User login
Alcohol: An unfortunate teratogen
Medical students learn early in their education that alcohol is a teratogen. Despite this widespread knowledge, many obstetricians counsel patients about the safety of low doses of alcohol in pregnancy.1 Indeed, the Royal College of Obstetricians and Gynaecologists’ position on this is, “while the safest approach may be to avoid any alcohol during pregnancy, it remains the case that there is no evidence of harm from low levels of alcohol consumption, defined as no more than one or two units of alcohol once or twice a week.”2
Like many providers, I was aware of this controversy, but it became truly personal when a beloved family member was diagnosed with fetal alcohol syndrome (FAS). In this paper, I will review some of the controversy regarding alcohol in pregnancy, highlight findings from the literature, provide tools for prevention, and identify new developments regarding this devastating, preventable condition.
Charlie
To know my nephew Charlie is to fall in love with my nephew Charlie. One of the happiest moments of my life was when I learned my brother and sister-in-law had adopted twins from Kazakhstan. When my little niece and nephew started their new life in the United States, certain medical issues seemed to merit additional attention. Although both were very small for their age and required significant nutritional support, Charlie seemed to be a bit more rambunctious and required additional supervision.
The children were fortunate enough to have incredibly loving, dedicated parents, who have access to exceptional medical care as residents of Philadelphia, Pennsylvania. After extensive testing, it became clear what was causing Charlie’s developmental delay; his pediatric team made the diagnosis of FAS. My brother and sister-in-law became incredibly well-read about this challenging disorder, and threw themselves into national advocacy work to help prevent this unnecessary tragedy.
Recent data point to teratogenicity, but media confuse the issue
Some recent media coverage3 of celebrities who apparently drank while pregnant was in response to an article in the Journal of Epidemiology and Community Health.4 The authors of this study concluded that, “at age 5 years, cohort members born to mothers who drank up to one to two drinks per week or per occasion during pregnancy were not at increased risk of clinically relevant behavioral difficulties or cognitive deficits, compared with children of mothers in the not-in-pregnancy group.”
This is certainly not the first occasion the popular press has covered a published study that seems to indicate no ill effects of alcohol use in pregnancy. A 2008 report by Kelly and colleagues,5 and its subsequent media coverage, prompted the Fetal Alcohol Spectrum Disorders Study Group to state that the panel of experts was “alarmed” by recent newspaper reports suggesting that light drinking during pregnancy may be beneficial for an unborn child.6 They noted misleading and irresponsible media reports of the findings, which suggested that 3-year-old children whose mothers drank “lightly” during pregnancy were not at risk for certain behavioral problems.
What the study authors proceeded to note, however (that the media did not mention), was that the light drinkers in their study had socioeconomic advantages, compared with nondrinkers.5 (Advantaged economic status is established to be beneficial for childhood development.) They also noted that the study involved preschool-aged children, stating “Generally the adverse effects of light drinking during pregnancy are subtle and may go undetected in young children. However, other group studies of more moderate or ‘social’ drinking levels during pregnancy have shown an adverse impact on multiple aspects of development through adolescence and young adulthood, even when important environmental factors are taken into account.” A sentence I thought was most compelling in their statement was, “It is an inconvenient fact of life that alcohol is a teratogen.” Now, this fact is well supported in the literature.7
There are animal studies regarding the use of “low-dose” or “moderate” alcohol in pregnancy that demonstrate adverse behavioral outcomes with exposure to even small doses of alcohol.8,9 It is an American tragedy that, according to the Centers for Disease Control and Prevention (CDC), rates of FAS in this country range from 0.2 to 2.0 cases per 1,000 live births. Indeed, the rates of fetal alcohol spectrum disorders (FASD) might be at least three times this rate.10 As is the case with other disorders, there are health disparities regarding the prevalence of this condition as well.11
FAS: A long history of preventable disease
1973: Identified. FAS was first described in a 1973 Lancet report, “Pattern of malformation in offspring of chronic alcoholic mothers.”12
1996: Call for prevention. In 1995, the US Surgeon General issued a statement regarding alcohol use in pregnancy, noting, “We do not know what, if any, amount of alcohol is safe.”13 In 1996, the Institute of Medicine released a paper calling FAS and FASD “completely preventable birth defects and neurodevelopmental abnormalities.”14
2000: The troubling effects gathered. The American Academy of Pediatrics (AAP) published a monograph on FAS in 2000, defining it as a constellation of physical, behavioral, and cognitive abnormalities.15
These features classically define FAS:
- dysmorphic facial features
- prenatal and postnatal growth abnormalities
- mental retardation.
Approximately 80% of children with this condition have:
- microcephaly
- behavioral abnormalities.
As many as 50% of affected children also exhibit:
- poor coordination
- hypotonia
- attention-deficit hyperactivity disorder
- decreased adipose tissue
- identifiable facial anomalies (such as maxillary hypoplasia, cleft palate, and micrognathia).
Also common:
- cardiac defects
- hemangiomas
- eye or ear abnormalities.
The AAP further noted that data current to the time (and still true today) did not support the concept of a safe level of alcohol consumption by pregnant women below which no damage to a fetus will occur.15
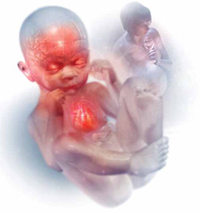
Alcohol intake during pregnancy puts the fetus at risk for cognitive and neuropsychological impairment and physical abnormalities, including dysmorphic facial features (such as micrognathia), restricted prenatal growth, cardiac defects, and eye and ear abnormalities. There is no threshold dose of alcohol that is safe during pregnancy, according to the American College of Obstetricians and Gynecologists.
Despite the knowledge we’ve gained, FAS persists
According to a 2006–2010 CDC analysis involving more than 345,000 women of reproductive age from all 50 states, 7.6% of pregnant women reported alcohol use and 1.4% (or 1 in 71) reported binge drinking (defined, respectively, as at least one alcoholic drink and four or more alcoholic drinks on one occasion in the past 30 days).16 The highest prevalence of obstetric alcohol consumption occurs in women who are:
- aged 35 to 44 years
- white
- college graduates
- employed.
The problem may be bigger than reported. The incidences of alcohol and binge drinking found in the CDC report include women’s self-report—but women drink alcohol without knowing they’re pregnant. Only 40% of women realize they’re pregnant at 4 weeks of gestation, a critical time for organogenesis, and approximately half of all births are unplanned.9
When my brother and sister-in law adopted my beautiful niece and nephew, they were very aware of the risk for conditions like FAS. In an evaluation of 71 children adopted from Eastern Europe at 5 years of age, FAS was diagnosed in 30% of children and “partial FAS” in another 9%.17 Birth defects attributed to alcohol were present in 11% of the children.
Are women’s health providers up to date on FAS education?
In recognition of alcohol’s potentially life-altering consequences for the developing fetus, the American College of Obstetricians and Gynecologists (ACOG) produced an FASD prevention tool kit in 2006 and published a 2011 committee opinion on at-risk drinking and alcohol dependence and their implications for obstetrics and gynecology.18,19 Both guidelines direct clinicians to advise patients to abstain from alcohol during pregnancy.
Results from a 2010 survey of 800 ACOG fellows revealed that only 78% of obstetricians advised abstinence from alcohol during pregnancy. Fifty-eight percent of respondents did not use a validated screening tool for alcohol use in their pregnant patients, and only 72% felt prepared to screen for risky or hazardous drinking.19 (Most were unaware of the ACOG tool kit, which had been published several years earlier.)
In a survey of pediatricians, obstetricians, and family physicians, clinicians said that about 67% of their patients asked about alcohol use in pregnancy, with about 2% of those patients specifically mentioning FAS. About 41% of these same physicians erroneously placed the threshold for FAS at one to three drinks per day,20 when in fact there is no threshold of drinking that has been proven to be safe.
A survey of 1,000 actively practicing ACOG fellows revealed that, while 97% of obstetricians routinely asked their patients about alcohol use, only 20% of providers reported to their patients that abstinence was safest, and 4% of providers didn’t believe that consumption of eight or more drinks weekly posed fetal risk.21
How can we educate our patients about the dangers of alcohol in pregnancy?
Fetal death. A recent Danish study of 79,216 pregnant women revealed that 45% had consumed some alcohol during pregnancy. Two percent reported at least four drinks per week, and 25% admitted to binge drinking during pregnancy. Term infants born to women in the latter two groups had increased neonatal mortality, with hazard ratios of 3.56 (95% confidence interval [CI], 1.15–8.43) and 2.69 (95% CI, 1.27–5.69), respectively.22
Decreased cognitive status. A study by Willford and colleagues evaluated the relationship between prenatal alcohol exposure and cognitive status of 1,360 10-year-old children.23 The authors utilized the Stanford-Binet Intelligence Test, including the composite scores and verbal, abstract/visual, quantitative, and short-term memory scores. After controlling for other variables, among African American offspring they found that, for each additional drink, the average composite score decreased by 1.9 points. This difference was more striking for second-trimester use, and was significant even for one drink daily versus abstention from alcohol.
Impaired neuropsychological development. Another study evaluating light to moderate amounts of prenatal alcohol exposure in 10- and 11-year-old children found significantly worse scores regarding a number of neuropsychological developmental assessments.24
No threshold dose of causation. Results of a 2012 prospective study in California, with data collected on 992 subjects from 1978 until 2005, revealed that many physical FAS features, including microcephaly, smooth philtrum, and thin vermillion border; reduced birth length; and reduced birth weight, were associated with alcohol exposure at specific gestational ages, and were dose-related.25 This paper didn’t reveal any evidence of a threshold dose of causation.
Neurobehavioral outcomes of FAS are not always considered
Another recent study that the media recently highlighted as finding “no association between low or moderate prenatal alcohol exposure and birth defects” was by O’Leary and colleagues.26 Like other similarly limited studies, this one involved only children younger than 6 years and didn’t assess any of the important neurobehavioral outcomes of FAS.
FAS encompasses much more than visible birth defects. As the aforementioned ACOG tool kit stated, “For every child born with FAS, many more children are born with neurobehavioral deficits caused by alcohol exposure but without the physical characteristics of FAS.”
The costs of FAS are felt with dollars, too
The financial cost to our nation is extraordinary. In 1991, Abel and Sokol estimated the incremental annual cost of treating FAS at nearly $75 million, with about three-quarters of that cost associated with FAS cases involving mental retardation.27
A 2002 assessment estimated the lifetime cost for each individual with FAS (adjusting for the change in the cost of medical care services, lost productivity, and inflation) at $2 million. This figure consists of $1.6 million for medical treatment, special education, and residential care for persons with mental retardation, and $0.4 million for productivity losses.28
Where human studies fall short, animal studies can help elucidate causation
Unquestionably, there are flaws in the existing literature on the causation of FAS. Many studies rely on self-reporting by pregnant women, and underreporting in these cases is a real concern. There often are other confounders potentially negatively affecting fetal development, making it difficult to differentiate causation. The animal studies that don’t share these limitations do suggest a causal relationship between antenatal alcohol exposure and poor obstetric outcomes, however.29 These studies suggest mechanisms such as altered gene expression, oxidative stress, and apoptosis (programmed cell death).30
Warren, Hewitt, and Thomas describe how intrauterine alcohol exposure interferes with the function of L1CAM, the L1 cell-adhesion molecule.31 They noted that just one drink could interfere with the ability of L1CAM to mediate cell adhesion and axonal growth. Prenatal alcohol exposure is also thought to contribute to interference in neurotransmitter and N-methyl-D-aspartate receptor coupling, which may have potential therapeutic implications.32
Considerations in FAS identification and treatment
There is a potential to identify alcohol exposure in the womb. The majority of ingested alcohol is eventually converted to carbon dioxide and water in both maternal and fetal circulations, which has hampered the identification of biomarkers for clinical use in FAS. Fatty acid ethyl esters (FAEEs), nonoxidative metabolites of ethanol, may prove to be such markers.33 FAEEs have been measured in a variety of tissues, including blood and meconium. FAEEs can be measured in both neonatal and maternal hair samples.
A study evaluating the utility of such testing in 324 at-risk pregnancies revealed 90% sensitivity and 90% specificity for identifying “excessive drinking” using a cutoff of 0.5 ng/mg.34
Research shows potential therapeutic approaches during pregnancy. While the use of biomarkers has the potential to assist with the identification of at-risk newborns, it merely identifies past alcohol use; it doesn’t necessarily permit identification and prevention of the known negative pediatric sequelae. Preliminary animal studies reveal the potential benefit of neuroprotective peptides to prevent brain damage in alcohol-exposed mice.35 Further research is ongoing.
Treatment: The earlier the better
Early diagnosis and a positive environment improve outcomes. It is well established that early intervention improves outcomes. One comprehensive review of 415 patients with FAS noted troubling outcomes in general for adolescents and adults.36 Over their life spans, the prevalence of such outcomes was:
- 61% for disrupted school experiences
- 60% for trouble with the law
- 50% for confinement (in detention, jail, prison, or a psychiatric or alcohol/drug inpatient setting)
- 49% for inappropriate sexual behaviors on repeated occasions
- 35% for alcohol/drug problems.
The odds of escaping these adverse life outcomes are increased up to fourfold when the individual receives a FAS or FASD diagnosis at an earlier age and is reared in a stable environment.36
Barrier to treatment: A mother’s guilt. One of the challenges I’ve learned from my sister-in-law is the stigma mothers face when they bring their child in for services once the diagnosis of FAS is suspected. While adoptive mothers obviously can’t be held accountable for the intrauterine environment to which a fetus is exposed, the same can’t be said of biologic mothers. Therefore, there is a real risk that a mother who is unwilling or unable to face the potentially devastating news that her baby’s issues might be related to choices she made during pregnancy, might not bring her child in for necessary assessment and treatment. Therefore, prevention is a key proponent of treatment.
Prevent FAS: Provide contraception, screen for alcohol use, intervene
While ObGyns aren’t likely to diagnose many children with FAS, we are in an excellent position to try to prevent this tragedy through our counseling of reproductive-aged women. I suspect that most obstetricians spend a considerable amount of time discussing much less frequent obstetric sequelae, such as listeriosis, in the prenatal care setting. Validated alcohol screening tools take moments to administer, and once patients who might have alcohol problems are identified, either a serious discussion about contraception or an honest discussion of FAS may be appropriate. There have been a number of screening tools developed.
The CAGE screen is frequently taught in medical schools, but it isn’t as sensitive for women or minorities.19
The T-ACE (Tolerance, Annoyed, Cut Down, Eye-opener) tool involves four questions that take less than 1 minute to administer (FIGURE 1).39
TWEAK is another potential tool identified by Russell and colleagues (Tolerance, Worry, Eye opener, Amnesia, and Cut down in drinking).39 Other methods utilized include an AUDIT screen and a CRAFFT screen.40 Regardless of which tool is utilized, screening is not time-consuming and is better than merely inquiring about alcohol consumption in general.

FIGURE 1 T-ACE validated alcohol screening tool
Source: American College of Obstetricians and Gynecologists. At risk drinking and illicit drug use: Ethical issues in obstetric and gynecologic practice. Obstet Gynecol. 2008;112(6):1449–1460.
When alcohol use is found, intervene
Once patients with at-risk behavior are identified, obstetric staff should offer brief interventions to influence problem drinking. Miller and Sanchez summarized the key elements that were most successful in these programs with the acronym FRAMES: Feedback, Responsibility, Advice, Menu, Empathy, Self-efficacy (FIGURE 2).41 This approach has been formally evaluated in the CDC’s multisite pilot study entitled Project CHOICES.42
In this motivational intervention, sexually active, fertile women of reproductive age underwent up to four motivational counseling sessions and one visit to a provider. At 6 months, 69% of women reduced their risk for an alcohol-exposed pregnancy—although the women who drank the least amount had the greatest benefit, primarily by choosing effective contraception, but also by reducing alcohol intake.

FIGURE 2 FRAMES model to deliver brief interventions
Source: American College of Obstetricians and Gynecologists. Drinking and reproductive health: A fetal alcohol spectrum disorders prevention tool kit. Washington, DC: ACOG; 2006.
A single, brief intervention is effective in already-pregnant women. Chang and colleagues conducted a randomized trial of a single-session brief intervention given to pregnant women with positive T-ACE screens and their partners (FIGURE 3).43 Either the study nurse or physician participated in the intervention, and each single session took 25 minutes on average. The pregnant women with the highest level of alcohol use reduced their drinking the most, and this effect was even larger when their partners participated. Other studies of brief interventions showed similar benefits.44,45
Another study evaluating a brief intervention involving training of health-care providers to improve screening rates revealed improved detection and therapy among at-risk patients.46

FIGURE 3 Single session, 25-minute intervention for patients and their partners
Source: Chang G, McNamara T, Orav J, et al. Brief intervention for prenatal alcohol use: a randomized trial. Obstet Gynecol. 2005;105(5 Pt 1):991–998.
FAS prevention begins with routine counseling and contraception
Although FAS is often thought of in relation to obstetric populations, appointments for preconception counseling or routine health maintenance among women of reproductive age are an essential tool in FAS prevention. As previously mentioned, since approximately half of all pregnancies in this country are unplanned, long-acting reversible contraception is widely available to facilitate improved family planning.
Other contraceptive options also should be discussed. ACOG has teamed up with the CDC to develop a phone app for providers to use at the patient’s bedside to assist with identification and treatment of women at risk for alcohol use during pregnancy.47
The stakes are high, it’s time to step up
As obstetricians, we are powerless to prevent many conditions—such as vasa previa, acute fatty liver of pregnancy, and amniotic band syndrome. FAS is 100% preventable.
There aren’t that many proven teratogens in our profession, and there are none that involve behavior that is more socially acceptable than alcohol consumption. It is time for our profession to encourage women to appreciate how small a percentage of one’s life is spent pregnant, how many more years there are to enjoy an occasional cocktail, and how very high the stakes are during this important period of their lives. Oh, how I wish someone had been able to communicate all of this to sweet Charlie’s biologic mother. I am so grateful he’s getting the exceptional care he’s getting and very optimistic regarding his future. I only hope others in his situation are given the same opportunities.
Prenatal counseling
Louise Wilkins-Haug, MD, PhD (January 2008)
Prevention of fetal alcohol syndrome requires routine screening of all women of reproductive age
We want to hear from you! Tell us what you think.
1. Baram M. Moms-to-be get mixed messages on drinking. ABC News. http://abcnews.go.com/Health/story?id=2654849&page=1#.UM9l-RyeARY. Published November 15 2006. Accessed December 14, 2012.
2. Royal College of Obstetricians and Gynaecologists. Alcohol consumption and the outcomes of pregnancy (RCOG Statement No. 5). London UK: Royal College of Obstetricians and Gynaecologists. January 3, 2006.
3. Pearson C. Alcohol during pregnancy: How dangerous is it really? The Huffington Post. http://www.huffingtonpost.com/2011/04/06/alcohol-during-pregnancy_n_845103.html. Published April 6 2011. Updated September 16, 2011. Accessed December 14, 2012.
4. Kelly YJ, Sacker A, Gray R, et al. Light drinking during pregnancy: still no increased risk for socioemotional difficulties or cognitive deficits at 5 years of age? J Epidemiol Community Health. 2012;66(1):41-48.Epub Oct 5, 2010.
5. Kelly Y, Sacker A, Gray R, Kelly J, Wolke D, Quigley MA. Light drinking in pregnancy a risk for behavioural problems and cognitive deficits at 3 years of age? Int J Epidemiol. 2009;38(1):129-140.Epub Oct 30, 2008.
6. Zhou F. Fetal Alcohol Spectrum Disorders Study Group (FASDSG). Research Society on Alcoholism. http://rsoa.org/fas.html. Updated September 9 2010. Accessed December 14, 2012.
7. Kelly S, Day N, Streissguth AP. Effects of prenatal alcohol exposure on social behavior in humans and other species. Neurotoxicol Teratol. 2000;22(2):143-149.
8. Vaglenova J, Petkov V. Fetal alcohol effects in rats exposed pre-and postnatally to a low dose of ethanol. Alcohol Clin Exp Res. 1998;22(3):697-703.
9. Schneider M, Moore C, Kraemer G. Moderate alcohol during pregnancy: learning and behavior in adolescent rhesus monkeys. Alcohol Clin Exp Res. 2001;25(9):1383-1392.
10. Centers for Disease Control and Prevention. Fetal alcohol spectrum disorders. Data and statistics in the United States. http://www.cdc.gov/ncbddd/fasd/data.html. Updated August 16 2012. Accessed December 14, 2012.
11. Egeland G, Perham-Hestere KA, Gessner BD, Ingle D, Berner JE, Middaugh J. Fetal alcohol syndrome in Alaska 1977 through 1992: an administrative prevalence derived from multiple data sources. Am J Pub Health. 1998;88(5):781-786.
12. Jones K, Smith D, Ulleland C, Streissguth A. Pattern of malformation in offspring of chronic alcoholic mothers. Lancet. 1973;1(7815):1267-1271.
13. Institute of Medicine. Fetal alcohol syndrome: diagnosis epidemiology, prevention, and treatment (1996). http://www.come-over.to/FAS/IOMsummary.htm. Accessed December 14, 2012.
14. Committee of Substance Abuse and Committee on Children with Disabilities. American Academy of Pediatrics. Fetal alcohol syndrome and alcohol-related neurodevelopmental disorders. Pediatrics. 2000;106(2):358-361.
15. US Department of Health & Human Services. US Surgeon General releases advisory on alcohol use in pregnancy. http://www.surgeongeneral.gov/news/2005/02/sg02222005.html. Published February 21 2005. Accessed December 13, 2012.
16. Centers for Disease Control and Prevention. Alcohol use and binge drinking among women of childbearing age–United States 2006–2010. MMWR. 2012;61(28):534-538.http://www.cdc.gov/mmwr/preview/mmwrhtml/mm6128a4.htm?s_cid=mm6128a4_w. Accessed December 17, 2012.
17. Landgren M, Svensson L, Stromland K, Gronlund M. Prenatal alcohol exposure and neurodvelopmental disorders in children adopted from Eastern Europe. Pediatrics. 2010;125(5):e1178-1185.doi:10.1542/peds.2009-0712.
18. American College of Obstetricians and Gynecologists. Drinking and reproductive health: A fetal alcohol spectrum disorders prevention tool kit. http://www.acog.org/~/media/Departments/Tobacco%20Alcohol%20and%20Substance%20Abuse/FASDToolKit.pdf?dmc=1&ts=20121217T1504384811. Published 2006. Accessed December 14 2012.
19. Anderson B, Dang E, Floyd R, Sokol R, Mahoney J, Schulkin J. Knowledge opinions, and practice patterns of obstetrician-gynecologist regarding their patients’ use of alcohol. J Addiction Med. 2010;4(2):114-121.
20. Abel EL, Kruger M. What do physicians know and say about fetal alcohol syndrome: a survey of obstetricians pediatricians, and family medicine physicians. Alcohol Clin Exp Res. 1998;22(9):1951-1954.
21. Diekman S, Floyd R, Decoufle P, Schulkin J, Ebrahim S, Sokol R. A survey of obstetrician-gynecologists on their patients’ alcohol use during pregnancy. Obstet Gynecol. 2000;95(5):756-763.
22. Strandberg-Larsen K, Gronboek M, Andersen A, Andersen P, Olsen J. Alcohol drinking pattern during pregnancy and risk of infant mortality. Epidemiology. 2009;20(6):884-891.
23. Willford J, Leech S, Day N. Moderate prenatal alcohol exposure and cognitive status of children at age 10. Alcohol Clin Exp Res. 2006;30(6):1051-1059.
24. Richardson G, Ryan C, Willford J, Day N, Goldschmidt. Prenatal alcohol and marijuana exposure: Effects on neuropsychological outcomes at 10 years. Neurotoxicol Teratol. 2002;24(3):309-320.
25. Feldman H, Jones K, Lindsay S, et al. Prenatal alcohol exposure patterns and alcohol-related birth defects and growth deficiencies: a prospective study. Alcohol Clin Exp Res. 2012;36(4):670-676.
26. O’Leary C, Nassar N, Kurinczuk J, et al. Prenatal alcohol exposure and risk of birth defects. Pediatrics. 2010;126(4):e843-850.doi:10.1542/peds.2010-0256.
27. Abel E, Sokol R. A revised conservative estimate of the incidence of FAS and its economic its impact. Alcohol Clin Exp Res. 1991;15(3):514-524.
28. Lupton C. The financial impact of fetal alcohol syndrome. Fetal Alcohol Spectrum Disorders Center for Excellence. www.fasdcenter.samhsa.gov/publications/cost.cfm. Accessed December 14 2012.
29. Bailey B, Sokol R. Prenatal alcohol exposure and miscarriage stillbirth, preterm delivery, and sudden infant death syndrome. Alcohol Res Health. 2011;34(1):86-91.
30. Yelin R, Kot H, Yelin D, Fainsod A. Early molecular effects of ethanol during vertebrate embryogenesis. Differentiation. 2007;75(5):393-403.
31. Warren K, Hewitt B, Thomas J. Fetal alcohol spectrum disorders: research challenges and opportunities. Alcohol Res Health. 2011;34(1):4-15.
32. Ramanathan R, Wilkemeyer M, Mittal B, Perides G, Chamess ME. Alcohol inhibits cell-cell adhesion mediated by human L1. J Cell Biol. 1996;133(2):381-390.
33. Burd L, Hofer R. Biomarkers for detection of prenatal alcohol exposure: a critical review of fatty acid ethyl estsers in meconium. Birth Defects Res A Clin Mol Teratol. 2008;82(7):487-493.
34. Kulaga V, Pragst F, Fulga N, Koren G. Hair análisis of fatty acid esters in the detection of excessive drinking in the context of fetal alcohol spectrum disorders. Ther Drug Monit. 2009;31(2):261-266.
35. Sari Y, Gozes I. Brain deficits associated with fetal alcohol exposure may be protected in part, by peptides derived from activity-dependent neurotrophic factor and activity-dependent neuroprotective protein. Brain Res Rev. 2006;52(1):107-118.
36. Streissguth A, Bookstein F, Barr H, Sampson P, O’malley K, Young J. Risk factors for adverse life outcomes in fetal alcohol syndrome and fetal alcohol effects. J Dev Behav Pediatr. 2004;25(4):228-238.
37. 19. Committee on Health Care for Underserved Women. American College of Obstetricians and Gynecologists. Committee Opinion No. 496: At-risk drinking and alcohol dependence: Obstetric and gynecologic implications. Obstet Gynecol. 2011;118(2 Pt 1):383-388.
38. Sokol R, Martier S, Ager J. The T-ACE questions: practical prenatal detection of risk-drinking. Am J Obstet Gynecol. 1989;160(4):863-868.
39. Chan A, Pristach E, Weite J, Russell M. Use of the TWEAK test in screening for alcoholism/ heavy drinking in three populations. Alcohol Clin Exp Res. 1993;17(6):1188-1192.
40. Floyd R, O’Connor M, Bertrand J, Sokol R. Reducing adverse outcomes from prenatal alcohol exposure: a clinical plan of action. Alcohol Clin Exp Res. 2006;30(8):1271-1275.
41. Miller W, Sanchez V. Motivating young adults for treatment and lifestyle change. In: Howard GS Nathan PE, eds. Alcohol use and misuse by young adults. Notre Dame, IN: University of Notre Dame Press; 1994:55–81.
42. Center for Disease Control and Prevention. Motivational intervention to reduce alcohol-exposed pregnancies—Florida Texas, and Virginia, 1997–2001. MMWR. 2003;52(19):441-444.
43. Chang G, McNamara T, Orav J, et al. Brief intervention for prenatal alcohol use: a randomized trial. Obstet Gynecol. 2005;105(5 Pt 1):991-998.
44. Manwell L, Fleming M, Mundt M, Stauffacher E, Barry K. Treatment of problem alcohol use in women of childbearing age: results of a brief intervention trial. Alcohol Clin Exp Res. 2000;24(10):1517-1524.
