User login
ObGyn: A leader in academic medicine, with progress still to be made in diversity
The nation’s population is quickly diversifying, making racial/ethnic disparities in health care outcomes even more apparent. Minority and non-English-speaking populations have grown and may become a majority in the next generation.1 A proposed strategy to reduce disparities in health care is to recruit more practitioners who better reflect the patient populations.2 Improved access to care with racial concordance between physicians and patients has been reported.3
Being increasingly aware of access-to-care data, more patients are advocating and asking for physicians of color to be their providers.4 Despite progress (ie, more women entering the medical profession), the proportion of physicians who are underrepresented in medicine (URiM—eg, Black, Hispanic, and Native American) still lags US population demographics.3
Why diversity in medicine matters
In addition to improving access to care, diversity in medicine offers other benefits. Working within diverse learning environments has demonstrated educational advantages.5,6 Medical students and residents from diverse backgrounds are less likely to report depression symptoms, regardless of their race. Diversity may accelerate advancements in health care as well, since it is well-established that diverse teams outperform nondiverse teams when it comes to innovation and productivity.7 Finally, as a profession committed to equity, advocacy, and justice, physicians are positioned to lead the way toward racial equity.
Overall, racial and gender diversity in all clinical specialties is improving, but not at the same pace. While the diversity of US medical students and residents by sex and race/ethnicity is greater than among faculty, change in racial diversity has been slow for all 3 groups.8 During the past 40 years the number of full-time faculty has increased 6-fold for females and more than tripled for males.8 However, this rise has not favored URiM faculty, because their proportion is still underrepresented relative to their group in the general population. Clinical departments that are making the most progress in recruiting URiM residents and faculty are often primary or preventive care specialties rather than surgical or service or hospital-based specialties.8,9 ObGyn has consistently had a proportion of URiM residents (18%) that is highest in the surgical specialties and comparable to family medicine and pediatrics.10
When examining physician workforce diversity, it is important to “drill down” to individual specialties to obtain a clearer understanding of trends. The continued need for increased resident and faculty diversity prompted us to examine ObGyn departments. The most recent nationwide data were gathered about full-time faculty from the 2021 AAMC Faculty Roster, residents from the 2021 Accreditation Counsel for Graduate Medical Education (ACGME) Data Resource Book, medical student matriculants from 2021 AAMC, and US adult women (defined arbitrarily as 15 years or older) from the 2019 American Community Survey.11-13
Increase in female faculty and residents
The expanding numbers of faculty and residents over a 40-year period (from 1973 to 2012) led to more women and underrepresented minorities in ObGyn than in other major clinical departments.14,15 Women now constitute two-thirds of all ObGyn faculty and are more likely to be junior rather than senior faculty.9 When looking at junior faculty, a higher proportion of junior faculty who are URiM are female. While more junior faculty and residents are female, male faculty are also racially and ethnically diverse.9
- ObGyn is a leader in racial/ethnic diversity in academic medicine.
- The rapid rise of faculty numbers in the past has not favored underrepresented faculty.
- The rise in ObGyn faculty and residents, who were predominantly female, has contributed to greater racial/ethnic diversity.
- Improved patient outcomes with racial concordance between physicians and patients have been reported.
- More patients are advocating and asking for physicians of color to be their clinicians.
- Racial/ethnic diversity of junior faculty and residents is similar to medical students.
- The most underrepresented group is Hispanic, due in part to its rapid growth in the US population.
Continue to: Growth of URiM physicians in ObGyn...
Growth of URiM physicians in ObGyn
The distribution of racial/ethnic groups in 2021 were compared between senior and junior ObGyn faculty and residents with the US adult female population.9 As shown in the FIGURE, the proportion of ObGyn faculty who are White approximates the White US adult female population. The most rapidly growing racial/ethnic group in the US population is Hispanic. Although Hispanic is the best represented ethnicity among junior faculty, the proportions of Hispanics among faculty and residents lag well behind the US population. The proportion of ObGyn faculty who are Black has consistently been less than in the US adult female population. ObGyns who are Asian constitute higher proportions of faculty and residents than in the US adult female population. This finding about Asians is consistent across all clinical specialties.7
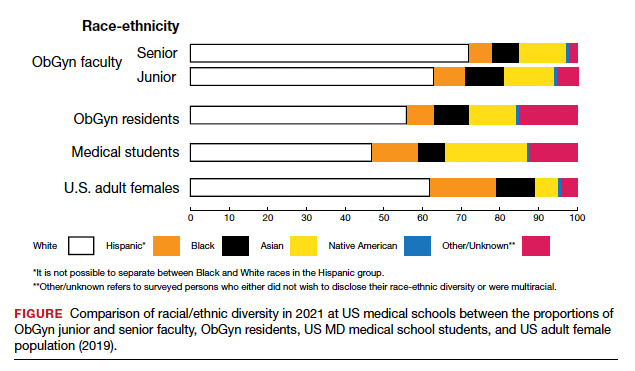
Recruiting URiM students into ObGyn is important. Racial and ethnic representation in surgical and nonsurgical residency programs has not substantially improved in the past decade and continues to lag the changing demographics of the US population.10 More students than residents and faculty are Hispanic, which represents a much-needed opportunity for recruitment. By contrast, junior ObGyn faculty are more likely to be Black than residents and students. Native Americans constitute less than 1% of all faculty, residents, students, and US adult females.9 Lastly, race/ethnicity being self-reported as “other” or “unknown” is most common among students and residents, which perhaps represents greater diversity.
Looking back
Increasing diversity in medicine and in ObGyn has not happened by accident. Transformational change requires rectifying any factors that detrimentally affect the racial/ethnic diversity of our medical students, residents, and faculty. For example, biases inherent in key residency application metrics are being recognized, and use of holistic review is increasing. Change is also accelerated by an explicit and public commitment from national organizations. In 2009, the Liaison Committee of Medical Education (LCME) mandated that medical schools engage in practices that focus on recruitment and retention of a diverse workforce. Increases in Black and Hispanic medical students were noted after implementation of this new mandate.16 The ACGME followed suit with similar guidelines in 2019.10
Diversity is one of the foundational strengths of the ObGyn specialty. Important aspects of the specialty are built upon the contributions of women of color, some voluntary and some not. One example is the knowledge of gynecology that was gained through the involuntary and nonanesthetized surgeries performed on
Moving forward
Advancing diversity in ObGyn offers advantages: better representation of patient populations, improving public health by better access to care, enhancing learning in medical education, building more comprehensive research agendas, and driving institutional excellence. While progress has been made, significant work is still to be done. We must continue to critically examine the role of biases and structural racism that are embedded in evaluating medical students, screening of residency applicants, and selecting and retaining faculty. In future work, we should explore the hypothesis that continued change in racial/ethnic diversity of faculty will only occur once more URiM students, especially the growing number of Hispanics, are admitted into medical schools and recruited for residency positions. We should also examine whether further diversity improves patient outcomes.
It is encouraging to realize that ObGyn departments are leaders in racial/ethnic diversity at US medical schools. It is also critical that the specialty commits to the progress that still needs to be made, including increasing diversity among faculty and institutional leadership. To maintain diversity that mirrors the US adult female population, the specialty of ObGyn will require active surveillance and continued recruitment of Black and, especially Hispanic, faculty and residents.19 The national strategies aimed at building medical student and residency diversity are beginning to yield results. For those gains to help faculty diversity, institutional and departmental leaders will need to implement best practices for recruiting, retaining, and advancing URiM faculty.19 Those practices would include making workforce diversity an explicit priority, building diverse applicant pools, and establishing infrastructure and mentorship to advance URiM faculty to senior leadership positions.20
In conclusion
Building a physician workforce that is more representative of the US population should aid in addressing inequalities in health and health care. Significant strides have been made in racial/ethnic diversity in ObGyn. This has resulted in a specialty that is among the most diverse in academic medicine. At the same time, there is more work to be done. For example, the specialty is far from reaching racial equity for Hispanic physicians. Also, continued efforts are necessary to advance URiM faculty to leadership positions. The legacy of racial/ethnic diversity in ObGyn did not happen by accident and will not be maintained without intention. ●
- Hummes KR, Jones NA, Ramierez RR. United States Census: overview of race and Hispanic origin: 2010. http//www. census.gov/prod/cen2010/briefs/c2010br-02.pdf. Accessed May 22, 2022.
- Xierali IM, Castillo-Page L, Zhang K, et al. AM last page: the urgency of physician workforce diversity. Acad Med. 2014;89:1192.
- Association of American Medical College. Diversity in the physician workforce. Facts & figures 2014. http://www .aamcdiversityfactsandfigures.org. Accessed April 9, 2022.
- Marrast LM, Zallman L, Woolhandler S, et al. Minority physicians’ role in the care of underserved patients: Diversifying the physician workforce may be key in addressing health disparities. JAMA Int Med. 2014;174:289-291.
- Amalba A, Abantanga FA, Scherpbier AJ, et al. Community-based education: The influence of role modeling on career choice and practice location. Med Teac. 2017;39:174-180.
- Umbach PD. The contribution of faculty of color to undergraduate education. Res High Educ. 2006;47:317-345.
- Gonzalo JD, Chuang CH, Glod SA, et al. General internists as change agents: opportunities and barriers to leadership in health systems and medical education transformation. J Gen Intern Med. 2020;35:1865-1869.
- Xierali IM, Fair MA, Nivet MA. Faculty diversity in U.S. medical schools: Progress and gaps coexist. AAMC Analysis in Brief. 2016;16. https://www.aamc.org/system/files/reports/1/decem ber2016facultydiversityinu.s.medicalschoolsprogressandga ps.pdf. Accessed May 4, 2022.
- Rayburn WF, Xierali IM, McDade WA. Racial-ethnic diversity of obstetrics and gynecology faculty at medical schools in the United States. Am J Obstet Gynecol. 2022;S00029378(22)00106-5. doi: 10.1016/j.ajog.2022.02.007.
- Hucko L, Al-khersan H, Lopez Dominguez J, et al. Racial and ethnic diversity of U.S. residency programs, 2011-2019. N Engl J Med. 2022;386:22-23.
- Accreditation Council for Graduate Medical Education. Data Resource Book Academic Year 2020-2021. https://www.acgme.org/globalassets/pfassets /publicationsbooks/2020-2021_acgme_databook _document.pdf. Accessed October 24, 2021
- United States Census Bureau. The 2019 American Community Survey 5-Year Public Use Microdata Sample (PUMS) Files.
- Accreditation Council for Graduate Medical Education. Data Resource Book Academic Year 2020-2021. https://www.acgme .org/globalassets/pfassets/publicationsbooks/2020-2021 _acgme_databook_document.pdf. Accessed October 24, 2021.
- Rayburn WF, Liu CQ, Elwell EC, et al. Diversity of physician faculty in obstetrics and gynecology. J Reprod Med. 2016;61:22-26.
- Xierali IM, Nivet MA, Rayburn WF. Full-time faculty in clinical and basic science departments by sex and underrepresented in medicine status: A 40-year review. Acad Med. 2021;96: 568-575.
- Boatright DH, Samuels EA, Cramer LJ, et al. Association between the Liaison Committee on Medical Education’s Diversity Standards and Changes in percentage of medical student sex, race, and ethnicity. JAMA. 2018;320:2267-2269.
- United States National Library of Medicine. Changing the face of medicine.
- https://cfmedicine.nlm.nih.gov/physicians/biography_82. html. Accessed May 5, 2022.
- Christmas M. #SayHerName: Should obstetrics and gynecology reckon with the legacy of JM Sims? Reprod Sci. 2021;28:3282-3284.
- Morgan HK, Winkel AF, Bands E, et al. Promoting diversity, equity, and inclusion in the selection of obstetrician-gynecologists. Obstet Gynecol. 2021;138:272-277.
- Peek ME, Kim KE, Johnson JK, et al. “URM candidates are encouraged to apply”: a national study to identify effective strategies to enhance racial and ethnic faculty diversity in academic departments of medicine. Acad Med. 2013;88:405-412.
The nation’s population is quickly diversifying, making racial/ethnic disparities in health care outcomes even more apparent. Minority and non-English-speaking populations have grown and may become a majority in the next generation.1 A proposed strategy to reduce disparities in health care is to recruit more practitioners who better reflect the patient populations.2 Improved access to care with racial concordance between physicians and patients has been reported.3
Being increasingly aware of access-to-care data, more patients are advocating and asking for physicians of color to be their providers.4 Despite progress (ie, more women entering the medical profession), the proportion of physicians who are underrepresented in medicine (URiM—eg, Black, Hispanic, and Native American) still lags US population demographics.3
Why diversity in medicine matters
In addition to improving access to care, diversity in medicine offers other benefits. Working within diverse learning environments has demonstrated educational advantages.5,6 Medical students and residents from diverse backgrounds are less likely to report depression symptoms, regardless of their race. Diversity may accelerate advancements in health care as well, since it is well-established that diverse teams outperform nondiverse teams when it comes to innovation and productivity.7 Finally, as a profession committed to equity, advocacy, and justice, physicians are positioned to lead the way toward racial equity.
Overall, racial and gender diversity in all clinical specialties is improving, but not at the same pace. While the diversity of US medical students and residents by sex and race/ethnicity is greater than among faculty, change in racial diversity has been slow for all 3 groups.8 During the past 40 years the number of full-time faculty has increased 6-fold for females and more than tripled for males.8 However, this rise has not favored URiM faculty, because their proportion is still underrepresented relative to their group in the general population. Clinical departments that are making the most progress in recruiting URiM residents and faculty are often primary or preventive care specialties rather than surgical or service or hospital-based specialties.8,9 ObGyn has consistently had a proportion of URiM residents (18%) that is highest in the surgical specialties and comparable to family medicine and pediatrics.10
When examining physician workforce diversity, it is important to “drill down” to individual specialties to obtain a clearer understanding of trends. The continued need for increased resident and faculty diversity prompted us to examine ObGyn departments. The most recent nationwide data were gathered about full-time faculty from the 2021 AAMC Faculty Roster, residents from the 2021 Accreditation Counsel for Graduate Medical Education (ACGME) Data Resource Book, medical student matriculants from 2021 AAMC, and US adult women (defined arbitrarily as 15 years or older) from the 2019 American Community Survey.11-13
Increase in female faculty and residents
The expanding numbers of faculty and residents over a 40-year period (from 1973 to 2012) led to more women and underrepresented minorities in ObGyn than in other major clinical departments.14,15 Women now constitute two-thirds of all ObGyn faculty and are more likely to be junior rather than senior faculty.9 When looking at junior faculty, a higher proportion of junior faculty who are URiM are female. While more junior faculty and residents are female, male faculty are also racially and ethnically diverse.9
- ObGyn is a leader in racial/ethnic diversity in academic medicine.
- The rapid rise of faculty numbers in the past has not favored underrepresented faculty.
- The rise in ObGyn faculty and residents, who were predominantly female, has contributed to greater racial/ethnic diversity.
- Improved patient outcomes with racial concordance between physicians and patients have been reported.
- More patients are advocating and asking for physicians of color to be their clinicians.
- Racial/ethnic diversity of junior faculty and residents is similar to medical students.
- The most underrepresented group is Hispanic, due in part to its rapid growth in the US population.
Continue to: Growth of URiM physicians in ObGyn...
Growth of URiM physicians in ObGyn
The distribution of racial/ethnic groups in 2021 were compared between senior and junior ObGyn faculty and residents with the US adult female population.9 As shown in the FIGURE, the proportion of ObGyn faculty who are White approximates the White US adult female population. The most rapidly growing racial/ethnic group in the US population is Hispanic. Although Hispanic is the best represented ethnicity among junior faculty, the proportions of Hispanics among faculty and residents lag well behind the US population. The proportion of ObGyn faculty who are Black has consistently been less than in the US adult female population. ObGyns who are Asian constitute higher proportions of faculty and residents than in the US adult female population. This finding about Asians is consistent across all clinical specialties.7

Recruiting URiM students into ObGyn is important. Racial and ethnic representation in surgical and nonsurgical residency programs has not substantially improved in the past decade and continues to lag the changing demographics of the US population.10 More students than residents and faculty are Hispanic, which represents a much-needed opportunity for recruitment. By contrast, junior ObGyn faculty are more likely to be Black than residents and students. Native Americans constitute less than 1% of all faculty, residents, students, and US adult females.9 Lastly, race/ethnicity being self-reported as “other” or “unknown” is most common among students and residents, which perhaps represents greater diversity.
Looking back
Increasing diversity in medicine and in ObGyn has not happened by accident. Transformational change requires rectifying any factors that detrimentally affect the racial/ethnic diversity of our medical students, residents, and faculty. For example, biases inherent in key residency application metrics are being recognized, and use of holistic review is increasing. Change is also accelerated by an explicit and public commitment from national organizations. In 2009, the Liaison Committee of Medical Education (LCME) mandated that medical schools engage in practices that focus on recruitment and retention of a diverse workforce. Increases in Black and Hispanic medical students were noted after implementation of this new mandate.16 The ACGME followed suit with similar guidelines in 2019.10
Diversity is one of the foundational strengths of the ObGyn specialty. Important aspects of the specialty are built upon the contributions of women of color, some voluntary and some not. One example is the knowledge of gynecology that was gained through the involuntary and nonanesthetized surgeries performed on
Moving forward
Advancing diversity in ObGyn offers advantages: better representation of patient populations, improving public health by better access to care, enhancing learning in medical education, building more comprehensive research agendas, and driving institutional excellence. While progress has been made, significant work is still to be done. We must continue to critically examine the role of biases and structural racism that are embedded in evaluating medical students, screening of residency applicants, and selecting and retaining faculty. In future work, we should explore the hypothesis that continued change in racial/ethnic diversity of faculty will only occur once more URiM students, especially the growing number of Hispanics, are admitted into medical schools and recruited for residency positions. We should also examine whether further diversity improves patient outcomes.
It is encouraging to realize that ObGyn departments are leaders in racial/ethnic diversity at US medical schools. It is also critical that the specialty commits to the progress that still needs to be made, including increasing diversity among faculty and institutional leadership. To maintain diversity that mirrors the US adult female population, the specialty of ObGyn will require active surveillance and continued recruitment of Black and, especially Hispanic, faculty and residents.19 The national strategies aimed at building medical student and residency diversity are beginning to yield results. For those gains to help faculty diversity, institutional and departmental leaders will need to implement best practices for recruiting, retaining, and advancing URiM faculty.19 Those practices would include making workforce diversity an explicit priority, building diverse applicant pools, and establishing infrastructure and mentorship to advance URiM faculty to senior leadership positions.20
In conclusion
Building a physician workforce that is more representative of the US population should aid in addressing inequalities in health and health care. Significant strides have been made in racial/ethnic diversity in ObGyn. This has resulted in a specialty that is among the most diverse in academic medicine. At the same time, there is more work to be done. For example, the specialty is far from reaching racial equity for Hispanic physicians. Also, continued efforts are necessary to advance URiM faculty to leadership positions. The legacy of racial/ethnic diversity in ObGyn did not happen by accident and will not be maintained without intention. ●
The nation’s population is quickly diversifying, making racial/ethnic disparities in health care outcomes even more apparent. Minority and non-English-speaking populations have grown and may become a majority in the next generation.1 A proposed strategy to reduce disparities in health care is to recruit more practitioners who better reflect the patient populations.2 Improved access to care with racial concordance between physicians and patients has been reported.3
Being increasingly aware of access-to-care data, more patients are advocating and asking for physicians of color to be their providers.4 Despite progress (ie, more women entering the medical profession), the proportion of physicians who are underrepresented in medicine (URiM—eg, Black, Hispanic, and Native American) still lags US population demographics.3
Why diversity in medicine matters
In addition to improving access to care, diversity in medicine offers other benefits. Working within diverse learning environments has demonstrated educational advantages.5,6 Medical students and residents from diverse backgrounds are less likely to report depression symptoms, regardless of their race. Diversity may accelerate advancements in health care as well, since it is well-established that diverse teams outperform nondiverse teams when it comes to innovation and productivity.7 Finally, as a profession committed to equity, advocacy, and justice, physicians are positioned to lead the way toward racial equity.
Overall, racial and gender diversity in all clinical specialties is improving, but not at the same pace. While the diversity of US medical students and residents by sex and race/ethnicity is greater than among faculty, change in racial diversity has been slow for all 3 groups.8 During the past 40 years the number of full-time faculty has increased 6-fold for females and more than tripled for males.8 However, this rise has not favored URiM faculty, because their proportion is still underrepresented relative to their group in the general population. Clinical departments that are making the most progress in recruiting URiM residents and faculty are often primary or preventive care specialties rather than surgical or service or hospital-based specialties.8,9 ObGyn has consistently had a proportion of URiM residents (18%) that is highest in the surgical specialties and comparable to family medicine and pediatrics.10
When examining physician workforce diversity, it is important to “drill down” to individual specialties to obtain a clearer understanding of trends. The continued need for increased resident and faculty diversity prompted us to examine ObGyn departments. The most recent nationwide data were gathered about full-time faculty from the 2021 AAMC Faculty Roster, residents from the 2021 Accreditation Counsel for Graduate Medical Education (ACGME) Data Resource Book, medical student matriculants from 2021 AAMC, and US adult women (defined arbitrarily as 15 years or older) from the 2019 American Community Survey.11-13
Increase in female faculty and residents
The expanding numbers of faculty and residents over a 40-year period (from 1973 to 2012) led to more women and underrepresented minorities in ObGyn than in other major clinical departments.14,15 Women now constitute two-thirds of all ObGyn faculty and are more likely to be junior rather than senior faculty.9 When looking at junior faculty, a higher proportion of junior faculty who are URiM are female. While more junior faculty and residents are female, male faculty are also racially and ethnically diverse.9
- ObGyn is a leader in racial/ethnic diversity in academic medicine.
- The rapid rise of faculty numbers in the past has not favored underrepresented faculty.
- The rise in ObGyn faculty and residents, who were predominantly female, has contributed to greater racial/ethnic diversity.
- Improved patient outcomes with racial concordance between physicians and patients have been reported.
- More patients are advocating and asking for physicians of color to be their clinicians.
- Racial/ethnic diversity of junior faculty and residents is similar to medical students.
- The most underrepresented group is Hispanic, due in part to its rapid growth in the US population.
Continue to: Growth of URiM physicians in ObGyn...
Growth of URiM physicians in ObGyn
The distribution of racial/ethnic groups in 2021 were compared between senior and junior ObGyn faculty and residents with the US adult female population.9 As shown in the FIGURE, the proportion of ObGyn faculty who are White approximates the White US adult female population. The most rapidly growing racial/ethnic group in the US population is Hispanic. Although Hispanic is the best represented ethnicity among junior faculty, the proportions of Hispanics among faculty and residents lag well behind the US population. The proportion of ObGyn faculty who are Black has consistently been less than in the US adult female population. ObGyns who are Asian constitute higher proportions of faculty and residents than in the US adult female population. This finding about Asians is consistent across all clinical specialties.7

Recruiting URiM students into ObGyn is important. Racial and ethnic representation in surgical and nonsurgical residency programs has not substantially improved in the past decade and continues to lag the changing demographics of the US population.10 More students than residents and faculty are Hispanic, which represents a much-needed opportunity for recruitment. By contrast, junior ObGyn faculty are more likely to be Black than residents and students. Native Americans constitute less than 1% of all faculty, residents, students, and US adult females.9 Lastly, race/ethnicity being self-reported as “other” or “unknown” is most common among students and residents, which perhaps represents greater diversity.
Looking back
Increasing diversity in medicine and in ObGyn has not happened by accident. Transformational change requires rectifying any factors that detrimentally affect the racial/ethnic diversity of our medical students, residents, and faculty. For example, biases inherent in key residency application metrics are being recognized, and use of holistic review is increasing. Change is also accelerated by an explicit and public commitment from national organizations. In 2009, the Liaison Committee of Medical Education (LCME) mandated that medical schools engage in practices that focus on recruitment and retention of a diverse workforce. Increases in Black and Hispanic medical students were noted after implementation of this new mandate.16 The ACGME followed suit with similar guidelines in 2019.10
Diversity is one of the foundational strengths of the ObGyn specialty. Important aspects of the specialty are built upon the contributions of women of color, some voluntary and some not. One example is the knowledge of gynecology that was gained through the involuntary and nonanesthetized surgeries performed on
Moving forward
Advancing diversity in ObGyn offers advantages: better representation of patient populations, improving public health by better access to care, enhancing learning in medical education, building more comprehensive research agendas, and driving institutional excellence. While progress has been made, significant work is still to be done. We must continue to critically examine the role of biases and structural racism that are embedded in evaluating medical students, screening of residency applicants, and selecting and retaining faculty. In future work, we should explore the hypothesis that continued change in racial/ethnic diversity of faculty will only occur once more URiM students, especially the growing number of Hispanics, are admitted into medical schools and recruited for residency positions. We should also examine whether further diversity improves patient outcomes.
It is encouraging to realize that ObGyn departments are leaders in racial/ethnic diversity at US medical schools. It is also critical that the specialty commits to the progress that still needs to be made, including increasing diversity among faculty and institutional leadership. To maintain diversity that mirrors the US adult female population, the specialty of ObGyn will require active surveillance and continued recruitment of Black and, especially Hispanic, faculty and residents.19 The national strategies aimed at building medical student and residency diversity are beginning to yield results. For those gains to help faculty diversity, institutional and departmental leaders will need to implement best practices for recruiting, retaining, and advancing URiM faculty.19 Those practices would include making workforce diversity an explicit priority, building diverse applicant pools, and establishing infrastructure and mentorship to advance URiM faculty to senior leadership positions.20
In conclusion
Building a physician workforce that is more representative of the US population should aid in addressing inequalities in health and health care. Significant strides have been made in racial/ethnic diversity in ObGyn. This has resulted in a specialty that is among the most diverse in academic medicine. At the same time, there is more work to be done. For example, the specialty is far from reaching racial equity for Hispanic physicians. Also, continued efforts are necessary to advance URiM faculty to leadership positions. The legacy of racial/ethnic diversity in ObGyn did not happen by accident and will not be maintained without intention. ●
- Hummes KR, Jones NA, Ramierez RR. United States Census: overview of race and Hispanic origin: 2010. http//www. census.gov/prod/cen2010/briefs/c2010br-02.pdf. Accessed May 22, 2022.
- Xierali IM, Castillo-Page L, Zhang K, et al. AM last page: the urgency of physician workforce diversity. Acad Med. 2014;89:1192.
- Association of American Medical College. Diversity in the physician workforce. Facts & figures 2014. http://www .aamcdiversityfactsandfigures.org. Accessed April 9, 2022.
- Marrast LM, Zallman L, Woolhandler S, et al. Minority physicians’ role in the care of underserved patients: Diversifying the physician workforce may be key in addressing health disparities. JAMA Int Med. 2014;174:289-291.
- Amalba A, Abantanga FA, Scherpbier AJ, et al. Community-based education: The influence of role modeling on career choice and practice location. Med Teac. 2017;39:174-180.
- Umbach PD. The contribution of faculty of color to undergraduate education. Res High Educ. 2006;47:317-345.
- Gonzalo JD, Chuang CH, Glod SA, et al. General internists as change agents: opportunities and barriers to leadership in health systems and medical education transformation. J Gen Intern Med. 2020;35:1865-1869.
- Xierali IM, Fair MA, Nivet MA. Faculty diversity in U.S. medical schools: Progress and gaps coexist. AAMC Analysis in Brief. 2016;16. https://www.aamc.org/system/files/reports/1/decem ber2016facultydiversityinu.s.medicalschoolsprogressandga ps.pdf. Accessed May 4, 2022.
- Rayburn WF, Xierali IM, McDade WA. Racial-ethnic diversity of obstetrics and gynecology faculty at medical schools in the United States. Am J Obstet Gynecol. 2022;S00029378(22)00106-5. doi: 10.1016/j.ajog.2022.02.007.
- Hucko L, Al-khersan H, Lopez Dominguez J, et al. Racial and ethnic diversity of U.S. residency programs, 2011-2019. N Engl J Med. 2022;386:22-23.
- Accreditation Council for Graduate Medical Education. Data Resource Book Academic Year 2020-2021. https://www.acgme.org/globalassets/pfassets /publicationsbooks/2020-2021_acgme_databook _document.pdf. Accessed October 24, 2021
- United States Census Bureau. The 2019 American Community Survey 5-Year Public Use Microdata Sample (PUMS) Files.
- Accreditation Council for Graduate Medical Education. Data Resource Book Academic Year 2020-2021. https://www.acgme .org/globalassets/pfassets/publicationsbooks/2020-2021 _acgme_databook_document.pdf. Accessed October 24, 2021.
- Rayburn WF, Liu CQ, Elwell EC, et al. Diversity of physician faculty in obstetrics and gynecology. J Reprod Med. 2016;61:22-26.
- Xierali IM, Nivet MA, Rayburn WF. Full-time faculty in clinical and basic science departments by sex and underrepresented in medicine status: A 40-year review. Acad Med. 2021;96: 568-575.
- Boatright DH, Samuels EA, Cramer LJ, et al. Association between the Liaison Committee on Medical Education’s Diversity Standards and Changes in percentage of medical student sex, race, and ethnicity. JAMA. 2018;320:2267-2269.
- United States National Library of Medicine. Changing the face of medicine.
- https://cfmedicine.nlm.nih.gov/physicians/biography_82. html. Accessed May 5, 2022.
- Christmas M. #SayHerName: Should obstetrics and gynecology reckon with the legacy of JM Sims? Reprod Sci. 2021;28:3282-3284.
- Morgan HK, Winkel AF, Bands E, et al. Promoting diversity, equity, and inclusion in the selection of obstetrician-gynecologists. Obstet Gynecol. 2021;138:272-277.
- Peek ME, Kim KE, Johnson JK, et al. “URM candidates are encouraged to apply”: a national study to identify effective strategies to enhance racial and ethnic faculty diversity in academic departments of medicine. Acad Med. 2013;88:405-412.
- Hummes KR, Jones NA, Ramierez RR. United States Census: overview of race and Hispanic origin: 2010. http//www. census.gov/prod/cen2010/briefs/c2010br-02.pdf. Accessed May 22, 2022.
- Xierali IM, Castillo-Page L, Zhang K, et al. AM last page: the urgency of physician workforce diversity. Acad Med. 2014;89:1192.
- Association of American Medical College. Diversity in the physician workforce. Facts & figures 2014. http://www .aamcdiversityfactsandfigures.org. Accessed April 9, 2022.
- Marrast LM, Zallman L, Woolhandler S, et al. Minority physicians’ role in the care of underserved patients: Diversifying the physician workforce may be key in addressing health disparities. JAMA Int Med. 2014;174:289-291.
- Amalba A, Abantanga FA, Scherpbier AJ, et al. Community-based education: The influence of role modeling on career choice and practice location. Med Teac. 2017;39:174-180.
- Umbach PD. The contribution of faculty of color to undergraduate education. Res High Educ. 2006;47:317-345.
- Gonzalo JD, Chuang CH, Glod SA, et al. General internists as change agents: opportunities and barriers to leadership in health systems and medical education transformation. J Gen Intern Med. 2020;35:1865-1869.
- Xierali IM, Fair MA, Nivet MA. Faculty diversity in U.S. medical schools: Progress and gaps coexist. AAMC Analysis in Brief. 2016;16. https://www.aamc.org/system/files/reports/1/decem ber2016facultydiversityinu.s.medicalschoolsprogressandga ps.pdf. Accessed May 4, 2022.
- Rayburn WF, Xierali IM, McDade WA. Racial-ethnic diversity of obstetrics and gynecology faculty at medical schools in the United States. Am J Obstet Gynecol. 2022;S00029378(22)00106-5. doi: 10.1016/j.ajog.2022.02.007.
- Hucko L, Al-khersan H, Lopez Dominguez J, et al. Racial and ethnic diversity of U.S. residency programs, 2011-2019. N Engl J Med. 2022;386:22-23.
- Accreditation Council for Graduate Medical Education. Data Resource Book Academic Year 2020-2021. https://www.acgme.org/globalassets/pfassets /publicationsbooks/2020-2021_acgme_databook _document.pdf. Accessed October 24, 2021
- United States Census Bureau. The 2019 American Community Survey 5-Year Public Use Microdata Sample (PUMS) Files.
- Accreditation Council for Graduate Medical Education. Data Resource Book Academic Year 2020-2021. https://www.acgme .org/globalassets/pfassets/publicationsbooks/2020-2021 _acgme_databook_document.pdf. Accessed October 24, 2021.
- Rayburn WF, Liu CQ, Elwell EC, et al. Diversity of physician faculty in obstetrics and gynecology. J Reprod Med. 2016;61:22-26.
- Xierali IM, Nivet MA, Rayburn WF. Full-time faculty in clinical and basic science departments by sex and underrepresented in medicine status: A 40-year review. Acad Med. 2021;96: 568-575.
- Boatright DH, Samuels EA, Cramer LJ, et al. Association between the Liaison Committee on Medical Education’s Diversity Standards and Changes in percentage of medical student sex, race, and ethnicity. JAMA. 2018;320:2267-2269.
- United States National Library of Medicine. Changing the face of medicine.
- https://cfmedicine.nlm.nih.gov/physicians/biography_82. html. Accessed May 5, 2022.
- Christmas M. #SayHerName: Should obstetrics and gynecology reckon with the legacy of JM Sims? Reprod Sci. 2021;28:3282-3284.
- Morgan HK, Winkel AF, Bands E, et al. Promoting diversity, equity, and inclusion in the selection of obstetrician-gynecologists. Obstet Gynecol. 2021;138:272-277.
- Peek ME, Kim KE, Johnson JK, et al. “URM candidates are encouraged to apply”: a national study to identify effective strategies to enhance racial and ethnic faculty diversity in academic departments of medicine. Acad Med. 2013;88:405-412.
Vismodegib for Basal Cell Carcinoma and Beyond: What Dermatologists Need to Know
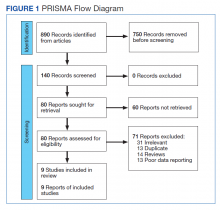
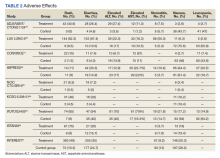
Basal cell carcinomas (BCCs) are considered the most common cutaneous cancers. Approximately 80% of nonmelanoma skin cancers are BCCs.1,2 Surgical management is the gold standard for early-stage and localized BCCs; it may include simple excision vs Mohs micrographic surgery.3,4 However, if left untreated, these lesions can progress to an advanced stage (locally advanced BCC) or infrequently may spread to distant sites (metastatic BCC). In the advanced stage, the lesions are no longer manageable by surgery or radiation therapy.5,6 Recently, inhibitors targeting the hedgehog (Hh) pathway have shown great promise for these patients. The first drug approved by the US Food and Drug Administration (FDA) for locally advanced and metastatic BCC is vismodegib.7 In this article, we provide a clinical review of vismodegib for the management of BCC, including a discussion of the Hh pathway in BCC, adverse effects of vismodegib, use of vismodegib in adnexal skin tumors, recommended doses for vismodegib therapy in BCC, and management of the side effects of treatment.
Hh Pathway in BCC
In embryonic development, the Hh signaling pathway is crucial across a broad spectrum of species, including humans. Various members of the Hh family have been recognized, all working as secreted regulatory proteins.8 The name of the Hh signaling pathway is derived from a polypeptide ligand called hedgehog found in some fruit flies. Mutations in the gene led to fruit fly larvae that had a spiky hairy pattern of denticles similar to hedgehogs, leading to the name of this molecule.9 The transmembrane protein smoothened (SMO) is the main component of the Hh signaling pathway and initiates a signaling cascade that in turn leads to an increased expression of target genes, such as GLI1. Patched (PTCH), also a transmembrane protein and a cell-surface receptor for the secreted Hh ligand, suppresses the signaling capacity of SMO. Upon binding of the Hh ligand to the PTCH receptor, the suppression of SMO is relieved and a signal is propagated, evoking a cellular response.10 Molecular and genetic studies have reported that genetic alterations in the Hh signaling pathway are almost universally present in all BCCs, leading to an aberrant activation of the pathway and an uncontrolled proliferation of the basal cells. Frequently, these alterations have been shown to cause loss of function of PTCH homologue 1, which usually acts to inhibit the SMO homologue signaling activity.11,12
Because of the potential importance of Hh signaling in other solid malignancies and the failure of topical inhibition of SMO,13 subsequent studies on the development of Hh pathway inhibitors have mostly focused on the systemic approach. A multitude of Hh pathway inhibitors have been developed thus far, such as SANT1-SANT4, GDC-0449, IPI-926, BMS-833923 (XL139), HhAntag-691, and MK-4101.14 Many of these inhibitors have been clinically investigated.13,15,16
Systemic SMO Inhibitor: Vismodegib
Vismodegib was the earliest systemic SMO inhibitor to fulfill early clinical evaluation15,16 and the first drug to receive FDA approval for the management of advanced or metastatic BCC. Vismodegib is a small-molecule SMO inhibitor used for the management of selected locally advanced BCC and metastatic BCC in adults.3,17 Although there is a possibility of recurrence following drug withdrawal, vismodegib constitutes a new therapeutic strategy presenting positive benefits to patients. It may provide superior improvement over sunitinib, which has shown efficacy in a few patients; however, the efficacy and tolerance of sunitinib have been shown to be limited.18,19
Adverse Effects of Vismodegib Therapy
Adverse events with vismodegib use have been reported in 98% of patients (N=491); most of these were mild to moderate.20 However, the frequency of adverse events could prove to be a therapeutic challenge for patients requiring extended treatment. The most frequently reported reversible side effects were muscle spasms (64%), alopecia (62%), weight loss (33%), fatigue (28%), decreased appetite (25%), diarrhea (17%), nausea (16%), dysgeusia (54%), and ageusia (22%).20 In clinical trials, amenorrhea was noticed in 30% (3/10) of females with reproductive potential.2 Apart from alopecia and possibly amenorrhea, these side effects are reversible.17 Alkeraye et al17 reported 3 clinical cases of persistent alopecia following the use of vismodegib. Amenorrhea is a possible side effect of unknown reversibility.7
Vismodegib is a pregnancy category D medication.4 Severe birth defects, including craniofacial abnormalities, retardations in normal growth, open perineum, and absence of digital fusion at a corresponding 20% of the recommended daily dose, were found in rat studies. Embryo-fetal death was noted when rats were exposedto concentrations comparable to the recommended human dose.4
Hepatic events with the use of vismodegib have been reported. The use of vismodegib in randomized controlled trials resulted in elevation of both alanine aminotransferase and aspartate aminotransferase levels compared with placebo.21 Moreover, severe hepatotoxicity with vismodegib has been reported.22-24 A study conducted by Edwards et al25 concluded that the use of vismodegib in patients with severe liver disease must include thorough risk-benefit assessment, with caution in using other concomitant hepatotoxic medications.
Rare adverse events also have been reported in the literature, including vismodegib-induced pancreatitis in a 79-year-old patient treated for locally advanced, recurrent BCC that was cleared following cessation of therapy.26 Additionally, atypical fibroxanthoma was observed in an 83-year-old patient after 30 days of treatment with vismodegib for multiple BCCs.27 The development of other secondary malignancies, such as squamous cell carcinoma, melanoma, keratoacanthomas, and pilomatricomas, during or after the long-term use of vismodegib also have been described.28-35
Use of Vismodegib for Adnexal Skin Tumors
The role of the sonic Hh–PTCH pathway in the pathogenesis of adnexal tumors varies in the literature. Some studies propose the involvement of this pathway in the formation of adnexal tumors such as trichoblastoma, trichoepithelioma, and cylindroma, as in BCC. Various lines of evidence support this involvement. Firstly, in mice, the spontaneous generation of numerous BCCs, trichoblastomas, trichoepitheliomas, and cylindromas has been observed following constitutive activation of the sonic Hh–PTCH pathway.36 Secondly, in trichoepitheliomas, there have been positive results in molecular research into the tumor suppressor gene PTCH homologue 1, PTCH1, whose mutations cause constitutive activation of the sonic Hh–PTCH pathway.37 Thirdly, GLI138 and SOX939 transcription factors associated with the signaling pathway of sonic Hh–PTCH appear to have increased levels in adnexal carcinomas.19 Lepesant et al19 reported a notable clinical response to vismodegib in trichoblastic carcinoma. Baur et al40 reported successful treatment of multiple familial trichoepitheliomas with vismodegib. Nonetheless, more studies are required to assess the efficacy and reliability of vismodegib in the management of adnexal tumors.
Recommended Dose of Vismodegib Therapy
The vismodegib dosage that is approved by the FDA is 150 mg/d until disease progression or the development of intolerable side effects.4 Higher dosing regimens were evaluated with 270 mg/d and 540 mg/d. No added therapeutic benefit was noted with the increase in the dose, and no dose-limiting toxic effects were observed.41
Management of Vismodegib Side Effects
Managing patient expectations is a crucial step in improving dysgeusia. The experience of dysgeusia varies among patients; thus, patients should be instructed to adjust their diets according to their level of dysgeusia, which can be achieved by changing ingredients or dressings used with their diet. This step has been proven to be effective in overcoming vismodegib-related dysgeusia. Also, fluid taste distortion may lead to dehydration and an increase in creatine level. Thus, patients should be encouraged to monitor fluid intake. Moreover, a treatment hiatus of 2 to 8 months results in near-complete improvement of taste distortion.
For muscle spasms, quinine, treatment break for 1 month, gentle exercise of affected areas, or muscle relaxants such as baclofen and temazepam all are effective methods. For vismodegib-related alopecia, managing patient expectations is key; patients should be aware that hair may take 6 to 12 months or even longer to regrow. In addition, shaving less frequently helps improve alopecia.
For gastrointestinal disorders, loperamide with or without codeine phosphate is effective in resolving diarrhea, and metoclopramide is mostly adequate in treating nausea. Another adverse event is weight loss; weight loss of 5% or more of total body weight prompts dietetic referral. If weight loss persists, a treatment break might be needed to regain weight.
Overall, treatment breaks are sufficient to resolve adverse events caused by vismodegib and do not compromise efficacy of treatment. The duration of a treatment break should be considered before initiation. In one clinical trial, a longer treatment break was associated with fewer adverse effects without affecting the efficacy of treatment.42
Conclusion
Vismodegib provides an effective alternative to surgical intervention in the management of BCC. However, patients must be monitored vigilantly, as adverse events are common (>90%).
- Sekulic A, Migden MR, Oro AE, et al. Efficacy and safety of vismodegib in advanced basal-cell carcinoma. N Engl J Med. 2012;366:2171-2179.
- Rogers HW, Weinstock MA, Harris AR, et al. Incidence estimate of nonmelanoma skin cancer in the United States, 2006. Arch Dermatol. 2010;146:283-287.
- Von Hoff DD, LoRusso PM, Rudin CM, et al. Inhibition of the hedgehog pathway in advanced basal-cell carcinoma. N Engl J Med. 2009;361:1164-1172.
- Cirrone F, Harris CS. Vismodegib and the hedgehog pathway: a new treatment for basal cell carcinoma. Clin Ther. 2012;34:2039-2050.
