User login
UPDATE ON CONTRACEPTION
- An appeal to the FDA: Remove the black-box warning for depot medroxyprogesterone acetate!
Andrew M. Kaunitz, MD; David A. Grimes, MD (August 2011) - 10 (+1) practical, evidence-based recommendations for you to improve contraceptive care now
Colleen Krajewski, MD; Mark D. Walters, MD (August 2011) - Levonorgestrel or ulipristal: Is one a better emergency contraceptive than the other?
Robert L. Barbieri, MD (Editorial; March 2011) - IUD use in nulliparous and adolescent women
Jennefer A. Russo, MD; Mitchell D. Creinin, MD (Update on Contraception, August 2010)
“Emergency contraception,” “the morning-after pill,” and “Plan B” are all phrases commonly used in most gynecologists’ offices. Regrettably, these phrases are not heard as frequently among patients. With half of all pregnancies unintended and 40% of these pregnancies ending in abortion, there is clearly an unmet need for both contraception and emergency contraception (EC). Although more women have turned to EC in recent years, this contraceptive approach remains highly underutilized in the US population. Despite some increase in usage, we have not yet realized a lower rate of unintended pregnancy or abortion.
Yuzpe and colleagues first published findings on the use of combined oral contraceptives (OCs) for postcoital contraception in 1974. Since then, researchers have been trying to manipulate various hormonal configurations in an attempt to best prevent pregnancy after unprotected intercourse. For years, we have quoted success rates as high as 85% when EC is initiated within 72 hours of unprotected intercourse1—but early studies may have overestimated the ability of EC to prevent unintended pregnancy. More recent investigations have shown that the magical “morning-after pill” and the physicians recommending it are long overdue for a wake-up call.
This installment of the Update on Contraception will review recent evidence on the efficacy of EC and make recommendations for practice, focusing on:
- the reasons EC has failed to reduce the rate of unintended pregnancy
- the efficacy of oral levonorgestrel (LNG) versus ulipristal acetate
- the impact of overweight and obesity on the efficacy of oral agents
- the overall superiority of the copper intrauterine device (IUD).
Half of all pregnancies are unintended, and 40% of unintended pregnancies end in abortion. These figures reflect an unmet need for both contraception and emergency contraception, which remains highly underutilized in the United States.
Access to EC is increasing, but women still lack basic information about it
Kavanaugh M, Schwarz EB. Counseling about and use of emergency contraception in the United States. Perspect Sex Reprod Health. 2008;40(2):81–86.
Kavanaugh M, Williams S, Schwarz EB. Emergency contraception use and counseling after changes in United States prescription status. Fertil Steril. 2011;95(8):2578–2581.
In 1974, Yuzpe and colleagues first published findings on the use of combined estrogen-progestin OCs for postcoital contraception.2 At the same time, Kesseru and colleagues were evaluating progestin-only regimens for the same purpose.3
For many subsequent years, combinations of common OC pills containing ethinyl estradiol and LNG were used for EC, until 1998, when a progestin-only method containing two 0.75-mg LNG pills was approved by the Food and Drug Administration (FDA) and marketed in the United States under the brand name Plan B. That approval was based on a double-blind, randomized trial by the World Health Organization that demonstrated an almost threefold higher incidence of pregnancy with use of the Yuzpe regimen, compared with this LNG regimen.1
Access to the LNG-only method in the United States increased when the product was given behind-the-counter status in 2006, making it possible for women 18 years and older to obtain the medication without a prescription. In 2009, access was approved—also without a prescription—for 17-year-old women. The same year, the FDA approved Plan B One-Step, allowing women to take both 0.75-mg tablets together as a single tablet, theoretically improving treatment adherence.
Seeking a way to further increase use of EC, many investigators explored the potential benefits of advance provision. The idea was not new, as it had been proposed even for the Yuzpe method, and utilization increased significantly after 2006. Reviews of data from the National Survey of Family Growth (NSFG) showed an increase in EC use among women who had ever had sexual intercourse with a man from 4.2% of women surveyed in 2002 to 9.7% of women surveyed in 2006 to 2008, as reported by Kavanaugh and colleagues. Regrettably, this increase did not reduce the number of unintended pregnancies during the same time periods. Clearly, men and women fail to use EC every time they are at risk of unintended pregnancy.4
One of the biggest barriers to EC use is probably the lack of information patients receive from providers. Only 3% of respondents to the 2006–2008 NSFG indicated that they had received any counseling about EC in the past year, a number relatively unchanged from the 2002 survey. This finding suggests that the increase in EC use is likely due to the publicity surrounding the EC status change in 2006.
Greater availability and less restrictive access to EC has not reduced the rate of unintended pregnancy in the United States. However, improvements in the counseling of women may have an impact on the pregnancy rate. As the National Survey of Family Growth reveals, only about 3% of women receive any counseling about EC in a given year. For utilization of EC to increase, women need to be aware that it exists. Providers must begin to change their practices and discuss EC at all routine appointments before the public health benefit of a decrease in unintended pregnancies can ever be realized.
Ulipristal acetate makes its debut—and demonstrates superiority to LNG
Glasier A, Cameron S, Fine P, et al. Ulipristal acetate versus levonorgestrel for emergency contraception: a randomized non-inferiority trial and meta-analysis. Lancet. 2010;375:555(9714)–562.
Fine P, Mathe H, Ginde S, et al. Ulipristal acetate taken 48–120 hours after intercourse for emergency contraception. Obstet Gynecol. 2010;115(2pt1):257–263.
In 1998, the first-generation antiprogestin mifepristone was approved for use in France in medical abortion. As early as 1991, researchers were already investigating mifepristone as a method of EC, with great success.5,6 Overall, mifepristone was more effective and had fewer side effects than oral LNG, although the onset of menses was delayed with mifepristone.7 Mifepristone is available as EC in Russia and China, but its use in other countries is limited by social and political constraints.
Enter ulipristal acetate (UPA), a second-generation progesterone receptor modulator. Unlike its predecessor mifepristone, UPA (brand name, ella) is not approved for pregnancy termination, and no studies have been performed to evaluate the effects of UPA on an existing pregnancy. Because effects on pregnancy are unknown, the manufacturer states that exclusion of pregnancy is a requirement before UPA can be prescribed for EC.
The data on UPA as emergency contraception
UPA has been evaluated for EC in two large randomized trials.8,9 In the first study, UPA was administered in a 50-mg dose as long as 72 hours after unprotected intercourse. In the second study, conducted by Glasier and colleagues, a 30-mg micronized dose (bioequivalent to the 50-mg nonmicronized dose) was used as long as 120 hours after unprotected intercourse. Participants in both studies were randomized to UPA or oral LNG.
The first study showed UPA to be at least as effective as LNG in preventing pregnancy when taken within 72 hours after unprotected intercourse. The efficacy of UPA did not appear to decline even when it was taken 48 to 72 hours after unprotected intercourse, unlike the efficacy of LNG.
The second study similarly found UPA to be non-inferior to LNG. Although neither study was powered to demonstrate superiority, both did show that UPA seemed to prevent more pregnancies than LNG.
Glasier and colleagues then performed a meta-analysis of both studies, demonstrating that UPA almost halved the risk of pregnancy, compared with LNG, in women who received treatment within 120 hours after intercourse, with a reduction of almost two thirds when UPA was taken within 24 hours of unprotected intercourse.
UPA has FDA approval for use within 120 hours after unprotected intercourse and requires a prescription. Although the data leading to this approval are incredibly encouraging, fewer than 200 of more than 2,000 women in three studies performed with UPA took EC 96 to 120 hours after intercourse. With such a small number of women actually tested in this time range, physicians should use caution when counseling patients about the efficacy of UPA when it is taken more than 96 hours after unprotected intercourse.8-10
UPA is more expensive than LNG, which may be a barrier to use by some women. However, because the probability of becoming pregnant when taking UPA within 120 hours of unprotected intercourse is lower than with LNG, the cost differential between drugs is much smaller when total costs—including the cost of unintended pregnancy—are consid-ered.11
Although the LNG-only method is the only EC that is available without a prescription, UPA appears to be more effective, particularly when it is taken more than 72 hours after unprotected intercourse. However, providers should be aware that a relatively small number of women have been studied with UPA beyond 72 hours after unprotected intercourse.
Although LNG-only EC is available behind the counter, the superiority of UPA means that physicians should discuss EC with patients during routine appointments and consider advance provision. For patients, cost and access will be important issues when deciding whether to use LNG or UPA.
EC is more likely to fail in overweight and obese women
Glasier A, Cameron S, Blithe D, et al. Can we identify women at risk of pregnancy despite using emergency contraception? Data from randomized trials of ulipristal acetate and levonorgestrel [published online ahead of print April 2, 2011]. Contraception. doi:10.1016/j.contraception.2011.02.009.
Edelman AB, Carlson NE, Cherala G, et al. Impact of obesity on oral contraceptive pharmacokinetics and hypothalamic-pituitary-ovarian activity. Contraception. 2009;80(2):119–127.
Westhoff CT, Torgal AL, Mayeda ER, et al. Ovarian suppression in normal-weight and obese women during oral contraceptive use. Obstet Gynecol. 2010;116(2 pt 2):275–283.
As we observed, despite more widespread use of EC after the LNG-only method was made available without a prescription, we have not realized the public health benefit of a decreased rate of unintended pregnancy or abortion.4 Studies have shown that, despite taking EC, women who have further acts of intercourse in the same cycle of EC use are more likely to conceive.12,13
We now have clear information about another specific population in which EC is more likely to fail: overweight and obese women. Compared with women of normal weight (body mass index [BMI] <25), overweight women (BMI 25–30) had a risk of pregnancy 1.5 times greater, and obese women (BMI ≥30) had a risk of pregnancy more than three times greater.13
Pregnancy rate among obese women using LNG was the same as the background rate
Obese women who used LNG as EC had a pregnancy rate of 5.8%, which is approximately equivalent to the overall pregnancy rate expected in the absence of EC. Overweight women in the LNG group had a relative risk of pregnancy that was double that of normal or underweight women, whereas overweight women taking UPA had the same risk as normal or underweight women taking the same medication.
When researchers compared pregnancy rates by weight instead of BMI, differences persisted between the two treatment options, with a limit of efficacy reached at a weight of 70 kg (154 lb) for LNG, compared with 88 kg (194 lb) for UPA.
OC hormone absorption is slower in obesity
Two recent studies—by Edelman and colleagues and Westhoff and coworkers—have demonstrated that OC hormone absorption is slower in obese women than it is in women of normal weight. With EC, immediate absorption is important; this delay could explain the lower efficacy in obese women. No studies have evaluated whether a higher or double dose of LNG would improve efficacy. Like women who experience repeated acts of unprotected intercourse, overweight and obese women are at high risk of EC failure and should be counseled about this risk.
As the incidence of obesity continues to increase exponentially in the United States, the efficacy of our commonly used methods of EC will continue to decline. At a minimum, overweight and obese women should be counseled to take UPA rather than LNG because of its increased efficacy in this population. We also need to inform overweight patients that their risk of pregnancy is higher than is commonly quoted.
Have we overlooked the best available emergency contraceptive?
Wu S, Godfrey EM, Wojdyla D, et al. Copper T380A intrauterine device for emergency contraception: a prospective, multicentre, cohort clinical trial. BJOG. 2010;117(10):1205–1210.
Turok D, Gurtcheff S, Handley E, et al. A pilot study of the Copper T380A IUD and levonorgestral for emergency contraception. Contraception. 2010;82(6):520–525.
The copper IUD has always been the most effective EC available. Not only does it prevent pregnancy when inserted as EC, but it continues to provide long-term, reversible contraception for 10 years or longer. Two large studies—one of them published within the past year—found efficacy rates of 96.9% and 100%, much higher than those associated with oral EC, with only two pregnancies occurring in more than 2,000 women.14,15
Although use of the IUD as EC was described as early as 1976, adoption of this method has been minimal in the United States.16 One reason may be the need for a clinician to insert the device, but many providers undoubtedly dismiss the IUD as an option for EC, believing that American women are unwilling to accept it. Some providers maintain the longstanding opinion that the IUD is an option only for parous women, although this notion has been cast aside by layers of medical evidence, as reviewed by current Centers for Disease Control and Prevention (CDC) medical eligibility criteria for contraception.17
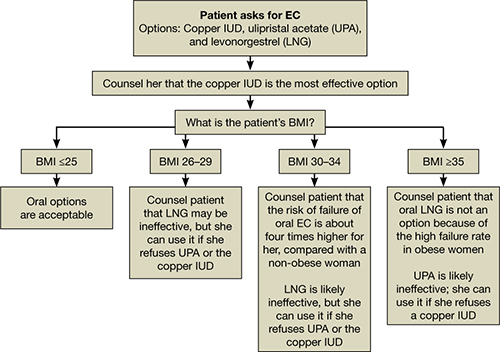
All women should be counseled about the long-term benefits of the copper IUD, the most reliable method of EC. The copper IUD not only provides effective emergency contraception but also long-term contraception for 10 years or more. Therefore, we should offer the copper IUD as first-line treatment for women seeking EC (FIGURE). This method is likely to be much more acceptable to patients than previously assumed.
Women are more accepting of the IUD than we thought
Schwarz and colleagues surveyed 412 women in Pittsburgh family planning clinics who were seeking EC or pregnancy testing and found that 15% of these women would be interested in same-day insertion of an IUD.18 This number increased if the IUD was free among women who reported difficulty with access to contraception.
In an observational study, Turok and colleagues offered women who were seeking EC a choice between the copper IUD and oral LNG and followed them for 6 months. Both methods were offered free of charge. They had assumed that, for every 20 women choosing oral LNG, one would choose the copper IUD. What they found was quite different: For every 1.5 women who chose oral LNG, one chose the copper IUD. Even more impressive was the number of women still using highly effective contraception (IUD, implant, or sterilization) 6 months later—4.5% in the oral LNG group and 61.5% in the IUD group. By the end of the 6-month period, two pregnancies had occurred in the oral LNG group and none in the IUD group.
How to counsel a patient seeking emergency contraceptionWe want to hear from you! Tell us what you think.
1. Task Force on Postovulatory Methods of Fertility Regulation. Randomised controlled trial of levonorgestrel versus the Yuzpe regimen of combined oral contraceptives for emergency contraception. Lancet. 1998;352(9126):428-433.
2. Yuzpe A, Thurlow H, Ramzy I, Leyshon J. Post coital contraception–A pilot study. J Reprod Med. 1974;13(2):53-58.
3. Kesseru E, Garmendia F, Westphal N, Parada J. The hormonal and peripheral effects of d-norgestrel in postcoital contraception. Contraception. 1974;10(4):411-424.
4. Polis CB, Schaffer K, Banchard K, et al. Advance provision of emergency contraception for pregnancy prevention (full review). Cochrane Database Syst Rev. 2007;(2):CD005497.-
5. Glasier A, Thong KJ, Dewar M, Mackie M, Baird DT. Postcoital contraception with mifepristone. Lancet. 1991;337(8754):1414-1415.
6. Webb AM. Alternative treatments in oral postcoital contraception: interim results. Adv Contracept. 1991;7(2–3):271-279.
7. Cheng L, Gülmezoglu AM, Piaggio G, Ezcurra E, Van Look PF. Interventions for emergency contraception. Cochrane Database Syst Rev. 2008;(2):CD001324.-
8. Creinin MD, Schlaff W, Archer D, et al. Progesterone receptor modulator for emergency contraception: a randomized controlled trial. Obstet Gynecol. 2006;108(5):1089-1097.
9. Glasier A, Cameron S, Fine P, et al. Ulipristal acetate versus levonorgestrel for emergency contraception: a randomized non-inferiority trial and meta-analysis. Lancet. 2010;375(9714):555-562.
10. Fine P, Mathe H, Ginde S, et al. Ulipristal acetate taken 48-120 hours after intercourse for emergency contraception. Obstet Gynecol. 2010;115(2 pt 1):257-263.
11. Thomas CM, Schmid R, Cameron S. Is it worth paying more for emergency hormonal contraception? The cost-effectiveness of ulipristal acetate versus levonorgestrel 1.5 mg. J Fam Plann Reprod Health Care. 2010;36(4):197-201.
12. von Hertzen H, Piaggio G, Ding J, et al. Low dose mifepris-tone and two regimens of levonorgestrel for emergency contraception: a WHO multicentre randomised trial. Lancet. 2002;360(9348):1803-1810.
13. Glasier A, Cameron S, Blithe D, et al. Can we identify women at risk of pregnancy despite using emergency contraception? Data from randomized trials of ulipristal acetate and levonorgestrel [published online ahead of print April 2 2011]. Contraception. doi:10.1016/j.contraception.2011.02.009.
14. Zhou LY, Xiao BL. Emergency contraception with Multiload Cu-375 SL IUD: A multicenter clinical trial. Contraception. 2001;64(2):107-112.
15. Wu S, Godfrey EM, Wojdyla D, et al. Copper T380A intrauterine device for emergency contraception: a prospective, multicentre, cohort clinical trial. BJOG. 2010;117(10):1205-1210.
16. Lippes J, Malik T, Tatum HJ. The postcoital copper-T. Adv Plan Parent. 1976;11(1):24-29.
17. Centers for Disease Control and Prevention. US medical eligibility criteria contraceptive use 2010. MMWR. 2010;59 (RR04):1-6.
18. Schwarz EB, Kavanaugh M, Douglas E, Dubowitz T, Creinin MD. Interest in intrauterine contraception among seekers of emergency contraception and pregnancy testing. Obstet Gynecol. 2009;113(4):833-839.
- An appeal to the FDA: Remove the black-box warning for depot medroxyprogesterone acetate!
Andrew M. Kaunitz, MD; David A. Grimes, MD (August 2011) - 10 (+1) practical, evidence-based recommendations for you to improve contraceptive care now
Colleen Krajewski, MD; Mark D. Walters, MD (August 2011) - Levonorgestrel or ulipristal: Is one a better emergency contraceptive than the other?
Robert L. Barbieri, MD (Editorial; March 2011) - IUD use in nulliparous and adolescent women
Jennefer A. Russo, MD; Mitchell D. Creinin, MD (Update on Contraception, August 2010)
“Emergency contraception,” “the morning-after pill,” and “Plan B” are all phrases commonly used in most gynecologists’ offices. Regrettably, these phrases are not heard as frequently among patients. With half of all pregnancies unintended and 40% of these pregnancies ending in abortion, there is clearly an unmet need for both contraception and emergency contraception (EC). Although more women have turned to EC in recent years, this contraceptive approach remains highly underutilized in the US population. Despite some increase in usage, we have not yet realized a lower rate of unintended pregnancy or abortion.
Yuzpe and colleagues first published findings on the use of combined oral contraceptives (OCs) for postcoital contraception in 1974. Since then, researchers have been trying to manipulate various hormonal configurations in an attempt to best prevent pregnancy after unprotected intercourse. For years, we have quoted success rates as high as 85% when EC is initiated within 72 hours of unprotected intercourse1—but early studies may have overestimated the ability of EC to prevent unintended pregnancy. More recent investigations have shown that the magical “morning-after pill” and the physicians recommending it are long overdue for a wake-up call.
This installment of the Update on Contraception will review recent evidence on the efficacy of EC and make recommendations for practice, focusing on:
- the reasons EC has failed to reduce the rate of unintended pregnancy
- the efficacy of oral levonorgestrel (LNG) versus ulipristal acetate
- the impact of overweight and obesity on the efficacy of oral agents
- the overall superiority of the copper intrauterine device (IUD).

Half of all pregnancies are unintended, and 40% of unintended pregnancies end in abortion. These figures reflect an unmet need for both contraception and emergency contraception, which remains highly underutilized in the United States.
Access to EC is increasing, but women still lack basic information about it
Kavanaugh M, Schwarz EB. Counseling about and use of emergency contraception in the United States. Perspect Sex Reprod Health. 2008;40(2):81–86.
Kavanaugh M, Williams S, Schwarz EB. Emergency contraception use and counseling after changes in United States prescription status. Fertil Steril. 2011;95(8):2578–2581.
In 1974, Yuzpe and colleagues first published findings on the use of combined estrogen-progestin OCs for postcoital contraception.2 At the same time, Kesseru and colleagues were evaluating progestin-only regimens for the same purpose.3
For many subsequent years, combinations of common OC pills containing ethinyl estradiol and LNG were used for EC, until 1998, when a progestin-only method containing two 0.75-mg LNG pills was approved by the Food and Drug Administration (FDA) and marketed in the United States under the brand name Plan B. That approval was based on a double-blind, randomized trial by the World Health Organization that demonstrated an almost threefold higher incidence of pregnancy with use of the Yuzpe regimen, compared with this LNG regimen.1
Access to the LNG-only method in the United States increased when the product was given behind-the-counter status in 2006, making it possible for women 18 years and older to obtain the medication without a prescription. In 2009, access was approved—also without a prescription—for 17-year-old women. The same year, the FDA approved Plan B One-Step, allowing women to take both 0.75-mg tablets together as a single tablet, theoretically improving treatment adherence.
Seeking a way to further increase use of EC, many investigators explored the potential benefits of advance provision. The idea was not new, as it had been proposed even for the Yuzpe method, and utilization increased significantly after 2006. Reviews of data from the National Survey of Family Growth (NSFG) showed an increase in EC use among women who had ever had sexual intercourse with a man from 4.2% of women surveyed in 2002 to 9.7% of women surveyed in 2006 to 2008, as reported by Kavanaugh and colleagues. Regrettably, this increase did not reduce the number of unintended pregnancies during the same time periods. Clearly, men and women fail to use EC every time they are at risk of unintended pregnancy.4
One of the biggest barriers to EC use is probably the lack of information patients receive from providers. Only 3% of respondents to the 2006–2008 NSFG indicated that they had received any counseling about EC in the past year, a number relatively unchanged from the 2002 survey. This finding suggests that the increase in EC use is likely due to the publicity surrounding the EC status change in 2006.
Greater availability and less restrictive access to EC has not reduced the rate of unintended pregnancy in the United States. However, improvements in the counseling of women may have an impact on the pregnancy rate. As the National Survey of Family Growth reveals, only about 3% of women receive any counseling about EC in a given year. For utilization of EC to increase, women need to be aware that it exists. Providers must begin to change their practices and discuss EC at all routine appointments before the public health benefit of a decrease in unintended pregnancies can ever be realized.
Ulipristal acetate makes its debut—and demonstrates superiority to LNG
Glasier A, Cameron S, Fine P, et al. Ulipristal acetate versus levonorgestrel for emergency contraception: a randomized non-inferiority trial and meta-analysis. Lancet. 2010;375:555(9714)–562.
Fine P, Mathe H, Ginde S, et al. Ulipristal acetate taken 48–120 hours after intercourse for emergency contraception. Obstet Gynecol. 2010;115(2pt1):257–263.
In 1998, the first-generation antiprogestin mifepristone was approved for use in France in medical abortion. As early as 1991, researchers were already investigating mifepristone as a method of EC, with great success.5,6 Overall, mifepristone was more effective and had fewer side effects than oral LNG, although the onset of menses was delayed with mifepristone.7 Mifepristone is available as EC in Russia and China, but its use in other countries is limited by social and political constraints.
Enter ulipristal acetate (UPA), a second-generation progesterone receptor modulator. Unlike its predecessor mifepristone, UPA (brand name, ella) is not approved for pregnancy termination, and no studies have been performed to evaluate the effects of UPA on an existing pregnancy. Because effects on pregnancy are unknown, the manufacturer states that exclusion of pregnancy is a requirement before UPA can be prescribed for EC.
The data on UPA as emergency contraception
UPA has been evaluated for EC in two large randomized trials.8,9 In the first study, UPA was administered in a 50-mg dose as long as 72 hours after unprotected intercourse. In the second study, conducted by Glasier and colleagues, a 30-mg micronized dose (bioequivalent to the 50-mg nonmicronized dose) was used as long as 120 hours after unprotected intercourse. Participants in both studies were randomized to UPA or oral LNG.
The first study showed UPA to be at least as effective as LNG in preventing pregnancy when taken within 72 hours after unprotected intercourse. The efficacy of UPA did not appear to decline even when it was taken 48 to 72 hours after unprotected intercourse, unlike the efficacy of LNG.
The second study similarly found UPA to be non-inferior to LNG. Although neither study was powered to demonstrate superiority, both did show that UPA seemed to prevent more pregnancies than LNG.
Glasier and colleagues then performed a meta-analysis of both studies, demonstrating that UPA almost halved the risk of pregnancy, compared with LNG, in women who received treatment within 120 hours after intercourse, with a reduction of almost two thirds when UPA was taken within 24 hours of unprotected intercourse.
UPA has FDA approval for use within 120 hours after unprotected intercourse and requires a prescription. Although the data leading to this approval are incredibly encouraging, fewer than 200 of more than 2,000 women in three studies performed with UPA took EC 96 to 120 hours after intercourse. With such a small number of women actually tested in this time range, physicians should use caution when counseling patients about the efficacy of UPA when it is taken more than 96 hours after unprotected intercourse.8-10
UPA is more expensive than LNG, which may be a barrier to use by some women. However, because the probability of becoming pregnant when taking UPA within 120 hours of unprotected intercourse is lower than with LNG, the cost differential between drugs is much smaller when total costs—including the cost of unintended pregnancy—are consid-ered.11
Although the LNG-only method is the only EC that is available without a prescription, UPA appears to be more effective, particularly when it is taken more than 72 hours after unprotected intercourse. However, providers should be aware that a relatively small number of women have been studied with UPA beyond 72 hours after unprotected intercourse.
Although LNG-only EC is available behind the counter, the superiority of UPA means that physicians should discuss EC with patients during routine appointments and consider advance provision. For patients, cost and access will be important issues when deciding whether to use LNG or UPA.
EC is more likely to fail in overweight and obese women
Glasier A, Cameron S, Blithe D, et al. Can we identify women at risk of pregnancy despite using emergency contraception? Data from randomized trials of ulipristal acetate and levonorgestrel [published online ahead of print April 2, 2011]. Contraception. doi:10.1016/j.contraception.2011.02.009.
Edelman AB, Carlson NE, Cherala G, et al. Impact of obesity on oral contraceptive pharmacokinetics and hypothalamic-pituitary-ovarian activity. Contraception. 2009;80(2):119–127.
Westhoff CT, Torgal AL, Mayeda ER, et al. Ovarian suppression in normal-weight and obese women during oral contraceptive use. Obstet Gynecol. 2010;116(2 pt 2):275–283.
As we observed, despite more widespread use of EC after the LNG-only method was made available without a prescription, we have not realized the public health benefit of a decreased rate of unintended pregnancy or abortion.4 Studies have shown that, despite taking EC, women who have further acts of intercourse in the same cycle of EC use are more likely to conceive.12,13
We now have clear information about another specific population in which EC is more likely to fail: overweight and obese women. Compared with women of normal weight (body mass index [BMI] <25), overweight women (BMI 25–30) had a risk of pregnancy 1.5 times greater, and obese women (BMI ≥30) had a risk of pregnancy more than three times greater.13
Pregnancy rate among obese women using LNG was the same as the background rate
Obese women who used LNG as EC had a pregnancy rate of 5.8%, which is approximately equivalent to the overall pregnancy rate expected in the absence of EC. Overweight women in the LNG group had a relative risk of pregnancy that was double that of normal or underweight women, whereas overweight women taking UPA had the same risk as normal or underweight women taking the same medication.
When researchers compared pregnancy rates by weight instead of BMI, differences persisted between the two treatment options, with a limit of efficacy reached at a weight of 70 kg (154 lb) for LNG, compared with 88 kg (194 lb) for UPA.
OC hormone absorption is slower in obesity
Two recent studies—by Edelman and colleagues and Westhoff and coworkers—have demonstrated that OC hormone absorption is slower in obese women than it is in women of normal weight. With EC, immediate absorption is important; this delay could explain the lower efficacy in obese women. No studies have evaluated whether a higher or double dose of LNG would improve efficacy. Like women who experience repeated acts of unprotected intercourse, overweight and obese women are at high risk of EC failure and should be counseled about this risk.
As the incidence of obesity continues to increase exponentially in the United States, the efficacy of our commonly used methods of EC will continue to decline. At a minimum, overweight and obese women should be counseled to take UPA rather than LNG because of its increased efficacy in this population. We also need to inform overweight patients that their risk of pregnancy is higher than is commonly quoted.
Have we overlooked the best available emergency contraceptive?
Wu S, Godfrey EM, Wojdyla D, et al. Copper T380A intrauterine device for emergency contraception: a prospective, multicentre, cohort clinical trial. BJOG. 2010;117(10):1205–1210.
Turok D, Gurtcheff S, Handley E, et al. A pilot study of the Copper T380A IUD and levonorgestral for emergency contraception. Contraception. 2010;82(6):520–525.
The copper IUD has always been the most effective EC available. Not only does it prevent pregnancy when inserted as EC, but it continues to provide long-term, reversible contraception for 10 years or longer. Two large studies—one of them published within the past year—found efficacy rates of 96.9% and 100%, much higher than those associated with oral EC, with only two pregnancies occurring in more than 2,000 women.14,15
Although use of the IUD as EC was described as early as 1976, adoption of this method has been minimal in the United States.16 One reason may be the need for a clinician to insert the device, but many providers undoubtedly dismiss the IUD as an option for EC, believing that American women are unwilling to accept it. Some providers maintain the longstanding opinion that the IUD is an option only for parous women, although this notion has been cast aside by layers of medical evidence, as reviewed by current Centers for Disease Control and Prevention (CDC) medical eligibility criteria for contraception.17
All women should be counseled about the long-term benefits of the copper IUD, the most reliable method of EC. The copper IUD not only provides effective emergency contraception but also long-term contraception for 10 years or more. Therefore, we should offer the copper IUD as first-line treatment for women seeking EC (FIGURE). This method is likely to be much more acceptable to patients than previously assumed.
Women are more accepting of the IUD than we thought
Schwarz and colleagues surveyed 412 women in Pittsburgh family planning clinics who were seeking EC or pregnancy testing and found that 15% of these women would be interested in same-day insertion of an IUD.18 This number increased if the IUD was free among women who reported difficulty with access to contraception.
In an observational study, Turok and colleagues offered women who were seeking EC a choice between the copper IUD and oral LNG and followed them for 6 months. Both methods were offered free of charge. They had assumed that, for every 20 women choosing oral LNG, one would choose the copper IUD. What they found was quite different: For every 1.5 women who chose oral LNG, one chose the copper IUD. Even more impressive was the number of women still using highly effective contraception (IUD, implant, or sterilization) 6 months later—4.5% in the oral LNG group and 61.5% in the IUD group. By the end of the 6-month period, two pregnancies had occurred in the oral LNG group and none in the IUD group.

How to counsel a patient seeking emergency contraceptionWe want to hear from you! Tell us what you think.
- An appeal to the FDA: Remove the black-box warning for depot medroxyprogesterone acetate!
Andrew M. Kaunitz, MD; David A. Grimes, MD (August 2011) - 10 (+1) practical, evidence-based recommendations for you to improve contraceptive care now
Colleen Krajewski, MD; Mark D. Walters, MD (August 2011) - Levonorgestrel or ulipristal: Is one a better emergency contraceptive than the other?
Robert L. Barbieri, MD (Editorial; March 2011) - IUD use in nulliparous and adolescent women
Jennefer A. Russo, MD; Mitchell D. Creinin, MD (Update on Contraception, August 2010)
“Emergency contraception,” “the morning-after pill,” and “Plan B” are all phrases commonly used in most gynecologists’ offices. Regrettably, these phrases are not heard as frequently among patients. With half of all pregnancies unintended and 40% of these pregnancies ending in abortion, there is clearly an unmet need for both contraception and emergency contraception (EC). Although more women have turned to EC in recent years, this contraceptive approach remains highly underutilized in the US population. Despite some increase in usage, we have not yet realized a lower rate of unintended pregnancy or abortion.
Yuzpe and colleagues first published findings on the use of combined oral contraceptives (OCs) for postcoital contraception in 1974. Since then, researchers have been trying to manipulate various hormonal configurations in an attempt to best prevent pregnancy after unprotected intercourse. For years, we have quoted success rates as high as 85% when EC is initiated within 72 hours of unprotected intercourse1—but early studies may have overestimated the ability of EC to prevent unintended pregnancy. More recent investigations have shown that the magical “morning-after pill” and the physicians recommending it are long overdue for a wake-up call.
This installment of the Update on Contraception will review recent evidence on the efficacy of EC and make recommendations for practice, focusing on:
- the reasons EC has failed to reduce the rate of unintended pregnancy
- the efficacy of oral levonorgestrel (LNG) versus ulipristal acetate
- the impact of overweight and obesity on the efficacy of oral agents
- the overall superiority of the copper intrauterine device (IUD).

Half of all pregnancies are unintended, and 40% of unintended pregnancies end in abortion. These figures reflect an unmet need for both contraception and emergency contraception, which remains highly underutilized in the United States.
Access to EC is increasing, but women still lack basic information about it
Kavanaugh M, Schwarz EB. Counseling about and use of emergency contraception in the United States. Perspect Sex Reprod Health. 2008;40(2):81–86.
Kavanaugh M, Williams S, Schwarz EB. Emergency contraception use and counseling after changes in United States prescription status. Fertil Steril. 2011;95(8):2578–2581.
