User login
Emergency Imaging: Abdominal Pain 6 Months After Cesarean Delivery
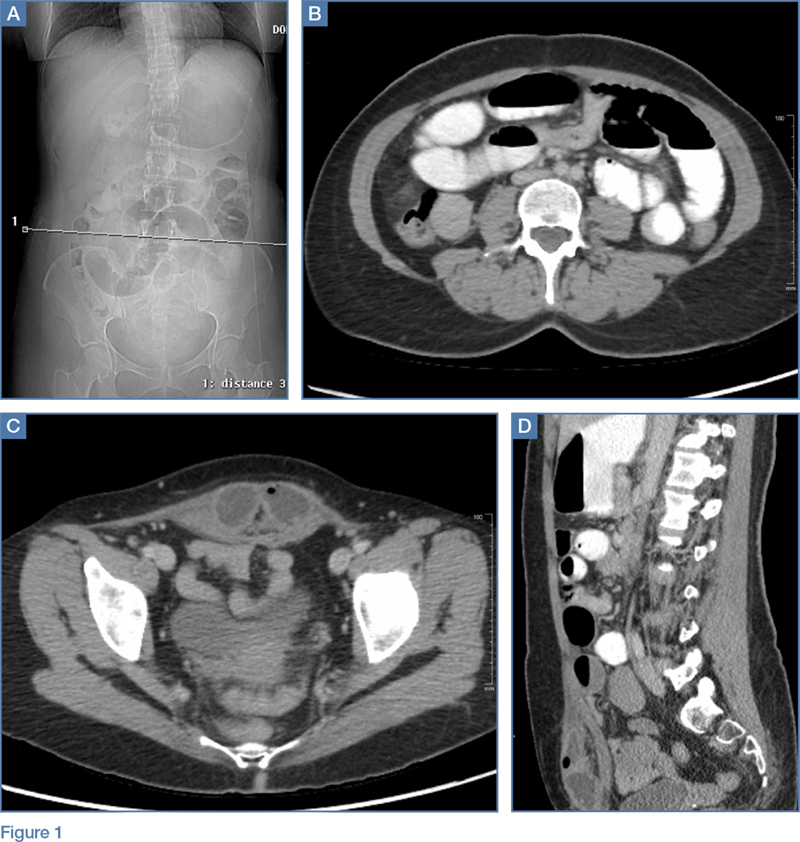
A 45-year-old woman with a history of polycystic ovary syndrome presented to the ED for evaluation of acute abdominal pain. The patient’s surgical history was significant for a cesarean delivery 6 months prior to presentation. Abdominal examination revealed a well-healed suprapubic cesarean incision scar, which was tender upon palpation. A computed tomography (CT) scan of the abdomen and pelvis with contrast were ordered; representative images are shown above (Figure 1a-1d).
What is the diagnosis? What are the associated complications and preferred management for this entity?
Answer
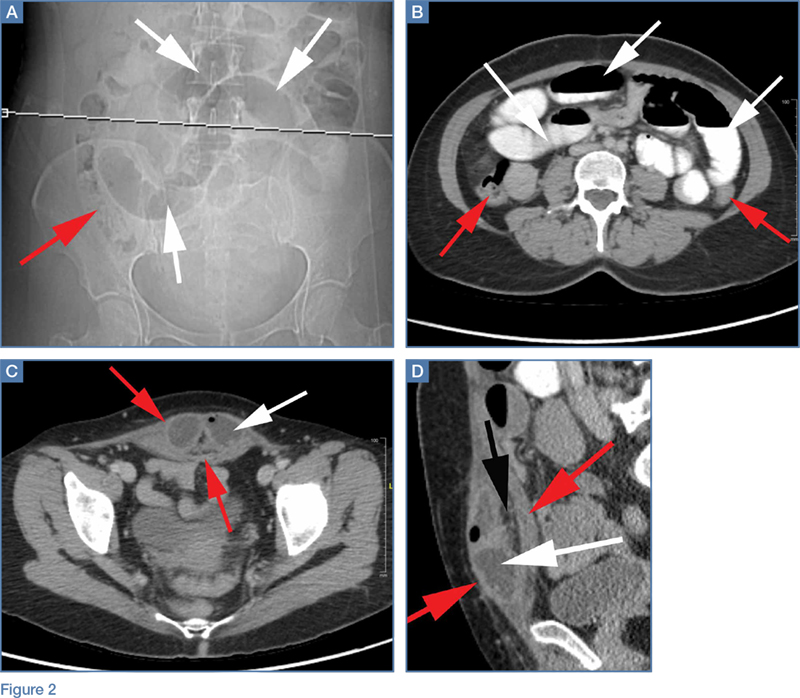
The scout image from the CT scan shows multiple dilated loops of small bowel (white arrows, Figure 2a) and only a small amount of air within a decompressed colon (red arrow, Figure 2a). The multiplanar CT image confirmed multiple dilated small bowel loops (white arrows, Figure 2b) and the decompressed large bowel (red arrows, Figure 2b), indicating the presence of a small bowel obstruction. A distal small bowel loop (white arrows, Figure 2c and 2d) was identified in a hernia sac within the walls of the rectus abdominis muscle (red arrows, Figure 2c and 2d). Mesenteric stranding within the hernia sac was suggestive of incarceration (black arrow, Figure 2d). No signs of intestinal ischemia, such as pneumatosis or wall thickening, were present.
An exploratory laparotomy was emergently performed, which confirmed the presence of incarcerated small bowel within the posterior rectus sheath defect without evidence of strangulation. Reduction of small bowel and primary closure of the hernia defect was subsequently performed without complication.
Abdominal Wall Hernias
Abdominal wall hernias are common in the United States, with more than 1 million abdominal wall hernia repairs performed annually.1 A posterior rectus sheath hernia is a rare type of abdominal wall hernia; the majority are postsurgical (as seen in this patient) or posttraumatic, with only a few reported congenital cases.2
Anatomy
The rectus sheath encloses the rectus abdominis muscle and is composed of the aponeuroses of the transversus abdominis, external oblique, and internal oblique muscles. The aponeuroses form an anterior and posterior sheath, which together serve as a strong barrier against the herniation of abdominal contents, accounting for the rarity of a spontaneous rectus sheath hernia. However, inferior to the umbilicus (below the arcuate line), the posterior rectus sheath is composed primarily of transversalis fascia, which may make this region more susceptible to herniation.3 Additional predisposing factors to herniation include increased muscle weakness and elevated intra-abdominal pressure, such as that which occurs during pregnancy or from ascites.4
Clinical Presentation
Like other abdominal wall hernias, the clinical presentation of posterior rectus sheath hernias is nonspecific. Patients may be asymptomatic or may develop abdominal pain, distension, and vomiting as a result of acute complications that necessitate emergent surgery. During history-taking, inquiry into a patient’s surgical history is crucial because it may raise clinical suspicion for an abdominal wall hernia, as was the case in our patient, who recently had a cesarean delivery.
Diagnosis
Because prompt and accurate diagnosis of acute complications of abdominal wall hernias is essential, imaging studies are typically required for diagnosis. Computed tomography is the modality of choice based on its ability to provide superior anatomic detail of the abdominal wall, permitting identification of hernias and differentiating them from other abdominal masses, such as hematomas, abscesses, or tumors. Additionally, CT is able to detect early signs of hernia sac complications, including bowel obstruction, incarceration, and strangulation.5
Treatment
Treatment for a posterior rectus sheath hernia is surgical with primary closure being the preferred method. Prosthetic repair may also be performed, particularly when the hernia defect is large, but it has been shown to be associated with an increased risk of intestinal strangulation.3
1. Rutkow IM. Demographic and socioeconomic aspects of hernia repair in the United States in 2003. Surg Clin North Am. 2003;83(5):1045-1051, v-vi. doi:10.1016/S0039-6109(03)00132-4.
2. Lenobel S, Lenobel R, Yu J. Posterior rectus sheath hernia causing intermittent small bowel obstruction. J Radiol Case Rep J. 2014;8(9):25-29. doi:10.3941/jrcr.v8i9.2081.
3. Losanoff JE, Basson MD, Gruber SA. Spontaneous hernia through the posterior rectus abdominis sheath: case report and review of the published literature 1937-2008. Hernia. 2009;13(5):555-558. doi:10.1007/s10029-009-0481-6.
4. Bentzon N, Adamsen S. Hernia of the posterior rectus sheath: a new entity? Eur J Surg. 1995;161(3):215-216.
5. Aguirre DA, Santosa AC, Casola G, Sirlin CB. Abdominal wall hernias: imaging features, complications, and diagnostic pitfalls at mutli-detector row CT. Radiographics. 2005;25(6):1501-1520. doi:10.1148/rg.256055018.
A 45-year-old woman with a history of polycystic ovary syndrome presented to the ED for evaluation of acute abdominal pain. The patient’s surgical history was significant for a cesarean delivery 6 months prior to presentation. Abdominal examination revealed a well-healed suprapubic cesarean incision scar, which was tender upon palpation. A computed tomography (CT) scan of the abdomen and pelvis with contrast were ordered; representative images are shown above (Figure 1a-1d).

What is the diagnosis? What are the associated complications and preferred management for this entity?
Answer
The scout image from the CT scan shows multiple dilated loops of small bowel (white arrows, Figure 2a) and only a small amount of air within a decompressed colon (red arrow, Figure 2a). The multiplanar CT image confirmed multiple dilated small bowel loops (white arrows, Figure 2b) and the decompressed large bowel (red arrows, Figure 2b), indicating the presence of a small bowel obstruction. A distal small bowel loop (white arrows, Figure 2c and 2d) was identified in a hernia sac within the walls of the rectus abdominis muscle (red arrows, Figure 2c and 2d). Mesenteric stranding within the hernia sac was suggestive of incarceration (black arrow, Figure 2d). No signs of intestinal ischemia, such as pneumatosis or wall thickening, were present.

An exploratory laparotomy was emergently performed, which confirmed the presence of incarcerated small bowel within the posterior rectus sheath defect without evidence of strangulation. Reduction of small bowel and primary closure of the hernia defect was subsequently performed without complication.
Abdominal Wall Hernias
Abdominal wall hernias are common in the United States, with more than 1 million abdominal wall hernia repairs performed annually.1 A posterior rectus sheath hernia is a rare type of abdominal wall hernia; the majority are postsurgical (as seen in this patient) or posttraumatic, with only a few reported congenital cases.2
Anatomy
The rectus sheath encloses the rectus abdominis muscle and is composed of the aponeuroses of the transversus abdominis, external oblique, and internal oblique muscles. The aponeuroses form an anterior and posterior sheath, which together serve as a strong barrier against the herniation of abdominal contents, accounting for the rarity of a spontaneous rectus sheath hernia. However, inferior to the umbilicus (below the arcuate line), the posterior rectus sheath is composed primarily of transversalis fascia, which may make this region more susceptible to herniation.3 Additional predisposing factors to herniation include increased muscle weakness and elevated intra-abdominal pressure, such as that which occurs during pregnancy or from ascites.4
Clinical Presentation
Like other abdominal wall hernias, the clinical presentation of posterior rectus sheath hernias is nonspecific. Patients may be asymptomatic or may develop abdominal pain, distension, and vomiting as a result of acute complications that necessitate emergent surgery. During history-taking, inquiry into a patient’s surgical history is crucial because it may raise clinical suspicion for an abdominal wall hernia, as was the case in our patient, who recently had a cesarean delivery.
Diagnosis
Because prompt and accurate diagnosis of acute complications of abdominal wall hernias is essential, imaging studies are typically required for diagnosis. Computed tomography is the modality of choice based on its ability to provide superior anatomic detail of the abdominal wall, permitting identification of hernias and differentiating them from other abdominal masses, such as hematomas, abscesses, or tumors. Additionally, CT is able to detect early signs of hernia sac complications, including bowel obstruction, incarceration, and strangulation.5
Treatment
Treatment for a posterior rectus sheath hernia is surgical with primary closure being the preferred method. Prosthetic repair may also be performed, particularly when the hernia defect is large, but it has been shown to be associated with an increased risk of intestinal strangulation.3
A 45-year-old woman with a history of polycystic ovary syndrome presented to the ED for evaluation of acute abdominal pain. The patient’s surgical history was significant for a cesarean delivery 6 months prior to presentation. Abdominal examination revealed a well-healed suprapubic cesarean incision scar, which was tender upon palpation. A computed tomography (CT) scan of the abdomen and pelvis with contrast were ordered; representative images are shown above (Figure 1a-1d).

What is the diagnosis? What are the associated complications and preferred management for this entity?
Answer
The scout image from the CT scan shows multiple dilated loops of small bowel (white arrows, Figure 2a) and only a small amount of air within a decompressed colon (red arrow, Figure 2a). The multiplanar CT image confirmed multiple dilated small bowel loops (white arrows, Figure 2b) and the decompressed large bowel (red arrows, Figure 2b), indicating the presence of a small bowel obstruction. A distal small bowel loop (white arrows, Figure 2c and 2d) was identified in a hernia sac within the walls of the rectus abdominis muscle (red arrows, Figure 2c and 2d). Mesenteric stranding within the hernia sac was suggestive of incarceration (black arrow, Figure 2d). No signs of intestinal ischemia, such as pneumatosis or wall thickening, were present.

An exploratory laparotomy was emergently performed, which confirmed the presence of incarcerated small bowel within the posterior rectus sheath defect without evidence of strangulation. Reduction of small bowel and primary closure of the hernia defect was subsequently performed without complication.
Abdominal Wall Hernias
Abdominal wall hernias are common in the United States, with more than 1 million abdominal wall hernia repairs performed annually.1 A posterior rectus sheath hernia is a rare type of abdominal wall hernia; the majority are postsurgical (as seen in this patient) or posttraumatic, with only a few reported congenital cases.2
Anatomy
The rectus sheath encloses the rectus abdominis muscle and is composed of the aponeuroses of the transversus abdominis, external oblique, and internal oblique muscles. The aponeuroses form an anterior and posterior sheath, which together serve as a strong barrier against the herniation of abdominal contents, accounting for the rarity of a spontaneous rectus sheath hernia. However, inferior to the umbilicus (below the arcuate line), the posterior rectus sheath is composed primarily of transversalis fascia, which may make this region more susceptible to herniation.3 Additional predisposing factors to herniation include increased muscle weakness and elevated intra-abdominal pressure, such as that which occurs during pregnancy or from ascites.4
Clinical Presentation
Like other abdominal wall hernias, the clinical presentation of posterior rectus sheath hernias is nonspecific. Patients may be asymptomatic or may develop abdominal pain, distension, and vomiting as a result of acute complications that necessitate emergent surgery. During history-taking, inquiry into a patient’s surgical history is crucial because it may raise clinical suspicion for an abdominal wall hernia, as was the case in our patient, who recently had a cesarean delivery.
Diagnosis
Because prompt and accurate diagnosis of acute complications of abdominal wall hernias is essential, imaging studies are typically required for diagnosis. Computed tomography is the modality of choice based on its ability to provide superior anatomic detail of the abdominal wall, permitting identification of hernias and differentiating them from other abdominal masses, such as hematomas, abscesses, or tumors. Additionally, CT is able to detect early signs of hernia sac complications, including bowel obstruction, incarceration, and strangulation.5
Treatment
Treatment for a posterior rectus sheath hernia is surgical with primary closure being the preferred method. Prosthetic repair may also be performed, particularly when the hernia defect is large, but it has been shown to be associated with an increased risk of intestinal strangulation.3
1. Rutkow IM. Demographic and socioeconomic aspects of hernia repair in the United States in 2003. Surg Clin North Am. 2003;83(5):1045-1051, v-vi. doi:10.1016/S0039-6109(03)00132-4.
2. Lenobel S, Lenobel R, Yu J. Posterior rectus sheath hernia causing intermittent small bowel obstruction. J Radiol Case Rep J. 2014;8(9):25-29. doi:10.3941/jrcr.v8i9.2081.
3. Losanoff JE, Basson MD, Gruber SA. Spontaneous hernia through the posterior rectus abdominis sheath: case report and review of the published literature 1937-2008. Hernia. 2009;13(5):555-558. doi:10.1007/s10029-009-0481-6.
4. Bentzon N, Adamsen S. Hernia of the posterior rectus sheath: a new entity? Eur J Surg. 1995;161(3):215-216.
5. Aguirre DA, Santosa AC, Casola G, Sirlin CB. Abdominal wall hernias: imaging features, complications, and diagnostic pitfalls at mutli-detector row CT. Radiographics. 2005;25(6):1501-1520. doi:10.1148/rg.256055018.
1. Rutkow IM. Demographic and socioeconomic aspects of hernia repair in the United States in 2003. Surg Clin North Am. 2003;83(5):1045-1051, v-vi. doi:10.1016/S0039-6109(03)00132-4.
2. Lenobel S, Lenobel R, Yu J. Posterior rectus sheath hernia causing intermittent small bowel obstruction. J Radiol Case Rep J. 2014;8(9):25-29. doi:10.3941/jrcr.v8i9.2081.
3. Losanoff JE, Basson MD, Gruber SA. Spontaneous hernia through the posterior rectus abdominis sheath: case report and review of the published literature 1937-2008. Hernia. 2009;13(5):555-558. doi:10.1007/s10029-009-0481-6.
4. Bentzon N, Adamsen S. Hernia of the posterior rectus sheath: a new entity? Eur J Surg. 1995;161(3):215-216.
5. Aguirre DA, Santosa AC, Casola G, Sirlin CB. Abdominal wall hernias: imaging features, complications, and diagnostic pitfalls at mutli-detector row CT. Radiographics. 2005;25(6):1501-1520. doi:10.1148/rg.256055018.
Nausea/vomiting • tachycardia • unintentional weight loss • Dx?
THE CASE
A 22-year-old woman presented to the emergency department (ED) with a 24-hour history of nausea, vomiting, diarrhea, generalized abdominal pain, and mild headache. She denied shortness of breath, chest pain, or anxiety, and didn’t have a history of cardiac problems. The physical examination revealed tachycardia (heart rate, 135 beats/min) and a respiratory rate of 24 breaths per minute. The patient was diagnosed with dehydration and was given 3 liters of intravenous (IV) fluids. After fluid administration, her heart rate decreased to 94 beats/min and she was discharged home.
The patient returned to the ED later that same day with recurrent nausea, vomiting, and a mild fever. This time she reported a several week history of palpitations, heat intolerance, agitation, mild cognitive impairment, and difficulty sleeping. Her mother accompanied her to this visit and added that the patient had unintentionally lost 13 pounds over the past 2 weeks. The patient denied pain or enlargement in her neck, obstructive symptoms, hives, pruritus, or changes in vision. Reexamination revealed tachycardia (132 beats/min) with no murmurs, rubs, or gallops; increased respiratory rate (26 breaths/min); and diffuse thyromegaly without distinct nodules. The thyroid was nontender to palpation. The patient was also found to have a fine resting tremor, hyperactive deep tendon reflexes, and clonus in her lower extremities. Bibasilar crackles were noted on lung exam.
THE DIAGNOSIS
An electrocardiogram (EKG) revealed sinus tachycardia with some sinus arrhythmia. A chest radiograph revealed prominent pulmonary vasculature and the presence of Kerley B lines consistent with marked pulmonary edema. Laboratory testing revealed an elevated N-terminal pro b-type natriuretic peptide level of 2420 pg/mL (normal range: <100 pg/mL). Evaluation of thyroid function revealed overt hyperthyroidism with an elevated free thyroxine of 4.6 ng/dL (normal range: 0.8-1.8 ng/dL), a total triiodothyronine of 199 ng/dL (normal range: 60-181 ng/dL), and a suppressed thyroid-stimulating hormone level of <0.02 mcU/mL (normal range: 0.35-5 mcU/mL). A subsequent thyroid ultrasound showed a diffusely enlarged thyroid gland with a thickened isthmus, but no nodules.
The patient’s results were discussed with the on-call endocrinology provider at the time of her revisit to the ED. The patient was started on antithyroid medications (methimazole 20 mg/d) and a beta-blocker (atenolol 25 mg/d). Arrangements were made for an outpatient endocrine consultation within 3 days of her visit to the ED.
Upon evaluation in the outpatient endocrinology clinic, a thyrotropin receptor antibody test was positive, confirming Graves’ disease. The patient was given a diagnosis of thyrotoxicosis secondary to hyperthyroidism due to Graves’ disease. Her marked pulmonary edema was secondary to thyrotoxicosis and aggressive hydration with IV fluids.
DISCUSSION
Hyperthyroidism is a common metabolic disorder with prominent cardiovascular manifestations.1 Classically, patients with hyperthyroidism develop irritability, heat intolerance, emotional lability, muscle weakness, menstrual abnormalities, and weight loss (despite an increased appetite). Cardiovascular manifestations include palpitations in up to 85% of patients, and dyspnea on exertion and fatigue in approximately 50% of patients.2 Hyperthyroidism has also been shown to produce changes in cardiac contractility, myocardial oxygen consumption, cardiac output, blood pressure, and systemic vascular resistance.3,4 Hyperthyroidism may complicate preexisting cardiac disease or may cause cardiac complications in individuals without structural abnormalities. (Our patient had no known structural abnormalities.)
In a small subset of patients with severe hyperthyroidism and exaggerated sinus tachycardia or atrial fibrillation, rate-related left ventricular dysfunction may cause heart failure.5 The assessment of thyrotoxic manifestations, especially potential cardiovascular complications, is essential to formulating an appropriate treatment plan.6 Cardiac evaluation may require an echocardiogram, EKG, Holter monitor, or myocardial perfusion studies.
Beta-blockers, diuretics among treatment options
Treatment with beta-blockers to reduce heart rate should be first-line therapy.7 In patients with overt heart failure involving pulmonary congestion, the use of diuretics may be appropriate.8
Our patient continued to take the medications prescribed during her ED visit: methimazole 20 mg/d and atenolol 25 mg/d for her Graves’ disease. A
THE TAKEAWAY
The cardiovascular manifestations of hyperthyroidism remain some of the most common signs and symptoms of thyroid disease. Pulmonary edema and congestive heart failure, however, are uncommon. Physicians need to be aware of this rare—but important—clinical presentation of a common condition.
1. Klein I, Ojamaa K. Thyroid hormone and the cardiovascular system. N Engl J Med. 2001;344:501-509.
2. Fadel BM, Ellahham S, Ringel MD, et al. Hyperthyroid heart disease. Clin Cardiol. 2000;23:402-408.
3. Biondi B, Palmieri EA, Lombardi G, et al. Effects of thyroid hormone on cardiac function: the relative importance of heart rate, loading conditions, and myocardial contractility in the regulation of cardiac performance in human hyperthyroidism. J Clin Endocrinol Metab. 2002;87:968-974.
4. Kahaly GJ, Dillmann WH. Thyroid hormone action in the heart. Endocr Rev. 2005;26:704-728.
5. Klein I, Danzi S. Thyroid disease and the heart. Circulation. 2007;116:1725-1735.
6. Bahn Chair RS, Burch HB, Cooper DS, et al; American Thyroid Association; American Association of Clinical Endocrinologists. Hyperthyroidism and other causes of thyrotoxicosis: management guidelines of the American Thyroid Association and American Association of Clinical Endocrinologists. Thyroid. 2011;21:593-646.
7. Klein I, Becker DV, Levey GS. Treatment of hyperthyroid disease. Ann Intern Med. 1994;121:281-288.
8. Danzi S, Klein I. Thyroid hormone and blood pressure regulation. Curr Hypertens Rep. 2003;5:513-520.
THE CASE
A 22-year-old woman presented to the emergency department (ED) with a 24-hour history of nausea, vomiting, diarrhea, generalized abdominal pain, and mild headache. She denied shortness of breath, chest pain, or anxiety, and didn’t have a history of cardiac problems. The physical examination revealed tachycardia (heart rate, 135 beats/min) and a respiratory rate of 24 breaths per minute. The patient was diagnosed with dehydration and was given 3 liters of intravenous (IV) fluids. After fluid administration, her heart rate decreased to 94 beats/min and she was discharged home.
The patient returned to the ED later that same day with recurrent nausea, vomiting, and a mild fever. This time she reported a several week history of palpitations, heat intolerance, agitation, mild cognitive impairment, and difficulty sleeping. Her mother accompanied her to this visit and added that the patient had unintentionally lost 13 pounds over the past 2 weeks. The patient denied pain or enlargement in her neck, obstructive symptoms, hives, pruritus, or changes in vision. Reexamination revealed tachycardia (132 beats/min) with no murmurs, rubs, or gallops; increased respiratory rate (26 breaths/min); and diffuse thyromegaly without distinct nodules. The thyroid was nontender to palpation. The patient was also found to have a fine resting tremor, hyperactive deep tendon reflexes, and clonus in her lower extremities. Bibasilar crackles were noted on lung exam.
THE DIAGNOSIS
An electrocardiogram (EKG) revealed sinus tachycardia with some sinus arrhythmia. A chest radiograph revealed prominent pulmonary vasculature and the presence of Kerley B lines consistent with marked pulmonary edema. Laboratory testing revealed an elevated N-terminal pro b-type natriuretic peptide level of 2420 pg/mL (normal range: <100 pg/mL). Evaluation of thyroid function revealed overt hyperthyroidism with an elevated free thyroxine of 4.6 ng/dL (normal range: 0.8-1.8 ng/dL), a total triiodothyronine of 199 ng/dL (normal range: 60-181 ng/dL), and a suppressed thyroid-stimulating hormone level of <0.02 mcU/mL (normal range: 0.35-5 mcU/mL). A subsequent thyroid ultrasound showed a diffusely enlarged thyroid gland with a thickened isthmus, but no nodules.
The patient’s results were discussed with the on-call endocrinology provider at the time of her revisit to the ED. The patient was started on antithyroid medications (methimazole 20 mg/d) and a beta-blocker (atenolol 25 mg/d). Arrangements were made for an outpatient endocrine consultation within 3 days of her visit to the ED.
Upon evaluation in the outpatient endocrinology clinic, a thyrotropin receptor antibody test was positive, confirming Graves’ disease. The patient was given a diagnosis of thyrotoxicosis secondary to hyperthyroidism due to Graves’ disease. Her marked pulmonary edema was secondary to thyrotoxicosis and aggressive hydration with IV fluids.
DISCUSSION
Hyperthyroidism is a common metabolic disorder with prominent cardiovascular manifestations.1 Classically, patients with hyperthyroidism develop irritability, heat intolerance, emotional lability, muscle weakness, menstrual abnormalities, and weight loss (despite an increased appetite). Cardiovascular manifestations include palpitations in up to 85% of patients, and dyspnea on exertion and fatigue in approximately 50% of patients.2 Hyperthyroidism has also been shown to produce changes in cardiac contractility, myocardial oxygen consumption, cardiac output, blood pressure, and systemic vascular resistance.3,4 Hyperthyroidism may complicate preexisting cardiac disease or may cause cardiac complications in individuals without structural abnormalities. (Our patient had no known structural abnormalities.)
In a small subset of patients with severe hyperthyroidism and exaggerated sinus tachycardia or atrial fibrillation, rate-related left ventricular dysfunction may cause heart failure.5 The assessment of thyrotoxic manifestations, especially potential cardiovascular complications, is essential to formulating an appropriate treatment plan.6 Cardiac evaluation may require an echocardiogram, EKG, Holter monitor, or myocardial perfusion studies.
Beta-blockers, diuretics among treatment options
Treatment with beta-blockers to reduce heart rate should be first-line therapy.7 In patients with overt heart failure involving pulmonary congestion, the use of diuretics may be appropriate.8
Our patient continued to take the medications prescribed during her ED visit: methimazole 20 mg/d and atenolol 25 mg/d for her Graves’ disease. A
THE TAKEAWAY
The cardiovascular manifestations of hyperthyroidism remain some of the most common signs and symptoms of thyroid disease. Pulmonary edema and congestive heart failure, however, are uncommon. Physicians need to be aware of this rare—but important—clinical presentation of a common condition.
THE CASE
A 22-year-old woman presented to the emergency department (ED) with a 24-hour history of nausea, vomiting, diarrhea, generalized abdominal pain, and mild headache. She denied shortness of breath, chest pain, or anxiety, and didn’t have a history of cardiac problems. The physical examination revealed tachycardia (heart rate, 135 beats/min) and a respiratory rate of 24 breaths per minute. The patient was diagnosed with dehydration and was given 3 liters of intravenous (IV) fluids. After fluid administration, her heart rate decreased to 94 beats/min and she was discharged home.
The patient returned to the ED later that same day with recurrent nausea, vomiting, and a mild fever. This time she reported a several week history of palpitations, heat intolerance, agitation, mild cognitive impairment, and difficulty sleeping. Her mother accompanied her to this visit and added that the patient had unintentionally lost 13 pounds over the past 2 weeks. The patient denied pain or enlargement in her neck, obstructive symptoms, hives, pruritus, or changes in vision. Reexamination revealed tachycardia (132 beats/min) with no murmurs, rubs, or gallops; increased respiratory rate (26 breaths/min); and diffuse thyromegaly without distinct nodules. The thyroid was nontender to palpation. The patient was also found to have a fine resting tremor, hyperactive deep tendon reflexes, and clonus in her lower extremities. Bibasilar crackles were noted on lung exam.
THE DIAGNOSIS
An electrocardiogram (EKG) revealed sinus tachycardia with some sinus arrhythmia. A chest radiograph revealed prominent pulmonary vasculature and the presence of Kerley B lines consistent with marked pulmonary edema. Laboratory testing revealed an elevated N-terminal pro b-type natriuretic peptide level of 2420 pg/mL (normal range: <100 pg/mL). Evaluation of thyroid function revealed overt hyperthyroidism with an elevated free thyroxine of 4.6 ng/dL (normal range: 0.8-1.8 ng/dL), a total triiodothyronine of 199 ng/dL (normal range: 60-181 ng/dL), and a suppressed thyroid-stimulating hormone level of <0.02 mcU/mL (normal range: 0.35-5 mcU/mL). A subsequent thyroid ultrasound showed a diffusely enlarged thyroid gland with a thickened isthmus, but no nodules.
The patient’s results were discussed with the on-call endocrinology provider at the time of her revisit to the ED. The patient was started on antithyroid medications (methimazole 20 mg/d) and a beta-blocker (atenolol 25 mg/d). Arrangements were made for an outpatient endocrine consultation within 3 days of her visit to the ED.
Upon evaluation in the outpatient endocrinology clinic, a thyrotropin receptor antibody test was positive, confirming Graves’ disease. The patient was given a diagnosis of thyrotoxicosis secondary to hyperthyroidism due to Graves’ disease. Her marked pulmonary edema was secondary to thyrotoxicosis and aggressive hydration with IV fluids.
DISCUSSION
Hyperthyroidism is a common metabolic disorder with prominent cardiovascular manifestations.1 Classically, patients with hyperthyroidism develop irritability, heat intolerance, emotional lability, muscle weakness, menstrual abnormalities, and weight loss (despite an increased appetite). Cardiovascular manifestations include palpitations in up to 85% of patients, and dyspnea on exertion and fatigue in approximately 50% of patients.2 Hyperthyroidism has also been shown to produce changes in cardiac contractility, myocardial oxygen consumption, cardiac output, blood pressure, and systemic vascular resistance.3,4 Hyperthyroidism may complicate preexisting cardiac disease or may cause cardiac complications in individuals without structural abnormalities. (Our patient had no known structural abnormalities.)