- Ruiz-Salas V, Alegre M, López-Ferrer A, et al. Vismodegib: a review [article in English, Spanish]. Actas Dermosifiliogr. 2014;105:744-751.
- Rubin AI, Chen EH, Ratner D. Basal-cell carcinoma. N Engl J Med. 2005;353:2262-2269.
- Cusack CA, Nijhawan R, Miller B, et al. Vismodegib for locally advanced basal cell carcinoma in a heart transplant patient. JAMA Dermatol. 2015;151:70-72.
- Aszterbaum M, Rothman A, Johnson RL, et al. Identification of mutations in the human PATCHED gene in sporadic basal cell carcinomas and in patients with the basal cell nevus syndrome. J Invest Dermatol. 1998;110:885-888.
- Abidi A. Hedgehog signaling pathway: a novel target for cancer therapy: vismodegib, a promising therapeutic option in treatment of basal cell carcinomas. Indian J Pharmacol. 2014;46:3-12.
- St-Jacques B, Dassule HR, Karavanova I, et al. Sonic hedgehog signaling is essential for hair development. Curr Biol. 1998;8:1058-1068.
- Gailani MR, Ståhle-Bäckdahl M, Leffell DJ, et al. The role of the human homologue of Drosophila patched in sporadic basal cell carcinomas. Nat Genet. 1996;14:78-81.
- Hall JM, Bell ML, Finger TE. Disruption of sonic hedgehog signaling alters growth and patterning of lingual taste papillae. Dev Biol. 2003;255:263-277.
- Bai CB, Stephen D, Joyner AL. All mouse ventral spinal cord patterning by hedgehog is Gli dependent and involves an activator function of Gli3. Dev Cell. 2004;6:103-115.
- Wang B, Fallon JF, Beachy PA. Hedgehog-regulated processing of Gli3 produces an anterior/posterior repressor gradient in the developing vertebrate limb. Cell. 2000;100:423-434.
- Sekulic A, Mangold AR, Northfelt DW, et al. Advanced basal cell carcinoma of the skin: targeting the hedgehog pathway. Curr Opin Oncol. 2013;25:218-223.
- Ingham PW, Placzek M. Orchestrating ontogenesis: variations on a theme by sonic hedgehog. Nature Rev Genet. 2006;7:841-850.
- Alkeraye S, Maire C, Desmedt E, et al. Persistent alopecia induced by vismodegib. Br J Dermatol. 2015;172:1671-1672.
- Battistella M, Mateus C, Lassau N, et al. Sunitinib efficacy in the treatment of metastatic skin adnexal carcinomas: report of two patients with hidradenocarcinoma and trichoblastic carcinoma. J Eur Acad Dermatol Venereol. 2010;24:199-203.
- Lepesant P, Crinquette M, Alkeraye S, et al. Vismodegib induces significant clinical response in locally advanced trichoblastic carcinoma. Br J Dermatol. 2015;173:1059-1062.
- Basset-Seguin N, Hauschild A, Grob JJ, et al. Vismodegib in patients with advanced basal cell carcinoma (STEVIE): a pre-plannedinterim analysis of an international, open-label trial. Lancet Oncol. 2015;16:729-736.
- Catenacci DV, Junttila MR, Karrison T, et al. Randomized phase Ib/II study of gemcitabine plus placebo or vismodegib, a hedgehog pathway inhibitor, in patients with metastatic pancreatic cancer. J Clin Oncol. 2015;33:4284-4292.
- Sanchez BE, Hajjafar L. Severe hepatotoxicity in a patient treated with hedgehog inhibitor: first case report. Gastroenterology. 2011;140:S974-S975.
- Ly P, Wolf K, Wilson J. A case of hepatotoxicity associated with vismodegib. JAAD Case Rep. 2018;5:57-59.
- Eiger-Moscovich M, Reich E, Tauber G, et al. Efficacy of vismodegib for the treatment of orbital and advanced periocular basal cell carcinoma. Am J Ophthalmol. 2019;207:62-70.
- Edwards BJ, Raisch DW, Saraykar SS, et al. Hepatotoxicity with vismodegib: an MD Anderson Cancer Center and Research on Adverse Drug Events and Reports Project. Drugs R D. 2017;17:211-218.
- Velter C, Blanc J, Robert C. Acute pancreatitis after vismodegib for basal cell carcinoma: a causal relation? Eur J Cancer. 2019;118:67-69.
- Giorgini C, Barbaccia V, Croci GA, et al. Rapid development of atypical fibroxanthoma during vismodegib treatment. Clin Exp Dermatol. 2019;44:86-88.
- Saintes C, Saint-Jean M, Brocard A, et al. Development of squamous cell carcinoma into basal cell carcinoma under treatment with vismodegib. J Eur Acad Dermatol Venereol. 2015;29:1006-1009.
- Zhu GA, Sundram U, Chang ALS. Two different scenarios of squamous cell carcinoma within advanced basal cell carcinomas: cases illustrating the importance of serial biopsy during vismodegib usage. JAMA Dermatol. 2014;150:970-973.
- Poulalhon N, Dalle S, Balme B, et al. Fast-growing cutaneous squamous cell carcinoma in a patient treated with vismodegib. Dermatology. 2015;230:101-104.
- Orouji A, Goerdt S, Utikal J, et al. Multiple highly and moderately differentiated squamous cell carcinomas of the skin during vismodegib treatment of inoperable basal cell carcinoma. Br J Dermatol. 2014;171:431-433.
- Iarrobino A, Messina JL, Kudchadkar R, et al. Emergence of a squamous cell carcinoma phenotype following treatment of metastatic basal cell carcinoma with vismodegib. J Am Acad Dermatol. 2013;69:E33-E34.
- Giuffrida R, Kashofer K, Dika E, et al. Fast growing melanoma following treatment with vismodegib for locally advanced basal cell carcinomas: report of two cases. Eur J Cancer. 2018;91:177-179.
- Aasi S, Silkiss R, Tang JY, et al. New onset of keratoacanthomas after vismodegib treatment for locally advanced basal cell carcinomas: a report of 2 cases. JAMA Dermatol. 2013;149:242-243.
- Magdaleno-Tapial J, Valenzuela-Oñate C, Ortiz-Salvador JM, et al. Pilomatricomas secondary to treatment with vismodegib. JAAD Case Rep. 2018;5:12-14.
- Nilsson M, Undèn AB, Krause D, et al. Induction of basal cell carcinomas and trichoepitheliomas in mice overexpressing GLI-1. Proc Natl Acad Sci U S A. 2000;97:3438-3443.
- Vorechovský I, Undén AB, Sandstedt B, et al. Trichoepitheliomas contain somatic mutations in the overexpressed PTCH gene: support for a gatekeeper mechanism in skin tumorigenesis. Cancer Res. 1997;57:4677-4681.
- Hatta N, Hirano T, Kimura T, et al. Molecular diagnosis of basal cell carcinoma and other basaloid cell neoplasms of the skin by the quantification of Gli1 transcript levels. J Cutan Pathol. 2005;32:131-136.
- Vidal VP, Ortonne N, Schedl A. SOX9 expression is a general marker of basal cell carcinoma and adnexal-related neoplasms. J Cutan Pathol. 2008;35:373-379.
- Baur V, Papadopoulos T, Kazakov DV, et al. A case of multiple familial trichoepitheliomas responding to treatment with the hedgehog signaling pathway inhibitor vismodegib. Virchows Arch. 2018;473:241-246.
- LoRusso PM, Rudin CM, Reddy JC, et al. Phase I trial of hedgehog pathway inhibitor vismodegib (GDC-0449) in patients with refractory, locally advanced or metastatic solid tumors. Clin Cancer Res. 2011;17:2502-2511.
- Fife K, Herd R, Lalondrelle S, et al. Managing adverse events associated with vismodegib in the treatment of basal cell carcinoma. Future Oncol. 2017;13:175-184.
Basal cell carcinomas (BCCs) are considered the most common cutaneous cancers. Approximately 80% of nonmelanoma skin cancers are BCCs.1,2 Surgical management is the gold standard for early-stage and localized BCCs; it may include simple excision vs Mohs micrographic surgery.3,4 However, if left untreated, these lesions can progress to an advanced stage (locally advanced BCC) or infrequently may spread to distant sites (metastatic BCC). In the advanced stage, the lesions are no longer manageable by surgery or radiation therapy.5,6 Recently, inhibitors targeting the hedgehog (Hh) pathway have shown great promise for these patients. The first drug approved by the US Food and Drug Administration (FDA) for locally advanced and metastatic BCC is vismodegib.7 In this article, we provide a clinical review of vismodegib for the management of BCC, including a discussion of the Hh pathway in BCC, adverse effects of vismodegib, use of vismodegib in adnexal skin tumors, recommended doses for vismodegib therapy in BCC, and management of the side effects of treatment.
Hh Pathway in BCC
In embryonic development, the Hh signaling pathway is crucial across a broad spectrum of species, including humans. Various members of the Hh family have been recognized, all working as secreted regulatory proteins.8 The name of the Hh signaling pathway is derived from a polypeptide ligand called hedgehog found in some fruit flies. Mutations in the gene led to fruit fly larvae that had a spiky hairy pattern of denticles similar to hedgehogs, leading to the name of this molecule.9 The transmembrane protein smoothened (SMO) is the main component of the Hh signaling pathway and initiates a signaling cascade that in turn leads to an increased expression of target genes, such as GLI1. Patched (PTCH), also a transmembrane protein and a cell-surface receptor for the secreted Hh ligand, suppresses the signaling capacity of SMO. Upon binding of the Hh ligand to the PTCH receptor, the suppression of SMO is relieved and a signal is propagated, evoking a cellular response.10 Molecular and genetic studies have reported that genetic alterations in the Hh signaling pathway are almost universally present in all BCCs, leading to an aberrant activation of the pathway and an uncontrolled proliferation of the basal cells. Frequently, these alterations have been shown to cause loss of function of PTCH homologue 1, which usually acts to inhibit the SMO homologue signaling activity.11,12
Because of the potential importance of Hh signaling in other solid malignancies and the failure of topical inhibition of SMO,13 subsequent studies on the development of Hh pathway inhibitors have mostly focused on the systemic approach. A multitude of Hh pathway inhibitors have been developed thus far, such as SANT1-SANT4, GDC-0449, IPI-926, BMS-833923 (XL139), HhAntag-691, and MK-4101.14 Many of these inhibitors have been clinically investigated.13,15,16
Systemic SMO Inhibitor: Vismodegib
Vismodegib was the earliest systemic SMO inhibitor to fulfill early clinical evaluation15,16 and the first drug to receive FDA approval for the management of advanced or metastatic BCC. Vismodegib is a small-molecule SMO inhibitor used for the management of selected locally advanced BCC and metastatic BCC in adults.3,17 Although there is a possibility of recurrence following drug withdrawal, vismodegib constitutes a new therapeutic strategy presenting positive benefits to patients. It may provide superior improvement over sunitinib, which has shown efficacy in a few patients; however, the efficacy and tolerance of sunitinib have been shown to be limited.18,19
Adverse Effects of Vismodegib Therapy
Adverse events with vismodegib use have been reported in 98% of patients (N=491); most of these were mild to moderate.20 However, the frequency of adverse events could prove to be a therapeutic challenge for patients requiring extended treatment. The most frequently reported reversible side effects were muscle spasms (64%), alopecia (62%), weight loss (33%), fatigue (28%), decreased appetite (25%), diarrhea (17%), nausea (16%), dysgeusia (54%), and ageusia (22%).20 In clinical trials, amenorrhea was noticed in 30% (3/10) of females with reproductive potential.2 Apart from alopecia and possibly amenorrhea, these side effects are reversible.17 Alkeraye et al17 reported 3 clinical cases of persistent alopecia following the use of vismodegib. Amenorrhea is a possible side effect of unknown reversibility.7
Vismodegib is a pregnancy category D medication.4 Severe birth defects, including craniofacial abnormalities, retardations in normal growth, open perineum, and absence of digital fusion at a corresponding 20% of the recommended daily dose, were found in rat studies. Embryo-fetal death was noted when rats were exposedto concentrations comparable to the recommended human dose.4
Hepatic events with the use of vismodegib have been reported. The use of vismodegib in randomized controlled trials resulted in elevation of both alanine aminotransferase and aspartate aminotransferase levels compared with placebo.21 Moreover, severe hepatotoxicity with vismodegib has been reported.22-24 A study conducted by Edwards et al25 concluded that the use of vismodegib in patients with severe liver disease must include thorough risk-benefit assessment, with caution in using other concomitant hepatotoxic medications.
Rare adverse events also have been reported in the literature, including vismodegib-induced pancreatitis in a 79-year-old patient treated for locally advanced, recurrent BCC that was cleared following cessation of therapy.26 Additionally, atypical fibroxanthoma was observed in an 83-year-old patient after 30 days of treatment with vismodegib for multiple BCCs.27 The development of other secondary malignancies, such as squamous cell carcinoma, melanoma, keratoacanthomas, and pilomatricomas, during or after the long-term use of vismodegib also have been described.28-35
Use of Vismodegib for Adnexal Skin Tumors
The role of the sonic Hh–PTCH pathway in the pathogenesis of adnexal tumors varies in the literature. Some studies propose the involvement of this pathway in the formation of adnexal tumors such as trichoblastoma, trichoepithelioma, and cylindroma, as in BCC. Various lines of evidence support this involvement. Firstly, in mice, the spontaneous generation of numerous BCCs, trichoblastomas, trichoepitheliomas, and cylindromas has been observed following constitutive activation of the sonic Hh–PTCH pathway.36 Secondly, in trichoepitheliomas, there have been positive results in molecular research into the tumor suppressor gene PTCH homologue 1, PTCH1, whose mutations cause constitutive activation of the sonic Hh–PTCH pathway.37 Thirdly, GLI138 and SOX939 transcription factors associated with the signaling pathway of sonic Hh–PTCH appear to have increased levels in adnexal carcinomas.19 Lepesant et al19 reported a notable clinical response to vismodegib in trichoblastic carcinoma. Baur et al40 reported successful treatment of multiple familial trichoepitheliomas with vismodegib. Nonetheless, more studies are required to assess the efficacy and reliability of vismodegib in the management of adnexal tumors.
Recommended Dose of Vismodegib Therapy
The vismodegib dosage that is approved by the FDA is 150 mg/d until disease progression or the development of intolerable side effects.4 Higher dosing regimens were evaluated with 270 mg/d and 540 mg/d. No added therapeutic benefit was noted with the increase in the dose, and no dose-limiting toxic effects were observed.41
Management of Vismodegib Side Effects
Managing patient expectations is a crucial step in improving dysgeusia. The experience of dysgeusia varies among patients; thus, patients should be instructed to adjust their diets according to their level of dysgeusia, which can be achieved by changing ingredients or dressings used with their diet. This step has been proven to be effective in overcoming vismodegib-related dysgeusia. Also, fluid taste distortion may lead to dehydration and an increase in creatine level. Thus, patients should be encouraged to monitor fluid intake. Moreover, a treatment hiatus of 2 to 8 months results in near-complete improvement of taste distortion.
For muscle spasms, quinine, treatment break for 1 month, gentle exercise of affected areas, or muscle relaxants such as baclofen and temazepam all are effective methods. For vismodegib-related alopecia, managing patient expectations is key; patients should be aware that hair may take 6 to 12 months or even longer to regrow. In addition, shaving less frequently helps improve alopecia.
For gastrointestinal disorders, loperamide with or without codeine phosphate is effective in resolving diarrhea, and metoclopramide is mostly adequate in treating nausea. Another adverse event is weight loss; weight loss of 5% or more of total body weight prompts dietetic referral. If weight loss persists, a treatment break might be needed to regain weight.
Overall, treatment breaks are sufficient to resolve adverse events caused by vismodegib and do not compromise efficacy of treatment. The duration of a treatment break should be considered before initiation. In one clinical trial, a longer treatment break was associated with fewer adverse effects without affecting the efficacy of treatment.42
Conclusion
Vismodegib provides an effective alternative to surgical intervention in the management of BCC. However, patients must be monitored vigilantly, as adverse events are common (>90%).
Basal cell carcinomas (BCCs) are considered the most common cutaneous cancers. Approximately 80% of nonmelanoma skin cancers are BCCs.1,2 Surgical management is the gold standard for early-stage and localized BCCs; it may include simple excision vs Mohs micrographic surgery.3,4 However, if left untreated, these lesions can progress to an advanced stage (locally advanced BCC) or infrequently may spread to distant sites (metastatic BCC). In the advanced stage, the lesions are no longer manageable by surgery or radiation therapy.5,6 Recently, inhibitors targeting the hedgehog (Hh) pathway have shown great promise for these patients. The first drug approved by the US Food and Drug Administration (FDA) for locally advanced and metastatic BCC is vismodegib.7 In this article, we provide a clinical review of vismodegib for the management of BCC, including a discussion of the Hh pathway in BCC, adverse effects of vismodegib, use of vismodegib in adnexal skin tumors, recommended doses for vismodegib therapy in BCC, and management of the side effects of treatment.
Hh Pathway in BCC
In embryonic development, the Hh signaling pathway is crucial across a broad spectrum of species, including humans. Various members of the Hh family have been recognized, all working as secreted regulatory proteins.8 The name of the Hh signaling pathway is derived from a polypeptide ligand called hedgehog found in some fruit flies. Mutations in the gene led to fruit fly larvae that had a spiky hairy pattern of denticles similar to hedgehogs, leading to the name of this molecule.9 The transmembrane protein smoothened (SMO) is the main component of the Hh signaling pathway and initiates a signaling cascade that in turn leads to an increased expression of target genes, such as GLI1. Patched (PTCH), also a transmembrane protein and a cell-surface receptor for the secreted Hh ligand, suppresses the signaling capacity of SMO. Upon binding of the Hh ligand to the PTCH receptor, the suppression of SMO is relieved and a signal is propagated, evoking a cellular response.10 Molecular and genetic studies have reported that genetic alterations in the Hh signaling pathway are almost universally present in all BCCs, leading to an aberrant activation of the pathway and an uncontrolled proliferation of the basal cells. Frequently, these alterations have been shown to cause loss of function of PTCH homologue 1, which usually acts to inhibit the SMO homologue signaling activity.11,12
Because of the potential importance of Hh signaling in other solid malignancies and the failure of topical inhibition of SMO,13 subsequent studies on the development of Hh pathway inhibitors have mostly focused on the systemic approach. A multitude of Hh pathway inhibitors have been developed thus far, such as SANT1-SANT4, GDC-0449, IPI-926, BMS-833923 (XL139), HhAntag-691, and MK-4101.14 Many of these inhibitors have been clinically investigated.13,15,16
Systemic SMO Inhibitor: Vismodegib
Vismodegib was the earliest systemic SMO inhibitor to fulfill early clinical evaluation15,16 and the first drug to receive FDA approval for the management of advanced or metastatic BCC. Vismodegib is a small-molecule SMO inhibitor used for the management of selected locally advanced BCC and metastatic BCC in adults.3,17 Although there is a possibility of recurrence following drug withdrawal, vismodegib constitutes a new therapeutic strategy presenting positive benefits to patients. It may provide superior improvement over sunitinib, which has shown efficacy in a few patients; however, the efficacy and tolerance of sunitinib have been shown to be limited.18,19
Adverse Effects of Vismodegib Therapy
Adverse events with vismodegib use have been reported in 98% of patients (N=491); most of these were mild to moderate.20 However, the frequency of adverse events could prove to be a therapeutic challenge for patients requiring extended treatment. The most frequently reported reversible side effects were muscle spasms (64%), alopecia (62%), weight loss (33%), fatigue (28%), decreased appetite (25%), diarrhea (17%), nausea (16%), dysgeusia (54%), and ageusia (22%).20 In clinical trials, amenorrhea was noticed in 30% (3/10) of females with reproductive potential.2 Apart from alopecia and possibly amenorrhea, these side effects are reversible.17 Alkeraye et al17 reported 3 clinical cases of persistent alopecia following the use of vismodegib. Amenorrhea is a possible side effect of unknown reversibility.7
Vismodegib is a pregnancy category D medication.4 Severe birth defects, including craniofacial abnormalities, retardations in normal growth, open perineum, and absence of digital fusion at a corresponding 20% of the recommended daily dose, were found in rat studies. Embryo-fetal death was noted when rats were exposedto concentrations comparable to the recommended human dose.4
Hepatic events with the use of vismodegib have been reported. The use of vismodegib in randomized controlled trials resulted in elevation of both alanine aminotransferase and aspartate aminotransferase levels compared with placebo.21 Moreover, severe hepatotoxicity with vismodegib has been reported.22-24 A study conducted by Edwards et al25 concluded that the use of vismodegib in patients with severe liver disease must include thorough risk-benefit assessment, with caution in using other concomitant hepatotoxic medications.
Rare adverse events also have been reported in the literature, including vismodegib-induced pancreatitis in a 79-year-old patient treated for locally advanced, recurrent BCC that was cleared following cessation of therapy.26 Additionally, atypical fibroxanthoma was observed in an 83-year-old patient after 30 days of treatment with vismodegib for multiple BCCs.27 The development of other secondary malignancies, such as squamous cell carcinoma, melanoma, keratoacanthomas, and pilomatricomas, during or after the long-term use of vismodegib also have been described.28-35
Use of Vismodegib for Adnexal Skin Tumors
The role of the sonic Hh–PTCH pathway in the pathogenesis of adnexal tumors varies in the literature. Some studies propose the involvement of this pathway in the formation of adnexal tumors such as trichoblastoma, trichoepithelioma, and cylindroma, as in BCC. Various lines of evidence support this involvement. Firstly, in mice, the spontaneous generation of numerous BCCs, trichoblastomas, trichoepitheliomas, and cylindromas has been observed following constitutive activation of the sonic Hh–PTCH pathway.36 Secondly, in trichoepitheliomas, there have been positive results in molecular research into the tumor suppressor gene PTCH homologue 1, PTCH1, whose mutations cause constitutive activation of the sonic Hh–PTCH pathway.37 Thirdly, GLI138 and SOX939 transcription factors associated with the signaling pathway of sonic Hh–PTCH appear to have increased levels in adnexal carcinomas.19 Lepesant et al19 reported a notable clinical response to vismodegib in trichoblastic carcinoma. Baur et al40 reported successful treatment of multiple familial trichoepitheliomas with vismodegib. Nonetheless, more studies are required to assess the efficacy and reliability of vismodegib in the management of adnexal tumors.
Recommended Dose of Vismodegib Therapy
The vismodegib dosage that is approved by the FDA is 150 mg/d until disease progression or the development of intolerable side effects.4 Higher dosing regimens were evaluated with 270 mg/d and 540 mg/d. No added therapeutic benefit was noted with the increase in the dose, and no dose-limiting toxic effects were observed.41
Management of Vismodegib Side Effects
Managing patient expectations is a crucial step in improving dysgeusia. The experience of dysgeusia varies among patients; thus, patients should be instructed to adjust their diets according to their level of dysgeusia, which can be achieved by changing ingredients or dressings used with their diet. This step has been proven to be effective in overcoming vismodegib-related dysgeusia. Also, fluid taste distortion may lead to dehydration and an increase in creatine level. Thus, patients should be encouraged to monitor fluid intake. Moreover, a treatment hiatus of 2 to 8 months results in near-complete improvement of taste distortion.
For muscle spasms, quinine, treatment break for 1 month, gentle exercise of affected areas, or muscle relaxants such as baclofen and temazepam all are effective methods. For vismodegib-related alopecia, managing patient expectations is key; patients should be aware that hair may take 6 to 12 months or even longer to regrow. In addition, shaving less frequently helps improve alopecia.
For gastrointestinal disorders, loperamide with or without codeine phosphate is effective in resolving diarrhea, and metoclopramide is mostly adequate in treating nausea. Another adverse event is weight loss; weight loss of 5% or more of total body weight prompts dietetic referral. If weight loss persists, a treatment break might be needed to regain weight.
Overall, treatment breaks are sufficient to resolve adverse events caused by vismodegib and do not compromise efficacy of treatment. The duration of a treatment break should be considered before initiation. In one clinical trial, a longer treatment break was associated with fewer adverse effects without affecting the efficacy of treatment.42
Conclusion
Vismodegib provides an effective alternative to surgical intervention in the management of BCC. However, patients must be monitored vigilantly, as adverse events are common (>90%).
- Sekulic A, Migden MR, Oro AE, et al. Efficacy and safety of vismodegib in advanced basal-cell carcinoma. N Engl J Med. 2012;366:2171-2179.
- Rogers HW, Weinstock MA, Harris AR, et al. Incidence estimate of nonmelanoma skin cancer in the United States, 2006. Arch Dermatol. 2010;146:283-287.
- Von Hoff DD, LoRusso PM, Rudin CM, et al. Inhibition of the hedgehog pathway in advanced basal-cell carcinoma. N Engl J Med. 2009;361:1164-1172.
- Cirrone F, Harris CS. Vismodegib and the hedgehog pathway: a new treatment for basal cell carcinoma. Clin Ther. 2012;34:2039-2050.
- Ruiz-Salas V, Alegre M, López-Ferrer A, et al. Vismodegib: a review [article in English, Spanish]. Actas Dermosifiliogr. 2014;105:744-751.
- Rubin AI, Chen EH, Ratner D. Basal-cell carcinoma. N Engl J Med. 2005;353:2262-2269.
- Cusack CA, Nijhawan R, Miller B, et al. Vismodegib for locally advanced basal cell carcinoma in a heart transplant patient. JAMA Dermatol. 2015;151:70-72.
- Aszterbaum M, Rothman A, Johnson RL, et al. Identification of mutations in the human PATCHED gene in sporadic basal cell carcinomas and in patients with the basal cell nevus syndrome. J Invest Dermatol. 1998;110:885-888.
- Abidi A. Hedgehog signaling pathway: a novel target for cancer therapy: vismodegib, a promising therapeutic option in treatment of basal cell carcinomas. Indian J Pharmacol. 2014;46:3-12.
- St-Jacques B, Dassule HR, Karavanova I, et al. Sonic hedgehog signaling is essential for hair development. Curr Biol. 1998;8:1058-1068.
- Gailani MR, Ståhle-Bäckdahl M, Leffell DJ, et al. The role of the human homologue of Drosophila patched in sporadic basal cell carcinomas. Nat Genet. 1996;14:78-81.
- Hall JM, Bell ML, Finger TE. Disruption of sonic hedgehog signaling alters growth and patterning of lingual taste papillae. Dev Biol. 2003;255:263-277.
- Bai CB, Stephen D, Joyner AL. All mouse ventral spinal cord patterning by hedgehog is Gli dependent and involves an activator function of Gli3. Dev Cell. 2004;6:103-115.
- Wang B, Fallon JF, Beachy PA. Hedgehog-regulated processing of Gli3 produces an anterior/posterior repressor gradient in the developing vertebrate limb. Cell. 2000;100:423-434.
- Sekulic A, Mangold AR, Northfelt DW, et al. Advanced basal cell carcinoma of the skin: targeting the hedgehog pathway. Curr Opin Oncol. 2013;25:218-223.
- Ingham PW, Placzek M. Orchestrating ontogenesis: variations on a theme by sonic hedgehog. Nature Rev Genet. 2006;7:841-850.
- Alkeraye S, Maire C, Desmedt E, et al. Persistent alopecia induced by vismodegib. Br J Dermatol. 2015;172:1671-1672.
- Battistella M, Mateus C, Lassau N, et al. Sunitinib efficacy in the treatment of metastatic skin adnexal carcinomas: report of two patients with hidradenocarcinoma and trichoblastic carcinoma. J Eur Acad Dermatol Venereol. 2010;24:199-203.
- Lepesant P, Crinquette M, Alkeraye S, et al. Vismodegib induces significant clinical response in locally advanced trichoblastic carcinoma. Br J Dermatol. 2015;173:1059-1062.
- Basset-Seguin N, Hauschild A, Grob JJ, et al. Vismodegib in patients with advanced basal cell carcinoma (STEVIE): a pre-plannedinterim analysis of an international, open-label trial. Lancet Oncol. 2015;16:729-736.
- Catenacci DV, Junttila MR, Karrison T, et al. Randomized phase Ib/II study of gemcitabine plus placebo or vismodegib, a hedgehog pathway inhibitor, in patients with metastatic pancreatic cancer. J Clin Oncol. 2015;33:4284-4292.
- Sanchez BE, Hajjafar L. Severe hepatotoxicity in a patient treated with hedgehog inhibitor: first case report. Gastroenterology. 2011;140:S974-S975.
- Ly P, Wolf K, Wilson J. A case of hepatotoxicity associated with vismodegib. JAAD Case Rep. 2018;5:57-59.
- Eiger-Moscovich M, Reich E, Tauber G, et al. Efficacy of vismodegib for the treatment of orbital and advanced periocular basal cell carcinoma. Am J Ophthalmol. 2019;207:62-70.
- Edwards BJ, Raisch DW, Saraykar SS, et al. Hepatotoxicity with vismodegib: an MD Anderson Cancer Center and Research on Adverse Drug Events and Reports Project. Drugs R D. 2017;17:211-218.
- Velter C, Blanc J, Robert C. Acute pancreatitis after vismodegib for basal cell carcinoma: a causal relation? Eur J Cancer. 2019;118:67-69.
- Giorgini C, Barbaccia V, Croci GA, et al. Rapid development of atypical fibroxanthoma during vismodegib treatment. Clin Exp Dermatol. 2019;44:86-88.
- Saintes C, Saint-Jean M, Brocard A, et al. Development of squamous cell carcinoma into basal cell carcinoma under treatment with vismodegib. J Eur Acad Dermatol Venereol. 2015;29:1006-1009.
- Zhu GA, Sundram U, Chang ALS. Two different scenarios of squamous cell carcinoma within advanced basal cell carcinomas: cases illustrating the importance of serial biopsy during vismodegib usage. JAMA Dermatol. 2014;150:970-973.
- Poulalhon N, Dalle S, Balme B, et al. Fast-growing cutaneous squamous cell carcinoma in a patient treated with vismodegib. Dermatology. 2015;230:101-104.
- Orouji A, Goerdt S, Utikal J, et al. Multiple highly and moderately differentiated squamous cell carcinomas of the skin during vismodegib treatment of inoperable basal cell carcinoma. Br J Dermatol. 2014;171:431-433.
- Iarrobino A, Messina JL, Kudchadkar R, et al. Emergence of a squamous cell carcinoma phenotype following treatment of metastatic basal cell carcinoma with vismodegib. J Am Acad Dermatol. 2013;69:E33-E34.
- Giuffrida R, Kashofer K, Dika E, et al. Fast growing melanoma following treatment with vismodegib for locally advanced basal cell carcinomas: report of two cases. Eur J Cancer. 2018;91:177-179.
- Aasi S, Silkiss R, Tang JY, et al. New onset of keratoacanthomas after vismodegib treatment for locally advanced basal cell carcinomas: a report of 2 cases. JAMA Dermatol. 2013;149:242-243.
- Magdaleno-Tapial J, Valenzuela-Oñate C, Ortiz-Salvador JM, et al. Pilomatricomas secondary to treatment with vismodegib. JAAD Case Rep. 2018;5:12-14.
- Nilsson M, Undèn AB, Krause D, et al. Induction of basal cell carcinomas and trichoepitheliomas in mice overexpressing GLI-1. Proc Natl Acad Sci U S A. 2000;97:3438-3443.
- Vorechovský I, Undén AB, Sandstedt B, et al. Trichoepitheliomas contain somatic mutations in the overexpressed PTCH gene: support for a gatekeeper mechanism in skin tumorigenesis. Cancer Res. 1997;57:4677-4681.
- Hatta N, Hirano T, Kimura T, et al. Molecular diagnosis of basal cell carcinoma and other basaloid cell neoplasms of the skin by the quantification of Gli1 transcript levels. J Cutan Pathol. 2005;32:131-136.
- Vidal VP, Ortonne N, Schedl A. SOX9 expression is a general marker of basal cell carcinoma and adnexal-related neoplasms. J Cutan Pathol. 2008;35:373-379.
- Baur V, Papadopoulos T, Kazakov DV, et al. A case of multiple familial trichoepitheliomas responding to treatment with the hedgehog signaling pathway inhibitor vismodegib. Virchows Arch. 2018;473:241-246.
- LoRusso PM, Rudin CM, Reddy JC, et al. Phase I trial of hedgehog pathway inhibitor vismodegib (GDC-0449) in patients with refractory, locally advanced or metastatic solid tumors. Clin Cancer Res. 2011;17:2502-2511.
- Fife K, Herd R, Lalondrelle S, et al. Managing adverse events associated with vismodegib in the treatment of basal cell carcinoma. Future Oncol. 2017;13:175-184.
- Sekulic A, Migden MR, Oro AE, et al. Efficacy and safety of vismodegib in advanced basal-cell carcinoma. N Engl J Med. 2012;366:2171-2179.
- Rogers HW, Weinstock MA, Harris AR, et al. Incidence estimate of nonmelanoma skin cancer in the United States, 2006. Arch Dermatol. 2010;146:283-287.
- Von Hoff DD, LoRusso PM, Rudin CM, et al. Inhibition of the hedgehog pathway in advanced basal-cell carcinoma. N Engl J Med. 2009;361:1164-1172.
- Cirrone F, Harris CS. Vismodegib and the hedgehog pathway: a new treatment for basal cell carcinoma. Clin Ther. 2012;34:2039-2050.
- Ruiz-Salas V, Alegre M, López-Ferrer A, et al. Vismodegib: a review [article in English, Spanish]. Actas Dermosifiliogr. 2014;105:744-751.
- Rubin AI, Chen EH, Ratner D. Basal-cell carcinoma. N Engl J Med. 2005;353:2262-2269.
- Cusack CA, Nijhawan R, Miller B, et al. Vismodegib for locally advanced basal cell carcinoma in a heart transplant patient. JAMA Dermatol. 2015;151:70-72.
- Aszterbaum M, Rothman A, Johnson RL, et al. Identification of mutations in the human PATCHED gene in sporadic basal cell carcinomas and in patients with the basal cell nevus syndrome. J Invest Dermatol. 1998;110:885-888.
- Abidi A. Hedgehog signaling pathway: a novel target for cancer therapy: vismodegib, a promising therapeutic option in treatment of basal cell carcinomas. Indian J Pharmacol. 2014;46:3-12.
- St-Jacques B, Dassule HR, Karavanova I, et al. Sonic hedgehog signaling is essential for hair development. Curr Biol. 1998;8:1058-1068.
- Gailani MR, Ståhle-Bäckdahl M, Leffell DJ, et al. The role of the human homologue of Drosophila patched in sporadic basal cell carcinomas. Nat Genet. 1996;14:78-81.
- Hall JM, Bell ML, Finger TE. Disruption of sonic hedgehog signaling alters growth and patterning of lingual taste papillae. Dev Biol. 2003;255:263-277.
- Bai CB, Stephen D, Joyner AL. All mouse ventral spinal cord patterning by hedgehog is Gli dependent and involves an activator function of Gli3. Dev Cell. 2004;6:103-115.
- Wang B, Fallon JF, Beachy PA. Hedgehog-regulated processing of Gli3 produces an anterior/posterior repressor gradient in the developing vertebrate limb. Cell. 2000;100:423-434.
- Sekulic A, Mangold AR, Northfelt DW, et al. Advanced basal cell carcinoma of the skin: targeting the hedgehog pathway. Curr Opin Oncol. 2013;25:218-223.
- Ingham PW, Placzek M. Orchestrating ontogenesis: variations on a theme by sonic hedgehog. Nature Rev Genet. 2006;7:841-850.
- Alkeraye S, Maire C, Desmedt E, et al. Persistent alopecia induced by vismodegib. Br J Dermatol. 2015;172:1671-1672.
- Battistella M, Mateus C, Lassau N, et al. Sunitinib efficacy in the treatment of metastatic skin adnexal carcinomas: report of two patients with hidradenocarcinoma and trichoblastic carcinoma. J Eur Acad Dermatol Venereol. 2010;24:199-203.
- Lepesant P, Crinquette M, Alkeraye S, et al. Vismodegib induces significant clinical response in locally advanced trichoblastic carcinoma. Br J Dermatol. 2015;173:1059-1062.
- Basset-Seguin N, Hauschild A, Grob JJ, et al. Vismodegib in patients with advanced basal cell carcinoma (STEVIE): a pre-plannedinterim analysis of an international, open-label trial. Lancet Oncol. 2015;16:729-736.
- Catenacci DV, Junttila MR, Karrison T, et al. Randomized phase Ib/II study of gemcitabine plus placebo or vismodegib, a hedgehog pathway inhibitor, in patients with metastatic pancreatic cancer. J Clin Oncol. 2015;33:4284-4292.
- Sanchez BE, Hajjafar L. Severe hepatotoxicity in a patient treated with hedgehog inhibitor: first case report. Gastroenterology. 2011;140:S974-S975.
- Ly P, Wolf K, Wilson J. A case of hepatotoxicity associated with vismodegib. JAAD Case Rep. 2018;5:57-59.
- Eiger-Moscovich M, Reich E, Tauber G, et al. Efficacy of vismodegib for the treatment of orbital and advanced periocular basal cell carcinoma. Am J Ophthalmol. 2019;207:62-70.
- Edwards BJ, Raisch DW, Saraykar SS, et al. Hepatotoxicity with vismodegib: an MD Anderson Cancer Center and Research on Adverse Drug Events and Reports Project. Drugs R D. 2017;17:211-218.
- Velter C, Blanc J, Robert C. Acute pancreatitis after vismodegib for basal cell carcinoma: a causal relation? Eur J Cancer. 2019;118:67-69.
- Giorgini C, Barbaccia V, Croci GA, et al. Rapid development of atypical fibroxanthoma during vismodegib treatment. Clin Exp Dermatol. 2019;44:86-88.
- Saintes C, Saint-Jean M, Brocard A, et al. Development of squamous cell carcinoma into basal cell carcinoma under treatment with vismodegib. J Eur Acad Dermatol Venereol. 2015;29:1006-1009.
- Zhu GA, Sundram U, Chang ALS. Two different scenarios of squamous cell carcinoma within advanced basal cell carcinomas: cases illustrating the importance of serial biopsy during vismodegib usage. JAMA Dermatol. 2014;150:970-973.
- Poulalhon N, Dalle S, Balme B, et al. Fast-growing cutaneous squamous cell carcinoma in a patient treated with vismodegib. Dermatology. 2015;230:101-104.
- Orouji A, Goerdt S, Utikal J, et al. Multiple highly and moderately differentiated squamous cell carcinomas of the skin during vismodegib treatment of inoperable basal cell carcinoma. Br J Dermatol. 2014;171:431-433.
- Iarrobino A, Messina JL, Kudchadkar R, et al. Emergence of a squamous cell carcinoma phenotype following treatment of metastatic basal cell carcinoma with vismodegib. J Am Acad Dermatol. 2013;69:E33-E34.
- Giuffrida R, Kashofer K, Dika E, et al. Fast growing melanoma following treatment with vismodegib for locally advanced basal cell carcinomas: report of two cases. Eur J Cancer. 2018;91:177-179.
- Aasi S, Silkiss R, Tang JY, et al. New onset of keratoacanthomas after vismodegib treatment for locally advanced basal cell carcinomas: a report of 2 cases. JAMA Dermatol. 2013;149:242-243.
- Magdaleno-Tapial J, Valenzuela-Oñate C, Ortiz-Salvador JM, et al. Pilomatricomas secondary to treatment with vismodegib. JAAD Case Rep. 2018;5:12-14.
- Nilsson M, Undèn AB, Krause D, et al. Induction of basal cell carcinomas and trichoepitheliomas in mice overexpressing GLI-1. Proc Natl Acad Sci U S A. 2000;97:3438-3443.
- Vorechovský I, Undén AB, Sandstedt B, et al. Trichoepitheliomas contain somatic mutations in the overexpressed PTCH gene: support for a gatekeeper mechanism in skin tumorigenesis. Cancer Res. 1997;57:4677-4681.
- Hatta N, Hirano T, Kimura T, et al. Molecular diagnosis of basal cell carcinoma and other basaloid cell neoplasms of the skin by the quantification of Gli1 transcript levels. J Cutan Pathol. 2005;32:131-136.
- Vidal VP, Ortonne N, Schedl A. SOX9 expression is a general marker of basal cell carcinoma and adnexal-related neoplasms. J Cutan Pathol. 2008;35:373-379.
- Baur V, Papadopoulos T, Kazakov DV, et al. A case of multiple familial trichoepitheliomas responding to treatment with the hedgehog signaling pathway inhibitor vismodegib. Virchows Arch. 2018;473:241-246.
- LoRusso PM, Rudin CM, Reddy JC, et al. Phase I trial of hedgehog pathway inhibitor vismodegib (GDC-0449) in patients with refractory, locally advanced or metastatic solid tumors. Clin Cancer Res. 2011;17:2502-2511.
- Fife K, Herd R, Lalondrelle S, et al. Managing adverse events associated with vismodegib in the treatment of basal cell carcinoma. Future Oncol. 2017;13:175-184.
Practice Points
- The recommended dosage of vismodegib is 150 mg/d until unendurable side effects develop or disease progression occurs.
- The efficacy of vismodegib in the management of locally advanced basal cell carcinoma (BCC) and metastatic BCC is promising. Thus, it is now considered an effective substitute to surgical therapy.
- Patients using vismodegib must be closely monitored, as it is commonly associated with adverse events.
Utilization and Clinical Benefit of Immune Checkpoint Inhibitor in Veterans With Microsatellite Instability-High Prostate Cancer
Background
The utilization of immune checkpoint inhibitors (ICI) in prostate cancer (PC) can be very effective for patients with mismatch repair-deficiency (as identified by MSI-H by PCR/NGS or dMMR IHC). The use of ICI in this patient population has been associated with high rates of durable response. There is limited published data on factors that may influence patient response and outcomes. The aim of this study is to describe the utilization of and tumor response to ICI in this patient population.
Methods
This is a retrospective study of men with MSI-H PC reported by somatic genomic testing from April 1, 2015 to March 31, 2022 through the VA National Precision Oncology Program (NPOP), who received at least one dose of ICI. The primary objectives are to describe the incidence of MSI-H PC and the utilization of ICI. Descriptive statistics and Kaplan- Meier estimator were used for secondary objectives to determine the prostate-specific antigen decline of at least 50% (PSA50), clinical progression free survival (cPFS), time on ICI as a function of number of prior therapies, the extent of metastasis prior to initiation of ICI, and the correlation of MMR genetic alterations with treatment response.
Results
66 patients with MSI-H PC were identified (1.5% of a total of 4267 patients with PC tested through NPOP). 23 patients (35%) received at least one dose of ICI. 12 of 23 patients (52%) had PSA response. PSA50 responses occurred in 6 patients (50%) and 5 continued to have durable PSA50 at six months. Median cPFS was 280 days (95% CI: 105 days-not reached) and the estimated PFS at six months was 72.2% (95% CI: 35.7%-90.2%). 8 of 12 (67%) responders have received multiple lines of therapy for M1 PC. 8 of 12 patients (67%) had high-volume disease at ICI initiation. Of those patients with a MMR genetic alteration, patients with MLH1 (3/3) and MSH2 (6/8) alterations responded more frequently than those with MSH6 alterations (1/4).
