User login
A Case Series of Rare Immune-Mediated Adverse Reactions at the New Mexico Veterans Affairs Medical Center
Immune checkpoint inhibitors (ICIs), often broadly referred to as immunotherapy, are being prescribed at increasing rates due to their effectiveness in treating a growing number of advanced solid tumors and hematologic malignancies.1 It has been well established that T-cell signaling mechanisms designed to combat foreign pathogens have been involved in the mitigation of tumor proliferation.2 This protective process can be supported or restricted by infection, medication, or mutations.
ICIs support T-cell–mediated destruction of tumor cells by inhibiting the mechanisms designed to limit autoimmunity, specifically the programmed cell death protein 1/programmed cell death ligand 1 (PD-1/PD-L1) and cytotoxic T-lymphocyte–associated antigen 4 (CTLA-4) pathways. The results have been impressive, leading to an expansive number of US Food and Drug Administration (FDA) approvals across a diverse set of malignancies. Consequently, the Nobel Prize in Physiology or Medicine was awarded for such work in 2018.3
BACKGROUND
While altering these pathways has been shown to hinder tumor growth, the lesser restrictions on the immune system can drive unwanted autoimmune inflammation to host tissue. These toxicities are collectively known as immune-mediated adverse reactions (IMARs). Clinically and histologically, IMARs frequently manifest similarly to other autoimmune conditions and may affect any organ, including skin, liver, lungs, heart, intestine (small and large), kidneys, eyes, endocrine glands, and neurologic tissue.4,5 According to recent studies, as many as 20% to 30% of patients receiving a single ICI will experience at least 1 clinically significant IMAR, and about 13% are classified as severe; however, < 10% of patients will have their ICIs discontinued due to these reactions.6
Though infrequent, a thorough understanding of the severity of IMARs to ICIs is critical for the diagnosis and management of these organ-threatening and potentially life-threatening toxicities. With the growing use of these agents and more FDA approvals for dual checkpoint blockage (concurrent use of CTLA-4 and PD-1/PD-L1 inhibitors), the absolute number of IMARs is expected to rise, thereby leading to more exposure of such events to both oncology and nononcology clinicians. Prior literature has clearly described the treatments and outcomes for many common severe toxicities; however, information regarding presentations and outcomes for rare IMARs is lacking.7
A few fascinating cases of rare toxicities have been observed at the New Mexico Veterans Affairs Medical Center (NMVAMC) in Albuquerque despite its relatively small size compared with other US Department of Veterans Affairs medical centers. As such, herein, the diagnostic evaluation, treatments, and outcomes of rare IMARs are reported for each case, and the related literature is reviewed.
Patient Selection
Patients who were required to discontinue or postpone treatment with any ICI blocking the CTLA-4 (ipilimumab), PD-1 (pembrolizumab, nivolumab, cemiplimab), or PD-L1 (atezolizumab, avelumab, durvalumab) pathways between 2015 to 2021 due to toxicity at the NMVAMC were eligible for inclusion. The electronic health record was reviewed for each eligible case, and the patient demographics, disease characteristics, toxicities, and outcomes were documented for each patient. For the 57 patients who received ICIs within the chosen period, 11 required a treatment break or discontinuation. Of these, 3 cases were selected for reporting due to the rare IMARs observed. This study was approved by the NMVAMC Institutional Review Board.
Case 1: Myocarditis
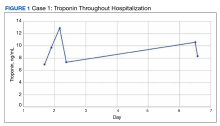
An 84-year-old man receiving a chemoimmunotherapy regimen consisting of carboplatin, pemetrexed, and pembrolizumab for recurrent, stage IV lung adenocarcinoma developed grade 4 cardiomyopathy, as defined by the Common Terminology Criteria for Adverse Events (CTCAE) v5.0, during his treatment.8 He was treated for 2 cycles before he began experiencing an increase in liver enzymes.
The patient’s presentation was concerning for myocarditis, and he was quickly admitted to NMVAMC. Cardiac catheterization did not reveal any signs of coronary occlusive disease. Prednisone 1 mg/kg was administered immediately; however, given continued chest pain and volume overload, he was quickly transitioned to solumedrol 1000 mg IV daily. After the initiation of his treatment, the patient’s transaminitis began to resolve, and troponin levels began to decrease; however, his symptoms continued to worsen, and his troponin rose again. By the fourth day of hospitalization, the patient was treated with infliximab, a tumor necrosis factor-α inhibitor shown to reverse ICI-induced autoimmune inflammation, with only mild improvement of his symptoms. The patient’s condition continued to deteriorate, his troponin levels remained elevated, and his family decided to withhold additional treatment. The patient died shortly thereafter.
Discussion
Cardiotoxicity resulting from ICI therapy is far less common than the other potential severe toxicities associated with ICIs. Nevertheless, many cases of ICI-induced cardiac inflammation have been reported, and it has been widely established that patients treated with ICIs are generally at higher risk for acute coronary syndrome.9-11 Acute cardiotoxicity secondary to autoimmune destruction of cardiac tissue includes myocarditis, pericarditis, and vasculitis, which may manifest with symptoms of heart failure and/or arrhythmia. Grading of ICI-induced cardiomyopathy has been defined by both CTCAE and the American Society of Clinical Oncology (ASCO), with grade 4 representing moderate to severe clinical decompensation requiring IV medications in the setting of life-threatening conditions.
Review articles have described the treatment options for severe cases.7,12 As detailed in prior reports, once ICI-induced cardiomyopathy is suspected, urgent admission and immediate evaluation to rule out acute coronary syndrome should be undertaken. Given the potential for deterioration despite the occasional insidious onset, aggressive cardiac monitoring, and close follow-up to measure response to interventions should be undertaken.
Case 2: Uveitis
A 70-year-old man who received pembrolizumab as a bladder-sparing approach for his superficial bladder cancer refractory to intravesical treatments developed uveitis. Approximately 3 months following the initiation of treatment, the patient reported bilateral itchy eyes, erythema, and tearing. He had a known history of allergic conjunctivitis that predated the ICI therapy, and consequently, it was unclear whether his symptoms were reflective of a more concerning issue. The patient’s symptoms continued to wax and wane for a few months, prompting a referral to ophthalmology colleagues at NMVAMC.
Ophthalmology evaluation identified uveitic glaucoma in the setting of his underlying chronic glaucoma. Pembrolizumab was discontinued, and the patient was counseled on choosing either cystectomy or locoregional therapies if further tumors arose. However, within a few weeks of administering topical steroid drops, his symptoms markedly improved, and he wished to be restarted on pembrolizumab. His uveitis remained in remission, and he has been treated with pembrolizumab for more than 1 year since this episode. He has had no clear findings of superficial bladder cancer recurrence while receiving ICI therapy.
Discussion
Uveitis is a known complication of pembrolizumab, and it has been shown to occur in 1% of patients with this treatment.13,14 It should be noted that most of the studies of this IMAR occurred in patients with metastatic melanoma; therefore the rate of this condition in other patients is less understood. Overall, ocular IMARs secondary to anti-PD-1 and anti-PD-L1 therapies are rare.
The most common IMAR is surface ocular disease, consisting of dry eye disease (DED), conjunctivitis, uveitis, and keratitis. Of these, the most common ocular surface disease is DED, which occurred in 1% to 4% of patients treated with ICI therapy; most of these reactions are mild and self-limiting.15 Atezolizumab has the highest association with ocular inflammation and ipilimumab has the highest association with uveitis, with reported odds ratios of 18.89 and 10.54, respectively.16 Treatment of ICI-induced uveitis generally includes topical steroids and treatment discontinuation or break.17 Oral or IV steroids, infliximab, and procedural involvement may be considered in refractory cases or those initially presenting with marked vision loss. Close communication with ophthalmology colleagues to monitor visual acuity and ocular pressure multiple times weekly during the acute phase is required for treatment titration.
Case 3: Organizing Pneumonia
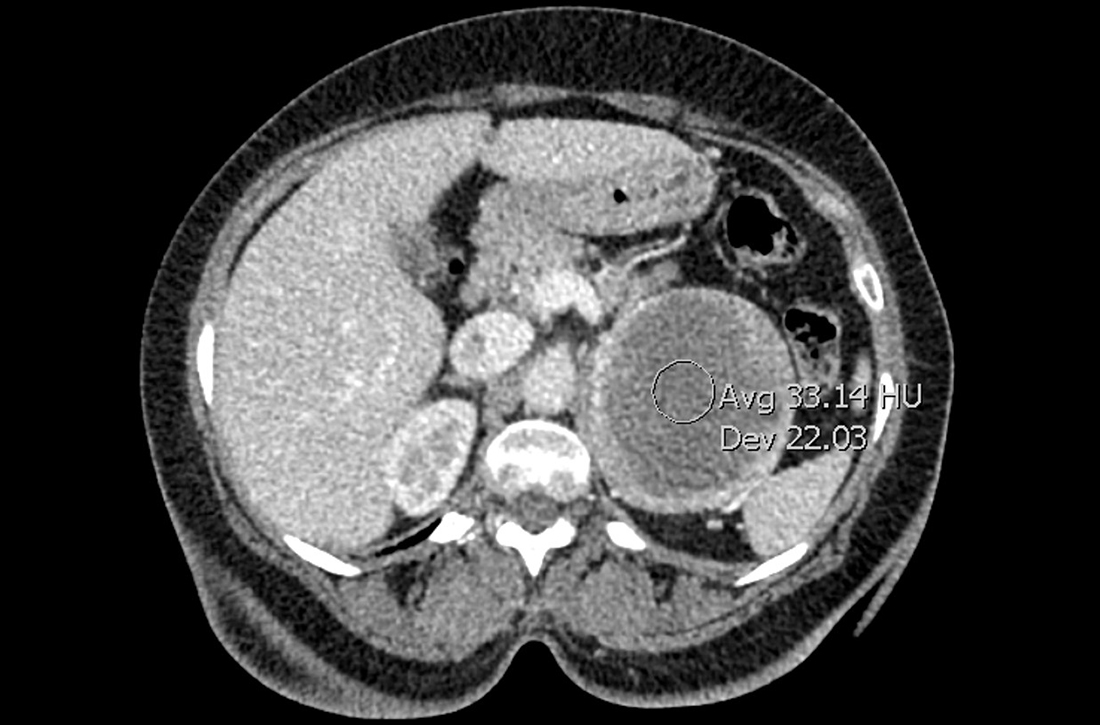
A man aged 63 years was diagnosed with malignant mesothelioma after incidentally noting a pleural effusion and thickening on routine low-dose computed tomography surveillance of pulmonary nodules. A biopsy was performed and was consistent with mesothelioma, and the patient was started on nivolumab (PD-1 inhibitor) and ipilimumab (CTLA-4 inhibitor). The patient was initiated on dual ICIs, and after 6 months of therapy, he had a promising complete response. However, after 9 months of therapy, he developed a new left upper lobe (LUL) pleural-based lesion (Figure 2A).
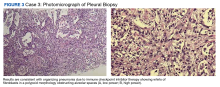
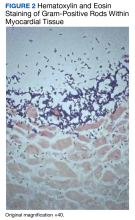
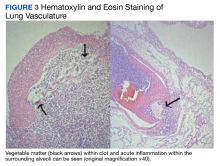
A biopsy was performed, and the histopathologic appearance was consistent with organizing pneumonia (OP) (Figure 3).
Discussion
ICIs can uncommonly drive pneumonitis, with the frequency adjusted based on the number of ICIs prescribed and the primary cancer involved. Across all cancers, up to 5% of patients treated with single-agent ICI therapy may experience pneumonitis, though often the findings may simply be radiographic without symptoms. Moreover, up to 10% of patients undergoing treatment for pulmonary cancer or those with dual ICI treatment regimens experience radiographic and/or clinical pneumonitis.18 The clinical manifestations include a broad spectrum of respiratory symptoms. Given the convoluting concerns of cancer progression and infection, a biopsy is often obtained. Histopathologic findings of pneumonitis may include diffuse alveolar damage and/or interstitial lung disease, with OP being a rare variant of ILD.
Among pulmonologists, OP is felt to have polymorphous imaging findings, and biopsy is required to confirm histology; however, histopathology cannot define etiology, and consequently, OP is somewhat of an umbrella diagnosis. The condition can be cryptogenic (idiopathic) or secondary to a multitude of conditions (infection, drug toxicity, or systemic disease). It is classically described as polypoid aggregations of fibroblasts that obstruct the alveolar spaces.19 This histopathologic pattern was demonstrated in our patient’s lung biopsy. Given a prior case description of ICIs, mesothelioma, OP development, and the unremarkable infectious workup, we felt that the patient’s OP was driven by his dual ICI therapy, thereby leading to the ultimate discontinuation of his ICIs and initiation of steroids.20 Thankfully, the patient had already obtained a complete response to his ICIs, and hopefully, he can attain a durable remission with the addition of maintenance chemotherapy.
CONCLUSIONS
ICIs have revolutionized the treatment of a myriad of solid tumors and hematologic malignancies, and their use internationally is expected to increase. With the alteration in immunology pathways, clinicians in all fields will need to be familiarized with IMARs secondary to these agents, including rare subtypes. In addition, the variability in presentations relative to the patients’ treatment course was significant (between 2-9 months), and this highlights that these IMARs can occur at any time point and clinicians should be ever vigilant to spot symptoms in their patients.
It was unexpected for the 3 aforementioned rare toxicities to arise at NMVAMC among only 57 treated patients, and we speculate that these findings may have been observed for 1 of 3 reasons. First, caring for 3 patients with this collection of rare toxicities may have been due to chance. Second, though there is sparse literature studying the topic, the regional environment, including sunlight exposure and air quality, may play a role in the development of one or all of these rare toxicities. Third, rates of these toxicities may be underreported in the literature or attributed to other conditions rather than due to ICIs at other sites, and the uncommon nature of these IMARs may be overstated. Investigations evaluating rates of toxicities, including those traditionally uncommonly seen, based on regional location should be conducted before any further conclusions are drawn.
1. Bagchi S, Yuan R, Engleman EG. Immune checkpoint inhibitors for the treatment of cancer: clinical impact and mechanisms of response and resistance. Published online 2020. doi:10.1146/annurev-pathol-042020
2. Chen DS, Mellman I. Oncology meets immunology: The cancer-immunity cycle. Immunity. 2013;39(1):1-10. doi:10.1016/j.immuni.2013.07.012
3. Smyth MJ, Teng MWL. 2018 Nobel Prize in physiology or medicine. Clin Transl Immunology. 2018;7(10). doi:10.1002/cti2.1041
4. Baxi S, Yang A, Gennarelli RL, et al. Immune-related adverse events for anti-PD-1 and anti-PD-L1 drugs: Systematic review and meta-analysis. BMJ (Online). 2018;360. doi:10.1136/bmj.k793
5. Ellithi M, Elnair R, Chang GV, Abdallah MA. Toxicities of immune checkpoint inhibitors: itis-ending adverse reactions and more. Cureus. Published online February 10, 2020. doi:10.7759/cureus.6935
6. Berti A, Bortolotti R, Dipasquale M, et al. Meta-analysis of immune-related adverse events in phase 3 clinical trials assessing immune checkpoint inhibitors for lung cancer. Crit Rev Oncol Hematol. 2021;162. doi:10.1016/j.critrevonc.2021.103351
7. Davies M, Duffield EA. Safety of checkpoint inhibitors for cancer treatment: strategies for patient monitoring and management of immune-mediated adverse events. Immunotargets Ther. 2017;Volume 6:51-71. doi:10.2147/itt.s141577
8. US Department of Health and Human Services. Common Terminology Criteria for Adverse Events V5.0. Accessed July 17, 2023. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5584920/
9. Johnson DB, Balko JM, Compton ML, et al. Fulminant myocarditis with combination immune checkpoint blockade. N Engl J Med. 2016;375(18):1749-1755. doi:10.1056/nejmoa1609214
10. Mahmood SS, Fradley MG, Cohen J V., et al. Myocarditis in patients treated with immune checkpoint inhibitors. J Am Coll Cardiol. 2018;71(16):1755-1764. doi:10.1016/j.jacc.2018.02.037
11. Wang DY, Salem JE, Cohen JV, et al. Fatal toxic effects associated with immune checkpoint inhibitors: a systematic review and meta-analysis. JAMA Oncol. 2018;4(12):1721-1728. doi:10.1001/jamaoncol.2018.3923
12. Brahmer JR, Lacchetti C, Schneider BJ, et al; National Comprehensive Cancer Network. Management of immune-related adverse events in patients treated with immune checkpoint inhibitor therapy: American Society of Clinical Oncology Clinical Practice Guideline. J Clin Onc. 2018;36(17):1714-1768. doi:10.1200/JCO
13. Ribas A, Hamid O, Daud A, et al. Association of pembrolizumab with tumor response and survival among patients with advanced melanoma. JAMA. 2016;315:1600-1609. doi:10.1001/jama.2016.4059
14. Dalvin LA, Shields CL, Orloff M, Sato T, Shields JA. Checkpoint inhibitor immune therapy: systemic indications and ophthalmic side effects. Retina. 2018;38(6):1063-1078. doi:10.1097/IAE.0000000000002181
15. Park RB, Jain S, Han H, Park J. Ocular surface disease associated with immune checkpoint inhibitor therapy. Ocular Surface. 2021;20:115-129. doi:10.1016/j.jtos.2021.02.004
16. Fang T, Maberley DA, Etminan M. Ocular adverse events with immune checkpoint inhibitors. J Curr Ophthalmol. 2019;31(3):319-322. doi:10.1016/j.joco.2019.05.002
17. Whist E, Symes RJ, Chang JH, et al. Uveitis caused by treatment for malignant melanoma: a case series. Retin Cases Brief Rep. 2021;15(6):718-723. doi:10.1097/ICB.0000000000000876
18. Naidoo J, Wang X, Woo KM, et al. Pneumonitis in patients treated with anti-programmed death-1/programmed death ligand 1 therapy. J Clin Onc. 2017;35(7):709-717. doi:10.1200/JCO.2016.68.2005
19. Yoshikawa A, Bychkov A, Sathirareuangchai S. Other nonneoplastic conditions, acute lung injury, organizing pneumonia. Accessed July 17, 2023. https://www.pathologyoutlines.com/topic/lungnontumorboop.html
20. Kuint R, Lotem M, Neuman T, et al. Organizing pneumonia following treatment with pembrolizumab for metastatic malignant melanoma–a case report. Respir Med Case Rep. 2017;20:95-97. doi:10.1016/j.rmcr.2017.01.003
Immune checkpoint inhibitors (ICIs), often broadly referred to as immunotherapy, are being prescribed at increasing rates due to their effectiveness in treating a growing number of advanced solid tumors and hematologic malignancies.1 It has been well established that T-cell signaling mechanisms designed to combat foreign pathogens have been involved in the mitigation of tumor proliferation.2 This protective process can be supported or restricted by infection, medication, or mutations.
ICIs support T-cell–mediated destruction of tumor cells by inhibiting the mechanisms designed to limit autoimmunity, specifically the programmed cell death protein 1/programmed cell death ligand 1 (PD-1/PD-L1) and cytotoxic T-lymphocyte–associated antigen 4 (CTLA-4) pathways. The results have been impressive, leading to an expansive number of US Food and Drug Administration (FDA) approvals across a diverse set of malignancies. Consequently, the Nobel Prize in Physiology or Medicine was awarded for such work in 2018.3
BACKGROUND
While altering these pathways has been shown to hinder tumor growth, the lesser restrictions on the immune system can drive unwanted autoimmune inflammation to host tissue. These toxicities are collectively known as immune-mediated adverse reactions (IMARs). Clinically and histologically, IMARs frequently manifest similarly to other autoimmune conditions and may affect any organ, including skin, liver, lungs, heart, intestine (small and large), kidneys, eyes, endocrine glands, and neurologic tissue.4,5 According to recent studies, as many as 20% to 30% of patients receiving a single ICI will experience at least 1 clinically significant IMAR, and about 13% are classified as severe; however, < 10% of patients will have their ICIs discontinued due to these reactions.6
Though infrequent, a thorough understanding of the severity of IMARs to ICIs is critical for the diagnosis and management of these organ-threatening and potentially life-threatening toxicities. With the growing use of these agents and more FDA approvals for dual checkpoint blockage (concurrent use of CTLA-4 and PD-1/PD-L1 inhibitors), the absolute number of IMARs is expected to rise, thereby leading to more exposure of such events to both oncology and nononcology clinicians. Prior literature has clearly described the treatments and outcomes for many common severe toxicities; however, information regarding presentations and outcomes for rare IMARs is lacking.7
A few fascinating cases of rare toxicities have been observed at the New Mexico Veterans Affairs Medical Center (NMVAMC) in Albuquerque despite its relatively small size compared with other US Department of Veterans Affairs medical centers. As such, herein, the diagnostic evaluation, treatments, and outcomes of rare IMARs are reported for each case, and the related literature is reviewed.
Patient Selection
Patients who were required to discontinue or postpone treatment with any ICI blocking the CTLA-4 (ipilimumab), PD-1 (pembrolizumab, nivolumab, cemiplimab), or PD-L1 (atezolizumab, avelumab, durvalumab) pathways between 2015 to 2021 due to toxicity at the NMVAMC were eligible for inclusion. The electronic health record was reviewed for each eligible case, and the patient demographics, disease characteristics, toxicities, and outcomes were documented for each patient. For the 57 patients who received ICIs within the chosen period, 11 required a treatment break or discontinuation. Of these, 3 cases were selected for reporting due to the rare IMARs observed. This study was approved by the NMVAMC Institutional Review Board.
Case 1: Myocarditis
An 84-year-old man receiving a chemoimmunotherapy regimen consisting of carboplatin, pemetrexed, and pembrolizumab for recurrent, stage IV lung adenocarcinoma developed grade 4 cardiomyopathy, as defined by the Common Terminology Criteria for Adverse Events (CTCAE) v5.0, during his treatment.8 He was treated for 2 cycles before he began experiencing an increase in liver enzymes.
The patient’s presentation was concerning for myocarditis, and he was quickly admitted to NMVAMC. Cardiac catheterization did not reveal any signs of coronary occlusive disease. Prednisone 1 mg/kg was administered immediately; however, given continued chest pain and volume overload, he was quickly transitioned to solumedrol 1000 mg IV daily. After the initiation of his treatment, the patient’s transaminitis began to resolve, and troponin levels began to decrease; however, his symptoms continued to worsen, and his troponin rose again. By the fourth day of hospitalization, the patient was treated with infliximab, a tumor necrosis factor-α inhibitor shown to reverse ICI-induced autoimmune inflammation, with only mild improvement of his symptoms. The patient’s condition continued to deteriorate, his troponin levels remained elevated, and his family decided to withhold additional treatment. The patient died shortly thereafter.
Discussion
Cardiotoxicity resulting from ICI therapy is far less common than the other potential severe toxicities associated with ICIs. Nevertheless, many cases of ICI-induced cardiac inflammation have been reported, and it has been widely established that patients treated with ICIs are generally at higher risk for acute coronary syndrome.9-11 Acute cardiotoxicity secondary to autoimmune destruction of cardiac tissue includes myocarditis, pericarditis, and vasculitis, which may manifest with symptoms of heart failure and/or arrhythmia. Grading of ICI-induced cardiomyopathy has been defined by both CTCAE and the American Society of Clinical Oncology (ASCO), with grade 4 representing moderate to severe clinical decompensation requiring IV medications in the setting of life-threatening conditions.
Review articles have described the treatment options for severe cases.7,12 As detailed in prior reports, once ICI-induced cardiomyopathy is suspected, urgent admission and immediate evaluation to rule out acute coronary syndrome should be undertaken. Given the potential for deterioration despite the occasional insidious onset, aggressive cardiac monitoring, and close follow-up to measure response to interventions should be undertaken.
Case 2: Uveitis
A 70-year-old man who received pembrolizumab as a bladder-sparing approach for his superficial bladder cancer refractory to intravesical treatments developed uveitis. Approximately 3 months following the initiation of treatment, the patient reported bilateral itchy eyes, erythema, and tearing. He had a known history of allergic conjunctivitis that predated the ICI therapy, and consequently, it was unclear whether his symptoms were reflective of a more concerning issue. The patient’s symptoms continued to wax and wane for a few months, prompting a referral to ophthalmology colleagues at NMVAMC.
Ophthalmology evaluation identified uveitic glaucoma in the setting of his underlying chronic glaucoma. Pembrolizumab was discontinued, and the patient was counseled on choosing either cystectomy or locoregional therapies if further tumors arose. However, within a few weeks of administering topical steroid drops, his symptoms markedly improved, and he wished to be restarted on pembrolizumab. His uveitis remained in remission, and he has been treated with pembrolizumab for more than 1 year since this episode. He has had no clear findings of superficial bladder cancer recurrence while receiving ICI therapy.
Discussion
Uveitis is a known complication of pembrolizumab, and it has been shown to occur in 1% of patients with this treatment.13,14 It should be noted that most of the studies of this IMAR occurred in patients with metastatic melanoma; therefore the rate of this condition in other patients is less understood. Overall, ocular IMARs secondary to anti-PD-1 and anti-PD-L1 therapies are rare.
The most common IMAR is surface ocular disease, consisting of dry eye disease (DED), conjunctivitis, uveitis, and keratitis. Of these, the most common ocular surface disease is DED, which occurred in 1% to 4% of patients treated with ICI therapy; most of these reactions are mild and self-limiting.15 Atezolizumab has the highest association with ocular inflammation and ipilimumab has the highest association with uveitis, with reported odds ratios of 18.89 and 10.54, respectively.16 Treatment of ICI-induced uveitis generally includes topical steroids and treatment discontinuation or break.17 Oral or IV steroids, infliximab, and procedural involvement may be considered in refractory cases or those initially presenting with marked vision loss. Close communication with ophthalmology colleagues to monitor visual acuity and ocular pressure multiple times weekly during the acute phase is required for treatment titration.
Case 3: Organizing Pneumonia
A man aged 63 years was diagnosed with malignant mesothelioma after incidentally noting a pleural effusion and thickening on routine low-dose computed tomography surveillance of pulmonary nodules. A biopsy was performed and was consistent with mesothelioma, and the patient was started on nivolumab (PD-1 inhibitor) and ipilimumab (CTLA-4 inhibitor). The patient was initiated on dual ICIs, and after 6 months of therapy, he had a promising complete response. However, after 9 months of therapy, he developed a new left upper lobe (LUL) pleural-based lesion (Figure 2A).
A biopsy was performed, and the histopathologic appearance was consistent with organizing pneumonia (OP) (Figure 3).
Discussion
ICIs can uncommonly drive pneumonitis, with the frequency adjusted based on the number of ICIs prescribed and the primary cancer involved. Across all cancers, up to 5% of patients treated with single-agent ICI therapy may experience pneumonitis, though often the findings may simply be radiographic without symptoms. Moreover, up to 10% of patients undergoing treatment for pulmonary cancer or those with dual ICI treatment regimens experience radiographic and/or clinical pneumonitis.18 The clinical manifestations include a broad spectrum of respiratory symptoms. Given the convoluting concerns of cancer progression and infection, a biopsy is often obtained. Histopathologic findings of pneumonitis may include diffuse alveolar damage and/or interstitial lung disease, with OP being a rare variant of ILD.
Among pulmonologists, OP is felt to have polymorphous imaging findings, and biopsy is required to confirm histology; however, histopathology cannot define etiology, and consequently, OP is somewhat of an umbrella diagnosis. The condition can be cryptogenic (idiopathic) or secondary to a multitude of conditions (infection, drug toxicity, or systemic disease). It is classically described as polypoid aggregations of fibroblasts that obstruct the alveolar spaces.19 This histopathologic pattern was demonstrated in our patient’s lung biopsy. Given a prior case description of ICIs, mesothelioma, OP development, and the unremarkable infectious workup, we felt that the patient’s OP was driven by his dual ICI therapy, thereby leading to the ultimate discontinuation of his ICIs and initiation of steroids.20 Thankfully, the patient had already obtained a complete response to his ICIs, and hopefully, he can attain a durable remission with the addition of maintenance chemotherapy.
CONCLUSIONS
ICIs have revolutionized the treatment of a myriad of solid tumors and hematologic malignancies, and their use internationally is expected to increase. With the alteration in immunology pathways, clinicians in all fields will need to be familiarized with IMARs secondary to these agents, including rare subtypes. In addition, the variability in presentations relative to the patients’ treatment course was significant (between 2-9 months), and this highlights that these IMARs can occur at any time point and clinicians should be ever vigilant to spot symptoms in their patients.
It was unexpected for the 3 aforementioned rare toxicities to arise at NMVAMC among only 57 treated patients, and we speculate that these findings may have been observed for 1 of 3 reasons. First, caring for 3 patients with this collection of rare toxicities may have been due to chance. Second, though there is sparse literature studying the topic, the regional environment, including sunlight exposure and air quality, may play a role in the development of one or all of these rare toxicities. Third, rates of these toxicities may be underreported in the literature or attributed to other conditions rather than due to ICIs at other sites, and the uncommon nature of these IMARs may be overstated. Investigations evaluating rates of toxicities, including those traditionally uncommonly seen, based on regional location should be conducted before any further conclusions are drawn.
Immune checkpoint inhibitors (ICIs), often broadly referred to as immunotherapy, are being prescribed at increasing rates due to their effectiveness in treating a growing number of advanced solid tumors and hematologic malignancies.1 It has been well established that T-cell signaling mechanisms designed to combat foreign pathogens have been involved in the mitigation of tumor proliferation.2 This protective process can be supported or restricted by infection, medication, or mutations.
ICIs support T-cell–mediated destruction of tumor cells by inhibiting the mechanisms designed to limit autoimmunity, specifically the programmed cell death protein 1/programmed cell death ligand 1 (PD-1/PD-L1) and cytotoxic T-lymphocyte–associated antigen 4 (CTLA-4) pathways. The results have been impressive, leading to an expansive number of US Food and Drug Administration (FDA) approvals across a diverse set of malignancies. Consequently, the Nobel Prize in Physiology or Medicine was awarded for such work in 2018.3
BACKGROUND
While altering these pathways has been shown to hinder tumor growth, the lesser restrictions on the immune system can drive unwanted autoimmune inflammation to host tissue. These toxicities are collectively known as immune-mediated adverse reactions (IMARs). Clinically and histologically, IMARs frequently manifest similarly to other autoimmune conditions and may affect any organ, including skin, liver, lungs, heart, intestine (small and large), kidneys, eyes, endocrine glands, and neurologic tissue.4,5 According to recent studies, as many as 20% to 30% of patients receiving a single ICI will experience at least 1 clinically significant IMAR, and about 13% are classified as severe; however, < 10% of patients will have their ICIs discontinued due to these reactions.6
Though infrequent, a thorough understanding of the severity of IMARs to ICIs is critical for the diagnosis and management of these organ-threatening and potentially life-threatening toxicities. With the growing use of these agents and more FDA approvals for dual checkpoint blockage (concurrent use of CTLA-4 and PD-1/PD-L1 inhibitors), the absolute number of IMARs is expected to rise, thereby leading to more exposure of such events to both oncology and nononcology clinicians. Prior literature has clearly described the treatments and outcomes for many common severe toxicities; however, information regarding presentations and outcomes for rare IMARs is lacking.7
A few fascinating cases of rare toxicities have been observed at the New Mexico Veterans Affairs Medical Center (NMVAMC) in Albuquerque despite its relatively small size compared with other US Department of Veterans Affairs medical centers. As such, herein, the diagnostic evaluation, treatments, and outcomes of rare IMARs are reported for each case, and the related literature is reviewed.
Patient Selection
Patients who were required to discontinue or postpone treatment with any ICI blocking the CTLA-4 (ipilimumab), PD-1 (pembrolizumab, nivolumab, cemiplimab), or PD-L1 (atezolizumab, avelumab, durvalumab) pathways between 2015 to 2021 due to toxicity at the NMVAMC were eligible for inclusion. The electronic health record was reviewed for each eligible case, and the patient demographics, disease characteristics, toxicities, and outcomes were documented for each patient. For the 57 patients who received ICIs within the chosen period, 11 required a treatment break or discontinuation. Of these, 3 cases were selected for reporting due to the rare IMARs observed. This study was approved by the NMVAMC Institutional Review Board.
Case 1: Myocarditis
An 84-year-old man receiving a chemoimmunotherapy regimen consisting of carboplatin, pemetrexed, and pembrolizumab for recurrent, stage IV lung adenocarcinoma developed grade 4 cardiomyopathy, as defined by the Common Terminology Criteria for Adverse Events (CTCAE) v5.0, during his treatment.8 He was treated for 2 cycles before he began experiencing an increase in liver enzymes.
The patient’s presentation was concerning for myocarditis, and he was quickly admitted to NMVAMC. Cardiac catheterization did not reveal any signs of coronary occlusive disease. Prednisone 1 mg/kg was administered immediately; however, given continued chest pain and volume overload, he was quickly transitioned to solumedrol 1000 mg IV daily. After the initiation of his treatment, the patient’s transaminitis began to resolve, and troponin levels began to decrease; however, his symptoms continued to worsen, and his troponin rose again. By the fourth day of hospitalization, the patient was treated with infliximab, a tumor necrosis factor-α inhibitor shown to reverse ICI-induced autoimmune inflammation, with only mild improvement of his symptoms. The patient’s condition continued to deteriorate, his troponin levels remained elevated, and his family decided to withhold additional treatment. The patient died shortly thereafter.
Discussion
Cardiotoxicity resulting from ICI therapy is far less common than the other potential severe toxicities associated with ICIs. Nevertheless, many cases of ICI-induced cardiac inflammation have been reported, and it has been widely established that patients treated with ICIs are generally at higher risk for acute coronary syndrome.9-11 Acute cardiotoxicity secondary to autoimmune destruction of cardiac tissue includes myocarditis, pericarditis, and vasculitis, which may manifest with symptoms of heart failure and/or arrhythmia. Grading of ICI-induced cardiomyopathy has been defined by both CTCAE and the American Society of Clinical Oncology (ASCO), with grade 4 representing moderate to severe clinical decompensation requiring IV medications in the setting of life-threatening conditions.
Review articles have described the treatment options for severe cases.7,12 As detailed in prior reports, once ICI-induced cardiomyopathy is suspected, urgent admission and immediate evaluation to rule out acute coronary syndrome should be undertaken. Given the potential for deterioration despite the occasional insidious onset, aggressive cardiac monitoring, and close follow-up to measure response to interventions should be undertaken.
Case 2: Uveitis
A 70-year-old man who received pembrolizumab as a bladder-sparing approach for his superficial bladder cancer refractory to intravesical treatments developed uveitis. Approximately 3 months following the initiation of treatment, the patient reported bilateral itchy eyes, erythema, and tearing. He had a known history of allergic conjunctivitis that predated the ICI therapy, and consequently, it was unclear whether his symptoms were reflective of a more concerning issue. The patient’s symptoms continued to wax and wane for a few months, prompting a referral to ophthalmology colleagues at NMVAMC.
Ophthalmology evaluation identified uveitic glaucoma in the setting of his underlying chronic glaucoma. Pembrolizumab was discontinued, and the patient was counseled on choosing either cystectomy or locoregional therapies if further tumors arose. However, within a few weeks of administering topical steroid drops, his symptoms markedly improved, and he wished to be restarted on pembrolizumab. His uveitis remained in remission, and he has been treated with pembrolizumab for more than 1 year since this episode. He has had no clear findings of superficial bladder cancer recurrence while receiving ICI therapy.
Discussion
Uveitis is a known complication of pembrolizumab, and it has been shown to occur in 1% of patients with this treatment.13,14 It should be noted that most of the studies of this IMAR occurred in patients with metastatic melanoma; therefore the rate of this condition in other patients is less understood. Overall, ocular IMARs secondary to anti-PD-1 and anti-PD-L1 therapies are rare.
The most common IMAR is surface ocular disease, consisting of dry eye disease (DED), conjunctivitis, uveitis, and keratitis. Of these, the most common ocular surface disease is DED, which occurred in 1% to 4% of patients treated with ICI therapy; most of these reactions are mild and self-limiting.15 Atezolizumab has the highest association with ocular inflammation and ipilimumab has the highest association with uveitis, with reported odds ratios of 18.89 and 10.54, respectively.16 Treatment of ICI-induced uveitis generally includes topical steroids and treatment discontinuation or break.17 Oral or IV steroids, infliximab, and procedural involvement may be considered in refractory cases or those initially presenting with marked vision loss. Close communication with ophthalmology colleagues to monitor visual acuity and ocular pressure multiple times weekly during the acute phase is required for treatment titration.
Case 3: Organizing Pneumonia
A man aged 63 years was diagnosed with malignant mesothelioma after incidentally noting a pleural effusion and thickening on routine low-dose computed tomography surveillance of pulmonary nodules. A biopsy was performed and was consistent with mesothelioma, and the patient was started on nivolumab (PD-1 inhibitor) and ipilimumab (CTLA-4 inhibitor). The patient was initiated on dual ICIs, and after 6 months of therapy, he had a promising complete response. However, after 9 months of therapy, he developed a new left upper lobe (LUL) pleural-based lesion (Figure 2A).
A biopsy was performed, and the histopathologic appearance was consistent with organizing pneumonia (OP) (Figure 3).
Discussion
ICIs can uncommonly drive pneumonitis, with the frequency adjusted based on the number of ICIs prescribed and the primary cancer involved. Across all cancers, up to 5% of patients treated with single-agent ICI therapy may experience pneumonitis, though often the findings may simply be radiographic without symptoms. Moreover, up to 10% of patients undergoing treatment for pulmonary cancer or those with dual ICI treatment regimens experience radiographic and/or clinical pneumonitis.18 The clinical manifestations include a broad spectrum of respiratory symptoms. Given the convoluting concerns of cancer progression and infection, a biopsy is often obtained. Histopathologic findings of pneumonitis may include diffuse alveolar damage and/or interstitial lung disease, with OP being a rare variant of ILD.
Among pulmonologists, OP is felt to have polymorphous imaging findings, and biopsy is required to confirm histology; however, histopathology cannot define etiology, and consequently, OP is somewhat of an umbrella diagnosis. The condition can be cryptogenic (idiopathic) or secondary to a multitude of conditions (infection, drug toxicity, or systemic disease). It is classically described as polypoid aggregations of fibroblasts that obstruct the alveolar spaces.19 This histopathologic pattern was demonstrated in our patient’s lung biopsy. Given a prior case description of ICIs, mesothelioma, OP development, and the unremarkable infectious workup, we felt that the patient’s OP was driven by his dual ICI therapy, thereby leading to the ultimate discontinuation of his ICIs and initiation of steroids.20 Thankfully, the patient had already obtained a complete response to his ICIs, and hopefully, he can attain a durable remission with the addition of maintenance chemotherapy.
CONCLUSIONS
ICIs have revolutionized the treatment of a myriad of solid tumors and hematologic malignancies, and their use internationally is expected to increase. With the alteration in immunology pathways, clinicians in all fields will need to be familiarized with IMARs secondary to these agents, including rare subtypes. In addition, the variability in presentations relative to the patients’ treatment course was significant (between 2-9 months), and this highlights that these IMARs can occur at any time point and clinicians should be ever vigilant to spot symptoms in their patients.
It was unexpected for the 3 aforementioned rare toxicities to arise at NMVAMC among only 57 treated patients, and we speculate that these findings may have been observed for 1 of 3 reasons. First, caring for 3 patients with this collection of rare toxicities may have been due to chance. Second, though there is sparse literature studying the topic, the regional environment, including sunlight exposure and air quality, may play a role in the development of one or all of these rare toxicities. Third, rates of these toxicities may be underreported in the literature or attributed to other conditions rather than due to ICIs at other sites, and the uncommon nature of these IMARs may be overstated. Investigations evaluating rates of toxicities, including those traditionally uncommonly seen, based on regional location should be conducted before any further conclusions are drawn.
1. Bagchi S, Yuan R, Engleman EG. Immune checkpoint inhibitors for the treatment of cancer: clinical impact and mechanisms of response and resistance. Published online 2020. doi:10.1146/annurev-pathol-042020
2. Chen DS, Mellman I. Oncology meets immunology: The cancer-immunity cycle. Immunity. 2013;39(1):1-10. doi:10.1016/j.immuni.2013.07.012
3. Smyth MJ, Teng MWL. 2018 Nobel Prize in physiology or medicine. Clin Transl Immunology. 2018;7(10). doi:10.1002/cti2.1041
4. Baxi S, Yang A, Gennarelli RL, et al. Immune-related adverse events for anti-PD-1 and anti-PD-L1 drugs: Systematic review and meta-analysis. BMJ (Online). 2018;360. doi:10.1136/bmj.k793
5. Ellithi M, Elnair R, Chang GV, Abdallah MA. Toxicities of immune checkpoint inhibitors: itis-ending adverse reactions and more. Cureus. Published online February 10, 2020. doi:10.7759/cureus.6935
6. Berti A, Bortolotti R, Dipasquale M, et al. Meta-analysis of immune-related adverse events in phase 3 clinical trials assessing immune checkpoint inhibitors for lung cancer. Crit Rev Oncol Hematol. 2021;162. doi:10.1016/j.critrevonc.2021.103351
7. Davies M, Duffield EA. Safety of checkpoint inhibitors for cancer treatment: strategies for patient monitoring and management of immune-mediated adverse events. Immunotargets Ther. 2017;Volume 6:51-71. doi:10.2147/itt.s141577
8. US Department of Health and Human Services. Common Terminology Criteria for Adverse Events V5.0. Accessed July 17, 2023. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5584920/
9. Johnson DB, Balko JM, Compton ML, et al. Fulminant myocarditis with combination immune checkpoint blockade. N Engl J Med. 2016;375(18):1749-1755. doi:10.1056/nejmoa1609214
10. Mahmood SS, Fradley MG, Cohen J V., et al. Myocarditis in patients treated with immune checkpoint inhibitors. J Am Coll Cardiol. 2018;71(16):1755-1764. doi:10.1016/j.jacc.2018.02.037
11. Wang DY, Salem JE, Cohen JV, et al. Fatal toxic effects associated with immune checkpoint inhibitors: a systematic review and meta-analysis. JAMA Oncol. 2018;4(12):1721-1728. doi:10.1001/jamaoncol.2018.3923
12. Brahmer JR, Lacchetti C, Schneider BJ, et al; National Comprehensive Cancer Network. Management of immune-related adverse events in patients treated with immune checkpoint inhibitor therapy: American Society of Clinical Oncology Clinical Practice Guideline. J Clin Onc. 2018;36(17):1714-1768. doi:10.1200/JCO
13. Ribas A, Hamid O, Daud A, et al. Association of pembrolizumab with tumor response and survival among patients with advanced melanoma. JAMA. 2016;315:1600-1609. doi:10.1001/jama.2016.4059
14. Dalvin LA, Shields CL, Orloff M, Sato T, Shields JA. Checkpoint inhibitor immune therapy: systemic indications and ophthalmic side effects. Retina. 2018;38(6):1063-1078. doi:10.1097/IAE.0000000000002181
15. Park RB, Jain S, Han H, Park J. Ocular surface disease associated with immune checkpoint inhibitor therapy. Ocular Surface. 2021;20:115-129. doi:10.1016/j.jtos.2021.02.004
16. Fang T, Maberley DA, Etminan M. Ocular adverse events with immune checkpoint inhibitors. J Curr Ophthalmol. 2019;31(3):319-322. doi:10.1016/j.joco.2019.05.002
17. Whist E, Symes RJ, Chang JH, et al. Uveitis caused by treatment for malignant melanoma: a case series. Retin Cases Brief Rep. 2021;15(6):718-723. doi:10.1097/ICB.0000000000000876
18. Naidoo J, Wang X, Woo KM, et al. Pneumonitis in patients treated with anti-programmed death-1/programmed death ligand 1 therapy. J Clin Onc. 2017;35(7):709-717. doi:10.1200/JCO.2016.68.2005
19. Yoshikawa A, Bychkov A, Sathirareuangchai S. Other nonneoplastic conditions, acute lung injury, organizing pneumonia. Accessed July 17, 2023. https://www.pathologyoutlines.com/topic/lungnontumorboop.html
20. Kuint R, Lotem M, Neuman T, et al. Organizing pneumonia following treatment with pembrolizumab for metastatic malignant melanoma–a case report. Respir Med Case Rep. 2017;20:95-97. doi:10.1016/j.rmcr.2017.01.003
1. Bagchi S, Yuan R, Engleman EG. Immune checkpoint inhibitors for the treatment of cancer: clinical impact and mechanisms of response and resistance. Published online 2020. doi:10.1146/annurev-pathol-042020
2. Chen DS, Mellman I. Oncology meets immunology: The cancer-immunity cycle. Immunity. 2013;39(1):1-10. doi:10.1016/j.immuni.2013.07.012
3. Smyth MJ, Teng MWL. 2018 Nobel Prize in physiology or medicine. Clin Transl Immunology. 2018;7(10). doi:10.1002/cti2.1041
4. Baxi S, Yang A, Gennarelli RL, et al. Immune-related adverse events for anti-PD-1 and anti-PD-L1 drugs: Systematic review and meta-analysis. BMJ (Online). 2018;360. doi:10.1136/bmj.k793
5. Ellithi M, Elnair R, Chang GV, Abdallah MA. Toxicities of immune checkpoint inhibitors: itis-ending adverse reactions and more. Cureus. Published online February 10, 2020. doi:10.7759/cureus.6935
6. Berti A, Bortolotti R, Dipasquale M, et al. Meta-analysis of immune-related adverse events in phase 3 clinical trials assessing immune checkpoint inhibitors for lung cancer. Crit Rev Oncol Hematol. 2021;162. doi:10.1016/j.critrevonc.2021.103351
7. Davies M, Duffield EA. Safety of checkpoint inhibitors for cancer treatment: strategies for patient monitoring and management of immune-mediated adverse events. Immunotargets Ther. 2017;Volume 6:51-71. doi:10.2147/itt.s141577
8. US Department of Health and Human Services. Common Terminology Criteria for Adverse Events V5.0. Accessed July 17, 2023. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5584920/
9. Johnson DB, Balko JM, Compton ML, et al. Fulminant myocarditis with combination immune checkpoint blockade. N Engl J Med. 2016;375(18):1749-1755. doi:10.1056/nejmoa1609214
10. Mahmood SS, Fradley MG, Cohen J V., et al. Myocarditis in patients treated with immune checkpoint inhibitors. J Am Coll Cardiol. 2018;71(16):1755-1764. doi:10.1016/j.jacc.2018.02.037
11. Wang DY, Salem JE, Cohen JV, et al. Fatal toxic effects associated with immune checkpoint inhibitors: a systematic review and meta-analysis. JAMA Oncol. 2018;4(12):1721-1728. doi:10.1001/jamaoncol.2018.3923
12. Brahmer JR, Lacchetti C, Schneider BJ, et al; National Comprehensive Cancer Network. Management of immune-related adverse events in patients treated with immune checkpoint inhibitor therapy: American Society of Clinical Oncology Clinical Practice Guideline. J Clin Onc. 2018;36(17):1714-1768. doi:10.1200/JCO
13. Ribas A, Hamid O, Daud A, et al. Association of pembrolizumab with tumor response and survival among patients with advanced melanoma. JAMA. 2016;315:1600-1609. doi:10.1001/jama.2016.4059
14. Dalvin LA, Shields CL, Orloff M, Sato T, Shields JA. Checkpoint inhibitor immune therapy: systemic indications and ophthalmic side effects. Retina. 2018;38(6):1063-1078. doi:10.1097/IAE.0000000000002181
15. Park RB, Jain S, Han H, Park J. Ocular surface disease associated with immune checkpoint inhibitor therapy. Ocular Surface. 2021;20:115-129. doi:10.1016/j.jtos.2021.02.004
16. Fang T, Maberley DA, Etminan M. Ocular adverse events with immune checkpoint inhibitors. J Curr Ophthalmol. 2019;31(3):319-322. doi:10.1016/j.joco.2019.05.002
17. Whist E, Symes RJ, Chang JH, et al. Uveitis caused by treatment for malignant melanoma: a case series. Retin Cases Brief Rep. 2021;15(6):718-723. doi:10.1097/ICB.0000000000000876
18. Naidoo J, Wang X, Woo KM, et al. Pneumonitis in patients treated with anti-programmed death-1/programmed death ligand 1 therapy. J Clin Onc. 2017;35(7):709-717. doi:10.1200/JCO.2016.68.2005
19. Yoshikawa A, Bychkov A, Sathirareuangchai S. Other nonneoplastic conditions, acute lung injury, organizing pneumonia. Accessed July 17, 2023. https://www.pathologyoutlines.com/topic/lungnontumorboop.html
20. Kuint R, Lotem M, Neuman T, et al. Organizing pneumonia following treatment with pembrolizumab for metastatic malignant melanoma–a case report. Respir Med Case Rep. 2017;20:95-97. doi:10.1016/j.rmcr.2017.01.003
Gastrointestinal Bleeding Caused by Large Intestine Amyloidosis
Gastrointestinal (GI) bleeding is a common cause of hospital admissions. The yearly incidence of upper GI bleeding is 80 to 150/100,000 people and lower GI bleeding is 87/100,000 people.1,2 The differential tends to initially be broad but narrows with good history followed by endoscopic findings. Getting an appropriate history can be difficult at times, which leads health care practitioners to rely more on interventional results.
Amyloidosis is a rare disorder of abnormal protein folding, leading to the deposition of insoluble fibrils that disrupt normal tissues and cause disease.3 There are 2 main types of amyloidosis, systemic and transthyretin, and 4 subtypes. Systemic amyloidosis includes amyloid light-chain (AL) deposition, caused by plasma cell dyscrasia, and amyloid A (AA) protein deposition, caused by systemic autoimmune illness or infections. Transthyretin amyloidosis is caused by changes and deposition of the transthyretin protein consisting of either unstable, mutant protein or wild type protein. Biopsy-proven amyloidosis of the GI tract is rare.4 About 60% of patients with AA amyloidosis and 8% with AL amyloidosis have GI involvement.5
We present a case of nonspecific symptoms that ultimately lined up perfectly with the official histologic confirmation of intestinal amyloidosis.
Case Presentation
A 79-year-old man with a history of type 2 diabetes mellitus, congestive heart failure, hyperlipidemia, obstructive sleep apnea, hypothyroidism, hypertension, coronary artery disease status postcoronary artery bypass grafting, and stent placements presented for 3 episodes of large, bright red bowel movements. He reported past bleeding and straining with stools, but bleeding of this amount had not been noted prior. He also reported dry heaves, lower abdominal pain, constipation with straining, early satiety with dysphagia, weakness, and decreased appetite. Lastly, he mentioned intentionally losing about 35 to 40 pounds in the past 3 to 4 months and over the past several months increased abdominal distention. However, he stated he had no history of alcohol misuse, liver or intestinal disease, cirrhosis, or other autoimmune diseases. His most recent colonoscopy was more than a decade prior and showed no acute process. The patient never had an esophagogastroduodenoscopy (EGD).
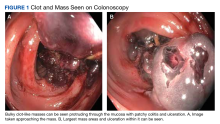
On initial presentation, the patient’s vital signs showed no acute findings. His physical examination noted a chronically ill–appearing male with decreased breath sounds to the bases bilaterally and noted abdominal distention with mild generalized tenderness. Laboratory findings were significant for a hemoglobin level, 9.4 g/dL (reference range, 11.6-15.3); iron, 23 ug/dL (reference range, 45-160); transferrin saturation, 8% (reference range, 15-50); ferritin level, 80 ng/mL (reference range, 30-300); and carcinoembryonic antigen level, 1.5 ng/mL (reference range, 0-2.9). Aspartate aminotransferase level was 54 IU/L (reference range, 0-40); alanine transaminase, 24 IU/L (reference range, 7-52); albumin, 2.7 g/dL (reference range, 3.4-5.7); international normalized ratio, 1.3 (reference range, 0-1.1); creatinine, 1.74 mg/dL (reference range, 0.44-1.27); alkaline phosphatase, 369 IU/L (reference range, 39-117). White blood cell count was 15.5 × 109/L (reference range, 3.5-10.3), and lactic acid was 2.5 mmol/L (reference range, 0.5-2.2). He was started on piperacillin/tazobactam in the emergency department and transitioned to ciprofloxacin and metronidazole for presumed intra-abdominal infection. Paracentesis showed a serum ascites albumin gradient of > 1.1 g/dL with no signs of spontaneous bacterial peritonitis. Computed tomography of the abdomen and pelvis with contrast was suspicious for colitis involving the proximal colon, and colonic mass could not be excluded. Also noted was hepatosplenomegaly with abdominopelvic ascites.
Based on these findings, an EGD and colonoscopy were done. The EGD showed mild portal hypertensive gastropathy.
After the biopsy results, the patient was officially diagnosed with intestinal amyloidosis (Figure 2).
He returned to the gastroenterology clinic 2 months later. At that point, he had worsening symptoms, liver function test results, and international normalized ratio. He was admitted for further investigation. A bone biopsy was done to confirm the histology and define the underlying disorder. The biopsy returned showing Waldenstrom macroglobulinemia, and he was started on bortezomib. Unfortunately, his clinical status rapidly worsened, leading to acute renal and hepatic failure and the development of encephalopathy. He eventually died under palliative care services.
Discussion
Amyloidosis is a rare disorder of abnormal protein folding, leading to the deposition of insoluble fibrils that disrupt normal tissues and cause disease.3 There are several variations of amyloid, but the most common type is AL amyloidosis, which affects several organs, including the heart, kidney, liver, nervous system, and GI tract. When AL amyloidosis involves the liver, the median survival time is about 8.5 months.6 There are different ways to diagnose the disease, but a tissue biopsy and Congo Red staining can confirm specific organ involvement as seen in our case.
This case adds another layer to our constantly expanding differential as health care practitioners and proves that atypical patient presentations may not be atypical after all. GI amyloidosis tends to present similarly to our patient with bleeding, malabsorption, dysmotility, and protein-losing gastroenteropathy as ascites, edema, pericardial effusions, and laboratory evidence of hypoalbuminemia.7 Because amyloidosis is a systemic illness, early recognition is important as intestinal complications tend to present as symptoms, but mortality is more often caused by renal failure, cardiomyopathy, or ischemic heart disease, making early multispecialty involvement very important.8
Conclusions
Health care practitioners in all specialties should be aware of and include intestinal amyloidosis in their differential diagnosis when working up GI bleeds with the hope of identifying the disease early. With early recognition, rapid biopsy identification, and early specialist involvement, patients will get the opportunity for expedited multidisciplinary treatment and potentially delay rapid decompensation as shown by the evidence in this case.
1. Antunes C, Copelin II EL. Upper gastrointestinal bleeding. StatPearls [internet]. Updated July 18, 2022. Accessed May 25, 2023. https://www.ncbi.nlm.nih.gov/books/NBK470300
2. Almaghrabi M, Gandhi M, Guizzetti L, et al. Comparison of risk scores for lower gastrointestinal bleeding: a systematic review and meta-analysis. JAMA Netw Open. 2022;5(5):e2214253. doi:10.1001/jamanetworkopen.2022.14253
3. Pepys MB. Pathogenesis, diagnosis and treatment of systemic amyloidosis. Philos Trans R Soc Lond B Biol Sci. 2001;356(1406):203-211. doi:10.1098/rstb.2000.0766
4. Cowan AJ, Skinner M, Seldin DC, et al. Amyloidosis of the gastrointestinal tract: a 13-year, single-center, referral experience. Haematologica. 2013;98(1):141-146. doi:10.3324/haematol.2012.068155
5. Lee BS, Chudasama Y, Chen AI, Lim BS, Taira MT. Colonoscopy leading to the diagnosis of AL amyloidosis in the gastrointestinal tract mimicking an acute ulcerative colitis flare. ACG Case Rep J. 2019;6(11):e00289. doi:10.14309/crj.0000000000000289
6. Zhao L, Ren G, Guo J, Chen W, Xu W, Huang X. The clinical features and outcomes of systemic light chain amyloidosis with hepatic involvement. Ann Med. 2022;54(1):1226-1232. doi:10.1080/07853890.2022.2069281
7. Rowe K, Pankow J, Nehme F, Salyers W. Gastrointestinal amyloidosis: review of the literature. Cureus. 2017;9(5):e1228. doi:10.7759/cureus.1228
8. Kyle RA, Greipp PR, O’Fallon WM. Primary systemic amyloidosis: multivariate analysis for prognostic factors in 168 cases. Blood. 1986;68(1):220-224.
Gastrointestinal (GI) bleeding is a common cause of hospital admissions. The yearly incidence of upper GI bleeding is 80 to 150/100,000 people and lower GI bleeding is 87/100,000 people.1,2 The differential tends to initially be broad but narrows with good history followed by endoscopic findings. Getting an appropriate history can be difficult at times, which leads health care practitioners to rely more on interventional results.
Amyloidosis is a rare disorder of abnormal protein folding, leading to the deposition of insoluble fibrils that disrupt normal tissues and cause disease.3 There are 2 main types of amyloidosis, systemic and transthyretin, and 4 subtypes. Systemic amyloidosis includes amyloid light-chain (AL) deposition, caused by plasma cell dyscrasia, and amyloid A (AA) protein deposition, caused by systemic autoimmune illness or infections. Transthyretin amyloidosis is caused by changes and deposition of the transthyretin protein consisting of either unstable, mutant protein or wild type protein. Biopsy-proven amyloidosis of the GI tract is rare.4 About 60% of patients with AA amyloidosis and 8% with AL amyloidosis have GI involvement.5
We present a case of nonspecific symptoms that ultimately lined up perfectly with the official histologic confirmation of intestinal amyloidosis.
Case Presentation
A 79-year-old man with a history of type 2 diabetes mellitus, congestive heart failure, hyperlipidemia, obstructive sleep apnea, hypothyroidism, hypertension, coronary artery disease status postcoronary artery bypass grafting, and stent placements presented for 3 episodes of large, bright red bowel movements. He reported past bleeding and straining with stools, but bleeding of this amount had not been noted prior. He also reported dry heaves, lower abdominal pain, constipation with straining, early satiety with dysphagia, weakness, and decreased appetite. Lastly, he mentioned intentionally losing about 35 to 40 pounds in the past 3 to 4 months and over the past several months increased abdominal distention. However, he stated he had no history of alcohol misuse, liver or intestinal disease, cirrhosis, or other autoimmune diseases. His most recent colonoscopy was more than a decade prior and showed no acute process. The patient never had an esophagogastroduodenoscopy (EGD).
On initial presentation, the patient’s vital signs showed no acute findings. His physical examination noted a chronically ill–appearing male with decreased breath sounds to the bases bilaterally and noted abdominal distention with mild generalized tenderness. Laboratory findings were significant for a hemoglobin level, 9.4 g/dL (reference range, 11.6-15.3); iron, 23 ug/dL (reference range, 45-160); transferrin saturation, 8% (reference range, 15-50); ferritin level, 80 ng/mL (reference range, 30-300); and carcinoembryonic antigen level, 1.5 ng/mL (reference range, 0-2.9). Aspartate aminotransferase level was 54 IU/L (reference range, 0-40); alanine transaminase, 24 IU/L (reference range, 7-52); albumin, 2.7 g/dL (reference range, 3.4-5.7); international normalized ratio, 1.3 (reference range, 0-1.1); creatinine, 1.74 mg/dL (reference range, 0.44-1.27); alkaline phosphatase, 369 IU/L (reference range, 39-117). White blood cell count was 15.5 × 109/L (reference range, 3.5-10.3), and lactic acid was 2.5 mmol/L (reference range, 0.5-2.2). He was started on piperacillin/tazobactam in the emergency department and transitioned to ciprofloxacin and metronidazole for presumed intra-abdominal infection. Paracentesis showed a serum ascites albumin gradient of > 1.1 g/dL with no signs of spontaneous bacterial peritonitis. Computed tomography of the abdomen and pelvis with contrast was suspicious for colitis involving the proximal colon, and colonic mass could not be excluded. Also noted was hepatosplenomegaly with abdominopelvic ascites.
Based on these findings, an EGD and colonoscopy were done. The EGD showed mild portal hypertensive gastropathy.
After the biopsy results, the patient was officially diagnosed with intestinal amyloidosis (Figure 2).
He returned to the gastroenterology clinic 2 months later. At that point, he had worsening symptoms, liver function test results, and international normalized ratio. He was admitted for further investigation. A bone biopsy was done to confirm the histology and define the underlying disorder. The biopsy returned showing Waldenstrom macroglobulinemia, and he was started on bortezomib. Unfortunately, his clinical status rapidly worsened, leading to acute renal and hepatic failure and the development of encephalopathy. He eventually died under palliative care services.
Discussion
Amyloidosis is a rare disorder of abnormal protein folding, leading to the deposition of insoluble fibrils that disrupt normal tissues and cause disease.3 There are several variations of amyloid, but the most common type is AL amyloidosis, which affects several organs, including the heart, kidney, liver, nervous system, and GI tract. When AL amyloidosis involves the liver, the median survival time is about 8.5 months.6 There are different ways to diagnose the disease, but a tissue biopsy and Congo Red staining can confirm specific organ involvement as seen in our case.
This case adds another layer to our constantly expanding differential as health care practitioners and proves that atypical patient presentations may not be atypical after all. GI amyloidosis tends to present similarly to our patient with bleeding, malabsorption, dysmotility, and protein-losing gastroenteropathy as ascites, edema, pericardial effusions, and laboratory evidence of hypoalbuminemia.7 Because amyloidosis is a systemic illness, early recognition is important as intestinal complications tend to present as symptoms, but mortality is more often caused by renal failure, cardiomyopathy, or ischemic heart disease, making early multispecialty involvement very important.8
Conclusions
Health care practitioners in all specialties should be aware of and include intestinal amyloidosis in their differential diagnosis when working up GI bleeds with the hope of identifying the disease early. With early recognition, rapid biopsy identification, and early specialist involvement, patients will get the opportunity for expedited multidisciplinary treatment and potentially delay rapid decompensation as shown by the evidence in this case.
Gastrointestinal (GI) bleeding is a common cause of hospital admissions. The yearly incidence of upper GI bleeding is 80 to 150/100,000 people and lower GI bleeding is 87/100,000 people.1,2 The differential tends to initially be broad but narrows with good history followed by endoscopic findings. Getting an appropriate history can be difficult at times, which leads health care practitioners to rely more on interventional results.
Amyloidosis is a rare disorder of abnormal protein folding, leading to the deposition of insoluble fibrils that disrupt normal tissues and cause disease.3 There are 2 main types of amyloidosis, systemic and transthyretin, and 4 subtypes. Systemic amyloidosis includes amyloid light-chain (AL) deposition, caused by plasma cell dyscrasia, and amyloid A (AA) protein deposition, caused by systemic autoimmune illness or infections. Transthyretin amyloidosis is caused by changes and deposition of the transthyretin protein consisting of either unstable, mutant protein or wild type protein. Biopsy-proven amyloidosis of the GI tract is rare.4 About 60% of patients with AA amyloidosis and 8% with AL amyloidosis have GI involvement.5
We present a case of nonspecific symptoms that ultimately lined up perfectly with the official histologic confirmation of intestinal amyloidosis.
Case Presentation
A 79-year-old man with a history of type 2 diabetes mellitus, congestive heart failure, hyperlipidemia, obstructive sleep apnea, hypothyroidism, hypertension, coronary artery disease status postcoronary artery bypass grafting, and stent placements presented for 3 episodes of large, bright red bowel movements. He reported past bleeding and straining with stools, but bleeding of this amount had not been noted prior. He also reported dry heaves, lower abdominal pain, constipation with straining, early satiety with dysphagia, weakness, and decreased appetite. Lastly, he mentioned intentionally losing about 35 to 40 pounds in the past 3 to 4 months and over the past several months increased abdominal distention. However, he stated he had no history of alcohol misuse, liver or intestinal disease, cirrhosis, or other autoimmune diseases. His most recent colonoscopy was more than a decade prior and showed no acute process. The patient never had an esophagogastroduodenoscopy (EGD).
On initial presentation, the patient’s vital signs showed no acute findings. His physical examination noted a chronically ill–appearing male with decreased breath sounds to the bases bilaterally and noted abdominal distention with mild generalized tenderness. Laboratory findings were significant for a hemoglobin level, 9.4 g/dL (reference range, 11.6-15.3); iron, 23 ug/dL (reference range, 45-160); transferrin saturation, 8% (reference range, 15-50); ferritin level, 80 ng/mL (reference range, 30-300); and carcinoembryonic antigen level, 1.5 ng/mL (reference range, 0-2.9). Aspartate aminotransferase level was 54 IU/L (reference range, 0-40); alanine transaminase, 24 IU/L (reference range, 7-52); albumin, 2.7 g/dL (reference range, 3.4-5.7); international normalized ratio, 1.3 (reference range, 0-1.1); creatinine, 1.74 mg/dL (reference range, 0.44-1.27); alkaline phosphatase, 369 IU/L (reference range, 39-117). White blood cell count was 15.5 × 109/L (reference range, 3.5-10.3), and lactic acid was 2.5 mmol/L (reference range, 0.5-2.2). He was started on piperacillin/tazobactam in the emergency department and transitioned to ciprofloxacin and metronidazole for presumed intra-abdominal infection. Paracentesis showed a serum ascites albumin gradient of > 1.1 g/dL with no signs of spontaneous bacterial peritonitis. Computed tomography of the abdomen and pelvis with contrast was suspicious for colitis involving the proximal colon, and colonic mass could not be excluded. Also noted was hepatosplenomegaly with abdominopelvic ascites.
Based on these findings, an EGD and colonoscopy were done. The EGD showed mild portal hypertensive gastropathy.
After the biopsy results, the patient was officially diagnosed with intestinal amyloidosis (Figure 2).
He returned to the gastroenterology clinic 2 months later. At that point, he had worsening symptoms, liver function test results, and international normalized ratio. He was admitted for further investigation. A bone biopsy was done to confirm the histology and define the underlying disorder. The biopsy returned showing Waldenstrom macroglobulinemia, and he was started on bortezomib. Unfortunately, his clinical status rapidly worsened, leading to acute renal and hepatic failure and the development of encephalopathy. He eventually died under palliative care services.
Discussion
Amyloidosis is a rare disorder of abnormal protein folding, leading to the deposition of insoluble fibrils that disrupt normal tissues and cause disease.3 There are several variations of amyloid, but the most common type is AL amyloidosis, which affects several organs, including the heart, kidney, liver, nervous system, and GI tract. When AL amyloidosis involves the liver, the median survival time is about 8.5 months.6 There are different ways to diagnose the disease, but a tissue biopsy and Congo Red staining can confirm specific organ involvement as seen in our case.
This case adds another layer to our constantly expanding differential as health care practitioners and proves that atypical patient presentations may not be atypical after all. GI amyloidosis tends to present similarly to our patient with bleeding, malabsorption, dysmotility, and protein-losing gastroenteropathy as ascites, edema, pericardial effusions, and laboratory evidence of hypoalbuminemia.7 Because amyloidosis is a systemic illness, early recognition is important as intestinal complications tend to present as symptoms, but mortality is more often caused by renal failure, cardiomyopathy, or ischemic heart disease, making early multispecialty involvement very important.8
Conclusions
Health care practitioners in all specialties should be aware of and include intestinal amyloidosis in their differential diagnosis when working up GI bleeds with the hope of identifying the disease early. With early recognition, rapid biopsy identification, and early specialist involvement, patients will get the opportunity for expedited multidisciplinary treatment and potentially delay rapid decompensation as shown by the evidence in this case.
1. Antunes C, Copelin II EL. Upper gastrointestinal bleeding. StatPearls [internet]. Updated July 18, 2022. Accessed May 25, 2023. https://www.ncbi.nlm.nih.gov/books/NBK470300
2. Almaghrabi M, Gandhi M, Guizzetti L, et al. Comparison of risk scores for lower gastrointestinal bleeding: a systematic review and meta-analysis. JAMA Netw Open. 2022;5(5):e2214253. doi:10.1001/jamanetworkopen.2022.14253
3. Pepys MB. Pathogenesis, diagnosis and treatment of systemic amyloidosis. Philos Trans R Soc Lond B Biol Sci. 2001;356(1406):203-211. doi:10.1098/rstb.2000.0766
4. Cowan AJ, Skinner M, Seldin DC, et al. Amyloidosis of the gastrointestinal tract: a 13-year, single-center, referral experience. Haematologica. 2013;98(1):141-146. doi:10.3324/haematol.2012.068155
5. Lee BS, Chudasama Y, Chen AI, Lim BS, Taira MT. Colonoscopy leading to the diagnosis of AL amyloidosis in the gastrointestinal tract mimicking an acute ulcerative colitis flare. ACG Case Rep J. 2019;6(11):e00289. doi:10.14309/crj.0000000000000289
6. Zhao L, Ren G, Guo J, Chen W, Xu W, Huang X. The clinical features and outcomes of systemic light chain amyloidosis with hepatic involvement. Ann Med. 2022;54(1):1226-1232. doi:10.1080/07853890.2022.2069281
7. Rowe K, Pankow J, Nehme F, Salyers W. Gastrointestinal amyloidosis: review of the literature. Cureus. 2017;9(5):e1228. doi:10.7759/cureus.1228
8. Kyle RA, Greipp PR, O’Fallon WM. Primary systemic amyloidosis: multivariate analysis for prognostic factors in 168 cases. Blood. 1986;68(1):220-224.
1. Antunes C, Copelin II EL. Upper gastrointestinal bleeding. StatPearls [internet]. Updated July 18, 2022. Accessed May 25, 2023. https://www.ncbi.nlm.nih.gov/books/NBK470300
2. Almaghrabi M, Gandhi M, Guizzetti L, et al. Comparison of risk scores for lower gastrointestinal bleeding: a systematic review and meta-analysis. JAMA Netw Open. 2022;5(5):e2214253. doi:10.1001/jamanetworkopen.2022.14253
3. Pepys MB. Pathogenesis, diagnosis and treatment of systemic amyloidosis. Philos Trans R Soc Lond B Biol Sci. 2001;356(1406):203-211. doi:10.1098/rstb.2000.0766
4. Cowan AJ, Skinner M, Seldin DC, et al. Amyloidosis of the gastrointestinal tract: a 13-year, single-center, referral experience. Haematologica. 2013;98(1):141-146. doi:10.3324/haematol.2012.068155
5. Lee BS, Chudasama Y, Chen AI, Lim BS, Taira MT. Colonoscopy leading to the diagnosis of AL amyloidosis in the gastrointestinal tract mimicking an acute ulcerative colitis flare. ACG Case Rep J. 2019;6(11):e00289. doi:10.14309/crj.0000000000000289
6. Zhao L, Ren G, Guo J, Chen W, Xu W, Huang X. The clinical features and outcomes of systemic light chain amyloidosis with hepatic involvement. Ann Med. 2022;54(1):1226-1232. doi:10.1080/07853890.2022.2069281
7. Rowe K, Pankow J, Nehme F, Salyers W. Gastrointestinal amyloidosis: review of the literature. Cureus. 2017;9(5):e1228. doi:10.7759/cureus.1228
8. Kyle RA, Greipp PR, O’Fallon WM. Primary systemic amyloidosis: multivariate analysis for prognostic factors in 168 cases. Blood. 1986;68(1):220-224.
Spider Bite Wound Care and Review of Traditional and Advanced Treatment Options
The costs for wound care play a significant role in total health care costs and are expected to rise dramatically. A 2018 Medicare analysis estimated chronic wound care cost $28.1 to $96.8 billion in supplies, hospitalization, and nursing care: Most costs were accrued in outpatient wound care.1 The global market for advanced wound care supplies is projected to reach $13.7 billion by 2027, and negative wound pressure therapy alone is projected to grow at a compound annual growth rate of 5% over the analysis period 2020 to 2027.2 Chronic wound care also impacts the patient physiologically, socially, and psychologically. One study compared the 5-year mortality of a patient with a diabetic foot ulcer (30.5%) as similar to those patients with cancer (31%).3 Yet the investment in cancer research far outstrips wound care research.
There is no perfect wound dressing for all chronic wounds, but there is expert consensus on interventions that facilitate wound healing. In 2021, Nuutila and Eriksson stated that wound dressings should fulfill the following criteria: protection against trauma, esthetically acceptable, painless to remove, easy to apply, protection for the wound from contamination and further trauma, a moist environment, and an optimal water vapor transmission rate.4 Balanced moisture control is considered essential for healing chronic wounds. Indeed, moisture control within the wound bed may be the most important factor in chronic wound management and healing. The body communicates through a liquid medium, and if that medium is compromised, communication and marshaling of the immune and healing responses may become inefficient.4 Too much moisture, exudate, or fluid in the wound, and the healing is slowed; too little moisture in the wound results in a compromised responses from the body’s immune system, thus delaying healing. In 1988, Dyson and colleagues demonstrated that moist wound care was superior for the inflammatory and proliferative phases of dermal repair compared with dry wound care. The results showed that 5 days after injury, 66% of the cells in the moist wound were fibroblasts and endothelial cells vs 48% of those in the dry wounds.5
The question of dry vs moist wound care has resulted in various wound dressings that produce favorable moisture balance. Moisture balance in a wound creates the ideal environment for wound healing. Sound wound care practices promote the following physiologic responses: increased probability of autolytic debridement; increased collagen synthesis; keratinocyte migration and reepithelization; decreased pain, inflammation, scarring, and necrosis;enhancement of cell-to-cell signaling; and increase in growth factors.5,6 All these processes are mediated through proper wound moisture control. In addition to proper moisture control, antibiotics added to the wound care milieu (either directly to the wound or systemically) may have a place in chronic wound care. In 2013, Junker and colleagues reported that low-dose antibiotics combined with appropriate moisture balance in wounds demonstrated less scar tissue compared with dry wound care.6
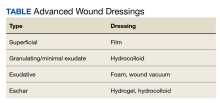
Approaches to chronic wound care are worlds apart: In developing nations the care of chronic wounds often involves traditional management with local products (eg, honey, boiled potato peels, aloe vera gel, banana leaves), whereas in developed nations, more expensive and technologically advanced products are available (eg, wound vacuum, saline wound chamber, hyperbaric oxygen therapy, antibacterial foam). Developing countries often do not have access to technologically advanced wound care products. Local products are often used by local healers, priests, and shamans. The use of these wound interventions in developing countries has produced satisfactory results. In contrast, developed countries have multiple chronic wound care products available (Table).
CASE Presentation
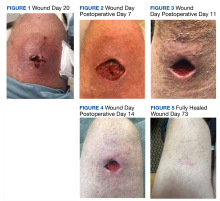
An athletic, healthy 60-year-old Utah National Guard member presented to the George E. Wahlen Department of Veterans Affairs Medical Center in Salt Lake City, Utah, 6 days after experiencing a spider bite. For the first 6 days, the patient applied bacitracin at home. On day 7, the patient noticed that the wound was enlarging and appeared to be fluctuant. The patient was prescribed clindamycin 300 mg 4 times daily on an outpatient basis, which was taken on days 7 to 14.
The wound’s total surface area continued to expand, and the patient returned to the Salt Lake City Veterans Hospital wound care clinic on day 17 stating that the wound was very painful and more fluctuant. The wound care nursing staff were consulted, the wound was debrided, and attempts to drain the wound resulted in minimal exudate expressed from the wound. Clindamycin was increased to 450 mg 4 times daily. However, the wound continued to enlarge and become more painful.
Discussion
Traditional Wound Care
Honey. Honey has been used as a treatment for wounds for almost 3000 years. It has antiseptic and antibacterial properties and contributes to a moist wound care environment. In 2011, Gupta and colleagues reported on the use of honey in 108 patients with burns of < 50% of the total body surface area.7 This report stated that delay in seeking medical care increased wound infection rates, contamination, time to sterilization, and healing. Compared with silver sulfadiazine cream, honey dressings improved the time to wound healing (33 days vs 18 days, respectively), decreased the time to wound sterilization (1 day vs no sterilization), and had better outcomes (37% vs 81%, respectively) with fewer hypertrophic scars and postburn contractures.7
Separate studies in 2011 and 2010 from Fukuda and colleagues and Majtan and colleagues, respectively, reported that honey eliminates pathogens from wounds, augments correct moisture balance, and elevates cytokine activity.8,9 Additional studies in 2006, 2008, and 2014 by Henriques and colleagues, Van den Berg and colleagues, and Majtan suggested that honey reduces reactive oxygen species, is responsible for direct antimicrobial effects in a healing wound, inhibits free radical production, and promotes antitumor activity, respectively.10-12 Van den Berg and colleagues suggested that buckwheat honey is the most effective honey in reducing reactive oxygen species.11
Sterile banana leaves. In medically underserved and rural areas, boiled banana leaves are used to treat burns and nonhealing wounds. In a 2015 study, Waffa and Hayah compared gauze dressings with sterile banana leaves wound dressing in patients with partial thickness burns. Topical antibiotics were added to each type of dressing. The results suggested that the banana leaf dressings were easier to remove, patients reported less pain overall, less pain with dressing changes, and demonstrated a decreased time to healing when contrasted with gauze.13 In 2003, Gore and Akolekar compared autoclaved banana leaves with boiled potato peels in the treatment of patients with partial thickness burns. The time to epithelialization, eschar formation, and skin graft healing were equal in both groups. However, banana leaves were 11 times cheaper and rated easier to prepare than boiled potato peels.14 In a study comparing petroleum gauze with sterile banana leaves, Chendake and colleagues reported that in measures of overall pain and trauma during dressing changes, patients with contused and sutured wounds on the face and neck achieved better outcomes with boiled banana leaves compared with petroleum gauze.15
Boiled potato peels. This treatment is used in rural areas of the world as an adjunct for wound care. In 2015, Manjunath and colleagues theorized that the use of boiled potato peels in patients with necrotizing fasciitis decreased the acidic environment created by the bacteria. Additionally, the study asserted that the toxic wound environment created by the bacteria was neutralized by the potassium content in the peel, and the flavonoids in the peel acted as a free radical scavenger.16 In 2011, Panda and colleagues, using povidone-iodine as a baseline control, reported that peel extract and a peel bandage of sweet potato showed an increased wound closure percentage measured by enhanced epithelialization.17 This increased epithelialization was attributed to the antioxidant effect of the peels enhancing collagen synthesis.17
In contrast, in 1996, a study by Subrahmanyam compared autoclaved potato peel bandages with honey dressings as adjuncts in burn patients with < 40% of the total body surface area affected. The author reported that 90% of the wounds treated with honey were sterile in 7 days, while infection persisted in the potato peel group after 7 days. In the same study, 100% of the wounds treated with honey were healed in 15 days vs 50% in the potato peel group.18 In 1990, Keswani and colleagues compared boiled potato peels with plain gauze as adjuncts in the treatment of burn patients and concluded that although the potato peels had no antibacterial effect, the wounds in both groups had identical bacterial species. But the wounds treated with the potato peels showed reduced desiccation, permitting the survival of skin cells, and enhanced epithelial regeneration.19
Aloe vera. First recorded by the Egyptians and Greeks, aloe vera gel has been used for centuries in many cultures for a variety of ailments, particularly burns and chronic wounds. In a 2016 wound healing study performed on rats, Oryan and colleagues demonstrated that aloe vera gel was superior to saline used as the baseline control. Aloe vera gel used in a dose-dependent fashion demonstrated increased tissue levels of collagen and glycosaminoglycans compared with controls. Aloe vera gel modulated wound inflammation, increased wound contraction, wound epithelialization, decreased scar tissue size, and increased alignment and organization of the scar tissue.20
Gauze. Iodoform gauze is a highly absorbent wound product. Sterile gauze promotes granulation and wound healing. It is well suited for wounds with minimal drainage. However, although gauze is inexpensive, it is easily overwhelmed by the moisture content in the wound, requiring frequent dressing changes (up to 3 times a day), ideally by nursing staff. The resulting increase in nursing care may actually increase the cost of wound care compared with other care modalities.
Petroleum gauze is often used in the care of acute and chronic wounds. However, petroleum-impregnated gauze has a water vapor transmission rate that needs to be remoistened every 4 hours. If the affected area is not remoistened during the exudative phase of wound healing, it may precipitate a delay in healing and increase pain and the prevalence of clinical infections compared with hydrocolloid, film, or foam dressings. Bolton suggested stopping the use of petroleum gauze as the control in studies because it does not provide a balanced and moist wound healing environment.21
Advanced Wound Treatments
Film products. Film products, including plastic food wrap, can be used as wound dressings and meet many of the necessary criteria for enhancing wound healing. These include moisture permeability, carbon dioxide, oxygen transfer, and wound protection. Transmission of moisture varies among products known as the moisture vapor transpiration rate. Film dressings have no absorptive qualities and are unsuited for highly exudative wounds.22,23 Adding polymers, antibacterial, and bioactive agents may increase the wound care properties of film dressings.22 Film dressings excel in protecting shallow nonexudative wounds, are waterproof, and help protect the wound. These products are transparent, allowing clinicians to monitor the progress of the wound without removing the covering, and allowing the dressing to remain in place longer, which decreases the repeated trauma that can occur with dressing changes. Film dressings for wounds differ from those used for IV dressings and should not be used interchangeably.23
Bioactive wound care. These solutions contribute to a moist wound-healing environment. Found naturally in brown seaweed, alginate-containing compounds were used by sailors for centuries to heal wounds. This was known in traditional medicine as the mariner’s cure. Alginate dressings are highly absorbent and can absorb up to 20 times their weight, which makes them desirable for use in highly exudative wounds. First synthesized more than 50 years ago, newer products contain bioactive compounds that prevent tissue damage, stimulate wound healing, improve cell proliferation and migration, and enhance metabolite formation.24-26
In 2018, Aderibigbe and Buyana reported that polymers in the form of hydrogels were able to absorb fluid, making them a suitable choice for minimally exudative wounds. However, in their distended state, the hydrogel subgroup of these products became unstable (perhaps making them a poor choice for extensively exudative wounds), tended to dehydrate, and often needed a secondary dressing, which could lead to wound maceration.22 Most commonly used for wounds with minimal exudate, these dressings shine when used in nominally exudative dry wounds to promote autolytic debridement and hydrate the wound that has formed an eschar.
Hydrocolloid dressings are another type of bioactive wound dressing. These dressings are composed of 2 layers: an inner hydrophilic layer and an outer vapor-permeable layer that promote a moist wound environment. Hydrocolloid dressings assist in hydrating dry eschar wounds and have slight absorbency for exudative wounds. These dressings are not designed to be changed daily and can remain in place for 3 to 6 days. In a 2008 extensive review article, Thomas compared the utility of these dressings in patients with superficial or partial thickness burns, donor sites, surgical wounds, and minor traumatic wounds with basic wound dressings. The results of the review suggested that hydrocolloid dressings conferred statistically significant advantages in measures of decreased pain, healing times (decreased in donor sites by 40%), mobility restriction, and number of dressing changes.27 Although more expensive than basic dressings, the longevity of the hydrocolloid dressing helps defray the original cost. Unfortunately, as these dressings remain in place and continue absorbing exudate, they can take on a very unpleasant odor.
A 2013 Cochrane database review comparing hydrocolloids with foams, alginate, basic wound dressing, and topical treatment found no statistical difference between hydrocolloids and basic wound dressings in patients with diabetes who have noncomplex foot ulcers.28 In 2014, Pott and colleagues suggested a slight superiority in the performance of polyurethane foam dressings over hydrocolloid dressings used in pressure ulcers in older adults.29 In a large pooled analysis in 2010, Davies compared foam to hydrocolloid dressings used in exudative wounds and reported that in 11 of 12 studies, foam dressings were superior to hydrocolloid in terms of exudate management, conformity to the wound, ease of use, decreased trauma and pain at dressing changes, and reduced odor of the wound.30
Foam dressings. These products are typically composed of silicone or polyurethane. Consisting of 2 to 3 layers with a hydrophilic surface, foams are cut to approximate the wound size and serve to wick the macerated wound products to a secondary dressing above the foam. The micropores in the foam matrix absorb exudate from the wound bed while maintaining moisture equilibrium in the wound by donating back moisture to the wound, creating an environment conducive to wound healing. Foam dressings can be combined with various antiseptics (silver, GV/MB, etc) and serve as a delivery vehicle of those products directly to the wound surface.
A 2011 review comparing 8 studies found no difference among foam products available at that time in the use for chronic wounds.31 However, newer products on the market today have produced intriguing results with chronic wounds.
In 2017, Woo and Heil observed that chronic wounds treated with foam products containing GV/MB produced significant improvement when measured at week 4 in the areas of mean wound surface area (42.5%), decrease in baseline Pressure Ulcer Scale for Healing scores (from 13.3 to 10.7), wound coverage by devitalized tissue reduced (from 52.6% to 11.4%), and mean upper and lower wound infection scores were reduced by 75%.32 Further, the researchers reported a moist wound bed was achieved at dressing changes with polyvinyl alcohol (PVA) foam dressing. This led to the presumption that adequate moisture balance and autolytic debridement were facilitated using GV/MB antibacterial PVA foam dressings.
Many foam products on the market today exert an antibacterial effect on the wound bed. Antibiotic properties of various foam dressings create a microenvironment hostile to bacterial growth.32 In addition, the antibacterial properties combined with foam products contribute to the following: autolytic debridement, absorptive qualities (which reduce the bioburden of the wound), and maintenance of moisture in the wound bed. These qualities contribute significantly to the effectiveness of foam products with antibacterial properties.32 The correct balance of moisture in the wound has been identified as a superior environment and perhaps the most important component in chronic wounds.4 Foam dressings are less painful to change, easier to change, and in this case report, contributed to faster wound healing than gauze alone. In 2016, a study by Lee and colleagues suggested that the makeup of the foam product, defined as smaller pore and uniform cell size (foam density), resulted in greater permeability and better moisture absorption and retention capacity, contributing to improved wound healing.33
In 2004, Sibbald and colleagues reported that in a 4-week study of nonhealing chronic wounds, foam wound dressing impregnated with sustained-release silver compared with foam dressing without silver resulted in a reduction in wound size (50% vs 30%, respectively), decreased fluid leakage (27% vs 44% respectively), and reduction in ulcer size measured from baseline (45% vs 25%, respectively).34
In a 2006 study, Varma and colleagues compared sterilized, saline-soaked, nonmedicated polyurethane industrial upholstery foam in nonhealing wounds used in patients with diabetes with conventional techniques using topical antibiotics, hydrocolloid or hydrogel dressings as necessary, and desloughing agents as controls. At the end of a 3-month follow-up period, 100% of the wounds of the foam group had healed compared with 29.2% of the control group. Additionally, the time to wound healing was less than half for the foam group (22.5 days) compared with the control group (52 days), and the time to granulation and epithelialization was faster in the foam group.35
In a 2012 meta-analysis, Aziz and colleagues reported that silver-impregnated dressings and topical silver were no better or worse than controls in preventing wound infection and promoting the healing of burn wounds.36 The authors also noted that the nonsilver dressing groups continuing povidone-iodine, ionic hydrogel, or silicone-coated dressing showed reduced healing time compared with the silver-containing group.36 This is intriguing because silver has long been used as a standard for the treatment of burn wounds.
Conclusions
Although there is no perfect wound dressing, some wound care products seem to perform better due to fewer adverse effects and a much lesser cost. Important aspects of wound care appear to be time from injury to wound care, cleanliness of the wound, moist wound environment, cost, ease of use, and pain of dressing changes.
Primitive wound care products perform admirably in many situations. Modern medicated foam dressings containing antibacterial properties may have beneficial properties compared with other wound care products; however, comparison studies are lacking and need broad-based, randomized, controlled trials to confirm utility. Finally, any choice of wound care product must be tailored to the particular wound and individual patient needs. More large, robust, randomized controlled trials are needed.
Acknowledgments
The authors thank Sarah Maria Paulsen and Rosemary Ellen Brown Smith for their editing, proofreading, and preparation of the manuscript.
1. Nussbaum SR, Carter MJ, Fife CE, et al. An economic evaluation of the impact, cost and Medicare policy implications of chronic non healing wounds. Value Health. 2018;21(1):27-32. doi:10.1016/j.jval.2017.07.007
3. Armstrong DG, Swerdlow MA, Armstrong AA, Conte MS, Padula WV, Bus SA. Five-year mortality and direct costs of care for people with diabetic foot complications are comparable to cancer. J Foot Ankle Res. 2020;13(1)16. doi:10.1186/s13047-020-00383-2
4. Nuutila K, Eriksson E. Moist wound healing with commonly available dressings. Adv Wound Care (New Rochelle). 2021;10(12):685-698. doi:10.1089/wound.2020.1232
5. Dyson M, Young S, Pendle CL, Webster DF, Lang SM. Comparison of the effects of moist and dry conditions on dermal repair. J Investig Dermatol. 1988;91:434-439. doi:10.1111/1523-1747.ep1247646
6. Junker JPE, Kamel RA, Caterson EJ, Eriksson E. Clinical impact upon wound healing and inflammation in moist, wet and dry environments. Adv Wound Care (New Rochelle). 2013;2(7):348-356. doi:10.1089/wound.2012.0412
7. Gupta SS, Singh O, Bhagel PS, Moses S, Shukla S, Mathur RK. Honey dressing versus silver sulfadiazine dressing for wound healing in burn patients: a retrospective study. J Cutan Aesthet Surg. 2011;4(3):183-187. doi:10.4103/0974-2077.91249
8. Fukuda M, Kobayashi K, Hirono Y, et al. Jungle honey enhances immune function and antitumor activity. Evid Based Complement Alternat Med. 2011;2011:1-8. doi:10.1093/ecam/nen086
9. Majtan J, Kumar P, Majtan T, Walls AF, Klaudiny J. Effect of honey and its major royal jelly protein 1 on cytokine and MMP-9 mRNA transcripts in human keratinocytes. Exp Dermatol. 2010;19(8):e73-e79. doi:10.1111/j.1600-0625.2009.00994.x
10. Henriques A, Jackson S, Cooper R, Burton N. Free radical production and quenching in honeys with wound healing potential. J Antimicrob Chemother. 2006;58(4):773-777. doi:10.1093/jac/dkl336
11. Van den Berg AJJ, Van den Worm E, Quarles van Ufford HC, Halkes SBA, M J Hoekstra MJ, Beukelman C J. An in vitro examination of the antioxidant and anti-inflammatory properties of buckwheat honey. J Wound Care. 2008;17(4):172-178. doi:10.12968/jowc.2008.17.4.28839
12. Majtan J. Honey: an immunomodulator in wound healing. Wound Repair Regen. 2014;22(2) 187-192. doi:10.1111/wrr.12117
13. Waffa GA, Hayah AEB. The effectiveness of using banana leaf dressing in management of partial thickness burns’ wound. IJND. 2015;5(4):22-27. doi:10.15520/ijnd.2015.vol5.iss04.70.
14. Gore MA, Akolekar D. Evaluation of banana leaf dressing for partial thickness burn wounds. Burns. 2003;29(5):487-492. doi:10.1016/s0305-4179(03)00050-0
15. Chendake S, Kale T, Manavadaria Y, Motimath AS. Evaluation of banana leaves (Musa paradisiaca) as an alternative wound dressing material compared to conventional petroleum jelly gauze dressing in contused, lacerated and sutured wounds over the head, neck and face region. Cureus. 2021;13(10):1-9. doi:10.7759/cureus.18552
16. Manjunath KS, Bhandage S, Kamat S. ‘Potato peel’ dressing: a novel adjunctive in the management of necrotizing fasciitis. J Maxillofacial Oral Surg. 2015;14(suppl 1):s352-s354. doi:10.1007/s12663-013-0590-8
17. Panda V, Sonkamble M, Patil S. Wound healing activity of Ipomoea batatas tubers (sweet potato). FFHDJ. 2011;1(10):403-415.
18. Subrahmanyam M. Honey dressing versus boiled potato peel in the treatment of burns: a prospective randomized study. Burns. 1996;22(6):491-493. doi:10.1016/0305-4179(96)00007-1.
19. Keswani M H, Vartak AM, Patil A, Davies JW. Histological and bacteriological studies of burn wounds treated with boiled potato peel dressings. Burns. 1990;16(2):137-143. doi:10.1016/0305-4179(90)90175-v
20. Oryan A, Mohammadalipour A, Moshiri A, MR Tabandeh. Topical application of aloe vera accelerated wound healing, modeling, and remodeling, an experimental study. Ann Plast Surg. 2016;77(1)37-46. doi:10.1097/SAP.0000000000000239
21. Bolton L. Evidence corner: April 2007. Wounds. 2007;19(4):A16-A22.
22. Aderibigbe BA, Buyana B. Alginate in wound dressings. Pharmaceutics. 2018;10(2):42. doi.10.3390/pharmaceutics10020042
23. Fletcher J. Using film dressings. Nurs Times. 2003;99(25):57.
24. Ranahewa TH, Premarathna AD, Wijesundara RMKK, Wijewardana V, Jayasooriya AP, Rajapakse RPVJ. Biochemical composition and anticancer effect of different seaweed species (in-vitro and in-vivo studies). Sustainable Marine Structures. 2019;1(2):5-11. doi:10.36956/sms.v1i2.94
25. El Gamal AA. Biological importance of marine algae. Saudi Pharmaceutical J. 2010;18(1):1-25. doi:10.1016/j.jsps.2009.12.001
26. Premarathna AD, Ranahewa TH, Wijesekera RRMKK, et al. Wound healing properties of aqueous extracts of Sargassum Illicifolium: an in vitro assay. Wound Medicine. 2019;24(1):1-7. doi:10.1016/j.wndm.2018.11.001
27. Thomas S. Hydrocolloid dressings in the management of acute wounds: a review of the literature. Int Wound J. 2008;5(5):602-613. doi:10.1111/j.1742-481X.2008.00541.x
28. Dumville JC, Deshpande S, O’Mera K, et al. Hydrocolloid dressing for healing diabetic foot ulcers. Cochrane Database Syst Rev. 2013(8): CD009099. doi:10.1002/14651858.CD009099.pub3
29. Pott FS, Meier MJ, Stocco JGD, Crozeta K, Dayane Ribas J. The effectiveness of hydrocolloid dressings versus other dressings in the healing of pressure ulcers in adults and older adults: a systematic review and meta-analysis. Rev Lat-Am Enfermagem. 2014;22(3):511-520. doi:10.1590/0104-1169.3480.2445
30. Davies P, Rippon M. Comparison of foam and hydrocolloid dressings in the management of wounds: a review of the published literature. Accessed May 17, 2023. http://www.worldwidewounds.com/2010/July/DaviesRippon/DaviesRippon.html
31. Bianchi J, Gray D, Timmons J. Meaume S. Do all foam dressings have the same efficacy in the treatment of chronic wounds? Wounds UK. 2011;7(1):62-67.

