User login
Management considerations for women with von Willebrand disease
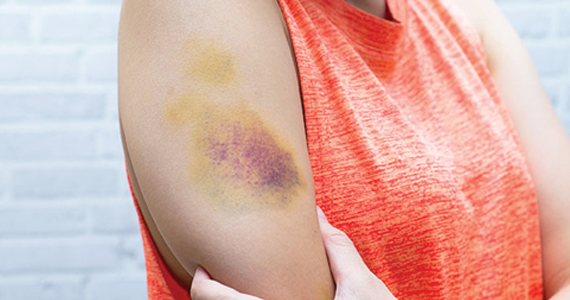
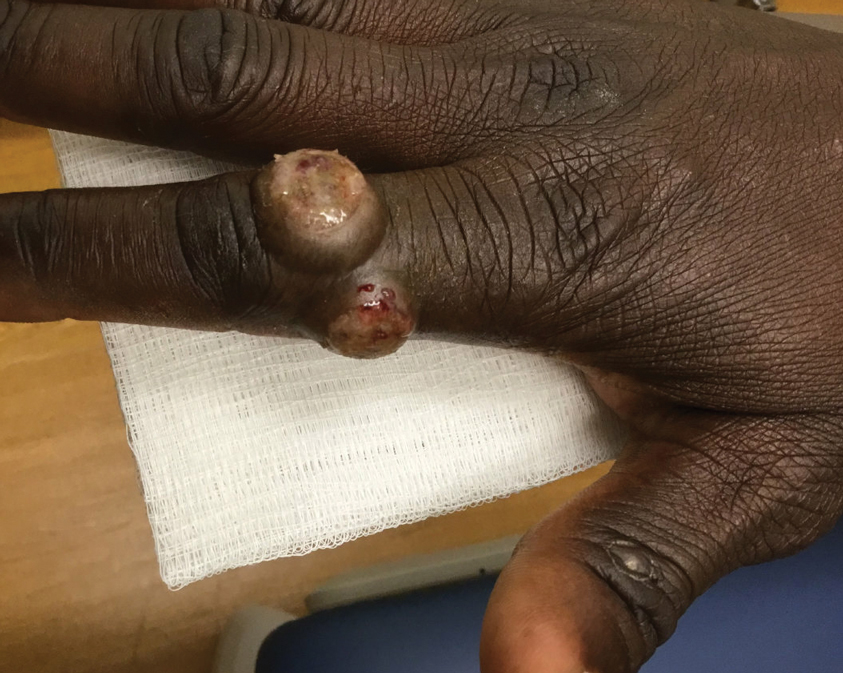
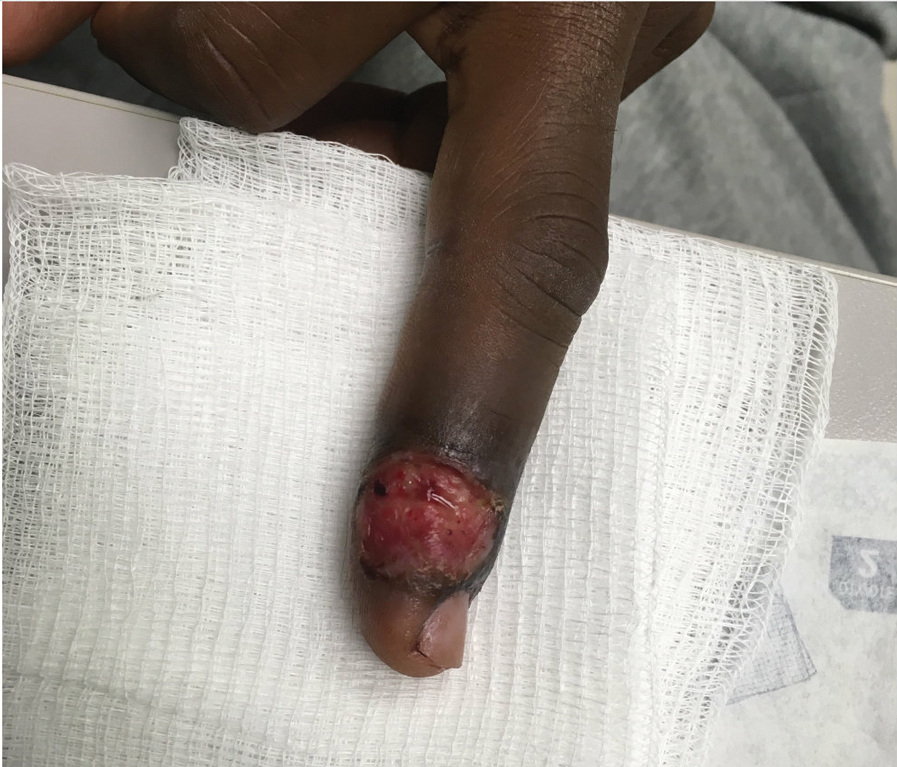
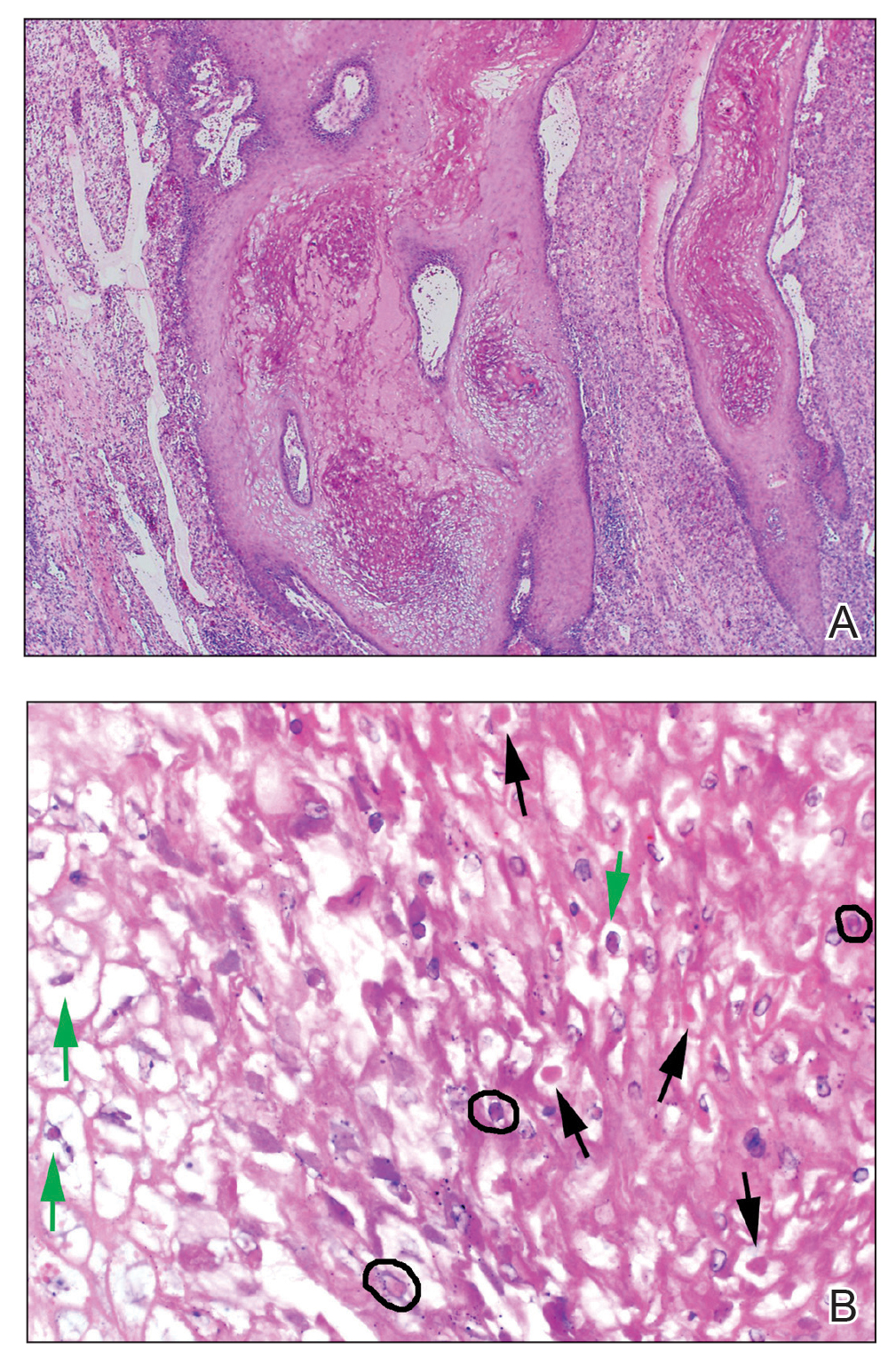
Von Willebrand disease (VWD) represents the most common inherited bleeding disorder, with a prevalence of approximately 1 in 1,000 people.
Bruising and mucocutaneous bleeding (epistaxis, gingival bleeding, and bleeding after dental extraction) are the most common presenting symptoms of VWD. Because VWD substantially increases the risk of heavy menstrual bleeding (HMB) and, to some extent, intrapartum bleeding complications, and postpartum hemorrhage, women experience a disproportionate burden from VWD. Thus, ObGyns are likely to be called on to make treatment recommendations in VWD patients with these concerns.1
In 2017, the American Society of Hematology, the International Society on Thrombosis and Haemostasis, the National Hemophilia Foundation, and the World Federation of Hemophilia determined that among clinical issues related to VWD, updating guidelines for women with VWD represented the highest priority.2 Accordingly, an international group of hematologists/coagulation specialists performed systematic literature reviews to address 3 questions faced by women with VWD and their clinicians:
- What are the most effective treatments for HMB?
- What is the safest approach for women desiring neuraxial analgesia for intrapartum pain?
- What is the impact of postpartum administration of tranexamic acid (TxA) on postpartum hemorrhage (PPH)?3
Evidence on management strategies for HMB in women with VWD
The prevalence of HMB in women with VWD ranges from 50% to 92%. Reports suggest that between 5% and 24% of women presenting with this symptom have VWD.3 However, the prevalence of VWD among women seeking care for HMB relates to referral patterns, with the prevalence of VWD substantially higher in patient populations who are referred to clinicians or centers that focus on care of patients with bleeding disorders.
The systematic review authors3 identified 2 comparative studies that assessed the treatment of HMB in women with VWD. One was a crossover trial that enrolled 116 VWD patients with HMB with a mean age of 36 years.4 All participants in this trial chose not to use combination oral contraceptives (COCs) as they had not experienced good results with prior COC use. Trial participants were randomly assigned to receive either intranasal desmopressin (DDAVP; a synthetic analog of the antidiuretic agent vasopressin, which stimulates the release of VWF from endothelial cells) or oral TxA therapy for 2 menstrual cycles. Participants then crossed over to the other drug for 2 additional cycles. Although both agents significantly reduced estimated menstrual blood loss, TxA was more effective in decreasing bleeding than intranasal DDAVP.4
In a retrospective cohort study, investigators compared COC use with intranasal DDAVP in 36 adolescents who had VWD and HMB.5 Participant follow-up ranged from 6 months to 4 years. The estimated efficacy of COCs and intranasal DDAVP was 86% and 77%, respectively, a difference that did not achieve statistical significance. Some of the adolescents who used intranasal DDAVP reported severe headaches and flushing.5
In addition, the systematic review authors3 identified 5 case series that described the use of the levonorgestrel (52 mg)-releasing intrauterine device (LNG 52 IUD) in women with VWD and HMB; 4 of these addressed the efficacy of progestin-releasing IUDs in reducing HMB in this patient population.6-9 Using different approaches to define HMB, the authors of these reports followed between 7 and 26 patients with bleeding disorders (most with confirmed VWD) and HMB for variable amounts of time after placement of an LNG 52 IUD. Many of the women described in these case series had tried other HMB treatments, including COCs, without success. Although these 4 reports assessed different outcomes, all reported that placement of the LNG 52 IUD substantially reduced menstrual blood loss, often resulting in amenorrhea. Several of these reports also noted important improvements in quality of life following LNG 52 IUD placement. One case series reported LNG 52 IUD placement in 13 adolescents with VWD and HMB. The mean time to achieve amenorrhea or occasional spotting was 94 days.6
The fifth report, which followed 20 women (median age, 31 years) with HMB associated with VWD or other bleeding disorders who underwent LNG 52 IUD placement, aimed to describe IUD expulsions and malpositioned IUDs in this population. In this small group of patients, 3 IUD expulsions and 2 malpositioned IUDs were observed. Furthermore, an additional 5 women had their device removed prematurely due to patient dissatisfaction. Accordingly, the IUD continuation rate in this case series was only 50%.10
Evidence on management of pregnancy, delivery, and the postpartum period
Heavy menstrual bleeding is not the only challenge for women with VWD. While pregnancy is accompanied by higher levels of VWF, potentially offsetting the risk of bleeding at the time of delivery, the levels do not achieve the same magnitude as they would in unaffected women.11 Women are at an increased risk of primary PPH12,13 and, importantly, since VWF levels fall exponentially after delivery when women are still experiencing lochia,11 they are at increased risk of secondary or delayed PPH.
Two questions arise frequently in the care of women with VWD at the time of delivery and during the postpartum period:
- What is the safest approach for women who desire neuraxial analgesia for intrapartum pain?
- What is the impact of postpartum administration of TxA on PPH?
The second systematic review the authors performed3 focused on VWF levels in women receiving neuraxial anesthesia during labor. After screening 27 studies, the authors included 5 case series, which did not describe outcomes based on VWF levels but rather described the outcomes of women with VWF levels of greater than 0.50 IU/mL (> 50% of normal compared with a normal standard).
Meta-analysis showed that the proportion of anesthesia complications was 6%, which sounds high, but the range of complications was what would be expected in any population (hypotension, accidental dural puncture, inadequate anesthesia, and bloody tap with no further complications). No spinal, subdural, or epidural hematomas were noted.3 Such hematomas are an extremely rare complication of neuraxial anesthesia, occurring in only 1 in 200,000 or 1 in 250,000 obstetric patients14,15; accordingly, an increase in the rate of hematomas among women with VWD could go undetected. The absence of hematomas among women with VWD as reported in the systematic review does not mean there is not an increase in the rate of hematomas in women with VWD. The relative risk is unknown and caution would be advised.
The third systematic review that the authors performed3 was on TxA treatment in the postpartum period. After screening 41 studies, the authors included 2 retrospective cohort studies.16,17 The majority of the participants had VWD. With very-low-certainty evidence, the authors found that TxA reduces the risk of:
- severe primary PPH (risk ratio [RR], 0.36; 95% confidence interval [CI], 0.05–2.59)
- primary PPH (RR, 0.25; 95% CI, 0.04–1.75)
- secondary PPH (RR, 0.42; 95% CI, 0.02–0.91—does not cross 1.0).
Note that the 95% confidence intervals for severe as well as primary PPH crossed 1.0 and therefore these reductions in risk did not achieve statistical significance. Additionally, there was very-low-certainty evidence on the effect of TxA on blood transfusions, vaginal hematomas, blood loss, and thrombotic complications.3
Continue to: Our recommendations for HMB management...
Our recommendations for HMB management
When first evaluating any woman with HMB, it is important to check a blood count and ferritin level, if not already done. If there is any suggestion of iron deficiency (with or without anemia), we recommend oral iron supplementation. This is best accomplished with slow-release iron supplement formulations (or less expensive generic or house brands that contain less than 65 mg of elemental iron per tablet) taken every other day. Such preparations may cause fewer gastrointestinal adverse effects than other oral iron formulations.18 Although it may appear counterintuitive, oral iron is better absorbed (and also may cause fewer gastrointestinal adverse effects) when taken every other day.19
Initial management of HMB, whether or not a bleeding disorder is present, often consists or oral hormonal management. If no contraindications are present, we recommend initiation of a COC with a short hormone-free interval (for example, a 24/4 formulation). If contraindications to contraceptive doses of estrogen are present, continuous use of norethindrone acetate 5-mg tablets or off-label use of combination tablets with 5 µg of ethinyl estradiol and 1 mg of norethindrone acetate (a formulation approved for the treatment of menopausal symptoms) is appropriate.20
Once a patient is established on oral hormonal management, placement of a levonorgestrel-releasing IUD should be considered. Given that expulsion rates may be higher in women with HMB, if feasible, consider using abdominal ultrasound guidance for IUD placement.
For women with VWD who fail first-line therapy (hormonal management) or are trying to become pregnant, TxA (two 650-mg tablets 3 times daily for up to 5 days during episodes of heavy flow) can reduce HMB.20,21
Our recommendations for management of pregnancy and delivery
The second and third systematic reviews discussed above provide very limited guidance on comprehensive management. The care of the pregnant patient with VWD starts with assessment of VWF levels and making an accurate diagnosis. This usually requires the input of a hematologist or other expert in hemostasis. If no recent VWF levels are available, the ObGyn can obtain a von Willebrand panel that includes VWF antigen, VWF activity (most commonly ristocetin cofactor), and factor VIII.
Levels should be reassessed around 36 weeks’ gestation in anticipation of delivery. VWF levels increase during pregnancy; accordingly, in mild, type 1 VWD, half the time treatment is not necessary.11 If VWF activity is less than 50 IU/dL (less than 50% of normal) at 36 weeks’ gestation, the patient should receive VWF concentrate (dosed in VWF units). This requires consultation with hematology and specialized pharmacy support.
For these reasons, the patient with a VWF level less than 50% should be delivered in a referral center with the necessary resources. Anesthesia should be aware of the patient. Unless
Due to the quantity of fluids administered during labor or at the time of delivery and the coexistent administration of oxytocin, desmopressin (synthetic vasopressin) should not be used without monitoring sodium levels, should not be dosed more than once, or should be avoided altogether due to the risk of water intoxication.
If the patient has sustained VWF and factor VIII levels greater than 50 IU/dL, she would be a candidate to deliver in her local hospital and receive neuraxial anesthesia.
Based on the best data we have for women with VWD, a patient with a VWF greater than 50 IU/dL is no more likely to experience PPH than other women.11 Intravenous TxA can be used for prevention or treatment of immediate postpartum bleeding per protocol (1 g after cord clamp and 1 g 30 minutes or more later).22 Oral TxA can be used for prevention or treatment of delayed postpartum bleeding as per HMB. Regardless of the outcome of any testing during pregnancy, nonsteroidal anti-inflammatory drugs should be avoided postpartum and the patient should be monitored closely for bleeding.
Neonatal care
As for the fetus/neonate, the parents should be aware that the infant has a 50% chance of inheriting VWD. If the baby’s father has no history of bleeding, it is unlikely that the infant would be any more affected than the patient herself. Nonetheless, cord blood (in one or more light blue top tubes) should be obtained at the time of delivery and sent for a von Willebrand panel. If the infant is male, a circumcision should be postponed until VWD is ruled out. In addition, fetal invasive procedures should be avoided during labor. Fetal scalp electrode placement should be avoided. Operative vaginal delivery also should be avoided. Cesarean delivery would be preferred to operative vaginal delivery, but if operative vaginal delivery is unavoidable, use of forceps is preferred to vacuum extraction. ●
- ACOG committee opinion no. 451: Von Willebrand disease in women. Obstet Gynecol. 2009;114:1439-1443. doi: 10.1097 /AOG.0b013e3181c6f975.
- Kalot MA, Al-Khatib M, Connell NT, et al; VWD Working Group. An international survey to inform priorities for new guidance on von Willebrand disease. Hemophilia. 2020;26:106-116. doi: 10.1111/hae.13881.
- Brignardello-Petersen R, El Alayli A, Husainat N, et al. Gynecologic and obstetric management of women with von Willebrand disease: summary of 3 systematic reviews of the literature. Blood Adv. 2022;6:228-237. doi: 10.1182 /bloodadvances.2021005589.
- Kouides PA, Byams VR, Philipp CS, et al. Multisite management study of menorrhagia with abnormal laboratory haemostasis: a prospective crossover study of intranasal desmopressin and oral tranexamic acid. Br J Haematol. 2009;145:212-220. doi: 10.1111/j.1365-2141.2009.07610.x.
- Amesse LS, Pfaff-Amesse T, Leonardi R, et al. Oral contraceptives and DDAVP nasal spray: patterns of use in managing vWD-associated menorrhagia: a single-institution study. J Pediatr Hematol Oncol. 2005;27:357-363. doi: 10.1097/01.mph.0000173175.95152.95.
- Adeyemi-Fowode OA, Santos XM, Dietrich JE, et al. Levonorgestrel-releasing intrauterine device use in female adolescents with heavy menstrual bleeding and bleeding disorders: single institution review. J Pediatr Adolesc Gynecol. 2017;30:479-483. doi: 10.1016/j.jpag.2016.04.001.
- Chi C, Huq FY, Kadir RA. Levonorgestrel-releasing intrauterine system for the management of heavy menstrual bleeding in women with inherited bleeding disorders: long-term follow-up. Contraception. 2011;83:242-247. doi: 10.1016/j.contraception.2010.07.010.
- Kingman CE, Kadir RA, Lee CA, et al. The use of levonorgestrel-releasing intrauterine system for treatment of menorrhagia in women with inherited bleeding disorders. BJOG. 2004;111:1425-1428. doi: 10.1111/j.1471-0528.2004.00305.x.
- Lukes AS, Reardon B, Arepally G. Use of the levonorgestrel-releasing intrauterine system in women with hemostatic disorders. Fertil Steril. 2008;90:673-677. doi: 10.1016 /j.fertnstert.2007.07.1315.
- Rimmer E, Jamieson MA, James P. Malposition and expulsion of the levonorgestrel intrauterine system among women with inherited bleeding disorders. Haemophilia. 2013;19:933-938. doi: 10.1111/hae.12184.
- James AH, Konkle BA, Kouides P, et al. Postpartum von Willebrand factor levels in women with and without von Willebrand disease and implications for prophylaxis. Haemophilia. 2015;21:81-87. doi: 10.1111/hae.12568.
- James AH, Jamison MG. Bleeding events and other complications during pregnancy and childbirth in women with von Willebrand disease. J Thromb Haemost. 2007;5: 1165-1169. doi: 10.1111/j.1538-7836.2007.02563.x.
- Al-Zirqi I, Vangen S, Forsen L, et al. Prevalence and risk factors of severe obstetric haemorrhage. BJOG. 2008;115:1265-1272. doi: 10.1111/j.1471-0528.2008.01859.x.
- Moen V, Dahlgren N, Irestedt L. Severe neurological complications after central neuraxial blockades in Sweden 1990-1999. Anesthesiology. 2004;101:950-959. doi: 10.1097/00000542-200410000-00021.
- D’Angelo R, Smiley RM, Riley ET, et al. Serious complications related to obstetric anesthesia: the serious complication repository project of the Society for Obstetric Anesthesia and Perinatology. Anesthesiology. 2014;120:1505-1512. doi: 10.1097/ALN.000000000000253.
- Govorov I, Lofgren S, Chaireti R, et al. Postpartum hemorrhage in women with von Willebrand disease—a retrospective observational study. PLos One. 2016;11:e0164683. doi: 10.1371/journal.pone.0164683.
- Hawke L, Grabell J, Sim W, et al. Obstetric bleeding among women with inherited bleeding disorders: a retrospective study. Haemophilia. 2016;22:906-911. doi: 10.1111/hae.13067.
- James AH. Iron deficiency anemia in pregnancy. Obstet Gynecol. 2021;138:663-674. doi:10.1097/AOG .000000000000.4559.
- Stoffel NU, Cercamondi CI, Brittenham G, et al. Iron absorption from oral iron supplements given on consecutive versus alternate days and as single morning doses versus twice-daily split dosing in iron-depleted women: two open-label, randomised controlled trials. Lancet Haematol. 2017;4:e524-e533. doi: 10.1016/S2352-3026(17)30182-5.
- Kaunitz AM. Abnormal uterine bleeding in reproductiveage women. JAMA. 2019;321:2126-2127. doi: 10.1001 /jama.2019.5248.
- James AH, Kouides PA, Abdul-Kadir R, et al. Von Willebrand disease and other bleeding disorders in women: consensus on diagnosis and management from an international expert panel. Am J Obstet Gynecol. 2009;201:12.e1-8. doi: 10.1016 /j.ajog.2009.04.024.
- WOMAN Trial Collaborators. Effect of early tranexamic acid administration on mortality, hysterectomy, and other morbidities in women with post-partum haemorrhage (WOMAN): an international, randomised, double-blind, placebo-controlled trial. Lancet. 2017;389:2105-2116. doi: 10.1016/S0140-6736(17)30638-4.
Von Willebrand disease (VWD) represents the most common inherited bleeding disorder, with a prevalence of approximately 1 in 1,000 people.
Bruising and mucocutaneous bleeding (epistaxis, gingival bleeding, and bleeding after dental extraction) are the most common presenting symptoms of VWD. Because VWD substantially increases the risk of heavy menstrual bleeding (HMB) and, to some extent, intrapartum bleeding complications, and postpartum hemorrhage, women experience a disproportionate burden from VWD. Thus, ObGyns are likely to be called on to make treatment recommendations in VWD patients with these concerns.1
In 2017, the American Society of Hematology, the International Society on Thrombosis and Haemostasis, the National Hemophilia Foundation, and the World Federation of Hemophilia determined that among clinical issues related to VWD, updating guidelines for women with VWD represented the highest priority.2 Accordingly, an international group of hematologists/coagulation specialists performed systematic literature reviews to address 3 questions faced by women with VWD and their clinicians:
- What are the most effective treatments for HMB?
- What is the safest approach for women desiring neuraxial analgesia for intrapartum pain?
- What is the impact of postpartum administration of tranexamic acid (TxA) on postpartum hemorrhage (PPH)?3
Evidence on management strategies for HMB in women with VWD
The prevalence of HMB in women with VWD ranges from 50% to 92%. Reports suggest that between 5% and 24% of women presenting with this symptom have VWD.3 However, the prevalence of VWD among women seeking care for HMB relates to referral patterns, with the prevalence of VWD substantially higher in patient populations who are referred to clinicians or centers that focus on care of patients with bleeding disorders.
The systematic review authors3 identified 2 comparative studies that assessed the treatment of HMB in women with VWD. One was a crossover trial that enrolled 116 VWD patients with HMB with a mean age of 36 years.4 All participants in this trial chose not to use combination oral contraceptives (COCs) as they had not experienced good results with prior COC use. Trial participants were randomly assigned to receive either intranasal desmopressin (DDAVP; a synthetic analog of the antidiuretic agent vasopressin, which stimulates the release of VWF from endothelial cells) or oral TxA therapy for 2 menstrual cycles. Participants then crossed over to the other drug for 2 additional cycles. Although both agents significantly reduced estimated menstrual blood loss, TxA was more effective in decreasing bleeding than intranasal DDAVP.4
In a retrospective cohort study, investigators compared COC use with intranasal DDAVP in 36 adolescents who had VWD and HMB.5 Participant follow-up ranged from 6 months to 4 years. The estimated efficacy of COCs and intranasal DDAVP was 86% and 77%, respectively, a difference that did not achieve statistical significance. Some of the adolescents who used intranasal DDAVP reported severe headaches and flushing.5
In addition, the systematic review authors3 identified 5 case series that described the use of the levonorgestrel (52 mg)-releasing intrauterine device (LNG 52 IUD) in women with VWD and HMB; 4 of these addressed the efficacy of progestin-releasing IUDs in reducing HMB in this patient population.6-9 Using different approaches to define HMB, the authors of these reports followed between 7 and 26 patients with bleeding disorders (most with confirmed VWD) and HMB for variable amounts of time after placement of an LNG 52 IUD. Many of the women described in these case series had tried other HMB treatments, including COCs, without success. Although these 4 reports assessed different outcomes, all reported that placement of the LNG 52 IUD substantially reduced menstrual blood loss, often resulting in amenorrhea. Several of these reports also noted important improvements in quality of life following LNG 52 IUD placement. One case series reported LNG 52 IUD placement in 13 adolescents with VWD and HMB. The mean time to achieve amenorrhea or occasional spotting was 94 days.6
The fifth report, which followed 20 women (median age, 31 years) with HMB associated with VWD or other bleeding disorders who underwent LNG 52 IUD placement, aimed to describe IUD expulsions and malpositioned IUDs in this population. In this small group of patients, 3 IUD expulsions and 2 malpositioned IUDs were observed. Furthermore, an additional 5 women had their device removed prematurely due to patient dissatisfaction. Accordingly, the IUD continuation rate in this case series was only 50%.10
Evidence on management of pregnancy, delivery, and the postpartum period
Heavy menstrual bleeding is not the only challenge for women with VWD. While pregnancy is accompanied by higher levels of VWF, potentially offsetting the risk of bleeding at the time of delivery, the levels do not achieve the same magnitude as they would in unaffected women.11 Women are at an increased risk of primary PPH12,13 and, importantly, since VWF levels fall exponentially after delivery when women are still experiencing lochia,11 they are at increased risk of secondary or delayed PPH.
Two questions arise frequently in the care of women with VWD at the time of delivery and during the postpartum period:
- What is the safest approach for women who desire neuraxial analgesia for intrapartum pain?
- What is the impact of postpartum administration of TxA on PPH?
The second systematic review the authors performed3 focused on VWF levels in women receiving neuraxial anesthesia during labor. After screening 27 studies, the authors included 5 case series, which did not describe outcomes based on VWF levels but rather described the outcomes of women with VWF levels of greater than 0.50 IU/mL (> 50% of normal compared with a normal standard).
Meta-analysis showed that the proportion of anesthesia complications was 6%, which sounds high, but the range of complications was what would be expected in any population (hypotension, accidental dural puncture, inadequate anesthesia, and bloody tap with no further complications). No spinal, subdural, or epidural hematomas were noted.3 Such hematomas are an extremely rare complication of neuraxial anesthesia, occurring in only 1 in 200,000 or 1 in 250,000 obstetric patients14,15; accordingly, an increase in the rate of hematomas among women with VWD could go undetected. The absence of hematomas among women with VWD as reported in the systematic review does not mean there is not an increase in the rate of hematomas in women with VWD. The relative risk is unknown and caution would be advised.
The third systematic review that the authors performed3 was on TxA treatment in the postpartum period. After screening 41 studies, the authors included 2 retrospective cohort studies.16,17 The majority of the participants had VWD. With very-low-certainty evidence, the authors found that TxA reduces the risk of:
- severe primary PPH (risk ratio [RR], 0.36; 95% confidence interval [CI], 0.05–2.59)
- primary PPH (RR, 0.25; 95% CI, 0.04–1.75)
- secondary PPH (RR, 0.42; 95% CI, 0.02–0.91—does not cross 1.0).
Note that the 95% confidence intervals for severe as well as primary PPH crossed 1.0 and therefore these reductions in risk did not achieve statistical significance. Additionally, there was very-low-certainty evidence on the effect of TxA on blood transfusions, vaginal hematomas, blood loss, and thrombotic complications.3
Continue to: Our recommendations for HMB management...
Our recommendations for HMB management
When first evaluating any woman with HMB, it is important to check a blood count and ferritin level, if not already done. If there is any suggestion of iron deficiency (with or without anemia), we recommend oral iron supplementation. This is best accomplished with slow-release iron supplement formulations (or less expensive generic or house brands that contain less than 65 mg of elemental iron per tablet) taken every other day. Such preparations may cause fewer gastrointestinal adverse effects than other oral iron formulations.18 Although it may appear counterintuitive, oral iron is better absorbed (and also may cause fewer gastrointestinal adverse effects) when taken every other day.19
Initial management of HMB, whether or not a bleeding disorder is present, often consists or oral hormonal management. If no contraindications are present, we recommend initiation of a COC with a short hormone-free interval (for example, a 24/4 formulation). If contraindications to contraceptive doses of estrogen are present, continuous use of norethindrone acetate 5-mg tablets or off-label use of combination tablets with 5 µg of ethinyl estradiol and 1 mg of norethindrone acetate (a formulation approved for the treatment of menopausal symptoms) is appropriate.20
Once a patient is established on oral hormonal management, placement of a levonorgestrel-releasing IUD should be considered. Given that expulsion rates may be higher in women with HMB, if feasible, consider using abdominal ultrasound guidance for IUD placement.
For women with VWD who fail first-line therapy (hormonal management) or are trying to become pregnant, TxA (two 650-mg tablets 3 times daily for up to 5 days during episodes of heavy flow) can reduce HMB.20,21
Our recommendations for management of pregnancy and delivery
The second and third systematic reviews discussed above provide very limited guidance on comprehensive management. The care of the pregnant patient with VWD starts with assessment of VWF levels and making an accurate diagnosis. This usually requires the input of a hematologist or other expert in hemostasis. If no recent VWF levels are available, the ObGyn can obtain a von Willebrand panel that includes VWF antigen, VWF activity (most commonly ristocetin cofactor), and factor VIII.
Levels should be reassessed around 36 weeks’ gestation in anticipation of delivery. VWF levels increase during pregnancy; accordingly, in mild, type 1 VWD, half the time treatment is not necessary.11 If VWF activity is less than 50 IU/dL (less than 50% of normal) at 36 weeks’ gestation, the patient should receive VWF concentrate (dosed in VWF units). This requires consultation with hematology and specialized pharmacy support.
For these reasons, the patient with a VWF level less than 50% should be delivered in a referral center with the necessary resources. Anesthesia should be aware of the patient. Unless
Due to the quantity of fluids administered during labor or at the time of delivery and the coexistent administration of oxytocin, desmopressin (synthetic vasopressin) should not be used without monitoring sodium levels, should not be dosed more than once, or should be avoided altogether due to the risk of water intoxication.
If the patient has sustained VWF and factor VIII levels greater than 50 IU/dL, she would be a candidate to deliver in her local hospital and receive neuraxial anesthesia.
Based on the best data we have for women with VWD, a patient with a VWF greater than 50 IU/dL is no more likely to experience PPH than other women.11 Intravenous TxA can be used for prevention or treatment of immediate postpartum bleeding per protocol (1 g after cord clamp and 1 g 30 minutes or more later).22 Oral TxA can be used for prevention or treatment of delayed postpartum bleeding as per HMB. Regardless of the outcome of any testing during pregnancy, nonsteroidal anti-inflammatory drugs should be avoided postpartum and the patient should be monitored closely for bleeding.
Neonatal care
As for the fetus/neonate, the parents should be aware that the infant has a 50% chance of inheriting VWD. If the baby’s father has no history of bleeding, it is unlikely that the infant would be any more affected than the patient herself. Nonetheless, cord blood (in one or more light blue top tubes) should be obtained at the time of delivery and sent for a von Willebrand panel. If the infant is male, a circumcision should be postponed until VWD is ruled out. In addition, fetal invasive procedures should be avoided during labor. Fetal scalp electrode placement should be avoided. Operative vaginal delivery also should be avoided. Cesarean delivery would be preferred to operative vaginal delivery, but if operative vaginal delivery is unavoidable, use of forceps is preferred to vacuum extraction. ●
Von Willebrand disease (VWD) represents the most common inherited bleeding disorder, with a prevalence of approximately 1 in 1,000 people.
Bruising and mucocutaneous bleeding (epistaxis, gingival bleeding, and bleeding after dental extraction) are the most common presenting symptoms of VWD. Because VWD substantially increases the risk of heavy menstrual bleeding (HMB) and, to some extent, intrapartum bleeding complications, and postpartum hemorrhage, women experience a disproportionate burden from VWD. Thus, ObGyns are likely to be called on to make treatment recommendations in VWD patients with these concerns.1
In 2017, the American Society of Hematology, the International Society on Thrombosis and Haemostasis, the National Hemophilia Foundation, and the World Federation of Hemophilia determined that among clinical issues related to VWD, updating guidelines for women with VWD represented the highest priority.2 Accordingly, an international group of hematologists/coagulation specialists performed systematic literature reviews to address 3 questions faced by women with VWD and their clinicians:
- What are the most effective treatments for HMB?
- What is the safest approach for women desiring neuraxial analgesia for intrapartum pain?
- What is the impact of postpartum administration of tranexamic acid (TxA) on postpartum hemorrhage (PPH)?3
Evidence on management strategies for HMB in women with VWD
The prevalence of HMB in women with VWD ranges from 50% to 92%. Reports suggest that between 5% and 24% of women presenting with this symptom have VWD.3 However, the prevalence of VWD among women seeking care for HMB relates to referral patterns, with the prevalence of VWD substantially higher in patient populations who are referred to clinicians or centers that focus on care of patients with bleeding disorders.
The systematic review authors3 identified 2 comparative studies that assessed the treatment of HMB in women with VWD. One was a crossover trial that enrolled 116 VWD patients with HMB with a mean age of 36 years.4 All participants in this trial chose not to use combination oral contraceptives (COCs) as they had not experienced good results with prior COC use. Trial participants were randomly assigned to receive either intranasal desmopressin (DDAVP; a synthetic analog of the antidiuretic agent vasopressin, which stimulates the release of VWF from endothelial cells) or oral TxA therapy for 2 menstrual cycles. Participants then crossed over to the other drug for 2 additional cycles. Although both agents significantly reduced estimated menstrual blood loss, TxA was more effective in decreasing bleeding than intranasal DDAVP.4
In a retrospective cohort study, investigators compared COC use with intranasal DDAVP in 36 adolescents who had VWD and HMB.5 Participant follow-up ranged from 6 months to 4 years. The estimated efficacy of COCs and intranasal DDAVP was 86% and 77%, respectively, a difference that did not achieve statistical significance. Some of the adolescents who used intranasal DDAVP reported severe headaches and flushing.5
In addition, the systematic review authors3 identified 5 case series that described the use of the levonorgestrel (52 mg)-releasing intrauterine device (LNG 52 IUD) in women with VWD and HMB; 4 of these addressed the efficacy of progestin-releasing IUDs in reducing HMB in this patient population.6-9 Using different approaches to define HMB, the authors of these reports followed between 7 and 26 patients with bleeding disorders (most with confirmed VWD) and HMB for variable amounts of time after placement of an LNG 52 IUD. Many of the women described in these case series had tried other HMB treatments, including COCs, without success. Although these 4 reports assessed different outcomes, all reported that placement of the LNG 52 IUD substantially reduced menstrual blood loss, often resulting in amenorrhea. Several of these reports also noted important improvements in quality of life following LNG 52 IUD placement. One case series reported LNG 52 IUD placement in 13 adolescents with VWD and HMB. The mean time to achieve amenorrhea or occasional spotting was 94 days.6
The fifth report, which followed 20 women (median age, 31 years) with HMB associated with VWD or other bleeding disorders who underwent LNG 52 IUD placement, aimed to describe IUD expulsions and malpositioned IUDs in this population. In this small group of patients, 3 IUD expulsions and 2 malpositioned IUDs were observed. Furthermore, an additional 5 women had their device removed prematurely due to patient dissatisfaction. Accordingly, the IUD continuation rate in this case series was only 50%.10
Evidence on management of pregnancy, delivery, and the postpartum period
Heavy menstrual bleeding is not the only challenge for women with VWD. While pregnancy is accompanied by higher levels of VWF, potentially offsetting the risk of bleeding at the time of delivery, the levels do not achieve the same magnitude as they would in unaffected women.11 Women are at an increased risk of primary PPH12,13 and, importantly, since VWF levels fall exponentially after delivery when women are still experiencing lochia,11 they are at increased risk of secondary or delayed PPH.
Two questions arise frequently in the care of women with VWD at the time of delivery and during the postpartum period:
- What is the safest approach for women who desire neuraxial analgesia for intrapartum pain?
- What is the impact of postpartum administration of TxA on PPH?
The second systematic review the authors performed3 focused on VWF levels in women receiving neuraxial anesthesia during labor. After screening 27 studies, the authors included 5 case series, which did not describe outcomes based on VWF levels but rather described the outcomes of women with VWF levels of greater than 0.50 IU/mL (> 50% of normal compared with a normal standard).
Meta-analysis showed that the proportion of anesthesia complications was 6%, which sounds high, but the range of complications was what would be expected in any population (hypotension, accidental dural puncture, inadequate anesthesia, and bloody tap with no further complications). No spinal, subdural, or epidural hematomas were noted.3 Such hematomas are an extremely rare complication of neuraxial anesthesia, occurring in only 1 in 200,000 or 1 in 250,000 obstetric patients14,15; accordingly, an increase in the rate of hematomas among women with VWD could go undetected. The absence of hematomas among women with VWD as reported in the systematic review does not mean there is not an increase in the rate of hematomas in women with VWD. The relative risk is unknown and caution would be advised.
The third systematic review that the authors performed3 was on TxA treatment in the postpartum period. After screening 41 studies, the authors included 2 retrospective cohort studies.16,17 The majority of the participants had VWD. With very-low-certainty evidence, the authors found that TxA reduces the risk of:
- severe primary PPH (risk ratio [RR], 0.36; 95% confidence interval [CI], 0.05–2.59)
- primary PPH (RR, 0.25; 95% CI, 0.04–1.75)
- secondary PPH (RR, 0.42; 95% CI, 0.02–0.91—does not cross 1.0).
Note that the 95% confidence intervals for severe as well as primary PPH crossed 1.0 and therefore these reductions in risk did not achieve statistical significance. Additionally, there was very-low-certainty evidence on the effect of TxA on blood transfusions, vaginal hematomas, blood loss, and thrombotic complications.3
Continue to: Our recommendations for HMB management...
Our recommendations for HMB management
When first evaluating any woman with HMB, it is important to check a blood count and ferritin level, if not already done. If there is any suggestion of iron deficiency (with or without anemia), we recommend oral iron supplementation. This is best accomplished with slow-release iron supplement formulations (or less expensive generic or house brands that contain less than 65 mg of elemental iron per tablet) taken every other day. Such preparations may cause fewer gastrointestinal adverse effects than other oral iron formulations.18 Although it may appear counterintuitive, oral iron is better absorbed (and also may cause fewer gastrointestinal adverse effects) when taken every other day.19
Initial management of HMB, whether or not a bleeding disorder is present, often consists or oral hormonal management. If no contraindications are present, we recommend initiation of a COC with a short hormone-free interval (for example, a 24/4 formulation). If contraindications to contraceptive doses of estrogen are present, continuous use of norethindrone acetate 5-mg tablets or off-label use of combination tablets with 5 µg of ethinyl estradiol and 1 mg of norethindrone acetate (a formulation approved for the treatment of menopausal symptoms) is appropriate.20
Once a patient is established on oral hormonal management, placement of a levonorgestrel-releasing IUD should be considered. Given that expulsion rates may be higher in women with HMB, if feasible, consider using abdominal ultrasound guidance for IUD placement.
For women with VWD who fail first-line therapy (hormonal management) or are trying to become pregnant, TxA (two 650-mg tablets 3 times daily for up to 5 days during episodes of heavy flow) can reduce HMB.20,21
Our recommendations for management of pregnancy and delivery
The second and third systematic reviews discussed above provide very limited guidance on comprehensive management. The care of the pregnant patient with VWD starts with assessment of VWF levels and making an accurate diagnosis. This usually requires the input of a hematologist or other expert in hemostasis. If no recent VWF levels are available, the ObGyn can obtain a von Willebrand panel that includes VWF antigen, VWF activity (most commonly ristocetin cofactor), and factor VIII.
Levels should be reassessed around 36 weeks’ gestation in anticipation of delivery. VWF levels increase during pregnancy; accordingly, in mild, type 1 VWD, half the time treatment is not necessary.11 If VWF activity is less than 50 IU/dL (less than 50% of normal) at 36 weeks’ gestation, the patient should receive VWF concentrate (dosed in VWF units). This requires consultation with hematology and specialized pharmacy support.
For these reasons, the patient with a VWF level less than 50% should be delivered in a referral center with the necessary resources. Anesthesia should be aware of the patient. Unless
Due to the quantity of fluids administered during labor or at the time of delivery and the coexistent administration of oxytocin, desmopressin (synthetic vasopressin) should not be used without monitoring sodium levels, should not be dosed more than once, or should be avoided altogether due to the risk of water intoxication.
If the patient has sustained VWF and factor VIII levels greater than 50 IU/dL, she would be a candidate to deliver in her local hospital and receive neuraxial anesthesia.
Based on the best data we have for women with VWD, a patient with a VWF greater than 50 IU/dL is no more likely to experience PPH than other women.11 Intravenous TxA can be used for prevention or treatment of immediate postpartum bleeding per protocol (1 g after cord clamp and 1 g 30 minutes or more later).22 Oral TxA can be used for prevention or treatment of delayed postpartum bleeding as per HMB. Regardless of the outcome of any testing during pregnancy, nonsteroidal anti-inflammatory drugs should be avoided postpartum and the patient should be monitored closely for bleeding.
Neonatal care
As for the fetus/neonate, the parents should be aware that the infant has a 50% chance of inheriting VWD. If the baby’s father has no history of bleeding, it is unlikely that the infant would be any more affected than the patient herself. Nonetheless, cord blood (in one or more light blue top tubes) should be obtained at the time of delivery and sent for a von Willebrand panel. If the infant is male, a circumcision should be postponed until VWD is ruled out. In addition, fetal invasive procedures should be avoided during labor. Fetal scalp electrode placement should be avoided. Operative vaginal delivery also should be avoided. Cesarean delivery would be preferred to operative vaginal delivery, but if operative vaginal delivery is unavoidable, use of forceps is preferred to vacuum extraction. ●
- ACOG committee opinion no. 451: Von Willebrand disease in women. Obstet Gynecol. 2009;114:1439-1443. doi: 10.1097 /AOG.0b013e3181c6f975.
- Kalot MA, Al-Khatib M, Connell NT, et al; VWD Working Group. An international survey to inform priorities for new guidance on von Willebrand disease. Hemophilia. 2020;26:106-116. doi: 10.1111/hae.13881.
- Brignardello-Petersen R, El Alayli A, Husainat N, et al. Gynecologic and obstetric management of women with von Willebrand disease: summary of 3 systematic reviews of the literature. Blood Adv. 2022;6:228-237. doi: 10.1182 /bloodadvances.2021005589.
- Kouides PA, Byams VR, Philipp CS, et al. Multisite management study of menorrhagia with abnormal laboratory haemostasis: a prospective crossover study of intranasal desmopressin and oral tranexamic acid. Br J Haematol. 2009;145:212-220. doi: 10.1111/j.1365-2141.2009.07610.x.
- Amesse LS, Pfaff-Amesse T, Leonardi R, et al. Oral contraceptives and DDAVP nasal spray: patterns of use in managing vWD-associated menorrhagia: a single-institution study. J Pediatr Hematol Oncol. 2005;27:357-363. doi: 10.1097/01.mph.0000173175.95152.95.
- Adeyemi-Fowode OA, Santos XM, Dietrich JE, et al. Levonorgestrel-releasing intrauterine device use in female adolescents with heavy menstrual bleeding and bleeding disorders: single institution review. J Pediatr Adolesc Gynecol. 2017;30:479-483. doi: 10.1016/j.jpag.2016.04.001.
- Chi C, Huq FY, Kadir RA. Levonorgestrel-releasing intrauterine system for the management of heavy menstrual bleeding in women with inherited bleeding disorders: long-term follow-up. Contraception. 2011;83:242-247. doi: 10.1016/j.contraception.2010.07.010.
- Kingman CE, Kadir RA, Lee CA, et al. The use of levonorgestrel-releasing intrauterine system for treatment of menorrhagia in women with inherited bleeding disorders. BJOG. 2004;111:1425-1428. doi: 10.1111/j.1471-0528.2004.00305.x.
- Lukes AS, Reardon B, Arepally G. Use of the levonorgestrel-releasing intrauterine system in women with hemostatic disorders. Fertil Steril. 2008;90:673-677. doi: 10.1016 /j.fertnstert.2007.07.1315.
- Rimmer E, Jamieson MA, James P. Malposition and expulsion of the levonorgestrel intrauterine system among women with inherited bleeding disorders. Haemophilia. 2013;19:933-938. doi: 10.1111/hae.12184.
- James AH, Konkle BA, Kouides P, et al. Postpartum von Willebrand factor levels in women with and without von Willebrand disease and implications for prophylaxis. Haemophilia. 2015;21:81-87. doi: 10.1111/hae.12568.
- James AH, Jamison MG. Bleeding events and other complications during pregnancy and childbirth in women with von Willebrand disease. J Thromb Haemost. 2007;5: 1165-1169. doi: 10.1111/j.1538-7836.2007.02563.x.
- Al-Zirqi I, Vangen S, Forsen L, et al. Prevalence and risk factors of severe obstetric haemorrhage. BJOG. 2008;115:1265-1272. doi: 10.1111/j.1471-0528.2008.01859.x.
- Moen V, Dahlgren N, Irestedt L. Severe neurological complications after central neuraxial blockades in Sweden 1990-1999. Anesthesiology. 2004;101:950-959. doi: 10.1097/00000542-200410000-00021.
- D’Angelo R, Smiley RM, Riley ET, et al. Serious complications related to obstetric anesthesia: the serious complication repository project of the Society for Obstetric Anesthesia and Perinatology. Anesthesiology. 2014;120:1505-1512. doi: 10.1097/ALN.000000000000253.
- Govorov I, Lofgren S, Chaireti R, et al. Postpartum hemorrhage in women with von Willebrand disease—a retrospective observational study. PLos One. 2016;11:e0164683. doi: 10.1371/journal.pone.0164683.
- Hawke L, Grabell J, Sim W, et al. Obstetric bleeding among women with inherited bleeding disorders: a retrospective study. Haemophilia. 2016;22:906-911. doi: 10.1111/hae.13067.
- James AH. Iron deficiency anemia in pregnancy. Obstet Gynecol. 2021;138:663-674. doi:10.1097/AOG .000000000000.4559.
- Stoffel NU, Cercamondi CI, Brittenham G, et al. Iron absorption from oral iron supplements given on consecutive versus alternate days and as single morning doses versus twice-daily split dosing in iron-depleted women: two open-label, randomised controlled trials. Lancet Haematol. 2017;4:e524-e533. doi: 10.1016/S2352-3026(17)30182-5.
- Kaunitz AM. Abnormal uterine bleeding in reproductiveage women. JAMA. 2019;321:2126-2127. doi: 10.1001 /jama.2019.5248.
- James AH, Kouides PA, Abdul-Kadir R, et al. Von Willebrand disease and other bleeding disorders in women: consensus on diagnosis and management from an international expert panel. Am J Obstet Gynecol. 2009;201:12.e1-8. doi: 10.1016 /j.ajog.2009.04.024.
- WOMAN Trial Collaborators. Effect of early tranexamic acid administration on mortality, hysterectomy, and other morbidities in women with post-partum haemorrhage (WOMAN): an international, randomised, double-blind, placebo-controlled trial. Lancet. 2017;389:2105-2116. doi: 10.1016/S0140-6736(17)30638-4.
- ACOG committee opinion no. 451: Von Willebrand disease in women. Obstet Gynecol. 2009;114:1439-1443. doi: 10.1097 /AOG.0b013e3181c6f975.
- Kalot MA, Al-Khatib M, Connell NT, et al; VWD Working Group. An international survey to inform priorities for new guidance on von Willebrand disease. Hemophilia. 2020;26:106-116. doi: 10.1111/hae.13881.
- Brignardello-Petersen R, El Alayli A, Husainat N, et al. Gynecologic and obstetric management of women with von Willebrand disease: summary of 3 systematic reviews of the literature. Blood Adv. 2022;6:228-237. doi: 10.1182 /bloodadvances.2021005589.
- Kouides PA, Byams VR, Philipp CS, et al. Multisite management study of menorrhagia with abnormal laboratory haemostasis: a prospective crossover study of intranasal desmopressin and oral tranexamic acid. Br J Haematol. 2009;145:212-220. doi: 10.1111/j.1365-2141.2009.07610.x.
- Amesse LS, Pfaff-Amesse T, Leonardi R, et al. Oral contraceptives and DDAVP nasal spray: patterns of use in managing vWD-associated menorrhagia: a single-institution study. J Pediatr Hematol Oncol. 2005;27:357-363. doi: 10.1097/01.mph.0000173175.95152.95.
- Adeyemi-Fowode OA, Santos XM, Dietrich JE, et al. Levonorgestrel-releasing intrauterine device use in female adolescents with heavy menstrual bleeding and bleeding disorders: single institution review. J Pediatr Adolesc Gynecol. 2017;30:479-483. doi: 10.1016/j.jpag.2016.04.001.
- Chi C, Huq FY, Kadir RA. Levonorgestrel-releasing intrauterine system for the management of heavy menstrual bleeding in women with inherited bleeding disorders: long-term follow-up. Contraception. 2011;83:242-247. doi: 10.1016/j.contraception.2010.07.010.
- Kingman CE, Kadir RA, Lee CA, et al. The use of levonorgestrel-releasing intrauterine system for treatment of menorrhagia in women with inherited bleeding disorders. BJOG. 2004;111:1425-1428. doi: 10.1111/j.1471-0528.2004.00305.x.
- Lukes AS, Reardon B, Arepally G. Use of the levonorgestrel-releasing intrauterine system in women with hemostatic disorders. Fertil Steril. 2008;90:673-677. doi: 10.1016 /j.fertnstert.2007.07.1315.
- Rimmer E, Jamieson MA, James P. Malposition and expulsion of the levonorgestrel intrauterine system among women with inherited bleeding disorders. Haemophilia. 2013;19:933-938. doi: 10.1111/hae.12184.
- James AH, Konkle BA, Kouides P, et al. Postpartum von Willebrand factor levels in women with and without von Willebrand disease and implications for prophylaxis. Haemophilia. 2015;21:81-87. doi: 10.1111/hae.12568.
- James AH, Jamison MG. Bleeding events and other complications during pregnancy and childbirth in women with von Willebrand disease. J Thromb Haemost. 2007;5: 1165-1169. doi: 10.1111/j.1538-7836.2007.02563.x.
- Al-Zirqi I, Vangen S, Forsen L, et al. Prevalence and risk factors of severe obstetric haemorrhage. BJOG. 2008;115:1265-1272. doi: 10.1111/j.1471-0528.2008.01859.x.
- Moen V, Dahlgren N, Irestedt L. Severe neurological complications after central neuraxial blockades in Sweden 1990-1999. Anesthesiology. 2004;101:950-959. doi: 10.1097/00000542-200410000-00021.
- D’Angelo R, Smiley RM, Riley ET, et al. Serious complications related to obstetric anesthesia: the serious complication repository project of the Society for Obstetric Anesthesia and Perinatology. Anesthesiology. 2014;120:1505-1512. doi: 10.1097/ALN.000000000000253.
- Govorov I, Lofgren S, Chaireti R, et al. Postpartum hemorrhage in women with von Willebrand disease—a retrospective observational study. PLos One. 2016;11:e0164683. doi: 10.1371/journal.pone.0164683.
- Hawke L, Grabell J, Sim W, et al. Obstetric bleeding among women with inherited bleeding disorders: a retrospective study. Haemophilia. 2016;22:906-911. doi: 10.1111/hae.13067.
- James AH. Iron deficiency anemia in pregnancy. Obstet Gynecol. 2021;138:663-674. doi:10.1097/AOG .000000000000.4559.
- Stoffel NU, Cercamondi CI, Brittenham G, et al. Iron absorption from oral iron supplements given on consecutive versus alternate days and as single morning doses versus twice-daily split dosing in iron-depleted women: two open-label, randomised controlled trials. Lancet Haematol. 2017;4:e524-e533. doi: 10.1016/S2352-3026(17)30182-5.
- Kaunitz AM. Abnormal uterine bleeding in reproductiveage women. JAMA. 2019;321:2126-2127. doi: 10.1001 /jama.2019.5248.
- James AH, Kouides PA, Abdul-Kadir R, et al. Von Willebrand disease and other bleeding disorders in women: consensus on diagnosis and management from an international expert panel. Am J Obstet Gynecol. 2009;201:12.e1-8. doi: 10.1016 /j.ajog.2009.04.024.
- WOMAN Trial Collaborators. Effect of early tranexamic acid administration on mortality, hysterectomy, and other morbidities in women with post-partum haemorrhage (WOMAN): an international, randomised, double-blind, placebo-controlled trial. Lancet. 2017;389:2105-2116. doi: 10.1016/S0140-6736(17)30638-4.
HIV management in pregnancy
Human immunodeficiency virus (HIV) is a single-stranded enveloped RNA retrovirus that was first described in the 1980s and is known for its severity of systemic immune dysregulation and associated opportunistic infections. It is transmitted through contact with blood or bodily fluids, and it can be transmitted vertically, most often at the time of delivery. Since the advent of antiretroviral therapy, the average life expectancy and natural course of HIV infection has improved notably.1
In 2019, just over 1 million adults and adolescents in the United States were living with the diagnosis of HIV.2 In the same year, the rate of new HIV diagnoses in the United States had stabilized at a rate of 13.2 new cases per 100,000 individuals.2 Among this cohort, individuals identifying as females at birth accounted for 19% of the total population living with HIV.2 Sexual contact was the most common route of transmission, followed by injection drug use—77% and 20%, respectively.2
It is important to note that the incidence and prevalence of HIV does not reflect the individuals who unknowingly are living with the disease. The disease burden associated with HIV infection and the availability of effective treatment modalities has led to the recommendation that all individuals undergo HIV screening at least once in their lifetime.3 Early identification of HIV infection is important to optimize the health of all individuals and future generations.
The interplay between high-risk sexual practices and the risk for HIV exposure and unintended pregnancy places the ObGyn at the forefront of HIV prevention and identification. Early diagnosis and standardized treatment with antiretroviral therapies have led to both a dramatic improvement in adult disease burden and a dramatic decrease in perinatal transmission.4,5 In 2019, perinatal transmission accounted for less than 1% of HIV transmission in the United States.2 This is a decrease of greater than 54% from 2014, which, again, emphasizes the role of the ObGyn in HIV management.6
Preconception care: Gynecologic screening, diagnosis, and management
The Centers for Disease Control and Prevention (CDC) recommends that an individual undergo HIV screening at least once in their lifetime.3 HIV screening algorithms have changed over the last 20 years to reduce the number of false-positive and/or false-negative results obtained through HIV antibody testing alone.7 HIV-1/2 antibody/antigen immunoassay is recommended as the initial screening test. If reactive, this should be followed by an HIV p24-specific antigen test. Reactivity for both the HIV-1/2 immunoassay and the HIV p24-specific antigen test confirms the diagnosis of HIV infection. However, if HIV p24-specific antigen testing is indeterminate or an acute HIV infection is suspected, an HIV nucleic acid test (NAT) should be performed.7,8
Upon a positive diagnosis, a multidisciplinary team approach is recommended to address the mental, social, and physical care of the patient. Team members should include an adult medicine clinician, an infectious disease clinician, an ObGyn, social services staff, and behavioral health support to achieve the goal of obtaining and maintaining the patient’s optimal health status.
TABLE 1 lists the recommended initial laboratory assessments that should follow a new diagnosis of HIV infection. Based on the laboratory results, the indicated vaccinations, antibiotic prophylaxis for opportunistic infections, and optimal combined antiretroviral therapy (cART) can be determined.9 The vaccinations listed in TABLE 2 should be up to date.10,11 Additionally, cervical cancer screening with cytology and human papillomavirus (HPV) testing and treatment should be performed in accordance with the 2019 American Society for Cervical Cancer Prevention (ASCCP) guidelines.12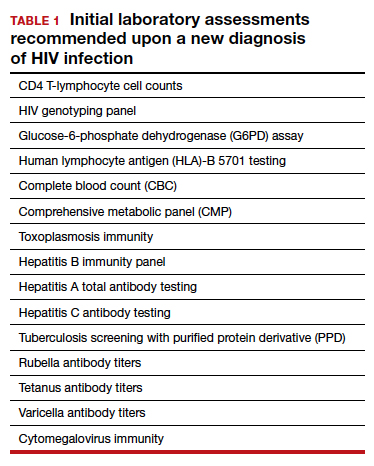
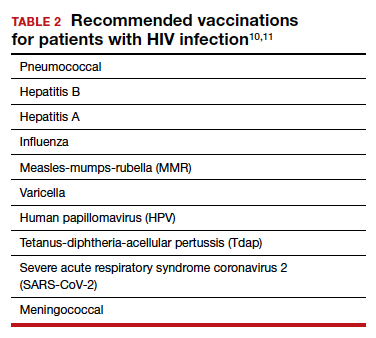
Promptly initiating cART is of utmost importance; this decreases the rate of HIV transmission via sexual contact and decreases the rate of perinatal transmission.5,13 Results of the initial laboratory assessment, hepatitis B status, and desire for pregnancy/contraception should be considered when initiating cART.3,14,15
It is imperative to discuss sharing the positive diagnostic results with the patient’s partner. The CDC provides guidance for these discussions,16 which should address the use of preexposure prophylaxis (PrEP) if partner screening establishes partner serodiscordance (that is, HIV positivity in one partner and HIV negativity in the other partner). PrEP is a single pill approved by the US Food and Drug Administration (FDA) that combines tenofovir 300 mg and emtricitabine 200 mg daily17 and has been recommended since 2012.18-20 PrEP also should be considered in sexually active individuals who have higher-risk behaviors within an area with high HIV prevalence.18-21 Despite the CDC’s strong recommendations for PrEP use, lack of insurance coverage and high cost are barriers to universal use. The National Alliance of State and Territorial AIDS Directors (NASTAD) provides a list of patient and copayment assistance programs that can be found at the NASTAD website: https://nastad.org/prepcost-resources/prep-assitance-programs.
Continue to: Preconception considerations...
Preconception considerations
In individuals with known HIV infection, preconception consultation with an ObGyn or maternal-fetal medicine (MFM) specialist should be recommended prior to conception.22 Preconception recommendations include addressing optimization of maternal medical comorbidities, addressing routine health screening and vaccinations, performing sexually transmitted infection screening, and optimizing HIV disease status.3,22,23
With the assistance of adult medicine and infectious disease clinicians, a cART regimen that is sufficient to reliably maintain viral suppression (that is, viral load < 50 copies/mL on 2 separate occasions at least 3 months apart) and is safe for use in pregnancy should be established.3 In serodiscordant couples, recommended mechanisms to prevent HIV transmission during conception include sustained viral suppression in the HIV-positive partner, PrEP use in the HIV-negative partner, and timing of unprotected intercourse during peak fertility only.3
Antepartum care
The initial prenatal visit
Women who have no prior screening for HIV or prior negative HIV results should undergo HIV screening at the first prenatal visit.3 Screening should be performed in accordance with the “opt out method.”6 Using this method, a woman without a known diagnosis of HIV infection is told that she will undergo HIV screening as a component of routine prenatal care unless she decides that she does not want this test performed.6,24,25 At the time of screening, all pregnant women should be provided with comprehensive information regarding HIV screening, HIV screening results, and the implications of HIV infection on pregnancy.26
In the pregnant patient with confirmed HIV infection, all preconception considerations should be addressed. If not already in place, referrals to appropriate providers (infectious disease specialist, ObGyn, MFM specialist) and ancillary support staff (social services, behavioral health support) should be arranged. All efforts should be implemented to optimize additional medical comorbidities. TABLE 3 lists additional prenatal testing requirements.22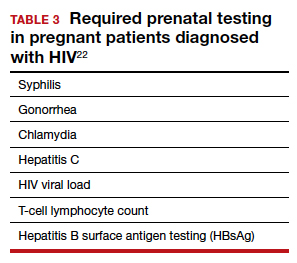
Antiretroviral therapy should be assessed for safety and efficacy in pregnancy and should comply with the CDC recommendations for cART in pregnancy.3 Patients with a T-lymphocyte cell count of less than 200 cells/mm3 and/or a viral load greater than 50 copies/mL despite adherent cART use should be referred to an infectious disease specialist to determine the need for alternative cART and/or the need for chemoprophylaxis against opportunistic infections.23
First and second trimester
Antiretroviral adherence and barriers to adherence should be addressed at every prenatal visit. If the patient is started on antiretroviral therapy in pregnancy or is switched to an alternative cART regimen, viral load assessment should be performed 2 to 4 weeks after the start or change in cART and then repeated monthly until undetectable levels are achieved.3,26 If an undetectable viral load cannot be obtained, cART adherence should be thoroughly evaluated, and the patient should be referred to an infectious disease or HIV treatment specialist.26
If the initial prenatal testing indicates an undetectable viral load, repeat viral load assessment can be performed every 3 months throughout the pregnancy.3 If initial prenatal testing indicates an undetectable HIV viral load and the T-lymphocyte count is greater than 200 cells/mm3, repeat viral load testing can be performed every 6 months to ensure stability.3
Early screening for gestational diabetes should be performed in patients receiving protease inhibitors because these agents may interfere with carbohydrate tolerance.22,26
Continue to: Third trimester...
Third trimester
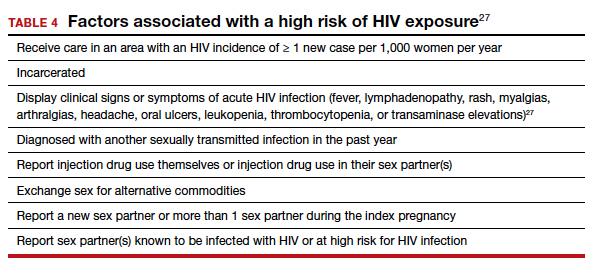
Women with negative HIV screening at the initial prenatal evaluation should undergo repeat HIV screening in the third trimester if they are at high risk for HIV exposure.25 Factors that determine high-risk status are listed in TABLE 4.27 Sexually transmitted infection screening should be repeated in the third trimester.26
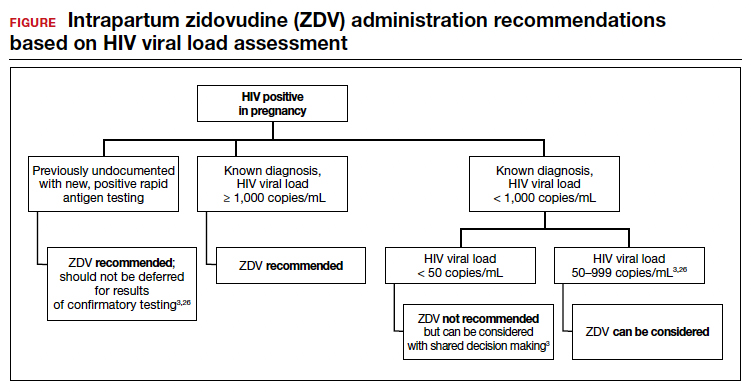
Repeat assessment of the viral load should be completed between 34 and 36 weeks’ gestation or sooner if additional indications for early term or late preterm delivery arise.3 Viral load assessments aid in determining delivery timing and route and the need for zidovudine (ZDV) treatment (FIGURE).
Studies that were performed prior to standardized cART use found higher rates of perinatal transmission associated with vaginal delivery when compared with cesarean delivery (CD).28-30 However, these studies did not account for measures of viral load within their study populations.28-30
In more recent studies performed in the era of standardized cART and viral load monitoring, CD does not provide protection from perinatal transmission when the maternal viral load is less than 1,000 copies/mL at the time of delivery.31 Similarly, delivery prior to 40 weeks’ gestation does not confer protection from perinatal transmission.32
Alternatively, if the maternal viral load is 1,000 copies/mL or greater, CD should be considered to reduce the risk of perinatal transmission. A scheduled, elective CD at 38 weeks’ gestation is recommended in those with a maternal viral load of 1,000 copies/mL or greater and no medical indication for earlier delivery in order to decrease the likelihood of labor onset and/or rupture of membranes prior to delivery.3,33
Intrapartum care
Rapid antigen testing (with follow-up confirmatory testing as indicated) is recommended in patients presenting to labor and delivery with no prior documentation of HIV status.3,8,26
Despite a significant decrease in perinatal transmission rates over the last 30 years, a large proportion of perinatal transmission cases are thought to result from intrapartum fetal exposure. While the mechanism of transmission is not known, a correlation between maternal viral load and risk for perinatal transmission has been shown. A maternal viral load of less than 1,000 copies/mL has been associated with a perinatal transmission risk of less than 2%.34,35 A maternal viral load between 50 and 999 copies/mL has been associated with a perinatal transmission rate of 1% to 2% compared with less than 1% for a maternal viral load of less than 50 copies/mL or undetectable measures.5,36,37
These differences in perinatal transmission rates have prompted the recommendation for administration of ZDV for a minimum of 3 hours prior to delivery in mothers with a viral load of 1,000 copies/mL or greater.4,38 The recommended ZDV dosing is: a 1-hour intravenous loading dose of 2 mg/kg followed by continuous infusion of 1 mg/kg per hour until delivery.39,40 Patients who opt for vaginal delivery despite nonsuppressed viral loads (≥1,000 copies/mL) after thorough perinatal counseling should receive ZDV at the start of labor through delivery.3 All patients should be continued on cART throughout their intrapartum and postpartum course.
The duration of membrane rupture and the use of invasive fetal monitoring (that is, fetal scalp electrodes) have been assessed as mechanisms of perinatal transmission. Although they were performed prior to the standardized use of cART, several studies demonstrated that increased perinatal transmission rates were associated with invasive fetal monitoring.34,41,42 While limited data have refuted this finding in women with suppressed viral loads (< 50 copies/mL), the American College of Obstetricians and Gynecologists recommends avoiding the use of invasive fetal monitoring in labor.26
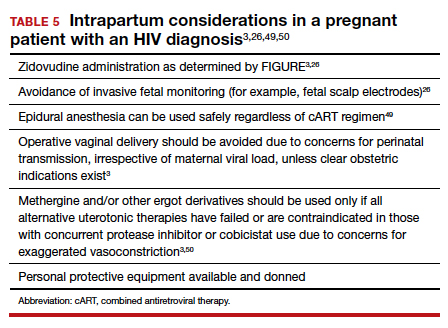
Pre-cART studies demonstrated increased rates of perinatal transmission with longer durations of membrane rupture prior to delivery.43,44 More recent studies have reevaluated this association and determined that the increased perinatal transmission rates are more likely associated with higher maternal viral loads at the time of delivery rather than duration of membrane rupture.45-47 No clear evidence describes when or if CD after the onset of labor or rupture of membranes provides protection from perinatal HIV transmission in pregnant women with HIV receiving no antiretroviral drugs or only ZDV during labor.43,48 CD can be considered for patients in whom scheduled, pre-labor CD was planned who present in labor or with rupture of membranes prior to scheduled CD.26 These, and additional intrapartum considerations, are listed in TABLE 5.49,50
Appropriate personal protective equipment should be available and donned for all providers present throughout intrapartum management and at delivery.23,26 Should any provider injury occur, immediate cleansing of the injury site should be performed, followed by referral to proper workplace supervisors for additional laboratory testing and antiretroviral prophylaxis.
Continue to: Postpartum care...
Postpartum care
Postpartum contraception should be offered and provided in accordance with patient request. Regardless of other birth control methods, strict condom use should be advised. PrEP should be discussed and offered for all partners of serodiscordant couples.
Upon outpatient follow-up, assessment and provision of routine health maintenance should be performed. Any abnormal cervical pathology encountered during prenatal care should be managed in accordance with ASCCP guidelines.12 Follow-up care should be established with adult medicine, infectious disease, and ObGyn clinicians.26
Neonatal considerations
Neonates born to mothers with positive or unknown HIV status should undergo expedited HIV testing.51,52 Consultation should be conducted with pediatric or neonatology colleagues to determine the antiretroviral regimen and duration of therapy based on presumed HIV status of the neonate. Ideally, antiretroviral therapy should be initiated within 6 hours of delivery.3,53
Formula feeding should be implemented as maternal HIV infection is one of the few contraindications to breastfeeding.54,55 The risk of late breast milk transmission, defined as postnatal transmission that occurs after 1 month of age, may vary based on maternal viral load, but it has been reported as high as 8.9 transmissions per 100 person-years of breastfeeding.56
Resources available
Care of the pregnant patient with HIV and the reduction of perinatal transmission both depend on early diagnosis of HIV and effective treatment with cART. Such patients benefit from a team-based care model that includes the ObGyn and/or MFM specialist, infectious disease clinician, pediatrician, and social worker. As guidelines evolve for care of these patients, a reference checklist, such as the examples provided at the Society for Maternal-Fetal Medicine website (smfm.org) or at HIV.gov, provide an outline for:
- management before, during, and after pregnancy
- suggestions for management teams of interest to successfully carry out the checklist requirements
- proposals for measurements of quality performance with the use of checklists in the management of HIV in pregnancy.
In addition, assistance with clinical decision making for patients with HIV in pregnancy can be obtained via telephone consultation with the National Clinician Consultation Center–Perinatal HIV/AIDS (888-448-8765), which is available 24 hours a day, 7 days a week. ●
- Samji H, Cescon A, Hogg RS, et al; North American AIDS Cohort Collaboration on Research and Design (NA-ACCORD) of IeDEA. Closing the gap: increases in life expectancy among treated HIV-positive individuals in the United States and Canada. PLoS One. 2013;8:e81355. doi: 10.1371/journal. pone.0081355.
- Centers for Disease Control and Prevention. May 1, 2021. HIV Surveillance Report, 2019, vol. 32: Diagnosis of HIV infection in the United States and dependent areas, 2019. Accessed February 15, 2022. http://www.cdc.gov/hiv/library/reports /hiv-surveillance.html
- Panel on Antiretroviral Therapy and Medical Management of Children Living with HIV. Guidelines for the use of antiretroviral agents in pediatric HIV infection. https: //clinicalinfo.hiv.gov/en/guidelines/pediatric-arv. Accessed February 15, 2022.
- Connor EM, Sperling RS, Gelber R, et al. Reduction of maternal-infant transmission of human immunodeficiency virus type 1 with zidovudine treatment. Pediatric AIDS Clinical Trials Group Protocol 076 Study Group. N Engl J Med. 1994;331:1173-1180.
- Townsend CL, Byrne L, Cortina-Borja M, et al. Earlier initiation of ART and further decline in mother-to-child HIV transmission rates, 2000-2011. AIDS. 2014;28:1049-1057.
- Centers for Disease Control and Prevention. January 26, 2022. HIV and pregnant women, infants, and children. Accessed February 15, 2022. https://www.cdc.gov/hiv/group/gender /pregnantwomen/index.html
- Centers for Disease Control and Prevention. 2018 Quick reference guide: Recommended laboratory HIV testing algorithm for serum or plasma specimens. National Center for HIV/AIDS, Viral Hepatitis, and TB Prevention (US); Division of HIV/AIDS Prevention; Association of Public Health Laboratories. Updated January 2018. https://stacks. cdc.gov/view/cdc/50872
- Centers for Disease Control and Prevention, Association of Public Health Laboratories. June 27, 2014. Laboratory testing for the diagnosis of HIV infection: updated recommendations. Accessed February 15, 2022. http://stacks.cdc.gov/view /cdc/23447
- Guidelines for the prevention and treatment of opportunistic infections in adults and adolescents with HIV. Updated April 12, 2022. Accessed July 6, 2022. https://clinicalinfo.hiv .gov/en/guidelines/adult-and-adolescent-opportunistic -infection/whats-new-guidelines
- Rubin LG, Levin MJ, Ljungman P, et al. 2013 IDSA clinical practice guideline for vaccination of the immunocompromised host. Clin Infect Dis. 2014;58: e44–e100. doi: 10.1093/cid/cit684.
- Centers for Disease Control and Prevention. ACIP: Guidance for vaccine recommendations for pregnant and breastfeeding women. Accessed July 5, 2022. https://www.cdc.gov /vaccines/hcp/acip-recs/rec-vac-preg.html?CDC_AA _refVal=https%3A%2F%2Fwww.cdc.gov%2Fvaccines%2Facip %2Fcommittee%2Fguidance%2Frec-vac-preg.html
- Perkins RB, Guido RS, Castle PE, et al; for the 2019 ASCCP Risk-Based Management Consensus Guidelines Committee. 2019 ASCCP risk-based management consensus guidelines for abnormal cervical cancer screening tests and cancer precursors. J Low Genit Tract Dis. 2020;24:102-131. doi: 10.1097/LGT.0000000000000525. Erratum in: J Low Genit Tract Dis. 2020;24:427.
- Cohen MS, Chen YQ, McCauley M, et al; HPTN 052 Study Team. Prevention of HIV-1 infection with early antiretroviral therapy. N Engl J Med. 2011;365:493-505.
- Drug interactions between antiretroviral agents and hormonal contraceptives. Accessed July 6, 2022. https://clinicalinfo .hiv.gov/en/table/table-3-drug-interactions-between -antiretroviral-agents-and-hormonal-contraceptives
- Panel on Treatment of HIV During Pregnancy and Prevention of Perinatal Transmission. Recommendations for use of antiretroviral drugs in pregnancy and interventions to reduce perinatal HIV transmission in the United States. Accessed July 7, 2022. https://clinicalinfo.hiv.gov/en/guidelines/perinatal /whats-new-guidelines
- Centers for Disease Control and Prevention. Recommendations for partner services programs for HIV infection, syphilis, gonorrhea, and chlamydial infection. MMWR Recomm Rep. 2008;57(RR-9):1–83.
- Gilead Sciences, Inc. Truvada (emtricitabine 200 mg/ tenofovir disoproxil fumarate 300 mg tablets). Accessed July 6, 2022. https://truvada.com
- Centers for Disease Control and Prevention. Interim guidance for clinicians considering the use of preexposure prophylaxis for the prevention of HIV infection in heterosexually active adults. MMWR Morb Mortal Wkly Rep. 2012;61:586-589.
- Baeten JM, Donnell D, Ndase P, et al; Partners PrEP Study Team. Antiretroviral prophylaxis for HIV prevention in heterosexual men and women. N Engl J Med. 2012;367: 399-410.
- Celum C, Baeten JM. Antiretroviral-based HIV-1 prevention: antiretroviral treatment and pre-exposure prophylaxis. Antivir Ther. 2012;17:1483-1493.
- Thigpen MC, Kebaabetswe PM, Paxton LA, et al; TDF2 Study Group. Antiretroviral preexposure prophylaxis for heterosexual HIV transmission in Botswana. N Engl J Med. 2012;367:423-434.
- Society for Maternal-Fetal Medicine. Special statement: updated checklists for pregnancy management in persons with HIV. Accessed July 5, 2022. https://www.smfm.org /publications/334-smfm-special-statement-updated -checklists-for-pregnancy-management-in-persons-with-hiv
- American College of Obstetricians and Gynecologists. ACOG committee opinion no. 752. Prenatal and perinatal human immunodeficiency virus testing. Obstet Gynecol. 2018;132:e138-e142.
- Human immunodeficiency virus screening. Joint statement of the American Academy of Pediatrics and the American College of Obstetricians and Gynecologists. Pediatrics. 1999;104(1 pt 1):128.
- Branson BM, Handsfield HH, Lampe MA, et al; Centers for Disease Control and Prevention. Revised recommendations for HIV testing of adults, adolescents, and pregnant women in health care settings. MMWR Recomm Rep. 2006; 55(RR-14):1-17.
- American College of Obstetricians and Gynecologists. ACOG committee opinion no. 751. Labor and delivery management of women with human immunodeficiency virus infection. Obstet Gynecol. 2018;132:e131-e137.
- Centers for Disease Control and Prevention. Factors increasing the risk of acquiring or transmitting HIV. November 12, 2019. Accessed July 29, 2022. https://www.cdc .gov/hiv/risk/estimates/riskfactors.html
- Mandelbrot L, Le Chenadec J, Berrebi A, et al. Perinatal HIV1 transmission: interaction between zidovudine prophylaxis and mode of delivery in the French Perinatal Cohort. JAMA. 1998;280:55-60.
- European Mode of Delivery Collaboration. Elective caesarean-section versus vaginal delivery in prevention of vertical HIV-1 transmission: a randomised clinical trial. Lancet. 1999;353:1035-1039.
- International Perinatal HIV Group; Andiman W, Bryson Y, de Martino M, et al. The mode of delivery and the risk of vertical transmission of human immunodeficiency virus type 1—a meta-analysis of 15 prospective cohort studies. N Engl J Med. 1999;340:977-987.
- Briand N, Jasseron C, Sibiude J, et al. Cesarean section for HIV-infected women in the combination antiretroviral therapies era, 2000–2010. Am J Obstet Gynecol. 2013;209: 335.e1-335.e12.
- Scott RK, Chakhtoura N, Burke MM, et al. Delivery after 40 weeks of gestation in pregnant women with well-controlled human immunodeficiency virus. Obstet Gynecol. 2017;130:502-510.
- American College of Obstetricians and Gynecologists. Committee opinion no. 560. Medically indicated late-preterm and early-term deliveries. Obstet Gynecol. 2013;121:908-910.
- Mofenson LM, Lambert JS, Stiehm ER, et al. Risk factors for perinatal transmission of human immunodeficiency virus type 1 in women treated with zidovudine. Pediatric AIDS Clinical Trials Group Study 185 Team. N Engl J Med. 1999;341:385-393.
- Garcia PM, Kalish LA, Pitt J, et al. Maternal levels of plasma human immunodeficiency virus type 1 RNA and the risk of perinatal transmission. Women and Infants Transmission Study Group. N Engl J Med. 1999;341:394-402.
- Briand N, Warszawski J, Mandelbrot L, et al; ANRS-EPF CO1CO11 Study Group. Is intrapartum intravenous zidovudine for prevention of mother-to-child HIV-1 transmission still useful in the combination antiretroviral therapy era? Clin Infect Dis. 2013;57:903-914.
- Myer L, Phillips TK, McIntyre JA, et al. HIV viraemia and mother-to-child transmission risk after antiretroviral therapy initiation in pregnancy in Cape Town, South Africa. HIV Med. 2017;18:80-88.
- Rodman JH, Flynn PM, Robbins B, et al. Systemic pharmacokinetics and cellular pharmacology of zidovudine in human immunodeficiency virus type 1-infected women and newborn infants. J Infect Dis. 1999;180:1844-1850.
- Wade NA, Birkhead GS, Warren BL, et al. Abbreviated regimens of zidovudine prophylaxis and perinatal transmission of the human immunodeficiency virus. N Engl J Med. 1998;339:1409-1414.
- Nielsen-Saines K, Watts HD, Veloso VS, et al; NICHD HPTN 040/PACTG 1043 Protocol Team. Three postpartum antiretroviral regimens to prevent intrapartum HIV infection. N Engl J Med. 2012;366:2368-2379.
- Mandelbrot L, Mayaux MJ, Bongain A, et al. Obstetric factors and mother-to-child transmission of human immunodeficiency virus type 1: the French perinatal cohorts. SEROGEST French Pediatric HIV Infection Study Group. Am J Obstet Gynecol. 1996;175(3 pt 1):661-667.
- Shapiro DE, Sperling RS, Mandelbrot L, et al. Risk factors for perinatal human immunodeficiency virus transmission in patients receiving zidovudine prophylaxis. Pediatric AIDS Clinical Trials Group protocol 076 Study Group. Obstet Gynecol. 1999;94:897-908.
- International Perinatal HIV Group. Duration of ruptured membranes and vertical transmission of HIV-1: a meta-analysis from 15 prospective cohort studies. AIDS. 2001;15:357-368.
- Nielsen TF, Hokegard KH. Postoperative cesarean section morbidity: a prospective study. Am J Obstet Gynecol. 1983;146:911-916.
- Mark S, Murphy KE, Read S, et al. HIV mother-to-child transmission, mode of delivery, and duration of rupture of membranes: experience in the current era. Infect Dis Obstet Gynecol. 2012;2012:267969.
- Cotter AM, Brookfield KF, Duthely LM, et al. Duration of membrane rupture and risk of perinatal transmission of HIV1 in the era of combination antiretroviral therapy. Am J Obstet Gynecol. 2012;207:482.e1-482.e5.
- Peters H, Byrne L, De Ruiter A, et al. Duration of ruptured membranes and mother-to-child HIV transmission: a prospective population-based surveillance study. BJOG. 2016;123:975-981.
- Jamieson DJ, Read JS, Kourtis AP, et al. Cesarean delivery for HIV-infected women: recommendations and controversies. Am J Obstet Gynecol. 2007;197(3 suppl):S96-S100.
- Cambic CR, Avram MJ, Gupta DK, et al. Effect of ritonavir-induced cytochrome P450 3A4 inhibition on plasma fentanyl concentrations during patient-controlled epidural labor analgesia: a pharmacokinetic simulation. Int J Obstet Anesth. 2014;23:45-51.
- Navarro J, Curran A, Burgos J, et al. Acute leg ischaemia in an HIV-infected patient receiving antiretroviral treatment. Antivir Ther. 2017;22:89-90.
- American Academy of Pediatrics, American College of Obstetricians and Gynecologists. Guidelines for Perinatal Care. 8th ed. American Academy of Pediatrics, American College of Obstetricians and Gynecologists; 2017.
- Siberry GK, Abzug MJ, Nachman S, et al; Panel on Opportunistic Infections in HIV-Exposed and HIV-Infected Children. Guidelines for the prevention and treatment of opportunistic infections in HIV-exposed and HIV-infected children: recommendations from the National Institutes of Health, Centers for Disease Control and Prevention, the HIV Medicine Association of the Infectious Diseases Society of America, the Pediatric Infectious Diseases Society, and the American Academy of Pediatrics. Pediatr Infect Dis J. 32(suppl 2[0 2]):i–KK4.
- Panel on Antiretroviral Therapy and Medical Management of Children Living with HIV. Guidelines for the use of antiretroviral agents in pediatric HIV infection. Accessed February 15, 2022. https://clinicalinfo.hiv.gov/en/guidelines /pediatric-arv
- Committee on Health Care for Underserved Women, American College of Obstetricians and Gynecologists. ACOG committee opinion no. 361. Breastfeeding: maternal and infant aspects. Obstet Gynecol. 2007;109(2 pt 1):479-480.
- Committee on Pediatric AIDS; Mofenson LM, Flynn PM, Aldrovandi GM, et al. Infant feeding and transmission of human immunodeficiency virus in the United States. Pediatrics. 2013;131:391-396.
- Breastfeeding and HIV International Transmission Study Group; Coutsoudis A, Dabis F, Fawzi W, et al. Late postnatal transmission of HIV-1 in breast-fed children: an individual patient data meta-analysis. J Infect Dis. 2004;189:2154-2166.
Human immunodeficiency virus (HIV) is a single-stranded enveloped RNA retrovirus that was first described in the 1980s and is known for its severity of systemic immune dysregulation and associated opportunistic infections. It is transmitted through contact with blood or bodily fluids, and it can be transmitted vertically, most often at the time of delivery. Since the advent of antiretroviral therapy, the average life expectancy and natural course of HIV infection has improved notably.1
In 2019, just over 1 million adults and adolescents in the United States were living with the diagnosis of HIV.2 In the same year, the rate of new HIV diagnoses in the United States had stabilized at a rate of 13.2 new cases per 100,000 individuals.2 Among this cohort, individuals identifying as females at birth accounted for 19% of the total population living with HIV.2 Sexual contact was the most common route of transmission, followed by injection drug use—77% and 20%, respectively.2
It is important to note that the incidence and prevalence of HIV does not reflect the individuals who unknowingly are living with the disease. The disease burden associated with HIV infection and the availability of effective treatment modalities has led to the recommendation that all individuals undergo HIV screening at least once in their lifetime.3 Early identification of HIV infection is important to optimize the health of all individuals and future generations.
The interplay between high-risk sexual practices and the risk for HIV exposure and unintended pregnancy places the ObGyn at the forefront of HIV prevention and identification. Early diagnosis and standardized treatment with antiretroviral therapies have led to both a dramatic improvement in adult disease burden and a dramatic decrease in perinatal transmission.4,5 In 2019, perinatal transmission accounted for less than 1% of HIV transmission in the United States.2 This is a decrease of greater than 54% from 2014, which, again, emphasizes the role of the ObGyn in HIV management.6
Preconception care: Gynecologic screening, diagnosis, and management
The Centers for Disease Control and Prevention (CDC) recommends that an individual undergo HIV screening at least once in their lifetime.3 HIV screening algorithms have changed over the last 20 years to reduce the number of false-positive and/or false-negative results obtained through HIV antibody testing alone.7 HIV-1/2 antibody/antigen immunoassay is recommended as the initial screening test. If reactive, this should be followed by an HIV p24-specific antigen test. Reactivity for both the HIV-1/2 immunoassay and the HIV p24-specific antigen test confirms the diagnosis of HIV infection. However, if HIV p24-specific antigen testing is indeterminate or an acute HIV infection is suspected, an HIV nucleic acid test (NAT) should be performed.7,8
Upon a positive diagnosis, a multidisciplinary team approach is recommended to address the mental, social, and physical care of the patient. Team members should include an adult medicine clinician, an infectious disease clinician, an ObGyn, social services staff, and behavioral health support to achieve the goal of obtaining and maintaining the patient’s optimal health status.
TABLE 1 lists the recommended initial laboratory assessments that should follow a new diagnosis of HIV infection. Based on the laboratory results, the indicated vaccinations, antibiotic prophylaxis for opportunistic infections, and optimal combined antiretroviral therapy (cART) can be determined.9 The vaccinations listed in TABLE 2 should be up to date.10,11 Additionally, cervical cancer screening with cytology and human papillomavirus (HPV) testing and treatment should be performed in accordance with the 2019 American Society for Cervical Cancer Prevention (ASCCP) guidelines.12

