners recognize the limitations of these metabolic panels on accurate estimation of kidney function. However, in 2022 an expert panel including VA physicians recommended the immediate use of eGFRCr-Cys or eGFRCys for confirmatory testing and potentially screening of CKD.26 A small number of VAs have since adopted this recommendation, which should lead to fewer misdiagnoses among US military members as clinicians should now have access to more accurate measurements of GFR.
The VA spends about $18 billion (excluding dialysis) for care for 1.1 to 2.5 million VA patients with CKD.27 The majority of these diagnoses were undoubtedly made using the eGFRCr equation, raising the question of how many may be misdiagnosed. Assessment with CysC is currently relatively expensive, but it will likely become more affordable as the use of CysC as a confirmatory test increases.5 The cost of a sCr test is about $2.50, while CysC costs about $10.60, with variation from laboratory to laboratory.28 By comparison, a renal ultrasound costs $99 to $140 for uninsured patients.29 Furthermore, the cost of CysC testing is likely to trend downward as more facilities adopt the use of CysC measurements, which can be run on the same analytical equipment currently used for Cr measurements. Currently, most laboratories do not have established assays to use in-house and thus require CysC to be sent out to a laboratory, which increases result time and makes Cr a more attractive option. As more laboratories adopt assays for CysC, the cost of reagents will further decrease.
Given such considerations, confirmation testing of kidney function with CysC in specific patient populations with decreased eGFRCr without other features of CKD can offer great medical and financial benefits. A 2023 KDIGO report noted that many individuals may be mistakenly diagnosed with CKD when using eGFRCr.3 KDIGO noted that a 2013 meta-analysis of 90,000 individuals found that with a Cr-based eGFR of 45 to 59 mL/min/1.73 m2 (42%) had a CysC-based eGFR of ≥ 60 mL/min/1.73 m2. An eGFRCr of 45 to 59 represents 54% of all patients with CKD, amounting to millions of people (including current and former military personnel).3,29-31 Correcting a misdiagnosis of CKD would bring significant relief to patients and save millions in health care spending.
Conclusions
In patients who meet CKD criteria using eGFRCr but without other features of CKD, we recommend using confirmatory CysC levels and the eGFRCr-Cys equation. This will align care with the KDIGO guidelines and could be a cost-effective step toward improving military patient care. Further work in this area should focus on determining the knowledge gaps in primary care practitioners’ understanding of the limits of eGFRCr, the potential mitigation of concomitant CysC testing in equivocal CKD cases, and the cost-effectiveness and increased utilization of CysC.
1. Gabriel R. Time to scrap creatinine clearance? Br Med J (Clin Res Ed). 1986;293(6555):1119-1120. doi:10.1136/bmj.293.6555.1119
2. Swan SK. The search continues—an ideal marker of GFR. Clin Chem. 1997;43(6):913-914.doi:10.1093/clinchem/43.6.913 3. KDIGO 2012 clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Int Suppl. 2013;3(1).
4. Wyss M, Kaddurah-Daouk R. Creatine and creatinine metabolism. Physiol Rev. 2000;80(3):1107-1213. doi:10.1152/physrev.2000.80.3.1107
5. Ferguson TW, Komenda P, Tangri N. Cystatin C as a biomarker for estimating glomerular filtration rate. Curr Opin Nephrol Hypertens. 2015;24(3):295-300. doi:10.1097/mnh.0000000000000115
6. Levey AS, Titan SM, Powe NR, Coresh J, Inker LA. Kidney disease, race, and GFR estimation. Clin J Am Soc Nephrol. 2020;15(8):1203-1212. doi:10.2215/cjn.12791019
7. Shlipak MG, Tummalapalli SL, Boulware LE, et al; Conference Participants. The case for early identification and intervention of chronic kidney disease: conclusions from a Kidney Disease: Improving Global Outcomes (KDIGO) controversies conference. Kidney Int. 2021;99(1):34-47. doi:10.1016/j.kint.2020.10.012
8. O’Riordan SE, Webb MC, Stowe HJ, et al. Cystatin C improves the detection of mild renal dysfunction in older patients. Ann Clin Biochem. 2003;40(pt 6):648-655. doi:10.1258/000456303770367243
9. Stevens LA, Schmid CH, Greene T, et al. Factors other than glomerular filtration rate affect serum cystatin C levels. Kidney Int. 2009;75(6):652-660. doi:10.1038/ki.2008.638
10. Levey AS, Coresh J, Greene T, et al; Chronic Kidney Disease Epidemiology Collaboration. Using standardized serum creatinine values in the modification of diet in renal disease study equation for estimating glomerular filtration rate. Ann Intern Med. 2006;145(4):247-254. doi:10.7326/0003-4819-145-4-200608150-00004
11. Levey AS, Stevens LA, Schmid CH, et al; Chronic Kidney Disease Epidemiology Collaboration. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150(9):604-612. doi:10.7326/0003-4819-150-9-200905050-00006
12. Pöge U, Gerhardt T, Stoffel-Wagner B, Klehr HU, Sauerbruch T, Woitas RP. Calculation of glomerular filtration rate based on cystatin C in cirrhotic patients. Nephrol Dial Transplant. 2006;21(3):660-664. doi:10.1093/ndt/gfi305
13. Larsson A, Malm J, Grubb A, Hansson LO. Calculation of glomerular filtration rate expressed in mL/min from plasma cystatin C values in mg/L. Scand J Clin Lab Invest. 2004;64(1):25-30. doi:10.1080/00365510410003723.
14. Macisaac RJ, Tsalamandris C, Thomas MC, et al. Estimating glomerular filtration rate in diabetes: a comparison of cystatin-C- and creatinine-based methods. Diabetologia. 2006;49(7):1686-1689. doi:10.1007/s00125-006-0275-7
15. Rule AD, Bergstralh EJ, Slezak JM, Bergert J, Larson TS. Glomerular filtration rate estimated by cystatin C among different clinical presentations. Kidney Int. 2006;69(2):399-405. doi:10.1038/sj.ki.5000073
16. Inker LA, Schmid CH, Tighiouart H, et al; Chronic Kidney Disease Epidemiology Collaboration Investigators. Estimating glomerular filtration rate from serum creatinine and cystatin C. N Engl J Med. 2012;367(1):20-29. doi:10.1056/NEJMoa1114248
17. Shlipak MG, Matsushita K, Ärnlöv J, et al; CKD Prognosis Consortium. Cystatin C versus creatinine in determining risk based on kidney function. N Engl J Med. 2013;369(10):932-943. doi:10.1056/NEJMoa1214234
18. Inker LA, Eneanya ND, Coresh J, et al; Chronic Kidney Disease Epidemiology Collaboration. New creatinine- and cystatin C–Based equations to estimate GFR without race. N Engl J Med. 2021;385(19):1737-1749. doi:10.1056/NEJMoa2102953
19. Oterdoom LH, Gansevoort RT, Schouten JP, de Jong PE, Gans ROB, Bakker SJL. Urinary creatinine excretion, an indirect measure of muscle mass, is an independent predictor of cardiovascular disease and mortality in the general population. Atherosclerosis. 2009;207(2):534-540. doi.10.1016/j.atherosclerosis.2009.05.010
20. National Kidney Foundation Inc. eGFR calculator. Accessed October 20, 2023. https://www.kidney.org/professionals/kdoqi/gfr_calculator
21. Ueaphongsukkit T, Gatechompol S, Avihingsanon A, et al. Tenofovir alafenamide nephrotoxicity: a case report and literature review. AIDS Res Ther. 2021;18(1):53. doi:10.1186/s12981-021-00380-w
22. D’Agati V, Appel GB. Renal pathology of human immunodeficiency virus infection. Semin Nephrol. 1998;18(4):406-421.
23. Glassock RJ, Winearls C. Ageing and the glomerular filtration rate: truths and consequences. Trans Am Clin Climatol Assoc. 2009;120:419-428.
24. Seape T, Gounden V, van Deventer HE, Candy GP, George JA. Cystatin C- and creatinine-based equations in the assessment of renal function in HIV-positive patients prior to commencing highly active antiretroviral therapy. Ann Clin Biochem. 2016;53(pt 1):58-66. doi:10.1177/0004563215579695
25. Tobin TW, Thurlow JS, Yuan CM. A healthy active duty soldier with an elevated serum creatinine. Mil Med. 2023;188(3-4):e866-e869. doi:10.1093/milmed/usab163
26. Delgado C, Baweja M, Crews DC, et al. A unifying approach for GFR estimation: recommendations of the NKF-ASN Task Force on Reassessing the Inclusion of Race in Diagnosing Kidney Disease. Am J Kidney Dis. 2022;79(2):268-288.e1. doi:10.1053/j.ajkd.2021.08.003
27. Saran R, Pearson A, Tilea A, et al; VA-REINS Steering Committee; VA Advisory Board. Burden and cost of caring for us veterans with CKD: initial findings from the VA Renal Information System (VA-REINS). Am J Kidney Dis. 2021;77(3):397-405. doi:10.1053/j.ajkd.2020.07.013
28. Zoler ML. Nephrologists make the case for cystatin C-based eGFR. Accessed October 20, 2023. https://www.medscape.com/viewarticle/951335#vp_2
29. Versaw N. How much does an ultrasound cost? Updated February 2022. Accessed October 20, 2023. https://www.compare.com/health/healthcare-resources/how-much-does-an-ultrasound-cost
30. Levey AS, Coresh J. Chronic kidney disease. Lancet. 2012;379(9811):165-180. doi:10.1016/S0140-6736(11)60178-5
31. Shlipak MG, Matsushita K, Ärnlöv J, et al; CKD Prognosis Consortium. Cystatin C versus creatinine in determining risk based on kidney function. N Engl J Med. 2013;369(10):932-943. doi:10.1056/NEJMoa1214234
1. Gabriel R. Time to scrap creatinine clearance? Br Med J (Clin Res Ed). 1986;293(6555):1119-1120. doi:10.1136/bmj.293.6555.1119
2. Swan SK. The search continues—an ideal marker of GFR. Clin Chem. 1997;43(6):913-914.doi:10.1093/clinchem/43.6.913 3. KDIGO 2012 clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Int Suppl. 2013;3(1).
4. Wyss M, Kaddurah-Daouk R. Creatine and creatinine metabolism. Physiol Rev. 2000;80(3):1107-1213. doi:10.1152/physrev.2000.80.3.1107
5. Ferguson TW, Komenda P, Tangri N. Cystatin C as a biomarker for estimating glomerular filtration rate. Curr Opin Nephrol Hypertens. 2015;24(3):295-300. doi:10.1097/mnh.0000000000000115
6. Levey AS, Titan SM, Powe NR, Coresh J, Inker LA. Kidney disease, race, and GFR estimation. Clin J Am Soc Nephrol. 2020;15(8):1203-1212. doi:10.2215/cjn.12791019
7. Shlipak MG, Tummalapalli SL, Boulware LE, et al; Conference Participants. The case for early identification and intervention of chronic kidney disease: conclusions from a Kidney Disease: Improving Global Outcomes (KDIGO) controversies conference. Kidney Int. 2021;99(1):34-47. doi:10.1016/j.kint.2020.10.012
8. O’Riordan SE, Webb MC, Stowe HJ, et al. Cystatin C improves the detection of mild renal dysfunction in older patients. Ann Clin Biochem. 2003;40(pt 6):648-655. doi:10.1258/000456303770367243
9. Stevens LA, Schmid CH, Greene T, et al. Factors other than glomerular filtration rate affect serum cystatin C levels. Kidney Int. 2009;75(6):652-660. doi:10.1038/ki.2008.638
10. Levey AS, Coresh J, Greene T, et al; Chronic Kidney Disease Epidemiology Collaboration. Using standardized serum creatinine values in the modification of diet in renal disease study equation for estimating glomerular filtration rate. Ann Intern Med. 2006;145(4):247-254. doi:10.7326/0003-4819-145-4-200608150-00004
11. Levey AS, Stevens LA, Schmid CH, et al; Chronic Kidney Disease Epidemiology Collaboration. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150(9):604-612. doi:10.7326/0003-4819-150-9-200905050-00006
12. Pöge U, Gerhardt T, Stoffel-Wagner B, Klehr HU, Sauerbruch T, Woitas RP. Calculation of glomerular filtration rate based on cystatin C in cirrhotic patients. Nephrol Dial Transplant. 2006;21(3):660-664. doi:10.1093/ndt/gfi305
13. Larsson A, Malm J, Grubb A, Hansson LO. Calculation of glomerular filtration rate expressed in mL/min from plasma cystatin C values in mg/L. Scand J Clin Lab Invest. 2004;64(1):25-30. doi:10.1080/00365510410003723.
14. Macisaac RJ, Tsalamandris C, Thomas MC, et al. Estimating glomerular filtration rate in diabetes: a comparison of cystatin-C- and creatinine-based methods. Diabetologia. 2006;49(7):1686-1689. doi:10.1007/s00125-006-0275-7
15. Rule AD, Bergstralh EJ, Slezak JM, Bergert J, Larson TS. Glomerular filtration rate estimated by cystatin C among different clinical presentations. Kidney Int. 2006;69(2):399-405. doi:10.1038/sj.ki.5000073
16. Inker LA, Schmid CH, Tighiouart H, et al; Chronic Kidney Disease Epidemiology Collaboration Investigators. Estimating glomerular filtration rate from serum creatinine and cystatin C. N Engl J Med. 2012;367(1):20-29. doi:10.1056/NEJMoa1114248
17. Shlipak MG, Matsushita K, Ärnlöv J, et al; CKD Prognosis Consortium. Cystatin C versus creatinine in determining risk based on kidney function. N Engl J Med. 2013;369(10):932-943. doi:10.1056/NEJMoa1214234
18. Inker LA, Eneanya ND, Coresh J, et al; Chronic Kidney Disease Epidemiology Collaboration. New creatinine- and cystatin C–Based equations to estimate GFR without race. N Engl J Med. 2021;385(19):1737-1749. doi:10.1056/NEJMoa2102953
19. Oterdoom LH, Gansevoort RT, Schouten JP, de Jong PE, Gans ROB, Bakker SJL. Urinary creatinine excretion, an indirect measure of muscle mass, is an independent predictor of cardiovascular disease and mortality in the general population. Atherosclerosis. 2009;207(2):534-540. doi.10.1016/j.atherosclerosis.2009.05.010
20. National Kidney Foundation Inc. eGFR calculator. Accessed October 20, 2023. https://www.kidney.org/professionals/kdoqi/gfr_calculator
21. Ueaphongsukkit T, Gatechompol S, Avihingsanon A, et al. Tenofovir alafenamide nephrotoxicity: a case report and literature review. AIDS Res Ther. 2021;18(1):53. doi:10.1186/s12981-021-00380-w
22. D’Agati V, Appel GB. Renal pathology of human immunodeficiency virus infection. Semin Nephrol. 1998;18(4):406-421.
23. Glassock RJ, Winearls C. Ageing and the glomerular filtration rate: truths and consequences. Trans Am Clin Climatol Assoc. 2009;120:419-428.
24. Seape T, Gounden V, van Deventer HE, Candy GP, George JA. Cystatin C- and creatinine-based equations in the assessment of renal function in HIV-positive patients prior to commencing highly active antiretroviral therapy. Ann Clin Biochem. 2016;53(pt 1):58-66. doi:10.1177/0004563215579695
25. Tobin TW, Thurlow JS, Yuan CM. A healthy active duty soldier with an elevated serum creatinine. Mil Med. 2023;188(3-4):e866-e869. doi:10.1093/milmed/usab163
26. Delgado C, Baweja M, Crews DC, et al. A unifying approach for GFR estimation: recommendations of the NKF-ASN Task Force on Reassessing the Inclusion of Race in Diagnosing Kidney Disease. Am J Kidney Dis. 2022;79(2):268-288.e1. doi:10.1053/j.ajkd.2021.08.003
27. Saran R, Pearson A, Tilea A, et al; VA-REINS Steering Committee; VA Advisory Board. Burden and cost of caring for us veterans with CKD: initial findings from the VA Renal Information System (VA-REINS). Am J Kidney Dis. 2021;77(3):397-405. doi:10.1053/j.ajkd.2020.07.013
28. Zoler ML. Nephrologists make the case for cystatin C-based eGFR. Accessed October 20, 2023. https://www.medscape.com/viewarticle/951335#vp_2
29. Versaw N. How much does an ultrasound cost? Updated February 2022. Accessed October 20, 2023. https://www.compare.com/health/healthcare-resources/how-much-does-an-ultrasound-cost
30. Levey AS, Coresh J. Chronic kidney disease. Lancet. 2012;379(9811):165-180. doi:10.1016/S0140-6736(11)60178-5
31. Shlipak MG, Matsushita K, Ärnlöv J, et al; CKD Prognosis Consortium. Cystatin C versus creatinine in determining risk based on kidney function. N Engl J Med. 2013;369(10):932-943. doi:10.1056/NEJMoa1214234
Thiazide-Induced Hyponatremia Presenting as a Fall in an Older Adult
Hypertension is a major risk factor for heart disease, stroke, and kidney disease. The prevalence of hypertension increases with age, primarily due to age-related changes in arterial physiology.1 For older adults, current guidelines regarding blood pressure (BP) treatment goals vary. The American Heart Association/American College of Cardiology 2017 clinical practice guidelines recommend a systolic BP (SBP) treatment goal of < 130 mm Hg for community-dwelling, ambulatory, noninstitutionalized adults aged ≥ 65 years; whereas the American College of Physicians/American Academy of Family Physicians recommend a goal of < 150 mm Hg for those aged ≥ 60 years without comorbidities and < 140 mm Hg for those with increased cardiovascular risk.1-3 Regardless of the specific threshold, agreement that some degree of BP control even in those with advanced age improves outcomes.2
First-line therapy for uncomplicated hypertension includes thiazide diuretics, long-acting calcium channel blockers, and renin-angiotensin system inhibitors. When choosing between these options, it is recommended to engage in shared decision making and to consider the patient’s comorbidities. Among patients who are likely to require a second agent (eg, if initial BP is > 20/10 mm Hg above goal), it is recommended to begin both drugs at the same time, preferably benazepril plus amlodipine due to the reduction in cardiovascular events reported in the ACCOMPLISH trial.4 If BP remains elevated despite 2 agents at moderate to maximum doses, it is important to investigate for secondary hypertension causes and to explore medication adherence as possible etiologies of treatment failure. Older adults are often at higher risk of adverse drug events due to age-related changes in pharmacodynamics. Despite this, there are no guidelines for choosing between different classes of antihypertensives in this population. We present a case of thiazide-induced hyponatremia in an older adult and review the risks of thiazide use in this population.
Case Presentation
A man aged > 90 years was admitted to the hospital after a syncopal episode. His history was significant for hypertension, hyperlipidemia, and vitamin D deficiency. At the time, his home medications were amlodipine 5 mg daily, atorvastatin 40 mg daily, ergocalciferol 50,000 IU weekly, and polyethylene glycol 17 g daily as needed. His syncope workup was unremarkable and included negative orthostatic vital signs, normal serial troponins, an electrocardiogram without ischemic changes, normal serum creatinine, sodium, and glucose, and a head computed tomography without any acute abnormality. Throughout the patient’s hospital stay, he had multiple elevated SBP readings, including many > 200 mm Hg. On discharge, in addition to continuing his home medications, he was started on valsartan 20 mg daily and enrolled in a remote BP monitoring program.
Three weeks later, the patient was seen by their primary care practitioner for follow-up. He reported adherence to his antihypertensive regimen. However, his remote BP monitoring revealed persistently elevated BPs, with an average of 179/79 mm Hg, a high of 205/85 mm Hg, and a low of 150/67 mm Hg over the previous 7 days. Laboratory tests obtained at the visit were notable for serum sodium of 138 mmol/L and potassium of 4.1 mmol/L. His weight was 87 kg. Given persistently elevated BP readings, in addition to continuing his amlodipine 5 mg daily and valsartan 20 mg daily, he was started on hydrochlorothiazide 25 mg daily, with plans to repeat a basic metabolic panel in 2 weeks.
Two weeks later, he fell after getting out of his bed. On examination, he was noted to have dry mucous membranes, and although no formal delirium screening was performed, he was able to repeat the months of the year backward. Vital signs were notable for positive postural hypertension, and his laboratory tests revealed a normal serum creatinine, serum sodium of 117 mmol/L
Discussion
Although thiazide diuretics are recommended as first-line therapy for uncomplicated hypertension, they are known to cause electrolyte abnormalities, including hypomagnesemia, hypokalemia, and hyponatremia.4 These metabolic derangements are more likely to occur in older adults. One study of adults aged ≥ 65 years found that at 9 months of follow-up, 14.3% of new thiazide users had developed a thiazide-related metabolic adverse event (hyponatremia < 135 mmol/L, hypokalemia < 3.5 mmol/L, and decrease in estimated glomerular filtration rate by > 25%) compared with 6.0% of nonusers (P < .001; number needed to harm [NNH] = 12).5 In addition, 3.8% of new thiazide users had an emergency department visit or were hospitalized for complications related to thiazides compared with only 2.0% of nonusers (P = .02; NNH = 56).5 Independent risk factors for thiazide-induced hyponatremia include high-comorbidity burden, low body weight, low-normal or unmeasured serum sodium, low potassium, and aged > 70 years.5-7 Each 10-year increment in age is associated with a 2-fold increase in risk, suggesting that older adults are at a much higher risk for hyponatremia than their younger peers.6
Despite their designation as a first-line option for uncomplicated hypertension, thiazide diuretics may cause more harm than good in some older adults, especially those with additional risk factors for thiazide-induced hyponatremia. In this population, these adverse effects should be discussed before starting thiazides for the treatment of hypertension. If thiazides are initiated, they should be started at the lowest possible dose, and plans made to monitor bloodwork within 1 to 2 weeks of initiation or dose change and periodically thereafter while the patient remains on the therapy.
Medication Management in Older Adults
Due to the risks of medication use in older adults, the phrase “start low, go slow” is commonly used in geriatric medicine to describe the optimal method for initiation and up-titration of new medication with the hope of mitigating adverse drug events. In our case, we started valsartan at 20 mg daily—one-fourth the recommended initial dose. Although this strategy is reasonable to “start low,” we were not surprised to find that the patient’s BP did not markedly improve on such a low dose. The team could have increased the valsartan dose to a therapeutically efficacious dose before choosing to add another hypertensive agent. In alignment with geriatric prescribing principles, starting at the lowest possible dose of hydrochlorothiazide is recommended.5 However, the clinician started hydrochlorothiazide at 25 mg daily, potentially increasing this patient’s risk of electrolyte abnormalities and eventual fall.
Managing hypertension also invites a discussion of polypharmacy and medication adherence. Older adults are at risk of polypharmacy, defined as the prescription of 5 or more medications.8 Polypharmacy is associated with increased hospitalizations, higher costs of care for individuals and health care systems, increased risks of adverse drug events, medication nonadherence, and lower quality of life for patients.9 In some situations, the risks of polypharmacy may outweigh the benefits of using multiple antihypertensives with different mechanisms of action if patients can reach their BP goal on the maximum dose of a single agent. For patients taking multiple antihypertensives, it is important to routinely monitor BP and assess whether deprescribing is indicated. Cognitive impairment and decreased social support may affect medication adherence for older adults.6 Clinicians should be aware of strategies, such as medication reminders and pillboxes, to increase antihypertensive medication adherence. Polypills that contain 2 antihypertensives can be another tool used to manage older adults to increase adherence and decrease health care costs.10
A current strategy that encompasses discussing many, if not all, of these noted elements is the Institute for Healthcare Improvement’s Age-Friendly Health System. This framework uses evidence-based tools to provide care for older adults across all clinical settings and highlights the 4Ms: what matters, medication, mentation, and mobility.11 Medication considers whether a medication is necessary, whether its use has benefits that outweigh the risks, and how it interacts with what matters, mentation, and mobility. In particular, what matters plays an important role in hypertension management in older adults given the recommended target BP differs, depending on which specialty organization guideline is followed. By better understanding what matters to patients, including their goals and priorities, clinicians can engage patients in shared decision making and provide individualized recommendations based on geriatric principles (eg, start low, go slow, principles of medication adherence) and patient comorbidities (eg, medical history and risk factors for hyponatremia) to help patients make a more informed choice about their antihypertensive treatment regimen (Figure).
Conclusions
This case illustrates the need for a specialized approach to hypertension management in older adults and the risks of thiazide diuretics in this population. Clinicians should consider BP goals, patient-specific factors, and principles of medication management in older adults. If initiating thiazide therapy, discuss the risks associated with use, start at the lowest possible dose, and monitor bloodwork within 1 to 2 weeks of initiation/dose change and periodically thereafter while the patient remains on the therapy to decrease the risk of adverse events. Finally, the Institute for Healthcare Improvement’s Age-Friendly Health System framework can be a useful when considering the addition of a new medication in an older adult’s treatment plan.
Acknowledgments
This material is the result of work supported with resources and the use of facilities at the New England Geriatrics Research, Education, and Clinical Center, Veterans Affairs Boston Healthcare System, and the Cincinnati VeteransAffairs Medical Center.
1. Whelton PK, Carey RM, Aronow WS, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the Prevention, Detection, Evaluation and Management of High Blood Pressure in Adults: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol. 2018;71(19):e127-e248. doi:10.1016/j.jacc.2017.11.006
2. Davis LL. Hypertension: how low to go when treating older adults. J Nurse Pract. 2019;15(1):1-6. doi:10.1016/j.nurpra.2018.10.010
3. Qaseem A, Wilt TJ, Rich R, et al. Pharmacologic Treatment of Hypertension in Adults Aged 60 Years or Older to Higher Versus Lower Blood Pressure Targets: A Clinical Practice Guideline From the American College of Physicians and the American Academy of Family Physicians. Ann Intern Med. 2017;166(6):430-437. doi:10.7326/M16-1785
4. Aronow WS, Fleg JL, Pepine CJ, et al. ACCF/AHA 2011 expert consensus document on hypertension in the elderly: a report of the American College of Cardiology Foundation Task Force on Clinical Expert Consensus documents developed in collaboration with the American Academy of Neurology, American Geriatrics Society, American Society for Preventive Cardiology, American Society of Hypertension, American Society of Nephrology, Association of Black Cardiologists, and European Society of Hypertension. J Am Coll Cardiol. 2011;57(20):2037-2114. doi:10.1016/j.jacc.2011.01.008
5. Makam AN, Boscardin WJ, Miao Y, Steinman MA. Risk of thiazide-induced metabolic adverse events in older adults. J Am Geriatr Soc. 2014;62(6):1039-1045. doi:10.1111/jgs.12839
6. Chow KM, Szeto CC, Wong TY, Leung CB, Li PK. Risk factors for thiazide-induced hyponatraemia. QJM. 2003;96(12):911-917. doi:10.1093/qjmed/hcg157
7. Clayton JA, Rodgers S, Blakey J, Avery A, Hall IP. Thiazide diuretic prescription and electrolyte abnormalities in primary care. Br J Clin Pharmacol. 2006;61(1):87-95. doi:10.1111/j.1365-2125.2005.02531.x
8. Shah BM, Hajjar ER. Polypharmacy, adverse drug reactions, and geriatric syndromes. Clin Geriatr Med. 2012;28(2):173-186. doi:10.1016/j.cger.2012.01.002
9. Benetos A, Petrovic M, Strandberg T. Hypertension management in older and frail older patients. Circ Res. 2019;124(7):1045-1060. doi:10.1161/CIRCRESAHA.118.313236
10. Sherrill B, Halpern M, Khan S, Zhang J, Panjabi S. Single-pill vs free-equivalent combination therapies for hypertension: a meta-analysis of health care costs and adherence. J Clin Hypertens (Greenwich). 2011;13(12):898-909. doi:10.1111/j.1751-7176.2011.00550.x
11. Mate K, Fulmer T, Pelton L, et al. Evidence for the 4Ms: interactions and outcomes across the care continuum. J Aging Health. 2021;33(7-8):469-481. doi:10.1177/0898264321991658
Hypertension is a major risk factor for heart disease, stroke, and kidney disease. The prevalence of hypertension increases with age, primarily due to age-related changes in arterial physiology.1 For older adults, current guidelines regarding blood pressure (BP) treatment goals vary. The American Heart Association/American College of Cardiology 2017 clinical practice guidelines recommend a systolic BP (SBP) treatment goal of < 130 mm Hg for community-dwelling, ambulatory, noninstitutionalized adults aged ≥ 65 years; whereas the American College of Physicians/American Academy of Family Physicians recommend a goal of < 150 mm Hg for those aged ≥ 60 years without comorbidities and < 140 mm Hg for those with increased cardiovascular risk.1-3 Regardless of the specific threshold, agreement that some degree of BP control even in those with advanced age improves outcomes.2
First-line therapy for uncomplicated hypertension includes thiazide diuretics, long-acting calcium channel blockers, and renin-angiotensin system inhibitors. When choosing between these options, it is recommended to engage in shared decision making and to consider the patient’s comorbidities. Among patients who are likely to require a second agent (eg, if initial BP is > 20/10 mm Hg above goal), it is recommended to begin both drugs at the same time, preferably benazepril plus amlodipine due to the reduction in cardiovascular events reported in the ACCOMPLISH trial.4 If BP remains elevated despite 2 agents at moderate to maximum doses, it is important to investigate for secondary hypertension causes and to explore medication adherence as possible etiologies of treatment failure. Older adults are often at higher risk of adverse drug events due to age-related changes in pharmacodynamics. Despite this, there are no guidelines for choosing between different classes of antihypertensives in this population. We present a case of thiazide-induced hyponatremia in an older adult and review the risks of thiazide use in this population.
Case Presentation
A man aged > 90 years was admitted to the hospital after a syncopal episode. His history was significant for hypertension, hyperlipidemia, and vitamin D deficiency. At the time, his home medications were amlodipine 5 mg daily, atorvastatin 40 mg daily, ergocalciferol 50,000 IU weekly, and polyethylene glycol 17 g daily as needed. His syncope workup was unremarkable and included negative orthostatic vital signs, normal serial troponins, an electrocardiogram without ischemic changes, normal serum creatinine, sodium, and glucose, and a head computed tomography without any acute abnormality. Throughout the patient’s hospital stay, he had multiple elevated SBP readings, including many > 200 mm Hg. On discharge, in addition to continuing his home medications, he was started on valsartan 20 mg daily and enrolled in a remote BP monitoring program.
Three weeks later, the patient was seen by their primary care practitioner for follow-up. He reported adherence to his antihypertensive regimen. However, his remote BP monitoring revealed persistently elevated BPs, with an average of 179/79 mm Hg, a high of 205/85 mm Hg, and a low of 150/67 mm Hg over the previous 7 days. Laboratory tests obtained at the visit were notable for serum sodium of 138 mmol/L and potassium of 4.1 mmol/L. His weight was 87 kg. Given persistently elevated BP readings, in addition to continuing his amlodipine 5 mg daily and valsartan 20 mg daily, he was started on hydrochlorothiazide 25 mg daily, with plans to repeat a basic metabolic panel in 2 weeks.
Two weeks later, he fell after getting out of his bed. On examination, he was noted to have dry mucous membranes, and although no formal delirium screening was performed, he was able to repeat the months of the year backward. Vital signs were notable for positive postural hypertension, and his laboratory tests revealed a normal serum creatinine, serum sodium of 117 mmol/L
Discussion
Although thiazide diuretics are recommended as first-line therapy for uncomplicated hypertension, they are known to cause electrolyte abnormalities, including hypomagnesemia, hypokalemia, and hyponatremia.4 These metabolic derangements are more likely to occur in older adults. One study of adults aged ≥ 65 years found that at 9 months of follow-up, 14.3% of new thiazide users had developed a thiazide-related metabolic adverse event (hyponatremia < 135 mmol/L, hypokalemia < 3.5 mmol/L, and decrease in estimated glomerular filtration rate by > 25%) compared with 6.0% of nonusers (P < .001; number needed to harm [NNH] = 12).5 In addition, 3.8% of new thiazide users had an emergency department visit or were hospitalized for complications related to thiazides compared with only 2.0% of nonusers (P = .02; NNH = 56).5 Independent risk factors for thiazide-induced hyponatremia include high-comorbidity burden, low body weight, low-normal or unmeasured serum sodium, low potassium, and aged > 70 years.5-7 Each 10-year increment in age is associated with a 2-fold increase in risk, suggesting that older adults are at a much higher risk for hyponatremia than their younger peers.6
Despite their designation as a first-line option for uncomplicated hypertension, thiazide diuretics may cause more harm than good in some older adults, especially those with additional risk factors for thiazide-induced hyponatremia. In this population, these adverse effects should be discussed before starting thiazides for the treatment of hypertension. If thiazides are initiated, they should be started at the lowest possible dose, and plans made to monitor bloodwork within 1 to 2 weeks of initiation or dose change and periodically thereafter while the patient remains on the therapy.
Medication Management in Older Adults
Due to the risks of medication use in older adults, the phrase “start low, go slow” is commonly used in geriatric medicine to describe the optimal method for initiation and up-titration of new medication with the hope of mitigating adverse drug events. In our case, we started valsartan at 20 mg daily—one-fourth the recommended initial dose. Although this strategy is reasonable to “start low,” we were not surprised to find that the patient’s BP did not markedly improve on such a low dose. The team could have increased the valsartan dose to a therapeutically efficacious dose before choosing to add another hypertensive agent. In alignment with geriatric prescribing principles, starting at the lowest possible dose of hydrochlorothiazide is recommended.5 However, the clinician started hydrochlorothiazide at 25 mg daily, potentially increasing this patient’s risk of electrolyte abnormalities and eventual fall.
Managing hypertension also invites a discussion of polypharmacy and medication adherence. Older adults are at risk of polypharmacy, defined as the prescription of 5 or more medications.8 Polypharmacy is associated with increased hospitalizations, higher costs of care for individuals and health care systems, increased risks of adverse drug events, medication nonadherence, and lower quality of life for patients.9 In some situations, the risks of polypharmacy may outweigh the benefits of using multiple antihypertensives with different mechanisms of action if patients can reach their BP goal on the maximum dose of a single agent. For patients taking multiple antihypertensives, it is important to routinely monitor BP and assess whether deprescribing is indicated. Cognitive impairment and decreased social support may affect medication adherence for older adults.6 Clinicians should be aware of strategies, such as medication reminders and pillboxes, to increase antihypertensive medication adherence. Polypills that contain 2 antihypertensives can be another tool used to manage older adults to increase adherence and decrease health care costs.10
A current strategy that encompasses discussing many, if not all, of these noted elements is the Institute for Healthcare Improvement’s Age-Friendly Health System. This framework uses evidence-based tools to provide care for older adults across all clinical settings and highlights the 4Ms: what matters, medication, mentation, and mobility.11 Medication considers whether a medication is necessary, whether its use has benefits that outweigh the risks, and how it interacts with what matters, mentation, and mobility. In particular, what matters plays an important role in hypertension management in older adults given the recommended target BP differs, depending on which specialty organization guideline is followed. By better understanding what matters to patients, including their goals and priorities, clinicians can engage patients in shared decision making and provide individualized recommendations based on geriatric principles (eg, start low, go slow, principles of medication adherence) and patient comorbidities (eg, medical history and risk factors for hyponatremia) to help patients make a more informed choice about their antihypertensive treatment regimen (Figure).
Conclusions
This case illustrates the need for a specialized approach to hypertension management in older adults and the risks of thiazide diuretics in this population. Clinicians should consider BP goals, patient-specific factors, and principles of medication management in older adults. If initiating thiazide therapy, discuss the risks associated with use, start at the lowest possible dose, and monitor bloodwork within 1 to 2 weeks of initiation/dose change and periodically thereafter while the patient remains on the therapy to decrease the risk of adverse events. Finally, the Institute for Healthcare Improvement’s Age-Friendly Health System framework can be a useful when considering the addition of a new medication in an older adult’s treatment plan.
Acknowledgments
This material is the result of work supported with resources and the use of facilities at the New England Geriatrics Research, Education, and Clinical Center, Veterans Affairs Boston Healthcare System, and the Cincinnati VeteransAffairs Medical Center.
Hypertension is a major risk factor for heart disease, stroke, and kidney disease. The prevalence of hypertension increases with age, primarily due to age-related changes in arterial physiology.1 For older adults, current guidelines regarding blood pressure (BP) treatment goals vary. The American Heart Association/American College of Cardiology 2017 clinical practice guidelines recommend a systolic BP (SBP) treatment goal of < 130 mm Hg for community-dwelling, ambulatory, noninstitutionalized adults aged ≥ 65 years; whereas the American College of Physicians/American Academy of Family Physicians recommend a goal of < 150 mm Hg for those aged ≥ 60 years without comorbidities and < 140 mm Hg for those with increased cardiovascular risk.1-3 Regardless of the specific threshold, agreement that some degree of BP control even in those with advanced age improves outcomes.2
First-line therapy for uncomplicated hypertension includes thiazide diuretics, long-acting calcium channel blockers, and renin-angiotensin system inhibitors. When choosing between these options, it is recommended to engage in shared decision making and to consider the patient’s comorbidities. Among patients who are likely to require a second agent (eg, if initial BP is > 20/10 mm Hg above goal), it is recommended to begin both drugs at the same time, preferably benazepril plus amlodipine due to the reduction in cardiovascular events reported in the ACCOMPLISH trial.4 If BP remains elevated despite 2 agents at moderate to maximum doses, it is important to investigate for secondary hypertension causes and to explore medication adherence as possible etiologies of treatment failure. Older adults are often at higher risk of adverse drug events due to age-related changes in pharmacodynamics. Despite this, there are no guidelines for choosing between different classes of antihypertensives in this population. We present a case of thiazide-induced hyponatremia in an older adult and review the risks of thiazide use in this population.
Case Presentation
A man aged > 90 years was admitted to the hospital after a syncopal episode. His history was significant for hypertension, hyperlipidemia, and vitamin D deficiency. At the time, his home medications were amlodipine 5 mg daily, atorvastatin 40 mg daily, ergocalciferol 50,000 IU weekly, and polyethylene glycol 17 g daily as needed. His syncope workup was unremarkable and included negative orthostatic vital signs, normal serial troponins, an electrocardiogram without ischemic changes, normal serum creatinine, sodium, and glucose, and a head computed tomography without any acute abnormality. Throughout the patient’s hospital stay, he had multiple elevated SBP readings, including many > 200 mm Hg. On discharge, in addition to continuing his home medications, he was started on valsartan 20 mg daily and enrolled in a remote BP monitoring program.
Three weeks later, the patient was seen by their primary care practitioner for follow-up. He reported adherence to his antihypertensive regimen. However, his remote BP monitoring revealed persistently elevated BPs, with an average of 179/79 mm Hg, a high of 205/85 mm Hg, and a low of 150/67 mm Hg over the previous 7 days. Laboratory tests obtained at the visit were notable for serum sodium of 138 mmol/L and potassium of 4.1 mmol/L. His weight was 87 kg. Given persistently elevated BP readings, in addition to continuing his amlodipine 5 mg daily and valsartan 20 mg daily, he was started on hydrochlorothiazide 25 mg daily, with plans to repeat a basic metabolic panel in 2 weeks.
Two weeks later, he fell after getting out of his bed. On examination, he was noted to have dry mucous membranes, and although no formal delirium screening was performed, he was able to repeat the months of the year backward. Vital signs were notable for positive postural hypertension, and his laboratory tests revealed a normal serum creatinine, serum sodium of 117 mmol/L
Discussion
Although thiazide diuretics are recommended as first-line therapy for uncomplicated hypertension, they are known to cause electrolyte abnormalities, including hypomagnesemia, hypokalemia, and hyponatremia.4 These metabolic derangements are more likely to occur in older adults. One study of adults aged ≥ 65 years found that at 9 months of follow-up, 14.3% of new thiazide users had developed a thiazide-related metabolic adverse event (hyponatremia < 135 mmol/L, hypokalemia < 3.5 mmol/L, and decrease in estimated glomerular filtration rate by > 25%) compared with 6.0% of nonusers (P < .001; number needed to harm [NNH] = 12).5 In addition, 3.8% of new thiazide users had an emergency department visit or were hospitalized for complications related to thiazides compared with only 2.0% of nonusers (P = .02; NNH = 56).5 Independent risk factors for thiazide-induced hyponatremia include high-comorbidity burden, low body weight, low-normal or unmeasured serum sodium, low potassium, and aged > 70 years.5-7 Each 10-year increment in age is associated with a 2-fold increase in risk, suggesting that older adults are at a much higher risk for hyponatremia than their younger peers.6
Despite their designation as a first-line option for uncomplicated hypertension, thiazide diuretics may cause more harm than good in some older adults, especially those with additional risk factors for thiazide-induced hyponatremia. In this population, these adverse effects should be discussed before starting thiazides for the treatment of hypertension. If thiazides are initiated, they should be started at the lowest possible dose, and plans made to monitor bloodwork within 1 to 2 weeks of initiation or dose change and periodically thereafter while the patient remains on the therapy.
Medication Management in Older Adults
Due to the risks of medication use in older adults, the phrase “start low, go slow” is commonly used in geriatric medicine to describe the optimal method for initiation and up-titration of new medication with the hope of mitigating adverse drug events. In our case, we started valsartan at 20 mg daily—one-fourth the recommended initial dose. Although this strategy is reasonable to “start low,” we were not surprised to find that the patient’s BP did not markedly improve on such a low dose. The team could have increased the valsartan dose to a therapeutically efficacious dose before choosing to add another hypertensive agent. In alignment with geriatric prescribing principles, starting at the lowest possible dose of hydrochlorothiazide is recommended.5 However, the clinician started hydrochlorothiazide at 25 mg daily, potentially increasing this patient’s risk of electrolyte abnormalities and eventual fall.
Managing hypertension also invites a discussion of polypharmacy and medication adherence. Older adults are at risk of polypharmacy, defined as the prescription of 5 or more medications.8 Polypharmacy is associated with increased hospitalizations, higher costs of care for individuals and health care systems, increased risks of adverse drug events, medication nonadherence, and lower quality of life for patients.9 In some situations, the risks of polypharmacy may outweigh the benefits of using multiple antihypertensives with different mechanisms of action if patients can reach their BP goal on the maximum dose of a single agent. For patients taking multiple antihypertensives, it is important to routinely monitor BP and assess whether deprescribing is indicated. Cognitive impairment and decreased social support may affect medication adherence for older adults.6 Clinicians should be aware of strategies, such as medication reminders and pillboxes, to increase antihypertensive medication adherence. Polypills that contain 2 antihypertensives can be another tool used to manage older adults to increase adherence and decrease health care costs.10
A current strategy that encompasses discussing many, if not all, of these noted elements is the Institute for Healthcare Improvement’s Age-Friendly Health System. This framework uses evidence-based tools to provide care for older adults across all clinical settings and highlights the 4Ms: what matters, medication, mentation, and mobility.11 Medication considers whether a medication is necessary, whether its use has benefits that outweigh the risks, and how it interacts with what matters, mentation, and mobility. In particular, what matters plays an important role in hypertension management in older adults given the recommended target BP differs, depending on which specialty organization guideline is followed. By better understanding what matters to patients, including their goals and priorities, clinicians can engage patients in shared decision making and provide individualized recommendations based on geriatric principles (eg, start low, go slow, principles of medication adherence) and patient comorbidities (eg, medical history and risk factors for hyponatremia) to help patients make a more informed choice about their antihypertensive treatment regimen (Figure).
Conclusions
This case illustrates the need for a specialized approach to hypertension management in older adults and the risks of thiazide diuretics in this population. Clinicians should consider BP goals, patient-specific factors, and principles of medication management in older adults. If initiating thiazide therapy, discuss the risks associated with use, start at the lowest possible dose, and monitor bloodwork within 1 to 2 weeks of initiation/dose change and periodically thereafter while the patient remains on the therapy to decrease the risk of adverse events. Finally, the Institute for Healthcare Improvement’s Age-Friendly Health System framework can be a useful when considering the addition of a new medication in an older adult’s treatment plan.
Acknowledgments
This material is the result of work supported with resources and the use of facilities at the New England Geriatrics Research, Education, and Clinical Center, Veterans Affairs Boston Healthcare System, and the Cincinnati VeteransAffairs Medical Center.
1. Whelton PK, Carey RM, Aronow WS, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the Prevention, Detection, Evaluation and Management of High Blood Pressure in Adults: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol. 2018;71(19):e127-e248. doi:10.1016/j.jacc.2017.11.006
2. Davis LL. Hypertension: how low to go when treating older adults. J Nurse Pract. 2019;15(1):1-6. doi:10.1016/j.nurpra.2018.10.010
3. Qaseem A, Wilt TJ, Rich R, et al. Pharmacologic Treatment of Hypertension in Adults Aged 60 Years or Older to Higher Versus Lower Blood Pressure Targets: A Clinical Practice Guideline From the American College of Physicians and the American Academy of Family Physicians. Ann Intern Med. 2017;166(6):430-437. doi:10.7326/M16-1785
4. Aronow WS, Fleg JL, Pepine CJ, et al. ACCF/AHA 2011 expert consensus document on hypertension in the elderly: a report of the American College of Cardiology Foundation Task Force on Clinical Expert Consensus documents developed in collaboration with the American Academy of Neurology, American Geriatrics Society, American Society for Preventive Cardiology, American Society of Hypertension, American Society of Nephrology, Association of Black Cardiologists, and European Society of Hypertension. J Am Coll Cardiol. 2011;57(20):2037-2114. doi:10.1016/j.jacc.2011.01.008
5. Makam AN, Boscardin WJ, Miao Y, Steinman MA. Risk of thiazide-induced metabolic adverse events in older adults. J Am Geriatr Soc. 2014;62(6):1039-1045. doi:10.1111/jgs.12839
6. Chow KM, Szeto CC, Wong TY, Leung CB, Li PK. Risk factors for thiazide-induced hyponatraemia. QJM. 2003;96(12):911-917. doi:10.1093/qjmed/hcg157
7. Clayton JA, Rodgers S, Blakey J, Avery A, Hall IP. Thiazide diuretic prescription and electrolyte abnormalities in primary care. Br J Clin Pharmacol. 2006;61(1):87-95. doi:10.1111/j.1365-2125.2005.02531.x
8. Shah BM, Hajjar ER. Polypharmacy, adverse drug reactions, and geriatric syndromes. Clin Geriatr Med. 2012;28(2):173-186. doi:10.1016/j.cger.2012.01.002
9. Benetos A, Petrovic M, Strandberg T. Hypertension management in older and frail older patients. Circ Res. 2019;124(7):1045-1060. doi:10.1161/CIRCRESAHA.118.313236
10. Sherrill B, Halpern M, Khan S, Zhang J, Panjabi S. Single-pill vs free-equivalent combination therapies for hypertension: a meta-analysis of health care costs and adherence. J Clin Hypertens (Greenwich). 2011;13(12):898-909. doi:10.1111/j.1751-7176.2011.00550.x
11. Mate K, Fulmer T, Pelton L, et al. Evidence for the 4Ms: interactions and outcomes across the care continuum. J Aging Health. 2021;33(7-8):469-481. doi:10.1177/0898264321991658
1. Whelton PK, Carey RM, Aronow WS, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the Prevention, Detection, Evaluation and Management of High Blood Pressure in Adults: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol. 2018;71(19):e127-e248. doi:10.1016/j.jacc.2017.11.006
2. Davis LL. Hypertension: how low to go when treating older adults. J Nurse Pract. 2019;15(1):1-6. doi:10.1016/j.nurpra.2018.10.010
3. Qaseem A, Wilt TJ, Rich R, et al. Pharmacologic Treatment of Hypertension in Adults Aged 60 Years or Older to Higher Versus Lower Blood Pressure Targets: A Clinical Practice Guideline From the American College of Physicians and the American Academy of Family Physicians. Ann Intern Med. 2017;166(6):430-437. doi:10.7326/M16-1785
4. Aronow WS, Fleg JL, Pepine CJ, et al. ACCF/AHA 2011 expert consensus document on hypertension in the elderly: a report of the American College of Cardiology Foundation Task Force on Clinical Expert Consensus documents developed in collaboration with the American Academy of Neurology, American Geriatrics Society, American Society for Preventive Cardiology, American Society of Hypertension, American Society of Nephrology, Association of Black Cardiologists, and European Society of Hypertension. J Am Coll Cardiol. 2011;57(20):2037-2114. doi:10.1016/j.jacc.2011.01.008
5. Makam AN, Boscardin WJ, Miao Y, Steinman MA. Risk of thiazide-induced metabolic adverse events in older adults. J Am Geriatr Soc. 2014;62(6):1039-1045. doi:10.1111/jgs.12839
6. Chow KM, Szeto CC, Wong TY, Leung CB, Li PK. Risk factors for thiazide-induced hyponatraemia. QJM. 2003;96(12):911-917. doi:10.1093/qjmed/hcg157
7. Clayton JA, Rodgers S, Blakey J, Avery A, Hall IP. Thiazide diuretic prescription and electrolyte abnormalities in primary care. Br J Clin Pharmacol. 2006;61(1):87-95. doi:10.1111/j.1365-2125.2005.02531.x
8. Shah BM, Hajjar ER. Polypharmacy, adverse drug reactions, and geriatric syndromes. Clin Geriatr Med. 2012;28(2):173-186. doi:10.1016/j.cger.2012.01.002
9. Benetos A, Petrovic M, Strandberg T. Hypertension management in older and frail older patients. Circ Res. 2019;124(7):1045-1060. doi:10.1161/CIRCRESAHA.118.313236
10. Sherrill B, Halpern M, Khan S, Zhang J, Panjabi S. Single-pill vs free-equivalent combination therapies for hypertension: a meta-analysis of health care costs and adherence. J Clin Hypertens (Greenwich). 2011;13(12):898-909. doi:10.1111/j.1751-7176.2011.00550.x
11. Mate K, Fulmer T, Pelton L, et al. Evidence for the 4Ms: interactions and outcomes across the care continuum. J Aging Health. 2021;33(7-8):469-481. doi:10.1177/0898264321991658
51-year-old woman • history of Graves disease • general fatigue, palpitations, and hand tremors • Dx?
THE CASE
A 51-year-old Japanese woman presented with fever, sore throat, and dyspnea of less than 1 day’s duration. Although she had developed general fatigue, palpitations, and tremors of the hands 2 months earlier, she had not sought medical care.
Her medical history included Graves disease, which had been diagnosed 13 years earlier. She reported that her only medication was methimazole 10 mg/d. She did not have any family history of endocrinopathies or hematologic diseases.
Physical examination revealed a body temperature of 99.7 °F; heart rate, 130 beats/min; blood pressure, 182/62 mm Hg; respiratory rate, 46 breaths/min; and oxygen saturation, 100% on room air. Pharyngeal erythema was seen. Lung sounds were clear. The patient had tremors in her hands, tenderness of the thyroid gland, and exophthalmos. No leg edema or jugular vein distension was seen.
Laboratory tests indicated hyperthyroidism, with a thyroid-stimulating hormone level < 0.01 µIU/mL (normal range, 0.5-5 µIU/mL); free T3 level, 4.87 pg/mL (normal range, 2.3-4.3 pg/mL); and free T4 level, 2.97 ng/dL (normal range, 0.9-1.7 ng/dL). The patient also had a white blood cell (WBC) count of 1020 cells/µL (normal range, 3500-9000 cells/µL) and neutrophil count of 5 cells/µL (normal range, 1500-6500 cells/µL).
Other blood cell counts were normal, and a chest x-ray did not reveal any abnormal findings. In addition, there was no evidence to suggest hematologic malignancies or congenital neutropenia.
THE DIAGNOSIS
Based on the patient’s low WBC and neutrophil counts, agranulocytosis due to antithyroid drug therapy was suspected; however, this diagnosis would be highly unusual in the context of a 13-year history of therapy. Further history taking revealed that, because of her lack of financial means, unstable living conditions, and lack of understanding of the necessity for medication adherence, the patient had not taken methimazole
In consideration of these factors, a diagnosis of exacerbation of hyperthyroidism and agranulocytosis (due to methimazole restart and upper respiratory infection) was made.
Continue to: DISCUSSION
DISCUSSION
Agranulocytosis is a severe adverse event of antithyroid agents and requires prompt diagnosis and treatment. In a 26-year study at one clinic, it occurred in approximately 0.4% of patients taking antithyroid agents.1 The possible mechanisms of agranulocytosis are the direct toxicity of drugs and immune-mediated responses.2 Older age, female sex, and some HLA genotypes are reported to be associated with susceptibility to agranulocytosis.2
Although the development of agranulocytosis tends to be dose related, a small dose of antithyroid agent can sometimes cause the condition.3,4 It usually occurs within the first 3 months of treatment initiation, but occasionally patients develop agranulocytosis after long-term therapy.5 Interruption and subsequent resumption of the same antithyroid drug treatment also can be a risk factor for agranulocytosis, as in this case.5
Treatment includes drug cessation, administration of broad-spectrum antibiotics if infection is suspected, and granulocyte-colony stimulating factor (G-CSF) therapy.5
Our patient was hospitalized, and methimazole was stopped immediately. Administration of potassium iodide 50 mg/d and G-CSF was started. Meropenem 3 g/d also was administered for neutropenic fever.
The patient’s condition improved, and her WBC count increased to 1640 cells/µL on Day 8 and 10,890 cells/µL on Day 9. G-CSF was stopped on Day 12 and meropenem on Day 13. Bone marrow aspiration was not performed because of improvement in lab values and her overall condition. Although monitoring of WBC count during methimazole therapy is controversial,5 we decided to routinely monitor this patient due to the possibility of drug cross-reactivity.
Continue to: Despite repeated explanations...
Despite repeated explanations that it was dangerous for a patient who had developed agranulocytosis to take another antithyroid medication, the patient refused surgical treatment or radioiodine ablation because of her financial situation. (While all Japanese citizens are covered by a national health insurance program, patients ages 6 to 70 years are required to pay approximately 30% of medical and pharmaceutical costs.) On Day 21, potassium iodide was stopped, and propylthiouracil 300 mg/d was administered with careful follow-up. Agranulocytosis did not recur.
Immediate problem solved, but what about the future?
During her hospital stay, the medical team spoke with the patient many times, during which she expressed anxiety about her health conditions and the difficulties that she had experienced in her life. The clinicians acknowledged her concerns and assured the patient of their continuing commitment to her well-being even after discharge. The patient also was advised that she should take her medication as prescribed and that if she had a fever or sore throat, she should stop the medication and seek medical care as soon as possible. The patient accepted the medical team’s advice and expressed hope for the future.
Conversations about medication adherence. In 1 survey, about 60% of patients taking antithyroid drugs were unfamiliar with the symptoms of agranulocytosis.6 To deliver safe and effective treatment and detect conditions such as agranulocytosis at an early stage, clinicians must communicate clearly with patients who have hyperthyroidism, providing sufficient explanation and ensuring understanding on the patient’s part.
Patients may be reluctant to provide the details of medication adherence.7 Although it is common for patients to need services for socioeconomic issues,8 health care professionals sometimes fail to adequately discuss these issues with patients, especially if the patients are marginalized and/or have lower economic status.9 Cases such as ours underscore the importance of improving clinicians’ awareness and sensitivity to patients’ socioeconomic challenges.10,11
Our patient received information about welfare and other government services from a medical social worker during her hospital stay. She also was informed that she could seek assistance from medical social workers in the future if needed.
Continue to: The patient was discharged...
The patient was discharged on Day 28. After discharge, she took propylthiouracil as prescribed (300 mg/d), and her Graves disease was well controlled. Outpatient follow-up visits were performed every 1 or 2 months. No adverse events of propylthiouracil were seen in the ensuing time.
THE TAKEAWAY
Patients with chronic conditions sometimes discontinue medications, and they may not talk about it with their medical team, especially if they have socioeconomic or other difficulties in their lives. Clinicians should consider medication nonadherence and its risk factors when patients with chronic conditions develop unexpected adverse events.
We thank Jane Charbonneau, DVM, from Edanz for doing an English-language review of a draft of this manuscript.
CORRESPONDENCE
Takuya Maejima, MD, Department of General Medicine and Primary Care, University of Tsukuba Hospital, 2-1-1 Amakubo, Tsukuba, Ibaraki 305-8576 Japan; [email protected]
1. Tajiri J, Noguchi S. Antithyroid drug-induced agranulocytosis: special reference to normal white blood cell count agranulocytosis. Thyroid. 2004;14:459-462. doi: 10.1089/105072504323150787
2. Vicente N, Cardoso L, Barros L, et al. Antithyroid drug-induced agranulocytosis: state of the art on diagnosis and management. Drugs R D. 2017;17:91-96. doi: 10.1007/s40268-017-0172-1
3. Takata K, Kubota S, Fukata S, et al. Methimazole-induced agranulocytosis in patients with Graves’ disease is more frequent with an initial dose of 30 mg daily than with 15 mg daily. Thyroid. 2009;19:559-563. doi: 10.1089/thy.2008.0364
4. Tsuboi K, Ueshiba H, Shimojo M, et al. The relation of initial methimazole dose to the incidence of methimazole-induced agranulocytosis in patients with Graves’ disease. Endocr J. 2007;54:39-43. doi: 10.1507/endocrj.k05-068
5. Burch HB, Cooper DS. Management of Graves disease: a review. J Am Med Assoc. 2015;314:2544-2554. doi: 10.1001/jama.2015.16535
6. Robinson J, Richardson M, Hickey J, et al. Patient knowledge of antithyroid drug-induced agranulocytosis. Eur Thyroid J. 2014;3:245-251. doi: https://doi.org/10.1159/000367990
7. Kini V, Ho PM. Interventions to improve medication adherence: a review. J Am Med Assoc. 2018;320:2461-2473. doi: 10.1001/jama.2018.19271
8. Vest JR, Grannis SJ, Haut DP, et al. Using structured and unstructured data to identify patients’ need for services that address the social determinants of health. Int J Med Inform. 2017;107:101-106. doi: 10.1016/j.ijmedinf.2017.09.008
9. Willems S, De Maesschalck S, Deveugele M, et al. Socio-economic status of the patient and doctor-patient communication: does it make a difference? Patient Educ Couns. 2005;56:139-146. doi: 10.1016/j.pec.2004.02.011
10. The College of Family Physicians of Canada. Best advice: social determinants of health. Accessed September 15, 2023. https://patientsmedicalhome.ca/resources/best-advice-guides/best-advice-guide-social-determinants-health/
11. Hunter K, Thomson B. A scoping review of social determinants of health curricula in post-graduate medical education. Can Med Educ J. 2019;10:e61-e71. doi: 10.36834/cmej.61709
THE CASE
A 51-year-old Japanese woman presented with fever, sore throat, and dyspnea of less than 1 day’s duration. Although she had developed general fatigue, palpitations, and tremors of the hands 2 months earlier, she had not sought medical care.
Her medical history included Graves disease, which had been diagnosed 13 years earlier. She reported that her only medication was methimazole 10 mg/d. She did not have any family history of endocrinopathies or hematologic diseases.
Physical examination revealed a body temperature of 99.7 °F; heart rate, 130 beats/min; blood pressure, 182/62 mm Hg; respiratory rate, 46 breaths/min; and oxygen saturation, 100% on room air. Pharyngeal erythema was seen. Lung sounds were clear. The patient had tremors in her hands, tenderness of the thyroid gland, and exophthalmos. No leg edema or jugular vein distension was seen.
Laboratory tests indicated hyperthyroidism, with a thyroid-stimulating hormone level < 0.01 µIU/mL (normal range, 0.5-5 µIU/mL); free T3 level, 4.87 pg/mL (normal range, 2.3-4.3 pg/mL); and free T4 level, 2.97 ng/dL (normal range, 0.9-1.7 ng/dL). The patient also had a white blood cell (WBC) count of 1020 cells/µL (normal range, 3500-9000 cells/µL) and neutrophil count of 5 cells/µL (normal range, 1500-6500 cells/µL).
Other blood cell counts were normal, and a chest x-ray did not reveal any abnormal findings. In addition, there was no evidence to suggest hematologic malignancies or congenital neutropenia.
THE DIAGNOSIS
Based on the patient’s low WBC and neutrophil counts, agranulocytosis due to antithyroid drug therapy was suspected; however, this diagnosis would be highly unusual in the context of a 13-year history of therapy. Further history taking revealed that, because of her lack of financial means, unstable living conditions, and lack of understanding of the necessity for medication adherence, the patient had not taken methimazole
In consideration of these factors, a diagnosis of exacerbation of hyperthyroidism and agranulocytosis (due to methimazole restart and upper respiratory infection) was made.
Continue to: DISCUSSION
DISCUSSION
Agranulocytosis is a severe adverse event of antithyroid agents and requires prompt diagnosis and treatment. In a 26-year study at one clinic, it occurred in approximately 0.4% of patients taking antithyroid agents.1 The possible mechanisms of agranulocytosis are the direct toxicity of drugs and immune-mediated responses.2 Older age, female sex, and some HLA genotypes are reported to be associated with susceptibility to agranulocytosis.2
Although the development of agranulocytosis tends to be dose related, a small dose of antithyroid agent can sometimes cause the condition.3,4 It usually occurs within the first 3 months of treatment initiation, but occasionally patients develop agranulocytosis after long-term therapy.5 Interruption and subsequent resumption of the same antithyroid drug treatment also can be a risk factor for agranulocytosis, as in this case.5
Treatment includes drug cessation, administration of broad-spectrum antibiotics if infection is suspected, and granulocyte-colony stimulating factor (G-CSF) therapy.5
Our patient was hospitalized, and methimazole was stopped immediately. Administration of potassium iodide 50 mg/d and G-CSF was started. Meropenem 3 g/d also was administered for neutropenic fever.
The patient’s condition improved, and her WBC count increased to 1640 cells/µL on Day 8 and 10,890 cells/µL on Day 9. G-CSF was stopped on Day 12 and meropenem on Day 13. Bone marrow aspiration was not performed because of improvement in lab values and her overall condition. Although monitoring of WBC count during methimazole therapy is controversial,5 we decided to routinely monitor this patient due to the possibility of drug cross-reactivity.
Continue to: Despite repeated explanations...
Despite repeated explanations that it was dangerous for a patient who had developed agranulocytosis to take another antithyroid medication, the patient refused surgical treatment or radioiodine ablation because of her financial situation. (While all Japanese citizens are covered by a national health insurance program, patients ages 6 to 70 years are required to pay approximately 30% of medical and pharmaceutical costs.) On Day 21, potassium iodide was stopped, and propylthiouracil 300 mg/d was administered with careful follow-up. Agranulocytosis did not recur.
Immediate problem solved, but what about the future?
During her hospital stay, the medical team spoke with the patient many times, during which she expressed anxiety about her health conditions and the difficulties that she had experienced in her life. The clinicians acknowledged her concerns and assured the patient of their continuing commitment to her well-being even after discharge. The patient also was advised that she should take her medication as prescribed and that if she had a fever or sore throat, she should stop the medication and seek medical care as soon as possible. The patient accepted the medical team’s advice and expressed hope for the future.
Conversations about medication adherence. In 1 survey, about 60% of patients taking antithyroid drugs were unfamiliar with the symptoms of agranulocytosis.6 To deliver safe and effective treatment and detect conditions such as agranulocytosis at an early stage, clinicians must communicate clearly with patients who have hyperthyroidism, providing sufficient explanation and ensuring understanding on the patient’s part.
Patients may be reluctant to provide the details of medication adherence.7 Although it is common for patients to need services for socioeconomic issues,8 health care professionals sometimes fail to adequately discuss these issues with patients, especially if the patients are marginalized and/or have lower economic status.9 Cases such as ours underscore the importance of improving clinicians’ awareness and sensitivity to patients’ socioeconomic challenges.10,11
Our patient received information about welfare and other government services from a medical social worker during her hospital stay. She also was informed that she could seek assistance from medical social workers in the future if needed.
Continue to: The patient was discharged...
The patient was discharged on Day 28. After discharge, she took propylthiouracil as prescribed (300 mg/d), and her Graves disease was well controlled. Outpatient follow-up visits were performed every 1 or 2 months. No adverse events of propylthiouracil were seen in the ensuing time.
THE TAKEAWAY
Patients with chronic conditions sometimes discontinue medications, and they may not talk about it with their medical team, especially if they have socioeconomic or other difficulties in their lives. Clinicians should consider medication nonadherence and its risk factors when patients with chronic conditions develop unexpected adverse events.
We thank Jane Charbonneau, DVM, from Edanz for doing an English-language review of a draft of this manuscript.
CORRESPONDENCE
Takuya Maejima, MD, Department of General Medicine and Primary Care, University of Tsukuba Hospital, 2-1-1 Amakubo, Tsukuba, Ibaraki 305-8576 Japan; [email protected]
THE CASE
A 51-year-old Japanese woman presented with fever, sore throat, and dyspnea of less than 1 day’s duration. Although she had developed general fatigue, palpitations, and tremors of the hands 2 months earlier, she had not sought medical care.
Her medical history included Graves disease, which had been diagnosed 13 years earlier. She reported that her only medication was methimazole 10 mg/d. She did not have any family history of endocrinopathies or hematologic diseases.
Physical examination revealed a body temperature of 99.7 °F; heart rate, 130 beats/min; blood pressure, 182/62 mm Hg; respiratory rate, 46 breaths/min; and oxygen saturation, 100% on room air. Pharyngeal erythema was seen. Lung sounds were clear. The patient had tremors in her hands, tenderness of the thyroid gland, and exophthalmos. No leg edema or jugular vein distension was seen.
Laboratory tests indicated hyperthyroidism, with a thyroid-stimulating hormone level < 0.01 µIU/mL (normal range, 0.5-5 µIU/mL); free T3 level, 4.87 pg/mL (normal range, 2.3-4.3 pg/mL); and free T4 level, 2.97 ng/dL (normal range, 0.9-1.7 ng/dL). The patient also had a white blood cell (WBC) count of 1020 cells/µL (normal range, 3500-9000 cells/µL) and neutrophil count of 5 cells/µL (normal range, 1500-6500 cells/µL).
Other blood cell counts were normal, and a chest x-ray did not reveal any abnormal findings. In addition, there was no evidence to suggest hematologic malignancies or congenital neutropenia.
THE DIAGNOSIS
Based on the patient’s low WBC and neutrophil counts, agranulocytosis due to antithyroid drug therapy was suspected; however, this diagnosis would be highly unusual in the context of a 13-year history of therapy. Further history taking revealed that, because of her lack of financial means, unstable living conditions, and lack of understanding of the necessity for medication adherence, the patient had not taken methimazole
In consideration of these factors, a diagnosis of exacerbation of hyperthyroidism and agranulocytosis (due to methimazole restart and upper respiratory infection) was made.
Continue to: DISCUSSION
DISCUSSION
Agranulocytosis is a severe adverse event of antithyroid agents and requires prompt diagnosis and treatment. In a 26-year study at one clinic, it occurred in approximately 0.4% of patients taking antithyroid agents.1 The possible mechanisms of agranulocytosis are the direct toxicity of drugs and immune-mediated responses.2 Older age, female sex, and some HLA genotypes are reported to be associated with susceptibility to agranulocytosis.2
Although the development of agranulocytosis tends to be dose related, a small dose of antithyroid agent can sometimes cause the condition.3,4 It usually occurs within the first 3 months of treatment initiation, but occasionally patients develop agranulocytosis after long-term therapy.5 Interruption and subsequent resumption of the same antithyroid drug treatment also can be a risk factor for agranulocytosis, as in this case.5
Treatment includes drug cessation, administration of broad-spectrum antibiotics if infection is suspected, and granulocyte-colony stimulating factor (G-CSF) therapy.5
Our patient was hospitalized, and methimazole was stopped immediately. Administration of potassium iodide 50 mg/d and G-CSF was started. Meropenem 3 g/d also was administered for neutropenic fever.
The patient’s condition improved, and her WBC count increased to 1640 cells/µL on Day 8 and 10,890 cells/µL on Day 9. G-CSF was stopped on Day 12 and meropenem on Day 13. Bone marrow aspiration was not performed because of improvement in lab values and her overall condition. Although monitoring of WBC count during methimazole therapy is controversial,5 we decided to routinely monitor this patient due to the possibility of drug cross-reactivity.
Continue to: Despite repeated explanations...
Despite repeated explanations that it was dangerous for a patient who had developed agranulocytosis to take another antithyroid medication, the patient refused surgical treatment or radioiodine ablation because of her financial situation. (While all Japanese citizens are covered by a national health insurance program, patients ages 6 to 70 years are required to pay approximately 30% of medical and pharmaceutical costs.) On Day 21, potassium iodide was stopped, and propylthiouracil 300 mg/d was administered with careful follow-up. Agranulocytosis did not recur.
Immediate problem solved, but what about the future?
During her hospital stay, the medical team spoke with the patient many times, during which she expressed anxiety about her health conditions and the difficulties that she had experienced in her life. The clinicians acknowledged her concerns and assured the patient of their continuing commitment to her well-being even after discharge. The patient also was advised that she should take her medication as prescribed and that if she had a fever or sore throat, she should stop the medication and seek medical care as soon as possible. The patient accepted the medical team’s advice and expressed hope for the future.
Conversations about medication adherence. In 1 survey, about 60% of patients taking antithyroid drugs were unfamiliar with the symptoms of agranulocytosis.6 To deliver safe and effective treatment and detect conditions such as agranulocytosis at an early stage, clinicians must communicate clearly with patients who have hyperthyroidism, providing sufficient explanation and ensuring understanding on the patient’s part.
Patients may be reluctant to provide the details of medication adherence.7 Although it is common for patients to need services for socioeconomic issues,8 health care professionals sometimes fail to adequately discuss these issues with patients, especially if the patients are marginalized and/or have lower economic status.9 Cases such as ours underscore the importance of improving clinicians’ awareness and sensitivity to patients’ socioeconomic challenges.10,11
Our patient received information about welfare and other government services from a medical social worker during her hospital stay. She also was informed that she could seek assistance from medical social workers in the future if needed.
Continue to: The patient was discharged...
The patient was discharged on Day 28. After discharge, she took propylthiouracil as prescribed (300 mg/d), and her Graves disease was well controlled. Outpatient follow-up visits were performed every 1 or 2 months. No adverse events of propylthiouracil were seen in the ensuing time.
THE TAKEAWAY
Patients with chronic conditions sometimes discontinue medications, and they may not talk about it with their medical team, especially if they have socioeconomic or other difficulties in their lives. Clinicians should consider medication nonadherence and its risk factors when patients with chronic conditions develop unexpected adverse events.
We thank Jane Charbonneau, DVM, from Edanz for doing an English-language review of a draft of this manuscript.
CORRESPONDENCE
Takuya Maejima, MD, Department of General Medicine and Primary Care, University of Tsukuba Hospital, 2-1-1 Amakubo, Tsukuba, Ibaraki 305-8576 Japan; [email protected]
1. Tajiri J, Noguchi S. Antithyroid drug-induced agranulocytosis: special reference to normal white blood cell count agranulocytosis. Thyroid. 2004;14:459-462. doi: 10.1089/105072504323150787
2. Vicente N, Cardoso L, Barros L, et al. Antithyroid drug-induced agranulocytosis: state of the art on diagnosis and management. Drugs R D. 2017;17:91-96. doi: 10.1007/s40268-017-0172-1
3. Takata K, Kubota S, Fukata S, et al. Methimazole-induced agranulocytosis in patients with Graves’ disease is more frequent with an initial dose of 30 mg daily than with 15 mg daily. Thyroid. 2009;19:559-563. doi: 10.1089/thy.2008.0364
4. Tsuboi K, Ueshiba H, Shimojo M, et al. The relation of initial methimazole dose to the incidence of methimazole-induced agranulocytosis in patients with Graves’ disease. Endocr J. 2007;54:39-43. doi: 10.1507/endocrj.k05-068
5. Burch HB, Cooper DS. Management of Graves disease: a review. J Am Med Assoc. 2015;314:2544-2554. doi: 10.1001/jama.2015.16535
6. Robinson J, Richardson M, Hickey J, et al. Patient knowledge of antithyroid drug-induced agranulocytosis. Eur Thyroid J. 2014;3:245-251. doi: https://doi.org/10.1159/000367990
7. Kini V, Ho PM. Interventions to improve medication adherence: a review. J Am Med Assoc. 2018;320:2461-2473. doi: 10.1001/jama.2018.19271
8. Vest JR, Grannis SJ, Haut DP, et al. Using structured and unstructured data to identify patients’ need for services that address the social determinants of health. Int J Med Inform. 2017;107:101-106. doi: 10.1016/j.ijmedinf.2017.09.008
9. Willems S, De Maesschalck S, Deveugele M, et al. Socio-economic status of the patient and doctor-patient communication: does it make a difference? Patient Educ Couns. 2005;56:139-146. doi: 10.1016/j.pec.2004.02.011
10. The College of Family Physicians of Canada. Best advice: social determinants of health. Accessed September 15, 2023. https://patientsmedicalhome.ca/resources/best-advice-guides/best-advice-guide-social-determinants-health/
11. Hunter K, Thomson B. A scoping review of social determinants of health curricula in post-graduate medical education. Can Med Educ J. 2019;10:e61-e71. doi: 10.36834/cmej.61709
1. Tajiri J, Noguchi S. Antithyroid drug-induced agranulocytosis: special reference to normal white blood cell count agranulocytosis. Thyroid. 2004;14:459-462. doi: 10.1089/105072504323150787
2. Vicente N, Cardoso L, Barros L, et al. Antithyroid drug-induced agranulocytosis: state of the art on diagnosis and management. Drugs R D. 2017;17:91-96. doi: 10.1007/s40268-017-0172-1
3. Takata K, Kubota S, Fukata S, et al. Methimazole-induced agranulocytosis in patients with Graves’ disease is more frequent with an initial dose of 30 mg daily than with 15 mg daily. Thyroid. 2009;19:559-563. doi: 10.1089/thy.2008.0364
4. Tsuboi K, Ueshiba H, Shimojo M, et al. The relation of initial methimazole dose to the incidence of methimazole-induced agranulocytosis in patients with Graves’ disease. Endocr J. 2007;54:39-43. doi: 10.1507/endocrj.k05-068
5. Burch HB, Cooper DS. Management of Graves disease: a review. J Am Med Assoc. 2015;314:2544-2554. doi: 10.1001/jama.2015.16535
6. Robinson J, Richardson M, Hickey J, et al. Patient knowledge of antithyroid drug-induced agranulocytosis. Eur Thyroid J. 2014;3:245-251. doi: https://doi.org/10.1159/000367990
7. Kini V, Ho PM. Interventions to improve medication adherence: a review. J Am Med Assoc. 2018;320:2461-2473. doi: 10.1001/jama.2018.19271
8. Vest JR, Grannis SJ, Haut DP, et al. Using structured and unstructured data to identify patients’ need for services that address the social determinants of health. Int J Med Inform. 2017;107:101-106. doi: 10.1016/j.ijmedinf.2017.09.008
9. Willems S, De Maesschalck S, Deveugele M, et al. Socio-economic status of the patient and doctor-patient communication: does it make a difference? Patient Educ Couns. 2005;56:139-146. doi: 10.1016/j.pec.2004.02.011
10. The College of Family Physicians of Canada. Best advice: social determinants of health. Accessed September 15, 2023. https://patientsmedicalhome.ca/resources/best-advice-guides/best-advice-guide-social-determinants-health/
11. Hunter K, Thomson B. A scoping review of social determinants of health curricula in post-graduate medical education. Can Med Educ J. 2019;10:e61-e71. doi: 10.36834/cmej.61709
► History of Graves disease
► General fatigue, palpitations, and hand tremors
55-year-old woman • myalgias and progressive symmetrical proximal weakness • history of type 2 diabetes and hyperlipidemia • Dx?
THE CASE
A 55-year-old woman developed subacute progression of myalgias and subjective weakness in her proximal extremities after starting a new exercise regimen. The patient had a history of unilateral renal agenesis, type 2 diabetes, and hyperlipidemia, for which she had taken atorvastatin 40 mg/d for several years before discontinuing it 2 years earlier for unknown reasons. She had been evaluated multiple times in the primary care clinic and emergency department over the previous month. Each time, her strength was minimally reduced in the upper extremities on examination, her renal function and electrolytes were normal, and her creatine kinase (CK) level was elevated (16,000-20,000 U/L; normal range, 26-192 U/L). She was managed conservatively with fluids and given return precautions each time.
After her myalgias and weakness increased in severity, she presented to the emergency department with a muscle strength score of 4/5 in both shoulders, triceps, hip flexors, hip extensors, abductors, and adductors. Her laboratory results were significant for the presence of blood without red blood cells on her urine dipstick test and a CK level of 25,070 U/L. She was admitted for further evaluation of progressive myopathy and given aggressive IV fluid hydration to prevent renal injury based on her history of unilateral renal agenesis.
Infectious disease testing, which included a respiratory virus panel, acute hepatitis panel, HIV screening, Lyme antibody testing, cytomegalovirus DNA detection by polymerase chain reaction, Epstein-Barr virus capsid immunoglobulin M, and anti-streptolysin O, were negative. Electrolytes, inflammatory markers, and kidney function were normal. However, high-sensitivity troponin-T levels were elevated, with a peak value of 216.3 ng/L (normal range, 0-19 ng/L). The patient denied having any chest pain, and her electrocardiogram and transthoracic echocardiogram were normal. By hospital Day 4, her myalgias and weakness had improved, CK had stabilized (19,000-21,000 U/L), cardiac enzymes had improved, and urinalysis had normalized. She was discharged with a referral to a rheumatologist.
However, 10 days later—before she could see a rheumatologist—she was readmitted to a community hospital for recurrence of severe myalgias, progressive weakness, positive blood on urine dipstick testing, and a rising CK level (to 24,580 U/L) found during a follow-up appointment with her primary care physician. At this point, Neurology and Rheumatology were consulted and myositis-specific and myositis-associated autoantibody tests were sent out. Magnetic resonance imaging (MRI) of her thighs was performed and showed diffusely increased T2 signal and short tau inversion recovery in multiple proximal muscles (FIGURE).
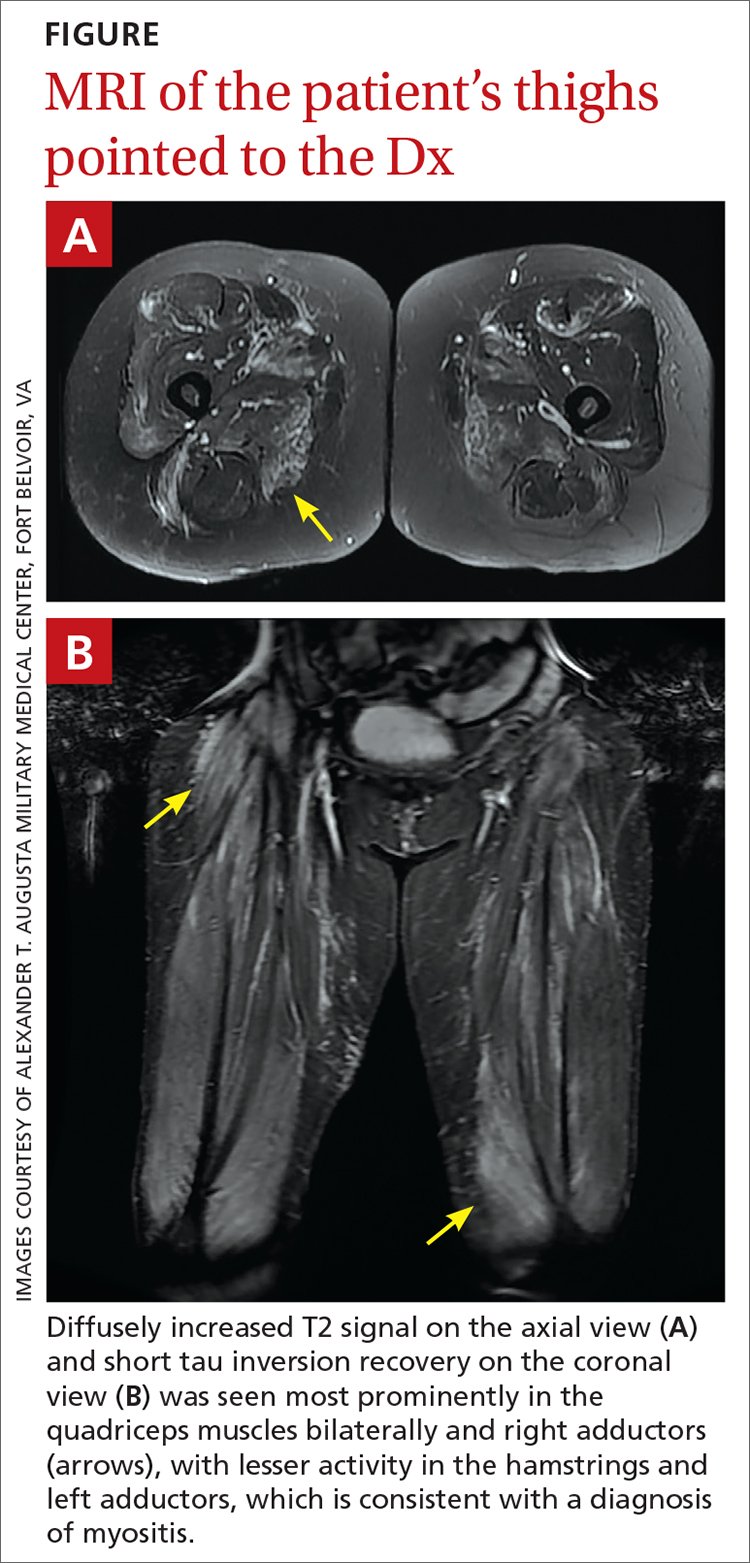
DIAGNOSIS
Given her symmetrical proximal muscle weakness (which was refractory to IV fluid resuscitation), MRI findings, and the exclusion of infection and metabolic derangements, the patient was given a working diagnosis of myositis and treated with 1-g IV methylprednisolone followed by a 4-month steroid taper, methotrexate 20 mg weekly, and physical therapy. This working diagnosis was later confirmed with the results of her autoantibody tests.
At her 1-month follow-up visit, the patient reported minimal improvement in her strength, new neck weakness, and dysphagia with solids. Testing revealed anti–3-hydroxy-3-methylglutaryl-coenzyme A reductase (anti-HMGCR) antibody levels of more than 200 U/L (negative < 20 U/L; positive > 59 U/L), which pointed to a more refined diagnosis of anti-HMGCR immune-mediated necrotizing myositis.
DISCUSSION
Myositis should be in the differential diagnosis for patients with symmetrical proximal muscle weakness. Bohan and Peter devised a 5-part set of criteria to help diagnose myositis, shown in the TABLE.1,2 This simple framework broadens the differential and guides diagnostic testing. Our patient’s presentation was fairly typical for anti-HMGCR myositis, a subset of immune-mediated necrotizing myositis,3 with a pretest probability of 62% per the European League Against Rheumatism/American College of Rheumatology classification criteria.2 Probability of this diagnosis was further increased by the high-titer anti-HMGCR, so biopsy and electromyography (EMG), as noted by Bohan and Peter, were not pursued.
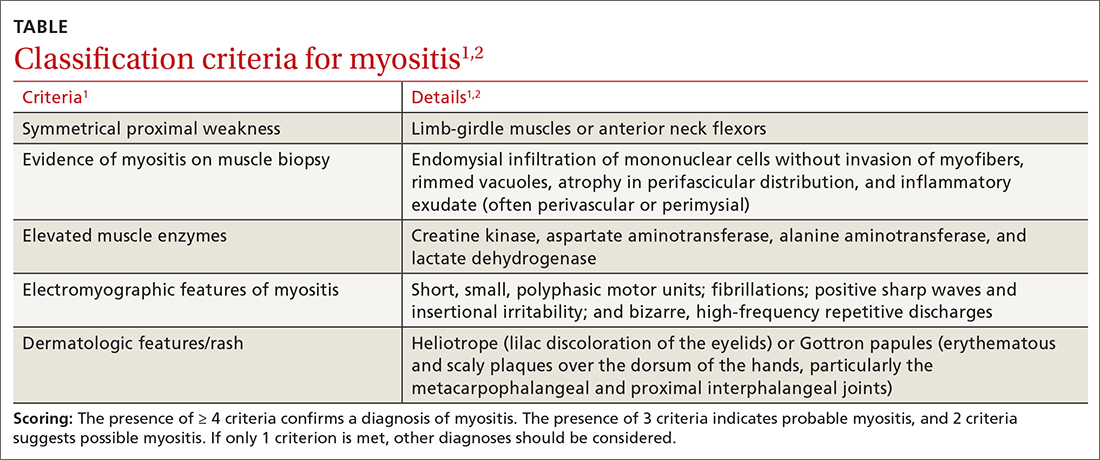
Continue to: Autoimmune myopathies...
Autoimmune myopathies occur in 9 to 14 per 100,000 people,4 with 6% of patients having anti-HMGCR auto-antibodies.5 Anti-HMGCR myositis is more prevalent in older women, patients with type 2 diabetes, and those with a history of atorvastatin use.3,6 Two-thirds of patients with anti-HMGCR myositis report current or prior statin use, and this increases to more than 90% in those age 50 years or older.5 Anti-HMGCR myositis causes significant muscle weakness that does not resolve with discontinuation of the statin and can occur years after the initiation or discontinuation of statin treatment.6 Cardiac involvement is rare4 but dysphagia is relatively common.7,8 Anti-HMGCR myositis also has a weak association with cancer, most commonly gastrointestinal and lung cancers.4,7
Distinguishing statin-induced myalgias from statin-induced myositis guides management. Statin-induced myalgias are associated with normal or slightly increased CK levels (typically < 1000 U/L) and resolve with discontinuation of the statin; the patient can often tolerate re-challenge with a statin.6 In contrast, CK elevation in patients with statin-induced myositis is typically more than 10,000 U/L6 and requires aggressive treatment with immunomodulatory medications to prevent permanent muscle damage.
Treatment recommendations are supported only by case series, observational studies, and expert opinion. Typical first-line treatment includes induction with high-dose corticosteroids followed by prolonged taper plus a conventional synthetic disease-modifying antirheumatic drug (csDMARD) such as methotrexate, azathioprine, or mycophenolate.4 Maintenance therapy often is achieved with csDMARD therapy for 2 years.4 Severe cases frequently are treated with combination csDMARD therapy (eg, methotrexate and azathioprine or methotrexate and mycophenolate).4 Rituximab and IV immunoglobulin (IVIG) are typically reserved for refractory cases.6 Usual monitoring for relapse includes muscle strength testing on examination and evaluation of trending CK levels.8
Our patient received monthly 2-g/kg IVIG infusions, which led to slow, consistent improvement in her strength and normalization of her CK levels to 181 U/L after 6 months.
THE TAKEAWAY
Anti-HMGCR myositis should be suspected in any patient currently or previously treated with a statin who presents with proximal muscle weakness, myalgias, or an elevated CK level. We suggest early subspecialty consultation to discuss whether antibody testing, EMG, or muscle biopsy are warranted. If anti-HMGCR myositis is confirmed, it is advisable to rule out comorbid malignancy and initiate early combination treatment to minimize relapses and permanent muscle damage.
CORRESPONDENCE
Daniel T. Schoenherr, MD, Family Medicine Residency, National Capital Consortium–Alexander T. Augusta Military Medical Center, 9300 DeWitt Loop, Fort Belvoir, VA 22060; [email protected]
1. Bohan A, Peter JB. Polymyositis and dermatomyositis (first of two parts). N Engl J Med. 1975;292:344-347. doi: 10.1056/NEJM197502132920706
2. Bottai M, Tjärnlund A, Santoni G, et al. EULAR/ACR classification criteria for adult and juvenile idiopathic inflammatory myopathies and their major subgroups: a methodology report. RMD Open. 2017;3:e000507. doi: 10.1136/rmdopen-2017-000507
3. Basharat P, Lahouti AH, Paik JJ, et al. Statin-induced anti-HMGCR-associated myopathy. J Am Coll Cardiol. 2016;68:234-235. doi: 10.1016/j.jacc.2016.04.037
4. Pinal-Fernandez I, Casal-Dominguez M, Mammen AL. Immune-mediated necrotizing myopathy. Curr Rheumatol Rep. 2018;20:21. doi: 10.1007/s11926-018-0732-6
5. Mammen AL, Chung T, Christopher-Stine L, et al. Autoantibodies against 3-hydroxy-3-methylglutaryl-coenzyme A reductase in patients with statin-associated autoimmune myopathy. Arthritis Rheum. 2011;63:713-721. doi: 10.1002/art.30156
6. Irvine NJ. Anti-HMGCR myopathy: a rare and serious side effect of statins. J Am Board Fam Med. 2020;33:785-788. doi: 10.3122/jabfm.2020.05.190450
7. Basharat P, Christopher-Stine L. Immune-mediated necrotizing myopathy: update on diagnosis and management. Curr Rheumatol Rep. 2015;17:72. doi: 10.1007/s11926-015-0548-6
8. Betteridge Z, McHugh N. Myositis-specific autoantibodies: an important tool to support diagnosis of myositis. J Int Med. 2016;280:8-23. doi: 10.1111/joim.12451
THE CASE
A 55-year-old woman developed subacute progression of myalgias and subjective weakness in her proximal extremities after starting a new exercise regimen. The patient had a history of unilateral renal agenesis, type 2 diabetes, and hyperlipidemia, for which she had taken atorvastatin 40 mg/d for several years before discontinuing it 2 years earlier for unknown reasons. She had been evaluated multiple times in the primary care clinic and emergency department over the previous month. Each time, her strength was minimally reduced in the upper extremities on examination, her renal function and electrolytes were normal, and her creatine kinase (CK) level was elevated (16,000-20,000 U/L; normal range, 26-192 U/L). She was managed conservatively with fluids and given return precautions each time.
After her myalgias and weakness increased in severity, she presented to the emergency department with a muscle strength score of 4/5 in both shoulders, triceps, hip flexors, hip extensors, abductors, and adductors. Her laboratory results were significant for the presence of blood without red blood cells on her urine dipstick test and a CK level of 25,070 U/L. She was admitted for further evaluation of progressive myopathy and given aggressive IV fluid hydration to prevent renal injury based on her history of unilateral renal agenesis.
Infectious disease testing, which included a respiratory virus panel, acute hepatitis panel, HIV screening, Lyme antibody testing, cytomegalovirus DNA detection by polymerase chain reaction, Epstein-Barr virus capsid immunoglobulin M, and anti-streptolysin O, were negative. Electrolytes, inflammatory markers, and kidney function were normal. However, high-sensitivity troponin-T levels were elevated, with a peak value of 216.3 ng/L (normal range, 0-19 ng/L). The patient denied having any chest pain, and her electrocardiogram and transthoracic echocardiogram were normal. By hospital Day 4, her myalgias and weakness had improved, CK had stabilized (19,000-21,000 U/L), cardiac enzymes had improved, and urinalysis had normalized. She was discharged with a referral to a rheumatologist.
However, 10 days later—before she could see a rheumatologist—she was readmitted to a community hospital for recurrence of severe myalgias, progressive weakness, positive blood on urine dipstick testing, and a rising CK level (to 24,580 U/L) found during a follow-up appointment with her primary care physician. At this point, Neurology and Rheumatology were consulted and myositis-specific and myositis-associated autoantibody tests were sent out. Magnetic resonance imaging (MRI) of her thighs was performed and showed diffusely increased T2 signal and short tau inversion recovery in multiple proximal muscles (FIGURE).