In a small subset of patients with severe hyperthyroidism and exaggerated sinus tachycardia or atrial fibrillation, rate-related left ventricular dysfunction may cause heart failure.5 The assessment of thyrotoxic manifestations, especially potential cardiovascular complications, is essential to formulating an appropriate treatment plan.6 Cardiac evaluation may require an echocardiogram, EKG, Holter monitor, or myocardial perfusion studies.
Beta-blockers, diuretics among treatment options
Treatment with beta-blockers to reduce heart rate should be first-line therapy.7 In patients with overt heart failure involving pulmonary congestion, the use of diuretics may be appropriate.8
Our patient continued to take the medications prescribed during her ED visit: methimazole 20 mg/d and atenolol 25 mg/d for her Graves’ disease. A
THE TAKEAWAY
The cardiovascular manifestations of hyperthyroidism remain some of the most common signs and symptoms of thyroid disease. Pulmonary edema and congestive heart failure, however, are uncommon. Physicians need to be aware of this rare—but important—clinical presentation of a common condition.
1. Klein I, Ojamaa K. Thyroid hormone and the cardiovascular system. N Engl J Med. 2001;344:501-509.
2. Fadel BM, Ellahham S, Ringel MD, et al. Hyperthyroid heart disease. Clin Cardiol. 2000;23:402-408.
3. Biondi B, Palmieri EA, Lombardi G, et al. Effects of thyroid hormone on cardiac function: the relative importance of heart rate, loading conditions, and myocardial contractility in the regulation of cardiac performance in human hyperthyroidism. J Clin Endocrinol Metab. 2002;87:968-974.
4. Kahaly GJ, Dillmann WH. Thyroid hormone action in the heart. Endocr Rev. 2005;26:704-728.
5. Klein I, Danzi S. Thyroid disease and the heart. Circulation. 2007;116:1725-1735.
6. Bahn Chair RS, Burch HB, Cooper DS, et al; American Thyroid Association; American Association of Clinical Endocrinologists. Hyperthyroidism and other causes of thyrotoxicosis: management guidelines of the American Thyroid Association and American Association of Clinical Endocrinologists. Thyroid. 2011;21:593-646.
7. Klein I, Becker DV, Levey GS. Treatment of hyperthyroid disease. Ann Intern Med. 1994;121:281-288.
8. Danzi S, Klein I. Thyroid hormone and blood pressure regulation. Curr Hypertens Rep. 2003;5:513-520.
1. Klein I, Ojamaa K. Thyroid hormone and the cardiovascular system. N Engl J Med. 2001;344:501-509.
2. Fadel BM, Ellahham S, Ringel MD, et al. Hyperthyroid heart disease. Clin Cardiol. 2000;23:402-408.
3. Biondi B, Palmieri EA, Lombardi G, et al. Effects of thyroid hormone on cardiac function: the relative importance of heart rate, loading conditions, and myocardial contractility in the regulation of cardiac performance in human hyperthyroidism. J Clin Endocrinol Metab. 2002;87:968-974.
4. Kahaly GJ, Dillmann WH. Thyroid hormone action in the heart. Endocr Rev. 2005;26:704-728.
5. Klein I, Danzi S. Thyroid disease and the heart. Circulation. 2007;116:1725-1735.
6. Bahn Chair RS, Burch HB, Cooper DS, et al; American Thyroid Association; American Association of Clinical Endocrinologists. Hyperthyroidism and other causes of thyrotoxicosis: management guidelines of the American Thyroid Association and American Association of Clinical Endocrinologists. Thyroid. 2011;21:593-646.
7. Klein I, Becker DV, Levey GS. Treatment of hyperthyroid disease. Ann Intern Med. 1994;121:281-288.
8. Danzi S, Klein I. Thyroid hormone and blood pressure regulation. Curr Hypertens Rep. 2003;5:513-520.
Muscle cramps/pain • weakness • muscle twitching • Dx?
THE CASE
A 39-year-old man who worked in construction presented to our clinic with complaints of muscle cramps and muscle pain that had been bothering him for several months. The cramps and pain started in both of his arms and subsequently became diffuse and generalized. He also reported an unintentional 15-pound weight loss.
His exam at that time was unremarkable. He was diagnosed with dehydration and cramping due to overexertion at work. A basic metabolic panel, hemogram, lipid panel, and thyroid stimulating hormone level were ordered. The patient’s triglyceride level, which was 227 mg/dL, was the only significant result (normal level: <150 mg/dL).
The patient’s symptoms continued to worsen until he returned to the clinic 6 months later, again complaining of muscle cramps and pain throughout his body. At that second visit, he also reported profound overall weakness and the development of diffuse muscle twitching, which his wife had observed while he was sleeping. As a result of these worrisome symptoms, he had become anxious and depressed.
A review of his medical record revealed a weight loss of about 20 pounds over the previous year. On exam, he had diffuse fasciculations in all the major muscle groups, including his tongue. The patient’s strength was 4/5 in all muscle groups. His deep tendon reflexes were 3+. He had a negative Babinski reflex (ie, he had downward facing toes with plantar stimulation), and cranial nerves II to XII were all intact. His rapid alternating movements and gait were slow.
THE DIAGNOSIS
Based on the exam, the primary diagnostic consideration for the patient was amyotrophic lateral sclerosis (ALS). Lab tests were ordered and revealed normal calcium and electrolyte levels, a normal erythrocyte sedimentation rate, a normal C-reactive protein level, and a negative test for acetylcholine receptor antibodies. However, the patient had an elevated creatine kinase level of 664 U/L (normal: 30-200 U/L). The patient was sent to a neuromuscular specialist, who identified signs of upper and lower motor neuron disease in all 4 of the patient’s extremities (he had foot drop that had not been present previously) and a very brisk jaw jerk. Along with the tongue fasciculations, the results of the specialist’s physical exam suggested ALS. Four-limb electromyography (EMG) showed widespread fasciculations and some large motor unit potentials and recruitment abnormalities, which were also consistent with ALS. It appeared that the patient’s weight loss was due to both muscle atrophy and the amount of calories burned from his constant twitching.
Extensive testing was done to rule out other potential causes of the patient’s symptoms, including magnetic resonance imaging (MRI) of the spine and brain (which was normal). In addition, the patient’s aldolase level and antineutrophil cytoplasmic antibodies were normal. The patient tested negative for human immunodeficiency virus and antibodies to double-stranded DNA. After serial neurologic exams, the final diagnosis of ALS was made.
DISCUSSION
ALS, also known as Lou Gehrig’s disease, is a degenerative motor neuron disease.1-3 The incidence in North America is 1.5 to 2.7 per 100,000 per year, and the prevalence is 2.7 to 7.4 per 100,000.4 The incidence of ALS increases with each decade of life, especially after age 40, and peaks at 74 years of age.4 The male to female ratio is 1:1.5-2.4 ALS affects upper and lower motor neurons and is progressive; however, the rate of progression and phenotype vary greatly between individuals.2 Most patients with ALS die within 2 to 5 years of onset.5
There is no specific test for ALS; the diagnosis is made clinically based on the revised El Escorial World Federation of Neurology criteria, also known as the Airlie House criteria.2,6,7 These criteria include evidence of lower motor neuron degeneration by clinical, electrophysiologic, or neuropathologic exam; evidence of upper motor neuron disease by clinical exam; progressive spread of symptoms or signs within a region or to other regions (by history or exam); and the absence of electrophysiologic, neuroimaging, or pathologic evidence of other disease processes that could explain the symptoms. If patients have evidence of upper and lower motor neuron disease, they should be reevaluated in 4 weeks to see if symptoms are improving or progressing.
Like our patient, many patients will have an elevated creatine kinase level (some with levels as high as 1000 U/L), and calcium may also be elevated because, rarely, ALS is associated with primary hyperparathyroidism.8 Electrophysiologic studies can be helpful in identifying active denervation of lower motor neurons.4,6,7
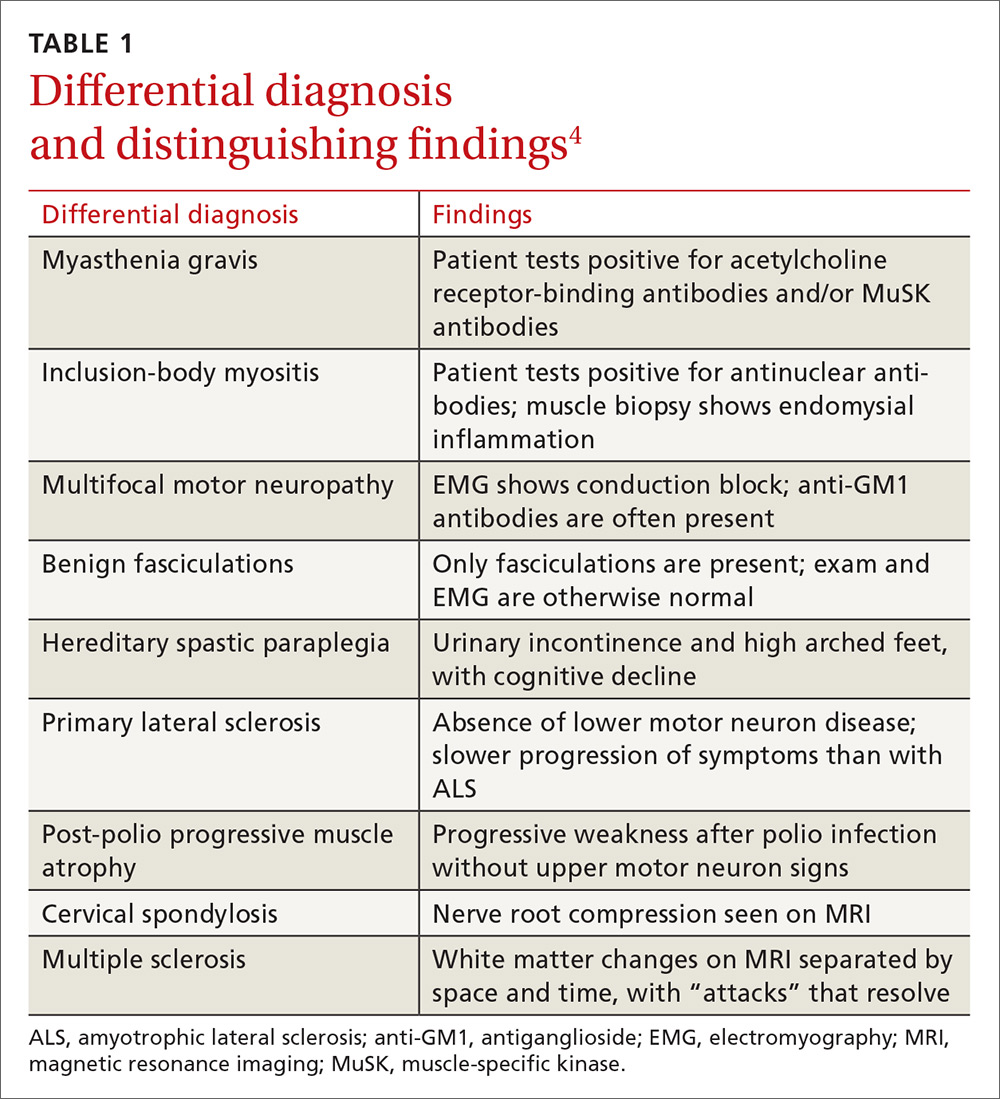
The differential diagnosis for ALS includes myasthenia gravis, inclusion-body myositis, multifocal motor neuropathy, benign fasciculations, hereditary spastic paraplegia, primary lateral sclerosis, post-polio progressive muscle atrophy, cervical spondylosis, and multiple sclerosis. A negative acetylcholine receptor antibody test will rule out myasthenia gravis, imaging of the spine can rule out cervical spondylosis, and electrophysiologic testing helps eliminate the other conditions (TABLE 14).
Treatment in specialty clinics can prolong survival
The mainstays of treatment are symptom management, multidisciplinary care (by physicians, physical/occupational/speech therapists, nutritionists, psychologists, psychotherapists, and genetic counselors), palliative care, and counseling about end-of-life issues for patients and family.1,5 Utilization of an ALS specialty clinic can provide access to all of these services and should be considered, as there is evidence that treatment in such clinics can prolong survival.5 The location of ALS specialty clinics can be found on the ALS Association’s Web site at http://www.alsa.org/community/.
Despite treatment, however, ALS is a progressive disease. The prognosis is poor, with a median survival of 2 to 5 years after diagnosis.9
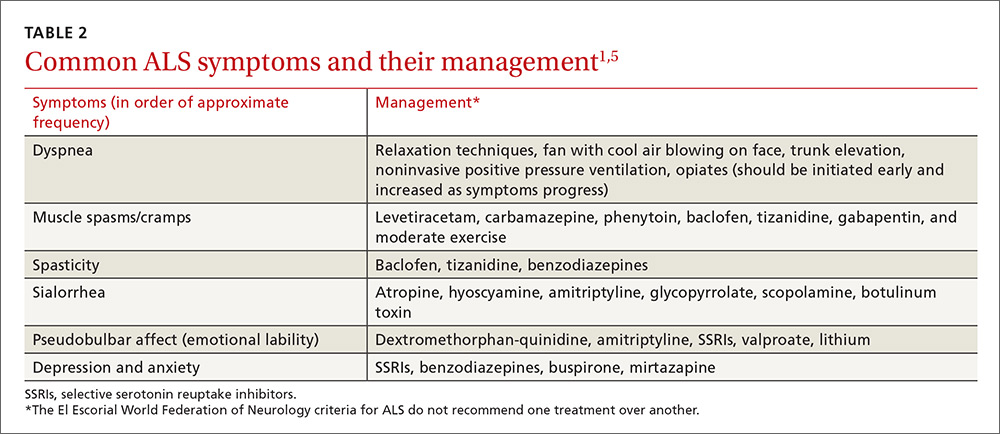
The El Escorial World Federation of Neurology criteria for the diagnosis of ALS address how to treat the most common symptoms of ALS that occur as the disease progresses. These symptoms include dyspnea, muscle spasms, spasticity, sialorrhea, and pseudobulbar affect (TABLE 21,5).
Our patient was started on baclofen 10 mg 3 times per day (titrated up as needed) for muscle spasms and cramps, which resulted in some improvement of his cramps, but no improvement in the spasms. He was also started on sertraline 50 mg for anxiety and depression. His overall weakness continued to progress, and we recommended that the patient get ankle-foot orthosis braces to help with the mobility impairment caused by foot drop.
We then referred him to an ALS specialty clinic recommended by the neuromuscular specialist. The patient is now enrolled in a clinical trial designed to test a cerebrospinal fluid marker for diagnosis and for a new drug aimed at symptom management.
THE TAKEAWAY
Muscle cramps and pain are early signs of ALS. Although ALS is uncommon, patients who present with muscle cramps and muscle pain should have a creatine kinase test ordered (which, if elevated, should prompt further investigation into ALS as the possible cause). Patients should also undergo a neurologic examination to seek evidence of upper and lower motor neuron disease. They should then be reevaluated in 4 weeks to see if symptoms are improving or progressing. If no improvement is seen and symptoms are progressive, a work-up for ALS should be considered.
The mainstay of treatment for patients with ALS is multidisciplinary symptom management and palliative care. Utilization of an ALS specialty clinic should also be recommended, as it can improve survival.5
1. Miller RG, Gelinas D, O’Connor P. Amyotrophic Lateral Sclerosis: American Academy of Neurology Press Quality of Life Guide Series. Demos Medical Publishing; 2004.
2. Simon NG, Turner MR, Vucic S, et al. Quantifying disease progression in amyotrophic lateral sclerosis. Ann Neurol. 2014;76:643-657.
3. Worms PM. The epidemiology of motor neuron diseases: a review of recent studies. J Neurol Sci. 2001;191:3-9.
4. Shaw PJ. ALS and other motor neuron diseases. In: Goldman L, Schafer AI, eds. Goldman’s Cecil Medicine. 24th ed. Philadelphia, PA: Elsevier Saunders; 2015:chap 418.
5. Miller RG, Jackson CE, Kasarskis EJ, et al. Practice Parameter update: The Care of the Patient with Amyotrophic Lateral Sclerosis: Multidisciplinary care, symptom management, and cognitive/behavioral impairment (an evidence-based review). Report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology. 2009;73:1227-1233.
6. Brooks BR. El Escorial World Federation of Neurology criteria for the diagnosis of amyotrophic lateral sclerosis. Subcommittee on Motor Neuron Diseases/Amyotrophic Lateral Sclerosis of the World Federation of Neurology Research Group on Neuromuscular Diseases and the El Escorial “Clinical limits of amyotrophic lateral sclerosis” workshop contributors. J Neurol Sci. 1994;124:96-107.
7. Brooks BR, Miller RG, Swash M, et al; World Federation of Neurology Research Group on Motor Neuron Diseases. El Escorial revisited: revised criteria for the diagnosis of amyotrophic lateral sclerosis. Amyotroph Lateral Scler Other Motor Neuron Disord. 2000;1:293-299.
8. Jackson CE, Amato AA, Bryan WW, et al. Primary hyperparathyroidism and ALS: is there a relation? Neurology. 1998;50:1795-1799.
9. Jablecki CK, Berry C, Leach J. Survival prediction in amyotrophic lateral sclerosis. Muscle Nerve. 1989;12:833-841.
THE CASE
A 39-year-old man who worked in construction presented to our clinic with complaints of muscle cramps and muscle pain that had been bothering him for several months. The cramps and pain started in both of his arms and subsequently became diffuse and generalized. He also reported an unintentional 15-pound weight loss.
His exam at that time was unremarkable. He was diagnosed with dehydration and cramping due to overexertion at work. A basic metabolic panel, hemogram, lipid panel, and thyroid stimulating hormone level were ordered. The patient’s triglyceride level, which was 227 mg/dL, was the only significant result (normal level: <150 mg/dL).
The patient’s symptoms continued to worsen until he returned to the clinic 6 months later, again complaining of muscle cramps and pain throughout his body. At that second visit, he also reported profound overall weakness and the development of diffuse muscle twitching, which his wife had observed while he was sleeping. As a result of these worrisome symptoms, he had become anxious and depressed.
A review of his medical record revealed a weight loss of about 20 pounds over the previous year. On exam, he had diffuse fasciculations in all the major muscle groups, including his tongue. The patient’s strength was 4/5 in all muscle groups. His deep tendon reflexes were 3+. He had a negative Babinski reflex (ie, he had downward facing toes with plantar stimulation), and cranial nerves II to XII were all intact. His rapid alternating movements and gait were slow.
THE DIAGNOSIS
Based on the exam, the primary diagnostic consideration for the patient was amyotrophic lateral sclerosis (ALS). Lab tests were ordered and revealed normal calcium and electrolyte levels, a normal erythrocyte sedimentation rate, a normal C-reactive protein level, and a negative test for acetylcholine receptor antibodies. However, the patient had an elevated creatine kinase level of 664 U/L (normal: 30-200 U/L). The patient was sent to a neuromuscular specialist, who identified signs of upper and lower motor neuron disease in all 4 of the patient’s extremities (he had foot drop that had not been present previously) and a very brisk jaw jerk. Along with the tongue fasciculations, the results of the specialist’s physical exam suggested ALS. Four-limb electromyography (EMG) showed widespread fasciculations and some large motor unit potentials and recruitment abnormalities, which were also consistent with ALS. It appeared that the patient’s weight loss was due to both muscle atrophy and the amount of calories burned from his constant twitching.
Extensive testing was done to rule out other potential causes of the patient’s symptoms, including magnetic resonance imaging (MRI) of the spine and brain (which was normal). In addition, the patient’s aldolase level and antineutrophil cytoplasmic antibodies were normal. The patient tested negative for human immunodeficiency virus and antibodies to double-stranded DNA. After serial neurologic exams, the final diagnosis of ALS was made.
DISCUSSION
ALS, also known as Lou Gehrig’s disease, is a degenerative motor neuron disease.1-3 The incidence in North America is 1.5 to 2.7 per 100,000 per year, and the prevalence is 2.7 to 7.4 per 100,000.4 The incidence of ALS increases with each decade of life, especially after age 40, and peaks at 74 years of age.4 The male to female ratio is 1:1.5-2.4 ALS affects upper and lower motor neurons and is progressive; however, the rate of progression and phenotype vary greatly between individuals.2 Most patients with ALS die within 2 to 5 years of onset.5
There is no specific test for ALS; the diagnosis is made clinically based on the revised El Escorial World Federation of Neurology criteria, also known as the Airlie House criteria.2,6,7 These criteria include evidence of lower motor neuron degeneration by clinical, electrophysiologic, or neuropathologic exam; evidence of upper motor neuron disease by clinical exam; progressive spread of symptoms or signs within a region or to other regions (by history or exam); and the absence of electrophysiologic, neuroimaging, or pathologic evidence of other disease processes that could explain the symptoms. If patients have evidence of upper and lower motor neuron disease, they should be reevaluated in 4 weeks to see if symptoms are improving or progressing.
Like our patient, many patients will have an elevated creatine kinase level (some with levels as high as 1000 U/L), and calcium may also be elevated because, rarely, ALS is associated with primary hyperparathyroidism.8 Electrophysiologic studies can be helpful in identifying active denervation of lower motor neurons.4,6,7
The differential diagnosis for ALS includes myasthenia gravis, inclusion-body myositis, multifocal motor neuropathy, benign fasciculations, hereditary spastic paraplegia, primary lateral sclerosis, post-polio progressive muscle atrophy, cervical spondylosis, and multiple sclerosis. A negative acetylcholine receptor antibody test will rule out myasthenia gravis, imaging of the spine can rule out cervical spondylosis, and electrophysiologic testing helps eliminate the other conditions (TABLE 14).

Treatment in specialty clinics can prolong survival
The mainstays of treatment are symptom management, multidisciplinary care (by physicians, physical/occupational/speech therapists, nutritionists, psychologists, psychotherapists, and genetic counselors), palliative care, and counseling about end-of-life issues for patients and family.1,5 Utilization of an ALS specialty clinic can provide access to all of these services and should be considered, as there is evidence that treatment in such clinics can prolong survival.5 The location of ALS specialty clinics can be found on the ALS Association’s Web site at http://www.alsa.org/community/.
Despite treatment, however, ALS is a progressive disease. The prognosis is poor, with a median survival of 2 to 5 years after diagnosis.9
The El Escorial World Federation of Neurology criteria for the diagnosis of ALS address how to treat the most common symptoms of ALS that occur as the disease progresses. These symptoms include dyspnea, muscle spasms, spasticity, sialorrhea, and pseudobulbar affect (TABLE 21,5).

Our patient was started on baclofen 10 mg 3 times per day (titrated up as needed) for muscle spasms and cramps, which resulted in some improvement of his cramps, but no improvement in the spasms. He was also started on sertraline 50 mg for anxiety and depression. His overall weakness continued to progress, and we recommended that the patient get ankle-foot orthosis braces to help with the mobility impairment caused by foot drop.
We then referred him to an ALS specialty clinic recommended by the neuromuscular specialist. The patient is now enrolled in a clinical trial designed to test a cerebrospinal fluid marker for diagnosis and for a new drug aimed at symptom management.
THE TAKEAWAY
Muscle cramps and pain are early signs of ALS. Although ALS is uncommon, patients who present with muscle cramps and muscle pain should have a creatine kinase test ordered (which, if elevated, should prompt further investigation into ALS as the possible cause). Patients should also undergo a neurologic examination to seek evidence of upper and lower motor neuron disease. They should then be reevaluated in 4 weeks to see if symptoms are improving or progressing. If no improvement is seen and symptoms are progressive, a work-up for ALS should be considered.
The mainstay of treatment for patients with ALS is multidisciplinary symptom management and palliative care. Utilization of an ALS specialty clinic should also be recommended, as it can improve survival.5
THE CASE
A 39-year-old man who worked in construction presented to our clinic with complaints of muscle cramps and muscle pain that had been bothering him for several months. The cramps and pain started in both of his arms and subsequently became diffuse and generalized. He also reported an unintentional 15-pound weight loss.
His exam at that time was unremarkable. He was diagnosed with dehydration and cramping due to overexertion at work. A basic metabolic panel, hemogram, lipid panel, and thyroid stimulating hormone level were ordered. The patient’s triglyceride level, which was 227 mg/dL, was the only significant result (normal level: <150 mg/dL).
The patient’s symptoms continued to worsen until he returned to the clinic 6 months later, again complaining of muscle cramps and pain throughout his body. At that second visit, he also reported profound overall weakness and the development of diffuse muscle twitching, which his wife had observed while he was sleeping. As a result of these worrisome symptoms, he had become anxious and depressed.
A review of his medical record revealed a weight loss of about 20 pounds over the previous year. On exam, he had diffuse fasciculations in all the major muscle groups, including his tongue. The patient’s strength was 4/5 in all muscle groups. His deep tendon reflexes were 3+. He had a negative Babinski reflex (ie, he had downward facing toes with plantar stimulation), and cranial nerves II to XII were all intact. His rapid alternating movements and gait were slow.
THE DIAGNOSIS
Based on the exam, the primary diagnostic consideration for the patient was amyotrophic lateral sclerosis (ALS). Lab tests were ordered and revealed normal calcium and electrolyte levels, a normal erythrocyte sedimentation rate, a normal C-reactive protein level, and a negative test for acetylcholine receptor antibodies. However, the patient had an elevated creatine kinase level of 664 U/L (normal: 30-200 U/L). The patient was sent to a neuromuscular specialist, who identified signs of upper and lower motor neuron disease in all 4 of the patient’s extremities (he had foot drop that had not been present previously) and a very brisk jaw jerk. Along with the tongue fasciculations, the results of the specialist’s physical exam suggested ALS. Four-limb electromyography (EMG) showed widespread fasciculations and some large motor unit potentials and recruitment abnormalities, which were also consistent with ALS. It appeared that the patient’s weight loss was due to both muscle atrophy and the amount of calories burned from his constant twitching.
Extensive testing was done to rule out other potential causes of the patient’s symptoms, including magnetic resonance imaging (MRI) of the spine and brain (which was normal). In addition, the patient’s aldolase level and antineutrophil cytoplasmic antibodies were normal. The patient tested negative for human immunodeficiency virus and antibodies to double-stranded DNA. After serial neurologic exams, the final diagnosis of ALS was made.
DISCUSSION
ALS, also known as Lou Gehrig’s disease, is a degenerative motor neuron disease.1-3 The incidence in North America is 1.5 to 2.7 per 100,000 per year, and the prevalence is 2.7 to 7.4 per 100,000.4 The incidence of ALS increases with each decade of life, especially after age 40, and peaks at 74 years of age.4 The male to female ratio is 1:1.5-2.4 ALS affects upper and lower motor neurons and is progressive; however, the rate of progression and phenotype vary greatly between individuals.2 Most patients with ALS die within 2 to 5 years of onset.5
There is no specific test for ALS; the diagnosis is made clinically based on the revised El Escorial World Federation of Neurology criteria, also known as the Airlie House criteria.2,6,7 These criteria include evidence of lower motor neuron degeneration by clinical, electrophysiologic, or neuropathologic exam; evidence of upper motor neuron disease by clinical exam; progressive spread of symptoms or signs within a region or to other regions (by history or exam); and the absence of electrophysiologic, neuroimaging, or pathologic evidence of other disease processes that could explain the symptoms. If patients have evidence of upper and lower motor neuron disease, they should be reevaluated in 4 weeks to see if symptoms are improving or progressing.
Like our patient, many patients will have an elevated creatine kinase level (some with levels as high as 1000 U/L), and calcium may also be elevated because, rarely, ALS is associated with primary hyperparathyroidism.8 Electrophysiologic studies can be helpful in identifying active denervation of lower motor neurons.4,6,7
The differential diagnosis for ALS includes myasthenia gravis, inclusion-body myositis, multifocal motor neuropathy, benign fasciculations, hereditary spastic paraplegia, primary lateral sclerosis, post-polio progressive muscle atrophy, cervical spondylosis, and multiple sclerosis. A negative acetylcholine receptor antibody test will rule out myasthenia gravis, imaging of the spine can rule out cervical spondylosis, and electrophysiologic testing helps eliminate the other conditions (TABLE 14).

Treatment in specialty clinics can prolong survival
The mainstays of treatment are symptom management, multidisciplinary care (by physicians, physical/occupational/speech therapists, nutritionists, psychologists, psychotherapists, and genetic counselors), palliative care, and counseling about end-of-life issues for patients and family.1,5 Utilization of an ALS specialty clinic can provide access to all of these services and should be considered, as there is evidence that treatment in such clinics can prolong survival.5 The location of ALS specialty clinics can be found on the ALS Association’s Web site at http://www.alsa.org/community/.
Despite treatment, however, ALS is a progressive disease. The prognosis is poor, with a median survival of 2 to 5 years after diagnosis.9
The El Escorial World Federation of Neurology criteria for the diagnosis of ALS address how to treat the most common symptoms of ALS that occur as the disease progresses. These symptoms include dyspnea, muscle spasms, spasticity, sialorrhea, and pseudobulbar affect (TABLE 21,5).

Our patient was started on baclofen 10 mg 3 times per day (titrated up as needed) for muscle spasms and cramps, which resulted in some improvement of his cramps, but no improvement in the spasms. He was also started on sertraline 50 mg for anxiety and depression. His overall weakness continued to progress, and we recommended that the patient get ankle-foot orthosis braces to help with the mobility impairment caused by foot drop.
We then referred him to an ALS specialty clinic recommended by the neuromuscular specialist. The patient is now enrolled in a clinical trial designed to test a cerebrospinal fluid marker for diagnosis and for a new drug aimed at symptom management.
THE TAKEAWAY
Muscle cramps and pain are early signs of ALS. Although ALS is uncommon, patients who present with muscle cramps and muscle pain should have a creatine kinase test ordered (which, if elevated, should prompt further investigation into ALS as the possible cause). Patients should also undergo a neurologic examination to seek evidence of upper and lower motor neuron disease. They should then be reevaluated in 4 weeks to see if symptoms are improving or progressing. If no improvement is seen and symptoms are progressive, a work-up for ALS should be considered.
The mainstay of treatment for patients with ALS is multidisciplinary symptom management and palliative care. Utilization of an ALS specialty clinic should also be recommended, as it can improve survival.5
1. Miller RG, Gelinas D, O’Connor P. Amyotrophic Lateral Sclerosis: American Academy of Neurology Press Quality of Life Guide Series. Demos Medical Publishing; 2004.
2. Simon NG, Turner MR, Vucic S, et al. Quantifying disease progression in amyotrophic lateral sclerosis. Ann Neurol. 2014;76:643-657.
3. Worms PM. The epidemiology of motor neuron diseases: a review of recent studies. J Neurol Sci. 2001;191:3-9.
4. Shaw PJ. ALS and other motor neuron diseases. In: Goldman L, Schafer AI, eds. Goldman’s Cecil Medicine. 24th ed. Philadelphia, PA: Elsevier Saunders; 2015:chap 418.
5. Miller RG, Jackson CE, Kasarskis EJ, et al. Practice Parameter update: The Care of the Patient with Amyotrophic Lateral Sclerosis: Multidisciplinary care, symptom management, and cognitive/behavioral impairment (an evidence-based review). Report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology. 2009;73:1227-1233.
6. Brooks BR. El Escorial World Federation of Neurology criteria for the diagnosis of amyotrophic lateral sclerosis. Subcommittee on Motor Neuron Diseases/Amyotrophic Lateral Sclerosis of the World Federation of Neurology Research Group on Neuromuscular Diseases and the El Escorial “Clinical limits of amyotrophic lateral sclerosis” workshop contributors. J Neurol Sci. 1994;124:96-107.