Promptly initiating cART is of utmost importance; this decreases the rate of HIV transmission via sexual contact and decreases the rate of perinatal transmission.5,13 Results of the initial laboratory assessment, hepatitis B status, and desire for pregnancy/contraception should be considered when initiating cART.3,14,15
It is imperative to discuss sharing the positive diagnostic results with the patient’s partner. The CDC provides guidance for these discussions,16 which should address the use of preexposure prophylaxis (PrEP) if partner screening establishes partner serodiscordance (that is, HIV positivity in one partner and HIV negativity in the other partner). PrEP is a single pill approved by the US Food and Drug Administration (FDA) that combines tenofovir 300 mg and emtricitabine 200 mg daily17 and has been recommended since 2012.18-20 PrEP also should be considered in sexually active individuals who have higher-risk behaviors within an area with high HIV prevalence.18-21 Despite the CDC’s strong recommendations for PrEP use, lack of insurance coverage and high cost are barriers to universal use. The National Alliance of State and Territorial AIDS Directors (NASTAD) provides a list of patient and copayment assistance programs that can be found at the NASTAD website: https://nastad.org/prepcost-resources/prep-assitance-programs.
Continue to: Preconception considerations...
Preconception considerations
In individuals with known HIV infection, preconception consultation with an ObGyn or maternal-fetal medicine (MFM) specialist should be recommended prior to conception.22 Preconception recommendations include addressing optimization of maternal medical comorbidities, addressing routine health screening and vaccinations, performing sexually transmitted infection screening, and optimizing HIV disease status.3,22,23
With the assistance of adult medicine and infectious disease clinicians, a cART regimen that is sufficient to reliably maintain viral suppression (that is, viral load < 50 copies/mL on 2 separate occasions at least 3 months apart) and is safe for use in pregnancy should be established.3 In serodiscordant couples, recommended mechanisms to prevent HIV transmission during conception include sustained viral suppression in the HIV-positive partner, PrEP use in the HIV-negative partner, and timing of unprotected intercourse during peak fertility only.3
Antepartum care
The initial prenatal visit
Women who have no prior screening for HIV or prior negative HIV results should undergo HIV screening at the first prenatal visit.3 Screening should be performed in accordance with the “opt out method.”6 Using this method, a woman without a known diagnosis of HIV infection is told that she will undergo HIV screening as a component of routine prenatal care unless she decides that she does not want this test performed.6,24,25 At the time of screening, all pregnant women should be provided with comprehensive information regarding HIV screening, HIV screening results, and the implications of HIV infection on pregnancy.26
In the pregnant patient with confirmed HIV infection, all preconception considerations should be addressed. If not already in place, referrals to appropriate providers (infectious disease specialist, ObGyn, MFM specialist) and ancillary support staff (social services, behavioral health support) should be arranged. All efforts should be implemented to optimize additional medical comorbidities. TABLE 3 lists additional prenatal testing requirements.22
Antiretroviral therapy should be assessed for safety and efficacy in pregnancy and should comply with the CDC recommendations for cART in pregnancy.3 Patients with a T-lymphocyte cell count of less than 200 cells/mm3 and/or a viral load greater than 50 copies/mL despite adherent cART use should be referred to an infectious disease specialist to determine the need for alternative cART and/or the need for chemoprophylaxis against opportunistic infections.23
First and second trimester
Antiretroviral adherence and barriers to adherence should be addressed at every prenatal visit. If the patient is started on antiretroviral therapy in pregnancy or is switched to an alternative cART regimen, viral load assessment should be performed 2 to 4 weeks after the start or change in cART and then repeated monthly until undetectable levels are achieved.3,26 If an undetectable viral load cannot be obtained, cART adherence should be thoroughly evaluated, and the patient should be referred to an infectious disease or HIV treatment specialist.26
If the initial prenatal testing indicates an undetectable viral load, repeat viral load assessment can be performed every 3 months throughout the pregnancy.3 If initial prenatal testing indicates an undetectable HIV viral load and the T-lymphocyte count is greater than 200 cells/mm3, repeat viral load testing can be performed every 6 months to ensure stability.3
Early screening for gestational diabetes should be performed in patients receiving protease inhibitors because these agents may interfere with carbohydrate tolerance.22,26
Continue to: Third trimester...
Third trimester
Women with negative HIV screening at the initial prenatal evaluation should undergo repeat HIV screening in the third trimester if they are at high risk for HIV exposure.25 Factors that determine high-risk status are listed in TABLE 4.27 Sexually transmitted infection screening should be repeated in the third trimester.26

Repeat assessment of the viral load should be completed between 34 and 36 weeks’ gestation or sooner if additional indications for early term or late preterm delivery arise.3 Viral load assessments aid in determining delivery timing and route and the need for zidovudine (ZDV) treatment (FIGURE).

Studies that were performed prior to standardized cART use found higher rates of perinatal transmission associated with vaginal delivery when compared with cesarean delivery (CD).28-30 However, these studies did not account for measures of viral load within their study populations.28-30
In more recent studies performed in the era of standardized cART and viral load monitoring, CD does not provide protection from perinatal transmission when the maternal viral load is less than 1,000 copies/mL at the time of delivery.31 Similarly, delivery prior to 40 weeks’ gestation does not confer protection from perinatal transmission.32
Alternatively, if the maternal viral load is 1,000 copies/mL or greater, CD should be considered to reduce the risk of perinatal transmission. A scheduled, elective CD at 38 weeks’ gestation is recommended in those with a maternal viral load of 1,000 copies/mL or greater and no medical indication for earlier delivery in order to decrease the likelihood of labor onset and/or rupture of membranes prior to delivery.3,33
Intrapartum care
Rapid antigen testing (with follow-up confirmatory testing as indicated) is recommended in patients presenting to labor and delivery with no prior documentation of HIV status.3,8,26
Despite a significant decrease in perinatal transmission rates over the last 30 years, a large proportion of perinatal transmission cases are thought to result from intrapartum fetal exposure. While the mechanism of transmission is not known, a correlation between maternal viral load and risk for perinatal transmission has been shown. A maternal viral load of less than 1,000 copies/mL has been associated with a perinatal transmission risk of less than 2%.34,35 A maternal viral load between 50 and 999 copies/mL has been associated with a perinatal transmission rate of 1% to 2% compared with less than 1% for a maternal viral load of less than 50 copies/mL or undetectable measures.5,36,37
These differences in perinatal transmission rates have prompted the recommendation for administration of ZDV for a minimum of 3 hours prior to delivery in mothers with a viral load of 1,000 copies/mL or greater.4,38 The recommended ZDV dosing is: a 1-hour intravenous loading dose of 2 mg/kg followed by continuous infusion of 1 mg/kg per hour until delivery.39,40 Patients who opt for vaginal delivery despite nonsuppressed viral loads (≥1,000 copies/mL) after thorough perinatal counseling should receive ZDV at the start of labor through delivery.3 All patients should be continued on cART throughout their intrapartum and postpartum course.
The duration of membrane rupture and the use of invasive fetal monitoring (that is, fetal scalp electrodes) have been assessed as mechanisms of perinatal transmission. Although they were performed prior to the standardized use of cART, several studies demonstrated that increased perinatal transmission rates were associated with invasive fetal monitoring.34,41,42 While limited data have refuted this finding in women with suppressed viral loads (< 50 copies/mL), the American College of Obstetricians and Gynecologists recommends avoiding the use of invasive fetal monitoring in labor.26
Pre-cART studies demonstrated increased rates of perinatal transmission with longer durations of membrane rupture prior to delivery.43,44 More recent studies have reevaluated this association and determined that the increased perinatal transmission rates are more likely associated with higher maternal viral loads at the time of delivery rather than duration of membrane rupture.45-47 No clear evidence describes when or if CD after the onset of labor or rupture of membranes provides protection from perinatal HIV transmission in pregnant women with HIV receiving no antiretroviral drugs or only ZDV during labor.43,48 CD can be considered for patients in whom scheduled, pre-labor CD was planned who present in labor or with rupture of membranes prior to scheduled CD.26 These, and additional intrapartum considerations, are listed in TABLE 5.49,50
Appropriate personal protective equipment should be available and donned for all providers present throughout intrapartum management and at delivery.23,26 Should any provider injury occur, immediate cleansing of the injury site should be performed, followed by referral to proper workplace supervisors for additional laboratory testing and antiretroviral prophylaxis.

Continue to: Postpartum care...
Postpartum care
Postpartum contraception should be offered and provided in accordance with patient request. Regardless of other birth control methods, strict condom use should be advised. PrEP should be discussed and offered for all partners of serodiscordant couples.
Upon outpatient follow-up, assessment and provision of routine health maintenance should be performed. Any abnormal cervical pathology encountered during prenatal care should be managed in accordance with ASCCP guidelines.12 Follow-up care should be established with adult medicine, infectious disease, and ObGyn clinicians.26
Neonatal considerations
Neonates born to mothers with positive or unknown HIV status should undergo expedited HIV testing.51,52 Consultation should be conducted with pediatric or neonatology colleagues to determine the antiretroviral regimen and duration of therapy based on presumed HIV status of the neonate. Ideally, antiretroviral therapy should be initiated within 6 hours of delivery.3,53
Formula feeding should be implemented as maternal HIV infection is one of the few contraindications to breastfeeding.54,55 The risk of late breast milk transmission, defined as postnatal transmission that occurs after 1 month of age, may vary based on maternal viral load, but it has been reported as high as 8.9 transmissions per 100 person-years of breastfeeding.56
Resources available
Care of the pregnant patient with HIV and the reduction of perinatal transmission both depend on early diagnosis of HIV and effective treatment with cART. Such patients benefit from a team-based care model that includes the ObGyn and/or MFM specialist, infectious disease clinician, pediatrician, and social worker. As guidelines evolve for care of these patients, a reference checklist, such as the examples provided at the Society for Maternal-Fetal Medicine website (smfm.org) or at HIV.gov, provide an outline for:
- management before, during, and after pregnancy
- suggestions for management teams of interest to successfully carry out the checklist requirements
- proposals for measurements of quality performance with the use of checklists in the management of HIV in pregnancy.
In addition, assistance with clinical decision making for patients with HIV in pregnancy can be obtained via telephone consultation with the National Clinician Consultation Center–Perinatal HIV/AIDS (888-448-8765), which is available 24 hours a day, 7 days a week. ●
Human immunodeficiency virus (HIV) is a single-stranded enveloped RNA retrovirus that was first described in the 1980s and is known for its severity of systemic immune dysregulation and associated opportunistic infections. It is transmitted through contact with blood or bodily fluids, and it can be transmitted vertically, most often at the time of delivery. Since the advent of antiretroviral therapy, the average life expectancy and natural course of HIV infection has improved notably.1
In 2019, just over 1 million adults and adolescents in the United States were living with the diagnosis of HIV.2 In the same year, the rate of new HIV diagnoses in the United States had stabilized at a rate of 13.2 new cases per 100,000 individuals.2 Among this cohort, individuals identifying as females at birth accounted for 19% of the total population living with HIV.2 Sexual contact was the most common route of transmission, followed by injection drug use—77% and 20%, respectively.2
It is important to note that the incidence and prevalence of HIV does not reflect the individuals who unknowingly are living with the disease. The disease burden associated with HIV infection and the availability of effective treatment modalities has led to the recommendation that all individuals undergo HIV screening at least once in their lifetime.3 Early identification of HIV infection is important to optimize the health of all individuals and future generations.
The interplay between high-risk sexual practices and the risk for HIV exposure and unintended pregnancy places the ObGyn at the forefront of HIV prevention and identification. Early diagnosis and standardized treatment with antiretroviral therapies have led to both a dramatic improvement in adult disease burden and a dramatic decrease in perinatal transmission.4,5 In 2019, perinatal transmission accounted for less than 1% of HIV transmission in the United States.2 This is a decrease of greater than 54% from 2014, which, again, emphasizes the role of the ObGyn in HIV management.6
Preconception care: Gynecologic screening, diagnosis, and management
The Centers for Disease Control and Prevention (CDC) recommends that an individual undergo HIV screening at least once in their lifetime.3 HIV screening algorithms have changed over the last 20 years to reduce the number of false-positive and/or false-negative results obtained through HIV antibody testing alone.7 HIV-1/2 antibody/antigen immunoassay is recommended as the initial screening test. If reactive, this should be followed by an HIV p24-specific antigen test. Reactivity for both the HIV-1/2 immunoassay and the HIV p24-specific antigen test confirms the diagnosis of HIV infection. However, if HIV p24-specific antigen testing is indeterminate or an acute HIV infection is suspected, an HIV nucleic acid test (NAT) should be performed.7,8
Upon a positive diagnosis, a multidisciplinary team approach is recommended to address the mental, social, and physical care of the patient. Team members should include an adult medicine clinician, an infectious disease clinician, an ObGyn, social services staff, and behavioral health support to achieve the goal of obtaining and maintaining the patient’s optimal health status.
TABLE 1 lists the recommended initial laboratory assessments that should follow a new diagnosis of HIV infection. Based on the laboratory results, the indicated vaccinations, antibiotic prophylaxis for opportunistic infections, and optimal combined antiretroviral therapy (cART) can be determined.9 The vaccinations listed in TABLE 2 should be up to date.10,11 Additionally, cervical cancer screening with cytology and human papillomavirus (HPV) testing and treatment should be performed in accordance with the 2019 American Society for Cervical Cancer Prevention (ASCCP) guidelines.12

Promptly initiating cART is of utmost importance; this decreases the rate of HIV transmission via sexual contact and decreases the rate of perinatal transmission.5,13 Results of the initial laboratory assessment, hepatitis B status, and desire for pregnancy/contraception should be considered when initiating cART.3,14,15
It is imperative to discuss sharing the positive diagnostic results with the patient’s partner. The CDC provides guidance for these discussions,16 which should address the use of preexposure prophylaxis (PrEP) if partner screening establishes partner serodiscordance (that is, HIV positivity in one partner and HIV negativity in the other partner). PrEP is a single pill approved by the US Food and Drug Administration (FDA) that combines tenofovir 300 mg and emtricitabine 200 mg daily17 and has been recommended since 2012.18-20 PrEP also should be considered in sexually active individuals who have higher-risk behaviors within an area with high HIV prevalence.18-21 Despite the CDC’s strong recommendations for PrEP use, lack of insurance coverage and high cost are barriers to universal use. The National Alliance of State and Territorial AIDS Directors (NASTAD) provides a list of patient and copayment assistance programs that can be found at the NASTAD website: https://nastad.org/prepcost-resources/prep-assitance-programs.
Continue to: Preconception considerations...
Preconception considerations
In individuals with known HIV infection, preconception consultation with an ObGyn or maternal-fetal medicine (MFM) specialist should be recommended prior to conception.22 Preconception recommendations include addressing optimization of maternal medical comorbidities, addressing routine health screening and vaccinations, performing sexually transmitted infection screening, and optimizing HIV disease status.3,22,23
With the assistance of adult medicine and infectious disease clinicians, a cART regimen that is sufficient to reliably maintain viral suppression (that is, viral load < 50 copies/mL on 2 separate occasions at least 3 months apart) and is safe for use in pregnancy should be established.3 In serodiscordant couples, recommended mechanisms to prevent HIV transmission during conception include sustained viral suppression in the HIV-positive partner, PrEP use in the HIV-negative partner, and timing of unprotected intercourse during peak fertility only.3
Antepartum care
The initial prenatal visit
Women who have no prior screening for HIV or prior negative HIV results should undergo HIV screening at the first prenatal visit.3 Screening should be performed in accordance with the “opt out method.”6 Using this method, a woman without a known diagnosis of HIV infection is told that she will undergo HIV screening as a component of routine prenatal care unless she decides that she does not want this test performed.6,24,25 At the time of screening, all pregnant women should be provided with comprehensive information regarding HIV screening, HIV screening results, and the implications of HIV infection on pregnancy.26
In the pregnant patient with confirmed HIV infection, all preconception considerations should be addressed. If not already in place, referrals to appropriate providers (infectious disease specialist, ObGyn, MFM specialist) and ancillary support staff (social services, behavioral health support) should be arranged. All efforts should be implemented to optimize additional medical comorbidities. TABLE 3 lists additional prenatal testing requirements.22
Antiretroviral therapy should be assessed for safety and efficacy in pregnancy and should comply with the CDC recommendations for cART in pregnancy.3 Patients with a T-lymphocyte cell count of less than 200 cells/mm3 and/or a viral load greater than 50 copies/mL despite adherent cART use should be referred to an infectious disease specialist to determine the need for alternative cART and/or the need for chemoprophylaxis against opportunistic infections.23
First and second trimester
Antiretroviral adherence and barriers to adherence should be addressed at every prenatal visit. If the patient is started on antiretroviral therapy in pregnancy or is switched to an alternative cART regimen, viral load assessment should be performed 2 to 4 weeks after the start or change in cART and then repeated monthly until undetectable levels are achieved.3,26 If an undetectable viral load cannot be obtained, cART adherence should be thoroughly evaluated, and the patient should be referred to an infectious disease or HIV treatment specialist.26
If the initial prenatal testing indicates an undetectable viral load, repeat viral load assessment can be performed every 3 months throughout the pregnancy.3 If initial prenatal testing indicates an undetectable HIV viral load and the T-lymphocyte count is greater than 200 cells/mm3, repeat viral load testing can be performed every 6 months to ensure stability.3
Early screening for gestational diabetes should be performed in patients receiving protease inhibitors because these agents may interfere with carbohydrate tolerance.22,26
Continue to: Third trimester...
Third trimester
Women with negative HIV screening at the initial prenatal evaluation should undergo repeat HIV screening in the third trimester if they are at high risk for HIV exposure.25 Factors that determine high-risk status are listed in TABLE 4.27 Sexually transmitted infection screening should be repeated in the third trimester.26

Repeat assessment of the viral load should be completed between 34 and 36 weeks’ gestation or sooner if additional indications for early term or late preterm delivery arise.3 Viral load assessments aid in determining delivery timing and route and the need for zidovudine (ZDV) treatment (FIGURE).

Studies that were performed prior to standardized cART use found higher rates of perinatal transmission associated with vaginal delivery when compared with cesarean delivery (CD).28-30 However, these studies did not account for measures of viral load within their study populations.28-30
In more recent studies performed in the era of standardized cART and viral load monitoring, CD does not provide protection from perinatal transmission when the maternal viral load is less than 1,000 copies/mL at the time of delivery.31 Similarly, delivery prior to 40 weeks’ gestation does not confer protection from perinatal transmission.32
Alternatively, if the maternal viral load is 1,000 copies/mL or greater, CD should be considered to reduce the risk of perinatal transmission. A scheduled, elective CD at 38 weeks’ gestation is recommended in those with a maternal viral load of 1,000 copies/mL or greater and no medical indication for earlier delivery in order to decrease the likelihood of labor onset and/or rupture of membranes prior to delivery.3,33
Intrapartum care
Rapid antigen testing (with follow-up confirmatory testing as indicated) is recommended in patients presenting to labor and delivery with no prior documentation of HIV status.3,8,26
Despite a significant decrease in perinatal transmission rates over the last 30 years, a large proportion of perinatal transmission cases are thought to result from intrapartum fetal exposure. While the mechanism of transmission is not known, a correlation between maternal viral load and risk for perinatal transmission has been shown. A maternal viral load of less than 1,000 copies/mL has been associated with a perinatal transmission risk of less than 2%.34,35 A maternal viral load between 50 and 999 copies/mL has been associated with a perinatal transmission rate of 1% to 2% compared with less than 1% for a maternal viral load of less than 50 copies/mL or undetectable measures.5,36,37
These differences in perinatal transmission rates have prompted the recommendation for administration of ZDV for a minimum of 3 hours prior to delivery in mothers with a viral load of 1,000 copies/mL or greater.4,38 The recommended ZDV dosing is: a 1-hour intravenous loading dose of 2 mg/kg followed by continuous infusion of 1 mg/kg per hour until delivery.39,40 Patients who opt for vaginal delivery despite nonsuppressed viral loads (≥1,000 copies/mL) after thorough perinatal counseling should receive ZDV at the start of labor through delivery.3 All patients should be continued on cART throughout their intrapartum and postpartum course.
The duration of membrane rupture and the use of invasive fetal monitoring (that is, fetal scalp electrodes) have been assessed as mechanisms of perinatal transmission. Although they were performed prior to the standardized use of cART, several studies demonstrated that increased perinatal transmission rates were associated with invasive fetal monitoring.34,41,42 While limited data have refuted this finding in women with suppressed viral loads (< 50 copies/mL), the American College of Obstetricians and Gynecologists recommends avoiding the use of invasive fetal monitoring in labor.26
Pre-cART studies demonstrated increased rates of perinatal transmission with longer durations of membrane rupture prior to delivery.43,44 More recent studies have reevaluated this association and determined that the increased perinatal transmission rates are more likely associated with higher maternal viral loads at the time of delivery rather than duration of membrane rupture.45-47 No clear evidence describes when or if CD after the onset of labor or rupture of membranes provides protection from perinatal HIV transmission in pregnant women with HIV receiving no antiretroviral drugs or only ZDV during labor.43,48 CD can be considered for patients in whom scheduled, pre-labor CD was planned who present in labor or with rupture of membranes prior to scheduled CD.26 These, and additional intrapartum considerations, are listed in TABLE 5.49,50
Appropriate personal protective equipment should be available and donned for all providers present throughout intrapartum management and at delivery.23,26 Should any provider injury occur, immediate cleansing of the injury site should be performed, followed by referral to proper workplace supervisors for additional laboratory testing and antiretroviral prophylaxis.

