User login
UPDATE ON OVARIAN CANCER
- Update on ovarian cancer screening
David G. Mutch, MD; Nora Kizer, MD (July 2010)
A majority of ovarian cancers are diagnosed at an advanced stage, requiring extensive surgical cytoreductive procedures.1 Because the presence of residual macroscopic disease correlates highly with decreased survival,2 these procedures can be lengthy, complicated, and risky for the patient. Many patients who undergo cytoreduction will be left with a suboptimal result despite surgery.
Better identification and improved treatment of patients who are at high risk of a suboptimal result are clearly needed. One treatment option is neoadjuvant chemotherapy, the administration of chemotherapy prior to the main treatment. Although early data suggested that it was associated with worse outcomes, recent studies have yielded new information:
- Neoadjuvant chemotherapy followed by interval debulking surgery is not inferior to primary debulking surgery followed by chemotherapy for patients who have bulky stage III or IV ovarian cancer
- In patients who have advanced ovarian cancer, neoadjuvant chemotherapy followed by surgical cytoreduction is associated with improved perioperative outcomes
- Postoperative intraperitoneal chemotherapy after neoadjuvant chemotherapy has not yet proved to be associated with improved survival.
Several questions prompted by these findings include:
- Will neoadjuvant chemotherapy improve surgical outcomes in patients who have advanced ovarian cancer and, thus, improve survival?
- Is neoadjuvant chemotherapy a better strategy for all patients?
- Will neoadjuvant chemotherapy reduce the surgical effort necessary to achieve an optimal result?
- What is the role of intraperitoneal chemotherapy in patients who undergo neoadjuvant chemotherapy?
Further national (or international) data are needed to confirm a survival advantage for patients who receive neoadjuvant chemotherapy, compared with those who undergo primary surgery before the administration of chemotherapy.
Neoadjuvant chemotherapy is an acceptable alternative to primary surgical cytoreduction
Vergote I, Tropé CG, Amant F, et al; European Organization for Research and Treatment of Cancer-Gynaecological Cancer Group; NCIC Clinical Trials Group. Neoadjuvant chemotherapy or primary surgery in stage IIIC or IV ovarian cancer. N Engl J Med. 2010;363(10):943–953.
Historically, the standard of care in ovarian cancer treatment has been surgical cytoreduction followed by chemotherapy.3-6 However, data from prospective randomized trials to support this practice are limited. Neoadjuvant chemotherapy is an alternative strategy that has been explored as a way to improve outcomes from interval surgical debulking in patients who have ovarian cancer in whom suboptimal cytoreduction is otherwise expected. Vergote and coworkers attempted to determine which strategy is better through a randomized trial of 632 patients.
Participants had to have biopsy-proven stage IIIc or IV ovarian, fallopian tube, or primary peritoneal cancer. The two treatment arms were:
- primary debulking surgery followed by at least 6 cycles of platinum-based chemotherapy
- 3 cycles of platinum-based neoadjuvant chemotherapy followed by interval debulking surgery in responders and those who had stable disease. These patients then received an additional 3 cycles of platinum-based chemotherapy post-operatively.
All surgical procedures were completed by qualified gynecologic oncologists, and all patients were evaluated for eligibility before randomization, with no additional selection criteria.
Postoperative death occurred in 2.5% of patients in the primary-surgery group, compared with 0.7% of patients in the neoadjuvant-chemotherapy group. Grade 3 or 4 hemorrhage occurred in 7.4% of patients after primary debulking, compared with 4.1% of patients after interval debulking. Patients who received neoadjuvant chemotherapy experienced a lower rate of infection (1.7% versus 8.1%) and venous complications (0% versus 2.6%).
Overall and progression-free survival rates were similar between the two groups. After multivariate analysis, the strongest predictors of survival were absence of residual disease after surgery (P<.001), small tumor size before randomization (P=.001), and endometrioid histology (P=.001)
Neoadjuvant chemotherapy is a preferred treatment strategy for patients who are expected to have a suboptimal result after surgery. Because neoadjuvant chemotherapy has a survival outcome similar to that of primary surgery followed by chemotherapy, it may be considered for all patients who have bulky stage IIIc or IV disease.
Although neoadjuvant chemotherapy improves the rate of optimal surgical cytoreduction, data are lacking to demonstrate that this improvement boosts survival.
Administration of neoadjuvant chemotherapy in these patients may improve perioperative morbidity and mortality, although no formal analysis was conducted in this study.
Neoadjuvant chemotherapy improves perioperative outcomes
Milam MR, Tao X, Coleman RL, et al. Neoadjuvant chemotherapy is associated with prolonged primary treatment intervals in patients with advanced epithelial ovarian cancer. Int J Gynecol Cancer. 2011;21(1):66–71.
Milam and coworkers investigated chemotherapy-associated morbidity and timing in two groups of patients who had advanced epithelial ovarian cancer:
- those undergoing neoadjuvant chemotherapy followed by maximal cytoreductive surgery
- those undergoing primary surgery followed by chemotherapy.
Their retrospective study involved 263 consecutive patients who were treated at MD Anderson Cancer Center from 1993 to 2005. In this cohort, 47 women (18%) received neoadjuvant chemotherapy. These patients experienced less blood loss (400 mL versus 750 mL) and a shorter hospital stay (6 versus 8 days). Time to the initiation of chemotherapy from the date of diagnosis did not differ between groups, and the amount of residual disease and rate of survival were also similar between arms. However, patients who received neoadjuvant chemotherapy underwent more cycles of chemotherapy over a longer treatment period.
Although neoadjuvant chemotherapy does not appear to offer a survival advantage, it is equivalent to primary surgery followed by adjuvant chemotherapy and may be associated with improved perioperative outcomes.
The results of the trial by Vergote and colleagues (page 25), should discourage oncologists from prescribing more than 6 cycles of chemotherapy in the neoadjuvant setting; patients from their study in the neoadjuvant group received a total of 6 cycles and had survival outcomes equivalent to those of women in the primary surgery group.
In the pipeline: Data on intraperitoneal chemotherapy after neoadjuvant chemotherapy
Le T, Latifah H, Jolicoeur L, et al. Does intraperitoneal chemotherapy benefit optimally debulked epithelial ovarian cancer patients after neoadjuvant chemotherapy? Gynecol Oncol. 2011;121(3):451–454.
Although several studies have demonstrated that intraperitoneal (IP) chemotherapy provides a survival advantage, compared with intravenous (IV) chemotherapy, after primary surgical debulking, it remains unclear whether IP chemotherapy would provide a similar superior survival outcome following neoadjuvant chemotherapy (FIGURE).
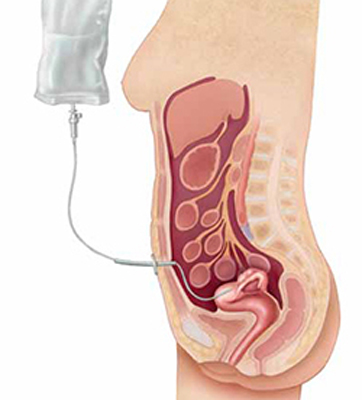
Intraperitoneal chemotherapy: How efficacious?
The jury is still out on whether intraperitoneal chemotherapy improves survival after neoadjuvant chemotherapy and interval debulking in stages III and IV ovarian cancer.The authors of this paper attempted to answer this question through a retrospective review of 71 patients. All patients were treated with neoadjuvant chemotherapy followed by interval debulking and either IP or IV chemotherapy. Overall, 17 patients (24%) received IP chemotherapy, and 54 patients (76%) received IV chemotherapy. The median number of cycles given prior to and after surgery was the same for both groups (3 for both neoadjuvant chemotherapy and chemotherapy following surgery).
Although patients who received IP chemotherapy had a higher overall response rate (82% versus 67%), there were no differences between groups in terms of progression-free (P=.42) and overall survival (P=.72).
One important limitation of this study was its small sample size and lack of statistical power. In addition, more patients in the IP group had macroscopic residual disease than in the IV group (71% versus 52%; P=.17).
A phase II/III study is under way to evaluate the use of IP chemotherapy following neoadjuvant chemotherapy in ovarian cancer patients.7 The two-stage randomized trial will compare IV chemotherapy with platinum-based IP chemotherapy in women who have undergone optimal surgical debulking (>1 cm) after 3 to 4 cycles of platinum-based neoadjuvant chemotherapy. This study is led by the US National Cancer Institute in collaboration with the Society of Gynecologic Oncologists of Canada, the UK National Cancer Research Institute, the Spanish Ovarian Cancer Research Group, and the US Southwest Oncology Group.
Data are limited on the use of intraperitoneal (IP) chemotherapy following neoadjuvant chemotherapy and interval surgical cytoreduction. We await the results of larger prospective studies to definitively determine whether there is a role for IP chemotherapy in this setting. For now, patients who receive neoadjuvant chemotherapy are limited to IV chemotherapy following surgery.
We want to hear from you! Tell us what you think.
1. Howlader N, Noone AM, Krapcho M, et all. eds. SEER Cancer Statistics Review 1975-2008. National Cancer Institute. http://seer.cancer.gov/csr/1975_2008. Published April 15, 2011. Accessed June 10, 2011.
2. du Bois A, Ruess A, Pujade-Lauraine E, Harter P, Ray-Coquard I, Pfisterer J. Role of surgical outcome as prognostic factor in advanced epithelial ovarian cancer: a combined exploratory analysis of 3 prospectively randomized phase 3 multicenter trials; by the Arbeitsgemeinschaft Gynaekologische Onkologie Studiengruppe Ovarialkarzinom (AGO-OVAR) and the Groupe d’Investigateurs Nationaux Pour les Etudes des Cancers de l’Ovaire (GINECO). Cancer. 2009;115(6):1234-1244.
3. Meigs JV. Tumors of the pelvic organs. New York: Macmillan: 1934.
4. Aure JC, Hoeg K, Kolstad P. Clinical and histologic studies of ovarian carcinoma. Long-term follow-up of 990 cases. Obstet Gynecol. 1971;37(1):1-9.
5. Griffiths CT, Fuller AF. Intensive surgical and chemotherapeutic management of advanced ovarian cancer. Surg Clin North Am. 1978;58(1):131-142.
6. du Bois A, Quinn M, Thigpen T, et al. 2004 Consensus statements on the management of ovarian cancer: final document of the 3rd International Gynecologic Cancer Intergroup Ovarian Cancer Consensus Conference (GCIG OCCC 2004). Ann Oncol. 2005;16(suppl 8):viii7-viii12.
7. Mackay HJ, Provencheur D, Heywood M, et al. Phase II/III study of intraperitoneal chemotherapy after neoadjuvant chemotherapy for ovarian cancer: ncic ctg ov.21. Curr Oncol. 2011;18(2):84-90.
- Update on ovarian cancer screening
David G. Mutch, MD; Nora Kizer, MD (July 2010)
A majority of ovarian cancers are diagnosed at an advanced stage, requiring extensive surgical cytoreductive procedures.1 Because the presence of residual macroscopic disease correlates highly with decreased survival,2 these procedures can be lengthy, complicated, and risky for the patient. Many patients who undergo cytoreduction will be left with a suboptimal result despite surgery.
Better identification and improved treatment of patients who are at high risk of a suboptimal result are clearly needed. One treatment option is neoadjuvant chemotherapy, the administration of chemotherapy prior to the main treatment. Although early data suggested that it was associated with worse outcomes, recent studies have yielded new information:
- Neoadjuvant chemotherapy followed by interval debulking surgery is not inferior to primary debulking surgery followed by chemotherapy for patients who have bulky stage III or IV ovarian cancer
- In patients who have advanced ovarian cancer, neoadjuvant chemotherapy followed by surgical cytoreduction is associated with improved perioperative outcomes
- Postoperative intraperitoneal chemotherapy after neoadjuvant chemotherapy has not yet proved to be associated with improved survival.
Several questions prompted by these findings include:
- Will neoadjuvant chemotherapy improve surgical outcomes in patients who have advanced ovarian cancer and, thus, improve survival?
- Is neoadjuvant chemotherapy a better strategy for all patients?
- Will neoadjuvant chemotherapy reduce the surgical effort necessary to achieve an optimal result?
- What is the role of intraperitoneal chemotherapy in patients who undergo neoadjuvant chemotherapy?
Further national (or international) data are needed to confirm a survival advantage for patients who receive neoadjuvant chemotherapy, compared with those who undergo primary surgery before the administration of chemotherapy.
Neoadjuvant chemotherapy is an acceptable alternative to primary surgical cytoreduction
Vergote I, Tropé CG, Amant F, et al; European Organization for Research and Treatment of Cancer-Gynaecological Cancer Group; NCIC Clinical Trials Group. Neoadjuvant chemotherapy or primary surgery in stage IIIC or IV ovarian cancer. N Engl J Med. 2010;363(10):943–953.
Historically, the standard of care in ovarian cancer treatment has been surgical cytoreduction followed by chemotherapy.3-6 However, data from prospective randomized trials to support this practice are limited. Neoadjuvant chemotherapy is an alternative strategy that has been explored as a way to improve outcomes from interval surgical debulking in patients who have ovarian cancer in whom suboptimal cytoreduction is otherwise expected. Vergote and coworkers attempted to determine which strategy is better through a randomized trial of 632 patients.
Participants had to have biopsy-proven stage IIIc or IV ovarian, fallopian tube, or primary peritoneal cancer. The two treatment arms were:
- primary debulking surgery followed by at least 6 cycles of platinum-based chemotherapy
- 3 cycles of platinum-based neoadjuvant chemotherapy followed by interval debulking surgery in responders and those who had stable disease. These patients then received an additional 3 cycles of platinum-based chemotherapy post-operatively.
All surgical procedures were completed by qualified gynecologic oncologists, and all patients were evaluated for eligibility before randomization, with no additional selection criteria.
Postoperative death occurred in 2.5% of patients in the primary-surgery group, compared with 0.7% of patients in the neoadjuvant-chemotherapy group. Grade 3 or 4 hemorrhage occurred in 7.4% of patients after primary debulking, compared with 4.1% of patients after interval debulking. Patients who received neoadjuvant chemotherapy experienced a lower rate of infection (1.7% versus 8.1%) and venous complications (0% versus 2.6%).
Overall and progression-free survival rates were similar between the two groups. After multivariate analysis, the strongest predictors of survival were absence of residual disease after surgery (P<.001), small tumor size before randomization (P=.001), and endometrioid histology (P=.001)
Neoadjuvant chemotherapy is a preferred treatment strategy for patients who are expected to have a suboptimal result after surgery. Because neoadjuvant chemotherapy has a survival outcome similar to that of primary surgery followed by chemotherapy, it may be considered for all patients who have bulky stage IIIc or IV disease.
Although neoadjuvant chemotherapy improves the rate of optimal surgical cytoreduction, data are lacking to demonstrate that this improvement boosts survival.
Administration of neoadjuvant chemotherapy in these patients may improve perioperative morbidity and mortality, although no formal analysis was conducted in this study.
Neoadjuvant chemotherapy improves perioperative outcomes
Milam MR, Tao X, Coleman RL, et al. Neoadjuvant chemotherapy is associated with prolonged primary treatment intervals in patients with advanced epithelial ovarian cancer. Int J Gynecol Cancer. 2011;21(1):66–71.
Milam and coworkers investigated chemotherapy-associated morbidity and timing in two groups of patients who had advanced epithelial ovarian cancer:
- those undergoing neoadjuvant chemotherapy followed by maximal cytoreductive surgery
- those undergoing primary surgery followed by chemotherapy.
Their retrospective study involved 263 consecutive patients who were treated at MD Anderson Cancer Center from 1993 to 2005. In this cohort, 47 women (18%) received neoadjuvant chemotherapy. These patients experienced less blood loss (400 mL versus 750 mL) and a shorter hospital stay (6 versus 8 days). Time to the initiation of chemotherapy from the date of diagnosis did not differ between groups, and the amount of residual disease and rate of survival were also similar between arms. However, patients who received neoadjuvant chemotherapy underwent more cycles of chemotherapy over a longer treatment period.
Although neoadjuvant chemotherapy does not appear to offer a survival advantage, it is equivalent to primary surgery followed by adjuvant chemotherapy and may be associated with improved perioperative outcomes.
The results of the trial by Vergote and colleagues (page 25), should discourage oncologists from prescribing more than 6 cycles of chemotherapy in the neoadjuvant setting; patients from their study in the neoadjuvant group received a total of 6 cycles and had survival outcomes equivalent to those of women in the primary surgery group.
In the pipeline: Data on intraperitoneal chemotherapy after neoadjuvant chemotherapy
Le T, Latifah H, Jolicoeur L, et al. Does intraperitoneal chemotherapy benefit optimally debulked epithelial ovarian cancer patients after neoadjuvant chemotherapy? Gynecol Oncol. 2011;121(3):451–454.
Although several studies have demonstrated that intraperitoneal (IP) chemotherapy provides a survival advantage, compared with intravenous (IV) chemotherapy, after primary surgical debulking, it remains unclear whether IP chemotherapy would provide a similar superior survival outcome following neoadjuvant chemotherapy (FIGURE).

Intraperitoneal chemotherapy: How efficacious?
The jury is still out on whether intraperitoneal chemotherapy improves survival after neoadjuvant chemotherapy and interval debulking in stages III and IV ovarian cancer.The authors of this paper attempted to answer this question through a retrospective review of 71 patients. All patients were treated with neoadjuvant chemotherapy followed by interval debulking and either IP or IV chemotherapy. Overall, 17 patients (24%) received IP chemotherapy, and 54 patients (76%) received IV chemotherapy. The median number of cycles given prior to and after surgery was the same for both groups (3 for both neoadjuvant chemotherapy and chemotherapy following surgery).
Although patients who received IP chemotherapy had a higher overall response rate (82% versus 67%), there were no differences between groups in terms of progression-free (P=.42) and overall survival (P=.72).
One important limitation of this study was its small sample size and lack of statistical power. In addition, more patients in the IP group had macroscopic residual disease than in the IV group (71% versus 52%; P=.17).
A phase II/III study is under way to evaluate the use of IP chemotherapy following neoadjuvant chemotherapy in ovarian cancer patients.7 The two-stage randomized trial will compare IV chemotherapy with platinum-based IP chemotherapy in women who have undergone optimal surgical debulking (>1 cm) after 3 to 4 cycles of platinum-based neoadjuvant chemotherapy. This study is led by the US National Cancer Institute in collaboration with the Society of Gynecologic Oncologists of Canada, the UK National Cancer Research Institute, the Spanish Ovarian Cancer Research Group, and the US Southwest Oncology Group.
Data are limited on the use of intraperitoneal (IP) chemotherapy following neoadjuvant chemotherapy and interval surgical cytoreduction. We await the results of larger prospective studies to definitively determine whether there is a role for IP chemotherapy in this setting. For now, patients who receive neoadjuvant chemotherapy are limited to IV chemotherapy following surgery.
We want to hear from you! Tell us what you think.
- Update on ovarian cancer screening
David G. Mutch, MD; Nora Kizer, MD (July 2010)
A majority of ovarian cancers are diagnosed at an advanced stage, requiring extensive surgical cytoreductive procedures.1 Because the presence of residual macroscopic disease correlates highly with decreased survival,2 these procedures can be lengthy, complicated, and risky for the patient. Many patients who undergo cytoreduction will be left with a suboptimal result despite surgery.
Better identification and improved treatment of patients who are at high risk of a suboptimal result are clearly needed. One treatment option is neoadjuvant chemotherapy, the administration of chemotherapy prior to the main treatment. Although early data suggested that it was associated with worse outcomes, recent studies have yielded new information:
- Neoadjuvant chemotherapy followed by interval debulking surgery is not inferior to primary debulking surgery followed by chemotherapy for patients who have bulky stage III or IV ovarian cancer
- In patients who have advanced ovarian cancer, neoadjuvant chemotherapy followed by surgical cytoreduction is associated with improved perioperative outcomes
- Postoperative intraperitoneal chemotherapy after neoadjuvant chemotherapy has not yet proved to be associated with improved survival.
Several questions prompted by these findings include:
- Will neoadjuvant chemotherapy improve surgical outcomes in patients who have advanced ovarian cancer and, thus, improve survival?
- Is neoadjuvant chemotherapy a better strategy for all patients?
- Will neoadjuvant chemotherapy reduce the surgical effort necessary to achieve an optimal result?
- What is the role of intraperitoneal chemotherapy in patients who undergo neoadjuvant chemotherapy?
Further national (or international) data are needed to confirm a survival advantage for patients who receive neoadjuvant chemotherapy, compared with those who undergo primary surgery before the administration of chemotherapy.
Neoadjuvant chemotherapy is an acceptable alternative to primary surgical cytoreduction
Vergote I, Tropé CG, Amant F, et al; European Organization for Research and Treatment of Cancer-Gynaecological Cancer Group; NCIC Clinical Trials Group. Neoadjuvant chemotherapy or primary surgery in stage IIIC or IV ovarian cancer. N Engl J Med. 2010;363(10):943–953.
Historically, the standard of care in ovarian cancer treatment has been surgical cytoreduction followed by chemotherapy.3-6 However, data from prospective randomized trials to support this practice are limited. Neoadjuvant chemotherapy is an alternative strategy that has been explored as a way to improve outcomes from interval surgical debulking in patients who have ovarian cancer in whom suboptimal cytoreduction is otherwise expected. Vergote and coworkers attempted to determine which strategy is better through a randomized trial of 632 patients.
Participants had to have biopsy-proven stage IIIc or IV ovarian, fallopian tube, or primary peritoneal cancer. The two treatment arms were:
- primary debulking surgery followed by at least 6 cycles of platinum-based chemotherapy
- 3 cycles of platinum-based neoadjuvant chemotherapy followed by interval debulking surgery in responders and those who had stable disease. These patients then received an additional 3 cycles of platinum-based chemotherapy post-operatively.
All surgical procedures were completed by qualified gynecologic oncologists, and all patients were evaluated for eligibility before randomization, with no additional selection criteria.
