User login
Systemic Targeted Treatments for Basal Cell Carcinoma
Basal cell carcinoma (BCC) is the most common keratinocyte carcinoma and affects more than 3 million individuals per year in the United States.1 Approximately 40% of patients diagnosed with BCC will develop another BCC within 5 years of the initial diagnosis.2 Most cases are successfully treated with surgical excision and occasionally topical therapy or radiotherapy. Despite the high cure rate with conventional treatments, BCC can recur and can cause substantial destruction of the surrounding tissue if left untreated.3-5 In some instances, BCC can even metastasize and lead to death.6 For patients who are poor candidates for surgical or topical treatment modalities because of locally advanced BCC (laBCC) or metastatic BCC (mBCC), systemic treatment may be indicated. Vismodegib, sonidegib, and cemiplimab are the only systemic medications approved by the US Food and Drug Administration (FDA) for the treatment of laBCC and/or mBCC. Vismodegib and sonidegib target the sonic hedgehog (SHH) signaling pathway that is abnormally activated in more than 90% of BCCs.7 Cemiplimab is an immune checkpoint inhibitor (ICI) that targets the programmed cell death protein 1 (PD-1) receptor.8 Herein, we review the clinical utility of these medications and their evolving roles in the treatment of BCC.
SHH Pathway Inhibitors
The SHH pathway is a key regulator of cell proliferation and differentiation during embryogenesis.7 During adulthood, SHH signaling decreases but still plays an important role in stem cell activation and in regulation of the hair follicle growth cycle.9,10 However, de novo mutations in the genes that comprise the SHH pathway can result in aberrant constitutive activation, leading to unrestricted cell proliferation. Genetic mutations resulting in activation of Smoothened (SMO), a G-protein–coupled receptor involved in the signal transduction and propagation of the SHH pathway, have been implicated in the pathogenesis of BCC. Inactivating mutations also are commonly observed in patched homolog 1, an upstream cell-surface protein that inhibits SMO.7 The mechanism by which vismodegib and sonidegib, 2 of the FDA-approved oral medications for the treatment of advanced BCC, block the SHH pathway is through the selective inhibition of SMO.7,11
Vismodegib first received FDA approval in 2012 for the treatment of laBCC and mBCC after initial results from the pivotal ERIVANCE phase 2 trial demonstrated an objective response rate (ORR) of 43% (27/63) and 30% (10/33) in patients with locally advanced and metastatic disease, respectively. In this single-arm study, all enrolled patients (63 with laBCC and 33 with mBCC) received 150 mg of oral vismodegib daily.12 Updated results at 39 months demonstrated improved ORRs of 60% (38/63) and 48% (16/33) for the laBCC and mBCC groups, respectively. A complete response (CR) and partial response (PR) were observed in 32% (n=20) and 29% (n=18) of patients with laBCC, respectively.13 These results have been confirmed in subsequent studies, including the large international open-label trial known as STEVIE, with ORRs of 68.5% for 1119 cases of laBCC and 37% for 96 cases of mBCC.14-17 The CR and PR rates were 33% and 35%, respectively, for the laBCC group. The CR and PR rates for the mBCC group were 5% and 32%, respectively.14
The FDA approval of sonidegib for laBCC—but not mBCC—occurred in 2015 after the pivotal BOLT randomized phase 2 trial demonstrated an initial ORR of 43% (18/42) for laBCC and 15% (2/13) for mBCC after administration of 200 mg of sonidegib daily.18 A final follow-up analysis at 42 months resulted in ORRs of 56% (37/66) and 8% (1/13) for the laBCC and mBCC groups, respectively.19 Additionally, improved efficacy was not observed in the 151 patients who were randomized to receive treatment with the higher 800-mg dose; however, they did experience a higher incidence of adverse events.18,19
Currently, the true clinical differences between vismodegib and sonidegib remain uncertain, as no head-to-head trials have been conducted. Moreover, direct comparison of the data from the ERIVANCE and BOLT trials is challenging owing to fundamental differences in methodologic design, including the criteria used to assess BCC severity. The ERIVANCE trial utilized the conventional Response Evaluation Criteria in Solid Tumors (RECIST), while BOLT used the rigorous modified RECIST. However, an expert consensus study attempted to compare the 2 trials by modifying the outcomes from BOLT with the former RECIST criteria. The expert group found that the 2 SHH inhibitors had comparable efficacy and adverse event profiles.20 Nevertheless, a recent meta-analysis found that although ORRs for laBCC were similar between the 2 drugs, the CR rate for vismodegib was 31% compared with 3% for sonidegib. Additionally, for mBCC, they reported the ORR of vismodegib to be 2.7 times higher than that of sonidegib (39% vs 15%).21
Immune Checkpoint Inhibitors
Immune checkpoint inhibitors have successfully been utilized in the treatment of cutaneous squamous cell carcinoma (cSCC); however, their use for treating BCC has been limited until recently.22-25 In February 2021, cemiplimab became the first and only ICI approved for the treatment of laBCC and mBCC in patients who did not respond to or were intolerant to prior SHH inhibitor therapy.26 Cemiplimab—a human monoclonal antibody against the PD-1 receptor expressed on T cells—blocks its interaction with programmed cell death ligand 1 and programmed cell death ligand 2 present on tumor cells. The blockade of the PD-1 pathway releases the inhibition of the antitumor immune response and enables appropriate cytotoxic T-cell activity to occur.8
The FDA approval of cemiplimab for the treatment of advanced BCC was based on an open-label, multicenter, single-arm phase 2 trial (NCT03132636) evaluating 84 patients with laBCC refractory or intolerant to SHH inhibitor therapy.26 Patients received an intravenous infusion of cemiplimab 350 mg every 3 weeks for up to 93 weeks or until disease progression or unacceptable toxicity. An ORR of 31% (26/84) was observed with a CR and PR of 6% (5/84) and 25% (21/84), respectively. The median duration of follow-up was 15 months.26 Given the clinically meaningful results of this trial, investigating the efficacy of other PD-1 inhibitors, such as pembrolizumab and nivolumab, for treatment of advanced BCC may prove worthwhile.
Adverse Effects of Systemic Treatments
The 2 approved SHH inhibitors—vismodegib and sonidegib—appear to have similar side-effect profiles, with the most common adverse effects being muscle spasms, dysgeusia, alopecia, nausea, vomiting, diarrhea, weight loss, and fatigue.20,21,27 These side effects occur at high frequencies (>40%) for both SHH inhibitors and often lead to discontinuation of the medication.21 Rates of treatment discontinuation range from 15% to 50% on average.12-14,18 Fortunately, the majority of these adverse effects do not appear to increase in severity or frequency with prolonged use of these medications.14,16,28
Various conservative and pharmacologic measures can be implemented to help manage side effects. For muscle spasms, which are the most commonly reported adverse effect, supplementation with magnesium, transcutaneous electrical nerve stimulation, acupuncture, massages, stretching, and thermal compresses can potentially be beneficial.29 Calcium channel blockers also may be effective, as one small prospective cohort study reported a reduction in the frequency of muscle cramps with amlodipine 10 mg daily.30 For alopecia, which typically is reversible and caused by SHH inhibition of the normal hair cycle, minoxidil theoretically can help, as it reduces telogen arrest and extends the anagen growth phase.31,32 Although usually mild and self-limiting, management of dysgeusia, weight loss, and gastrointestinal upset often can be managed with dietary changes, such as smaller, more frequent meals.33,34 Finally, alternative dosing strategies and drug holidays have been employed to mitigate these side effects and increase drug tolerability.35,36 These are discussed in the Alternative Dosing section.
Given the essential role of the SHH pathway in embryologic development, SHH inhibitors carry a black box warning of embryofetal teratogenicity and are contraindicated in females who are pregnant or breastfeeding. For females of reproductive potential, verification of pregnancy status should be performed prior to initiating treatment with an SHH inhibitor. These patients should be counseled on the use of contraception during treatment and for at least 24 months and 20 months after cessation of vismodegib and sonidegib, respectively.27,37,38 Male patients, even after a vasectomy, should use barrier contraception during treatment and for at least 3 months and 8 months after the final dose of vismodegib and sonidegib, respectively.37,38
Laboratory abnormalities commonly associated with SHH inhibitors include elevated hepatic enzymes, particularly with vismodegib, and elevated creatine kinase levels, particularly with sonidegib.28,39 Other laboratory abnormalities that can occur include hypercholesterolemia, hypercreatininemia, hyperglycemia, and increased serum lipase levels.19,28 Although these laboratory abnormalities usually are asymptomatic and self-limiting, regular monitoring should be performed.
There also is concern that SHH inhibitors may induce the development of cSCC. A case-control study of 55 cases and 125 control patients found an increased risk for cSCC in those previously treated with vismodegib, with a hazard ratio of 8.12.40 However, a subsequent retrospective cohort study of 1675 patients with BCC failed to find any association with cSCC among those treated with vismodegib compared to those who received standard surgical therapy.41 Clinical data for sonidegib are lacking, but the BOLT trial found that cSCC occurred in 3 patients receiving treatment with the SHH inhibitor.18 Thus, further studies are needed to more thoroughly assess this concern. Close monitoring for cSCC may be warranted in patients prescribed SHH inhibitors at this time.
Cemiplimab has demonstrated an acceptable safety profile and is generally well tolerated. In the phase 2 trial of cemiplimab for cSCC, approximately 5% of patients discontinued treatment because of adverse effects. The most commonly reported side effects of cemiplimab were diarrhea (27%), fatigue (24%), nausea (17%), constipation (15%), and rash (15%).23 In the phase 2 trial for laBCC, grade 3 or 4 adverse events occurred in 48% of patients, with hypertension (5%) being the most common.26 Although rare, immune-mediated adverse reactions also can occur, given the mechanism of action of ICIs. These side effects, ranging in severity from mild to fatal, include pneumonitis, colitis, hepatitis, nephritis, myocarditis, and hypophysitis. Therefore, close monitoring for these immune-mediated reactions is critical, but most can be managed with corticosteroids or treatment interruption if they occur.42,43
No absolute contraindications exist for cemiplimab; however, extreme caution should be taken in immunosuppressed individuals, such as solid organ transplant recipients and those with chronic lymphocytic leukemia (CLL), as safety data are limited in these patients.44,45 Although small retrospective studies have reported reasonable tolerability in solid organ transplant recipients treated with ICIs, an allograft rejection rate of 41% was found in a meta-analysis of 64 patients.46-48 In CLL patients with keratinocyte carcinomas, ICIs have been safely used and have even demonstrated efficacy for CLL in some cases.49-52
Alternative Dosing
The side effects of SHH inhibitors have led to alternative dosing strategies to prevent medication discontinuation and improve adherence. In patients with basal cell nevus syndrome, multimonth drug holidays have been shown to increase drug tolerability without compromising efficacy.35,36 Weekly intermittent dosing regimens of vismodegib ranging from 1 week on followed by 1 to 3 weeks off demonstrated efficacy in a retrospective study of 7 patients with advanced BCC.53 All 7 patients experienced improvement in their BCCs, with 3 patients experiencing CR. Importantly, treatment-related adverse effects were mild and well tolerated, with no patients terminating the medication.53 Two other retrospective case series of patients with advanced BCC treated with vismodegib reported similar findings for those placed on an intermittent dosing schedule ranging from once every other day to once per week.54,55
In the large phase 2 randomized trial known as MIKIE, 2 different intermittent dosing regimens of 150 mg vismodegib daily for patients with multiple BCCs were found to have good activity and tolerability.56 The first group (n=116) received vismodegib for 12 weeks, then 3 rounds of 8 weeks of placebo, followed by 12 weeks of vismodegib; there was a 63% reduction in clinically evident BCCs after 73 weeks. The second group (n=113) received the medication for 24 weeks, then 3 rounds of 8 weeks of placebo, followed by 8 weeks of vismodegib; there was a 54% reduction at the end of 73 weeks.56 Subsequent analyses found improvements in health-related quality-of-life outcomes that were similar for both groups.57
Consequently, alternative dosing schedules appear to be a viable option for patients at risk of discontinuing treatment because of adverse effects, and current data support the recently approved recommendations of dose interruptions of up to 8 weeks to manage adverse effects in patients with laBCC or mBCC.58 Nevertheless, further clinical studies are required to determine the optimal intermittent dosing regimen for patients treated with SHH inhibitors.
Neoadjuvant Administration
Recently, vismodegib has been studied as a neoadjuvant therapy for BCC with promising results. Several small retrospective studies and case reports have documented successful treatment of both operable and inoperable periocular laBCC, with preservation of the eye in all patients.59-61 An open-label trial of 15 patients with advanced BCC who received neoadjuvant vismodegib for 3 to 6 months prior to surgical excision reported a mean reduction of 35% in the final surgical defect size, with no recurrence at 22 months.62,63 The latest and largest study performed was a phase 2 open-label trial known as VISMONEO, where 44 of 55 laBCC patients (80%) receiving neoadjuvant vismodegib for a mean duration of 6 months (range, 4–10 months) achieved the primary end point of tumor surgical downstaging.64 Of the 44 patients who had tumor downstaging, 27 (61%) experienced histologically proven CRs. Additionally, a 66% reduction in the average target lesion size was reported in this group compared to29% in the 11 patients who did not have tumor downstaging (P=.0002).64 Thus, SHH inhibitors may hold an important neoadjuvant role in the treatment of BCC by decreasing surgical defect size and allowing for surgical management of previously inoperable cases.
Synergism With Radiation
Preliminary data suggest SHH inhibitors may help potentiate the effects of radiation therapy for the treatment of BCC. Currently, the evidence primarily is limited to case studies, with several reports describing complete remission in patients with advanced BCCs who were considered unsuitable candidates for surgery. In these cases, vismodegib was administered either prior to or concurrently with radiation treatment.65-69 An in vitro study also documented the radiation-sensitizing effects of vismodegib in a BCC cell line.70 Recently, a phase 2 trial (ClinicalTrials.gov identifier NCT01835626) evaluating the concurrent use of vismodegib and radiotherapy for patients with advanced BCC was completed, but data has yet to be published.
Synergism With and Benefit of Antifungal Therapy
The antifungal drug itraconazole is a potent inhibitor of the SHH pathway and may have an adjunctive role in the treatment of BCC. Similar to vismodegib and sonidegib, itraconazole acts as a direct antagonist of SMO. However, it is thought to bind to a distinct site on SMO.71,72 An open-label, exploratory phase 2 trial of 19 patients with BCC found that oral itraconazole 200 to 400 mg daily decreased tumor proliferative index by 45% (P=.04), as measured by Ki-67; SHH activity by 65% (P=.03), as measured by GLI1 messenger RNA; and mean tumor area by 24%.73 In a case series of 5 patients with mBCC refractory to conventional SHH inhibitor therapy, combined treatment with itraconazole and arsenic trioxide resulted in stable disease and a 75% reduction in SHH activity (P<.001).74 One case report documented tumor regression leading to stable disease for 15 months in a patient with laBCC treated with itraconazole monotherapy due to being unable to afford vismodegib or sonidegib. However, within 2 months of treatment discontinuation, the lesion progressed considerably.75 The efficacy of a topical formulation of itraconazole also has been tested in an open-label, placebo-controlled phase 2 trial, but no benefit was observed.76
Posaconazole is a second-generation antifungal agent that may serve as a potential alternative to itraconazole.77 Although clinical data are lacking, a basic science study found that posaconazole could inhibit the growth of SHH-dependent BCC in vivo (in mice).78 Furthermore, posaconazole has demonstrated a better safety profile with fewer and more mild side effects than itraconazole and does not require dose adjustment for those with hepatic or renal failure.79,80 Thus, posaconazole may be a safer alternative to itraconazole for the treatment of BCC. Further clinical studies are needed to elucidate the potential synergistic effects of these antifungal agents with the 2 currently approved SHH inhibitors for the treatment of advanced BCC.
Drug Resistance
Treatment resistance to SHH inhibitors, though uncommon, is a growing concern. Acquired mutations in the SMO binding site or downstream mediators of the SHH pathway have been shown to confer resistance to vismodegib and sonidegib.72,81-83 In addition, it appears that there may be shared resistance among the drugs in this class. One study assessing the efficacy of sonidegib in 9 patients with laBCC resistant to vismodegib found that these patients also did not respond to sonidegib.84 Interestingly, 1 case report documented tumor regression of an intracranial BCC in a patient treated with sonidegib and itraconazole after failure with vismodegib.85 An in vitro study also found that itraconazole maintained SHH inhibitory activity for all drug-resistant SMO mutations that have been reported.72 Therefore, itraconazole monotherapy or combination therapy with a canonical SHH inhibitor may be considered for patients with recalcitrant BCC and warrants further investigation.
Taladegib is a newly developed SMO inhibitor that may serve as another promising alternative for patients who develop resistance to vismodegib or sonidegib. A phase 1 trial of taladegib for advanced BCC found an ORR of 69% (11/16) in the SHH inhibitor–naïve group and an ORR of 36% (11/32) in the group previously treated with a SHH inhibitor.86 Additionally, the safety profile and frequency of adverse effects appear to be similar to those associated with vismodegib and sonidegib.86,87 Unfortunately, no clinical trials evaluating taladegib for BCC are ongoing or in development at this time.
Recurrence
There appears to be a relatively high rate of recurrence for BCC patients who achieve a CR to SHH inhibitors. In a retrospective study of 116 laBCC patients who experienced a CR after vismodegib therapy, 54 patients (47%) relapsed at 36 months. Among the 54 patients that relapsed, 27 were re-treated with vismodegib, which resulted in an ORR of 85% (23/27), a CR rate of 37% (10/27), and a PR rate of 48% (13/27).88 Another retrospective study of 35 laBCC patients who relapsed after vismodegib treatment reported a 31% (11/35) clinical recurrence rate at 6-month follow-up.89 An observational retrospective study also assessed the efficacy of SHH inhibitor maintenance therapy for advanced BCC patients who achieved a CR.90 In the study, 27 (64%) patients received a maintenance dose of 150 mg vismodegib once per week for 1 year, while 15 (36%) patients decided not to take a maintenance dose following CR of their BCC. All patients who took the maintenance therapy did not experience clinical recurrence at 1-year follow-up, whereas 26% of patients not on the maintenance dose relapsed.90 Consequently, these results indicate that BCC recurrence is frequent after SHH inhibitor therapy and highlights the importance of close surveillance after CR is attained. Nevertheless, most patients still respond to treatment with SHH inhibitors after relapsing, and intermittent maintenance doses may be an effective means to reduce risk of recurrence.
Conclusion
Vismodegib and sonidegib are SHH inhibitors approved for the treatment of laBCC and mBCC. Cemiplimab is now also approved for patients who do not respond to SHH inhibitors or for whom SHH inhibitors are not tolerable. Although these systemic targeted therapies can lead to notable tumor shrinkage and even complete regression in some patients, recurrence is common, and adverse effects may limit their use. Drug resistance is an emerging issue that requires additional examination. Further clinical studies are needed to determine which patients are likely to respond to these targeted treatments.
Various intermittent and maintenance drug regimens should be evaluated for their potential to mitigate adverse effects and reduce risk of recurrence, respectively. The synergistic effects of these medications with other therapies as well as their neoadjuvant and adjuvant roles should be further investigated. For example, administration of an SHH inhibitor prior to surgical excision of a BCC may allow for a smaller surgical defect size, thereby improving cosmetic and functional outcomes. Moreover, these systemic targeted medications may allow for previously inoperable tumors to become amenable to surgical treatment.
Although SHH inhibitors and PD-1 inhibitors represent a major advancement in the field of oncodermatology, real-world efficacy and safety data in the upcoming years will be important for elucidating their true benefit for patients with BCC.
- Rogers HW, Weinstock MA, Feldman SR, et al. Incidence estimate of nonmelanoma skin cancer (keratinocyte carcinomas) in the U.S. population, 2012. JAMA Dermatol. 2015;151:1081-1086.
- Cameron MC, Lee E, Hibler BP, et al. Basal cell carcinoma: epidemiology; pathophysiology; clinical and histological subtypes; and disease associations. J Am Acad Dermatol. 2019;80:303-317.
- Rees JR, Zens MS, Celaya MO, et al. Survival after squamous cell and basal cell carcinoma of the skin: a retrospective cohort analysis. Int J Cancer. 2015;137:878-884.
- Kasumagic-Halilovic E, Hasic M, Ovcina-Kurtovic N. A clinical study of basal cell carcinoma. Med Arch. 2019;73:394-398.
- Goldenberg G, Karagiannis T, Palmer JB, et al. Incidence and prevalence of basal cell carcinoma (BCC) and locally advanced BCC (LABCC) in a large commercially insured population in the United States: a retrospective cohort study. J Am Acad Dermatol. 2016;75:957.e952-966.e952.
- Laga AC, Schaefer IM, Sholl LM, et al. Metastatic basal cell carcinoma. Am J Clin Pathol. 2019;152:706-717.
- Rimkus TK, Carpenter RL, Qasem S, et al. Targeting the sonic hedgehog signaling pathway: review of smoothened and GLI inhibitors. Cancers (Basel). 2016;8:22.
- Burova E, Hermann A, Waite J, et al. Characterization of the anti–PD-1 antibody REGN2810 and its antitumor activity in human PD-1 knock-in mice. Mol Cancer Ther. 2017;16:861-870.
- Bhardwaj G, Murdoch B, Wu D, et al. Sonic hedgehog induces the proliferation of primitive human hematopoietic cells via BMP regulation. Nat Immunol. 2001;2:172-180.
- Paladini RD, Saleh J, Qian C, et al. Modulation of hair growth with small molecule agonists of the hedgehog signaling pathway. J Invest Dermatol. 2005;125:638-646.
- Von Hoff DD, LoRusso PM, Rudin CM, et al. Inhibition of the hedgehog pathway in advanced basal-cell carcinoma. N Engl J Med. 2009;361:1164-1172.
- Sekulic A, Migden MR, Oro AE, et al. Efficacy and safety of vismodegib in advanced basal-cell carcinoma. N Engl J Med. 2012;366:2171-2179.
- Sekulic A, Migden MR, Basset-Seguin N, et al. Long-term safety and efficacy of vismodegib in patients with advanced basal cell carcinoma: final update of the pivotal ERIVANCE BCC study. BMC Cancer. 2017;17:332.
- Basset-Séguin N, Hauschild A, Kunstfeld R, et al. Vismodegib in patients with advanced basal cell carcinoma: primary analysis of STEVIE, an international, open-label trial. Eur J Cancer. 2017;86:334-348.
- Cozzani R, Del Aguila R, Carrizo M, et al. Efficacy and safety profile of vismodegib in a real-world setting cohort of patients with advanced basal cell carcinoma in Argentina. Int J Dermatol. 2020;59:627-632.
- Spallone G, Sollena P, Ventura A, et al. Efficacy and safety of vismodegib treatment in patients with advanced basal cell carcinoma and multiple comorbidities. Dermatol Ther. 2019;32:E13108.
- Fosko SW, Chu MB, Armbrecht E, et al. Efficacy, rate of tumor response, and safety of a short course (12-24 weeks) of oral vismodegib in various histologic subtypes (infiltrative, nodular, and superficial) of high-risk or locally advanced basal cell carcinoma, in an open-label, prospective case series clinical trial. J Am Acad Dermatol. 2020;82:946-954.
- Migden MR, Guminski A, Gutzmer R, et al. Treatment with two different doses of sonidegib in patients with locally advanced or metastatic basal cell carcinoma (BOLT): a multicentre, randomised, double-blind phase 2 trial. Lancet Oncol. 2015;16:716-728.
- Dummer R, Guminksi A, Gutzmer R, et al. Long-term efficacy and safety of sonidegib in patients with advanced basal cell carcinoma: 42-month analysis of the phase II randomized, double-blind BOLT study. Br J Dermatol. 2020;182:1369-1378.
- Dummer R, Ascierto PA, Basset-Seguin N, et al. Sonidegib and vismodegib in the treatment of patients with locally advanced basal cell carcinoma: a joint expert opinion. J Eur Acad Dermatol Venereol. 2020;34:1944-1956.
- Xie P, Lefrançois P. Efficacy, safety, and comparison of sonic hedgehog inhibitors in basal cell carcinomas: a systematic review and meta-analysis. J Am Acad Dermatol. 2018;79:1089-1100.e1017.
- Gentzler R, Hall R, Kunk PR, et al. Beyond melanoma: inhibiting the PD-1/PD-L1 pathway in solid tumors. Immunotherapy. 2016;8:583-600.
- Guminski AD, Lim AML, Khushalani NI, et al. Phase 2 study of cemiplimab, a human monoclonal anti-PD-1, in patients (pts) with metastatic cutaneous squamous cell carcinoma (mCSCC; group 1): 12-month follow-up [abstract]. J Clin Oncol. 2019;37(15 suppl):9526.
- Grob JJ, Gonzalez Mendoza R, Basset-Seguin N, et al. Pembrolizumab for recurrent/metastatic cutaneous squamous cell carcinoma (cSCC): efficacy and safety results from the phase II KEYNOTE-629 study [abstract]. Ann Oncol. 2019;30 (suppl 5):v908.
- Maubec E, Boubaya M, Petrow P, et al. Pembrolizumab as first-line therapy in patients with unresectable cutaneous squamous cell carcinoma (cSCC): phase 2 results from CARSKIN [abstract]. J Clin Oncol. 2019;37(15 suppl):9547.
- Stratigos AJ, Sekulic A, Peris K, et al. Cemiplimab in locally advanced basal cell carcinoma after hedgehog inhibitor therapy: an open-label, multi-centre, single-arm, phase 2 trial. Lancet Oncol. 2021;22:848-857.
- Carpenter RL, Ray H. Safety and tolerability of sonic hedgehog pathway inhibitors in cancer. Drug Saf. 2019;42:263-279.
- Villani A, Fabbrocini G, Costa C, et al. Sonidegib: safety and efficacy in treatment of advanced basal cell carcinoma. Dermatol Ther (Heidelb). 2020;10:401-412.
- Wright A, Sluka KA. Nonpharmacological treatments for musculoskeletal pain. Clin J Pain. 2001;17:33-46.
- Ally MS, Tang JY, Lindgren J, et al. Effect of calcium channel blockade on vismodegib-induced muscle cramps. JAMA Dermatol. 2015;151:1132-1134.
- Yang X, Thai K-E. Treatment of permanent chemotherapy-induced alopecia with low dose oral minoxidil. Australas J Dermatol. 2016;57:e130-e132.
- Ferguson JS, Hannam S, Toholka R, et al. Hair loss and hedgehog inhibitors: a class effect? Br J Dermatol. 2015;173:262-264.
- Kumbargere Nagraj S, George RP, Shetty N, et al. Interventions for managing taste disturbances. Cochrane Database Syst Rev. 2017;12:CD010470.
- Jacobsen AA, Kydd AR, Strasswimmer J. Practical management of the adverse effects of hedgehog pathway inhibitor therapy for basal cell carcinoma. J Am Acad Dermatol. 2017;76:767-768.
- Ally MS, Tang JY, Joseph T, et al. The use of vismodegib to shrink keratocystic odontogenic tumors in patients with basal cell nevus syndrome. JAMA Dermatol. 2014;150:542-545.
- Yang X, Dinehart SM. Intermittent vismodegib therapy in basal cell nevus syndrome. JAMA Dermatol. 2016;152:223-224.
- Erivedge. Prescribing information. Genentech; 2015.
- Odomzo. Prescribing information. Novartis; 2015.
- Ventarola DJ, Silverstein DI. Vismodegib-associated hepatotoxicity: a potential side effect detected in postmarketing surveillance. J Am Acad Dermatol. 2014;71:397-398.
- Mohan SV, Chang J, Li S, et al. Increased risk of cutaneous squamous cell carcinoma after vismodegib therapy for basal cell carcinoma. JAMA Dermatol. 2016;152:527-532.
- Bhutani T, Abrouk M, Sima CS, et al. Risk of cutaneous squamous cell carcinoma after treatment of basal cell carcinoma with vismodegib. J Am Acad Dermatol. 2017;77:713-718.
- Morgado M, Plácido A, Morgado S, et al. Management of the adverse effects of immune checkpoint inhibitors. Vaccines (Basel). 2020;8:575.
- Martins F, Sofiya L, Sykiotis GP, et al. Adverse effects of immune-checkpoint inhibitors: epidemiology, management and surveillance. Nat Rev Clin Oncol. 2019;16:563-580.
- Ntsethe A, Dludla PV, Nyambuya TM, et al. The impact of immune checkpoint inhibitors in patients with chronic lymphocytic leukemia (CLL): a protocol for a systematic review and meta-analysis of randomized controlled trials. Medicine (Baltimore). 2020;99:E21167.
- Johnson DB, Sullivan RJ, Menzies AM. Immune checkpoint inhibitors in challenging populations. Cancer. 2017;123:1904-1911.
- Tsung I, Worden FP, Fontana RJ. Safety and efficacy of checkpoint inhibitors in solid organ transplant recipients with cutaneous squamous cell carcinoma [abstract]. J Clin Oncol. 2020;38(15 suppl):E22014.
- Owoyemi I, Vaughan LE, Costello CM, et al. Clinical outcomes of solid organ transplant recipients with metastatic cancers who are treated with immune checkpoint inhibitors: a single-center analysis. Cancer. 2020;126:4780-4787.
- Kumar V, Shinagare AB, Rennke HG, et al. The safety and efficacy of checkpoint inhibitors in transplant recipients: a case series and systematic review of literature. Oncologist. 2020;25:505-514.
- Arenbergerova M, Fialova A, Arenberger P, et al. Killing two birds with one stone: response to pembrolizumab in a patient with metastatic melanoma and B-cell chronic lymphocytic leukaemia. J Eur Acad Dermatol Venereol. 2018;32:E72-E74.
- Archibald WJ, Meacham PJ, Williams AM, et al. Management of melanoma in patients with chronic lymphocytic leukemia. Leuk Res. 2018;71:43-46.
- Ding W, LaPlant BR, Call TG, et al. Pembrolizumab in patients with CLL and Richter transformation or with relapsed CLL. Blood. 2017;129:3419-3427.
- Leiter U, Loquai C, Reinhardt L, et al. Immune checkpoint inhibition therapy for advanced skin cancer in patients with concomitant hematological malignancy: a retrospective multicenter DeCOG study of 84 patients. J Immunother Cancer. 2020;8:E000897.
- Becker LR, Aakhus AE, Reich HC, et al. A novel alternate dosing of vismodegib for treatment of patients with advanced basal cell carcinomas. JAMA Dermatol. 2017;153:321-322.
- Woltsche N, Pichler N, Wolf I, et al. Managing adverse effects by dose reduction during routine treatment of locally advanced basal cell carcinoma with the hedgehog inhibitor vismodegib: a single centre experience. J Eur Acad Dermatol Venereol. 2019;33:E144-E145.
- Wong C, Poblete-Lopez C, Vidimos A. Comparison of daily dosing versus Monday through Friday dosing of vismodegib for locally advanced basal cell carcinoma and basal cell nevus syndrome: a retrospective case series. J Am Acad Dermatol. 2020;82:1539-1542.
- Dréno B, Kunstfeld R, Hauschild A, et al. Two intermittent vismodegib dosing regimens in patients with multiple basal-cell carcinomas (MIKIE): a randomised, regimen-controlled, double-blind, phase 2 trial. Lancet Oncol. 2017;18:404-412.
- Schadendorf D, Hauschild A, Fosko S, et al. Quality-of-life analysis with intermittent vismodegib regimens in patients with multiple basal cell carcinomas: patient-reported outcomes from the MIKIE study. J Eur Acad Dermatol Venereol. 2020;34:E526-E529.
- Chanu P, Musib L, Wang X, et al. Vismodegib efficacy in advanced basal cell carcinoma maintained with 8-week dose interruptions: a model-based evaluation. J Invest Dermatol. 2021;141:930-933.
- Su MG, Potts LB, Tsai JH. Treatment of periocular basal cell carcinoma with neoadjuvant vismodegib. Am J Ophthalmol Case Rep. 2020;19:100755.
- González AR, Etchichury D, Gil ME, et al. Neoadjuvant vismodegib and Mohs micrographic surgery for locally advanced periocular basal cell carcinoma. Ophthalmic Plast Reconstr Surg. 2019;35:56-61.
- Sagiv O, Nagarajan P, Ferrarotto R, et al. Ocular preservation with neoadjuvant vismodegib in patients with locally advanced periocular basal cell carcinoma. Br J Ophthalmol. 2019;103:775-780.
- Ally MS, Aasi S, Wysong A, et al. An investigator-initiated open-label clinical trial of vismodegib as a neoadjuvant to surgery for high-risk basal cell carcinoma. J Am Acad Dermatol. 2014;71:904-911.e1.
- Kwon GP, Ally MS, Bailey-Healy I, et al. Update to an open-label clinical trial of vismodegib as neoadjuvant before surgery for high-risk basal cell carcinoma (BCC). J Am Acad Dermatol. 2016;75:213-215.
- Mortier L, Bertrand N, Basset-Seguin N, et al. Vismodegib in neoadjuvant treatment of locally advanced basal cell carcinoma: first results of a multicenter, open-label, phase 2 trial (VISMONEO study) [abstract]. J Clin Oncol. 2018;36(15 suppl):9509.
- Strasswimmer JM. Potential synergy of radiation therapy with vismodegib for basal cell carcinoma. JAMA Dermatol. 2015;151:925-926.
- Gathings RM, Orscheln CS, Huang WW. Compassionate use of vismodegib and adjuvant radiotherapy in the treatment of multiple locally advanced and inoperable basal cell carcinomas and squamous cell carcinomas of the skin. J Am Acad Dermatol. 2014;70:E88-E89.
- Franco AI, Eastwick G, Farah R, et al. Upfront radiotherapy with concurrent and adjuvant vismodegib is effective and well-tolerated in a patient with advanced, multifocal basal cell carcinoma. Case Rep Dermatol Med. 2018;2018:2354146.
- Pollom EL, Bui TT, Chang AL, et al. Concurrent vismodegib and radiotherapy for recurrent, advanced basal cell carcinoma. JAMA Dermatol. 2015;151:998-1001.
- Janela-Lapert R, Dubray B, Duval-Modeste A, et al. Treatment of advanced basal cell carcinoma with vismodegib followed by radiotherapy [in French]. Ann Dermatol Venereol. 2020;147:780-782.
- Hehlgans S, Booms P, Güllülü Ö, et al. Radiation sensitization of basal cell and head and neck squamous cell carcinoma by the hedgehog pathway inhibitor vismodegib. Int J Mol Sci. 2018;19:2485.
- Kim J, Tang JY, Gong R, et al. Itraconazole, a commonly used antifungal that inhibits hedgehog pathway activity and cancer growth. Cancer Cell. 2010;17:388-399.
- Kim J, Aftab BT, Tang JY, et al. Itraconazole and arsenic trioxide inhibit hedgehog pathway activation and tumor growth associated with acquired resistance to smoothened antagonists. Cancer Cell. 2013;23:23-34.
- Kim DJ, Kim J, Spaunhurst K, et al. Open-label, exploratory phase II trial of oral itraconazole for the treatment of basal cell carcinoma. J Clin Oncol. 2014;32:745-751.
- Ally MS, Ransohoff K, Sarin K, et al. Effects of combined treatment with arsenic trioxide and itraconazole in patients with refractory metastatic basal cell carcinoma. JAMA Dermatol. 2016;152:452-456.
- Cia˛z˙yn´yska M, Narbutt J, Skibin´ska M, et al. Itraconazole—a new player in the therapy of advanced basal cell carcinoma: a case report. JCO Oncol Pract. 2020;16:837-838.
- Sohn GK, Kwon GP, Bailey-Healy I, et al. Topical itraconazole for the treatment of basal cell carcinoma in patients with basal cell nevus syndrome or high-frequency basal cell carcinomas: a phase 2, open-label, placebo-controlled trial. JAMA Dermatol. 2019;155:1078-1080.
- Lass-Flörl C. Triazole antifungal agents in invasive fungal infections: a comparative review. Drugs. 2011;71:2405-2419.
- Chen B, Trang V, Lee A, et al. Posaconazole, a second-generation triazole antifungal drug, inhibits the hedgehog signaling pathway and progression of basal cell carcinoma. Mol Cancer Ther. 2016;15:866-876.
- Katragkou A, Tsikopoulou F, Roilides E, et al. Posaconazole: when and how? the clinician’s view. Mycoses. 2012;55:110-122.
- Raad II, Graybill JR, Bustamante AB, et al. Safety of long-term oral posaconazole use in the treatment of refractory invasive fungal infections. Clin Infect Dis. 2006;42:1726-1734.
- Atwood SX, Sarin KY, Whitson RJ, et al. Smoothened variants explain the majority of drug resistance in basal cell carcinoma. Cancer Cell. 2015;27:342-353.
- Sun Q, Atzmony L, Zaki T, et al. Clues to primary vismodegib resistance lie in histology and genetics. J Clin Pathol. 2020;73:678-680.
- Verkouteren BJA, Wakkee M, van Geel M, et al. Molecular testing in metastatic basal cell carcinoma. J Am Acad Dermatol. 2021;85:1135-1142.
- Danial C, Sarin KY, Oro AE, et al. An investigator-initiated open-label trial of sonidegib in advanced basal cell carcinoma patients resistant to vismodegib. Clin Cancer Res. 2016;22:1325-1329.
- Yoon J, Apicelli AJ 3rd, Pavlopoulos TV. Intracranial regression of an advanced basal cell carcinoma using sonidegib and itraconazole after failure with vismodegib. JAAD Case Rep. 2017;4:10-12.
- Bendell J, Andre V, Ho A, et al. Phase I study of LY2940680, a Smo antagonist, in patients with advanced cancer including treatment-naïve and previously treated basal cell carcinoma. Clin Cancer Res. 2018;24:2082-2091.
- Ueno H, Kondo S, Yoshikawa S, et al. A phase I and pharmacokinetic study of taladegib, a Smoothened inhibitor, in Japanese patients with advanced solid tumors. Invest New Drugs. 2018;36:647-656.
- Herms F, Lambert J, Grob JJ, et al. Follow-up of patients with complete remission of locally advanced basal cell carcinoma after vismodegib discontinuation: a multicenter French study of 116 patients. J Clin Oncol. 2019;37:3275-3282.
- Villani A, Megna M, Fabbrocini G, et al. Long-term efficacy of vismodegib after its withdrawal and patients’ health-related quality of life using the Dermatology Life Quality Index (DLQI). Dermatol Ther (Heidelb). 2019;9:719-724.
- Scalvenzi M, Cappello M, Costa C, et al. Low-dose vismodegib as maintenance therapy after locally advanced basal cell carcinoma complete remission: high efficacy with minimal toxicity. Dermatol Ther (Heidelb). 2020;10:465-468.
Basal cell carcinoma (BCC) is the most common keratinocyte carcinoma and affects more than 3 million individuals per year in the United States.1 Approximately 40% of patients diagnosed with BCC will develop another BCC within 5 years of the initial diagnosis.2 Most cases are successfully treated with surgical excision and occasionally topical therapy or radiotherapy. Despite the high cure rate with conventional treatments, BCC can recur and can cause substantial destruction of the surrounding tissue if left untreated.3-5 In some instances, BCC can even metastasize and lead to death.6 For patients who are poor candidates for surgical or topical treatment modalities because of locally advanced BCC (laBCC) or metastatic BCC (mBCC), systemic treatment may be indicated. Vismodegib, sonidegib, and cemiplimab are the only systemic medications approved by the US Food and Drug Administration (FDA) for the treatment of laBCC and/or mBCC. Vismodegib and sonidegib target the sonic hedgehog (SHH) signaling pathway that is abnormally activated in more than 90% of BCCs.7 Cemiplimab is an immune checkpoint inhibitor (ICI) that targets the programmed cell death protein 1 (PD-1) receptor.8 Herein, we review the clinical utility of these medications and their evolving roles in the treatment of BCC.
SHH Pathway Inhibitors
The SHH pathway is a key regulator of cell proliferation and differentiation during embryogenesis.7 During adulthood, SHH signaling decreases but still plays an important role in stem cell activation and in regulation of the hair follicle growth cycle.9,10 However, de novo mutations in the genes that comprise the SHH pathway can result in aberrant constitutive activation, leading to unrestricted cell proliferation. Genetic mutations resulting in activation of Smoothened (SMO), a G-protein–coupled receptor involved in the signal transduction and propagation of the SHH pathway, have been implicated in the pathogenesis of BCC. Inactivating mutations also are commonly observed in patched homolog 1, an upstream cell-surface protein that inhibits SMO.7 The mechanism by which vismodegib and sonidegib, 2 of the FDA-approved oral medications for the treatment of advanced BCC, block the SHH pathway is through the selective inhibition of SMO.7,11
Vismodegib first received FDA approval in 2012 for the treatment of laBCC and mBCC after initial results from the pivotal ERIVANCE phase 2 trial demonstrated an objective response rate (ORR) of 43% (27/63) and 30% (10/33) in patients with locally advanced and metastatic disease, respectively. In this single-arm study, all enrolled patients (63 with laBCC and 33 with mBCC) received 150 mg of oral vismodegib daily.12 Updated results at 39 months demonstrated improved ORRs of 60% (38/63) and 48% (16/33) for the laBCC and mBCC groups, respectively. A complete response (CR) and partial response (PR) were observed in 32% (n=20) and 29% (n=18) of patients with laBCC, respectively.13 These results have been confirmed in subsequent studies, including the large international open-label trial known as STEVIE, with ORRs of 68.5% for 1119 cases of laBCC and 37% for 96 cases of mBCC.14-17 The CR and PR rates were 33% and 35%, respectively, for the laBCC group. The CR and PR rates for the mBCC group were 5% and 32%, respectively.14
The FDA approval of sonidegib for laBCC—but not mBCC—occurred in 2015 after the pivotal BOLT randomized phase 2 trial demonstrated an initial ORR of 43% (18/42) for laBCC and 15% (2/13) for mBCC after administration of 200 mg of sonidegib daily.18 A final follow-up analysis at 42 months resulted in ORRs of 56% (37/66) and 8% (1/13) for the laBCC and mBCC groups, respectively.19 Additionally, improved efficacy was not observed in the 151 patients who were randomized to receive treatment with the higher 800-mg dose; however, they did experience a higher incidence of adverse events.18,19
Currently, the true clinical differences between vismodegib and sonidegib remain uncertain, as no head-to-head trials have been conducted. Moreover, direct comparison of the data from the ERIVANCE and BOLT trials is challenging owing to fundamental differences in methodologic design, including the criteria used to assess BCC severity. The ERIVANCE trial utilized the conventional Response Evaluation Criteria in Solid Tumors (RECIST), while BOLT used the rigorous modified RECIST. However, an expert consensus study attempted to compare the 2 trials by modifying the outcomes from BOLT with the former RECIST criteria. The expert group found that the 2 SHH inhibitors had comparable efficacy and adverse event profiles.20 Nevertheless, a recent meta-analysis found that although ORRs for laBCC were similar between the 2 drugs, the CR rate for vismodegib was 31% compared with 3% for sonidegib. Additionally, for mBCC, they reported the ORR of vismodegib to be 2.7 times higher than that of sonidegib (39% vs 15%).21
Immune Checkpoint Inhibitors
Immune checkpoint inhibitors have successfully been utilized in the treatment of cutaneous squamous cell carcinoma (cSCC); however, their use for treating BCC has been limited until recently.22-25 In February 2021, cemiplimab became the first and only ICI approved for the treatment of laBCC and mBCC in patients who did not respond to or were intolerant to prior SHH inhibitor therapy.26 Cemiplimab—a human monoclonal antibody against the PD-1 receptor expressed on T cells—blocks its interaction with programmed cell death ligand 1 and programmed cell death ligand 2 present on tumor cells. The blockade of the PD-1 pathway releases the inhibition of the antitumor immune response and enables appropriate cytotoxic T-cell activity to occur.8
The FDA approval of cemiplimab for the treatment of advanced BCC was based on an open-label, multicenter, single-arm phase 2 trial (NCT03132636) evaluating 84 patients with laBCC refractory or intolerant to SHH inhibitor therapy.26 Patients received an intravenous infusion of cemiplimab 350 mg every 3 weeks for up to 93 weeks or until disease progression or unacceptable toxicity. An ORR of 31% (26/84) was observed with a CR and PR of 6% (5/84) and 25% (21/84), respectively. The median duration of follow-up was 15 months.26 Given the clinically meaningful results of this trial, investigating the efficacy of other PD-1 inhibitors, such as pembrolizumab and nivolumab, for treatment of advanced BCC may prove worthwhile.
Adverse Effects of Systemic Treatments
The 2 approved SHH inhibitors—vismodegib and sonidegib—appear to have similar side-effect profiles, with the most common adverse effects being muscle spasms, dysgeusia, alopecia, nausea, vomiting, diarrhea, weight loss, and fatigue.20,21,27 These side effects occur at high frequencies (>40%) for both SHH inhibitors and often lead to discontinuation of the medication.21 Rates of treatment discontinuation range from 15% to 50% on average.12-14,18 Fortunately, the majority of these adverse effects do not appear to increase in severity or frequency with prolonged use of these medications.14,16,28
Various conservative and pharmacologic measures can be implemented to help manage side effects. For muscle spasms, which are the most commonly reported adverse effect, supplementation with magnesium, transcutaneous electrical nerve stimulation, acupuncture, massages, stretching, and thermal compresses can potentially be beneficial.29 Calcium channel blockers also may be effective, as one small prospective cohort study reported a reduction in the frequency of muscle cramps with amlodipine 10 mg daily.30 For alopecia, which typically is reversible and caused by SHH inhibition of the normal hair cycle, minoxidil theoretically can help, as it reduces telogen arrest and extends the anagen growth phase.31,32 Although usually mild and self-limiting, management of dysgeusia, weight loss, and gastrointestinal upset often can be managed with dietary changes, such as smaller, more frequent meals.33,34 Finally, alternative dosing strategies and drug holidays have been employed to mitigate these side effects and increase drug tolerability.35,36 These are discussed in the Alternative Dosing section.
Given the essential role of the SHH pathway in embryologic development, SHH inhibitors carry a black box warning of embryofetal teratogenicity and are contraindicated in females who are pregnant or breastfeeding. For females of reproductive potential, verification of pregnancy status should be performed prior to initiating treatment with an SHH inhibitor. These patients should be counseled on the use of contraception during treatment and for at least 24 months and 20 months after cessation of vismodegib and sonidegib, respectively.27,37,38 Male patients, even after a vasectomy, should use barrier contraception during treatment and for at least 3 months and 8 months after the final dose of vismodegib and sonidegib, respectively.37,38
Laboratory abnormalities commonly associated with SHH inhibitors include elevated hepatic enzymes, particularly with vismodegib, and elevated creatine kinase levels, particularly with sonidegib.28,39 Other laboratory abnormalities that can occur include hypercholesterolemia, hypercreatininemia, hyperglycemia, and increased serum lipase levels.19,28 Although these laboratory abnormalities usually are asymptomatic and self-limiting, regular monitoring should be performed.
There also is concern that SHH inhibitors may induce the development of cSCC. A case-control study of 55 cases and 125 control patients found an increased risk for cSCC in those previously treated with vismodegib, with a hazard ratio of 8.12.40 However, a subsequent retrospective cohort study of 1675 patients with BCC failed to find any association with cSCC among those treated with vismodegib compared to those who received standard surgical therapy.41 Clinical data for sonidegib are lacking, but the BOLT trial found that cSCC occurred in 3 patients receiving treatment with the SHH inhibitor.18 Thus, further studies are needed to more thoroughly assess this concern. Close monitoring for cSCC may be warranted in patients prescribed SHH inhibitors at this time.
Cemiplimab has demonstrated an acceptable safety profile and is generally well tolerated. In the phase 2 trial of cemiplimab for cSCC, approximately 5% of patients discontinued treatment because of adverse effects. The most commonly reported side effects of cemiplimab were diarrhea (27%), fatigue (24%), nausea (17%), constipation (15%), and rash (15%).23 In the phase 2 trial for laBCC, grade 3 or 4 adverse events occurred in 48% of patients, with hypertension (5%) being the most common.26 Although rare, immune-mediated adverse reactions also can occur, given the mechanism of action of ICIs. These side effects, ranging in severity from mild to fatal, include pneumonitis, colitis, hepatitis, nephritis, myocarditis, and hypophysitis. Therefore, close monitoring for these immune-mediated reactions is critical, but most can be managed with corticosteroids or treatment interruption if they occur.42,43
No absolute contraindications exist for cemiplimab; however, extreme caution should be taken in immunosuppressed individuals, such as solid organ transplant recipients and those with chronic lymphocytic leukemia (CLL), as safety data are limited in these patients.44,45 Although small retrospective studies have reported reasonable tolerability in solid organ transplant recipients treated with ICIs, an allograft rejection rate of 41% was found in a meta-analysis of 64 patients.46-48 In CLL patients with keratinocyte carcinomas, ICIs have been safely used and have even demonstrated efficacy for CLL in some cases.49-52
Alternative Dosing
The side effects of SHH inhibitors have led to alternative dosing strategies to prevent medication discontinuation and improve adherence. In patients with basal cell nevus syndrome, multimonth drug holidays have been shown to increase drug tolerability without compromising efficacy.35,36 Weekly intermittent dosing regimens of vismodegib ranging from 1 week on followed by 1 to 3 weeks off demonstrated efficacy in a retrospective study of 7 patients with advanced BCC.53 All 7 patients experienced improvement in their BCCs, with 3 patients experiencing CR. Importantly, treatment-related adverse effects were mild and well tolerated, with no patients terminating the medication.53 Two other retrospective case series of patients with advanced BCC treated with vismodegib reported similar findings for those placed on an intermittent dosing schedule ranging from once every other day to once per week.54,55
In the large phase 2 randomized trial known as MIKIE, 2 different intermittent dosing regimens of 150 mg vismodegib daily for patients with multiple BCCs were found to have good activity and tolerability.56 The first group (n=116) received vismodegib for 12 weeks, then 3 rounds of 8 weeks of placebo, followed by 12 weeks of vismodegib; there was a 63% reduction in clinically evident BCCs after 73 weeks. The second group (n=113) received the medication for 24 weeks, then 3 rounds of 8 weeks of placebo, followed by 8 weeks of vismodegib; there was a 54% reduction at the end of 73 weeks.56 Subsequent analyses found improvements in health-related quality-of-life outcomes that were similar for both groups.57
Consequently, alternative dosing schedules appear to be a viable option for patients at risk of discontinuing treatment because of adverse effects, and current data support the recently approved recommendations of dose interruptions of up to 8 weeks to manage adverse effects in patients with laBCC or mBCC.58 Nevertheless, further clinical studies are required to determine the optimal intermittent dosing regimen for patients treated with SHH inhibitors.
Neoadjuvant Administration
Recently, vismodegib has been studied as a neoadjuvant therapy for BCC with promising results. Several small retrospective studies and case reports have documented successful treatment of both operable and inoperable periocular laBCC, with preservation of the eye in all patients.59-61 An open-label trial of 15 patients with advanced BCC who received neoadjuvant vismodegib for 3 to 6 months prior to surgical excision reported a mean reduction of 35% in the final surgical defect size, with no recurrence at 22 months.62,63 The latest and largest study performed was a phase 2 open-label trial known as VISMONEO, where 44 of 55 laBCC patients (80%) receiving neoadjuvant vismodegib for a mean duration of 6 months (range, 4–10 months) achieved the primary end point of tumor surgical downstaging.64 Of the 44 patients who had tumor downstaging, 27 (61%) experienced histologically proven CRs. Additionally, a 66% reduction in the average target lesion size was reported in this group compared to29% in the 11 patients who did not have tumor downstaging (P=.0002).64 Thus, SHH inhibitors may hold an important neoadjuvant role in the treatment of BCC by decreasing surgical defect size and allowing for surgical management of previously inoperable cases.
Synergism With Radiation
Preliminary data suggest SHH inhibitors may help potentiate the effects of radiation therapy for the treatment of BCC. Currently, the evidence primarily is limited to case studies, with several reports describing complete remission in patients with advanced BCCs who were considered unsuitable candidates for surgery. In these cases, vismodegib was administered either prior to or concurrently with radiation treatment.65-69 An in vitro study also documented the radiation-sensitizing effects of vismodegib in a BCC cell line.70 Recently, a phase 2 trial (ClinicalTrials.gov identifier NCT01835626) evaluating the concurrent use of vismodegib and radiotherapy for patients with advanced BCC was completed, but data has yet to be published.
Synergism With and Benefit of Antifungal Therapy
The antifungal drug itraconazole is a potent inhibitor of the SHH pathway and may have an adjunctive role in the treatment of BCC. Similar to vismodegib and sonidegib, itraconazole acts as a direct antagonist of SMO. However, it is thought to bind to a distinct site on SMO.71,72 An open-label, exploratory phase 2 trial of 19 patients with BCC found that oral itraconazole 200 to 400 mg daily decreased tumor proliferative index by 45% (P=.04), as measured by Ki-67; SHH activity by 65% (P=.03), as measured by GLI1 messenger RNA; and mean tumor area by 24%.73 In a case series of 5 patients with mBCC refractory to conventional SHH inhibitor therapy, combined treatment with itraconazole and arsenic trioxide resulted in stable disease and a 75% reduction in SHH activity (P<.001).74 One case report documented tumor regression leading to stable disease for 15 months in a patient with laBCC treated with itraconazole monotherapy due to being unable to afford vismodegib or sonidegib. However, within 2 months of treatment discontinuation, the lesion progressed considerably.75 The efficacy of a topical formulation of itraconazole also has been tested in an open-label, placebo-controlled phase 2 trial, but no benefit was observed.76
Posaconazole is a second-generation antifungal agent that may serve as a potential alternative to itraconazole.77 Although clinical data are lacking, a basic science study found that posaconazole could inhibit the growth of SHH-dependent BCC in vivo (in mice).78 Furthermore, posaconazole has demonstrated a better safety profile with fewer and more mild side effects than itraconazole and does not require dose adjustment for those with hepatic or renal failure.79,80 Thus, posaconazole may be a safer alternative to itraconazole for the treatment of BCC. Further clinical studies are needed to elucidate the potential synergistic effects of these antifungal agents with the 2 currently approved SHH inhibitors for the treatment of advanced BCC.
Drug Resistance
Treatment resistance to SHH inhibitors, though uncommon, is a growing concern. Acquired mutations in the SMO binding site or downstream mediators of the SHH pathway have been shown to confer resistance to vismodegib and sonidegib.72,81-83 In addition, it appears that there may be shared resistance among the drugs in this class. One study assessing the efficacy of sonidegib in 9 patients with laBCC resistant to vismodegib found that these patients also did not respond to sonidegib.84 Interestingly, 1 case report documented tumor regression of an intracranial BCC in a patient treated with sonidegib and itraconazole after failure with vismodegib.85 An in vitro study also found that itraconazole maintained SHH inhibitory activity for all drug-resistant SMO mutations that have been reported.72 Therefore, itraconazole monotherapy or combination therapy with a canonical SHH inhibitor may be considered for patients with recalcitrant BCC and warrants further investigation.
Taladegib is a newly developed SMO inhibitor that may serve as another promising alternative for patients who develop resistance to vismodegib or sonidegib. A phase 1 trial of taladegib for advanced BCC found an ORR of 69% (11/16) in the SHH inhibitor–naïve group and an ORR of 36% (11/32) in the group previously treated with a SHH inhibitor.86 Additionally, the safety profile and frequency of adverse effects appear to be similar to those associated with vismodegib and sonidegib.86,87 Unfortunately, no clinical trials evaluating taladegib for BCC are ongoing or in development at this time.
Recurrence
There appears to be a relatively high rate of recurrence for BCC patients who achieve a CR to SHH inhibitors. In a retrospective study of 116 laBCC patients who experienced a CR after vismodegib therapy, 54 patients (47%) relapsed at 36 months. Among the 54 patients that relapsed, 27 were re-treated with vismodegib, which resulted in an ORR of 85% (23/27), a CR rate of 37% (10/27), and a PR rate of 48% (13/27).88 Another retrospective study of 35 laBCC patients who relapsed after vismodegib treatment reported a 31% (11/35) clinical recurrence rate at 6-month follow-up.89 An observational retrospective study also assessed the efficacy of SHH inhibitor maintenance therapy for advanced BCC patients who achieved a CR.90 In the study, 27 (64%) patients received a maintenance dose of 150 mg vismodegib once per week for 1 year, while 15 (36%) patients decided not to take a maintenance dose following CR of their BCC. All patients who took the maintenance therapy did not experience clinical recurrence at 1-year follow-up, whereas 26% of patients not on the maintenance dose relapsed.90 Consequently, these results indicate that BCC recurrence is frequent after SHH inhibitor therapy and highlights the importance of close surveillance after CR is attained. Nevertheless, most patients still respond to treatment with SHH inhibitors after relapsing, and intermittent maintenance doses may be an effective means to reduce risk of recurrence.
Conclusion
Vismodegib and sonidegib are SHH inhibitors approved for the treatment of laBCC and mBCC. Cemiplimab is now also approved for patients who do not respond to SHH inhibitors or for whom SHH inhibitors are not tolerable. Although these systemic targeted therapies can lead to notable tumor shrinkage and even complete regression in some patients, recurrence is common, and adverse effects may limit their use. Drug resistance is an emerging issue that requires additional examination. Further clinical studies are needed to determine which patients are likely to respond to these targeted treatments.
Various intermittent and maintenance drug regimens should be evaluated for their potential to mitigate adverse effects and reduce risk of recurrence, respectively. The synergistic effects of these medications with other therapies as well as their neoadjuvant and adjuvant roles should be further investigated. For example, administration of an SHH inhibitor prior to surgical excision of a BCC may allow for a smaller surgical defect size, thereby improving cosmetic and functional outcomes. Moreover, these systemic targeted medications may allow for previously inoperable tumors to become amenable to surgical treatment.
Although SHH inhibitors and PD-1 inhibitors represent a major advancement in the field of oncodermatology, real-world efficacy and safety data in the upcoming years will be important for elucidating their true benefit for patients with BCC.
Basal cell carcinoma (BCC) is the most common keratinocyte carcinoma and affects more than 3 million individuals per year in the United States.1 Approximately 40% of patients diagnosed with BCC will develop another BCC within 5 years of the initial diagnosis.2 Most cases are successfully treated with surgical excision and occasionally topical therapy or radiotherapy. Despite the high cure rate with conventional treatments, BCC can recur and can cause substantial destruction of the surrounding tissue if left untreated.3-5 In some instances, BCC can even metastasize and lead to death.6 For patients who are poor candidates for surgical or topical treatment modalities because of locally advanced BCC (laBCC) or metastatic BCC (mBCC), systemic treatment may be indicated. Vismodegib, sonidegib, and cemiplimab are the only systemic medications approved by the US Food and Drug Administration (FDA) for the treatment of laBCC and/or mBCC. Vismodegib and sonidegib target the sonic hedgehog (SHH) signaling pathway that is abnormally activated in more than 90% of BCCs.7 Cemiplimab is an immune checkpoint inhibitor (ICI) that targets the programmed cell death protein 1 (PD-1) receptor.8 Herein, we review the clinical utility of these medications and their evolving roles in the treatment of BCC.
SHH Pathway Inhibitors
The SHH pathway is a key regulator of cell proliferation and differentiation during embryogenesis.7 During adulthood, SHH signaling decreases but still plays an important role in stem cell activation and in regulation of the hair follicle growth cycle.9,10 However, de novo mutations in the genes that comprise the SHH pathway can result in aberrant constitutive activation, leading to unrestricted cell proliferation. Genetic mutations resulting in activation of Smoothened (SMO), a G-protein–coupled receptor involved in the signal transduction and propagation of the SHH pathway, have been implicated in the pathogenesis of BCC. Inactivating mutations also are commonly observed in patched homolog 1, an upstream cell-surface protein that inhibits SMO.7 The mechanism by which vismodegib and sonidegib, 2 of the FDA-approved oral medications for the treatment of advanced BCC, block the SHH pathway is through the selective inhibition of SMO.7,11
Vismodegib first received FDA approval in 2012 for the treatment of laBCC and mBCC after initial results from the pivotal ERIVANCE phase 2 trial demonstrated an objective response rate (ORR) of 43% (27/63) and 30% (10/33) in patients with locally advanced and metastatic disease, respectively. In this single-arm study, all enrolled patients (63 with laBCC and 33 with mBCC) received 150 mg of oral vismodegib daily.12 Updated results at 39 months demonstrated improved ORRs of 60% (38/63) and 48% (16/33) for the laBCC and mBCC groups, respectively. A complete response (CR) and partial response (PR) were observed in 32% (n=20) and 29% (n=18) of patients with laBCC, respectively.13 These results have been confirmed in subsequent studies, including the large international open-label trial known as STEVIE, with ORRs of 68.5% for 1119 cases of laBCC and 37% for 96 cases of mBCC.14-17 The CR and PR rates were 33% and 35%, respectively, for the laBCC group. The CR and PR rates for the mBCC group were 5% and 32%, respectively.14
The FDA approval of sonidegib for laBCC—but not mBCC—occurred in 2015 after the pivotal BOLT randomized phase 2 trial demonstrated an initial ORR of 43% (18/42) for laBCC and 15% (2/13) for mBCC after administration of 200 mg of sonidegib daily.18 A final follow-up analysis at 42 months resulted in ORRs of 56% (37/66) and 8% (1/13) for the laBCC and mBCC groups, respectively.19 Additionally, improved efficacy was not observed in the 151 patients who were randomized to receive treatment with the higher 800-mg dose; however, they did experience a higher incidence of adverse events.18,19
Currently, the true clinical differences between vismodegib and sonidegib remain uncertain, as no head-to-head trials have been conducted. Moreover, direct comparison of the data from the ERIVANCE and BOLT trials is challenging owing to fundamental differences in methodologic design, including the criteria used to assess BCC severity. The ERIVANCE trial utilized the conventional Response Evaluation Criteria in Solid Tumors (RECIST), while BOLT used the rigorous modified RECIST. However, an expert consensus study attempted to compare the 2 trials by modifying the outcomes from BOLT with the former RECIST criteria. The expert group found that the 2 SHH inhibitors had comparable efficacy and adverse event profiles.20 Nevertheless, a recent meta-analysis found that although ORRs for laBCC were similar between the 2 drugs, the CR rate for vismodegib was 31% compared with 3% for sonidegib. Additionally, for mBCC, they reported the ORR of vismodegib to be 2.7 times higher than that of sonidegib (39% vs 15%).21
Immune Checkpoint Inhibitors
Immune checkpoint inhibitors have successfully been utilized in the treatment of cutaneous squamous cell carcinoma (cSCC); however, their use for treating BCC has been limited until recently.22-25 In February 2021, cemiplimab became the first and only ICI approved for the treatment of laBCC and mBCC in patients who did not respond to or were intolerant to prior SHH inhibitor therapy.26 Cemiplimab—a human monoclonal antibody against the PD-1 receptor expressed on T cells—blocks its interaction with programmed cell death ligand 1 and programmed cell death ligand 2 present on tumor cells. The blockade of the PD-1 pathway releases the inhibition of the antitumor immune response and enables appropriate cytotoxic T-cell activity to occur.8
The FDA approval of cemiplimab for the treatment of advanced BCC was based on an open-label, multicenter, single-arm phase 2 trial (NCT03132636) evaluating 84 patients with laBCC refractory or intolerant to SHH inhibitor therapy.26 Patients received an intravenous infusion of cemiplimab 350 mg every 3 weeks for up to 93 weeks or until disease progression or unacceptable toxicity. An ORR of 31% (26/84) was observed with a CR and PR of 6% (5/84) and 25% (21/84), respectively. The median duration of follow-up was 15 months.26 Given the clinically meaningful results of this trial, investigating the efficacy of other PD-1 inhibitors, such as pembrolizumab and nivolumab, for treatment of advanced BCC may prove worthwhile.
Adverse Effects of Systemic Treatments
The 2 approved SHH inhibitors—vismodegib and sonidegib—appear to have similar side-effect profiles, with the most common adverse effects being muscle spasms, dysgeusia, alopecia, nausea, vomiting, diarrhea, weight loss, and fatigue.20,21,27 These side effects occur at high frequencies (>40%) for both SHH inhibitors and often lead to discontinuation of the medication.21 Rates of treatment discontinuation range from 15% to 50% on average.12-14,18 Fortunately, the majority of these adverse effects do not appear to increase in severity or frequency with prolonged use of these medications.14,16,28
Various conservative and pharmacologic measures can be implemented to help manage side effects. For muscle spasms, which are the most commonly reported adverse effect, supplementation with magnesium, transcutaneous electrical nerve stimulation, acupuncture, massages, stretching, and thermal compresses can potentially be beneficial.29 Calcium channel blockers also may be effective, as one small prospective cohort study reported a reduction in the frequency of muscle cramps with amlodipine 10 mg daily.30 For alopecia, which typically is reversible and caused by SHH inhibition of the normal hair cycle, minoxidil theoretically can help, as it reduces telogen arrest and extends the anagen growth phase.31,32 Although usually mild and self-limiting, management of dysgeusia, weight loss, and gastrointestinal upset often can be managed with dietary changes, such as smaller, more frequent meals.33,34 Finally, alternative dosing strategies and drug holidays have been employed to mitigate these side effects and increase drug tolerability.35,36 These are discussed in the Alternative Dosing section.
Given the essential role of the SHH pathway in embryologic development, SHH inhibitors carry a black box warning of embryofetal teratogenicity and are contraindicated in females who are pregnant or breastfeeding. For females of reproductive potential, verification of pregnancy status should be performed prior to initiating treatment with an SHH inhibitor. These patients should be counseled on the use of contraception during treatment and for at least 24 months and 20 months after cessation of vismodegib and sonidegib, respectively.27,37,38 Male patients, even after a vasectomy, should use barrier contraception during treatment and for at least 3 months and 8 months after the final dose of vismodegib and sonidegib, respectively.37,38
Laboratory abnormalities commonly associated with SHH inhibitors include elevated hepatic enzymes, particularly with vismodegib, and elevated creatine kinase levels, particularly with sonidegib.28,39 Other laboratory abnormalities that can occur include hypercholesterolemia, hypercreatininemia, hyperglycemia, and increased serum lipase levels.19,28 Although these laboratory abnormalities usually are asymptomatic and self-limiting, regular monitoring should be performed.
There also is concern that SHH inhibitors may induce the development of cSCC. A case-control study of 55 cases and 125 control patients found an increased risk for cSCC in those previously treated with vismodegib, with a hazard ratio of 8.12.40 However, a subsequent retrospective cohort study of 1675 patients with BCC failed to find any association with cSCC among those treated with vismodegib compared to those who received standard surgical therapy.41 Clinical data for sonidegib are lacking, but the BOLT trial found that cSCC occurred in 3 patients receiving treatment with the SHH inhibitor.18 Thus, further studies are needed to more thoroughly assess this concern. Close monitoring for cSCC may be warranted in patients prescribed SHH inhibitors at this time.
Cemiplimab has demonstrated an acceptable safety profile and is generally well tolerated. In the phase 2 trial of cemiplimab for cSCC, approximately 5% of patients discontinued treatment because of adverse effects. The most commonly reported side effects of cemiplimab were diarrhea (27%), fatigue (24%), nausea (17%), constipation (15%), and rash (15%).23 In the phase 2 trial for laBCC, grade 3 or 4 adverse events occurred in 48% of patients, with hypertension (5%) being the most common.26 Although rare, immune-mediated adverse reactions also can occur, given the mechanism of action of ICIs. These side effects, ranging in severity from mild to fatal, include pneumonitis, colitis, hepatitis, nephritis, myocarditis, and hypophysitis. Therefore, close monitoring for these immune-mediated reactions is critical, but most can be managed with corticosteroids or treatment interruption if they occur.42,43
No absolute contraindications exist for cemiplimab; however, extreme caution should be taken in immunosuppressed individuals, such as solid organ transplant recipients and those with chronic lymphocytic leukemia (CLL), as safety data are limited in these patients.44,45 Although small retrospective studies have reported reasonable tolerability in solid organ transplant recipients treated with ICIs, an allograft rejection rate of 41% was found in a meta-analysis of 64 patients.46-48 In CLL patients with keratinocyte carcinomas, ICIs have been safely used and have even demonstrated efficacy for CLL in some cases.49-52
Alternative Dosing
The side effects of SHH inhibitors have led to alternative dosing strategies to prevent medication discontinuation and improve adherence. In patients with basal cell nevus syndrome, multimonth drug holidays have been shown to increase drug tolerability without compromising efficacy.35,36 Weekly intermittent dosing regimens of vismodegib ranging from 1 week on followed by 1 to 3 weeks off demonstrated efficacy in a retrospective study of 7 patients with advanced BCC.53 All 7 patients experienced improvement in their BCCs, with 3 patients experiencing CR. Importantly, treatment-related adverse effects were mild and well tolerated, with no patients terminating the medication.53 Two other retrospective case series of patients with advanced BCC treated with vismodegib reported similar findings for those placed on an intermittent dosing schedule ranging from once every other day to once per week.54,55
In the large phase 2 randomized trial known as MIKIE, 2 different intermittent dosing regimens of 150 mg vismodegib daily for patients with multiple BCCs were found to have good activity and tolerability.56 The first group (n=116) received vismodegib for 12 weeks, then 3 rounds of 8 weeks of placebo, followed by 12 weeks of vismodegib; there was a 63% reduction in clinically evident BCCs after 73 weeks. The second group (n=113) received the medication for 24 weeks, then 3 rounds of 8 weeks of placebo, followed by 8 weeks of vismodegib; there was a 54% reduction at the end of 73 weeks.56 Subsequent analyses found improvements in health-related quality-of-life outcomes that were similar for both groups.57
Consequently, alternative dosing schedules appear to be a viable option for patients at risk of discontinuing treatment because of adverse effects, and current data support the recently approved recommendations of dose interruptions of up to 8 weeks to manage adverse effects in patients with laBCC or mBCC.58 Nevertheless, further clinical studies are required to determine the optimal intermittent dosing regimen for patients treated with SHH inhibitors.
Neoadjuvant Administration
Recently, vismodegib has been studied as a neoadjuvant therapy for BCC with promising results. Several small retrospective studies and case reports have documented successful treatment of both operable and inoperable periocular laBCC, with preservation of the eye in all patients.59-61 An open-label trial of 15 patients with advanced BCC who received neoadjuvant vismodegib for 3 to 6 months prior to surgical excision reported a mean reduction of 35% in the final surgical defect size, with no recurrence at 22 months.62,63 The latest and largest study performed was a phase 2 open-label trial known as VISMONEO, where 44 of 55 laBCC patients (80%) receiving neoadjuvant vismodegib for a mean duration of 6 months (range, 4–10 months) achieved the primary end point of tumor surgical downstaging.64 Of the 44 patients who had tumor downstaging, 27 (61%) experienced histologically proven CRs. Additionally, a 66% reduction in the average target lesion size was reported in this group compared to29% in the 11 patients who did not have tumor downstaging (P=.0002).64 Thus, SHH inhibitors may hold an important neoadjuvant role in the treatment of BCC by decreasing surgical defect size and allowing for surgical management of previously inoperable cases.
Synergism With Radiation
Preliminary data suggest SHH inhibitors may help potentiate the effects of radiation therapy for the treatment of BCC. Currently, the evidence primarily is limited to case studies, with several reports describing complete remission in patients with advanced BCCs who were considered unsuitable candidates for surgery. In these cases, vismodegib was administered either prior to or concurrently with radiation treatment.65-69 An in vitro study also documented the radiation-sensitizing effects of vismodegib in a BCC cell line.70 Recently, a phase 2 trial (ClinicalTrials.gov identifier NCT01835626) evaluating the concurrent use of vismodegib and radiotherapy for patients with advanced BCC was completed, but data has yet to be published.
Synergism With and Benefit of Antifungal Therapy
The antifungal drug itraconazole is a potent inhibitor of the SHH pathway and may have an adjunctive role in the treatment of BCC. Similar to vismodegib and sonidegib, itraconazole acts as a direct antagonist of SMO. However, it is thought to bind to a distinct site on SMO.71,72 An open-label, exploratory phase 2 trial of 19 patients with BCC found that oral itraconazole 200 to 400 mg daily decreased tumor proliferative index by 45% (P=.04), as measured by Ki-67; SHH activity by 65% (P=.03), as measured by GLI1 messenger RNA; and mean tumor area by 24%.73 In a case series of 5 patients with mBCC refractory to conventional SHH inhibitor therapy, combined treatment with itraconazole and arsenic trioxide resulted in stable disease and a 75% reduction in SHH activity (P<.001).74 One case report documented tumor regression leading to stable disease for 15 months in a patient with laBCC treated with itraconazole monotherapy due to being unable to afford vismodegib or sonidegib. However, within 2 months of treatment discontinuation, the lesion progressed considerably.75 The efficacy of a topical formulation of itraconazole also has been tested in an open-label, placebo-controlled phase 2 trial, but no benefit was observed.76
Posaconazole is a second-generation antifungal agent that may serve as a potential alternative to itraconazole.77 Although clinical data are lacking, a basic science study found that posaconazole could inhibit the growth of SHH-dependent BCC in vivo (in mice).78 Furthermore, posaconazole has demonstrated a better safety profile with fewer and more mild side effects than itraconazole and does not require dose adjustment for those with hepatic or renal failure.79,80 Thus, posaconazole may be a safer alternative to itraconazole for the treatment of BCC. Further clinical studies are needed to elucidate the potential synergistic effects of these antifungal agents with the 2 currently approved SHH inhibitors for the treatment of advanced BCC.
Drug Resistance
Treatment resistance to SHH inhibitors, though uncommon, is a growing concern. Acquired mutations in the SMO binding site or downstream mediators of the SHH pathway have been shown to confer resistance to vismodegib and sonidegib.72,81-83 In addition, it appears that there may be shared resistance among the drugs in this class. One study assessing the efficacy of sonidegib in 9 patients with laBCC resistant to vismodegib found that these patients also did not respond to sonidegib.84 Interestingly, 1 case report documented tumor regression of an intracranial BCC in a patient treated with sonidegib and itraconazole after failure with vismodegib.85 An in vitro study also found that itraconazole maintained SHH inhibitory activity for all drug-resistant SMO mutations that have been reported.72 Therefore, itraconazole monotherapy or combination therapy with a canonical SHH inhibitor may be considered for patients with recalcitrant BCC and warrants further investigation.
Taladegib is a newly developed SMO inhibitor that may serve as another promising alternative for patients who develop resistance to vismodegib or sonidegib. A phase 1 trial of taladegib for advanced BCC found an ORR of 69% (11/16) in the SHH inhibitor–naïve group and an ORR of 36% (11/32) in the group previously treated with a SHH inhibitor.86 Additionally, the safety profile and frequency of adverse effects appear to be similar to those associated with vismodegib and sonidegib.86,87 Unfortunately, no clinical trials evaluating taladegib for BCC are ongoing or in development at this time.
Recurrence
There appears to be a relatively high rate of recurrence for BCC patients who achieve a CR to SHH inhibitors. In a retrospective study of 116 laBCC patients who experienced a CR after vismodegib therapy, 54 patients (47%) relapsed at 36 months. Among the 54 patients that relapsed, 27 were re-treated with vismodegib, which resulted in an ORR of 85% (23/27), a CR rate of 37% (10/27), and a PR rate of 48% (13/27).88 Another retrospective study of 35 laBCC patients who relapsed after vismodegib treatment reported a 31% (11/35) clinical recurrence rate at 6-month follow-up.89 An observational retrospective study also assessed the efficacy of SHH inhibitor maintenance therapy for advanced BCC patients who achieved a CR.90 In the study, 27 (64%) patients received a maintenance dose of 150 mg vismodegib once per week for 1 year, while 15 (36%) patients decided not to take a maintenance dose following CR of their BCC. All patients who took the maintenance therapy did not experience clinical recurrence at 1-year follow-up, whereas 26% of patients not on the maintenance dose relapsed.90 Consequently, these results indicate that BCC recurrence is frequent after SHH inhibitor therapy and highlights the importance of close surveillance after CR is attained. Nevertheless, most patients still respond to treatment with SHH inhibitors after relapsing, and intermittent maintenance doses may be an effective means to reduce risk of recurrence.
Conclusion
Vismodegib and sonidegib are SHH inhibitors approved for the treatment of laBCC and mBCC. Cemiplimab is now also approved for patients who do not respond to SHH inhibitors or for whom SHH inhibitors are not tolerable. Although these systemic targeted therapies can lead to notable tumor shrinkage and even complete regression in some patients, recurrence is common, and adverse effects may limit their use. Drug resistance is an emerging issue that requires additional examination. Further clinical studies are needed to determine which patients are likely to respond to these targeted treatments.
Various intermittent and maintenance drug regimens should be evaluated for their potential to mitigate adverse effects and reduce risk of recurrence, respectively. The synergistic effects of these medications with other therapies as well as their neoadjuvant and adjuvant roles should be further investigated. For example, administration of an SHH inhibitor prior to surgical excision of a BCC may allow for a smaller surgical defect size, thereby improving cosmetic and functional outcomes. Moreover, these systemic targeted medications may allow for previously inoperable tumors to become amenable to surgical treatment.
Although SHH inhibitors and PD-1 inhibitors represent a major advancement in the field of oncodermatology, real-world efficacy and safety data in the upcoming years will be important for elucidating their true benefit for patients with BCC.
- Rogers HW, Weinstock MA, Feldman SR, et al. Incidence estimate of nonmelanoma skin cancer (keratinocyte carcinomas) in the U.S. population, 2012. JAMA Dermatol. 2015;151:1081-1086.
- Cameron MC, Lee E, Hibler BP, et al. Basal cell carcinoma: epidemiology; pathophysiology; clinical and histological subtypes; and disease associations. J Am Acad Dermatol. 2019;80:303-317.
- Rees JR, Zens MS, Celaya MO, et al. Survival after squamous cell and basal cell carcinoma of the skin: a retrospective cohort analysis. Int J Cancer. 2015;137:878-884.
- Kasumagic-Halilovic E, Hasic M, Ovcina-Kurtovic N. A clinical study of basal cell carcinoma. Med Arch. 2019;73:394-398.
- Goldenberg G, Karagiannis T, Palmer JB, et al. Incidence and prevalence of basal cell carcinoma (BCC) and locally advanced BCC (LABCC) in a large commercially insured population in the United States: a retrospective cohort study. J Am Acad Dermatol. 2016;75:957.e952-966.e952.
- Laga AC, Schaefer IM, Sholl LM, et al. Metastatic basal cell carcinoma. Am J Clin Pathol. 2019;152:706-717.
- Rimkus TK, Carpenter RL, Qasem S, et al. Targeting the sonic hedgehog signaling pathway: review of smoothened and GLI inhibitors. Cancers (Basel). 2016;8:22.
- Burova E, Hermann A, Waite J, et al. Characterization of the anti–PD-1 antibody REGN2810 and its antitumor activity in human PD-1 knock-in mice. Mol Cancer Ther. 2017;16:861-870.
- Bhardwaj G, Murdoch B, Wu D, et al. Sonic hedgehog induces the proliferation of primitive human hematopoietic cells via BMP regulation. Nat Immunol. 2001;2:172-180.
- Paladini RD, Saleh J, Qian C, et al. Modulation of hair growth with small molecule agonists of the hedgehog signaling pathway. J Invest Dermatol. 2005;125:638-646.
- Von Hoff DD, LoRusso PM, Rudin CM, et al. Inhibition of the hedgehog pathway in advanced basal-cell carcinoma. N Engl J Med. 2009;361:1164-1172.
- Sekulic A, Migden MR, Oro AE, et al. Efficacy and safety of vismodegib in advanced basal-cell carcinoma. N Engl J Med. 2012;366:2171-2179.
- Sekulic A, Migden MR, Basset-Seguin N, et al. Long-term safety and efficacy of vismodegib in patients with advanced basal cell carcinoma: final update of the pivotal ERIVANCE BCC study. BMC Cancer. 2017;17:332.
- Basset-Séguin N, Hauschild A, Kunstfeld R, et al. Vismodegib in patients with advanced basal cell carcinoma: primary analysis of STEVIE, an international, open-label trial. Eur J Cancer. 2017;86:334-348.
- Cozzani R, Del Aguila R, Carrizo M, et al. Efficacy and safety profile of vismodegib in a real-world setting cohort of patients with advanced basal cell carcinoma in Argentina. Int J Dermatol. 2020;59:627-632.
- Spallone G, Sollena P, Ventura A, et al. Efficacy and safety of vismodegib treatment in patients with advanced basal cell carcinoma and multiple comorbidities. Dermatol Ther. 2019;32:E13108.
- Fosko SW, Chu MB, Armbrecht E, et al. Efficacy, rate of tumor response, and safety of a short course (12-24 weeks) of oral vismodegib in various histologic subtypes (infiltrative, nodular, and superficial) of high-risk or locally advanced basal cell carcinoma, in an open-label, prospective case series clinical trial. J Am Acad Dermatol. 2020;82:946-954.
- Migden MR, Guminski A, Gutzmer R, et al. Treatment with two different doses of sonidegib in patients with locally advanced or metastatic basal cell carcinoma (BOLT): a multicentre, randomised, double-blind phase 2 trial. Lancet Oncol. 2015;16:716-728.
- Dummer R, Guminksi A, Gutzmer R, et al. Long-term efficacy and safety of sonidegib in patients with advanced basal cell carcinoma: 42-month analysis of the phase II randomized, double-blind BOLT study. Br J Dermatol. 2020;182:1369-1378.
- Dummer R, Ascierto PA, Basset-Seguin N, et al. Sonidegib and vismodegib in the treatment of patients with locally advanced basal cell carcinoma: a joint expert opinion. J Eur Acad Dermatol Venereol. 2020;34:1944-1956.
- Xie P, Lefrançois P. Efficacy, safety, and comparison of sonic hedgehog inhibitors in basal cell carcinomas: a systematic review and meta-analysis. J Am Acad Dermatol. 2018;79:1089-1100.e1017.
- Gentzler R, Hall R, Kunk PR, et al. Beyond melanoma: inhibiting the PD-1/PD-L1 pathway in solid tumors. Immunotherapy. 2016;8:583-600.
- Guminski AD, Lim AML, Khushalani NI, et al. Phase 2 study of cemiplimab, a human monoclonal anti-PD-1, in patients (pts) with metastatic cutaneous squamous cell carcinoma (mCSCC; group 1): 12-month follow-up [abstract]. J Clin Oncol. 2019;37(15 suppl):9526.
- Grob JJ, Gonzalez Mendoza R, Basset-Seguin N, et al. Pembrolizumab for recurrent/metastatic cutaneous squamous cell carcinoma (cSCC): efficacy and safety results from the phase II KEYNOTE-629 study [abstract]. Ann Oncol. 2019;30 (suppl 5):v908.
- Maubec E, Boubaya M, Petrow P, et al. Pembrolizumab as first-line therapy in patients with unresectable cutaneous squamous cell carcinoma (cSCC): phase 2 results from CARSKIN [abstract]. J Clin Oncol. 2019;37(15 suppl):9547.
- Stratigos AJ, Sekulic A, Peris K, et al. Cemiplimab in locally advanced basal cell carcinoma after hedgehog inhibitor therapy: an open-label, multi-centre, single-arm, phase 2 trial. Lancet Oncol. 2021;22:848-857.
- Carpenter RL, Ray H. Safety and tolerability of sonic hedgehog pathway inhibitors in cancer. Drug Saf. 2019;42:263-279.
- Villani A, Fabbrocini G, Costa C, et al. Sonidegib: safety and efficacy in treatment of advanced basal cell carcinoma. Dermatol Ther (Heidelb). 2020;10:401-412.
- Wright A, Sluka KA. Nonpharmacological treatments for musculoskeletal pain. Clin J Pain. 2001;17:33-46.
- Ally MS, Tang JY, Lindgren J, et al. Effect of calcium channel blockade on vismodegib-induced muscle cramps. JAMA Dermatol. 2015;151:1132-1134.
- Yang X, Thai K-E. Treatment of permanent chemotherapy-induced alopecia with low dose oral minoxidil. Australas J Dermatol. 2016;57:e130-e132.
- Ferguson JS, Hannam S, Toholka R, et al. Hair loss and hedgehog inhibitors: a class effect? Br J Dermatol. 2015;173:262-264.
- Kumbargere Nagraj S, George RP, Shetty N, et al. Interventions for managing taste disturbances. Cochrane Database Syst Rev. 2017;12:CD010470.
- Jacobsen AA, Kydd AR, Strasswimmer J. Practical management of the adverse effects of hedgehog pathway inhibitor therapy for basal cell carcinoma. J Am Acad Dermatol. 2017;76:767-768.
- Ally MS, Tang JY, Joseph T, et al. The use of vismodegib to shrink keratocystic odontogenic tumors in patients with basal cell nevus syndrome. JAMA Dermatol. 2014;150:542-545.
- Yang X, Dinehart SM. Intermittent vismodegib therapy in basal cell nevus syndrome. JAMA Dermatol. 2016;152:223-224.
- Erivedge. Prescribing information. Genentech; 2015.
- Odomzo. Prescribing information. Novartis; 2015.
- Ventarola DJ, Silverstein DI. Vismodegib-associated hepatotoxicity: a potential side effect detected in postmarketing surveillance. J Am Acad Dermatol. 2014;71:397-398.
- Mohan SV, Chang J, Li S, et al. Increased risk of cutaneous squamous cell carcinoma after vismodegib therapy for basal cell carcinoma. JAMA Dermatol. 2016;152:527-532.
- Bhutani T, Abrouk M, Sima CS, et al. Risk of cutaneous squamous cell carcinoma after treatment of basal cell carcinoma with vismodegib. J Am Acad Dermatol. 2017;77:713-718.
- Morgado M, Plácido A, Morgado S, et al. Management of the adverse effects of immune checkpoint inhibitors. Vaccines (Basel). 2020;8:575.
- Martins F, Sofiya L, Sykiotis GP, et al. Adverse effects of immune-checkpoint inhibitors: epidemiology, management and surveillance. Nat Rev Clin Oncol. 2019;16:563-580.
- Ntsethe A, Dludla PV, Nyambuya TM, et al. The impact of immune checkpoint inhibitors in patients with chronic lymphocytic leukemia (CLL): a protocol for a systematic review and meta-analysis of randomized controlled trials. Medicine (Baltimore). 2020;99:E21167.
- Johnson DB, Sullivan RJ, Menzies AM. Immune checkpoint inhibitors in challenging populations. Cancer. 2017;123:1904-1911.
- Tsung I, Worden FP, Fontana RJ. Safety and efficacy of checkpoint inhibitors in solid organ transplant recipients with cutaneous squamous cell carcinoma [abstract]. J Clin Oncol. 2020;38(15 suppl):E22014.
- Owoyemi I, Vaughan LE, Costello CM, et al. Clinical outcomes of solid organ transplant recipients with metastatic cancers who are treated with immune checkpoint inhibitors: a single-center analysis. Cancer. 2020;126:4780-4787.
- Kumar V, Shinagare AB, Rennke HG, et al. The safety and efficacy of checkpoint inhibitors in transplant recipients: a case series and systematic review of literature. Oncologist. 2020;25:505-514.
- Arenbergerova M, Fialova A, Arenberger P, et al. Killing two birds with one stone: response to pembrolizumab in a patient with metastatic melanoma and B-cell chronic lymphocytic leukaemia. J Eur Acad Dermatol Venereol. 2018;32:E72-E74.
- Archibald WJ, Meacham PJ, Williams AM, et al. Management of melanoma in patients with chronic lymphocytic leukemia. Leuk Res. 2018;71:43-46.
- Ding W, LaPlant BR, Call TG, et al. Pembrolizumab in patients with CLL and Richter transformation or with relapsed CLL. Blood. 2017;129:3419-3427.
- Leiter U, Loquai C, Reinhardt L, et al. Immune checkpoint inhibition therapy for advanced skin cancer in patients with concomitant hematological malignancy: a retrospective multicenter DeCOG study of 84 patients. J Immunother Cancer. 2020;8:E000897.
- Becker LR, Aakhus AE, Reich HC, et al. A novel alternate dosing of vismodegib for treatment of patients with advanced basal cell carcinomas. JAMA Dermatol. 2017;153:321-322.
- Woltsche N, Pichler N, Wolf I, et al. Managing adverse effects by dose reduction during routine treatment of locally advanced basal cell carcinoma with the hedgehog inhibitor vismodegib: a single centre experience. J Eur Acad Dermatol Venereol. 2019;33:E144-E145.
- Wong C, Poblete-Lopez C, Vidimos A. Comparison of daily dosing versus Monday through Friday dosing of vismodegib for locally advanced basal cell carcinoma and basal cell nevus syndrome: a retrospective case series. J Am Acad Dermatol. 2020;82:1539-1542.
- Dréno B, Kunstfeld R, Hauschild A, et al. Two intermittent vismodegib dosing regimens in patients with multiple basal-cell carcinomas (MIKIE): a randomised, regimen-controlled, double-blind, phase 2 trial. Lancet Oncol. 2017;18:404-412.
- Schadendorf D, Hauschild A, Fosko S, et al. Quality-of-life analysis with intermittent vismodegib regimens in patients with multiple basal cell carcinomas: patient-reported outcomes from the MIKIE study. J Eur Acad Dermatol Venereol. 2020;34:E526-E529.
- Chanu P, Musib L, Wang X, et al. Vismodegib efficacy in advanced basal cell carcinoma maintained with 8-week dose interruptions: a model-based evaluation. J Invest Dermatol. 2021;141:930-933.
- Su MG, Potts LB, Tsai JH. Treatment of periocular basal cell carcinoma with neoadjuvant vismodegib. Am J Ophthalmol Case Rep. 2020;19:100755.
- González AR, Etchichury D, Gil ME, et al. Neoadjuvant vismodegib and Mohs micrographic surgery for locally advanced periocular basal cell carcinoma. Ophthalmic Plast Reconstr Surg. 2019;35:56-61.
- Sagiv O, Nagarajan P, Ferrarotto R, et al. Ocular preservation with neoadjuvant vismodegib in patients with locally advanced periocular basal cell carcinoma. Br J Ophthalmol. 2019;103:775-780.
- Ally MS, Aasi S, Wysong A, et al. An investigator-initiated open-label clinical trial of vismodegib as a neoadjuvant to surgery for high-risk basal cell carcinoma. J Am Acad Dermatol. 2014;71:904-911.e1.
- Kwon GP, Ally MS, Bailey-Healy I, et al. Update to an open-label clinical trial of vismodegib as neoadjuvant before surgery for high-risk basal cell carcinoma (BCC). J Am Acad Dermatol. 2016;75:213-215.
- Mortier L, Bertrand N, Basset-Seguin N, et al. Vismodegib in neoadjuvant treatment of locally advanced basal cell carcinoma: first results of a multicenter, open-label, phase 2 trial (VISMONEO study) [abstract]. J Clin Oncol. 2018;36(15 suppl):9509.
- Strasswimmer JM. Potential synergy of radiation therapy with vismodegib for basal cell carcinoma. JAMA Dermatol. 2015;151:925-926.
- Gathings RM, Orscheln CS, Huang WW. Compassionate use of vismodegib and adjuvant radiotherapy in the treatment of multiple locally advanced and inoperable basal cell carcinomas and squamous cell carcinomas of the skin. J Am Acad Dermatol. 2014;70:E88-E89.
- Franco AI, Eastwick G, Farah R, et al. Upfront radiotherapy with concurrent and adjuvant vismodegib is effective and well-tolerated in a patient with advanced, multifocal basal cell carcinoma. Case Rep Dermatol Med. 2018;2018:2354146.
- Pollom EL, Bui TT, Chang AL, et al. Concurrent vismodegib and radiotherapy for recurrent, advanced basal cell carcinoma. JAMA Dermatol. 2015;151:998-1001.
- Janela-Lapert R, Dubray B, Duval-Modeste A, et al. Treatment of advanced basal cell carcinoma with vismodegib followed by radiotherapy [in French]. Ann Dermatol Venereol. 2020;147:780-782.
- Hehlgans S, Booms P, Güllülü Ö, et al. Radiation sensitization of basal cell and head and neck squamous cell carcinoma by the hedgehog pathway inhibitor vismodegib. Int J Mol Sci. 2018;19:2485.
- Kim J, Tang JY, Gong R, et al. Itraconazole, a commonly used antifungal that inhibits hedgehog pathway activity and cancer growth. Cancer Cell. 2010;17:388-399.
- Kim J, Aftab BT, Tang JY, et al. Itraconazole and arsenic trioxide inhibit hedgehog pathway activation and tumor growth associated with acquired resistance to smoothened antagonists. Cancer Cell. 2013;23:23-34.
- Kim DJ, Kim J, Spaunhurst K, et al. Open-label, exploratory phase II trial of oral itraconazole for the treatment of basal cell carcinoma. J Clin Oncol. 2014;32:745-751.
- Ally MS, Ransohoff K, Sarin K, et al. Effects of combined treatment with arsenic trioxide and itraconazole in patients with refractory metastatic basal cell carcinoma. JAMA Dermatol. 2016;152:452-456.
- Cia˛z˙yn´yska M, Narbutt J, Skibin´ska M, et al. Itraconazole—a new player in the therapy of advanced basal cell carcinoma: a case report. JCO Oncol Pract. 2020;16:837-838.
- Sohn GK, Kwon GP, Bailey-Healy I, et al. Topical itraconazole for the treatment of basal cell carcinoma in patients with basal cell nevus syndrome or high-frequency basal cell carcinomas: a phase 2, open-label, placebo-controlled trial. JAMA Dermatol. 2019;155:1078-1080.
- Lass-Flörl C. Triazole antifungal agents in invasive fungal infections: a comparative review. Drugs. 2011;71:2405-2419.
- Chen B, Trang V, Lee A, et al. Posaconazole, a second-generation triazole antifungal drug, inhibits the hedgehog signaling pathway and progression of basal cell carcinoma. Mol Cancer Ther. 2016;15:866-876.
- Katragkou A, Tsikopoulou F, Roilides E, et al. Posaconazole: when and how? the clinician’s view. Mycoses. 2012;55:110-122.
- Raad II, Graybill JR, Bustamante AB, et al. Safety of long-term oral posaconazole use in the treatment of refractory invasive fungal infections. Clin Infect Dis. 2006;42:1726-1734.
- Atwood SX, Sarin KY, Whitson RJ, et al. Smoothened variants explain the majority of drug resistance in basal cell carcinoma. Cancer Cell. 2015;27:342-353.
- Sun Q, Atzmony L, Zaki T, et al. Clues to primary vismodegib resistance lie in histology and genetics. J Clin Pathol. 2020;73:678-680.
- Verkouteren BJA, Wakkee M, van Geel M, et al. Molecular testing in metastatic basal cell carcinoma. J Am Acad Dermatol. 2021;85:1135-1142.
- Danial C, Sarin KY, Oro AE, et al. An investigator-initiated open-label trial of sonidegib in advanced basal cell carcinoma patients resistant to vismodegib. Clin Cancer Res. 2016;22:1325-1329.
- Yoon J, Apicelli AJ 3rd, Pavlopoulos TV. Intracranial regression of an advanced basal cell carcinoma using sonidegib and itraconazole after failure with vismodegib. JAAD Case Rep. 2017;4:10-12.
- Bendell J, Andre V, Ho A, et al. Phase I study of LY2940680, a Smo antagonist, in patients with advanced cancer including treatment-naïve and previously treated basal cell carcinoma. Clin Cancer Res. 2018;24:2082-2091.
- Ueno H, Kondo S, Yoshikawa S, et al. A phase I and pharmacokinetic study of taladegib, a Smoothened inhibitor, in Japanese patients with advanced solid tumors. Invest New Drugs. 2018;36:647-656.
- Herms F, Lambert J, Grob JJ, et al. Follow-up of patients with complete remission of locally advanced basal cell carcinoma after vismodegib discontinuation: a multicenter French study of 116 patients. J Clin Oncol. 2019;37:3275-3282.
- Villani A, Megna M, Fabbrocini G, et al. Long-term efficacy of vismodegib after its withdrawal and patients’ health-related quality of life using the Dermatology Life Quality Index (DLQI). Dermatol Ther (Heidelb). 2019;9:719-724.
- Scalvenzi M, Cappello M, Costa C, et al. Low-dose vismodegib as maintenance therapy after locally advanced basal cell carcinoma complete remission: high efficacy with minimal toxicity. Dermatol Ther (Heidelb). 2020;10:465-468.
- Rogers HW, Weinstock MA, Feldman SR, et al. Incidence estimate of nonmelanoma skin cancer (keratinocyte carcinomas) in the U.S. population, 2012. JAMA Dermatol. 2015;151:1081-1086.
- Cameron MC, Lee E, Hibler BP, et al. Basal cell carcinoma: epidemiology; pathophysiology; clinical and histological subtypes; and disease associations. J Am Acad Dermatol. 2019;80:303-317.
- Rees JR, Zens MS, Celaya MO, et al. Survival after squamous cell and basal cell carcinoma of the skin: a retrospective cohort analysis. Int J Cancer. 2015;137:878-884.
- Kasumagic-Halilovic E, Hasic M, Ovcina-Kurtovic N. A clinical study of basal cell carcinoma. Med Arch. 2019;73:394-398.
- Goldenberg G, Karagiannis T, Palmer JB, et al. Incidence and prevalence of basal cell carcinoma (BCC) and locally advanced BCC (LABCC) in a large commercially insured population in the United States: a retrospective cohort study. J Am Acad Dermatol. 2016;75:957.e952-966.e952.
- Laga AC, Schaefer IM, Sholl LM, et al. Metastatic basal cell carcinoma. Am J Clin Pathol. 2019;152:706-717.
- Rimkus TK, Carpenter RL, Qasem S, et al. Targeting the sonic hedgehog signaling pathway: review of smoothened and GLI inhibitors. Cancers (Basel). 2016;8:22.
- Burova E, Hermann A, Waite J, et al. Characterization of the anti–PD-1 antibody REGN2810 and its antitumor activity in human PD-1 knock-in mice. Mol Cancer Ther. 2017;16:861-870.
- Bhardwaj G, Murdoch B, Wu D, et al. Sonic hedgehog induces the proliferation of primitive human hematopoietic cells via BMP regulation. Nat Immunol. 2001;2:172-180.
- Paladini RD, Saleh J, Qian C, et al. Modulation of hair growth with small molecule agonists of the hedgehog signaling pathway. J Invest Dermatol. 2005;125:638-646.
- Von Hoff DD, LoRusso PM, Rudin CM, et al. Inhibition of the hedgehog pathway in advanced basal-cell carcinoma. N Engl J Med. 2009;361:1164-1172.
- Sekulic A, Migden MR, Oro AE, et al. Efficacy and safety of vismodegib in advanced basal-cell carcinoma. N Engl J Med. 2012;366:2171-2179.
- Sekulic A, Migden MR, Basset-Seguin N, et al. Long-term safety and efficacy of vismodegib in patients with advanced basal cell carcinoma: final update of the pivotal ERIVANCE BCC study. BMC Cancer. 2017;17:332.
- Basset-Séguin N, Hauschild A, Kunstfeld R, et al. Vismodegib in patients with advanced basal cell carcinoma: primary analysis of STEVIE, an international, open-label trial. Eur J Cancer. 2017;86:334-348.
- Cozzani R, Del Aguila R, Carrizo M, et al. Efficacy and safety profile of vismodegib in a real-world setting cohort of patients with advanced basal cell carcinoma in Argentina. Int J Dermatol. 2020;59:627-632.
- Spallone G, Sollena P, Ventura A, et al. Efficacy and safety of vismodegib treatment in patients with advanced basal cell carcinoma and multiple comorbidities. Dermatol Ther. 2019;32:E13108.
- Fosko SW, Chu MB, Armbrecht E, et al. Efficacy, rate of tumor response, and safety of a short course (12-24 weeks) of oral vismodegib in various histologic subtypes (infiltrative, nodular, and superficial) of high-risk or locally advanced basal cell carcinoma, in an open-label, prospective case series clinical trial. J Am Acad Dermatol. 2020;82:946-954.
- Migden MR, Guminski A, Gutzmer R, et al. Treatment with two different doses of sonidegib in patients with locally advanced or metastatic basal cell carcinoma (BOLT): a multicentre, randomised, double-blind phase 2 trial. Lancet Oncol. 2015;16:716-728.
- Dummer R, Guminksi A, Gutzmer R, et al. Long-term efficacy and safety of sonidegib in patients with advanced basal cell carcinoma: 42-month analysis of the phase II randomized, double-blind BOLT study. Br J Dermatol. 2020;182:1369-1378.
- Dummer R, Ascierto PA, Basset-Seguin N, et al. Sonidegib and vismodegib in the treatment of patients with locally advanced basal cell carcinoma: a joint expert opinion. J Eur Acad Dermatol Venereol. 2020;34:1944-1956.
- Xie P, Lefrançois P. Efficacy, safety, and comparison of sonic hedgehog inhibitors in basal cell carcinomas: a systematic review and meta-analysis. J Am Acad Dermatol. 2018;79:1089-1100.e1017.
- Gentzler R, Hall R, Kunk PR, et al. Beyond melanoma: inhibiting the PD-1/PD-L1 pathway in solid tumors. Immunotherapy. 2016;8:583-600.
- Guminski AD, Lim AML, Khushalani NI, et al. Phase 2 study of cemiplimab, a human monoclonal anti-PD-1, in patients (pts) with metastatic cutaneous squamous cell carcinoma (mCSCC; group 1): 12-month follow-up [abstract]. J Clin Oncol. 2019;37(15 suppl):9526.
- Grob JJ, Gonzalez Mendoza R, Basset-Seguin N, et al. Pembrolizumab for recurrent/metastatic cutaneous squamous cell carcinoma (cSCC): efficacy and safety results from the phase II KEYNOTE-629 study [abstract]. Ann Oncol. 2019;30 (suppl 5):v908.
- Maubec E, Boubaya M, Petrow P, et al. Pembrolizumab as first-line therapy in patients with unresectable cutaneous squamous cell carcinoma (cSCC): phase 2 results from CARSKIN [abstract]. J Clin Oncol. 2019;37(15 suppl):9547.
- Stratigos AJ, Sekulic A, Peris K, et al. Cemiplimab in locally advanced basal cell carcinoma after hedgehog inhibitor therapy: an open-label, multi-centre, single-arm, phase 2 trial. Lancet Oncol. 2021;22:848-857.
- Carpenter RL, Ray H. Safety and tolerability of sonic hedgehog pathway inhibitors in cancer. Drug Saf. 2019;42:263-279.
- Villani A, Fabbrocini G, Costa C, et al. Sonidegib: safety and efficacy in treatment of advanced basal cell carcinoma. Dermatol Ther (Heidelb). 2020;10:401-412.
- Wright A, Sluka KA. Nonpharmacological treatments for musculoskeletal pain. Clin J Pain. 2001;17:33-46.
- Ally MS, Tang JY, Lindgren J, et al. Effect of calcium channel blockade on vismodegib-induced muscle cramps. JAMA Dermatol. 2015;151:1132-1134.
- Yang X, Thai K-E. Treatment of permanent chemotherapy-induced alopecia with low dose oral minoxidil. Australas J Dermatol. 2016;57:e130-e132.
- Ferguson JS, Hannam S, Toholka R, et al. Hair loss and hedgehog inhibitors: a class effect? Br J Dermatol. 2015;173:262-264.
- Kumbargere Nagraj S, George RP, Shetty N, et al. Interventions for managing taste disturbances. Cochrane Database Syst Rev. 2017;12:CD010470.
- Jacobsen AA, Kydd AR, Strasswimmer J. Practical management of the adverse effects of hedgehog pathway inhibitor therapy for basal cell carcinoma. J Am Acad Dermatol. 2017;76:767-768.
- Ally MS, Tang JY, Joseph T, et al. The use of vismodegib to shrink keratocystic odontogenic tumors in patients with basal cell nevus syndrome. JAMA Dermatol. 2014;150:542-545.
- Yang X, Dinehart SM. Intermittent vismodegib therapy in basal cell nevus syndrome. JAMA Dermatol. 2016;152:223-224.
- Erivedge. Prescribing information. Genentech; 2015.
- Odomzo. Prescribing information. Novartis; 2015.
- Ventarola DJ, Silverstein DI. Vismodegib-associated hepatotoxicity: a potential side effect detected in postmarketing surveillance. J Am Acad Dermatol. 2014;71:397-398.
- Mohan SV, Chang J, Li S, et al. Increased risk of cutaneous squamous cell carcinoma after vismodegib therapy for basal cell carcinoma. JAMA Dermatol. 2016;152:527-532.
- Bhutani T, Abrouk M, Sima CS, et al. Risk of cutaneous squamous cell carcinoma after treatment of basal cell carcinoma with vismodegib. J Am Acad Dermatol. 2017;77:713-718.
- Morgado M, Plácido A, Morgado S, et al. Management of the adverse effects of immune checkpoint inhibitors. Vaccines (Basel). 2020;8:575.
- Martins F, Sofiya L, Sykiotis GP, et al. Adverse effects of immune-checkpoint inhibitors: epidemiology, management and surveillance. Nat Rev Clin Oncol. 2019;16:563-580.
- Ntsethe A, Dludla PV, Nyambuya TM, et al. The impact of immune checkpoint inhibitors in patients with chronic lymphocytic leukemia (CLL): a protocol for a systematic review and meta-analysis of randomized controlled trials. Medicine (Baltimore). 2020;99:E21167.
- Johnson DB, Sullivan RJ, Menzies AM. Immune checkpoint inhibitors in challenging populations. Cancer. 2017;123:1904-1911.
- Tsung I, Worden FP, Fontana RJ. Safety and efficacy of checkpoint inhibitors in solid organ transplant recipients with cutaneous squamous cell carcinoma [abstract]. J Clin Oncol. 2020;38(15 suppl):E22014.
- Owoyemi I, Vaughan LE, Costello CM, et al. Clinical outcomes of solid organ transplant recipients with metastatic cancers who are treated with immune checkpoint inhibitors: a single-center analysis. Cancer. 2020;126:4780-4787.
- Kumar V, Shinagare AB, Rennke HG, et al. The safety and efficacy of checkpoint inhibitors in transplant recipients: a case series and systematic review of literature. Oncologist. 2020;25:505-514.
- Arenbergerova M, Fialova A, Arenberger P, et al. Killing two birds with one stone: response to pembrolizumab in a patient with metastatic melanoma and B-cell chronic lymphocytic leukaemia. J Eur Acad Dermatol Venereol. 2018;32:E72-E74.
- Archibald WJ, Meacham PJ, Williams AM, et al. Management of melanoma in patients with chronic lymphocytic leukemia. Leuk Res. 2018;71:43-46.
- Ding W, LaPlant BR, Call TG, et al. Pembrolizumab in patients with CLL and Richter transformation or with relapsed CLL. Blood. 2017;129:3419-3427.
- Leiter U, Loquai C, Reinhardt L, et al. Immune checkpoint inhibition therapy for advanced skin cancer in patients with concomitant hematological malignancy: a retrospective multicenter DeCOG study of 84 patients. J Immunother Cancer. 2020;8:E000897.
- Becker LR, Aakhus AE, Reich HC, et al. A novel alternate dosing of vismodegib for treatment of patients with advanced basal cell carcinomas. JAMA Dermatol. 2017;153:321-322.
- Woltsche N, Pichler N, Wolf I, et al. Managing adverse effects by dose reduction during routine treatment of locally advanced basal cell carcinoma with the hedgehog inhibitor vismodegib: a single centre experience. J Eur Acad Dermatol Venereol. 2019;33:E144-E145.
- Wong C, Poblete-Lopez C, Vidimos A. Comparison of daily dosing versus Monday through Friday dosing of vismodegib for locally advanced basal cell carcinoma and basal cell nevus syndrome: a retrospective case series. J Am Acad Dermatol. 2020;82:1539-1542.
- Dréno B, Kunstfeld R, Hauschild A, et al. Two intermittent vismodegib dosing regimens in patients with multiple basal-cell carcinomas (MIKIE): a randomised, regimen-controlled, double-blind, phase 2 trial. Lancet Oncol. 2017;18:404-412.
- Schadendorf D, Hauschild A, Fosko S, et al. Quality-of-life analysis with intermittent vismodegib regimens in patients with multiple basal cell carcinomas: patient-reported outcomes from the MIKIE study. J Eur Acad Dermatol Venereol. 2020;34:E526-E529.
- Chanu P, Musib L, Wang X, et al. Vismodegib efficacy in advanced basal cell carcinoma maintained with 8-week dose interruptions: a model-based evaluation. J Invest Dermatol. 2021;141:930-933.
- Su MG, Potts LB, Tsai JH. Treatment of periocular basal cell carcinoma with neoadjuvant vismodegib. Am J Ophthalmol Case Rep. 2020;19:100755.
- González AR, Etchichury D, Gil ME, et al. Neoadjuvant vismodegib and Mohs micrographic surgery for locally advanced periocular basal cell carcinoma. Ophthalmic Plast Reconstr Surg. 2019;35:56-61.
- Sagiv O, Nagarajan P, Ferrarotto R, et al. Ocular preservation with neoadjuvant vismodegib in patients with locally advanced periocular basal cell carcinoma. Br J Ophthalmol. 2019;103:775-780.
- Ally MS, Aasi S, Wysong A, et al. An investigator-initiated open-label clinical trial of vismodegib as a neoadjuvant to surgery for high-risk basal cell carcinoma. J Am Acad Dermatol. 2014;71:904-911.e1.
- Kwon GP, Ally MS, Bailey-Healy I, et al. Update to an open-label clinical trial of vismodegib as neoadjuvant before surgery for high-risk basal cell carcinoma (BCC). J Am Acad Dermatol. 2016;75:213-215.
- Mortier L, Bertrand N, Basset-Seguin N, et al. Vismodegib in neoadjuvant treatment of locally advanced basal cell carcinoma: first results of a multicenter, open-label, phase 2 trial (VISMONEO study) [abstract]. J Clin Oncol. 2018;36(15 suppl):9509.
- Strasswimmer JM. Potential synergy of radiation therapy with vismodegib for basal cell carcinoma. JAMA Dermatol. 2015;151:925-926.
- Gathings RM, Orscheln CS, Huang WW. Compassionate use of vismodegib and adjuvant radiotherapy in the treatment of multiple locally advanced and inoperable basal cell carcinomas and squamous cell carcinomas of the skin. J Am Acad Dermatol. 2014;70:E88-E89.
- Franco AI, Eastwick G, Farah R, et al. Upfront radiotherapy with concurrent and adjuvant vismodegib is effective and well-tolerated in a patient with advanced, multifocal basal cell carcinoma. Case Rep Dermatol Med. 2018;2018:2354146.
- Pollom EL, Bui TT, Chang AL, et al. Concurrent vismodegib and radiotherapy for recurrent, advanced basal cell carcinoma. JAMA Dermatol. 2015;151:998-1001.
- Janela-Lapert R, Dubray B, Duval-Modeste A, et al. Treatment of advanced basal cell carcinoma with vismodegib followed by radiotherapy [in French]. Ann Dermatol Venereol. 2020;147:780-782.
- Hehlgans S, Booms P, Güllülü Ö, et al. Radiation sensitization of basal cell and head and neck squamous cell carcinoma by the hedgehog pathway inhibitor vismodegib. Int J Mol Sci. 2018;19:2485.
- Kim J, Tang JY, Gong R, et al. Itraconazole, a commonly used antifungal that inhibits hedgehog pathway activity and cancer growth. Cancer Cell. 2010;17:388-399.
- Kim J, Aftab BT, Tang JY, et al. Itraconazole and arsenic trioxide inhibit hedgehog pathway activation and tumor growth associated with acquired resistance to smoothened antagonists. Cancer Cell. 2013;23:23-34.
- Kim DJ, Kim J, Spaunhurst K, et al. Open-label, exploratory phase II trial of oral itraconazole for the treatment of basal cell carcinoma. J Clin Oncol. 2014;32:745-751.
- Ally MS, Ransohoff K, Sarin K, et al. Effects of combined treatment with arsenic trioxide and itraconazole in patients with refractory metastatic basal cell carcinoma. JAMA Dermatol. 2016;152:452-456.
- Cia˛z˙yn´yska M, Narbutt J, Skibin´ska M, et al. Itraconazole—a new player in the therapy of advanced basal cell carcinoma: a case report. JCO Oncol Pract. 2020;16:837-838.
- Sohn GK, Kwon GP, Bailey-Healy I, et al. Topical itraconazole for the treatment of basal cell carcinoma in patients with basal cell nevus syndrome or high-frequency basal cell carcinomas: a phase 2, open-label, placebo-controlled trial. JAMA Dermatol. 2019;155:1078-1080.
- Lass-Flörl C. Triazole antifungal agents in invasive fungal infections: a comparative review. Drugs. 2011;71:2405-2419.
- Chen B, Trang V, Lee A, et al. Posaconazole, a second-generation triazole antifungal drug, inhibits the hedgehog signaling pathway and progression of basal cell carcinoma. Mol Cancer Ther. 2016;15:866-876.
- Katragkou A, Tsikopoulou F, Roilides E, et al. Posaconazole: when and how? the clinician’s view. Mycoses. 2012;55:110-122.
- Raad II, Graybill JR, Bustamante AB, et al. Safety of long-term oral posaconazole use in the treatment of refractory invasive fungal infections. Clin Infect Dis. 2006;42:1726-1734.
- Atwood SX, Sarin KY, Whitson RJ, et al. Smoothened variants explain the majority of drug resistance in basal cell carcinoma. Cancer Cell. 2015;27:342-353.
- Sun Q, Atzmony L, Zaki T, et al. Clues to primary vismodegib resistance lie in histology and genetics. J Clin Pathol. 2020;73:678-680.
- Verkouteren BJA, Wakkee M, van Geel M, et al. Molecular testing in metastatic basal cell carcinoma. J Am Acad Dermatol. 2021;85:1135-1142.
- Danial C, Sarin KY, Oro AE, et al. An investigator-initiated open-label trial of sonidegib in advanced basal cell carcinoma patients resistant to vismodegib. Clin Cancer Res. 2016;22:1325-1329.
- Yoon J, Apicelli AJ 3rd, Pavlopoulos TV. Intracranial regression of an advanced basal cell carcinoma using sonidegib and itraconazole after failure with vismodegib. JAAD Case Rep. 2017;4:10-12.
- Bendell J, Andre V, Ho A, et al. Phase I study of LY2940680, a Smo antagonist, in patients with advanced cancer including treatment-naïve and previously treated basal cell carcinoma. Clin Cancer Res. 2018;24:2082-2091.
- Ueno H, Kondo S, Yoshikawa S, et al. A phase I and pharmacokinetic study of taladegib, a Smoothened inhibitor, in Japanese patients with advanced solid tumors. Invest New Drugs. 2018;36:647-656.
- Herms F, Lambert J, Grob JJ, et al. Follow-up of patients with complete remission of locally advanced basal cell carcinoma after vismodegib discontinuation: a multicenter French study of 116 patients. J Clin Oncol. 2019;37:3275-3282.
- Villani A, Megna M, Fabbrocini G, et al. Long-term efficacy of vismodegib after its withdrawal and patients’ health-related quality of life using the Dermatology Life Quality Index (DLQI). Dermatol Ther (Heidelb). 2019;9:719-724.
- Scalvenzi M, Cappello M, Costa C, et al. Low-dose vismodegib as maintenance therapy after locally advanced basal cell carcinoma complete remission: high efficacy with minimal toxicity. Dermatol Ther (Heidelb). 2020;10:465-468.
Practice Points
- The sonic hedgehog (SHH) inhibitors vismodegib and sonidegib currently are the only 2 oral medications approved by the US Food and Drug Administration for the first-line treatment of locally advanced basal cell carcinoma (BCC). Vismodegib also is approved for metastatic BCC.
- Cemiplimab, a programmed cell death protein 1 inhibitor, is now an approved treatment for patients with advanced BCC refractory or intolerant to SHH inhibitor therapy.
- Adverse effects of SHH inhibitors, most commonly muscle spasms, often lead to treatment discontinuation, but intermittent dosing regimens can be used to increase tolerability and adherence.
- Combining SHH inhibitors with radiotherapy or antifungal therapy as well as maintenance dosing strategies may help reduce the risk of recurrence.
- Neoadjuvant administration of a SHH inhibitor may enable surgical excision of previously inoperable cases through tumor shrinkage.
2022 Update: Beyond prenatal exome sequencing
Last year, our Update focused on the expansion of sequencing in prenatal diagnosis. This year, we are taking a step sideways to remember the many diagnoses we may miss if we rely on exome sequencing alone. A recent case report in Prenatal Diagnosis describes a pregnancy affected by fetal akinesia sequence and polyhydramnios in which sequencing did not reveal a diagnosis. Expansion of the differential to include congenital myotonic dystrophy and subsequent triplet repeat testing led the clinicians to the diagnosis and identification of a triplet repeat expansion in the DMPK gene. This case serves as our first example of how complementary testing and technologies should continue to help us make critical diagnoses.
What is the yield of exome sequencing vs panels in nonimmune hydrops?
Rogers R, Moyer K, Moise KJ Jr. Congenital myotonic dystrophy: an overlooked diagnosis not amenable to detection by sequencing. Prenat Diagn. 2022;42:233-235. doi:10.1002/pd.6105.
Norton ME, Ziffle JV, Lianoglou BR, et al. Exome sequencing vs targeted gene panels for the evaluation of nonimmune hydrops fetalis. Am J Obstet Gynecol. 2021;28:S0002-9378(21)00828-0. doi:10.1016/j.ajog.2021.07.014.
We have had several illuminating discussions with our colleagues about the merits of exome sequencing (ES) versus panels and other modalities for fetal diagnosis. Many obstetricians practicing at the leading edge may feel like ES should be utilized uniformly for fetal anomalies with nondiagnostic karyotype or microarray. However, for well-defined phenotypes with clear and narrow lists of implicated genes (eg, skeletal dysplasias) or patients without insurance coverage, panel sequencing still has utility in prenatal diagnosis. The question of which phenotypes most benefit from ES versus panel sequencing is an area of interesting, ongoing research for several investigators.
Secondary analysis of nonimmune hydrops cohort
Norton and colleagues tackled one such cohort in a study presented in the American Journal of Obstetrics and Gynecology. They compared the proportion of diagnoses that would have been identified in commercial lab panels with their research of phenotype-driven ES in a cohort of 127 fetuses with features of nonimmune hydrops fetalis (NIHF). NIHF can be caused by a variety of single-gene disorders in addition to chromosomal disorders and copy number variants on chromosomal microarray. Patients were eligible for inclusion in the cohort if they had a nondiagnostic karyotype or microarray and any of the following features: nuchal translucency of 3.5 mm or greater, cystic hygroma, pleural effusion, pericardial effusion, ascites, or skin edema. Standard sequencing methods and variant analysis were performed. They assumed 100% analytical sensitivity and specificity of the panels for variant detection and collected cost information on the targeted gene panels.
Study outcomes
In the ES analysis of cases, 37 of 127 cases (29%) had a pathogenic or likely pathogenic variant in 1 of 29 genes, and another 12 of 127 cases (9%) had variants of uncertain significance that were strongly suspected to be the etiology during clinical analysis. The types of disorders that were identified are listed in the TABLE. In addition to a feature of NIHF, 50% of the cases had a structural anomaly.
There were 10 identified clinical panels from 7 clinical laboratories. These panels ranged in size from 11 to 128 genes. The highest simulated yield of any commercial panel was only 62% of the pathogenic variants identified by ES. The other commercial laboratory panels detection yield ranged from 11% to 62% of pathogenic variants detected by ES. For overall yield, the largest panel would have a diagnostic yield of 18% of diagnoses relative to the 29% diagnostic yield from ES.
The largest panel included 128 genes prior to the publication of the original cohort and was updated after publication to include 148 genes. The larger updated panel would have identified all of the patients in the ES cohort. However, many of the other panels listed would have identified a smaller fraction of the variants identified by ES (range, 11%-62%). At the time of publication, the cost of the panels ranged from $640 to $3,500, and the cost of prenatal ES ranged from $2,458 to $7,500.
Continue to: Strengths and limitations...
Strengths and limitations
Twenty-three percent of the patients who were sequenced had an increased fetal nuchal translucency or cystic hygroma, and another 17% had a single fetal effusion. This inclusivity makes this study more applicable to broader fetal anomaly populations. However, it is worth noting that only 61% of patients had NIHF by the definition of 2 or more fluid collections or skin thickening.
The authors assumed 100% sensitivity and specificity for the panel tests relative to diagnostic ES results, but this may not reflect real-life analysis. There is inherent subjectivity and subsequent differences in variant calling (deciding which genetic changes are pathogenic) between institutions and companies despite efforts to standardize this process. Due to the simulated nature of this study, these differences are not captured. Additionally, although the authors note that the research ES had at least 30 times the coverage (an adequate number of sequence reads for accurate testing) than did the commercial lab panels, some gene panels have additional sequencing of intronic regions, copy number analysis, and up to 10 times more coverage than ES, which could lead to more diagnoses.
This study illustrates that there is nuance involved in selecting which type of gene sequencing and which clinical laboratory to use for prenatal diagnosis. Labs with more updated literature searches and more inclusive gene panels may be excellent options for patients in whom ES is not covered by insurance or with phenotypes with a narrow range of suspected causative genes. However, there is a lag time in updating the genes offered on each panel, and new genedisease associations will not be captured by existing panels.
From a cost, speed-of-analysis, and depth-of-sequencing perspective, panel sequencing can have advantages that should be considered in some patients, particularly if the panels are large and regularly updated. However, the authors summarize our sentiments and their findings with the following:
“For disorders, such as NIHF with marked genetic heterogeneity and less clear in utero phenotypes of underlying genetic diseases, the broader coverage of exome sequencing makes it a superior option to targeted panel testing.”
We look forward to the publication of further anomaly-specific cohorts and secondary analyses of the utility of current panels and ES that may follow.
Frequency of Beckwith-Widemann syndrome in prenatally diagnosed omphaloceles
Abbasi N, Moore A, Chiu P, et al. Prenatally diagnosed omphaloceles: report of 92 cases and association with Beckwith-Wiedemann syndrome. Prenat Diagn. 2021;41:798-816. doi:10.1002/pd.5930.
An omphalocele is diagnosed prenatally on ultrasound when an anterior midline mass, often containing abdominal contents, is seen herniating into the base of the umbilical cord. Omphaloceles are often associated with additional structural abnormalities and underlying genetic syndromes, thus a thorough fetal assessment is required for accurate prenatal counseling and neonatal care.
Identification of Beckwith-Widemann syndrome (BWS) in the setting of a prenatally diagnosed omphalocele is difficult because of its wide range of clinical features and its unique genetic basis. Unlike many genetic disorders that are caused by specific genetic variants, or spelling changes in the genes, BWS results from a change in the expression of one or more of the genes in a specific region of chromosome 11. A high index of clinical suspicion as well as an understanding of the various genetic and epigenetics alterations that cause BWS is required for prenatal diagnosis.
Retrospective cohort at a single center
The authors in this study reviewed all pregnancies in which an omphalocele was diagnosed prenatally at a single center between 2010 and 2015. They describe a standard prenatal evaluation following identification of an omphalocele including echocardiogram, detailed anatomic survey, and availability of an amniocentesis to facilitate aneuploidy screening and testing for BWS. This review also includes an overview of perinatal and long-term outcomes for cases of BWS diagnosed at their center between 2000 and 2015.
Study outcomes
Results of prenatal genetic testing in this cohort were divided between cases of an isolated omphalocele (without other structural changes) and cases of nonisolated omphaloceles. In the group of pregnancies with an isolated omphalocele, 2 of 27 pregnancies (7.4%) were found to have an abnormal karyotype, and 6 of 16 of the remaining pregnancies (37.5%) were diagnosed with BWS. Among the group of pregnancies with a nonisolated omphalocele, 23 of 59 pregnancies (39%) were found to have an abnormal karyotype, and 1 of 20 pregnancies (5%) were diagnosed with BWS.
Prenatal sonographic features associated with cases of BWS included polyhydramnios in 12 of 19 cases (63%) and macrosomia in 8 of 19 cases (42%). Macroglossia is another characteristic feature of the disorder, which was identified in 4 of 19 cases (21%) prenatally and in an additional 10 of 19 cases (52.6%) postnatally. Interestingly, only 1 of the cases of BWS was caused by a microdeletion at 11p15.4—a change that was identified on microarray. The additional 6 cases of BWS were caused by imprinting changes in the region, which are only detectable with a specific methylation-analysis technique.
Among the 19 cases of BWS identified over a 15-year period, there was 1 intrauterine demise. Preterm birth occurred in 10 of 19 cases (52.6%), including 8 of 19 cases (42.1%) of spontaneous preterm labor. Respiratory distress (27.8%), hypoglycemia (61%), and gastrointestinal reflux (59%) were common neonatal complications. Embryonal tumors were diagnosed in 2 of 16 patients (12.5%). Although neurodevelopmental outcomes were incomplete, their data suggested normal development in 75% of children. There were 2 neonatal deaths in this cohort and 1 childhood death at age 2 years.
Study strengths and limitations
As with many studies investigating a rare disorder, this study is limited by its retrospective nature and small sample size. Nevertheless, it adds an important cohort of patients with a prenatally diagnosed omphalocele to the literature and illuminates the utility of a standardized approach to testing for BWS in this population.
In this cohort with prenatally diagnosed omphaloceles with standardized testing for BWS, the prevalence of the disorder was approximately 8% and more common in cases of an isolated omphalocele. The most common supporting sonographic features of BWS may not be detected until later in gestation, including polyhydramnios and macrosomia. This demonstrates the importance of both sonographic follow-up as well as universal testing for BWS in euploid cases of a prenatally diagnosed omphalocele. Almost all cases of BWS in this cohort required specialized molecular techniques for diagnosis, and the diagnosis would have been missed on karyotype, microarray, and ES.
Continue to: Genetic diagnoses that could have been identified by expanded carrier screening...
Genetic diagnoses that could have been identified by expanded carrier screening
Stevens BK, Nunley PB, Wagner C, et al. Utility of expanded carrier screening in pregnancies with ultrasound abnormalities. Prenat Diagn. 2022;42:60-78. doi:10.1002/pd.6069.
This series is a thorough retrospective review of patients evaluated in a pediatric genetics clinic from 2014 through 2017. Patients were included if they were evaluated in the first 6 months of life and had a structural abnormality that might be detected on prenatal ultrasonography. The genetic testing results were analyzed and categorized according to types of genetic disorders, with the goal of identifying how many patients might have been identified by expanded carrier screening (ECS) panels.
Study outcomes
A total of 931 charts were reviewed, and 85% (791 of 931) of patients evaluated in the first 6 months of life were determined to have a structural anomaly that might be detected on prenatal ultrasonography. Of those patients, 691 went on to have genetic testing and 32.1% (222 of 691) of them had a diagnostic (pathogenic) genetic testing result related to the phenotype. The types of diagnostic testing results are shown in the FIGURE. Notably, 42 single-gene disorders were detected.
FIGURE Diagnostic test result in pediatric patients evaluated under age 6 months
Of those 222 patients with diagnostic results, there were 8 patients with autosomal recessive and X-linked conditions that could be detected using a 500-gene ECS panel. Five patients could be detected with a 271-gene panel. After nondiagnostic microarray, 11.3% of patients had a condition that could be detected by using a 500-gene ECS panel. The identified conditions included cystic fibrosis, CYP21‐related congenital adrenal hyperplasia, autosomal recessive polycystic kidney disease, Antley‐Bixler syndrome, and Morquio syndrome type A.
Furthermore, the authors conducted a literature review of 271 conditions and found that 32% (88 of 271) of conditions may be associated with ultrasound findings.
Study strengths and limitations
When applying these data to prenatal populations, the authors acknowledge several notable limitations. There is a selection bias toward less-severe phenotypes for many patients choosing to continue rather than to interrupt a pregnancy. Additionally, only 23% of the patients in the study had a microarray and ES, which may lead to an underrepresentation of single-gene disorders and an underestimation of the utility of ECS. Finally, a retrospective classification of structural abnormalities that may be detectable by ultrasonography may not always reflect what is actually reported in prenatal imaging.
However, the work that the authors put forth to evaluate and categorize 931 participants by the results of genetic testing and structural anomalies is appreciated, and the level of detail is impressive for this retrospective chart review. Additionally, the tables itemizing the authors’ review of 271 ECS disorders that may have ultrasonography findings categorized by disorder and system are helpful and quick diagnostic references for clinicians providing prenatal care. ●
This study of potentially detectable prenatal findings from the lens of a pediatric genetics clinic lends an interesting perspective: Exome sequencing is not the primary route to establish a diagnosis; karyotype, microarray, methylation disorders, and triplet repeat disorders all have an established role in the diagnostic toolkit. Keeping in mind the contribution of these modalities to pediatric testing may shorten the diagnostic odyssey to continue pregnancies or help to fully counsel patients on expectations and decision-making after birth.
Carrier screening is not a substitute for diagnostic testing in pregnancy. However, in appropriately selected patients, a broad carrier screening panel may have added utility. ECS can be conducted while awaiting microarray results to help target testing and may be particularly useful for patients who decline diagnostic testing until the postnatal period. It is important to counsel patients that carrier screening is not a diagnostic test, and results will only report likely pathogenic or pathogenic variants, not variants of uncertain significance that may be of clinical relevance. However, our practice has had several insightful diagnoses reached through ECS, in conjunction with microarray testing that allowed for faster and more targeted sequencing and precise fetal diagnosis. This readily available molecular tool (often covered by insurance) deserves a spot in your fetal diagnosis tool belt based on available evidence.
Last year, our Update focused on the expansion of sequencing in prenatal diagnosis. This year, we are taking a step sideways to remember the many diagnoses we may miss if we rely on exome sequencing alone. A recent case report in Prenatal Diagnosis describes a pregnancy affected by fetal akinesia sequence and polyhydramnios in which sequencing did not reveal a diagnosis. Expansion of the differential to include congenital myotonic dystrophy and subsequent triplet repeat testing led the clinicians to the diagnosis and identification of a triplet repeat expansion in the DMPK gene. This case serves as our first example of how complementary testing and technologies should continue to help us make critical diagnoses.
What is the yield of exome sequencing vs panels in nonimmune hydrops?
Rogers R, Moyer K, Moise KJ Jr. Congenital myotonic dystrophy: an overlooked diagnosis not amenable to detection by sequencing. Prenat Diagn. 2022;42:233-235. doi:10.1002/pd.6105.
Norton ME, Ziffle JV, Lianoglou BR, et al. Exome sequencing vs targeted gene panels for the evaluation of nonimmune hydrops fetalis. Am J Obstet Gynecol. 2021;28:S0002-9378(21)00828-0. doi:10.1016/j.ajog.2021.07.014.
We have had several illuminating discussions with our colleagues about the merits of exome sequencing (ES) versus panels and other modalities for fetal diagnosis. Many obstetricians practicing at the leading edge may feel like ES should be utilized uniformly for fetal anomalies with nondiagnostic karyotype or microarray. However, for well-defined phenotypes with clear and narrow lists of implicated genes (eg, skeletal dysplasias) or patients without insurance coverage, panel sequencing still has utility in prenatal diagnosis. The question of which phenotypes most benefit from ES versus panel sequencing is an area of interesting, ongoing research for several investigators.
Secondary analysis of nonimmune hydrops cohort
Norton and colleagues tackled one such cohort in a study presented in the American Journal of Obstetrics and Gynecology. They compared the proportion of diagnoses that would have been identified in commercial lab panels with their research of phenotype-driven ES in a cohort of 127 fetuses with features of nonimmune hydrops fetalis (NIHF). NIHF can be caused by a variety of single-gene disorders in addition to chromosomal disorders and copy number variants on chromosomal microarray. Patients were eligible for inclusion in the cohort if they had a nondiagnostic karyotype or microarray and any of the following features: nuchal translucency of 3.5 mm or greater, cystic hygroma, pleural effusion, pericardial effusion, ascites, or skin edema. Standard sequencing methods and variant analysis were performed. They assumed 100% analytical sensitivity and specificity of the panels for variant detection and collected cost information on the targeted gene panels.
Study outcomes
In the ES analysis of cases, 37 of 127 cases (29%) had a pathogenic or likely pathogenic variant in 1 of 29 genes, and another 12 of 127 cases (9%) had variants of uncertain significance that were strongly suspected to be the etiology during clinical analysis. The types of disorders that were identified are listed in the TABLE. In addition to a feature of NIHF, 50% of the cases had a structural anomaly.
There were 10 identified clinical panels from 7 clinical laboratories. These panels ranged in size from 11 to 128 genes. The highest simulated yield of any commercial panel was only 62% of the pathogenic variants identified by ES. The other commercial laboratory panels detection yield ranged from 11% to 62% of pathogenic variants detected by ES. For overall yield, the largest panel would have a diagnostic yield of 18% of diagnoses relative to the 29% diagnostic yield from ES.
The largest panel included 128 genes prior to the publication of the original cohort and was updated after publication to include 148 genes. The larger updated panel would have identified all of the patients in the ES cohort. However, many of the other panels listed would have identified a smaller fraction of the variants identified by ES (range, 11%-62%). At the time of publication, the cost of the panels ranged from $640 to $3,500, and the cost of prenatal ES ranged from $2,458 to $7,500.
Continue to: Strengths and limitations...
Strengths and limitations
Twenty-three percent of the patients who were sequenced had an increased fetal nuchal translucency or cystic hygroma, and another 17% had a single fetal effusion. This inclusivity makes this study more applicable to broader fetal anomaly populations. However, it is worth noting that only 61% of patients had NIHF by the definition of 2 or more fluid collections or skin thickening.
The authors assumed 100% sensitivity and specificity for the panel tests relative to diagnostic ES results, but this may not reflect real-life analysis. There is inherent subjectivity and subsequent differences in variant calling (deciding which genetic changes are pathogenic) between institutions and companies despite efforts to standardize this process. Due to the simulated nature of this study, these differences are not captured. Additionally, although the authors note that the research ES had at least 30 times the coverage (an adequate number of sequence reads for accurate testing) than did the commercial lab panels, some gene panels have additional sequencing of intronic regions, copy number analysis, and up to 10 times more coverage than ES, which could lead to more diagnoses.
This study illustrates that there is nuance involved in selecting which type of gene sequencing and which clinical laboratory to use for prenatal diagnosis. Labs with more updated literature searches and more inclusive gene panels may be excellent options for patients in whom ES is not covered by insurance or with phenotypes with a narrow range of suspected causative genes. However, there is a lag time in updating the genes offered on each panel, and new genedisease associations will not be captured by existing panels.
From a cost, speed-of-analysis, and depth-of-sequencing perspective, panel sequencing can have advantages that should be considered in some patients, particularly if the panels are large and regularly updated. However, the authors summarize our sentiments and their findings with the following:
“For disorders, such as NIHF with marked genetic heterogeneity and less clear in utero phenotypes of underlying genetic diseases, the broader coverage of exome sequencing makes it a superior option to targeted panel testing.”
We look forward to the publication of further anomaly-specific cohorts and secondary analyses of the utility of current panels and ES that may follow.
Frequency of Beckwith-Widemann syndrome in prenatally diagnosed omphaloceles
Abbasi N, Moore A, Chiu P, et al. Prenatally diagnosed omphaloceles: report of 92 cases and association with Beckwith-Wiedemann syndrome. Prenat Diagn. 2021;41:798-816. doi:10.1002/pd.5930.
An omphalocele is diagnosed prenatally on ultrasound when an anterior midline mass, often containing abdominal contents, is seen herniating into the base of the umbilical cord. Omphaloceles are often associated with additional structural abnormalities and underlying genetic syndromes, thus a thorough fetal assessment is required for accurate prenatal counseling and neonatal care.
Identification of Beckwith-Widemann syndrome (BWS) in the setting of a prenatally diagnosed omphalocele is difficult because of its wide range of clinical features and its unique genetic basis. Unlike many genetic disorders that are caused by specific genetic variants, or spelling changes in the genes, BWS results from a change in the expression of one or more of the genes in a specific region of chromosome 11. A high index of clinical suspicion as well as an understanding of the various genetic and epigenetics alterations that cause BWS is required for prenatal diagnosis.
Retrospective cohort at a single center
The authors in this study reviewed all pregnancies in which an omphalocele was diagnosed prenatally at a single center between 2010 and 2015. They describe a standard prenatal evaluation following identification of an omphalocele including echocardiogram, detailed anatomic survey, and availability of an amniocentesis to facilitate aneuploidy screening and testing for BWS. This review also includes an overview of perinatal and long-term outcomes for cases of BWS diagnosed at their center between 2000 and 2015.
Study outcomes
Results of prenatal genetic testing in this cohort were divided between cases of an isolated omphalocele (without other structural changes) and cases of nonisolated omphaloceles. In the group of pregnancies with an isolated omphalocele, 2 of 27 pregnancies (7.4%) were found to have an abnormal karyotype, and 6 of 16 of the remaining pregnancies (37.5%) were diagnosed with BWS. Among the group of pregnancies with a nonisolated omphalocele, 23 of 59 pregnancies (39%) were found to have an abnormal karyotype, and 1 of 20 pregnancies (5%) were diagnosed with BWS.
Prenatal sonographic features associated with cases of BWS included polyhydramnios in 12 of 19 cases (63%) and macrosomia in 8 of 19 cases (42%). Macroglossia is another characteristic feature of the disorder, which was identified in 4 of 19 cases (21%) prenatally and in an additional 10 of 19 cases (52.6%) postnatally. Interestingly, only 1 of the cases of BWS was caused by a microdeletion at 11p15.4—a change that was identified on microarray. The additional 6 cases of BWS were caused by imprinting changes in the region, which are only detectable with a specific methylation-analysis technique.
Among the 19 cases of BWS identified over a 15-year period, there was 1 intrauterine demise. Preterm birth occurred in 10 of 19 cases (52.6%), including 8 of 19 cases (42.1%) of spontaneous preterm labor. Respiratory distress (27.8%), hypoglycemia (61%), and gastrointestinal reflux (59%) were common neonatal complications. Embryonal tumors were diagnosed in 2 of 16 patients (12.5%). Although neurodevelopmental outcomes were incomplete, their data suggested normal development in 75% of children. There were 2 neonatal deaths in this cohort and 1 childhood death at age 2 years.
Study strengths and limitations
As with many studies investigating a rare disorder, this study is limited by its retrospective nature and small sample size. Nevertheless, it adds an important cohort of patients with a prenatally diagnosed omphalocele to the literature and illuminates the utility of a standardized approach to testing for BWS in this population.
In this cohort with prenatally diagnosed omphaloceles with standardized testing for BWS, the prevalence of the disorder was approximately 8% and more common in cases of an isolated omphalocele. The most common supporting sonographic features of BWS may not be detected until later in gestation, including polyhydramnios and macrosomia. This demonstrates the importance of both sonographic follow-up as well as universal testing for BWS in euploid cases of a prenatally diagnosed omphalocele. Almost all cases of BWS in this cohort required specialized molecular techniques for diagnosis, and the diagnosis would have been missed on karyotype, microarray, and ES.
Continue to: Genetic diagnoses that could have been identified by expanded carrier screening...
Genetic diagnoses that could have been identified by expanded carrier screening
Stevens BK, Nunley PB, Wagner C, et al. Utility of expanded carrier screening in pregnancies with ultrasound abnormalities. Prenat Diagn. 2022;42:60-78. doi:10.1002/pd.6069.
This series is a thorough retrospective review of patients evaluated in a pediatric genetics clinic from 2014 through 2017. Patients were included if they were evaluated in the first 6 months of life and had a structural abnormality that might be detected on prenatal ultrasonography. The genetic testing results were analyzed and categorized according to types of genetic disorders, with the goal of identifying how many patients might have been identified by expanded carrier screening (ECS) panels.
Study outcomes
A total of 931 charts were reviewed, and 85% (791 of 931) of patients evaluated in the first 6 months of life were determined to have a structural anomaly that might be detected on prenatal ultrasonography. Of those patients, 691 went on to have genetic testing and 32.1% (222 of 691) of them had a diagnostic (pathogenic) genetic testing result related to the phenotype. The types of diagnostic testing results are shown in the FIGURE. Notably, 42 single-gene disorders were detected.
FIGURE Diagnostic test result in pediatric patients evaluated under age 6 months