DIAGNOSIS
Given her symmetrical proximal muscle weakness (which was refractory to IV fluid resuscitation), MRI findings, and the exclusion of infection and metabolic derangements, the patient was given a working diagnosis of myositis and treated with 1-g IV methylprednisolone followed by a 4-month steroid taper, methotrexate 20 mg weekly, and physical therapy. This working diagnosis was later confirmed with the results of her autoantibody tests.
At her 1-month follow-up visit, the patient reported minimal improvement in her strength, new neck weakness, and dysphagia with solids. Testing revealed anti–3-hydroxy-3-methylglutaryl-coenzyme A reductase (anti-HMGCR) antibody levels of more than 200 U/L (negative < 20 U/L; positive > 59 U/L), which pointed to a more refined diagnosis of anti-HMGCR immune-mediated necrotizing myositis.
DISCUSSION
Myositis should be in the differential diagnosis for patients with symmetrical proximal muscle weakness. Bohan and Peter devised a 5-part set of criteria to help diagnose myositis, shown in the TABLE.1,2 This simple framework broadens the differential and guides diagnostic testing. Our patient’s presentation was fairly typical for anti-HMGCR myositis, a subset of immune-mediated necrotizing myositis,3 with a pretest probability of 62% per the European League Against Rheumatism/American College of Rheumatology classification criteria.2 Probability of this diagnosis was further increased by the high-titer anti-HMGCR, so biopsy and electromyography (EMG), as noted by Bohan and Peter, were not pursued.