Postoperative death occurred in 2.5% of patients in the primary-surgery group, compared with 0.7% of patients in the neoadjuvant-chemotherapy group. Grade 3 or 4 hemorrhage occurred in 7.4% of patients after primary debulking, compared with 4.1% of patients after interval debulking. Patients who received neoadjuvant chemotherapy experienced a lower rate of infection (1.7% versus 8.1%) and venous complications (0% versus 2.6%).
Overall and progression-free survival rates were similar between the two groups. After multivariate analysis, the strongest predictors of survival were absence of residual disease after surgery (P<.001), small tumor size before randomization (P=.001), and endometrioid histology (P=.001)
Neoadjuvant chemotherapy is a preferred treatment strategy for patients who are expected to have a suboptimal result after surgery. Because neoadjuvant chemotherapy has a survival outcome similar to that of primary surgery followed by chemotherapy, it may be considered for all patients who have bulky stage IIIc or IV disease.
Although neoadjuvant chemotherapy improves the rate of optimal surgical cytoreduction, data are lacking to demonstrate that this improvement boosts survival.
Administration of neoadjuvant chemotherapy in these patients may improve perioperative morbidity and mortality, although no formal analysis was conducted in this study.
Neoadjuvant chemotherapy improves perioperative outcomes
Milam MR, Tao X, Coleman RL, et al. Neoadjuvant chemotherapy is associated with prolonged primary treatment intervals in patients with advanced epithelial ovarian cancer. Int J Gynecol Cancer. 2011;21(1):66–71.
Milam and coworkers investigated chemotherapy-associated morbidity and timing in two groups of patients who had advanced epithelial ovarian cancer:
- those undergoing neoadjuvant chemotherapy followed by maximal cytoreductive surgery
- those undergoing primary surgery followed by chemotherapy.
Their retrospective study involved 263 consecutive patients who were treated at MD Anderson Cancer Center from 1993 to 2005. In this cohort, 47 women (18%) received neoadjuvant chemotherapy. These patients experienced less blood loss (400 mL versus 750 mL) and a shorter hospital stay (6 versus 8 days). Time to the initiation of chemotherapy from the date of diagnosis did not differ between groups, and the amount of residual disease and rate of survival were also similar between arms. However, patients who received neoadjuvant chemotherapy underwent more cycles of chemotherapy over a longer treatment period.
Although neoadjuvant chemotherapy does not appear to offer a survival advantage, it is equivalent to primary surgery followed by adjuvant chemotherapy and may be associated with improved perioperative outcomes.
The results of the trial by Vergote and colleagues (page 25), should discourage oncologists from prescribing more than 6 cycles of chemotherapy in the neoadjuvant setting; patients from their study in the neoadjuvant group received a total of 6 cycles and had survival outcomes equivalent to those of women in the primary surgery group.
In the pipeline: Data on intraperitoneal chemotherapy after neoadjuvant chemotherapy
Le T, Latifah H, Jolicoeur L, et al. Does intraperitoneal chemotherapy benefit optimally debulked epithelial ovarian cancer patients after neoadjuvant chemotherapy? Gynecol Oncol. 2011;121(3):451–454.
Although several studies have demonstrated that intraperitoneal (IP) chemotherapy provides a survival advantage, compared with intravenous (IV) chemotherapy, after primary surgical debulking, it remains unclear whether IP chemotherapy would provide a similar superior survival outcome following neoadjuvant chemotherapy (FIGURE).

Intraperitoneal chemotherapy: How efficacious?
The jury is still out on whether intraperitoneal chemotherapy improves survival after neoadjuvant chemotherapy and interval debulking in stages III and IV ovarian cancer.The authors of this paper attempted to answer this question through a retrospective review of 71 patients. All patients were treated with neoadjuvant chemotherapy followed by interval debulking and either IP or IV chemotherapy. Overall, 17 patients (24%) received IP chemotherapy, and 54 patients (76%) received IV chemotherapy. The median number of cycles given prior to and after surgery was the same for both groups (3 for both neoadjuvant chemotherapy and chemotherapy following surgery).
Although patients who received IP chemotherapy had a higher overall response rate (82% versus 67%), there were no differences between groups in terms of progression-free (P=.42) and overall survival (P=.72).
One important limitation of this study was its small sample size and lack of statistical power. In addition, more patients in the IP group had macroscopic residual disease than in the IV group (71% versus 52%; P=.17).
A phase II/III study is under way to evaluate the use of IP chemotherapy following neoadjuvant chemotherapy in ovarian cancer patients.7 The two-stage randomized trial will compare IV chemotherapy with platinum-based IP chemotherapy in women who have undergone optimal surgical debulking (>1 cm) after 3 to 4 cycles of platinum-based neoadjuvant chemotherapy. This study is led by the US National Cancer Institute in collaboration with the Society of Gynecologic Oncologists of Canada, the UK National Cancer Research Institute, the Spanish Ovarian Cancer Research Group, and the US Southwest Oncology Group.
Data are limited on the use of intraperitoneal (IP) chemotherapy following neoadjuvant chemotherapy and interval surgical cytoreduction. We await the results of larger prospective studies to definitively determine whether there is a role for IP chemotherapy in this setting. For now, patients who receive neoadjuvant chemotherapy are limited to IV chemotherapy following surgery.
We want to hear from you! Tell us what you think.
1. Howlader N, Noone AM, Krapcho M, et all. eds. SEER Cancer Statistics Review 1975-2008. National Cancer Institute. http://seer.cancer.gov/csr/1975_2008. Published April 15, 2011. Accessed June 10, 2011.
2. du Bois A, Ruess A, Pujade-Lauraine E, Harter P, Ray-Coquard I, Pfisterer J. Role of surgical outcome as prognostic factor in advanced epithelial ovarian cancer: a combined exploratory analysis of 3 prospectively randomized phase 3 multicenter trials; by the Arbeitsgemeinschaft Gynaekologische Onkologie Studiengruppe Ovarialkarzinom (AGO-OVAR) and the Groupe d’Investigateurs Nationaux Pour les Etudes des Cancers de l’Ovaire (GINECO). Cancer. 2009;115(6):1234-1244.
3. Meigs JV. Tumors of the pelvic organs. New York: Macmillan: 1934.
4. Aure JC, Hoeg K, Kolstad P. Clinical and histologic studies of ovarian carcinoma. Long-term follow-up of 990 cases. Obstet Gynecol. 1971;37(1):1-9.
5. Griffiths CT, Fuller AF. Intensive surgical and chemotherapeutic management of advanced ovarian cancer. Surg Clin North Am. 1978;58(1):131-142.
6. du Bois A, Quinn M, Thigpen T, et al. 2004 Consensus statements on the management of ovarian cancer: final document of the 3rd International Gynecologic Cancer Intergroup Ovarian Cancer Consensus Conference (GCIG OCCC 2004). Ann Oncol. 2005;16(suppl 8):viii7-viii12.
7. Mackay HJ, Provencheur D, Heywood M, et al. Phase II/III study of intraperitoneal chemotherapy after neoadjuvant chemotherapy for ovarian cancer: ncic ctg ov.21. Curr Oncol. 2011;18(2):84-90.
1. Howlader N, Noone AM, Krapcho M, et all. eds. SEER Cancer Statistics Review 1975-2008. National Cancer Institute. http://seer.cancer.gov/csr/1975_2008. Published April 15, 2011. Accessed June 10, 2011.
2. du Bois A, Ruess A, Pujade-Lauraine E, Harter P, Ray-Coquard I, Pfisterer J. Role of surgical outcome as prognostic factor in advanced epithelial ovarian cancer: a combined exploratory analysis of 3 prospectively randomized phase 3 multicenter trials; by the Arbeitsgemeinschaft Gynaekologische Onkologie Studiengruppe Ovarialkarzinom (AGO-OVAR) and the Groupe d’Investigateurs Nationaux Pour les Etudes des Cancers de l’Ovaire (GINECO). Cancer. 2009;115(6):1234-1244.
3. Meigs JV. Tumors of the pelvic organs. New York: Macmillan: 1934.
4. Aure JC, Hoeg K, Kolstad P. Clinical and histologic studies of ovarian carcinoma. Long-term follow-up of 990 cases. Obstet Gynecol. 1971;37(1):1-9.
5. Griffiths CT, Fuller AF. Intensive surgical and chemotherapeutic management of advanced ovarian cancer. Surg Clin North Am. 1978;58(1):131-142.
6. du Bois A, Quinn M, Thigpen T, et al. 2004 Consensus statements on the management of ovarian cancer: final document of the 3rd International Gynecologic Cancer Intergroup Ovarian Cancer Consensus Conference (GCIG OCCC 2004). Ann Oncol. 2005;16(suppl 8):viii7-viii12.
7. Mackay HJ, Provencheur D, Heywood M, et al. Phase II/III study of intraperitoneal chemotherapy after neoadjuvant chemotherapy for ovarian cancer: ncic ctg ov.21. Curr Oncol. 2011;18(2):84-90.
How much vitamin D should you recommend to your nonpregnant patients?
No question: Vitamin D plays a vital role in bone health. In recent years, the possibility that it plays a role in other aspects of health has prompted considerable speculation, fueled by both widespread media coverage and dissemination of conflicting information about the potential nonskeletal benefits of high-dose vitamin D supplementation. Controversy has emerged about:
- the appropriate criteria for defining vitamin D deficiency
- the extent to which vitamin D influences nonskeletal health conditions
- the optimal level of vitamin D supplementation.
In 2010, the Institute of Medicine (IOM) released a report that provided recommendations for vitamin D intake, which were also summarized in a recent article for clinicians.1,2 The IOM report provided much-needed clinical guidance, but it has also fueled additional questions.
This article describes the IOM recommendations, explains what we know now about the effect of vitamin D on various health outcomes, and offers concrete recommendations on vitamin D measurement, intake, and supplementation.
How the Institute of Medicine formulated its recommendations
The Institute of Medicine (IOM) committee conducted a comprehensive review of the literature to date on the relationship between vitamin D (and calcium) intake and several health outcomes. In terms of skeletal health, the IOM committee concluded that a 25OHD level of at least 20 ng/mL is sufficient to meet the needs of at least 97.5% of the population. The vitamin D intake thought to be necessary to achieve this 25OHD level for at least 97.5% of the population was provided for different age groups (TABLE 2).
The Recommended Dietary Allowance (RDA) of vitamin D is 600 IU daily for all adults up to age 70 years, and 800 IU daily for adults older than 70 years. These values were based on an assumption of minimal sun exposure, due to wide variability in vitamin D synthesis from ultraviolet light, as well as the risk of skin cancer. The IOM concluded that there is no compelling evidence that a 25OHD level above 20 ng/mL or a vitamin D intake greater than 600 IU (800Â IU for adults over 70) affords greater skeletal or nonskeletal benefits.
The IOM recommendations were based on the integration of bone health outcomes. The evidence supporting causal relationships between vitamin D insufficiency and nonskeletal outcomes such as cancer, cardiovascular disease, diabetes, impaired physical performance, autoimmune disorders, and other chronic diseases was found to be inconsistent and inconclusive.
The IOM report also noted the emergence of a “U”-shaped curve in regard to vitamin D and several health outcomes, which has fueled concern about attainment of a 25OHD level above 50 ng/mL. The IOM committee designated 4,000 IU daily as the tolerable upper intake but emphasized that research into long-term outcomes and safety at intakes above the RDA is limited. Therefore, this upper limit should not be interpreted as a target intake level.
How is vitamin D metabolized?
Vitamin D is produced endogenously in the skin in the form of vitamin D3 (cholecalciferol). It also can be ingested exogenously in the form of vitamin D3 or vitamin D2 (ergocalciferol). Cutaneous synthesis of vitamin D is stimulated by solar ultraviolet radiation.
Vitamin D2 and D3 are hydroxylated in the liver to form 25-hydroxyvitamin D (25OHD). Measurement of the serum 25OHD level is thought to be the most reliable indicator of vitamin D exposure.3 25OHD is hydroxylated again, primarily in the kidneys, to the most active form of vitamin D (1,25-dihydroxyvitamin D).
The adverse skeletal effects of severe vitamin D deficiency are well established; those effects include calcium malabsorption, secondary hyperparathyroidism, bone loss, and increased risk of fracture. In this setting, secondary hyperparathyroidism results from both decreased gastrointestinal calcium absorption and decreased suppression of parathyroid hormone (PTH) production by the parathyroid glands from vitamin D metabolites. Secondary hyperparathyroidism leads to increased bone resorption and bone loss. Rickets, osteomalacia, hypocalcemia, hypophosphatemia, muscle weakness, and bone pain are less common effects that can occur with severe vitamin D deficiency.
It is worth noting that women of color are at increased risk of vitamin D deficiency as a result of greater skin pigmentation.3 Obesity is also a risk factor for vitamin D deficiency.3 Additional risk factors for vitamin D insufficiency are listed in TABLE 1.
TABLE 1
Risk factors for vitamin D insufficiency
| Obesity |
| Dark skin pigmentation |
Decreased sun exposure
|
| Low dietary intake of vitamin D |
| Malabsorption of ingested vitamin D |
Increased hepatic degradation of 25-hydroxyvitamin D
|
| Decreased hepatic hydroxylation of vitamin D (occurs only with severe hepatic disease) |
| Impaired renal hydroxylation of vitamin D (renal insufficiency) |
| Osteoporosis or osteopenia |
How should vitamin D insufficiency be defined?
Biochemical criteria for defining vitamin D insufficiency vary. That makes it difficult to estimate the prevalence of vitamin D insufficiency.
Severe vitamin D deficiency is commonly defined as a serum 25OHD level below 10 ng/mL.3 Vitamin D insufficiency has been variably defined as a serum 25OHD level below 20 to 32 ng/mL,3,4 and the lower limit of normal in most clinical laboratories is now typically 30 to 32 ng/mL. Many patients become concerned when their serum 25OHD level is flagged as “low” on a laboratory report, and it’s likely that you are called on from time to time to interpret and make recommendations about the appropriate response to this “abnormal” finding.
The broad definition of vitamin D insufficiency stems, in part, from the assessment of a wide range of outcomes. Measures that have been used include fracture risk, calcium absorptive capacity, and the serum concentration of PTH. In regard to calcium absorption, most studies suggest that maximal dietary calcium absorption occurs when the 25OHD level reaches 20 ng/mL, although some studies suggest a higher threshold.1,3
The optimal level of 25OHD for PTH suppression remains unclear. Several studies have suggested that the PTH level increases when the 25OHD concentration falls below 30 ng/mL,4,5 although this threshold has varied substantially across studies.6
How prevalent is vitamin D insufficiency?
Estimates of the prevalence of vitamin D insufficiency vary by the criteria used to define the condition. A recent report using data from the National Health and Nutrition Examination Survey (NHANES) estimated that approximately 30% of US adults 20 years of age or older have a 25OHD level below 20Â ng/mL, and more than 70% of this age group has a 25OHD level below 32 ng/mL.7
The IOM committee noted that several reports have most likely overestimated the prevalence of vitamin D insufficiency through the use of 25OHD cut points higher than 20Â ng/mL.
The data on vitamin D insufficiency and skeletal health
Many studies have examined the relationship between vitamin D supplementation or the 25OHD level and fracture risk, and conflicting results have emerged. Many trials have examined the combination of calcium and vitamin D supplementation, the effects of which are tightly interwoven, confounding interpretation.
Interpretation of large observational studies is further confounded by the inability to attribute association to causation. In the Women’s Health Initiative (WHI) study of calcium with vitamin D, treatment of healthy postmenopausal women with 1,000 mg of calcium and 400 IU of vitamin D daily led to improved bone density at the hip but no statistically significant reduction in hip fracture.8 However, a reduced risk of hip fracture was demonstrated in secondary analyses among women who adhered to treatment and among women 60 years or older. Meta-analyses of clinical trials have reported that treatment with varying doses of vitamin D (more than 400 IU daily) reduces the risk of vertebral,9 nonvertebral,10 and hip fractures.10
Several studies have examined the relationship between the 25OHD level and fracture risk, with inconsistent findings:
- A nested case-control study from the WHI found that the risk of hip fracture was significantly increased among postmenopausal women who had a 25OHD level of 19 ng/mL or lower.11
- A 2009 report from the Agency for Healthcare Research and Quality (AHRQ) concluded that the association between the 25OHD level and the risk of fracture was inconsistent.12
After a comprehensive review of the available research, the IOM committee concluded that a serum 25OHD level of 20 ng/mL would meet the needs for bone health for at least 97.5% of the US and Canadian populations.
TABLE 2
Calcium and vitamin D dietary reference intakes for adults, by life stage
| Life stage (gender) | Calcium | Vitamin D | |||
|---|---|---|---|---|---|
| RDA (mg/d) | Tolerable upper intake level (mg/d)* | RDA (IU/d) | Serum 25OHD level (ng/mL) (corresponding to the RDA)† | Tolerable upper intake level (IU/d)* | |
| 19–50 yr (male and female) | 1,000 | 2,500 | 600 | 20 | 4,000 |
| 51–70 yr (male) | 1,000 | 2,000 | 600 | 20 | 4,000 |
| 51–70 yr (female) | 1,200 | 2,000 | 600 | 20 | 4,000 |
| 71+ yr (male and female) | 1,200 | 2,000 | 800 | 20 | 4,000 |
| Adapted from: Ross AC, Manson JE, Abrams SA, et al. J Clin Endocrinol Metab. 2011;96(1):53–58. RDA = Recommended Dietary Allowance, 25OHD=25-hydroxyvitamin D * The tolerable upper intake level is the threshold above which is a risk of adverse events. The upper intake level is not intended to be a target intake. There is no consistent evidence of greater benefit at intake levels above the RDA. The serum 25OHD level corresponding to the upper intake level is 50 ng/mL. † Measures of the serum 25OHD level corresponding to the RDA and covering the requirements of at least 97.5% of the population. | |||||
The data on vitamin D insufficiency and nonskeletal outcomes
Many observational studies have reported relationships between vitamin D insufficiency and myriad nonskeletal health outcomes, particularly cardiovascular disease, cancer, diabetes, and autoimmune disorders.3 However, well-designed randomized clinical trials that examine nonskeletal outcomes as primary pre-specified outcomes are lacking.13 Such studies will be essential to elucidate the relationship between vitamin D insufficiency and nonskeletal chronic diseases. The VITamin D and OmegA-3 TriaL (VITAL) is an ongoing large-scale, randomized clinical trial designed to evaluate the role of supplementation with 2,000 IU of vitamin D3 daily in the primary prevention of cancer and cardiovascular disease.14
- Vitamin D plays a vital role in bone health
- The Institute of Medicine released a 2010 report that provided public health recommendations for vitamin D intake based on bone health outcomes
- Many observational studies have reported a relationship between vitamin D insufficiency and adverse nonskeletal health outcomes, including cardiovascular disease, cancer, diabetes, and autoimmune disorders, but evidence from randomized clinical trials on the potential nonskeletal benefits of vitamin D is sparse
- Excessive vitamin D intake should be avoided because of the potential for harm and the lack of evidence from well-designed clinical trials that vitamin D intake beyond the recommended amount affords greater skeletal or nonskeletal health benefits
- Among women who have an increased risk of vitamin D insufficiency or bone loss, 25OHD concentration should be measured and vitamin D supplementation should be provided as necessary to achieve the target 25OHD level
What we recommend for treatment
The IOM report provided the medical community with evidence-based recommendations for vitamin D intake at the population level, based on a public health perspective.1,2 However, the public health guideline model must be distinguished from the medical model, in which shared clinical decision-making between physician and patient occurs on an individual level and is informed by individual clinical risk factors. The public health recommendations detailed in the IOM report are not intended to replace or interfere with clinical judgment or preclude individualized clinical decision-making.
The debate over optimal levels of vitamin D supplementation for individual patients who have osteoporosis or other health conditions continues.15 Here, we provide general guidelines for treatment, based on the evidence available to date.
Clear benefits of vitamin D in bone health notwithstanding, advise your patients to avoid excessive intake because it can cause harm. See “More is not necessarily better”.
Recommendations for healthy adult nonpregnant women
Vitamin D intake: We recommend a daily vitamin D intake of 600 IU for healthy nonpregnant women up to age 70 years (and 800 Â IU daily for women older than 70 years) who are at average risk of vitamin D insufficiency and bone loss, consistent with the IOM recommendations. The IOM guidelines assume minimal to no sun exposure.
Measurement of 25OHD: It is not necessary to routinely measure the 25OHD level in these women. However, it is prudent to measure 25OHD in women who have risk factors for vitamin D insufficiency (TABLE 1) or a clinical condition associated with severe vitamin D deficiency. In these cases, if the 25OHD level is found to be below 20 ng/mL, vitamin D therapy should be initiated, with the goal of boosting the 25OHD level above the threshold of 20 ng/mL.
Treatment of vitamin D insufficiency: Options include daily vitamin D supplementation and higher-dose weekly preparations.
Many clinicians treat severe vitamin D insufficiency with 50,000 IU of vitamin D2 once weekly for 8 weeks, followed by a maintenance dose (described below) of vitamin D to preserve the target 25OHD level.5 An alternative is daily vitamin D supplementation, with the dosage based on the degree of insufficiency.
A general rule of thumb, for persons who have normal vitamin D absorption, is that every 1,000 IU of vitamin D3 ingested daily increases the 25OHD level by approximately 6 to 10 ng/mL.4,16 However, the incremental increase in the 25OHD concentration varies among individuals, depending on the baseline 25OHD level, with a greater incremental increase occurring at lower baseline 25OHD levels.
Monitoring of the 25OHD level after adjustment of the dosage is necessary to ensure that the target level is achieved.
Maintaining an adequate vitamin D level: Once vitamin D insufficiency has been corrected, a maintenance dosage of vitamin D should be selected—commonly 800 to 1,000 Â IU daily. A higher maintenance dosage may be required for persons who have genetic or ongoing environmental factors that predispose them to vitamin D insufficiency.
Vitamin D3 is reportedly more potent than D2 in increasing the 25OHD level,17 although this finding has not been universal.18 Monthly or twice-monthly administration of 50,000 IU of vitamin D2 is another option for maintenance of vitamin D sufficiency,5,16 although daily doses are more commonly used and are readily available in over-the-counter preparations.