7. Brooks BR, Miller RG, Swash M, et al; World Federation of Neurology Research Group on Motor Neuron Diseases. El Escorial revisited: revised criteria for the diagnosis of amyotrophic lateral sclerosis. Amyotroph Lateral Scler Other Motor Neuron Disord. 2000;1:293-299.
8. Jackson CE, Amato AA, Bryan WW, et al. Primary hyperparathyroidism and ALS: is there a relation? Neurology. 1998;50:1795-1799.
9. Jablecki CK, Berry C, Leach J. Survival prediction in amyotrophic lateral sclerosis. Muscle Nerve. 1989;12:833-841.
1. Miller RG, Gelinas D, O’Connor P. Amyotrophic Lateral Sclerosis: American Academy of Neurology Press Quality of Life Guide Series. Demos Medical Publishing; 2004.
2. Simon NG, Turner MR, Vucic S, et al. Quantifying disease progression in amyotrophic lateral sclerosis. Ann Neurol. 2014;76:643-657.
3. Worms PM. The epidemiology of motor neuron diseases: a review of recent studies. J Neurol Sci. 2001;191:3-9.
4. Shaw PJ. ALS and other motor neuron diseases. In: Goldman L, Schafer AI, eds. Goldman’s Cecil Medicine. 24th ed. Philadelphia, PA: Elsevier Saunders; 2015:chap 418.
5. Miller RG, Jackson CE, Kasarskis EJ, et al. Practice Parameter update: The Care of the Patient with Amyotrophic Lateral Sclerosis: Multidisciplinary care, symptom management, and cognitive/behavioral impairment (an evidence-based review). Report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology. 2009;73:1227-1233.
6. Brooks BR. El Escorial World Federation of Neurology criteria for the diagnosis of amyotrophic lateral sclerosis. Subcommittee on Motor Neuron Diseases/Amyotrophic Lateral Sclerosis of the World Federation of Neurology Research Group on Neuromuscular Diseases and the El Escorial “Clinical limits of amyotrophic lateral sclerosis” workshop contributors. J Neurol Sci. 1994;124:96-107.
7. Brooks BR, Miller RG, Swash M, et al; World Federation of Neurology Research Group on Motor Neuron Diseases. El Escorial revisited: revised criteria for the diagnosis of amyotrophic lateral sclerosis. Amyotroph Lateral Scler Other Motor Neuron Disord. 2000;1:293-299.
8. Jackson CE, Amato AA, Bryan WW, et al. Primary hyperparathyroidism and ALS: is there a relation? Neurology. 1998;50:1795-1799.
9. Jablecki CK, Berry C, Leach J. Survival prediction in amyotrophic lateral sclerosis. Muscle Nerve. 1989;12:833-841.
Torsades de Pointes in Severe Alcohol Withdrawal and Cirrhosis: Implications for Risk Stratification and Management
Torsades de pointes (TdP) is a life-threatening ventricular arrhythmia that is associated with both congenital and acquired QT interval prolongation. QT interval prolongation is commonly observed in acute alcohol withdrawal and cirrhotic cardiomyopathy.1-3 In both conditions, there is a positive correlation between the degree of QT interval prolongation and disease severity.4,5 The precise mechanisms of QT interval prolongation in these conditions are not well understood. One hypothesis is that autonomic hyperexcitability results in altered ventricular repolarization and QT interval prolongation. This mechanism of QT prolongation has been found in acute alcohol withdrawal independent of electrolyte abnormalities, use of QT-prolonging medications, and cirrhosis.1,2,6
The authors report the case of a veteran who was hospitalized for acute alcohol withdrawal and decompensated cirrhosis and was found to have a newly prolonged QT interval. On hospital day 3, the patient developed TdP, which required external defibrillation. Despite correction of electrolyte abnormalities, abstinence from alcohol, avoidance of QT-prolonging medications, and exclusion of cardiac ischemia, there was significant and persistent prolongation of the QT interval—ultimately attributed to cirrhotic cardiomyopathy. Acquired QT interval prolongation is common in both acute alcohol withdrawal and cirrhosis.This case highlights the importance of close monitoring of the QT interval and TdP susceptibility in patients being treated for acute alcohol withdrawal, particularly those with cirrhosis.
Case Report
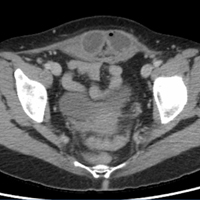
A 66-year-old male veteran with a 35-year history of alcohol dependence presented for alcohol detoxification. He reported having drunk at least 32 ounces of vodka every day of the preceding 5 years and reported having unsuccessfully attempted self-detoxification several times. Prior detoxification efforts were unsuccessful because of intractable nausea and tremulousness. Additional presenting symptoms included lethargy, anorexia, and a fall with transient right-side hemiparesis (findings on magnetic resonance imaging of the head had been normal).
His medical history included type 2 diabetes, tobacco dependence, and macular degeneration. The only medication being taken was glargine 25 units daily. On admission, the patient was afebrile (98.1°F), normotensive (103/77 mm Hg), and oriented to person, place, and time. Examination also revealed a protuberant abdomen with caput medusae, and no shifting dullness or lower extremity edema. The neurologic examination was nonfocal.
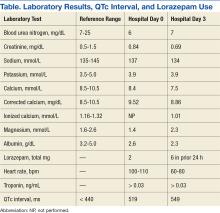
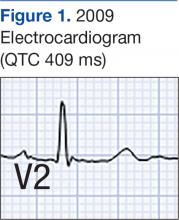
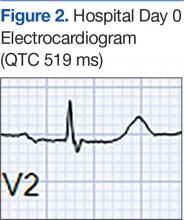
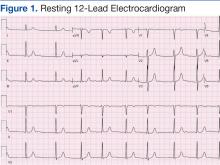
Laboratory test results on admission were significant for elevated serum alcohol level (243.8 mg/dL); elevated levels of aspartate aminotransferase (144 units/L) alanine aminotransferase (25 units/L), and total bilirubin (4.2 mg/L); hypoalbuminemia; normocalemia; hypomagnesemia; normal corrected calcium level; and normal renal function (0.84 mg/dL)(Table). The patient’s admission Child-Pugh score of 10 indicated class C liver disease. Admission electrocardiogram (EKG) revealed normal sinus rhythm, first-degree atrioventricular block, and prolongation of the QTc interval (519 ms). Six years earlier, the patient’s QTc interval had been 409 ms (Figures 1 and 2). As QT interval depends on heart rate, it is most commonly expressed as corrected QT, or QTc, where QTc = QT/(√RR).
Symptom-triggered therapy for alcohol withdrawal was instituted, and the patient’s electrolyte abnormalities were corrected. Telemetry monitoring demonstrated polymorphic ventricular ectopy, including a 6.8-s run of polymorphic ventricular tachycardia and several shorter runs (4-10 beats) of nonsustained ventricular tachycardia, prompting initiation of a low-dose beta blocker. Based on elevated scores on the symptom-triggered scale for alcohol withdrawal, the Clinical Institute Withdrawal Assessement for Alcohol Withdrawal (CIWA), several doses of oral lorazepam were given for withdrawal symptoms.
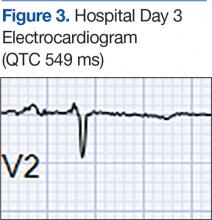
The patient became increasingly confused, and new-onset nystagmus was noted. These findings raised concern for Wernicke encephalopathy, so the patient was empirically started on IV high-dose thiamine supplementation. The CIWA scores remained high, and there were frequent episodes of ventricular ectopy during the first 2 hospital days. Interval EKG revealed further prolongation of the QTc interval (549 ms) without evidence of cardiac ischemia (Figure 3). Cardiac enzymes were negative, and electrolyte levels were within normal limits.
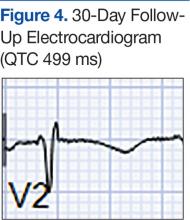
The month-long hospitalization was notable for development of significant ascites, continual electrolyte repletion in the setting of diuresis, formal diagnosis of alcoholic cirrhoisis, cognitive and physical rehabilitation. During the hospitalization, QTc interval remained prolonged (range, 460-500 ms), despite electrolyte repletion, and he was discharged with a wearable cardioverter defibrillator. A month
Discussion
Alcohol dependence is a common chronic and relapsing disease that often requires controlled detoxification. Investigators have found a high incidence of QT interval prolongation in alcohol withdrawal and hepatic disease.3,6 Common causes of QT interval prolongation in this setting are poor nutrition, electrolyte abnormalities (particularly hypocalcemia and hypomagnesemia), and use of certain medications.1-3,7,8 In addition, alcohol is directly toxic to the renal tubules, resulting in renal wasting of divalent cations, which may persist up to 30 days after the most recent alcohol exposure.9,10
The patient in this case report was initially thought to have hypomagnesemia-induced long QT syndrome (leading to TdP cardiac arrest), but the authors’ review of laboratory test results revealed the QT interval remained markedly prolonged, despite adequate correction of hypomagnesemia implicating the hyperadrenergic state of acute alcohol withdrawal in QT interval prolongation and TdP cardiac arrest. Interestingly, the QT interval remained prolonged 2 months after TdP arrest, despite sustained normalization of electrolyte levels and the absence of active ischemia or use of QT-prolonging medications.
Given the exclusion of other causes of QT interval prolongation, the authors hypothesized that autonomic hyperactivity of acute alcohol withdrawal and resultant QT interval prolongation were potentiated by underlying cirrhotic cardiomyopathy, a well described cause of QT interval prolongation. Cirrhotic cardiomyopathy is thought to cause QT interval prolongation by delayed repolarization of cardiomyocytes and promotion of sympatho-adrenergic hyperactivity.6 In other case series, TdP development has been associated with severe withdrawal symptoms, particularly delirium tremens.2 In cirrhosis, QT interval prolongation often is described as an early manifestation of cirrhotic cardiomyopathy, irrespective of the underlying etiology, and precedes systolic and diastolic dysfunction.6
The magnitude of QT prolongation has been associated with severity of liver disease as expressed by Child-Pugh score, with reports of QT normalization after liver transplantation.4,5 Patients with higher Child-Pugh scores should be considered to be at elevated risk for malignant ventricular arrhythmias. The authors recommend checking an EKG on admission of any patient who has liver disease or has presented for alcohol withdrawal. Patients with a prolonged QTc interval should be monitored on telemetry. The authors also recommend aggressive repletion of electrolytes, particularly potassium and magnesium, in patients who present with cirrhosis and alcohol withdrawal.
Avoidance of QT-prolonging medications is advisable for all patients with a long QT interval. Beta blockers shorten the QT interval in cirrhotic patients, but the role of beta blockers in preventing malignant arrhythmias in this group of patients is not yet clear.11 The present patient’s QT interval had been normal before he developed cirrhotic liver disease. His presentation was suggestive of acquired long QT syndrome, likely caused by cirrhotic cardiomyopathy given the exhaustive exclusion of other causes of QT interval prolongation.
Conclusion
This case highlights the importance of close monitoring of the QT interval in patients being treated for acute alcohol withdrawal, particularly those with cirrhosis, and suggests that timely and aggressive management of withdrawal, repletion of electrolytes, and telemetry monitoring may prevent life-threatening arrhythmia.
1. Otero-Antón E, González-Quintela A, Saborido J, Torre JA, Virgós A, Barrio E. Prolongation of the QTc interval during alcohol withdrawal syndrome. Acta Cardiol. 1997;52(3):285-294.
2. Cuculi F, Kobza R, Ehmann T, Erne P. ECG changes amongst patients with alcohol withdrawal seizures and delirium tremens. Swiss Med Wkly. 2006;136(13-14):223-227.
3. Mimidis K, Thomopoulos K, Tziakas D, et al. Prolongation of the QTc interval in patients with cirrhosis. Ann Gastroenterol. 2003;16(2):155-158.
4. Bernardi M, Calandra S, Colantoni A, et al. Q-T interval prolongation in cirrhosis: prevalence, relationship with severity, and etiology of the disease and possible pathogenetic factors. Hepatology. 1998;27(1):28-34.
5. Bal JS, Thuluvath PJ. Prolongation of QTc interval: relationship with etiology and severity of liver disease, mortality and liver transplantation. Liver Int. 2003;23(4):243-248.
6. Zardi EM, Abbate A, Zardi DM, et al. Cirrhotic cardiomyopathy. J Am Coll Cardiol. 2010;56(7):539-549.
7. Faigel DO, Metz DC, Kochman ML. Torsade de pointes complicating the treatment of bleeding esophageal varices: association with neuroleptics, vasopressin, and electrolyte imbalance. Am J Gastroenterol. 1995;90(5):822-824.
8. Kotsia AP, Dimitriadis G, Baltogiannis GG, Kolettis TM. Torsade de pointes and persistent QTc prolongation after intravenous amiodarone. Case Rep Med. 2012;2012:673019.
9. Denison H, Jern S, Jagenburg R, Wendestam C, Wallerstedt S. Influence of increased adrenergic activity and magnesium depletion on cardiac rhythm in alcohol withdrawal. Br Heart J. 1994;72(6):554-560.
10. Plaza de los Reyes M, Orozco R, Rosemblitt M, Rendic Y, Espinace M. Renal secretion of magnesium and other electrolytes under the influence of acute ingestion of alcohol, in normal subjects [in Spanish]. Rev Med Chil. 1968;96(3):138-141.
11. Bernardi M, Maggioli C, Dibra V, Zaccherini G. QT interval prolongation in liver cirrhosis: innocent bystander or serious threat? Expert Rev Gastroenterol Hepatol. 2012;6(1):57-66.
Torsades de pointes (TdP) is a life-threatening ventricular arrhythmia that is associated with both congenital and acquired QT interval prolongation. QT interval prolongation is commonly observed in acute alcohol withdrawal and cirrhotic cardiomyopathy.1-3 In both conditions, there is a positive correlation between the degree of QT interval prolongation and disease severity.4,5 The precise mechanisms of QT interval prolongation in these conditions are not well understood. One hypothesis is that autonomic hyperexcitability results in altered ventricular repolarization and QT interval prolongation. This mechanism of QT prolongation has been found in acute alcohol withdrawal independent of electrolyte abnormalities, use of QT-prolonging medications, and cirrhosis.1,2,6
The authors report the case of a veteran who was hospitalized for acute alcohol withdrawal and decompensated cirrhosis and was found to have a newly prolonged QT interval. On hospital day 3, the patient developed TdP, which required external defibrillation. Despite correction of electrolyte abnormalities, abstinence from alcohol, avoidance of QT-prolonging medications, and exclusion of cardiac ischemia, there was significant and persistent prolongation of the QT interval—ultimately attributed to cirrhotic cardiomyopathy. Acquired QT interval prolongation is common in both acute alcohol withdrawal and cirrhosis.This case highlights the importance of close monitoring of the QT interval and TdP susceptibility in patients being treated for acute alcohol withdrawal, particularly those with cirrhosis.
Case Report
A 66-year-old male veteran with a 35-year history of alcohol dependence presented for alcohol detoxification. He reported having drunk at least 32 ounces of vodka every day of the preceding 5 years and reported having unsuccessfully attempted self-detoxification several times. Prior detoxification efforts were unsuccessful because of intractable nausea and tremulousness. Additional presenting symptoms included lethargy, anorexia, and a fall with transient right-side hemiparesis (findings on magnetic resonance imaging of the head had been normal).
His medical history included type 2 diabetes, tobacco dependence, and macular degeneration. The only medication being taken was glargine 25 units daily. On admission, the patient was afebrile (98.1°F), normotensive (103/77 mm Hg), and oriented to person, place, and time. Examination also revealed a protuberant abdomen with caput medusae, and no shifting dullness or lower extremity edema. The neurologic examination was nonfocal.
Laboratory test results on admission were significant for elevated serum alcohol level (243.8 mg/dL); elevated levels of aspartate aminotransferase (144 units/L) alanine aminotransferase (25 units/L), and total bilirubin (4.2 mg/L); hypoalbuminemia; normocalemia; hypomagnesemia; normal corrected calcium level; and normal renal function (0.84 mg/dL)(Table). The patient’s admission Child-Pugh score of 10 indicated class C liver disease. Admission electrocardiogram (EKG) revealed normal sinus rhythm, first-degree atrioventricular block, and prolongation of the QTc interval (519 ms). Six years earlier, the patient’s QTc interval had been 409 ms (Figures 1 and 2). As QT interval depends on heart rate, it is most commonly expressed as corrected QT, or QTc, where QTc = QT/(√RR).
Symptom-triggered therapy for alcohol withdrawal was instituted, and the patient’s electrolyte abnormalities were corrected. Telemetry monitoring demonstrated polymorphic ventricular ectopy, including a 6.8-s run of polymorphic ventricular tachycardia and several shorter runs (4-10 beats) of nonsustained ventricular tachycardia, prompting initiation of a low-dose beta blocker. Based on elevated scores on the symptom-triggered scale for alcohol withdrawal, the Clinical Institute Withdrawal Assessement for Alcohol Withdrawal (CIWA), several doses of oral lorazepam were given for withdrawal symptoms.
The patient became increasingly confused, and new-onset nystagmus was noted. These findings raised concern for Wernicke encephalopathy, so the patient was empirically started on IV high-dose thiamine supplementation. The CIWA scores remained high, and there were frequent episodes of ventricular ectopy during the first 2 hospital days. Interval EKG revealed further prolongation of the QTc interval (549 ms) without evidence of cardiac ischemia (Figure 3). Cardiac enzymes were negative, and electrolyte levels were within normal limits.
The month-long hospitalization was notable for development of significant ascites, continual electrolyte repletion in the setting of diuresis, formal diagnosis of alcoholic cirrhoisis, cognitive and physical rehabilitation. During the hospitalization, QTc interval remained prolonged (range, 460-500 ms), despite electrolyte repletion, and he was discharged with a wearable cardioverter defibrillator. A month
Discussion
Alcohol dependence is a common chronic and relapsing disease that often requires controlled detoxification. Investigators have found a high incidence of QT interval prolongation in alcohol withdrawal and hepatic disease.3,6 Common causes of QT interval prolongation in this setting are poor nutrition, electrolyte abnormalities (particularly hypocalcemia and hypomagnesemia), and use of certain medications.1-3,7,8 In addition, alcohol is directly toxic to the renal tubules, resulting in renal wasting of divalent cations, which may persist up to 30 days after the most recent alcohol exposure.9,10
The patient in this case report was initially thought to have hypomagnesemia-induced long QT syndrome (leading to TdP cardiac arrest), but the authors’ review of laboratory test results revealed the QT interval remained markedly prolonged, despite adequate correction of hypomagnesemia implicating the hyperadrenergic state of acute alcohol withdrawal in QT interval prolongation and TdP cardiac arrest. Interestingly, the QT interval remained prolonged 2 months after TdP arrest, despite sustained normalization of electrolyte levels and the absence of active ischemia or use of QT-prolonging medications.
Given the exclusion of other causes of QT interval prolongation, the authors hypothesized that autonomic hyperactivity of acute alcohol withdrawal and resultant QT interval prolongation were potentiated by underlying cirrhotic cardiomyopathy, a well described cause of QT interval prolongation. Cirrhotic cardiomyopathy is thought to cause QT interval prolongation by delayed repolarization of cardiomyocytes and promotion of sympatho-adrenergic hyperactivity.6 In other case series, TdP development has been associated with severe withdrawal symptoms, particularly delirium tremens.2 In cirrhosis, QT interval prolongation often is described as an early manifestation of cirrhotic cardiomyopathy, irrespective of the underlying etiology, and precedes systolic and diastolic dysfunction.6
The magnitude of QT prolongation has been associated with severity of liver disease as expressed by Child-Pugh score, with reports of QT normalization after liver transplantation.4,5 Patients with higher Child-Pugh scores should be considered to be at elevated risk for malignant ventricular arrhythmias. The authors recommend checking an EKG on admission of any patient who has liver disease or has presented for alcohol withdrawal. Patients with a prolonged QTc interval should be monitored on telemetry. The authors also recommend aggressive repletion of electrolytes, particularly potassium and magnesium, in patients who present with cirrhosis and alcohol withdrawal.
Avoidance of QT-prolonging medications is advisable for all patients with a long QT interval. Beta blockers shorten the QT interval in cirrhotic patients, but the role of beta blockers in preventing malignant arrhythmias in this group of patients is not yet clear.11 The present patient’s QT interval had been normal before he developed cirrhotic liver disease. His presentation was suggestive of acquired long QT syndrome, likely caused by cirrhotic cardiomyopathy given the exhaustive exclusion of other causes of QT interval prolongation.
Conclusion
This case highlights the importance of close monitoring of the QT interval in patients being treated for acute alcohol withdrawal, particularly those with cirrhosis, and suggests that timely and aggressive management of withdrawal, repletion of electrolytes, and telemetry monitoring may prevent life-threatening arrhythmia.
Torsades de pointes (TdP) is a life-threatening ventricular arrhythmia that is associated with both congenital and acquired QT interval prolongation. QT interval prolongation is commonly observed in acute alcohol withdrawal and cirrhotic cardiomyopathy.1-3 In both conditions, there is a positive correlation between the degree of QT interval prolongation and disease severity.4,5 The precise mechanisms of QT interval prolongation in these conditions are not well understood. One hypothesis is that autonomic hyperexcitability results in altered ventricular repolarization and QT interval prolongation. This mechanism of QT prolongation has been found in acute alcohol withdrawal independent of electrolyte abnormalities, use of QT-prolonging medications, and cirrhosis.1,2,6
The authors report the case of a veteran who was hospitalized for acute alcohol withdrawal and decompensated cirrhosis and was found to have a newly prolonged QT interval. On hospital day 3, the patient developed TdP, which required external defibrillation. Despite correction of electrolyte abnormalities, abstinence from alcohol, avoidance of QT-prolonging medications, and exclusion of cardiac ischemia, there was significant and persistent prolongation of the QT interval—ultimately attributed to cirrhotic cardiomyopathy. Acquired QT interval prolongation is common in both acute alcohol withdrawal and cirrhosis.This case highlights the importance of close monitoring of the QT interval and TdP susceptibility in patients being treated for acute alcohol withdrawal, particularly those with cirrhosis.
Case Report
A 66-year-old male veteran with a 35-year history of alcohol dependence presented for alcohol detoxification. He reported having drunk at least 32 ounces of vodka every day of the preceding 5 years and reported having unsuccessfully attempted self-detoxification several times. Prior detoxification efforts were unsuccessful because of intractable nausea and tremulousness. Additional presenting symptoms included lethargy, anorexia, and a fall with transient right-side hemiparesis (findings on magnetic resonance imaging of the head had been normal).
His medical history included type 2 diabetes, tobacco dependence, and macular degeneration. The only medication being taken was glargine 25 units daily. On admission, the patient was afebrile (98.1°F), normotensive (103/77 mm Hg), and oriented to person, place, and time. Examination also revealed a protuberant abdomen with caput medusae, and no shifting dullness or lower extremity edema. The neurologic examination was nonfocal.
Laboratory test results on admission were significant for elevated serum alcohol level (243.8 mg/dL); elevated levels of aspartate aminotransferase (144 units/L) alanine aminotransferase (25 units/L), and total bilirubin (4.2 mg/L); hypoalbuminemia; normocalemia; hypomagnesemia; normal corrected calcium level; and normal renal function (0.84 mg/dL)(Table). The patient’s admission Child-Pugh score of 10 indicated class C liver disease. Admission electrocardiogram (EKG) revealed normal sinus rhythm, first-degree atrioventricular block, and prolongation of the QTc interval (519 ms). Six years earlier, the patient’s QTc interval had been 409 ms (Figures 1 and 2). As QT interval depends on heart rate, it is most commonly expressed as corrected QT, or QTc, where QTc = QT/(√RR).
Symptom-triggered therapy for alcohol withdrawal was instituted, and the patient’s electrolyte abnormalities were corrected. Telemetry monitoring demonstrated polymorphic ventricular ectopy, including a 6.8-s run of polymorphic ventricular tachycardia and several shorter runs (4-10 beats) of nonsustained ventricular tachycardia, prompting initiation of a low-dose beta blocker. Based on elevated scores on the symptom-triggered scale for alcohol withdrawal, the Clinical Institute Withdrawal Assessement for Alcohol Withdrawal (CIWA), several doses of oral lorazepam were given for withdrawal symptoms.
The patient became increasingly confused, and new-onset nystagmus was noted. These findings raised concern for Wernicke encephalopathy, so the patient was empirically started on IV high-dose thiamine supplementation. The CIWA scores remained high, and there were frequent episodes of ventricular ectopy during the first 2 hospital days. Interval EKG revealed further prolongation of the QTc interval (549 ms) without evidence of cardiac ischemia (Figure 3). Cardiac enzymes were negative, and electrolyte levels were within normal limits.
The month-long hospitalization was notable for development of significant ascites, continual electrolyte repletion in the setting of diuresis, formal diagnosis of alcoholic cirrhoisis, cognitive and physical rehabilitation. During the hospitalization, QTc interval remained prolonged (range, 460-500 ms), despite electrolyte repletion, and he was discharged with a wearable cardioverter defibrillator. A month
Discussion
Alcohol dependence is a common chronic and relapsing disease that often requires controlled detoxification. Investigators have found a high incidence of QT interval prolongation in alcohol withdrawal and hepatic disease.3,6 Common causes of QT interval prolongation in this setting are poor nutrition, electrolyte abnormalities (particularly hypocalcemia and hypomagnesemia), and use of certain medications.1-3,7,8 In addition, alcohol is directly toxic to the renal tubules, resulting in renal wasting of divalent cations, which may persist up to 30 days after the most recent alcohol exposure.9,10
The patient in this case report was initially thought to have hypomagnesemia-induced long QT syndrome (leading to TdP cardiac arrest), but the authors’ review of laboratory test results revealed the QT interval remained markedly prolonged, despite adequate correction of hypomagnesemia implicating the hyperadrenergic state of acute alcohol withdrawal in QT interval prolongation and TdP cardiac arrest. Interestingly, the QT interval remained prolonged 2 months after TdP arrest, despite sustained normalization of electrolyte levels and the absence of active ischemia or use of QT-prolonging medications.
Given the exclusion of other causes of QT interval prolongation, the authors hypothesized that autonomic hyperactivity of acute alcohol withdrawal and resultant QT interval prolongation were potentiated by underlying cirrhotic cardiomyopathy, a well described cause of QT interval prolongation. Cirrhotic cardiomyopathy is thought to cause QT interval prolongation by delayed repolarization of cardiomyocytes and promotion of sympatho-adrenergic hyperactivity.6 In other case series, TdP development has been associated with severe withdrawal symptoms, particularly delirium tremens.2 In cirrhosis, QT interval prolongation often is described as an early manifestation of cirrhotic cardiomyopathy, irrespective of the underlying etiology, and precedes systolic and diastolic dysfunction.6
The magnitude of QT prolongation has been associated with severity of liver disease as expressed by Child-Pugh score, with reports of QT normalization after liver transplantation.4,5 Patients with higher Child-Pugh scores should be considered to be at elevated risk for malignant ventricular arrhythmias. The authors recommend checking an EKG on admission of any patient who has liver disease or has presented for alcohol withdrawal. Patients with a prolonged QTc interval should be monitored on telemetry. The authors also recommend aggressive repletion of electrolytes, particularly potassium and magnesium, in patients who present with cirrhosis and alcohol withdrawal.
Avoidance of QT-prolonging medications is advisable for all patients with a long QT interval. Beta blockers shorten the QT interval in cirrhotic patients, but the role of beta blockers in preventing malignant arrhythmias in this group of patients is not yet clear.11 The present patient’s QT interval had been normal before he developed cirrhotic liver disease. His presentation was suggestive of acquired long QT syndrome, likely caused by cirrhotic cardiomyopathy given the exhaustive exclusion of other causes of QT interval prolongation.
Conclusion
This case highlights the importance of close monitoring of the QT interval in patients being treated for acute alcohol withdrawal, particularly those with cirrhosis, and suggests that timely and aggressive management of withdrawal, repletion of electrolytes, and telemetry monitoring may prevent life-threatening arrhythmia.
1. Otero-Antón E, González-Quintela A, Saborido J, Torre JA, Virgós A, Barrio E. Prolongation of the QTc interval during alcohol withdrawal syndrome. Acta Cardiol. 1997;52(3):285-294.
2. Cuculi F, Kobza R, Ehmann T, Erne P. ECG changes amongst patients with alcohol withdrawal seizures and delirium tremens. Swiss Med Wkly. 2006;136(13-14):223-227.
3. Mimidis K, Thomopoulos K, Tziakas D, et al. Prolongation of the QTc interval in patients with cirrhosis. Ann Gastroenterol. 2003;16(2):155-158.
4. Bernardi M, Calandra S, Colantoni A, et al. Q-T interval prolongation in cirrhosis: prevalence, relationship with severity, and etiology of the disease and possible pathogenetic factors. Hepatology. 1998;27(1):28-34.
5. Bal JS, Thuluvath PJ. Prolongation of QTc interval: relationship with etiology and severity of liver disease, mortality and liver transplantation. Liver Int. 2003;23(4):243-248.
6. Zardi EM, Abbate A, Zardi DM, et al. Cirrhotic cardiomyopathy. J Am Coll Cardiol. 2010;56(7):539-549.
7. Faigel DO, Metz DC, Kochman ML. Torsade de pointes complicating the treatment of bleeding esophageal varices: association with neuroleptics, vasopressin, and electrolyte imbalance. Am J Gastroenterol. 1995;90(5):822-824.
8. Kotsia AP, Dimitriadis G, Baltogiannis GG, Kolettis TM. Torsade de pointes and persistent QTc prolongation after intravenous amiodarone. Case Rep Med. 2012;2012:673019.
9. Denison H, Jern S, Jagenburg R, Wendestam C, Wallerstedt S. Influence of increased adrenergic activity and magnesium depletion on cardiac rhythm in alcohol withdrawal. Br Heart J. 1994;72(6):554-560.
10. Plaza de los Reyes M, Orozco R, Rosemblitt M, Rendic Y, Espinace M. Renal secretion of magnesium and other electrolytes under the influence of acute ingestion of alcohol, in normal subjects [in Spanish]. Rev Med Chil. 1968;96(3):138-141.
11. Bernardi M, Maggioli C, Dibra V, Zaccherini G. QT interval prolongation in liver cirrhosis: innocent bystander or serious threat? Expert Rev Gastroenterol Hepatol. 2012;6(1):57-66.
1. Otero-Antón E, González-Quintela A, Saborido J, Torre JA, Virgós A, Barrio E. Prolongation of the QTc interval during alcohol withdrawal syndrome. Acta Cardiol. 1997;52(3):285-294.
2. Cuculi F, Kobza R, Ehmann T, Erne P. ECG changes amongst patients with alcohol withdrawal seizures and delirium tremens. Swiss Med Wkly. 2006;136(13-14):223-227.
3. Mimidis K, Thomopoulos K, Tziakas D, et al. Prolongation of the QTc interval in patients with cirrhosis. Ann Gastroenterol. 2003;16(2):155-158.
4. Bernardi M, Calandra S, Colantoni A, et al. Q-T interval prolongation in cirrhosis: prevalence, relationship with severity, and etiology of the disease and possible pathogenetic factors. Hepatology. 1998;27(1):28-34.
5. Bal JS, Thuluvath PJ. Prolongation of QTc interval: relationship with etiology and severity of liver disease, mortality and liver transplantation. Liver Int. 2003;23(4):243-248.