Of those 222 patients with diagnostic results, there were 8 patients with autosomal recessive and X-linked conditions that could be detected using a 500-gene ECS panel. Five patients could be detected with a 271-gene panel. After nondiagnostic microarray, 11.3% of patients had a condition that could be detected by using a 500-gene ECS panel. The identified conditions included cystic fibrosis, CYP21‐related congenital adrenal hyperplasia, autosomal recessive polycystic kidney disease, Antley‐Bixler syndrome, and Morquio syndrome type A.
Furthermore, the authors conducted a literature review of 271 conditions and found that 32% (88 of 271) of conditions may be associated with ultrasound findings.
Study strengths and limitations
When applying these data to prenatal populations, the authors acknowledge several notable limitations. There is a selection bias toward less-severe phenotypes for many patients choosing to continue rather than to interrupt a pregnancy. Additionally, only 23% of the patients in the study had a microarray and ES, which may lead to an underrepresentation of single-gene disorders and an underestimation of the utility of ECS. Finally, a retrospective classification of structural abnormalities that may be detectable by ultrasonography may not always reflect what is actually reported in prenatal imaging.
However, the work that the authors put forth to evaluate and categorize 931 participants by the results of genetic testing and structural anomalies is appreciated, and the level of detail is impressive for this retrospective chart review. Additionally, the tables itemizing the authors’ review of 271 ECS disorders that may have ultrasonography findings categorized by disorder and system are helpful and quick diagnostic references for clinicians providing prenatal care. ●
This study of potentially detectable prenatal findings from the lens of a pediatric genetics clinic lends an interesting perspective: Exome sequencing is not the primary route to establish a diagnosis; karyotype, microarray, methylation disorders, and triplet repeat disorders all have an established role in the diagnostic toolkit. Keeping in mind the contribution of these modalities to pediatric testing may shorten the diagnostic odyssey to continue pregnancies or help to fully counsel patients on expectations and decision-making after birth.
Carrier screening is not a substitute for diagnostic testing in pregnancy. However, in appropriately selected patients, a broad carrier screening panel may have added utility. ECS can be conducted while awaiting microarray results to help target testing and may be particularly useful for patients who decline diagnostic testing until the postnatal period. It is important to counsel patients that carrier screening is not a diagnostic test, and results will only report likely pathogenic or pathogenic variants, not variants of uncertain significance that may be of clinical relevance. However, our practice has had several insightful diagnoses reached through ECS, in conjunction with microarray testing that allowed for faster and more targeted sequencing and precise fetal diagnosis. This readily available molecular tool (often covered by insurance) deserves a spot in your fetal diagnosis tool belt based on available evidence.
Last year, our Update focused on the expansion of sequencing in prenatal diagnosis. This year, we are taking a step sideways to remember the many diagnoses we may miss if we rely on exome sequencing alone. A recent case report in Prenatal Diagnosis describes a pregnancy affected by fetal akinesia sequence and polyhydramnios in which sequencing did not reveal a diagnosis. Expansion of the differential to include congenital myotonic dystrophy and subsequent triplet repeat testing led the clinicians to the diagnosis and identification of a triplet repeat expansion in the DMPK gene. This case serves as our first example of how complementary testing and technologies should continue to help us make critical diagnoses.
What is the yield of exome sequencing vs panels in nonimmune hydrops?
Rogers R, Moyer K, Moise KJ Jr. Congenital myotonic dystrophy: an overlooked diagnosis not amenable to detection by sequencing. Prenat Diagn. 2022;42:233-235. doi:10.1002/pd.6105.
Norton ME, Ziffle JV, Lianoglou BR, et al. Exome sequencing vs targeted gene panels for the evaluation of nonimmune hydrops fetalis. Am J Obstet Gynecol. 2021;28:S0002-9378(21)00828-0. doi:10.1016/j.ajog.2021.07.014.
We have had several illuminating discussions with our colleagues about the merits of exome sequencing (ES) versus panels and other modalities for fetal diagnosis. Many obstetricians practicing at the leading edge may feel like ES should be utilized uniformly for fetal anomalies with nondiagnostic karyotype or microarray. However, for well-defined phenotypes with clear and narrow lists of implicated genes (eg, skeletal dysplasias) or patients without insurance coverage, panel sequencing still has utility in prenatal diagnosis. The question of which phenotypes most benefit from ES versus panel sequencing is an area of interesting, ongoing research for several investigators.
Secondary analysis of nonimmune hydrops cohort
Norton and colleagues tackled one such cohort in a study presented in the American Journal of Obstetrics and Gynecology. They compared the proportion of diagnoses that would have been identified in commercial lab panels with their research of phenotype-driven ES in a cohort of 127 fetuses with features of nonimmune hydrops fetalis (NIHF). NIHF can be caused by a variety of single-gene disorders in addition to chromosomal disorders and copy number variants on chromosomal microarray. Patients were eligible for inclusion in the cohort if they had a nondiagnostic karyotype or microarray and any of the following features: nuchal translucency of 3.5 mm or greater, cystic hygroma, pleural effusion, pericardial effusion, ascites, or skin edema. Standard sequencing methods and variant analysis were performed. They assumed 100% analytical sensitivity and specificity of the panels for variant detection and collected cost information on the targeted gene panels.
Study outcomes
In the ES analysis of cases, 37 of 127 cases (29%) had a pathogenic or likely pathogenic variant in 1 of 29 genes, and another 12 of 127 cases (9%) had variants of uncertain significance that were strongly suspected to be the etiology during clinical analysis. The types of disorders that were identified are listed in the TABLE. In addition to a feature of NIHF, 50% of the cases had a structural anomaly.
There were 10 identified clinical panels from 7 clinical laboratories. These panels ranged in size from 11 to 128 genes. The highest simulated yield of any commercial panel was only 62% of the pathogenic variants identified by ES. The other commercial laboratory panels detection yield ranged from 11% to 62% of pathogenic variants detected by ES. For overall yield, the largest panel would have a diagnostic yield of 18% of diagnoses relative to the 29% diagnostic yield from ES.
The largest panel included 128 genes prior to the publication of the original cohort and was updated after publication to include 148 genes. The larger updated panel would have identified all of the patients in the ES cohort. However, many of the other panels listed would have identified a smaller fraction of the variants identified by ES (range, 11%-62%). At the time of publication, the cost of the panels ranged from $640 to $3,500, and the cost of prenatal ES ranged from $2,458 to $7,500.
Continue to: Strengths and limitations...
Strengths and limitations
Twenty-three percent of the patients who were sequenced had an increased fetal nuchal translucency or cystic hygroma, and another 17% had a single fetal effusion. This inclusivity makes this study more applicable to broader fetal anomaly populations. However, it is worth noting that only 61% of patients had NIHF by the definition of 2 or more fluid collections or skin thickening.
The authors assumed 100% sensitivity and specificity for the panel tests relative to diagnostic ES results, but this may not reflect real-life analysis. There is inherent subjectivity and subsequent differences in variant calling (deciding which genetic changes are pathogenic) between institutions and companies despite efforts to standardize this process. Due to the simulated nature of this study, these differences are not captured. Additionally, although the authors note that the research ES had at least 30 times the coverage (an adequate number of sequence reads for accurate testing) than did the commercial lab panels, some gene panels have additional sequencing of intronic regions, copy number analysis, and up to 10 times more coverage than ES, which could lead to more diagnoses.
This study illustrates that there is nuance involved in selecting which type of gene sequencing and which clinical laboratory to use for prenatal diagnosis. Labs with more updated literature searches and more inclusive gene panels may be excellent options for patients in whom ES is not covered by insurance or with phenotypes with a narrow range of suspected causative genes. However, there is a lag time in updating the genes offered on each panel, and new genedisease associations will not be captured by existing panels.
From a cost, speed-of-analysis, and depth-of-sequencing perspective, panel sequencing can have advantages that should be considered in some patients, particularly if the panels are large and regularly updated. However, the authors summarize our sentiments and their findings with the following:
“For disorders, such as NIHF with marked genetic heterogeneity and less clear in utero phenotypes of underlying genetic diseases, the broader coverage of exome sequencing makes it a superior option to targeted panel testing.”
We look forward to the publication of further anomaly-specific cohorts and secondary analyses of the utility of current panels and ES that may follow.
Frequency of Beckwith-Widemann syndrome in prenatally diagnosed omphaloceles
Abbasi N, Moore A, Chiu P, et al. Prenatally diagnosed omphaloceles: report of 92 cases and association with Beckwith-Wiedemann syndrome. Prenat Diagn. 2021;41:798-816. doi:10.1002/pd.5930.
An omphalocele is diagnosed prenatally on ultrasound when an anterior midline mass, often containing abdominal contents, is seen herniating into the base of the umbilical cord. Omphaloceles are often associated with additional structural abnormalities and underlying genetic syndromes, thus a thorough fetal assessment is required for accurate prenatal counseling and neonatal care.
Identification of Beckwith-Widemann syndrome (BWS) in the setting of a prenatally diagnosed omphalocele is difficult because of its wide range of clinical features and its unique genetic basis. Unlike many genetic disorders that are caused by specific genetic variants, or spelling changes in the genes, BWS results from a change in the expression of one or more of the genes in a specific region of chromosome 11. A high index of clinical suspicion as well as an understanding of the various genetic and epigenetics alterations that cause BWS is required for prenatal diagnosis.
Retrospective cohort at a single center
The authors in this study reviewed all pregnancies in which an omphalocele was diagnosed prenatally at a single center between 2010 and 2015. They describe a standard prenatal evaluation following identification of an omphalocele including echocardiogram, detailed anatomic survey, and availability of an amniocentesis to facilitate aneuploidy screening and testing for BWS. This review also includes an overview of perinatal and long-term outcomes for cases of BWS diagnosed at their center between 2000 and 2015.
Study outcomes
Results of prenatal genetic testing in this cohort were divided between cases of an isolated omphalocele (without other structural changes) and cases of nonisolated omphaloceles. In the group of pregnancies with an isolated omphalocele, 2 of 27 pregnancies (7.4%) were found to have an abnormal karyotype, and 6 of 16 of the remaining pregnancies (37.5%) were diagnosed with BWS. Among the group of pregnancies with a nonisolated omphalocele, 23 of 59 pregnancies (39%) were found to have an abnormal karyotype, and 1 of 20 pregnancies (5%) were diagnosed with BWS.
Prenatal sonographic features associated with cases of BWS included polyhydramnios in 12 of 19 cases (63%) and macrosomia in 8 of 19 cases (42%). Macroglossia is another characteristic feature of the disorder, which was identified in 4 of 19 cases (21%) prenatally and in an additional 10 of 19 cases (52.6%) postnatally. Interestingly, only 1 of the cases of BWS was caused by a microdeletion at 11p15.4—a change that was identified on microarray. The additional 6 cases of BWS were caused by imprinting changes in the region, which are only detectable with a specific methylation-analysis technique.
Among the 19 cases of BWS identified over a 15-year period, there was 1 intrauterine demise. Preterm birth occurred in 10 of 19 cases (52.6%), including 8 of 19 cases (42.1%) of spontaneous preterm labor. Respiratory distress (27.8%), hypoglycemia (61%), and gastrointestinal reflux (59%) were common neonatal complications. Embryonal tumors were diagnosed in 2 of 16 patients (12.5%). Although neurodevelopmental outcomes were incomplete, their data suggested normal development in 75% of children. There were 2 neonatal deaths in this cohort and 1 childhood death at age 2 years.
Study strengths and limitations
As with many studies investigating a rare disorder, this study is limited by its retrospective nature and small sample size. Nevertheless, it adds an important cohort of patients with a prenatally diagnosed omphalocele to the literature and illuminates the utility of a standardized approach to testing for BWS in this population.
In this cohort with prenatally diagnosed omphaloceles with standardized testing for BWS, the prevalence of the disorder was approximately 8% and more common in cases of an isolated omphalocele. The most common supporting sonographic features of BWS may not be detected until later in gestation, including polyhydramnios and macrosomia. This demonstrates the importance of both sonographic follow-up as well as universal testing for BWS in euploid cases of a prenatally diagnosed omphalocele. Almost all cases of BWS in this cohort required specialized molecular techniques for diagnosis, and the diagnosis would have been missed on karyotype, microarray, and ES.
Continue to: Genetic diagnoses that could have been identified by expanded carrier screening...
Genetic diagnoses that could have been identified by expanded carrier screening
Stevens BK, Nunley PB, Wagner C, et al. Utility of expanded carrier screening in pregnancies with ultrasound abnormalities. Prenat Diagn. 2022;42:60-78. doi:10.1002/pd.6069.
This series is a thorough retrospective review of patients evaluated in a pediatric genetics clinic from 2014 through 2017. Patients were included if they were evaluated in the first 6 months of life and had a structural abnormality that might be detected on prenatal ultrasonography. The genetic testing results were analyzed and categorized according to types of genetic disorders, with the goal of identifying how many patients might have been identified by expanded carrier screening (ECS) panels.
Study outcomes
A total of 931 charts were reviewed, and 85% (791 of 931) of patients evaluated in the first 6 months of life were determined to have a structural anomaly that might be detected on prenatal ultrasonography. Of those patients, 691 went on to have genetic testing and 32.1% (222 of 691) of them had a diagnostic (pathogenic) genetic testing result related to the phenotype. The types of diagnostic testing results are shown in the FIGURE. Notably, 42 single-gene disorders were detected.
FIGURE Diagnostic test result in pediatric patients evaluated under age 6 months