45. O’Connor M, Whaley S. Brief intervention for alcohol use by pregnant women. Am J Pub Health. 2007;97(2):252-258.
46. Mwansa-Kambafwile J, Rendall-Mkosi K, Jacobs R, Nel E, London L. Evaluation of a service provider short course for prevention of fetal alcohol syndrome. J Stud Alcohol Drugs. 2011;72(4):530-535.
47. American College of Obstetricians and Gynecologists. At-risk alcohol use screening and intervention. http://198.87.1.43/womenalcohol/index.html. Published 2011. Accessed December 16 2012.
Medical students learn early in their education that alcohol is a teratogen. Despite this widespread knowledge, many obstetricians counsel patients about the safety of low doses of alcohol in pregnancy.1 Indeed, the Royal College of Obstetricians and Gynaecologists’ position on this is, “while the safest approach may be to avoid any alcohol during pregnancy, it remains the case that there is no evidence of harm from low levels of alcohol consumption, defined as no more than one or two units of alcohol once or twice a week.”2
Like many providers, I was aware of this controversy, but it became truly personal when a beloved family member was diagnosed with fetal alcohol syndrome (FAS). In this paper, I will review some of the controversy regarding alcohol in pregnancy, highlight findings from the literature, provide tools for prevention, and identify new developments regarding this devastating, preventable condition.
Charlie
To know my nephew Charlie is to fall in love with my nephew Charlie. One of the happiest moments of my life was when I learned my brother and sister-in-law had adopted twins from Kazakhstan. When my little niece and nephew started their new life in the United States, certain medical issues seemed to merit additional attention. Although both were very small for their age and required significant nutritional support, Charlie seemed to be a bit more rambunctious and required additional supervision.
The children were fortunate enough to have incredibly loving, dedicated parents, who have access to exceptional medical care as residents of Philadelphia, Pennsylvania. After extensive testing, it became clear what was causing Charlie’s developmental delay; his pediatric team made the diagnosis of FAS. My brother and sister-in-law became incredibly well-read about this challenging disorder, and threw themselves into national advocacy work to help prevent this unnecessary tragedy.
Recent data point to teratogenicity, but media confuse the issue
Some recent media coverage3 of celebrities who apparently drank while pregnant was in response to an article in the Journal of Epidemiology and Community Health.4 The authors of this study concluded that, “at age 5 years, cohort members born to mothers who drank up to one to two drinks per week or per occasion during pregnancy were not at increased risk of clinically relevant behavioral difficulties or cognitive deficits, compared with children of mothers in the not-in-pregnancy group.”
This is certainly not the first occasion the popular press has covered a published study that seems to indicate no ill effects of alcohol use in pregnancy. A 2008 report by Kelly and colleagues,5 and its subsequent media coverage, prompted the Fetal Alcohol Spectrum Disorders Study Group to state that the panel of experts was “alarmed” by recent newspaper reports suggesting that light drinking during pregnancy may be beneficial for an unborn child.6 They noted misleading and irresponsible media reports of the findings, which suggested that 3-year-old children whose mothers drank “lightly” during pregnancy were not at risk for certain behavioral problems.
What the study authors proceeded to note, however (that the media did not mention), was that the light drinkers in their study had socioeconomic advantages, compared with nondrinkers.5 (Advantaged economic status is established to be beneficial for childhood development.) They also noted that the study involved preschool-aged children, stating “Generally the adverse effects of light drinking during pregnancy are subtle and may go undetected in young children. However, other group studies of more moderate or ‘social’ drinking levels during pregnancy have shown an adverse impact on multiple aspects of development through adolescence and young adulthood, even when important environmental factors are taken into account.” A sentence I thought was most compelling in their statement was, “It is an inconvenient fact of life that alcohol is a teratogen.” Now, this fact is well supported in the literature.7
There are animal studies regarding the use of “low-dose” or “moderate” alcohol in pregnancy that demonstrate adverse behavioral outcomes with exposure to even small doses of alcohol.8,9 It is an American tragedy that, according to the Centers for Disease Control and Prevention (CDC), rates of FAS in this country range from 0.2 to 2.0 cases per 1,000 live births. Indeed, the rates of fetal alcohol spectrum disorders (FASD) might be at least three times this rate.10 As is the case with other disorders, there are health disparities regarding the prevalence of this condition as well.11
FAS: A long history of preventable disease
1973: Identified. FAS was first described in a 1973 Lancet report, “Pattern of malformation in offspring of chronic alcoholic mothers.”12
1996: Call for prevention. In 1995, the US Surgeon General issued a statement regarding alcohol use in pregnancy, noting, “We do not know what, if any, amount of alcohol is safe.”13 In 1996, the Institute of Medicine released a paper calling FAS and FASD “completely preventable birth defects and neurodevelopmental abnormalities.”14
2000: The troubling effects gathered. The American Academy of Pediatrics (AAP) published a monograph on FAS in 2000, defining it as a constellation of physical, behavioral, and cognitive abnormalities.15
These features classically define FAS:
- dysmorphic facial features
- prenatal and postnatal growth abnormalities
- mental retardation.
Approximately 80% of children with this condition have:
- microcephaly
- behavioral abnormalities.
As many as 50% of affected children also exhibit:
- poor coordination
- hypotonia
- attention-deficit hyperactivity disorder
- decreased adipose tissue
- identifiable facial anomalies (such as maxillary hypoplasia, cleft palate, and micrognathia).
Also common:
- cardiac defects
- hemangiomas
- eye or ear abnormalities.
The AAP further noted that data current to the time (and still true today) did not support the concept of a safe level of alcohol consumption by pregnant women below which no damage to a fetus will occur.15

Alcohol intake during pregnancy puts the fetus at risk for cognitive and neuropsychological impairment and physical abnormalities, including dysmorphic facial features (such as micrognathia), restricted prenatal growth, cardiac defects, and eye and ear abnormalities. There is no threshold dose of alcohol that is safe during pregnancy, according to the American College of Obstetricians and Gynecologists.
Despite the knowledge we’ve gained, FAS persists
According to a 2006–2010 CDC analysis involving more than 345,000 women of reproductive age from all 50 states, 7.6% of pregnant women reported alcohol use and 1.4% (or 1 in 71) reported binge drinking (defined, respectively, as at least one alcoholic drink and four or more alcoholic drinks on one occasion in the past 30 days).16 The highest prevalence of obstetric alcohol consumption occurs in women who are:
- aged 35 to 44 years
- white
- college graduates
- employed.
The problem may be bigger than reported. The incidences of alcohol and binge drinking found in the CDC report include women’s self-report—but women drink alcohol without knowing they’re pregnant. Only 40% of women realize they’re pregnant at 4 weeks of gestation, a critical time for organogenesis, and approximately half of all births are unplanned.9
When my brother and sister-in law adopted my beautiful niece and nephew, they were very aware of the risk for conditions like FAS. In an evaluation of 71 children adopted from Eastern Europe at 5 years of age, FAS was diagnosed in 30% of children and “partial FAS” in another 9%.17 Birth defects attributed to alcohol were present in 11% of the children.
Are women’s health providers up to date on FAS education?
In recognition of alcohol’s potentially life-altering consequences for the developing fetus, the American College of Obstetricians and Gynecologists (ACOG) produced an FASD prevention tool kit in 2006 and published a 2011 committee opinion on at-risk drinking and alcohol dependence and their implications for obstetrics and gynecology.18,19 Both guidelines direct clinicians to advise patients to abstain from alcohol during pregnancy.
Results from a 2010 survey of 800 ACOG fellows revealed that only 78% of obstetricians advised abstinence from alcohol during pregnancy. Fifty-eight percent of respondents did not use a validated screening tool for alcohol use in their pregnant patients, and only 72% felt prepared to screen for risky or hazardous drinking.19 (Most were unaware of the ACOG tool kit, which had been published several years earlier.)
In a survey of pediatricians, obstetricians, and family physicians, clinicians said that about 67% of their patients asked about alcohol use in pregnancy, with about 2% of those patients specifically mentioning FAS. About 41% of these same physicians erroneously placed the threshold for FAS at one to three drinks per day,20 when in fact there is no threshold of drinking that has been proven to be safe.
A survey of 1,000 actively practicing ACOG fellows revealed that, while 97% of obstetricians routinely asked their patients about alcohol use, only 20% of providers reported to their patients that abstinence was safest, and 4% of providers didn’t believe that consumption of eight or more drinks weekly posed fetal risk.21
How can we educate our patients about the dangers of alcohol in pregnancy?
Fetal death. A recent Danish study of 79,216 pregnant women revealed that 45% had consumed some alcohol during pregnancy. Two percent reported at least four drinks per week, and 25% admitted to binge drinking during pregnancy. Term infants born to women in the latter two groups had increased neonatal mortality, with hazard ratios of 3.56 (95% confidence interval [CI], 1.15–8.43) and 2.69 (95% CI, 1.27–5.69), respectively.22
Decreased cognitive status. A study by Willford and colleagues evaluated the relationship between prenatal alcohol exposure and cognitive status of 1,360 10-year-old children.23 The authors utilized the Stanford-Binet Intelligence Test, including the composite scores and verbal, abstract/visual, quantitative, and short-term memory scores. After controlling for other variables, among African American offspring they found that, for each additional drink, the average composite score decreased by 1.9 points. This difference was more striking for second-trimester use, and was significant even for one drink daily versus abstention from alcohol.
Impaired neuropsychological development. Another study evaluating light to moderate amounts of prenatal alcohol exposure in 10- and 11-year-old children found significantly worse scores regarding a number of neuropsychological developmental assessments.24
No threshold dose of causation. Results of a 2012 prospective study in California, with data collected on 992 subjects from 1978 until 2005, revealed that many physical FAS features, including microcephaly, smooth philtrum, and thin vermillion border; reduced birth length; and reduced birth weight, were associated with alcohol exposure at specific gestational ages, and were dose-related.25 This paper didn’t reveal any evidence of a threshold dose of causation.
Neurobehavioral outcomes of FAS are not always considered
Another recent study that the media recently highlighted as finding “no association between low or moderate prenatal alcohol exposure and birth defects” was by O’Leary and colleagues.26 Like other similarly limited studies, this one involved only children younger than 6 years and didn’t assess any of the important neurobehavioral outcomes of FAS.
FAS encompasses much more than visible birth defects. As the aforementioned ACOG tool kit stated, “For every child born with FAS, many more children are born with neurobehavioral deficits caused by alcohol exposure but without the physical characteristics of FAS.”
The costs of FAS are felt with dollars, too
The financial cost to our nation is extraordinary. In 1991, Abel and Sokol estimated the incremental annual cost of treating FAS at nearly $75 million, with about three-quarters of that cost associated with FAS cases involving mental retardation.27
A 2002 assessment estimated the lifetime cost for each individual with FAS (adjusting for the change in the cost of medical care services, lost productivity, and inflation) at $2 million. This figure consists of $1.6 million for medical treatment, special education, and residential care for persons with mental retardation, and $0.4 million for productivity losses.28
Where human studies fall short, animal studies can help elucidate causation
Unquestionably, there are flaws in the existing literature on the causation of FAS. Many studies rely on self-reporting by pregnant women, and underreporting in these cases is a real concern. There often are other confounders potentially negatively affecting fetal development, making it difficult to differentiate causation. The animal studies that don’t share these limitations do suggest a causal relationship between antenatal alcohol exposure and poor obstetric outcomes, however.29 These studies suggest mechanisms such as altered gene expression, oxidative stress, and apoptosis (programmed cell death).30
Warren, Hewitt, and Thomas describe how intrauterine alcohol exposure interferes with the function of L1CAM, the L1 cell-adhesion molecule.31 They noted that just one drink could interfere with the ability of L1CAM to mediate cell adhesion and axonal growth. Prenatal alcohol exposure is also thought to contribute to interference in neurotransmitter and N-methyl-D-aspartate receptor coupling, which may have potential therapeutic implications.32
Considerations in FAS identification and treatment
There is a potential to identify alcohol exposure in the womb. The majority of ingested alcohol is eventually converted to carbon dioxide and water in both maternal and fetal circulations, which has hampered the identification of biomarkers for clinical use in FAS. Fatty acid ethyl esters (FAEEs), nonoxidative metabolites of ethanol, may prove to be such markers.33 FAEEs have been measured in a variety of tissues, including blood and meconium. FAEEs can be measured in both neonatal and maternal hair samples.
A study evaluating the utility of such testing in 324 at-risk pregnancies revealed 90% sensitivity and 90% specificity for identifying “excessive drinking” using a cutoff of 0.5 ng/mg.34
Research shows potential therapeutic approaches during pregnancy. While the use of biomarkers has the potential to assist with the identification of at-risk newborns, it merely identifies past alcohol use; it doesn’t necessarily permit identification and prevention of the known negative pediatric sequelae. Preliminary animal studies reveal the potential benefit of neuroprotective peptides to prevent brain damage in alcohol-exposed mice.35 Further research is ongoing.
Treatment: The earlier the better
Early diagnosis and a positive environment improve outcomes. It is well established that early intervention improves outcomes. One comprehensive review of 415 patients with FAS noted troubling outcomes in general for adolescents and adults.36 Over their life spans, the prevalence of such outcomes was:
- 61% for disrupted school experiences
- 60% for trouble with the law
- 50% for confinement (in detention, jail, prison, or a psychiatric or alcohol/drug inpatient setting)
- 49% for inappropriate sexual behaviors on repeated occasions
- 35% for alcohol/drug problems.
The odds of escaping these adverse life outcomes are increased up to fourfold when the individual receives a FAS or FASD diagnosis at an earlier age and is reared in a stable environment.36
Barrier to treatment: A mother’s guilt. One of the challenges I’ve learned from my sister-in-law is the stigma mothers face when they bring their child in for services once the diagnosis of FAS is suspected. While adoptive mothers obviously can’t be held accountable for the intrauterine environment to which a fetus is exposed, the same can’t be said of biologic mothers. Therefore, there is a real risk that a mother who is unwilling or unable to face the potentially devastating news that her baby’s issues might be related to choices she made during pregnancy, might not bring her child in for necessary assessment and treatment. Therefore, prevention is a key proponent of treatment.
Prevent FAS: Provide contraception, screen for alcohol use, intervene
While ObGyns aren’t likely to diagnose many children with FAS, we are in an excellent position to try to prevent this tragedy through our counseling of reproductive-aged women. I suspect that most obstetricians spend a considerable amount of time discussing much less frequent obstetric sequelae, such as listeriosis, in the prenatal care setting. Validated alcohol screening tools take moments to administer, and once patients who might have alcohol problems are identified, either a serious discussion about contraception or an honest discussion of FAS may be appropriate. There have been a number of screening tools developed.
The CAGE screen is frequently taught in medical schools, but it isn’t as sensitive for women or minorities.19
The T-ACE (Tolerance, Annoyed, Cut Down, Eye-opener) tool involves four questions that take less than 1 minute to administer (FIGURE 1).39
TWEAK is another potential tool identified by Russell and colleagues (Tolerance, Worry, Eye opener, Amnesia, and Cut down in drinking).39 Other methods utilized include an AUDIT screen and a CRAFFT screen.40 Regardless of which tool is utilized, screening is not time-consuming and is better than merely inquiring about alcohol consumption in general.

FIGURE 1 T-ACE validated alcohol screening tool
Source: American College of Obstetricians and Gynecologists. At risk drinking and illicit drug use: Ethical issues in obstetric and gynecologic practice. Obstet Gynecol. 2008;112(6):1449–1460.
When alcohol use is found, intervene
Once patients with at-risk behavior are identified, obstetric staff should offer brief interventions to influence problem drinking. Miller and Sanchez summarized the key elements that were most successful in these programs with the acronym FRAMES: Feedback, Responsibility, Advice, Menu, Empathy, Self-efficacy (FIGURE 2).41 This approach has been formally evaluated in the CDC’s multisite pilot study entitled Project CHOICES.42
In this motivational intervention, sexually active, fertile women of reproductive age underwent up to four motivational counseling sessions and one visit to a provider. At 6 months, 69% of women reduced their risk for an alcohol-exposed pregnancy—although the women who drank the least amount had the greatest benefit, primarily by choosing effective contraception, but also by reducing alcohol intake.

FIGURE 2 FRAMES model to deliver brief interventions
Source: American College of Obstetricians and Gynecologists. Drinking and reproductive health: A fetal alcohol spectrum disorders prevention tool kit. Washington, DC: ACOG; 2006.
A single, brief intervention is effective in already-pregnant women. Chang and colleagues conducted a randomized trial of a single-session brief intervention given to pregnant women with positive T-ACE screens and their partners (FIGURE 3).43 Either the study nurse or physician participated in the intervention, and each single session took 25 minutes on average. The pregnant women with the highest level of alcohol use reduced their drinking the most, and this effect was even larger when their partners participated. Other studies of brief interventions showed similar benefits.44,45
Another study evaluating a brief intervention involving training of health-care providers to improve screening rates revealed improved detection and therapy among at-risk patients.46

FIGURE 3 Single session, 25-minute intervention for patients and their partners
Source: Chang G, McNamara T, Orav J, et al. Brief intervention for prenatal alcohol use: a randomized trial. Obstet Gynecol. 2005;105(5 Pt 1):991–998.
FAS prevention begins with routine counseling and contraception
Although FAS is often thought of in relation to obstetric populations, appointments for preconception counseling or routine health maintenance among women of reproductive age are an essential tool in FAS prevention. As previously mentioned, since approximately half of all pregnancies in this country are unplanned, long-acting reversible contraception is widely available to facilitate improved family planning.
Other contraceptive options also should be discussed. ACOG has teamed up with the CDC to develop a phone app for providers to use at the patient’s bedside to assist with identification and treatment of women at risk for alcohol use during pregnancy.47
The stakes are high, it’s time to step up
As obstetricians, we are powerless to prevent many conditions—such as vasa previa, acute fatty liver of pregnancy, and amniotic band syndrome. FAS is 100% preventable.
There aren’t that many proven teratogens in our profession, and there are none that involve behavior that is more socially acceptable than alcohol consumption. It is time for our profession to encourage women to appreciate how small a percentage of one’s life is spent pregnant, how many more years there are to enjoy an occasional cocktail, and how very high the stakes are during this important period of their lives. Oh, how I wish someone had been able to communicate all of this to sweet Charlie’s biologic mother. I am so grateful he’s getting the exceptional care he’s getting and very optimistic regarding his future. I only hope others in his situation are given the same opportunities.
Prenatal counseling
Louise Wilkins-Haug, MD, PhD (January 2008)
Prevention of fetal alcohol syndrome requires routine screening of all women of reproductive age
We want to hear from you! Tell us what you think.
Medical students learn early in their education that alcohol is a teratogen. Despite this widespread knowledge, many obstetricians counsel patients about the safety of low doses of alcohol in pregnancy.1 Indeed, the Royal College of Obstetricians and Gynaecologists’ position on this is, “while the safest approach may be to avoid any alcohol during pregnancy, it remains the case that there is no evidence of harm from low levels of alcohol consumption, defined as no more than one or two units of alcohol once or twice a week.”2
Like many providers, I was aware of this controversy, but it became truly personal when a beloved family member was diagnosed with fetal alcohol syndrome (FAS). In this paper, I will review some of the controversy regarding alcohol in pregnancy, highlight findings from the literature, provide tools for prevention, and identify new developments regarding this devastating, preventable condition.
Charlie
To know my nephew Charlie is to fall in love with my nephew Charlie. One of the happiest moments of my life was when I learned my brother and sister-in-law had adopted twins from Kazakhstan. When my little niece and nephew started their new life in the United States, certain medical issues seemed to merit additional attention. Although both were very small for their age and required significant nutritional support, Charlie seemed to be a bit more rambunctious and required additional supervision.
The children were fortunate enough to have incredibly loving, dedicated parents, who have access to exceptional medical care as residents of Philadelphia, Pennsylvania. After extensive testing, it became clear what was causing Charlie’s developmental delay; his pediatric team made the diagnosis of FAS. My brother and sister-in-law became incredibly well-read about this challenging disorder, and threw themselves into national advocacy work to help prevent this unnecessary tragedy.
Recent data point to teratogenicity, but media confuse the issue
Some recent media coverage3 of celebrities who apparently drank while pregnant was in response to an article in the Journal of Epidemiology and Community Health.4 The authors of this study concluded that, “at age 5 years, cohort members born to mothers who drank up to one to two drinks per week or per occasion during pregnancy were not at increased risk of clinically relevant behavioral difficulties or cognitive deficits, compared with children of mothers in the not-in-pregnancy group.”
This is certainly not the first occasion the popular press has covered a published study that seems to indicate no ill effects of alcohol use in pregnancy. A 2008 report by Kelly and colleagues,5 and its subsequent media coverage, prompted the Fetal Alcohol Spectrum Disorders Study Group to state that the panel of experts was “alarmed” by recent newspaper reports suggesting that light drinking during pregnancy may be beneficial for an unborn child.6 They noted misleading and irresponsible media reports of the findings, which suggested that 3-year-old children whose mothers drank “lightly” during pregnancy were not at risk for certain behavioral problems.
What the study authors proceeded to note, however (that the media did not mention), was that the light drinkers in their study had socioeconomic advantages, compared with nondrinkers.5 (Advantaged economic status is established to be beneficial for childhood development.) They also noted that the study involved preschool-aged children, stating “Generally the adverse effects of light drinking during pregnancy are subtle and may go undetected in young children. However, other group studies of more moderate or ‘social’ drinking levels during pregnancy have shown an adverse impact on multiple aspects of development through adolescence and young adulthood, even when important environmental factors are taken into account.” A sentence I thought was most compelling in their statement was, “It is an inconvenient fact of life that alcohol is a teratogen.” Now, this fact is well supported in the literature.7
There are animal studies regarding the use of “low-dose” or “moderate” alcohol in pregnancy that demonstrate adverse behavioral outcomes with exposure to even small doses of alcohol.8,9 It is an American tragedy that, according to the Centers for Disease Control and Prevention (CDC), rates of FAS in this country range from 0.2 to 2.0 cases per 1,000 live births. Indeed, the rates of fetal alcohol spectrum disorders (FASD) might be at least three times this rate.10 As is the case with other disorders, there are health disparities regarding the prevalence of this condition as well.11
FAS: A long history of preventable disease
1973: Identified. FAS was first described in a 1973 Lancet report, “Pattern of malformation in offspring of chronic alcoholic mothers.”12
1996: Call for prevention. In 1995, the US Surgeon General issued a statement regarding alcohol use in pregnancy, noting, “We do not know what, if any, amount of alcohol is safe.”13 In 1996, the Institute of Medicine released a paper calling FAS and FASD “completely preventable birth defects and neurodevelopmental abnormalities.”14
2000: The troubling effects gathered. The American Academy of Pediatrics (AAP) published a monograph on FAS in 2000, defining it as a constellation of physical, behavioral, and cognitive abnormalities.15
These features classically define FAS:
- dysmorphic facial features
- prenatal and postnatal growth abnormalities
- mental retardation.
Approximately 80% of children with this condition have:
- microcephaly
- behavioral abnormalities.
As many as 50% of affected children also exhibit:
- poor coordination
- hypotonia
- attention-deficit hyperactivity disorder
- decreased adipose tissue
- identifiable facial anomalies (such as maxillary hypoplasia, cleft palate, and micrognathia).
Also common:
- cardiac defects
- hemangiomas
- eye or ear abnormalities.
The AAP further noted that data current to the time (and still true today) did not support the concept of a safe level of alcohol consumption by pregnant women below which no damage to a fetus will occur.15

Alcohol intake during pregnancy puts the fetus at risk for cognitive and neuropsychological impairment and physical abnormalities, including dysmorphic facial features (such as micrognathia), restricted prenatal growth, cardiac defects, and eye and ear abnormalities. There is no threshold dose of alcohol that is safe during pregnancy, according to the American College of Obstetricians and Gynecologists.
Despite the knowledge we’ve gained, FAS persists
According to a 2006–2010 CDC analysis involving more than 345,000 women of reproductive age from all 50 states, 7.6% of pregnant women reported alcohol use and 1.4% (or 1 in 71) reported binge drinking (defined, respectively, as at least one alcoholic drink and four or more alcoholic drinks on one occasion in the past 30 days).16 The highest prevalence of obstetric alcohol consumption occurs in women who are:
- aged 35 to 44 years
- white
- college graduates
- employed.
The problem may be bigger than reported. The incidences of alcohol and binge drinking found in the CDC report include women’s self-report—but women drink alcohol without knowing they’re pregnant. Only 40% of women realize they’re pregnant at 4 weeks of gestation, a critical time for organogenesis, and approximately half of all births are unplanned.9
When my brother and sister-in law adopted my beautiful niece and nephew, they were very aware of the risk for conditions like FAS. In an evaluation of 71 children adopted from Eastern Europe at 5 years of age, FAS was diagnosed in 30% of children and “partial FAS” in another 9%.17 Birth defects attributed to alcohol were present in 11% of the children.
Are women’s health providers up to date on FAS education?
In recognition of alcohol’s potentially life-altering consequences for the developing fetus, the American College of Obstetricians and Gynecologists (ACOG) produced an FASD prevention tool kit in 2006 and published a 2011 committee opinion on at-risk drinking and alcohol dependence and their implications for obstetrics and gynecology.18,19 Both guidelines direct clinicians to advise patients to abstain from alcohol during pregnancy.
Results from a 2010 survey of 800 ACOG fellows revealed that only 78% of obstetricians advised abstinence from alcohol during pregnancy. Fifty-eight percent of respondents did not use a validated screening tool for alcohol use in their pregnant patients, and only 72% felt prepared to screen for risky or hazardous drinking.19 (Most were unaware of the ACOG tool kit, which had been published several years earlier.)
In a survey of pediatricians, obstetricians, and family physicians, clinicians said that about 67% of their patients asked about alcohol use in pregnancy, with about 2% of those patients specifically mentioning FAS. About 41% of these same physicians erroneously placed the threshold for FAS at one to three drinks per day,20 when in fact there is no threshold of drinking that has been proven to be safe.
A survey of 1,000 actively practicing ACOG fellows revealed that, while 97% of obstetricians routinely asked their patients about alcohol use, only 20% of providers reported to their patients that abstinence was safest, and 4% of providers didn’t believe that consumption of eight or more drinks weekly posed fetal risk.21
How can we educate our patients about the dangers of alcohol in pregnancy?
Fetal death. A recent Danish study of 79,216 pregnant women revealed that 45% had consumed some alcohol during pregnancy. Two percent reported at least four drinks per week, and 25% admitted to binge drinking during pregnancy. Term infants born to women in the latter two groups had increased neonatal mortality, with hazard ratios of 3.56 (95% confidence interval [CI], 1.15–8.43) and 2.69 (95% CI, 1.27–5.69), respectively.22
Decreased cognitive status. A study by Willford and colleagues evaluated the relationship between prenatal alcohol exposure and cognitive status of 1,360 10-year-old children.23 The authors utilized the Stanford-Binet Intelligence Test, including the composite scores and verbal, abstract/visual, quantitative, and short-term memory scores. After controlling for other variables, among African American offspring they found that, for each additional drink, the average composite score decreased by 1.9 points. This difference was more striking for second-trimester use, and was significant even for one drink daily versus abstention from alcohol.
Impaired neuropsychological development. Another study evaluating light to moderate amounts of prenatal alcohol exposure in 10- and 11-year-old children found significantly worse scores regarding a number of neuropsychological developmental assessments.24
No threshold dose of causation. Results of a 2012 prospective study in California, with data collected on 992 subjects from 1978 until 2005, revealed that many physical FAS features, including microcephaly, smooth philtrum, and thin vermillion border; reduced birth length; and reduced birth weight, were associated with alcohol exposure at specific gestational ages, and were dose-related.25 This paper didn’t reveal any evidence of a threshold dose of causation.
Neurobehavioral outcomes of FAS are not always considered
Another recent study that the media recently highlighted as finding “no association between low or moderate prenatal alcohol exposure and birth defects” was by O’Leary and colleagues.26 Like other similarly limited studies, this one involved only children younger than 6 years and didn’t assess any of the important neurobehavioral outcomes of FAS.