Conclusions
MSI-H PC is rare but response rates to ICI are high and durable. Patients with MLH1 and MSH2 alterations appeared to respond more frequently than those with MSH6. Additional follow-up is ongoing.
Background
The utilization of immune checkpoint inhibitors (ICI) in prostate cancer (PC) can be very effective for patients with mismatch repair-deficiency (as identified by MSI-H by PCR/NGS or dMMR IHC). The use of ICI in this patient population has been associated with high rates of durable response. There is limited published data on factors that may influence patient response and outcomes. The aim of this study is to describe the utilization of and tumor response to ICI in this patient population.
Methods
This is a retrospective study of men with MSI-H PC reported by somatic genomic testing from April 1, 2015 to March 31, 2022 through the VA National Precision Oncology Program (NPOP), who received at least one dose of ICI. The primary objectives are to describe the incidence of MSI-H PC and the utilization of ICI. Descriptive statistics and Kaplan- Meier estimator were used for secondary objectives to determine the prostate-specific antigen decline of at least 50% (PSA50), clinical progression free survival (cPFS), time on ICI as a function of number of prior therapies, the extent of metastasis prior to initiation of ICI, and the correlation of MMR genetic alterations with treatment response.
Results
66 patients with MSI-H PC were identified (1.5% of a total of 4267 patients with PC tested through NPOP). 23 patients (35%) received at least one dose of ICI. 12 of 23 patients (52%) had PSA response. PSA50 responses occurred in 6 patients (50%) and 5 continued to have durable PSA50 at six months. Median cPFS was 280 days (95% CI: 105 days-not reached) and the estimated PFS at six months was 72.2% (95% CI: 35.7%-90.2%). 8 of 12 (67%) responders have received multiple lines of therapy for M1 PC. 8 of 12 patients (67%) had high-volume disease at ICI initiation. Of those patients with a MMR genetic alteration, patients with MLH1 (3/3) and MSH2 (6/8) alterations responded more frequently than those with MSH6 alterations (1/4).
Conclusions
MSI-H PC is rare but response rates to ICI are high and durable. Patients with MLH1 and MSH2 alterations appeared to respond more frequently than those with MSH6. Additional follow-up is ongoing.
Background
The utilization of immune checkpoint inhibitors (ICI) in prostate cancer (PC) can be very effective for patients with mismatch repair-deficiency (as identified by MSI-H by PCR/NGS or dMMR IHC). The use of ICI in this patient population has been associated with high rates of durable response. There is limited published data on factors that may influence patient response and outcomes. The aim of this study is to describe the utilization of and tumor response to ICI in this patient population.
Methods
This is a retrospective study of men with MSI-H PC reported by somatic genomic testing from April 1, 2015 to March 31, 2022 through the VA National Precision Oncology Program (NPOP), who received at least one dose of ICI. The primary objectives are to describe the incidence of MSI-H PC and the utilization of ICI. Descriptive statistics and Kaplan- Meier estimator were used for secondary objectives to determine the prostate-specific antigen decline of at least 50% (PSA50), clinical progression free survival (cPFS), time on ICI as a function of number of prior therapies, the extent of metastasis prior to initiation of ICI, and the correlation of MMR genetic alterations with treatment response.
Results
66 patients with MSI-H PC were identified (1.5% of a total of 4267 patients with PC tested through NPOP). 23 patients (35%) received at least one dose of ICI. 12 of 23 patients (52%) had PSA response. PSA50 responses occurred in 6 patients (50%) and 5 continued to have durable PSA50 at six months. Median cPFS was 280 days (95% CI: 105 days-not reached) and the estimated PFS at six months was 72.2% (95% CI: 35.7%-90.2%). 8 of 12 (67%) responders have received multiple lines of therapy for M1 PC. 8 of 12 patients (67%) had high-volume disease at ICI initiation. Of those patients with a MMR genetic alteration, patients with MLH1 (3/3) and MSH2 (6/8) alterations responded more frequently than those with MSH6 alterations (1/4).
Conclusions
MSI-H PC is rare but response rates to ICI are high and durable. Patients with MLH1 and MSH2 alterations appeared to respond more frequently than those with MSH6. Additional follow-up is ongoing.
Castration-Resistant Prostate Cancer—Not Only Challenging to Treat, but Difficult to Define
Purpose
Examine the impact of different definitions of castration resistance used to identify patients with castration-resistant prostate cancer (CRPC) using electronic health records (EHR).
Background
CRPC is a form of prostate cancer that is resistant to treatment with androgen deprivation therapy (ADT) and is associated with higher morbidity and mortality. Widely used guidelines like the Prostate Cancer Working Group 3 (PCWG 3), the American Urological Association (AUA), and many others differ in their definitions of castration-resistance. Until now, the feasibility of identifying CRPC using different definitions from EHR data has not been studied.
Methods/Data Analyisis
EHR data from the Veterans Health Administration (01/2006-12/2020) were used to identify veterans with CRPC according to the following criteria: 1) PCWG 3—a PSA increase ?25% from the nadir with a minimum rise of 2 ng/mL, while castrate (testosterone < 50 ng/mL); 2) AUA—2 consecutive PSA rises of ?0.2 ng/mL; 3) CRPC screening—a PSA rise of > 0.0 ng/mL within a window of 7–90 days.
Results
36,101 unique patients were identified using 1 of (or a combination of) the 3 CRPC criteria. Approximately 12,775 (35%) patients met all 3 criteria, while 8,589 (24%) were identified by AUA, 4,785 (13%) by CRPC screening, and 145 (0.4%) by PCWG3. A total of 8,377 (23%) patients met both the AUA and CRPC screening criteria, 1,219 (3%) patients met the AUA and PCWG3 criteria, and 211 (1%) met the PCWG3 and CRPC screening criteria.
Conculsions/Implications
Although several definitions can be used to identify CRPC patients, a combination of these definitions results in the greatest yield of CRPC patients identified using EHR data. Even though the PCWG3 criterion is frequently used in both clinical trials research and retrospective observational research, PCWG3 may miss many patients meeting other criteria and should not be used by itself when studying patients with CRPC identified from EHR data.
Purpose
Examine the impact of different definitions of castration resistance used to identify patients with castration-resistant prostate cancer (CRPC) using electronic health records (EHR).
Background
CRPC is a form of prostate cancer that is resistant to treatment with androgen deprivation therapy (ADT) and is associated with higher morbidity and mortality. Widely used guidelines like the Prostate Cancer Working Group 3 (PCWG 3), the American Urological Association (AUA), and many others differ in their definitions of castration-resistance. Until now, the feasibility of identifying CRPC using different definitions from EHR data has not been studied.
Methods/Data Analyisis
EHR data from the Veterans Health Administration (01/2006-12/2020) were used to identify veterans with CRPC according to the following criteria: 1) PCWG 3—a PSA increase ?25% from the nadir with a minimum rise of 2 ng/mL, while castrate (testosterone < 50 ng/mL); 2) AUA—2 consecutive PSA rises of ?0.2 ng/mL; 3) CRPC screening—a PSA rise of > 0.0 ng/mL within a window of 7–90 days.
Results
36,101 unique patients were identified using 1 of (or a combination of) the 3 CRPC criteria. Approximately 12,775 (35%) patients met all 3 criteria, while 8,589 (24%) were identified by AUA, 4,785 (13%) by CRPC screening, and 145 (0.4%) by PCWG3. A total of 8,377 (23%) patients met both the AUA and CRPC screening criteria, 1,219 (3%) patients met the AUA and PCWG3 criteria, and 211 (1%) met the PCWG3 and CRPC screening criteria.
Conculsions/Implications
Although several definitions can be used to identify CRPC patients, a combination of these definitions results in the greatest yield of CRPC patients identified using EHR data. Even though the PCWG3 criterion is frequently used in both clinical trials research and retrospective observational research, PCWG3 may miss many patients meeting other criteria and should not be used by itself when studying patients with CRPC identified from EHR data.
Purpose
Examine the impact of different definitions of castration resistance used to identify patients with castration-resistant prostate cancer (CRPC) using electronic health records (EHR).
Background
CRPC is a form of prostate cancer that is resistant to treatment with androgen deprivation therapy (ADT) and is associated with higher morbidity and mortality. Widely used guidelines like the Prostate Cancer Working Group 3 (PCWG 3), the American Urological Association (AUA), and many others differ in their definitions of castration-resistance. Until now, the feasibility of identifying CRPC using different definitions from EHR data has not been studied.
Methods/Data Analyisis
EHR data from the Veterans Health Administration (01/2006-12/2020) were used to identify veterans with CRPC according to the following criteria: 1) PCWG 3—a PSA increase ?25% from the nadir with a minimum rise of 2 ng/mL, while castrate (testosterone < 50 ng/mL); 2) AUA—2 consecutive PSA rises of ?0.2 ng/mL; 3) CRPC screening—a PSA rise of > 0.0 ng/mL within a window of 7–90 days.
Results
36,101 unique patients were identified using 1 of (or a combination of) the 3 CRPC criteria. Approximately 12,775 (35%) patients met all 3 criteria, while 8,589 (24%) were identified by AUA, 4,785 (13%) by CRPC screening, and 145 (0.4%) by PCWG3. A total of 8,377 (23%) patients met both the AUA and CRPC screening criteria, 1,219 (3%) patients met the AUA and PCWG3 criteria, and 211 (1%) met the PCWG3 and CRPC screening criteria.
Conculsions/Implications
Although several definitions can be used to identify CRPC patients, a combination of these definitions results in the greatest yield of CRPC patients identified using EHR data. Even though the PCWG3 criterion is frequently used in both clinical trials research and retrospective observational research, PCWG3 may miss many patients meeting other criteria and should not be used by itself when studying patients with CRPC identified from EHR data.
MYO1E DNA Methylation in U.S. Military Veterans With Adenocarcinoma of the Lung Is Associated With Increased Mortality Risk
Project Purpose
The aim is to assess the role of MYO1E in survival among veterans with lung adenocarcinoma (LUAD).
Background
Veterans have a higher smoking exposure than civilians; a higher incidence of lung cancer; and a younger age at diagnosis of lung cancer. We recently showed that MYO1E DNA methylation and RNA expression in LUAD are associated with survival among civilians.
Methods
This is a retrospective cohort study involving LUAD among civilians and veterans with biopsy or pathologically proven LUAD from surgical specimens. DNA extraction and isolation from FFPE cancer tissues was performed using methylation-onbeads as previously published, followed by qMSP with bisulfite treatment to quantify DNA methylation. RNA extraction and quantification from lung tissues was obtained as described in previous publications.
Data Analysis
Differences were assessed with Wilcoxon rank sum test for continuous variables and Fisher’s exact test for categorical. Two-tailed log-rank test was used to estimate overall survival differences and Cox hazard models, to quantify risk of mortality using hazard ratios (HRs) with 95% confidence intervals (CIs).
Results
There were 91 LUAD patients, 27 veterans and 64 civilians. Veterans were older than civilians, aged 70 years vs aged 66 years (P = .003); with higher proportions of males, 93% vs 69% (P = .03); higher proportion of African Americans, 67% vs 39% (P = .03); smoking more, 50 pack-year vs 40 (0.005), and having a higher proportion of grade I, 78% vs 55% (P = .036). Survival was statistically longer for MYO1E high DNA methylation group 48 months vs 33 for low methylation (P = .049). MYO1E RNA expression did not show statistically significant differences (P = .32). Multivariate Cox regression analysis adjusted by age, veteran/civil status, gender, race, packyear, and stage showed that DNA methylation was significantly associated with mortality risk (HR 5.14; 95% CI, 1.12-23.60) (P = .035).
Conclusions/Implications
This study suggests the utility of MYO1E DNA methylation as a prognostic biomarker for veterans with LUAD. Further studies are necessary to understand the role of MYO1E in chemotherapy resistance and microenvironment immune modulation. Given the low expression of MYO1E in blood cells, MYO1E DNA methylation has the potential to be used as circulating tumor marker in liquid biopsies.
Project Purpose
The aim is to assess the role of MYO1E in survival among veterans with lung adenocarcinoma (LUAD).
Background
Veterans have a higher smoking exposure than civilians; a higher incidence of lung cancer; and a younger age at diagnosis of lung cancer. We recently showed that MYO1E DNA methylation and RNA expression in LUAD are associated with survival among civilians.
Methods
This is a retrospective cohort study involving LUAD among civilians and veterans with biopsy or pathologically proven LUAD from surgical specimens. DNA extraction and isolation from FFPE cancer tissues was performed using methylation-onbeads as previously published, followed by qMSP with bisulfite treatment to quantify DNA methylation. RNA extraction and quantification from lung tissues was obtained as described in previous publications.
Data Analysis
Differences were assessed with Wilcoxon rank sum test for continuous variables and Fisher’s exact test for categorical. Two-tailed log-rank test was used to estimate overall survival differences and Cox hazard models, to quantify risk of mortality using hazard ratios (HRs) with 95% confidence intervals (CIs).
Results
There were 91 LUAD patients, 27 veterans and 64 civilians. Veterans were older than civilians, aged 70 years vs aged 66 years (P = .003); with higher proportions of males, 93% vs 69% (P = .03); higher proportion of African Americans, 67% vs 39% (P = .03); smoking more, 50 pack-year vs 40 (0.005), and having a higher proportion of grade I, 78% vs 55% (P = .036). Survival was statistically longer for MYO1E high DNA methylation group 48 months vs 33 for low methylation (P = .049). MYO1E RNA expression did not show statistically significant differences (P = .32). Multivariate Cox regression analysis adjusted by age, veteran/civil status, gender, race, packyear, and stage showed that DNA methylation was significantly associated with mortality risk (HR 5.14; 95% CI, 1.12-23.60) (P = .035).
Conclusions/Implications
This study suggests the utility of MYO1E DNA methylation as a prognostic biomarker for veterans with LUAD. Further studies are necessary to understand the role of MYO1E in chemotherapy resistance and microenvironment immune modulation. Given the low expression of MYO1E in blood cells, MYO1E DNA methylation has the potential to be used as circulating tumor marker in liquid biopsies.
Project Purpose
The aim is to assess the role of MYO1E in survival among veterans with lung adenocarcinoma (LUAD).
Background
Veterans have a higher smoking exposure than civilians; a higher incidence of lung cancer; and a younger age at diagnosis of lung cancer. We recently showed that MYO1E DNA methylation and RNA expression in LUAD are associated with survival among civilians.
Methods
This is a retrospective cohort study involving LUAD among civilians and veterans with biopsy or pathologically proven LUAD from surgical specimens. DNA extraction and isolation from FFPE cancer tissues was performed using methylation-onbeads as previously published, followed by qMSP with bisulfite treatment to quantify DNA methylation. RNA extraction and quantification from lung tissues was obtained as described in previous publications.
Data Analysis
Differences were assessed with Wilcoxon rank sum test for continuous variables and Fisher’s exact test for categorical. Two-tailed log-rank test was used to estimate overall survival differences and Cox hazard models, to quantify risk of mortality using hazard ratios (HRs) with 95% confidence intervals (CIs).
Results
There were 91 LUAD patients, 27 veterans and 64 civilians. Veterans were older than civilians, aged 70 years vs aged 66 years (P = .003); with higher proportions of males, 93% vs 69% (P = .03); higher proportion of African Americans, 67% vs 39% (P = .03); smoking more, 50 pack-year vs 40 (0.005), and having a higher proportion of grade I, 78% vs 55% (P = .036). Survival was statistically longer for MYO1E high DNA methylation group 48 months vs 33 for low methylation (P = .049). MYO1E RNA expression did not show statistically significant differences (P = .32). Multivariate Cox regression analysis adjusted by age, veteran/civil status, gender, race, packyear, and stage showed that DNA methylation was significantly associated with mortality risk (HR 5.14; 95% CI, 1.12-23.60) (P = .035).
Conclusions/Implications
This study suggests the utility of MYO1E DNA methylation as a prognostic biomarker for veterans with LUAD. Further studies are necessary to understand the role of MYO1E in chemotherapy resistance and microenvironment immune modulation. Given the low expression of MYO1E in blood cells, MYO1E DNA methylation has the potential to be used as circulating tumor marker in liquid biopsies.
Safety Profile of Mutant EGFR-TK Inhibitors in Advanced Non–Small Cell Lung Cancer: A Meta-analysis
Lung cancer has been the leading cause of cancer-related mortality for decades. It is also predicted to remain as the leading cause of cancer-related mortality through 2030.1 Platinum-based chemotherapy, including carboplatin and paclitaxel, was introduced 3 decades ago and revolutionized the management of advanced non–small cell lung cancer (NSCLC). A more recent advancement has been mutant epidermal growth factor receptor–tyrosine kinase (EGFR-TK) inhibitors.1 EGFR is a transmembrane protein that functions by transducing essential growth factor signaling from the extracellular milieu to the cell. As 60% of the advanced NSCLC expresses this receptor, blocking the mutant EGFR receptor was a groundbreaking development in the management of advanced NSCLC.2 Development of mutant EGFR-TK inhibitors has revolutionized the management of advanced NSCLC. This study was conducted to determine the safety profile of mutant EGFR-TK inhibitors in the management of advanced NSCLC.
Methods
This meta-analysis was conducted according to Cochrane Collaboration guidelines and reported as per Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines. The findings are summarized in the PRISMA flow diagram (Figure 1). Two authors (MZ and MM) performed a systematic literature search using databases such as MEDLINE (via PubMed), Embase, and Cochrane Library using the medical search terms and their respective entry words with the following search strategy: safety, “mutant EGFR-TK inhibitors,” advanced, “non–small cell,” “lung cancer,” “adverse effect,” and literature. Additionally, unpublished trials were identified from clinicaltrials.gov, and references of all pertinent articles were also scrutinized to ensure the inclusion of all relevant studies. The search was completed on June 1, 2021, and we only included studies available in English. Two authors (MM and MZ) independently screened the search results in a 2-step process based on predetermined inclusion/exclusion criteria. First, 890 articles were evaluated for relevance on title and abstract level, followed by full-text screening of the final list of 140 articles. Any disagreements were resolved by discussion or third-party review, and a total of 9 articles were included in the study.
The following eligibility criteria were used: original articles reporting adverse effects (AEs) of mutant EGFR-TK inhibitors in patients with advanced NSCLC compared with control groups receiving platinum-based chemotherapy. All the patients included in the study had an EGFR mutation but randomly assigned to either treatment or control group. All articles with subjective data on mutant EGFR-TK inhibitors AEs in patients with advanced NSCLC compared with control groups receiving platinum-based chemotherapy were included in the analysis. Only 9 articles qualified the aforementioned selection criteria for eligibility. All qualifying studies were nationwide inpatient or pooled clinical trials data. The reasons for exclusion of the other 71 articles were irrelevant (n = 31), duplicate (n = 13), reviews (n = 14), and poor data reporting (n = 12). Out of the 9 included studies, 9 studies showed correlation of AEs, including rash, diarrhea, nausea, and fatigue. Seven studies showed correlation of AEs including neutropenia, anorexia, and vomiting. Six studies showed correlation of anemia, cough, and stomatitis. Five studies showed correlation of elevated aspartate aminotransferase (AST), alanine aminotransferase (ALT), and leucopenia. Four studies showed correlation of fever between mutant EGFR-TK inhibitors and platinum-based chemotherapy.
The primary endpoints were reported AEs including rash, diarrhea, elevated ALT, elevated AST, stomatitis, nausea, leucopenia, fatigue, neutropenia, anorexia, anemia, cough, vomiting, and fever, respectively. Data on baseline characteristics and clinical outcomes were then extracted, and summary tables were created. Summary estimates of the clinical endpoints were then calculated with risk ratio (RR) and 95% confidence intervals (CIs) using the random-effects model. Heterogeneity between studies was examined with the Cochran Q I2 statistic which can be defined as low (25% to 50%), moderate (50% to 75%), or high (> 75%). Statistical analysis was performed using Comprehensive Meta-Analysis Software CMA Version 3.0.
Results
A total of 9 studies including 3415 patients (1775 in EGFR-TK inhibitor treatment group while 1640 patients in platinum-based chemotherapy control group) were included in the study. All 9 studies were phase III randomized control clinical trials conducted to compare the safety profile of mutant EGFR-TK inhibitors in patients with advanced NSCLC. Mean age was 61 years in both treatment and control groups. Further details on study and participant characteristics and safety profile including AEs are summarized in Tables 1 and 2. No evidence of publication bias was found.
Rash developed in 45.8% of patients in the treatment group receiving mutant EGFR-TK inhibitors vs only 5.6% of patients in the control group receiving platinum-based chemotherapy. Overall RR of 7.38 with the 95% CI noted, which was statistically significant, confirming higher rash event rates in patients receiving EGFR-TK inhibitors for their advanced NSCLC (Figure 2).
Diarrhea occurred in 33.6% of patients in the mutant EGFR-TK inhibitors treatment group vs 13.5% of patients in the control group receiving platinum-based chemotherapy. Overall RR of 2.63 and 95% CI was noted, which was statistically significant, confirming higher diarrheal rates in patients receiving EGFR-TK inhibitors for their advanced NSCLC (Figure 3).
Elevated ALT levels developed in 27.9% of patients in the treatment group receiving mutant EGFR-TK inhibitors compared with 15.1% of patients in the control group receiving platinum-based chemotherapy. Overall RR of 1.37 and 95% CI was noted, which was statistically significant, confirming higher ALT levels in patients receiving EGFR-TK inhibitors for their advanced NSCLC (Figure 4).
Elevated AST levels occurred in 40.7% of patients in the mutant EGFR-TK inhibitors treatment group vs 12.8% of patients in the control group receiving platinum-based chemotherapy. Overall RR of 1.77 and 95% CI was noted, which was statistically significant, confirming elevated AST levels in patients receiving EGFR-TK inhibitors for their advanced NSCLC (Figure 5).
Stomatitis developed in 17.2% of patients in the treatment group receiving mutant EGFR-TK inhibitors compared with 7.9% of patients in the control group receiving platinum-based chemotherapy. Overall RR of 1.53 and 95% CI was noted, which was statistically significant, confirming higher stomatitis event rates in patients receiving EGFR-TK inhibitors for their advanced NSCLC (Figure 6).
Nausea occurred in 16.5% of patients in the mutant EGFR-TK inhibitors group vs 42.5% of patients in the control group receiving platinum-based chemotherapy. Overall RR of 0.37 and 95% CI was noted, which was statistically significant, confirming higher nausea rates in patients receiving platinum-based chemotherapy compared with treatment group for their advanced NSCLC (Figure 7).
Leucopenia developed in 9.7% of patients in the mutant EGFR-TK inhibitors group compared with 51.3% of patients in the control group receiving platinum-based chemotherapy. Overall RR of 0.18 and 95% CI was noted, which was statistically significant, confirming higher leucopenia incidence in patients receiving platinum-based chemotherapy compared with treatment group for their advanced NSCLC (Figure 8).
Fatigue was reported in 17% of patients in the mutant EGFR-TK inhibitors group compared with 29.5% of patients in the control group receiving platinum-based chemotherapy. Overall RR of 0.59 and 95% CI was noted, which was statistically significant, confirming higher fatigue rates in patients receiving platinum-based chemotherapy compared with treatment group for their advanced NSCLC (Figure 9).
Neutropenia developed in 6.1% of patients in the mutant EGFR-TK inhibitors group vs 48.2% of patients in the control group receiving platinum-based chemotherapy. Overall RR of 0.11 and 95% CI was noted, which was statistically significant, confirming higher neutropenia rates in patients receiving platinum-based chemotherapy compared with the treatment group for their advanced NSCLC (Figure 10).
Anorexia developed in 21.3% of patients in the mutant EGFR-TK inhibitors group vs 31.4% of patients in the control group receiving platinum-based chemotherapy. Overall RR of 0.44 and 95% CI was noted, which was statistically significant, confirming higher anorexia rates in patients receiving platinum-based chemotherapy compared with the treatment group for their advanced NSCLC (Figure 11).
Anemia occurred in 8.7% of patients in the mutant EGFR-TK inhibitors group compared with 32.1% of patients in the control group receiving platinum-based chemotherapy. Overall RR of 0.24 and 95% CI was noted, which was statistically significant, confirming higher anorexia rates in patients receiving platinum-based chemotherapy compared with treatment for their advanced NSCLC (Figure 12).
Cough was reported in 17.8% of patients in the mutant EGFR-TK inhibitors group compared with 18.9% of patients in the control group receiving platinum-based chemotherapy. Overall RR of 0.99 and 95% CI was noted, which was statistically significant, confirming slightly higher cough rates in patients receiving platinum-based chemotherapy compared with treatment for their advanced NSCLC (Figure 13).
Vomiting developed in 11% of patients in the mutant EGFR-TK inhibitors group vs 30.1% of patients in the control group receiving platinum-based chemotherapy. Overall RR of 0.35 and 95% CI was noted, which was statistically significant, confirming higher vomiting rates in patients receiving platinum-based chemotherapy compared with the treatment group for their advanced NSCLC (Figure 14).
Fever occurred in 5.6% of patients in the mutant EGFR-TK inhibitors group compared with 30.1% of patients in the control group receiving platinum-based chemotherapy. Overall RR of 0.41 and 95% CI was noted, which was statistically significant, confirming higher fever rates in patients receiving platinum-based chemotherapy compared with the treatment group for their advanced NSCLC (Figure 15).
Discussion
Despite the advancement in the treatment of metastatic NSCLC, lung cancer stays as most common cause of cancer-related death in North America and European countries, as patients usually have an advanced disease at the time of diagnosis.3 In the past, platinum-based chemotherapy remained the standard of care for most of the patients affected with advanced NSCLC, but the higher recurrence rate and increase in frequency and intensity of AEs with platinum-based chemotherapy led to the development of targeted therapy for NSCLC, one of which includes
Smoking is the most common reversible risk factor associated with lung cancer. The EURTAC trial was the first perspective study in this regard, which compared safety and efficacy of mutant EGFR-TK inhibitors with platinum-based chemotherapy. Results analyzed in this study were in favor of mutant EGFR-TK inhibitors except in the group of former smokers.5 On the contrary, the OPTIMAL trial showed results in favor of mutant EGFR-TK inhibitors both in active and former smokers; this trial also confirmed the efficacy of mutant EGFR-TK inhibitors in European and Asian populations, confirming the rationale for routine testing of EGFR mutation in all the patients being diagnosed with advanced NSCLC.6 Similarly, osimertinib is one of the most recent mutant EGFR-TK inhibitors developed for the treatment of advanced NSCLC in patients with EGFR-positive receptors.
According to the FLAURA trial, patients receiving osimertinib showed significantly longer progression-free survival compared with platinum-based chemotherapy and early mutant EGFR-TK inhibitors. Median progression-free survival was noted to be 18.9 months, which showed 54% lower risk of disease progression in the treatment group receiving osimertinib.7 The ARCHER study emphasized a significant improvement in overall survival as well as progression-free survival among a patient population receiving dacomitinib compared with platinum-based chemotherapy.8,9
Being a potent targeted therapy, mutant EGFR-TK inhibitors do come with some AEs including diarrhea, which was seen in 33.6% of the patients receiving mutant EGFR-TK inhibitors in our study vs 53% in the chemotherapy group, as was observed in the study conducted by Pless and colleagues.10 Similarly, only 16.5% of patients receiving mutant EGFR-TK inhibitors developed nausea compared with 66% being observed in patients receiving chemotherapy. Correspondingly, only a small fraction of patients (9.7%) receiving mutant EGFR-TK inhibitors developed leucopenia, which was 10 times less reported in mutant EGFR-TK inhibitors compared with patients receiving chemotherapy having a percentage of 100%. A similar trend was reported for neutropenia and anemia in mutant EGFR-TK inhibitors with an incidence of 6.1% and 8.7%, compared with the platinum-based chemotherapy group in which the incidence was found to be 80% and 100%, respectively. It was concluded that platinum-based chemotherapy had played a vital role in the treatment of advanced NSCLC but at an expense of serious and severe AEs which led to discontinuation or withdrawal of treatment, leading to relapse and recurrence of lung cancer.10,11
Zhong and colleagues conducted a phase 2 randomized clinical trial comparing mutant EGFR-TK inhibitors with platinum-based chemotherapy. They concluded that in patients receiving platinum-based chemotherapy, incidence of rash, vomiting, anorexia, neutropenia, and nausea were 29.4%, 47%, 41.2%, 55.8%, and 32.4% compared with 45.8%, 11%, 21.3%, 6.1%, and 16.5%, respectively, reported in patients receiving mutant EGFR-TK inhibitors for their advanced NSCLC.12
Another study was conducted in 2019 by Noronha and colleagues to determine the impact of platinum-based chemotherapy combined with gefitinib on patients with advanced NSCLC.13 They concluded that 70% of the patients receiving combination treatment developed rash, which was significantly higher compared with 45.8% patients receiving the mutant EGFR-TK inhibitors alone in our study. Also, 56% of patients receiving combination therapy developed diarrhea vs 33.6% of patients receiving mutant EGFR-TK inhibitors only. Similarly, 96% of patients in the combination therapy group developed some degree of anemia compared with only 8.7% patients in the mutant EGFR-TK inhibitors group included in our study. In the same way, neutropenia was observed in 55% of patients receiving combination therapy vs 6.1% in patients receiving mutant EGFR-TK inhibitors solely. They concluded that mutant EGFR-TK inhibitors when combined with platinum-based chemotherapy increase the incidence of AEs of chemotherapy by many folds.13,14
Kato and colleagues conducted a study to determine the impact on AEs when erlotinib was combined with anti–vascular endothelial growth factor (VEGF) inhibitors like bevacizumab, they stated that 98.7% of patient in combination therapy developed rash, the incidence of which was only 45.8% in patients receiving mutant EGFR-TK inhibitors as was observed in our study. Similar trends were noticed with other AEs, including diarrhea, fatigue, nausea, and elevated liver enzymes.15
With the latest advancements in the management of advanced NSCLC, nivolumab, a programmed death ligand 1 (PD-L1) inhibitor, was developed and either used as monotherapy in patients with PD-L1 expression or was combined with platinum-based chemotherapy regardless of PD-L1 expression.16,17 Patients expressing lower PD-L1 levels were not omitted from receiving nivolumab as no significant difference was noted in progression-free span and overall survival in patients receiving nivolumab irrespective of PD-L1 levels.15 Rash developed in 17% of patients after receiving nivolumab vs 45.8% patients being observed in our study. A similar trend was observed with diarrhea as only 17% of the population receiving nivolumab developed diarrhea compared with 33.6% of the population receiving mutant EGFR-TK inhibitors in our study. Likewise, only 9.9% of the patients receiving nivolumab developed nausea as an AE compared with 16.5% being observed in mutant EGFR-TK inhibitors in our study. Also, fatigue was observed in 14.4% of the population receiving nivolumab vs 17% observed in patients receiving mutant EGFR-TK inhibitors as was noticed in our study.7,8
Rizvi and colleagues conducted a study on the role of nivolumab when combined with platinum-based chemotherapy in patients with advanced NSCLC and reported that 40% of patients included in the study developed rash compared with 45.8% reported in mutant EGFR-TK inhibitors in our study. Similarly, only 13% of patients in the nivolumab group developed diarrhea vs 33.6% cases reported in the mutant EGFR-TK inhibitors group included in our study. Also, 7% of patients in the nivolumab group developed elevated ALT levels vs 27.9% of patients receiving mutant EGFR-TK inhibitors included in our study, concluding that addition of immune checkpoint inhibitors like nivolumab to platinum-based chemotherapy does not increase the frequency of AEs.18
Conclusions
Our study focused on the safety profile of mutant EGFR-TK inhibitors vs platinum-based chemotherapy in the treatment of advanced NSCLC. Mutant EGFR-TK inhibitors are safer than platinum-based chemotherapy when compared for nausea, leucopenia, fatigue, neutropenia, anorexia, anemia, cough, vomiting, and fever. On the other end, mutant EGFR-TK inhibitors cause slightly higher AEs, including rash, diarrhea, elevated AST and ALT levels, and stomatitis. However, considering that the development of mutant EGFR-TK inhibitors laid a foundation of targeted therapy, we recommend continuing using mutant EGFR-TK inhibitors in patients with advanced NSCLC especially in patients having mutant EGFR receptors. AEs caused by mutant EGFR-TK inhibitors are significant but are usually tolerable and can be avoided by reducing the dosage of it with each cycle or by skipping or delaying the dose until the patient is symptomatic.
1. Rahib L, Smith BD, Aizenberg R, Rosenzweig AB, Fleshman JM, Matrisian LM. Projecting cancer incidence and deaths to 2030: the unexpected burden of thyroid, liver, and pancreas cancers in the United States. Cancer Res. 2014;74(11):2913-2921. doi:10.1158/0008-5472.CAN-14-0155
2. da Cunha Santos G, Shepherd FA, Tsao MS. EGFR mutations and lung cancer. Annu Rev Pathol. 2011;6:49-69. doi:10.1146/annurev-pathol-011110-130206
3. Sgambato A, Casaluce F, Maione P, et al. The role of EGFR tyrosine kinase inhibitors in the first-line treatment of advanced non small cell lung cancer patients harboring EGFR mutation. Curr Med Chem. 2012;19(20):3337-3352. doi:10.2174/092986712801215973
4. Rossi A, Di Maio M. Platinum-based chemotherapy in advanced non–small-cell lung cancer: optimal number of treatment cycles. Expert Rev Anticancer Ther. 2016;16(6):653-660. doi:10.1586/14737140.2016.1170596
5. Rosell R, Carcereny E, Gervais R, et al. Erlotinib versus standard chemotherapy as first-line treatment for European patients with advanced EGFR mutation-positive non–small-cell lung cancer (EURTAC): a multicentre, open-label, randomised phase 3 trial. Lancet Oncol. 2012;13(3):239-246. doi:10.1016/S1470-2045(11)70393-X
6. Zhou C, Wu YL, Chen G, et al. Erlotinib versus chemotherapy as first-line treatment for patients with advanced EGFR mutation-positive non–small-cell lung cancer (OPTIMAL, CTONG-0802): a multicentre, open-label, randomised, phase 3 study. Lancet Oncol. 2011;12(8):735-742. doi:10.1016/S1470-2045(11)70184-X
7. Soria JC, Ohe Y, Vansteenkiste J, et al. Osimertinib in untreated EGFR-mutated advanced non–small-cell lung cancer. N Engl J Med. 2018;378(2):113-125. doi:10.1056/NEJMoa1713137
8. Mok TS, Cheng Y, Zhou X, et al. Improvement in overall survival in a randomized study that compared dacomitinib with gefitinib in patients with advanced non–small-cell lung cancer and EGFR-activating mutations. J Clin Oncol. 2018;36(22):2244-2250. doi:10.1200/JCO.2018.78.7994
9. Mok TS, Wu YL, Thongprasert S, et al. Gefitinib or carboplatin-paclitaxel in pulmonary adenocarcinoma. N Engl J Med. 2009;361(10):947-957. doi:10.1056/NEJMoa0810699
10. Pless M, Stupp R, Ris HB, et al. Induction chemoradiation in stage IIIA/N2 non–small-cell lung cancer: a phase 3 randomised trial. Lancet. 2015;386(9998):1049-1056. doi:10.1016/S0140-6736(15)60294-X
11. Albain KS, Rusch VW, Crowley JJ, et al. Concurrent cisplatin/etoposide plus chest radiotherapy followed by surgery for stages IIIA (N2) and IIIB non–small-cell lung cancer: mature results of Southwest Oncology Group phase II study 8805. J Clin Oncol. 1995;13(8):1880-1892. doi:10.1200/JCO.1995.13.8.1880
12. Zhong WZ, Chen KN, Chen C, et al. Erlotinib versus gemcitabine plus cisplatin as neoadjuvant treatment of Stage IIIA-N2 EGFR-mutant non–small-cell lung cancer (EMERGING-CTONG 1103): a randomized phase II study. J Clin Oncol. 2019;37(25):2235-2245. doi:10.1200/JCO.19.00075
13. Noronha V, Patil VM, Joshi A, et al. Gefitinib versus gefitinib plus pemetrexed and carboplatin chemotherapy in EGFR-mutated lung cancer. J Clin Oncol. 2020;38(2):124-136. doi:10.1200/JCO.19.01154
14. Noronha V, Prabhash K, Thavamani A, et al. EGFR mutations in Indian lung cancer patients: clinical correlation and outcome to EGFR targeted therapy. PLoS One. 2013;8(4):e61561. Published 2013 Apr 19. doi:10.1371/journal.pone.0061561
15. Kato T, Seto T, Nishio M, et al. Erlotinib plus bevacizumab phase ll study in patients with advanced non–small-cell lung cancer (JO25567): updated safety results. Drug Saf. 2018;41(2):229-237. doi:10.1007/s40264-017-0596-0
16. Hellmann MD, Paz-Ares L, Bernabe Caro R, et al. Nivolumab plus ipilimumab in advanced non–small-cell lung cancer. N Engl J Med. 2019;381(21):2020-2031. doi:10.1056/NEJMoa1910231
17. Hellmann MD, Ciuleanu TE, Pluzanski A, et al. Nivolumab plus ipilimumab in lung cancer with a high tumor mutational burden. N Engl J Med. 2018;378(22):2093-2104. doi:10.1056/NEJMoa1801946
18. Rizvi NA, Hellmann MD, Brahmer JR, et al. Nivolumab in combination with platinum-based doublet chemotherapy for first-line treatment of advanced non–small-cell lung cancer. J Clin Oncol. 2016;34(25):2969-2979. doi:10.1200/JCO.2016.66.9861
19. Zhong WZ, Wang Q, Mao WM, et al. Gefitinib versus vinorelbine plus cisplatin as adjuvant treatment for stage II-IIIA (N1-N2) EGFR-mutant NSCLC: final overall survival analysis of CTONG1104 Phase III Trial. J Clin Oncol. 2021;39(7):713-722. doi:10.1200/JCO.20.01820
20. Yang JC, Sequist LV, Geater SL, et al. Clinical activity of afatinib in patients with advanced non-small-cell lung cancer harbouring uncommon EGFR mutations: a combined post-hoc analysis of LUX-Lung 2, LUX-Lung 3, and LUX-Lung 6. Lancet Oncol. 2015;16(7):830-838. doi:10.1016/S1470-2045(15)00026-1
21. Shi YK, Wang L, Han BH, et al. First-line icotinib versus cisplatin/pemetrexed plus pemetrexed maintenance therapy for patients with advanced EGFR mutation-positive lung adenocarcinoma (CONVINCE): a phase 3, open-label, randomized study. Ann Oncol. 2017;28(10):2443-2450. doi:10.1093/annonc/mdx359
22. Soria JC, Wu YL, Nakagawa K, et al. Gefitinib plus chemotherapy versus placebo plus chemotherapy in EGFR-mutation-positive non-small-cell lung cancer after progression on first-line gefitinib (IMPRESS): a phase 3 randomized trial. Lancet Oncol. 2015;16(8):990-998 doi:10.1016/S1470-2045(15)00121-7
23. Goss GD, O’Callaghan C, Lorimer I, et al. Gefitinib versus placebo in completely resected non-small-cell lung cancer: results of the NCIC CTG BR19 study. J Clin Oncol. 2013;31(27):3320-3326. doi:10.1200/JCO.2013.51.1816
24. Sun JM, Lee KH, Kim SW, et al. Gefitinib versus pemetrexed as second-line treatment in patients with non-small cell lung cancer previously treated with platinum-based chemotherapy (KCSG-LU08-01): an open-label, phase 3 trial. Cancer. 2012;118(24):6234-6242. doi:10.1200/JCO.2013.51.1816
25. Mitsudomi T, Morita S, Yatabe Y, et al. Gefitinib versus cisplatin plus docetaxel in patients with non-small-cell lung cancer harbouring mutations of the epidermal growth factor receptor (WJTOG3405): an open label, randomized phase 3 trial. Lancet Oncol. 2010;11(2):121-128. doi:10.1016/S1470-2045(09)70364-X
26. Lee DH, Park K, Kim JH, Lee JS, et al. Randomized phase III trial of gefitinib versus docetaxel in non-small cell lung cancer patients who have previously received platinum-based chemotherapy. Clin Cancer Res. 2010;16(4):1307-1314. doi:10.1158/1078-0432.CCR-09-1903
27. Kim ES, Hirsh V, Mok T, et al. Gefitinib versus docetaxel in previously treated non-small-cell lung cancer (INTEREST): a randomized phase III trial. Lancet. 2008;22;372(9652):1809-1818. doi:10.1016/S0140-6736(08)61758-4
Lung cancer has been the leading cause of cancer-related mortality for decades. It is also predicted to remain as the leading cause of cancer-related mortality through 2030.1 Platinum-based chemotherapy, including carboplatin and paclitaxel, was introduced 3 decades ago and revolutionized the management of advanced non–small cell lung cancer (NSCLC). A more recent advancement has been mutant epidermal growth factor receptor–tyrosine kinase (EGFR-TK) inhibitors.1 EGFR is a transmembrane protein that functions by transducing essential growth factor signaling from the extracellular milieu to the cell. As 60% of the advanced NSCLC expresses this receptor, blocking the mutant EGFR receptor was a groundbreaking development in the management of advanced NSCLC.2 Development of mutant EGFR-TK inhibitors has revolutionized the management of advanced NSCLC. This study was conducted to determine the safety profile of mutant EGFR-TK inhibitors in the management of advanced NSCLC.
Methods
This meta-analysis was conducted according to Cochrane Collaboration guidelines and reported as per Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines. The findings are summarized in the PRISMA flow diagram (Figure 1). Two authors (MZ and MM) performed a systematic literature search using databases such as MEDLINE (via PubMed), Embase, and Cochrane Library using the medical search terms and their respective entry words with the following search strategy: safety, “mutant EGFR-TK inhibitors,” advanced, “non–small cell,” “lung cancer,” “adverse effect,” and literature. Additionally, unpublished trials were identified from clinicaltrials.gov, and references of all pertinent articles were also scrutinized to ensure the inclusion of all relevant studies. The search was completed on June 1, 2021, and we only included studies available in English. Two authors (MM and MZ) independently screened the search results in a 2-step process based on predetermined inclusion/exclusion criteria. First, 890 articles were evaluated for relevance on title and abstract level, followed by full-text screening of the final list of 140 articles. Any disagreements were resolved by discussion or third-party review, and a total of 9 articles were included in the study.
The following eligibility criteria were used: original articles reporting adverse effects (AEs) of mutant EGFR-TK inhibitors in patients with advanced NSCLC compared with control groups receiving platinum-based chemotherapy. All the patients included in the study had an EGFR mutation but randomly assigned to either treatment or control group. All articles with subjective data on mutant EGFR-TK inhibitors AEs in patients with advanced NSCLC compared with control groups receiving platinum-based chemotherapy were included in the analysis. Only 9 articles qualified the aforementioned selection criteria for eligibility. All qualifying studies were nationwide inpatient or pooled clinical trials data. The reasons for exclusion of the other 71 articles were irrelevant (n = 31), duplicate (n = 13), reviews (n = 14), and poor data reporting (n = 12). Out of the 9 included studies, 9 studies showed correlation of AEs, including rash, diarrhea, nausea, and fatigue. Seven studies showed correlation of AEs including neutropenia, anorexia, and vomiting. Six studies showed correlation of anemia, cough, and stomatitis. Five studies showed correlation of elevated aspartate aminotransferase (AST), alanine aminotransferase (ALT), and leucopenia. Four studies showed correlation of fever between mutant EGFR-TK inhibitors and platinum-based chemotherapy.