Continue to: Autoimmune myopathies...
Autoimmune myopathies occur in 9 to 14 per 100,000 people,4 with 6% of patients having anti-HMGCR auto-antibodies.5 Anti-HMGCR myositis is more prevalent in older women, patients with type 2 diabetes, and those with a history of atorvastatin use.3,6 Two-thirds of patients with anti-HMGCR myositis report current or prior statin use, and this increases to more than 90% in those age 50 years or older.5 Anti-HMGCR myositis causes significant muscle weakness that does not resolve with discontinuation of the statin and can occur years after the initiation or discontinuation of statin treatment.6 Cardiac involvement is rare4 but dysphagia is relatively common.7,8 Anti-HMGCR myositis also has a weak association with cancer, most commonly gastrointestinal and lung cancers.4,7
Distinguishing statin-induced myalgias from statin-induced myositis guides management. Statin-induced myalgias are associated with normal or slightly increased CK levels (typically < 1000 U/L) and resolve with discontinuation of the statin; the patient can often tolerate re-challenge with a statin.6 In contrast, CK elevation in patients with statin-induced myositis is typically more than 10,000 U/L6 and requires aggressive treatment with immunomodulatory medications to prevent permanent muscle damage.
Treatment recommendations are supported only by case series, observational studies, and expert opinion. Typical first-line treatment includes induction with high-dose corticosteroids followed by prolonged taper plus a conventional synthetic disease-modifying antirheumatic drug (csDMARD) such as methotrexate, azathioprine, or mycophenolate.4 Maintenance therapy often is achieved with csDMARD therapy for 2 years.4 Severe cases frequently are treated with combination csDMARD therapy (eg, methotrexate and azathioprine or methotrexate and mycophenolate).4 Rituximab and IV immunoglobulin (IVIG) are typically reserved for refractory cases.6 Usual monitoring for relapse includes muscle strength testing on examination and evaluation of trending CK levels.8
Our patient received monthly 2-g/kg IVIG infusions, which led to slow, consistent improvement in her strength and normalization of her CK levels to 181 U/L after 6 months.
THE TAKEAWAY
Anti-HMGCR myositis should be suspected in any patient currently or previously treated with a statin who presents with proximal muscle weakness, myalgias, or an elevated CK level. We suggest early subspecialty consultation to discuss whether antibody testing, EMG, or muscle biopsy are warranted. If anti-HMGCR myositis is confirmed, it is advisable to rule out comorbid malignancy and initiate early combination treatment to minimize relapses and permanent muscle damage.
CORRESPONDENCE
Daniel T. Schoenherr, MD, Family Medicine Residency, National Capital Consortium–Alexander T. Augusta Military Medical Center, 9300 DeWitt Loop, Fort Belvoir, VA 22060; [email protected]
THE CASE
A 55-year-old woman developed subacute progression of myalgias and subjective weakness in her proximal extremities after starting a new exercise regimen. The patient had a history of unilateral renal agenesis, type 2 diabetes, and hyperlipidemia, for which she had taken atorvastatin 40 mg/d for several years before discontinuing it 2 years earlier for unknown reasons. She had been evaluated multiple times in the primary care clinic and emergency department over the previous month. Each time, her strength was minimally reduced in the upper extremities on examination, her renal function and electrolytes were normal, and her creatine kinase (CK) level was elevated (16,000-20,000 U/L; normal range, 26-192 U/L). She was managed conservatively with fluids and given return precautions each time.
After her myalgias and weakness increased in severity, she presented to the emergency department with a muscle strength score of 4/5 in both shoulders, triceps, hip flexors, hip extensors, abductors, and adductors. Her laboratory results were significant for the presence of blood without red blood cells on her urine dipstick test and a CK level of 25,070 U/L. She was admitted for further evaluation of progressive myopathy and given aggressive IV fluid hydration to prevent renal injury based on her history of unilateral renal agenesis.
Infectious disease testing, which included a respiratory virus panel, acute hepatitis panel, HIV screening, Lyme antibody testing, cytomegalovirus DNA detection by polymerase chain reaction, Epstein-Barr virus capsid immunoglobulin M, and anti-streptolysin O, were negative. Electrolytes, inflammatory markers, and kidney function were normal. However, high-sensitivity troponin-T levels were elevated, with a peak value of 216.3 ng/L (normal range, 0-19 ng/L). The patient denied having any chest pain, and her electrocardiogram and transthoracic echocardiogram were normal. By hospital Day 4, her myalgias and weakness had improved, CK had stabilized (19,000-21,000 U/L), cardiac enzymes had improved, and urinalysis had normalized. She was discharged with a referral to a rheumatologist.
However, 10 days later—before she could see a rheumatologist—she was readmitted to a community hospital for recurrence of severe myalgias, progressive weakness, positive blood on urine dipstick testing, and a rising CK level (to 24,580 U/L) found during a follow-up appointment with her primary care physician. At this point, Neurology and Rheumatology were consulted and myositis-specific and myositis-associated autoantibody tests were sent out. Magnetic resonance imaging (MRI) of her thighs was performed and showed diffusely increased T2 signal and short tau inversion recovery in multiple proximal muscles (FIGURE).