FAS encompasses much more than visible birth defects. As the aforementioned ACOG tool kit stated, “For every child born with FAS, many more children are born with neurobehavioral deficits caused by alcohol exposure but without the physical characteristics of FAS.”
The costs of FAS are felt with dollars, too
The financial cost to our nation is extraordinary. In 1991, Abel and Sokol estimated the incremental annual cost of treating FAS at nearly $75 million, with about three-quarters of that cost associated with FAS cases involving mental retardation.27
A 2002 assessment estimated the lifetime cost for each individual with FAS (adjusting for the change in the cost of medical care services, lost productivity, and inflation) at $2 million. This figure consists of $1.6 million for medical treatment, special education, and residential care for persons with mental retardation, and $0.4 million for productivity losses.28
Where human studies fall short, animal studies can help elucidate causation
Unquestionably, there are flaws in the existing literature on the causation of FAS. Many studies rely on self-reporting by pregnant women, and underreporting in these cases is a real concern. There often are other confounders potentially negatively affecting fetal development, making it difficult to differentiate causation. The animal studies that don’t share these limitations do suggest a causal relationship between antenatal alcohol exposure and poor obstetric outcomes, however.29 These studies suggest mechanisms such as altered gene expression, oxidative stress, and apoptosis (programmed cell death).30
Warren, Hewitt, and Thomas describe how intrauterine alcohol exposure interferes with the function of L1CAM, the L1 cell-adhesion molecule.31 They noted that just one drink could interfere with the ability of L1CAM to mediate cell adhesion and axonal growth. Prenatal alcohol exposure is also thought to contribute to interference in neurotransmitter and N-methyl-D-aspartate receptor coupling, which may have potential therapeutic implications.32
Considerations in FAS identification and treatment
There is a potential to identify alcohol exposure in the womb. The majority of ingested alcohol is eventually converted to carbon dioxide and water in both maternal and fetal circulations, which has hampered the identification of biomarkers for clinical use in FAS. Fatty acid ethyl esters (FAEEs), nonoxidative metabolites of ethanol, may prove to be such markers.33 FAEEs have been measured in a variety of tissues, including blood and meconium. FAEEs can be measured in both neonatal and maternal hair samples.
A study evaluating the utility of such testing in 324 at-risk pregnancies revealed 90% sensitivity and 90% specificity for identifying “excessive drinking” using a cutoff of 0.5 ng/mg.34
Research shows potential therapeutic approaches during pregnancy. While the use of biomarkers has the potential to assist with the identification of at-risk newborns, it merely identifies past alcohol use; it doesn’t necessarily permit identification and prevention of the known negative pediatric sequelae. Preliminary animal studies reveal the potential benefit of neuroprotective peptides to prevent brain damage in alcohol-exposed mice.35 Further research is ongoing.
Treatment: The earlier the better
Early diagnosis and a positive environment improve outcomes. It is well established that early intervention improves outcomes. One comprehensive review of 415 patients with FAS noted troubling outcomes in general for adolescents and adults.36 Over their life spans, the prevalence of such outcomes was:
- 61% for disrupted school experiences
- 60% for trouble with the law
- 50% for confinement (in detention, jail, prison, or a psychiatric or alcohol/drug inpatient setting)
- 49% for inappropriate sexual behaviors on repeated occasions
- 35% for alcohol/drug problems.
The odds of escaping these adverse life outcomes are increased up to fourfold when the individual receives a FAS or FASD diagnosis at an earlier age and is reared in a stable environment.36
Barrier to treatment: A mother’s guilt. One of the challenges I’ve learned from my sister-in-law is the stigma mothers face when they bring their child in for services once the diagnosis of FAS is suspected. While adoptive mothers obviously can’t be held accountable for the intrauterine environment to which a fetus is exposed, the same can’t be said of biologic mothers. Therefore, there is a real risk that a mother who is unwilling or unable to face the potentially devastating news that her baby’s issues might be related to choices she made during pregnancy, might not bring her child in for necessary assessment and treatment. Therefore, prevention is a key proponent of treatment.
Prevent FAS: Provide contraception, screen for alcohol use, intervene
While ObGyns aren’t likely to diagnose many children with FAS, we are in an excellent position to try to prevent this tragedy through our counseling of reproductive-aged women. I suspect that most obstetricians spend a considerable amount of time discussing much less frequent obstetric sequelae, such as listeriosis, in the prenatal care setting. Validated alcohol screening tools take moments to administer, and once patients who might have alcohol problems are identified, either a serious discussion about contraception or an honest discussion of FAS may be appropriate. There have been a number of screening tools developed.
The CAGE screen is frequently taught in medical schools, but it isn’t as sensitive for women or minorities.19
The T-ACE (Tolerance, Annoyed, Cut Down, Eye-opener) tool involves four questions that take less than 1 minute to administer (FIGURE 1).39
TWEAK is another potential tool identified by Russell and colleagues (Tolerance, Worry, Eye opener, Amnesia, and Cut down in drinking).39 Other methods utilized include an AUDIT screen and a CRAFFT screen.40 Regardless of which tool is utilized, screening is not time-consuming and is better than merely inquiring about alcohol consumption in general.

FIGURE 1 T-ACE validated alcohol screening tool
Source: American College of Obstetricians and Gynecologists. At risk drinking and illicit drug use: Ethical issues in obstetric and gynecologic practice. Obstet Gynecol. 2008;112(6):1449–1460.
When alcohol use is found, intervene
Once patients with at-risk behavior are identified, obstetric staff should offer brief interventions to influence problem drinking. Miller and Sanchez summarized the key elements that were most successful in these programs with the acronym FRAMES: Feedback, Responsibility, Advice, Menu, Empathy, Self-efficacy (FIGURE 2).41 This approach has been formally evaluated in the CDC’s multisite pilot study entitled Project CHOICES.42
In this motivational intervention, sexually active, fertile women of reproductive age underwent up to four motivational counseling sessions and one visit to a provider. At 6 months, 69% of women reduced their risk for an alcohol-exposed pregnancy—although the women who drank the least amount had the greatest benefit, primarily by choosing effective contraception, but also by reducing alcohol intake.

FIGURE 2 FRAMES model to deliver brief interventions
Source: American College of Obstetricians and Gynecologists. Drinking and reproductive health: A fetal alcohol spectrum disorders prevention tool kit. Washington, DC: ACOG; 2006.
A single, brief intervention is effective in already-pregnant women. Chang and colleagues conducted a randomized trial of a single-session brief intervention given to pregnant women with positive T-ACE screens and their partners (FIGURE 3).43 Either the study nurse or physician participated in the intervention, and each single session took 25 minutes on average. The pregnant women with the highest level of alcohol use reduced their drinking the most, and this effect was even larger when their partners participated. Other studies of brief interventions showed similar benefits.44,45
Another study evaluating a brief intervention involving training of health-care providers to improve screening rates revealed improved detection and therapy among at-risk patients.46

FIGURE 3 Single session, 25-minute intervention for patients and their partners
Source: Chang G, McNamara T, Orav J, et al. Brief intervention for prenatal alcohol use: a randomized trial. Obstet Gynecol. 2005;105(5 Pt 1):991–998.
FAS prevention begins with routine counseling and contraception
Although FAS is often thought of in relation to obstetric populations, appointments for preconception counseling or routine health maintenance among women of reproductive age are an essential tool in FAS prevention. As previously mentioned, since approximately half of all pregnancies in this country are unplanned, long-acting reversible contraception is widely available to facilitate improved family planning.
Other contraceptive options also should be discussed. ACOG has teamed up with the CDC to develop a phone app for providers to use at the patient’s bedside to assist with identification and treatment of women at risk for alcohol use during pregnancy.47
The stakes are high, it’s time to step up
As obstetricians, we are powerless to prevent many conditions—such as vasa previa, acute fatty liver of pregnancy, and amniotic band syndrome. FAS is 100% preventable.
There aren’t that many proven teratogens in our profession, and there are none that involve behavior that is more socially acceptable than alcohol consumption. It is time for our profession to encourage women to appreciate how small a percentage of one’s life is spent pregnant, how many more years there are to enjoy an occasional cocktail, and how very high the stakes are during this important period of their lives. Oh, how I wish someone had been able to communicate all of this to sweet Charlie’s biologic mother. I am so grateful he’s getting the exceptional care he’s getting and very optimistic regarding his future. I only hope others in his situation are given the same opportunities.
Prenatal counseling
Louise Wilkins-Haug, MD, PhD (January 2008)
Prevention of fetal alcohol syndrome requires routine screening of all women of reproductive age
We want to hear from you! Tell us what you think.
1. Baram M. Moms-to-be get mixed messages on drinking. ABC News. http://abcnews.go.com/Health/story?id=2654849&page=1#.UM9l-RyeARY. Published November 15 2006. Accessed December 14, 2012.
2. Royal College of Obstetricians and Gynaecologists. Alcohol consumption and the outcomes of pregnancy (RCOG Statement No. 5). London UK: Royal College of Obstetricians and Gynaecologists. January 3, 2006.
3. Pearson C. Alcohol during pregnancy: How dangerous is it really? The Huffington Post. http://www.huffingtonpost.com/2011/04/06/alcohol-during-pregnancy_n_845103.html. Published April 6 2011. Updated September 16, 2011. Accessed December 14, 2012.
4. Kelly YJ, Sacker A, Gray R, et al. Light drinking during pregnancy: still no increased risk for socioemotional difficulties or cognitive deficits at 5 years of age? J Epidemiol Community Health. 2012;66(1):41-48.Epub Oct 5, 2010.
5. Kelly Y, Sacker A, Gray R, Kelly J, Wolke D, Quigley MA. Light drinking in pregnancy a risk for behavioural problems and cognitive deficits at 3 years of age? Int J Epidemiol. 2009;38(1):129-140.Epub Oct 30, 2008.
6. Zhou F. Fetal Alcohol Spectrum Disorders Study Group (FASDSG). Research Society on Alcoholism. http://rsoa.org/fas.html. Updated September 9 2010. Accessed December 14, 2012.
7. Kelly S, Day N, Streissguth AP. Effects of prenatal alcohol exposure on social behavior in humans and other species. Neurotoxicol Teratol. 2000;22(2):143-149.
8. Vaglenova J, Petkov V. Fetal alcohol effects in rats exposed pre-and postnatally to a low dose of ethanol. Alcohol Clin Exp Res. 1998;22(3):697-703.
9. Schneider M, Moore C, Kraemer G. Moderate alcohol during pregnancy: learning and behavior in adolescent rhesus monkeys. Alcohol Clin Exp Res. 2001;25(9):1383-1392.
10. Centers for Disease Control and Prevention. Fetal alcohol spectrum disorders. Data and statistics in the United States. http://www.cdc.gov/ncbddd/fasd/data.html. Updated August 16 2012. Accessed December 14, 2012.
11. Egeland G, Perham-Hestere KA, Gessner BD, Ingle D, Berner JE, Middaugh J. Fetal alcohol syndrome in Alaska 1977 through 1992: an administrative prevalence derived from multiple data sources. Am J Pub Health. 1998;88(5):781-786.
12. Jones K, Smith D, Ulleland C, Streissguth A. Pattern of malformation in offspring of chronic alcoholic mothers. Lancet. 1973;1(7815):1267-1271.
13. Institute of Medicine. Fetal alcohol syndrome: diagnosis epidemiology, prevention, and treatment (1996). http://www.come-over.to/FAS/IOMsummary.htm. Accessed December 14, 2012.
14. Committee of Substance Abuse and Committee on Children with Disabilities. American Academy of Pediatrics. Fetal alcohol syndrome and alcohol-related neurodevelopmental disorders. Pediatrics. 2000;106(2):358-361.
15. US Department of Health & Human Services. US Surgeon General releases advisory on alcohol use in pregnancy. http://www.surgeongeneral.gov/news/2005/02/sg02222005.html. Published February 21 2005. Accessed December 13, 2012.
16. Centers for Disease Control and Prevention. Alcohol use and binge drinking among women of childbearing age–United States 2006–2010. MMWR. 2012;61(28):534-538.http://www.cdc.gov/mmwr/preview/mmwrhtml/mm6128a4.htm?s_cid=mm6128a4_w. Accessed December 17, 2012.
17. Landgren M, Svensson L, Stromland K, Gronlund M. Prenatal alcohol exposure and neurodvelopmental disorders in children adopted from Eastern Europe. Pediatrics. 2010;125(5):e1178-1185.doi:10.1542/peds.2009-0712.
18. American College of Obstetricians and Gynecologists. Drinking and reproductive health: A fetal alcohol spectrum disorders prevention tool kit. http://www.acog.org/~/media/Departments/Tobacco%20Alcohol%20and%20Substance%20Abuse/FASDToolKit.pdf?dmc=1&ts=20121217T1504384811. Published 2006. Accessed December 14 2012.
19. Anderson B, Dang E, Floyd R, Sokol R, Mahoney J, Schulkin J. Knowledge opinions, and practice patterns of obstetrician-gynecologist regarding their patients’ use of alcohol. J Addiction Med. 2010;4(2):114-121.
20. Abel EL, Kruger M. What do physicians know and say about fetal alcohol syndrome: a survey of obstetricians pediatricians, and family medicine physicians. Alcohol Clin Exp Res. 1998;22(9):1951-1954.
21. Diekman S, Floyd R, Decoufle P, Schulkin J, Ebrahim S, Sokol R. A survey of obstetrician-gynecologists on their patients’ alcohol use during pregnancy. Obstet Gynecol. 2000;95(5):756-763.
22. Strandberg-Larsen K, Gronboek M, Andersen A, Andersen P, Olsen J. Alcohol drinking pattern during pregnancy and risk of infant mortality. Epidemiology. 2009;20(6):884-891.
23. Willford J, Leech S, Day N. Moderate prenatal alcohol exposure and cognitive status of children at age 10. Alcohol Clin Exp Res. 2006;30(6):1051-1059.
24. Richardson G, Ryan C, Willford J, Day N, Goldschmidt. Prenatal alcohol and marijuana exposure: Effects on neuropsychological outcomes at 10 years. Neurotoxicol Teratol. 2002;24(3):309-320.
25. Feldman H, Jones K, Lindsay S, et al. Prenatal alcohol exposure patterns and alcohol-related birth defects and growth deficiencies: a prospective study. Alcohol Clin Exp Res. 2012;36(4):670-676.
26. O’Leary C, Nassar N, Kurinczuk J, et al. Prenatal alcohol exposure and risk of birth defects. Pediatrics. 2010;126(4):e843-850.doi:10.1542/peds.2010-0256.
27. Abel E, Sokol R. A revised conservative estimate of the incidence of FAS and its economic its impact. Alcohol Clin Exp Res. 1991;15(3):514-524.
28. Lupton C. The financial impact of fetal alcohol syndrome. Fetal Alcohol Spectrum Disorders Center for Excellence. www.fasdcenter.samhsa.gov/publications/cost.cfm. Accessed December 14 2012.
29. Bailey B, Sokol R. Prenatal alcohol exposure and miscarriage stillbirth, preterm delivery, and sudden infant death syndrome. Alcohol Res Health. 2011;34(1):86-91.
30. Yelin R, Kot H, Yelin D, Fainsod A. Early molecular effects of ethanol during vertebrate embryogenesis. Differentiation. 2007;75(5):393-403.
31. Warren K, Hewitt B, Thomas J. Fetal alcohol spectrum disorders: research challenges and opportunities. Alcohol Res Health. 2011;34(1):4-15.
32. Ramanathan R, Wilkemeyer M, Mittal B, Perides G, Chamess ME. Alcohol inhibits cell-cell adhesion mediated by human L1. J Cell Biol. 1996;133(2):381-390.
33. Burd L, Hofer R. Biomarkers for detection of prenatal alcohol exposure: a critical review of fatty acid ethyl estsers in meconium. Birth Defects Res A Clin Mol Teratol. 2008;82(7):487-493.
34. Kulaga V, Pragst F, Fulga N, Koren G. Hair análisis of fatty acid esters in the detection of excessive drinking in the context of fetal alcohol spectrum disorders. Ther Drug Monit. 2009;31(2):261-266.
35. Sari Y, Gozes I. Brain deficits associated with fetal alcohol exposure may be protected in part, by peptides derived from activity-dependent neurotrophic factor and activity-dependent neuroprotective protein. Brain Res Rev. 2006;52(1):107-118.
36. Streissguth A, Bookstein F, Barr H, Sampson P, O’malley K, Young J. Risk factors for adverse life outcomes in fetal alcohol syndrome and fetal alcohol effects. J Dev Behav Pediatr. 2004;25(4):228-238.
37. 19. Committee on Health Care for Underserved Women. American College of Obstetricians and Gynecologists. Committee Opinion No. 496: At-risk drinking and alcohol dependence: Obstetric and gynecologic implications. Obstet Gynecol. 2011;118(2 Pt 1):383-388.
38. Sokol R, Martier S, Ager J. The T-ACE questions: practical prenatal detection of risk-drinking. Am J Obstet Gynecol. 1989;160(4):863-868.
39. Chan A, Pristach E, Weite J, Russell M. Use of the TWEAK test in screening for alcoholism/ heavy drinking in three populations. Alcohol Clin Exp Res. 1993;17(6):1188-1192.
40. Floyd R, O’Connor M, Bertrand J, Sokol R. Reducing adverse outcomes from prenatal alcohol exposure: a clinical plan of action. Alcohol Clin Exp Res. 2006;30(8):1271-1275.
41. Miller W, Sanchez V. Motivating young adults for treatment and lifestyle change. In: Howard GS Nathan PE, eds. Alcohol use and misuse by young adults. Notre Dame, IN: University of Notre Dame Press; 1994:55–81.
42. Center for Disease Control and Prevention. Motivational intervention to reduce alcohol-exposed pregnancies—Florida Texas, and Virginia, 1997–2001. MMWR. 2003;52(19):441-444.
43. Chang G, McNamara T, Orav J, et al. Brief intervention for prenatal alcohol use: a randomized trial. Obstet Gynecol. 2005;105(5 Pt 1):991-998.
44. Manwell L, Fleming M, Mundt M, Stauffacher E, Barry K. Treatment of problem alcohol use in women of childbearing age: results of a brief intervention trial. Alcohol Clin Exp Res. 2000;24(10):1517-1524.
45. O’Connor M, Whaley S. Brief intervention for alcohol use by pregnant women. Am J Pub Health. 2007;97(2):252-258.
46. Mwansa-Kambafwile J, Rendall-Mkosi K, Jacobs R, Nel E, London L. Evaluation of a service provider short course for prevention of fetal alcohol syndrome. J Stud Alcohol Drugs. 2011;72(4):530-535.
47. American College of Obstetricians and Gynecologists. At-risk alcohol use screening and intervention. http://198.87.1.43/womenalcohol/index.html. Published 2011. Accessed December 16 2012.
1. Baram M. Moms-to-be get mixed messages on drinking. ABC News. http://abcnews.go.com/Health/story?id=2654849&page=1#.UM9l-RyeARY. Published November 15 2006. Accessed December 14, 2012.
2. Royal College of Obstetricians and Gynaecologists. Alcohol consumption and the outcomes of pregnancy (RCOG Statement No. 5). London UK: Royal College of Obstetricians and Gynaecologists. January 3, 2006.
3. Pearson C. Alcohol during pregnancy: How dangerous is it really? The Huffington Post. http://www.huffingtonpost.com/2011/04/06/alcohol-during-pregnancy_n_845103.html. Published April 6 2011. Updated September 16, 2011. Accessed December 14, 2012.
4. Kelly YJ, Sacker A, Gray R, et al. Light drinking during pregnancy: still no increased risk for socioemotional difficulties or cognitive deficits at 5 years of age? J Epidemiol Community Health. 2012;66(1):41-48.Epub Oct 5, 2010.
5. Kelly Y, Sacker A, Gray R, Kelly J, Wolke D, Quigley MA. Light drinking in pregnancy a risk for behavioural problems and cognitive deficits at 3 years of age? Int J Epidemiol. 2009;38(1):129-140.Epub Oct 30, 2008.
6. Zhou F. Fetal Alcohol Spectrum Disorders Study Group (FASDSG). Research Society on Alcoholism. http://rsoa.org/fas.html. Updated September 9 2010. Accessed December 14, 2012.
7. Kelly S, Day N, Streissguth AP. Effects of prenatal alcohol exposure on social behavior in humans and other species. Neurotoxicol Teratol. 2000;22(2):143-149.
8. Vaglenova J, Petkov V. Fetal alcohol effects in rats exposed pre-and postnatally to a low dose of ethanol. Alcohol Clin Exp Res. 1998;22(3):697-703.
9. Schneider M, Moore C, Kraemer G. Moderate alcohol during pregnancy: learning and behavior in adolescent rhesus monkeys. Alcohol Clin Exp Res. 2001;25(9):1383-1392.
10. Centers for Disease Control and Prevention. Fetal alcohol spectrum disorders. Data and statistics in the United States. http://www.cdc.gov/ncbddd/fasd/data.html. Updated August 16 2012. Accessed December 14, 2012.
11. Egeland G, Perham-Hestere KA, Gessner BD, Ingle D, Berner JE, Middaugh J. Fetal alcohol syndrome in Alaska 1977 through 1992: an administrative prevalence derived from multiple data sources. Am J Pub Health. 1998;88(5):781-786.
12. Jones K, Smith D, Ulleland C, Streissguth A. Pattern of malformation in offspring of chronic alcoholic mothers. Lancet. 1973;1(7815):1267-1271.
13. Institute of Medicine. Fetal alcohol syndrome: diagnosis epidemiology, prevention, and treatment (1996). http://www.come-over.to/FAS/IOMsummary.htm. Accessed December 14, 2012.
14. Committee of Substance Abuse and Committee on Children with Disabilities. American Academy of Pediatrics. Fetal alcohol syndrome and alcohol-related neurodevelopmental disorders. Pediatrics. 2000;106(2):358-361.
15. US Department of Health & Human Services. US Surgeon General releases advisory on alcohol use in pregnancy. http://www.surgeongeneral.gov/news/2005/02/sg02222005.html. Published February 21 2005. Accessed December 13, 2012.
16. Centers for Disease Control and Prevention. Alcohol use and binge drinking among women of childbearing age–United States 2006–2010. MMWR. 2012;61(28):534-538.http://www.cdc.gov/mmwr/preview/mmwrhtml/mm6128a4.htm?s_cid=mm6128a4_w. Accessed December 17, 2012.
17. Landgren M, Svensson L, Stromland K, Gronlund M. Prenatal alcohol exposure and neurodvelopmental disorders in children adopted from Eastern Europe. Pediatrics. 2010;125(5):e1178-1185.doi:10.1542/peds.2009-0712.
18. American College of Obstetricians and Gynecologists. Drinking and reproductive health: A fetal alcohol spectrum disorders prevention tool kit. http://www.acog.org/~/media/Departments/Tobacco%20Alcohol%20and%20Substance%20Abuse/FASDToolKit.pdf?dmc=1&ts=20121217T1504384811. Published 2006. Accessed December 14 2012.
19. Anderson B, Dang E, Floyd R, Sokol R, Mahoney J, Schulkin J. Knowledge opinions, and practice patterns of obstetrician-gynecologist regarding their patients’ use of alcohol. J Addiction Med. 2010;4(2):114-121.
20. Abel EL, Kruger M. What do physicians know and say about fetal alcohol syndrome: a survey of obstetricians pediatricians, and family medicine physicians. Alcohol Clin Exp Res. 1998;22(9):1951-1954.
21. Diekman S, Floyd R, Decoufle P, Schulkin J, Ebrahim S, Sokol R. A survey of obstetrician-gynecologists on their patients’ alcohol use during pregnancy. Obstet Gynecol. 2000;95(5):756-763.
22. Strandberg-Larsen K, Gronboek M, Andersen A, Andersen P, Olsen J. Alcohol drinking pattern during pregnancy and risk of infant mortality. Epidemiology. 2009;20(6):884-891.
23. Willford J, Leech S, Day N. Moderate prenatal alcohol exposure and cognitive status of children at age 10. Alcohol Clin Exp Res. 2006;30(6):1051-1059.
24. Richardson G, Ryan C, Willford J, Day N, Goldschmidt. Prenatal alcohol and marijuana exposure: Effects on neuropsychological outcomes at 10 years. Neurotoxicol Teratol. 2002;24(3):309-320.
25. Feldman H, Jones K, Lindsay S, et al. Prenatal alcohol exposure patterns and alcohol-related birth defects and growth deficiencies: a prospective study. Alcohol Clin Exp Res. 2012;36(4):670-676.
26. O’Leary C, Nassar N, Kurinczuk J, et al. Prenatal alcohol exposure and risk of birth defects. Pediatrics. 2010;126(4):e843-850.doi:10.1542/peds.2010-0256.
27. Abel E, Sokol R. A revised conservative estimate of the incidence of FAS and its economic its impact. Alcohol Clin Exp Res. 1991;15(3):514-524.
28. Lupton C. The financial impact of fetal alcohol syndrome. Fetal Alcohol Spectrum Disorders Center for Excellence. www.fasdcenter.samhsa.gov/publications/cost.cfm. Accessed December 14 2012.
29. Bailey B, Sokol R. Prenatal alcohol exposure and miscarriage stillbirth, preterm delivery, and sudden infant death syndrome. Alcohol Res Health. 2011;34(1):86-91.
30. Yelin R, Kot H, Yelin D, Fainsod A. Early molecular effects of ethanol during vertebrate embryogenesis. Differentiation. 2007;75(5):393-403.
31. Warren K, Hewitt B, Thomas J. Fetal alcohol spectrum disorders: research challenges and opportunities. Alcohol Res Health. 2011;34(1):4-15.
32. Ramanathan R, Wilkemeyer M, Mittal B, Perides G, Chamess ME. Alcohol inhibits cell-cell adhesion mediated by human L1. J Cell Biol. 1996;133(2):381-390.
33. Burd L, Hofer R. Biomarkers for detection of prenatal alcohol exposure: a critical review of fatty acid ethyl estsers in meconium. Birth Defects Res A Clin Mol Teratol. 2008;82(7):487-493.
34. Kulaga V, Pragst F, Fulga N, Koren G. Hair análisis of fatty acid esters in the detection of excessive drinking in the context of fetal alcohol spectrum disorders. Ther Drug Monit. 2009;31(2):261-266.
35. Sari Y, Gozes I. Brain deficits associated with fetal alcohol exposure may be protected in part, by peptides derived from activity-dependent neurotrophic factor and activity-dependent neuroprotective protein. Brain Res Rev. 2006;52(1):107-118.
36. Streissguth A, Bookstein F, Barr H, Sampson P, O’malley K, Young J. Risk factors for adverse life outcomes in fetal alcohol syndrome and fetal alcohol effects. J Dev Behav Pediatr. 2004;25(4):228-238.
37. 19. Committee on Health Care for Underserved Women. American College of Obstetricians and Gynecologists. Committee Opinion No. 496: At-risk drinking and alcohol dependence: Obstetric and gynecologic implications. Obstet Gynecol. 2011;118(2 Pt 1):383-388.
38. Sokol R, Martier S, Ager J. The T-ACE questions: practical prenatal detection of risk-drinking. Am J Obstet Gynecol. 1989;160(4):863-868.
39. Chan A, Pristach E, Weite J, Russell M. Use of the TWEAK test in screening for alcoholism/ heavy drinking in three populations. Alcohol Clin Exp Res. 1993;17(6):1188-1192.
40. Floyd R, O’Connor M, Bertrand J, Sokol R. Reducing adverse outcomes from prenatal alcohol exposure: a clinical plan of action. Alcohol Clin Exp Res. 2006;30(8):1271-1275.
41. Miller W, Sanchez V. Motivating young adults for treatment and lifestyle change. In: Howard GS Nathan PE, eds. Alcohol use and misuse by young adults. Notre Dame, IN: University of Notre Dame Press; 1994:55–81.
42. Center for Disease Control and Prevention. Motivational intervention to reduce alcohol-exposed pregnancies—Florida Texas, and Virginia, 1997–2001. MMWR. 2003;52(19):441-444.