Of those 222 patients with diagnostic results, there were 8 patients with autosomal recessive and X-linked conditions that could be detected using a 500-gene ECS panel. Five patients could be detected with a 271-gene panel. After nondiagnostic microarray, 11.3% of patients had a condition that could be detected by using a 500-gene ECS panel. The identified conditions included cystic fibrosis, CYP21‐related congenital adrenal hyperplasia, autosomal recessive polycystic kidney disease, Antley‐Bixler syndrome, and Morquio syndrome type A.
Furthermore, the authors conducted a literature review of 271 conditions and found that 32% (88 of 271) of conditions may be associated with ultrasound findings.
Study strengths and limitations
When applying these data to prenatal populations, the authors acknowledge several notable limitations. There is a selection bias toward less-severe phenotypes for many patients choosing to continue rather than to interrupt a pregnancy. Additionally, only 23% of the patients in the study had a microarray and ES, which may lead to an underrepresentation of single-gene disorders and an underestimation of the utility of ECS. Finally, a retrospective classification of structural abnormalities that may be detectable by ultrasonography may not always reflect what is actually reported in prenatal imaging.
However, the work that the authors put forth to evaluate and categorize 931 participants by the results of genetic testing and structural anomalies is appreciated, and the level of detail is impressive for this retrospective chart review. Additionally, the tables itemizing the authors’ review of 271 ECS disorders that may have ultrasonography findings categorized by disorder and system are helpful and quick diagnostic references for clinicians providing prenatal care. ●
This study of potentially detectable prenatal findings from the lens of a pediatric genetics clinic lends an interesting perspective: Exome sequencing is not the primary route to establish a diagnosis; karyotype, microarray, methylation disorders, and triplet repeat disorders all have an established role in the diagnostic toolkit. Keeping in mind the contribution of these modalities to pediatric testing may shorten the diagnostic odyssey to continue pregnancies or help to fully counsel patients on expectations and decision-making after birth.
Carrier screening is not a substitute for diagnostic testing in pregnancy. However, in appropriately selected patients, a broad carrier screening panel may have added utility. ECS can be conducted while awaiting microarray results to help target testing and may be particularly useful for patients who decline diagnostic testing until the postnatal period. It is important to counsel patients that carrier screening is not a diagnostic test, and results will only report likely pathogenic or pathogenic variants, not variants of uncertain significance that may be of clinical relevance. However, our practice has had several insightful diagnoses reached through ECS, in conjunction with microarray testing that allowed for faster and more targeted sequencing and precise fetal diagnosis. This readily available molecular tool (often covered by insurance) deserves a spot in your fetal diagnosis tool belt based on available evidence.
Knowledge gaps and challenges in care for menopausal women
The transition to menopause begins with ovarian fluctuation and hormonal changes, often beginning before significant changes in menstruation. Reproductive aging with loss of follicular activity progresses over a wide age range (42 to 58 years) with an average onset at approximately age 47, ranging from 4 to 8 years. Although most women have heard about menopause, defined as 12 months after the last period, they often lack understanding about perimenopause or that the menopausal transition usually begins 5 years before menopause.1
Perimenopause, defined as early and late menopause transition stages, may be viewed as a window of potential vulnerability for women who develop or have worsening menstrual-related mood disorders. Over time, hormonal fluctuations often lead to menstrual cycle irregularity (either shorter or longer). Changes occurring during perimenopause may be confusing as it may not be clear whether symptoms are related to menopause, aging, or stress. Often not recognized or treated adequately, perimenopausal symptoms may be challenging to navigate for both women and clinicians.
The perimenopausal process is often even more confusing for women with early menopause—whether due to bilateral oophorectomy, chemotherapy or radiation therapy, genetics, or an autoimmune process—because of lack of recognition that an early menopausal transition is occurring or what solutions are available for symptoms. While there is support in the workplace for women during pregnancy and breastfeeding, there remains little support or recognition for the oft challenging perimenopausal transition leading to menopause.
Perimenopause: Common symptoms and treatments
Symptoms may be related to either estrogen level deficiency or excess during perimenopause, and these level changes may even occur within the same cycle.
Cyclic breast tenderness may develop, worsened by caffeine or high salt intake (which can be potentially improved, although without clinical trial evidence, with decreased caffeine or a trial of evening primrose oil or vitamin E).
Changes in menstrual flow and frequency of menses are typical. Flow may be lighter or heavier, longer or shorter, and there may be cycle variability, missed menses, or midcycle spotting.2 Bleeding may be heavy, with or without cramping. In addition to imaging with vaginal ultrasonography or hysteroscopy to identify structural issues, symptoms may be managed with nonsteroidal anti-inflammatory drugs (NSAIDs), hormonal therapy (HT) with short hormone-free interval contraceptives, oral progestogens, or progestin intrauterine systems. Newer medical treatments include antifibrinolytic drugs and selective progesterone-receptor modulators. Uterine ablation to decrease or stop bleeding is effective if there are no structural abnormalities, such as fibroids or polyps or the presence of adenomyosis, where glands will regrow into the endometrium after ablation. Endometrial biopsy is indicated for persistent abnormal uterine bleeding or those with risk factors such as chronic anovulation.
Worsening headaches or menstrual migraines may be triggered by hormonal changes, which may respond to NSAIDs; dihydroergotamine; triptans; the combination of aspirin, acetaminophen, and caffeine; or estrogen the week before menses. For women taking oral contraceptives (OCPs), adding estradiol the week before menses, or using the OCP continuously, may decrease headache frequency. These short-term prophylactic strategies during the perimenstrual time are often effective. If not, preventive therapy is available for women with frequent, severe headaches.
Mood complaints and poor sleep are independently associated with menstrual irregularity, and can lead to fatigue or anxiety, worsening premenstrual syndrome, or depressive moods. Sleep is disrupted premenstrually for up to one-third of women, and sleep disruption is particularly prevalent in those with premenstrual mood disorders and worsens during perimenopause.3
Reproductive hormones act on the neurotransmitter systems in the brain involved in mood regulation and emotion. The fluctuating hormones occurring during perimenopause may exacerbate pre-existing menstrual-related mood disorders. A subset of women experience depressive moods due to perimenopausal elevations in ovarian hormones.4 Others may exhibit increased mood sensitivity with the ovarian hormone withdrawal accompanying late menopause transition and early postmenopausal phase.5 There is significant comorbidity between premenstrual mood disorder (PMDD) and postpartum depression.6 During perimenopause and early menopause, clinicians should ask about prior hormonally-related depression (puberty, postpartum) and recognize that current or past premenstrual syndrome may worsen into a more severe premenstrual dysphoric disorder. Evidence-based treatments for PMDD include selective serotonin reuptake inhibitors (SSRIs); either taken continuously or only during the luteal phase; drospirenone-containing oral contraceptives, often with shorter pill-free intervals; GnRH analogues with or without hormone add-back; and cognitive behavioral therapy.7 For women whose perimenopausal moods improve with HT or develop worsened mood sensitivity with ovarian hormone withdrawal, clinicians should recognize that mood may worsen when treatment is ceased.5
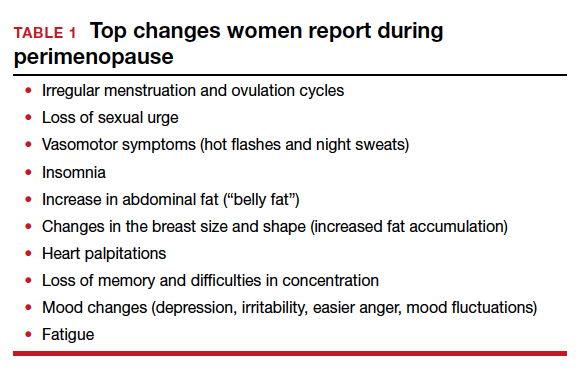
Continue to: Menopausal symptoms...
Menopausal symptoms
Vasomotor symptoms (VMS), hot flashes, or night sweats occur in up to 75% of women as they develop more menstrual irregularity and move closer to their final period and menopause.
Hot flashes are transient episodes of flushing with the sensation of warmth (up to intense heat) on the upper body and face or head, often associated with sweating, chills or flushing, an increase in heart rate, and lowered blood pressure. Hot flashes can sometimes be preceded by an intense feeling of dread, followed by rapid heat dissipation. The etiology of hot flashes is still not clear, but the neurokinin receptors are involved. They are related to small fluctuations in core body temperature superimposed on a narrow thermoneutral zone in symptomatic women. Hot flashes are triggered when core body temperature rises above the upper (sweating) threshold. Shivering occurs if the core body temperature falls below the lower threshold. Sleep may be disrupted, with less rapid eye movement (REM) sleep, and associated with throwing covers on and off or changing sheets or nightclothes. On average, hot flashes last 7.2 years,8 and they are more bothersome if night sweats interfere with sleep or disrupt performance during the day.
In the Stages of Reproductive Aging Workshop (STRAW + 10), women reported VMS within 1-3 years after the menopausal transition.8 Four trajectories of hot flashes were identified in the Study of Women’s Health Across the Nation (SWAN) trial,9 including low levels throughout the menopause transition, early onset, late onset, and a group which had frequent hot flashes, starting early and lasting longer. Serum estrogen levels were not predictive of hot flash frequency or severity.
Hot flashes have been associated with low levels of exercise, cigarette smoking, high follicle-stimulating hormone levels and low estradiol levels, increasing body mass index, ethnicity (with hot flashes more common among Black and Hispanic women), low socioeconomic status, prior PMDD, anxiety, perceived stress, and depression.8 Women with a history of premenstrual syndrome, stress, sexual dysfunction, physical inactivity, or hot flashes are more vulnerable to depressive symptoms during perimenopause and early menopause.5
Depression may co-occur or overlap with menopause symptoms. Diagnosis involves menopausal stage, co-occurring psychiatric and menopause symptoms, psychosocial stressors, and a validated screening tool such as PQ9. Treatments for perimenopausal depression, such as antidepressants, psychotherapy, or cognitive behavioral therapy, are recommended first line for perimenopausal depression. Estrogen therapy has not been approved to treat perimenopausal depression but appears to have antidepressant effects in perimenopausal women, particularly those with bothersome vasomotor symptoms.5
Anxiety can worsen during menopause, and may respond to calming apps, meditation, cognitive behavioral therapy, hypnosis, yoga or tai chi, HT, or antianxiety medications.
Weight gain around the abdomen (ie, belly fat) is a common complaint during the menopausal transition, despite women reporting not changing their eating or exercise patterns. Increasing exercise or bursts of higher intensity, decreasing portion sizes or limiting carbohydrates and alcohol may help.
Memory and concentration problems, described as brain fog, tend to be more of an issue in perimenopause and level out after menopause. Counsel midlife women that these changes are not due to dementia but are related to normal aging, hormonal changes, mood, stress, or other life circumstances. Identifying and addressing sleep issues and mood disorders may help mitigate brain fog, as can advising women to avoid excess caffeine, alcohol, nicotine, and eating before bed. Improvements in memory, cognition, and health have been found with the Mediterranean diet, regular exercise, avoiding multitasking, and engaging in mentally stimulating activities.
Sleeping concerns in peri- and postmenopausal women include sleeping less and more frequent insomnia. Women are more likely to use prescription sleeping aids during these times of their lives. The data from SWAN8 show that the menopausal transition is related to self-reported difficulty sleeping, independent of age. Sleep latency interval is increased while REM sleep decreases. Night sweats can trigger awakenings in the first half of the night. The perceived decline in sleep quality also may be attributed to general aging effects, nocturnal urination, sleep-related disorders such as sleep apnea or restless legs, or chronic pain, stress, or depression.10 Suggestions for management include sleep apps, cognitive behavioral therapy, low-dose antidepressant therapy, addressing sleep routines, and HT. Hypnotics should be avoided.
Sexuality issues are common complaints during the menopausal transition. Cross-sectional data reported from a longitudinal, population-based Australian cohort of women aged 45 to 55 years, found a decrease in sexual responsivity, sexual frequency, libido, vaginal dyspareunia, and more partner problems.11 Low libido may be related to relationship issues, dyspareunia with vaginal narrowing, loss of lubrication, levator spasm, stress, anxiety, exhaustion or mood disorder, lowered hormone levels, excess alcohol intake, underlying health concerns, or a side effect of medications for depression or pain. There is no direct correlation between testosterone levels and libido.
When HT at menopause may be helpful
For healthy symptomatic women without contraindications who are younger than age 60, or within 10 years of menopause onset, the benefits of initiating HT most likely outweigh the risks to relieve bothersome hot flashes and night sweats.12-17 For older women, or for those further from menopause, the greater absolute risks of coronary heart disease, stroke, venous thromboembolism, and dementia, in general, outweigh the potential benefits.12-17 Extended durations of HT have less safety and efficacy data and should be considered primarily for those with persistent menopausal symptoms, with periodic re-evaluation.13,14 For bothersome genitourinary syndrome of menopause symptoms that do not respond to vaginal moisturizers or lubricants, low-dose vaginal HTs are encouraged.13-17
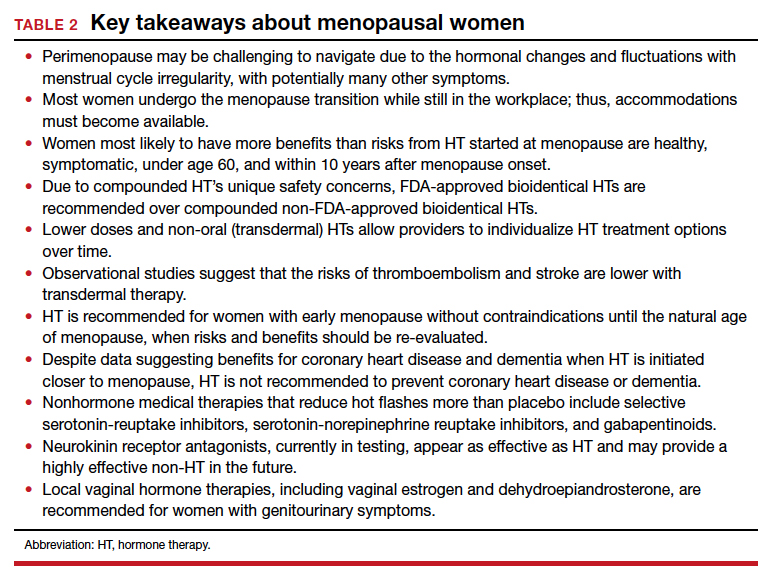
Continue to: Early-onset menopause...
Early-onset menopause
According to observational studies,18 early menopause is associated with a higher risk of osteoporosis, coronary heart disease, cognitive changes, vaginal dryness, loss of libido, and mood changes. Studies have shown that women with early menopause who take HT, without contraindications, to the average age of menopause (age 52) decrease the health risks of early menopause (bone loss, heart disease, mood, and cognition changes).13,14,18
Women with early menopause, whether spontaneous or following bilateral oophorectomy or cancer treatment, should be counseled to get adequate calcium (dietary recommended over supplementation) and vitamin D intake, eat a healthy diet, and exercise regularly. Evaluation should include risk for bone loss, heart disease, mood changes, and vaginal changes.
Extended use of HT
Up to 8% of women have hot flashes for 20 years or more after menopause.19 The decision to continue or to stop HT is not always clear for women:
- with persistent hot flashes after a trial period of HT discontinuation
- with bone loss that cannot be treated with bone-specific medications
- who request continuation for quality of life.
Extended use of HT should include an ongoing assessment of its risks and benefits, periodic trials off of HT, and documentation of rationale and informed discussions about continuing. Lower doses and transdermal therapies appear safer, as does micronized progesterone instead of more potent synthetic progestins.13-17
Genitourinary syndrome of menopause
Once women are further into menopause, they may notice vaginal dryness, vulvar itching or burning, bothersome vaginal discharge, or urinary urgency or frequency. The development of painful intercourse frequently occurs, a combination of the loss of estrogen with thinning of the vaginal mucosa, a loss of the acidic vaginal milieu with less elasticity, and spasm of the levator muscles. Some women develop urinary tract infections after intercourse or have more frequent reoccurrences. First-line therapy is often vaginal moisturizers and lubricants. Vaginal therapies (estradiol, conjugated estrogen, or dehydroepiandrosterone) or oral selective estrogen-receptor modulators (SERMs; ospemifene) improve vaginal dryness and dyspareunia.13,14 Pelvic therapy has also proved valuable for incontinence, pelvic floor dysfunction, and levator spasms.20
Where are there gaps in clinician knowledge?
Studies on emotional health, mood, and sleep need to incorporate measures of menstrual timing into data collection and analyses. Does the sleep disruption occurring premenstrually during perimenopause disproportionately contribute to a woman’s vulnerability to depressive disorders? The risk of clinically significant depressive symptoms increases 1.5- to 2.9-fold in the menopause transition.5 Research into premenstrual dysphoria during the menopause transition may identify different trajectories in the timing of symptoms related to either cycle itself or the ovarian hormone fluctuations or both.21 Gamma-aminobutyric acid (GABA)-modulating drugs, such as sepranolone, which blocks allopregnanolone’s actions at the GABAA receptor, may allow treatment of menstrual-related mood disorders without the need for hormonal interventions.21
Despite extended observational trial data, more data are needed to inform us about the long-term risks and benefits of using menopausal HT, particularly when initiated at menopause and to help address the timing of HT discontinuation. Furthermore, there are many unanswered questions. For instance:
- How much safer are lower dose and transdermal therapies?
- Do untreated hot flashes increase the risk of cardiovascular disease or dementia?
- Will newer non-HT options, such as the neurokinin receptor antagonists that are in testing but are not yet available, lower cardiovascular or dementia risks?
- What will be the risks and benefits for the newer estrogen in testing (estetrol, or E4), considered a natural estrogen and which appears to have lower thrombotic risks?
- What will be the role of intravaginal energy-based therapies, such as vaginal laser or radiofrequency devices?
- How do we address diverse populations and the effects of menopause on race, gender, culture, prior trauma, and socioeconomic status?
Lack of recognition of menopausal symptoms, particularly in the workplace
Clinicians need to understand the varied physical and emotional symptoms that may occur with hormonal changes as women traverse perimenopause and early menopause. We need to recognize that the lack of discussion about women’s health during this time may make women feel ashamed and fearful of bringing up their symptoms due to fear of being dismissed or stigmatized.22 Women may not seek help until a crisis at home or work occurs, as they may fear that admitting symptoms or a need for help or time away from work will threaten how they are viewed at work or affect their chances of promotion. Although there are economic costs around menopause for appointments, tests, therapies, and missed time at work, not addressing menopausal health leads to poorer performance, workplace absences, and additional medical costs.22
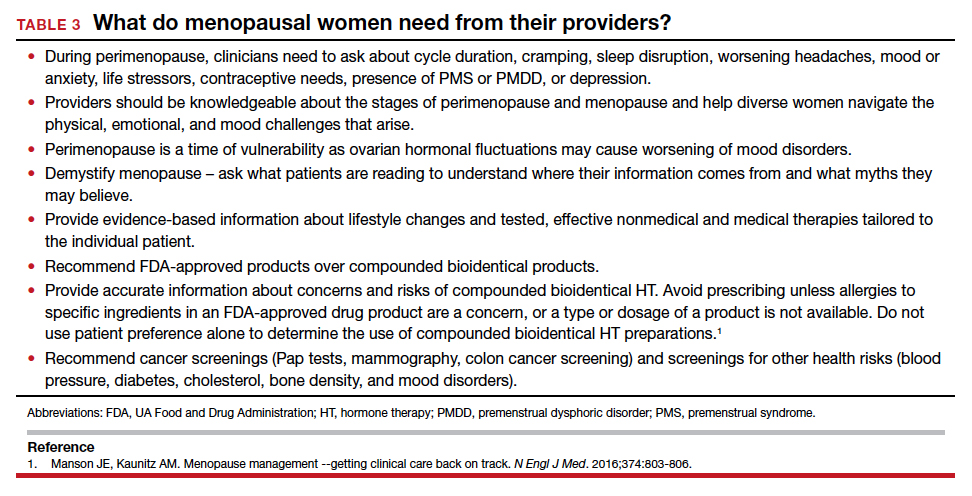
Conclusion
Menopause occurs naturally as a part of a woman’s life cycle. However, women need assistance navigating perimenopausal hormonal fluctuations and decisions about HT once in menopause. Increased awareness and education about perimenopause and menopause will allow compassionate, individualized, informed care, including lifestyle changes, behavioral or complementary strategies, or medical therapies, hormonal or nonhormonal.27 As a medical society, we need to challenge the stigma associated with aging and menopause and educate ourselves and our patients to help women navigate this challenging time. ●
Myth 1: All hot flashes are the same
The truth: Seventy-five percent of women will have hot flashes, but only 25% are severe enough to cause women to seek treatment. Duration varies with identified patterns, including starting early or late, being mild or starting early, and going late. Ethnicity affects the duration of hot flashes, with longer durations seen in Black and Hispanic women. About 15% of women have had hot flashes for more than 15 or 20 years.1,2
Myth 2: There is no help for hot flashes
The truth: For some women, lifestyle changes are helpful, such as dressing in layers, turning down the thermostat at night, avoiding hot beverages or alcohol, or using technology (Femtech) for cooling devices. Over-the-counter products that are available, but are not clearly proven to help more than placebo, include soy (which may be estrogenic), black cohosh supplements, and nutritional supplements. Cognitive behavioral therapy, hypnosis, weight loss, or mindfulness may help.3 Nonhormone medications such as low-dose antidepressants or gabapentin have shown benefit. Newer treatments in testing, including neurokinin receptor antagonists, appear to work quickly and as effectively as HT. When initiating HT, healthy women with bothersome hot flashes under age 60 or within 10 years of menopause are the best candidates for HT; many lower doses and oral and non-oral therapies are available.
Myth 3: Compounded bioidentical hormones made by a compounding pharmacy are safer and more effective than FDA-approved ones
The truth: Compounded bioidentical hormones are touted as safer or more effective, but there is no good evidence to back up those claims. Whether US Food and Drug Administration (FDA)-approved or compounded, hormones come from the same precursors and have potential risks. With custom compounded HT, there is additional concern about precisely what is in the compounded product, whether levels are similar batch to batch, and the degree of absorption of progesterone, which is better absorbed oral.4-6 FDA-approved bioidentical HTs have been tested for safety, proven to contain consistent, effective levels of hormones, and are monitored by the FDA. For menopausal symptoms, FDA-approved therapies are available as estradiol (oral, patch, spray, gel, lotion, and vaginal ring) and progesterone (as an oral compound or combined with estradiol). Pellets made of compounded hormones have shown higher serum levels and more adverse events.5,7
Myth 4: Menopause causes weight gain
The truth is that fluctuating and declining hormones and the slowing of metabolism affect weight. Weight gain is not inevitable, just harder to prevent. Many women gain an average of 5 lb (2.27 kg) at midlife, which is mainly related to aging and lifestyle and not to menopause or HT. However, menopause may be related to body composition and fat distribution changes. Counsel women to decrease portion sizes, limit carbs, and increase exercise intensity, including strength training. The goal is 30 minutes of moderate aerobic activity per day, all at once or through smaller time increments, to improve their energy, mood, and sleep.
References
1. The NAMS 2017 HT Position Statement Advisory Panel. The 2017 HT position statement of The North American Menopause Society. Menopause. 2017;24:728-753.
2. Pinkerton JV. HT for postmenopausal women. N Engl J Med. 2020;382:446-455.
3. Paramsothy P. Duration of the menopausal transition is longer in women with young age at onset: the multiethnic Study of Women’s Health Across the Nation. Menopause. 2017;24:142-149.
4. Kingsberg SA, Schaffir J, Faught BM, et al. Female sexual health: barriers to optimal outcomes and a roadmap for improved patient-clinician communications. J Womens Health (Larchmt). 2019;28:432-443.
5. Eisenlohr-Moul TA, Kaiser G, Weise C, et al. Are there temporal subtypes of premenstrual dysphoric disorder? Using group-based trajectory modeling to identify individual differences in symptom change. Psychol Med. 2020;50:964-972.
6. Seibel M, Seibel S. Working through Menopause: The Impact on Women, Businesses and the Bottom Line. Bookbaby. March 8, 2022.
7. Kingsberg SA, Schaffir J, Faught BM, et al. Female sexual health: barriers to optimal outcomes and a roadmap for improved patient-clinician communications. J Womens Health (Larchmt). 2019;28:432-443.
- Paramsothy P. Duration of the menopausal transition is longer in women with young age at onset: the multiethnic Study of Women’s Health Across the Nation. Menopause. 2017;24:142–149.
- Harlow SD, Gass M, Hall JE, et al. STRAW 10 Collaborative Group. Executive summary of the Stages of Reproductive Aging Workshop + 10: addressing the unfinished agenda of staging reproductive aging. Menopause. 2012;19:387-95.
- Meers JM, Nowakowski S. Sleep, premenstrual mood disorder, and women’s health. Curr Opin Psychol. 2020;34:43-49.
- Sander B, Gordon JL. Premenstrual mood symptoms in the perimenopause. Curr Psychiatry Rep. 2021;23:73.
- Maki PM, Kornstein SG, Joffe H, et al. Guidelines for the evaluation and treatment of perimenopausal depression: summary and recommendations. J Women’s Health. 2019;28:117–134.
- Cao S, Jones M, Tooth L, et al. History of premenstrual syndrome and development of postpartum depression: a systematic review and meta-analysis. J Psychiatr Res. 2020;121:82–90.
- Rapkin AJ, Korotkaya Y, Taylor KC. Contraception counseling for women with premenstrual dysphoric disorder (PMDD): current perspectives. Open Access J Contracept. 2019;10:27–39.
- Avis NE, Crawford SL, Greendale G, et al; Study of Women's Health Across the Nation. Duration of menopausal vasomotor symptoms over the menopause transition. JAMA Intern Med. 2015;175:531.
- Tepper PG, Brooks MM, Randolph JF Jr, et al. Characterizing the trajectories of vasomotor symptoms across the menopausal transition. Menopause. 2016;23:1067-1074.
- Kravitz HM, Ganz PA, Bromberger J, et al. Sleep difficulty in women at midlife: a community survey of sleep and the menopausal transition. Menopause. 2003;10:19-28.
- Dennerstein L, Dudley EC, Hopper JL, et al. A prospective population-based study of menopausal symptoms. Obstet Gynecol. 2000;96:351-358.
- Manson JE, Chlebowski RT, Stefanick ML, et al. Menopausal HT and health outcomes during the intervention and extended poststopping phases of the Women’s Health Initiative randomized trials. JAMA. 2013;310:1353-1368.
- The NAMS 2017 HT Position Statement Advisory Panel. T he 2017 HT position statement of The North American Menopause Society. Menopause. 2017;24:728-753.
- Pinkerton JV. HT for postmenopausal women. N Engl J Med. 2020;382:446-455.
- Stuenkel CA, Davis SR, Gompel A, et al. Treatment of symptoms of the menopause: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2015;100:39754011.
- Manson JE, Kaunitz AM. Menopause management—getting clinical care back on track. N Engl J Med. 2016;374:803–806.
- American College of Obstetricians and Gynecologists. Practice Bulletin No. 141: Management of menopausal symptoms. Obstet Gynecol. 2014;123:202-216.
- Shuster LT, Rhodes DJ, Gostout BS, et al. Premature menopause or early menopause: long-term health consequences. Maturitas. 2010;65:161-166.
- Zeleke BM, Davis SR, Fradkin P, et al. Vasomotor symptoms and urogenital atrophy in older women: a systematic review. Climacteric. 2015;18:112-120.
- Kingsberg SA, Schaffir J, Faught BM, et al. Female sexual health: barriers to optimal outcomes and a roadmap for improved patient-clinician communications. J Womens Health (Larchmt). 2019;28:432-443.
- Eisenlohr-Moul TA, Kaiser G, Weise C, et al. Are there temporal subtypes of pre- menstrual dysphoric disorder? Using group-based trajectory modeling to identify individual differences in symptom change. Psychol Med. 2020;50: 964-972.
- Seibel M, Seibel S. Working through Menopause: The Impact on Women, Businesses and the Bottom Line. Bookbaby. March 8, 2022.
- Jackson LM, Parker RM, Mattison DR, eds. The Clinical Utility of Compounded Bioidentical HT: A Review of Safety, Effectiveness, and Use. Washington, DC: National Academies Press; 2020.
- Pinkerton JV. Concerns about safety and efficacy of compounded bioidentical HT. Menopause. 2021;28:847-849.
- Liu JH, Pinkerton JV. Prescription therapies. In: CJ Crandall, ed. Menopause Practice: A Clinician’s Guide, 6th ed. Pepper Pike, OH: The North American Menopause Society; 2019: 277-309.
- Jiang X, Bossert A, Parthasarathy KN, et al. Safety assessment of compounded non-FDA-approved hormonal therapy versus FDA-approved hormonal therapy in treating postmenopausal women. Menopause. 2021;28:867-874.
- Aninye IO, Laitner MH, Chinnappan S; Society for Women’s Health Research Menopause Working Group. Menopause preparedness: perspectives for patient, provider, and policymaker consideration. Menopause. 2021;28:1186-1191.
The transition to menopause begins with ovarian fluctuation and hormonal changes, often beginning before significant changes in menstruation. Reproductive aging with loss of follicular activity progresses over a wide age range (42 to 58 years) with an average onset at approximately age 47, ranging from 4 to 8 years. Although most women have heard about menopause, defined as 12 months after the last period, they often lack understanding about perimenopause or that the menopausal transition usually begins 5 years before menopause.1
Perimenopause, defined as early and late menopause transition stages, may be viewed as a window of potential vulnerability for women who develop or have worsening menstrual-related mood disorders. Over time, hormonal fluctuations often lead to menstrual cycle irregularity (either shorter or longer). Changes occurring during perimenopause may be confusing as it may not be clear whether symptoms are related to menopause, aging, or stress. Often not recognized or treated adequately, perimenopausal symptoms may be challenging to navigate for both women and clinicians.
The perimenopausal process is often even more confusing for women with early menopause—whether due to bilateral oophorectomy, chemotherapy or radiation therapy, genetics, or an autoimmune process—because of lack of recognition that an early menopausal transition is occurring or what solutions are available for symptoms. While there is support in the workplace for women during pregnancy and breastfeeding, there remains little support or recognition for the oft challenging perimenopausal transition leading to menopause.
Perimenopause: Common symptoms and treatments
Symptoms may be related to either estrogen level deficiency or excess during perimenopause, and these level changes may even occur within the same cycle.
Cyclic breast tenderness may develop, worsened by caffeine or high salt intake (which can be potentially improved, although without clinical trial evidence, with decreased caffeine or a trial of evening primrose oil or vitamin E).
Changes in menstrual flow and frequency of menses are typical. Flow may be lighter or heavier, longer or shorter, and there may be cycle variability, missed menses, or midcycle spotting.2 Bleeding may be heavy, with or without cramping. In addition to imaging with vaginal ultrasonography or hysteroscopy to identify structural issues, symptoms may be managed with nonsteroidal anti-inflammatory drugs (NSAIDs), hormonal therapy (HT) with short hormone-free interval contraceptives, oral progestogens, or progestin intrauterine systems. Newer medical treatments include antifibrinolytic drugs and selective progesterone-receptor modulators. Uterine ablation to decrease or stop bleeding is effective if there are no structural abnormalities, such as fibroids or polyps or the presence of adenomyosis, where glands will regrow into the endometrium after ablation. Endometrial biopsy is indicated for persistent abnormal uterine bleeding or those with risk factors such as chronic anovulation.
Worsening headaches or menstrual migraines may be triggered by hormonal changes, which may respond to NSAIDs; dihydroergotamine; triptans; the combination of aspirin, acetaminophen, and caffeine; or estrogen the week before menses. For women taking oral contraceptives (OCPs), adding estradiol the week before menses, or using the OCP continuously, may decrease headache frequency. These short-term prophylactic strategies during the perimenstrual time are often effective. If not, preventive therapy is available for women with frequent, severe headaches.
Mood complaints and poor sleep are independently associated with menstrual irregularity, and can lead to fatigue or anxiety, worsening premenstrual syndrome, or depressive moods. Sleep is disrupted premenstrually for up to one-third of women, and sleep disruption is particularly prevalent in those with premenstrual mood disorders and worsens during perimenopause.3
Reproductive hormones act on the neurotransmitter systems in the brain involved in mood regulation and emotion. The fluctuating hormones occurring during perimenopause may exacerbate pre-existing menstrual-related mood disorders. A subset of women experience depressive moods due to perimenopausal elevations in ovarian hormones.4 Others may exhibit increased mood sensitivity with the ovarian hormone withdrawal accompanying late menopause transition and early postmenopausal phase.5 There is significant comorbidity between premenstrual mood disorder (PMDD) and postpartum depression.6 During perimenopause and early menopause, clinicians should ask about prior hormonally-related depression (puberty, postpartum) and recognize that current or past premenstrual syndrome may worsen into a more severe premenstrual dysphoric disorder. Evidence-based treatments for PMDD include selective serotonin reuptake inhibitors (SSRIs); either taken continuously or only during the luteal phase; drospirenone-containing oral contraceptives, often with shorter pill-free intervals; GnRH analogues with or without hormone add-back; and cognitive behavioral therapy.7 For women whose perimenopausal moods improve with HT or develop worsened mood sensitivity with ovarian hormone withdrawal, clinicians should recognize that mood may worsen when treatment is ceased.5