DIAGNOSIS
Given her symmetrical proximal muscle weakness (which was refractory to IV fluid resuscitation), MRI findings, and the exclusion of infection and metabolic derangements, the patient was given a working diagnosis of myositis and treated with 1-g IV methylprednisolone followed by a 4-month steroid taper, methotrexate 20 mg weekly, and physical therapy. This working diagnosis was later confirmed with the results of her autoantibody tests.
At her 1-month follow-up visit, the patient reported minimal improvement in her strength, new neck weakness, and dysphagia with solids. Testing revealed anti–3-hydroxy-3-methylglutaryl-coenzyme A reductase (anti-HMGCR) antibody levels of more than 200 U/L (negative < 20 U/L; positive > 59 U/L), which pointed to a more refined diagnosis of anti-HMGCR immune-mediated necrotizing myositis.
DISCUSSION
Myositis should be in the differential diagnosis for patients with symmetrical proximal muscle weakness. Bohan and Peter devised a 5-part set of criteria to help diagnose myositis, shown in the TABLE.1,2 This simple framework broadens the differential and guides diagnostic testing. Our patient’s presentation was fairly typical for anti-HMGCR myositis, a subset of immune-mediated necrotizing myositis,3 with a pretest probability of 62% per the European League Against Rheumatism/American College of Rheumatology classification criteria.2 Probability of this diagnosis was further increased by the high-titer anti-HMGCR, so biopsy and electromyography (EMG), as noted by Bohan and Peter, were not pursued.