43. Chang G, McNamara T, Orav J, et al. Brief intervention for prenatal alcohol use: a randomized trial. Obstet Gynecol. 2005;105(5 Pt 1):991-998.
44. Manwell L, Fleming M, Mundt M, Stauffacher E, Barry K. Treatment of problem alcohol use in women of childbearing age: results of a brief intervention trial. Alcohol Clin Exp Res. 2000;24(10):1517-1524.
45. O’Connor M, Whaley S. Brief intervention for alcohol use by pregnant women. Am J Pub Health. 2007;97(2):252-258.
46. Mwansa-Kambafwile J, Rendall-Mkosi K, Jacobs R, Nel E, London L. Evaluation of a service provider short course for prevention of fetal alcohol syndrome. J Stud Alcohol Drugs. 2011;72(4):530-535.
47. American College of Obstetricians and Gynecologists. At-risk alcohol use screening and intervention. http://198.87.1.43/womenalcohol/index.html. Published 2011. Accessed December 16 2012.
Porphyria after Bariatric Surgery
Prolotherapy for Management of Myofascial Pain Syndrome
Utilization of Antipsychotics in Ambulatory Elderly With Dementia in an Outpatient Setting
Lateral Epicondylitis in Occupational Settings: Prevention and Treatment
Lateral epicondylitis, also commonly referred to as tennis elbow, is a cumulative trauma disorder (CTD) that affects the extensor tendons of the forearm. Its causative mechanism is repetitive tension and movement of the extensor tendons of the wrist. Characteristic associated movements include supination and pronation of the forearm; these occur frequently during the performance of tasks that require repeated gripping and twisting.1
Among the extensor tendons affected by lateral epicondylitis, the extensor carpi radialis brevis is most commonly identified as the injured tendon.2 While the term epicondylitis implies inflammation as the pathological phenomenon behind this disorder, histologic evidence supports a degenerative process in which a noninflammatory angiofibroblastic tendinosis develops, one that is characterized by degenerative processes, including neovascularization and a disordered collagen matrix.3 In this discussion, therefore, while the disorder will be referred to as lateral epicondylitis (the more common nomenclature), lateral epicondylosis may be a more accurate term.
Pain symptoms described by patients with lateral epicondylitis are thought to be attributable to increasing numbers of free nerve endings in the newly developed granulomatous tissue, in addition to associated synovitis.4 The presence of microvascular damage, a histologic finding in lateral epicondylitis, has also led investigators to suggest several contributing risk factors for tennis elbow, including diabetes and smoking.5
More obscure contributing factors, including psychosocial and socioeconomic concerns, have also been suggested.6
EPIDEMIOLOGY
Though not uncommon in the general population, lateral epicondylitis has a peak incidence in the occupational setting, where its prevalence ranges from 4% to 30%.7 Lateral epicondylitis and other upper-extremity CTD account for 56% of all occupational injuries,8 making them a paramount source of concern for employers and employees alike. In a study of workers’ compensation claims in the United States, van Tulder et al6 found that the mean cost per repetitive strain injury ranged from $5,000 to $8,000. The medical costs and lost work time associated with lateral and medial epicondylitis have been estimated to total more than $22 billion per year in the US alone.7
DIAGNOSIS
Making a diagnosis of lateral epicondylitis is usually straightforward, requiring very little in the way of ancillary testing, such as x-rays or MRI.9
Patient History
A thorough patient history will typically reveal complaints of occupation- or activity-related pain in the lateral elbow, possibly affecting the muscle mass of the forearm.9 Often, the patient will report that symptoms are aggravated by repetitive movements that require strenuous use of the hand, wrist, and/or forearm.10 Examples may include using a screwdriver, grasping a lever, or lifting a heavy load. Occupations associated with risk for lateral epicondylitis include those in the construction industry (eg, plumbers, carpenters) and in manufacturing.
Because a positive association has been established between lateral epicondylitis and ipsilateral rotator cuff tears, carpal tunnel syndrome, and De Quervain’s syndrome, it is important to inquire about any prior history of musculoskeletal injury and coexisting musculoskeletal symptoms. Previous use of oral corticosteroids and a history of smoking are also considered risk factors for lateral epicondylitis.5
Physical Examination
Physical exam findings include reproducible pain at the lateral elbow with resisted supination or wrist dorsiflexion when the forearm is fully extended.9 The examiner may also elicit pain by palpating just distal to the lateral epicondyle over the extensor tendon mass (see Figure 1). Range of motion in the wrist may be diminished due to pain, as when the patient is asked to extend the wrist against resistance. As the condition progresses, the patient may also experience pain and weakness of the forearm with resisted finger motion.
TREATMENT
A number of researchers have investigated the most efficacious therapies for lateral epicondylitis (see Table 111-21); as in the case of other musculoskeletal injuries, rest is a cornerstone of treatment. NSAIDs, too, are often indicated, but the clinician must thoroughly evaluate risk versus benefit before initiating NSAID use.11 Additionally, the patient should be educated regarding NSAIDs’ potential side effects and recommended safety precautions.
Two bracing interventions commonly used to treat lateral epicondylitis are the wrist extension splint and the counterforce forearm strap (see Figure 2). In one small prospective, randomized trial (n = 44) in which these devices were compared, no significant between-group differences were found in terms of Mayo Elbow Performance scores, but according to scores derived from American Shoulder and Elbow Society assessments, pain relief was significantly better in patients who wore the wrist extension splint.12 In another study of orthotic management of lateral epicondylosis, immediate improvement in pain-free grip strength was reported in subjects wearing either an elbow strap or an elbow sleeve, but wearers of a wrist splint experienced no immediate change, making it inadvisable as a first-choice orthotic modality.22
Other treatment modalities to be considered include corticosteroid injections, acupuncture, autologous blood and other injection therapies, botulinum injection, topical nitrates, laser therapy, and surgery.
Corticosteroid Injections Versus Physical Therapy
In a randomized controlled trial contrasting the use of corticosteroid injections, physical therapy, and a wait-and-see approach for patients with lateral epicondylitis, corticosteroid injections were found superior in the short term (defined as six weeks after treatment initiation). In the long term, however (ie, at 52 weeks), physical therapy was found more effective than either of the alternative approaches. Outcome measures included general improvement, reduction in severity of the main complaint, alleviation of pain, improved elbow function, and patient satisfaction.13
A less invasive alternative to steroid injections for patients with lateral epicondylitis, though not yet FDA approved, has yielded promising results: administration of transdermal dexamethasone by way of iontophoresis.14,15 This delivery method (in which administration of ionic, water-soluble agents is facilitated using a weak electric current23) was recently studied by Stefanou et al14 in the form of a transdermal patch, activated by a 24-hour battery.
In the short term (ie, upon completion of therapy), patients who received iontophoretic dexamethasone had significantly better grip strength and were better able to return to work than those treated with injections of dexamethasone or triamcinolone. By six months, outcome measures were comparable among the three groups.14 However, possible advantages to iontophoresis are that it is painless, noninvasive, and less likely to cause adverse effects.15,23
Acupuncture
While few sources support the use of complementary modalities to treat lateral epicondylitis,24 findings from one randomized controlled trial offered modest support for acupuncture use. Compared with a sham procedure (in which nonspecific points were targeted), “real” acupuncture—selection and stimulation of specific acupuncture points—provided reductions in pain intensity and improvements in function and strength. Evidence of these improvements became even more robust at two-week follow-up. At two months, however, only improvements in function remained significant.16
Four Injection Therapies
Injections of autologous whole blood or platelet-rich plasma, prolotherapy (injections with hyperosmolar dextrose and sodium morrhuate25), and polidocanol injections were examined in a systematic literature review of treatment strategies for lateral epicondylosis published in 2009 by Rabago et al.3
Although most of the study cohorts involved were small, significant improvements were reported for all modalities and outcome measures, particularly pain: reduction in pain scores by as much as 88% among patients injected with autologous whole blood17; a 55% improvement at eight months in patients treated with the sclerosing agent polidocanol26; improvement as great as 90%, 16 weeks after treatment with prolotherapy (compared with 22% in controls)25; and, in a nonrandomized trial, pain reduction of 93% in patients who had received platelet-rich plasma injections about 26 months earlier.27 Nevertheless, the researchers note, further study is required in larger trials examining specified biomarkers in addition to clinical, biomechanical, and radiologic means of measurement to assure the long-term safety and effectiveness of each of these modalities.3
Botulinum Toxin
Injecting botulinum toxin is another treatment strategy that may be considered when results of more traditional approaches are unsatisfactory. In two double-blind, placebo-controlled, randomized studies, patients injected once with botulinum toxin A experienced significant reductions in pain within four to six weeks, compared with those given placebo.18,28 A notable but expected complication of this treatment reported by Placzek et al18 was weakness of the third finger of the treated hand, two weeks after treatment. However, normal strength was regained in all affected patients by week 18 of follow-up.
Nitrates
Use of topical nitric oxide showed promising results in a 2003 study by Australian researchers.19 Eighty-six patients with chronic extensor tendinosis, all of whom underwent a standard tendon rehabilitation program (ie, rest and stretching and strengthening exercises) and used a forearm counterforce brace, were randomized to receive glyceryl trinitrate transdermal patches or sham patches. One-quarter of a patch was applied just distal to the lateral epicondyle of the humerus for 24 hours, then replaced by another in a rotating fashion around the target spot.
Treated patients reported significant reduction in elbow pain within two weeks. At six months, 81% of patients with active patches were able to perform activities of daily living with no symptoms (versus 60% of controls, with the rehabilitation program and brace use alone).19
Laser Therapy
After completing a 2010 study of low-energy gallium-arsenide (GaAs) laser therapy for the treatment of lateral epicondylitis, researchers found no short-term advantages of laser therapy, compared with a sham procedure—but long-term results were more promising.20 Study participants underwent 15 sessions of treatment (application of a laser probe to the two most sensitive points, with a specified dose given) over a three-week period. Shortly after the treatment period ended, patients in both groups had significant improvements.
At 12 weeks’ follow-up, however, patients in the active treatment group had significantly better long-term results than did controls (about 60% vs 40% improvement from baseline, respectively), especially in functional outcome measures. As the study authors noted, low-energy laser therapy carries a low risk for adverse effects, making it a long-term treatment strategy to be considered for patients with intractable lateral epicondylitis.20
Surgery
When conservative strategies implemented for several months do not resolve symptoms of lateral epicondylitis, surgical intervention may be required.2,21 Whether in open, percutaneous, or arthroscopic procedures, tendinopathic tissue is excised at the origin of the extensor carpi radialis brevis tendon in an effort to re-approximate the healthy tendon.21
Another less frequently used surgical procedure for lateral epicondylitis is extensor tendon release. In one long-term study investigating outpatient open extensor tendon release in 77 affected patients, researchers noted predominately good to excellent outcomes (ie, symptom relief, functional improvement), coupled with a low perioperative complication rate.29
PREVENTION
Prevention strategies are designed to mitigate the risk factors and minimize behaviors associated with each CTD. The science of ergonomics has produced numerous strategies intended to reduce the incidence of CTD through proper body mechanics, work habits, and equipment. In the workplace, CTD-specific ergonomic guidelines include posture training, reduction of excessive force and unnecessary repetition, and provision of adequate rest intervals. In addition to improving symptoms and possibly resolving some cases of lateral epicondylitis, ergonomic interventions may even reduce the incidence of new CTD in similarly exposed, asymptomatic workers30 (see “Proven Ergonomic Interventions”31,32).
Regarding specific prevention guidelines for lateral epicondylitis, several ergonomic recommendations can be offered to the patient and the patient’s employer. Consultation with a physical therapist may be helpful in developing specific recommendations focused on prevention of workplace injuries. Regarding CTD, physical therapists may recommend several tendon protection techniques that the clinician can then provide to the patient and his/her employer.33,34
Tendon protection techniques encourage proper body mechanics, avoidance of excessive force and repetition, as well as proper tool selection. Patients should be advised to avoid tasks that require unnecessary repetitive wrist flexion and extension, forearm pronation and supination, and strong, forceful gripping of objects. They should also avoid lifting objects with forearms pronated and wrists extended, as this will place increased tension and stress on the extensor tendon. Rather, patients should be advised to lift objects with their forearm supinated in a scooping motion (see Table 233).
Force is considered a significant contributor to the development of lateral epicondylitis. Thus, patients should be instructed to minimize forceful gestures at work, particularly with the forearm in full extension.34 Patients and employers should be advised to encourage use of proper tools for the tasks performed. Use of hand tools with lighter handles and larger grips requires less force than do tools with heavier, smaller grips, and are more likely to protect the upper-extremity tendons.
Finally, the worker who is required to perform a repetitive task for a sustained amount of time (eg, certain tasks performed by an electrical contractor; steady use of a computer workstation) is likely to benefit from small “micro-breaks” of a few minutes each hour. This habit may reduce cumulative stress, and possibly the incidence of CTD, including lateral epicondylitis.32,34
CONCLUSION
Primary care and occupational medicine clinicians, as well as providers in other frontline specialties, have a great deal to offer in regard to stemming the disabling symptoms and fiscal strain associated with lateral epicondylitis. While early recognition and intervention for patients with symptoms of lateral epicondylitis are essential to the clinician’s role, providers must also focus on key preventive measures. Involving key stakeholders, including patients and their employers and managers, in establishing appropriate safety measures in the workplace is an optimal strategy. The formation of a multidisciplinary health care team that includes ergonomists, physical therapists, and orthopedic specialists may also prove beneficial in both treatment and prevention.
REFERENCES
1. Noteboom T, Cruver R, Keller J, et al. Tennis elbow: a review. J Orthop Sports Phys Ther. 1994;19(6):357-366.
2. Cohen MS, Romeo AA. Lateral epicondylitis: open and arthroscopic treatment. J Am Soc Surg Hand. 2001;1(3):172-176.
3. Rabago D, Best TM, Zgierska AE, et al. A systematic review of four injection therapies for lateral epicondylosis: prolotherapy, polidocanol, whole blood and platelet-rich plasma. Br J Sports Med. 2009;43(7):471-481.
4. Calfee RP, Patel A, DaSilva MF, Akelman E. Management of lateral epicondylitis: current concepts. J Am Acad Orthop Surg. 2008;16(1):19-29.
5. Titchener AG, Fakis A, Tambe AA, et al. Risk factors in lateral epicondylitis (tennis elbow): a case-control study. J Hand Surg Eur Vol. 2012 Apr 4. [Epub ahead of print]
6. van Tulder M, Malmivaara A, Koes B. Repetitive strain injury. Lancet. 2007;369(9575):1815-1822.
7. Werner RA, Franzblau A, Gell N, et al. Predictors of persistent elbow tendonitis among auto assembly workers. J Occup Rehabil. 2005; 15(3):393-400.
8. Melhorn MJ. A prospective study for upper-extremity cumulative trauma disorders of workers in aircraft manufacturing. J Occup Environ Med. 1996;38(12):1264-1271.
9. Johnson GW, Cadwallader K, Scheffel SB, Epperly TD. Treatment of lateral epicondylitis. Am Fam Physician. 2007;76(6):843-848.
10. Haahr JP, Andersen JH. Physical and psychosocial risk factors for lateral epicondylitis: a population based case-referent study. Occup Environ Med. 2003;60(5):322-329.
11. Green S, Buchbinder R, Barnsley L, et al. Non-steroidal anti-inflammatory drugs (NSAIDs) for treating lateral elbow pain in adults. Cochrane Database Syst Rev. 2002;(2):CD003686.
12. Garg R, Adamson GJ, Dawson PA, et al. A prospective randomized study comparing a forearm strap brace versus a wrist splint for the treatment of lateral epicondylitis. J Shoulder Elbow Surg. 2010;19(4):508-512.
13. Smidt N, van der Windt DA, Assendelft WJ, et al. Corticosteroid injections, physiotherapy, or a wait-and-see policy for lateral epicondylitis: a randomised controlled trial. Lancet. 2002;359 (9307):657-662.
14. Stefanou A, Marshall N, Holdan W, Siddiqui A. A randomized study comparing corticosteroid injection to corticosteroid iontophoresis for lateral epicondylitis. J Hand Surg Am. 2012;37(1):104-109.
15. Runeson L, Haker E. Iontophoresis with cortisone in the treatment of lateral epicondylalgia (tennis elbow): a double-blind study. Scand J Med Sci Sports. 2002;12(3):136-142.
16. Fink M, Wolkenstein E, Krst M, Gehrke A. Acupuncture in chronic epicondylitis: a randomized controlled trial. Rheumatology (Oxford). 2002;41(2):205-209.
17. Edwards SG, Calandruccio JH. Autologous blood injections for refractory lateral epicondylitis. J Hand Surgery Am. 2003;28(2):272-278.
18. Placzek R, Drescher W, Deuretzbacher G. Treatment of chronic radial epicondylitis with botulinum toxin A: a double-blind, placebo-controlled, randomized multicenter study. J Bone Joint Surg Am. 2007;89(2):255-260.
19. Paoloni JA, Appleyard RC, Nelson J, Murrell GA. Topical nitric oxide application in the treatment of chronic extensor tendinosis at the elbow: a randomized, double-blinded, placebo-controlled clinical trial. Am J Sports Med. 2003;31(6):915-920.
20. Emanet SK, Altan LI, Yurtkuran M. Investigation of the effect of GaAs laser therapy on lateral epicondylitis. Photomed Laser Surg. 2010;28(3):397-403.
21. Buchbinder R, Johnston RV, Barnsley L, et al. Surgery for lateral elbow pain. Cochrane Database Syst Rev. 2011;(3):CD003525.
22. Jafarian FS, Demneh ES, Tyson SF. The immediate effect of orthotic management on grip strength of patients with lateral epicondylosis.
J Orthop Sports Phys Ther. 2009;39(6):484-489.
23. Grewal R, King GJW. Commentary on “Iontophoresis for the Treatment of Lateral Epicondylitis of the Elbow.” J Hand Surg Am. 2012;37(1):110-111.
24. Green S, Buchbinder R, Barnsley L, et al. Acupuncture for lateral elbow pain. Cochrane Database Syst Rev. 2002;(1):CD003527.
25. Scarpone M, Rabago DP, Zgierska A, et al. The efficacy of prolotherapy for lateral epicondylosis: a pilot study. Clin J Sport Med. 2008;18(3):248-254.
26. Zeisig E, Ohberg L, Alfredson H. Sclerosing polidocanol injections in chronic painful tennis elbow: promising results in a pilot study. Knee Surg Sports Traumatol Arthrosc. 2006;14(11):1218-1224.
27. Mishra A, Pavelko T. Treatment of chronic elbow tendinosis with buffered platelet-rich plasma. Am J Sports Med. 2006;34(11):1774-1778.
28. Wong SM, Hui AC, Tong PY, et al. Treatment of lateral epicondylitis with botulinum toxin: a randomized, double-blind, placebo-controlled trial. Ann Intern Med. 2005;143(11):793-797.
29. Solheim E, Hegna J, Øyen J. Extensor tendon release in tennis elbow: results and prognostic factors in 80 elbows. Knee Surg Sports Traumatol Arthrosc. 2011;19(6):1023-1027.
30. Piligian G, Herbert R, Hearns M, et al. Evaluation and management of chronic work-related musculoskeletal disorders of the distal upper extremity. Am J Ind Med. 2000;37(1):75-93.
31. Bernacki EJ, Guidera JA, Schaefer JA, et al. An ergonomics program designed to reduce the incidence of upper extremity work related musculoskeletal disorders. J Occup Environ Med. 1999;41(12):1032-1040.
32. Motamedzade M, Mohseni M, Golmohammadi R, Mahjoob H. Ergonomics intervention in an Iranian television manufacturing industry. Work. 2011;38(3):257-263.
33. Fallon Clinic, Rehabilitation and Sports Medicine. Lateral epicondylitis: protection techniques. http://www.fallonclinic.org/internet/medical/rehab/exercisesheets/lateral_epicondylitis.pdf. Accessed November 27, 2012.
34. Occupational Safety and Health Administration, US Department of Labor. Computer workstations, additional information: awkward postures, contact stress, force, general controls, repetition. www.osha.gov/SLTC/etools/computer workstations/more.html. Accessed November 29, 2012.
Lateral epicondylitis, also commonly referred to as tennis elbow, is a cumulative trauma disorder (CTD) that affects the extensor tendons of the forearm. Its causative mechanism is repetitive tension and movement of the extensor tendons of the wrist. Characteristic associated movements include supination and pronation of the forearm; these occur frequently during the performance of tasks that require repeated gripping and twisting.1
Among the extensor tendons affected by lateral epicondylitis, the extensor carpi radialis brevis is most commonly identified as the injured tendon.2 While the term epicondylitis implies inflammation as the pathological phenomenon behind this disorder, histologic evidence supports a degenerative process in which a noninflammatory angiofibroblastic tendinosis develops, one that is characterized by degenerative processes, including neovascularization and a disordered collagen matrix.3 In this discussion, therefore, while the disorder will be referred to as lateral epicondylitis (the more common nomenclature), lateral epicondylosis may be a more accurate term.
Pain symptoms described by patients with lateral epicondylitis are thought to be attributable to increasing numbers of free nerve endings in the newly developed granulomatous tissue, in addition to associated synovitis.4 The presence of microvascular damage, a histologic finding in lateral epicondylitis, has also led investigators to suggest several contributing risk factors for tennis elbow, including diabetes and smoking.5
More obscure contributing factors, including psychosocial and socioeconomic concerns, have also been suggested.6
EPIDEMIOLOGY
Though not uncommon in the general population, lateral epicondylitis has a peak incidence in the occupational setting, where its prevalence ranges from 4% to 30%.7 Lateral epicondylitis and other upper-extremity CTD account for 56% of all occupational injuries,8 making them a paramount source of concern for employers and employees alike. In a study of workers’ compensation claims in the United States, van Tulder et al6 found that the mean cost per repetitive strain injury ranged from $5,000 to $8,000. The medical costs and lost work time associated with lateral and medial epicondylitis have been estimated to total more than $22 billion per year in the US alone.7
DIAGNOSIS
Making a diagnosis of lateral epicondylitis is usually straightforward, requiring very little in the way of ancillary testing, such as x-rays or MRI.9
Patient History
A thorough patient history will typically reveal complaints of occupation- or activity-related pain in the lateral elbow, possibly affecting the muscle mass of the forearm.9 Often, the patient will report that symptoms are aggravated by repetitive movements that require strenuous use of the hand, wrist, and/or forearm.10 Examples may include using a screwdriver, grasping a lever, or lifting a heavy load. Occupations associated with risk for lateral epicondylitis include those in the construction industry (eg, plumbers, carpenters) and in manufacturing.
Because a positive association has been established between lateral epicondylitis and ipsilateral rotator cuff tears, carpal tunnel syndrome, and De Quervain’s syndrome, it is important to inquire about any prior history of musculoskeletal injury and coexisting musculoskeletal symptoms. Previous use of oral corticosteroids and a history of smoking are also considered risk factors for lateral epicondylitis.5
Physical Examination
Physical exam findings include reproducible pain at the lateral elbow with resisted supination or wrist dorsiflexion when the forearm is fully extended.9 The examiner may also elicit pain by palpating just distal to the lateral epicondyle over the extensor tendon mass (see Figure 1). Range of motion in the wrist may be diminished due to pain, as when the patient is asked to extend the wrist against resistance. As the condition progresses, the patient may also experience pain and weakness of the forearm with resisted finger motion.
TREATMENT
A number of researchers have investigated the most efficacious therapies for lateral epicondylitis (see Table 111-21); as in the case of other musculoskeletal injuries, rest is a cornerstone of treatment. NSAIDs, too, are often indicated, but the clinician must thoroughly evaluate risk versus benefit before initiating NSAID use.11 Additionally, the patient should be educated regarding NSAIDs’ potential side effects and recommended safety precautions.
Two bracing interventions commonly used to treat lateral epicondylitis are the wrist extension splint and the counterforce forearm strap (see Figure 2). In one small prospective, randomized trial (n = 44) in which these devices were compared, no significant between-group differences were found in terms of Mayo Elbow Performance scores, but according to scores derived from American Shoulder and Elbow Society assessments, pain relief was significantly better in patients who wore the wrist extension splint.12 In another study of orthotic management of lateral epicondylosis, immediate improvement in pain-free grip strength was reported in subjects wearing either an elbow strap or an elbow sleeve, but wearers of a wrist splint experienced no immediate change, making it inadvisable as a first-choice orthotic modality.22
Other treatment modalities to be considered include corticosteroid injections, acupuncture, autologous blood and other injection therapies, botulinum injection, topical nitrates, laser therapy, and surgery.
Corticosteroid Injections Versus Physical Therapy
In a randomized controlled trial contrasting the use of corticosteroid injections, physical therapy, and a wait-and-see approach for patients with lateral epicondylitis, corticosteroid injections were found superior in the short term (defined as six weeks after treatment initiation). In the long term, however (ie, at 52 weeks), physical therapy was found more effective than either of the alternative approaches. Outcome measures included general improvement, reduction in severity of the main complaint, alleviation of pain, improved elbow function, and patient satisfaction.13
A less invasive alternative to steroid injections for patients with lateral epicondylitis, though not yet FDA approved, has yielded promising results: administration of transdermal dexamethasone by way of iontophoresis.14,15 This delivery method (in which administration of ionic, water-soluble agents is facilitated using a weak electric current23) was recently studied by Stefanou et al14 in the form of a transdermal patch, activated by a 24-hour battery.
In the short term (ie, upon completion of therapy), patients who received iontophoretic dexamethasone had significantly better grip strength and were better able to return to work than those treated with injections of dexamethasone or triamcinolone. By six months, outcome measures were comparable among the three groups.14 However, possible advantages to iontophoresis are that it is painless, noninvasive, and less likely to cause adverse effects.15,23
Acupuncture
While few sources support the use of complementary modalities to treat lateral epicondylitis,24 findings from one randomized controlled trial offered modest support for acupuncture use. Compared with a sham procedure (in which nonspecific points were targeted), “real” acupuncture—selection and stimulation of specific acupuncture points—provided reductions in pain intensity and improvements in function and strength. Evidence of these improvements became even more robust at two-week follow-up. At two months, however, only improvements in function remained significant.16
Four Injection Therapies
Injections of autologous whole blood or platelet-rich plasma, prolotherapy (injections with hyperosmolar dextrose and sodium morrhuate25), and polidocanol injections were examined in a systematic literature review of treatment strategies for lateral epicondylosis published in 2009 by Rabago et al.3
Although most of the study cohorts involved were small, significant improvements were reported for all modalities and outcome measures, particularly pain: reduction in pain scores by as much as 88% among patients injected with autologous whole blood17; a 55% improvement at eight months in patients treated with the sclerosing agent polidocanol26; improvement as great as 90%, 16 weeks after treatment with prolotherapy (compared with 22% in controls)25; and, in a nonrandomized trial, pain reduction of 93% in patients who had received platelet-rich plasma injections about 26 months earlier.27 Nevertheless, the researchers note, further study is required in larger trials examining specified biomarkers in addition to clinical, biomechanical, and radiologic means of measurement to assure the long-term safety and effectiveness of each of these modalities.3
Botulinum Toxin
Injecting botulinum toxin is another treatment strategy that may be considered when results of more traditional approaches are unsatisfactory. In two double-blind, placebo-controlled, randomized studies, patients injected once with botulinum toxin A experienced significant reductions in pain within four to six weeks, compared with those given placebo.18,28 A notable but expected complication of this treatment reported by Placzek et al18 was weakness of the third finger of the treated hand, two weeks after treatment. However, normal strength was regained in all affected patients by week 18 of follow-up.
Nitrates
Use of topical nitric oxide showed promising results in a 2003 study by Australian researchers.19 Eighty-six patients with chronic extensor tendinosis, all of whom underwent a standard tendon rehabilitation program (ie, rest and stretching and strengthening exercises) and used a forearm counterforce brace, were randomized to receive glyceryl trinitrate transdermal patches or sham patches. One-quarter of a patch was applied just distal to the lateral epicondyle of the humerus for 24 hours, then replaced by another in a rotating fashion around the target spot.