The primary endpoints were reported AEs including rash, diarrhea, elevated ALT, elevated AST, stomatitis, nausea, leucopenia, fatigue, neutropenia, anorexia, anemia, cough, vomiting, and fever, respectively. Data on baseline characteristics and clinical outcomes were then extracted, and summary tables were created. Summary estimates of the clinical endpoints were then calculated with risk ratio (RR) and 95% confidence intervals (CIs) using the random-effects model. Heterogeneity between studies was examined with the Cochran Q I2 statistic which can be defined as low (25% to 50%), moderate (50% to 75%), or high (> 75%). Statistical analysis was performed using Comprehensive Meta-Analysis Software CMA Version 3.0.
Results
A total of 9 studies including 3415 patients (1775 in EGFR-TK inhibitor treatment group while 1640 patients in platinum-based chemotherapy control group) were included in the study. All 9 studies were phase III randomized control clinical trials conducted to compare the safety profile of mutant EGFR-TK inhibitors in patients with advanced NSCLC. Mean age was 61 years in both treatment and control groups. Further details on study and participant characteristics and safety profile including AEs are summarized in Tables 1 and 2. No evidence of publication bias was found.
Rash developed in 45.8% of patients in the treatment group receiving mutant EGFR-TK inhibitors vs only 5.6% of patients in the control group receiving platinum-based chemotherapy. Overall RR of 7.38 with the 95% CI noted, which was statistically significant, confirming higher rash event rates in patients receiving EGFR-TK inhibitors for their advanced NSCLC (Figure 2).
Diarrhea occurred in 33.6% of patients in the mutant EGFR-TK inhibitors treatment group vs 13.5% of patients in the control group receiving platinum-based chemotherapy. Overall RR of 2.63 and 95% CI was noted, which was statistically significant, confirming higher diarrheal rates in patients receiving EGFR-TK inhibitors for their advanced NSCLC (Figure 3).
Elevated ALT levels developed in 27.9% of patients in the treatment group receiving mutant EGFR-TK inhibitors compared with 15.1% of patients in the control group receiving platinum-based chemotherapy. Overall RR of 1.37 and 95% CI was noted, which was statistically significant, confirming higher ALT levels in patients receiving EGFR-TK inhibitors for their advanced NSCLC (Figure 4).
Elevated AST levels occurred in 40.7% of patients in the mutant EGFR-TK inhibitors treatment group vs 12.8% of patients in the control group receiving platinum-based chemotherapy. Overall RR of 1.77 and 95% CI was noted, which was statistically significant, confirming elevated AST levels in patients receiving EGFR-TK inhibitors for their advanced NSCLC (Figure 5).
Stomatitis developed in 17.2% of patients in the treatment group receiving mutant EGFR-TK inhibitors compared with 7.9% of patients in the control group receiving platinum-based chemotherapy. Overall RR of 1.53 and 95% CI was noted, which was statistically significant, confirming higher stomatitis event rates in patients receiving EGFR-TK inhibitors for their advanced NSCLC (Figure 6).
Nausea occurred in 16.5% of patients in the mutant EGFR-TK inhibitors group vs 42.5% of patients in the control group receiving platinum-based chemotherapy. Overall RR of 0.37 and 95% CI was noted, which was statistically significant, confirming higher nausea rates in patients receiving platinum-based chemotherapy compared with treatment group for their advanced NSCLC (Figure 7).
Leucopenia developed in 9.7% of patients in the mutant EGFR-TK inhibitors group compared with 51.3% of patients in the control group receiving platinum-based chemotherapy. Overall RR of 0.18 and 95% CI was noted, which was statistically significant, confirming higher leucopenia incidence in patients receiving platinum-based chemotherapy compared with treatment group for their advanced NSCLC (Figure 8).
Fatigue was reported in 17% of patients in the mutant EGFR-TK inhibitors group compared with 29.5% of patients in the control group receiving platinum-based chemotherapy. Overall RR of 0.59 and 95% CI was noted, which was statistically significant, confirming higher fatigue rates in patients receiving platinum-based chemotherapy compared with treatment group for their advanced NSCLC (Figure 9).
Neutropenia developed in 6.1% of patients in the mutant EGFR-TK inhibitors group vs 48.2% of patients in the control group receiving platinum-based chemotherapy. Overall RR of 0.11 and 95% CI was noted, which was statistically significant, confirming higher neutropenia rates in patients receiving platinum-based chemotherapy compared with the treatment group for their advanced NSCLC (Figure 10).
Anorexia developed in 21.3% of patients in the mutant EGFR-TK inhibitors group vs 31.4% of patients in the control group receiving platinum-based chemotherapy. Overall RR of 0.44 and 95% CI was noted, which was statistically significant, confirming higher anorexia rates in patients receiving platinum-based chemotherapy compared with the treatment group for their advanced NSCLC (Figure 11).
Anemia occurred in 8.7% of patients in the mutant EGFR-TK inhibitors group compared with 32.1% of patients in the control group receiving platinum-based chemotherapy. Overall RR of 0.24 and 95% CI was noted, which was statistically significant, confirming higher anorexia rates in patients receiving platinum-based chemotherapy compared with treatment for their advanced NSCLC (Figure 12).
Cough was reported in 17.8% of patients in the mutant EGFR-TK inhibitors group compared with 18.9% of patients in the control group receiving platinum-based chemotherapy. Overall RR of 0.99 and 95% CI was noted, which was statistically significant, confirming slightly higher cough rates in patients receiving platinum-based chemotherapy compared with treatment for their advanced NSCLC (Figure 13).
Vomiting developed in 11% of patients in the mutant EGFR-TK inhibitors group vs 30.1% of patients in the control group receiving platinum-based chemotherapy. Overall RR of 0.35 and 95% CI was noted, which was statistically significant, confirming higher vomiting rates in patients receiving platinum-based chemotherapy compared with the treatment group for their advanced NSCLC (Figure 14).
Fever occurred in 5.6% of patients in the mutant EGFR-TK inhibitors group compared with 30.1% of patients in the control group receiving platinum-based chemotherapy. Overall RR of 0.41 and 95% CI was noted, which was statistically significant, confirming higher fever rates in patients receiving platinum-based chemotherapy compared with the treatment group for their advanced NSCLC (Figure 15).
Discussion
Despite the advancement in the treatment of metastatic NSCLC, lung cancer stays as most common cause of cancer-related death in North America and European countries, as patients usually have an advanced disease at the time of diagnosis.3 In the past, platinum-based chemotherapy remained the standard of care for most of the patients affected with advanced NSCLC, but the higher recurrence rate and increase in frequency and intensity of AEs with platinum-based chemotherapy led to the development of targeted therapy for NSCLC, one of which includes
Smoking is the most common reversible risk factor associated with lung cancer. The EURTAC trial was the first perspective study in this regard, which compared safety and efficacy of mutant EGFR-TK inhibitors with platinum-based chemotherapy. Results analyzed in this study were in favor of mutant EGFR-TK inhibitors except in the group of former smokers.5 On the contrary, the OPTIMAL trial showed results in favor of mutant EGFR-TK inhibitors both in active and former smokers; this trial also confirmed the efficacy of mutant EGFR-TK inhibitors in European and Asian populations, confirming the rationale for routine testing of EGFR mutation in all the patients being diagnosed with advanced NSCLC.6 Similarly, osimertinib is one of the most recent mutant EGFR-TK inhibitors developed for the treatment of advanced NSCLC in patients with EGFR-positive receptors.
According to the FLAURA trial, patients receiving osimertinib showed significantly longer progression-free survival compared with platinum-based chemotherapy and early mutant EGFR-TK inhibitors. Median progression-free survival was noted to be 18.9 months, which showed 54% lower risk of disease progression in the treatment group receiving osimertinib.7 The ARCHER study emphasized a significant improvement in overall survival as well as progression-free survival among a patient population receiving dacomitinib compared with platinum-based chemotherapy.8,9
Being a potent targeted therapy, mutant EGFR-TK inhibitors do come with some AEs including diarrhea, which was seen in 33.6% of the patients receiving mutant EGFR-TK inhibitors in our study vs 53% in the chemotherapy group, as was observed in the study conducted by Pless and colleagues.10 Similarly, only 16.5% of patients receiving mutant EGFR-TK inhibitors developed nausea compared with 66% being observed in patients receiving chemotherapy. Correspondingly, only a small fraction of patients (9.7%) receiving mutant EGFR-TK inhibitors developed leucopenia, which was 10 times less reported in mutant EGFR-TK inhibitors compared with patients receiving chemotherapy having a percentage of 100%. A similar trend was reported for neutropenia and anemia in mutant EGFR-TK inhibitors with an incidence of 6.1% and 8.7%, compared with the platinum-based chemotherapy group in which the incidence was found to be 80% and 100%, respectively. It was concluded that platinum-based chemotherapy had played a vital role in the treatment of advanced NSCLC but at an expense of serious and severe AEs which led to discontinuation or withdrawal of treatment, leading to relapse and recurrence of lung cancer.10,11
Zhong and colleagues conducted a phase 2 randomized clinical trial comparing mutant EGFR-TK inhibitors with platinum-based chemotherapy. They concluded that in patients receiving platinum-based chemotherapy, incidence of rash, vomiting, anorexia, neutropenia, and nausea were 29.4%, 47%, 41.2%, 55.8%, and 32.4% compared with 45.8%, 11%, 21.3%, 6.1%, and 16.5%, respectively, reported in patients receiving mutant EGFR-TK inhibitors for their advanced NSCLC.12
Another study was conducted in 2019 by Noronha and colleagues to determine the impact of platinum-based chemotherapy combined with gefitinib on patients with advanced NSCLC.13 They concluded that 70% of the patients receiving combination treatment developed rash, which was significantly higher compared with 45.8% patients receiving the mutant EGFR-TK inhibitors alone in our study. Also, 56% of patients receiving combination therapy developed diarrhea vs 33.6% of patients receiving mutant EGFR-TK inhibitors only. Similarly, 96% of patients in the combination therapy group developed some degree of anemia compared with only 8.7% patients in the mutant EGFR-TK inhibitors group included in our study. In the same way, neutropenia was observed in 55% of patients receiving combination therapy vs 6.1% in patients receiving mutant EGFR-TK inhibitors solely. They concluded that mutant EGFR-TK inhibitors when combined with platinum-based chemotherapy increase the incidence of AEs of chemotherapy by many folds.13,14
Kato and colleagues conducted a study to determine the impact on AEs when erlotinib was combined with anti–vascular endothelial growth factor (VEGF) inhibitors like bevacizumab, they stated that 98.7% of patient in combination therapy developed rash, the incidence of which was only 45.8% in patients receiving mutant EGFR-TK inhibitors as was observed in our study. Similar trends were noticed with other AEs, including diarrhea, fatigue, nausea, and elevated liver enzymes.15
With the latest advancements in the management of advanced NSCLC, nivolumab, a programmed death ligand 1 (PD-L1) inhibitor, was developed and either used as monotherapy in patients with PD-L1 expression or was combined with platinum-based chemotherapy regardless of PD-L1 expression.16,17 Patients expressing lower PD-L1 levels were not omitted from receiving nivolumab as no significant difference was noted in progression-free span and overall survival in patients receiving nivolumab irrespective of PD-L1 levels.15 Rash developed in 17% of patients after receiving nivolumab vs 45.8% patients being observed in our study. A similar trend was observed with diarrhea as only 17% of the population receiving nivolumab developed diarrhea compared with 33.6% of the population receiving mutant EGFR-TK inhibitors in our study. Likewise, only 9.9% of the patients receiving nivolumab developed nausea as an AE compared with 16.5% being observed in mutant EGFR-TK inhibitors in our study. Also, fatigue was observed in 14.4% of the population receiving nivolumab vs 17% observed in patients receiving mutant EGFR-TK inhibitors as was noticed in our study.7,8
Rizvi and colleagues conducted a study on the role of nivolumab when combined with platinum-based chemotherapy in patients with advanced NSCLC and reported that 40% of patients included in the study developed rash compared with 45.8% reported in mutant EGFR-TK inhibitors in our study. Similarly, only 13% of patients in the nivolumab group developed diarrhea vs 33.6% cases reported in the mutant EGFR-TK inhibitors group included in our study. Also, 7% of patients in the nivolumab group developed elevated ALT levels vs 27.9% of patients receiving mutant EGFR-TK inhibitors included in our study, concluding that addition of immune checkpoint inhibitors like nivolumab to platinum-based chemotherapy does not increase the frequency of AEs.18
Conclusions
Our study focused on the safety profile of mutant EGFR-TK inhibitors vs platinum-based chemotherapy in the treatment of advanced NSCLC. Mutant EGFR-TK inhibitors are safer than platinum-based chemotherapy when compared for nausea, leucopenia, fatigue, neutropenia, anorexia, anemia, cough, vomiting, and fever. On the other end, mutant EGFR-TK inhibitors cause slightly higher AEs, including rash, diarrhea, elevated AST and ALT levels, and stomatitis. However, considering that the development of mutant EGFR-TK inhibitors laid a foundation of targeted therapy, we recommend continuing using mutant EGFR-TK inhibitors in patients with advanced NSCLC especially in patients having mutant EGFR receptors. AEs caused by mutant EGFR-TK inhibitors are significant but are usually tolerable and can be avoided by reducing the dosage of it with each cycle or by skipping or delaying the dose until the patient is symptomatic.
Lung cancer has been the leading cause of cancer-related mortality for decades. It is also predicted to remain as the leading cause of cancer-related mortality through 2030.1 Platinum-based chemotherapy, including carboplatin and paclitaxel, was introduced 3 decades ago and revolutionized the management of advanced non–small cell lung cancer (NSCLC). A more recent advancement has been mutant epidermal growth factor receptor–tyrosine kinase (EGFR-TK) inhibitors.1 EGFR is a transmembrane protein that functions by transducing essential growth factor signaling from the extracellular milieu to the cell. As 60% of the advanced NSCLC expresses this receptor, blocking the mutant EGFR receptor was a groundbreaking development in the management of advanced NSCLC.2 Development of mutant EGFR-TK inhibitors has revolutionized the management of advanced NSCLC. This study was conducted to determine the safety profile of mutant EGFR-TK inhibitors in the management of advanced NSCLC.
Methods
This meta-analysis was conducted according to Cochrane Collaboration guidelines and reported as per Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines. The findings are summarized in the PRISMA flow diagram (Figure 1). Two authors (MZ and MM) performed a systematic literature search using databases such as MEDLINE (via PubMed), Embase, and Cochrane Library using the medical search terms and their respective entry words with the following search strategy: safety, “mutant EGFR-TK inhibitors,” advanced, “non–small cell,” “lung cancer,” “adverse effect,” and literature. Additionally, unpublished trials were identified from clinicaltrials.gov, and references of all pertinent articles were also scrutinized to ensure the inclusion of all relevant studies. The search was completed on June 1, 2021, and we only included studies available in English. Two authors (MM and MZ) independently screened the search results in a 2-step process based on predetermined inclusion/exclusion criteria. First, 890 articles were evaluated for relevance on title and abstract level, followed by full-text screening of the final list of 140 articles. Any disagreements were resolved by discussion or third-party review, and a total of 9 articles were included in the study.
The following eligibility criteria were used: original articles reporting adverse effects (AEs) of mutant EGFR-TK inhibitors in patients with advanced NSCLC compared with control groups receiving platinum-based chemotherapy. All the patients included in the study had an EGFR mutation but randomly assigned to either treatment or control group. All articles with subjective data on mutant EGFR-TK inhibitors AEs in patients with advanced NSCLC compared with control groups receiving platinum-based chemotherapy were included in the analysis. Only 9 articles qualified the aforementioned selection criteria for eligibility. All qualifying studies were nationwide inpatient or pooled clinical trials data. The reasons for exclusion of the other 71 articles were irrelevant (n = 31), duplicate (n = 13), reviews (n = 14), and poor data reporting (n = 12). Out of the 9 included studies, 9 studies showed correlation of AEs, including rash, diarrhea, nausea, and fatigue. Seven studies showed correlation of AEs including neutropenia, anorexia, and vomiting. Six studies showed correlation of anemia, cough, and stomatitis. Five studies showed correlation of elevated aspartate aminotransferase (AST), alanine aminotransferase (ALT), and leucopenia. Four studies showed correlation of fever between mutant EGFR-TK inhibitors and platinum-based chemotherapy.
The primary endpoints were reported AEs including rash, diarrhea, elevated ALT, elevated AST, stomatitis, nausea, leucopenia, fatigue, neutropenia, anorexia, anemia, cough, vomiting, and fever, respectively. Data on baseline characteristics and clinical outcomes were then extracted, and summary tables were created. Summary estimates of the clinical endpoints were then calculated with risk ratio (RR) and 95% confidence intervals (CIs) using the random-effects model. Heterogeneity between studies was examined with the Cochran Q I2 statistic which can be defined as low (25% to 50%), moderate (50% to 75%), or high (> 75%). Statistical analysis was performed using Comprehensive Meta-Analysis Software CMA Version 3.0.
Results
A total of 9 studies including 3415 patients (1775 in EGFR-TK inhibitor treatment group while 1640 patients in platinum-based chemotherapy control group) were included in the study. All 9 studies were phase III randomized control clinical trials conducted to compare the safety profile of mutant EGFR-TK inhibitors in patients with advanced NSCLC. Mean age was 61 years in both treatment and control groups. Further details on study and participant characteristics and safety profile including AEs are summarized in Tables 1 and 2. No evidence of publication bias was found.
Rash developed in 45.8% of patients in the treatment group receiving mutant EGFR-TK inhibitors vs only 5.6% of patients in the control group receiving platinum-based chemotherapy. Overall RR of 7.38 with the 95% CI noted, which was statistically significant, confirming higher rash event rates in patients receiving EGFR-TK inhibitors for their advanced NSCLC (Figure 2).
Diarrhea occurred in 33.6% of patients in the mutant EGFR-TK inhibitors treatment group vs 13.5% of patients in the control group receiving platinum-based chemotherapy. Overall RR of 2.63 and 95% CI was noted, which was statistically significant, confirming higher diarrheal rates in patients receiving EGFR-TK inhibitors for their advanced NSCLC (Figure 3).
Elevated ALT levels developed in 27.9% of patients in the treatment group receiving mutant EGFR-TK inhibitors compared with 15.1% of patients in the control group receiving platinum-based chemotherapy. Overall RR of 1.37 and 95% CI was noted, which was statistically significant, confirming higher ALT levels in patients receiving EGFR-TK inhibitors for their advanced NSCLC (Figure 4).
Elevated AST levels occurred in 40.7% of patients in the mutant EGFR-TK inhibitors treatment group vs 12.8% of patients in the control group receiving platinum-based chemotherapy. Overall RR of 1.77 and 95% CI was noted, which was statistically significant, confirming elevated AST levels in patients receiving EGFR-TK inhibitors for their advanced NSCLC (Figure 5).
Stomatitis developed in 17.2% of patients in the treatment group receiving mutant EGFR-TK inhibitors compared with 7.9% of patients in the control group receiving platinum-based chemotherapy. Overall RR of 1.53 and 95% CI was noted, which was statistically significant, confirming higher stomatitis event rates in patients receiving EGFR-TK inhibitors for their advanced NSCLC (Figure 6).
Nausea occurred in 16.5% of patients in the mutant EGFR-TK inhibitors group vs 42.5% of patients in the control group receiving platinum-based chemotherapy. Overall RR of 0.37 and 95% CI was noted, which was statistically significant, confirming higher nausea rates in patients receiving platinum-based chemotherapy compared with treatment group for their advanced NSCLC (Figure 7).
Leucopenia developed in 9.7% of patients in the mutant EGFR-TK inhibitors group compared with 51.3% of patients in the control group receiving platinum-based chemotherapy. Overall RR of 0.18 and 95% CI was noted, which was statistically significant, confirming higher leucopenia incidence in patients receiving platinum-based chemotherapy compared with treatment group for their advanced NSCLC (Figure 8).
Fatigue was reported in 17% of patients in the mutant EGFR-TK inhibitors group compared with 29.5% of patients in the control group receiving platinum-based chemotherapy. Overall RR of 0.59 and 95% CI was noted, which was statistically significant, confirming higher fatigue rates in patients receiving platinum-based chemotherapy compared with treatment group for their advanced NSCLC (Figure 9).
Neutropenia developed in 6.1% of patients in the mutant EGFR-TK inhibitors group vs 48.2% of patients in the control group receiving platinum-based chemotherapy. Overall RR of 0.11 and 95% CI was noted, which was statistically significant, confirming higher neutropenia rates in patients receiving platinum-based chemotherapy compared with the treatment group for their advanced NSCLC (Figure 10).
Anorexia developed in 21.3% of patients in the mutant EGFR-TK inhibitors group vs 31.4% of patients in the control group receiving platinum-based chemotherapy. Overall RR of 0.44 and 95% CI was noted, which was statistically significant, confirming higher anorexia rates in patients receiving platinum-based chemotherapy compared with the treatment group for their advanced NSCLC (Figure 11).
Anemia occurred in 8.7% of patients in the mutant EGFR-TK inhibitors group compared with 32.1% of patients in the control group receiving platinum-based chemotherapy. Overall RR of 0.24 and 95% CI was noted, which was statistically significant, confirming higher anorexia rates in patients receiving platinum-based chemotherapy compared with treatment for their advanced NSCLC (Figure 12).
Cough was reported in 17.8% of patients in the mutant EGFR-TK inhibitors group compared with 18.9% of patients in the control group receiving platinum-based chemotherapy. Overall RR of 0.99 and 95% CI was noted, which was statistically significant, confirming slightly higher cough rates in patients receiving platinum-based chemotherapy compared with treatment for their advanced NSCLC (Figure 13).
Vomiting developed in 11% of patients in the mutant EGFR-TK inhibitors group vs 30.1% of patients in the control group receiving platinum-based chemotherapy. Overall RR of 0.35 and 95% CI was noted, which was statistically significant, confirming higher vomiting rates in patients receiving platinum-based chemotherapy compared with the treatment group for their advanced NSCLC (Figure 14).
Fever occurred in 5.6% of patients in the mutant EGFR-TK inhibitors group compared with 30.1% of patients in the control group receiving platinum-based chemotherapy. Overall RR of 0.41 and 95% CI was noted, which was statistically significant, confirming higher fever rates in patients receiving platinum-based chemotherapy compared with the treatment group for their advanced NSCLC (Figure 15).
Discussion
Despite the advancement in the treatment of metastatic NSCLC, lung cancer stays as most common cause of cancer-related death in North America and European countries, as patients usually have an advanced disease at the time of diagnosis.3 In the past, platinum-based chemotherapy remained the standard of care for most of the patients affected with advanced NSCLC, but the higher recurrence rate and increase in frequency and intensity of AEs with platinum-based chemotherapy led to the development of targeted therapy for NSCLC, one of which includes
Smoking is the most common reversible risk factor associated with lung cancer. The EURTAC trial was the first perspective study in this regard, which compared safety and efficacy of mutant EGFR-TK inhibitors with platinum-based chemotherapy. Results analyzed in this study were in favor of mutant EGFR-TK inhibitors except in the group of former smokers.5 On the contrary, the OPTIMAL trial showed results in favor of mutant EGFR-TK inhibitors both in active and former smokers; this trial also confirmed the efficacy of mutant EGFR-TK inhibitors in European and Asian populations, confirming the rationale for routine testing of EGFR mutation in all the patients being diagnosed with advanced NSCLC.6 Similarly, osimertinib is one of the most recent mutant EGFR-TK inhibitors developed for the treatment of advanced NSCLC in patients with EGFR-positive receptors.
According to the FLAURA trial, patients receiving osimertinib showed significantly longer progression-free survival compared with platinum-based chemotherapy and early mutant EGFR-TK inhibitors. Median progression-free survival was noted to be 18.9 months, which showed 54% lower risk of disease progression in the treatment group receiving osimertinib.7 The ARCHER study emphasized a significant improvement in overall survival as well as progression-free survival among a patient population receiving dacomitinib compared with platinum-based chemotherapy.8,9
Being a potent targeted therapy, mutant EGFR-TK inhibitors do come with some AEs including diarrhea, which was seen in 33.6% of the patients receiving mutant EGFR-TK inhibitors in our study vs 53% in the chemotherapy group, as was observed in the study conducted by Pless and colleagues.10 Similarly, only 16.5% of patients receiving mutant EGFR-TK inhibitors developed nausea compared with 66% being observed in patients receiving chemotherapy. Correspondingly, only a small fraction of patients (9.7%) receiving mutant EGFR-TK inhibitors developed leucopenia, which was 10 times less reported in mutant EGFR-TK inhibitors compared with patients receiving chemotherapy having a percentage of 100%. A similar trend was reported for neutropenia and anemia in mutant EGFR-TK inhibitors with an incidence of 6.1% and 8.7%, compared with the platinum-based chemotherapy group in which the incidence was found to be 80% and 100%, respectively. It was concluded that platinum-based chemotherapy had played a vital role in the treatment of advanced NSCLC but at an expense of serious and severe AEs which led to discontinuation or withdrawal of treatment, leading to relapse and recurrence of lung cancer.10,11
Zhong and colleagues conducted a phase 2 randomized clinical trial comparing mutant EGFR-TK inhibitors with platinum-based chemotherapy. They concluded that in patients receiving platinum-based chemotherapy, incidence of rash, vomiting, anorexia, neutropenia, and nausea were 29.4%, 47%, 41.2%, 55.8%, and 32.4% compared with 45.8%, 11%, 21.3%, 6.1%, and 16.5%, respectively, reported in patients receiving mutant EGFR-TK inhibitors for their advanced NSCLC.12
Another study was conducted in 2019 by Noronha and colleagues to determine the impact of platinum-based chemotherapy combined with gefitinib on patients with advanced NSCLC.13 They concluded that 70% of the patients receiving combination treatment developed rash, which was significantly higher compared with 45.8% patients receiving the mutant EGFR-TK inhibitors alone in our study. Also, 56% of patients receiving combination therapy developed diarrhea vs 33.6% of patients receiving mutant EGFR-TK inhibitors only. Similarly, 96% of patients in the combination therapy group developed some degree of anemia compared with only 8.7% patients in the mutant EGFR-TK inhibitors group included in our study. In the same way, neutropenia was observed in 55% of patients receiving combination therapy vs 6.1% in patients receiving mutant EGFR-TK inhibitors solely. They concluded that mutant EGFR-TK inhibitors when combined with platinum-based chemotherapy increase the incidence of AEs of chemotherapy by many folds.13,14
Kato and colleagues conducted a study to determine the impact on AEs when erlotinib was combined with anti–vascular endothelial growth factor (VEGF) inhibitors like bevacizumab, they stated that 98.7% of patient in combination therapy developed rash, the incidence of which was only 45.8% in patients receiving mutant EGFR-TK inhibitors as was observed in our study. Similar trends were noticed with other AEs, including diarrhea, fatigue, nausea, and elevated liver enzymes.15
With the latest advancements in the management of advanced NSCLC, nivolumab, a programmed death ligand 1 (PD-L1) inhibitor, was developed and either used as monotherapy in patients with PD-L1 expression or was combined with platinum-based chemotherapy regardless of PD-L1 expression.16,17 Patients expressing lower PD-L1 levels were not omitted from receiving nivolumab as no significant difference was noted in progression-free span and overall survival in patients receiving nivolumab irrespective of PD-L1 levels.15 Rash developed in 17% of patients after receiving nivolumab vs 45.8% patients being observed in our study. A similar trend was observed with diarrhea as only 17% of the population receiving nivolumab developed diarrhea compared with 33.6% of the population receiving mutant EGFR-TK inhibitors in our study. Likewise, only 9.9% of the patients receiving nivolumab developed nausea as an AE compared with 16.5% being observed in mutant EGFR-TK inhibitors in our study. Also, fatigue was observed in 14.4% of the population receiving nivolumab vs 17% observed in patients receiving mutant EGFR-TK inhibitors as was noticed in our study.7,8
Rizvi and colleagues conducted a study on the role of nivolumab when combined with platinum-based chemotherapy in patients with advanced NSCLC and reported that 40% of patients included in the study developed rash compared with 45.8% reported in mutant EGFR-TK inhibitors in our study. Similarly, only 13% of patients in the nivolumab group developed diarrhea vs 33.6% cases reported in the mutant EGFR-TK inhibitors group included in our study. Also, 7% of patients in the nivolumab group developed elevated ALT levels vs 27.9% of patients receiving mutant EGFR-TK inhibitors included in our study, concluding that addition of immune checkpoint inhibitors like nivolumab to platinum-based chemotherapy does not increase the frequency of AEs.18
Conclusions
Our study focused on the safety profile of mutant EGFR-TK inhibitors vs platinum-based chemotherapy in the treatment of advanced NSCLC. Mutant EGFR-TK inhibitors are safer than platinum-based chemotherapy when compared for nausea, leucopenia, fatigue, neutropenia, anorexia, anemia, cough, vomiting, and fever. On the other end, mutant EGFR-TK inhibitors cause slightly higher AEs, including rash, diarrhea, elevated AST and ALT levels, and stomatitis. However, considering that the development of mutant EGFR-TK inhibitors laid a foundation of targeted therapy, we recommend continuing using mutant EGFR-TK inhibitors in patients with advanced NSCLC especially in patients having mutant EGFR receptors. AEs caused by mutant EGFR-TK inhibitors are significant but are usually tolerable and can be avoided by reducing the dosage of it with each cycle or by skipping or delaying the dose until the patient is symptomatic.
1. Rahib L, Smith BD, Aizenberg R, Rosenzweig AB, Fleshman JM, Matrisian LM. Projecting cancer incidence and deaths to 2030: the unexpected burden of thyroid, liver, and pancreas cancers in the United States. Cancer Res. 2014;74(11):2913-2921. doi:10.1158/0008-5472.CAN-14-0155
2. da Cunha Santos G, Shepherd FA, Tsao MS. EGFR mutations and lung cancer. Annu Rev Pathol. 2011;6:49-69. doi:10.1146/annurev-pathol-011110-130206
3. Sgambato A, Casaluce F, Maione P, et al. The role of EGFR tyrosine kinase inhibitors in the first-line treatment of advanced non small cell lung cancer patients harboring EGFR mutation. Curr Med Chem. 2012;19(20):3337-3352. doi:10.2174/092986712801215973
4. Rossi A, Di Maio M. Platinum-based chemotherapy in advanced non–small-cell lung cancer: optimal number of treatment cycles. Expert Rev Anticancer Ther. 2016;16(6):653-660. doi:10.1586/14737140.2016.1170596
5. Rosell R, Carcereny E, Gervais R, et al. Erlotinib versus standard chemotherapy as first-line treatment for European patients with advanced EGFR mutation-positive non–small-cell lung cancer (EURTAC): a multicentre, open-label, randomised phase 3 trial. Lancet Oncol. 2012;13(3):239-246. doi:10.1016/S1470-2045(11)70393-X
6. Zhou C, Wu YL, Chen G, et al. Erlotinib versus chemotherapy as first-line treatment for patients with advanced EGFR mutation-positive non–small-cell lung cancer (OPTIMAL, CTONG-0802): a multicentre, open-label, randomised, phase 3 study. Lancet Oncol. 2011;12(8):735-742. doi:10.1016/S1470-2045(11)70184-X
7. Soria JC, Ohe Y, Vansteenkiste J, et al. Osimertinib in untreated EGFR-mutated advanced non–small-cell lung cancer. N Engl J Med. 2018;378(2):113-125. doi:10.1056/NEJMoa1713137
8. Mok TS, Cheng Y, Zhou X, et al. Improvement in overall survival in a randomized study that compared dacomitinib with gefitinib in patients with advanced non–small-cell lung cancer and EGFR-activating mutations. J Clin Oncol. 2018;36(22):2244-2250. doi:10.1200/JCO.2018.78.7994
9. Mok TS, Wu YL, Thongprasert S, et al. Gefitinib or carboplatin-paclitaxel in pulmonary adenocarcinoma. N Engl J Med. 2009;361(10):947-957. doi:10.1056/NEJMoa0810699
10. Pless M, Stupp R, Ris HB, et al. Induction chemoradiation in stage IIIA/N2 non–small-cell lung cancer: a phase 3 randomised trial. Lancet. 2015;386(9998):1049-1056. doi:10.1016/S0140-6736(15)60294-X
11. Albain KS, Rusch VW, Crowley JJ, et al. Concurrent cisplatin/etoposide plus chest radiotherapy followed by surgery for stages IIIA (N2) and IIIB non–small-cell lung cancer: mature results of Southwest Oncology Group phase II study 8805. J Clin Oncol. 1995;13(8):1880-1892. doi:10.1200/JCO.1995.13.8.1880
12. Zhong WZ, Chen KN, Chen C, et al. Erlotinib versus gemcitabine plus cisplatin as neoadjuvant treatment of Stage IIIA-N2 EGFR-mutant non–small-cell lung cancer (EMERGING-CTONG 1103): a randomized phase II study. J Clin Oncol. 2019;37(25):2235-2245. doi:10.1200/JCO.19.00075
13. Noronha V, Patil VM, Joshi A, et al. Gefitinib versus gefitinib plus pemetrexed and carboplatin chemotherapy in EGFR-mutated lung cancer. J Clin Oncol. 2020;38(2):124-136. doi:10.1200/JCO.19.01154
14. Noronha V, Prabhash K, Thavamani A, et al. EGFR mutations in Indian lung cancer patients: clinical correlation and outcome to EGFR targeted therapy. PLoS One. 2013;8(4):e61561. Published 2013 Apr 19. doi:10.1371/journal.pone.0061561
15. Kato T, Seto T, Nishio M, et al. Erlotinib plus bevacizumab phase ll study in patients with advanced non–small-cell lung cancer (JO25567): updated safety results. Drug Saf. 2018;41(2):229-237. doi:10.1007/s40264-017-0596-0
16. Hellmann MD, Paz-Ares L, Bernabe Caro R, et al. Nivolumab plus ipilimumab in advanced non–small-cell lung cancer. N Engl J Med. 2019;381(21):2020-2031. doi:10.1056/NEJMoa1910231
17. Hellmann MD, Ciuleanu TE, Pluzanski A, et al. Nivolumab plus ipilimumab in lung cancer with a high tumor mutational burden. N Engl J Med. 2018;378(22):2093-2104. doi:10.1056/NEJMoa1801946
18. Rizvi NA, Hellmann MD, Brahmer JR, et al. Nivolumab in combination with platinum-based doublet chemotherapy for first-line treatment of advanced non–small-cell lung cancer. J Clin Oncol. 2016;34(25):2969-2979. doi:10.1200/JCO.2016.66.9861
19. Zhong WZ, Wang Q, Mao WM, et al. Gefitinib versus vinorelbine plus cisplatin as adjuvant treatment for stage II-IIIA (N1-N2) EGFR-mutant NSCLC: final overall survival analysis of CTONG1104 Phase III Trial. J Clin Oncol. 2021;39(7):713-722. doi:10.1200/JCO.20.01820
20. Yang JC, Sequist LV, Geater SL, et al. Clinical activity of afatinib in patients with advanced non-small-cell lung cancer harbouring uncommon EGFR mutations: a combined post-hoc analysis of LUX-Lung 2, LUX-Lung 3, and LUX-Lung 6. Lancet Oncol. 2015;16(7):830-838. doi:10.1016/S1470-2045(15)00026-1
21. Shi YK, Wang L, Han BH, et al. First-line icotinib versus cisplatin/pemetrexed plus pemetrexed maintenance therapy for patients with advanced EGFR mutation-positive lung adenocarcinoma (CONVINCE): a phase 3, open-label, randomized study. Ann Oncol. 2017;28(10):2443-2450. doi:10.1093/annonc/mdx359
22. Soria JC, Wu YL, Nakagawa K, et al. Gefitinib plus chemotherapy versus placebo plus chemotherapy in EGFR-mutation-positive non-small-cell lung cancer after progression on first-line gefitinib (IMPRESS): a phase 3 randomized trial. Lancet Oncol. 2015;16(8):990-998 doi:10.1016/S1470-2045(15)00121-7
23. Goss GD, O’Callaghan C, Lorimer I, et al. Gefitinib versus placebo in completely resected non-small-cell lung cancer: results of the NCIC CTG BR19 study. J Clin Oncol. 2013;31(27):3320-3326. doi:10.1200/JCO.2013.51.1816
24. Sun JM, Lee KH, Kim SW, et al. Gefitinib versus pemetrexed as second-line treatment in patients with non-small cell lung cancer previously treated with platinum-based chemotherapy (KCSG-LU08-01): an open-label, phase 3 trial. Cancer. 2012;118(24):6234-6242. doi:10.1200/JCO.2013.51.1816
25. Mitsudomi T, Morita S, Yatabe Y, et al. Gefitinib versus cisplatin plus docetaxel in patients with non-small-cell lung cancer harbouring mutations of the epidermal growth factor receptor (WJTOG3405): an open label, randomized phase 3 trial. Lancet Oncol. 2010;11(2):121-128. doi:10.1016/S1470-2045(09)70364-X
26. Lee DH, Park K, Kim JH, Lee JS, et al. Randomized phase III trial of gefitinib versus docetaxel in non-small cell lung cancer patients who have previously received platinum-based chemotherapy. Clin Cancer Res. 2010;16(4):1307-1314. doi:10.1158/1078-0432.CCR-09-1903
27. Kim ES, Hirsh V, Mok T, et al. Gefitinib versus docetaxel in previously treated non-small-cell lung cancer (INTEREST): a randomized phase III trial. Lancet. 2008;22;372(9652):1809-1818. doi:10.1016/S0140-6736(08)61758-4
1. Rahib L, Smith BD, Aizenberg R, Rosenzweig AB, Fleshman JM, Matrisian LM. Projecting cancer incidence and deaths to 2030: the unexpected burden of thyroid, liver, and pancreas cancers in the United States. Cancer Res. 2014;74(11):2913-2921. doi:10.1158/0008-5472.CAN-14-0155
2. da Cunha Santos G, Shepherd FA, Tsao MS. EGFR mutations and lung cancer. Annu Rev Pathol. 2011;6:49-69. doi:10.1146/annurev-pathol-011110-130206
3. Sgambato A, Casaluce F, Maione P, et al. The role of EGFR tyrosine kinase inhibitors in the first-line treatment of advanced non small cell lung cancer patients harboring EGFR mutation. Curr Med Chem. 2012;19(20):3337-3352. doi:10.2174/092986712801215973
4. Rossi A, Di Maio M. Platinum-based chemotherapy in advanced non–small-cell lung cancer: optimal number of treatment cycles. Expert Rev Anticancer Ther. 2016;16(6):653-660. doi:10.1586/14737140.2016.1170596
5. Rosell R, Carcereny E, Gervais R, et al. Erlotinib versus standard chemotherapy as first-line treatment for European patients with advanced EGFR mutation-positive non–small-cell lung cancer (EURTAC): a multicentre, open-label, randomised phase 3 trial. Lancet Oncol. 2012;13(3):239-246. doi:10.1016/S1470-2045(11)70393-X
6. Zhou C, Wu YL, Chen G, et al. Erlotinib versus chemotherapy as first-line treatment for patients with advanced EGFR mutation-positive non–small-cell lung cancer (OPTIMAL, CTONG-0802): a multicentre, open-label, randomised, phase 3 study. Lancet Oncol. 2011;12(8):735-742. doi:10.1016/S1470-2045(11)70184-X
7. Soria JC, Ohe Y, Vansteenkiste J, et al. Osimertinib in untreated EGFR-mutated advanced non–small-cell lung cancer. N Engl J Med. 2018;378(2):113-125. doi:10.1056/NEJMoa1713137
8. Mok TS, Cheng Y, Zhou X, et al. Improvement in overall survival in a randomized study that compared dacomitinib with gefitinib in patients with advanced non–small-cell lung cancer and EGFR-activating mutations. J Clin Oncol. 2018;36(22):2244-2250. doi:10.1200/JCO.2018.78.7994
9. Mok TS, Wu YL, Thongprasert S, et al. Gefitinib or carboplatin-paclitaxel in pulmonary adenocarcinoma. N Engl J Med. 2009;361(10):947-957. doi:10.1056/NEJMoa0810699
10. Pless M, Stupp R, Ris HB, et al. Induction chemoradiation in stage IIIA/N2 non–small-cell lung cancer: a phase 3 randomised trial. Lancet. 2015;386(9998):1049-1056. doi:10.1016/S0140-6736(15)60294-X
11. Albain KS, Rusch VW, Crowley JJ, et al. Concurrent cisplatin/etoposide plus chest radiotherapy followed by surgery for stages IIIA (N2) and IIIB non–small-cell lung cancer: mature results of Southwest Oncology Group phase II study 8805. J Clin Oncol. 1995;13(8):1880-1892. doi:10.1200/JCO.1995.13.8.1880
12. Zhong WZ, Chen KN, Chen C, et al. Erlotinib versus gemcitabine plus cisplatin as neoadjuvant treatment of Stage IIIA-N2 EGFR-mutant non–small-cell lung cancer (EMERGING-CTONG 1103): a randomized phase II study. J Clin Oncol. 2019;37(25):2235-2245. doi:10.1200/JCO.19.00075
13. Noronha V, Patil VM, Joshi A, et al. Gefitinib versus gefitinib plus pemetrexed and carboplatin chemotherapy in EGFR-mutated lung cancer. J Clin Oncol. 2020;38(2):124-136. doi:10.1200/JCO.19.01154
14. Noronha V, Prabhash K, Thavamani A, et al. EGFR mutations in Indian lung cancer patients: clinical correlation and outcome to EGFR targeted therapy. PLoS One. 2013;8(4):e61561. Published 2013 Apr 19. doi:10.1371/journal.pone.0061561
15. Kato T, Seto T, Nishio M, et al. Erlotinib plus bevacizumab phase ll study in patients with advanced non–small-cell lung cancer (JO25567): updated safety results. Drug Saf. 2018;41(2):229-237. doi:10.1007/s40264-017-0596-0
16. Hellmann MD, Paz-Ares L, Bernabe Caro R, et al. Nivolumab plus ipilimumab in advanced non–small-cell lung cancer. N Engl J Med. 2019;381(21):2020-2031. doi:10.1056/NEJMoa1910231
17. Hellmann MD, Ciuleanu TE, Pluzanski A, et al. Nivolumab plus ipilimumab in lung cancer with a high tumor mutational burden. N Engl J Med. 2018;378(22):2093-2104. doi:10.1056/NEJMoa1801946
18. Rizvi NA, Hellmann MD, Brahmer JR, et al. Nivolumab in combination with platinum-based doublet chemotherapy for first-line treatment of advanced non–small-cell lung cancer. J Clin Oncol. 2016;34(25):2969-2979. doi:10.1200/JCO.2016.66.9861
19. Zhong WZ, Wang Q, Mao WM, et al. Gefitinib versus vinorelbine plus cisplatin as adjuvant treatment for stage II-IIIA (N1-N2) EGFR-mutant NSCLC: final overall survival analysis of CTONG1104 Phase III Trial. J Clin Oncol. 2021;39(7):713-722. doi:10.1200/JCO.20.01820
20. Yang JC, Sequist LV, Geater SL, et al. Clinical activity of afatinib in patients with advanced non-small-cell lung cancer harbouring uncommon EGFR mutations: a combined post-hoc analysis of LUX-Lung 2, LUX-Lung 3, and LUX-Lung 6. Lancet Oncol. 2015;16(7):830-838. doi:10.1016/S1470-2045(15)00026-1
21. Shi YK, Wang L, Han BH, et al. First-line icotinib versus cisplatin/pemetrexed plus pemetrexed maintenance therapy for patients with advanced EGFR mutation-positive lung adenocarcinoma (CONVINCE): a phase 3, open-label, randomized study. Ann Oncol. 2017;28(10):2443-2450. doi:10.1093/annonc/mdx359
22. Soria JC, Wu YL, Nakagawa K, et al. Gefitinib plus chemotherapy versus placebo plus chemotherapy in EGFR-mutation-positive non-small-cell lung cancer after progression on first-line gefitinib (IMPRESS): a phase 3 randomized trial. Lancet Oncol. 2015;16(8):990-998 doi:10.1016/S1470-2045(15)00121-7
23. Goss GD, O’Callaghan C, Lorimer I, et al. Gefitinib versus placebo in completely resected non-small-cell lung cancer: results of the NCIC CTG BR19 study. J Clin Oncol. 2013;31(27):3320-3326. doi:10.1200/JCO.2013.51.1816
24. Sun JM, Lee KH, Kim SW, et al. Gefitinib versus pemetrexed as second-line treatment in patients with non-small cell lung cancer previously treated with platinum-based chemotherapy (KCSG-LU08-01): an open-label, phase 3 trial. Cancer. 2012;118(24):6234-6242. doi:10.1200/JCO.2013.51.1816
25. Mitsudomi T, Morita S, Yatabe Y, et al. Gefitinib versus cisplatin plus docetaxel in patients with non-small-cell lung cancer harbouring mutations of the epidermal growth factor receptor (WJTOG3405): an open label, randomized phase 3 trial. Lancet Oncol. 2010;11(2):121-128. doi:10.1016/S1470-2045(09)70364-X
26. Lee DH, Park K, Kim JH, Lee JS, et al. Randomized phase III trial of gefitinib versus docetaxel in non-small cell lung cancer patients who have previously received platinum-based chemotherapy. Clin Cancer Res. 2010;16(4):1307-1314. doi:10.1158/1078-0432.CCR-09-1903
27. Kim ES, Hirsh V, Mok T, et al. Gefitinib versus docetaxel in previously treated non-small-cell lung cancer (INTEREST): a randomized phase III trial. Lancet. 2008;22;372(9652):1809-1818. doi:10.1016/S0140-6736(08)61758-4
Generalized Pustular Psoriasis: A Review of the Pathophysiology, Clinical Manifestations, Diagnosis, and Treatment
Acute generalized pustular psoriasis (GPP) is a rare severe variant of psoriasis characterized by the sudden widespread eruption of sterile pustules.1,2 The cutaneous manifestations of GPP also may be accompanied by signs of systemic inflammation, including fever, malaise, and leukocytosis.2 Complications are common and may be life-threatening, especially in older patients with comorbid diseases.3 Generalized pustular psoriasis most commonly occurs in patients with a preceding history of psoriasis, but it also may occur de novo.4 Generalized pustular psoriasis is associated with notable morbidity and mortality, and relapses are common.3,4 Many triggers of GPP have been identified, including initiation and withdrawal of various medications, infections, pregnancy, and other conditions.5,6 Although GPP most often occurs in adults, it also may arise in children and infants.3 In pregnancy, GPP is referred to as impetigo herpetiformis, despite having no etiologic ties with either herpes simplex virus or staphylococcal or streptococcal infection. Impetigo herpetiformis is considered one of the most dangerous dermatoses of pregnancy because of high rates of associated maternal and fetal morbidity.6,7
Acute GPP has proven to be a challenging disease to treat due to the rarity and relapsing-remitting nature of the disease; additionally, there are relatively few randomized controlled trials investigating the efficacy and safety of treatments for GPP. This review summarizes the features of GPP, including the pathophysiology of the disease, clinical and histological manifestations, and recommendations for management based on a PubMed search of articles indexed for MEDLINE using MeSH terms pertaining to the disease, including generalized pustular psoriasis, impetigo herpetiformis, and von Zumbusch psoriasis.