Regardless of the regimen selected, the 25OHD level should be measured again approximately 3 months after a change in dosage to ensure that the target level has been achieved, with further dosage adjustments as indicated.
Recommendations for adult women at increased risk of skeletal disease
Measurement of 25OHD: The 25OHD level should be measured among women at increased risk of vitamin D insufficiency, bone loss, or fracture and among women who have established skeletal disease.
Vitamin D intake: We recommend that women at increased risk of osteoporosis and women older than 70 years receive at least 800 IU daily and, potentially, more if necessary to achieve the target 25OHD level.
Although the evidence to date does not support routine achievement of a 25OHD level substantially above 20 ng/mL in most women, many clinicians recommend that women in this higher-risk group maintain a 25OHD level above 30 ng/mL because of the possibly greater (although unproven) skeletal and nonskeletal benefits. As more data become available regarding the benefits and safety of vitamin D doses higher than those recommended by the IOM, these recommendations may be revised.
In 2010, the National Osteoporosis Foundation (NOF) recommended a vitamin D intake of 800 to 1,000 IU daily for all adults 50 years and older. Among persons at risk of deficiency, the NOF also recommended measurement of the serum 25OHD level, with vitamin D supplementation, as necessary, to achieve a 25OHD level of 30 ng/mL or higher.19 Also in 2010, the International Osteoporosis Foundation (IOF) recommended a target 25OHD level above 30 ng/mL for all older adults. The IOF also estimated that the average dosage required to achieve this level in older adults is 800 to 1,000 IU daily, noting that upward adjustment may be required in some people.4 It is unclear whether these guidelines will be revised in the future, based on the IOM report.
We recommend against achieving a 25OHD level above 50 ng/mL, based on evidence suggesting potential adverse health effects above this level.
Excessive vitamin D intake should be avoided because of the potential for harm and the lack of evidence from well-designed clinical trials that vitamin D intake beyond the currently recommended amount affords greater skeletal or nonskeletal health benefits. Although moderate vitamin D supplementation has proven skeletal benefits, a “U-shaped” curve for some outcomes has emerged, suggesting that excessive vitamin D supplementation may pose health risks. Notably, a recent clinical trial reported a higher risk of fracture (and falls) among elderly women treated annually with high-dose (500,000 IU) oral vitamin D3 versus placebo.20
A suggestion of adverse effects associated with 25OHD levels above 50 ng/mL has also emerged, from observational studies, for several nonskeletal health outcomes, including pancreatic cancer,21 cardiovascular disease,1 and all-cause mortality.22
Limited evidence is available regarding the safety and overall risk-benefit profile of long-term maintenance of 25OHD levels above the recommended dietary allowance (RDA) range. Therefore, you should remind your patients that, despite the importance of both prevention and treatment of vitamin D insufficiency, more is not necessarily better.
We want to hear from you! Tell us what you think.
1. Institute of Medicine. 2011 Dietary Reference Intakes for Calcium and Vitamin D. Washington DC: National Academies Press; 2011.
2. Ross AC, Manson JE, Abrams SA, et al. The 2011 report on dietary reference intakes for calcium and vitamin D from the Institute of Medicine: what clinicians need to know. J Clin Endocrinol Metab. 2011;96(1):53-58.
3. Rosen CJ. Clinical practice. Vitamin D insufficiency. N Engl J Med. 2011;364(3):248-254.
4. Dawson-Hughes B, Mithal A, Bonjour JP, et al. IOF position statement: vitamin D recommendations for older adults. Osteoporos Int. 2010;21(7):1151-1154.
5. Holick MF. Vitamin D deficiency. N Engl J Med. 2007;357(3):266-281.
6. Sai AJ, Walters RW, Fang X, Gallagher JC. Relationship between vitamin D parathyroid hormone, and bone health. J Clin Endocrinol Metab. 2011;96(3):E436-446.
7. Yetley EA. Assessing the vitamin D status of the US population. Am J Clin Nutr. 2008;88(2):558S-564S.
8. Jackson RD, LaCroix AZ, Gass M, et al. Women’s Health Initiative Investigators. Calcium plus vitamin D supplementation and the risk of fractures. N Engl J Med. 2006;354(7):669-683.
9. Papadimitropoulos E, Wells G, Shea B, et al. Osteoporosis Methodology Group and The Osteoporosis Research Advisory Group. Meta-analyses of therapies for postmenopausal osteoporosis. VIII: Meta-analysis of the efficacy of vitamin D treatment in preventing osteoporosis in postmenopausal women. Endocr Rev. 2002;23(4):560-569.
10. Bischoff-Ferrari HA, Willett WC, Wong JB, et al. Prevention of nonvertebral fractures with oral vitamin D and dose dependency: a meta-analysis of randomized controlled trials. Arch Intern Med. 2009;169(6):551-561.
11. Cauley JA, Lacroix AZ, Wu L, et al. Serum 25-hydroxyvitamin D concentrations and risk for hip fractures. Ann Intern Med. 2008;149(4):242-250.
12. Chung M, Balk EM, Brendel M, et al. Vitamin D and calcium: a systematic review of health outcomes. Evid Rep Technol Assess (Full Rep). 2009;(183):1-420.
13. Manson JE, Mayne ST, Clinton SK. Vitamin D and prevention of cancer—ready for prime time? N Engl J Med. 2011;364(15):1385-1387.
14. Manson JE. Vitamin D and the heart: why we need large-scale clinical trials. Cleve Clin J Med. 2010;77(12):903-910.
15. The Forum at Harvard School of Public Health. Boosting Vitamin D: Not enough or too much? The Andelot Series on Current Science Controversies. http://www.hsph.harvard.edu/forum/boosting-vitamin-d-not-enough-or-too-much.cfm. Published March 29 2011. Accessed April 22, 2011.
16. Binkley N, Gemar D, Engelke J, et al. Evaluation of ergocalciferol or cholecalciferol dosing, 1,600 IU daily or 50,000 IU monthly in older adults. J Clin Endocrinol Metab. 2011;96(4):981-988.
17. Heaney RP, Recker RR, Grote J, Horst RL, Armas LA. Vitamin D(3) is more potent than vitamin D(2) in humans. J Clin Endocrinol Metab. 2011;96(3):E447-452.
18. Holick MF, Biancuzzo RM, Chen TC, et al. Vitamin D2 is as effective as vitamin D3 in maintaining circulating concentrations of 25-hydroxyvitamin D. J Clin Endocrinol Metab. 2008;93(3):677-681.
19. National Osteoporosis Foundation. Clinician’s Guide to Prevention and Treatment of Osteoporosis. Washington DC: National Osteoporosis Foundation; 2010. http://www.nof.org/professionals/clinical-guidelines. Accessed June 7, 2011.
20. Sanders KM, Stuart AL, Williamson EJ, et al. Annual high-dose oral vitamin D and falls and fractures in older women: a randomized controlled trial. JAMA. 2010;303(18):1815-1822.
21. Stolzenberg-Solomon RZ, Jacobs EJ, Arslan AA, et al. Circulating 25-hydroxyvitamin D and risk of pancreatic cancer: Cohort Consortium Vitamin D Pooling Project of Rarer Cancers. Am J Epidemiol. 2010;172(1):81-93.
22. Melamed ML, Michos ED, Post W, Astor B. 25-hydroxyvitamin D levels and the risk of mortality in the general population. Arch Intern Med. 2008;168(15):1629-1637.
No question: Vitamin D plays a vital role in bone health. In recent years, the possibility that it plays a role in other aspects of health has prompted considerable speculation, fueled by both widespread media coverage and dissemination of conflicting information about the potential nonskeletal benefits of high-dose vitamin D supplementation. Controversy has emerged about:
- the appropriate criteria for defining vitamin D deficiency
- the extent to which vitamin D influences nonskeletal health conditions
- the optimal level of vitamin D supplementation.
In 2010, the Institute of Medicine (IOM) released a report that provided recommendations for vitamin D intake, which were also summarized in a recent article for clinicians.1,2 The IOM report provided much-needed clinical guidance, but it has also fueled additional questions.
This article describes the IOM recommendations, explains what we know now about the effect of vitamin D on various health outcomes, and offers concrete recommendations on vitamin D measurement, intake, and supplementation.
How the Institute of Medicine formulated its recommendations
The Institute of Medicine (IOM) committee conducted a comprehensive review of the literature to date on the relationship between vitamin D (and calcium) intake and several health outcomes. In terms of skeletal health, the IOM committee concluded that a 25OHD level of at least 20 ng/mL is sufficient to meet the needs of at least 97.5% of the population. The vitamin D intake thought to be necessary to achieve this 25OHD level for at least 97.5% of the population was provided for different age groups (TABLE 2).
The Recommended Dietary Allowance (RDA) of vitamin D is 600 IU daily for all adults up to age 70 years, and 800 IU daily for adults older than 70 years. These values were based on an assumption of minimal sun exposure, due to wide variability in vitamin D synthesis from ultraviolet light, as well as the risk of skin cancer. The IOM concluded that there is no compelling evidence that a 25OHD level above 20 ng/mL or a vitamin D intake greater than 600 IU (800Â IU for adults over 70) affords greater skeletal or nonskeletal benefits.
The IOM recommendations were based on the integration of bone health outcomes. The evidence supporting causal relationships between vitamin D insufficiency and nonskeletal outcomes such as cancer, cardiovascular disease, diabetes, impaired physical performance, autoimmune disorders, and other chronic diseases was found to be inconsistent and inconclusive.
The IOM report also noted the emergence of a “U”-shaped curve in regard to vitamin D and several health outcomes, which has fueled concern about attainment of a 25OHD level above 50 ng/mL. The IOM committee designated 4,000 IU daily as the tolerable upper intake but emphasized that research into long-term outcomes and safety at intakes above the RDA is limited. Therefore, this upper limit should not be interpreted as a target intake level.
How is vitamin D metabolized?
Vitamin D is produced endogenously in the skin in the form of vitamin D3 (cholecalciferol). It also can be ingested exogenously in the form of vitamin D3 or vitamin D2 (ergocalciferol). Cutaneous synthesis of vitamin D is stimulated by solar ultraviolet radiation.
Vitamin D2 and D3 are hydroxylated in the liver to form 25-hydroxyvitamin D (25OHD). Measurement of the serum 25OHD level is thought to be the most reliable indicator of vitamin D exposure.3 25OHD is hydroxylated again, primarily in the kidneys, to the most active form of vitamin D (1,25-dihydroxyvitamin D).
The adverse skeletal effects of severe vitamin D deficiency are well established; those effects include calcium malabsorption, secondary hyperparathyroidism, bone loss, and increased risk of fracture. In this setting, secondary hyperparathyroidism results from both decreased gastrointestinal calcium absorption and decreased suppression of parathyroid hormone (PTH) production by the parathyroid glands from vitamin D metabolites. Secondary hyperparathyroidism leads to increased bone resorption and bone loss. Rickets, osteomalacia, hypocalcemia, hypophosphatemia, muscle weakness, and bone pain are less common effects that can occur with severe vitamin D deficiency.
It is worth noting that women of color are at increased risk of vitamin D deficiency as a result of greater skin pigmentation.3 Obesity is also a risk factor for vitamin D deficiency.3 Additional risk factors for vitamin D insufficiency are listed in TABLE 1.
TABLE 1
Risk factors for vitamin D insufficiency
| Obesity |
| Dark skin pigmentation |
Decreased sun exposure
|
| Low dietary intake of vitamin D |
| Malabsorption of ingested vitamin D |
Increased hepatic degradation of 25-hydroxyvitamin D
|
| Decreased hepatic hydroxylation of vitamin D (occurs only with severe hepatic disease) |
| Impaired renal hydroxylation of vitamin D (renal insufficiency) |
| Osteoporosis or osteopenia |
How should vitamin D insufficiency be defined?
Biochemical criteria for defining vitamin D insufficiency vary. That makes it difficult to estimate the prevalence of vitamin D insufficiency.
Severe vitamin D deficiency is commonly defined as a serum 25OHD level below 10 ng/mL.3 Vitamin D insufficiency has been variably defined as a serum 25OHD level below 20 to 32 ng/mL,3,4 and the lower limit of normal in most clinical laboratories is now typically 30 to 32 ng/mL. Many patients become concerned when their serum 25OHD level is flagged as “low” on a laboratory report, and it’s likely that you are called on from time to time to interpret and make recommendations about the appropriate response to this “abnormal” finding.
The broad definition of vitamin D insufficiency stems, in part, from the assessment of a wide range of outcomes. Measures that have been used include fracture risk, calcium absorptive capacity, and the serum concentration of PTH. In regard to calcium absorption, most studies suggest that maximal dietary calcium absorption occurs when the 25OHD level reaches 20 ng/mL, although some studies suggest a higher threshold.1,3
The optimal level of 25OHD for PTH suppression remains unclear. Several studies have suggested that the PTH level increases when the 25OHD concentration falls below 30 ng/mL,4,5 although this threshold has varied substantially across studies.6
How prevalent is vitamin D insufficiency?
Estimates of the prevalence of vitamin D insufficiency vary by the criteria used to define the condition. A recent report using data from the National Health and Nutrition Examination Survey (NHANES) estimated that approximately 30% of US adults 20 years of age or older have a 25OHD level below 20Â ng/mL, and more than 70% of this age group has a 25OHD level below 32 ng/mL.7
The IOM committee noted that several reports have most likely overestimated the prevalence of vitamin D insufficiency through the use of 25OHD cut points higher than 20Â ng/mL.
The data on vitamin D insufficiency and skeletal health
Many studies have examined the relationship between vitamin D supplementation or the 25OHD level and fracture risk, and conflicting results have emerged. Many trials have examined the combination of calcium and vitamin D supplementation, the effects of which are tightly interwoven, confounding interpretation.
Interpretation of large observational studies is further confounded by the inability to attribute association to causation. In the Women’s Health Initiative (WHI) study of calcium with vitamin D, treatment of healthy postmenopausal women with 1,000 mg of calcium and 400 IU of vitamin D daily led to improved bone density at the hip but no statistically significant reduction in hip fracture.8 However, a reduced risk of hip fracture was demonstrated in secondary analyses among women who adhered to treatment and among women 60 years or older. Meta-analyses of clinical trials have reported that treatment with varying doses of vitamin D (more than 400 IU daily) reduces the risk of vertebral,9 nonvertebral,10 and hip fractures.10
Several studies have examined the relationship between the 25OHD level and fracture risk, with inconsistent findings:
- A nested case-control study from the WHI found that the risk of hip fracture was significantly increased among postmenopausal women who had a 25OHD level of 19 ng/mL or lower.11
- A 2009 report from the Agency for Healthcare Research and Quality (AHRQ) concluded that the association between the 25OHD level and the risk of fracture was inconsistent.12
After a comprehensive review of the available research, the IOM committee concluded that a serum 25OHD level of 20 ng/mL would meet the needs for bone health for at least 97.5% of the US and Canadian populations.
TABLE 2
Calcium and vitamin D dietary reference intakes for adults, by life stage
| Life stage (gender) | Calcium | Vitamin D | |||
|---|---|---|---|---|---|
| RDA (mg/d) | Tolerable upper intake level (mg/d)* | RDA (IU/d) | Serum 25OHD level (ng/mL) (corresponding to the RDA)† | Tolerable upper intake level (IU/d)* | |
| 19–50 yr (male and female) | 1,000 | 2,500 | 600 | 20 | 4,000 |
| 51–70 yr (male) | 1,000 | 2,000 | 600 | 20 | 4,000 |
| 51–70 yr (female) | 1,200 | 2,000 | 600 | 20 | 4,000 |
| 71+ yr (male and female) | 1,200 | 2,000 | 800 | 20 | 4,000 |
| Adapted from: Ross AC, Manson JE, Abrams SA, et al. J Clin Endocrinol Metab. 2011;96(1):53–58. RDA = Recommended Dietary Allowance, 25OHD=25-hydroxyvitamin D * The tolerable upper intake level is the threshold above which is a risk of adverse events. The upper intake level is not intended to be a target intake. There is no consistent evidence of greater benefit at intake levels above the RDA. The serum 25OHD level corresponding to the upper intake level is 50 ng/mL. † Measures of the serum 25OHD level corresponding to the RDA and covering the requirements of at least 97.5% of the population. | |||||
The data on vitamin D insufficiency and nonskeletal outcomes
Many observational studies have reported relationships between vitamin D insufficiency and myriad nonskeletal health outcomes, particularly cardiovascular disease, cancer, diabetes, and autoimmune disorders.3 However, well-designed randomized clinical trials that examine nonskeletal outcomes as primary pre-specified outcomes are lacking.13 Such studies will be essential to elucidate the relationship between vitamin D insufficiency and nonskeletal chronic diseases. The VITamin D and OmegA-3 TriaL (VITAL) is an ongoing large-scale, randomized clinical trial designed to evaluate the role of supplementation with 2,000 IU of vitamin D3 daily in the primary prevention of cancer and cardiovascular disease.14
- Vitamin D plays a vital role in bone health
- The Institute of Medicine released a 2010 report that provided public health recommendations for vitamin D intake based on bone health outcomes
- Many observational studies have reported a relationship between vitamin D insufficiency and adverse nonskeletal health outcomes, including cardiovascular disease, cancer, diabetes, and autoimmune disorders, but evidence from randomized clinical trials on the potential nonskeletal benefits of vitamin D is sparse
- Excessive vitamin D intake should be avoided because of the potential for harm and the lack of evidence from well-designed clinical trials that vitamin D intake beyond the recommended amount affords greater skeletal or nonskeletal health benefits
- Among women who have an increased risk of vitamin D insufficiency or bone loss, 25OHD concentration should be measured and vitamin D supplementation should be provided as necessary to achieve the target 25OHD level
What we recommend for treatment
The IOM report provided the medical community with evidence-based recommendations for vitamin D intake at the population level, based on a public health perspective.1,2 However, the public health guideline model must be distinguished from the medical model, in which shared clinical decision-making between physician and patient occurs on an individual level and is informed by individual clinical risk factors. The public health recommendations detailed in the IOM report are not intended to replace or interfere with clinical judgment or preclude individualized clinical decision-making.
The debate over optimal levels of vitamin D supplementation for individual patients who have osteoporosis or other health conditions continues.15 Here, we provide general guidelines for treatment, based on the evidence available to date.

Clear benefits of vitamin D in bone health notwithstanding, advise your patients to avoid excessive intake because it can cause harm. See “More is not necessarily better”.
Recommendations for healthy adult nonpregnant women
Vitamin D intake: We recommend a daily vitamin D intake of 600 IU for healthy nonpregnant women up to age 70 years (and 800 Â IU daily for women older than 70 years) who are at average risk of vitamin D insufficiency and bone loss, consistent with the IOM recommendations. The IOM guidelines assume minimal to no sun exposure.
Measurement of 25OHD: It is not necessary to routinely measure the 25OHD level in these women. However, it is prudent to measure 25OHD in women who have risk factors for vitamin D insufficiency (TABLE 1) or a clinical condition associated with severe vitamin D deficiency. In these cases, if the 25OHD level is found to be below 20 ng/mL, vitamin D therapy should be initiated, with the goal of boosting the 25OHD level above the threshold of 20 ng/mL.
Treatment of vitamin D insufficiency: Options include daily vitamin D supplementation and higher-dose weekly preparations.
Many clinicians treat severe vitamin D insufficiency with 50,000 IU of vitamin D2 once weekly for 8 weeks, followed by a maintenance dose (described below) of vitamin D to preserve the target 25OHD level.5 An alternative is daily vitamin D supplementation, with the dosage based on the degree of insufficiency.
A general rule of thumb, for persons who have normal vitamin D absorption, is that every 1,000 IU of vitamin D3 ingested daily increases the 25OHD level by approximately 6 to 10 ng/mL.4,16 However, the incremental increase in the 25OHD concentration varies among individuals, depending on the baseline 25OHD level, with a greater incremental increase occurring at lower baseline 25OHD levels.
Monitoring of the 25OHD level after adjustment of the dosage is necessary to ensure that the target level is achieved.
Maintaining an adequate vitamin D level: Once vitamin D insufficiency has been corrected, a maintenance dosage of vitamin D should be selected—commonly 800 to 1,000 Â IU daily. A higher maintenance dosage may be required for persons who have genetic or ongoing environmental factors that predispose them to vitamin D insufficiency.
Vitamin D3 is reportedly more potent than D2 in increasing the 25OHD level,17 although this finding has not been universal.18 Monthly or twice-monthly administration of 50,000 IU of vitamin D2 is another option for maintenance of vitamin D sufficiency,5,16 although daily doses are more commonly used and are readily available in over-the-counter preparations.
Regardless of the regimen selected, the 25OHD level should be measured again approximately 3 months after a change in dosage to ensure that the target level has been achieved, with further dosage adjustments as indicated.
Recommendations for adult women at increased risk of skeletal disease
Measurement of 25OHD: The 25OHD level should be measured among women at increased risk of vitamin D insufficiency, bone loss, or fracture and among women who have established skeletal disease.
Vitamin D intake: We recommend that women at increased risk of osteoporosis and women older than 70 years receive at least 800 IU daily and, potentially, more if necessary to achieve the target 25OHD level.
Although the evidence to date does not support routine achievement of a 25OHD level substantially above 20 ng/mL in most women, many clinicians recommend that women in this higher-risk group maintain a 25OHD level above 30 ng/mL because of the possibly greater (although unproven) skeletal and nonskeletal benefits. As more data become available regarding the benefits and safety of vitamin D doses higher than those recommended by the IOM, these recommendations may be revised.
In 2010, the National Osteoporosis Foundation (NOF) recommended a vitamin D intake of 800 to 1,000 IU daily for all adults 50 years and older. Among persons at risk of deficiency, the NOF also recommended measurement of the serum 25OHD level, with vitamin D supplementation, as necessary, to achieve a 25OHD level of 30 ng/mL or higher.19 Also in 2010, the International Osteoporosis Foundation (IOF) recommended a target 25OHD level above 30 ng/mL for all older adults. The IOF also estimated that the average dosage required to achieve this level in older adults is 800 to 1,000 IU daily, noting that upward adjustment may be required in some people.4 It is unclear whether these guidelines will be revised in the future, based on the IOM report.