Continue to: Postpartum care...
Postpartum care
Postpartum contraception should be offered and provided in accordance with patient request. Regardless of other birth control methods, strict condom use should be advised. PrEP should be discussed and offered for all partners of serodiscordant couples.
Upon outpatient follow-up, assessment and provision of routine health maintenance should be performed. Any abnormal cervical pathology encountered during prenatal care should be managed in accordance with ASCCP guidelines.12 Follow-up care should be established with adult medicine, infectious disease, and ObGyn clinicians.26
Neonatal considerations
Neonates born to mothers with positive or unknown HIV status should undergo expedited HIV testing.51,52 Consultation should be conducted with pediatric or neonatology colleagues to determine the antiretroviral regimen and duration of therapy based on presumed HIV status of the neonate. Ideally, antiretroviral therapy should be initiated within 6 hours of delivery.3,53
Formula feeding should be implemented as maternal HIV infection is one of the few contraindications to breastfeeding.54,55 The risk of late breast milk transmission, defined as postnatal transmission that occurs after 1 month of age, may vary based on maternal viral load, but it has been reported as high as 8.9 transmissions per 100 person-years of breastfeeding.56
Resources available
Care of the pregnant patient with HIV and the reduction of perinatal transmission both depend on early diagnosis of HIV and effective treatment with cART. Such patients benefit from a team-based care model that includes the ObGyn and/or MFM specialist, infectious disease clinician, pediatrician, and social worker. As guidelines evolve for care of these patients, a reference checklist, such as the examples provided at the Society for Maternal-Fetal Medicine website (smfm.org) or at HIV.gov, provide an outline for:
- management before, during, and after pregnancy
- suggestions for management teams of interest to successfully carry out the checklist requirements
- proposals for measurements of quality performance with the use of checklists in the management of HIV in pregnancy.
In addition, assistance with clinical decision making for patients with HIV in pregnancy can be obtained via telephone consultation with the National Clinician Consultation Center–Perinatal HIV/AIDS (888-448-8765), which is available 24 hours a day, 7 days a week. ●
- Samji H, Cescon A, Hogg RS, et al; North American AIDS Cohort Collaboration on Research and Design (NA-ACCORD) of IeDEA. Closing the gap: increases in life expectancy among treated HIV-positive individuals in the United States and Canada. PLoS One. 2013;8:e81355. doi: 10.1371/journal. pone.0081355.
- Centers for Disease Control and Prevention. May 1, 2021. HIV Surveillance Report, 2019, vol. 32: Diagnosis of HIV infection in the United States and dependent areas, 2019. Accessed February 15, 2022. http://www.cdc.gov/hiv/library/reports /hiv-surveillance.html
- Panel on Antiretroviral Therapy and Medical Management of Children Living with HIV. Guidelines for the use of antiretroviral agents in pediatric HIV infection. https: //clinicalinfo.hiv.gov/en/guidelines/pediatric-arv. Accessed February 15, 2022.
- Connor EM, Sperling RS, Gelber R, et al. Reduction of maternal-infant transmission of human immunodeficiency virus type 1 with zidovudine treatment. Pediatric AIDS Clinical Trials Group Protocol 076 Study Group. N Engl J Med. 1994;331:1173-1180.
- Townsend CL, Byrne L, Cortina-Borja M, et al. Earlier initiation of ART and further decline in mother-to-child HIV transmission rates, 2000-2011. AIDS. 2014;28:1049-1057.
- Centers for Disease Control and Prevention. January 26, 2022. HIV and pregnant women, infants, and children. Accessed February 15, 2022. https://www.cdc.gov/hiv/group/gender /pregnantwomen/index.html
- Centers for Disease Control and Prevention. 2018 Quick reference guide: Recommended laboratory HIV testing algorithm for serum or plasma specimens. National Center for HIV/AIDS, Viral Hepatitis, and TB Prevention (US); Division of HIV/AIDS Prevention; Association of Public Health Laboratories. Updated January 2018. https://stacks. cdc.gov/view/cdc/50872
- Centers for Disease Control and Prevention, Association of Public Health Laboratories. June 27, 2014. Laboratory testing for the diagnosis of HIV infection: updated recommendations. Accessed February 15, 2022. http://stacks.cdc.gov/view /cdc/23447
- Guidelines for the prevention and treatment of opportunistic infections in adults and adolescents with HIV. Updated April 12, 2022. Accessed July 6, 2022. https://clinicalinfo.hiv .gov/en/guidelines/adult-and-adolescent-opportunistic -infection/whats-new-guidelines
- Rubin LG, Levin MJ, Ljungman P, et al. 2013 IDSA clinical practice guideline for vaccination of the immunocompromised host. Clin Infect Dis. 2014;58: e44–e100. doi: 10.1093/cid/cit684.
- Centers for Disease Control and Prevention. ACIP: Guidance for vaccine recommendations for pregnant and breastfeeding women. Accessed July 5, 2022. https://www.cdc.gov /vaccines/hcp/acip-recs/rec-vac-preg.html?CDC_AA _refVal=https%3A%2F%2Fwww.cdc.gov%2Fvaccines%2Facip %2Fcommittee%2Fguidance%2Frec-vac-preg.html
- Perkins RB, Guido RS, Castle PE, et al; for the 2019 ASCCP Risk-Based Management Consensus Guidelines Committee. 2019 ASCCP risk-based management consensus guidelines for abnormal cervical cancer screening tests and cancer precursors. J Low Genit Tract Dis. 2020;24:102-131. doi: 10.1097/LGT.0000000000000525. Erratum in: J Low Genit Tract Dis. 2020;24:427.
- Cohen MS, Chen YQ, McCauley M, et al; HPTN 052 Study Team. Prevention of HIV-1 infection with early antiretroviral therapy. N Engl J Med. 2011;365:493-505.
- Drug interactions between antiretroviral agents and hormonal contraceptives. Accessed July 6, 2022. https://clinicalinfo .hiv.gov/en/table/table-3-drug-interactions-between -antiretroviral-agents-and-hormonal-contraceptives
- Panel on Treatment of HIV During Pregnancy and Prevention of Perinatal Transmission. Recommendations for use of antiretroviral drugs in pregnancy and interventions to reduce perinatal HIV transmission in the United States. Accessed July 7, 2022. https://clinicalinfo.hiv.gov/en/guidelines/perinatal /whats-new-guidelines
- Centers for Disease Control and Prevention. Recommendations for partner services programs for HIV infection, syphilis, gonorrhea, and chlamydial infection. MMWR Recomm Rep. 2008;57(RR-9):1–83.
- Gilead Sciences, Inc. Truvada (emtricitabine 200 mg/ tenofovir disoproxil fumarate 300 mg tablets). Accessed July 6, 2022. https://truvada.com
- Centers for Disease Control and Prevention. Interim guidance for clinicians considering the use of preexposure prophylaxis for the prevention of HIV infection in heterosexually active adults. MMWR Morb Mortal Wkly Rep. 2012;61:586-589.
- Baeten JM, Donnell D, Ndase P, et al; Partners PrEP Study Team. Antiretroviral prophylaxis for HIV prevention in heterosexual men and women. N Engl J Med. 2012;367: 399-410.
- Celum C, Baeten JM. Antiretroviral-based HIV-1 prevention: antiretroviral treatment and pre-exposure prophylaxis. Antivir Ther. 2012;17:1483-1493.
- Thigpen MC, Kebaabetswe PM, Paxton LA, et al; TDF2 Study Group. Antiretroviral preexposure prophylaxis for heterosexual HIV transmission in Botswana. N Engl J Med. 2012;367:423-434.
- Society for Maternal-Fetal Medicine. Special statement: updated checklists for pregnancy management in persons with HIV. Accessed July 5, 2022. https://www.smfm.org /publications/334-smfm-special-statement-updated -checklists-for-pregnancy-management-in-persons-with-hiv
- American College of Obstetricians and Gynecologists. ACOG committee opinion no. 752. Prenatal and perinatal human immunodeficiency virus testing. Obstet Gynecol. 2018;132:e138-e142.
- Human immunodeficiency virus screening. Joint statement of the American Academy of Pediatrics and the American College of Obstetricians and Gynecologists. Pediatrics. 1999;104(1 pt 1):128.
- Branson BM, Handsfield HH, Lampe MA, et al; Centers for Disease Control and Prevention. Revised recommendations for HIV testing of adults, adolescents, and pregnant women in health care settings. MMWR Recomm Rep. 2006; 55(RR-14):1-17.
- American College of Obstetricians and Gynecologists. ACOG committee opinion no. 751. Labor and delivery management of women with human immunodeficiency virus infection. Obstet Gynecol. 2018;132:e131-e137.
- Centers for Disease Control and Prevention. Factors increasing the risk of acquiring or transmitting HIV. November 12, 2019. Accessed July 29, 2022. https://www.cdc .gov/hiv/risk/estimates/riskfactors.html
- Mandelbrot L, Le Chenadec J, Berrebi A, et al. Perinatal HIV1 transmission: interaction between zidovudine prophylaxis and mode of delivery in the French Perinatal Cohort. JAMA. 1998;280:55-60.
- European Mode of Delivery Collaboration. Elective caesarean-section versus vaginal delivery in prevention of vertical HIV-1 transmission: a randomised clinical trial. Lancet. 1999;353:1035-1039.
- International Perinatal HIV Group; Andiman W, Bryson Y, de Martino M, et al. The mode of delivery and the risk of vertical transmission of human immunodeficiency virus type 1—a meta-analysis of 15 prospective cohort studies. N Engl J Med. 1999;340:977-987.
- Briand N, Jasseron C, Sibiude J, et al. Cesarean section for HIV-infected women in the combination antiretroviral therapies era, 2000–2010. Am J Obstet Gynecol. 2013;209: 335.e1-335.e12.
- Scott RK, Chakhtoura N, Burke MM, et al. Delivery after 40 weeks of gestation in pregnant women with well-controlled human immunodeficiency virus. Obstet Gynecol. 2017;130:502-510.
- American College of Obstetricians and Gynecologists. Committee opinion no. 560. Medically indicated late-preterm and early-term deliveries. Obstet Gynecol. 2013;121:908-910.
- Mofenson LM, Lambert JS, Stiehm ER, et al. Risk factors for perinatal transmission of human immunodeficiency virus type 1 in women treated with zidovudine. Pediatric AIDS Clinical Trials Group Study 185 Team. N Engl J Med. 1999;341:385-393.
- Garcia PM, Kalish LA, Pitt J, et al. Maternal levels of plasma human immunodeficiency virus type 1 RNA and the risk of perinatal transmission. Women and Infants Transmission Study Group. N Engl J Med. 1999;341:394-402.
- Briand N, Warszawski J, Mandelbrot L, et al; ANRS-EPF CO1CO11 Study Group. Is intrapartum intravenous zidovudine for prevention of mother-to-child HIV-1 transmission still useful in the combination antiretroviral therapy era? Clin Infect Dis. 2013;57:903-914.
- Myer L, Phillips TK, McIntyre JA, et al. HIV viraemia and mother-to-child transmission risk after antiretroviral therapy initiation in pregnancy in Cape Town, South Africa. HIV Med. 2017;18:80-88.
- Rodman JH, Flynn PM, Robbins B, et al. Systemic pharmacokinetics and cellular pharmacology of zidovudine in human immunodeficiency virus type 1-infected women and newborn infants. J Infect Dis. 1999;180:1844-1850.
- Wade NA, Birkhead GS, Warren BL, et al. Abbreviated regimens of zidovudine prophylaxis and perinatal transmission of the human immunodeficiency virus. N Engl J Med. 1998;339:1409-1414.
- Nielsen-Saines K, Watts HD, Veloso VS, et al; NICHD HPTN 040/PACTG 1043 Protocol Team. Three postpartum antiretroviral regimens to prevent intrapartum HIV infection. N Engl J Med. 2012;366:2368-2379.
- Mandelbrot L, Mayaux MJ, Bongain A, et al. Obstetric factors and mother-to-child transmission of human immunodeficiency virus type 1: the French perinatal cohorts. SEROGEST French Pediatric HIV Infection Study Group. Am J Obstet Gynecol. 1996;175(3 pt 1):661-667.
- Shapiro DE, Sperling RS, Mandelbrot L, et al. Risk factors for perinatal human immunodeficiency virus transmission in patients receiving zidovudine prophylaxis. Pediatric AIDS Clinical Trials Group protocol 076 Study Group. Obstet Gynecol. 1999;94:897-908.
- International Perinatal HIV Group. Duration of ruptured membranes and vertical transmission of HIV-1: a meta-analysis from 15 prospective cohort studies. AIDS. 2001;15:357-368.
- Nielsen TF, Hokegard KH. Postoperative cesarean section morbidity: a prospective study. Am J Obstet Gynecol. 1983;146:911-916.
- Mark S, Murphy KE, Read S, et al. HIV mother-to-child transmission, mode of delivery, and duration of rupture of membranes: experience in the current era. Infect Dis Obstet Gynecol. 2012;2012:267969.
- Cotter AM, Brookfield KF, Duthely LM, et al. Duration of membrane rupture and risk of perinatal transmission of HIV1 in the era of combination antiretroviral therapy. Am J Obstet Gynecol. 2012;207:482.e1-482.e5.
- Peters H, Byrne L, De Ruiter A, et al. Duration of ruptured membranes and mother-to-child HIV transmission: a prospective population-based surveillance study. BJOG. 2016;123:975-981.
- Jamieson DJ, Read JS, Kourtis AP, et al. Cesarean delivery for HIV-infected women: recommendations and controversies. Am J Obstet Gynecol. 2007;197(3 suppl):S96-S100.
- Cambic CR, Avram MJ, Gupta DK, et al. Effect of ritonavir-induced cytochrome P450 3A4 inhibition on plasma fentanyl concentrations during patient-controlled epidural labor analgesia: a pharmacokinetic simulation. Int J Obstet Anesth. 2014;23:45-51.
- Navarro J, Curran A, Burgos J, et al. Acute leg ischaemia in an HIV-infected patient receiving antiretroviral treatment. Antivir Ther. 2017;22:89-90.
- American Academy of Pediatrics, American College of Obstetricians and Gynecologists. Guidelines for Perinatal Care. 8th ed. American Academy of Pediatrics, American College of Obstetricians and Gynecologists; 2017.
- Siberry GK, Abzug MJ, Nachman S, et al; Panel on Opportunistic Infections in HIV-Exposed and HIV-Infected Children. Guidelines for the prevention and treatment of opportunistic infections in HIV-exposed and HIV-infected children: recommendations from the National Institutes of Health, Centers for Disease Control and Prevention, the HIV Medicine Association of the Infectious Diseases Society of America, the Pediatric Infectious Diseases Society, and the American Academy of Pediatrics. Pediatr Infect Dis J. 32(suppl 2[0 2]):i–KK4.
- Panel on Antiretroviral Therapy and Medical Management of Children Living with HIV. Guidelines for the use of antiretroviral agents in pediatric HIV infection. Accessed February 15, 2022. https://clinicalinfo.hiv.gov/en/guidelines /pediatric-arv
- Committee on Health Care for Underserved Women, American College of Obstetricians and Gynecologists. ACOG committee opinion no. 361. Breastfeeding: maternal and infant aspects. Obstet Gynecol. 2007;109(2 pt 1):479-480.
- Committee on Pediatric AIDS; Mofenson LM, Flynn PM, Aldrovandi GM, et al. Infant feeding and transmission of human immunodeficiency virus in the United States. Pediatrics. 2013;131:391-396.
- Breastfeeding and HIV International Transmission Study Group; Coutsoudis A, Dabis F, Fawzi W, et al. Late postnatal transmission of HIV-1 in breast-fed children: an individual patient data meta-analysis. J Infect Dis. 2004;189:2154-2166.
- Samji H, Cescon A, Hogg RS, et al; North American AIDS Cohort Collaboration on Research and Design (NA-ACCORD) of IeDEA. Closing the gap: increases in life expectancy among treated HIV-positive individuals in the United States and Canada. PLoS One. 2013;8:e81355. doi: 10.1371/journal. pone.0081355.
- Centers for Disease Control and Prevention. May 1, 2021. HIV Surveillance Report, 2019, vol. 32: Diagnosis of HIV infection in the United States and dependent areas, 2019. Accessed February 15, 2022. http://www.cdc.gov/hiv/library/reports /hiv-surveillance.html
- Panel on Antiretroviral Therapy and Medical Management of Children Living with HIV. Guidelines for the use of antiretroviral agents in pediatric HIV infection. https: //clinicalinfo.hiv.gov/en/guidelines/pediatric-arv. Accessed February 15, 2022.
- Connor EM, Sperling RS, Gelber R, et al. Reduction of maternal-infant transmission of human immunodeficiency virus type 1 with zidovudine treatment. Pediatric AIDS Clinical Trials Group Protocol 076 Study Group. N Engl J Med. 1994;331:1173-1180.
- Townsend CL, Byrne L, Cortina-Borja M, et al. Earlier initiation of ART and further decline in mother-to-child HIV transmission rates, 2000-2011. AIDS. 2014;28:1049-1057.
- Centers for Disease Control and Prevention. January 26, 2022. HIV and pregnant women, infants, and children. Accessed February 15, 2022. https://www.cdc.gov/hiv/group/gender /pregnantwomen/index.html
- Centers for Disease Control and Prevention. 2018 Quick reference guide: Recommended laboratory HIV testing algorithm for serum or plasma specimens. National Center for HIV/AIDS, Viral Hepatitis, and TB Prevention (US); Division of HIV/AIDS Prevention; Association of Public Health Laboratories. Updated January 2018. https://stacks. cdc.gov/view/cdc/50872
- Centers for Disease Control and Prevention, Association of Public Health Laboratories. June 27, 2014. Laboratory testing for the diagnosis of HIV infection: updated recommendations. Accessed February 15, 2022. http://stacks.cdc.gov/view /cdc/23447
- Guidelines for the prevention and treatment of opportunistic infections in adults and adolescents with HIV. Updated April 12, 2022. Accessed July 6, 2022. https://clinicalinfo.hiv .gov/en/guidelines/adult-and-adolescent-opportunistic -infection/whats-new-guidelines
- Rubin LG, Levin MJ, Ljungman P, et al. 2013 IDSA clinical practice guideline for vaccination of the immunocompromised host. Clin Infect Dis. 2014;58: e44–e100. doi: 10.1093/cid/cit684.
- Centers for Disease Control and Prevention. ACIP: Guidance for vaccine recommendations for pregnant and breastfeeding women. Accessed July 5, 2022. https://www.cdc.gov /vaccines/hcp/acip-recs/rec-vac-preg.html?CDC_AA _refVal=https%3A%2F%2Fwww.cdc.gov%2Fvaccines%2Facip %2Fcommittee%2Fguidance%2Frec-vac-preg.html
- Perkins RB, Guido RS, Castle PE, et al; for the 2019 ASCCP Risk-Based Management Consensus Guidelines Committee. 2019 ASCCP risk-based management consensus guidelines for abnormal cervical cancer screening tests and cancer precursors. J Low Genit Tract Dis. 2020;24:102-131. doi: 10.1097/LGT.0000000000000525. Erratum in: J Low Genit Tract Dis. 2020;24:427.
- Cohen MS, Chen YQ, McCauley M, et al; HPTN 052 Study Team. Prevention of HIV-1 infection with early antiretroviral therapy. N Engl J Med. 2011;365:493-505.
- Drug interactions between antiretroviral agents and hormonal contraceptives. Accessed July 6, 2022. https://clinicalinfo .hiv.gov/en/table/table-3-drug-interactions-between -antiretroviral-agents-and-hormonal-contraceptives
- Panel on Treatment of HIV During Pregnancy and Prevention of Perinatal Transmission. Recommendations for use of antiretroviral drugs in pregnancy and interventions to reduce perinatal HIV transmission in the United States. Accessed July 7, 2022. https://clinicalinfo.hiv.gov/en/guidelines/perinatal /whats-new-guidelines
- Centers for Disease Control and Prevention. Recommendations for partner services programs for HIV infection, syphilis, gonorrhea, and chlamydial infection. MMWR Recomm Rep. 2008;57(RR-9):1–83.
- Gilead Sciences, Inc. Truvada (emtricitabine 200 mg/ tenofovir disoproxil fumarate 300 mg tablets). Accessed July 6, 2022. https://truvada.com
- Centers for Disease Control and Prevention. Interim guidance for clinicians considering the use of preexposure prophylaxis for the prevention of HIV infection in heterosexually active adults. MMWR Morb Mortal Wkly Rep. 2012;61:586-589.
- Baeten JM, Donnell D, Ndase P, et al; Partners PrEP Study Team. Antiretroviral prophylaxis for HIV prevention in heterosexual men and women. N Engl J Med. 2012;367: 399-410.
- Celum C, Baeten JM. Antiretroviral-based HIV-1 prevention: antiretroviral treatment and pre-exposure prophylaxis. Antivir Ther. 2012;17:1483-1493.
- Thigpen MC, Kebaabetswe PM, Paxton LA, et al; TDF2 Study Group. Antiretroviral preexposure prophylaxis for heterosexual HIV transmission in Botswana. N Engl J Med. 2012;367:423-434.
- Society for Maternal-Fetal Medicine. Special statement: updated checklists for pregnancy management in persons with HIV. Accessed July 5, 2022. https://www.smfm.org /publications/334-smfm-special-statement-updated -checklists-for-pregnancy-management-in-persons-with-hiv
- American College of Obstetricians and Gynecologists. ACOG committee opinion no. 752. Prenatal and perinatal human immunodeficiency virus testing. Obstet Gynecol. 2018;132:e138-e142.
- Human immunodeficiency virus screening. Joint statement of the American Academy of Pediatrics and the American College of Obstetricians and Gynecologists. Pediatrics. 1999;104(1 pt 1):128.
- Branson BM, Handsfield HH, Lampe MA, et al; Centers for Disease Control and Prevention. Revised recommendations for HIV testing of adults, adolescents, and pregnant women in health care settings. MMWR Recomm Rep. 2006; 55(RR-14):1-17.
- American College of Obstetricians and Gynecologists. ACOG committee opinion no. 751. Labor and delivery management of women with human immunodeficiency virus infection. Obstet Gynecol. 2018;132:e131-e137.
- Centers for Disease Control and Prevention. Factors increasing the risk of acquiring or transmitting HIV. November 12, 2019. Accessed July 29, 2022. https://www.cdc .gov/hiv/risk/estimates/riskfactors.html
- Mandelbrot L, Le Chenadec J, Berrebi A, et al. Perinatal HIV1 transmission: interaction between zidovudine prophylaxis and mode of delivery in the French Perinatal Cohort. JAMA. 1998;280:55-60.
- European Mode of Delivery Collaboration. Elective caesarean-section versus vaginal delivery in prevention of vertical HIV-1 transmission: a randomised clinical trial. Lancet. 1999;353:1035-1039.
- International Perinatal HIV Group; Andiman W, Bryson Y, de Martino M, et al. The mode of delivery and the risk of vertical transmission of human immunodeficiency virus type 1—a meta-analysis of 15 prospective cohort studies. N Engl J Med. 1999;340:977-987.
- Briand N, Jasseron C, Sibiude J, et al. Cesarean section for HIV-infected women in the combination antiretroviral therapies era, 2000–2010. Am J Obstet Gynecol. 2013;209: 335.e1-335.e12.
- Scott RK, Chakhtoura N, Burke MM, et al. Delivery after 40 weeks of gestation in pregnant women with well-controlled human immunodeficiency virus. Obstet Gynecol. 2017;130:502-510.
- American College of Obstetricians and Gynecologists. Committee opinion no. 560. Medically indicated late-preterm and early-term deliveries. Obstet Gynecol. 2013;121:908-910.
- Mofenson LM, Lambert JS, Stiehm ER, et al. Risk factors for perinatal transmission of human immunodeficiency virus type 1 in women treated with zidovudine. Pediatric AIDS Clinical Trials Group Study 185 Team. N Engl J Med. 1999;341:385-393.
- Garcia PM, Kalish LA, Pitt J, et al. Maternal levels of plasma human immunodeficiency virus type 1 RNA and the risk of perinatal transmission. Women and Infants Transmission Study Group. N Engl J Med. 1999;341:394-402.
- Briand N, Warszawski J, Mandelbrot L, et al; ANRS-EPF CO1CO11 Study Group. Is intrapartum intravenous zidovudine for prevention of mother-to-child HIV-1 transmission still useful in the combination antiretroviral therapy era? Clin Infect Dis. 2013;57:903-914.
- Myer L, Phillips TK, McIntyre JA, et al. HIV viraemia and mother-to-child transmission risk after antiretroviral therapy initiation in pregnancy in Cape Town, South Africa. HIV Med. 2017;18:80-88.
- Rodman JH, Flynn PM, Robbins B, et al. Systemic pharmacokinetics and cellular pharmacology of zidovudine in human immunodeficiency virus type 1-infected women and newborn infants. J Infect Dis. 1999;180:1844-1850.
- Wade NA, Birkhead GS, Warren BL, et al. Abbreviated regimens of zidovudine prophylaxis and perinatal transmission of the human immunodeficiency virus. N Engl J Med. 1998;339:1409-1414.
- Nielsen-Saines K, Watts HD, Veloso VS, et al; NICHD HPTN 040/PACTG 1043 Protocol Team. Three postpartum antiretroviral regimens to prevent intrapartum HIV infection. N Engl J Med. 2012;366:2368-2379.
- Mandelbrot L, Mayaux MJ, Bongain A, et al. Obstetric factors and mother-to-child transmission of human immunodeficiency virus type 1: the French perinatal cohorts. SEROGEST French Pediatric HIV Infection Study Group. Am J Obstet Gynecol. 1996;175(3 pt 1):661-667.
- Shapiro DE, Sperling RS, Mandelbrot L, et al. Risk factors for perinatal human immunodeficiency virus transmission in patients receiving zidovudine prophylaxis. Pediatric AIDS Clinical Trials Group protocol 076 Study Group. Obstet Gynecol. 1999;94:897-908.
- International Perinatal HIV Group. Duration of ruptured membranes and vertical transmission of HIV-1: a meta-analysis from 15 prospective cohort studies. AIDS. 2001;15:357-368.
- Nielsen TF, Hokegard KH. Postoperative cesarean section morbidity: a prospective study. Am J Obstet Gynecol. 1983;146:911-916.
- Mark S, Murphy KE, Read S, et al. HIV mother-to-child transmission, mode of delivery, and duration of rupture of membranes: experience in the current era. Infect Dis Obstet Gynecol. 2012;2012:267969.
- Cotter AM, Brookfield KF, Duthely LM, et al. Duration of membrane rupture and risk of perinatal transmission of HIV1 in the era of combination antiretroviral therapy. Am J Obstet Gynecol. 2012;207:482.e1-482.e5.
- Peters H, Byrne L, De Ruiter A, et al. Duration of ruptured membranes and mother-to-child HIV transmission: a prospective population-based surveillance study. BJOG. 2016;123:975-981.
- Jamieson DJ, Read JS, Kourtis AP, et al. Cesarean delivery for HIV-infected women: recommendations and controversies. Am J Obstet Gynecol. 2007;197(3 suppl):S96-S100.
- Cambic CR, Avram MJ, Gupta DK, et al. Effect of ritonavir-induced cytochrome P450 3A4 inhibition on plasma fentanyl concentrations during patient-controlled epidural labor analgesia: a pharmacokinetic simulation. Int J Obstet Anesth. 2014;23:45-51.
- Navarro J, Curran A, Burgos J, et al. Acute leg ischaemia in an HIV-infected patient receiving antiretroviral treatment. Antivir Ther. 2017;22:89-90.
- American Academy of Pediatrics, American College of Obstetricians and Gynecologists. Guidelines for Perinatal Care. 8th ed. American Academy of Pediatrics, American College of Obstetricians and Gynecologists; 2017.
- Siberry GK, Abzug MJ, Nachman S, et al; Panel on Opportunistic Infections in HIV-Exposed and HIV-Infected Children. Guidelines for the prevention and treatment of opportunistic infections in HIV-exposed and HIV-infected children: recommendations from the National Institutes of Health, Centers for Disease Control and Prevention, the HIV Medicine Association of the Infectious Diseases Society of America, the Pediatric Infectious Diseases Society, and the American Academy of Pediatrics. Pediatr Infect Dis J. 32(suppl 2[0 2]):i–KK4.
- Panel on Antiretroviral Therapy and Medical Management of Children Living with HIV. Guidelines for the use of antiretroviral agents in pediatric HIV infection. Accessed February 15, 2022. https://clinicalinfo.hiv.gov/en/guidelines /pediatric-arv
- Committee on Health Care for Underserved Women, American College of Obstetricians and Gynecologists. ACOG committee opinion no. 361. Breastfeeding: maternal and infant aspects. Obstet Gynecol. 2007;109(2 pt 1):479-480.
- Committee on Pediatric AIDS; Mofenson LM, Flynn PM, Aldrovandi GM, et al. Infant feeding and transmission of human immunodeficiency virus in the United States. Pediatrics. 2013;131:391-396.
- Breastfeeding and HIV International Transmission Study Group; Coutsoudis A, Dabis F, Fawzi W, et al. Late postnatal transmission of HIV-1 in breast-fed children: an individual patient data meta-analysis. J Infect Dis. 2004;189:2154-2166.
Monkeypox: Another emerging threat?
CASE Pregnant woman’s husband is ill after traveling
A 29-year-old primigravid woman at 18 weeks’ gestation just returned from a 10-day trip to Nigeria with her husband. While in Nigeria, the couple went on safari. On several occasions during the safari, they consumed bushmeat prepared by their guides. Her husband now has severe malaise, fever, chills, myalgias, cough, and prominent submandibular, cervical, and inguinal adenopathy. In addition, he has developed a diffuse papular-vesicular rash on his trunk and extremities.
- What is the most likely diagnosis?
- Does this condition pose a danger to his wife?
- What treatment is indicated for his wife?
What we know
In recent weeks, the specter of another poorly understood biological threat has emerged in the medical literature and lay press: monkeypox. This article will first review the epidemiology, clinical manifestations, and diagnosis of this infection, followed by a discussion of how to prevent and treat the condition, with special emphasis on the risks that this infection poses in pregnant women.
Virology
The monkeypox virus is a member of the orthopoxvirus genus. The variola (smallpox) virus and vaccinia virus are included in this genus. It is one of the largest of all viruses, measuring 200-250 nm. It is enveloped and contains double-stranded DNA. Its natural reservoir is probably African rodents. Two distinct strains of monkeypox exist in different geographical regions of Africa: the Central African clade and the West African clade. The Central African clade is significantly more virulent than the latter, with a mortality rate approaching 10%, versus 1% in the West African clade. The incubation period of the virus ranges from 4-20 days and averages 12 days.1,2
Epidemiology
Monkeypox was first discovered in 1958 by Preben von Magnus in a colony of research monkeys in Copenhagen, Denmark. The first case of monkeypox in humans occurred in the Democratic Republic of Congo in 1970 in a 9-year-old boy. Subsequently, cases were reported in the Ivory Coast, Liberia, Nigeria, and Sierra Leone. The infection was limited to the rain forests of central and western Africa until 2003. At that time, the first cases in the United States were reported. The US cases occurred in the Midwest and were traced to exposure to pet prairie dogs. These animals all came from a single distributor, and they apparently were infected when they were housed in the same space with Gambian rats, which are well recognized reservoirs of monkeypox in their native habitat in Africa.1-3
A limited outbreak of monkeypox occurred in the United Kingdom in 2018. Seventy-one cases, with no fatalities, were reported. In 2021 another US case of monkeypox was reported in Dallas, Texas, in an individual who had recently traveled to the United States from Nigeria. A second US case was reported in November 2021 from a patient in Maryland who had returned from a visit to Nigeria. Those were the only 2 reported cases of monkeypox in the United States in 2021.1-3
Then in early May 2022, the United Kingdom reported 9 cases of monkeypox. The first infected patient had recently traveled to Nigeria and, subsequently, infected 2 members of his family.4 On May 18, the Massachusetts Department of Public Health confirmed a case of monkeypox in an adult man who had recently traveled to Canada. As of July 7, 6,027 cases have been reported from at least 39 countries.
The current outbreak is unusual in that, previously, almost all cases occurred in western and central Africa in remote tropical rain forests. Infection usually resulted from close exposure to rats, rabbits, squirrels, monkeys, porcupines, and gazelles. Exposure occurred when persons captured, slaughtered, prepared, and then ate these animals for food without properly cooking the flesh.
The leading theory is that the present outbreak originated among men who had sex with men at 2 raves held in Spain and Belgium. The virus appears to have been spread by skin-to-skin contact, by respiratory droplets, by contact with contaminated bedding, and probably by sperm.2,4,6
Continue to: Clinical manifestations...
Clinical manifestations
Monkeypox evolves through 2 stages: a pre-eruptive stage and an eruptive stage. Prodromal symptoms include malaise, severe headache, myalgias, fever, drenching sweats, backache, fatigue, sore throat, dyspnea, and cough. Within 2-3 days, the characteristic skin eruption develops. The lesions usually begin on the face and then spread in a centrifugal manner to the trunk and extremities, including the palms of the hands and soles of the feet. The lesions typically progress from macules to papules to vesicles to pustules. They then crust and scab over. An interesting additional finding is the presence of prominent lymphadenopathy behind the ear, beneath the mandible, in the neck, and in the groin.1
Several different illnesses must be considered in the differential diagnosis of monkeypox infection. They include measles, scabies, secondary syphilis, and medication-associated allergic reactions. However, the 2 conditions most likely to be confused with monkeypox are chickenpox (varicella) and smallpox. Lymphadenopathy is much more prominent in monkeypox compared with chickenpox. Moreover, with monkeypox, all lesions tend to be at the same stage of evolution as opposed to appearing in crops as they do in chickenpox. Smallpox would be extremely unlikely in the absence of a recognized laboratory accident or a bioterrorism incident.7
Diagnosis
The presumptive diagnosis of monkeypox infection is made primarily based on clinical examination. However, laboratory testing is indicated to definitively differentiate monkeypox from other orthopoxvirus infections such as varicella and smallpox.
In specialized laboratories that employ highly trained personnel and maintain strict safety precautions, the virus can be isolated in mammalian cell cultures. Electron microscopy is a valuable tool for identifying the characteristic brick-shaped poxvirus virions. Routine histologic examination of a lesion will show ballooning degeneration of keratinocytes, prominent spongiosis, dermal edema, and acute inflammation, although these findings are not unique to monkeypox.1
The Centers for Disease Control and Prevention (CDC) has developed serologic tests that detect immunoglobulin (Ig) M- and IgG-specific antibody. However, the most useful and practical diagnostic test is assessment of a skin scraping by polymerase chain reaction (PCR). This test is more sensitive than assessment of serum PCR.1
When the diagnosis of monkeypox is being considered, the clinician should coordinate testing through the local and state public health departments and through the CDC. Effective communication with all agencies will ensure that laboratory specimens are processed in a timely and efficient manner. The CDC website presents information on specimen collection.8
How do we manage monkeypox?
Prevention
The first step in prevention of infection is to isolate infected individuals until all lesions have dried and crusted over. Susceptible people should avoid close contact with skin lesions, respiratory and genital secretions, and bedding of patients who are infected.
The ultimate preventive measure, however, is vaccination of susceptible people either immediately before exposure (eg, military personnel, first responders, infection control investigators, health care workers) or immediately after exposure (general population). Older individuals who received the original smallpox vaccine likely have immunity to monkeypox infection. Unfortunately, very few women who currently are of reproductive age received this vaccine because its use was discontinued in the United States in the early 1970s. Therefore, the vast majority of our patients are uniquely susceptible to this infection and should be vaccinated if there is an outbreak of monkeypox in their locality.7,9
The current preferred vaccine for prevention of both smallpox and monkeypox is the Jynneos (Bavarian Nordic A/S) vaccine.10 This agent incorporates a replication-deficient live virus and does not pose the same risk for adverse events as the original versions of the smallpox vaccine. Jynneos is administered subcutaneously rather than by scarification. Two 0.5-mL doses, delivered 28 days apart, are required for optimal effect. The vaccine must be obtained from local and state health departments, in consultation with the CDC.7,9
There is very little published information on the safety of the Jynneos vaccine in pregnant or lactating women, although animal data are reassuring. Moreover, the dangers of monkeypox infection are significant, and in the event of an outbreak, vaccination of susceptible individuals, including pregnant women, is indicated.
- Monkeypox is a member of the orthopoxvirus genus and is closely related to the smallpox virus. It is a large, double-stranded, enveloped DNA virus.
- The virus is transmitted primarily by close contact with infected animals or other humans or by consumption of contaminated bushmeat.
- The infection evolves in 2 phases. The pre-eruptive phase is characterized by severe flu-like symptoms and signs. The eruptive phase is distinguished by a diffuse papular-vesicular rash.
- The most valuable test for confirming the diagnosis is a polymerase chain reaction test of a fresh skin lesion.
- In women who are pregnant, monkeypox has been associated with spontaneous abortion and fetal death.
- Three antiviral agents may be of value in treating infected patients: cidofovir, brincidofovir, and tecovirimat. Only the latter has an acceptable safety profile for women who are pregnant or lactating.
- The new nonreplicating smallpox vaccine Jynneos (Bavarian Nordic A/S) is of great value for pre- and post-exposure prophylaxis.
Continue to: Treatment...
Treatment
Infected pregnant women should receive acetaminophen 1,000 mg orally every 8 hours, to control fever and provide analgesia. An antihistamine such as diphenhydramine 25 mg orally every 6-8 hours, may be used to control pruritus and provide mild sedation. Adequate fluid intake and optimal nutrition should be encouraged. Skin lesions should be inspected regularly to detect signs of superimposed bacterial infections. Small, localized bacterial skin infections can be treated with topical application of mupirocin ointment 2%, 3 times daily for 7-14 days. For diffuse and more severe bacterial skin infections, a systemic antibiotic may be necessary. Reasonable choices include amoxicillin-clavulanate 875 mg/125 mg orally every 12 hours, or trimethoprim-sulfamethoxazole double strength 800 mg/160 mg orally every 12 hours.11 The latter agent should be avoided in the first trimester of pregnancy because of potential teratogenic effects.
Several specific agents are available through the CDC for treatment of orthopoxvirus infections such as smallpox and monkeypox. Information about these agents is summarized in the TABLE.12-16
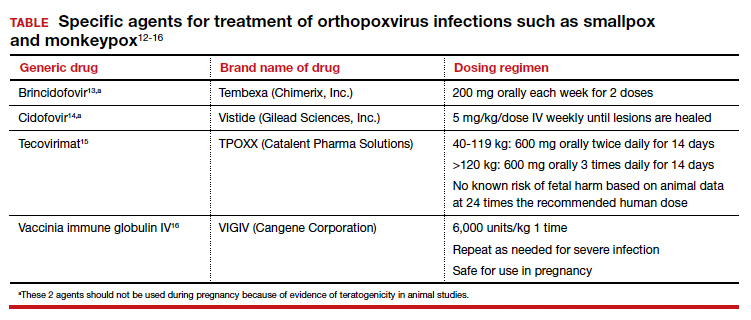
Unique considerations in pregnancy
Because monkeypox is so rare, there is very little information about the effects of this infection in pregnant women. The report most commonly cited in the literature is that by Mbala et al, which was published in 2017.17 These authors described 4 pregnant patients in the Democratic Republic of Congo who contracted monkeypox infection over a 4-year period. All 4 women were hospitalized and treated with systemic antibiotics, antiparasitic medications, and analgesics. One patient delivered a healthy infant. Two women had spontaneous abortions in the first trimester. The fourth patient experienced a stillbirth at 22 weeks’ gestation. At postmortem examination, the fetus had diffuse cutaneous lesions, prominent hepatomegaly, and hydrops. No structural malformations were noted. The placenta demonstrated numerous punctate hemorrhages, and high concentrations of virus were recovered from the placenta and from fetal tissue.
Although the information on pregnancy outcome is quite limited, it seems clear that the virus can cross the placenta and cause adverse effects such as spontaneous abortion and fetal death. Accordingly, I think the following guidelines are a reasonable approach to a pregnant patient who has been exposed to monkeypox or who has developed manifestations of infection.3,7,9
- In the event of a community outbreak, bioterrorism event, or exposure to a person with suspected or confirmed monkeypox infection, the pregnant patient should receive the Jynneos vaccine.
- The pregnant patient should be isolated from any individual with suspected or confirmed monkeypox.
- If infection develops despite these measures, the patient should be treated with either tecovirimat or vaccinia immune globulin IV. Hospitalization may be necessary for seriously ill individuals.
- Within 2 weeks of infection, a comprehensive ultrasound examination should be performed to assess for structural abnormalities in the fetus.
- Subsequently, serial ultrasound examinations should be performed at intervals of 4-6 weeks to assess fetal growth and re-evaluate fetal anatomy.
- Following delivery, a detailed neonatal examination should be performed to assess for evidence of viral injury. Neonatal skin lesions and neonatal serum can be assessed by PCR for monkeypox virus. The newborn should be isolated from the mother until all the mother’s lesions have dried and crusted over.
CASE Resolved
Given the husband’s recent travel to Nigeria and consumption of bushmeat, he most likely has monkeypox. The infection can be spread from person to person by close contact; thus, his wife is at risk. The couple should isolate until all of his lesions have dried and crusted over. The woman also should receive the Jynneos vaccine. If she becomes symptomatic, she should be treated with tecovirimat or vaccinia immune globulin IV. ●
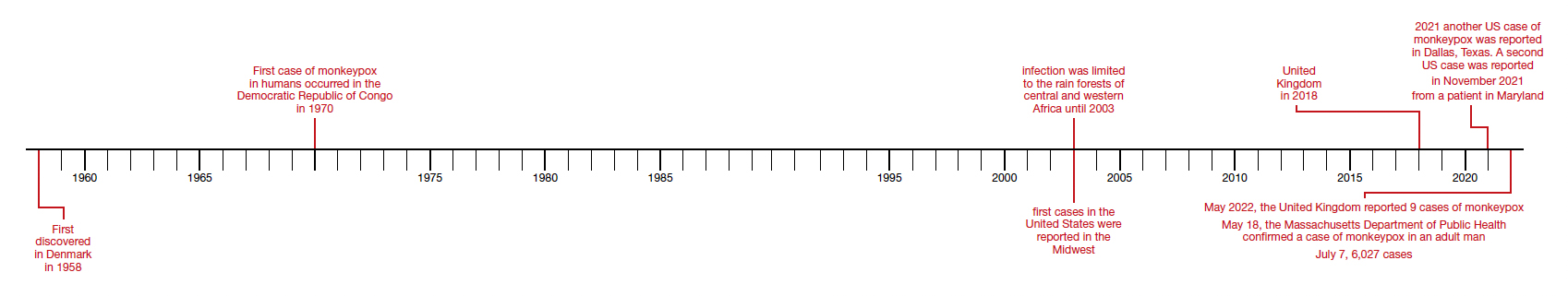
- Isaacs SN, Shenoy ES. Monkeypox. UpToDate. Updated June 28,2022. Accessed July 1, 2022. https://www.uptodate.com /contents/monkeypox?topicRef=8349&source=see_link
- Graham MB. Monkeypox. Medscape. Updated June 29, 2022. Accessed July 1, 2022. https://emedicine.medscape.com /article/1134714-overview.
- Khalil A, Samara A, O’Brien P, et al. Monkeypox and pregnancy: what do obstetricians need to know? Ultrasound Obstet Gynecol. 2022;60:22-27. doi:10.1002/uog.24968.
- World Health Organization. Monkeypox-United Kingdom of Great Britain and Northern Ireland. May 18, 2022. Accessed July 1, 2022. https://www.who.int/emergencies/diseaseoutbreak-news/item/2022-DON383.
- WHO reports two new monkeypox deaths, cases in new areas. Reuters. July 7, 2022. https://www.reuters.com/world /who-reports-two-new-monkeypox-deaths-2022-07-07/. Accessed July 19, 2022.
- World Health Organization. Multi-country monkeypox outbreak in non-endemic countries: update. May 29, 2022. Accessed July 1, 2022. https://www.who.int /emergencies/disease-outbreak-news/item/2022 -DON388#:~:text=Multi%2Dcountry%20monkeypox%20 outbreak%20in%20non%2Dendemic%20countries%3A%20 Update,-29%20May%202022&text=Since%2013%20 May%202022%2C%20monkeypox,Epidemiological%20 investigations%20are%20ongoing.
- Cono J, Cragan JD, Jamieson DJ, Rasmussen SA. Prophylaxis and treatment of pregnant women for emerging infections andbioterrorism emergencies. Emerg Infect Dis. 2006;12:16311637. doi:10.3201/eid1211.060618.
- Centers for Disease Control and Prevention. Preparation and collection of specimens. Reviewed June 29, 2022. Accessed July 6, 2022. https://www.cdc.gov/poxvirus /monkeypox/clinicians/prep-collection-specimens.html.
- Rao AK, Petersen BW, Whitehill F, et al. Monkeypox vaccination. MMWR Morb Mortal Wkly Rep. 2022;71:734-742. doi:10.15585/mmwr.mm7122e1.
- Smallpox and monkeypox vaccine, live, nonreplicating. Package insert. Bavarian Nordic A/S; 2021. Accessed July 1, 2022. https://www.fda.gov/media/131078/download.
- Duff P. Commonly used antibiotics in ObGyn practice. OBG Manag. 2022;34:29, 36-40. doi:10.12788/obgm.0191.
- Centers for Disease Control and Prevention. Treatment information for healthcare professionals: interim clinical guidance for the treatment of monkeypox. Reviewed June 17, 2022. Accessed July 1, 2022. https://www.cdc.gov/poxvirus /monkeypox/clinicians/treatment.html.
- Brincidofovir. Prescribing information. Chimerix, Inc.; 2021. Accessed July 1, 2022. https://www.accessdata.fda.gov /drugsatfda_docs/label/2021/214460s000,214461s000lbl.pdf.
- Cidofovir. Package insert. Gilead Sciences, Inc.; 2010. Accessed July 1, 2022. https://www.gilead.com/~/media /Files/pdfs/medicines/other/vistide/vistide.pdf.
- Tecovirimat. Prescribing information. Catalent Pharma Solutions; 2022. Accessed July 1, 2022. https://www.accessdata.fda.gov/drugsatfda_docs /label/2022/214518s000lbl.pdf.
- Vaccinia immune globulin IV. Prescribing information. Cangene Corporation; 2010. Accessed July 1, 2022. https: //www.fda.gov/media/77004/download.
- Mbala PK, Huggins JW, Riu-Rovira T, et al. Maternal and fetal outcomes among pregnant women with human monkeypox infection in the Democratic Republic of Congo. J Infect Dis. 2017;216:824-828. doi:10.1093/infdis/jix260.
CASE Pregnant woman’s husband is ill after traveling
A 29-year-old primigravid woman at 18 weeks’ gestation just returned from a 10-day trip to Nigeria with her husband. While in Nigeria, the couple went on safari. On several occasions during the safari, they consumed bushmeat prepared by their guides. Her husband now has severe malaise, fever, chills, myalgias, cough, and prominent submandibular, cervical, and inguinal adenopathy. In addition, he has developed a diffuse papular-vesicular rash on his trunk and extremities.
- What is the most likely diagnosis?
- Does this condition pose a danger to his wife?
- What treatment is indicated for his wife?
What we know
In recent weeks, the specter of another poorly understood biological threat has emerged in the medical literature and lay press: monkeypox. This article will first review the epidemiology, clinical manifestations, and diagnosis of this infection, followed by a discussion of how to prevent and treat the condition, with special emphasis on the risks that this infection poses in pregnant women.
Virology
The monkeypox virus is a member of the orthopoxvirus genus. The variola (smallpox) virus and vaccinia virus are included in this genus. It is one of the largest of all viruses, measuring 200-250 nm. It is enveloped and contains double-stranded DNA. Its natural reservoir is probably African rodents. Two distinct strains of monkeypox exist in different geographical regions of Africa: the Central African clade and the West African clade. The Central African clade is significantly more virulent than the latter, with a mortality rate approaching 10%, versus 1% in the West African clade. The incubation period of the virus ranges from 4-20 days and averages 12 days.1,2
Epidemiology
Monkeypox was first discovered in 1958 by Preben von Magnus in a colony of research monkeys in Copenhagen, Denmark. The first case of monkeypox in humans occurred in the Democratic Republic of Congo in 1970 in a 9-year-old boy. Subsequently, cases were reported in the Ivory Coast, Liberia, Nigeria, and Sierra Leone. The infection was limited to the rain forests of central and western Africa until 2003. At that time, the first cases in the United States were reported. The US cases occurred in the Midwest and were traced to exposure to pet prairie dogs. These animals all came from a single distributor, and they apparently were infected when they were housed in the same space with Gambian rats, which are well recognized reservoirs of monkeypox in their native habitat in Africa.1-3
A limited outbreak of monkeypox occurred in the United Kingdom in 2018. Seventy-one cases, with no fatalities, were reported. In 2021 another US case of monkeypox was reported in Dallas, Texas, in an individual who had recently traveled to the United States from Nigeria. A second US case was reported in November 2021 from a patient in Maryland who had returned from a visit to Nigeria. Those were the only 2 reported cases of monkeypox in the United States in 2021.1-3
Then in early May 2022, the United Kingdom reported 9 cases of monkeypox. The first infected patient had recently traveled to Nigeria and, subsequently, infected 2 members of his family.4 On May 18, the Massachusetts Department of Public Health confirmed a case of monkeypox in an adult man who had recently traveled to Canada. As of July 7, 6,027 cases have been reported from at least 39 countries.
The current outbreak is unusual in that, previously, almost all cases occurred in western and central Africa in remote tropical rain forests. Infection usually resulted from close exposure to rats, rabbits, squirrels, monkeys, porcupines, and gazelles. Exposure occurred when persons captured, slaughtered, prepared, and then ate these animals for food without properly cooking the flesh.
The leading theory is that the present outbreak originated among men who had sex with men at 2 raves held in Spain and Belgium. The virus appears to have been spread by skin-to-skin contact, by respiratory droplets, by contact with contaminated bedding, and probably by sperm.2,4,6
Continue to: Clinical manifestations...
Clinical manifestations
Monkeypox evolves through 2 stages: a pre-eruptive stage and an eruptive stage. Prodromal symptoms include malaise, severe headache, myalgias, fever, drenching sweats, backache, fatigue, sore throat, dyspnea, and cough. Within 2-3 days, the characteristic skin eruption develops. The lesions usually begin on the face and then spread in a centrifugal manner to the trunk and extremities, including the palms of the hands and soles of the feet. The lesions typically progress from macules to papules to vesicles to pustules. They then crust and scab over. An interesting additional finding is the presence of prominent lymphadenopathy behind the ear, beneath the mandible, in the neck, and in the groin.1
Several different illnesses must be considered in the differential diagnosis of monkeypox infection. They include measles, scabies, secondary syphilis, and medication-associated allergic reactions. However, the 2 conditions most likely to be confused with monkeypox are chickenpox (varicella) and smallpox. Lymphadenopathy is much more prominent in monkeypox compared with chickenpox. Moreover, with monkeypox, all lesions tend to be at the same stage of evolution as opposed to appearing in crops as they do in chickenpox. Smallpox would be extremely unlikely in the absence of a recognized laboratory accident or a bioterrorism incident.7
Diagnosis
The presumptive diagnosis of monkeypox infection is made primarily based on clinical examination. However, laboratory testing is indicated to definitively differentiate monkeypox from other orthopoxvirus infections such as varicella and smallpox.
In specialized laboratories that employ highly trained personnel and maintain strict safety precautions, the virus can be isolated in mammalian cell cultures. Electron microscopy is a valuable tool for identifying the characteristic brick-shaped poxvirus virions. Routine histologic examination of a lesion will show ballooning degeneration of keratinocytes, prominent spongiosis, dermal edema, and acute inflammation, although these findings are not unique to monkeypox.1
The Centers for Disease Control and Prevention (CDC) has developed serologic tests that detect immunoglobulin (Ig) M- and IgG-specific antibody. However, the most useful and practical diagnostic test is assessment of a skin scraping by polymerase chain reaction (PCR). This test is more sensitive than assessment of serum PCR.1
When the diagnosis of monkeypox is being considered, the clinician should coordinate testing through the local and state public health departments and through the CDC. Effective communication with all agencies will ensure that laboratory specimens are processed in a timely and efficient manner. The CDC website presents information on specimen collection.8
How do we manage monkeypox?
Prevention
The first step in prevention of infection is to isolate infected individuals until all lesions have dried and crusted over. Susceptible people should avoid close contact with skin lesions, respiratory and genital secretions, and bedding of patients who are infected.
The ultimate preventive measure, however, is vaccination of susceptible people either immediately before exposure (eg, military personnel, first responders, infection control investigators, health care workers) or immediately after exposure (general population). Older individuals who received the original smallpox vaccine likely have immunity to monkeypox infection. Unfortunately, very few women who currently are of reproductive age received this vaccine because its use was discontinued in the United States in the early 1970s. Therefore, the vast majority of our patients are uniquely susceptible to this infection and should be vaccinated if there is an outbreak of monkeypox in their locality.7,9
The current preferred vaccine for prevention of both smallpox and monkeypox is the Jynneos (Bavarian Nordic A/S) vaccine.10 This agent incorporates a replication-deficient live virus and does not pose the same risk for adverse events as the original versions of the smallpox vaccine. Jynneos is administered subcutaneously rather than by scarification. Two 0.5-mL doses, delivered 28 days apart, are required for optimal effect. The vaccine must be obtained from local and state health departments, in consultation with the CDC.7,9
There is very little published information on the safety of the Jynneos vaccine in pregnant or lactating women, although animal data are reassuring. Moreover, the dangers of monkeypox infection are significant, and in the event of an outbreak, vaccination of susceptible individuals, including pregnant women, is indicated.
- Monkeypox is a member of the orthopoxvirus genus and is closely related to the smallpox virus. It is a large, double-stranded, enveloped DNA virus.
- The virus is transmitted primarily by close contact with infected animals or other humans or by consumption of contaminated bushmeat.
- The infection evolves in 2 phases. The pre-eruptive phase is characterized by severe flu-like symptoms and signs. The eruptive phase is distinguished by a diffuse papular-vesicular rash.
- The most valuable test for confirming the diagnosis is a polymerase chain reaction test of a fresh skin lesion.
- In women who are pregnant, monkeypox has been associated with spontaneous abortion and fetal death.
- Three antiviral agents may be of value in treating infected patients: cidofovir, brincidofovir, and tecovirimat. Only the latter has an acceptable safety profile for women who are pregnant or lactating.
- The new nonreplicating smallpox vaccine Jynneos (Bavarian Nordic A/S) is of great value for pre- and post-exposure prophylaxis.
Continue to: Treatment...
Treatment
Infected pregnant women should receive acetaminophen 1,000 mg orally every 8 hours, to control fever and provide analgesia. An antihistamine such as diphenhydramine 25 mg orally every 6-8 hours, may be used to control pruritus and provide mild sedation. Adequate fluid intake and optimal nutrition should be encouraged. Skin lesions should be inspected regularly to detect signs of superimposed bacterial infections. Small, localized bacterial skin infections can be treated with topical application of mupirocin ointment 2%, 3 times daily for 7-14 days. For diffuse and more severe bacterial skin infections, a systemic antibiotic may be necessary. Reasonable choices include amoxicillin-clavulanate 875 mg/125 mg orally every 12 hours, or trimethoprim-sulfamethoxazole double strength 800 mg/160 mg orally every 12 hours.11 The latter agent should be avoided in the first trimester of pregnancy because of potential teratogenic effects.
Several specific agents are available through the CDC for treatment of orthopoxvirus infections such as smallpox and monkeypox. Information about these agents is summarized in the TABLE.12-16

Unique considerations in pregnancy
Because monkeypox is so rare, there is very little information about the effects of this infection in pregnant women. The report most commonly cited in the literature is that by Mbala et al, which was published in 2017.17 These authors described 4 pregnant patients in the Democratic Republic of Congo who contracted monkeypox infection over a 4-year period. All 4 women were hospitalized and treated with systemic antibiotics, antiparasitic medications, and analgesics. One patient delivered a healthy infant. Two women had spontaneous abortions in the first trimester. The fourth patient experienced a stillbirth at 22 weeks’ gestation. At postmortem examination, the fetus had diffuse cutaneous lesions, prominent hepatomegaly, and hydrops. No structural malformations were noted. The placenta demonstrated numerous punctate hemorrhages, and high concentrations of virus were recovered from the placenta and from fetal tissue.
Although the information on pregnancy outcome is quite limited, it seems clear that the virus can cross the placenta and cause adverse effects such as spontaneous abortion and fetal death. Accordingly, I think the following guidelines are a reasonable approach to a pregnant patient who has been exposed to monkeypox or who has developed manifestations of infection.3,7,9
- In the event of a community outbreak, bioterrorism event, or exposure to a person with suspected or confirmed monkeypox infection, the pregnant patient should receive the Jynneos vaccine.
- The pregnant patient should be isolated from any individual with suspected or confirmed monkeypox.
- If infection develops despite these measures, the patient should be treated with either tecovirimat or vaccinia immune globulin IV. Hospitalization may be necessary for seriously ill individuals.
- Within 2 weeks of infection, a comprehensive ultrasound examination should be performed to assess for structural abnormalities in the fetus.
- Subsequently, serial ultrasound examinations should be performed at intervals of 4-6 weeks to assess fetal growth and re-evaluate fetal anatomy.
- Following delivery, a detailed neonatal examination should be performed to assess for evidence of viral injury. Neonatal skin lesions and neonatal serum can be assessed by PCR for monkeypox virus. The newborn should be isolated from the mother until all the mother’s lesions have dried and crusted over.
CASE Resolved
Given the husband’s recent travel to Nigeria and consumption of bushmeat, he most likely has monkeypox. The infection can be spread from person to person by close contact; thus, his wife is at risk. The couple should isolate until all of his lesions have dried and crusted over. The woman also should receive the Jynneos vaccine. If she becomes symptomatic, she should be treated with tecovirimat or vaccinia immune globulin IV. ●

CASE Pregnant woman’s husband is ill after traveling
A 29-year-old primigravid woman at 18 weeks’ gestation just returned from a 10-day trip to Nigeria with her husband. While in Nigeria, the couple went on safari. On several occasions during the safari, they consumed bushmeat prepared by their guides. Her husband now has severe malaise, fever, chills, myalgias, cough, and prominent submandibular, cervical, and inguinal adenopathy. In addition, he has developed a diffuse papular-vesicular rash on his trunk and extremities.
- What is the most likely diagnosis?
- Does this condition pose a danger to his wife?
- What treatment is indicated for his wife?
What we know
In recent weeks, the specter of another poorly understood biological threat has emerged in the medical literature and lay press: monkeypox. This article will first review the epidemiology, clinical manifestations, and diagnosis of this infection, followed by a discussion of how to prevent and treat the condition, with special emphasis on the risks that this infection poses in pregnant women.
Virology
The monkeypox virus is a member of the orthopoxvirus genus. The variola (smallpox) virus and vaccinia virus are included in this genus. It is one of the largest of all viruses, measuring 200-250 nm. It is enveloped and contains double-stranded DNA. Its natural reservoir is probably African rodents. Two distinct strains of monkeypox exist in different geographical regions of Africa: the Central African clade and the West African clade. The Central African clade is significantly more virulent than the latter, with a mortality rate approaching 10%, versus 1% in the West African clade. The incubation period of the virus ranges from 4-20 days and averages 12 days.1,2
Epidemiology
Monkeypox was first discovered in 1958 by Preben von Magnus in a colony of research monkeys in Copenhagen, Denmark. The first case of monkeypox in humans occurred in the Democratic Republic of Congo in 1970 in a 9-year-old boy. Subsequently, cases were reported in the Ivory Coast, Liberia, Nigeria, and Sierra Leone. The infection was limited to the rain forests of central and western Africa until 2003. At that time, the first cases in the United States were reported. The US cases occurred in the Midwest and were traced to exposure to pet prairie dogs. These animals all came from a single distributor, and they apparently were infected when they were housed in the same space with Gambian rats, which are well recognized reservoirs of monkeypox in their native habitat in Africa.1-3
A limited outbreak of monkeypox occurred in the United Kingdom in 2018. Seventy-one cases, with no fatalities, were reported. In 2021 another US case of monkeypox was reported in Dallas, Texas, in an individual who had recently traveled to the United States from Nigeria. A second US case was reported in November 2021 from a patient in Maryland who had returned from a visit to Nigeria. Those were the only 2 reported cases of monkeypox in the United States in 2021.1-3
Then in early May 2022, the United Kingdom reported 9 cases of monkeypox. The first infected patient had recently traveled to Nigeria and, subsequently, infected 2 members of his family.4 On May 18, the Massachusetts Department of Public Health confirmed a case of monkeypox in an adult man who had recently traveled to Canada. As of July 7, 6,027 cases have been reported from at least 39 countries.
The current outbreak is unusual in that, previously, almost all cases occurred in western and central Africa in remote tropical rain forests. Infection usually resulted from close exposure to rats, rabbits, squirrels, monkeys, porcupines, and gazelles. Exposure occurred when persons captured, slaughtered, prepared, and then ate these animals for food without properly cooking the flesh.
The leading theory is that the present outbreak originated among men who had sex with men at 2 raves held in Spain and Belgium. The virus appears to have been spread by skin-to-skin contact, by respiratory droplets, by contact with contaminated bedding, and probably by sperm.2,4,6
Continue to: Clinical manifestations...
Clinical manifestations
Monkeypox evolves through 2 stages: a pre-eruptive stage and an eruptive stage. Prodromal symptoms include malaise, severe headache, myalgias, fever, drenching sweats, backache, fatigue, sore throat, dyspnea, and cough. Within 2-3 days, the characteristic skin eruption develops. The lesions usually begin on the face and then spread in a centrifugal manner to the trunk and extremities, including the palms of the hands and soles of the feet. The lesions typically progress from macules to papules to vesicles to pustules. They then crust and scab over. An interesting additional finding is the presence of prominent lymphadenopathy behind the ear, beneath the mandible, in the neck, and in the groin.1
Several different illnesses must be considered in the differential diagnosis of monkeypox infection. They include measles, scabies, secondary syphilis, and medication-associated allergic reactions. However, the 2 conditions most likely to be confused with monkeypox are chickenpox (varicella) and smallpox. Lymphadenopathy is much more prominent in monkeypox compared with chickenpox. Moreover, with monkeypox, all lesions tend to be at the same stage of evolution as opposed to appearing in crops as they do in chickenpox. Smallpox would be extremely unlikely in the absence of a recognized laboratory accident or a bioterrorism incident.7
Diagnosis
The presumptive diagnosis of monkeypox infection is made primarily based on clinical examination. However, laboratory testing is indicated to definitively differentiate monkeypox from other orthopoxvirus infections such as varicella and smallpox.
In specialized laboratories that employ highly trained personnel and maintain strict safety precautions, the virus can be isolated in mammalian cell cultures. Electron microscopy is a valuable tool for identifying the characteristic brick-shaped poxvirus virions. Routine histologic examination of a lesion will show ballooning degeneration of keratinocytes, prominent spongiosis, dermal edema, and acute inflammation, although these findings are not unique to monkeypox.1
The Centers for Disease Control and Prevention (CDC) has developed serologic tests that detect immunoglobulin (Ig) M- and IgG-specific antibody. However, the most useful and practical diagnostic test is assessment of a skin scraping by polymerase chain reaction (PCR). This test is more sensitive than assessment of serum PCR.1
When the diagnosis of monkeypox is being considered, the clinician should coordinate testing through the local and state public health departments and through the CDC. Effective communication with all agencies will ensure that laboratory specimens are processed in a timely and efficient manner. The CDC website presents information on specimen collection.8
How do we manage monkeypox?
Prevention
The first step in prevention of infection is to isolate infected individuals until all lesions have dried and crusted over. Susceptible people should avoid close contact with skin lesions, respiratory and genital secretions, and bedding of patients who are infected.
The ultimate preventive measure, however, is vaccination of susceptible people either immediately before exposure (eg, military personnel, first responders, infection control investigators, health care workers) or immediately after exposure (general population). Older individuals who received the original smallpox vaccine likely have immunity to monkeypox infection. Unfortunately, very few women who currently are of reproductive age received this vaccine because its use was discontinued in the United States in the early 1970s. Therefore, the vast majority of our patients are uniquely susceptible to this infection and should be vaccinated if there is an outbreak of monkeypox in their locality.7,9
The current preferred vaccine for prevention of both smallpox and monkeypox is the Jynneos (Bavarian Nordic A/S) vaccine.10 This agent incorporates a replication-deficient live virus and does not pose the same risk for adverse events as the original versions of the smallpox vaccine. Jynneos is administered subcutaneously rather than by scarification. Two 0.5-mL doses, delivered 28 days apart, are required for optimal effect. The vaccine must be obtained from local and state health departments, in consultation with the CDC.7,9
There is very little published information on the safety of the Jynneos vaccine in pregnant or lactating women, although animal data are reassuring. Moreover, the dangers of monkeypox infection are significant, and in the event of an outbreak, vaccination of susceptible individuals, including pregnant women, is indicated.
- Monkeypox is a member of the orthopoxvirus genus and is closely related to the smallpox virus. It is a large, double-stranded, enveloped DNA virus.
- The virus is transmitted primarily by close contact with infected animals or other humans or by consumption of contaminated bushmeat.
- The infection evolves in 2 phases. The pre-eruptive phase is characterized by severe flu-like symptoms and signs. The eruptive phase is distinguished by a diffuse papular-vesicular rash.
- The most valuable test for confirming the diagnosis is a polymerase chain reaction test of a fresh skin lesion.
- In women who are pregnant, monkeypox has been associated with spontaneous abortion and fetal death.
- Three antiviral agents may be of value in treating infected patients: cidofovir, brincidofovir, and tecovirimat. Only the latter has an acceptable safety profile for women who are pregnant or lactating.
- The new nonreplicating smallpox vaccine Jynneos (Bavarian Nordic A/S) is of great value for pre- and post-exposure prophylaxis.
Continue to: Treatment...
Treatment
Infected pregnant women should receive acetaminophen 1,000 mg orally every 8 hours, to control fever and provide analgesia. An antihistamine such as diphenhydramine 25 mg orally every 6-8 hours, may be used to control pruritus and provide mild sedation. Adequate fluid intake and optimal nutrition should be encouraged. Skin lesions should be inspected regularly to detect signs of superimposed bacterial infections. Small, localized bacterial skin infections can be treated with topical application of mupirocin ointment 2%, 3 times daily for 7-14 days. For diffuse and more severe bacterial skin infections, a systemic antibiotic may be necessary. Reasonable choices include amoxicillin-clavulanate 875 mg/125 mg orally every 12 hours, or trimethoprim-sulfamethoxazole double strength 800 mg/160 mg orally every 12 hours.11 The latter agent should be avoided in the first trimester of pregnancy because of potential teratogenic effects.
Several specific agents are available through the CDC for treatment of orthopoxvirus infections such as smallpox and monkeypox. Information about these agents is summarized in the TABLE.12-16

Unique considerations in pregnancy
Because monkeypox is so rare, there is very little information about the effects of this infection in pregnant women. The report most commonly cited in the literature is that by Mbala et al, which was published in 2017.17 These authors described 4 pregnant patients in the Democratic Republic of Congo who contracted monkeypox infection over a 4-year period. All 4 women were hospitalized and treated with systemic antibiotics, antiparasitic medications, and analgesics. One patient delivered a healthy infant. Two women had spontaneous abortions in the first trimester. The fourth patient experienced a stillbirth at 22 weeks’ gestation. At postmortem examination, the fetus had diffuse cutaneous lesions, prominent hepatomegaly, and hydrops. No structural malformations were noted. The placenta demonstrated numerous punctate hemorrhages, and high concentrations of virus were recovered from the placenta and from fetal tissue.
Although the information on pregnancy outcome is quite limited, it seems clear that the virus can cross the placenta and cause adverse effects such as spontaneous abortion and fetal death. Accordingly, I think the following guidelines are a reasonable approach to a pregnant patient who has been exposed to monkeypox or who has developed manifestations of infection.3,7,9
- In the event of a community outbreak, bioterrorism event, or exposure to a person with suspected or confirmed monkeypox infection, the pregnant patient should receive the Jynneos vaccine.
- The pregnant patient should be isolated from any individual with suspected or confirmed monkeypox.
- If infection develops despite these measures, the patient should be treated with either tecovirimat or vaccinia immune globulin IV. Hospitalization may be necessary for seriously ill individuals.
- Within 2 weeks of infection, a comprehensive ultrasound examination should be performed to assess for structural abnormalities in the fetus.
- Subsequently, serial ultrasound examinations should be performed at intervals of 4-6 weeks to assess fetal growth and re-evaluate fetal anatomy.
- Following delivery, a detailed neonatal examination should be performed to assess for evidence of viral injury. Neonatal skin lesions and neonatal serum can be assessed by PCR for monkeypox virus. The newborn should be isolated from the mother until all the mother’s lesions have dried and crusted over.
CASE Resolved
Given the husband’s recent travel to Nigeria and consumption of bushmeat, he most likely has monkeypox. The infection can be spread from person to person by close contact; thus, his wife is at risk. The couple should isolate until all of his lesions have dried and crusted over. The woman also should receive the Jynneos vaccine. If she becomes symptomatic, she should be treated with tecovirimat or vaccinia immune globulin IV. ●