Pathophysiology
The pathophysiology of GPP is only partially understood, but it is thought to have a distinct pattern of immune activation compared with plaque psoriasis.8 Although there is a considerable amount of overlap and cross-talk among cytokine pathways, GPP generally is driven by innate immunity and unrestrained IL-36 cytokine activity. In contrast, adaptive immune responses—namely the tumor necrosis factor (TNF) α, IL-23, IL-17, and IL-22 axes—underlie plaque psoriasis.8-10
Proinflammatory IL-36 cytokines α, β, and γ, which are all part of the IL-1 superfamily, bind to the IL-36 receptor (IL-36R) to recruit and activate immune cells via various mediators, including IL-1β; IL-8; and chemokines CXCL1, CXCL2, and CXCL8.3 The IL-36 receptor antagonist (IL-36ra) acts to inhibit this inflammatory cascade.3,8 Microarray analyses of skin biopsy samples have shown that overexpression of IL-17A, TNF-α, IL-1, and IL-36 are seen in both GPP and plaque psoriasis lesions, but GPP lesions had higher expression of IL-1β, IL-36α, and IL-36γ and elevated neutrophil chemokines—CXCL1, CXCL2, and CXCL8—compared with plaque psoriasis lesions.8
Gene Mutations Associated With GPP
There are 3 gene mutations that have been associated with pustular variants of psoriasis, though these mutations account for a minority of cases of GPP.4 Genetic screenings are not routinely indicated in patients with GPP, but they may be warranted in severe cases when a familial pattern of inheritance is suspected.4
IL36RN—The gene IL36RN codes the anti-inflammatory IL-36ra. Loss-of-function mutations in IL36RN lead to impairment of IL-36ra and consequently hyperactivity of the proinflammatory responses triggered by IL-36.3 Homozygous and heterozygous mutations in IL36RN have been observed in both familial and sporadic cases of GPP.11-13 Subsequent retrospective analyses have identified the presence of IL36RN mutations in patients with GPP with frequencies ranging from 23% to 37%.14-17IL36RN mutations are thought to be more common in patients without concomitant plaque psoriasis and have been associated with severe disease and early disease onset.15
CARD14—A gain-of-function mutation in CARD14 results in overactivation of the proinflammatory nuclear factor κB pathway and has been implicated in cases of GPP with concurrent psoriasis vulgaris. Interestingly, this may suggest distinct etiologies underlying GPP de novo and GPP in patients with a history of psoriasis.18,19
AP1S3—A loss-of-function mutation in AP1S3 results in abnormal endosomal trafficking and autophagy as well as increased expression of IL-36α.20,21
Clinical Presentation and Diagnosis Cutaneous Manifestations of GPP
Generalized pustular psoriasis is characterized by the onset of widespread 2- to 3-mm sterile pustules on erythematous skin or within psoriasiform plaques4 (Figure). In patients with skin of color, the erythema may appear less obvious or perhaps slightly violaceous compared to White skin. Pustules may coalesce to form “lakes” of pus.5 Cutaneous symptoms include pain, burning, and pruritus. Associated mucosal findings may include cheilitis, geographic tongue, conjunctivitis, and uveitis.4
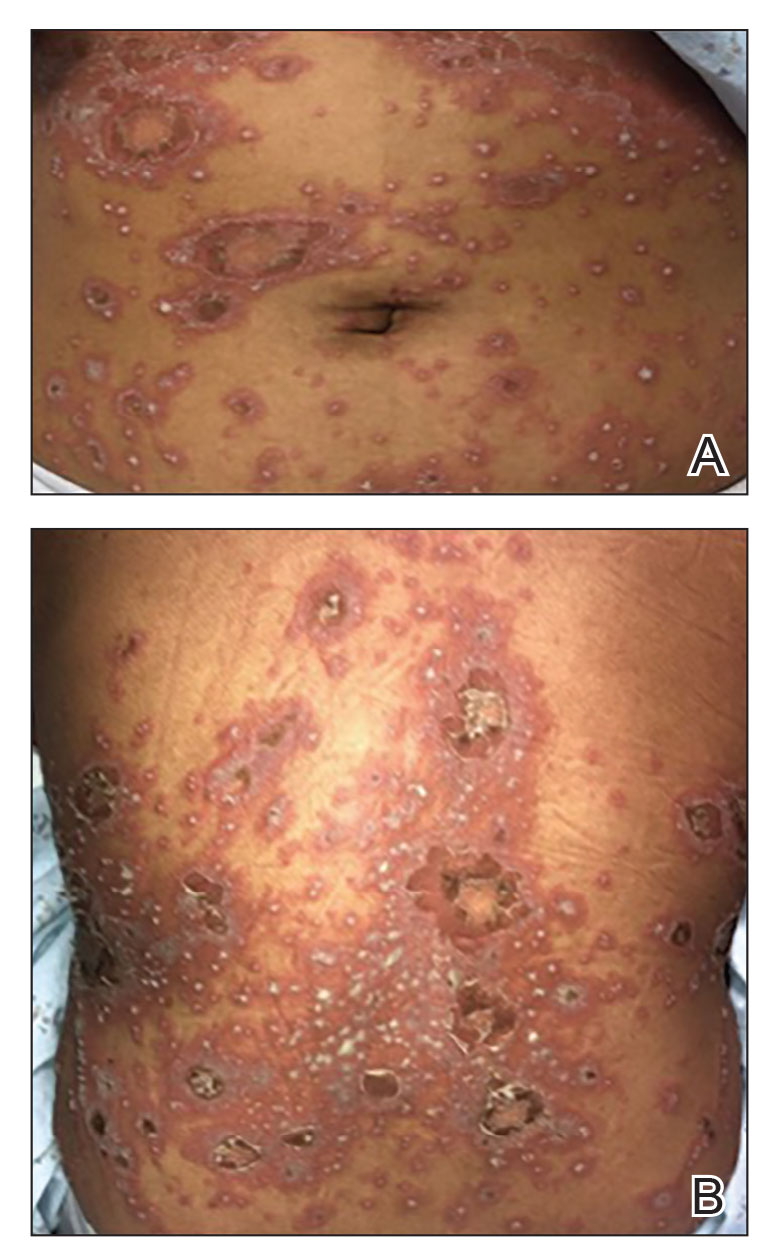
The severity of symptoms can vary greatly among patients as well as between flares within the same patient.2,3 Four distinct patterns of GPP have been described. The von Zumbusch pattern is characterized by a rapid, generalized, painful, erythematous and pustular eruption accompanied by fever and asthenia. The pustules usually resolve after several days with extensive scaling. The annular pattern is characterized by annular, erythematous, scaly lesions with pustules present centrifugally. The lesions enlarge by centrifugal expansion over a period of hours to days, while healing occurs centrally. The exanthematic type is an acute eruption of small pustules that abruptly appear and disappear within a few days, usually from infection or medication initiation. Sometimes pustules appear within or at the edge of existing psoriatic plaques in a localized pattern—the fourth pattern—often following the exposure to irritants (eg, tars, anthralin).5
Impetigo Herpetiformis—Impetigo herpetiformis is a form of GPP associated with pregnancy. It generally presents early in the third trimester with symmetric erythematous plaques in flexural and intertriginous areas with pustules present at lesion margins. Lesions expand centrifugally, with pustulation present at the advancing edge.6,7 Patients often are acutely ill with fever, delirium, vomiting, and tetany. Mucous membranes, including the tongue, mouth, and esophagus, also may be involved. The eruption typically resolves after delivery, though it often recurs with subsequent pregnancies, with the morbidity risk rising with each successive pregnancy.7
Systemic and Extracutaneous Manifestations of GPP
Although the severity of GPP is highly variable, skin manifestations often are accompanied by systemic manifestations of inflammation, including fever and malaise. Common laboratory abnormalities include leukocytosis with peripheral neutrophilia, a high serum C-reactive protein level, hypocalcemia, and hypoalbuminemia.22 Abnormal liver enzymes often are present and result from neutrophilic cholangitis, with alternating strictures and dilations of biliary ducts observed on magnetic resonance imaging.23 Additional laboratory abnormalities are provided in Table 2. Other extracutaneous findings associated with GPP include arthralgia, edema, and characteristic psoriatic nail changes.4 Fatal complications include acute respiratory distress syndrome, renal dysfunction, cardiovascular shock, and sepsis.24,25
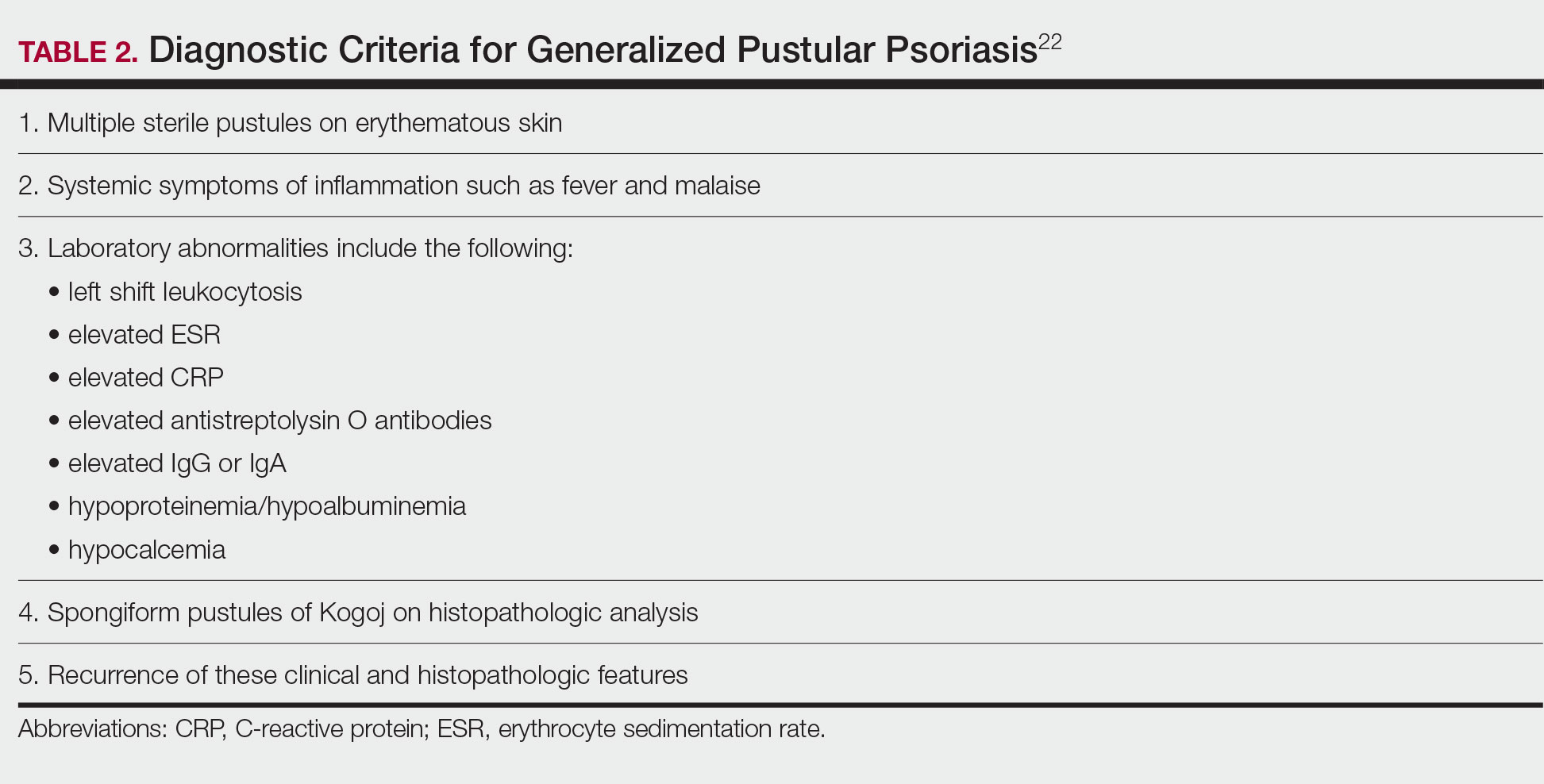
Histologic Features
Given the potential for the skin manifestations of GPP to mimic other disorders, a skin biopsy is warranted to confirm the diagnosis. Generalized pustular psoriasis is histologically characterized by the presence of subcorneal macropustules (ie, spongiform pustules of Kogoj) formed by neutrophil infiltration into the spongelike network of the epidermis.6 Otherwise, the architecture of the epithelium in GPP is similar to that seen with plaque psoriasis, with parakeratosis, acanthosis, rete-ridge elongation, diminished stratum granulosum, and thinning of the suprapapillary epidermis, though the inflammatory cell infiltrate and edema are markedly more severe in GPP than plaque psoriasis.3,4
Differential Diagnosis
There are many other cutaneous pustular diagnoses that must be ruled out when evaluating a patient with GPP (Table 1).26 Acute generalized exanthematous pustulosis (AGEP) is a common mimicker of GPP that is differentiated histologically by the presence of eosinophils and necrotic keratinocytes.4 In addition to its distinct histopathologic findings, AGEP is classically associated with recent initiation of certain medications, most commonly penicillins, macrolides, quinolones, sulfonamides, terbinafine, and diltiazem.27 In contrast, GPP more commonly is related to withdrawal of corticosteroids as well as initiation of some biologic medications, including anti-TNF agents.3 Generalized pustular psoriasis should be suspected over AGEP in patients with a personal or family history of psoriasis, though GPP may arise in patients with or without a history of psoriasis. Acute generalized exanthematous pustulosis usually is more abrupt in both onset and resolution compared with GPP, with clearance of pustules within a few days to weeks following cessation of the triggering factor.4
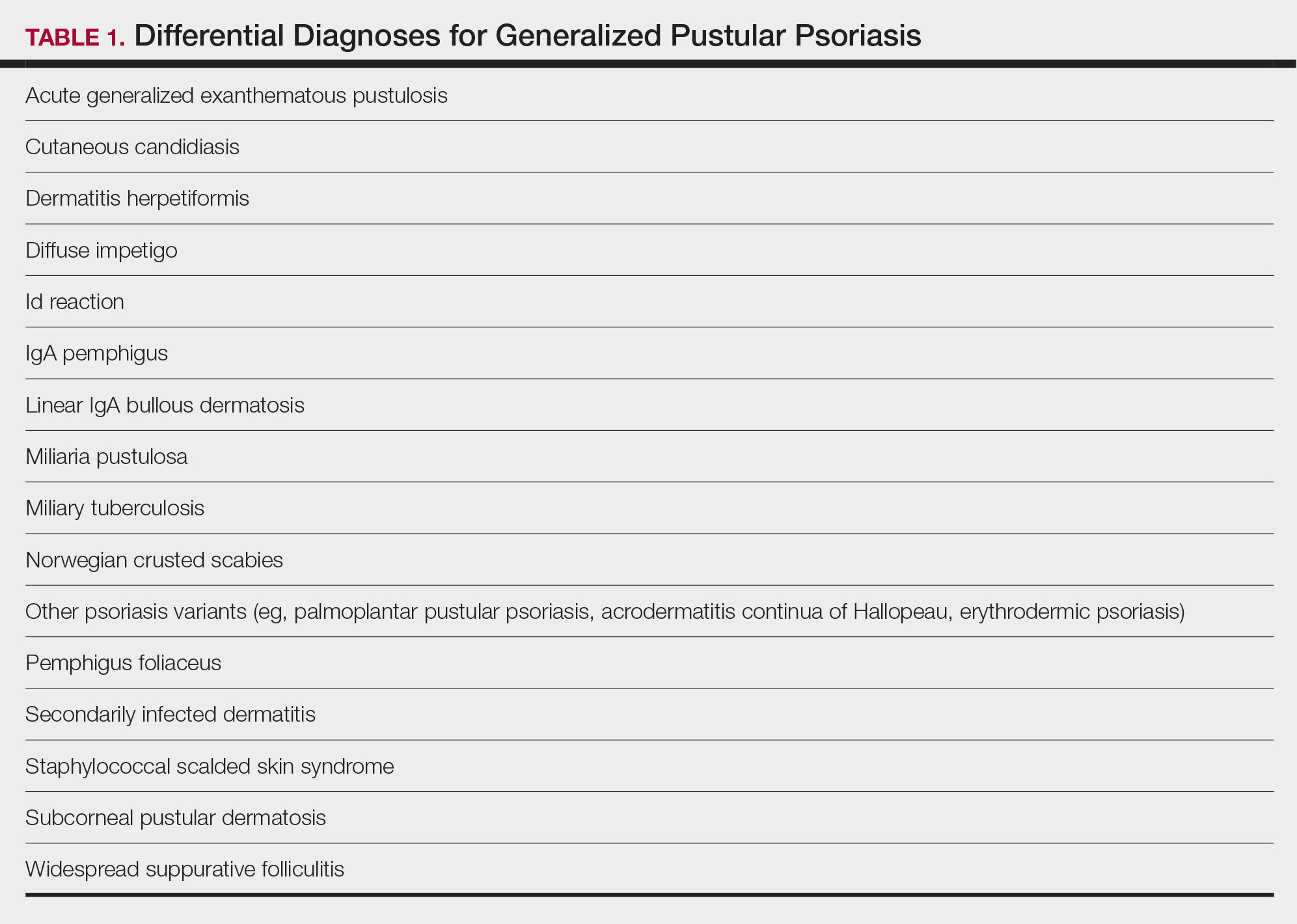
Other pustular variants of psoriasis (eg, palmoplantar pustular psoriasis, acrodermatitis continua of Hallopeau) are differentiated from GPP by their chronicity and localization to palmoplantar and/or ungual surfaces.5 Other differential diagnoses are listed in Table 1.
Diagnostic Criteria for GPP
Diagnostic criteria have been proposed for GPP (Table 2), including (1) the presence of sterile pustules, (2) systemic signs of inflammation, (3) laboratory abnormalities, (4) histopathologic confirmation of spongiform pustules of Kogoj, and (5) recurrence of symptoms.22 To definitively diagnose GPP, all 5 criteria must be met. To rule out mimickers, it may be worthwhile to perform Gram staining, potassium hydroxide preparation, in vitro cultures, and/or immunofluorescence testing.6
Treatment
Given the high potential for mortality associated with GPP, the most essential component of management is to ensure adequate supportive care. Any temperature, fluid, or electrolyte imbalances should be corrected as they arise. Secondary infections also must be identified and treated, if present, to reduce the risk for fatal complications, including systemic infection and sepsis. Precautions must be taken to ensure that serious end-organ damage, including hepatic, renal, and respiratory dysfunction, is avoided.
Adjunctive topical intervention often is initiated with bland emollients, corticosteroids, calcineurin inhibitors, and/or vitamin D derivatives to help soothe skin symptoms, but treatment with systemic therapies usually is warranted to achieve symptom control.2,25 Importantly, there are no systemic or topical agents that have specifically been approved for the treatment of GPP in Europe or the United States.3 Given the absence of universally accepted treatment guidelines, therapeutic agents for GPP usually are selected based on clinical experience while also taking the extent of involvement and disease severity into consideration.3
Treatment Recommendations for Adults
Oral Systemic Agents—Treatment guidelines set forth by the National Psoriasis Foundation (NPF) in 2012 proposed that first-line therapies for GPP should be acitretin, cyclosporine, methotrexate, and infliximab.28 However, since those guidelines were established, many new biologic therapies have been approved for the treatment of psoriasis and often are considered in the treatment of psoriasis subtypes, including GPP.29 Although retinoids previously were considered to be a preferred first-line therapy, they are associated with a high incidence of adverse effects and must be used with caution in women of childbearing age.6 Oral acitretin at a dosage of 0.75 to 1.0 mg/kg/d has been shown to result in clinical improvement within 1 to 2 weeks, and a maintenance dosage of 0.125 to 0.25 mg/kg/d is required for several months to prevent recurrence.30 Methotrexate—5.0 to 15.0 mg/wk, or perhaps higher in patients with refractory disease, increased by 2.5-mg intervals until symptoms improve—is recommended by the NPF in patients who are unresponsive or cannot tolerate retinoids, though close monitoring for hematologic abnormalities is required. Cyclosporine 2.5 to 5.0 mg/kg/d is considered an alternative to methotrexate and retinoids; it has a faster onset of action, with improvement reported as early as 2 weeks after initiation of therapy.1,28 Although cyclosporine may be effective in the acute phase, especially in severe cases of GPP, long-term use of cyclosporine is not recommended because of the potential for renal dysfunction and hypertension.31
Biologic Agents—More recent evidence has accumulated supporting the efficacy of anti-TNF agents in the treatment of GPP, suggesting the positioning of these agents as first line. A number of case series have shown dramatic and rapid improvement of GPP with intravenous infliximab 3 to 5 mg/kg, with results observed hours to days after the first infusion.32-37 Thus, infliximab is recommended as first-line treatment in severe acute cases, though its efficacy as a maintenance therapy has not been sufficiently investigated.6 Case reports and case series document the safety and efficacy of adalimumab 40 to 80 mg every 1 to 2 weeks38,39 and etanercept 25 to 50 mg twice weekly40-42 in patients with recalcitrantGPP. Therefore, these anti-TNF agents may be considered in patients who are nonresponsive to treatment with infliximab.
Rarely, there have been reports of paradoxical induction of GPP with the use of some anti-TNF agents,43-45 which may be due to a cytokine imbalance characterized by unopposed IFN-α activation.6 In patients with a history of GPP after initiation of a biologic, treatment with agents from within the offending class should be avoided.
The IL-17A monoclonal antibodies secukinumab, ixekizumab, and brodalumab have been shown in open-label phase 3 studies to result in disease remission at 12 weeks.46-48 Treatment with guselkumab, an IL-23 monoclonal antibody, also has demonstrated efficacy in patients with GPP.49 Ustekinumab, an IL-12/23 inhibitor, in combination with acitretin also has been shown to be successful in achieving disease remission after a few weeks of treatment.50
More recent case reports have shown the efficacy of IL-1 inhibitors including gevokizumab, canakinumab, and anakinra in achieving GPP clearance, though more prospective studies are needed to evaluate their efficacy.51-53 Given the etiologic association between IL-1 disinhibition and GPP, future investigations of these therapies as well as those that target the IL-36 pathway may prove to be particularly interesting.
Phototherapy and Combination Therapies—Phototherapy may be considered as maintenance therapy after disease control is achieved, though it is not considered appropriate for acute cases.28 Combination therapies with a biologic plus a nonbiologic systemic agent or alternating among various biologics may allow physicians to maximize benefits and minimize adverse effects in the long term, though there is insufficient evidence to suggest any specific combination treatment algorithm for GPP.28
Treatment Recommendations for Pediatric Patients
Based on a small number of case series and case reports, the first-line treatment strategy for children with GPP is similar to adults. Given the notable adverse events of most oral systemic agents, biologic therapies may emerge as first-line therapy in the pediatric population as more evidence accumulates.28
Treatment Recommendations for Pregnant Patients
Systemic corticosteroids are widely considered to be the first-line treatments for the management of impetigo herpetiformis.7 Low-dose prednisone (15–30 mg/d) usually is effective, but severe cases may require increasing the dosage to 60 mg/d.6 Given the potential for rebound flares upon withdrawal of systemic corticosteroids, these agents must be gradually tapered after the resolution of symptoms.
Certolizumab pegol also is an attractive option in pregnant patients with impetigo herpetiformis because of its favorable safety profile and negligible mother-to-infant transfer through the placenta or breast milk. It has been shown to be effective in treating GPP and impetigo herpetiformis during pregnancy in recently published case reports.54,55 In refractory cases, other TNF-α inhibitors (eg, adalimumab, infliximab, etanercept) or cyclosporine may be considered. However, cautious medical monitoring is warranted, as little is known about the potential adverse effects of these agents to the mother and fetus.28,56 Data from transplant recipients along with several case reports indicate that cyclosporine is not associated with an increased risk for adverse effects during pregnancy at a dose of 2 to 3 mg/kg.57-59 Both methotrexate and retinoids are known teratogens and are therefore contraindicated in pregnant patients.56
If pustules do not resolve in the postpartum period, patients should be treated with standard GPP therapies. However, long-term and population studies are lacking regarding the potential for infant exposure to systemic agents in breast milk. Therefore, the NPF recommends avoiding breastfeeding while taking systemic medications, if possible.56
Limitations of Treatment Recommendations
The ability to generate an evidence-based treatment strategy for GPP is limited by a lack of high-quality studies investigating the efficacy and safety of treatments in patients with GPP due to the rarity and relapsing-remitting nature of the disease, which makes randomized controlled trials difficult to conduct. The quality of the available research is further limited by the lack of validated outcome measures to specifically assess improvements in patients with GPP, such that results are difficult to synthesize and compare among studies.31
Conclusion
Although limited, the available research suggests that treatment with various biologics, especially infliximab, is effective in achieving rapid clearance in patients with GPP. In general, biologics may be the most appropriate treatment option in patients with GPP given their relatively favorable safety profiles. Other oral systemic agents, including acitretin, cyclosporine, and methotrexate, have limited evidence to support their use in the acute phase, but their safety profiles often limit their utility in the long-term. Emerging evidence regarding the association of GPP with IL36RN mutations suggests a unique role for agents targeting the IL-36 or IL-1 pathways, though this has yet to be thoroughly investigated.
- Benjegerdes KE, Hyde K, Kivelevitch D, et al. Pustular psoriasis: pathophysiology and current treatment perspectives. Psoriasis (Auckl). 2016;6:131‐144.
- Bachelez H. Pustular psoriasis and related pustular skin diseases. Br J Dermatol. 2018;178:614‐618.
- Gooderham MJ, Van Voorhees AS, Lebwohl MG. An update on generalized pustular psoriasis. Expert Rev Clin Immunol. 2019;15:907‐919.
- Ly K, Beck KM, Smith MP, et al. Diagnosis and screening of patients with generalized pustular psoriasis. Psoriasis (Auckl). 2019;9:37‐42.
- van de Kerkhof PCM, Nestle FO. Psoriasis. In: Bolognia JL, Jorizzo JJ, Schaffer JV, eds. Dermatology. 3rd ed. Elsevier; 2012:138-160.
- Hoegler KM, John AM, Handler MZ, et al. Generalized pustular psoriasis: a review and update on treatment. J Eur Acad Dermatol Venereol. 2018;32:1645‐1651.
- Oumeish OY, Parish JL. Impetigo herpetiformis. Clin Dermatol. 2006;24:101‐104.
- Johnston A, Xing X, Wolterink L, et al. IL-1 and IL-36 are dominant cytokines in generalized pustular psoriasis. J Allergy Clin Immunol. 2017;140:109-120.
- Furue K, Yamamura K, Tsuji G, et al. Highlighting interleukin-36 signalling in plaque psoriasis and pustular psoriasis. Acta Derm Venereol. 2018;98:5-13.
- Ogawa E, Sato Y, Minagawa A, et al. Pathogenesis of psoriasis and development of treatment. J Dermatol. 2018;45:264-272.
- Marrakchi S, Guigue P, Renshaw BR, et al. Interleukin-36-receptor antagonist deficiency and generalized pustular psoriasis. N Engl J Med. 2011;365:620-628.
- Onoufriadis A, Simpson MA, Pink AE, et al. Mutations in IL36RN/IL1F5 are associated with the severe episodic inflammatory skin disease known as generalized pustular psoriasis. Am J Hum Genet. 2011;89:432-437.
- Setta-Kaffetzi N, Navarini AA, Patel VM, et al. Rare pathogenic variants in IL36RN underlie a spectrum of psoriasis-associated pustular phenotypes. J Invest Dermatol. 2013;133:1366-1369.
- Sugiura K, Takemoto A, Yamaguchi M, et al. The majority of generalized pustular psoriasis without psoriasis vulgaris is caused by deficiency of interleukin-36 receptor antagonist. J Invest Dermatol. 2013;133:2514-2521.
- Hussain S, Berki DM, Choon SE, et al. IL36RN mutations define a severe autoinflammatory phenotype of generalized pustular psoriasis. J Allergy Clin Immunol. 2015;135:1067-1070.e9.
- Körber A, Mossner R, Renner R, et al. Mutations in IL36RN in patients with generalized pustular psoriasis. J Invest Dermatol. 2013;133:2634-2637.
- Twelves S, Mostafa A, Dand N, et al. Clinical and genetic differences between pustular psoriasis subtypes. J Allergy Clin Immunol. 2019;143:1021-1026.
- Sugiura K. The genetic background of generalized pustular psoriasis: IL36RN mutations and CARD14 gain-of-function variants. J Dermatol Sci. 2014;74:187-192
- Wang Y, Cheng R, Lu Z, et al. Clinical profiles of pediatric patients with GPP alone and with different IL36RN genotypes. J Dermatol Sci. 2017;85:235-240.
- Setta-Kaffetzi N, Simpson MA, Navarini AA, et al. AP1S3 mutations are associated with pustular psoriasis and impaired Toll-like receptor 3 trafficking. Am J Hum Genet. 2014;94:790-797.
- Mahil SK, Twelves S, Farkas K, et al. AP1S3 mutations cause skin autoinflammation by disrupting keratinocyte autophagy and upregulating IL-36 production. J Invest Dermatol. 2016;136:2251-2259.
- Umezawa Y, Ozawa A, Kawasima T, et al. Therapeutic guidelines for the treatment of generalized pustular psoriasis (GPP) based on a proposed classification of disease severity. Arch Dermatol Res. 2003;295(suppl 1):S43-S54.
- Viguier M, Allez M, Zagdanski AM, et al. High frequency of cholestasis in generalized pustular psoriasis: evidence for neutrophilic involvement of the biliary tract. Hepatology. 2004;40:452-458.
- Ryan TJ, Baker H. The prognosis of generalized pustular psoriasis. Br J Dermatol. 1971;85:407-411.
- Kalb RE. Pustular psoriasis: management. In: Ofori AO, Duffin KC, eds. UpToDate. UpToDate; 2014. Accessed July 20, 2022. https://www.uptodate.com/contents/pustular-psoriasis-management/print
- Naik HB, Cowen EW. Autoinflammatory pustular neutrophilic diseases. Dermatol Clin. 2013;31:405-425.
- Sidoroff A, Dunant A, Viboud C, et al. Risk factors for acute generalized exanthematous pustulosis (AGEP)—results of a multinational case-control study (EuroSCAR). Br J Dermatol. 2007;157:989-996.
- Robinson A, Van Voorhees AS, Hsu S, et al. Treatment of pustular psoriasis: from the Medical Board of the National Psoriasis Foundation. J Am Acad Dermatol. 2012;67:279‐288.
- Menter A, Strober BE, Kaplan DH, et al. Joint AAD-NPF guidelines of care for the management and treatment of psoriasis with biologics. J Am Acad Dermatol. 2019;80:1029-1072.
- Mengesha YM, Bennett ML. Pustular skin disorders: diagnosis and treatment. Am J Clin Dermatol 2002;3:389-400.
- Zhou LL, Georgakopoulos JR, Ighani A, et al. Systemic monotherapy treatments for generalized pustular psoriasis: a systematic review. J Cutan Med Surg. 2018;22:591‐601.
- Elewski BE. Infliximab for the treatment of severe pustular psoriasis. J Am Acad Dermatol. 2002;47:796-797.
- Kim HS, You HS, Cho HH, et al. Two cases of generalized pustular psoriasis: successful treatment with infliximab. Ann Dermatol. 2014;26:787-788.
- Trent JT, Kerdel FA. Successful treatment of Von Zumbusch pustular psoriasis with infliximab. J Cutan Med Surg. 2004;8:224-228.
- Poulalhon N, Begon E, Lebbé C, et al. A follow-up study in 28 patients treated with infliximab for severe recalcitrant psoriasis: evidence for efficacy and high incidence of biological autoimmunity. Br J Dermatol. 2007;156:329-336.
- Routhouska S, Sheth PB, Korman NJ. Long-term management of generalized pustular psoriasis with infliximab: case series. J Cutan Med Surg. 2008;12:184-188.
- Lisby S, Gniadecki R. Infliximab (Remicade) for acute, severe pustular and erythrodermic psoriasis. Acta Derm Venereol. 2004;84:247-248.
- Zangrilli A, Papoutsaki M, Talamonti M, et al. Long-term efficacy of adalimumab in generalized pustular psoriasis. J Dermatol Treat. 2008;19:185-187.
- Matsumoto A, Komine M, Karakawa M, et al. Adalimumab administration after infliximab therapy is a successful treatment strategy for generalized pustular psoriasis. J Dermatol. 2017;44:202-204.
- Kamarashev J, Lor P, Forster A, et al. Generalized pustular psoriasis induced by cyclosporin in a withdrawal responding to the tumour necrosis factor alpha inhibitor etanercept. Dermatology. 2002;205:213-216.
- Esposito M, Mazzotta A, Casciello C, et al. Etanercept at different dosages in the treatment of generalized pustular psoriasis: a case series. Dermatology. 2008;216:355-360.
- Lo Schiavo A, Brancaccio G, Puca RV, et al. Etanercept in the treatment of generalized annular pustular psoriasis. Ann Dermatol. 2012;24:233-234.
- Goiriz R, Daudén E, Pérez-Gala S, et al. Flare and change of psoriasis morphology during the course of treatment with tumor necrosis factor blockers. Clin Exp Dermatol. 2006;32:176-179.
- Collamer AN, Battafarano DF. Psoriatic skin lesions induced by tumor necrosis factor antagonist therapy: clinical features and possible immunopathogenesis. Semin Arthritis Rheum. 2010;40:233-240.
- Almutairi D, Sheasgreen C, Weizman A, et al. Generalized pustular psoriasis induced by infliximab in a patient with inflammatory bowel disease. J Cutan Med Surg. 2018;1:507-510.
- Imafuku S, Honma M, Okubo Y, et al. Efficacy and safety of secukinumab in patients with generalized pustular psoriasis: a 52-week analysis from phase III open-label multicenter Japanese study. J Dermatol. 2016;43:1011-1017
- Saeki H, Nakagawa H, Ishii T, et al. Efficacy and safety of open-label ixekizumab treatment in Japanese patients with moderate-to-severe plaque psoriasis, erythrodermic psoriasis, and generalized pustular psoriasis. J Eur Acad Dermatol Venereol. 2015;29:1148-1155.
- Yamasaki K, Nakagawa H, Kubo Y, et al. Efficacy and safety of brodalumab in patients with generalized pustular psoriasis and psoriatic erythroderma: results from a 52-week, open-label study. Br J Dermatol. 2017;176:741-751.
- Sano S, Kubo H, Morishima H, et al. Guselkumab, a human interleukin-23 monoclonal antibody in Japanese patients with generalized pustular psoriasis and erythrodermic psoriasis: efficacy and safety analyses of a 52-week, phase 3, multicenter, open-label study. J Dermatol. 2018;45:529‐539.
- Arakawa A, Ruzicka T, Prinz JC. Therapeutic efficacy of interleukin 12/interleukin 23 blockade in generalized pustular psoriasis regardless of IL36RN mutation status. JAMA Dermatol. 2016;152:825-828.
- Mansouri B, Richards L, Menter A. Treatment of two patients with generalized pustular psoriasis with the interleukin-1beta inhibitor gevokizumab. Br J Dermatol. 2015;173:239-241.
- Skendros P, Papagoras C, Lefaki I, et al. Successful response in a case of severe pustular psoriasis after interleukin-1 beta inhibition. Br J Dermatol. 2017;176:212-215.
- Viguier M, Guigue P, Pagès C, et al. Successful treatment of generalized pustular psoriasis with the interleukin-1-receptor antagonist Anakinra: lack of correlation with IL1RN mutations. Ann Intern Med. 2010;153:66-67.
- Fukushima H, Iwata Y, Arima M, et al. Efficacy and safety of treatment with anti-tumor necrosis factor‐α drugs for severe impetigo herpetiformis. J Dermatol. 2021;48:207-210.
- Mizutani Y, Mizutani YH, Matsuyama K, et al. Generalized pustular psoriasis in pregnancy, successfully treated with certolizumab pegol. J Dermatol. 2021;47:e262-e263.
- Bae YS, Van Voorhees AS, Hsu S, et al. Review of treatment options for psoriasis in pregnant or lactating women: from the Medical Board of the National Psoriasis Foundation. J Am Acad Dermatol. 2012;67:459‐477.
- Finch TM, Tan CY. Pustular psoriasis exacerbated by pregnancy and controlled by cyclosporin A. Br J Dermatol. 2000;142:582-584.
- Gaughan WJ, Moritz MJ, Radomski JS, et al. National Transplantation Pregnancy Registry: report on outcomes of cyclosporine-treated female kidney transplant recipients with an interval from transplantation to pregnancy of greater than five years. Am J Kidney Dis. 1996;28:266-269.
- Kura MM, Surjushe AU. Generalized pustular psoriasis of pregnancy treated with oral cyclosporin. Indian J Dermatol Venereol Leprol. 2006;72:458-459.
Acute generalized pustular psoriasis (GPP) is a rare severe variant of psoriasis characterized by the sudden widespread eruption of sterile pustules.1,2 The cutaneous manifestations of GPP also may be accompanied by signs of systemic inflammation, including fever, malaise, and leukocytosis.2 Complications are common and may be life-threatening, especially in older patients with comorbid diseases.3 Generalized pustular psoriasis most commonly occurs in patients with a preceding history of psoriasis, but it also may occur de novo.4 Generalized pustular psoriasis is associated with notable morbidity and mortality, and relapses are common.3,4 Many triggers of GPP have been identified, including initiation and withdrawal of various medications, infections, pregnancy, and other conditions.5,6 Although GPP most often occurs in adults, it also may arise in children and infants.3 In pregnancy, GPP is referred to as impetigo herpetiformis, despite having no etiologic ties with either herpes simplex virus or staphylococcal or streptococcal infection. Impetigo herpetiformis is considered one of the most dangerous dermatoses of pregnancy because of high rates of associated maternal and fetal morbidity.6,7
Acute GPP has proven to be a challenging disease to treat due to the rarity and relapsing-remitting nature of the disease; additionally, there are relatively few randomized controlled trials investigating the efficacy and safety of treatments for GPP. This review summarizes the features of GPP, including the pathophysiology of the disease, clinical and histological manifestations, and recommendations for management based on a PubMed search of articles indexed for MEDLINE using MeSH terms pertaining to the disease, including generalized pustular psoriasis, impetigo herpetiformis, and von Zumbusch psoriasis.