33. Lee SM, Park IK, Kim HJ, et al. Physical, morphological, and wound healing properties of a polyurethane foam-film dressing. Biomaterials Res. 2016;20(15):1-11. doi:10.1186/s40824-016-0063-5
34. Sibbald RG, Meaume S, Kirsner RS, et al. Review of the clinical RCT evidence and cost-effectiveness data of a sustained-release silver foam dressing in the healing of critically colonized wounds. December 2005. Updated January 2006. Accessed May 18, 2023. http://www.worldwidewounds.com/2005/december/Sibbald/Silver-Foam-Dressings-Colonised-Wounds.html
35. Varma AK, Kumar H, Kesav Raiesh. Efficacy of polyurethane foam dressing in debrided diabetic lower limb wounds. Wounds. 2006;18(10):300-306.
36. Aziz Z, Abu SF, Chong NJ. A systematic review of silver-containing dressings and topical silver agents (used with dressings) for burn wounds. Burns. 2012;38(3):307-318. doi:10.1016/j.burns.2011.09.020
37. Agarwal P, Kukrele R, Sharma D. Vacuum assisted closure (VAC)/negative pressure wound therapy (NPWT) for difficult wounds: a review. J Clin Orthop Trauma. 2019;10(5):845-848. doi:10.1016/j.jcot.2019.06.015
The costs for wound care play a significant role in total health care costs and are expected to rise dramatically. A 2018 Medicare analysis estimated chronic wound care cost $28.1 to $96.8 billion in supplies, hospitalization, and nursing care: Most costs were accrued in outpatient wound care.1 The global market for advanced wound care supplies is projected to reach $13.7 billion by 2027, and negative wound pressure therapy alone is projected to grow at a compound annual growth rate of 5% over the analysis period 2020 to 2027.2 Chronic wound care also impacts the patient physiologically, socially, and psychologically. One study compared the 5-year mortality of a patient with a diabetic foot ulcer (30.5%) as similar to those patients with cancer (31%).3 Yet the investment in cancer research far outstrips wound care research.
There is no perfect wound dressing for all chronic wounds, but there is expert consensus on interventions that facilitate wound healing. In 2021, Nuutila and Eriksson stated that wound dressings should fulfill the following criteria: protection against trauma, esthetically acceptable, painless to remove, easy to apply, protection for the wound from contamination and further trauma, a moist environment, and an optimal water vapor transmission rate.4 Balanced moisture control is considered essential for healing chronic wounds. Indeed, moisture control within the wound bed may be the most important factor in chronic wound management and healing. The body communicates through a liquid medium, and if that medium is compromised, communication and marshaling of the immune and healing responses may become inefficient.4 Too much moisture, exudate, or fluid in the wound, and the healing is slowed; too little moisture in the wound results in a compromised responses from the body’s immune system, thus delaying healing. In 1988, Dyson and colleagues demonstrated that moist wound care was superior for the inflammatory and proliferative phases of dermal repair compared with dry wound care. The results showed that 5 days after injury, 66% of the cells in the moist wound were fibroblasts and endothelial cells vs 48% of those in the dry wounds.5
The question of dry vs moist wound care has resulted in various wound dressings that produce favorable moisture balance. Moisture balance in a wound creates the ideal environment for wound healing. Sound wound care practices promote the following physiologic responses: increased probability of autolytic debridement; increased collagen synthesis; keratinocyte migration and reepithelization; decreased pain, inflammation, scarring, and necrosis;enhancement of cell-to-cell signaling; and increase in growth factors.5,6 All these processes are mediated through proper wound moisture control. In addition to proper moisture control, antibiotics added to the wound care milieu (either directly to the wound or systemically) may have a place in chronic wound care. In 2013, Junker and colleagues reported that low-dose antibiotics combined with appropriate moisture balance in wounds demonstrated less scar tissue compared with dry wound care.6
Approaches to chronic wound care are worlds apart: In developing nations the care of chronic wounds often involves traditional management with local products (eg, honey, boiled potato peels, aloe vera gel, banana leaves), whereas in developed nations, more expensive and technologically advanced products are available (eg, wound vacuum, saline wound chamber, hyperbaric oxygen therapy, antibacterial foam). Developing countries often do not have access to technologically advanced wound care products. Local products are often used by local healers, priests, and shamans. The use of these wound interventions in developing countries has produced satisfactory results. In contrast, developed countries have multiple chronic wound care products available (Table).
CASE Presentation
An athletic, healthy 60-year-old Utah National Guard member presented to the George E. Wahlen Department of Veterans Affairs Medical Center in Salt Lake City, Utah, 6 days after experiencing a spider bite. For the first 6 days, the patient applied bacitracin at home. On day 7, the patient noticed that the wound was enlarging and appeared to be fluctuant. The patient was prescribed clindamycin 300 mg 4 times daily on an outpatient basis, which was taken on days 7 to 14.
The wound’s total surface area continued to expand, and the patient returned to the Salt Lake City Veterans Hospital wound care clinic on day 17 stating that the wound was very painful and more fluctuant. The wound care nursing staff were consulted, the wound was debrided, and attempts to drain the wound resulted in minimal exudate expressed from the wound. Clindamycin was increased to 450 mg 4 times daily. However, the wound continued to enlarge and become more painful.
Discussion
Traditional Wound Care
Honey. Honey has been used as a treatment for wounds for almost 3000 years. It has antiseptic and antibacterial properties and contributes to a moist wound care environment. In 2011, Gupta and colleagues reported on the use of honey in 108 patients with burns of < 50% of the total body surface area.7 This report stated that delay in seeking medical care increased wound infection rates, contamination, time to sterilization, and healing. Compared with silver sulfadiazine cream, honey dressings improved the time to wound healing (33 days vs 18 days, respectively), decreased the time to wound sterilization (1 day vs no sterilization), and had better outcomes (37% vs 81%, respectively) with fewer hypertrophic scars and postburn contractures.7
Separate studies in 2011 and 2010 from Fukuda and colleagues and Majtan and colleagues, respectively, reported that honey eliminates pathogens from wounds, augments correct moisture balance, and elevates cytokine activity.8,9 Additional studies in 2006, 2008, and 2014 by Henriques and colleagues, Van den Berg and colleagues, and Majtan suggested that honey reduces reactive oxygen species, is responsible for direct antimicrobial effects in a healing wound, inhibits free radical production, and promotes antitumor activity, respectively.10-12 Van den Berg and colleagues suggested that buckwheat honey is the most effective honey in reducing reactive oxygen species.11
Sterile banana leaves. In medically underserved and rural areas, boiled banana leaves are used to treat burns and nonhealing wounds. In a 2015 study, Waffa and Hayah compared gauze dressings with sterile banana leaves wound dressing in patients with partial thickness burns. Topical antibiotics were added to each type of dressing. The results suggested that the banana leaf dressings were easier to remove, patients reported less pain overall, less pain with dressing changes, and demonstrated a decreased time to healing when contrasted with gauze.13 In 2003, Gore and Akolekar compared autoclaved banana leaves with boiled potato peels in the treatment of patients with partial thickness burns. The time to epithelialization, eschar formation, and skin graft healing were equal in both groups. However, banana leaves were 11 times cheaper and rated easier to prepare than boiled potato peels.14 In a study comparing petroleum gauze with sterile banana leaves, Chendake and colleagues reported that in measures of overall pain and trauma during dressing changes, patients with contused and sutured wounds on the face and neck achieved better outcomes with boiled banana leaves compared with petroleum gauze.15
Boiled potato peels. This treatment is used in rural areas of the world as an adjunct for wound care. In 2015, Manjunath and colleagues theorized that the use of boiled potato peels in patients with necrotizing fasciitis decreased the acidic environment created by the bacteria. Additionally, the study asserted that the toxic wound environment created by the bacteria was neutralized by the potassium content in the peel, and the flavonoids in the peel acted as a free radical scavenger.16 In 2011, Panda and colleagues, using povidone-iodine as a baseline control, reported that peel extract and a peel bandage of sweet potato showed an increased wound closure percentage measured by enhanced epithelialization.17 This increased epithelialization was attributed to the antioxidant effect of the peels enhancing collagen synthesis.17
In contrast, in 1996, a study by Subrahmanyam compared autoclaved potato peel bandages with honey dressings as adjuncts in burn patients with < 40% of the total body surface area affected. The author reported that 90% of the wounds treated with honey were sterile in 7 days, while infection persisted in the potato peel group after 7 days. In the same study, 100% of the wounds treated with honey were healed in 15 days vs 50% in the potato peel group.18 In 1990, Keswani and colleagues compared boiled potato peels with plain gauze as adjuncts in the treatment of burn patients and concluded that although the potato peels had no antibacterial effect, the wounds in both groups had identical bacterial species. But the wounds treated with the potato peels showed reduced desiccation, permitting the survival of skin cells, and enhanced epithelial regeneration.19
Aloe vera. First recorded by the Egyptians and Greeks, aloe vera gel has been used for centuries in many cultures for a variety of ailments, particularly burns and chronic wounds. In a 2016 wound healing study performed on rats, Oryan and colleagues demonstrated that aloe vera gel was superior to saline used as the baseline control. Aloe vera gel used in a dose-dependent fashion demonstrated increased tissue levels of collagen and glycosaminoglycans compared with controls. Aloe vera gel modulated wound inflammation, increased wound contraction, wound epithelialization, decreased scar tissue size, and increased alignment and organization of the scar tissue.20
Gauze. Iodoform gauze is a highly absorbent wound product. Sterile gauze promotes granulation and wound healing. It is well suited for wounds with minimal drainage. However, although gauze is inexpensive, it is easily overwhelmed by the moisture content in the wound, requiring frequent dressing changes (up to 3 times a day), ideally by nursing staff. The resulting increase in nursing care may actually increase the cost of wound care compared with other care modalities.
Petroleum gauze is often used in the care of acute and chronic wounds. However, petroleum-impregnated gauze has a water vapor transmission rate that needs to be remoistened every 4 hours. If the affected area is not remoistened during the exudative phase of wound healing, it may precipitate a delay in healing and increase pain and the prevalence of clinical infections compared with hydrocolloid, film, or foam dressings. Bolton suggested stopping the use of petroleum gauze as the control in studies because it does not provide a balanced and moist wound healing environment.21
Advanced Wound Treatments
Film products. Film products, including plastic food wrap, can be used as wound dressings and meet many of the necessary criteria for enhancing wound healing. These include moisture permeability, carbon dioxide, oxygen transfer, and wound protection. Transmission of moisture varies among products known as the moisture vapor transpiration rate. Film dressings have no absorptive qualities and are unsuited for highly exudative wounds.22,23 Adding polymers, antibacterial, and bioactive agents may increase the wound care properties of film dressings.22 Film dressings excel in protecting shallow nonexudative wounds, are waterproof, and help protect the wound. These products are transparent, allowing clinicians to monitor the progress of the wound without removing the covering, and allowing the dressing to remain in place longer, which decreases the repeated trauma that can occur with dressing changes. Film dressings for wounds differ from those used for IV dressings and should not be used interchangeably.23
Bioactive wound care. These solutions contribute to a moist wound-healing environment. Found naturally in brown seaweed, alginate-containing compounds were used by sailors for centuries to heal wounds. This was known in traditional medicine as the mariner’s cure. Alginate dressings are highly absorbent and can absorb up to 20 times their weight, which makes them desirable for use in highly exudative wounds. First synthesized more than 50 years ago, newer products contain bioactive compounds that prevent tissue damage, stimulate wound healing, improve cell proliferation and migration, and enhance metabolite formation.24-26
In 2018, Aderibigbe and Buyana reported that polymers in the form of hydrogels were able to absorb fluid, making them a suitable choice for minimally exudative wounds. However, in their distended state, the hydrogel subgroup of these products became unstable (perhaps making them a poor choice for extensively exudative wounds), tended to dehydrate, and often needed a secondary dressing, which could lead to wound maceration.22 Most commonly used for wounds with minimal exudate, these dressings shine when used in nominally exudative dry wounds to promote autolytic debridement and hydrate the wound that has formed an eschar.
Hydrocolloid dressings are another type of bioactive wound dressing. These dressings are composed of 2 layers: an inner hydrophilic layer and an outer vapor-permeable layer that promote a moist wound environment. Hydrocolloid dressings assist in hydrating dry eschar wounds and have slight absorbency for exudative wounds. These dressings are not designed to be changed daily and can remain in place for 3 to 6 days. In a 2008 extensive review article, Thomas compared the utility of these dressings in patients with superficial or partial thickness burns, donor sites, surgical wounds, and minor traumatic wounds with basic wound dressings. The results of the review suggested that hydrocolloid dressings conferred statistically significant advantages in measures of decreased pain, healing times (decreased in donor sites by 40%), mobility restriction, and number of dressing changes.27 Although more expensive than basic dressings, the longevity of the hydrocolloid dressing helps defray the original cost. Unfortunately, as these dressings remain in place and continue absorbing exudate, they can take on a very unpleasant odor.
A 2013 Cochrane database review comparing hydrocolloids with foams, alginate, basic wound dressing, and topical treatment found no statistical difference between hydrocolloids and basic wound dressings in patients with diabetes who have noncomplex foot ulcers.28 In 2014, Pott and colleagues suggested a slight superiority in the performance of polyurethane foam dressings over hydrocolloid dressings used in pressure ulcers in older adults.29 In a large pooled analysis in 2010, Davies compared foam to hydrocolloid dressings used in exudative wounds and reported that in 11 of 12 studies, foam dressings were superior to hydrocolloid in terms of exudate management, conformity to the wound, ease of use, decreased trauma and pain at dressing changes, and reduced odor of the wound.30
Foam dressings. These products are typically composed of silicone or polyurethane. Consisting of 2 to 3 layers with a hydrophilic surface, foams are cut to approximate the wound size and serve to wick the macerated wound products to a secondary dressing above the foam. The micropores in the foam matrix absorb exudate from the wound bed while maintaining moisture equilibrium in the wound by donating back moisture to the wound, creating an environment conducive to wound healing. Foam dressings can be combined with various antiseptics (silver, GV/MB, etc) and serve as a delivery vehicle of those products directly to the wound surface.
A 2011 review comparing 8 studies found no difference among foam products available at that time in the use for chronic wounds.31 However, newer products on the market today have produced intriguing results with chronic wounds.
In 2017, Woo and Heil observed that chronic wounds treated with foam products containing GV/MB produced significant improvement when measured at week 4 in the areas of mean wound surface area (42.5%), decrease in baseline Pressure Ulcer Scale for Healing scores (from 13.3 to 10.7), wound coverage by devitalized tissue reduced (from 52.6% to 11.4%), and mean upper and lower wound infection scores were reduced by 75%.32 Further, the researchers reported a moist wound bed was achieved at dressing changes with polyvinyl alcohol (PVA) foam dressing. This led to the presumption that adequate moisture balance and autolytic debridement were facilitated using GV/MB antibacterial PVA foam dressings.
Many foam products on the market today exert an antibacterial effect on the wound bed. Antibiotic properties of various foam dressings create a microenvironment hostile to bacterial growth.32 In addition, the antibacterial properties combined with foam products contribute to the following: autolytic debridement, absorptive qualities (which reduce the bioburden of the wound), and maintenance of moisture in the wound bed. These qualities contribute significantly to the effectiveness of foam products with antibacterial properties.32 The correct balance of moisture in the wound has been identified as a superior environment and perhaps the most important component in chronic wounds.4 Foam dressings are less painful to change, easier to change, and in this case report, contributed to faster wound healing than gauze alone. In 2016, a study by Lee and colleagues suggested that the makeup of the foam product, defined as smaller pore and uniform cell size (foam density), resulted in greater permeability and better moisture absorption and retention capacity, contributing to improved wound healing.33
In 2004, Sibbald and colleagues reported that in a 4-week study of nonhealing chronic wounds, foam wound dressing impregnated with sustained-release silver compared with foam dressing without silver resulted in a reduction in wound size (50% vs 30%, respectively), decreased fluid leakage (27% vs 44% respectively), and reduction in ulcer size measured from baseline (45% vs 25%, respectively).34
In a 2006 study, Varma and colleagues compared sterilized, saline-soaked, nonmedicated polyurethane industrial upholstery foam in nonhealing wounds used in patients with diabetes with conventional techniques using topical antibiotics, hydrocolloid or hydrogel dressings as necessary, and desloughing agents as controls. At the end of a 3-month follow-up period, 100% of the wounds of the foam group had healed compared with 29.2% of the control group. Additionally, the time to wound healing was less than half for the foam group (22.5 days) compared with the control group (52 days), and the time to granulation and epithelialization was faster in the foam group.35
In a 2012 meta-analysis, Aziz and colleagues reported that silver-impregnated dressings and topical silver were no better or worse than controls in preventing wound infection and promoting the healing of burn wounds.36 The authors also noted that the nonsilver dressing groups continuing povidone-iodine, ionic hydrogel, or silicone-coated dressing showed reduced healing time compared with the silver-containing group.36 This is intriguing because silver has long been used as a standard for the treatment of burn wounds.
Conclusions
Although there is no perfect wound dressing, some wound care products seem to perform better due to fewer adverse effects and a much lesser cost. Important aspects of wound care appear to be time from injury to wound care, cleanliness of the wound, moist wound environment, cost, ease of use, and pain of dressing changes.
Primitive wound care products perform admirably in many situations. Modern medicated foam dressings containing antibacterial properties may have beneficial properties compared with other wound care products; however, comparison studies are lacking and need broad-based, randomized, controlled trials to confirm utility. Finally, any choice of wound care product must be tailored to the particular wound and individual patient needs. More large, robust, randomized controlled trials are needed.
Acknowledgments
The authors thank Sarah Maria Paulsen and Rosemary Ellen Brown Smith for their editing, proofreading, and preparation of the manuscript.
The costs for wound care play a significant role in total health care costs and are expected to rise dramatically. A 2018 Medicare analysis estimated chronic wound care cost $28.1 to $96.8 billion in supplies, hospitalization, and nursing care: Most costs were accrued in outpatient wound care.1 The global market for advanced wound care supplies is projected to reach $13.7 billion by 2027, and negative wound pressure therapy alone is projected to grow at a compound annual growth rate of 5% over the analysis period 2020 to 2027.2 Chronic wound care also impacts the patient physiologically, socially, and psychologically. One study compared the 5-year mortality of a patient with a diabetic foot ulcer (30.5%) as similar to those patients with cancer (31%).3 Yet the investment in cancer research far outstrips wound care research.
There is no perfect wound dressing for all chronic wounds, but there is expert consensus on interventions that facilitate wound healing. In 2021, Nuutila and Eriksson stated that wound dressings should fulfill the following criteria: protection against trauma, esthetically acceptable, painless to remove, easy to apply, protection for the wound from contamination and further trauma, a moist environment, and an optimal water vapor transmission rate.4 Balanced moisture control is considered essential for healing chronic wounds. Indeed, moisture control within the wound bed may be the most important factor in chronic wound management and healing. The body communicates through a liquid medium, and if that medium is compromised, communication and marshaling of the immune and healing responses may become inefficient.4 Too much moisture, exudate, or fluid in the wound, and the healing is slowed; too little moisture in the wound results in a compromised responses from the body’s immune system, thus delaying healing. In 1988, Dyson and colleagues demonstrated that moist wound care was superior for the inflammatory and proliferative phases of dermal repair compared with dry wound care. The results showed that 5 days after injury, 66% of the cells in the moist wound were fibroblasts and endothelial cells vs 48% of those in the dry wounds.5
The question of dry vs moist wound care has resulted in various wound dressings that produce favorable moisture balance. Moisture balance in a wound creates the ideal environment for wound healing. Sound wound care practices promote the following physiologic responses: increased probability of autolytic debridement; increased collagen synthesis; keratinocyte migration and reepithelization; decreased pain, inflammation, scarring, and necrosis;enhancement of cell-to-cell signaling; and increase in growth factors.5,6 All these processes are mediated through proper wound moisture control. In addition to proper moisture control, antibiotics added to the wound care milieu (either directly to the wound or systemically) may have a place in chronic wound care. In 2013, Junker and colleagues reported that low-dose antibiotics combined with appropriate moisture balance in wounds demonstrated less scar tissue compared with dry wound care.6
Approaches to chronic wound care are worlds apart: In developing nations the care of chronic wounds often involves traditional management with local products (eg, honey, boiled potato peels, aloe vera gel, banana leaves), whereas in developed nations, more expensive and technologically advanced products are available (eg, wound vacuum, saline wound chamber, hyperbaric oxygen therapy, antibacterial foam). Developing countries often do not have access to technologically advanced wound care products. Local products are often used by local healers, priests, and shamans. The use of these wound interventions in developing countries has produced satisfactory results. In contrast, developed countries have multiple chronic wound care products available (Table).
CASE Presentation
An athletic, healthy 60-year-old Utah National Guard member presented to the George E. Wahlen Department of Veterans Affairs Medical Center in Salt Lake City, Utah, 6 days after experiencing a spider bite. For the first 6 days, the patient applied bacitracin at home. On day 7, the patient noticed that the wound was enlarging and appeared to be fluctuant. The patient was prescribed clindamycin 300 mg 4 times daily on an outpatient basis, which was taken on days 7 to 14.
The wound’s total surface area continued to expand, and the patient returned to the Salt Lake City Veterans Hospital wound care clinic on day 17 stating that the wound was very painful and more fluctuant. The wound care nursing staff were consulted, the wound was debrided, and attempts to drain the wound resulted in minimal exudate expressed from the wound. Clindamycin was increased to 450 mg 4 times daily. However, the wound continued to enlarge and become more painful.
Discussion
Traditional Wound Care
Honey. Honey has been used as a treatment for wounds for almost 3000 years. It has antiseptic and antibacterial properties and contributes to a moist wound care environment. In 2011, Gupta and colleagues reported on the use of honey in 108 patients with burns of < 50% of the total body surface area.7 This report stated that delay in seeking medical care increased wound infection rates, contamination, time to sterilization, and healing. Compared with silver sulfadiazine cream, honey dressings improved the time to wound healing (33 days vs 18 days, respectively), decreased the time to wound sterilization (1 day vs no sterilization), and had better outcomes (37% vs 81%, respectively) with fewer hypertrophic scars and postburn contractures.7
Separate studies in 2011 and 2010 from Fukuda and colleagues and Majtan and colleagues, respectively, reported that honey eliminates pathogens from wounds, augments correct moisture balance, and elevates cytokine activity.8,9 Additional studies in 2006, 2008, and 2014 by Henriques and colleagues, Van den Berg and colleagues, and Majtan suggested that honey reduces reactive oxygen species, is responsible for direct antimicrobial effects in a healing wound, inhibits free radical production, and promotes antitumor activity, respectively.10-12 Van den Berg and colleagues suggested that buckwheat honey is the most effective honey in reducing reactive oxygen species.11
Sterile banana leaves. In medically underserved and rural areas, boiled banana leaves are used to treat burns and nonhealing wounds. In a 2015 study, Waffa and Hayah compared gauze dressings with sterile banana leaves wound dressing in patients with partial thickness burns. Topical antibiotics were added to each type of dressing. The results suggested that the banana leaf dressings were easier to remove, patients reported less pain overall, less pain with dressing changes, and demonstrated a decreased time to healing when contrasted with gauze.13 In 2003, Gore and Akolekar compared autoclaved banana leaves with boiled potato peels in the treatment of patients with partial thickness burns. The time to epithelialization, eschar formation, and skin graft healing were equal in both groups. However, banana leaves were 11 times cheaper and rated easier to prepare than boiled potato peels.14 In a study comparing petroleum gauze with sterile banana leaves, Chendake and colleagues reported that in measures of overall pain and trauma during dressing changes, patients with contused and sutured wounds on the face and neck achieved better outcomes with boiled banana leaves compared with petroleum gauze.15
Boiled potato peels. This treatment is used in rural areas of the world as an adjunct for wound care. In 2015, Manjunath and colleagues theorized that the use of boiled potato peels in patients with necrotizing fasciitis decreased the acidic environment created by the bacteria. Additionally, the study asserted that the toxic wound environment created by the bacteria was neutralized by the potassium content in the peel, and the flavonoids in the peel acted as a free radical scavenger.16 In 2011, Panda and colleagues, using povidone-iodine as a baseline control, reported that peel extract and a peel bandage of sweet potato showed an increased wound closure percentage measured by enhanced epithelialization.17 This increased epithelialization was attributed to the antioxidant effect of the peels enhancing collagen synthesis.17
In contrast, in 1996, a study by Subrahmanyam compared autoclaved potato peel bandages with honey dressings as adjuncts in burn patients with < 40% of the total body surface area affected. The author reported that 90% of the wounds treated with honey were sterile in 7 days, while infection persisted in the potato peel group after 7 days. In the same study, 100% of the wounds treated with honey were healed in 15 days vs 50% in the potato peel group.18 In 1990, Keswani and colleagues compared boiled potato peels with plain gauze as adjuncts in the treatment of burn patients and concluded that although the potato peels had no antibacterial effect, the wounds in both groups had identical bacterial species. But the wounds treated with the potato peels showed reduced desiccation, permitting the survival of skin cells, and enhanced epithelial regeneration.19
Aloe vera. First recorded by the Egyptians and Greeks, aloe vera gel has been used for centuries in many cultures for a variety of ailments, particularly burns and chronic wounds. In a 2016 wound healing study performed on rats, Oryan and colleagues demonstrated that aloe vera gel was superior to saline used as the baseline control. Aloe vera gel used in a dose-dependent fashion demonstrated increased tissue levels of collagen and glycosaminoglycans compared with controls. Aloe vera gel modulated wound inflammation, increased wound contraction, wound epithelialization, decreased scar tissue size, and increased alignment and organization of the scar tissue.20
Gauze. Iodoform gauze is a highly absorbent wound product. Sterile gauze promotes granulation and wound healing. It is well suited for wounds with minimal drainage. However, although gauze is inexpensive, it is easily overwhelmed by the moisture content in the wound, requiring frequent dressing changes (up to 3 times a day), ideally by nursing staff. The resulting increase in nursing care may actually increase the cost of wound care compared with other care modalities.
Petroleum gauze is often used in the care of acute and chronic wounds. However, petroleum-impregnated gauze has a water vapor transmission rate that needs to be remoistened every 4 hours. If the affected area is not remoistened during the exudative phase of wound healing, it may precipitate a delay in healing and increase pain and the prevalence of clinical infections compared with hydrocolloid, film, or foam dressings. Bolton suggested stopping the use of petroleum gauze as the control in studies because it does not provide a balanced and moist wound healing environment.21
Advanced Wound Treatments
Film products. Film products, including plastic food wrap, can be used as wound dressings and meet many of the necessary criteria for enhancing wound healing. These include moisture permeability, carbon dioxide, oxygen transfer, and wound protection. Transmission of moisture varies among products known as the moisture vapor transpiration rate. Film dressings have no absorptive qualities and are unsuited for highly exudative wounds.22,23 Adding polymers, antibacterial, and bioactive agents may increase the wound care properties of film dressings.22 Film dressings excel in protecting shallow nonexudative wounds, are waterproof, and help protect the wound. These products are transparent, allowing clinicians to monitor the progress of the wound without removing the covering, and allowing the dressing to remain in place longer, which decreases the repeated trauma that can occur with dressing changes. Film dressings for wounds differ from those used for IV dressings and should not be used interchangeably.23
Bioactive wound care. These solutions contribute to a moist wound-healing environment. Found naturally in brown seaweed, alginate-containing compounds were used by sailors for centuries to heal wounds. This was known in traditional medicine as the mariner’s cure. Alginate dressings are highly absorbent and can absorb up to 20 times their weight, which makes them desirable for use in highly exudative wounds. First synthesized more than 50 years ago, newer products contain bioactive compounds that prevent tissue damage, stimulate wound healing, improve cell proliferation and migration, and enhance metabolite formation.24-26
In 2018, Aderibigbe and Buyana reported that polymers in the form of hydrogels were able to absorb fluid, making them a suitable choice for minimally exudative wounds. However, in their distended state, the hydrogel subgroup of these products became unstable (perhaps making them a poor choice for extensively exudative wounds), tended to dehydrate, and often needed a secondary dressing, which could lead to wound maceration.22 Most commonly used for wounds with minimal exudate, these dressings shine when used in nominally exudative dry wounds to promote autolytic debridement and hydrate the wound that has formed an eschar.
Hydrocolloid dressings are another type of bioactive wound dressing. These dressings are composed of 2 layers: an inner hydrophilic layer and an outer vapor-permeable layer that promote a moist wound environment. Hydrocolloid dressings assist in hydrating dry eschar wounds and have slight absorbency for exudative wounds. These dressings are not designed to be changed daily and can remain in place for 3 to 6 days. In a 2008 extensive review article, Thomas compared the utility of these dressings in patients with superficial or partial thickness burns, donor sites, surgical wounds, and minor traumatic wounds with basic wound dressings. The results of the review suggested that hydrocolloid dressings conferred statistically significant advantages in measures of decreased pain, healing times (decreased in donor sites by 40%), mobility restriction, and number of dressing changes.27 Although more expensive than basic dressings, the longevity of the hydrocolloid dressing helps defray the original cost. Unfortunately, as these dressings remain in place and continue absorbing exudate, they can take on a very unpleasant odor.
A 2013 Cochrane database review comparing hydrocolloids with foams, alginate, basic wound dressing, and topical treatment found no statistical difference between hydrocolloids and basic wound dressings in patients with diabetes who have noncomplex foot ulcers.28 In 2014, Pott and colleagues suggested a slight superiority in the performance of polyurethane foam dressings over hydrocolloid dressings used in pressure ulcers in older adults.29 In a large pooled analysis in 2010, Davies compared foam to hydrocolloid dressings used in exudative wounds and reported that in 11 of 12 studies, foam dressings were superior to hydrocolloid in terms of exudate management, conformity to the wound, ease of use, decreased trauma and pain at dressing changes, and reduced odor of the wound.30
Foam dressings. These products are typically composed of silicone or polyurethane. Consisting of 2 to 3 layers with a hydrophilic surface, foams are cut to approximate the wound size and serve to wick the macerated wound products to a secondary dressing above the foam. The micropores in the foam matrix absorb exudate from the wound bed while maintaining moisture equilibrium in the wound by donating back moisture to the wound, creating an environment conducive to wound healing. Foam dressings can be combined with various antiseptics (silver, GV/MB, etc) and serve as a delivery vehicle of those products directly to the wound surface.
A 2011 review comparing 8 studies found no difference among foam products available at that time in the use for chronic wounds.31 However, newer products on the market today have produced intriguing results with chronic wounds.
In 2017, Woo and Heil observed that chronic wounds treated with foam products containing GV/MB produced significant improvement when measured at week 4 in the areas of mean wound surface area (42.5%), decrease in baseline Pressure Ulcer Scale for Healing scores (from 13.3 to 10.7), wound coverage by devitalized tissue reduced (from 52.6% to 11.4%), and mean upper and lower wound infection scores were reduced by 75%.32 Further, the researchers reported a moist wound bed was achieved at dressing changes with polyvinyl alcohol (PVA) foam dressing. This led to the presumption that adequate moisture balance and autolytic debridement were facilitated using GV/MB antibacterial PVA foam dressings.
Many foam products on the market today exert an antibacterial effect on the wound bed. Antibiotic properties of various foam dressings create a microenvironment hostile to bacterial growth.32 In addition, the antibacterial properties combined with foam products contribute to the following: autolytic debridement, absorptive qualities (which reduce the bioburden of the wound), and maintenance of moisture in the wound bed. These qualities contribute significantly to the effectiveness of foam products with antibacterial properties.32 The correct balance of moisture in the wound has been identified as a superior environment and perhaps the most important component in chronic wounds.4 Foam dressings are less painful to change, easier to change, and in this case report, contributed to faster wound healing than gauze alone. In 2016, a study by Lee and colleagues suggested that the makeup of the foam product, defined as smaller pore and uniform cell size (foam density), resulted in greater permeability and better moisture absorption and retention capacity, contributing to improved wound healing.33
In 2004, Sibbald and colleagues reported that in a 4-week study of nonhealing chronic wounds, foam wound dressing impregnated with sustained-release silver compared with foam dressing without silver resulted in a reduction in wound size (50% vs 30%, respectively), decreased fluid leakage (27% vs 44% respectively), and reduction in ulcer size measured from baseline (45% vs 25%, respectively).34
In a 2006 study, Varma and colleagues compared sterilized, saline-soaked, nonmedicated polyurethane industrial upholstery foam in nonhealing wounds used in patients with diabetes with conventional techniques using topical antibiotics, hydrocolloid or hydrogel dressings as necessary, and desloughing agents as controls. At the end of a 3-month follow-up period, 100% of the wounds of the foam group had healed compared with 29.2% of the control group. Additionally, the time to wound healing was less than half for the foam group (22.5 days) compared with the control group (52 days), and the time to granulation and epithelialization was faster in the foam group.35
In a 2012 meta-analysis, Aziz and colleagues reported that silver-impregnated dressings and topical silver were no better or worse than controls in preventing wound infection and promoting the healing of burn wounds.36 The authors also noted that the nonsilver dressing groups continuing povidone-iodine, ionic hydrogel, or silicone-coated dressing showed reduced healing time compared with the silver-containing group.36 This is intriguing because silver has long been used as a standard for the treatment of burn wounds.
Conclusions
Although there is no perfect wound dressing, some wound care products seem to perform better due to fewer adverse effects and a much lesser cost. Important aspects of wound care appear to be time from injury to wound care, cleanliness of the wound, moist wound environment, cost, ease of use, and pain of dressing changes.
Primitive wound care products perform admirably in many situations. Modern medicated foam dressings containing antibacterial properties may have beneficial properties compared with other wound care products; however, comparison studies are lacking and need broad-based, randomized, controlled trials to confirm utility. Finally, any choice of wound care product must be tailored to the particular wound and individual patient needs. More large, robust, randomized controlled trials are needed.
Acknowledgments
The authors thank Sarah Maria Paulsen and Rosemary Ellen Brown Smith for their editing, proofreading, and preparation of the manuscript.
1. Nussbaum SR, Carter MJ, Fife CE, et al. An economic evaluation of the impact, cost and Medicare policy implications of chronic non healing wounds. Value Health. 2018;21(1):27-32. doi:10.1016/j.jval.2017.07.007
3. Armstrong DG, Swerdlow MA, Armstrong AA, Conte MS, Padula WV, Bus SA. Five-year mortality and direct costs of care for people with diabetic foot complications are comparable to cancer. J Foot Ankle Res. 2020;13(1)16. doi:10.1186/s13047-020-00383-2
4. Nuutila K, Eriksson E. Moist wound healing with commonly available dressings. Adv Wound Care (New Rochelle). 2021;10(12):685-698. doi:10.1089/wound.2020.1232
5. Dyson M, Young S, Pendle CL, Webster DF, Lang SM. Comparison of the effects of moist and dry conditions on dermal repair. J Investig Dermatol. 1988;91:434-439. doi:10.1111/1523-1747.ep1247646
6. Junker JPE, Kamel RA, Caterson EJ, Eriksson E. Clinical impact upon wound healing and inflammation in moist, wet and dry environments. Adv Wound Care (New Rochelle). 2013;2(7):348-356. doi:10.1089/wound.2012.0412
7. Gupta SS, Singh O, Bhagel PS, Moses S, Shukla S, Mathur RK. Honey dressing versus silver sulfadiazine dressing for wound healing in burn patients: a retrospective study. J Cutan Aesthet Surg. 2011;4(3):183-187. doi:10.4103/0974-2077.91249
8. Fukuda M, Kobayashi K, Hirono Y, et al. Jungle honey enhances immune function and antitumor activity. Evid Based Complement Alternat Med. 2011;2011:1-8. doi:10.1093/ecam/nen086
9. Majtan J, Kumar P, Majtan T, Walls AF, Klaudiny J. Effect of honey and its major royal jelly protein 1 on cytokine and MMP-9 mRNA transcripts in human keratinocytes. Exp Dermatol. 2010;19(8):e73-e79. doi:10.1111/j.1600-0625.2009.00994.x
10. Henriques A, Jackson S, Cooper R, Burton N. Free radical production and quenching in honeys with wound healing potential. J Antimicrob Chemother. 2006;58(4):773-777. doi:10.1093/jac/dkl336
11. Van den Berg AJJ, Van den Worm E, Quarles van Ufford HC, Halkes SBA, M J Hoekstra MJ, Beukelman C J. An in vitro examination of the antioxidant and anti-inflammatory properties of buckwheat honey. J Wound Care. 2008;17(4):172-178. doi:10.12968/jowc.2008.17.4.28839
12. Majtan J. Honey: an immunomodulator in wound healing. Wound Repair Regen. 2014;22(2) 187-192. doi:10.1111/wrr.12117
13. Waffa GA, Hayah AEB. The effectiveness of using banana leaf dressing in management of partial thickness burns’ wound. IJND. 2015;5(4):22-27. doi:10.15520/ijnd.2015.vol5.iss04.70.
14. Gore MA, Akolekar D. Evaluation of banana leaf dressing for partial thickness burn wounds. Burns. 2003;29(5):487-492. doi:10.1016/s0305-4179(03)00050-0
15. Chendake S, Kale T, Manavadaria Y, Motimath AS. Evaluation of banana leaves (Musa paradisiaca) as an alternative wound dressing material compared to conventional petroleum jelly gauze dressing in contused, lacerated and sutured wounds over the head, neck and face region. Cureus. 2021;13(10):1-9. doi:10.7759/cureus.18552
16. Manjunath KS, Bhandage S, Kamat S. ‘Potato peel’ dressing: a novel adjunctive in the management of necrotizing fasciitis. J Maxillofacial Oral Surg. 2015;14(suppl 1):s352-s354. doi:10.1007/s12663-013-0590-8
17. Panda V, Sonkamble M, Patil S. Wound healing activity of Ipomoea batatas tubers (sweet potato). FFHDJ. 2011;1(10):403-415.
18. Subrahmanyam M. Honey dressing versus boiled potato peel in the treatment of burns: a prospective randomized study. Burns. 1996;22(6):491-493. doi:10.1016/0305-4179(96)00007-1.
19. Keswani M H, Vartak AM, Patil A, Davies JW. Histological and bacteriological studies of burn wounds treated with boiled potato peel dressings. Burns. 1990;16(2):137-143. doi:10.1016/0305-4179(90)90175-v
20. Oryan A, Mohammadalipour A, Moshiri A, MR Tabandeh. Topical application of aloe vera accelerated wound healing, modeling, and remodeling, an experimental study. Ann Plast Surg. 2016;77(1)37-46. doi:10.1097/SAP.0000000000000239
21. Bolton L. Evidence corner: April 2007. Wounds. 2007;19(4):A16-A22.
22. Aderibigbe BA, Buyana B. Alginate in wound dressings. Pharmaceutics. 2018;10(2):42. doi.10.3390/pharmaceutics10020042
23. Fletcher J. Using film dressings. Nurs Times. 2003;99(25):57.
24. Ranahewa TH, Premarathna AD, Wijesundara RMKK, Wijewardana V, Jayasooriya AP, Rajapakse RPVJ. Biochemical composition and anticancer effect of different seaweed species (in-vitro and in-vivo studies). Sustainable Marine Structures. 2019;1(2):5-11. doi:10.36956/sms.v1i2.94
25. El Gamal AA. Biological importance of marine algae. Saudi Pharmaceutical J. 2010;18(1):1-25. doi:10.1016/j.jsps.2009.12.001
26. Premarathna AD, Ranahewa TH, Wijesekera RRMKK, et al. Wound healing properties of aqueous extracts of Sargassum Illicifolium: an in vitro assay. Wound Medicine. 2019;24(1):1-7. doi:10.1016/j.wndm.2018.11.001
27. Thomas S. Hydrocolloid dressings in the management of acute wounds: a review of the literature. Int Wound J. 2008;5(5):602-613. doi:10.1111/j.1742-481X.2008.00541.x
28. Dumville JC, Deshpande S, O’Mera K, et al. Hydrocolloid dressing for healing diabetic foot ulcers. Cochrane Database Syst Rev. 2013(8): CD009099. doi:10.1002/14651858.CD009099.pub3
29. Pott FS, Meier MJ, Stocco JGD, Crozeta K, Dayane Ribas J. The effectiveness of hydrocolloid dressings versus other dressings in the healing of pressure ulcers in adults and older adults: a systematic review and meta-analysis. Rev Lat-Am Enfermagem. 2014;22(3):511-520. doi:10.1590/0104-1169.3480.2445
30. Davies P, Rippon M. Comparison of foam and hydrocolloid dressings in the management of wounds: a review of the published literature. Accessed May 17, 2023. http://www.worldwidewounds.com/2010/July/DaviesRippon/DaviesRippon.html
31. Bianchi J, Gray D, Timmons J. Meaume S. Do all foam dressings have the same efficacy in the treatment of chronic wounds? Wounds UK. 2011;7(1):62-67.