In 1974, Yuzpe and colleagues first published findings on the use of combined estrogen-progestin OCs for postcoital contraception.2 At the same time, Kesseru and colleagues were evaluating progestin-only regimens for the same purpose.3
For many subsequent years, combinations of common OC pills containing ethinyl estradiol and LNG were used for EC, until 1998, when a progestin-only method containing two 0.75-mg LNG pills was approved by the Food and Drug Administration (FDA) and marketed in the United States under the brand name Plan B. That approval was based on a double-blind, randomized trial by the World Health Organization that demonstrated an almost threefold higher incidence of pregnancy with use of the Yuzpe regimen, compared with this LNG regimen.1
Access to the LNG-only method in the United States increased when the product was given behind-the-counter status in 2006, making it possible for women 18 years and older to obtain the medication without a prescription. In 2009, access was approved—also without a prescription—for 17-year-old women. The same year, the FDA approved Plan B One-Step, allowing women to take both 0.75-mg tablets together as a single tablet, theoretically improving treatment adherence.
Seeking a way to further increase use of EC, many investigators explored the potential benefits of advance provision. The idea was not new, as it had been proposed even for the Yuzpe method, and utilization increased significantly after 2006. Reviews of data from the National Survey of Family Growth (NSFG) showed an increase in EC use among women who had ever had sexual intercourse with a man from 4.2% of women surveyed in 2002 to 9.7% of women surveyed in 2006 to 2008, as reported by Kavanaugh and colleagues. Regrettably, this increase did not reduce the number of unintended pregnancies during the same time periods. Clearly, men and women fail to use EC every time they are at risk of unintended pregnancy.4
One of the biggest barriers to EC use is probably the lack of information patients receive from providers. Only 3% of respondents to the 2006–2008 NSFG indicated that they had received any counseling about EC in the past year, a number relatively unchanged from the 2002 survey. This finding suggests that the increase in EC use is likely due to the publicity surrounding the EC status change in 2006.
Greater availability and less restrictive access to EC has not reduced the rate of unintended pregnancy in the United States. However, improvements in the counseling of women may have an impact on the pregnancy rate. As the National Survey of Family Growth reveals, only about 3% of women receive any counseling about EC in a given year. For utilization of EC to increase, women need to be aware that it exists. Providers must begin to change their practices and discuss EC at all routine appointments before the public health benefit of a decrease in unintended pregnancies can ever be realized.
Ulipristal acetate makes its debut—and demonstrates superiority to LNG
Glasier A, Cameron S, Fine P, et al. Ulipristal acetate versus levonorgestrel for emergency contraception: a randomized non-inferiority trial and meta-analysis. Lancet. 2010;375:555(9714)–562.
Fine P, Mathe H, Ginde S, et al. Ulipristal acetate taken 48–120 hours after intercourse for emergency contraception. Obstet Gynecol. 2010;115(2pt1):257–263.
In 1998, the first-generation antiprogestin mifepristone was approved for use in France in medical abortion. As early as 1991, researchers were already investigating mifepristone as a method of EC, with great success.5,6 Overall, mifepristone was more effective and had fewer side effects than oral LNG, although the onset of menses was delayed with mifepristone.7 Mifepristone is available as EC in Russia and China, but its use in other countries is limited by social and political constraints.
Enter ulipristal acetate (UPA), a second-generation progesterone receptor modulator. Unlike its predecessor mifepristone, UPA (brand name, ella) is not approved for pregnancy termination, and no studies have been performed to evaluate the effects of UPA on an existing pregnancy. Because effects on pregnancy are unknown, the manufacturer states that exclusion of pregnancy is a requirement before UPA can be prescribed for EC.
The data on UPA as emergency contraception
UPA has been evaluated for EC in two large randomized trials.8,9 In the first study, UPA was administered in a 50-mg dose as long as 72 hours after unprotected intercourse. In the second study, conducted by Glasier and colleagues, a 30-mg micronized dose (bioequivalent to the 50-mg nonmicronized dose) was used as long as 120 hours after unprotected intercourse. Participants in both studies were randomized to UPA or oral LNG.
The first study showed UPA to be at least as effective as LNG in preventing pregnancy when taken within 72 hours after unprotected intercourse. The efficacy of UPA did not appear to decline even when it was taken 48 to 72 hours after unprotected intercourse, unlike the efficacy of LNG.
The second study similarly found UPA to be non-inferior to LNG. Although neither study was powered to demonstrate superiority, both did show that UPA seemed to prevent more pregnancies than LNG.
Glasier and colleagues then performed a meta-analysis of both studies, demonstrating that UPA almost halved the risk of pregnancy, compared with LNG, in women who received treatment within 120 hours after intercourse, with a reduction of almost two thirds when UPA was taken within 24 hours of unprotected intercourse.
UPA has FDA approval for use within 120 hours after unprotected intercourse and requires a prescription. Although the data leading to this approval are incredibly encouraging, fewer than 200 of more than 2,000 women in three studies performed with UPA took EC 96 to 120 hours after intercourse. With such a small number of women actually tested in this time range, physicians should use caution when counseling patients about the efficacy of UPA when it is taken more than 96 hours after unprotected intercourse.8-10
UPA is more expensive than LNG, which may be a barrier to use by some women. However, because the probability of becoming pregnant when taking UPA within 120 hours of unprotected intercourse is lower than with LNG, the cost differential between drugs is much smaller when total costs—including the cost of unintended pregnancy—are consid-ered.11
Although the LNG-only method is the only EC that is available without a prescription, UPA appears to be more effective, particularly when it is taken more than 72 hours after unprotected intercourse. However, providers should be aware that a relatively small number of women have been studied with UPA beyond 72 hours after unprotected intercourse.
Although LNG-only EC is available behind the counter, the superiority of UPA means that physicians should discuss EC with patients during routine appointments and consider advance provision. For patients, cost and access will be important issues when deciding whether to use LNG or UPA.
EC is more likely to fail in overweight and obese women
Glasier A, Cameron S, Blithe D, et al. Can we identify women at risk of pregnancy despite using emergency contraception? Data from randomized trials of ulipristal acetate and levonorgestrel [published online ahead of print April 2, 2011]. Contraception. doi:10.1016/j.contraception.2011.02.009.
Edelman AB, Carlson NE, Cherala G, et al. Impact of obesity on oral contraceptive pharmacokinetics and hypothalamic-pituitary-ovarian activity. Contraception. 2009;80(2):119–127.
Westhoff CT, Torgal AL, Mayeda ER, et al. Ovarian suppression in normal-weight and obese women during oral contraceptive use. Obstet Gynecol. 2010;116(2 pt 2):275–283.
As we observed, despite more widespread use of EC after the LNG-only method was made available without a prescription, we have not realized the public health benefit of a decreased rate of unintended pregnancy or abortion.4 Studies have shown that, despite taking EC, women who have further acts of intercourse in the same cycle of EC use are more likely to conceive.12,13
We now have clear information about another specific population in which EC is more likely to fail: overweight and obese women. Compared with women of normal weight (body mass index [BMI] <25), overweight women (BMI 25–30) had a risk of pregnancy 1.5 times greater, and obese women (BMI ≥30) had a risk of pregnancy more than three times greater.13
Pregnancy rate among obese women using LNG was the same as the background rate
Obese women who used LNG as EC had a pregnancy rate of 5.8%, which is approximately equivalent to the overall pregnancy rate expected in the absence of EC. Overweight women in the LNG group had a relative risk of pregnancy that was double that of normal or underweight women, whereas overweight women taking UPA had the same risk as normal or underweight women taking the same medication.
When researchers compared pregnancy rates by weight instead of BMI, differences persisted between the two treatment options, with a limit of efficacy reached at a weight of 70 kg (154 lb) for LNG, compared with 88 kg (194 lb) for UPA.
OC hormone absorption is slower in obesity
Two recent studies—by Edelman and colleagues and Westhoff and coworkers—have demonstrated that OC hormone absorption is slower in obese women than it is in women of normal weight. With EC, immediate absorption is important; this delay could explain the lower efficacy in obese women. No studies have evaluated whether a higher or double dose of LNG would improve efficacy. Like women who experience repeated acts of unprotected intercourse, overweight and obese women are at high risk of EC failure and should be counseled about this risk.
As the incidence of obesity continues to increase exponentially in the United States, the efficacy of our commonly used methods of EC will continue to decline. At a minimum, overweight and obese women should be counseled to take UPA rather than LNG because of its increased efficacy in this population. We also need to inform overweight patients that their risk of pregnancy is higher than is commonly quoted.
Have we overlooked the best available emergency contraceptive?
Wu S, Godfrey EM, Wojdyla D, et al. Copper T380A intrauterine device for emergency contraception: a prospective, multicentre, cohort clinical trial. BJOG. 2010;117(10):1205–1210.
Turok D, Gurtcheff S, Handley E, et al. A pilot study of the Copper T380A IUD and levonorgestral for emergency contraception. Contraception. 2010;82(6):520–525.
The copper IUD has always been the most effective EC available. Not only does it prevent pregnancy when inserted as EC, but it continues to provide long-term, reversible contraception for 10 years or longer. Two large studies—one of them published within the past year—found efficacy rates of 96.9% and 100%, much higher than those associated with oral EC, with only two pregnancies occurring in more than 2,000 women.14,15
Although use of the IUD as EC was described as early as 1976, adoption of this method has been minimal in the United States.16 One reason may be the need for a clinician to insert the device, but many providers undoubtedly dismiss the IUD as an option for EC, believing that American women are unwilling to accept it. Some providers maintain the longstanding opinion that the IUD is an option only for parous women, although this notion has been cast aside by layers of medical evidence, as reviewed by current Centers for Disease Control and Prevention (CDC) medical eligibility criteria for contraception.17
All women should be counseled about the long-term benefits of the copper IUD, the most reliable method of EC. The copper IUD not only provides effective emergency contraception but also long-term contraception for 10 years or more. Therefore, we should offer the copper IUD as first-line treatment for women seeking EC (FIGURE). This method is likely to be much more acceptable to patients than previously assumed.
Women are more accepting of the IUD than we thought
Schwarz and colleagues surveyed 412 women in Pittsburgh family planning clinics who were seeking EC or pregnancy testing and found that 15% of these women would be interested in same-day insertion of an IUD.18 This number increased if the IUD was free among women who reported difficulty with access to contraception.
In an observational study, Turok and colleagues offered women who were seeking EC a choice between the copper IUD and oral LNG and followed them for 6 months. Both methods were offered free of charge. They had assumed that, for every 20 women choosing oral LNG, one would choose the copper IUD. What they found was quite different: For every 1.5 women who chose oral LNG, one chose the copper IUD. Even more impressive was the number of women still using highly effective contraception (IUD, implant, or sterilization) 6 months later—4.5% in the oral LNG group and 61.5% in the IUD group. By the end of the 6-month period, two pregnancies had occurred in the oral LNG group and none in the IUD group.

How to counsel a patient seeking emergency contraceptionWe want to hear from you! Tell us what you think.
1. Task Force on Postovulatory Methods of Fertility Regulation. Randomised controlled trial of levonorgestrel versus the Yuzpe regimen of combined oral contraceptives for emergency contraception. Lancet. 1998;352(9126):428-433.
2. Yuzpe A, Thurlow H, Ramzy I, Leyshon J. Post coital contraception–A pilot study. J Reprod Med. 1974;13(2):53-58.
3. Kesseru E, Garmendia F, Westphal N, Parada J. The hormonal and peripheral effects of d-norgestrel in postcoital contraception. Contraception. 1974;10(4):411-424.
4. Polis CB, Schaffer K, Banchard K, et al. Advance provision of emergency contraception for pregnancy prevention (full review). Cochrane Database Syst Rev. 2007;(2):CD005497.-
5. Glasier A, Thong KJ, Dewar M, Mackie M, Baird DT. Postcoital contraception with mifepristone. Lancet. 1991;337(8754):1414-1415.
6. Webb AM. Alternative treatments in oral postcoital contraception: interim results. Adv Contracept. 1991;7(2–3):271-279.
7. Cheng L, Gülmezoglu AM, Piaggio G, Ezcurra E, Van Look PF. Interventions for emergency contraception. Cochrane Database Syst Rev. 2008;(2):CD001324.-
8. Creinin MD, Schlaff W, Archer D, et al. Progesterone receptor modulator for emergency contraception: a randomized controlled trial. Obstet Gynecol. 2006;108(5):1089-1097.
9. Glasier A, Cameron S, Fine P, et al. Ulipristal acetate versus levonorgestrel for emergency contraception: a randomized non-inferiority trial and meta-analysis. Lancet. 2010;375(9714):555-562.
10. Fine P, Mathe H, Ginde S, et al. Ulipristal acetate taken 48-120 hours after intercourse for emergency contraception. Obstet Gynecol. 2010;115(2 pt 1):257-263.
11. Thomas CM, Schmid R, Cameron S. Is it worth paying more for emergency hormonal contraception? The cost-effectiveness of ulipristal acetate versus levonorgestrel 1.5 mg. J Fam Plann Reprod Health Care. 2010;36(4):197-201.
12. von Hertzen H, Piaggio G, Ding J, et al. Low dose mifepris-tone and two regimens of levonorgestrel for emergency contraception: a WHO multicentre randomised trial. Lancet. 2002;360(9348):1803-1810.
13. Glasier A, Cameron S, Blithe D, et al. Can we identify women at risk of pregnancy despite using emergency contraception? Data from randomized trials of ulipristal acetate and levonorgestrel [published online ahead of print April 2 2011]. Contraception. doi:10.1016/j.contraception.2011.02.009.
14. Zhou LY, Xiao BL. Emergency contraception with Multiload Cu-375 SL IUD: A multicenter clinical trial. Contraception. 2001;64(2):107-112.
15. Wu S, Godfrey EM, Wojdyla D, et al. Copper T380A intrauterine device for emergency contraception: a prospective, multicentre, cohort clinical trial. BJOG. 2010;117(10):1205-1210.
16. Lippes J, Malik T, Tatum HJ. The postcoital copper-T. Adv Plan Parent. 1976;11(1):24-29.
17. Centers for Disease Control and Prevention. US medical eligibility criteria contraceptive use 2010. MMWR. 2010;59 (RR04):1-6.
18. Schwarz EB, Kavanaugh M, Douglas E, Dubowitz T, Creinin MD. Interest in intrauterine contraception among seekers of emergency contraception and pregnancy testing. Obstet Gynecol. 2009;113(4):833-839.
1. Task Force on Postovulatory Methods of Fertility Regulation. Randomised controlled trial of levonorgestrel versus the Yuzpe regimen of combined oral contraceptives for emergency contraception. Lancet. 1998;352(9126):428-433.
2. Yuzpe A, Thurlow H, Ramzy I, Leyshon J. Post coital contraception–A pilot study. J Reprod Med. 1974;13(2):53-58.
3. Kesseru E, Garmendia F, Westphal N, Parada J. The hormonal and peripheral effects of d-norgestrel in postcoital contraception. Contraception. 1974;10(4):411-424.
4. Polis CB, Schaffer K, Banchard K, et al. Advance provision of emergency contraception for pregnancy prevention (full review). Cochrane Database Syst Rev. 2007;(2):CD005497.-
5. Glasier A, Thong KJ, Dewar M, Mackie M, Baird DT. Postcoital contraception with mifepristone. Lancet. 1991;337(8754):1414-1415.
6. Webb AM. Alternative treatments in oral postcoital contraception: interim results. Adv Contracept. 1991;7(2–3):271-279.
7. Cheng L, Gülmezoglu AM, Piaggio G, Ezcurra E, Van Look PF. Interventions for emergency contraception. Cochrane Database Syst Rev. 2008;(2):CD001324.-
8. Creinin MD, Schlaff W, Archer D, et al. Progesterone receptor modulator for emergency contraception: a randomized controlled trial. Obstet Gynecol. 2006;108(5):1089-1097.
9. Glasier A, Cameron S, Fine P, et al. Ulipristal acetate versus levonorgestrel for emergency contraception: a randomized non-inferiority trial and meta-analysis. Lancet. 2010;375(9714):555-562.
10. Fine P, Mathe H, Ginde S, et al. Ulipristal acetate taken 48-120 hours after intercourse for emergency contraception. Obstet Gynecol. 2010;115(2 pt 1):257-263.
11. Thomas CM, Schmid R, Cameron S. Is it worth paying more for emergency hormonal contraception? The cost-effectiveness of ulipristal acetate versus levonorgestrel 1.5 mg. J Fam Plann Reprod Health Care. 2010;36(4):197-201.
12. von Hertzen H, Piaggio G, Ding J, et al. Low dose mifepris-tone and two regimens of levonorgestrel for emergency contraception: a WHO multicentre randomised trial. Lancet. 2002;360(9348):1803-1810.
13. Glasier A, Cameron S, Blithe D, et al. Can we identify women at risk of pregnancy despite using emergency contraception? Data from randomized trials of ulipristal acetate and levonorgestrel [published online ahead of print April 2 2011]. Contraception. doi:10.1016/j.contraception.2011.02.009.
14. Zhou LY, Xiao BL. Emergency contraception with Multiload Cu-375 SL IUD: A multicenter clinical trial. Contraception. 2001;64(2):107-112.
15. Wu S, Godfrey EM, Wojdyla D, et al. Copper T380A intrauterine device for emergency contraception: a prospective, multicentre, cohort clinical trial. BJOG. 2010;117(10):1205-1210.
16. Lippes J, Malik T, Tatum HJ. The postcoital copper-T. Adv Plan Parent. 1976;11(1):24-29.
17. Centers for Disease Control and Prevention. US medical eligibility criteria contraceptive use 2010. MMWR. 2010;59 (RR04):1-6.
18. Schwarz EB, Kavanaugh M, Douglas E, Dubowitz T, Creinin MD. Interest in intrauterine contraception among seekers of emergency contraception and pregnancy testing. Obstet Gynecol. 2009;113(4):833-839.
Vitamin D and pregnancy: 9 things you need to know
- How much vitamin D should you recommend to your nonpregnant patients?
Emily D. Szmuilowicz, MD, MS; JoAnn E. Manson, MD, DrPH (July 2011)
With all the publicity surrounding vitamin D lately, it’s no surprise that you have lots of questions. Should you test your patients for deficiency? When? What numbers should you use? And how do you treat a low vitamin D level?
In pregnancy, these issues become critical because there are not one but two patients to consider. Despite the lack of clear guidelines, there is sufficient evidence to suggest that you should at least consider monitoring the vitamin D status of your pregnant patients.
Fetal needs for vitamin D increase during the latter half of pregnancy, when bone growth and ossification are most prominent. Vitamin D travels to the fetus by passive transfer, and the fetus is entirely dependent on maternal stores.1 Therefore, maternal status is a direct reflection of fetal nutritional status.
The vitamin D level in breast milk also correlates with the maternal serum level, and a low vitamin D level in breast milk can exert a harmful effect on a newborn.
In this article, I address nine questions regarding vitamin D and pregnancy:
- Is vitamin D really a vitamin?
- Why do the numbers vary?
- Does the vitamin D level affect pregnancy outcomes?
- Can’t people get enough vitamin D through their diet?
- What level signals deficiency?
- How many women are deficient?
- Should you test all pregnant patients?
- How should you treat vitamin D deficiency in pregnancy?
- Can a person get too much vitamin D?
1. Is vitamin D really a vitamin?
For years, vitamin D was discussed solely in relation to bone metabolism and absorption, and deficiency states were the purview of endocrinologists and gynecologists who treated menopausal patients at risk of osteoporosis. Recent studies demonstrate that vitamin D plays a role in multiple endocrine systems. Indeed, vitamin D may be more correctly considered a hormone because it is a substance produced by one organ (skin) that travels through the bloodstream to target end organs. Vitamin D receptors have been found in bone, breast, brain, colon, muscle, and pancreatic tissues. Not only does vitamin D affect bone metabolism, it also modulates immune responses and even glucose metabolism.2 Vitamin D receptors have also been found in the placenta; their role in that organ remains to be elucidated.
2. Why do the numbers vary?
Some of the confusion surrounding vitamin D concerns the units used to measure and discuss it. Vitamin D can be measured in nanograms per milliliter (ng/mL) or in nanomoles per liter (nmol/L). A measurement of 1 ng/mL equals approximately 2.44 nmol/L. Therefore, deficiency in some articles is described as a vitamin D level below 20 ng/mL and in other articles as a level below 50 nmol/L. As for normal range, it may be listed as a level above 32 ng/mL or as a level above 75 nmol/L.
Compounding the confusion, vitamin D in supplement form can be written in two different measurements—using micrograms or international units. A measurement of 1 μg equals 40 IU, so a supplement of 150 μg/day is the same as one of 6,000 IU/day.
3. Does the vitamin D level affect pregnancy outcomes?
Vitamin D’s role in pregnancy outcomes has yet to be fully described, making it an exciting field to explore. Research into vitamin D and its effects on pregnancy is still in its infancy, but many intriguing associations have been noted. For example, lower levels of vitamin D have been associated with increased rates of cesarean delivery,3 bacterial vaginosis,4 and preeclampsia,5 as well as less efficient glucose metabolism.6
There is biological plausibility for vitamin D to play a role in pregnancy outcomes, given the presence of receptors in gestational tissues. Vitamin D receptors in uterine muscle could affect contractile strength, and vitamin D has been shown to have immunomodulatory effects, thereby potentially protecting the host from infection.
As I mentioned, placental vitamin D receptors and their role need further exploration.
4. Can’t people get enough vitamin D through their diet?
Very few foods contain a large amount of vitamin D, and the few that do (herring, cod liver oil) are not standard fare. Even fortified foods such as milk lack a substantial amount. TABLE 1 lists the amount of vitamin D in various foods.7
TABLE 1
In food, the vitamin D level is generally low
| Source | Amount of vitamin D (IU) |
|---|---|
| Egg yolk | 25 |
| Cereal, fortified with vitamin D, 1 cup | 40–50 |
| Cow’s milk, fortified with vitamin D, 8 oz | 98 |
| Soy milk, fortified with vitamin D, 8 oz | 100 |
| Orange juice, fortified with vitamin D, 8 oz | 100 |
| Quaker Nutrition for Women instant oatmeal, 1 packet | 154 |
| Tuna, canned in oil, 3 oz | 200 |
| Sardines, canned, 3 oz | 231 |
| Mackerel, 3 oz | 306 |
| Most multivitamins | 400 |
| Tri-Vi-Sol infant supplements, 1 drop | 400 |
| Prenatal vitamins | 400 |
| Catfish, 3 oz | 425 |
| Pink salmon, canned, 3 oz | 530 |
| Cod liver oil, 1 tablespoon | 1,360 |
| Herring, 3 oz | 1,383 |
| Over-the-counter vitamin D3 supplements | 2,000 (maximum) |
| Typical prescription of vitamin D2 for deficiency | 50,000 (given weekly until replete) |
5. What level signals deficiency?
Experts disagree about the level of vitamin D that signals deficiency. Many labs report a reference range of 32 to 100 ng/mL as normal. However, in November 2010, the Institute of Medicine (IOM) weighed in on the matter. After examining the data, the IOM suggested that a vitamin D level of 20 ng/mL is sufficient to prevent bone loss and changes seen in rickets and osteoporosis.
This level is hotly contested by experts in other fields, who argue that, although 20 ng/mL may be considered the bare minimum level to prevent negative bone resorption changes, it can hardly be construed as a normal level.
Nor did the IOM recommendation take pregnancy into consideration. Therefore, the IOM made no comment as to whether a level of 20 ng/mL is sufficient for a pregnant woman, given that the fetus will be actively soliciting maternal vitamin D for its own development. Indeed, some researchers have indicated that the actual daily recommended intake for pregnancy and lactation may be as high as 6,000 IU/day.8
6. How many women are deficient?
The rate of deficiency varies, but studies have documented rates as high as 97% in some pregnant populations; the rates vary by race and latitude.9-11
The high prevalence of deficiency in the population is due, in large part, to vitamin D’s mode of production and changes in human lifestyle and culture. Vitamin D is produced primarily through direct exposure of the skin to the sun. Over the past 50 years, as more and more people have come to spend their days in an office or factory instead of on a farm, the opportunity to produce vitamin D has greatly diminished.
Other entities or practices that reduce the production of vitamin D:
- Sunscreen SPF 50 may prevent skin cancer, but it also blocks vitamin D production.
- Fat cells Obese patients produce vitamin D less rapidly than patients of normal weight.
- Melanin Darker-skinned people produce vitamin D at a slower rate than those who have fair skin.
- Cultural practices Some religious and cultural practices mandate full skin coverage in public, particularly for women, leading to minimal sun exposure.
- Age Older people also produce vitamin D more slowly. Among the population of reproductive age, however, the effect of age is minimal.
- Latitude Northern latitudes, with their longer winters and shorter summers, provide less opportunity for sun exposure.
Because vitamin D is, in essence, a “seasonal” vitamin, it makes evolutionary sense that the human body has developed a wide normal range to “store up” vitamin D when sunshine is plentiful and then use its stores during times of scarcity, such as winter. This seasonal variability is another reason why the rate of deficiency can vary, depending on the time and location of study.
Because vitamin D deficiency is clinically silent until severe events such as rickets occur, the best way to check for it is to measure total levels of the two forms of vitamin D found in the body—D2 and D3. The recommended test is total 25-hydroxy vitamin D (25-OHD). Measurement of the activated form of vitamin D—1,25-OHD—will not tell you whether a person’s overall stores are lacking, because the body maintains a normal 1,25-OHD level over a wide range until severe deficiency occurs.
7. Should you test all pregnant patients for deficiency?
ACOG does not recommend that vitamin D be measured routinely in pregnant women.12 In a Committee Opinion published in July 2011, ACOG determined that “there is insufficient evidence to support a recommendation for screening all pregnant women for vitamin D deficiency.”12
Many experts disagree, however, citing the increased rate of rickets being found in the United States.6,8 Pediatricians in the United States have found such a high rate of deficiency in the neonatal population that the American Academy of Pediatricians now recommends that all exclusively breastfed babies be given a supplement of 400 IU of vitamin D daily, beginning in the first few days of life.13
ACOG acknowledged that, for pregnant patients “thought to be at increased risk, measurement of total levels can be considered with “high-risk groups” that have many of the risk factors cited earlier.12
If you want to test your patients, no single plan is recommended. A sample algorithm includes the following steps:
- Measure total 25-OHD at the time of prenatal registration labs
- Select a level of supplementation, based on the findings (see TABLE 2)
- Recheck the 25-OHD level after 3 months. For most patients, this would be around the time of a standard glucose screening test
- Adjust the supplementation level, as needed
- Measure 25-OHD at admission to labor and delivery.
TABLE 2
When (and with how much “D”) to treat pregnant patients
| If the 25-OHD level is… | …then supplement with* |
|---|---|
| <20 ng/mL | 50,000 IU oral vitamin D weekly for 12 weeks |
| 20–32 ng/mL | 2,000–4,000 IU oral vitamin D daily (~15,000–30,000 IU weekly) |
| >32 ng/mL | No action needed |
| *Assuming that the patient will continue taking a prenatal vitamin containing 400 IU/tablet. | |
8. How should you treat vitamin D deficiency in pregnancy?
Here, again, there is a lack of solid evidence. No guidelines exist for pregnant patients. In its Commitee Opinion, ACOG points out that higher-dose regimens have not been studied in pregnancy, but cites studies using up to 4,000 IU daily.12 The question becomes: Can guidelines that have been established for nonpregnant patients be used safely in pregnancy?
Although there is no evidence-based consensus, physiology and previous studies suggest that they can.
In one study, pregnant women were given doses as high as 200,000 IU in the third trimester to treat vitamin D deficiency.14 That investigation produced two key findings:
- There were no signs or symptoms of toxicity in patients or newborns, demonstrating that a single dose of a large amount of vitamin D can be administered safely.
- Despite the treatment, many of the women in this study remained deficient, indicating that continued supplementation would be required beyond the initial dose.
Although the dosage administered in this study seems like a large amount, it should be viewed in context: a Caucasian female can produce 50,000 IU of vitamin D from 30 minutes of sun exposure at midday.14
The IOM acknowledged that it underestimated the amount of vitamin D that can be taken safely and increased its upper limit of normal to 4,000 IU daily. Note that this upper limit is for people who are presumed to have a normal level to begin with. Therefore, it would be expected that a deficiency would require a greater amount for treatment.
As for treatment, both daily and weekly regimens are acceptable. Because vitamin D is fat-soluble, a daily dose of 1,000 IU is equivalent to a weekly dose of 7,000 IU. Many patients prefer the convenience of weekly dosing, which can also improve compliance.
See TABLE 2 for a proposed guideline on how to treat a pregnant patient, based on the 25-OHD level.
9. Can a person get too much vitamin D?
Vitamin D is fat-soluble. Should you worry about toxicity?
Because there is such a wide normal range for vitamin D, a person would have to be taking massive amounts of the nutrient for a substantial time before hypervitaminosis and a potential impact on calcium metabolism occur. Pharmacokinetic data demonstrate that toxicity may not occur until a vitamin D level of 300 ng/mL or higher is reached, which is three times the upper limit of normal for most reference ranges.15 A 2007 review found no cases of toxicity reported in the literature at a total serum level below 200 ng/mL (twice the normal limit) or a dose of less than 30,000 IU/day.16
Last words
Many questions and research opportunities remain regarding optimal vitamin D levels and supplementation in pregnancy, as well as the impact of vitamin D not only on pregnancy-related outcomes but on neonatal and infant health. One thing is certain: No one can argue that a nutritionally deficient state is preferred in pregnancy for maternal or fetal health. As advocates for women’s health, it behooves us to address this situation for the benefit of our patients and their children.
How do you manage the vitamin D requirements of pregnant and nonpregnant patients? Do you agree with the IOM that a vitamin D level of 20 ng/mL is sufficient for most individuals? Do you routinely measure the vitamin D level of your patients? Do you recommend vitamin D supplementation in pregnancy?
To tell us, click here
Study finds vitamin D supplementation in pregnancy to be safe and effective
Daily 4,000-IU vitamin D supplementation from 12 to 16 weeks of gestation is safe and effective in achieving vitamin D sufficiency in pregnant women and their neonates, according to a study published in the July 2011 issue of the Journal of Bone and Mineral Research.
Bruce W. Hollis, PhD, from the Medical University of South Carolina in Charleston, and colleagues assessed the need, safety, and effectiveness of vitamin D supplementation in 350 women with singleton pregnancies at 12 to 16 weeks of gestation. Participants were randomly assigned to receive 400 IU, 2,000 IU, or 4,000 IU vitamin D3 daily until delivery. The outcomes studied included maternal/neonatal circulating serum vitamin D (25-OHD) levels at delivery, achieving 25-OHD of 80 nmol/L or more, and achieving 25-OHD concentration for maximal 1,25-dihydroxycholecalciferol (1,25-OH2D) production.
The investigators found that the percentage of participants who achieved vitamin D sufficiency was significantly different between groups, with the 4,000-IU group having the highest percentage. Within 1 month of delivery, the relative risk (RR) of achieving 25-OHD of 80 nmol/L or more differed significantly between the 2,000-IU versus 400-IU groups and 4,000-IU versus 400-IU groups (RR, 1.52 and 1.60, respectively). There was no significant difference between the 2,000-IU and 4,000-IU groups. Circulatory 25-OHD directly influenced 1,25-OH2D levels throughout pregnancy, with maximal production of 1,25-OH2D in the 4,000-IU group. Vitamin D supplementation was not associated with adverse events, and safety measures were similar between the groups.
“A daily vitamin D dose of 4,000 IU was associated with improved vitamin D status throughout pregnancy, one month prior, and at delivery in both mother and neonate,” the authors write.
One of the study authors disclosed financial ties with the Diasorin Corporation.
Copyright © 2011 HealthDay. All rights reserved.
1. Dror DK, Allen LH. Vitamin D inadequacy in pregnancy: biology outcomes, and interventions. Nutr Rev. 2010;68(8):465-477.
2. Verstuyf A, Carmeliet G, Bouillon R, Mathieu C. Vitamin D: a pleiotropic hormone. Kidney Int. 2010;78(2):140-145.
3. Merewood A, Mehta SD, Chen TC, Bauchner H, Holick MF. Association between vitamin D deficiency and primary cesarean section. J Clin Endocrinol Metab. 2009;94(3):940-945.
4. Bodnar LM, Krohn MA, Simhan HN. Maternal vitamin D deficiency is associated with bacterial vaginosis in the first trimester of pregnancy. J Nutr. 2009;139(6):1157-1161.
5. Robinson CJ, Alanis MC, Wagner CL, Hollis BW, Johnson DD. Plasma 25-hydroxyvitamin D levels in early-onset severe preeclampsia. Am J Obstet Gynecol. 2010;203(4):366.e1-6.
6. Lau SL, Gunton JE, Athayde NP, Byth K, Cheung NW. Serum 25-hydroxyvitamin D and glycated haemoglobin levels in women with gestational diabetes mellitus. Med J Aust. 2011;194(7):334-337.
7. Mulligan ML, Felton SK, Riek AE, Bernal-Mizrachi C. Implications of vitamin D deficiency in pregnancy and lactation. Am J Obstet Gynecol. 2010;202(5):429.e1-9.
8. Hollis BW. Vitamin D requirement during pregnancy and lactation. J Bone Miner Res. 2007;22 (suppl 2):V39-44.
9. Johnson DD, Wagner CL, Hulsey TC, McNeil RB, Ebeling M, Hollis BW. Vitamin D deficiency and insufficiency is common during pregnancy. Am J Perinatol. 2010;28(1):7-12.
10. Bodnar LM, Simhan HN, Powers RW, Frank MP, Cooperstein E, Roberts JM. High prevalence of vitamin D insufficiency in black and white pregnant women residing in the northern United States and their neonates. J Nutr. 2007;137(2):447-452.
11. Ginde AA, Sullivan AF, Mansbach JM, Camargo CA, Jr. Vitamin D insufficiency in pregnant and nonpregnant women of childbearing age in the United States. Am J Obstet Gynecol. 2010;202(5):436.e1-8.
12. ACOG Committee Opinion No. 495: Vitamin D: Screening and supplementation during pregnancy. Obstet Gynecol. 2011;118(1):197-198.