Continue to: Autoimmune myopathies...
Autoimmune myopathies occur in 9 to 14 per 100,000 people,4 with 6% of patients having anti-HMGCR auto-antibodies.5 Anti-HMGCR myositis is more prevalent in older women, patients with type 2 diabetes, and those with a history of atorvastatin use.3,6 Two-thirds of patients with anti-HMGCR myositis report current or prior statin use, and this increases to more than 90% in those age 50 years or older.5 Anti-HMGCR myositis causes significant muscle weakness that does not resolve with discontinuation of the statin and can occur years after the initiation or discontinuation of statin treatment.6 Cardiac involvement is rare4 but dysphagia is relatively common.7,8 Anti-HMGCR myositis also has a weak association with cancer, most commonly gastrointestinal and lung cancers.4,7
Distinguishing statin-induced myalgias from statin-induced myositis guides management. Statin-induced myalgias are associated with normal or slightly increased CK levels (typically < 1000 U/L) and resolve with discontinuation of the statin; the patient can often tolerate re-challenge with a statin.6 In contrast, CK elevation in patients with statin-induced myositis is typically more than 10,000 U/L6 and requires aggressive treatment with immunomodulatory medications to prevent permanent muscle damage.
Treatment recommendations are supported only by case series, observational studies, and expert opinion. Typical first-line treatment includes induction with high-dose corticosteroids followed by prolonged taper plus a conventional synthetic disease-modifying antirheumatic drug (csDMARD) such as methotrexate, azathioprine, or mycophenolate.4 Maintenance therapy often is achieved with csDMARD therapy for 2 years.4 Severe cases frequently are treated with combination csDMARD therapy (eg, methotrexate and azathioprine or methotrexate and mycophenolate).4 Rituximab and IV immunoglobulin (IVIG) are typically reserved for refractory cases.6 Usual monitoring for relapse includes muscle strength testing on examination and evaluation of trending CK levels.8
Our patient received monthly 2-g/kg IVIG infusions, which led to slow, consistent improvement in her strength and normalization of her CK levels to 181 U/L after 6 months.
THE TAKEAWAY
Anti-HMGCR myositis should be suspected in any patient currently or previously treated with a statin who presents with proximal muscle weakness, myalgias, or an elevated CK level. We suggest early subspecialty consultation to discuss whether antibody testing, EMG, or muscle biopsy are warranted. If anti-HMGCR myositis is confirmed, it is advisable to rule out comorbid malignancy and initiate early combination treatment to minimize relapses and permanent muscle damage.
CORRESPONDENCE
Daniel T. Schoenherr, MD, Family Medicine Residency, National Capital Consortium–Alexander T. Augusta Military Medical Center, 9300 DeWitt Loop, Fort Belvoir, VA 22060; [email protected]
1. Bohan A, Peter JB. Polymyositis and dermatomyositis (first of two parts). N Engl J Med. 1975;292:344-347. doi: 10.1056/NEJM197502132920706
2. Bottai M, Tjärnlund A, Santoni G, et al. EULAR/ACR classification criteria for adult and juvenile idiopathic inflammatory myopathies and their major subgroups: a methodology report. RMD Open. 2017;3:e000507. doi: 10.1136/rmdopen-2017-000507
3. Basharat P, Lahouti AH, Paik JJ, et al. Statin-induced anti-HMGCR-associated myopathy. J Am Coll Cardiol. 2016;68:234-235. doi: 10.1016/j.jacc.2016.04.037
4. Pinal-Fernandez I, Casal-Dominguez M, Mammen AL. Immune-mediated necrotizing myopathy. Curr Rheumatol Rep. 2018;20:21. doi: 10.1007/s11926-018-0732-6
5. Mammen AL, Chung T, Christopher-Stine L, et al. Autoantibodies against 3-hydroxy-3-methylglutaryl-coenzyme A reductase in patients with statin-associated autoimmune myopathy. Arthritis Rheum. 2011;63:713-721. doi: 10.1002/art.30156
6. Irvine NJ. Anti-HMGCR myopathy: a rare and serious side effect of statins. J Am Board Fam Med. 2020;33:785-788. doi: 10.3122/jabfm.2020.05.190450
7. Basharat P, Christopher-Stine L. Immune-mediated necrotizing myopathy: update on diagnosis and management. Curr Rheumatol Rep. 2015;17:72. doi: 10.1007/s11926-015-0548-6
8. Betteridge Z, McHugh N. Myositis-specific autoantibodies: an important tool to support diagnosis of myositis. J Int Med. 2016;280:8-23. doi: 10.1111/joim.12451
1. Bohan A, Peter JB. Polymyositis and dermatomyositis (first of two parts). N Engl J Med. 1975;292:344-347. doi: 10.1056/NEJM197502132920706
2. Bottai M, Tjärnlund A, Santoni G, et al. EULAR/ACR classification criteria for adult and juvenile idiopathic inflammatory myopathies and their major subgroups: a methodology report. RMD Open. 2017;3:e000507. doi: 10.1136/rmdopen-2017-000507
3. Basharat P, Lahouti AH, Paik JJ, et al. Statin-induced anti-HMGCR-associated myopathy. J Am Coll Cardiol. 2016;68:234-235. doi: 10.1016/j.jacc.2016.04.037
4. Pinal-Fernandez I, Casal-Dominguez M, Mammen AL. Immune-mediated necrotizing myopathy. Curr Rheumatol Rep. 2018;20:21. doi: 10.1007/s11926-018-0732-6
5. Mammen AL, Chung T, Christopher-Stine L, et al. Autoantibodies against 3-hydroxy-3-methylglutaryl-coenzyme A reductase in patients with statin-associated autoimmune myopathy. Arthritis Rheum. 2011;63:713-721. doi: 10.1002/art.30156
6. Irvine NJ. Anti-HMGCR myopathy: a rare and serious side effect of statins. J Am Board Fam Med. 2020;33:785-788. doi: 10.3122/jabfm.2020.05.190450
7. Basharat P, Christopher-Stine L. Immune-mediated necrotizing myopathy: update on diagnosis and management. Curr Rheumatol Rep. 2015;17:72. doi: 10.1007/s11926-015-0548-6
8. Betteridge Z, McHugh N. Myositis-specific autoantibodies: an important tool to support diagnosis of myositis. J Int Med. 2016;280:8-23. doi: 10.1111/joim.12451
► Myalgias and progressive symmetrical proximal weakness
► History of unilateral renal agenesis, type 2 diabetes, and hyperlipidemia
Alopecia Universalis Treated With Tofacitinib: The Role of JAK/STAT Inhibitors in Hair Regrowth
Alopecia areata (AA) is an autoimmune disease that immunopathogenetically is thought to be due to breakdown of the immune privilege of the proximal hair follicle during the anagen growth phase. Alopecia areata has been reported to have a lifetime prevalence of 1.7%.1 Recent studies have specifically identified cytotoxic CD8+ NKG2D+ T cells as being responsible for the activation of AA.2-4 Two interleukins—IL-2 and IL-15—have been implicated to be cytotoxic sensitizers allowing CD8+ T cells to secrete IFN-γ and recognize autoantigens via major histocompatibility complex class I.5,6 Janus kinases (JAKs) are enzymes that play major roles in many different molecular processes. Specifically, JAK1/3 has been determined to arbitrate IL-15 activation of receptors on CD8+ T cells.7 These cells then interact with CD4 T cells, mast cells, and other inflammatory cells to cause destruction of the hair follicle without damage to the keratinocyte and melanocyte stem cells, allowing for reversible yet relapsing hair loss.8
Treatment of AA is difficult, requiring patience and strict compliance while taking into account duration of disease, age at presentation, site involvement, patient expectations, cost and insurance coverage, prior therapies, and any comorbidities. At the time of this case, no US Food and Drug Administration–approved drug regimen existed for the treatment of AA, and, to date, no treatment is preventative.4 We present a case of a patient with alopecia universalis of 11 years’ duration that was refractory to intralesional triamcinolone, clobetasol, minoxidil, and UVB brush therapy yet was successfully treated with tofacitinib.
Case Report
A 29-year-old otherwise-healthy woman presented to our clinic for treatment of alopecia universalis of 11 years’ duration that flared intermittently despite various treatments. Her medical history was unremarkable; however, she had a brother with alopecia universalis. She had no family history of any other autoimmune disorders. At the current presentation, the patient was known to have alopecia universalis with scant evidence of exclamation-point hairs on dermoscopy. Her treatment plan at this point consisted of intralesional triamcinolone to the active areas at 10 mg/mL every 4 weeks, plus clobetasol foam 0.05% at bedtime, minoxidil foam 5% at bedtime, and a UVB brush 3 times a week for 6 months before progressing to universalis type because of hair loss in the eyebrows and eyelashes. This treatment plan continued for 1 year with minimal improvement of the alopecia (Figure 1).
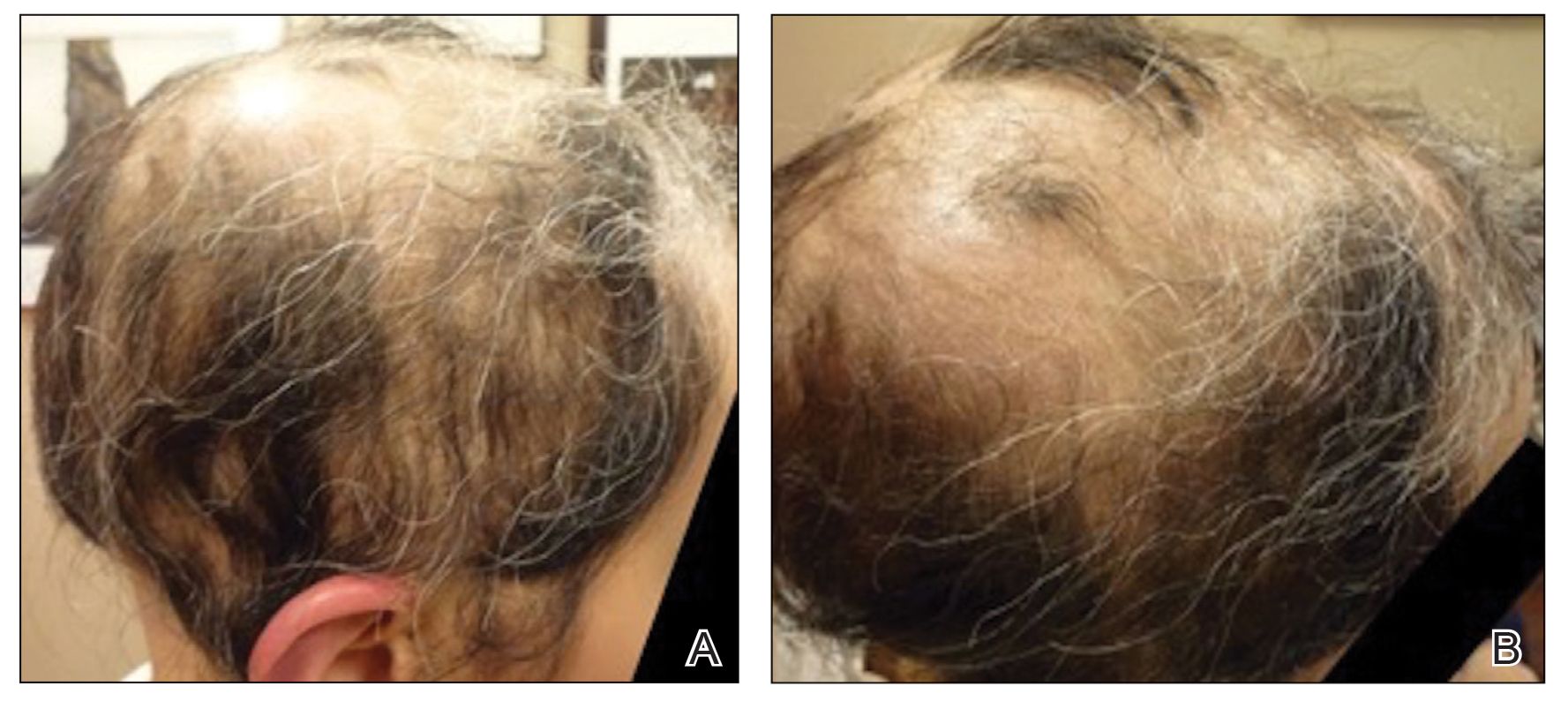
The patient was dissatisfied and wanted to discontinue therapy. Because these treatment options were exhausted with minimal benefit, the patient was then considered for treatment with tofacitinib. Baseline studies were performed, including purified protein derivative, complete blood cell count with differential, comprehensive metabolic panel, lipid profile, and liver function tests, all of which were within reference range. Insurance initially denied coverage of this therapy; a prior authorization was subsequently submitted and denied. A letter of medical necessity was then proposed, and approval for tofacitinib was finally granted. The patient was started on tofacitinib 5 mg twice daily and was monitored every 2 months with a complete blood cell count, comprehensive metabolic panel, lipid panels, and liver function tests. She had a platelet count of 112,000/μL (reference range, 150,000–450,000/μL) at baseline, and continued monitoring revealed a platelet count of 83,000 after 7 months of treatment. This platelet abnormality was evaluated by a hematologist and found to be within reference range; subsequent monitoring did not reveal any abnormalities.
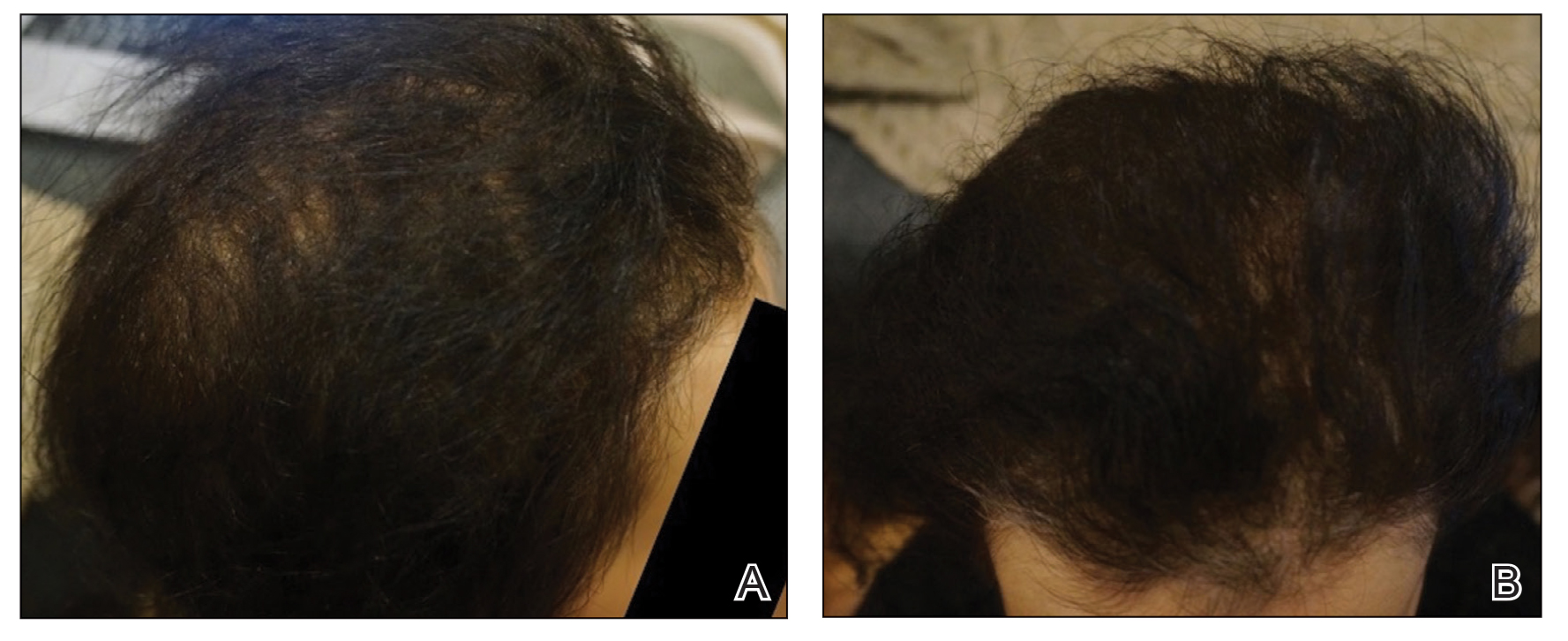
Initial hair growth on the scalp was diffuse with thin, white to light brown hairs in areas of hair loss at months 1 and 2, with progressive hair growth over months 3 to 7. Eyebrow hair growth was noted beginning at month 6. One year later, only hair regrowth occurred without any adverse events (Figure 2). After 5 years of treatment, the patient had a full head of thick hair (Figure 3). The tofacitinib dosage was 5 mg twice daily at initiation, and after 1 year increased to 10 mg twice daily. Her medical insurance subsequently changed and the regimen was adjusted to an 11-mg tablet and 5-mg tablet daily. She remained on this regimen with success.
Comment
Use of JAK Inhibitors—Reports and studies have shed light on the use and efficacy of JAK inhibitors in AA (Table).5-11 Tofacitinib is a selective JAK1/3 inhibitor that predominantly inhibits JAK3 but also inhibits JAK1, albeit to a lesser degree, which interferes with the JAK/STAT (signal transducer and activator of transcription) cascade responsible for the production, differentiation, and function of various B cells, T cells, and natural killer cells.2 Although it was developed for the management of allograft rejection, tofacitinib has made headway in rheumatology for treatment of patients with moderate to severe rheumatoid arthritis who are unable to take or are not responding to methotrexate.2 Since 2014, tofacitinib has been introduced to the therapeutic realm for AA but is not yet approved by the US Food and Drug Administration.3,4
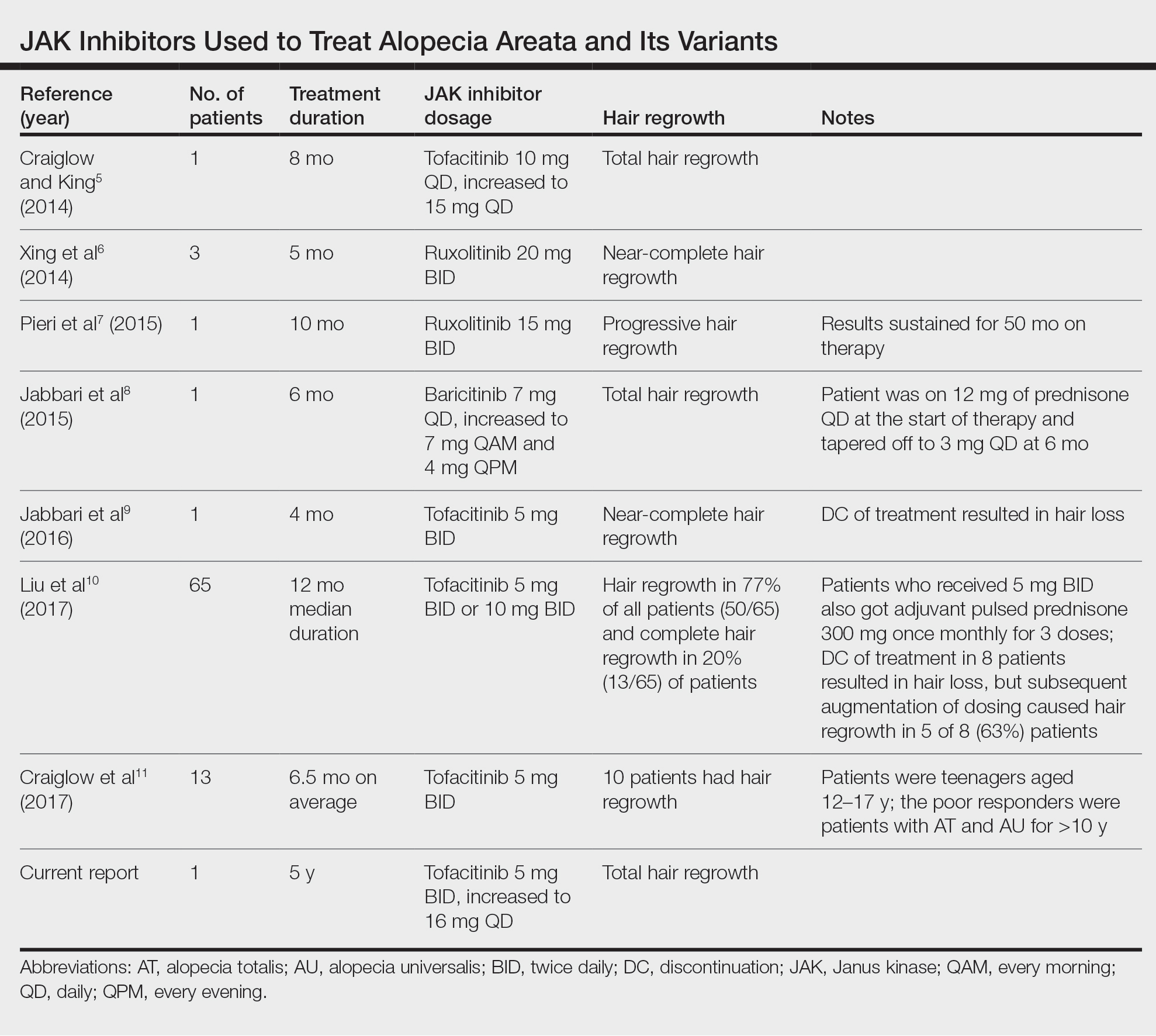
In 2014, Craiglow and King5 reported use of tofacitinib with dosages beginning at 10 mg/d and increasing to 15 mg/d in a patient with alopecia universalis and psoriasis. Total hair regrowth was noted after 8 months of therapy.5 Xing et al6 described 3 patients treated with ruxolitinib, a JAK1/2 inhibitor approved for the treatment of myelofibrosis, at an oral dose of 20 mg twice daily with near-complete hair regrowth after 5 months of treatment.6 Biopsies from lesions at baseline and after 3 months of therapy revealed a reduction in perifollicular T cells and in HLA class I and II expression in follicles.6 A patient in Italy with essential thrombocythemia and concurrent alopecia universalis was enrolled in a clinical trial with ruxolitinib and was treated with 15 mg twice daily. After 10 months of treatment, the patient had progressive hair regrowth that was sustained for more than 50 months of therapy.7 Baricitinib, a JAK1/2 inhibitor, was used in a 17-year-old adolescent boy to assess efficacy of the drug in
A recent retrospective study assessing response to tofacitinib in adults with AA (>40% hair loss), alopecia totalis, alopecia universalis, and stable or progressive diseases for at least 6 months determined a clinical response in 50 of 65 (77%) patients, with 13 patients exhibiting a complete response.10 Patients in this study were started on tofacitinib 5 mg twice daily with the addition of adjuvant pulsed prednisone (300 mg once monthly for 3 doses) with or without doubled dosing of tofacitinib if they had a halt in hair regrowth. This study demonstrated some benefit when pulsed prednisone was combined with the daily tofacitinib therapy. However, the study emphasized the importance of maintenance therapy, as 8 patients experienced hair loss with discontinuation after previously having hair regrowth; 5 (63%) of these patients experienced regrowth with augmentation of dosing or addition of adjuvant therapy.10
Another group of investigators assessed the efficacy of tofacitinib 5 mg in 13 adolescents aged 12 to 17 years, most with alopecia universalis (46% [6/13]); 10 of 13 (77%) patients responded to treatment with a mean duration of 6.5 months. The patients who had alopecia totalis and alopecia universalis for more than 10 years were poor responders to tofacitinib, and in fact, 1 of 13 (33%) patients in the study who did not respond to therapy had disease for 12 years.11 Therefore, starting tofacitinib either long-term or intermittently should be considered in children diagnosed early with severe AA, alopecia totalis, or alopecia universalis to prevent irreversible hair loss or progressive disease12,13; however, further data are required to assess efficacy and long-term benefits of this type of regimen.
Safety Profile—Widespread use of a medication is determined not only by its efficacy profile but also its safety profile. With any medication that exhibits immunosuppressive effects, adverse events must be considered and thoroughly discussed with patients and their primary care physicians. A prospective, open-label, single-arm trial examined the efficacy and safety of tofacitinib 5 mg twice daily in the treatment of AA and its more severe forms over 3 months.12 Of the 66 patients who completed the trial, 64% (42/66) exhibited a positive response to tofacitinib. Relapse was noted in 8.5 weeks after discontinuation of tofacitinib, reiterating the potential need for a maintenance regimen. In this study, 25.8% (17/66) of patients experienced infections as adverse events including (in decreasing order) upper respiratory tract infections, urinary tract infections, herpes zoster, conjunctivitis, bronchitis, mononucleosis, and paronychia. No reports of new or recurrent malignancy were noted. Other more constitutional adverse events were noted including headaches, abdominal pain, acne, diarrhea, fatigue, nausea, pruritus, hot flashes, cough, folliculitis, weight gain, dry eyes, and amenorrhea. One patient with a pre-existing liver condition experienced transaminitis that resolved with weight loss. There also were noted increases in low- and high-density lipoprotein levels.12 Our patient with baseline thrombocytopenia had mild drops in platelet count that subsequently stabilized and did not result in any bleeding abnormalities.
Duration of Therapy—Tofacitinib has demonstrated some preliminary success in the management of AA, but the appropriate duration of treatment requires further investigation. Our patient has been on tofacitinib for more than 5 years. She started at a total dosage of 10 mg/d, which increased to 16 mg/d. Initial dosing with maintenance regimens needs to be established for further widespread use to maximize benefit and minimize harm.
At what point do we decide to continue or stop treatment in patients who do not respond as expected or plateau? This is another critical question; our patient had periods of slowed growth and plateauing, but knowing the risks and benefits, she continued the medication and eventually experienced improved regrowth again.
Conclusion
Throughout the literature and in our patient, tofacitinib has demonstrated efficacy in treating AA. When other conventional therapies have failed, use of tofacitinib should be considered.
- Safavi KH, Muller SA, Suman VJ, et al. Incidence of alopecia areata in Olmstead County, Minnesota, 1975 through 1989. Mayo Clin Proc. 1995;70:628-633.
- Borazan NH, Furst DE. Nonsteroidal anti-inflammatory drugs, disease-modifying antirheumatic drugs, nonopioid analgesics, & drugs used in gout. In: Katzung BG, Trevor AJ, eds. Basic & Clinical Pharmacology. 13th ed. McGraw-Hill; 2015:618-642.
- Shapiro J. Current treatment of alopecia areata. J Investig Dermatol Symp Proc. 2013;16:S42-S44.
- Shapiro J. Dermatologic therapy: alopecia areata update. Dermatol Ther. 2011;24:301.
- Craiglow BG, King BA. Killing two birds with one stone: oral tofacitinib reverses alopecia universalis in a patient with plaque psoriasis. J Invest Dermatol. 2014;134:2988-2990.
- Xing L, Dai Z, Jabbari A, et al. Alopecia areata is driven by cytotoxic T lymphocytes and is reversed by JAK inhibition. Nat Med. 2014;20:1043-1049.
- Pieri L, Guglielmelli P, Vannucchi AM. Ruxolitinib-induced reversal of alopecia universalis in a patient with essential thrombocythemia. Am J Hematol. 2015;90:82-83.
- Jabbari A, Dai Z, Xing L, et al. Reversal of alopecia areata following treatment with the JAK1/2 inhibitor baricitinib. EbioMedicine. 2015;2:351-355.
- Jabbari A, Nguyen N, Cerise JE, et al. Treatment of an alopecia areata patient with tofacitinib results in regrowth of hair and changes in serum and skin biomarkers. Exp Dermatol. 2016;25:642-643.
- Liu LY, Craiglow BG, Dai F, et al. Tofacitinib for the treatment of severe alopecia areata and variants: a study of 90 patients. J Am Acad Dermatol. 2017;76:22-28.
- Craiglow BG, Liu LY, King BA. Tofacitinib for the treatment of alopecia areata and variants in adolescents. J Am Acad Dermatol. 2017;76:29-32.
- Kennedy Crispin M, Ko JM, Craiglow BG, et al. Safety and efficacy of the JAK inhibitor tofacitinib citrate in patients with alopecia areata. JCI Insight. 2016;1:E89776.
- Iorizzo M, Tosti A. Emerging drugs for alopecia areata: JAK inhibitors. Expert Opin Emerg Drugs. 2018;23:77-81.
Alopecia areata (AA) is an autoimmune disease that immunopathogenetically is thought to be due to breakdown of the immune privilege of the proximal hair follicle during the anagen growth phase. Alopecia areata has been reported to have a lifetime prevalence of 1.7%.1 Recent studies have specifically identified cytotoxic CD8+ NKG2D+ T cells as being responsible for the activation of AA.2-4 Two interleukins—IL-2 and IL-15—have been implicated to be cytotoxic sensitizers allowing CD8+ T cells to secrete IFN-γ and recognize autoantigens via major histocompatibility complex class I.5,6 Janus kinases (JAKs) are enzymes that play major roles in many different molecular processes. Specifically, JAK1/3 has been determined to arbitrate IL-15 activation of receptors on CD8+ T cells.7 These cells then interact with CD4 T cells, mast cells, and other inflammatory cells to cause destruction of the hair follicle without damage to the keratinocyte and melanocyte stem cells, allowing for reversible yet relapsing hair loss.8
Treatment of AA is difficult, requiring patience and strict compliance while taking into account duration of disease, age at presentation, site involvement, patient expectations, cost and insurance coverage, prior therapies, and any comorbidities. At the time of this case, no US Food and Drug Administration–approved drug regimen existed for the treatment of AA, and, to date, no treatment is preventative.4 We present a case of a patient with alopecia universalis of 11 years’ duration that was refractory to intralesional triamcinolone, clobetasol, minoxidil, and UVB brush therapy yet was successfully treated with tofacitinib.
Case Report
A 29-year-old otherwise-healthy woman presented to our clinic for treatment of alopecia universalis of 11 years’ duration that flared intermittently despite various treatments. Her medical history was unremarkable; however, she had a brother with alopecia universalis. She had no family history of any other autoimmune disorders. At the current presentation, the patient was known to have alopecia universalis with scant evidence of exclamation-point hairs on dermoscopy. Her treatment plan at this point consisted of intralesional triamcinolone to the active areas at 10 mg/mL every 4 weeks, plus clobetasol foam 0.05% at bedtime, minoxidil foam 5% at bedtime, and a UVB brush 3 times a week for 6 months before progressing to universalis type because of hair loss in the eyebrows and eyelashes. This treatment plan continued for 1 year with minimal improvement of the alopecia (Figure 1).

The patient was dissatisfied and wanted to discontinue therapy. Because these treatment options were exhausted with minimal benefit, the patient was then considered for treatment with tofacitinib. Baseline studies were performed, including purified protein derivative, complete blood cell count with differential, comprehensive metabolic panel, lipid profile, and liver function tests, all of which were within reference range. Insurance initially denied coverage of this therapy; a prior authorization was subsequently submitted and denied. A letter of medical necessity was then proposed, and approval for tofacitinib was finally granted. The patient was started on tofacitinib 5 mg twice daily and was monitored every 2 months with a complete blood cell count, comprehensive metabolic panel, lipid panels, and liver function tests. She had a platelet count of 112,000/μL (reference range, 150,000–450,000/μL) at baseline, and continued monitoring revealed a platelet count of 83,000 after 7 months of treatment. This platelet abnormality was evaluated by a hematologist and found to be within reference range; subsequent monitoring did not reveal any abnormalities.

Initial hair growth on the scalp was diffuse with thin, white to light brown hairs in areas of hair loss at months 1 and 2, with progressive hair growth over months 3 to 7. Eyebrow hair growth was noted beginning at month 6. One year later, only hair regrowth occurred without any adverse events (Figure 2). After 5 years of treatment, the patient had a full head of thick hair (Figure 3). The tofacitinib dosage was 5 mg twice daily at initiation, and after 1 year increased to 10 mg twice daily. Her medical insurance subsequently changed and the regimen was adjusted to an 11-mg tablet and 5-mg tablet daily. She remained on this regimen with success.

Comment
Use of JAK Inhibitors—Reports and studies have shed light on the use and efficacy of JAK inhibitors in AA (Table).5-11 Tofacitinib is a selective JAK1/3 inhibitor that predominantly inhibits JAK3 but also inhibits JAK1, albeit to a lesser degree, which interferes with the JAK/STAT (signal transducer and activator of transcription) cascade responsible for the production, differentiation, and function of various B cells, T cells, and natural killer cells.2 Although it was developed for the management of allograft rejection, tofacitinib has made headway in rheumatology for treatment of patients with moderate to severe rheumatoid arthritis who are unable to take or are not responding to methotrexate.2 Since 2014, tofacitinib has been introduced to the therapeutic realm for AA but is not yet approved by the US Food and Drug Administration.3,4

In 2014, Craiglow and King5 reported use of tofacitinib with dosages beginning at 10 mg/d and increasing to 15 mg/d in a patient with alopecia universalis and psoriasis. Total hair regrowth was noted after 8 months of therapy.5 Xing et al6 described 3 patients treated with ruxolitinib, a JAK1/2 inhibitor approved for the treatment of myelofibrosis, at an oral dose of 20 mg twice daily with near-complete hair regrowth after 5 months of treatment.6 Biopsies from lesions at baseline and after 3 months of therapy revealed a reduction in perifollicular T cells and in HLA class I and II expression in follicles.6 A patient in Italy with essential thrombocythemia and concurrent alopecia universalis was enrolled in a clinical trial with ruxolitinib and was treated with 15 mg twice daily. After 10 months of treatment, the patient had progressive hair regrowth that was sustained for more than 50 months of therapy.7 Baricitinib, a JAK1/2 inhibitor, was used in a 17-year-old adolescent boy to assess efficacy of the drug in
A recent retrospective study assessing response to tofacitinib in adults with AA (>40% hair loss), alopecia totalis, alopecia universalis, and stable or progressive diseases for at least 6 months determined a clinical response in 50 of 65 (77%) patients, with 13 patients exhibiting a complete response.10 Patients in this study were started on tofacitinib 5 mg twice daily with the addition of adjuvant pulsed prednisone (300 mg once monthly for 3 doses) with or without doubled dosing of tofacitinib if they had a halt in hair regrowth. This study demonstrated some benefit when pulsed prednisone was combined with the daily tofacitinib therapy. However, the study emphasized the importance of maintenance therapy, as 8 patients experienced hair loss with discontinuation after previously having hair regrowth; 5 (63%) of these patients experienced regrowth with augmentation of dosing or addition of adjuvant therapy.10
Another group of investigators assessed the efficacy of tofacitinib 5 mg in 13 adolescents aged 12 to 17 years, most with alopecia universalis (46% [6/13]); 10 of 13 (77%) patients responded to treatment with a mean duration of 6.5 months. The patients who had alopecia totalis and alopecia universalis for more than 10 years were poor responders to tofacitinib, and in fact, 1 of 13 (33%) patients in the study who did not respond to therapy had disease for 12 years.11 Therefore, starting tofacitinib either long-term or intermittently should be considered in children diagnosed early with severe AA, alopecia totalis, or alopecia universalis to prevent irreversible hair loss or progressive disease12,13; however, further data are required to assess efficacy and long-term benefits of this type of regimen.
Safety Profile—Widespread use of a medication is determined not only by its efficacy profile but also its safety profile. With any medication that exhibits immunosuppressive effects, adverse events must be considered and thoroughly discussed with patients and their primary care physicians. A prospective, open-label, single-arm trial examined the efficacy and safety of tofacitinib 5 mg twice daily in the treatment of AA and its more severe forms over 3 months.12 Of the 66 patients who completed the trial, 64% (42/66) exhibited a positive response to tofacitinib. Relapse was noted in 8.5 weeks after discontinuation of tofacitinib, reiterating the potential need for a maintenance regimen. In this study, 25.8% (17/66) of patients experienced infections as adverse events including (in decreasing order) upper respiratory tract infections, urinary tract infections, herpes zoster, conjunctivitis, bronchitis, mononucleosis, and paronychia. No reports of new or recurrent malignancy were noted. Other more constitutional adverse events were noted including headaches, abdominal pain, acne, diarrhea, fatigue, nausea, pruritus, hot flashes, cough, folliculitis, weight gain, dry eyes, and amenorrhea. One patient with a pre-existing liver condition experienced transaminitis that resolved with weight loss. There also were noted increases in low- and high-density lipoprotein levels.12 Our patient with baseline thrombocytopenia had mild drops in platelet count that subsequently stabilized and did not result in any bleeding abnormalities.
Duration of Therapy—Tofacitinib has demonstrated some preliminary success in the management of AA, but the appropriate duration of treatment requires further investigation. Our patient has been on tofacitinib for more than 5 years. She started at a total dosage of 10 mg/d, which increased to 16 mg/d. Initial dosing with maintenance regimens needs to be established for further widespread use to maximize benefit and minimize harm.
At what point do we decide to continue or stop treatment in patients who do not respond as expected or plateau? This is another critical question; our patient had periods of slowed growth and plateauing, but knowing the risks and benefits, she continued the medication and eventually experienced improved regrowth again.
Conclusion
Throughout the literature and in our patient, tofacitinib has demonstrated efficacy in treating AA. When other conventional therapies have failed, use of tofacitinib should be considered.
Alopecia areata (AA) is an autoimmune disease that immunopathogenetically is thought to be due to breakdown of the immune privilege of the proximal hair follicle during the anagen growth phase. Alopecia areata has been reported to have a lifetime prevalence of 1.7%.1 Recent studies have specifically identified cytotoxic CD8+ NKG2D+ T cells as being responsible for the activation of AA.2-4 Two interleukins—IL-2 and IL-15—have been implicated to be cytotoxic sensitizers allowing CD8+ T cells to secrete IFN-γ and recognize autoantigens via major histocompatibility complex class I.5,6 Janus kinases (JAKs) are enzymes that play major roles in many different molecular processes. Specifically, JAK1/3 has been determined to arbitrate IL-15 activation of receptors on CD8+ T cells.7 These cells then interact with CD4 T cells, mast cells, and other inflammatory cells to cause destruction of the hair follicle without damage to the keratinocyte and melanocyte stem cells, allowing for reversible yet relapsing hair loss.8
Treatment of AA is difficult, requiring patience and strict compliance while taking into account duration of disease, age at presentation, site involvement, patient expectations, cost and insurance coverage, prior therapies, and any comorbidities. At the time of this case, no US Food and Drug Administration–approved drug regimen existed for the treatment of AA, and, to date, no treatment is preventative.4 We present a case of a patient with alopecia universalis of 11 years’ duration that was refractory to intralesional triamcinolone, clobetasol, minoxidil, and UVB brush therapy yet was successfully treated with tofacitinib.
Case Report
A 29-year-old otherwise-healthy woman presented to our clinic for treatment of alopecia universalis of 11 years’ duration that flared intermittently despite various treatments. Her medical history was unremarkable; however, she had a brother with alopecia universalis. She had no family history of any other autoimmune disorders. At the current presentation, the patient was known to have alopecia universalis with scant evidence of exclamation-point hairs on dermoscopy. Her treatment plan at this point consisted of intralesional triamcinolone to the active areas at 10 mg/mL every 4 weeks, plus clobetasol foam 0.05% at bedtime, minoxidil foam 5% at bedtime, and a UVB brush 3 times a week for 6 months before progressing to universalis type because of hair loss in the eyebrows and eyelashes. This treatment plan continued for 1 year with minimal improvement of the alopecia (Figure 1).