33. Lee SM, Park IK, Kim HJ, et al. Physical, morphological, and wound healing properties of a polyurethane foam-film dressing. Biomaterials Res. 2016;20(15):1-11. doi:10.1186/s40824-016-0063-5
34. Sibbald RG, Meaume S, Kirsner RS, et al. Review of the clinical RCT evidence and cost-effectiveness data of a sustained-release silver foam dressing in the healing of critically colonized wounds. December 2005. Updated January 2006. Accessed May 18, 2023. http://www.worldwidewounds.com/2005/december/Sibbald/Silver-Foam-Dressings-Colonised-Wounds.html
35. Varma AK, Kumar H, Kesav Raiesh. Efficacy of polyurethane foam dressing in debrided diabetic lower limb wounds. Wounds. 2006;18(10):300-306.
36. Aziz Z, Abu SF, Chong NJ. A systematic review of silver-containing dressings and topical silver agents (used with dressings) for burn wounds. Burns. 2012;38(3):307-318. doi:10.1016/j.burns.2011.09.020
37. Agarwal P, Kukrele R, Sharma D. Vacuum assisted closure (VAC)/negative pressure wound therapy (NPWT) for difficult wounds: a review. J Clin Orthop Trauma. 2019;10(5):845-848. doi:10.1016/j.jcot.2019.06.015
1. Nussbaum SR, Carter MJ, Fife CE, et al. An economic evaluation of the impact, cost and Medicare policy implications of chronic non healing wounds. Value Health. 2018;21(1):27-32. doi:10.1016/j.jval.2017.07.007
3. Armstrong DG, Swerdlow MA, Armstrong AA, Conte MS, Padula WV, Bus SA. Five-year mortality and direct costs of care for people with diabetic foot complications are comparable to cancer. J Foot Ankle Res. 2020;13(1)16. doi:10.1186/s13047-020-00383-2
4. Nuutila K, Eriksson E. Moist wound healing with commonly available dressings. Adv Wound Care (New Rochelle). 2021;10(12):685-698. doi:10.1089/wound.2020.1232
5. Dyson M, Young S, Pendle CL, Webster DF, Lang SM. Comparison of the effects of moist and dry conditions on dermal repair. J Investig Dermatol. 1988;91:434-439. doi:10.1111/1523-1747.ep1247646
6. Junker JPE, Kamel RA, Caterson EJ, Eriksson E. Clinical impact upon wound healing and inflammation in moist, wet and dry environments. Adv Wound Care (New Rochelle). 2013;2(7):348-356. doi:10.1089/wound.2012.0412
7. Gupta SS, Singh O, Bhagel PS, Moses S, Shukla S, Mathur RK. Honey dressing versus silver sulfadiazine dressing for wound healing in burn patients: a retrospective study. J Cutan Aesthet Surg. 2011;4(3):183-187. doi:10.4103/0974-2077.91249
8. Fukuda M, Kobayashi K, Hirono Y, et al. Jungle honey enhances immune function and antitumor activity. Evid Based Complement Alternat Med. 2011;2011:1-8. doi:10.1093/ecam/nen086
9. Majtan J, Kumar P, Majtan T, Walls AF, Klaudiny J. Effect of honey and its major royal jelly protein 1 on cytokine and MMP-9 mRNA transcripts in human keratinocytes. Exp Dermatol. 2010;19(8):e73-e79. doi:10.1111/j.1600-0625.2009.00994.x
10. Henriques A, Jackson S, Cooper R, Burton N. Free radical production and quenching in honeys with wound healing potential. J Antimicrob Chemother. 2006;58(4):773-777. doi:10.1093/jac/dkl336
11. Van den Berg AJJ, Van den Worm E, Quarles van Ufford HC, Halkes SBA, M J Hoekstra MJ, Beukelman C J. An in vitro examination of the antioxidant and anti-inflammatory properties of buckwheat honey. J Wound Care. 2008;17(4):172-178. doi:10.12968/jowc.2008.17.4.28839
12. Majtan J. Honey: an immunomodulator in wound healing. Wound Repair Regen. 2014;22(2) 187-192. doi:10.1111/wrr.12117
13. Waffa GA, Hayah AEB. The effectiveness of using banana leaf dressing in management of partial thickness burns’ wound. IJND. 2015;5(4):22-27. doi:10.15520/ijnd.2015.vol5.iss04.70.
14. Gore MA, Akolekar D. Evaluation of banana leaf dressing for partial thickness burn wounds. Burns. 2003;29(5):487-492. doi:10.1016/s0305-4179(03)00050-0
15. Chendake S, Kale T, Manavadaria Y, Motimath AS. Evaluation of banana leaves (Musa paradisiaca) as an alternative wound dressing material compared to conventional petroleum jelly gauze dressing in contused, lacerated and sutured wounds over the head, neck and face region. Cureus. 2021;13(10):1-9. doi:10.7759/cureus.18552
16. Manjunath KS, Bhandage S, Kamat S. ‘Potato peel’ dressing: a novel adjunctive in the management of necrotizing fasciitis. J Maxillofacial Oral Surg. 2015;14(suppl 1):s352-s354. doi:10.1007/s12663-013-0590-8
17. Panda V, Sonkamble M, Patil S. Wound healing activity of Ipomoea batatas tubers (sweet potato). FFHDJ. 2011;1(10):403-415.
18. Subrahmanyam M. Honey dressing versus boiled potato peel in the treatment of burns: a prospective randomized study. Burns. 1996;22(6):491-493. doi:10.1016/0305-4179(96)00007-1.
19. Keswani M H, Vartak AM, Patil A, Davies JW. Histological and bacteriological studies of burn wounds treated with boiled potato peel dressings. Burns. 1990;16(2):137-143. doi:10.1016/0305-4179(90)90175-v
20. Oryan A, Mohammadalipour A, Moshiri A, MR Tabandeh. Topical application of aloe vera accelerated wound healing, modeling, and remodeling, an experimental study. Ann Plast Surg. 2016;77(1)37-46. doi:10.1097/SAP.0000000000000239
21. Bolton L. Evidence corner: April 2007. Wounds. 2007;19(4):A16-A22.
22. Aderibigbe BA, Buyana B. Alginate in wound dressings. Pharmaceutics. 2018;10(2):42. doi.10.3390/pharmaceutics10020042
23. Fletcher J. Using film dressings. Nurs Times. 2003;99(25):57.
24. Ranahewa TH, Premarathna AD, Wijesundara RMKK, Wijewardana V, Jayasooriya AP, Rajapakse RPVJ. Biochemical composition and anticancer effect of different seaweed species (in-vitro and in-vivo studies). Sustainable Marine Structures. 2019;1(2):5-11. doi:10.36956/sms.v1i2.94
25. El Gamal AA. Biological importance of marine algae. Saudi Pharmaceutical J. 2010;18(1):1-25. doi:10.1016/j.jsps.2009.12.001
26. Premarathna AD, Ranahewa TH, Wijesekera RRMKK, et al. Wound healing properties of aqueous extracts of Sargassum Illicifolium: an in vitro assay. Wound Medicine. 2019;24(1):1-7. doi:10.1016/j.wndm.2018.11.001
27. Thomas S. Hydrocolloid dressings in the management of acute wounds: a review of the literature. Int Wound J. 2008;5(5):602-613. doi:10.1111/j.1742-481X.2008.00541.x
28. Dumville JC, Deshpande S, O’Mera K, et al. Hydrocolloid dressing for healing diabetic foot ulcers. Cochrane Database Syst Rev. 2013(8): CD009099. doi:10.1002/14651858.CD009099.pub3
29. Pott FS, Meier MJ, Stocco JGD, Crozeta K, Dayane Ribas J. The effectiveness of hydrocolloid dressings versus other dressings in the healing of pressure ulcers in adults and older adults: a systematic review and meta-analysis. Rev Lat-Am Enfermagem. 2014;22(3):511-520. doi:10.1590/0104-1169.3480.2445
30. Davies P, Rippon M. Comparison of foam and hydrocolloid dressings in the management of wounds: a review of the published literature. Accessed May 17, 2023. http://www.worldwidewounds.com/2010/July/DaviesRippon/DaviesRippon.html
31. Bianchi J, Gray D, Timmons J. Meaume S. Do all foam dressings have the same efficacy in the treatment of chronic wounds? Wounds UK. 2011;7(1):62-67.