- Isaacs SN, Shenoy ES. Monkeypox. UpToDate. Updated June 28,2022. Accessed July 1, 2022. https://www.uptodate.com /contents/monkeypox?topicRef=8349&source=see_link
- Graham MB. Monkeypox. Medscape. Updated June 29, 2022. Accessed July 1, 2022. https://emedicine.medscape.com /article/1134714-overview.
- Khalil A, Samara A, O’Brien P, et al. Monkeypox and pregnancy: what do obstetricians need to know? Ultrasound Obstet Gynecol. 2022;60:22-27. doi:10.1002/uog.24968.
- World Health Organization. Monkeypox-United Kingdom of Great Britain and Northern Ireland. May 18, 2022. Accessed July 1, 2022. https://www.who.int/emergencies/diseaseoutbreak-news/item/2022-DON383.
- WHO reports two new monkeypox deaths, cases in new areas. Reuters. July 7, 2022. https://www.reuters.com/world /who-reports-two-new-monkeypox-deaths-2022-07-07/. Accessed July 19, 2022.
- World Health Organization. Multi-country monkeypox outbreak in non-endemic countries: update. May 29, 2022. Accessed July 1, 2022. https://www.who.int /emergencies/disease-outbreak-news/item/2022 -DON388#:~:text=Multi%2Dcountry%20monkeypox%20 outbreak%20in%20non%2Dendemic%20countries%3A%20 Update,-29%20May%202022&text=Since%2013%20 May%202022%2C%20monkeypox,Epidemiological%20 investigations%20are%20ongoing.
- Cono J, Cragan JD, Jamieson DJ, Rasmussen SA. Prophylaxis and treatment of pregnant women for emerging infections andbioterrorism emergencies. Emerg Infect Dis. 2006;12:16311637. doi:10.3201/eid1211.060618.
- Centers for Disease Control and Prevention. Preparation and collection of specimens. Reviewed June 29, 2022. Accessed July 6, 2022. https://www.cdc.gov/poxvirus /monkeypox/clinicians/prep-collection-specimens.html.
- Rao AK, Petersen BW, Whitehill F, et al. Monkeypox vaccination. MMWR Morb Mortal Wkly Rep. 2022;71:734-742. doi:10.15585/mmwr.mm7122e1.
- Smallpox and monkeypox vaccine, live, nonreplicating. Package insert. Bavarian Nordic A/S; 2021. Accessed July 1, 2022. https://www.fda.gov/media/131078/download.
- Duff P. Commonly used antibiotics in ObGyn practice. OBG Manag. 2022;34:29, 36-40. doi:10.12788/obgm.0191.
- Centers for Disease Control and Prevention. Treatment information for healthcare professionals: interim clinical guidance for the treatment of monkeypox. Reviewed June 17, 2022. Accessed July 1, 2022. https://www.cdc.gov/poxvirus /monkeypox/clinicians/treatment.html.
- Brincidofovir. Prescribing information. Chimerix, Inc.; 2021. Accessed July 1, 2022. https://www.accessdata.fda.gov /drugsatfda_docs/label/2021/214460s000,214461s000lbl.pdf.
- Cidofovir. Package insert. Gilead Sciences, Inc.; 2010. Accessed July 1, 2022. https://www.gilead.com/~/media /Files/pdfs/medicines/other/vistide/vistide.pdf.
- Tecovirimat. Prescribing information. Catalent Pharma Solutions; 2022. Accessed July 1, 2022. https://www.accessdata.fda.gov/drugsatfda_docs /label/2022/214518s000lbl.pdf.
- Vaccinia immune globulin IV. Prescribing information. Cangene Corporation; 2010. Accessed July 1, 2022. https: //www.fda.gov/media/77004/download.
- Mbala PK, Huggins JW, Riu-Rovira T, et al. Maternal and fetal outcomes among pregnant women with human monkeypox infection in the Democratic Republic of Congo. J Infect Dis. 2017;216:824-828. doi:10.1093/infdis/jix260.
- Isaacs SN, Shenoy ES. Monkeypox. UpToDate. Updated June 28,2022. Accessed July 1, 2022. https://www.uptodate.com /contents/monkeypox?topicRef=8349&source=see_link
- Graham MB. Monkeypox. Medscape. Updated June 29, 2022. Accessed July 1, 2022. https://emedicine.medscape.com /article/1134714-overview.
- Khalil A, Samara A, O’Brien P, et al. Monkeypox and pregnancy: what do obstetricians need to know? Ultrasound Obstet Gynecol. 2022;60:22-27. doi:10.1002/uog.24968.
- World Health Organization. Monkeypox-United Kingdom of Great Britain and Northern Ireland. May 18, 2022. Accessed July 1, 2022. https://www.who.int/emergencies/diseaseoutbreak-news/item/2022-DON383.
- WHO reports two new monkeypox deaths, cases in new areas. Reuters. July 7, 2022. https://www.reuters.com/world /who-reports-two-new-monkeypox-deaths-2022-07-07/. Accessed July 19, 2022.
- World Health Organization. Multi-country monkeypox outbreak in non-endemic countries: update. May 29, 2022. Accessed July 1, 2022. https://www.who.int /emergencies/disease-outbreak-news/item/2022 -DON388#:~:text=Multi%2Dcountry%20monkeypox%20 outbreak%20in%20non%2Dendemic%20countries%3A%20 Update,-29%20May%202022&text=Since%2013%20 May%202022%2C%20monkeypox,Epidemiological%20 investigations%20are%20ongoing.
- Cono J, Cragan JD, Jamieson DJ, Rasmussen SA. Prophylaxis and treatment of pregnant women for emerging infections andbioterrorism emergencies. Emerg Infect Dis. 2006;12:16311637. doi:10.3201/eid1211.060618.
- Centers for Disease Control and Prevention. Preparation and collection of specimens. Reviewed June 29, 2022. Accessed July 6, 2022. https://www.cdc.gov/poxvirus /monkeypox/clinicians/prep-collection-specimens.html.
- Rao AK, Petersen BW, Whitehill F, et al. Monkeypox vaccination. MMWR Morb Mortal Wkly Rep. 2022;71:734-742. doi:10.15585/mmwr.mm7122e1.
- Smallpox and monkeypox vaccine, live, nonreplicating. Package insert. Bavarian Nordic A/S; 2021. Accessed July 1, 2022. https://www.fda.gov/media/131078/download.
- Duff P. Commonly used antibiotics in ObGyn practice. OBG Manag. 2022;34:29, 36-40. doi:10.12788/obgm.0191.
- Centers for Disease Control and Prevention. Treatment information for healthcare professionals: interim clinical guidance for the treatment of monkeypox. Reviewed June 17, 2022. Accessed July 1, 2022. https://www.cdc.gov/poxvirus /monkeypox/clinicians/treatment.html.
- Brincidofovir. Prescribing information. Chimerix, Inc.; 2021. Accessed July 1, 2022. https://www.accessdata.fda.gov /drugsatfda_docs/label/2021/214460s000,214461s000lbl.pdf.
- Cidofovir. Package insert. Gilead Sciences, Inc.; 2010. Accessed July 1, 2022. https://www.gilead.com/~/media /Files/pdfs/medicines/other/vistide/vistide.pdf.
- Tecovirimat. Prescribing information. Catalent Pharma Solutions; 2022. Accessed July 1, 2022. https://www.accessdata.fda.gov/drugsatfda_docs /label/2022/214518s000lbl.pdf.
- Vaccinia immune globulin IV. Prescribing information. Cangene Corporation; 2010. Accessed July 1, 2022. https: //www.fda.gov/media/77004/download.
- Mbala PK, Huggins JW, Riu-Rovira T, et al. Maternal and fetal outcomes among pregnant women with human monkeypox infection in the Democratic Republic of Congo. J Infect Dis. 2017;216:824-828. doi:10.1093/infdis/jix260.
Nail Changes Associated With Thyroid Disease
The major classifications of thyroid disease include hyperthyroidism, which is seen in Graves disease, and hypothyroidism due to iodine deficiency and Hashimoto thyroiditis, which have potentially devastating health consequences. The prevalence of hyperthyroidism ranges from 0.2% to 1.3% in iodine-sufficient parts of the world, and the prevalence of hypothyroidism in the general population is 5.3% in Europe and 3.7% in the United States.1 Thyroid hormones physiologically potentiate α- and β-adrenergic receptors by increasing their sensitivity to catecholamines. Excess thyroid hormones manifest as tachycardia, increased cardiac output, increased body temperature, hyperhidrosis, and warm moist skin. Reduced sensitivity of adrenergic receptors to catecholamines from insufficient thyroid hormones results in a lower metabolic rate and decreases response to the sympathetic nervous system.2 Nail changes in thyroid patients have not been well studied.3 Our objectives were to characterize nail findings in patients with thyroid disease. Early diagnosis of thyroid disease and prompt referral for treatment may be instrumental in preventing serious morbidities and permanent sequelae.
Methods
PubMed, Scopus, Web of Science, and Google Scholar were searched for the terms nail + thyroid, nail + hyperthyroid, nail + hypothyroid, nail + Graves, and nail + Hashimoto on June 10, 2020, and then updated on November 18, 2020. All English-language articles were included. Non–English-language articles and those that did not describe clinical trials of nail changes in patients with thyroid disease were excluded. One study that utilized survey-based data for nail changes without corroboration with physical examination findings was excluded. Hypothyroidism/hyperthyroidism was defined by all authors as measurement of serum thyroid hormones triiodothyronine, thyroxine, and thyroid-stimulating hormone outside of the normal range. Eight studies were included in the final analysis. Patient demographics, thyroid disease type, physical examination findings, nail clinical findings, age at diagnosis, age at onset of nail changes, treatments/medications, and comorbidities were recorded and analyzed.
Results
Nail changes in patients with thyroid disease were reported in 8 studies (7 cross-sectional, 1 retrospective cohort) and are summarized in the Table.4-11 The mean age was 41.2 years (range, 5–80 years), with a higher representation of females (range, 70%–94% female). The most common nail changes in thyroid patients were koilonychia, clubbing, and nail brittleness. Other changes included onycholysis, thin nails, dryness, and changes in nail growth rate. Frequent physical findings were xerosis, pruritus, and alopecia.
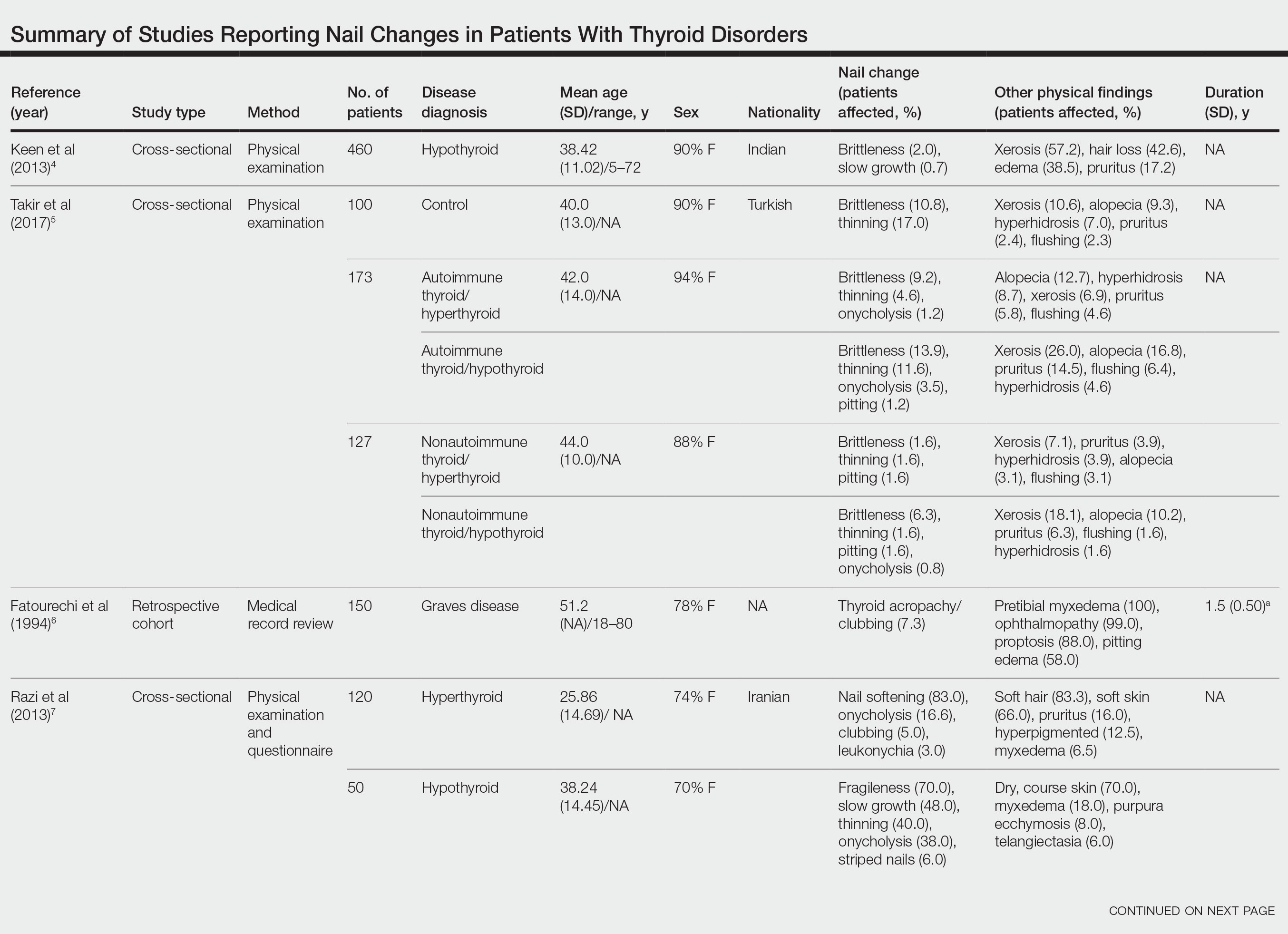
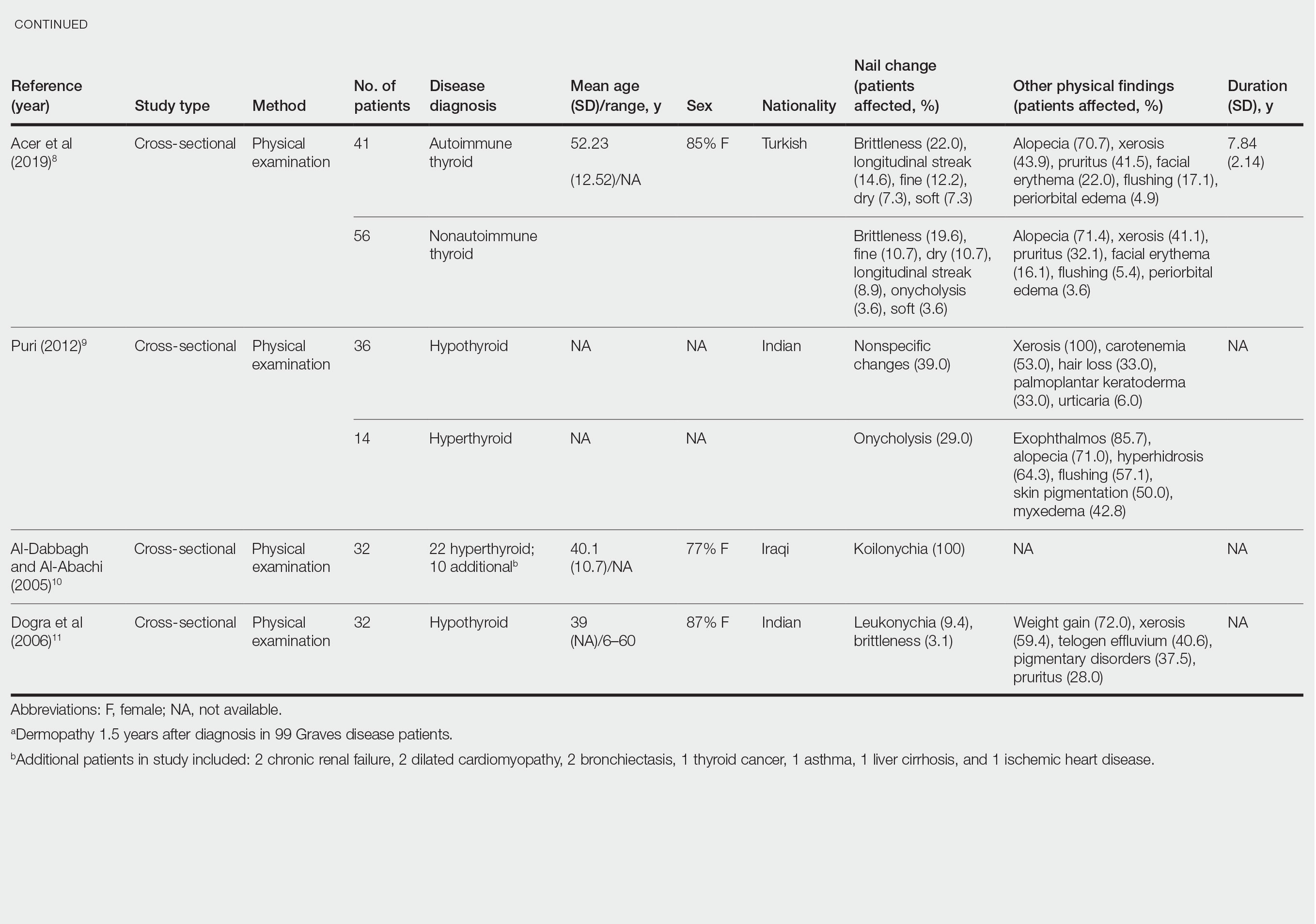
Both koilonychia and clubbing were reported in patients with hyperthyroidism. In a study of 32 patients with koilonychia, 22 (68.8%) were diagnosed with hyperthyroidism.10 Nail clubbing affected 7.3% of Graves disease patients (n=150)6 and 5.0% of hyperthyroid patients (n=120).7 Dermopathy presented more than 1 year after diagnosis of Graves disease in 99 (66%) of 150 patients as a late manifestation of thyrotoxicosis.6 Additional physical features in patients with Graves disease (n=150) were pretibial myxedema (100%), ophthalmopathy (99.0%), and proptosis (88.0%). Non–Graves hyperthyroid patients showed physical features of soft hair (83.3%) and soft skin (66.0%).7
Nail brittleness was a frequently reported nail change in thyroid patients (4/8 studies, 50%), most often seen in 22% of autoimmune patients, 19.6% of nonautoimmune patients, 13.9% of hypothyroid patients, and 9.2% of hyperthyroid patients.5,8 For comparison, brittle nails presented in 10.8% of participants in a control group.5 Brittle nails in thyroid patients often are accompanied by other nail findings such as thinning, onycholysis, and pitting.
Among hypothyroid patients, nail changes included fragility (70%; n=50), slow growth (48%; n=50), thinning (40%; n=50), onycholysis (38%; n=50),7 and brittleness (13.9%; n=173).5 Less common nail changes in hypothyroid patients were leukonychia (9.4%; n=32), striped nails (6%; n=50), and pitting (1.2%; n=173).5,7,11 Among hyperthyroid patients, the most common nail changes were koilonychia (100%; n=22), softening (83%; n=120), onycholysis (29%; n=14), and brittleness (9.2%; n=173).5,7,9,10 Less common nail changes in hyperthyroid patients were clubbing (5%; n=120), thinning (4.6%; n=173), and leukonychia (3%; n=120).5,7
Additional cutaneous findings of thyroid disorder included xerosis, alopecia, pruritus, and weight change. Xerosis was most common in hypothyroid disease (57.2%; n=460).4 In 2 studies,8,9 alopecia affected approximately 70% of autoimmune, nonautoimmune, and hyperthyroid patients. Hair loss was reported in 42.6% (n=460)4 and 33.0% (n=36)9 of hypothyroid patients. Additionally, pruritus affected up to 28% (n=32)11 of hypothyroid and 16.0% (n=120)7 of hyperthyroid patients and was more common in autoimmune (41%) vs nonautoimmune (32%) thyroid patients.8 Weight gain was seen in 72% of hypothyroid patients (n=32),11 and soft hair and skin were reported in 83.3% and 66% of hyperthyroid patients (n=120), respectively.7 Flushing was a less common physical finding in thyroid patients (usually affecting <10%); however, it also was reported in 17.1% of autoimmune and 57.1% of hyperthyroid patients from 2 separate studies.8,9
Comment
There are limited data describing nail changes with thyroid disease. Singal and Arora3 reported in their clinical review of nail changes in systemic disease that koilonychia, onycholysis, and melanonychia are associated with thyroid disorders. We similarly found that koilonychia and onycholysis are associated with thyroid disorders without an association with melanonychia.
In his clinical review of thyroid hormone action on the skin, Safer12 described hypothyroid patients having coarse, dull, thin, and brittle nails, whereas in thyrotoxicosis, patients had shiny, soft, and concave nails with onycholysis; however, the author commented that there were limited data on the clinical findings in thyroid disorders. These nail findings are consistent with our results, but onycholysis was more common in hypothyroid patients than in hyperthyroid patients in our review. Fox13 reported on 30 cases of onycholysis, stating that it affected patients with hypothyroidism and improved with thyroid treatment. In a clinical review of 8 commonly seen nail abnormalities, Fowler et al14 reported that hyperthyroidism was associated with nail findings in 5% of cases and may result in onycholysis of the fourth and fifth nails or all nails. They also reported that onychorrhexis may be seen in patients with hypothyroidism, a finding that differed from our results.14
The mechanism of nail changes in thyroid disease has not been well studied. A protein/amino acid–deficiency state may contribute to the development of koilonychia. Hyperthyroid patients, who have high metabolic activity, may have hypoalbuminemia, leading to koilonychia.15 Hypothyroidism causes hypothermia from decreased metabolic rate and secondary compensatory vasoconstriction. Vasoconstriction decreases blood flow of nutrients and oxygen to cutaneous structures and may cause slow-growing, brittle nails. In hyperthyroidism, vasodilation alternatively may contribute to the fast-growing nails. Anti–thyroid-stimulating hormone receptor antibodies in Graves disease may increase the synthesis of hyaluronic acid and glycosaminoglycans from fibroblasts, keratinocytes, adipocytes, or endothelial cells in the dermis and may contribute to development of clubbing.16
Our review is subject to several limitations. We recorded nail findings as they were described in the original studies; however, we could not confirm the accuracy of these descriptions. In addition, some specific nail changes were not described in sufficient detail. In all but 1 study, dermatologists performed the physical examination. In the study by Al-Dabbagh and Al-Abachi,10 the physical examinations were performed by general medicine physicians, but they selected only for patients with koilonychia and did not assess for other skin findings. Fragile nails and brittle nails were described in hypothyroid and hyperthyroid patients, but these nail changes were not described in detail. There also were studies describing nail changes in thyroid patients; some studies had small numbers of patients, and many did not have a control group.
Conclusion
Nail changes may be early clinical presenting signs of thyroid disorders and may be the clue to prompt diagnosis of thyroid disease. Dermatologists should be mindful that fragile, slow-growing, thin nails and onycholysis are associated with hypothyroidism and that koilonychia, softening, onycholysis, and brittle nail changes may be seen in hyperthyroidism. Our review aimed to describe nail changes associated with thyroid disease to guide dermatologists on diagnosis and promote future research on dermatologic manifestations of thyroid disease. Future research is necessary to explore the association between koilonychia and hyperthyroidism as well as the association of nail changes with thyroid disease duration and severity.
- Taylor PN, Albrecht D, Scholz A, et al. Global epidemiology of hyperthyroidism and hypothyroidism. Nat Rev Endocrinol. 2018;14:301-316.
- Lause M, Kamboj A, Faith EF. Dermatologic manifestations of endocrine disorders. Transl Pediatr. 2017;6:300-312.
- Singal A, Arora R. Nail as a window of systemic diseases. Indian Dermatol Online J. 2015;6:67-74.
- Keen MA, Hassan I, Bhat MH. A clinical study of the cutaneous manifestations of hypothyroidism in Kashmir Valley. Indian J Dermatol. 2013;58:326.
- Takir M, Özlü E, Köstek O, et al. Skin findings in autoimmune and nonautoimmune thyroid disease with respect to thyroid functional status and healthy controls. Turk J Med Sci. 2017;47:764-770.
- Fatourechi V, Pajouhi M, Fransway AF. Dermopathy of Graves disease (pretibial myxedema). review of 150 cases. Medicine (Baltimore). 1994;73:1-7.
- Razi A, Golforoushan F, Nejad AB, et al. Evaluation of dermal symptoms in hypothyroidism and hyperthyroidism. Pak J Biol Sci. 2013;16:541-544.
- Acer E, Ag˘aog˘lu E, Yorulmaz G, et al. Evaluation of cutaneous manifestations in patients under treatment with thyroid disease. Turkderm-Turk Arch Dermatol Venereol. 2019;54:46-50.
- Puri N. A study on cutaneous manifestations of thyroid disease. Indian J Dermatol. 2012;57:247-248.
- Al-Dabbagh TQ, Al-Abachi KG. Nutritional koilonychia in 32 Iraqi subjects. Ann Saudi Med. 2005;25:154-157.
- Dogra A, Dua A, Singh P. Thyroid and skin. Indian J Dermatol. 2006;51:96-99.
- Safer JD. Thyroid hormone action on skin. Dermatoendocrinol. 2011;3:211-215.
- Fox EC. Diseases of the nails: report of cases of onycholysis. Arch Derm Syphilol. 1940;41:98-112.
- Fowler JR, Stern E, English JC 3rd, et al. A hand surgeon’s guide to common onychodystrophies. Hand (N Y). 2014;9:24-28.
- Truswell AS. Nutritional factors in disease. In: Edwards CRW, Bouchier IAD, Haslett C, et al, eds. Davidson’s Principles and Practice of Medicine. 17th ed. Churchill Livingstone; 1995:554.
- Heymann WR. Cutaneous manifestations of thyroid disease. J Am Acad Dermatol. 1992;26:885-902.
The major classifications of thyroid disease include hyperthyroidism, which is seen in Graves disease, and hypothyroidism due to iodine deficiency and Hashimoto thyroiditis, which have potentially devastating health consequences. The prevalence of hyperthyroidism ranges from 0.2% to 1.3% in iodine-sufficient parts of the world, and the prevalence of hypothyroidism in the general population is 5.3% in Europe and 3.7% in the United States.1 Thyroid hormones physiologically potentiate α- and β-adrenergic receptors by increasing their sensitivity to catecholamines. Excess thyroid hormones manifest as tachycardia, increased cardiac output, increased body temperature, hyperhidrosis, and warm moist skin. Reduced sensitivity of adrenergic receptors to catecholamines from insufficient thyroid hormones results in a lower metabolic rate and decreases response to the sympathetic nervous system.2 Nail changes in thyroid patients have not been well studied.3 Our objectives were to characterize nail findings in patients with thyroid disease. Early diagnosis of thyroid disease and prompt referral for treatment may be instrumental in preventing serious morbidities and permanent sequelae.
Methods
PubMed, Scopus, Web of Science, and Google Scholar were searched for the terms nail + thyroid, nail + hyperthyroid, nail + hypothyroid, nail + Graves, and nail + Hashimoto on June 10, 2020, and then updated on November 18, 2020. All English-language articles were included. Non–English-language articles and those that did not describe clinical trials of nail changes in patients with thyroid disease were excluded. One study that utilized survey-based data for nail changes without corroboration with physical examination findings was excluded. Hypothyroidism/hyperthyroidism was defined by all authors as measurement of serum thyroid hormones triiodothyronine, thyroxine, and thyroid-stimulating hormone outside of the normal range. Eight studies were included in the final analysis. Patient demographics, thyroid disease type, physical examination findings, nail clinical findings, age at diagnosis, age at onset of nail changes, treatments/medications, and comorbidities were recorded and analyzed.
Results
Nail changes in patients with thyroid disease were reported in 8 studies (7 cross-sectional, 1 retrospective cohort) and are summarized in the Table.4-11 The mean age was 41.2 years (range, 5–80 years), with a higher representation of females (range, 70%–94% female). The most common nail changes in thyroid patients were koilonychia, clubbing, and nail brittleness. Other changes included onycholysis, thin nails, dryness, and changes in nail growth rate. Frequent physical findings were xerosis, pruritus, and alopecia.


Both koilonychia and clubbing were reported in patients with hyperthyroidism. In a study of 32 patients with koilonychia, 22 (68.8%) were diagnosed with hyperthyroidism.10 Nail clubbing affected 7.3% of Graves disease patients (n=150)6 and 5.0% of hyperthyroid patients (n=120).7 Dermopathy presented more than 1 year after diagnosis of Graves disease in 99 (66%) of 150 patients as a late manifestation of thyrotoxicosis.6 Additional physical features in patients with Graves disease (n=150) were pretibial myxedema (100%), ophthalmopathy (99.0%), and proptosis (88.0%). Non–Graves hyperthyroid patients showed physical features of soft hair (83.3%) and soft skin (66.0%).7
Nail brittleness was a frequently reported nail change in thyroid patients (4/8 studies, 50%), most often seen in 22% of autoimmune patients, 19.6% of nonautoimmune patients, 13.9% of hypothyroid patients, and 9.2% of hyperthyroid patients.5,8 For comparison, brittle nails presented in 10.8% of participants in a control group.5 Brittle nails in thyroid patients often are accompanied by other nail findings such as thinning, onycholysis, and pitting.
Among hypothyroid patients, nail changes included fragility (70%; n=50), slow growth (48%; n=50), thinning (40%; n=50), onycholysis (38%; n=50),7 and brittleness (13.9%; n=173).5 Less common nail changes in hypothyroid patients were leukonychia (9.4%; n=32), striped nails (6%; n=50), and pitting (1.2%; n=173).5,7,11 Among hyperthyroid patients, the most common nail changes were koilonychia (100%; n=22), softening (83%; n=120), onycholysis (29%; n=14), and brittleness (9.2%; n=173).5,7,9,10 Less common nail changes in hyperthyroid patients were clubbing (5%; n=120), thinning (4.6%; n=173), and leukonychia (3%; n=120).5,7
Additional cutaneous findings of thyroid disorder included xerosis, alopecia, pruritus, and weight change. Xerosis was most common in hypothyroid disease (57.2%; n=460).4 In 2 studies,8,9 alopecia affected approximately 70% of autoimmune, nonautoimmune, and hyperthyroid patients. Hair loss was reported in 42.6% (n=460)4 and 33.0% (n=36)9 of hypothyroid patients. Additionally, pruritus affected up to 28% (n=32)11 of hypothyroid and 16.0% (n=120)7 of hyperthyroid patients and was more common in autoimmune (41%) vs nonautoimmune (32%) thyroid patients.8 Weight gain was seen in 72% of hypothyroid patients (n=32),11 and soft hair and skin were reported in 83.3% and 66% of hyperthyroid patients (n=120), respectively.7 Flushing was a less common physical finding in thyroid patients (usually affecting <10%); however, it also was reported in 17.1% of autoimmune and 57.1% of hyperthyroid patients from 2 separate studies.8,9
Comment
There are limited data describing nail changes with thyroid disease. Singal and Arora3 reported in their clinical review of nail changes in systemic disease that koilonychia, onycholysis, and melanonychia are associated with thyroid disorders. We similarly found that koilonychia and onycholysis are associated with thyroid disorders without an association with melanonychia.
In his clinical review of thyroid hormone action on the skin, Safer12 described hypothyroid patients having coarse, dull, thin, and brittle nails, whereas in thyrotoxicosis, patients had shiny, soft, and concave nails with onycholysis; however, the author commented that there were limited data on the clinical findings in thyroid disorders. These nail findings are consistent with our results, but onycholysis was more common in hypothyroid patients than in hyperthyroid patients in our review. Fox13 reported on 30 cases of onycholysis, stating that it affected patients with hypothyroidism and improved with thyroid treatment. In a clinical review of 8 commonly seen nail abnormalities, Fowler et al14 reported that hyperthyroidism was associated with nail findings in 5% of cases and may result in onycholysis of the fourth and fifth nails or all nails. They also reported that onychorrhexis may be seen in patients with hypothyroidism, a finding that differed from our results.14
The mechanism of nail changes in thyroid disease has not been well studied. A protein/amino acid–deficiency state may contribute to the development of koilonychia. Hyperthyroid patients, who have high metabolic activity, may have hypoalbuminemia, leading to koilonychia.15 Hypothyroidism causes hypothermia from decreased metabolic rate and secondary compensatory vasoconstriction. Vasoconstriction decreases blood flow of nutrients and oxygen to cutaneous structures and may cause slow-growing, brittle nails. In hyperthyroidism, vasodilation alternatively may contribute to the fast-growing nails. Anti–thyroid-stimulating hormone receptor antibodies in Graves disease may increase the synthesis of hyaluronic acid and glycosaminoglycans from fibroblasts, keratinocytes, adipocytes, or endothelial cells in the dermis and may contribute to development of clubbing.16
Our review is subject to several limitations. We recorded nail findings as they were described in the original studies; however, we could not confirm the accuracy of these descriptions. In addition, some specific nail changes were not described in sufficient detail. In all but 1 study, dermatologists performed the physical examination. In the study by Al-Dabbagh and Al-Abachi,10 the physical examinations were performed by general medicine physicians, but they selected only for patients with koilonychia and did not assess for other skin findings. Fragile nails and brittle nails were described in hypothyroid and hyperthyroid patients, but these nail changes were not described in detail. There also were studies describing nail changes in thyroid patients; some studies had small numbers of patients, and many did not have a control group.
Conclusion
Nail changes may be early clinical presenting signs of thyroid disorders and may be the clue to prompt diagnosis of thyroid disease. Dermatologists should be mindful that fragile, slow-growing, thin nails and onycholysis are associated with hypothyroidism and that koilonychia, softening, onycholysis, and brittle nail changes may be seen in hyperthyroidism. Our review aimed to describe nail changes associated with thyroid disease to guide dermatologists on diagnosis and promote future research on dermatologic manifestations of thyroid disease. Future research is necessary to explore the association between koilonychia and hyperthyroidism as well as the association of nail changes with thyroid disease duration and severity.
The major classifications of thyroid disease include hyperthyroidism, which is seen in Graves disease, and hypothyroidism due to iodine deficiency and Hashimoto thyroiditis, which have potentially devastating health consequences. The prevalence of hyperthyroidism ranges from 0.2% to 1.3% in iodine-sufficient parts of the world, and the prevalence of hypothyroidism in the general population is 5.3% in Europe and 3.7% in the United States.1 Thyroid hormones physiologically potentiate α- and β-adrenergic receptors by increasing their sensitivity to catecholamines. Excess thyroid hormones manifest as tachycardia, increased cardiac output, increased body temperature, hyperhidrosis, and warm moist skin. Reduced sensitivity of adrenergic receptors to catecholamines from insufficient thyroid hormones results in a lower metabolic rate and decreases response to the sympathetic nervous system.2 Nail changes in thyroid patients have not been well studied.3 Our objectives were to characterize nail findings in patients with thyroid disease. Early diagnosis of thyroid disease and prompt referral for treatment may be instrumental in preventing serious morbidities and permanent sequelae.
Methods
PubMed, Scopus, Web of Science, and Google Scholar were searched for the terms nail + thyroid, nail + hyperthyroid, nail + hypothyroid, nail + Graves, and nail + Hashimoto on June 10, 2020, and then updated on November 18, 2020. All English-language articles were included. Non–English-language articles and those that did not describe clinical trials of nail changes in patients with thyroid disease were excluded. One study that utilized survey-based data for nail changes without corroboration with physical examination findings was excluded. Hypothyroidism/hyperthyroidism was defined by all authors as measurement of serum thyroid hormones triiodothyronine, thyroxine, and thyroid-stimulating hormone outside of the normal range. Eight studies were included in the final analysis. Patient demographics, thyroid disease type, physical examination findings, nail clinical findings, age at diagnosis, age at onset of nail changes, treatments/medications, and comorbidities were recorded and analyzed.
Results
Nail changes in patients with thyroid disease were reported in 8 studies (7 cross-sectional, 1 retrospective cohort) and are summarized in the Table.4-11 The mean age was 41.2 years (range, 5–80 years), with a higher representation of females (range, 70%–94% female). The most common nail changes in thyroid patients were koilonychia, clubbing, and nail brittleness. Other changes included onycholysis, thin nails, dryness, and changes in nail growth rate. Frequent physical findings were xerosis, pruritus, and alopecia.


Both koilonychia and clubbing were reported in patients with hyperthyroidism. In a study of 32 patients with koilonychia, 22 (68.8%) were diagnosed with hyperthyroidism.10 Nail clubbing affected 7.3% of Graves disease patients (n=150)6 and 5.0% of hyperthyroid patients (n=120).7 Dermopathy presented more than 1 year after diagnosis of Graves disease in 99 (66%) of 150 patients as a late manifestation of thyrotoxicosis.6 Additional physical features in patients with Graves disease (n=150) were pretibial myxedema (100%), ophthalmopathy (99.0%), and proptosis (88.0%). Non–Graves hyperthyroid patients showed physical features of soft hair (83.3%) and soft skin (66.0%).7
Nail brittleness was a frequently reported nail change in thyroid patients (4/8 studies, 50%), most often seen in 22% of autoimmune patients, 19.6% of nonautoimmune patients, 13.9% of hypothyroid patients, and 9.2% of hyperthyroid patients.5,8 For comparison, brittle nails presented in 10.8% of participants in a control group.5 Brittle nails in thyroid patients often are accompanied by other nail findings such as thinning, onycholysis, and pitting.
Among hypothyroid patients, nail changes included fragility (70%; n=50), slow growth (48%; n=50), thinning (40%; n=50), onycholysis (38%; n=50),7 and brittleness (13.9%; n=173).5 Less common nail changes in hypothyroid patients were leukonychia (9.4%; n=32), striped nails (6%; n=50), and pitting (1.2%; n=173).5,7,11 Among hyperthyroid patients, the most common nail changes were koilonychia (100%; n=22), softening (83%; n=120), onycholysis (29%; n=14), and brittleness (9.2%; n=173).5,7,9,10 Less common nail changes in hyperthyroid patients were clubbing (5%; n=120), thinning (4.6%; n=173), and leukonychia (3%; n=120).5,7
Additional cutaneous findings of thyroid disorder included xerosis, alopecia, pruritus, and weight change. Xerosis was most common in hypothyroid disease (57.2%; n=460).4 In 2 studies,8,9 alopecia affected approximately 70% of autoimmune, nonautoimmune, and hyperthyroid patients. Hair loss was reported in 42.6% (n=460)4 and 33.0% (n=36)9 of hypothyroid patients. Additionally, pruritus affected up to 28% (n=32)11 of hypothyroid and 16.0% (n=120)7 of hyperthyroid patients and was more common in autoimmune (41%) vs nonautoimmune (32%) thyroid patients.8 Weight gain was seen in 72% of hypothyroid patients (n=32),11 and soft hair and skin were reported in 83.3% and 66% of hyperthyroid patients (n=120), respectively.7 Flushing was a less common physical finding in thyroid patients (usually affecting <10%); however, it also was reported in 17.1% of autoimmune and 57.1% of hyperthyroid patients from 2 separate studies.8,9
Comment
There are limited data describing nail changes with thyroid disease. Singal and Arora3 reported in their clinical review of nail changes in systemic disease that koilonychia, onycholysis, and melanonychia are associated with thyroid disorders. We similarly found that koilonychia and onycholysis are associated with thyroid disorders without an association with melanonychia.
In his clinical review of thyroid hormone action on the skin, Safer12 described hypothyroid patients having coarse, dull, thin, and brittle nails, whereas in thyrotoxicosis, patients had shiny, soft, and concave nails with onycholysis; however, the author commented that there were limited data on the clinical findings in thyroid disorders. These nail findings are consistent with our results, but onycholysis was more common in hypothyroid patients than in hyperthyroid patients in our review. Fox13 reported on 30 cases of onycholysis, stating that it affected patients with hypothyroidism and improved with thyroid treatment. In a clinical review of 8 commonly seen nail abnormalities, Fowler et al14 reported that hyperthyroidism was associated with nail findings in 5% of cases and may result in onycholysis of the fourth and fifth nails or all nails. They also reported that onychorrhexis may be seen in patients with hypothyroidism, a finding that differed from our results.14
The mechanism of nail changes in thyroid disease has not been well studied. A protein/amino acid–deficiency state may contribute to the development of koilonychia. Hyperthyroid patients, who have high metabolic activity, may have hypoalbuminemia, leading to koilonychia.15 Hypothyroidism causes hypothermia from decreased metabolic rate and secondary compensatory vasoconstriction. Vasoconstriction decreases blood flow of nutrients and oxygen to cutaneous structures and may cause slow-growing, brittle nails. In hyperthyroidism, vasodilation alternatively may contribute to the fast-growing nails. Anti–thyroid-stimulating hormone receptor antibodies in Graves disease may increase the synthesis of hyaluronic acid and glycosaminoglycans from fibroblasts, keratinocytes, adipocytes, or endothelial cells in the dermis and may contribute to development of clubbing.16
Our review is subject to several limitations. We recorded nail findings as they were described in the original studies; however, we could not confirm the accuracy of these descriptions. In addition, some specific nail changes were not described in sufficient detail. In all but 1 study, dermatologists performed the physical examination. In the study by Al-Dabbagh and Al-Abachi,10 the physical examinations were performed by general medicine physicians, but they selected only for patients with koilonychia and did not assess for other skin findings. Fragile nails and brittle nails were described in hypothyroid and hyperthyroid patients, but these nail changes were not described in detail. There also were studies describing nail changes in thyroid patients; some studies had small numbers of patients, and many did not have a control group.
Conclusion
Nail changes may be early clinical presenting signs of thyroid disorders and may be the clue to prompt diagnosis of thyroid disease. Dermatologists should be mindful that fragile, slow-growing, thin nails and onycholysis are associated with hypothyroidism and that koilonychia, softening, onycholysis, and brittle nail changes may be seen in hyperthyroidism. Our review aimed to describe nail changes associated with thyroid disease to guide dermatologists on diagnosis and promote future research on dermatologic manifestations of thyroid disease. Future research is necessary to explore the association between koilonychia and hyperthyroidism as well as the association of nail changes with thyroid disease duration and severity.
- Taylor PN, Albrecht D, Scholz A, et al. Global epidemiology of hyperthyroidism and hypothyroidism. Nat Rev Endocrinol. 2018;14:301-316.
- Lause M, Kamboj A, Faith EF. Dermatologic manifestations of endocrine disorders. Transl Pediatr. 2017;6:300-312.
- Singal A, Arora R. Nail as a window of systemic diseases. Indian Dermatol Online J. 2015;6:67-74.
- Keen MA, Hassan I, Bhat MH. A clinical study of the cutaneous manifestations of hypothyroidism in Kashmir Valley. Indian J Dermatol. 2013;58:326.
- Takir M, Özlü E, Köstek O, et al. Skin findings in autoimmune and nonautoimmune thyroid disease with respect to thyroid functional status and healthy controls. Turk J Med Sci. 2017;47:764-770.
- Fatourechi V, Pajouhi M, Fransway AF. Dermopathy of Graves disease (pretibial myxedema). review of 150 cases. Medicine (Baltimore). 1994;73:1-7.
- Razi A, Golforoushan F, Nejad AB, et al. Evaluation of dermal symptoms in hypothyroidism and hyperthyroidism. Pak J Biol Sci. 2013;16:541-544.
- Acer E, Ag˘aog˘lu E, Yorulmaz G, et al. Evaluation of cutaneous manifestations in patients under treatment with thyroid disease. Turkderm-Turk Arch Dermatol Venereol. 2019;54:46-50.
- Puri N. A study on cutaneous manifestations of thyroid disease. Indian J Dermatol. 2012;57:247-248.
- Al-Dabbagh TQ, Al-Abachi KG. Nutritional koilonychia in 32 Iraqi subjects. Ann Saudi Med. 2005;25:154-157.
- Dogra A, Dua A, Singh P. Thyroid and skin. Indian J Dermatol. 2006;51:96-99.
- Safer JD. Thyroid hormone action on skin. Dermatoendocrinol. 2011;3:211-215.
- Fox EC. Diseases of the nails: report of cases of onycholysis. Arch Derm Syphilol. 1940;41:98-112.
- Fowler JR, Stern E, English JC 3rd, et al. A hand surgeon’s guide to common onychodystrophies. Hand (N Y). 2014;9:24-28.
- Truswell AS. Nutritional factors in disease. In: Edwards CRW, Bouchier IAD, Haslett C, et al, eds. Davidson’s Principles and Practice of Medicine. 17th ed. Churchill Livingstone; 1995:554.
- Heymann WR. Cutaneous manifestations of thyroid disease. J Am Acad Dermatol. 1992;26:885-902.
- Taylor PN, Albrecht D, Scholz A, et al. Global epidemiology of hyperthyroidism and hypothyroidism. Nat Rev Endocrinol. 2018;14:301-316.
- Lause M, Kamboj A, Faith EF. Dermatologic manifestations of endocrine disorders. Transl Pediatr. 2017;6:300-312.
- Singal A, Arora R. Nail as a window of systemic diseases. Indian Dermatol Online J. 2015;6:67-74.
- Keen MA, Hassan I, Bhat MH. A clinical study of the cutaneous manifestations of hypothyroidism in Kashmir Valley. Indian J Dermatol. 2013;58:326.
- Takir M, Özlü E, Köstek O, et al. Skin findings in autoimmune and nonautoimmune thyroid disease with respect to thyroid functional status and healthy controls. Turk J Med Sci. 2017;47:764-770.
- Fatourechi V, Pajouhi M, Fransway AF. Dermopathy of Graves disease (pretibial myxedema). review of 150 cases. Medicine (Baltimore). 1994;73:1-7.
- Razi A, Golforoushan F, Nejad AB, et al. Evaluation of dermal symptoms in hypothyroidism and hyperthyroidism. Pak J Biol Sci. 2013;16:541-544.
- Acer E, Ag˘aog˘lu E, Yorulmaz G, et al. Evaluation of cutaneous manifestations in patients under treatment with thyroid disease. Turkderm-Turk Arch Dermatol Venereol. 2019;54:46-50.
- Puri N. A study on cutaneous manifestations of thyroid disease. Indian J Dermatol. 2012;57:247-248.
- Al-Dabbagh TQ, Al-Abachi KG. Nutritional koilonychia in 32 Iraqi subjects. Ann Saudi Med. 2005;25:154-157.
- Dogra A, Dua A, Singh P. Thyroid and skin. Indian J Dermatol. 2006;51:96-99.
- Safer JD. Thyroid hormone action on skin. Dermatoendocrinol. 2011;3:211-215.
- Fox EC. Diseases of the nails: report of cases of onycholysis. Arch Derm Syphilol. 1940;41:98-112.
- Fowler JR, Stern E, English JC 3rd, et al. A hand surgeon’s guide to common onychodystrophies. Hand (N Y). 2014;9:24-28.
- Truswell AS. Nutritional factors in disease. In: Edwards CRW, Bouchier IAD, Haslett C, et al, eds. Davidson’s Principles and Practice of Medicine. 17th ed. Churchill Livingstone; 1995:554.
- Heymann WR. Cutaneous manifestations of thyroid disease. J Am Acad Dermatol. 1992;26:885-902.
Practice Points
- Koilonychia is associated with hyperthyroidism.
- Clubbing is a manifestation of thyroid acropachy in Graves disease and also affects other patients with hyperthyroidism.
- Onycholysis improves in patients with hypothyroidism treated with thyroid hormone replacement therapy.
Medical assistants identify strategies and barriers to clinic efficiency
ABSTRACT
Background: Medical assistant (MA) roles have expanded rapidly as primary care has evolved and MAs take on new patient care duties. Research that looks at the MA experience and factors that enhance or reduce efficiency among MAs is limited.
Methods: We surveyed all MAs working in 6 clinics run by a large academic family medicine department in Ann Arbor, Michigan. MAs deemed by peers as “most efficient” were selected for follow-up interviews. We evaluated personal strategies for efficiency, barriers to efficient care, impact of physician actions on efficiency, and satisfaction.
Results: A total of 75/86 MAs (87%) responded to at least some survey questions and 61/86 (71%) completed the full survey. We interviewed 18 MAs face to face. Most saw their role as essential to clinic functioning and viewed health care as a personal calling. MAs identified common strategies to improve efficiency and described the MA role to orchestrate the flow of the clinic day. Staff recognized differing priorities of patients, staff, and physicians and articulated frustrations with hierarchy and competing priorities as well as behaviors that impeded clinic efficiency. Respondents emphasized the importance of feeling valued by others on their team.
Conclusions: With the evolving demands made on MAs’ time, it is critical to understand how the most effective staff members manage their role and highlight the strategies they employ to provide efficient clinical care. Understanding factors that increase or decrease MA job satisfaction can help identify high-efficiency practices and promote a clinic culture that values and supports all staff.
As primary care continues to evolve into more team-based practice, the role of the medical assistant (MA) has rapidly transformed.1 Staff may assist with patient management, documentation in the electronic medical record, order entry, pre-visit planning, and fulfillment of quality metrics, particularly in a Primary Care Medical Home (PCMH).2 From 2012 through 2014, MA job postings per graduate increased from 1.3 to 2.3, suggesting twice as many job postings as graduates.3 As the demand for experienced MAs increases, the ability to recruit and retain high-performing staff members will be critical.
MAs are referenced in medical literature as early as the 1800s.4 The American Association of Medical Assistants was founded in 1956, which led to educational standardization and certifications.5 Despite the important role that MAs have long played in the proper functioning of a medical clinic—and the knowledge that team configurations impact a clinic’s efficiency and quality6,7—few investigations have sought out the MA’s perspective.8,9 Given the increasing clinical demands placed on all members of the primary care team (and the burnout that often results), it seems that MA insights into clinic efficiency could be valuable.
Continue to: Methods...
METHODS
This cross-sectional study was conducted from February to April 2019 at a large academic institution with 6 regional ambulatory care family medicine clinics, each one with 11,000 to 18,000 patient visits annually. Faculty work at all 6 clinics and residents at 2 of them. All MAs are hired, paid, and managed by a central administrative department rather than by the family medicine department. The family medicine clinics are currently PCMH certified, with a mix of fee-for-service and capitated reimbursement.
We developed and piloted a voluntary, anonymous 39-question (29 closed-ended and 10 brief open-ended) online Qualtrics survey, which we distributed via an email link to all the MAs in the department. The survey included clinic site, years as an MA, perceptions of the clinic environment, perception of teamwork at their site, identification of efficient practices, and feedback for physicians to improve efficiency and flow. Most questions were Likert-style with 5 choices ranging from “strongly agree” to “strongly disagree” or short answer. Age and gender were omitted to protect confidentiality, as most MAs in the department are female. Participants could opt to enter in a drawing for three $25 gift cards. The survey was reviewed by the University of Michigan Institutional Review Board and deemed exempt.
We asked MAs to nominate peers in their clinic who were “especially efficient and do their jobs well—people that others can learn from.” The staff members who were nominated most frequently by their peers were invited to share additional perspectives via a 10- to 30-minute semi-structured interview with the first author. Interviews covered highly efficient practices, barriers and facilitators to efficient care, and physician behaviors that impaired efficiency. We interviewed a minimum of 2 MAs per clinic and increased the number of interviews through snowball sampling, as needed, to reach data saturation (eg, the point at which we were no longer hearing new content). MAs were assured that all comments would be anonymized. There was no monetary incentive for the interviews. The interviewer had previously met only 3 of the 18 MAs interviewed.
Analysis. Summary statistics were calculated for quantitative data. To compare subgroups (such as individual clinics), a chi-square test was used. In cases when there were small cell sizes (< 5 subjects), we used the Fisher’s Exact test. Qualitative data was collected with real-time typewritten notes during the interviews to capture ideas and verbatim quotes when possible. We also included open-ended comments shared on the Qualtrics survey. Data were organized by theme using a deductive coding approach. Both authors reviewed and discussed observations, and coding was conducted by the first author. Reporting followed the STROBE Statement checklist for cross-sectional studies.10 Results were shared with MAs, supervisory staff, and physicians, which allowed for feedback and comments and served as “member-checking.” MAs reported that the data reflected their lived experiences.
Continue to: RESULTS...
RESULTS
Surveys were distributed to all 86 MAs working in family medicine clinics. A total of 75 (87%) responded to at least some questions (typically just demographics). We used those who completed the full survey (n = 61; 71%) for data analysis. Eighteen MAs participated in face-to-face interviews. Among respondents, 35 (47%) had worked at least 10 years as an MA and 21 (28%) had worked at least a decade in the family medicine department.
Perception of role
All respondents (n = 61; 100%) somewhat or strongly agreed that the MA role was “very important to keep the clinic functioning” and 58 (95%) reported that working in health care was “a calling” for them. Only 7 (11%) agreed that family medicine was an easier environment for MAs compared to a specialty clinic; 30 (49%) disagreed with this. Among respondents, 32 (53%) strongly or somewhat agreed that their work was very stressful and just half (n = 28; 46%) agreed there were adequate MA staff at their clinic.
Efficiency and competing priorities
MAs described important work values that increased their efficiency. These included clinic culture (good communication and strong teamwork), as well as individual strategies such as multitasking, limiting patient conversations, and doing tasks in a consistent way to improve accuracy. (See TABLE 1.) They identified ways physicians bolster or hurt efficiency and ways in which the relationship between the physician and the MA shapes the MA’s perception of their value in clinic.
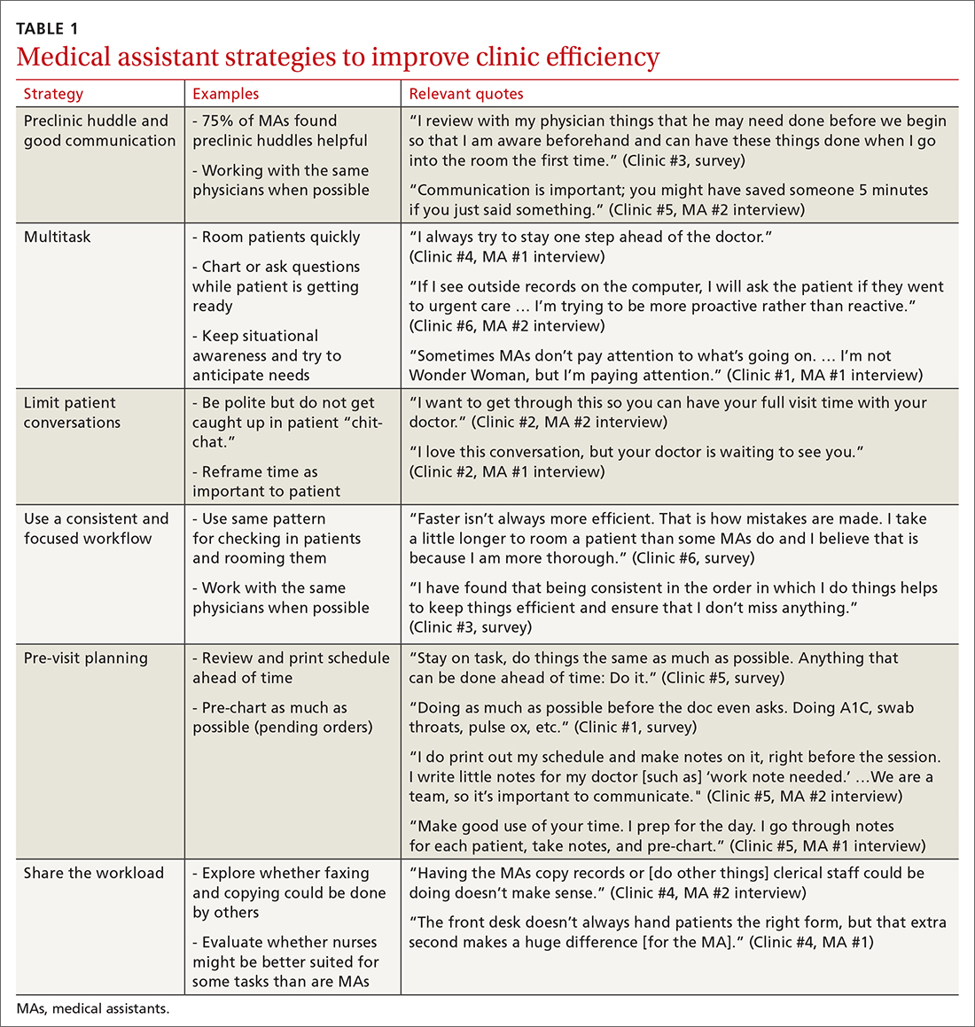
Communication was emphasized as critical for efficient care, and MAs encouraged the use of preclinic huddles and communication as priorities. Seventy-five percent of MAs reported preclinic huddles to plan for patient care were helpful, but only half said huddles took place “always” or “most of the time.” Many described reviewing the schedule and completing tasks ahead of patient arrival as critical to efficiency.
Participants described the tension between their identified role of orchestrating clinic flow and responding to directives by others that disrupted the flow. Several MAs found it challenging when physicians agreed to see very late patients and felt frustrated when decisions that changed the flow were made by the physician or front desk staff without including the MA. MAs were also able to articulate how they managed competing priorities within the clinic, such as when a patient- or physician-driven need to extend appointments was at odds with maintaining a timely schedule. They were eager to share personal tips for time management and prided themselves on careful and accurate performance and skills they had learned on the job. MAs also described how efficiency could be adversely affected by the behaviors or attitudes of physicians. (See TABLE 2.)
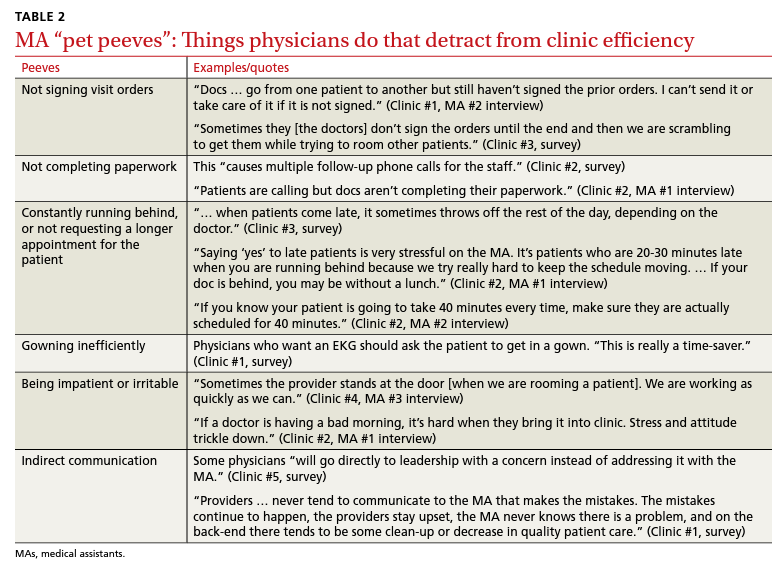
Continue to: Clinic environment...
Clinic environment
Thirty-six MAs (59%) reported that other MAs on their team were willing to help them out in clinic “a great deal” or “a lot” of the time, by helping to room a patient, acting as a chaperone for an exam, or doing a point-of-care lab. This sense of support varied across clinics (38% to 91% reported good support), suggesting that cultures vary by site. Some MAs expressed frustration at peers they saw as resistant to helping, exemplified by this verbatim quote from an interview:
“Some don’t want to help out. They may sigh. It’s how they react—you just know.” (Clinic #1, MA #2 interview)
Efficient MAs stressed the need for situational awareness to recognize when co-workers need help:
“[Peers often] are not aware that another MA is drowning. There’s 5 people who could have done that, and here I am running around and nobody budged.” (Clinic #5, MA #2 interview)
A minority of staff used the open-ended survey sections to describe clinic hierarchy. When asked about “pet peeves,” a few advised that physicians should not “talk down” to staff and should try to teach rather than criticize. Another asked that physicians not “bark orders” or have “low gratitude” for staff work. MAs found micromanaging stressful—particularly when the physician prompted the MA about patient arrivals:
“[I don’t like] when providers will make a comment about a patient arriving when you already know this information. You then rush to put [the] patient in [a] room, then [the] provider ends up making [the] patient wait an extensive amount of time. I’m perfectly capable of knowing when a patient arrives.” (Clinic #6, survey)
MAs did not like physicians “talking bad about us” or blaming the MA if the clinic is running behind.
Despite these concerns, most MAs reported feeling appreciated for the job they do. Only 10 (16%) reported that the people they work with rarely say “thank you,” and 2 (3%) stated they were not well supported by the physicians in clinic. Most (n = 38; 62%) strongly agreed or agreed that they felt part of the team and that their opinions matter. In the interviews, many expanded on this idea:
“I really feel like I’m valued, so I want to do everything I can to make [my doctor’s] day go better. If you want a good clinic, the best thing a doc can do is make the MA feel valued.” (Clinic #1, MA #1 interview)
Continue to: DISCUSSION...
DISCUSSION
Participants described their role much as an orchestra director, with MAs as the key to clinic flow and timeliness.9 Respondents articulated multiple common strategies used to increase their own efficiency and clinic flow; these may be considered best practices and incorporated as part of the basic training. Most MAs reported their day-to-day jobs were stressful and believed this was underrecognized, so efficiency strategies are critical. With staff completing multiple time-sensitive tasks during clinic, consistent co-worker support is crucial and may impact efficiency.8 Proper training of managers to provide that support and ensure equitable workloads may be one strategy to ensure that staff members feel the workplace is fair and collegial.
Several comments reflected the power differential within medical offices. One study reported that MAs and physicians “occupy roles at opposite ends of social and occupational hierarchies.”11 It’s important for physicians to be cognizant of these patterns and clinic culture, as reducing a hierarchy-based environment will be appreciated by MAs.9 Prior research has found that MAs have higher perceptions of their own competence than do the physicians working with them.12 If there is a fundamental lack of trust between the 2 groups, this will undoubtedly hinder team-building. Attention to this issue is key to a more favorable work environment.
Almost all respondents reported health care was a “calling,” which mirrors physician research that suggests seeing work as a “calling” is protective against burnout.13,14 Open-ended comments indicated great pride in contributions, and most staff members felt appreciated by their teams. Many described the working relationships with physicians as critical to their satisfaction at work and indicated that strong partnerships motivated them to do their best to make the physician’s day easier. Staff job satisfaction is linked to improved quality of care, so treating staff well contributes to high-value care for patients.15 We also uncovered some MA “pet peeves” that hinder efficiency and could be shared with physicians to emphasize the importance of patience and civility.
One barrier to expansion of MA roles within PCMH practices is the limited pay and career ladder for MAs who adopt new job responsibilities that require advanced skills or training.1,2 The mean MA salary at our institution ($37,372) is higher than in our state overall ($33,760), which may impact satisfaction.16 In addition, 93% of MAs are women; thus, they may continue to struggle more with lower pay than do workers in male- dominated professions.17,18 Expected job growth from 2018-2028 is predicted at 23%, which may help to boost salaries. 19 Prior studies describe the lack of a job ladder or promotion opportunities as a challenge1,20; this was not formally assessed in our study.
MAs see work in family medicine as much harder than it is in other specialty clinics. Being trusted with more responsibility, greater autonomy,21-23 and expanded patient care roles can boost MA self-efficacy, which can reduce burnout for both physicians and MAs. 8,24 However, new responsibilities should include appropriate training, support, and compensation, and match staff interests.7
Study limitations. The study was limited to 6 clinics in 1 department at a large academic medical center. Interviewed participants were selected by convenience and snowball sampling and thus, the results cannot be generalized to the population of MAs as a whole. As the initial interview goal was simply to gather efficiency tips, the project was not designed to be formal qualitative research. However, the discussions built on open-ended comments from the written survey helped contextualize our quantitative findings about efficiency. Notes were documented in real time by a single interviewer with rapid typing skills, which allowed capture of quotes verbatim. Subsequent studies would benefit from more formal qualitative research methods (recording and transcribing interviews, multiple coders to reduce risk of bias, and more complex thematic analysis).
Our research demonstrated how MAs perceive their roles in primary care and the facilitators and barriers to high efficiency in the workplace, which begins to fill an important knowledge gap in primary care. Disseminating practices that staff members themselves have identified as effective, and being attentive to how staff members are treated, may increase individual efficiency while improving staff retention and satisfaction.
CORRESPONDENCE Katherine J. Gold, MD, MSW, MS, Department of Family Medicine and Department of Obstetrics and Gynecology, University of Michigan, 1018 Fuller Street, Ann Arbor, MI 48104-1213; [email protected]
- Chapman SA, Blash LK. New roles for medical assistants in innovative primary care practices. Health Serv Res. 2017;52(suppl 1):383-406.
- Ferrante JM, Shaw EK, Bayly JE, et al. Barriers and facilitators to expanding roles of medical assistants in patient-centered medical homes (PCMHs). J Am Board Fam Med. 2018;31:226-235.
- Atkins B. The outlook for medical assisting in 2016 and beyond. Accessed January 27, 2022. www.medicalassistantdegrees.net/ articles/medical-assisting-trends/
- Unqualified medical “assistants.” Hospital (Lond 1886). 1897;23:163-164.
- Ameritech College of Healthcare. The origins of the AAMA. Accessed January 27, 2022. www.ameritech.edu/blog/medicalassisting-history/
- Dai M, Willard-Grace R, Knox M, et al. Team configurations, efficiency, and family physician burnout. J Am Board Fam Med. 2020;33:368-377.
- Harper PG, Van Riper K, Ramer T, et al. Team-based care: an expanded medical assistant role—enhanced rooming and visit assistance. J Interprof Care. 2018:1-7.
- Sheridan B, Chien AT, Peters AS, et al. Team-based primary care: the medical assistant perspective. Health Care Manage Rev. 2018;43:115-125.
- Tache S, Hill-Sakurai L. Medical assistants: the invisible “glue” of primary health care practices in the United States? J Health Organ Manag. 2010;24:288-305.
- STROBE checklist for cohort, case-control, and cross-sectional studies. Accessed January 27, 2022. www.strobe-statement.org/ fileadmin/Strobe/uploads/checklists/STROBE_checklist_v4_ combined.pdf
- Gray CP, Harrison MI, Hung D. Medical assistants as flow managers in primary care: challenges and recommendations. J Healthc Manag. 2016;61:181-191.
- Elder NC, Jacobson CJ, Bolon SK, et al. Patterns of relating between physicians and medical assistants in small family medicine offices. Ann Fam Med. 2014;12:150-157.
- Jager AJ, Tutty MA, Kao AC. Association between physician burnout and identification with medicine as a calling. Mayo Clinic Proc. 2017;92:415-422.
- Yoon JD, Daley BM, Curlin FA. The association between a sense of calling and physician well-being: a national study of primary care physicians and psychiatrists. Acad Psychiatry. 2017;41:167-173.
- Mohr DC, Young GJ, Meterko M, et al. Job satisfaction of primary care team members and quality of care. Am J Med Qual. 2011;26:18-25.
- US Bureau of Labor Statistics. Occupational employment and wage statistics. Accessed January 27, 2022. https://www.bls.gov/ oes/current/oes319092.htm
- Chapman SA, Marks A, Dower C. Positioning medical assistants for a greater role in the era of health reform. Acad Med. 2015;90:1347-1352.
- Mandel H. The role of occupational attributes in gender earnings inequality, 1970-2010. Soc Sci Res. 2016;55:122-138.
- US Bureau of Labor Statistics. Occupational outlook handbook: medical assistants. Accessed January 27, 2022. www.bls.gov/ooh/ healthcare/medical-assistants.htm
- Skillman SM, Dahal A, Frogner BK, et al. Frontline workers’ career pathways: a detailed look at Washington state’s medical assistant workforce. Med Care Res Rev. 2018:1077558718812950.
- Morse G, Salyers MP, Rollins AL, et al. Burnout in mental health services: a review of the problem and its remediation. Adm Policy Ment Health. 2012;39:341-352.
- Dubois CA, Bentein K, Ben Mansour JB, et al. Why some employees adopt or resist reorganization of work practices in health care: associations between perceived loss of resources, burnout, and attitudes to change. Int J Environ Res Pub Health. 2014;11: 187-201.
- Aronsson G, Theorell T, Grape T, et al. A systematic review including meta-analysis of work environment and burnout symptoms. BMC Public Health. 2017;17:264.
- O’Malley AS, Gourevitch R, Draper K, et al. Overcoming challenges to teamwork in patient-centered medical homes: a qualitative study. J Gen Intern Med. 2015;30:183-192.
ABSTRACT
Background: Medical assistant (MA) roles have expanded rapidly as primary care has evolved and MAs take on new patient care duties. Research that looks at the MA experience and factors that enhance or reduce efficiency among MAs is limited.
Methods: We surveyed all MAs working in 6 clinics run by a large academic family medicine department in Ann Arbor, Michigan. MAs deemed by peers as “most efficient” were selected for follow-up interviews. We evaluated personal strategies for efficiency, barriers to efficient care, impact of physician actions on efficiency, and satisfaction.
Results: A total of 75/86 MAs (87%) responded to at least some survey questions and 61/86 (71%) completed the full survey. We interviewed 18 MAs face to face. Most saw their role as essential to clinic functioning and viewed health care as a personal calling. MAs identified common strategies to improve efficiency and described the MA role to orchestrate the flow of the clinic day. Staff recognized differing priorities of patients, staff, and physicians and articulated frustrations with hierarchy and competing priorities as well as behaviors that impeded clinic efficiency. Respondents emphasized the importance of feeling valued by others on their team.
Conclusions: With the evolving demands made on MAs’ time, it is critical to understand how the most effective staff members manage their role and highlight the strategies they employ to provide efficient clinical care. Understanding factors that increase or decrease MA job satisfaction can help identify high-efficiency practices and promote a clinic culture that values and supports all staff.
As primary care continues to evolve into more team-based practice, the role of the medical assistant (MA) has rapidly transformed.1 Staff may assist with patient management, documentation in the electronic medical record, order entry, pre-visit planning, and fulfillment of quality metrics, particularly in a Primary Care Medical Home (PCMH).2 From 2012 through 2014, MA job postings per graduate increased from 1.3 to 2.3, suggesting twice as many job postings as graduates.3 As the demand for experienced MAs increases, the ability to recruit and retain high-performing staff members will be critical.
MAs are referenced in medical literature as early as the 1800s.4 The American Association of Medical Assistants was founded in 1956, which led to educational standardization and certifications.5 Despite the important role that MAs have long played in the proper functioning of a medical clinic—and the knowledge that team configurations impact a clinic’s efficiency and quality6,7—few investigations have sought out the MA’s perspective.8,9 Given the increasing clinical demands placed on all members of the primary care team (and the burnout that often results), it seems that MA insights into clinic efficiency could be valuable.
Continue to: Methods...
METHODS
This cross-sectional study was conducted from February to April 2019 at a large academic institution with 6 regional ambulatory care family medicine clinics, each one with 11,000 to 18,000 patient visits annually. Faculty work at all 6 clinics and residents at 2 of them. All MAs are hired, paid, and managed by a central administrative department rather than by the family medicine department. The family medicine clinics are currently PCMH certified, with a mix of fee-for-service and capitated reimbursement.
We developed and piloted a voluntary, anonymous 39-question (29 closed-ended and 10 brief open-ended) online Qualtrics survey, which we distributed via an email link to all the MAs in the department. The survey included clinic site, years as an MA, perceptions of the clinic environment, perception of teamwork at their site, identification of efficient practices, and feedback for physicians to improve efficiency and flow. Most questions were Likert-style with 5 choices ranging from “strongly agree” to “strongly disagree” or short answer. Age and gender were omitted to protect confidentiality, as most MAs in the department are female. Participants could opt to enter in a drawing for three $25 gift cards. The survey was reviewed by the University of Michigan Institutional Review Board and deemed exempt.
We asked MAs to nominate peers in their clinic who were “especially efficient and do their jobs well—people that others can learn from.” The staff members who were nominated most frequently by their peers were invited to share additional perspectives via a 10- to 30-minute semi-structured interview with the first author. Interviews covered highly efficient practices, barriers and facilitators to efficient care, and physician behaviors that impaired efficiency. We interviewed a minimum of 2 MAs per clinic and increased the number of interviews through snowball sampling, as needed, to reach data saturation (eg, the point at which we were no longer hearing new content). MAs were assured that all comments would be anonymized. There was no monetary incentive for the interviews. The interviewer had previously met only 3 of the 18 MAs interviewed.
Analysis. Summary statistics were calculated for quantitative data. To compare subgroups (such as individual clinics), a chi-square test was used. In cases when there were small cell sizes (< 5 subjects), we used the Fisher’s Exact test. Qualitative data was collected with real-time typewritten notes during the interviews to capture ideas and verbatim quotes when possible. We also included open-ended comments shared on the Qualtrics survey. Data were organized by theme using a deductive coding approach. Both authors reviewed and discussed observations, and coding was conducted by the first author. Reporting followed the STROBE Statement checklist for cross-sectional studies.10 Results were shared with MAs, supervisory staff, and physicians, which allowed for feedback and comments and served as “member-checking.” MAs reported that the data reflected their lived experiences.
Continue to: RESULTS...
RESULTS
Surveys were distributed to all 86 MAs working in family medicine clinics. A total of 75 (87%) responded to at least some questions (typically just demographics). We used those who completed the full survey (n = 61; 71%) for data analysis. Eighteen MAs participated in face-to-face interviews. Among respondents, 35 (47%) had worked at least 10 years as an MA and 21 (28%) had worked at least a decade in the family medicine department.
Perception of role
All respondents (n = 61; 100%) somewhat or strongly agreed that the MA role was “very important to keep the clinic functioning” and 58 (95%) reported that working in health care was “a calling” for them. Only 7 (11%) agreed that family medicine was an easier environment for MAs compared to a specialty clinic; 30 (49%) disagreed with this. Among respondents, 32 (53%) strongly or somewhat agreed that their work was very stressful and just half (n = 28; 46%) agreed there were adequate MA staff at their clinic.
Efficiency and competing priorities
MAs described important work values that increased their efficiency. These included clinic culture (good communication and strong teamwork), as well as individual strategies such as multitasking, limiting patient conversations, and doing tasks in a consistent way to improve accuracy. (See TABLE 1.) They identified ways physicians bolster or hurt efficiency and ways in which the relationship between the physician and the MA shapes the MA’s perception of their value in clinic.

Communication was emphasized as critical for efficient care, and MAs encouraged the use of preclinic huddles and communication as priorities. Seventy-five percent of MAs reported preclinic huddles to plan for patient care were helpful, but only half said huddles took place “always” or “most of the time.” Many described reviewing the schedule and completing tasks ahead of patient arrival as critical to efficiency.
Participants described the tension between their identified role of orchestrating clinic flow and responding to directives by others that disrupted the flow. Several MAs found it challenging when physicians agreed to see very late patients and felt frustrated when decisions that changed the flow were made by the physician or front desk staff without including the MA. MAs were also able to articulate how they managed competing priorities within the clinic, such as when a patient- or physician-driven need to extend appointments was at odds with maintaining a timely schedule. They were eager to share personal tips for time management and prided themselves on careful and accurate performance and skills they had learned on the job. MAs also described how efficiency could be adversely affected by the behaviors or attitudes of physicians. (See TABLE 2.)