The patient was dissatisfied and wanted to discontinue therapy. Because these treatment options were exhausted with minimal benefit, the patient was then considered for treatment with tofacitinib. Baseline studies were performed, including purified protein derivative, complete blood cell count with differential, comprehensive metabolic panel, lipid profile, and liver function tests, all of which were within reference range. Insurance initially denied coverage of this therapy; a prior authorization was subsequently submitted and denied. A letter of medical necessity was then proposed, and approval for tofacitinib was finally granted. The patient was started on tofacitinib 5 mg twice daily and was monitored every 2 months with a complete blood cell count, comprehensive metabolic panel, lipid panels, and liver function tests. She had a platelet count of 112,000/μL (reference range, 150,000–450,000/μL) at baseline, and continued monitoring revealed a platelet count of 83,000 after 7 months of treatment. This platelet abnormality was evaluated by a hematologist and found to be within reference range; subsequent monitoring did not reveal any abnormalities.

Initial hair growth on the scalp was diffuse with thin, white to light brown hairs in areas of hair loss at months 1 and 2, with progressive hair growth over months 3 to 7. Eyebrow hair growth was noted beginning at month 6. One year later, only hair regrowth occurred without any adverse events (Figure 2). After 5 years of treatment, the patient had a full head of thick hair (Figure 3). The tofacitinib dosage was 5 mg twice daily at initiation, and after 1 year increased to 10 mg twice daily. Her medical insurance subsequently changed and the regimen was adjusted to an 11-mg tablet and 5-mg tablet daily. She remained on this regimen with success.

Comment
Use of JAK Inhibitors—Reports and studies have shed light on the use and efficacy of JAK inhibitors in AA (Table).5-11 Tofacitinib is a selective JAK1/3 inhibitor that predominantly inhibits JAK3 but also inhibits JAK1, albeit to a lesser degree, which interferes with the JAK/STAT (signal transducer and activator of transcription) cascade responsible for the production, differentiation, and function of various B cells, T cells, and natural killer cells.2 Although it was developed for the management of allograft rejection, tofacitinib has made headway in rheumatology for treatment of patients with moderate to severe rheumatoid arthritis who are unable to take or are not responding to methotrexate.2 Since 2014, tofacitinib has been introduced to the therapeutic realm for AA but is not yet approved by the US Food and Drug Administration.3,4

In 2014, Craiglow and King5 reported use of tofacitinib with dosages beginning at 10 mg/d and increasing to 15 mg/d in a patient with alopecia universalis and psoriasis. Total hair regrowth was noted after 8 months of therapy.5 Xing et al6 described 3 patients treated with ruxolitinib, a JAK1/2 inhibitor approved for the treatment of myelofibrosis, at an oral dose of 20 mg twice daily with near-complete hair regrowth after 5 months of treatment.6 Biopsies from lesions at baseline and after 3 months of therapy revealed a reduction in perifollicular T cells and in HLA class I and II expression in follicles.6 A patient in Italy with essential thrombocythemia and concurrent alopecia universalis was enrolled in a clinical trial with ruxolitinib and was treated with 15 mg twice daily. After 10 months of treatment, the patient had progressive hair regrowth that was sustained for more than 50 months of therapy.7 Baricitinib, a JAK1/2 inhibitor, was used in a 17-year-old adolescent boy to assess efficacy of the drug in
A recent retrospective study assessing response to tofacitinib in adults with AA (>40% hair loss), alopecia totalis, alopecia universalis, and stable or progressive diseases for at least 6 months determined a clinical response in 50 of 65 (77%) patients, with 13 patients exhibiting a complete response.10 Patients in this study were started on tofacitinib 5 mg twice daily with the addition of adjuvant pulsed prednisone (300 mg once monthly for 3 doses) with or without doubled dosing of tofacitinib if they had a halt in hair regrowth. This study demonstrated some benefit when pulsed prednisone was combined with the daily tofacitinib therapy. However, the study emphasized the importance of maintenance therapy, as 8 patients experienced hair loss with discontinuation after previously having hair regrowth; 5 (63%) of these patients experienced regrowth with augmentation of dosing or addition of adjuvant therapy.10
Another group of investigators assessed the efficacy of tofacitinib 5 mg in 13 adolescents aged 12 to 17 years, most with alopecia universalis (46% [6/13]); 10 of 13 (77%) patients responded to treatment with a mean duration of 6.5 months. The patients who had alopecia totalis and alopecia universalis for more than 10 years were poor responders to tofacitinib, and in fact, 1 of 13 (33%) patients in the study who did not respond to therapy had disease for 12 years.11 Therefore, starting tofacitinib either long-term or intermittently should be considered in children diagnosed early with severe AA, alopecia totalis, or alopecia universalis to prevent irreversible hair loss or progressive disease12,13; however, further data are required to assess efficacy and long-term benefits of this type of regimen.
Safety Profile—Widespread use of a medication is determined not only by its efficacy profile but also its safety profile. With any medication that exhibits immunosuppressive effects, adverse events must be considered and thoroughly discussed with patients and their primary care physicians. A prospective, open-label, single-arm trial examined the efficacy and safety of tofacitinib 5 mg twice daily in the treatment of AA and its more severe forms over 3 months.12 Of the 66 patients who completed the trial, 64% (42/66) exhibited a positive response to tofacitinib. Relapse was noted in 8.5 weeks after discontinuation of tofacitinib, reiterating the potential need for a maintenance regimen. In this study, 25.8% (17/66) of patients experienced infections as adverse events including (in decreasing order) upper respiratory tract infections, urinary tract infections, herpes zoster, conjunctivitis, bronchitis, mononucleosis, and paronychia. No reports of new or recurrent malignancy were noted. Other more constitutional adverse events were noted including headaches, abdominal pain, acne, diarrhea, fatigue, nausea, pruritus, hot flashes, cough, folliculitis, weight gain, dry eyes, and amenorrhea. One patient with a pre-existing liver condition experienced transaminitis that resolved with weight loss. There also were noted increases in low- and high-density lipoprotein levels.12 Our patient with baseline thrombocytopenia had mild drops in platelet count that subsequently stabilized and did not result in any bleeding abnormalities.
Duration of Therapy—Tofacitinib has demonstrated some preliminary success in the management of AA, but the appropriate duration of treatment requires further investigation. Our patient has been on tofacitinib for more than 5 years. She started at a total dosage of 10 mg/d, which increased to 16 mg/d. Initial dosing with maintenance regimens needs to be established for further widespread use to maximize benefit and minimize harm.
At what point do we decide to continue or stop treatment in patients who do not respond as expected or plateau? This is another critical question; our patient had periods of slowed growth and plateauing, but knowing the risks and benefits, she continued the medication and eventually experienced improved regrowth again.
Conclusion
Throughout the literature and in our patient, tofacitinib has demonstrated efficacy in treating AA. When other conventional therapies have failed, use of tofacitinib should be considered.
- Safavi KH, Muller SA, Suman VJ, et al. Incidence of alopecia areata in Olmstead County, Minnesota, 1975 through 1989. Mayo Clin Proc. 1995;70:628-633.
- Borazan NH, Furst DE. Nonsteroidal anti-inflammatory drugs, disease-modifying antirheumatic drugs, nonopioid analgesics, & drugs used in gout. In: Katzung BG, Trevor AJ, eds. Basic & Clinical Pharmacology. 13th ed. McGraw-Hill; 2015:618-642.
- Shapiro J. Current treatment of alopecia areata. J Investig Dermatol Symp Proc. 2013;16:S42-S44.
- Shapiro J. Dermatologic therapy: alopecia areata update. Dermatol Ther. 2011;24:301.
- Craiglow BG, King BA. Killing two birds with one stone: oral tofacitinib reverses alopecia universalis in a patient with plaque psoriasis. J Invest Dermatol. 2014;134:2988-2990.
- Xing L, Dai Z, Jabbari A, et al. Alopecia areata is driven by cytotoxic T lymphocytes and is reversed by JAK inhibition. Nat Med. 2014;20:1043-1049.
- Pieri L, Guglielmelli P, Vannucchi AM. Ruxolitinib-induced reversal of alopecia universalis in a patient with essential thrombocythemia. Am J Hematol. 2015;90:82-83.
- Jabbari A, Dai Z, Xing L, et al. Reversal of alopecia areata following treatment with the JAK1/2 inhibitor baricitinib. EbioMedicine. 2015;2:351-355.
- Jabbari A, Nguyen N, Cerise JE, et al. Treatment of an alopecia areata patient with tofacitinib results in regrowth of hair and changes in serum and skin biomarkers. Exp Dermatol. 2016;25:642-643.
- Liu LY, Craiglow BG, Dai F, et al. Tofacitinib for the treatment of severe alopecia areata and variants: a study of 90 patients. J Am Acad Dermatol. 2017;76:22-28.
- Craiglow BG, Liu LY, King BA. Tofacitinib for the treatment of alopecia areata and variants in adolescents. J Am Acad Dermatol. 2017;76:29-32.
- Kennedy Crispin M, Ko JM, Craiglow BG, et al. Safety and efficacy of the JAK inhibitor tofacitinib citrate in patients with alopecia areata. JCI Insight. 2016;1:E89776.
- Iorizzo M, Tosti A. Emerging drugs for alopecia areata: JAK inhibitors. Expert Opin Emerg Drugs. 2018;23:77-81.
- Safavi KH, Muller SA, Suman VJ, et al. Incidence of alopecia areata in Olmstead County, Minnesota, 1975 through 1989. Mayo Clin Proc. 1995;70:628-633.
- Borazan NH, Furst DE. Nonsteroidal anti-inflammatory drugs, disease-modifying antirheumatic drugs, nonopioid analgesics, & drugs used in gout. In: Katzung BG, Trevor AJ, eds. Basic & Clinical Pharmacology. 13th ed. McGraw-Hill; 2015:618-642.
- Shapiro J. Current treatment of alopecia areata. J Investig Dermatol Symp Proc. 2013;16:S42-S44.
- Shapiro J. Dermatologic therapy: alopecia areata update. Dermatol Ther. 2011;24:301.
- Craiglow BG, King BA. Killing two birds with one stone: oral tofacitinib reverses alopecia universalis in a patient with plaque psoriasis. J Invest Dermatol. 2014;134:2988-2990.
- Xing L, Dai Z, Jabbari A, et al. Alopecia areata is driven by cytotoxic T lymphocytes and is reversed by JAK inhibition. Nat Med. 2014;20:1043-1049.
- Pieri L, Guglielmelli P, Vannucchi AM. Ruxolitinib-induced reversal of alopecia universalis in a patient with essential thrombocythemia. Am J Hematol. 2015;90:82-83.
- Jabbari A, Dai Z, Xing L, et al. Reversal of alopecia areata following treatment with the JAK1/2 inhibitor baricitinib. EbioMedicine. 2015;2:351-355.
- Jabbari A, Nguyen N, Cerise JE, et al. Treatment of an alopecia areata patient with tofacitinib results in regrowth of hair and changes in serum and skin biomarkers. Exp Dermatol. 2016;25:642-643.
- Liu LY, Craiglow BG, Dai F, et al. Tofacitinib for the treatment of severe alopecia areata and variants: a study of 90 patients. J Am Acad Dermatol. 2017;76:22-28.
- Craiglow BG, Liu LY, King BA. Tofacitinib for the treatment of alopecia areata and variants in adolescents. J Am Acad Dermatol. 2017;76:29-32.
- Kennedy Crispin M, Ko JM, Craiglow BG, et al. Safety and efficacy of the JAK inhibitor tofacitinib citrate in patients with alopecia areata. JCI Insight. 2016;1:E89776.
- Iorizzo M, Tosti A. Emerging drugs for alopecia areata: JAK inhibitors. Expert Opin Emerg Drugs. 2018;23:77-81.
Practice Points
- Janus kinase inhibitors target one of the cellular pathogeneses of alopecia areata.
- Janus kinase inhibitors may be an option for patients who have exhausted other treatment modalities for alopecia.
Pediatric Primary Cutaneous Marginal Zone Lymphoma Treated With Doxycycline
Case Report
An otherwise healthy 13-year-old boy was referred to pediatric dermatology with multiple asymptomatic erythematous papules throughout the trunk and arms of 6 months’ duration. He denied fevers, night sweats, or weight loss. A punch biopsy revealed a dense atypical lymphoid infiltrate with follicular prominence extending periadnexally and perivascularly, which was most consistent with extranodal marginal zone lymphoma of mucosa-associated lymphoid tissue (Figures 1A and 1B). Cells were positive for Bcl-2, CD23, and CD20 (Figure 1C). Polymerase chain reaction analysis of the immunoglobulin heavy and κ chain gene rearrangements were positive, indicating the presence of a clonal B-cell expansion. The patient’s complete blood cell count, complete metabolic profile, serum lactate dehydrogenase, and erythrocyte sedimentation rate were within reference range. Lyme disease antibodies, Helicobacter pylori testing, thyroid function testing, thyroid antibodies, anti–Sjogren syndrome–related antigen A antibody, and anti–Sjogren syndrome–related antigen B were negative. Additionally, positron emission tomography (PET) with computed tomography (CT) revealed no abnormalities. He was diagnosed with stage T3b primary cutaneous marginal zone lymphoma (PCMZL) due to cutaneous involvement of 3 or more body regions.
The patient was started on clobetasol ointment 0.05% twice daily to the affected areas. After 2 months, he had progression of cutaneous disease, including increased number of lesions; erythema; and induration of lesions on the chest, back, and arms (Figure 2A) and was started on oral doxycycline 100 mg twice daily with subsequent notable improvement of the skin lesions at 2-week follow-up, including decreased erythema and induration of all lesions. He then received intralesional triamcinolone 20 mg/mL injections to 4 residual lesions; clobetasol ointment 0.05% twice daily was continued for the remaining lesions as needed for pruritus. He continued doxycycline for 4 months with further improvement of lesions (Figure 2B). Six months after discontinuing doxycycline, 2 small residual lesions remained on the left arm and back, but the patient did not develop any new or recurrent lesions.
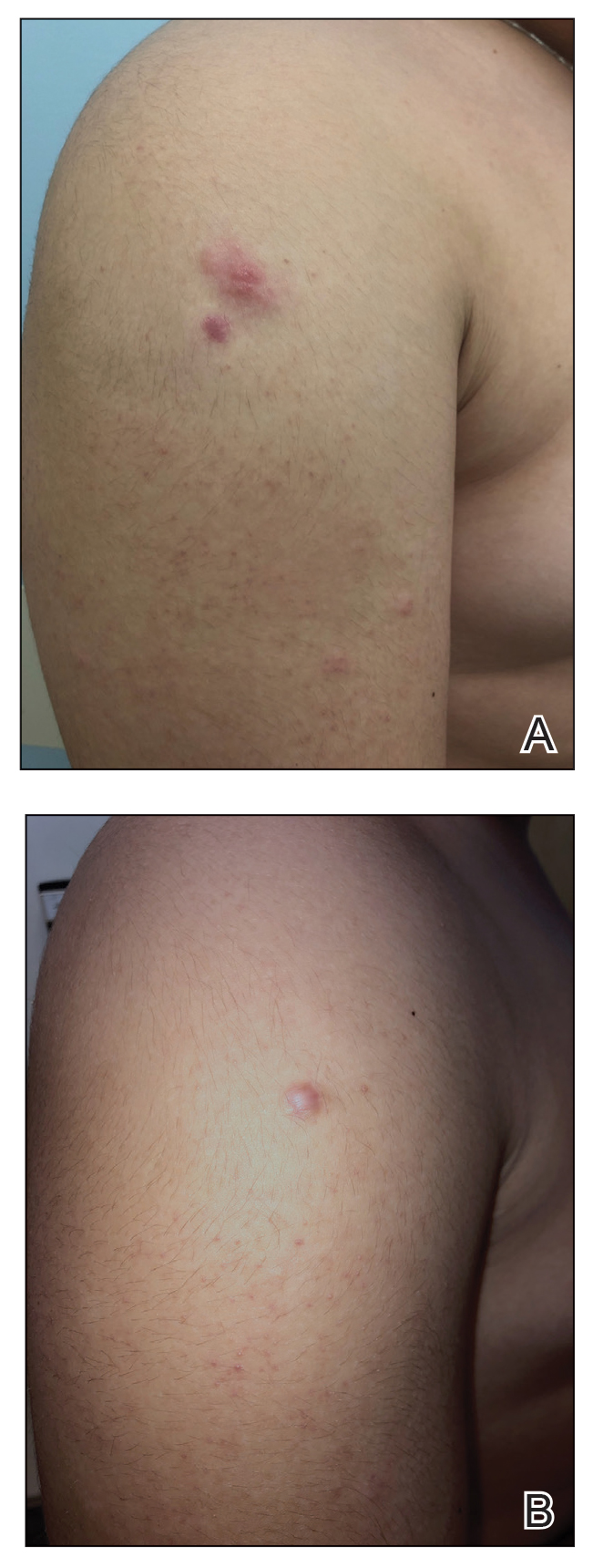
Comment
Clinical Presentation—Primary cutaneous B-cell lymphomas include PCMZL, primary cutaneous follicle center lymphoma, and primary cutaneous large B-cell lymphoma. Primary cutaneous marginal zone lymphoma is an indolent extranodal B-cell lymphoma composed of small B cells, marginal zone cells, lymphoplasmacytoid cells, and mature plasma cells.1
Primary cutaneous marginal zone lymphoma typically presents in the fourth to sixth decades of life and is rare in children, with fewer than 40 cases in patients younger than 20 years.2 Amitay-Laish and colleagues2 reported 29 patients with pediatric PCMZL ranging in age from 1 to 19.5 years at diagnosis, with the majority of patients diagnosed after 10 years of age. Clinically, patients present with multifocal, erythematous to brown, dermal papules, plaques, and nodules most commonly distributed on the trunk and arms. A retrospective review of 11 pediatric patients with PCMZL over a median of 5.5 years demonstrated that the clinical presentation, histopathology, molecular findings, and prognosis of pediatric PCMZL appears similar to adult PCMZL.2 Cutaneous relapse is common, but extracutaneous spread is rare. The prognosis is excellent, with a disease-free survival rate of 93%.3
Diagnosis—The diagnosis of PCMZL requires histopathologic analysis of involved skin as well as exclusion of extracutaneous disease at the time of diagnosis during initial staging evaluation. Histologically there are nodular infiltrates of small lymphocytes in interfollicular compartments, reactive germinal centers, and clonality with monotypic immunoglobulin heavy chain genes.4 Laboratory workup should include complete blood cell count with differential, complete metabolic panel, and serum lactate dehydrogenase level. If lymphocytosis is present, flow cytometry of peripheral blood cells should be performed. Radiographic imaging with contrast-enhanced CT or PET/CT of the chest, abdomen, and pelvis should be performed for routine staging in most patients, with imaging of the neck recommended when cervical lymphadenopathy is detected.5 Patients with multifocal skin lesions should receive PET/CT to exclude systemic disease and assess lymph nodes. Bone marrow studies are not required for diagnosis.5,6
Associated Conditions—Systemic marginal zone lymphoma has been associated with autoimmune conditions, including Hashimoto thyroiditis and Sjögren syndrome; however, this association has not been shown in PCMZL and was not found in our patient.7,8 Borrelia-positive serology has been described in cases of PCMZL in Europe. The pathogenesis has been speculated to be due to chronic antigen stimulation related to the geographic distribution of Borrelia species.9 In endemic areas, Borrelia testing with serology or DNA testing of skin is recommended; however, there has been no strong correlation between Borrelia burgdorferi and PCMZL found in North America or Asia.9,10 Helicobacter pylori has been associated with gastric mucosal-associated lymphatic tissue lymphoma, which responds well to antibiotic therapy. However, an association between PCMZL and H pylori has not been well described.11
Management—Several treatment modalities have been attempted in patients with PCMZL with varying efficacy. Given the rarity of this disease, there is no standard therapy. Treatment options include radiation therapy, excision, topical steroids, intralesional steroids, intralesional rituximab, and antibiotics.2,12-14 Case reports of pediatric patients have demonstrated improvement with excision,15-19 intralesional steroids,20,21 intralesional rituximab,22 and clobetasol cream.23,24 In asymptomatic patients, watchful waiting often is employed given the overall indolent nature of PCMZL. Antibiotic therapy may be favored in Borrelia-positive cases. However, even in B burgdorferi–negative patients, there have been cases where there is response to antibiotics, particularly doxycycline.2,15,25 We elected for a trial of doxycycline in our patient based on these prior reports, along with the overall favorable side-effect profile of doxycycline for adolescents and our patient’s widespread cutaneous involvement.
Doxycycline is utilized in pediatric patients 8 years and older for numerous indications, including treatment of acne, Rocky Mountain spotted fever, and Lyme disease. Use of doxycycline in younger patients typically is avoided given the risk for dental enamel hypoplasia, tooth discoloration, and possible delays in skeletal development. Originally utilized for its antibacterial effects as an intracellular inhibitor of protein synthesis, doxycycline has been repurposed for oncologic therapies. It has been shown to have cytotoxic and antiproliferative properties in various cancer cells and also may inhibit leukemic cell migration.26 In PCMZL, doxycycline initially was utilized in Borrelia-positive patients in Europe and found to improve disease clearance.27 In patients without Borrelia infection, doxycycline is thought to enhance apoptosis through caspase-3 activation along with p53 and Bax upregulation.28
Intralesional triamcinolone alone may not be feasible in pediatric PCMZL patients because of widespread involvement, and doxycycline may be considered as a treatment option. Multiple low-risk treatment modalities may be used in conjunction to clear disease in pediatric patients, as demonstrated in our case.
Acknowledgment—We thank Ali Nael Amzajerdi, MD (Orange, California), for his contributions to the pathologic imaging in this report.
- Willemze R, Cerroni L, Kempf W, et al. The 2018 update of the WHO-EORTC classification for primary cutaneous lymphomas. Blood. 2019;133:1703-1714.
- Amitay-Laish I, Tavallaee M, Kim J, et al. Paediatric primary cutaneous marginal zone B-cell lymphoma: does it differ from its adult counterpart? Br J Dermatol. 2017;176:1010-1020.
- Servitje O, Muniesa C, Benavente Y, et al. Primary cutaneous marginal zone B-cell lymphoma: response to treatment and disease-free survival in a series of 137 patients. J Am Acad Dermatol. 2013;69:357-365.
- Vitiello P, Sica A, Ronchi A, et al. Primary cutaneous B-cell lymphomas: an update. Front Oncol. 2020;10:651.
- Tadiotto Cicogna G, Ferranti M, Alaibac M. Diagnostic workup of primary cutaneous B cell lymphomas: a clinician’s approach. Front Oncol. 2020;10:988.
- Willemze R, Hodak E, Zinzani PL, et al. Primary cutaneous lymphomas: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2013;24:149-154.
- Pereira FO, Graf H, Nomura LM, et al. Concomitant presentation of Hashimoto’s thyroiditis and maltoma of the thyroid in a twenty-year-old man with a rapidly growing mass in the neck. Thyroid. 2000;10:833-835.
- Ekström Smedby K, Vajdic CM, Falster M, et al. Autoimmune disorders and risk of non-Hodgkin lymphoma subtypes: a pooled analysis within the InterLymph Consortium. Blood. 2008;111:4029-4038.
- Slater DN. Borrelia burgdorferi-associated primary cutaneous B-cell lymphoma. Histopathology. 2001;38:73-77.
- Wood GS, Kamath NV, Guitart J, et al. Absence of Borrelia burgdorferi DNA in cutaneous B-cell lymphomas from the United States. J Cutan Pathol. 2001;28:502-507.
- Dalle S, Thomas L, Balme B, et al. Primary cutaneous marginal zone lymphoma. Crit Rev Oncol Hematol. 2010;74:156-162.
- Senff NJ, Noordijk EM, Kim YH, et al. European Organization for Research and Treatment of Cancer and International Society for Cutaneous Lymphoma consensus recommendations for the management of cutaneous B-cell lymphomas. Blood. 2008;112:1600-1609.
- Hamilton SN, Wai ES, Tan K, et al. Treatment and outcomes in patients with primary cutaneous B-cell lymphoma: the BC Cancer Agency experience. Int J Radiat Oncol Biol Phys. 2013;87:719-725.
- Peñate Y, Hernández-Machín B, Pérez-Méndez LI, et al. Intralesional rituximab in the treatment of indolent primary cutaneous B-cell lymphomas: an epidemiological observational multicentre study. The Spanish Working Group on Cutaneous Lymphoma. Br J Dermatol. 2012;167:174-179.
- Kempf W, Kazakov DV, Buechner SA, et al. Primary cutaneous marginal zone lymphoma in children: a report of 3 cases and review of the literature. Am J Dermatopathol. 2014;36:661-666.
- Ghatalia P, Porter J, Wroblewski D, et al. Primary cutaneous marginal zone lymphoma associated with juxta-articular fibrotic nodules in a teenager. J Cutan Pathol. 2013;40:477-484.
- Dargent JL, Devalck C, De Mey A, et al. Primary cutaneous marginal zone B-cell lymphoma of MALT type in a child. Pediatr Dev Pathol. 2006;9:468-473.
- Sroa N, Magro CM. Pediatric primary cutaneous marginal zone lymphoma: in association with chronic antihistamine use. J Cutan Pathol. 2006;33(suppl 2):1-5.
- Zambrano E, Mejıa-Mejıa O, Bifulco C, et al. Extranodal marginal zone B-cell lymphoma/maltoma of the lip in a child: case report and review of cutaneous lymphoid proliferations in childhood. Int J Surg Pathol. 2006;14:163-169.
- Kollipara R, Hans A, Hall J, et al. A case report of primary cutaneous marginal zone lymphoma treated with intralesional steroids. Dermatol Online J. 2015;21:13030/qt9s15929m.
- Skaljic M, Cotton CH, Reilly AF, et al. Complete resolution of primary cutaneous marginal zone B-cell lymphoma on the cheek of a 7-year-old boy with intralesional triamcinolone and tincture of time. Pediatr Dermatol. 2020;37:228-229.
- Park MY, Jung HJ, Park JE, et al. Pediatric primary cutaneous marginal zone B-cell lymphoma treated with intralesional rituximab. Eur J Dermatol. 2010;20:533-534.
- Amitay-Laish I, Feinmesser M, Ben-Amitai D, et al. Juvenile onset of primary low-grade cutaneous B-cell lymphoma. Br J Dermatol. 2009;161:140-147.
- Sharon V, Mecca PS, Steinherz PG, et al. Two pediatric cases of primary cutaneous B-cell lymphoma and review of the literature. Pediatr Dermatol. 2009;26:34-39.
- Jothishankar B, Di Raimondo C, Mueller L, et al. Primary cutaneous marginal zone lymphoma treated with doxycycline in a pediatric patient. Pediatr Dermatol. 2020;37:759-761.
- Markowska A, Kaysiewicz J, Markowska J, et al. Doxycycline, salinomycin, monensin and ivermectin repositioned as cancer drugs. Bioorg Med Chem Lett. 2019;29:1549-1554.
- Kutting B, Bonsmann G, Metze D, et al. Borrelia burgdorferi-associated primary cutaneous B-cell lymphoma: complete clearing of skin lesions after antibiotic pulse therapy or intralesional injection of interferon alfa-2a. J Am Acad Dermatol. 1997;36:311-314.
- Protasoni M, Kroon AM, Taanman JW. Mitochondria as oncotarget: a comparison between the tetracycline analogs doxycycline and COL-3. Oncotarget. 2018;9:33818-33831.
Case Report
An otherwise healthy 13-year-old boy was referred to pediatric dermatology with multiple asymptomatic erythematous papules throughout the trunk and arms of 6 months’ duration. He denied fevers, night sweats, or weight loss. A punch biopsy revealed a dense atypical lymphoid infiltrate with follicular prominence extending periadnexally and perivascularly, which was most consistent with extranodal marginal zone lymphoma of mucosa-associated lymphoid tissue (Figures 1A and 1B). Cells were positive for Bcl-2, CD23, and CD20 (Figure 1C). Polymerase chain reaction analysis of the immunoglobulin heavy and κ chain gene rearrangements were positive, indicating the presence of a clonal B-cell expansion. The patient’s complete blood cell count, complete metabolic profile, serum lactate dehydrogenase, and erythrocyte sedimentation rate were within reference range. Lyme disease antibodies, Helicobacter pylori testing, thyroid function testing, thyroid antibodies, anti–Sjogren syndrome–related antigen A antibody, and anti–Sjogren syndrome–related antigen B were negative. Additionally, positron emission tomography (PET) with computed tomography (CT) revealed no abnormalities. He was diagnosed with stage T3b primary cutaneous marginal zone lymphoma (PCMZL) due to cutaneous involvement of 3 or more body regions.