We recommend against achieving a 25OHD level above 50 ng/mL, based on evidence suggesting potential adverse health effects above this level.
Excessive vitamin D intake should be avoided because of the potential for harm and the lack of evidence from well-designed clinical trials that vitamin D intake beyond the currently recommended amount affords greater skeletal or nonskeletal health benefits. Although moderate vitamin D supplementation has proven skeletal benefits, a “U-shaped” curve for some outcomes has emerged, suggesting that excessive vitamin D supplementation may pose health risks. Notably, a recent clinical trial reported a higher risk of fracture (and falls) among elderly women treated annually with high-dose (500,000 IU) oral vitamin D3 versus placebo.20
A suggestion of adverse effects associated with 25OHD levels above 50 ng/mL has also emerged, from observational studies, for several nonskeletal health outcomes, including pancreatic cancer,21 cardiovascular disease,1 and all-cause mortality.22
Limited evidence is available regarding the safety and overall risk-benefit profile of long-term maintenance of 25OHD levels above the recommended dietary allowance (RDA) range. Therefore, you should remind your patients that, despite the importance of both prevention and treatment of vitamin D insufficiency, more is not necessarily better.
We want to hear from you! Tell us what you think.
No question: Vitamin D plays a vital role in bone health. In recent years, the possibility that it plays a role in other aspects of health has prompted considerable speculation, fueled by both widespread media coverage and dissemination of conflicting information about the potential nonskeletal benefits of high-dose vitamin D supplementation. Controversy has emerged about:
- the appropriate criteria for defining vitamin D deficiency
- the extent to which vitamin D influences nonskeletal health conditions
- the optimal level of vitamin D supplementation.
In 2010, the Institute of Medicine (IOM) released a report that provided recommendations for vitamin D intake, which were also summarized in a recent article for clinicians.1,2 The IOM report provided much-needed clinical guidance, but it has also fueled additional questions.
This article describes the IOM recommendations, explains what we know now about the effect of vitamin D on various health outcomes, and offers concrete recommendations on vitamin D measurement, intake, and supplementation.
How the Institute of Medicine formulated its recommendations
The Institute of Medicine (IOM) committee conducted a comprehensive review of the literature to date on the relationship between vitamin D (and calcium) intake and several health outcomes. In terms of skeletal health, the IOM committee concluded that a 25OHD level of at least 20 ng/mL is sufficient to meet the needs of at least 97.5% of the population. The vitamin D intake thought to be necessary to achieve this 25OHD level for at least 97.5% of the population was provided for different age groups (TABLE 2).
The Recommended Dietary Allowance (RDA) of vitamin D is 600 IU daily for all adults up to age 70 years, and 800 IU daily for adults older than 70 years. These values were based on an assumption of minimal sun exposure, due to wide variability in vitamin D synthesis from ultraviolet light, as well as the risk of skin cancer. The IOM concluded that there is no compelling evidence that a 25OHD level above 20 ng/mL or a vitamin D intake greater than 600 IU (800Â IU for adults over 70) affords greater skeletal or nonskeletal benefits.
The IOM recommendations were based on the integration of bone health outcomes. The evidence supporting causal relationships between vitamin D insufficiency and nonskeletal outcomes such as cancer, cardiovascular disease, diabetes, impaired physical performance, autoimmune disorders, and other chronic diseases was found to be inconsistent and inconclusive.
The IOM report also noted the emergence of a “U”-shaped curve in regard to vitamin D and several health outcomes, which has fueled concern about attainment of a 25OHD level above 50 ng/mL. The IOM committee designated 4,000 IU daily as the tolerable upper intake but emphasized that research into long-term outcomes and safety at intakes above the RDA is limited. Therefore, this upper limit should not be interpreted as a target intake level.
How is vitamin D metabolized?
Vitamin D is produced endogenously in the skin in the form of vitamin D3 (cholecalciferol). It also can be ingested exogenously in the form of vitamin D3 or vitamin D2 (ergocalciferol). Cutaneous synthesis of vitamin D is stimulated by solar ultraviolet radiation.
Vitamin D2 and D3 are hydroxylated in the liver to form 25-hydroxyvitamin D (25OHD). Measurement of the serum 25OHD level is thought to be the most reliable indicator of vitamin D exposure.3 25OHD is hydroxylated again, primarily in the kidneys, to the most active form of vitamin D (1,25-dihydroxyvitamin D).
The adverse skeletal effects of severe vitamin D deficiency are well established; those effects include calcium malabsorption, secondary hyperparathyroidism, bone loss, and increased risk of fracture. In this setting, secondary hyperparathyroidism results from both decreased gastrointestinal calcium absorption and decreased suppression of parathyroid hormone (PTH) production by the parathyroid glands from vitamin D metabolites. Secondary hyperparathyroidism leads to increased bone resorption and bone loss. Rickets, osteomalacia, hypocalcemia, hypophosphatemia, muscle weakness, and bone pain are less common effects that can occur with severe vitamin D deficiency.
It is worth noting that women of color are at increased risk of vitamin D deficiency as a result of greater skin pigmentation.3 Obesity is also a risk factor for vitamin D deficiency.3 Additional risk factors for vitamin D insufficiency are listed in TABLE 1.
TABLE 1
Risk factors for vitamin D insufficiency
| Obesity |
| Dark skin pigmentation |
Decreased sun exposure
|
| Low dietary intake of vitamin D |
| Malabsorption of ingested vitamin D |
Increased hepatic degradation of 25-hydroxyvitamin D
|
| Decreased hepatic hydroxylation of vitamin D (occurs only with severe hepatic disease) |
| Impaired renal hydroxylation of vitamin D (renal insufficiency) |
| Osteoporosis or osteopenia |
How should vitamin D insufficiency be defined?
Biochemical criteria for defining vitamin D insufficiency vary. That makes it difficult to estimate the prevalence of vitamin D insufficiency.
Severe vitamin D deficiency is commonly defined as a serum 25OHD level below 10 ng/mL.3 Vitamin D insufficiency has been variably defined as a serum 25OHD level below 20 to 32 ng/mL,3,4 and the lower limit of normal in most clinical laboratories is now typically 30 to 32 ng/mL. Many patients become concerned when their serum 25OHD level is flagged as “low” on a laboratory report, and it’s likely that you are called on from time to time to interpret and make recommendations about the appropriate response to this “abnormal” finding.
The broad definition of vitamin D insufficiency stems, in part, from the assessment of a wide range of outcomes. Measures that have been used include fracture risk, calcium absorptive capacity, and the serum concentration of PTH. In regard to calcium absorption, most studies suggest that maximal dietary calcium absorption occurs when the 25OHD level reaches 20 ng/mL, although some studies suggest a higher threshold.1,3
The optimal level of 25OHD for PTH suppression remains unclear. Several studies have suggested that the PTH level increases when the 25OHD concentration falls below 30 ng/mL,4,5 although this threshold has varied substantially across studies.6
How prevalent is vitamin D insufficiency?
Estimates of the prevalence of vitamin D insufficiency vary by the criteria used to define the condition. A recent report using data from the National Health and Nutrition Examination Survey (NHANES) estimated that approximately 30% of US adults 20 years of age or older have a 25OHD level below 20Â ng/mL, and more than 70% of this age group has a 25OHD level below 32 ng/mL.7
The IOM committee noted that several reports have most likely overestimated the prevalence of vitamin D insufficiency through the use of 25OHD cut points higher than 20Â ng/mL.
The data on vitamin D insufficiency and skeletal health
Many studies have examined the relationship between vitamin D supplementation or the 25OHD level and fracture risk, and conflicting results have emerged. Many trials have examined the combination of calcium and vitamin D supplementation, the effects of which are tightly interwoven, confounding interpretation.
Interpretation of large observational studies is further confounded by the inability to attribute association to causation. In the Women’s Health Initiative (WHI) study of calcium with vitamin D, treatment of healthy postmenopausal women with 1,000 mg of calcium and 400 IU of vitamin D daily led to improved bone density at the hip but no statistically significant reduction in hip fracture.8 However, a reduced risk of hip fracture was demonstrated in secondary analyses among women who adhered to treatment and among women 60 years or older. Meta-analyses of clinical trials have reported that treatment with varying doses of vitamin D (more than 400 IU daily) reduces the risk of vertebral,9 nonvertebral,10 and hip fractures.10
Several studies have examined the relationship between the 25OHD level and fracture risk, with inconsistent findings:
- A nested case-control study from the WHI found that the risk of hip fracture was significantly increased among postmenopausal women who had a 25OHD level of 19 ng/mL or lower.11
- A 2009 report from the Agency for Healthcare Research and Quality (AHRQ) concluded that the association between the 25OHD level and the risk of fracture was inconsistent.12
After a comprehensive review of the available research, the IOM committee concluded that a serum 25OHD level of 20 ng/mL would meet the needs for bone health for at least 97.5% of the US and Canadian populations.
TABLE 2
Calcium and vitamin D dietary reference intakes for adults, by life stage
| Life stage (gender) | Calcium | Vitamin D | |||
|---|---|---|---|---|---|
| RDA (mg/d) | Tolerable upper intake level (mg/d)* | RDA (IU/d) | Serum 25OHD level (ng/mL) (corresponding to the RDA)† | Tolerable upper intake level (IU/d)* | |
| 19–50 yr (male and female) | 1,000 | 2,500 | 600 | 20 | 4,000 |
| 51–70 yr (male) | 1,000 | 2,000 | 600 | 20 | 4,000 |
| 51–70 yr (female) | 1,200 | 2,000 | 600 | 20 | 4,000 |
| 71+ yr (male and female) | 1,200 | 2,000 | 800 | 20 | 4,000 |
| Adapted from: Ross AC, Manson JE, Abrams SA, et al. J Clin Endocrinol Metab. 2011;96(1):53–58. RDA = Recommended Dietary Allowance, 25OHD=25-hydroxyvitamin D * The tolerable upper intake level is the threshold above which is a risk of adverse events. The upper intake level is not intended to be a target intake. There is no consistent evidence of greater benefit at intake levels above the RDA. The serum 25OHD level corresponding to the upper intake level is 50 ng/mL. † Measures of the serum 25OHD level corresponding to the RDA and covering the requirements of at least 97.5% of the population. | |||||
The data on vitamin D insufficiency and nonskeletal outcomes
Many observational studies have reported relationships between vitamin D insufficiency and myriad nonskeletal health outcomes, particularly cardiovascular disease, cancer, diabetes, and autoimmune disorders.3 However, well-designed randomized clinical trials that examine nonskeletal outcomes as primary pre-specified outcomes are lacking.13 Such studies will be essential to elucidate the relationship between vitamin D insufficiency and nonskeletal chronic diseases. The VITamin D and OmegA-3 TriaL (VITAL) is an ongoing large-scale, randomized clinical trial designed to evaluate the role of supplementation with 2,000 IU of vitamin D3 daily in the primary prevention of cancer and cardiovascular disease.14
- Vitamin D plays a vital role in bone health
- The Institute of Medicine released a 2010 report that provided public health recommendations for vitamin D intake based on bone health outcomes
- Many observational studies have reported a relationship between vitamin D insufficiency and adverse nonskeletal health outcomes, including cardiovascular disease, cancer, diabetes, and autoimmune disorders, but evidence from randomized clinical trials on the potential nonskeletal benefits of vitamin D is sparse
- Excessive vitamin D intake should be avoided because of the potential for harm and the lack of evidence from well-designed clinical trials that vitamin D intake beyond the recommended amount affords greater skeletal or nonskeletal health benefits
- Among women who have an increased risk of vitamin D insufficiency or bone loss, 25OHD concentration should be measured and vitamin D supplementation should be provided as necessary to achieve the target 25OHD level
What we recommend for treatment
The IOM report provided the medical community with evidence-based recommendations for vitamin D intake at the population level, based on a public health perspective.1,2 However, the public health guideline model must be distinguished from the medical model, in which shared clinical decision-making between physician and patient occurs on an individual level and is informed by individual clinical risk factors. The public health recommendations detailed in the IOM report are not intended to replace or interfere with clinical judgment or preclude individualized clinical decision-making.
The debate over optimal levels of vitamin D supplementation for individual patients who have osteoporosis or other health conditions continues.15 Here, we provide general guidelines for treatment, based on the evidence available to date.

Clear benefits of vitamin D in bone health notwithstanding, advise your patients to avoid excessive intake because it can cause harm. See “More is not necessarily better”.
Recommendations for healthy adult nonpregnant women
Vitamin D intake: We recommend a daily vitamin D intake of 600 IU for healthy nonpregnant women up to age 70 years (and 800 Â IU daily for women older than 70 years) who are at average risk of vitamin D insufficiency and bone loss, consistent with the IOM recommendations. The IOM guidelines assume minimal to no sun exposure.
Measurement of 25OHD: It is not necessary to routinely measure the 25OHD level in these women. However, it is prudent to measure 25OHD in women who have risk factors for vitamin D insufficiency (TABLE 1) or a clinical condition associated with severe vitamin D deficiency. In these cases, if the 25OHD level is found to be below 20 ng/mL, vitamin D therapy should be initiated, with the goal of boosting the 25OHD level above the threshold of 20 ng/mL.
Treatment of vitamin D insufficiency: Options include daily vitamin D supplementation and higher-dose weekly preparations.
Many clinicians treat severe vitamin D insufficiency with 50,000 IU of vitamin D2 once weekly for 8 weeks, followed by a maintenance dose (described below) of vitamin D to preserve the target 25OHD level.5 An alternative is daily vitamin D supplementation, with the dosage based on the degree of insufficiency.
A general rule of thumb, for persons who have normal vitamin D absorption, is that every 1,000 IU of vitamin D3 ingested daily increases the 25OHD level by approximately 6 to 10 ng/mL.4,16 However, the incremental increase in the 25OHD concentration varies among individuals, depending on the baseline 25OHD level, with a greater incremental increase occurring at lower baseline 25OHD levels.
Monitoring of the 25OHD level after adjustment of the dosage is necessary to ensure that the target level is achieved.
Maintaining an adequate vitamin D level: Once vitamin D insufficiency has been corrected, a maintenance dosage of vitamin D should be selected—commonly 800 to 1,000 Â IU daily. A higher maintenance dosage may be required for persons who have genetic or ongoing environmental factors that predispose them to vitamin D insufficiency.
Vitamin D3 is reportedly more potent than D2 in increasing the 25OHD level,17 although this finding has not been universal.18 Monthly or twice-monthly administration of 50,000 IU of vitamin D2 is another option for maintenance of vitamin D sufficiency,5,16 although daily doses are more commonly used and are readily available in over-the-counter preparations.
Regardless of the regimen selected, the 25OHD level should be measured again approximately 3 months after a change in dosage to ensure that the target level has been achieved, with further dosage adjustments as indicated.
Recommendations for adult women at increased risk of skeletal disease
Measurement of 25OHD: The 25OHD level should be measured among women at increased risk of vitamin D insufficiency, bone loss, or fracture and among women who have established skeletal disease.
Vitamin D intake: We recommend that women at increased risk of osteoporosis and women older than 70 years receive at least 800 IU daily and, potentially, more if necessary to achieve the target 25OHD level.
Although the evidence to date does not support routine achievement of a 25OHD level substantially above 20 ng/mL in most women, many clinicians recommend that women in this higher-risk group maintain a 25OHD level above 30 ng/mL because of the possibly greater (although unproven) skeletal and nonskeletal benefits. As more data become available regarding the benefits and safety of vitamin D doses higher than those recommended by the IOM, these recommendations may be revised.
In 2010, the National Osteoporosis Foundation (NOF) recommended a vitamin D intake of 800 to 1,000 IU daily for all adults 50 years and older. Among persons at risk of deficiency, the NOF also recommended measurement of the serum 25OHD level, with vitamin D supplementation, as necessary, to achieve a 25OHD level of 30 ng/mL or higher.19 Also in 2010, the International Osteoporosis Foundation (IOF) recommended a target 25OHD level above 30 ng/mL for all older adults. The IOF also estimated that the average dosage required to achieve this level in older adults is 800 to 1,000 IU daily, noting that upward adjustment may be required in some people.4 It is unclear whether these guidelines will be revised in the future, based on the IOM report.
We recommend against achieving a 25OHD level above 50 ng/mL, based on evidence suggesting potential adverse health effects above this level.
Excessive vitamin D intake should be avoided because of the potential for harm and the lack of evidence from well-designed clinical trials that vitamin D intake beyond the currently recommended amount affords greater skeletal or nonskeletal health benefits. Although moderate vitamin D supplementation has proven skeletal benefits, a “U-shaped” curve for some outcomes has emerged, suggesting that excessive vitamin D supplementation may pose health risks. Notably, a recent clinical trial reported a higher risk of fracture (and falls) among elderly women treated annually with high-dose (500,000 IU) oral vitamin D3 versus placebo.20
A suggestion of adverse effects associated with 25OHD levels above 50 ng/mL has also emerged, from observational studies, for several nonskeletal health outcomes, including pancreatic cancer,21 cardiovascular disease,1 and all-cause mortality.22
Limited evidence is available regarding the safety and overall risk-benefit profile of long-term maintenance of 25OHD levels above the recommended dietary allowance (RDA) range. Therefore, you should remind your patients that, despite the importance of both prevention and treatment of vitamin D insufficiency, more is not necessarily better.
We want to hear from you! Tell us what you think.
1. Institute of Medicine. 2011 Dietary Reference Intakes for Calcium and Vitamin D. Washington DC: National Academies Press; 2011.
2. Ross AC, Manson JE, Abrams SA, et al. The 2011 report on dietary reference intakes for calcium and vitamin D from the Institute of Medicine: what clinicians need to know. J Clin Endocrinol Metab. 2011;96(1):53-58.
3. Rosen CJ. Clinical practice. Vitamin D insufficiency. N Engl J Med. 2011;364(3):248-254.
4. Dawson-Hughes B, Mithal A, Bonjour JP, et al. IOF position statement: vitamin D recommendations for older adults. Osteoporos Int. 2010;21(7):1151-1154.
5. Holick MF. Vitamin D deficiency. N Engl J Med. 2007;357(3):266-281.
6. Sai AJ, Walters RW, Fang X, Gallagher JC. Relationship between vitamin D parathyroid hormone, and bone health. J Clin Endocrinol Metab. 2011;96(3):E436-446.
7. Yetley EA. Assessing the vitamin D status of the US population. Am J Clin Nutr. 2008;88(2):558S-564S.
8. Jackson RD, LaCroix AZ, Gass M, et al. Women’s Health Initiative Investigators. Calcium plus vitamin D supplementation and the risk of fractures. N Engl J Med. 2006;354(7):669-683.
9. Papadimitropoulos E, Wells G, Shea B, et al. Osteoporosis Methodology Group and The Osteoporosis Research Advisory Group. Meta-analyses of therapies for postmenopausal osteoporosis. VIII: Meta-analysis of the efficacy of vitamin D treatment in preventing osteoporosis in postmenopausal women. Endocr Rev. 2002;23(4):560-569.
10. Bischoff-Ferrari HA, Willett WC, Wong JB, et al. Prevention of nonvertebral fractures with oral vitamin D and dose dependency: a meta-analysis of randomized controlled trials. Arch Intern Med. 2009;169(6):551-561.
11. Cauley JA, Lacroix AZ, Wu L, et al. Serum 25-hydroxyvitamin D concentrations and risk for hip fractures. Ann Intern Med. 2008;149(4):242-250.
12. Chung M, Balk EM, Brendel M, et al. Vitamin D and calcium: a systematic review of health outcomes. Evid Rep Technol Assess (Full Rep). 2009;(183):1-420.
13. Manson JE, Mayne ST, Clinton SK. Vitamin D and prevention of cancer—ready for prime time? N Engl J Med. 2011;364(15):1385-1387.
14. Manson JE. Vitamin D and the heart: why we need large-scale clinical trials. Cleve Clin J Med. 2010;77(12):903-910.
15. The Forum at Harvard School of Public Health. Boosting Vitamin D: Not enough or too much? The Andelot Series on Current Science Controversies. http://www.hsph.harvard.edu/forum/boosting-vitamin-d-not-enough-or-too-much.cfm. Published March 29 2011. Accessed April 22, 2011.
16. Binkley N, Gemar D, Engelke J, et al. Evaluation of ergocalciferol or cholecalciferol dosing, 1,600 IU daily or 50,000 IU monthly in older adults. J Clin Endocrinol Metab. 2011;96(4):981-988.
17. Heaney RP, Recker RR, Grote J, Horst RL, Armas LA. Vitamin D(3) is more potent than vitamin D(2) in humans. J Clin Endocrinol Metab. 2011;96(3):E447-452.
18. Holick MF, Biancuzzo RM, Chen TC, et al. Vitamin D2 is as effective as vitamin D3 in maintaining circulating concentrations of 25-hydroxyvitamin D. J Clin Endocrinol Metab. 2008;93(3):677-681.
19. National Osteoporosis Foundation. Clinician’s Guide to Prevention and Treatment of Osteoporosis. Washington DC: National Osteoporosis Foundation; 2010. http://www.nof.org/professionals/clinical-guidelines. Accessed June 7, 2011.
20. Sanders KM, Stuart AL, Williamson EJ, et al. Annual high-dose oral vitamin D and falls and fractures in older women: a randomized controlled trial. JAMA. 2010;303(18):1815-1822.
21. Stolzenberg-Solomon RZ, Jacobs EJ, Arslan AA, et al. Circulating 25-hydroxyvitamin D and risk of pancreatic cancer: Cohort Consortium Vitamin D Pooling Project of Rarer Cancers. Am J Epidemiol. 2010;172(1):81-93.
22. Melamed ML, Michos ED, Post W, Astor B. 25-hydroxyvitamin D levels and the risk of mortality in the general population. Arch Intern Med. 2008;168(15):1629-1637.