13. Wagner CL, Greer FR. American Academy of Pediatrics Section on Breastfeeding; American Academy of Pediatrics Committee on Nutrition. Prevention of rickets and vitamin D deficiency in infants children, and adolescents. Pediatrics. 2008;122(5):1142-1152.
14. Yu CK, Sykes L, Sethi M, Teoh TG, Robinson S. Vitamin D deficiency and supplementation during pregnancy. Clin Endocrinol (Oxf). 2009;70(5):685-690.
15. Jones G. Pharmacokinetics of vitamin D toxicity. Am J Clin Nutr. 2008;88(2):582S-586S.
16. Hathcock JN, Shao A, Vieth R, Heaney R. Risk assessment for vitamin D. Am J Clin Nutr. 2007;85(1):6-18.
17. Hollis BW. Vitamin D requirement during pregnancy and lactation. J Bone Miner Res. 2007;22 (suppl 2):V39-44.
- How much vitamin D should you recommend to your nonpregnant patients?
Emily D. Szmuilowicz, MD, MS; JoAnn E. Manson, MD, DrPH (July 2011)
With all the publicity surrounding vitamin D lately, it’s no surprise that you have lots of questions. Should you test your patients for deficiency? When? What numbers should you use? And how do you treat a low vitamin D level?
In pregnancy, these issues become critical because there are not one but two patients to consider. Despite the lack of clear guidelines, there is sufficient evidence to suggest that you should at least consider monitoring the vitamin D status of your pregnant patients.
Fetal needs for vitamin D increase during the latter half of pregnancy, when bone growth and ossification are most prominent. Vitamin D travels to the fetus by passive transfer, and the fetus is entirely dependent on maternal stores.1 Therefore, maternal status is a direct reflection of fetal nutritional status.
The vitamin D level in breast milk also correlates with the maternal serum level, and a low vitamin D level in breast milk can exert a harmful effect on a newborn.
In this article, I address nine questions regarding vitamin D and pregnancy:
- Is vitamin D really a vitamin?
- Why do the numbers vary?
- Does the vitamin D level affect pregnancy outcomes?
- Can’t people get enough vitamin D through their diet?
- What level signals deficiency?
- How many women are deficient?
- Should you test all pregnant patients?
- How should you treat vitamin D deficiency in pregnancy?
- Can a person get too much vitamin D?

1. Is vitamin D really a vitamin?
For years, vitamin D was discussed solely in relation to bone metabolism and absorption, and deficiency states were the purview of endocrinologists and gynecologists who treated menopausal patients at risk of osteoporosis. Recent studies demonstrate that vitamin D plays a role in multiple endocrine systems. Indeed, vitamin D may be more correctly considered a hormone because it is a substance produced by one organ (skin) that travels through the bloodstream to target end organs. Vitamin D receptors have been found in bone, breast, brain, colon, muscle, and pancreatic tissues. Not only does vitamin D affect bone metabolism, it also modulates immune responses and even glucose metabolism.2 Vitamin D receptors have also been found in the placenta; their role in that organ remains to be elucidated.
2. Why do the numbers vary?
Some of the confusion surrounding vitamin D concerns the units used to measure and discuss it. Vitamin D can be measured in nanograms per milliliter (ng/mL) or in nanomoles per liter (nmol/L). A measurement of 1 ng/mL equals approximately 2.44 nmol/L. Therefore, deficiency in some articles is described as a vitamin D level below 20 ng/mL and in other articles as a level below 50 nmol/L. As for normal range, it may be listed as a level above 32 ng/mL or as a level above 75 nmol/L.
Compounding the confusion, vitamin D in supplement form can be written in two different measurements—using micrograms or international units. A measurement of 1 μg equals 40 IU, so a supplement of 150 μg/day is the same as one of 6,000 IU/day.
3. Does the vitamin D level affect pregnancy outcomes?
Vitamin D’s role in pregnancy outcomes has yet to be fully described, making it an exciting field to explore. Research into vitamin D and its effects on pregnancy is still in its infancy, but many intriguing associations have been noted. For example, lower levels of vitamin D have been associated with increased rates of cesarean delivery,3 bacterial vaginosis,4 and preeclampsia,5 as well as less efficient glucose metabolism.6
There is biological plausibility for vitamin D to play a role in pregnancy outcomes, given the presence of receptors in gestational tissues. Vitamin D receptors in uterine muscle could affect contractile strength, and vitamin D has been shown to have immunomodulatory effects, thereby potentially protecting the host from infection.
As I mentioned, placental vitamin D receptors and their role need further exploration.
4. Can’t people get enough vitamin D through their diet?
Very few foods contain a large amount of vitamin D, and the few that do (herring, cod liver oil) are not standard fare. Even fortified foods such as milk lack a substantial amount. TABLE 1 lists the amount of vitamin D in various foods.7
TABLE 1
In food, the vitamin D level is generally low
| Source | Amount of vitamin D (IU) |
|---|---|
| Egg yolk | 25 |
| Cereal, fortified with vitamin D, 1 cup | 40–50 |
| Cow’s milk, fortified with vitamin D, 8 oz | 98 |
| Soy milk, fortified with vitamin D, 8 oz | 100 |
| Orange juice, fortified with vitamin D, 8 oz | 100 |
| Quaker Nutrition for Women instant oatmeal, 1 packet | 154 |
| Tuna, canned in oil, 3 oz | 200 |
| Sardines, canned, 3 oz | 231 |
| Mackerel, 3 oz | 306 |
| Most multivitamins | 400 |
| Tri-Vi-Sol infant supplements, 1 drop | 400 |
| Prenatal vitamins | 400 |
| Catfish, 3 oz | 425 |
| Pink salmon, canned, 3 oz | 530 |
| Cod liver oil, 1 tablespoon | 1,360 |
| Herring, 3 oz | 1,383 |
| Over-the-counter vitamin D3 supplements | 2,000 (maximum) |
| Typical prescription of vitamin D2 for deficiency | 50,000 (given weekly until replete) |
5. What level signals deficiency?
Experts disagree about the level of vitamin D that signals deficiency. Many labs report a reference range of 32 to 100 ng/mL as normal. However, in November 2010, the Institute of Medicine (IOM) weighed in on the matter. After examining the data, the IOM suggested that a vitamin D level of 20 ng/mL is sufficient to prevent bone loss and changes seen in rickets and osteoporosis.
This level is hotly contested by experts in other fields, who argue that, although 20 ng/mL may be considered the bare minimum level to prevent negative bone resorption changes, it can hardly be construed as a normal level.
Nor did the IOM recommendation take pregnancy into consideration. Therefore, the IOM made no comment as to whether a level of 20 ng/mL is sufficient for a pregnant woman, given that the fetus will be actively soliciting maternal vitamin D for its own development. Indeed, some researchers have indicated that the actual daily recommended intake for pregnancy and lactation may be as high as 6,000 IU/day.8
6. How many women are deficient?
The rate of deficiency varies, but studies have documented rates as high as 97% in some pregnant populations; the rates vary by race and latitude.9-11
The high prevalence of deficiency in the population is due, in large part, to vitamin D’s mode of production and changes in human lifestyle and culture. Vitamin D is produced primarily through direct exposure of the skin to the sun. Over the past 50 years, as more and more people have come to spend their days in an office or factory instead of on a farm, the opportunity to produce vitamin D has greatly diminished.
Other entities or practices that reduce the production of vitamin D:
- Sunscreen SPF 50 may prevent skin cancer, but it also blocks vitamin D production.
- Fat cells Obese patients produce vitamin D less rapidly than patients of normal weight.
- Melanin Darker-skinned people produce vitamin D at a slower rate than those who have fair skin.
- Cultural practices Some religious and cultural practices mandate full skin coverage in public, particularly for women, leading to minimal sun exposure.
- Age Older people also produce vitamin D more slowly. Among the population of reproductive age, however, the effect of age is minimal.
- Latitude Northern latitudes, with their longer winters and shorter summers, provide less opportunity for sun exposure.
Because vitamin D is, in essence, a “seasonal” vitamin, it makes evolutionary sense that the human body has developed a wide normal range to “store up” vitamin D when sunshine is plentiful and then use its stores during times of scarcity, such as winter. This seasonal variability is another reason why the rate of deficiency can vary, depending on the time and location of study.
Because vitamin D deficiency is clinically silent until severe events such as rickets occur, the best way to check for it is to measure total levels of the two forms of vitamin D found in the body—D2 and D3. The recommended test is total 25-hydroxy vitamin D (25-OHD). Measurement of the activated form of vitamin D—1,25-OHD—will not tell you whether a person’s overall stores are lacking, because the body maintains a normal 1,25-OHD level over a wide range until severe deficiency occurs.
7. Should you test all pregnant patients for deficiency?
ACOG does not recommend that vitamin D be measured routinely in pregnant women.12 In a Committee Opinion published in July 2011, ACOG determined that “there is insufficient evidence to support a recommendation for screening all pregnant women for vitamin D deficiency.”12
Many experts disagree, however, citing the increased rate of rickets being found in the United States.6,8 Pediatricians in the United States have found such a high rate of deficiency in the neonatal population that the American Academy of Pediatricians now recommends that all exclusively breastfed babies be given a supplement of 400 IU of vitamin D daily, beginning in the first few days of life.13
ACOG acknowledged that, for pregnant patients “thought to be at increased risk, measurement of total levels can be considered with “high-risk groups” that have many of the risk factors cited earlier.12
If you want to test your patients, no single plan is recommended. A sample algorithm includes the following steps:
- Measure total 25-OHD at the time of prenatal registration labs
- Select a level of supplementation, based on the findings (see TABLE 2)
- Recheck the 25-OHD level after 3 months. For most patients, this would be around the time of a standard glucose screening test
- Adjust the supplementation level, as needed
- Measure 25-OHD at admission to labor and delivery.
TABLE 2
When (and with how much “D”) to treat pregnant patients
| If the 25-OHD level is… | …then supplement with* |
|---|---|
| <20 ng/mL | 50,000 IU oral vitamin D weekly for 12 weeks |
| 20–32 ng/mL | 2,000–4,000 IU oral vitamin D daily (~15,000–30,000 IU weekly) |
| >32 ng/mL | No action needed |
| *Assuming that the patient will continue taking a prenatal vitamin containing 400 IU/tablet. | |
8. How should you treat vitamin D deficiency in pregnancy?
Here, again, there is a lack of solid evidence. No guidelines exist for pregnant patients. In its Commitee Opinion, ACOG points out that higher-dose regimens have not been studied in pregnancy, but cites studies using up to 4,000 IU daily.12 The question becomes: Can guidelines that have been established for nonpregnant patients be used safely in pregnancy?
Although there is no evidence-based consensus, physiology and previous studies suggest that they can.
In one study, pregnant women were given doses as high as 200,000 IU in the third trimester to treat vitamin D deficiency.14 That investigation produced two key findings:
- There were no signs or symptoms of toxicity in patients or newborns, demonstrating that a single dose of a large amount of vitamin D can be administered safely.
- Despite the treatment, many of the women in this study remained deficient, indicating that continued supplementation would be required beyond the initial dose.
Although the dosage administered in this study seems like a large amount, it should be viewed in context: a Caucasian female can produce 50,000 IU of vitamin D from 30 minutes of sun exposure at midday.14
The IOM acknowledged that it underestimated the amount of vitamin D that can be taken safely and increased its upper limit of normal to 4,000 IU daily. Note that this upper limit is for people who are presumed to have a normal level to begin with. Therefore, it would be expected that a deficiency would require a greater amount for treatment.
As for treatment, both daily and weekly regimens are acceptable. Because vitamin D is fat-soluble, a daily dose of 1,000 IU is equivalent to a weekly dose of 7,000 IU. Many patients prefer the convenience of weekly dosing, which can also improve compliance.
See TABLE 2 for a proposed guideline on how to treat a pregnant patient, based on the 25-OHD level.
9. Can a person get too much vitamin D?
Vitamin D is fat-soluble. Should you worry about toxicity?
Because there is such a wide normal range for vitamin D, a person would have to be taking massive amounts of the nutrient for a substantial time before hypervitaminosis and a potential impact on calcium metabolism occur. Pharmacokinetic data demonstrate that toxicity may not occur until a vitamin D level of 300 ng/mL or higher is reached, which is three times the upper limit of normal for most reference ranges.15 A 2007 review found no cases of toxicity reported in the literature at a total serum level below 200 ng/mL (twice the normal limit) or a dose of less than 30,000 IU/day.16
Last words
Many questions and research opportunities remain regarding optimal vitamin D levels and supplementation in pregnancy, as well as the impact of vitamin D not only on pregnancy-related outcomes but on neonatal and infant health. One thing is certain: No one can argue that a nutritionally deficient state is preferred in pregnancy for maternal or fetal health. As advocates for women’s health, it behooves us to address this situation for the benefit of our patients and their children.
How do you manage the vitamin D requirements of pregnant and nonpregnant patients? Do you agree with the IOM that a vitamin D level of 20 ng/mL is sufficient for most individuals? Do you routinely measure the vitamin D level of your patients? Do you recommend vitamin D supplementation in pregnancy?
To tell us, click here
Study finds vitamin D supplementation in pregnancy to be safe and effective
Daily 4,000-IU vitamin D supplementation from 12 to 16 weeks of gestation is safe and effective in achieving vitamin D sufficiency in pregnant women and their neonates, according to a study published in the July 2011 issue of the Journal of Bone and Mineral Research.
Bruce W. Hollis, PhD, from the Medical University of South Carolina in Charleston, and colleagues assessed the need, safety, and effectiveness of vitamin D supplementation in 350 women with singleton pregnancies at 12 to 16 weeks of gestation. Participants were randomly assigned to receive 400 IU, 2,000 IU, or 4,000 IU vitamin D3 daily until delivery. The outcomes studied included maternal/neonatal circulating serum vitamin D (25-OHD) levels at delivery, achieving 25-OHD of 80 nmol/L or more, and achieving 25-OHD concentration for maximal 1,25-dihydroxycholecalciferol (1,25-OH2D) production.
The investigators found that the percentage of participants who achieved vitamin D sufficiency was significantly different between groups, with the 4,000-IU group having the highest percentage. Within 1 month of delivery, the relative risk (RR) of achieving 25-OHD of 80 nmol/L or more differed significantly between the 2,000-IU versus 400-IU groups and 4,000-IU versus 400-IU groups (RR, 1.52 and 1.60, respectively). There was no significant difference between the 2,000-IU and 4,000-IU groups. Circulatory 25-OHD directly influenced 1,25-OH2D levels throughout pregnancy, with maximal production of 1,25-OH2D in the 4,000-IU group. Vitamin D supplementation was not associated with adverse events, and safety measures were similar between the groups.
“A daily vitamin D dose of 4,000 IU was associated with improved vitamin D status throughout pregnancy, one month prior, and at delivery in both mother and neonate,” the authors write.
One of the study authors disclosed financial ties with the Diasorin Corporation.
Copyright © 2011 HealthDay. All rights reserved.
- How much vitamin D should you recommend to your nonpregnant patients?
Emily D. Szmuilowicz, MD, MS; JoAnn E. Manson, MD, DrPH (July 2011)
With all the publicity surrounding vitamin D lately, it’s no surprise that you have lots of questions. Should you test your patients for deficiency? When? What numbers should you use? And how do you treat a low vitamin D level?
In pregnancy, these issues become critical because there are not one but two patients to consider. Despite the lack of clear guidelines, there is sufficient evidence to suggest that you should at least consider monitoring the vitamin D status of your pregnant patients.
Fetal needs for vitamin D increase during the latter half of pregnancy, when bone growth and ossification are most prominent. Vitamin D travels to the fetus by passive transfer, and the fetus is entirely dependent on maternal stores.1 Therefore, maternal status is a direct reflection of fetal nutritional status.
The vitamin D level in breast milk also correlates with the maternal serum level, and a low vitamin D level in breast milk can exert a harmful effect on a newborn.
In this article, I address nine questions regarding vitamin D and pregnancy:
- Is vitamin D really a vitamin?
- Why do the numbers vary?
- Does the vitamin D level affect pregnancy outcomes?
- Can’t people get enough vitamin D through their diet?
- What level signals deficiency?
- How many women are deficient?
- Should you test all pregnant patients?
- How should you treat vitamin D deficiency in pregnancy?
- Can a person get too much vitamin D?

1. Is vitamin D really a vitamin?
For years, vitamin D was discussed solely in relation to bone metabolism and absorption, and deficiency states were the purview of endocrinologists and gynecologists who treated menopausal patients at risk of osteoporosis. Recent studies demonstrate that vitamin D plays a role in multiple endocrine systems. Indeed, vitamin D may be more correctly considered a hormone because it is a substance produced by one organ (skin) that travels through the bloodstream to target end organs. Vitamin D receptors have been found in bone, breast, brain, colon, muscle, and pancreatic tissues. Not only does vitamin D affect bone metabolism, it also modulates immune responses and even glucose metabolism.2 Vitamin D receptors have also been found in the placenta; their role in that organ remains to be elucidated.
2. Why do the numbers vary?
Some of the confusion surrounding vitamin D concerns the units used to measure and discuss it. Vitamin D can be measured in nanograms per milliliter (ng/mL) or in nanomoles per liter (nmol/L). A measurement of 1 ng/mL equals approximately 2.44 nmol/L. Therefore, deficiency in some articles is described as a vitamin D level below 20 ng/mL and in other articles as a level below 50 nmol/L. As for normal range, it may be listed as a level above 32 ng/mL or as a level above 75 nmol/L.
Compounding the confusion, vitamin D in supplement form can be written in two different measurements—using micrograms or international units. A measurement of 1 μg equals 40 IU, so a supplement of 150 μg/day is the same as one of 6,000 IU/day.
3. Does the vitamin D level affect pregnancy outcomes?
Vitamin D’s role in pregnancy outcomes has yet to be fully described, making it an exciting field to explore. Research into vitamin D and its effects on pregnancy is still in its infancy, but many intriguing associations have been noted. For example, lower levels of vitamin D have been associated with increased rates of cesarean delivery,3 bacterial vaginosis,4 and preeclampsia,5 as well as less efficient glucose metabolism.6
There is biological plausibility for vitamin D to play a role in pregnancy outcomes, given the presence of receptors in gestational tissues. Vitamin D receptors in uterine muscle could affect contractile strength, and vitamin D has been shown to have immunomodulatory effects, thereby potentially protecting the host from infection.
As I mentioned, placental vitamin D receptors and their role need further exploration.
4. Can’t people get enough vitamin D through their diet?
Very few foods contain a large amount of vitamin D, and the few that do (herring, cod liver oil) are not standard fare. Even fortified foods such as milk lack a substantial amount. TABLE 1 lists the amount of vitamin D in various foods.7
TABLE 1
In food, the vitamin D level is generally low
| Source | Amount of vitamin D (IU) |
|---|---|
| Egg yolk | 25 |
| Cereal, fortified with vitamin D, 1 cup | 40–50 |
| Cow’s milk, fortified with vitamin D, 8 oz | 98 |
| Soy milk, fortified with vitamin D, 8 oz | 100 |
| Orange juice, fortified with vitamin D, 8 oz | 100 |
| Quaker Nutrition for Women instant oatmeal, 1 packet | 154 |
| Tuna, canned in oil, 3 oz | 200 |
| Sardines, canned, 3 oz | 231 |
| Mackerel, 3 oz | 306 |
| Most multivitamins | 400 |
| Tri-Vi-Sol infant supplements, 1 drop | 400 |
| Prenatal vitamins | 400 |
| Catfish, 3 oz | 425 |
| Pink salmon, canned, 3 oz | 530 |
| Cod liver oil, 1 tablespoon | 1,360 |
| Herring, 3 oz | 1,383 |
| Over-the-counter vitamin D3 supplements | 2,000 (maximum) |
| Typical prescription of vitamin D2 for deficiency | 50,000 (given weekly until replete) |
5. What level signals deficiency?
Experts disagree about the level of vitamin D that signals deficiency. Many labs report a reference range of 32 to 100 ng/mL as normal. However, in November 2010, the Institute of Medicine (IOM) weighed in on the matter. After examining the data, the IOM suggested that a vitamin D level of 20 ng/mL is sufficient to prevent bone loss and changes seen in rickets and osteoporosis.
This level is hotly contested by experts in other fields, who argue that, although 20 ng/mL may be considered the bare minimum level to prevent negative bone resorption changes, it can hardly be construed as a normal level.
Nor did the IOM recommendation take pregnancy into consideration. Therefore, the IOM made no comment as to whether a level of 20 ng/mL is sufficient for a pregnant woman, given that the fetus will be actively soliciting maternal vitamin D for its own development. Indeed, some researchers have indicated that the actual daily recommended intake for pregnancy and lactation may be as high as 6,000 IU/day.8
6. How many women are deficient?
The rate of deficiency varies, but studies have documented rates as high as 97% in some pregnant populations; the rates vary by race and latitude.9-11
The high prevalence of deficiency in the population is due, in large part, to vitamin D’s mode of production and changes in human lifestyle and culture. Vitamin D is produced primarily through direct exposure of the skin to the sun. Over the past 50 years, as more and more people have come to spend their days in an office or factory instead of on a farm, the opportunity to produce vitamin D has greatly diminished.
Other entities or practices that reduce the production of vitamin D:
- Sunscreen SPF 50 may prevent skin cancer, but it also blocks vitamin D production.
- Fat cells Obese patients produce vitamin D less rapidly than patients of normal weight.
- Melanin Darker-skinned people produce vitamin D at a slower rate than those who have fair skin.
- Cultural practices Some religious and cultural practices mandate full skin coverage in public, particularly for women, leading to minimal sun exposure.
- Age Older people also produce vitamin D more slowly. Among the population of reproductive age, however, the effect of age is minimal.
- Latitude Northern latitudes, with their longer winters and shorter summers, provide less opportunity for sun exposure.
Because vitamin D is, in essence, a “seasonal” vitamin, it makes evolutionary sense that the human body has developed a wide normal range to “store up” vitamin D when sunshine is plentiful and then use its stores during times of scarcity, such as winter. This seasonal variability is another reason why the rate of deficiency can vary, depending on the time and location of study.
Because vitamin D deficiency is clinically silent until severe events such as rickets occur, the best way to check for it is to measure total levels of the two forms of vitamin D found in the body—D2 and D3. The recommended test is total 25-hydroxy vitamin D (25-OHD). Measurement of the activated form of vitamin D—1,25-OHD—will not tell you whether a person’s overall stores are lacking, because the body maintains a normal 1,25-OHD level over a wide range until severe deficiency occurs.
7. Should you test all pregnant patients for deficiency?
ACOG does not recommend that vitamin D be measured routinely in pregnant women.12 In a Committee Opinion published in July 2011, ACOG determined that “there is insufficient evidence to support a recommendation for screening all pregnant women for vitamin D deficiency.”12
Many experts disagree, however, citing the increased rate of rickets being found in the United States.6,8 Pediatricians in the United States have found such a high rate of deficiency in the neonatal population that the American Academy of Pediatricians now recommends that all exclusively breastfed babies be given a supplement of 400 IU of vitamin D daily, beginning in the first few days of life.13
ACOG acknowledged that, for pregnant patients “thought to be at increased risk, measurement of total levels can be considered with “high-risk groups” that have many of the risk factors cited earlier.12
If you want to test your patients, no single plan is recommended. A sample algorithm includes the following steps:
- Measure total 25-OHD at the time of prenatal registration labs
- Select a level of supplementation, based on the findings (see TABLE 2)
- Recheck the 25-OHD level after 3 months. For most patients, this would be around the time of a standard glucose screening test
- Adjust the supplementation level, as needed
- Measure 25-OHD at admission to labor and delivery.
TABLE 2
When (and with how much “D”) to treat pregnant patients
| If the 25-OHD level is… | …then supplement with* |
|---|---|
| <20 ng/mL | 50,000 IU oral vitamin D weekly for 12 weeks |
| 20–32 ng/mL | 2,000–4,000 IU oral vitamin D daily (~15,000–30,000 IU weekly) |
| >32 ng/mL | No action needed |
| *Assuming that the patient will continue taking a prenatal vitamin containing 400 IU/tablet. | |
8. How should you treat vitamin D deficiency in pregnancy?
Here, again, there is a lack of solid evidence. No guidelines exist for pregnant patients. In its Commitee Opinion, ACOG points out that higher-dose regimens have not been studied in pregnancy, but cites studies using up to 4,000 IU daily.12 The question becomes: Can guidelines that have been established for nonpregnant patients be used safely in pregnancy?
Although there is no evidence-based consensus, physiology and previous studies suggest that they can.
In one study, pregnant women were given doses as high as 200,000 IU in the third trimester to treat vitamin D deficiency.14 That investigation produced two key findings:
- There were no signs or symptoms of toxicity in patients or newborns, demonstrating that a single dose of a large amount of vitamin D can be administered safely.
- Despite the treatment, many of the women in this study remained deficient, indicating that continued supplementation would be required beyond the initial dose.
Although the dosage administered in this study seems like a large amount, it should be viewed in context: a Caucasian female can produce 50,000 IU of vitamin D from 30 minutes of sun exposure at midday.14
The IOM acknowledged that it underestimated the amount of vitamin D that can be taken safely and increased its upper limit of normal to 4,000 IU daily. Note that this upper limit is for people who are presumed to have a normal level to begin with. Therefore, it would be expected that a deficiency would require a greater amount for treatment.
As for treatment, both daily and weekly regimens are acceptable. Because vitamin D is fat-soluble, a daily dose of 1,000 IU is equivalent to a weekly dose of 7,000 IU. Many patients prefer the convenience of weekly dosing, which can also improve compliance.
See TABLE 2 for a proposed guideline on how to treat a pregnant patient, based on the 25-OHD level.
9. Can a person get too much vitamin D?
Vitamin D is fat-soluble. Should you worry about toxicity?
Because there is such a wide normal range for vitamin D, a person would have to be taking massive amounts of the nutrient for a substantial time before hypervitaminosis and a potential impact on calcium metabolism occur. Pharmacokinetic data demonstrate that toxicity may not occur until a vitamin D level of 300 ng/mL or higher is reached, which is three times the upper limit of normal for most reference ranges.15 A 2007 review found no cases of toxicity reported in the literature at a total serum level below 200 ng/mL (twice the normal limit) or a dose of less than 30,000 IU/day.16
Last words
Many questions and research opportunities remain regarding optimal vitamin D levels and supplementation in pregnancy, as well as the impact of vitamin D not only on pregnancy-related outcomes but on neonatal and infant health. One thing is certain: No one can argue that a nutritionally deficient state is preferred in pregnancy for maternal or fetal health. As advocates for women’s health, it behooves us to address this situation for the benefit of our patients and their children.
How do you manage the vitamin D requirements of pregnant and nonpregnant patients? Do you agree with the IOM that a vitamin D level of 20 ng/mL is sufficient for most individuals? Do you routinely measure the vitamin D level of your patients? Do you recommend vitamin D supplementation in pregnancy?
To tell us, click here
Study finds vitamin D supplementation in pregnancy to be safe and effective
Daily 4,000-IU vitamin D supplementation from 12 to 16 weeks of gestation is safe and effective in achieving vitamin D sufficiency in pregnant women and their neonates, according to a study published in the July 2011 issue of the Journal of Bone and Mineral Research.
Bruce W. Hollis, PhD, from the Medical University of South Carolina in Charleston, and colleagues assessed the need, safety, and effectiveness of vitamin D supplementation in 350 women with singleton pregnancies at 12 to 16 weeks of gestation. Participants were randomly assigned to receive 400 IU, 2,000 IU, or 4,000 IU vitamin D3 daily until delivery. The outcomes studied included maternal/neonatal circulating serum vitamin D (25-OHD) levels at delivery, achieving 25-OHD of 80 nmol/L or more, and achieving 25-OHD concentration for maximal 1,25-dihydroxycholecalciferol (1,25-OH2D) production.
The investigators found that the percentage of participants who achieved vitamin D sufficiency was significantly different between groups, with the 4,000-IU group having the highest percentage. Within 1 month of delivery, the relative risk (RR) of achieving 25-OHD of 80 nmol/L or more differed significantly between the 2,000-IU versus 400-IU groups and 4,000-IU versus 400-IU groups (RR, 1.52 and 1.60, respectively). There was no significant difference between the 2,000-IU and 4,000-IU groups. Circulatory 25-OHD directly influenced 1,25-OH2D levels throughout pregnancy, with maximal production of 1,25-OH2D in the 4,000-IU group. Vitamin D supplementation was not associated with adverse events, and safety measures were similar between the groups.
“A daily vitamin D dose of 4,000 IU was associated with improved vitamin D status throughout pregnancy, one month prior, and at delivery in both mother and neonate,” the authors write.
One of the study authors disclosed financial ties with the Diasorin Corporation.
Copyright © 2011 HealthDay. All rights reserved.
1. Dror DK, Allen LH. Vitamin D inadequacy in pregnancy: biology outcomes, and interventions. Nutr Rev. 2010;68(8):465-477.
2. Verstuyf A, Carmeliet G, Bouillon R, Mathieu C. Vitamin D: a pleiotropic hormone. Kidney Int. 2010;78(2):140-145.
3. Merewood A, Mehta SD, Chen TC, Bauchner H, Holick MF. Association between vitamin D deficiency and primary cesarean section. J Clin Endocrinol Metab. 2009;94(3):940-945.
4. Bodnar LM, Krohn MA, Simhan HN. Maternal vitamin D deficiency is associated with bacterial vaginosis in the first trimester of pregnancy. J Nutr. 2009;139(6):1157-1161.
5. Robinson CJ, Alanis MC, Wagner CL, Hollis BW, Johnson DD. Plasma 25-hydroxyvitamin D levels in early-onset severe preeclampsia. Am J Obstet Gynecol. 2010;203(4):366.e1-6.
6. Lau SL, Gunton JE, Athayde NP, Byth K, Cheung NW. Serum 25-hydroxyvitamin D and glycated haemoglobin levels in women with gestational diabetes mellitus. Med J Aust. 2011;194(7):334-337.
7. Mulligan ML, Felton SK, Riek AE, Bernal-Mizrachi C. Implications of vitamin D deficiency in pregnancy and lactation. Am J Obstet Gynecol. 2010;202(5):429.e1-9.
8. Hollis BW. Vitamin D requirement during pregnancy and lactation. J Bone Miner Res. 2007;22 (suppl 2):V39-44.
9. Johnson DD, Wagner CL, Hulsey TC, McNeil RB, Ebeling M, Hollis BW. Vitamin D deficiency and insufficiency is common during pregnancy. Am J Perinatol. 2010;28(1):7-12.
10. Bodnar LM, Simhan HN, Powers RW, Frank MP, Cooperstein E, Roberts JM. High prevalence of vitamin D insufficiency in black and white pregnant women residing in the northern United States and their neonates. J Nutr. 2007;137(2):447-452.
11. Ginde AA, Sullivan AF, Mansbach JM, Camargo CA, Jr. Vitamin D insufficiency in pregnant and nonpregnant women of childbearing age in the United States. Am J Obstet Gynecol. 2010;202(5):436.e1-8.
12. ACOG Committee Opinion No. 495: Vitamin D: Screening and supplementation during pregnancy. Obstet Gynecol. 2011;118(1):197-198.
13. Wagner CL, Greer FR. American Academy of Pediatrics Section on Breastfeeding; American Academy of Pediatrics Committee on Nutrition. Prevention of rickets and vitamin D deficiency in infants children, and adolescents. Pediatrics. 2008;122(5):1142-1152.
14. Yu CK, Sykes L, Sethi M, Teoh TG, Robinson S. Vitamin D deficiency and supplementation during pregnancy. Clin Endocrinol (Oxf). 2009;70(5):685-690.
15. Jones G. Pharmacokinetics of vitamin D toxicity. Am J Clin Nutr. 2008;88(2):582S-586S.
16. Hathcock JN, Shao A, Vieth R, Heaney R. Risk assessment for vitamin D. Am J Clin Nutr. 2007;85(1):6-18.
17. Hollis BW. Vitamin D requirement during pregnancy and lactation. J Bone Miner Res. 2007;22 (suppl 2):V39-44.
1. Dror DK, Allen LH. Vitamin D inadequacy in pregnancy: biology outcomes, and interventions. Nutr Rev. 2010;68(8):465-477.
2. Verstuyf A, Carmeliet G, Bouillon R, Mathieu C. Vitamin D: a pleiotropic hormone. Kidney Int. 2010;78(2):140-145.
3. Merewood A, Mehta SD, Chen TC, Bauchner H, Holick MF. Association between vitamin D deficiency and primary cesarean section. J Clin Endocrinol Metab. 2009;94(3):940-945.
4. Bodnar LM, Krohn MA, Simhan HN. Maternal vitamin D deficiency is associated with bacterial vaginosis in the first trimester of pregnancy. J Nutr. 2009;139(6):1157-1161.
5. Robinson CJ, Alanis MC, Wagner CL, Hollis BW, Johnson DD. Plasma 25-hydroxyvitamin D levels in early-onset severe preeclampsia. Am J Obstet Gynecol. 2010;203(4):366.e1-6.
6. Lau SL, Gunton JE, Athayde NP, Byth K, Cheung NW. Serum 25-hydroxyvitamin D and glycated haemoglobin levels in women with gestational diabetes mellitus. Med J Aust. 2011;194(7):334-337.
7. Mulligan ML, Felton SK, Riek AE, Bernal-Mizrachi C. Implications of vitamin D deficiency in pregnancy and lactation. Am J Obstet Gynecol. 2010;202(5):429.e1-9.
8. Hollis BW. Vitamin D requirement during pregnancy and lactation. J Bone Miner Res. 2007;22 (suppl 2):V39-44.
9. Johnson DD, Wagner CL, Hulsey TC, McNeil RB, Ebeling M, Hollis BW. Vitamin D deficiency and insufficiency is common during pregnancy. Am J Perinatol. 2010;28(1):7-12.