The patient was started on clobetasol ointment 0.05% twice daily to the affected areas. After 2 months, he had progression of cutaneous disease, including increased number of lesions; erythema; and induration of lesions on the chest, back, and arms (Figure 2A) and was started on oral doxycycline 100 mg twice daily with subsequent notable improvement of the skin lesions at 2-week follow-up, including decreased erythema and induration of all lesions. He then received intralesional triamcinolone 20 mg/mL injections to 4 residual lesions; clobetasol ointment 0.05% twice daily was continued for the remaining lesions as needed for pruritus. He continued doxycycline for 4 months with further improvement of lesions (Figure 2B). Six months after discontinuing doxycycline, 2 small residual lesions remained on the left arm and back, but the patient did not develop any new or recurrent lesions.

Comment
Clinical Presentation—Primary cutaneous B-cell lymphomas include PCMZL, primary cutaneous follicle center lymphoma, and primary cutaneous large B-cell lymphoma. Primary cutaneous marginal zone lymphoma is an indolent extranodal B-cell lymphoma composed of small B cells, marginal zone cells, lymphoplasmacytoid cells, and mature plasma cells.1
Primary cutaneous marginal zone lymphoma typically presents in the fourth to sixth decades of life and is rare in children, with fewer than 40 cases in patients younger than 20 years.2 Amitay-Laish and colleagues2 reported 29 patients with pediatric PCMZL ranging in age from 1 to 19.5 years at diagnosis, with the majority of patients diagnosed after 10 years of age. Clinically, patients present with multifocal, erythematous to brown, dermal papules, plaques, and nodules most commonly distributed on the trunk and arms. A retrospective review of 11 pediatric patients with PCMZL over a median of 5.5 years demonstrated that the clinical presentation, histopathology, molecular findings, and prognosis of pediatric PCMZL appears similar to adult PCMZL.2 Cutaneous relapse is common, but extracutaneous spread is rare. The prognosis is excellent, with a disease-free survival rate of 93%.3
Diagnosis—The diagnosis of PCMZL requires histopathologic analysis of involved skin as well as exclusion of extracutaneous disease at the time of diagnosis during initial staging evaluation. Histologically there are nodular infiltrates of small lymphocytes in interfollicular compartments, reactive germinal centers, and clonality with monotypic immunoglobulin heavy chain genes.4 Laboratory workup should include complete blood cell count with differential, complete metabolic panel, and serum lactate dehydrogenase level. If lymphocytosis is present, flow cytometry of peripheral blood cells should be performed. Radiographic imaging with contrast-enhanced CT or PET/CT of the chest, abdomen, and pelvis should be performed for routine staging in most patients, with imaging of the neck recommended when cervical lymphadenopathy is detected.5 Patients with multifocal skin lesions should receive PET/CT to exclude systemic disease and assess lymph nodes. Bone marrow studies are not required for diagnosis.5,6
Associated Conditions—Systemic marginal zone lymphoma has been associated with autoimmune conditions, including Hashimoto thyroiditis and Sjögren syndrome; however, this association has not been shown in PCMZL and was not found in our patient.7,8 Borrelia-positive serology has been described in cases of PCMZL in Europe. The pathogenesis has been speculated to be due to chronic antigen stimulation related to the geographic distribution of Borrelia species.9 In endemic areas, Borrelia testing with serology or DNA testing of skin is recommended; however, there has been no strong correlation between Borrelia burgdorferi and PCMZL found in North America or Asia.9,10 Helicobacter pylori has been associated with gastric mucosal-associated lymphatic tissue lymphoma, which responds well to antibiotic therapy. However, an association between PCMZL and H pylori has not been well described.11
Management—Several treatment modalities have been attempted in patients with PCMZL with varying efficacy. Given the rarity of this disease, there is no standard therapy. Treatment options include radiation therapy, excision, topical steroids, intralesional steroids, intralesional rituximab, and antibiotics.2,12-14 Case reports of pediatric patients have demonstrated improvement with excision,15-19 intralesional steroids,20,21 intralesional rituximab,22 and clobetasol cream.23,24 In asymptomatic patients, watchful waiting often is employed given the overall indolent nature of PCMZL. Antibiotic therapy may be favored in Borrelia-positive cases. However, even in B burgdorferi–negative patients, there have been cases where there is response to antibiotics, particularly doxycycline.2,15,25 We elected for a trial of doxycycline in our patient based on these prior reports, along with the overall favorable side-effect profile of doxycycline for adolescents and our patient’s widespread cutaneous involvement.
Doxycycline is utilized in pediatric patients 8 years and older for numerous indications, including treatment of acne, Rocky Mountain spotted fever, and Lyme disease. Use of doxycycline in younger patients typically is avoided given the risk for dental enamel hypoplasia, tooth discoloration, and possible delays in skeletal development. Originally utilized for its antibacterial effects as an intracellular inhibitor of protein synthesis, doxycycline has been repurposed for oncologic therapies. It has been shown to have cytotoxic and antiproliferative properties in various cancer cells and also may inhibit leukemic cell migration.26 In PCMZL, doxycycline initially was utilized in Borrelia-positive patients in Europe and found to improve disease clearance.27 In patients without Borrelia infection, doxycycline is thought to enhance apoptosis through caspase-3 activation along with p53 and Bax upregulation.28
Intralesional triamcinolone alone may not be feasible in pediatric PCMZL patients because of widespread involvement, and doxycycline may be considered as a treatment option. Multiple low-risk treatment modalities may be used in conjunction to clear disease in pediatric patients, as demonstrated in our case.
Acknowledgment—We thank Ali Nael Amzajerdi, MD (Orange, California), for his contributions to the pathologic imaging in this report.
Case Report
An otherwise healthy 13-year-old boy was referred to pediatric dermatology with multiple asymptomatic erythematous papules throughout the trunk and arms of 6 months’ duration. He denied fevers, night sweats, or weight loss. A punch biopsy revealed a dense atypical lymphoid infiltrate with follicular prominence extending periadnexally and perivascularly, which was most consistent with extranodal marginal zone lymphoma of mucosa-associated lymphoid tissue (Figures 1A and 1B). Cells were positive for Bcl-2, CD23, and CD20 (Figure 1C). Polymerase chain reaction analysis of the immunoglobulin heavy and κ chain gene rearrangements were positive, indicating the presence of a clonal B-cell expansion. The patient’s complete blood cell count, complete metabolic profile, serum lactate dehydrogenase, and erythrocyte sedimentation rate were within reference range. Lyme disease antibodies, Helicobacter pylori testing, thyroid function testing, thyroid antibodies, anti–Sjogren syndrome–related antigen A antibody, and anti–Sjogren syndrome–related antigen B were negative. Additionally, positron emission tomography (PET) with computed tomography (CT) revealed no abnormalities. He was diagnosed with stage T3b primary cutaneous marginal zone lymphoma (PCMZL) due to cutaneous involvement of 3 or more body regions.

The patient was started on clobetasol ointment 0.05% twice daily to the affected areas. After 2 months, he had progression of cutaneous disease, including increased number of lesions; erythema; and induration of lesions on the chest, back, and arms (Figure 2A) and was started on oral doxycycline 100 mg twice daily with subsequent notable improvement of the skin lesions at 2-week follow-up, including decreased erythema and induration of all lesions. He then received intralesional triamcinolone 20 mg/mL injections to 4 residual lesions; clobetasol ointment 0.05% twice daily was continued for the remaining lesions as needed for pruritus. He continued doxycycline for 4 months with further improvement of lesions (Figure 2B). Six months after discontinuing doxycycline, 2 small residual lesions remained on the left arm and back, but the patient did not develop any new or recurrent lesions.