1. Institute of Medicine. 2011 Dietary Reference Intakes for Calcium and Vitamin D. Washington DC: National Academies Press; 2011.
2. Ross AC, Manson JE, Abrams SA, et al. The 2011 report on dietary reference intakes for calcium and vitamin D from the Institute of Medicine: what clinicians need to know. J Clin Endocrinol Metab. 2011;96(1):53-58.
3. Rosen CJ. Clinical practice. Vitamin D insufficiency. N Engl J Med. 2011;364(3):248-254.
4. Dawson-Hughes B, Mithal A, Bonjour JP, et al. IOF position statement: vitamin D recommendations for older adults. Osteoporos Int. 2010;21(7):1151-1154.
5. Holick MF. Vitamin D deficiency. N Engl J Med. 2007;357(3):266-281.
6. Sai AJ, Walters RW, Fang X, Gallagher JC. Relationship between vitamin D parathyroid hormone, and bone health. J Clin Endocrinol Metab. 2011;96(3):E436-446.
7. Yetley EA. Assessing the vitamin D status of the US population. Am J Clin Nutr. 2008;88(2):558S-564S.
8. Jackson RD, LaCroix AZ, Gass M, et al. Women’s Health Initiative Investigators. Calcium plus vitamin D supplementation and the risk of fractures. N Engl J Med. 2006;354(7):669-683.
9. Papadimitropoulos E, Wells G, Shea B, et al. Osteoporosis Methodology Group and The Osteoporosis Research Advisory Group. Meta-analyses of therapies for postmenopausal osteoporosis. VIII: Meta-analysis of the efficacy of vitamin D treatment in preventing osteoporosis in postmenopausal women. Endocr Rev. 2002;23(4):560-569.
10. Bischoff-Ferrari HA, Willett WC, Wong JB, et al. Prevention of nonvertebral fractures with oral vitamin D and dose dependency: a meta-analysis of randomized controlled trials. Arch Intern Med. 2009;169(6):551-561.
11. Cauley JA, Lacroix AZ, Wu L, et al. Serum 25-hydroxyvitamin D concentrations and risk for hip fractures. Ann Intern Med. 2008;149(4):242-250.
12. Chung M, Balk EM, Brendel M, et al. Vitamin D and calcium: a systematic review of health outcomes. Evid Rep Technol Assess (Full Rep). 2009;(183):1-420.
13. Manson JE, Mayne ST, Clinton SK. Vitamin D and prevention of cancer—ready for prime time? N Engl J Med. 2011;364(15):1385-1387.
14. Manson JE. Vitamin D and the heart: why we need large-scale clinical trials. Cleve Clin J Med. 2010;77(12):903-910.
15. The Forum at Harvard School of Public Health. Boosting Vitamin D: Not enough or too much? The Andelot Series on Current Science Controversies. http://www.hsph.harvard.edu/forum/boosting-vitamin-d-not-enough-or-too-much.cfm. Published March 29 2011. Accessed April 22, 2011.
16. Binkley N, Gemar D, Engelke J, et al. Evaluation of ergocalciferol or cholecalciferol dosing, 1,600 IU daily or 50,000 IU monthly in older adults. J Clin Endocrinol Metab. 2011;96(4):981-988.
17. Heaney RP, Recker RR, Grote J, Horst RL, Armas LA. Vitamin D(3) is more potent than vitamin D(2) in humans. J Clin Endocrinol Metab. 2011;96(3):E447-452.
18. Holick MF, Biancuzzo RM, Chen TC, et al. Vitamin D2 is as effective as vitamin D3 in maintaining circulating concentrations of 25-hydroxyvitamin D. J Clin Endocrinol Metab. 2008;93(3):677-681.
19. National Osteoporosis Foundation. Clinician’s Guide to Prevention and Treatment of Osteoporosis. Washington DC: National Osteoporosis Foundation; 2010. http://www.nof.org/professionals/clinical-guidelines. Accessed June 7, 2011.
20. Sanders KM, Stuart AL, Williamson EJ, et al. Annual high-dose oral vitamin D and falls and fractures in older women: a randomized controlled trial. JAMA. 2010;303(18):1815-1822.
21. Stolzenberg-Solomon RZ, Jacobs EJ, Arslan AA, et al. Circulating 25-hydroxyvitamin D and risk of pancreatic cancer: Cohort Consortium Vitamin D Pooling Project of Rarer Cancers. Am J Epidemiol. 2010;172(1):81-93.
22. Melamed ML, Michos ED, Post W, Astor B. 25-hydroxyvitamin D levels and the risk of mortality in the general population. Arch Intern Med. 2008;168(15):1629-1637.
Immunizing the Adult Patient
Reported incidence rates of certain vaccine-preventable diseases—measles, rubella, diphtheria, polio, and tetanus—are low in the United States.1 However, as was demonstrated during the 2009-2010 flu season and the outbreak of H1N1 influenza,2 we cannot afford to be complacent in our attitudes toward vaccines and vaccination. New virus strains exist and can become endemic quickly and ravenously. Furthermore, certain vaccine-preventable illnesses are frequently reported among adult patients, including hepatitis B, herpes zoster, human papillomavirus infection, influenza, pertussis, and pneumococcal infection.3
Vigilance regarding vaccination of children and adults was addressed by the CDC’s Office of Disease Prevention and Health Promotion in Healthy People 2010.4 These target objectives are proposed to be retained in Healthy People 2020,5 as the objectives from 2010 have not been met. The Healthy People 2010 target for administration of the pneumococcal vaccine in adults ages 65 and older, for example, is 90%. As of 2008, research has shown, only 60% of that population was immunized.6
There are subgroups of immunocompromised people who will probably never achieve adequate antibody levels to ensure immunity to vaccine-preventable diseases (for example, measles and influenza can be deadly to the immunocompromised person, as they can be to the very young and the very old). This is an important reason why vaccination of the healthy population is essential: the concept of herd immunity.7 Herd immunity (or community immunity) suggests that if most people around you are immune to an infection and do not become ill, then there is no one who can infect you—even if you are not immune to the infection.
WHY SOME ADULTS MIGHT NEED VACCINES
Adults who were vaccinated as children may incorrectly assume that they are protected from disease for life. In the case of some diseases, this may be true. However:
• Some adults were never vaccinated as children
• Newer vaccines have been developed since many adults were children
• Immunity can begin to fade over time
• As we age, we become more susceptible to serious disease caused by common infections (eg, influenza, pneumococcus).8
Barriers to Vaccination
Barriers to vaccination are varied, but none is insurmountable. Some of these barriers include8-15:
Missed opportunities. Providers should address vaccination needs for both adults and children at each visit or encounter. According to the CDC, studies have shown that eliminating missed opportunities could increase vaccination coverage by as much as 20%.8,11
Provider misconceptions regarding vaccine contraindications, schedules, and simultaneous vaccine administration. These misconceptions may prompt providers to forego an opportunity to vaccinate. Up-to-date information about vaccinations and ongoing provider education are imperative to improve immunity among both adults and children against vaccine-preventable disease.8 Numerous Web sites and publications are instrumental and essential in furnishing the health care provider with the most current information about vaccinations (see Table 1, above, and Table 2,10,12-14).
A belief on patients’ part that they are fully vaccinated when they are not. It is important to provide a vaccination record and a return date at every vaccination encounter, even if just one vaccination has been administered on a given day. Participating in Immunization Information System,15 if one is available, is an efficient way to access computerized vaccine records easily at the point of contact.
Just as parents should be encouraged to bring their child’s vaccine record with them to every health care visit, adults are also called upon to maintain a record of all their vaccinations. Each entry in the immunization record should include:
• The type of vaccine and dose
• The site and route of administration
• The date that the vaccine was administered
• The date that the next dose is due
• The manufacturer and lot number
• The name, address, and title of the person who administered the vaccine.15
PRINCIPLES OF VACCINATION
There are two ways to acquire immunity: active and passive.
Active immunity is produced by the individual’s own immune system and usually represents a permanent immunity toward the antigen.10,16
Passive immunity is produced when the individual receives products of immunity made by another animal or a human and transferred to the host. Passive immunity can be accomplished by injection of these products or through the placenta in infants. This type of immunity is not permanent and wanes over time—usually within weeks or months.10,16
This article will concentrate on active immunity, acquired through the administration of vaccines.
CLASSIFICATION OF VACCINES
Vaccines are classified as either live, attenuated vaccine (viral or bacterial) or inactivated vaccine.
Live, attenuated vaccines are derived from “wild” or disease-causing viruses and bacteria. Through procedures conducted in the laboratory, these wild organisms are weakened or attenuated. The live, attenuated vaccine must grow and replicate in the vaccinated person in order to stimulate an immune response. However, because the organism has been weakened, it usually does not cause disease or illness.10,16
The immune response to a live, attenuated vaccine is virtually identical to a response produced by natural infection. In rare instances, however, live, attenuated vaccines may cause severe or fatal reactions as a result of uncontrolled replication of the organism. This occurs only in individuals who are significantly immunocompromised.10,16
Inactivated vaccines are produced by growing the viral or bacterial organism in a culture medium, then using heat and/or chemicals to inactivate the organism. Because inactivated vaccines are not alive, they cannot replicate, and therefore cannot cause disease, even in an immunodeficient patient. The immune response to an inactivated vaccine is mostly humoral (in contrast to the natural infection response of a live vaccine), and little or no cellular immunity is produced.10,16
Inactivated vaccines always require multiple doses, gradually building up a protective immune response. The antibody titers diminish over time, so some inactivated vaccines may require periodic doses to “boost” or increase the titers.16
Some vaccines, such as hepatitis B vaccine, lead to the development of immune memory, which stays intact for at least 20 years following immunization. Immune memory occurs during replication of B cells and T cells; some cells will become long-lived memory cells that will “remember” the pathogen and produce an immune response if the pathogen is detected again. In this case, boosters are not recommended.10
Toxoids are a type of vaccine made from the inactivated toxin of a bacterium—not the bacterium itself. Tetanus and diphtheria vaccines are examples of toxoid vaccines.10,16
Subunit and conjugate vaccines are segments of the pathogen. A subunit vaccine can be created via genetic engineering. The end result is a recombinant vaccine that can stimulate cell memory (eg, hepatitis B vaccine).10,16 Conjugate vaccines, which are similar to recombinant vaccines, are made by combining two different components to prompt a more powerful, combined immune response.10
Spacing of Live-Virus and Inactivated Vaccines
There are almost no spacing requirements between two or more inactivated vaccines8 (see Table 38,14 for spacing recommendations from the Advisory Committee on Immunization Practices [ACIP]). The only vaccines that must be spaced at least four weeks apart are live-virus vaccines—that is, if they are not given on the same day. Studies have shown that the immune response to a live-virus vaccine may be impaired if it is administered within 30 days of another live-virus vaccine. Inactivated vaccines, on the other hand, may usually be administered at any time after or before a live-virus vaccine.8
One exception to this statement is the administration of Zostavax (zoster live-virus vaccine) with Pneumovax 23 (inactivated pneumococcal vaccine, polyvalent, MSD).17-19 The manufacturer of the two vaccines recommends a spacing of at least four weeks between them, based on research showing that concomitant use may result in reduced immunogenicity for Zostavax.20 However, as of this writing, ACIP has not revised its statement that both vaccines can be given at the same time or at any time before or after each other.21
Live-virus vaccines currently licensed in the US provide protection against diseases including measles/mumps/rubella, varicella, zoster (ie, shingles), influenza, and yellow fever.
ADULT VACCINATION HIGHLIGHTS
A summary of 2011 recommendations for adult immunization from ACIP is shown in Table 412,21. The following information is specific to each of the vaccine-preventable illnesses of concern in adults.12,13,22
Seasonal Influenza
The options to protect the adult patient against seasonal influenza are a trivalent, inactivated influenza vaccine (TIV; Fluzone, high-dose Fluzone for adults ages 65 and older, Fluvirin, Fluarix, FluLaval, Afluria, Agriflu) or a live, attenuated influenza vaccine (LAIV; FluMist).2,23 Dosage of TIV for adults is 0.5 mL IM in the deltoid once annually. For adults ages 49 and younger, LAIV is administered at 0.2 mL intranasally, once per year.
ACIP now recommends universal influenza vaccination for all persons ages 6 months and older with no contraindications.2,8 Strong consideration should be given to concurrent administration of influenza vaccine and pneumococcal vaccine to high-risk persons not previously vaccinated against pneumococcal disease.12
Note: When influenza and pneumococcal vaccines are given at the same time, they should be administered in opposite arms to reduce the risk of adverse reactions or a decreased antibody response to either vaccine.18,19
Pneumococcal Polysaccharide (PPSV)
Pneumovax 2319 is administered as a 0.5-mL dose IM in the deltoid or subcutaneously in the upper arm. The vaccine is recommended for8,19,24:
• Adults ages 65 and older who have not been previously vaccinated
• Adults now 65 and older who received PPSV vaccine at least five years ago and were younger than 65 at that time
• Adults ages 19 through 64 years who have asthma or who smoke24
• Any adult with the following underlying medical conditions: chronic heart or lung disease, diabetes mellitus, cerebrospinal fluid leaks, cochlear implants, chronic liver disease, cirrhosis, chronic alcoholism, functional or anatomic asplenia, and immunocompromising conditions (HIV infection, diseases that require immunosuppressive therapy, chemotherapy, or radiation therapy; congenital immunodeficiency).24
A one-time revaccination is recommended after five years for persons ages 19 to 64 who have chronic renal failure, nephrotic syndrome, or functional or anatomic asplenia, and for those who are immunocompromised.24
Note: According to the manufacturer of Zostavax18 and Pneumovax,23,19 these vaccines should not be given at the same time, as research has shown that Zostavax immunogenicity is reduced as a result.18-20
Zoster
For adults ages 60 and older, Zostavax18 is administered in a single 0.65-mL dose, subcutaneously in the upper arm. Providers are not required to ask about varicella vaccination history or history of varicella disease before administering the vaccine. Adults ages 60 and older who have previously had shingles can still be vaccinated during a routine health care visit.10,21,22
Immunization is contraindicated in adults with a previous anaphylactic reaction to neomycin or gelatin, although a nonanaphylactic reaction to neomycin (most commonly, contact dermatitis) is not considered a contraindication.8
Any adult patient who has close household or occupational contact with persons at risk for severe varicella (eg, infants) need not take precautions after receiving the zoster vaccine, except in the rare case in which a varicella-like rash develops.10,21,22
Note: Review the note appearing in “Pneumococcal Polysaccharide (PPSV),” above, regarding coadministration of Zostavax and Pneumovax 23.18-20
Varicella
Adults who were born in the US before 1980 are considered immune to varicella and don’t need to be vaccinated, with the exception of health care workers, pregnant women, and immunocompromised persons. Nonimmune healthy adults who have not previously undergone vaccination should receive two 0.5-mL doses of Varivax, administered subcutaneously, four to eight weeks apart.25
Immunization is contraindicated in adults with a previous anaphylactic reaction to neomycin or gelatin, although a nonanaphylactic reaction to neomycin (eg, contact dermatitis) is not considered a contraindication.8
Measles, Mumps, Rubella (MMR)
The MMR vaccine is administered at 0.5 mL, given subcutaneously in the posterolateral fat of the upper arm.8
MMR-susceptible adults who were born during or since 1957 and are not at increased risk (see below) need only one dose of the MMR vaccine; those considered at increased risk need two doses, and a second dose can also be considered during an outbreak. Adults who require two doses should wait at least four weeks between the first and second doses.12
The following factors place adults at increased risk for MMR:
• Anticipated international travel
• Being a student in a post–secondary educational setting
• Working in a health care facility
• Recent exposure to measles, or an outbreak of measles or mumps
• Previous vaccination with killed measles vaccine
• Previous vaccination with an unknown measles vaccine between 1963 and 1967.
Also at risk are health care workers born before 1957 who have no evidence of immunity, and women who plan to become pregnant and have no evidence of immunity.8,12
Tetanus, Diphtheria, Pertussis
Options for adults include a vaccine against tetanus and diphtheria (Td; Decavac); or a vaccine that protects against tetanus, diphtheria, and acellular pertussis (Tdap; Adacel, Boostrix). Adults who have not been previously vaccinated should receive one dose of Tdap and two doses of Td (the first, one month after Tdap; the second at six to 12 months after the Tdap). Each is administered as a 0.5-mL dose IM in the deltoid. A booster dose is recommended every 10 years but can be given earlier in patients who sustain wounds or who anticipate international travel.8,12
Adults ages 19 through 64 should receive a single dose of Tdap in place of a booster dose if the last Td dose was administered at least 10 years earlier and the patient has not previously received Tdap. Additionally, a dose of Tdap (if not previously given) is recommended for postpartum women, close contacts of infants younger than 12 months, and all health care workers with direct patient contact. An interval as short as two years from the last Td is suggested; shorter intervals may be appropriate.8,12
According to the new 2011 recommendations, persons ages 65 and older who have close contact with an infant younger than 12 months should be vaccinated with Tdap, and any person age 65 or older may be vaccinated with Tdap. Also added is a recommendation to administer Tdap, regardless of the interval since the patient received his or her most recent Td-containing vaccine.8,12
Human Papillomavirus (HPV)
Gardasil26 protects both female and male patients against HPV infection; Cervarix27 is indicated only for female patients. Either quadrivalent vaccine or bivalent vaccine is recommended for female patients.12
In women ages 26 and younger, Gardasil (0.5 mL IM, administered in the deltoid at 0 month, 2 months, and 6 months) provides protection against diseases caused by HPV types 6, 11, 16, and 18 (including cervical, vaginal, and vulvar cancer caused by HPV types 16 and 18). In men ages 26 and younger, Gardasil provides protection against genital warts caused by HPV types 6 and 11.26
Cervarix,27 administered at 0 month, 1 month, and 6 months (0.5 mL IM in the deltoid), provides protection for women ages 25 and younger against cervical cancer and precancerous lesions caused by HPV types 16 and 18.
Caution: Patients should be advised to sit or lie down when the HPV vaccine is administered, and they should be observed for the subsequent 15 minutes. Syncope can occur after vaccination—most commonly among adolescents and young adults.28 Convulsive syncope has been reported.
Meningococcal Disease
Two vaccines are available to protect against meningococcal disease: Menactra29 (meningococcal groups A, C, Y, and W-135 polysaccharide diphtheria toxoids conjugate vaccine); and Menveo30 (meningococcal groups A, C, Y, and W-135 oligosaccharide diphtheria CRM197 conjugate vaccine). Both are administered in the deltoid, 0.5 mL IM.
The following patients should be considered for vaccination:
• College freshmen living in dormitories, as well as college students with immune deficiencies, as they are at higher risk for meningococcal disease
• Patients who travel to or reside in countries in which Neisseria meningitidis is epidemic (particularly those with the potential for prolonged contact with the local population)
• Travelers to Saudi Arabia for pilgrimage to Mecca (the Hajj)
• Patients with anatomical or functional asplenia (two-dose series).
A two-dose series of meningococcal conjugate vaccine is also recommended for adults with persistent complement component deficiencies, and for those with HIV infection who are vaccinated.12
Hepatitis A
Two hepatitis A vaccines (both inactivated) can be used interchangeably: Havrix31 and Vaqta.32 Dosing for both vaccines in 18-year-old patients is 0.5 mL IM in the deltoid at 0 months, then at 6 to 12 months. In patients ages 19 and older, administration is the same, with the exception of increased dosing (1.0 mL IM).
Vaccination against hepatitis A is recommended for men who have sex with men, and for all adult patients who12:
• Travel to or work in areas where risk for hepatitis A transmission is high (especially those who take frequent trips or experience prolonged stays)
• Use injection drugs
• Have chronic liver disease
• Receive clotting factor concentrates for treatment of a blood-clotting disorder
• May have been exposed to hepatitis A in the previous two weeks
• Wish to be vaccinated against hepatitis A to avoid future infection.
Hepatitis B
Recombivax HB33 and Engerix-B34 are the two vaccines available to protect patients against hepatitis B (HBV), and they can be used interchangeably.12 In patients from birth through age 19, Recombivax HB33 or Engerix-B34 is given as 0.5 mL IM in the deltoid at 0, 1, and 6 months; patients ages 20 and older receive an increased dose (1.0 mL IM), with administration otherwise the same. According to the manufacturer of Recombivax HB,33 patients age 11 through 15 may be given either three doses of 0.5 mL or two doses of 1.0 mL.
The following adults are advised to undergo vaccination for HBV:
• At-risk, unvaccinated adults
• Those requesting protection against HBV infection
• Those planning to travel to areas where HBV is common
• Household contacts of a patient with chronic HBV infection, and sexual partners of a patient with HBV infection
• Adults with chronic liver disease
• Men who have sex with men
• Sexually active adults who are not in a long-term, mutually monogamous relationship
• Adults who are being evaluated or treated for a sexually transmitted disease, including HIV infection
• Health care or public safety workers who may be exposed to blood or blood-contaminated body fluids
• Workers and residents in facilities for developmentally disabled persons
• Patients undergoing or anticipating dialysis
• Adults who inject illegal drugs or who have done so recently.12
CONTRAINDICATIONS AND PRECAUTIONS FOR VACCINES COMMONLY USED IN ADULTS
See Table 5,8,22 for a summary of contraindications and precautions from ACIP and the Immunization Action Coalition that are associated with vaccinations mentioned in this article. A more complete summary can be found at www.im munize.org/catg.d/p3072a.pdf.
Conclusion
The adult patient’s vaccination status should be addressed at each health care encounter, and current recommendations should be followed. The duration of efficacy for vaccines is not an exact science. Many vaccines licensed in the US are relatively new, and recommendations for boosters for some of these vaccines will be forthcoming as more data are gathered. For example, the recommendation that a booster dose of Tdap be given to adults resulted from the recent increase in reported pertussis cases.35
Providers armed with the most current information and resources represent the forefront in ensuring that the US adult population is adequately immunized.
REFERENCES
1. World Health Organization. Immunization surveillance, assessment, and monitoring (2010). www.who.int/immunization_monitor ing/en. Accessed May 12, 2011.