33. Lee SM, Park IK, Kim HJ, et al. Physical, morphological, and wound healing properties of a polyurethane foam-film dressing. Biomaterials Res. 2016;20(15):1-11. doi:10.1186/s40824-016-0063-5
34. Sibbald RG, Meaume S, Kirsner RS, et al. Review of the clinical RCT evidence and cost-effectiveness data of a sustained-release silver foam dressing in the healing of critically colonized wounds. December 2005. Updated January 2006. Accessed May 18, 2023. http://www.worldwidewounds.com/2005/december/Sibbald/Silver-Foam-Dressings-Colonised-Wounds.html
35. Varma AK, Kumar H, Kesav Raiesh. Efficacy of polyurethane foam dressing in debrided diabetic lower limb wounds. Wounds. 2006;18(10):300-306.
36. Aziz Z, Abu SF, Chong NJ. A systematic review of silver-containing dressings and topical silver agents (used with dressings) for burn wounds. Burns. 2012;38(3):307-318. doi:10.1016/j.burns.2011.09.020
37. Agarwal P, Kukrele R, Sharma D. Vacuum assisted closure (VAC)/negative pressure wound therapy (NPWT) for difficult wounds: a review. J Clin Orthop Trauma. 2019;10(5):845-848. doi:10.1016/j.jcot.2019.06.015
30-year-old woman • progressive dyspnea and peripheral edema • 35th week of gestation with a history of mild preeclampsia • Dx?
THE CASE
A 30-year-old woman sought care at her rural family physician’s office for progressive dyspnea and peripheral edema, which she had been experiencing for several weeks. She was G1P0 and in her 35th week of gestation.
Her medical history was remarkable for mild preeclampsia, which was being managed observantly by her obstetrician in consultation with a maternal-fetal medicine specialist. She had been evaluated by her local hospital’s labor and delivery department and her maternal-fetal medicine specialist earlier in the week and seen the previous day by her obstetrician for these signs and symptoms. They all reassured her and told her these symptoms were normal during pregnancy. No diagnostic studies were performed. However, she remained concerned and decided to see her family physician for another opinion.
Upon presentation to her family physician, the patient was afebrile. Her blood pressure was 135/98 mm Hg; heart rate, 96 beats/min; and respiration, 20 breaths/min and slightly labored. Edema of 2 to 3+ was noted in her lower extremities, hands, and face. Bibasilar breath sounds were diminished, and her abdomen was nontender.
The family physician suspected left ventricular systolic dysfunction. He worked in a small office that lacked access to a laboratory or radiographic studies. However, he did have an ultrasound machine available, and although he was not skilled in echocardiography to assess cardiac function, he was able to obtain a bedside lung ultrasound.
THE DIAGNOSIS
While no B-lines were seen on the lung ultrasound, bilateral plural effusions were noted (FIGURE). This finding, paired with the patient’s signs and symptoms, prompted the family physician to suspect a diagnosis of acute decompensated heart failure with presumptive peripartum cardiomyopathy. The patient was immediately driven to the hospital by her family physician for emergency admission with stat obstetric and cardiology consultations.
An in-hospital echocardiogram revealed severe global hypokinesia with a left ventricular ejection fraction of 25% to 30%, which confirmed the family physician’s suspicions. Laboratory studies were significant for elevated N-terminal pro-brain natriuretic peptide (43,449 pg/mL; normal, < 125 pg/mL), troponin (1.12 ng/mL; normal range, 0-0.10 ng/mL), and white blood cell count (27.6 x 103/µL). She also had evidence of acute renal injury, with blood urea nitrogen of 46 mg/dL (normal range, 7-18 mg/dL), creatinine of 2.0 mg/dL (normal range, 0.5-1.0 mg/dL), and potassium of 7.6 mmol/L (normal range, 3.5-5.1 mmol/L). Emergency delivery was induced by amniotomy, resulting in the birth of a baby girl weighing 5 lb 4 oz (Apgar scores 6, 8, and 9).
Following delivery, the patient was placed on a milrinone infusion and required dialysis. She was emergently transferred to a tertiary care hospital, where she was admitted to the cardiac intensive care unit by the cardiology/heart transplant service with nephrology and obstetric consultations. Hematology and infectious disease specialists were consulted to rule out HELLP (hemolysis, elevated liver enzymes, and low platelets) syndrome and sepsis, respectively. Her course of care remained complicated with further testing, including cardiac catheterization and biopsy, which was negative for additional pathology.
Continue to: One week after admission...
One week after admission, she was discharged home with a 24-hour wearable external cardiac defibrillator and a confirmed diagnosis of peripartum cardiomyopathy. Her medication regimen included digoxin (125 µg 3 times/wk), spironolactone (25 mg/d), carvedilol (3.125 mg twice daily), sacubitril/valsartan (24 mg/26 mg twice daily), furosemide (20 mg/d as needed for weight gain > 3-4 lb or leg swelling), magnesium oxide (400 mg twice daily), and ferrous sulfate (325 mg/d).
DISCUSSION
Peripartum cardiomyopathy is a rare, life-threatening, idiopathic cardiomyopathy that is responsible for one-half to two-thirds of cardiovascular disease–related maternal deaths in the United States.1,2 It manifests in late pregnancy or early in the postpartum period and is characterized by left ventricular systolic dysfunction with resultant heart failure and an ejection fraction of less than 45%.1,2
Recognized as early as the 1800s by Virchow,2,3 the incidence of peripartum cardiomyopathy in the United States ranges from 1 in 1000 to 4000 live births and is increasing worldwide.1,2 While the cause of peripartum cardiomyopathy remains unknown, risk factors include advanced maternal age, African descent, hypertension, preeclampsia, and multiple gestation pregnancy.1,2
Early diagnosis of peripartum cardiomyopathy is imperative for survival of both mother and baby.4 This may be difficult because the signs and symptoms of heart failure—such as dyspnea, edema, orthopnea, cough, and chest and abdominal pain—overlap with those of a typical pregnancy, resulting in it often being missed on evaluation.1,2
Dx with echocardiography; in a pinch, consider lung ultrasound
Usually a diagnosis of peripartum cardiomyopathy is established with echocardiography.1,2 Thus, this case is of significant importance because it illustrates the successful use of lung ultrasound—a simple and easy test—by a rural family doctor to identify this potentially fatal, elusive condition with no additional studies.
Continue to: Use of lung ultrasound...
Use of lung ultrasound in the detection of acute decompensated heart failure is accepted in the medical literature.5-7 Given clinical correlation, a positive scan is defined by the presence of at least 3 B-lines on a longitudinal plane between 2 ribs or, as seen in our case, by the presence of pleural effusion.5-8 Lung ultrasound is readily available worldwide, is completely safe in pregnancy, and is considered one of the easiest studies to perform.7-10
At the patient’s 9-month follow-up visit, she had made a full clinical recovery. Her ejection fraction was 59.8%, and she had stopped all medications. The patient and her child did not experience any continued complications.
THE TAKEAWAY
Family physicians should be aware of peripartum cardiomyopathy—one of the most elusive and life-threatening diseases of pregnancy. When managing a pregnant patient, it is imperative to follow up on complaints such as dyspnea, peripheral edema, and chest and/or abdominal pain. While these symptoms are not unusual during pregnancy, they should always prompt a more thorough evaluation. If peripartum cardiomyopathy is suspected, lung ultrasound is a valuable diagnostic tool for family physicians. Further research is needed before the findings of this case report can be universally applied in the routine prenatal care of women at risk for peripartum cardiomyopathy.
The authors thank their daughter, Nickel Cielo Abarbanell, for her help in the preparation of this manuscript.
CORRESPONDENCE
Neal Robert Abarbanell, MD, First Choice Healthcare, 1867 20th Avenue, Vero Beach, FL 32960; neal.abarbanell@ gmail.com
1. Honigberg MC, Givertz MM. Peripartum cardiomyopathy. BMJ. 2019;364:k5287. doi: 10.1136/bmj.k5287
2. Arany Z, Elkayam U. Peripartum cardiomyopathy. Circulation. 2016;133:1397-1409. doi: 10.1161/CIRCULATIONAHA.115.020491
3. Porak C. De L’influence reciproque de la grossesse et del maladies du Coceur [thesis]. Medical Faculty of Paris, France: 1880.
4. Lewey J, Levine LD, Elovitz MA, et al. Importance of early diagnosis in peripartum cardiomyopathy. Hypertension. 2020;75:91-97. doi: 10.1161/HYPERTENSIONAHA.119.13291
5. Volpicelli G, Caramello V, Cardinale L, et al. Bedside ultrasound of the lung for the monitoring of acute decompensated heart failure. Am J Emerg Med. 2008;26:585-591. doi: 10.1016/j.ajem.2007.09.014
6. Muniz RT, Mesquita ET, Souza CV Jr, et al. Pulmonary ultrasound in patients with heart failure-systematic review. Arq Bras Cardiol. 2018;110:577-584. doi: 10.5935/abc.20180097
7. Russell FM, Rutz M, Pang PS. Focused ultrasound in the emergency department for patients with acute heart failure. Card Fail Rev. 2015;1:83-86. doi: 10.15420/cfr.2015.1.2.83
8. Gustafsson M, Alehagen U, Johansson P. Imaging congestion with a pocket ultrasound device: prognostic implications in patients with chronic heart failure. J Card Fail. 2015;21:548-554. doi: 10.1016/j.cardfail.2015.02.004
9. Ntusi NA, Samuels P, Moosa S, et al. Diagnosing cardiac disease during pregnancy: imaging modalities. Cardiovasc J Afr. 2016;27:95-103. doi: 10.5830/CVJA-2016-022
10. Kimberly HH, Murray A, Mennicke M, et al. Focused maternal ultrasound by midwives in rural Zambia. Ultrasound Med Biol. 2010;36:1267-1272. doi: 10.1016/j.ultrasmedbio.2010.05.017
THE CASE
A 30-year-old woman sought care at her rural family physician’s office for progressive dyspnea and peripheral edema, which she had been experiencing for several weeks. She was G1P0 and in her 35th week of gestation.
Her medical history was remarkable for mild preeclampsia, which was being managed observantly by her obstetrician in consultation with a maternal-fetal medicine specialist. She had been evaluated by her local hospital’s labor and delivery department and her maternal-fetal medicine specialist earlier in the week and seen the previous day by her obstetrician for these signs and symptoms. They all reassured her and told her these symptoms were normal during pregnancy. No diagnostic studies were performed. However, she remained concerned and decided to see her family physician for another opinion.
Upon presentation to her family physician, the patient was afebrile. Her blood pressure was 135/98 mm Hg; heart rate, 96 beats/min; and respiration, 20 breaths/min and slightly labored. Edema of 2 to 3+ was noted in her lower extremities, hands, and face. Bibasilar breath sounds were diminished, and her abdomen was nontender.
The family physician suspected left ventricular systolic dysfunction. He worked in a small office that lacked access to a laboratory or radiographic studies. However, he did have an ultrasound machine available, and although he was not skilled in echocardiography to assess cardiac function, he was able to obtain a bedside lung ultrasound.
THE DIAGNOSIS
While no B-lines were seen on the lung ultrasound, bilateral plural effusions were noted (FIGURE). This finding, paired with the patient’s signs and symptoms, prompted the family physician to suspect a diagnosis of acute decompensated heart failure with presumptive peripartum cardiomyopathy. The patient was immediately driven to the hospital by her family physician for emergency admission with stat obstetric and cardiology consultations.

An in-hospital echocardiogram revealed severe global hypokinesia with a left ventricular ejection fraction of 25% to 30%, which confirmed the family physician’s suspicions. Laboratory studies were significant for elevated N-terminal pro-brain natriuretic peptide (43,449 pg/mL; normal, < 125 pg/mL), troponin (1.12 ng/mL; normal range, 0-0.10 ng/mL), and white blood cell count (27.6 x 103/µL). She also had evidence of acute renal injury, with blood urea nitrogen of 46 mg/dL (normal range, 7-18 mg/dL), creatinine of 2.0 mg/dL (normal range, 0.5-1.0 mg/dL), and potassium of 7.6 mmol/L (normal range, 3.5-5.1 mmol/L). Emergency delivery was induced by amniotomy, resulting in the birth of a baby girl weighing 5 lb 4 oz (Apgar scores 6, 8, and 9).
Following delivery, the patient was placed on a milrinone infusion and required dialysis. She was emergently transferred to a tertiary care hospital, where she was admitted to the cardiac intensive care unit by the cardiology/heart transplant service with nephrology and obstetric consultations. Hematology and infectious disease specialists were consulted to rule out HELLP (hemolysis, elevated liver enzymes, and low platelets) syndrome and sepsis, respectively. Her course of care remained complicated with further testing, including cardiac catheterization and biopsy, which was negative for additional pathology.
Continue to: One week after admission...
One week after admission, she was discharged home with a 24-hour wearable external cardiac defibrillator and a confirmed diagnosis of peripartum cardiomyopathy. Her medication regimen included digoxin (125 µg 3 times/wk), spironolactone (25 mg/d), carvedilol (3.125 mg twice daily), sacubitril/valsartan (24 mg/26 mg twice daily), furosemide (20 mg/d as needed for weight gain > 3-4 lb or leg swelling), magnesium oxide (400 mg twice daily), and ferrous sulfate (325 mg/d).
DISCUSSION
Peripartum cardiomyopathy is a rare, life-threatening, idiopathic cardiomyopathy that is responsible for one-half to two-thirds of cardiovascular disease–related maternal deaths in the United States.1,2 It manifests in late pregnancy or early in the postpartum period and is characterized by left ventricular systolic dysfunction with resultant heart failure and an ejection fraction of less than 45%.1,2
Recognized as early as the 1800s by Virchow,2,3 the incidence of peripartum cardiomyopathy in the United States ranges from 1 in 1000 to 4000 live births and is increasing worldwide.1,2 While the cause of peripartum cardiomyopathy remains unknown, risk factors include advanced maternal age, African descent, hypertension, preeclampsia, and multiple gestation pregnancy.1,2
Early diagnosis of peripartum cardiomyopathy is imperative for survival of both mother and baby.4 This may be difficult because the signs and symptoms of heart failure—such as dyspnea, edema, orthopnea, cough, and chest and abdominal pain—overlap with those of a typical pregnancy, resulting in it often being missed on evaluation.1,2
Dx with echocardiography; in a pinch, consider lung ultrasound
Usually a diagnosis of peripartum cardiomyopathy is established with echocardiography.1,2 Thus, this case is of significant importance because it illustrates the successful use of lung ultrasound—a simple and easy test—by a rural family doctor to identify this potentially fatal, elusive condition with no additional studies.
Continue to: Use of lung ultrasound...
Use of lung ultrasound in the detection of acute decompensated heart failure is accepted in the medical literature.5-7 Given clinical correlation, a positive scan is defined by the presence of at least 3 B-lines on a longitudinal plane between 2 ribs or, as seen in our case, by the presence of pleural effusion.5-8 Lung ultrasound is readily available worldwide, is completely safe in pregnancy, and is considered one of the easiest studies to perform.7-10
At the patient’s 9-month follow-up visit, she had made a full clinical recovery. Her ejection fraction was 59.8%, and she had stopped all medications. The patient and her child did not experience any continued complications.
THE TAKEAWAY
Family physicians should be aware of peripartum cardiomyopathy—one of the most elusive and life-threatening diseases of pregnancy. When managing a pregnant patient, it is imperative to follow up on complaints such as dyspnea, peripheral edema, and chest and/or abdominal pain. While these symptoms are not unusual during pregnancy, they should always prompt a more thorough evaluation. If peripartum cardiomyopathy is suspected, lung ultrasound is a valuable diagnostic tool for family physicians. Further research is needed before the findings of this case report can be universally applied in the routine prenatal care of women at risk for peripartum cardiomyopathy.
The authors thank their daughter, Nickel Cielo Abarbanell, for her help in the preparation of this manuscript.
CORRESPONDENCE
Neal Robert Abarbanell, MD, First Choice Healthcare, 1867 20th Avenue, Vero Beach, FL 32960; neal.abarbanell@ gmail.com
THE CASE
A 30-year-old woman sought care at her rural family physician’s office for progressive dyspnea and peripheral edema, which she had been experiencing for several weeks. She was G1P0 and in her 35th week of gestation.
Her medical history was remarkable for mild preeclampsia, which was being managed observantly by her obstetrician in consultation with a maternal-fetal medicine specialist. She had been evaluated by her local hospital’s labor and delivery department and her maternal-fetal medicine specialist earlier in the week and seen the previous day by her obstetrician for these signs and symptoms. They all reassured her and told her these symptoms were normal during pregnancy. No diagnostic studies were performed. However, she remained concerned and decided to see her family physician for another opinion.
Upon presentation to her family physician, the patient was afebrile. Her blood pressure was 135/98 mm Hg; heart rate, 96 beats/min; and respiration, 20 breaths/min and slightly labored. Edema of 2 to 3+ was noted in her lower extremities, hands, and face. Bibasilar breath sounds were diminished, and her abdomen was nontender.
The family physician suspected left ventricular systolic dysfunction. He worked in a small office that lacked access to a laboratory or radiographic studies. However, he did have an ultrasound machine available, and although he was not skilled in echocardiography to assess cardiac function, he was able to obtain a bedside lung ultrasound.
THE DIAGNOSIS
While no B-lines were seen on the lung ultrasound, bilateral plural effusions were noted (FIGURE). This finding, paired with the patient’s signs and symptoms, prompted the family physician to suspect a diagnosis of acute decompensated heart failure with presumptive peripartum cardiomyopathy. The patient was immediately driven to the hospital by her family physician for emergency admission with stat obstetric and cardiology consultations.