6. Zardi EM, Abbate A, Zardi DM, et al. Cirrhotic cardiomyopathy. J Am Coll Cardiol. 2010;56(7):539-549.
7. Faigel DO, Metz DC, Kochman ML. Torsade de pointes complicating the treatment of bleeding esophageal varices: association with neuroleptics, vasopressin, and electrolyte imbalance. Am J Gastroenterol. 1995;90(5):822-824.
8. Kotsia AP, Dimitriadis G, Baltogiannis GG, Kolettis TM. Torsade de pointes and persistent QTc prolongation after intravenous amiodarone. Case Rep Med. 2012;2012:673019.
9. Denison H, Jern S, Jagenburg R, Wendestam C, Wallerstedt S. Influence of increased adrenergic activity and magnesium depletion on cardiac rhythm in alcohol withdrawal. Br Heart J. 1994;72(6):554-560.
10. Plaza de los Reyes M, Orozco R, Rosemblitt M, Rendic Y, Espinace M. Renal secretion of magnesium and other electrolytes under the influence of acute ingestion of alcohol, in normal subjects [in Spanish]. Rev Med Chil. 1968;96(3):138-141.
11. Bernardi M, Maggioli C, Dibra V, Zaccherini G. QT interval prolongation in liver cirrhosis: innocent bystander or serious threat? Expert Rev Gastroenterol Hepatol. 2012;6(1):57-66.
Questioning the Specificity and Sensitivity of ELISA for Bullous Pemphigoid Diagnosis
Bullous pemphigoid (BP) is the most common autoimmune blistering disease. The classic presentation of BP is a generalized, pruritic, bullous eruption in elderly patients, which is occasionally preceded by an urticarial prodrome. Immunopathologically, BP is characterized by IgG and sometimes IgE autoantibodies that target basement membrane zone proteins BP180 and BP230 of the epidermis.1
The diagnosis of BP should be suspected when an elderly patient presents with tense blisters and can be confirmed via diagnostic testing, including tissue histology and direct immunofluorescence (DIF) as the gold standard, as well as indirect immunofluorescence (IIF), enzyme-linked immunosorbent assay (ELISA), and most recently biochip technology as supportive tests.2 Since its advent, ELISA has gained popularity as a trustworthy diagnostic test for BP. The specificity of ELISA for BP diagnosis is reported to be 98% to 100%, which leads clinicians to believe that a positive ELISA equals certain diagnosis of BP; however, misdiagnosis of BP based on a positive ELISA result can occur.3-13 The treatment of BP often involves lifelong immunosuppressive therapy. Complications of immunosuppressive therapy contribute to morbidity and mortality in these patients, thus an accurate diagnosis is paramount before introducing therapy.14
We present the case of a 74-year-old man with a history of a pruritic nonbullous eruption who was diagnosed with BP and treated for 3 years based on positive ELISA results in the absence of confirmatory histology or DIF.
Case Report
A 74-year-old man with diabetes mellitus, hypertension, hyperlipidemia, benign prostatic hypertrophy, and obstructive sleep apnea presented for further evaluation and confirmation of a prior diagnosis of BP by an outside dermatologist. He reported a pruritic rash on the trunk, back, and extremities of 3 years’ duration. He denied occurrence of blisters at any time.
On presentation to an outside dermatologist 3 years ago, a biopsy was performed along with serologic studies due to the patient’s age and the possibility of an urticarial prodrome in BP. The biopsy revealed epidermal acanthosis, subepidermal separation, and a perivascular and interstitial infiltrate of lymphocytes and eosinophils in the papillary dermis. Direct immunofluorescence was nondiagnostic with a weak discontinuous pattern of IgG and IgA linearly along the basement membrane zone as well as few scattered and clumped cytoid bodies of IgM and IgA. Indirect immunofluoresence revealed a positive IgG titer of 1:40 on monkey esophagus substrate and a positive epidermal pattern on human split-skin substrate with a titer of 1:80. An ELISA for IgG autoantibodies against BP180 and BP230 yielded 15 U and 6 U, respectively (cut off value, 9 U). Based on the positive ELISA for IgG against BP180, a diagnosis of BP was made.
Over the following 3 years, the treatment included prednisone, tetracycline, nicotinamide, doxycycline, and dapsone. Therapy was suboptimal due to the patient’s comorbidities and socioeconomic status. Poorly controlled diabetes mellitus precluded consistent use of prednisone as recommended for BP. Tetracycline and nicotinamide were transiently effective in controlling the patient’s symptoms but were discontinued due to changes in his health insurance. Doxycycline and dapsone were ineffective. Throughout this 3-year period, the patient remained blister free, but the pruritic eruption was persistent.
The patient presented to our clinic due to his frustration with the lack of improvement and doubts about the BP diagnosis given the persistent absence of bullous lesions. Physical examination revealed numerous eroded, scaly, crusted papules on erythematous edematous plaques on all extremities, trunk, and back (Figure 1). The head, neck, face, and oral mucosa were spared. His history and clinical findings were atypical for BP and skin biopsies were performed. Histology revealed epidermal erosion with parakeratosis, spongiosis, and superficial perivascular lymphocytic inflammation with rare eosinophils without subepidermal split (Figure 2). Direct immunofluorescence was negative for IgG, IgA, IgM, C3, and C1q. Additionally, further review of the initial histology by another dermatopathologist revealed that the subepidermal separation reported was more likely artifactual clefts. These findings were not consistent with BP.

Given the patient’s clinical history, lack of bullae, and twice-negative DIF, the diagnosis was determined to be more consistent with eczematous spongiotic dermatitis. He refused a referral for phototherapy due to scheduling inconvenience. The patient was started on cyclosporine 0.5 mg/kg twice daily. After 10 days of treatment, he returned for follow-up and reported notable improvement in the pruritus. On physical examination, his dermatitis was improved with decreased erythema and inflammation.
The patient is being continued on extensive dry skin care with thick moisturizers and additional topical corticosteroid application on an as-needed basis.
Comment
Chronic immunosuppression contributes to morbidity and mortality in patients with BP; therefore, accurate diagnosis of BP is of utmost importance.14 A meta-analysis described ELISA as a test with high sensitivity and specificity (87% and 98%–100%, respectively) for diagnosis of BP.3 Nevertheless, there are opportunities for misdiagnosis using ELISA, as demonstrated in our case. To determine if the reported sensitivity and specificity of ELISA is accurate and reliable for clinical use, individual studies from the meta-analysis were reviewed.4,5,7-10,13,15 Issues identified in our review included dissimilar diagnostic procedures and patient populations among individual studies, several reports of positive ELISA in patients without BP, and a lack of explanation for these false-positive results.
There are notable differences in diagnostic procedures and patient populations among reports that establish the sensitivity and specificity of ELISA for BP diagnosis.3-13 Studies have detected IgG that targets the NC16A domain of the BP180 kD antigen, the C-terminal of the BP180 kD antigen, or the entire ectodomain of the BP180 kD antigen. Study patient populations varied in disease activity, stage, and treatment. Control patients included healthy patients as well as those with many dermatoses, including pemphigus vulgaris, systemic scleroderma, systemic lupus erythematosus, rheumatoid arthritis, lichen planus, and discoid lupus erythematosus.3-13 Due to these differences between individual studies, we believe the results that determine the overall sensitivity and specificity of ELISA for BP diagnosis must be interpreted with caution. For ELISA statistics to be clinically applicable to a specific patient, he/she should be similar to the patients studied. Therefore, we believe each study must be evaluated individually for applicability, given the differences that exist between them.
Furthermore, there have been several reports of false-positive ELISA results in patients with other dermatologic disorders, specifically in elderly patients with pruritus who do not fulfill clinical criteria for diagnosis with BP.16-18 In a population of elderly patients with pruritus for which no specific dermatological or systemic cause was identified, Hofmann et al18 found that 12% (3/25) of patients showed IgG reactivity to BP180 despite having negative DIF results. In another study of elderly patients with pruritic dermatoses, Feliciani et al17 found that 33% (5/15) of patients had IgG reactivity against BP230 or BP180, though they did not fulfill BP criteria based on clinical presentation and showed negative DIF and IIF results. These findings suggest that IgG reactivity against BP autoantibodies as determined by ELISA is not uncommon in pruritic diseases of the elderly.
Explanations for false-positive ELISA results were rare. A few authors suggested that false-positives could be attributed to an excessively low cutoff value,7-9 which was consistent with reports that the titer of autoantibodies to BP180 correlates with disease severity, suggesting that the higher titer of antibodies correlates with more severe disease and likely more accurate diagnosis.10,19,20 It is important to consider that patients who have low titers of BP180 autoantibodies with inconsistent clinical characteristics and DIF results may not truly have BP. Furthermore, to determine the clinical value of ELISA in identifying patients in the initial phase of BP, sera of BP patients should be compared with sera of elderly patients with pruritic skin disorders because they comprise the patient population that often requires diagnosis.18
Given the issues identified in our review of the literature, the published sensitivity and specificity of ELISA for BP diagnosis are likely overstated. In conclusion, ELISA should not be relied on as a single criterion adequate for diagnosis of BP.12,21 Rather, the diagnosis of BP can be obtained with a positive predictive value of 95% when a patient meets 3 of 4 clinical criteria (ie, absence of atrophic scars, absence of head and neck involvement, absence of mucosal involvement, and older than 70 years) and demonstrates linear deposits of predominantly IgG and/or C3 along the basement membrane zone of a perilesional biopsy on DIF.15 The gold standard for diagnosis of BP remains clinical presentation along with DIF, which can be supported by histology, IIF, and ELISA.22
- Delaporte E, Dubost-Brama A, Ghohestani R, et al. IgE autoantibodies directed against the major bullous pemphigoid antigen in patients with a severe form of pemphigoid. J Immunol. 1996;157:3642-3647.
- Schmidt E, Zillikens D. Diagnosis and clinical severity markers of bullous pemphigoid. F1000 Med Rep. 2009;1:15.
- Tampoia M, Giavarina D, Di Giorgio C, et al. Diagnostic accuracy of enzyme-linked immunosorbent assays (ELISA) to detect anti-skin autoantibodies in autoimmune blistering diseases: a systematic review and meta-analysis. Autoimmun Rev. 2012;12:121-126.
- Zillikens D, Mascaro JM, Rose PA, et al. A highly sensitive enzyme-linked immunosorbent assay for the detection of circulating anti-BP180 autoantibodies in patients with bullous pemphigoid. J Invest Dermatol. 1997;109:679-683.
- Sitaru C, Dahnrich C, Probst C, et al. Enzyme-linked immunosorbent assay using multimers of the 16th non-collagenous domain of the BP180 antigen for sensitive and specific detection of pemphigoid autoantibodies. Exp Dermatol. 2007;16:770-777.
- Yang B, Wang C, Chen S, et al. Evaluation of the combination of BP180-NC16a enzyme-linked immunosorbent assay and BP230 enzyme-linked immunosorbent assay in the diagnosis of bullous pemphigoid. Indian J Dermatol Venereol Leprol. 2012;78:722-727.
- Sakuma-Oyama Y, Powell AM, Oyama N, et al. Evaluation of a BP180-NC16a enzyme-linked immunosorbent assay in the initial diagnosis of bullous pemphigoid. Br J Dermatol. 2004;151:126-131.
- Tampoia M, Lattanzi V, Zucano A, et al. Evaluation of a new ELISA assay for detection of BP230 autoantibodies in bullous pemphigoid. Ann N Y Acad Sci. 2009;1173:15-20.
- Feng S, Lin L, Jin P, et al. Role of BP180NC16a-enzyme-linked immunosorbent assay (ELISA) in the diagnosis of bullous pemphigoid in China. Int J Dermatol. 2008;47:24-28.
- Kobayashi M, Amagai M, Kuroda-Kinoshita K, et al. BP180 ELISA using bacterial recombinant NC16a protein as a diagnostic and monitoring tool for bullous pemphigoid. J Dermatol Sci. 2002;30:224-232.
- Roussel A, Benichou J, Arivelo Randriamanantany Z, et al. Enzyme-linked immunosorbent assay for the combination of bullous pemphigoid antigens 1 and 2 in the diagnosis of bullous pemphigoid. Arch Dermatol. 2011;147:293-298.
- Chan, Lawrence S. ELISA instead of indirect IF in patients with BP. Arch Dermatol. 2011;147:291-292.
- Barnadas MA, Rubiales V, González J, et al. Enzyme-linked immunosorbent assay (ELISA) and indirect immunofluorescence testing in a bullous pemphigoid and pemphigoid gestationis. Int J Dermatol. 2008;47:1245-1249.
- Borradori L, Bernard P. Pemphigoid group. In: Bolognia JL, Jorizzo JL, Rapini RP, eds. Dermatology. New York, NY: Mosby; 2003:469.
- Vaillant L, Bernard P, Joly P, et al. Evaluation of clinical criteria for diagnosis of bullous pemphigoid. Arch Dermatol. 1998;134:1075-1080.
- Fania L, Caldarola G, Muller R, et al. IgE recognition of bullous pemphigoid (BP)180 and BP230 in BP patients and elderly individuals with pruritic dermatoses. Clin Immunol. 2012;143:236-245.
- Feliciani C, Caldarola G, Kneisel A, et al. IgG autoantibody reactivity against bullous pemphigoid (BP) 180 and BP230 in elderly patients with pruritic dermatoses. Br J Dermatol. 2009;61:306-312.
- Hofmann SC, Tamm K, Hertl M, et al. Diagnostic value of an enzyme-linked immunosorbent assay using BP180 recombinant proteins in elderly patients with pruritic skin disorders. Br J Dermatol. 2003;149:910-911.
- Schmidt E, Obe K, Brocker EB, et al. Serum levels of autoantibodies to BP180 correlate with disease activity in patients with bullous pemphigoid. Arch Dermatol. 2000;136:174-178.
- Feng S, Wu Q, Jin P, et al. Serum levels of autoantibodies to BP180 correlate with disease activity in patients with bullous pemphigoid. Int J Dermatol. 2008;47:225-228.
- Di Zenzo G, Joly P, Zambruno G, et al. Sensitivity of immunofluorescence studies vs enzyme-linked immunosorbent assay for diagnosis of bullous pemphigoid. Arch Dermatol. 2011;147:1454-1456.
- Schmidt E, Zillikens D. Modern diagnosis of autoimmune blistering skin diseases. Autoimmun Rev. 2010;10:84-89.
Bullous pemphigoid (BP) is the most common autoimmune blistering disease. The classic presentation of BP is a generalized, pruritic, bullous eruption in elderly patients, which is occasionally preceded by an urticarial prodrome. Immunopathologically, BP is characterized by IgG and sometimes IgE autoantibodies that target basement membrane zone proteins BP180 and BP230 of the epidermis.1
The diagnosis of BP should be suspected when an elderly patient presents with tense blisters and can be confirmed via diagnostic testing, including tissue histology and direct immunofluorescence (DIF) as the gold standard, as well as indirect immunofluorescence (IIF), enzyme-linked immunosorbent assay (ELISA), and most recently biochip technology as supportive tests.2 Since its advent, ELISA has gained popularity as a trustworthy diagnostic test for BP. The specificity of ELISA for BP diagnosis is reported to be 98% to 100%, which leads clinicians to believe that a positive ELISA equals certain diagnosis of BP; however, misdiagnosis of BP based on a positive ELISA result can occur.3-13 The treatment of BP often involves lifelong immunosuppressive therapy. Complications of immunosuppressive therapy contribute to morbidity and mortality in these patients, thus an accurate diagnosis is paramount before introducing therapy.14
We present the case of a 74-year-old man with a history of a pruritic nonbullous eruption who was diagnosed with BP and treated for 3 years based on positive ELISA results in the absence of confirmatory histology or DIF.
Case Report
A 74-year-old man with diabetes mellitus, hypertension, hyperlipidemia, benign prostatic hypertrophy, and obstructive sleep apnea presented for further evaluation and confirmation of a prior diagnosis of BP by an outside dermatologist. He reported a pruritic rash on the trunk, back, and extremities of 3 years’ duration. He denied occurrence of blisters at any time.
On presentation to an outside dermatologist 3 years ago, a biopsy was performed along with serologic studies due to the patient’s age and the possibility of an urticarial prodrome in BP. The biopsy revealed epidermal acanthosis, subepidermal separation, and a perivascular and interstitial infiltrate of lymphocytes and eosinophils in the papillary dermis. Direct immunofluorescence was nondiagnostic with a weak discontinuous pattern of IgG and IgA linearly along the basement membrane zone as well as few scattered and clumped cytoid bodies of IgM and IgA. Indirect immunofluoresence revealed a positive IgG titer of 1:40 on monkey esophagus substrate and a positive epidermal pattern on human split-skin substrate with a titer of 1:80. An ELISA for IgG autoantibodies against BP180 and BP230 yielded 15 U and 6 U, respectively (cut off value, 9 U). Based on the positive ELISA for IgG against BP180, a diagnosis of BP was made.
Over the following 3 years, the treatment included prednisone, tetracycline, nicotinamide, doxycycline, and dapsone. Therapy was suboptimal due to the patient’s comorbidities and socioeconomic status. Poorly controlled diabetes mellitus precluded consistent use of prednisone as recommended for BP. Tetracycline and nicotinamide were transiently effective in controlling the patient’s symptoms but were discontinued due to changes in his health insurance. Doxycycline and dapsone were ineffective. Throughout this 3-year period, the patient remained blister free, but the pruritic eruption was persistent.
The patient presented to our clinic due to his frustration with the lack of improvement and doubts about the BP diagnosis given the persistent absence of bullous lesions. Physical examination revealed numerous eroded, scaly, crusted papules on erythematous edematous plaques on all extremities, trunk, and back (Figure 1). The head, neck, face, and oral mucosa were spared. His history and clinical findings were atypical for BP and skin biopsies were performed. Histology revealed epidermal erosion with parakeratosis, spongiosis, and superficial perivascular lymphocytic inflammation with rare eosinophils without subepidermal split (Figure 2). Direct immunofluorescence was negative for IgG, IgA, IgM, C3, and C1q. Additionally, further review of the initial histology by another dermatopathologist revealed that the subepidermal separation reported was more likely artifactual clefts. These findings were not consistent with BP.


Given the patient’s clinical history, lack of bullae, and twice-negative DIF, the diagnosis was determined to be more consistent with eczematous spongiotic dermatitis. He refused a referral for phototherapy due to scheduling inconvenience. The patient was started on cyclosporine 0.5 mg/kg twice daily. After 10 days of treatment, he returned for follow-up and reported notable improvement in the pruritus. On physical examination, his dermatitis was improved with decreased erythema and inflammation.
The patient is being continued on extensive dry skin care with thick moisturizers and additional topical corticosteroid application on an as-needed basis.
Comment
Chronic immunosuppression contributes to morbidity and mortality in patients with BP; therefore, accurate diagnosis of BP is of utmost importance.14 A meta-analysis described ELISA as a test with high sensitivity and specificity (87% and 98%–100%, respectively) for diagnosis of BP.3 Nevertheless, there are opportunities for misdiagnosis using ELISA, as demonstrated in our case. To determine if the reported sensitivity and specificity of ELISA is accurate and reliable for clinical use, individual studies from the meta-analysis were reviewed.4,5,7-10,13,15 Issues identified in our review included dissimilar diagnostic procedures and patient populations among individual studies, several reports of positive ELISA in patients without BP, and a lack of explanation for these false-positive results.
There are notable differences in diagnostic procedures and patient populations among reports that establish the sensitivity and specificity of ELISA for BP diagnosis.3-13 Studies have detected IgG that targets the NC16A domain of the BP180 kD antigen, the C-terminal of the BP180 kD antigen, or the entire ectodomain of the BP180 kD antigen. Study patient populations varied in disease activity, stage, and treatment. Control patients included healthy patients as well as those with many dermatoses, including pemphigus vulgaris, systemic scleroderma, systemic lupus erythematosus, rheumatoid arthritis, lichen planus, and discoid lupus erythematosus.3-13 Due to these differences between individual studies, we believe the results that determine the overall sensitivity and specificity of ELISA for BP diagnosis must be interpreted with caution. For ELISA statistics to be clinically applicable to a specific patient, he/she should be similar to the patients studied. Therefore, we believe each study must be evaluated individually for applicability, given the differences that exist between them.
Furthermore, there have been several reports of false-positive ELISA results in patients with other dermatologic disorders, specifically in elderly patients with pruritus who do not fulfill clinical criteria for diagnosis with BP.16-18 In a population of elderly patients with pruritus for which no specific dermatological or systemic cause was identified, Hofmann et al18 found that 12% (3/25) of patients showed IgG reactivity to BP180 despite having negative DIF results. In another study of elderly patients with pruritic dermatoses, Feliciani et al17 found that 33% (5/15) of patients had IgG reactivity against BP230 or BP180, though they did not fulfill BP criteria based on clinical presentation and showed negative DIF and IIF results. These findings suggest that IgG reactivity against BP autoantibodies as determined by ELISA is not uncommon in pruritic diseases of the elderly.
Explanations for false-positive ELISA results were rare. A few authors suggested that false-positives could be attributed to an excessively low cutoff value,7-9 which was consistent with reports that the titer of autoantibodies to BP180 correlates with disease severity, suggesting that the higher titer of antibodies correlates with more severe disease and likely more accurate diagnosis.10,19,20 It is important to consider that patients who have low titers of BP180 autoantibodies with inconsistent clinical characteristics and DIF results may not truly have BP. Furthermore, to determine the clinical value of ELISA in identifying patients in the initial phase of BP, sera of BP patients should be compared with sera of elderly patients with pruritic skin disorders because they comprise the patient population that often requires diagnosis.18
Given the issues identified in our review of the literature, the published sensitivity and specificity of ELISA for BP diagnosis are likely overstated. In conclusion, ELISA should not be relied on as a single criterion adequate for diagnosis of BP.12,21 Rather, the diagnosis of BP can be obtained with a positive predictive value of 95% when a patient meets 3 of 4 clinical criteria (ie, absence of atrophic scars, absence of head and neck involvement, absence of mucosal involvement, and older than 70 years) and demonstrates linear deposits of predominantly IgG and/or C3 along the basement membrane zone of a perilesional biopsy on DIF.15 The gold standard for diagnosis of BP remains clinical presentation along with DIF, which can be supported by histology, IIF, and ELISA.22
Bullous pemphigoid (BP) is the most common autoimmune blistering disease. The classic presentation of BP is a generalized, pruritic, bullous eruption in elderly patients, which is occasionally preceded by an urticarial prodrome. Immunopathologically, BP is characterized by IgG and sometimes IgE autoantibodies that target basement membrane zone proteins BP180 and BP230 of the epidermis.1
The diagnosis of BP should be suspected when an elderly patient presents with tense blisters and can be confirmed via diagnostic testing, including tissue histology and direct immunofluorescence (DIF) as the gold standard, as well as indirect immunofluorescence (IIF), enzyme-linked immunosorbent assay (ELISA), and most recently biochip technology as supportive tests.2 Since its advent, ELISA has gained popularity as a trustworthy diagnostic test for BP. The specificity of ELISA for BP diagnosis is reported to be 98% to 100%, which leads clinicians to believe that a positive ELISA equals certain diagnosis of BP; however, misdiagnosis of BP based on a positive ELISA result can occur.3-13 The treatment of BP often involves lifelong immunosuppressive therapy. Complications of immunosuppressive therapy contribute to morbidity and mortality in these patients, thus an accurate diagnosis is paramount before introducing therapy.14
We present the case of a 74-year-old man with a history of a pruritic nonbullous eruption who was diagnosed with BP and treated for 3 years based on positive ELISA results in the absence of confirmatory histology or DIF.
Case Report
A 74-year-old man with diabetes mellitus, hypertension, hyperlipidemia, benign prostatic hypertrophy, and obstructive sleep apnea presented for further evaluation and confirmation of a prior diagnosis of BP by an outside dermatologist. He reported a pruritic rash on the trunk, back, and extremities of 3 years’ duration. He denied occurrence of blisters at any time.
On presentation to an outside dermatologist 3 years ago, a biopsy was performed along with serologic studies due to the patient’s age and the possibility of an urticarial prodrome in BP. The biopsy revealed epidermal acanthosis, subepidermal separation, and a perivascular and interstitial infiltrate of lymphocytes and eosinophils in the papillary dermis. Direct immunofluorescence was nondiagnostic with a weak discontinuous pattern of IgG and IgA linearly along the basement membrane zone as well as few scattered and clumped cytoid bodies of IgM and IgA. Indirect immunofluoresence revealed a positive IgG titer of 1:40 on monkey esophagus substrate and a positive epidermal pattern on human split-skin substrate with a titer of 1:80. An ELISA for IgG autoantibodies against BP180 and BP230 yielded 15 U and 6 U, respectively (cut off value, 9 U). Based on the positive ELISA for IgG against BP180, a diagnosis of BP was made.
Over the following 3 years, the treatment included prednisone, tetracycline, nicotinamide, doxycycline, and dapsone. Therapy was suboptimal due to the patient’s comorbidities and socioeconomic status. Poorly controlled diabetes mellitus precluded consistent use of prednisone as recommended for BP. Tetracycline and nicotinamide were transiently effective in controlling the patient’s symptoms but were discontinued due to changes in his health insurance. Doxycycline and dapsone were ineffective. Throughout this 3-year period, the patient remained blister free, but the pruritic eruption was persistent.
The patient presented to our clinic due to his frustration with the lack of improvement and doubts about the BP diagnosis given the persistent absence of bullous lesions. Physical examination revealed numerous eroded, scaly, crusted papules on erythematous edematous plaques on all extremities, trunk, and back (Figure 1). The head, neck, face, and oral mucosa were spared. His history and clinical findings were atypical for BP and skin biopsies were performed. Histology revealed epidermal erosion with parakeratosis, spongiosis, and superficial perivascular lymphocytic inflammation with rare eosinophils without subepidermal split (Figure 2). Direct immunofluorescence was negative for IgG, IgA, IgM, C3, and C1q. Additionally, further review of the initial histology by another dermatopathologist revealed that the subepidermal separation reported was more likely artifactual clefts. These findings were not consistent with BP.


Given the patient’s clinical history, lack of bullae, and twice-negative DIF, the diagnosis was determined to be more consistent with eczematous spongiotic dermatitis. He refused a referral for phototherapy due to scheduling inconvenience. The patient was started on cyclosporine 0.5 mg/kg twice daily. After 10 days of treatment, he returned for follow-up and reported notable improvement in the pruritus. On physical examination, his dermatitis was improved with decreased erythema and inflammation.
The patient is being continued on extensive dry skin care with thick moisturizers and additional topical corticosteroid application on an as-needed basis.
Comment
Chronic immunosuppression contributes to morbidity and mortality in patients with BP; therefore, accurate diagnosis of BP is of utmost importance.14 A meta-analysis described ELISA as a test with high sensitivity and specificity (87% and 98%–100%, respectively) for diagnosis of BP.3 Nevertheless, there are opportunities for misdiagnosis using ELISA, as demonstrated in our case. To determine if the reported sensitivity and specificity of ELISA is accurate and reliable for clinical use, individual studies from the meta-analysis were reviewed.4,5,7-10,13,15 Issues identified in our review included dissimilar diagnostic procedures and patient populations among individual studies, several reports of positive ELISA in patients without BP, and a lack of explanation for these false-positive results.
There are notable differences in diagnostic procedures and patient populations among reports that establish the sensitivity and specificity of ELISA for BP diagnosis.3-13 Studies have detected IgG that targets the NC16A domain of the BP180 kD antigen, the C-terminal of the BP180 kD antigen, or the entire ectodomain of the BP180 kD antigen. Study patient populations varied in disease activity, stage, and treatment. Control patients included healthy patients as well as those with many dermatoses, including pemphigus vulgaris, systemic scleroderma, systemic lupus erythematosus, rheumatoid arthritis, lichen planus, and discoid lupus erythematosus.3-13 Due to these differences between individual studies, we believe the results that determine the overall sensitivity and specificity of ELISA for BP diagnosis must be interpreted with caution. For ELISA statistics to be clinically applicable to a specific patient, he/she should be similar to the patients studied. Therefore, we believe each study must be evaluated individually for applicability, given the differences that exist between them.
Furthermore, there have been several reports of false-positive ELISA results in patients with other dermatologic disorders, specifically in elderly patients with pruritus who do not fulfill clinical criteria for diagnosis with BP.16-18 In a population of elderly patients with pruritus for which no specific dermatological or systemic cause was identified, Hofmann et al18 found that 12% (3/25) of patients showed IgG reactivity to BP180 despite having negative DIF results. In another study of elderly patients with pruritic dermatoses, Feliciani et al17 found that 33% (5/15) of patients had IgG reactivity against BP230 or BP180, though they did not fulfill BP criteria based on clinical presentation and showed negative DIF and IIF results. These findings suggest that IgG reactivity against BP autoantibodies as determined by ELISA is not uncommon in pruritic diseases of the elderly.
Explanations for false-positive ELISA results were rare. A few authors suggested that false-positives could be attributed to an excessively low cutoff value,7-9 which was consistent with reports that the titer of autoantibodies to BP180 correlates with disease severity, suggesting that the higher titer of antibodies correlates with more severe disease and likely more accurate diagnosis.10,19,20 It is important to consider that patients who have low titers of BP180 autoantibodies with inconsistent clinical characteristics and DIF results may not truly have BP. Furthermore, to determine the clinical value of ELISA in identifying patients in the initial phase of BP, sera of BP patients should be compared with sera of elderly patients with pruritic skin disorders because they comprise the patient population that often requires diagnosis.18
Given the issues identified in our review of the literature, the published sensitivity and specificity of ELISA for BP diagnosis are likely overstated. In conclusion, ELISA should not be relied on as a single criterion adequate for diagnosis of BP.12,21 Rather, the diagnosis of BP can be obtained with a positive predictive value of 95% when a patient meets 3 of 4 clinical criteria (ie, absence of atrophic scars, absence of head and neck involvement, absence of mucosal involvement, and older than 70 years) and demonstrates linear deposits of predominantly IgG and/or C3 along the basement membrane zone of a perilesional biopsy on DIF.15 The gold standard for diagnosis of BP remains clinical presentation along with DIF, which can be supported by histology, IIF, and ELISA.22
- Delaporte E, Dubost-Brama A, Ghohestani R, et al. IgE autoantibodies directed against the major bullous pemphigoid antigen in patients with a severe form of pemphigoid. J Immunol. 1996;157:3642-3647.
- Schmidt E, Zillikens D. Diagnosis and clinical severity markers of bullous pemphigoid. F1000 Med Rep. 2009;1:15.
- Tampoia M, Giavarina D, Di Giorgio C, et al. Diagnostic accuracy of enzyme-linked immunosorbent assays (ELISA) to detect anti-skin autoantibodies in autoimmune blistering diseases: a systematic review and meta-analysis. Autoimmun Rev. 2012;12:121-126.
- Zillikens D, Mascaro JM, Rose PA, et al. A highly sensitive enzyme-linked immunosorbent assay for the detection of circulating anti-BP180 autoantibodies in patients with bullous pemphigoid. J Invest Dermatol. 1997;109:679-683.
- Sitaru C, Dahnrich C, Probst C, et al. Enzyme-linked immunosorbent assay using multimers of the 16th non-collagenous domain of the BP180 antigen for sensitive and specific detection of pemphigoid autoantibodies. Exp Dermatol. 2007;16:770-777.