Continue to: Clinic environment...
Clinic environment
Thirty-six MAs (59%) reported that other MAs on their team were willing to help them out in clinic “a great deal” or “a lot” of the time, by helping to room a patient, acting as a chaperone for an exam, or doing a point-of-care lab. This sense of support varied across clinics (38% to 91% reported good support), suggesting that cultures vary by site. Some MAs expressed frustration at peers they saw as resistant to helping, exemplified by this verbatim quote from an interview:
“Some don’t want to help out. They may sigh. It’s how they react—you just know.” (Clinic #1, MA #2 interview)
Efficient MAs stressed the need for situational awareness to recognize when co-workers need help:
“[Peers often] are not aware that another MA is drowning. There’s 5 people who could have done that, and here I am running around and nobody budged.” (Clinic #5, MA #2 interview)
A minority of staff used the open-ended survey sections to describe clinic hierarchy. When asked about “pet peeves,” a few advised that physicians should not “talk down” to staff and should try to teach rather than criticize. Another asked that physicians not “bark orders” or have “low gratitude” for staff work. MAs found micromanaging stressful—particularly when the physician prompted the MA about patient arrivals:
“[I don’t like] when providers will make a comment about a patient arriving when you already know this information. You then rush to put [the] patient in [a] room, then [the] provider ends up making [the] patient wait an extensive amount of time. I’m perfectly capable of knowing when a patient arrives.” (Clinic #6, survey)
MAs did not like physicians “talking bad about us” or blaming the MA if the clinic is running behind.
Despite these concerns, most MAs reported feeling appreciated for the job they do. Only 10 (16%) reported that the people they work with rarely say “thank you,” and 2 (3%) stated they were not well supported by the physicians in clinic. Most (n = 38; 62%) strongly agreed or agreed that they felt part of the team and that their opinions matter. In the interviews, many expanded on this idea:
“I really feel like I’m valued, so I want to do everything I can to make [my doctor’s] day go better. If you want a good clinic, the best thing a doc can do is make the MA feel valued.” (Clinic #1, MA #1 interview)
Continue to: DISCUSSION...
DISCUSSION
Participants described their role much as an orchestra director, with MAs as the key to clinic flow and timeliness.9 Respondents articulated multiple common strategies used to increase their own efficiency and clinic flow; these may be considered best practices and incorporated as part of the basic training. Most MAs reported their day-to-day jobs were stressful and believed this was underrecognized, so efficiency strategies are critical. With staff completing multiple time-sensitive tasks during clinic, consistent co-worker support is crucial and may impact efficiency.8 Proper training of managers to provide that support and ensure equitable workloads may be one strategy to ensure that staff members feel the workplace is fair and collegial.
Several comments reflected the power differential within medical offices. One study reported that MAs and physicians “occupy roles at opposite ends of social and occupational hierarchies.”11 It’s important for physicians to be cognizant of these patterns and clinic culture, as reducing a hierarchy-based environment will be appreciated by MAs.9 Prior research has found that MAs have higher perceptions of their own competence than do the physicians working with them.12 If there is a fundamental lack of trust between the 2 groups, this will undoubtedly hinder team-building. Attention to this issue is key to a more favorable work environment.
Almost all respondents reported health care was a “calling,” which mirrors physician research that suggests seeing work as a “calling” is protective against burnout.13,14 Open-ended comments indicated great pride in contributions, and most staff members felt appreciated by their teams. Many described the working relationships with physicians as critical to their satisfaction at work and indicated that strong partnerships motivated them to do their best to make the physician’s day easier. Staff job satisfaction is linked to improved quality of care, so treating staff well contributes to high-value care for patients.15 We also uncovered some MA “pet peeves” that hinder efficiency and could be shared with physicians to emphasize the importance of patience and civility.
One barrier to expansion of MA roles within PCMH practices is the limited pay and career ladder for MAs who adopt new job responsibilities that require advanced skills or training.1,2 The mean MA salary at our institution ($37,372) is higher than in our state overall ($33,760), which may impact satisfaction.16 In addition, 93% of MAs are women; thus, they may continue to struggle more with lower pay than do workers in male- dominated professions.17,18 Expected job growth from 2018-2028 is predicted at 23%, which may help to boost salaries. 19 Prior studies describe the lack of a job ladder or promotion opportunities as a challenge1,20; this was not formally assessed in our study.
MAs see work in family medicine as much harder than it is in other specialty clinics. Being trusted with more responsibility, greater autonomy,21-23 and expanded patient care roles can boost MA self-efficacy, which can reduce burnout for both physicians and MAs. 8,24 However, new responsibilities should include appropriate training, support, and compensation, and match staff interests.7
Study limitations. The study was limited to 6 clinics in 1 department at a large academic medical center. Interviewed participants were selected by convenience and snowball sampling and thus, the results cannot be generalized to the population of MAs as a whole. As the initial interview goal was simply to gather efficiency tips, the project was not designed to be formal qualitative research. However, the discussions built on open-ended comments from the written survey helped contextualize our quantitative findings about efficiency. Notes were documented in real time by a single interviewer with rapid typing skills, which allowed capture of quotes verbatim. Subsequent studies would benefit from more formal qualitative research methods (recording and transcribing interviews, multiple coders to reduce risk of bias, and more complex thematic analysis).
Our research demonstrated how MAs perceive their roles in primary care and the facilitators and barriers to high efficiency in the workplace, which begins to fill an important knowledge gap in primary care. Disseminating practices that staff members themselves have identified as effective, and being attentive to how staff members are treated, may increase individual efficiency while improving staff retention and satisfaction.
CORRESPONDENCE Katherine J. Gold, MD, MSW, MS, Department of Family Medicine and Department of Obstetrics and Gynecology, University of Michigan, 1018 Fuller Street, Ann Arbor, MI 48104-1213; [email protected]
ABSTRACT
Background: Medical assistant (MA) roles have expanded rapidly as primary care has evolved and MAs take on new patient care duties. Research that looks at the MA experience and factors that enhance or reduce efficiency among MAs is limited.
Methods: We surveyed all MAs working in 6 clinics run by a large academic family medicine department in Ann Arbor, Michigan. MAs deemed by peers as “most efficient” were selected for follow-up interviews. We evaluated personal strategies for efficiency, barriers to efficient care, impact of physician actions on efficiency, and satisfaction.
Results: A total of 75/86 MAs (87%) responded to at least some survey questions and 61/86 (71%) completed the full survey. We interviewed 18 MAs face to face. Most saw their role as essential to clinic functioning and viewed health care as a personal calling. MAs identified common strategies to improve efficiency and described the MA role to orchestrate the flow of the clinic day. Staff recognized differing priorities of patients, staff, and physicians and articulated frustrations with hierarchy and competing priorities as well as behaviors that impeded clinic efficiency. Respondents emphasized the importance of feeling valued by others on their team.
Conclusions: With the evolving demands made on MAs’ time, it is critical to understand how the most effective staff members manage their role and highlight the strategies they employ to provide efficient clinical care. Understanding factors that increase or decrease MA job satisfaction can help identify high-efficiency practices and promote a clinic culture that values and supports all staff.
As primary care continues to evolve into more team-based practice, the role of the medical assistant (MA) has rapidly transformed.1 Staff may assist with patient management, documentation in the electronic medical record, order entry, pre-visit planning, and fulfillment of quality metrics, particularly in a Primary Care Medical Home (PCMH).2 From 2012 through 2014, MA job postings per graduate increased from 1.3 to 2.3, suggesting twice as many job postings as graduates.3 As the demand for experienced MAs increases, the ability to recruit and retain high-performing staff members will be critical.
MAs are referenced in medical literature as early as the 1800s.4 The American Association of Medical Assistants was founded in 1956, which led to educational standardization and certifications.5 Despite the important role that MAs have long played in the proper functioning of a medical clinic—and the knowledge that team configurations impact a clinic’s efficiency and quality6,7—few investigations have sought out the MA’s perspective.8,9 Given the increasing clinical demands placed on all members of the primary care team (and the burnout that often results), it seems that MA insights into clinic efficiency could be valuable.
Continue to: Methods...
METHODS
This cross-sectional study was conducted from February to April 2019 at a large academic institution with 6 regional ambulatory care family medicine clinics, each one with 11,000 to 18,000 patient visits annually. Faculty work at all 6 clinics and residents at 2 of them. All MAs are hired, paid, and managed by a central administrative department rather than by the family medicine department. The family medicine clinics are currently PCMH certified, with a mix of fee-for-service and capitated reimbursement.
We developed and piloted a voluntary, anonymous 39-question (29 closed-ended and 10 brief open-ended) online Qualtrics survey, which we distributed via an email link to all the MAs in the department. The survey included clinic site, years as an MA, perceptions of the clinic environment, perception of teamwork at their site, identification of efficient practices, and feedback for physicians to improve efficiency and flow. Most questions were Likert-style with 5 choices ranging from “strongly agree” to “strongly disagree” or short answer. Age and gender were omitted to protect confidentiality, as most MAs in the department are female. Participants could opt to enter in a drawing for three $25 gift cards. The survey was reviewed by the University of Michigan Institutional Review Board and deemed exempt.
We asked MAs to nominate peers in their clinic who were “especially efficient and do their jobs well—people that others can learn from.” The staff members who were nominated most frequently by their peers were invited to share additional perspectives via a 10- to 30-minute semi-structured interview with the first author. Interviews covered highly efficient practices, barriers and facilitators to efficient care, and physician behaviors that impaired efficiency. We interviewed a minimum of 2 MAs per clinic and increased the number of interviews through snowball sampling, as needed, to reach data saturation (eg, the point at which we were no longer hearing new content). MAs were assured that all comments would be anonymized. There was no monetary incentive for the interviews. The interviewer had previously met only 3 of the 18 MAs interviewed.
Analysis. Summary statistics were calculated for quantitative data. To compare subgroups (such as individual clinics), a chi-square test was used. In cases when there were small cell sizes (< 5 subjects), we used the Fisher’s Exact test. Qualitative data was collected with real-time typewritten notes during the interviews to capture ideas and verbatim quotes when possible. We also included open-ended comments shared on the Qualtrics survey. Data were organized by theme using a deductive coding approach. Both authors reviewed and discussed observations, and coding was conducted by the first author. Reporting followed the STROBE Statement checklist for cross-sectional studies.10 Results were shared with MAs, supervisory staff, and physicians, which allowed for feedback and comments and served as “member-checking.” MAs reported that the data reflected their lived experiences.
Continue to: RESULTS...
RESULTS
Surveys were distributed to all 86 MAs working in family medicine clinics. A total of 75 (87%) responded to at least some questions (typically just demographics). We used those who completed the full survey (n = 61; 71%) for data analysis. Eighteen MAs participated in face-to-face interviews. Among respondents, 35 (47%) had worked at least 10 years as an MA and 21 (28%) had worked at least a decade in the family medicine department.
Perception of role
All respondents (n = 61; 100%) somewhat or strongly agreed that the MA role was “very important to keep the clinic functioning” and 58 (95%) reported that working in health care was “a calling” for them. Only 7 (11%) agreed that family medicine was an easier environment for MAs compared to a specialty clinic; 30 (49%) disagreed with this. Among respondents, 32 (53%) strongly or somewhat agreed that their work was very stressful and just half (n = 28; 46%) agreed there were adequate MA staff at their clinic.
Efficiency and competing priorities
MAs described important work values that increased their efficiency. These included clinic culture (good communication and strong teamwork), as well as individual strategies such as multitasking, limiting patient conversations, and doing tasks in a consistent way to improve accuracy. (See TABLE 1.) They identified ways physicians bolster or hurt efficiency and ways in which the relationship between the physician and the MA shapes the MA’s perception of their value in clinic.

Communication was emphasized as critical for efficient care, and MAs encouraged the use of preclinic huddles and communication as priorities. Seventy-five percent of MAs reported preclinic huddles to plan for patient care were helpful, but only half said huddles took place “always” or “most of the time.” Many described reviewing the schedule and completing tasks ahead of patient arrival as critical to efficiency.
Participants described the tension between their identified role of orchestrating clinic flow and responding to directives by others that disrupted the flow. Several MAs found it challenging when physicians agreed to see very late patients and felt frustrated when decisions that changed the flow were made by the physician or front desk staff without including the MA. MAs were also able to articulate how they managed competing priorities within the clinic, such as when a patient- or physician-driven need to extend appointments was at odds with maintaining a timely schedule. They were eager to share personal tips for time management and prided themselves on careful and accurate performance and skills they had learned on the job. MAs also described how efficiency could be adversely affected by the behaviors or attitudes of physicians. (See TABLE 2.)