Continue to: Menopausal symptoms...
Menopausal symptoms
Vasomotor symptoms (VMS), hot flashes, or night sweats occur in up to 75% of women as they develop more menstrual irregularity and move closer to their final period and menopause.
Hot flashes are transient episodes of flushing with the sensation of warmth (up to intense heat) on the upper body and face or head, often associated with sweating, chills or flushing, an increase in heart rate, and lowered blood pressure. Hot flashes can sometimes be preceded by an intense feeling of dread, followed by rapid heat dissipation. The etiology of hot flashes is still not clear, but the neurokinin receptors are involved. They are related to small fluctuations in core body temperature superimposed on a narrow thermoneutral zone in symptomatic women. Hot flashes are triggered when core body temperature rises above the upper (sweating) threshold. Shivering occurs if the core body temperature falls below the lower threshold. Sleep may be disrupted, with less rapid eye movement (REM) sleep, and associated with throwing covers on and off or changing sheets or nightclothes. On average, hot flashes last 7.2 years,8 and they are more bothersome if night sweats interfere with sleep or disrupt performance during the day.
In the Stages of Reproductive Aging Workshop (STRAW + 10), women reported VMS within 1-3 years after the menopausal transition.8 Four trajectories of hot flashes were identified in the Study of Women’s Health Across the Nation (SWAN) trial,9 including low levels throughout the menopause transition, early onset, late onset, and a group which had frequent hot flashes, starting early and lasting longer. Serum estrogen levels were not predictive of hot flash frequency or severity.
Hot flashes have been associated with low levels of exercise, cigarette smoking, high follicle-stimulating hormone levels and low estradiol levels, increasing body mass index, ethnicity (with hot flashes more common among Black and Hispanic women), low socioeconomic status, prior PMDD, anxiety, perceived stress, and depression.8 Women with a history of premenstrual syndrome, stress, sexual dysfunction, physical inactivity, or hot flashes are more vulnerable to depressive symptoms during perimenopause and early menopause.5
Depression may co-occur or overlap with menopause symptoms. Diagnosis involves menopausal stage, co-occurring psychiatric and menopause symptoms, psychosocial stressors, and a validated screening tool such as PQ9. Treatments for perimenopausal depression, such as antidepressants, psychotherapy, or cognitive behavioral therapy, are recommended first line for perimenopausal depression. Estrogen therapy has not been approved to treat perimenopausal depression but appears to have antidepressant effects in perimenopausal women, particularly those with bothersome vasomotor symptoms.5
Anxiety can worsen during menopause, and may respond to calming apps, meditation, cognitive behavioral therapy, hypnosis, yoga or tai chi, HT, or antianxiety medications.
Weight gain around the abdomen (ie, belly fat) is a common complaint during the menopausal transition, despite women reporting not changing their eating or exercise patterns. Increasing exercise or bursts of higher intensity, decreasing portion sizes or limiting carbohydrates and alcohol may help.
Memory and concentration problems, described as brain fog, tend to be more of an issue in perimenopause and level out after menopause. Counsel midlife women that these changes are not due to dementia but are related to normal aging, hormonal changes, mood, stress, or other life circumstances. Identifying and addressing sleep issues and mood disorders may help mitigate brain fog, as can advising women to avoid excess caffeine, alcohol, nicotine, and eating before bed. Improvements in memory, cognition, and health have been found with the Mediterranean diet, regular exercise, avoiding multitasking, and engaging in mentally stimulating activities.
Sleeping concerns in peri- and postmenopausal women include sleeping less and more frequent insomnia. Women are more likely to use prescription sleeping aids during these times of their lives. The data from SWAN8 show that the menopausal transition is related to self-reported difficulty sleeping, independent of age. Sleep latency interval is increased while REM sleep decreases. Night sweats can trigger awakenings in the first half of the night. The perceived decline in sleep quality also may be attributed to general aging effects, nocturnal urination, sleep-related disorders such as sleep apnea or restless legs, or chronic pain, stress, or depression.10 Suggestions for management include sleep apps, cognitive behavioral therapy, low-dose antidepressant therapy, addressing sleep routines, and HT. Hypnotics should be avoided.
Sexuality issues are common complaints during the menopausal transition. Cross-sectional data reported from a longitudinal, population-based Australian cohort of women aged 45 to 55 years, found a decrease in sexual responsivity, sexual frequency, libido, vaginal dyspareunia, and more partner problems.11 Low libido may be related to relationship issues, dyspareunia with vaginal narrowing, loss of lubrication, levator spasm, stress, anxiety, exhaustion or mood disorder, lowered hormone levels, excess alcohol intake, underlying health concerns, or a side effect of medications for depression or pain. There is no direct correlation between testosterone levels and libido.
When HT at menopause may be helpful
For healthy symptomatic women without contraindications who are younger than age 60, or within 10 years of menopause onset, the benefits of initiating HT most likely outweigh the risks to relieve bothersome hot flashes and night sweats.12-17 For older women, or for those further from menopause, the greater absolute risks of coronary heart disease, stroke, venous thromboembolism, and dementia, in general, outweigh the potential benefits.12-17 Extended durations of HT have less safety and efficacy data and should be considered primarily for those with persistent menopausal symptoms, with periodic re-evaluation.13,14 For bothersome genitourinary syndrome of menopause symptoms that do not respond to vaginal moisturizers or lubricants, low-dose vaginal HTs are encouraged.13-17

Continue to: Early-onset menopause...
Early-onset menopause
According to observational studies,18 early menopause is associated with a higher risk of osteoporosis, coronary heart disease, cognitive changes, vaginal dryness, loss of libido, and mood changes. Studies have shown that women with early menopause who take HT, without contraindications, to the average age of menopause (age 52) decrease the health risks of early menopause (bone loss, heart disease, mood, and cognition changes).13,14,18
Women with early menopause, whether spontaneous or following bilateral oophorectomy or cancer treatment, should be counseled to get adequate calcium (dietary recommended over supplementation) and vitamin D intake, eat a healthy diet, and exercise regularly. Evaluation should include risk for bone loss, heart disease, mood changes, and vaginal changes.
Extended use of HT
Up to 8% of women have hot flashes for 20 years or more after menopause.19 The decision to continue or to stop HT is not always clear for women:
- with persistent hot flashes after a trial period of HT discontinuation
- with bone loss that cannot be treated with bone-specific medications
- who request continuation for quality of life.
Extended use of HT should include an ongoing assessment of its risks and benefits, periodic trials off of HT, and documentation of rationale and informed discussions about continuing. Lower doses and transdermal therapies appear safer, as does micronized progesterone instead of more potent synthetic progestins.13-17
Genitourinary syndrome of menopause
Once women are further into menopause, they may notice vaginal dryness, vulvar itching or burning, bothersome vaginal discharge, or urinary urgency or frequency. The development of painful intercourse frequently occurs, a combination of the loss of estrogen with thinning of the vaginal mucosa, a loss of the acidic vaginal milieu with less elasticity, and spasm of the levator muscles. Some women develop urinary tract infections after intercourse or have more frequent reoccurrences. First-line therapy is often vaginal moisturizers and lubricants. Vaginal therapies (estradiol, conjugated estrogen, or dehydroepiandrosterone) or oral selective estrogen-receptor modulators (SERMs; ospemifene) improve vaginal dryness and dyspareunia.13,14 Pelvic therapy has also proved valuable for incontinence, pelvic floor dysfunction, and levator spasms.20
Where are there gaps in clinician knowledge?
Studies on emotional health, mood, and sleep need to incorporate measures of menstrual timing into data collection and analyses. Does the sleep disruption occurring premenstrually during perimenopause disproportionately contribute to a woman’s vulnerability to depressive disorders? The risk of clinically significant depressive symptoms increases 1.5- to 2.9-fold in the menopause transition.5 Research into premenstrual dysphoria during the menopause transition may identify different trajectories in the timing of symptoms related to either cycle itself or the ovarian hormone fluctuations or both.21 Gamma-aminobutyric acid (GABA)-modulating drugs, such as sepranolone, which blocks allopregnanolone’s actions at the GABAA receptor, may allow treatment of menstrual-related mood disorders without the need for hormonal interventions.21
Despite extended observational trial data, more data are needed to inform us about the long-term risks and benefits of using menopausal HT, particularly when initiated at menopause and to help address the timing of HT discontinuation. Furthermore, there are many unanswered questions. For instance:
- How much safer are lower dose and transdermal therapies?
- Do untreated hot flashes increase the risk of cardiovascular disease or dementia?
- Will newer non-HT options, such as the neurokinin receptor antagonists that are in testing but are not yet available, lower cardiovascular or dementia risks?
- What will be the risks and benefits for the newer estrogen in testing (estetrol, or E4), considered a natural estrogen and which appears to have lower thrombotic risks?
- What will be the role of intravaginal energy-based therapies, such as vaginal laser or radiofrequency devices?
- How do we address diverse populations and the effects of menopause on race, gender, culture, prior trauma, and socioeconomic status?
Lack of recognition of menopausal symptoms, particularly in the workplace
Clinicians need to understand the varied physical and emotional symptoms that may occur with hormonal changes as women traverse perimenopause and early menopause. We need to recognize that the lack of discussion about women’s health during this time may make women feel ashamed and fearful of bringing up their symptoms due to fear of being dismissed or stigmatized.22 Women may not seek help until a crisis at home or work occurs, as they may fear that admitting symptoms or a need for help or time away from work will threaten how they are viewed at work or affect their chances of promotion. Although there are economic costs around menopause for appointments, tests, therapies, and missed time at work, not addressing menopausal health leads to poorer performance, workplace absences, and additional medical costs.22

Conclusion
Menopause occurs naturally as a part of a woman’s life cycle. However, women need assistance navigating perimenopausal hormonal fluctuations and decisions about HT once in menopause. Increased awareness and education about perimenopause and menopause will allow compassionate, individualized, informed care, including lifestyle changes, behavioral or complementary strategies, or medical therapies, hormonal or nonhormonal.27 As a medical society, we need to challenge the stigma associated with aging and menopause and educate ourselves and our patients to help women navigate this challenging time. ●
Myth 1: All hot flashes are the same
The truth: Seventy-five percent of women will have hot flashes, but only 25% are severe enough to cause women to seek treatment. Duration varies with identified patterns, including starting early or late, being mild or starting early, and going late. Ethnicity affects the duration of hot flashes, with longer durations seen in Black and Hispanic women. About 15% of women have had hot flashes for more than 15 or 20 years.1,2
Myth 2: There is no help for hot flashes
The truth: For some women, lifestyle changes are helpful, such as dressing in layers, turning down the thermostat at night, avoiding hot beverages or alcohol, or using technology (Femtech) for cooling devices. Over-the-counter products that are available, but are not clearly proven to help more than placebo, include soy (which may be estrogenic), black cohosh supplements, and nutritional supplements. Cognitive behavioral therapy, hypnosis, weight loss, or mindfulness may help.3 Nonhormone medications such as low-dose antidepressants or gabapentin have shown benefit. Newer treatments in testing, including neurokinin receptor antagonists, appear to work quickly and as effectively as HT. When initiating HT, healthy women with bothersome hot flashes under age 60 or within 10 years of menopause are the best candidates for HT; many lower doses and oral and non-oral therapies are available.
Myth 3: Compounded bioidentical hormones made by a compounding pharmacy are safer and more effective than FDA-approved ones
The truth: Compounded bioidentical hormones are touted as safer or more effective, but there is no good evidence to back up those claims. Whether US Food and Drug Administration (FDA)-approved or compounded, hormones come from the same precursors and have potential risks. With custom compounded HT, there is additional concern about precisely what is in the compounded product, whether levels are similar batch to batch, and the degree of absorption of progesterone, which is better absorbed oral.4-6 FDA-approved bioidentical HTs have been tested for safety, proven to contain consistent, effective levels of hormones, and are monitored by the FDA. For menopausal symptoms, FDA-approved therapies are available as estradiol (oral, patch, spray, gel, lotion, and vaginal ring) and progesterone (as an oral compound or combined with estradiol). Pellets made of compounded hormones have shown higher serum levels and more adverse events.5,7
Myth 4: Menopause causes weight gain
The truth is that fluctuating and declining hormones and the slowing of metabolism affect weight. Weight gain is not inevitable, just harder to prevent. Many women gain an average of 5 lb (2.27 kg) at midlife, which is mainly related to aging and lifestyle and not to menopause or HT. However, menopause may be related to body composition and fat distribution changes. Counsel women to decrease portion sizes, limit carbs, and increase exercise intensity, including strength training. The goal is 30 minutes of moderate aerobic activity per day, all at once or through smaller time increments, to improve their energy, mood, and sleep.
References
1. The NAMS 2017 HT Position Statement Advisory Panel. The 2017 HT position statement of The North American Menopause Society. Menopause. 2017;24:728-753.
2. Pinkerton JV. HT for postmenopausal women. N Engl J Med. 2020;382:446-455.
3. Paramsothy P. Duration of the menopausal transition is longer in women with young age at onset: the multiethnic Study of Women’s Health Across the Nation. Menopause. 2017;24:142-149.
4. Kingsberg SA, Schaffir J, Faught BM, et al. Female sexual health: barriers to optimal outcomes and a roadmap for improved patient-clinician communications. J Womens Health (Larchmt). 2019;28:432-443.
5. Eisenlohr-Moul TA, Kaiser G, Weise C, et al. Are there temporal subtypes of premenstrual dysphoric disorder? Using group-based trajectory modeling to identify individual differences in symptom change. Psychol Med. 2020;50:964-972.
6. Seibel M, Seibel S. Working through Menopause: The Impact on Women, Businesses and the Bottom Line. Bookbaby. March 8, 2022.
7. Kingsberg SA, Schaffir J, Faught BM, et al. Female sexual health: barriers to optimal outcomes and a roadmap for improved patient-clinician communications. J Womens Health (Larchmt). 2019;28:432-443.
The transition to menopause begins with ovarian fluctuation and hormonal changes, often beginning before significant changes in menstruation. Reproductive aging with loss of follicular activity progresses over a wide age range (42 to 58 years) with an average onset at approximately age 47, ranging from 4 to 8 years. Although most women have heard about menopause, defined as 12 months after the last period, they often lack understanding about perimenopause or that the menopausal transition usually begins 5 years before menopause.1
Perimenopause, defined as early and late menopause transition stages, may be viewed as a window of potential vulnerability for women who develop or have worsening menstrual-related mood disorders. Over time, hormonal fluctuations often lead to menstrual cycle irregularity (either shorter or longer). Changes occurring during perimenopause may be confusing as it may not be clear whether symptoms are related to menopause, aging, or stress. Often not recognized or treated adequately, perimenopausal symptoms may be challenging to navigate for both women and clinicians.
The perimenopausal process is often even more confusing for women with early menopause—whether due to bilateral oophorectomy, chemotherapy or radiation therapy, genetics, or an autoimmune process—because of lack of recognition that an early menopausal transition is occurring or what solutions are available for symptoms. While there is support in the workplace for women during pregnancy and breastfeeding, there remains little support or recognition for the oft challenging perimenopausal transition leading to menopause.
Perimenopause: Common symptoms and treatments
Symptoms may be related to either estrogen level deficiency or excess during perimenopause, and these level changes may even occur within the same cycle.
Cyclic breast tenderness may develop, worsened by caffeine or high salt intake (which can be potentially improved, although without clinical trial evidence, with decreased caffeine or a trial of evening primrose oil or vitamin E).
Changes in menstrual flow and frequency of menses are typical. Flow may be lighter or heavier, longer or shorter, and there may be cycle variability, missed menses, or midcycle spotting.2 Bleeding may be heavy, with or without cramping. In addition to imaging with vaginal ultrasonography or hysteroscopy to identify structural issues, symptoms may be managed with nonsteroidal anti-inflammatory drugs (NSAIDs), hormonal therapy (HT) with short hormone-free interval contraceptives, oral progestogens, or progestin intrauterine systems. Newer medical treatments include antifibrinolytic drugs and selective progesterone-receptor modulators. Uterine ablation to decrease or stop bleeding is effective if there are no structural abnormalities, such as fibroids or polyps or the presence of adenomyosis, where glands will regrow into the endometrium after ablation. Endometrial biopsy is indicated for persistent abnormal uterine bleeding or those with risk factors such as chronic anovulation.
Worsening headaches or menstrual migraines may be triggered by hormonal changes, which may respond to NSAIDs; dihydroergotamine; triptans; the combination of aspirin, acetaminophen, and caffeine; or estrogen the week before menses. For women taking oral contraceptives (OCPs), adding estradiol the week before menses, or using the OCP continuously, may decrease headache frequency. These short-term prophylactic strategies during the perimenstrual time are often effective. If not, preventive therapy is available for women with frequent, severe headaches.
Mood complaints and poor sleep are independently associated with menstrual irregularity, and can lead to fatigue or anxiety, worsening premenstrual syndrome, or depressive moods. Sleep is disrupted premenstrually for up to one-third of women, and sleep disruption is particularly prevalent in those with premenstrual mood disorders and worsens during perimenopause.3
Reproductive hormones act on the neurotransmitter systems in the brain involved in mood regulation and emotion. The fluctuating hormones occurring during perimenopause may exacerbate pre-existing menstrual-related mood disorders. A subset of women experience depressive moods due to perimenopausal elevations in ovarian hormones.4 Others may exhibit increased mood sensitivity with the ovarian hormone withdrawal accompanying late menopause transition and early postmenopausal phase.5 There is significant comorbidity between premenstrual mood disorder (PMDD) and postpartum depression.6 During perimenopause and early menopause, clinicians should ask about prior hormonally-related depression (puberty, postpartum) and recognize that current or past premenstrual syndrome may worsen into a more severe premenstrual dysphoric disorder. Evidence-based treatments for PMDD include selective serotonin reuptake inhibitors (SSRIs); either taken continuously or only during the luteal phase; drospirenone-containing oral contraceptives, often with shorter pill-free intervals; GnRH analogues with or without hormone add-back; and cognitive behavioral therapy.7 For women whose perimenopausal moods improve with HT or develop worsened mood sensitivity with ovarian hormone withdrawal, clinicians should recognize that mood may worsen when treatment is ceased.5

Continue to: Menopausal symptoms...
Menopausal symptoms
Vasomotor symptoms (VMS), hot flashes, or night sweats occur in up to 75% of women as they develop more menstrual irregularity and move closer to their final period and menopause.
Hot flashes are transient episodes of flushing with the sensation of warmth (up to intense heat) on the upper body and face or head, often associated with sweating, chills or flushing, an increase in heart rate, and lowered blood pressure. Hot flashes can sometimes be preceded by an intense feeling of dread, followed by rapid heat dissipation. The etiology of hot flashes is still not clear, but the neurokinin receptors are involved. They are related to small fluctuations in core body temperature superimposed on a narrow thermoneutral zone in symptomatic women. Hot flashes are triggered when core body temperature rises above the upper (sweating) threshold. Shivering occurs if the core body temperature falls below the lower threshold. Sleep may be disrupted, with less rapid eye movement (REM) sleep, and associated with throwing covers on and off or changing sheets or nightclothes. On average, hot flashes last 7.2 years,8 and they are more bothersome if night sweats interfere with sleep or disrupt performance during the day.
In the Stages of Reproductive Aging Workshop (STRAW + 10), women reported VMS within 1-3 years after the menopausal transition.8 Four trajectories of hot flashes were identified in the Study of Women’s Health Across the Nation (SWAN) trial,9 including low levels throughout the menopause transition, early onset, late onset, and a group which had frequent hot flashes, starting early and lasting longer. Serum estrogen levels were not predictive of hot flash frequency or severity.
Hot flashes have been associated with low levels of exercise, cigarette smoking, high follicle-stimulating hormone levels and low estradiol levels, increasing body mass index, ethnicity (with hot flashes more common among Black and Hispanic women), low socioeconomic status, prior PMDD, anxiety, perceived stress, and depression.8 Women with a history of premenstrual syndrome, stress, sexual dysfunction, physical inactivity, or hot flashes are more vulnerable to depressive symptoms during perimenopause and early menopause.5
Depression may co-occur or overlap with menopause symptoms. Diagnosis involves menopausal stage, co-occurring psychiatric and menopause symptoms, psychosocial stressors, and a validated screening tool such as PQ9. Treatments for perimenopausal depression, such as antidepressants, psychotherapy, or cognitive behavioral therapy, are recommended first line for perimenopausal depression. Estrogen therapy has not been approved to treat perimenopausal depression but appears to have antidepressant effects in perimenopausal women, particularly those with bothersome vasomotor symptoms.5
Anxiety can worsen during menopause, and may respond to calming apps, meditation, cognitive behavioral therapy, hypnosis, yoga or tai chi, HT, or antianxiety medications.
Weight gain around the abdomen (ie, belly fat) is a common complaint during the menopausal transition, despite women reporting not changing their eating or exercise patterns. Increasing exercise or bursts of higher intensity, decreasing portion sizes or limiting carbohydrates and alcohol may help.
Memory and concentration problems, described as brain fog, tend to be more of an issue in perimenopause and level out after menopause. Counsel midlife women that these changes are not due to dementia but are related to normal aging, hormonal changes, mood, stress, or other life circumstances. Identifying and addressing sleep issues and mood disorders may help mitigate brain fog, as can advising women to avoid excess caffeine, alcohol, nicotine, and eating before bed. Improvements in memory, cognition, and health have been found with the Mediterranean diet, regular exercise, avoiding multitasking, and engaging in mentally stimulating activities.
Sleeping concerns in peri- and postmenopausal women include sleeping less and more frequent insomnia. Women are more likely to use prescription sleeping aids during these times of their lives. The data from SWAN8 show that the menopausal transition is related to self-reported difficulty sleeping, independent of age. Sleep latency interval is increased while REM sleep decreases. Night sweats can trigger awakenings in the first half of the night. The perceived decline in sleep quality also may be attributed to general aging effects, nocturnal urination, sleep-related disorders such as sleep apnea or restless legs, or chronic pain, stress, or depression.10 Suggestions for management include sleep apps, cognitive behavioral therapy, low-dose antidepressant therapy, addressing sleep routines, and HT. Hypnotics should be avoided.
Sexuality issues are common complaints during the menopausal transition. Cross-sectional data reported from a longitudinal, population-based Australian cohort of women aged 45 to 55 years, found a decrease in sexual responsivity, sexual frequency, libido, vaginal dyspareunia, and more partner problems.11 Low libido may be related to relationship issues, dyspareunia with vaginal narrowing, loss of lubrication, levator spasm, stress, anxiety, exhaustion or mood disorder, lowered hormone levels, excess alcohol intake, underlying health concerns, or a side effect of medications for depression or pain. There is no direct correlation between testosterone levels and libido.
When HT at menopause may be helpful
For healthy symptomatic women without contraindications who are younger than age 60, or within 10 years of menopause onset, the benefits of initiating HT most likely outweigh the risks to relieve bothersome hot flashes and night sweats.12-17 For older women, or for those further from menopause, the greater absolute risks of coronary heart disease, stroke, venous thromboembolism, and dementia, in general, outweigh the potential benefits.12-17 Extended durations of HT have less safety and efficacy data and should be considered primarily for those with persistent menopausal symptoms, with periodic re-evaluation.13,14 For bothersome genitourinary syndrome of menopause symptoms that do not respond to vaginal moisturizers or lubricants, low-dose vaginal HTs are encouraged.13-17

Continue to: Early-onset menopause...
Early-onset menopause
According to observational studies,18 early menopause is associated with a higher risk of osteoporosis, coronary heart disease, cognitive changes, vaginal dryness, loss of libido, and mood changes. Studies have shown that women with early menopause who take HT, without contraindications, to the average age of menopause (age 52) decrease the health risks of early menopause (bone loss, heart disease, mood, and cognition changes).13,14,18
Women with early menopause, whether spontaneous or following bilateral oophorectomy or cancer treatment, should be counseled to get adequate calcium (dietary recommended over supplementation) and vitamin D intake, eat a healthy diet, and exercise regularly. Evaluation should include risk for bone loss, heart disease, mood changes, and vaginal changes.
Extended use of HT
Up to 8% of women have hot flashes for 20 years or more after menopause.19 The decision to continue or to stop HT is not always clear for women:
- with persistent hot flashes after a trial period of HT discontinuation
- with bone loss that cannot be treated with bone-specific medications
- who request continuation for quality of life.
Extended use of HT should include an ongoing assessment of its risks and benefits, periodic trials off of HT, and documentation of rationale and informed discussions about continuing. Lower doses and transdermal therapies appear safer, as does micronized progesterone instead of more potent synthetic progestins.13-17
Genitourinary syndrome of menopause
Once women are further into menopause, they may notice vaginal dryness, vulvar itching or burning, bothersome vaginal discharge, or urinary urgency or frequency. The development of painful intercourse frequently occurs, a combination of the loss of estrogen with thinning of the vaginal mucosa, a loss of the acidic vaginal milieu with less elasticity, and spasm of the levator muscles. Some women develop urinary tract infections after intercourse or have more frequent reoccurrences. First-line therapy is often vaginal moisturizers and lubricants. Vaginal therapies (estradiol, conjugated estrogen, or dehydroepiandrosterone) or oral selective estrogen-receptor modulators (SERMs; ospemifene) improve vaginal dryness and dyspareunia.13,14 Pelvic therapy has also proved valuable for incontinence, pelvic floor dysfunction, and levator spasms.20
Where are there gaps in clinician knowledge?
Studies on emotional health, mood, and sleep need to incorporate measures of menstrual timing into data collection and analyses. Does the sleep disruption occurring premenstrually during perimenopause disproportionately contribute to a woman’s vulnerability to depressive disorders? The risk of clinically significant depressive symptoms increases 1.5- to 2.9-fold in the menopause transition.5 Research into premenstrual dysphoria during the menopause transition may identify different trajectories in the timing of symptoms related to either cycle itself or the ovarian hormone fluctuations or both.21 Gamma-aminobutyric acid (GABA)-modulating drugs, such as sepranolone, which blocks allopregnanolone’s actions at the GABAA receptor, may allow treatment of menstrual-related mood disorders without the need for hormonal interventions.21
Despite extended observational trial data, more data are needed to inform us about the long-term risks and benefits of using menopausal HT, particularly when initiated at menopause and to help address the timing of HT discontinuation. Furthermore, there are many unanswered questions. For instance:
- How much safer are lower dose and transdermal therapies?
- Do untreated hot flashes increase the risk of cardiovascular disease or dementia?
- Will newer non-HT options, such as the neurokinin receptor antagonists that are in testing but are not yet available, lower cardiovascular or dementia risks?
- What will be the risks and benefits for the newer estrogen in testing (estetrol, or E4), considered a natural estrogen and which appears to have lower thrombotic risks?
- What will be the role of intravaginal energy-based therapies, such as vaginal laser or radiofrequency devices?
- How do we address diverse populations and the effects of menopause on race, gender, culture, prior trauma, and socioeconomic status?
Lack of recognition of menopausal symptoms, particularly in the workplace
Clinicians need to understand the varied physical and emotional symptoms that may occur with hormonal changes as women traverse perimenopause and early menopause. We need to recognize that the lack of discussion about women’s health during this time may make women feel ashamed and fearful of bringing up their symptoms due to fear of being dismissed or stigmatized.22 Women may not seek help until a crisis at home or work occurs, as they may fear that admitting symptoms or a need for help or time away from work will threaten how they are viewed at work or affect their chances of promotion. Although there are economic costs around menopause for appointments, tests, therapies, and missed time at work, not addressing menopausal health leads to poorer performance, workplace absences, and additional medical costs.22

Conclusion
Menopause occurs naturally as a part of a woman’s life cycle. However, women need assistance navigating perimenopausal hormonal fluctuations and decisions about HT once in menopause. Increased awareness and education about perimenopause and menopause will allow compassionate, individualized, informed care, including lifestyle changes, behavioral or complementary strategies, or medical therapies, hormonal or nonhormonal.27 As a medical society, we need to challenge the stigma associated with aging and menopause and educate ourselves and our patients to help women navigate this challenging time. ●
Myth 1: All hot flashes are the same
The truth: Seventy-five percent of women will have hot flashes, but only 25% are severe enough to cause women to seek treatment. Duration varies with identified patterns, including starting early or late, being mild or starting early, and going late. Ethnicity affects the duration of hot flashes, with longer durations seen in Black and Hispanic women. About 15% of women have had hot flashes for more than 15 or 20 years.1,2
Myth 2: There is no help for hot flashes
The truth: For some women, lifestyle changes are helpful, such as dressing in layers, turning down the thermostat at night, avoiding hot beverages or alcohol, or using technology (Femtech) for cooling devices. Over-the-counter products that are available, but are not clearly proven to help more than placebo, include soy (which may be estrogenic), black cohosh supplements, and nutritional supplements. Cognitive behavioral therapy, hypnosis, weight loss, or mindfulness may help.3 Nonhormone medications such as low-dose antidepressants or gabapentin have shown benefit. Newer treatments in testing, including neurokinin receptor antagonists, appear to work quickly and as effectively as HT. When initiating HT, healthy women with bothersome hot flashes under age 60 or within 10 years of menopause are the best candidates for HT; many lower doses and oral and non-oral therapies are available.
Myth 3: Compounded bioidentical hormones made by a compounding pharmacy are safer and more effective than FDA-approved ones
The truth: Compounded bioidentical hormones are touted as safer or more effective, but there is no good evidence to back up those claims. Whether US Food and Drug Administration (FDA)-approved or compounded, hormones come from the same precursors and have potential risks. With custom compounded HT, there is additional concern about precisely what is in the compounded product, whether levels are similar batch to batch, and the degree of absorption of progesterone, which is better absorbed oral.4-6 FDA-approved bioidentical HTs have been tested for safety, proven to contain consistent, effective levels of hormones, and are monitored by the FDA. For menopausal symptoms, FDA-approved therapies are available as estradiol (oral, patch, spray, gel, lotion, and vaginal ring) and progesterone (as an oral compound or combined with estradiol). Pellets made of compounded hormones have shown higher serum levels and more adverse events.5,7
Myth 4: Menopause causes weight gain
The truth is that fluctuating and declining hormones and the slowing of metabolism affect weight. Weight gain is not inevitable, just harder to prevent. Many women gain an average of 5 lb (2.27 kg) at midlife, which is mainly related to aging and lifestyle and not to menopause or HT. However, menopause may be related to body composition and fat distribution changes. Counsel women to decrease portion sizes, limit carbs, and increase exercise intensity, including strength training. The goal is 30 minutes of moderate aerobic activity per day, all at once or through smaller time increments, to improve their energy, mood, and sleep.
References
1. The NAMS 2017 HT Position Statement Advisory Panel. The 2017 HT position statement of The North American Menopause Society. Menopause. 2017;24:728-753.
2. Pinkerton JV. HT for postmenopausal women. N Engl J Med. 2020;382:446-455.
3. Paramsothy P. Duration of the menopausal transition is longer in women with young age at onset: the multiethnic Study of Women’s Health Across the Nation. Menopause. 2017;24:142-149.
4. Kingsberg SA, Schaffir J, Faught BM, et al. Female sexual health: barriers to optimal outcomes and a roadmap for improved patient-clinician communications. J Womens Health (Larchmt). 2019;28:432-443.
5. Eisenlohr-Moul TA, Kaiser G, Weise C, et al. Are there temporal subtypes of premenstrual dysphoric disorder? Using group-based trajectory modeling to identify individual differences in symptom change. Psychol Med. 2020;50:964-972.
6. Seibel M, Seibel S. Working through Menopause: The Impact on Women, Businesses and the Bottom Line. Bookbaby. March 8, 2022.
7. Kingsberg SA, Schaffir J, Faught BM, et al. Female sexual health: barriers to optimal outcomes and a roadmap for improved patient-clinician communications. J Womens Health (Larchmt). 2019;28:432-443.
- Paramsothy P. Duration of the menopausal transition is longer in women with young age at onset: the multiethnic Study of Women’s Health Across the Nation. Menopause. 2017;24:142–149.
- Harlow SD, Gass M, Hall JE, et al. STRAW 10 Collaborative Group. Executive summary of the Stages of Reproductive Aging Workshop + 10: addressing the unfinished agenda of staging reproductive aging. Menopause. 2012;19:387-95.
- Meers JM, Nowakowski S. Sleep, premenstrual mood disorder, and women’s health. Curr Opin Psychol. 2020;34:43-49.
- Sander B, Gordon JL. Premenstrual mood symptoms in the perimenopause. Curr Psychiatry Rep. 2021;23:73.
- Maki PM, Kornstein SG, Joffe H, et al. Guidelines for the evaluation and treatment of perimenopausal depression: summary and recommendations. J Women’s Health. 2019;28:117–134.
- Cao S, Jones M, Tooth L, et al. History of premenstrual syndrome and development of postpartum depression: a systematic review and meta-analysis. J Psychiatr Res. 2020;121:82–90.
- Rapkin AJ, Korotkaya Y, Taylor KC. Contraception counseling for women with premenstrual dysphoric disorder (PMDD): current perspectives. Open Access J Contracept. 2019;10:27–39.
- Avis NE, Crawford SL, Greendale G, et al; Study of Women's Health Across the Nation. Duration of menopausal vasomotor symptoms over the menopause transition. JAMA Intern Med. 2015;175:531.
- Tepper PG, Brooks MM, Randolph JF Jr, et al. Characterizing the trajectories of vasomotor symptoms across the menopausal transition. Menopause. 2016;23:1067-1074.
- Kravitz HM, Ganz PA, Bromberger J, et al. Sleep difficulty in women at midlife: a community survey of sleep and the menopausal transition. Menopause. 2003;10:19-28.
- Dennerstein L, Dudley EC, Hopper JL, et al. A prospective population-based study of menopausal symptoms. Obstet Gynecol. 2000;96:351-358.
- Manson JE, Chlebowski RT, Stefanick ML, et al. Menopausal HT and health outcomes during the intervention and extended poststopping phases of the Women’s Health Initiative randomized trials. JAMA. 2013;310:1353-1368.
- The NAMS 2017 HT Position Statement Advisory Panel. T he 2017 HT position statement of The North American Menopause Society. Menopause. 2017;24:728-753.
- Pinkerton JV. HT for postmenopausal women. N Engl J Med. 2020;382:446-455.
- Stuenkel CA, Davis SR, Gompel A, et al. Treatment of symptoms of the menopause: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2015;100:39754011.
- Manson JE, Kaunitz AM. Menopause management—getting clinical care back on track. N Engl J Med. 2016;374:803–806.
- American College of Obstetricians and Gynecologists. Practice Bulletin No. 141: Management of menopausal symptoms. Obstet Gynecol. 2014;123:202-216.
- Shuster LT, Rhodes DJ, Gostout BS, et al. Premature menopause or early menopause: long-term health consequences. Maturitas. 2010;65:161-166.
- Zeleke BM, Davis SR, Fradkin P, et al. Vasomotor symptoms and urogenital atrophy in older women: a systematic review. Climacteric. 2015;18:112-120.
- Kingsberg SA, Schaffir J, Faught BM, et al. Female sexual health: barriers to optimal outcomes and a roadmap for improved patient-clinician communications. J Womens Health (Larchmt). 2019;28:432-443.
- Eisenlohr-Moul TA, Kaiser G, Weise C, et al. Are there temporal subtypes of pre- menstrual dysphoric disorder? Using group-based trajectory modeling to identify individual differences in symptom change. Psychol Med. 2020;50: 964-972.
- Seibel M, Seibel S. Working through Menopause: The Impact on Women, Businesses and the Bottom Line. Bookbaby. March 8, 2022.
- Jackson LM, Parker RM, Mattison DR, eds. The Clinical Utility of Compounded Bioidentical HT: A Review of Safety, Effectiveness, and Use. Washington, DC: National Academies Press; 2020.
- Pinkerton JV. Concerns about safety and efficacy of compounded bioidentical HT. Menopause. 2021;28:847-849.
- Liu JH, Pinkerton JV. Prescription therapies. In: CJ Crandall, ed. Menopause Practice: A Clinician’s Guide, 6th ed. Pepper Pike, OH: The North American Menopause Society; 2019: 277-309.
- Jiang X, Bossert A, Parthasarathy KN, et al. Safety assessment of compounded non-FDA-approved hormonal therapy versus FDA-approved hormonal therapy in treating postmenopausal women. Menopause. 2021;28:867-874.
- Aninye IO, Laitner MH, Chinnappan S; Society for Women’s Health Research Menopause Working Group. Menopause preparedness: perspectives for patient, provider, and policymaker consideration. Menopause. 2021;28:1186-1191.
- Paramsothy P. Duration of the menopausal transition is longer in women with young age at onset: the multiethnic Study of Women’s Health Across the Nation. Menopause. 2017;24:142–149.
- Harlow SD, Gass M, Hall JE, et al. STRAW 10 Collaborative Group. Executive summary of the Stages of Reproductive Aging Workshop + 10: addressing the unfinished agenda of staging reproductive aging. Menopause. 2012;19:387-95.
- Meers JM, Nowakowski S. Sleep, premenstrual mood disorder, and women’s health. Curr Opin Psychol. 2020;34:43-49.
- Sander B, Gordon JL. Premenstrual mood symptoms in the perimenopause. Curr Psychiatry Rep. 2021;23:73.
- Maki PM, Kornstein SG, Joffe H, et al. Guidelines for the evaluation and treatment of perimenopausal depression: summary and recommendations. J Women’s Health. 2019;28:117–134.
- Cao S, Jones M, Tooth L, et al. History of premenstrual syndrome and development of postpartum depression: a systematic review and meta-analysis. J Psychiatr Res. 2020;121:82–90.
- Rapkin AJ, Korotkaya Y, Taylor KC. Contraception counseling for women with premenstrual dysphoric disorder (PMDD): current perspectives. Open Access J Contracept. 2019;10:27–39.
- Avis NE, Crawford SL, Greendale G, et al; Study of Women's Health Across the Nation. Duration of menopausal vasomotor symptoms over the menopause transition. JAMA Intern Med. 2015;175:531.
- Tepper PG, Brooks MM, Randolph JF Jr, et al. Characterizing the trajectories of vasomotor symptoms across the menopausal transition. Menopause. 2016;23:1067-1074.
- Kravitz HM, Ganz PA, Bromberger J, et al. Sleep difficulty in women at midlife: a community survey of sleep and the menopausal transition. Menopause. 2003;10:19-28.
- Dennerstein L, Dudley EC, Hopper JL, et al. A prospective population-based study of menopausal symptoms. Obstet Gynecol. 2000;96:351-358.
- Manson JE, Chlebowski RT, Stefanick ML, et al. Menopausal HT and health outcomes during the intervention and extended poststopping phases of the Women’s Health Initiative randomized trials. JAMA. 2013;310:1353-1368.
- The NAMS 2017 HT Position Statement Advisory Panel. T he 2017 HT position statement of The North American Menopause Society. Menopause. 2017;24:728-753.
- Pinkerton JV. HT for postmenopausal women. N Engl J Med. 2020;382:446-455.
- Stuenkel CA, Davis SR, Gompel A, et al. Treatment of symptoms of the menopause: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2015;100:39754011.
- Manson JE, Kaunitz AM. Menopause management—getting clinical care back on track. N Engl J Med. 2016;374:803–806.
- American College of Obstetricians and Gynecologists. Practice Bulletin No. 141: Management of menopausal symptoms. Obstet Gynecol. 2014;123:202-216.
- Shuster LT, Rhodes DJ, Gostout BS, et al. Premature menopause or early menopause: long-term health consequences. Maturitas. 2010;65:161-166.
- Zeleke BM, Davis SR, Fradkin P, et al. Vasomotor symptoms and urogenital atrophy in older women: a systematic review. Climacteric. 2015;18:112-120.
- Kingsberg SA, Schaffir J, Faught BM, et al. Female sexual health: barriers to optimal outcomes and a roadmap for improved patient-clinician communications. J Womens Health (Larchmt). 2019;28:432-443.
- Eisenlohr-Moul TA, Kaiser G, Weise C, et al. Are there temporal subtypes of pre- menstrual dysphoric disorder? Using group-based trajectory modeling to identify individual differences in symptom change. Psychol Med. 2020;50: 964-972.
- Seibel M, Seibel S. Working through Menopause: The Impact on Women, Businesses and the Bottom Line. Bookbaby. March 8, 2022.
- Jackson LM, Parker RM, Mattison DR, eds. The Clinical Utility of Compounded Bioidentical HT: A Review of Safety, Effectiveness, and Use. Washington, DC: National Academies Press; 2020.
- Pinkerton JV. Concerns about safety and efficacy of compounded bioidentical HT. Menopause. 2021;28:847-849.
- Liu JH, Pinkerton JV. Prescription therapies. In: CJ Crandall, ed. Menopause Practice: A Clinician’s Guide, 6th ed. Pepper Pike, OH: The North American Menopause Society; 2019: 277-309.
- Jiang X, Bossert A, Parthasarathy KN, et al. Safety assessment of compounded non-FDA-approved hormonal therapy versus FDA-approved hormonal therapy in treating postmenopausal women. Menopause. 2021;28:867-874.
- Aninye IO, Laitner MH, Chinnappan S; Society for Women’s Health Research Menopause Working Group. Menopause preparedness: perspectives for patient, provider, and policymaker consideration. Menopause. 2021;28:1186-1191.
Steps to minimize morbidity from unanticipated placenta accreta spectrum
CASE Placenta accreta spectrum following uncomplicated vaginal delivery
Imagine you are an obstetric hospitalist taking call at a level II maternal level of care hospital. Your patient is a 35-year-old woman, gravida 2, para 1, with a past history of retained placenta requiring dilation and curettage and intravenous antibiotics for endomyometritis. This is an in vitro fertilization pregnancy that has progressed normally, and the patient labored spontaneously at 38 weeks’ gestation. Following an uncomplicated vaginal delivery, the placenta has not delivered, and you attempt a manual placental extraction after a 40-minute third stage. While there is epidural analgesia and you can reach the uterine fundus, you are unable to create a separation plane between the placenta and uterus.
What do you do next?
Placenta accreta spectrum (PAS) includes a broad range of clinical scenarios with abnormal placental attachment as their common denominator. The condition has classically been defined pathologically, with chorionic villi attaching directly to the myometrium (“accreta”) or extending more deeply into the myometrium (“increta”) or attaching to surrounding tissues and structures (“percreta”).1 It is most commonly encountered in patients with low placental implantation on a prior cesarean section scar; indeed, placenta previa, particularly with a history of cesarean delivery, is the strongest risk factor for the development of PAS.2 In addition to abnormal placental attachment, these placental attachments are often hypervascular and can lead to catastrophic hemorrhage if not managed appropriately. For this reason, patients with sonographic or radiologic signs of PAS should be referred to specialized centers for further workup, counseling, and delivery planning.3
Although delivery at a specialized PAS center has been associated with improved patient outcomes,4 not all patients with PAS will be identified in the antepartum period. Ultrasonography may miss up to 40% to 50% of PAS cases, particularly when the sonologist has not been advised to look for the condition,5 and not all patients with PAS will have a previa implanted in a prior cesarean scar. A recent study found that these patients with nonprevia PAS were identified by imaging less than 40% of the time and were significantly less likely to be managed by a specialized team of clinicians.6 Thus, it falls upon every obstetric care provider to be aware of this diagnosis, promptly recognize its unanticipated presentations, and have a plan to optimize patient safety.
Step 1: Recognition
While PAS is classically defined as a pathologic condition, no clinician has the luxury of histology in the delivery room. Researchers have variously defined PAS clinically, with the common trait of abnormal placental adherence.7-9 The TABLE compares published definitions that have been used in the literature. While some definitions include hemorrhage, no clinician wants to induce significant hemorrhage to confirm their patient’s diagnosis. Thus, practically, the clinical PAS diagnosis comes down to abnormal placental attachment: If it is apparent that some or all of the placenta will not separate from the uterine wall with digital manipulation or careful curettage, then PAS should be suspected, and appropriate steps should be taken before further removal attempts.
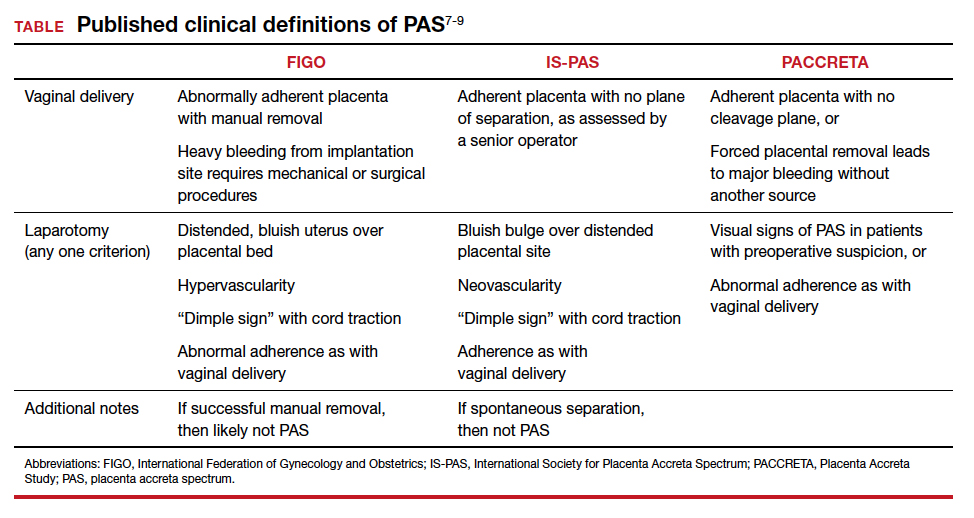
At cesarean delivery, the PAS diagnosis may be aided by visual cues. With placenta previa, the lower uterine segment may bulge and take on a bluish hue, distinctly different from the upper healthy myometrium. PAS may also manifest with neovascularization, particularly behind the bladder. As with vaginal births, the placenta will fail to separate after the delivery, and controlled traction on the umbilical cord can produce a “dimple sign,” or visible myometrial retraction at the site of implantation (FIGURE 1). Finally, if the diagnosis is still in doubt, attempts to gently form a cleavage plane between the placenta and myometrium will be unsuccessful if PAS is present.8
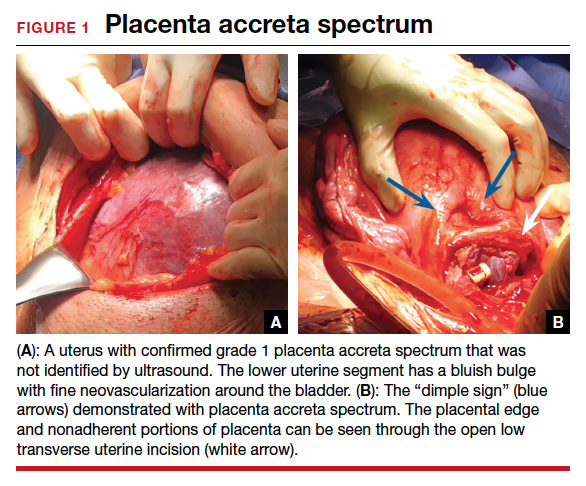
Step 2: Initial management—pause, plan
Most importantly, do not attempt to forcibly remove the placenta. It can be left attached to the uterus until appropriate resources are secured. Efforts to forcibly remove an adherent placenta may well lead to major hemorrhage, and thus it falls on the patient’s care team to pause and plan for PAS care at this point. FIGURE 2 displays an algorithm for patient management. Further steps depend primarily on whether or not the patient is already hemorrhaging. In a stable situation, the patient should be counseled regarding the abnormal findings and the suspected PAS diagnosis. This includes the possibility of further procedures, blood transfusion, and hysterectomy. Local resources, including nursing, anesthesia, and the blood bank, should be notified about the situation and for the potential to call in specialized services. If on-site experienced specialists are not available, then patient transfer to a PAS specialty center should be strongly considered. While awaiting additional help or transport, the patient requires close monitoring for gross and physiologic signs of hemorrhage. If pursued, transport to a PAS specialty center should be expedited.

If the patient is already hemorrhaging or unstable, then appropriate local resources must be activated. At a minimum, this requires an obstetrician and anesthesiologist at the bedside and activation of hemorrhage protocols (eg, a massive transfusion protocol). If blood products are unavailable, consider whether they can be transported from other nearby blood banks, and start that process promptly. Next, contact backup services. Based on local resources and clinical severity, this may include maternal-fetal medicine specialists, pelvic surgeons, general and trauma surgeons, intensivists, interventional radiologists, and transfusion specialists. Even if the patient cannot be safely transferred to another hospital, the obstetrician can call an outside PAS specialist to discuss next steps in care and begin transfer plans, assuming the patient can be stabilized. Based on the Maternal Levels of Care definitions published by the American College of Obstetricians and Gynecologists and the Society of Maternal-Fetal Medicine,10 patients with PAS should be managed at level III or level IV centers. However, delivery units at every level of maternal care should have a protocol for securing local help and reaching an appropriate consultant if a PAS case is encountered. Know which center in your area specializes in PAS so that when an unanticipated case arises, you know who to call.
Continue to: Step 3: Ultimate management—mobilize and prepare for bleeding...
Step 3: Ultimate management—mobilize and prepare for bleeding
If diagnosis occurs intraoperatively at a PAS specialty center, or if safe transport is not possible, then the team should mobilize for the possibility of hysterectomy and prepare for massive bleeding, which can occur regardless of the treatment chosen. Many patients require or will opt for hysterectomy. For example, a patient who has finished childbearing may consent to a hysterectomy upon hearing she likely has PAS. In patients with suspected PAS who are actively hemorrhaging or are unstable, hysterectomy is required.
Uterine conservation may be considered in stable patients who strongly desire future childbearing or uterine retention. This often requires leaving densely adherent placental tissue in situ and thus requires thorough counseling regarding the risks of delayed hemorrhage, infection, and emergent hysterectomy.11 This may not be desirable or safe for some patients, so informed consent is crucial. In such cases, we strongly recommend consultation with a PAS specialist, even if that requires immediate control of the placental blood supply (such as with arterial embolization), and transfer to a PAS specialty center.
Clinical scenarios
Vaginal delivery
The patient in the opening case was never expected to have PAS given her normal placental location and absence of a uterine scar. Even though she had some possible PAS risk factors (past retained placenta with instrumentation and in vitro fertilization), her absolute risk for the condition was low. Nevertheless, inability to create a separation plane should be considered PAS until proven otherwise. Although at this point many obstetricians would move to an operating room for uterine curettage, we recommend that the care team pause and put measures in place for possible PAS and hemorrhage. This involves notification of the blood bank, crossmatching of blood products, alerting the anesthesia team, and having a clear plan in place should a major hemorrhage ensue. This may involve use of balloon tamponade, activation of an interventional radiology team, or possible laparotomy with arterial ligations or hysterectomy. Avoidance of a prolonged third stage should be balanced against the need for preparation with these cases.
It is important for clinicians to bear in mind, and communicate to the patient, that hysterectomy is the standard of care for PAS. Significant delays in performing an indicated hysterectomy can lead to coagulopathy and patient instability. Timeliness is key; we find that delays in the decision to perform an indicated hysterectomy are often at the root of the cause for worsened morbidity in patients with unanticipated PAS. With an unscarred uterus and no placenta previa, a postpartum hysterectomy can be performed by many obstetrician-gynecologists experienced in this abdominal procedure.
Cesarean delivery
Undiagnosed PAS may present at cesarean delivery with or without placenta previa and a prior uterine scar. With this combination, PAS is often visually apparent upon opening the abdominal cavity (TABLE and FIGURE 1). Such surgical findings call for a clinical pause, as further actions at this point can lead to catastrophic hemorrhage. The obstetrician should consider a series of questions:
1. Are appropriate surgical and transfusion resources immediately available? If yes, they should be notified in case they are needed urgently. If not, then the obstetrician should ask whether the delivery must occur now.
2. Is this a scheduled delivery with a stable patient and fetus? If so, then closing the abdominal incision, monitoring the patient and fetus, and either transferring the patient to a PAS center or awaiting appropriate local specialists may be a lifesaving step.
3. Is immediate delivery required? If the fetus must be delivered, then it is imperative to create a hysterotomy out of the way of the placenta. Disrupting the adherent placenta with either an incision or manual manipulation may trigger a massive hemorrhage and should be avoided. This may require rectus muscle transection or creating a “T” incision on the skin to reach the uterine fundus and creating a hysterotomy over the top or even the back of the uterus. Once the fetus is delivered and lack of uterine hemorrhage confirmed (both abdominally and vaginally), the hysterotomy and abdomen can be closed with anticipation of urgent patient transfer to a PAS team or center.
4. Is the patient hemorrhaging? If the patient is hemorrhaging and closure is not an option, then recruitment of local emergent surgical teams is warranted, even if that requires packing the abdomen until an appropriate surgeon can arrive.
Diagnosis at cesarean delivery requires expedited and complex patient counseling. A patient who is unstable or hemorrhaging needs to be told that hysterectomy is lifesaving in this situation. For patients who are stable, it may be appropriate to close the abdomen and leave the placenta in situ, perform comprehensive counseling, and assess the possibility of transfer to a specialty center.
Summary
All obstetric care providers should be familiar with the clinical presentation of undiagnosed accreta spectrum. While hemorrhage is often part of the diagnosis, recognition of abnormal placental adherence and PAS-focused management should ideally be undertaken before this occurs. Once PAS is suspected, avoidance of further placental disruption may save significant morbidity, even if that means leaving the placenta attached until appropriate resources can be obtained. A local protocol for consultation, emergency transfer, and deployment of local resources should be part of every delivery unit’s emergency preparedness plan.
CASE Outcome
This patient is stabilized, with an adherent, retained placenta and no signs of hemorrhage. You administer uterotonics and notify your anesthesiologist and backup obstetrician that you have a likely case of accreta spectrum. A second intravenous line is placed, and blood products are crossmatched. The closest level III hospital is called, and they accept your patient for transfer. There, she is counseled about PAS, and she expresses no desire for future childbearing. After again confirming no placental separation in the operating room, the patient is moved immediately to perform laparotomy and total abdominal hysterectomy through a Pfannenstiel incision. She does not require a blood transfusion, and the pathology returns with grade I placenta accreta spectrum. ●
- American College of Obstetricians and Gynecologists, Society for Maternal-Fetal Medicine. Obstetric Care Consensus No. 7: placenta accreta spectrum. Obstet Gynecol. 2018; 132:e259-e275. doi:10.1097/AOG.0000000000002983.
- Carusi DA. The placenta accreta spectrum: epidemiology and risk factors. Clin Obstet Gynecol. 2018;61:733-742. doi:10.1097/GRF.0000000000000391.
- Silver RM, Fox KA, Barton JR, et al. Center of excellence for placenta accreta. Am J Obstet Gynecol. 2015;212:561-568. doi:10.1016/j.ajog.2014.11.018.
- Shamshirsaz AA, Fox KA, Salmanian B, et al. Maternal morbidity in patients with morbidly adherent placenta treated with and without a standardized multidisciplinary approach. Am J Obstet Gynecol. 2015;212:218.e1-9. doi:10.1016/j.ajog.2014.08.019.
- Bowman ZS, Eller AG, Kennedy AM, et al. Accuracy of ultrasound for the prediction of placenta accreta. Am J Obstet Gynecol. 2014;211:177.e1-7. doi:10.1016/j.ajog.2014.03.029.
- Carusi DA, Fox KA, Lyell DJ, et al. Placenta accreta spectrum without placenta previa. Obstet Gynecol. 2020;136:458-465. doi:10.1097/AOG.0000000000003970.
- Kayem G, Seco A, Beucher G, et al. Clinical profiles of placenta accreta spectrum: the PACCRETA population-based study. BJOG. 2021;128:1646-1655. doi:10.1111/1471-0528.16647.
- Jauniaux E, Ayres-de-Campos D, Langhoff-Roos J, et al. FIGO classification for the clinical diagnosis of placenta accreta spectrum disorders. Int J Gynaecol Obstet. 2019;146:20-24. doi:10.1002/ijgo.12761.
- Collins SL, Alemdar B, van Beekhuizen HJ, et al. Evidence-based guidelines for the management of abnormally invasive placenta: recommendations from the International Society for Abnormally Invasive Placenta. Am J Obstet Gynecol. 2019;220(6):511-526. doi:10.1016/j.ajog.2019.02.054.
- American College of Obstetricians and Gynecologists; Society for Maternal-Fetal Medicine. Obstetric care consensus. No. 7: placenta accreta spectrum. Obstet Gynecol. 2018;132:e259-e275. doi: 10.1097/AOG.0000000000002983.
- Sentilhes L, Kayem G, Silver RM. Conservative management of placenta accreta spectrum. Clin Obstet Gynecol. 2018; 61(4):783-794. doi:10.1097/GRF.0000000000000395.
CASE Placenta accreta spectrum following uncomplicated vaginal delivery
Imagine you are an obstetric hospitalist taking call at a level II maternal level of care hospital. Your patient is a 35-year-old woman, gravida 2, para 1, with a past history of retained placenta requiring dilation and curettage and intravenous antibiotics for endomyometritis. This is an in vitro fertilization pregnancy that has progressed normally, and the patient labored spontaneously at 38 weeks’ gestation. Following an uncomplicated vaginal delivery, the placenta has not delivered, and you attempt a manual placental extraction after a 40-minute third stage. While there is epidural analgesia and you can reach the uterine fundus, you are unable to create a separation plane between the placenta and uterus.
What do you do next?
Placenta accreta spectrum (PAS) includes a broad range of clinical scenarios with abnormal placental attachment as their common denominator. The condition has classically been defined pathologically, with chorionic villi attaching directly to the myometrium (“accreta”) or extending more deeply into the myometrium (“increta”) or attaching to surrounding tissues and structures (“percreta”).1 It is most commonly encountered in patients with low placental implantation on a prior cesarean section scar; indeed, placenta previa, particularly with a history of cesarean delivery, is the strongest risk factor for the development of PAS.2 In addition to abnormal placental attachment, these placental attachments are often hypervascular and can lead to catastrophic hemorrhage if not managed appropriately. For this reason, patients with sonographic or radiologic signs of PAS should be referred to specialized centers for further workup, counseling, and delivery planning.3
Although delivery at a specialized PAS center has been associated with improved patient outcomes,4 not all patients with PAS will be identified in the antepartum period. Ultrasonography may miss up to 40% to 50% of PAS cases, particularly when the sonologist has not been advised to look for the condition,5 and not all patients with PAS will have a previa implanted in a prior cesarean scar. A recent study found that these patients with nonprevia PAS were identified by imaging less than 40% of the time and were significantly less likely to be managed by a specialized team of clinicians.6 Thus, it falls upon every obstetric care provider to be aware of this diagnosis, promptly recognize its unanticipated presentations, and have a plan to optimize patient safety.
Step 1: Recognition
While PAS is classically defined as a pathologic condition, no clinician has the luxury of histology in the delivery room. Researchers have variously defined PAS clinically, with the common trait of abnormal placental adherence.7-9 The TABLE compares published definitions that have been used in the literature. While some definitions include hemorrhage, no clinician wants to induce significant hemorrhage to confirm their patient’s diagnosis. Thus, practically, the clinical PAS diagnosis comes down to abnormal placental attachment: If it is apparent that some or all of the placenta will not separate from the uterine wall with digital manipulation or careful curettage, then PAS should be suspected, and appropriate steps should be taken before further removal attempts.