- Yang B, Wang C, Chen S, et al. Evaluation of the combination of BP180-NC16a enzyme-linked immunosorbent assay and BP230 enzyme-linked immunosorbent assay in the diagnosis of bullous pemphigoid. Indian J Dermatol Venereol Leprol. 2012;78:722-727.
- Sakuma-Oyama Y, Powell AM, Oyama N, et al. Evaluation of a BP180-NC16a enzyme-linked immunosorbent assay in the initial diagnosis of bullous pemphigoid. Br J Dermatol. 2004;151:126-131.
- Tampoia M, Lattanzi V, Zucano A, et al. Evaluation of a new ELISA assay for detection of BP230 autoantibodies in bullous pemphigoid. Ann N Y Acad Sci. 2009;1173:15-20.
- Feng S, Lin L, Jin P, et al. Role of BP180NC16a-enzyme-linked immunosorbent assay (ELISA) in the diagnosis of bullous pemphigoid in China. Int J Dermatol. 2008;47:24-28.
- Kobayashi M, Amagai M, Kuroda-Kinoshita K, et al. BP180 ELISA using bacterial recombinant NC16a protein as a diagnostic and monitoring tool for bullous pemphigoid. J Dermatol Sci. 2002;30:224-232.
- Roussel A, Benichou J, Arivelo Randriamanantany Z, et al. Enzyme-linked immunosorbent assay for the combination of bullous pemphigoid antigens 1 and 2 in the diagnosis of bullous pemphigoid. Arch Dermatol. 2011;147:293-298.
- Chan, Lawrence S. ELISA instead of indirect IF in patients with BP. Arch Dermatol. 2011;147:291-292.
- Barnadas MA, Rubiales V, González J, et al. Enzyme-linked immunosorbent assay (ELISA) and indirect immunofluorescence testing in a bullous pemphigoid and pemphigoid gestationis. Int J Dermatol. 2008;47:1245-1249.
- Borradori L, Bernard P. Pemphigoid group. In: Bolognia JL, Jorizzo JL, Rapini RP, eds. Dermatology. New York, NY: Mosby; 2003:469.
- Vaillant L, Bernard P, Joly P, et al. Evaluation of clinical criteria for diagnosis of bullous pemphigoid. Arch Dermatol. 1998;134:1075-1080.
- Fania L, Caldarola G, Muller R, et al. IgE recognition of bullous pemphigoid (BP)180 and BP230 in BP patients and elderly individuals with pruritic dermatoses. Clin Immunol. 2012;143:236-245.
- Feliciani C, Caldarola G, Kneisel A, et al. IgG autoantibody reactivity against bullous pemphigoid (BP) 180 and BP230 in elderly patients with pruritic dermatoses. Br J Dermatol. 2009;61:306-312.
- Hofmann SC, Tamm K, Hertl M, et al. Diagnostic value of an enzyme-linked immunosorbent assay using BP180 recombinant proteins in elderly patients with pruritic skin disorders. Br J Dermatol. 2003;149:910-911.
- Schmidt E, Obe K, Brocker EB, et al. Serum levels of autoantibodies to BP180 correlate with disease activity in patients with bullous pemphigoid. Arch Dermatol. 2000;136:174-178.
- Feng S, Wu Q, Jin P, et al. Serum levels of autoantibodies to BP180 correlate with disease activity in patients with bullous pemphigoid. Int J Dermatol. 2008;47:225-228.
- Di Zenzo G, Joly P, Zambruno G, et al. Sensitivity of immunofluorescence studies vs enzyme-linked immunosorbent assay for diagnosis of bullous pemphigoid. Arch Dermatol. 2011;147:1454-1456.
- Schmidt E, Zillikens D. Modern diagnosis of autoimmune blistering skin diseases. Autoimmun Rev. 2010;10:84-89.
- Delaporte E, Dubost-Brama A, Ghohestani R, et al. IgE autoantibodies directed against the major bullous pemphigoid antigen in patients with a severe form of pemphigoid. J Immunol. 1996;157:3642-3647.
- Schmidt E, Zillikens D. Diagnosis and clinical severity markers of bullous pemphigoid. F1000 Med Rep. 2009;1:15.
- Tampoia M, Giavarina D, Di Giorgio C, et al. Diagnostic accuracy of enzyme-linked immunosorbent assays (ELISA) to detect anti-skin autoantibodies in autoimmune blistering diseases: a systematic review and meta-analysis. Autoimmun Rev. 2012;12:121-126.
- Zillikens D, Mascaro JM, Rose PA, et al. A highly sensitive enzyme-linked immunosorbent assay for the detection of circulating anti-BP180 autoantibodies in patients with bullous pemphigoid. J Invest Dermatol. 1997;109:679-683.
- Sitaru C, Dahnrich C, Probst C, et al. Enzyme-linked immunosorbent assay using multimers of the 16th non-collagenous domain of the BP180 antigen for sensitive and specific detection of pemphigoid autoantibodies. Exp Dermatol. 2007;16:770-777.
- Yang B, Wang C, Chen S, et al. Evaluation of the combination of BP180-NC16a enzyme-linked immunosorbent assay and BP230 enzyme-linked immunosorbent assay in the diagnosis of bullous pemphigoid. Indian J Dermatol Venereol Leprol. 2012;78:722-727.
- Sakuma-Oyama Y, Powell AM, Oyama N, et al. Evaluation of a BP180-NC16a enzyme-linked immunosorbent assay in the initial diagnosis of bullous pemphigoid. Br J Dermatol. 2004;151:126-131.
- Tampoia M, Lattanzi V, Zucano A, et al. Evaluation of a new ELISA assay for detection of BP230 autoantibodies in bullous pemphigoid. Ann N Y Acad Sci. 2009;1173:15-20.
- Feng S, Lin L, Jin P, et al. Role of BP180NC16a-enzyme-linked immunosorbent assay (ELISA) in the diagnosis of bullous pemphigoid in China. Int J Dermatol. 2008;47:24-28.
- Kobayashi M, Amagai M, Kuroda-Kinoshita K, et al. BP180 ELISA using bacterial recombinant NC16a protein as a diagnostic and monitoring tool for bullous pemphigoid. J Dermatol Sci. 2002;30:224-232.
- Roussel A, Benichou J, Arivelo Randriamanantany Z, et al. Enzyme-linked immunosorbent assay for the combination of bullous pemphigoid antigens 1 and 2 in the diagnosis of bullous pemphigoid. Arch Dermatol. 2011;147:293-298.
- Chan, Lawrence S. ELISA instead of indirect IF in patients with BP. Arch Dermatol. 2011;147:291-292.
- Barnadas MA, Rubiales V, González J, et al. Enzyme-linked immunosorbent assay (ELISA) and indirect immunofluorescence testing in a bullous pemphigoid and pemphigoid gestationis. Int J Dermatol. 2008;47:1245-1249.
- Borradori L, Bernard P. Pemphigoid group. In: Bolognia JL, Jorizzo JL, Rapini RP, eds. Dermatology. New York, NY: Mosby; 2003:469.
- Vaillant L, Bernard P, Joly P, et al. Evaluation of clinical criteria for diagnosis of bullous pemphigoid. Arch Dermatol. 1998;134:1075-1080.
- Fania L, Caldarola G, Muller R, et al. IgE recognition of bullous pemphigoid (BP)180 and BP230 in BP patients and elderly individuals with pruritic dermatoses. Clin Immunol. 2012;143:236-245.
- Feliciani C, Caldarola G, Kneisel A, et al. IgG autoantibody reactivity against bullous pemphigoid (BP) 180 and BP230 in elderly patients with pruritic dermatoses. Br J Dermatol. 2009;61:306-312.
- Hofmann SC, Tamm K, Hertl M, et al. Diagnostic value of an enzyme-linked immunosorbent assay using BP180 recombinant proteins in elderly patients with pruritic skin disorders. Br J Dermatol. 2003;149:910-911.
- Schmidt E, Obe K, Brocker EB, et al. Serum levels of autoantibodies to BP180 correlate with disease activity in patients with bullous pemphigoid. Arch Dermatol. 2000;136:174-178.
- Feng S, Wu Q, Jin P, et al. Serum levels of autoantibodies to BP180 correlate with disease activity in patients with bullous pemphigoid. Int J Dermatol. 2008;47:225-228.
- Di Zenzo G, Joly P, Zambruno G, et al. Sensitivity of immunofluorescence studies vs enzyme-linked immunosorbent assay for diagnosis of bullous pemphigoid. Arch Dermatol. 2011;147:1454-1456.
- Schmidt E, Zillikens D. Modern diagnosis of autoimmune blistering skin diseases. Autoimmun Rev. 2010;10:84-89.
Practice Points
- A low serum level of autoantibodies to BP180 should be interpreted with caution because it is more likely to represent a false-positive than a high serum level.
- Rely on the gold standard for diagnosis of bullous pemphigoid: clinical presentation along with direct immunofluorescence, which can be supported by histology, indirect immunofluorescence, and enzyme-linked immunosorbent assay (ELISA) rather than ELISA alone.
Plesiomonas shigelloides Periprosthetic Knee Infection After Consumption of Raw Oysters
Take-Home Points
- History and physical examination are key in identifying possible etiologies of orthopedic infections.
- If identified in the acute setting, periprosthetic infections can successfully be treated with irrigation, débridement, and polyethylene liner exchange.
- Discussion with an interdisciplinary medical team, including infectious disease specialists, can aide in improved diagnosis and treatment of periprosthetic infections.
Periprosthetic infection is a leading cause of morbidity after total joint arthroplasty.1 Despite advances in modern surgical practices, infection rates continue to range from 1% to 3% among all arthroplasty procedures performed in the United States.2-5 The most common causes of periprosthetic infection include Staphylococcus aureus, streptococcus, enterococcus, Escherichia coli, and Pseudomonas aeruginosa.6 However, many other pathogens that cause periprosthetic infection should be considered in the clinical setting. In this case report, periprosthetic knee infection with P shigelloides occurred after consumption of raw oysters.
P shigelloides is a gram-negative facultative anaerobic organism in the Vibrionaceae family,7 which also includes Vibrio vulnificus and Vibrio parahaemolyticus. P shigelloides is most well-known for causing diarrhea and septicemia in people who have consumed raw oysters or shellfish in the United States.8,9 Although P shigelloides infection is rare, there have been clinically significant outbreaks from contaminated water in Japan,10 consumption of freshwater fish in the Democratic Republic of the Congo,11 and consumption of raw oysters in the United States.8,9 Children and immunosuppressed people are most susceptible to the disease, which most commonly manifests as self-limiting watery diarrhea, with septicemia only in advanced cases.12There are very few reports of P shigelloides in the orthopedic population. In the medical literature, we found only 1 case of septic arthritis in a native knee; disease progression resulted in the patient’s death.13In this article, we report a case of P shigelloides septicemia that caused periprosthetic knee infection in a chemically and biologically immunosuppressed patient. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
Out of concern about a periprosthetic knee infection, a 66-year-old man was transferred from a regional medical center to our tertiary referral center. The patient reported a 3-day history of significant knee pain, swelling, and erythema that started the day after he consumed raw oysters at a seafood bar. He was unable to bear weight on the right knee and remained at home 1 day before presenting to the regional medical center.
The patient had undergone elective right total knee arthroplasty 18 months earlier, without previous issue (Figures A, B), and had a medical history of type 2 diabetes mellitus, psoriatic arthritis, hypertension, hyperlipidemia, hypothyroidism, and benign prostatic hypertrophy.
On presentation to our facility, the patient described pain in the right knee. Physical examination revealed swelling and erythema of the knee. Vital signs were within normal limits, with a temperature of 98.5°F. Laboratory work-up revealed white blood cell count of 17,700 with 79% neutrophils and 9% lymphocytes, serum C-reactive protein level of 270 mg/L, and erythrocyte sedimentation rate of 46 mm/h. Aspiration of the knee yielded about 100 mL of thick, brownish synovial fluid. Gram stain of the knee aspirate revealed gram-negative rods and many white blood cells. Nucleated cell count of the aspirate was 22,400 with 88% neutrophils. Blood cultures were obtained, and broad-spectrum antibiotics (vancomycin and ceftriaxone) were started in preparation for surgery.
Within 24 hours, the patient was taken for irrigation and débridement with polyethylene exchange of the right knee. Surgical exploration revealed brownish purulent fluid in the knee. The polyethylene insert was removed, and a complete synovectomy was performed for knee débridement. Nine liters of triple antibiotic (utilized bacitracin, polymyxin, and gentamicin) saline were used to copiously clean the metal surfaces of the implant, and a new polyethylene liner was inserted. Absorbable calcium sulfate antimicrobial beads, stimulant beads with 1 gram of vancomycin and 1.2 grams of tobramycin, were implanted both inside and over the knee capsule during closure.
Blood cultures, knee aspirate, and surgical cultures were all positive for P shigelloides. Of note, the patient did not describe having diarrhea, a symptom common in P shigelloides infection. After final cultures were received, the patient was placed on intravenous ceftriaxone and oral levofloxacin for 6 weeks. Three months later, he reported full return to activity and clearance of the infection.
Discussion
This case is a reminder that periprosthetic knee infection can occur from a variety of pathologic organisms and that obtaining a complete history is an important part of any diagnostic work-up. Although P shigelloides infection is rare, our patient had important historical findings that led to suspicion of Vibrionaceae infection: recent consumption of raw oysters, immunosuppression with etanercept and prednisone for psoriatic arthritis, and diabetes with hemoglobin A1c of 9.9% and presenting blood sugar of 338 mg/dL. His positive blood cultures represented P shigelloides septicemia, which seeded the knee prosthesis and led to acute periprosthetic infection. To our knowledge, this is the first report of P shigelloides periprosthetic infection in the orthopedic literature. The only other reported case of P shigelloides septicemia leading to septic arthritis in a native knee occurred in a 68-year-old Australian man who had end-stage liver disease and eventually died from complications of the P shigelloides infection.13
Although P shigelloides infection is rare, outbreaks have occurred around the world.7-11,14 Infections are most commonly associated with consumption of raw shellfish or freshwater fish or with water contamination.12 In the United States, the only described vector for disease has been consumption of raw oysters and shellfish—in particular, those harvested from the warm waters of the Gulf Coast.8,9P shigelloides usually causes a self-limiting watery diarrhea. However, in children and immunosuppressed patients, P shigelloides can lead to life-threatening septicemia.12 In the United States, P shigelloides cases often occur in the summer, likely related to the easy growth of the bacteria from shellfish in the Gulf Coast’s warm water and mud.8 This predilection for summer infections has been documented around the world.15Our patient reported eating raw oysters imported to the US Southwest from an unknown location. He likely was susceptible to P shigelloides infection, as he was immunosuppressed with etanercept and prednisone. However, there were no traditional diarrheal symptoms. Case reports have described nondiarrheal symptoms in children and other immunosuppressed people.12There is much to learn from this case report. Most important, it highlights the need to obtain a complete history and perform a thorough physical examination. Our patient’s 2 key historical findings, immunosuppressive medication use and raw oyster consumption, point strongly toward Vibrionaceae infection. Although a majority of periprosthetic infections are caused by common organisms, such as Staphylococcus and Streptococcus species, orthopedic clinicians should continue to expand their knowledge of periprosthetic infections, as many other pathogens can cause disease.
Am J Orthop. 2017;46(1):E32-E34. Copyright Frontline Medical Communications Inc. 2017. All rights reserved.
1. Parvizi J, Adeli B, Zmistowski B, Restrepo C, Greenwald AS. Management of periprosthetic joint infection: the current knowledge: AAOS exhibit selection. J Bone Joint Surg Am. 2012;94(14):e104.
2. Fehring TK, Odum S, Griffin WL, Mason JB, Nadaud M. Early failures in total knee arthroplasty. Clin Orthop. 2001;(392):315-318.
3. Kurtz SM, Lau E, Schmier J, Ong KL, Zhao K, Parvizi J. Infection burden for hip and knee arthroplasty in the United States. J Arthroplasty. 2008;23(7):984-991.
4. Clohisy JC, Calvert G, Tull F, McDonald D, Maloney WJ. Reasons for revision hip surgery: a retrospective review. Clin Orthop. 2004;(429):188-192.
5. Vessely MB, Whaley AL, Harmsen WS, Schleck CD, Berry DJ. The Chitranjan Ranawat Award: long-term survivorship and failure modes of 1000 cemented condylar total knee arthroplasties. Clin Orthop. 2006;(452):28-34.
6. Peel TN, Cheng AC, Buising KL, Choong PF. Microbiological aetiology, epidemiology, and clinical profile of prosthetic joint infections: are current antibiotic prophylaxis guidelines effective? Antimicrob Agents Chemother. 2012;56(5):2386-2391.
7. Wong TY, Tsui HY, So MK, et al. Plesiomonas shigelloides infection in Hong Kong: retrospective study of 167 laboratory-confirmed cases. Hong Kong Med J. 2000;6(4):375-380.
8. Holmberg SD, Wachsmuth IK, Hickman-Brenner FW, Blake PA, Farmer JJ 3rd. Plesiomonas enteric infections in the United States. Ann Intern Med. 1986;105(5):690-694.
9. Rutala WA, Sarubi FA Jr, Finch CS, McCormack JN, Steinkraus GE. Oyster-associated outbreak of diarrhoeal disease possibly caused by Plesiomonas shigelloides. Lancet. 1982;1(8274):739.
10. Tsukamoto T, Kinoshita Y, Shimada T, Sakazaki R. Two epidemics of diarrhoeal disease possibly caused by Plesiomonas shigelloides. J Hyg (Lond). 1978;80(2):275-280.
11. Van Damme LR, Vandepitte J. Frequent isolation of Edwardsiella tarda and Plesiomonas shigelloides from healthy Zairese freshwater fish: a possible source of sporadic diarrhea in the tropics. Appl Environ Microbiol. 1980;39(3):475-479.
12. Brenden RA, Miller MA, Janda JM. Clinical disease spectrum and pathogenic factors associated with Plesiomonas shigelloides infections in humans. Rev Infect Dis. 1988;10(2):303-316.
13. Gordon DL, Philpot CR, McGuire C. Plesiomonas shigelloides septic arthritis complicating rheumatoid arthritis. Aust N Z J Med. 1983;13(3):275-276.
14. Medema G, Schets C. Occurrence of Plesiomonas shigelloides in surface water: relationship with faecal pollution and trophic state. Zentralbl Hyg Umweltmed. 1993;194(4):398-404.
15. Huq MI, Islam MR. Microbiological & clinical studies in diarrhoea due to Plesiomonas shigelloides. Indian J Med Res. 1983;77:793-797.
Take-Home Points
- History and physical examination are key in identifying possible etiologies of orthopedic infections.
- If identified in the acute setting, periprosthetic infections can successfully be treated with irrigation, débridement, and polyethylene liner exchange.
- Discussion with an interdisciplinary medical team, including infectious disease specialists, can aide in improved diagnosis and treatment of periprosthetic infections.
Periprosthetic infection is a leading cause of morbidity after total joint arthroplasty.1 Despite advances in modern surgical practices, infection rates continue to range from 1% to 3% among all arthroplasty procedures performed in the United States.2-5 The most common causes of periprosthetic infection include Staphylococcus aureus, streptococcus, enterococcus, Escherichia coli, and Pseudomonas aeruginosa.6 However, many other pathogens that cause periprosthetic infection should be considered in the clinical setting. In this case report, periprosthetic knee infection with P shigelloides occurred after consumption of raw oysters.
P shigelloides is a gram-negative facultative anaerobic organism in the Vibrionaceae family,7 which also includes Vibrio vulnificus and Vibrio parahaemolyticus. P shigelloides is most well-known for causing diarrhea and septicemia in people who have consumed raw oysters or shellfish in the United States.8,9 Although P shigelloides infection is rare, there have been clinically significant outbreaks from contaminated water in Japan,10 consumption of freshwater fish in the Democratic Republic of the Congo,11 and consumption of raw oysters in the United States.8,9 Children and immunosuppressed people are most susceptible to the disease, which most commonly manifests as self-limiting watery diarrhea, with septicemia only in advanced cases.12There are very few reports of P shigelloides in the orthopedic population. In the medical literature, we found only 1 case of septic arthritis in a native knee; disease progression resulted in the patient’s death.13In this article, we report a case of P shigelloides septicemia that caused periprosthetic knee infection in a chemically and biologically immunosuppressed patient. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
Out of concern about a periprosthetic knee infection, a 66-year-old man was transferred from a regional medical center to our tertiary referral center. The patient reported a 3-day history of significant knee pain, swelling, and erythema that started the day after he consumed raw oysters at a seafood bar. He was unable to bear weight on the right knee and remained at home 1 day before presenting to the regional medical center.
The patient had undergone elective right total knee arthroplasty 18 months earlier, without previous issue (Figures A, B), and had a medical history of type 2 diabetes mellitus, psoriatic arthritis, hypertension, hyperlipidemia, hypothyroidism, and benign prostatic hypertrophy.
On presentation to our facility, the patient described pain in the right knee. Physical examination revealed swelling and erythema of the knee. Vital signs were within normal limits, with a temperature of 98.5°F. Laboratory work-up revealed white blood cell count of 17,700 with 79% neutrophils and 9% lymphocytes, serum C-reactive protein level of 270 mg/L, and erythrocyte sedimentation rate of 46 mm/h. Aspiration of the knee yielded about 100 mL of thick, brownish synovial fluid. Gram stain of the knee aspirate revealed gram-negative rods and many white blood cells. Nucleated cell count of the aspirate was 22,400 with 88% neutrophils. Blood cultures were obtained, and broad-spectrum antibiotics (vancomycin and ceftriaxone) were started in preparation for surgery.
Within 24 hours, the patient was taken for irrigation and débridement with polyethylene exchange of the right knee. Surgical exploration revealed brownish purulent fluid in the knee. The polyethylene insert was removed, and a complete synovectomy was performed for knee débridement. Nine liters of triple antibiotic (utilized bacitracin, polymyxin, and gentamicin) saline were used to copiously clean the metal surfaces of the implant, and a new polyethylene liner was inserted. Absorbable calcium sulfate antimicrobial beads, stimulant beads with 1 gram of vancomycin and 1.2 grams of tobramycin, were implanted both inside and over the knee capsule during closure.
Blood cultures, knee aspirate, and surgical cultures were all positive for P shigelloides. Of note, the patient did not describe having diarrhea, a symptom common in P shigelloides infection. After final cultures were received, the patient was placed on intravenous ceftriaxone and oral levofloxacin for 6 weeks. Three months later, he reported full return to activity and clearance of the infection.
Discussion
This case is a reminder that periprosthetic knee infection can occur from a variety of pathologic organisms and that obtaining a complete history is an important part of any diagnostic work-up. Although P shigelloides infection is rare, our patient had important historical findings that led to suspicion of Vibrionaceae infection: recent consumption of raw oysters, immunosuppression with etanercept and prednisone for psoriatic arthritis, and diabetes with hemoglobin A1c of 9.9% and presenting blood sugar of 338 mg/dL. His positive blood cultures represented P shigelloides septicemia, which seeded the knee prosthesis and led to acute periprosthetic infection. To our knowledge, this is the first report of P shigelloides periprosthetic infection in the orthopedic literature. The only other reported case of P shigelloides septicemia leading to septic arthritis in a native knee occurred in a 68-year-old Australian man who had end-stage liver disease and eventually died from complications of the P shigelloides infection.13
Although P shigelloides infection is rare, outbreaks have occurred around the world.7-11,14 Infections are most commonly associated with consumption of raw shellfish or freshwater fish or with water contamination.12 In the United States, the only described vector for disease has been consumption of raw oysters and shellfish—in particular, those harvested from the warm waters of the Gulf Coast.8,9P shigelloides usually causes a self-limiting watery diarrhea. However, in children and immunosuppressed patients, P shigelloides can lead to life-threatening septicemia.12 In the United States, P shigelloides cases often occur in the summer, likely related to the easy growth of the bacteria from shellfish in the Gulf Coast’s warm water and mud.8 This predilection for summer infections has been documented around the world.15Our patient reported eating raw oysters imported to the US Southwest from an unknown location. He likely was susceptible to P shigelloides infection, as he was immunosuppressed with etanercept and prednisone. However, there were no traditional diarrheal symptoms. Case reports have described nondiarrheal symptoms in children and other immunosuppressed people.12There is much to learn from this case report. Most important, it highlights the need to obtain a complete history and perform a thorough physical examination. Our patient’s 2 key historical findings, immunosuppressive medication use and raw oyster consumption, point strongly toward Vibrionaceae infection. Although a majority of periprosthetic infections are caused by common organisms, such as Staphylococcus and Streptococcus species, orthopedic clinicians should continue to expand their knowledge of periprosthetic infections, as many other pathogens can cause disease.
Am J Orthop. 2017;46(1):E32-E34. Copyright Frontline Medical Communications Inc. 2017. All rights reserved.
Take-Home Points
- History and physical examination are key in identifying possible etiologies of orthopedic infections.
- If identified in the acute setting, periprosthetic infections can successfully be treated with irrigation, débridement, and polyethylene liner exchange.
- Discussion with an interdisciplinary medical team, including infectious disease specialists, can aide in improved diagnosis and treatment of periprosthetic infections.
Periprosthetic infection is a leading cause of morbidity after total joint arthroplasty.1 Despite advances in modern surgical practices, infection rates continue to range from 1% to 3% among all arthroplasty procedures performed in the United States.2-5 The most common causes of periprosthetic infection include Staphylococcus aureus, streptococcus, enterococcus, Escherichia coli, and Pseudomonas aeruginosa.6 However, many other pathogens that cause periprosthetic infection should be considered in the clinical setting. In this case report, periprosthetic knee infection with P shigelloides occurred after consumption of raw oysters.
P shigelloides is a gram-negative facultative anaerobic organism in the Vibrionaceae family,7 which also includes Vibrio vulnificus and Vibrio parahaemolyticus. P shigelloides is most well-known for causing diarrhea and septicemia in people who have consumed raw oysters or shellfish in the United States.8,9 Although P shigelloides infection is rare, there have been clinically significant outbreaks from contaminated water in Japan,10 consumption of freshwater fish in the Democratic Republic of the Congo,11 and consumption of raw oysters in the United States.8,9 Children and immunosuppressed people are most susceptible to the disease, which most commonly manifests as self-limiting watery diarrhea, with septicemia only in advanced cases.12There are very few reports of P shigelloides in the orthopedic population. In the medical literature, we found only 1 case of septic arthritis in a native knee; disease progression resulted in the patient’s death.13In this article, we report a case of P shigelloides septicemia that caused periprosthetic knee infection in a chemically and biologically immunosuppressed patient. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
Out of concern about a periprosthetic knee infection, a 66-year-old man was transferred from a regional medical center to our tertiary referral center. The patient reported a 3-day history of significant knee pain, swelling, and erythema that started the day after he consumed raw oysters at a seafood bar. He was unable to bear weight on the right knee and remained at home 1 day before presenting to the regional medical center.
The patient had undergone elective right total knee arthroplasty 18 months earlier, without previous issue (Figures A, B), and had a medical history of type 2 diabetes mellitus, psoriatic arthritis, hypertension, hyperlipidemia, hypothyroidism, and benign prostatic hypertrophy.
On presentation to our facility, the patient described pain in the right knee. Physical examination revealed swelling and erythema of the knee. Vital signs were within normal limits, with a temperature of 98.5°F. Laboratory work-up revealed white blood cell count of 17,700 with 79% neutrophils and 9% lymphocytes, serum C-reactive protein level of 270 mg/L, and erythrocyte sedimentation rate of 46 mm/h. Aspiration of the knee yielded about 100 mL of thick, brownish synovial fluid. Gram stain of the knee aspirate revealed gram-negative rods and many white blood cells. Nucleated cell count of the aspirate was 22,400 with 88% neutrophils. Blood cultures were obtained, and broad-spectrum antibiotics (vancomycin and ceftriaxone) were started in preparation for surgery.
Within 24 hours, the patient was taken for irrigation and débridement with polyethylene exchange of the right knee. Surgical exploration revealed brownish purulent fluid in the knee. The polyethylene insert was removed, and a complete synovectomy was performed for knee débridement. Nine liters of triple antibiotic (utilized bacitracin, polymyxin, and gentamicin) saline were used to copiously clean the metal surfaces of the implant, and a new polyethylene liner was inserted. Absorbable calcium sulfate antimicrobial beads, stimulant beads with 1 gram of vancomycin and 1.2 grams of tobramycin, were implanted both inside and over the knee capsule during closure.
Blood cultures, knee aspirate, and surgical cultures were all positive for P shigelloides. Of note, the patient did not describe having diarrhea, a symptom common in P shigelloides infection. After final cultures were received, the patient was placed on intravenous ceftriaxone and oral levofloxacin for 6 weeks. Three months later, he reported full return to activity and clearance of the infection.
Discussion
This case is a reminder that periprosthetic knee infection can occur from a variety of pathologic organisms and that obtaining a complete history is an important part of any diagnostic work-up. Although P shigelloides infection is rare, our patient had important historical findings that led to suspicion of Vibrionaceae infection: recent consumption of raw oysters, immunosuppression with etanercept and prednisone for psoriatic arthritis, and diabetes with hemoglobin A1c of 9.9% and presenting blood sugar of 338 mg/dL. His positive blood cultures represented P shigelloides septicemia, which seeded the knee prosthesis and led to acute periprosthetic infection. To our knowledge, this is the first report of P shigelloides periprosthetic infection in the orthopedic literature. The only other reported case of P shigelloides septicemia leading to septic arthritis in a native knee occurred in a 68-year-old Australian man who had end-stage liver disease and eventually died from complications of the P shigelloides infection.13
Although P shigelloides infection is rare, outbreaks have occurred around the world.7-11,14 Infections are most commonly associated with consumption of raw shellfish or freshwater fish or with water contamination.12 In the United States, the only described vector for disease has been consumption of raw oysters and shellfish—in particular, those harvested from the warm waters of the Gulf Coast.8,9P shigelloides usually causes a self-limiting watery diarrhea. However, in children and immunosuppressed patients, P shigelloides can lead to life-threatening septicemia.12 In the United States, P shigelloides cases often occur in the summer, likely related to the easy growth of the bacteria from shellfish in the Gulf Coast’s warm water and mud.8 This predilection for summer infections has been documented around the world.15Our patient reported eating raw oysters imported to the US Southwest from an unknown location. He likely was susceptible to P shigelloides infection, as he was immunosuppressed with etanercept and prednisone. However, there were no traditional diarrheal symptoms. Case reports have described nondiarrheal symptoms in children and other immunosuppressed people.12There is much to learn from this case report. Most important, it highlights the need to obtain a complete history and perform a thorough physical examination. Our patient’s 2 key historical findings, immunosuppressive medication use and raw oyster consumption, point strongly toward Vibrionaceae infection. Although a majority of periprosthetic infections are caused by common organisms, such as Staphylococcus and Streptococcus species, orthopedic clinicians should continue to expand their knowledge of periprosthetic infections, as many other pathogens can cause disease.
Am J Orthop. 2017;46(1):E32-E34. Copyright Frontline Medical Communications Inc. 2017. All rights reserved.
1. Parvizi J, Adeli B, Zmistowski B, Restrepo C, Greenwald AS. Management of periprosthetic joint infection: the current knowledge: AAOS exhibit selection. J Bone Joint Surg Am. 2012;94(14):e104.
2. Fehring TK, Odum S, Griffin WL, Mason JB, Nadaud M. Early failures in total knee arthroplasty. Clin Orthop. 2001;(392):315-318.