Continue to: Clinic environment...
Clinic environment
Thirty-six MAs (59%) reported that other MAs on their team were willing to help them out in clinic “a great deal” or “a lot” of the time, by helping to room a patient, acting as a chaperone for an exam, or doing a point-of-care lab. This sense of support varied across clinics (38% to 91% reported good support), suggesting that cultures vary by site. Some MAs expressed frustration at peers they saw as resistant to helping, exemplified by this verbatim quote from an interview:
“Some don’t want to help out. They may sigh. It’s how they react—you just know.” (Clinic #1, MA #2 interview)
Efficient MAs stressed the need for situational awareness to recognize when co-workers need help:
“[Peers often] are not aware that another MA is drowning. There’s 5 people who could have done that, and here I am running around and nobody budged.” (Clinic #5, MA #2 interview)
A minority of staff used the open-ended survey sections to describe clinic hierarchy. When asked about “pet peeves,” a few advised that physicians should not “talk down” to staff and should try to teach rather than criticize. Another asked that physicians not “bark orders” or have “low gratitude” for staff work. MAs found micromanaging stressful—particularly when the physician prompted the MA about patient arrivals:
“[I don’t like] when providers will make a comment about a patient arriving when you already know this information. You then rush to put [the] patient in [a] room, then [the] provider ends up making [the] patient wait an extensive amount of time. I’m perfectly capable of knowing when a patient arrives.” (Clinic #6, survey)
MAs did not like physicians “talking bad about us” or blaming the MA if the clinic is running behind.
Despite these concerns, most MAs reported feeling appreciated for the job they do. Only 10 (16%) reported that the people they work with rarely say “thank you,” and 2 (3%) stated they were not well supported by the physicians in clinic. Most (n = 38; 62%) strongly agreed or agreed that they felt part of the team and that their opinions matter. In the interviews, many expanded on this idea:
“I really feel like I’m valued, so I want to do everything I can to make [my doctor’s] day go better. If you want a good clinic, the best thing a doc can do is make the MA feel valued.” (Clinic #1, MA #1 interview)
Continue to: DISCUSSION...
DISCUSSION
Participants described their role much as an orchestra director, with MAs as the key to clinic flow and timeliness.9 Respondents articulated multiple common strategies used to increase their own efficiency and clinic flow; these may be considered best practices and incorporated as part of the basic training. Most MAs reported their day-to-day jobs were stressful and believed this was underrecognized, so efficiency strategies are critical. With staff completing multiple time-sensitive tasks during clinic, consistent co-worker support is crucial and may impact efficiency.8 Proper training of managers to provide that support and ensure equitable workloads may be one strategy to ensure that staff members feel the workplace is fair and collegial.
Several comments reflected the power differential within medical offices. One study reported that MAs and physicians “occupy roles at opposite ends of social and occupational hierarchies.”11 It’s important for physicians to be cognizant of these patterns and clinic culture, as reducing a hierarchy-based environment will be appreciated by MAs.9 Prior research has found that MAs have higher perceptions of their own competence than do the physicians working with them.12 If there is a fundamental lack of trust between the 2 groups, this will undoubtedly hinder team-building. Attention to this issue is key to a more favorable work environment.
Almost all respondents reported health care was a “calling,” which mirrors physician research that suggests seeing work as a “calling” is protective against burnout.13,14 Open-ended comments indicated great pride in contributions, and most staff members felt appreciated by their teams. Many described the working relationships with physicians as critical to their satisfaction at work and indicated that strong partnerships motivated them to do their best to make the physician’s day easier. Staff job satisfaction is linked to improved quality of care, so treating staff well contributes to high-value care for patients.15 We also uncovered some MA “pet peeves” that hinder efficiency and could be shared with physicians to emphasize the importance of patience and civility.
One barrier to expansion of MA roles within PCMH practices is the limited pay and career ladder for MAs who adopt new job responsibilities that require advanced skills or training.1,2 The mean MA salary at our institution ($37,372) is higher than in our state overall ($33,760), which may impact satisfaction.16 In addition, 93% of MAs are women; thus, they may continue to struggle more with lower pay than do workers in male- dominated professions.17,18 Expected job growth from 2018-2028 is predicted at 23%, which may help to boost salaries. 19 Prior studies describe the lack of a job ladder or promotion opportunities as a challenge1,20; this was not formally assessed in our study.
MAs see work in family medicine as much harder than it is in other specialty clinics. Being trusted with more responsibility, greater autonomy,21-23 and expanded patient care roles can boost MA self-efficacy, which can reduce burnout for both physicians and MAs. 8,24 However, new responsibilities should include appropriate training, support, and compensation, and match staff interests.7
Study limitations. The study was limited to 6 clinics in 1 department at a large academic medical center. Interviewed participants were selected by convenience and snowball sampling and thus, the results cannot be generalized to the population of MAs as a whole. As the initial interview goal was simply to gather efficiency tips, the project was not designed to be formal qualitative research. However, the discussions built on open-ended comments from the written survey helped contextualize our quantitative findings about efficiency. Notes were documented in real time by a single interviewer with rapid typing skills, which allowed capture of quotes verbatim. Subsequent studies would benefit from more formal qualitative research methods (recording and transcribing interviews, multiple coders to reduce risk of bias, and more complex thematic analysis).
Our research demonstrated how MAs perceive their roles in primary care and the facilitators and barriers to high efficiency in the workplace, which begins to fill an important knowledge gap in primary care. Disseminating practices that staff members themselves have identified as effective, and being attentive to how staff members are treated, may increase individual efficiency while improving staff retention and satisfaction.
CORRESPONDENCE Katherine J. Gold, MD, MSW, MS, Department of Family Medicine and Department of Obstetrics and Gynecology, University of Michigan, 1018 Fuller Street, Ann Arbor, MI 48104-1213; [email protected]
- Chapman SA, Blash LK. New roles for medical assistants in innovative primary care practices. Health Serv Res. 2017;52(suppl 1):383-406.
- Ferrante JM, Shaw EK, Bayly JE, et al. Barriers and facilitators to expanding roles of medical assistants in patient-centered medical homes (PCMHs). J Am Board Fam Med. 2018;31:226-235.
- Atkins B. The outlook for medical assisting in 2016 and beyond. Accessed January 27, 2022. www.medicalassistantdegrees.net/ articles/medical-assisting-trends/
- Unqualified medical “assistants.” Hospital (Lond 1886). 1897;23:163-164.
- Ameritech College of Healthcare. The origins of the AAMA. Accessed January 27, 2022. www.ameritech.edu/blog/medicalassisting-history/
- Dai M, Willard-Grace R, Knox M, et al. Team configurations, efficiency, and family physician burnout. J Am Board Fam Med. 2020;33:368-377.
- Harper PG, Van Riper K, Ramer T, et al. Team-based care: an expanded medical assistant role—enhanced rooming and visit assistance. J Interprof Care. 2018:1-7.
- Sheridan B, Chien AT, Peters AS, et al. Team-based primary care: the medical assistant perspective. Health Care Manage Rev. 2018;43:115-125.
- Tache S, Hill-Sakurai L. Medical assistants: the invisible “glue” of primary health care practices in the United States? J Health Organ Manag. 2010;24:288-305.
- STROBE checklist for cohort, case-control, and cross-sectional studies. Accessed January 27, 2022. www.strobe-statement.org/ fileadmin/Strobe/uploads/checklists/STROBE_checklist_v4_ combined.pdf
- Gray CP, Harrison MI, Hung D. Medical assistants as flow managers in primary care: challenges and recommendations. J Healthc Manag. 2016;61:181-191.
- Elder NC, Jacobson CJ, Bolon SK, et al. Patterns of relating between physicians and medical assistants in small family medicine offices. Ann Fam Med. 2014;12:150-157.
- Jager AJ, Tutty MA, Kao AC. Association between physician burnout and identification with medicine as a calling. Mayo Clinic Proc. 2017;92:415-422.
- Yoon JD, Daley BM, Curlin FA. The association between a sense of calling and physician well-being: a national study of primary care physicians and psychiatrists. Acad Psychiatry. 2017;41:167-173.
- Mohr DC, Young GJ, Meterko M, et al. Job satisfaction of primary care team members and quality of care. Am J Med Qual. 2011;26:18-25.
- US Bureau of Labor Statistics. Occupational employment and wage statistics. Accessed January 27, 2022. https://www.bls.gov/ oes/current/oes319092.htm
- Chapman SA, Marks A, Dower C. Positioning medical assistants for a greater role in the era of health reform. Acad Med. 2015;90:1347-1352.
- Mandel H. The role of occupational attributes in gender earnings inequality, 1970-2010. Soc Sci Res. 2016;55:122-138.
- US Bureau of Labor Statistics. Occupational outlook handbook: medical assistants. Accessed January 27, 2022. www.bls.gov/ooh/ healthcare/medical-assistants.htm
- Skillman SM, Dahal A, Frogner BK, et al. Frontline workers’ career pathways: a detailed look at Washington state’s medical assistant workforce. Med Care Res Rev. 2018:1077558718812950.
- Morse G, Salyers MP, Rollins AL, et al. Burnout in mental health services: a review of the problem and its remediation. Adm Policy Ment Health. 2012;39:341-352.
- Dubois CA, Bentein K, Ben Mansour JB, et al. Why some employees adopt or resist reorganization of work practices in health care: associations between perceived loss of resources, burnout, and attitudes to change. Int J Environ Res Pub Health. 2014;11: 187-201.
- Aronsson G, Theorell T, Grape T, et al. A systematic review including meta-analysis of work environment and burnout symptoms. BMC Public Health. 2017;17:264.
- O’Malley AS, Gourevitch R, Draper K, et al. Overcoming challenges to teamwork in patient-centered medical homes: a qualitative study. J Gen Intern Med. 2015;30:183-192.
- Chapman SA, Blash LK. New roles for medical assistants in innovative primary care practices. Health Serv Res. 2017;52(suppl 1):383-406.
- Ferrante JM, Shaw EK, Bayly JE, et al. Barriers and facilitators to expanding roles of medical assistants in patient-centered medical homes (PCMHs). J Am Board Fam Med. 2018;31:226-235.
- Atkins B. The outlook for medical assisting in 2016 and beyond. Accessed January 27, 2022. www.medicalassistantdegrees.net/ articles/medical-assisting-trends/
- Unqualified medical “assistants.” Hospital (Lond 1886). 1897;23:163-164.
- Ameritech College of Healthcare. The origins of the AAMA. Accessed January 27, 2022. www.ameritech.edu/blog/medicalassisting-history/
- Dai M, Willard-Grace R, Knox M, et al. Team configurations, efficiency, and family physician burnout. J Am Board Fam Med. 2020;33:368-377.
- Harper PG, Van Riper K, Ramer T, et al. Team-based care: an expanded medical assistant role—enhanced rooming and visit assistance. J Interprof Care. 2018:1-7.
- Sheridan B, Chien AT, Peters AS, et al. Team-based primary care: the medical assistant perspective. Health Care Manage Rev. 2018;43:115-125.
- Tache S, Hill-Sakurai L. Medical assistants: the invisible “glue” of primary health care practices in the United States? J Health Organ Manag. 2010;24:288-305.
- STROBE checklist for cohort, case-control, and cross-sectional studies. Accessed January 27, 2022. www.strobe-statement.org/ fileadmin/Strobe/uploads/checklists/STROBE_checklist_v4_ combined.pdf
- Gray CP, Harrison MI, Hung D. Medical assistants as flow managers in primary care: challenges and recommendations. J Healthc Manag. 2016;61:181-191.
- Elder NC, Jacobson CJ, Bolon SK, et al. Patterns of relating between physicians and medical assistants in small family medicine offices. Ann Fam Med. 2014;12:150-157.
- Jager AJ, Tutty MA, Kao AC. Association between physician burnout and identification with medicine as a calling. Mayo Clinic Proc. 2017;92:415-422.
- Yoon JD, Daley BM, Curlin FA. The association between a sense of calling and physician well-being: a national study of primary care physicians and psychiatrists. Acad Psychiatry. 2017;41:167-173.
- Mohr DC, Young GJ, Meterko M, et al. Job satisfaction of primary care team members and quality of care. Am J Med Qual. 2011;26:18-25.
- US Bureau of Labor Statistics. Occupational employment and wage statistics. Accessed January 27, 2022. https://www.bls.gov/ oes/current/oes319092.htm
- Chapman SA, Marks A, Dower C. Positioning medical assistants for a greater role in the era of health reform. Acad Med. 2015;90:1347-1352.
- Mandel H. The role of occupational attributes in gender earnings inequality, 1970-2010. Soc Sci Res. 2016;55:122-138.
- US Bureau of Labor Statistics. Occupational outlook handbook: medical assistants. Accessed January 27, 2022. www.bls.gov/ooh/ healthcare/medical-assistants.htm
- Skillman SM, Dahal A, Frogner BK, et al. Frontline workers’ career pathways: a detailed look at Washington state’s medical assistant workforce. Med Care Res Rev. 2018:1077558718812950.
- Morse G, Salyers MP, Rollins AL, et al. Burnout in mental health services: a review of the problem and its remediation. Adm Policy Ment Health. 2012;39:341-352.
- Dubois CA, Bentein K, Ben Mansour JB, et al. Why some employees adopt or resist reorganization of work practices in health care: associations between perceived loss of resources, burnout, and attitudes to change. Int J Environ Res Pub Health. 2014;11: 187-201.
- Aronsson G, Theorell T, Grape T, et al. A systematic review including meta-analysis of work environment and burnout symptoms. BMC Public Health. 2017;17:264.
- O’Malley AS, Gourevitch R, Draper K, et al. Overcoming challenges to teamwork in patient-centered medical homes: a qualitative study. J Gen Intern Med. 2015;30:183-192.
2022 Update on menopause
This year’s Menopause Update focuses on 2 menopause-related issues relevant to ObGyns and our menopausal patients:
- choosing the safest regimens, particularly with respect to risk of breast cancer, when prescribing hormone therapy (HT) to menopausal women
- reviewing the risks and benefits of premenopausal bilateral salpingo-oophorectomy and the pros and cons of replacement HT in surgically menopausal patients.
We hope that you find this updated information useful as you care for menopausal women.
Revisiting menopausal HT and the risk of breast cancer: What we know now
Abenhaim HA, Suissa S, Azoulay L, et al. Menopausal hormone therapy formulation and breast cancer risk. Obstet Gynecol. 2022;139:1103-1110. doi: 10.1097/AOG.0000000000004723.
Reevaluation of the Women’s Health Initiative randomized controlled trials (WHI RCTs), long-term (median follow-up more than 20 years) cumulative follow-up data, and results from additional studies have suggested that estrogen therapy (ET) alone in menopausal women with prior hysterectomy does not increase the risk of breast cancer. By contrast, estrogen with progestin (synthetic progestogens that include medroxyprogesterone acetate [MPA] and norethindrone acetate) slightly increases the risk of breast cancer. In the past 10 years, several publications have shed light on whether the type of progestogen affects the risk of breast cancer and can help provide evidence-based information to guide clinicians.
Breast cancer risk with combined HT and synthetic progestin
In the first part of the WHI RCT, women were randomly assigned to receive either conjugated equine estrogen (CEE) plus synthetic progestin (MPA) or a placebo. Combined estrogen-progestin therapy (EPT) was associated with a modestly elevated risk of breast cancer.1 In the second part of the WHI trial, CEE only (estrogen alone, ET) was compared with placebo among women with prior hysterectomy, with no effect found on breast cancer incidence.2
Most older observational studies published in 2003 to 2005 found that neither CEE nor estradiol appeared to increase the risk of breast cancer when used alone.3-5 However, estrogen use in combination with synthetic progestins (MPA, norethindrone, levonorgestrel, and norgestrel) has been associated with an increased risk of breast cancer,4,6 while the elevated risk of breast cancer with micronized progesterone has been less substantial.7,8
Continue to: Newer data suggest the type of progestogen used affects risk...
Newer data suggest the type of progestogen used affects risk
In a report published in the June 2022 issue of Obstetrics and Gynecology, Abenhaim and colleagues used a nested population-based case-control study of administrative data available in the UK Clinical Practice Research Datalink and provider prescriptions to evaluate the additive effect on the risk of breast cancer of the type of progestogen (micronized progesterone or synthetic progestins) when combined with estradiol for the treatment of menopausal symptoms.9 A cohort of 561,379 women was included in the case-control study (10:1 ratio), 43,183 in the case group (patients diagnosed with invasive breast cancer), and 431,830 in the matched control group.
Overall, in the stratified analysis, a small but significant increase in the risk of breast cancer was found in ever users of menopausal HT (odds ratio [OR], 1.12; 95% confidence interval [CI], 1.09–1.15). Neither estradiol (OR, 1.04; 95% CI, 1.00–1.09) nor CEE (OR, 1.01; 95% CI, 0.96–1.06) was associated with an elevated risk of being diagnosed with invasive breast cancer. Of note, no elevated risk of breast cancer was associated with combination estrogen-progesterone therapy. However, the risk of breast cancer for women who had used synthetic progestins, mostly MPA, was significantly elevated (OR, 1.28; 95% CI, 1.22-1.35). Notably, this modestly elevated odds ratio with the use of estrogen-progestin HT is almost identical to that observed with CEE/ MPA in the WHI.1 Similar findings were found in women aged 50 to 60 years.
The adjusted analyses from the large WHI RCTs provide additional support: the synthetic progestin MPA combined with CEE showed a higher risk of breast cancer than CEE alone in women with prior hysterectomy.10
In the long-term follow-up of the WHI RCTs, after a median of 20.3 years postrandomization, prior randomization to CEE alone for postmenopausal women with prior hysterectomy was associated with a significantly lowered risk of breast cancer incidence and mortality.11 By contrast, prior randomization to CEE plus MPA (EPT) for women with an intact uterus was associated with a small but significantly increased incidence of breast cancer but no significant difference in breast cancer mortality.
In the French E3N EPIC population-based prospective cohort study, Fournier and colleagues4,5 found that women who received estrogen combined with synthetic progestins (mostly MPA) had a higher risk of breast cancer, with an age-adjusted relative risk of 1.4 (95% CI, 1.2–1.7), a finding not seen in women who received estrogen combined with micronized progesterone, similar to findings by Cordina-Duverger and colleagues and Simin and colleagues.12,13 In the E3N study, only 948 women were identified with breast cancer; 268 of these had used synthetic progestins.4,5
Both the Abenhaim cohort9 and the longterm outcomes of WHI RCT trial data11 found a significant contributing effect of MPA (synthetic progestin) in the risk of breast cancer. Progestogens are not thought to exert a class effect. Although it is clear that progestogens (progesterone or progestins) prevent estrogeninduced endometrial neoplasia when dosed adequately, different types of progestogens have a differential risk of breast epithelium proliferation and carcinogenic potential.14 A systematic review by Stute and colleagues found that micronized progesterone did not appear to alter mammographic breast density assessments or breast biopsy results.15
Progesterone capsules, available in generic form in 100-mg and 200-mg doses, are formulated with peanut oil, and they should be taken at bedtime as progesterone can induce drowsiness.
When combined with standard-dose estrogen, including oral estradiol 1.0 mg, transdermal estradiol 0.05 mg, or oral conjugated equine estrogen 0.625 mg, the appropriate dose of progesterone is 100 mg if used continuously or 200 mg if used as cyclic therapy. With higher doses of estrogen, progesterone 200 mg should be taken continuously.
An oral formulation that combines estradiol 1 mg and progesterone 100 mg does not contain peanut oil and, accordingly, can be used safely by those with peanut allergies. This combination product is marketed under the name Bijuva (TherapeuticsMD, Boca Raton, Florida).1
Reference
1. Lobo RA, Archer DF, Kagan R, et al. A 17β-estradiol-progesterone oral capsule for vasomotor symptoms in postmenopausal women: a randomized controlled trial. Obstet Gynecol. 2018;132:161-170. doi: 10.1097/AOG.0000000000002645. Erratum in: Obstet Gynecol. 2018;132:786.
Race considerations
The study by Abenhaim and colleagues was unable to address the issues of race or ethnicity.9 However, in the racially diverse WHI trial of women with prior hysterectomy, estrogen-alone use significantly reduced breast cancer incidence in all participants.10,16 Post hoc analysis of the 1,616 Black women with prior hysterectomy in the WHI RCT showed a significantly decreased breast cancer incidence with use of estrogen alone (hazard ratio [HR], 0.47; 95% CI, 0.26–0.82).1 When race was evaluated in the long-term cumulative follow-up of the WHI trial, estrogen-alone use significantly reduced breast cancer incidence in Black women, with no adverse effect on coronary heart disease, global index, or all-cause mortality, and with fewer cases of venous thromboembolism.17 The global index findings were favorable for Black women in their 50s and those with vasomotor symptoms.
Continue to: Impact of HT in women with an elevated risk of breast cancer...
Impact of HT in women with an elevated risk of breast cancer
Abenhaim and colleagues could not evaluate the effect of HT in women with a baseline elevated risk of breast cancer.9 For these women, HT may be recommended after premature surgical menopause due to increased risks for coronary heart disease, osteoporosis, genitourinary syndrome of menopause, and cognitive changes when estrogen is not taken postsurgery through to at least the average age of menopause, considered age 51.18,19
Marchetti and colleagues reviewed 3 clinical trials that assessed breast cancer events in 1,100 BRCA gene mutation carriers with intact breasts who underwent risk-reducing salpingo-oophorectomy (RRSO) who used or did not use HT.20 For BRCA1 and BRCA2 mutation carriers who received HT after RRSO, no elevated risk of breast cancer risk was seen (HR, 0.98; 95% CI, 0.63–1.52). There was a nonsignificant reduction in breast cancer risk for the estrogen-alone users compared with EPT HT (OR, 0.53; 95% CI, 0.25–1.15). Thus, short-term use of HT, estrogen alone or EPT, does not appear to elevate the risk of breast cancer after RRSO in these high-risk women.
Individualizing HT for menopausal symptoms
The data presented provide reassuring evidence that longer-term use of ET does not appear to increase breast cancer risk, regardless of the type of estrogen (CEE or estradiol).4,5,9,11 For women with a uterus, micronized progesterone has less (if any) effect on breast cancer risk. By contrast, the use of synthetic progestins (such as MPA), when combined with estrogen, has been associated with a small but real increased breast cancer risk.
The most evident benefit of HT is in treating vasomotor symptoms and preventing bone loss for those at elevated risk in healthy women without contraindications who initiate systemic HT when younger than age 60 or within 10 years of menopause onset. Benefit and risk ratio depends on age and time from menopause onset when HT is initiated. Hormone therapy safety varies depending on type, dose, duration, route of administration, timing of initiation, and whether, and type, of progestogen is used. Transdermal estradiol, particularly when dosed at 0.05 mg or less, has been shown to have less thrombotic and stroke risk than oral estrogen.21
Individualizing treatment includes using the best available evidence to maximize benefits and minimize risks, with periodic reevaluation of benefits and risks of continuing or discontinuing HT or changing to lower doses. ObGyns who follow best practices in prescribing systemic HT can now help menopausal patients with bothersome symptoms take advantage of systemic HT’s benefits while providing reassurance regarding menopausal HT’s safety.18 Transdermal therapy is a safer option for women at elevated baseline risk of venous thrombosis (for example, obese women) and older patients. Likewise, given its safety with respect to risk of breast cancer, the use of micronized progesterone over synthetic progestins should be considered when prescribing EPT to women with an intact uterus.
We can replace fear of HT with evidence-based discussions.22 For women with prior hysterectomy who have menopausal symptoms that impact their quality of life, ET at menopause does not appear to increase the risk of breast cancer. For women with an intact uterus who are considering use of estrogen and progestogen, extended-duration use of combination HT with synthetic progestins slightly elevates the risk of breast cancer, while the use of micronized progesterone does not appear to elevate breast cancer risk. Likewise, transdermal estrogen does not appear to elevate thrombosis risk.
Continue to: Benefits of avoiding BSO in women at average risk of ovarian cancer...
Benefits of avoiding BSO in women at average risk of ovarian cancer
Erickson Z, Rocca WA, Smith CY, et al. Time trends in unilateral and bilateral oophorectomy in a geographically defined American population. Obstet Gynecol. 2022;139:724-734. doi: 10.1097/ AOG.0000000000004728.
In 2005, gynecologist William Parker, MD, and colleagues used modeling methodology to assess the long-term risks and benefits of performing bilateral salpingo-oophorectomy (BSO) at the time of hysterectomy for benign disease in women at average risk for ovarian cancer.23 They concluded that practicing ovarian conservation until age 65 increased women’s long-term survival. Among their findings were that women with BSO before age 55 had an 8.6% excess overall mortality by age 80, while those with oophorectomy before age 59 had 3.9% excess mortality. They noted a sustained, but decreasing, mortality benefit until the age of 75 and stated that at no age did their model suggest higher mortality in women who chose ovarian conservation. Parker and colleagues concluded that ovarian conservation until at least age 65 benefited long-term survival for women at average risk for ovarian cancer when undergoing hysterectomy for benign disease.23
Certain risks decreased, others increased
A second report in 2009 by Parker and colleagues from the large prospective Nurses’ Health Study found that, while BSO at the time of hysterectomy for benign disease was associated with a decreased risk of breast and ovarian cancer, BSO was associated with an increased risk of all-cause mortality, fatal and nonfatal coronary heart disease, and lung cancer.24 Similar to the findings of the 2005 report, the authors noted that in no analysis or age group was BSO associated with increased survival. They also noted that compared with those who underwent BSO before age 50 and used ET, women with no history of ET use had an approximately 2-fold elevated risk of new onset coronary heart disease (HR, 1.98; 95% CI, 1.18–3.32).24
In 2007, Walter Rocca, MD, a Mayo Clinic neurologist with a particular interest in the epidemiology of dementia, and colleagues at the Mayo Clinic published results of a study that assessed a cohort of women who had undergone unilateral oophorectomy or BSO prior to the onset of menopause.25 The risk of cognitive impairment or dementia was higher in these women compared with women who had intact ovaries (HR, 1.46; 95% CI, 1.13-1.90). Of note, this elevated risk was confined to those who underwent oophorectomy before 49 years of age and were not prescribed estrogen until age 50 or older.25
In a subsequent publication, Rocca and colleagues pointed out that BSO prior to menopause not only is associated with higher rates of all-cause mortality and cognitive impairment but also with coronary heart disease, parkinsonism, osteoporosis, and other chronic conditions associated with aging, including metabolic, mental health, and arthritic disorders.26
Oophorectomy trends tracked
Given these and other reports27 that highlighted the health risks of premenopausal BSO in women at average risk for ovarian cancer, Rocca and colleagues recently assessed trends in the occurrence of unilateral oophorectomy or BSO versus ovarian conservation among all women residing in the Minnesota county (Olmsted) in which Mayo Clinic is located, and who underwent gynecologic surgery between 1950 and 2018.28
The investigators limited their analysis to women who had undergone unilateral oophorectomy or BSO between ages 18 and 49 years (these women are assumed to have been premenopausal). The authors considered as indications for oophorectomy primary or metastatic ovarian cancer, risk-reducing BSO for women at elevated risk for ovarian cancer (for example, strong family history or known BRCA gene mutation), adnexal mass, endometriosis, torsion, and other benign gynecologic conditions that included pelvic pain, abscess, oophoritis, or ectopic pregnancy. When more than 1 indication for ovarian surgery was present, the authors used the most clinically important indication. Unilateral oophorectomy or BSO was considered not indicated if the surgery was performed during another primary procedure (usually hysterectomy) without indication, or if the surgeon referred to the ovarian surgery as elective.
Results. Among 5,154 women who had oophorectomies between 1950 and 2018, the proportion of these women who underwent unilateral oophorectomy and BSO was 40.6% and 59.4%, respectively.
For most years between 1950 and 1979, the incidence of unilateral oophorectomy was higher than BSO. However, from 1980 to 2004, the incidence of BSO increased more than 2-fold while the incidence of unilateral surgery declined. After 2005, however, both types of ovarian surgery declined. During the years 2005–2018, a marked decline in BSO occurred, with the reduced incidence in premenopausal BSO most notable among women undergoing hysterectomy or those without an indication for oophorectomy.
Historically, ObGyns were taught that the benefits of removing normal ovaries (to prevent ovarian cancer) in average-risk women at the time of hysterectomy outweighed the risks. We agree with the authors’ speculation that beginning with Parker’s 2005 publication,23 ObGyns have become more conservative in performing unindicated BSO in women at average risk for ovarian cancer, now recognizing that the harms of this procedure often outweigh any benefits.28
Women with BRCA1 and BRCA2 gene mutations are at elevated risk for ovarian, tubal, and breast malignancies. In this population, risk-reducing BSO dramatically lowers future risk of ovarian and tubal cancer.
Data addressing the effect of RRSO in BRCA1 and BRCA2 gene mutation carriers continue to be evaluated, with differences between the 2 mutations, but they suggest that the surgery reduces not only ovarian cancer and tubal cancer but also possibly breast cancer.29
Many of our patients are fearful regarding the possibility that they could be diagnosed with breast or ovarian cancer, and in their minds, fears regarding these 2 potentially deadly diseases outweigh concerns about more common causes of death in women, including cardiovascular disease. Accordingly, counseling women at average risk for ovarian cancer who are planning hysterectomy for benign indications can be challenging. In recent years, ObGyns have increasingly been performing opportunistic bilateral salpingectomy (OS) in women at average risk of ovarian cancer at the time of hysterectomy for benign disease. It is important to note that the studies we refer to in this Update addressed BSO, not OS. We hope that the findings we have reviewed here assist clinicians in helping women to understand the risks and benefits associated with premenopausal BSO and the need to discuss the pros and cons of HT for these women before surgery.
Continue to: Trends show decline in ET use in surgically menopausal women...
Trends show decline in ET use in surgically menopausal women
Suzuki Y, Huang Y, Melamed A, et al. Use of estrogen therapy after surgical menopause in women who are premenopausal. Obstet Gynecol. 2022;139:756-763. doi: 10.1097/AOG.0000000000004762.
In addition to highlighting the risks associated with premenopausal BSO in women at average risk for ovarian cancer, the reports referred to above also underscore that the use of replacement menopausal HT in premenopausal women who undergo BSO prevents morbidity and mortality that otherwise accompanies surgical menopause. In addition, the North American Menopause Society (NAMS) recommends replacement menopausal HT in the setting of induced early menopause when no contraindications are present.18
To assess the prevalence of HT use in surgically menopausal women, investigators at Columbia University College of Physicians and Surgeons used a national database that captures health insurance claims for some 280 million US patients, focusing on women aged 18 to 50 years who underwent BSO from 2008 to 2019.30 The great majority of women in this database have private insurance. Although the authors used the term estrogen therapy in their article, this term refers to systemic estrogen alone or with progestogen, as well as vaginal ET (personal communication with Jason Wright, MD, a coauthor of the study, May 19, 2022). In this Update section, we use the term HT to include use of any systemic HT or vaginal estrogen.
Prevalence of HT use changed over time period and patient age range
Among almost 61,980 evaluable women who had undergone BSO (median age, 45 years; 75.1% with concomitant hysterectomy; median follow-up time, 27 months), with no history of gynecologic or breast cancer, HT was used within 3 years of BSO by 64.5%. The highest percentage of women in this cohort who used HT peaked in 2008 (69.5%), declining to 58.2% by 2016. The median duration of HT use was 5.3 months. The prevalence of HT use 3 years after BSO declined with age, from 79.1% in women aged 18–29 to 60.0% in women aged 45–50.30
This report, published in the June 2022 issue of Obstetrics and Gynecology, makes several sobering observations: Many surgically menopausal women aged 50 years and younger are not prescribed HT, the proportion of such women receiving a prescription for HT is declining over time, and the duration of HT use following BSO is short. ●
As ObGyn physicians, we can play an important role by educating healthy women with induced menopause who are younger than the average age of spontaneous menopause, and who have no contraindications, that the benefits of HT far outweigh risks. Many of these women will benefit from longer-term HT, using doses substantially higher than are used in women who undergo spontaneous menopause.31,32 After reaching the age of menopause, healthy women without contraindications may continue to benefit from HT into their 50s or beyond if they have vasomotor symptoms, bone loss, or other indications for treatment.18,19
- Chlebowski RT, Hendrix SL, Langer RD, et al; WHI Investigators. Influence of estrogen plus progestin on breast cancer and mammography in healthy postmenopausal women: the Women’s Health Initiative randomized trial. JAMA. 2003;289:3243-3253. doi: 10.1001/jama.289.24.3243.
- Anderson GL, Limacher M, Assaf AR, et al; Women’s Health Initiative Steering Committee. Effects of conjugated equine estrogen in postmenopausal women with hysterectomy: the Women’s Health Initiative randomized controlled trial. JAMA. 2004;291:1701-1712. doi: 10.1001/jama.291.14.1701.
- Opatrny L, Dell’Aniello S, Assouline S, et al. Hormone replacement therapy use and variations in the risk of breast cancer. BJOG. 2008;115:169-175. doi: 10.1111/j.14710528.2007.01520.x.
- Fournier A, Berrino F, Riboli E, et al. Breast cancer risk in relation to different types of hormone replacement therapy in the E3N-EPIC cohort. Int J Cancer. 2005;114:448-454. doi: 10.1002/ijc.20710.
- Fournier A, Berrino F, Clavel-Chapelon F. Unequal risks for breast cancer associated with different hormone replacement therapies: results from the E3N cohort study. Breast Cancer Res Treat. 2008;107:103-111. doi: 10.1007/s10549-007-9523-x.
- Beral V; Million Women Study Collaborators. Breast cancer and hormone-replacement therapy in the million women study. Lancet. 2003;362:419–27. doi: 10.1016/s01406736(03)14065-2.
- Yang Z, Hu Y, Zhang J, et al. Estradiol therapy and breast cancer risk in perimenopausal and postmenopausal women: a systematic review and meta-analysis. Gynecol Endocrinol. 2017;33:87-92. doi: 10.1080/09513590.2016.1248932.
- Asi N, Mohammed K, Haydour Q, et al. Progesterone vs synthetic progestins and the risk of breast cancer: a systematic review and meta-analysis. Syst Rev. 2016;5:121. doi: 10.1186/ s13643-016-0294-5.
- Abenhaim HA, Suissa S, Azoulay L, et al. Menopausal hormone therapy formulation and breast cancer risk. Obstet Gynecol. 2022;139:1103-1110. doi: 10.1097/AOG.0000000000004723.
- Chlebowski RT, Rohan TE, Manson JE, et al. Breast cancer after use of estrogen plus progestin and estrogen alone: analyses of data from 2 Women’s Health Initiative randomized clinical trials. JAMA Oncol. 2015;1:296-305. doi: 10.1001/ jamaoncol.2015.0494.
- Chlebowski RT, Anderson GL, Aragaki A, et al. Association of menopausal hormone therapy with breast cancer incidence and mortality during long-term follow-up of the Women’s Health Initiative randomized clinical trials. JAMA. 2020;324:369-380. doi: 10.1001/jama.2020.9482.
- Cordina-Duverger E, Truong T, Anger A, et al. Risk of breast cancer by type of menopausal hormone therapy: a case-control study among postmenopausal women in France. PLoS One. 2013;8:e78016. doi: 10.1371/journal.pone.0078016.
- Simin J, Tamimi R, Lagergren J, et al. Menopausal hormone therapy and cancer risk: an overestimated risk? Eur J Cancer. 2017;84:60–8. doi: 10.1016/j.ejca. 2017.07.012.
- Stanczyk FZ, Hapgood JP, Winer S, et al. Progestogens used in postmenopausal hormone therapy: differences in their pharmacological properties, intracellular actions, and clinical effects. Endocr Rev. 2013;34:171-208. doi: 10.1210/er.20121008.
- Stute P, Wildt L, Neulen J. The impact of micronized progesterone on breast cancer risk: a systematic review. Climacteric. 2018;21:111-122. doi: 10.1080/13697137.2017.1421925.
- Anderson GL, Chlebowski RT, Aragaki A, et al. Conjugated equine oestrogen and breast cancer incidence and mortality in postmenopausal women with hysterectomy: extended follow-up of the Women’s Health Initiative randomised placebo-controlled trial. Lancet Oncol. 2012;13:476-486. doi: 10.1016/S1470-2045(12)70075-X.
- Chlebowski RT, Barrington W, Aragaki AK, et al. Estrogen alone and health outcomes in black women by African ancestry: a secondary analyses of a randomized controlled trial. Menopause. 2017;24:133-141. doi: 10.1097/ GME.0000000000000733.
- The NAMS 2017 Hormone Therapy Position Statement Advisory Panel. The 2017 hormone therapy position statement of The North American Menopause Society. Menopause. 2017;24:728-753. doi: 10.1097/GME.0000000000000921.
- Pinkerton JV. Hormone therapy for postmenopausal women. N Engl J Med. 2020;382(5):446-455. doi: 10.1056/ NEJMcp1714787.
- Marchetti C, De Felice F, Boccia S, et al. Hormone replacement therapy after prophylactic risk-reducing salpingooophorectomy and breast cancer risk in BRCA1 and BRCA2 mutation carriers: a meta-analysis. Crit Rev Oncol Hematol. 2018;132:111-115. doi: 10.1016/j.critrevonc.2018.09.018.
- Vinogradova Y, Coupland C, Hippisley-Cox J. Use of hormone replacement therapy and risk of venous thromboembolism: nested case-control studies using the QResearch and CPRD databases. BMJ. 2019;364:k4810. doi: 10.1136/bmj.k4810.
- Pinkerton JV. Hormone therapy: key points from NAMS 2017 Position Statement. Clin Obstet Gynecol. 2018;61:447453. doi: 10.1097/GRF.0000000000000383.
- Parker WH, Broder MS, Liu Z, et al. Ovarian conservation at the time of hysterectomy for benign disease. Obstet Gynecol. 2005;106:219-226. doi: 10.1097/01. AOG.0000167394.38215.56.
- Parker WH, Broder MS, Chang E, et al. Ovarian conservation at the time of hysterectomy and long-term health outcomes in the Nurses’ Health Study. Obstet Gynecol. 2009;113:10271037. doi: 10.1097/AOG.0b013e3181a11c64.
- Rocca WA, Bower JH, Maraganore DM, et al. Increased risk of cognitive impairment or dementia in women who underwent oophorectomy before menopause. Neurology. 2007;69:10741083. doi: 10.1212/01.wnl.0000276984.19542.e6.
- Rocca WA, Gazzuola Rocca L, Smith CY, et al Loss of ovarian hormones and accelerated somatic and mental aging. Physiology (Bethesda). 2018;33:374-383. doi: 10.1152/ physiol.00024.2018.
- Mytton J, Evison F, Chilton PJ, et al. Removal of all ovarian tissue versus conserving ovarian tissue at time of hysterectomy in premenopausal patients with benign disease: study using routine data and data linkage. BMJ. 2017;356:j372. doi: 10.1136/bmj.j372.
- Erickson Z, Rocca WA, Smith CY, et al. Time trends in unilateral and bilateral oophorectomy in a geographically defined American population. Obstet Gynecol. 2022;139:724-734. doi: 10.1097/AOG.0000000000004728.
- Choi YH, Terry MB, Daly MB, et al. Association of risk-reducing salpingo-oophorectomy with breast cancer risk in women with BRCA1 and BRCA2 pathogenic variants. JAMA Oncol. 2021;7:585-592. doi: 10.1001/jamaoncol.2020 .7995.
- Suzuki Y, Huang Y, Melamed A, et al. Use of estrogen therapy after surgical menopause in women who are premenopausal. Obstet Gynecol. 2022;139:756-763. doi: 10.1097/ AOG.0000000000004762.
- Faubion S, Kaunitz AM, Kapoor E. HT for women who have had BSO before the age of natural menopause: discerning the nuances. OBG Manag. 2022;34(2):20-27, 45. doi: 10.12788/ obgm.0174.
- Kaunitz AM, Kapoor E, Faubion S. Treatment of women after bilateral salpingo-oophorectomy performed prior to natural menopause. JAMA. 2021;326:1429-1430. doi: 10.1001/ jama.2021.3305.
This year’s Menopause Update focuses on 2 menopause-related issues relevant to ObGyns and our menopausal patients:
- choosing the safest regimens, particularly with respect to risk of breast cancer, when prescribing hormone therapy (HT) to menopausal women
- reviewing the risks and benefits of premenopausal bilateral salpingo-oophorectomy and the pros and cons of replacement HT in surgically menopausal patients.
We hope that you find this updated information useful as you care for menopausal women.
Revisiting menopausal HT and the risk of breast cancer: What we know now
Abenhaim HA, Suissa S, Azoulay L, et al. Menopausal hormone therapy formulation and breast cancer risk. Obstet Gynecol. 2022;139:1103-1110. doi: 10.1097/AOG.0000000000004723.
Reevaluation of the Women’s Health Initiative randomized controlled trials (WHI RCTs), long-term (median follow-up more than 20 years) cumulative follow-up data, and results from additional studies have suggested that estrogen therapy (ET) alone in menopausal women with prior hysterectomy does not increase the risk of breast cancer. By contrast, estrogen with progestin (synthetic progestogens that include medroxyprogesterone acetate [MPA] and norethindrone acetate) slightly increases the risk of breast cancer. In the past 10 years, several publications have shed light on whether the type of progestogen affects the risk of breast cancer and can help provide evidence-based information to guide clinicians.
Breast cancer risk with combined HT and synthetic progestin
In the first part of the WHI RCT, women were randomly assigned to receive either conjugated equine estrogen (CEE) plus synthetic progestin (MPA) or a placebo. Combined estrogen-progestin therapy (EPT) was associated with a modestly elevated risk of breast cancer.1 In the second part of the WHI trial, CEE only (estrogen alone, ET) was compared with placebo among women with prior hysterectomy, with no effect found on breast cancer incidence.2
Most older observational studies published in 2003 to 2005 found that neither CEE nor estradiol appeared to increase the risk of breast cancer when used alone.3-5 However, estrogen use in combination with synthetic progestins (MPA, norethindrone, levonorgestrel, and norgestrel) has been associated with an increased risk of breast cancer,4,6 while the elevated risk of breast cancer with micronized progesterone has been less substantial.7,8
Continue to: Newer data suggest the type of progestogen used affects risk...
Newer data suggest the type of progestogen used affects risk
In a report published in the June 2022 issue of Obstetrics and Gynecology, Abenhaim and colleagues used a nested population-based case-control study of administrative data available in the UK Clinical Practice Research Datalink and provider prescriptions to evaluate the additive effect on the risk of breast cancer of the type of progestogen (micronized progesterone or synthetic progestins) when combined with estradiol for the treatment of menopausal symptoms.9 A cohort of 561,379 women was included in the case-control study (10:1 ratio), 43,183 in the case group (patients diagnosed with invasive breast cancer), and 431,830 in the matched control group.
Overall, in the stratified analysis, a small but significant increase in the risk of breast cancer was found in ever users of menopausal HT (odds ratio [OR], 1.12; 95% confidence interval [CI], 1.09–1.15). Neither estradiol (OR, 1.04; 95% CI, 1.00–1.09) nor CEE (OR, 1.01; 95% CI, 0.96–1.06) was associated with an elevated risk of being diagnosed with invasive breast cancer. Of note, no elevated risk of breast cancer was associated with combination estrogen-progesterone therapy. However, the risk of breast cancer for women who had used synthetic progestins, mostly MPA, was significantly elevated (OR, 1.28; 95% CI, 1.22-1.35). Notably, this modestly elevated odds ratio with the use of estrogen-progestin HT is almost identical to that observed with CEE/ MPA in the WHI.1 Similar findings were found in women aged 50 to 60 years.
The adjusted analyses from the large WHI RCTs provide additional support: the synthetic progestin MPA combined with CEE showed a higher risk of breast cancer than CEE alone in women with prior hysterectomy.10
In the long-term follow-up of the WHI RCTs, after a median of 20.3 years postrandomization, prior randomization to CEE alone for postmenopausal women with prior hysterectomy was associated with a significantly lowered risk of breast cancer incidence and mortality.11 By contrast, prior randomization to CEE plus MPA (EPT) for women with an intact uterus was associated with a small but significantly increased incidence of breast cancer but no significant difference in breast cancer mortality.
In the French E3N EPIC population-based prospective cohort study, Fournier and colleagues4,5 found that women who received estrogen combined with synthetic progestins (mostly MPA) had a higher risk of breast cancer, with an age-adjusted relative risk of 1.4 (95% CI, 1.2–1.7), a finding not seen in women who received estrogen combined with micronized progesterone, similar to findings by Cordina-Duverger and colleagues and Simin and colleagues.12,13 In the E3N study, only 948 women were identified with breast cancer; 268 of these had used synthetic progestins.4,5
Both the Abenhaim cohort9 and the longterm outcomes of WHI RCT trial data11 found a significant contributing effect of MPA (synthetic progestin) in the risk of breast cancer. Progestogens are not thought to exert a class effect. Although it is clear that progestogens (progesterone or progestins) prevent estrogeninduced endometrial neoplasia when dosed adequately, different types of progestogens have a differential risk of breast epithelium proliferation and carcinogenic potential.14 A systematic review by Stute and colleagues found that micronized progesterone did not appear to alter mammographic breast density assessments or breast biopsy results.15
Progesterone capsules, available in generic form in 100-mg and 200-mg doses, are formulated with peanut oil, and they should be taken at bedtime as progesterone can induce drowsiness.
When combined with standard-dose estrogen, including oral estradiol 1.0 mg, transdermal estradiol 0.05 mg, or oral conjugated equine estrogen 0.625 mg, the appropriate dose of progesterone is 100 mg if used continuously or 200 mg if used as cyclic therapy. With higher doses of estrogen, progesterone 200 mg should be taken continuously.
An oral formulation that combines estradiol 1 mg and progesterone 100 mg does not contain peanut oil and, accordingly, can be used safely by those with peanut allergies. This combination product is marketed under the name Bijuva (TherapeuticsMD, Boca Raton, Florida).1
Reference
1. Lobo RA, Archer DF, Kagan R, et al. A 17β-estradiol-progesterone oral capsule for vasomotor symptoms in postmenopausal women: a randomized controlled trial. Obstet Gynecol. 2018;132:161-170. doi: 10.1097/AOG.0000000000002645. Erratum in: Obstet Gynecol. 2018;132:786.
Race considerations
The study by Abenhaim and colleagues was unable to address the issues of race or ethnicity.9 However, in the racially diverse WHI trial of women with prior hysterectomy, estrogen-alone use significantly reduced breast cancer incidence in all participants.10,16 Post hoc analysis of the 1,616 Black women with prior hysterectomy in the WHI RCT showed a significantly decreased breast cancer incidence with use of estrogen alone (hazard ratio [HR], 0.47; 95% CI, 0.26–0.82).1 When race was evaluated in the long-term cumulative follow-up of the WHI trial, estrogen-alone use significantly reduced breast cancer incidence in Black women, with no adverse effect on coronary heart disease, global index, or all-cause mortality, and with fewer cases of venous thromboembolism.17 The global index findings were favorable for Black women in their 50s and those with vasomotor symptoms.
Continue to: Impact of HT in women with an elevated risk of breast cancer...
Impact of HT in women with an elevated risk of breast cancer
Abenhaim and colleagues could not evaluate the effect of HT in women with a baseline elevated risk of breast cancer.9 For these women, HT may be recommended after premature surgical menopause due to increased risks for coronary heart disease, osteoporosis, genitourinary syndrome of menopause, and cognitive changes when estrogen is not taken postsurgery through to at least the average age of menopause, considered age 51.18,19
Marchetti and colleagues reviewed 3 clinical trials that assessed breast cancer events in 1,100 BRCA gene mutation carriers with intact breasts who underwent risk-reducing salpingo-oophorectomy (RRSO) who used or did not use HT.20 For BRCA1 and BRCA2 mutation carriers who received HT after RRSO, no elevated risk of breast cancer risk was seen (HR, 0.98; 95% CI, 0.63–1.52). There was a nonsignificant reduction in breast cancer risk for the estrogen-alone users compared with EPT HT (OR, 0.53; 95% CI, 0.25–1.15). Thus, short-term use of HT, estrogen alone or EPT, does not appear to elevate the risk of breast cancer after RRSO in these high-risk women.
Individualizing HT for menopausal symptoms
The data presented provide reassuring evidence that longer-term use of ET does not appear to increase breast cancer risk, regardless of the type of estrogen (CEE or estradiol).4,5,9,11 For women with a uterus, micronized progesterone has less (if any) effect on breast cancer risk. By contrast, the use of synthetic progestins (such as MPA), when combined with estrogen, has been associated with a small but real increased breast cancer risk.
The most evident benefit of HT is in treating vasomotor symptoms and preventing bone loss for those at elevated risk in healthy women without contraindications who initiate systemic HT when younger than age 60 or within 10 years of menopause onset. Benefit and risk ratio depends on age and time from menopause onset when HT is initiated. Hormone therapy safety varies depending on type, dose, duration, route of administration, timing of initiation, and whether, and type, of progestogen is used. Transdermal estradiol, particularly when dosed at 0.05 mg or less, has been shown to have less thrombotic and stroke risk than oral estrogen.21
Individualizing treatment includes using the best available evidence to maximize benefits and minimize risks, with periodic reevaluation of benefits and risks of continuing or discontinuing HT or changing to lower doses. ObGyns who follow best practices in prescribing systemic HT can now help menopausal patients with bothersome symptoms take advantage of systemic HT’s benefits while providing reassurance regarding menopausal HT’s safety.18 Transdermal therapy is a safer option for women at elevated baseline risk of venous thrombosis (for example, obese women) and older patients. Likewise, given its safety with respect to risk of breast cancer, the use of micronized progesterone over synthetic progestins should be considered when prescribing EPT to women with an intact uterus.
We can replace fear of HT with evidence-based discussions.22 For women with prior hysterectomy who have menopausal symptoms that impact their quality of life, ET at menopause does not appear to increase the risk of breast cancer. For women with an intact uterus who are considering use of estrogen and progestogen, extended-duration use of combination HT with synthetic progestins slightly elevates the risk of breast cancer, while the use of micronized progesterone does not appear to elevate breast cancer risk. Likewise, transdermal estrogen does not appear to elevate thrombosis risk.
Continue to: Benefits of avoiding BSO in women at average risk of ovarian cancer...
Benefits of avoiding BSO in women at average risk of ovarian cancer
Erickson Z, Rocca WA, Smith CY, et al. Time trends in unilateral and bilateral oophorectomy in a geographically defined American population. Obstet Gynecol. 2022;139:724-734. doi: 10.1097/ AOG.0000000000004728.
In 2005, gynecologist William Parker, MD, and colleagues used modeling methodology to assess the long-term risks and benefits of performing bilateral salpingo-oophorectomy (BSO) at the time of hysterectomy for benign disease in women at average risk for ovarian cancer.23 They concluded that practicing ovarian conservation until age 65 increased women’s long-term survival. Among their findings were that women with BSO before age 55 had an 8.6% excess overall mortality by age 80, while those with oophorectomy before age 59 had 3.9% excess mortality. They noted a sustained, but decreasing, mortality benefit until the age of 75 and stated that at no age did their model suggest higher mortality in women who chose ovarian conservation. Parker and colleagues concluded that ovarian conservation until at least age 65 benefited long-term survival for women at average risk for ovarian cancer when undergoing hysterectomy for benign disease.23
Certain risks decreased, others increased
A second report in 2009 by Parker and colleagues from the large prospective Nurses’ Health Study found that, while BSO at the time of hysterectomy for benign disease was associated with a decreased risk of breast and ovarian cancer, BSO was associated with an increased risk of all-cause mortality, fatal and nonfatal coronary heart disease, and lung cancer.24 Similar to the findings of the 2005 report, the authors noted that in no analysis or age group was BSO associated with increased survival. They also noted that compared with those who underwent BSO before age 50 and used ET, women with no history of ET use had an approximately 2-fold elevated risk of new onset coronary heart disease (HR, 1.98; 95% CI, 1.18–3.32).24
In 2007, Walter Rocca, MD, a Mayo Clinic neurologist with a particular interest in the epidemiology of dementia, and colleagues at the Mayo Clinic published results of a study that assessed a cohort of women who had undergone unilateral oophorectomy or BSO prior to the onset of menopause.25 The risk of cognitive impairment or dementia was higher in these women compared with women who had intact ovaries (HR, 1.46; 95% CI, 1.13-1.90). Of note, this elevated risk was confined to those who underwent oophorectomy before 49 years of age and were not prescribed estrogen until age 50 or older.25
In a subsequent publication, Rocca and colleagues pointed out that BSO prior to menopause not only is associated with higher rates of all-cause mortality and cognitive impairment but also with coronary heart disease, parkinsonism, osteoporosis, and other chronic conditions associated with aging, including metabolic, mental health, and arthritic disorders.26
Oophorectomy trends tracked
Given these and other reports27 that highlighted the health risks of premenopausal BSO in women at average risk for ovarian cancer, Rocca and colleagues recently assessed trends in the occurrence of unilateral oophorectomy or BSO versus ovarian conservation among all women residing in the Minnesota county (Olmsted) in which Mayo Clinic is located, and who underwent gynecologic surgery between 1950 and 2018.28
The investigators limited their analysis to women who had undergone unilateral oophorectomy or BSO between ages 18 and 49 years (these women are assumed to have been premenopausal). The authors considered as indications for oophorectomy primary or metastatic ovarian cancer, risk-reducing BSO for women at elevated risk for ovarian cancer (for example, strong family history or known BRCA gene mutation), adnexal mass, endometriosis, torsion, and other benign gynecologic conditions that included pelvic pain, abscess, oophoritis, or ectopic pregnancy. When more than 1 indication for ovarian surgery was present, the authors used the most clinically important indication. Unilateral oophorectomy or BSO was considered not indicated if the surgery was performed during another primary procedure (usually hysterectomy) without indication, or if the surgeon referred to the ovarian surgery as elective.
Results. Among 5,154 women who had oophorectomies between 1950 and 2018, the proportion of these women who underwent unilateral oophorectomy and BSO was 40.6% and 59.4%, respectively.
For most years between 1950 and 1979, the incidence of unilateral oophorectomy was higher than BSO. However, from 1980 to 2004, the incidence of BSO increased more than 2-fold while the incidence of unilateral surgery declined. After 2005, however, both types of ovarian surgery declined. During the years 2005–2018, a marked decline in BSO occurred, with the reduced incidence in premenopausal BSO most notable among women undergoing hysterectomy or those without an indication for oophorectomy.
Historically, ObGyns were taught that the benefits of removing normal ovaries (to prevent ovarian cancer) in average-risk women at the time of hysterectomy outweighed the risks. We agree with the authors’ speculation that beginning with Parker’s 2005 publication,23 ObGyns have become more conservative in performing unindicated BSO in women at average risk for ovarian cancer, now recognizing that the harms of this procedure often outweigh any benefits.28
Women with BRCA1 and BRCA2 gene mutations are at elevated risk for ovarian, tubal, and breast malignancies. In this population, risk-reducing BSO dramatically lowers future risk of ovarian and tubal cancer.
Data addressing the effect of RRSO in BRCA1 and BRCA2 gene mutation carriers continue to be evaluated, with differences between the 2 mutations, but they suggest that the surgery reduces not only ovarian cancer and tubal cancer but also possibly breast cancer.29
Many of our patients are fearful regarding the possibility that they could be diagnosed with breast or ovarian cancer, and in their minds, fears regarding these 2 potentially deadly diseases outweigh concerns about more common causes of death in women, including cardiovascular disease. Accordingly, counseling women at average risk for ovarian cancer who are planning hysterectomy for benign indications can be challenging. In recent years, ObGyns have increasingly been performing opportunistic bilateral salpingectomy (OS) in women at average risk of ovarian cancer at the time of hysterectomy for benign disease. It is important to note that the studies we refer to in this Update addressed BSO, not OS. We hope that the findings we have reviewed here assist clinicians in helping women to understand the risks and benefits associated with premenopausal BSO and the need to discuss the pros and cons of HT for these women before surgery.
Continue to: Trends show decline in ET use in surgically menopausal women...
Trends show decline in ET use in surgically menopausal women
Suzuki Y, Huang Y, Melamed A, et al. Use of estrogen therapy after surgical menopause in women who are premenopausal. Obstet Gynecol. 2022;139:756-763. doi: 10.1097/AOG.0000000000004762.
In addition to highlighting the risks associated with premenopausal BSO in women at average risk for ovarian cancer, the reports referred to above also underscore that the use of replacement menopausal HT in premenopausal women who undergo BSO prevents morbidity and mortality that otherwise accompanies surgical menopause. In addition, the North American Menopause Society (NAMS) recommends replacement menopausal HT in the setting of induced early menopause when no contraindications are present.18
To assess the prevalence of HT use in surgically menopausal women, investigators at Columbia University College of Physicians and Surgeons used a national database that captures health insurance claims for some 280 million US patients, focusing on women aged 18 to 50 years who underwent BSO from 2008 to 2019.30 The great majority of women in this database have private insurance. Although the authors used the term estrogen therapy in their article, this term refers to systemic estrogen alone or with progestogen, as well as vaginal ET (personal communication with Jason Wright, MD, a coauthor of the study, May 19, 2022). In this Update section, we use the term HT to include use of any systemic HT or vaginal estrogen.
Prevalence of HT use changed over time period and patient age range
Among almost 61,980 evaluable women who had undergone BSO (median age, 45 years; 75.1% with concomitant hysterectomy; median follow-up time, 27 months), with no history of gynecologic or breast cancer, HT was used within 3 years of BSO by 64.5%. The highest percentage of women in this cohort who used HT peaked in 2008 (69.5%), declining to 58.2% by 2016. The median duration of HT use was 5.3 months. The prevalence of HT use 3 years after BSO declined with age, from 79.1% in women aged 18–29 to 60.0% in women aged 45–50.30
This report, published in the June 2022 issue of Obstetrics and Gynecology, makes several sobering observations: Many surgically menopausal women aged 50 years and younger are not prescribed HT, the proportion of such women receiving a prescription for HT is declining over time, and the duration of HT use following BSO is short. ●
As ObGyn physicians, we can play an important role by educating healthy women with induced menopause who are younger than the average age of spontaneous menopause, and who have no contraindications, that the benefits of HT far outweigh risks. Many of these women will benefit from longer-term HT, using doses substantially higher than are used in women who undergo spontaneous menopause.31,32 After reaching the age of menopause, healthy women without contraindications may continue to benefit from HT into their 50s or beyond if they have vasomotor symptoms, bone loss, or other indications for treatment.18,19
This year’s Menopause Update focuses on 2 menopause-related issues relevant to ObGyns and our menopausal patients:
- choosing the safest regimens, particularly with respect to risk of breast cancer, when prescribing hormone therapy (HT) to menopausal women
- reviewing the risks and benefits of premenopausal bilateral salpingo-oophorectomy and the pros and cons of replacement HT in surgically menopausal patients.
We hope that you find this updated information useful as you care for menopausal women.
Revisiting menopausal HT and the risk of breast cancer: What we know now
Abenhaim HA, Suissa S, Azoulay L, et al. Menopausal hormone therapy formulation and breast cancer risk. Obstet Gynecol. 2022;139:1103-1110. doi: 10.1097/AOG.0000000000004723.
Reevaluation of the Women’s Health Initiative randomized controlled trials (WHI RCTs), long-term (median follow-up more than 20 years) cumulative follow-up data, and results from additional studies have suggested that estrogen therapy (ET) alone in menopausal women with prior hysterectomy does not increase the risk of breast cancer. By contrast, estrogen with progestin (synthetic progestogens that include medroxyprogesterone acetate [MPA] and norethindrone acetate) slightly increases the risk of breast cancer. In the past 10 years, several publications have shed light on whether the type of progestogen affects the risk of breast cancer and can help provide evidence-based information to guide clinicians.
Breast cancer risk with combined HT and synthetic progestin
In the first part of the WHI RCT, women were randomly assigned to receive either conjugated equine estrogen (CEE) plus synthetic progestin (MPA) or a placebo. Combined estrogen-progestin therapy (EPT) was associated with a modestly elevated risk of breast cancer.1 In the second part of the WHI trial, CEE only (estrogen alone, ET) was compared with placebo among women with prior hysterectomy, with no effect found on breast cancer incidence.2
Most older observational studies published in 2003 to 2005 found that neither CEE nor estradiol appeared to increase the risk of breast cancer when used alone.3-5 However, estrogen use in combination with synthetic progestins (MPA, norethindrone, levonorgestrel, and norgestrel) has been associated with an increased risk of breast cancer,4,6 while the elevated risk of breast cancer with micronized progesterone has been less substantial.7,8
Continue to: Newer data suggest the type of progestogen used affects risk...
Newer data suggest the type of progestogen used affects risk
In a report published in the June 2022 issue of Obstetrics and Gynecology, Abenhaim and colleagues used a nested population-based case-control study of administrative data available in the UK Clinical Practice Research Datalink and provider prescriptions to evaluate the additive effect on the risk of breast cancer of the type of progestogen (micronized progesterone or synthetic progestins) when combined with estradiol for the treatment of menopausal symptoms.9 A cohort of 561,379 women was included in the case-control study (10:1 ratio), 43,183 in the case group (patients diagnosed with invasive breast cancer), and 431,830 in the matched control group.
Overall, in the stratified analysis, a small but significant increase in the risk of breast cancer was found in ever users of menopausal HT (odds ratio [OR], 1.12; 95% confidence interval [CI], 1.09–1.15). Neither estradiol (OR, 1.04; 95% CI, 1.00–1.09) nor CEE (OR, 1.01; 95% CI, 0.96–1.06) was associated with an elevated risk of being diagnosed with invasive breast cancer. Of note, no elevated risk of breast cancer was associated with combination estrogen-progesterone therapy. However, the risk of breast cancer for women who had used synthetic progestins, mostly MPA, was significantly elevated (OR, 1.28; 95% CI, 1.22-1.35). Notably, this modestly elevated odds ratio with the use of estrogen-progestin HT is almost identical to that observed with CEE/ MPA in the WHI.1 Similar findings were found in women aged 50 to 60 years.
The adjusted analyses from the large WHI RCTs provide additional support: the synthetic progestin MPA combined with CEE showed a higher risk of breast cancer than CEE alone in women with prior hysterectomy.10
In the long-term follow-up of the WHI RCTs, after a median of 20.3 years postrandomization, prior randomization to CEE alone for postmenopausal women with prior hysterectomy was associated with a significantly lowered risk of breast cancer incidence and mortality.11 By contrast, prior randomization to CEE plus MPA (EPT) for women with an intact uterus was associated with a small but significantly increased incidence of breast cancer but no significant difference in breast cancer mortality.
In the French E3N EPIC population-based prospective cohort study, Fournier and colleagues4,5 found that women who received estrogen combined with synthetic progestins (mostly MPA) had a higher risk of breast cancer, with an age-adjusted relative risk of 1.4 (95% CI, 1.2–1.7), a finding not seen in women who received estrogen combined with micronized progesterone, similar to findings by Cordina-Duverger and colleagues and Simin and colleagues.12,13 In the E3N study, only 948 women were identified with breast cancer; 268 of these had used synthetic progestins.4,5
Both the Abenhaim cohort9 and the longterm outcomes of WHI RCT trial data11 found a significant contributing effect of MPA (synthetic progestin) in the risk of breast cancer. Progestogens are not thought to exert a class effect. Although it is clear that progestogens (progesterone or progestins) prevent estrogeninduced endometrial neoplasia when dosed adequately, different types of progestogens have a differential risk of breast epithelium proliferation and carcinogenic potential.14 A systematic review by Stute and colleagues found that micronized progesterone did not appear to alter mammographic breast density assessments or breast biopsy results.15
Progesterone capsules, available in generic form in 100-mg and 200-mg doses, are formulated with peanut oil, and they should be taken at bedtime as progesterone can induce drowsiness.
When combined with standard-dose estrogen, including oral estradiol 1.0 mg, transdermal estradiol 0.05 mg, or oral conjugated equine estrogen 0.625 mg, the appropriate dose of progesterone is 100 mg if used continuously or 200 mg if used as cyclic therapy. With higher doses of estrogen, progesterone 200 mg should be taken continuously.
An oral formulation that combines estradiol 1 mg and progesterone 100 mg does not contain peanut oil and, accordingly, can be used safely by those with peanut allergies. This combination product is marketed under the name Bijuva (TherapeuticsMD, Boca Raton, Florida).1
Reference
1. Lobo RA, Archer DF, Kagan R, et al. A 17β-estradiol-progesterone oral capsule for vasomotor symptoms in postmenopausal women: a randomized controlled trial. Obstet Gynecol. 2018;132:161-170. doi: 10.1097/AOG.0000000000002645. Erratum in: Obstet Gynecol. 2018;132:786.
Race considerations
The study by Abenhaim and colleagues was unable to address the issues of race or ethnicity.9 However, in the racially diverse WHI trial of women with prior hysterectomy, estrogen-alone use significantly reduced breast cancer incidence in all participants.10,16 Post hoc analysis of the 1,616 Black women with prior hysterectomy in the WHI RCT showed a significantly decreased breast cancer incidence with use of estrogen alone (hazard ratio [HR], 0.47; 95% CI, 0.26–0.82).1 When race was evaluated in the long-term cumulative follow-up of the WHI trial, estrogen-alone use significantly reduced breast cancer incidence in Black women, with no adverse effect on coronary heart disease, global index, or all-cause mortality, and with fewer cases of venous thromboembolism.17 The global index findings were favorable for Black women in their 50s and those with vasomotor symptoms.
Continue to: Impact of HT in women with an elevated risk of breast cancer...
Impact of HT in women with an elevated risk of breast cancer
Abenhaim and colleagues could not evaluate the effect of HT in women with a baseline elevated risk of breast cancer.9 For these women, HT may be recommended after premature surgical menopause due to increased risks for coronary heart disease, osteoporosis, genitourinary syndrome of menopause, and cognitive changes when estrogen is not taken postsurgery through to at least the average age of menopause, considered age 51.18,19
Marchetti and colleagues reviewed 3 clinical trials that assessed breast cancer events in 1,100 BRCA gene mutation carriers with intact breasts who underwent risk-reducing salpingo-oophorectomy (RRSO) who used or did not use HT.20 For BRCA1 and BRCA2 mutation carriers who received HT after RRSO, no elevated risk of breast cancer risk was seen (HR, 0.98; 95% CI, 0.63–1.52). There was a nonsignificant reduction in breast cancer risk for the estrogen-alone users compared with EPT HT (OR, 0.53; 95% CI, 0.25–1.15). Thus, short-term use of HT, estrogen alone or EPT, does not appear to elevate the risk of breast cancer after RRSO in these high-risk women.
Individualizing HT for menopausal symptoms
The data presented provide reassuring evidence that longer-term use of ET does not appear to increase breast cancer risk, regardless of the type of estrogen (CEE or estradiol).4,5,9,11 For women with a uterus, micronized progesterone has less (if any) effect on breast cancer risk. By contrast, the use of synthetic progestins (such as MPA), when combined with estrogen, has been associated with a small but real increased breast cancer risk.
The most evident benefit of HT is in treating vasomotor symptoms and preventing bone loss for those at elevated risk in healthy women without contraindications who initiate systemic HT when younger than age 60 or within 10 years of menopause onset. Benefit and risk ratio depends on age and time from menopause onset when HT is initiated. Hormone therapy safety varies depending on type, dose, duration, route of administration, timing of initiation, and whether, and type, of progestogen is used. Transdermal estradiol, particularly when dosed at 0.05 mg or less, has been shown to have less thrombotic and stroke risk than oral estrogen.21
Individualizing treatment includes using the best available evidence to maximize benefits and minimize risks, with periodic reevaluation of benefits and risks of continuing or discontinuing HT or changing to lower doses. ObGyns who follow best practices in prescribing systemic HT can now help menopausal patients with bothersome symptoms take advantage of systemic HT’s benefits while providing reassurance regarding menopausal HT’s safety.18 Transdermal therapy is a safer option for women at elevated baseline risk of venous thrombosis (for example, obese women) and older patients. Likewise, given its safety with respect to risk of breast cancer, the use of micronized progesterone over synthetic progestins should be considered when prescribing EPT to women with an intact uterus.
We can replace fear of HT with evidence-based discussions.22 For women with prior hysterectomy who have menopausal symptoms that impact their quality of life, ET at menopause does not appear to increase the risk of breast cancer. For women with an intact uterus who are considering use of estrogen and progestogen, extended-duration use of combination HT with synthetic progestins slightly elevates the risk of breast cancer, while the use of micronized progesterone does not appear to elevate breast cancer risk. Likewise, transdermal estrogen does not appear to elevate thrombosis risk.
Continue to: Benefits of avoiding BSO in women at average risk of ovarian cancer...
Benefits of avoiding BSO in women at average risk of ovarian cancer
Erickson Z, Rocca WA, Smith CY, et al. Time trends in unilateral and bilateral oophorectomy in a geographically defined American population. Obstet Gynecol. 2022;139:724-734. doi: 10.1097/ AOG.0000000000004728.
In 2005, gynecologist William Parker, MD, and colleagues used modeling methodology to assess the long-term risks and benefits of performing bilateral salpingo-oophorectomy (BSO) at the time of hysterectomy for benign disease in women at average risk for ovarian cancer.23 They concluded that practicing ovarian conservation until age 65 increased women’s long-term survival. Among their findings were that women with BSO before age 55 had an 8.6% excess overall mortality by age 80, while those with oophorectomy before age 59 had 3.9% excess mortality. They noted a sustained, but decreasing, mortality benefit until the age of 75 and stated that at no age did their model suggest higher mortality in women who chose ovarian conservation. Parker and colleagues concluded that ovarian conservation until at least age 65 benefited long-term survival for women at average risk for ovarian cancer when undergoing hysterectomy for benign disease.23
Certain risks decreased, others increased
A second report in 2009 by Parker and colleagues from the large prospective Nurses’ Health Study found that, while BSO at the time of hysterectomy for benign disease was associated with a decreased risk of breast and ovarian cancer, BSO was associated with an increased risk of all-cause mortality, fatal and nonfatal coronary heart disease, and lung cancer.24 Similar to the findings of the 2005 report, the authors noted that in no analysis or age group was BSO associated with increased survival. They also noted that compared with those who underwent BSO before age 50 and used ET, women with no history of ET use had an approximately 2-fold elevated risk of new onset coronary heart disease (HR, 1.98; 95% CI, 1.18–3.32).24
In 2007, Walter Rocca, MD, a Mayo Clinic neurologist with a particular interest in the epidemiology of dementia, and colleagues at the Mayo Clinic published results of a study that assessed a cohort of women who had undergone unilateral oophorectomy or BSO prior to the onset of menopause.25 The risk of cognitive impairment or dementia was higher in these women compared with women who had intact ovaries (HR, 1.46; 95% CI, 1.13-1.90). Of note, this elevated risk was confined to those who underwent oophorectomy before 49 years of age and were not prescribed estrogen until age 50 or older.25
In a subsequent publication, Rocca and colleagues pointed out that BSO prior to menopause not only is associated with higher rates of all-cause mortality and cognitive impairment but also with coronary heart disease, parkinsonism, osteoporosis, and other chronic conditions associated with aging, including metabolic, mental health, and arthritic disorders.26
Oophorectomy trends tracked
Given these and other reports27 that highlighted the health risks of premenopausal BSO in women at average risk for ovarian cancer, Rocca and colleagues recently assessed trends in the occurrence of unilateral oophorectomy or BSO versus ovarian conservation among all women residing in the Minnesota county (Olmsted) in which Mayo Clinic is located, and who underwent gynecologic surgery between 1950 and 2018.28
The investigators limited their analysis to women who had undergone unilateral oophorectomy or BSO between ages 18 and 49 years (these women are assumed to have been premenopausal). The authors considered as indications for oophorectomy primary or metastatic ovarian cancer, risk-reducing BSO for women at elevated risk for ovarian cancer (for example, strong family history or known BRCA gene mutation), adnexal mass, endometriosis, torsion, and other benign gynecologic conditions that included pelvic pain, abscess, oophoritis, or ectopic pregnancy. When more than 1 indication for ovarian surgery was present, the authors used the most clinically important indication. Unilateral oophorectomy or BSO was considered not indicated if the surgery was performed during another primary procedure (usually hysterectomy) without indication, or if the surgeon referred to the ovarian surgery as elective.
Results. Among 5,154 women who had oophorectomies between 1950 and 2018, the proportion of these women who underwent unilateral oophorectomy and BSO was 40.6% and 59.4%, respectively.
For most years between 1950 and 1979, the incidence of unilateral oophorectomy was higher than BSO. However, from 1980 to 2004, the incidence of BSO increased more than 2-fold while the incidence of unilateral surgery declined. After 2005, however, both types of ovarian surgery declined. During the years 2005–2018, a marked decline in BSO occurred, with the reduced incidence in premenopausal BSO most notable among women undergoing hysterectomy or those without an indication for oophorectomy.
Historically, ObGyns were taught that the benefits of removing normal ovaries (to prevent ovarian cancer) in average-risk women at the time of hysterectomy outweighed the risks. We agree with the authors’ speculation that beginning with Parker’s 2005 publication,23 ObGyns have become more conservative in performing unindicated BSO in women at average risk for ovarian cancer, now recognizing that the harms of this procedure often outweigh any benefits.28
Women with BRCA1 and BRCA2 gene mutations are at elevated risk for ovarian, tubal, and breast malignancies. In this population, risk-reducing BSO dramatically lowers future risk of ovarian and tubal cancer.
Data addressing the effect of RRSO in BRCA1 and BRCA2 gene mutation carriers continue to be evaluated, with differences between the 2 mutations, but they suggest that the surgery reduces not only ovarian cancer and tubal cancer but also possibly breast cancer.29
Many of our patients are fearful regarding the possibility that they could be diagnosed with breast or ovarian cancer, and in their minds, fears regarding these 2 potentially deadly diseases outweigh concerns about more common causes of death in women, including cardiovascular disease. Accordingly, counseling women at average risk for ovarian cancer who are planning hysterectomy for benign indications can be challenging. In recent years, ObGyns have increasingly been performing opportunistic bilateral salpingectomy (OS) in women at average risk of ovarian cancer at the time of hysterectomy for benign disease. It is important to note that the studies we refer to in this Update addressed BSO, not OS. We hope that the findings we have reviewed here assist clinicians in helping women to understand the risks and benefits associated with premenopausal BSO and the need to discuss the pros and cons of HT for these women before surgery.
Continue to: Trends show decline in ET use in surgically menopausal women...
Trends show decline in ET use in surgically menopausal women
Suzuki Y, Huang Y, Melamed A, et al. Use of estrogen therapy after surgical menopause in women who are premenopausal. Obstet Gynecol. 2022;139:756-763. doi: 10.1097/AOG.0000000000004762.
In addition to highlighting the risks associated with premenopausal BSO in women at average risk for ovarian cancer, the reports referred to above also underscore that the use of replacement menopausal HT in premenopausal women who undergo BSO prevents morbidity and mortality that otherwise accompanies surgical menopause. In addition, the North American Menopause Society (NAMS) recommends replacement menopausal HT in the setting of induced early menopause when no contraindications are present.18
To assess the prevalence of HT use in surgically menopausal women, investigators at Columbia University College of Physicians and Surgeons used a national database that captures health insurance claims for some 280 million US patients, focusing on women aged 18 to 50 years who underwent BSO from 2008 to 2019.30 The great majority of women in this database have private insurance. Although the authors used the term estrogen therapy in their article, this term refers to systemic estrogen alone or with progestogen, as well as vaginal ET (personal communication with Jason Wright, MD, a coauthor of the study, May 19, 2022). In this Update section, we use the term HT to include use of any systemic HT or vaginal estrogen.
Prevalence of HT use changed over time period and patient age range
Among almost 61,980 evaluable women who had undergone BSO (median age, 45 years; 75.1% with concomitant hysterectomy; median follow-up time, 27 months), with no history of gynecologic or breast cancer, HT was used within 3 years of BSO by 64.5%. The highest percentage of women in this cohort who used HT peaked in 2008 (69.5%), declining to 58.2% by 2016. The median duration of HT use was 5.3 months. The prevalence of HT use 3 years after BSO declined with age, from 79.1% in women aged 18–29 to 60.0% in women aged 45–50.30
This report, published in the June 2022 issue of Obstetrics and Gynecology, makes several sobering observations: Many surgically menopausal women aged 50 years and younger are not prescribed HT, the proportion of such women receiving a prescription for HT is declining over time, and the duration of HT use following BSO is short. ●
As ObGyn physicians, we can play an important role by educating healthy women with induced menopause who are younger than the average age of spontaneous menopause, and who have no contraindications, that the benefits of HT far outweigh risks. Many of these women will benefit from longer-term HT, using doses substantially higher than are used in women who undergo spontaneous menopause.31,32 After reaching the age of menopause, healthy women without contraindications may continue to benefit from HT into their 50s or beyond if they have vasomotor symptoms, bone loss, or other indications for treatment.18,19
- Chlebowski RT, Hendrix SL, Langer RD, et al; WHI Investigators. Influence of estrogen plus progestin on breast cancer and mammography in healthy postmenopausal women: the Women’s Health Initiative randomized trial. JAMA. 2003;289:3243-3253. doi: 10.1001/jama.289.24.3243.
- Anderson GL, Limacher M, Assaf AR, et al; Women’s Health Initiative Steering Committee. Effects of conjugated equine estrogen in postmenopausal women with hysterectomy: the Women’s Health Initiative randomized controlled trial. JAMA. 2004;291:1701-1712. doi: 10.1001/jama.291.14.1701.
- Opatrny L, Dell’Aniello S, Assouline S, et al. Hormone replacement therapy use and variations in the risk of breast cancer. BJOG. 2008;115:169-175. doi: 10.1111/j.14710528.2007.01520.x.
- Fournier A, Berrino F, Riboli E, et al. Breast cancer risk in relation to different types of hormone replacement therapy in the E3N-EPIC cohort. Int J Cancer. 2005;114:448-454. doi: 10.1002/ijc.20710.
- Fournier A, Berrino F, Clavel-Chapelon F. Unequal risks for breast cancer associated with different hormone replacement therapies: results from the E3N cohort study. Breast Cancer Res Treat. 2008;107:103-111. doi: 10.1007/s10549-007-9523-x.
- Beral V; Million Women Study Collaborators. Breast cancer and hormone-replacement therapy in the million women study. Lancet. 2003;362:419–27. doi: 10.1016/s01406736(03)14065-2.
- Yang Z, Hu Y, Zhang J, et al. Estradiol therapy and breast cancer risk in perimenopausal and postmenopausal women: a systematic review and meta-analysis. Gynecol Endocrinol. 2017;33:87-92. doi: 10.1080/09513590.2016.1248932.
- Asi N, Mohammed K, Haydour Q, et al. Progesterone vs synthetic progestins and the risk of breast cancer: a systematic review and meta-analysis. Syst Rev. 2016;5:121. doi: 10.1186/ s13643-016-0294-5.
- Abenhaim HA, Suissa S, Azoulay L, et al. Menopausal hormone therapy formulation and breast cancer risk. Obstet Gynecol. 2022;139:1103-1110. doi: 10.1097/AOG.0000000000004723.
- Chlebowski RT, Rohan TE, Manson JE, et al. Breast cancer after use of estrogen plus progestin and estrogen alone: analyses of data from 2 Women’s Health Initiative randomized clinical trials. JAMA Oncol. 2015;1:296-305. doi: 10.1001/ jamaoncol.2015.0494.
- Chlebowski RT, Anderson GL, Aragaki A, et al. Association of menopausal hormone therapy with breast cancer incidence and mortality during long-term follow-up of the Women’s Health Initiative randomized clinical trials. JAMA. 2020;324:369-380. doi: 10.1001/jama.2020.9482.
- Cordina-Duverger E, Truong T, Anger A, et al. Risk of breast cancer by type of menopausal hormone therapy: a case-control study among postmenopausal women in France. PLoS One. 2013;8:e78016. doi: 10.1371/journal.pone.0078016.
- Simin J, Tamimi R, Lagergren J, et al. Menopausal hormone therapy and cancer risk: an overestimated risk? Eur J Cancer. 2017;84:60–8. doi: 10.1016/j.ejca. 2017.07.012.
- Stanczyk FZ, Hapgood JP, Winer S, et al. Progestogens used in postmenopausal hormone therapy: differences in their pharmacological properties, intracellular actions, and clinical effects. Endocr Rev. 2013;34:171-208. doi: 10.1210/er.20121008.
- Stute P, Wildt L, Neulen J. The impact of micronized progesterone on breast cancer risk: a systematic review. Climacteric. 2018;21:111-122. doi: 10.1080/13697137.2017.1421925.
- Anderson GL, Chlebowski RT, Aragaki A, et al. Conjugated equine oestrogen and breast cancer incidence and mortality in postmenopausal women with hysterectomy: extended follow-up of the Women’s Health Initiative randomised placebo-controlled trial. Lancet Oncol. 2012;13:476-486. doi: 10.1016/S1470-2045(12)70075-X.
- Chlebowski RT, Barrington W, Aragaki AK, et al. Estrogen alone and health outcomes in black women by African ancestry: a secondary analyses of a randomized controlled trial. Menopause. 2017;24:133-141. doi: 10.1097/ GME.0000000000000733.
- The NAMS 2017 Hormone Therapy Position Statement Advisory Panel. The 2017 hormone therapy position statement of The North American Menopause Society. Menopause. 2017;24:728-753. doi: 10.1097/GME.0000000000000921.
- Pinkerton JV. Hormone therapy for postmenopausal women. N Engl J Med. 2020;382(5):446-455. doi: 10.1056/ NEJMcp1714787.
- Marchetti C, De Felice F, Boccia S, et al. Hormone replacement therapy after prophylactic risk-reducing salpingooophorectomy and breast cancer risk in BRCA1 and BRCA2 mutation carriers: a meta-analysis. Crit Rev Oncol Hematol. 2018;132:111-115. doi: 10.1016/j.critrevonc.2018.09.018.
- Vinogradova Y, Coupland C, Hippisley-Cox J. Use of hormone replacement therapy and risk of venous thromboembolism: nested case-control studies using the QResearch and CPRD databases. BMJ. 2019;364:k4810. doi: 10.1136/bmj.k4810.
- Pinkerton JV. Hormone therapy: key points from NAMS 2017 Position Statement. Clin Obstet Gynecol. 2018;61:447453. doi: 10.1097/GRF.0000000000000383.
- Parker WH, Broder MS, Liu Z, et al. Ovarian conservation at the time of hysterectomy for benign disease. Obstet Gynecol. 2005;106:219-226. doi: 10.1097/01. AOG.0000167394.38215.56.
- Parker WH, Broder MS, Chang E, et al. Ovarian conservation at the time of hysterectomy and long-term health outcomes in the Nurses’ Health Study. Obstet Gynecol. 2009;113:10271037. doi: 10.1097/AOG.0b013e3181a11c64.
- Rocca WA, Bower JH, Maraganore DM, et al. Increased risk of cognitive impairment or dementia in women who underwent oophorectomy before menopause. Neurology. 2007;69:10741083. doi: 10.1212/01.wnl.0000276984.19542.e6.
- Rocca WA, Gazzuola Rocca L, Smith CY, et al Loss of ovarian hormones and accelerated somatic and mental aging. Physiology (Bethesda). 2018;33:374-383. doi: 10.1152/ physiol.00024.2018.
- Mytton J, Evison F, Chilton PJ, et al. Removal of all ovarian tissue versus conserving ovarian tissue at time of hysterectomy in premenopausal patients with benign disease: study using routine data and data linkage. BMJ. 2017;356:j372. doi: 10.1136/bmj.j372.
- Erickson Z, Rocca WA, Smith CY, et al. Time trends in unilateral and bilateral oophorectomy in a geographically defined American population. Obstet Gynecol. 2022;139:724-734. doi: 10.1097/AOG.0000000000004728.
- Choi YH, Terry MB, Daly MB, et al. Association of risk-reducing salpingo-oophorectomy with breast cancer risk in women with BRCA1 and BRCA2 pathogenic variants. JAMA Oncol. 2021;7:585-592. doi: 10.1001/jamaoncol.2020 .7995.
- Suzuki Y, Huang Y, Melamed A, et al. Use of estrogen therapy after surgical menopause in women who are premenopausal. Obstet Gynecol. 2022;139:756-763. doi: 10.1097/ AOG.0000000000004762.
- Faubion S, Kaunitz AM, Kapoor E. HT for women who have had BSO before the age of natural menopause: discerning the nuances. OBG Manag. 2022;34(2):20-27, 45. doi: 10.12788/ obgm.0174.
- Kaunitz AM, Kapoor E, Faubion S. Treatment of women after bilateral salpingo-oophorectomy performed prior to natural menopause. JAMA. 2021;326:1429-1430. doi: 10.1001/ jama.2021.3305.
- Chlebowski RT, Hendrix SL, Langer RD, et al; WHI Investigators. Influence of estrogen plus progestin on breast cancer and mammography in healthy postmenopausal women: the Women’s Health Initiative randomized trial. JAMA. 2003;289:3243-3253. doi: 10.1001/jama.289.24.3243.
- Anderson GL, Limacher M, Assaf AR, et al; Women’s Health Initiative Steering Committee. Effects of conjugated equine estrogen in postmenopausal women with hysterectomy: the Women’s Health Initiative randomized controlled trial. JAMA. 2004;291:1701-1712. doi: 10.1001/jama.291.14.1701.
- Opatrny L, Dell’Aniello S, Assouline S, et al. Hormone replacement therapy use and variations in the risk of breast cancer. BJOG. 2008;115:169-175. doi: 10.1111/j.14710528.2007.01520.x.
- Fournier A, Berrino F, Riboli E, et al. Breast cancer risk in relation to different types of hormone replacement therapy in the E3N-EPIC cohort. Int J Cancer. 2005;114:448-454. doi: 10.1002/ijc.20710.
- Fournier A, Berrino F, Clavel-Chapelon F. Unequal risks for breast cancer associated with different hormone replacement therapies: results from the E3N cohort study. Breast Cancer Res Treat. 2008;107:103-111. doi: 10.1007/s10549-007-9523-x.
- Beral V; Million Women Study Collaborators. Breast cancer and hormone-replacement therapy in the million women study. Lancet. 2003;362:419–27. doi: 10.1016/s01406736(03)14065-2.
- Yang Z, Hu Y, Zhang J, et al. Estradiol therapy and breast cancer risk in perimenopausal and postmenopausal women: a systematic review and meta-analysis. Gynecol Endocrinol. 2017;33:87-92. doi: 10.1080/09513590.2016.1248932.
- Asi N, Mohammed K, Haydour Q, et al. Progesterone vs synthetic progestins and the risk of breast cancer: a systematic review and meta-analysis. Syst Rev. 2016;5:121. doi: 10.1186/ s13643-016-0294-5.
- Abenhaim HA, Suissa S, Azoulay L, et al. Menopausal hormone therapy formulation and breast cancer risk. Obstet Gynecol. 2022;139:1103-1110. doi: 10.1097/AOG.0000000000004723.
- Chlebowski RT, Rohan TE, Manson JE, et al. Breast cancer after use of estrogen plus progestin and estrogen alone: analyses of data from 2 Women’s Health Initiative randomized clinical trials. JAMA Oncol. 2015;1:296-305. doi: 10.1001/ jamaoncol.2015.0494.
- Chlebowski RT, Anderson GL, Aragaki A, et al. Association of menopausal hormone therapy with breast cancer incidence and mortality during long-term follow-up of the Women’s Health Initiative randomized clinical trials. JAMA. 2020;324:369-380. doi: 10.1001/jama.2020.9482.
- Cordina-Duverger E, Truong T, Anger A, et al. Risk of breast cancer by type of menopausal hormone therapy: a case-control study among postmenopausal women in France. PLoS One. 2013;8:e78016. doi: 10.1371/journal.pone.0078016.
- Simin J, Tamimi R, Lagergren J, et al. Menopausal hormone therapy and cancer risk: an overestimated risk? Eur J Cancer. 2017;84:60–8. doi: 10.1016/j.ejca. 2017.07.012.
- Stanczyk FZ, Hapgood JP, Winer S, et al. Progestogens used in postmenopausal hormone therapy: differences in their pharmacological properties, intracellular actions, and clinical effects. Endocr Rev. 2013;34:171-208. doi: 10.1210/er.20121008.
- Stute P, Wildt L, Neulen J. The impact of micronized progesterone on breast cancer risk: a systematic review. Climacteric. 2018;21:111-122. doi: 10.1080/13697137.2017.1421925.
- Anderson GL, Chlebowski RT, Aragaki A, et al. Conjugated equine oestrogen and breast cancer incidence and mortality in postmenopausal women with hysterectomy: extended follow-up of the Women’s Health Initiative randomised placebo-controlled trial. Lancet Oncol. 2012;13:476-486. doi: 10.1016/S1470-2045(12)70075-X.
- Chlebowski RT, Barrington W, Aragaki AK, et al. Estrogen alone and health outcomes in black women by African ancestry: a secondary analyses of a randomized controlled trial. Menopause. 2017;24:133-141. doi: 10.1097/ GME.0000000000000733.
- The NAMS 2017 Hormone Therapy Position Statement Advisory Panel. The 2017 hormone therapy position statement of The North American Menopause Society. Menopause. 2017;24:728-753. doi: 10.1097/GME.0000000000000921.
- Pinkerton JV. Hormone therapy for postmenopausal women. N Engl J Med. 2020;382(5):446-455. doi: 10.1056/ NEJMcp1714787.
- Marchetti C, De Felice F, Boccia S, et al. Hormone replacement therapy after prophylactic risk-reducing salpingooophorectomy and breast cancer risk in BRCA1 and BRCA2 mutation carriers: a meta-analysis. Crit Rev Oncol Hematol. 2018;132:111-115. doi: 10.1016/j.critrevonc.2018.09.018.
- Vinogradova Y, Coupland C, Hippisley-Cox J. Use of hormone replacement therapy and risk of venous thromboembolism: nested case-control studies using the QResearch and CPRD databases. BMJ. 2019;364:k4810. doi: 10.1136/bmj.k4810.
- Pinkerton JV. Hormone therapy: key points from NAMS 2017 Position Statement. Clin Obstet Gynecol. 2018;61:447453. doi: 10.1097/GRF.0000000000000383.
- Parker WH, Broder MS, Liu Z, et al. Ovarian conservation at the time of hysterectomy for benign disease. Obstet Gynecol. 2005;106:219-226. doi: 10.1097/01. AOG.0000167394.38215.56.
- Parker WH, Broder MS, Chang E, et al. Ovarian conservation at the time of hysterectomy and long-term health outcomes in the Nurses’ Health Study. Obstet Gynecol. 2009;113:10271037. doi: 10.1097/AOG.0b013e3181a11c64.
- Rocca WA, Bower JH, Maraganore DM, et al. Increased risk of cognitive impairment or dementia in women who underwent oophorectomy before menopause. Neurology. 2007;69:10741083. doi: 10.1212/01.wnl.0000276984.19542.e6.
- Rocca WA, Gazzuola Rocca L, Smith CY, et al Loss of ovarian hormones and accelerated somatic and mental aging. Physiology (Bethesda). 2018;33:374-383. doi: 10.1152/ physiol.00024.2018.
- Mytton J, Evison F, Chilton PJ, et al. Removal of all ovarian tissue versus conserving ovarian tissue at time of hysterectomy in premenopausal patients with benign disease: study using routine data and data linkage. BMJ. 2017;356:j372. doi: 10.1136/bmj.j372.
- Erickson Z, Rocca WA, Smith CY, et al. Time trends in unilateral and bilateral oophorectomy in a geographically defined American population. Obstet Gynecol. 2022;139:724-734. doi: 10.1097/AOG.0000000000004728.
- Choi YH, Terry MB, Daly MB, et al. Association of risk-reducing salpingo-oophorectomy with breast cancer risk in women with BRCA1 and BRCA2 pathogenic variants. JAMA Oncol. 2021;7:585-592. doi: 10.1001/jamaoncol.2020 .7995.
- Suzuki Y, Huang Y, Melamed A, et al. Use of estrogen therapy after surgical menopause in women who are premenopausal. Obstet Gynecol. 2022;139:756-763. doi: 10.1097/ AOG.0000000000004762.
- Faubion S, Kaunitz AM, Kapoor E. HT for women who have had BSO before the age of natural menopause: discerning the nuances. OBG Manag. 2022;34(2):20-27, 45. doi: 10.12788/ obgm.0174.
- Kaunitz AM, Kapoor E, Faubion S. Treatment of women after bilateral salpingo-oophorectomy performed prior to natural menopause. JAMA. 2021;326:1429-1430. doi: 10.1001/ jama.2021.3305.
Best practices for evaluating pelvic pain in patients with Essure tubal occlusion devices
The evaluation and management of chronic pelvic pain in patients with a history of Essure device (Bayer HealthCare Pharmaceuticals Inc, Whippany, New Jersey) insertion have posed many challenges for both clinicians and patients. The availability of high-quality, evidence-based clinical guidance has been limited. We have reviewed the currently available published data, and here provide an overview of takeaways, as well as share our perspective and approach on evaluating and managing chronic pelvic pain in this unique patient population.
The device
The Essure microinsert is a hysteroscopically placed device that facilitates permanent sterilization by occluding the bilateral proximal fallopian tubes. The microinsert has an inner and outer nitinol coil that attaches the device to the proximal fallopian tube to ensure retention. The inner coil releases polyethylene terephthalate fibers that cause tubal fiber proliferation to occlude the lumen of the fallopian tube and achieve sterilization.
The device was first approved by the US Food and Drug Administration (FDA) in 2002. In subsequent years, the device was well received and widely used, with approximately 750,000 women worldwide undergoing Essure placement.1,2 Shortly after approval, many adverse events (AEs), including pelvic pain and abnormal uterine bleeding, were reported, resulting in a public meeting of the FDA Obstetrics and Gynecology Devices Panel in September 2015. A postmarket surveillance study on the device ensued to assess complication rates including unplanned pregnancy, pelvic pain, and surgery for removal. In February 2016, the FDA issued a black box warning and a patient decision checklist.3,4 In December 2018, Bayer stopped selling and distributing Essure in the United States.5 A 4-year follow-up surveillance study on Essure was submitted to the FDA in March 2020.
Adverse outcomes
Common AEs related to the Essure device include heavy uterine bleeding, pelvic pain, and other quality-of-life symptoms such as fatigue and weight gain.6-8 The main safety endpoints for the mandated FDA postmarket 522 surveillance studies were chronic lower abdominal and pelvic pain; abnormal uterine bleeding; hypersensitivity; allergic reaction, as well as autoimmune disorders incorporating inflammatory markers and human leukocyte antigen; and gynecologic surgery for device removal.9 Postmarket surveillence has shown that most AEs are related to placement complications or pelvic pain after Essure insertion. However, there have been several reports of autoimmune diseases categorized as serious AEs, such as new-onset systemic lupus erythematosus, rheumatoid arthritis, and worsening ulcerative colitis, after Essure insertion.5
Evaluation of symptoms
Prevalence of pelvic pain following device placement
We conducted a PubMed and MEDLINE search from January 2000 to May 2020, which identified 43 studies citing AEs related to device placement, including pelvic or abdominal pain, abnormal uterine bleeding, hypersensitivity, and autoimmune disorders. A particularly debilitating and frequently cited AE was new-onset pelvic pain or worsening of preexisting pelvic pain. Perforation of the uterus or fallopian tube, resulting in displacement of the device into the peritoneal cavity, or fragmentation of the microinsert was reported as a serious AE that occurred after device placement. However, due to the complexity of chronic pelvic pain pathogenesis, the effect of the insert on patients with existing chronic pelvic pain remains unknown.
Authors of a large retrospective study found that approximately 2.7% of 1,430 patients developed new-onset or worsening pelvic pain after device placement. New-onset pelvic pain in 1% of patients was thought to be secondary to device placement, without a coexisting pathology or diagnosis.10
In a retrospective study by Clark and colleagues, 22 of 50 women (44%) with pelvic pain after microinsert placement were found to have at least one other cause of pelvic pain. The most common alternative diagnoses were endometriosis, adenomyosis, salpingitis, and adhesive disease. Nine of the 50 patients (18%) were found to have endometriosis upon surgical removal of the microinsert.7
Another case series examined outcomes in 29 patients undergoing laparoscopic device removal due to new-onset pelvic pain. Intraoperative findings included endometriosis in 5 patients (17.2%) and pelvic adhesions in 3 (10.3%).2 Chronic pelvic pain secondary to endometriosis may be exacerbated with Essure insertion due to discontinuation of hormonal birth control after device placement,7 and this diagnosis along with adenomyosis should be strongly considered in patients whose pelvic pain began when hormonal contraception was discontinued after placement of the device.
Continue to: Risk factors...
Risk factors
Authors of a retrospective cohort study found that patients with prior diagnosis of a chronic pain syndrome, low back pain, headaches, or fibromyalgia were 5 to 6 times more likely to report acute and chronic pain after hysteroscopic sterilization with Essure.11 Since chronic pain is often thought to be driven by a hyperalgesic state of the central nervous system, as previously shown in patients with conditions such as vulvodynia, interstitial cystitis, and fibromyalgia,12 a hyperalgesic state can potentially explain why some patients are more susceptible to developing worsening pain.
Van Limburg and colleagues conducted a retrospective cohort study with prospective follow-up on 284 women who underwent Essure sterilization. Among these patients, 48% reported negative AEs; risk factors included young age at placement, increasing gravidity, and no prior abdominal surgery.13
Onset of pain
The timing and onset of pelvic pain vary widely, suggesting there is no particular time frame for this AE after device placement.2,6,14-18 A case series by Arjona and colleagues analyzed the incidence of chronic pelvic pain in 4,274 patients after Essure sterilization. Seven patients (0.16%) reported chronic pelvic pain that necessitated device removal. In 6 of the women, the pelvic pain began within 1 week of device placement. In 3 of the 6 cases, the surgeon reported the removal procedures as “difficult.” In all 6 cases, the level of pelvic pain increased with time and was not alleviated with standard analgesic medications.6
In another case series of 26 patients, the authors evaluated patients undergoing laparoscopic removal of Essure secondary to pelvic pain and reported that the time range for symptom presentation was immediate to 85 months. Thirteen of 26 patients (50%) reported pain onset within less than 1 month of device placement, 5 of 26 patients (19.2%) reported pain between 1 and 12 months after device placement, and 8 of 26 patients (30.8%) reported pain onset more than 12 months after microinsert placement.2 In this study, 17.2% of operative reports indicated difficulty with device placement. It is unclear whether difficulty with placement was associated with development of subsequent abdominal or pelvic pain; however, the relevance of initial insertion difficulty diminished with longer follow-up.
Workup and evaluation
We found 5 studies that provided some framework for evaluating a patient with new-onset or worsening pelvic pain after microinsert placement. Overall, correct placement and functionality of the device should be confirmed by either hysterosalpingogram (HSG) or transvaginal ultrasonography (TVUS). The gold standard to determine tubal occlusion is the HSG. However, TVUS may be a dependable alternative, and either test can accurately demonstrate Essure location.19 Patients often prefer TVUS over HSG due to the low cost, minimal discomfort, and short examination time.1 TVUS is a noninvasive and reasonable test to start the initial assessment. The Essure devices are highly echogenic on pelvic ultrasound and easily identifiable by the proximity of the device to the uterotubal junction and its relationship with the surrounding soft tissue. If the device perforates the peritoneal cavity, then the echogenic bowel can impede adequate visualization of the Essure microinsert. If the Essure insert is not visualized on TVUS, an HSG will not only confirm placement but also test insert functionality. After confirming correct placement of the device, the provider can proceed with standard workup for chronic pelvic pain.
If one or more of the devices are malpositioned, the devices are generally presumed to be the etiology of the new pain. Multiple case reports demonstrate pain due to Essure misconfiguration or perforation with subsequent resolution of symptoms after device removal.18,20,21 A case study by Alcantara and colleagues described a patient with chronic pelvic pain and an Essure coil that was curved in an elliptical shape, not adhering to the anatomic course of the fallopian tube. The patient reported pain resolution after laparoscopic removal of the device.20 Another case report by Mahmoud et al described a subserosal malpositioned device that caused acute pelvic pain 4 months after sterilization. The patient reported resolution of pain after the microinsert was removed via laparoscopy.21 These reports highlight the importance of considering malpositioned devices as the etiology of new pelvic pain after Essure placement.
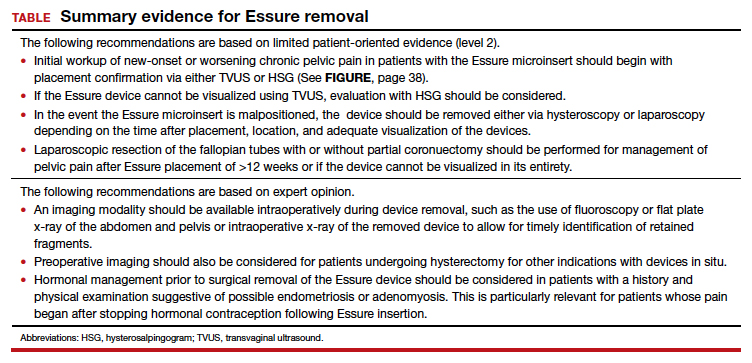
Continue to: Device removal and patient outcomes...
Device removal and patient outcomes
Removal
Several studies that we evaluated included a discussion on the methods for Essure removal. which are divided into 2 general categories: hysteroscopy and laparoscopy.
Hysteroscopic removal is generally used when the device was placed less than 12 weeks prior to removal.7,19 After 12 weeks, removal is more difficult due to fibrosis within the fallopian tubes. A risk with hysteroscopic removal is failure to remove all fibers, which allows inflammation and fibrosis to continue.7 This risk is mitigated via laparoscopic hysterectomy or mini-cornuectomy with bilateral salpingectomy, where the devices can be removed en bloc and without excessive traction.
Laparoscopic Essure removal procedures described in the literature include salpingostomy and traction on the device, salpingectomy, and salpingectomy with mini-cornuectomy. The incision and traction method is typically performed via a 2- to 3-cm incision on the antimesial edge of the fallopian tube along with a circumferential incision to surround the interstitial tubal area. The implant is carefully extracted from the fallopian tube and cornua, and a salpingectomy is then performed.22 The implant is removed prior to the salpingectomy to ensure that the Essure device is removed in its entirety prior to performing a salpingectomy.
A prospective observational study evaluated laparoscopic removal of Essure devices in 80 women with or without cornual excision. Results suggest that the incision and traction method poses more technical difficulties than the cornuectomy approach.23 Surgeons reported significant difficulty controlling the tensile pressure with traction, whereas use of the cornuectomy approach eliminated this risk and decreased the risk of fragmentation and incomplete removal.23,24
Charavil and colleagues demonstrated in a prospective observational study that a vaginal hysterectomy with bilateral salpingectomy is a feasible approach to Essure removal. Twenty-six vaginal hysterectomies with bilateral salpingectomy and Essure removal were performed without conversion to laparoscopy or laparotomy. The surgeons performed an en bloc removal of each hemiuterus along with the ipsilateral tube, which ensured complete removal of the Essure device. Each case was confirmed with an x-ray of the surgical specimen.25
If device fragmentation occurs, there are different methods recommended for locating fragments. A case report of bilateral uterine perforation after uncomplicated Essure placement used a preoperative computed tomography (CT) scan to locate the Essure fragments, but no intraoperative imaging was performed to confirm complete fragment removal.26 The patient continued reporting chronic pelvic pain and ultimately underwent exploratory laparotomy with intraoperative fluoroscopy. Using fluoroscopy, investigators identified omental fragments that were missed on preoperative CT imaging. Fluoroscopy is not commonly used intraoperatively, but it may have added benefit for localizing retained fragments.
A retrospective cohort study reviewed the use of intraoperative x-ray of the removed specimen to confirm complete Essure removal.27 If an x-ray of the removed specimen showed incomplete removal, an intraoperative pelvic x-ray was performed to locate missing fragments. X-ray of the removed devices confirmed complete removal in 63 of 72 patients (87.5%). Six of 9 women with an unsatisfactory specimen x-ray had no residual fragments identified during pelvic x-ray, and the device removal was deemed adequate. The remaining 3 women had radiologic evidence of incomplete device removal and required additional dissection for complete removal. Overall, use of x-ray or fluoroscopy is a relatively safe and accessible way to ensure complete removal of the Essure device and is worth consideration, especially when retained device fragments are suspected.
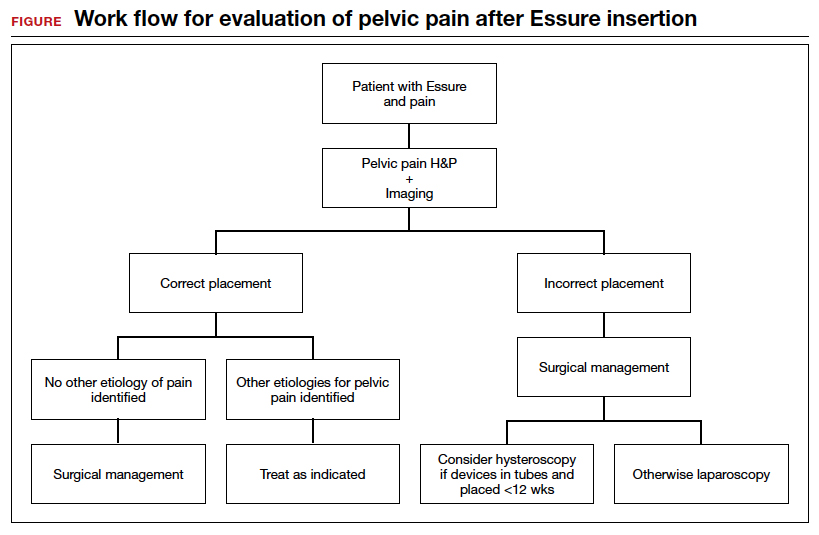
Symptom resolution
We reviewed 5 studies that examined pain outcomes after removal of the Essure devices. Casey et al found that 23 of 26 patients (88.5%) reported significant pain relief at the postoperative visit, while 3 of 26 (11.5%) reported persistent pelvic pain.2 Two of 3 case series examined other outcomes in addition to postoperative pelvic pain, including sexual function and activities of daily living.7,14 In the first case series by Brito and colleagues, 8 of 11 patients (72.7%) reported an improvement in pelvic pain, ability to perform daily activities, sexual life, and overall quality of life after Essure removal. For the remaining 3 patients with persistent pelvic pain after surgical removal of the device, 2 patients reported worsening pain symptoms and dyspareunia.14 In this study, 5 of 11 patients reported a history of chronic pelvic pain at baseline. In a retrospective case series by Clark et al, 28 of 32 women (87.5%) reported some improvement in all domains, with 24 of 32 patients (75%) reporting almost total or complete improvement in quality of life, sexual life, pelvic pain, and scores related to activities of daily living. Pain and quality-of-life scores were similar for women who underwent uterine-preserving surgery and for those who underwent hysterectomy. Ten of 32 women (31.3%) reported persistent or worsening symptoms after the Essure removal surgery. In these patients, the authors recommended consideration of other autoimmune and hypersensitivity etiologies.7
In a retrospective cohort study by Kamencic et al from 2002 to 2013 of 1,430 patients who underwent Essure placement with postplacement imaging, 62 patients (4.3%) required a second surgery after Essure placement due to pelvic pain.10 This study also found that 4 of 62 patients (0.3%) had no other obvious cause for the pelvic pain. All 4 of these women had complete resolution of their pain with removal of the Essure microinsert device. A prospective observational study by Chene e
Summary
Although Essure products were withdrawn from the market in the United States in 2018, many patients still experience significant AEs associated with the device. The goal of the perspectives and data presented here is to assist clinicians in addressing and managing the pain experienced by patients after device insertion. ●
- Connor VF. Essure: a review six years later. J Minim Invasive Gynecol. 2009;16:282-290. doi:10.1016/j.jmig.2009.02.009.
- Casey J, Aguirre F, Yunker A. Outcomes of laparoscopic removal of the Essure sterilization device for pelvic pain: a case series. Contraception. 2016;94:190-192. doi:10.1016/j.contraception.2016.03.017.
- Jackson I. Essure device removed entirely from market, with 99% of unused birth control implants retrieved: FDA. AboutLawsuits.com. January 13, 2020. https://www.aboutlawsuits.com/Essure-removal-update-166509. Accessed June 7, 2022.
- US Food and Drug Administration. Labeling for permanent hysteroscopically-placed tubal implants intended for sterilization. October 31, 2016. https://www.fda.gov/media/96315/download. Accessed June 7, 2022.
- US Food and Drug Administration. FDA activities related to Essure. March 14, 2022. https://www.fda.gov/medical-devices/essure-permanent-birth-control/fda-activities-related-essure. Accessed June 8, 2022.
- Arjona Berral JE, Rodríguez Jiménez B, Velasco Sánchez E, et al. Essure and chronic pelvic pain: a population-based cohort. J Obstet Gynaecol. 2014;34:712-713. doi:10.3109/01443615.2014.92075.
- Clark NV, Rademaker D, Mushinski AA, et al. Essure removal for the treatment of device-attributed symptoms: an expanded case series and follow-up survey. J Minim Invasive Gynecol. 2017;24:971-976. doi:10.1016/j.jmig.2017.05.015.
- Sills ES, Rickers NS, Li X. Surgical management after hysteroscopic sterilization: minimally invasive approach incorporating intraoperative fluoroscopy for symptomatic patients with >2 Essure devices. Surg Technol Int. 2018;32:156-161.
- Administration USF and D. 522 Postmarket Surveillance Studies. Center for Devices and Radiological Health; 2020.
- Kamencic H, Thiel L, Karreman E, et al. Does Essure cause significant de novo pain? A retrospective review of indications for second surgeries after Essure placement. J Minim Invasive Gynecol. 2016;23:1158-1162. doi:10.1016/j.jmig.2016.08.823.
- Yunker AC, Ritch JM, Robinson EF, et al. Incidence and risk factors for chronic pelvic pain after hysteroscopic sterilization. J Minim Invasive Gynecol. 2015;22:390-994. doi:10.1016/j.jmig.2014.06.007.
- Phillips K, Clauw DJ. Central pain mechanisms in chronic pain states--maybe it is all in their head. Best Pract Res Clin Rheumatol. 2011;25:141-154. doi:10.1016/j.berh.2011.02.005.
- van Limburg Stirum EVJ, Clark NV, Lindsey A, et al. Factors associated with negative patient experiences with Essure sterilization. JSLS. 2020;24(1):e2019.00065. doi:10.4293/JSLS.2019.00065.
- Brito LG, Cohen SL, Goggins ER, et al. Essure surgical removal and subsequent symptom resolution: case series and follow-up survey. J Minim Invasive Gynecol. 2015;22:910-913. doi:10.1016/j.jmig.2015.03.018.
- Maassen LW, van Gastel DM, Haveman I, et al. Removal of Essure sterilization devices: a retrospective cohort study in the Netherlands. J Minim Invasive Gynecol. 2019;26:1056-1062. doi:10.1016/j.jmig.2018.10.009.
- Sills ES, Palermo GD. Surgical excision of Essure devices with ESHRE class IIb uterine malformation: sequential hysteroscopic-laparoscopic approach to the septate uterus. Facts Views Vis Obgyn. 2016;8:49-52.
- Ricci G, Restaino S, Di Lorenzo G, et al. Risk of Essure microinsert abdominal migration: case report and review of literature. Ther Clin Risk Manag. 2014;10:963-968. doi:10.2147/TCRM.S65634.
- Borley J, Shabajee N, Tan TL. A kink is not always a perforation: assessing Essure hysteroscopic sterilization placement. Fertil Steril. 2011;95:2429.e15-7. doi:10.1016/j.fertnstert.2011.02.006.
- Djeffal H, Blouet M, Pizzoferato AC, et al. Imaging findings in Essure-related complications: a pictorial review.7Br J Radiol. 2018;91(1090):20170686. doi:10.1259/bjr.20170686.
- Lora Alcantara I, Rezai S, Kirby C, et al. Essure surgical removal and subsequent resolution of chronic pelvic pain: a case report and review of the literature. Case Rep Obstet Gynecol. 2016;2016:6961202. doi:10.1155/2016/6961202.
- Mahmoud MS, Fridman D, Merhi ZO. Subserosal misplacement of Essure device manifested by late-onset acute pelvic pain. Fertil Steril. 2009;92:2038.e1-3. doi:10.1016/j.fertnstert.2009.07.1677.
- Tissot M, Petry S, Lecointre L, et al. Two surgical techniques for Essure device ablation: the hysteroscopic way and the laparoscopic way by salpingectomy with tubal interstitial resection. J Minim Invasive Gynecol. 2019;26(4):603. doi:10.1016/j.jmig.2018.07.017.
- Chene G, Cerruto E, Moret S, et al. Quality of life after laparoscopic removal of Essure sterilization devices. Eur J Obstet Gynecol Reprod Biol X. 2019;3:100054. doi:10.1016/j.eurox.2019.100054.
- Thiel L, Rattray D, Thiel J. Laparoscopic cornuectomy as a technique for removal of Essure microinserts. J Minim Invasive Gynecol. 2017;24(1):10. doi:10.1016/j.jmig.2016.07.004.
- Charavil A, Agostini A, Rambeaud C, et al. Vaginal hysterectomy with salpingectomy for Essure insert removal. J Minim Invasive Gynecol. 2019;2:695-701. doi:10.1016/j.jmig.2018.07.019.
- Howard DL, Christenson PJ, Strickland JL. Use of intraoperative fluoroscopy during laparotomy to identify fragments of retained Essure microinserts: case report. J Minim Invasive Gynecol. 2012;19:667-670. doi:10.1016/j.jmig.2012.04.007.
- Miquel L, Crochet P, Francini S, et al. Laparoscopic Essure device removal by en bloc salpingectomy-cornuectomy with intraoperative x-ray checking: a retrospective cohort study. J Minim Invasive Gynecol. 2020;27:697-703. doi:10.1016/j. jmig.2019.06.006.
The evaluation and management of chronic pelvic pain in patients with a history of Essure device (Bayer HealthCare Pharmaceuticals Inc, Whippany, New Jersey) insertion have posed many challenges for both clinicians and patients. The availability of high-quality, evidence-based clinical guidance has been limited. We have reviewed the currently available published data, and here provide an overview of takeaways, as well as share our perspective and approach on evaluating and managing chronic pelvic pain in this unique patient population.
The device
The Essure microinsert is a hysteroscopically placed device that facilitates permanent sterilization by occluding the bilateral proximal fallopian tubes. The microinsert has an inner and outer nitinol coil that attaches the device to the proximal fallopian tube to ensure retention. The inner coil releases polyethylene terephthalate fibers that cause tubal fiber proliferation to occlude the lumen of the fallopian tube and achieve sterilization.
The device was first approved by the US Food and Drug Administration (FDA) in 2002. In subsequent years, the device was well received and widely used, with approximately 750,000 women worldwide undergoing Essure placement.1,2 Shortly after approval, many adverse events (AEs), including pelvic pain and abnormal uterine bleeding, were reported, resulting in a public meeting of the FDA Obstetrics and Gynecology Devices Panel in September 2015. A postmarket surveillance study on the device ensued to assess complication rates including unplanned pregnancy, pelvic pain, and surgery for removal. In February 2016, the FDA issued a black box warning and a patient decision checklist.3,4 In December 2018, Bayer stopped selling and distributing Essure in the United States.5 A 4-year follow-up surveillance study on Essure was submitted to the FDA in March 2020.
Adverse outcomes
Common AEs related to the Essure device include heavy uterine bleeding, pelvic pain, and other quality-of-life symptoms such as fatigue and weight gain.6-8 The main safety endpoints for the mandated FDA postmarket 522 surveillance studies were chronic lower abdominal and pelvic pain; abnormal uterine bleeding; hypersensitivity; allergic reaction, as well as autoimmune disorders incorporating inflammatory markers and human leukocyte antigen; and gynecologic surgery for device removal.9 Postmarket surveillence has shown that most AEs are related to placement complications or pelvic pain after Essure insertion. However, there have been several reports of autoimmune diseases categorized as serious AEs, such as new-onset systemic lupus erythematosus, rheumatoid arthritis, and worsening ulcerative colitis, after Essure insertion.5
Evaluation of symptoms
Prevalence of pelvic pain following device placement
We conducted a PubMed and MEDLINE search from January 2000 to May 2020, which identified 43 studies citing AEs related to device placement, including pelvic or abdominal pain, abnormal uterine bleeding, hypersensitivity, and autoimmune disorders. A particularly debilitating and frequently cited AE was new-onset pelvic pain or worsening of preexisting pelvic pain. Perforation of the uterus or fallopian tube, resulting in displacement of the device into the peritoneal cavity, or fragmentation of the microinsert was reported as a serious AE that occurred after device placement. However, due to the complexity of chronic pelvic pain pathogenesis, the effect of the insert on patients with existing chronic pelvic pain remains unknown.
Authors of a large retrospective study found that approximately 2.7% of 1,430 patients developed new-onset or worsening pelvic pain after device placement. New-onset pelvic pain in 1% of patients was thought to be secondary to device placement, without a coexisting pathology or diagnosis.10
In a retrospective study by Clark and colleagues, 22 of 50 women (44%) with pelvic pain after microinsert placement were found to have at least one other cause of pelvic pain. The most common alternative diagnoses were endometriosis, adenomyosis, salpingitis, and adhesive disease. Nine of the 50 patients (18%) were found to have endometriosis upon surgical removal of the microinsert.7
Another case series examined outcomes in 29 patients undergoing laparoscopic device removal due to new-onset pelvic pain. Intraoperative findings included endometriosis in 5 patients (17.2%) and pelvic adhesions in 3 (10.3%).2 Chronic pelvic pain secondary to endometriosis may be exacerbated with Essure insertion due to discontinuation of hormonal birth control after device placement,7 and this diagnosis along with adenomyosis should be strongly considered in patients whose pelvic pain began when hormonal contraception was discontinued after placement of the device.
Continue to: Risk factors...
Risk factors
Authors of a retrospective cohort study found that patients with prior diagnosis of a chronic pain syndrome, low back pain, headaches, or fibromyalgia were 5 to 6 times more likely to report acute and chronic pain after hysteroscopic sterilization with Essure.11 Since chronic pain is often thought to be driven by a hyperalgesic state of the central nervous system, as previously shown in patients with conditions such as vulvodynia, interstitial cystitis, and fibromyalgia,12 a hyperalgesic state can potentially explain why some patients are more susceptible to developing worsening pain.
Van Limburg and colleagues conducted a retrospective cohort study with prospective follow-up on 284 women who underwent Essure sterilization. Among these patients, 48% reported negative AEs; risk factors included young age at placement, increasing gravidity, and no prior abdominal surgery.13
Onset of pain
The timing and onset of pelvic pain vary widely, suggesting there is no particular time frame for this AE after device placement.2,6,14-18 A case series by Arjona and colleagues analyzed the incidence of chronic pelvic pain in 4,274 patients after Essure sterilization. Seven patients (0.16%) reported chronic pelvic pain that necessitated device removal. In 6 of the women, the pelvic pain began within 1 week of device placement. In 3 of the 6 cases, the surgeon reported the removal procedures as “difficult.” In all 6 cases, the level of pelvic pain increased with time and was not alleviated with standard analgesic medications.6
In another case series of 26 patients, the authors evaluated patients undergoing laparoscopic removal of Essure secondary to pelvic pain and reported that the time range for symptom presentation was immediate to 85 months. Thirteen of 26 patients (50%) reported pain onset within less than 1 month of device placement, 5 of 26 patients (19.2%) reported pain between 1 and 12 months after device placement, and 8 of 26 patients (30.8%) reported pain onset more than 12 months after microinsert placement.2 In this study, 17.2% of operative reports indicated difficulty with device placement. It is unclear whether difficulty with placement was associated with development of subsequent abdominal or pelvic pain; however, the relevance of initial insertion difficulty diminished with longer follow-up.
Workup and evaluation
We found 5 studies that provided some framework for evaluating a patient with new-onset or worsening pelvic pain after microinsert placement. Overall, correct placement and functionality of the device should be confirmed by either hysterosalpingogram (HSG) or transvaginal ultrasonography (TVUS). The gold standard to determine tubal occlusion is the HSG. However, TVUS may be a dependable alternative, and either test can accurately demonstrate Essure location.19 Patients often prefer TVUS over HSG due to the low cost, minimal discomfort, and short examination time.1 TVUS is a noninvasive and reasonable test to start the initial assessment. The Essure devices are highly echogenic on pelvic ultrasound and easily identifiable by the proximity of the device to the uterotubal junction and its relationship with the surrounding soft tissue. If the device perforates the peritoneal cavity, then the echogenic bowel can impede adequate visualization of the Essure microinsert. If the Essure insert is not visualized on TVUS, an HSG will not only confirm placement but also test insert functionality. After confirming correct placement of the device, the provider can proceed with standard workup for chronic pelvic pain.
If one or more of the devices are malpositioned, the devices are generally presumed to be the etiology of the new pain. Multiple case reports demonstrate pain due to Essure misconfiguration or perforation with subsequent resolution of symptoms after device removal.18,20,21 A case study by Alcantara and colleagues described a patient with chronic pelvic pain and an Essure coil that was curved in an elliptical shape, not adhering to the anatomic course of the fallopian tube. The patient reported pain resolution after laparoscopic removal of the device.20 Another case report by Mahmoud et al described a subserosal malpositioned device that caused acute pelvic pain 4 months after sterilization. The patient reported resolution of pain after the microinsert was removed via laparoscopy.21 These reports highlight the importance of considering malpositioned devices as the etiology of new pelvic pain after Essure placement.