Treated patients reported significant reduction in elbow pain within two weeks. At six months, 81% of patients with active patches were able to perform activities of daily living with no symptoms (versus 60% of controls, with the rehabilitation program and brace use alone).19
Laser Therapy
After completing a 2010 study of low-energy gallium-arsenide (GaAs) laser therapy for the treatment of lateral epicondylitis, researchers found no short-term advantages of laser therapy, compared with a sham procedure—but long-term results were more promising.20 Study participants underwent 15 sessions of treatment (application of a laser probe to the two most sensitive points, with a specified dose given) over a three-week period. Shortly after the treatment period ended, patients in both groups had significant improvements.
At 12 weeks’ follow-up, however, patients in the active treatment group had significantly better long-term results than did controls (about 60% vs 40% improvement from baseline, respectively), especially in functional outcome measures. As the study authors noted, low-energy laser therapy carries a low risk for adverse effects, making it a long-term treatment strategy to be considered for patients with intractable lateral epicondylitis.20
Surgery
When conservative strategies implemented for several months do not resolve symptoms of lateral epicondylitis, surgical intervention may be required.2,21 Whether in open, percutaneous, or arthroscopic procedures, tendinopathic tissue is excised at the origin of the extensor carpi radialis brevis tendon in an effort to re-approximate the healthy tendon.21
Another less frequently used surgical procedure for lateral epicondylitis is extensor tendon release. In one long-term study investigating outpatient open extensor tendon release in 77 affected patients, researchers noted predominately good to excellent outcomes (ie, symptom relief, functional improvement), coupled with a low perioperative complication rate.29
PREVENTION
Prevention strategies are designed to mitigate the risk factors and minimize behaviors associated with each CTD. The science of ergonomics has produced numerous strategies intended to reduce the incidence of CTD through proper body mechanics, work habits, and equipment. In the workplace, CTD-specific ergonomic guidelines include posture training, reduction of excessive force and unnecessary repetition, and provision of adequate rest intervals. In addition to improving symptoms and possibly resolving some cases of lateral epicondylitis, ergonomic interventions may even reduce the incidence of new CTD in similarly exposed, asymptomatic workers30 (see “Proven Ergonomic Interventions”31,32).
Regarding specific prevention guidelines for lateral epicondylitis, several ergonomic recommendations can be offered to the patient and the patient’s employer. Consultation with a physical therapist may be helpful in developing specific recommendations focused on prevention of workplace injuries. Regarding CTD, physical therapists may recommend several tendon protection techniques that the clinician can then provide to the patient and his/her employer.33,34
Tendon protection techniques encourage proper body mechanics, avoidance of excessive force and repetition, as well as proper tool selection. Patients should be advised to avoid tasks that require unnecessary repetitive wrist flexion and extension, forearm pronation and supination, and strong, forceful gripping of objects. They should also avoid lifting objects with forearms pronated and wrists extended, as this will place increased tension and stress on the extensor tendon. Rather, patients should be advised to lift objects with their forearm supinated in a scooping motion (see Table 233).
Force is considered a significant contributor to the development of lateral epicondylitis. Thus, patients should be instructed to minimize forceful gestures at work, particularly with the forearm in full extension.34 Patients and employers should be advised to encourage use of proper tools for the tasks performed. Use of hand tools with lighter handles and larger grips requires less force than do tools with heavier, smaller grips, and are more likely to protect the upper-extremity tendons.
Finally, the worker who is required to perform a repetitive task for a sustained amount of time (eg, certain tasks performed by an electrical contractor; steady use of a computer workstation) is likely to benefit from small “micro-breaks” of a few minutes each hour. This habit may reduce cumulative stress, and possibly the incidence of CTD, including lateral epicondylitis.32,34
CONCLUSION
Primary care and occupational medicine clinicians, as well as providers in other frontline specialties, have a great deal to offer in regard to stemming the disabling symptoms and fiscal strain associated with lateral epicondylitis. While early recognition and intervention for patients with symptoms of lateral epicondylitis are essential to the clinician’s role, providers must also focus on key preventive measures. Involving key stakeholders, including patients and their employers and managers, in establishing appropriate safety measures in the workplace is an optimal strategy. The formation of a multidisciplinary health care team that includes ergonomists, physical therapists, and orthopedic specialists may also prove beneficial in both treatment and prevention.
REFERENCES
1. Noteboom T, Cruver R, Keller J, et al. Tennis elbow: a review. J Orthop Sports Phys Ther. 1994;19(6):357-366.
2. Cohen MS, Romeo AA. Lateral epicondylitis: open and arthroscopic treatment. J Am Soc Surg Hand. 2001;1(3):172-176.
3. Rabago D, Best TM, Zgierska AE, et al. A systematic review of four injection therapies for lateral epicondylosis: prolotherapy, polidocanol, whole blood and platelet-rich plasma. Br J Sports Med. 2009;43(7):471-481.
4. Calfee RP, Patel A, DaSilva MF, Akelman E. Management of lateral epicondylitis: current concepts. J Am Acad Orthop Surg. 2008;16(1):19-29.
5. Titchener AG, Fakis A, Tambe AA, et al. Risk factors in lateral epicondylitis (tennis elbow): a case-control study. J Hand Surg Eur Vol. 2012 Apr 4. [Epub ahead of print]
6. van Tulder M, Malmivaara A, Koes B. Repetitive strain injury. Lancet. 2007;369(9575):1815-1822.
7. Werner RA, Franzblau A, Gell N, et al. Predictors of persistent elbow tendonitis among auto assembly workers. J Occup Rehabil. 2005; 15(3):393-400.
8. Melhorn MJ. A prospective study for upper-extremity cumulative trauma disorders of workers in aircraft manufacturing. J Occup Environ Med. 1996;38(12):1264-1271.
9. Johnson GW, Cadwallader K, Scheffel SB, Epperly TD. Treatment of lateral epicondylitis. Am Fam Physician. 2007;76(6):843-848.
10. Haahr JP, Andersen JH. Physical and psychosocial risk factors for lateral epicondylitis: a population based case-referent study. Occup Environ Med. 2003;60(5):322-329.
11. Green S, Buchbinder R, Barnsley L, et al. Non-steroidal anti-inflammatory drugs (NSAIDs) for treating lateral elbow pain in adults. Cochrane Database Syst Rev. 2002;(2):CD003686.
12. Garg R, Adamson GJ, Dawson PA, et al. A prospective randomized study comparing a forearm strap brace versus a wrist splint for the treatment of lateral epicondylitis. J Shoulder Elbow Surg. 2010;19(4):508-512.
13. Smidt N, van der Windt DA, Assendelft WJ, et al. Corticosteroid injections, physiotherapy, or a wait-and-see policy for lateral epicondylitis: a randomised controlled trial. Lancet. 2002;359 (9307):657-662.
14. Stefanou A, Marshall N, Holdan W, Siddiqui A. A randomized study comparing corticosteroid injection to corticosteroid iontophoresis for lateral epicondylitis. J Hand Surg Am. 2012;37(1):104-109.
15. Runeson L, Haker E. Iontophoresis with cortisone in the treatment of lateral epicondylalgia (tennis elbow): a double-blind study. Scand J Med Sci Sports. 2002;12(3):136-142.
16. Fink M, Wolkenstein E, Krst M, Gehrke A. Acupuncture in chronic epicondylitis: a randomized controlled trial. Rheumatology (Oxford). 2002;41(2):205-209.
17. Edwards SG, Calandruccio JH. Autologous blood injections for refractory lateral epicondylitis. J Hand Surgery Am. 2003;28(2):272-278.
18. Placzek R, Drescher W, Deuretzbacher G. Treatment of chronic radial epicondylitis with botulinum toxin A: a double-blind, placebo-controlled, randomized multicenter study. J Bone Joint Surg Am. 2007;89(2):255-260.
19. Paoloni JA, Appleyard RC, Nelson J, Murrell GA. Topical nitric oxide application in the treatment of chronic extensor tendinosis at the elbow: a randomized, double-blinded, placebo-controlled clinical trial. Am J Sports Med. 2003;31(6):915-920.
20. Emanet SK, Altan LI, Yurtkuran M. Investigation of the effect of GaAs laser therapy on lateral epicondylitis. Photomed Laser Surg. 2010;28(3):397-403.
21. Buchbinder R, Johnston RV, Barnsley L, et al. Surgery for lateral elbow pain. Cochrane Database Syst Rev. 2011;(3):CD003525.
22. Jafarian FS, Demneh ES, Tyson SF. The immediate effect of orthotic management on grip strength of patients with lateral epicondylosis.
J Orthop Sports Phys Ther. 2009;39(6):484-489.
23. Grewal R, King GJW. Commentary on “Iontophoresis for the Treatment of Lateral Epicondylitis of the Elbow.” J Hand Surg Am. 2012;37(1):110-111.
24. Green S, Buchbinder R, Barnsley L, et al. Acupuncture for lateral elbow pain. Cochrane Database Syst Rev. 2002;(1):CD003527.
25. Scarpone M, Rabago DP, Zgierska A, et al. The efficacy of prolotherapy for lateral epicondylosis: a pilot study. Clin J Sport Med. 2008;18(3):248-254.
26. Zeisig E, Ohberg L, Alfredson H. Sclerosing polidocanol injections in chronic painful tennis elbow: promising results in a pilot study. Knee Surg Sports Traumatol Arthrosc. 2006;14(11):1218-1224.
27. Mishra A, Pavelko T. Treatment of chronic elbow tendinosis with buffered platelet-rich plasma. Am J Sports Med. 2006;34(11):1774-1778.
28. Wong SM, Hui AC, Tong PY, et al. Treatment of lateral epicondylitis with botulinum toxin: a randomized, double-blind, placebo-controlled trial. Ann Intern Med. 2005;143(11):793-797.
29. Solheim E, Hegna J, Øyen J. Extensor tendon release in tennis elbow: results and prognostic factors in 80 elbows. Knee Surg Sports Traumatol Arthrosc. 2011;19(6):1023-1027.
30. Piligian G, Herbert R, Hearns M, et al. Evaluation and management of chronic work-related musculoskeletal disorders of the distal upper extremity. Am J Ind Med. 2000;37(1):75-93.
31. Bernacki EJ, Guidera JA, Schaefer JA, et al. An ergonomics program designed to reduce the incidence of upper extremity work related musculoskeletal disorders. J Occup Environ Med. 1999;41(12):1032-1040.
32. Motamedzade M, Mohseni M, Golmohammadi R, Mahjoob H. Ergonomics intervention in an Iranian television manufacturing industry. Work. 2011;38(3):257-263.
33. Fallon Clinic, Rehabilitation and Sports Medicine. Lateral epicondylitis: protection techniques. http://www.fallonclinic.org/internet/medical/rehab/exercisesheets/lateral_epicondylitis.pdf. Accessed November 27, 2012.
34. Occupational Safety and Health Administration, US Department of Labor. Computer workstations, additional information: awkward postures, contact stress, force, general controls, repetition. www.osha.gov/SLTC/etools/computer workstations/more.html. Accessed November 29, 2012.
Lateral epicondylitis, also commonly referred to as tennis elbow, is a cumulative trauma disorder (CTD) that affects the extensor tendons of the forearm. Its causative mechanism is repetitive tension and movement of the extensor tendons of the wrist. Characteristic associated movements include supination and pronation of the forearm; these occur frequently during the performance of tasks that require repeated gripping and twisting.1
Among the extensor tendons affected by lateral epicondylitis, the extensor carpi radialis brevis is most commonly identified as the injured tendon.2 While the term epicondylitis implies inflammation as the pathological phenomenon behind this disorder, histologic evidence supports a degenerative process in which a noninflammatory angiofibroblastic tendinosis develops, one that is characterized by degenerative processes, including neovascularization and a disordered collagen matrix.3 In this discussion, therefore, while the disorder will be referred to as lateral epicondylitis (the more common nomenclature), lateral epicondylosis may be a more accurate term.
Pain symptoms described by patients with lateral epicondylitis are thought to be attributable to increasing numbers of free nerve endings in the newly developed granulomatous tissue, in addition to associated synovitis.4 The presence of microvascular damage, a histologic finding in lateral epicondylitis, has also led investigators to suggest several contributing risk factors for tennis elbow, including diabetes and smoking.5
More obscure contributing factors, including psychosocial and socioeconomic concerns, have also been suggested.6
EPIDEMIOLOGY
Though not uncommon in the general population, lateral epicondylitis has a peak incidence in the occupational setting, where its prevalence ranges from 4% to 30%.7 Lateral epicondylitis and other upper-extremity CTD account for 56% of all occupational injuries,8 making them a paramount source of concern for employers and employees alike. In a study of workers’ compensation claims in the United States, van Tulder et al6 found that the mean cost per repetitive strain injury ranged from $5,000 to $8,000. The medical costs and lost work time associated with lateral and medial epicondylitis have been estimated to total more than $22 billion per year in the US alone.7
DIAGNOSIS
Making a diagnosis of lateral epicondylitis is usually straightforward, requiring very little in the way of ancillary testing, such as x-rays or MRI.9
Patient History
A thorough patient history will typically reveal complaints of occupation- or activity-related pain in the lateral elbow, possibly affecting the muscle mass of the forearm.9 Often, the patient will report that symptoms are aggravated by repetitive movements that require strenuous use of the hand, wrist, and/or forearm.10 Examples may include using a screwdriver, grasping a lever, or lifting a heavy load. Occupations associated with risk for lateral epicondylitis include those in the construction industry (eg, plumbers, carpenters) and in manufacturing.
Because a positive association has been established between lateral epicondylitis and ipsilateral rotator cuff tears, carpal tunnel syndrome, and De Quervain’s syndrome, it is important to inquire about any prior history of musculoskeletal injury and coexisting musculoskeletal symptoms. Previous use of oral corticosteroids and a history of smoking are also considered risk factors for lateral epicondylitis.5
Physical Examination
Physical exam findings include reproducible pain at the lateral elbow with resisted supination or wrist dorsiflexion when the forearm is fully extended.9 The examiner may also elicit pain by palpating just distal to the lateral epicondyle over the extensor tendon mass (see Figure 1). Range of motion in the wrist may be diminished due to pain, as when the patient is asked to extend the wrist against resistance. As the condition progresses, the patient may also experience pain and weakness of the forearm with resisted finger motion.
TREATMENT
A number of researchers have investigated the most efficacious therapies for lateral epicondylitis (see Table 111-21); as in the case of other musculoskeletal injuries, rest is a cornerstone of treatment. NSAIDs, too, are often indicated, but the clinician must thoroughly evaluate risk versus benefit before initiating NSAID use.11 Additionally, the patient should be educated regarding NSAIDs’ potential side effects and recommended safety precautions.
Two bracing interventions commonly used to treat lateral epicondylitis are the wrist extension splint and the counterforce forearm strap (see Figure 2). In one small prospective, randomized trial (n = 44) in which these devices were compared, no significant between-group differences were found in terms of Mayo Elbow Performance scores, but according to scores derived from American Shoulder and Elbow Society assessments, pain relief was significantly better in patients who wore the wrist extension splint.12 In another study of orthotic management of lateral epicondylosis, immediate improvement in pain-free grip strength was reported in subjects wearing either an elbow strap or an elbow sleeve, but wearers of a wrist splint experienced no immediate change, making it inadvisable as a first-choice orthotic modality.22
Other treatment modalities to be considered include corticosteroid injections, acupuncture, autologous blood and other injection therapies, botulinum injection, topical nitrates, laser therapy, and surgery.
Corticosteroid Injections Versus Physical Therapy
In a randomized controlled trial contrasting the use of corticosteroid injections, physical therapy, and a wait-and-see approach for patients with lateral epicondylitis, corticosteroid injections were found superior in the short term (defined as six weeks after treatment initiation). In the long term, however (ie, at 52 weeks), physical therapy was found more effective than either of the alternative approaches. Outcome measures included general improvement, reduction in severity of the main complaint, alleviation of pain, improved elbow function, and patient satisfaction.13
A less invasive alternative to steroid injections for patients with lateral epicondylitis, though not yet FDA approved, has yielded promising results: administration of transdermal dexamethasone by way of iontophoresis.14,15 This delivery method (in which administration of ionic, water-soluble agents is facilitated using a weak electric current23) was recently studied by Stefanou et al14 in the form of a transdermal patch, activated by a 24-hour battery.
In the short term (ie, upon completion of therapy), patients who received iontophoretic dexamethasone had significantly better grip strength and were better able to return to work than those treated with injections of dexamethasone or triamcinolone. By six months, outcome measures were comparable among the three groups.14 However, possible advantages to iontophoresis are that it is painless, noninvasive, and less likely to cause adverse effects.15,23
Acupuncture
While few sources support the use of complementary modalities to treat lateral epicondylitis,24 findings from one randomized controlled trial offered modest support for acupuncture use. Compared with a sham procedure (in which nonspecific points were targeted), “real” acupuncture—selection and stimulation of specific acupuncture points—provided reductions in pain intensity and improvements in function and strength. Evidence of these improvements became even more robust at two-week follow-up. At two months, however, only improvements in function remained significant.16
Four Injection Therapies
Injections of autologous whole blood or platelet-rich plasma, prolotherapy (injections with hyperosmolar dextrose and sodium morrhuate25), and polidocanol injections were examined in a systematic literature review of treatment strategies for lateral epicondylosis published in 2009 by Rabago et al.3
Although most of the study cohorts involved were small, significant improvements were reported for all modalities and outcome measures, particularly pain: reduction in pain scores by as much as 88% among patients injected with autologous whole blood17; a 55% improvement at eight months in patients treated with the sclerosing agent polidocanol26; improvement as great as 90%, 16 weeks after treatment with prolotherapy (compared with 22% in controls)25; and, in a nonrandomized trial, pain reduction of 93% in patients who had received platelet-rich plasma injections about 26 months earlier.27 Nevertheless, the researchers note, further study is required in larger trials examining specified biomarkers in addition to clinical, biomechanical, and radiologic means of measurement to assure the long-term safety and effectiveness of each of these modalities.3
Botulinum Toxin
Injecting botulinum toxin is another treatment strategy that may be considered when results of more traditional approaches are unsatisfactory. In two double-blind, placebo-controlled, randomized studies, patients injected once with botulinum toxin A experienced significant reductions in pain within four to six weeks, compared with those given placebo.18,28 A notable but expected complication of this treatment reported by Placzek et al18 was weakness of the third finger of the treated hand, two weeks after treatment. However, normal strength was regained in all affected patients by week 18 of follow-up.
Nitrates
Use of topical nitric oxide showed promising results in a 2003 study by Australian researchers.19 Eighty-six patients with chronic extensor tendinosis, all of whom underwent a standard tendon rehabilitation program (ie, rest and stretching and strengthening exercises) and used a forearm counterforce brace, were randomized to receive glyceryl trinitrate transdermal patches or sham patches. One-quarter of a patch was applied just distal to the lateral epicondyle of the humerus for 24 hours, then replaced by another in a rotating fashion around the target spot.
Treated patients reported significant reduction in elbow pain within two weeks. At six months, 81% of patients with active patches were able to perform activities of daily living with no symptoms (versus 60% of controls, with the rehabilitation program and brace use alone).19
Laser Therapy
After completing a 2010 study of low-energy gallium-arsenide (GaAs) laser therapy for the treatment of lateral epicondylitis, researchers found no short-term advantages of laser therapy, compared with a sham procedure—but long-term results were more promising.20 Study participants underwent 15 sessions of treatment (application of a laser probe to the two most sensitive points, with a specified dose given) over a three-week period. Shortly after the treatment period ended, patients in both groups had significant improvements.
At 12 weeks’ follow-up, however, patients in the active treatment group had significantly better long-term results than did controls (about 60% vs 40% improvement from baseline, respectively), especially in functional outcome measures. As the study authors noted, low-energy laser therapy carries a low risk for adverse effects, making it a long-term treatment strategy to be considered for patients with intractable lateral epicondylitis.20
Surgery
When conservative strategies implemented for several months do not resolve symptoms of lateral epicondylitis, surgical intervention may be required.2,21 Whether in open, percutaneous, or arthroscopic procedures, tendinopathic tissue is excised at the origin of the extensor carpi radialis brevis tendon in an effort to re-approximate the healthy tendon.21
Another less frequently used surgical procedure for lateral epicondylitis is extensor tendon release. In one long-term study investigating outpatient open extensor tendon release in 77 affected patients, researchers noted predominately good to excellent outcomes (ie, symptom relief, functional improvement), coupled with a low perioperative complication rate.29
PREVENTION
Prevention strategies are designed to mitigate the risk factors and minimize behaviors associated with each CTD. The science of ergonomics has produced numerous strategies intended to reduce the incidence of CTD through proper body mechanics, work habits, and equipment. In the workplace, CTD-specific ergonomic guidelines include posture training, reduction of excessive force and unnecessary repetition, and provision of adequate rest intervals. In addition to improving symptoms and possibly resolving some cases of lateral epicondylitis, ergonomic interventions may even reduce the incidence of new CTD in similarly exposed, asymptomatic workers30 (see “Proven Ergonomic Interventions”31,32).
Regarding specific prevention guidelines for lateral epicondylitis, several ergonomic recommendations can be offered to the patient and the patient’s employer. Consultation with a physical therapist may be helpful in developing specific recommendations focused on prevention of workplace injuries. Regarding CTD, physical therapists may recommend several tendon protection techniques that the clinician can then provide to the patient and his/her employer.33,34
Tendon protection techniques encourage proper body mechanics, avoidance of excessive force and repetition, as well as proper tool selection. Patients should be advised to avoid tasks that require unnecessary repetitive wrist flexion and extension, forearm pronation and supination, and strong, forceful gripping of objects. They should also avoid lifting objects with forearms pronated and wrists extended, as this will place increased tension and stress on the extensor tendon. Rather, patients should be advised to lift objects with their forearm supinated in a scooping motion (see Table 233).
Force is considered a significant contributor to the development of lateral epicondylitis. Thus, patients should be instructed to minimize forceful gestures at work, particularly with the forearm in full extension.34 Patients and employers should be advised to encourage use of proper tools for the tasks performed. Use of hand tools with lighter handles and larger grips requires less force than do tools with heavier, smaller grips, and are more likely to protect the upper-extremity tendons.
Finally, the worker who is required to perform a repetitive task for a sustained amount of time (eg, certain tasks performed by an electrical contractor; steady use of a computer workstation) is likely to benefit from small “micro-breaks” of a few minutes each hour. This habit may reduce cumulative stress, and possibly the incidence of CTD, including lateral epicondylitis.32,34
CONCLUSION
Primary care and occupational medicine clinicians, as well as providers in other frontline specialties, have a great deal to offer in regard to stemming the disabling symptoms and fiscal strain associated with lateral epicondylitis. While early recognition and intervention for patients with symptoms of lateral epicondylitis are essential to the clinician’s role, providers must also focus on key preventive measures. Involving key stakeholders, including patients and their employers and managers, in establishing appropriate safety measures in the workplace is an optimal strategy. The formation of a multidisciplinary health care team that includes ergonomists, physical therapists, and orthopedic specialists may also prove beneficial in both treatment and prevention.
REFERENCES
1. Noteboom T, Cruver R, Keller J, et al. Tennis elbow: a review. J Orthop Sports Phys Ther. 1994;19(6):357-366.
2. Cohen MS, Romeo AA. Lateral epicondylitis: open and arthroscopic treatment. J Am Soc Surg Hand. 2001;1(3):172-176.
3. Rabago D, Best TM, Zgierska AE, et al. A systematic review of four injection therapies for lateral epicondylosis: prolotherapy, polidocanol, whole blood and platelet-rich plasma. Br J Sports Med. 2009;43(7):471-481.
4. Calfee RP, Patel A, DaSilva MF, Akelman E. Management of lateral epicondylitis: current concepts. J Am Acad Orthop Surg. 2008;16(1):19-29.
5. Titchener AG, Fakis A, Tambe AA, et al. Risk factors in lateral epicondylitis (tennis elbow): a case-control study. J Hand Surg Eur Vol. 2012 Apr 4. [Epub ahead of print]
6. van Tulder M, Malmivaara A, Koes B. Repetitive strain injury. Lancet. 2007;369(9575):1815-1822.
7. Werner RA, Franzblau A, Gell N, et al. Predictors of persistent elbow tendonitis among auto assembly workers. J Occup Rehabil. 2005; 15(3):393-400.
8. Melhorn MJ. A prospective study for upper-extremity cumulative trauma disorders of workers in aircraft manufacturing. J Occup Environ Med. 1996;38(12):1264-1271.
9. Johnson GW, Cadwallader K, Scheffel SB, Epperly TD. Treatment of lateral epicondylitis. Am Fam Physician. 2007;76(6):843-848.
10. Haahr JP, Andersen JH. Physical and psychosocial risk factors for lateral epicondylitis: a population based case-referent study. Occup Environ Med. 2003;60(5):322-329.
11. Green S, Buchbinder R, Barnsley L, et al. Non-steroidal anti-inflammatory drugs (NSAIDs) for treating lateral elbow pain in adults. Cochrane Database Syst Rev. 2002;(2):CD003686.
12. Garg R, Adamson GJ, Dawson PA, et al. A prospective randomized study comparing a forearm strap brace versus a wrist splint for the treatment of lateral epicondylitis. J Shoulder Elbow Surg. 2010;19(4):508-512.
13. Smidt N, van der Windt DA, Assendelft WJ, et al. Corticosteroid injections, physiotherapy, or a wait-and-see policy for lateral epicondylitis: a randomised controlled trial. Lancet. 2002;359 (9307):657-662.
14. Stefanou A, Marshall N, Holdan W, Siddiqui A. A randomized study comparing corticosteroid injection to corticosteroid iontophoresis for lateral epicondylitis. J Hand Surg Am. 2012;37(1):104-109.
15. Runeson L, Haker E. Iontophoresis with cortisone in the treatment of lateral epicondylalgia (tennis elbow): a double-blind study. Scand J Med Sci Sports. 2002;12(3):136-142.
16. Fink M, Wolkenstein E, Krst M, Gehrke A. Acupuncture in chronic epicondylitis: a randomized controlled trial. Rheumatology (Oxford). 2002;41(2):205-209.
17. Edwards SG, Calandruccio JH. Autologous blood injections for refractory lateral epicondylitis. J Hand Surgery Am. 2003;28(2):272-278.
18. Placzek R, Drescher W, Deuretzbacher G. Treatment of chronic radial epicondylitis with botulinum toxin A: a double-blind, placebo-controlled, randomized multicenter study. J Bone Joint Surg Am. 2007;89(2):255-260.
19. Paoloni JA, Appleyard RC, Nelson J, Murrell GA. Topical nitric oxide application in the treatment of chronic extensor tendinosis at the elbow: a randomized, double-blinded, placebo-controlled clinical trial. Am J Sports Med. 2003;31(6):915-920.
20. Emanet SK, Altan LI, Yurtkuran M. Investigation of the effect of GaAs laser therapy on lateral epicondylitis. Photomed Laser Surg. 2010;28(3):397-403.
21. Buchbinder R, Johnston RV, Barnsley L, et al. Surgery for lateral elbow pain. Cochrane Database Syst Rev. 2011;(3):CD003525.
22. Jafarian FS, Demneh ES, Tyson SF. The immediate effect of orthotic management on grip strength of patients with lateral epicondylosis.
J Orthop Sports Phys Ther. 2009;39(6):484-489.
23. Grewal R, King GJW. Commentary on “Iontophoresis for the Treatment of Lateral Epicondylitis of the Elbow.” J Hand Surg Am. 2012;37(1):110-111.
24. Green S, Buchbinder R, Barnsley L, et al. Acupuncture for lateral elbow pain. Cochrane Database Syst Rev. 2002;(1):CD003527.
25. Scarpone M, Rabago DP, Zgierska A, et al. The efficacy of prolotherapy for lateral epicondylosis: a pilot study. Clin J Sport Med. 2008;18(3):248-254.