10. Bodnar LM, Simhan HN, Powers RW, Frank MP, Cooperstein E, Roberts JM. High prevalence of vitamin D insufficiency in black and white pregnant women residing in the northern United States and their neonates. J Nutr. 2007;137(2):447-452.
11. Ginde AA, Sullivan AF, Mansbach JM, Camargo CA, Jr. Vitamin D insufficiency in pregnant and nonpregnant women of childbearing age in the United States. Am J Obstet Gynecol. 2010;202(5):436.e1-8.
12. ACOG Committee Opinion No. 495: Vitamin D: Screening and supplementation during pregnancy. Obstet Gynecol. 2011;118(1):197-198.
13. Wagner CL, Greer FR. American Academy of Pediatrics Section on Breastfeeding; American Academy of Pediatrics Committee on Nutrition. Prevention of rickets and vitamin D deficiency in infants children, and adolescents. Pediatrics. 2008;122(5):1142-1152.
14. Yu CK, Sykes L, Sethi M, Teoh TG, Robinson S. Vitamin D deficiency and supplementation during pregnancy. Clin Endocrinol (Oxf). 2009;70(5):685-690.
15. Jones G. Pharmacokinetics of vitamin D toxicity. Am J Clin Nutr. 2008;88(2):582S-586S.
16. Hathcock JN, Shao A, Vieth R, Heaney R. Risk assessment for vitamin D. Am J Clin Nutr. 2007;85(1):6-18.
17. Hollis BW. Vitamin D requirement during pregnancy and lactation. J Bone Miner Res. 2007;22 (suppl 2):V39-44.
Gout: A Clinical Overview
Gouty arthritis is a common form of inflammatory arthritis, occurring more frequently in men than in women. The condition has a male–female ratio of 3 or 4 to 1, although that ratio narrows as adults age; because the uricosuric effects of estrogen decline with menopause, the risk for gout increases in postmenopausal women.1,2 Mean age at disease onset is 40 to 60 in men,3,4 with onset in women averaging seven years later.5
Apart from the pain and loss of function associated with this disorder of purine metabolism6 and the risk for a chronic form of the disease, gout is almost universally linked with serious comorbidities that require timely intervention. These include hypertension, dyslipidemia, hyperglycemia and diabetes, obesity, metabolic syndrome, cardiovascular disease (CVD), renal insufficiency, and coronary heart disease (CHD).2,3,7 The presence of gout is independently associated with a risk for acute myocardial infarction (AMI) and increased rates of all-cause mortality.3,8-10
EPIDEMIOLOGY
In 2007, the National Arthritis Data Workgroup11,12 estimated that about three million US adults had had “self-reported gout” in the previous year. An estimated six million US adults have been diagnosed with gout,2,11 and its incidence and prevalence are increasing. The incidence of primary gout more than doubled between 1977-1978 and 1995-1996,13 especially affecting the aging population. The prevalence of gout among 1,000 managed care patients ages 65 to 74 increased by at least 30% between 1990 and 1999, while prevalence among those older than 75 almost doubled during this same period.14
Numerous factors appear to contribute to these trends, including aging of the population, dietary trends (ie, increased consumption of red meat, organ food, game, and shellfish and reduced consumption of low-fat dairy products), presence of certain comorbid conditions (ie, hypertension, dyslipidemia, diabetes, metabolic syndrome, end-stage renal disease), the increasing prevalence of obesity in younger adults, use of specific prescription medications, and increased incidence of organ transplantation.1,7,8,15-19
The body’s underexcretion or overproduction of uric acid (a byproduct of purine metabolism12) can lead to hyperuricemia. This condition, defined as a serum urate level exceeding 7.0 mg/dL in men or 6.0 mg/dL in women20,21 (levels above 9.0 mg/dL are considered very high22), is the primary risk factor for gout.8,23,24 As with gout, the incidence of hyperuricemia has increased in recent years,20 with researchers attributing the trend to worldwide popularization of the Westernized diet (particularly use of high-fructose corn syrup20,25) and increased use of certain medications, including thiazide diuretics, cyclosporine, and low-dose aspirin.2,20,25,26
As serum urate levels rise, the patient with hyperuricemia may experience urate supersaturation, often followed by crystallization of the excess urate into monosodium urate (MSU) crystals. Subsequently, circulating MSU crystals may deposit in body tissues, especially in the joint spaces. The body’s ensuing inflammatory response to the MSU deposits is gout.20
In addition to hyperuricemia, risk factors for gout include a high-purine diet, habitual alcohol consumption (especially beer and fortified wines27), diuretic therapy (particularly in patients with heart failure or renal insufficiency), obesity, hypertension, and high levels of fructose consumption.7,28 Additionally, cyclosporine use in an organ transplant recipient, poorly controlled uric acid levels, and a long history of gout increase the patient’s risk for chronic tophaceous gout.24,29 Tophi may be more common in a patient with a history of organ transplantation.16
Genetic variants are currently being investigated to possibly identify a predisposition to gout. The most significant genetic factors appear to involve mechanisms that regulate serum uric acid levels—particularly urate underexcretion.23 Other factors that contribute to underexcretion or overproduction of uric acid are shown in Table 1.1,15,26,30-33
A dynamic relationship exists between gout and a number of pathologic processes. According to researchers investigating nearly 178,000 patients with gout in a managed care database, 36% had hypertension, 27% had dyslipidemia, and 15% had diabetes.8 In a smaller cohort study conducted in Spain and Mexico, it was demonstrated that 93% of patients with gout had one or more associated diseases, in order of decreasing frequency: hypertriglyceridemia, obesity, hypertension, metabolic syndrome, hyperglycemia, chronic renal failure, diabetes, and ischemic heart disease.3
Of particular clinical importance in this study was a finding that the first gout attack generally preceded the diagnosis of the associated diseases.3 Thus, a diagnosis of gout should lead the primary care provider to discuss modifiable risk factors with the patient—but also to investigate for comorbid illnesses that may require timely management.2
In a 12-year-long prospective study of more than 50,000 men participating in the Health Professionals Follow-Up Study,9 it was found that men with gout had a 28% increased risk for all-cause mortality, a 38% increased risk for CVD-related death, and a 55% increased risk for CHD-related death, compared with men who did not have gout (excluding other risk factors).9 Similarly, researchers for the Multiple Risk Factor Intervention Trial10 demonstrated a clinically significant association between gout and an increased risk for AMI: 10.5% of men with gout, compared with 8.43% of men without gout, had an AMI during mean follow-up of 6.5 years.10
THE STAGES
The four stages of gout are asymptomatic hyperuricemia, acute gout, intercritical gout, and chronic tophaceous gout.20
Only a small percentage (0.5% to 4.5%) of patients with asymptomatic hyperuricemia will develop acute gout.28 Nevertheless, any patient with serum urate greater than 6.8 mg/dL is at risk for the deposition of MSU crystals into body tissues and the potential associated organ damage—even patients without symptoms. There is currently no evidence-based method to determine which patients with asymptomatic hyperuricemia will experience disease progression.16
Acute gout develops when deposition of MSU crystals in the joints initiates an inflammatory response. In the typical history, the patient experiences sudden-onset severe pain, swelling, and erythema. The pain often starts in the middle of the night or early morning,34 waking the patient from sleep and peaking within 24 hours of onset. At this time, the patient is often unable to bear weight comfortably on the affected joint. The patient may also report fever and flu-like malaise resulting from the release of interleukin 1- (IL-1), IL-1 receptor, cytokines, and prostaglandins.16,24,35 Usually in these early attacks, symptoms resolve spontaneously within three to 14 days.16,24
After resolution of an acute attack, the patient enters the intercritical stage, another asymptomatic stage that may last for months or years—or indefinitely. During the intercritical stage, MSU crystal deposition continues, adding crystals in and around the affected joint or joints, possibly continuing to inflict damage (in some patients, substantial), and in many cases resulting in additional attacks and pain.16 Any subsequent acute gout attacks the patient may experience are likely to last longer than the initial attack and to involve additional joints or tendons.24
Some patients, especially those who do not receive adequate treatment for hyperuricemia,2 progress to develop chronic tophaceous gout. This is a deforming disease process in which the joints may become stiff and swollen, and subcutaneous nodules or whitish-yellow intradermal deposits may be present under taut skin, anywhere in the body.16
PATIENT PRESENTATION AND HISTORY
Typically, a patient with gout will present with a chief complaint of a painful, tender, inflamed joint (classically described in Latin as calor, rubor, dolor, et tumor6). However, clinicians must also be aware of unusual presentations and consider gout in the differential whenever a patient with a history of gout or pertinent risk factors presents with unexplained clinical findings.32 The history of present illness will vary according to the stage of the disease.
About 90% of recognized initial attacks of gout are monoarticular, usually occurring in one of the lower extremities.16 While the first metatarsophalangeal (MTP) joint is affected in about 50% of gout cases (podagra, the Greek term for gout),2,35 eventually patients with gout have a 90% chance of involvement with the MTP joint (see Figure 1). According to Zhang et al,26 patients with hyperuricemia and an affected MTP joint have an 82% chance of having gout.2,26
Because such a large proportion of patients have the classic presentation of rapid-onset warmth, redness, and tenderness at the MTP, knee, or ankle and surrounding soft tissue, cases with a differing presentation are likely to be misdiagnosed or overlooked, or a correct diagnosis is delayed.26 In many documented cases, gout was the ultimate diagnosis—but one that was reached only incidentally because of unusual clinical presentation, ranging from entrapment neuropathy to a pancreatic mass.32
The presence of hyperuricemia and other risk factors must be investigated. Also relevant in the history of an acute gout attack may be a preceding event that has caused damage or stress to the joint, such as infection, trauma, or surgery. Other possible triggers for an attack include alcohol ingestion, acidosis, use of IV contrast media, diuretic therapy, chemotherapy, recent hospitalization or surgery, and initiation or termination of urate-lowering therapy with the xanthine oxidase inhibitor allopurinol.2,30 According to Primatesta et al,8 the risk for flares is increased in patients with cardiometabolic comorbidities.
The medication history of a patient with gout may include low-dose aspirin (but not standard-dose aspirin, which is uricosuric2), diuretics, cyclosporine, cytotoxic agents, and vitamin B12, which may contribute to hyperuricemia.24 Additionally, ethambutol, pyrazinamide, levodopa, nicotinic acid, didanosine, niacin, and warfarin may raise uric acid levels.15,30,33
Medical history should include a thorough assessment of the comorbidities associated with gout. In addition to the conditions mentioned previously, patients with a history of polycystic kidney disease, dehydration, lactic acidosis, hyperparathyroidism, toxemia of pregnancy, hypothyroidism, or sarcoidosis may have elevated urate levels due to underexcretion—and thus may be vulnerable to gout.30 History of gout in a first-degree relative is associated with an increased risk for gout.36
The social history should address alcohol use or abuse. The clinician should also inquire about how gout is impacting the daily life of the patient. Diet and exercise habits should be assessed37 (see “Patient Education,” below).
PHYSICAL EXAMINATION
The physical exam begins with evaluation of the skin and extremities for the classic features of gout. Affected joints will be exquisitely tender, and patients may be febrile. Most cases are monoarticular, but polyarticular involvement is likely in patients with advanced disease (and can, particularly in women, be mistaken for rheumatoid arthritis).2,16 In patients with chronic tophaceous gout, there may be whitish-yellow skin deposits, subcutaneous nodules, and areas of taut skin. The lower-extremity joints and tendons, as well as the wrists, fingers, and elbows, are commonly affected16 (see Figure 2).
DIAGNOSIS
Diagnostic criteria that are currently available (and have long been in use) include the American College of Rheumatology/American Rheumatism Association (ACR/ARA) preliminary criteria,34 the New York criteria,21 and the Rome criteria.38 The specifics of each are listed in Table 2.21,34,38,39
The gold standard for gout diagnosis is detection of MSU crystals in a sample of synovial fluid aspirated from the affected joint or from a tophus and examined by polarized light microscopy.24 This is of significant importance to the clinician who is faced with a questionable diagnosis.16 However, crystal visualization is not ordinarily available to the primary care clinician,2,17,40 and it is not always necessary if a careful history and physical exam are conducted in a patient with hyperuricemia or other risk factors for gout. A presumptive diagnosis may be acceptable in a patient with the classic presentation of acute gout: rapid onset of severe pain in a swollen, erythematous joint and symptoms peaking within 24 hours. The presence of tophi is pathognomonic for chronic tophaceous gout.41
In cases of questionable or unusual manifestation of gout, however, various imaging techniques and crystal visualization may be indicated.32
In order to compare the effectiveness of the latter technique with conventional diagnostic criteria for gout, Malik et al39 conducted a pilot study involving 82 patients who had undergone synovial fluid analysis with polarized light microscopy. Patients were surveyed about the clinical features of their disease, as listed in the three standard sets of criteria for diagnosis of gout. Compared with the “gold standard” of urate crystal detection (which is one of the Rome criteria38), the study authors found the ACR/ARA preliminary criteria,34 the New York criteria,21 and the Rome criteria38 generally unsatisfactory.
In the study, among patients with confirmed presence of MSU crystals:
• 87% reported more than one attack of acute arthritis (ACR/ARA34)
• 86% reported monoarthritis attack (ACR/ARA34)
• 89% had hyperuricemia (ACR/ARA34 and Rome,38 with the latter giving effective, specific parameters)
• 100% had negative results on joint fluid culture (ACR/ARA34)
• 90% reported an attack starting at night (ACR/ARA34).
The positive predictive values for these signs and symptoms are 38%, 39%, 74%, 50%, and 45%, respectively, according to Malik et al.39 The presence of tophi (cited by all three sets of criteria but “proven or suspected” in the ACR/ARA34) had the highest positive predictive value for gout (91%) and a likelihood ratio of 15.56, which was at least three times higher than any of the other listed criteria. A verified response to colchicine, one of the New York criteria,21 had the second highest positive predictive value at 86%.39
In summary, the ACR/ARA,34 the New York,21 and the Rome criteria38 had specificity of 79%, 83%, and 89%, respectively; sensitivity of 70%, 70%, and 67%, respectively; and positive predictive values for gout of 66%, 70%, and 77%, respectively. The Rome criteria38 had the highest specificity and highest positive predictive value, perhaps making them most helpful for clinicians who lack access to synovial fluid analysis.
DIFFERENTIAL DIAGNOSIS
Conditions to be considered and ruled out before a diagnosis of gout can be made are:
• Pseudogout
• Septic arthritis
• Psoriatic arthritis
• Rheumatoid arthritis
• Erosive osteoarthritis
• Bacterial cellulitis
• Sarcoid arthropathy.16,28,42,43
Unlike gout (in which compensated polarized light microscopy reveals needle-shaped urate crystals with strong negative birefringence), pseudogout is characterized by calcium pyrophosphate dihydrate crystals; these are rhomboid-shaped, with weak positive birefringence.42 Additionally, radiographic imaging will reveal soft tissue swelling and chondrocalcinosis of the joint in pseudogout.44
The patient with septic arthritis, most likely affecting the knee, will have a white blood cell (WBC) count exceeding 50,000/mm3 and a positive culture of the synovial fluid, with absence of crystals.28
Chronic tophaceous gout can mimic rheumatoid arthritis in appearance and joint distribution, and patients affected by either condition may develop a positive rheumatoid factor. Examination of synovial fluid for MSU crystals and radiographic imaging will be of value in making a distinction.
Osteoarthritis is usually evidenced by joint space narrowing on x-ray.42
Bacterial cellulitis will present similarly to gout, but the erythema of bacterial cellulitis will more likely extend beyond the involved joint.16
Sarcoid arthropathy often presents as a polyarthritis, as in advanced gouty arthritis. However, in sarcoid arthropathy, serum calcium and angiotensin-converting enzyme will likely be elevated.43 Synovial or tendon sheath biopsy will show non-caseating granulomas, which are the hallmark for sarcoid disease. Additionally, joint fluid analysis will demonstrate a predominance of mononuclear or polymorphonuclear cells.43
Diagnostic Tests
Diagnostic tests to consider are analysis and culture of the synovial fluid, complete blood count (CBC), blood urea nitrogen (BUN), creatinine, radiography, ultrasonography, serum uric acid, and blood culture if septic arthritis is suspected.28 While serum urate levels may be normal during an acute gout attack, measurement may still be helpful for comparison, since elevation is a likely finding two weeks after an attack—if the patient was, in fact, experiencing an acute gout attack.42
Since renal dialysis increases the risk for gout, pseudogout, and septic arthritis, synovial fluid analysis is essential in patients undergoing renal dialysis.42
Various imaging techniques may aid in confirming a diagnosis of gout and monitoring its progression, but further studies are needed to more clearly define the role of these techniques in management of gout.45 Plain radiographic evidence of asymmetric swelling in a joint (one of the ACR/ARA preliminary criteria34) was shown to have a 60% positive predictive value for a diagnosis of gout.39 Late in the disease process, an affected joint may be affected by characteristic “punched out” intra-articular lesions, with a normal amount of joint space.45
Ultrasound is a safe and inexpensive test that can reveal soft tissue edema and increased vascularity during an acute gout attack. Chronic changes include the double contour sign and tophus-like lesions surrounded by a thin, anechoic rim.45
CT will also show tophi and bony erosion. While CT is more specific than other techniques, it is also more expensive and exposes the patient to increased radiation. MRI can help monitor the complications of gout, especially entrapment neuropathies.45
TREATMENT/MANAGEMENT
According to current evidence, treatment is not indicated for asymptomatic hyperuricemia.16
Acute Gout Management
Pharmacologic treatments available for an acute gout attack include NSAIDs, colchicine, and local or systemic corticosteroids.24,46 At the onset of an attack, patients should start high-dose NSAID therapy, and continue for two to three days after symptoms are resolved.6 Oral indomethacin (50 mg tid) or oral ibuprofen (800 mg tid) are both reasonable options.6 It may be prudent to consider a proton pump inhibitor (eg, omeprazole) to protect the gastric mucosa in patients who are susceptible to gastrointestinal problems.27
In addition to high-dose NSAID therapy, adding colchicine (1.2 mg by mouth at onset of symptoms, followed by 0.6 mg one hour later) has proven to be effective in relieving the symptoms of gout, but its serious gastrointestinal adverse effects, particularly diarrhea, must be considered.6,47
In patients with monoarticular gout who cannot tolerate NSAIDs, intra-articular aspiration and corticosteroid injections may provide relief.
Long-acting triamcinolone, administered by intra-articular injection, has been found to relieve pain and inflammation in patients with gout. Septic arthritis must be ruled out by way of joint aspiration and culture before injection of corticosteroids.47
Oral or IM-administered corticosteroids may be considered for patients with polyarticular involvement. Prednisone (60 mg/d, tapered over 10 days) is an appropriate option for outpatients or inpatients; methylprednisone (80 to 120 mg IM) may be suitable for inpatients.6 Again, septic arthritis must be ruled out before corticosteroids are administered.47
For the patient who is currently taking a thiazide diuretic for hypertension, substituting a different medication may be warranted; the angiotensin receptor blocker losartan, for example, has uricosuric action.27,29,47 Nonpharmacologic strategies, such as rest, ice, elevation, and avoiding trauma to the affected joint, are also recommended.27
Of note, allopurinol therapy should be neither initiated nor discontinued during an acute gout attack.27
Management of Chronic and Intercritical Gout
Urate-lowering therapy, such as allopurinol (50 to 300 mg/d29), should be considered for patients who experience frequent attacks (ie, three or more per year), patients with chronic tophi, patients with radiographically demonstrated joint damage,47 or patients with a documented state of uric acid overproduction.29
Allopurinol dosage should be adjusted based on creatinine clearance; dosing as high as 800 mg/d has been recommended in patients with normal renal function.2 Again, allopurinol should never be started or discontinued during an acute attack,27 because abrupt fluctuations in uric acid levels may heighten the inflammation. The target serum urate level is 6.0 mg/dL.29
Febuxostat, which received FDA approval in 2009, was the first oral urate-lowering treatment to be approved since the 1960s. Like allopurinol, this nonpurine xanthine oxidase inhibitor blocks uric acid synthesis.48,49 In a trial reported by Becker et al,50 67% of patients who took febuxostat 80 mg/d reached the target serum urate level (ie, < 6.0 mg/dL), compared with 45% of those who took 40 mg/d of febuxostat and 42% of those taking 300 mg/d of allopurinol. While incidence of adverse events was low in all treatment groups, Hu and Tomlinson51 report that febuxostat is tolerable in patients who are hypersensitive to allopurinol. As with other urate-lowering medications, gout flares are common during the early period of febuxostat use.51
For patients with gout that does not respond to conventional urate-lowering therapy, new options are being introduced. Two agents, each a recombinant form of the enzyme urate oxidase, are designed to convert uric acid into allantoin, which can then be excreted in the urine. Late in 2010, one of these agents, pegloticase, was approved for use in patients with refractory gout.48 In one clinical trial, tophi were reported dissolved in 40% of patients who took pegloticase, but 58% of patients did not achieve the targeted response (ie, serum urate < 6.0 mg/dL), and 77% of patients experienced gout flares.52 Infusion reactions occurred in 26% to 31% of patients, and Reinders and Jansen52 recommended the clinical evaluation of glucocorticoids and other anti-inflammatory agents to prevent the formation of antibodies involved in these reactions.
The second agent, rasburicase, has been approved for treatment and prevention of acute hyperuricemia in adult cancer patients. Rasburicase is now being investigated for use in patients with nonresponsive tophaceous gout.53-55 It can be administered in the form of monthly infusions.54
Patient Education
Educating the patient about modifiable risk factors, such as diet, alcohol consumption, and adherence to the medication regimen, should be a priority.
Patients should be encouraged to target and maintain an ideal body weight, through diet and moderate physical exercise, as a strategy to normalize serum urate levels.27,47 However, they should be advised to avoid “crash dieting,” as this may precipitate a gout attack.27 In the recommended low-purine diet, consumption of red meat and shellfish is restricted,17 whereas consumption of soy, nonfat milk and other low-fat dairy products, cherries and other fruits, and increased vegetable protein is encouraged.31,37 Consumption of alcohol, especially beer and fortified wines, should be limited.27,47
Avoiding trauma to joints affected by gout (including the stress of bearing excess weight) can help patients limit future attacks.7,27
CONCLUSION
Patients with gout often have the characteristic presentation of an acutely tender, inflamed joint, but since gout is a systemic disorder, the clinician must also consider the possibility of gout in almost any organ system. Gout is a common disease, and its diagnosis can alert the astute clinician to investigate for certain metabolic disorders requiring intervention. Hyperlipidemia, metabolic syndrome, hypertension, chronic kidney disease, obesity, cardiovascular disease, and diabetes are all conditions associated with gout.
Recognizing the opportunity to offer preventive care measures and recommend lifestyle modifications to the patient with gout allows the clinician to play an important role in the patient’s care.
REFERENCES
1. Bhole V, de Vera M, Rahman MM, et al. Epidemiology of gout in women: fifty-two–year followup of a prospective cohort. Arthritis Rheum. 2010;62(4):1069-1076.
2. Neogi T. Clinical practice: gout. N Engl J Med. 2011;364(5):443-452.
3. Hernández-Cuevas CB, Roque LH, Huerta-Sil G, et al. First acute gout attacks commonly precede features of the metabolic syndrome. J Clin Rheumatol. 2009;15(2):65-67.
4. Louthrenoo W, Kasitanon N, Sukitawut W, Wichainun R. A clinical study of crystal-proven gouty arthritis in a university hospital. J Med Assoc Thai. 2003;86(9):868-875.
5. De Souza AW, Fernandes V, Ferrari AJ. Female gout: clinical and laboratory features. J Rheumatol. 2005;32(11):2186-2188.
6. Kurakula PC, Keenan RT. Diagnosis and management of gout: an update. J Musculoskel Med. 2010;27(10). www.musculoskeletalnet work.com/display/article/1145622/1692895. Accessed June 14, 2011.
7. Choi HK, Atkinson K, Karlson EW, Curhan G. Obesity, weight change, hypertension, diuretic use, and risk of gout in men: the Health Professionals Follow-up Study. Arch Intern Med. 2005;165(7):742-748.
8. Primatesta P, Plana E, Rothenbacher D. Gout treatment and comorbidities: a retrospective cohort study in a large US managed care population. BMC Musculoskelet Disord. 2011 May 20;12(1):103. [Epub ahead of print]
9. Choi HK, Curhan G. Independent impact of gout on mortality and risk for coronary heart disease. Circulation. 2007;116(8):894-900.
10. Krishnan E, Baker JF, Furst DE, Schumacher HR. Gout and the risk of acute myocardial infarction. Arthritis Rheum. 2006;54(8):2688-2696.
11. Lawrence RC, Felson DT, Helmick CG, et al; National Arthritis Data Workgroup. Estimates of the prevalence of arthritis and other rheumatic conditions in the United States. Part II. Arthritis Rheum. 2008;58(1):26-35.
12. CDC. Gout. www.cdc.gov/arthritis/basics/gout.htm. Accessed June 14, 2011.
13. Arromdee E, Michet CJ, Crowson CS, et al. Epidemiology of gout: is the incidence rising? J Rheumatol. 2002;29(11):2403-2406.
14. Wallace KL, Riedel AA, Joseph-Ridge N, Wortmann R. Increasing prevalence of gout and hyperuricemia over 10 years among older adults in a managed care population. J Rheumatol. 2004; 31(8):1582-1587.
15. Weaver AL. Epidemiology of gout. Cleve Clin J Med. 2008;75 suppl 5:S9-S12.
16. Mandell BF. Clinical manifestations of hyperuricemia and gout. Cleve Clin J Med. 2008;75 suppl 5:S5-S8.
17. Choi HK, Atkinson K, Karlson EW, et al. Purine-rich foods, dairy and protein intake, and the risk of gout in men. N Engl J Med. 2004;350(11):1093-1103.
18. Demarco MA, Maynard JW, Huizinga MM, et al. Younger age at gout onset is related to obesity in a community-based cohort. Arthritis Care Res (Hoboken). 2011 Apr 11; [Epub ahead of print].
19. Brook RA, Forsythe A, Smeeding JE, Lawrence Edwards N. Chronic gout: epidemiology, disease progression treatment and disease burden. Curr Med Res Opin. 2010;26(12):2813-2821.
20. Sachs L, Batra KL, Zimmermann B. Medical implications of hyperuricemia. Med Health R I. 2009;92(11):353-355.
21. Kellgren JH, Jeffrey MR, Ball J, eds. The Epidemiology of Chronic Rheumatism: Atlas of Standard Radiographs of Arthritis. Oxford: Blackwell; 1963:327.
22. Wu EQ, Patel PA, Mody RR, et al. Frequency, risk, and cost of gout-related episodes among the elderly: does serum uric acid level matter? J Rheumatol. 2009;36(5):1032-1040.
23. Riches PL, Wright AF, Ralston SH. Recent insights into the pathogenesis of hyperuricaemia and gout. Hum Mol Genet. 2009;18(R2):R177-R184.
24. Schumacher HR Jr. The pathogenesis of gout. Cleve Clin J Med. 2008;75 suppl 5:S2-S4.
25. Nakagawa T, Hu H, Zharikov S, et al. A causal role for uric acid in fructose-induced metabolic syndrome. Am J Physiol Renal Physiol. 2006;290(3):F625-F631.
26. Zhang W, Doherty M, Pascual E, et al; EULAR (European League Against Rheumatism) Standing Committee for International Clinical Studies Including Therapeutics. EULAR evidence based recommendations for gout. Part I: Diagnosis. Ann Rheum Dis. 2006;65(10):1301-1311.
27. Jordan KM, Cameron JS, Snaith M, et al. British Society for Rheumatology and British Health Professionals in Rheumatology guideline for the management of gout. Rheumatology. 2007;46(8):1372-1374.
28. Eggebeen AT. Gout: an update. Am Fam Physician. 2007;76(6):801-808.
29. Terkeltaub RA. Gout. N Engl J Med. 2003; 349(17):1647-1655.
30. Harris MD, Siegel LB, Alloway JA. Gout and hyperuricemia. Am Fam Physician. 1999;59(4): 925-934.
31. Schlesinger N. Dietary factors and hyperuricemia. Curr Pharm Des. 2005;11(32):4133-4138.
32. Ning TC, Keenan RT. Unusual presentations of gout. Curr Opin Rheumatol. 2010;22(2): 181-187.
33. Menon RK, Mikhailidis DP, Bell JL, et al. Warfarin administration increases uric acid concentrations in plasma. Clin Chem. 1986;32(8):
1557-1559.
34. Wallace SL, Robinson H, Masi AT, et al. Preliminary criteria for the classification of the acute arthritis of primary gout. Arthritis Rheum. 1977;20(3):895-900.
35. Martinon F, Glincher LH. Gout: new insights into an old disease. J Clin Invest. 2006;116 (8):2073-2075.
36. Zampogna G, Andracco R, Parodi M, Cimmino MA. Clinical features of gout in a cohort of Italian patients [in Italian]. Reumatismo. 2009; 61(1):41-47.
37. Choi HK. A prescription for lifestyle change in patients with hyperuricemia and gout. Curr Opin Rheumatol. 2010;22(2):165-172.
38. Bennett PH, Wood PH, eds. Population studies of the rheumatic diseases: proceedings of the Third International Symposium; June 5-10, 1966; New York, NY. Amsterdam: Excerpta Medica Foundation; 1968:457-458.
39. Malik A, Schumacher HR, Dinnella JE, Clayburne GM. Clinical diagnostic criteria for gout: comparison with the gold standard of synovial fluid crystal analysis. J Clin Rheumatol. 2009;15 (1):22-24.
40. Wijnands JMA, Boonen A, Arts ICW, et al. Large epidemiologic studies of gout: challenges in diagnosis and diagnostic criteria. Curr Rheumatol Rep. 2011;13(2):167-174.
41. Dodd LG, Major NM. Fine-needle aspiration cytology of articular and periarticular lesions. Cancer. 2002;96(3):157-165.
42. Dore RK. The gout diagnosis. Cleve Clin J Med. 2008;75 suppl 5:S17-S21.
43. Pettersson T. Sarcoid and erythema nodosum arthropathies. Baillieres Best Pract Res Clin Rheumatol. 2000;14(3):461-476.
44. Córdoba-Fernández A, Rayo-Rosado R. Pseudogout of the first metatarsophalangeal joint associated with hallux valgus: an atypical bilateral case. J Am Podiatr Med Assoc. 2010;100(2):138-142.
45. Dalbeth N, McQueen FM. Use of imaging to evaluate gout and other crystal deposition disorders. Curr Opin Rheumatol. 2009;21(2):124-131.
46. Wu EQ, Forsythe A, Guérin A, et al. Comorbidity burden healthcare resource utilization, and costs in chronic gout patients refractory to conventional urate-lowering therapy. Am J Ther. 2011 Feb 10; [Epub ahead of print].
47. Zhang W, Doherty M, Pascual E, et al; EULAR (European League Against Rheumatism) Standing Committee for International Clinical Studies Including Therapeutics. EULAR evidence based recommendations for gout. Part II: Management. Ann Rheum Dis. 2006;65(10):1312-1324.
48. Schlesinger N, Yasothan U, Kirkpatrick P. Pegloticase [published correction appears in Nat Rev Drug Discov. 2011;10(2):156]. Nat Rev Drug Discov. 2011;10(1):17-18.
49. Pascual E, Sivera F, Yasothan U, Kurkpatrick P. Febuxostat. Nat Rev Drug Discov. 2009;8(3): 191-192.
50. Becker MA, Schumacher HR, Espinoza LR, et al. The urate-lowering efficacy and safety of febuxostat in the treatment of the hyperuricemia of gout; the CONFIRMS trial. Arthritis Res Ther. 2010;12(2):R63.
51. Hu M, Tomlinson B. Febuxostat in the management of hyperuricemia and chronic gout: a review. Ther Clin Risk Manag. 2008;4(6):1209-1220.
52. Reinders MK, Jansen TL. New advances in the treatment of gout: review of pegloticase. Ther Clin Risk Manag. 2010;6:543-550.
53. Cammalleri L, Malaguarnera M. Rasburicase represents a new tool for hyperuricemia in tumor lysis syndrome and in gout. Int J Med Sci. 2007; 4(2):83-93.
54. Richette P, Brière C, Hoenen-Clavert V, et al. Rasburicase for topaceous gout not treatable with allopurinol: an exploratory study. J Rheumatol. 2007;34(10):2093-2098.
55. Moolenburgh JD, Reinders MK, Jansen TL. Rasburicase treatment in severe tophaceous gout: a novel therapeutic option. Clin Rheumatol. 2006;25(5):749-752.