3. Kurtz SM, Lau E, Schmier J, Ong KL, Zhao K, Parvizi J. Infection burden for hip and knee arthroplasty in the United States. J Arthroplasty. 2008;23(7):984-991.
4. Clohisy JC, Calvert G, Tull F, McDonald D, Maloney WJ. Reasons for revision hip surgery: a retrospective review. Clin Orthop. 2004;(429):188-192.
5. Vessely MB, Whaley AL, Harmsen WS, Schleck CD, Berry DJ. The Chitranjan Ranawat Award: long-term survivorship and failure modes of 1000 cemented condylar total knee arthroplasties. Clin Orthop. 2006;(452):28-34.
6. Peel TN, Cheng AC, Buising KL, Choong PF. Microbiological aetiology, epidemiology, and clinical profile of prosthetic joint infections: are current antibiotic prophylaxis guidelines effective? Antimicrob Agents Chemother. 2012;56(5):2386-2391.
7. Wong TY, Tsui HY, So MK, et al. Plesiomonas shigelloides infection in Hong Kong: retrospective study of 167 laboratory-confirmed cases. Hong Kong Med J. 2000;6(4):375-380.
8. Holmberg SD, Wachsmuth IK, Hickman-Brenner FW, Blake PA, Farmer JJ 3rd. Plesiomonas enteric infections in the United States. Ann Intern Med. 1986;105(5):690-694.
9. Rutala WA, Sarubi FA Jr, Finch CS, McCormack JN, Steinkraus GE. Oyster-associated outbreak of diarrhoeal disease possibly caused by Plesiomonas shigelloides. Lancet. 1982;1(8274):739.
10. Tsukamoto T, Kinoshita Y, Shimada T, Sakazaki R. Two epidemics of diarrhoeal disease possibly caused by Plesiomonas shigelloides. J Hyg (Lond). 1978;80(2):275-280.
11. Van Damme LR, Vandepitte J. Frequent isolation of Edwardsiella tarda and Plesiomonas shigelloides from healthy Zairese freshwater fish: a possible source of sporadic diarrhea in the tropics. Appl Environ Microbiol. 1980;39(3):475-479.
12. Brenden RA, Miller MA, Janda JM. Clinical disease spectrum and pathogenic factors associated with Plesiomonas shigelloides infections in humans. Rev Infect Dis. 1988;10(2):303-316.
13. Gordon DL, Philpot CR, McGuire C. Plesiomonas shigelloides septic arthritis complicating rheumatoid arthritis. Aust N Z J Med. 1983;13(3):275-276.
14. Medema G, Schets C. Occurrence of Plesiomonas shigelloides in surface water: relationship with faecal pollution and trophic state. Zentralbl Hyg Umweltmed. 1993;194(4):398-404.
15. Huq MI, Islam MR. Microbiological & clinical studies in diarrhoea due to Plesiomonas shigelloides. Indian J Med Res. 1983;77:793-797.
1. Parvizi J, Adeli B, Zmistowski B, Restrepo C, Greenwald AS. Management of periprosthetic joint infection: the current knowledge: AAOS exhibit selection. J Bone Joint Surg Am. 2012;94(14):e104.
2. Fehring TK, Odum S, Griffin WL, Mason JB, Nadaud M. Early failures in total knee arthroplasty. Clin Orthop. 2001;(392):315-318.
3. Kurtz SM, Lau E, Schmier J, Ong KL, Zhao K, Parvizi J. Infection burden for hip and knee arthroplasty in the United States. J Arthroplasty. 2008;23(7):984-991.
4. Clohisy JC, Calvert G, Tull F, McDonald D, Maloney WJ. Reasons for revision hip surgery: a retrospective review. Clin Orthop. 2004;(429):188-192.
5. Vessely MB, Whaley AL, Harmsen WS, Schleck CD, Berry DJ. The Chitranjan Ranawat Award: long-term survivorship and failure modes of 1000 cemented condylar total knee arthroplasties. Clin Orthop. 2006;(452):28-34.
6. Peel TN, Cheng AC, Buising KL, Choong PF. Microbiological aetiology, epidemiology, and clinical profile of prosthetic joint infections: are current antibiotic prophylaxis guidelines effective? Antimicrob Agents Chemother. 2012;56(5):2386-2391.
7. Wong TY, Tsui HY, So MK, et al. Plesiomonas shigelloides infection in Hong Kong: retrospective study of 167 laboratory-confirmed cases. Hong Kong Med J. 2000;6(4):375-380.
8. Holmberg SD, Wachsmuth IK, Hickman-Brenner FW, Blake PA, Farmer JJ 3rd. Plesiomonas enteric infections in the United States. Ann Intern Med. 1986;105(5):690-694.
9. Rutala WA, Sarubi FA Jr, Finch CS, McCormack JN, Steinkraus GE. Oyster-associated outbreak of diarrhoeal disease possibly caused by Plesiomonas shigelloides. Lancet. 1982;1(8274):739.
10. Tsukamoto T, Kinoshita Y, Shimada T, Sakazaki R. Two epidemics of diarrhoeal disease possibly caused by Plesiomonas shigelloides. J Hyg (Lond). 1978;80(2):275-280.
11. Van Damme LR, Vandepitte J. Frequent isolation of Edwardsiella tarda and Plesiomonas shigelloides from healthy Zairese freshwater fish: a possible source of sporadic diarrhea in the tropics. Appl Environ Microbiol. 1980;39(3):475-479.
12. Brenden RA, Miller MA, Janda JM. Clinical disease spectrum and pathogenic factors associated with Plesiomonas shigelloides infections in humans. Rev Infect Dis. 1988;10(2):303-316.
13. Gordon DL, Philpot CR, McGuire C. Plesiomonas shigelloides septic arthritis complicating rheumatoid arthritis. Aust N Z J Med. 1983;13(3):275-276.
14. Medema G, Schets C. Occurrence of Plesiomonas shigelloides in surface water: relationship with faecal pollution and trophic state. Zentralbl Hyg Umweltmed. 1993;194(4):398-404.
15. Huq MI, Islam MR. Microbiological & clinical studies in diarrhoea due to Plesiomonas shigelloides. Indian J Med Res. 1983;77:793-797.
The Importance of Subclavian Angiography in the Evaluation of Chest Pain: Coronary-Subclavian Steal Syndrome
Coronary-subclavian steal syndrome (CSSS) is a rare clinical entity with an incidence of 0.2% to 0.7%.1 Despite its scarcity, CSSS is a condition that can result in devastating clinical consequences, such as myocardial ischemia, ranging from angina to myocardial infarction (MI) and ischemic cardiomyopathy.2
In 1974, Harjola and Valle first reported the angiographic and physiologic descriptions of CSSS in an asymptomatic patient who was found to have flow reversal in the left internal mammary artery (LIMA) graft in a follow-up coronary angiography performed 11 months after coronary artery bypass grafting (CABG).3 Because of the similarity in the pathophysiology of this condition with vertebral-subclavian steal syndrome, this clinical entity was named coronary-subclavian steal syndrome (CSSS).4,5
In steal-syndrome phenomena, there is a significant stenosis in the subclavian artery proximal to the origin of an arterial branch, either LIMA or vertebral artery, resulting in lower pressure in the distal subclavian artery. As a result, the negative pressure gradient might be sufficient to cause retrograde flow; consequently causing arterial branch “flow reversal,” and then “steal” flow from the organ—either heart or brain—supplied by that artery.3,6
Coronary-subclavian steal syndrome is caused by a reversal of flow in a previously constructed internal mammary artery (IMA)-coronary conduit graft. It typically results from hemodynamically significant subclavian artery stenosis proximal to the ipsilateral IMA. The reversal of flow will “steal” the blood from the coronary territory supplied by the IMA conduit.4,5 The absence of proximal subclavian artery stenosis does not preclude the presence of this syndrome; reversal in the IMA conduit can occur in association with upper extremity hemodialysis fistulae or anomalous connection of the left subclavian artery to the pulmonary artery in d-transposition of the great arteries.2 Although the stenosis is most commonly caused by atherosclerotic disease, other clinical entities, including Takayasu vasculitis, radiation, and giant cell arteritis, have been described.6 Patients with CSSS usually present with stable or unstable angina as well as arm claudication and various neurologic symptoms.5 The consequence of CSSS can include ischemic cardiomyopathy, acute MI,7 stroke, and death.5,8
Case Presentation
A 66-year-old man with a previous MI managed with CABG, permanent atrial fibrillation (AF), and moderate aortic stenosis presented to the ambulatory clinic with recurrent symptoms of stable angina despite being on maximal anti-anginal therapy. A coronary angiogram performed 4 years earlier had revealed significant left main artery disease and total occlusion of the right coronary artery.
Cardiovascular examination revealed an irregular rhythm with a normal S1, variable S2, and a 3/6 systolic ejection murmur heard best at the right second intercostal space with radiation to the carotids. His peripheral pulses were equal and symmetric in the lower extremities, and no peripheral edema was noted. The remainder of the physical examination was otherwise unremarkable. The resting 12-lead electrocardiogram showed AF at a rate of 60 bpm (Figure 1).
A stress test was performed to elucidate a possible coronary distribution for the cause of the chest pain.
Consequently, coronary angiography was performed and showed 95% left main stenosis and total occlusion of the mid-right coronary artery with right dominance, patent LIMA to mid-LAD and patent saphenous venous graft to posterior descending artery grafts (Figure 3)
The patient underwent percutaneous transluminal angioplasty (PTA) of the subclavian stenosis with insertion of an 8 mm x 27 mm balloon-expandable peripheral stent (Figure 5) (Supplemental video 6). The patient tolerated the procedure well without complications and with resolution of his symptoms at a 6-month follow-up.
Discussion
Long-term follow-up of LIMA as a conduit to LAD has shown a 10-year patency of 95% compared with 76% for saphenous vein and an associated 10-year survival of 93.4% for LIMA compared with 88% for saphenous vein graft.9,10 Because of the superiority of LIMA outcomes, it has become the preferred graft in CABG. However, this approach is associated with 0.1% to 0.2% risk of ischemia related to flow reversal in the LIMA b
Greater awareness and improvement in diagnostic imaging have contributed to the increased incidence of CSSS and its consequences.2 Although symptoms related to myocardial ischemia, as in this case, are the most dominant in CSSS, other brachiocephalic symptoms, including vertebral-subclavian steal, transient ischemic attacks, and strokes, have been reported.11 Additionally, the same disease might compromise distal flow, resulting in extremity claudication or even distal microembolization.12
It is important to recognize that significant brachiocephalic stenosis has been reported in about 0.2% to 2.5% of patients undergoing elective CABG.6,8 Therefore, it is essential to screen for brachiocephalic artery disease before undergoing CABG. Different strategies have been suggested, including assessing pressure gradient between the upper extremities as the initial step; CSSS should be considered when the pressure gradient is > 20 mm Hg.
Other strategies include ultrasonic duplex scanning with provocation test using arm exercise or reactive hyperemia.13 Many high-volume centers are performing screening by proximal subclavian angiography in all patients undergoing coronary angiography. When significant disease is detected, arch aortography and 4-vessel cerebral angiography is performed.6 In addition, other centers have adopted the routine use of computerized tomographic angiography before CABG.14
Surgical correction of CSSS is considered to be the gold standard and can be accomplished by performing aorta-subclavian bypass, carotid-subclavian bypass, axillo-axillary bypass, or relocation of the IMA graft.2 Although this approach is invasive and carries many disadvantages related to patient comfort,surgical revascularization can be performed safely at the time of CABG and may not carry additional risk of morbidity or mortality.15 Moreover, surgical correction is the preferred modality for treatment of CSSS when the anatomy is not favorable for percutaneous intervention, such as chronic total occlusion of the subclavian artery.15Alternatively, CSSS can effectively be managed less invasively by percutaneous intervention, including PTA with stent placement,16,17 thrombectomy18 or atherectomy of the stenotic subclavian artery.19
In this patient, PTA was performed with primary stent placement. The lesion was crossed with a sheath, using combined femoral and radial access. After proper positioning, a balloon-expandable stent was deployed that resulted in complete angiographic resolution of the lesion and improvement of symptoms at 6-month follow-up. In line with previous reports, this case demonstrated that percutaneous intervention is a feasible and less invasive approach for management of CSSS.16,17 The effectiveness of the percutaneous approach has effectiveness equivalent to surgical bypass with minimal complications and good long-term success. Therefore, it has been suggested as first-line therapy in CSSS.8,16
Although preoperative screening for brachiocephalic disease before undergoing ipsilateral IMA coronary artery bypass can prevent the development of CSSS, there is controversy about the best approach for managing these concomitant conditions. Many institutions use all-vein coronary conduits, but that forgoes the benefit of a LIMA graft. Therefore, others still perform an IMA conduit after brachiocephalic reconstruction. An alternative method is to use free IMA or radial artery conduit. Currently, there are limited data about the use of endovascular treatment for brachiocephalic disease with a CABG.2
Conclusion
Coronary-subclavian steal syndrome is an important clinical condition that is associated with significant morbidity and mortality. In the Sullivan and colleagues report of 27 patients with CSSS, 59.3% had stable angina and 40.7% had acute coronary syndrome, among which 14.8% presented with acute MI.7 Therefore, early recognition is essential to prevent catastrophic consequences.
Patients with CSSS usually present with cardiac symptoms, but symptoms related to vertebral-subclavian steal and posterior cerebral insufficiency can coexist. The authors suggest routine preoperative screening for the presence of brachiocephalic disease, using ultrasonic duplex or angiography. This practice is cost-effective and essential to prevent the development of CSSS. Optimal management of brachiocephalic disease prior to CABG is debatable; however, IMA grafting and reconstruction of the brachiocephalic system seems to be a promising approach.
When CSSS develops after CABG, the condition can be successfully treated with percutaneous intervention and outcomes comparable with those of surgical bypass.
Acknowledgments
Special thanks to the division of cardiology at New Jersey VA Health Care System, in particular Steve Tsai, MD; Ronald L. Vaillancourt, RN, and Preciosa Yap, RN.
1. Marques KM, Ernst SM, Mast EG, Bal ET, Suttorp MJ, Plokker HW. Percutaneous transluminal angioplasty of the left subclavian artery to prevent or treat the coronary-subclavian steal syndrome. Am J Cardiol. 1996;78(6):687-690.
2. Takach TJ, Reul GJ, Cooley DA, et al. Myocardial thievery: the coronary-subclavian steal syndrome. Ann Thorac Surg. 2006;81(1):386-392.
3. Harjola PT, Valle M. The importance of aortic arch or subclavian angiography before coronary reconstruction. Chest. 1974;66(4):436-438.
4. Tyras DH, Barner HB. Coronary-subclavian steal. Arch Surg. 1977;112(9):1125-1127.
5. Brown AH. Coronary steal by internal mammary graft with subclavian stenosis. J Thorac Cardiovasc Surg. 1977;73(5):690-693.
6. Takach TJ, Reul GJ, Duncan JM, et al. Concomitant brachiocephalic and coronary artery disease: outcome and decision analysis. Ann Thorac Surg. 2005;80(2):564-569.
7. Sullivan TM, Gray BH, Bacharach JM, et al. Angioplasty and primary stenting of the subclavian, innominate, and common carotid arteries in 83 patients. J Vasc Surg. 1998;28(6):1059-1065.
8. Hwang HY, Kim JH, Lee W, Park JH, Kim KB. Left subclavian artery stenosis in coronary artery bypass: prevalence and revascularization strategies. Ann Thorac Surg. 2010;89(4):1146-11 50.
9. Zeff RH, Kongtahworn C, Iannone LA, et al. Internal mammary artery versus saphenous vein graft to the left anterior descending coronary artery: prospective randomized study with 10-year follow-up. Ann Thorac Surg.1988;45(5):533-536.
10. Loop FD, Lytle BW, Cosgrove DM, et al. Influence of the internal-mammary-artery graft on 10-year survival and other cardiac events. N Engl J Med. 1986;314(1):1-6.
11. Lee SR, Jeong MH, Rhew JY, et al. Simultaneous coronary-subclavian and vertebral-subclavian steal syndrome. Circ J. 2003;67(5):464-466.
12. Takach TJ, Beggs ML, Nykamp VJ, Reul GJ Jr. Concomitant cerebral and coronary subclavian steal. Ann Thorac Surg. 1997;63(3):853-854.
13. Branchereau A, Magnan PE, Espinoza H, Bartoli JM. Subclavian artery stenosis: hemodynamic aspects and surgical outcome. J Cardiovasc Surg (Torino). 1991;32(5):604-661.
14. Park KH, Lee HY, Lim C, et al. Clinical impact of computerised tomographic angiography performed for preoperative evaluation before coronary artery bypass grafting. Eur J Cardiothorac Surg. 2010;37(6):1346-1352.
15. Sintek M, Coverstone E, Singh J. Coronary subclavian steal syndrome. Curr Opin Cardiol. 2014;29(6):506-513.
16. Eisenhauer AC. Subclavian and innominate revascularization: surgical therapy versus catheter-based intervention. Curr Interv Cardiol Rep. 2000;2(2):101-110.
17. Bates MC, Broce M, Lavigne PS, Stone P. Subclavian artery stenting: factors influencing long-term outcome. Catheter Cardiovasc Interv. 2004;61(1):5-11.
18. Zeller T, Frank U, Burgelin K, Sinn L, Horn B, Roskamm H. Acute thrombotic subclavian artery occlusion treated with a new rotational thrombectomy device. J Endovasc Ther. 2002;9(6):917-921.
19. Breall JA, Grossman W, Stillman IE, Gianturco LE, Kim D. Atherectomy of the subclavian artery for patients with symptomatic coronary-subclavian steal syndrome. J Am Coll Cardiol. 1993;21(7):1564-1567.
Coronary-subclavian steal syndrome (CSSS) is a rare clinical entity with an incidence of 0.2% to 0.7%.1 Despite its scarcity, CSSS is a condition that can result in devastating clinical consequences, such as myocardial ischemia, ranging from angina to myocardial infarction (MI) and ischemic cardiomyopathy.2
In 1974, Harjola and Valle first reported the angiographic and physiologic descriptions of CSSS in an asymptomatic patient who was found to have flow reversal in the left internal mammary artery (LIMA) graft in a follow-up coronary angiography performed 11 months after coronary artery bypass grafting (CABG).3 Because of the similarity in the pathophysiology of this condition with vertebral-subclavian steal syndrome, this clinical entity was named coronary-subclavian steal syndrome (CSSS).4,5
In steal-syndrome phenomena, there is a significant stenosis in the subclavian artery proximal to the origin of an arterial branch, either LIMA or vertebral artery, resulting in lower pressure in the distal subclavian artery. As a result, the negative pressure gradient might be sufficient to cause retrograde flow; consequently causing arterial branch “flow reversal,” and then “steal” flow from the organ—either heart or brain—supplied by that artery.3,6
Coronary-subclavian steal syndrome is caused by a reversal of flow in a previously constructed internal mammary artery (IMA)-coronary conduit graft. It typically results from hemodynamically significant subclavian artery stenosis proximal to the ipsilateral IMA. The reversal of flow will “steal” the blood from the coronary territory supplied by the IMA conduit.4,5 The absence of proximal subclavian artery stenosis does not preclude the presence of this syndrome; reversal in the IMA conduit can occur in association with upper extremity hemodialysis fistulae or anomalous connection of the left subclavian artery to the pulmonary artery in d-transposition of the great arteries.2 Although the stenosis is most commonly caused by atherosclerotic disease, other clinical entities, including Takayasu vasculitis, radiation, and giant cell arteritis, have been described.6 Patients with CSSS usually present with stable or unstable angina as well as arm claudication and various neurologic symptoms.5 The consequence of CSSS can include ischemic cardiomyopathy, acute MI,7 stroke, and death.5,8
Case Presentation
A 66-year-old man with a previous MI managed with CABG, permanent atrial fibrillation (AF), and moderate aortic stenosis presented to the ambulatory clinic with recurrent symptoms of stable angina despite being on maximal anti-anginal therapy. A coronary angiogram performed 4 years earlier had revealed significant left main artery disease and total occlusion of the right coronary artery.
Cardiovascular examination revealed an irregular rhythm with a normal S1, variable S2, and a 3/6 systolic ejection murmur heard best at the right second intercostal space with radiation to the carotids. His peripheral pulses were equal and symmetric in the lower extremities, and no peripheral edema was noted. The remainder of the physical examination was otherwise unremarkable. The resting 12-lead electrocardiogram showed AF at a rate of 60 bpm (Figure 1).
A stress test was performed to elucidate a possible coronary distribution for the cause of the chest pain.
Consequently, coronary angiography was performed and showed 95% left main stenosis and total occlusion of the mid-right coronary artery with right dominance, patent LIMA to mid-LAD and patent saphenous venous graft to posterior descending artery grafts (Figure 3)
The patient underwent percutaneous transluminal angioplasty (PTA) of the subclavian stenosis with insertion of an 8 mm x 27 mm balloon-expandable peripheral stent (Figure 5) (Supplemental video 6). The patient tolerated the procedure well without complications and with resolution of his symptoms at a 6-month follow-up.
Discussion
Long-term follow-up of LIMA as a conduit to LAD has shown a 10-year patency of 95% compared with 76% for saphenous vein and an associated 10-year survival of 93.4% for LIMA compared with 88% for saphenous vein graft.9,10 Because of the superiority of LIMA outcomes, it has become the preferred graft in CABG. However, this approach is associated with 0.1% to 0.2% risk of ischemia related to flow reversal in the LIMA b
Greater awareness and improvement in diagnostic imaging have contributed to the increased incidence of CSSS and its consequences.2 Although symptoms related to myocardial ischemia, as in this case, are the most dominant in CSSS, other brachiocephalic symptoms, including vertebral-subclavian steal, transient ischemic attacks, and strokes, have been reported.11 Additionally, the same disease might compromise distal flow, resulting in extremity claudication or even distal microembolization.12
It is important to recognize that significant brachiocephalic stenosis has been reported in about 0.2% to 2.5% of patients undergoing elective CABG.6,8 Therefore, it is essential to screen for brachiocephalic artery disease before undergoing CABG. Different strategies have been suggested, including assessing pressure gradient between the upper extremities as the initial step; CSSS should be considered when the pressure gradient is > 20 mm Hg.
Other strategies include ultrasonic duplex scanning with provocation test using arm exercise or reactive hyperemia.13 Many high-volume centers are performing screening by proximal subclavian angiography in all patients undergoing coronary angiography. When significant disease is detected, arch aortography and 4-vessel cerebral angiography is performed.6 In addition, other centers have adopted the routine use of computerized tomographic angiography before CABG.14
Surgical correction of CSSS is considered to be the gold standard and can be accomplished by performing aorta-subclavian bypass, carotid-subclavian bypass, axillo-axillary bypass, or relocation of the IMA graft.2 Although this approach is invasive and carries many disadvantages related to patient comfort,surgical revascularization can be performed safely at the time of CABG and may not carry additional risk of morbidity or mortality.15 Moreover, surgical correction is the preferred modality for treatment of CSSS when the anatomy is not favorable for percutaneous intervention, such as chronic total occlusion of the subclavian artery.15Alternatively, CSSS can effectively be managed less invasively by percutaneous intervention, including PTA with stent placement,16,17 thrombectomy18 or atherectomy of the stenotic subclavian artery.19
In this patient, PTA was performed with primary stent placement. The lesion was crossed with a sheath, using combined femoral and radial access. After proper positioning, a balloon-expandable stent was deployed that resulted in complete angiographic resolution of the lesion and improvement of symptoms at 6-month follow-up. In line with previous reports, this case demonstrated that percutaneous intervention is a feasible and less invasive approach for management of CSSS.16,17 The effectiveness of the percutaneous approach has effectiveness equivalent to surgical bypass with minimal complications and good long-term success. Therefore, it has been suggested as first-line therapy in CSSS.8,16
Although preoperative screening for brachiocephalic disease before undergoing ipsilateral IMA coronary artery bypass can prevent the development of CSSS, there is controversy about the best approach for managing these concomitant conditions. Many institutions use all-vein coronary conduits, but that forgoes the benefit of a LIMA graft. Therefore, others still perform an IMA conduit after brachiocephalic reconstruction. An alternative method is to use free IMA or radial artery conduit. Currently, there are limited data about the use of endovascular treatment for brachiocephalic disease with a CABG.2
Conclusion
Coronary-subclavian steal syndrome is an important clinical condition that is associated with significant morbidity and mortality. In the Sullivan and colleagues report of 27 patients with CSSS, 59.3% had stable angina and 40.7% had acute coronary syndrome, among which 14.8% presented with acute MI.7 Therefore, early recognition is essential to prevent catastrophic consequences.
Patients with CSSS usually present with cardiac symptoms, but symptoms related to vertebral-subclavian steal and posterior cerebral insufficiency can coexist. The authors suggest routine preoperative screening for the presence of brachiocephalic disease, using ultrasonic duplex or angiography. This practice is cost-effective and essential to prevent the development of CSSS. Optimal management of brachiocephalic disease prior to CABG is debatable; however, IMA grafting and reconstruction of the brachiocephalic system seems to be a promising approach.
When CSSS develops after CABG, the condition can be successfully treated with percutaneous intervention and outcomes comparable with those of surgical bypass.
Acknowledgments
Special thanks to the division of cardiology at New Jersey VA Health Care System, in particular Steve Tsai, MD; Ronald L. Vaillancourt, RN, and Preciosa Yap, RN.
Coronary-subclavian steal syndrome (CSSS) is a rare clinical entity with an incidence of 0.2% to 0.7%.1 Despite its scarcity, CSSS is a condition that can result in devastating clinical consequences, such as myocardial ischemia, ranging from angina to myocardial infarction (MI) and ischemic cardiomyopathy.2
In 1974, Harjola and Valle first reported the angiographic and physiologic descriptions of CSSS in an asymptomatic patient who was found to have flow reversal in the left internal mammary artery (LIMA) graft in a follow-up coronary angiography performed 11 months after coronary artery bypass grafting (CABG).3 Because of the similarity in the pathophysiology of this condition with vertebral-subclavian steal syndrome, this clinical entity was named coronary-subclavian steal syndrome (CSSS).4,5
In steal-syndrome phenomena, there is a significant stenosis in the subclavian artery proximal to the origin of an arterial branch, either LIMA or vertebral artery, resulting in lower pressure in the distal subclavian artery. As a result, the negative pressure gradient might be sufficient to cause retrograde flow; consequently causing arterial branch “flow reversal,” and then “steal” flow from the organ—either heart or brain—supplied by that artery.3,6
Coronary-subclavian steal syndrome is caused by a reversal of flow in a previously constructed internal mammary artery (IMA)-coronary conduit graft. It typically results from hemodynamically significant subclavian artery stenosis proximal to the ipsilateral IMA. The reversal of flow will “steal” the blood from the coronary territory supplied by the IMA conduit.4,5 The absence of proximal subclavian artery stenosis does not preclude the presence of this syndrome; reversal in the IMA conduit can occur in association with upper extremity hemodialysis fistulae or anomalous connection of the left subclavian artery to the pulmonary artery in d-transposition of the great arteries.2 Although the stenosis is most commonly caused by atherosclerotic disease, other clinical entities, including Takayasu vasculitis, radiation, and giant cell arteritis, have been described.6 Patients with CSSS usually present with stable or unstable angina as well as arm claudication and various neurologic symptoms.5 The consequence of CSSS can include ischemic cardiomyopathy, acute MI,7 stroke, and death.5,8
Case Presentation
A 66-year-old man with a previous MI managed with CABG, permanent atrial fibrillation (AF), and moderate aortic stenosis presented to the ambulatory clinic with recurrent symptoms of stable angina despite being on maximal anti-anginal therapy. A coronary angiogram performed 4 years earlier had revealed significant left main artery disease and total occlusion of the right coronary artery.
Cardiovascular examination revealed an irregular rhythm with a normal S1, variable S2, and a 3/6 systolic ejection murmur heard best at the right second intercostal space with radiation to the carotids. His peripheral pulses were equal and symmetric in the lower extremities, and no peripheral edema was noted. The remainder of the physical examination was otherwise unremarkable. The resting 12-lead electrocardiogram showed AF at a rate of 60 bpm (Figure 1).
A stress test was performed to elucidate a possible coronary distribution for the cause of the chest pain.
Consequently, coronary angiography was performed and showed 95% left main stenosis and total occlusion of the mid-right coronary artery with right dominance, patent LIMA to mid-LAD and patent saphenous venous graft to posterior descending artery grafts (Figure 3)
The patient underwent percutaneous transluminal angioplasty (PTA) of the subclavian stenosis with insertion of an 8 mm x 27 mm balloon-expandable peripheral stent (Figure 5) (Supplemental video 6). The patient tolerated the procedure well without complications and with resolution of his symptoms at a 6-month follow-up.
Discussion
Long-term follow-up of LIMA as a conduit to LAD has shown a 10-year patency of 95% compared with 76% for saphenous vein and an associated 10-year survival of 93.4% for LIMA compared with 88% for saphenous vein graft.9,10 Because of the superiority of LIMA outcomes, it has become the preferred graft in CABG. However, this approach is associated with 0.1% to 0.2% risk of ischemia related to flow reversal in the LIMA b
Greater awareness and improvement in diagnostic imaging have contributed to the increased incidence of CSSS and its consequences.2 Although symptoms related to myocardial ischemia, as in this case, are the most dominant in CSSS, other brachiocephalic symptoms, including vertebral-subclavian steal, transient ischemic attacks, and strokes, have been reported.11 Additionally, the same disease might compromise distal flow, resulting in extremity claudication or even distal microembolization.12
It is important to recognize that significant brachiocephalic stenosis has been reported in about 0.2% to 2.5% of patients undergoing elective CABG.6,8 Therefore, it is essential to screen for brachiocephalic artery disease before undergoing CABG. Different strategies have been suggested, including assessing pressure gradient between the upper extremities as the initial step; CSSS should be considered when the pressure gradient is > 20 mm Hg.
Other strategies include ultrasonic duplex scanning with provocation test using arm exercise or reactive hyperemia.13 Many high-volume centers are performing screening by proximal subclavian angiography in all patients undergoing coronary angiography. When significant disease is detected, arch aortography and 4-vessel cerebral angiography is performed.6 In addition, other centers have adopted the routine use of computerized tomographic angiography before CABG.14
Surgical correction of CSSS is considered to be the gold standard and can be accomplished by performing aorta-subclavian bypass, carotid-subclavian bypass, axillo-axillary bypass, or relocation of the IMA graft.2 Although this approach is invasive and carries many disadvantages related to patient comfort,surgical revascularization can be performed safely at the time of CABG and may not carry additional risk of morbidity or mortality.15 Moreover, surgical correction is the preferred modality for treatment of CSSS when the anatomy is not favorable for percutaneous intervention, such as chronic total occlusion of the subclavian artery.15Alternatively, CSSS can effectively be managed less invasively by percutaneous intervention, including PTA with stent placement,16,17 thrombectomy18 or atherectomy of the stenotic subclavian artery.19
In this patient, PTA was performed with primary stent placement. The lesion was crossed with a sheath, using combined femoral and radial access. After proper positioning, a balloon-expandable stent was deployed that resulted in complete angiographic resolution of the lesion and improvement of symptoms at 6-month follow-up. In line with previous reports, this case demonstrated that percutaneous intervention is a feasible and less invasive approach for management of CSSS.16,17 The effectiveness of the percutaneous approach has effectiveness equivalent to surgical bypass with minimal complications and good long-term success. Therefore, it has been suggested as first-line therapy in CSSS.8,16
Although preoperative screening for brachiocephalic disease before undergoing ipsilateral IMA coronary artery bypass can prevent the development of CSSS, there is controversy about the best approach for managing these concomitant conditions. Many institutions use all-vein coronary conduits, but that forgoes the benefit of a LIMA graft. Therefore, others still perform an IMA conduit after brachiocephalic reconstruction. An alternative method is to use free IMA or radial artery conduit. Currently, there are limited data about the use of endovascular treatment for brachiocephalic disease with a CABG.2
Conclusion
Coronary-subclavian steal syndrome is an important clinical condition that is associated with significant morbidity and mortality. In the Sullivan and colleagues report of 27 patients with CSSS, 59.3% had stable angina and 40.7% had acute coronary syndrome, among which 14.8% presented with acute MI.7 Therefore, early recognition is essential to prevent catastrophic consequences.