26. Zeisig E, Ohberg L, Alfredson H. Sclerosing polidocanol injections in chronic painful tennis elbow: promising results in a pilot study. Knee Surg Sports Traumatol Arthrosc. 2006;14(11):1218-1224.
27. Mishra A, Pavelko T. Treatment of chronic elbow tendinosis with buffered platelet-rich plasma. Am J Sports Med. 2006;34(11):1774-1778.
28. Wong SM, Hui AC, Tong PY, et al. Treatment of lateral epicondylitis with botulinum toxin: a randomized, double-blind, placebo-controlled trial. Ann Intern Med. 2005;143(11):793-797.
29. Solheim E, Hegna J, Øyen J. Extensor tendon release in tennis elbow: results and prognostic factors in 80 elbows. Knee Surg Sports Traumatol Arthrosc. 2011;19(6):1023-1027.
30. Piligian G, Herbert R, Hearns M, et al. Evaluation and management of chronic work-related musculoskeletal disorders of the distal upper extremity. Am J Ind Med. 2000;37(1):75-93.
31. Bernacki EJ, Guidera JA, Schaefer JA, et al. An ergonomics program designed to reduce the incidence of upper extremity work related musculoskeletal disorders. J Occup Environ Med. 1999;41(12):1032-1040.
32. Motamedzade M, Mohseni M, Golmohammadi R, Mahjoob H. Ergonomics intervention in an Iranian television manufacturing industry. Work. 2011;38(3):257-263.
33. Fallon Clinic, Rehabilitation and Sports Medicine. Lateral epicondylitis: protection techniques. http://www.fallonclinic.org/internet/medical/rehab/exercisesheets/lateral_epicondylitis.pdf. Accessed November 27, 2012.
34. Occupational Safety and Health Administration, US Department of Labor. Computer workstations, additional information: awkward postures, contact stress, force, general controls, repetition. www.osha.gov/SLTC/etools/computer workstations/more.html. Accessed November 29, 2012.
Encountering the Victim of Sexual Assault
Each year in the United States, between 300,000 and 700,000 adult women are estimated to experience sexual assault, with 40,000 of such victims typically seeking treatment in an emergency department (ED).1 In a survey of hospital EDs published in 2008, only 9.6% of the 117 responding hospitals provided to presenting victims of sexual assault all of the following elements of comprehensive medical care management2:
• Acute medical care
•
History and physical examination
•
Acute and long-term rape crisis counseling
•
Prophylactic and therapeutic management for HIV or other sexually transmitted infection (STI)
•
Provision of emergency contraception, with appropriate counseling.2
Specific data from a similar survey included these findings: appropriate, CDC-recommended prophylaxis against STI prescribed in only 6.7% of cases, HIV serology testing in only 13%, and information about follow-up care given to only 31% of patients. Nearly 80% of sexual assault victims treated in the responding hospital EDs received less than optimal care.3,4
In the ED, where victims of sexual assault are most likely to be evaluated, the responsibilities involved in managing the department may hamper emergency physicians’ ability to provide the detailed, time-consuming, one-on-one care such patients require; often, this care is entrusted to an NP or an RN.1 Clinicians in this setting, as well as those who practice in student health, primary care, and women’s health, must be competent in assessing and treating the injuries assaulted patients have sustained, providing STI prophylaxis and pregnancy prevention, collecting forensic evidence in order to facilitate prosecution of the perpetrator, and providing appropriate referrals to promote physical and emotional recovery through counseling and other follow-up care—in short, meeting these patients’ medical, legal, and psychosocial needs.5,6 (See “Specialized Training, a Team Response.”5-7)
DEFINITIONS: RAPE AND SEXUAL ASSAULT
The definition of rape varies from state to state, but three criteria are typically present:
•
Sexual penetration of the victim’s vagina, mouth, or rectum
•
Absence of consent from the victim
• The use or threat of force.8
Sexual assault is a less restrictive term, referring to the sexual contact of one person with another without appropriate consent. Specified manifestations vary state by state but typically include child sexual assault, incest, marital rape, and other forced sexual acts.7
“Julie,” 18, presents to the ED, accompanied by a female friend, after being sexually assaulted by a male student from the college Julie attends. Earlier that evening, Julie was drinking alcohol at a party in the suspect’s apartment. While everyone else was dancing, he invited Julie to his room. She admits that she was willing to “fool around” with him, but when he asked to have intercourse, she said “no.” The suspect insisted that she “wanted it” and proceeded to engage in unprotected intercourse with her. Julie is distressed because she was a virgin until the encounter and had not been using any form of birth control.
On presentation of a victim of sexual assault, local law enforcement and an advocate from the local rape crisis center should be promptly notified; however, the patient’s permission must be obtained before the police department is contacted. A victim may not want to report a sexual assault to the police for a number of reasons, including:
•
A belief that the police are limited in their ability to intervene effectively
•
A perception that victims of sexual assault are often considered at fault
•
Fear that the assailant may assault the victim again
•
Misplaced feelings of fear and shame.5
The NP or PA who performs the initial examination should make every effort to interview the patient while both law enforcement and the advocate are present so that the victim is not required to describe and relive the traumatic situation repeatedly. The advocate is present to support the victim throughout the ED or office visit and evidence collection process; and to provide referrals for follow-up care.
The clinician must strive to remain objective during the evaluation and evidence collection process. For example, the detection of another person’s DNA on the body of the patient is not proof, in and of itself, of that person’s guilt, but only the presence of his or her DNA.9
HISTORY AND PHYSICAL
A thorough medical history and assessment should always be completed, either before or after the forensic examination, depending on the patient’s condition.
Evidence collection is begun by obtaining consent and interviewing the patient. The patient’s account of the assault will guide the practitioner to specific areas of the body where evidence may be found (for example, the case patient said the suspect had kissed her neck, which was swabbed to corroborate her story). Whatever the patient’s age, the presence of a family member or friend is not recommended during the interview, as this could cause the victim to withhold information, and any emotional reaction may be a distraction for the patient. Additionally, having a family member or friend present during the interview process puts that individual at risk for subpoena and court appearance.7
The interview should be concise, with the patient’s account of the assault recorded in some way so that he or she can later be quoted as closely as possible. The clinician should avoid using medical or legal terms or abbreviations, or altering the patient’s own words.
Before the physical examination is begun, the patient’s clothing must be collected and each piece packaged in a separate paper bag. Women’s underpants are the garment most likely to contain “transfer from the perpetrator.”10
Julie had changed her clothes before coming to the ED but was wearing the same underpants she had on at the time of the assault. This garment was collected in a paper bag.
The physical exam is conducted in a head-to-toe manner. Each marking found on the victim must be charted on a diagram of the body or the genitalia (see Figure 1). Injuries should be described using the mnemonic TEARS: tissue integrity, ecchymoses, abrasions, redness, and swelling.11 The most common descriptors include abrasions that are tangential or patterned, fingernail markings, contusions, and lacerations.
When the clinician examines the patient’s genitalia and anal area, it is important to report a thorough description of any injuries. The most common area of injury in the female sexual assault victim is a small tear to the posterior fourchette. Visualization can be enhanced by use of toluidine blue dye—but this must be applied before use of a speculum,12 and not until after any photographs of the outer genitalia have been taken.
Photographs should be taken of all injuries, then presented to the police. It is suggested that each injury be photographed from a medium distance, and up close with a ruler or other scale.7
Julie stated that she had been a virgin prior to the assault. She was placed in the lithotomy position, and a careful internal inspection was performed. Gentle retraction of the labia with a good light source allowed adequate visualization. Photographs were taken of an acute laceration of the hymen at the 5:00 position.
PHYSICAL EVIDENCE
Next, evidence is collected from the patient’s body. Fingernails are clipped and saved for possible DNA from the suspect, especially if the victim reports having tried to fight back. The fingernail trimmings from each hand should be packaged separately, with labels.7
Debris is combed from the head hair and pubic hair. This can be significant for confirming details from the victim’s story, such as being attacked and thrown into the mud. Next, the patient’s head hair is collected. When plucking the hair, the examiner must ensure that the root is intact, since the patient’s DNA is contained therein. This can be important for distinguishing the patient’s hair from that of the suspect. Hairs should be chosen from a few different areas of the patient’s head.7
Oral, genital, and anal swabs are collected. For collection of evidence from a female patient, a speculum exam is required.12 The vagina is swabbed with at least four different cotton swabs: one for the cervix, and the other three to collect visualized secretions.10 For each area, a clean, sterile swab should be moistened with distilled water and used to swab lightly, rotating downward. A dry sterile swab is then used to re-swab the area lightly and lift the DNA. Collected swabs should be allowed to dry completely before the packaging is sealed to minimize the risk for contamination by bacterial growth.
Use of a Wood’s lamp can help the examiner detect semen and saliva on the patient’s body.10 However, a recent examination of alternate light sources with appropriate wavelengths has demonstrated improved detection of trace DNA evidence.13
Once the steps in evidence collection have been completed, the patient can be permitted to urinate, shower, brush his or her teeth, and make any necessary phone calls.
Storing and Protecting the Evidence
It is imperative for the NP or PA who completes the kit to maintain the chain of custody—that is, never leaving the evidence unattended until the police collect it. This will eliminate the possibility of tampering or any other reason for the legal system to designate the evidence as inadmissible. If it is not feasible for the responsible clinician to guard the evidence, it must be placed under lock and key, with limited availability to others.10
Evidence that cannot be thoroughly dried during the examination (eg, tampon, condom, tissues) should be collected in a sterile specimen cup and sent to the crime lab immediately.7 Otherwise, if such a sample is packaged and left to sit, the risk increases for any DNA to become contaminated by bacterial growth.
To verify that the chain of custody was maintained, several items must be signed or initialed by both the provider and the law enforcement officer who receives the kit:
•
The evidence log sheet. This should be included in the original kit (see Figure 2 for a sample). It should be removed from the kit, completed, and affixed to the outside of the kit before the kit is sealed. A copy of this log should be kept attached to the patient chart.
•
The evidence kit itself. The lid bears a form to be completed by the practitioner.
•
The components of evidence other than the kit (ie, clothing bags, sterile specimen cups containing collected specimens). These bear labels, preprinted with the patient’s name, date of birth, and medical record number, which are signed by the practitioner.
TREATMENT AND
PROPHYLAXIS
The likelihood for a sexual assault victim to have contracted an STI is 26.3%.3 Current recommendations from the CDC,4 including postexposure vaccination against hepatitis B, must be followed for prevention of and treatment for STI. Prophylactic treatment for gonorrhea, chlamydia, and trichomonas should be offered to all victims of sexual assault, as cultures are not taken until patient follow-up at the primary care provider’s office or the county health department.4 Prophylactic treatment for hepatitis B or HIV may be discussed with the patient; he or she must be fully informed about the rigorous follow-up treatment regimens required, as well as the associated adverse effects.
According to the CDC,4 baseline test results for HIV, hepatitis B, and syphilis may be negative, but antibodies can develop over time; thus, reexamination with re-testing should be performed at three months, six months, and 12 months postassault.
Progestin-only emergency contraceptive tablets should be offered through 72 hours postassault to all female sexual assault victims with a negative pregnancy test result in the ED.14
Julie was treated with intramuscular ceftriaxone 250 mg for prevention of gonorrhea, azithromycin 1 g by mouth for prevention of chlamydia, and progestin for pregnancy prevention. She had undergone the hepatitis B vaccination series as a child and had a positive titer drawn before the current school year. Julie declined prophylaxis for HIV because she felt the suspect was at low risk for HIV; however, she was encouraged to undergo HIV testing at her follow-up visit at the local health department.
FOLLOW-UP
Follow-up counseling is a vital component of care for the victim of sexual assault. The police will arrange to ensure the patient’s safety at home before he or she is discharged. A victim of sexual assault should never be discharged if suicidal ideation is evident; in this case, a psychiatry consult must be arranged. For survivors of sexual assault who reside in remote or rural areas, treatment via videoconferencing-based technology has been shown to reduce measures of depression and posttraumatic stress.15
Information regarding rape crisis services should be provided before patients are discharged; the advocate present during the exam should be familiar with services offered in the area. These centers offer emotional support, helpful medical and legal information, and post-rape counseling.7
CONCLUSION
Although the ED is ordinarily the first medical entry point for a sexual assault victim, clinicians in other settings, too, must be prepared to offer medical care to these patients and collect forensic evidence appropriately. Comprehensive care of a sexual assault victim must be completed in a timely and sensitive manner, with documentation that can withstand the exacting requirements of the court system.
REFERENCES
1. Sampsel K, Szobota L, Joyce D, et al. The impact of a sexual assault/domestic violence program on ED care. J Emerg Nurs. 2009;35(4): 282-289.
2. Patel A, Panchal H, Piotrowski ZH, Patel D. Comprehensive medical care for victims of sexual assault: a survey of Illinois hospital emergency departments. Contraception. 2008;77(6):426-430.
3. Straight JD, Heaton PC. Emergency department care for victims of sexual offense. Am J Health Syst Pharm. 2007;64(17):1845-1850.
4. CDC. Sexually transmitted disease treatment guidelines, 2010: sexual assault and STDs (2010). www.cdc.gov/std/treat ment/2010/sexual-assault.htm. Accessed November 26, 2012.
5. Stermac L, Dunlap H, Bainbridge D. Sexual assault services delivered by SANEs. J Forensic Nurs. 2005;1(3):124-128.
6. Plichta SB, Clements PT, Houseman C. Why SANEs matter: models of care for sexual violence victims in the emergency department.
J Forensic Nurs. 2007;3(1):15-23.
7. National Criminal Justice Reference Services. A national protocol for sexual assault medical forensic examinations: adults/adolescents (2004). www.ncjrs.gov/pdffiles1/ovw/206554.pdf. Accessed November 26, 2012.
8. Burgess AW, Hazelwood RR. Victim care services and the Comprehensive Sexual Assault Assessment Tool (CSAAT). In: Hazelwood RR, Burgess AW, eds. Practical Aspects of Rape Investigation: A Multidisciplinary Approach. 4th ed. Boca Raton, FL: CRC Press; 2009:47-68.
9. Burg A, Kahn R, Welch K. DNA testing of sexual assault evidence: the laboratory perspective. J Forensic Nurs. 2011;7(3):145-152.
10. Brown K. Forensic examination of sexual assault victims. In: Hazelwood RR, Burgess AW, eds. Practical Aspects of Rape Investigation: A Multidisciplinary Approach. 4th ed. Boca Raton, FL: CRC Press; 2009:365-381.
11. Slaughter L, Brown CR, Crowley S, Peck R. Patterns of genital injury in female sexual assault victims. Am J Obstet Gynecol. 1997; 176(3):609-616.
12. Jones JS, Dunnuck C, Rossman L, et al. Significance of toluidine blue positive findings after speculum examination for sexual assault. Am J Emerg Med. 2004;22(3):201-203.
13. Eldredge K, Huggins E, Pugh LC. Alternate light sources in sexual assault examinations: an evidence-based practice project. J Forensic Nurs. 2012;8(1):39-44.
14. Ledray LE. Evidence collection and care of the sexual assault survivor: the SANE/SART response (2001). www.mincava.umn.edu/documents/commissioned/2forensicevidence/2forensicevidence.pdf. Accessed November 26, 2012.
15. Hassija C, Gray MJ. The effectiveness and feasibility of videoconferencing technology to provide evidence-based treatment to rural domestic violence and sexual assault populations. Telemed J E Health. 2011;17(4):30
Each year in the United States, between 300,000 and 700,000 adult women are estimated to experience sexual assault, with 40,000 of such victims typically seeking treatment in an emergency department (ED).1 In a survey of hospital EDs published in 2008, only 9.6% of the 117 responding hospitals provided to presenting victims of sexual assault all of the following elements of comprehensive medical care management2:
• Acute medical care
•
History and physical examination
•
Acute and long-term rape crisis counseling
•
Prophylactic and therapeutic management for HIV or other sexually transmitted infection (STI)
•
Provision of emergency contraception, with appropriate counseling.2
Specific data from a similar survey included these findings: appropriate, CDC-recommended prophylaxis against STI prescribed in only 6.7% of cases, HIV serology testing in only 13%, and information about follow-up care given to only 31% of patients. Nearly 80% of sexual assault victims treated in the responding hospital EDs received less than optimal care.3,4
In the ED, where victims of sexual assault are most likely to be evaluated, the responsibilities involved in managing the department may hamper emergency physicians’ ability to provide the detailed, time-consuming, one-on-one care such patients require; often, this care is entrusted to an NP or an RN.1 Clinicians in this setting, as well as those who practice in student health, primary care, and women’s health, must be competent in assessing and treating the injuries assaulted patients have sustained, providing STI prophylaxis and pregnancy prevention, collecting forensic evidence in order to facilitate prosecution of the perpetrator, and providing appropriate referrals to promote physical and emotional recovery through counseling and other follow-up care—in short, meeting these patients’ medical, legal, and psychosocial needs.5,6 (See “Specialized Training, a Team Response.”5-7)
DEFINITIONS: RAPE AND SEXUAL ASSAULT
The definition of rape varies from state to state, but three criteria are typically present:
•
Sexual penetration of the victim’s vagina, mouth, or rectum
•
Absence of consent from the victim
• The use or threat of force.8
Sexual assault is a less restrictive term, referring to the sexual contact of one person with another without appropriate consent. Specified manifestations vary state by state but typically include child sexual assault, incest, marital rape, and other forced sexual acts.7
“Julie,” 18, presents to the ED, accompanied by a female friend, after being sexually assaulted by a male student from the college Julie attends. Earlier that evening, Julie was drinking alcohol at a party in the suspect’s apartment. While everyone else was dancing, he invited Julie to his room. She admits that she was willing to “fool around” with him, but when he asked to have intercourse, she said “no.” The suspect insisted that she “wanted it” and proceeded to engage in unprotected intercourse with her. Julie is distressed because she was a virgin until the encounter and had not been using any form of birth control.
On presentation of a victim of sexual assault, local law enforcement and an advocate from the local rape crisis center should be promptly notified; however, the patient’s permission must be obtained before the police department is contacted. A victim may not want to report a sexual assault to the police for a number of reasons, including:
•
A belief that the police are limited in their ability to intervene effectively
•
A perception that victims of sexual assault are often considered at fault
•
Fear that the assailant may assault the victim again
•
Misplaced feelings of fear and shame.5
The NP or PA who performs the initial examination should make every effort to interview the patient while both law enforcement and the advocate are present so that the victim is not required to describe and relive the traumatic situation repeatedly. The advocate is present to support the victim throughout the ED or office visit and evidence collection process; and to provide referrals for follow-up care.
The clinician must strive to remain objective during the evaluation and evidence collection process. For example, the detection of another person’s DNA on the body of the patient is not proof, in and of itself, of that person’s guilt, but only the presence of his or her DNA.9
HISTORY AND PHYSICAL
A thorough medical history and assessment should always be completed, either before or after the forensic examination, depending on the patient’s condition.
Evidence collection is begun by obtaining consent and interviewing the patient. The patient’s account of the assault will guide the practitioner to specific areas of the body where evidence may be found (for example, the case patient said the suspect had kissed her neck, which was swabbed to corroborate her story). Whatever the patient’s age, the presence of a family member or friend is not recommended during the interview, as this could cause the victim to withhold information, and any emotional reaction may be a distraction for the patient. Additionally, having a family member or friend present during the interview process puts that individual at risk for subpoena and court appearance.7
The interview should be concise, with the patient’s account of the assault recorded in some way so that he or she can later be quoted as closely as possible. The clinician should avoid using medical or legal terms or abbreviations, or altering the patient’s own words.
Before the physical examination is begun, the patient’s clothing must be collected and each piece packaged in a separate paper bag. Women’s underpants are the garment most likely to contain “transfer from the perpetrator.”10
Julie had changed her clothes before coming to the ED but was wearing the same underpants she had on at the time of the assault. This garment was collected in a paper bag.
The physical exam is conducted in a head-to-toe manner. Each marking found on the victim must be charted on a diagram of the body or the genitalia (see Figure 1). Injuries should be described using the mnemonic TEARS: tissue integrity, ecchymoses, abrasions, redness, and swelling.11 The most common descriptors include abrasions that are tangential or patterned, fingernail markings, contusions, and lacerations.
When the clinician examines the patient’s genitalia and anal area, it is important to report a thorough description of any injuries. The most common area of injury in the female sexual assault victim is a small tear to the posterior fourchette. Visualization can be enhanced by use of toluidine blue dye—but this must be applied before use of a speculum,12 and not until after any photographs of the outer genitalia have been taken.
Photographs should be taken of all injuries, then presented to the police. It is suggested that each injury be photographed from a medium distance, and up close with a ruler or other scale.7
Julie stated that she had been a virgin prior to the assault. She was placed in the lithotomy position, and a careful internal inspection was performed. Gentle retraction of the labia with a good light source allowed adequate visualization. Photographs were taken of an acute laceration of the hymen at the 5:00 position.
PHYSICAL EVIDENCE
Next, evidence is collected from the patient’s body. Fingernails are clipped and saved for possible DNA from the suspect, especially if the victim reports having tried to fight back. The fingernail trimmings from each hand should be packaged separately, with labels.7
Debris is combed from the head hair and pubic hair. This can be significant for confirming details from the victim’s story, such as being attacked and thrown into the mud. Next, the patient’s head hair is collected. When plucking the hair, the examiner must ensure that the root is intact, since the patient’s DNA is contained therein. This can be important for distinguishing the patient’s hair from that of the suspect. Hairs should be chosen from a few different areas of the patient’s head.7
Oral, genital, and anal swabs are collected. For collection of evidence from a female patient, a speculum exam is required.12 The vagina is swabbed with at least four different cotton swabs: one for the cervix, and the other three to collect visualized secretions.10 For each area, a clean, sterile swab should be moistened with distilled water and used to swab lightly, rotating downward. A dry sterile swab is then used to re-swab the area lightly and lift the DNA. Collected swabs should be allowed to dry completely before the packaging is sealed to minimize the risk for contamination by bacterial growth.
Use of a Wood’s lamp can help the examiner detect semen and saliva on the patient’s body.10 However, a recent examination of alternate light sources with appropriate wavelengths has demonstrated improved detection of trace DNA evidence.13
Once the steps in evidence collection have been completed, the patient can be permitted to urinate, shower, brush his or her teeth, and make any necessary phone calls.
Storing and Protecting the Evidence
It is imperative for the NP or PA who completes the kit to maintain the chain of custody—that is, never leaving the evidence unattended until the police collect it. This will eliminate the possibility of tampering or any other reason for the legal system to designate the evidence as inadmissible. If it is not feasible for the responsible clinician to guard the evidence, it must be placed under lock and key, with limited availability to others.10
Evidence that cannot be thoroughly dried during the examination (eg, tampon, condom, tissues) should be collected in a sterile specimen cup and sent to the crime lab immediately.7 Otherwise, if such a sample is packaged and left to sit, the risk increases for any DNA to become contaminated by bacterial growth.
To verify that the chain of custody was maintained, several items must be signed or initialed by both the provider and the law enforcement officer who receives the kit:
•
The evidence log sheet. This should be included in the original kit (see Figure 2 for a sample). It should be removed from the kit, completed, and affixed to the outside of the kit before the kit is sealed. A copy of this log should be kept attached to the patient chart.
•
The evidence kit itself. The lid bears a form to be completed by the practitioner.
•
The components of evidence other than the kit (ie, clothing bags, sterile specimen cups containing collected specimens). These bear labels, preprinted with the patient’s name, date of birth, and medical record number, which are signed by the practitioner.
TREATMENT AND
PROPHYLAXIS
The likelihood for a sexual assault victim to have contracted an STI is 26.3%.3 Current recommendations from the CDC,4 including postexposure vaccination against hepatitis B, must be followed for prevention of and treatment for STI. Prophylactic treatment for gonorrhea, chlamydia, and trichomonas should be offered to all victims of sexual assault, as cultures are not taken until patient follow-up at the primary care provider’s office or the county health department.4 Prophylactic treatment for hepatitis B or HIV may be discussed with the patient; he or she must be fully informed about the rigorous follow-up treatment regimens required, as well as the associated adverse effects.
According to the CDC,4 baseline test results for HIV, hepatitis B, and syphilis may be negative, but antibodies can develop over time; thus, reexamination with re-testing should be performed at three months, six months, and 12 months postassault.
Progestin-only emergency contraceptive tablets should be offered through 72 hours postassault to all female sexual assault victims with a negative pregnancy test result in the ED.14
Julie was treated with intramuscular ceftriaxone 250 mg for prevention of gonorrhea, azithromycin 1 g by mouth for prevention of chlamydia, and progestin for pregnancy prevention. She had undergone the hepatitis B vaccination series as a child and had a positive titer drawn before the current school year. Julie declined prophylaxis for HIV because she felt the suspect was at low risk for HIV; however, she was encouraged to undergo HIV testing at her follow-up visit at the local health department.
FOLLOW-UP
Follow-up counseling is a vital component of care for the victim of sexual assault. The police will arrange to ensure the patient’s safety at home before he or she is discharged. A victim of sexual assault should never be discharged if suicidal ideation is evident; in this case, a psychiatry consult must be arranged. For survivors of sexual assault who reside in remote or rural areas, treatment via videoconferencing-based technology has been shown to reduce measures of depression and posttraumatic stress.15
Information regarding rape crisis services should be provided before patients are discharged; the advocate present during the exam should be familiar with services offered in the area. These centers offer emotional support, helpful medical and legal information, and post-rape counseling.7
CONCLUSION
Although the ED is ordinarily the first medical entry point for a sexual assault victim, clinicians in other settings, too, must be prepared to offer medical care to these patients and collect forensic evidence appropriately. Comprehensive care of a sexual assault victim must be completed in a timely and sensitive manner, with documentation that can withstand the exacting requirements of the court system.
REFERENCES
1. Sampsel K, Szobota L, Joyce D, et al. The impact of a sexual assault/domestic violence program on ED care. J Emerg Nurs. 2009;35(4): 282-289.
2. Patel A, Panchal H, Piotrowski ZH, Patel D. Comprehensive medical care for victims of sexual assault: a survey of Illinois hospital emergency departments. Contraception. 2008;77(6):426-430.
3. Straight JD, Heaton PC. Emergency department care for victims of sexual offense. Am J Health Syst Pharm. 2007;64(17):1845-1850.
4. CDC. Sexually transmitted disease treatment guidelines, 2010: sexual assault and STDs (2010). www.cdc.gov/std/treat ment/2010/sexual-assault.htm. Accessed November 26, 2012.
5. Stermac L, Dunlap H, Bainbridge D. Sexual assault services delivered by SANEs. J Forensic Nurs. 2005;1(3):124-128.
6. Plichta SB, Clements PT, Houseman C. Why SANEs matter: models of care for sexual violence victims in the emergency department.
J Forensic Nurs. 2007;3(1):15-23.
7. National Criminal Justice Reference Services. A national protocol for sexual assault medical forensic examinations: adults/adolescents (2004). www.ncjrs.gov/pdffiles1/ovw/206554.pdf. Accessed November 26, 2012.
8. Burgess AW, Hazelwood RR. Victim care services and the Comprehensive Sexual Assault Assessment Tool (CSAAT). In: Hazelwood RR, Burgess AW, eds. Practical Aspects of Rape Investigation: A Multidisciplinary Approach. 4th ed. Boca Raton, FL: CRC Press; 2009:47-68.
9. Burg A, Kahn R, Welch K. DNA testing of sexual assault evidence: the laboratory perspective. J Forensic Nurs. 2011;7(3):145-152.
10. Brown K. Forensic examination of sexual assault victims. In: Hazelwood RR, Burgess AW, eds. Practical Aspects of Rape Investigation: A Multidisciplinary Approach. 4th ed. Boca Raton, FL: CRC Press; 2009:365-381.
11. Slaughter L, Brown CR, Crowley S, Peck R. Patterns of genital injury in female sexual assault victims. Am J Obstet Gynecol. 1997; 176(3):609-616.
12. Jones JS, Dunnuck C, Rossman L, et al. Significance of toluidine blue positive findings after speculum examination for sexual assault. Am J Emerg Med. 2004;22(3):201-203.