An in-hospital echocardiogram revealed severe global hypokinesia with a left ventricular ejection fraction of 25% to 30%, which confirmed the family physician’s suspicions. Laboratory studies were significant for elevated N-terminal pro-brain natriuretic peptide (43,449 pg/mL; normal, < 125 pg/mL), troponin (1.12 ng/mL; normal range, 0-0.10 ng/mL), and white blood cell count (27.6 x 103/µL). She also had evidence of acute renal injury, with blood urea nitrogen of 46 mg/dL (normal range, 7-18 mg/dL), creatinine of 2.0 mg/dL (normal range, 0.5-1.0 mg/dL), and potassium of 7.6 mmol/L (normal range, 3.5-5.1 mmol/L). Emergency delivery was induced by amniotomy, resulting in the birth of a baby girl weighing 5 lb 4 oz (Apgar scores 6, 8, and 9).
Following delivery, the patient was placed on a milrinone infusion and required dialysis. She was emergently transferred to a tertiary care hospital, where she was admitted to the cardiac intensive care unit by the cardiology/heart transplant service with nephrology and obstetric consultations. Hematology and infectious disease specialists were consulted to rule out HELLP (hemolysis, elevated liver enzymes, and low platelets) syndrome and sepsis, respectively. Her course of care remained complicated with further testing, including cardiac catheterization and biopsy, which was negative for additional pathology.
Continue to: One week after admission...
One week after admission, she was discharged home with a 24-hour wearable external cardiac defibrillator and a confirmed diagnosis of peripartum cardiomyopathy. Her medication regimen included digoxin (125 µg 3 times/wk), spironolactone (25 mg/d), carvedilol (3.125 mg twice daily), sacubitril/valsartan (24 mg/26 mg twice daily), furosemide (20 mg/d as needed for weight gain > 3-4 lb or leg swelling), magnesium oxide (400 mg twice daily), and ferrous sulfate (325 mg/d).
DISCUSSION
Peripartum cardiomyopathy is a rare, life-threatening, idiopathic cardiomyopathy that is responsible for one-half to two-thirds of cardiovascular disease–related maternal deaths in the United States.1,2 It manifests in late pregnancy or early in the postpartum period and is characterized by left ventricular systolic dysfunction with resultant heart failure and an ejection fraction of less than 45%.1,2
Recognized as early as the 1800s by Virchow,2,3 the incidence of peripartum cardiomyopathy in the United States ranges from 1 in 1000 to 4000 live births and is increasing worldwide.1,2 While the cause of peripartum cardiomyopathy remains unknown, risk factors include advanced maternal age, African descent, hypertension, preeclampsia, and multiple gestation pregnancy.1,2
Early diagnosis of peripartum cardiomyopathy is imperative for survival of both mother and baby.4 This may be difficult because the signs and symptoms of heart failure—such as dyspnea, edema, orthopnea, cough, and chest and abdominal pain—overlap with those of a typical pregnancy, resulting in it often being missed on evaluation.1,2
Dx with echocardiography; in a pinch, consider lung ultrasound
Usually a diagnosis of peripartum cardiomyopathy is established with echocardiography.1,2 Thus, this case is of significant importance because it illustrates the successful use of lung ultrasound—a simple and easy test—by a rural family doctor to identify this potentially fatal, elusive condition with no additional studies.
Continue to: Use of lung ultrasound...
Use of lung ultrasound in the detection of acute decompensated heart failure is accepted in the medical literature.5-7 Given clinical correlation, a positive scan is defined by the presence of at least 3 B-lines on a longitudinal plane between 2 ribs or, as seen in our case, by the presence of pleural effusion.5-8 Lung ultrasound is readily available worldwide, is completely safe in pregnancy, and is considered one of the easiest studies to perform.7-10
At the patient’s 9-month follow-up visit, she had made a full clinical recovery. Her ejection fraction was 59.8%, and she had stopped all medications. The patient and her child did not experience any continued complications.
THE TAKEAWAY
Family physicians should be aware of peripartum cardiomyopathy—one of the most elusive and life-threatening diseases of pregnancy. When managing a pregnant patient, it is imperative to follow up on complaints such as dyspnea, peripheral edema, and chest and/or abdominal pain. While these symptoms are not unusual during pregnancy, they should always prompt a more thorough evaluation. If peripartum cardiomyopathy is suspected, lung ultrasound is a valuable diagnostic tool for family physicians. Further research is needed before the findings of this case report can be universally applied in the routine prenatal care of women at risk for peripartum cardiomyopathy.
The authors thank their daughter, Nickel Cielo Abarbanell, for her help in the preparation of this manuscript.
CORRESPONDENCE
Neal Robert Abarbanell, MD, First Choice Healthcare, 1867 20th Avenue, Vero Beach, FL 32960; neal.abarbanell@ gmail.com
1. Honigberg MC, Givertz MM. Peripartum cardiomyopathy. BMJ. 2019;364:k5287. doi: 10.1136/bmj.k5287
2. Arany Z, Elkayam U. Peripartum cardiomyopathy. Circulation. 2016;133:1397-1409. doi: 10.1161/CIRCULATIONAHA.115.020491
3. Porak C. De L’influence reciproque de la grossesse et del maladies du Coceur [thesis]. Medical Faculty of Paris, France: 1880.
4. Lewey J, Levine LD, Elovitz MA, et al. Importance of early diagnosis in peripartum cardiomyopathy. Hypertension. 2020;75:91-97. doi: 10.1161/HYPERTENSIONAHA.119.13291
5. Volpicelli G, Caramello V, Cardinale L, et al. Bedside ultrasound of the lung for the monitoring of acute decompensated heart failure. Am J Emerg Med. 2008;26:585-591. doi: 10.1016/j.ajem.2007.09.014
6. Muniz RT, Mesquita ET, Souza CV Jr, et al. Pulmonary ultrasound in patients with heart failure-systematic review. Arq Bras Cardiol. 2018;110:577-584. doi: 10.5935/abc.20180097
7. Russell FM, Rutz M, Pang PS. Focused ultrasound in the emergency department for patients with acute heart failure. Card Fail Rev. 2015;1:83-86. doi: 10.15420/cfr.2015.1.2.83
8. Gustafsson M, Alehagen U, Johansson P. Imaging congestion with a pocket ultrasound device: prognostic implications in patients with chronic heart failure. J Card Fail. 2015;21:548-554. doi: 10.1016/j.cardfail.2015.02.004
9. Ntusi NA, Samuels P, Moosa S, et al. Diagnosing cardiac disease during pregnancy: imaging modalities. Cardiovasc J Afr. 2016;27:95-103. doi: 10.5830/CVJA-2016-022
10. Kimberly HH, Murray A, Mennicke M, et al. Focused maternal ultrasound by midwives in rural Zambia. Ultrasound Med Biol. 2010;36:1267-1272. doi: 10.1016/j.ultrasmedbio.2010.05.017
1. Honigberg MC, Givertz MM. Peripartum cardiomyopathy. BMJ. 2019;364:k5287. doi: 10.1136/bmj.k5287
2. Arany Z, Elkayam U. Peripartum cardiomyopathy. Circulation. 2016;133:1397-1409. doi: 10.1161/CIRCULATIONAHA.115.020491
3. Porak C. De L’influence reciproque de la grossesse et del maladies du Coceur [thesis]. Medical Faculty of Paris, France: 1880.
4. Lewey J, Levine LD, Elovitz MA, et al. Importance of early diagnosis in peripartum cardiomyopathy. Hypertension. 2020;75:91-97. doi: 10.1161/HYPERTENSIONAHA.119.13291
5. Volpicelli G, Caramello V, Cardinale L, et al. Bedside ultrasound of the lung for the monitoring of acute decompensated heart failure. Am J Emerg Med. 2008;26:585-591. doi: 10.1016/j.ajem.2007.09.014
6. Muniz RT, Mesquita ET, Souza CV Jr, et al. Pulmonary ultrasound in patients with heart failure-systematic review. Arq Bras Cardiol. 2018;110:577-584. doi: 10.5935/abc.20180097
7. Russell FM, Rutz M, Pang PS. Focused ultrasound in the emergency department for patients with acute heart failure. Card Fail Rev. 2015;1:83-86. doi: 10.15420/cfr.2015.1.2.83
8. Gustafsson M, Alehagen U, Johansson P. Imaging congestion with a pocket ultrasound device: prognostic implications in patients with chronic heart failure. J Card Fail. 2015;21:548-554. doi: 10.1016/j.cardfail.2015.02.004
9. Ntusi NA, Samuels P, Moosa S, et al. Diagnosing cardiac disease during pregnancy: imaging modalities. Cardiovasc J Afr. 2016;27:95-103. doi: 10.5830/CVJA-2016-022
10. Kimberly HH, Murray A, Mennicke M, et al. Focused maternal ultrasound by midwives in rural Zambia. Ultrasound Med Biol. 2010;36:1267-1272. doi: 10.1016/j.ultrasmedbio.2010.05.017
► Progressive dyspnea and peripheral edema
► 35th week of gestation with a history of mild preeclampsia
A Case of Duodenocaval Fistula in the Setting of Respiratory Failure Initially Confused for Transfusion-Related Acute Lung Injury
A duodenocaval fistula (DCF) is seen when a connection exists between the duodenum and the inferior vena cava. It is a rare entity that is commonly missed and presents a diagnostic challenge due to its nonspecific presenting symptoms.1,2 Patients commonly present with gastrointestinal (GI) bleeding or sepsis. Here we present a case of a 37-year-old man who presented to the hospital for a workup related to melena but went into cardiac arrest prior to an esophagogastroduodenoscopy. Unfortunately, on autopsy, the patient was found to have a DCF. We highlight the diagnostic challenge associated with DCF and how in this case the presentation was confused by a diagnosis of possible transfusion-related acute lung injury (TRALI). To the best of our knowledge, this is also the first description of a case of DCF associated with food embolism to the lungs causing respiratory failure.
Case Presentation
A 37-year-old man with a history significant for bulimia presented to the hospital with a 3-day history of melena and reports of dizziness. The patient did not report being on any prescribed medications but noted that he took 4 aspirin daily to “calm his nerves.” The rest of the patient’s history was unremarkable aside from a reported history of induced emesis 3 to 4 times per week for an extended period up until 2 weeks before admission.
On admission, his vital signs demonstrated tachycardia and orthostatic hypotension. Pertinent findings on physical examination were skin pallor, a normal lung examination, mild epigastric tenderness, and guaiac-positive stools. He was alert and oriented to person, place, and time with no focal deficits. His admission laboratory tests were notable for a hemoglobin (Hb) level of 4.6 g/dL (reference range, 14-17.9), a white blood cell count of 13.5 K/cm (reference range, 4.5-11), an international normalized ratio of 1.21, a blood urea nitrogen of 61 mg/dL (reference range, 10-20), and a creatinine of 2.3 mg/dL (reference range, 0.8-1.4). The patient was placed on 2 L of oxygen via nasal cannula for comfort rather than true hypoxia. A chest X-ray on admission was negative with no signs of infiltrate, edema, or widened mediastinum. An abdominal X-ray was significant for a dilated stomach consistent with bulimia with no abdominal free air or signs of obstruction. The case was discussed with the gastroenterology service who felt that the patient needed to be more hemodynamically stable before pursuing endoscopic evaluation.
He was admitted to the intensive care unit and give a transfusion of 4 units of fresh frozen plasma and 2 packed red blood cells (PRBCs) without any issues. During the infusion of a third PRBC, he developed chills, tachycardia, and hypertension with accompanying respiratory distress characterized by wheezing, decreased breath sounds bilaterally, and a decrease in oxygen saturation to 70% on 2 L supplemental oxygen. He responded to treatment with meperidine, methylprednisolone sodium succinate, albuterol nebulizer, and acetaminophen. A new chest X-ray was read as “development of pulmonary edema vs bilateral pneumonitis.” A transfusion reaction was reported to the blood bank and a diagnosis of TRALI was considered. That evening, he completed a dose of platelets and another PRBC without difficulty after he was premedicated with meperidine, methylprednisolone sodium succinate, and acetaminophen. During the night, the patient spiked a temperature of 40.3 °C that was successfully treated with a cooling blanket and acetaminophen.
The following morning the patient was found to be tachypneic and tachycardic with his face mask off. His symptoms were corrected by replacing his face mask. He claimed he felt anxious about getting more transfusions and that he had breathing problems like this at home in the recent past. The patient requested an aspirin to calm his nerves. Over the course of the day, his Hb level dropped from 6.6 g/dL to 5.9 g/dL, and 2 washed leukopoor PRBCs were ordered.
The first unit was infused uneventfully, but after 125 cc of the second unit, the patient developed respiratory distress, rigors, and hypotension to 70/58 mm Hg despite premedication. He again was treated successfully with increased face mask support. A few rales were noted, but his fluid balance was even. A second transfusion reaction was filed with the blood bank and based on the 2 transfusion-associated events with no other clear explanation for his symptoms, the clinical team favored the TRALI diagnosis. However, the blood bank was suspicious this might not be TRALI as the previous night the patient had 2 episodes of respiratory distress with drops in oxygen saturation unassociated with transfusions. The patient was clinically stable for the remainder of the night.
Early the following morning the patient was scheduled for an esophagogastroduodenoscopy to evaluate for a source of his bleeding. At the beginning of the procedure, a unit of washed leukoreduced PRBCs was hung for a Hb level of 6.9 g/dL. No bleeding source was noted in the stomach, but as the endoscope was passed into the duodenum, and after an infusion of only 25 cc of RBCs, the patient became cyanotic and went into cardiac arrest. Despite advanced resuscitation efforts over 90 minutes, the patient could not be successfully resuscitated and died while in the endoscopy suite. A transfusion reaction workup was initiated but was unremarkable. The transfusion medicine staff was suspicious that something other than TRALI was the cause of the patient’s respiratory distress as he had respiratory distress remote to the transfusions and the unit was prepared correctly before administration. The patient’s family agreed to an autopsy.
Pathology
A full autopsy was performed 22 hours after the patient died. The lungs were congested and of increased weight: The right lung was 800 g, and the left was 750 g. The right lower lobe had a wedge-shaped infarction measuring 6 cm × 5 cm fed by a thrombosed vessel. Multiple small hemorrhagic wedge-shaped areas were noted in the left lung. An ulcer measuring 6 cm × 5 cm was noted just distal to the pylorus. At the base of this ulcer was a 1.5 cm × 0.5 cm tract that communicated with the inferior vena cava (Figure 1).
A postmortem blood culture was positive for Clostridium perfringens (C perfringens) and Candida albicans (C Albicans). Interestingly, one of the collected blood culture vials exploded en route to the laboratory, presumably due to the presence of many gas-forming C perfringens bacteria.
On microscopic examination of the autopsy samples, gram-positive rods were observed in the tissue of multiple organs, including the heart, lungs, liver, and kidneys (Figure 2).
Serology
Fourteen days after the patient’s death, both PRBC units infused during transfusion reactions were positive for granulocyte antibodies by immunofluorescence and agglutination techniques. Human leukocyte antigen antibody testing was also sent but was not found in either the donor or patient.
Discussion
Our case illustrates the unique and challenging diagnosis of DCF given the rarity of presentation and how quickly patients may clinically decompensate. After an extensive search of the medical literature, we were only able to identify about 40 previous cases of DCF, of which 37 were described in one review.1 DCF, although rare, should be considered at risk for forming in the following settings: migrating inferior vena cava filter, right nephrectomy and radiotherapy, duodenal peptic ulcer, abdominal trauma, and oncologic settings involving metastatic malignancy requiring radiation and/or surgical grafting of the inferior vena cava.1-4 When the diagnosis is considered, computed tomography (CT) is the best initial imaging modality as it allows for noninvasive evaluation of both the inferior vena cava and nonadjacent structures. A commonality of our case and those described in the literature is the diagnostic mystery and nonspecific symptoms patients present with, thus making CT an appropriate diagnostic modality. Endoscopy is useful for the further workup of GI bleeding and the diagnosis of peptic ulcer disease.5 In our case, given the patient’s autopsy findings and history of extensive nonsteroidal anti-inflammatory drug use, the duodenal peptic ulcer was likely the precipitating factor for his DCF.
The most challenging aspect in diagnosing DCF is that many times patients present with nonspecific symptoms, and given its rarity it is not something that is usually at the forefront of most differentials.2 This diagnostic difficulty may elucidate why there is such a relatively high mortality rate—nearly 40%—associated with DCF and why many times accurate diagnosis is not made until autopsy.1,3 The most common presenting manifestations are sepsis and/or GI bleeding; in less than half the cases described in the literature patients had both sepsis and GI bleeding. In our case, the patient had signs of melena but was not felt to be septic as his presenting signs were felt to be in the setting of blood loss and dehydration (given his history of bulimia), not an acute infectious source.
In retrospect, one of the more confounding aspects of this case is the clinical picture concerning for TRALI. The patient required supplemental oxygen throughout his hospitalization and decompensated while or after receiving a transfusion, thus having TRALI on the differential was not felt inappropriate at that time. However, this case also illustrates the power of an anchoring bias, and perhaps the clinical team anchored on the diagnosis of TRALI too quickly before considering other possible etiologies for the patient’s respiratory distress. TRALI can be one of the most challenging diagnoses to make in the field of transfusion medicine as there are no definitive diagnostic criteria.6 It is felt to be a clinical diagnosis of exclusion as there is no pathognomonic sign or diagnostic test to confirm it as the cause of the patient’s respiratory distress, though anti–human leukocyte antigen antibodies commonly are present.6,7 Considering how quickly the patient decompensated on day 2 of hospitalization and the presence of C perfringens bacteremia, which
Conclusions
Our investigation reports a case of a DCF in the setting of significant duodenal peptic ulcer disease. We highlight the diagnostic challenge that this commonly lethal etiology presents. We believe ours is the first case in which it was confused for TRALI and associated with food embolism to the lungs causing hypoxic respiratory failure. We want to highlight that DCF, though rare, should be considered for patients who present with GI bleeding and hypoxic respiratory failure.
1. Guillem PG, Binot D, Dupuy-Cuny J, et al. Duodenocaval fistula: a life-threatening condition of various origins. J Vasc Surg. 2001;33(3):643-645. doi:10.1067/mva.2001.111741
2. Ippolito D, Querques G, Drago SG, Bonaffini PA, Sironi S. Duodenocaval fistula in a patient with inferior vena cava leiomyosarcoma treated by surgical resection and caval polytetrafluoroethylene prosthesis. Case Rep Radiol. 2015;2015:1-5. doi:10.1155/2015/575961
3. Guo Y, Zhang YQ, Lin W. Radiological diagnosis of duodenocaval fistula: a case report and literature review. World J Gastroenterol. 2010;16(18):2314-2316. doi:10.3748/wjg.v16.i18.2314
4. Perera GB, Wilson SE, Barie PS, Butler JA. Duodenocaval fistula: A late complication of retroperitoneal irradiation and vena cava replacement. Ann Vasc Surg. 2004;18(1):52-58. doi:10.1007/s10016-003-0097-8
5. Addeo P, Rosso E, Oussoultzoglou E, Jaeck D, Pessaux P, Bachellier P. Inferior vena cava graft-enteric fistula after extended hepatectomy with caval replacement. J Vasc Surg. 2012;55(1):226-229. doi:10.1016/j.jvs.2011.05.118
6. Chapman CE, Stainsby D, Jones H, et al. Ten years of hemovigilance reports of transfusion-related acute lung injury in the United Kingdom and the impact of preferential use of male donor plasma. Transfusion. 2009;49(3):440-452. doi:10.1111/j.1537-2995.2008.01948.x
7. Fontaine MJ, Malone J, Mullins FM, Grumet FC. Diagnosis of transfusion-related acute lung injury: TRALI or not TRALI? Ann Clin Lab Sci. 2006;36(1):53-58.
8. Yang C-C, Hsu P-C, Chang H-J, Cheng C-W, Lee M-H. Clinical significance and outcomes of clostridium perfringens bacteremia—a 10-year experience at a tertiary care hospital. Int J Infect Dis. 2013;17(11):e9of55-e960. doi:10.1016/j.ijid.2013.03.001
A duodenocaval fistula (DCF) is seen when a connection exists between the duodenum and the inferior vena cava. It is a rare entity that is commonly missed and presents a diagnostic challenge due to its nonspecific presenting symptoms.1,2 Patients commonly present with gastrointestinal (GI) bleeding or sepsis. Here we present a case of a 37-year-old man who presented to the hospital for a workup related to melena but went into cardiac arrest prior to an esophagogastroduodenoscopy. Unfortunately, on autopsy, the patient was found to have a DCF. We highlight the diagnostic challenge associated with DCF and how in this case the presentation was confused by a diagnosis of possible transfusion-related acute lung injury (TRALI). To the best of our knowledge, this is also the first description of a case of DCF associated with food embolism to the lungs causing respiratory failure.
Case Presentation
A 37-year-old man with a history significant for bulimia presented to the hospital with a 3-day history of melena and reports of dizziness. The patient did not report being on any prescribed medications but noted that he took 4 aspirin daily to “calm his nerves.” The rest of the patient’s history was unremarkable aside from a reported history of induced emesis 3 to 4 times per week for an extended period up until 2 weeks before admission.
On admission, his vital signs demonstrated tachycardia and orthostatic hypotension. Pertinent findings on physical examination were skin pallor, a normal lung examination, mild epigastric tenderness, and guaiac-positive stools. He was alert and oriented to person, place, and time with no focal deficits. His admission laboratory tests were notable for a hemoglobin (Hb) level of 4.6 g/dL (reference range, 14-17.9), a white blood cell count of 13.5 K/cm (reference range, 4.5-11), an international normalized ratio of 1.21, a blood urea nitrogen of 61 mg/dL (reference range, 10-20), and a creatinine of 2.3 mg/dL (reference range, 0.8-1.4). The patient was placed on 2 L of oxygen via nasal cannula for comfort rather than true hypoxia. A chest X-ray on admission was negative with no signs of infiltrate, edema, or widened mediastinum. An abdominal X-ray was significant for a dilated stomach consistent with bulimia with no abdominal free air or signs of obstruction. The case was discussed with the gastroenterology service who felt that the patient needed to be more hemodynamically stable before pursuing endoscopic evaluation.
He was admitted to the intensive care unit and give a transfusion of 4 units of fresh frozen plasma and 2 packed red blood cells (PRBCs) without any issues. During the infusion of a third PRBC, he developed chills, tachycardia, and hypertension with accompanying respiratory distress characterized by wheezing, decreased breath sounds bilaterally, and a decrease in oxygen saturation to 70% on 2 L supplemental oxygen. He responded to treatment with meperidine, methylprednisolone sodium succinate, albuterol nebulizer, and acetaminophen. A new chest X-ray was read as “development of pulmonary edema vs bilateral pneumonitis.” A transfusion reaction was reported to the blood bank and a diagnosis of TRALI was considered. That evening, he completed a dose of platelets and another PRBC without difficulty after he was premedicated with meperidine, methylprednisolone sodium succinate, and acetaminophen. During the night, the patient spiked a temperature of 40.3 °C that was successfully treated with a cooling blanket and acetaminophen.
The following morning the patient was found to be tachypneic and tachycardic with his face mask off. His symptoms were corrected by replacing his face mask. He claimed he felt anxious about getting more transfusions and that he had breathing problems like this at home in the recent past. The patient requested an aspirin to calm his nerves. Over the course of the day, his Hb level dropped from 6.6 g/dL to 5.9 g/dL, and 2 washed leukopoor PRBCs were ordered.
The first unit was infused uneventfully, but after 125 cc of the second unit, the patient developed respiratory distress, rigors, and hypotension to 70/58 mm Hg despite premedication. He again was treated successfully with increased face mask support. A few rales were noted, but his fluid balance was even. A second transfusion reaction was filed with the blood bank and based on the 2 transfusion-associated events with no other clear explanation for his symptoms, the clinical team favored the TRALI diagnosis. However, the blood bank was suspicious this might not be TRALI as the previous night the patient had 2 episodes of respiratory distress with drops in oxygen saturation unassociated with transfusions. The patient was clinically stable for the remainder of the night.
Early the following morning the patient was scheduled for an esophagogastroduodenoscopy to evaluate for a source of his bleeding. At the beginning of the procedure, a unit of washed leukoreduced PRBCs was hung for a Hb level of 6.9 g/dL. No bleeding source was noted in the stomach, but as the endoscope was passed into the duodenum, and after an infusion of only 25 cc of RBCs, the patient became cyanotic and went into cardiac arrest. Despite advanced resuscitation efforts over 90 minutes, the patient could not be successfully resuscitated and died while in the endoscopy suite. A transfusion reaction workup was initiated but was unremarkable. The transfusion medicine staff was suspicious that something other than TRALI was the cause of the patient’s respiratory distress as he had respiratory distress remote to the transfusions and the unit was prepared correctly before administration. The patient’s family agreed to an autopsy.
Pathology
A full autopsy was performed 22 hours after the patient died. The lungs were congested and of increased weight: The right lung was 800 g, and the left was 750 g. The right lower lobe had a wedge-shaped infarction measuring 6 cm × 5 cm fed by a thrombosed vessel. Multiple small hemorrhagic wedge-shaped areas were noted in the left lung. An ulcer measuring 6 cm × 5 cm was noted just distal to the pylorus. At the base of this ulcer was a 1.5 cm × 0.5 cm tract that communicated with the inferior vena cava (Figure 1).
A postmortem blood culture was positive for Clostridium perfringens (C perfringens) and Candida albicans (C Albicans). Interestingly, one of the collected blood culture vials exploded en route to the laboratory, presumably due to the presence of many gas-forming C perfringens bacteria.
On microscopic examination of the autopsy samples, gram-positive rods were observed in the tissue of multiple organs, including the heart, lungs, liver, and kidneys (Figure 2).
Serology
Fourteen days after the patient’s death, both PRBC units infused during transfusion reactions were positive for granulocyte antibodies by immunofluorescence and agglutination techniques. Human leukocyte antigen antibody testing was also sent but was not found in either the donor or patient.
Discussion
Our case illustrates the unique and challenging diagnosis of DCF given the rarity of presentation and how quickly patients may clinically decompensate. After an extensive search of the medical literature, we were only able to identify about 40 previous cases of DCF, of which 37 were described in one review.1 DCF, although rare, should be considered at risk for forming in the following settings: migrating inferior vena cava filter, right nephrectomy and radiotherapy, duodenal peptic ulcer, abdominal trauma, and oncologic settings involving metastatic malignancy requiring radiation and/or surgical grafting of the inferior vena cava.1-4 When the diagnosis is considered, computed tomography (CT) is the best initial imaging modality as it allows for noninvasive evaluation of both the inferior vena cava and nonadjacent structures. A commonality of our case and those described in the literature is the diagnostic mystery and nonspecific symptoms patients present with, thus making CT an appropriate diagnostic modality. Endoscopy is useful for the further workup of GI bleeding and the diagnosis of peptic ulcer disease.5 In our case, given the patient’s autopsy findings and history of extensive nonsteroidal anti-inflammatory drug use, the duodenal peptic ulcer was likely the precipitating factor for his DCF.
The most challenging aspect in diagnosing DCF is that many times patients present with nonspecific symptoms, and given its rarity it is not something that is usually at the forefront of most differentials.2 This diagnostic difficulty may elucidate why there is such a relatively high mortality rate—nearly 40%—associated with DCF and why many times accurate diagnosis is not made until autopsy.1,3 The most common presenting manifestations are sepsis and/or GI bleeding; in less than half the cases described in the literature patients had both sepsis and GI bleeding. In our case, the patient had signs of melena but was not felt to be septic as his presenting signs were felt to be in the setting of blood loss and dehydration (given his history of bulimia), not an acute infectious source.
In retrospect, one of the more confounding aspects of this case is the clinical picture concerning for TRALI. The patient required supplemental oxygen throughout his hospitalization and decompensated while or after receiving a transfusion, thus having TRALI on the differential was not felt inappropriate at that time. However, this case also illustrates the power of an anchoring bias, and perhaps the clinical team anchored on the diagnosis of TRALI too quickly before considering other possible etiologies for the patient’s respiratory distress. TRALI can be one of the most challenging diagnoses to make in the field of transfusion medicine as there are no definitive diagnostic criteria.6 It is felt to be a clinical diagnosis of exclusion as there is no pathognomonic sign or diagnostic test to confirm it as the cause of the patient’s respiratory distress, though anti–human leukocyte antigen antibodies commonly are present.6,7 Considering how quickly the patient decompensated on day 2 of hospitalization and the presence of C perfringens bacteremia, which
Conclusions
Our investigation reports a case of a DCF in the setting of significant duodenal peptic ulcer disease. We highlight the diagnostic challenge that this commonly lethal etiology presents. We believe ours is the first case in which it was confused for TRALI and associated with food embolism to the lungs causing hypoxic respiratory failure. We want to highlight that DCF, though rare, should be considered for patients who present with GI bleeding and hypoxic respiratory failure.
A duodenocaval fistula (DCF) is seen when a connection exists between the duodenum and the inferior vena cava. It is a rare entity that is commonly missed and presents a diagnostic challenge due to its nonspecific presenting symptoms.1,2 Patients commonly present with gastrointestinal (GI) bleeding or sepsis. Here we present a case of a 37-year-old man who presented to the hospital for a workup related to melena but went into cardiac arrest prior to an esophagogastroduodenoscopy. Unfortunately, on autopsy, the patient was found to have a DCF. We highlight the diagnostic challenge associated with DCF and how in this case the presentation was confused by a diagnosis of possible transfusion-related acute lung injury (TRALI). To the best of our knowledge, this is also the first description of a case of DCF associated with food embolism to the lungs causing respiratory failure.
Case Presentation
A 37-year-old man with a history significant for bulimia presented to the hospital with a 3-day history of melena and reports of dizziness. The patient did not report being on any prescribed medications but noted that he took 4 aspirin daily to “calm his nerves.” The rest of the patient’s history was unremarkable aside from a reported history of induced emesis 3 to 4 times per week for an extended period up until 2 weeks before admission.
On admission, his vital signs demonstrated tachycardia and orthostatic hypotension. Pertinent findings on physical examination were skin pallor, a normal lung examination, mild epigastric tenderness, and guaiac-positive stools. He was alert and oriented to person, place, and time with no focal deficits. His admission laboratory tests were notable for a hemoglobin (Hb) level of 4.6 g/dL (reference range, 14-17.9), a white blood cell count of 13.5 K/cm (reference range, 4.5-11), an international normalized ratio of 1.21, a blood urea nitrogen of 61 mg/dL (reference range, 10-20), and a creatinine of 2.3 mg/dL (reference range, 0.8-1.4). The patient was placed on 2 L of oxygen via nasal cannula for comfort rather than true hypoxia. A chest X-ray on admission was negative with no signs of infiltrate, edema, or widened mediastinum. An abdominal X-ray was significant for a dilated stomach consistent with bulimia with no abdominal free air or signs of obstruction. The case was discussed with the gastroenterology service who felt that the patient needed to be more hemodynamically stable before pursuing endoscopic evaluation.
He was admitted to the intensive care unit and give a transfusion of 4 units of fresh frozen plasma and 2 packed red blood cells (PRBCs) without any issues. During the infusion of a third PRBC, he developed chills, tachycardia, and hypertension with accompanying respiratory distress characterized by wheezing, decreased breath sounds bilaterally, and a decrease in oxygen saturation to 70% on 2 L supplemental oxygen. He responded to treatment with meperidine, methylprednisolone sodium succinate, albuterol nebulizer, and acetaminophen. A new chest X-ray was read as “development of pulmonary edema vs bilateral pneumonitis.” A transfusion reaction was reported to the blood bank and a diagnosis of TRALI was considered. That evening, he completed a dose of platelets and another PRBC without difficulty after he was premedicated with meperidine, methylprednisolone sodium succinate, and acetaminophen. During the night, the patient spiked a temperature of 40.3 °C that was successfully treated with a cooling blanket and acetaminophen.
The following morning the patient was found to be tachypneic and tachycardic with his face mask off. His symptoms were corrected by replacing his face mask. He claimed he felt anxious about getting more transfusions and that he had breathing problems like this at home in the recent past. The patient requested an aspirin to calm his nerves. Over the course of the day, his Hb level dropped from 6.6 g/dL to 5.9 g/dL, and 2 washed leukopoor PRBCs were ordered.
The first unit was infused uneventfully, but after 125 cc of the second unit, the patient developed respiratory distress, rigors, and hypotension to 70/58 mm Hg despite premedication. He again was treated successfully with increased face mask support. A few rales were noted, but his fluid balance was even. A second transfusion reaction was filed with the blood bank and based on the 2 transfusion-associated events with no other clear explanation for his symptoms, the clinical team favored the TRALI diagnosis. However, the blood bank was suspicious this might not be TRALI as the previous night the patient had 2 episodes of respiratory distress with drops in oxygen saturation unassociated with transfusions. The patient was clinically stable for the remainder of the night.
Early the following morning the patient was scheduled for an esophagogastroduodenoscopy to evaluate for a source of his bleeding. At the beginning of the procedure, a unit of washed leukoreduced PRBCs was hung for a Hb level of 6.9 g/dL. No bleeding source was noted in the stomach, but as the endoscope was passed into the duodenum, and after an infusion of only 25 cc of RBCs, the patient became cyanotic and went into cardiac arrest. Despite advanced resuscitation efforts over 90 minutes, the patient could not be successfully resuscitated and died while in the endoscopy suite. A transfusion reaction workup was initiated but was unremarkable. The transfusion medicine staff was suspicious that something other than TRALI was the cause of the patient’s respiratory distress as he had respiratory distress remote to the transfusions and the unit was prepared correctly before administration. The patient’s family agreed to an autopsy.
Pathology
A full autopsy was performed 22 hours after the patient died. The lungs were congested and of increased weight: The right lung was 800 g, and the left was 750 g. The right lower lobe had a wedge-shaped infarction measuring 6 cm × 5 cm fed by a thrombosed vessel. Multiple small hemorrhagic wedge-shaped areas were noted in the left lung. An ulcer measuring 6 cm × 5 cm was noted just distal to the pylorus. At the base of this ulcer was a 1.5 cm × 0.5 cm tract that communicated with the inferior vena cava (Figure 1).
A postmortem blood culture was positive for Clostridium perfringens (C perfringens) and Candida albicans (C Albicans). Interestingly, one of the collected blood culture vials exploded en route to the laboratory, presumably due to the presence of many gas-forming C perfringens bacteria.
On microscopic examination of the autopsy samples, gram-positive rods were observed in the tissue of multiple organs, including the heart, lungs, liver, and kidneys (Figure 2).
Serology
Fourteen days after the patient’s death, both PRBC units infused during transfusion reactions were positive for granulocyte antibodies by immunofluorescence and agglutination techniques. Human leukocyte antigen antibody testing was also sent but was not found in either the donor or patient.
Discussion
Our case illustrates the unique and challenging diagnosis of DCF given the rarity of presentation and how quickly patients may clinically decompensate. After an extensive search of the medical literature, we were only able to identify about 40 previous cases of DCF, of which 37 were described in one review.1 DCF, although rare, should be considered at risk for forming in the following settings: migrating inferior vena cava filter, right nephrectomy and radiotherapy, duodenal peptic ulcer, abdominal trauma, and oncologic settings involving metastatic malignancy requiring radiation and/or surgical grafting of the inferior vena cava.1-4 When the diagnosis is considered, computed tomography (CT) is the best initial imaging modality as it allows for noninvasive evaluation of both the inferior vena cava and nonadjacent structures. A commonality of our case and those described in the literature is the diagnostic mystery and nonspecific symptoms patients present with, thus making CT an appropriate diagnostic modality. Endoscopy is useful for the further workup of GI bleeding and the diagnosis of peptic ulcer disease.5 In our case, given the patient’s autopsy findings and history of extensive nonsteroidal anti-inflammatory drug use, the duodenal peptic ulcer was likely the precipitating factor for his DCF.
The most challenging aspect in diagnosing DCF is that many times patients present with nonspecific symptoms, and given its rarity it is not something that is usually at the forefront of most differentials.2 This diagnostic difficulty may elucidate why there is such a relatively high mortality rate—nearly 40%—associated with DCF and why many times accurate diagnosis is not made until autopsy.1,3 The most common presenting manifestations are sepsis and/or GI bleeding; in less than half the cases described in the literature patients had both sepsis and GI bleeding. In our case, the patient had signs of melena but was not felt to be septic as his presenting signs were felt to be in the setting of blood loss and dehydration (given his history of bulimia), not an acute infectious source.
In retrospect, one of the more confounding aspects of this case is the clinical picture concerning for TRALI. The patient required supplemental oxygen throughout his hospitalization and decompensated while or after receiving a transfusion, thus having TRALI on the differential was not felt inappropriate at that time. However, this case also illustrates the power of an anchoring bias, and perhaps the clinical team anchored on the diagnosis of TRALI too quickly before considering other possible etiologies for the patient’s respiratory distress. TRALI can be one of the most challenging diagnoses to make in the field of transfusion medicine as there are no definitive diagnostic criteria.6 It is felt to be a clinical diagnosis of exclusion as there is no pathognomonic sign or diagnostic test to confirm it as the cause of the patient’s respiratory distress, though anti–human leukocyte antigen antibodies commonly are present.6,7 Considering how quickly the patient decompensated on day 2 of hospitalization and the presence of C perfringens bacteremia, which
Conclusions
Our investigation reports a case of a DCF in the setting of significant duodenal peptic ulcer disease. We highlight the diagnostic challenge that this commonly lethal etiology presents. We believe ours is the first case in which it was confused for TRALI and associated with food embolism to the lungs causing hypoxic respiratory failure. We want to highlight that DCF, though rare, should be considered for patients who present with GI bleeding and hypoxic respiratory failure.
1. Guillem PG, Binot D, Dupuy-Cuny J, et al. Duodenocaval fistula: a life-threatening condition of various origins. J Vasc Surg. 2001;33(3):643-645. doi:10.1067/mva.2001.111741
2. Ippolito D, Querques G, Drago SG, Bonaffini PA, Sironi S. Duodenocaval fistula in a patient with inferior vena cava leiomyosarcoma treated by surgical resection and caval polytetrafluoroethylene prosthesis. Case Rep Radiol. 2015;2015:1-5. doi:10.1155/2015/575961
3. Guo Y, Zhang YQ, Lin W. Radiological diagnosis of duodenocaval fistula: a case report and literature review. World J Gastroenterol. 2010;16(18):2314-2316. doi:10.3748/wjg.v16.i18.2314
4. Perera GB, Wilson SE, Barie PS, Butler JA. Duodenocaval fistula: A late complication of retroperitoneal irradiation and vena cava replacement. Ann Vasc Surg. 2004;18(1):52-58. doi:10.1007/s10016-003-0097-8
5. Addeo P, Rosso E, Oussoultzoglou E, Jaeck D, Pessaux P, Bachellier P. Inferior vena cava graft-enteric fistula after extended hepatectomy with caval replacement. J Vasc Surg. 2012;55(1):226-229. doi:10.1016/j.jvs.2011.05.118
6. Chapman CE, Stainsby D, Jones H, et al. Ten years of hemovigilance reports of transfusion-related acute lung injury in the United Kingdom and the impact of preferential use of male donor plasma. Transfusion. 2009;49(3):440-452. doi:10.1111/j.1537-2995.2008.01948.x
7. Fontaine MJ, Malone J, Mullins FM, Grumet FC. Diagnosis of transfusion-related acute lung injury: TRALI or not TRALI? Ann Clin Lab Sci. 2006;36(1):53-58.
8. Yang C-C, Hsu P-C, Chang H-J, Cheng C-W, Lee M-H. Clinical significance and outcomes of clostridium perfringens bacteremia—a 10-year experience at a tertiary care hospital. Int J Infect Dis. 2013;17(11):e9of55-e960. doi:10.1016/j.ijid.2013.03.001
1. Guillem PG, Binot D, Dupuy-Cuny J, et al. Duodenocaval fistula: a life-threatening condition of various origins. J Vasc Surg. 2001;33(3):643-645. doi:10.1067/mva.2001.111741
2. Ippolito D, Querques G, Drago SG, Bonaffini PA, Sironi S. Duodenocaval fistula in a patient with inferior vena cava leiomyosarcoma treated by surgical resection and caval polytetrafluoroethylene prosthesis. Case Rep Radiol. 2015;2015:1-5. doi:10.1155/2015/575961
3. Guo Y, Zhang YQ, Lin W. Radiological diagnosis of duodenocaval fistula: a case report and literature review. World J Gastroenterol. 2010;16(18):2314-2316. doi:10.3748/wjg.v16.i18.2314
4. Perera GB, Wilson SE, Barie PS, Butler JA. Duodenocaval fistula: A late complication of retroperitoneal irradiation and vena cava replacement. Ann Vasc Surg. 2004;18(1):52-58. doi:10.1007/s10016-003-0097-8
5. Addeo P, Rosso E, Oussoultzoglou E, Jaeck D, Pessaux P, Bachellier P. Inferior vena cava graft-enteric fistula after extended hepatectomy with caval replacement. J Vasc Surg. 2012;55(1):226-229. doi:10.1016/j.jvs.2011.05.118
6. Chapman CE, Stainsby D, Jones H, et al. Ten years of hemovigilance reports of transfusion-related acute lung injury in the United Kingdom and the impact of preferential use of male donor plasma. Transfusion. 2009;49(3):440-452. doi:10.1111/j.1537-2995.2008.01948.x
7. Fontaine MJ, Malone J, Mullins FM, Grumet FC. Diagnosis of transfusion-related acute lung injury: TRALI or not TRALI? Ann Clin Lab Sci. 2006;36(1):53-58.
8. Yang C-C, Hsu P-C, Chang H-J, Cheng C-W, Lee M-H. Clinical significance and outcomes of clostridium perfringens bacteremia—a 10-year experience at a tertiary care hospital. Int J Infect Dis. 2013;17(11):e9of55-e960. doi:10.1016/j.ijid.2013.03.001
Dupilumab-Associated Sweet Syndrome
Sweet syndrome (SS), also known as acute febrile neutrophilic dermatosis, was first described in 1964. 1 Since then, several subtypes of SS have been recognized, including classic or idiopathic, which typically follows an acute viral illness; cancer related, typically in the form of a paraneoplastic syndrome; and drug induced. 2 Drug-induced SS is defined by the following: (1) an abrupt onset of painful erythematous plaques or nodules; (2) histopathologic evidence of a dense neutrophilic infiltrate without evidence of leukocytoclastic vasculitis; (3) pyrexia above 38 ° C; (4) temporal relationship between drug and clinical presentation or temporally related recurrence after rechallenge; and (5) temporally related resolution of lesions after drug withdrawal or treatment with systemic corticosteroids. 3 All 5 criteria must be met to make a diagnosis of drug-induced SS. Since these criteria were established by Walker and Cohen, 3 various drugs have been identified as causative agents, including antibiotics, antiepileptics, antiretrovirals, antineoplastic agents, antipsychotics, oral contraceptives, nonsteroidal anti-inflammatory agents, and retinoids. 4 W e present a rare case of SS caused by dupilumab, a monoclonal antibody therapy, used in the treatment of severe eosinophilic asthma and atopic dermatitis.
Case Report
A 53-year-old woman presented with painful skin lesions, arthralgia, fever, and leukocytosis following initiation of dupilumab. She had a history of adult-onset, severe, persistent eosinophilic asthma, as well as chronic rhinosinusitis with nasal polyps, plaque psoriasis, and hypertrophic cardiomyopathy. She started mepolizumab 3 years prior to the current presentation for persistently uncontrolled asthma with a baseline peripheral eosinophil count of 860 cells/µL. After 3 years of minimal response to mepolizumab, she was started on dupilumab. Within 2 weeks of the first dose of dupilumab, she started experiencing bilateral knee pain. She subsequently developed daily fevers (temperature, 38.3 °C to 39.4 °C), fatigue, and pain in the back of the neck and head. After the second dose of dupilumab, she started experiencing painful skin lesions on the bilateral knuckles, elbows, and abdomen (Figure 1). She had difficulty using her hands and walking secondary to intense arthralgia involving the bilateral finger joints, elbows, and knees. Her primary care physician obtained a laboratory evaluation, which revealed an elevated total white blood cell count of 20×103/mm3 (reference range, 4–11×103/mm3) with 27.5% neutrophils and severely elevated eosinophils above her baseline to 57.3% with an absolute eosinophil count of 11,700 cells/µL (reference range, <400 cells/µL). Further assessment revealed an elevated erythrocyte sedimentation rate of 64 mm/h (reference range, 0–30 mm/h) and C-reactive protein level of 34 mg/dL (reference range, ≤0.80 mg/dL), with negative antinuclear antibody, rheumatoid factor, antineutrophilic cytoplasmic antibody, and Lyme antibody. IgG, IgA, and IgM levels were within reference range, and the IgE level was not elevated above her baseline. She had normal serum tryptase, and a peripheral D816V c-KIT mutation was not detected. She was subsequently hospitalized for further evaluation, at which time there was no fever or localizing infectious signs or symptoms. An infectious evaluation including urinalysis; respiratory swab for adenovirus, coronaviruses, human metapneumovirus, rhinovirus/enterovirus, influenza A and B, parainfluenza viruses, respiratory syncytial virus, Chlamydophila pneumoniae, and Mycoplasma pneumoniae; Lyme serology; and a computed tomography (CT) scan of the chest, abdomen, and pelvis revealed no evidence of infection. A parasite evaluation was ordered but was not performed. There was no evidence of malignancy on CT of the chest, abdomen, and pelvis or CT of the head without contrast. A lumbar puncture was considered but was ultimately deferred.

At the current presentation, the patient was following up in the dermatology clinic shortly after discharge. The lesions on the fingers and arms were described by the dermatologist as deep, erythematous, 0.5-cm bullous papules. The differential diagnosis at this time included a cutaneous or systemic infection, vasculitis, drug eruption, or cutaneous manifestation of an autoimmune condition. A shave biopsy of a skin lesion on the right hand demonstrated epidermal necrosis with a dense dermal neutrophilic infiltrate consistent with a neutrophilic dermatosis (Figure 2). There was no evidence of leukocytoclastic vasculitis. The histologic differential diagnosis included cutaneous infection, neutrophilic dermatosis of the hand, and SS. Special stains for infectious organisms including Gram, Grocott methenamine-silver, and auramine-rhodamine stains were negative for bacterial, fungal, and mycobacterial organisms, ruling out cutaneous infection. A diagnosis of drug-induced SS was made based on the histologic findings, diffuse distribution of the lesions, negative infectious evaluation, lack of underlying malignancy or autoimmune conditions, and onset following initiation of dupilumab.
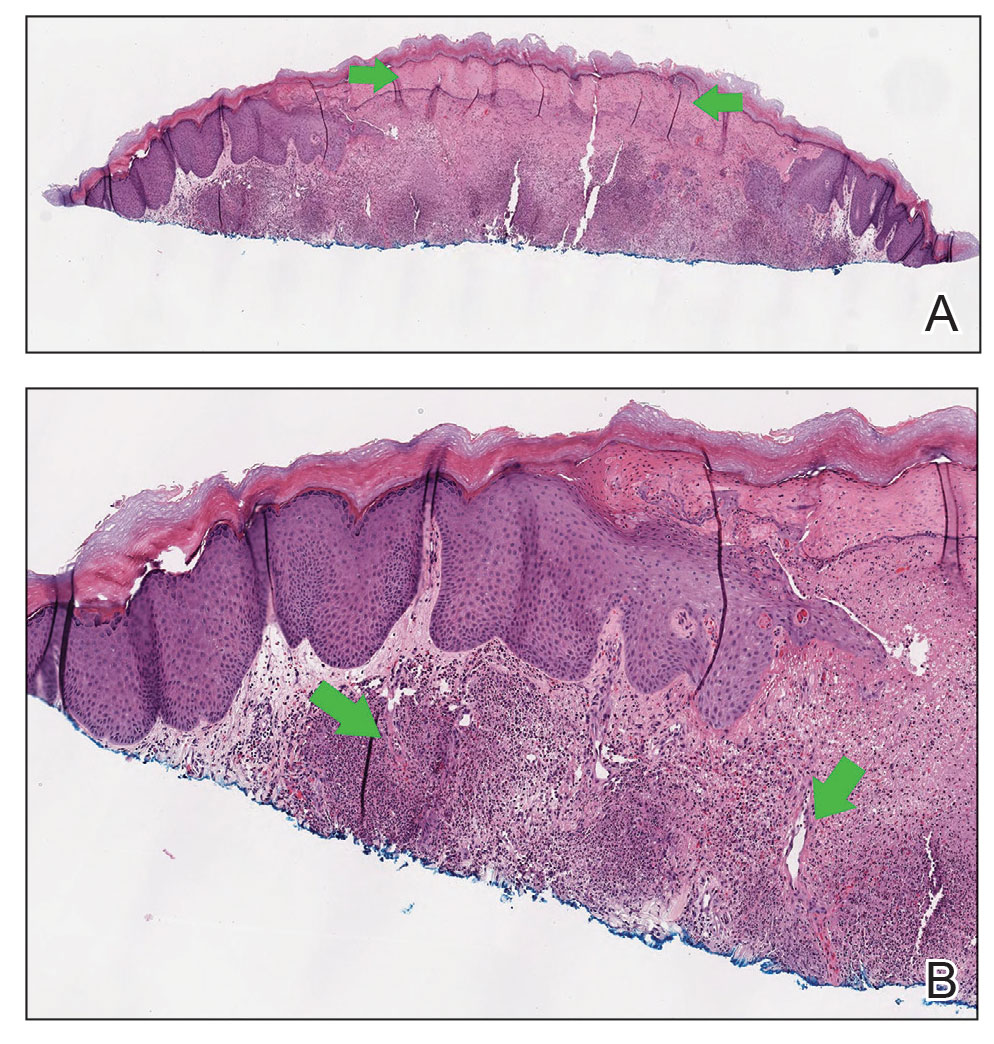
Dupilumab was discontinued, and the patient was started on prednisone with rapid improvement in the symptoms. She underwent a slow taper of the prednisone over approximately 2 months with a slow downtrend of eosinophils. She was transitioned to a different biologic agent, benralizumab, with no further recurrence of the rash or arthralgia.
Comment
Dupilumab is a human monoclonal IgG4 antibody that inhibits IL-4 and IL-13 signaling by binding to the IL-4Rα subunit. By blocking IL-4Rα, dupilumab inhibits IL-4 and IL-13 cytokine-induced inflammatory responses, including the release of proinflammatory cytokines, chemokines, nitric oxide, and IgE. Currently, dupilumab is approved to treat refractory forms of moderate to severe asthma characterized by an eosinophilic phenotype or with corticosteroid-dependent asthma, moderate to severe atopic dermatitis, chronic rhinosinusitis with nasal polyposis, and eosinophilic esophagitis. The most common adverse events (incidence ≥1%) are injection-site reactions, oropharyngeal pain, and eosinophilia.5 Interestingly, our patient did exhibit a high degree of eosinophilia; however, she met all criteria for drug-induced SS, and the skin biopsy was not consistent with an eosinophilic process. Notably, the peripheral neutrophils were not elevated. Neutrophilia often is seen in classic SS but is not required for a diagnosis of drug-induced SS. Rare cases of dupilumab-associated arthritis and serum sickness–like reaction have been described,6-8 but our patient’s presentation was distinct, given other described signs, symptoms, and skin biopsy results. Histopathology results were not consistent with leukocytoclastic vasculitis, a potential mimicker of SS. Although the infectious and paraneoplastic evaluation was not exhaustive, the negative imaging from head to pelvis, the lack of recurrence of skin lesions, and the laboratory abnormalities after dupilumab discontinuation supported the conclusion that the culprit was not an infection or underlying malignancy. She had not started any other medications during this time frame, leaving dupilumab as the most likely offending agent. The mechanism for this reaction is not clear. It is possible that inhibition of IL-4 and IL-13 in the T helper 2 (TH2) cell pathway may have led to upregulated IL-17–mediated inflammation9 as well as a neutrophilic process in the skin, but this would not explain the concurrent peripheral eosinophilia that was noted. Further studies are needed to investigate the pathophysiology of SS.
Conclusion
We report a rare case of dupilumab-induced SS. Corticosteroids accompanied by cessation of the medication proved to be an effective treatment.
- Sweet RB. An acute febrile neutrophilic dermatosis. Br J Dermatol. 1964;76:349-356.
- Cohen PR, Kurzrock R. Sweet’s syndrome revisited: a review of disease concepts. Int J Dermatol. 2003;42:761-778.
- Walker DC, Cohen PR. Trimethoprim-sulfamethoxazole-associated acute febrile neutrophilic dermatosis: case report and review of drug-induced Sweet’s syndrome. J Am Acad Dermatol. 1996;34:918-923.
- Cohen PR. Sweet’s syndrome—a comprehensive review of an acute febrile neutrophilic dermatosis. Orphanet J Rare Dis. 2007;2:34.
- Jackson K, Bahna SL. Hypersensitivity and adverse reactions to biologics for asthma and allergic diseases. Expert Rev Clin Immunol. 2020;16:311-319.
- Willsmore ZN, Woolf RT, Hughes C, et al. Development of inflammatory arthritis and enthesitis in patients on dupilumab: a case series. Br J Dermatol. 2019;181:1068-1070.
- de Wijs LEM, van der Waa JD, de Jong PHP, et al. Acute arthritis and arthralgia as an adverse drug reaction to dupilumab. Clin Exp Dermatol. 2020;45:262-263.
- Treudler R, Delaroque N, Puder M, et al. Dupilumab-induced serum sickness-like reaction: an unusual adverse effect in a patient with atopic eczema. J Eur Acad Dermatol Venereol. 2021;35:E30-E32.
- Guenova E, Skabytska Y, Hoetzenecker W, et al. IL-4 abrogates TH17 cell-mediated inflammation by selective silencing of IL-23 in antigen-presenting cells. Proc Natl Acad Sci U S A. 2015;112:2163-2168.
Sweet syndrome (SS), also known as acute febrile neutrophilic dermatosis, was first described in 1964. 1 Since then, several subtypes of SS have been recognized, including classic or idiopathic, which typically follows an acute viral illness; cancer related, typically in the form of a paraneoplastic syndrome; and drug induced. 2 Drug-induced SS is defined by the following: (1) an abrupt onset of painful erythematous plaques or nodules; (2) histopathologic evidence of a dense neutrophilic infiltrate without evidence of leukocytoclastic vasculitis; (3) pyrexia above 38 ° C; (4) temporal relationship between drug and clinical presentation or temporally related recurrence after rechallenge; and (5) temporally related resolution of lesions after drug withdrawal or treatment with systemic corticosteroids. 3 All 5 criteria must be met to make a diagnosis of drug-induced SS. Since these criteria were established by Walker and Cohen, 3 various drugs have been identified as causative agents, including antibiotics, antiepileptics, antiretrovirals, antineoplastic agents, antipsychotics, oral contraceptives, nonsteroidal anti-inflammatory agents, and retinoids. 4 W e present a rare case of SS caused by dupilumab, a monoclonal antibody therapy, used in the treatment of severe eosinophilic asthma and atopic dermatitis.
Case Report
A 53-year-old woman presented with painful skin lesions, arthralgia, fever, and leukocytosis following initiation of dupilumab. She had a history of adult-onset, severe, persistent eosinophilic asthma, as well as chronic rhinosinusitis with nasal polyps, plaque psoriasis, and hypertrophic cardiomyopathy. She started mepolizumab 3 years prior to the current presentation for persistently uncontrolled asthma with a baseline peripheral eosinophil count of 860 cells/µL. After 3 years of minimal response to mepolizumab, she was started on dupilumab. Within 2 weeks of the first dose of dupilumab, she started experiencing bilateral knee pain. She subsequently developed daily fevers (temperature, 38.3 °C to 39.4 °C), fatigue, and pain in the back of the neck and head. After the second dose of dupilumab, she started experiencing painful skin lesions on the bilateral knuckles, elbows, and abdomen (Figure 1). She had difficulty using her hands and walking secondary to intense arthralgia involving the bilateral finger joints, elbows, and knees. Her primary care physician obtained a laboratory evaluation, which revealed an elevated total white blood cell count of 20×103/mm3 (reference range, 4–11×103/mm3) with 27.5% neutrophils and severely elevated eosinophils above her baseline to 57.3% with an absolute eosinophil count of 11,700 cells/µL (reference range, <400 cells/µL). Further assessment revealed an elevated erythrocyte sedimentation rate of 64 mm/h (reference range, 0–30 mm/h) and C-reactive protein level of 34 mg/dL (reference range, ≤0.80 mg/dL), with negative antinuclear antibody, rheumatoid factor, antineutrophilic cytoplasmic antibody, and Lyme antibody. IgG, IgA, and IgM levels were within reference range, and the IgE level was not elevated above her baseline. She had normal serum tryptase, and a peripheral D816V c-KIT mutation was not detected. She was subsequently hospitalized for further evaluation, at which time there was no fever or localizing infectious signs or symptoms. An infectious evaluation including urinalysis; respiratory swab for adenovirus, coronaviruses, human metapneumovirus, rhinovirus/enterovirus, influenza A and B, parainfluenza viruses, respiratory syncytial virus, Chlamydophila pneumoniae, and Mycoplasma pneumoniae; Lyme serology; and a computed tomography (CT) scan of the chest, abdomen, and pelvis revealed no evidence of infection. A parasite evaluation was ordered but was not performed. There was no evidence of malignancy on CT of the chest, abdomen, and pelvis or CT of the head without contrast. A lumbar puncture was considered but was ultimately deferred.