2. Fiore AE, Uyeki TM, Broder K, et al; Centers for Disease Control and Prevention. Prevention and control of influenza with vaccines: recommendations of the Advisory Committee on Immunization Practices (ACIP), 2010. MMWR Morb Mortal Wkly Rep. 2010;59(RR-08);1-62.
3. Schaffner W. Update on vaccine-preventable diseases: are adults in your community adequately protected? J Fam Pract. 2008;57(4 suppl):S1-S11.
4. CDC. Healthy People 2010: Objectives for Improving Health. www.healthypeople.gov. Accessed May 6, 2011.
5. US Department of Health and Human Services. Developing Healthy People 2020: immunization and infectious diseases. www.healthy people.gov/2020. Accessed May 12, 2011.
6. Lu PJ, Nuorti JP. Pneumococcal polysaccharide vaccination among adults aged 65 years and older, United States, 1989-2008. Am J Prev Med. 2010;39(4):287-295.
7. National Institute of Allergy and Infectious Diseases, NIH. Community immunity (“herd” immunity) (2010). www.niaid.nih.gov/topics/pages/communityimmunity.aspx. Accessed May 12, 2011.
8. National Center for Immunization and Respiratory Diseases. General recommendations on immunization: recommendations of the Advisory Committee on Immunization Practices (ADIP). MMWR Recomm Rep. 2011;60(2):1-64.
9. High KP. Overcoming barriers to adult immunization. J Am Osteopath Assoc. 2009;109(6): 525-528.
10. CDC; Atkinson W, Wolfe S, Hamborsky J, eds. Epidemiology and Prevention of Vaccine-Preventable Diseases (Pink Book). 12th ed. Washington, DC: Public Health Foundation, 2011.
11. CDC. Vaccine-preventable diseases: improving vaccination coverage in children, adolescents, and adults: a report on recommendations from the Task Force on Community Preventive Services. MMWR Recomm Rep. 1999;48(RR-8):1-15.
12. CDC. Recommended adult immunization schedule: United States, 2011. MMWR Morb Mortal Wkly Rep. 2011;60(4):1-4.
13. Thompson RF. Travel & Routine Immunizations: A Practical Guide for the Medical Office. 19th ed. Milwaukee, WI: Shoreland, Inc: 2001.
14. American Academy of Pediatrics. Pertussis. In: Pickering LK, Backer, CJ, Long SS, McMillan J, eds. Red Book: 2006 Report of the Committee on Infectious Diseases. 27th ed. Elk Grove Village, IL: American Academy of Pediatrics.
15. CDC. Immunization information systems (IIS). www.cdc.gov/vaccines/programs/iis/default.htm. Accessed May 12, 2011.
16. College of Physicians of Philadelphia. The history of vaccines: a project of the College of Physicians of Philadelphia (2011). www.history ofvaccines.org/content/articles/different-types-vaccines. Accessed May 12, 2011.
17. US Food and Drug Administration. Vaccines, blood, and biologics: December 18, 2009 Approval Letter—Zostavax. www.fda.gov/
BiologicsBloodVaccines/Vaccines/Approved Products/ucm195993.htm. Accessed May 12, 2011.
18. Merck & Co, Inc. Zostavax® (zoster vaccine live; product insert). www.fda.gov/downloads/BiologicsBloodVaccines/Vaccines/Approved Products/UCM132831.pdf. Accessed May 12, 2011.
19. Merck & Co, Inc. Pneumovax® 23 (pneumococcal vaccine, polyvalent, MSD; product information). www.merck.com/product/usa/pi_circulars/p/pneumovax_23/pneumovax_pi.pdf. Accessed May 16, 2011.
20. Macintyre CR, Egerton T, McCaughey M, et al. Concomitant administration of zoster and pneumococcal vaccines in adults ≥60 years old. Hum Vaccin. 2010;6(11):18-26.
21. CDC. Prevention of herpes zoster: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep. 2008;57(RR-5):1–30.
22. Immunization Action Coalition. Vaccinate Adults. 2010 Aug;14(5). www.immunize.org/va. Accessed May 12, 2011.
23. CDC. Update: recommendations of the Advisory Committee on Immunization Practices (ACIP) regarding use of CSL seasonal influenza vaccine (Afluria) in the United States during 2010–2011. MMWR Morb Mortal Wkly Rep. 2010;59(31);989-992.
24. CDC. Updated recommendations for prevention of invasive pneumococcal disease among adults using the 23-valent pneumococcal polysaccharide vaccine (PPSV23). MMWR Morb Mortal Wkly Rep. 2010;59(RR-34):1102-1106.
25. Merck & Co, Inc. Varivax® varicella virus vaccine live (product information). www.merck
.com/product/usa/pi_circulars/v/varivax/varivax_pi.pdf. Accessed May 12, 2011.
26. Merck & Co, Inc. Gardasil® (human papillomavirus quadrivalent [types 6, 11, 16, and 18] vaccine, recombinant; product information). www.merck.com/product/usa/pi_circulars/g/gardasil/gardasil_ppi.pdf. Accessed May 12, 2011.
27. GlaxoSmithKline Biologicals. Cervarix (human papillomavirus bivalent [types 16 and 18] vaccine, recombinant; product information). http://us.gsk.com/products/assets/us_cervarix.pdf. Accessed May 12, 2011.
28. CDC. Syncope after vaccination—United States, January 2005-July 2007. MMWR Morb Mortal Wkly Rep. 2008;57(17):457-460.
29. Sanofi Pasteur. Meningococcal (groups A, C, Y, and W-135) polysaccharide diphtheria
toxoids conjugate vaccine Menactra® for intramuscular injection. www.fda.gov/downloads/BiologicsBloodVaccines/Vaccines/Approved
Products/UCM131170.pdf. Accessed May 12, 2011.
30. Novartis Vaccines and Diagnostics, Inc. Menveo® (meningococcal [groups A, C, Y and W-135] oligosaccharide diphtheria CRM197 conjugate vaccine solution for intramuscular injection; prescribing information highlights). www .fda.gov/downloads/biologicsbloodvaccines/vaccines/approvedproducts/ucm201349.pdf. Accessed May 12, 2011.
31. GlaxoSmithKline Biologicals. Havrix (hepatitis A vaccine, suspension for intramuscular injection; prescribing information highlights). http://us.gsk.com/products/assets/us_havrix .pdf. Accessed May 12, 2011.
32. Merck & Co, Inc. Vaqta (hepatitis A vaccine, inactivated; suspension for intramuscular injection; highlighted prescribing information). www.merck.com/product/usa/pi_circulars/v/vaqta/vaqta_pi.pdf. Accessed May 12, 2011.
33. Merck & Co, Inc. Recombivax HB® hepatitis B vaccine (recombinant; product information). www.merck.com/product/usa/pi_circulars/r/recombivax_hb/recombivax_pi.pdf. Accessed May 12, 2011.
34. GlaxoSmithKline Biologicals. Engerix-B® (hepatitis B vaccine, recombinant; prescribing information). http://us.gsk.com/products/assets/us_engerixb.pdf. Accessed May 12, 2011.
35. CDC. Tetanus and pertussis vaccination coverage among adults aged ≥ 18 years—United States, 1999 and 2008. MMWR Morb Mortal Wkly Rep. 2010;59(40):1302-1306.
Reported incidence rates of certain vaccine-preventable diseases—measles, rubella, diphtheria, polio, and tetanus—are low in the United States.1 However, as was demonstrated during the 2009-2010 flu season and the outbreak of H1N1 influenza,2 we cannot afford to be complacent in our attitudes toward vaccines and vaccination. New virus strains exist and can become endemic quickly and ravenously. Furthermore, certain vaccine-preventable illnesses are frequently reported among adult patients, including hepatitis B, herpes zoster, human papillomavirus infection, influenza, pertussis, and pneumococcal infection.3
Vigilance regarding vaccination of children and adults was addressed by the CDC’s Office of Disease Prevention and Health Promotion in Healthy People 2010.4 These target objectives are proposed to be retained in Healthy People 2020,5 as the objectives from 2010 have not been met. The Healthy People 2010 target for administration of the pneumococcal vaccine in adults ages 65 and older, for example, is 90%. As of 2008, research has shown, only 60% of that population was immunized.6
There are subgroups of immunocompromised people who will probably never achieve adequate antibody levels to ensure immunity to vaccine-preventable diseases (for example, measles and influenza can be deadly to the immunocompromised person, as they can be to the very young and the very old). This is an important reason why vaccination of the healthy population is essential: the concept of herd immunity.7 Herd immunity (or community immunity) suggests that if most people around you are immune to an infection and do not become ill, then there is no one who can infect you—even if you are not immune to the infection.
WHY SOME ADULTS MIGHT NEED VACCINES
Adults who were vaccinated as children may incorrectly assume that they are protected from disease for life. In the case of some diseases, this may be true. However:
• Some adults were never vaccinated as children
• Newer vaccines have been developed since many adults were children
• Immunity can begin to fade over time
• As we age, we become more susceptible to serious disease caused by common infections (eg, influenza, pneumococcus).8
Barriers to Vaccination
Barriers to vaccination are varied, but none is insurmountable. Some of these barriers include8-15:
Missed opportunities. Providers should address vaccination needs for both adults and children at each visit or encounter. According to the CDC, studies have shown that eliminating missed opportunities could increase vaccination coverage by as much as 20%.8,11
Provider misconceptions regarding vaccine contraindications, schedules, and simultaneous vaccine administration. These misconceptions may prompt providers to forego an opportunity to vaccinate. Up-to-date information about vaccinations and ongoing provider education are imperative to improve immunity among both adults and children against vaccine-preventable disease.8 Numerous Web sites and publications are instrumental and essential in furnishing the health care provider with the most current information about vaccinations (see Table 1, above, and Table 2,10,12-14).
A belief on patients’ part that they are fully vaccinated when they are not. It is important to provide a vaccination record and a return date at every vaccination encounter, even if just one vaccination has been administered on a given day. Participating in Immunization Information System,15 if one is available, is an efficient way to access computerized vaccine records easily at the point of contact.
Just as parents should be encouraged to bring their child’s vaccine record with them to every health care visit, adults are also called upon to maintain a record of all their vaccinations. Each entry in the immunization record should include:
• The type of vaccine and dose
• The site and route of administration
• The date that the vaccine was administered
• The date that the next dose is due
• The manufacturer and lot number
• The name, address, and title of the person who administered the vaccine.15
PRINCIPLES OF VACCINATION
There are two ways to acquire immunity: active and passive.
Active immunity is produced by the individual’s own immune system and usually represents a permanent immunity toward the antigen.10,16
Passive immunity is produced when the individual receives products of immunity made by another animal or a human and transferred to the host. Passive immunity can be accomplished by injection of these products or through the placenta in infants. This type of immunity is not permanent and wanes over time—usually within weeks or months.10,16
This article will concentrate on active immunity, acquired through the administration of vaccines.
CLASSIFICATION OF VACCINES
Vaccines are classified as either live, attenuated vaccine (viral or bacterial) or inactivated vaccine.
Live, attenuated vaccines are derived from “wild” or disease-causing viruses and bacteria. Through procedures conducted in the laboratory, these wild organisms are weakened or attenuated. The live, attenuated vaccine must grow and replicate in the vaccinated person in order to stimulate an immune response. However, because the organism has been weakened, it usually does not cause disease or illness.10,16
The immune response to a live, attenuated vaccine is virtually identical to a response produced by natural infection. In rare instances, however, live, attenuated vaccines may cause severe or fatal reactions as a result of uncontrolled replication of the organism. This occurs only in individuals who are significantly immunocompromised.10,16
Inactivated vaccines are produced by growing the viral or bacterial organism in a culture medium, then using heat and/or chemicals to inactivate the organism. Because inactivated vaccines are not alive, they cannot replicate, and therefore cannot cause disease, even in an immunodeficient patient. The immune response to an inactivated vaccine is mostly humoral (in contrast to the natural infection response of a live vaccine), and little or no cellular immunity is produced.10,16
Inactivated vaccines always require multiple doses, gradually building up a protective immune response. The antibody titers diminish over time, so some inactivated vaccines may require periodic doses to “boost” or increase the titers.16
Some vaccines, such as hepatitis B vaccine, lead to the development of immune memory, which stays intact for at least 20 years following immunization. Immune memory occurs during replication of B cells and T cells; some cells will become long-lived memory cells that will “remember” the pathogen and produce an immune response if the pathogen is detected again. In this case, boosters are not recommended.10
Toxoids are a type of vaccine made from the inactivated toxin of a bacterium—not the bacterium itself. Tetanus and diphtheria vaccines are examples of toxoid vaccines.10,16
Subunit and conjugate vaccines are segments of the pathogen. A subunit vaccine can be created via genetic engineering. The end result is a recombinant vaccine that can stimulate cell memory (eg, hepatitis B vaccine).10,16 Conjugate vaccines, which are similar to recombinant vaccines, are made by combining two different components to prompt a more powerful, combined immune response.10
Spacing of Live-Virus and Inactivated Vaccines
There are almost no spacing requirements between two or more inactivated vaccines8 (see Table 38,14 for spacing recommendations from the Advisory Committee on Immunization Practices [ACIP]). The only vaccines that must be spaced at least four weeks apart are live-virus vaccines—that is, if they are not given on the same day. Studies have shown that the immune response to a live-virus vaccine may be impaired if it is administered within 30 days of another live-virus vaccine. Inactivated vaccines, on the other hand, may usually be administered at any time after or before a live-virus vaccine.8
One exception to this statement is the administration of Zostavax (zoster live-virus vaccine) with Pneumovax 23 (inactivated pneumococcal vaccine, polyvalent, MSD).17-19 The manufacturer of the two vaccines recommends a spacing of at least four weeks between them, based on research showing that concomitant use may result in reduced immunogenicity for Zostavax.20 However, as of this writing, ACIP has not revised its statement that both vaccines can be given at the same time or at any time before or after each other.21
Live-virus vaccines currently licensed in the US provide protection against diseases including measles/mumps/rubella, varicella, zoster (ie, shingles), influenza, and yellow fever.
ADULT VACCINATION HIGHLIGHTS
A summary of 2011 recommendations for adult immunization from ACIP is shown in Table 412,21. The following information is specific to each of the vaccine-preventable illnesses of concern in adults.12,13,22
Seasonal Influenza
The options to protect the adult patient against seasonal influenza are a trivalent, inactivated influenza vaccine (TIV; Fluzone, high-dose Fluzone for adults ages 65 and older, Fluvirin, Fluarix, FluLaval, Afluria, Agriflu) or a live, attenuated influenza vaccine (LAIV; FluMist).2,23 Dosage of TIV for adults is 0.5 mL IM in the deltoid once annually. For adults ages 49 and younger, LAIV is administered at 0.2 mL intranasally, once per year.
ACIP now recommends universal influenza vaccination for all persons ages 6 months and older with no contraindications.2,8 Strong consideration should be given to concurrent administration of influenza vaccine and pneumococcal vaccine to high-risk persons not previously vaccinated against pneumococcal disease.12
Note: When influenza and pneumococcal vaccines are given at the same time, they should be administered in opposite arms to reduce the risk of adverse reactions or a decreased antibody response to either vaccine.18,19
Pneumococcal Polysaccharide (PPSV)
Pneumovax 2319 is administered as a 0.5-mL dose IM in the deltoid or subcutaneously in the upper arm. The vaccine is recommended for8,19,24:
• Adults ages 65 and older who have not been previously vaccinated
• Adults now 65 and older who received PPSV vaccine at least five years ago and were younger than 65 at that time
• Adults ages 19 through 64 years who have asthma or who smoke24
• Any adult with the following underlying medical conditions: chronic heart or lung disease, diabetes mellitus, cerebrospinal fluid leaks, cochlear implants, chronic liver disease, cirrhosis, chronic alcoholism, functional or anatomic asplenia, and immunocompromising conditions (HIV infection, diseases that require immunosuppressive therapy, chemotherapy, or radiation therapy; congenital immunodeficiency).24
A one-time revaccination is recommended after five years for persons ages 19 to 64 who have chronic renal failure, nephrotic syndrome, or functional or anatomic asplenia, and for those who are immunocompromised.24
Note: According to the manufacturer of Zostavax18 and Pneumovax,23,19 these vaccines should not be given at the same time, as research has shown that Zostavax immunogenicity is reduced as a result.18-20
Zoster
For adults ages 60 and older, Zostavax18 is administered in a single 0.65-mL dose, subcutaneously in the upper arm. Providers are not required to ask about varicella vaccination history or history of varicella disease before administering the vaccine. Adults ages 60 and older who have previously had shingles can still be vaccinated during a routine health care visit.10,21,22
Immunization is contraindicated in adults with a previous anaphylactic reaction to neomycin or gelatin, although a nonanaphylactic reaction to neomycin (most commonly, contact dermatitis) is not considered a contraindication.8
Any adult patient who has close household or occupational contact with persons at risk for severe varicella (eg, infants) need not take precautions after receiving the zoster vaccine, except in the rare case in which a varicella-like rash develops.10,21,22
Note: Review the note appearing in “Pneumococcal Polysaccharide (PPSV),” above, regarding coadministration of Zostavax and Pneumovax 23.18-20
Varicella
Adults who were born in the US before 1980 are considered immune to varicella and don’t need to be vaccinated, with the exception of health care workers, pregnant women, and immunocompromised persons. Nonimmune healthy adults who have not previously undergone vaccination should receive two 0.5-mL doses of Varivax, administered subcutaneously, four to eight weeks apart.25
Immunization is contraindicated in adults with a previous anaphylactic reaction to neomycin or gelatin, although a nonanaphylactic reaction to neomycin (eg, contact dermatitis) is not considered a contraindication.8
Measles, Mumps, Rubella (MMR)
The MMR vaccine is administered at 0.5 mL, given subcutaneously in the posterolateral fat of the upper arm.8
MMR-susceptible adults who were born during or since 1957 and are not at increased risk (see below) need only one dose of the MMR vaccine; those considered at increased risk need two doses, and a second dose can also be considered during an outbreak. Adults who require two doses should wait at least four weeks between the first and second doses.12
The following factors place adults at increased risk for MMR:
• Anticipated international travel
• Being a student in a post–secondary educational setting
• Working in a health care facility
• Recent exposure to measles, or an outbreak of measles or mumps
• Previous vaccination with killed measles vaccine
• Previous vaccination with an unknown measles vaccine between 1963 and 1967.
Also at risk are health care workers born before 1957 who have no evidence of immunity, and women who plan to become pregnant and have no evidence of immunity.8,12
Tetanus, Diphtheria, Pertussis
Options for adults include a vaccine against tetanus and diphtheria (Td; Decavac); or a vaccine that protects against tetanus, diphtheria, and acellular pertussis (Tdap; Adacel, Boostrix). Adults who have not been previously vaccinated should receive one dose of Tdap and two doses of Td (the first, one month after Tdap; the second at six to 12 months after the Tdap). Each is administered as a 0.5-mL dose IM in the deltoid. A booster dose is recommended every 10 years but can be given earlier in patients who sustain wounds or who anticipate international travel.8,12
Adults ages 19 through 64 should receive a single dose of Tdap in place of a booster dose if the last Td dose was administered at least 10 years earlier and the patient has not previously received Tdap. Additionally, a dose of Tdap (if not previously given) is recommended for postpartum women, close contacts of infants younger than 12 months, and all health care workers with direct patient contact. An interval as short as two years from the last Td is suggested; shorter intervals may be appropriate.8,12
According to the new 2011 recommendations, persons ages 65 and older who have close contact with an infant younger than 12 months should be vaccinated with Tdap, and any person age 65 or older may be vaccinated with Tdap. Also added is a recommendation to administer Tdap, regardless of the interval since the patient received his or her most recent Td-containing vaccine.8,12
Human Papillomavirus (HPV)
Gardasil26 protects both female and male patients against HPV infection; Cervarix27 is indicated only for female patients. Either quadrivalent vaccine or bivalent vaccine is recommended for female patients.12
In women ages 26 and younger, Gardasil (0.5 mL IM, administered in the deltoid at 0 month, 2 months, and 6 months) provides protection against diseases caused by HPV types 6, 11, 16, and 18 (including cervical, vaginal, and vulvar cancer caused by HPV types 16 and 18). In men ages 26 and younger, Gardasil provides protection against genital warts caused by HPV types 6 and 11.26
Cervarix,27 administered at 0 month, 1 month, and 6 months (0.5 mL IM in the deltoid), provides protection for women ages 25 and younger against cervical cancer and precancerous lesions caused by HPV types 16 and 18.
Caution: Patients should be advised to sit or lie down when the HPV vaccine is administered, and they should be observed for the subsequent 15 minutes. Syncope can occur after vaccination—most commonly among adolescents and young adults.28 Convulsive syncope has been reported.
Meningococcal Disease
Two vaccines are available to protect against meningococcal disease: Menactra29 (meningococcal groups A, C, Y, and W-135 polysaccharide diphtheria toxoids conjugate vaccine); and Menveo30 (meningococcal groups A, C, Y, and W-135 oligosaccharide diphtheria CRM197 conjugate vaccine). Both are administered in the deltoid, 0.5 mL IM.
The following patients should be considered for vaccination:
• College freshmen living in dormitories, as well as college students with immune deficiencies, as they are at higher risk for meningococcal disease
• Patients who travel to or reside in countries in which Neisseria meningitidis is epidemic (particularly those with the potential for prolonged contact with the local population)
• Travelers to Saudi Arabia for pilgrimage to Mecca (the Hajj)
• Patients with anatomical or functional asplenia (two-dose series).
A two-dose series of meningococcal conjugate vaccine is also recommended for adults with persistent complement component deficiencies, and for those with HIV infection who are vaccinated.12
Hepatitis A
Two hepatitis A vaccines (both inactivated) can be used interchangeably: Havrix31 and Vaqta.32 Dosing for both vaccines in 18-year-old patients is 0.5 mL IM in the deltoid at 0 months, then at 6 to 12 months. In patients ages 19 and older, administration is the same, with the exception of increased dosing (1.0 mL IM).