Pathophysiology
The pathophysiology of GPP is only partially understood, but it is thought to have a distinct pattern of immune activation compared with plaque psoriasis.8 Although there is a considerable amount of overlap and cross-talk among cytokine pathways, GPP generally is driven by innate immunity and unrestrained IL-36 cytokine activity. In contrast, adaptive immune responses—namely the tumor necrosis factor (TNF) α, IL-23, IL-17, and IL-22 axes—underlie plaque psoriasis.8-10
Proinflammatory IL-36 cytokines α, β, and γ, which are all part of the IL-1 superfamily, bind to the IL-36 receptor (IL-36R) to recruit and activate immune cells via various mediators, including IL-1β; IL-8; and chemokines CXCL1, CXCL2, and CXCL8.3 The IL-36 receptor antagonist (IL-36ra) acts to inhibit this inflammatory cascade.3,8 Microarray analyses of skin biopsy samples have shown that overexpression of IL-17A, TNF-α, IL-1, and IL-36 are seen in both GPP and plaque psoriasis lesions, but GPP lesions had higher expression of IL-1β, IL-36α, and IL-36γ and elevated neutrophil chemokines—CXCL1, CXCL2, and CXCL8—compared with plaque psoriasis lesions.8
Gene Mutations Associated With GPP
There are 3 gene mutations that have been associated with pustular variants of psoriasis, though these mutations account for a minority of cases of GPP.4 Genetic screenings are not routinely indicated in patients with GPP, but they may be warranted in severe cases when a familial pattern of inheritance is suspected.4
IL36RN—The gene IL36RN codes the anti-inflammatory IL-36ra. Loss-of-function mutations in IL36RN lead to impairment of IL-36ra and consequently hyperactivity of the proinflammatory responses triggered by IL-36.3 Homozygous and heterozygous mutations in IL36RN have been observed in both familial and sporadic cases of GPP.11-13 Subsequent retrospective analyses have identified the presence of IL36RN mutations in patients with GPP with frequencies ranging from 23% to 37%.14-17IL36RN mutations are thought to be more common in patients without concomitant plaque psoriasis and have been associated with severe disease and early disease onset.15
CARD14—A gain-of-function mutation in CARD14 results in overactivation of the proinflammatory nuclear factor κB pathway and has been implicated in cases of GPP with concurrent psoriasis vulgaris. Interestingly, this may suggest distinct etiologies underlying GPP de novo and GPP in patients with a history of psoriasis.18,19
AP1S3—A loss-of-function mutation in AP1S3 results in abnormal endosomal trafficking and autophagy as well as increased expression of IL-36α.20,21
Clinical Presentation and Diagnosis Cutaneous Manifestations of GPP
Generalized pustular psoriasis is characterized by the onset of widespread 2- to 3-mm sterile pustules on erythematous skin or within psoriasiform plaques4 (Figure). In patients with skin of color, the erythema may appear less obvious or perhaps slightly violaceous compared to White skin. Pustules may coalesce to form “lakes” of pus.5 Cutaneous symptoms include pain, burning, and pruritus. Associated mucosal findings may include cheilitis, geographic tongue, conjunctivitis, and uveitis.4

The severity of symptoms can vary greatly among patients as well as between flares within the same patient.2,3 Four distinct patterns of GPP have been described. The von Zumbusch pattern is characterized by a rapid, generalized, painful, erythematous and pustular eruption accompanied by fever and asthenia. The pustules usually resolve after several days with extensive scaling. The annular pattern is characterized by annular, erythematous, scaly lesions with pustules present centrifugally. The lesions enlarge by centrifugal expansion over a period of hours to days, while healing occurs centrally. The exanthematic type is an acute eruption of small pustules that abruptly appear and disappear within a few days, usually from infection or medication initiation. Sometimes pustules appear within or at the edge of existing psoriatic plaques in a localized pattern—the fourth pattern—often following the exposure to irritants (eg, tars, anthralin).5
Impetigo Herpetiformis—Impetigo herpetiformis is a form of GPP associated with pregnancy. It generally presents early in the third trimester with symmetric erythematous plaques in flexural and intertriginous areas with pustules present at lesion margins. Lesions expand centrifugally, with pustulation present at the advancing edge.6,7 Patients often are acutely ill with fever, delirium, vomiting, and tetany. Mucous membranes, including the tongue, mouth, and esophagus, also may be involved. The eruption typically resolves after delivery, though it often recurs with subsequent pregnancies, with the morbidity risk rising with each successive pregnancy.7
Systemic and Extracutaneous Manifestations of GPP
Although the severity of GPP is highly variable, skin manifestations often are accompanied by systemic manifestations of inflammation, including fever and malaise. Common laboratory abnormalities include leukocytosis with peripheral neutrophilia, a high serum C-reactive protein level, hypocalcemia, and hypoalbuminemia.22 Abnormal liver enzymes often are present and result from neutrophilic cholangitis, with alternating strictures and dilations of biliary ducts observed on magnetic resonance imaging.23 Additional laboratory abnormalities are provided in Table 2. Other extracutaneous findings associated with GPP include arthralgia, edema, and characteristic psoriatic nail changes.4 Fatal complications include acute respiratory distress syndrome, renal dysfunction, cardiovascular shock, and sepsis.24,25

Histologic Features
Given the potential for the skin manifestations of GPP to mimic other disorders, a skin biopsy is warranted to confirm the diagnosis. Generalized pustular psoriasis is histologically characterized by the presence of subcorneal macropustules (ie, spongiform pustules of Kogoj) formed by neutrophil infiltration into the spongelike network of the epidermis.6 Otherwise, the architecture of the epithelium in GPP is similar to that seen with plaque psoriasis, with parakeratosis, acanthosis, rete-ridge elongation, diminished stratum granulosum, and thinning of the suprapapillary epidermis, though the inflammatory cell infiltrate and edema are markedly more severe in GPP than plaque psoriasis.3,4
Differential Diagnosis
There are many other cutaneous pustular diagnoses that must be ruled out when evaluating a patient with GPP (Table 1).26 Acute generalized exanthematous pustulosis (AGEP) is a common mimicker of GPP that is differentiated histologically by the presence of eosinophils and necrotic keratinocytes.4 In addition to its distinct histopathologic findings, AGEP is classically associated with recent initiation of certain medications, most commonly penicillins, macrolides, quinolones, sulfonamides, terbinafine, and diltiazem.27 In contrast, GPP more commonly is related to withdrawal of corticosteroids as well as initiation of some biologic medications, including anti-TNF agents.3 Generalized pustular psoriasis should be suspected over AGEP in patients with a personal or family history of psoriasis, though GPP may arise in patients with or without a history of psoriasis. Acute generalized exanthematous pustulosis usually is more abrupt in both onset and resolution compared with GPP, with clearance of pustules within a few days to weeks following cessation of the triggering factor.4

Other pustular variants of psoriasis (eg, palmoplantar pustular psoriasis, acrodermatitis continua of Hallopeau) are differentiated from GPP by their chronicity and localization to palmoplantar and/or ungual surfaces.5 Other differential diagnoses are listed in Table 1.
Diagnostic Criteria for GPP
Diagnostic criteria have been proposed for GPP (Table 2), including (1) the presence of sterile pustules, (2) systemic signs of inflammation, (3) laboratory abnormalities, (4) histopathologic confirmation of spongiform pustules of Kogoj, and (5) recurrence of symptoms.22 To definitively diagnose GPP, all 5 criteria must be met. To rule out mimickers, it may be worthwhile to perform Gram staining, potassium hydroxide preparation, in vitro cultures, and/or immunofluorescence testing.6
Treatment
Given the high potential for mortality associated with GPP, the most essential component of management is to ensure adequate supportive care. Any temperature, fluid, or electrolyte imbalances should be corrected as they arise. Secondary infections also must be identified and treated, if present, to reduce the risk for fatal complications, including systemic infection and sepsis. Precautions must be taken to ensure that serious end-organ damage, including hepatic, renal, and respiratory dysfunction, is avoided.
Adjunctive topical intervention often is initiated with bland emollients, corticosteroids, calcineurin inhibitors, and/or vitamin D derivatives to help soothe skin symptoms, but treatment with systemic therapies usually is warranted to achieve symptom control.2,25 Importantly, there are no systemic or topical agents that have specifically been approved for the treatment of GPP in Europe or the United States.3 Given the absence of universally accepted treatment guidelines, therapeutic agents for GPP usually are selected based on clinical experience while also taking the extent of involvement and disease severity into consideration.3
Treatment Recommendations for Adults
Oral Systemic Agents—Treatment guidelines set forth by the National Psoriasis Foundation (NPF) in 2012 proposed that first-line therapies for GPP should be acitretin, cyclosporine, methotrexate, and infliximab.28 However, since those guidelines were established, many new biologic therapies have been approved for the treatment of psoriasis and often are considered in the treatment of psoriasis subtypes, including GPP.29 Although retinoids previously were considered to be a preferred first-line therapy, they are associated with a high incidence of adverse effects and must be used with caution in women of childbearing age.6 Oral acitretin at a dosage of 0.75 to 1.0 mg/kg/d has been shown to result in clinical improvement within 1 to 2 weeks, and a maintenance dosage of 0.125 to 0.25 mg/kg/d is required for several months to prevent recurrence.30 Methotrexate—5.0 to 15.0 mg/wk, or perhaps higher in patients with refractory disease, increased by 2.5-mg intervals until symptoms improve—is recommended by the NPF in patients who are unresponsive or cannot tolerate retinoids, though close monitoring for hematologic abnormalities is required. Cyclosporine 2.5 to 5.0 mg/kg/d is considered an alternative to methotrexate and retinoids; it has a faster onset of action, with improvement reported as early as 2 weeks after initiation of therapy.1,28 Although cyclosporine may be effective in the acute phase, especially in severe cases of GPP, long-term use of cyclosporine is not recommended because of the potential for renal dysfunction and hypertension.31
Biologic Agents—More recent evidence has accumulated supporting the efficacy of anti-TNF agents in the treatment of GPP, suggesting the positioning of these agents as first line. A number of case series have shown dramatic and rapid improvement of GPP with intravenous infliximab 3 to 5 mg/kg, with results observed hours to days after the first infusion.32-37 Thus, infliximab is recommended as first-line treatment in severe acute cases, though its efficacy as a maintenance therapy has not been sufficiently investigated.6 Case reports and case series document the safety and efficacy of adalimumab 40 to 80 mg every 1 to 2 weeks38,39 and etanercept 25 to 50 mg twice weekly40-42 in patients with recalcitrantGPP. Therefore, these anti-TNF agents may be considered in patients who are nonresponsive to treatment with infliximab.
Rarely, there have been reports of paradoxical induction of GPP with the use of some anti-TNF agents,43-45 which may be due to a cytokine imbalance characterized by unopposed IFN-α activation.6 In patients with a history of GPP after initiation of a biologic, treatment with agents from within the offending class should be avoided.
The IL-17A monoclonal antibodies secukinumab, ixekizumab, and brodalumab have been shown in open-label phase 3 studies to result in disease remission at 12 weeks.46-48 Treatment with guselkumab, an IL-23 monoclonal antibody, also has demonstrated efficacy in patients with GPP.49 Ustekinumab, an IL-12/23 inhibitor, in combination with acitretin also has been shown to be successful in achieving disease remission after a few weeks of treatment.50
More recent case reports have shown the efficacy of IL-1 inhibitors including gevokizumab, canakinumab, and anakinra in achieving GPP clearance, though more prospective studies are needed to evaluate their efficacy.51-53 Given the etiologic association between IL-1 disinhibition and GPP, future investigations of these therapies as well as those that target the IL-36 pathway may prove to be particularly interesting.
Phototherapy and Combination Therapies—Phototherapy may be considered as maintenance therapy after disease control is achieved, though it is not considered appropriate for acute cases.28 Combination therapies with a biologic plus a nonbiologic systemic agent or alternating among various biologics may allow physicians to maximize benefits and minimize adverse effects in the long term, though there is insufficient evidence to suggest any specific combination treatment algorithm for GPP.28
Treatment Recommendations for Pediatric Patients
Based on a small number of case series and case reports, the first-line treatment strategy for children with GPP is similar to adults. Given the notable adverse events of most oral systemic agents, biologic therapies may emerge as first-line therapy in the pediatric population as more evidence accumulates.28
Treatment Recommendations for Pregnant Patients
Systemic corticosteroids are widely considered to be the first-line treatments for the management of impetigo herpetiformis.7 Low-dose prednisone (15–30 mg/d) usually is effective, but severe cases may require increasing the dosage to 60 mg/d.6 Given the potential for rebound flares upon withdrawal of systemic corticosteroids, these agents must be gradually tapered after the resolution of symptoms.
Certolizumab pegol also is an attractive option in pregnant patients with impetigo herpetiformis because of its favorable safety profile and negligible mother-to-infant transfer through the placenta or breast milk. It has been shown to be effective in treating GPP and impetigo herpetiformis during pregnancy in recently published case reports.54,55 In refractory cases, other TNF-α inhibitors (eg, adalimumab, infliximab, etanercept) or cyclosporine may be considered. However, cautious medical monitoring is warranted, as little is known about the potential adverse effects of these agents to the mother and fetus.28,56 Data from transplant recipients along with several case reports indicate that cyclosporine is not associated with an increased risk for adverse effects during pregnancy at a dose of 2 to 3 mg/kg.57-59 Both methotrexate and retinoids are known teratogens and are therefore contraindicated in pregnant patients.56
If pustules do not resolve in the postpartum period, patients should be treated with standard GPP therapies. However, long-term and population studies are lacking regarding the potential for infant exposure to systemic agents in breast milk. Therefore, the NPF recommends avoiding breastfeeding while taking systemic medications, if possible.56
Limitations of Treatment Recommendations
The ability to generate an evidence-based treatment strategy for GPP is limited by a lack of high-quality studies investigating the efficacy and safety of treatments in patients with GPP due to the rarity and relapsing-remitting nature of the disease, which makes randomized controlled trials difficult to conduct. The quality of the available research is further limited by the lack of validated outcome measures to specifically assess improvements in patients with GPP, such that results are difficult to synthesize and compare among studies.31
Conclusion
Although limited, the available research suggests that treatment with various biologics, especially infliximab, is effective in achieving rapid clearance in patients with GPP. In general, biologics may be the most appropriate treatment option in patients with GPP given their relatively favorable safety profiles. Other oral systemic agents, including acitretin, cyclosporine, and methotrexate, have limited evidence to support their use in the acute phase, but their safety profiles often limit their utility in the long-term. Emerging evidence regarding the association of GPP with IL36RN mutations suggests a unique role for agents targeting the IL-36 or IL-1 pathways, though this has yet to be thoroughly investigated.
Acute generalized pustular psoriasis (GPP) is a rare severe variant of psoriasis characterized by the sudden widespread eruption of sterile pustules.1,2 The cutaneous manifestations of GPP also may be accompanied by signs of systemic inflammation, including fever, malaise, and leukocytosis.2 Complications are common and may be life-threatening, especially in older patients with comorbid diseases.3 Generalized pustular psoriasis most commonly occurs in patients with a preceding history of psoriasis, but it also may occur de novo.4 Generalized pustular psoriasis is associated with notable morbidity and mortality, and relapses are common.3,4 Many triggers of GPP have been identified, including initiation and withdrawal of various medications, infections, pregnancy, and other conditions.5,6 Although GPP most often occurs in adults, it also may arise in children and infants.3 In pregnancy, GPP is referred to as impetigo herpetiformis, despite having no etiologic ties with either herpes simplex virus or staphylococcal or streptococcal infection. Impetigo herpetiformis is considered one of the most dangerous dermatoses of pregnancy because of high rates of associated maternal and fetal morbidity.6,7
Acute GPP has proven to be a challenging disease to treat due to the rarity and relapsing-remitting nature of the disease; additionally, there are relatively few randomized controlled trials investigating the efficacy and safety of treatments for GPP. This review summarizes the features of GPP, including the pathophysiology of the disease, clinical and histological manifestations, and recommendations for management based on a PubMed search of articles indexed for MEDLINE using MeSH terms pertaining to the disease, including generalized pustular psoriasis, impetigo herpetiformis, and von Zumbusch psoriasis.
Pathophysiology
The pathophysiology of GPP is only partially understood, but it is thought to have a distinct pattern of immune activation compared with plaque psoriasis.8 Although there is a considerable amount of overlap and cross-talk among cytokine pathways, GPP generally is driven by innate immunity and unrestrained IL-36 cytokine activity. In contrast, adaptive immune responses—namely the tumor necrosis factor (TNF) α, IL-23, IL-17, and IL-22 axes—underlie plaque psoriasis.8-10
Proinflammatory IL-36 cytokines α, β, and γ, which are all part of the IL-1 superfamily, bind to the IL-36 receptor (IL-36R) to recruit and activate immune cells via various mediators, including IL-1β; IL-8; and chemokines CXCL1, CXCL2, and CXCL8.3 The IL-36 receptor antagonist (IL-36ra) acts to inhibit this inflammatory cascade.3,8 Microarray analyses of skin biopsy samples have shown that overexpression of IL-17A, TNF-α, IL-1, and IL-36 are seen in both GPP and plaque psoriasis lesions, but GPP lesions had higher expression of IL-1β, IL-36α, and IL-36γ and elevated neutrophil chemokines—CXCL1, CXCL2, and CXCL8—compared with plaque psoriasis lesions.8
Gene Mutations Associated With GPP
There are 3 gene mutations that have been associated with pustular variants of psoriasis, though these mutations account for a minority of cases of GPP.4 Genetic screenings are not routinely indicated in patients with GPP, but they may be warranted in severe cases when a familial pattern of inheritance is suspected.4
IL36RN—The gene IL36RN codes the anti-inflammatory IL-36ra. Loss-of-function mutations in IL36RN lead to impairment of IL-36ra and consequently hyperactivity of the proinflammatory responses triggered by IL-36.3 Homozygous and heterozygous mutations in IL36RN have been observed in both familial and sporadic cases of GPP.11-13 Subsequent retrospective analyses have identified the presence of IL36RN mutations in patients with GPP with frequencies ranging from 23% to 37%.14-17IL36RN mutations are thought to be more common in patients without concomitant plaque psoriasis and have been associated with severe disease and early disease onset.15
CARD14—A gain-of-function mutation in CARD14 results in overactivation of the proinflammatory nuclear factor κB pathway and has been implicated in cases of GPP with concurrent psoriasis vulgaris. Interestingly, this may suggest distinct etiologies underlying GPP de novo and GPP in patients with a history of psoriasis.18,19
AP1S3—A loss-of-function mutation in AP1S3 results in abnormal endosomal trafficking and autophagy as well as increased expression of IL-36α.20,21
Clinical Presentation and Diagnosis Cutaneous Manifestations of GPP
Generalized pustular psoriasis is characterized by the onset of widespread 2- to 3-mm sterile pustules on erythematous skin or within psoriasiform plaques4 (Figure). In patients with skin of color, the erythema may appear less obvious or perhaps slightly violaceous compared to White skin. Pustules may coalesce to form “lakes” of pus.5 Cutaneous symptoms include pain, burning, and pruritus. Associated mucosal findings may include cheilitis, geographic tongue, conjunctivitis, and uveitis.4

The severity of symptoms can vary greatly among patients as well as between flares within the same patient.2,3 Four distinct patterns of GPP have been described. The von Zumbusch pattern is characterized by a rapid, generalized, painful, erythematous and pustular eruption accompanied by fever and asthenia. The pustules usually resolve after several days with extensive scaling. The annular pattern is characterized by annular, erythematous, scaly lesions with pustules present centrifugally. The lesions enlarge by centrifugal expansion over a period of hours to days, while healing occurs centrally. The exanthematic type is an acute eruption of small pustules that abruptly appear and disappear within a few days, usually from infection or medication initiation. Sometimes pustules appear within or at the edge of existing psoriatic plaques in a localized pattern—the fourth pattern—often following the exposure to irritants (eg, tars, anthralin).5
Impetigo Herpetiformis—Impetigo herpetiformis is a form of GPP associated with pregnancy. It generally presents early in the third trimester with symmetric erythematous plaques in flexural and intertriginous areas with pustules present at lesion margins. Lesions expand centrifugally, with pustulation present at the advancing edge.6,7 Patients often are acutely ill with fever, delirium, vomiting, and tetany. Mucous membranes, including the tongue, mouth, and esophagus, also may be involved. The eruption typically resolves after delivery, though it often recurs with subsequent pregnancies, with the morbidity risk rising with each successive pregnancy.7
Systemic and Extracutaneous Manifestations of GPP
Although the severity of GPP is highly variable, skin manifestations often are accompanied by systemic manifestations of inflammation, including fever and malaise. Common laboratory abnormalities include leukocytosis with peripheral neutrophilia, a high serum C-reactive protein level, hypocalcemia, and hypoalbuminemia.22 Abnormal liver enzymes often are present and result from neutrophilic cholangitis, with alternating strictures and dilations of biliary ducts observed on magnetic resonance imaging.23 Additional laboratory abnormalities are provided in Table 2. Other extracutaneous findings associated with GPP include arthralgia, edema, and characteristic psoriatic nail changes.4 Fatal complications include acute respiratory distress syndrome, renal dysfunction, cardiovascular shock, and sepsis.24,25

Histologic Features
Given the potential for the skin manifestations of GPP to mimic other disorders, a skin biopsy is warranted to confirm the diagnosis. Generalized pustular psoriasis is histologically characterized by the presence of subcorneal macropustules (ie, spongiform pustules of Kogoj) formed by neutrophil infiltration into the spongelike network of the epidermis.6 Otherwise, the architecture of the epithelium in GPP is similar to that seen with plaque psoriasis, with parakeratosis, acanthosis, rete-ridge elongation, diminished stratum granulosum, and thinning of the suprapapillary epidermis, though the inflammatory cell infiltrate and edema are markedly more severe in GPP than plaque psoriasis.3,4
Differential Diagnosis
There are many other cutaneous pustular diagnoses that must be ruled out when evaluating a patient with GPP (Table 1).26 Acute generalized exanthematous pustulosis (AGEP) is a common mimicker of GPP that is differentiated histologically by the presence of eosinophils and necrotic keratinocytes.4 In addition to its distinct histopathologic findings, AGEP is classically associated with recent initiation of certain medications, most commonly penicillins, macrolides, quinolones, sulfonamides, terbinafine, and diltiazem.27 In contrast, GPP more commonly is related to withdrawal of corticosteroids as well as initiation of some biologic medications, including anti-TNF agents.3 Generalized pustular psoriasis should be suspected over AGEP in patients with a personal or family history of psoriasis, though GPP may arise in patients with or without a history of psoriasis. Acute generalized exanthematous pustulosis usually is more abrupt in both onset and resolution compared with GPP, with clearance of pustules within a few days to weeks following cessation of the triggering factor.4

Other pustular variants of psoriasis (eg, palmoplantar pustular psoriasis, acrodermatitis continua of Hallopeau) are differentiated from GPP by their chronicity and localization to palmoplantar and/or ungual surfaces.5 Other differential diagnoses are listed in Table 1.
Diagnostic Criteria for GPP
Diagnostic criteria have been proposed for GPP (Table 2), including (1) the presence of sterile pustules, (2) systemic signs of inflammation, (3) laboratory abnormalities, (4) histopathologic confirmation of spongiform pustules of Kogoj, and (5) recurrence of symptoms.22 To definitively diagnose GPP, all 5 criteria must be met. To rule out mimickers, it may be worthwhile to perform Gram staining, potassium hydroxide preparation, in vitro cultures, and/or immunofluorescence testing.6
Treatment
Given the high potential for mortality associated with GPP, the most essential component of management is to ensure adequate supportive care. Any temperature, fluid, or electrolyte imbalances should be corrected as they arise. Secondary infections also must be identified and treated, if present, to reduce the risk for fatal complications, including systemic infection and sepsis. Precautions must be taken to ensure that serious end-organ damage, including hepatic, renal, and respiratory dysfunction, is avoided.
Adjunctive topical intervention often is initiated with bland emollients, corticosteroids, calcineurin inhibitors, and/or vitamin D derivatives to help soothe skin symptoms, but treatment with systemic therapies usually is warranted to achieve symptom control.2,25 Importantly, there are no systemic or topical agents that have specifically been approved for the treatment of GPP in Europe or the United States.3 Given the absence of universally accepted treatment guidelines, therapeutic agents for GPP usually are selected based on clinical experience while also taking the extent of involvement and disease severity into consideration.3
Treatment Recommendations for Adults
Oral Systemic Agents—Treatment guidelines set forth by the National Psoriasis Foundation (NPF) in 2012 proposed that first-line therapies for GPP should be acitretin, cyclosporine, methotrexate, and infliximab.28 However, since those guidelines were established, many new biologic therapies have been approved for the treatment of psoriasis and often are considered in the treatment of psoriasis subtypes, including GPP.29 Although retinoids previously were considered to be a preferred first-line therapy, they are associated with a high incidence of adverse effects and must be used with caution in women of childbearing age.6 Oral acitretin at a dosage of 0.75 to 1.0 mg/kg/d has been shown to result in clinical improvement within 1 to 2 weeks, and a maintenance dosage of 0.125 to 0.25 mg/kg/d is required for several months to prevent recurrence.30 Methotrexate—5.0 to 15.0 mg/wk, or perhaps higher in patients with refractory disease, increased by 2.5-mg intervals until symptoms improve—is recommended by the NPF in patients who are unresponsive or cannot tolerate retinoids, though close monitoring for hematologic abnormalities is required. Cyclosporine 2.5 to 5.0 mg/kg/d is considered an alternative to methotrexate and retinoids; it has a faster onset of action, with improvement reported as early as 2 weeks after initiation of therapy.1,28 Although cyclosporine may be effective in the acute phase, especially in severe cases of GPP, long-term use of cyclosporine is not recommended because of the potential for renal dysfunction and hypertension.31
Biologic Agents—More recent evidence has accumulated supporting the efficacy of anti-TNF agents in the treatment of GPP, suggesting the positioning of these agents as first line. A number of case series have shown dramatic and rapid improvement of GPP with intravenous infliximab 3 to 5 mg/kg, with results observed hours to days after the first infusion.32-37 Thus, infliximab is recommended as first-line treatment in severe acute cases, though its efficacy as a maintenance therapy has not been sufficiently investigated.6 Case reports and case series document the safety and efficacy of adalimumab 40 to 80 mg every 1 to 2 weeks38,39 and etanercept 25 to 50 mg twice weekly40-42 in patients with recalcitrantGPP. Therefore, these anti-TNF agents may be considered in patients who are nonresponsive to treatment with infliximab.
Rarely, there have been reports of paradoxical induction of GPP with the use of some anti-TNF agents,43-45 which may be due to a cytokine imbalance characterized by unopposed IFN-α activation.6 In patients with a history of GPP after initiation of a biologic, treatment with agents from within the offending class should be avoided.
The IL-17A monoclonal antibodies secukinumab, ixekizumab, and brodalumab have been shown in open-label phase 3 studies to result in disease remission at 12 weeks.46-48 Treatment with guselkumab, an IL-23 monoclonal antibody, also has demonstrated efficacy in patients with GPP.49 Ustekinumab, an IL-12/23 inhibitor, in combination with acitretin also has been shown to be successful in achieving disease remission after a few weeks of treatment.50
More recent case reports have shown the efficacy of IL-1 inhibitors including gevokizumab, canakinumab, and anakinra in achieving GPP clearance, though more prospective studies are needed to evaluate their efficacy.51-53 Given the etiologic association between IL-1 disinhibition and GPP, future investigations of these therapies as well as those that target the IL-36 pathway may prove to be particularly interesting.
Phototherapy and Combination Therapies—Phototherapy may be considered as maintenance therapy after disease control is achieved, though it is not considered appropriate for acute cases.28 Combination therapies with a biologic plus a nonbiologic systemic agent or alternating among various biologics may allow physicians to maximize benefits and minimize adverse effects in the long term, though there is insufficient evidence to suggest any specific combination treatment algorithm for GPP.28
Treatment Recommendations for Pediatric Patients
Based on a small number of case series and case reports, the first-line treatment strategy for children with GPP is similar to adults. Given the notable adverse events of most oral systemic agents, biologic therapies may emerge as first-line therapy in the pediatric population as more evidence accumulates.28
Treatment Recommendations for Pregnant Patients
Systemic corticosteroids are widely considered to be the first-line treatments for the management of impetigo herpetiformis.7 Low-dose prednisone (15–30 mg/d) usually is effective, but severe cases may require increasing the dosage to 60 mg/d.6 Given the potential for rebound flares upon withdrawal of systemic corticosteroids, these agents must be gradually tapered after the resolution of symptoms.
Certolizumab pegol also is an attractive option in pregnant patients with impetigo herpetiformis because of its favorable safety profile and negligible mother-to-infant transfer through the placenta or breast milk. It has been shown to be effective in treating GPP and impetigo herpetiformis during pregnancy in recently published case reports.54,55 In refractory cases, other TNF-α inhibitors (eg, adalimumab, infliximab, etanercept) or cyclosporine may be considered. However, cautious medical monitoring is warranted, as little is known about the potential adverse effects of these agents to the mother and fetus.28,56 Data from transplant recipients along with several case reports indicate that cyclosporine is not associated with an increased risk for adverse effects during pregnancy at a dose of 2 to 3 mg/kg.57-59 Both methotrexate and retinoids are known teratogens and are therefore contraindicated in pregnant patients.56
If pustules do not resolve in the postpartum period, patients should be treated with standard GPP therapies. However, long-term and population studies are lacking regarding the potential for infant exposure to systemic agents in breast milk. Therefore, the NPF recommends avoiding breastfeeding while taking systemic medications, if possible.56
Limitations of Treatment Recommendations
The ability to generate an evidence-based treatment strategy for GPP is limited by a lack of high-quality studies investigating the efficacy and safety of treatments in patients with GPP due to the rarity and relapsing-remitting nature of the disease, which makes randomized controlled trials difficult to conduct. The quality of the available research is further limited by the lack of validated outcome measures to specifically assess improvements in patients with GPP, such that results are difficult to synthesize and compare among studies.31
Conclusion
Although limited, the available research suggests that treatment with various biologics, especially infliximab, is effective in achieving rapid clearance in patients with GPP. In general, biologics may be the most appropriate treatment option in patients with GPP given their relatively favorable safety profiles. Other oral systemic agents, including acitretin, cyclosporine, and methotrexate, have limited evidence to support their use in the acute phase, but their safety profiles often limit their utility in the long-term. Emerging evidence regarding the association of GPP with IL36RN mutations suggests a unique role for agents targeting the IL-36 or IL-1 pathways, though this has yet to be thoroughly investigated.
- Benjegerdes KE, Hyde K, Kivelevitch D, et al. Pustular psoriasis: pathophysiology and current treatment perspectives. Psoriasis (Auckl). 2016;6:131‐144.
- Bachelez H. Pustular psoriasis and related pustular skin diseases. Br J Dermatol. 2018;178:614‐618.
- Gooderham MJ, Van Voorhees AS, Lebwohl MG. An update on generalized pustular psoriasis. Expert Rev Clin Immunol. 2019;15:907‐919.
- Ly K, Beck KM, Smith MP, et al. Diagnosis and screening of patients with generalized pustular psoriasis. Psoriasis (Auckl). 2019;9:37‐42.
- van de Kerkhof PCM, Nestle FO. Psoriasis. In: Bolognia JL, Jorizzo JJ, Schaffer JV, eds. Dermatology. 3rd ed. Elsevier; 2012:138-160.
- Hoegler KM, John AM, Handler MZ, et al. Generalized pustular psoriasis: a review and update on treatment. J Eur Acad Dermatol Venereol. 2018;32:1645‐1651.
- Oumeish OY, Parish JL. Impetigo herpetiformis. Clin Dermatol. 2006;24:101‐104.
- Johnston A, Xing X, Wolterink L, et al. IL-1 and IL-36 are dominant cytokines in generalized pustular psoriasis. J Allergy Clin Immunol. 2017;140:109-120.
- Furue K, Yamamura K, Tsuji G, et al. Highlighting interleukin-36 signalling in plaque psoriasis and pustular psoriasis. Acta Derm Venereol. 2018;98:5-13.
- Ogawa E, Sato Y, Minagawa A, et al. Pathogenesis of psoriasis and development of treatment. J Dermatol. 2018;45:264-272.
- Marrakchi S, Guigue P, Renshaw BR, et al. Interleukin-36-receptor antagonist deficiency and generalized pustular psoriasis. N Engl J Med. 2011;365:620-628.
- Onoufriadis A, Simpson MA, Pink AE, et al. Mutations in IL36RN/IL1F5 are associated with the severe episodic inflammatory skin disease known as generalized pustular psoriasis. Am J Hum Genet. 2011;89:432-437.
- Setta-Kaffetzi N, Navarini AA, Patel VM, et al. Rare pathogenic variants in IL36RN underlie a spectrum of psoriasis-associated pustular phenotypes. J Invest Dermatol. 2013;133:1366-1369.
- Sugiura K, Takemoto A, Yamaguchi M, et al. The majority of generalized pustular psoriasis without psoriasis vulgaris is caused by deficiency of interleukin-36 receptor antagonist. J Invest Dermatol. 2013;133:2514-2521.
- Hussain S, Berki DM, Choon SE, et al. IL36RN mutations define a severe autoinflammatory phenotype of generalized pustular psoriasis. J Allergy Clin Immunol. 2015;135:1067-1070.e9.
- Körber A, Mossner R, Renner R, et al. Mutations in IL36RN in patients with generalized pustular psoriasis. J Invest Dermatol. 2013;133:2634-2637.
- Twelves S, Mostafa A, Dand N, et al. Clinical and genetic differences between pustular psoriasis subtypes. J Allergy Clin Immunol. 2019;143:1021-1026.
- Sugiura K. The genetic background of generalized pustular psoriasis: IL36RN mutations and CARD14 gain-of-function variants. J Dermatol Sci. 2014;74:187-192
- Wang Y, Cheng R, Lu Z, et al. Clinical profiles of pediatric patients with GPP alone and with different IL36RN genotypes. J Dermatol Sci. 2017;85:235-240.
- Setta-Kaffetzi N, Simpson MA, Navarini AA, et al. AP1S3 mutations are associated with pustular psoriasis and impaired Toll-like receptor 3 trafficking. Am J Hum Genet. 2014;94:790-797.
- Mahil SK, Twelves S, Farkas K, et al. AP1S3 mutations cause skin autoinflammation by disrupting keratinocyte autophagy and upregulating IL-36 production. J Invest Dermatol. 2016;136:2251-2259.
- Umezawa Y, Ozawa A, Kawasima T, et al. Therapeutic guidelines for the treatment of generalized pustular psoriasis (GPP) based on a proposed classification of disease severity. Arch Dermatol Res. 2003;295(suppl 1):S43-S54.
- Viguier M, Allez M, Zagdanski AM, et al. High frequency of cholestasis in generalized pustular psoriasis: evidence for neutrophilic involvement of the biliary tract. Hepatology. 2004;40:452-458.
- Ryan TJ, Baker H. The prognosis of generalized pustular psoriasis. Br J Dermatol. 1971;85:407-411.
- Kalb RE. Pustular psoriasis: management. In: Ofori AO, Duffin KC, eds. UpToDate. UpToDate; 2014. Accessed July 20, 2022. https://www.uptodate.com/contents/pustular-psoriasis-management/print
- Naik HB, Cowen EW. Autoinflammatory pustular neutrophilic diseases. Dermatol Clin. 2013;31:405-425.
- Sidoroff A, Dunant A, Viboud C, et al. Risk factors for acute generalized exanthematous pustulosis (AGEP)—results of a multinational case-control study (EuroSCAR). Br J Dermatol. 2007;157:989-996.
- Robinson A, Van Voorhees AS, Hsu S, et al. Treatment of pustular psoriasis: from the Medical Board of the National Psoriasis Foundation. J Am Acad Dermatol. 2012;67:279‐288.
- Menter A, Strober BE, Kaplan DH, et al. Joint AAD-NPF guidelines of care for the management and treatment of psoriasis with biologics. J Am Acad Dermatol. 2019;80:1029-1072.
- Mengesha YM, Bennett ML. Pustular skin disorders: diagnosis and treatment. Am J Clin Dermatol 2002;3:389-400.
- Zhou LL, Georgakopoulos JR, Ighani A, et al. Systemic monotherapy treatments for generalized pustular psoriasis: a systematic review. J Cutan Med Surg. 2018;22:591‐601.
- Elewski BE. Infliximab for the treatment of severe pustular psoriasis. J Am Acad Dermatol. 2002;47:796-797.
- Kim HS, You HS, Cho HH, et al. Two cases of generalized pustular psoriasis: successful treatment with infliximab. Ann Dermatol. 2014;26:787-788.
- Trent JT, Kerdel FA. Successful treatment of Von Zumbusch pustular psoriasis with infliximab. J Cutan Med Surg. 2004;8:224-228.
- Poulalhon N, Begon E, Lebbé C, et al. A follow-up study in 28 patients treated with infliximab for severe recalcitrant psoriasis: evidence for efficacy and high incidence of biological autoimmunity. Br J Dermatol. 2007;156:329-336.
- Routhouska S, Sheth PB, Korman NJ. Long-term management of generalized pustular psoriasis with infliximab: case series. J Cutan Med Surg. 2008;12:184-188.
- Lisby S, Gniadecki R. Infliximab (Remicade) for acute, severe pustular and erythrodermic psoriasis. Acta Derm Venereol. 2004;84:247-248.
- Zangrilli A, Papoutsaki M, Talamonti M, et al. Long-term efficacy of adalimumab in generalized pustular psoriasis. J Dermatol Treat. 2008;19:185-187.
- Matsumoto A, Komine M, Karakawa M, et al. Adalimumab administration after infliximab therapy is a successful treatment strategy for generalized pustular psoriasis. J Dermatol. 2017;44:202-204.
- Kamarashev J, Lor P, Forster A, et al. Generalized pustular psoriasis induced by cyclosporin in a withdrawal responding to the tumour necrosis factor alpha inhibitor etanercept. Dermatology. 2002;205:213-216.
- Esposito M, Mazzotta A, Casciello C, et al. Etanercept at different dosages in the treatment of generalized pustular psoriasis: a case series. Dermatology. 2008;216:355-360.
- Lo Schiavo A, Brancaccio G, Puca RV, et al. Etanercept in the treatment of generalized annular pustular psoriasis. Ann Dermatol. 2012;24:233-234.
- Goiriz R, Daudén E, Pérez-Gala S, et al. Flare and change of psoriasis morphology during the course of treatment with tumor necrosis factor blockers. Clin Exp Dermatol. 2006;32:176-179.
- Collamer AN, Battafarano DF. Psoriatic skin lesions induced by tumor necrosis factor antagonist therapy: clinical features and possible immunopathogenesis. Semin Arthritis Rheum. 2010;40:233-240.
- Almutairi D, Sheasgreen C, Weizman A, et al. Generalized pustular psoriasis induced by infliximab in a patient with inflammatory bowel disease. J Cutan Med Surg. 2018;1:507-510.
- Imafuku S, Honma M, Okubo Y, et al. Efficacy and safety of secukinumab in patients with generalized pustular psoriasis: a 52-week analysis from phase III open-label multicenter Japanese study. J Dermatol. 2016;43:1011-1017
- Saeki H, Nakagawa H, Ishii T, et al. Efficacy and safety of open-label ixekizumab treatment in Japanese patients with moderate-to-severe plaque psoriasis, erythrodermic psoriasis, and generalized pustular psoriasis. J Eur Acad Dermatol Venereol. 2015;29:1148-1155.
- Yamasaki K, Nakagawa H, Kubo Y, et al. Efficacy and safety of brodalumab in patients with generalized pustular psoriasis and psoriatic erythroderma: results from a 52-week, open-label study. Br J Dermatol. 2017;176:741-751.
- Sano S, Kubo H, Morishima H, et al. Guselkumab, a human interleukin-23 monoclonal antibody in Japanese patients with generalized pustular psoriasis and erythrodermic psoriasis: efficacy and safety analyses of a 52-week, phase 3, multicenter, open-label study. J Dermatol. 2018;45:529‐539.
- Arakawa A, Ruzicka T, Prinz JC. Therapeutic efficacy of interleukin 12/interleukin 23 blockade in generalized pustular psoriasis regardless of IL36RN mutation status. JAMA Dermatol. 2016;152:825-828.
- Mansouri B, Richards L, Menter A. Treatment of two patients with generalized pustular psoriasis with the interleukin-1beta inhibitor gevokizumab. Br J Dermatol. 2015;173:239-241.
- Skendros P, Papagoras C, Lefaki I, et al. Successful response in a case of severe pustular psoriasis after interleukin-1 beta inhibition. Br J Dermatol. 2017;176:212-215.
- Viguier M, Guigue P, Pagès C, et al. Successful treatment of generalized pustular psoriasis with the interleukin-1-receptor antagonist Anakinra: lack of correlation with IL1RN mutations. Ann Intern Med. 2010;153:66-67.
- Fukushima H, Iwata Y, Arima M, et al. Efficacy and safety of treatment with anti-tumor necrosis factor‐α drugs for severe impetigo herpetiformis. J Dermatol. 2021;48:207-210.
- Mizutani Y, Mizutani YH, Matsuyama K, et al. Generalized pustular psoriasis in pregnancy, successfully treated with certolizumab pegol. J Dermatol. 2021;47:e262-e263.
- Bae YS, Van Voorhees AS, Hsu S, et al. Review of treatment options for psoriasis in pregnant or lactating women: from the Medical Board of the National Psoriasis Foundation. J Am Acad Dermatol. 2012;67:459‐477.
- Finch TM, Tan CY. Pustular psoriasis exacerbated by pregnancy and controlled by cyclosporin A. Br J Dermatol. 2000;142:582-584.
- Gaughan WJ, Moritz MJ, Radomski JS, et al. National Transplantation Pregnancy Registry: report on outcomes of cyclosporine-treated female kidney transplant recipients with an interval from transplantation to pregnancy of greater than five years. Am J Kidney Dis. 1996;28:266-269.
- Kura MM, Surjushe AU. Generalized pustular psoriasis of pregnancy treated with oral cyclosporin. Indian J Dermatol Venereol Leprol. 2006;72:458-459.
- Benjegerdes KE, Hyde K, Kivelevitch D, et al. Pustular psoriasis: pathophysiology and current treatment perspectives. Psoriasis (Auckl). 2016;6:131‐144.
- Bachelez H. Pustular psoriasis and related pustular skin diseases. Br J Dermatol. 2018;178:614‐618.
- Gooderham MJ, Van Voorhees AS, Lebwohl MG. An update on generalized pustular psoriasis. Expert Rev Clin Immunol. 2019;15:907‐919.
- Ly K, Beck KM, Smith MP, et al. Diagnosis and screening of patients with generalized pustular psoriasis. Psoriasis (Auckl). 2019;9:37‐42.
- van de Kerkhof PCM, Nestle FO. Psoriasis. In: Bolognia JL, Jorizzo JJ, Schaffer JV, eds. Dermatology. 3rd ed. Elsevier; 2012:138-160.
- Hoegler KM, John AM, Handler MZ, et al. Generalized pustular psoriasis: a review and update on treatment. J Eur Acad Dermatol Venereol. 2018;32:1645‐1651.
- Oumeish OY, Parish JL. Impetigo herpetiformis. Clin Dermatol. 2006;24:101‐104.