At the current presentation, the patient was following up in the dermatology clinic shortly after discharge. The lesions on the fingers and arms were described by the dermatologist as deep, erythematous, 0.5-cm bullous papules. The differential diagnosis at this time included a cutaneous or systemic infection, vasculitis, drug eruption, or cutaneous manifestation of an autoimmune condition. A shave biopsy of a skin lesion on the right hand demonstrated epidermal necrosis with a dense dermal neutrophilic infiltrate consistent with a neutrophilic dermatosis (Figure 2). There was no evidence of leukocytoclastic vasculitis. The histologic differential diagnosis included cutaneous infection, neutrophilic dermatosis of the hand, and SS. Special stains for infectious organisms including Gram, Grocott methenamine-silver, and auramine-rhodamine stains were negative for bacterial, fungal, and mycobacterial organisms, ruling out cutaneous infection. A diagnosis of drug-induced SS was made based on the histologic findings, diffuse distribution of the lesions, negative infectious evaluation, lack of underlying malignancy or autoimmune conditions, and onset following initiation of dupilumab.

Dupilumab was discontinued, and the patient was started on prednisone with rapid improvement in the symptoms. She underwent a slow taper of the prednisone over approximately 2 months with a slow downtrend of eosinophils. She was transitioned to a different biologic agent, benralizumab, with no further recurrence of the rash or arthralgia.
Comment
Dupilumab is a human monoclonal IgG4 antibody that inhibits IL-4 and IL-13 signaling by binding to the IL-4Rα subunit. By blocking IL-4Rα, dupilumab inhibits IL-4 and IL-13 cytokine-induced inflammatory responses, including the release of proinflammatory cytokines, chemokines, nitric oxide, and IgE. Currently, dupilumab is approved to treat refractory forms of moderate to severe asthma characterized by an eosinophilic phenotype or with corticosteroid-dependent asthma, moderate to severe atopic dermatitis, chronic rhinosinusitis with nasal polyposis, and eosinophilic esophagitis. The most common adverse events (incidence ≥1%) are injection-site reactions, oropharyngeal pain, and eosinophilia.5 Interestingly, our patient did exhibit a high degree of eosinophilia; however, she met all criteria for drug-induced SS, and the skin biopsy was not consistent with an eosinophilic process. Notably, the peripheral neutrophils were not elevated. Neutrophilia often is seen in classic SS but is not required for a diagnosis of drug-induced SS. Rare cases of dupilumab-associated arthritis and serum sickness–like reaction have been described,6-8 but our patient’s presentation was distinct, given other described signs, symptoms, and skin biopsy results. Histopathology results were not consistent with leukocytoclastic vasculitis, a potential mimicker of SS. Although the infectious and paraneoplastic evaluation was not exhaustive, the negative imaging from head to pelvis, the lack of recurrence of skin lesions, and the laboratory abnormalities after dupilumab discontinuation supported the conclusion that the culprit was not an infection or underlying malignancy. She had not started any other medications during this time frame, leaving dupilumab as the most likely offending agent. The mechanism for this reaction is not clear. It is possible that inhibition of IL-4 and IL-13 in the T helper 2 (TH2) cell pathway may have led to upregulated IL-17–mediated inflammation9 as well as a neutrophilic process in the skin, but this would not explain the concurrent peripheral eosinophilia that was noted. Further studies are needed to investigate the pathophysiology of SS.
Conclusion
We report a rare case of dupilumab-induced SS. Corticosteroids accompanied by cessation of the medication proved to be an effective treatment.
Sweet syndrome (SS), also known as acute febrile neutrophilic dermatosis, was first described in 1964. 1 Since then, several subtypes of SS have been recognized, including classic or idiopathic, which typically follows an acute viral illness; cancer related, typically in the form of a paraneoplastic syndrome; and drug induced. 2 Drug-induced SS is defined by the following: (1) an abrupt onset of painful erythematous plaques or nodules; (2) histopathologic evidence of a dense neutrophilic infiltrate without evidence of leukocytoclastic vasculitis; (3) pyrexia above 38 ° C; (4) temporal relationship between drug and clinical presentation or temporally related recurrence after rechallenge; and (5) temporally related resolution of lesions after drug withdrawal or treatment with systemic corticosteroids. 3 All 5 criteria must be met to make a diagnosis of drug-induced SS. Since these criteria were established by Walker and Cohen, 3 various drugs have been identified as causative agents, including antibiotics, antiepileptics, antiretrovirals, antineoplastic agents, antipsychotics, oral contraceptives, nonsteroidal anti-inflammatory agents, and retinoids. 4 W e present a rare case of SS caused by dupilumab, a monoclonal antibody therapy, used in the treatment of severe eosinophilic asthma and atopic dermatitis.
Case Report
A 53-year-old woman presented with painful skin lesions, arthralgia, fever, and leukocytosis following initiation of dupilumab. She had a history of adult-onset, severe, persistent eosinophilic asthma, as well as chronic rhinosinusitis with nasal polyps, plaque psoriasis, and hypertrophic cardiomyopathy. She started mepolizumab 3 years prior to the current presentation for persistently uncontrolled asthma with a baseline peripheral eosinophil count of 860 cells/µL. After 3 years of minimal response to mepolizumab, she was started on dupilumab. Within 2 weeks of the first dose of dupilumab, she started experiencing bilateral knee pain. She subsequently developed daily fevers (temperature, 38.3 °C to 39.4 °C), fatigue, and pain in the back of the neck and head. After the second dose of dupilumab, she started experiencing painful skin lesions on the bilateral knuckles, elbows, and abdomen (Figure 1). She had difficulty using her hands and walking secondary to intense arthralgia involving the bilateral finger joints, elbows, and knees. Her primary care physician obtained a laboratory evaluation, which revealed an elevated total white blood cell count of 20×103/mm3 (reference range, 4–11×103/mm3) with 27.5% neutrophils and severely elevated eosinophils above her baseline to 57.3% with an absolute eosinophil count of 11,700 cells/µL (reference range, <400 cells/µL). Further assessment revealed an elevated erythrocyte sedimentation rate of 64 mm/h (reference range, 0–30 mm/h) and C-reactive protein level of 34 mg/dL (reference range, ≤0.80 mg/dL), with negative antinuclear antibody, rheumatoid factor, antineutrophilic cytoplasmic antibody, and Lyme antibody. IgG, IgA, and IgM levels were within reference range, and the IgE level was not elevated above her baseline. She had normal serum tryptase, and a peripheral D816V c-KIT mutation was not detected. She was subsequently hospitalized for further evaluation, at which time there was no fever or localizing infectious signs or symptoms. An infectious evaluation including urinalysis; respiratory swab for adenovirus, coronaviruses, human metapneumovirus, rhinovirus/enterovirus, influenza A and B, parainfluenza viruses, respiratory syncytial virus, Chlamydophila pneumoniae, and Mycoplasma pneumoniae; Lyme serology; and a computed tomography (CT) scan of the chest, abdomen, and pelvis revealed no evidence of infection. A parasite evaluation was ordered but was not performed. There was no evidence of malignancy on CT of the chest, abdomen, and pelvis or CT of the head without contrast. A lumbar puncture was considered but was ultimately deferred.

At the current presentation, the patient was following up in the dermatology clinic shortly after discharge. The lesions on the fingers and arms were described by the dermatologist as deep, erythematous, 0.5-cm bullous papules. The differential diagnosis at this time included a cutaneous or systemic infection, vasculitis, drug eruption, or cutaneous manifestation of an autoimmune condition. A shave biopsy of a skin lesion on the right hand demonstrated epidermal necrosis with a dense dermal neutrophilic infiltrate consistent with a neutrophilic dermatosis (Figure 2). There was no evidence of leukocytoclastic vasculitis. The histologic differential diagnosis included cutaneous infection, neutrophilic dermatosis of the hand, and SS. Special stains for infectious organisms including Gram, Grocott methenamine-silver, and auramine-rhodamine stains were negative for bacterial, fungal, and mycobacterial organisms, ruling out cutaneous infection. A diagnosis of drug-induced SS was made based on the histologic findings, diffuse distribution of the lesions, negative infectious evaluation, lack of underlying malignancy or autoimmune conditions, and onset following initiation of dupilumab.

Dupilumab was discontinued, and the patient was started on prednisone with rapid improvement in the symptoms. She underwent a slow taper of the prednisone over approximately 2 months with a slow downtrend of eosinophils. She was transitioned to a different biologic agent, benralizumab, with no further recurrence of the rash or arthralgia.
Comment
Dupilumab is a human monoclonal IgG4 antibody that inhibits IL-4 and IL-13 signaling by binding to the IL-4Rα subunit. By blocking IL-4Rα, dupilumab inhibits IL-4 and IL-13 cytokine-induced inflammatory responses, including the release of proinflammatory cytokines, chemokines, nitric oxide, and IgE. Currently, dupilumab is approved to treat refractory forms of moderate to severe asthma characterized by an eosinophilic phenotype or with corticosteroid-dependent asthma, moderate to severe atopic dermatitis, chronic rhinosinusitis with nasal polyposis, and eosinophilic esophagitis. The most common adverse events (incidence ≥1%) are injection-site reactions, oropharyngeal pain, and eosinophilia.5 Interestingly, our patient did exhibit a high degree of eosinophilia; however, she met all criteria for drug-induced SS, and the skin biopsy was not consistent with an eosinophilic process. Notably, the peripheral neutrophils were not elevated. Neutrophilia often is seen in classic SS but is not required for a diagnosis of drug-induced SS. Rare cases of dupilumab-associated arthritis and serum sickness–like reaction have been described,6-8 but our patient’s presentation was distinct, given other described signs, symptoms, and skin biopsy results. Histopathology results were not consistent with leukocytoclastic vasculitis, a potential mimicker of SS. Although the infectious and paraneoplastic evaluation was not exhaustive, the negative imaging from head to pelvis, the lack of recurrence of skin lesions, and the laboratory abnormalities after dupilumab discontinuation supported the conclusion that the culprit was not an infection or underlying malignancy. She had not started any other medications during this time frame, leaving dupilumab as the most likely offending agent. The mechanism for this reaction is not clear. It is possible that inhibition of IL-4 and IL-13 in the T helper 2 (TH2) cell pathway may have led to upregulated IL-17–mediated inflammation9 as well as a neutrophilic process in the skin, but this would not explain the concurrent peripheral eosinophilia that was noted. Further studies are needed to investigate the pathophysiology of SS.
Conclusion
We report a rare case of dupilumab-induced SS. Corticosteroids accompanied by cessation of the medication proved to be an effective treatment.
- Sweet RB. An acute febrile neutrophilic dermatosis. Br J Dermatol. 1964;76:349-356.
- Cohen PR, Kurzrock R. Sweet’s syndrome revisited: a review of disease concepts. Int J Dermatol. 2003;42:761-778.
- Walker DC, Cohen PR. Trimethoprim-sulfamethoxazole-associated acute febrile neutrophilic dermatosis: case report and review of drug-induced Sweet’s syndrome. J Am Acad Dermatol. 1996;34:918-923.
- Cohen PR. Sweet’s syndrome—a comprehensive review of an acute febrile neutrophilic dermatosis. Orphanet J Rare Dis. 2007;2:34.
- Jackson K, Bahna SL. Hypersensitivity and adverse reactions to biologics for asthma and allergic diseases. Expert Rev Clin Immunol. 2020;16:311-319.
- Willsmore ZN, Woolf RT, Hughes C, et al. Development of inflammatory arthritis and enthesitis in patients on dupilumab: a case series. Br J Dermatol. 2019;181:1068-1070.
- de Wijs LEM, van der Waa JD, de Jong PHP, et al. Acute arthritis and arthralgia as an adverse drug reaction to dupilumab. Clin Exp Dermatol. 2020;45:262-263.
- Treudler R, Delaroque N, Puder M, et al. Dupilumab-induced serum sickness-like reaction: an unusual adverse effect in a patient with atopic eczema. J Eur Acad Dermatol Venereol. 2021;35:E30-E32.
- Guenova E, Skabytska Y, Hoetzenecker W, et al. IL-4 abrogates TH17 cell-mediated inflammation by selective silencing of IL-23 in antigen-presenting cells. Proc Natl Acad Sci U S A. 2015;112:2163-2168.
- Sweet RB. An acute febrile neutrophilic dermatosis. Br J Dermatol. 1964;76:349-356.
- Cohen PR, Kurzrock R. Sweet’s syndrome revisited: a review of disease concepts. Int J Dermatol. 2003;42:761-778.
- Walker DC, Cohen PR. Trimethoprim-sulfamethoxazole-associated acute febrile neutrophilic dermatosis: case report and review of drug-induced Sweet’s syndrome. J Am Acad Dermatol. 1996;34:918-923.
- Cohen PR. Sweet’s syndrome—a comprehensive review of an acute febrile neutrophilic dermatosis. Orphanet J Rare Dis. 2007;2:34.
- Jackson K, Bahna SL. Hypersensitivity and adverse reactions to biologics for asthma and allergic diseases. Expert Rev Clin Immunol. 2020;16:311-319.
- Willsmore ZN, Woolf RT, Hughes C, et al. Development of inflammatory arthritis and enthesitis in patients on dupilumab: a case series. Br J Dermatol. 2019;181:1068-1070.
- de Wijs LEM, van der Waa JD, de Jong PHP, et al. Acute arthritis and arthralgia as an adverse drug reaction to dupilumab. Clin Exp Dermatol. 2020;45:262-263.
- Treudler R, Delaroque N, Puder M, et al. Dupilumab-induced serum sickness-like reaction: an unusual adverse effect in a patient with atopic eczema. J Eur Acad Dermatol Venereol. 2021;35:E30-E32.
- Guenova E, Skabytska Y, Hoetzenecker W, et al. IL-4 abrogates TH17 cell-mediated inflammation by selective silencing of IL-23 in antigen-presenting cells. Proc Natl Acad Sci U S A. 2015;112:2163-2168.
Practice Points
- Prescribers of dupilumab should be aware that Sweet syndrome is a potential adverse reaction.
- Sweet syndrome should be suspected if there is abrupt onset of painful erythematous plaques or nodules accompanied by pyrexia following injection of dupilumab. Biopsy of the nodules should be obtained to confirm the diagnosis.
- Systemic corticosteroids with cessation of dupilumab are effective treatments.
- Following treatment, dupilumab should not be reinitiated, and alternative therapies should be used.
Long-term Remission of Pyoderma Gangrenosum, Acne, and Hidradenitis Suppurativa Syndrome
Pyoderma gangrenosum (PG), acne, and hidradenitis suppurativa (HS)(PASH) syndrome is a recently identified disease process within the spectrum of autoinflammatory diseases (AIDs), which are distinct from autoimmune, infectious, and allergic syndromes and are gaining increasing interest given their complex pathophysiology and therapeutic resistance.1 Autoinflammatory diseases are defined by a dysregulation of the innate immune system in the absence of typical autoimmune features, including autoantibodies and antigen-specific T lymphocytes.2 Mutations affecting proteins of the inflammasome or proteins involved in regulating inflammasome function have been associated with these AIDs.2
Many AIDs have cutaneous involvement, as seen in PASH syndrome. Pyoderma gangrenosum is a neutrophilic dermatosis presenting as skin ulcers with undermined, erythematous, violaceous borders. It can be isolated, syndromic, or associated with inflammatory conditions (eg, inflammatory bowel disease, rheumatologic disorders, hematologic disorders).1 Acne vulgaris develops because of chronic obstruction of hair follicles as a result of disordered keratinization and abnormal sebaceous stem cell differentiation.2Propionibacterium acnes can reside and replicate within the biofilm community of the hair follicle and activate the inflammasome.2,3 Hidradenitis suppurativa, a chronic relapsing neutrophilic dermatosis, is a debilitating inflammatory disease of the hair follicles involving apocrine gland–bearing skin (ie, the axillary, inguinal, and anogenital regions).2 Onset often occurs between the ages of 20 and 40 years, with a 3-fold higher incidence in women compared to men.3 Patients experience painful, deep-seated nodules that drain into sinus tracts and abscesses. The condition can be isolated or associated with inflammatory conditions, such as inflammatory bowel disease.4
PASH syndrome has been described as a polygenic autoinflammatory condition that most commonly presents in young adults, with onset of acne beginning years prior to other manifestations. A study analyzing 5 patients with PASH syndrome reported an average age of 32.2 years at diagnosis with a disease duration of 3 to 7 years.5 Pathophysiology of this condition is not well understood, with many hypotheses calling upon dysregulation of the innate immune system, a commonality this syndrome may share with other AIDs. Given its poorly understood pathophysiology, treating PASH syndrome can be especially difficult. We report a novel case of disease remission lasting more than 4 years using adalimumab and cyclosporine. We also discuss prior treatment successes and hypotheses regarding etiologic factors in PASH syndrome.
Case Report
A 36-year-old woman presented for evaluation of open draining ulcerations on the back of 18 months’ duration. She had a 16-year history of scarring cystic acne of the face and HS of the groin. The patient’s family history was remarkable for severe cystic acne in her brother and son as well as HS in her mother and another brother. Her treatment history included isotretinoin, doxycycline, and topical steroids.
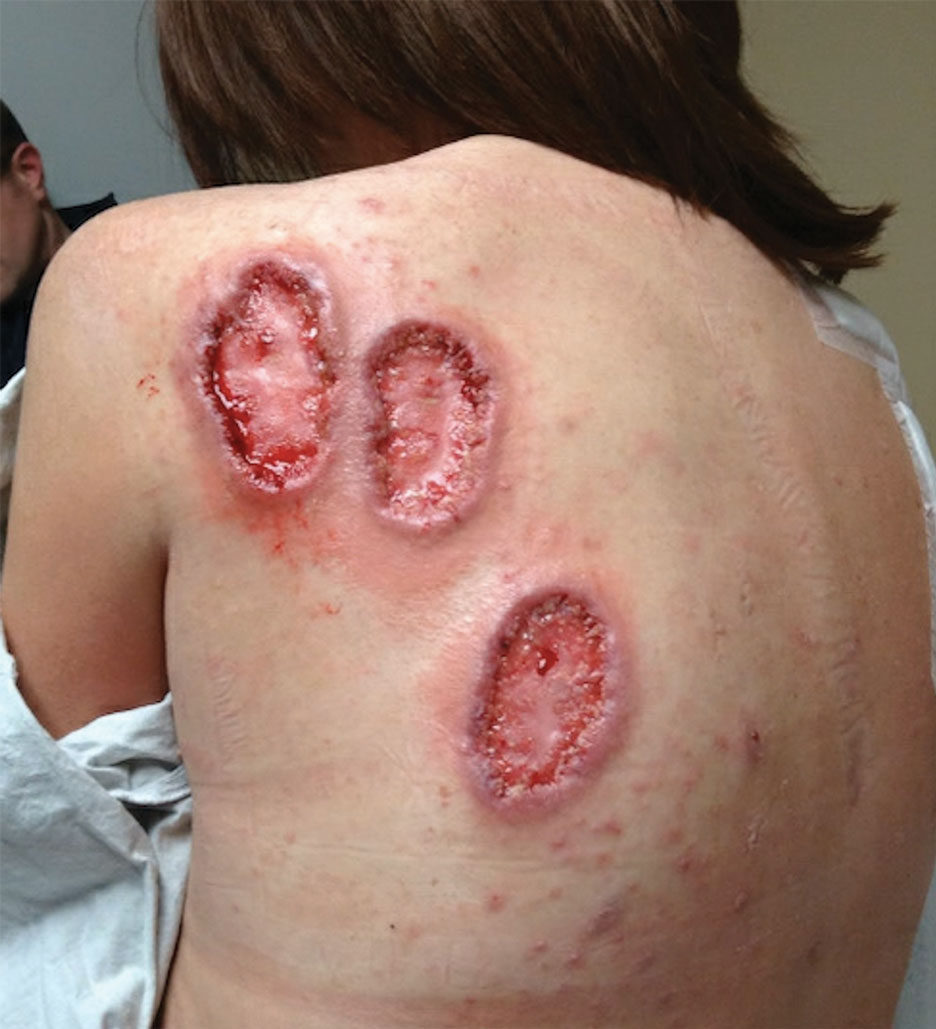
Physical examination revealed 2 ulcerations with violaceous borders involving the left upper back (greatest diameter, 5×7 cm)(Figure 1). Evidence of papular and cystic acne with residual scarring was noted on the cheeks. Scarring from HS was noted in the axillae and right groin. A biopsy from the edge of an ulceration on the back demonstrated epidermal spongiosis with acute and chronic inflammation and fibrosis (Figure 2). The clinicopathologic findings were most consistent with PG, and the patient was diagnosed with PASH syndrome, given the constellation of cutaneous lesions.
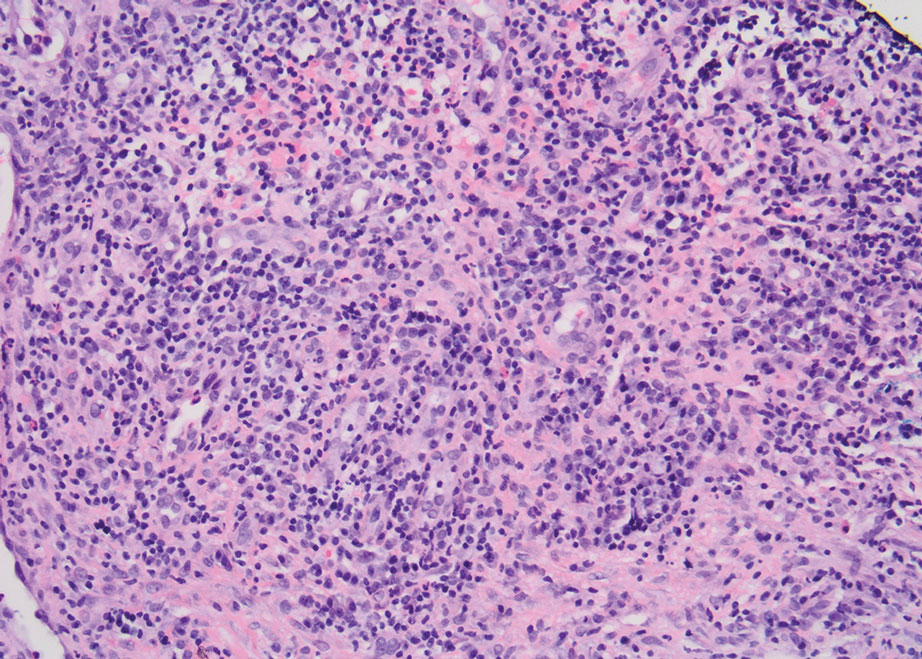
After treatment with topical and systemic antibiotics for acne and HS for more than 1 year failed, the patient was started on adalimumab. The initial dose was 160 mg subcutaneously, then 80 mg 2 weeks later, then 40 mg weekly thereafter. Doxycycline was continued for treatment of the acne and HS. After 6 weeks of adalimumab, the PG worsened and prednisone was added. She developed tender furuncles on the back, and cultures grew Pseudomonas aeruginosa and methicillin-sensitive Staphylococcus aureus that responded to ciprofloxacin and cephalexin.
Due to progression of PG on adalimumab, switching to an infliximab infusion or anakinra was considered, but these options were not covered by the patient’s health insurance. Three months after the initial presentation, the patient was started on cyclosporine 100 mg 3 times daily (5 mg/kg/d) while adalimumab was continued; the ulcers started to improve within 2.5 weeks. After 3 months (Figure 3), the cyclosporine was reduced to 100 mg twice daily, and adalimumab was continued. She had a slight flare of PG after 8 months of treatment when adalimumab was unavailable to her for 2 months. After 8 months on cyclosporine, the dosage was tapered to 100 mg/d and then completely discontinued after 12 months.
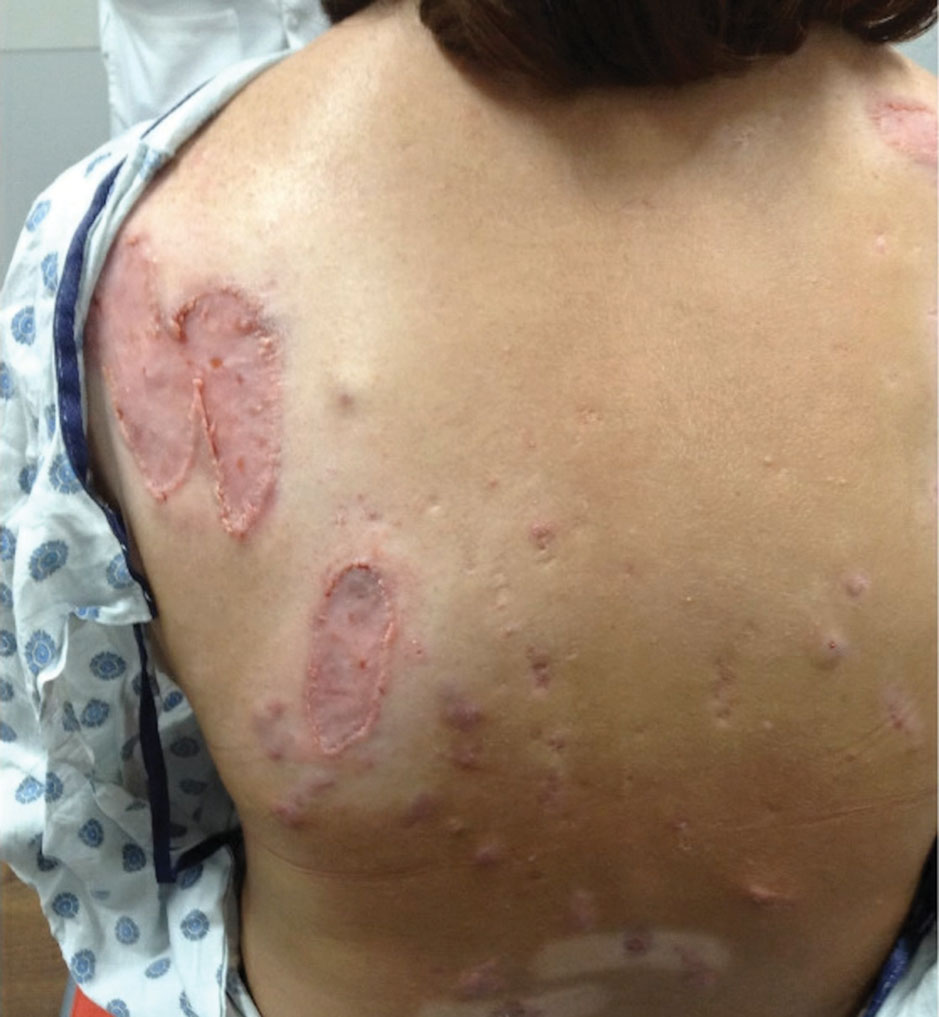
The patient has continued on adalimumab 40 mg weekly with excellent control of the PG (Figure 4), although she did have one HS flare in the left axilla 11 months after the initial treatment. The patient’s cystic acne has intermittently flared and has been managed with spironolactone 100 mg/d for 3 years. After 4 years of management, the patient’s PG and HS remain well controlled on adalimumab.
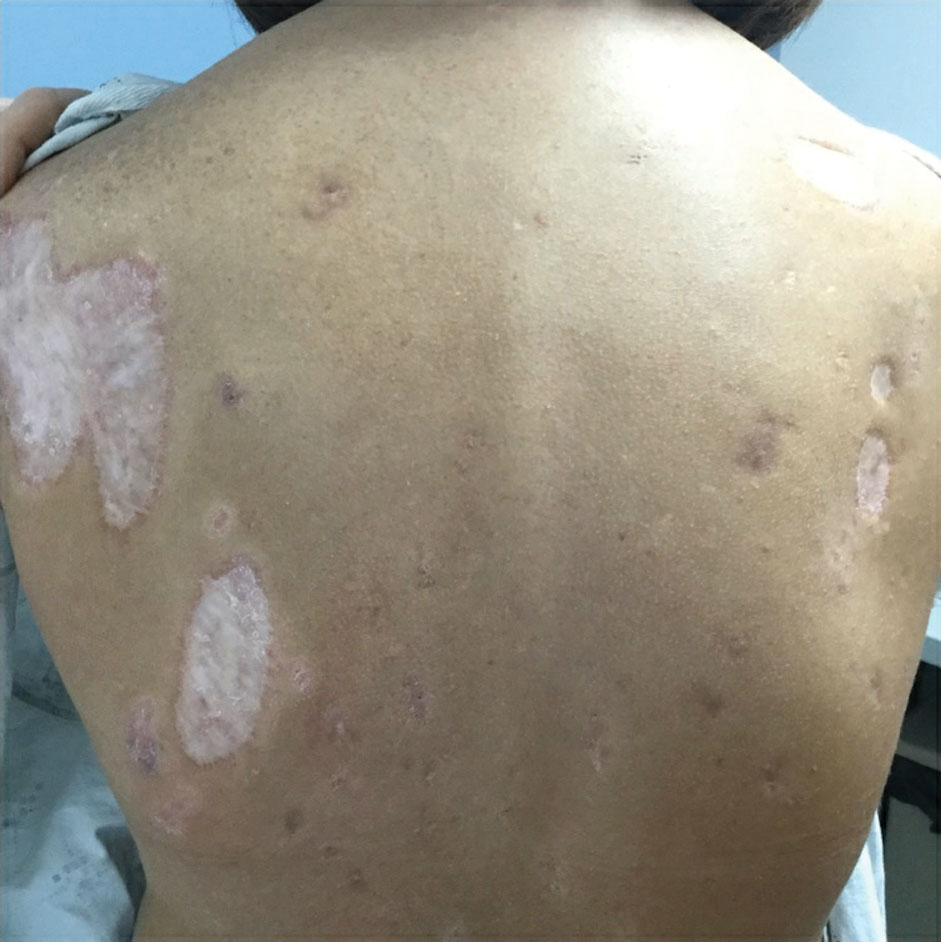
Comment
Our case represents a major step in refining long-term treatment approaches for PASH syndrome due to the 4-year remission. Prior cases have reported use of anakinra, anakinra-cyclosporine combination, prednisone, azathioprine, topical tacrolimus, etanercept, and dapsone without sustainable success.1-6 The case studies discussed below have achieved remission via alternative drug combinations.
Staub et al4 found greatest success with a combination of infliximab, dapsone, and cyclosporine, and their patient had been in remission for 20 months at time of publication. Their hypothesis proposed that multiple inflammatory signaling pathways are involved in PASH syndrome, and this is why combination therapy is required for remission.4 In 2018, Lamiaux et al7 demonstrated successful treatment with rifampicin and clindamycin. Their patient had been in remission for 22 months at the time of publication—this time frame included 12 months of combination therapy and 10 months without medication. The authors hypothesized that, because of the autoinflammatory nature of these antibiotics, this pharmacologic combination could eradicate pathogenic bacteria from host microbiota while also inhibiting neutrophil function and synthesis of chemokines and cytokines.7
More recently, reports have been published regarding the success of tildrakizumab, an IL-23 antagonist, and ixekizumab, an IL-17 antagonist, in the treatment of PASH syndrome.6,8 Ixekizumab was used in combination with doxycycline, and remission was achieved in 12 months.8 However, tildrakizumab was used alone and achieved greater than 75% improvement in disease manifestations within 2 months.
Marzano et al5 conducted protein arrays and enzyme-linked immunosorbent assay to analyze the expression of cytokine, chemokine, and effector molecule profiles in PASH syndrome. It was determined that serum analysis displayed a normal cytokine/chemokine profile, with the only abnormalities being anemia and elevated C-reactive protein. There were no statistically significant differences in serum levels of IL-1β, tumor necrosis factor (TNF) α, or IL-17 between PASH syndrome and healthy controls. However, cutaneous analysis revealed extensive cytokine and chemokine hyperactivity for IL-1β and IL-1β receptor; TNF-α; C-X-C motif ligands 1, 2, and 3; C-X-C motif ligand 16;
Ead et al3 presented a unique perspective focusing on cutaneous biofilm involvement in PASH syndrome. Microbes within these biofilms induce the migration and proliferation of inflammatory cells that consume factors normally utilized for tissue catabolism. These organisms deplete necessary biochemical cofactors used during healing. This lack of nutrients needed for healing not only slows the process but also promotes favorable conditions for the growth of anerobic species. In conjunction, biofilm formation restricts bacterial access to oxygen and nutrients, thus decreasing the bacterial metabolic rate and preventing the effects of antibiotic therapy. These features of biofilm communities contribute to inflammation and possibly the troubling resistance to many therapeutic options for PASH syndrome.
Each component of PASH syndrome has been associated with biofilm formation. As previously described, PG manifests in the skin as painful ulcerations, often with slough. This slough is hypothesized to be a consequence of increased vascular permeability and exudative byproducts that accompany the inflammatory nature of biofilms.3 Acne vulgaris has well-described associations with P acnes. Ead et al3 described P acnes as a component of the biofilm community within the microcomedone of hair follicles. This biofilm allows for antibiotic resistance occasionally seen in the treatment of acne and is potentially the pathogenic factor that both impedes healing and enhances the inflammatory state. Hidradenitis suppurativa has been associated with biofilm formation.3
In further pursuit of PASH syndrome pathophysiology, many experts have sought to uncover the relationship between PASH syndrome and the previously described pyogenic arthritis, PG, and acne (PAPA) syndrome, another entity within the AIDs spectrum (Table). This condition was first recognized in 1997 in a 3-generation family with 10 affected members.1 It is characterized by PG and acne, similar to PASH; however, PAPA syndrome includes PG arthritis and lacks HS. Pyogenic arthritis manifests as recurrent aseptic inflammation of the joints, mainly the elbows, knees, and ankles. Pyogenic arthritis commonly is the presenting symptom of PAPA syndrome, with onset in childhood.2 As patients age, the arthritic symptoms decrease, and skin manifestations become more prominent.
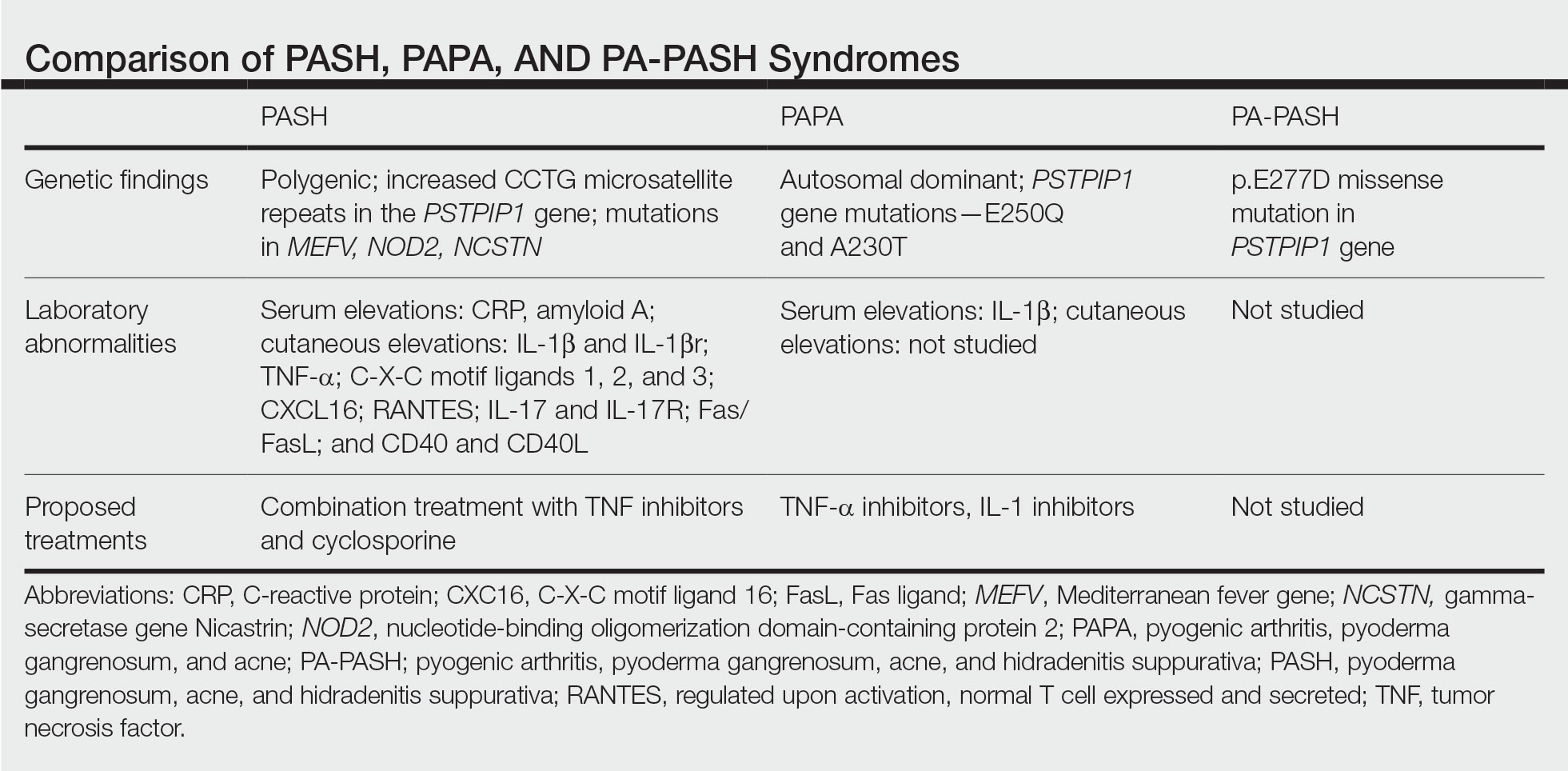
PAPA syndrome has autosomal-dominant inheritance with mutations on chromosome 15 in the proline-serine-threonine phosphatase interacting protein 1 (PSTPIP1) gene.1 This mutation induces hyperphosphorylation of PSTPIP1, allowing for increased binding affinity to pyrin. Both PSTPIP1 and pyrin are co-expressed as parts of the NLRP3 inflammasome in granulocytes and monocytes.1 As a result, pyrin is more highly bound and loses its inhibitory effect on the NLRP3 inflammasome pathway. This lack of inhibition allows for uninhibited cleavage of pro–IL-1β to active IL-1β by the inflammasome.1
Elevated concentrations of IL-1β in patients with PAPA syndrome result in a dysregulation of the innate immune system. IL-1β induces the release of proinflammatory cytokines, namely TNF-α; interferon γ; IL-8; and regulated on activation, normal T cell expressed and secreted (RANTES), all of which activate neutrophils and induce neutrophilic inflammation.2 IL-1β not only initiates this entire cascade but also acts as an antiapoptotic signal for neutrophils.2 When IL-1β reaches a critical threshold, it induces enough inflammation to cause severe tissue damage, thus causing joint and cutaneous disease in PAPA syndrome. IL-1 inhibitors (anakinra) or TNF-α inhibitors (etanercept, adalimumab, infliximab) have been used many times to successfully treat PAPA syndrome, with TNF-α inhibitors providing the most consistent results.
Another AIDs entity with similarities to both PAPA syndrome and PASH syndrome is pyogenic arthritis, PG, acne, and HS (PA-PASH) syndrome. First identified in 2012 by Bruzzese,9 genetic analyses revealed a p.E277D missense mutation in PSTPIP1 in PA-PASH syndrome. Research has suggested that the key molecular feature is neutrophil activation by TH17 cells and the TNF-α axis.9 This syndrome has not been further characterized, and little is known regarding adequate treatment for PA-PASH syndrome.
Although it is similar in phenotype to aspects of PAPA and PA-PASH syndromes, PASH syndrome has distinct genotypic and immunologic abnormalities. Genetic analysis of this condition has shown an increased number of CCTG repeats in proximity to the PSTPIP1 promoter. It is hypothesized that these additional repeats predispose patients to neutrophilic inflammation in a similar manner to a condition described in France, termed aseptic abscess syndrome.1,5 Other mutations have been identified, including those in IL-1N, PSMB8, MEFV, NOD2, NCSTN, and more.2,7 However, it has been determined that the majority of these variants have already been filed in the Single Nucleotide Polymorphism Database or in the Registry of Hereditary Auto-inflammatory Disorders Mutations.2 The question remains regarding the origin of inflammation seen in PASH syndrome; the potential role of biofilms; and the relationship between PASH, PAPA, and PA-PASH syndromes. Much work remains to be done in refining therapeutic options for PASH syndrome. Continued biochemical research is necessary, as well as collaboration among dermatologists worldwide who find success in treating this condition.
Conclusion
There are genotypic and phenotypic similarities between PASH, PAPA, and PA-PASH syndromes, with various mutations within or near the PSTPIP1 gene; however, their genetic discrepancies seem to play a major role in the pathophysiology of each syndrome. Much work remains to be done in PA-PASH syndrome, which has not yet been well described. Meanwhile, PAPA syndrome has been well characterized with mutations affecting proteins of the NLRP3 inflammasome, resulting in elevated IL-1β and excess neutrophilic inflammation. In PASH syndrome, the importance of increased repeats near the PSTPIP1 promoter is yet to be elucidated. It has been shown that these abnormalities predispose individuals to neutrophilic inflammation, but the mechanism by which they do so is unknown. In addition, consideration of biofilms and their predisposition to inflammation within the pathophysiology of PASH syndrome is a possibility that must be considered when discussing therapeutic options. Based on our case study and previous successes in treating PASH syndrome, it is clear that a multidrug approach is necessary for remission. It is likely that the etiology of PASH syndrome is multifaceted and involves hyperactivity in multiple arms of the innate immune system.
Patients with PASH syndrome have severely impaired quality of life and often experience social withdrawal due to the disfiguring sequelae and limited treatment options available. To improve patient outcomes, it is essential for physicians and scientists to report on successful treatment strategies and advances in immunologic understanding. Improved understanding of PASH syndrome calls for further genetic exploration into the role of additional genomic repeats and how these affect the PSTPIP1 gene and inflammasome activity. As medical advances improve understanding of the pathophysiology of this disease entity, it will likely become clear which mechanisms are most important in disease progression and how clinicians can best optimize treatment.
- Braun-Falco M, Kovnerystyy O, Lohse P, et al. Pyoderma gangrenosum, acne, and suppurative hidradenitis (PASH)—a new autoinflammatory syndrome distinct from PAPA syndrome. J Am Acad Dermatol. 2012;66:409-415.
- Cugno M, Borghi A, Marzano AV. PAPA, PASH and PAPASH syndromes: pathophysiology, presentation and treatment. Am J Clin Dermatol. 2017;18:555-562.
- Ead JK, Snyder RJ, Wise J, et al. Is PASH syndrome a biofilm disease?: a case series and review of the literature. Wounds. 2018;30:216-223.
- Staub J, Pfannschmidt N, Strohal R, et al. Successful treatment of PASH syndrome with infliximab, cyclosporine and dapsone. J Eur Acad Dermatol Venereol. 2015;29:2243-2247.
- Marzano AV, Ceccherini I, Gattorno M, et al. Association of pyoderma gangrenosum, acne, and suppurative hidradenitis (PASH) shares genetic and cytokine profiles with other autoinflammatory diseases. Medicine (Baltimore). 2014;93:E187.
- Kok Y, Nicolopoulos J, Varigos G, et al. Tildrakizumab in the treatment of PASH syndrome: a potential novel therapeutic target. Australas J Dermatol. 2020;61:E373-E374.
- Lamiaux M, Dabouz F, Wantz M, et al. Successful combined antibiotic therapy with oral clindamycin and oral rifampicin for pyoderma gangrenosum in patient with PASH syndrome. JAAD Case Rep. 2018;4:17-21.
- Gul MI, Singam V, Hanson C, et al. Remission of refractory PASH syndrome using ixekizumab and doxycycline. J Drugs Dermatol. 2020;19:1123.
- Bruzzese V. Pyoderma gangrenosum, acne conglobata, suppurative hidradenitis, and axial spondyloarthritis: efficacy of anti-tumor necrosis factor α therapy. J Clin Rheumatol. 2012;18:413-415.
Pyoderma gangrenosum (PG), acne, and hidradenitis suppurativa (HS)(PASH) syndrome is a recently identified disease process within the spectrum of autoinflammatory diseases (AIDs), which are distinct from autoimmune, infectious, and allergic syndromes and are gaining increasing interest given their complex pathophysiology and therapeutic resistance.1 Autoinflammatory diseases are defined by a dysregulation of the innate immune system in the absence of typical autoimmune features, including autoantibodies and antigen-specific T lymphocytes.2 Mutations affecting proteins of the inflammasome or proteins involved in regulating inflammasome function have been associated with these AIDs.2
Many AIDs have cutaneous involvement, as seen in PASH syndrome. Pyoderma gangrenosum is a neutrophilic dermatosis presenting as skin ulcers with undermined, erythematous, violaceous borders. It can be isolated, syndromic, or associated with inflammatory conditions (eg, inflammatory bowel disease, rheumatologic disorders, hematologic disorders).1 Acne vulgaris develops because of chronic obstruction of hair follicles as a result of disordered keratinization and abnormal sebaceous stem cell differentiation.2Propionibacterium acnes can reside and replicate within the biofilm community of the hair follicle and activate the inflammasome.2,3 Hidradenitis suppurativa, a chronic relapsing neutrophilic dermatosis, is a debilitating inflammatory disease of the hair follicles involving apocrine gland–bearing skin (ie, the axillary, inguinal, and anogenital regions).2 Onset often occurs between the ages of 20 and 40 years, with a 3-fold higher incidence in women compared to men.3 Patients experience painful, deep-seated nodules that drain into sinus tracts and abscesses. The condition can be isolated or associated with inflammatory conditions, such as inflammatory bowel disease.4
PASH syndrome has been described as a polygenic autoinflammatory condition that most commonly presents in young adults, with onset of acne beginning years prior to other manifestations. A study analyzing 5 patients with PASH syndrome reported an average age of 32.2 years at diagnosis with a disease duration of 3 to 7 years.5 Pathophysiology of this condition is not well understood, with many hypotheses calling upon dysregulation of the innate immune system, a commonality this syndrome may share with other AIDs. Given its poorly understood pathophysiology, treating PASH syndrome can be especially difficult. We report a novel case of disease remission lasting more than 4 years using adalimumab and cyclosporine. We also discuss prior treatment successes and hypotheses regarding etiologic factors in PASH syndrome.
Case Report
A 36-year-old woman presented for evaluation of open draining ulcerations on the back of 18 months’ duration. She had a 16-year history of scarring cystic acne of the face and HS of the groin. The patient’s family history was remarkable for severe cystic acne in her brother and son as well as HS in her mother and another brother. Her treatment history included isotretinoin, doxycycline, and topical steroids.