Gouty arthritis is a common form of inflammatory arthritis, occurring more frequently in men than in women. The condition has a male–female ratio of 3 or 4 to 1, although that ratio narrows as adults age; because the uricosuric effects of estrogen decline with menopause, the risk for gout increases in postmenopausal women.1,2 Mean age at disease onset is 40 to 60 in men,3,4 with onset in women averaging seven years later.5
Apart from the pain and loss of function associated with this disorder of purine metabolism6 and the risk for a chronic form of the disease, gout is almost universally linked with serious comorbidities that require timely intervention. These include hypertension, dyslipidemia, hyperglycemia and diabetes, obesity, metabolic syndrome, cardiovascular disease (CVD), renal insufficiency, and coronary heart disease (CHD).2,3,7 The presence of gout is independently associated with a risk for acute myocardial infarction (AMI) and increased rates of all-cause mortality.3,8-10
EPIDEMIOLOGY
In 2007, the National Arthritis Data Workgroup11,12 estimated that about three million US adults had had “self-reported gout” in the previous year. An estimated six million US adults have been diagnosed with gout,2,11 and its incidence and prevalence are increasing. The incidence of primary gout more than doubled between 1977-1978 and 1995-1996,13 especially affecting the aging population. The prevalence of gout among 1,000 managed care patients ages 65 to 74 increased by at least 30% between 1990 and 1999, while prevalence among those older than 75 almost doubled during this same period.14
Numerous factors appear to contribute to these trends, including aging of the population, dietary trends (ie, increased consumption of red meat, organ food, game, and shellfish and reduced consumption of low-fat dairy products), presence of certain comorbid conditions (ie, hypertension, dyslipidemia, diabetes, metabolic syndrome, end-stage renal disease), the increasing prevalence of obesity in younger adults, use of specific prescription medications, and increased incidence of organ transplantation.1,7,8,15-19
The body’s underexcretion or overproduction of uric acid (a byproduct of purine metabolism12) can lead to hyperuricemia. This condition, defined as a serum urate level exceeding 7.0 mg/dL in men or 6.0 mg/dL in women20,21 (levels above 9.0 mg/dL are considered very high22), is the primary risk factor for gout.8,23,24 As with gout, the incidence of hyperuricemia has increased in recent years,20 with researchers attributing the trend to worldwide popularization of the Westernized diet (particularly use of high-fructose corn syrup20,25) and increased use of certain medications, including thiazide diuretics, cyclosporine, and low-dose aspirin.2,20,25,26
As serum urate levels rise, the patient with hyperuricemia may experience urate supersaturation, often followed by crystallization of the excess urate into monosodium urate (MSU) crystals. Subsequently, circulating MSU crystals may deposit in body tissues, especially in the joint spaces. The body’s ensuing inflammatory response to the MSU deposits is gout.20
In addition to hyperuricemia, risk factors for gout include a high-purine diet, habitual alcohol consumption (especially beer and fortified wines27), diuretic therapy (particularly in patients with heart failure or renal insufficiency), obesity, hypertension, and high levels of fructose consumption.7,28 Additionally, cyclosporine use in an organ transplant recipient, poorly controlled uric acid levels, and a long history of gout increase the patient’s risk for chronic tophaceous gout.24,29 Tophi may be more common in a patient with a history of organ transplantation.16
Genetic variants are currently being investigated to possibly identify a predisposition to gout. The most significant genetic factors appear to involve mechanisms that regulate serum uric acid levels—particularly urate underexcretion.23 Other factors that contribute to underexcretion or overproduction of uric acid are shown in Table 1.1,15,26,30-33
A dynamic relationship exists between gout and a number of pathologic processes. According to researchers investigating nearly 178,000 patients with gout in a managed care database, 36% had hypertension, 27% had dyslipidemia, and 15% had diabetes.8 In a smaller cohort study conducted in Spain and Mexico, it was demonstrated that 93% of patients with gout had one or more associated diseases, in order of decreasing frequency: hypertriglyceridemia, obesity, hypertension, metabolic syndrome, hyperglycemia, chronic renal failure, diabetes, and ischemic heart disease.3
Of particular clinical importance in this study was a finding that the first gout attack generally preceded the diagnosis of the associated diseases.3 Thus, a diagnosis of gout should lead the primary care provider to discuss modifiable risk factors with the patient—but also to investigate for comorbid illnesses that may require timely management.2
In a 12-year-long prospective study of more than 50,000 men participating in the Health Professionals Follow-Up Study,9 it was found that men with gout had a 28% increased risk for all-cause mortality, a 38% increased risk for CVD-related death, and a 55% increased risk for CHD-related death, compared with men who did not have gout (excluding other risk factors).9 Similarly, researchers for the Multiple Risk Factor Intervention Trial10 demonstrated a clinically significant association between gout and an increased risk for AMI: 10.5% of men with gout, compared with 8.43% of men without gout, had an AMI during mean follow-up of 6.5 years.10
THE STAGES
The four stages of gout are asymptomatic hyperuricemia, acute gout, intercritical gout, and chronic tophaceous gout.20
Only a small percentage (0.5% to 4.5%) of patients with asymptomatic hyperuricemia will develop acute gout.28 Nevertheless, any patient with serum urate greater than 6.8 mg/dL is at risk for the deposition of MSU crystals into body tissues and the potential associated organ damage—even patients without symptoms. There is currently no evidence-based method to determine which patients with asymptomatic hyperuricemia will experience disease progression.16
Acute gout develops when deposition of MSU crystals in the joints initiates an inflammatory response. In the typical history, the patient experiences sudden-onset severe pain, swelling, and erythema. The pain often starts in the middle of the night or early morning,34 waking the patient from sleep and peaking within 24 hours of onset. At this time, the patient is often unable to bear weight comfortably on the affected joint. The patient may also report fever and flu-like malaise resulting from the release of interleukin 1- (IL-1), IL-1 receptor, cytokines, and prostaglandins.16,24,35 Usually in these early attacks, symptoms resolve spontaneously within three to 14 days.16,24
After resolution of an acute attack, the patient enters the intercritical stage, another asymptomatic stage that may last for months or years—or indefinitely. During the intercritical stage, MSU crystal deposition continues, adding crystals in and around the affected joint or joints, possibly continuing to inflict damage (in some patients, substantial), and in many cases resulting in additional attacks and pain.16 Any subsequent acute gout attacks the patient may experience are likely to last longer than the initial attack and to involve additional joints or tendons.24
Some patients, especially those who do not receive adequate treatment for hyperuricemia,2 progress to develop chronic tophaceous gout. This is a deforming disease process in which the joints may become stiff and swollen, and subcutaneous nodules or whitish-yellow intradermal deposits may be present under taut skin, anywhere in the body.16
PATIENT PRESENTATION AND HISTORY
Typically, a patient with gout will present with a chief complaint of a painful, tender, inflamed joint (classically described in Latin as calor, rubor, dolor, et tumor6). However, clinicians must also be aware of unusual presentations and consider gout in the differential whenever a patient with a history of gout or pertinent risk factors presents with unexplained clinical findings.32 The history of present illness will vary according to the stage of the disease.
About 90% of recognized initial attacks of gout are monoarticular, usually occurring in one of the lower extremities.16 While the first metatarsophalangeal (MTP) joint is affected in about 50% of gout cases (podagra, the Greek term for gout),2,35 eventually patients with gout have a 90% chance of involvement with the MTP joint (see Figure 1). According to Zhang et al,26 patients with hyperuricemia and an affected MTP joint have an 82% chance of having gout.2,26
Because such a large proportion of patients have the classic presentation of rapid-onset warmth, redness, and tenderness at the MTP, knee, or ankle and surrounding soft tissue, cases with a differing presentation are likely to be misdiagnosed or overlooked, or a correct diagnosis is delayed.26 In many documented cases, gout was the ultimate diagnosis—but one that was reached only incidentally because of unusual clinical presentation, ranging from entrapment neuropathy to a pancreatic mass.32
The presence of hyperuricemia and other risk factors must be investigated. Also relevant in the history of an acute gout attack may be a preceding event that has caused damage or stress to the joint, such as infection, trauma, or surgery. Other possible triggers for an attack include alcohol ingestion, acidosis, use of IV contrast media, diuretic therapy, chemotherapy, recent hospitalization or surgery, and initiation or termination of urate-lowering therapy with the xanthine oxidase inhibitor allopurinol.2,30 According to Primatesta et al,8 the risk for flares is increased in patients with cardiometabolic comorbidities.
The medication history of a patient with gout may include low-dose aspirin (but not standard-dose aspirin, which is uricosuric2), diuretics, cyclosporine, cytotoxic agents, and vitamin B12, which may contribute to hyperuricemia.24 Additionally, ethambutol, pyrazinamide, levodopa, nicotinic acid, didanosine, niacin, and warfarin may raise uric acid levels.15,30,33
Medical history should include a thorough assessment of the comorbidities associated with gout. In addition to the conditions mentioned previously, patients with a history of polycystic kidney disease, dehydration, lactic acidosis, hyperparathyroidism, toxemia of pregnancy, hypothyroidism, or sarcoidosis may have elevated urate levels due to underexcretion—and thus may be vulnerable to gout.30 History of gout in a first-degree relative is associated with an increased risk for gout.36
The social history should address alcohol use or abuse. The clinician should also inquire about how gout is impacting the daily life of the patient. Diet and exercise habits should be assessed37 (see “Patient Education,” below).
PHYSICAL EXAMINATION
The physical exam begins with evaluation of the skin and extremities for the classic features of gout. Affected joints will be exquisitely tender, and patients may be febrile. Most cases are monoarticular, but polyarticular involvement is likely in patients with advanced disease (and can, particularly in women, be mistaken for rheumatoid arthritis).2,16 In patients with chronic tophaceous gout, there may be whitish-yellow skin deposits, subcutaneous nodules, and areas of taut skin. The lower-extremity joints and tendons, as well as the wrists, fingers, and elbows, are commonly affected16 (see Figure 2).
DIAGNOSIS
Diagnostic criteria that are currently available (and have long been in use) include the American College of Rheumatology/American Rheumatism Association (ACR/ARA) preliminary criteria,34 the New York criteria,21 and the Rome criteria.38 The specifics of each are listed in Table 2.21,34,38,39
The gold standard for gout diagnosis is detection of MSU crystals in a sample of synovial fluid aspirated from the affected joint or from a tophus and examined by polarized light microscopy.24 This is of significant importance to the clinician who is faced with a questionable diagnosis.16 However, crystal visualization is not ordinarily available to the primary care clinician,2,17,40 and it is not always necessary if a careful history and physical exam are conducted in a patient with hyperuricemia or other risk factors for gout. A presumptive diagnosis may be acceptable in a patient with the classic presentation of acute gout: rapid onset of severe pain in a swollen, erythematous joint and symptoms peaking within 24 hours. The presence of tophi is pathognomonic for chronic tophaceous gout.41
In cases of questionable or unusual manifestation of gout, however, various imaging techniques and crystal visualization may be indicated.32
In order to compare the effectiveness of the latter technique with conventional diagnostic criteria for gout, Malik et al39 conducted a pilot study involving 82 patients who had undergone synovial fluid analysis with polarized light microscopy. Patients were surveyed about the clinical features of their disease, as listed in the three standard sets of criteria for diagnosis of gout. Compared with the “gold standard” of urate crystal detection (which is one of the Rome criteria38), the study authors found the ACR/ARA preliminary criteria,34 the New York criteria,21 and the Rome criteria38 generally unsatisfactory.
In the study, among patients with confirmed presence of MSU crystals:
• 87% reported more than one attack of acute arthritis (ACR/ARA34)
• 86% reported monoarthritis attack (ACR/ARA34)
• 89% had hyperuricemia (ACR/ARA34 and Rome,38 with the latter giving effective, specific parameters)
• 100% had negative results on joint fluid culture (ACR/ARA34)
• 90% reported an attack starting at night (ACR/ARA34).
The positive predictive values for these signs and symptoms are 38%, 39%, 74%, 50%, and 45%, respectively, according to Malik et al.39 The presence of tophi (cited by all three sets of criteria but “proven or suspected” in the ACR/ARA34) had the highest positive predictive value for gout (91%) and a likelihood ratio of 15.56, which was at least three times higher than any of the other listed criteria. A verified response to colchicine, one of the New York criteria,21 had the second highest positive predictive value at 86%.39
In summary, the ACR/ARA,34 the New York,21 and the Rome criteria38 had specificity of 79%, 83%, and 89%, respectively; sensitivity of 70%, 70%, and 67%, respectively; and positive predictive values for gout of 66%, 70%, and 77%, respectively. The Rome criteria38 had the highest specificity and highest positive predictive value, perhaps making them most helpful for clinicians who lack access to synovial fluid analysis.
DIFFERENTIAL DIAGNOSIS
Conditions to be considered and ruled out before a diagnosis of gout can be made are:
• Pseudogout
• Septic arthritis
• Psoriatic arthritis
• Rheumatoid arthritis
• Erosive osteoarthritis
• Bacterial cellulitis
• Sarcoid arthropathy.16,28,42,43
Unlike gout (in which compensated polarized light microscopy reveals needle-shaped urate crystals with strong negative birefringence), pseudogout is characterized by calcium pyrophosphate dihydrate crystals; these are rhomboid-shaped, with weak positive birefringence.42 Additionally, radiographic imaging will reveal soft tissue swelling and chondrocalcinosis of the joint in pseudogout.44
The patient with septic arthritis, most likely affecting the knee, will have a white blood cell (WBC) count exceeding 50,000/mm3 and a positive culture of the synovial fluid, with absence of crystals.28
Chronic tophaceous gout can mimic rheumatoid arthritis in appearance and joint distribution, and patients affected by either condition may develop a positive rheumatoid factor. Examination of synovial fluid for MSU crystals and radiographic imaging will be of value in making a distinction.
Osteoarthritis is usually evidenced by joint space narrowing on x-ray.42
Bacterial cellulitis will present similarly to gout, but the erythema of bacterial cellulitis will more likely extend beyond the involved joint.16
Sarcoid arthropathy often presents as a polyarthritis, as in advanced gouty arthritis. However, in sarcoid arthropathy, serum calcium and angiotensin-converting enzyme will likely be elevated.43 Synovial or tendon sheath biopsy will show non-caseating granulomas, which are the hallmark for sarcoid disease. Additionally, joint fluid analysis will demonstrate a predominance of mononuclear or polymorphonuclear cells.43
Diagnostic Tests
Diagnostic tests to consider are analysis and culture of the synovial fluid, complete blood count (CBC), blood urea nitrogen (BUN), creatinine, radiography, ultrasonography, serum uric acid, and blood culture if septic arthritis is suspected.28 While serum urate levels may be normal during an acute gout attack, measurement may still be helpful for comparison, since elevation is a likely finding two weeks after an attack—if the patient was, in fact, experiencing an acute gout attack.42
Since renal dialysis increases the risk for gout, pseudogout, and septic arthritis, synovial fluid analysis is essential in patients undergoing renal dialysis.42
Various imaging techniques may aid in confirming a diagnosis of gout and monitoring its progression, but further studies are needed to more clearly define the role of these techniques in management of gout.45 Plain radiographic evidence of asymmetric swelling in a joint (one of the ACR/ARA preliminary criteria34) was shown to have a 60% positive predictive value for a diagnosis of gout.39 Late in the disease process, an affected joint may be affected by characteristic “punched out” intra-articular lesions, with a normal amount of joint space.45
Ultrasound is a safe and inexpensive test that can reveal soft tissue edema and increased vascularity during an acute gout attack. Chronic changes include the double contour sign and tophus-like lesions surrounded by a thin, anechoic rim.45
CT will also show tophi and bony erosion. While CT is more specific than other techniques, it is also more expensive and exposes the patient to increased radiation. MRI can help monitor the complications of gout, especially entrapment neuropathies.45
TREATMENT/MANAGEMENT
According to current evidence, treatment is not indicated for asymptomatic hyperuricemia.16
Acute Gout Management
Pharmacologic treatments available for an acute gout attack include NSAIDs, colchicine, and local or systemic corticosteroids.24,46 At the onset of an attack, patients should start high-dose NSAID therapy, and continue for two to three days after symptoms are resolved.6 Oral indomethacin (50 mg tid) or oral ibuprofen (800 mg tid) are both reasonable options.6 It may be prudent to consider a proton pump inhibitor (eg, omeprazole) to protect the gastric mucosa in patients who are susceptible to gastrointestinal problems.27
In addition to high-dose NSAID therapy, adding colchicine (1.2 mg by mouth at onset of symptoms, followed by 0.6 mg one hour later) has proven to be effective in relieving the symptoms of gout, but its serious gastrointestinal adverse effects, particularly diarrhea, must be considered.6,47
In patients with monoarticular gout who cannot tolerate NSAIDs, intra-articular aspiration and corticosteroid injections may provide relief.
Long-acting triamcinolone, administered by intra-articular injection, has been found to relieve pain and inflammation in patients with gout. Septic arthritis must be ruled out by way of joint aspiration and culture before injection of corticosteroids.47
Oral or IM-administered corticosteroids may be considered for patients with polyarticular involvement. Prednisone (60 mg/d, tapered over 10 days) is an appropriate option for outpatients or inpatients; methylprednisone (80 to 120 mg IM) may be suitable for inpatients.6 Again, septic arthritis must be ruled out before corticosteroids are administered.47
For the patient who is currently taking a thiazide diuretic for hypertension, substituting a different medication may be warranted; the angiotensin receptor blocker losartan, for example, has uricosuric action.27,29,47 Nonpharmacologic strategies, such as rest, ice, elevation, and avoiding trauma to the affected joint, are also recommended.27
Of note, allopurinol therapy should be neither initiated nor discontinued during an acute gout attack.27
Management of Chronic and Intercritical Gout
Urate-lowering therapy, such as allopurinol (50 to 300 mg/d29), should be considered for patients who experience frequent attacks (ie, three or more per year), patients with chronic tophi, patients with radiographically demonstrated joint damage,47 or patients with a documented state of uric acid overproduction.29
Allopurinol dosage should be adjusted based on creatinine clearance; dosing as high as 800 mg/d has been recommended in patients with normal renal function.2 Again, allopurinol should never be started or discontinued during an acute attack,27 because abrupt fluctuations in uric acid levels may heighten the inflammation. The target serum urate level is 6.0 mg/dL.29
Febuxostat, which received FDA approval in 2009, was the first oral urate-lowering treatment to be approved since the 1960s. Like allopurinol, this nonpurine xanthine oxidase inhibitor blocks uric acid synthesis.48,49 In a trial reported by Becker et al,50 67% of patients who took febuxostat 80 mg/d reached the target serum urate level (ie, < 6.0 mg/dL), compared with 45% of those who took 40 mg/d of febuxostat and 42% of those taking 300 mg/d of allopurinol. While incidence of adverse events was low in all treatment groups, Hu and Tomlinson51 report that febuxostat is tolerable in patients who are hypersensitive to allopurinol. As with other urate-lowering medications, gout flares are common during the early period of febuxostat use.51
For patients with gout that does not respond to conventional urate-lowering therapy, new options are being introduced. Two agents, each a recombinant form of the enzyme urate oxidase, are designed to convert uric acid into allantoin, which can then be excreted in the urine. Late in 2010, one of these agents, pegloticase, was approved for use in patients with refractory gout.48 In one clinical trial, tophi were reported dissolved in 40% of patients who took pegloticase, but 58% of patients did not achieve the targeted response (ie, serum urate < 6.0 mg/dL), and 77% of patients experienced gout flares.52 Infusion reactions occurred in 26% to 31% of patients, and Reinders and Jansen52 recommended the clinical evaluation of glucocorticoids and other anti-inflammatory agents to prevent the formation of antibodies involved in these reactions.
The second agent, rasburicase, has been approved for treatment and prevention of acute hyperuricemia in adult cancer patients. Rasburicase is now being investigated for use in patients with nonresponsive tophaceous gout.53-55 It can be administered in the form of monthly infusions.54
Patient Education
Educating the patient about modifiable risk factors, such as diet, alcohol consumption, and adherence to the medication regimen, should be a priority.
Patients should be encouraged to target and maintain an ideal body weight, through diet and moderate physical exercise, as a strategy to normalize serum urate levels.27,47 However, they should be advised to avoid “crash dieting,” as this may precipitate a gout attack.27 In the recommended low-purine diet, consumption of red meat and shellfish is restricted,17 whereas consumption of soy, nonfat milk and other low-fat dairy products, cherries and other fruits, and increased vegetable protein is encouraged.31,37 Consumption of alcohol, especially beer and fortified wines, should be limited.27,47
Avoiding trauma to joints affected by gout (including the stress of bearing excess weight) can help patients limit future attacks.7,27
CONCLUSION
Patients with gout often have the characteristic presentation of an acutely tender, inflamed joint, but since gout is a systemic disorder, the clinician must also consider the possibility of gout in almost any organ system. Gout is a common disease, and its diagnosis can alert the astute clinician to investigate for certain metabolic disorders requiring intervention. Hyperlipidemia, metabolic syndrome, hypertension, chronic kidney disease, obesity, cardiovascular disease, and diabetes are all conditions associated with gout.
Recognizing the opportunity to offer preventive care measures and recommend lifestyle modifications to the patient with gout allows the clinician to play an important role in the patient’s care.
REFERENCES
1. Bhole V, de Vera M, Rahman MM, et al. Epidemiology of gout in women: fifty-two–year followup of a prospective cohort. Arthritis Rheum. 2010;62(4):1069-1076.
2. Neogi T. Clinical practice: gout. N Engl J Med. 2011;364(5):443-452.
3. Hernández-Cuevas CB, Roque LH, Huerta-Sil G, et al. First acute gout attacks commonly precede features of the metabolic syndrome. J Clin Rheumatol. 2009;15(2):65-67.
4. Louthrenoo W, Kasitanon N, Sukitawut W, Wichainun R. A clinical study of crystal-proven gouty arthritis in a university hospital. J Med Assoc Thai. 2003;86(9):868-875.
5. De Souza AW, Fernandes V, Ferrari AJ. Female gout: clinical and laboratory features. J Rheumatol. 2005;32(11):2186-2188.
6. Kurakula PC, Keenan RT. Diagnosis and management of gout: an update. J Musculoskel Med. 2010;27(10). www.musculoskeletalnet work.com/display/article/1145622/1692895. Accessed June 14, 2011.
7. Choi HK, Atkinson K, Karlson EW, Curhan G. Obesity, weight change, hypertension, diuretic use, and risk of gout in men: the Health Professionals Follow-up Study. Arch Intern Med. 2005;165(7):742-748.
8. Primatesta P, Plana E, Rothenbacher D. Gout treatment and comorbidities: a retrospective cohort study in a large US managed care population. BMC Musculoskelet Disord. 2011 May 20;12(1):103. [Epub ahead of print]
9. Choi HK, Curhan G. Independent impact of gout on mortality and risk for coronary heart disease. Circulation. 2007;116(8):894-900.
10. Krishnan E, Baker JF, Furst DE, Schumacher HR. Gout and the risk of acute myocardial infarction. Arthritis Rheum. 2006;54(8):2688-2696.
11. Lawrence RC, Felson DT, Helmick CG, et al; National Arthritis Data Workgroup. Estimates of the prevalence of arthritis and other rheumatic conditions in the United States. Part II. Arthritis Rheum. 2008;58(1):26-35.
12. CDC. Gout. www.cdc.gov/arthritis/basics/gout.htm. Accessed June 14, 2011.
13. Arromdee E, Michet CJ, Crowson CS, et al. Epidemiology of gout: is the incidence rising? J Rheumatol. 2002;29(11):2403-2406.
14. Wallace KL, Riedel AA, Joseph-Ridge N, Wortmann R. Increasing prevalence of gout and hyperuricemia over 10 years among older adults in a managed care population. J Rheumatol. 2004; 31(8):1582-1587.
15. Weaver AL. Epidemiology of gout. Cleve Clin J Med. 2008;75 suppl 5:S9-S12.
16. Mandell BF. Clinical manifestations of hyperuricemia and gout. Cleve Clin J Med. 2008;75 suppl 5:S5-S8.
17. Choi HK, Atkinson K, Karlson EW, et al. Purine-rich foods, dairy and protein intake, and the risk of gout in men. N Engl J Med. 2004;350(11):1093-1103.
18. Demarco MA, Maynard JW, Huizinga MM, et al. Younger age at gout onset is related to obesity in a community-based cohort. Arthritis Care Res (Hoboken). 2011 Apr 11; [Epub ahead of print].
19. Brook RA, Forsythe A, Smeeding JE, Lawrence Edwards N. Chronic gout: epidemiology, disease progression treatment and disease burden. Curr Med Res Opin. 2010;26(12):2813-2821.
20. Sachs L, Batra KL, Zimmermann B. Medical implications of hyperuricemia. Med Health R I. 2009;92(11):353-355.
21. Kellgren JH, Jeffrey MR, Ball J, eds. The Epidemiology of Chronic Rheumatism: Atlas of Standard Radiographs of Arthritis. Oxford: Blackwell; 1963:327.
22. Wu EQ, Patel PA, Mody RR, et al. Frequency, risk, and cost of gout-related episodes among the elderly: does serum uric acid level matter? J Rheumatol. 2009;36(5):1032-1040.
23. Riches PL, Wright AF, Ralston SH. Recent insights into the pathogenesis of hyperuricaemia and gout. Hum Mol Genet. 2009;18(R2):R177-R184.
24. Schumacher HR Jr. The pathogenesis of gout. Cleve Clin J Med. 2008;75 suppl 5:S2-S4.
25. Nakagawa T, Hu H, Zharikov S, et al. A causal role for uric acid in fructose-induced metabolic syndrome. Am J Physiol Renal Physiol. 2006;290(3):F625-F631.
26. Zhang W, Doherty M, Pascual E, et al; EULAR (European League Against Rheumatism) Standing Committee for International Clinical Studies Including Therapeutics. EULAR evidence based recommendations for gout. Part I: Diagnosis. Ann Rheum Dis. 2006;65(10):1301-1311.
27. Jordan KM, Cameron JS, Snaith M, et al. British Society for Rheumatology and British Health Professionals in Rheumatology guideline for the management of gout. Rheumatology. 2007;46(8):1372-1374.
28. Eggebeen AT. Gout: an update. Am Fam Physician. 2007;76(6):801-808.
29. Terkeltaub RA. Gout. N Engl J Med. 2003; 349(17):1647-1655.
30. Harris MD, Siegel LB, Alloway JA. Gout and hyperuricemia. Am Fam Physician. 1999;59(4): 925-934.
31. Schlesinger N. Dietary factors and hyperuricemia. Curr Pharm Des. 2005;11(32):4133-4138.
32. Ning TC, Keenan RT. Unusual presentations of gout. Curr Opin Rheumatol. 2010;22(2): 181-187.
33. Menon RK, Mikhailidis DP, Bell JL, et al. Warfarin administration increases uric acid concentrations in plasma. Clin Chem. 1986;32(8):
1557-1559.
34. Wallace SL, Robinson H, Masi AT, et al. Preliminary criteria for the classification of the acute arthritis of primary gout. Arthritis Rheum. 1977;20(3):895-900.
35. Martinon F, Glincher LH. Gout: new insights into an old disease. J Clin Invest. 2006;116 (8):2073-2075.
36. Zampogna G, Andracco R, Parodi M, Cimmino MA. Clinical features of gout in a cohort of Italian patients [in Italian]. Reumatismo. 2009; 61(1):41-47.
37. Choi HK. A prescription for lifestyle change in patients with hyperuricemia and gout. Curr Opin Rheumatol. 2010;22(2):165-172.
38. Bennett PH, Wood PH, eds. Population studies of the rheumatic diseases: proceedings of the Third International Symposium; June 5-10, 1966; New York, NY. Amsterdam: Excerpta Medica Foundation; 1968:457-458.
39. Malik A, Schumacher HR, Dinnella JE, Clayburne GM. Clinical diagnostic criteria for gout: comparison with the gold standard of synovial fluid crystal analysis. J Clin Rheumatol. 2009;15 (1):22-24.
40. Wijnands JMA, Boonen A, Arts ICW, et al. Large epidemiologic studies of gout: challenges in diagnosis and diagnostic criteria. Curr Rheumatol Rep. 2011;13(2):167-174.
41. Dodd LG, Major NM. Fine-needle aspiration cytology of articular and periarticular lesions. Cancer. 2002;96(3):157-165.
42. Dore RK. The gout diagnosis. Cleve Clin J Med. 2008;75 suppl 5:S17-S21.
43. Pettersson T. Sarcoid and erythema nodosum arthropathies. Baillieres Best Pract Res Clin Rheumatol. 2000;14(3):461-476.
44. Córdoba-Fernández A, Rayo-Rosado R. Pseudogout of the first metatarsophalangeal joint associated with hallux valgus: an atypical bilateral case. J Am Podiatr Med Assoc. 2010;100(2):138-142.
45. Dalbeth N, McQueen FM. Use of imaging to evaluate gout and other crystal deposition disorders. Curr Opin Rheumatol. 2009;21(2):124-131.
46. Wu EQ, Forsythe A, Guérin A, et al. Comorbidity burden healthcare resource utilization, and costs in chronic gout patients refractory to conventional urate-lowering therapy. Am J Ther. 2011 Feb 10; [Epub ahead of print].
47. Zhang W, Doherty M, Pascual E, et al; EULAR (European League Against Rheumatism) Standing Committee for International Clinical Studies Including Therapeutics. EULAR evidence based recommendations for gout. Part II: Management. Ann Rheum Dis. 2006;65(10):1312-1324.
48. Schlesinger N, Yasothan U, Kirkpatrick P. Pegloticase [published correction appears in Nat Rev Drug Discov. 2011;10(2):156]. Nat Rev Drug Discov. 2011;10(1):17-18.
49. Pascual E, Sivera F, Yasothan U, Kurkpatrick P. Febuxostat. Nat Rev Drug Discov. 2009;8(3): 191-192.
50. Becker MA, Schumacher HR, Espinoza LR, et al. The urate-lowering efficacy and safety of febuxostat in the treatment of the hyperuricemia of gout; the CONFIRMS trial. Arthritis Res Ther. 2010;12(2):R63.
51. Hu M, Tomlinson B. Febuxostat in the management of hyperuricemia and chronic gout: a review. Ther Clin Risk Manag. 2008;4(6):1209-1220.
52. Reinders MK, Jansen TL. New advances in the treatment of gout: review of pegloticase. Ther Clin Risk Manag. 2010;6:543-550.
53. Cammalleri L, Malaguarnera M. Rasburicase represents a new tool for hyperuricemia in tumor lysis syndrome and in gout. Int J Med Sci. 2007; 4(2):83-93.
54. Richette P, Brière C, Hoenen-Clavert V, et al. Rasburicase for topaceous gout not treatable with allopurinol: an exploratory study. J Rheumatol. 2007;34(10):2093-2098.
55. Moolenburgh JD, Reinders MK, Jansen TL. Rasburicase treatment in severe tophaceous gout: a novel therapeutic option. Clin Rheumatol. 2006;25(5):749-752.
Gouty arthritis is a common form of inflammatory arthritis, occurring more frequently in men than in women. The condition has a male–female ratio of 3 or 4 to 1, although that ratio narrows as adults age; because the uricosuric effects of estrogen decline with menopause, the risk for gout increases in postmenopausal women.1,2 Mean age at disease onset is 40 to 60 in men,3,4 with onset in women averaging seven years later.5
Apart from the pain and loss of function associated with this disorder of purine metabolism6 and the risk for a chronic form of the disease, gout is almost universally linked with serious comorbidities that require timely intervention. These include hypertension, dyslipidemia, hyperglycemia and diabetes, obesity, metabolic syndrome, cardiovascular disease (CVD), renal insufficiency, and coronary heart disease (CHD).2,3,7 The presence of gout is independently associated with a risk for acute myocardial infarction (AMI) and increased rates of all-cause mortality.3,8-10
EPIDEMIOLOGY
In 2007, the National Arthritis Data Workgroup11,12 estimated that about three million US adults had had “self-reported gout” in the previous year. An estimated six million US adults have been diagnosed with gout,2,11 and its incidence and prevalence are increasing. The incidence of primary gout more than doubled between 1977-1978 and 1995-1996,13 especially affecting the aging population. The prevalence of gout among 1,000 managed care patients ages 65 to 74 increased by at least 30% between 1990 and 1999, while prevalence among those older than 75 almost doubled during this same period.14
Numerous factors appear to contribute to these trends, including aging of the population, dietary trends (ie, increased consumption of red meat, organ food, game, and shellfish and reduced consumption of low-fat dairy products), presence of certain comorbid conditions (ie, hypertension, dyslipidemia, diabetes, metabolic syndrome, end-stage renal disease), the increasing prevalence of obesity in younger adults, use of specific prescription medications, and increased incidence of organ transplantation.1,7,8,15-19
The body’s underexcretion or overproduction of uric acid (a byproduct of purine metabolism12) can lead to hyperuricemia. This condition, defined as a serum urate level exceeding 7.0 mg/dL in men or 6.0 mg/dL in women20,21 (levels above 9.0 mg/dL are considered very high22), is the primary risk factor for gout.8,23,24 As with gout, the incidence of hyperuricemia has increased in recent years,20 with researchers attributing the trend to worldwide popularization of the Westernized diet (particularly use of high-fructose corn syrup20,25) and increased use of certain medications, including thiazide diuretics, cyclosporine, and low-dose aspirin.2,20,25,26
As serum urate levels rise, the patient with hyperuricemia may experience urate supersaturation, often followed by crystallization of the excess urate into monosodium urate (MSU) crystals. Subsequently, circulating MSU crystals may deposit in body tissues, especially in the joint spaces. The body’s ensuing inflammatory response to the MSU deposits is gout.20
In addition to hyperuricemia, risk factors for gout include a high-purine diet, habitual alcohol consumption (especially beer and fortified wines27), diuretic therapy (particularly in patients with heart failure or renal insufficiency), obesity, hypertension, and high levels of fructose consumption.7,28 Additionally, cyclosporine use in an organ transplant recipient, poorly controlled uric acid levels, and a long history of gout increase the patient’s risk for chronic tophaceous gout.24,29 Tophi may be more common in a patient with a history of organ transplantation.16
Genetic variants are currently being investigated to possibly identify a predisposition to gout. The most significant genetic factors appear to involve mechanisms that regulate serum uric acid levels—particularly urate underexcretion.23 Other factors that contribute to underexcretion or overproduction of uric acid are shown in Table 1.1,15,26,30-33
A dynamic relationship exists between gout and a number of pathologic processes. According to researchers investigating nearly 178,000 patients with gout in a managed care database, 36% had hypertension, 27% had dyslipidemia, and 15% had diabetes.8 In a smaller cohort study conducted in Spain and Mexico, it was demonstrated that 93% of patients with gout had one or more associated diseases, in order of decreasing frequency: hypertriglyceridemia, obesity, hypertension, metabolic syndrome, hyperglycemia, chronic renal failure, diabetes, and ischemic heart disease.3
Of particular clinical importance in this study was a finding that the first gout attack generally preceded the diagnosis of the associated diseases.3 Thus, a diagnosis of gout should lead the primary care provider to discuss modifiable risk factors with the patient—but also to investigate for comorbid illnesses that may require timely management.2
In a 12-year-long prospective study of more than 50,000 men participating in the Health Professionals Follow-Up Study,9 it was found that men with gout had a 28% increased risk for all-cause mortality, a 38% increased risk for CVD-related death, and a 55% increased risk for CHD-related death, compared with men who did not have gout (excluding other risk factors).9 Similarly, researchers for the Multiple Risk Factor Intervention Trial10 demonstrated a clinically significant association between gout and an increased risk for AMI: 10.5% of men with gout, compared with 8.43% of men without gout, had an AMI during mean follow-up of 6.5 years.10
THE STAGES
The four stages of gout are asymptomatic hyperuricemia, acute gout, intercritical gout, and chronic tophaceous gout.20
Only a small percentage (0.5% to 4.5%) of patients with asymptomatic hyperuricemia will develop acute gout.28 Nevertheless, any patient with serum urate greater than 6.8 mg/dL is at risk for the deposition of MSU crystals into body tissues and the potential associated organ damage—even patients without symptoms. There is currently no evidence-based method to determine which patients with asymptomatic hyperuricemia will experience disease progression.16
Acute gout develops when deposition of MSU crystals in the joints initiates an inflammatory response. In the typical history, the patient experiences sudden-onset severe pain, swelling, and erythema. The pain often starts in the middle of the night or early morning,34 waking the patient from sleep and peaking within 24 hours of onset. At this time, the patient is often unable to bear weight comfortably on the affected joint. The patient may also report fever and flu-like malaise resulting from the release of interleukin 1- (IL-1), IL-1 receptor, cytokines, and prostaglandins.16,24,35 Usually in these early attacks, symptoms resolve spontaneously within three to 14 days.16,24
After resolution of an acute attack, the patient enters the intercritical stage, another asymptomatic stage that may last for months or years—or indefinitely. During the intercritical stage, MSU crystal deposition continues, adding crystals in and around the affected joint or joints, possibly continuing to inflict damage (in some patients, substantial), and in many cases resulting in additional attacks and pain.16 Any subsequent acute gout attacks the patient may experience are likely to last longer than the initial attack and to involve additional joints or tendons.24
Some patients, especially those who do not receive adequate treatment for hyperuricemia,2 progress to develop chronic tophaceous gout. This is a deforming disease process in which the joints may become stiff and swollen, and subcutaneous nodules or whitish-yellow intradermal deposits may be present under taut skin, anywhere in the body.16
PATIENT PRESENTATION AND HISTORY
Typically, a patient with gout will present with a chief complaint of a painful, tender, inflamed joint (classically described in Latin as calor, rubor, dolor, et tumor6). However, clinicians must also be aware of unusual presentations and consider gout in the differential whenever a patient with a history of gout or pertinent risk factors presents with unexplained clinical findings.32 The history of present illness will vary according to the stage of the disease.