13. Eldredge K, Huggins E, Pugh LC. Alternate light sources in sexual assault examinations: an evidence-based practice project. J Forensic Nurs. 2012;8(1):39-44.
14. Ledray LE. Evidence collection and care of the sexual assault survivor: the SANE/SART response (2001). www.mincava.umn.edu/documents/commissioned/2forensicevidence/2forensicevidence.pdf. Accessed November 26, 2012.
15. Hassija C, Gray MJ. The effectiveness and feasibility of videoconferencing technology to provide evidence-based treatment to rural domestic violence and sexual assault populations. Telemed J E Health. 2011;17(4):30
Each year in the United States, between 300,000 and 700,000 adult women are estimated to experience sexual assault, with 40,000 of such victims typically seeking treatment in an emergency department (ED).1 In a survey of hospital EDs published in 2008, only 9.6% of the 117 responding hospitals provided to presenting victims of sexual assault all of the following elements of comprehensive medical care management2:
• Acute medical care
•
History and physical examination
•
Acute and long-term rape crisis counseling
•
Prophylactic and therapeutic management for HIV or other sexually transmitted infection (STI)
•
Provision of emergency contraception, with appropriate counseling.2
Specific data from a similar survey included these findings: appropriate, CDC-recommended prophylaxis against STI prescribed in only 6.7% of cases, HIV serology testing in only 13%, and information about follow-up care given to only 31% of patients. Nearly 80% of sexual assault victims treated in the responding hospital EDs received less than optimal care.3,4
In the ED, where victims of sexual assault are most likely to be evaluated, the responsibilities involved in managing the department may hamper emergency physicians’ ability to provide the detailed, time-consuming, one-on-one care such patients require; often, this care is entrusted to an NP or an RN.1 Clinicians in this setting, as well as those who practice in student health, primary care, and women’s health, must be competent in assessing and treating the injuries assaulted patients have sustained, providing STI prophylaxis and pregnancy prevention, collecting forensic evidence in order to facilitate prosecution of the perpetrator, and providing appropriate referrals to promote physical and emotional recovery through counseling and other follow-up care—in short, meeting these patients’ medical, legal, and psychosocial needs.5,6 (See “Specialized Training, a Team Response.”5-7)
DEFINITIONS: RAPE AND SEXUAL ASSAULT
The definition of rape varies from state to state, but three criteria are typically present:
•
Sexual penetration of the victim’s vagina, mouth, or rectum
•
Absence of consent from the victim
• The use or threat of force.8
Sexual assault is a less restrictive term, referring to the sexual contact of one person with another without appropriate consent. Specified manifestations vary state by state but typically include child sexual assault, incest, marital rape, and other forced sexual acts.7
“Julie,” 18, presents to the ED, accompanied by a female friend, after being sexually assaulted by a male student from the college Julie attends. Earlier that evening, Julie was drinking alcohol at a party in the suspect’s apartment. While everyone else was dancing, he invited Julie to his room. She admits that she was willing to “fool around” with him, but when he asked to have intercourse, she said “no.” The suspect insisted that she “wanted it” and proceeded to engage in unprotected intercourse with her. Julie is distressed because she was a virgin until the encounter and had not been using any form of birth control.
On presentation of a victim of sexual assault, local law enforcement and an advocate from the local rape crisis center should be promptly notified; however, the patient’s permission must be obtained before the police department is contacted. A victim may not want to report a sexual assault to the police for a number of reasons, including:
•
A belief that the police are limited in their ability to intervene effectively
•
A perception that victims of sexual assault are often considered at fault
•
Fear that the assailant may assault the victim again
•
Misplaced feelings of fear and shame.5
The NP or PA who performs the initial examination should make every effort to interview the patient while both law enforcement and the advocate are present so that the victim is not required to describe and relive the traumatic situation repeatedly. The advocate is present to support the victim throughout the ED or office visit and evidence collection process; and to provide referrals for follow-up care.
The clinician must strive to remain objective during the evaluation and evidence collection process. For example, the detection of another person’s DNA on the body of the patient is not proof, in and of itself, of that person’s guilt, but only the presence of his or her DNA.9
HISTORY AND PHYSICAL
A thorough medical history and assessment should always be completed, either before or after the forensic examination, depending on the patient’s condition.
Evidence collection is begun by obtaining consent and interviewing the patient. The patient’s account of the assault will guide the practitioner to specific areas of the body where evidence may be found (for example, the case patient said the suspect had kissed her neck, which was swabbed to corroborate her story). Whatever the patient’s age, the presence of a family member or friend is not recommended during the interview, as this could cause the victim to withhold information, and any emotional reaction may be a distraction for the patient. Additionally, having a family member or friend present during the interview process puts that individual at risk for subpoena and court appearance.7
The interview should be concise, with the patient’s account of the assault recorded in some way so that he or she can later be quoted as closely as possible. The clinician should avoid using medical or legal terms or abbreviations, or altering the patient’s own words.
Before the physical examination is begun, the patient’s clothing must be collected and each piece packaged in a separate paper bag. Women’s underpants are the garment most likely to contain “transfer from the perpetrator.”10
Julie had changed her clothes before coming to the ED but was wearing the same underpants she had on at the time of the assault. This garment was collected in a paper bag.
The physical exam is conducted in a head-to-toe manner. Each marking found on the victim must be charted on a diagram of the body or the genitalia (see Figure 1). Injuries should be described using the mnemonic TEARS: tissue integrity, ecchymoses, abrasions, redness, and swelling.11 The most common descriptors include abrasions that are tangential or patterned, fingernail markings, contusions, and lacerations.
When the clinician examines the patient’s genitalia and anal area, it is important to report a thorough description of any injuries. The most common area of injury in the female sexual assault victim is a small tear to the posterior fourchette. Visualization can be enhanced by use of toluidine blue dye—but this must be applied before use of a speculum,12 and not until after any photographs of the outer genitalia have been taken.
Photographs should be taken of all injuries, then presented to the police. It is suggested that each injury be photographed from a medium distance, and up close with a ruler or other scale.7
Julie stated that she had been a virgin prior to the assault. She was placed in the lithotomy position, and a careful internal inspection was performed. Gentle retraction of the labia with a good light source allowed adequate visualization. Photographs were taken of an acute laceration of the hymen at the 5:00 position.
PHYSICAL EVIDENCE
Next, evidence is collected from the patient’s body. Fingernails are clipped and saved for possible DNA from the suspect, especially if the victim reports having tried to fight back. The fingernail trimmings from each hand should be packaged separately, with labels.7
Debris is combed from the head hair and pubic hair. This can be significant for confirming details from the victim’s story, such as being attacked and thrown into the mud. Next, the patient’s head hair is collected. When plucking the hair, the examiner must ensure that the root is intact, since the patient’s DNA is contained therein. This can be important for distinguishing the patient’s hair from that of the suspect. Hairs should be chosen from a few different areas of the patient’s head.7
Oral, genital, and anal swabs are collected. For collection of evidence from a female patient, a speculum exam is required.12 The vagina is swabbed with at least four different cotton swabs: one for the cervix, and the other three to collect visualized secretions.10 For each area, a clean, sterile swab should be moistened with distilled water and used to swab lightly, rotating downward. A dry sterile swab is then used to re-swab the area lightly and lift the DNA. Collected swabs should be allowed to dry completely before the packaging is sealed to minimize the risk for contamination by bacterial growth.
Use of a Wood’s lamp can help the examiner detect semen and saliva on the patient’s body.10 However, a recent examination of alternate light sources with appropriate wavelengths has demonstrated improved detection of trace DNA evidence.13
Once the steps in evidence collection have been completed, the patient can be permitted to urinate, shower, brush his or her teeth, and make any necessary phone calls.
Storing and Protecting the Evidence
It is imperative for the NP or PA who completes the kit to maintain the chain of custody—that is, never leaving the evidence unattended until the police collect it. This will eliminate the possibility of tampering or any other reason for the legal system to designate the evidence as inadmissible. If it is not feasible for the responsible clinician to guard the evidence, it must be placed under lock and key, with limited availability to others.10
Evidence that cannot be thoroughly dried during the examination (eg, tampon, condom, tissues) should be collected in a sterile specimen cup and sent to the crime lab immediately.7 Otherwise, if such a sample is packaged and left to sit, the risk increases for any DNA to become contaminated by bacterial growth.
To verify that the chain of custody was maintained, several items must be signed or initialed by both the provider and the law enforcement officer who receives the kit:
•
The evidence log sheet. This should be included in the original kit (see Figure 2 for a sample). It should be removed from the kit, completed, and affixed to the outside of the kit before the kit is sealed. A copy of this log should be kept attached to the patient chart.
•
The evidence kit itself. The lid bears a form to be completed by the practitioner.
•
The components of evidence other than the kit (ie, clothing bags, sterile specimen cups containing collected specimens). These bear labels, preprinted with the patient’s name, date of birth, and medical record number, which are signed by the practitioner.
TREATMENT AND
PROPHYLAXIS
The likelihood for a sexual assault victim to have contracted an STI is 26.3%.3 Current recommendations from the CDC,4 including postexposure vaccination against hepatitis B, must be followed for prevention of and treatment for STI. Prophylactic treatment for gonorrhea, chlamydia, and trichomonas should be offered to all victims of sexual assault, as cultures are not taken until patient follow-up at the primary care provider’s office or the county health department.4 Prophylactic treatment for hepatitis B or HIV may be discussed with the patient; he or she must be fully informed about the rigorous follow-up treatment regimens required, as well as the associated adverse effects.
According to the CDC,4 baseline test results for HIV, hepatitis B, and syphilis may be negative, but antibodies can develop over time; thus, reexamination with re-testing should be performed at three months, six months, and 12 months postassault.
Progestin-only emergency contraceptive tablets should be offered through 72 hours postassault to all female sexual assault victims with a negative pregnancy test result in the ED.14
Julie was treated with intramuscular ceftriaxone 250 mg for prevention of gonorrhea, azithromycin 1 g by mouth for prevention of chlamydia, and progestin for pregnancy prevention. She had undergone the hepatitis B vaccination series as a child and had a positive titer drawn before the current school year. Julie declined prophylaxis for HIV because she felt the suspect was at low risk for HIV; however, she was encouraged to undergo HIV testing at her follow-up visit at the local health department.
FOLLOW-UP
Follow-up counseling is a vital component of care for the victim of sexual assault. The police will arrange to ensure the patient’s safety at home before he or she is discharged. A victim of sexual assault should never be discharged if suicidal ideation is evident; in this case, a psychiatry consult must be arranged. For survivors of sexual assault who reside in remote or rural areas, treatment via videoconferencing-based technology has been shown to reduce measures of depression and posttraumatic stress.15
Information regarding rape crisis services should be provided before patients are discharged; the advocate present during the exam should be familiar with services offered in the area. These centers offer emotional support, helpful medical and legal information, and post-rape counseling.7
CONCLUSION
Although the ED is ordinarily the first medical entry point for a sexual assault victim, clinicians in other settings, too, must be prepared to offer medical care to these patients and collect forensic evidence appropriately. Comprehensive care of a sexual assault victim must be completed in a timely and sensitive manner, with documentation that can withstand the exacting requirements of the court system.
REFERENCES
1. Sampsel K, Szobota L, Joyce D, et al. The impact of a sexual assault/domestic violence program on ED care. J Emerg Nurs. 2009;35(4): 282-289.
2. Patel A, Panchal H, Piotrowski ZH, Patel D. Comprehensive medical care for victims of sexual assault: a survey of Illinois hospital emergency departments. Contraception. 2008;77(6):426-430.
3. Straight JD, Heaton PC. Emergency department care for victims of sexual offense. Am J Health Syst Pharm. 2007;64(17):1845-1850.
4. CDC. Sexually transmitted disease treatment guidelines, 2010: sexual assault and STDs (2010). www.cdc.gov/std/treat ment/2010/sexual-assault.htm. Accessed November 26, 2012.
5. Stermac L, Dunlap H, Bainbridge D. Sexual assault services delivered by SANEs. J Forensic Nurs. 2005;1(3):124-128.
6. Plichta SB, Clements PT, Houseman C. Why SANEs matter: models of care for sexual violence victims in the emergency department.
J Forensic Nurs. 2007;3(1):15-23.
7. National Criminal Justice Reference Services. A national protocol for sexual assault medical forensic examinations: adults/adolescents (2004). www.ncjrs.gov/pdffiles1/ovw/206554.pdf. Accessed November 26, 2012.
8. Burgess AW, Hazelwood RR. Victim care services and the Comprehensive Sexual Assault Assessment Tool (CSAAT). In: Hazelwood RR, Burgess AW, eds. Practical Aspects of Rape Investigation: A Multidisciplinary Approach. 4th ed. Boca Raton, FL: CRC Press; 2009:47-68.
9. Burg A, Kahn R, Welch K. DNA testing of sexual assault evidence: the laboratory perspective. J Forensic Nurs. 2011;7(3):145-152.
10. Brown K. Forensic examination of sexual assault victims. In: Hazelwood RR, Burgess AW, eds. Practical Aspects of Rape Investigation: A Multidisciplinary Approach. 4th ed. Boca Raton, FL: CRC Press; 2009:365-381.
11. Slaughter L, Brown CR, Crowley S, Peck R. Patterns of genital injury in female sexual assault victims. Am J Obstet Gynecol. 1997; 176(3):609-616.
12. Jones JS, Dunnuck C, Rossman L, et al. Significance of toluidine blue positive findings after speculum examination for sexual assault. Am J Emerg Med. 2004;22(3):201-203.
13. Eldredge K, Huggins E, Pugh LC. Alternate light sources in sexual assault examinations: an evidence-based practice project. J Forensic Nurs. 2012;8(1):39-44.
14. Ledray LE. Evidence collection and care of the sexual assault survivor: the SANE/SART response (2001). www.mincava.umn.edu/documents/commissioned/2forensicevidence/2forensicevidence.pdf. Accessed November 26, 2012.
15. Hassija C, Gray MJ. The effectiveness and feasibility of videoconferencing technology to provide evidence-based treatment to rural domestic violence and sexual assault populations. Telemed J E Health. 2011;17(4):30
Grand Rounds: Woman, 38, With Pulseless Electrical Activity
On an autumn day, a 38-year-old woman with a history of asthma presented to the emergency department (ED) with the chief complaint of shortness of breath (SOB). The patient described her SOB as sudden in onset and not relieved by use of her albuterol inhaler; hence the ED visit.
She denied any chest pain, palpitations, dizziness, orthopnea, upper respiratory tract infection, cough, wheezing, fever or chills, headache, vision changes, body aches, sick contacts, or pets at home. She said she uses her albuterol inhaler as needed, and that she had used it that day for the first time in “a few months.” She denied any history of intubation or steroid use. Additionally, she had not been seen by a primary care provider in years.
The woman, a native of Ghana, had been living in the United States for many years. She denied any recent travel or exposure to toxic chemicals; any use of tobacco, alcohol, or illicit drugs; or any history of sexually transmitted disease.
The patient was afebrile (temperature, 98.6°F), with a respiratory rate of 20 breaths/min; blood pressure, 144/69 mm Hg; and ventricular rate, 125 beats/min. On physical examination, her extraocular movements were intact; pupils were equal, round, reactive to light and accommodation; and sclera were nonicteric. The patient’s head was normocephalic and atraumatic, and the neck was supple with normal range of motion and no jugular venous distension or lymphadenopathy. Her mucous membranes were moist with no pharyngeal erythema or exudates. Cardiovascular examination, including ECG, revealed tachycardia but no murmurs or gallops.
While being evaluated in the ED, the patient became tachypneic and began to experience respiratory distress. She was intubated for airway protection, at which time she developed pulseless electrical activity (PEA), with 30 beats/min. She responded to atropine and epinephrine injections. A repeat ECG showed sinus tachycardia and right atrial enlargement with right-axis deviation. Chest x-ray (see Figure 1) showed no consolidation, pleural effusion, or pneumothorax.
Results from the patient’s lab work are shown in the table, above. Negative results were reported for a urine pregnancy test.
Since there was no clear etiology for the patient’s PEA, she underwent pan-culturing, with the following tests ordered: HIV antibody testing, immunovirology for influenza A and B viruses, and urine toxicology. Doppler ultrasound of the bilateral lower extremities was also ordered, in addition to chest CT and transthoracic and transesophageal echocardiography (TTE and TEE, respectively). The patient was intubated and transferred to the medical ICU for further management.
The differential diagnosis included cardiac tamponade, acute MI, acute pulmonary embolus (PE), tension pneumothorax, hypovolemia, and asthma exacerbated by viral or bacterial infection.1,2 Although the case patient presented with PEA, she did not have the presenting signs of cardiac tamponade known as Beck’s triad: hypotension, jugular venous distension, and muffled heart sounds.3 TTE showed an ejection fraction of 65% and grade 2 diastolic dysfunction but no pericardial effusions (which accumulate rapidly in the patient with cardiac tamponade, resulting from fluid buildup in the pericardial layers),4 and TEE showed no atrial thrombi (which can masquerade as cardiac tamponade5). The patient had no signs of trauma and denied any history of malignancy (both potential causes of cardiac tamponade). Chest x-ray showed normal heart size and no pneumothorax, consolidations, or pleural effusions.4,6-8 Thus, the diagnosis of cardiac tamponade was ruled out.
Common presenting symptoms of acute MI include sudden-onset chest pain, SOB, palpitations, dizziness, nausea, and/or vomiting. Women may experience less dramatic symptoms—often little more than SOB and fatigue.9 According to a 2000 consensus document from a joint European Society of Cardiology/American College of Cardiology committee10 in which MI was redefined, the diagnosis of MI relies on a rise in cardiac troponin levels, typical MI symptoms, and changes in ECG showing pathological Q waves or ST elevation or depression. The case patient’s troponin I level was less than 0.02 ng/mL, and ECG did not reveal Q waves or ST-T wave changes; additionally, since the patient had no chest pain, palpitations, diaphoresis, nausea, or vomiting, acute MI was ruled out.
Blood clots capable of blocking the pulmonary artery usually originate in the deep veins of the lower extremities.11 Three main factors, called Virchow’s triad, are known to contribute to these deep vein thromboses (DVTs): venous stasis, endothelial injury, and a hypercoagulability state.12,13 The patient had denied any trauma, recent travel, history of malignancy, or use of tobacco or oral contraceptives, and the result of her urine pregnancy test was negative. Even though the patient presented with tachypnea and acute SOB, with ECG showing right-axis deviation and tachycardia (common presenting signs and symptoms for PE), her chest CT showed no evidence of PE (see Figure 2); additionally, Doppler ultrasound of the bilateral lower extremities revealed no DVTs. Thus, PE was also excluded.
Tension pneumothorax was also ruled out, as chest x-ray showed neither mediastinal shift nor tracheal deviation, and the patient had denied any trauma. Laboratory analyses did not indicate hyponatremia, and the patient’s hemoglobin and hematocrit were satisfactory. She was tachycardic on admission, but her blood pressure was stable. As the patient denied any use of vasodilators or diuretics, hypovolemia was ruled out.
Patients experiencing asthma exacerbation can present with acute SOB, which usually resolves following use of IV steroids, nebulizer therapy, and inhaler treatments. Despite being administered IV methylprednisolone and magnesium sulfate in the ED, the patient experienced PEA and respiratory distress and required intubation for airway protection.
The HIV test was nonreactive, and blood and urine cultures did not show any growth. Results of tests for Legionella urinary antigen and Streptococcus pneumoniae antigen were negative. Sputum culture showed normal flora. Immunovirology testing, however, was positive for both influenza A and B antigens.
Chest X-ray showed no acute pulmonary pathology, nor did chest CT show any central, interlobar, or segmental embolism or mediastinal lymphadenopathy. It was determined that the patient’s acute SOB might represent asthma exacerbation secondary to influenza viral infection. Her PEA was attributed to possible acute pericarditis secondary to concomitant influenza A and B viral infection.
DISCUSSION
Currently, the CDC recognizes three types of influenza virus: A, B, and C.14 Only influenza A viruses are further classified into subtypes, based on the presence of surface proteins called hemagglutinin (HA) or neuraminidase (NA) glycoproteins. Humans can be infected by influenza A subtypes H1N1 and H3N2.14 Influenza B viruses, found mostly in humans, are associated with significant morbidity and mortality.
Influenza A and B viruses are further classified into strains that change with each flu season—thus, the need to update vaccinations against influenza A and B each year. No vaccination exists against influenza C virus, which is known to cause only mild illness in humans.15
In patients with asthma (as in the case patient), chronic bronchitis, or emphysema, infection with the influenza virus can manifest with SOB, in addition to the more common symptoms of fever, sore throat, headache, rhinorrhea, chills, muscle aches, and general discomfort.16 Patients with coronary artery disease, congestive heart failure (CHF), and/or a history of smoking may experience more severe symptoms and increased risk for influenza-associated mortality than do other patients.17,18
Rare cardiac complications of influenza infections are myocarditis and benign acute pericarditis; myocarditis can progress to CHF and death.19,20 A case of acute myopericarditis was reported by Proby et al21 in a patient with acute influenza A infection who developed pericardial effusions, myositis, tamponade, and pleurisy. That patient recovered after pericardiocentesis and administration of inotropic drugs.
In the literature, a few cases of acute pericarditis have been reported in association with administration of the influenza vaccination.22,23
In the case patient, the diagnosis of influenza A and B was made following testing of nasal and nasopharyngeal swabs with an immunochromatographic assay that uses highly sensitive monoclonal antibodies to detect influenza A and B nucleoprotein antigens.24,25
According to reports in the literature, two-thirds of cases of acute pericarditis are caused by infection, most commonly viral infection (including influenza virus, adenovirus, enterovirus, cytomegalovirus, hepatitis B virus, and herpes simplex virus).26,27 Other etiologies for acute pericarditis are autoimmune (accounting for less than 10% of cases) and neoplastic conditions (5% to 7% of cases).26
PATIENT OUTCOME
Consultation with an infectious disease specialist was obtained. The patient was placed under droplet isolation precautions and was started on a nebulizer, IV steroid treatments, and oseltamivir 75 mg by mouth every 12 hours. She was transferred to a medical floor, where she completed a five-day course of oseltamivir.
As a result of timely intervention, the patient was discharged in stable condition on a therapeutic regimen that included albuterol, fluticasone, and salmeterol inhalation, in addition to tapered-dose steroids. She was advised to follow up with her primary care provider and at the pulmonary clinic.
CONCLUSION
To our knowledge, this is the first reported case of acute pericarditis in a patient with concomitant acute infections with influenza A and B. According to conclusions reached in recent literature, further research is needed to explain the pathophysiology of influenza viral infections, associated cardiovascular morbidity and mortality, and the degree to which these can be prevented by influenza vaccination.1,28 Also to be pursued through research is a better understanding of the morbidity and mortality associated with influenza viruses, especially in children and in adults affected by asthma, cardiac disease, and/or obesity.
REFERENCES
1. Finelli L, Chaves SS. Influenza and acute myocardial infarction. J Infect Dis. 2011;203(12):
1701-1704.
2. Steiger HV, Rimbach K, Müller E, Breitkreutz R. Focused emergency echocardiography: lifesaving tool for a 14-year-old girl suffering out-of-hospital pulseless electrical activity arrest because of cardiac tamponade. Eur J Emerg Med. 2009;16(2): 103-105.
3. Goodman A, Perera P, Mailhot T, Mandavia D. The role of bedside ultrasound in the diagnosis of pericardial effusion and cardiac tamponade.
J Emerg Trauma Shock. 2012;5(1):72-75.
4. Restrepo CS, Lemos DF, Lemos JA, et al. Imaging findings in cardiac tamponade with emphasis on CT. Radiographics. 2007;27(6):1595-1610.
5. Papanagnou D, Stone MB. Massive right atrial thrombus masquerading as cardiac tamponade. Acad Emerg Med. 2010;17(2):E11.
6. Saito Y, Donohue A, Attai S, et al. The syndrome of cardiac tamponade with “small” pericardial effusion. Echocardiography. 2008;25(3): 321-327.
7. Lin E, Boire A, Hemmige V, et al. Cardiac tamponade mimicking tuberculous pericarditis as the initial presentation of chronic lymphocytic leukemia in a 58-year-old woman: a case report. J Med Case Rep. 2010;4:246.
8. Meniconi A, Attenhofer Jost CH, Jenni R. How to survive myocardial rupture after myocardial infarction. Heart. 2000;84(5):552.
9. Kosuge M, Kimura K, Ishikawa T, et al. Differences between men and women in terms of clinical features of ST-segment elevation acute myocardial infarction. Circ J. 2006;70(3):222-226.
10. Alpert JS, Thygesen K, Antman E, Bassand JP. Myocardial infarction redefined: a consensus document of the Joint European Society of Cardiology/American College of Cardiology Committee for the redefinition of myocardial infarction. J Am Coll Cardiol. 2000;36(3):959-969.
11. Goldhaber SZ. Deep venous thrombosis and pulmonary thromboembolism. In: Fauci AS, Braunwald E, Kasper DL, et al. Harrison’s Principles of Internal Medicine. 17th ed. New York, NY: McGraw-Hill Medical; 2008:1651–1657.
12. Brooks EG, Trotman W, Wadsworth MP, et al. Valves of the deep venous system: an overlooked risk factor. Blood. 2009;114(6):1276-1279.
13. Kyrle PA, Eichinger S. Is Virchow’s triad complete? Blood. 2009;114(6):1138-1139.
14. CDC. Seasonal influenza (flu): types of influenza viruses (2012). www.cdc.gov/flu/about/viruses/types.htm. Accessed October 24, 2012.
15. CDC. Seasonal influenza (flu)(2012). www.cdc .gov/flu. Accessed October 24, 2012.
16. Eccles R. Understanding the symptoms of the common cold and influenza. Lancet Infect Dis. 2005;5(11):718-725.
17. Angelo SJ, Marshall PS, Chrissoheris MP, Chaves AM. Clinical characteristics associated with poor outcome in patients acutely infected with Influenza A. Conn Med. 2004;68(4):199-205.
18. Murin S, Bilello K. Respiratory tract infections: another reason not to smoke. Cleve Clin J Med. 2005;72(10):916-920.
19. Ray CG, Icenogle TB, Minnich LL, et al. The use of intravenous ribavirin to treat influenza virus–associated acute myocarditis. J Infect Dis. 1989; 159(5):829-836.
20. Fairley CK, Ryan M, Wall PG, Weinberg J. The organism reported to cause infective myocarditis and pericarditis in England and Wales. J Infect. 1996;32(3):223-225.
21. Proby CM, Hackett D, Gupta S, Cox TM. Acute myopericarditis in influenza A infection. Q J Med. 1986;60(233):887-892.
22. Streifler JJ, Dux S, Garty M, Rosenfeld JB. Recurrent pericarditis: a rare complication of influenza vaccination. Br Med J (Clin Res Ed). 1981; 283(6290):526-527.
23. Desson JF, Leprévost M, Vabret F, Davy A. Acute benign pericarditis after anti-influenza vaccination [in French]. Presse Med. 1997;26 (9):415.
24. BinaxNOW® Influenza A&B Test Kit (product instructions). www.diagnosticsdirect2u.com/images/PDF/Binax%20Now%20416-022%20PPI .pdf. Accessed October 24, 2012.
25. 510(k) Substantial Equivalence Determination Decision Summary [BinaxNow® Influenza A & B Test] (2009). www.accessdata.fda.gov/cdrh_docs/reviews/K062109.pdf. Accessed October 24, 2012.
26. Imazio M, Spodick DH, Brucato A, et al. Controversial issues in the management of pericardial diseases. Circulation. 2010;121(7):916-928.
27. Maisch B, Seferovic PM, Ristic AD, et al; Task Force on the Diagnosis and Management of Pericardial Diseases of the European Society of Cardiology. Guidelines on the diagnosis and management of pericardial diseases: executive summary. Eur Heart J. 2004;25(7):587-610.