At cesarean delivery, the PAS diagnosis may be aided by visual cues. With placenta previa, the lower uterine segment may bulge and take on a bluish hue, distinctly different from the upper healthy myometrium. PAS may also manifest with neovascularization, particularly behind the bladder. As with vaginal births, the placenta will fail to separate after the delivery, and controlled traction on the umbilical cord can produce a “dimple sign,” or visible myometrial retraction at the site of implantation (FIGURE 1). Finally, if the diagnosis is still in doubt, attempts to gently form a cleavage plane between the placenta and myometrium will be unsuccessful if PAS is present.8

Step 2: Initial management—pause, plan
Most importantly, do not attempt to forcibly remove the placenta. It can be left attached to the uterus until appropriate resources are secured. Efforts to forcibly remove an adherent placenta may well lead to major hemorrhage, and thus it falls on the patient’s care team to pause and plan for PAS care at this point. FIGURE 2 displays an algorithm for patient management. Further steps depend primarily on whether or not the patient is already hemorrhaging. In a stable situation, the patient should be counseled regarding the abnormal findings and the suspected PAS diagnosis. This includes the possibility of further procedures, blood transfusion, and hysterectomy. Local resources, including nursing, anesthesia, and the blood bank, should be notified about the situation and for the potential to call in specialized services. If on-site experienced specialists are not available, then patient transfer to a PAS specialty center should be strongly considered. While awaiting additional help or transport, the patient requires close monitoring for gross and physiologic signs of hemorrhage. If pursued, transport to a PAS specialty center should be expedited.

If the patient is already hemorrhaging or unstable, then appropriate local resources must be activated. At a minimum, this requires an obstetrician and anesthesiologist at the bedside and activation of hemorrhage protocols (eg, a massive transfusion protocol). If blood products are unavailable, consider whether they can be transported from other nearby blood banks, and start that process promptly. Next, contact backup services. Based on local resources and clinical severity, this may include maternal-fetal medicine specialists, pelvic surgeons, general and trauma surgeons, intensivists, interventional radiologists, and transfusion specialists. Even if the patient cannot be safely transferred to another hospital, the obstetrician can call an outside PAS specialist to discuss next steps in care and begin transfer plans, assuming the patient can be stabilized. Based on the Maternal Levels of Care definitions published by the American College of Obstetricians and Gynecologists and the Society of Maternal-Fetal Medicine,10 patients with PAS should be managed at level III or level IV centers. However, delivery units at every level of maternal care should have a protocol for securing local help and reaching an appropriate consultant if a PAS case is encountered. Know which center in your area specializes in PAS so that when an unanticipated case arises, you know who to call.
Continue to: Step 3: Ultimate management—mobilize and prepare for bleeding...
Step 3: Ultimate management—mobilize and prepare for bleeding
If diagnosis occurs intraoperatively at a PAS specialty center, or if safe transport is not possible, then the team should mobilize for the possibility of hysterectomy and prepare for massive bleeding, which can occur regardless of the treatment chosen. Many patients require or will opt for hysterectomy. For example, a patient who has finished childbearing may consent to a hysterectomy upon hearing she likely has PAS. In patients with suspected PAS who are actively hemorrhaging or are unstable, hysterectomy is required.
Uterine conservation may be considered in stable patients who strongly desire future childbearing or uterine retention. This often requires leaving densely adherent placental tissue in situ and thus requires thorough counseling regarding the risks of delayed hemorrhage, infection, and emergent hysterectomy.11 This may not be desirable or safe for some patients, so informed consent is crucial. In such cases, we strongly recommend consultation with a PAS specialist, even if that requires immediate control of the placental blood supply (such as with arterial embolization), and transfer to a PAS specialty center.
Clinical scenarios
Vaginal delivery
The patient in the opening case was never expected to have PAS given her normal placental location and absence of a uterine scar. Even though she had some possible PAS risk factors (past retained placenta with instrumentation and in vitro fertilization), her absolute risk for the condition was low. Nevertheless, inability to create a separation plane should be considered PAS until proven otherwise. Although at this point many obstetricians would move to an operating room for uterine curettage, we recommend that the care team pause and put measures in place for possible PAS and hemorrhage. This involves notification of the blood bank, crossmatching of blood products, alerting the anesthesia team, and having a clear plan in place should a major hemorrhage ensue. This may involve use of balloon tamponade, activation of an interventional radiology team, or possible laparotomy with arterial ligations or hysterectomy. Avoidance of a prolonged third stage should be balanced against the need for preparation with these cases.
It is important for clinicians to bear in mind, and communicate to the patient, that hysterectomy is the standard of care for PAS. Significant delays in performing an indicated hysterectomy can lead to coagulopathy and patient instability. Timeliness is key; we find that delays in the decision to perform an indicated hysterectomy are often at the root of the cause for worsened morbidity in patients with unanticipated PAS. With an unscarred uterus and no placenta previa, a postpartum hysterectomy can be performed by many obstetrician-gynecologists experienced in this abdominal procedure.
Cesarean delivery
Undiagnosed PAS may present at cesarean delivery with or without placenta previa and a prior uterine scar. With this combination, PAS is often visually apparent upon opening the abdominal cavity (TABLE and FIGURE 1). Such surgical findings call for a clinical pause, as further actions at this point can lead to catastrophic hemorrhage. The obstetrician should consider a series of questions:
1. Are appropriate surgical and transfusion resources immediately available? If yes, they should be notified in case they are needed urgently. If not, then the obstetrician should ask whether the delivery must occur now.
2. Is this a scheduled delivery with a stable patient and fetus? If so, then closing the abdominal incision, monitoring the patient and fetus, and either transferring the patient to a PAS center or awaiting appropriate local specialists may be a lifesaving step.
3. Is immediate delivery required? If the fetus must be delivered, then it is imperative to create a hysterotomy out of the way of the placenta. Disrupting the adherent placenta with either an incision or manual manipulation may trigger a massive hemorrhage and should be avoided. This may require rectus muscle transection or creating a “T” incision on the skin to reach the uterine fundus and creating a hysterotomy over the top or even the back of the uterus. Once the fetus is delivered and lack of uterine hemorrhage confirmed (both abdominally and vaginally), the hysterotomy and abdomen can be closed with anticipation of urgent patient transfer to a PAS team or center.
4. Is the patient hemorrhaging? If the patient is hemorrhaging and closure is not an option, then recruitment of local emergent surgical teams is warranted, even if that requires packing the abdomen until an appropriate surgeon can arrive.
Diagnosis at cesarean delivery requires expedited and complex patient counseling. A patient who is unstable or hemorrhaging needs to be told that hysterectomy is lifesaving in this situation. For patients who are stable, it may be appropriate to close the abdomen and leave the placenta in situ, perform comprehensive counseling, and assess the possibility of transfer to a specialty center.
Summary
All obstetric care providers should be familiar with the clinical presentation of undiagnosed accreta spectrum. While hemorrhage is often part of the diagnosis, recognition of abnormal placental adherence and PAS-focused management should ideally be undertaken before this occurs. Once PAS is suspected, avoidance of further placental disruption may save significant morbidity, even if that means leaving the placenta attached until appropriate resources can be obtained. A local protocol for consultation, emergency transfer, and deployment of local resources should be part of every delivery unit’s emergency preparedness plan.
CASE Outcome
This patient is stabilized, with an adherent, retained placenta and no signs of hemorrhage. You administer uterotonics and notify your anesthesiologist and backup obstetrician that you have a likely case of accreta spectrum. A second intravenous line is placed, and blood products are crossmatched. The closest level III hospital is called, and they accept your patient for transfer. There, she is counseled about PAS, and she expresses no desire for future childbearing. After again confirming no placental separation in the operating room, the patient is moved immediately to perform laparotomy and total abdominal hysterectomy through a Pfannenstiel incision. She does not require a blood transfusion, and the pathology returns with grade I placenta accreta spectrum. ●
CASE Placenta accreta spectrum following uncomplicated vaginal delivery
Imagine you are an obstetric hospitalist taking call at a level II maternal level of care hospital. Your patient is a 35-year-old woman, gravida 2, para 1, with a past history of retained placenta requiring dilation and curettage and intravenous antibiotics for endomyometritis. This is an in vitro fertilization pregnancy that has progressed normally, and the patient labored spontaneously at 38 weeks’ gestation. Following an uncomplicated vaginal delivery, the placenta has not delivered, and you attempt a manual placental extraction after a 40-minute third stage. While there is epidural analgesia and you can reach the uterine fundus, you are unable to create a separation plane between the placenta and uterus.
What do you do next?
Placenta accreta spectrum (PAS) includes a broad range of clinical scenarios with abnormal placental attachment as their common denominator. The condition has classically been defined pathologically, with chorionic villi attaching directly to the myometrium (“accreta”) or extending more deeply into the myometrium (“increta”) or attaching to surrounding tissues and structures (“percreta”).1 It is most commonly encountered in patients with low placental implantation on a prior cesarean section scar; indeed, placenta previa, particularly with a history of cesarean delivery, is the strongest risk factor for the development of PAS.2 In addition to abnormal placental attachment, these placental attachments are often hypervascular and can lead to catastrophic hemorrhage if not managed appropriately. For this reason, patients with sonographic or radiologic signs of PAS should be referred to specialized centers for further workup, counseling, and delivery planning.3
Although delivery at a specialized PAS center has been associated with improved patient outcomes,4 not all patients with PAS will be identified in the antepartum period. Ultrasonography may miss up to 40% to 50% of PAS cases, particularly when the sonologist has not been advised to look for the condition,5 and not all patients with PAS will have a previa implanted in a prior cesarean scar. A recent study found that these patients with nonprevia PAS were identified by imaging less than 40% of the time and were significantly less likely to be managed by a specialized team of clinicians.6 Thus, it falls upon every obstetric care provider to be aware of this diagnosis, promptly recognize its unanticipated presentations, and have a plan to optimize patient safety.
Step 1: Recognition
While PAS is classically defined as a pathologic condition, no clinician has the luxury of histology in the delivery room. Researchers have variously defined PAS clinically, with the common trait of abnormal placental adherence.7-9 The TABLE compares published definitions that have been used in the literature. While some definitions include hemorrhage, no clinician wants to induce significant hemorrhage to confirm their patient’s diagnosis. Thus, practically, the clinical PAS diagnosis comes down to abnormal placental attachment: If it is apparent that some or all of the placenta will not separate from the uterine wall with digital manipulation or careful curettage, then PAS should be suspected, and appropriate steps should be taken before further removal attempts.

At cesarean delivery, the PAS diagnosis may be aided by visual cues. With placenta previa, the lower uterine segment may bulge and take on a bluish hue, distinctly different from the upper healthy myometrium. PAS may also manifest with neovascularization, particularly behind the bladder. As with vaginal births, the placenta will fail to separate after the delivery, and controlled traction on the umbilical cord can produce a “dimple sign,” or visible myometrial retraction at the site of implantation (FIGURE 1). Finally, if the diagnosis is still in doubt, attempts to gently form a cleavage plane between the placenta and myometrium will be unsuccessful if PAS is present.8

Step 2: Initial management—pause, plan
Most importantly, do not attempt to forcibly remove the placenta. It can be left attached to the uterus until appropriate resources are secured. Efforts to forcibly remove an adherent placenta may well lead to major hemorrhage, and thus it falls on the patient’s care team to pause and plan for PAS care at this point. FIGURE 2 displays an algorithm for patient management. Further steps depend primarily on whether or not the patient is already hemorrhaging. In a stable situation, the patient should be counseled regarding the abnormal findings and the suspected PAS diagnosis. This includes the possibility of further procedures, blood transfusion, and hysterectomy. Local resources, including nursing, anesthesia, and the blood bank, should be notified about the situation and for the potential to call in specialized services. If on-site experienced specialists are not available, then patient transfer to a PAS specialty center should be strongly considered. While awaiting additional help or transport, the patient requires close monitoring for gross and physiologic signs of hemorrhage. If pursued, transport to a PAS specialty center should be expedited.