- Johnston A, Xing X, Wolterink L, et al. IL-1 and IL-36 are dominant cytokines in generalized pustular psoriasis. J Allergy Clin Immunol. 2017;140:109-120.
- Furue K, Yamamura K, Tsuji G, et al. Highlighting interleukin-36 signalling in plaque psoriasis and pustular psoriasis. Acta Derm Venereol. 2018;98:5-13.
- Ogawa E, Sato Y, Minagawa A, et al. Pathogenesis of psoriasis and development of treatment. J Dermatol. 2018;45:264-272.
- Marrakchi S, Guigue P, Renshaw BR, et al. Interleukin-36-receptor antagonist deficiency and generalized pustular psoriasis. N Engl J Med. 2011;365:620-628.
- Onoufriadis A, Simpson MA, Pink AE, et al. Mutations in IL36RN/IL1F5 are associated with the severe episodic inflammatory skin disease known as generalized pustular psoriasis. Am J Hum Genet. 2011;89:432-437.
- Setta-Kaffetzi N, Navarini AA, Patel VM, et al. Rare pathogenic variants in IL36RN underlie a spectrum of psoriasis-associated pustular phenotypes. J Invest Dermatol. 2013;133:1366-1369.
- Sugiura K, Takemoto A, Yamaguchi M, et al. The majority of generalized pustular psoriasis without psoriasis vulgaris is caused by deficiency of interleukin-36 receptor antagonist. J Invest Dermatol. 2013;133:2514-2521.
- Hussain S, Berki DM, Choon SE, et al. IL36RN mutations define a severe autoinflammatory phenotype of generalized pustular psoriasis. J Allergy Clin Immunol. 2015;135:1067-1070.e9.
- Körber A, Mossner R, Renner R, et al. Mutations in IL36RN in patients with generalized pustular psoriasis. J Invest Dermatol. 2013;133:2634-2637.
- Twelves S, Mostafa A, Dand N, et al. Clinical and genetic differences between pustular psoriasis subtypes. J Allergy Clin Immunol. 2019;143:1021-1026.
- Sugiura K. The genetic background of generalized pustular psoriasis: IL36RN mutations and CARD14 gain-of-function variants. J Dermatol Sci. 2014;74:187-192
- Wang Y, Cheng R, Lu Z, et al. Clinical profiles of pediatric patients with GPP alone and with different IL36RN genotypes. J Dermatol Sci. 2017;85:235-240.
- Setta-Kaffetzi N, Simpson MA, Navarini AA, et al. AP1S3 mutations are associated with pustular psoriasis and impaired Toll-like receptor 3 trafficking. Am J Hum Genet. 2014;94:790-797.
- Mahil SK, Twelves S, Farkas K, et al. AP1S3 mutations cause skin autoinflammation by disrupting keratinocyte autophagy and upregulating IL-36 production. J Invest Dermatol. 2016;136:2251-2259.
- Umezawa Y, Ozawa A, Kawasima T, et al. Therapeutic guidelines for the treatment of generalized pustular psoriasis (GPP) based on a proposed classification of disease severity. Arch Dermatol Res. 2003;295(suppl 1):S43-S54.
- Viguier M, Allez M, Zagdanski AM, et al. High frequency of cholestasis in generalized pustular psoriasis: evidence for neutrophilic involvement of the biliary tract. Hepatology. 2004;40:452-458.
- Ryan TJ, Baker H. The prognosis of generalized pustular psoriasis. Br J Dermatol. 1971;85:407-411.
- Kalb RE. Pustular psoriasis: management. In: Ofori AO, Duffin KC, eds. UpToDate. UpToDate; 2014. Accessed July 20, 2022. https://www.uptodate.com/contents/pustular-psoriasis-management/print
- Naik HB, Cowen EW. Autoinflammatory pustular neutrophilic diseases. Dermatol Clin. 2013;31:405-425.
- Sidoroff A, Dunant A, Viboud C, et al. Risk factors for acute generalized exanthematous pustulosis (AGEP)—results of a multinational case-control study (EuroSCAR). Br J Dermatol. 2007;157:989-996.
- Robinson A, Van Voorhees AS, Hsu S, et al. Treatment of pustular psoriasis: from the Medical Board of the National Psoriasis Foundation. J Am Acad Dermatol. 2012;67:279‐288.
- Menter A, Strober BE, Kaplan DH, et al. Joint AAD-NPF guidelines of care for the management and treatment of psoriasis with biologics. J Am Acad Dermatol. 2019;80:1029-1072.
- Mengesha YM, Bennett ML. Pustular skin disorders: diagnosis and treatment. Am J Clin Dermatol 2002;3:389-400.
- Zhou LL, Georgakopoulos JR, Ighani A, et al. Systemic monotherapy treatments for generalized pustular psoriasis: a systematic review. J Cutan Med Surg. 2018;22:591‐601.
- Elewski BE. Infliximab for the treatment of severe pustular psoriasis. J Am Acad Dermatol. 2002;47:796-797.
- Kim HS, You HS, Cho HH, et al. Two cases of generalized pustular psoriasis: successful treatment with infliximab. Ann Dermatol. 2014;26:787-788.
- Trent JT, Kerdel FA. Successful treatment of Von Zumbusch pustular psoriasis with infliximab. J Cutan Med Surg. 2004;8:224-228.
- Poulalhon N, Begon E, Lebbé C, et al. A follow-up study in 28 patients treated with infliximab for severe recalcitrant psoriasis: evidence for efficacy and high incidence of biological autoimmunity. Br J Dermatol. 2007;156:329-336.
- Routhouska S, Sheth PB, Korman NJ. Long-term management of generalized pustular psoriasis with infliximab: case series. J Cutan Med Surg. 2008;12:184-188.
- Lisby S, Gniadecki R. Infliximab (Remicade) for acute, severe pustular and erythrodermic psoriasis. Acta Derm Venereol. 2004;84:247-248.
- Zangrilli A, Papoutsaki M, Talamonti M, et al. Long-term efficacy of adalimumab in generalized pustular psoriasis. J Dermatol Treat. 2008;19:185-187.
- Matsumoto A, Komine M, Karakawa M, et al. Adalimumab administration after infliximab therapy is a successful treatment strategy for generalized pustular psoriasis. J Dermatol. 2017;44:202-204.
- Kamarashev J, Lor P, Forster A, et al. Generalized pustular psoriasis induced by cyclosporin in a withdrawal responding to the tumour necrosis factor alpha inhibitor etanercept. Dermatology. 2002;205:213-216.
- Esposito M, Mazzotta A, Casciello C, et al. Etanercept at different dosages in the treatment of generalized pustular psoriasis: a case series. Dermatology. 2008;216:355-360.
- Lo Schiavo A, Brancaccio G, Puca RV, et al. Etanercept in the treatment of generalized annular pustular psoriasis. Ann Dermatol. 2012;24:233-234.
- Goiriz R, Daudén E, Pérez-Gala S, et al. Flare and change of psoriasis morphology during the course of treatment with tumor necrosis factor blockers. Clin Exp Dermatol. 2006;32:176-179.
- Collamer AN, Battafarano DF. Psoriatic skin lesions induced by tumor necrosis factor antagonist therapy: clinical features and possible immunopathogenesis. Semin Arthritis Rheum. 2010;40:233-240.
- Almutairi D, Sheasgreen C, Weizman A, et al. Generalized pustular psoriasis induced by infliximab in a patient with inflammatory bowel disease. J Cutan Med Surg. 2018;1:507-510.
- Imafuku S, Honma M, Okubo Y, et al. Efficacy and safety of secukinumab in patients with generalized pustular psoriasis: a 52-week analysis from phase III open-label multicenter Japanese study. J Dermatol. 2016;43:1011-1017
- Saeki H, Nakagawa H, Ishii T, et al. Efficacy and safety of open-label ixekizumab treatment in Japanese patients with moderate-to-severe plaque psoriasis, erythrodermic psoriasis, and generalized pustular psoriasis. J Eur Acad Dermatol Venereol. 2015;29:1148-1155.
- Yamasaki K, Nakagawa H, Kubo Y, et al. Efficacy and safety of brodalumab in patients with generalized pustular psoriasis and psoriatic erythroderma: results from a 52-week, open-label study. Br J Dermatol. 2017;176:741-751.
- Sano S, Kubo H, Morishima H, et al. Guselkumab, a human interleukin-23 monoclonal antibody in Japanese patients with generalized pustular psoriasis and erythrodermic psoriasis: efficacy and safety analyses of a 52-week, phase 3, multicenter, open-label study. J Dermatol. 2018;45:529‐539.
- Arakawa A, Ruzicka T, Prinz JC. Therapeutic efficacy of interleukin 12/interleukin 23 blockade in generalized pustular psoriasis regardless of IL36RN mutation status. JAMA Dermatol. 2016;152:825-828.
- Mansouri B, Richards L, Menter A. Treatment of two patients with generalized pustular psoriasis with the interleukin-1beta inhibitor gevokizumab. Br J Dermatol. 2015;173:239-241.
- Skendros P, Papagoras C, Lefaki I, et al. Successful response in a case of severe pustular psoriasis after interleukin-1 beta inhibition. Br J Dermatol. 2017;176:212-215.
- Viguier M, Guigue P, Pagès C, et al. Successful treatment of generalized pustular psoriasis with the interleukin-1-receptor antagonist Anakinra: lack of correlation with IL1RN mutations. Ann Intern Med. 2010;153:66-67.
- Fukushima H, Iwata Y, Arima M, et al. Efficacy and safety of treatment with anti-tumor necrosis factor‐α drugs for severe impetigo herpetiformis. J Dermatol. 2021;48:207-210.
- Mizutani Y, Mizutani YH, Matsuyama K, et al. Generalized pustular psoriasis in pregnancy, successfully treated with certolizumab pegol. J Dermatol. 2021;47:e262-e263.
- Bae YS, Van Voorhees AS, Hsu S, et al. Review of treatment options for psoriasis in pregnant or lactating women: from the Medical Board of the National Psoriasis Foundation. J Am Acad Dermatol. 2012;67:459‐477.
- Finch TM, Tan CY. Pustular psoriasis exacerbated by pregnancy and controlled by cyclosporin A. Br J Dermatol. 2000;142:582-584.
- Gaughan WJ, Moritz MJ, Radomski JS, et al. National Transplantation Pregnancy Registry: report on outcomes of cyclosporine-treated female kidney transplant recipients with an interval from transplantation to pregnancy of greater than five years. Am J Kidney Dis. 1996;28:266-269.
- Kura MM, Surjushe AU. Generalized pustular psoriasis of pregnancy treated with oral cyclosporin. Indian J Dermatol Venereol Leprol. 2006;72:458-459.
Practice Points
- Generalized pustular psoriasis (GPP) is a rare severe variant of psoriasis that is characterized by the abrupt widespread onset of small pustules.
- Although no treatments have specifically been approved for GPP, various biologics, especially infliximab, may be effective in achieving rapid clearance in patients with GPP. Other oral systemic agents including acitretin, cyclosporine, and methotrexate also have been shown to be effective.
Management of Psoriasis With Topicals: Applying the 2020 AAD-NPF Guidelines of Care to Clinical Practice
Psoriasis is a chronic inflammatory skin disease characterized by erythematous scaly plaques that can invoke substantial pain, pruritus, and quality-of-life disturbance in patients. Topical therapies are the most commonly used medications for treating psoriasis, with one study (N = 128,308) showing that more than 85% of patients with psoriasis were managed solely with topical medications. 1 For patients with mild to moderate psoriasis, topical agents alone may be able to control disease completely. For those with more severe disease, topical agents are used adjunctively with systemic or biologic agents to optimize disease control in localized areas.
The American Academy of Dermatology (AAD) and National Psoriasis Foundation (NPF) published guidelines in 2020 for managing psoriasis with topical agents in adults.2 This review presents the most up-to-date clinical recommendations for topical agent use in adult patients with psoriasis and elaborates on each drug’s pharmacologic and safety profile. Specifically, evidence-based treatment recommendations for topical steroids, calcineurin inhibitors (CNIs), vitamin D analogues, retinoids (tazarotene), emollients, keratolytics (salicylic acid), anthracenes (anthralin), and keratoplastics (coal tar) will be addressed (Table 1). Recommendations for combination therapy with other treatment modalities including UVB light therapy, biologics, and systemic nonbiologic agents also will be discussed.
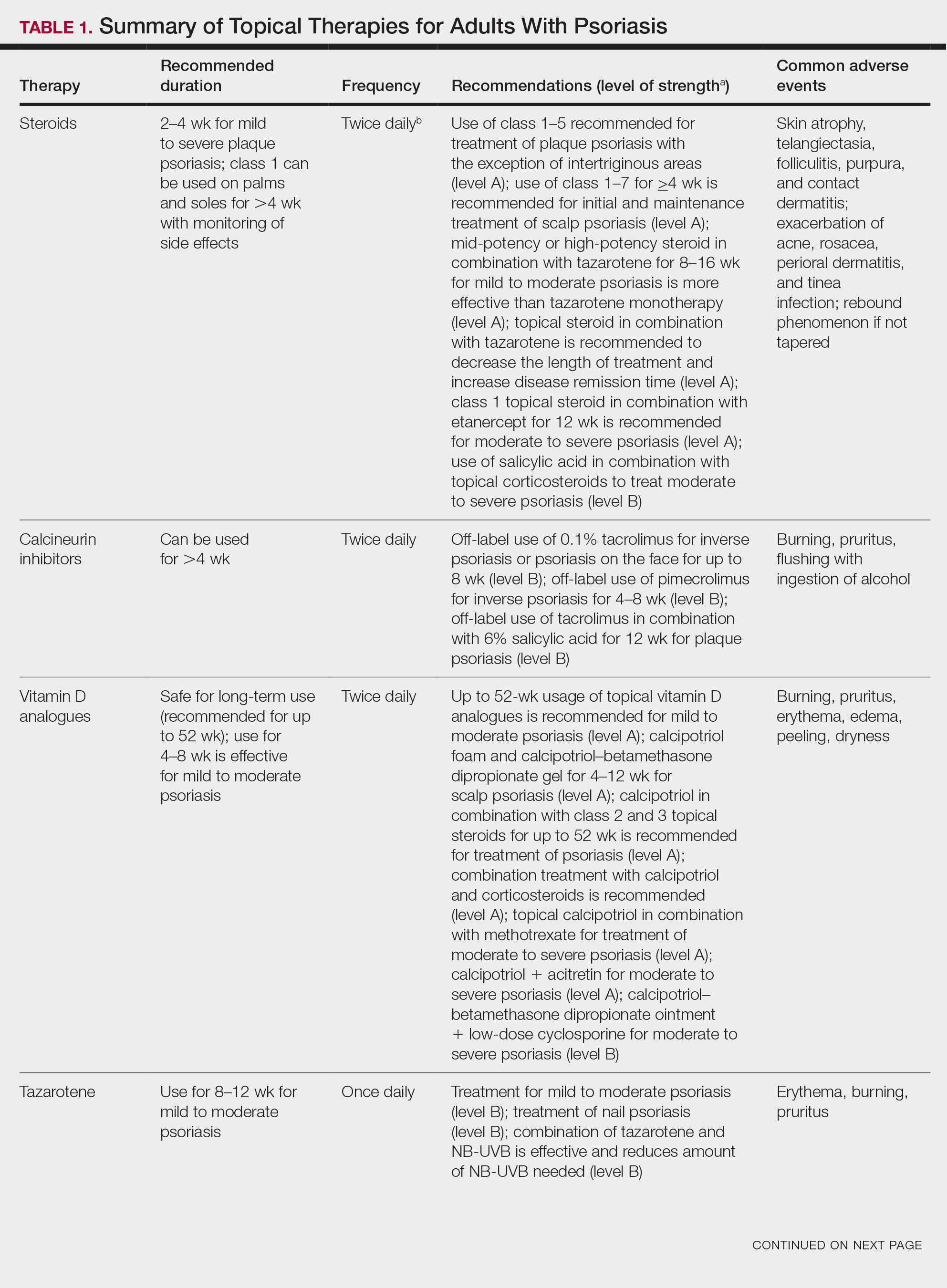
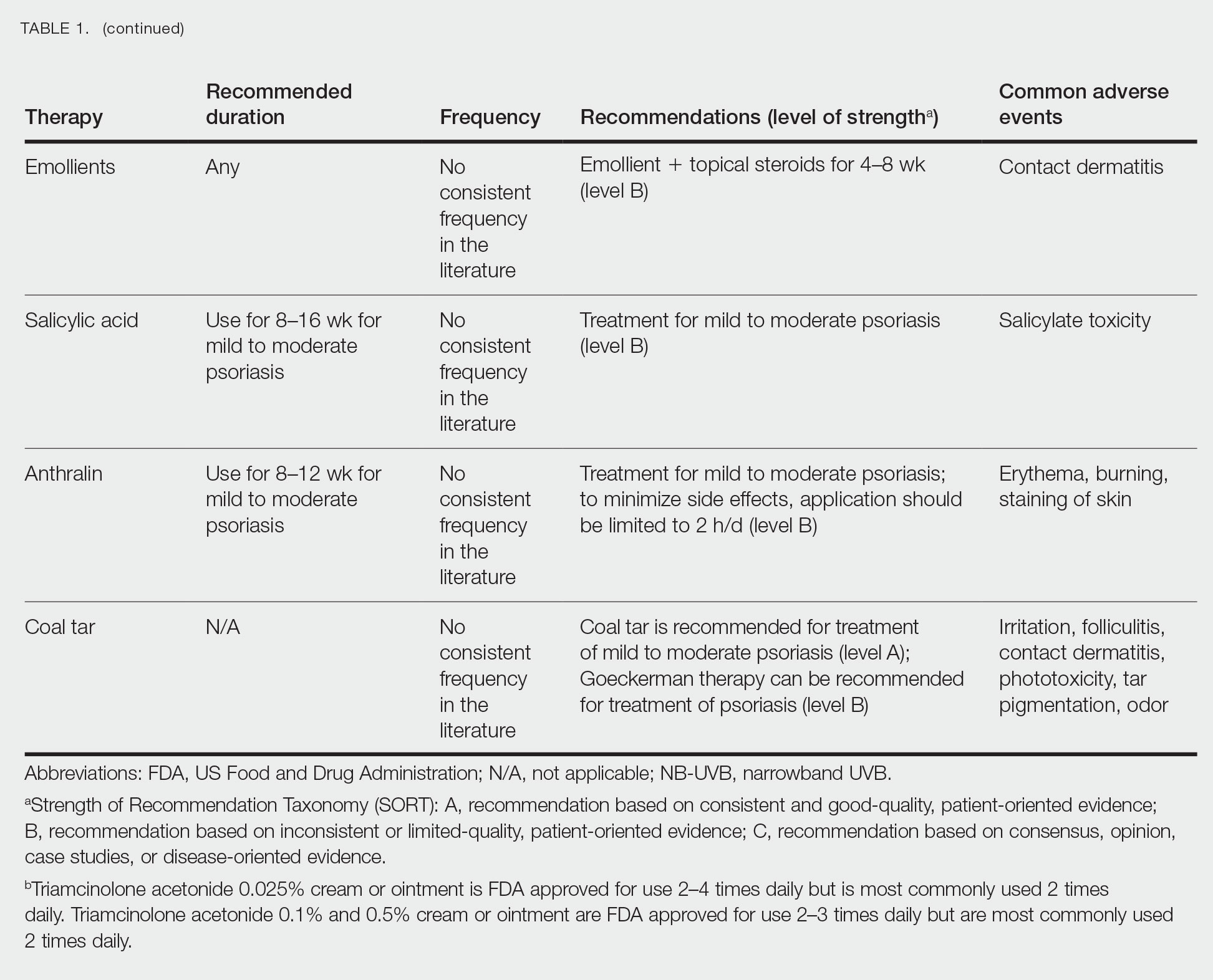
Selecting a Topical Agent Based on Disease Localization
When treating patients with psoriasis with topical therapies, clinicians should take into consideration drug potency, as it determines how effective a treatment will be in penetrating the skin barrier. Plaque characteristics, such as distribution (localized vs widespread), anatomical localization (flexural, scalp, palms/soles/nails), size (large vs small), and thickness (thick vs thin), not only influence treatment effectiveness but also the incidence of drug-related adverse events. Furthermore, preferred topical therapies are tailored to each patient based on disease characteristics and activity. Coal tar and anthralin have been used less frequently than other topical therapies for psoriasis because of their undesirable side-effect profiles (Table 1).3
Face and Intertriginous Regions—The face and intertriginous areas are sensitive because skin tends to be thin in these regions. Emollients are recommended for disease in these locations given their safety and flexibility in use for most areas. Conversely, anthralin should be avoided on the face, intertriginous areas, and even highly visible locations because of the potential for skin staining. Low-potency corticosteroids also have utility in psoriasis distributed on the face and intertriginous regions. Additionally, application of steroids around the eyes should be cautioned because topical steroids can induce ocular complications such as glaucoma and cataracts in rare circumstances.4
Off-label use of CNIs for psoriasis on the face and intertriginous areas also is effective. Currently, there is a level B recommendation for off-label use of 0.1% tacrolimus for up to 8 weeks for inverse psoriasis or psoriasis on the face. Off-label use of pimecrolimus for 4 to 8 weeks also can be considered for inverse psoriasis. Combination therapy consisting of hydrocortisone with calcipotriol ointment is another effective regimen.5 One study also suggested that use of crisaborole for 4 to 8 weeks in intertriginous psoriasis can be effective and well tolerated.6
Scalp—The vehicle of medication administration is especially important in hair-bearing areas such as the scalp, as these areas are challenging for medication application and patient adherence. Thus, patient preferences for the vehicle must be considered. Several studies have been conducted to assess preference for various vehicles in scalp psoriasis. A foam or solution may be preferable to ointments, gels, or creams.7 Gels may be preferred over ointments.8 There is a level A recommendation supporting the use of class 1 to 7 topical steroids for a minimum of 4 weeks as initial and maintenance treatment of scalp psoriasis. The highest level of evidence (level A) also supports the use of calcipotriol foam or combination therapy of calcipotriol–betamethasone dipropionate gel for 4 to 12 weeks as treatment of mild to moderate scalp psoriasis.
Nails—Several options for topical medications have been recommended for the treatment of nail psoriasis. Currently, there is a level B recommendation for the use of tazarotene for the treatment of nail psoriasis. Another effective regimen is combination therapy with vitamin D analogues and betamethasone dipropionate.9 Topical steroid use for nail psoriasis should be limited to 12 weeks because of the risk for bone atrophy with chronic steroid use.
Palmoplantar—The palms and soles have a thicker epidermal layer than other areas of the body. As a result, class 1 corticosteroids can be used for palmoplantar psoriasis for more than 4 weeks with vigilant monitoring for adverse effects such as skin atrophy, tachyphylaxis, or tinea infection. Tazarotene also has been shown to be helpful in treating palmoplantar psoriasis.
Resistant Disease—Intralesional steroids are beneficial treatment options for recalcitrant psoriasis in glabrous areas, as well as for palmoplantar, nail, and scalp psoriasis. Up to 10 mg/mL of triamcinolone acetonide used every 3 to 4 weeks is an effective regimen.10Pregnancy/Breastfeeding—Women of childbearing potential have additional safety precautions that should be considered during medication selection. Emollients have been shown to be safe during pregnancy and lactation. Currently, there is little known about CNI use during pregnancy. During lactation, CNIs can be used by breastfeeding mothers in most areas, excluding the breasts. Evaluation of the safety of anthralin and vitamin D analogues during pregnancy and lactation have not been studied. For these agents, dermatologists need to use their clinical judgment to weigh the risks and benefits of medication, particularly in patients requiring occlusion, higher medication doses, or treatment over a large surface area. Salicylic acid should be used with caution in pregnant and breastfeeding mothers because it is a pregnancy category C drug. Lower-potency corticosteroids may be used with caution during pregnancy and breastfeeding. More potent corticosteroids and coal tar, however, should be avoided. Similarly, tazarotene use is contraindicated in pregnancy. According to the US Food and Drug Administration labels for all forms of topical tazarotene, a pregnancy test must be obtained 2 weeks prior to tazarotene treatment initiation in women of childbearing potential because of the risk for serious fetal malformations and toxicity.
Recommendations, Risks, and Benefits of Topical Therapy for the Management of Psoriasis
Topical Corticosteroids—Topical corticosteroids (TCs) are widely used for inflammatory skin conditions and are available in a variety of strengths (Table 2). They are thought to exert their action by regulating the gene transcription of proinflammatory mediators. For psoriasis, steroids are recommended for 2 to 4 weeks, depending on disease severity. Although potent and superpotent steroids are more effective than mild- to moderate-strength TCs, use of lower-potency TCs may be warranted depending on disease distribution and localization.11 For treatment of psoriasis with no involvement of the intertriginous areas, use of class 1 to 5 TCs for up to 4 weeks is recommended.
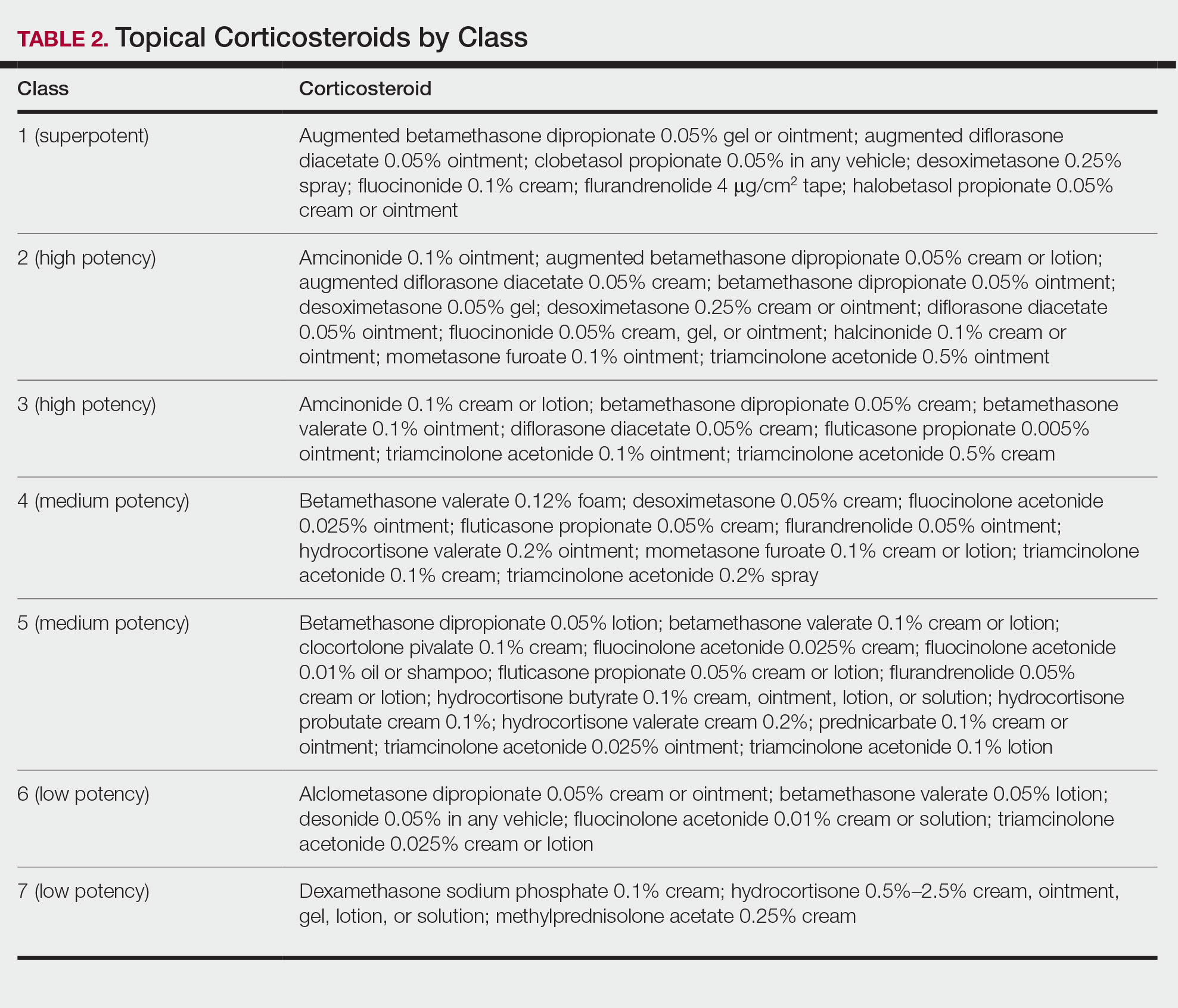
For moderate to severe psoriasis with 20% or less body surface area (BSA) affected, combination therapy consisting of mometasone and salicylic acid has been shown to be more effective than mometasone alone.12,13 There currently is a level A recommendation for the use of combination therapy with class 1 TCs and etanercept for 12 weeks in patients with moderate to severe psoriasis who require both systemic and topical therapies for disease control. Similarly, combination therapy with infliximab and high-potency TCs has a level B recommendation to enhance efficacy for the treatment of moderate to severe psoriasis.14 High-quality studies on the use of TCs with anti–IL-12/IL-23, anti–IL-23, and anti–IL-17 currently are unavailable, but the combination is not expected to be unsafe.14,15 Combination therapy of betamethasone dipropionate ointment and low-dose cyclosporine is an alternative regimen with a level B recommendation.
The most common adverse effects with use of TCs are skin thinning and atrophy, telangiectasia, and striae (Table 1). With clinical improvement of disease, it is recommended that clinicians taper TCs to prevent rebound effect. To decrease TC-related adverse effects, clinicians should use combination therapy with steroid-sparing agents for disease maintenance, transition to lower-potency corticosteroids, or use intermittent steroid therapy. Systemic effects of TC use include hypothalamic-pituitary-adrenal axis suppression, Cushing syndrome, and osteonecrosis of the femoral head.16-18 These systemic effects with TC use are rare unless treatment is for disease involving greater than 20% BSA or occlusion for more than 4 weeks.
Calcineurin Inhibitors—Calcineurin inhibitors inhibit calcineurin phosphorylation and T-cell activation, subsequently decreasing the expression of proinflammatory cytokines. Currently, they are not approved by the US Food and Drug Administration to treat psoriasis but have demonstrated efficacy in randomized control trials (RCTs) for facial and intertriginous psoriasis. In RCTs, 71% of patients using pimecrolimus cream 0.1% twice daily for 8 weeks achieved an investigator global assessment score of clear (0) or almost clear (1) compared with 21% of placebo-treated patients (N=57).19 Other trials have shown that 65% of patients receiving tacrolimus ointment 0.1% for 8 weeks achieved an investigator global assessment score of 0 or 1 compared with 31% of placebo-treated patients (N=167).20 Because of their efficacy in RCTs, CNIs commonly are used off label to treat psoriasis.
The most common adverse effects with CNI use are burning, pruritus, and flushing with alcohol ingestion (Table 1). Additionally, CNIs have a black box warning that use may increase the risk for malignancy, but this risk has not been demonstrated with topical use in humans.21Vitamin D Analogues—The class of vitamin D analogues—calcipotriol/calcipotriene and calcitriol—frequently are used to treat psoriasis. Vitamin D analogues exert their beneficial effects by inhibiting keratinocyte proliferation and enhancing keratinocyte differentiation. They also are ideal for long-term use (up to 52 weeks) in mild to moderate psoriasis and can be used in combination with class 2 and 3 TCs. There is a level A recommendation that supports the use of combination therapy with calcipotriol and TCs for the treatment of mild to moderate psoriasis.
For severe psoriasis, many studies have investigated the efficacy of combination therapy with vitamin D analogues and systemic treatments. Combination therapy with calcipotriol and methotrexate or calcipotriol and acitretin are effective treatment regimens with level A recommendations. Calcipotriol–betamethasone dipropionate ointment in combination with low-dose cyclosporine is an alternative option with a level B recommendation. Because vitamin D analogues are inactivated by UVA and UVB radiation, clinicians should advise their patients to use vitamin D analogues after receiving UVB phototherapy.22
Common adverse effects of vitamin D analogues include burning, pruritus, erythema, and dryness (Table 1). Hypercalcemia and parathyroid hormone suppression are extremely rare unless treatment occurs over a large surface area (>30% BSA) or the patient has concurrent renal disease or impairments in calcium metabolism.
Tazarotene—Tazarotene is a topical retinoid that acts by decreasing keratinocyte proliferation, facilitating keratinocyte differentiation, and inhibiting inflammation. Patients with mild to moderate psoriasis are recommended to receive tazarotene treatment for 8 to 12 weeks. In several RCTs, tazarotene gel 0.1% and tazarotene cream 0.1% and 0.05% achieved treatment success in treating plaque psoriasis.23,24
For increased efficacy, clinicians can recommend combination therapy with tazarotene and a TC. Combination therapy with tazarotene and a mid- or high-potency TC for 8 to 16 weeks has been shown to be more effective than treatment with tazarotene alone.25 Thus, there is a level A recommendation for use of this combination to treat mild to moderate psoriasis. Agents used in combination therapy work synergistically to decrease the length of treatment and increase the duration of remission. The frequency of adverse effects, such as irritation from tazarotene and skin atrophy from TCs, also are reduced.26 Combination therapy with tazarotene and narrowband UVB (NB-UVB) is another effective option that requires less UV radiation than NB-UVB alone because of the synergistic effects of both treatment modalities.27 Clinicians should counsel patients on the adverse effects of tazarotene, which include local irritation, burning, pruritus, and erythema (Table 1).
Emollients—Emollients are nonmedicated moisturizers that decrease the amount of transepidermal water loss. There is a level B recommendation for use of emollients and TCs in combination for 4 to 8 weeks to treat psoriasis. In fact, combination therapy with mometasone and emollients has demonstrated greater improvement in symptoms of palmoplantar psoriasis (ie, erythema, desquamation, infiltration, BSA involvement) than mometasone alone.28 Emollients are safe options that can be used on all areas of the body and during pregnancy and lactation. Although adverse effects of emollients are rare, clinicians should counsel patients on the risk for contact dermatitis if specific allergies to ingredients/fragrances exist (Table 1).
Salicylic Acid—Salicylic acid is a topical keratolytic that can be used to treat psoriatic plaques. Use of salicylic acid for 8 to 16 weeks has been shown to be effective for mild to moderate psoriasis. Combination therapy of salicylic acid and TCs in patients with 20% or less BSA affected is a safe and effective option with a level B recommendation. Combination therapy with salicylic acid and calcipotriene, however, should be avoided because calcipotriene is inactivated by salicylic acid. It also is recommended that salicylic acid application follow phototherapy when both treatment modalities are used in combination.29,30 Clinicians should be cautious about using salicylic acid in patients with renal or hepatic disease because of the increased risk for salicylate toxicity (Table 1).
Anthralin—Anthralin is a synthetic hydrocarbon derivative that has been shown to reduce inflammation and normalize keratinocyte proliferation through an unknown mechanism. It is recommended that patients with mild to moderate psoriasis receive anthralin treatment for 8 to 12 weeks, with a maximum application time of 2 hours per day. Combination therapy of excimer laser and anthralin has been shown to be more effective in treating psoriasis than anthralin alone.31 Therefore, clinicians have the option of including excimer laser therapy for additional disease control. Anthralin should be avoided on the face, flexural regions, and highly visible areas because of potential skin staining (Table 1). Other adverse effects include application-site burning and erythema.
Coal Tar—Coal tar is a heterogenous mixture of aromatic hydrocarbons that is an effective treatment of psoriasis because of its inherent anti-inflammatory and keratoplastic properties. There is high-quality evidence supporting a level A recommendation for coal tar use in mild to moderate psoriasis. Combination therapy with NB-UVB and coal tar (also known as Goeckerman therapy) is a recommended treatment option with a quicker onset of action and improved outcomes compared with NB-UVB therapy alone.32,33 Adverse events of coal tar include application-site irritation, folliculitis, contact dermatitis, phototoxicity, and skin pigmentation (Table 1).
Conclusion
Topical medications are versatile treatment options that can be utilized as monotherapy or adjunct therapy for mild to severe psoriasis. Benefits of topical agents include minimal required monitoring, few contraindications, and direct localized effect on plaques. Therefore, side effects with topical agent use rarely are systemic. Medication interactions are less of a concern with topical therapies; thus, they have better safety profiles compared with systemic therapies. This clinical review summarizes the recently published evidence-based guidelines from the AAD and NPF on the use of topical agents in psoriasis and may be a useful guiding framework for clinicians in their everyday practice.
- Murage MJ, Kern DM, Chang L, et al. Treatment patterns among patients with psoriasis using a large national payer database in the United States: a retrospective study. J Med Econ. 2018:1-9.
- Elmets CA, Korman NJ, Prater EF, et al. Joint AAD-NPF Guidelines of care for the management and treatment of psoriasis with topical therapy and alternative medicine modalities for psoriasis severity measures. J Am Acad Dermatol. 2021;84:432-470.
- Svendsen MT, Jeyabalan J, Andersen KE, et al. Worldwide utilization of topical remedies in treatment of psoriasis: a systematic review. J Dermatolog Treat. 2017;28:374-383.
- Day A, Abramson AK, Patel M, et al. The spectrum of oculocutaneous disease: part II. neoplastic and drug-related causes of oculocutaneous disease. J Am Acad Dermatol. 2014;70:821.e821-819.
- Choi JW, Choi JW, Kwon IH, et al. High-concentration (20 μg g-¹) tacalcitol ointment in the treatment of facial psoriasis: an 8-week open-label clinical trial. Br J Dermatol. 2010;162:1359-1364.
- Hashim PW, Chima M, Kim HJ, et al. Crisaborole 2% ointment for the treatment of intertriginous, anogenital, and facial psoriasis: a double-blind, randomized, vehicle-controlled trial. J Am Acad Dermatol. 2020;82:360-365.
- Housman TS, Mellen BG, Rapp SR, et al. Patients with psoriasis prefer solution and foam vehicles: a quantitative assessment of vehicle preference. Cutis. 2002;70:327-332.
- Iversen L, Jakobsen HB. Patient preferences for topical psoriasis treatments are diverse and difficult to predict. Dermatol Ther. 2016;6:273-285.
- Clobex Package insert. Galderma Laboratories, LP; 2012.
- Kenalog-10 Injection. Package insert. Bristol-Myers Squibb Company; 2018.
- Mason J, Mason AR, Cork MJ. Topical preparations for the treatment of psoriasis: a systematic review. Br J Dermatol. 2002;146:351-364.
- Koo J, Cuffie CA, Tanner DJ, et al. Mometasone furoate 0.1%-salicylic acid 5% ointment versus mometasone furoate 0.1% ointment in the treatment of moderate-to-severe psoriasis: a multicenter study. Clin Ther. 1998;20:283-291.
- Tiplica GS, Salavastru CM. Mometasone furoate 0.1% and salicylic acid 5% vs. mometasone furoate 0.1% as sequential local therapy in psoriasis vulgaris. J Eur Acad Dermatol Venereol. 2009;23:905-912.
- Menter A, Strober BE, Kaplan DH, et al. Joint AAD-NPF guidelines of care for the management and treatment of psoriasis with biologics. J Am Acad Dermatol. 2019;80:1029-1072.
- Strober BE, Bissonnette R, Fiorentino D, et al. Comparative effectiveness of biologic agents for the treatment of psoriasis in a real-world setting: results from a large, prospective, observational study (Psoriasis Longitudinal Assessment and Registry [PSOLAR]). J Am Acad Dermatol. 2016;74:851-861.e854.
- Castela E, Archier E, Devaux S, et al. Topical corticosteroids in plaque psoriasis: a systematic review of risk of adrenal axis suppression and skin atrophy. J Eur Acad Dermatol Venereol. 2012;26(suppl 3):47-51.
- Takahashi H, Tsuji H, Honma M, et al. Femoral head osteonecrosis after long-term topical corticosteroid treatment in a psoriasis patient. J Dermatol. 2012;39:887-888.
- el Maghraoui A, Tabache F, Bezza A, et al. Femoral head osteonecrosis after topical corticosteroid therapy. Clin Exp Rheumatol. 2001;19:233.
- Gribetz C, Ling M, Lebwohl M, et al. Pimecrolimus cream 1% in the treatment of intertriginous psoriasis: a double-blind, randomized study. J Am Acad Dermatol. 2004;51:731-738.
- Lebwohl M, Freeman AK, Chapman MS, et al. Tacrolimus ointment is effective for facial and intertriginous psoriasis. J Am Acad Dermatol. 2004;51:723-730.
- Paller AS, Fölster-Holst R, Chen SC, et al. No evidence of increased cancer incidence in children using topical tacrolimus for atopic dermatitis. J Am Acad Dermatol. 2020;83:375-381.
- Elmets CA, Lim HW, Stoff B, et al. Joint American Academy of Dermatology-National Psoriasis Foundation guidelines of care for the management and treatment of psoriasis with phototherapy. J Am Acad Dermatol. 2019;81:775-804.
- Lebwohl M, Ast E, Callen JP, et al. Once-daily tazarotene gel versus twice-daily fluocinonide cream in the treatment of plaque psoriasis. J Am Acad Dermatol. 1998;38:705-711.
- Weinstein GD, Koo JY, Krueger GG, et al. Tazarotene cream in the treatment of psoriasis: two multicenter, double-blind, randomized, vehicle-controlled studies of the safety and efficacy of tazarotene creams 0.05% and 0.1% applied once daily for 12 weeks. J Am Acad Dermatol. 2003;48:760-767.
- Lebwohl M, Lombardi K, Tan MH. Duration of improvement in psoriasis after treatment with tazarotene 0.1% gel plus clobetasol propionate 0.05% ointment: comparison of maintenance treatments. Int J Dermatol. 2001;40:64-66.
- Sugarman JL, Weiss J, Tanghetti EA, et al. Safety and efficacy of a fixed combination halobetasol and tazarotene lotion in the treatment of moderate-to-severe plaque psoriasis: a pooled analysis of two phase 3 studies. J Drugs Dermatol. 2018;17:855-861.
- Koo JY, Lowe NJ, Lew-Kaya DA, et al. Tazarotene plus UVB phototherapy in the treatment of psoriasis. J Am Acad Dermatol. 2000;43:821-828.
- Cassano N, Mantegazza R, Battaglini S, et al. Adjuvant role of a new emollient cream in patients with palmar and/or plantar psoriasis: a pilot randomized open-label study. G Ital Dermatol Venereol. 2010;145:789-792.
- Kristensen B, Kristensen O. Topical salicylic acid interferes with UVB therapy for psoriasis. Acta Derm Venereol. 1991;71:37-40.
- Menter A, Korman NJ, Elmets CA, et al. Guidelines of care for the management of psoriasis and psoriatic arthritis. section 3. guidelines of care for the management and treatment of psoriasis with topical therapies. J Am Acad Dermatol. 2009;60:643-659.
- Rogalski C, Grunewald S, Schetschorke M, et al. Treatment of plaque-type psoriasis with the 308 nm excimer laser in combination with dithranol or calcipotriol. Int J Hyperthermia. 2012;28:184-190.
- Bagel J. LCD plus NB-UVB reduces time to improvement of psoriasis vs. NB-UVB alone. J Drugs Dermatol. 2009;8:351-357.