Vaccination against hepatitis A is recommended for men who have sex with men, and for all adult patients who12:
• Travel to or work in areas where risk for hepatitis A transmission is high (especially those who take frequent trips or experience prolonged stays)
• Use injection drugs
• Have chronic liver disease
• Receive clotting factor concentrates for treatment of a blood-clotting disorder
• May have been exposed to hepatitis A in the previous two weeks
• Wish to be vaccinated against hepatitis A to avoid future infection.
Hepatitis B
Recombivax HB33 and Engerix-B34 are the two vaccines available to protect patients against hepatitis B (HBV), and they can be used interchangeably.12 In patients from birth through age 19, Recombivax HB33 or Engerix-B34 is given as 0.5 mL IM in the deltoid at 0, 1, and 6 months; patients ages 20 and older receive an increased dose (1.0 mL IM), with administration otherwise the same. According to the manufacturer of Recombivax HB,33 patients age 11 through 15 may be given either three doses of 0.5 mL or two doses of 1.0 mL.
The following adults are advised to undergo vaccination for HBV:
• At-risk, unvaccinated adults
• Those requesting protection against HBV infection
• Those planning to travel to areas where HBV is common
• Household contacts of a patient with chronic HBV infection, and sexual partners of a patient with HBV infection
• Adults with chronic liver disease
• Men who have sex with men
• Sexually active adults who are not in a long-term, mutually monogamous relationship
• Adults who are being evaluated or treated for a sexually transmitted disease, including HIV infection
• Health care or public safety workers who may be exposed to blood or blood-contaminated body fluids
• Workers and residents in facilities for developmentally disabled persons
• Patients undergoing or anticipating dialysis
• Adults who inject illegal drugs or who have done so recently.12
CONTRAINDICATIONS AND PRECAUTIONS FOR VACCINES COMMONLY USED IN ADULTS
See Table 5,8,22 for a summary of contraindications and precautions from ACIP and the Immunization Action Coalition that are associated with vaccinations mentioned in this article. A more complete summary can be found at www.im munize.org/catg.d/p3072a.pdf.
Conclusion
The adult patient’s vaccination status should be addressed at each health care encounter, and current recommendations should be followed. The duration of efficacy for vaccines is not an exact science. Many vaccines licensed in the US are relatively new, and recommendations for boosters for some of these vaccines will be forthcoming as more data are gathered. For example, the recommendation that a booster dose of Tdap be given to adults resulted from the recent increase in reported pertussis cases.35
Providers armed with the most current information and resources represent the forefront in ensuring that the US adult population is adequately immunized.
REFERENCES
1. World Health Organization. Immunization surveillance, assessment, and monitoring (2010). www.who.int/immunization_monitor ing/en. Accessed May 12, 2011.
2. Fiore AE, Uyeki TM, Broder K, et al; Centers for Disease Control and Prevention. Prevention and control of influenza with vaccines: recommendations of the Advisory Committee on Immunization Practices (ACIP), 2010. MMWR Morb Mortal Wkly Rep. 2010;59(RR-08);1-62.
3. Schaffner W. Update on vaccine-preventable diseases: are adults in your community adequately protected? J Fam Pract. 2008;57(4 suppl):S1-S11.
4. CDC. Healthy People 2010: Objectives for Improving Health. www.healthypeople.gov. Accessed May 6, 2011.
5. US Department of Health and Human Services. Developing Healthy People 2020: immunization and infectious diseases. www.healthy people.gov/2020. Accessed May 12, 2011.
6. Lu PJ, Nuorti JP. Pneumococcal polysaccharide vaccination among adults aged 65 years and older, United States, 1989-2008. Am J Prev Med. 2010;39(4):287-295.
7. National Institute of Allergy and Infectious Diseases, NIH. Community immunity (“herd” immunity) (2010). www.niaid.nih.gov/topics/pages/communityimmunity.aspx. Accessed May 12, 2011.
8. National Center for Immunization and Respiratory Diseases. General recommendations on immunization: recommendations of the Advisory Committee on Immunization Practices (ADIP). MMWR Recomm Rep. 2011;60(2):1-64.
9. High KP. Overcoming barriers to adult immunization. J Am Osteopath Assoc. 2009;109(6): 525-528.
10. CDC; Atkinson W, Wolfe S, Hamborsky J, eds. Epidemiology and Prevention of Vaccine-Preventable Diseases (Pink Book). 12th ed. Washington, DC: Public Health Foundation, 2011.
11. CDC. Vaccine-preventable diseases: improving vaccination coverage in children, adolescents, and adults: a report on recommendations from the Task Force on Community Preventive Services. MMWR Recomm Rep. 1999;48(RR-8):1-15.
12. CDC. Recommended adult immunization schedule: United States, 2011. MMWR Morb Mortal Wkly Rep. 2011;60(4):1-4.
13. Thompson RF. Travel & Routine Immunizations: A Practical Guide for the Medical Office. 19th ed. Milwaukee, WI: Shoreland, Inc: 2001.
14. American Academy of Pediatrics. Pertussis. In: Pickering LK, Backer, CJ, Long SS, McMillan J, eds. Red Book: 2006 Report of the Committee on Infectious Diseases. 27th ed. Elk Grove Village, IL: American Academy of Pediatrics.
15. CDC. Immunization information systems (IIS). www.cdc.gov/vaccines/programs/iis/default.htm. Accessed May 12, 2011.
16. College of Physicians of Philadelphia. The history of vaccines: a project of the College of Physicians of Philadelphia (2011). www.history ofvaccines.org/content/articles/different-types-vaccines. Accessed May 12, 2011.
17. US Food and Drug Administration. Vaccines, blood, and biologics: December 18, 2009 Approval Letter—Zostavax. www.fda.gov/
BiologicsBloodVaccines/Vaccines/Approved Products/ucm195993.htm. Accessed May 12, 2011.
18. Merck & Co, Inc. Zostavax® (zoster vaccine live; product insert). www.fda.gov/downloads/BiologicsBloodVaccines/Vaccines/Approved Products/UCM132831.pdf. Accessed May 12, 2011.
19. Merck & Co, Inc. Pneumovax® 23 (pneumococcal vaccine, polyvalent, MSD; product information). www.merck.com/product/usa/pi_circulars/p/pneumovax_23/pneumovax_pi.pdf. Accessed May 16, 2011.
20. Macintyre CR, Egerton T, McCaughey M, et al. Concomitant administration of zoster and pneumococcal vaccines in adults ≥60 years old. Hum Vaccin. 2010;6(11):18-26.
21. CDC. Prevention of herpes zoster: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep. 2008;57(RR-5):1–30.
22. Immunization Action Coalition. Vaccinate Adults. 2010 Aug;14(5). www.immunize.org/va. Accessed May 12, 2011.
23. CDC. Update: recommendations of the Advisory Committee on Immunization Practices (ACIP) regarding use of CSL seasonal influenza vaccine (Afluria) in the United States during 2010–2011. MMWR Morb Mortal Wkly Rep. 2010;59(31);989-992.
24. CDC. Updated recommendations for prevention of invasive pneumococcal disease among adults using the 23-valent pneumococcal polysaccharide vaccine (PPSV23). MMWR Morb Mortal Wkly Rep. 2010;59(RR-34):1102-1106.
25. Merck & Co, Inc. Varivax® varicella virus vaccine live (product information). www.merck
.com/product/usa/pi_circulars/v/varivax/varivax_pi.pdf. Accessed May 12, 2011.
26. Merck & Co, Inc. Gardasil® (human papillomavirus quadrivalent [types 6, 11, 16, and 18] vaccine, recombinant; product information). www.merck.com/product/usa/pi_circulars/g/gardasil/gardasil_ppi.pdf. Accessed May 12, 2011.
27. GlaxoSmithKline Biologicals. Cervarix (human papillomavirus bivalent [types 16 and 18] vaccine, recombinant; product information). http://us.gsk.com/products/assets/us_cervarix.pdf. Accessed May 12, 2011.
28. CDC. Syncope after vaccination—United States, January 2005-July 2007. MMWR Morb Mortal Wkly Rep. 2008;57(17):457-460.
29. Sanofi Pasteur. Meningococcal (groups A, C, Y, and W-135) polysaccharide diphtheria
toxoids conjugate vaccine Menactra® for intramuscular injection. www.fda.gov/downloads/BiologicsBloodVaccines/Vaccines/Approved
Products/UCM131170.pdf. Accessed May 12, 2011.
30. Novartis Vaccines and Diagnostics, Inc. Menveo® (meningococcal [groups A, C, Y and W-135] oligosaccharide diphtheria CRM197 conjugate vaccine solution for intramuscular injection; prescribing information highlights). www .fda.gov/downloads/biologicsbloodvaccines/vaccines/approvedproducts/ucm201349.pdf. Accessed May 12, 2011.
31. GlaxoSmithKline Biologicals. Havrix (hepatitis A vaccine, suspension for intramuscular injection; prescribing information highlights). http://us.gsk.com/products/assets/us_havrix .pdf. Accessed May 12, 2011.
32. Merck & Co, Inc. Vaqta (hepatitis A vaccine, inactivated; suspension for intramuscular injection; highlighted prescribing information). www.merck.com/product/usa/pi_circulars/v/vaqta/vaqta_pi.pdf. Accessed May 12, 2011.
33. Merck & Co, Inc. Recombivax HB® hepatitis B vaccine (recombinant; product information). www.merck.com/product/usa/pi_circulars/r/recombivax_hb/recombivax_pi.pdf. Accessed May 12, 2011.
34. GlaxoSmithKline Biologicals. Engerix-B® (hepatitis B vaccine, recombinant; prescribing information). http://us.gsk.com/products/assets/us_engerixb.pdf. Accessed May 12, 2011.
35. CDC. Tetanus and pertussis vaccination coverage among adults aged ≥ 18 years—United States, 1999 and 2008. MMWR Morb Mortal Wkly Rep. 2010;59(40):1302-1306.
Reported incidence rates of certain vaccine-preventable diseases—measles, rubella, diphtheria, polio, and tetanus—are low in the United States.1 However, as was demonstrated during the 2009-2010 flu season and the outbreak of H1N1 influenza,2 we cannot afford to be complacent in our attitudes toward vaccines and vaccination. New virus strains exist and can become endemic quickly and ravenously. Furthermore, certain vaccine-preventable illnesses are frequently reported among adult patients, including hepatitis B, herpes zoster, human papillomavirus infection, influenza, pertussis, and pneumococcal infection.3
Vigilance regarding vaccination of children and adults was addressed by the CDC’s Office of Disease Prevention and Health Promotion in Healthy People 2010.4 These target objectives are proposed to be retained in Healthy People 2020,5 as the objectives from 2010 have not been met. The Healthy People 2010 target for administration of the pneumococcal vaccine in adults ages 65 and older, for example, is 90%. As of 2008, research has shown, only 60% of that population was immunized.6
There are subgroups of immunocompromised people who will probably never achieve adequate antibody levels to ensure immunity to vaccine-preventable diseases (for example, measles and influenza can be deadly to the immunocompromised person, as they can be to the very young and the very old). This is an important reason why vaccination of the healthy population is essential: the concept of herd immunity.7 Herd immunity (or community immunity) suggests that if most people around you are immune to an infection and do not become ill, then there is no one who can infect you—even if you are not immune to the infection.
WHY SOME ADULTS MIGHT NEED VACCINES
Adults who were vaccinated as children may incorrectly assume that they are protected from disease for life. In the case of some diseases, this may be true. However:
• Some adults were never vaccinated as children
• Newer vaccines have been developed since many adults were children
• Immunity can begin to fade over time
• As we age, we become more susceptible to serious disease caused by common infections (eg, influenza, pneumococcus).8
Barriers to Vaccination
Barriers to vaccination are varied, but none is insurmountable. Some of these barriers include8-15:
Missed opportunities. Providers should address vaccination needs for both adults and children at each visit or encounter. According to the CDC, studies have shown that eliminating missed opportunities could increase vaccination coverage by as much as 20%.8,11
Provider misconceptions regarding vaccine contraindications, schedules, and simultaneous vaccine administration. These misconceptions may prompt providers to forego an opportunity to vaccinate. Up-to-date information about vaccinations and ongoing provider education are imperative to improve immunity among both adults and children against vaccine-preventable disease.8 Numerous Web sites and publications are instrumental and essential in furnishing the health care provider with the most current information about vaccinations (see Table 1, above, and Table 2,10,12-14).
A belief on patients’ part that they are fully vaccinated when they are not. It is important to provide a vaccination record and a return date at every vaccination encounter, even if just one vaccination has been administered on a given day. Participating in Immunization Information System,15 if one is available, is an efficient way to access computerized vaccine records easily at the point of contact.
Just as parents should be encouraged to bring their child’s vaccine record with them to every health care visit, adults are also called upon to maintain a record of all their vaccinations. Each entry in the immunization record should include:
• The type of vaccine and dose
• The site and route of administration
• The date that the vaccine was administered
• The date that the next dose is due
• The manufacturer and lot number
• The name, address, and title of the person who administered the vaccine.15
PRINCIPLES OF VACCINATION
There are two ways to acquire immunity: active and passive.
Active immunity is produced by the individual’s own immune system and usually represents a permanent immunity toward the antigen.10,16
Passive immunity is produced when the individual receives products of immunity made by another animal or a human and transferred to the host. Passive immunity can be accomplished by injection of these products or through the placenta in infants. This type of immunity is not permanent and wanes over time—usually within weeks or months.10,16
This article will concentrate on active immunity, acquired through the administration of vaccines.
CLASSIFICATION OF VACCINES
Vaccines are classified as either live, attenuated vaccine (viral or bacterial) or inactivated vaccine.
Live, attenuated vaccines are derived from “wild” or disease-causing viruses and bacteria. Through procedures conducted in the laboratory, these wild organisms are weakened or attenuated. The live, attenuated vaccine must grow and replicate in the vaccinated person in order to stimulate an immune response. However, because the organism has been weakened, it usually does not cause disease or illness.10,16
The immune response to a live, attenuated vaccine is virtually identical to a response produced by natural infection. In rare instances, however, live, attenuated vaccines may cause severe or fatal reactions as a result of uncontrolled replication of the organism. This occurs only in individuals who are significantly immunocompromised.10,16
Inactivated vaccines are produced by growing the viral or bacterial organism in a culture medium, then using heat and/or chemicals to inactivate the organism. Because inactivated vaccines are not alive, they cannot replicate, and therefore cannot cause disease, even in an immunodeficient patient. The immune response to an inactivated vaccine is mostly humoral (in contrast to the natural infection response of a live vaccine), and little or no cellular immunity is produced.10,16
Inactivated vaccines always require multiple doses, gradually building up a protective immune response. The antibody titers diminish over time, so some inactivated vaccines may require periodic doses to “boost” or increase the titers.16
Some vaccines, such as hepatitis B vaccine, lead to the development of immune memory, which stays intact for at least 20 years following immunization. Immune memory occurs during replication of B cells and T cells; some cells will become long-lived memory cells that will “remember” the pathogen and produce an immune response if the pathogen is detected again. In this case, boosters are not recommended.10
Toxoids are a type of vaccine made from the inactivated toxin of a bacterium—not the bacterium itself. Tetanus and diphtheria vaccines are examples of toxoid vaccines.10,16
Subunit and conjugate vaccines are segments of the pathogen. A subunit vaccine can be created via genetic engineering. The end result is a recombinant vaccine that can stimulate cell memory (eg, hepatitis B vaccine).10,16 Conjugate vaccines, which are similar to recombinant vaccines, are made by combining two different components to prompt a more powerful, combined immune response.10
Spacing of Live-Virus and Inactivated Vaccines
There are almost no spacing requirements between two or more inactivated vaccines8 (see Table 38,14 for spacing recommendations from the Advisory Committee on Immunization Practices [ACIP]). The only vaccines that must be spaced at least four weeks apart are live-virus vaccines—that is, if they are not given on the same day. Studies have shown that the immune response to a live-virus vaccine may be impaired if it is administered within 30 days of another live-virus vaccine. Inactivated vaccines, on the other hand, may usually be administered at any time after or before a live-virus vaccine.8
One exception to this statement is the administration of Zostavax (zoster live-virus vaccine) with Pneumovax 23 (inactivated pneumococcal vaccine, polyvalent, MSD).17-19 The manufacturer of the two vaccines recommends a spacing of at least four weeks between them, based on research showing that concomitant use may result in reduced immunogenicity for Zostavax.20 However, as of this writing, ACIP has not revised its statement that both vaccines can be given at the same time or at any time before or after each other.21
Live-virus vaccines currently licensed in the US provide protection against diseases including measles/mumps/rubella, varicella, zoster (ie, shingles), influenza, and yellow fever.
ADULT VACCINATION HIGHLIGHTS
A summary of 2011 recommendations for adult immunization from ACIP is shown in Table 412,21. The following information is specific to each of the vaccine-preventable illnesses of concern in adults.12,13,22
Seasonal Influenza
The options to protect the adult patient against seasonal influenza are a trivalent, inactivated influenza vaccine (TIV; Fluzone, high-dose Fluzone for adults ages 65 and older, Fluvirin, Fluarix, FluLaval, Afluria, Agriflu) or a live, attenuated influenza vaccine (LAIV; FluMist).2,23 Dosage of TIV for adults is 0.5 mL IM in the deltoid once annually. For adults ages 49 and younger, LAIV is administered at 0.2 mL intranasally, once per year.
ACIP now recommends universal influenza vaccination for all persons ages 6 months and older with no contraindications.2,8 Strong consideration should be given to concurrent administration of influenza vaccine and pneumococcal vaccine to high-risk persons not previously vaccinated against pneumococcal disease.12
Note: When influenza and pneumococcal vaccines are given at the same time, they should be administered in opposite arms to reduce the risk of adverse reactions or a decreased antibody response to either vaccine.18,19
Pneumococcal Polysaccharide (PPSV)
Pneumovax 2319 is administered as a 0.5-mL dose IM in the deltoid or subcutaneously in the upper arm. The vaccine is recommended for8,19,24:
• Adults ages 65 and older who have not been previously vaccinated
• Adults now 65 and older who received PPSV vaccine at least five years ago and were younger than 65 at that time
• Adults ages 19 through 64 years who have asthma or who smoke24
• Any adult with the following underlying medical conditions: chronic heart or lung disease, diabetes mellitus, cerebrospinal fluid leaks, cochlear implants, chronic liver disease, cirrhosis, chronic alcoholism, functional or anatomic asplenia, and immunocompromising conditions (HIV infection, diseases that require immunosuppressive therapy, chemotherapy, or radiation therapy; congenital immunodeficiency).24
A one-time revaccination is recommended after five years for persons ages 19 to 64 who have chronic renal failure, nephrotic syndrome, or functional or anatomic asplenia, and for those who are immunocompromised.24
Note: According to the manufacturer of Zostavax18 and Pneumovax,23,19 these vaccines should not be given at the same time, as research has shown that Zostavax immunogenicity is reduced as a result.18-20
Zoster
For adults ages 60 and older, Zostavax18 is administered in a single 0.65-mL dose, subcutaneously in the upper arm. Providers are not required to ask about varicella vaccination history or history of varicella disease before administering the vaccine. Adults ages 60 and older who have previously had shingles can still be vaccinated during a routine health care visit.10,21,22
Immunization is contraindicated in adults with a previous anaphylactic reaction to neomycin or gelatin, although a nonanaphylactic reaction to neomycin (most commonly, contact dermatitis) is not considered a contraindication.8
Any adult patient who has close household or occupational contact with persons at risk for severe varicella (eg, infants) need not take precautions after receiving the zoster vaccine, except in the rare case in which a varicella-like rash develops.10,21,22
Note: Review the note appearing in “Pneumococcal Polysaccharide (PPSV),” above, regarding coadministration of Zostavax and Pneumovax 23.18-20
Varicella
Adults who were born in the US before 1980 are considered immune to varicella and don’t need to be vaccinated, with the exception of health care workers, pregnant women, and immunocompromised persons. Nonimmune healthy adults who have not previously undergone vaccination should receive two 0.5-mL doses of Varivax, administered subcutaneously, four to eight weeks apart.25
Immunization is contraindicated in adults with a previous anaphylactic reaction to neomycin or gelatin, although a nonanaphylactic reaction to neomycin (eg, contact dermatitis) is not considered a contraindication.8
Measles, Mumps, Rubella (MMR)
The MMR vaccine is administered at 0.5 mL, given subcutaneously in the posterolateral fat of the upper arm.8
MMR-susceptible adults who were born during or since 1957 and are not at increased risk (see below) need only one dose of the MMR vaccine; those considered at increased risk need two doses, and a second dose can also be considered during an outbreak. Adults who require two doses should wait at least four weeks between the first and second doses.12
The following factors place adults at increased risk for MMR:
• Anticipated international travel
• Being a student in a post–secondary educational setting
• Working in a health care facility
• Recent exposure to measles, or an outbreak of measles or mumps
• Previous vaccination with killed measles vaccine
• Previous vaccination with an unknown measles vaccine between 1963 and 1967.
Also at risk are health care workers born before 1957 who have no evidence of immunity, and women who plan to become pregnant and have no evidence of immunity.8,12
Tetanus, Diphtheria, Pertussis
Options for adults include a vaccine against tetanus and diphtheria (Td; Decavac); or a vaccine that protects against tetanus, diphtheria, and acellular pertussis (Tdap; Adacel, Boostrix). Adults who have not been previously vaccinated should receive one dose of Tdap and two doses of Td (the first, one month after Tdap; the second at six to 12 months after the Tdap). Each is administered as a 0.5-mL dose IM in the deltoid. A booster dose is recommended every 10 years but can be given earlier in patients who sustain wounds or who anticipate international travel.8,12
Adults ages 19 through 64 should receive a single dose of Tdap in place of a booster dose if the last Td dose was administered at least 10 years earlier and the patient has not previously received Tdap. Additionally, a dose of Tdap (if not previously given) is recommended for postpartum women, close contacts of infants younger than 12 months, and all health care workers with direct patient contact. An interval as short as two years from the last Td is suggested; shorter intervals may be appropriate.8,12
According to the new 2011 recommendations, persons ages 65 and older who have close contact with an infant younger than 12 months should be vaccinated with Tdap, and any person age 65 or older may be vaccinated with Tdap. Also added is a recommendation to administer Tdap, regardless of the interval since the patient received his or her most recent Td-containing vaccine.8,12
Human Papillomavirus (HPV)
Gardasil26 protects both female and male patients against HPV infection; Cervarix27 is indicated only for female patients. Either quadrivalent vaccine or bivalent vaccine is recommended for female patients.12
In women ages 26 and younger, Gardasil (0.5 mL IM, administered in the deltoid at 0 month, 2 months, and 6 months) provides protection against diseases caused by HPV types 6, 11, 16, and 18 (including cervical, vaginal, and vulvar cancer caused by HPV types 16 and 18). In men ages 26 and younger, Gardasil provides protection against genital warts caused by HPV types 6 and 11.26
Cervarix,27 administered at 0 month, 1 month, and 6 months (0.5 mL IM in the deltoid), provides protection for women ages 25 and younger against cervical cancer and precancerous lesions caused by HPV types 16 and 18.