Continue to: Device removal and patient outcomes...
Device removal and patient outcomes
Removal
Several studies that we evaluated included a discussion on the methods for Essure removal. which are divided into 2 general categories: hysteroscopy and laparoscopy.
Hysteroscopic removal is generally used when the device was placed less than 12 weeks prior to removal.7,19 After 12 weeks, removal is more difficult due to fibrosis within the fallopian tubes. A risk with hysteroscopic removal is failure to remove all fibers, which allows inflammation and fibrosis to continue.7 This risk is mitigated via laparoscopic hysterectomy or mini-cornuectomy with bilateral salpingectomy, where the devices can be removed en bloc and without excessive traction.
Laparoscopic Essure removal procedures described in the literature include salpingostomy and traction on the device, salpingectomy, and salpingectomy with mini-cornuectomy. The incision and traction method is typically performed via a 2- to 3-cm incision on the antimesial edge of the fallopian tube along with a circumferential incision to surround the interstitial tubal area. The implant is carefully extracted from the fallopian tube and cornua, and a salpingectomy is then performed.22 The implant is removed prior to the salpingectomy to ensure that the Essure device is removed in its entirety prior to performing a salpingectomy.
A prospective observational study evaluated laparoscopic removal of Essure devices in 80 women with or without cornual excision. Results suggest that the incision and traction method poses more technical difficulties than the cornuectomy approach.23 Surgeons reported significant difficulty controlling the tensile pressure with traction, whereas use of the cornuectomy approach eliminated this risk and decreased the risk of fragmentation and incomplete removal.23,24
Charavil and colleagues demonstrated in a prospective observational study that a vaginal hysterectomy with bilateral salpingectomy is a feasible approach to Essure removal. Twenty-six vaginal hysterectomies with bilateral salpingectomy and Essure removal were performed without conversion to laparoscopy or laparotomy. The surgeons performed an en bloc removal of each hemiuterus along with the ipsilateral tube, which ensured complete removal of the Essure device. Each case was confirmed with an x-ray of the surgical specimen.25
If device fragmentation occurs, there are different methods recommended for locating fragments. A case report of bilateral uterine perforation after uncomplicated Essure placement used a preoperative computed tomography (CT) scan to locate the Essure fragments, but no intraoperative imaging was performed to confirm complete fragment removal.26 The patient continued reporting chronic pelvic pain and ultimately underwent exploratory laparotomy with intraoperative fluoroscopy. Using fluoroscopy, investigators identified omental fragments that were missed on preoperative CT imaging. Fluoroscopy is not commonly used intraoperatively, but it may have added benefit for localizing retained fragments.
A retrospective cohort study reviewed the use of intraoperative x-ray of the removed specimen to confirm complete Essure removal.27 If an x-ray of the removed specimen showed incomplete removal, an intraoperative pelvic x-ray was performed to locate missing fragments. X-ray of the removed devices confirmed complete removal in 63 of 72 patients (87.5%). Six of 9 women with an unsatisfactory specimen x-ray had no residual fragments identified during pelvic x-ray, and the device removal was deemed adequate. The remaining 3 women had radiologic evidence of incomplete device removal and required additional dissection for complete removal. Overall, use of x-ray or fluoroscopy is a relatively safe and accessible way to ensure complete removal of the Essure device and is worth consideration, especially when retained device fragments are suspected.

Symptom resolution
We reviewed 5 studies that examined pain outcomes after removal of the Essure devices. Casey et al found that 23 of 26 patients (88.5%) reported significant pain relief at the postoperative visit, while 3 of 26 (11.5%) reported persistent pelvic pain.2 Two of 3 case series examined other outcomes in addition to postoperative pelvic pain, including sexual function and activities of daily living.7,14 In the first case series by Brito and colleagues, 8 of 11 patients (72.7%) reported an improvement in pelvic pain, ability to perform daily activities, sexual life, and overall quality of life after Essure removal. For the remaining 3 patients with persistent pelvic pain after surgical removal of the device, 2 patients reported worsening pain symptoms and dyspareunia.14 In this study, 5 of 11 patients reported a history of chronic pelvic pain at baseline. In a retrospective case series by Clark et al, 28 of 32 women (87.5%) reported some improvement in all domains, with 24 of 32 patients (75%) reporting almost total or complete improvement in quality of life, sexual life, pelvic pain, and scores related to activities of daily living. Pain and quality-of-life scores were similar for women who underwent uterine-preserving surgery and for those who underwent hysterectomy. Ten of 32 women (31.3%) reported persistent or worsening symptoms after the Essure removal surgery. In these patients, the authors recommended consideration of other autoimmune and hypersensitivity etiologies.7
In a retrospective cohort study by Kamencic et al from 2002 to 2013 of 1,430 patients who underwent Essure placement with postplacement imaging, 62 patients (4.3%) required a second surgery after Essure placement due to pelvic pain.10 This study also found that 4 of 62 patients (0.3%) had no other obvious cause for the pelvic pain. All 4 of these women had complete resolution of their pain with removal of the Essure microinsert device. A prospective observational study by Chene e
Summary
Although Essure products were withdrawn from the market in the United States in 2018, many patients still experience significant AEs associated with the device. The goal of the perspectives and data presented here is to assist clinicians in addressing and managing the pain experienced by patients after device insertion. ●
The evaluation and management of chronic pelvic pain in patients with a history of Essure device (Bayer HealthCare Pharmaceuticals Inc, Whippany, New Jersey) insertion have posed many challenges for both clinicians and patients. The availability of high-quality, evidence-based clinical guidance has been limited. We have reviewed the currently available published data, and here provide an overview of takeaways, as well as share our perspective and approach on evaluating and managing chronic pelvic pain in this unique patient population.
The device
The Essure microinsert is a hysteroscopically placed device that facilitates permanent sterilization by occluding the bilateral proximal fallopian tubes. The microinsert has an inner and outer nitinol coil that attaches the device to the proximal fallopian tube to ensure retention. The inner coil releases polyethylene terephthalate fibers that cause tubal fiber proliferation to occlude the lumen of the fallopian tube and achieve sterilization.
The device was first approved by the US Food and Drug Administration (FDA) in 2002. In subsequent years, the device was well received and widely used, with approximately 750,000 women worldwide undergoing Essure placement.1,2 Shortly after approval, many adverse events (AEs), including pelvic pain and abnormal uterine bleeding, were reported, resulting in a public meeting of the FDA Obstetrics and Gynecology Devices Panel in September 2015. A postmarket surveillance study on the device ensued to assess complication rates including unplanned pregnancy, pelvic pain, and surgery for removal. In February 2016, the FDA issued a black box warning and a patient decision checklist.3,4 In December 2018, Bayer stopped selling and distributing Essure in the United States.5 A 4-year follow-up surveillance study on Essure was submitted to the FDA in March 2020.
Adverse outcomes
Common AEs related to the Essure device include heavy uterine bleeding, pelvic pain, and other quality-of-life symptoms such as fatigue and weight gain.6-8 The main safety endpoints for the mandated FDA postmarket 522 surveillance studies were chronic lower abdominal and pelvic pain; abnormal uterine bleeding; hypersensitivity; allergic reaction, as well as autoimmune disorders incorporating inflammatory markers and human leukocyte antigen; and gynecologic surgery for device removal.9 Postmarket surveillence has shown that most AEs are related to placement complications or pelvic pain after Essure insertion. However, there have been several reports of autoimmune diseases categorized as serious AEs, such as new-onset systemic lupus erythematosus, rheumatoid arthritis, and worsening ulcerative colitis, after Essure insertion.5
Evaluation of symptoms
Prevalence of pelvic pain following device placement
We conducted a PubMed and MEDLINE search from January 2000 to May 2020, which identified 43 studies citing AEs related to device placement, including pelvic or abdominal pain, abnormal uterine bleeding, hypersensitivity, and autoimmune disorders. A particularly debilitating and frequently cited AE was new-onset pelvic pain or worsening of preexisting pelvic pain. Perforation of the uterus or fallopian tube, resulting in displacement of the device into the peritoneal cavity, or fragmentation of the microinsert was reported as a serious AE that occurred after device placement. However, due to the complexity of chronic pelvic pain pathogenesis, the effect of the insert on patients with existing chronic pelvic pain remains unknown.
Authors of a large retrospective study found that approximately 2.7% of 1,430 patients developed new-onset or worsening pelvic pain after device placement. New-onset pelvic pain in 1% of patients was thought to be secondary to device placement, without a coexisting pathology or diagnosis.10
In a retrospective study by Clark and colleagues, 22 of 50 women (44%) with pelvic pain after microinsert placement were found to have at least one other cause of pelvic pain. The most common alternative diagnoses were endometriosis, adenomyosis, salpingitis, and adhesive disease. Nine of the 50 patients (18%) were found to have endometriosis upon surgical removal of the microinsert.7
Another case series examined outcomes in 29 patients undergoing laparoscopic device removal due to new-onset pelvic pain. Intraoperative findings included endometriosis in 5 patients (17.2%) and pelvic adhesions in 3 (10.3%).2 Chronic pelvic pain secondary to endometriosis may be exacerbated with Essure insertion due to discontinuation of hormonal birth control after device placement,7 and this diagnosis along with adenomyosis should be strongly considered in patients whose pelvic pain began when hormonal contraception was discontinued after placement of the device.
Continue to: Risk factors...
Risk factors
Authors of a retrospective cohort study found that patients with prior diagnosis of a chronic pain syndrome, low back pain, headaches, or fibromyalgia were 5 to 6 times more likely to report acute and chronic pain after hysteroscopic sterilization with Essure.11 Since chronic pain is often thought to be driven by a hyperalgesic state of the central nervous system, as previously shown in patients with conditions such as vulvodynia, interstitial cystitis, and fibromyalgia,12 a hyperalgesic state can potentially explain why some patients are more susceptible to developing worsening pain.
Van Limburg and colleagues conducted a retrospective cohort study with prospective follow-up on 284 women who underwent Essure sterilization. Among these patients, 48% reported negative AEs; risk factors included young age at placement, increasing gravidity, and no prior abdominal surgery.13
Onset of pain
The timing and onset of pelvic pain vary widely, suggesting there is no particular time frame for this AE after device placement.2,6,14-18 A case series by Arjona and colleagues analyzed the incidence of chronic pelvic pain in 4,274 patients after Essure sterilization. Seven patients (0.16%) reported chronic pelvic pain that necessitated device removal. In 6 of the women, the pelvic pain began within 1 week of device placement. In 3 of the 6 cases, the surgeon reported the removal procedures as “difficult.” In all 6 cases, the level of pelvic pain increased with time and was not alleviated with standard analgesic medications.6
In another case series of 26 patients, the authors evaluated patients undergoing laparoscopic removal of Essure secondary to pelvic pain and reported that the time range for symptom presentation was immediate to 85 months. Thirteen of 26 patients (50%) reported pain onset within less than 1 month of device placement, 5 of 26 patients (19.2%) reported pain between 1 and 12 months after device placement, and 8 of 26 patients (30.8%) reported pain onset more than 12 months after microinsert placement.2 In this study, 17.2% of operative reports indicated difficulty with device placement. It is unclear whether difficulty with placement was associated with development of subsequent abdominal or pelvic pain; however, the relevance of initial insertion difficulty diminished with longer follow-up.
Workup and evaluation
We found 5 studies that provided some framework for evaluating a patient with new-onset or worsening pelvic pain after microinsert placement. Overall, correct placement and functionality of the device should be confirmed by either hysterosalpingogram (HSG) or transvaginal ultrasonography (TVUS). The gold standard to determine tubal occlusion is the HSG. However, TVUS may be a dependable alternative, and either test can accurately demonstrate Essure location.19 Patients often prefer TVUS over HSG due to the low cost, minimal discomfort, and short examination time.1 TVUS is a noninvasive and reasonable test to start the initial assessment. The Essure devices are highly echogenic on pelvic ultrasound and easily identifiable by the proximity of the device to the uterotubal junction and its relationship with the surrounding soft tissue. If the device perforates the peritoneal cavity, then the echogenic bowel can impede adequate visualization of the Essure microinsert. If the Essure insert is not visualized on TVUS, an HSG will not only confirm placement but also test insert functionality. After confirming correct placement of the device, the provider can proceed with standard workup for chronic pelvic pain.
If one or more of the devices are malpositioned, the devices are generally presumed to be the etiology of the new pain. Multiple case reports demonstrate pain due to Essure misconfiguration or perforation with subsequent resolution of symptoms after device removal.18,20,21 A case study by Alcantara and colleagues described a patient with chronic pelvic pain and an Essure coil that was curved in an elliptical shape, not adhering to the anatomic course of the fallopian tube. The patient reported pain resolution after laparoscopic removal of the device.20 Another case report by Mahmoud et al described a subserosal malpositioned device that caused acute pelvic pain 4 months after sterilization. The patient reported resolution of pain after the microinsert was removed via laparoscopy.21 These reports highlight the importance of considering malpositioned devices as the etiology of new pelvic pain after Essure placement.

Continue to: Device removal and patient outcomes...
Device removal and patient outcomes
Removal
Several studies that we evaluated included a discussion on the methods for Essure removal. which are divided into 2 general categories: hysteroscopy and laparoscopy.
Hysteroscopic removal is generally used when the device was placed less than 12 weeks prior to removal.7,19 After 12 weeks, removal is more difficult due to fibrosis within the fallopian tubes. A risk with hysteroscopic removal is failure to remove all fibers, which allows inflammation and fibrosis to continue.7 This risk is mitigated via laparoscopic hysterectomy or mini-cornuectomy with bilateral salpingectomy, where the devices can be removed en bloc and without excessive traction.
Laparoscopic Essure removal procedures described in the literature include salpingostomy and traction on the device, salpingectomy, and salpingectomy with mini-cornuectomy. The incision and traction method is typically performed via a 2- to 3-cm incision on the antimesial edge of the fallopian tube along with a circumferential incision to surround the interstitial tubal area. The implant is carefully extracted from the fallopian tube and cornua, and a salpingectomy is then performed.22 The implant is removed prior to the salpingectomy to ensure that the Essure device is removed in its entirety prior to performing a salpingectomy.
A prospective observational study evaluated laparoscopic removal of Essure devices in 80 women with or without cornual excision. Results suggest that the incision and traction method poses more technical difficulties than the cornuectomy approach.23 Surgeons reported significant difficulty controlling the tensile pressure with traction, whereas use of the cornuectomy approach eliminated this risk and decreased the risk of fragmentation and incomplete removal.23,24
Charavil and colleagues demonstrated in a prospective observational study that a vaginal hysterectomy with bilateral salpingectomy is a feasible approach to Essure removal. Twenty-six vaginal hysterectomies with bilateral salpingectomy and Essure removal were performed without conversion to laparoscopy or laparotomy. The surgeons performed an en bloc removal of each hemiuterus along with the ipsilateral tube, which ensured complete removal of the Essure device. Each case was confirmed with an x-ray of the surgical specimen.25
If device fragmentation occurs, there are different methods recommended for locating fragments. A case report of bilateral uterine perforation after uncomplicated Essure placement used a preoperative computed tomography (CT) scan to locate the Essure fragments, but no intraoperative imaging was performed to confirm complete fragment removal.26 The patient continued reporting chronic pelvic pain and ultimately underwent exploratory laparotomy with intraoperative fluoroscopy. Using fluoroscopy, investigators identified omental fragments that were missed on preoperative CT imaging. Fluoroscopy is not commonly used intraoperatively, but it may have added benefit for localizing retained fragments.
A retrospective cohort study reviewed the use of intraoperative x-ray of the removed specimen to confirm complete Essure removal.27 If an x-ray of the removed specimen showed incomplete removal, an intraoperative pelvic x-ray was performed to locate missing fragments. X-ray of the removed devices confirmed complete removal in 63 of 72 patients (87.5%). Six of 9 women with an unsatisfactory specimen x-ray had no residual fragments identified during pelvic x-ray, and the device removal was deemed adequate. The remaining 3 women had radiologic evidence of incomplete device removal and required additional dissection for complete removal. Overall, use of x-ray or fluoroscopy is a relatively safe and accessible way to ensure complete removal of the Essure device and is worth consideration, especially when retained device fragments are suspected.

Symptom resolution
We reviewed 5 studies that examined pain outcomes after removal of the Essure devices. Casey et al found that 23 of 26 patients (88.5%) reported significant pain relief at the postoperative visit, while 3 of 26 (11.5%) reported persistent pelvic pain.2 Two of 3 case series examined other outcomes in addition to postoperative pelvic pain, including sexual function and activities of daily living.7,14 In the first case series by Brito and colleagues, 8 of 11 patients (72.7%) reported an improvement in pelvic pain, ability to perform daily activities, sexual life, and overall quality of life after Essure removal. For the remaining 3 patients with persistent pelvic pain after surgical removal of the device, 2 patients reported worsening pain symptoms and dyspareunia.14 In this study, 5 of 11 patients reported a history of chronic pelvic pain at baseline. In a retrospective case series by Clark et al, 28 of 32 women (87.5%) reported some improvement in all domains, with 24 of 32 patients (75%) reporting almost total or complete improvement in quality of life, sexual life, pelvic pain, and scores related to activities of daily living. Pain and quality-of-life scores were similar for women who underwent uterine-preserving surgery and for those who underwent hysterectomy. Ten of 32 women (31.3%) reported persistent or worsening symptoms after the Essure removal surgery. In these patients, the authors recommended consideration of other autoimmune and hypersensitivity etiologies.7
In a retrospective cohort study by Kamencic et al from 2002 to 2013 of 1,430 patients who underwent Essure placement with postplacement imaging, 62 patients (4.3%) required a second surgery after Essure placement due to pelvic pain.10 This study also found that 4 of 62 patients (0.3%) had no other obvious cause for the pelvic pain. All 4 of these women had complete resolution of their pain with removal of the Essure microinsert device. A prospective observational study by Chene e
Summary
Although Essure products were withdrawn from the market in the United States in 2018, many patients still experience significant AEs associated with the device. The goal of the perspectives and data presented here is to assist clinicians in addressing and managing the pain experienced by patients after device insertion. ●
- Connor VF. Essure: a review six years later. J Minim Invasive Gynecol. 2009;16:282-290. doi:10.1016/j.jmig.2009.02.009.
- Casey J, Aguirre F, Yunker A. Outcomes of laparoscopic removal of the Essure sterilization device for pelvic pain: a case series. Contraception. 2016;94:190-192. doi:10.1016/j.contraception.2016.03.017.
- Jackson I. Essure device removed entirely from market, with 99% of unused birth control implants retrieved: FDA. AboutLawsuits.com. January 13, 2020. https://www.aboutlawsuits.com/Essure-removal-update-166509. Accessed June 7, 2022.
- US Food and Drug Administration. Labeling for permanent hysteroscopically-placed tubal implants intended for sterilization. October 31, 2016. https://www.fda.gov/media/96315/download. Accessed June 7, 2022.
- US Food and Drug Administration. FDA activities related to Essure. March 14, 2022. https://www.fda.gov/medical-devices/essure-permanent-birth-control/fda-activities-related-essure. Accessed June 8, 2022.
- Arjona Berral JE, Rodríguez Jiménez B, Velasco Sánchez E, et al. Essure and chronic pelvic pain: a population-based cohort. J Obstet Gynaecol. 2014;34:712-713. doi:10.3109/01443615.2014.92075.
- Clark NV, Rademaker D, Mushinski AA, et al. Essure removal for the treatment of device-attributed symptoms: an expanded case series and follow-up survey. J Minim Invasive Gynecol. 2017;24:971-976. doi:10.1016/j.jmig.2017.05.015.
- Sills ES, Rickers NS, Li X. Surgical management after hysteroscopic sterilization: minimally invasive approach incorporating intraoperative fluoroscopy for symptomatic patients with >2 Essure devices. Surg Technol Int. 2018;32:156-161.
- Administration USF and D. 522 Postmarket Surveillance Studies. Center for Devices and Radiological Health; 2020.
- Kamencic H, Thiel L, Karreman E, et al. Does Essure cause significant de novo pain? A retrospective review of indications for second surgeries after Essure placement. J Minim Invasive Gynecol. 2016;23:1158-1162. doi:10.1016/j.jmig.2016.08.823.
- Yunker AC, Ritch JM, Robinson EF, et al. Incidence and risk factors for chronic pelvic pain after hysteroscopic sterilization. J Minim Invasive Gynecol. 2015;22:390-994. doi:10.1016/j.jmig.2014.06.007.
- Phillips K, Clauw DJ. Central pain mechanisms in chronic pain states--maybe it is all in their head. Best Pract Res Clin Rheumatol. 2011;25:141-154. doi:10.1016/j.berh.2011.02.005.
- van Limburg Stirum EVJ, Clark NV, Lindsey A, et al. Factors associated with negative patient experiences with Essure sterilization. JSLS. 2020;24(1):e2019.00065. doi:10.4293/JSLS.2019.00065.
- Brito LG, Cohen SL, Goggins ER, et al. Essure surgical removal and subsequent symptom resolution: case series and follow-up survey. J Minim Invasive Gynecol. 2015;22:910-913. doi:10.1016/j.jmig.2015.03.018.
- Maassen LW, van Gastel DM, Haveman I, et al. Removal of Essure sterilization devices: a retrospective cohort study in the Netherlands. J Minim Invasive Gynecol. 2019;26:1056-1062. doi:10.1016/j.jmig.2018.10.009.
- Sills ES, Palermo GD. Surgical excision of Essure devices with ESHRE class IIb uterine malformation: sequential hysteroscopic-laparoscopic approach to the septate uterus. Facts Views Vis Obgyn. 2016;8:49-52.
- Ricci G, Restaino S, Di Lorenzo G, et al. Risk of Essure microinsert abdominal migration: case report and review of literature. Ther Clin Risk Manag. 2014;10:963-968. doi:10.2147/TCRM.S65634.
- Borley J, Shabajee N, Tan TL. A kink is not always a perforation: assessing Essure hysteroscopic sterilization placement. Fertil Steril. 2011;95:2429.e15-7. doi:10.1016/j.fertnstert.2011.02.006.
- Djeffal H, Blouet M, Pizzoferato AC, et al. Imaging findings in Essure-related complications: a pictorial review.7Br J Radiol. 2018;91(1090):20170686. doi:10.1259/bjr.20170686.
- Lora Alcantara I, Rezai S, Kirby C, et al. Essure surgical removal and subsequent resolution of chronic pelvic pain: a case report and review of the literature. Case Rep Obstet Gynecol. 2016;2016:6961202. doi:10.1155/2016/6961202.
- Mahmoud MS, Fridman D, Merhi ZO. Subserosal misplacement of Essure device manifested by late-onset acute pelvic pain. Fertil Steril. 2009;92:2038.e1-3. doi:10.1016/j.fertnstert.2009.07.1677.
- Tissot M, Petry S, Lecointre L, et al. Two surgical techniques for Essure device ablation: the hysteroscopic way and the laparoscopic way by salpingectomy with tubal interstitial resection. J Minim Invasive Gynecol. 2019;26(4):603. doi:10.1016/j.jmig.2018.07.017.
- Chene G, Cerruto E, Moret S, et al. Quality of life after laparoscopic removal of Essure sterilization devices. Eur J Obstet Gynecol Reprod Biol X. 2019;3:100054. doi:10.1016/j.eurox.2019.100054.
- Thiel L, Rattray D, Thiel J. Laparoscopic cornuectomy as a technique for removal of Essure microinserts. J Minim Invasive Gynecol. 2017;24(1):10. doi:10.1016/j.jmig.2016.07.004.
- Charavil A, Agostini A, Rambeaud C, et al. Vaginal hysterectomy with salpingectomy for Essure insert removal. J Minim Invasive Gynecol. 2019;2:695-701. doi:10.1016/j.jmig.2018.07.019.
- Howard DL, Christenson PJ, Strickland JL. Use of intraoperative fluoroscopy during laparotomy to identify fragments of retained Essure microinserts: case report. J Minim Invasive Gynecol. 2012;19:667-670. doi:10.1016/j.jmig.2012.04.007.
- Miquel L, Crochet P, Francini S, et al. Laparoscopic Essure device removal by en bloc salpingectomy-cornuectomy with intraoperative x-ray checking: a retrospective cohort study. J Minim Invasive Gynecol. 2020;27:697-703. doi:10.1016/j. jmig.2019.06.006.
- Connor VF. Essure: a review six years later. J Minim Invasive Gynecol. 2009;16:282-290. doi:10.1016/j.jmig.2009.02.009.
- Casey J, Aguirre F, Yunker A. Outcomes of laparoscopic removal of the Essure sterilization device for pelvic pain: a case series. Contraception. 2016;94:190-192. doi:10.1016/j.contraception.2016.03.017.
- Jackson I. Essure device removed entirely from market, with 99% of unused birth control implants retrieved: FDA. AboutLawsuits.com. January 13, 2020. https://www.aboutlawsuits.com/Essure-removal-update-166509. Accessed June 7, 2022.
- US Food and Drug Administration. Labeling for permanent hysteroscopically-placed tubal implants intended for sterilization. October 31, 2016. https://www.fda.gov/media/96315/download. Accessed June 7, 2022.
- US Food and Drug Administration. FDA activities related to Essure. March 14, 2022. https://www.fda.gov/medical-devices/essure-permanent-birth-control/fda-activities-related-essure. Accessed June 8, 2022.
- Arjona Berral JE, Rodríguez Jiménez B, Velasco Sánchez E, et al. Essure and chronic pelvic pain: a population-based cohort. J Obstet Gynaecol. 2014;34:712-713. doi:10.3109/01443615.2014.92075.
- Clark NV, Rademaker D, Mushinski AA, et al. Essure removal for the treatment of device-attributed symptoms: an expanded case series and follow-up survey. J Minim Invasive Gynecol. 2017;24:971-976. doi:10.1016/j.jmig.2017.05.015.
- Sills ES, Rickers NS, Li X. Surgical management after hysteroscopic sterilization: minimally invasive approach incorporating intraoperative fluoroscopy for symptomatic patients with >2 Essure devices. Surg Technol Int. 2018;32:156-161.
- Administration USF and D. 522 Postmarket Surveillance Studies. Center for Devices and Radiological Health; 2020.
- Kamencic H, Thiel L, Karreman E, et al. Does Essure cause significant de novo pain? A retrospective review of indications for second surgeries after Essure placement. J Minim Invasive Gynecol. 2016;23:1158-1162. doi:10.1016/j.jmig.2016.08.823.
- Yunker AC, Ritch JM, Robinson EF, et al. Incidence and risk factors for chronic pelvic pain after hysteroscopic sterilization. J Minim Invasive Gynecol. 2015;22:390-994. doi:10.1016/j.jmig.2014.06.007.
- Phillips K, Clauw DJ. Central pain mechanisms in chronic pain states--maybe it is all in their head. Best Pract Res Clin Rheumatol. 2011;25:141-154. doi:10.1016/j.berh.2011.02.005.
- van Limburg Stirum EVJ, Clark NV, Lindsey A, et al. Factors associated with negative patient experiences with Essure sterilization. JSLS. 2020;24(1):e2019.00065. doi:10.4293/JSLS.2019.00065.
- Brito LG, Cohen SL, Goggins ER, et al. Essure surgical removal and subsequent symptom resolution: case series and follow-up survey. J Minim Invasive Gynecol. 2015;22:910-913. doi:10.1016/j.jmig.2015.03.018.
- Maassen LW, van Gastel DM, Haveman I, et al. Removal of Essure sterilization devices: a retrospective cohort study in the Netherlands. J Minim Invasive Gynecol. 2019;26:1056-1062. doi:10.1016/j.jmig.2018.10.009.
- Sills ES, Palermo GD. Surgical excision of Essure devices with ESHRE class IIb uterine malformation: sequential hysteroscopic-laparoscopic approach to the septate uterus. Facts Views Vis Obgyn. 2016;8:49-52.
- Ricci G, Restaino S, Di Lorenzo G, et al. Risk of Essure microinsert abdominal migration: case report and review of literature. Ther Clin Risk Manag. 2014;10:963-968. doi:10.2147/TCRM.S65634.
- Borley J, Shabajee N, Tan TL. A kink is not always a perforation: assessing Essure hysteroscopic sterilization placement. Fertil Steril. 2011;95:2429.e15-7. doi:10.1016/j.fertnstert.2011.02.006.
- Djeffal H, Blouet M, Pizzoferato AC, et al. Imaging findings in Essure-related complications: a pictorial review.7Br J Radiol. 2018;91(1090):20170686. doi:10.1259/bjr.20170686.
- Lora Alcantara I, Rezai S, Kirby C, et al. Essure surgical removal and subsequent resolution of chronic pelvic pain: a case report and review of the literature. Case Rep Obstet Gynecol. 2016;2016:6961202. doi:10.1155/2016/6961202.
- Mahmoud MS, Fridman D, Merhi ZO. Subserosal misplacement of Essure device manifested by late-onset acute pelvic pain. Fertil Steril. 2009;92:2038.e1-3. doi:10.1016/j.fertnstert.2009.07.1677.
- Tissot M, Petry S, Lecointre L, et al. Two surgical techniques for Essure device ablation: the hysteroscopic way and the laparoscopic way by salpingectomy with tubal interstitial resection. J Minim Invasive Gynecol. 2019;26(4):603. doi:10.1016/j.jmig.2018.07.017.
- Chene G, Cerruto E, Moret S, et al. Quality of life after laparoscopic removal of Essure sterilization devices. Eur J Obstet Gynecol Reprod Biol X. 2019;3:100054. doi:10.1016/j.eurox.2019.100054.
- Thiel L, Rattray D, Thiel J. Laparoscopic cornuectomy as a technique for removal of Essure microinserts. J Minim Invasive Gynecol. 2017;24(1):10. doi:10.1016/j.jmig.2016.07.004.
- Charavil A, Agostini A, Rambeaud C, et al. Vaginal hysterectomy with salpingectomy for Essure insert removal. J Minim Invasive Gynecol. 2019;2:695-701. doi:10.1016/j.jmig.2018.07.019.
- Howard DL, Christenson PJ, Strickland JL. Use of intraoperative fluoroscopy during laparotomy to identify fragments of retained Essure microinserts: case report. J Minim Invasive Gynecol. 2012;19:667-670. doi:10.1016/j.jmig.2012.04.007.
- Miquel L, Crochet P, Francini S, et al. Laparoscopic Essure device removal by en bloc salpingectomy-cornuectomy with intraoperative x-ray checking: a retrospective cohort study. J Minim Invasive Gynecol. 2020;27:697-703. doi:10.1016/j. jmig.2019.06.006.
Amniotic fluid embolism: Management using a checklist
CASE Part 1: CPR initiated during induction of labor
A 32-year-old gravida 4 para 3-0-0-3 is undergoing induction of labor with intravenous (IV) oxytocin at 39 weeks of gestation. She has no significant medical or obstetric history. Fifteen minutes after reaching complete cervical dilation, she says “I don’t feel right,” then suddenly loses consciousness. The nurse finds no detectable pulse, calls a “code blue,” and initiates cardiopulmonary resuscitation (CPR). The obstetrician is notified, appears promptly, assesses the situation, and delivers a 3.6-kg baby via vacuum extraction. Apgar score is 2/10 at 1 minute and 6/10 at 5 minutes. After delivery of the placenta, there is uterine atony and brisk hemorrhage with 2 L of blood loss.
Management of AFE: A rare complication
This case demonstrates a classic presentation of amniotic fluid embolism (AFE) syndrome—a patient in labor or within 30 minutes after delivery has sudden onset of cardiorespiratory collapse followed by disseminated intravascular coagulation (DIC). AFE is rare, affecting only about 2 to 6 per 100,000 births, but classic cases have a reported maternal mortality rate that exceeds 50%.1 It is thought to reflect a complex, systemic proinflammatory response to maternal intravasation of pregnancy material, such as trophoblast, thromboplastins, fetal cells, or amniotic fluid. Because the syndrome is not necessarily directly caused by emboli or by amniotic fluid per se,2 it has been proposed that AFE be called “anaphylactoid syndrome of pregnancy,” but this terminology has not yet been widely adopted.3
Guidelines from the Society for Maternal-Fetal Medicine (SMFM) recommend several time-critical steps for the initial stabilization and management of patients with AFE.4 However, because AFE is rare, most obstetric providers may not encounter a case for many years or even decades after they have received training, so it is unrealistic to expect that they will remember these guidelines when they are needed. For this reason, when AFE occurs, it is important to have a readily accessible cognitive aid, such as a checklist that summarizes the key management steps. The SMFM provides a checklist for initial management of AFE that can be used at your institution; it is presented in the FIGURE and provides the outline for this discussion.5

Provide CPR immediately
Most AFE cases are accompanied by cardiorespiratory arrest. If the patient has no pulse, call a “code” to mobilize additional help and immediately start CPR. Use a backboard to make cardiac compressions most effective and manually displace the uterus or tilt the patient to avoid supine hypotension. Designate a timekeeper to call out 1-minute intervals and record critical data, such as medication administration and laboratory orders/results.
Expedite delivery
Immediate delivery is needed if maternal cardiac activity is not restored within 4 minutes of starting CPR, with a target to have delivery completed within 5 minutes. Operative vaginal delivery may be an option if delivery is imminent, as in the case presented, but cesarean delivery (CD) will be needed in most cases. This was previously called “perimortem cesarean” delivery, but the term “resuscitative hysterotomy” has been proposed because the primary goal is to improve the effectiveness of CPR6 and prevent both maternal and perinatal death. CPR is less effective in pregnant women because the pregnant uterus takes a substantial fraction of the maternal cardiac output, as well as compresses the vena cava. Some experts suggest that, rather than waiting 4 minutes, CD should be started as soon as an obstetrician or other surgeon is present, unless there is an immediate response to electrical cardioversion.6,7
In most cases, immediate CD should be performed wherever the patient is located rather than using precious minutes to move the patient to an operating room. Antiseptic preparation is expedited by simply pouring povidone-iodine or chlorhexidine over the lower abdomen if readily available; if not available, skip this step. Enter the abdomen and uterus as rapidly as possible using only a scalpel to make generous midline incisions.
If CPR is not required, proceed with cesarean or operative vaginal delivery as soon as the mother has been stabilized. These procedures should be performed using standard safety precautions outlined in the SMFM patient safety checklists for cesarean or operative vaginal delivery.8,9
Continue to: Anticipate hemorrhage...
Anticipate hemorrhage
Be prepared for uterine atony, coagulopathy, and catastrophic hemorrhage. Initiate IV oxytocin prophylaxis as soon as the infant is delivered. Have a low threshold for giving other uterotonic agents such as methylergonovine, carboprost, or misoprostol. If hemorrhage or DIC occurs, give tranexamic acid. Have the anesthesiologist or trauma team (if available) insert an intraosseous line for fluid resuscitation if peripheral IV access is inadequate.
Massive transfusion is often needed to treat DIC, which occurs in most AFE cases. Anticipate—do not wait—for DIC to occur. We propose activating your hospital’s massive transfusion protocol (MTP) as soon as you diagnose AFE so that blood products will be available as soon as possible. A typical MTP provides several units of red blood cells, a pheresis pack of platelets, and fresh/frozen plasma (FFP). If clinically indicated, administer cryoprecipitate instead of FFP to minimize volume overload, which may occur with FFP.
CASE Part 2: MTP initiated to treat DIC
The MTP is initiated. Laboratory results immediately pre-transfusion include hemoglobin 11.3 g/dL, platelet count 46,000 per mm3, fibrinogen 87 mg/dL, and an elevated prothrombin time international normalized ratio.
Expect heart failure
The initial hemodynamic picture in AFE is right heart failure, which should optimally be managed by a specialist from anesthesiology, cardiology, or critical care as soon as they are available. An emergency department physician may manage the hemodynamics until a specialist arrives. Avoidance of fluid overload is one important principle. If fluid challenges are needed for hypovolemic shock, boluses should be restricted to 500 mL rather than the traditional 1000 mL.
Pharmacologic treatment may include vasopressors, inotropic agents, and pulmonary vasodilators. Example medications and dosages recommended by SMFM are summarized in the checklist (FIGURE).5
After the initial phase of recovery, the hemodynamic picture often changes from right heart failure to left heart failure. Management of left heart failure is not covered in the SMFM checklist because, by the time it appears, the patient will usually be in the intensive care unit, managed by the critical care team. Management of left heart failure generally includes diuresis as needed for cardiogenic pulmonary edema, optimization of cardiac preload, and inotropic agents or vasopressors if needed to maintain cardiac output or perfusion pressure.4
Debrief, learning opportunities
Complex emergencies such as AFE are rarely handled 100% perfectly, even those with a good outcome, so they present opportunities for team learning and improvement. The team should conduct a 10- to 15-minute debrief soon after the patient is stabilized. Make an explicit statement that the main goal of the debrief is to gather suggestions as to how systems and processes could be improved for next time, not to find fault or lay blame on individuals. Encourage all personnel involved in the initial management to attend and discuss what went well and what did not. Another goal is to provide support for individuals who may feel traumatized by the dramatic, frightening events surrounding an AFE and by the poor patient outcome or guarded prognosis that frequently follows. Another goal is to discuss the plan for providing support and disclosure to the patient and family.
The vast majority of AFE cases meet criteria to be designated as “sentinel events,” because of patient transfer to the intensive care unit, multi-unit blood transfusion, other severe maternal morbidities, or maternal death. Therefore, most AFE cases will trigger a root cause analysis (RCA) or other formal sentinel event analysis conducted by the hospital’s Safety or Quality Department. As with the immediate post-event debrief, the first goal of the RCA is to identify systems issues that may have resulted in suboptimal care and that can be modified to improve future care. Specific issues regarding the checklist should also be addressed:
- Was the checklist used?
- Was the checklist available?
- Are there items on the checklist that need to be modified, added, or deleted?
The RCA concludes with the development of a performance improvement plan.
Ultimately, we encourage all AFE cases be reported to the registry maintained by the Amniotic Fluid Embolism Foundation at https://www.afesupport.org/, regardless of whether the outcome was favorable for the mother and newborn. The registry includes over 130 AFE cases since 2013 from around the world. Researchers periodically report on the registry findings.10 If providers report cases with both good and bad outcomes, the registry may provide future insights regarding which adjunctive or empiric treatments may or may not be promising.
Continue to: Empiric treatments...
Empiric treatments
From time-to-time, new regimens for empiric treatment of AFE are reported. It is important to recognize that these reports are generally uncontrolled case reports of favorable outcomes and that, without a control group, it is impossible to determine to what extent the treatment contributed to the outcome or was merely incidental. Given the rarity of AFE, it seems unlikely that there will ever be a randomized clinical trial or even a controlled prospective study comparing treatment regimens.
The “A-OK” regimen is an empiric treatment that has garnered some interest after an initial case report.11 It consists of an anticholinergic agent (atropine 0.2 mg IV), a selective 5-HT3 receptor antagonist (ondansetron 8 mg IV), and a nonsteroidal anti-inflammatory drug (ketorolac 15 mg IV). We have some reservations about this regimen, however, because atropine is relatively contraindicated if the patient has tachycardia (which is common in patients with hemorrhage) and ketorolac may suppress platelet function, which might be harmful for patients with DIC or thrombocytopenia.
Another empiric treatment is the “50-50-500” regimen, which includes an H1 antihistamine (diphenhydramine 50 mg IV), an H2 antihistamine (famotidine 50 mg IV), and a corticosteroid (hydrocortisone 500 mg IV). This regimen aims to suppress histamine-mediated and cell-mediated inflammatory responses, based on the notion that proinflammatory responses likely mediate much of the underlying pathophysiology of the AFE syndrome.
We would emphasize that these empiric regimens are not clinically validated, US Food and Drug Administration approved for treatment of AFE, or considered standard of care. Future reports of these and other regimens will be needed to evaluate their efficacy, limitations, and risks. Again, we encourage providers to report all AFE cases to the AFE Foundation registry, regardless of whether the treatments are successful.
CASE Conclusion
The hemorrhage stops after administration of oxytocin, carboprost, 6 units of cryoprecipitate, and a 6-unit platelet pheresis pack. The patient is transferred to the intensive care unit where she eventually requires a total of 10 units of red cells, 8 more units of cryoprecipitate, and another platelet pheresis pack. She is discharged to home in stable condition on postpartum day 4.
Be prepared, have the checklist ready
Because AFE is rare, most members of the health care team will have no prior experience managing a real case. It may have been years or decades since they had any education on AFE or they last read a review article such as this one. It is even possible the anesthesiologist, cardiologist, or critical care specialist has never heard of AFE. Thus if they rely on memory alone, there is substantial risk of forgetting items, getting dosages wrong, or other errors. With this in mind, what is the best way to prepare the team to expeditiously employ the management steps outlined here?
Use of a checklist that summarizes these key steps for early management, such as the SMFM checklist in the FIGURE, will help ensure that all relevant steps are performed in every AFE case. It is designed to be printed on a single sheet of letter-sized paper, and we propose that every labor and delivery (L&D) unit keep laminated copies of this checklist in several places where they will be immediately available should an AFE occur. Copies can be kept on the anesthesia carts in the L&D operating rooms, in an emergency procedures binder on the unit, and on the “crash carts” and hemorrhage supply carts in the L&D unit. Effective implementation of an AFE checklist requires all personnel know where to readily find it and have some familiarity with its contents.
An interdisciplinary team comprising representatives from nursing, obstetrics, and anesthesia should meet to discuss whether the checklist needs to be modified to fit the local hospital formulary or other unique local circumstances. The team should develop an implementation plan that includes where to keep checklist copies, a process to periodically ensure that the copies are still present and readable, a roll-out plan to inform all personnel about the checklist process, and most importantly a training plan that includes incorporating AFE cases into the schedule of multidisciplinary simulations and drills for obstetric emergencies. Other implementation strategies are outlined in the SMFM document.5
Ultimately an organized, systematic approach is recommended for management of AFE. There is no single best treatment of AFE; it is supportive and directed toward the underlying pathophysiology, which may vary from patient to patient. Therefore, although a checklist, in conjunction with regular education and simulation activities, may help optimize care and improve outcomes, there is still a high risk of maternal morbidity and mortality from AFE. ●
- Clark SL. Amniotic fluid embolism. Obstet Gynecol. 2014;123(2 Pt 1):337-348. doi:10.1097/AOG.0000000000000107.
- Funk M, Damron A, Bandi V, et al. Pulmonary vascular obstruction by squamous cells is not involved in amniotic fluid embolism. Am J Obstet Gynecol. 2018;218:460-461. doi:10.1016/j.ajog.2017.12.225.
- Gilmore DA, Wakim J, Secrest J, et al. Anaphylactoid syndrome of pregnancy: a review of the literature with latest management and outcome data. AANA J. 2003;71:120-126.
- Society for Maternal-Fetal Medicine, Pacheco LD, Saade G, et al. Amniotic fluid embolism: diagnosis and management. Am J Obstet Gynecol. 2016;215:B16-24. doi:10.1016/j.ajog.2016.03.012.
- Patient Safety and Quality Committee, Society for Maternal-Fetal Medicine; Combs CA, Montgomery DM, et al. Society for Maternal-Fetal Medicine Special Statement: checklist for initial management of amniotic fluid embolism. Am J Obstet Gynecol. 2021;224:B29-B32. doi:10.1016/j.ajog.2021.01.001.
- Rose CH, Faksh A, Traynor KD, et al. Challenging the 4- to 5-minute rule: from perimortem cesarean to resuscitative hysterotomy. Am J Obstet Gynecol. 2015;213:653-6, 653.e1. doi:10.1016/j.ajog.2015.07.019.
- Pacheco LD, Clark SL, Klassen M, et al. Amniotic fluid embolism: principles of early clinical management. Am J Obstet Gynecol. 2020;222:48-52. doi:10.1016/j.ajog.2019.07.036.
- Combs CA, Einerson BD, Toner LE, SMFM Patient Safety and Quality Committee. SMFM Special Statement: surgical safety checklists for cesarean delivery. Am J Obstet Gynecol. 2021;225:B43-B49. doi:10.1016/j.ajog.2021.07.011.
- SMFM Patient Safety and Quality Committee, Staat B, Combs CA. SMFM Special Statement: operative vaginal delivery: checklists for performance and documentation. Am J Obstet Gynecol. 2020;222:B15-B21. doi:10.1016/j.ajog.2020.02.011.
- Stafford IA, Moaddab A, Dildy GA, et al. Amniotic fluid embolism syndrome: analysis of the United States international registry. Am J Obstet Gynecol MFM. 2020;2:100083. doi:10.1016/j.ajogmf.2019.100083.
- Rezai S, Hughes AZC, Larsen TB, et al. Atypical amniotic f luid embolism managed with a novel therapeutic regimen. Case Rep Obstet Gynecol. 2017; 2017:8458375. doi:10.1155/2017/8458375.

CASE Part 1: CPR initiated during induction of labor
A 32-year-old gravida 4 para 3-0-0-3 is undergoing induction of labor with intravenous (IV) oxytocin at 39 weeks of gestation. She has no significant medical or obstetric history. Fifteen minutes after reaching complete cervical dilation, she says “I don’t feel right,” then suddenly loses consciousness. The nurse finds no detectable pulse, calls a “code blue,” and initiates cardiopulmonary resuscitation (CPR). The obstetrician is notified, appears promptly, assesses the situation, and delivers a 3.6-kg baby via vacuum extraction. Apgar score is 2/10 at 1 minute and 6/10 at 5 minutes. After delivery of the placenta, there is uterine atony and brisk hemorrhage with 2 L of blood loss.
Management of AFE: A rare complication
This case demonstrates a classic presentation of amniotic fluid embolism (AFE) syndrome—a patient in labor or within 30 minutes after delivery has sudden onset of cardiorespiratory collapse followed by disseminated intravascular coagulation (DIC). AFE is rare, affecting only about 2 to 6 per 100,000 births, but classic cases have a reported maternal mortality rate that exceeds 50%.1 It is thought to reflect a complex, systemic proinflammatory response to maternal intravasation of pregnancy material, such as trophoblast, thromboplastins, fetal cells, or amniotic fluid. Because the syndrome is not necessarily directly caused by emboli or by amniotic fluid per se,2 it has been proposed that AFE be called “anaphylactoid syndrome of pregnancy,” but this terminology has not yet been widely adopted.3
Guidelines from the Society for Maternal-Fetal Medicine (SMFM) recommend several time-critical steps for the initial stabilization and management of patients with AFE.4 However, because AFE is rare, most obstetric providers may not encounter a case for many years or even decades after they have received training, so it is unrealistic to expect that they will remember these guidelines when they are needed. For this reason, when AFE occurs, it is important to have a readily accessible cognitive aid, such as a checklist that summarizes the key management steps. The SMFM provides a checklist for initial management of AFE that can be used at your institution; it is presented in the FIGURE and provides the outline for this discussion.5

Provide CPR immediately
Most AFE cases are accompanied by cardiorespiratory arrest. If the patient has no pulse, call a “code” to mobilize additional help and immediately start CPR. Use a backboard to make cardiac compressions most effective and manually displace the uterus or tilt the patient to avoid supine hypotension. Designate a timekeeper to call out 1-minute intervals and record critical data, such as medication administration and laboratory orders/results.
Expedite delivery
Immediate delivery is needed if maternal cardiac activity is not restored within 4 minutes of starting CPR, with a target to have delivery completed within 5 minutes. Operative vaginal delivery may be an option if delivery is imminent, as in the case presented, but cesarean delivery (CD) will be needed in most cases. This was previously called “perimortem cesarean” delivery, but the term “resuscitative hysterotomy” has been proposed because the primary goal is to improve the effectiveness of CPR6 and prevent both maternal and perinatal death. CPR is less effective in pregnant women because the pregnant uterus takes a substantial fraction of the maternal cardiac output, as well as compresses the vena cava. Some experts suggest that, rather than waiting 4 minutes, CD should be started as soon as an obstetrician or other surgeon is present, unless there is an immediate response to electrical cardioversion.6,7
In most cases, immediate CD should be performed wherever the patient is located rather than using precious minutes to move the patient to an operating room. Antiseptic preparation is expedited by simply pouring povidone-iodine or chlorhexidine over the lower abdomen if readily available; if not available, skip this step. Enter the abdomen and uterus as rapidly as possible using only a scalpel to make generous midline incisions.
If CPR is not required, proceed with cesarean or operative vaginal delivery as soon as the mother has been stabilized. These procedures should be performed using standard safety precautions outlined in the SMFM patient safety checklists for cesarean or operative vaginal delivery.8,9
Continue to: Anticipate hemorrhage...
Anticipate hemorrhage
Be prepared for uterine atony, coagulopathy, and catastrophic hemorrhage. Initiate IV oxytocin prophylaxis as soon as the infant is delivered. Have a low threshold for giving other uterotonic agents such as methylergonovine, carboprost, or misoprostol. If hemorrhage or DIC occurs, give tranexamic acid. Have the anesthesiologist or trauma team (if available) insert an intraosseous line for fluid resuscitation if peripheral IV access is inadequate.
Massive transfusion is often needed to treat DIC, which occurs in most AFE cases. Anticipate—do not wait—for DIC to occur. We propose activating your hospital’s massive transfusion protocol (MTP) as soon as you diagnose AFE so that blood products will be available as soon as possible. A typical MTP provides several units of red blood cells, a pheresis pack of platelets, and fresh/frozen plasma (FFP). If clinically indicated, administer cryoprecipitate instead of FFP to minimize volume overload, which may occur with FFP.
CASE Part 2: MTP initiated to treat DIC
The MTP is initiated. Laboratory results immediately pre-transfusion include hemoglobin 11.3 g/dL, platelet count 46,000 per mm3, fibrinogen 87 mg/dL, and an elevated prothrombin time international normalized ratio.
Expect heart failure
The initial hemodynamic picture in AFE is right heart failure, which should optimally be managed by a specialist from anesthesiology, cardiology, or critical care as soon as they are available. An emergency department physician may manage the hemodynamics until a specialist arrives. Avoidance of fluid overload is one important principle. If fluid challenges are needed for hypovolemic shock, boluses should be restricted to 500 mL rather than the traditional 1000 mL.
Pharmacologic treatment may include vasopressors, inotropic agents, and pulmonary vasodilators. Example medications and dosages recommended by SMFM are summarized in the checklist (FIGURE).5
After the initial phase of recovery, the hemodynamic picture often changes from right heart failure to left heart failure. Management of left heart failure is not covered in the SMFM checklist because, by the time it appears, the patient will usually be in the intensive care unit, managed by the critical care team. Management of left heart failure generally includes diuresis as needed for cardiogenic pulmonary edema, optimization of cardiac preload, and inotropic agents or vasopressors if needed to maintain cardiac output or perfusion pressure.4
Debrief, learning opportunities
Complex emergencies such as AFE are rarely handled 100% perfectly, even those with a good outcome, so they present opportunities for team learning and improvement. The team should conduct a 10- to 15-minute debrief soon after the patient is stabilized. Make an explicit statement that the main goal of the debrief is to gather suggestions as to how systems and processes could be improved for next time, not to find fault or lay blame on individuals. Encourage all personnel involved in the initial management to attend and discuss what went well and what did not. Another goal is to provide support for individuals who may feel traumatized by the dramatic, frightening events surrounding an AFE and by the poor patient outcome or guarded prognosis that frequently follows. Another goal is to discuss the plan for providing support and disclosure to the patient and family.
The vast majority of AFE cases meet criteria to be designated as “sentinel events,” because of patient transfer to the intensive care unit, multi-unit blood transfusion, other severe maternal morbidities, or maternal death. Therefore, most AFE cases will trigger a root cause analysis (RCA) or other formal sentinel event analysis conducted by the hospital’s Safety or Quality Department. As with the immediate post-event debrief, the first goal of the RCA is to identify systems issues that may have resulted in suboptimal care and that can be modified to improve future care. Specific issues regarding the checklist should also be addressed:
- Was the checklist used?
- Was the checklist available?
- Are there items on the checklist that need to be modified, added, or deleted?
The RCA concludes with the development of a performance improvement plan.
Ultimately, we encourage all AFE cases be reported to the registry maintained by the Amniotic Fluid Embolism Foundation at https://www.afesupport.org/, regardless of whether the outcome was favorable for the mother and newborn. The registry includes over 130 AFE cases since 2013 from around the world. Researchers periodically report on the registry findings.10 If providers report cases with both good and bad outcomes, the registry may provide future insights regarding which adjunctive or empiric treatments may or may not be promising.
Continue to: Empiric treatments...
Empiric treatments
From time-to-time, new regimens for empiric treatment of AFE are reported. It is important to recognize that these reports are generally uncontrolled case reports of favorable outcomes and that, without a control group, it is impossible to determine to what extent the treatment contributed to the outcome or was merely incidental. Given the rarity of AFE, it seems unlikely that there will ever be a randomized clinical trial or even a controlled prospective study comparing treatment regimens.
The “A-OK” regimen is an empiric treatment that has garnered some interest after an initial case report.11 It consists of an anticholinergic agent (atropine 0.2 mg IV), a selective 5-HT3 receptor antagonist (ondansetron 8 mg IV), and a nonsteroidal anti-inflammatory drug (ketorolac 15 mg IV). We have some reservations about this regimen, however, because atropine is relatively contraindicated if the patient has tachycardia (which is common in patients with hemorrhage) and ketorolac may suppress platelet function, which might be harmful for patients with DIC or thrombocytopenia.
Another empiric treatment is the “50-50-500” regimen, which includes an H1 antihistamine (diphenhydramine 50 mg IV), an H2 antihistamine (famotidine 50 mg IV), and a corticosteroid (hydrocortisone 500 mg IV). This regimen aims to suppress histamine-mediated and cell-mediated inflammatory responses, based on the notion that proinflammatory responses likely mediate much of the underlying pathophysiology of the AFE syndrome.
We would emphasize that these empiric regimens are not clinically validated, US Food and Drug Administration approved for treatment of AFE, or considered standard of care. Future reports of these and other regimens will be needed to evaluate their efficacy, limitations, and risks. Again, we encourage providers to report all AFE cases to the AFE Foundation registry, regardless of whether the treatments are successful.
CASE Conclusion
The hemorrhage stops after administration of oxytocin, carboprost, 6 units of cryoprecipitate, and a 6-unit platelet pheresis pack. The patient is transferred to the intensive care unit where she eventually requires a total of 10 units of red cells, 8 more units of cryoprecipitate, and another platelet pheresis pack. She is discharged to home in stable condition on postpartum day 4.
Be prepared, have the checklist ready
Because AFE is rare, most members of the health care team will have no prior experience managing a real case. It may have been years or decades since they had any education on AFE or they last read a review article such as this one. It is even possible the anesthesiologist, cardiologist, or critical care specialist has never heard of AFE. Thus if they rely on memory alone, there is substantial risk of forgetting items, getting dosages wrong, or other errors. With this in mind, what is the best way to prepare the team to expeditiously employ the management steps outlined here?
Use of a checklist that summarizes these key steps for early management, such as the SMFM checklist in the FIGURE, will help ensure that all relevant steps are performed in every AFE case. It is designed to be printed on a single sheet of letter-sized paper, and we propose that every labor and delivery (L&D) unit keep laminated copies of this checklist in several places where they will be immediately available should an AFE occur. Copies can be kept on the anesthesia carts in the L&D operating rooms, in an emergency procedures binder on the unit, and on the “crash carts” and hemorrhage supply carts in the L&D unit. Effective implementation of an AFE checklist requires all personnel know where to readily find it and have some familiarity with its contents.
An interdisciplinary team comprising representatives from nursing, obstetrics, and anesthesia should meet to discuss whether the checklist needs to be modified to fit the local hospital formulary or other unique local circumstances. The team should develop an implementation plan that includes where to keep checklist copies, a process to periodically ensure that the copies are still present and readable, a roll-out plan to inform all personnel about the checklist process, and most importantly a training plan that includes incorporating AFE cases into the schedule of multidisciplinary simulations and drills for obstetric emergencies. Other implementation strategies are outlined in the SMFM document.5
Ultimately an organized, systematic approach is recommended for management of AFE. There is no single best treatment of AFE; it is supportive and directed toward the underlying pathophysiology, which may vary from patient to patient. Therefore, although a checklist, in conjunction with regular education and simulation activities, may help optimize care and improve outcomes, there is still a high risk of maternal morbidity and mortality from AFE. ●