Physical examination revealed 2 ulcerations with violaceous borders involving the left upper back (greatest diameter, 5×7 cm)(Figure 1). Evidence of papular and cystic acne with residual scarring was noted on the cheeks. Scarring from HS was noted in the axillae and right groin. A biopsy from the edge of an ulceration on the back demonstrated epidermal spongiosis with acute and chronic inflammation and fibrosis (Figure 2). The clinicopathologic findings were most consistent with PG, and the patient was diagnosed with PASH syndrome, given the constellation of cutaneous lesions.

After treatment with topical and systemic antibiotics for acne and HS for more than 1 year failed, the patient was started on adalimumab. The initial dose was 160 mg subcutaneously, then 80 mg 2 weeks later, then 40 mg weekly thereafter. Doxycycline was continued for treatment of the acne and HS. After 6 weeks of adalimumab, the PG worsened and prednisone was added. She developed tender furuncles on the back, and cultures grew Pseudomonas aeruginosa and methicillin-sensitive Staphylococcus aureus that responded to ciprofloxacin and cephalexin.
Due to progression of PG on adalimumab, switching to an infliximab infusion or anakinra was considered, but these options were not covered by the patient’s health insurance. Three months after the initial presentation, the patient was started on cyclosporine 100 mg 3 times daily (5 mg/kg/d) while adalimumab was continued; the ulcers started to improve within 2.5 weeks. After 3 months (Figure 3), the cyclosporine was reduced to 100 mg twice daily, and adalimumab was continued. She had a slight flare of PG after 8 months of treatment when adalimumab was unavailable to her for 2 months. After 8 months on cyclosporine, the dosage was tapered to 100 mg/d and then completely discontinued after 12 months.

The patient has continued on adalimumab 40 mg weekly with excellent control of the PG (Figure 4), although she did have one HS flare in the left axilla 11 months after the initial treatment. The patient’s cystic acne has intermittently flared and has been managed with spironolactone 100 mg/d for 3 years. After 4 years of management, the patient’s PG and HS remain well controlled on adalimumab.

Comment
Our case represents a major step in refining long-term treatment approaches for PASH syndrome due to the 4-year remission. Prior cases have reported use of anakinra, anakinra-cyclosporine combination, prednisone, azathioprine, topical tacrolimus, etanercept, and dapsone without sustainable success.1-6 The case studies discussed below have achieved remission via alternative drug combinations.
Staub et al4 found greatest success with a combination of infliximab, dapsone, and cyclosporine, and their patient had been in remission for 20 months at time of publication. Their hypothesis proposed that multiple inflammatory signaling pathways are involved in PASH syndrome, and this is why combination therapy is required for remission.4 In 2018, Lamiaux et al7 demonstrated successful treatment with rifampicin and clindamycin. Their patient had been in remission for 22 months at the time of publication—this time frame included 12 months of combination therapy and 10 months without medication. The authors hypothesized that, because of the autoinflammatory nature of these antibiotics, this pharmacologic combination could eradicate pathogenic bacteria from host microbiota while also inhibiting neutrophil function and synthesis of chemokines and cytokines.7
More recently, reports have been published regarding the success of tildrakizumab, an IL-23 antagonist, and ixekizumab, an IL-17 antagonist, in the treatment of PASH syndrome.6,8 Ixekizumab was used in combination with doxycycline, and remission was achieved in 12 months.8 However, tildrakizumab was used alone and achieved greater than 75% improvement in disease manifestations within 2 months.
Marzano et al5 conducted protein arrays and enzyme-linked immunosorbent assay to analyze the expression of cytokine, chemokine, and effector molecule profiles in PASH syndrome. It was determined that serum analysis displayed a normal cytokine/chemokine profile, with the only abnormalities being anemia and elevated C-reactive protein. There were no statistically significant differences in serum levels of IL-1β, tumor necrosis factor (TNF) α, or IL-17 between PASH syndrome and healthy controls. However, cutaneous analysis revealed extensive cytokine and chemokine hyperactivity for IL-1β and IL-1β receptor; TNF-α; C-X-C motif ligands 1, 2, and 3; C-X-C motif ligand 16;
Ead et al3 presented a unique perspective focusing on cutaneous biofilm involvement in PASH syndrome. Microbes within these biofilms induce the migration and proliferation of inflammatory cells that consume factors normally utilized for tissue catabolism. These organisms deplete necessary biochemical cofactors used during healing. This lack of nutrients needed for healing not only slows the process but also promotes favorable conditions for the growth of anerobic species. In conjunction, biofilm formation restricts bacterial access to oxygen and nutrients, thus decreasing the bacterial metabolic rate and preventing the effects of antibiotic therapy. These features of biofilm communities contribute to inflammation and possibly the troubling resistance to many therapeutic options for PASH syndrome.
Each component of PASH syndrome has been associated with biofilm formation. As previously described, PG manifests in the skin as painful ulcerations, often with slough. This slough is hypothesized to be a consequence of increased vascular permeability and exudative byproducts that accompany the inflammatory nature of biofilms.3 Acne vulgaris has well-described associations with P acnes. Ead et al3 described P acnes as a component of the biofilm community within the microcomedone of hair follicles. This biofilm allows for antibiotic resistance occasionally seen in the treatment of acne and is potentially the pathogenic factor that both impedes healing and enhances the inflammatory state. Hidradenitis suppurativa has been associated with biofilm formation.3
In further pursuit of PASH syndrome pathophysiology, many experts have sought to uncover the relationship between PASH syndrome and the previously described pyogenic arthritis, PG, and acne (PAPA) syndrome, another entity within the AIDs spectrum (Table). This condition was first recognized in 1997 in a 3-generation family with 10 affected members.1 It is characterized by PG and acne, similar to PASH; however, PAPA syndrome includes PG arthritis and lacks HS. Pyogenic arthritis manifests as recurrent aseptic inflammation of the joints, mainly the elbows, knees, and ankles. Pyogenic arthritis commonly is the presenting symptom of PAPA syndrome, with onset in childhood.2 As patients age, the arthritic symptoms decrease, and skin manifestations become more prominent.

PAPA syndrome has autosomal-dominant inheritance with mutations on chromosome 15 in the proline-serine-threonine phosphatase interacting protein 1 (PSTPIP1) gene.1 This mutation induces hyperphosphorylation of PSTPIP1, allowing for increased binding affinity to pyrin. Both PSTPIP1 and pyrin are co-expressed as parts of the NLRP3 inflammasome in granulocytes and monocytes.1 As a result, pyrin is more highly bound and loses its inhibitory effect on the NLRP3 inflammasome pathway. This lack of inhibition allows for uninhibited cleavage of pro–IL-1β to active IL-1β by the inflammasome.1
Elevated concentrations of IL-1β in patients with PAPA syndrome result in a dysregulation of the innate immune system. IL-1β induces the release of proinflammatory cytokines, namely TNF-α; interferon γ; IL-8; and regulated on activation, normal T cell expressed and secreted (RANTES), all of which activate neutrophils and induce neutrophilic inflammation.2 IL-1β not only initiates this entire cascade but also acts as an antiapoptotic signal for neutrophils.2 When IL-1β reaches a critical threshold, it induces enough inflammation to cause severe tissue damage, thus causing joint and cutaneous disease in PAPA syndrome. IL-1 inhibitors (anakinra) or TNF-α inhibitors (etanercept, adalimumab, infliximab) have been used many times to successfully treat PAPA syndrome, with TNF-α inhibitors providing the most consistent results.
Another AIDs entity with similarities to both PAPA syndrome and PASH syndrome is pyogenic arthritis, PG, acne, and HS (PA-PASH) syndrome. First identified in 2012 by Bruzzese,9 genetic analyses revealed a p.E277D missense mutation in PSTPIP1 in PA-PASH syndrome. Research has suggested that the key molecular feature is neutrophil activation by TH17 cells and the TNF-α axis.9 This syndrome has not been further characterized, and little is known regarding adequate treatment for PA-PASH syndrome.
Although it is similar in phenotype to aspects of PAPA and PA-PASH syndromes, PASH syndrome has distinct genotypic and immunologic abnormalities. Genetic analysis of this condition has shown an increased number of CCTG repeats in proximity to the PSTPIP1 promoter. It is hypothesized that these additional repeats predispose patients to neutrophilic inflammation in a similar manner to a condition described in France, termed aseptic abscess syndrome.1,5 Other mutations have been identified, including those in IL-1N, PSMB8, MEFV, NOD2, NCSTN, and more.2,7 However, it has been determined that the majority of these variants have already been filed in the Single Nucleotide Polymorphism Database or in the Registry of Hereditary Auto-inflammatory Disorders Mutations.2 The question remains regarding the origin of inflammation seen in PASH syndrome; the potential role of biofilms; and the relationship between PASH, PAPA, and PA-PASH syndromes. Much work remains to be done in refining therapeutic options for PASH syndrome. Continued biochemical research is necessary, as well as collaboration among dermatologists worldwide who find success in treating this condition.
Conclusion
There are genotypic and phenotypic similarities between PASH, PAPA, and PA-PASH syndromes, with various mutations within or near the PSTPIP1 gene; however, their genetic discrepancies seem to play a major role in the pathophysiology of each syndrome. Much work remains to be done in PA-PASH syndrome, which has not yet been well described. Meanwhile, PAPA syndrome has been well characterized with mutations affecting proteins of the NLRP3 inflammasome, resulting in elevated IL-1β and excess neutrophilic inflammation. In PASH syndrome, the importance of increased repeats near the PSTPIP1 promoter is yet to be elucidated. It has been shown that these abnormalities predispose individuals to neutrophilic inflammation, but the mechanism by which they do so is unknown. In addition, consideration of biofilms and their predisposition to inflammation within the pathophysiology of PASH syndrome is a possibility that must be considered when discussing therapeutic options. Based on our case study and previous successes in treating PASH syndrome, it is clear that a multidrug approach is necessary for remission. It is likely that the etiology of PASH syndrome is multifaceted and involves hyperactivity in multiple arms of the innate immune system.
Patients with PASH syndrome have severely impaired quality of life and often experience social withdrawal due to the disfiguring sequelae and limited treatment options available. To improve patient outcomes, it is essential for physicians and scientists to report on successful treatment strategies and advances in immunologic understanding. Improved understanding of PASH syndrome calls for further genetic exploration into the role of additional genomic repeats and how these affect the PSTPIP1 gene and inflammasome activity. As medical advances improve understanding of the pathophysiology of this disease entity, it will likely become clear which mechanisms are most important in disease progression and how clinicians can best optimize treatment.
Pyoderma gangrenosum (PG), acne, and hidradenitis suppurativa (HS)(PASH) syndrome is a recently identified disease process within the spectrum of autoinflammatory diseases (AIDs), which are distinct from autoimmune, infectious, and allergic syndromes and are gaining increasing interest given their complex pathophysiology and therapeutic resistance.1 Autoinflammatory diseases are defined by a dysregulation of the innate immune system in the absence of typical autoimmune features, including autoantibodies and antigen-specific T lymphocytes.2 Mutations affecting proteins of the inflammasome or proteins involved in regulating inflammasome function have been associated with these AIDs.2
Many AIDs have cutaneous involvement, as seen in PASH syndrome. Pyoderma gangrenosum is a neutrophilic dermatosis presenting as skin ulcers with undermined, erythematous, violaceous borders. It can be isolated, syndromic, or associated with inflammatory conditions (eg, inflammatory bowel disease, rheumatologic disorders, hematologic disorders).1 Acne vulgaris develops because of chronic obstruction of hair follicles as a result of disordered keratinization and abnormal sebaceous stem cell differentiation.2Propionibacterium acnes can reside and replicate within the biofilm community of the hair follicle and activate the inflammasome.2,3 Hidradenitis suppurativa, a chronic relapsing neutrophilic dermatosis, is a debilitating inflammatory disease of the hair follicles involving apocrine gland–bearing skin (ie, the axillary, inguinal, and anogenital regions).2 Onset often occurs between the ages of 20 and 40 years, with a 3-fold higher incidence in women compared to men.3 Patients experience painful, deep-seated nodules that drain into sinus tracts and abscesses. The condition can be isolated or associated with inflammatory conditions, such as inflammatory bowel disease.4
PASH syndrome has been described as a polygenic autoinflammatory condition that most commonly presents in young adults, with onset of acne beginning years prior to other manifestations. A study analyzing 5 patients with PASH syndrome reported an average age of 32.2 years at diagnosis with a disease duration of 3 to 7 years.5 Pathophysiology of this condition is not well understood, with many hypotheses calling upon dysregulation of the innate immune system, a commonality this syndrome may share with other AIDs. Given its poorly understood pathophysiology, treating PASH syndrome can be especially difficult. We report a novel case of disease remission lasting more than 4 years using adalimumab and cyclosporine. We also discuss prior treatment successes and hypotheses regarding etiologic factors in PASH syndrome.
Case Report
A 36-year-old woman presented for evaluation of open draining ulcerations on the back of 18 months’ duration. She had a 16-year history of scarring cystic acne of the face and HS of the groin. The patient’s family history was remarkable for severe cystic acne in her brother and son as well as HS in her mother and another brother. Her treatment history included isotretinoin, doxycycline, and topical steroids.

Physical examination revealed 2 ulcerations with violaceous borders involving the left upper back (greatest diameter, 5×7 cm)(Figure 1). Evidence of papular and cystic acne with residual scarring was noted on the cheeks. Scarring from HS was noted in the axillae and right groin. A biopsy from the edge of an ulceration on the back demonstrated epidermal spongiosis with acute and chronic inflammation and fibrosis (Figure 2). The clinicopathologic findings were most consistent with PG, and the patient was diagnosed with PASH syndrome, given the constellation of cutaneous lesions.

After treatment with topical and systemic antibiotics for acne and HS for more than 1 year failed, the patient was started on adalimumab. The initial dose was 160 mg subcutaneously, then 80 mg 2 weeks later, then 40 mg weekly thereafter. Doxycycline was continued for treatment of the acne and HS. After 6 weeks of adalimumab, the PG worsened and prednisone was added. She developed tender furuncles on the back, and cultures grew Pseudomonas aeruginosa and methicillin-sensitive Staphylococcus aureus that responded to ciprofloxacin and cephalexin.
Due to progression of PG on adalimumab, switching to an infliximab infusion or anakinra was considered, but these options were not covered by the patient’s health insurance. Three months after the initial presentation, the patient was started on cyclosporine 100 mg 3 times daily (5 mg/kg/d) while adalimumab was continued; the ulcers started to improve within 2.5 weeks. After 3 months (Figure 3), the cyclosporine was reduced to 100 mg twice daily, and adalimumab was continued. She had a slight flare of PG after 8 months of treatment when adalimumab was unavailable to her for 2 months. After 8 months on cyclosporine, the dosage was tapered to 100 mg/d and then completely discontinued after 12 months.

The patient has continued on adalimumab 40 mg weekly with excellent control of the PG (Figure 4), although she did have one HS flare in the left axilla 11 months after the initial treatment. The patient’s cystic acne has intermittently flared and has been managed with spironolactone 100 mg/d for 3 years. After 4 years of management, the patient’s PG and HS remain well controlled on adalimumab.

Comment
Our case represents a major step in refining long-term treatment approaches for PASH syndrome due to the 4-year remission. Prior cases have reported use of anakinra, anakinra-cyclosporine combination, prednisone, azathioprine, topical tacrolimus, etanercept, and dapsone without sustainable success.1-6 The case studies discussed below have achieved remission via alternative drug combinations.
Staub et al4 found greatest success with a combination of infliximab, dapsone, and cyclosporine, and their patient had been in remission for 20 months at time of publication. Their hypothesis proposed that multiple inflammatory signaling pathways are involved in PASH syndrome, and this is why combination therapy is required for remission.4 In 2018, Lamiaux et al7 demonstrated successful treatment with rifampicin and clindamycin. Their patient had been in remission for 22 months at the time of publication—this time frame included 12 months of combination therapy and 10 months without medication. The authors hypothesized that, because of the autoinflammatory nature of these antibiotics, this pharmacologic combination could eradicate pathogenic bacteria from host microbiota while also inhibiting neutrophil function and synthesis of chemokines and cytokines.7
More recently, reports have been published regarding the success of tildrakizumab, an IL-23 antagonist, and ixekizumab, an IL-17 antagonist, in the treatment of PASH syndrome.6,8 Ixekizumab was used in combination with doxycycline, and remission was achieved in 12 months.8 However, tildrakizumab was used alone and achieved greater than 75% improvement in disease manifestations within 2 months.
Marzano et al5 conducted protein arrays and enzyme-linked immunosorbent assay to analyze the expression of cytokine, chemokine, and effector molecule profiles in PASH syndrome. It was determined that serum analysis displayed a normal cytokine/chemokine profile, with the only abnormalities being anemia and elevated C-reactive protein. There were no statistically significant differences in serum levels of IL-1β, tumor necrosis factor (TNF) α, or IL-17 between PASH syndrome and healthy controls. However, cutaneous analysis revealed extensive cytokine and chemokine hyperactivity for IL-1β and IL-1β receptor; TNF-α; C-X-C motif ligands 1, 2, and 3; C-X-C motif ligand 16;
Ead et al3 presented a unique perspective focusing on cutaneous biofilm involvement in PASH syndrome. Microbes within these biofilms induce the migration and proliferation of inflammatory cells that consume factors normally utilized for tissue catabolism. These organisms deplete necessary biochemical cofactors used during healing. This lack of nutrients needed for healing not only slows the process but also promotes favorable conditions for the growth of anerobic species. In conjunction, biofilm formation restricts bacterial access to oxygen and nutrients, thus decreasing the bacterial metabolic rate and preventing the effects of antibiotic therapy. These features of biofilm communities contribute to inflammation and possibly the troubling resistance to many therapeutic options for PASH syndrome.
Each component of PASH syndrome has been associated with biofilm formation. As previously described, PG manifests in the skin as painful ulcerations, often with slough. This slough is hypothesized to be a consequence of increased vascular permeability and exudative byproducts that accompany the inflammatory nature of biofilms.3 Acne vulgaris has well-described associations with P acnes. Ead et al3 described P acnes as a component of the biofilm community within the microcomedone of hair follicles. This biofilm allows for antibiotic resistance occasionally seen in the treatment of acne and is potentially the pathogenic factor that both impedes healing and enhances the inflammatory state. Hidradenitis suppurativa has been associated with biofilm formation.3
In further pursuit of PASH syndrome pathophysiology, many experts have sought to uncover the relationship between PASH syndrome and the previously described pyogenic arthritis, PG, and acne (PAPA) syndrome, another entity within the AIDs spectrum (Table). This condition was first recognized in 1997 in a 3-generation family with 10 affected members.1 It is characterized by PG and acne, similar to PASH; however, PAPA syndrome includes PG arthritis and lacks HS. Pyogenic arthritis manifests as recurrent aseptic inflammation of the joints, mainly the elbows, knees, and ankles. Pyogenic arthritis commonly is the presenting symptom of PAPA syndrome, with onset in childhood.2 As patients age, the arthritic symptoms decrease, and skin manifestations become more prominent.