If the patient is already hemorrhaging or unstable, then appropriate local resources must be activated. At a minimum, this requires an obstetrician and anesthesiologist at the bedside and activation of hemorrhage protocols (eg, a massive transfusion protocol). If blood products are unavailable, consider whether they can be transported from other nearby blood banks, and start that process promptly. Next, contact backup services. Based on local resources and clinical severity, this may include maternal-fetal medicine specialists, pelvic surgeons, general and trauma surgeons, intensivists, interventional radiologists, and transfusion specialists. Even if the patient cannot be safely transferred to another hospital, the obstetrician can call an outside PAS specialist to discuss next steps in care and begin transfer plans, assuming the patient can be stabilized. Based on the Maternal Levels of Care definitions published by the American College of Obstetricians and Gynecologists and the Society of Maternal-Fetal Medicine,10 patients with PAS should be managed at level III or level IV centers. However, delivery units at every level of maternal care should have a protocol for securing local help and reaching an appropriate consultant if a PAS case is encountered. Know which center in your area specializes in PAS so that when an unanticipated case arises, you know who to call.
Continue to: Step 3: Ultimate management—mobilize and prepare for bleeding...
Step 3: Ultimate management—mobilize and prepare for bleeding
If diagnosis occurs intraoperatively at a PAS specialty center, or if safe transport is not possible, then the team should mobilize for the possibility of hysterectomy and prepare for massive bleeding, which can occur regardless of the treatment chosen. Many patients require or will opt for hysterectomy. For example, a patient who has finished childbearing may consent to a hysterectomy upon hearing she likely has PAS. In patients with suspected PAS who are actively hemorrhaging or are unstable, hysterectomy is required.
Uterine conservation may be considered in stable patients who strongly desire future childbearing or uterine retention. This often requires leaving densely adherent placental tissue in situ and thus requires thorough counseling regarding the risks of delayed hemorrhage, infection, and emergent hysterectomy.11 This may not be desirable or safe for some patients, so informed consent is crucial. In such cases, we strongly recommend consultation with a PAS specialist, even if that requires immediate control of the placental blood supply (such as with arterial embolization), and transfer to a PAS specialty center.
Clinical scenarios
Vaginal delivery
The patient in the opening case was never expected to have PAS given her normal placental location and absence of a uterine scar. Even though she had some possible PAS risk factors (past retained placenta with instrumentation and in vitro fertilization), her absolute risk for the condition was low. Nevertheless, inability to create a separation plane should be considered PAS until proven otherwise. Although at this point many obstetricians would move to an operating room for uterine curettage, we recommend that the care team pause and put measures in place for possible PAS and hemorrhage. This involves notification of the blood bank, crossmatching of blood products, alerting the anesthesia team, and having a clear plan in place should a major hemorrhage ensue. This may involve use of balloon tamponade, activation of an interventional radiology team, or possible laparotomy with arterial ligations or hysterectomy. Avoidance of a prolonged third stage should be balanced against the need for preparation with these cases.
It is important for clinicians to bear in mind, and communicate to the patient, that hysterectomy is the standard of care for PAS. Significant delays in performing an indicated hysterectomy can lead to coagulopathy and patient instability. Timeliness is key; we find that delays in the decision to perform an indicated hysterectomy are often at the root of the cause for worsened morbidity in patients with unanticipated PAS. With an unscarred uterus and no placenta previa, a postpartum hysterectomy can be performed by many obstetrician-gynecologists experienced in this abdominal procedure.
Cesarean delivery
Undiagnosed PAS may present at cesarean delivery with or without placenta previa and a prior uterine scar. With this combination, PAS is often visually apparent upon opening the abdominal cavity (TABLE and FIGURE 1). Such surgical findings call for a clinical pause, as further actions at this point can lead to catastrophic hemorrhage. The obstetrician should consider a series of questions:
1. Are appropriate surgical and transfusion resources immediately available? If yes, they should be notified in case they are needed urgently. If not, then the obstetrician should ask whether the delivery must occur now.
2. Is this a scheduled delivery with a stable patient and fetus? If so, then closing the abdominal incision, monitoring the patient and fetus, and either transferring the patient to a PAS center or awaiting appropriate local specialists may be a lifesaving step.
3. Is immediate delivery required? If the fetus must be delivered, then it is imperative to create a hysterotomy out of the way of the placenta. Disrupting the adherent placenta with either an incision or manual manipulation may trigger a massive hemorrhage and should be avoided. This may require rectus muscle transection or creating a “T” incision on the skin to reach the uterine fundus and creating a hysterotomy over the top or even the back of the uterus. Once the fetus is delivered and lack of uterine hemorrhage confirmed (both abdominally and vaginally), the hysterotomy and abdomen can be closed with anticipation of urgent patient transfer to a PAS team or center.
4. Is the patient hemorrhaging? If the patient is hemorrhaging and closure is not an option, then recruitment of local emergent surgical teams is warranted, even if that requires packing the abdomen until an appropriate surgeon can arrive.
Diagnosis at cesarean delivery requires expedited and complex patient counseling. A patient who is unstable or hemorrhaging needs to be told that hysterectomy is lifesaving in this situation. For patients who are stable, it may be appropriate to close the abdomen and leave the placenta in situ, perform comprehensive counseling, and assess the possibility of transfer to a specialty center.
Summary
All obstetric care providers should be familiar with the clinical presentation of undiagnosed accreta spectrum. While hemorrhage is often part of the diagnosis, recognition of abnormal placental adherence and PAS-focused management should ideally be undertaken before this occurs. Once PAS is suspected, avoidance of further placental disruption may save significant morbidity, even if that means leaving the placenta attached until appropriate resources can be obtained. A local protocol for consultation, emergency transfer, and deployment of local resources should be part of every delivery unit’s emergency preparedness plan.
CASE Outcome
This patient is stabilized, with an adherent, retained placenta and no signs of hemorrhage. You administer uterotonics and notify your anesthesiologist and backup obstetrician that you have a likely case of accreta spectrum. A second intravenous line is placed, and blood products are crossmatched. The closest level III hospital is called, and they accept your patient for transfer. There, she is counseled about PAS, and she expresses no desire for future childbearing. After again confirming no placental separation in the operating room, the patient is moved immediately to perform laparotomy and total abdominal hysterectomy through a Pfannenstiel incision. She does not require a blood transfusion, and the pathology returns with grade I placenta accreta spectrum. ●
- American College of Obstetricians and Gynecologists, Society for Maternal-Fetal Medicine. Obstetric Care Consensus No. 7: placenta accreta spectrum. Obstet Gynecol. 2018; 132:e259-e275. doi:10.1097/AOG.0000000000002983.
- Carusi DA. The placenta accreta spectrum: epidemiology and risk factors. Clin Obstet Gynecol. 2018;61:733-742. doi:10.1097/GRF.0000000000000391.
- Silver RM, Fox KA, Barton JR, et al. Center of excellence for placenta accreta. Am J Obstet Gynecol. 2015;212:561-568. doi:10.1016/j.ajog.2014.11.018.
- Shamshirsaz AA, Fox KA, Salmanian B, et al. Maternal morbidity in patients with morbidly adherent placenta treated with and without a standardized multidisciplinary approach. Am J Obstet Gynecol. 2015;212:218.e1-9. doi:10.1016/j.ajog.2014.08.019.
- Bowman ZS, Eller AG, Kennedy AM, et al. Accuracy of ultrasound for the prediction of placenta accreta. Am J Obstet Gynecol. 2014;211:177.e1-7. doi:10.1016/j.ajog.2014.03.029.
- Carusi DA, Fox KA, Lyell DJ, et al. Placenta accreta spectrum without placenta previa. Obstet Gynecol. 2020;136:458-465. doi:10.1097/AOG.0000000000003970.
- Kayem G, Seco A, Beucher G, et al. Clinical profiles of placenta accreta spectrum: the PACCRETA population-based study. BJOG. 2021;128:1646-1655. doi:10.1111/1471-0528.16647.
- Jauniaux E, Ayres-de-Campos D, Langhoff-Roos J, et al. FIGO classification for the clinical diagnosis of placenta accreta spectrum disorders. Int J Gynaecol Obstet. 2019;146:20-24. doi:10.1002/ijgo.12761.
- Collins SL, Alemdar B, van Beekhuizen HJ, et al. Evidence-based guidelines for the management of abnormally invasive placenta: recommendations from the International Society for Abnormally Invasive Placenta. Am J Obstet Gynecol. 2019;220(6):511-526. doi:10.1016/j.ajog.2019.02.054.
- American College of Obstetricians and Gynecologists; Society for Maternal-Fetal Medicine. Obstetric care consensus. No. 7: placenta accreta spectrum. Obstet Gynecol. 2018;132:e259-e275. doi: 10.1097/AOG.0000000000002983.
- Sentilhes L, Kayem G, Silver RM. Conservative management of placenta accreta spectrum. Clin Obstet Gynecol. 2018; 61(4):783-794. doi:10.1097/GRF.0000000000000395.
- American College of Obstetricians and Gynecologists, Society for Maternal-Fetal Medicine. Obstetric Care Consensus No. 7: placenta accreta spectrum. Obstet Gynecol. 2018; 132:e259-e275. doi:10.1097/AOG.0000000000002983.
- Carusi DA. The placenta accreta spectrum: epidemiology and risk factors. Clin Obstet Gynecol. 2018;61:733-742. doi:10.1097/GRF.0000000000000391.
- Silver RM, Fox KA, Barton JR, et al. Center of excellence for placenta accreta. Am J Obstet Gynecol. 2015;212:561-568. doi:10.1016/j.ajog.2014.11.018.
- Shamshirsaz AA, Fox KA, Salmanian B, et al. Maternal morbidity in patients with morbidly adherent placenta treated with and without a standardized multidisciplinary approach. Am J Obstet Gynecol. 2015;212:218.e1-9. doi:10.1016/j.ajog.2014.08.019.
- Bowman ZS, Eller AG, Kennedy AM, et al. Accuracy of ultrasound for the prediction of placenta accreta. Am J Obstet Gynecol. 2014;211:177.e1-7. doi:10.1016/j.ajog.2014.03.029.
- Carusi DA, Fox KA, Lyell DJ, et al. Placenta accreta spectrum without placenta previa. Obstet Gynecol. 2020;136:458-465. doi:10.1097/AOG.0000000000003970.
- Kayem G, Seco A, Beucher G, et al. Clinical profiles of placenta accreta spectrum: the PACCRETA population-based study. BJOG. 2021;128:1646-1655. doi:10.1111/1471-0528.16647.
- Jauniaux E, Ayres-de-Campos D, Langhoff-Roos J, et al. FIGO classification for the clinical diagnosis of placenta accreta spectrum disorders. Int J Gynaecol Obstet. 2019;146:20-24. doi:10.1002/ijgo.12761.
- Collins SL, Alemdar B, van Beekhuizen HJ, et al. Evidence-based guidelines for the management of abnormally invasive placenta: recommendations from the International Society for Abnormally Invasive Placenta. Am J Obstet Gynecol. 2019;220(6):511-526. doi:10.1016/j.ajog.2019.02.054.
- American College of Obstetricians and Gynecologists; Society for Maternal-Fetal Medicine. Obstetric care consensus. No. 7: placenta accreta spectrum. Obstet Gynecol. 2018;132:e259-e275. doi: 10.1097/AOG.0000000000002983.
- Sentilhes L, Kayem G, Silver RM. Conservative management of placenta accreta spectrum. Clin Obstet Gynecol. 2018; 61(4):783-794. doi:10.1097/GRF.0000000000000395.
Lupus Erythematosus Tumidus Clinical Characteristics and Treatment: A Retrospective Review of 25 Patients
Lupus erythematosus tumidus (LET) is a rare photosensitive dermatosis1 that previously was considered a subtype of chronic cutaneous lupus erythematosus; however, the clinical course and favorable prognosis of LET led to its reclassification into another category, called intermittent cutaneous lupus erythematosus.2 Although known about for more than 100 years, the association of LET with systemic lupus erythematosus (SLE), its autoantibody profile, and its prognosis are not well characterized. The purpose of this study was to describe the demographics, clinical characteristics, autoantibody profile, comorbidities, and treatment of LET based on a retrospective review of patients with LET.
Methods
A retrospective review was conducted in patients with histologically diagnosed LET who presented to the Department of Dermatology at the Wake Forest School of Medicine (Winston-Salem, North Carolina) over 6 years (July 2012 to July 2018). Inclusion criteria included males or females aged 18 to 75 years with clinical and histopathology-proven LET, which was defined as a superficial and deep lymphocytic infiltrate with abundant mucin deposition in the reticular dermis and absent or focal dermoepidermal junction alterations. Exclusion criteria included males or females younger than 18 years or older than 75 years or patients without clinical and histopathologically proven LET. Medical records were evaluated for demographics, clinical characteristics, diagnoses, autoantibodies, treatment, and recurrence. Photosensitivity was confirmed by clinical history. This study was approved by the Wake Forest School of Medicine institutional review board.
Results
Twenty-five patients were included in the study (eTable). The mean age (SD) at diagnosis was 46 (10.9) years, with a male to female ratio of 1:4. Twenty-two (88%) patients were White non-Hispanic, whereas 3 (12%) were Black. Lupus erythematosus tumidus most commonly affected the trunk (18/25 [72%]) and upper extremities (18/25 [72%]), followed by the head and neck (15/25 [60%]) and lower extremities (8/25 [32%])(Figure 1). The most common morphologies were plaques (18/25 [72%]), papules (17/25 [68%]), and nodules (6/25 [24%])(Figures 2 and 3). Most patients experienced painful (14/25 [56%]) or pruritic (13/25 [52%]) lesions as well as photosensitivity (13/25 [52%]). Of all measured autoantibodies, 5 of 22 (23%) patients had positive antinuclear antibody (ANA) titers greater than 1:80, 1 of 14 (7%) patients had positive anti-Ro (anti-SSA), 1 of 14 (7%) had positive anti-La (anti-SSB), 2 of 10 (20%) had positive anti–double-stranded DNA, and 0 of 6 (0%) patients had positive anti-Smith antibodies. Four (16%) patients with SLE had skin and joint involvement, whereas 1 had lupus nephritis. One (4%) patient had discoid lupus erythematosus (DLE). Seventeen (68%) patients reported recurrences or flares. The mean duration of symptoms (SD) was 28 (44) months.
Topical corticosteroids (21/25 [84%]) and hydroxychloroquine (20/25 [80%]) were the most commonly prescribed treatments. Hydroxychloroquine monotherapy achieved clearance or almost clearance in 12 (60%) patients. Four patients were prescribed thalidomide after hydroxychloroquine monotherapy failed; 2 achieved complete clearance with thalidomide and hydroxychloroquine, 1 achieved complete clearance with thalidomide monotherapy, and 1 improved but did not clear. Four patients were concurrently started on quinacrine (mepacrine) after hydroxychloroquine monotherapy failed; 1 patient had no clearance, 1 discontinued because of allergy, 1 improved, and 1 cleared. Four patients had short courses of prednisone lasting 1 to 4 weeks. Three of 4 patients treated with methotrexate discontinued because of adverse effects, and 1 patient improved. Other prescribed treatments included topical calcineurin inhibitors (10/25 [40%]), dapsone (1/25 [4%]), and clofazimine (1/25 [4%]).
Comment
Prevalence of LET—Although other European LET case series reported a male predominance or equal male to female ratio, our case series reported female predominance (1:4).1,3-5 Our male to female ratio resembles similar ratios in DLE and subacute lupus erythematosus, whereas relative to our study, SLE male to female ratios favored females over males.6,7
Clinical Distribution of LET—In one study enrolling 24 patients with LET, 79% (19/24) of patients had facial involvement, 50% (12/24) had V-neck involvement, 50% (12/24) had back involvement, and 46% (11/24) had arm involvement,2 whereas our study reported 72% involvement of the trunk, 72% involvement of the upper extremities, 60% involvement of the head and neck region, and 32% involvement of the lower extremities. Although our study reported more lower extremity involvement, the aforementioned study used precise topographic locations, whereas we used more generalized topographic locations. Therefore, it was difficult to compare disease distribution between both studies.2
Presence of Autoantibodies and Comorbidities—Of the 22 patients tested for ANA, 23% reported titers greater than 1:80, similar to the 20% positive ANA prevalence in an LET case series of 25 patients.5 Of 4 patients diagnosed with SLE, 3 had articular and skin involvement, and 1 had renal involvement. These findings resemble a similar LET case series.2 Nonetheless, given the numerous skin criteria in the American College of Rheumatology SLE classification criteria, patients with predominant skin disease and positive autoantibodies are diagnosed as having SLE without notable extracutaneous involvement.2 Therefore, SLE diagnosis in the setting of LET could be reassessed periodically in this population. One patient in our study was diagnosed with DLE several years later. It is uncommon for LET to be reported concomitantly with DLE.8
Treatment of LET—Evidence supporting efficacious treatment options for LET is limited to case series. Sun protection is recommended in all patients with LET. Earlier case series reported a high response rate with sun protection and topical corticosteroids, with 19% to 55% of patients requiring subsequent systemic antimalarials.3,4 However, one case series presented a need for systemic antimalarials,5 similar to our study. Hydroxychloroquine 200 to 400 mg daily is considered the first-line systemic treatment for LET. Its response rate varies among studies and may be influenced by dosage.1,3 Second-line treatments include methotrexate 7.5 to 25 mg once weekly, thalidomide 50 to 100 mg daily, and quinacrine. However, quinacrine is not currently commercially available. Thalidomide and quinacrine represented useful alternatives when hydroxychloroquine monotherapy failed. As with other immunomodulators, adverse effects should be monitored periodically.
Conclusion
Lupus erythematosus tumidus is characterized by erythematous papules and plaques that may be tender or pruritic. It follows an intermittent course and rarely is associated with SLE. Hydroxychloroquine is considered the first-line systemic treatment; however, recalcitrant disease could be managed with other immunomodulators, including methotrexate, thalidomide, or quinacrine.
- Kuhn A, Bein D, Bonsmann G. The 100th anniversary of lupus erythematosus tumidus. Autoimmun Rev. 2009;8:441-448.
- Schmitt V, Meuth AM, Amler S, et al. Lupus erythematosus tumidus is a separate subtype of cutaneous lupus erythematosus. Br J Dermatol. 2010;162:64-73.
- Kuhn A, Richter-Hintz D, Oslislo C, et al. Lupus erythematosus tumidus—a neglected subset of cutaneous lupus erythematosus: report of 40 cases. Arch Dermatol. 2000;136:1033-1041.
- Vieira V, Del Pozo J, Yebra-Pimentel MT, et al. Lupus erythematosus tumidus: a series of 26 cases. Int J Dermatol. 2006;45:512-517.
- Rodriguez-Caruncho C, Bielsa I, Fernandez-Figueras MT, et al. Lupus erythematosus tumidus: a clinical and histological study of 25 cases. Lupus. 2015;24:751-755.
- Patsinakidis N, Gambichler T, Lahner N, et al. Cutaneous characteristics and association with antinuclear antibodies in 402 patients with different subtypes of lupus erythematosus. J Eur Acad Dermatol Venereol. 2016;30:2097-2104.
- Petersen MP, Moller S, Bygum A, et al. Epidemiology of cutaneous lupus erythematosus and the associated risk of systemic lupus erythematosus: a nationwide cohort study in Denmark. Lupus. 2018;27:1424-1430.
- Dekle CL, Mannes KD, Davis LS, et al. Lupus tumidus. J Am AcadDermatol. 1999;41:250-253.
Lupus erythematosus tumidus (LET) is a rare photosensitive dermatosis1 that previously was considered a subtype of chronic cutaneous lupus erythematosus; however, the clinical course and favorable prognosis of LET led to its reclassification into another category, called intermittent cutaneous lupus erythematosus.2 Although known about for more than 100 years, the association of LET with systemic lupus erythematosus (SLE), its autoantibody profile, and its prognosis are not well characterized. The purpose of this study was to describe the demographics, clinical characteristics, autoantibody profile, comorbidities, and treatment of LET based on a retrospective review of patients with LET.
Methods
A retrospective review was conducted in patients with histologically diagnosed LET who presented to the Department of Dermatology at the Wake Forest School of Medicine (Winston-Salem, North Carolina) over 6 years (July 2012 to July 2018). Inclusion criteria included males or females aged 18 to 75 years with clinical and histopathology-proven LET, which was defined as a superficial and deep lymphocytic infiltrate with abundant mucin deposition in the reticular dermis and absent or focal dermoepidermal junction alterations. Exclusion criteria included males or females younger than 18 years or older than 75 years or patients without clinical and histopathologically proven LET. Medical records were evaluated for demographics, clinical characteristics, diagnoses, autoantibodies, treatment, and recurrence. Photosensitivity was confirmed by clinical history. This study was approved by the Wake Forest School of Medicine institutional review board.
Results
Twenty-five patients were included in the study (eTable). The mean age (SD) at diagnosis was 46 (10.9) years, with a male to female ratio of 1:4. Twenty-two (88%) patients were White non-Hispanic, whereas 3 (12%) were Black. Lupus erythematosus tumidus most commonly affected the trunk (18/25 [72%]) and upper extremities (18/25 [72%]), followed by the head and neck (15/25 [60%]) and lower extremities (8/25 [32%])(Figure 1). The most common morphologies were plaques (18/25 [72%]), papules (17/25 [68%]), and nodules (6/25 [24%])(Figures 2 and 3). Most patients experienced painful (14/25 [56%]) or pruritic (13/25 [52%]) lesions as well as photosensitivity (13/25 [52%]). Of all measured autoantibodies, 5 of 22 (23%) patients had positive antinuclear antibody (ANA) titers greater than 1:80, 1 of 14 (7%) patients had positive anti-Ro (anti-SSA), 1 of 14 (7%) had positive anti-La (anti-SSB), 2 of 10 (20%) had positive anti–double-stranded DNA, and 0 of 6 (0%) patients had positive anti-Smith antibodies. Four (16%) patients with SLE had skin and joint involvement, whereas 1 had lupus nephritis. One (4%) patient had discoid lupus erythematosus (DLE). Seventeen (68%) patients reported recurrences or flares. The mean duration of symptoms (SD) was 28 (44) months.
Topical corticosteroids (21/25 [84%]) and hydroxychloroquine (20/25 [80%]) were the most commonly prescribed treatments. Hydroxychloroquine monotherapy achieved clearance or almost clearance in 12 (60%) patients. Four patients were prescribed thalidomide after hydroxychloroquine monotherapy failed; 2 achieved complete clearance with thalidomide and hydroxychloroquine, 1 achieved complete clearance with thalidomide monotherapy, and 1 improved but did not clear. Four patients were concurrently started on quinacrine (mepacrine) after hydroxychloroquine monotherapy failed; 1 patient had no clearance, 1 discontinued because of allergy, 1 improved, and 1 cleared. Four patients had short courses of prednisone lasting 1 to 4 weeks. Three of 4 patients treated with methotrexate discontinued because of adverse effects, and 1 patient improved. Other prescribed treatments included topical calcineurin inhibitors (10/25 [40%]), dapsone (1/25 [4%]), and clofazimine (1/25 [4%]).
Comment
Prevalence of LET—Although other European LET case series reported a male predominance or equal male to female ratio, our case series reported female predominance (1:4).1,3-5 Our male to female ratio resembles similar ratios in DLE and subacute lupus erythematosus, whereas relative to our study, SLE male to female ratios favored females over males.6,7
Clinical Distribution of LET—In one study enrolling 24 patients with LET, 79% (19/24) of patients had facial involvement, 50% (12/24) had V-neck involvement, 50% (12/24) had back involvement, and 46% (11/24) had arm involvement,2 whereas our study reported 72% involvement of the trunk, 72% involvement of the upper extremities, 60% involvement of the head and neck region, and 32% involvement of the lower extremities. Although our study reported more lower extremity involvement, the aforementioned study used precise topographic locations, whereas we used more generalized topographic locations. Therefore, it was difficult to compare disease distribution between both studies.2
Presence of Autoantibodies and Comorbidities—Of the 22 patients tested for ANA, 23% reported titers greater than 1:80, similar to the 20% positive ANA prevalence in an LET case series of 25 patients.5 Of 4 patients diagnosed with SLE, 3 had articular and skin involvement, and 1 had renal involvement. These findings resemble a similar LET case series.2 Nonetheless, given the numerous skin criteria in the American College of Rheumatology SLE classification criteria, patients with predominant skin disease and positive autoantibodies are diagnosed as having SLE without notable extracutaneous involvement.2 Therefore, SLE diagnosis in the setting of LET could be reassessed periodically in this population. One patient in our study was diagnosed with DLE several years later. It is uncommon for LET to be reported concomitantly with DLE.8
Treatment of LET—Evidence supporting efficacious treatment options for LET is limited to case series. Sun protection is recommended in all patients with LET. Earlier case series reported a high response rate with sun protection and topical corticosteroids, with 19% to 55% of patients requiring subsequent systemic antimalarials.3,4 However, one case series presented a need for systemic antimalarials,5 similar to our study. Hydroxychloroquine 200 to 400 mg daily is considered the first-line systemic treatment for LET. Its response rate varies among studies and may be influenced by dosage.1,3 Second-line treatments include methotrexate 7.5 to 25 mg once weekly, thalidomide 50 to 100 mg daily, and quinacrine. However, quinacrine is not currently commercially available. Thalidomide and quinacrine represented useful alternatives when hydroxychloroquine monotherapy failed. As with other immunomodulators, adverse effects should be monitored periodically.
Conclusion
Lupus erythematosus tumidus is characterized by erythematous papules and plaques that may be tender or pruritic. It follows an intermittent course and rarely is associated with SLE. Hydroxychloroquine is considered the first-line systemic treatment; however, recalcitrant disease could be managed with other immunomodulators, including methotrexate, thalidomide, or quinacrine.
Lupus erythematosus tumidus (LET) is a rare photosensitive dermatosis1 that previously was considered a subtype of chronic cutaneous lupus erythematosus; however, the clinical course and favorable prognosis of LET led to its reclassification into another category, called intermittent cutaneous lupus erythematosus.2 Although known about for more than 100 years, the association of LET with systemic lupus erythematosus (SLE), its autoantibody profile, and its prognosis are not well characterized. The purpose of this study was to describe the demographics, clinical characteristics, autoantibody profile, comorbidities, and treatment of LET based on a retrospective review of patients with LET.
Methods
A retrospective review was conducted in patients with histologically diagnosed LET who presented to the Department of Dermatology at the Wake Forest School of Medicine (Winston-Salem, North Carolina) over 6 years (July 2012 to July 2018). Inclusion criteria included males or females aged 18 to 75 years with clinical and histopathology-proven LET, which was defined as a superficial and deep lymphocytic infiltrate with abundant mucin deposition in the reticular dermis and absent or focal dermoepidermal junction alterations. Exclusion criteria included males or females younger than 18 years or older than 75 years or patients without clinical and histopathologically proven LET. Medical records were evaluated for demographics, clinical characteristics, diagnoses, autoantibodies, treatment, and recurrence. Photosensitivity was confirmed by clinical history. This study was approved by the Wake Forest School of Medicine institutional review board.
Results
Twenty-five patients were included in the study (eTable). The mean age (SD) at diagnosis was 46 (10.9) years, with a male to female ratio of 1:4. Twenty-two (88%) patients were White non-Hispanic, whereas 3 (12%) were Black. Lupus erythematosus tumidus most commonly affected the trunk (18/25 [72%]) and upper extremities (18/25 [72%]), followed by the head and neck (15/25 [60%]) and lower extremities (8/25 [32%])(Figure 1). The most common morphologies were plaques (18/25 [72%]), papules (17/25 [68%]), and nodules (6/25 [24%])(Figures 2 and 3). Most patients experienced painful (14/25 [56%]) or pruritic (13/25 [52%]) lesions as well as photosensitivity (13/25 [52%]). Of all measured autoantibodies, 5 of 22 (23%) patients had positive antinuclear antibody (ANA) titers greater than 1:80, 1 of 14 (7%) patients had positive anti-Ro (anti-SSA), 1 of 14 (7%) had positive anti-La (anti-SSB), 2 of 10 (20%) had positive anti–double-stranded DNA, and 0 of 6 (0%) patients had positive anti-Smith antibodies. Four (16%) patients with SLE had skin and joint involvement, whereas 1 had lupus nephritis. One (4%) patient had discoid lupus erythematosus (DLE). Seventeen (68%) patients reported recurrences or flares. The mean duration of symptoms (SD) was 28 (44) months.
Topical corticosteroids (21/25 [84%]) and hydroxychloroquine (20/25 [80%]) were the most commonly prescribed treatments. Hydroxychloroquine monotherapy achieved clearance or almost clearance in 12 (60%) patients. Four patients were prescribed thalidomide after hydroxychloroquine monotherapy failed; 2 achieved complete clearance with thalidomide and hydroxychloroquine, 1 achieved complete clearance with thalidomide monotherapy, and 1 improved but did not clear. Four patients were concurrently started on quinacrine (mepacrine) after hydroxychloroquine monotherapy failed; 1 patient had no clearance, 1 discontinued because of allergy, 1 improved, and 1 cleared. Four patients had short courses of prednisone lasting 1 to 4 weeks. Three of 4 patients treated with methotrexate discontinued because of adverse effects, and 1 patient improved. Other prescribed treatments included topical calcineurin inhibitors (10/25 [40%]), dapsone (1/25 [4%]), and clofazimine (1/25 [4%]).
Comment
Prevalence of LET—Although other European LET case series reported a male predominance or equal male to female ratio, our case series reported female predominance (1:4).1,3-5 Our male to female ratio resembles similar ratios in DLE and subacute lupus erythematosus, whereas relative to our study, SLE male to female ratios favored females over males.6,7
Clinical Distribution of LET—In one study enrolling 24 patients with LET, 79% (19/24) of patients had facial involvement, 50% (12/24) had V-neck involvement, 50% (12/24) had back involvement, and 46% (11/24) had arm involvement,2 whereas our study reported 72% involvement of the trunk, 72% involvement of the upper extremities, 60% involvement of the head and neck region, and 32% involvement of the lower extremities. Although our study reported more lower extremity involvement, the aforementioned study used precise topographic locations, whereas we used more generalized topographic locations. Therefore, it was difficult to compare disease distribution between both studies.2
Presence of Autoantibodies and Comorbidities—Of the 22 patients tested for ANA, 23% reported titers greater than 1:80, similar to the 20% positive ANA prevalence in an LET case series of 25 patients.5 Of 4 patients diagnosed with SLE, 3 had articular and skin involvement, and 1 had renal involvement. These findings resemble a similar LET case series.2 Nonetheless, given the numerous skin criteria in the American College of Rheumatology SLE classification criteria, patients with predominant skin disease and positive autoantibodies are diagnosed as having SLE without notable extracutaneous involvement.2 Therefore, SLE diagnosis in the setting of LET could be reassessed periodically in this population. One patient in our study was diagnosed with DLE several years later. It is uncommon for LET to be reported concomitantly with DLE.8
Treatment of LET—Evidence supporting efficacious treatment options for LET is limited to case series. Sun protection is recommended in all patients with LET. Earlier case series reported a high response rate with sun protection and topical corticosteroids, with 19% to 55% of patients requiring subsequent systemic antimalarials.3,4 However, one case series presented a need for systemic antimalarials,5 similar to our study. Hydroxychloroquine 200 to 400 mg daily is considered the first-line systemic treatment for LET. Its response rate varies among studies and may be influenced by dosage.1,3 Second-line treatments include methotrexate 7.5 to 25 mg once weekly, thalidomide 50 to 100 mg daily, and quinacrine. However, quinacrine is not currently commercially available. Thalidomide and quinacrine represented useful alternatives when hydroxychloroquine monotherapy failed. As with other immunomodulators, adverse effects should be monitored periodically.
Conclusion
Lupus erythematosus tumidus is characterized by erythematous papules and plaques that may be tender or pruritic. It follows an intermittent course and rarely is associated with SLE. Hydroxychloroquine is considered the first-line systemic treatment; however, recalcitrant disease could be managed with other immunomodulators, including methotrexate, thalidomide, or quinacrine.
- Kuhn A, Bein D, Bonsmann G. The 100th anniversary of lupus erythematosus tumidus. Autoimmun Rev. 2009;8:441-448.
- Schmitt V, Meuth AM, Amler S, et al. Lupus erythematosus tumidus is a separate subtype of cutaneous lupus erythematosus. Br J Dermatol. 2010;162:64-73.
- Kuhn A, Richter-Hintz D, Oslislo C, et al. Lupus erythematosus tumidus—a neglected subset of cutaneous lupus erythematosus: report of 40 cases. Arch Dermatol. 2000;136:1033-1041.
- Vieira V, Del Pozo J, Yebra-Pimentel MT, et al. Lupus erythematosus tumidus: a series of 26 cases. Int J Dermatol. 2006;45:512-517.
- Rodriguez-Caruncho C, Bielsa I, Fernandez-Figueras MT, et al. Lupus erythematosus tumidus: a clinical and histological study of 25 cases. Lupus. 2015;24:751-755.
- Patsinakidis N, Gambichler T, Lahner N, et al. Cutaneous characteristics and association with antinuclear antibodies in 402 patients with different subtypes of lupus erythematosus. J Eur Acad Dermatol Venereol. 2016;30:2097-2104.
- Petersen MP, Moller S, Bygum A, et al. Epidemiology of cutaneous lupus erythematosus and the associated risk of systemic lupus erythematosus: a nationwide cohort study in Denmark. Lupus. 2018;27:1424-1430.
- Dekle CL, Mannes KD, Davis LS, et al. Lupus tumidus. J Am AcadDermatol. 1999;41:250-253.
- Kuhn A, Bein D, Bonsmann G. The 100th anniversary of lupus erythematosus tumidus. Autoimmun Rev. 2009;8:441-448.
- Schmitt V, Meuth AM, Amler S, et al. Lupus erythematosus tumidus is a separate subtype of cutaneous lupus erythematosus. Br J Dermatol. 2010;162:64-73.
- Kuhn A, Richter-Hintz D, Oslislo C, et al. Lupus erythematosus tumidus—a neglected subset of cutaneous lupus erythematosus: report of 40 cases. Arch Dermatol. 2000;136:1033-1041.
- Vieira V, Del Pozo J, Yebra-Pimentel MT, et al. Lupus erythematosus tumidus: a series of 26 cases. Int J Dermatol. 2006;45:512-517.
- Rodriguez-Caruncho C, Bielsa I, Fernandez-Figueras MT, et al. Lupus erythematosus tumidus: a clinical and histological study of 25 cases. Lupus. 2015;24:751-755.
- Patsinakidis N, Gambichler T, Lahner N, et al. Cutaneous characteristics and association with antinuclear antibodies in 402 patients with different subtypes of lupus erythematosus. J Eur Acad Dermatol Venereol. 2016;30:2097-2104.
- Petersen MP, Moller S, Bygum A, et al. Epidemiology of cutaneous lupus erythematosus and the associated risk of systemic lupus erythematosus: a nationwide cohort study in Denmark. Lupus. 2018;27:1424-1430.
- Dekle CL, Mannes KD, Davis LS, et al. Lupus tumidus. J Am AcadDermatol. 1999;41:250-253.
Practice Points
- Approximately 20% of patients with lupus erythematosus tumidus (LET) will have positive antinuclear antibody titers.
- Along with cutaneous manifestations, approximately 50% of patients with LET also will have pruritus, tenderness, and photosensitivity.
- If LET is resistant to hydroxychloroquine, consider using quinacrine, methotrexate, or thalidomide.
Ordering and Interpreting Precision Oncology Studies for Adults With Advanced Solid Tumors: A Primer
The ability to find and target specific biomarkers in the DNA of advanced cancers is rapidly changing options and outcomes for patients with locally advanced and metastatic solid tumors. This strategy is the basis for precision oncology, defined here as using predictive biomarkers from tumor and/or germline sequencing to guide therapies. This article focuses specifically on the use of DNA sequencing to find those biomarkers and provides guidance about which test is optimal in a specific situation, as well as interpretation of the results. We emphasize the identification of biomarkers that provide adult patients with advanced solid tumors access to therapies that would not be an option had sequencing not been performed and that have the potential for significant clinical benefit. The best approach is to have an expert team with experience in precision oncology to assist in the interpretation of results.
Which test?
Deciding what test of the array of assays available to use and which tissue to test can be overwhelming, and uncertainty may prevent oncology practitioners from ordering germline or somatic sequencing. For the purposes of this article, we will focus on DNA sequencing for inherited/germline alterations (including mutations, copy number changes, or fusions), which may inform treatment, or alterations that arise in the process of carcinogenesis and tumor evolution (somatic alterations in tumor DNA). This focus is not meant to exclude any specific test but to focus on DNA-based tests in patients with locally advanced or metastatic malignancy.
Germline Testing
Germline testing is the sequencing of inherited DNA in noncancerous cells to find alterations that may play a role in the development of cancers and are actionable in some cases. Germline alterations can inform therapeutic decisions, predict future cancer risk, and provide information that can help family members to better manage their risks of malignancy. Detailed discussions of the importance of germline testing to inform cancer surveillance, risk-reducing interventions, and the testing of relatives to determine who carries inherited alterations (cascade testing) is extremely important with several advantages and is covered in a number of excellent reviews elsewhere.1-3 Testing of germline DNA in patients with a metastatic malignancy can provide treatment options otherwise not available for patients, particularly for BRCA1/2 and Lynch syndrome–related cancers. Recent studies have shown that 10 to 15% of patients with advanced malignancies of many types have a pathogenic germline alteration.4,5
Germline DNA is usually acquired from peripheral blood, a buccal swab, or saliva collection and is therefore readily available. This is advantageous because it does not require a biopsy to identify relevant alterations. Germline testing is also less susceptible to the rare situations in which artifacts occur in formalin-fixed tissues and obscure relevant alterations.
The cost of germline testing varies, but most commercial vendor assays for germline testing are significantly less expensive than the cost of somatic testing. The disadvantages include the inability of germline testing to find any alterations that arise solely in tumor tissue and the smaller gene panels included in germline testing as compared to somatic testing panels. Other considerations relate to the inherited nature of pathogenic germline variants and its implications for family members that may affect the patient’s psychosocial health and potentially change the family dynamics.
Deciding who is appropriate for germline testing and when to perform the testing should be individualized to the patient’s wishes and disease status. Treatment planning may be less complicated if testing has been performed and germline status is known. In some cases urgent germline testing is indicated to inform pending procedures and/or surgical decisions for risk reduction, including more extensive tissue resection, such as the removal of additional organs or contralateral tissue. A minor point regarding germline testing is that the DNA of patients with hematologic malignancies may be difficult to sequence because of sample contamination by the circulating malignancy. For this reason, most laboratories will not accept peripheral blood or saliva samples for germline testing in patients with active hematologic malignancies; they often require DNA from another source such as fibroblasts from a skin biopsy or cells from a muscle biopsy. Germline testing is recommended for all patients with metastatic prostate cancer, as well as any patient with any stage of pancreatic cancer or ovarian cancer and patients with breast cancer diagnosed at age ≤ 45 years. More detailed criteria for who is appropriate for germline testing outside of these groups can be found in the appropriate National Comprehensive Cancer Network (NCCN) guidelines.6-8 In patients with some malignancies such as prostate and pancreatic cancer, approximately half of patients who have a BRCA-related cancer developed that malignancy because of a germline BRCA alteration.9-11 Testing germline DNA is therefore an easy way to quickly find almost half of all targetable alterations with a treatment approved by the US Food and Drug Administration (FDA) and at low cost, with the added benefit of providing critical information for families who may be unaware that members carry a relevant pathogenic germline alteration. In those families, cascade testing can provide surveillance and intervention strategies that can be lifesaving.
A related and particularly relevant question is when should a result found on a somatic testing panel prompt follow-up germline testing? Some institutions have algorithms in place to automate referral for germline testing based on specific genetic criteria.12 Excellent reviews are available that outline the following considerations in more detail.13 Typically, somatic testing results that would trigger follow-up germline testing would be truncating or deleterious or likely deleterious mutations per germline datasets in high-risk genes associated with highly penetrant autosomal dominant conditions (BRCA1, BRCA2, PALB2, MLH1, MSH2, and MSH6), selected moderate-risk genes (BRIP1, RAD51C, and RAD51D), and specific variants with a high probability of being germline because they are common germline founder mutations. Although the actionability and significance of specific genes remains a matter of some discussion, generally finding a somatic pathogenic sequencing result included in the 59-gene list of the American College of Medical Genetics and Genomics (ACMG) guidelines would be an indication for germline testing. Another indication for germline testing would be finding genes with germline mutations for which the NCCN has specific management guidelines, or the presence of alterations consistent with known founder mutations.14 When a patient’s tumor has microsatellite instability or is hypermutated (defined as > 10 mutations per megabase), a search for germline alterations is warranted given that about 15% of these patients with these tumors carry a Lynch syndrome gene.15 Genes that are commonly found as somatic alterations alone (eg, TP53 or APC) are generally not an indication for germline testing unless family history is compelling.
Although some clinicians use the variant allele fraction in the somatic sequencing report to decide whether to conduct germline testing, this approach is suboptimal, as allele fraction may be confounded by assay conditions and a high allele fraction may be found in pure tumors with loss of heterozygosity (LOH) of the other allele. There is also evidence that for a variety of reasons, somatic sequencing panels do not always detect germline alterations in somatic tissues.16 Reasons for this may include discordance between the genes being tested in the germline vs the somatic panel, technical differences such as interference of formalin-fixed paraffin-embedded (FFPE) artifact with detecting the germline variant, lack of expertise in germline variant interpretation among laboratories doing tumor-only sequencing, and, in rare cases, large deletions in tumor tissue masking a germline point mutation.
Variant Interpretation of Germline Testing
A general understanding of the terminology used for germline variant interpretation allows for the ordering health care practitioner (HCP) to provide the best quality care and an appreciation for the limitations of current molecular testing. Not all variants are associated with disease; the clinical significance of a genetic variant falls on a spectrum. The criteria for determining pathogenicity differ between molecular laboratories, but most are influenced by the standards and guidelines set forth by the ACMG.14 The clinical molecular laboratory determines variant classification, and a detailed discussion is beyond the scope of this primer. In brief, variant classification is based on evidence of varying strength in different categories including population data, computational and predictive data, functional data, segregation data, de novo data, allelic data, and information from various databases. The ACMG has proposed a 5-tiered classification system, by which most molecular laboratories adhere to in their genetic test reports (Table 1).14
Pathogenic and likely pathogenic variants are clinically actionable, whereas variants of uncertain significance (VUS) require additional data and/or functional studies before making clinical decisions. Depending on the clinical context and existing supporting evidence, it may be prudent to continue monitoring for worsening or new signs of disease in patients with one or more VUS while additional efforts are underway to understand the variant’s significance.
In some cases, variants are reclassified, which may alter the management and treatment of patients. Reclassification can occur with VUS, and in rare instances, can also occur with variants previously classified as pathogenic/likely pathogenic or benign/likely benign. In such a case, the reporting laboratory will typically make concerted efforts to alert the ordering HCP. However, variant reclassifications are not always communicated to the care team. Thus, it is important to periodically contact the molecular laboratory of interest to obtain updated test interpretations.
Somatic Testing
Testing of somatic (tumor) tissue is critical and is the approach most commonly taken in medical oncology (Table 2).
The advantages of primary tumor are that it is usually in hand as a diagnostic biopsy, acquisition is standard of care, and several targetable alterations are truncal, defined as driver mutations present at the time of tumor development. Also, the potential that the tumor arose in the background of a predisposing germline alteration can be suggested by sequencing primary tumor as discussed above. Moreover, sequencing the primary tumor can be done at any time unless the biopsy sample is considered too old or degraded (per specific platform requirements). The information gained can be used to anticipate additional treatment options that are relevant when patients experience disease progression. Disadvantages include the problem that primary specimens may be old or have limited tumor content, both of which increase the likelihood that sequencing will not be technically successful.
Alterations that are targetable and arise as a result of either treatment pressure or clonal evolution are considered evolutionary. If evolutionary alterations are the main focus for sequencing, then metastasis biopsy or ctDNA are better choices. The advantages of a metastasis biopsy are that tissue is contemporary, tumor content may be higher than in primary tumor, and both truncal and evolutionary alterations can be detected.
For specific tumors, continued analysis of evolving genomic alterations can play a critical role in management. In non–small cell lung cancer (NSCLC), somatic testing is conducted again at progression on repeat biopsies to evaluate for emerging resistance mutations. In epidermal growth factor receptor (EGFR)–mutated lung cancer, the resistance mutation, exon 20 p.T790M (point mutation), can present in patients after treatment with first- or second-generation EGFR tyrosine kinase inhibitors (TKI). Even in patients who are treated with the third-generation EGFR TKI osimertinib that can treat T790M-mutated lung cancer, multiple possible evolutionary mutations can occur at progression, including other EGFR mutations, MET/HER2 amplification, and BRAF V600E, to name a few.20 Resistance mechanisms develop due to treatment selection pressure and the molecular heterogeneity seen in lung cancer.
Disadvantages for metastatic biopsy include the inability to safely access a metastatic site, the time considerations for preauthorization and arrangement of biopsy, and a lower-than-average likelihood of successful sequencing from sites such as bone.21,22 In addition, there is some concern that a single metastatic site may not capture all relevant alterations for multiple reasons, including tumor heterogeneity.
Significant advances in the past decade have dramatically improved the ability to use ctDNA to guide therapy. Advantages include ease of acquisition as acquiring a sample requires only a blood draw, and the potential that the pool of ctDNA is a better reflection of the relevant biology as it potentially reflects all metastatic tissues. Disadvantages are that sequencing attempts may not be productive if the sample is acquired at a time when the tumor is either quiescent or tumor burden is so low that only limited amounts of DNA are being shed. Performing ctDNA analysis when a tumor is not progressing is less likely to be productive for a number of tumor types.23,24 Sequencing ctDNA is also more susceptible than sequencing tumor biopsies to detection of alterations that are not from the tumor of interest but from clonal hematopoiesis of indeterminate potential (CHIP) or other clonal hematopoietic disorders (see Confounders section below).
Selecting the Tissue
Deciding on the tissue to analyze is a critical part of the decision process (Table 3). If the primary tumor tissue is old the likelihood of productive sequencing is lower, although age alone is not the only consideration and the methods of fixation may be just as relevant.
For prostate cancer in particular, the ability to successfully sequence primary tumor tissue decreases as the amount of tumor decreases in low-volume biopsies such as prostate needle biopsies. Generally, if tumor content is < 10% of the biopsy specimen, then sequencing is less likely to be productive.25 Also, if the alteration of interest is not known to be truncal, then a relevant target might be missed by sequencing tissue that does not reflect current biology. Metastasis biopsy may be the most appropriate tissue, particularly if this specimen has already been acquired. As above, a metastasis biopsy may have a higher tumor content, and it should reflect relevant biology if it is recent. However, bone biopsies have a relatively low yield for successful sequencing, so a soft tissue lesion (eg, liver or lymph node metastasis) is generally preferred.
The inability to safely access tissue is often a consideration. Proximity to vital structures such as large blood vessels or the potential for significant morbidity in the event of a complication (liver or lung biopsies, particularly in patients on anticoagulation medications) may make the risk/benefit ratio too high. The inability to conduct somatic testing has been reported to often be due to inadequate tissue sampling.26 ctDNA is an attractive alternative but should typically be drawn when a tumor is progressing with a reasonable tumor burden that is more likely to be shedding DNA. Performing ctDNA analysis in patients without obvious radiographic metastasis or in patients whose tumor is under good control is unlikely to produce interpretable results.
Interpreting the Results
The intent of sequencing tumor tissue is to identify alterations that are biologically important and may provide a point of therapeutic leverage. However, deciding which alterations are relevant is not always straightforward. For example, any normal individual genome contains around 10,000 missense variants, hundreds of insertion/deletion variants, and dozens of protein-truncating variants. Distinguishing these alterations, which are part of the individual, from those that are tumor-specific and have functional significance can be difficult in the absence of paired sequencing of both normal and tissue samples.
Specific Alterations
Although most commercial vendors provide important information in sequencing reports to assist oncology HCPs in deciding which alterations are relevant, the reports are not always clear. In many cases the report will specifically indicate whether the alteration has been reported previously as pathogenic or benign. However, some platforms will report alterations that are not known to be drivers of tumor biology. It is critical to be aware that if variants are not reported as pathogenic, they should not be assumed to be pathogenic simply because they are included in the report. Alterations more likely to be drivers of relevant biology are those that change gene and protein structure and include frameshift (fs*), nonsense (denoted by sequence ending in “X” or “*”), or specific fusions or insertions/deletions (indel) that occur in important domains of the gene.
For some genes, only specific alterations are targetable and not all alterations have the same effect on protein function. Although overexpression of certain genes and proteins are actionable (eg, HER2), amplification of a gene does not necessarily indicate that it is targetable. In NSCLC, specific alterations convey sensitivity to targeted therapies. For example, in EGFR-mutated NSCLC, the sensitizing mutations to EGFR TKIs are exon19 deletions and exon 21 L858R point mutations (the most common mutations), as well as less common mutations found in exon 18-21. Exon 20 mutations, however, are not responsive to EGFR TKIs with a few exceptions.27 Patients who have tumors that do not harbor a sensitizing EGFR mutation should not be treated with an EGFR TKI. In a variety of solid tumors, gene fusions of the NTRK 1/2/3, act as oncogenic drivers. The chromosomal fusion events involving the carboxy-terminal kinase domain of TRK and upstream amino-terminal partners lead to overexpression of the chimeric proteins tropomyosin receptor kinase (TRK) A/B/C, resulting in constitutively active, ligand-independent downstream signaling. In patients with NTRK 1/2/3 gene fusions, larotrectinib and entrectinib, small molecule inhibitors to TRK, have shown antitumor activity.28,29 No alterations beyond these fusions are known to be targetable.
Allele Fraction
Knowing the fraction (or proportion) of the alteration of interest in the sequenced tissue relative to the estimated tumor content can assist in decision making. Not all platforms will provide this information, which is referred to as mutation allele fraction (MAF) or variant allele fraction (VAF), but sometimes will provide it on request. Platforms will usually provide an estimate of the percent tumor in the tissue being sampled if it is from a biopsy. If the MAF is around 50% in the sequenced tissue (including ctDNA), then there is a reasonable chance that it is a germline variant. However, there are nuances as germline alterations in some genes, such as BRCA1/2, can be accompanied by loss of the other allele of the gene (LOH). In that case, if most of the circulating DNA is from tumor, then the MAF can be > 50%.
If there are 2 alterations of the same gene with MAF percentages that are each half of the total percent tumor, there is a high likelihood of biallelic alteration. These sorts of paired alterations or one mutation with apparent LOH or copy loss would again indicate a high likelihood that the alteration is in fact pathogenic and a relevant driver. Not all pathogenic alterations have to be biallelic to be driver mutations but in BRCA1/2, or mismatch repair deficiency genes, the presence of biallelic alterations increases the likelihood of their being pathogenic.
Tumors that are hypermutated—containing sometimes hundreds of mutations per megabyte of DNA—can be particularly complicated to interpret, because the likelihood increases that many of the alterations are a function of the hypermutation and not a driver mutation. This is particularly important when there are concurrent mutations in mismatch repair genes and genes, such as BRCA1/2. If the tumor is
Confounders
In some situations, interpretation can be particularly challenging. For example, several alterations for which there are FDA on-label indications (such as ATM or BRCA2) can be detected in ctDNA that may not be due to the tumor but to CHIP. CHIP represents hematopoietic clones that are dysplastic as a result of exposure to DNA-damaging agents (eg, platinum chemotherapy) or as a result of aging and arise when mutations in hematopoietic stem cells provide a competitive advantage.31 The most common CHIP clones that can be detected are DNMT3A, ASXL1, or TET2; because these alterations are not targetable, their importance lies primarily in whether patients have evidence of hematologic abnormality, which might represent an evolving hematopoietic disorder. Because CHIP alterations can overlap with somatic alterations for which FDA-approved drugs exist, such as ATM or CHEK2 (olaparib for prostate cancer) and BRCA2 (poly-ADP-ribose polymerase inhibitors in a range of indications) there is concern that CHIP might result in patient harm from inappropriate treatment of CHIP rather than the tumor, with no likelihood that the treatment would affect the tumor, causing treatment delays.32 General considerations for deciding whether an alteration represents CHIP include excluding alteration in which the VAF is < 1% and when the VAF in the alteration of interest is < 20% of the estimated tumor fraction in the sample. Exceptions to this are found in patients with true myelodysplasia or chronic lymphocytic leukemia, in whom the VAF can be well over 50% because of circulating tumor burden. The only way to be certain that an alteration detected on ctDNA reflects tumor rather than CHIP is to utilize an assay with matched tumor-normal sequencing.
Resources for Assistance
For oncology HCPs, perhaps the best resource to help in selecting and interpreting the appropriate testing is through a dedicated molecular oncology tumor board and subject matter experts who contribute to those tumor boards. In the US Department of Veterans Affairs, the national precision oncology program and its affiliated clinical services, such as the option to order a national consultation and molecular tumor board education, are easily accessible to all HCPs (www.cancer.va.gov). Many commercial vendors provide support to assist with questions of interpretation and to inform clinical decision-making. Other resources that can assist with deciding whether an alteration is pathogenic include extensive curated databases such as ClinVar (www.ncbi.nlm.nih.gov/clinvar) and the Human Genetic Mutation Database (www.hgmd.cf.ac.uk/ac/index.php) for germline alterations or COSMIC (cancer.sanger.ac.uk/cosmic) for somatic alterations. OncoKB (www.oncokb.org) is a resource for assistance in defining levels of evidence for the use of agents to target specific alterations and to assist in assigning pathogenicity to specific alterations. Additional educational resources for training in genomics and genetics are also included in the Appendix.
The rapid growth in technology and ability to enhance understanding of relevant tumor biology continues to improve the therapeutic landscape for men and women dealing with malignancy and our ability to find targetable genetic alterations with the potential for meaningful clinical benefit.
Acknowledgments
Dedicated to Neil Spector.
1. Domchek SM, Mardis E, Carlisle JW, Owonikoko TK. Integrating genetic and genomic testing into oncology practice. Am Soc Clin Oncol Educ Book. 2020;40:e259-e263. doi:10.1200/EDBK_280607
2. Stoffel EM, Carethers JM. Current approaches to germline cancer genetic testing. Annu Rev Med. 2020;71:85-102. doi:10.1146/annurev-med-052318-101009
3. Lappalainen T, Scott AJ, Brandt M, Hall IM. Genomic analysis in the age of human genome sequencing. Cell. 2019;177(1):70-84. doi:10.1016/j.cell.2019.02.032
4. Samadder NJ, Riegert-Johnson D, Boardman L, et al. Comparison of universal genetic testing vs guideline-directed targeted testing for patients with hereditary cancer syndrome. JAMA Oncol. 2021;7(2):230-237. doi:10.1001/jamaoncol.2020.6252
5. Schneider BP, Stout L, Philips S, et al. Implications of incidental germline findings identified in the context of clinical whole exome sequencing for guiding cancer therapy. JCO Precis Oncol. 2020;4:1109-1121. doi:10.1200/PO.19.00354
6. National Comprehensive Cancer Network. Pancreatic cancer (Version 1.2022). Updated February 24, 2022. Accessed April 13, 2022. https://www.nccn.org/professionals/physician_gls/pdf/pancreatic.pdf
7. National Comprehensive Cancer Network. Prostate cancer (Version 3.2022). Updated January 10, 2022. Accessed April 13, 2022. https://www.nccn.org/professionals/physician_gls/pdf/prostate.pdf
8. National Comprehensive Cancer Network. Genetic/familial high-risk assessment: breast, ovarian, and pancreatic (Version 2.2022). Updated March 9, 2022. Accessed April 13, 2022. https://www.nccn.org/professionals/physician_gls/pdf/genetics_bop.pdf
9. Robinson D, Van Allen EM, Wu YM, et al. Integrative clinical genomics of advanced prostate cancer. Cell. 2015;161(5):1215-1228. doi:10.1016/j.cell.2015.05.001
10. Pritchard CC, Mateo J, Walsh MF, et al. Inherited DNA-repair gene mutations in men with metastatic prostate cancer. N Engl J Med. 2016;375(5):443-453. doi:10.1056/NEJMoa1603144
11. Cancer Genome Atlas Research Network. Integrated genomic characterization of pancreatic ductal adenocarcinoma. Cancer Cell. 2017;32(2):185-203.e13. doi:10.1016/j.ccell.2017.07.007
12. Clark DF, Maxwell KN, Powers J, et al. Identification and confirmation of potentially actionable germline mutations in tumor-only genomic sequencing. JCO Precis Oncol. 2019;3:PO.19.00076. doi:10.1200/PO.19.00076
13. DeLeonardis K, Hogan L, Cannistra SA, Rangachari D, Tung N. When should tumor genomic profiling prompt consideration of germline testing? J Oncol Pract. 2019;15(9):465-473. doi:10.1200/JOP.19.00201
14. Richards S, Aziz N, Bale S, et al. Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet Med. 2015;17(5):405-424. doi:10.1038/gim.2015.30
15. Latham A, Srinivasan P, Kemel Y, et al. Microsatellite instability is associated with the presence of Lynch syndrome pan-cancer. J Clin Oncol. 2019;37(4):286-295. doi:10.1200/JCO.18.00283
16. Lincoln SE, Nussbaum RL, Kurian AW, et al. Yield and utility of germline testing following tumor sequencing in patients with cancer. JAMA Netw Open. 2020;3(10):e2019452. doi:10.1001/jamanetworkopen.2020.19452
17. National Comprehensive Cancer Network. Non-small cell lung cancer (Version: 3.2022). Updated March 16, 2022. Accessed April 13, 2022. https://www.nccn.org/professionals/physician_gls/pdf/nscl.pdf
18. National Comprehensive Cancer Network. Colon cancer (Version 1.2022). February 25, 2022. Accessed April 13, 2022. https://www.nccn.org/professionals/physician_gls/pdf/colon.pdf
19. National Comprehensive Cancer Network. Melanoma: cutaneous (Version 3.2022). April 11, 2022. Accessed April 13, 2022. https://www.nccn.org/professionals/physician_gls/pdf/cutaneous_melanoma.pdf
20. Leonetti A, Sharma S, Minari R, Perego P, Giovannetti E, Tiseo M. Resistance mechanisms to osimertinib in EGFR-mutated non-small cell lung cancer. Br J Cancer. 2019;121(9):725-737. doi:10.1038/s41416-019-0573-8
21. Zheng G, Lin MT, Lokhandwala PM, et al. Clinical mutational profiling of bone metastases of lung and colon carcinoma and malignant melanoma using next-generation sequencing. Cancer Cytopathol. 2016;124(10):744-753. doi:10.1002/cncy.21743
22. Spritzer CE, Afonso PD, Vinson EN, et al. Bone marrow biopsy: RNA isolation with expression profiling in men with metastatic castration-resistant prostate cancer—factors affecting diagnostic success. Radiology. 2013;269(3):816-823. doi:10.1148/radiol.13121782
23. Schweizer MT, Gulati R, Beightol M, et al. Clinical determinants for successful circulating tumor DNA analysis in prostate cancer. Prostate. 2019;79(7):701-708. doi:10.1002/pros.23778
24. Bettegowda C, Sausen M, Leary RJ, et al. Detection of circulating tumor DNA in early- and late-stage human malignancies. Sci Transl Med. 2014;6(224):224ra224. doi:10.1126/scitranslmed.3007094
25. Pritchard CC, Salipante SJ, Koehler K, et al. Validation and implementation of targeted capture and sequencing for the detection of actionable mutation, copy number variation, and gene rearrangement in clinical cancer specimens. J Mol Diagn. 2014;16(1):56-67. doi:10.1016/j.jmoldx.2013.08.004
26. Gutierrez ME, Choi K, Lanman RB, et al. Genomic profiling of advanced non-small cell lung cancer in community settings: gaps and opportunities. Clin Lung Cancer. 2017;18(6):651-659. doi:10.1016/j.cllc.2017.04.004
27. Malapelle U, Pilotto S, Passiglia F, et al. Dealing with NSCLC EGFR mutation testing and treatment: a comprehensive review with an Italian real-world perspective. Crit Rev Oncol Hematol. 2021;160:103300. doi:10.1016/j.critrevonc.2021.103300
28. Drilon A, Laetsch TW, Kummar S, et al. Efficacy of larotrectinib in TRK fusion-positive cancers in adults and children. N Engl J Med. 2018;378(8):731-739. doi:10.1056/NEJMoa1714448
29. Doebele RC, Drilon A, Paz-Ares L, et al. Entrectinib in patients with advanced or metastatic NTRK fusion-positive solid tumours: integrated analysis of three phase 1-2 trials. Lancet Oncol. 2020;21(2):271-282. doi:10.1016/S1470-2045(19)30691-6
30. Jonsson P, Bandlamudi C, Cheng ML, et al. Tumour lineage shapes BRCA-mediated phenotypes. Nature. 2019;571(7766):576-579. doi:10.1038/s41586-019-1382-1
31. Steensma DP. Clinical consequences of clonal hematopoiesis of indeterminate potential. Hematology Am Soc Hematol Educ Program. 2018;2018(1):264-269. doi:10.1182/asheducation-2018.1.264
32. Jensen K, Konnick EQ, Schweizer MT, et al. Association of clonal hematopoiesis in DNA repair genes with prostate cancer plasma cell-free DNA testing interference. JAMA Oncol. 2021;7(1):107-110. doi:10.1001/jamaoncol.2020.5161
The ability to find and target specific biomarkers in the DNA of advanced cancers is rapidly changing options and outcomes for patients with locally advanced and metastatic solid tumors. This strategy is the basis for precision oncology, defined here as using predictive biomarkers from tumor and/or germline sequencing to guide therapies. This article focuses specifically on the use of DNA sequencing to find those biomarkers and provides guidance about which test is optimal in a specific situation, as well as interpretation of the results. We emphasize the identification of biomarkers that provide adult patients with advanced solid tumors access to therapies that would not be an option had sequencing not been performed and that have the potential for significant clinical benefit. The best approach is to have an expert team with experience in precision oncology to assist in the interpretation of results.
Which test?
Deciding what test of the array of assays available to use and which tissue to test can be overwhelming, and uncertainty may prevent oncology practitioners from ordering germline or somatic sequencing. For the purposes of this article, we will focus on DNA sequencing for inherited/germline alterations (including mutations, copy number changes, or fusions), which may inform treatment, or alterations that arise in the process of carcinogenesis and tumor evolution (somatic alterations in tumor DNA). This focus is not meant to exclude any specific test but to focus on DNA-based tests in patients with locally advanced or metastatic malignancy.
Germline Testing
Germline testing is the sequencing of inherited DNA in noncancerous cells to find alterations that may play a role in the development of cancers and are actionable in some cases. Germline alterations can inform therapeutic decisions, predict future cancer risk, and provide information that can help family members to better manage their risks of malignancy. Detailed discussions of the importance of germline testing to inform cancer surveillance, risk-reducing interventions, and the testing of relatives to determine who carries inherited alterations (cascade testing) is extremely important with several advantages and is covered in a number of excellent reviews elsewhere.1-3 Testing of germline DNA in patients with a metastatic malignancy can provide treatment options otherwise not available for patients, particularly for BRCA1/2 and Lynch syndrome–related cancers. Recent studies have shown that 10 to 15% of patients with advanced malignancies of many types have a pathogenic germline alteration.4,5
Germline DNA is usually acquired from peripheral blood, a buccal swab, or saliva collection and is therefore readily available. This is advantageous because it does not require a biopsy to identify relevant alterations. Germline testing is also less susceptible to the rare situations in which artifacts occur in formalin-fixed tissues and obscure relevant alterations.
The cost of germline testing varies, but most commercial vendor assays for germline testing are significantly less expensive than the cost of somatic testing. The disadvantages include the inability of germline testing to find any alterations that arise solely in tumor tissue and the smaller gene panels included in germline testing as compared to somatic testing panels. Other considerations relate to the inherited nature of pathogenic germline variants and its implications for family members that may affect the patient’s psychosocial health and potentially change the family dynamics.
Deciding who is appropriate for germline testing and when to perform the testing should be individualized to the patient’s wishes and disease status. Treatment planning may be less complicated if testing has been performed and germline status is known. In some cases urgent germline testing is indicated to inform pending procedures and/or surgical decisions for risk reduction, including more extensive tissue resection, such as the removal of additional organs or contralateral tissue. A minor point regarding germline testing is that the DNA of patients with hematologic malignancies may be difficult to sequence because of sample contamination by the circulating malignancy. For this reason, most laboratories will not accept peripheral blood or saliva samples for germline testing in patients with active hematologic malignancies; they often require DNA from another source such as fibroblasts from a skin biopsy or cells from a muscle biopsy. Germline testing is recommended for all patients with metastatic prostate cancer, as well as any patient with any stage of pancreatic cancer or ovarian cancer and patients with breast cancer diagnosed at age ≤ 45 years. More detailed criteria for who is appropriate for germline testing outside of these groups can be found in the appropriate National Comprehensive Cancer Network (NCCN) guidelines.6-8 In patients with some malignancies such as prostate and pancreatic cancer, approximately half of patients who have a BRCA-related cancer developed that malignancy because of a germline BRCA alteration.9-11 Testing germline DNA is therefore an easy way to quickly find almost half of all targetable alterations with a treatment approved by the US Food and Drug Administration (FDA) and at low cost, with the added benefit of providing critical information for families who may be unaware that members carry a relevant pathogenic germline alteration. In those families, cascade testing can provide surveillance and intervention strategies that can be lifesaving.
A related and particularly relevant question is when should a result found on a somatic testing panel prompt follow-up germline testing? Some institutions have algorithms in place to automate referral for germline testing based on specific genetic criteria.12 Excellent reviews are available that outline the following considerations in more detail.13 Typically, somatic testing results that would trigger follow-up germline testing would be truncating or deleterious or likely deleterious mutations per germline datasets in high-risk genes associated with highly penetrant autosomal dominant conditions (BRCA1, BRCA2, PALB2, MLH1, MSH2, and MSH6), selected moderate-risk genes (BRIP1, RAD51C, and RAD51D), and specific variants with a high probability of being germline because they are common germline founder mutations. Although the actionability and significance of specific genes remains a matter of some discussion, generally finding a somatic pathogenic sequencing result included in the 59-gene list of the American College of Medical Genetics and Genomics (ACMG) guidelines would be an indication for germline testing. Another indication for germline testing would be finding genes with germline mutations for which the NCCN has specific management guidelines, or the presence of alterations consistent with known founder mutations.14 When a patient’s tumor has microsatellite instability or is hypermutated (defined as > 10 mutations per megabase), a search for germline alterations is warranted given that about 15% of these patients with these tumors carry a Lynch syndrome gene.15 Genes that are commonly found as somatic alterations alone (eg, TP53 or APC) are generally not an indication for germline testing unless family history is compelling.
Although some clinicians use the variant allele fraction in the somatic sequencing report to decide whether to conduct germline testing, this approach is suboptimal, as allele fraction may be confounded by assay conditions and a high allele fraction may be found in pure tumors with loss of heterozygosity (LOH) of the other allele. There is also evidence that for a variety of reasons, somatic sequencing panels do not always detect germline alterations in somatic tissues.16 Reasons for this may include discordance between the genes being tested in the germline vs the somatic panel, technical differences such as interference of formalin-fixed paraffin-embedded (FFPE) artifact with detecting the germline variant, lack of expertise in germline variant interpretation among laboratories doing tumor-only sequencing, and, in rare cases, large deletions in tumor tissue masking a germline point mutation.
Variant Interpretation of Germline Testing
A general understanding of the terminology used for germline variant interpretation allows for the ordering health care practitioner (HCP) to provide the best quality care and an appreciation for the limitations of current molecular testing. Not all variants are associated with disease; the clinical significance of a genetic variant falls on a spectrum. The criteria for determining pathogenicity differ between molecular laboratories, but most are influenced by the standards and guidelines set forth by the ACMG.14 The clinical molecular laboratory determines variant classification, and a detailed discussion is beyond the scope of this primer. In brief, variant classification is based on evidence of varying strength in different categories including population data, computational and predictive data, functional data, segregation data, de novo data, allelic data, and information from various databases. The ACMG has proposed a 5-tiered classification system, by which most molecular laboratories adhere to in their genetic test reports (Table 1).14
Pathogenic and likely pathogenic variants are clinically actionable, whereas variants of uncertain significance (VUS) require additional data and/or functional studies before making clinical decisions. Depending on the clinical context and existing supporting evidence, it may be prudent to continue monitoring for worsening or new signs of disease in patients with one or more VUS while additional efforts are underway to understand the variant’s significance.
In some cases, variants are reclassified, which may alter the management and treatment of patients. Reclassification can occur with VUS, and in rare instances, can also occur with variants previously classified as pathogenic/likely pathogenic or benign/likely benign. In such a case, the reporting laboratory will typically make concerted efforts to alert the ordering HCP. However, variant reclassifications are not always communicated to the care team. Thus, it is important to periodically contact the molecular laboratory of interest to obtain updated test interpretations.
Somatic Testing
Testing of somatic (tumor) tissue is critical and is the approach most commonly taken in medical oncology (Table 2).
The advantages of primary tumor are that it is usually in hand as a diagnostic biopsy, acquisition is standard of care, and several targetable alterations are truncal, defined as driver mutations present at the time of tumor development. Also, the potential that the tumor arose in the background of a predisposing germline alteration can be suggested by sequencing primary tumor as discussed above. Moreover, sequencing the primary tumor can be done at any time unless the biopsy sample is considered too old or degraded (per specific platform requirements). The information gained can be used to anticipate additional treatment options that are relevant when patients experience disease progression. Disadvantages include the problem that primary specimens may be old or have limited tumor content, both of which increase the likelihood that sequencing will not be technically successful.
Alterations that are targetable and arise as a result of either treatment pressure or clonal evolution are considered evolutionary. If evolutionary alterations are the main focus for sequencing, then metastasis biopsy or ctDNA are better choices. The advantages of a metastasis biopsy are that tissue is contemporary, tumor content may be higher than in primary tumor, and both truncal and evolutionary alterations can be detected.
For specific tumors, continued analysis of evolving genomic alterations can play a critical role in management. In non–small cell lung cancer (NSCLC), somatic testing is conducted again at progression on repeat biopsies to evaluate for emerging resistance mutations. In epidermal growth factor receptor (EGFR)–mutated lung cancer, the resistance mutation, exon 20 p.T790M (point mutation), can present in patients after treatment with first- or second-generation EGFR tyrosine kinase inhibitors (TKI). Even in patients who are treated with the third-generation EGFR TKI osimertinib that can treat T790M-mutated lung cancer, multiple possible evolutionary mutations can occur at progression, including other EGFR mutations, MET/HER2 amplification, and BRAF V600E, to name a few.20 Resistance mechanisms develop due to treatment selection pressure and the molecular heterogeneity seen in lung cancer.
Disadvantages for metastatic biopsy include the inability to safely access a metastatic site, the time considerations for preauthorization and arrangement of biopsy, and a lower-than-average likelihood of successful sequencing from sites such as bone.21,22 In addition, there is some concern that a single metastatic site may not capture all relevant alterations for multiple reasons, including tumor heterogeneity.
Significant advances in the past decade have dramatically improved the ability to use ctDNA to guide therapy. Advantages include ease of acquisition as acquiring a sample requires only a blood draw, and the potential that the pool of ctDNA is a better reflection of the relevant biology as it potentially reflects all metastatic tissues. Disadvantages are that sequencing attempts may not be productive if the sample is acquired at a time when the tumor is either quiescent or tumor burden is so low that only limited amounts of DNA are being shed. Performing ctDNA analysis when a tumor is not progressing is less likely to be productive for a number of tumor types.23,24 Sequencing ctDNA is also more susceptible than sequencing tumor biopsies to detection of alterations that are not from the tumor of interest but from clonal hematopoiesis of indeterminate potential (CHIP) or other clonal hematopoietic disorders (see Confounders section below).
Selecting the Tissue
Deciding on the tissue to analyze is a critical part of the decision process (Table 3). If the primary tumor tissue is old the likelihood of productive sequencing is lower, although age alone is not the only consideration and the methods of fixation may be just as relevant.
For prostate cancer in particular, the ability to successfully sequence primary tumor tissue decreases as the amount of tumor decreases in low-volume biopsies such as prostate needle biopsies. Generally, if tumor content is < 10% of the biopsy specimen, then sequencing is less likely to be productive.25 Also, if the alteration of interest is not known to be truncal, then a relevant target might be missed by sequencing tissue that does not reflect current biology. Metastasis biopsy may be the most appropriate tissue, particularly if this specimen has already been acquired. As above, a metastasis biopsy may have a higher tumor content, and it should reflect relevant biology if it is recent. However, bone biopsies have a relatively low yield for successful sequencing, so a soft tissue lesion (eg, liver or lymph node metastasis) is generally preferred.
The inability to safely access tissue is often a consideration. Proximity to vital structures such as large blood vessels or the potential for significant morbidity in the event of a complication (liver or lung biopsies, particularly in patients on anticoagulation medications) may make the risk/benefit ratio too high. The inability to conduct somatic testing has been reported to often be due to inadequate tissue sampling.26 ctDNA is an attractive alternative but should typically be drawn when a tumor is progressing with a reasonable tumor burden that is more likely to be shedding DNA. Performing ctDNA analysis in patients without obvious radiographic metastasis or in patients whose tumor is under good control is unlikely to produce interpretable results.
Interpreting the Results
The intent of sequencing tumor tissue is to identify alterations that are biologically important and may provide a point of therapeutic leverage. However, deciding which alterations are relevant is not always straightforward. For example, any normal individual genome contains around 10,000 missense variants, hundreds of insertion/deletion variants, and dozens of protein-truncating variants. Distinguishing these alterations, which are part of the individual, from those that are tumor-specific and have functional significance can be difficult in the absence of paired sequencing of both normal and tissue samples.
Specific Alterations
Although most commercial vendors provide important information in sequencing reports to assist oncology HCPs in deciding which alterations are relevant, the reports are not always clear. In many cases the report will specifically indicate whether the alteration has been reported previously as pathogenic or benign. However, some platforms will report alterations that are not known to be drivers of tumor biology. It is critical to be aware that if variants are not reported as pathogenic, they should not be assumed to be pathogenic simply because they are included in the report. Alterations more likely to be drivers of relevant biology are those that change gene and protein structure and include frameshift (fs*), nonsense (denoted by sequence ending in “X” or “*”), or specific fusions or insertions/deletions (indel) that occur in important domains of the gene.
For some genes, only specific alterations are targetable and not all alterations have the same effect on protein function. Although overexpression of certain genes and proteins are actionable (eg, HER2), amplification of a gene does not necessarily indicate that it is targetable. In NSCLC, specific alterations convey sensitivity to targeted therapies. For example, in EGFR-mutated NSCLC, the sensitizing mutations to EGFR TKIs are exon19 deletions and exon 21 L858R point mutations (the most common mutations), as well as less common mutations found in exon 18-21. Exon 20 mutations, however, are not responsive to EGFR TKIs with a few exceptions.27 Patients who have tumors that do not harbor a sensitizing EGFR mutation should not be treated with an EGFR TKI. In a variety of solid tumors, gene fusions of the NTRK 1/2/3, act as oncogenic drivers. The chromosomal fusion events involving the carboxy-terminal kinase domain of TRK and upstream amino-terminal partners lead to overexpression of the chimeric proteins tropomyosin receptor kinase (TRK) A/B/C, resulting in constitutively active, ligand-independent downstream signaling. In patients with NTRK 1/2/3 gene fusions, larotrectinib and entrectinib, small molecule inhibitors to TRK, have shown antitumor activity.28,29 No alterations beyond these fusions are known to be targetable.
Allele Fraction
Knowing the fraction (or proportion) of the alteration of interest in the sequenced tissue relative to the estimated tumor content can assist in decision making. Not all platforms will provide this information, which is referred to as mutation allele fraction (MAF) or variant allele fraction (VAF), but sometimes will provide it on request. Platforms will usually provide an estimate of the percent tumor in the tissue being sampled if it is from a biopsy. If the MAF is around 50% in the sequenced tissue (including ctDNA), then there is a reasonable chance that it is a germline variant. However, there are nuances as germline alterations in some genes, such as BRCA1/2, can be accompanied by loss of the other allele of the gene (LOH). In that case, if most of the circulating DNA is from tumor, then the MAF can be > 50%.
If there are 2 alterations of the same gene with MAF percentages that are each half of the total percent tumor, there is a high likelihood of biallelic alteration. These sorts of paired alterations or one mutation with apparent LOH or copy loss would again indicate a high likelihood that the alteration is in fact pathogenic and a relevant driver. Not all pathogenic alterations have to be biallelic to be driver mutations but in BRCA1/2, or mismatch repair deficiency genes, the presence of biallelic alterations increases the likelihood of their being pathogenic.
Tumors that are hypermutated—containing sometimes hundreds of mutations per megabyte of DNA—can be particularly complicated to interpret, because the likelihood increases that many of the alterations are a function of the hypermutation and not a driver mutation. This is particularly important when there are concurrent mutations in mismatch repair genes and genes, such as BRCA1/2. If the tumor is
Confounders
In some situations, interpretation can be particularly challenging. For example, several alterations for which there are FDA on-label indications (such as ATM or BRCA2) can be detected in ctDNA that may not be due to the tumor but to CHIP. CHIP represents hematopoietic clones that are dysplastic as a result of exposure to DNA-damaging agents (eg, platinum chemotherapy) or as a result of aging and arise when mutations in hematopoietic stem cells provide a competitive advantage.31 The most common CHIP clones that can be detected are DNMT3A, ASXL1, or TET2; because these alterations are not targetable, their importance lies primarily in whether patients have evidence of hematologic abnormality, which might represent an evolving hematopoietic disorder. Because CHIP alterations can overlap with somatic alterations for which FDA-approved drugs exist, such as ATM or CHEK2 (olaparib for prostate cancer) and BRCA2 (poly-ADP-ribose polymerase inhibitors in a range of indications) there is concern that CHIP might result in patient harm from inappropriate treatment of CHIP rather than the tumor, with no likelihood that the treatment would affect the tumor, causing treatment delays.32 General considerations for deciding whether an alteration represents CHIP include excluding alteration in which the VAF is < 1% and when the VAF in the alteration of interest is < 20% of the estimated tumor fraction in the sample. Exceptions to this are found in patients with true myelodysplasia or chronic lymphocytic leukemia, in whom the VAF can be well over 50% because of circulating tumor burden. The only way to be certain that an alteration detected on ctDNA reflects tumor rather than CHIP is to utilize an assay with matched tumor-normal sequencing.
Resources for Assistance
For oncology HCPs, perhaps the best resource to help in selecting and interpreting the appropriate testing is through a dedicated molecular oncology tumor board and subject matter experts who contribute to those tumor boards. In the US Department of Veterans Affairs, the national precision oncology program and its affiliated clinical services, such as the option to order a national consultation and molecular tumor board education, are easily accessible to all HCPs (www.cancer.va.gov). Many commercial vendors provide support to assist with questions of interpretation and to inform clinical decision-making. Other resources that can assist with deciding whether an alteration is pathogenic include extensive curated databases such as ClinVar (www.ncbi.nlm.nih.gov/clinvar) and the Human Genetic Mutation Database (www.hgmd.cf.ac.uk/ac/index.php) for germline alterations or COSMIC (cancer.sanger.ac.uk/cosmic) for somatic alterations. OncoKB (www.oncokb.org) is a resource for assistance in defining levels of evidence for the use of agents to target specific alterations and to assist in assigning pathogenicity to specific alterations. Additional educational resources for training in genomics and genetics are also included in the Appendix.
The rapid growth in technology and ability to enhance understanding of relevant tumor biology continues to improve the therapeutic landscape for men and women dealing with malignancy and our ability to find targetable genetic alterations with the potential for meaningful clinical benefit.
Acknowledgments
Dedicated to Neil Spector.
The ability to find and target specific biomarkers in the DNA of advanced cancers is rapidly changing options and outcomes for patients with locally advanced and metastatic solid tumors. This strategy is the basis for precision oncology, defined here as using predictive biomarkers from tumor and/or germline sequencing to guide therapies. This article focuses specifically on the use of DNA sequencing to find those biomarkers and provides guidance about which test is optimal in a specific situation, as well as interpretation of the results. We emphasize the identification of biomarkers that provide adult patients with advanced solid tumors access to therapies that would not be an option had sequencing not been performed and that have the potential for significant clinical benefit. The best approach is to have an expert team with experience in precision oncology to assist in the interpretation of results.
Which test?
Deciding what test of the array of assays available to use and which tissue to test can be overwhelming, and uncertainty may prevent oncology practitioners from ordering germline or somatic sequencing. For the purposes of this article, we will focus on DNA sequencing for inherited/germline alterations (including mutations, copy number changes, or fusions), which may inform treatment, or alterations that arise in the process of carcinogenesis and tumor evolution (somatic alterations in tumor DNA). This focus is not meant to exclude any specific test but to focus on DNA-based tests in patients with locally advanced or metastatic malignancy.
Germline Testing
Germline testing is the sequencing of inherited DNA in noncancerous cells to find alterations that may play a role in the development of cancers and are actionable in some cases. Germline alterations can inform therapeutic decisions, predict future cancer risk, and provide information that can help family members to better manage their risks of malignancy. Detailed discussions of the importance of germline testing to inform cancer surveillance, risk-reducing interventions, and the testing of relatives to determine who carries inherited alterations (cascade testing) is extremely important with several advantages and is covered in a number of excellent reviews elsewhere.1-3 Testing of germline DNA in patients with a metastatic malignancy can provide treatment options otherwise not available for patients, particularly for BRCA1/2 and Lynch syndrome–related cancers. Recent studies have shown that 10 to 15% of patients with advanced malignancies of many types have a pathogenic germline alteration.4,5
Germline DNA is usually acquired from peripheral blood, a buccal swab, or saliva collection and is therefore readily available. This is advantageous because it does not require a biopsy to identify relevant alterations. Germline testing is also less susceptible to the rare situations in which artifacts occur in formalin-fixed tissues and obscure relevant alterations.
The cost of germline testing varies, but most commercial vendor assays for germline testing are significantly less expensive than the cost of somatic testing. The disadvantages include the inability of germline testing to find any alterations that arise solely in tumor tissue and the smaller gene panels included in germline testing as compared to somatic testing panels. Other considerations relate to the inherited nature of pathogenic germline variants and its implications for family members that may affect the patient’s psychosocial health and potentially change the family dynamics.
Deciding who is appropriate for germline testing and when to perform the testing should be individualized to the patient’s wishes and disease status. Treatment planning may be less complicated if testing has been performed and germline status is known. In some cases urgent germline testing is indicated to inform pending procedures and/or surgical decisions for risk reduction, including more extensive tissue resection, such as the removal of additional organs or contralateral tissue. A minor point regarding germline testing is that the DNA of patients with hematologic malignancies may be difficult to sequence because of sample contamination by the circulating malignancy. For this reason, most laboratories will not accept peripheral blood or saliva samples for germline testing in patients with active hematologic malignancies; they often require DNA from another source such as fibroblasts from a skin biopsy or cells from a muscle biopsy. Germline testing is recommended for all patients with metastatic prostate cancer, as well as any patient with any stage of pancreatic cancer or ovarian cancer and patients with breast cancer diagnosed at age ≤ 45 years. More detailed criteria for who is appropriate for germline testing outside of these groups can be found in the appropriate National Comprehensive Cancer Network (NCCN) guidelines.6-8 In patients with some malignancies such as prostate and pancreatic cancer, approximately half of patients who have a BRCA-related cancer developed that malignancy because of a germline BRCA alteration.9-11 Testing germline DNA is therefore an easy way to quickly find almost half of all targetable alterations with a treatment approved by the US Food and Drug Administration (FDA) and at low cost, with the added benefit of providing critical information for families who may be unaware that members carry a relevant pathogenic germline alteration. In those families, cascade testing can provide surveillance and intervention strategies that can be lifesaving.
A related and particularly relevant question is when should a result found on a somatic testing panel prompt follow-up germline testing? Some institutions have algorithms in place to automate referral for germline testing based on specific genetic criteria.12 Excellent reviews are available that outline the following considerations in more detail.13 Typically, somatic testing results that would trigger follow-up germline testing would be truncating or deleterious or likely deleterious mutations per germline datasets in high-risk genes associated with highly penetrant autosomal dominant conditions (BRCA1, BRCA2, PALB2, MLH1, MSH2, and MSH6), selected moderate-risk genes (BRIP1, RAD51C, and RAD51D), and specific variants with a high probability of being germline because they are common germline founder mutations. Although the actionability and significance of specific genes remains a matter of some discussion, generally finding a somatic pathogenic sequencing result included in the 59-gene list of the American College of Medical Genetics and Genomics (ACMG) guidelines would be an indication for germline testing. Another indication for germline testing would be finding genes with germline mutations for which the NCCN has specific management guidelines, or the presence of alterations consistent with known founder mutations.14 When a patient’s tumor has microsatellite instability or is hypermutated (defined as > 10 mutations per megabase), a search for germline alterations is warranted given that about 15% of these patients with these tumors carry a Lynch syndrome gene.15 Genes that are commonly found as somatic alterations alone (eg, TP53 or APC) are generally not an indication for germline testing unless family history is compelling.
Although some clinicians use the variant allele fraction in the somatic sequencing report to decide whether to conduct germline testing, this approach is suboptimal, as allele fraction may be confounded by assay conditions and a high allele fraction may be found in pure tumors with loss of heterozygosity (LOH) of the other allele. There is also evidence that for a variety of reasons, somatic sequencing panels do not always detect germline alterations in somatic tissues.16 Reasons for this may include discordance between the genes being tested in the germline vs the somatic panel, technical differences such as interference of formalin-fixed paraffin-embedded (FFPE) artifact with detecting the germline variant, lack of expertise in germline variant interpretation among laboratories doing tumor-only sequencing, and, in rare cases, large deletions in tumor tissue masking a germline point mutation.
Variant Interpretation of Germline Testing
A general understanding of the terminology used for germline variant interpretation allows for the ordering health care practitioner (HCP) to provide the best quality care and an appreciation for the limitations of current molecular testing. Not all variants are associated with disease; the clinical significance of a genetic variant falls on a spectrum. The criteria for determining pathogenicity differ between molecular laboratories, but most are influenced by the standards and guidelines set forth by the ACMG.14 The clinical molecular laboratory determines variant classification, and a detailed discussion is beyond the scope of this primer. In brief, variant classification is based on evidence of varying strength in different categories including population data, computational and predictive data, functional data, segregation data, de novo data, allelic data, and information from various databases. The ACMG has proposed a 5-tiered classification system, by which most molecular laboratories adhere to in their genetic test reports (Table 1).14
Pathogenic and likely pathogenic variants are clinically actionable, whereas variants of uncertain significance (VUS) require additional data and/or functional studies before making clinical decisions. Depending on the clinical context and existing supporting evidence, it may be prudent to continue monitoring for worsening or new signs of disease in patients with one or more VUS while additional efforts are underway to understand the variant’s significance.
In some cases, variants are reclassified, which may alter the management and treatment of patients. Reclassification can occur with VUS, and in rare instances, can also occur with variants previously classified as pathogenic/likely pathogenic or benign/likely benign. In such a case, the reporting laboratory will typically make concerted efforts to alert the ordering HCP. However, variant reclassifications are not always communicated to the care team. Thus, it is important to periodically contact the molecular laboratory of interest to obtain updated test interpretations.
Somatic Testing
Testing of somatic (tumor) tissue is critical and is the approach most commonly taken in medical oncology (Table 2).
The advantages of primary tumor are that it is usually in hand as a diagnostic biopsy, acquisition is standard of care, and several targetable alterations are truncal, defined as driver mutations present at the time of tumor development. Also, the potential that the tumor arose in the background of a predisposing germline alteration can be suggested by sequencing primary tumor as discussed above. Moreover, sequencing the primary tumor can be done at any time unless the biopsy sample is considered too old or degraded (per specific platform requirements). The information gained can be used to anticipate additional treatment options that are relevant when patients experience disease progression. Disadvantages include the problem that primary specimens may be old or have limited tumor content, both of which increase the likelihood that sequencing will not be technically successful.
Alterations that are targetable and arise as a result of either treatment pressure or clonal evolution are considered evolutionary. If evolutionary alterations are the main focus for sequencing, then metastasis biopsy or ctDNA are better choices. The advantages of a metastasis biopsy are that tissue is contemporary, tumor content may be higher than in primary tumor, and both truncal and evolutionary alterations can be detected.
For specific tumors, continued analysis of evolving genomic alterations can play a critical role in management. In non–small cell lung cancer (NSCLC), somatic testing is conducted again at progression on repeat biopsies to evaluate for emerging resistance mutations. In epidermal growth factor receptor (EGFR)–mutated lung cancer, the resistance mutation, exon 20 p.T790M (point mutation), can present in patients after treatment with first- or second-generation EGFR tyrosine kinase inhibitors (TKI). Even in patients who are treated with the third-generation EGFR TKI osimertinib that can treat T790M-mutated lung cancer, multiple possible evolutionary mutations can occur at progression, including other EGFR mutations, MET/HER2 amplification, and BRAF V600E, to name a few.20 Resistance mechanisms develop due to treatment selection pressure and the molecular heterogeneity seen in lung cancer.
Disadvantages for metastatic biopsy include the inability to safely access a metastatic site, the time considerations for preauthorization and arrangement of biopsy, and a lower-than-average likelihood of successful sequencing from sites such as bone.21,22 In addition, there is some concern that a single metastatic site may not capture all relevant alterations for multiple reasons, including tumor heterogeneity.
Significant advances in the past decade have dramatically improved the ability to use ctDNA to guide therapy. Advantages include ease of acquisition as acquiring a sample requires only a blood draw, and the potential that the pool of ctDNA is a better reflection of the relevant biology as it potentially reflects all metastatic tissues. Disadvantages are that sequencing attempts may not be productive if the sample is acquired at a time when the tumor is either quiescent or tumor burden is so low that only limited amounts of DNA are being shed. Performing ctDNA analysis when a tumor is not progressing is less likely to be productive for a number of tumor types.23,24 Sequencing ctDNA is also more susceptible than sequencing tumor biopsies to detection of alterations that are not from the tumor of interest but from clonal hematopoiesis of indeterminate potential (CHIP) or other clonal hematopoietic disorders (see Confounders section below).
Selecting the Tissue
Deciding on the tissue to analyze is a critical part of the decision process (Table 3). If the primary tumor tissue is old the likelihood of productive sequencing is lower, although age alone is not the only consideration and the methods of fixation may be just as relevant.
For prostate cancer in particular, the ability to successfully sequence primary tumor tissue decreases as the amount of tumor decreases in low-volume biopsies such as prostate needle biopsies. Generally, if tumor content is < 10% of the biopsy specimen, then sequencing is less likely to be productive.25 Also, if the alteration of interest is not known to be truncal, then a relevant target might be missed by sequencing tissue that does not reflect current biology. Metastasis biopsy may be the most appropriate tissue, particularly if this specimen has already been acquired. As above, a metastasis biopsy may have a higher tumor content, and it should reflect relevant biology if it is recent. However, bone biopsies have a relatively low yield for successful sequencing, so a soft tissue lesion (eg, liver or lymph node metastasis) is generally preferred.
The inability to safely access tissue is often a consideration. Proximity to vital structures such as large blood vessels or the potential for significant morbidity in the event of a complication (liver or lung biopsies, particularly in patients on anticoagulation medications) may make the risk/benefit ratio too high. The inability to conduct somatic testing has been reported to often be due to inadequate tissue sampling.26 ctDNA is an attractive alternative but should typically be drawn when a tumor is progressing with a reasonable tumor burden that is more likely to be shedding DNA. Performing ctDNA analysis in patients without obvious radiographic metastasis or in patients whose tumor is under good control is unlikely to produce interpretable results.
Interpreting the Results
The intent of sequencing tumor tissue is to identify alterations that are biologically important and may provide a point of therapeutic leverage. However, deciding which alterations are relevant is not always straightforward. For example, any normal individual genome contains around 10,000 missense variants, hundreds of insertion/deletion variants, and dozens of protein-truncating variants. Distinguishing these alterations, which are part of the individual, from those that are tumor-specific and have functional significance can be difficult in the absence of paired sequencing of both normal and tissue samples.
Specific Alterations
Although most commercial vendors provide important information in sequencing reports to assist oncology HCPs in deciding which alterations are relevant, the reports are not always clear. In many cases the report will specifically indicate whether the alteration has been reported previously as pathogenic or benign. However, some platforms will report alterations that are not known to be drivers of tumor biology. It is critical to be aware that if variants are not reported as pathogenic, they should not be assumed to be pathogenic simply because they are included in the report. Alterations more likely to be drivers of relevant biology are those that change gene and protein structure and include frameshift (fs*), nonsense (denoted by sequence ending in “X” or “*”), or specific fusions or insertions/deletions (indel) that occur in important domains of the gene.
For some genes, only specific alterations are targetable and not all alterations have the same effect on protein function. Although overexpression of certain genes and proteins are actionable (eg, HER2), amplification of a gene does not necessarily indicate that it is targetable. In NSCLC, specific alterations convey sensitivity to targeted therapies. For example, in EGFR-mutated NSCLC, the sensitizing mutations to EGFR TKIs are exon19 deletions and exon 21 L858R point mutations (the most common mutations), as well as less common mutations found in exon 18-21. Exon 20 mutations, however, are not responsive to EGFR TKIs with a few exceptions.27 Patients who have tumors that do not harbor a sensitizing EGFR mutation should not be treated with an EGFR TKI. In a variety of solid tumors, gene fusions of the NTRK 1/2/3, act as oncogenic drivers. The chromosomal fusion events involving the carboxy-terminal kinase domain of TRK and upstream amino-terminal partners lead to overexpression of the chimeric proteins tropomyosin receptor kinase (TRK) A/B/C, resulting in constitutively active, ligand-independent downstream signaling. In patients with NTRK 1/2/3 gene fusions, larotrectinib and entrectinib, small molecule inhibitors to TRK, have shown antitumor activity.28,29 No alterations beyond these fusions are known to be targetable.
Allele Fraction
Knowing the fraction (or proportion) of the alteration of interest in the sequenced tissue relative to the estimated tumor content can assist in decision making. Not all platforms will provide this information, which is referred to as mutation allele fraction (MAF) or variant allele fraction (VAF), but sometimes will provide it on request. Platforms will usually provide an estimate of the percent tumor in the tissue being sampled if it is from a biopsy. If the MAF is around 50% in the sequenced tissue (including ctDNA), then there is a reasonable chance that it is a germline variant. However, there are nuances as germline alterations in some genes, such as BRCA1/2, can be accompanied by loss of the other allele of the gene (LOH). In that case, if most of the circulating DNA is from tumor, then the MAF can be > 50%.
If there are 2 alterations of the same gene with MAF percentages that are each half of the total percent tumor, there is a high likelihood of biallelic alteration. These sorts of paired alterations or one mutation with apparent LOH or copy loss would again indicate a high likelihood that the alteration is in fact pathogenic and a relevant driver. Not all pathogenic alterations have to be biallelic to be driver mutations but in BRCA1/2, or mismatch repair deficiency genes, the presence of biallelic alterations increases the likelihood of their being pathogenic.
Tumors that are hypermutated—containing sometimes hundreds of mutations per megabyte of DNA—can be particularly complicated to interpret, because the likelihood increases that many of the alterations are a function of the hypermutation and not a driver mutation. This is particularly important when there are concurrent mutations in mismatch repair genes and genes, such as BRCA1/2. If the tumor is
Confounders
In some situations, interpretation can be particularly challenging. For example, several alterations for which there are FDA on-label indications (such as ATM or BRCA2) can be detected in ctDNA that may not be due to the tumor but to CHIP. CHIP represents hematopoietic clones that are dysplastic as a result of exposure to DNA-damaging agents (eg, platinum chemotherapy) or as a result of aging and arise when mutations in hematopoietic stem cells provide a competitive advantage.31 The most common CHIP clones that can be detected are DNMT3A, ASXL1, or TET2; because these alterations are not targetable, their importance lies primarily in whether patients have evidence of hematologic abnormality, which might represent an evolving hematopoietic disorder. Because CHIP alterations can overlap with somatic alterations for which FDA-approved drugs exist, such as ATM or CHEK2 (olaparib for prostate cancer) and BRCA2 (poly-ADP-ribose polymerase inhibitors in a range of indications) there is concern that CHIP might result in patient harm from inappropriate treatment of CHIP rather than the tumor, with no likelihood that the treatment would affect the tumor, causing treatment delays.32 General considerations for deciding whether an alteration represents CHIP include excluding alteration in which the VAF is < 1% and when the VAF in the alteration of interest is < 20% of the estimated tumor fraction in the sample. Exceptions to this are found in patients with true myelodysplasia or chronic lymphocytic leukemia, in whom the VAF can be well over 50% because of circulating tumor burden. The only way to be certain that an alteration detected on ctDNA reflects tumor rather than CHIP is to utilize an assay with matched tumor-normal sequencing.
Resources for Assistance
For oncology HCPs, perhaps the best resource to help in selecting and interpreting the appropriate testing is through a dedicated molecular oncology tumor board and subject matter experts who contribute to those tumor boards. In the US Department of Veterans Affairs, the national precision oncology program and its affiliated clinical services, such as the option to order a national consultation and molecular tumor board education, are easily accessible to all HCPs (www.cancer.va.gov). Many commercial vendors provide support to assist with questions of interpretation and to inform clinical decision-making. Other resources that can assist with deciding whether an alteration is pathogenic include extensive curated databases such as ClinVar (www.ncbi.nlm.nih.gov/clinvar) and the Human Genetic Mutation Database (www.hgmd.cf.ac.uk/ac/index.php) for germline alterations or COSMIC (cancer.sanger.ac.uk/cosmic) for somatic alterations. OncoKB (www.oncokb.org) is a resource for assistance in defining levels of evidence for the use of agents to target specific alterations and to assist in assigning pathogenicity to specific alterations. Additional educational resources for training in genomics and genetics are also included in the Appendix.
The rapid growth in technology and ability to enhance understanding of relevant tumor biology continues to improve the therapeutic landscape for men and women dealing with malignancy and our ability to find targetable genetic alterations with the potential for meaningful clinical benefit.
Acknowledgments
Dedicated to Neil Spector.
1. Domchek SM, Mardis E, Carlisle JW, Owonikoko TK. Integrating genetic and genomic testing into oncology practice. Am Soc Clin Oncol Educ Book. 2020;40:e259-e263. doi:10.1200/EDBK_280607
2. Stoffel EM, Carethers JM. Current approaches to germline cancer genetic testing. Annu Rev Med. 2020;71:85-102. doi:10.1146/annurev-med-052318-101009
3. Lappalainen T, Scott AJ, Brandt M, Hall IM. Genomic analysis in the age of human genome sequencing. Cell. 2019;177(1):70-84. doi:10.1016/j.cell.2019.02.032
4. Samadder NJ, Riegert-Johnson D, Boardman L, et al. Comparison of universal genetic testing vs guideline-directed targeted testing for patients with hereditary cancer syndrome. JAMA Oncol. 2021;7(2):230-237. doi:10.1001/jamaoncol.2020.6252
5. Schneider BP, Stout L, Philips S, et al. Implications of incidental germline findings identified in the context of clinical whole exome sequencing for guiding cancer therapy. JCO Precis Oncol. 2020;4:1109-1121. doi:10.1200/PO.19.00354
6. National Comprehensive Cancer Network. Pancreatic cancer (Version 1.2022). Updated February 24, 2022. Accessed April 13, 2022. https://www.nccn.org/professionals/physician_gls/pdf/pancreatic.pdf
7. National Comprehensive Cancer Network. Prostate cancer (Version 3.2022). Updated January 10, 2022. Accessed April 13, 2022. https://www.nccn.org/professionals/physician_gls/pdf/prostate.pdf
8. National Comprehensive Cancer Network. Genetic/familial high-risk assessment: breast, ovarian, and pancreatic (Version 2.2022). Updated March 9, 2022. Accessed April 13, 2022. https://www.nccn.org/professionals/physician_gls/pdf/genetics_bop.pdf
9. Robinson D, Van Allen EM, Wu YM, et al. Integrative clinical genomics of advanced prostate cancer. Cell. 2015;161(5):1215-1228. doi:10.1016/j.cell.2015.05.001
10. Pritchard CC, Mateo J, Walsh MF, et al. Inherited DNA-repair gene mutations in men with metastatic prostate cancer. N Engl J Med. 2016;375(5):443-453. doi:10.1056/NEJMoa1603144
11. Cancer Genome Atlas Research Network. Integrated genomic characterization of pancreatic ductal adenocarcinoma. Cancer Cell. 2017;32(2):185-203.e13. doi:10.1016/j.ccell.2017.07.007
12. Clark DF, Maxwell KN, Powers J, et al. Identification and confirmation of potentially actionable germline mutations in tumor-only genomic sequencing. JCO Precis Oncol. 2019;3:PO.19.00076. doi:10.1200/PO.19.00076
13. DeLeonardis K, Hogan L, Cannistra SA, Rangachari D, Tung N. When should tumor genomic profiling prompt consideration of germline testing? J Oncol Pract. 2019;15(9):465-473. doi:10.1200/JOP.19.00201
14. Richards S, Aziz N, Bale S, et al. Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet Med. 2015;17(5):405-424. doi:10.1038/gim.2015.30
15. Latham A, Srinivasan P, Kemel Y, et al. Microsatellite instability is associated with the presence of Lynch syndrome pan-cancer. J Clin Oncol. 2019;37(4):286-295. doi:10.1200/JCO.18.00283
16. Lincoln SE, Nussbaum RL, Kurian AW, et al. Yield and utility of germline testing following tumor sequencing in patients with cancer. JAMA Netw Open. 2020;3(10):e2019452. doi:10.1001/jamanetworkopen.2020.19452
17. National Comprehensive Cancer Network. Non-small cell lung cancer (Version: 3.2022). Updated March 16, 2022. Accessed April 13, 2022. https://www.nccn.org/professionals/physician_gls/pdf/nscl.pdf
18. National Comprehensive Cancer Network. Colon cancer (Version 1.2022). February 25, 2022. Accessed April 13, 2022. https://www.nccn.org/professionals/physician_gls/pdf/colon.pdf
19. National Comprehensive Cancer Network. Melanoma: cutaneous (Version 3.2022). April 11, 2022. Accessed April 13, 2022. https://www.nccn.org/professionals/physician_gls/pdf/cutaneous_melanoma.pdf
20. Leonetti A, Sharma S, Minari R, Perego P, Giovannetti E, Tiseo M. Resistance mechanisms to osimertinib in EGFR-mutated non-small cell lung cancer. Br J Cancer. 2019;121(9):725-737. doi:10.1038/s41416-019-0573-8
21. Zheng G, Lin MT, Lokhandwala PM, et al. Clinical mutational profiling of bone metastases of lung and colon carcinoma and malignant melanoma using next-generation sequencing. Cancer Cytopathol. 2016;124(10):744-753. doi:10.1002/cncy.21743
22. Spritzer CE, Afonso PD, Vinson EN, et al. Bone marrow biopsy: RNA isolation with expression profiling in men with metastatic castration-resistant prostate cancer—factors affecting diagnostic success. Radiology. 2013;269(3):816-823. doi:10.1148/radiol.13121782
23. Schweizer MT, Gulati R, Beightol M, et al. Clinical determinants for successful circulating tumor DNA analysis in prostate cancer. Prostate. 2019;79(7):701-708. doi:10.1002/pros.23778
24. Bettegowda C, Sausen M, Leary RJ, et al. Detection of circulating tumor DNA in early- and late-stage human malignancies. Sci Transl Med. 2014;6(224):224ra224. doi:10.1126/scitranslmed.3007094
25. Pritchard CC, Salipante SJ, Koehler K, et al. Validation and implementation of targeted capture and sequencing for the detection of actionable mutation, copy number variation, and gene rearrangement in clinical cancer specimens. J Mol Diagn. 2014;16(1):56-67. doi:10.1016/j.jmoldx.2013.08.004
26. Gutierrez ME, Choi K, Lanman RB, et al. Genomic profiling of advanced non-small cell lung cancer in community settings: gaps and opportunities. Clin Lung Cancer. 2017;18(6):651-659. doi:10.1016/j.cllc.2017.04.004
27. Malapelle U, Pilotto S, Passiglia F, et al. Dealing with NSCLC EGFR mutation testing and treatment: a comprehensive review with an Italian real-world perspective. Crit Rev Oncol Hematol. 2021;160:103300. doi:10.1016/j.critrevonc.2021.103300
28. Drilon A, Laetsch TW, Kummar S, et al. Efficacy of larotrectinib in TRK fusion-positive cancers in adults and children. N Engl J Med. 2018;378(8):731-739. doi:10.1056/NEJMoa1714448
29. Doebele RC, Drilon A, Paz-Ares L, et al. Entrectinib in patients with advanced or metastatic NTRK fusion-positive solid tumours: integrated analysis of three phase 1-2 trials. Lancet Oncol. 2020;21(2):271-282. doi:10.1016/S1470-2045(19)30691-6
30. Jonsson P, Bandlamudi C, Cheng ML, et al. Tumour lineage shapes BRCA-mediated phenotypes. Nature. 2019;571(7766):576-579. doi:10.1038/s41586-019-1382-1
31. Steensma DP. Clinical consequences of clonal hematopoiesis of indeterminate potential. Hematology Am Soc Hematol Educ Program. 2018;2018(1):264-269. doi:10.1182/asheducation-2018.1.264
32. Jensen K, Konnick EQ, Schweizer MT, et al. Association of clonal hematopoiesis in DNA repair genes with prostate cancer plasma cell-free DNA testing interference. JAMA Oncol. 2021;7(1):107-110. doi:10.1001/jamaoncol.2020.5161
1. Domchek SM, Mardis E, Carlisle JW, Owonikoko TK. Integrating genetic and genomic testing into oncology practice. Am Soc Clin Oncol Educ Book. 2020;40:e259-e263. doi:10.1200/EDBK_280607
2. Stoffel EM, Carethers JM. Current approaches to germline cancer genetic testing. Annu Rev Med. 2020;71:85-102. doi:10.1146/annurev-med-052318-101009
3. Lappalainen T, Scott AJ, Brandt M, Hall IM. Genomic analysis in the age of human genome sequencing. Cell. 2019;177(1):70-84. doi:10.1016/j.cell.2019.02.032
4. Samadder NJ, Riegert-Johnson D, Boardman L, et al. Comparison of universal genetic testing vs guideline-directed targeted testing for patients with hereditary cancer syndrome. JAMA Oncol. 2021;7(2):230-237. doi:10.1001/jamaoncol.2020.6252
5. Schneider BP, Stout L, Philips S, et al. Implications of incidental germline findings identified in the context of clinical whole exome sequencing for guiding cancer therapy. JCO Precis Oncol. 2020;4:1109-1121. doi:10.1200/PO.19.00354
6. National Comprehensive Cancer Network. Pancreatic cancer (Version 1.2022). Updated February 24, 2022. Accessed April 13, 2022. https://www.nccn.org/professionals/physician_gls/pdf/pancreatic.pdf
7. National Comprehensive Cancer Network. Prostate cancer (Version 3.2022). Updated January 10, 2022. Accessed April 13, 2022. https://www.nccn.org/professionals/physician_gls/pdf/prostate.pdf
8. National Comprehensive Cancer Network. Genetic/familial high-risk assessment: breast, ovarian, and pancreatic (Version 2.2022). Updated March 9, 2022. Accessed April 13, 2022. https://www.nccn.org/professionals/physician_gls/pdf/genetics_bop.pdf
9. Robinson D, Van Allen EM, Wu YM, et al. Integrative clinical genomics of advanced prostate cancer. Cell. 2015;161(5):1215-1228. doi:10.1016/j.cell.2015.05.001
10. Pritchard CC, Mateo J, Walsh MF, et al. Inherited DNA-repair gene mutations in men with metastatic prostate cancer. N Engl J Med. 2016;375(5):443-453. doi:10.1056/NEJMoa1603144
11. Cancer Genome Atlas Research Network. Integrated genomic characterization of pancreatic ductal adenocarcinoma. Cancer Cell. 2017;32(2):185-203.e13. doi:10.1016/j.ccell.2017.07.007
12. Clark DF, Maxwell KN, Powers J, et al. Identification and confirmation of potentially actionable germline mutations in tumor-only genomic sequencing. JCO Precis Oncol. 2019;3:PO.19.00076. doi:10.1200/PO.19.00076
13. DeLeonardis K, Hogan L, Cannistra SA, Rangachari D, Tung N. When should tumor genomic profiling prompt consideration of germline testing? J Oncol Pract. 2019;15(9):465-473. doi:10.1200/JOP.19.00201
14. Richards S, Aziz N, Bale S, et al. Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet Med. 2015;17(5):405-424. doi:10.1038/gim.2015.30
15. Latham A, Srinivasan P, Kemel Y, et al. Microsatellite instability is associated with the presence of Lynch syndrome pan-cancer. J Clin Oncol. 2019;37(4):286-295. doi:10.1200/JCO.18.00283
16. Lincoln SE, Nussbaum RL, Kurian AW, et al. Yield and utility of germline testing following tumor sequencing in patients with cancer. JAMA Netw Open. 2020;3(10):e2019452. doi:10.1001/jamanetworkopen.2020.19452
17. National Comprehensive Cancer Network. Non-small cell lung cancer (Version: 3.2022). Updated March 16, 2022. Accessed April 13, 2022. https://www.nccn.org/professionals/physician_gls/pdf/nscl.pdf
18. National Comprehensive Cancer Network. Colon cancer (Version 1.2022). February 25, 2022. Accessed April 13, 2022. https://www.nccn.org/professionals/physician_gls/pdf/colon.pdf
19. National Comprehensive Cancer Network. Melanoma: cutaneous (Version 3.2022). April 11, 2022. Accessed April 13, 2022. https://www.nccn.org/professionals/physician_gls/pdf/cutaneous_melanoma.pdf
20. Leonetti A, Sharma S, Minari R, Perego P, Giovannetti E, Tiseo M. Resistance mechanisms to osimertinib in EGFR-mutated non-small cell lung cancer. Br J Cancer. 2019;121(9):725-737. doi:10.1038/s41416-019-0573-8
21. Zheng G, Lin MT, Lokhandwala PM, et al. Clinical mutational profiling of bone metastases of lung and colon carcinoma and malignant melanoma using next-generation sequencing. Cancer Cytopathol. 2016;124(10):744-753. doi:10.1002/cncy.21743
22. Spritzer CE, Afonso PD, Vinson EN, et al. Bone marrow biopsy: RNA isolation with expression profiling in men with metastatic castration-resistant prostate cancer—factors affecting diagnostic success. Radiology. 2013;269(3):816-823. doi:10.1148/radiol.13121782
23. Schweizer MT, Gulati R, Beightol M, et al. Clinical determinants for successful circulating tumor DNA analysis in prostate cancer. Prostate. 2019;79(7):701-708. doi:10.1002/pros.23778
24. Bettegowda C, Sausen M, Leary RJ, et al. Detection of circulating tumor DNA in early- and late-stage human malignancies. Sci Transl Med. 2014;6(224):224ra224. doi:10.1126/scitranslmed.3007094
25. Pritchard CC, Salipante SJ, Koehler K, et al. Validation and implementation of targeted capture and sequencing for the detection of actionable mutation, copy number variation, and gene rearrangement in clinical cancer specimens. J Mol Diagn. 2014;16(1):56-67. doi:10.1016/j.jmoldx.2013.08.004
26. Gutierrez ME, Choi K, Lanman RB, et al. Genomic profiling of advanced non-small cell lung cancer in community settings: gaps and opportunities. Clin Lung Cancer. 2017;18(6):651-659. doi:10.1016/j.cllc.2017.04.004
27. Malapelle U, Pilotto S, Passiglia F, et al. Dealing with NSCLC EGFR mutation testing and treatment: a comprehensive review with an Italian real-world perspective. Crit Rev Oncol Hematol. 2021;160:103300. doi:10.1016/j.critrevonc.2021.103300
28. Drilon A, Laetsch TW, Kummar S, et al. Efficacy of larotrectinib in TRK fusion-positive cancers in adults and children. N Engl J Med. 2018;378(8):731-739. doi:10.1056/NEJMoa1714448
29. Doebele RC, Drilon A, Paz-Ares L, et al. Entrectinib in patients with advanced or metastatic NTRK fusion-positive solid tumours: integrated analysis of three phase 1-2 trials. Lancet Oncol. 2020;21(2):271-282. doi:10.1016/S1470-2045(19)30691-6
30. Jonsson P, Bandlamudi C, Cheng ML, et al. Tumour lineage shapes BRCA-mediated phenotypes. Nature. 2019;571(7766):576-579. doi:10.1038/s41586-019-1382-1
31. Steensma DP. Clinical consequences of clonal hematopoiesis of indeterminate potential. Hematology Am Soc Hematol Educ Program. 2018;2018(1):264-269. doi:10.1182/asheducation-2018.1.264
32. Jensen K, Konnick EQ, Schweizer MT, et al. Association of clonal hematopoiesis in DNA repair genes with prostate cancer plasma cell-free DNA testing interference. JAMA Oncol. 2021;7(1):107-110. doi:10.1001/jamaoncol.2020.5161
2022 Update on cervical disease
Cervical cancer is an important global health problem with an estimated 604,127 new cases and 341,831 deaths in 2020.1 Nearly 85% of the disease burden affects individuals from low and middle-income countries. The World Health Organization (WHO) set forth the goal for all countries to reach and maintain an incidence rate of below 4 per 100,000 women by 2030 as part of the Global Strategy to Accelerate the Elimination of Cervical Cancer.
Although traditional Pap cytology has been the cornerstone of screening programs, its poor sensitivity of approximately 50% and limitations in accessibility require new strategies to achieve the elimination of cervical cancer.2 The discovery that persistent infection with oncogenic human papillomavirus (HPV) is an essential step in the development of cervical cancer led to the development of diagnostic HPV tests, which have higher sensitivity than cytology (96.1% vs 53.0%) but somewhat lower specificity (90.7% vs 96.3%) for the detection of cervical intraepithelial neoplasia (CIN) 2 or worse lesions.2 Initially, HPV testing was incorporated as a method to triage atypical squamous cells of undetermined significance (ASCUS) cytology results.3 Later, the concept of cotesting with cytology emerged,4,5 and since then, several clinical trials have demonstrated the effectiveness of primary HPV screening.6-9
In 2020, the WHO recommended HPV DNA testing as the primary screening method starting at the age of 30 years, with regular testing every 5 to 10 years, for the general population.10 Currently, primary HPV has been adopted in multiple countries, including Australia, the Netherlands, Turkey, England, and Argentina.
In the United States, there are 3 currently acceptable screening strategies: cytology, cytology plus HPV (cotesting), and primary HPV testing (TABLE). The American Cancer Society (ACS) specifically states that HPV testing alone every 5 years is preferred starting at age 25 years; cotesting every 5 years or cytology alone every 3 years are also acceptable.11 The US Preventive Services Task Force (USPSTF) states that cytology alone every 3 years starting at 21 years and then HPV testing alone or cotesting every 5 years or cytology every 3 years starting at age 30 are all acceptable strategies.12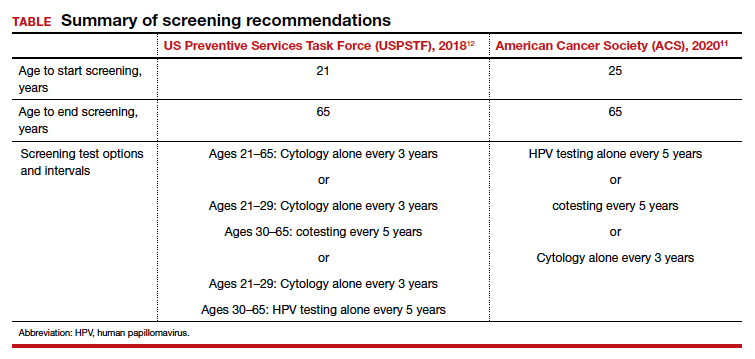
When applying these guidelines, it is important to note that they are intended for the screening of patients with all prior normal results with no symptoms. These routine screening guidelines do not apply to special populations, such as those with a history of abnormal results or treatment, a history of immunosuppression,13 a history of HPV-related vulvar or vaginal dysplasia,14-16 or a history of hysterectomy with removal of the cervix and no prior history of cervical dysplasia.17,18 By contrast, surveillance is interval testing for those who have either an abnormal prior test result or treatment; these may be managed per risk-based estimates provided by the American Society for Colposcopy and Cervical Pathology (ASCCP).18,19 Finally, diagnosis is evaluation (which may include diagnostic cytology) of a patient with abnormal signs and/or symptoms (such as bleeding, pain, discharge, or cervical mass).
In this Update, we present the evidence for primary HPV testing, the management options for a positive result in the United States, and research that will improve uptake of primary HPV testing as well as accessibility.
Change in screening paradigm: Evidence for primary HPV testing
HPV DNA tests are multiplex assays that detect the DNA of targeted high-risk HPV types, using multiple probes, either by direct genomic detection or by amplification of a viral DNA fragment using polymerase chain reaction (PCR).20,21 Alternatively, HPV mRNA-based tests detect the expression of E6 and E7 oncoproteins, a marker of viral integration.20 In examining the data from well-conducted clinical trials, 2 important observations are that different HPV assays were used and that direct comparison may not be valid. In addition, not all tests used in the studies are approved by the US Food and Drug Administration (FDA) for primary HPV testing.
Continue to: FDA-approved HPV tests...
FDA-approved HPV tests
Currently, 2 tests are FDA approved for primary HPV screening. The Cobas HPV test (Roche Molecular Diagnostics) was the first FDA-approved test for primary HPV screening in women aged 25 years and older.6 This test reports pooled results from 12 high-risk (hr) HPV types (31/33/35/39/45/51/52/56/58/59/66/68) with reflex genotyping for HPV 16/18, and thus it provides an immediate triage option for HPV-positive women. Of note, it is also approved for cotesting. The second FDA-approved test is the BD Onclarity HPV assay (Becton, Dickinson and Company) for primary HPV screening.22 It detects 14 hrHPV types, types 16/18/45 specifically as well as types 31/33/35/39/51/52/56/58/59/66/68.
Other HPV tests are FDA approved for cotesting and reflex testing but not for primary HPV testing. The Hybrid Capture test, or HC2 (Qiagen Inc), was the first HPV test to be approved by the FDA in 1997 for reflex testing of women with ASCUS cytology. In 2003, it was approved for cotesting along with cytology in women aged 30 years and older.20,21 In 2009, the Cervista HPV HR test (Hologic Inc) was approved for cotesting. The Aptima HPV assay (Hologic Inc), which is also approved for cotesting, is an RNA-based assay that allows detection of E6/E7 mRNA transcripts of 14 HPV types.23
Comparing HPV testing with cytology
Ronco and colleagues pooled data from 4 European randomized controlled trials (RCTs)—Swedescreen, POBASCAM, NTCC, ARTISTIC—with a total of 176,464 participants randomly assigned to HPV or cytology screening.24 Swedescreen and POBASCAM used GP5/GP6 PCR, while ARTISTIC and NTCC used HC2 for primary HPV screening. The screening interval was 3 years in all except 5 years in POBASCAM. The pooled detection rate of invasive disease was similar in the 2 arms, with pooled rate ratio for cancer detection being 0.79 (95% confidence interval [CI], 0.46–1.36) in the first 2.5 years, but was 0.45 (95% CI, 0.25–0.81), favoring the HPV arm, after 2.5 years. HPV testing was more effective in preventing cases of adenocarcinoma than squamous cell carcinoma (0.31 [95% CI, 0.14–0.69] vs 0.78 [95% CI, 0.49–1.25]). The authors concluded that HPV-based screening from age 30 years provided 60% to 70% better protection than cytology.
The result of the above meta-analysis was confirmed by the HPV FOCAL RCT that investigated the efficacy of HPV testing (HC2) in comparison with cytology.25 The detection rates for CIN 3 lesions supported primary HPV screening, with an absolute difference in incidence rate of 2.67/1,000 (95% CI, 0.53–4.88) at study randomization and 3.22/1,000 (95% CI, 5.12–1.48) at study exit 4 years later.
Cotesting using HPV and cytology: Marginal benefit
Dillner and colleagues were one of the first groups to report on the risk of CIN 3 based on both HPV and cytology status.26 Using pooled analysis of data from multiple countries, these investigators reported that the cumulative incidence rates (CIR) of CIN 3 after 6 years of follow-up increased consistently in HPV-positive subjects, and an HPV-positive result more accurately predicted CIN 3+ at 5 years than cytology alone. Furthermore, HPV negativity provided greater reassurance than cytology alone. At 5 years of follow-up, the rates of CIN 3+ were 0.25% (0.12%–0.41%) for women negative for HPV compared with 0.83% (0.50%–1.13%) for women with negative cytology results. There was little difference in rates for CIN 3+ between women with negative results on both tests and women who were negative for HPV.
The important question is then the marginal benefit of cotesting, which is the most costly screening option. A study of 331,818 women enrolled for cotesting at Kaiser Permanente found that the risk of CIN 3+ predicted by HPV testing alone when compared with cytology was significantly higher at both 3 years (5.0% vs 3.8%; P = .046) and 5 years (7.6% vs 4.7%; P = .001).27 A negative cytology result did not decrease the risk of CIN 3 further for HPV-negative patients (3 years: 0.047% vs 0.063%, P = .6; 5 years: 0.16% vs 0.17%, P = .8). They concluded that a negative HPV test was enough reassurance for low risk of CIN 3+ and that an additional negative cytology result does not provide extra reassurance.
Furthermore, a systematic meta-analysis of 48 studies, including 8 RCTs, found that the addition of cytology to HPV testing raised the sensitivity by 2% for CIN 3 compared with HPV testing alone. This improvement in sensitivity was at the expense of considerable loss of specificity, with a ratio of 0.93 (95% CI, 0.92–0.95) for CIN 3.28 Schiffman and colleagues also assessed the relative contribution of HPV testing and cytology in detection of CIN 3 and cancer.29 The HPV component alone identified a significantly higher proportion of preinvasive and invasive disease than cytology. Only 3.5% of precancers and 5.9% of cancers were preceded by HPV-negative, cytology-positive results. Thus, cytology contributed only 5 cases per million women per year to the sensitivity of the combined test, at the cost of significantly more colposcopies. Hence, the evidence suggests that there is limited benefit of adding cytology to HPV testing.30
Continue to: Triage of a positive HPV result...
Triage of a positive HPV result
An important limitation of HPV testing is its inability to discriminate between transient and persistent infections. Referral of all HPV-positive cases to colposcopy would overburden the system with associated unnecessary procedures. Hence, a triage strategy is essential to identify clinically important infections that truly require colposcopic evaluation. The FIGURE illustrates the management of a primary HPV test result performed for screening.
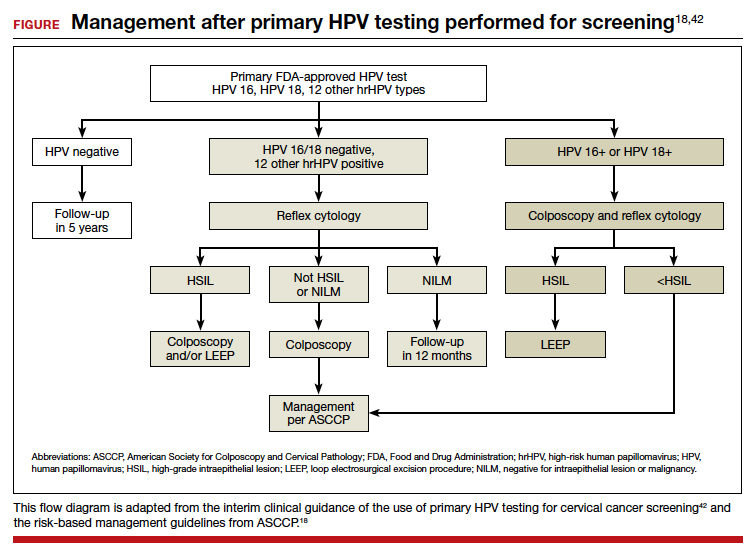
HPV genotyping
One strategy for triaging a positive HPV test result is genotyping. HPV 16 and 18 have the highest risk of persistence and progression and merit immediate referral to colposcopy. In the ATHENA trial, CIN 3 was identified in 17.8% (95% CI, 14.8–20.7%) of HPV 16 positive women at baseline, and the CIR increased to 25.2% (95% CI, 21.7–28.7%) after 3 years. The 3-year CIR of CIN 3 was only 5.4% (95% CI, 4.5–6.3%) in women with HPV genotypes other than 16/18. HPV 18–positive women had a 3-year CIR that was intermediate between women with HPV 16 and women with the 12 other genotypes.6 Hence, HPV 16/18–positive cases should be referred for immediate colposcopy, and negative cases should be followed up with cytology and referred for colposcopy if the cytology is ASCUS or worse.31
In July 2020, extended genotyping was approved by the FDA with individual detection of HPV 31, 51, 52 (in addition to 16, 18, and 45) and pooled detection of 33/58, 35/39/68, and 56/59/66. One study found that individual genotypes HPV 16 and 31 carry baseline risk values for CIN 3+ (8.1% and 7.5%, respectively) that are above the 5-year risk threshold for referral to colposcopy following the ASCCP risk-based management guideline.32
Cytology
The higher specificity of cytology makes it an option for triaging HPV-positive cases, and current management guidelines recommend triage to both genotyping and cytology for all patients who are HPV positive, and especially if they are HPV positive but HPV 16/18 negative. Of note, cytology results remain more subjective than those of primary HPV testing, but the combination of initial HPV testing with reflex to cytology is a reasonable and cost effective next step.18 The VASCAR trial found higher colposcopy referrals in the HPV screening and cytology triage group compared with the cytology alone group (19.36 vs 14.54 per 1,000 women).33 The ATHENA trial investigated various triage strategies for HPV-positive cases and its impact on colposcopy referrals.6 Using HPV genotyping and reflex cytology, if HPV 16/18 was positive, colposcopy was advised, but if any of the other 12 HPV types were positive, reflex cytology was done. If reported as ASCUS or worse, colposcopy was performed; conversely, if it was normal, women were rescreened with cotesting after 1 year. Although this strategy led to a reduction in the number of colposcopies, referrals were still higher in the primary HPV arm (3,769 colposcopies per 294 cases) compared with cytology (1,934 colposcopies per 179 cases) or cotesting (3,097 colposcopies per 240 cases) in women aged 25 years.14
p16/Ki-67 Dual-Stain
Diffused p16 immunohistochemical staining, as opposed to focal staining, is associated with active HPV infection but can be present in low-grade as well as high-grade lesions.34 Ki-67 is a marker of cellular proliferation. Coexpression of p16 and Ki-67 indicates a loss of cell cycle regulation and is a hallmark of neoplastic transformation. When positive, these tests are supportive of active HPV infection and of a high-grade lesion. Incorporation of these stains to cytology alone provides additional objective reassurance to cytology, where there is much inter- and intra-observer variability. These stains can be done by laboratories using the stains alone or they can use the FDA-approved p16/Ki-67 Dual-Stain immunohistochemistry (DS), CINtec PLUS Cytology (Roche Diagnostics). However, DS is not yet formally incorporated into triage algorithms by national guidelines.
The IMPACT trial assessed the performance of DS compared with cytology in the triage of HPV-positive results, with or without HPV 16/18 genotyping.35 This was a prospective observational screening study of 35,263 women aged 25 to 65 years across 32 sites in the United States. Of the 4,927 HPV-positive patients with DS results, the sensitivity of DS for CIN 3+ was 91.9% (95% CI, 86.1%–95.4%) and 86.0% (95% CI, 77.5%–91.6%) in HPV 16/18–positive and in the 12 other genotypes, respectively. Using DS alone to triage HPV-positive results showed significantly higher sensitivity and specificity than HPV 16/18 genotyping with cytology triage of 12 “other” genotypes, and substantially higher sensitivity but lower specificity than using cytology alone. Of note, triage with DS alone would have referred significantly fewer women to colposcopy than HPV 16/18 genotyping with cytology triage for the 12 other genotypes (48.6% vs 56.0%; P< .0001).
Similarly, a retrospective analysis of the ATHENA trial cohort of HPV-positive results of 7,727 patients aged 25 years or older also demonstrated increased sensitivity of DS compared with cytology (74.9% vs 51.9%; P<.0001) and similar specificities (74.1% vs 75%; P = .3198).36 The European PALMS study, which included 27,349 women aged 18 years or older across 5 countries who underwent routine screening with HPV testing, cytology, and DS, confirmed these findings.37 The sensitivity of DS was higher than that of cytology (86.7% vs 68.5%; P<.001) for CIN 3+ with comparable specificities (95.2% vs 95.4%; P = .15).
Challenges and opportunities to improve access to primary HPV screening
The historical success of the Pap test in reducing the incidence of cervical cancer relied on individuals having access to the test. This remains true as screening transitions to primary HPV testing. Limitations of HPV-based screening include provider and patient knowledge; access to tests; cost; need for new laboratory infrastructure; need to leverage the electronic health record to record results, calculate a patient’s risk and determine next steps; and the need to re-educate patients and providers about this new model of care. The American Cancer Society and the Centers for Disease Control and Prevention are currently leading initiatives to help adopt primary HPV screening in the United States and to facilitate new care approaches.
Self-collection and independence from subjective cytology would further improve access. Multiple effectiveness studies and patient acceptability studies have shown that primary HPV screening via self-collection is effective, cost effective, and acceptable to women, especially among underscreened populations.38 Sensitivity is comparable to clinician-obtained samples with polymerase chain reaction–based HPV tests. Furthermore, newer molecular tests that detect methylated target host genes or methylated viral genome can be used to triage HPV-positive cases. Several host methylation markers that identify the specific host genes (for example, CADM1, MAL, and miR-124-2) have been shown to be more specific, reproducible, and can be used in self-collected samples as they are based on molecular methylation analysis.39 The ASCCP monitors these new developments and will incorporate promising tests and approaches once validated and FDA approved into the risk-based management guidelines. An erratum was recently published, and the risk-calculator is also available on the ASCCP website free of charge (https://app.asccp.org).40
In conclusion, transition to primary HPV testing from Pap cytology in cervical cancer screening has many challenges but also opportunities. Learning from the experience of countries that have already adopted primary HPV testing is crucial to successful implementation of this new screening paradigm.41 The evidence supporting primary HPV screening with its improved sensitivity is clear, and the existing triage options and innovations will continue to improve triage of patients with clinically important lesions as well as accessibility. With strong advocacy and sound implementation, the WHO goal of cervical cancer elimination and 70% of women being screened with a high-performance test by age 35 and again by age 45 is achievable. ●
- Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71: 209-249.
- Cuzick J, Clavel C, Petry KU, et al. Overview of the European and North American studies on HPV testing in primary cervical cancer screening. Int J Cancer. 2006;119:1095-1101.
- Wright TC Jr, Massad LS, Dunton CJ, et al. 2006 consensus guidelines for the management of women with abnormal cervical cancer screening tests. Am J Obstet Gynecol. 2007;197:346-355.
- Tota JE, Bentley J, Blake J, et al. Introduction of molecular HPV testing as the primary technology in cervical cancer screening: acting on evidence to change the current paradigm. Prev Med. 2017;98:5-14.
- Ronco G, Giorgi Rossi P. Role of HPV DNA testing in modern gynaecological practice. Best Prac Res Clin Obstet Gynaecol. 2018;47:107-118.
- Wright TC, Stoler MH, Behrens CM, et al. Primary cervical cancer screening with human papillomavirus: end of study results from the ATHENA study using HPV as the first-line screening test. Gynecol Oncol. 2015;136:189-197.
- Mayrand MH, Duarte-Franco E, Rodrigues I, et al. Human papillomavirus DNA versus Papanicolaou screening tests for cervical cancer. N Engl J Med. 2007;357:1579-1588.
- Ronco G, Giorgi-Rossi P, Carozzi F, et al. Efficacy of human papillomavirus testing for the detection of invasive cervical cancers and cervical intraepithelial neoplasia: a randomised controlled trial. Lancet Oncol. 2010;11:249-257.
- Bulkmans NW, Rozendaal L, Snijders PJ, et al. POBASCAM, a population-based randomized controlled trial for implementation of high-risk HPV testing in cervical screening: design, methods and baseline data of 44,102 women. Int J Cancer. 2004;110:94-101.
- World Health Organization. WHO guideline for screening and treatment of cervical pre-cancer lesions for cervical cancer prevention. 2nd edition. Geneva: 2021. https://www .who.int/publications/i/item/9789240030824. Accessed April 28, 2022.
- American Cancer Society. The American Cancer Society guidelines for the prevention and early detection of cervical cancer. American Cancer Society; 2020. https://www.cancer .org/cancer/cervical-cancer/detection-diagnosis-staging /cervical-cancer-screening-guidelines.html. Accessed April 28, 2022.
- US Preventive Services Task Force; Curry SJ, Krist AH, Owens KD, et al. Screening for cervical cancer: US Preventive Services Task Force recommendation statement. JAMA. 2018;320:674-686.
- Moscicki AB, Flowers L, Huchko MJ, et al. Guidelines for cervical cancer screening in immunosuppressed women without HIV infection. J Low Gen Tract Dis. 2019;23:87-101.
- Committee opinion no. 675. Management of vulvar intraepithelial neoplasia. Obstet Gynecol. 2016;128:e178-e182.
- Satmary W, Holschneider CH, Brunette LL, et al. Vulvar intraepithelial neoplasia: risk factors for recurrence. Gynecol Oncol. 2018;148:126-131.
- Preti M, Scurry J, Marchitelli CE, et al. Vulvar intraepithelial neoplasia. Best Pract Res Clin Obstet Gynaecol. 2014;28:10511062.
- Khan MJ, Massad LS, Kinney W, et al. A common clinical dilemma: management of abnormal vaginal cytology and human papillomavirus test results. Gynecol Oncol. 2016;141:364-370.
- Perkins RB, Guido RS, Castle PE, et al. 2019 ASCCP risk-based management consensus guidelines for abnormal cervical cancer screening tests and cancer precursors. J Low Gen Tract Dis. 2020;24:102-131.
- Egemen D, Cheung LC, Chen X, et al. Risk estimates supporting the 2019 ASCCP risk-based management consensus guidelines. J Low Gen Tract Dis. 2020;24:132-143.
- Bhatla N, Singla S, Awasthi D. Human papillomavirus deoxyribonucleic acid testing in developed countries. Best Pract Res Clin Obstet Gynaecol. 2012;26:209-220.
- Meijer CJ, Berkhof J, Castle PE, et al. Guidelines for human papillomavirus DNA test requirements for primary cervical cancer screening in women 30 years and older. Int J Cancer. 2009;124:516-520.
- Ejegod D, Bottari F, Pedersen H, et al. The BD Onclarity HPV assay on samples collected in SurePath medium meets the international guidelines for human papillomavirus test requirements for cervical screening. J Clin Microbiol. 2016;54:2267-2272.
- Richardson LA, Tota J, Franco EL. Optimizing technology for cervical cancer screening in high-resource settings. Expert Rev Obstet Gynecol. 2011;6:343-353.
- Ronco G, Dillner J, Elfström KM, et al. Efficacy of HPV-based screening for prevention of invasive cervical cancer: followup of four European randomised controlled trials. Lancet. 2014;383:524-532.
- Ogilvie GS, van Niekerk D, Krajden M, et al. Effect of screening with primary cervical HPV testing vs cytology testing on high-grade cervical intraepithelial neoplasia at 48 months: the HPV FOCAL randomized clinical trial. JAMA. 2018;320:43-52.
- Dillner J, Rebolj M, Birembaut P, et al. Long term predictive values of cytology and human papillomavirus testing in cervical cancer screening: joint European cohort study. BMJ. 2008;337:a1754.
- Katki HA, Kinney WK, Fetterman B, et al. Cervical cancer risk for women undergoing concurrent testing for human papillomavirus and cervical cytology: a population-based study in routine clinical practice. Lancet Oncol. 2011;12:663-672.
- Arbyn M, Ronco G, Anttila A, et al. Evidence regarding human papillomavirus testing in secondary prevention of cervical cancer. Vaccine. 2012;30(suppl 5):F88-99.
- Schiffman M, Kinney WK, et al. Relative performance of HPV and cytology components of cotesting in cervical screening. J Nat Cancer Inst. 2018;110:501-508.
- Jin XW, Lipold L, Foucher J, et al. Cost-effectiveness of primary HPV testing, cytology and co-testing as cervical cancer screening for women above age 30 years. J Gen Intern Med. 2016;31:1338-1344.
- Tota JE, Bentley J, Blake J, et al. Approaches for triaging women who test positive for human papillomavirus in cervical cancer screening. Prev Med. 2017;98:15-20.
- Stoler MH, Wright TC Jr, Parvu V, et al. Stratified risk of high-grade cervical disease using onclarity HPV extended genotyping in women, ≥25 years of age, with NILM cytology. Gynecol Oncol. 2019;153:26-33.
- Louvanto K, Chevarie-Davis M, Ramanakumar AV, et al. HPV testing with cytology triage for cervical cancer screening in routine practice. Am J Obstet Gynecol. 2014;210:474.e1-7.
- Keating JT, Cviko A, Riethdorf S, et al. Ki-67, cyclin E, and p16INK4 are complimentary surrogate biomarkers for human papilloma virus-related cervical neoplasia. Am J Surg Pathol. 2001;25:884-891.
- Wright TC Jr, Stoler MH, Ranger-Moore J, et al. Clinical validation of p16/Ki-67 dual-stained cytology triage of HPV-positive women: results from the IMPACT trial. Int J Cancer. 2022;150:461-471.
- Wright TC Jr, Behrens CM, Ranger-Moore J, et al. Triaging HPV-positive women with p16/Ki-67 dual-stained cytology: results from a sub-study nested into the ATHENA trial. Gynecol Oncol. 2017;144:51-56.
- Ikenberg H, Bergeron C, Schmidt D, et al. Screening for cervical cancer precursors with p16/Ki-67 dual-stained cytology: results of the PALMS study. J Nat Cancer Inst. 2013;105:15501557.
- Arbyn M, Smith SB, Temin S, et al. Detecting cervical precancer and reaching underscreened women by using HPV testing on self samples: updated meta-analyses. BMJ. 2018;363:k4823.
- Verhoef VMJ, Bosgraaf RP, van Kemenade FJ, et al. Triage by methylation-marker testing versus cytology in women who test HPV-positive on self-collected cervicovaginal specimens (PROHTECT-3): a randomised controlled non-inferiority trial. Lancet Oncol. 2014;15:315-322.
- Perkins RB, Guido RS, Castle PE, et al. Erratum: 2019 ASCCP risk-based management consensus guidelines for abnormal cervical cancer screening tests and cancer precursors. J Low Gen Tract Dis. 2021;25:330-331.
- Hall MT, Simms KT, Lew JB, et al. The projected timeframe until cervical cancer elimination in Australia: a modelling study. Lancet Public Health. 2019;4:e19-e27.
- Huh WK, Ault KA, Chelmow D, et al. Use of primary high-risk human papillomavirus testing for cervical cancer screening: interim clinical guidance. Gynecol Oncol. 2015;136:178-182.
Cervical cancer is an important global health problem with an estimated 604,127 new cases and 341,831 deaths in 2020.1 Nearly 85% of the disease burden affects individuals from low and middle-income countries. The World Health Organization (WHO) set forth the goal for all countries to reach and maintain an incidence rate of below 4 per 100,000 women by 2030 as part of the Global Strategy to Accelerate the Elimination of Cervical Cancer.
Although traditional Pap cytology has been the cornerstone of screening programs, its poor sensitivity of approximately 50% and limitations in accessibility require new strategies to achieve the elimination of cervical cancer.2 The discovery that persistent infection with oncogenic human papillomavirus (HPV) is an essential step in the development of cervical cancer led to the development of diagnostic HPV tests, which have higher sensitivity than cytology (96.1% vs 53.0%) but somewhat lower specificity (90.7% vs 96.3%) for the detection of cervical intraepithelial neoplasia (CIN) 2 or worse lesions.2 Initially, HPV testing was incorporated as a method to triage atypical squamous cells of undetermined significance (ASCUS) cytology results.3 Later, the concept of cotesting with cytology emerged,4,5 and since then, several clinical trials have demonstrated the effectiveness of primary HPV screening.6-9
In 2020, the WHO recommended HPV DNA testing as the primary screening method starting at the age of 30 years, with regular testing every 5 to 10 years, for the general population.10 Currently, primary HPV has been adopted in multiple countries, including Australia, the Netherlands, Turkey, England, and Argentina.
In the United States, there are 3 currently acceptable screening strategies: cytology, cytology plus HPV (cotesting), and primary HPV testing (TABLE). The American Cancer Society (ACS) specifically states that HPV testing alone every 5 years is preferred starting at age 25 years; cotesting every 5 years or cytology alone every 3 years are also acceptable.11 The US Preventive Services Task Force (USPSTF) states that cytology alone every 3 years starting at 21 years and then HPV testing alone or cotesting every 5 years or cytology every 3 years starting at age 30 are all acceptable strategies.12
When applying these guidelines, it is important to note that they are intended for the screening of patients with all prior normal results with no symptoms. These routine screening guidelines do not apply to special populations, such as those with a history of abnormal results or treatment, a history of immunosuppression,13 a history of HPV-related vulvar or vaginal dysplasia,14-16 or a history of hysterectomy with removal of the cervix and no prior history of cervical dysplasia.17,18 By contrast, surveillance is interval testing for those who have either an abnormal prior test result or treatment; these may be managed per risk-based estimates provided by the American Society for Colposcopy and Cervical Pathology (ASCCP).18,19 Finally, diagnosis is evaluation (which may include diagnostic cytology) of a patient with abnormal signs and/or symptoms (such as bleeding, pain, discharge, or cervical mass).
In this Update, we present the evidence for primary HPV testing, the management options for a positive result in the United States, and research that will improve uptake of primary HPV testing as well as accessibility.
Change in screening paradigm: Evidence for primary HPV testing
HPV DNA tests are multiplex assays that detect the DNA of targeted high-risk HPV types, using multiple probes, either by direct genomic detection or by amplification of a viral DNA fragment using polymerase chain reaction (PCR).20,21 Alternatively, HPV mRNA-based tests detect the expression of E6 and E7 oncoproteins, a marker of viral integration.20 In examining the data from well-conducted clinical trials, 2 important observations are that different HPV assays were used and that direct comparison may not be valid. In addition, not all tests used in the studies are approved by the US Food and Drug Administration (FDA) for primary HPV testing.
Continue to: FDA-approved HPV tests...
FDA-approved HPV tests
Currently, 2 tests are FDA approved for primary HPV screening. The Cobas HPV test (Roche Molecular Diagnostics) was the first FDA-approved test for primary HPV screening in women aged 25 years and older.6 This test reports pooled results from 12 high-risk (hr) HPV types (31/33/35/39/45/51/52/56/58/59/66/68) with reflex genotyping for HPV 16/18, and thus it provides an immediate triage option for HPV-positive women. Of note, it is also approved for cotesting. The second FDA-approved test is the BD Onclarity HPV assay (Becton, Dickinson and Company) for primary HPV screening.22 It detects 14 hrHPV types, types 16/18/45 specifically as well as types 31/33/35/39/51/52/56/58/59/66/68.
Other HPV tests are FDA approved for cotesting and reflex testing but not for primary HPV testing. The Hybrid Capture test, or HC2 (Qiagen Inc), was the first HPV test to be approved by the FDA in 1997 for reflex testing of women with ASCUS cytology. In 2003, it was approved for cotesting along with cytology in women aged 30 years and older.20,21 In 2009, the Cervista HPV HR test (Hologic Inc) was approved for cotesting. The Aptima HPV assay (Hologic Inc), which is also approved for cotesting, is an RNA-based assay that allows detection of E6/E7 mRNA transcripts of 14 HPV types.23
Comparing HPV testing with cytology
Ronco and colleagues pooled data from 4 European randomized controlled trials (RCTs)—Swedescreen, POBASCAM, NTCC, ARTISTIC—with a total of 176,464 participants randomly assigned to HPV or cytology screening.24 Swedescreen and POBASCAM used GP5/GP6 PCR, while ARTISTIC and NTCC used HC2 for primary HPV screening. The screening interval was 3 years in all except 5 years in POBASCAM. The pooled detection rate of invasive disease was similar in the 2 arms, with pooled rate ratio for cancer detection being 0.79 (95% confidence interval [CI], 0.46–1.36) in the first 2.5 years, but was 0.45 (95% CI, 0.25–0.81), favoring the HPV arm, after 2.5 years. HPV testing was more effective in preventing cases of adenocarcinoma than squamous cell carcinoma (0.31 [95% CI, 0.14–0.69] vs 0.78 [95% CI, 0.49–1.25]). The authors concluded that HPV-based screening from age 30 years provided 60% to 70% better protection than cytology.
The result of the above meta-analysis was confirmed by the HPV FOCAL RCT that investigated the efficacy of HPV testing (HC2) in comparison with cytology.25 The detection rates for CIN 3 lesions supported primary HPV screening, with an absolute difference in incidence rate of 2.67/1,000 (95% CI, 0.53–4.88) at study randomization and 3.22/1,000 (95% CI, 5.12–1.48) at study exit 4 years later.
Cotesting using HPV and cytology: Marginal benefit
Dillner and colleagues were one of the first groups to report on the risk of CIN 3 based on both HPV and cytology status.26 Using pooled analysis of data from multiple countries, these investigators reported that the cumulative incidence rates (CIR) of CIN 3 after 6 years of follow-up increased consistently in HPV-positive subjects, and an HPV-positive result more accurately predicted CIN 3+ at 5 years than cytology alone. Furthermore, HPV negativity provided greater reassurance than cytology alone. At 5 years of follow-up, the rates of CIN 3+ were 0.25% (0.12%–0.41%) for women negative for HPV compared with 0.83% (0.50%–1.13%) for women with negative cytology results. There was little difference in rates for CIN 3+ between women with negative results on both tests and women who were negative for HPV.
The important question is then the marginal benefit of cotesting, which is the most costly screening option. A study of 331,818 women enrolled for cotesting at Kaiser Permanente found that the risk of CIN 3+ predicted by HPV testing alone when compared with cytology was significantly higher at both 3 years (5.0% vs 3.8%; P = .046) and 5 years (7.6% vs 4.7%; P = .001).27 A negative cytology result did not decrease the risk of CIN 3 further for HPV-negative patients (3 years: 0.047% vs 0.063%, P = .6; 5 years: 0.16% vs 0.17%, P = .8). They concluded that a negative HPV test was enough reassurance for low risk of CIN 3+ and that an additional negative cytology result does not provide extra reassurance.
Furthermore, a systematic meta-analysis of 48 studies, including 8 RCTs, found that the addition of cytology to HPV testing raised the sensitivity by 2% for CIN 3 compared with HPV testing alone. This improvement in sensitivity was at the expense of considerable loss of specificity, with a ratio of 0.93 (95% CI, 0.92–0.95) for CIN 3.28 Schiffman and colleagues also assessed the relative contribution of HPV testing and cytology in detection of CIN 3 and cancer.29 The HPV component alone identified a significantly higher proportion of preinvasive and invasive disease than cytology. Only 3.5% of precancers and 5.9% of cancers were preceded by HPV-negative, cytology-positive results. Thus, cytology contributed only 5 cases per million women per year to the sensitivity of the combined test, at the cost of significantly more colposcopies. Hence, the evidence suggests that there is limited benefit of adding cytology to HPV testing.30
Continue to: Triage of a positive HPV result...
Triage of a positive HPV result
An important limitation of HPV testing is its inability to discriminate between transient and persistent infections. Referral of all HPV-positive cases to colposcopy would overburden the system with associated unnecessary procedures. Hence, a triage strategy is essential to identify clinically important infections that truly require colposcopic evaluation. The FIGURE illustrates the management of a primary HPV test result performed for screening.