Patients with CSSS usually present with cardiac symptoms, but symptoms related to vertebral-subclavian steal and posterior cerebral insufficiency can coexist. The authors suggest routine preoperative screening for the presence of brachiocephalic disease, using ultrasonic duplex or angiography. This practice is cost-effective and essential to prevent the development of CSSS. Optimal management of brachiocephalic disease prior to CABG is debatable; however, IMA grafting and reconstruction of the brachiocephalic system seems to be a promising approach.
When CSSS develops after CABG, the condition can be successfully treated with percutaneous intervention and outcomes comparable with those of surgical bypass.
Acknowledgments
Special thanks to the division of cardiology at New Jersey VA Health Care System, in particular Steve Tsai, MD; Ronald L. Vaillancourt, RN, and Preciosa Yap, RN.
1. Marques KM, Ernst SM, Mast EG, Bal ET, Suttorp MJ, Plokker HW. Percutaneous transluminal angioplasty of the left subclavian artery to prevent or treat the coronary-subclavian steal syndrome. Am J Cardiol. 1996;78(6):687-690.
2. Takach TJ, Reul GJ, Cooley DA, et al. Myocardial thievery: the coronary-subclavian steal syndrome. Ann Thorac Surg. 2006;81(1):386-392.
3. Harjola PT, Valle M. The importance of aortic arch or subclavian angiography before coronary reconstruction. Chest. 1974;66(4):436-438.
4. Tyras DH, Barner HB. Coronary-subclavian steal. Arch Surg. 1977;112(9):1125-1127.
5. Brown AH. Coronary steal by internal mammary graft with subclavian stenosis. J Thorac Cardiovasc Surg. 1977;73(5):690-693.
6. Takach TJ, Reul GJ, Duncan JM, et al. Concomitant brachiocephalic and coronary artery disease: outcome and decision analysis. Ann Thorac Surg. 2005;80(2):564-569.
7. Sullivan TM, Gray BH, Bacharach JM, et al. Angioplasty and primary stenting of the subclavian, innominate, and common carotid arteries in 83 patients. J Vasc Surg. 1998;28(6):1059-1065.
8. Hwang HY, Kim JH, Lee W, Park JH, Kim KB. Left subclavian artery stenosis in coronary artery bypass: prevalence and revascularization strategies. Ann Thorac Surg. 2010;89(4):1146-11 50.
9. Zeff RH, Kongtahworn C, Iannone LA, et al. Internal mammary artery versus saphenous vein graft to the left anterior descending coronary artery: prospective randomized study with 10-year follow-up. Ann Thorac Surg.1988;45(5):533-536.
10. Loop FD, Lytle BW, Cosgrove DM, et al. Influence of the internal-mammary-artery graft on 10-year survival and other cardiac events. N Engl J Med. 1986;314(1):1-6.
11. Lee SR, Jeong MH, Rhew JY, et al. Simultaneous coronary-subclavian and vertebral-subclavian steal syndrome. Circ J. 2003;67(5):464-466.
12. Takach TJ, Beggs ML, Nykamp VJ, Reul GJ Jr. Concomitant cerebral and coronary subclavian steal. Ann Thorac Surg. 1997;63(3):853-854.
13. Branchereau A, Magnan PE, Espinoza H, Bartoli JM. Subclavian artery stenosis: hemodynamic aspects and surgical outcome. J Cardiovasc Surg (Torino). 1991;32(5):604-661.
14. Park KH, Lee HY, Lim C, et al. Clinical impact of computerised tomographic angiography performed for preoperative evaluation before coronary artery bypass grafting. Eur J Cardiothorac Surg. 2010;37(6):1346-1352.
15. Sintek M, Coverstone E, Singh J. Coronary subclavian steal syndrome. Curr Opin Cardiol. 2014;29(6):506-513.
16. Eisenhauer AC. Subclavian and innominate revascularization: surgical therapy versus catheter-based intervention. Curr Interv Cardiol Rep. 2000;2(2):101-110.
17. Bates MC, Broce M, Lavigne PS, Stone P. Subclavian artery stenting: factors influencing long-term outcome. Catheter Cardiovasc Interv. 2004;61(1):5-11.
18. Zeller T, Frank U, Burgelin K, Sinn L, Horn B, Roskamm H. Acute thrombotic subclavian artery occlusion treated with a new rotational thrombectomy device. J Endovasc Ther. 2002;9(6):917-921.
19. Breall JA, Grossman W, Stillman IE, Gianturco LE, Kim D. Atherectomy of the subclavian artery for patients with symptomatic coronary-subclavian steal syndrome. J Am Coll Cardiol. 1993;21(7):1564-1567.
1. Marques KM, Ernst SM, Mast EG, Bal ET, Suttorp MJ, Plokker HW. Percutaneous transluminal angioplasty of the left subclavian artery to prevent or treat the coronary-subclavian steal syndrome. Am J Cardiol. 1996;78(6):687-690.
2. Takach TJ, Reul GJ, Cooley DA, et al. Myocardial thievery: the coronary-subclavian steal syndrome. Ann Thorac Surg. 2006;81(1):386-392.
3. Harjola PT, Valle M. The importance of aortic arch or subclavian angiography before coronary reconstruction. Chest. 1974;66(4):436-438.
4. Tyras DH, Barner HB. Coronary-subclavian steal. Arch Surg. 1977;112(9):1125-1127.
5. Brown AH. Coronary steal by internal mammary graft with subclavian stenosis. J Thorac Cardiovasc Surg. 1977;73(5):690-693.
6. Takach TJ, Reul GJ, Duncan JM, et al. Concomitant brachiocephalic and coronary artery disease: outcome and decision analysis. Ann Thorac Surg. 2005;80(2):564-569.
7. Sullivan TM, Gray BH, Bacharach JM, et al. Angioplasty and primary stenting of the subclavian, innominate, and common carotid arteries in 83 patients. J Vasc Surg. 1998;28(6):1059-1065.
8. Hwang HY, Kim JH, Lee W, Park JH, Kim KB. Left subclavian artery stenosis in coronary artery bypass: prevalence and revascularization strategies. Ann Thorac Surg. 2010;89(4):1146-11 50.
9. Zeff RH, Kongtahworn C, Iannone LA, et al. Internal mammary artery versus saphenous vein graft to the left anterior descending coronary artery: prospective randomized study with 10-year follow-up. Ann Thorac Surg.1988;45(5):533-536.
10. Loop FD, Lytle BW, Cosgrove DM, et al. Influence of the internal-mammary-artery graft on 10-year survival and other cardiac events. N Engl J Med. 1986;314(1):1-6.
11. Lee SR, Jeong MH, Rhew JY, et al. Simultaneous coronary-subclavian and vertebral-subclavian steal syndrome. Circ J. 2003;67(5):464-466.
12. Takach TJ, Beggs ML, Nykamp VJ, Reul GJ Jr. Concomitant cerebral and coronary subclavian steal. Ann Thorac Surg. 1997;63(3):853-854.
13. Branchereau A, Magnan PE, Espinoza H, Bartoli JM. Subclavian artery stenosis: hemodynamic aspects and surgical outcome. J Cardiovasc Surg (Torino). 1991;32(5):604-661.
14. Park KH, Lee HY, Lim C, et al. Clinical impact of computerised tomographic angiography performed for preoperative evaluation before coronary artery bypass grafting. Eur J Cardiothorac Surg. 2010;37(6):1346-1352.
15. Sintek M, Coverstone E, Singh J. Coronary subclavian steal syndrome. Curr Opin Cardiol. 2014;29(6):506-513.
16. Eisenhauer AC. Subclavian and innominate revascularization: surgical therapy versus catheter-based intervention. Curr Interv Cardiol Rep. 2000;2(2):101-110.
17. Bates MC, Broce M, Lavigne PS, Stone P. Subclavian artery stenting: factors influencing long-term outcome. Catheter Cardiovasc Interv. 2004;61(1):5-11.
18. Zeller T, Frank U, Burgelin K, Sinn L, Horn B, Roskamm H. Acute thrombotic subclavian artery occlusion treated with a new rotational thrombectomy device. J Endovasc Ther. 2002;9(6):917-921.
19. Breall JA, Grossman W, Stillman IE, Gianturco LE, Kim D. Atherectomy of the subclavian artery for patients with symptomatic coronary-subclavian steal syndrome. J Am Coll Cardiol. 1993;21(7):1564-1567.
Diagnosis at a Glance: Partial Hydatidiform Molar Pregnancy
Case
A 26-year-old gravida 3, para 2-0-0-2, aborta 0 whose last menstrual period was 15 weeks 5 days, presented to the ED with complaints of mild vaginal spotting, which she first noted postcoitally the previous day. The patient denied fatigue, lightheadedness, dyspnea, abdominal pain, nausea, or vomiting.
Physical examination revealed a well-appearing patient with normal vital signs. The abdomen was soft and nontender, and the fundus was palpable at the level of the umbilicus. A speculum examination was unremarkable, with normal external genitalia, a closed cervical os, no adnexal masses or tenderness, and no blood in the vaginal vault. Laboratory studies were significant for a serum beta human chorionic gonadotropin (beta-hCG) of 7,442 mIU/mL (reference range for 15 weeks: 12,039-70,971 mIU/mL). The patient was Rh positive with a stable hematocrit.
A bedside ultrasound, performed by an ultrasound-]trained emergency physician (EP), was noted to demonstrate a complex intrauterine mass comprised of several small, rounded anechoic clusters (Figure).
An obstetric consultation was made and the patient was taken to the operating room the following day for a dilation and curettage (D&C) procedure. She was discharged home the next day without complications. The products of conception were sent to pathology, and confirmed a triploid karyotype and p57 trophoblastic immunopositivity, diagnostic of a partial hydatidiform mole.
Discussion
Hydatidiform moles are a subset of abnormal pregnancies termed gestational trophoblastic disease (GTD). The two greatest risk factors for GTD are previous GTD and extremis of maternal age.1 Patients often present to the ED because of painless heavy vaginal bleeding, hyperemesis gravidarum, symptoms of hyperthyroidism, or preeclampsia before 20 weeks.2 Clinically, these patients present with an enlarged uterus for gestational age and very high beta-hCG levels, often greater than 100,000 mIU/mL.3 The high beta-hCG levels can lead the patient to present with symptoms of hyperthyroidism, such as severe hypertension, given the similar chemical structures of beta-hCG and thyroid-stimulating hormone.4
After a D&C, interval beta-hCG levels need to be obtained to ensure resolution. A patient with beta-hCG levels that do not fall by 10% after 3 weeks, or are still present after 6 months, should be referred to a gynecologic oncologist.5,6 Furthermore, a chest X-ray is strongly suggested, as the lungs are often the first place of metastasis.7
Partial hydatidiform moles are formed by a dispermic fertilization of a normal ovum leading to a triploid pattern, and are clinically distinguished from complete molar pregnancies because affected patients have a uterus that is often small for gestational age.8 Also, while the beta-hCG is also abnormally elevated, the median value is more modest at approximately 50,000 mIU/mL.3
According to the American College of Radiology’s Appropriateness Criteria, ultrasound is the gold standard for evaluating gestational trophoblastic disease. While the classic sonographic appearance of a molar pregnancy is described as a “snowstorm” appearance, advancement in technology more clearly demonstrates a “cluster of grapes” or “honeycomb” appearance.9 On Doppler mode, increased vascularity peripherally can also be detected due to engorgement of the spiral arteries. While partial moles tend to have more focal lesions, the greatest distinguishing factor is the presence of embryonic or fetal tissue, which is not seen in complete moles. However, due to the heterogeneous appearance of the uterus in all GTD, molar pregnancies can sometimes be misinterpreted as missed abortions or clotted blood, so that pathological confirmation is mandatory for all products of conception in the United States and Canada.2,10
Summary
This case is of particular interest because it demonstrates an atypical presentation of a partial hydatidiform mole. While most classic presentations include older patients with heavy vaginal bleeding, a smaller uterus than expected, significantly elevated beta-hCGs, and hyperemesis gravidarum, our patient was relatively young with no history of molar pregnancies in the past, a larger-than-expected uterus, and no vaginal bleeding noted. Laboratory values also indicated a significantly lower-than-expected beta-hCG level. As such, bedside ultrasound findings were unexpected but resulted in the prompt diagnosis, an emergent obstetric consultation, and confirmatory radiology imaging. The ED bedside ultrasound findings did demonstrate the characteristic “cluster of grapes” appearance surrounded by the hyperechoic appearance of the spiral arteries (Figure). An intrauterine yolk sac was also identified by ultrasound, which strongly suggested a partial rather than a complete hydatidiform molar pregnancy.
While hydatidiform pregnancies are relatively rare, EPs should be aware of the clinical and sonographic features of these diseases. This case, particularly given the atypical clinical presentation for a partial molar pregnancy, highlights the importance of ultrasound in pregnancy, and the utility of bedside ultrasound in the evaluation of the etiology of vaginal bleeding in the early pregnant patient that presents to the ED.
1. Ngan H, Bender H, Benedet JL, et al. Gestational trophoblastic neoplasia, FIGO 2000 staging and classification. Int J Gynaecol Obstet. 2003;83 Suppl 1:175-177.
2. Tie W, Tajnert K, Plavsic SK. Ultrasound imaging of gestational trophoblastic disease. Donald School J Ultrasound Obstet Gynecol. 2013;7(1):105-112.
3. Berkowitz RS, Goldstein DP. Current advances in the management of gestational trophoblastic disease. Gynecol Oncol. 2013;128(1):3-5.
4. Cole LA, Butler S. Detection of hCG in trophoblastic disease: The USA hCG reference service experience. J Reprod Med. 2002;47(6):433-444.
5. Lavie I, Rao GG, Castrillon DH, Miller DS, Schorge JO. Duration of human chorionic gonadotropin surveillance for partial hydatidiform moles. Am J Obstet Gynecol. 2005;192(5):1362-1364.
6. Kenny L, Seckl MJ. Treatments for gestational trophoblastic disease. Expert Rev of Obstet Gynecol. 2010;5(2):215-225.
7. Soto-Wright V, Bernstein M, Goldstein DP, Berkowitz RS. The changing clinical presentation of complete molar pregnancy. Obstet Gynecol. 1995;86(5):775-779.
8. Berkowitz RS, Goldstein DP. Clinical practice. Molar pregnancy. N Engl J Med. 2009;360(16):1639-1645. doi: 10.1056/NEJMcp0900696.
9. Kirk E, Papageorghiou AT, Condous G, Bottomley C, Bourne T. The accuracy of first trimester ultrasound in the diagnosis of hydatidiform mole. Ultrasound Obstet Gynecol. 2007;29(1):70-75.
10. Wang Y, Zhao S. Vascular Biology of the Placenta. Chapter 4. Cell Types of the Placenta. San Rafael, CA: Morgan & Claypool Life Sciences; 2010.
Case
A 26-year-old gravida 3, para 2-0-0-2, aborta 0 whose last menstrual period was 15 weeks 5 days, presented to the ED with complaints of mild vaginal spotting, which she first noted postcoitally the previous day. The patient denied fatigue, lightheadedness, dyspnea, abdominal pain, nausea, or vomiting.
Physical examination revealed a well-appearing patient with normal vital signs. The abdomen was soft and nontender, and the fundus was palpable at the level of the umbilicus. A speculum examination was unremarkable, with normal external genitalia, a closed cervical os, no adnexal masses or tenderness, and no blood in the vaginal vault. Laboratory studies were significant for a serum beta human chorionic gonadotropin (beta-hCG) of 7,442 mIU/mL (reference range for 15 weeks: 12,039-70,971 mIU/mL). The patient was Rh positive with a stable hematocrit.
A bedside ultrasound, performed by an ultrasound-]trained emergency physician (EP), was noted to demonstrate a complex intrauterine mass comprised of several small, rounded anechoic clusters (Figure).
An obstetric consultation was made and the patient was taken to the operating room the following day for a dilation and curettage (D&C) procedure. She was discharged home the next day without complications. The products of conception were sent to pathology, and confirmed a triploid karyotype and p57 trophoblastic immunopositivity, diagnostic of a partial hydatidiform mole.
Discussion
Hydatidiform moles are a subset of abnormal pregnancies termed gestational trophoblastic disease (GTD). The two greatest risk factors for GTD are previous GTD and extremis of maternal age.1 Patients often present to the ED because of painless heavy vaginal bleeding, hyperemesis gravidarum, symptoms of hyperthyroidism, or preeclampsia before 20 weeks.2 Clinically, these patients present with an enlarged uterus for gestational age and very high beta-hCG levels, often greater than 100,000 mIU/mL.3 The high beta-hCG levels can lead the patient to present with symptoms of hyperthyroidism, such as severe hypertension, given the similar chemical structures of beta-hCG and thyroid-stimulating hormone.4
After a D&C, interval beta-hCG levels need to be obtained to ensure resolution. A patient with beta-hCG levels that do not fall by 10% after 3 weeks, or are still present after 6 months, should be referred to a gynecologic oncologist.5,6 Furthermore, a chest X-ray is strongly suggested, as the lungs are often the first place of metastasis.7
Partial hydatidiform moles are formed by a dispermic fertilization of a normal ovum leading to a triploid pattern, and are clinically distinguished from complete molar pregnancies because affected patients have a uterus that is often small for gestational age.8 Also, while the beta-hCG is also abnormally elevated, the median value is more modest at approximately 50,000 mIU/mL.3
According to the American College of Radiology’s Appropriateness Criteria, ultrasound is the gold standard for evaluating gestational trophoblastic disease. While the classic sonographic appearance of a molar pregnancy is described as a “snowstorm” appearance, advancement in technology more clearly demonstrates a “cluster of grapes” or “honeycomb” appearance.9 On Doppler mode, increased vascularity peripherally can also be detected due to engorgement of the spiral arteries. While partial moles tend to have more focal lesions, the greatest distinguishing factor is the presence of embryonic or fetal tissue, which is not seen in complete moles. However, due to the heterogeneous appearance of the uterus in all GTD, molar pregnancies can sometimes be misinterpreted as missed abortions or clotted blood, so that pathological confirmation is mandatory for all products of conception in the United States and Canada.2,10
Summary
This case is of particular interest because it demonstrates an atypical presentation of a partial hydatidiform mole. While most classic presentations include older patients with heavy vaginal bleeding, a smaller uterus than expected, significantly elevated beta-hCGs, and hyperemesis gravidarum, our patient was relatively young with no history of molar pregnancies in the past, a larger-than-expected uterus, and no vaginal bleeding noted. Laboratory values also indicated a significantly lower-than-expected beta-hCG level. As such, bedside ultrasound findings were unexpected but resulted in the prompt diagnosis, an emergent obstetric consultation, and confirmatory radiology imaging. The ED bedside ultrasound findings did demonstrate the characteristic “cluster of grapes” appearance surrounded by the hyperechoic appearance of the spiral arteries (Figure). An intrauterine yolk sac was also identified by ultrasound, which strongly suggested a partial rather than a complete hydatidiform molar pregnancy.
While hydatidiform pregnancies are relatively rare, EPs should be aware of the clinical and sonographic features of these diseases. This case, particularly given the atypical clinical presentation for a partial molar pregnancy, highlights the importance of ultrasound in pregnancy, and the utility of bedside ultrasound in the evaluation of the etiology of vaginal bleeding in the early pregnant patient that presents to the ED.
Case
A 26-year-old gravida 3, para 2-0-0-2, aborta 0 whose last menstrual period was 15 weeks 5 days, presented to the ED with complaints of mild vaginal spotting, which she first noted postcoitally the previous day. The patient denied fatigue, lightheadedness, dyspnea, abdominal pain, nausea, or vomiting.
Physical examination revealed a well-appearing patient with normal vital signs. The abdomen was soft and nontender, and the fundus was palpable at the level of the umbilicus. A speculum examination was unremarkable, with normal external genitalia, a closed cervical os, no adnexal masses or tenderness, and no blood in the vaginal vault. Laboratory studies were significant for a serum beta human chorionic gonadotropin (beta-hCG) of 7,442 mIU/mL (reference range for 15 weeks: 12,039-70,971 mIU/mL). The patient was Rh positive with a stable hematocrit.
A bedside ultrasound, performed by an ultrasound-]trained emergency physician (EP), was noted to demonstrate a complex intrauterine mass comprised of several small, rounded anechoic clusters (Figure).
An obstetric consultation was made and the patient was taken to the operating room the following day for a dilation and curettage (D&C) procedure. She was discharged home the next day without complications. The products of conception were sent to pathology, and confirmed a triploid karyotype and p57 trophoblastic immunopositivity, diagnostic of a partial hydatidiform mole.
Discussion
Hydatidiform moles are a subset of abnormal pregnancies termed gestational trophoblastic disease (GTD). The two greatest risk factors for GTD are previous GTD and extremis of maternal age.1 Patients often present to the ED because of painless heavy vaginal bleeding, hyperemesis gravidarum, symptoms of hyperthyroidism, or preeclampsia before 20 weeks.2 Clinically, these patients present with an enlarged uterus for gestational age and very high beta-hCG levels, often greater than 100,000 mIU/mL.3 The high beta-hCG levels can lead the patient to present with symptoms of hyperthyroidism, such as severe hypertension, given the similar chemical structures of beta-hCG and thyroid-stimulating hormone.4
After a D&C, interval beta-hCG levels need to be obtained to ensure resolution. A patient with beta-hCG levels that do not fall by 10% after 3 weeks, or are still present after 6 months, should be referred to a gynecologic oncologist.5,6 Furthermore, a chest X-ray is strongly suggested, as the lungs are often the first place of metastasis.7
Partial hydatidiform moles are formed by a dispermic fertilization of a normal ovum leading to a triploid pattern, and are clinically distinguished from complete molar pregnancies because affected patients have a uterus that is often small for gestational age.8 Also, while the beta-hCG is also abnormally elevated, the median value is more modest at approximately 50,000 mIU/mL.3
According to the American College of Radiology’s Appropriateness Criteria, ultrasound is the gold standard for evaluating gestational trophoblastic disease. While the classic sonographic appearance of a molar pregnancy is described as a “snowstorm” appearance, advancement in technology more clearly demonstrates a “cluster of grapes” or “honeycomb” appearance.9 On Doppler mode, increased vascularity peripherally can also be detected due to engorgement of the spiral arteries. While partial moles tend to have more focal lesions, the greatest distinguishing factor is the presence of embryonic or fetal tissue, which is not seen in complete moles. However, due to the heterogeneous appearance of the uterus in all GTD, molar pregnancies can sometimes be misinterpreted as missed abortions or clotted blood, so that pathological confirmation is mandatory for all products of conception in the United States and Canada.2,10
Summary
This case is of particular interest because it demonstrates an atypical presentation of a partial hydatidiform mole. While most classic presentations include older patients with heavy vaginal bleeding, a smaller uterus than expected, significantly elevated beta-hCGs, and hyperemesis gravidarum, our patient was relatively young with no history of molar pregnancies in the past, a larger-than-expected uterus, and no vaginal bleeding noted. Laboratory values also indicated a significantly lower-than-expected beta-hCG level. As such, bedside ultrasound findings were unexpected but resulted in the prompt diagnosis, an emergent obstetric consultation, and confirmatory radiology imaging. The ED bedside ultrasound findings did demonstrate the characteristic “cluster of grapes” appearance surrounded by the hyperechoic appearance of the spiral arteries (Figure). An intrauterine yolk sac was also identified by ultrasound, which strongly suggested a partial rather than a complete hydatidiform molar pregnancy.
While hydatidiform pregnancies are relatively rare, EPs should be aware of the clinical and sonographic features of these diseases. This case, particularly given the atypical clinical presentation for a partial molar pregnancy, highlights the importance of ultrasound in pregnancy, and the utility of bedside ultrasound in the evaluation of the etiology of vaginal bleeding in the early pregnant patient that presents to the ED.
1. Ngan H, Bender H, Benedet JL, et al. Gestational trophoblastic neoplasia, FIGO 2000 staging and classification. Int J Gynaecol Obstet. 2003;83 Suppl 1:175-177.
2. Tie W, Tajnert K, Plavsic SK. Ultrasound imaging of gestational trophoblastic disease. Donald School J Ultrasound Obstet Gynecol. 2013;7(1):105-112.
3. Berkowitz RS, Goldstein DP. Current advances in the management of gestational trophoblastic disease. Gynecol Oncol. 2013;128(1):3-5.
4. Cole LA, Butler S. Detection of hCG in trophoblastic disease: The USA hCG reference service experience. J Reprod Med. 2002;47(6):433-444.
5. Lavie I, Rao GG, Castrillon DH, Miller DS, Schorge JO. Duration of human chorionic gonadotropin surveillance for partial hydatidiform moles. Am J Obstet Gynecol. 2005;192(5):1362-1364.
6. Kenny L, Seckl MJ. Treatments for gestational trophoblastic disease. Expert Rev of Obstet Gynecol. 2010;5(2):215-225.
7. Soto-Wright V, Bernstein M, Goldstein DP, Berkowitz RS. The changing clinical presentation of complete molar pregnancy. Obstet Gynecol. 1995;86(5):775-779.
8. Berkowitz RS, Goldstein DP. Clinical practice. Molar pregnancy. N Engl J Med. 2009;360(16):1639-1645. doi: 10.1056/NEJMcp0900696.
9. Kirk E, Papageorghiou AT, Condous G, Bottomley C, Bourne T. The accuracy of first trimester ultrasound in the diagnosis of hydatidiform mole. Ultrasound Obstet Gynecol. 2007;29(1):70-75.
10. Wang Y, Zhao S. Vascular Biology of the Placenta. Chapter 4. Cell Types of the Placenta. San Rafael, CA: Morgan & Claypool Life Sciences; 2010.
1. Ngan H, Bender H, Benedet JL, et al. Gestational trophoblastic neoplasia, FIGO 2000 staging and classification. Int J Gynaecol Obstet. 2003;83 Suppl 1:175-177.
2. Tie W, Tajnert K, Plavsic SK. Ultrasound imaging of gestational trophoblastic disease. Donald School J Ultrasound Obstet Gynecol. 2013;7(1):105-112.
3. Berkowitz RS, Goldstein DP. Current advances in the management of gestational trophoblastic disease. Gynecol Oncol. 2013;128(1):3-5.
4. Cole LA, Butler S. Detection of hCG in trophoblastic disease: The USA hCG reference service experience. J Reprod Med. 2002;47(6):433-444.
5. Lavie I, Rao GG, Castrillon DH, Miller DS, Schorge JO. Duration of human chorionic gonadotropin surveillance for partial hydatidiform moles. Am J Obstet Gynecol. 2005;192(5):1362-1364.
6. Kenny L, Seckl MJ. Treatments for gestational trophoblastic disease. Expert Rev of Obstet Gynecol. 2010;5(2):215-225.
7. Soto-Wright V, Bernstein M, Goldstein DP, Berkowitz RS. The changing clinical presentation of complete molar pregnancy. Obstet Gynecol. 1995;86(5):775-779.
8. Berkowitz RS, Goldstein DP. Clinical practice. Molar pregnancy. N Engl J Med. 2009;360(16):1639-1645. doi: 10.1056/NEJMcp0900696.
9. Kirk E, Papageorghiou AT, Condous G, Bottomley C, Bourne T. The accuracy of first trimester ultrasound in the diagnosis of hydatidiform mole. Ultrasound Obstet Gynecol. 2007;29(1):70-75.
10. Wang Y, Zhao S. Vascular Biology of the Placenta. Chapter 4. Cell Types of the Placenta. San Rafael, CA: Morgan & Claypool Life Sciences; 2010.
Bedside Cardiac Ultrasound to Aid in Diagnosing Takotsubo Cardiomyopathy
Cardiac ultrasound is among the many beneficial applications of point-of-care (POC) ultrasound in the ED. This modality can prove extremely beneficial in evaluating the critically ill patient. For example, POC cardiac ultrasound not only permits the emergency physician (EP) to diagnose a pericardial effusion and cardiac tamponade, but also perform a pericardiocentesis.1 The EP can also employ beside ultrasound to estimate an ejection fraction (EF) almost as well as cardiology services,2 look for signs of right-heart strain in patients with pulmonary embolism (PE),3 and guide fluid management in patients who have septic shock.4 In addition to only taking a few minutes to perform, POC cardiac ultrasound can also drastically change the course of management in some patients. Our case illustrates the use of POC ultrasound to diagnose Takotsubo cardiomyopathy in a 64-year-old patient and guide management when she became unstable prior to cardiac catheterization.
Case
A 64-year-old white woman with a medical history of diabetes, obesity, and nephrolithiasis presented to the ED with chest pain and shortness of breath, which she stated had begun earlier in the day. The patient’s chest pain did not intensify upon exertion, but the shortness of breath worsened when she was in the supine position.
Three months prior, the patient had also presented to our ED with chest pain. Evaluation during that visit included a negative stress echocardiogram with an EF of 55%. At this second visit, an electrocardiogram (ECG) showed new T-wave inversions in the anterior, lateral, and inferior leads. Vital signs at presentation were: blood pressure, 107/63 mm Hg; heart rate, 100 beats/min; respiratory rate, 18 breaths/min; and temperature, 97.9°F. Oxygen saturation was 97% on room air when patient was sitting upright, but decreased to 90% when she was supine. A chest X-ray showed left basilar atelectasis with a trace effusion. Laboratory evaluation was remarkable for the following: troponin I, 2.99 ng/mL; D-dimer, 294 ng/mL; and brain natriuretic peptide, 559 pg/mL.
Given the patient’s vital signs and positive troponin I level, a computed tomography (CT) scan was ordered to assess for a PE. This was done despite the patient’s negative D-dimer results, as it was felt that she was not low-risk for PE. At the same time the CT scan was ordered, a POC cardiac ultrasound was performed to assess for signs of right heart strain.
Based on the ultrasound findings and a normal EF 3 months prior, there was concern for Takotsubo cardiomyopathy. The patient was further questioned as to the events surrounding the onset of her chest pain. She informed the EP the pain started when she learned that she might be evicted from her home.
The CT scan was negative for PE. The consulting cardiologist was informed of the results of the ultrasound findings, and the patient was given aspirin, heparin, morphine, and furosemide, and was admitted to the cardiac progressive unit. She was also initially given morphine for pain management, but due to intolerance, she was switched to nitroglycerin.
During the first evening of her inpatient stay, the patient experienced acute changes in her chest pain that resulted in activating the rapid response team. Secondary to the information gathered in the ED, the patient was managed conservatively and was evaluated by a physician extender who repeated laboratory studies, provided supplemental potassium and magnesium, and ordered another ECG in consultation with the cardiologist (who was caring for the patient via telephone). In the morning, the patient continued to have chest pain, and a repeat ECG showed worsening of previous T-wave inversions. Based on these findings, the cardiologist ordered cardiac catheterization.