Comment
Clinical Presentation—Primary cutaneous B-cell lymphomas include PCMZL, primary cutaneous follicle center lymphoma, and primary cutaneous large B-cell lymphoma. Primary cutaneous marginal zone lymphoma is an indolent extranodal B-cell lymphoma composed of small B cells, marginal zone cells, lymphoplasmacytoid cells, and mature plasma cells.1
Primary cutaneous marginal zone lymphoma typically presents in the fourth to sixth decades of life and is rare in children, with fewer than 40 cases in patients younger than 20 years.2 Amitay-Laish and colleagues2 reported 29 patients with pediatric PCMZL ranging in age from 1 to 19.5 years at diagnosis, with the majority of patients diagnosed after 10 years of age. Clinically, patients present with multifocal, erythematous to brown, dermal papules, plaques, and nodules most commonly distributed on the trunk and arms. A retrospective review of 11 pediatric patients with PCMZL over a median of 5.5 years demonstrated that the clinical presentation, histopathology, molecular findings, and prognosis of pediatric PCMZL appears similar to adult PCMZL.2 Cutaneous relapse is common, but extracutaneous spread is rare. The prognosis is excellent, with a disease-free survival rate of 93%.3
Diagnosis—The diagnosis of PCMZL requires histopathologic analysis of involved skin as well as exclusion of extracutaneous disease at the time of diagnosis during initial staging evaluation. Histologically there are nodular infiltrates of small lymphocytes in interfollicular compartments, reactive germinal centers, and clonality with monotypic immunoglobulin heavy chain genes.4 Laboratory workup should include complete blood cell count with differential, complete metabolic panel, and serum lactate dehydrogenase level. If lymphocytosis is present, flow cytometry of peripheral blood cells should be performed. Radiographic imaging with contrast-enhanced CT or PET/CT of the chest, abdomen, and pelvis should be performed for routine staging in most patients, with imaging of the neck recommended when cervical lymphadenopathy is detected.5 Patients with multifocal skin lesions should receive PET/CT to exclude systemic disease and assess lymph nodes. Bone marrow studies are not required for diagnosis.5,6
Associated Conditions—Systemic marginal zone lymphoma has been associated with autoimmune conditions, including Hashimoto thyroiditis and Sjögren syndrome; however, this association has not been shown in PCMZL and was not found in our patient.7,8 Borrelia-positive serology has been described in cases of PCMZL in Europe. The pathogenesis has been speculated to be due to chronic antigen stimulation related to the geographic distribution of Borrelia species.9 In endemic areas, Borrelia testing with serology or DNA testing of skin is recommended; however, there has been no strong correlation between Borrelia burgdorferi and PCMZL found in North America or Asia.9,10 Helicobacter pylori has been associated with gastric mucosal-associated lymphatic tissue lymphoma, which responds well to antibiotic therapy. However, an association between PCMZL and H pylori has not been well described.11
Management—Several treatment modalities have been attempted in patients with PCMZL with varying efficacy. Given the rarity of this disease, there is no standard therapy. Treatment options include radiation therapy, excision, topical steroids, intralesional steroids, intralesional rituximab, and antibiotics.2,12-14 Case reports of pediatric patients have demonstrated improvement with excision,15-19 intralesional steroids,20,21 intralesional rituximab,22 and clobetasol cream.23,24 In asymptomatic patients, watchful waiting often is employed given the overall indolent nature of PCMZL. Antibiotic therapy may be favored in Borrelia-positive cases. However, even in B burgdorferi–negative patients, there have been cases where there is response to antibiotics, particularly doxycycline.2,15,25 We elected for a trial of doxycycline in our patient based on these prior reports, along with the overall favorable side-effect profile of doxycycline for adolescents and our patient’s widespread cutaneous involvement.
Doxycycline is utilized in pediatric patients 8 years and older for numerous indications, including treatment of acne, Rocky Mountain spotted fever, and Lyme disease. Use of doxycycline in younger patients typically is avoided given the risk for dental enamel hypoplasia, tooth discoloration, and possible delays in skeletal development. Originally utilized for its antibacterial effects as an intracellular inhibitor of protein synthesis, doxycycline has been repurposed for oncologic therapies. It has been shown to have cytotoxic and antiproliferative properties in various cancer cells and also may inhibit leukemic cell migration.26 In PCMZL, doxycycline initially was utilized in Borrelia-positive patients in Europe and found to improve disease clearance.27 In patients without Borrelia infection, doxycycline is thought to enhance apoptosis through caspase-3 activation along with p53 and Bax upregulation.28
Intralesional triamcinolone alone may not be feasible in pediatric PCMZL patients because of widespread involvement, and doxycycline may be considered as a treatment option. Multiple low-risk treatment modalities may be used in conjunction to clear disease in pediatric patients, as demonstrated in our case.
Acknowledgment—We thank Ali Nael Amzajerdi, MD (Orange, California), for his contributions to the pathologic imaging in this report.
- Willemze R, Cerroni L, Kempf W, et al. The 2018 update of the WHO-EORTC classification for primary cutaneous lymphomas. Blood. 2019;133:1703-1714.
- Amitay-Laish I, Tavallaee M, Kim J, et al. Paediatric primary cutaneous marginal zone B-cell lymphoma: does it differ from its adult counterpart? Br J Dermatol. 2017;176:1010-1020.
- Servitje O, Muniesa C, Benavente Y, et al. Primary cutaneous marginal zone B-cell lymphoma: response to treatment and disease-free survival in a series of 137 patients. J Am Acad Dermatol. 2013;69:357-365.
- Vitiello P, Sica A, Ronchi A, et al. Primary cutaneous B-cell lymphomas: an update. Front Oncol. 2020;10:651.
- Tadiotto Cicogna G, Ferranti M, Alaibac M. Diagnostic workup of primary cutaneous B cell lymphomas: a clinician’s approach. Front Oncol. 2020;10:988.
- Willemze R, Hodak E, Zinzani PL, et al. Primary cutaneous lymphomas: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2013;24:149-154.
- Pereira FO, Graf H, Nomura LM, et al. Concomitant presentation of Hashimoto’s thyroiditis and maltoma of the thyroid in a twenty-year-old man with a rapidly growing mass in the neck. Thyroid. 2000;10:833-835.
- Ekström Smedby K, Vajdic CM, Falster M, et al. Autoimmune disorders and risk of non-Hodgkin lymphoma subtypes: a pooled analysis within the InterLymph Consortium. Blood. 2008;111:4029-4038.
- Slater DN. Borrelia burgdorferi-associated primary cutaneous B-cell lymphoma. Histopathology. 2001;38:73-77.
- Wood GS, Kamath NV, Guitart J, et al. Absence of Borrelia burgdorferi DNA in cutaneous B-cell lymphomas from the United States. J Cutan Pathol. 2001;28:502-507.
- Dalle S, Thomas L, Balme B, et al. Primary cutaneous marginal zone lymphoma. Crit Rev Oncol Hematol. 2010;74:156-162.
- Senff NJ, Noordijk EM, Kim YH, et al. European Organization for Research and Treatment of Cancer and International Society for Cutaneous Lymphoma consensus recommendations for the management of cutaneous B-cell lymphomas. Blood. 2008;112:1600-1609.
- Hamilton SN, Wai ES, Tan K, et al. Treatment and outcomes in patients with primary cutaneous B-cell lymphoma: the BC Cancer Agency experience. Int J Radiat Oncol Biol Phys. 2013;87:719-725.
- Peñate Y, Hernández-Machín B, Pérez-Méndez LI, et al. Intralesional rituximab in the treatment of indolent primary cutaneous B-cell lymphomas: an epidemiological observational multicentre study. The Spanish Working Group on Cutaneous Lymphoma. Br J Dermatol. 2012;167:174-179.
- Kempf W, Kazakov DV, Buechner SA, et al. Primary cutaneous marginal zone lymphoma in children: a report of 3 cases and review of the literature. Am J Dermatopathol. 2014;36:661-666.
- Ghatalia P, Porter J, Wroblewski D, et al. Primary cutaneous marginal zone lymphoma associated with juxta-articular fibrotic nodules in a teenager. J Cutan Pathol. 2013;40:477-484.
- Dargent JL, Devalck C, De Mey A, et al. Primary cutaneous marginal zone B-cell lymphoma of MALT type in a child. Pediatr Dev Pathol. 2006;9:468-473.
- Sroa N, Magro CM. Pediatric primary cutaneous marginal zone lymphoma: in association with chronic antihistamine use. J Cutan Pathol. 2006;33(suppl 2):1-5.
- Zambrano E, Mejıa-Mejıa O, Bifulco C, et al. Extranodal marginal zone B-cell lymphoma/maltoma of the lip in a child: case report and review of cutaneous lymphoid proliferations in childhood. Int J Surg Pathol. 2006;14:163-169.
- Kollipara R, Hans A, Hall J, et al. A case report of primary cutaneous marginal zone lymphoma treated with intralesional steroids. Dermatol Online J. 2015;21:13030/qt9s15929m.
- Skaljic M, Cotton CH, Reilly AF, et al. Complete resolution of primary cutaneous marginal zone B-cell lymphoma on the cheek of a 7-year-old boy with intralesional triamcinolone and tincture of time. Pediatr Dermatol. 2020;37:228-229.
- Park MY, Jung HJ, Park JE, et al. Pediatric primary cutaneous marginal zone B-cell lymphoma treated with intralesional rituximab. Eur J Dermatol. 2010;20:533-534.
- Amitay-Laish I, Feinmesser M, Ben-Amitai D, et al. Juvenile onset of primary low-grade cutaneous B-cell lymphoma. Br J Dermatol. 2009;161:140-147.
- Sharon V, Mecca PS, Steinherz PG, et al. Two pediatric cases of primary cutaneous B-cell lymphoma and review of the literature. Pediatr Dermatol. 2009;26:34-39.
- Jothishankar B, Di Raimondo C, Mueller L, et al. Primary cutaneous marginal zone lymphoma treated with doxycycline in a pediatric patient. Pediatr Dermatol. 2020;37:759-761.
- Markowska A, Kaysiewicz J, Markowska J, et al. Doxycycline, salinomycin, monensin and ivermectin repositioned as cancer drugs. Bioorg Med Chem Lett. 2019;29:1549-1554.
- Kutting B, Bonsmann G, Metze D, et al. Borrelia burgdorferi-associated primary cutaneous B-cell lymphoma: complete clearing of skin lesions after antibiotic pulse therapy or intralesional injection of interferon alfa-2a. J Am Acad Dermatol. 1997;36:311-314.
- Protasoni M, Kroon AM, Taanman JW. Mitochondria as oncotarget: a comparison between the tetracycline analogs doxycycline and COL-3. Oncotarget. 2018;9:33818-33831.
- Willemze R, Cerroni L, Kempf W, et al. The 2018 update of the WHO-EORTC classification for primary cutaneous lymphomas. Blood. 2019;133:1703-1714.
- Amitay-Laish I, Tavallaee M, Kim J, et al. Paediatric primary cutaneous marginal zone B-cell lymphoma: does it differ from its adult counterpart? Br J Dermatol. 2017;176:1010-1020.
- Servitje O, Muniesa C, Benavente Y, et al. Primary cutaneous marginal zone B-cell lymphoma: response to treatment and disease-free survival in a series of 137 patients. J Am Acad Dermatol. 2013;69:357-365.
- Vitiello P, Sica A, Ronchi A, et al. Primary cutaneous B-cell lymphomas: an update. Front Oncol. 2020;10:651.
- Tadiotto Cicogna G, Ferranti M, Alaibac M. Diagnostic workup of primary cutaneous B cell lymphomas: a clinician’s approach. Front Oncol. 2020;10:988.
- Willemze R, Hodak E, Zinzani PL, et al. Primary cutaneous lymphomas: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2013;24:149-154.
- Pereira FO, Graf H, Nomura LM, et al. Concomitant presentation of Hashimoto’s thyroiditis and maltoma of the thyroid in a twenty-year-old man with a rapidly growing mass in the neck. Thyroid. 2000;10:833-835.
- Ekström Smedby K, Vajdic CM, Falster M, et al. Autoimmune disorders and risk of non-Hodgkin lymphoma subtypes: a pooled analysis within the InterLymph Consortium. Blood. 2008;111:4029-4038.
- Slater DN. Borrelia burgdorferi-associated primary cutaneous B-cell lymphoma. Histopathology. 2001;38:73-77.
- Wood GS, Kamath NV, Guitart J, et al. Absence of Borrelia burgdorferi DNA in cutaneous B-cell lymphomas from the United States. J Cutan Pathol. 2001;28:502-507.
- Dalle S, Thomas L, Balme B, et al. Primary cutaneous marginal zone lymphoma. Crit Rev Oncol Hematol. 2010;74:156-162.
- Senff NJ, Noordijk EM, Kim YH, et al. European Organization for Research and Treatment of Cancer and International Society for Cutaneous Lymphoma consensus recommendations for the management of cutaneous B-cell lymphomas. Blood. 2008;112:1600-1609.
- Hamilton SN, Wai ES, Tan K, et al. Treatment and outcomes in patients with primary cutaneous B-cell lymphoma: the BC Cancer Agency experience. Int J Radiat Oncol Biol Phys. 2013;87:719-725.
- Peñate Y, Hernández-Machín B, Pérez-Méndez LI, et al. Intralesional rituximab in the treatment of indolent primary cutaneous B-cell lymphomas: an epidemiological observational multicentre study. The Spanish Working Group on Cutaneous Lymphoma. Br J Dermatol. 2012;167:174-179.
- Kempf W, Kazakov DV, Buechner SA, et al. Primary cutaneous marginal zone lymphoma in children: a report of 3 cases and review of the literature. Am J Dermatopathol. 2014;36:661-666.
- Ghatalia P, Porter J, Wroblewski D, et al. Primary cutaneous marginal zone lymphoma associated with juxta-articular fibrotic nodules in a teenager. J Cutan Pathol. 2013;40:477-484.
- Dargent JL, Devalck C, De Mey A, et al. Primary cutaneous marginal zone B-cell lymphoma of MALT type in a child. Pediatr Dev Pathol. 2006;9:468-473.
- Sroa N, Magro CM. Pediatric primary cutaneous marginal zone lymphoma: in association with chronic antihistamine use. J Cutan Pathol. 2006;33(suppl 2):1-5.
- Zambrano E, Mejıa-Mejıa O, Bifulco C, et al. Extranodal marginal zone B-cell lymphoma/maltoma of the lip in a child: case report and review of cutaneous lymphoid proliferations in childhood. Int J Surg Pathol. 2006;14:163-169.
- Kollipara R, Hans A, Hall J, et al. A case report of primary cutaneous marginal zone lymphoma treated with intralesional steroids. Dermatol Online J. 2015;21:13030/qt9s15929m.
- Skaljic M, Cotton CH, Reilly AF, et al. Complete resolution of primary cutaneous marginal zone B-cell lymphoma on the cheek of a 7-year-old boy with intralesional triamcinolone and tincture of time. Pediatr Dermatol. 2020;37:228-229.
- Park MY, Jung HJ, Park JE, et al. Pediatric primary cutaneous marginal zone B-cell lymphoma treated with intralesional rituximab. Eur J Dermatol. 2010;20:533-534.
- Amitay-Laish I, Feinmesser M, Ben-Amitai D, et al. Juvenile onset of primary low-grade cutaneous B-cell lymphoma. Br J Dermatol. 2009;161:140-147.
- Sharon V, Mecca PS, Steinherz PG, et al. Two pediatric cases of primary cutaneous B-cell lymphoma and review of the literature. Pediatr Dermatol. 2009;26:34-39.
- Jothishankar B, Di Raimondo C, Mueller L, et al. Primary cutaneous marginal zone lymphoma treated with doxycycline in a pediatric patient. Pediatr Dermatol. 2020;37:759-761.
- Markowska A, Kaysiewicz J, Markowska J, et al. Doxycycline, salinomycin, monensin and ivermectin repositioned as cancer drugs. Bioorg Med Chem Lett. 2019;29:1549-1554.
- Kutting B, Bonsmann G, Metze D, et al. Borrelia burgdorferi-associated primary cutaneous B-cell lymphoma: complete clearing of skin lesions after antibiotic pulse therapy or intralesional injection of interferon alfa-2a. J Am Acad Dermatol. 1997;36:311-314.
- Protasoni M, Kroon AM, Taanman JW. Mitochondria as oncotarget: a comparison between the tetracycline analogs doxycycline and COL-3. Oncotarget. 2018;9:33818-33831.
Practice Points
- When skin biopsy reveals marginal zone lymphoma, laboratory workup should include a complete blood cell count, chemistry, and serum lactate dehydrogenase levels. If lymphocytosis is present, flow cytometry of peripheral blood cells should be performed.
- For patients with multifocal skin lesions, positive emission tomography with computed tomography is utilized to exclude systemic disease and assess lymph node involvement.
- Treatments for primary cutaneous marginal zone lymphoma include excision, topical steroids, intralesional steroids, intralesional rituximab, radiation therapy, and antibiotics.
- Doxycycline can be considered as a treatment option for pediatric patients with widespread cutaneous involvement.
52-year-old man • intermittent fevers • recently received second dose of COVID-19 vaccine • tremors in all 4 extremities • Dx?
THE CASE
A 52-year-old man sought care at the emergency department for intermittent fevers that started within 6 days of receiving his second dose of the BNT162b2 mRNA COVID-19 vaccine (Pfizer/BioNTech). After an unremarkable work-up, he was discharged home. Six days later, he returned to the emergency department with a fever of 102 °F and new-onset, progressive tremors in all 4 of his extremities.
The patient had a history of rheumatoid arthritis, for which he was taking oral methotrexate 15 mg once weekly and golimumab 50 mg SQ once monthly, and atrial fibrillation. He’d also had mechanical aortic and mitral valves implanted and was taking warfarin (9 mg/d on weekdays, 6 mg/d on Saturday and Sunday). Aside from his fever, his vital signs were normal. He also had horizontal nystagmus (chronically present) and diffuse tremors/myoclonic movements throughout his upper and lower extremities. The tremors were present at rest and worsened with intention/activity, which affected the patient’s ability to walk and perform activities of daily living.
He was admitted the next day to the family medicine service for further evaluation. Neurology and infectious disease consultations were requested, and a broad initial work-up was undertaken. Hyperreflexia was present in all of his extremities, but his neurologic examination was otherwise normal. Initial laboratory tests demonstrated leukocytosis and elevated liver transaminases. His international normalized ratio (INR) and prothrombin time (PT) also were elevated (> 8 [goal, 2.5-3.5 for mechanical heart valves] and > 90 seconds [normal range, 9.7-13.0 seconds], respectively), thus his warfarin was held and oral vitamin K was started (initial dose of 2.5 mg, which was increased to 5 mg when his INR did not decrease enough).
By Day 2, his INR and PT had normalized enough to reinitiate his warfarin dosing. Results from the viral antibody and polymerase chain reaction testing indicated the presence of cytomegalovirus (CMV) infection with viremia; blood cultures for bacterial infection were negative. Brain magnetic resonance imaging was ordered and identified a small, acute left-side cerebellar stroke. Lumbar puncture also was ordered but deferred until his INR was below 1.5 (on Day 8), at which point it confirmed the absence of CMV or herpes simplex virus in his central nervous system.
THE DIAGNOSIS
The patient started oral valganciclovir 900 mg twice daily to ameliorate his tremors, but he did not tolerate it well, vomiting after dosing. He was switched to IV ganciclovir 5 mg/kg every 12 hours; however, his tremors were not improving, leading the team to suspect an etiology other than viral infection. A presumptive diagnosis of autoimmune movement disorder was made, and serum tests were ordered; the results were positive for antiphospholipid antibodies, including anticardiolipin and anti-ß2 glycoprotein-I antibodies. A final diagnosis of autoimmune antiphospholipid antibody syndrome (APS)–related movement disorder1 with coagulopathy was reached, and the patient was started on methylprednisolone 1 g/d IV.
We suspected the CMV viremia was reactivated by the COVID-19 vaccine and caused the APS that led to the movement disorder, coagulopathy, and likely, the thrombotic cerebellar stroke. The case was reported to the Vaccine Adverse Event Reporting System (VAERS).2
DISCUSSION
Continue to: The development of antiphospholipid antibodies...
The development of antiphospholipid antibodies has been independently associated with rheumatoid arthritis,5 COVID-19,6 and CMV infection,7 as well as with vaccination for influenza and tetanus.8 There also are reports of antiphospholipid antibodies occurring in patients who have received adenovirus-vectored and mRNA COVID-19 vaccines.9-11
Movement disorders occurring with APS are unusual, with approximately 1.3% to 4.5% of patients with APS demonstrating this manifestation.12 One of multiple autoimmune-related movement disorders, APS-related movement disorder is most commonly associated with systemic lupus erythematosus (SLE), although it can occur outside an SLE diagnosis.4
While APS-related movement disorder occurs with the presence of antiphospholipid antibodies, the pathogenesis of the movement disorder is unclear.4 Patients are typically young women, and the associated movements are choreiform. The condition often occurs with coagulopathy and arterial thrombosis.4 Psychiatric manifestations also can occur, including changes in behavior—up to and including psychosis.4
Evidence of COVID-19 vaccination reactivating herpesviruses exists, although it is rare and usually does not cause serious health outcomes.13 The annual incidence of reactivation related to vaccination is estimated to be 0.7 per 100,000 for varicella zoster virus and 0.03 per 100,000 for herpes simplex virus.13 The literature also suggests that the occurrence of Bell palsy—the onset of which may be related to the reactivation of a latent virus—may increase in relation to particular COVID-19 vaccines.14,15 Although there is no confirmed explanation for these reactivation events at this time, different theories related to altering the focus of immune cells from latent disease to the newly generated antigen have been suggested.16
To date, reactivation has not been demonstrated with CMV specifically. However, based on the literature reviewed here on the reactivation of herpesviruses and the temporal relationship to infection in our patient, we propose that the BNT162b2 mRNA vaccination reactivated his CMV infection and led to his APS-related movement disorder.
Continue to: Treatment is focused on resolved the autoimmune condition
Treatment is focused on resolving the autoimmune condition, usually with corticosteroids. Longer-term treatment of the movement disorder with antiepileptics such as carbamazepine and valproic acid may be necessary.4
Our patient received methylprednisolone IV 1 g/d for 3 days and responded quickly to the treatment. He was discharged to a post-acute rehabilitation hospital on Day 16 with a plan for 21 days of antiviral treatment for an acute CMV infection, 1 month of oral steroid taper for the APS, and continued warfarin treatment. This regimen resulted in complete resolution of his movement disorder and negative testing of antiphospholipid antibodies 16 days after he was discharged from the hospital.
THE TAKEAWAY
This case illustrates the possible reactivation of a herpesvirus (CMV) related to COVID-19 vaccination, as well as the development of APS-related movement disorder and coagulopathy related to acute CMV infection with viremia. Vaccination for the COVID-19 virus is seen as the best intervention available for preventing serious illness and death associated with COVID-19 infection. Thus, it is important to be aware of these unusual events when vaccinating large populations. This case also demonstrates the need to understand the interplay of immune status and possible disorders associated with autoimmune conditions. Keeping an open mind when evaluating patients with post-vaccination complaints is beneficial—especially given the volume of distrust and misinformation associated with COVID-19 vaccination.
CORRESPONDENCE
Aaron Lear, MD, MSc, CAQ, Cleveland Clinic Akron General Center for Family Medicine, 1 Akron General Avenue, Building 301, Akron, OH 44307; [email protected]
1. Martino D, Chew N-K, Mir P, et al. Atypical movement disorders in antiphospholipid syndrome. 2006;21:944-949. doi: 10.1002/mds.20842
2. Vaccine Adverse Event Reporting System. Accessed February 9, 2022. vaers.hhs.gov
3. Duarte-García A, Pham MM, Crowson CS, et al. The epidemiology of antiphospholipid syndrome: a population-based Study. Arthritis Rheumatol. 2019;71:1545-1552. doi: 10.1002/art.40901
4. Baizabal-Carvallo JF, Jankovic J. Autoimmune and paraneoplastic movement disorders: an update. J Neurol Sci. 2018;385:175-184. doi: 10.1016/j.jns.2017.12.035
5. O’Leary RE, Hsiao JL, Worswick SD. Antiphospholipid syndrome in a patient with rheumatoid arthritis. Cutis. 2017;99:E21-E24.
6. Taha M, Samavati L. Antiphospholipid antibodies in COVID-19: a meta-analysis and systematic review. RMD Open. 2021;7:e001580. doi: 10.1136/rmdopen-2021-001580
7. Nakayama T, Akahoshi M, Irino K, et al. Transient antiphospholipid syndrome associated with primary cytomegalovirus infection: a case report and literature review. Case Rep Rheumatol. 2014;2014:27154. doi: 10.1155/2014/271548
8. Cruz-Tapias P, Blank M, Anaya J-M, et al. Infections and vaccines in the etiology of antiphospholipid syndrome. Curr Opin Rheumatol. 2012;24:389-393. doi: 10.1097/BOR.0b013e32835448b8
9. Schultz NH, Sørvoll IH, Michelsen AE, et al. Thrombosis and thrombocytopenia after ChAdOx1 nCoV-19 vaccination. N Engl J Med. 2021;384:2124-2130. doi: 10.1056/nejmoa2104882
10. Cimolai N. Untangling the intricacies of infection, thrombosis, vaccination, and antiphospholipid antibodies for COVID-19. SN Compr Clin Med. 2021;3:2093-2108. doi: 10.1007/s42399-021-00992-3
11. Jinno S, Naka I, Nakazawa T. Catastrophic antiphospholipid syndrome complicated with essential thrombocythaemia after COVID-19 vaccination: in search of the underlying mechanism. Rheumatol Adv Pract. 2021;5:rkab096. doi: 10.1093/rap/rkab096
12. Ricarte IF, Dutra LA, Abrantes FF, et al. Neurologic manifestations of antiphospholipid syndrome. Lupus. 2018;27:1404-1414. doi: 10.1177/0961203318776110
13. Gringeri M, Battini V, Cammarata G, et al. Herpes zoster and simplex reactivation following COVID-19 vaccination: new insights from a vaccine adverse event reporting system (VAERS) database analysis. Expert Rev Vaccines. 2022;21:675-684. doi: 10.1080/14760584.2022.2044799
14. Cirillo N, Doan R. The association between COVID-19 vaccination and Bell’s palsy. Lancet Infect Dis. 2022;22:5-6. doi: 10.1016/s1473-3099(21)00467-9
15. Poudel S, Nepali P, Baniya S, et al. Bell’s palsy as a possible complication of mRNA-1273 (Moderna) vaccine against COVID-19. Ann Med Surg (Lond). 2022;78:103897. doi: 10.1016/j.amsu.2022.103897
16. Furer V, Zisman D, Kibari A, et al. Herpes zoster following BNT162b2 mRNA COVID-19 vaccination in patients with autoimmune inflammatory rheumatic diseases: a case series. Rheumatology (Oxford). 2021;60:SI90-SI95. doi: 10.1093/rheumatology/keab345
THE CASE
A 52-year-old man sought care at the emergency department for intermittent fevers that started within 6 days of receiving his second dose of the BNT162b2 mRNA COVID-19 vaccine (Pfizer/BioNTech). After an unremarkable work-up, he was discharged home. Six days later, he returned to the emergency department with a fever of 102 °F and new-onset, progressive tremors in all 4 of his extremities.
The patient had a history of rheumatoid arthritis, for which he was taking oral methotrexate 15 mg once weekly and golimumab 50 mg SQ once monthly, and atrial fibrillation. He’d also had mechanical aortic and mitral valves implanted and was taking warfarin (9 mg/d on weekdays, 6 mg/d on Saturday and Sunday). Aside from his fever, his vital signs were normal. He also had horizontal nystagmus (chronically present) and diffuse tremors/myoclonic movements throughout his upper and lower extremities. The tremors were present at rest and worsened with intention/activity, which affected the patient’s ability to walk and perform activities of daily living.
He was admitted the next day to the family medicine service for further evaluation. Neurology and infectious disease consultations were requested, and a broad initial work-up was undertaken. Hyperreflexia was present in all of his extremities, but his neurologic examination was otherwise normal. Initial laboratory tests demonstrated leukocytosis and elevated liver transaminases. His international normalized ratio (INR) and prothrombin time (PT) also were elevated (> 8 [goal, 2.5-3.5 for mechanical heart valves] and > 90 seconds [normal range, 9.7-13.0 seconds], respectively), thus his warfarin was held and oral vitamin K was started (initial dose of 2.5 mg, which was increased to 5 mg when his INR did not decrease enough).
By Day 2, his INR and PT had normalized enough to reinitiate his warfarin dosing. Results from the viral antibody and polymerase chain reaction testing indicated the presence of cytomegalovirus (CMV) infection with viremia; blood cultures for bacterial infection were negative. Brain magnetic resonance imaging was ordered and identified a small, acute left-side cerebellar stroke. Lumbar puncture also was ordered but deferred until his INR was below 1.5 (on Day 8), at which point it confirmed the absence of CMV or herpes simplex virus in his central nervous system.
THE DIAGNOSIS
The patient started oral valganciclovir 900 mg twice daily to ameliorate his tremors, but he did not tolerate it well, vomiting after dosing. He was switched to IV ganciclovir 5 mg/kg every 12 hours; however, his tremors were not improving, leading the team to suspect an etiology other than viral infection. A presumptive diagnosis of autoimmune movement disorder was made, and serum tests were ordered; the results were positive for antiphospholipid antibodies, including anticardiolipin and anti-ß2 glycoprotein-I antibodies. A final diagnosis of autoimmune antiphospholipid antibody syndrome (APS)–related movement disorder1 with coagulopathy was reached, and the patient was started on methylprednisolone 1 g/d IV.
We suspected the CMV viremia was reactivated by the COVID-19 vaccine and caused the APS that led to the movement disorder, coagulopathy, and likely, the thrombotic cerebellar stroke. The case was reported to the Vaccine Adverse Event Reporting System (VAERS).2
DISCUSSION
Continue to: The development of antiphospholipid antibodies...
The development of antiphospholipid antibodies has been independently associated with rheumatoid arthritis,5 COVID-19,6 and CMV infection,7 as well as with vaccination for influenza and tetanus.8 There also are reports of antiphospholipid antibodies occurring in patients who have received adenovirus-vectored and mRNA COVID-19 vaccines.9-11
Movement disorders occurring with APS are unusual, with approximately 1.3% to 4.5% of patients with APS demonstrating this manifestation.12 One of multiple autoimmune-related movement disorders, APS-related movement disorder is most commonly associated with systemic lupus erythematosus (SLE), although it can occur outside an SLE diagnosis.4
While APS-related movement disorder occurs with the presence of antiphospholipid antibodies, the pathogenesis of the movement disorder is unclear.4 Patients are typically young women, and the associated movements are choreiform. The condition often occurs with coagulopathy and arterial thrombosis.4 Psychiatric manifestations also can occur, including changes in behavior—up to and including psychosis.4
Evidence of COVID-19 vaccination reactivating herpesviruses exists, although it is rare and usually does not cause serious health outcomes.13 The annual incidence of reactivation related to vaccination is estimated to be 0.7 per 100,000 for varicella zoster virus and 0.03 per 100,000 for herpes simplex virus.13 The literature also suggests that the occurrence of Bell palsy—the onset of which may be related to the reactivation of a latent virus—may increase in relation to particular COVID-19 vaccines.14,15 Although there is no confirmed explanation for these reactivation events at this time, different theories related to altering the focus of immune cells from latent disease to the newly generated antigen have been suggested.16
To date, reactivation has not been demonstrated with CMV specifically. However, based on the literature reviewed here on the reactivation of herpesviruses and the temporal relationship to infection in our patient, we propose that the BNT162b2 mRNA vaccination reactivated his CMV infection and led to his APS-related movement disorder.
Continue to: Treatment is focused on resolved the autoimmune condition
Treatment is focused on resolving the autoimmune condition, usually with corticosteroids. Longer-term treatment of the movement disorder with antiepileptics such as carbamazepine and valproic acid may be necessary.4
Our patient received methylprednisolone IV 1 g/d for 3 days and responded quickly to the treatment. He was discharged to a post-acute rehabilitation hospital on Day 16 with a plan for 21 days of antiviral treatment for an acute CMV infection, 1 month of oral steroid taper for the APS, and continued warfarin treatment. This regimen resulted in complete resolution of his movement disorder and negative testing of antiphospholipid antibodies 16 days after he was discharged from the hospital.
THE TAKEAWAY
This case illustrates the possible reactivation of a herpesvirus (CMV) related to COVID-19 vaccination, as well as the development of APS-related movement disorder and coagulopathy related to acute CMV infection with viremia. Vaccination for the COVID-19 virus is seen as the best intervention available for preventing serious illness and death associated with COVID-19 infection. Thus, it is important to be aware of these unusual events when vaccinating large populations. This case also demonstrates the need to understand the interplay of immune status and possible disorders associated with autoimmune conditions. Keeping an open mind when evaluating patients with post-vaccination complaints is beneficial—especially given the volume of distrust and misinformation associated with COVID-19 vaccination.
CORRESPONDENCE
Aaron Lear, MD, MSc, CAQ, Cleveland Clinic Akron General Center for Family Medicine, 1 Akron General Avenue, Building 301, Akron, OH 44307; [email protected]
THE CASE
A 52-year-old man sought care at the emergency department for intermittent fevers that started within 6 days of receiving his second dose of the BNT162b2 mRNA COVID-19 vaccine (Pfizer/BioNTech). After an unremarkable work-up, he was discharged home. Six days later, he returned to the emergency department with a fever of 102 °F and new-onset, progressive tremors in all 4 of his extremities.
The patient had a history of rheumatoid arthritis, for which he was taking oral methotrexate 15 mg once weekly and golimumab 50 mg SQ once monthly, and atrial fibrillation. He’d also had mechanical aortic and mitral valves implanted and was taking warfarin (9 mg/d on weekdays, 6 mg/d on Saturday and Sunday). Aside from his fever, his vital signs were normal. He also had horizontal nystagmus (chronically present) and diffuse tremors/myoclonic movements throughout his upper and lower extremities. The tremors were present at rest and worsened with intention/activity, which affected the patient’s ability to walk and perform activities of daily living.
He was admitted the next day to the family medicine service for further evaluation. Neurology and infectious disease consultations were requested, and a broad initial work-up was undertaken. Hyperreflexia was present in all of his extremities, but his neurologic examination was otherwise normal. Initial laboratory tests demonstrated leukocytosis and elevated liver transaminases. His international normalized ratio (INR) and prothrombin time (PT) also were elevated (> 8 [goal, 2.5-3.5 for mechanical heart valves] and > 90 seconds [normal range, 9.7-13.0 seconds], respectively), thus his warfarin was held and oral vitamin K was started (initial dose of 2.5 mg, which was increased to 5 mg when his INR did not decrease enough).
By Day 2, his INR and PT had normalized enough to reinitiate his warfarin dosing. Results from the viral antibody and polymerase chain reaction testing indicated the presence of cytomegalovirus (CMV) infection with viremia; blood cultures for bacterial infection were negative. Brain magnetic resonance imaging was ordered and identified a small, acute left-side cerebellar stroke. Lumbar puncture also was ordered but deferred until his INR was below 1.5 (on Day 8), at which point it confirmed the absence of CMV or herpes simplex virus in his central nervous system.
THE DIAGNOSIS
The patient started oral valganciclovir 900 mg twice daily to ameliorate his tremors, but he did not tolerate it well, vomiting after dosing. He was switched to IV ganciclovir 5 mg/kg every 12 hours; however, his tremors were not improving, leading the team to suspect an etiology other than viral infection. A presumptive diagnosis of autoimmune movement disorder was made, and serum tests were ordered; the results were positive for antiphospholipid antibodies, including anticardiolipin and anti-ß2 glycoprotein-I antibodies. A final diagnosis of autoimmune antiphospholipid antibody syndrome (APS)–related movement disorder1 with coagulopathy was reached, and the patient was started on methylprednisolone 1 g/d IV.
We suspected the CMV viremia was reactivated by the COVID-19 vaccine and caused the APS that led to the movement disorder, coagulopathy, and likely, the thrombotic cerebellar stroke. The case was reported to the Vaccine Adverse Event Reporting System (VAERS).2
DISCUSSION
Continue to: The development of antiphospholipid antibodies...
The development of antiphospholipid antibodies has been independently associated with rheumatoid arthritis,5 COVID-19,6 and CMV infection,7 as well as with vaccination for influenza and tetanus.8 There also are reports of antiphospholipid antibodies occurring in patients who have received adenovirus-vectored and mRNA COVID-19 vaccines.9-11
Movement disorders occurring with APS are unusual, with approximately 1.3% to 4.5% of patients with APS demonstrating this manifestation.12 One of multiple autoimmune-related movement disorders, APS-related movement disorder is most commonly associated with systemic lupus erythematosus (SLE), although it can occur outside an SLE diagnosis.4
While APS-related movement disorder occurs with the presence of antiphospholipid antibodies, the pathogenesis of the movement disorder is unclear.4 Patients are typically young women, and the associated movements are choreiform. The condition often occurs with coagulopathy and arterial thrombosis.4 Psychiatric manifestations also can occur, including changes in behavior—up to and including psychosis.4
Evidence of COVID-19 vaccination reactivating herpesviruses exists, although it is rare and usually does not cause serious health outcomes.13 The annual incidence of reactivation related to vaccination is estimated to be 0.7 per 100,000 for varicella zoster virus and 0.03 per 100,000 for herpes simplex virus.13 The literature also suggests that the occurrence of Bell palsy—the onset of which may be related to the reactivation of a latent virus—may increase in relation to particular COVID-19 vaccines.14,15 Although there is no confirmed explanation for these reactivation events at this time, different theories related to altering the focus of immune cells from latent disease to the newly generated antigen have been suggested.16
To date, reactivation has not been demonstrated with CMV specifically. However, based on the literature reviewed here on the reactivation of herpesviruses and the temporal relationship to infection in our patient, we propose that the BNT162b2 mRNA vaccination reactivated his CMV infection and led to his APS-related movement disorder.
Continue to: Treatment is focused on resolved the autoimmune condition
Treatment is focused on resolving the autoimmune condition, usually with corticosteroids. Longer-term treatment of the movement disorder with antiepileptics such as carbamazepine and valproic acid may be necessary.4
Our patient received methylprednisolone IV 1 g/d for 3 days and responded quickly to the treatment. He was discharged to a post-acute rehabilitation hospital on Day 16 with a plan for 21 days of antiviral treatment for an acute CMV infection, 1 month of oral steroid taper for the APS, and continued warfarin treatment. This regimen resulted in complete resolution of his movement disorder and negative testing of antiphospholipid antibodies 16 days after he was discharged from the hospital.
THE TAKEAWAY
This case illustrates the possible reactivation of a herpesvirus (CMV) related to COVID-19 vaccination, as well as the development of APS-related movement disorder and coagulopathy related to acute CMV infection with viremia. Vaccination for the COVID-19 virus is seen as the best intervention available for preventing serious illness and death associated with COVID-19 infection. Thus, it is important to be aware of these unusual events when vaccinating large populations. This case also demonstrates the need to understand the interplay of immune status and possible disorders associated with autoimmune conditions. Keeping an open mind when evaluating patients with post-vaccination complaints is beneficial—especially given the volume of distrust and misinformation associated with COVID-19 vaccination.
CORRESPONDENCE
Aaron Lear, MD, MSc, CAQ, Cleveland Clinic Akron General Center for Family Medicine, 1 Akron General Avenue, Building 301, Akron, OH 44307; [email protected]
1. Martino D, Chew N-K, Mir P, et al. Atypical movement disorders in antiphospholipid syndrome. 2006;21:944-949. doi: 10.1002/mds.20842
2. Vaccine Adverse Event Reporting System. Accessed February 9, 2022. vaers.hhs.gov
3. Duarte-García A, Pham MM, Crowson CS, et al. The epidemiology of antiphospholipid syndrome: a population-based Study. Arthritis Rheumatol. 2019;71:1545-1552. doi: 10.1002/art.40901
4. Baizabal-Carvallo JF, Jankovic J. Autoimmune and paraneoplastic movement disorders: an update. J Neurol Sci. 2018;385:175-184. doi: 10.1016/j.jns.2017.12.035
5. O’Leary RE, Hsiao JL, Worswick SD. Antiphospholipid syndrome in a patient with rheumatoid arthritis. Cutis. 2017;99:E21-E24.
6. Taha M, Samavati L. Antiphospholipid antibodies in COVID-19: a meta-analysis and systematic review. RMD Open. 2021;7:e001580. doi: 10.1136/rmdopen-2021-001580
7. Nakayama T, Akahoshi M, Irino K, et al. Transient antiphospholipid syndrome associated with primary cytomegalovirus infection: a case report and literature review. Case Rep Rheumatol. 2014;2014:27154. doi: 10.1155/2014/271548
8. Cruz-Tapias P, Blank M, Anaya J-M, et al. Infections and vaccines in the etiology of antiphospholipid syndrome. Curr Opin Rheumatol. 2012;24:389-393. doi: 10.1097/BOR.0b013e32835448b8
9. Schultz NH, Sørvoll IH, Michelsen AE, et al. Thrombosis and thrombocytopenia after ChAdOx1 nCoV-19 vaccination. N Engl J Med. 2021;384:2124-2130. doi: 10.1056/nejmoa2104882
10. Cimolai N. Untangling the intricacies of infection, thrombosis, vaccination, and antiphospholipid antibodies for COVID-19. SN Compr Clin Med. 2021;3:2093-2108. doi: 10.1007/s42399-021-00992-3
11. Jinno S, Naka I, Nakazawa T. Catastrophic antiphospholipid syndrome complicated with essential thrombocythaemia after COVID-19 vaccination: in search of the underlying mechanism. Rheumatol Adv Pract. 2021;5:rkab096. doi: 10.1093/rap/rkab096
12. Ricarte IF, Dutra LA, Abrantes FF, et al. Neurologic manifestations of antiphospholipid syndrome. Lupus. 2018;27:1404-1414. doi: 10.1177/0961203318776110
13. Gringeri M, Battini V, Cammarata G, et al. Herpes zoster and simplex reactivation following COVID-19 vaccination: new insights from a vaccine adverse event reporting system (VAERS) database analysis. Expert Rev Vaccines. 2022;21:675-684. doi: 10.1080/14760584.2022.2044799
14. Cirillo N, Doan R. The association between COVID-19 vaccination and Bell’s palsy. Lancet Infect Dis. 2022;22:5-6. doi: 10.1016/s1473-3099(21)00467-9
15. Poudel S, Nepali P, Baniya S, et al. Bell’s palsy as a possible complication of mRNA-1273 (Moderna) vaccine against COVID-19. Ann Med Surg (Lond). 2022;78:103897. doi: 10.1016/j.amsu.2022.103897
16. Furer V, Zisman D, Kibari A, et al. Herpes zoster following BNT162b2 mRNA COVID-19 vaccination in patients with autoimmune inflammatory rheumatic diseases: a case series. Rheumatology (Oxford). 2021;60:SI90-SI95. doi: 10.1093/rheumatology/keab345
1. Martino D, Chew N-K, Mir P, et al. Atypical movement disorders in antiphospholipid syndrome. 2006;21:944-949. doi: 10.1002/mds.20842
2. Vaccine Adverse Event Reporting System. Accessed February 9, 2022. vaers.hhs.gov
3. Duarte-García A, Pham MM, Crowson CS, et al. The epidemiology of antiphospholipid syndrome: a population-based Study. Arthritis Rheumatol. 2019;71:1545-1552. doi: 10.1002/art.40901
4. Baizabal-Carvallo JF, Jankovic J. Autoimmune and paraneoplastic movement disorders: an update. J Neurol Sci. 2018;385:175-184. doi: 10.1016/j.jns.2017.12.035
5. O’Leary RE, Hsiao JL, Worswick SD. Antiphospholipid syndrome in a patient with rheumatoid arthritis. Cutis. 2017;99:E21-E24.
6. Taha M, Samavati L. Antiphospholipid antibodies in COVID-19: a meta-analysis and systematic review. RMD Open. 2021;7:e001580. doi: 10.1136/rmdopen-2021-001580
7. Nakayama T, Akahoshi M, Irino K, et al. Transient antiphospholipid syndrome associated with primary cytomegalovirus infection: a case report and literature review. Case Rep Rheumatol. 2014;2014:27154. doi: 10.1155/2014/271548
8. Cruz-Tapias P, Blank M, Anaya J-M, et al. Infections and vaccines in the etiology of antiphospholipid syndrome. Curr Opin Rheumatol. 2012;24:389-393. doi: 10.1097/BOR.0b013e32835448b8
9. Schultz NH, Sørvoll IH, Michelsen AE, et al. Thrombosis and thrombocytopenia after ChAdOx1 nCoV-19 vaccination. N Engl J Med. 2021;384:2124-2130. doi: 10.1056/nejmoa2104882
10. Cimolai N. Untangling the intricacies of infection, thrombosis, vaccination, and antiphospholipid antibodies for COVID-19. SN Compr Clin Med. 2021;3:2093-2108. doi: 10.1007/s42399-021-00992-3
11. Jinno S, Naka I, Nakazawa T. Catastrophic antiphospholipid syndrome complicated with essential thrombocythaemia after COVID-19 vaccination: in search of the underlying mechanism. Rheumatol Adv Pract. 2021;5:rkab096. doi: 10.1093/rap/rkab096
12. Ricarte IF, Dutra LA, Abrantes FF, et al. Neurologic manifestations of antiphospholipid syndrome. Lupus. 2018;27:1404-1414. doi: 10.1177/0961203318776110
13. Gringeri M, Battini V, Cammarata G, et al. Herpes zoster and simplex reactivation following COVID-19 vaccination: new insights from a vaccine adverse event reporting system (VAERS) database analysis. Expert Rev Vaccines. 2022;21:675-684. doi: 10.1080/14760584.2022.2044799
14. Cirillo N, Doan R. The association between COVID-19 vaccination and Bell’s palsy. Lancet Infect Dis. 2022;22:5-6. doi: 10.1016/s1473-3099(21)00467-9
15. Poudel S, Nepali P, Baniya S, et al. Bell’s palsy as a possible complication of mRNA-1273 (Moderna) vaccine against COVID-19. Ann Med Surg (Lond). 2022;78:103897. doi: 10.1016/j.amsu.2022.103897
16. Furer V, Zisman D, Kibari A, et al. Herpes zoster following BNT162b2 mRNA COVID-19 vaccination in patients with autoimmune inflammatory rheumatic diseases: a case series. Rheumatology (Oxford). 2021;60:SI90-SI95. doi: 10.1093/rheumatology/keab345
► Intermittent fevers
► Recently received second dose of COVID-19 vaccine
► Tremors in all 4 extremities