CASE Part 1: CPR initiated during induction of labor
A 32-year-old gravida 4 para 3-0-0-3 is undergoing induction of labor with intravenous (IV) oxytocin at 39 weeks of gestation. She has no significant medical or obstetric history. Fifteen minutes after reaching complete cervical dilation, she says “I don’t feel right,” then suddenly loses consciousness. The nurse finds no detectable pulse, calls a “code blue,” and initiates cardiopulmonary resuscitation (CPR). The obstetrician is notified, appears promptly, assesses the situation, and delivers a 3.6-kg baby via vacuum extraction. Apgar score is 2/10 at 1 minute and 6/10 at 5 minutes. After delivery of the placenta, there is uterine atony and brisk hemorrhage with 2 L of blood loss.
Management of AFE: A rare complication
This case demonstrates a classic presentation of amniotic fluid embolism (AFE) syndrome—a patient in labor or within 30 minutes after delivery has sudden onset of cardiorespiratory collapse followed by disseminated intravascular coagulation (DIC). AFE is rare, affecting only about 2 to 6 per 100,000 births, but classic cases have a reported maternal mortality rate that exceeds 50%.1 It is thought to reflect a complex, systemic proinflammatory response to maternal intravasation of pregnancy material, such as trophoblast, thromboplastins, fetal cells, or amniotic fluid. Because the syndrome is not necessarily directly caused by emboli or by amniotic fluid per se,2 it has been proposed that AFE be called “anaphylactoid syndrome of pregnancy,” but this terminology has not yet been widely adopted.3
Guidelines from the Society for Maternal-Fetal Medicine (SMFM) recommend several time-critical steps for the initial stabilization and management of patients with AFE.4 However, because AFE is rare, most obstetric providers may not encounter a case for many years or even decades after they have received training, so it is unrealistic to expect that they will remember these guidelines when they are needed. For this reason, when AFE occurs, it is important to have a readily accessible cognitive aid, such as a checklist that summarizes the key management steps. The SMFM provides a checklist for initial management of AFE that can be used at your institution; it is presented in the FIGURE and provides the outline for this discussion.5

Provide CPR immediately
Most AFE cases are accompanied by cardiorespiratory arrest. If the patient has no pulse, call a “code” to mobilize additional help and immediately start CPR. Use a backboard to make cardiac compressions most effective and manually displace the uterus or tilt the patient to avoid supine hypotension. Designate a timekeeper to call out 1-minute intervals and record critical data, such as medication administration and laboratory orders/results.
Expedite delivery
Immediate delivery is needed if maternal cardiac activity is not restored within 4 minutes of starting CPR, with a target to have delivery completed within 5 minutes. Operative vaginal delivery may be an option if delivery is imminent, as in the case presented, but cesarean delivery (CD) will be needed in most cases. This was previously called “perimortem cesarean” delivery, but the term “resuscitative hysterotomy” has been proposed because the primary goal is to improve the effectiveness of CPR6 and prevent both maternal and perinatal death. CPR is less effective in pregnant women because the pregnant uterus takes a substantial fraction of the maternal cardiac output, as well as compresses the vena cava. Some experts suggest that, rather than waiting 4 minutes, CD should be started as soon as an obstetrician or other surgeon is present, unless there is an immediate response to electrical cardioversion.6,7
In most cases, immediate CD should be performed wherever the patient is located rather than using precious minutes to move the patient to an operating room. Antiseptic preparation is expedited by simply pouring povidone-iodine or chlorhexidine over the lower abdomen if readily available; if not available, skip this step. Enter the abdomen and uterus as rapidly as possible using only a scalpel to make generous midline incisions.
If CPR is not required, proceed with cesarean or operative vaginal delivery as soon as the mother has been stabilized. These procedures should be performed using standard safety precautions outlined in the SMFM patient safety checklists for cesarean or operative vaginal delivery.8,9
Continue to: Anticipate hemorrhage...
Anticipate hemorrhage
Be prepared for uterine atony, coagulopathy, and catastrophic hemorrhage. Initiate IV oxytocin prophylaxis as soon as the infant is delivered. Have a low threshold for giving other uterotonic agents such as methylergonovine, carboprost, or misoprostol. If hemorrhage or DIC occurs, give tranexamic acid. Have the anesthesiologist or trauma team (if available) insert an intraosseous line for fluid resuscitation if peripheral IV access is inadequate.
Massive transfusion is often needed to treat DIC, which occurs in most AFE cases. Anticipate—do not wait—for DIC to occur. We propose activating your hospital’s massive transfusion protocol (MTP) as soon as you diagnose AFE so that blood products will be available as soon as possible. A typical MTP provides several units of red blood cells, a pheresis pack of platelets, and fresh/frozen plasma (FFP). If clinically indicated, administer cryoprecipitate instead of FFP to minimize volume overload, which may occur with FFP.
CASE Part 2: MTP initiated to treat DIC
The MTP is initiated. Laboratory results immediately pre-transfusion include hemoglobin 11.3 g/dL, platelet count 46,000 per mm3, fibrinogen 87 mg/dL, and an elevated prothrombin time international normalized ratio.
Expect heart failure
The initial hemodynamic picture in AFE is right heart failure, which should optimally be managed by a specialist from anesthesiology, cardiology, or critical care as soon as they are available. An emergency department physician may manage the hemodynamics until a specialist arrives. Avoidance of fluid overload is one important principle. If fluid challenges are needed for hypovolemic shock, boluses should be restricted to 500 mL rather than the traditional 1000 mL.
Pharmacologic treatment may include vasopressors, inotropic agents, and pulmonary vasodilators. Example medications and dosages recommended by SMFM are summarized in the checklist (FIGURE).5
After the initial phase of recovery, the hemodynamic picture often changes from right heart failure to left heart failure. Management of left heart failure is not covered in the SMFM checklist because, by the time it appears, the patient will usually be in the intensive care unit, managed by the critical care team. Management of left heart failure generally includes diuresis as needed for cardiogenic pulmonary edema, optimization of cardiac preload, and inotropic agents or vasopressors if needed to maintain cardiac output or perfusion pressure.4
Debrief, learning opportunities
Complex emergencies such as AFE are rarely handled 100% perfectly, even those with a good outcome, so they present opportunities for team learning and improvement. The team should conduct a 10- to 15-minute debrief soon after the patient is stabilized. Make an explicit statement that the main goal of the debrief is to gather suggestions as to how systems and processes could be improved for next time, not to find fault or lay blame on individuals. Encourage all personnel involved in the initial management to attend and discuss what went well and what did not. Another goal is to provide support for individuals who may feel traumatized by the dramatic, frightening events surrounding an AFE and by the poor patient outcome or guarded prognosis that frequently follows. Another goal is to discuss the plan for providing support and disclosure to the patient and family.
The vast majority of AFE cases meet criteria to be designated as “sentinel events,” because of patient transfer to the intensive care unit, multi-unit blood transfusion, other severe maternal morbidities, or maternal death. Therefore, most AFE cases will trigger a root cause analysis (RCA) or other formal sentinel event analysis conducted by the hospital’s Safety or Quality Department. As with the immediate post-event debrief, the first goal of the RCA is to identify systems issues that may have resulted in suboptimal care and that can be modified to improve future care. Specific issues regarding the checklist should also be addressed:
- Was the checklist used?
- Was the checklist available?
- Are there items on the checklist that need to be modified, added, or deleted?
The RCA concludes with the development of a performance improvement plan.
Ultimately, we encourage all AFE cases be reported to the registry maintained by the Amniotic Fluid Embolism Foundation at https://www.afesupport.org/, regardless of whether the outcome was favorable for the mother and newborn. The registry includes over 130 AFE cases since 2013 from around the world. Researchers periodically report on the registry findings.10 If providers report cases with both good and bad outcomes, the registry may provide future insights regarding which adjunctive or empiric treatments may or may not be promising.
Continue to: Empiric treatments...
Empiric treatments
From time-to-time, new regimens for empiric treatment of AFE are reported. It is important to recognize that these reports are generally uncontrolled case reports of favorable outcomes and that, without a control group, it is impossible to determine to what extent the treatment contributed to the outcome or was merely incidental. Given the rarity of AFE, it seems unlikely that there will ever be a randomized clinical trial or even a controlled prospective study comparing treatment regimens.
The “A-OK” regimen is an empiric treatment that has garnered some interest after an initial case report.11 It consists of an anticholinergic agent (atropine 0.2 mg IV), a selective 5-HT3 receptor antagonist (ondansetron 8 mg IV), and a nonsteroidal anti-inflammatory drug (ketorolac 15 mg IV). We have some reservations about this regimen, however, because atropine is relatively contraindicated if the patient has tachycardia (which is common in patients with hemorrhage) and ketorolac may suppress platelet function, which might be harmful for patients with DIC or thrombocytopenia.
Another empiric treatment is the “50-50-500” regimen, which includes an H1 antihistamine (diphenhydramine 50 mg IV), an H2 antihistamine (famotidine 50 mg IV), and a corticosteroid (hydrocortisone 500 mg IV). This regimen aims to suppress histamine-mediated and cell-mediated inflammatory responses, based on the notion that proinflammatory responses likely mediate much of the underlying pathophysiology of the AFE syndrome.
We would emphasize that these empiric regimens are not clinically validated, US Food and Drug Administration approved for treatment of AFE, or considered standard of care. Future reports of these and other regimens will be needed to evaluate their efficacy, limitations, and risks. Again, we encourage providers to report all AFE cases to the AFE Foundation registry, regardless of whether the treatments are successful.
CASE Conclusion
The hemorrhage stops after administration of oxytocin, carboprost, 6 units of cryoprecipitate, and a 6-unit platelet pheresis pack. The patient is transferred to the intensive care unit where she eventually requires a total of 10 units of red cells, 8 more units of cryoprecipitate, and another platelet pheresis pack. She is discharged to home in stable condition on postpartum day 4.
Be prepared, have the checklist ready
Because AFE is rare, most members of the health care team will have no prior experience managing a real case. It may have been years or decades since they had any education on AFE or they last read a review article such as this one. It is even possible the anesthesiologist, cardiologist, or critical care specialist has never heard of AFE. Thus if they rely on memory alone, there is substantial risk of forgetting items, getting dosages wrong, or other errors. With this in mind, what is the best way to prepare the team to expeditiously employ the management steps outlined here?
Use of a checklist that summarizes these key steps for early management, such as the SMFM checklist in the FIGURE, will help ensure that all relevant steps are performed in every AFE case. It is designed to be printed on a single sheet of letter-sized paper, and we propose that every labor and delivery (L&D) unit keep laminated copies of this checklist in several places where they will be immediately available should an AFE occur. Copies can be kept on the anesthesia carts in the L&D operating rooms, in an emergency procedures binder on the unit, and on the “crash carts” and hemorrhage supply carts in the L&D unit. Effective implementation of an AFE checklist requires all personnel know where to readily find it and have some familiarity with its contents.
An interdisciplinary team comprising representatives from nursing, obstetrics, and anesthesia should meet to discuss whether the checklist needs to be modified to fit the local hospital formulary or other unique local circumstances. The team should develop an implementation plan that includes where to keep checklist copies, a process to periodically ensure that the copies are still present and readable, a roll-out plan to inform all personnel about the checklist process, and most importantly a training plan that includes incorporating AFE cases into the schedule of multidisciplinary simulations and drills for obstetric emergencies. Other implementation strategies are outlined in the SMFM document.5
Ultimately an organized, systematic approach is recommended for management of AFE. There is no single best treatment of AFE; it is supportive and directed toward the underlying pathophysiology, which may vary from patient to patient. Therefore, although a checklist, in conjunction with regular education and simulation activities, may help optimize care and improve outcomes, there is still a high risk of maternal morbidity and mortality from AFE. ●
- Clark SL. Amniotic fluid embolism. Obstet Gynecol. 2014;123(2 Pt 1):337-348. doi:10.1097/AOG.0000000000000107.
- Funk M, Damron A, Bandi V, et al. Pulmonary vascular obstruction by squamous cells is not involved in amniotic fluid embolism. Am J Obstet Gynecol. 2018;218:460-461. doi:10.1016/j.ajog.2017.12.225.
- Gilmore DA, Wakim J, Secrest J, et al. Anaphylactoid syndrome of pregnancy: a review of the literature with latest management and outcome data. AANA J. 2003;71:120-126.
- Society for Maternal-Fetal Medicine, Pacheco LD, Saade G, et al. Amniotic fluid embolism: diagnosis and management. Am J Obstet Gynecol. 2016;215:B16-24. doi:10.1016/j.ajog.2016.03.012.
- Patient Safety and Quality Committee, Society for Maternal-Fetal Medicine; Combs CA, Montgomery DM, et al. Society for Maternal-Fetal Medicine Special Statement: checklist for initial management of amniotic fluid embolism. Am J Obstet Gynecol. 2021;224:B29-B32. doi:10.1016/j.ajog.2021.01.001.
- Rose CH, Faksh A, Traynor KD, et al. Challenging the 4- to 5-minute rule: from perimortem cesarean to resuscitative hysterotomy. Am J Obstet Gynecol. 2015;213:653-6, 653.e1. doi:10.1016/j.ajog.2015.07.019.
- Pacheco LD, Clark SL, Klassen M, et al. Amniotic fluid embolism: principles of early clinical management. Am J Obstet Gynecol. 2020;222:48-52. doi:10.1016/j.ajog.2019.07.036.
- Combs CA, Einerson BD, Toner LE, SMFM Patient Safety and Quality Committee. SMFM Special Statement: surgical safety checklists for cesarean delivery. Am J Obstet Gynecol. 2021;225:B43-B49. doi:10.1016/j.ajog.2021.07.011.
- SMFM Patient Safety and Quality Committee, Staat B, Combs CA. SMFM Special Statement: operative vaginal delivery: checklists for performance and documentation. Am J Obstet Gynecol. 2020;222:B15-B21. doi:10.1016/j.ajog.2020.02.011.
- Stafford IA, Moaddab A, Dildy GA, et al. Amniotic fluid embolism syndrome: analysis of the United States international registry. Am J Obstet Gynecol MFM. 2020;2:100083. doi:10.1016/j.ajogmf.2019.100083.
- Rezai S, Hughes AZC, Larsen TB, et al. Atypical amniotic f luid embolism managed with a novel therapeutic regimen. Case Rep Obstet Gynecol. 2017; 2017:8458375. doi:10.1155/2017/8458375.
- Clark SL. Amniotic fluid embolism. Obstet Gynecol. 2014;123(2 Pt 1):337-348. doi:10.1097/AOG.0000000000000107.
- Funk M, Damron A, Bandi V, et al. Pulmonary vascular obstruction by squamous cells is not involved in amniotic fluid embolism. Am J Obstet Gynecol. 2018;218:460-461. doi:10.1016/j.ajog.2017.12.225.
- Gilmore DA, Wakim J, Secrest J, et al. Anaphylactoid syndrome of pregnancy: a review of the literature with latest management and outcome data. AANA J. 2003;71:120-126.
- Society for Maternal-Fetal Medicine, Pacheco LD, Saade G, et al. Amniotic fluid embolism: diagnosis and management. Am J Obstet Gynecol. 2016;215:B16-24. doi:10.1016/j.ajog.2016.03.012.
- Patient Safety and Quality Committee, Society for Maternal-Fetal Medicine; Combs CA, Montgomery DM, et al. Society for Maternal-Fetal Medicine Special Statement: checklist for initial management of amniotic fluid embolism. Am J Obstet Gynecol. 2021;224:B29-B32. doi:10.1016/j.ajog.2021.01.001.
- Rose CH, Faksh A, Traynor KD, et al. Challenging the 4- to 5-minute rule: from perimortem cesarean to resuscitative hysterotomy. Am J Obstet Gynecol. 2015;213:653-6, 653.e1. doi:10.1016/j.ajog.2015.07.019.
- Pacheco LD, Clark SL, Klassen M, et al. Amniotic fluid embolism: principles of early clinical management. Am J Obstet Gynecol. 2020;222:48-52. doi:10.1016/j.ajog.2019.07.036.
- Combs CA, Einerson BD, Toner LE, SMFM Patient Safety and Quality Committee. SMFM Special Statement: surgical safety checklists for cesarean delivery. Am J Obstet Gynecol. 2021;225:B43-B49. doi:10.1016/j.ajog.2021.07.011.
- SMFM Patient Safety and Quality Committee, Staat B, Combs CA. SMFM Special Statement: operative vaginal delivery: checklists for performance and documentation. Am J Obstet Gynecol. 2020;222:B15-B21. doi:10.1016/j.ajog.2020.02.011.
- Stafford IA, Moaddab A, Dildy GA, et al. Amniotic fluid embolism syndrome: analysis of the United States international registry. Am J Obstet Gynecol MFM. 2020;2:100083. doi:10.1016/j.ajogmf.2019.100083.
- Rezai S, Hughes AZC, Larsen TB, et al. Atypical amniotic f luid embolism managed with a novel therapeutic regimen. Case Rep Obstet Gynecol. 2017; 2017:8458375. doi:10.1155/2017/8458375.
Orf Virus in Humans: Case Series and Clinical Review
A patient presenting with a hand pustule is a phenomenon encountered worldwide requiring careful history-taking. Some occupations, activities, and various religious practices (eg, Eid al-Adha, Passover, Easter) have been implicated worldwide in orf infection. In the United States, orf virus usually is spread from infected animal hosts to humans. Herein, we review the differential for a single hand pustule, which includes both infectious and noninfectious causes. Recognizing orf virus as the etiology of a cutaneous hand pustule in patients is important, as misdiagnosis can lead to unnecessary invasive testing and/or treatments with suboptimal clinical outcomes.
Case Series
When conducting a search for orf virus cases at our institution (University of Iowa Hospitals and Clinics, Iowa City, Iowa), 5 patient cases were identified.
Patient 1—A 27-year-old otherwise healthy woman presented to clinic with a tender red bump on the right ring finger that had been slowly growing over the course of 2 weeks and had recently started to bleed. A social history revealed that she owned several goats, which she frequently milked; 1 of the goats had a cyst on the mouth, which she popped approximately 1 to 2 weeks prior to the appearance of the lesion on the finger. She also endorsed that she owned several cattle and various other animals with which she had frequent contact. A biopsy was obtained with features consistent with orf virus.
Patient 2—A 33-year-old man presented to clinic with a lesion of concern on the left index finger. Several days prior to presentation, the patient had visited the emergency department for swelling and erythema of the same finger after cutting himself with a knife while preparing sheep meat. Radiographs were normal, and the patient was referred to dermatology. In clinic, there was a 0.5-cm fluctuant mass on the distal interphalangeal joint of the third finger. The patient declined a biopsy, and the lesion healed over 4 to 6 weeks without complication.
Patient 3—A 38-year-old man presented to clinic with 2 painless, large, round nodules on the right proximal index finger, with open friable centers noted on physical examination (Figure 1). The patient reported cutting the finger while preparing sheep meat several days prior. The nodules had been present for a few weeks and continued to grow. A punch biopsy revealed evidence of parapoxvirus infection consistent with a diagnosis of orf.
Patient 4—A 48-year-old man was referred to our dermatology clinic for evaluation of a bleeding lesion on the left middle finger. Physical examination revealed an exophytic, friable, ulcerated nodule on the dorsal aspect of the left middle finger (Figure 2). Upon further questioning, the patient mentioned that he handled raw lamb meat after cutting the finger. A punch biopsy was obtained and was consistent with orf virus infection.
Patient 5—A 43-year-old woman presented to clinic with a chronic wound on the mid lower back that was noted to drain and crust over. She thought the lesion was improving, but it had become painful over the last few weeks. A shave biopsy of the lesion was consistent with orf virus. At follow-up, the patient was unable to identify any recent contact with animals.
Comment
Transmission From Animals to Humans—Orf virus is a member of the Parapoxvirus genus of the Poxviridae family.1 This virus is highly contagious among animals and has been described around the globe. The resulting disease also is known as contagious pustular dermatitis,2 soremuzzle,3 ecthyma contagiosum of sheep,4 and scabby mouth.5 This virus most commonly infects young lambs and manifests as raw to crusty papules, pustules, or vesicles around the mouth and nose of the animal.4 Additional signs include excessive salivation and weight loss or starvation from the inability to suckle because of the lesions.5 Although ecthyma contagiosum infection of sheep and goats has been well known for centuries, human infection was first reported in the literature in 1934.6
Transmission of orf to humans can occur when direct contact with an infected animal exhibiting active lesions occurs.7 Orf virus also can be transmitted through fomites (eg, from knives, wool, buildings, equipment) that previously were in contact with infected animals, making it relevant to ask all farmers about any animals with pustules around the mouth, nose, udders, or other commonly affected areas. Although sanitation efforts are important for prevention, orf virus is hardy, and fomites can remain on surfaces for many months.8 Transmission among animals and from animals to humans frequently occurs; however, human-to-human transmission is less common.9 Ecthyma contagiosum is considered an occupational hazard, with the disease being most prevalent in shepherds, veterinarians, and butchers.1,8 Disease prevalence in these occupations has been reported to be as high as 50%.10 Infections also are seen in patients who attend petting zoos or who slaughter goats and sheep for cultural practices.8
Clinical Characteristics in Humans—The clinical diagnosis of orf is dependent on taking a thorough patient history that includes social, occupational, and religious activities. Development of a nodule or papule on a patient’s hand with recent exposure to fomites or direct contact with a goat or sheep up to 1 week prior is extremely suggestive of an orf virus infection.
Clinically, orf most often begins as an individual papule or nodule on the dorsal surface of the patient’s finger or hand and ranges from completely asymptomatic to pruritic or even painful.1,8 Depending on how the infection was inoculated, lesions can vary in size and number. Other sites that have been reported less frequently include the genitals, legs, axillae, and head.11,12 Lesions are roughly 1 cm in diameter but can vary in size. Ecthyma contagiosum is not a static disease but changes in appearance over the course of infection. Typically, lesions will appear 3 to 7 days after inoculation with the orf virus and will self-resolve 6 to 8 weeks later.
Orf lesions have been described to progress through 6 distinct phases before resolving: maculopapular (erythematous macule or papule forms), targetoid (formation of a necrotic center with red outer halo), acute (lesion begins to weep), regenerative (lesion becomes dry), papilloma (dry crust becomes papillomatous), and regression (skin returns to normal appearance).1,8,9 Each phase of ecthyma contagiosum is unique and will last up to 1 week before progressing. Because of this prolonged clinical course, patients can present at any stage.
Reports of systemic symptoms are uncommon but can include lymphadenopathy, fever, and malaise.13 Although the disease course in immunocompetent individuals is quite mild, immunocompromised patients may experience persistent orf lesions that are painful and can be much larger, with reports of several centimeters in diameter.14
Dermatopathology and Molecular Studies—When a clinical diagnosis is not possible, biopsy or molecular studies can be helpful.8 Histopathology can vary depending on the phase of the lesion. Early stages are characterized by spongiform degeneration of the epidermis with variable vesiculation of the superficial epidermis and eosinophilic cytoplasmic inclusion bodies of keratinocytes (Figure 3). Later stages demonstrate full-thickness necrosis with epidermal balloon degeneration and dense inflammation of the dermis with edema and extravasated erythrocytes from dilated blood vessels. Both early- and late-stage disease commonly show characteristic elongated thin rete ridges.8
Molecular studies are another reliable method for diagnosis, though these are not always readily available. Polymerase chain reaction can be used for sensitive and rapid diagnosis.15 Less commonly, electron microscopy, Western blot, or enzyme-linked immunosorbent assays are used.16 Laboratory studies, such as complete blood cell count with differential, erythrocyte sedimentation rate, and C-reactive protein, often are unnecessary but may be helpful in ruling out other infectious causes. Tissue culture can be considered if bacterial, fungal, or acid-fast bacilli are in the differential; however, no growth will be seen in the case of orf viral infection.
Differential Diagnosis—The differential diagnosis for patients presenting with a large pustule on the hand or fingers can depend on geographic location, as the potential etiology may vary widely around the world. Several zoonotic viral infections other than orf can present with pustular lesions on the hands (Table).17-24
Clinically, infection with these named viruses can be hard to distinguish; however, appropriate social history or polymerase chain reaction can be obtained to differentiate them. Other infectious entities include herpetic whitlow, giant molluscum, and anthrax (eTable).24-26 Biopsy of the lesion with bacterial tissue culture may lead to definitive diagnosis.26
Treatment—Because of the self-resolving nature of orf, treatment usually is not needed in immunocompetent patients with a solitary lesion. However, wound care is essential to prevent secondary infections of the lesion. If secondarily infected, topical or oral antibiotics may be prescribed. Immunocompromised individuals are at increased risk for developing large persistent lesions and sometimes require intervention for successful treatment. Several successful treatment methods have been described and include intralesional interferon injections, electrocautery, topical imiquimod, topical cidofovir, and cryotherapy.8,14,27-30 Infections that continue to be refractory to less-invasive treatment can be considered for wide local excision; however, recurrence is possible.8 Vaccinations are available for animals to prevent the spread of infection in the flock, but there are no formulations of vaccines for human use. Prevention of spread to humans can be done through animal vaccination, careful handling of animal products while wearing nonporous gloves, and proper sanitation techniques.
Complications—Orf has an excellent long-term prognosis in immunocompetent patients, as the virus is epitheliotropic, and inoculation does not lead to viremia.2 Although lesions typically are asymptomatic in most patients, complications can occur, especially in immunosuppressed individuals. These complications include systemic symptoms, giant persistent lesions prone to infection or scarring, erysipelas, lymphadenitis, and erythema multiforme.8,31 Common systemic symptoms of ecthyma contagiosum include fever, fatigue, and myalgia. Lymphadenitis can occur along with local swelling and lymphatic streaking. Although erythema multiforme is a rare complication occurring after initial ecthyma contagiosum infection, this hypersensitivity reaction is postulated to be in response to the immunologic clearing of the orf virus.32,33 Patients receiving systemic immunosuppressive medications are at an increased risk of developing complications from infection and may even be required to pause systemic treatment for complete resolution of orf lesions.34 Other cutaneous diseases that decrease the skin’s barrier protection, such as bullous pemphigoid or eczema, also can place patients at an increased risk for complications.35 Although human-to-human orf virus transmission is exceptionally rare, there is a case report of this phenomenon in immunosuppressed patients residing in a burn unit.36 Transplant recipients on immunosuppressive medications also can experience orf lesions with exaggerated presentations that continue to grow up to several centimeters in diameter.31 Long-term prognosis is still good in these patients with appropriate disease recognition and treatment. Reinfection is not uncommon with repeated exposure to the source, but lesions are less severe and resolve faster than with initial infection.1,8
Conclusion
The contagious hand pustule caused by orf virus is a distinct clinical entity that is prevalent worldwide and requires thorough evaluation of the clinical course of the lesion and the patient’s social history. Several zoonotic viral infections have been implicated in this presentation. Although biopsy and molecular studies can be helpful, the expert diagnostician can make a clinical diagnosis with careful attention to social history, geographic location, and cultural practices.
- Haig DM, Mercer AA. Ovine diseases. orf. Vet Res. 1998;29:311-326.
- Glover RE. Contagious pustular dermatitis of the sheep. J Comp Pathol Ther. 1928;41:318-340.
- Hardy WT, Price DA. Soremuzzle of sheep.
J Am Vet Med Assoc. 1952;120:23-25. - Boughton IB, Hardy WT. Contagious ecthyma (sore mouth) of sheep and goats. J Am Vet Med Assoc. 1934;85:150-178.
- Gardiner MR, Craig VMD, Nairn ME. An unusual outbreak of contagious ecthyma (scabby mouth) in sheep. Aust Vet J. 1967;43:163-165.
- Newsome IE, Cross F. Sore mouth in sheep transmissible to man. J Am Vet Med Assoc. 1934;84:790-802.
- Demiraslan H, Dinc G, Doganay M. An overview of orf virus infection in humans and animals. Recent Pat Anti Infect Drug Discov. 2017;12:21-30.
- Bergqvist C, Kurban M, Abbas O. Orf virus infection. Rev Med Virol. 2017;27:E1932.
- Duchateau NC, Aerts O, Lambert J. Autoinoculation with orf virus (ecthyma contagiosum). Int J Dermatol. 2014;53:E60-E62.
- Paiba GA, Thomas DR, Morgan KL, et al. Orf (contagious pustular dermatitis) in farmworkers: prevalence and risk factors in three areas of England. Vet Rec. 1999;145:7-11
- Kandemir H, Ciftcioglu MA, Yilmaz E. Genital orf. Eur J Dermatol. 2008;18:460-461.
- Weide B, Metzler G, Eigentler TK, et al. Inflammatory nodules around the axilla: an uncommon localization of orf virus infection. Clin Exp Dermatol. 2009;34:240-242.
- Wilkinson JD. Orf: a family with unusual complications. Br J Dermatol. 1977;97:447-450.
- Zaharia D, Kanitakis J, Pouteil-Noble C, et al. Rapidly growing orf in a renal transplant recipient: favourable outcome with reduction of immunosuppression and imiquimod. Transpl Int. 2010;23:E62-E64.
- Bora DP, Venkatesan G, Bhanuprakash V, et al. TaqMan real-time PCR assay based on DNA polymerase gene for rapid detection of orf infection. J Virol Methods. 2011;178:249-252.
- Töndury B, Kühne A, Kutzner H, et al. Molecular diagnostics of parapox virus infections. J Dtsch Dermatol Ges. 2010;8:681-684.
- Handler NS, Handler MZ, Rubins A, et al. Milker’s nodule: an occupational infection and threat to the immunocompromised. J Eur Acad Dermatol Venereol. 2018;32:537-541.
- Groves RW, Wilson-Jones E, MacDonald DM. Human orf and milkers’ nodule: a clinicopathologic study. J Am Acad Dermatol. 1991;25:706-711.
- Bowman KF, Barbery RT, Swango LJ, et al. Cutaneous form of bovine papular stomatitis in man. JAMA. 1981;246;1813-1818.
- Nagington J, Lauder IM, Smith JS. Bovine papular stomatitis, pseudocowpox and milker’s nodules. Vet Rec. 1967;79:306-313.
- Clark C, McIntyre PG, Evans A, et al. Human sealpox resulting from a seal bite: confirmation that sealpox virus is zoonotic. Br J Dermatol. 2005;152:791-793.
- Downie AW, Espana C. A comparative study of tanapox and yaba viruses. J Gen Virol. 1973;19:37-49.
- Zimmermann P, Thordsen I, Frangoulidis D, et al. Real-time PCR assay for the detection of tanapox virus and yaba-like disease virus. J Virol Methods. 2005;130:149-153.
- Bolognia J, Schaffer J, Cerroni L. Dermatology. 4th ed. Elsevier Saunders; 2018.
- Wenner KA, Kenner JR. Anthrax. Dermatol Clin. 2004;22:247-256.
- Brachman P, Kaufmann A. Anthrax. In: Evans A, Brachman P, eds. Bacterial Infections of Humans: Epidemiology and Control. 3rd ed. Plenum Publishing; 1998:95.
- Ran M, Lee M, Gong J, et al. Oral acyclovir and intralesional interferon injections for treatment of giant pyogenic granuloma-like lesions in an immunocompromised patient with human orf. JAMA Dermatol. 2015;151:1032-1034.
- Degraeve C, De Coninck A, Senneseael J, et al. Recurrent contagious ecthyma (orf) in an immunocompromised host successfully treated with cryotherapy. Dermatology. 1999;198:162-163.
- Geerinck K, Lukito G, Snoeck R, et al. A case of human orf in an immunocompromised patient treated successfully with cidofovir cream. J Med Virol. 2001;64:543-549.
- Ertekin S, Gurel M, Erdemir A, et al. Systemic interferon alfa injections for the treatment of a giant orf. Cutis. 2017;99:E19-E21.
- Hunskaar S. Giant orf in a patient with chronic lymphocytic leukaemia. Br J Dermatol. 1986;114:631-634.
- Ozturk P, Sayar H, Karakas T, et al. Erythema multiforme as a result of orf disease. Acta Dermatovenereol Alp Pannonica Adriat. 2012;21:45-46.
- Shahmoradi Z, Abtahi-Naeini B, Pourazizi M, et al. Orf disease following ‘eid ul-adha’: a rare cause of erythema multiforme. Int J Prev Med. 2014;5:912-914.
- Kostopoulos M, Gerodimos C, Batsila E, et al. Orf disease in a patient with rheumatoid arthritis. Mediterr J Rheumatol. 2018;29:89-91.
- Murphy JK, Ralphs IG. Bullous pemphigoid complicating human orf. Br J Dermatol. 1996;134:929-930.
- Midilli K, Erkiliç A, Kus¸kucu M, et al. Nosocomial outbreak of disseminated orf infection in a burn unit, Gaziantep, Turkey, October to December 2012. Euro Surveill. 2013;18:20425.
A patient presenting with a hand pustule is a phenomenon encountered worldwide requiring careful history-taking. Some occupations, activities, and various religious practices (eg, Eid al-Adha, Passover, Easter) have been implicated worldwide in orf infection. In the United States, orf virus usually is spread from infected animal hosts to humans. Herein, we review the differential for a single hand pustule, which includes both infectious and noninfectious causes. Recognizing orf virus as the etiology of a cutaneous hand pustule in patients is important, as misdiagnosis can lead to unnecessary invasive testing and/or treatments with suboptimal clinical outcomes.
Case Series
When conducting a search for orf virus cases at our institution (University of Iowa Hospitals and Clinics, Iowa City, Iowa), 5 patient cases were identified.
Patient 1—A 27-year-old otherwise healthy woman presented to clinic with a tender red bump on the right ring finger that had been slowly growing over the course of 2 weeks and had recently started to bleed. A social history revealed that she owned several goats, which she frequently milked; 1 of the goats had a cyst on the mouth, which she popped approximately 1 to 2 weeks prior to the appearance of the lesion on the finger. She also endorsed that she owned several cattle and various other animals with which she had frequent contact. A biopsy was obtained with features consistent with orf virus.
Patient 2—A 33-year-old man presented to clinic with a lesion of concern on the left index finger. Several days prior to presentation, the patient had visited the emergency department for swelling and erythema of the same finger after cutting himself with a knife while preparing sheep meat. Radiographs were normal, and the patient was referred to dermatology. In clinic, there was a 0.5-cm fluctuant mass on the distal interphalangeal joint of the third finger. The patient declined a biopsy, and the lesion healed over 4 to 6 weeks without complication.
Patient 3—A 38-year-old man presented to clinic with 2 painless, large, round nodules on the right proximal index finger, with open friable centers noted on physical examination (Figure 1). The patient reported cutting the finger while preparing sheep meat several days prior. The nodules had been present for a few weeks and continued to grow. A punch biopsy revealed evidence of parapoxvirus infection consistent with a diagnosis of orf.
Patient 4—A 48-year-old man was referred to our dermatology clinic for evaluation of a bleeding lesion on the left middle finger. Physical examination revealed an exophytic, friable, ulcerated nodule on the dorsal aspect of the left middle finger (Figure 2). Upon further questioning, the patient mentioned that he handled raw lamb meat after cutting the finger. A punch biopsy was obtained and was consistent with orf virus infection.
Patient 5—A 43-year-old woman presented to clinic with a chronic wound on the mid lower back that was noted to drain and crust over. She thought the lesion was improving, but it had become painful over the last few weeks. A shave biopsy of the lesion was consistent with orf virus. At follow-up, the patient was unable to identify any recent contact with animals.
Comment
Transmission From Animals to Humans—Orf virus is a member of the Parapoxvirus genus of the Poxviridae family.1 This virus is highly contagious among animals and has been described around the globe. The resulting disease also is known as contagious pustular dermatitis,2 soremuzzle,3 ecthyma contagiosum of sheep,4 and scabby mouth.5 This virus most commonly infects young lambs and manifests as raw to crusty papules, pustules, or vesicles around the mouth and nose of the animal.4 Additional signs include excessive salivation and weight loss or starvation from the inability to suckle because of the lesions.5 Although ecthyma contagiosum infection of sheep and goats has been well known for centuries, human infection was first reported in the literature in 1934.6
Transmission of orf to humans can occur when direct contact with an infected animal exhibiting active lesions occurs.7 Orf virus also can be transmitted through fomites (eg, from knives, wool, buildings, equipment) that previously were in contact with infected animals, making it relevant to ask all farmers about any animals with pustules around the mouth, nose, udders, or other commonly affected areas. Although sanitation efforts are important for prevention, orf virus is hardy, and fomites can remain on surfaces for many months.8 Transmission among animals and from animals to humans frequently occurs; however, human-to-human transmission is less common.9 Ecthyma contagiosum is considered an occupational hazard, with the disease being most prevalent in shepherds, veterinarians, and butchers.1,8 Disease prevalence in these occupations has been reported to be as high as 50%.10 Infections also are seen in patients who attend petting zoos or who slaughter goats and sheep for cultural practices.8
Clinical Characteristics in Humans—The clinical diagnosis of orf is dependent on taking a thorough patient history that includes social, occupational, and religious activities. Development of a nodule or papule on a patient’s hand with recent exposure to fomites or direct contact with a goat or sheep up to 1 week prior is extremely suggestive of an orf virus infection.
Clinically, orf most often begins as an individual papule or nodule on the dorsal surface of the patient’s finger or hand and ranges from completely asymptomatic to pruritic or even painful.1,8 Depending on how the infection was inoculated, lesions can vary in size and number. Other sites that have been reported less frequently include the genitals, legs, axillae, and head.11,12 Lesions are roughly 1 cm in diameter but can vary in size. Ecthyma contagiosum is not a static disease but changes in appearance over the course of infection. Typically, lesions will appear 3 to 7 days after inoculation with the orf virus and will self-resolve 6 to 8 weeks later.
Orf lesions have been described to progress through 6 distinct phases before resolving: maculopapular (erythematous macule or papule forms), targetoid (formation of a necrotic center with red outer halo), acute (lesion begins to weep), regenerative (lesion becomes dry), papilloma (dry crust becomes papillomatous), and regression (skin returns to normal appearance).1,8,9 Each phase of ecthyma contagiosum is unique and will last up to 1 week before progressing. Because of this prolonged clinical course, patients can present at any stage.
Reports of systemic symptoms are uncommon but can include lymphadenopathy, fever, and malaise.13 Although the disease course in immunocompetent individuals is quite mild, immunocompromised patients may experience persistent orf lesions that are painful and can be much larger, with reports of several centimeters in diameter.14
Dermatopathology and Molecular Studies—When a clinical diagnosis is not possible, biopsy or molecular studies can be helpful.8 Histopathology can vary depending on the phase of the lesion. Early stages are characterized by spongiform degeneration of the epidermis with variable vesiculation of the superficial epidermis and eosinophilic cytoplasmic inclusion bodies of keratinocytes (Figure 3). Later stages demonstrate full-thickness necrosis with epidermal balloon degeneration and dense inflammation of the dermis with edema and extravasated erythrocytes from dilated blood vessels. Both early- and late-stage disease commonly show characteristic elongated thin rete ridges.8
Molecular studies are another reliable method for diagnosis, though these are not always readily available. Polymerase chain reaction can be used for sensitive and rapid diagnosis.15 Less commonly, electron microscopy, Western blot, or enzyme-linked immunosorbent assays are used.16 Laboratory studies, such as complete blood cell count with differential, erythrocyte sedimentation rate, and C-reactive protein, often are unnecessary but may be helpful in ruling out other infectious causes. Tissue culture can be considered if bacterial, fungal, or acid-fast bacilli are in the differential; however, no growth will be seen in the case of orf viral infection.
Differential Diagnosis—The differential diagnosis for patients presenting with a large pustule on the hand or fingers can depend on geographic location, as the potential etiology may vary widely around the world. Several zoonotic viral infections other than orf can present with pustular lesions on the hands (Table).17-24
Clinically, infection with these named viruses can be hard to distinguish; however, appropriate social history or polymerase chain reaction can be obtained to differentiate them. Other infectious entities include herpetic whitlow, giant molluscum, and anthrax (eTable).24-26 Biopsy of the lesion with bacterial tissue culture may lead to definitive diagnosis.26
Treatment—Because of the self-resolving nature of orf, treatment usually is not needed in immunocompetent patients with a solitary lesion. However, wound care is essential to prevent secondary infections of the lesion. If secondarily infected, topical or oral antibiotics may be prescribed. Immunocompromised individuals are at increased risk for developing large persistent lesions and sometimes require intervention for successful treatment. Several successful treatment methods have been described and include intralesional interferon injections, electrocautery, topical imiquimod, topical cidofovir, and cryotherapy.8,14,27-30 Infections that continue to be refractory to less-invasive treatment can be considered for wide local excision; however, recurrence is possible.8 Vaccinations are available for animals to prevent the spread of infection in the flock, but there are no formulations of vaccines for human use. Prevention of spread to humans can be done through animal vaccination, careful handling of animal products while wearing nonporous gloves, and proper sanitation techniques.
Complications—Orf has an excellent long-term prognosis in immunocompetent patients, as the virus is epitheliotropic, and inoculation does not lead to viremia.2 Although lesions typically are asymptomatic in most patients, complications can occur, especially in immunosuppressed individuals. These complications include systemic symptoms, giant persistent lesions prone to infection or scarring, erysipelas, lymphadenitis, and erythema multiforme.8,31 Common systemic symptoms of ecthyma contagiosum include fever, fatigue, and myalgia. Lymphadenitis can occur along with local swelling and lymphatic streaking. Although erythema multiforme is a rare complication occurring after initial ecthyma contagiosum infection, this hypersensitivity reaction is postulated to be in response to the immunologic clearing of the orf virus.32,33 Patients receiving systemic immunosuppressive medications are at an increased risk of developing complications from infection and may even be required to pause systemic treatment for complete resolution of orf lesions.34 Other cutaneous diseases that decrease the skin’s barrier protection, such as bullous pemphigoid or eczema, also can place patients at an increased risk for complications.35 Although human-to-human orf virus transmission is exceptionally rare, there is a case report of this phenomenon in immunosuppressed patients residing in a burn unit.36 Transplant recipients on immunosuppressive medications also can experience orf lesions with exaggerated presentations that continue to grow up to several centimeters in diameter.31 Long-term prognosis is still good in these patients with appropriate disease recognition and treatment. Reinfection is not uncommon with repeated exposure to the source, but lesions are less severe and resolve faster than with initial infection.1,8
Conclusion
The contagious hand pustule caused by orf virus is a distinct clinical entity that is prevalent worldwide and requires thorough evaluation of the clinical course of the lesion and the patient’s social history. Several zoonotic viral infections have been implicated in this presentation. Although biopsy and molecular studies can be helpful, the expert diagnostician can make a clinical diagnosis with careful attention to social history, geographic location, and cultural practices.
A patient presenting with a hand pustule is a phenomenon encountered worldwide requiring careful history-taking. Some occupations, activities, and various religious practices (eg, Eid al-Adha, Passover, Easter) have been implicated worldwide in orf infection. In the United States, orf virus usually is spread from infected animal hosts to humans. Herein, we review the differential for a single hand pustule, which includes both infectious and noninfectious causes. Recognizing orf virus as the etiology of a cutaneous hand pustule in patients is important, as misdiagnosis can lead to unnecessary invasive testing and/or treatments with suboptimal clinical outcomes.
Case Series
When conducting a search for orf virus cases at our institution (University of Iowa Hospitals and Clinics, Iowa City, Iowa), 5 patient cases were identified.
Patient 1—A 27-year-old otherwise healthy woman presented to clinic with a tender red bump on the right ring finger that had been slowly growing over the course of 2 weeks and had recently started to bleed. A social history revealed that she owned several goats, which she frequently milked; 1 of the goats had a cyst on the mouth, which she popped approximately 1 to 2 weeks prior to the appearance of the lesion on the finger. She also endorsed that she owned several cattle and various other animals with which she had frequent contact. A biopsy was obtained with features consistent with orf virus.
Patient 2—A 33-year-old man presented to clinic with a lesion of concern on the left index finger. Several days prior to presentation, the patient had visited the emergency department for swelling and erythema of the same finger after cutting himself with a knife while preparing sheep meat. Radiographs were normal, and the patient was referred to dermatology. In clinic, there was a 0.5-cm fluctuant mass on the distal interphalangeal joint of the third finger. The patient declined a biopsy, and the lesion healed over 4 to 6 weeks without complication.
Patient 3—A 38-year-old man presented to clinic with 2 painless, large, round nodules on the right proximal index finger, with open friable centers noted on physical examination (Figure 1). The patient reported cutting the finger while preparing sheep meat several days prior. The nodules had been present for a few weeks and continued to grow. A punch biopsy revealed evidence of parapoxvirus infection consistent with a diagnosis of orf.
Patient 4—A 48-year-old man was referred to our dermatology clinic for evaluation of a bleeding lesion on the left middle finger. Physical examination revealed an exophytic, friable, ulcerated nodule on the dorsal aspect of the left middle finger (Figure 2). Upon further questioning, the patient mentioned that he handled raw lamb meat after cutting the finger. A punch biopsy was obtained and was consistent with orf virus infection.
Patient 5—A 43-year-old woman presented to clinic with a chronic wound on the mid lower back that was noted to drain and crust over. She thought the lesion was improving, but it had become painful over the last few weeks. A shave biopsy of the lesion was consistent with orf virus. At follow-up, the patient was unable to identify any recent contact with animals.
Comment
Transmission From Animals to Humans—Orf virus is a member of the Parapoxvirus genus of the Poxviridae family.1 This virus is highly contagious among animals and has been described around the globe. The resulting disease also is known as contagious pustular dermatitis,2 soremuzzle,3 ecthyma contagiosum of sheep,4 and scabby mouth.5 This virus most commonly infects young lambs and manifests as raw to crusty papules, pustules, or vesicles around the mouth and nose of the animal.4 Additional signs include excessive salivation and weight loss or starvation from the inability to suckle because of the lesions.5 Although ecthyma contagiosum infection of sheep and goats has been well known for centuries, human infection was first reported in the literature in 1934.6
Transmission of orf to humans can occur when direct contact with an infected animal exhibiting active lesions occurs.7 Orf virus also can be transmitted through fomites (eg, from knives, wool, buildings, equipment) that previously were in contact with infected animals, making it relevant to ask all farmers about any animals with pustules around the mouth, nose, udders, or other commonly affected areas. Although sanitation efforts are important for prevention, orf virus is hardy, and fomites can remain on surfaces for many months.8 Transmission among animals and from animals to humans frequently occurs; however, human-to-human transmission is less common.9 Ecthyma contagiosum is considered an occupational hazard, with the disease being most prevalent in shepherds, veterinarians, and butchers.1,8 Disease prevalence in these occupations has been reported to be as high as 50%.10 Infections also are seen in patients who attend petting zoos or who slaughter goats and sheep for cultural practices.8
Clinical Characteristics in Humans—The clinical diagnosis of orf is dependent on taking a thorough patient history that includes social, occupational, and religious activities. Development of a nodule or papule on a patient’s hand with recent exposure to fomites or direct contact with a goat or sheep up to 1 week prior is extremely suggestive of an orf virus infection.
Clinically, orf most often begins as an individual papule or nodule on the dorsal surface of the patient’s finger or hand and ranges from completely asymptomatic to pruritic or even painful.1,8 Depending on how the infection was inoculated, lesions can vary in size and number. Other sites that have been reported less frequently include the genitals, legs, axillae, and head.11,12 Lesions are roughly 1 cm in diameter but can vary in size. Ecthyma contagiosum is not a static disease but changes in appearance over the course of infection. Typically, lesions will appear 3 to 7 days after inoculation with the orf virus and will self-resolve 6 to 8 weeks later.
Orf lesions have been described to progress through 6 distinct phases before resolving: maculopapular (erythematous macule or papule forms), targetoid (formation of a necrotic center with red outer halo), acute (lesion begins to weep), regenerative (lesion becomes dry), papilloma (dry crust becomes papillomatous), and regression (skin returns to normal appearance).1,8,9 Each phase of ecthyma contagiosum is unique and will last up to 1 week before progressing. Because of this prolonged clinical course, patients can present at any stage.
Reports of systemic symptoms are uncommon but can include lymphadenopathy, fever, and malaise.13 Although the disease course in immunocompetent individuals is quite mild, immunocompromised patients may experience persistent orf lesions that are painful and can be much larger, with reports of several centimeters in diameter.14
Dermatopathology and Molecular Studies—When a clinical diagnosis is not possible, biopsy or molecular studies can be helpful.8 Histopathology can vary depending on the phase of the lesion. Early stages are characterized by spongiform degeneration of the epidermis with variable vesiculation of the superficial epidermis and eosinophilic cytoplasmic inclusion bodies of keratinocytes (Figure 3). Later stages demonstrate full-thickness necrosis with epidermal balloon degeneration and dense inflammation of the dermis with edema and extravasated erythrocytes from dilated blood vessels. Both early- and late-stage disease commonly show characteristic elongated thin rete ridges.8
Molecular studies are another reliable method for diagnosis, though these are not always readily available. Polymerase chain reaction can be used for sensitive and rapid diagnosis.15 Less commonly, electron microscopy, Western blot, or enzyme-linked immunosorbent assays are used.16 Laboratory studies, such as complete blood cell count with differential, erythrocyte sedimentation rate, and C-reactive protein, often are unnecessary but may be helpful in ruling out other infectious causes. Tissue culture can be considered if bacterial, fungal, or acid-fast bacilli are in the differential; however, no growth will be seen in the case of orf viral infection.
Differential Diagnosis—The differential diagnosis for patients presenting with a large pustule on the hand or fingers can depend on geographic location, as the potential etiology may vary widely around the world. Several zoonotic viral infections other than orf can present with pustular lesions on the hands (Table).17-24
Clinically, infection with these named viruses can be hard to distinguish; however, appropriate social history or polymerase chain reaction can be obtained to differentiate them. Other infectious entities include herpetic whitlow, giant molluscum, and anthrax (eTable).24-26 Biopsy of the lesion with bacterial tissue culture may lead to definitive diagnosis.26
Treatment—Because of the self-resolving nature of orf, treatment usually is not needed in immunocompetent patients with a solitary lesion. However, wound care is essential to prevent secondary infections of the lesion. If secondarily infected, topical or oral antibiotics may be prescribed. Immunocompromised individuals are at increased risk for developing large persistent lesions and sometimes require intervention for successful treatment. Several successful treatment methods have been described and include intralesional interferon injections, electrocautery, topical imiquimod, topical cidofovir, and cryotherapy.8,14,27-30 Infections that continue to be refractory to less-invasive treatment can be considered for wide local excision; however, recurrence is possible.8 Vaccinations are available for animals to prevent the spread of infection in the flock, but there are no formulations of vaccines for human use. Prevention of spread to humans can be done through animal vaccination, careful handling of animal products while wearing nonporous gloves, and proper sanitation techniques.
Complications—Orf has an excellent long-term prognosis in immunocompetent patients, as the virus is epitheliotropic, and inoculation does not lead to viremia.2 Although lesions typically are asymptomatic in most patients, complications can occur, especially in immunosuppressed individuals. These complications include systemic symptoms, giant persistent lesions prone to infection or scarring, erysipelas, lymphadenitis, and erythema multiforme.8,31 Common systemic symptoms of ecthyma contagiosum include fever, fatigue, and myalgia. Lymphadenitis can occur along with local swelling and lymphatic streaking. Although erythema multiforme is a rare complication occurring after initial ecthyma contagiosum infection, this hypersensitivity reaction is postulated to be in response to the immunologic clearing of the orf virus.32,33 Patients receiving systemic immunosuppressive medications are at an increased risk of developing complications from infection and may even be required to pause systemic treatment for complete resolution of orf lesions.34 Other cutaneous diseases that decrease the skin’s barrier protection, such as bullous pemphigoid or eczema, also can place patients at an increased risk for complications.35 Although human-to-human orf virus transmission is exceptionally rare, there is a case report of this phenomenon in immunosuppressed patients residing in a burn unit.36 Transplant recipients on immunosuppressive medications also can experience orf lesions with exaggerated presentations that continue to grow up to several centimeters in diameter.31 Long-term prognosis is still good in these patients with appropriate disease recognition and treatment. Reinfection is not uncommon with repeated exposure to the source, but lesions are less severe and resolve faster than with initial infection.1,8
Conclusion
The contagious hand pustule caused by orf virus is a distinct clinical entity that is prevalent worldwide and requires thorough evaluation of the clinical course of the lesion and the patient’s social history. Several zoonotic viral infections have been implicated in this presentation. Although biopsy and molecular studies can be helpful, the expert diagnostician can make a clinical diagnosis with careful attention to social history, geographic location, and cultural practices.
- Haig DM, Mercer AA. Ovine diseases. orf. Vet Res. 1998;29:311-326.
- Glover RE. Contagious pustular dermatitis of the sheep. J Comp Pathol Ther. 1928;41:318-340.
- Hardy WT, Price DA. Soremuzzle of sheep.
J Am Vet Med Assoc. 1952;120:23-25. - Boughton IB, Hardy WT. Contagious ecthyma (sore mouth) of sheep and goats. J Am Vet Med Assoc. 1934;85:150-178.
- Gardiner MR, Craig VMD, Nairn ME. An unusual outbreak of contagious ecthyma (scabby mouth) in sheep. Aust Vet J. 1967;43:163-165.
- Newsome IE, Cross F. Sore mouth in sheep transmissible to man. J Am Vet Med Assoc. 1934;84:790-802.
- Demiraslan H, Dinc G, Doganay M. An overview of orf virus infection in humans and animals. Recent Pat Anti Infect Drug Discov. 2017;12:21-30.
- Bergqvist C, Kurban M, Abbas O. Orf virus infection. Rev Med Virol. 2017;27:E1932.
- Duchateau NC, Aerts O, Lambert J. Autoinoculation with orf virus (ecthyma contagiosum). Int J Dermatol. 2014;53:E60-E62.
- Paiba GA, Thomas DR, Morgan KL, et al. Orf (contagious pustular dermatitis) in farmworkers: prevalence and risk factors in three areas of England. Vet Rec. 1999;145:7-11
- Kandemir H, Ciftcioglu MA, Yilmaz E. Genital orf. Eur J Dermatol. 2008;18:460-461.
- Weide B, Metzler G, Eigentler TK, et al. Inflammatory nodules around the axilla: an uncommon localization of orf virus infection. Clin Exp Dermatol. 2009;34:240-242.
- Wilkinson JD. Orf: a family with unusual complications. Br J Dermatol. 1977;97:447-450.
- Zaharia D, Kanitakis J, Pouteil-Noble C, et al. Rapidly growing orf in a renal transplant recipient: favourable outcome with reduction of immunosuppression and imiquimod. Transpl Int. 2010;23:E62-E64.
- Bora DP, Venkatesan G, Bhanuprakash V, et al. TaqMan real-time PCR assay based on DNA polymerase gene for rapid detection of orf infection. J Virol Methods. 2011;178:249-252.
- Töndury B, Kühne A, Kutzner H, et al. Molecular diagnostics of parapox virus infections. J Dtsch Dermatol Ges. 2010;8:681-684.
- Handler NS, Handler MZ, Rubins A, et al. Milker’s nodule: an occupational infection and threat to the immunocompromised. J Eur Acad Dermatol Venereol. 2018;32:537-541.
- Groves RW, Wilson-Jones E, MacDonald DM. Human orf and milkers’ nodule: a clinicopathologic study. J Am Acad Dermatol. 1991;25:706-711.
- Bowman KF, Barbery RT, Swango LJ, et al. Cutaneous form of bovine papular stomatitis in man. JAMA. 1981;246;1813-1818.
- Nagington J, Lauder IM, Smith JS. Bovine papular stomatitis, pseudocowpox and milker’s nodules. Vet Rec. 1967;79:306-313.
- Clark C, McIntyre PG, Evans A, et al. Human sealpox resulting from a seal bite: confirmation that sealpox virus is zoonotic. Br J Dermatol. 2005;152:791-793.
- Downie AW, Espana C. A comparative study of tanapox and yaba viruses. J Gen Virol. 1973;19:37-49.
- Zimmermann P, Thordsen I, Frangoulidis D, et al. Real-time PCR assay for the detection of tanapox virus and yaba-like disease virus. J Virol Methods. 2005;130:149-153.
- Bolognia J, Schaffer J, Cerroni L. Dermatology. 4th ed. Elsevier Saunders; 2018.
- Wenner KA, Kenner JR. Anthrax. Dermatol Clin. 2004;22:247-256.
- Brachman P, Kaufmann A. Anthrax. In: Evans A, Brachman P, eds. Bacterial Infections of Humans: Epidemiology and Control. 3rd ed. Plenum Publishing; 1998:95.
- Ran M, Lee M, Gong J, et al. Oral acyclovir and intralesional interferon injections for treatment of giant pyogenic granuloma-like lesions in an immunocompromised patient with human orf. JAMA Dermatol. 2015;151:1032-1034.
- Degraeve C, De Coninck A, Senneseael J, et al. Recurrent contagious ecthyma (orf) in an immunocompromised host successfully treated with cryotherapy. Dermatology. 1999;198:162-163.
- Geerinck K, Lukito G, Snoeck R, et al. A case of human orf in an immunocompromised patient treated successfully with cidofovir cream. J Med Virol. 2001;64:543-549.
- Ertekin S, Gurel M, Erdemir A, et al. Systemic interferon alfa injections for the treatment of a giant orf. Cutis. 2017;99:E19-E21.
- Hunskaar S. Giant orf in a patient with chronic lymphocytic leukaemia. Br J Dermatol. 1986;114:631-634.
- Ozturk P, Sayar H, Karakas T, et al. Erythema multiforme as a result of orf disease. Acta Dermatovenereol Alp Pannonica Adriat. 2012;21:45-46.
- Shahmoradi Z, Abtahi-Naeini B, Pourazizi M, et al. Orf disease following ‘eid ul-adha’: a rare cause of erythema multiforme. Int J Prev Med. 2014;5:912-914.
- Kostopoulos M, Gerodimos C, Batsila E, et al. Orf disease in a patient with rheumatoid arthritis. Mediterr J Rheumatol. 2018;29:89-91.
- Murphy JK, Ralphs IG. Bullous pemphigoid complicating human orf. Br J Dermatol. 1996;134:929-930.
- Midilli K, Erkiliç A, Kus¸kucu M, et al. Nosocomial outbreak of disseminated orf infection in a burn unit, Gaziantep, Turkey, October to December 2012. Euro Surveill. 2013;18:20425.
- Haig DM, Mercer AA. Ovine diseases. orf. Vet Res. 1998;29:311-326.
- Glover RE. Contagious pustular dermatitis of the sheep. J Comp Pathol Ther. 1928;41:318-340.
- Hardy WT, Price DA. Soremuzzle of sheep.
J Am Vet Med Assoc. 1952;120:23-25. - Boughton IB, Hardy WT. Contagious ecthyma (sore mouth) of sheep and goats. J Am Vet Med Assoc. 1934;85:150-178.
- Gardiner MR, Craig VMD, Nairn ME. An unusual outbreak of contagious ecthyma (scabby mouth) in sheep. Aust Vet J. 1967;43:163-165.
- Newsome IE, Cross F. Sore mouth in sheep transmissible to man. J Am Vet Med Assoc. 1934;84:790-802.
- Demiraslan H, Dinc G, Doganay M. An overview of orf virus infection in humans and animals. Recent Pat Anti Infect Drug Discov. 2017;12:21-30.
- Bergqvist C, Kurban M, Abbas O. Orf virus infection. Rev Med Virol. 2017;27:E1932.
- Duchateau NC, Aerts O, Lambert J. Autoinoculation with orf virus (ecthyma contagiosum). Int J Dermatol. 2014;53:E60-E62.
- Paiba GA, Thomas DR, Morgan KL, et al. Orf (contagious pustular dermatitis) in farmworkers: prevalence and risk factors in three areas of England. Vet Rec. 1999;145:7-11
- Kandemir H, Ciftcioglu MA, Yilmaz E. Genital orf. Eur J Dermatol. 2008;18:460-461.
- Weide B, Metzler G, Eigentler TK, et al. Inflammatory nodules around the axilla: an uncommon localization of orf virus infection. Clin Exp Dermatol. 2009;34:240-242.
- Wilkinson JD. Orf: a family with unusual complications. Br J Dermatol. 1977;97:447-450.
- Zaharia D, Kanitakis J, Pouteil-Noble C, et al. Rapidly growing orf in a renal transplant recipient: favourable outcome with reduction of immunosuppression and imiquimod. Transpl Int. 2010;23:E62-E64.
- Bora DP, Venkatesan G, Bhanuprakash V, et al. TaqMan real-time PCR assay based on DNA polymerase gene for rapid detection of orf infection. J Virol Methods. 2011;178:249-252.
- Töndury B, Kühne A, Kutzner H, et al. Molecular diagnostics of parapox virus infections. J Dtsch Dermatol Ges. 2010;8:681-684.
- Handler NS, Handler MZ, Rubins A, et al. Milker’s nodule: an occupational infection and threat to the immunocompromised. J Eur Acad Dermatol Venereol. 2018;32:537-541.
- Groves RW, Wilson-Jones E, MacDonald DM. Human orf and milkers’ nodule: a clinicopathologic study. J Am Acad Dermatol. 1991;25:706-711.
- Bowman KF, Barbery RT, Swango LJ, et al. Cutaneous form of bovine papular stomatitis in man. JAMA. 1981;246;1813-1818.
- Nagington J, Lauder IM, Smith JS. Bovine papular stomatitis, pseudocowpox and milker’s nodules. Vet Rec. 1967;79:306-313.
- Clark C, McIntyre PG, Evans A, et al. Human sealpox resulting from a seal bite: confirmation that sealpox virus is zoonotic. Br J Dermatol. 2005;152:791-793.
- Downie AW, Espana C. A comparative study of tanapox and yaba viruses. J Gen Virol. 1973;19:37-49.
- Zimmermann P, Thordsen I, Frangoulidis D, et al. Real-time PCR assay for the detection of tanapox virus and yaba-like disease virus. J Virol Methods. 2005;130:149-153.
- Bolognia J, Schaffer J, Cerroni L. Dermatology. 4th ed. Elsevier Saunders; 2018.
- Wenner KA, Kenner JR. Anthrax. Dermatol Clin. 2004;22:247-256.
- Brachman P, Kaufmann A. Anthrax. In: Evans A, Brachman P, eds. Bacterial Infections of Humans: Epidemiology and Control. 3rd ed. Plenum Publishing; 1998:95.
- Ran M, Lee M, Gong J, et al. Oral acyclovir and intralesional interferon injections for treatment of giant pyogenic granuloma-like lesions in an immunocompromised patient with human orf. JAMA Dermatol. 2015;151:1032-1034.
- Degraeve C, De Coninck A, Senneseael J, et al. Recurrent contagious ecthyma (orf) in an immunocompromised host successfully treated with cryotherapy. Dermatology. 1999;198:162-163.
- Geerinck K, Lukito G, Snoeck R, et al. A case of human orf in an immunocompromised patient treated successfully with cidofovir cream. J Med Virol. 2001;64:543-549.
- Ertekin S, Gurel M, Erdemir A, et al. Systemic interferon alfa injections for the treatment of a giant orf. Cutis. 2017;99:E19-E21.
- Hunskaar S. Giant orf in a patient with chronic lymphocytic leukaemia. Br J Dermatol. 1986;114:631-634.
- Ozturk P, Sayar H, Karakas T, et al. Erythema multiforme as a result of orf disease. Acta Dermatovenereol Alp Pannonica Adriat. 2012;21:45-46.
- Shahmoradi Z, Abtahi-Naeini B, Pourazizi M, et al. Orf disease following ‘eid ul-adha’: a rare cause of erythema multiforme. Int J Prev Med. 2014;5:912-914.
- Kostopoulos M, Gerodimos C, Batsila E, et al. Orf disease in a patient with rheumatoid arthritis. Mediterr J Rheumatol. 2018;29:89-91.
- Murphy JK, Ralphs IG. Bullous pemphigoid complicating human orf. Br J Dermatol. 1996;134:929-930.
- Midilli K, Erkiliç A, Kus¸kucu M, et al. Nosocomial outbreak of disseminated orf infection in a burn unit, Gaziantep, Turkey, October to December 2012. Euro Surveill. 2013;18:20425.
Practice Points
- Ecthyma contagiosum is a discrete clinical entity that occurs worldwide and demands careful attention to clinical course and social history.
- Ecthyma contagiosum is caused by orf virus, an epitheliotropic zoonotic infection that spreads from ruminants to humans.
- Early and rapid diagnosis of this classic condition is critical to prevent unnecessary biopsies or extensive testing, and determination of etiology can be important in preventing reinfection or spread to other humans by the same infected animal.