About 90% of recognized initial attacks of gout are monoarticular, usually occurring in one of the lower extremities.16 While the first metatarsophalangeal (MTP) joint is affected in about 50% of gout cases (podagra, the Greek term for gout),2,35 eventually patients with gout have a 90% chance of involvement with the MTP joint (see Figure 1). According to Zhang et al,26 patients with hyperuricemia and an affected MTP joint have an 82% chance of having gout.2,26
Because such a large proportion of patients have the classic presentation of rapid-onset warmth, redness, and tenderness at the MTP, knee, or ankle and surrounding soft tissue, cases with a differing presentation are likely to be misdiagnosed or overlooked, or a correct diagnosis is delayed.26 In many documented cases, gout was the ultimate diagnosis—but one that was reached only incidentally because of unusual clinical presentation, ranging from entrapment neuropathy to a pancreatic mass.32
The presence of hyperuricemia and other risk factors must be investigated. Also relevant in the history of an acute gout attack may be a preceding event that has caused damage or stress to the joint, such as infection, trauma, or surgery. Other possible triggers for an attack include alcohol ingestion, acidosis, use of IV contrast media, diuretic therapy, chemotherapy, recent hospitalization or surgery, and initiation or termination of urate-lowering therapy with the xanthine oxidase inhibitor allopurinol.2,30 According to Primatesta et al,8 the risk for flares is increased in patients with cardiometabolic comorbidities.
The medication history of a patient with gout may include low-dose aspirin (but not standard-dose aspirin, which is uricosuric2), diuretics, cyclosporine, cytotoxic agents, and vitamin B12, which may contribute to hyperuricemia.24 Additionally, ethambutol, pyrazinamide, levodopa, nicotinic acid, didanosine, niacin, and warfarin may raise uric acid levels.15,30,33
Medical history should include a thorough assessment of the comorbidities associated with gout. In addition to the conditions mentioned previously, patients with a history of polycystic kidney disease, dehydration, lactic acidosis, hyperparathyroidism, toxemia of pregnancy, hypothyroidism, or sarcoidosis may have elevated urate levels due to underexcretion—and thus may be vulnerable to gout.30 History of gout in a first-degree relative is associated with an increased risk for gout.36
The social history should address alcohol use or abuse. The clinician should also inquire about how gout is impacting the daily life of the patient. Diet and exercise habits should be assessed37 (see “Patient Education,” below).
PHYSICAL EXAMINATION
The physical exam begins with evaluation of the skin and extremities for the classic features of gout. Affected joints will be exquisitely tender, and patients may be febrile. Most cases are monoarticular, but polyarticular involvement is likely in patients with advanced disease (and can, particularly in women, be mistaken for rheumatoid arthritis).2,16 In patients with chronic tophaceous gout, there may be whitish-yellow skin deposits, subcutaneous nodules, and areas of taut skin. The lower-extremity joints and tendons, as well as the wrists, fingers, and elbows, are commonly affected16 (see Figure 2).
DIAGNOSIS
Diagnostic criteria that are currently available (and have long been in use) include the American College of Rheumatology/American Rheumatism Association (ACR/ARA) preliminary criteria,34 the New York criteria,21 and the Rome criteria.38 The specifics of each are listed in Table 2.21,34,38,39
The gold standard for gout diagnosis is detection of MSU crystals in a sample of synovial fluid aspirated from the affected joint or from a tophus and examined by polarized light microscopy.24 This is of significant importance to the clinician who is faced with a questionable diagnosis.16 However, crystal visualization is not ordinarily available to the primary care clinician,2,17,40 and it is not always necessary if a careful history and physical exam are conducted in a patient with hyperuricemia or other risk factors for gout. A presumptive diagnosis may be acceptable in a patient with the classic presentation of acute gout: rapid onset of severe pain in a swollen, erythematous joint and symptoms peaking within 24 hours. The presence of tophi is pathognomonic for chronic tophaceous gout.41
In cases of questionable or unusual manifestation of gout, however, various imaging techniques and crystal visualization may be indicated.32
In order to compare the effectiveness of the latter technique with conventional diagnostic criteria for gout, Malik et al39 conducted a pilot study involving 82 patients who had undergone synovial fluid analysis with polarized light microscopy. Patients were surveyed about the clinical features of their disease, as listed in the three standard sets of criteria for diagnosis of gout. Compared with the “gold standard” of urate crystal detection (which is one of the Rome criteria38), the study authors found the ACR/ARA preliminary criteria,34 the New York criteria,21 and the Rome criteria38 generally unsatisfactory.
In the study, among patients with confirmed presence of MSU crystals:
• 87% reported more than one attack of acute arthritis (ACR/ARA34)
• 86% reported monoarthritis attack (ACR/ARA34)
• 89% had hyperuricemia (ACR/ARA34 and Rome,38 with the latter giving effective, specific parameters)
• 100% had negative results on joint fluid culture (ACR/ARA34)
• 90% reported an attack starting at night (ACR/ARA34).
The positive predictive values for these signs and symptoms are 38%, 39%, 74%, 50%, and 45%, respectively, according to Malik et al.39 The presence of tophi (cited by all three sets of criteria but “proven or suspected” in the ACR/ARA34) had the highest positive predictive value for gout (91%) and a likelihood ratio of 15.56, which was at least three times higher than any of the other listed criteria. A verified response to colchicine, one of the New York criteria,21 had the second highest positive predictive value at 86%.39
In summary, the ACR/ARA,34 the New York,21 and the Rome criteria38 had specificity of 79%, 83%, and 89%, respectively; sensitivity of 70%, 70%, and 67%, respectively; and positive predictive values for gout of 66%, 70%, and 77%, respectively. The Rome criteria38 had the highest specificity and highest positive predictive value, perhaps making them most helpful for clinicians who lack access to synovial fluid analysis.
DIFFERENTIAL DIAGNOSIS
Conditions to be considered and ruled out before a diagnosis of gout can be made are:
• Pseudogout
• Septic arthritis
• Psoriatic arthritis
• Rheumatoid arthritis
• Erosive osteoarthritis
• Bacterial cellulitis
• Sarcoid arthropathy.16,28,42,43
Unlike gout (in which compensated polarized light microscopy reveals needle-shaped urate crystals with strong negative birefringence), pseudogout is characterized by calcium pyrophosphate dihydrate crystals; these are rhomboid-shaped, with weak positive birefringence.42 Additionally, radiographic imaging will reveal soft tissue swelling and chondrocalcinosis of the joint in pseudogout.44
The patient with septic arthritis, most likely affecting the knee, will have a white blood cell (WBC) count exceeding 50,000/mm3 and a positive culture of the synovial fluid, with absence of crystals.28
Chronic tophaceous gout can mimic rheumatoid arthritis in appearance and joint distribution, and patients affected by either condition may develop a positive rheumatoid factor. Examination of synovial fluid for MSU crystals and radiographic imaging will be of value in making a distinction.
Osteoarthritis is usually evidenced by joint space narrowing on x-ray.42
Bacterial cellulitis will present similarly to gout, but the erythema of bacterial cellulitis will more likely extend beyond the involved joint.16
Sarcoid arthropathy often presents as a polyarthritis, as in advanced gouty arthritis. However, in sarcoid arthropathy, serum calcium and angiotensin-converting enzyme will likely be elevated.43 Synovial or tendon sheath biopsy will show non-caseating granulomas, which are the hallmark for sarcoid disease. Additionally, joint fluid analysis will demonstrate a predominance of mononuclear or polymorphonuclear cells.43
Diagnostic Tests
Diagnostic tests to consider are analysis and culture of the synovial fluid, complete blood count (CBC), blood urea nitrogen (BUN), creatinine, radiography, ultrasonography, serum uric acid, and blood culture if septic arthritis is suspected.28 While serum urate levels may be normal during an acute gout attack, measurement may still be helpful for comparison, since elevation is a likely finding two weeks after an attack—if the patient was, in fact, experiencing an acute gout attack.42
Since renal dialysis increases the risk for gout, pseudogout, and septic arthritis, synovial fluid analysis is essential in patients undergoing renal dialysis.42
Various imaging techniques may aid in confirming a diagnosis of gout and monitoring its progression, but further studies are needed to more clearly define the role of these techniques in management of gout.45 Plain radiographic evidence of asymmetric swelling in a joint (one of the ACR/ARA preliminary criteria34) was shown to have a 60% positive predictive value for a diagnosis of gout.39 Late in the disease process, an affected joint may be affected by characteristic “punched out” intra-articular lesions, with a normal amount of joint space.45
Ultrasound is a safe and inexpensive test that can reveal soft tissue edema and increased vascularity during an acute gout attack. Chronic changes include the double contour sign and tophus-like lesions surrounded by a thin, anechoic rim.45
CT will also show tophi and bony erosion. While CT is more specific than other techniques, it is also more expensive and exposes the patient to increased radiation. MRI can help monitor the complications of gout, especially entrapment neuropathies.45
TREATMENT/MANAGEMENT
According to current evidence, treatment is not indicated for asymptomatic hyperuricemia.16
Acute Gout Management
Pharmacologic treatments available for an acute gout attack include NSAIDs, colchicine, and local or systemic corticosteroids.24,46 At the onset of an attack, patients should start high-dose NSAID therapy, and continue for two to three days after symptoms are resolved.6 Oral indomethacin (50 mg tid) or oral ibuprofen (800 mg tid) are both reasonable options.6 It may be prudent to consider a proton pump inhibitor (eg, omeprazole) to protect the gastric mucosa in patients who are susceptible to gastrointestinal problems.27
In addition to high-dose NSAID therapy, adding colchicine (1.2 mg by mouth at onset of symptoms, followed by 0.6 mg one hour later) has proven to be effective in relieving the symptoms of gout, but its serious gastrointestinal adverse effects, particularly diarrhea, must be considered.6,47
In patients with monoarticular gout who cannot tolerate NSAIDs, intra-articular aspiration and corticosteroid injections may provide relief.
Long-acting triamcinolone, administered by intra-articular injection, has been found to relieve pain and inflammation in patients with gout. Septic arthritis must be ruled out by way of joint aspiration and culture before injection of corticosteroids.47
Oral or IM-administered corticosteroids may be considered for patients with polyarticular involvement. Prednisone (60 mg/d, tapered over 10 days) is an appropriate option for outpatients or inpatients; methylprednisone (80 to 120 mg IM) may be suitable for inpatients.6 Again, septic arthritis must be ruled out before corticosteroids are administered.47
For the patient who is currently taking a thiazide diuretic for hypertension, substituting a different medication may be warranted; the angiotensin receptor blocker losartan, for example, has uricosuric action.27,29,47 Nonpharmacologic strategies, such as rest, ice, elevation, and avoiding trauma to the affected joint, are also recommended.27
Of note, allopurinol therapy should be neither initiated nor discontinued during an acute gout attack.27
Management of Chronic and Intercritical Gout
Urate-lowering therapy, such as allopurinol (50 to 300 mg/d29), should be considered for patients who experience frequent attacks (ie, three or more per year), patients with chronic tophi, patients with radiographically demonstrated joint damage,47 or patients with a documented state of uric acid overproduction.29
Allopurinol dosage should be adjusted based on creatinine clearance; dosing as high as 800 mg/d has been recommended in patients with normal renal function.2 Again, allopurinol should never be started or discontinued during an acute attack,27 because abrupt fluctuations in uric acid levels may heighten the inflammation. The target serum urate level is 6.0 mg/dL.29
Febuxostat, which received FDA approval in 2009, was the first oral urate-lowering treatment to be approved since the 1960s. Like allopurinol, this nonpurine xanthine oxidase inhibitor blocks uric acid synthesis.48,49 In a trial reported by Becker et al,50 67% of patients who took febuxostat 80 mg/d reached the target serum urate level (ie, < 6.0 mg/dL), compared with 45% of those who took 40 mg/d of febuxostat and 42% of those taking 300 mg/d of allopurinol. While incidence of adverse events was low in all treatment groups, Hu and Tomlinson51 report that febuxostat is tolerable in patients who are hypersensitive to allopurinol. As with other urate-lowering medications, gout flares are common during the early period of febuxostat use.51
For patients with gout that does not respond to conventional urate-lowering therapy, new options are being introduced. Two agents, each a recombinant form of the enzyme urate oxidase, are designed to convert uric acid into allantoin, which can then be excreted in the urine. Late in 2010, one of these agents, pegloticase, was approved for use in patients with refractory gout.48 In one clinical trial, tophi were reported dissolved in 40% of patients who took pegloticase, but 58% of patients did not achieve the targeted response (ie, serum urate < 6.0 mg/dL), and 77% of patients experienced gout flares.52 Infusion reactions occurred in 26% to 31% of patients, and Reinders and Jansen52 recommended the clinical evaluation of glucocorticoids and other anti-inflammatory agents to prevent the formation of antibodies involved in these reactions.
The second agent, rasburicase, has been approved for treatment and prevention of acute hyperuricemia in adult cancer patients. Rasburicase is now being investigated for use in patients with nonresponsive tophaceous gout.53-55 It can be administered in the form of monthly infusions.54
Patient Education
Educating the patient about modifiable risk factors, such as diet, alcohol consumption, and adherence to the medication regimen, should be a priority.
Patients should be encouraged to target and maintain an ideal body weight, through diet and moderate physical exercise, as a strategy to normalize serum urate levels.27,47 However, they should be advised to avoid “crash dieting,” as this may precipitate a gout attack.27 In the recommended low-purine diet, consumption of red meat and shellfish is restricted,17 whereas consumption of soy, nonfat milk and other low-fat dairy products, cherries and other fruits, and increased vegetable protein is encouraged.31,37 Consumption of alcohol, especially beer and fortified wines, should be limited.27,47
Avoiding trauma to joints affected by gout (including the stress of bearing excess weight) can help patients limit future attacks.7,27
CONCLUSION
Patients with gout often have the characteristic presentation of an acutely tender, inflamed joint, but since gout is a systemic disorder, the clinician must also consider the possibility of gout in almost any organ system. Gout is a common disease, and its diagnosis can alert the astute clinician to investigate for certain metabolic disorders requiring intervention. Hyperlipidemia, metabolic syndrome, hypertension, chronic kidney disease, obesity, cardiovascular disease, and diabetes are all conditions associated with gout.
Recognizing the opportunity to offer preventive care measures and recommend lifestyle modifications to the patient with gout allows the clinician to play an important role in the patient’s care.
REFERENCES
1. Bhole V, de Vera M, Rahman MM, et al. Epidemiology of gout in women: fifty-two–year followup of a prospective cohort. Arthritis Rheum. 2010;62(4):1069-1076.
2. Neogi T. Clinical practice: gout. N Engl J Med. 2011;364(5):443-452.
3. Hernández-Cuevas CB, Roque LH, Huerta-Sil G, et al. First acute gout attacks commonly precede features of the metabolic syndrome. J Clin Rheumatol. 2009;15(2):65-67.
4. Louthrenoo W, Kasitanon N, Sukitawut W, Wichainun R. A clinical study of crystal-proven gouty arthritis in a university hospital. J Med Assoc Thai. 2003;86(9):868-875.
5. De Souza AW, Fernandes V, Ferrari AJ. Female gout: clinical and laboratory features. J Rheumatol. 2005;32(11):2186-2188.
6. Kurakula PC, Keenan RT. Diagnosis and management of gout: an update. J Musculoskel Med. 2010;27(10). www.musculoskeletalnet work.com/display/article/1145622/1692895. Accessed June 14, 2011.
7. Choi HK, Atkinson K, Karlson EW, Curhan G. Obesity, weight change, hypertension, diuretic use, and risk of gout in men: the Health Professionals Follow-up Study. Arch Intern Med. 2005;165(7):742-748.
8. Primatesta P, Plana E, Rothenbacher D. Gout treatment and comorbidities: a retrospective cohort study in a large US managed care population. BMC Musculoskelet Disord. 2011 May 20;12(1):103. [Epub ahead of print]
9. Choi HK, Curhan G. Independent impact of gout on mortality and risk for coronary heart disease. Circulation. 2007;116(8):894-900.
10. Krishnan E, Baker JF, Furst DE, Schumacher HR. Gout and the risk of acute myocardial infarction. Arthritis Rheum. 2006;54(8):2688-2696.
11. Lawrence RC, Felson DT, Helmick CG, et al; National Arthritis Data Workgroup. Estimates of the prevalence of arthritis and other rheumatic conditions in the United States. Part II. Arthritis Rheum. 2008;58(1):26-35.
12. CDC. Gout. www.cdc.gov/arthritis/basics/gout.htm. Accessed June 14, 2011.
13. Arromdee E, Michet CJ, Crowson CS, et al. Epidemiology of gout: is the incidence rising? J Rheumatol. 2002;29(11):2403-2406.
14. Wallace KL, Riedel AA, Joseph-Ridge N, Wortmann R. Increasing prevalence of gout and hyperuricemia over 10 years among older adults in a managed care population. J Rheumatol. 2004; 31(8):1582-1587.
15. Weaver AL. Epidemiology of gout. Cleve Clin J Med. 2008;75 suppl 5:S9-S12.
16. Mandell BF. Clinical manifestations of hyperuricemia and gout. Cleve Clin J Med. 2008;75 suppl 5:S5-S8.
17. Choi HK, Atkinson K, Karlson EW, et al. Purine-rich foods, dairy and protein intake, and the risk of gout in men. N Engl J Med. 2004;350(11):1093-1103.
18. Demarco MA, Maynard JW, Huizinga MM, et al. Younger age at gout onset is related to obesity in a community-based cohort. Arthritis Care Res (Hoboken). 2011 Apr 11; [Epub ahead of print].
19. Brook RA, Forsythe A, Smeeding JE, Lawrence Edwards N. Chronic gout: epidemiology, disease progression treatment and disease burden. Curr Med Res Opin. 2010;26(12):2813-2821.
20. Sachs L, Batra KL, Zimmermann B. Medical implications of hyperuricemia. Med Health R I. 2009;92(11):353-355.
21. Kellgren JH, Jeffrey MR, Ball J, eds. The Epidemiology of Chronic Rheumatism: Atlas of Standard Radiographs of Arthritis. Oxford: Blackwell; 1963:327.
22. Wu EQ, Patel PA, Mody RR, et al. Frequency, risk, and cost of gout-related episodes among the elderly: does serum uric acid level matter? J Rheumatol. 2009;36(5):1032-1040.
23. Riches PL, Wright AF, Ralston SH. Recent insights into the pathogenesis of hyperuricaemia and gout. Hum Mol Genet. 2009;18(R2):R177-R184.
24. Schumacher HR Jr. The pathogenesis of gout. Cleve Clin J Med. 2008;75 suppl 5:S2-S4.
25. Nakagawa T, Hu H, Zharikov S, et al. A causal role for uric acid in fructose-induced metabolic syndrome. Am J Physiol Renal Physiol. 2006;290(3):F625-F631.
26. Zhang W, Doherty M, Pascual E, et al; EULAR (European League Against Rheumatism) Standing Committee for International Clinical Studies Including Therapeutics. EULAR evidence based recommendations for gout. Part I: Diagnosis. Ann Rheum Dis. 2006;65(10):1301-1311.
27. Jordan KM, Cameron JS, Snaith M, et al. British Society for Rheumatology and British Health Professionals in Rheumatology guideline for the management of gout. Rheumatology. 2007;46(8):1372-1374.
28. Eggebeen AT. Gout: an update. Am Fam Physician. 2007;76(6):801-808.
29. Terkeltaub RA. Gout. N Engl J Med. 2003; 349(17):1647-1655.
30. Harris MD, Siegel LB, Alloway JA. Gout and hyperuricemia. Am Fam Physician. 1999;59(4): 925-934.
31. Schlesinger N. Dietary factors and hyperuricemia. Curr Pharm Des. 2005;11(32):4133-4138.
32. Ning TC, Keenan RT. Unusual presentations of gout. Curr Opin Rheumatol. 2010;22(2): 181-187.
33. Menon RK, Mikhailidis DP, Bell JL, et al. Warfarin administration increases uric acid concentrations in plasma. Clin Chem. 1986;32(8):
1557-1559.
34. Wallace SL, Robinson H, Masi AT, et al. Preliminary criteria for the classification of the acute arthritis of primary gout. Arthritis Rheum. 1977;20(3):895-900.
35. Martinon F, Glincher LH. Gout: new insights into an old disease. J Clin Invest. 2006;116 (8):2073-2075.
36. Zampogna G, Andracco R, Parodi M, Cimmino MA. Clinical features of gout in a cohort of Italian patients [in Italian]. Reumatismo. 2009; 61(1):41-47.
37. Choi HK. A prescription for lifestyle change in patients with hyperuricemia and gout. Curr Opin Rheumatol. 2010;22(2):165-172.
38. Bennett PH, Wood PH, eds. Population studies of the rheumatic diseases: proceedings of the Third International Symposium; June 5-10, 1966; New York, NY. Amsterdam: Excerpta Medica Foundation; 1968:457-458.
39. Malik A, Schumacher HR, Dinnella JE, Clayburne GM. Clinical diagnostic criteria for gout: comparison with the gold standard of synovial fluid crystal analysis. J Clin Rheumatol. 2009;15 (1):22-24.
40. Wijnands JMA, Boonen A, Arts ICW, et al. Large epidemiologic studies of gout: challenges in diagnosis and diagnostic criteria. Curr Rheumatol Rep. 2011;13(2):167-174.
41. Dodd LG, Major NM. Fine-needle aspiration cytology of articular and periarticular lesions. Cancer. 2002;96(3):157-165.
42. Dore RK. The gout diagnosis. Cleve Clin J Med. 2008;75 suppl 5:S17-S21.
43. Pettersson T. Sarcoid and erythema nodosum arthropathies. Baillieres Best Pract Res Clin Rheumatol. 2000;14(3):461-476.
44. Córdoba-Fernández A, Rayo-Rosado R. Pseudogout of the first metatarsophalangeal joint associated with hallux valgus: an atypical bilateral case. J Am Podiatr Med Assoc. 2010;100(2):138-142.
45. Dalbeth N, McQueen FM. Use of imaging to evaluate gout and other crystal deposition disorders. Curr Opin Rheumatol. 2009;21(2):124-131.
46. Wu EQ, Forsythe A, Guérin A, et al. Comorbidity burden healthcare resource utilization, and costs in chronic gout patients refractory to conventional urate-lowering therapy. Am J Ther. 2011 Feb 10; [Epub ahead of print].
47. Zhang W, Doherty M, Pascual E, et al; EULAR (European League Against Rheumatism) Standing Committee for International Clinical Studies Including Therapeutics. EULAR evidence based recommendations for gout. Part II: Management. Ann Rheum Dis. 2006;65(10):1312-1324.
48. Schlesinger N, Yasothan U, Kirkpatrick P. Pegloticase [published correction appears in Nat Rev Drug Discov. 2011;10(2):156]. Nat Rev Drug Discov. 2011;10(1):17-18.
49. Pascual E, Sivera F, Yasothan U, Kurkpatrick P. Febuxostat. Nat Rev Drug Discov. 2009;8(3): 191-192.
50. Becker MA, Schumacher HR, Espinoza LR, et al. The urate-lowering efficacy and safety of febuxostat in the treatment of the hyperuricemia of gout; the CONFIRMS trial. Arthritis Res Ther. 2010;12(2):R63.
51. Hu M, Tomlinson B. Febuxostat in the management of hyperuricemia and chronic gout: a review. Ther Clin Risk Manag. 2008;4(6):1209-1220.
52. Reinders MK, Jansen TL. New advances in the treatment of gout: review of pegloticase. Ther Clin Risk Manag. 2010;6:543-550.
53. Cammalleri L, Malaguarnera M. Rasburicase represents a new tool for hyperuricemia in tumor lysis syndrome and in gout. Int J Med Sci. 2007; 4(2):83-93.
54. Richette P, Brière C, Hoenen-Clavert V, et al. Rasburicase for topaceous gout not treatable with allopurinol: an exploratory study. J Rheumatol. 2007;34(10):2093-2098.
55. Moolenburgh JD, Reinders MK, Jansen TL. Rasburicase treatment in severe tophaceous gout: a novel therapeutic option. Clin Rheumatol. 2006;25(5):749-752.
GERD: Two Options Offer Similar Long-Term Relief
The Nurse–Physician Liaison: How a New Position Can Reduce House Staff Workload
Epstein-Barr Virus–Induced Adrenal Insufficiency, Cardiac Tamponade, and Pleural Effusions
Evaluating Adherence With the GOLD Guidelines for Treating Stage II (Moderate) COPD at a Single Tribal Facility
Grand Rounds: Woman, 20, With Difficulty Walking
A 20-year-old woman presented to her primary care clinic with a chief complaint of lower leg weakness and difficulty walking. The weakness she described had been worsening over the previous four days, with progressively worsening tingling and numbness of her toes bilaterally.
The day before the patient presented, she noticed numbness and paresthesia in both calves. At the time of her presentation to the clinic, she complained of low back ache, paresthesia of both hands, numbness bilaterally to her groin, difficulty sitting upright, ataxia, and a numb, thick-feeling tongue. She denied fever, neck stiffness, shortness of breath, headache, or visual changes.
The patient stated that 10 days earlier, she had developed an upper respiratory infection for which she was seen at the clinic and treated with a seven-day course of amoxicillin/clavulanate 875/125 mg twice daily. She said that she had recovered completely.
A review of the patient’s systems revealed proximal muscle weakness bilaterally (2/5) and loss of touch-pressure in the lower extremities. She was experiencing paresthesia of the hands and mild weakness bilaterally (4/5). She also walked with an ataxic gait and had reduced deep tendon reflexes in the lower limbs. All cranial nerves were intact, and her vital signs were stable.
The woman’s medical history was positive only for asthma. Her family history included ischemic stroke in the maternal grandfather and brain tumor in the paternal grandfather. Social history was positive for alcohol intake (ranging from four to 12 beers per week). The patient said she had never smoked or used illicit drugs. She was an unmarried college student, living in a dorm on campus. She participated in track at school.
The patient was admitted to the hospital telemetry step-down unit, and a neurology consultation was requested. Tests were ordered, among them MRI of the head and spine and comprehensive blood work, to rule out neurologic, infectious, or metabolic causes of the patient’s weakness; urinalysis was also obtained. These tests all yielded negative results.
A lumbar puncture performed the following day revealed a cerebrospinal fluid (CSF) protein level of 570 mg/L (normal range, 150 to 450 mg/L). Leukocytes numbered 2 cells/mm3 (normal count, 0 to 10 cells/mm3).
Based on the patient’s presentation, history, and symptoms, a neurologist made a diagnosis of Guillain-Barré syndrome. It was decided that no electromyographic (EMG) study was required to rule out other disease processes (eg, spinal cord disease, multiple sclerosis, tumors).
The patient underwent a five-dose course of immunomodulatory therapy with IV immunoglobulin (IVIG). In the step-down unit, she experienced one incident of sinus bradycardia (ie, resting heart rate between 40 and 50 beats/min). Her blood pressure remained stable, as did her respiratory status, according to peak expiratory flow measured frequently at her bedside.
Physical therapy was initiated, consisting of passive and active range of motion, crossovers with the patient’s feet, and stair training. This was done in response to a complaint of ankle weakness, and it helped to strengthen weakened muscles and improve alignment while the patient was bedridden and in a weakened, fatigued state. Additionally, the patient was given enoxaparin, wore antiembolic hose, and used sequential compression devices while in bed. As a result of these measures, she never experienced a pulmonary embolus or deep vein thrombosis (DVT) as a result of being immobilized.
By the seventh day of hospitalization, the patient had stable vital signs and improved lower limb strength, and numbness was resolving in her hands and lower extremities. She was discharged to home, with physical therapy to resume on an outpatient basis.
Discussion
Guillain-Barré syndrome (GBS), an acute immune-mediated paralytic disorder,1 manifests in the form of weakness and diminished reflexes. Affecting the peripheral nerves, GBS is characterized by progressive symmetrical ascending weakness with varying degrees of sensory complaints.2,3
GBS occurs worldwide, and incidence is estimated between 1.1 and 1.8 cases per 100,000 persons.4 In the United States, GBS can be found in all age-groups, with peak incidence noted in elderly persons and young adults.5,6 Even with treatment, 3% to 10% of patients are reported to die of this illness, and 20% cannot walk six months after symptom onset.7 In one prospective population-based study of patients with confirmed GBS, 6% of patients died within 30 days of symptom onset, often as a result of respiratory complications.8
GBS is a postinfectious disorder, with cases developing several days or weeks after a viral or bacterial illness—most commonly, an upper respiratory infection or diarrhea (see Table 19-13). The most common trigger of GBS is infection with the bacterial microorganism Campylobacter jejuni (occurring in 15% to 40% of patients with GBS),9,14 a pathogen that can produce demyelination-causing antibodies. Other responsible pathogens include cytomegalovirus and Epstein-Barr virus.9 In a process called molecular mimicry, the immune system is unable to distinguish the amino acid of an infectious organism from the proteinaceous content of the peripheral nerve.15 Subsequently, the immune system attacks and destroys the myelin sheath.