28. McCullers JA, Hayden FG. Fatal influenza B infections: time to reexamine influenza research priorities. J Infect Dis. 2012;205(6):870-872.
On an autumn day, a 38-year-old woman with a history of asthma presented to the emergency department (ED) with the chief complaint of shortness of breath (SOB). The patient described her SOB as sudden in onset and not relieved by use of her albuterol inhaler; hence the ED visit.
She denied any chest pain, palpitations, dizziness, orthopnea, upper respiratory tract infection, cough, wheezing, fever or chills, headache, vision changes, body aches, sick contacts, or pets at home. She said she uses her albuterol inhaler as needed, and that she had used it that day for the first time in “a few months.” She denied any history of intubation or steroid use. Additionally, she had not been seen by a primary care provider in years.
The woman, a native of Ghana, had been living in the United States for many years. She denied any recent travel or exposure to toxic chemicals; any use of tobacco, alcohol, or illicit drugs; or any history of sexually transmitted disease.
The patient was afebrile (temperature, 98.6°F), with a respiratory rate of 20 breaths/min; blood pressure, 144/69 mm Hg; and ventricular rate, 125 beats/min. On physical examination, her extraocular movements were intact; pupils were equal, round, reactive to light and accommodation; and sclera were nonicteric. The patient’s head was normocephalic and atraumatic, and the neck was supple with normal range of motion and no jugular venous distension or lymphadenopathy. Her mucous membranes were moist with no pharyngeal erythema or exudates. Cardiovascular examination, including ECG, revealed tachycardia but no murmurs or gallops.
While being evaluated in the ED, the patient became tachypneic and began to experience respiratory distress. She was intubated for airway protection, at which time she developed pulseless electrical activity (PEA), with 30 beats/min. She responded to atropine and epinephrine injections. A repeat ECG showed sinus tachycardia and right atrial enlargement with right-axis deviation. Chest x-ray (see Figure 1) showed no consolidation, pleural effusion, or pneumothorax.
Results from the patient’s lab work are shown in the table, above. Negative results were reported for a urine pregnancy test.
Since there was no clear etiology for the patient’s PEA, she underwent pan-culturing, with the following tests ordered: HIV antibody testing, immunovirology for influenza A and B viruses, and urine toxicology. Doppler ultrasound of the bilateral lower extremities was also ordered, in addition to chest CT and transthoracic and transesophageal echocardiography (TTE and TEE, respectively). The patient was intubated and transferred to the medical ICU for further management.
The differential diagnosis included cardiac tamponade, acute MI, acute pulmonary embolus (PE), tension pneumothorax, hypovolemia, and asthma exacerbated by viral or bacterial infection.1,2 Although the case patient presented with PEA, she did not have the presenting signs of cardiac tamponade known as Beck’s triad: hypotension, jugular venous distension, and muffled heart sounds.3 TTE showed an ejection fraction of 65% and grade 2 diastolic dysfunction but no pericardial effusions (which accumulate rapidly in the patient with cardiac tamponade, resulting from fluid buildup in the pericardial layers),4 and TEE showed no atrial thrombi (which can masquerade as cardiac tamponade5). The patient had no signs of trauma and denied any history of malignancy (both potential causes of cardiac tamponade). Chest x-ray showed normal heart size and no pneumothorax, consolidations, or pleural effusions.4,6-8 Thus, the diagnosis of cardiac tamponade was ruled out.
Common presenting symptoms of acute MI include sudden-onset chest pain, SOB, palpitations, dizziness, nausea, and/or vomiting. Women may experience less dramatic symptoms—often little more than SOB and fatigue.9 According to a 2000 consensus document from a joint European Society of Cardiology/American College of Cardiology committee10 in which MI was redefined, the diagnosis of MI relies on a rise in cardiac troponin levels, typical MI symptoms, and changes in ECG showing pathological Q waves or ST elevation or depression. The case patient’s troponin I level was less than 0.02 ng/mL, and ECG did not reveal Q waves or ST-T wave changes; additionally, since the patient had no chest pain, palpitations, diaphoresis, nausea, or vomiting, acute MI was ruled out.
Blood clots capable of blocking the pulmonary artery usually originate in the deep veins of the lower extremities.11 Three main factors, called Virchow’s triad, are known to contribute to these deep vein thromboses (DVTs): venous stasis, endothelial injury, and a hypercoagulability state.12,13 The patient had denied any trauma, recent travel, history of malignancy, or use of tobacco or oral contraceptives, and the result of her urine pregnancy test was negative. Even though the patient presented with tachypnea and acute SOB, with ECG showing right-axis deviation and tachycardia (common presenting signs and symptoms for PE), her chest CT showed no evidence of PE (see Figure 2); additionally, Doppler ultrasound of the bilateral lower extremities revealed no DVTs. Thus, PE was also excluded.
Tension pneumothorax was also ruled out, as chest x-ray showed neither mediastinal shift nor tracheal deviation, and the patient had denied any trauma. Laboratory analyses did not indicate hyponatremia, and the patient’s hemoglobin and hematocrit were satisfactory. She was tachycardic on admission, but her blood pressure was stable. As the patient denied any use of vasodilators or diuretics, hypovolemia was ruled out.
Patients experiencing asthma exacerbation can present with acute SOB, which usually resolves following use of IV steroids, nebulizer therapy, and inhaler treatments. Despite being administered IV methylprednisolone and magnesium sulfate in the ED, the patient experienced PEA and respiratory distress and required intubation for airway protection.
The HIV test was nonreactive, and blood and urine cultures did not show any growth. Results of tests for Legionella urinary antigen and Streptococcus pneumoniae antigen were negative. Sputum culture showed normal flora. Immunovirology testing, however, was positive for both influenza A and B antigens.
Chest X-ray showed no acute pulmonary pathology, nor did chest CT show any central, interlobar, or segmental embolism or mediastinal lymphadenopathy. It was determined that the patient’s acute SOB might represent asthma exacerbation secondary to influenza viral infection. Her PEA was attributed to possible acute pericarditis secondary to concomitant influenza A and B viral infection.
DISCUSSION
Currently, the CDC recognizes three types of influenza virus: A, B, and C.14 Only influenza A viruses are further classified into subtypes, based on the presence of surface proteins called hemagglutinin (HA) or neuraminidase (NA) glycoproteins. Humans can be infected by influenza A subtypes H1N1 and H3N2.14 Influenza B viruses, found mostly in humans, are associated with significant morbidity and mortality.
Influenza A and B viruses are further classified into strains that change with each flu season—thus, the need to update vaccinations against influenza A and B each year. No vaccination exists against influenza C virus, which is known to cause only mild illness in humans.15
In patients with asthma (as in the case patient), chronic bronchitis, or emphysema, infection with the influenza virus can manifest with SOB, in addition to the more common symptoms of fever, sore throat, headache, rhinorrhea, chills, muscle aches, and general discomfort.16 Patients with coronary artery disease, congestive heart failure (CHF), and/or a history of smoking may experience more severe symptoms and increased risk for influenza-associated mortality than do other patients.17,18
Rare cardiac complications of influenza infections are myocarditis and benign acute pericarditis; myocarditis can progress to CHF and death.19,20 A case of acute myopericarditis was reported by Proby et al21 in a patient with acute influenza A infection who developed pericardial effusions, myositis, tamponade, and pleurisy. That patient recovered after pericardiocentesis and administration of inotropic drugs.
In the literature, a few cases of acute pericarditis have been reported in association with administration of the influenza vaccination.22,23
In the case patient, the diagnosis of influenza A and B was made following testing of nasal and nasopharyngeal swabs with an immunochromatographic assay that uses highly sensitive monoclonal antibodies to detect influenza A and B nucleoprotein antigens.24,25
According to reports in the literature, two-thirds of cases of acute pericarditis are caused by infection, most commonly viral infection (including influenza virus, adenovirus, enterovirus, cytomegalovirus, hepatitis B virus, and herpes simplex virus).26,27 Other etiologies for acute pericarditis are autoimmune (accounting for less than 10% of cases) and neoplastic conditions (5% to 7% of cases).26
PATIENT OUTCOME
Consultation with an infectious disease specialist was obtained. The patient was placed under droplet isolation precautions and was started on a nebulizer, IV steroid treatments, and oseltamivir 75 mg by mouth every 12 hours. She was transferred to a medical floor, where she completed a five-day course of oseltamivir.
As a result of timely intervention, the patient was discharged in stable condition on a therapeutic regimen that included albuterol, fluticasone, and salmeterol inhalation, in addition to tapered-dose steroids. She was advised to follow up with her primary care provider and at the pulmonary clinic.
CONCLUSION
To our knowledge, this is the first reported case of acute pericarditis in a patient with concomitant acute infections with influenza A and B. According to conclusions reached in recent literature, further research is needed to explain the pathophysiology of influenza viral infections, associated cardiovascular morbidity and mortality, and the degree to which these can be prevented by influenza vaccination.1,28 Also to be pursued through research is a better understanding of the morbidity and mortality associated with influenza viruses, especially in children and in adults affected by asthma, cardiac disease, and/or obesity.
REFERENCES
1. Finelli L, Chaves SS. Influenza and acute myocardial infarction. J Infect Dis. 2011;203(12):
1701-1704.
2. Steiger HV, Rimbach K, Müller E, Breitkreutz R. Focused emergency echocardiography: lifesaving tool for a 14-year-old girl suffering out-of-hospital pulseless electrical activity arrest because of cardiac tamponade. Eur J Emerg Med. 2009;16(2): 103-105.
3. Goodman A, Perera P, Mailhot T, Mandavia D. The role of bedside ultrasound in the diagnosis of pericardial effusion and cardiac tamponade.
J Emerg Trauma Shock. 2012;5(1):72-75.
4. Restrepo CS, Lemos DF, Lemos JA, et al. Imaging findings in cardiac tamponade with emphasis on CT. Radiographics. 2007;27(6):1595-1610.
5. Papanagnou D, Stone MB. Massive right atrial thrombus masquerading as cardiac tamponade. Acad Emerg Med. 2010;17(2):E11.
6. Saito Y, Donohue A, Attai S, et al. The syndrome of cardiac tamponade with “small” pericardial effusion. Echocardiography. 2008;25(3): 321-327.
7. Lin E, Boire A, Hemmige V, et al. Cardiac tamponade mimicking tuberculous pericarditis as the initial presentation of chronic lymphocytic leukemia in a 58-year-old woman: a case report. J Med Case Rep. 2010;4:246.
8. Meniconi A, Attenhofer Jost CH, Jenni R. How to survive myocardial rupture after myocardial infarction. Heart. 2000;84(5):552.
9. Kosuge M, Kimura K, Ishikawa T, et al. Differences between men and women in terms of clinical features of ST-segment elevation acute myocardial infarction. Circ J. 2006;70(3):222-226.
10. Alpert JS, Thygesen K, Antman E, Bassand JP. Myocardial infarction redefined: a consensus document of the Joint European Society of Cardiology/American College of Cardiology Committee for the redefinition of myocardial infarction. J Am Coll Cardiol. 2000;36(3):959-969.
11. Goldhaber SZ. Deep venous thrombosis and pulmonary thromboembolism. In: Fauci AS, Braunwald E, Kasper DL, et al. Harrison’s Principles of Internal Medicine. 17th ed. New York, NY: McGraw-Hill Medical; 2008:1651–1657.
12. Brooks EG, Trotman W, Wadsworth MP, et al. Valves of the deep venous system: an overlooked risk factor. Blood. 2009;114(6):1276-1279.
13. Kyrle PA, Eichinger S. Is Virchow’s triad complete? Blood. 2009;114(6):1138-1139.
14. CDC. Seasonal influenza (flu): types of influenza viruses (2012). www.cdc.gov/flu/about/viruses/types.htm. Accessed October 24, 2012.
15. CDC. Seasonal influenza (flu)(2012). www.cdc .gov/flu. Accessed October 24, 2012.
16. Eccles R. Understanding the symptoms of the common cold and influenza. Lancet Infect Dis. 2005;5(11):718-725.
17. Angelo SJ, Marshall PS, Chrissoheris MP, Chaves AM. Clinical characteristics associated with poor outcome in patients acutely infected with Influenza A. Conn Med. 2004;68(4):199-205.
18. Murin S, Bilello K. Respiratory tract infections: another reason not to smoke. Cleve Clin J Med. 2005;72(10):916-920.
19. Ray CG, Icenogle TB, Minnich LL, et al. The use of intravenous ribavirin to treat influenza virus–associated acute myocarditis. J Infect Dis. 1989; 159(5):829-836.
20. Fairley CK, Ryan M, Wall PG, Weinberg J. The organism reported to cause infective myocarditis and pericarditis in England and Wales. J Infect. 1996;32(3):223-225.
21. Proby CM, Hackett D, Gupta S, Cox TM. Acute myopericarditis in influenza A infection. Q J Med. 1986;60(233):887-892.
22. Streifler JJ, Dux S, Garty M, Rosenfeld JB. Recurrent pericarditis: a rare complication of influenza vaccination. Br Med J (Clin Res Ed). 1981; 283(6290):526-527.
23. Desson JF, Leprévost M, Vabret F, Davy A. Acute benign pericarditis after anti-influenza vaccination [in French]. Presse Med. 1997;26 (9):415.
24. BinaxNOW® Influenza A&B Test Kit (product instructions). www.diagnosticsdirect2u.com/images/PDF/Binax%20Now%20416-022%20PPI .pdf. Accessed October 24, 2012.
25. 510(k) Substantial Equivalence Determination Decision Summary [BinaxNow® Influenza A & B Test] (2009). www.accessdata.fda.gov/cdrh_docs/reviews/K062109.pdf. Accessed October 24, 2012.
26. Imazio M, Spodick DH, Brucato A, et al. Controversial issues in the management of pericardial diseases. Circulation. 2010;121(7):916-928.
27. Maisch B, Seferovic PM, Ristic AD, et al; Task Force on the Diagnosis and Management of Pericardial Diseases of the European Society of Cardiology. Guidelines on the diagnosis and management of pericardial diseases: executive summary. Eur Heart J. 2004;25(7):587-610.
28. McCullers JA, Hayden FG. Fatal influenza B infections: time to reexamine influenza research priorities. J Infect Dis. 2012;205(6):870-872.
On an autumn day, a 38-year-old woman with a history of asthma presented to the emergency department (ED) with the chief complaint of shortness of breath (SOB). The patient described her SOB as sudden in onset and not relieved by use of her albuterol inhaler; hence the ED visit.
She denied any chest pain, palpitations, dizziness, orthopnea, upper respiratory tract infection, cough, wheezing, fever or chills, headache, vision changes, body aches, sick contacts, or pets at home. She said she uses her albuterol inhaler as needed, and that she had used it that day for the first time in “a few months.” She denied any history of intubation or steroid use. Additionally, she had not been seen by a primary care provider in years.
The woman, a native of Ghana, had been living in the United States for many years. She denied any recent travel or exposure to toxic chemicals; any use of tobacco, alcohol, or illicit drugs; or any history of sexually transmitted disease.
The patient was afebrile (temperature, 98.6°F), with a respiratory rate of 20 breaths/min; blood pressure, 144/69 mm Hg; and ventricular rate, 125 beats/min. On physical examination, her extraocular movements were intact; pupils were equal, round, reactive to light and accommodation; and sclera were nonicteric. The patient’s head was normocephalic and atraumatic, and the neck was supple with normal range of motion and no jugular venous distension or lymphadenopathy. Her mucous membranes were moist with no pharyngeal erythema or exudates. Cardiovascular examination, including ECG, revealed tachycardia but no murmurs or gallops.
While being evaluated in the ED, the patient became tachypneic and began to experience respiratory distress. She was intubated for airway protection, at which time she developed pulseless electrical activity (PEA), with 30 beats/min. She responded to atropine and epinephrine injections. A repeat ECG showed sinus tachycardia and right atrial enlargement with right-axis deviation. Chest x-ray (see Figure 1) showed no consolidation, pleural effusion, or pneumothorax.
Results from the patient’s lab work are shown in the table, above. Negative results were reported for a urine pregnancy test.
Since there was no clear etiology for the patient’s PEA, she underwent pan-culturing, with the following tests ordered: HIV antibody testing, immunovirology for influenza A and B viruses, and urine toxicology. Doppler ultrasound of the bilateral lower extremities was also ordered, in addition to chest CT and transthoracic and transesophageal echocardiography (TTE and TEE, respectively). The patient was intubated and transferred to the medical ICU for further management.
The differential diagnosis included cardiac tamponade, acute MI, acute pulmonary embolus (PE), tension pneumothorax, hypovolemia, and asthma exacerbated by viral or bacterial infection.1,2 Although the case patient presented with PEA, she did not have the presenting signs of cardiac tamponade known as Beck’s triad: hypotension, jugular venous distension, and muffled heart sounds.3 TTE showed an ejection fraction of 65% and grade 2 diastolic dysfunction but no pericardial effusions (which accumulate rapidly in the patient with cardiac tamponade, resulting from fluid buildup in the pericardial layers),4 and TEE showed no atrial thrombi (which can masquerade as cardiac tamponade5). The patient had no signs of trauma and denied any history of malignancy (both potential causes of cardiac tamponade). Chest x-ray showed normal heart size and no pneumothorax, consolidations, or pleural effusions.4,6-8 Thus, the diagnosis of cardiac tamponade was ruled out.
Common presenting symptoms of acute MI include sudden-onset chest pain, SOB, palpitations, dizziness, nausea, and/or vomiting. Women may experience less dramatic symptoms—often little more than SOB and fatigue.9 According to a 2000 consensus document from a joint European Society of Cardiology/American College of Cardiology committee10 in which MI was redefined, the diagnosis of MI relies on a rise in cardiac troponin levels, typical MI symptoms, and changes in ECG showing pathological Q waves or ST elevation or depression. The case patient’s troponin I level was less than 0.02 ng/mL, and ECG did not reveal Q waves or ST-T wave changes; additionally, since the patient had no chest pain, palpitations, diaphoresis, nausea, or vomiting, acute MI was ruled out.
Blood clots capable of blocking the pulmonary artery usually originate in the deep veins of the lower extremities.11 Three main factors, called Virchow’s triad, are known to contribute to these deep vein thromboses (DVTs): venous stasis, endothelial injury, and a hypercoagulability state.12,13 The patient had denied any trauma, recent travel, history of malignancy, or use of tobacco or oral contraceptives, and the result of her urine pregnancy test was negative. Even though the patient presented with tachypnea and acute SOB, with ECG showing right-axis deviation and tachycardia (common presenting signs and symptoms for PE), her chest CT showed no evidence of PE (see Figure 2); additionally, Doppler ultrasound of the bilateral lower extremities revealed no DVTs. Thus, PE was also excluded.
Tension pneumothorax was also ruled out, as chest x-ray showed neither mediastinal shift nor tracheal deviation, and the patient had denied any trauma. Laboratory analyses did not indicate hyponatremia, and the patient’s hemoglobin and hematocrit were satisfactory. She was tachycardic on admission, but her blood pressure was stable. As the patient denied any use of vasodilators or diuretics, hypovolemia was ruled out.
Patients experiencing asthma exacerbation can present with acute SOB, which usually resolves following use of IV steroids, nebulizer therapy, and inhaler treatments. Despite being administered IV methylprednisolone and magnesium sulfate in the ED, the patient experienced PEA and respiratory distress and required intubation for airway protection.
The HIV test was nonreactive, and blood and urine cultures did not show any growth. Results of tests for Legionella urinary antigen and Streptococcus pneumoniae antigen were negative. Sputum culture showed normal flora. Immunovirology testing, however, was positive for both influenza A and B antigens.
Chest X-ray showed no acute pulmonary pathology, nor did chest CT show any central, interlobar, or segmental embolism or mediastinal lymphadenopathy. It was determined that the patient’s acute SOB might represent asthma exacerbation secondary to influenza viral infection. Her PEA was attributed to possible acute pericarditis secondary to concomitant influenza A and B viral infection.
DISCUSSION
Currently, the CDC recognizes three types of influenza virus: A, B, and C.14 Only influenza A viruses are further classified into subtypes, based on the presence of surface proteins called hemagglutinin (HA) or neuraminidase (NA) glycoproteins. Humans can be infected by influenza A subtypes H1N1 and H3N2.14 Influenza B viruses, found mostly in humans, are associated with significant morbidity and mortality.
Influenza A and B viruses are further classified into strains that change with each flu season—thus, the need to update vaccinations against influenza A and B each year. No vaccination exists against influenza C virus, which is known to cause only mild illness in humans.15
In patients with asthma (as in the case patient), chronic bronchitis, or emphysema, infection with the influenza virus can manifest with SOB, in addition to the more common symptoms of fever, sore throat, headache, rhinorrhea, chills, muscle aches, and general discomfort.16 Patients with coronary artery disease, congestive heart failure (CHF), and/or a history of smoking may experience more severe symptoms and increased risk for influenza-associated mortality than do other patients.17,18
Rare cardiac complications of influenza infections are myocarditis and benign acute pericarditis; myocarditis can progress to CHF and death.19,20 A case of acute myopericarditis was reported by Proby et al21 in a patient with acute influenza A infection who developed pericardial effusions, myositis, tamponade, and pleurisy. That patient recovered after pericardiocentesis and administration of inotropic drugs.
In the literature, a few cases of acute pericarditis have been reported in association with administration of the influenza vaccination.22,23
In the case patient, the diagnosis of influenza A and B was made following testing of nasal and nasopharyngeal swabs with an immunochromatographic assay that uses highly sensitive monoclonal antibodies to detect influenza A and B nucleoprotein antigens.24,25
According to reports in the literature, two-thirds of cases of acute pericarditis are caused by infection, most commonly viral infection (including influenza virus, adenovirus, enterovirus, cytomegalovirus, hepatitis B virus, and herpes simplex virus).26,27 Other etiologies for acute pericarditis are autoimmune (accounting for less than 10% of cases) and neoplastic conditions (5% to 7% of cases).26
PATIENT OUTCOME
Consultation with an infectious disease specialist was obtained. The patient was placed under droplet isolation precautions and was started on a nebulizer, IV steroid treatments, and oseltamivir 75 mg by mouth every 12 hours. She was transferred to a medical floor, where she completed a five-day course of oseltamivir.
As a result of timely intervention, the patient was discharged in stable condition on a therapeutic regimen that included albuterol, fluticasone, and salmeterol inhalation, in addition to tapered-dose steroids. She was advised to follow up with her primary care provider and at the pulmonary clinic.
CONCLUSION
To our knowledge, this is the first reported case of acute pericarditis in a patient with concomitant acute infections with influenza A and B. According to conclusions reached in recent literature, further research is needed to explain the pathophysiology of influenza viral infections, associated cardiovascular morbidity and mortality, and the degree to which these can be prevented by influenza vaccination.1,28 Also to be pursued through research is a better understanding of the morbidity and mortality associated with influenza viruses, especially in children and in adults affected by asthma, cardiac disease, and/or obesity.
REFERENCES
1. Finelli L, Chaves SS. Influenza and acute myocardial infarction. J Infect Dis. 2011;203(12):
1701-1704.
2. Steiger HV, Rimbach K, Müller E, Breitkreutz R. Focused emergency echocardiography: lifesaving tool for a 14-year-old girl suffering out-of-hospital pulseless electrical activity arrest because of cardiac tamponade. Eur J Emerg Med. 2009;16(2): 103-105.
3. Goodman A, Perera P, Mailhot T, Mandavia D. The role of bedside ultrasound in the diagnosis of pericardial effusion and cardiac tamponade.
J Emerg Trauma Shock. 2012;5(1):72-75.
4. Restrepo CS, Lemos DF, Lemos JA, et al. Imaging findings in cardiac tamponade with emphasis on CT. Radiographics. 2007;27(6):1595-1610.
5. Papanagnou D, Stone MB. Massive right atrial thrombus masquerading as cardiac tamponade. Acad Emerg Med. 2010;17(2):E11.
6. Saito Y, Donohue A, Attai S, et al. The syndrome of cardiac tamponade with “small” pericardial effusion. Echocardiography. 2008;25(3): 321-327.
7. Lin E, Boire A, Hemmige V, et al. Cardiac tamponade mimicking tuberculous pericarditis as the initial presentation of chronic lymphocytic leukemia in a 58-year-old woman: a case report. J Med Case Rep. 2010;4:246.
8. Meniconi A, Attenhofer Jost CH, Jenni R. How to survive myocardial rupture after myocardial infarction. Heart. 2000;84(5):552.
9. Kosuge M, Kimura K, Ishikawa T, et al. Differences between men and women in terms of clinical features of ST-segment elevation acute myocardial infarction. Circ J. 2006;70(3):222-226.
10. Alpert JS, Thygesen K, Antman E, Bassand JP. Myocardial infarction redefined: a consensus document of the Joint European Society of Cardiology/American College of Cardiology Committee for the redefinition of myocardial infarction. J Am Coll Cardiol. 2000;36(3):959-969.
11. Goldhaber SZ. Deep venous thrombosis and pulmonary thromboembolism. In: Fauci AS, Braunwald E, Kasper DL, et al. Harrison’s Principles of Internal Medicine. 17th ed. New York, NY: McGraw-Hill Medical; 2008:1651–1657.
12. Brooks EG, Trotman W, Wadsworth MP, et al. Valves of the deep venous system: an overlooked risk factor. Blood. 2009;114(6):1276-1279.
13. Kyrle PA, Eichinger S. Is Virchow’s triad complete? Blood. 2009;114(6):1138-1139.
14. CDC. Seasonal influenza (flu): types of influenza viruses (2012). www.cdc.gov/flu/about/viruses/types.htm. Accessed October 24, 2012.
15. CDC. Seasonal influenza (flu)(2012). www.cdc .gov/flu. Accessed October 24, 2012.
16. Eccles R. Understanding the symptoms of the common cold and influenza. Lancet Infect Dis. 2005;5(11):718-725.
17. Angelo SJ, Marshall PS, Chrissoheris MP, Chaves AM. Clinical characteristics associated with poor outcome in patients acutely infected with Influenza A. Conn Med. 2004;68(4):199-205.
18. Murin S, Bilello K. Respiratory tract infections: another reason not to smoke. Cleve Clin J Med. 2005;72(10):916-920.
19. Ray CG, Icenogle TB, Minnich LL, et al. The use of intravenous ribavirin to treat influenza virus–associated acute myocarditis. J Infect Dis. 1989; 159(5):829-836.
20. Fairley CK, Ryan M, Wall PG, Weinberg J. The organism reported to cause infective myocarditis and pericarditis in England and Wales. J Infect. 1996;32(3):223-225.
21. Proby CM, Hackett D, Gupta S, Cox TM. Acute myopericarditis in influenza A infection. Q J Med. 1986;60(233):887-892.
22. Streifler JJ, Dux S, Garty M, Rosenfeld JB. Recurrent pericarditis: a rare complication of influenza vaccination. Br Med J (Clin Res Ed). 1981; 283(6290):526-527.
23. Desson JF, Leprévost M, Vabret F, Davy A. Acute benign pericarditis after anti-influenza vaccination [in French]. Presse Med. 1997;26 (9):415.
24. BinaxNOW® Influenza A&B Test Kit (product instructions). www.diagnosticsdirect2u.com/images/PDF/Binax%20Now%20416-022%20PPI .pdf. Accessed October 24, 2012.
25. 510(k) Substantial Equivalence Determination Decision Summary [BinaxNow® Influenza A & B Test] (2009). www.accessdata.fda.gov/cdrh_docs/reviews/K062109.pdf. Accessed October 24, 2012.
26. Imazio M, Spodick DH, Brucato A, et al. Controversial issues in the management of pericardial diseases. Circulation. 2010;121(7):916-928.
27. Maisch B, Seferovic PM, Ristic AD, et al; Task Force on the Diagnosis and Management of Pericardial Diseases of the European Society of Cardiology. Guidelines on the diagnosis and management of pericardial diseases: executive summary. Eur Heart J. 2004;25(7):587-610.
28. McCullers JA, Hayden FG. Fatal influenza B infections: time to reexamine influenza research priorities. J Infect Dis. 2012;205(6):870-872.