PAPA syndrome has autosomal-dominant inheritance with mutations on chromosome 15 in the proline-serine-threonine phosphatase interacting protein 1 (PSTPIP1) gene.1 This mutation induces hyperphosphorylation of PSTPIP1, allowing for increased binding affinity to pyrin. Both PSTPIP1 and pyrin are co-expressed as parts of the NLRP3 inflammasome in granulocytes and monocytes.1 As a result, pyrin is more highly bound and loses its inhibitory effect on the NLRP3 inflammasome pathway. This lack of inhibition allows for uninhibited cleavage of pro–IL-1β to active IL-1β by the inflammasome.1
Elevated concentrations of IL-1β in patients with PAPA syndrome result in a dysregulation of the innate immune system. IL-1β induces the release of proinflammatory cytokines, namely TNF-α; interferon γ; IL-8; and regulated on activation, normal T cell expressed and secreted (RANTES), all of which activate neutrophils and induce neutrophilic inflammation.2 IL-1β not only initiates this entire cascade but also acts as an antiapoptotic signal for neutrophils.2 When IL-1β reaches a critical threshold, it induces enough inflammation to cause severe tissue damage, thus causing joint and cutaneous disease in PAPA syndrome. IL-1 inhibitors (anakinra) or TNF-α inhibitors (etanercept, adalimumab, infliximab) have been used many times to successfully treat PAPA syndrome, with TNF-α inhibitors providing the most consistent results.
Another AIDs entity with similarities to both PAPA syndrome and PASH syndrome is pyogenic arthritis, PG, acne, and HS (PA-PASH) syndrome. First identified in 2012 by Bruzzese,9 genetic analyses revealed a p.E277D missense mutation in PSTPIP1 in PA-PASH syndrome. Research has suggested that the key molecular feature is neutrophil activation by TH17 cells and the TNF-α axis.9 This syndrome has not been further characterized, and little is known regarding adequate treatment for PA-PASH syndrome.
Although it is similar in phenotype to aspects of PAPA and PA-PASH syndromes, PASH syndrome has distinct genotypic and immunologic abnormalities. Genetic analysis of this condition has shown an increased number of CCTG repeats in proximity to the PSTPIP1 promoter. It is hypothesized that these additional repeats predispose patients to neutrophilic inflammation in a similar manner to a condition described in France, termed aseptic abscess syndrome.1,5 Other mutations have been identified, including those in IL-1N, PSMB8, MEFV, NOD2, NCSTN, and more.2,7 However, it has been determined that the majority of these variants have already been filed in the Single Nucleotide Polymorphism Database or in the Registry of Hereditary Auto-inflammatory Disorders Mutations.2 The question remains regarding the origin of inflammation seen in PASH syndrome; the potential role of biofilms; and the relationship between PASH, PAPA, and PA-PASH syndromes. Much work remains to be done in refining therapeutic options for PASH syndrome. Continued biochemical research is necessary, as well as collaboration among dermatologists worldwide who find success in treating this condition.
Conclusion
There are genotypic and phenotypic similarities between PASH, PAPA, and PA-PASH syndromes, with various mutations within or near the PSTPIP1 gene; however, their genetic discrepancies seem to play a major role in the pathophysiology of each syndrome. Much work remains to be done in PA-PASH syndrome, which has not yet been well described. Meanwhile, PAPA syndrome has been well characterized with mutations affecting proteins of the NLRP3 inflammasome, resulting in elevated IL-1β and excess neutrophilic inflammation. In PASH syndrome, the importance of increased repeats near the PSTPIP1 promoter is yet to be elucidated. It has been shown that these abnormalities predispose individuals to neutrophilic inflammation, but the mechanism by which they do so is unknown. In addition, consideration of biofilms and their predisposition to inflammation within the pathophysiology of PASH syndrome is a possibility that must be considered when discussing therapeutic options. Based on our case study and previous successes in treating PASH syndrome, it is clear that a multidrug approach is necessary for remission. It is likely that the etiology of PASH syndrome is multifaceted and involves hyperactivity in multiple arms of the innate immune system.
Patients with PASH syndrome have severely impaired quality of life and often experience social withdrawal due to the disfiguring sequelae and limited treatment options available. To improve patient outcomes, it is essential for physicians and scientists to report on successful treatment strategies and advances in immunologic understanding. Improved understanding of PASH syndrome calls for further genetic exploration into the role of additional genomic repeats and how these affect the PSTPIP1 gene and inflammasome activity. As medical advances improve understanding of the pathophysiology of this disease entity, it will likely become clear which mechanisms are most important in disease progression and how clinicians can best optimize treatment.
- Braun-Falco M, Kovnerystyy O, Lohse P, et al. Pyoderma gangrenosum, acne, and suppurative hidradenitis (PASH)—a new autoinflammatory syndrome distinct from PAPA syndrome. J Am Acad Dermatol. 2012;66:409-415.
- Cugno M, Borghi A, Marzano AV. PAPA, PASH and PAPASH syndromes: pathophysiology, presentation and treatment. Am J Clin Dermatol. 2017;18:555-562.
- Ead JK, Snyder RJ, Wise J, et al. Is PASH syndrome a biofilm disease?: a case series and review of the literature. Wounds. 2018;30:216-223.
- Staub J, Pfannschmidt N, Strohal R, et al. Successful treatment of PASH syndrome with infliximab, cyclosporine and dapsone. J Eur Acad Dermatol Venereol. 2015;29:2243-2247.
- Marzano AV, Ceccherini I, Gattorno M, et al. Association of pyoderma gangrenosum, acne, and suppurative hidradenitis (PASH) shares genetic and cytokine profiles with other autoinflammatory diseases. Medicine (Baltimore). 2014;93:E187.
- Kok Y, Nicolopoulos J, Varigos G, et al. Tildrakizumab in the treatment of PASH syndrome: a potential novel therapeutic target. Australas J Dermatol. 2020;61:E373-E374.
- Lamiaux M, Dabouz F, Wantz M, et al. Successful combined antibiotic therapy with oral clindamycin and oral rifampicin for pyoderma gangrenosum in patient with PASH syndrome. JAAD Case Rep. 2018;4:17-21.
- Gul MI, Singam V, Hanson C, et al. Remission of refractory PASH syndrome using ixekizumab and doxycycline. J Drugs Dermatol. 2020;19:1123.
- Bruzzese V. Pyoderma gangrenosum, acne conglobata, suppurative hidradenitis, and axial spondyloarthritis: efficacy of anti-tumor necrosis factor α therapy. J Clin Rheumatol. 2012;18:413-415.
- Braun-Falco M, Kovnerystyy O, Lohse P, et al. Pyoderma gangrenosum, acne, and suppurative hidradenitis (PASH)—a new autoinflammatory syndrome distinct from PAPA syndrome. J Am Acad Dermatol. 2012;66:409-415.
- Cugno M, Borghi A, Marzano AV. PAPA, PASH and PAPASH syndromes: pathophysiology, presentation and treatment. Am J Clin Dermatol. 2017;18:555-562.
- Ead JK, Snyder RJ, Wise J, et al. Is PASH syndrome a biofilm disease?: a case series and review of the literature. Wounds. 2018;30:216-223.
- Staub J, Pfannschmidt N, Strohal R, et al. Successful treatment of PASH syndrome with infliximab, cyclosporine and dapsone. J Eur Acad Dermatol Venereol. 2015;29:2243-2247.
- Marzano AV, Ceccherini I, Gattorno M, et al. Association of pyoderma gangrenosum, acne, and suppurative hidradenitis (PASH) shares genetic and cytokine profiles with other autoinflammatory diseases. Medicine (Baltimore). 2014;93:E187.
- Kok Y, Nicolopoulos J, Varigos G, et al. Tildrakizumab in the treatment of PASH syndrome: a potential novel therapeutic target. Australas J Dermatol. 2020;61:E373-E374.
- Lamiaux M, Dabouz F, Wantz M, et al. Successful combined antibiotic therapy with oral clindamycin and oral rifampicin for pyoderma gangrenosum in patient with PASH syndrome. JAAD Case Rep. 2018;4:17-21.
- Gul MI, Singam V, Hanson C, et al. Remission of refractory PASH syndrome using ixekizumab and doxycycline. J Drugs Dermatol. 2020;19:1123.
- Bruzzese V. Pyoderma gangrenosum, acne conglobata, suppurative hidradenitis, and axial spondyloarthritis: efficacy of anti-tumor necrosis factor α therapy. J Clin Rheumatol. 2012;18:413-415.
Practice Points
- Despite phenotypic similarities among pyoderma gangrenosum (PG), acne, and hidradenitis suppurativa (PASH) syndrome; pyogenic arthritis, PG, and acne syndrome; and pyogenic arthritis–PASH syndrome, there are genotypic differences that contribute to unique inflammatory cytokine patterns and the need for distinct pharmacologic considerations within each entity.
- When formulating therapeutic regimens for patients with PASH syndrome, it is essential for dermatologists to consider the likelihood of hyperactivity in multiple pathways of the innate immune system and utilize a combination of multimodal antiinflammatory therapies.
55-year-old woman • unilateral nasal drainage • salty taste • nasal redness • recent COVID-19 nasal swabs • Dx?
THE CASE
A 55-year-old woman was evaluated in a family medicine clinic for clear, right-side nasal drainage. She stated that the drainage began 5 months earlier after 2 hospitalizations for severe anxiety leading to emesis and hypokalemia. She reported 3 different COVID-19 nasal swab tests performed on the right nare. Chart review showed 2 negative COVID-19 tests, 6 days apart. Since the hospitalizations, the patient had been given antihistamines for rhinorrhea at an urgent care visit. Despite this treatment, the patient reported a constant drip from the right nare with a salty taste. She also reported experiencing occasional headaches but denied nausea/vomiting.
The patient’s history included uncontrolled hypertension, treatment-resistant anxiety and depression, obstructive sleep apnea, chronic sinus disease (observed on computed tomography [CT] scans), and type 2 diabetes. She was on amlodipine 10 mg/d for hypertension and was not taking any medication for diabetes.
On examination, the patient’s vital signs were within normal limits except for an elevated blood pressure of 158/88 mm Hg. The patient had persistent clear rhinorrhea fluid draining from the right nostril that was exacerbated when she looked down. Right nasal erythema was present.
THE DIAGNOSIS
The patient’s negative COVID-19 tests, lack of improvement on antihistamines, and description of the nasal fluid as salty tasting prompted us to suspect a cerebrospinal fluid (CSF) leak. The clinical work-up included a halo (“double-ring”) sign test, a β-2 transferrin test, and a sinus x-ray.
The halo sign test was negative for CSF fluid. Sinus/skull x-ray did not show a cribriform or other fracture. However, a sample of the nasal fluid collected in a sterile container was positive for β-2 transferrin, the gold-standard laboratory test to confirm a CSF leak.
The patient was sent for a maxillofacial CT scan without contrast. Results showed a 3-mm defect over the right ethmoid roof associated with a 10 × 16–mm low-attenuation structure in the right ethmoid labyrinth, suspicious for encephalocele. This defect, in the setting of the patient’s history of chronic sinus disease, furthered our suspicion of a CSF leak secondary to COVID-19 testing. Radiology confirmed the diagnosis.
DISCUSSION
CSF rhinorrhea is CSF leakage through the nasal cavity due to abnormal communication between the arachnoid membrane and nasal mucosa.1 The most commonly reported risk factors for this include female sex, middle age (fourth to fifth decade), obesity (body mass index > 40), intracranial hypertension, and obstructive sleep apnea.1,2
Continue to: Clear, unilateral rhinorrhea...
Clear, unilateral rhinorrhea drainage that increases at times of relatively increased intracranial pressure and has a metallic or salty taste is suspicious for CSF rhinorrhea.3 It can occur following skull‐base trauma (eg, cribriform plate, temporal bone), endoscopic sinus surgery, or neurosurgical procedures, or have a spontaneous etiology.3,4
Modalities to confirm CSF rhinorrhea include radionuclide cisternography and testing of fluid for the halo sign, glucose, and the CSF-specific proteins β‐2 transferrin and β-trace protein.3,4 High‐resolution CT is the imaging method most commonly used for localizing a CSF leak.4
Treatment is provided in the hospital
Patients with CSF rhinorrhea typically require inpatient management with bed rest, head-of-bed elevation, and frequent neurologic evaluation, as persistent CSF rhinorrhea increases the risk for meningitis, thus necessitating surgical intervention.3,5 Some cases resolve with bed rest alone. Endonasal endoscopic repair of CSF leaks has become the standard of care because of its high success rate and lower morbidity profile.4
The preferred treatment method for encephalocele is surgical removal after diagnosis is confirmed with CT or magnetic resonance imaging.6
Our patient underwent surgery to remove the encephalocele. The surgeons reported no evidence of fracture.
The final cause of her CSF leak is still uncertain. The surgeons felt confident it was due to ethmoidal encephalocele, a form of neural tube defect in which brain tissue herniates through structural weaknesses of the skull.6-8 While more common in infants, encephalocele can manifest in adulthood due to traumatic or iatrogenic causes.7,8
There is a previous report of encephalocele with CSF leak after COVID-19 testing.9 This case report suggests the possibility of a nasal swab causing trauma to a patient’s pre‐existing encephalocele—a probability in our patient’s case. It is unlikely, however, that the nasal swab itself violated the bony skull base.
THE TAKEAWAY
This case exemplifies how unexplained local symptoms, a high index of suspicion, and adequate work-up can lead to a rare diagnosis. Diagnostic strategies employed for cases of CSF rhinorrhea vary widely due to limited evidence-based guidance.4 Unilateral rhinorrhea with clear fluid that increases at times of increased intracranial pressure, such as bending over, should prompt suspicion for CSF rhinorrhea. With millions of people getting nasal swabs daily during the COVID-19 pandemic, it is even more important to keep CSF leak in our differential diagnosis.
CORRESPONDENCE
Eliana Lizeth Garcia, MD, BS, BA, University of New Mexico Health Sciences Center, 1209 University Boulevard NE, Albuquerque, NM 87131-5001; [email protected]
1. Keshri A, Jain R, Manogaran RS, et al. Management of spontaneous CSF rhinorrhea: an institutional experience. J Neurol Surg B Skull Base. 2019;80:493-499. doi: 10.1055/s-0038-1676334
2. Lobo BC, Baumanis MM, Nelson RF. Surgical repair of spontaneous cerebrospinal fluid (CSF) leaks: a systematic review. Laryngoscope Investig Otolaryngol. 2017;2:215-224. doi: 10.1002/lio2.75
3. Van Zele T, Dewaele F. Traumatic CSF leaks of the anterior skull base. B-ENT. 2016;suppl 26:19-27.
4. Oakley GM, Alt JA, Schlosser RJ, et al. Diagnosis of cerebrospinal fluid rhinorrhea: an evidence-based review with recommendations. Int Forum Allergy Rhinol. 2016;6:8-16. doi: 10.1002/alr.21637
5. Friedman JA, Ebersold MJ, Quast LM. Post-traumatic cerebrospinal fluid leakage. World J Surg. 2001;25:1062-1066. doi: 10.1007/s00268-001-0059-7
6. Tirumandas M, Sharma A, Gbenimacho I, et al. Nasal encephaloceles: a review of etiology, pathophysiology, clinical presentations, diagnosis, treatment, and complications. Childs Nerv Syst. 2013;29:739-744. doi: 10.1007/s00381-012-1998-z
7. Junaid M, Sobani ZU, Shamim AA, et al. Nasal encephaloceles presenting at later ages: experience of otorhinolaryngology department at a tertiary care center in Karachi, Pakistan. J Pak Med Assoc. 2012;62:74-76.
8. Dhirawani RB, Gupta R, Pathak S, et al. Frontoethmoidal encephalocele: case report and review on management. Ann Maxillofac Surg. 2014;4:195-197. doi: 10.4103/2231-0746.147140
9. Paquin R, Ryan L, Vale FL, et al. CSF leak after COVID-19 nasopharyngeal swab: a case report. Laryngoscope. 2021;131:1927-1929. doi: 10.1002/lary.29462
THE CASE
A 55-year-old woman was evaluated in a family medicine clinic for clear, right-side nasal drainage. She stated that the drainage began 5 months earlier after 2 hospitalizations for severe anxiety leading to emesis and hypokalemia. She reported 3 different COVID-19 nasal swab tests performed on the right nare. Chart review showed 2 negative COVID-19 tests, 6 days apart. Since the hospitalizations, the patient had been given antihistamines for rhinorrhea at an urgent care visit. Despite this treatment, the patient reported a constant drip from the right nare with a salty taste. She also reported experiencing occasional headaches but denied nausea/vomiting.
The patient’s history included uncontrolled hypertension, treatment-resistant anxiety and depression, obstructive sleep apnea, chronic sinus disease (observed on computed tomography [CT] scans), and type 2 diabetes. She was on amlodipine 10 mg/d for hypertension and was not taking any medication for diabetes.
On examination, the patient’s vital signs were within normal limits except for an elevated blood pressure of 158/88 mm Hg. The patient had persistent clear rhinorrhea fluid draining from the right nostril that was exacerbated when she looked down. Right nasal erythema was present.
THE DIAGNOSIS
The patient’s negative COVID-19 tests, lack of improvement on antihistamines, and description of the nasal fluid as salty tasting prompted us to suspect a cerebrospinal fluid (CSF) leak. The clinical work-up included a halo (“double-ring”) sign test, a β-2 transferrin test, and a sinus x-ray.
The halo sign test was negative for CSF fluid. Sinus/skull x-ray did not show a cribriform or other fracture. However, a sample of the nasal fluid collected in a sterile container was positive for β-2 transferrin, the gold-standard laboratory test to confirm a CSF leak.
The patient was sent for a maxillofacial CT scan without contrast. Results showed a 3-mm defect over the right ethmoid roof associated with a 10 × 16–mm low-attenuation structure in the right ethmoid labyrinth, suspicious for encephalocele. This defect, in the setting of the patient’s history of chronic sinus disease, furthered our suspicion of a CSF leak secondary to COVID-19 testing. Radiology confirmed the diagnosis.
DISCUSSION
CSF rhinorrhea is CSF leakage through the nasal cavity due to abnormal communication between the arachnoid membrane and nasal mucosa.1 The most commonly reported risk factors for this include female sex, middle age (fourth to fifth decade), obesity (body mass index > 40), intracranial hypertension, and obstructive sleep apnea.1,2
Continue to: Clear, unilateral rhinorrhea...
Clear, unilateral rhinorrhea drainage that increases at times of relatively increased intracranial pressure and has a metallic or salty taste is suspicious for CSF rhinorrhea.3 It can occur following skull‐base trauma (eg, cribriform plate, temporal bone), endoscopic sinus surgery, or neurosurgical procedures, or have a spontaneous etiology.3,4
Modalities to confirm CSF rhinorrhea include radionuclide cisternography and testing of fluid for the halo sign, glucose, and the CSF-specific proteins β‐2 transferrin and β-trace protein.3,4 High‐resolution CT is the imaging method most commonly used for localizing a CSF leak.4
Treatment is provided in the hospital
Patients with CSF rhinorrhea typically require inpatient management with bed rest, head-of-bed elevation, and frequent neurologic evaluation, as persistent CSF rhinorrhea increases the risk for meningitis, thus necessitating surgical intervention.3,5 Some cases resolve with bed rest alone. Endonasal endoscopic repair of CSF leaks has become the standard of care because of its high success rate and lower morbidity profile.4
The preferred treatment method for encephalocele is surgical removal after diagnosis is confirmed with CT or magnetic resonance imaging.6
Our patient underwent surgery to remove the encephalocele. The surgeons reported no evidence of fracture.
The final cause of her CSF leak is still uncertain. The surgeons felt confident it was due to ethmoidal encephalocele, a form of neural tube defect in which brain tissue herniates through structural weaknesses of the skull.6-8 While more common in infants, encephalocele can manifest in adulthood due to traumatic or iatrogenic causes.7,8
There is a previous report of encephalocele with CSF leak after COVID-19 testing.9 This case report suggests the possibility of a nasal swab causing trauma to a patient’s pre‐existing encephalocele—a probability in our patient’s case. It is unlikely, however, that the nasal swab itself violated the bony skull base.
THE TAKEAWAY
This case exemplifies how unexplained local symptoms, a high index of suspicion, and adequate work-up can lead to a rare diagnosis. Diagnostic strategies employed for cases of CSF rhinorrhea vary widely due to limited evidence-based guidance.4 Unilateral rhinorrhea with clear fluid that increases at times of increased intracranial pressure, such as bending over, should prompt suspicion for CSF rhinorrhea. With millions of people getting nasal swabs daily during the COVID-19 pandemic, it is even more important to keep CSF leak in our differential diagnosis.
CORRESPONDENCE
Eliana Lizeth Garcia, MD, BS, BA, University of New Mexico Health Sciences Center, 1209 University Boulevard NE, Albuquerque, NM 87131-5001; [email protected]
THE CASE
A 55-year-old woman was evaluated in a family medicine clinic for clear, right-side nasal drainage. She stated that the drainage began 5 months earlier after 2 hospitalizations for severe anxiety leading to emesis and hypokalemia. She reported 3 different COVID-19 nasal swab tests performed on the right nare. Chart review showed 2 negative COVID-19 tests, 6 days apart. Since the hospitalizations, the patient had been given antihistamines for rhinorrhea at an urgent care visit. Despite this treatment, the patient reported a constant drip from the right nare with a salty taste. She also reported experiencing occasional headaches but denied nausea/vomiting.
The patient’s history included uncontrolled hypertension, treatment-resistant anxiety and depression, obstructive sleep apnea, chronic sinus disease (observed on computed tomography [CT] scans), and type 2 diabetes. She was on amlodipine 10 mg/d for hypertension and was not taking any medication for diabetes.
On examination, the patient’s vital signs were within normal limits except for an elevated blood pressure of 158/88 mm Hg. The patient had persistent clear rhinorrhea fluid draining from the right nostril that was exacerbated when she looked down. Right nasal erythema was present.
THE DIAGNOSIS
The patient’s negative COVID-19 tests, lack of improvement on antihistamines, and description of the nasal fluid as salty tasting prompted us to suspect a cerebrospinal fluid (CSF) leak. The clinical work-up included a halo (“double-ring”) sign test, a β-2 transferrin test, and a sinus x-ray.
The halo sign test was negative for CSF fluid. Sinus/skull x-ray did not show a cribriform or other fracture. However, a sample of the nasal fluid collected in a sterile container was positive for β-2 transferrin, the gold-standard laboratory test to confirm a CSF leak.
The patient was sent for a maxillofacial CT scan without contrast. Results showed a 3-mm defect over the right ethmoid roof associated with a 10 × 16–mm low-attenuation structure in the right ethmoid labyrinth, suspicious for encephalocele. This defect, in the setting of the patient’s history of chronic sinus disease, furthered our suspicion of a CSF leak secondary to COVID-19 testing. Radiology confirmed the diagnosis.
DISCUSSION
CSF rhinorrhea is CSF leakage through the nasal cavity due to abnormal communication between the arachnoid membrane and nasal mucosa.1 The most commonly reported risk factors for this include female sex, middle age (fourth to fifth decade), obesity (body mass index > 40), intracranial hypertension, and obstructive sleep apnea.1,2
Continue to: Clear, unilateral rhinorrhea...
Clear, unilateral rhinorrhea drainage that increases at times of relatively increased intracranial pressure and has a metallic or salty taste is suspicious for CSF rhinorrhea.3 It can occur following skull‐base trauma (eg, cribriform plate, temporal bone), endoscopic sinus surgery, or neurosurgical procedures, or have a spontaneous etiology.3,4
Modalities to confirm CSF rhinorrhea include radionuclide cisternography and testing of fluid for the halo sign, glucose, and the CSF-specific proteins β‐2 transferrin and β-trace protein.3,4 High‐resolution CT is the imaging method most commonly used for localizing a CSF leak.4
Treatment is provided in the hospital
Patients with CSF rhinorrhea typically require inpatient management with bed rest, head-of-bed elevation, and frequent neurologic evaluation, as persistent CSF rhinorrhea increases the risk for meningitis, thus necessitating surgical intervention.3,5 Some cases resolve with bed rest alone. Endonasal endoscopic repair of CSF leaks has become the standard of care because of its high success rate and lower morbidity profile.4
The preferred treatment method for encephalocele is surgical removal after diagnosis is confirmed with CT or magnetic resonance imaging.6
Our patient underwent surgery to remove the encephalocele. The surgeons reported no evidence of fracture.
The final cause of her CSF leak is still uncertain. The surgeons felt confident it was due to ethmoidal encephalocele, a form of neural tube defect in which brain tissue herniates through structural weaknesses of the skull.6-8 While more common in infants, encephalocele can manifest in adulthood due to traumatic or iatrogenic causes.7,8
There is a previous report of encephalocele with CSF leak after COVID-19 testing.9 This case report suggests the possibility of a nasal swab causing trauma to a patient’s pre‐existing encephalocele—a probability in our patient’s case. It is unlikely, however, that the nasal swab itself violated the bony skull base.
THE TAKEAWAY
This case exemplifies how unexplained local symptoms, a high index of suspicion, and adequate work-up can lead to a rare diagnosis. Diagnostic strategies employed for cases of CSF rhinorrhea vary widely due to limited evidence-based guidance.4 Unilateral rhinorrhea with clear fluid that increases at times of increased intracranial pressure, such as bending over, should prompt suspicion for CSF rhinorrhea. With millions of people getting nasal swabs daily during the COVID-19 pandemic, it is even more important to keep CSF leak in our differential diagnosis.
CORRESPONDENCE
Eliana Lizeth Garcia, MD, BS, BA, University of New Mexico Health Sciences Center, 1209 University Boulevard NE, Albuquerque, NM 87131-5001; [email protected]
1. Keshri A, Jain R, Manogaran RS, et al. Management of spontaneous CSF rhinorrhea: an institutional experience. J Neurol Surg B Skull Base. 2019;80:493-499. doi: 10.1055/s-0038-1676334
2. Lobo BC, Baumanis MM, Nelson RF. Surgical repair of spontaneous cerebrospinal fluid (CSF) leaks: a systematic review. Laryngoscope Investig Otolaryngol. 2017;2:215-224. doi: 10.1002/lio2.75
3. Van Zele T, Dewaele F. Traumatic CSF leaks of the anterior skull base. B-ENT. 2016;suppl 26:19-27.
4. Oakley GM, Alt JA, Schlosser RJ, et al. Diagnosis of cerebrospinal fluid rhinorrhea: an evidence-based review with recommendations. Int Forum Allergy Rhinol. 2016;6:8-16. doi: 10.1002/alr.21637
5. Friedman JA, Ebersold MJ, Quast LM. Post-traumatic cerebrospinal fluid leakage. World J Surg. 2001;25:1062-1066. doi: 10.1007/s00268-001-0059-7
6. Tirumandas M, Sharma A, Gbenimacho I, et al. Nasal encephaloceles: a review of etiology, pathophysiology, clinical presentations, diagnosis, treatment, and complications. Childs Nerv Syst. 2013;29:739-744. doi: 10.1007/s00381-012-1998-z
7. Junaid M, Sobani ZU, Shamim AA, et al. Nasal encephaloceles presenting at later ages: experience of otorhinolaryngology department at a tertiary care center in Karachi, Pakistan. J Pak Med Assoc. 2012;62:74-76.
8. Dhirawani RB, Gupta R, Pathak S, et al. Frontoethmoidal encephalocele: case report and review on management. Ann Maxillofac Surg. 2014;4:195-197. doi: 10.4103/2231-0746.147140
9. Paquin R, Ryan L, Vale FL, et al. CSF leak after COVID-19 nasopharyngeal swab: a case report. Laryngoscope. 2021;131:1927-1929. doi: 10.1002/lary.29462
1. Keshri A, Jain R, Manogaran RS, et al. Management of spontaneous CSF rhinorrhea: an institutional experience. J Neurol Surg B Skull Base. 2019;80:493-499. doi: 10.1055/s-0038-1676334
2. Lobo BC, Baumanis MM, Nelson RF. Surgical repair of spontaneous cerebrospinal fluid (CSF) leaks: a systematic review. Laryngoscope Investig Otolaryngol. 2017;2:215-224. doi: 10.1002/lio2.75
3. Van Zele T, Dewaele F. Traumatic CSF leaks of the anterior skull base. B-ENT. 2016;suppl 26:19-27.
4. Oakley GM, Alt JA, Schlosser RJ, et al. Diagnosis of cerebrospinal fluid rhinorrhea: an evidence-based review with recommendations. Int Forum Allergy Rhinol. 2016;6:8-16. doi: 10.1002/alr.21637
5. Friedman JA, Ebersold MJ, Quast LM. Post-traumatic cerebrospinal fluid leakage. World J Surg. 2001;25:1062-1066. doi: 10.1007/s00268-001-0059-7
6. Tirumandas M, Sharma A, Gbenimacho I, et al. Nasal encephaloceles: a review of etiology, pathophysiology, clinical presentations, diagnosis, treatment, and complications. Childs Nerv Syst. 2013;29:739-744. doi: 10.1007/s00381-012-1998-z
7. Junaid M, Sobani ZU, Shamim AA, et al. Nasal encephaloceles presenting at later ages: experience of otorhinolaryngology department at a tertiary care center in Karachi, Pakistan. J Pak Med Assoc. 2012;62:74-76.
8. Dhirawani RB, Gupta R, Pathak S, et al. Frontoethmoidal encephalocele: case report and review on management. Ann Maxillofac Surg. 2014;4:195-197. doi: 10.4103/2231-0746.147140
9. Paquin R, Ryan L, Vale FL, et al. CSF leak after COVID-19 nasopharyngeal swab: a case report. Laryngoscope. 2021;131:1927-1929. doi: 10.1002/lary.29462
► Unilateral nasal drainage
► Salty taste
► Nasal redness
► Recent COVID-19 nasal swabs
64-year-old woman • hot flashes, facial flushing, excessive sweating, and palpitations • daily headaches • history of hypertension • Dx?
THE CASE
A 64-year-old woman sought care after having hot flashes, facial flushing, excessive sweating, palpitations, and daily headaches for 1 month. She had a history of hypertension that was well controlled with hydrochlorothiazide 25 mg/d but over the previous month, it had become more difficult to control. Her blood pressure remained elevated to 150/100 mm Hg despite the addition of lisinopril 40 mg/d and amlodipine 10 mg/d, indicating resistant hypertension. She had no family history of hypertension, diabetes, or obesity or any other pertinent medical or surgical history. Physical examination was negative for weight gain, stretch marks, or muscle weakness.
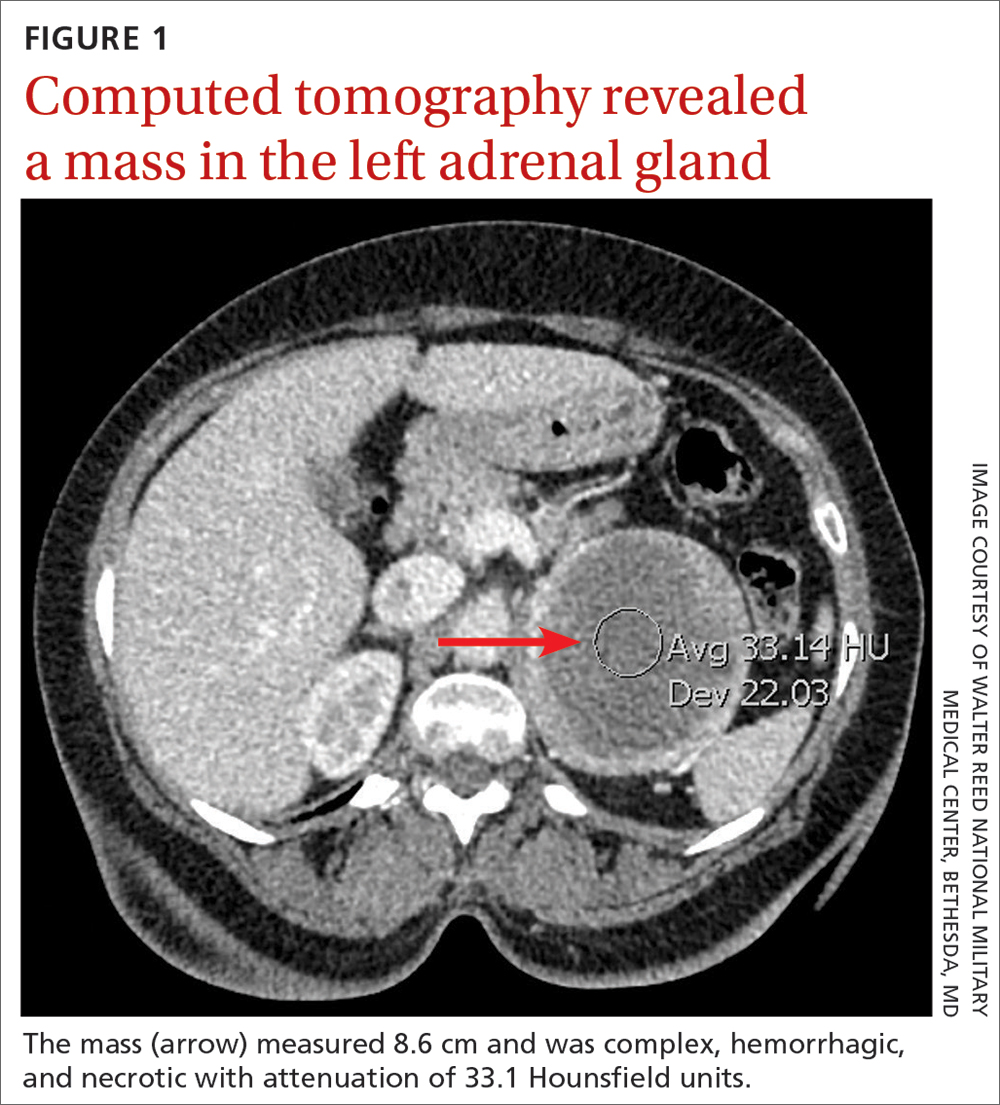
Laboratory tests revealed a normal serum aldosterone-renin ratio, renal function, and thyroid function; however, she had elevated levels of normetanephrine (2429 pg/mL; normal range, 0-145 pg/mL) and metanephrine (143 pg/mL; normal range, 0-62 pg/mL). Computed tomography (CT) revealed an 8.6-cm complex, hemorrhagic, necrotic left adrenal mass with attenuation of 33.1 Hounsfield units (HU) (FIGURE 1). Magnetic resonance imaging (MRI) demonstrated a T2 hyperintense left adrenal mass. An evaluation for Cushing syndrome was negative, and positron emission tomography (PET)/CT with gallium-68 dotatate was ordered. It showed intense radiotracer uptake in the left adrenal gland, with a maximum standardized uptake value of 70.1 (FIGURE 2).
THE DIAGNOSIS
After appropriate preparation with alpha blockade (phenoxybenzamine 20 mg twice daily for 7 days) and fluid resuscitation (normal saline run over 12 hours preoperatively), the patient underwent successful open surgical resection of the adrenal mass, during which her blood pressure was controlled with a nitroprusside infusion and boluses of esmolol and labetalol. Pathology results showed cells in a nested pattern with round to oval nuclei in a vascular background. There was no necrosis, increased mitotic figures, capsular invasion, or increased cellularity. Chromogranin immunohistochemical staining was positive. Given her resistant hypertension, clinical symptoms, and pathology results, the patient was given a diagnosis of pheochromocytoma.
DISCUSSION
Resistant hypertension is defined as blood pressure that is elevated above goal despite the use of 3 maximally titrated antihypertensive agents from different classes or that is well controlled with at least 4 antihypertensive medications.1 The prevalence of resistant hypertension is 12% to 18% in adults being treated for hypertension.1 Patients with resistant hypertension have a higher risk for cardiovascular events and death, are more likely to have a secondary cause of hypertension, and may benefit from special diagnostic testing or treatment approaches to control their blood pressure.1
There are many causes of resistant hypertension; primary aldosteronism is the most common cause (prevalence as high as 20%).2 Given the increased risk for cardiovascular/cerebrovascular disease, all patients with resistant hypertension should be screened for this condition.2 Other causes of resistant hypertension include renal parenchymal disease, renal artery stenosis, coarctation of the aorta, thyroid dysfunction, Cushing syndrome, paraganglioma, and as seen in our case, pheochromocytoma. Although pheochromocytoma is a rare cause of resistant hypertension (0.01%-4%),1 it is associated with high rates of morbidity and mortality if left untreated and may be inherited, making it an essential diagnosis to consider in all patients with resistant hypertension.1,3
Common symptoms of pheochromocytoma are hypertension (paroxysmal or sustained), headaches, palpitations, pallor, and piloerection (or cold sweats).1 Patients with pheochromocytoma typically exhibit metanephrine levels that are more than 4 times the upper limit of normal.4 Therefore, measurement of plasma free metanephrines or urinary fractionated metanephrines is recommended.5 Elevated metanephrine levels also are caused by obesity, obstructive sleep apnea, and certain medications and should be ruled out.5
All pheochromocytomas are potentially malignant. Despite the existence of pathologic scoring systems6,7 and radiographic features that suggest malignancy,8,9 no single risk-stratification tool is recommended in the current literature.10 Ultimately, the only way to confirm malignancy is to see metastases where chromaffin tissue is not normally found on imaging.10
Continue to: Pathologic features to look for...
Pathologic features to look for include capsular/periadrenal adipose invasion, increased cellularity, necrosis, tumor cell spindling, increased/atypical mitotic figures, and nuclear pleomorphism. Radiographic features include larger size (≥ 4-6 cm),11 an irregular shape, necrosis, calcifications, attenuation of 10 HU or higher on noncontrast CT, absolute washout of 60% or lower, and relative washout of 40% or lower.8,12 On MRI, malignant lesions appear hypointense on T1-weighted imaging and hyperintense on T2-weighted imaging.9 Fluorodeoxyglucose avidity on PET scan also is indicative of malignancy.8,9
Treatment for pheochromocytoma is surgical resection. An experienced surgical team and proper preoperative preparation are necessary because the induction of anesthesia, endotracheal intubation, and tumor manipulation can lead to a release of catecholamines, potentially resulting in an intraoperative hypertensive crisis, cardiac arrhythmias, and multiorgan failure.
Proper preoperative preparation includes taking an alpha-adrenergic blocker, such as phenoxybenzamine, prazosin, terazosin, or doxazosin, for at least 7 days to normalize the patient’s blood pressure. Patients should be counseled that they may experience nasal congestion, orthostasis, and fatigue while taking these medications. Volume expansion with intravenous fluids also should be performed and a high-salt diet considered. Beta-adrenergic blockade can be initiated once appropriate alpha-adrenergic blockade is achieved to control the patient’s heart rate; beta-blockers should never be started first because of the risk for severe hypertension. Careful hemodynamic monitoring is vital intraoperatively and postoperatively.5,13 Because metastatic lesions can occur decades after resection, long-term follow-up is critical.5,10
Following tumor resection, our patient’s blood pressure was supported with intravenous fluids and phenylephrine. She was able to discontinue all her antihypertensive medications postoperatively, and her plasma free and urinary fractionated metanephrine levels returned to within normal limits 8 weeks after surgery. Five years after surgery, she continues to have no signs of recurrence, as evidenced by annual negative plasma free metanephrines testing and abdominal/pelvic CT.
THE TAKEAWAY
This case highlights the importance of recognizing resistant hypertension and a potential secondary cause of this disease—pheochromocytoma. Although rare, pheochromocytomas confer increased risk for cardiovascular disease and death. Thus, swift recognition and proper preparation for surgical resection are necessary. Malignant lesions can be diagnosed only upon discovery of metastatic disease and can recur for decades after surgical resection, making diligent long-term follow-up imperative.
CORRESPONDENCE
Nicole O. Vietor, MD, Division of Endocrinology, Walter Reed National Military Medical Center, 8901 Wisconsin Avenue, Bethesda, MD 20889; [email protected]
1. Carey RM, Calhoun DA, Bakris GL, et al. Resistant hypertension: detection, evaluation, and management: a scientific statement from the American Heart Association. Hypertension. 2018;72:e53-e90. doi: 10.1161/HYP.0000000000000084
2. Young WF Jr. Diagnosis and treatment of primary aldosteronism: practical clinical perspectives. J Intern Med. 2019;285:126-148. doi: 10.1111/joim.12831
3. Young WF Jr, Calhoun DA, Lenders JWM, et al. Screening for endocrine hypertension: an Endocrine Society Scientific Statement. Endocr Rev. 2017;38:103-122. doi: 10.1210/er.2017-00054
4. Lenders JWM, Pacak K, Walther MM, et al. Biochemical diagnosis of pheochromocytoma: which test is best? JAMA. 2002;287:1427-1434. doi: 10.1001/jama.287.11.1427
5. Lenders JW, Duh Q-Y, Eisenhofer G, et al. Pheochromocytoma and paraganglioma: an Endocrine Society Clinical Practice Guideline. J Clin Endocrinol Metab. 2014;99:1915-1942. doi: 10.1210/jc.2014-1498
6. Kimura N, Takayanagi R, Takizawa N, et al. Pathological grading for predicting metastasis in phaeochromocytoma and paraganglioma. Endocr Relat Cancer. 2014;21:405-414. doi: 10.1530/ERC-13-0494
7. Thompson LDR. Pheochromocytoma of the Adrenal gland Scaled Score (PASS) to separate benign from malignant neoplasms: a clinicopathologic and immunophenotypic study of 100 cases. Am J Surg Pathol. 2002;26:551-566. doi: 10.1097/00000478-200205000-00002
8. Vaidya A, Hamrahian A, Bancos I, et al. The evaluation of incidentally discovered adrenal masses. Endocr Pract. 2019;25:178-192. doi: 10.4158/DSCR-2018-0565
9. Young WF Jr. Conventional imaging in adrenocortical carcinoma: update and perspectives. Horm Cancer. 2011;2:341-347. doi: 10.1007/s12672-011-0089-z
10. Neumann HPH, Young WF Jr, Eng C. Pheochromocytoma and paraganglioma. N Engl J Med. 2019;381:552-565. doi: 10.1056/NEJMra1806651
11. Iñiguez-Ariza NM, Kohlenberg JD, Delivanis DA, et al. Clinical, biochemical, and radiological characteristics of a single-center retrospective cohort of 705 large adrenal tumors. Mayo Clin Proc Innov Qual Outcomes. 2017;2:30-39. doi: 10.1016/j.mayocpiqo.2017.11.002
12. Marty M, Gaye D, Perez P, et al. Diagnostic accuracy of computed tomography to identify adenomas among adrenal incidentalomas in an endocrinological population. Eur J Endocrinol. 2018;178:439-446. doi: 10.1530/EJE-17-1056
13. Pacak K. Preoperative management of the pheochromocytoma patient. J Clin Endocrinol Metab. 2007;92:4069-4079. doi: 10.1210/jc.2007-1720
THE CASE
A 64-year-old woman sought care after having hot flashes, facial flushing, excessive sweating, palpitations, and daily headaches for 1 month. She had a history of hypertension that was well controlled with hydrochlorothiazide 25 mg/d but over the previous month, it had become more difficult to control. Her blood pressure remained elevated to 150/100 mm Hg despite the addition of lisinopril 40 mg/d and amlodipine 10 mg/d, indicating resistant hypertension. She had no family history of hypertension, diabetes, or obesity or any other pertinent medical or surgical history. Physical examination was negative for weight gain, stretch marks, or muscle weakness.

Laboratory tests revealed a normal serum aldosterone-renin ratio, renal function, and thyroid function; however, she had elevated levels of normetanephrine (2429 pg/mL; normal range, 0-145 pg/mL) and metanephrine (143 pg/mL; normal range, 0-62 pg/mL). Computed tomography (CT) revealed an 8.6-cm complex, hemorrhagic, necrotic left adrenal mass with attenuation of 33.1 Hounsfield units (HU) (FIGURE 1). Magnetic resonance imaging (MRI) demonstrated a T2 hyperintense left adrenal mass. An evaluation for Cushing syndrome was negative, and positron emission tomography (PET)/CT with gallium-68 dotatate was ordered. It showed intense radiotracer uptake in the left adrenal gland, with a maximum standardized uptake value of 70.1 (FIGURE 2).

THE DIAGNOSIS
After appropriate preparation with alpha blockade (phenoxybenzamine 20 mg twice daily for 7 days) and fluid resuscitation (normal saline run over 12 hours preoperatively), the patient underwent successful open surgical resection of the adrenal mass, during which her blood pressure was controlled with a nitroprusside infusion and boluses of esmolol and labetalol. Pathology results showed cells in a nested pattern with round to oval nuclei in a vascular background. There was no necrosis, increased mitotic figures, capsular invasion, or increased cellularity. Chromogranin immunohistochemical staining was positive. Given her resistant hypertension, clinical symptoms, and pathology results, the patient was given a diagnosis of pheochromocytoma.
DISCUSSION
Resistant hypertension is defined as blood pressure that is elevated above goal despite the use of 3 maximally titrated antihypertensive agents from different classes or that is well controlled with at least 4 antihypertensive medications.1 The prevalence of resistant hypertension is 12% to 18% in adults being treated for hypertension.1 Patients with resistant hypertension have a higher risk for cardiovascular events and death, are more likely to have a secondary cause of hypertension, and may benefit from special diagnostic testing or treatment approaches to control their blood pressure.1
There are many causes of resistant hypertension; primary aldosteronism is the most common cause (prevalence as high as 20%).2 Given the increased risk for cardiovascular/cerebrovascular disease, all patients with resistant hypertension should be screened for this condition.2 Other causes of resistant hypertension include renal parenchymal disease, renal artery stenosis, coarctation of the aorta, thyroid dysfunction, Cushing syndrome, paraganglioma, and as seen in our case, pheochromocytoma. Although pheochromocytoma is a rare cause of resistant hypertension (0.01%-4%),1 it is associated with high rates of morbidity and mortality if left untreated and may be inherited, making it an essential diagnosis to consider in all patients with resistant hypertension.1,3
Common symptoms of pheochromocytoma are hypertension (paroxysmal or sustained), headaches, palpitations, pallor, and piloerection (or cold sweats).1 Patients with pheochromocytoma typically exhibit metanephrine levels that are more than 4 times the upper limit of normal.4 Therefore, measurement of plasma free metanephrines or urinary fractionated metanephrines is recommended.5 Elevated metanephrine levels also are caused by obesity, obstructive sleep apnea, and certain medications and should be ruled out.5
All pheochromocytomas are potentially malignant. Despite the existence of pathologic scoring systems6,7 and radiographic features that suggest malignancy,8,9 no single risk-stratification tool is recommended in the current literature.10 Ultimately, the only way to confirm malignancy is to see metastases where chromaffin tissue is not normally found on imaging.10
Continue to: Pathologic features to look for...
Pathologic features to look for include capsular/periadrenal adipose invasion, increased cellularity, necrosis, tumor cell spindling, increased/atypical mitotic figures, and nuclear pleomorphism. Radiographic features include larger size (≥ 4-6 cm),11 an irregular shape, necrosis, calcifications, attenuation of 10 HU or higher on noncontrast CT, absolute washout of 60% or lower, and relative washout of 40% or lower.8,12 On MRI, malignant lesions appear hypointense on T1-weighted imaging and hyperintense on T2-weighted imaging.9 Fluorodeoxyglucose avidity on PET scan also is indicative of malignancy.8,9
Treatment for pheochromocytoma is surgical resection. An experienced surgical team and proper preoperative preparation are necessary because the induction of anesthesia, endotracheal intubation, and tumor manipulation can lead to a release of catecholamines, potentially resulting in an intraoperative hypertensive crisis, cardiac arrhythmias, and multiorgan failure.
Proper preoperative preparation includes taking an alpha-adrenergic blocker, such as phenoxybenzamine, prazosin, terazosin, or doxazosin, for at least 7 days to normalize the patient’s blood pressure. Patients should be counseled that they may experience nasal congestion, orthostasis, and fatigue while taking these medications. Volume expansion with intravenous fluids also should be performed and a high-salt diet considered. Beta-adrenergic blockade can be initiated once appropriate alpha-adrenergic blockade is achieved to control the patient’s heart rate; beta-blockers should never be started first because of the risk for severe hypertension. Careful hemodynamic monitoring is vital intraoperatively and postoperatively.5,13 Because metastatic lesions can occur decades after resection, long-term follow-up is critical.5,10
Following tumor resection, our patient’s blood pressure was supported with intravenous fluids and phenylephrine. She was able to discontinue all her antihypertensive medications postoperatively, and her plasma free and urinary fractionated metanephrine levels returned to within normal limits 8 weeks after surgery. Five years after surgery, she continues to have no signs of recurrence, as evidenced by annual negative plasma free metanephrines testing and abdominal/pelvic CT.
THE TAKEAWAY
This case highlights the importance of recognizing resistant hypertension and a potential secondary cause of this disease—pheochromocytoma. Although rare, pheochromocytomas confer increased risk for cardiovascular disease and death. Thus, swift recognition and proper preparation for surgical resection are necessary. Malignant lesions can be diagnosed only upon discovery of metastatic disease and can recur for decades after surgical resection, making diligent long-term follow-up imperative.
CORRESPONDENCE
Nicole O. Vietor, MD, Division of Endocrinology, Walter Reed National Military Medical Center, 8901 Wisconsin Avenue, Bethesda, MD 20889; [email protected]
THE CASE
A 64-year-old woman sought care after having hot flashes, facial flushing, excessive sweating, palpitations, and daily headaches for 1 month. She had a history of hypertension that was well controlled with hydrochlorothiazide 25 mg/d but over the previous month, it had become more difficult to control. Her blood pressure remained elevated to 150/100 mm Hg despite the addition of lisinopril 40 mg/d and amlodipine 10 mg/d, indicating resistant hypertension. She had no family history of hypertension, diabetes, or obesity or any other pertinent medical or surgical history. Physical examination was negative for weight gain, stretch marks, or muscle weakness.

Laboratory tests revealed a normal serum aldosterone-renin ratio, renal function, and thyroid function; however, she had elevated levels of normetanephrine (2429 pg/mL; normal range, 0-145 pg/mL) and metanephrine (143 pg/mL; normal range, 0-62 pg/mL). Computed tomography (CT) revealed an 8.6-cm complex, hemorrhagic, necrotic left adrenal mass with attenuation of 33.1 Hounsfield units (HU) (FIGURE 1). Magnetic resonance imaging (MRI) demonstrated a T2 hyperintense left adrenal mass. An evaluation for Cushing syndrome was negative, and positron emission tomography (PET)/CT with gallium-68 dotatate was ordered. It showed intense radiotracer uptake in the left adrenal gland, with a maximum standardized uptake value of 70.1 (FIGURE 2).

THE DIAGNOSIS
After appropriate preparation with alpha blockade (phenoxybenzamine 20 mg twice daily for 7 days) and fluid resuscitation (normal saline run over 12 hours preoperatively), the patient underwent successful open surgical resection of the adrenal mass, during which her blood pressure was controlled with a nitroprusside infusion and boluses of esmolol and labetalol. Pathology results showed cells in a nested pattern with round to oval nuclei in a vascular background. There was no necrosis, increased mitotic figures, capsular invasion, or increased cellularity. Chromogranin immunohistochemical staining was positive. Given her resistant hypertension, clinical symptoms, and pathology results, the patient was given a diagnosis of pheochromocytoma.
DISCUSSION
Resistant hypertension is defined as blood pressure that is elevated above goal despite the use of 3 maximally titrated antihypertensive agents from different classes or that is well controlled with at least 4 antihypertensive medications.1 The prevalence of resistant hypertension is 12% to 18% in adults being treated for hypertension.1 Patients with resistant hypertension have a higher risk for cardiovascular events and death, are more likely to have a secondary cause of hypertension, and may benefit from special diagnostic testing or treatment approaches to control their blood pressure.1
There are many causes of resistant hypertension; primary aldosteronism is the most common cause (prevalence as high as 20%).2 Given the increased risk for cardiovascular/cerebrovascular disease, all patients with resistant hypertension should be screened for this condition.2 Other causes of resistant hypertension include renal parenchymal disease, renal artery stenosis, coarctation of the aorta, thyroid dysfunction, Cushing syndrome, paraganglioma, and as seen in our case, pheochromocytoma. Although pheochromocytoma is a rare cause of resistant hypertension (0.01%-4%),1 it is associated with high rates of morbidity and mortality if left untreated and may be inherited, making it an essential diagnosis to consider in all patients with resistant hypertension.1,3
Common symptoms of pheochromocytoma are hypertension (paroxysmal or sustained), headaches, palpitations, pallor, and piloerection (or cold sweats).1 Patients with pheochromocytoma typically exhibit metanephrine levels that are more than 4 times the upper limit of normal.4 Therefore, measurement of plasma free metanephrines or urinary fractionated metanephrines is recommended.5 Elevated metanephrine levels also are caused by obesity, obstructive sleep apnea, and certain medications and should be ruled out.5
All pheochromocytomas are potentially malignant. Despite the existence of pathologic scoring systems6,7 and radiographic features that suggest malignancy,8,9 no single risk-stratification tool is recommended in the current literature.10 Ultimately, the only way to confirm malignancy is to see metastases where chromaffin tissue is not normally found on imaging.10
Continue to: Pathologic features to look for...
Pathologic features to look for include capsular/periadrenal adipose invasion, increased cellularity, necrosis, tumor cell spindling, increased/atypical mitotic figures, and nuclear pleomorphism. Radiographic features include larger size (≥ 4-6 cm),11 an irregular shape, necrosis, calcifications, attenuation of 10 HU or higher on noncontrast CT, absolute washout of 60% or lower, and relative washout of 40% or lower.8,12 On MRI, malignant lesions appear hypointense on T1-weighted imaging and hyperintense on T2-weighted imaging.9 Fluorodeoxyglucose avidity on PET scan also is indicative of malignancy.8,9
Treatment for pheochromocytoma is surgical resection. An experienced surgical team and proper preoperative preparation are necessary because the induction of anesthesia, endotracheal intubation, and tumor manipulation can lead to a release of catecholamines, potentially resulting in an intraoperative hypertensive crisis, cardiac arrhythmias, and multiorgan failure.
Proper preoperative preparation includes taking an alpha-adrenergic blocker, such as phenoxybenzamine, prazosin, terazosin, or doxazosin, for at least 7 days to normalize the patient’s blood pressure. Patients should be counseled that they may experience nasal congestion, orthostasis, and fatigue while taking these medications. Volume expansion with intravenous fluids also should be performed and a high-salt diet considered. Beta-adrenergic blockade can be initiated once appropriate alpha-adrenergic blockade is achieved to control the patient’s heart rate; beta-blockers should never be started first because of the risk for severe hypertension. Careful hemodynamic monitoring is vital intraoperatively and postoperatively.5,13 Because metastatic lesions can occur decades after resection, long-term follow-up is critical.5,10
Following tumor resection, our patient’s blood pressure was supported with intravenous fluids and phenylephrine. She was able to discontinue all her antihypertensive medications postoperatively, and her plasma free and urinary fractionated metanephrine levels returned to within normal limits 8 weeks after surgery. Five years after surgery, she continues to have no signs of recurrence, as evidenced by annual negative plasma free metanephrines testing and abdominal/pelvic CT.
THE TAKEAWAY
This case highlights the importance of recognizing resistant hypertension and a potential secondary cause of this disease—pheochromocytoma. Although rare, pheochromocytomas confer increased risk for cardiovascular disease and death. Thus, swift recognition and proper preparation for surgical resection are necessary. Malignant lesions can be diagnosed only upon discovery of metastatic disease and can recur for decades after surgical resection, making diligent long-term follow-up imperative.
CORRESPONDENCE
Nicole O. Vietor, MD, Division of Endocrinology, Walter Reed National Military Medical Center, 8901 Wisconsin Avenue, Bethesda, MD 20889; [email protected]
1. Carey RM, Calhoun DA, Bakris GL, et al. Resistant hypertension: detection, evaluation, and management: a scientific statement from the American Heart Association. Hypertension. 2018;72:e53-e90. doi: 10.1161/HYP.0000000000000084
2. Young WF Jr. Diagnosis and treatment of primary aldosteronism: practical clinical perspectives. J Intern Med. 2019;285:126-148. doi: 10.1111/joim.12831
3. Young WF Jr, Calhoun DA, Lenders JWM, et al. Screening for endocrine hypertension: an Endocrine Society Scientific Statement. Endocr Rev. 2017;38:103-122. doi: 10.1210/er.2017-00054
4. Lenders JWM, Pacak K, Walther MM, et al. Biochemical diagnosis of pheochromocytoma: which test is best? JAMA. 2002;287:1427-1434. doi: 10.1001/jama.287.11.1427
5. Lenders JW, Duh Q-Y, Eisenhofer G, et al. Pheochromocytoma and paraganglioma: an Endocrine Society Clinical Practice Guideline. J Clin Endocrinol Metab. 2014;99:1915-1942. doi: 10.1210/jc.2014-1498
6. Kimura N, Takayanagi R, Takizawa N, et al. Pathological grading for predicting metastasis in phaeochromocytoma and paraganglioma. Endocr Relat Cancer. 2014;21:405-414. doi: 10.1530/ERC-13-0494
7. Thompson LDR. Pheochromocytoma of the Adrenal gland Scaled Score (PASS) to separate benign from malignant neoplasms: a clinicopathologic and immunophenotypic study of 100 cases. Am J Surg Pathol. 2002;26:551-566. doi: 10.1097/00000478-200205000-00002
8. Vaidya A, Hamrahian A, Bancos I, et al. The evaluation of incidentally discovered adrenal masses. Endocr Pract. 2019;25:178-192. doi: 10.4158/DSCR-2018-0565
9. Young WF Jr. Conventional imaging in adrenocortical carcinoma: update and perspectives. Horm Cancer. 2011;2:341-347. doi: 10.1007/s12672-011-0089-z
10. Neumann HPH, Young WF Jr, Eng C. Pheochromocytoma and paraganglioma. N Engl J Med. 2019;381:552-565. doi: 10.1056/NEJMra1806651
11. Iñiguez-Ariza NM, Kohlenberg JD, Delivanis DA, et al. Clinical, biochemical, and radiological characteristics of a single-center retrospective cohort of 705 large adrenal tumors. Mayo Clin Proc Innov Qual Outcomes. 2017;2:30-39. doi: 10.1016/j.mayocpiqo.2017.11.002
12. Marty M, Gaye D, Perez P, et al. Diagnostic accuracy of computed tomography to identify adenomas among adrenal incidentalomas in an endocrinological population. Eur J Endocrinol. 2018;178:439-446. doi: 10.1530/EJE-17-1056
13. Pacak K. Preoperative management of the pheochromocytoma patient. J Clin Endocrinol Metab. 2007;92:4069-4079. doi: 10.1210/jc.2007-1720
1. Carey RM, Calhoun DA, Bakris GL, et al. Resistant hypertension: detection, evaluation, and management: a scientific statement from the American Heart Association. Hypertension. 2018;72:e53-e90. doi: 10.1161/HYP.0000000000000084
2. Young WF Jr. Diagnosis and treatment of primary aldosteronism: practical clinical perspectives. J Intern Med. 2019;285:126-148. doi: 10.1111/joim.12831
3. Young WF Jr, Calhoun DA, Lenders JWM, et al. Screening for endocrine hypertension: an Endocrine Society Scientific Statement. Endocr Rev. 2017;38:103-122. doi: 10.1210/er.2017-00054
4. Lenders JWM, Pacak K, Walther MM, et al. Biochemical diagnosis of pheochromocytoma: which test is best? JAMA. 2002;287:1427-1434. doi: 10.1001/jama.287.11.1427
5. Lenders JW, Duh Q-Y, Eisenhofer G, et al. Pheochromocytoma and paraganglioma: an Endocrine Society Clinical Practice Guideline. J Clin Endocrinol Metab. 2014;99:1915-1942. doi: 10.1210/jc.2014-1498
6. Kimura N, Takayanagi R, Takizawa N, et al. Pathological grading for predicting metastasis in phaeochromocytoma and paraganglioma. Endocr Relat Cancer. 2014;21:405-414. doi: 10.1530/ERC-13-0494
7. Thompson LDR. Pheochromocytoma of the Adrenal gland Scaled Score (PASS) to separate benign from malignant neoplasms: a clinicopathologic and immunophenotypic study of 100 cases. Am J Surg Pathol. 2002;26:551-566. doi: 10.1097/00000478-200205000-00002
8. Vaidya A, Hamrahian A, Bancos I, et al. The evaluation of incidentally discovered adrenal masses. Endocr Pract. 2019;25:178-192. doi: 10.4158/DSCR-2018-0565
9. Young WF Jr. Conventional imaging in adrenocortical carcinoma: update and perspectives. Horm Cancer. 2011;2:341-347. doi: 10.1007/s12672-011-0089-z
10. Neumann HPH, Young WF Jr, Eng C. Pheochromocytoma and paraganglioma. N Engl J Med. 2019;381:552-565. doi: 10.1056/NEJMra1806651
11. Iñiguez-Ariza NM, Kohlenberg JD, Delivanis DA, et al. Clinical, biochemical, and radiological characteristics of a single-center retrospective cohort of 705 large adrenal tumors. Mayo Clin Proc Innov Qual Outcomes. 2017;2:30-39. doi: 10.1016/j.mayocpiqo.2017.11.002
12. Marty M, Gaye D, Perez P, et al. Diagnostic accuracy of computed tomography to identify adenomas among adrenal incidentalomas in an endocrinological population. Eur J Endocrinol. 2018;178:439-446. doi: 10.1530/EJE-17-1056
13. Pacak K. Preoperative management of the pheochromocytoma patient. J Clin Endocrinol Metab. 2007;92:4069-4079. doi: 10.1210/jc.2007-1720
► Hot flashes, facial flushing, excessive sweating, and palpitations
► Daily headaches
► History of hypertension