On hospital day 2, the cardiologist performed another echocardiogram, which confirmed the low EF of 20% with severe global hypokinesis with sparing of the basal segments. Cardiac catheterization showed no significant disease (20% lesion in the mid-left anterior descending artery) with the left ventriculogram showing an EF of 10%, cardiac output of 3.7, and cardiac index of 1.8, confirming the diagnosis of Takotsubo cardiomyopathy. The patient remained in the hospital for a total of 8 days while awaiting a life vest; however, a repeat echocardiogram on hospital day 8 showed an EF of 55%.
Discussion
Takotsubo cardiomyopathy is an acute, stress-induced cardiomyopathy that was first described in Japan in the early 1990s.5 It is thought to be due to catecholamine-induced dysfunction from a stressful event,6-8 such as the death of a loved one, which is why it is often referred to as “broken heart syndrome.” However there are case reports highlighting other causes of Takotsubo cardiomyopathy, such as cocaine use,9 scuba diving,10 and diabetic ketoacidosis combined with hypothermia.11
Patients with Takotsubo cardiomyopathy will frequently have ECG abnormalities, including ST-segment elevation or depression, or T-wave changes; troponin levels also may be elevated. The majority of patients (>80%) are postmenopausal women, typically aged 50 to 75 years.6,12 Echocardiogram findings in Takotsubo cardiomyopathy show significant left ventricular (LV) dysfunction or regional dysfunction that is not in one coronary artery distribution.12,13 There will often be apical dilation or ballooning with dyskinesia but more preserved function at the base and normal dimensions.14,15 A negative cardiac catheterization or catheterization in the absence of significant disease is required to confirm the diagnosis.16 The LV function usually returns to baseline in 1 to 4 weeks, but there can be recurrence in some patients.6,17 The condition is also associated with a large burden of morbidity and mortality.6,18 In a case series by Gopalakrishnan et al6 of 56 patients, there was an 8.9% in-hospital mortality rate and an additional 17.9% out-of-hospital mortality rate even in patients in whom LV function had returned to normal.
In a review by Gianni et al,19 4.2% of patients with Takotsubo cardiomyopathy present with or go into cardiogenic shock at some point during admission, and up to 2% of patients who present with acute myocardial infarction have Takotsubo cardiomyopathy. Patients can go into cardiogenic shock due to depressed EF or LV outflow tract obstruction from hyperkinesis of the basilar segments. Some of these patients may be sent directly to the catheter laboratory based on ST elevations on ECG, in which case the diagnosis is made there. Our patient, however, did not have ST elevation and later became unstable on the floor. Citro et al20 suggest that a patient with a predisposition for Takotsubo cardiomyopathy (eg, postmenopausal patients, those who experienced a trigger event), in the right clinical setting and without ST-segment elevation on ECG, could be managed more conservatively with delayed cardiac angiography or CT angiography (CTA) evaluation of the coronary arteries (sparing the patient an invasive procedure)—as long as ultrasound was consistent with typical Takotsubo cardiomyopathy findings. However, CTA is still needed to make the diagnosis.
At this time, Takotsubo cardiomyopathy should remain an important part of the differential diagnosis for emergency patients who have chest pain—especially for postmenopausal women with a history of significant stressor—as early recognition can lead to better patient care.
Conclusion
This case highlights the importance of POC ultrasound in the management of patients in the ED and after admission. The care of our patient was enhanced by the ability to take a real-time look at her EF and cardiac function at the time of admission through bedside ultrasound. This information guided her management and optimized stabilization.
1. Goodman A, Perera P, Mailhot T, Mandavia D. The role of bedside ultrasound in the diagnosis of pericardial effusion and cardiac tamponade. J Emerg Trauma Shock. 2012;5(1):72-75. doi:10.4103/0974-2700.93118.
2. Unlüer EE, Karagöz A, Akoğlu H, Bayata S. Visual estimation of bedside echocardiographic ejection fraction by emergency physicians. West J Emerg Med. 2014;15(2):221-226. doi:10.5811/westjem.2013.9.16185.
3. McConnell MV, Solomon SD, Rayan ME, Come PC, Goldhaber SZ, Lee RT. Regional right ventricular dysfunction detected by echocardiography in acute pulmonary embolism. Am J Cardiol. 1996;78(4):469-473.
4. Coen D, Cortellaro F, Pasini S, et al. Towards a less invasive approach to the early goal-directed treatment of septic shock in the ED. Am J Emerg Med. 2014;32(6):563-568. doi:10.1016/j.ajem.2014.02.011.
5. Dote K, Sato H, Tateishi H, Uchida T, Ishihara M. [Myocardial stunning due to simultaneous multivessel coronary spasms: a review of 5 cases.] J Cardiol. 1991;21(2):203-214.
6. Gopalakrishnan M, Hassan A, Villines D, Nasr S, Chandrasekaran M, Klein LW. Predictors of short- and long-term outcomes of Takotsubo cardiomyopathy. Am J Cardiol. 2015;116(10):1586-1590. doi:10.1016/j.amjcard.2015.08.024.
7. Paur H, Wright PT, Sikkel MB, et al. High levels of circulating epinephrine trigger apical cardiodepression in a β2-adrenergic receptor/Gi-dependent manner: a new model of Takotsubo cardiomyopathy. Circulation. 2012;126(6):697-706. doi:10.1161/CIRCULATIONAHA.112.111591.
8. Wittstein IS, Thiemann DR, Lima JA, et al. Neurohumoral features of myocardial stunning due to sudden emotional stress. N Engl J Med. 2005;352(6):539-548. doi:10.1056/NEJMoa043046.
9. Butterfield M, Riguzzi C, Frenkel O, Nagdev A. Stimulant-related Takotsubo cardiomyopathy. Am J Emerg Med. 2015;33(3):476.e1-e3. doi:10.1016/j.ajem.2014.08.058.
10. Baber A, Nair SU, Duggal S, Bhatti S, Sundlof DW. Stress cardiomyopathy caused by diving: case report and review of the literature. J Emerg Med. 2016;50(2):277-280. doi:10.1016/j.jemermed.2015.09.045.
11. Katayama Y, Hifumi T, Inoue J, Koido Y. A case of Takotsubo cardiomyopathy induced by accidental hypothermia and diabetic ketoacidosis. BMJ Case Rep. 2013;2013:1-3. doi:10.1136/bcr-2012-008143.
12. Bybee KA, Kara T, Prasad A, et al. Systematic review: transient left ventricular apical ballooning: a syndrome that mimics ST-segment elevation myocardial infarction. Ann Intern Med. 2004;141(11):858-865.
13. Virani SS, Khan AN, Mendoza CE, Ferreira AC, de Marchena E. Takotsubo cardiomyopathy, or brokenheart syndrome. Tex Heart Inst J. 2007;34(1):76-79.
14. Okura H. Echocardiographic assessment of takotsubo cardiomyopathy: beyond apical ballooning. J Echocardiogr. 2016;14(1):13-20. doi:10.1007/s12574-015-0271-3.
15. Naser N, Buksa M, Kusljugic Z, Terzic I, Sokolovic S, Hodzic E. The role of echocardiography in diagnosis and follow up of patients with takotsubo cardiomyopathy or acute ballooning syndrome. Med Arh. 2011;65(5):287-290.
16. Ono R, Falcão LM. Takotsubo cardiomyopathy systematic review: Pathophysiologic process, clinical presentation and diagnostic approach to Takotsubo cardiomyopathy. Int J Cardiol. 2016;209:196-205. doi:10.1016/j.ijcard.2016.02.012.
17. Opolski G, Budnik M, Kochanowski J, Kowalik R, Piatkowski R, Kochman J. Four episodes of takotsubo cardiomyopathy in one patient. Int J Cardiol. 2016;203:53-54. doi:10.1016/j.ijcard.2015.10.048.
18. Templin C, Ghadri JR, Diekmann J, et al. Clinical features and outcomes of Takotsubo (stress) cardiomyopathy. N Engl J Med. 2015;373(10):929-938.
19. Gianni M, Dentali F, Grandi AM, Sumner G, Hiralal R, Lonn E. Apical ballooning syndrome or takotsubo cardiomyopathy: a systematic review. Eur Heart J. 2006;27(13):1523-1529. doi:10.1093/eurheartj/ehl032.
20. Citro R, Lyon AR, Meimoun P, et al. Standard and advanced echocardiography in Takotsubo (stress) cardiomyopathy: clinical and prognostic implications. J Am Soc Echocardiogr. 2015;28(1):57-74. doi:10.1016/j.echo.2014.08.020.
Cardiac ultrasound is among the many beneficial applications of point-of-care (POC) ultrasound in the ED. This modality can prove extremely beneficial in evaluating the critically ill patient. For example, POC cardiac ultrasound not only permits the emergency physician (EP) to diagnose a pericardial effusion and cardiac tamponade, but also perform a pericardiocentesis.1 The EP can also employ beside ultrasound to estimate an ejection fraction (EF) almost as well as cardiology services,2 look for signs of right-heart strain in patients with pulmonary embolism (PE),3 and guide fluid management in patients who have septic shock.4 In addition to only taking a few minutes to perform, POC cardiac ultrasound can also drastically change the course of management in some patients. Our case illustrates the use of POC ultrasound to diagnose Takotsubo cardiomyopathy in a 64-year-old patient and guide management when she became unstable prior to cardiac catheterization.
Case
A 64-year-old white woman with a medical history of diabetes, obesity, and nephrolithiasis presented to the ED with chest pain and shortness of breath, which she stated had begun earlier in the day. The patient’s chest pain did not intensify upon exertion, but the shortness of breath worsened when she was in the supine position.
Three months prior, the patient had also presented to our ED with chest pain. Evaluation during that visit included a negative stress echocardiogram with an EF of 55%. At this second visit, an electrocardiogram (ECG) showed new T-wave inversions in the anterior, lateral, and inferior leads. Vital signs at presentation were: blood pressure, 107/63 mm Hg; heart rate, 100 beats/min; respiratory rate, 18 breaths/min; and temperature, 97.9°F. Oxygen saturation was 97% on room air when patient was sitting upright, but decreased to 90% when she was supine. A chest X-ray showed left basilar atelectasis with a trace effusion. Laboratory evaluation was remarkable for the following: troponin I, 2.99 ng/mL; D-dimer, 294 ng/mL; and brain natriuretic peptide, 559 pg/mL.
Given the patient’s vital signs and positive troponin I level, a computed tomography (CT) scan was ordered to assess for a PE. This was done despite the patient’s negative D-dimer results, as it was felt that she was not low-risk for PE. At the same time the CT scan was ordered, a POC cardiac ultrasound was performed to assess for signs of right heart strain.
Based on the ultrasound findings and a normal EF 3 months prior, there was concern for Takotsubo cardiomyopathy. The patient was further questioned as to the events surrounding the onset of her chest pain. She informed the EP the pain started when she learned that she might be evicted from her home.
The CT scan was negative for PE. The consulting cardiologist was informed of the results of the ultrasound findings, and the patient was given aspirin, heparin, morphine, and furosemide, and was admitted to the cardiac progressive unit. She was also initially given morphine for pain management, but due to intolerance, she was switched to nitroglycerin.
During the first evening of her inpatient stay, the patient experienced acute changes in her chest pain that resulted in activating the rapid response team. Secondary to the information gathered in the ED, the patient was managed conservatively and was evaluated by a physician extender who repeated laboratory studies, provided supplemental potassium and magnesium, and ordered another ECG in consultation with the cardiologist (who was caring for the patient via telephone). In the morning, the patient continued to have chest pain, and a repeat ECG showed worsening of previous T-wave inversions. Based on these findings, the cardiologist ordered cardiac catheterization.
On hospital day 2, the cardiologist performed another echocardiogram, which confirmed the low EF of 20% with severe global hypokinesis with sparing of the basal segments. Cardiac catheterization showed no significant disease (20% lesion in the mid-left anterior descending artery) with the left ventriculogram showing an EF of 10%, cardiac output of 3.7, and cardiac index of 1.8, confirming the diagnosis of Takotsubo cardiomyopathy. The patient remained in the hospital for a total of 8 days while awaiting a life vest; however, a repeat echocardiogram on hospital day 8 showed an EF of 55%.
Discussion
Takotsubo cardiomyopathy is an acute, stress-induced cardiomyopathy that was first described in Japan in the early 1990s.5 It is thought to be due to catecholamine-induced dysfunction from a stressful event,6-8 such as the death of a loved one, which is why it is often referred to as “broken heart syndrome.” However there are case reports highlighting other causes of Takotsubo cardiomyopathy, such as cocaine use,9 scuba diving,10 and diabetic ketoacidosis combined with hypothermia.11
Patients with Takotsubo cardiomyopathy will frequently have ECG abnormalities, including ST-segment elevation or depression, or T-wave changes; troponin levels also may be elevated. The majority of patients (>80%) are postmenopausal women, typically aged 50 to 75 years.6,12 Echocardiogram findings in Takotsubo cardiomyopathy show significant left ventricular (LV) dysfunction or regional dysfunction that is not in one coronary artery distribution.12,13 There will often be apical dilation or ballooning with dyskinesia but more preserved function at the base and normal dimensions.14,15 A negative cardiac catheterization or catheterization in the absence of significant disease is required to confirm the diagnosis.16 The LV function usually returns to baseline in 1 to 4 weeks, but there can be recurrence in some patients.6,17 The condition is also associated with a large burden of morbidity and mortality.6,18 In a case series by Gopalakrishnan et al6 of 56 patients, there was an 8.9% in-hospital mortality rate and an additional 17.9% out-of-hospital mortality rate even in patients in whom LV function had returned to normal.
In a review by Gianni et al,19 4.2% of patients with Takotsubo cardiomyopathy present with or go into cardiogenic shock at some point during admission, and up to 2% of patients who present with acute myocardial infarction have Takotsubo cardiomyopathy. Patients can go into cardiogenic shock due to depressed EF or LV outflow tract obstruction from hyperkinesis of the basilar segments. Some of these patients may be sent directly to the catheter laboratory based on ST elevations on ECG, in which case the diagnosis is made there. Our patient, however, did not have ST elevation and later became unstable on the floor. Citro et al20 suggest that a patient with a predisposition for Takotsubo cardiomyopathy (eg, postmenopausal patients, those who experienced a trigger event), in the right clinical setting and without ST-segment elevation on ECG, could be managed more conservatively with delayed cardiac angiography or CT angiography (CTA) evaluation of the coronary arteries (sparing the patient an invasive procedure)—as long as ultrasound was consistent with typical Takotsubo cardiomyopathy findings. However, CTA is still needed to make the diagnosis.
At this time, Takotsubo cardiomyopathy should remain an important part of the differential diagnosis for emergency patients who have chest pain—especially for postmenopausal women with a history of significant stressor—as early recognition can lead to better patient care.
Conclusion
This case highlights the importance of POC ultrasound in the management of patients in the ED and after admission. The care of our patient was enhanced by the ability to take a real-time look at her EF and cardiac function at the time of admission through bedside ultrasound. This information guided her management and optimized stabilization.
Cardiac ultrasound is among the many beneficial applications of point-of-care (POC) ultrasound in the ED. This modality can prove extremely beneficial in evaluating the critically ill patient. For example, POC cardiac ultrasound not only permits the emergency physician (EP) to diagnose a pericardial effusion and cardiac tamponade, but also perform a pericardiocentesis.1 The EP can also employ beside ultrasound to estimate an ejection fraction (EF) almost as well as cardiology services,2 look for signs of right-heart strain in patients with pulmonary embolism (PE),3 and guide fluid management in patients who have septic shock.4 In addition to only taking a few minutes to perform, POC cardiac ultrasound can also drastically change the course of management in some patients. Our case illustrates the use of POC ultrasound to diagnose Takotsubo cardiomyopathy in a 64-year-old patient and guide management when she became unstable prior to cardiac catheterization.
Case
A 64-year-old white woman with a medical history of diabetes, obesity, and nephrolithiasis presented to the ED with chest pain and shortness of breath, which she stated had begun earlier in the day. The patient’s chest pain did not intensify upon exertion, but the shortness of breath worsened when she was in the supine position.
Three months prior, the patient had also presented to our ED with chest pain. Evaluation during that visit included a negative stress echocardiogram with an EF of 55%. At this second visit, an electrocardiogram (ECG) showed new T-wave inversions in the anterior, lateral, and inferior leads. Vital signs at presentation were: blood pressure, 107/63 mm Hg; heart rate, 100 beats/min; respiratory rate, 18 breaths/min; and temperature, 97.9°F. Oxygen saturation was 97% on room air when patient was sitting upright, but decreased to 90% when she was supine. A chest X-ray showed left basilar atelectasis with a trace effusion. Laboratory evaluation was remarkable for the following: troponin I, 2.99 ng/mL; D-dimer, 294 ng/mL; and brain natriuretic peptide, 559 pg/mL.
Given the patient’s vital signs and positive troponin I level, a computed tomography (CT) scan was ordered to assess for a PE. This was done despite the patient’s negative D-dimer results, as it was felt that she was not low-risk for PE. At the same time the CT scan was ordered, a POC cardiac ultrasound was performed to assess for signs of right heart strain.
Based on the ultrasound findings and a normal EF 3 months prior, there was concern for Takotsubo cardiomyopathy. The patient was further questioned as to the events surrounding the onset of her chest pain. She informed the EP the pain started when she learned that she might be evicted from her home.
The CT scan was negative for PE. The consulting cardiologist was informed of the results of the ultrasound findings, and the patient was given aspirin, heparin, morphine, and furosemide, and was admitted to the cardiac progressive unit. She was also initially given morphine for pain management, but due to intolerance, she was switched to nitroglycerin.
During the first evening of her inpatient stay, the patient experienced acute changes in her chest pain that resulted in activating the rapid response team. Secondary to the information gathered in the ED, the patient was managed conservatively and was evaluated by a physician extender who repeated laboratory studies, provided supplemental potassium and magnesium, and ordered another ECG in consultation with the cardiologist (who was caring for the patient via telephone). In the morning, the patient continued to have chest pain, and a repeat ECG showed worsening of previous T-wave inversions. Based on these findings, the cardiologist ordered cardiac catheterization.
On hospital day 2, the cardiologist performed another echocardiogram, which confirmed the low EF of 20% with severe global hypokinesis with sparing of the basal segments. Cardiac catheterization showed no significant disease (20% lesion in the mid-left anterior descending artery) with the left ventriculogram showing an EF of 10%, cardiac output of 3.7, and cardiac index of 1.8, confirming the diagnosis of Takotsubo cardiomyopathy. The patient remained in the hospital for a total of 8 days while awaiting a life vest; however, a repeat echocardiogram on hospital day 8 showed an EF of 55%.
Discussion
Takotsubo cardiomyopathy is an acute, stress-induced cardiomyopathy that was first described in Japan in the early 1990s.5 It is thought to be due to catecholamine-induced dysfunction from a stressful event,6-8 such as the death of a loved one, which is why it is often referred to as “broken heart syndrome.” However there are case reports highlighting other causes of Takotsubo cardiomyopathy, such as cocaine use,9 scuba diving,10 and diabetic ketoacidosis combined with hypothermia.11
Patients with Takotsubo cardiomyopathy will frequently have ECG abnormalities, including ST-segment elevation or depression, or T-wave changes; troponin levels also may be elevated. The majority of patients (>80%) are postmenopausal women, typically aged 50 to 75 years.6,12 Echocardiogram findings in Takotsubo cardiomyopathy show significant left ventricular (LV) dysfunction or regional dysfunction that is not in one coronary artery distribution.12,13 There will often be apical dilation or ballooning with dyskinesia but more preserved function at the base and normal dimensions.14,15 A negative cardiac catheterization or catheterization in the absence of significant disease is required to confirm the diagnosis.16 The LV function usually returns to baseline in 1 to 4 weeks, but there can be recurrence in some patients.6,17 The condition is also associated with a large burden of morbidity and mortality.6,18 In a case series by Gopalakrishnan et al6 of 56 patients, there was an 8.9% in-hospital mortality rate and an additional 17.9% out-of-hospital mortality rate even in patients in whom LV function had returned to normal.
In a review by Gianni et al,19 4.2% of patients with Takotsubo cardiomyopathy present with or go into cardiogenic shock at some point during admission, and up to 2% of patients who present with acute myocardial infarction have Takotsubo cardiomyopathy. Patients can go into cardiogenic shock due to depressed EF or LV outflow tract obstruction from hyperkinesis of the basilar segments. Some of these patients may be sent directly to the catheter laboratory based on ST elevations on ECG, in which case the diagnosis is made there. Our patient, however, did not have ST elevation and later became unstable on the floor. Citro et al20 suggest that a patient with a predisposition for Takotsubo cardiomyopathy (eg, postmenopausal patients, those who experienced a trigger event), in the right clinical setting and without ST-segment elevation on ECG, could be managed more conservatively with delayed cardiac angiography or CT angiography (CTA) evaluation of the coronary arteries (sparing the patient an invasive procedure)—as long as ultrasound was consistent with typical Takotsubo cardiomyopathy findings. However, CTA is still needed to make the diagnosis.
At this time, Takotsubo cardiomyopathy should remain an important part of the differential diagnosis for emergency patients who have chest pain—especially for postmenopausal women with a history of significant stressor—as early recognition can lead to better patient care.
Conclusion
This case highlights the importance of POC ultrasound in the management of patients in the ED and after admission. The care of our patient was enhanced by the ability to take a real-time look at her EF and cardiac function at the time of admission through bedside ultrasound. This information guided her management and optimized stabilization.
1. Goodman A, Perera P, Mailhot T, Mandavia D. The role of bedside ultrasound in the diagnosis of pericardial effusion and cardiac tamponade. J Emerg Trauma Shock. 2012;5(1):72-75. doi:10.4103/0974-2700.93118.
2. Unlüer EE, Karagöz A, Akoğlu H, Bayata S. Visual estimation of bedside echocardiographic ejection fraction by emergency physicians. West J Emerg Med. 2014;15(2):221-226. doi:10.5811/westjem.2013.9.16185.
3. McConnell MV, Solomon SD, Rayan ME, Come PC, Goldhaber SZ, Lee RT. Regional right ventricular dysfunction detected by echocardiography in acute pulmonary embolism. Am J Cardiol. 1996;78(4):469-473.
4. Coen D, Cortellaro F, Pasini S, et al. Towards a less invasive approach to the early goal-directed treatment of septic shock in the ED. Am J Emerg Med. 2014;32(6):563-568. doi:10.1016/j.ajem.2014.02.011.
5. Dote K, Sato H, Tateishi H, Uchida T, Ishihara M. [Myocardial stunning due to simultaneous multivessel coronary spasms: a review of 5 cases.] J Cardiol. 1991;21(2):203-214.
6. Gopalakrishnan M, Hassan A, Villines D, Nasr S, Chandrasekaran M, Klein LW. Predictors of short- and long-term outcomes of Takotsubo cardiomyopathy. Am J Cardiol. 2015;116(10):1586-1590. doi:10.1016/j.amjcard.2015.08.024.
7. Paur H, Wright PT, Sikkel MB, et al. High levels of circulating epinephrine trigger apical cardiodepression in a β2-adrenergic receptor/Gi-dependent manner: a new model of Takotsubo cardiomyopathy. Circulation. 2012;126(6):697-706. doi:10.1161/CIRCULATIONAHA.112.111591.
8. Wittstein IS, Thiemann DR, Lima JA, et al. Neurohumoral features of myocardial stunning due to sudden emotional stress. N Engl J Med. 2005;352(6):539-548. doi:10.1056/NEJMoa043046.
9. Butterfield M, Riguzzi C, Frenkel O, Nagdev A. Stimulant-related Takotsubo cardiomyopathy. Am J Emerg Med. 2015;33(3):476.e1-e3. doi:10.1016/j.ajem.2014.08.058.
10. Baber A, Nair SU, Duggal S, Bhatti S, Sundlof DW. Stress cardiomyopathy caused by diving: case report and review of the literature. J Emerg Med. 2016;50(2):277-280. doi:10.1016/j.jemermed.2015.09.045.
11. Katayama Y, Hifumi T, Inoue J, Koido Y. A case of Takotsubo cardiomyopathy induced by accidental hypothermia and diabetic ketoacidosis. BMJ Case Rep. 2013;2013:1-3. doi:10.1136/bcr-2012-008143.
12. Bybee KA, Kara T, Prasad A, et al. Systematic review: transient left ventricular apical ballooning: a syndrome that mimics ST-segment elevation myocardial infarction. Ann Intern Med. 2004;141(11):858-865.
13. Virani SS, Khan AN, Mendoza CE, Ferreira AC, de Marchena E. Takotsubo cardiomyopathy, or brokenheart syndrome. Tex Heart Inst J. 2007;34(1):76-79.
14. Okura H. Echocardiographic assessment of takotsubo cardiomyopathy: beyond apical ballooning. J Echocardiogr. 2016;14(1):13-20. doi:10.1007/s12574-015-0271-3.
15. Naser N, Buksa M, Kusljugic Z, Terzic I, Sokolovic S, Hodzic E. The role of echocardiography in diagnosis and follow up of patients with takotsubo cardiomyopathy or acute ballooning syndrome. Med Arh. 2011;65(5):287-290.
16. Ono R, Falcão LM. Takotsubo cardiomyopathy systematic review: Pathophysiologic process, clinical presentation and diagnostic approach to Takotsubo cardiomyopathy. Int J Cardiol. 2016;209:196-205. doi:10.1016/j.ijcard.2016.02.012.
17. Opolski G, Budnik M, Kochanowski J, Kowalik R, Piatkowski R, Kochman J. Four episodes of takotsubo cardiomyopathy in one patient. Int J Cardiol. 2016;203:53-54. doi:10.1016/j.ijcard.2015.10.048.
18. Templin C, Ghadri JR, Diekmann J, et al. Clinical features and outcomes of Takotsubo (stress) cardiomyopathy. N Engl J Med. 2015;373(10):929-938.
19. Gianni M, Dentali F, Grandi AM, Sumner G, Hiralal R, Lonn E. Apical ballooning syndrome or takotsubo cardiomyopathy: a systematic review. Eur Heart J. 2006;27(13):1523-1529. doi:10.1093/eurheartj/ehl032.
20. Citro R, Lyon AR, Meimoun P, et al. Standard and advanced echocardiography in Takotsubo (stress) cardiomyopathy: clinical and prognostic implications. J Am Soc Echocardiogr. 2015;28(1):57-74. doi:10.1016/j.echo.2014.08.020.
1. Goodman A, Perera P, Mailhot T, Mandavia D. The role of bedside ultrasound in the diagnosis of pericardial effusion and cardiac tamponade. J Emerg Trauma Shock. 2012;5(1):72-75. doi:10.4103/0974-2700.93118.
2. Unlüer EE, Karagöz A, Akoğlu H, Bayata S. Visual estimation of bedside echocardiographic ejection fraction by emergency physicians. West J Emerg Med. 2014;15(2):221-226. doi:10.5811/westjem.2013.9.16185.
3. McConnell MV, Solomon SD, Rayan ME, Come PC, Goldhaber SZ, Lee RT. Regional right ventricular dysfunction detected by echocardiography in acute pulmonary embolism. Am J Cardiol. 1996;78(4):469-473.
4. Coen D, Cortellaro F, Pasini S, et al. Towards a less invasive approach to the early goal-directed treatment of septic shock in the ED. Am J Emerg Med. 2014;32(6):563-568. doi:10.1016/j.ajem.2014.02.011.
5. Dote K, Sato H, Tateishi H, Uchida T, Ishihara M. [Myocardial stunning due to simultaneous multivessel coronary spasms: a review of 5 cases.] J Cardiol. 1991;21(2):203-214.
6. Gopalakrishnan M, Hassan A, Villines D, Nasr S, Chandrasekaran M, Klein LW. Predictors of short- and long-term outcomes of Takotsubo cardiomyopathy. Am J Cardiol. 2015;116(10):1586-1590. doi:10.1016/j.amjcard.2015.08.024.
7. Paur H, Wright PT, Sikkel MB, et al. High levels of circulating epinephrine trigger apical cardiodepression in a β2-adrenergic receptor/Gi-dependent manner: a new model of Takotsubo cardiomyopathy. Circulation. 2012;126(6):697-706. doi:10.1161/CIRCULATIONAHA.112.111591.
8. Wittstein IS, Thiemann DR, Lima JA, et al. Neurohumoral features of myocardial stunning due to sudden emotional stress. N Engl J Med. 2005;352(6):539-548. doi:10.1056/NEJMoa043046.
9. Butterfield M, Riguzzi C, Frenkel O, Nagdev A. Stimulant-related Takotsubo cardiomyopathy. Am J Emerg Med. 2015;33(3):476.e1-e3. doi:10.1016/j.ajem.2014.08.058.
10. Baber A, Nair SU, Duggal S, Bhatti S, Sundlof DW. Stress cardiomyopathy caused by diving: case report and review of the literature. J Emerg Med. 2016;50(2):277-280. doi:10.1016/j.jemermed.2015.09.045.
11. Katayama Y, Hifumi T, Inoue J, Koido Y. A case of Takotsubo cardiomyopathy induced by accidental hypothermia and diabetic ketoacidosis. BMJ Case Rep. 2013;2013:1-3. doi:10.1136/bcr-2012-008143.
12. Bybee KA, Kara T, Prasad A, et al. Systematic review: transient left ventricular apical ballooning: a syndrome that mimics ST-segment elevation myocardial infarction. Ann Intern Med. 2004;141(11):858-865.
13. Virani SS, Khan AN, Mendoza CE, Ferreira AC, de Marchena E. Takotsubo cardiomyopathy, or brokenheart syndrome. Tex Heart Inst J. 2007;34(1):76-79.
14. Okura H. Echocardiographic assessment of takotsubo cardiomyopathy: beyond apical ballooning. J Echocardiogr. 2016;14(1):13-20. doi:10.1007/s12574-015-0271-3.
15. Naser N, Buksa M, Kusljugic Z, Terzic I, Sokolovic S, Hodzic E. The role of echocardiography in diagnosis and follow up of patients with takotsubo cardiomyopathy or acute ballooning syndrome. Med Arh. 2011;65(5):287-290.
16. Ono R, Falcão LM. Takotsubo cardiomyopathy systematic review: Pathophysiologic process, clinical presentation and diagnostic approach to Takotsubo cardiomyopathy. Int J Cardiol. 2016;209:196-205. doi:10.1016/j.ijcard.2016.02.012.
17. Opolski G, Budnik M, Kochanowski J, Kowalik R, Piatkowski R, Kochman J. Four episodes of takotsubo cardiomyopathy in one patient. Int J Cardiol. 2016;203:53-54. doi:10.1016/j.ijcard.2015.10.048.
18. Templin C, Ghadri JR, Diekmann J, et al. Clinical features and outcomes of Takotsubo (stress) cardiomyopathy. N Engl J Med. 2015;373(10):929-938.
19. Gianni M, Dentali F, Grandi AM, Sumner G, Hiralal R, Lonn E. Apical ballooning syndrome or takotsubo cardiomyopathy: a systematic review. Eur Heart J. 2006;27(13):1523-1529. doi:10.1093/eurheartj/ehl032.
20. Citro R, Lyon AR, Meimoun P, et al. Standard and advanced echocardiography in Takotsubo (stress) cardiomyopathy: clinical and prognostic implications. J Am Soc Echocardiogr. 2015;28(1):57-74. doi:10.1016/j.echo.2014.08.020.