An example of this is the apparent cross-reaction of the ganglioside GM1 with C jejuni lipopolysaccharide antigens.14,15 The resulting effect is immunologic damage to the peripheral nervous system. The flaccid paralysis that occurs in patients with GBS is thought to be caused by lymphocytic infiltration and complement activation of the spinal roots and peripheral nerves, where macrophages strip the myelin.5,15,16
Stages and Variants
Three stages characterize the course of GBS. The acute phase, which lasts one to four weeks, begins with onset of symptoms and persists until the associated neurologic deterioration has ceased. During the second phase, the plateau period, symptoms persist with no further deterioration; this stage can last several days to several weeks or months. The final phase, the recovery period, can last from four months to two years after symptom onset.15,17,18
The clinical course of GBS is highly variable and in many cases difficult to predict. Certain factors have been associated with a poor outcome: advancing age, previous presence of diarrhea, need for mechanical ventilation, an extended plateau phase, and a lower patient score on the Erasmus GBS Outcome Scale,19 when measured two weeks after GBS onset.8,20 This score can help predict the patient’s chance of independent walking after six months.15,19
Although the classic presenting symptom of GBS is symmetric ascending weakness, several disease variants have been identified, with differing symptoms and degrees of recovery. These variants also differ in terms of the muscle groups affected; in some, visual defects may be present at onset. GBS variants include21:
• Acute motor axonal neuropathy (AMAN)1,22
• Acute inflammatory demyelinating polyneuropathy (AIDP)1
• Pharyngeal-cervical-brachial variant23
• Purely sensory variant24
• Miller-Fisher syndrome, which manifests with ophthalmoplegia, in addition to ataxia and areflexia25
• Axonal form.5,21
AMAN and AIDP are the most common subtypes of GBS.1
Symptoms, Signs, and Disease Manifestations
Limb weakness, the classic presenting symptom of GBS, is both symmetrical and ascending. Weakness can develop acutely and progress over days to weeks.2,15 Hughes and Cornblath26 also note pain, numbness, and paresthesias among the initial symptoms of GBS. Others include sensory changes, cranial nerve involvement, various autonomic changes, and respiratory or oropharyngeal weakness. Reflexes, particularly the tendon reflexes, may be diminished or absent.15,18,21 In many cases, sensory changes (ie, pain) may precede the onset of weakness, often making diagnosis difficult.15
Cranial nerves most commonly affected are V, VI, VII, X, XI, and XII, with manifestations that include dysphagia, dysarthria, diplopia, limitation to eye movements, and facial droop and weakness. Usually facial and oropharyngeal weakness occur after the extremities and trunk are affected. Blindness may occur if demyelination of the optic nerve occurs; this is seen in Miller-Fisher syndrome.10,15,25,27
In GBS, many patients report pain, which can present as bilateral sciatica or as throbbing or aching in the large muscles of the upper legs, flanks, or back.28 This pain, which results from the demyelination of the sensory nerve fibers, can be severe.10
Patients with GBS may experience manifestations of autonomic nervous system dysfunction—for example, arrhythmias, hypotension or hypertension, urinary retention, cardiomyopathy, and paralytic ileus.10,20 Dysautonomia often impedes patients’ progress in inpatient rehabilitation. Patients may have persistent problems involving postural hypotension, hypertension, excessive sympathetic outflow, or bladder and bowel dysfunction.29
Blood pressure fluctuations, often attributed to changes in catecholamine levels and disturbances in the baroreceptor reflex pathway, are common and are considered characteristic of GBS. Transient or persistent hypotension is caused by the dysregulation of the parasympathetic and sympathetic systems, with subsequent alterations in venomotor tone.3 Additionally, an increased sensitivity to catecholamine can lead to cardiovascular disturbances, resulting in denervation hypersensitivity and impairment of the carotid sinus reflex.
Arrhythmias occur in perhaps half of patients with GBS. The most common is sustained sinus tachycardia, which usually requires no treatment. Bradycardia leading to atrioventricular blocks and asystole is believed to result from afferent baroreceptor reflex failure. Treatment may be required—either administration of atropine or insertion of a pacemaker, depending on the severity of the arrhythmia.3,10
Myocardial involvement can range from asymptomatic mycocarditis to neurogenic stunned myocardium and heart failure. Patients with ECG abnormalities should undergo two-dimensional echocardiographic studies and other testing to explore cardiac involvement. Acute coronary syndromes, including ST-segment elevation MI, have been reported, in some cases associated with IVIG treatment. In one patient, coronary spasm was reported, with clean coronary arteries found on cardiac catheterization.3
Patients with GBS are at risk for compromised neuromuscular respiratory function; demyelination of the nerves that innervate the intercostal muscles and the diaphragm can result in respiratory failure. Key clinical indicators of respiratory muscle fatigue include tachypnea, diaphoresis, and asynchronous movements of the abdomen and chest;10 other symptoms relevant to respiratory or oropharyngeal weakness include slurred speech, dyspnea (with or without exertion), difficulty swallowing, and inability to cough.2,10 Serial respiratory function testing is advisable to detect patients at risk for respiratory failure.30
Diagnosis
Guillain-Barré is a syndrome diagnosed by a collection of symptoms (see Table 22,21,31), including subacute developing paralysis, symmetrical bilateral weakness beginning at onset, and diminishing to absent reflexes.21,31 Other causes for rapidly developing weaknesses should be ruled out (see Table 310,21,26,31). Lumbar puncture typically shows increased protein levels with a normal white cell count; however, neither this test nor electrophysiologic evaluation offers significant value for diagnosis of GBS.21,26,31
During the acute phase of GBS (within three weeks of onset), there is found an elevation of CSF protein (> 550 mg/L) without an elevation in white blood cells. This phenomenon, called albuminocytologic dissociation, reflects inflammation of the nerve roots and is considered the hallmark of GBS.2
MRI can also facilitate the diagnosis of GBS; it demonstrates anterior and posterior intrathecal spinal nerve roots and cauda equina.32 In patients with GBS, evidence supporting breakdown of the blood–nerve barrier can be seen in abnormal gadolinium enhancement of the intrathecal nerve roots on MRI.33
When electrophysiologic studies are performed, they typically reveal slowing nerve conduction, prolonged distal latencies, and partial motor conduction block.34 The characteristic finding of early demyelination is conduction block, a reduction in the amplitude of the muscle action potential after stimulation of the distal, as opposed to the proximal, nerve.28 Nerve conduction studies may help in the diagnosis and classification of GBS—and, to a limited extent, formulation of a prognosis. Such alternative diagnoses as myositis and myasthenia gravis may be excluded by neurophysiology.26 Early in GBS, neurophysiologic abnormalities may be very mild or occasionally normal; test results may not correlate with clinical disability.35,36
The clinician cannot depend on clinical features alone to predict respiratory decline.31 Frequent evaluations of respiratory effort, by measurement of maximal inspiratory pressures and vital capacity, should be performed at the bedside to monitor diaphragmatic strength. Respiratory ventilation should be initiated if the patient becomes hypoxic or experiences a rapid decline in vital capacity (ie, below 60% of predicted value).10 Mechanical ventilation is more likely to be required in patients with a negative inspiratory force of less than 30 cm H2O.31
Treatment
Guillain-Barré syndrome has an acute onset and progression. Patients quickly become nonambulatory and may require total ventilation due to paralysis. Therapeutic options are IVIG or plasmapheresis (plasma exchange).37-40 Corticosteroids do not appear to benefit patients with GBS.41,42
Several mechanisms appear to contribute to the effectiveness of immunoglobulin.38,39 Infused IVIG interferes with antigen presentation, inhibits antibody production, neutralizes pathologic autoantibodies, and modulates other immunologic events involved in the pathogenesis of autoimmune neuromuscular diseases, including GBS.43 Adverse reactions, which are usually minor, include headache, fever, chills, myalgia, and malaise. In rare instances, anaphylaxis or renal failure may occur.15,44
In plasmapheresis, blood is removed from the body and dialyzed, with circulating antibodies and immunoglobulins removed from the plasma; fresh frozen plasma, albumin, or saline is administered. This treatment, performed via central venous catheter, should be initiated as soon as possible after onset of symptoms but can be implemented as late as 30 days after GBS onset. Plasmapheresis requires personnel trained in dialysis, which may not be performed in all hospitals. Possible adverse events include infection and hemorrhage. Laboratory values must be monitored for hypokalemia and hypocalcemia.45,46
Supportive Care
Patients with GBS require intensive care and very close monitoring for complications of respiratory difficulty and autonomic dysfunction. Individualized programs should be initiated for patients in the acute phase of GBS, aimed at the prevention of contractures and skin breakdown.10 Exercise programs, as conducted with the case patient, should also help relieve the fatigue syndromes that accompany GBS.
Immobilization associated with bed rest incurs a risk for pulmonary emboli and DVT; this has been found true during the first 12 weeks after symptom onset in patients with GBS who remain immobile.47 The use of antiembolic hose and sequential compression devices can help reduce the risk for thrombotic events.10 Use of enoxaparin or heparin is recommended for nonambulating patients until they are able to walk, with Gaber et al47 specifying the use of low-molecular-weight heparin to reduce, but not eliminate, the risk for DVT.
The pain associated with GBS can be severe. Narcotic analgesics may be administered with careful monitoring of autonomic denervation. Long-term management of neuropathic pain may require adjuvant therapy, such as tricyclic antidepressants, gabapentin, or tramadol hydrochloride.10 According to Pandey et al,48 gabapentin alone may suffice for pain control in GBS, with minimal adverse effects. In certain rehabilitation facilities, tricyclic antidepressants, capsaicin, and transcutaneous nerve stimulation have been reported effective; during the early stages of treatment, until these treatments reach their full effect, pain medications such as tramadol or narcotics can provide temporary relief.29
More than one-half of patients with GBS in the acute phase can develop ileus. Constipation can also occur as a result of pain medication use, prolonged bed rest, and poor intake. Auscultation of bowel sounds and abdominal assessment should be performed daily to monitor for ileus. Hughes et al10 do not recommend the use of promotility drugs in patients with dysautonomia.
After hospital discharge, easy fatigability can affect work and social activities. With continued physical therapy, occupational therapy, and monitoring, however, patients with GBS can expect to return to an optimal level of functioning. Speed of recovery varies with these patients from a few months to several years, depending on such factors as age and the extent to which axonal degeneration has occurred.6,49
The Case Patient
For several weeks after discharge, the case patient continued to experience fatigue, low back pain, and general muscle pain. With her family’s support, she continued to receive outpatient physical therapy, and within one month she had regained her ankle strength. She was soon able to resume her classes, despite some lingering fatigue.
Conclusion
Guillain-Barré syndrome is a potentially life-threatening disease whose symptoms health care providers need to recognize quickly to provide prompt treatment. Supportive care for both patient and family is of key importance for maximum rehabilitation and return to the previous lifestyle. The clinical course of GBS is highly variable and difficult to predict. The patient’s outcome depends on several factors, including age and severity of illness. GBS patients can experience long-term psychosocial effects.
References
1. Magira EE, Papaioakim M, Nachamkin I, et al. Differential distribution of HLA-DQ beta/DR beta epitopes in the two forms of Guillain-Barré syndrome, acute motor axonal neuropathy and acute inflammatory demyelinating polyneuropathy (AIDP): identification of DQ beta epitopes associated with susceptibility to and protection from AIDP. J Immunol. 2003;170(6):3074-3080.
2. Tremblay ME, Closon A, D’Anjou G, Bussières JF. Guillain-Barré syndrome following H1N1 immunization in a pediatric patient. Ann Pharmacother. 2010;44(7-8):1330-1333.
3. Mukerji S, Aloka F, Farooq MU, et al. Cardiovascular complications of the Guillain-Barré syndrome. Am J Cardiol. 2009;104(10):1452-1455.
4. McGrogan A, Madle GC, Seaman HE, de Vries CS. The epidemiology of Guillain-Barré syndrome worldwide: a systematic literature review. Neuroepidemiology. 2009;32(2):150-163.
5. Haber P, Sejvar J, Mikaeloff Y, DeStefano F. Vaccines and Guillain-Barré syndrome. Drug Saf. 2009; 32(4):309-323.
6. van Doorn PA. What’s new in Guillain-Barré syndrome in 2007-2008? J Periph Nerv Syst. 2009;14(2):72-74.
7. van Doorn PA, Ruts L, Jacobs BC. Clinical features, pathogenesis, and treatment of Guillain-Barré syndrome. Lancet Neurol. 2008;7(10):939-950.
8. Chiò A, Cocito D, Leone M, et al; Piemonte and alle d’Aosta Register for Guillain-Barré Syndrome. Guillain-Barré syndrome: a prospective, population-based incidence and outcome survey. Neurology. 2003; 60(7):1146-1150.
9. Hadden RD, Karch H, Hartung HP, et al. Preceding infections, immune factors, and outcome in Guillain-Barré syndrome. Neurology. 2001;56(6):758-765.
10. Hughes RA, Wijdicks EF, Benson E, et al. Supportive care for patients with Guillain-Barré syndrome. Arch Neurol. 2005;62(8):1194-1198.
11. Aluka KJ, Turner PL, Fullum TM. Guillain-Barré syndrome and postbariatric surgery polyneuropathies. JSLS. 2009;13(2):250-253.
12. Brannagan TH 3rd, Zhou Y. HIV-associated Guillain-Barré syndrome. J Neurol Sci. 2003;208(1-2):39-42.
13. Lin WC, Lee PI, Lu CY, et al. Mycoplasma pneumoniae encephalitis in childhood. J Microbiol Immunol Infect. 2002;35(3):173-178.
14. Sivadon-Tardy V, Orlikowski D, Porcher R, et al. Detection of Campylobacter jejuni by culture and real-time PCR in a French cohort of patients with Guillain-Barre syndrome. J Clin Microbiol. 2010;48 (6):2278-2281.
15. van Doorn PA, Kuitwaard K, Walgaard C, et al. IVIG treatment and prognosis in Guillain-Barré syndrome. J Clin Immunol. 2010;30 suppl 1:S74-S78.
16. Kaida K, Kusunoki S. Guillan-Barré syndrome: update on immunobiology and treatment. Expert Rev Neurother. 2009;9(9):1307-1319.
17. Forsberg A, Press R, Einarsson U, et al. Disability and health-related quality of life in Guillain-Barré syndrome during the first two years after onset: a prospective study. Clin Rehabil. 2005;19(8):900-909.
18. Criteria for diagnosis of Guillain-Barré syndrome. Ann Neurol. 1978;3(6):565-566.
19. van Koningsveld R, Steyerberg EW, Hughes RA, et al. A clinical progostic scoring system for Guillain-Barré syndrome. Lancet Neurol. 2007;6(7):589-594.
20. Koeppen S, Kraywinkel K, Wessendorf TE, et al. Long-term outcome of Guillain-Barré syndrome. Neurocrit Care. 2006;5(3)235-242.
21. Sheridan JM, Smith D. Atypical Guillain-Barré in the emergency department. West J Emerg Med. 2010;11(1):80-82.
22. Ogawara K, Kuwabara S, Koga M, et al. Anti-GM1b IgG antibody is associated with acute motor axonal neuropathy and Campylobacter jejuni infection. J Neurol Sci. 2003;210(1-2):41-45.
23. Nagashima T, Koga M, Odaka M, et al. Continuous spectrum of pharyngeal-cervical-brachial variant of Guillain-Barré syndrome. Arch Neurol. 2007;64(10):1519-1523.
24. Oh SJ, LaGanke C, Claussen GC. Sensory Guillain-Barré syndrome. Neurology. 2001;56(1):82-86.
25. Aráranyi Z, Kovács T, Sipos I, Bereczki D. Miller Fisher syndrome: brief overview and update with a focus on electrophysiological findings. Eur J Neurol. 2011 Jun 1. [Epub ahead of print]
26. Hughes RA, Cornblath, DR. Guillain-Barré syndrome. Lancet. 2005;366(9497):1653-1666.
27. Snyder LA, Rismondo V, Miller NR. The Fisher variant of Guillain-Barré syndrome (Fisher syndrome). J Neuroophthalmol. 2009;29(4):312-324.
28. Ropper AH. The Guillain-Barré syndrome. N Engl J Med.1992;326(17):1130-1136.
29. Meythaler JM. Rehabilitation of Guillain-Barré syndrome. Arch Phys Med Rehabil.1997;78(8):872-879.
30. Sharshar T, Chevret S, Bourdain F, et al; French Cooperative Group on Plasma Exchange in Guillain-Barré syndrome. Early predictors of mechanical ventilation in Guillain-Barré syndrome. Crit Care Med. 2003; 31(1):278-283.
31. McGillicuddy DC, Walker O, Shapiro NI, et al. Guillain-Barré syndrome in the emergency department. Ann Emerg Med. 2006;47(4):390-393.
32. Yikilmaz A, Doganay S, Gumus H, et al. Magnetic resonance imaging of childhood Guillain-Barré syndrome. Childs Nerv Syst. 2010;26(8):1103-1108.
33. Gonzalez-Quevedo A, Carriera RF, O’Farrill ZL, et al. An appraisal of blood-cerebrospinal fluid barrier dysfunction during the course of Guillain-Barré syndrome. Neurol India. 2009;57(3):288-294.
34. Abai S, Kim SB, Kim JP, Lim YJ. Guillan-Barré syndrome combined with acute cervical myelopathy. J Korean Neurosurg Soc. 2010;48(3):298-300.
35. Uncini A, Yuki N. Electrophysiologic and immunopathologic correlates in Guillain-Barré syndrome subtypes. Expert Rev Neurother. 2009;9(6):869-884.
36. Hadden RD, Hughes RA. Management of inflammatory neuropathies. J Neurol Neurosurg Psychiatry. 2003;74 suppl 2:ii9-ii14.
37. Raphaël JC, Chevret S, Hughes RA, Annane D. Plasma exchange for Guillain-Barré syndrome. Cochrane Database Syst Rev. 2002;(2):CD001798.
38. Hughes RA, Swan AV, van Doorn PA. Intravenous immunoglobulin for Guillain-Barré syndrome. Cochrane Database Syst Rev. 2010 Jun 16; (6):CD002063.
39. Human immunoglobulin and the Guillain-Barré syndrome: new indication. An alternative to plasmapheresis. Prescrire Int. 2000;9(49):142-143.
40. van der Meché FG, Schmitz PI; Dutch Guillain-Barré Study Group. A randomized trial comparing intravenous immune globulin and plasma exchange in Guillain-Barré syndrome. N Engl J Med. 1992;327(17):1123-1129.
41. Hughes RA, Swan AV, van Doorn PA. Corticosteroids for Guillain-Barré syndrome. Cochrane Database Syst Rev. 2010 Feb 16;(2):CD001446.
42. Hahn AF. Guillain-Barré syndrome. Lancet. 1998; 352(9128):635-641.
43. Dalakas MC. Intravenous immunoglobulin in autoimmune neuromuscular diseases. JAMA. 2004;291(19):2367-2375.
44. Kuitwaard K, de Gelder J, Tio-Gillen AP, et al. Pharmacokenetics of intravenous immunoglobulin and outcome in Guillain-Barré syndrome. Ann Neurol. 2009;66(5):597-603.
45. Atkinson SB, Carr RL, Maybee P, Haynes D. The challenges of managing and treating Guillain-Barré syndrome during the acute phase. Dimens Crit Care Nurs. 2006;25(6):256-263.
46. van Doorn PA. Treatment of Guillain-Barré syndrome and CIDP. J Periph Nerv Syst. 2005;10(2):113-127.
47. Gaber TA, Kirker SGB, Jenner JR. Current practice of prophylactic anticoagulation in Guillain-Barré syndrome. Clin Rehabil. 2002;16(2):190-193.
48. Pandey CK, Bose N, Garg G, et al. Gabapentin for the treatment of pain in Guillain-Barré syndrome: a double-blinded, placebo-controlled, crossover study. Anesth Analg. 2002;95(6):1719-1723.
49. de Vries JM, Hagemans ML, Bussmann JB, et al. Fatigue in neuromuscular disorders: focus on Guillain-Barré syndrome and Pompe disease. Cell Mol Life Sci. 2010;67(5):701-713.
A 20-year-old woman presented to her primary care clinic with a chief complaint of lower leg weakness and difficulty walking. The weakness she described had been worsening over the previous four days, with progressively worsening tingling and numbness of her toes bilaterally.
The day before the patient presented, she noticed numbness and paresthesia in both calves. At the time of her presentation to the clinic, she complained of low back ache, paresthesia of both hands, numbness bilaterally to her groin, difficulty sitting upright, ataxia, and a numb, thick-feeling tongue. She denied fever, neck stiffness, shortness of breath, headache, or visual changes.
The patient stated that 10 days earlier, she had developed an upper respiratory infection for which she was seen at the clinic and treated with a seven-day course of amoxicillin/clavulanate 875/125 mg twice daily. She said that she had recovered completely.
A review of the patient’s systems revealed proximal muscle weakness bilaterally (2/5) and loss of touch-pressure in the lower extremities. She was experiencing paresthesia of the hands and mild weakness bilaterally (4/5). She also walked with an ataxic gait and had reduced deep tendon reflexes in the lower limbs. All cranial nerves were intact, and her vital signs were stable.
The woman’s medical history was positive only for asthma. Her family history included ischemic stroke in the maternal grandfather and brain tumor in the paternal grandfather. Social history was positive for alcohol intake (ranging from four to 12 beers per week). The patient said she had never smoked or used illicit drugs. She was an unmarried college student, living in a dorm on campus. She participated in track at school.
The patient was admitted to the hospital telemetry step-down unit, and a neurology consultation was requested. Tests were ordered, among them MRI of the head and spine and comprehensive blood work, to rule out neurologic, infectious, or metabolic causes of the patient’s weakness; urinalysis was also obtained. These tests all yielded negative results.
A lumbar puncture performed the following day revealed a cerebrospinal fluid (CSF) protein level of 570 mg/L (normal range, 150 to 450 mg/L). Leukocytes numbered 2 cells/mm3 (normal count, 0 to 10 cells/mm3).
Based on the patient’s presentation, history, and symptoms, a neurologist made a diagnosis of Guillain-Barré syndrome. It was decided that no electromyographic (EMG) study was required to rule out other disease processes (eg, spinal cord disease, multiple sclerosis, tumors).
The patient underwent a five-dose course of immunomodulatory therapy with IV immunoglobulin (IVIG). In the step-down unit, she experienced one incident of sinus bradycardia (ie, resting heart rate between 40 and 50 beats/min). Her blood pressure remained stable, as did her respiratory status, according to peak expiratory flow measured frequently at her bedside.
Physical therapy was initiated, consisting of passive and active range of motion, crossovers with the patient’s feet, and stair training. This was done in response to a complaint of ankle weakness, and it helped to strengthen weakened muscles and improve alignment while the patient was bedridden and in a weakened, fatigued state. Additionally, the patient was given enoxaparin, wore antiembolic hose, and used sequential compression devices while in bed. As a result of these measures, she never experienced a pulmonary embolus or deep vein thrombosis (DVT) as a result of being immobilized.
By the seventh day of hospitalization, the patient had stable vital signs and improved lower limb strength, and numbness was resolving in her hands and lower extremities. She was discharged to home, with physical therapy to resume on an outpatient basis.
Discussion
Guillain-Barré syndrome (GBS), an acute immune-mediated paralytic disorder,1 manifests in the form of weakness and diminished reflexes. Affecting the peripheral nerves, GBS is characterized by progressive symmetrical ascending weakness with varying degrees of sensory complaints.2,3
GBS occurs worldwide, and incidence is estimated between 1.1 and 1.8 cases per 100,000 persons.4 In the United States, GBS can be found in all age-groups, with peak incidence noted in elderly persons and young adults.5,6 Even with treatment, 3% to 10% of patients are reported to die of this illness, and 20% cannot walk six months after symptom onset.7 In one prospective population-based study of patients with confirmed GBS, 6% of patients died within 30 days of symptom onset, often as a result of respiratory complications.8
GBS is a postinfectious disorder, with cases developing several days or weeks after a viral or bacterial illness—most commonly, an upper respiratory infection or diarrhea (see Table 19-13). The most common trigger of GBS is infection with the bacterial microorganism Campylobacter jejuni (occurring in 15% to 40% of patients with GBS),9,14 a pathogen that can produce demyelination-causing antibodies. Other responsible pathogens include cytomegalovirus and Epstein-Barr virus.9 In a process called molecular mimicry, the immune system is unable to distinguish the amino acid of an infectious organism from the proteinaceous content of the peripheral nerve.15 Subsequently, the immune system attacks and destroys the myelin sheath.
An example of this is the apparent cross-reaction of the ganglioside GM1 with C jejuni lipopolysaccharide antigens.14,15 The resulting effect is immunologic damage to the peripheral nervous system. The flaccid paralysis that occurs in patients with GBS is thought to be caused by lymphocytic infiltration and complement activation of the spinal roots and peripheral nerves, where macrophages strip the myelin.5,15,16
Stages and Variants
Three stages characterize the course of GBS. The acute phase, which lasts one to four weeks, begins with onset of symptoms and persists until the associated neurologic deterioration has ceased. During the second phase, the plateau period, symptoms persist with no further deterioration; this stage can last several days to several weeks or months. The final phase, the recovery period, can last from four months to two years after symptom onset.15,17,18
The clinical course of GBS is highly variable and in many cases difficult to predict. Certain factors have been associated with a poor outcome: advancing age, previous presence of diarrhea, need for mechanical ventilation, an extended plateau phase, and a lower patient score on the Erasmus GBS Outcome Scale,19 when measured two weeks after GBS onset.8,20 This score can help predict the patient’s chance of independent walking after six months.15,19
Although the classic presenting symptom of GBS is symmetric ascending weakness, several disease variants have been identified, with differing symptoms and degrees of recovery. These variants also differ in terms of the muscle groups affected; in some, visual defects may be present at onset. GBS variants include21:
• Acute motor axonal neuropathy (AMAN)1,22
• Acute inflammatory demyelinating polyneuropathy (AIDP)1
• Pharyngeal-cervical-brachial variant23
• Purely sensory variant24
• Miller-Fisher syndrome, which manifests with ophthalmoplegia, in addition to ataxia and areflexia25
• Axonal form.5,21
AMAN and AIDP are the most common subtypes of GBS.1
Symptoms, Signs, and Disease Manifestations
Limb weakness, the classic presenting symptom of GBS, is both symmetrical and ascending. Weakness can develop acutely and progress over days to weeks.2,15 Hughes and Cornblath26 also note pain, numbness, and paresthesias among the initial symptoms of GBS. Others include sensory changes, cranial nerve involvement, various autonomic changes, and respiratory or oropharyngeal weakness. Reflexes, particularly the tendon reflexes, may be diminished or absent.15,18,21 In many cases, sensory changes (ie, pain) may precede the onset of weakness, often making diagnosis difficult.15
Cranial nerves most commonly affected are V, VI, VII, X, XI, and XII, with manifestations that include dysphagia, dysarthria, diplopia, limitation to eye movements, and facial droop and weakness. Usually facial and oropharyngeal weakness occur after the extremities and trunk are affected. Blindness may occur if demyelination of the optic nerve occurs; this is seen in Miller-Fisher syndrome.10,15,25,27
In GBS, many patients report pain, which can present as bilateral sciatica or as throbbing or aching in the large muscles of the upper legs, flanks, or back.28 This pain, which results from the demyelination of the sensory nerve fibers, can be severe.10
Patients with GBS may experience manifestations of autonomic nervous system dysfunction—for example, arrhythmias, hypotension or hypertension, urinary retention, cardiomyopathy, and paralytic ileus.10,20 Dysautonomia often impedes patients’ progress in inpatient rehabilitation. Patients may have persistent problems involving postural hypotension, hypertension, excessive sympathetic outflow, or bladder and bowel dysfunction.29
Blood pressure fluctuations, often attributed to changes in catecholamine levels and disturbances in the baroreceptor reflex pathway, are common and are considered characteristic of GBS. Transient or persistent hypotension is caused by the dysregulation of the parasympathetic and sympathetic systems, with subsequent alterations in venomotor tone.3 Additionally, an increased sensitivity to catecholamine can lead to cardiovascular disturbances, resulting in denervation hypersensitivity and impairment of the carotid sinus reflex.
Arrhythmias occur in perhaps half of patients with GBS. The most common is sustained sinus tachycardia, which usually requires no treatment. Bradycardia leading to atrioventricular blocks and asystole is believed to result from afferent baroreceptor reflex failure. Treatment may be required—either administration of atropine or insertion of a pacemaker, depending on the severity of the arrhythmia.3,10
Myocardial involvement can range from asymptomatic mycocarditis to neurogenic stunned myocardium and heart failure. Patients with ECG abnormalities should undergo two-dimensional echocardiographic studies and other testing to explore cardiac involvement. Acute coronary syndromes, including ST-segment elevation MI, have been reported, in some cases associated with IVIG treatment. In one patient, coronary spasm was reported, with clean coronary arteries found on cardiac catheterization.3
Patients with GBS are at risk for compromised neuromuscular respiratory function; demyelination of the nerves that innervate the intercostal muscles and the diaphragm can result in respiratory failure. Key clinical indicators of respiratory muscle fatigue include tachypnea, diaphoresis, and asynchronous movements of the abdomen and chest;10 other symptoms relevant to respiratory or oropharyngeal weakness include slurred speech, dyspnea (with or without exertion), difficulty swallowing, and inability to cough.2,10 Serial respiratory function testing is advisable to detect patients at risk for respiratory failure.30
Diagnosis
Guillain-Barré is a syndrome diagnosed by a collection of symptoms (see Table 22,21,31), including subacute developing paralysis, symmetrical bilateral weakness beginning at onset, and diminishing to absent reflexes.21,31 Other causes for rapidly developing weaknesses should be ruled out (see Table 310,21,26,31). Lumbar puncture typically shows increased protein levels with a normal white cell count; however, neither this test nor electrophysiologic evaluation offers significant value for diagnosis of GBS.21,26,31
During the acute phase of GBS (within three weeks of onset), there is found an elevation of CSF protein (> 550 mg/L) without an elevation in white blood cells. This phenomenon, called albuminocytologic dissociation, reflects inflammation of the nerve roots and is considered the hallmark of GBS.2
MRI can also facilitate the diagnosis of GBS; it demonstrates anterior and posterior intrathecal spinal nerve roots and cauda equina.32 In patients with GBS, evidence supporting breakdown of the blood–nerve barrier can be seen in abnormal gadolinium enhancement of the intrathecal nerve roots on MRI.33
When electrophysiologic studies are performed, they typically reveal slowing nerve conduction, prolonged distal latencies, and partial motor conduction block.34 The characteristic finding of early demyelination is conduction block, a reduction in the amplitude of the muscle action potential after stimulation of the distal, as opposed to the proximal, nerve.28 Nerve conduction studies may help in the diagnosis and classification of GBS—and, to a limited extent, formulation of a prognosis. Such alternative diagnoses as myositis and myasthenia gravis may be excluded by neurophysiology.26 Early in GBS, neurophysiologic abnormalities may be very mild or occasionally normal; test results may not correlate with clinical disability.35,36
The clinician cannot depend on clinical features alone to predict respiratory decline.31 Frequent evaluations of respiratory effort, by measurement of maximal inspiratory pressures and vital capacity, should be performed at the bedside to monitor diaphragmatic strength. Respiratory ventilation should be initiated if the patient becomes hypoxic or experiences a rapid decline in vital capacity (ie, below 60% of predicted value).10 Mechanical ventilation is more likely to be required in patients with a negative inspiratory force of less than 30 cm H2O.31
Treatment
Guillain-Barré syndrome has an acute onset and progression. Patients quickly become nonambulatory and may require total ventilation due to paralysis. Therapeutic options are IVIG or plasmapheresis (plasma exchange).37-40 Corticosteroids do not appear to benefit patients with GBS.41,42
Several mechanisms appear to contribute to the effectiveness of immunoglobulin.38,39 Infused IVIG interferes with antigen presentation, inhibits antibody production, neutralizes pathologic autoantibodies, and modulates other immunologic events involved in the pathogenesis of autoimmune neuromuscular diseases, including GBS.43 Adverse reactions, which are usually minor, include headache, fever, chills, myalgia, and malaise. In rare instances, anaphylaxis or renal failure may occur.15,44
In plasmapheresis, blood is removed from the body and dialyzed, with circulating antibodies and immunoglobulins removed from the plasma; fresh frozen plasma, albumin, or saline is administered. This treatment, performed via central venous catheter, should be initiated as soon as possible after onset of symptoms but can be implemented as late as 30 days after GBS onset. Plasmapheresis requires personnel trained in dialysis, which may not be performed in all hospitals. Possible adverse events include infection and hemorrhage. Laboratory values must be monitored for hypokalemia and hypocalcemia.45,46
Supportive Care
Patients with GBS require intensive care and very close monitoring for complications of respiratory difficulty and autonomic dysfunction. Individualized programs should be initiated for patients in the acute phase of GBS, aimed at the prevention of contractures and skin breakdown.10 Exercise programs, as conducted with the case patient, should also help relieve the fatigue syndromes that accompany GBS.
Immobilization associated with bed rest incurs a risk for pulmonary emboli and DVT; this has been found true during the first 12 weeks after symptom onset in patients with GBS who remain immobile.47 The use of antiembolic hose and sequential compression devices can help reduce the risk for thrombotic events.10 Use of enoxaparin or heparin is recommended for nonambulating patients until they are able to walk, with Gaber et al47 specifying the use of low-molecular-weight heparin to reduce, but not eliminate, the risk for DVT.
The pain associated with GBS can be severe. Narcotic analgesics may be administered with careful monitoring of autonomic denervation. Long-term management of neuropathic pain may require adjuvant therapy, such as tricyclic antidepressants, gabapentin, or tramadol hydrochloride.10 According to Pandey et al,48 gabapentin alone may suffice for pain control in GBS, with minimal adverse effects. In certain rehabilitation facilities, tricyclic antidepressants, capsaicin, and transcutaneous nerve stimulation have been reported effective; during the early stages of treatment, until these treatments reach their full effect, pain medications such as tramadol or narcotics can provide temporary relief.29
More than one-half of patients with GBS in the acute phase can develop ileus. Constipation can also occur as a result of pain medication use, prolonged bed rest, and poor intake. Auscultation of bowel sounds and abdominal assessment should be performed daily to monitor for ileus. Hughes et al10 do not recommend the use of promotility drugs in patients with dysautonomia.
After hospital discharge, easy fatigability can affect work and social activities. With continued physical therapy, occupational therapy, and monitoring, however, patients with GBS can expect to return to an optimal level of functioning. Speed of recovery varies with these patients from a few months to several years, depending on such factors as age and the extent to which axonal degeneration has occurred.6,49
The Case Patient
For several weeks after discharge, the case patient continued to experience fatigue, low back pain, and general muscle pain. With her family’s support, she continued to receive outpatient physical therapy, and within one month she had regained her ankle strength. She was soon able to resume her classes, despite some lingering fatigue.
Conclusion
Guillain-Barré syndrome is a potentially life-threatening disease whose symptoms health care providers need to recognize quickly to provide prompt treatment. Supportive care for both patient and family is of key importance for maximum rehabilitation and return to the previous lifestyle. The clinical course of GBS is highly variable and difficult to predict. The patient’s outcome depends on several factors, including age and severity of illness. GBS patients can experience long-term psychosocial effects.