- Abdallah MA, El-Khateeb EA, Abdel-Rahman SH. The influence of psoriatic plaques pretreatment with crude coal tar vs. petrolatum on the efficacy of narrow-band ultraviolet B: a half-vs.-half intra-individual double-blinded comparative study. Photodermatol Photoimmunol Photomed. 2011;27:226-230.
Psoriasis is a chronic inflammatory skin disease characterized by erythematous scaly plaques that can invoke substantial pain, pruritus, and quality-of-life disturbance in patients. Topical therapies are the most commonly used medications for treating psoriasis, with one study (N = 128,308) showing that more than 85% of patients with psoriasis were managed solely with topical medications. 1 For patients with mild to moderate psoriasis, topical agents alone may be able to control disease completely. For those with more severe disease, topical agents are used adjunctively with systemic or biologic agents to optimize disease control in localized areas.
The American Academy of Dermatology (AAD) and National Psoriasis Foundation (NPF) published guidelines in 2020 for managing psoriasis with topical agents in adults.2 This review presents the most up-to-date clinical recommendations for topical agent use in adult patients with psoriasis and elaborates on each drug’s pharmacologic and safety profile. Specifically, evidence-based treatment recommendations for topical steroids, calcineurin inhibitors (CNIs), vitamin D analogues, retinoids (tazarotene), emollients, keratolytics (salicylic acid), anthracenes (anthralin), and keratoplastics (coal tar) will be addressed (Table 1). Recommendations for combination therapy with other treatment modalities including UVB light therapy, biologics, and systemic nonbiologic agents also will be discussed.


Selecting a Topical Agent Based on Disease Localization
When treating patients with psoriasis with topical therapies, clinicians should take into consideration drug potency, as it determines how effective a treatment will be in penetrating the skin barrier. Plaque characteristics, such as distribution (localized vs widespread), anatomical localization (flexural, scalp, palms/soles/nails), size (large vs small), and thickness (thick vs thin), not only influence treatment effectiveness but also the incidence of drug-related adverse events. Furthermore, preferred topical therapies are tailored to each patient based on disease characteristics and activity. Coal tar and anthralin have been used less frequently than other topical therapies for psoriasis because of their undesirable side-effect profiles (Table 1).3
Face and Intertriginous Regions—The face and intertriginous areas are sensitive because skin tends to be thin in these regions. Emollients are recommended for disease in these locations given their safety and flexibility in use for most areas. Conversely, anthralin should be avoided on the face, intertriginous areas, and even highly visible locations because of the potential for skin staining. Low-potency corticosteroids also have utility in psoriasis distributed on the face and intertriginous regions. Additionally, application of steroids around the eyes should be cautioned because topical steroids can induce ocular complications such as glaucoma and cataracts in rare circumstances.4
Off-label use of CNIs for psoriasis on the face and intertriginous areas also is effective. Currently, there is a level B recommendation for off-label use of 0.1% tacrolimus for up to 8 weeks for inverse psoriasis or psoriasis on the face. Off-label use of pimecrolimus for 4 to 8 weeks also can be considered for inverse psoriasis. Combination therapy consisting of hydrocortisone with calcipotriol ointment is another effective regimen.5 One study also suggested that use of crisaborole for 4 to 8 weeks in intertriginous psoriasis can be effective and well tolerated.6
Scalp—The vehicle of medication administration is especially important in hair-bearing areas such as the scalp, as these areas are challenging for medication application and patient adherence. Thus, patient preferences for the vehicle must be considered. Several studies have been conducted to assess preference for various vehicles in scalp psoriasis. A foam or solution may be preferable to ointments, gels, or creams.7 Gels may be preferred over ointments.8 There is a level A recommendation supporting the use of class 1 to 7 topical steroids for a minimum of 4 weeks as initial and maintenance treatment of scalp psoriasis. The highest level of evidence (level A) also supports the use of calcipotriol foam or combination therapy of calcipotriol–betamethasone dipropionate gel for 4 to 12 weeks as treatment of mild to moderate scalp psoriasis.
Nails—Several options for topical medications have been recommended for the treatment of nail psoriasis. Currently, there is a level B recommendation for the use of tazarotene for the treatment of nail psoriasis. Another effective regimen is combination therapy with vitamin D analogues and betamethasone dipropionate.9 Topical steroid use for nail psoriasis should be limited to 12 weeks because of the risk for bone atrophy with chronic steroid use.
Palmoplantar—The palms and soles have a thicker epidermal layer than other areas of the body. As a result, class 1 corticosteroids can be used for palmoplantar psoriasis for more than 4 weeks with vigilant monitoring for adverse effects such as skin atrophy, tachyphylaxis, or tinea infection. Tazarotene also has been shown to be helpful in treating palmoplantar psoriasis.
Resistant Disease—Intralesional steroids are beneficial treatment options for recalcitrant psoriasis in glabrous areas, as well as for palmoplantar, nail, and scalp psoriasis. Up to 10 mg/mL of triamcinolone acetonide used every 3 to 4 weeks is an effective regimen.10Pregnancy/Breastfeeding—Women of childbearing potential have additional safety precautions that should be considered during medication selection. Emollients have been shown to be safe during pregnancy and lactation. Currently, there is little known about CNI use during pregnancy. During lactation, CNIs can be used by breastfeeding mothers in most areas, excluding the breasts. Evaluation of the safety of anthralin and vitamin D analogues during pregnancy and lactation have not been studied. For these agents, dermatologists need to use their clinical judgment to weigh the risks and benefits of medication, particularly in patients requiring occlusion, higher medication doses, or treatment over a large surface area. Salicylic acid should be used with caution in pregnant and breastfeeding mothers because it is a pregnancy category C drug. Lower-potency corticosteroids may be used with caution during pregnancy and breastfeeding. More potent corticosteroids and coal tar, however, should be avoided. Similarly, tazarotene use is contraindicated in pregnancy. According to the US Food and Drug Administration labels for all forms of topical tazarotene, a pregnancy test must be obtained 2 weeks prior to tazarotene treatment initiation in women of childbearing potential because of the risk for serious fetal malformations and toxicity.
Recommendations, Risks, and Benefits of Topical Therapy for the Management of Psoriasis
Topical Corticosteroids—Topical corticosteroids (TCs) are widely used for inflammatory skin conditions and are available in a variety of strengths (Table 2). They are thought to exert their action by regulating the gene transcription of proinflammatory mediators. For psoriasis, steroids are recommended for 2 to 4 weeks, depending on disease severity. Although potent and superpotent steroids are more effective than mild- to moderate-strength TCs, use of lower-potency TCs may be warranted depending on disease distribution and localization.11 For treatment of psoriasis with no involvement of the intertriginous areas, use of class 1 to 5 TCs for up to 4 weeks is recommended.

For moderate to severe psoriasis with 20% or less body surface area (BSA) affected, combination therapy consisting of mometasone and salicylic acid has been shown to be more effective than mometasone alone.12,13 There currently is a level A recommendation for the use of combination therapy with class 1 TCs and etanercept for 12 weeks in patients with moderate to severe psoriasis who require both systemic and topical therapies for disease control. Similarly, combination therapy with infliximab and high-potency TCs has a level B recommendation to enhance efficacy for the treatment of moderate to severe psoriasis.14 High-quality studies on the use of TCs with anti–IL-12/IL-23, anti–IL-23, and anti–IL-17 currently are unavailable, but the combination is not expected to be unsafe.14,15 Combination therapy of betamethasone dipropionate ointment and low-dose cyclosporine is an alternative regimen with a level B recommendation.
The most common adverse effects with use of TCs are skin thinning and atrophy, telangiectasia, and striae (Table 1). With clinical improvement of disease, it is recommended that clinicians taper TCs to prevent rebound effect. To decrease TC-related adverse effects, clinicians should use combination therapy with steroid-sparing agents for disease maintenance, transition to lower-potency corticosteroids, or use intermittent steroid therapy. Systemic effects of TC use include hypothalamic-pituitary-adrenal axis suppression, Cushing syndrome, and osteonecrosis of the femoral head.16-18 These systemic effects with TC use are rare unless treatment is for disease involving greater than 20% BSA or occlusion for more than 4 weeks.
Calcineurin Inhibitors—Calcineurin inhibitors inhibit calcineurin phosphorylation and T-cell activation, subsequently decreasing the expression of proinflammatory cytokines. Currently, they are not approved by the US Food and Drug Administration to treat psoriasis but have demonstrated efficacy in randomized control trials (RCTs) for facial and intertriginous psoriasis. In RCTs, 71% of patients using pimecrolimus cream 0.1% twice daily for 8 weeks achieved an investigator global assessment score of clear (0) or almost clear (1) compared with 21% of placebo-treated patients (N=57).19 Other trials have shown that 65% of patients receiving tacrolimus ointment 0.1% for 8 weeks achieved an investigator global assessment score of 0 or 1 compared with 31% of placebo-treated patients (N=167).20 Because of their efficacy in RCTs, CNIs commonly are used off label to treat psoriasis.
The most common adverse effects with CNI use are burning, pruritus, and flushing with alcohol ingestion (Table 1). Additionally, CNIs have a black box warning that use may increase the risk for malignancy, but this risk has not been demonstrated with topical use in humans.21Vitamin D Analogues—The class of vitamin D analogues—calcipotriol/calcipotriene and calcitriol—frequently are used to treat psoriasis. Vitamin D analogues exert their beneficial effects by inhibiting keratinocyte proliferation and enhancing keratinocyte differentiation. They also are ideal for long-term use (up to 52 weeks) in mild to moderate psoriasis and can be used in combination with class 2 and 3 TCs. There is a level A recommendation that supports the use of combination therapy with calcipotriol and TCs for the treatment of mild to moderate psoriasis.
For severe psoriasis, many studies have investigated the efficacy of combination therapy with vitamin D analogues and systemic treatments. Combination therapy with calcipotriol and methotrexate or calcipotriol and acitretin are effective treatment regimens with level A recommendations. Calcipotriol–betamethasone dipropionate ointment in combination with low-dose cyclosporine is an alternative option with a level B recommendation. Because vitamin D analogues are inactivated by UVA and UVB radiation, clinicians should advise their patients to use vitamin D analogues after receiving UVB phototherapy.22
Common adverse effects of vitamin D analogues include burning, pruritus, erythema, and dryness (Table 1). Hypercalcemia and parathyroid hormone suppression are extremely rare unless treatment occurs over a large surface area (>30% BSA) or the patient has concurrent renal disease or impairments in calcium metabolism.
Tazarotene—Tazarotene is a topical retinoid that acts by decreasing keratinocyte proliferation, facilitating keratinocyte differentiation, and inhibiting inflammation. Patients with mild to moderate psoriasis are recommended to receive tazarotene treatment for 8 to 12 weeks. In several RCTs, tazarotene gel 0.1% and tazarotene cream 0.1% and 0.05% achieved treatment success in treating plaque psoriasis.23,24
For increased efficacy, clinicians can recommend combination therapy with tazarotene and a TC. Combination therapy with tazarotene and a mid- or high-potency TC for 8 to 16 weeks has been shown to be more effective than treatment with tazarotene alone.25 Thus, there is a level A recommendation for use of this combination to treat mild to moderate psoriasis. Agents used in combination therapy work synergistically to decrease the length of treatment and increase the duration of remission. The frequency of adverse effects, such as irritation from tazarotene and skin atrophy from TCs, also are reduced.26 Combination therapy with tazarotene and narrowband UVB (NB-UVB) is another effective option that requires less UV radiation than NB-UVB alone because of the synergistic effects of both treatment modalities.27 Clinicians should counsel patients on the adverse effects of tazarotene, which include local irritation, burning, pruritus, and erythema (Table 1).
Emollients—Emollients are nonmedicated moisturizers that decrease the amount of transepidermal water loss. There is a level B recommendation for use of emollients and TCs in combination for 4 to 8 weeks to treat psoriasis. In fact, combination therapy with mometasone and emollients has demonstrated greater improvement in symptoms of palmoplantar psoriasis (ie, erythema, desquamation, infiltration, BSA involvement) than mometasone alone.28 Emollients are safe options that can be used on all areas of the body and during pregnancy and lactation. Although adverse effects of emollients are rare, clinicians should counsel patients on the risk for contact dermatitis if specific allergies to ingredients/fragrances exist (Table 1).
Salicylic Acid—Salicylic acid is a topical keratolytic that can be used to treat psoriatic plaques. Use of salicylic acid for 8 to 16 weeks has been shown to be effective for mild to moderate psoriasis. Combination therapy of salicylic acid and TCs in patients with 20% or less BSA affected is a safe and effective option with a level B recommendation. Combination therapy with salicylic acid and calcipotriene, however, should be avoided because calcipotriene is inactivated by salicylic acid. It also is recommended that salicylic acid application follow phototherapy when both treatment modalities are used in combination.29,30 Clinicians should be cautious about using salicylic acid in patients with renal or hepatic disease because of the increased risk for salicylate toxicity (Table 1).
Anthralin—Anthralin is a synthetic hydrocarbon derivative that has been shown to reduce inflammation and normalize keratinocyte proliferation through an unknown mechanism. It is recommended that patients with mild to moderate psoriasis receive anthralin treatment for 8 to 12 weeks, with a maximum application time of 2 hours per day. Combination therapy of excimer laser and anthralin has been shown to be more effective in treating psoriasis than anthralin alone.31 Therefore, clinicians have the option of including excimer laser therapy for additional disease control. Anthralin should be avoided on the face, flexural regions, and highly visible areas because of potential skin staining (Table 1). Other adverse effects include application-site burning and erythema.
Coal Tar—Coal tar is a heterogenous mixture of aromatic hydrocarbons that is an effective treatment of psoriasis because of its inherent anti-inflammatory and keratoplastic properties. There is high-quality evidence supporting a level A recommendation for coal tar use in mild to moderate psoriasis. Combination therapy with NB-UVB and coal tar (also known as Goeckerman therapy) is a recommended treatment option with a quicker onset of action and improved outcomes compared with NB-UVB therapy alone.32,33 Adverse events of coal tar include application-site irritation, folliculitis, contact dermatitis, phototoxicity, and skin pigmentation (Table 1).
Conclusion
Topical medications are versatile treatment options that can be utilized as monotherapy or adjunct therapy for mild to severe psoriasis. Benefits of topical agents include minimal required monitoring, few contraindications, and direct localized effect on plaques. Therefore, side effects with topical agent use rarely are systemic. Medication interactions are less of a concern with topical therapies; thus, they have better safety profiles compared with systemic therapies. This clinical review summarizes the recently published evidence-based guidelines from the AAD and NPF on the use of topical agents in psoriasis and may be a useful guiding framework for clinicians in their everyday practice.
Psoriasis is a chronic inflammatory skin disease characterized by erythematous scaly plaques that can invoke substantial pain, pruritus, and quality-of-life disturbance in patients. Topical therapies are the most commonly used medications for treating psoriasis, with one study (N = 128,308) showing that more than 85% of patients with psoriasis were managed solely with topical medications. 1 For patients with mild to moderate psoriasis, topical agents alone may be able to control disease completely. For those with more severe disease, topical agents are used adjunctively with systemic or biologic agents to optimize disease control in localized areas.
The American Academy of Dermatology (AAD) and National Psoriasis Foundation (NPF) published guidelines in 2020 for managing psoriasis with topical agents in adults.2 This review presents the most up-to-date clinical recommendations for topical agent use in adult patients with psoriasis and elaborates on each drug’s pharmacologic and safety profile. Specifically, evidence-based treatment recommendations for topical steroids, calcineurin inhibitors (CNIs), vitamin D analogues, retinoids (tazarotene), emollients, keratolytics (salicylic acid), anthracenes (anthralin), and keratoplastics (coal tar) will be addressed (Table 1). Recommendations for combination therapy with other treatment modalities including UVB light therapy, biologics, and systemic nonbiologic agents also will be discussed.


Selecting a Topical Agent Based on Disease Localization
When treating patients with psoriasis with topical therapies, clinicians should take into consideration drug potency, as it determines how effective a treatment will be in penetrating the skin barrier. Plaque characteristics, such as distribution (localized vs widespread), anatomical localization (flexural, scalp, palms/soles/nails), size (large vs small), and thickness (thick vs thin), not only influence treatment effectiveness but also the incidence of drug-related adverse events. Furthermore, preferred topical therapies are tailored to each patient based on disease characteristics and activity. Coal tar and anthralin have been used less frequently than other topical therapies for psoriasis because of their undesirable side-effect profiles (Table 1).3
Face and Intertriginous Regions—The face and intertriginous areas are sensitive because skin tends to be thin in these regions. Emollients are recommended for disease in these locations given their safety and flexibility in use for most areas. Conversely, anthralin should be avoided on the face, intertriginous areas, and even highly visible locations because of the potential for skin staining. Low-potency corticosteroids also have utility in psoriasis distributed on the face and intertriginous regions. Additionally, application of steroids around the eyes should be cautioned because topical steroids can induce ocular complications such as glaucoma and cataracts in rare circumstances.4
Off-label use of CNIs for psoriasis on the face and intertriginous areas also is effective. Currently, there is a level B recommendation for off-label use of 0.1% tacrolimus for up to 8 weeks for inverse psoriasis or psoriasis on the face. Off-label use of pimecrolimus for 4 to 8 weeks also can be considered for inverse psoriasis. Combination therapy consisting of hydrocortisone with calcipotriol ointment is another effective regimen.5 One study also suggested that use of crisaborole for 4 to 8 weeks in intertriginous psoriasis can be effective and well tolerated.6
Scalp—The vehicle of medication administration is especially important in hair-bearing areas such as the scalp, as these areas are challenging for medication application and patient adherence. Thus, patient preferences for the vehicle must be considered. Several studies have been conducted to assess preference for various vehicles in scalp psoriasis. A foam or solution may be preferable to ointments, gels, or creams.7 Gels may be preferred over ointments.8 There is a level A recommendation supporting the use of class 1 to 7 topical steroids for a minimum of 4 weeks as initial and maintenance treatment of scalp psoriasis. The highest level of evidence (level A) also supports the use of calcipotriol foam or combination therapy of calcipotriol–betamethasone dipropionate gel for 4 to 12 weeks as treatment of mild to moderate scalp psoriasis.
Nails—Several options for topical medications have been recommended for the treatment of nail psoriasis. Currently, there is a level B recommendation for the use of tazarotene for the treatment of nail psoriasis. Another effective regimen is combination therapy with vitamin D analogues and betamethasone dipropionate.9 Topical steroid use for nail psoriasis should be limited to 12 weeks because of the risk for bone atrophy with chronic steroid use.
Palmoplantar—The palms and soles have a thicker epidermal layer than other areas of the body. As a result, class 1 corticosteroids can be used for palmoplantar psoriasis for more than 4 weeks with vigilant monitoring for adverse effects such as skin atrophy, tachyphylaxis, or tinea infection. Tazarotene also has been shown to be helpful in treating palmoplantar psoriasis.
Resistant Disease—Intralesional steroids are beneficial treatment options for recalcitrant psoriasis in glabrous areas, as well as for palmoplantar, nail, and scalp psoriasis. Up to 10 mg/mL of triamcinolone acetonide used every 3 to 4 weeks is an effective regimen.10Pregnancy/Breastfeeding—Women of childbearing potential have additional safety precautions that should be considered during medication selection. Emollients have been shown to be safe during pregnancy and lactation. Currently, there is little known about CNI use during pregnancy. During lactation, CNIs can be used by breastfeeding mothers in most areas, excluding the breasts. Evaluation of the safety of anthralin and vitamin D analogues during pregnancy and lactation have not been studied. For these agents, dermatologists need to use their clinical judgment to weigh the risks and benefits of medication, particularly in patients requiring occlusion, higher medication doses, or treatment over a large surface area. Salicylic acid should be used with caution in pregnant and breastfeeding mothers because it is a pregnancy category C drug. Lower-potency corticosteroids may be used with caution during pregnancy and breastfeeding. More potent corticosteroids and coal tar, however, should be avoided. Similarly, tazarotene use is contraindicated in pregnancy. According to the US Food and Drug Administration labels for all forms of topical tazarotene, a pregnancy test must be obtained 2 weeks prior to tazarotene treatment initiation in women of childbearing potential because of the risk for serious fetal malformations and toxicity.
Recommendations, Risks, and Benefits of Topical Therapy for the Management of Psoriasis
Topical Corticosteroids—Topical corticosteroids (TCs) are widely used for inflammatory skin conditions and are available in a variety of strengths (Table 2). They are thought to exert their action by regulating the gene transcription of proinflammatory mediators. For psoriasis, steroids are recommended for 2 to 4 weeks, depending on disease severity. Although potent and superpotent steroids are more effective than mild- to moderate-strength TCs, use of lower-potency TCs may be warranted depending on disease distribution and localization.11 For treatment of psoriasis with no involvement of the intertriginous areas, use of class 1 to 5 TCs for up to 4 weeks is recommended.

For moderate to severe psoriasis with 20% or less body surface area (BSA) affected, combination therapy consisting of mometasone and salicylic acid has been shown to be more effective than mometasone alone.12,13 There currently is a level A recommendation for the use of combination therapy with class 1 TCs and etanercept for 12 weeks in patients with moderate to severe psoriasis who require both systemic and topical therapies for disease control. Similarly, combination therapy with infliximab and high-potency TCs has a level B recommendation to enhance efficacy for the treatment of moderate to severe psoriasis.14 High-quality studies on the use of TCs with anti–IL-12/IL-23, anti–IL-23, and anti–IL-17 currently are unavailable, but the combination is not expected to be unsafe.14,15 Combination therapy of betamethasone dipropionate ointment and low-dose cyclosporine is an alternative regimen with a level B recommendation.
The most common adverse effects with use of TCs are skin thinning and atrophy, telangiectasia, and striae (Table 1). With clinical improvement of disease, it is recommended that clinicians taper TCs to prevent rebound effect. To decrease TC-related adverse effects, clinicians should use combination therapy with steroid-sparing agents for disease maintenance, transition to lower-potency corticosteroids, or use intermittent steroid therapy. Systemic effects of TC use include hypothalamic-pituitary-adrenal axis suppression, Cushing syndrome, and osteonecrosis of the femoral head.16-18 These systemic effects with TC use are rare unless treatment is for disease involving greater than 20% BSA or occlusion for more than 4 weeks.
Calcineurin Inhibitors—Calcineurin inhibitors inhibit calcineurin phosphorylation and T-cell activation, subsequently decreasing the expression of proinflammatory cytokines. Currently, they are not approved by the US Food and Drug Administration to treat psoriasis but have demonstrated efficacy in randomized control trials (RCTs) for facial and intertriginous psoriasis. In RCTs, 71% of patients using pimecrolimus cream 0.1% twice daily for 8 weeks achieved an investigator global assessment score of clear (0) or almost clear (1) compared with 21% of placebo-treated patients (N=57).19 Other trials have shown that 65% of patients receiving tacrolimus ointment 0.1% for 8 weeks achieved an investigator global assessment score of 0 or 1 compared with 31% of placebo-treated patients (N=167).20 Because of their efficacy in RCTs, CNIs commonly are used off label to treat psoriasis.
The most common adverse effects with CNI use are burning, pruritus, and flushing with alcohol ingestion (Table 1). Additionally, CNIs have a black box warning that use may increase the risk for malignancy, but this risk has not been demonstrated with topical use in humans.21Vitamin D Analogues—The class of vitamin D analogues—calcipotriol/calcipotriene and calcitriol—frequently are used to treat psoriasis. Vitamin D analogues exert their beneficial effects by inhibiting keratinocyte proliferation and enhancing keratinocyte differentiation. They also are ideal for long-term use (up to 52 weeks) in mild to moderate psoriasis and can be used in combination with class 2 and 3 TCs. There is a level A recommendation that supports the use of combination therapy with calcipotriol and TCs for the treatment of mild to moderate psoriasis.
For severe psoriasis, many studies have investigated the efficacy of combination therapy with vitamin D analogues and systemic treatments. Combination therapy with calcipotriol and methotrexate or calcipotriol and acitretin are effective treatment regimens with level A recommendations. Calcipotriol–betamethasone dipropionate ointment in combination with low-dose cyclosporine is an alternative option with a level B recommendation. Because vitamin D analogues are inactivated by UVA and UVB radiation, clinicians should advise their patients to use vitamin D analogues after receiving UVB phototherapy.22
Common adverse effects of vitamin D analogues include burning, pruritus, erythema, and dryness (Table 1). Hypercalcemia and parathyroid hormone suppression are extremely rare unless treatment occurs over a large surface area (>30% BSA) or the patient has concurrent renal disease or impairments in calcium metabolism.
Tazarotene—Tazarotene is a topical retinoid that acts by decreasing keratinocyte proliferation, facilitating keratinocyte differentiation, and inhibiting inflammation. Patients with mild to moderate psoriasis are recommended to receive tazarotene treatment for 8 to 12 weeks. In several RCTs, tazarotene gel 0.1% and tazarotene cream 0.1% and 0.05% achieved treatment success in treating plaque psoriasis.23,24
For increased efficacy, clinicians can recommend combination therapy with tazarotene and a TC. Combination therapy with tazarotene and a mid- or high-potency TC for 8 to 16 weeks has been shown to be more effective than treatment with tazarotene alone.25 Thus, there is a level A recommendation for use of this combination to treat mild to moderate psoriasis. Agents used in combination therapy work synergistically to decrease the length of treatment and increase the duration of remission. The frequency of adverse effects, such as irritation from tazarotene and skin atrophy from TCs, also are reduced.26 Combination therapy with tazarotene and narrowband UVB (NB-UVB) is another effective option that requires less UV radiation than NB-UVB alone because of the synergistic effects of both treatment modalities.27 Clinicians should counsel patients on the adverse effects of tazarotene, which include local irritation, burning, pruritus, and erythema (Table 1).
Emollients—Emollients are nonmedicated moisturizers that decrease the amount of transepidermal water loss. There is a level B recommendation for use of emollients and TCs in combination for 4 to 8 weeks to treat psoriasis. In fact, combination therapy with mometasone and emollients has demonstrated greater improvement in symptoms of palmoplantar psoriasis (ie, erythema, desquamation, infiltration, BSA involvement) than mometasone alone.28 Emollients are safe options that can be used on all areas of the body and during pregnancy and lactation. Although adverse effects of emollients are rare, clinicians should counsel patients on the risk for contact dermatitis if specific allergies to ingredients/fragrances exist (Table 1).
Salicylic Acid—Salicylic acid is a topical keratolytic that can be used to treat psoriatic plaques. Use of salicylic acid for 8 to 16 weeks has been shown to be effective for mild to moderate psoriasis. Combination therapy of salicylic acid and TCs in patients with 20% or less BSA affected is a safe and effective option with a level B recommendation. Combination therapy with salicylic acid and calcipotriene, however, should be avoided because calcipotriene is inactivated by salicylic acid. It also is recommended that salicylic acid application follow phototherapy when both treatment modalities are used in combination.29,30 Clinicians should be cautious about using salicylic acid in patients with renal or hepatic disease because of the increased risk for salicylate toxicity (Table 1).
Anthralin—Anthralin is a synthetic hydrocarbon derivative that has been shown to reduce inflammation and normalize keratinocyte proliferation through an unknown mechanism. It is recommended that patients with mild to moderate psoriasis receive anthralin treatment for 8 to 12 weeks, with a maximum application time of 2 hours per day. Combination therapy of excimer laser and anthralin has been shown to be more effective in treating psoriasis than anthralin alone.31 Therefore, clinicians have the option of including excimer laser therapy for additional disease control. Anthralin should be avoided on the face, flexural regions, and highly visible areas because of potential skin staining (Table 1). Other adverse effects include application-site burning and erythema.
Coal Tar—Coal tar is a heterogenous mixture of aromatic hydrocarbons that is an effective treatment of psoriasis because of its inherent anti-inflammatory and keratoplastic properties. There is high-quality evidence supporting a level A recommendation for coal tar use in mild to moderate psoriasis. Combination therapy with NB-UVB and coal tar (also known as Goeckerman therapy) is a recommended treatment option with a quicker onset of action and improved outcomes compared with NB-UVB therapy alone.32,33 Adverse events of coal tar include application-site irritation, folliculitis, contact dermatitis, phototoxicity, and skin pigmentation (Table 1).
Conclusion
Topical medications are versatile treatment options that can be utilized as monotherapy or adjunct therapy for mild to severe psoriasis. Benefits of topical agents include minimal required monitoring, few contraindications, and direct localized effect on plaques. Therefore, side effects with topical agent use rarely are systemic. Medication interactions are less of a concern with topical therapies; thus, they have better safety profiles compared with systemic therapies. This clinical review summarizes the recently published evidence-based guidelines from the AAD and NPF on the use of topical agents in psoriasis and may be a useful guiding framework for clinicians in their everyday practice.
- Murage MJ, Kern DM, Chang L, et al. Treatment patterns among patients with psoriasis using a large national payer database in the United States: a retrospective study. J Med Econ. 2018:1-9.
- Elmets CA, Korman NJ, Prater EF, et al. Joint AAD-NPF Guidelines of care for the management and treatment of psoriasis with topical therapy and alternative medicine modalities for psoriasis severity measures. J Am Acad Dermatol. 2021;84:432-470.
- Svendsen MT, Jeyabalan J, Andersen KE, et al. Worldwide utilization of topical remedies in treatment of psoriasis: a systematic review. J Dermatolog Treat. 2017;28:374-383.
- Day A, Abramson AK, Patel M, et al. The spectrum of oculocutaneous disease: part II. neoplastic and drug-related causes of oculocutaneous disease. J Am Acad Dermatol. 2014;70:821.e821-819.
- Choi JW, Choi JW, Kwon IH, et al. High-concentration (20 μg g-¹) tacalcitol ointment in the treatment of facial psoriasis: an 8-week open-label clinical trial. Br J Dermatol. 2010;162:1359-1364.
- Hashim PW, Chima M, Kim HJ, et al. Crisaborole 2% ointment for the treatment of intertriginous, anogenital, and facial psoriasis: a double-blind, randomized, vehicle-controlled trial. J Am Acad Dermatol. 2020;82:360-365.
- Housman TS, Mellen BG, Rapp SR, et al. Patients with psoriasis prefer solution and foam vehicles: a quantitative assessment of vehicle preference. Cutis. 2002;70:327-332.
- Iversen L, Jakobsen HB. Patient preferences for topical psoriasis treatments are diverse and difficult to predict. Dermatol Ther. 2016;6:273-285.
- Clobex Package insert. Galderma Laboratories, LP; 2012.
- Kenalog-10 Injection. Package insert. Bristol-Myers Squibb Company; 2018.
- Mason J, Mason AR, Cork MJ. Topical preparations for the treatment of psoriasis: a systematic review. Br J Dermatol. 2002;146:351-364.
- Koo J, Cuffie CA, Tanner DJ, et al. Mometasone furoate 0.1%-salicylic acid 5% ointment versus mometasone furoate 0.1% ointment in the treatment of moderate-to-severe psoriasis: a multicenter study. Clin Ther. 1998;20:283-291.
- Tiplica GS, Salavastru CM. Mometasone furoate 0.1% and salicylic acid 5% vs. mometasone furoate 0.1% as sequential local therapy in psoriasis vulgaris. J Eur Acad Dermatol Venereol. 2009;23:905-912.
- Menter A, Strober BE, Kaplan DH, et al. Joint AAD-NPF guidelines of care for the management and treatment of psoriasis with biologics. J Am Acad Dermatol. 2019;80:1029-1072.
- Strober BE, Bissonnette R, Fiorentino D, et al. Comparative effectiveness of biologic agents for the treatment of psoriasis in a real-world setting: results from a large, prospective, observational study (Psoriasis Longitudinal Assessment and Registry [PSOLAR]). J Am Acad Dermatol. 2016;74:851-861.e854.
- Castela E, Archier E, Devaux S, et al. Topical corticosteroids in plaque psoriasis: a systematic review of risk of adrenal axis suppression and skin atrophy. J Eur Acad Dermatol Venereol. 2012;26(suppl 3):47-51.
- Takahashi H, Tsuji H, Honma M, et al. Femoral head osteonecrosis after long-term topical corticosteroid treatment in a psoriasis patient. J Dermatol. 2012;39:887-888.
- el Maghraoui A, Tabache F, Bezza A, et al. Femoral head osteonecrosis after topical corticosteroid therapy. Clin Exp Rheumatol. 2001;19:233.
- Gribetz C, Ling M, Lebwohl M, et al. Pimecrolimus cream 1% in the treatment of intertriginous psoriasis: a double-blind, randomized study. J Am Acad Dermatol. 2004;51:731-738.
- Lebwohl M, Freeman AK, Chapman MS, et al. Tacrolimus ointment is effective for facial and intertriginous psoriasis. J Am Acad Dermatol. 2004;51:723-730.
- Paller AS, Fölster-Holst R, Chen SC, et al. No evidence of increased cancer incidence in children using topical tacrolimus for atopic dermatitis. J Am Acad Dermatol. 2020;83:375-381.
- Elmets CA, Lim HW, Stoff B, et al. Joint American Academy of Dermatology-National Psoriasis Foundation guidelines of care for the management and treatment of psoriasis with phototherapy. J Am Acad Dermatol. 2019;81:775-804.
- Lebwohl M, Ast E, Callen JP, et al. Once-daily tazarotene gel versus twice-daily fluocinonide cream in the treatment of plaque psoriasis. J Am Acad Dermatol. 1998;38:705-711.
- Weinstein GD, Koo JY, Krueger GG, et al. Tazarotene cream in the treatment of psoriasis: two multicenter, double-blind, randomized, vehicle-controlled studies of the safety and efficacy of tazarotene creams 0.05% and 0.1% applied once daily for 12 weeks. J Am Acad Dermatol. 2003;48:760-767.
- Lebwohl M, Lombardi K, Tan MH. Duration of improvement in psoriasis after treatment with tazarotene 0.1% gel plus clobetasol propionate 0.05% ointment: comparison of maintenance treatments. Int J Dermatol. 2001;40:64-66.
- Sugarman JL, Weiss J, Tanghetti EA, et al. Safety and efficacy of a fixed combination halobetasol and tazarotene lotion in the treatment of moderate-to-severe plaque psoriasis: a pooled analysis of two phase 3 studies. J Drugs Dermatol. 2018;17:855-861.
- Koo JY, Lowe NJ, Lew-Kaya DA, et al. Tazarotene plus UVB phototherapy in the treatment of psoriasis. J Am Acad Dermatol. 2000;43:821-828.
- Cassano N, Mantegazza R, Battaglini S, et al. Adjuvant role of a new emollient cream in patients with palmar and/or plantar psoriasis: a pilot randomized open-label study. G Ital Dermatol Venereol. 2010;145:789-792.
- Kristensen B, Kristensen O. Topical salicylic acid interferes with UVB therapy for psoriasis. Acta Derm Venereol. 1991;71:37-40.
- Menter A, Korman NJ, Elmets CA, et al. Guidelines of care for the management of psoriasis and psoriatic arthritis. section 3. guidelines of care for the management and treatment of psoriasis with topical therapies. J Am Acad Dermatol. 2009;60:643-659.
- Rogalski C, Grunewald S, Schetschorke M, et al. Treatment of plaque-type psoriasis with the 308 nm excimer laser in combination with dithranol or calcipotriol. Int J Hyperthermia. 2012;28:184-190.
- Bagel J. LCD plus NB-UVB reduces time to improvement of psoriasis vs. NB-UVB alone. J Drugs Dermatol. 2009;8:351-357.
- Abdallah MA, El-Khateeb EA, Abdel-Rahman SH. The influence of psoriatic plaques pretreatment with crude coal tar vs. petrolatum on the efficacy of narrow-band ultraviolet B: a half-vs.-half intra-individual double-blinded comparative study. Photodermatol Photoimmunol Photomed. 2011;27:226-230.
- Murage MJ, Kern DM, Chang L, et al. Treatment patterns among patients with psoriasis using a large national payer database in the United States: a retrospective study. J Med Econ. 2018:1-9.
- Elmets CA, Korman NJ, Prater EF, et al. Joint AAD-NPF Guidelines of care for the management and treatment of psoriasis with topical therapy and alternative medicine modalities for psoriasis severity measures. J Am Acad Dermatol. 2021;84:432-470.
- Svendsen MT, Jeyabalan J, Andersen KE, et al. Worldwide utilization of topical remedies in treatment of psoriasis: a systematic review. J Dermatolog Treat. 2017;28:374-383.
- Day A, Abramson AK, Patel M, et al. The spectrum of oculocutaneous disease: part II. neoplastic and drug-related causes of oculocutaneous disease. J Am Acad Dermatol. 2014;70:821.e821-819.
- Choi JW, Choi JW, Kwon IH, et al. High-concentration (20 μg g-¹) tacalcitol ointment in the treatment of facial psoriasis: an 8-week open-label clinical trial. Br J Dermatol. 2010;162:1359-1364.
- Hashim PW, Chima M, Kim HJ, et al. Crisaborole 2% ointment for the treatment of intertriginous, anogenital, and facial psoriasis: a double-blind, randomized, vehicle-controlled trial. J Am Acad Dermatol. 2020;82:360-365.
- Housman TS, Mellen BG, Rapp SR, et al. Patients with psoriasis prefer solution and foam vehicles: a quantitative assessment of vehicle preference. Cutis. 2002;70:327-332.
- Iversen L, Jakobsen HB. Patient preferences for topical psoriasis treatments are diverse and difficult to predict. Dermatol Ther. 2016;6:273-285.
- Clobex Package insert. Galderma Laboratories, LP; 2012.
- Kenalog-10 Injection. Package insert. Bristol-Myers Squibb Company; 2018.
- Mason J, Mason AR, Cork MJ. Topical preparations for the treatment of psoriasis: a systematic review. Br J Dermatol. 2002;146:351-364.
- Koo J, Cuffie CA, Tanner DJ, et al. Mometasone furoate 0.1%-salicylic acid 5% ointment versus mometasone furoate 0.1% ointment in the treatment of moderate-to-severe psoriasis: a multicenter study. Clin Ther. 1998;20:283-291.
- Tiplica GS, Salavastru CM. Mometasone furoate 0.1% and salicylic acid 5% vs. mometasone furoate 0.1% as sequential local therapy in psoriasis vulgaris. J Eur Acad Dermatol Venereol. 2009;23:905-912.
- Menter A, Strober BE, Kaplan DH, et al. Joint AAD-NPF guidelines of care for the management and treatment of psoriasis with biologics. J Am Acad Dermatol. 2019;80:1029-1072.
- Strober BE, Bissonnette R, Fiorentino D, et al. Comparative effectiveness of biologic agents for the treatment of psoriasis in a real-world setting: results from a large, prospective, observational study (Psoriasis Longitudinal Assessment and Registry [PSOLAR]). J Am Acad Dermatol. 2016;74:851-861.e854.
- Castela E, Archier E, Devaux S, et al. Topical corticosteroids in plaque psoriasis: a systematic review of risk of adrenal axis suppression and skin atrophy. J Eur Acad Dermatol Venereol. 2012;26(suppl 3):47-51.
- Takahashi H, Tsuji H, Honma M, et al. Femoral head osteonecrosis after long-term topical corticosteroid treatment in a psoriasis patient. J Dermatol. 2012;39:887-888.
- el Maghraoui A, Tabache F, Bezza A, et al. Femoral head osteonecrosis after topical corticosteroid therapy. Clin Exp Rheumatol. 2001;19:233.
- Gribetz C, Ling M, Lebwohl M, et al. Pimecrolimus cream 1% in the treatment of intertriginous psoriasis: a double-blind, randomized study. J Am Acad Dermatol. 2004;51:731-738.
- Lebwohl M, Freeman AK, Chapman MS, et al. Tacrolimus ointment is effective for facial and intertriginous psoriasis. J Am Acad Dermatol. 2004;51:723-730.
- Paller AS, Fölster-Holst R, Chen SC, et al. No evidence of increased cancer incidence in children using topical tacrolimus for atopic dermatitis. J Am Acad Dermatol. 2020;83:375-381.
- Elmets CA, Lim HW, Stoff B, et al. Joint American Academy of Dermatology-National Psoriasis Foundation guidelines of care for the management and treatment of psoriasis with phototherapy. J Am Acad Dermatol. 2019;81:775-804.
- Lebwohl M, Ast E, Callen JP, et al. Once-daily tazarotene gel versus twice-daily fluocinonide cream in the treatment of plaque psoriasis. J Am Acad Dermatol. 1998;38:705-711.
- Weinstein GD, Koo JY, Krueger GG, et al. Tazarotene cream in the treatment of psoriasis: two multicenter, double-blind, randomized, vehicle-controlled studies of the safety and efficacy of tazarotene creams 0.05% and 0.1% applied once daily for 12 weeks. J Am Acad Dermatol. 2003;48:760-767.
- Lebwohl M, Lombardi K, Tan MH. Duration of improvement in psoriasis after treatment with tazarotene 0.1% gel plus clobetasol propionate 0.05% ointment: comparison of maintenance treatments. Int J Dermatol. 2001;40:64-66.
- Sugarman JL, Weiss J, Tanghetti EA, et al. Safety and efficacy of a fixed combination halobetasol and tazarotene lotion in the treatment of moderate-to-severe plaque psoriasis: a pooled analysis of two phase 3 studies. J Drugs Dermatol. 2018;17:855-861.
- Koo JY, Lowe NJ, Lew-Kaya DA, et al. Tazarotene plus UVB phototherapy in the treatment of psoriasis. J Am Acad Dermatol. 2000;43:821-828.
- Cassano N, Mantegazza R, Battaglini S, et al. Adjuvant role of a new emollient cream in patients with palmar and/or plantar psoriasis: a pilot randomized open-label study. G Ital Dermatol Venereol. 2010;145:789-792.
- Kristensen B, Kristensen O. Topical salicylic acid interferes with UVB therapy for psoriasis. Acta Derm Venereol. 1991;71:37-40.
- Menter A, Korman NJ, Elmets CA, et al. Guidelines of care for the management of psoriasis and psoriatic arthritis. section 3. guidelines of care for the management and treatment of psoriasis with topical therapies. J Am Acad Dermatol. 2009;60:643-659.
- Rogalski C, Grunewald S, Schetschorke M, et al. Treatment of plaque-type psoriasis with the 308 nm excimer laser in combination with dithranol or calcipotriol. Int J Hyperthermia. 2012;28:184-190.
- Bagel J. LCD plus NB-UVB reduces time to improvement of psoriasis vs. NB-UVB alone. J Drugs Dermatol. 2009;8:351-357.
- Abdallah MA, El-Khateeb EA, Abdel-Rahman SH. The influence of psoriatic plaques pretreatment with crude coal tar vs. petrolatum on the efficacy of narrow-band ultraviolet B: a half-vs.-half intra-individual double-blinded comparative study. Photodermatol Photoimmunol Photomed. 2011;27:226-230.
Practice Points
- Topical medications collectively represent the most common form of psoriasis treatment. Depending on disease severity and distribution, topical agents can be used as monotherapy or adjunct therapy, offering the benefit of localized treatment without systemic side effects.
- Dermatologists should base the selection of an appropriate topical medication on factors including adverse effects, potency, vehicle, and anatomic localization of disease.