HPV genotyping
One strategy for triaging a positive HPV test result is genotyping. HPV 16 and 18 have the highest risk of persistence and progression and merit immediate referral to colposcopy. In the ATHENA trial, CIN 3 was identified in 17.8% (95% CI, 14.8–20.7%) of HPV 16 positive women at baseline, and the CIR increased to 25.2% (95% CI, 21.7–28.7%) after 3 years. The 3-year CIR of CIN 3 was only 5.4% (95% CI, 4.5–6.3%) in women with HPV genotypes other than 16/18. HPV 18–positive women had a 3-year CIR that was intermediate between women with HPV 16 and women with the 12 other genotypes.6 Hence, HPV 16/18–positive cases should be referred for immediate colposcopy, and negative cases should be followed up with cytology and referred for colposcopy if the cytology is ASCUS or worse.31
In July 2020, extended genotyping was approved by the FDA with individual detection of HPV 31, 51, 52 (in addition to 16, 18, and 45) and pooled detection of 33/58, 35/39/68, and 56/59/66. One study found that individual genotypes HPV 16 and 31 carry baseline risk values for CIN 3+ (8.1% and 7.5%, respectively) that are above the 5-year risk threshold for referral to colposcopy following the ASCCP risk-based management guideline.32
Cytology
The higher specificity of cytology makes it an option for triaging HPV-positive cases, and current management guidelines recommend triage to both genotyping and cytology for all patients who are HPV positive, and especially if they are HPV positive but HPV 16/18 negative. Of note, cytology results remain more subjective than those of primary HPV testing, but the combination of initial HPV testing with reflex to cytology is a reasonable and cost effective next step.18 The VASCAR trial found higher colposcopy referrals in the HPV screening and cytology triage group compared with the cytology alone group (19.36 vs 14.54 per 1,000 women).33 The ATHENA trial investigated various triage strategies for HPV-positive cases and its impact on colposcopy referrals.6 Using HPV genotyping and reflex cytology, if HPV 16/18 was positive, colposcopy was advised, but if any of the other 12 HPV types were positive, reflex cytology was done. If reported as ASCUS or worse, colposcopy was performed; conversely, if it was normal, women were rescreened with cotesting after 1 year. Although this strategy led to a reduction in the number of colposcopies, referrals were still higher in the primary HPV arm (3,769 colposcopies per 294 cases) compared with cytology (1,934 colposcopies per 179 cases) or cotesting (3,097 colposcopies per 240 cases) in women aged 25 years.14
p16/Ki-67 Dual-Stain
Diffused p16 immunohistochemical staining, as opposed to focal staining, is associated with active HPV infection but can be present in low-grade as well as high-grade lesions.34 Ki-67 is a marker of cellular proliferation. Coexpression of p16 and Ki-67 indicates a loss of cell cycle regulation and is a hallmark of neoplastic transformation. When positive, these tests are supportive of active HPV infection and of a high-grade lesion. Incorporation of these stains to cytology alone provides additional objective reassurance to cytology, where there is much inter- and intra-observer variability. These stains can be done by laboratories using the stains alone or they can use the FDA-approved p16/Ki-67 Dual-Stain immunohistochemistry (DS), CINtec PLUS Cytology (Roche Diagnostics). However, DS is not yet formally incorporated into triage algorithms by national guidelines.
The IMPACT trial assessed the performance of DS compared with cytology in the triage of HPV-positive results, with or without HPV 16/18 genotyping.35 This was a prospective observational screening study of 35,263 women aged 25 to 65 years across 32 sites in the United States. Of the 4,927 HPV-positive patients with DS results, the sensitivity of DS for CIN 3+ was 91.9% (95% CI, 86.1%–95.4%) and 86.0% (95% CI, 77.5%–91.6%) in HPV 16/18–positive and in the 12 other genotypes, respectively. Using DS alone to triage HPV-positive results showed significantly higher sensitivity and specificity than HPV 16/18 genotyping with cytology triage of 12 “other” genotypes, and substantially higher sensitivity but lower specificity than using cytology alone. Of note, triage with DS alone would have referred significantly fewer women to colposcopy than HPV 16/18 genotyping with cytology triage for the 12 other genotypes (48.6% vs 56.0%; P< .0001).
Similarly, a retrospective analysis of the ATHENA trial cohort of HPV-positive results of 7,727 patients aged 25 years or older also demonstrated increased sensitivity of DS compared with cytology (74.9% vs 51.9%; P<.0001) and similar specificities (74.1% vs 75%; P = .3198).36 The European PALMS study, which included 27,349 women aged 18 years or older across 5 countries who underwent routine screening with HPV testing, cytology, and DS, confirmed these findings.37 The sensitivity of DS was higher than that of cytology (86.7% vs 68.5%; P<.001) for CIN 3+ with comparable specificities (95.2% vs 95.4%; P = .15).
Challenges and opportunities to improve access to primary HPV screening
The historical success of the Pap test in reducing the incidence of cervical cancer relied on individuals having access to the test. This remains true as screening transitions to primary HPV testing. Limitations of HPV-based screening include provider and patient knowledge; access to tests; cost; need for new laboratory infrastructure; need to leverage the electronic health record to record results, calculate a patient’s risk and determine next steps; and the need to re-educate patients and providers about this new model of care. The American Cancer Society and the Centers for Disease Control and Prevention are currently leading initiatives to help adopt primary HPV screening in the United States and to facilitate new care approaches.
Self-collection and independence from subjective cytology would further improve access. Multiple effectiveness studies and patient acceptability studies have shown that primary HPV screening via self-collection is effective, cost effective, and acceptable to women, especially among underscreened populations.38 Sensitivity is comparable to clinician-obtained samples with polymerase chain reaction–based HPV tests. Furthermore, newer molecular tests that detect methylated target host genes or methylated viral genome can be used to triage HPV-positive cases. Several host methylation markers that identify the specific host genes (for example, CADM1, MAL, and miR-124-2) have been shown to be more specific, reproducible, and can be used in self-collected samples as they are based on molecular methylation analysis.39 The ASCCP monitors these new developments and will incorporate promising tests and approaches once validated and FDA approved into the risk-based management guidelines. An erratum was recently published, and the risk-calculator is also available on the ASCCP website free of charge (https://app.asccp.org).40
In conclusion, transition to primary HPV testing from Pap cytology in cervical cancer screening has many challenges but also opportunities. Learning from the experience of countries that have already adopted primary HPV testing is crucial to successful implementation of this new screening paradigm.41 The evidence supporting primary HPV screening with its improved sensitivity is clear, and the existing triage options and innovations will continue to improve triage of patients with clinically important lesions as well as accessibility. With strong advocacy and sound implementation, the WHO goal of cervical cancer elimination and 70% of women being screened with a high-performance test by age 35 and again by age 45 is achievable. ●
Cervical cancer is an important global health problem with an estimated 604,127 new cases and 341,831 deaths in 2020.1 Nearly 85% of the disease burden affects individuals from low and middle-income countries. The World Health Organization (WHO) set forth the goal for all countries to reach and maintain an incidence rate of below 4 per 100,000 women by 2030 as part of the Global Strategy to Accelerate the Elimination of Cervical Cancer.
Although traditional Pap cytology has been the cornerstone of screening programs, its poor sensitivity of approximately 50% and limitations in accessibility require new strategies to achieve the elimination of cervical cancer.2 The discovery that persistent infection with oncogenic human papillomavirus (HPV) is an essential step in the development of cervical cancer led to the development of diagnostic HPV tests, which have higher sensitivity than cytology (96.1% vs 53.0%) but somewhat lower specificity (90.7% vs 96.3%) for the detection of cervical intraepithelial neoplasia (CIN) 2 or worse lesions.2 Initially, HPV testing was incorporated as a method to triage atypical squamous cells of undetermined significance (ASCUS) cytology results.3 Later, the concept of cotesting with cytology emerged,4,5 and since then, several clinical trials have demonstrated the effectiveness of primary HPV screening.6-9
In 2020, the WHO recommended HPV DNA testing as the primary screening method starting at the age of 30 years, with regular testing every 5 to 10 years, for the general population.10 Currently, primary HPV has been adopted in multiple countries, including Australia, the Netherlands, Turkey, England, and Argentina.
In the United States, there are 3 currently acceptable screening strategies: cytology, cytology plus HPV (cotesting), and primary HPV testing (TABLE). The American Cancer Society (ACS) specifically states that HPV testing alone every 5 years is preferred starting at age 25 years; cotesting every 5 years or cytology alone every 3 years are also acceptable.11 The US Preventive Services Task Force (USPSTF) states that cytology alone every 3 years starting at 21 years and then HPV testing alone or cotesting every 5 years or cytology every 3 years starting at age 30 are all acceptable strategies.12
When applying these guidelines, it is important to note that they are intended for the screening of patients with all prior normal results with no symptoms. These routine screening guidelines do not apply to special populations, such as those with a history of abnormal results or treatment, a history of immunosuppression,13 a history of HPV-related vulvar or vaginal dysplasia,14-16 or a history of hysterectomy with removal of the cervix and no prior history of cervical dysplasia.17,18 By contrast, surveillance is interval testing for those who have either an abnormal prior test result or treatment; these may be managed per risk-based estimates provided by the American Society for Colposcopy and Cervical Pathology (ASCCP).18,19 Finally, diagnosis is evaluation (which may include diagnostic cytology) of a patient with abnormal signs and/or symptoms (such as bleeding, pain, discharge, or cervical mass).
In this Update, we present the evidence for primary HPV testing, the management options for a positive result in the United States, and research that will improve uptake of primary HPV testing as well as accessibility.
Change in screening paradigm: Evidence for primary HPV testing
HPV DNA tests are multiplex assays that detect the DNA of targeted high-risk HPV types, using multiple probes, either by direct genomic detection or by amplification of a viral DNA fragment using polymerase chain reaction (PCR).20,21 Alternatively, HPV mRNA-based tests detect the expression of E6 and E7 oncoproteins, a marker of viral integration.20 In examining the data from well-conducted clinical trials, 2 important observations are that different HPV assays were used and that direct comparison may not be valid. In addition, not all tests used in the studies are approved by the US Food and Drug Administration (FDA) for primary HPV testing.
Continue to: FDA-approved HPV tests...
FDA-approved HPV tests
Currently, 2 tests are FDA approved for primary HPV screening. The Cobas HPV test (Roche Molecular Diagnostics) was the first FDA-approved test for primary HPV screening in women aged 25 years and older.6 This test reports pooled results from 12 high-risk (hr) HPV types (31/33/35/39/45/51/52/56/58/59/66/68) with reflex genotyping for HPV 16/18, and thus it provides an immediate triage option for HPV-positive women. Of note, it is also approved for cotesting. The second FDA-approved test is the BD Onclarity HPV assay (Becton, Dickinson and Company) for primary HPV screening.22 It detects 14 hrHPV types, types 16/18/45 specifically as well as types 31/33/35/39/51/52/56/58/59/66/68.
Other HPV tests are FDA approved for cotesting and reflex testing but not for primary HPV testing. The Hybrid Capture test, or HC2 (Qiagen Inc), was the first HPV test to be approved by the FDA in 1997 for reflex testing of women with ASCUS cytology. In 2003, it was approved for cotesting along with cytology in women aged 30 years and older.20,21 In 2009, the Cervista HPV HR test (Hologic Inc) was approved for cotesting. The Aptima HPV assay (Hologic Inc), which is also approved for cotesting, is an RNA-based assay that allows detection of E6/E7 mRNA transcripts of 14 HPV types.23
Comparing HPV testing with cytology
Ronco and colleagues pooled data from 4 European randomized controlled trials (RCTs)—Swedescreen, POBASCAM, NTCC, ARTISTIC—with a total of 176,464 participants randomly assigned to HPV or cytology screening.24 Swedescreen and POBASCAM used GP5/GP6 PCR, while ARTISTIC and NTCC used HC2 for primary HPV screening. The screening interval was 3 years in all except 5 years in POBASCAM. The pooled detection rate of invasive disease was similar in the 2 arms, with pooled rate ratio for cancer detection being 0.79 (95% confidence interval [CI], 0.46–1.36) in the first 2.5 years, but was 0.45 (95% CI, 0.25–0.81), favoring the HPV arm, after 2.5 years. HPV testing was more effective in preventing cases of adenocarcinoma than squamous cell carcinoma (0.31 [95% CI, 0.14–0.69] vs 0.78 [95% CI, 0.49–1.25]). The authors concluded that HPV-based screening from age 30 years provided 60% to 70% better protection than cytology.
The result of the above meta-analysis was confirmed by the HPV FOCAL RCT that investigated the efficacy of HPV testing (HC2) in comparison with cytology.25 The detection rates for CIN 3 lesions supported primary HPV screening, with an absolute difference in incidence rate of 2.67/1,000 (95% CI, 0.53–4.88) at study randomization and 3.22/1,000 (95% CI, 5.12–1.48) at study exit 4 years later.
Cotesting using HPV and cytology: Marginal benefit
Dillner and colleagues were one of the first groups to report on the risk of CIN 3 based on both HPV and cytology status.26 Using pooled analysis of data from multiple countries, these investigators reported that the cumulative incidence rates (CIR) of CIN 3 after 6 years of follow-up increased consistently in HPV-positive subjects, and an HPV-positive result more accurately predicted CIN 3+ at 5 years than cytology alone. Furthermore, HPV negativity provided greater reassurance than cytology alone. At 5 years of follow-up, the rates of CIN 3+ were 0.25% (0.12%–0.41%) for women negative for HPV compared with 0.83% (0.50%–1.13%) for women with negative cytology results. There was little difference in rates for CIN 3+ between women with negative results on both tests and women who were negative for HPV.
The important question is then the marginal benefit of cotesting, which is the most costly screening option. A study of 331,818 women enrolled for cotesting at Kaiser Permanente found that the risk of CIN 3+ predicted by HPV testing alone when compared with cytology was significantly higher at both 3 years (5.0% vs 3.8%; P = .046) and 5 years (7.6% vs 4.7%; P = .001).27 A negative cytology result did not decrease the risk of CIN 3 further for HPV-negative patients (3 years: 0.047% vs 0.063%, P = .6; 5 years: 0.16% vs 0.17%, P = .8). They concluded that a negative HPV test was enough reassurance for low risk of CIN 3+ and that an additional negative cytology result does not provide extra reassurance.
Furthermore, a systematic meta-analysis of 48 studies, including 8 RCTs, found that the addition of cytology to HPV testing raised the sensitivity by 2% for CIN 3 compared with HPV testing alone. This improvement in sensitivity was at the expense of considerable loss of specificity, with a ratio of 0.93 (95% CI, 0.92–0.95) for CIN 3.28 Schiffman and colleagues also assessed the relative contribution of HPV testing and cytology in detection of CIN 3 and cancer.29 The HPV component alone identified a significantly higher proportion of preinvasive and invasive disease than cytology. Only 3.5% of precancers and 5.9% of cancers were preceded by HPV-negative, cytology-positive results. Thus, cytology contributed only 5 cases per million women per year to the sensitivity of the combined test, at the cost of significantly more colposcopies. Hence, the evidence suggests that there is limited benefit of adding cytology to HPV testing.30
Continue to: Triage of a positive HPV result...
Triage of a positive HPV result
An important limitation of HPV testing is its inability to discriminate between transient and persistent infections. Referral of all HPV-positive cases to colposcopy would overburden the system with associated unnecessary procedures. Hence, a triage strategy is essential to identify clinically important infections that truly require colposcopic evaluation. The FIGURE illustrates the management of a primary HPV test result performed for screening.