References
1. Magira EE, Papaioakim M, Nachamkin I, et al. Differential distribution of HLA-DQ beta/DR beta epitopes in the two forms of Guillain-Barré syndrome, acute motor axonal neuropathy and acute inflammatory demyelinating polyneuropathy (AIDP): identification of DQ beta epitopes associated with susceptibility to and protection from AIDP. J Immunol. 2003;170(6):3074-3080.
2. Tremblay ME, Closon A, D’Anjou G, Bussières JF. Guillain-Barré syndrome following H1N1 immunization in a pediatric patient. Ann Pharmacother. 2010;44(7-8):1330-1333.
3. Mukerji S, Aloka F, Farooq MU, et al. Cardiovascular complications of the Guillain-Barré syndrome. Am J Cardiol. 2009;104(10):1452-1455.
4. McGrogan A, Madle GC, Seaman HE, de Vries CS. The epidemiology of Guillain-Barré syndrome worldwide: a systematic literature review. Neuroepidemiology. 2009;32(2):150-163.
5. Haber P, Sejvar J, Mikaeloff Y, DeStefano F. Vaccines and Guillain-Barré syndrome. Drug Saf. 2009; 32(4):309-323.
6. van Doorn PA. What’s new in Guillain-Barré syndrome in 2007-2008? J Periph Nerv Syst. 2009;14(2):72-74.
7. van Doorn PA, Ruts L, Jacobs BC. Clinical features, pathogenesis, and treatment of Guillain-Barré syndrome. Lancet Neurol. 2008;7(10):939-950.
8. Chiò A, Cocito D, Leone M, et al; Piemonte and alle d’Aosta Register for Guillain-Barré Syndrome. Guillain-Barré syndrome: a prospective, population-based incidence and outcome survey. Neurology. 2003; 60(7):1146-1150.
9. Hadden RD, Karch H, Hartung HP, et al. Preceding infections, immune factors, and outcome in Guillain-Barré syndrome. Neurology. 2001;56(6):758-765.
10. Hughes RA, Wijdicks EF, Benson E, et al. Supportive care for patients with Guillain-Barré syndrome. Arch Neurol. 2005;62(8):1194-1198.
11. Aluka KJ, Turner PL, Fullum TM. Guillain-Barré syndrome and postbariatric surgery polyneuropathies. JSLS. 2009;13(2):250-253.
12. Brannagan TH 3rd, Zhou Y. HIV-associated Guillain-Barré syndrome. J Neurol Sci. 2003;208(1-2):39-42.
13. Lin WC, Lee PI, Lu CY, et al. Mycoplasma pneumoniae encephalitis in childhood. J Microbiol Immunol Infect. 2002;35(3):173-178.
14. Sivadon-Tardy V, Orlikowski D, Porcher R, et al. Detection of Campylobacter jejuni by culture and real-time PCR in a French cohort of patients with Guillain-Barre syndrome. J Clin Microbiol. 2010;48 (6):2278-2281.
15. van Doorn PA, Kuitwaard K, Walgaard C, et al. IVIG treatment and prognosis in Guillain-Barré syndrome. J Clin Immunol. 2010;30 suppl 1:S74-S78.
16. Kaida K, Kusunoki S. Guillan-Barré syndrome: update on immunobiology and treatment. Expert Rev Neurother. 2009;9(9):1307-1319.
17. Forsberg A, Press R, Einarsson U, et al. Disability and health-related quality of life in Guillain-Barré syndrome during the first two years after onset: a prospective study. Clin Rehabil. 2005;19(8):900-909.
18. Criteria for diagnosis of Guillain-Barré syndrome. Ann Neurol. 1978;3(6):565-566.
19. van Koningsveld R, Steyerberg EW, Hughes RA, et al. A clinical progostic scoring system for Guillain-Barré syndrome. Lancet Neurol. 2007;6(7):589-594.
20. Koeppen S, Kraywinkel K, Wessendorf TE, et al. Long-term outcome of Guillain-Barré syndrome. Neurocrit Care. 2006;5(3)235-242.
21. Sheridan JM, Smith D. Atypical Guillain-Barré in the emergency department. West J Emerg Med. 2010;11(1):80-82.
22. Ogawara K, Kuwabara S, Koga M, et al. Anti-GM1b IgG antibody is associated with acute motor axonal neuropathy and Campylobacter jejuni infection. J Neurol Sci. 2003;210(1-2):41-45.
23. Nagashima T, Koga M, Odaka M, et al. Continuous spectrum of pharyngeal-cervical-brachial variant of Guillain-Barré syndrome. Arch Neurol. 2007;64(10):1519-1523.
24. Oh SJ, LaGanke C, Claussen GC. Sensory Guillain-Barré syndrome. Neurology. 2001;56(1):82-86.
25. Aráranyi Z, Kovács T, Sipos I, Bereczki D. Miller Fisher syndrome: brief overview and update with a focus on electrophysiological findings. Eur J Neurol. 2011 Jun 1. [Epub ahead of print]
26. Hughes RA, Cornblath, DR. Guillain-Barré syndrome. Lancet. 2005;366(9497):1653-1666.
27. Snyder LA, Rismondo V, Miller NR. The Fisher variant of Guillain-Barré syndrome (Fisher syndrome). J Neuroophthalmol. 2009;29(4):312-324.
28. Ropper AH. The Guillain-Barré syndrome. N Engl J Med.1992;326(17):1130-1136.
29. Meythaler JM. Rehabilitation of Guillain-Barré syndrome. Arch Phys Med Rehabil.1997;78(8):872-879.
30. Sharshar T, Chevret S, Bourdain F, et al; French Cooperative Group on Plasma Exchange in Guillain-Barré syndrome. Early predictors of mechanical ventilation in Guillain-Barré syndrome. Crit Care Med. 2003; 31(1):278-283.
31. McGillicuddy DC, Walker O, Shapiro NI, et al. Guillain-Barré syndrome in the emergency department. Ann Emerg Med. 2006;47(4):390-393.
32. Yikilmaz A, Doganay S, Gumus H, et al. Magnetic resonance imaging of childhood Guillain-Barré syndrome. Childs Nerv Syst. 2010;26(8):1103-1108.
33. Gonzalez-Quevedo A, Carriera RF, O’Farrill ZL, et al. An appraisal of blood-cerebrospinal fluid barrier dysfunction during the course of Guillain-Barré syndrome. Neurol India. 2009;57(3):288-294.
34. Abai S, Kim SB, Kim JP, Lim YJ. Guillan-Barré syndrome combined with acute cervical myelopathy. J Korean Neurosurg Soc. 2010;48(3):298-300.
35. Uncini A, Yuki N. Electrophysiologic and immunopathologic correlates in Guillain-Barré syndrome subtypes. Expert Rev Neurother. 2009;9(6):869-884.
36. Hadden RD, Hughes RA. Management of inflammatory neuropathies. J Neurol Neurosurg Psychiatry. 2003;74 suppl 2:ii9-ii14.
37. Raphaël JC, Chevret S, Hughes RA, Annane D. Plasma exchange for Guillain-Barré syndrome. Cochrane Database Syst Rev. 2002;(2):CD001798.
38. Hughes RA, Swan AV, van Doorn PA. Intravenous immunoglobulin for Guillain-Barré syndrome. Cochrane Database Syst Rev. 2010 Jun 16; (6):CD002063.
39. Human immunoglobulin and the Guillain-Barré syndrome: new indication. An alternative to plasmapheresis. Prescrire Int. 2000;9(49):142-143.
40. van der Meché FG, Schmitz PI; Dutch Guillain-Barré Study Group. A randomized trial comparing intravenous immune globulin and plasma exchange in Guillain-Barré syndrome. N Engl J Med. 1992;327(17):1123-1129.
41. Hughes RA, Swan AV, van Doorn PA. Corticosteroids for Guillain-Barré syndrome. Cochrane Database Syst Rev. 2010 Feb 16;(2):CD001446.
42. Hahn AF. Guillain-Barré syndrome. Lancet. 1998; 352(9128):635-641.
43. Dalakas MC. Intravenous immunoglobulin in autoimmune neuromuscular diseases. JAMA. 2004;291(19):2367-2375.
44. Kuitwaard K, de Gelder J, Tio-Gillen AP, et al. Pharmacokenetics of intravenous immunoglobulin and outcome in Guillain-Barré syndrome. Ann Neurol. 2009;66(5):597-603.
45. Atkinson SB, Carr RL, Maybee P, Haynes D. The challenges of managing and treating Guillain-Barré syndrome during the acute phase. Dimens Crit Care Nurs. 2006;25(6):256-263.
46. van Doorn PA. Treatment of Guillain-Barré syndrome and CIDP. J Periph Nerv Syst. 2005;10(2):113-127.
47. Gaber TA, Kirker SGB, Jenner JR. Current practice of prophylactic anticoagulation in Guillain-Barré syndrome. Clin Rehabil. 2002;16(2):190-193.
48. Pandey CK, Bose N, Garg G, et al. Gabapentin for the treatment of pain in Guillain-Barré syndrome: a double-blinded, placebo-controlled, crossover study. Anesth Analg. 2002;95(6):1719-1723.
49. de Vries JM, Hagemans ML, Bussmann JB, et al. Fatigue in neuromuscular disorders: focus on Guillain-Barré syndrome and Pompe disease. Cell Mol Life Sci. 2010;67(5):701-713.
A 20-year-old woman presented to her primary care clinic with a chief complaint of lower leg weakness and difficulty walking. The weakness she described had been worsening over the previous four days, with progressively worsening tingling and numbness of her toes bilaterally.
The day before the patient presented, she noticed numbness and paresthesia in both calves. At the time of her presentation to the clinic, she complained of low back ache, paresthesia of both hands, numbness bilaterally to her groin, difficulty sitting upright, ataxia, and a numb, thick-feeling tongue. She denied fever, neck stiffness, shortness of breath, headache, or visual changes.
The patient stated that 10 days earlier, she had developed an upper respiratory infection for which she was seen at the clinic and treated with a seven-day course of amoxicillin/clavulanate 875/125 mg twice daily. She said that she had recovered completely.
A review of the patient’s systems revealed proximal muscle weakness bilaterally (2/5) and loss of touch-pressure in the lower extremities. She was experiencing paresthesia of the hands and mild weakness bilaterally (4/5). She also walked with an ataxic gait and had reduced deep tendon reflexes in the lower limbs. All cranial nerves were intact, and her vital signs were stable.
The woman’s medical history was positive only for asthma. Her family history included ischemic stroke in the maternal grandfather and brain tumor in the paternal grandfather. Social history was positive for alcohol intake (ranging from four to 12 beers per week). The patient said she had never smoked or used illicit drugs. She was an unmarried college student, living in a dorm on campus. She participated in track at school.
The patient was admitted to the hospital telemetry step-down unit, and a neurology consultation was requested. Tests were ordered, among them MRI of the head and spine and comprehensive blood work, to rule out neurologic, infectious, or metabolic causes of the patient’s weakness; urinalysis was also obtained. These tests all yielded negative results.
A lumbar puncture performed the following day revealed a cerebrospinal fluid (CSF) protein level of 570 mg/L (normal range, 150 to 450 mg/L). Leukocytes numbered 2 cells/mm3 (normal count, 0 to 10 cells/mm3).
Based on the patient’s presentation, history, and symptoms, a neurologist made a diagnosis of Guillain-Barré syndrome. It was decided that no electromyographic (EMG) study was required to rule out other disease processes (eg, spinal cord disease, multiple sclerosis, tumors).
The patient underwent a five-dose course of immunomodulatory therapy with IV immunoglobulin (IVIG). In the step-down unit, she experienced one incident of sinus bradycardia (ie, resting heart rate between 40 and 50 beats/min). Her blood pressure remained stable, as did her respiratory status, according to peak expiratory flow measured frequently at her bedside.
Physical therapy was initiated, consisting of passive and active range of motion, crossovers with the patient’s feet, and stair training. This was done in response to a complaint of ankle weakness, and it helped to strengthen weakened muscles and improve alignment while the patient was bedridden and in a weakened, fatigued state. Additionally, the patient was given enoxaparin, wore antiembolic hose, and used sequential compression devices while in bed. As a result of these measures, she never experienced a pulmonary embolus or deep vein thrombosis (DVT) as a result of being immobilized.
By the seventh day of hospitalization, the patient had stable vital signs and improved lower limb strength, and numbness was resolving in her hands and lower extremities. She was discharged to home, with physical therapy to resume on an outpatient basis.
Discussion
Guillain-Barré syndrome (GBS), an acute immune-mediated paralytic disorder,1 manifests in the form of weakness and diminished reflexes. Affecting the peripheral nerves, GBS is characterized by progressive symmetrical ascending weakness with varying degrees of sensory complaints.2,3
GBS occurs worldwide, and incidence is estimated between 1.1 and 1.8 cases per 100,000 persons.4 In the United States, GBS can be found in all age-groups, with peak incidence noted in elderly persons and young adults.5,6 Even with treatment, 3% to 10% of patients are reported to die of this illness, and 20% cannot walk six months after symptom onset.7 In one prospective population-based study of patients with confirmed GBS, 6% of patients died within 30 days of symptom onset, often as a result of respiratory complications.8
GBS is a postinfectious disorder, with cases developing several days or weeks after a viral or bacterial illness—most commonly, an upper respiratory infection or diarrhea (see Table 19-13). The most common trigger of GBS is infection with the bacterial microorganism Campylobacter jejuni (occurring in 15% to 40% of patients with GBS),9,14 a pathogen that can produce demyelination-causing antibodies. Other responsible pathogens include cytomegalovirus and Epstein-Barr virus.9 In a process called molecular mimicry, the immune system is unable to distinguish the amino acid of an infectious organism from the proteinaceous content of the peripheral nerve.15 Subsequently, the immune system attacks and destroys the myelin sheath.
An example of this is the apparent cross-reaction of the ganglioside GM1 with C jejuni lipopolysaccharide antigens.14,15 The resulting effect is immunologic damage to the peripheral nervous system. The flaccid paralysis that occurs in patients with GBS is thought to be caused by lymphocytic infiltration and complement activation of the spinal roots and peripheral nerves, where macrophages strip the myelin.5,15,16
Stages and Variants
Three stages characterize the course of GBS. The acute phase, which lasts one to four weeks, begins with onset of symptoms and persists until the associated neurologic deterioration has ceased. During the second phase, the plateau period, symptoms persist with no further deterioration; this stage can last several days to several weeks or months. The final phase, the recovery period, can last from four months to two years after symptom onset.15,17,18
The clinical course of GBS is highly variable and in many cases difficult to predict. Certain factors have been associated with a poor outcome: advancing age, previous presence of diarrhea, need for mechanical ventilation, an extended plateau phase, and a lower patient score on the Erasmus GBS Outcome Scale,19 when measured two weeks after GBS onset.8,20 This score can help predict the patient’s chance of independent walking after six months.15,19
Although the classic presenting symptom of GBS is symmetric ascending weakness, several disease variants have been identified, with differing symptoms and degrees of recovery. These variants also differ in terms of the muscle groups affected; in some, visual defects may be present at onset. GBS variants include21:
• Acute motor axonal neuropathy (AMAN)1,22
• Acute inflammatory demyelinating polyneuropathy (AIDP)1
• Pharyngeal-cervical-brachial variant23
• Purely sensory variant24
• Miller-Fisher syndrome, which manifests with ophthalmoplegia, in addition to ataxia and areflexia25
• Axonal form.5,21
AMAN and AIDP are the most common subtypes of GBS.1
Symptoms, Signs, and Disease Manifestations
Limb weakness, the classic presenting symptom of GBS, is both symmetrical and ascending. Weakness can develop acutely and progress over days to weeks.2,15 Hughes and Cornblath26 also note pain, numbness, and paresthesias among the initial symptoms of GBS. Others include sensory changes, cranial nerve involvement, various autonomic changes, and respiratory or oropharyngeal weakness. Reflexes, particularly the tendon reflexes, may be diminished or absent.15,18,21 In many cases, sensory changes (ie, pain) may precede the onset of weakness, often making diagnosis difficult.15
Cranial nerves most commonly affected are V, VI, VII, X, XI, and XII, with manifestations that include dysphagia, dysarthria, diplopia, limitation to eye movements, and facial droop and weakness. Usually facial and oropharyngeal weakness occur after the extremities and trunk are affected. Blindness may occur if demyelination of the optic nerve occurs; this is seen in Miller-Fisher syndrome.10,15,25,27
In GBS, many patients report pain, which can present as bilateral sciatica or as throbbing or aching in the large muscles of the upper legs, flanks, or back.28 This pain, which results from the demyelination of the sensory nerve fibers, can be severe.10
Patients with GBS may experience manifestations of autonomic nervous system dysfunction—for example, arrhythmias, hypotension or hypertension, urinary retention, cardiomyopathy, and paralytic ileus.10,20 Dysautonomia often impedes patients’ progress in inpatient rehabilitation. Patients may have persistent problems involving postural hypotension, hypertension, excessive sympathetic outflow, or bladder and bowel dysfunction.29
Blood pressure fluctuations, often attributed to changes in catecholamine levels and disturbances in the baroreceptor reflex pathway, are common and are considered characteristic of GBS. Transient or persistent hypotension is caused by the dysregulation of the parasympathetic and sympathetic systems, with subsequent alterations in venomotor tone.3 Additionally, an increased sensitivity to catecholamine can lead to cardiovascular disturbances, resulting in denervation hypersensitivity and impairment of the carotid sinus reflex.
Arrhythmias occur in perhaps half of patients with GBS. The most common is sustained sinus tachycardia, which usually requires no treatment. Bradycardia leading to atrioventricular blocks and asystole is believed to result from afferent baroreceptor reflex failure. Treatment may be required—either administration of atropine or insertion of a pacemaker, depending on the severity of the arrhythmia.3,10
Myocardial involvement can range from asymptomatic mycocarditis to neurogenic stunned myocardium and heart failure. Patients with ECG abnormalities should undergo two-dimensional echocardiographic studies and other testing to explore cardiac involvement. Acute coronary syndromes, including ST-segment elevation MI, have been reported, in some cases associated with IVIG treatment. In one patient, coronary spasm was reported, with clean coronary arteries found on cardiac catheterization.3
Patients with GBS are at risk for compromised neuromuscular respiratory function; demyelination of the nerves that innervate the intercostal muscles and the diaphragm can result in respiratory failure. Key clinical indicators of respiratory muscle fatigue include tachypnea, diaphoresis, and asynchronous movements of the abdomen and chest;10 other symptoms relevant to respiratory or oropharyngeal weakness include slurred speech, dyspnea (with or without exertion), difficulty swallowing, and inability to cough.2,10 Serial respiratory function testing is advisable to detect patients at risk for respiratory failure.30
Diagnosis
Guillain-Barré is a syndrome diagnosed by a collection of symptoms (see Table 22,21,31), including subacute developing paralysis, symmetrical bilateral weakness beginning at onset, and diminishing to absent reflexes.21,31 Other causes for rapidly developing weaknesses should be ruled out (see Table 310,21,26,31). Lumbar puncture typically shows increased protein levels with a normal white cell count; however, neither this test nor electrophysiologic evaluation offers significant value for diagnosis of GBS.21,26,31
During the acute phase of GBS (within three weeks of onset), there is found an elevation of CSF protein (> 550 mg/L) without an elevation in white blood cells. This phenomenon, called albuminocytologic dissociation, reflects inflammation of the nerve roots and is considered the hallmark of GBS.2
MRI can also facilitate the diagnosis of GBS; it demonstrates anterior and posterior intrathecal spinal nerve roots and cauda equina.32 In patients with GBS, evidence supporting breakdown of the blood–nerve barrier can be seen in abnormal gadolinium enhancement of the intrathecal nerve roots on MRI.33
When electrophysiologic studies are performed, they typically reveal slowing nerve conduction, prolonged distal latencies, and partial motor conduction block.34 The characteristic finding of early demyelination is conduction block, a reduction in the amplitude of the muscle action potential after stimulation of the distal, as opposed to the proximal, nerve.28 Nerve conduction studies may help in the diagnosis and classification of GBS—and, to a limited extent, formulation of a prognosis. Such alternative diagnoses as myositis and myasthenia gravis may be excluded by neurophysiology.26 Early in GBS, neurophysiologic abnormalities may be very mild or occasionally normal; test results may not correlate with clinical disability.35,36
The clinician cannot depend on clinical features alone to predict respiratory decline.31 Frequent evaluations of respiratory effort, by measurement of maximal inspiratory pressures and vital capacity, should be performed at the bedside to monitor diaphragmatic strength. Respiratory ventilation should be initiated if the patient becomes hypoxic or experiences a rapid decline in vital capacity (ie, below 60% of predicted value).10 Mechanical ventilation is more likely to be required in patients with a negative inspiratory force of less than 30 cm H2O.31
Treatment
Guillain-Barré syndrome has an acute onset and progression. Patients quickly become nonambulatory and may require total ventilation due to paralysis. Therapeutic options are IVIG or plasmapheresis (plasma exchange).37-40 Corticosteroids do not appear to benefit patients with GBS.41,42
Several mechanisms appear to contribute to the effectiveness of immunoglobulin.38,39 Infused IVIG interferes with antigen presentation, inhibits antibody production, neutralizes pathologic autoantibodies, and modulates other immunologic events involved in the pathogenesis of autoimmune neuromuscular diseases, including GBS.43 Adverse reactions, which are usually minor, include headache, fever, chills, myalgia, and malaise. In rare instances, anaphylaxis or renal failure may occur.15,44
In plasmapheresis, blood is removed from the body and dialyzed, with circulating antibodies and immunoglobulins removed from the plasma; fresh frozen plasma, albumin, or saline is administered. This treatment, performed via central venous catheter, should be initiated as soon as possible after onset of symptoms but can be implemented as late as 30 days after GBS onset. Plasmapheresis requires personnel trained in dialysis, which may not be performed in all hospitals. Possible adverse events include infection and hemorrhage. Laboratory values must be monitored for hypokalemia and hypocalcemia.45,46
Supportive Care
Patients with GBS require intensive care and very close monitoring for complications of respiratory difficulty and autonomic dysfunction. Individualized programs should be initiated for patients in the acute phase of GBS, aimed at the prevention of contractures and skin breakdown.10 Exercise programs, as conducted with the case patient, should also help relieve the fatigue syndromes that accompany GBS.
Immobilization associated with bed rest incurs a risk for pulmonary emboli and DVT; this has been found true during the first 12 weeks after symptom onset in patients with GBS who remain immobile.47 The use of antiembolic hose and sequential compression devices can help reduce the risk for thrombotic events.10 Use of enoxaparin or heparin is recommended for nonambulating patients until they are able to walk, with Gaber et al47 specifying the use of low-molecular-weight heparin to reduce, but not eliminate, the risk for DVT.
The pain associated with GBS can be severe. Narcotic analgesics may be administered with careful monitoring of autonomic denervation. Long-term management of neuropathic pain may require adjuvant therapy, such as tricyclic antidepressants, gabapentin, or tramadol hydrochloride.10 According to Pandey et al,48 gabapentin alone may suffice for pain control in GBS, with minimal adverse effects. In certain rehabilitation facilities, tricyclic antidepressants, capsaicin, and transcutaneous nerve stimulation have been reported effective; during the early stages of treatment, until these treatments reach their full effect, pain medications such as tramadol or narcotics can provide temporary relief.29
More than one-half of patients with GBS in the acute phase can develop ileus. Constipation can also occur as a result of pain medication use, prolonged bed rest, and poor intake. Auscultation of bowel sounds and abdominal assessment should be performed daily to monitor for ileus. Hughes et al10 do not recommend the use of promotility drugs in patients with dysautonomia.
After hospital discharge, easy fatigability can affect work and social activities. With continued physical therapy, occupational therapy, and monitoring, however, patients with GBS can expect to return to an optimal level of functioning. Speed of recovery varies with these patients from a few months to several years, depending on such factors as age and the extent to which axonal degeneration has occurred.6,49
The Case Patient
For several weeks after discharge, the case patient continued to experience fatigue, low back pain, and general muscle pain. With her family’s support, she continued to receive outpatient physical therapy, and within one month she had regained her ankle strength. She was soon able to resume her classes, despite some lingering fatigue.
Conclusion
Guillain-Barré syndrome is a potentially life-threatening disease whose symptoms health care providers need to recognize quickly to provide prompt treatment. Supportive care for both patient and family is of key importance for maximum rehabilitation and return to the previous lifestyle. The clinical course of GBS is highly variable and difficult to predict. The patient’s outcome depends on several factors, including age and severity of illness. GBS patients can experience long-term psychosocial effects.
References
1. Magira EE, Papaioakim M, Nachamkin I, et al. Differential distribution of HLA-DQ beta/DR beta epitopes in the two forms of Guillain-Barré syndrome, acute motor axonal neuropathy and acute inflammatory demyelinating polyneuropathy (AIDP): identification of DQ beta epitopes associated with susceptibility to and protection from AIDP. J Immunol. 2003;170(6):3074-3080.
2. Tremblay ME, Closon A, D’Anjou G, Bussières JF. Guillain-Barré syndrome following H1N1 immunization in a pediatric patient. Ann Pharmacother. 2010;44(7-8):1330-1333.
3. Mukerji S, Aloka F, Farooq MU, et al. Cardiovascular complications of the Guillain-Barré syndrome. Am J Cardiol. 2009;104(10):1452-1455.
4. McGrogan A, Madle GC, Seaman HE, de Vries CS. The epidemiology of Guillain-Barré syndrome worldwide: a systematic literature review. Neuroepidemiology. 2009;32(2):150-163.
5. Haber P, Sejvar J, Mikaeloff Y, DeStefano F. Vaccines and Guillain-Barré syndrome. Drug Saf. 2009; 32(4):309-323.
6. van Doorn PA. What’s new in Guillain-Barré syndrome in 2007-2008? J Periph Nerv Syst. 2009;14(2):72-74.
7. van Doorn PA, Ruts L, Jacobs BC. Clinical features, pathogenesis, and treatment of Guillain-Barré syndrome. Lancet Neurol. 2008;7(10):939-950.
8. Chiò A, Cocito D, Leone M, et al; Piemonte and alle d’Aosta Register for Guillain-Barré Syndrome. Guillain-Barré syndrome: a prospective, population-based incidence and outcome survey. Neurology. 2003; 60(7):1146-1150.
9. Hadden RD, Karch H, Hartung HP, et al. Preceding infections, immune factors, and outcome in Guillain-Barré syndrome. Neurology. 2001;56(6):758-765.
10. Hughes RA, Wijdicks EF, Benson E, et al. Supportive care for patients with Guillain-Barré syndrome. Arch Neurol. 2005;62(8):1194-1198.
11. Aluka KJ, Turner PL, Fullum TM. Guillain-Barré syndrome and postbariatric surgery polyneuropathies. JSLS. 2009;13(2):250-253.
12. Brannagan TH 3rd, Zhou Y. HIV-associated Guillain-Barré syndrome. J Neurol Sci. 2003;208(1-2):39-42.
13. Lin WC, Lee PI, Lu CY, et al. Mycoplasma pneumoniae encephalitis in childhood. J Microbiol Immunol Infect. 2002;35(3):173-178.
14. Sivadon-Tardy V, Orlikowski D, Porcher R, et al. Detection of Campylobacter jejuni by culture and real-time PCR in a French cohort of patients with Guillain-Barre syndrome. J Clin Microbiol. 2010;48 (6):2278-2281.
15. van Doorn PA, Kuitwaard K, Walgaard C, et al. IVIG treatment and prognosis in Guillain-Barré syndrome. J Clin Immunol. 2010;30 suppl 1:S74-S78.
16. Kaida K, Kusunoki S. Guillan-Barré syndrome: update on immunobiology and treatment. Expert Rev Neurother. 2009;9(9):1307-1319.
17. Forsberg A, Press R, Einarsson U, et al. Disability and health-related quality of life in Guillain-Barré syndrome during the first two years after onset: a prospective study. Clin Rehabil. 2005;19(8):900-909.
18. Criteria for diagnosis of Guillain-Barré syndrome. Ann Neurol. 1978;3(6):565-566.
19. van Koningsveld R, Steyerberg EW, Hughes RA, et al. A clinical progostic scoring system for Guillain-Barré syndrome. Lancet Neurol. 2007;6(7):589-594.
20. Koeppen S, Kraywinkel K, Wessendorf TE, et al. Long-term outcome of Guillain-Barré syndrome. Neurocrit Care. 2006;5(3)235-242.
21. Sheridan JM, Smith D. Atypical Guillain-Barré in the emergency department. West J Emerg Med. 2010;11(1):80-82.
22. Ogawara K, Kuwabara S, Koga M, et al. Anti-GM1b IgG antibody is associated with acute motor axonal neuropathy and Campylobacter jejuni infection. J Neurol Sci. 2003;210(1-2):41-45.
23. Nagashima T, Koga M, Odaka M, et al. Continuous spectrum of pharyngeal-cervical-brachial variant of Guillain-Barré syndrome. Arch Neurol. 2007;64(10):1519-1523.
24. Oh SJ, LaGanke C, Claussen GC. Sensory Guillain-Barré syndrome. Neurology. 2001;56(1):82-86.
25. Aráranyi Z, Kovács T, Sipos I, Bereczki D. Miller Fisher syndrome: brief overview and update with a focus on electrophysiological findings. Eur J Neurol. 2011 Jun 1. [Epub ahead of print]
26. Hughes RA, Cornblath, DR. Guillain-Barré syndrome. Lancet. 2005;366(9497):1653-1666.
27. Snyder LA, Rismondo V, Miller NR. The Fisher variant of Guillain-Barré syndrome (Fisher syndrome). J Neuroophthalmol. 2009;29(4):312-324.
28. Ropper AH. The Guillain-Barré syndrome. N Engl J Med.1992;326(17):1130-1136.
29. Meythaler JM. Rehabilitation of Guillain-Barré syndrome. Arch Phys Med Rehabil.1997;78(8):872-879.
30. Sharshar T, Chevret S, Bourdain F, et al; French Cooperative Group on Plasma Exchange in Guillain-Barré syndrome. Early predictors of mechanical ventilation in Guillain-Barré syndrome. Crit Care Med. 2003; 31(1):278-283.
31. McGillicuddy DC, Walker O, Shapiro NI, et al. Guillain-Barré syndrome in the emergency department. Ann Emerg Med. 2006;47(4):390-393.
32. Yikilmaz A, Doganay S, Gumus H, et al. Magnetic resonance imaging of childhood Guillain-Barré syndrome. Childs Nerv Syst. 2010;26(8):1103-1108.
33. Gonzalez-Quevedo A, Carriera RF, O’Farrill ZL, et al. An appraisal of blood-cerebrospinal fluid barrier dysfunction during the course of Guillain-Barré syndrome. Neurol India. 2009;57(3):288-294.
34. Abai S, Kim SB, Kim JP, Lim YJ. Guillan-Barré syndrome combined with acute cervical myelopathy. J Korean Neurosurg Soc. 2010;48(3):298-300.
35. Uncini A, Yuki N. Electrophysiologic and immunopathologic correlates in Guillain-Barré syndrome subtypes. Expert Rev Neurother. 2009;9(6):869-884.
36. Hadden RD, Hughes RA. Management of inflammatory neuropathies. J Neurol Neurosurg Psychiatry. 2003;74 suppl 2:ii9-ii14.
37. Raphaël JC, Chevret S, Hughes RA, Annane D. Plasma exchange for Guillain-Barré syndrome. Cochrane Database Syst Rev. 2002;(2):CD001798.
38. Hughes RA, Swan AV, van Doorn PA. Intravenous immunoglobulin for Guillain-Barré syndrome. Cochrane Database Syst Rev. 2010 Jun 16; (6):CD002063.
39. Human immunoglobulin and the Guillain-Barré syndrome: new indication. An alternative to plasmapheresis. Prescrire Int. 2000;9(49):142-143.
40. van der Meché FG, Schmitz PI; Dutch Guillain-Barré Study Group. A randomized trial comparing intravenous immune globulin and plasma exchange in Guillain-Barré syndrome. N Engl J Med. 1992;327(17):1123-1129.
41. Hughes RA, Swan AV, van Doorn PA. Corticosteroids for Guillain-Barré syndrome. Cochrane Database Syst Rev. 2010 Feb 16;(2):CD001446.
42. Hahn AF. Guillain-Barré syndrome. Lancet. 1998; 352(9128):635-641.
43. Dalakas MC. Intravenous immunoglobulin in autoimmune neuromuscular diseases. JAMA. 2004;291(19):2367-2375.
44. Kuitwaard K, de Gelder J, Tio-Gillen AP, et al. Pharmacokenetics of intravenous immunoglobulin and outcome in Guillain-Barré syndrome. Ann Neurol. 2009;66(5):597-603.
45. Atkinson SB, Carr RL, Maybee P, Haynes D. The challenges of managing and treating Guillain-Barré syndrome during the acute phase. Dimens Crit Care Nurs. 2006;25(6):256-263.
46. van Doorn PA. Treatment of Guillain-Barré syndrome and CIDP. J Periph Nerv Syst. 2005;10(2):113-127.
47. Gaber TA, Kirker SGB, Jenner JR. Current practice of prophylactic anticoagulation in Guillain-Barré syndrome. Clin Rehabil. 2002;16(2):190-193.
48. Pandey CK, Bose N, Garg G, et al. Gabapentin for the treatment of pain in Guillain-Barré syndrome: a double-blinded, placebo-controlled, crossover study. Anesth Analg. 2002;95(6):1719-1723.
49. de Vries JM, Hagemans ML, Bussmann JB, et al. Fatigue in neuromuscular disorders: focus on Guillain-Barré syndrome and Pompe disease. Cell Mol Life Sci. 2010;67(5):701-713.