HPV genotyping
One strategy for triaging a positive HPV test result is genotyping. HPV 16 and 18 have the highest risk of persistence and progression and merit immediate referral to colposcopy. In the ATHENA trial, CIN 3 was identified in 17.8% (95% CI, 14.8–20.7%) of HPV 16 positive women at baseline, and the CIR increased to 25.2% (95% CI, 21.7–28.7%) after 3 years. The 3-year CIR of CIN 3 was only 5.4% (95% CI, 4.5–6.3%) in women with HPV genotypes other than 16/18. HPV 18–positive women had a 3-year CIR that was intermediate between women with HPV 16 and women with the 12 other genotypes.6 Hence, HPV 16/18–positive cases should be referred for immediate colposcopy, and negative cases should be followed up with cytology and referred for colposcopy if the cytology is ASCUS or worse.31
In July 2020, extended genotyping was approved by the FDA with individual detection of HPV 31, 51, 52 (in addition to 16, 18, and 45) and pooled detection of 33/58, 35/39/68, and 56/59/66. One study found that individual genotypes HPV 16 and 31 carry baseline risk values for CIN 3+ (8.1% and 7.5%, respectively) that are above the 5-year risk threshold for referral to colposcopy following the ASCCP risk-based management guideline.32
Cytology
The higher specificity of cytology makes it an option for triaging HPV-positive cases, and current management guidelines recommend triage to both genotyping and cytology for all patients who are HPV positive, and especially if they are HPV positive but HPV 16/18 negative. Of note, cytology results remain more subjective than those of primary HPV testing, but the combination of initial HPV testing with reflex to cytology is a reasonable and cost effective next step.18 The VASCAR trial found higher colposcopy referrals in the HPV screening and cytology triage group compared with the cytology alone group (19.36 vs 14.54 per 1,000 women).33 The ATHENA trial investigated various triage strategies for HPV-positive cases and its impact on colposcopy referrals.6 Using HPV genotyping and reflex cytology, if HPV 16/18 was positive, colposcopy was advised, but if any of the other 12 HPV types were positive, reflex cytology was done. If reported as ASCUS or worse, colposcopy was performed; conversely, if it was normal, women were rescreened with cotesting after 1 year. Although this strategy led to a reduction in the number of colposcopies, referrals were still higher in the primary HPV arm (3,769 colposcopies per 294 cases) compared with cytology (1,934 colposcopies per 179 cases) or cotesting (3,097 colposcopies per 240 cases) in women aged 25 years.14
p16/Ki-67 Dual-Stain
Diffused p16 immunohistochemical staining, as opposed to focal staining, is associated with active HPV infection but can be present in low-grade as well as high-grade lesions.34 Ki-67 is a marker of cellular proliferation. Coexpression of p16 and Ki-67 indicates a loss of cell cycle regulation and is a hallmark of neoplastic transformation. When positive, these tests are supportive of active HPV infection and of a high-grade lesion. Incorporation of these stains to cytology alone provides additional objective reassurance to cytology, where there is much inter- and intra-observer variability. These stains can be done by laboratories using the stains alone or they can use the FDA-approved p16/Ki-67 Dual-Stain immunohistochemistry (DS), CINtec PLUS Cytology (Roche Diagnostics). However, DS is not yet formally incorporated into triage algorithms by national guidelines.
The IMPACT trial assessed the performance of DS compared with cytology in the triage of HPV-positive results, with or without HPV 16/18 genotyping.35 This was a prospective observational screening study of 35,263 women aged 25 to 65 years across 32 sites in the United States. Of the 4,927 HPV-positive patients with DS results, the sensitivity of DS for CIN 3+ was 91.9% (95% CI, 86.1%–95.4%) and 86.0% (95% CI, 77.5%–91.6%) in HPV 16/18–positive and in the 12 other genotypes, respectively. Using DS alone to triage HPV-positive results showed significantly higher sensitivity and specificity than HPV 16/18 genotyping with cytology triage of 12 “other” genotypes, and substantially higher sensitivity but lower specificity than using cytology alone. Of note, triage with DS alone would have referred significantly fewer women to colposcopy than HPV 16/18 genotyping with cytology triage for the 12 other genotypes (48.6% vs 56.0%; P< .0001).
Similarly, a retrospective analysis of the ATHENA trial cohort of HPV-positive results of 7,727 patients aged 25 years or older also demonstrated increased sensitivity of DS compared with cytology (74.9% vs 51.9%; P<.0001) and similar specificities (74.1% vs 75%; P = .3198).36 The European PALMS study, which included 27,349 women aged 18 years or older across 5 countries who underwent routine screening with HPV testing, cytology, and DS, confirmed these findings.37 The sensitivity of DS was higher than that of cytology (86.7% vs 68.5%; P<.001) for CIN 3+ with comparable specificities (95.2% vs 95.4%; P = .15).
Challenges and opportunities to improve access to primary HPV screening
The historical success of the Pap test in reducing the incidence of cervical cancer relied on individuals having access to the test. This remains true as screening transitions to primary HPV testing. Limitations of HPV-based screening include provider and patient knowledge; access to tests; cost; need for new laboratory infrastructure; need to leverage the electronic health record to record results, calculate a patient’s risk and determine next steps; and the need to re-educate patients and providers about this new model of care. The American Cancer Society and the Centers for Disease Control and Prevention are currently leading initiatives to help adopt primary HPV screening in the United States and to facilitate new care approaches.
Self-collection and independence from subjective cytology would further improve access. Multiple effectiveness studies and patient acceptability studies have shown that primary HPV screening via self-collection is effective, cost effective, and acceptable to women, especially among underscreened populations.38 Sensitivity is comparable to clinician-obtained samples with polymerase chain reaction–based HPV tests. Furthermore, newer molecular tests that detect methylated target host genes or methylated viral genome can be used to triage HPV-positive cases. Several host methylation markers that identify the specific host genes (for example, CADM1, MAL, and miR-124-2) have been shown to be more specific, reproducible, and can be used in self-collected samples as they are based on molecular methylation analysis.39 The ASCCP monitors these new developments and will incorporate promising tests and approaches once validated and FDA approved into the risk-based management guidelines. An erratum was recently published, and the risk-calculator is also available on the ASCCP website free of charge (https://app.asccp.org).40
In conclusion, transition to primary HPV testing from Pap cytology in cervical cancer screening has many challenges but also opportunities. Learning from the experience of countries that have already adopted primary HPV testing is crucial to successful implementation of this new screening paradigm.41 The evidence supporting primary HPV screening with its improved sensitivity is clear, and the existing triage options and innovations will continue to improve triage of patients with clinically important lesions as well as accessibility. With strong advocacy and sound implementation, the WHO goal of cervical cancer elimination and 70% of women being screened with a high-performance test by age 35 and again by age 45 is achievable. ●
- Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71: 209-249.
- Cuzick J, Clavel C, Petry KU, et al. Overview of the European and North American studies on HPV testing in primary cervical cancer screening. Int J Cancer. 2006;119:1095-1101.
- Wright TC Jr, Massad LS, Dunton CJ, et al. 2006 consensus guidelines for the management of women with abnormal cervical cancer screening tests. Am J Obstet Gynecol. 2007;197:346-355.
- Tota JE, Bentley J, Blake J, et al. Introduction of molecular HPV testing as the primary technology in cervical cancer screening: acting on evidence to change the current paradigm. Prev Med. 2017;98:5-14.
- Ronco G, Giorgi Rossi P. Role of HPV DNA testing in modern gynaecological practice. Best Prac Res Clin Obstet Gynaecol. 2018;47:107-118.
- Wright TC, Stoler MH, Behrens CM, et al. Primary cervical cancer screening with human papillomavirus: end of study results from the ATHENA study using HPV as the first-line screening test. Gynecol Oncol. 2015;136:189-197.
- Mayrand MH, Duarte-Franco E, Rodrigues I, et al. Human papillomavirus DNA versus Papanicolaou screening tests for cervical cancer. N Engl J Med. 2007;357:1579-1588.
- Ronco G, Giorgi-Rossi P, Carozzi F, et al. Efficacy of human papillomavirus testing for the detection of invasive cervical cancers and cervical intraepithelial neoplasia: a randomised controlled trial. Lancet Oncol. 2010;11:249-257.
- Bulkmans NW, Rozendaal L, Snijders PJ, et al. POBASCAM, a population-based randomized controlled trial for implementation of high-risk HPV testing in cervical screening: design, methods and baseline data of 44,102 women. Int J Cancer. 2004;110:94-101.
- World Health Organization. WHO guideline for screening and treatment of cervical pre-cancer lesions for cervical cancer prevention. 2nd edition. Geneva: 2021. https://www .who.int/publications/i/item/9789240030824. Accessed April 28, 2022.
- American Cancer Society. The American Cancer Society guidelines for the prevention and early detection of cervical cancer. American Cancer Society; 2020. https://www.cancer .org/cancer/cervical-cancer/detection-diagnosis-staging /cervical-cancer-screening-guidelines.html. Accessed April 28, 2022.
- US Preventive Services Task Force; Curry SJ, Krist AH, Owens KD, et al. Screening for cervical cancer: US Preventive Services Task Force recommendation statement. JAMA. 2018;320:674-686.
- Moscicki AB, Flowers L, Huchko MJ, et al. Guidelines for cervical cancer screening in immunosuppressed women without HIV infection. J Low Gen Tract Dis. 2019;23:87-101.
- Committee opinion no. 675. Management of vulvar intraepithelial neoplasia. Obstet Gynecol. 2016;128:e178-e182.
- Satmary W, Holschneider CH, Brunette LL, et al. Vulvar intraepithelial neoplasia: risk factors for recurrence. Gynecol Oncol. 2018;148:126-131.
- Preti M, Scurry J, Marchitelli CE, et al. Vulvar intraepithelial neoplasia. Best Pract Res Clin Obstet Gynaecol. 2014;28:10511062.
- Khan MJ, Massad LS, Kinney W, et al. A common clinical dilemma: management of abnormal vaginal cytology and human papillomavirus test results. Gynecol Oncol. 2016;141:364-370.
- Perkins RB, Guido RS, Castle PE, et al. 2019 ASCCP risk-based management consensus guidelines for abnormal cervical cancer screening tests and cancer precursors. J Low Gen Tract Dis. 2020;24:102-131.
- Egemen D, Cheung LC, Chen X, et al. Risk estimates supporting the 2019 ASCCP risk-based management consensus guidelines. J Low Gen Tract Dis. 2020;24:132-143.
- Bhatla N, Singla S, Awasthi D. Human papillomavirus deoxyribonucleic acid testing in developed countries. Best Pract Res Clin Obstet Gynaecol. 2012;26:209-220.
- Meijer CJ, Berkhof J, Castle PE, et al. Guidelines for human papillomavirus DNA test requirements for primary cervical cancer screening in women 30 years and older. Int J Cancer. 2009;124:516-520.
- Ejegod D, Bottari F, Pedersen H, et al. The BD Onclarity HPV assay on samples collected in SurePath medium meets the international guidelines for human papillomavirus test requirements for cervical screening. J Clin Microbiol. 2016;54:2267-2272.
- Richardson LA, Tota J, Franco EL. Optimizing technology for cervical cancer screening in high-resource settings. Expert Rev Obstet Gynecol. 2011;6:343-353.
- Ronco G, Dillner J, Elfström KM, et al. Efficacy of HPV-based screening for prevention of invasive cervical cancer: followup of four European randomised controlled trials. Lancet. 2014;383:524-532.
- Ogilvie GS, van Niekerk D, Krajden M, et al. Effect of screening with primary cervical HPV testing vs cytology testing on high-grade cervical intraepithelial neoplasia at 48 months: the HPV FOCAL randomized clinical trial. JAMA. 2018;320:43-52.
- Dillner J, Rebolj M, Birembaut P, et al. Long term predictive values of cytology and human papillomavirus testing in cervical cancer screening: joint European cohort study. BMJ. 2008;337:a1754.
- Katki HA, Kinney WK, Fetterman B, et al. Cervical cancer risk for women undergoing concurrent testing for human papillomavirus and cervical cytology: a population-based study in routine clinical practice. Lancet Oncol. 2011;12:663-672.
- Arbyn M, Ronco G, Anttila A, et al. Evidence regarding human papillomavirus testing in secondary prevention of cervical cancer. Vaccine. 2012;30(suppl 5):F88-99.
- Schiffman M, Kinney WK, et al. Relative performance of HPV and cytology components of cotesting in cervical screening. J Nat Cancer Inst. 2018;110:501-508.
- Jin XW, Lipold L, Foucher J, et al. Cost-effectiveness of primary HPV testing, cytology and co-testing as cervical cancer screening for women above age 30 years. J Gen Intern Med. 2016;31:1338-1344.
- Tota JE, Bentley J, Blake J, et al. Approaches for triaging women who test positive for human papillomavirus in cervical cancer screening. Prev Med. 2017;98:15-20.
- Stoler MH, Wright TC Jr, Parvu V, et al. Stratified risk of high-grade cervical disease using onclarity HPV extended genotyping in women, ≥25 years of age, with NILM cytology. Gynecol Oncol. 2019;153:26-33.
- Louvanto K, Chevarie-Davis M, Ramanakumar AV, et al. HPV testing with cytology triage for cervical cancer screening in routine practice. Am J Obstet Gynecol. 2014;210:474.e1-7.
- Keating JT, Cviko A, Riethdorf S, et al. Ki-67, cyclin E, and p16INK4 are complimentary surrogate biomarkers for human papilloma virus-related cervical neoplasia. Am J Surg Pathol. 2001;25:884-891.
- Wright TC Jr, Stoler MH, Ranger-Moore J, et al. Clinical validation of p16/Ki-67 dual-stained cytology triage of HPV-positive women: results from the IMPACT trial. Int J Cancer. 2022;150:461-471.
- Wright TC Jr, Behrens CM, Ranger-Moore J, et al. Triaging HPV-positive women with p16/Ki-67 dual-stained cytology: results from a sub-study nested into the ATHENA trial. Gynecol Oncol. 2017;144:51-56.
- Ikenberg H, Bergeron C, Schmidt D, et al. Screening for cervical cancer precursors with p16/Ki-67 dual-stained cytology: results of the PALMS study. J Nat Cancer Inst. 2013;105:15501557.
- Arbyn M, Smith SB, Temin S, et al. Detecting cervical precancer and reaching underscreened women by using HPV testing on self samples: updated meta-analyses. BMJ. 2018;363:k4823.
- Verhoef VMJ, Bosgraaf RP, van Kemenade FJ, et al. Triage by methylation-marker testing versus cytology in women who test HPV-positive on self-collected cervicovaginal specimens (PROHTECT-3): a randomised controlled non-inferiority trial. Lancet Oncol. 2014;15:315-322.
- Perkins RB, Guido RS, Castle PE, et al. Erratum: 2019 ASCCP risk-based management consensus guidelines for abnormal cervical cancer screening tests and cancer precursors. J Low Gen Tract Dis. 2021;25:330-331.
- Hall MT, Simms KT, Lew JB, et al. The projected timeframe until cervical cancer elimination in Australia: a modelling study. Lancet Public Health. 2019;4:e19-e27.
- Huh WK, Ault KA, Chelmow D, et al. Use of primary high-risk human papillomavirus testing for cervical cancer screening: interim clinical guidance. Gynecol Oncol. 2015;136:178-182.
- Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71: 209-249.
- Cuzick J, Clavel C, Petry KU, et al. Overview of the European and North American studies on HPV testing in primary cervical cancer screening. Int J Cancer. 2006;119:1095-1101.
- Wright TC Jr, Massad LS, Dunton CJ, et al. 2006 consensus guidelines for the management of women with abnormal cervical cancer screening tests. Am J Obstet Gynecol. 2007;197:346-355.
- Tota JE, Bentley J, Blake J, et al. Introduction of molecular HPV testing as the primary technology in cervical cancer screening: acting on evidence to change the current paradigm. Prev Med. 2017;98:5-14.
- Ronco G, Giorgi Rossi P. Role of HPV DNA testing in modern gynaecological practice. Best Prac Res Clin Obstet Gynaecol. 2018;47:107-118.
- Wright TC, Stoler MH, Behrens CM, et al. Primary cervical cancer screening with human papillomavirus: end of study results from the ATHENA study using HPV as the first-line screening test. Gynecol Oncol. 2015;136:189-197.
- Mayrand MH, Duarte-Franco E, Rodrigues I, et al. Human papillomavirus DNA versus Papanicolaou screening tests for cervical cancer. N Engl J Med. 2007;357:1579-1588.
- Ronco G, Giorgi-Rossi P, Carozzi F, et al. Efficacy of human papillomavirus testing for the detection of invasive cervical cancers and cervical intraepithelial neoplasia: a randomised controlled trial. Lancet Oncol. 2010;11:249-257.
- Bulkmans NW, Rozendaal L, Snijders PJ, et al. POBASCAM, a population-based randomized controlled trial for implementation of high-risk HPV testing in cervical screening: design, methods and baseline data of 44,102 women. Int J Cancer. 2004;110:94-101.
- World Health Organization. WHO guideline for screening and treatment of cervical pre-cancer lesions for cervical cancer prevention. 2nd edition. Geneva: 2021. https://www .who.int/publications/i/item/9789240030824. Accessed April 28, 2022.
- American Cancer Society. The American Cancer Society guidelines for the prevention and early detection of cervical cancer. American Cancer Society; 2020. https://www.cancer .org/cancer/cervical-cancer/detection-diagnosis-staging /cervical-cancer-screening-guidelines.html. Accessed April 28, 2022.
- US Preventive Services Task Force; Curry SJ, Krist AH, Owens KD, et al. Screening for cervical cancer: US Preventive Services Task Force recommendation statement. JAMA. 2018;320:674-686.
- Moscicki AB, Flowers L, Huchko MJ, et al. Guidelines for cervical cancer screening in immunosuppressed women without HIV infection. J Low Gen Tract Dis. 2019;23:87-101.
- Committee opinion no. 675. Management of vulvar intraepithelial neoplasia. Obstet Gynecol. 2016;128:e178-e182.
- Satmary W, Holschneider CH, Brunette LL, et al. Vulvar intraepithelial neoplasia: risk factors for recurrence. Gynecol Oncol. 2018;148:126-131.
- Preti M, Scurry J, Marchitelli CE, et al. Vulvar intraepithelial neoplasia. Best Pract Res Clin Obstet Gynaecol. 2014;28:10511062.
- Khan MJ, Massad LS, Kinney W, et al. A common clinical dilemma: management of abnormal vaginal cytology and human papillomavirus test results. Gynecol Oncol. 2016;141:364-370.
- Perkins RB, Guido RS, Castle PE, et al. 2019 ASCCP risk-based management consensus guidelines for abnormal cervical cancer screening tests and cancer precursors. J Low Gen Tract Dis. 2020;24:102-131.
- Egemen D, Cheung LC, Chen X, et al. Risk estimates supporting the 2019 ASCCP risk-based management consensus guidelines. J Low Gen Tract Dis. 2020;24:132-143.
- Bhatla N, Singla S, Awasthi D. Human papillomavirus deoxyribonucleic acid testing in developed countries. Best Pract Res Clin Obstet Gynaecol. 2012;26:209-220.
- Meijer CJ, Berkhof J, Castle PE, et al. Guidelines for human papillomavirus DNA test requirements for primary cervical cancer screening in women 30 years and older. Int J Cancer. 2009;124:516-520.
- Ejegod D, Bottari F, Pedersen H, et al. The BD Onclarity HPV assay on samples collected in SurePath medium meets the international guidelines for human papillomavirus test requirements for cervical screening. J Clin Microbiol. 2016;54:2267-2272.
- Richardson LA, Tota J, Franco EL. Optimizing technology for cervical cancer screening in high-resource settings. Expert Rev Obstet Gynecol. 2011;6:343-353.
- Ronco G, Dillner J, Elfström KM, et al. Efficacy of HPV-based screening for prevention of invasive cervical cancer: followup of four European randomised controlled trials. Lancet. 2014;383:524-532.
- Ogilvie GS, van Niekerk D, Krajden M, et al. Effect of screening with primary cervical HPV testing vs cytology testing on high-grade cervical intraepithelial neoplasia at 48 months: the HPV FOCAL randomized clinical trial. JAMA. 2018;320:43-52.
- Dillner J, Rebolj M, Birembaut P, et al. Long term predictive values of cytology and human papillomavirus testing in cervical cancer screening: joint European cohort study. BMJ. 2008;337:a1754.
- Katki HA, Kinney WK, Fetterman B, et al. Cervical cancer risk for women undergoing concurrent testing for human papillomavirus and cervical cytology: a population-based study in routine clinical practice. Lancet Oncol. 2011;12:663-672.
- Arbyn M, Ronco G, Anttila A, et al. Evidence regarding human papillomavirus testing in secondary prevention of cervical cancer. Vaccine. 2012;30(suppl 5):F88-99.
- Schiffman M, Kinney WK, et al. Relative performance of HPV and cytology components of cotesting in cervical screening. J Nat Cancer Inst. 2018;110:501-508.
- Jin XW, Lipold L, Foucher J, et al. Cost-effectiveness of primary HPV testing, cytology and co-testing as cervical cancer screening for women above age 30 years. J Gen Intern Med. 2016;31:1338-1344.
- Tota JE, Bentley J, Blake J, et al. Approaches for triaging women who test positive for human papillomavirus in cervical cancer screening. Prev Med. 2017;98:15-20.
- Stoler MH, Wright TC Jr, Parvu V, et al. Stratified risk of high-grade cervical disease using onclarity HPV extended genotyping in women, ≥25 years of age, with NILM cytology. Gynecol Oncol. 2019;153:26-33.
- Louvanto K, Chevarie-Davis M, Ramanakumar AV, et al. HPV testing with cytology triage for cervical cancer screening in routine practice. Am J Obstet Gynecol. 2014;210:474.e1-7.
- Keating JT, Cviko A, Riethdorf S, et al. Ki-67, cyclin E, and p16INK4 are complimentary surrogate biomarkers for human papilloma virus-related cervical neoplasia. Am J Surg Pathol. 2001;25:884-891.
- Wright TC Jr, Stoler MH, Ranger-Moore J, et al. Clinical validation of p16/Ki-67 dual-stained cytology triage of HPV-positive women: results from the IMPACT trial. Int J Cancer. 2022;150:461-471.
- Wright TC Jr, Behrens CM, Ranger-Moore J, et al. Triaging HPV-positive women with p16/Ki-67 dual-stained cytology: results from a sub-study nested into the ATHENA trial. Gynecol Oncol. 2017;144:51-56.
- Ikenberg H, Bergeron C, Schmidt D, et al. Screening for cervical cancer precursors with p16/Ki-67 dual-stained cytology: results of the PALMS study. J Nat Cancer Inst. 2013;105:15501557.
- Arbyn M, Smith SB, Temin S, et al. Detecting cervical precancer and reaching underscreened women by using HPV testing on self samples: updated meta-analyses. BMJ. 2018;363:k4823.
- Verhoef VMJ, Bosgraaf RP, van Kemenade FJ, et al. Triage by methylation-marker testing versus cytology in women who test HPV-positive on self-collected cervicovaginal specimens (PROHTECT-3): a randomised controlled non-inferiority trial. Lancet Oncol. 2014;15:315-322.
- Perkins RB, Guido RS, Castle PE, et al. Erratum: 2019 ASCCP risk-based management consensus guidelines for abnormal cervical cancer screening tests and cancer precursors. J Low Gen Tract Dis. 2021;25:330-331.
- Hall MT, Simms KT, Lew JB, et al. The projected timeframe until cervical cancer elimination in Australia: a modelling study. Lancet Public Health. 2019;4:e19-e27.
- Huh WK, Ault KA, Chelmow D, et al. Use of primary high-risk human papillomavirus testing for cervical cancer screening: interim clinical guidance. Gynecol Oncol. 2015;136:178-182.
Cervical cancer: A path to eradication
David G. Mutch, MD: The cervical cancer screening guidelines, using Pap testing, have changed significantly since the times of yearly Paps and exams. Coupled with vaccination and new management guidelines (recommending HPV testing, etc), we actually hope that we are on the way to eradicating cervical cancer from our environment.
Screening: Current recommendations
Dr. Mutch: Warner, the American Society of Colposcopy and Cervical Pathology (ASCCP)1 endorses the cervical cancer screening guidelines for several professional organizations, including the American Cancer Society (ACS),2 the US Preventive Services Task Force (USPSTF),3 and the American College of Obstetricians and Gynecologists (ACOG).4 What are the current screening recommendations, as these organizations have disparate views?
Warner Huh, MD: There was a time, around 2012-2013, when for the first time ever, we had significant harmonization of the guidelines between ACOG and the USPSTF and ACS. But in the last 10 years there has been an explosion of data in terms of how to best screen patients.
The move to primary HPV testing. The USPSTF3 initially had recommended looking into primary HPV screening, which is just using HPV testing by itself as the screening modality. But there was a lot of pushback at that time. I think a lot of people thought that we were not prepared to make that leap. Therefore, they endorsed screening with cytology by itself as well as HPV testing by itself, as well as co-testing; but in their recommendations, they made it very clear that they were leaning toward primary HPV screening.
A new patient age to begin screening. In 2020, the ACS put out their new guidelines,2 which are a significant departure from what we are used to—they are recommending that we start screening at 25 years of age. Like you said, Dr. Mutch, it doesn’t seem that long ago when we were screening people at age 18, or within 3 years of sexual intercourse. But the reason for it is that the rate of cervical cancer is extremely low under age 25, and other countries like the United Kingdom already do this.
The other major departure in the ACS guidelines is that they really are asking clinicians and screeners to focus on primary HPV screening. Overall, they have sort of doubled down on why they think primary HPV screening is so important.
ACOG sits sort of in the middle of the other recommendations. ACOG understands the value of primary HPV screening, but I don’t think that they are quite ready to recommend screening at age 25. If you look at their updated guidelines from April 2021,4 they state that we should continue a screening-starting age of 21 years. So there are some disparate views, but I am confident, Dr. Mutch, that in the next 2 to 3 years, there will be greater harmonization of these guidelines and less confusion for our providers. The greatest barrier is understanding the science and the comfort level of clinicians to go with just an HPV test, since for the last 40 years the Pap test has anchored gynecologic care in this country. And it took at least 10 years to get to what I consider to be widespread adoption to co-testing. The other thing that readers should recognize is that the Task Force is actually revisiting their cervical cancer screening guidance now, so expect another major revision.
Reimbursement and access are barriers. Reimbursement is a further real issue. We are now using one less test, but insurance companies may not reimburse when just the HPV test is used. The other issue is access to labs that can do the HPV testing.
Dr. Mutch: We used to see patients yearly and picked up a lot of adjunctive or additional illnesses. Now they are not being seen yearly it could impact negatively their overall health care. We need to understand that cervical cancer screening is simply a test, which should not eliminate other health care.
Dr. Huh: Yes, I think the extended interval between recommended HPV screenings scares people. I have been involved in these screening guidelines (and I can only speak for myself, not for my colleagues), but even I do think we made a leap to a longer interval way too quickly in this country. Screening changes are slow, and sometimes a glacial process. I think it can worry providers when we make rapid changes.
But this is a test that should not anchor the yearly visit. There are plenty of other reasons—and ACOG actually states this4—why patients should come for a wellness exam on a yearly basis. So I think our ObGyns in the United States need to recognize that, but I understand there are underlying concerns that if you extend intervals too long, (a) will patients come back, and (b), as a consequence, is the interval going to miss something in between? Those are real legitimate concerns.
Continue to: Management guidelines...
Management guidelines: The latest
Dr. Mutch: The ASCCP issued new management guidelines in 2019.5 Can you address what you feel are the most important updates?
Dr. Huh: Going back to 2002, we have revised these guidelines every 5 years. For this one, the revision came out a little bit later for various reasons, but the reason we revised it is because we collect new data that we think markedly changes our understanding of the disease process and natural history and the interventions for women that have preinvasive disease of the cervix.
Briefly, I think the biggest changes based on what we were hearing from our providers and users of our apps and algorithms was that our algorithms were becoming way too complicated, and they were. If you look over the last 10 years, the number of branch points on our algorithms basically quadrupled. If we incorporated the new data this time, the algorithms would be unworkable, and you could not use them on your phone because they would be too complicated.
So, we created a system where, in essence, providers have 5 choices for patients:
- treatment
- colposcopy
- follow-up in 1 year
- follow-up in 3 years
- follow-up in 5 years.
Those recommendations are based on what we call “clinically actionable thresholds”—basically, the percent chance of developing immediate CIN3 or worse. That threshold will probably change over time, but what we did is create a system that (a) makes it easier for the provider, (although they have to trust the system—and they can look under the hood and understand how we did this) and (b) allows us to create a foundation where we can add future technologies that use the same rubric or paradigm so that they still wind up getting the same result without having to go to another algorithm.
This new system is probably the most marked change in the history of the ASCCP management guidelines, but we did it to make it ultimately easier for providers going forward for the next 10 to 20 years. There are real opportunities, Dr. Mutch, in terms of how do we integrate this into the electronic medical record (EMR), and how do we pull data so clinicians don’t have to manually enter it.
The other difference is now there is a web-based application. Back in 2012, there were a lot of people that were not using EMRs. Now the majority of the country is, and so they actually are on a browser more than they are on their phone. We actually have an equally robust web platform that allows them to get the information that they need.
Dr. Mutch: I think that is really important—the utility of utilizing a mobile app, if you will, for triaging your patient with a specific test result so that patients are followed up at the proper interval, and that ultimately becomes cost-effective.
Dr. Huh: Yes, the app now is very different than the app that I think people are used to using for the last almost 10 years. You don’t put inputs, pull up the algorithm, and look at the outcome. This is different. You enter the patient’s age. You add their cytology, their HPV results, the clinical scenario that you are in, and then it puts out a recommendation of what to do next. Over time, we want to get away from an algorithm and for our providers to understand what the risk is and how that risk calculation then translates into a clinical recommendation.
Dr. Mutch: I think to utilize an app is almost necessary given the complexity of the triaging process so that it does become, in fact, the most cost-effective way to screen patients.
Dr. Huh: I would agree with that. There is a learning curve for whenever you see new technology. There was a learning curve for even ASCCP leadership as they tried to educate providers. I think people will ultimately see that this is a much better way of managing patients with cervical abnormalities, and I am hoping actually that we will use a similar platform for many other diseases that we manage in women’s health.
Continue to: Chipping away of the yearly exam...
Chipping away of the yearly exam
Dr. Mutch: With this moving away from the yearly exam and Pap test, women may not get yearly examinations. Do you feel that this could affect a stage migration to a higher stage at diagnosis, for instance, of a cervical cancer? Or that it might adversely impact other health issues?
Dr. Huh: I think that’s a good question. I am worried about the interval—I think 5 years is a bit long. I am more worried that patients will miss out on visits because they may think that they need to only come back for their Pap, even though they should be re-educated on that.
COVID-19 has made this a little hard for us to analyze because, clearly, we have had access to care issues. But I am a little concerned that we could see an uptick in invasive cancer rates in this country, including an uptick in the stage and more locally advanced cervical cancer because of the changes in the screening paradigms. But we don’t know that to be the case.
As with all screenings, the bottom line is you have to worry about what the false-negative and false-positive rates of screening are, and that affects everything. I want the readers to know that primary HPV should be used for screenings. It is not perfect, but it is much better than cytology alone. We need to think about how to better adapt screening in the age that we live in.
HPV self-sampling
Dr. Mutch: Could self-sampling for HPV testing, which obviously would be easier for the patient, and certainly useful in terms of screening, address some health care disparities with regard to cervical cancer?
Dr. Huh: The short answer is, yes. Self-sampling is not US Food and Drug Administration-approved in this country. It’s not being widely used without that approval. But there are multiple countries, including the United States, that have done lots of studies on this topic. There are many public health experts and champions for HPV self-sampling. I think we have learned, based on some studies, that the sensitivity is reasonable.6
I live in a part of the country that is woefully underserved; where you are there are pockets in Missouri that are woefully underserved as well. So the issue is, can we reduce these disparities and access to care with something like self-sampling? My personal feeling is I think that we can make a dent in that, and it is never going to fully replace screening, but it at least will allow us to reallocate our resources and attention to those women that are at highest risk for developing cervical cancer or precancer based on the self-sampling result.
I don’t think it will ever replace screening per se, but if we have an abnormal self-sampling test, we might say to that patient, “You really do need to come in to get re-tested or to get re-evaluated.” So it could be a better resource and use of our health care dollars and investments in terms of trying to reduce the incidence of cervical cancer. Of course the verdict is out, but I think there are a lot of people who would love to see this scenario.
If we screen and treat perfectly in this country, we would not even need the HPV vaccine when it comes to cervical cancer. That is how effective screening is. But, up to 50% to 60% of women in this country now still are underscreened or unscreened. We were talking about that number almost 25-30 years ago, Dr. Mutch. So access to screening is a big problem, but the other problem is how do you get patients in to be seen if they have an abnormal screening test? It’s not just about screening. It’s about screening, evaluation, and treatment; all 3 components are really important.
Continue to: Where do we stand with HPV vaccination?...
Where do we stand with HPV vaccination?
Dr. Mutch: Those are great points.
You brought up vaccination. We have a long way to go with regard to that, certainly in the United States, because of the various factions opposed to vaccination and so on. But do you think that vaccination has allowed us to decrease the incidence of cervical cancer?
Dr. Huh: Yes. There is clear evidence from the Nordic countries.7 There is emerging evidence from Australia.8 There is emerging evidence from other industrialized nations that clearly demonstrate vaccination’s positive effect in reducing the incidence of cervical cancer. None of this should be a surprise. Every population-based study that has been published with the HPV vaccine in populations that have a low frequency of vaccination have demonstrated substantial reductions in things like genital warts, abnormal Pap tests, precancer, and now evidence that there is a downward trend in terms of the incidence of cervical cancer.9
I don’t think that there is any debate anymore that vaccination is the way to go. Our challenge is about implementation and getting the vaccine to people. We still have a long way to go with that. There are parts of the world that are so affected by invasive cervical cancers; we need to get the vaccine to those parts of the world.
Dr. Mutch: What are the barriers to vaccination? How can we overcome those barriers?
Dr. Huh: There is a lot of criticism that we are not vaccinating more in the United States. However, the rates of vaccination are going up every single year. The pandemic may have blunted that rise a bit, but if you look at the vaccination curves, they are going up, not down. We need to continue to educate patients, parents, and pediatricians on the importance of vaccination.
Boys still get vaccinated less frequently than girls, so we have some work to do there. I think globally it is the issue of getting the vaccine to people, making sure that vaccine is available. The thing that I think will be the game-changer going forward is whether or not we will have evidence to indicate that 1 dose is as effective as 2 doses or 3 doses. If we can vaccinate boys and girls with just 1 dose, then in the next generation or two, we seriously might eradicate not just cervical cancer but a lot of HPV-related malignancies worldwide.
Educating patients, clinicians is key
Dr. Mutch: So it seems education, education, education, with regard to screening guidelines, with regard to the need for continued examinations, and that HPV testing is only a test, it does not supplant overall care. Finally, education regarding eradication of cervical cancer through vaccination.
Dr. Huh: That summarizes it well. We are still going to screen for cervical cancer. We are still going to vaccinate, and providers are still going to manage abnormal Pap tests. It is confusing because we are changing it up it seems every year or 2, so this conversation you and I are having is particularly important for clinicians to understand the basis of that. There has been an explosion of data that has come out in this area in the last decade.
Dr. Mutch: Thank you, Dr. Huh. I really appreciate your thoughts on this. As you all know, Dr. Huh has been President of the ASCCP and is instrumental in writing and disseminating these guidelines, so we are very grateful that he has consented to agree to come and talk with us today.
Dr. Huh: My pleasure. Thank you for inviting me. This was fun, and I have really enjoyed talking to you and participating. ●
- ASCCP Screening Guidelines. https://www.asccp.org /screening-guidelines. Accessed April 25, 2021.
- Fontham ET, Wolf AM, Church TR, et al. Cervical cancer screening for individuals at average risk: 2020 guidelines update from the American Cancer Society. CA Cancer J Clin. 2020;70:321-346. doi:10.3322/caac.21628.
- US Preventive Services Task Force. Screening for cervical cancer. US Preventive Services Task Force Recommendation Statement. JAMA. 2018;320:674-686. doi:10.1001/jama .2018.10897.
- American College of Obstetricians and Gynecologists. Practice Advisory: Updated cervical cancer screening guidelines. April 2022. https://www.acog.org/clinical/clinical-guidance /practice-advisory/articles/2021/04/updated-cervical -cancer-screening-guidelines. Accessed April 25, 2022.
- Perkins RB, Guido RS, Castle PE, et al; 2019 ASCCP RiskBased Management Consensus Guidelines Committee. 2019 ASCCP risk-based management consensus guidelines for abnormal cervical cancer screening tests and cancer precursors. J Low Genit Tract Dis. 2020;24:102-131. doi: 10 34 .1097/LGT.0000000000000525.
- Yeh PT, Kennedy CE, de Vuyst H, et al. Self-sampling for human papillomavirus (HPV) testing: a systematic review and meta-analysis. BMJ Global Health. 2019;4:e001351. doi:10.1136/ bmjgh-2018-001351.
- Kjaer SK, Nygard M, Dillner J, et al. A 12-year follow-up on the long-term effectiveness of the quadrivalent human papillomavirus vaccine in 4 Nordic countries. Clin Infect Dis. 2018;66:339-345. doi: 10.1093/cid/cix797.
- Patel C, Brotherton JM, Pillsbury A, et al. The impact of 10 years of human papillomavirus (HPV) vaccination in Australia: what additional disease burden will a nonvalent vaccine prevent? Euro Surveill. 2018;23:1700737. doi: 10 .2807/1560-7917.
- Falcaro M, Castanon A, Ndlela B, et al. The effects of the national HPV vaccination programme in England, UK, on cervical cancer and grade 3 cervical intraepithelial neoplasia incidence: a register-based observational study. 2021;398:2084-2092. doi.org/10.1016/S0140-6736(21) 02178-4.

David G. Mutch, MD: The cervical cancer screening guidelines, using Pap testing, have changed significantly since the times of yearly Paps and exams. Coupled with vaccination and new management guidelines (recommending HPV testing, etc), we actually hope that we are on the way to eradicating cervical cancer from our environment.
Screening: Current recommendations
Dr. Mutch: Warner, the American Society of Colposcopy and Cervical Pathology (ASCCP)1 endorses the cervical cancer screening guidelines for several professional organizations, including the American Cancer Society (ACS),2 the US Preventive Services Task Force (USPSTF),3 and the American College of Obstetricians and Gynecologists (ACOG).4 What are the current screening recommendations, as these organizations have disparate views?
Warner Huh, MD: There was a time, around 2012-2013, when for the first time ever, we had significant harmonization of the guidelines between ACOG and the USPSTF and ACS. But in the last 10 years there has been an explosion of data in terms of how to best screen patients.
The move to primary HPV testing. The USPSTF3 initially had recommended looking into primary HPV screening, which is just using HPV testing by itself as the screening modality. But there was a lot of pushback at that time. I think a lot of people thought that we were not prepared to make that leap. Therefore, they endorsed screening with cytology by itself as well as HPV testing by itself, as well as co-testing; but in their recommendations, they made it very clear that they were leaning toward primary HPV screening.
A new patient age to begin screening. In 2020, the ACS put out their new guidelines,2 which are a significant departure from what we are used to—they are recommending that we start screening at 25 years of age. Like you said, Dr. Mutch, it doesn’t seem that long ago when we were screening people at age 18, or within 3 years of sexual intercourse. But the reason for it is that the rate of cervical cancer is extremely low under age 25, and other countries like the United Kingdom already do this.
The other major departure in the ACS guidelines is that they really are asking clinicians and screeners to focus on primary HPV screening. Overall, they have sort of doubled down on why they think primary HPV screening is so important.
ACOG sits sort of in the middle of the other recommendations. ACOG understands the value of primary HPV screening, but I don’t think that they are quite ready to recommend screening at age 25. If you look at their updated guidelines from April 2021,4 they state that we should continue a screening-starting age of 21 years. So there are some disparate views, but I am confident, Dr. Mutch, that in the next 2 to 3 years, there will be greater harmonization of these guidelines and less confusion for our providers. The greatest barrier is understanding the science and the comfort level of clinicians to go with just an HPV test, since for the last 40 years the Pap test has anchored gynecologic care in this country. And it took at least 10 years to get to what I consider to be widespread adoption to co-testing. The other thing that readers should recognize is that the Task Force is actually revisiting their cervical cancer screening guidance now, so expect another major revision.
Reimbursement and access are barriers. Reimbursement is a further real issue. We are now using one less test, but insurance companies may not reimburse when just the HPV test is used. The other issue is access to labs that can do the HPV testing.
Dr. Mutch: We used to see patients yearly and picked up a lot of adjunctive or additional illnesses. Now they are not being seen yearly it could impact negatively their overall health care. We need to understand that cervical cancer screening is simply a test, which should not eliminate other health care.
Dr. Huh: Yes, I think the extended interval between recommended HPV screenings scares people. I have been involved in these screening guidelines (and I can only speak for myself, not for my colleagues), but even I do think we made a leap to a longer interval way too quickly in this country. Screening changes are slow, and sometimes a glacial process. I think it can worry providers when we make rapid changes.
But this is a test that should not anchor the yearly visit. There are plenty of other reasons—and ACOG actually states this4—why patients should come for a wellness exam on a yearly basis. So I think our ObGyns in the United States need to recognize that, but I understand there are underlying concerns that if you extend intervals too long, (a) will patients come back, and (b), as a consequence, is the interval going to miss something in between? Those are real legitimate concerns.
Continue to: Management guidelines...
Management guidelines: The latest
Dr. Mutch: The ASCCP issued new management guidelines in 2019.5 Can you address what you feel are the most important updates?
Dr. Huh: Going back to 2002, we have revised these guidelines every 5 years. For this one, the revision came out a little bit later for various reasons, but the reason we revised it is because we collect new data that we think markedly changes our understanding of the disease process and natural history and the interventions for women that have preinvasive disease of the cervix.
Briefly, I think the biggest changes based on what we were hearing from our providers and users of our apps and algorithms was that our algorithms were becoming way too complicated, and they were. If you look over the last 10 years, the number of branch points on our algorithms basically quadrupled. If we incorporated the new data this time, the algorithms would be unworkable, and you could not use them on your phone because they would be too complicated.
So, we created a system where, in essence, providers have 5 choices for patients:
- treatment
- colposcopy
- follow-up in 1 year
- follow-up in 3 years
- follow-up in 5 years.
Those recommendations are based on what we call “clinically actionable thresholds”—basically, the percent chance of developing immediate CIN3 or worse. That threshold will probably change over time, but what we did is create a system that (a) makes it easier for the provider, (although they have to trust the system—and they can look under the hood and understand how we did this) and (b) allows us to create a foundation where we can add future technologies that use the same rubric or paradigm so that they still wind up getting the same result without having to go to another algorithm.
This new system is probably the most marked change in the history of the ASCCP management guidelines, but we did it to make it ultimately easier for providers going forward for the next 10 to 20 years. There are real opportunities, Dr. Mutch, in terms of how do we integrate this into the electronic medical record (EMR), and how do we pull data so clinicians don’t have to manually enter it.
The other difference is now there is a web-based application. Back in 2012, there were a lot of people that were not using EMRs. Now the majority of the country is, and so they actually are on a browser more than they are on their phone. We actually have an equally robust web platform that allows them to get the information that they need.
Dr. Mutch: I think that is really important—the utility of utilizing a mobile app, if you will, for triaging your patient with a specific test result so that patients are followed up at the proper interval, and that ultimately becomes cost-effective.
Dr. Huh: Yes, the app now is very different than the app that I think people are used to using for the last almost 10 years. You don’t put inputs, pull up the algorithm, and look at the outcome. This is different. You enter the patient’s age. You add their cytology, their HPV results, the clinical scenario that you are in, and then it puts out a recommendation of what to do next. Over time, we want to get away from an algorithm and for our providers to understand what the risk is and how that risk calculation then translates into a clinical recommendation.
Dr. Mutch: I think to utilize an app is almost necessary given the complexity of the triaging process so that it does become, in fact, the most cost-effective way to screen patients.
Dr. Huh: I would agree with that. There is a learning curve for whenever you see new technology. There was a learning curve for even ASCCP leadership as they tried to educate providers. I think people will ultimately see that this is a much better way of managing patients with cervical abnormalities, and I am hoping actually that we will use a similar platform for many other diseases that we manage in women’s health.
Continue to: Chipping away of the yearly exam...
Chipping away of the yearly exam
Dr. Mutch: With this moving away from the yearly exam and Pap test, women may not get yearly examinations. Do you feel that this could affect a stage migration to a higher stage at diagnosis, for instance, of a cervical cancer? Or that it might adversely impact other health issues?
Dr. Huh: I think that’s a good question. I am worried about the interval—I think 5 years is a bit long. I am more worried that patients will miss out on visits because they may think that they need to only come back for their Pap, even though they should be re-educated on that.
COVID-19 has made this a little hard for us to analyze because, clearly, we have had access to care issues. But I am a little concerned that we could see an uptick in invasive cancer rates in this country, including an uptick in the stage and more locally advanced cervical cancer because of the changes in the screening paradigms. But we don’t know that to be the case.
As with all screenings, the bottom line is you have to worry about what the false-negative and false-positive rates of screening are, and that affects everything. I want the readers to know that primary HPV should be used for screenings. It is not perfect, but it is much better than cytology alone. We need to think about how to better adapt screening in the age that we live in.
HPV self-sampling
Dr. Mutch: Could self-sampling for HPV testing, which obviously would be easier for the patient, and certainly useful in terms of screening, address some health care disparities with regard to cervical cancer?
Dr. Huh: The short answer is, yes. Self-sampling is not US Food and Drug Administration-approved in this country. It’s not being widely used without that approval. But there are multiple countries, including the United States, that have done lots of studies on this topic. There are many public health experts and champions for HPV self-sampling. I think we have learned, based on some studies, that the sensitivity is reasonable.6
I live in a part of the country that is woefully underserved; where you are there are pockets in Missouri that are woefully underserved as well. So the issue is, can we reduce these disparities and access to care with something like self-sampling? My personal feeling is I think that we can make a dent in that, and it is never going to fully replace screening, but it at least will allow us to reallocate our resources and attention to those women that are at highest risk for developing cervical cancer or precancer based on the self-sampling result.
I don’t think it will ever replace screening per se, but if we have an abnormal self-sampling test, we might say to that patient, “You really do need to come in to get re-tested or to get re-evaluated.” So it could be a better resource and use of our health care dollars and investments in terms of trying to reduce the incidence of cervical cancer. Of course the verdict is out, but I think there are a lot of people who would love to see this scenario.
If we screen and treat perfectly in this country, we would not even need the HPV vaccine when it comes to cervical cancer. That is how effective screening is. But, up to 50% to 60% of women in this country now still are underscreened or unscreened. We were talking about that number almost 25-30 years ago, Dr. Mutch. So access to screening is a big problem, but the other problem is how do you get patients in to be seen if they have an abnormal screening test? It’s not just about screening. It’s about screening, evaluation, and treatment; all 3 components are really important.
Continue to: Where do we stand with HPV vaccination?...
Where do we stand with HPV vaccination?
Dr. Mutch: Those are great points.
You brought up vaccination. We have a long way to go with regard to that, certainly in the United States, because of the various factions opposed to vaccination and so on. But do you think that vaccination has allowed us to decrease the incidence of cervical cancer?
Dr. Huh: Yes. There is clear evidence from the Nordic countries.7 There is emerging evidence from Australia.8 There is emerging evidence from other industrialized nations that clearly demonstrate vaccination’s positive effect in reducing the incidence of cervical cancer. None of this should be a surprise. Every population-based study that has been published with the HPV vaccine in populations that have a low frequency of vaccination have demonstrated substantial reductions in things like genital warts, abnormal Pap tests, precancer, and now evidence that there is a downward trend in terms of the incidence of cervical cancer.9
I don’t think that there is any debate anymore that vaccination is the way to go. Our challenge is about implementation and getting the vaccine to people. We still have a long way to go with that. There are parts of the world that are so affected by invasive cervical cancers; we need to get the vaccine to those parts of the world.
Dr. Mutch: What are the barriers to vaccination? How can we overcome those barriers?
Dr. Huh: There is a lot of criticism that we are not vaccinating more in the United States. However, the rates of vaccination are going up every single year. The pandemic may have blunted that rise a bit, but if you look at the vaccination curves, they are going up, not down. We need to continue to educate patients, parents, and pediatricians on the importance of vaccination.
Boys still get vaccinated less frequently than girls, so we have some work to do there. I think globally it is the issue of getting the vaccine to people, making sure that vaccine is available. The thing that I think will be the game-changer going forward is whether or not we will have evidence to indicate that 1 dose is as effective as 2 doses or 3 doses. If we can vaccinate boys and girls with just 1 dose, then in the next generation or two, we seriously might eradicate not just cervical cancer but a lot of HPV-related malignancies worldwide.
Educating patients, clinicians is key
Dr. Mutch: So it seems education, education, education, with regard to screening guidelines, with regard to the need for continued examinations, and that HPV testing is only a test, it does not supplant overall care. Finally, education regarding eradication of cervical cancer through vaccination.
Dr. Huh: That summarizes it well. We are still going to screen for cervical cancer. We are still going to vaccinate, and providers are still going to manage abnormal Pap tests. It is confusing because we are changing it up it seems every year or 2, so this conversation you and I are having is particularly important for clinicians to understand the basis of that. There has been an explosion of data that has come out in this area in the last decade.
Dr. Mutch: Thank you, Dr. Huh. I really appreciate your thoughts on this. As you all know, Dr. Huh has been President of the ASCCP and is instrumental in writing and disseminating these guidelines, so we are very grateful that he has consented to agree to come and talk with us today.
Dr. Huh: My pleasure. Thank you for inviting me. This was fun, and I have really enjoyed talking to you and participating. ●

David G. Mutch, MD: The cervical cancer screening guidelines, using Pap testing, have changed significantly since the times of yearly Paps and exams. Coupled with vaccination and new management guidelines (recommending HPV testing, etc), we actually hope that we are on the way to eradicating cervical cancer from our environment.
Screening: Current recommendations
Dr. Mutch: Warner, the American Society of Colposcopy and Cervical Pathology (ASCCP)1 endorses the cervical cancer screening guidelines for several professional organizations, including the American Cancer Society (ACS),2 the US Preventive Services Task Force (USPSTF),3 and the American College of Obstetricians and Gynecologists (ACOG).4 What are the current screening recommendations, as these organizations have disparate views?
Warner Huh, MD: There was a time, around 2012-2013, when for the first time ever, we had significant harmonization of the guidelines between ACOG and the USPSTF and ACS. But in the last 10 years there has been an explosion of data in terms of how to best screen patients.
The move to primary HPV testing. The USPSTF3 initially had recommended looking into primary HPV screening, which is just using HPV testing by itself as the screening modality. But there was a lot of pushback at that time. I think a lot of people thought that we were not prepared to make that leap. Therefore, they endorsed screening with cytology by itself as well as HPV testing by itself, as well as co-testing; but in their recommendations, they made it very clear that they were leaning toward primary HPV screening.
A new patient age to begin screening. In 2020, the ACS put out their new guidelines,2 which are a significant departure from what we are used to—they are recommending that we start screening at 25 years of age. Like you said, Dr. Mutch, it doesn’t seem that long ago when we were screening people at age 18, or within 3 years of sexual intercourse. But the reason for it is that the rate of cervical cancer is extremely low under age 25, and other countries like the United Kingdom already do this.
The other major departure in the ACS guidelines is that they really are asking clinicians and screeners to focus on primary HPV screening. Overall, they have sort of doubled down on why they think primary HPV screening is so important.
ACOG sits sort of in the middle of the other recommendations. ACOG understands the value of primary HPV screening, but I don’t think that they are quite ready to recommend screening at age 25. If you look at their updated guidelines from April 2021,4 they state that we should continue a screening-starting age of 21 years. So there are some disparate views, but I am confident, Dr. Mutch, that in the next 2 to 3 years, there will be greater harmonization of these guidelines and less confusion for our providers. The greatest barrier is understanding the science and the comfort level of clinicians to go with just an HPV test, since for the last 40 years the Pap test has anchored gynecologic care in this country. And it took at least 10 years to get to what I consider to be widespread adoption to co-testing. The other thing that readers should recognize is that the Task Force is actually revisiting their cervical cancer screening guidance now, so expect another major revision.
Reimbursement and access are barriers. Reimbursement is a further real issue. We are now using one less test, but insurance companies may not reimburse when just the HPV test is used. The other issue is access to labs that can do the HPV testing.
Dr. Mutch: We used to see patients yearly and picked up a lot of adjunctive or additional illnesses. Now they are not being seen yearly it could impact negatively their overall health care. We need to understand that cervical cancer screening is simply a test, which should not eliminate other health care.
Dr. Huh: Yes, I think the extended interval between recommended HPV screenings scares people. I have been involved in these screening guidelines (and I can only speak for myself, not for my colleagues), but even I do think we made a leap to a longer interval way too quickly in this country. Screening changes are slow, and sometimes a glacial process. I think it can worry providers when we make rapid changes.
But this is a test that should not anchor the yearly visit. There are plenty of other reasons—and ACOG actually states this4—why patients should come for a wellness exam on a yearly basis. So I think our ObGyns in the United States need to recognize that, but I understand there are underlying concerns that if you extend intervals too long, (a) will patients come back, and (b), as a consequence, is the interval going to miss something in between? Those are real legitimate concerns.
Continue to: Management guidelines...
Management guidelines: The latest
Dr. Mutch: The ASCCP issued new management guidelines in 2019.5 Can you address what you feel are the most important updates?
Dr. Huh: Going back to 2002, we have revised these guidelines every 5 years. For this one, the revision came out a little bit later for various reasons, but the reason we revised it is because we collect new data that we think markedly changes our understanding of the disease process and natural history and the interventions for women that have preinvasive disease of the cervix.
Briefly, I think the biggest changes based on what we were hearing from our providers and users of our apps and algorithms was that our algorithms were becoming way too complicated, and they were. If you look over the last 10 years, the number of branch points on our algorithms basically quadrupled. If we incorporated the new data this time, the algorithms would be unworkable, and you could not use them on your phone because they would be too complicated.
So, we created a system where, in essence, providers have 5 choices for patients:
- treatment
- colposcopy
- follow-up in 1 year
- follow-up in 3 years
- follow-up in 5 years.
Those recommendations are based on what we call “clinically actionable thresholds”—basically, the percent chance of developing immediate CIN3 or worse. That threshold will probably change over time, but what we did is create a system that (a) makes it easier for the provider, (although they have to trust the system—and they can look under the hood and understand how we did this) and (b) allows us to create a foundation where we can add future technologies that use the same rubric or paradigm so that they still wind up getting the same result without having to go to another algorithm.
This new system is probably the most marked change in the history of the ASCCP management guidelines, but we did it to make it ultimately easier for providers going forward for the next 10 to 20 years. There are real opportunities, Dr. Mutch, in terms of how do we integrate this into the electronic medical record (EMR), and how do we pull data so clinicians don’t have to manually enter it.
The other difference is now there is a web-based application. Back in 2012, there were a lot of people that were not using EMRs. Now the majority of the country is, and so they actually are on a browser more than they are on their phone. We actually have an equally robust web platform that allows them to get the information that they need.
Dr. Mutch: I think that is really important—the utility of utilizing a mobile app, if you will, for triaging your patient with a specific test result so that patients are followed up at the proper interval, and that ultimately becomes cost-effective.
Dr. Huh: Yes, the app now is very different than the app that I think people are used to using for the last almost 10 years. You don’t put inputs, pull up the algorithm, and look at the outcome. This is different. You enter the patient’s age. You add their cytology, their HPV results, the clinical scenario that you are in, and then it puts out a recommendation of what to do next. Over time, we want to get away from an algorithm and for our providers to understand what the risk is and how that risk calculation then translates into a clinical recommendation.
Dr. Mutch: I think to utilize an app is almost necessary given the complexity of the triaging process so that it does become, in fact, the most cost-effective way to screen patients.
Dr. Huh: I would agree with that. There is a learning curve for whenever you see new technology. There was a learning curve for even ASCCP leadership as they tried to educate providers. I think people will ultimately see that this is a much better way of managing patients with cervical abnormalities, and I am hoping actually that we will use a similar platform for many other diseases that we manage in women’s health.
Continue to: Chipping away of the yearly exam...
Chipping away of the yearly exam
Dr. Mutch: With this moving away from the yearly exam and Pap test, women may not get yearly examinations. Do you feel that this could affect a stage migration to a higher stage at diagnosis, for instance, of a cervical cancer? Or that it might adversely impact other health issues?
Dr. Huh: I think that’s a good question. I am worried about the interval—I think 5 years is a bit long. I am more worried that patients will miss out on visits because they may think that they need to only come back for their Pap, even though they should be re-educated on that.
COVID-19 has made this a little hard for us to analyze because, clearly, we have had access to care issues. But I am a little concerned that we could see an uptick in invasive cancer rates in this country, including an uptick in the stage and more locally advanced cervical cancer because of the changes in the screening paradigms. But we don’t know that to be the case.
As with all screenings, the bottom line is you have to worry about what the false-negative and false-positive rates of screening are, and that affects everything. I want the readers to know that primary HPV should be used for screenings. It is not perfect, but it is much better than cytology alone. We need to think about how to better adapt screening in the age that we live in.
HPV self-sampling
Dr. Mutch: Could self-sampling for HPV testing, which obviously would be easier for the patient, and certainly useful in terms of screening, address some health care disparities with regard to cervical cancer?
Dr. Huh: The short answer is, yes. Self-sampling is not US Food and Drug Administration-approved in this country. It’s not being widely used without that approval. But there are multiple countries, including the United States, that have done lots of studies on this topic. There are many public health experts and champions for HPV self-sampling. I think we have learned, based on some studies, that the sensitivity is reasonable.6
I live in a part of the country that is woefully underserved; where you are there are pockets in Missouri that are woefully underserved as well. So the issue is, can we reduce these disparities and access to care with something like self-sampling? My personal feeling is I think that we can make a dent in that, and it is never going to fully replace screening, but it at least will allow us to reallocate our resources and attention to those women that are at highest risk for developing cervical cancer or precancer based on the self-sampling result.
I don’t think it will ever replace screening per se, but if we have an abnormal self-sampling test, we might say to that patient, “You really do need to come in to get re-tested or to get re-evaluated.” So it could be a better resource and use of our health care dollars and investments in terms of trying to reduce the incidence of cervical cancer. Of course the verdict is out, but I think there are a lot of people who would love to see this scenario.
If we screen and treat perfectly in this country, we would not even need the HPV vaccine when it comes to cervical cancer. That is how effective screening is. But, up to 50% to 60% of women in this country now still are underscreened or unscreened. We were talking about that number almost 25-30 years ago, Dr. Mutch. So access to screening is a big problem, but the other problem is how do you get patients in to be seen if they have an abnormal screening test? It’s not just about screening. It’s about screening, evaluation, and treatment; all 3 components are really important.
Continue to: Where do we stand with HPV vaccination?...
Where do we stand with HPV vaccination?
Dr. Mutch: Those are great points.
You brought up vaccination. We have a long way to go with regard to that, certainly in the United States, because of the various factions opposed to vaccination and so on. But do you think that vaccination has allowed us to decrease the incidence of cervical cancer?
Dr. Huh: Yes. There is clear evidence from the Nordic countries.7 There is emerging evidence from Australia.8 There is emerging evidence from other industrialized nations that clearly demonstrate vaccination’s positive effect in reducing the incidence of cervical cancer. None of this should be a surprise. Every population-based study that has been published with the HPV vaccine in populations that have a low frequency of vaccination have demonstrated substantial reductions in things like genital warts, abnormal Pap tests, precancer, and now evidence that there is a downward trend in terms of the incidence of cervical cancer.9
I don’t think that there is any debate anymore that vaccination is the way to go. Our challenge is about implementation and getting the vaccine to people. We still have a long way to go with that. There are parts of the world that are so affected by invasive cervical cancers; we need to get the vaccine to those parts of the world.
Dr. Mutch: What are the barriers to vaccination? How can we overcome those barriers?
Dr. Huh: There is a lot of criticism that we are not vaccinating more in the United States. However, the rates of vaccination are going up every single year. The pandemic may have blunted that rise a bit, but if you look at the vaccination curves, they are going up, not down. We need to continue to educate patients, parents, and pediatricians on the importance of vaccination.
Boys still get vaccinated less frequently than girls, so we have some work to do there. I think globally it is the issue of getting the vaccine to people, making sure that vaccine is available. The thing that I think will be the game-changer going forward is whether or not we will have evidence to indicate that 1 dose is as effective as 2 doses or 3 doses. If we can vaccinate boys and girls with just 1 dose, then in the next generation or two, we seriously might eradicate not just cervical cancer but a lot of HPV-related malignancies worldwide.
Educating patients, clinicians is key
Dr. Mutch: So it seems education, education, education, with regard to screening guidelines, with regard to the need for continued examinations, and that HPV testing is only a test, it does not supplant overall care. Finally, education regarding eradication of cervical cancer through vaccination.
Dr. Huh: That summarizes it well. We are still going to screen for cervical cancer. We are still going to vaccinate, and providers are still going to manage abnormal Pap tests. It is confusing because we are changing it up it seems every year or 2, so this conversation you and I are having is particularly important for clinicians to understand the basis of that. There has been an explosion of data that has come out in this area in the last decade.
Dr. Mutch: Thank you, Dr. Huh. I really appreciate your thoughts on this. As you all know, Dr. Huh has been President of the ASCCP and is instrumental in writing and disseminating these guidelines, so we are very grateful that he has consented to agree to come and talk with us today.
Dr. Huh: My pleasure. Thank you for inviting me. This was fun, and I have really enjoyed talking to you and participating. ●
- ASCCP Screening Guidelines. https://www.asccp.org /screening-guidelines. Accessed April 25, 2021.
- Fontham ET, Wolf AM, Church TR, et al. Cervical cancer screening for individuals at average risk: 2020 guidelines update from the American Cancer Society. CA Cancer J Clin. 2020;70:321-346. doi:10.3322/caac.21628.
- US Preventive Services Task Force. Screening for cervical cancer. US Preventive Services Task Force Recommendation Statement. JAMA. 2018;320:674-686. doi:10.1001/jama .2018.10897.
- American College of Obstetricians and Gynecologists. Practice Advisory: Updated cervical cancer screening guidelines. April 2022. https://www.acog.org/clinical/clinical-guidance /practice-advisory/articles/2021/04/updated-cervical -cancer-screening-guidelines. Accessed April 25, 2022.
- Perkins RB, Guido RS, Castle PE, et al; 2019 ASCCP RiskBased Management Consensus Guidelines Committee. 2019 ASCCP risk-based management consensus guidelines for abnormal cervical cancer screening tests and cancer precursors. J Low Genit Tract Dis. 2020;24:102-131. doi: 10 34 .1097/LGT.0000000000000525.
- Yeh PT, Kennedy CE, de Vuyst H, et al. Self-sampling for human papillomavirus (HPV) testing: a systematic review and meta-analysis. BMJ Global Health. 2019;4:e001351. doi:10.1136/ bmjgh-2018-001351.
- Kjaer SK, Nygard M, Dillner J, et al. A 12-year follow-up on the long-term effectiveness of the quadrivalent human papillomavirus vaccine in 4 Nordic countries. Clin Infect Dis. 2018;66:339-345. doi: 10.1093/cid/cix797.
- Patel C, Brotherton JM, Pillsbury A, et al. The impact of 10 years of human papillomavirus (HPV) vaccination in Australia: what additional disease burden will a nonvalent vaccine prevent? Euro Surveill. 2018;23:1700737. doi: 10 .2807/1560-7917.
- Falcaro M, Castanon A, Ndlela B, et al. The effects of the national HPV vaccination programme in England, UK, on cervical cancer and grade 3 cervical intraepithelial neoplasia incidence: a register-based observational study. 2021;398:2084-2092. doi.org/10.1016/S0140-6736(21) 02178-4.
- ASCCP Screening Guidelines. https://www.asccp.org /screening-guidelines. Accessed April 25, 2021.
- Fontham ET, Wolf AM, Church TR, et al. Cervical cancer screening for individuals at average risk: 2020 guidelines update from the American Cancer Society. CA Cancer J Clin. 2020;70:321-346. doi:10.3322/caac.21628.
- US Preventive Services Task Force. Screening for cervical cancer. US Preventive Services Task Force Recommendation Statement. JAMA. 2018;320:674-686. doi:10.1001/jama .2018.10897.
- American College of Obstetricians and Gynecologists. Practice Advisory: Updated cervical cancer screening guidelines. April 2022. https://www.acog.org/clinical/clinical-guidance /practice-advisory/articles/2021/04/updated-cervical -cancer-screening-guidelines. Accessed April 25, 2022.
- Perkins RB, Guido RS, Castle PE, et al; 2019 ASCCP RiskBased Management Consensus Guidelines Committee. 2019 ASCCP risk-based management consensus guidelines for abnormal cervical cancer screening tests and cancer precursors. J Low Genit Tract Dis. 2020;24:102-131. doi: 10 34 .1097/LGT.0000000000000525.
- Yeh PT, Kennedy CE, de Vuyst H, et al. Self-sampling for human papillomavirus (HPV) testing: a systematic review and meta-analysis. BMJ Global Health. 2019;4:e001351. doi:10.1136/ bmjgh-2018-001351.
- Kjaer SK, Nygard M, Dillner J, et al. A 12-year follow-up on the long-term effectiveness of the quadrivalent human papillomavirus vaccine in 4 Nordic countries. Clin Infect Dis. 2018;66:339-345. doi: 10.1093/cid/cix797.
- Patel C, Brotherton JM, Pillsbury A, et al. The impact of 10 years of human papillomavirus (HPV) vaccination in Australia: what additional disease burden will a nonvalent vaccine prevent? Euro Surveill. 2018;23:1700737. doi: 10 .2807/1560-7917.
- Falcaro M, Castanon A, Ndlela B, et al. The effects of the national HPV vaccination programme in England, UK, on cervical cancer and grade 3 cervical intraepithelial neoplasia incidence: a register-based observational study. 2021;398:2084-2092. doi.org/10.1016/S0140-6736(21) 02178-4.