Caution: Patients should be advised to sit or lie down when the HPV vaccine is administered, and they should be observed for the subsequent 15 minutes. Syncope can occur after vaccination—most commonly among adolescents and young adults.28 Convulsive syncope has been reported.
Meningococcal Disease
Two vaccines are available to protect against meningococcal disease: Menactra29 (meningococcal groups A, C, Y, and W-135 polysaccharide diphtheria toxoids conjugate vaccine); and Menveo30 (meningococcal groups A, C, Y, and W-135 oligosaccharide diphtheria CRM197 conjugate vaccine). Both are administered in the deltoid, 0.5 mL IM.
The following patients should be considered for vaccination:
• College freshmen living in dormitories, as well as college students with immune deficiencies, as they are at higher risk for meningococcal disease
• Patients who travel to or reside in countries in which Neisseria meningitidis is epidemic (particularly those with the potential for prolonged contact with the local population)
• Travelers to Saudi Arabia for pilgrimage to Mecca (the Hajj)
• Patients with anatomical or functional asplenia (two-dose series).
A two-dose series of meningococcal conjugate vaccine is also recommended for adults with persistent complement component deficiencies, and for those with HIV infection who are vaccinated.12
Hepatitis A
Two hepatitis A vaccines (both inactivated) can be used interchangeably: Havrix31 and Vaqta.32 Dosing for both vaccines in 18-year-old patients is 0.5 mL IM in the deltoid at 0 months, then at 6 to 12 months. In patients ages 19 and older, administration is the same, with the exception of increased dosing (1.0 mL IM).
Vaccination against hepatitis A is recommended for men who have sex with men, and for all adult patients who12:
• Travel to or work in areas where risk for hepatitis A transmission is high (especially those who take frequent trips or experience prolonged stays)
• Use injection drugs
• Have chronic liver disease
• Receive clotting factor concentrates for treatment of a blood-clotting disorder
• May have been exposed to hepatitis A in the previous two weeks
• Wish to be vaccinated against hepatitis A to avoid future infection.
Hepatitis B
Recombivax HB33 and Engerix-B34 are the two vaccines available to protect patients against hepatitis B (HBV), and they can be used interchangeably.12 In patients from birth through age 19, Recombivax HB33 or Engerix-B34 is given as 0.5 mL IM in the deltoid at 0, 1, and 6 months; patients ages 20 and older receive an increased dose (1.0 mL IM), with administration otherwise the same. According to the manufacturer of Recombivax HB,33 patients age 11 through 15 may be given either three doses of 0.5 mL or two doses of 1.0 mL.
The following adults are advised to undergo vaccination for HBV:
• At-risk, unvaccinated adults
• Those requesting protection against HBV infection
• Those planning to travel to areas where HBV is common
• Household contacts of a patient with chronic HBV infection, and sexual partners of a patient with HBV infection
• Adults with chronic liver disease
• Men who have sex with men
• Sexually active adults who are not in a long-term, mutually monogamous relationship
• Adults who are being evaluated or treated for a sexually transmitted disease, including HIV infection
• Health care or public safety workers who may be exposed to blood or blood-contaminated body fluids
• Workers and residents in facilities for developmentally disabled persons
• Patients undergoing or anticipating dialysis
• Adults who inject illegal drugs or who have done so recently.12
CONTRAINDICATIONS AND PRECAUTIONS FOR VACCINES COMMONLY USED IN ADULTS
See Table 5,8,22 for a summary of contraindications and precautions from ACIP and the Immunization Action Coalition that are associated with vaccinations mentioned in this article. A more complete summary can be found at www.im munize.org/catg.d/p3072a.pdf.
Conclusion
The adult patient’s vaccination status should be addressed at each health care encounter, and current recommendations should be followed. The duration of efficacy for vaccines is not an exact science. Many vaccines licensed in the US are relatively new, and recommendations for boosters for some of these vaccines will be forthcoming as more data are gathered. For example, the recommendation that a booster dose of Tdap be given to adults resulted from the recent increase in reported pertussis cases.35
Providers armed with the most current information and resources represent the forefront in ensuring that the US adult population is adequately immunized.
REFERENCES
1. World Health Organization. Immunization surveillance, assessment, and monitoring (2010). www.who.int/immunization_monitor ing/en. Accessed May 12, 2011.
2. Fiore AE, Uyeki TM, Broder K, et al; Centers for Disease Control and Prevention. Prevention and control of influenza with vaccines: recommendations of the Advisory Committee on Immunization Practices (ACIP), 2010. MMWR Morb Mortal Wkly Rep. 2010;59(RR-08);1-62.
3. Schaffner W. Update on vaccine-preventable diseases: are adults in your community adequately protected? J Fam Pract. 2008;57(4 suppl):S1-S11.
4. CDC. Healthy People 2010: Objectives for Improving Health. www.healthypeople.gov. Accessed May 6, 2011.
5. US Department of Health and Human Services. Developing Healthy People 2020: immunization and infectious diseases. www.healthy people.gov/2020. Accessed May 12, 2011.
6. Lu PJ, Nuorti JP. Pneumococcal polysaccharide vaccination among adults aged 65 years and older, United States, 1989-2008. Am J Prev Med. 2010;39(4):287-295.
7. National Institute of Allergy and Infectious Diseases, NIH. Community immunity (“herd” immunity) (2010). www.niaid.nih.gov/topics/pages/communityimmunity.aspx. Accessed May 12, 2011.
8. National Center for Immunization and Respiratory Diseases. General recommendations on immunization: recommendations of the Advisory Committee on Immunization Practices (ADIP). MMWR Recomm Rep. 2011;60(2):1-64.
9. High KP. Overcoming barriers to adult immunization. J Am Osteopath Assoc. 2009;109(6): 525-528.
10. CDC; Atkinson W, Wolfe S, Hamborsky J, eds. Epidemiology and Prevention of Vaccine-Preventable Diseases (Pink Book). 12th ed. Washington, DC: Public Health Foundation, 2011.
11. CDC. Vaccine-preventable diseases: improving vaccination coverage in children, adolescents, and adults: a report on recommendations from the Task Force on Community Preventive Services. MMWR Recomm Rep. 1999;48(RR-8):1-15.
12. CDC. Recommended adult immunization schedule: United States, 2011. MMWR Morb Mortal Wkly Rep. 2011;60(4):1-4.
13. Thompson RF. Travel & Routine Immunizations: A Practical Guide for the Medical Office. 19th ed. Milwaukee, WI: Shoreland, Inc: 2001.
14. American Academy of Pediatrics. Pertussis. In: Pickering LK, Backer, CJ, Long SS, McMillan J, eds. Red Book: 2006 Report of the Committee on Infectious Diseases. 27th ed. Elk Grove Village, IL: American Academy of Pediatrics.
15. CDC. Immunization information systems (IIS). www.cdc.gov/vaccines/programs/iis/default.htm. Accessed May 12, 2011.
16. College of Physicians of Philadelphia. The history of vaccines: a project of the College of Physicians of Philadelphia (2011). www.history ofvaccines.org/content/articles/different-types-vaccines. Accessed May 12, 2011.
17. US Food and Drug Administration. Vaccines, blood, and biologics: December 18, 2009 Approval Letter—Zostavax. www.fda.gov/
BiologicsBloodVaccines/Vaccines/Approved Products/ucm195993.htm. Accessed May 12, 2011.
18. Merck & Co, Inc. Zostavax® (zoster vaccine live; product insert). www.fda.gov/downloads/BiologicsBloodVaccines/Vaccines/Approved Products/UCM132831.pdf. Accessed May 12, 2011.
19. Merck & Co, Inc. Pneumovax® 23 (pneumococcal vaccine, polyvalent, MSD; product information). www.merck.com/product/usa/pi_circulars/p/pneumovax_23/pneumovax_pi.pdf. Accessed May 16, 2011.
20. Macintyre CR, Egerton T, McCaughey M, et al. Concomitant administration of zoster and pneumococcal vaccines in adults ≥60 years old. Hum Vaccin. 2010;6(11):18-26.
21. CDC. Prevention of herpes zoster: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep. 2008;57(RR-5):1–30.
22. Immunization Action Coalition. Vaccinate Adults. 2010 Aug;14(5). www.immunize.org/va. Accessed May 12, 2011.
23. CDC. Update: recommendations of the Advisory Committee on Immunization Practices (ACIP) regarding use of CSL seasonal influenza vaccine (Afluria) in the United States during 2010–2011. MMWR Morb Mortal Wkly Rep. 2010;59(31);989-992.
24. CDC. Updated recommendations for prevention of invasive pneumococcal disease among adults using the 23-valent pneumococcal polysaccharide vaccine (PPSV23). MMWR Morb Mortal Wkly Rep. 2010;59(RR-34):1102-1106.
25. Merck & Co, Inc. Varivax® varicella virus vaccine live (product information). www.merck
.com/product/usa/pi_circulars/v/varivax/varivax_pi.pdf. Accessed May 12, 2011.
26. Merck & Co, Inc. Gardasil® (human papillomavirus quadrivalent [types 6, 11, 16, and 18] vaccine, recombinant; product information). www.merck.com/product/usa/pi_circulars/g/gardasil/gardasil_ppi.pdf. Accessed May 12, 2011.
27. GlaxoSmithKline Biologicals. Cervarix (human papillomavirus bivalent [types 16 and 18] vaccine, recombinant; product information). http://us.gsk.com/products/assets/us_cervarix.pdf. Accessed May 12, 2011.
28. CDC. Syncope after vaccination—United States, January 2005-July 2007. MMWR Morb Mortal Wkly Rep. 2008;57(17):457-460.
29. Sanofi Pasteur. Meningococcal (groups A, C, Y, and W-135) polysaccharide diphtheria
toxoids conjugate vaccine Menactra® for intramuscular injection. www.fda.gov/downloads/BiologicsBloodVaccines/Vaccines/Approved
Products/UCM131170.pdf. Accessed May 12, 2011.
30. Novartis Vaccines and Diagnostics, Inc. Menveo® (meningococcal [groups A, C, Y and W-135] oligosaccharide diphtheria CRM197 conjugate vaccine solution for intramuscular injection; prescribing information highlights). www .fda.gov/downloads/biologicsbloodvaccines/vaccines/approvedproducts/ucm201349.pdf. Accessed May 12, 2011.
31. GlaxoSmithKline Biologicals. Havrix (hepatitis A vaccine, suspension for intramuscular injection; prescribing information highlights). http://us.gsk.com/products/assets/us_havrix .pdf. Accessed May 12, 2011.
32. Merck & Co, Inc. Vaqta (hepatitis A vaccine, inactivated; suspension for intramuscular injection; highlighted prescribing information). www.merck.com/product/usa/pi_circulars/v/vaqta/vaqta_pi.pdf. Accessed May 12, 2011.
33. Merck & Co, Inc. Recombivax HB® hepatitis B vaccine (recombinant; product information). www.merck.com/product/usa/pi_circulars/r/recombivax_hb/recombivax_pi.pdf. Accessed May 12, 2011.
34. GlaxoSmithKline Biologicals. Engerix-B® (hepatitis B vaccine, recombinant; prescribing information). http://us.gsk.com/products/assets/us_engerixb.pdf. Accessed May 12, 2011.
35. CDC. Tetanus and pertussis vaccination coverage among adults aged ≥ 18 years—United States, 1999 and 2008. MMWR Morb Mortal Wkly Rep. 2010;59(40):1302-1306.
Insulin Resistance: Another Clue to Endometrial Cancer?
Evidence-Based Management of Diabetic Dyslipidemia in a VA Population: Beyond the LDL Target
Comparing Depression Screening Tests in Older veterans
Nocardia Bacteremia Associated With Pulmonary Alveolar Proteinosis
Grand Rounds: Woman, 22, With Dizziness and Headache
A 22-year-old student was brought in to a college student health center in a wheelchair by campus safety personnel. She appeared drowsy and was crying softly. She complained of a severe headache and said she was “tired of going through this all the time.” The woman said she had seen spots and become dizzy, then had gotten “the worst headache of my life” while sitting in class. She rated the headache pain at 8 on a 10-point scale and also complained of nausea and photophobia.
The history revealed dizziness that made her “feel as if I’m tipping over” and similar headaches during the previous year. The patient said she had seen “a few doctors” for her symptoms, but that they “could never find anything.” The headaches usually occurred on the left side of her head, lasted hours to days, and were only partially relieved with acetaminophen. The patient could not remember whether she had eaten breakfast and was unsure of what day it was. She described herself as frustrated and began to weep again.
She was currently under the care of a psychologist but seemed uncertain why; she said that she was sexually active and used condoms. She had undergone an appendectomy at age 12. She denied taking any medications besides acetaminophen. She denied smoking or drug use, history of migraine headaches, vision or hearing changes, facial weakness, depression, or anxiety. Her family history included a grandfather with diabetes and hypertension and an uncle with heart disease. The family history was negative for migraine or psychiatric illness.
Because of the patient’s weakness, she was assisted onto the examination table by a nurse. Physical exam revealed a pale, slightly sweaty, overweight, tearful young woman who was slow to respond. Her blood pressure was measured at 134/104 mm Hg; pulse, 100 beats/min; respirations, 14 breaths/min; and temperature, 97.0ºF. Point-of-care testing of blood glucose was 91 mg/dL, and hemoglobin was measured at 12.3 g/dL. The ophthalmologic exam was positive for photophobia and revealed slightly disconjugate gaze with horizontal nystagmus during testing of cranial nerves (CN) III, IV, and VI. The otoscopic exam revealed a slightly injected right tympanic membrane, and there were no apparent hearing deficits.
The neurologic exam showed patellar and brachial deep tendon reflexes equal, grips weak and equal, and the pupillary response intact. The patient was able to stand without assistance, although her gait was slightly unsteady. Because the patient was of college age, the clinician ruled out meningitis by negative Kernig’s and Brudzinski’s signs and absence of fever. Subarachnoid hemorrhage was also a concern when the patient mentioned the “worst headache of my life,” indicating the need for emergent imaging.
The patient’s presentation, it was felt, warranted a 911 call. The emergency medical team arrived, and its members began to question the patient. Discrepancies in the patient’s history during the paramedics’ reexamination led them to question whether an emergency department (ED) visit was necessary, but at the clinician’s insistence, they agreed to transport the student to the ED.
The following day, the student health center clinician was contacted by a member of the hospital ED staff with an update on the patient’s status. Shortly after her arrival at the hospital, she underwent MRI and was diagnosed with a vestibular schwannoma. She had surgery that same evening, during which the surgeon removed most of the tumor. Although the ED staff was not at liberty to provide more complete information, they did inform the clinician that the patient would require radiation for the remainder of the tumor.
DISCUSSION
Vestibular schwannoma is also known as acoustic schwannoma, acoustic neuroma, acoustic neurinoma, or vestibular neurilemmoma. These tumors arise from perineural elements of Schwann cells, which commonly form and lead to myelination in the vestibular area of CN VIII1 (see figure). They occur with equal frequency on the superior and inferior branches of the vestibular nerve and originate only rarely at the cochlear portion of the eighth cranial nerve. Vestibular schwannomas represent approximately 8% to 10% of brain tumors and 80% to 90% of tumors in the cerebellopontine angle in adults.2 Tumors are distributed evenly across genders, but the majority of diagnosed patients are white.3
Most likely because of improvements in diagnostic technology, the incidence of vestibular schwannoma has increased over the past 30 years. One British research team predicts that one in 1,000 persons will receive a diagnosis of vestibular schwannoma in their lifetime.4 These tumors are most commonly diagnosed in people ages 30 to 60, with a median age of 55.5
A relationship has been demonstrated between neurofibromatosis type 2 (NF2), an autosomal-dominant disease, and the development of vestibular schwannomas.6,7 NF2 has a birth prevalence of one in about 25,000 persons,4,8 and those who inherit the responsible gene inevitably develop vestibular schwannomas.9 Patients with a confirmed diagnosis of vestibular schwannoma should be screened by a geneticist for the NF2 gene; although the tumors are benign, they can cause compression of the vestibular nerve, leading to deafness and balance disorders.10 Schwannomas of the spinal nerves can also occur in persons with NF2.11 Compression of the spinal nerves in these patients can lead to significant morbidity and a shortened average life span.10
NF2 is diagnosed using the following criteria:
1) Bilateral vestibular schwannomas
2) Diagnosis of a family member with either NF2 or unilateral vestibular schwannoma, and
3) Juvenile posterior subscapular lens opacities.9,12,13
Because schwannomas grow slowly, the vestibular system can adapt to the slow destruction of CN VIII. For this reason, patients typically present with unilateral deafness or hearing impairment rather than dizziness.11 Many patients also present with tinnitus and/or vertigo.14,15
Some vestibular tumors remain stable or even regress; others progress, in some cases causing life-threatening complications.16 An extremely rare complication of a vestibular schwannoma was reported in one patient: an intratumoral hemorrhage that led to acute neurologic deterioration and death.17
Since the case patient underwent immediate surgical intervention, it appears she was experiencing significant involvement and it was likely anticipated that without surgical intervention, clinical progression would occur. Her young age could be considered a risk factor for a faster-growing neuroma.18
Clinical Presentation and Diagnosis
Primary care clinicians commonly see patients with complaints of dizziness, lightheadedness, faintness, or a sensation of spinning or tilting. Vestibular schwannoma should be considered in the differential diagnosis of the patient who presents with these complaints, as well as tinnitus or hearing loss.9 The patient with vestibular schwannoma may also have a history of headache, unsteady gait, facial pain, and numbness.19 A partial differential diagnosis is listed in the table20,21). The astute clinician will systematically rule out many of these conditions, since certain other features that may be present (eg, rapid onset, vomiting, fever) do not typically occur in the patient with vestibular schwannoma.
Because the symptoms typically associated with vestibular schwannoma are likely to occur bilaterally in patients with other conditions, unilateral symptoms should alert the clinician to investigate further. The patterns and growth rates of vestibular schwannomas are highly variable and currently unpredictable18 (according to Fortnum et al,14 at least 50% of tumors do not grow within several years after diagnosis); thus, no clear predictors of tumor growth have been identified to assist in the evaluation of an affected patient,16 although faster tumor growth rates have been reported in young patients, and Baser et al18 have called for additional research involving younger persons with vestibular schwannomas.
Standard testing is audiometry followed by MRI, which is considered the most effective means to confirm a diagnosis of vestibular schwannoma.5,14,22
Treatment for Vestibular Schwannoma
Treatment, whether with surgery or radiation, is associated with significant morbidity and possibly decreased quality of life.16 Therefore, distinguishing patients whose tumors will grow and pose a threat to them from those whose tumors are likely to remain stable is central to appropriate management.23
Treatment modalities are considered based on tumor size, growth, presence or absence of tinnitus, and the patient’s preferences and life expectancy.23 In most cases, decision making is complex and should be customized to meet the patient’s individual circumstances. Patients with similar clinical scenarios have been reported to opt for different treatment choices.24
Four treatment options are currently available for patients with vestibular schwannoma:
Serial observation with periodic MRI studies. Since vestibular schwannomas are benign and slow-growing, conservative management can be a reasonable option, particularly if the patient is elderly, the tumor is small, and/or little hearing loss has taken place. However, use of observation is associated with a risk for progressive and permanent hearing loss.2 Between 15% and 50% of patients who opt for serial observation will undergo subsequent surgical intervention, particularly in cases involving worsening tinnitus, balance problems, or hearing loss.23-25
Chemotherapy. Agents including bevacizumab (a humanized monoclonal antibody against vascular endothelial growth factor)8,26,27 and erlotinib (an epidermal growth factor receptor inhibitor) may delay progression or even facilitate regression of vestibular schwannomas.28 Hearing improvement has also been reported in patients with NF2 who were treated with bevacizumab8; research is ongoing.26
Fractionated radiotherapy. Hearing may be preserved in 60% to 95% of patients, depending on levels of dosing to the cochlea, but 3% to 7% of patients will need further treatment.29-31 Radiation treatment is a likely choice in patients with tumors measuring 2.0 cm or less. Larger tumors are considered a surgical disease, and directed radiotherapy may be administered postoperatively (as in the case patient) for residual portions of the tumor.16
Microsurgery. Compared with other treatment modalities, the emphasis of microsurgery is on removing tumors (particularly larger tumors) rather than controlling their growth.29 The three common approaches are retrosigmoid, middle fossa, or translabyrinthine.32-34 Preservation of hearing is reportedly better following retrosigmoid or middle fossa microsurgery, compared with a translabyrinthine procedure (because in the latter, the tumor cannot be exposed without damage to the inner ear).32,35
With any such surgery, risks include cranial nerve damage, leakage of cerebrospinal fluid, and infection.29,32 Postsurgically, about half of patients report frequent headaches, which are persistent in about half of these cases.36-38 Another concern is preservation of the facial nerves, with a risk for temporary facial weakness or dysfunction.3,24,39 Less than 2% of patients who undergo microsurgery require additional treatment.29
Stereotactic radiosurgery. These procedures, which are performed using the Gamma Knife,® the CyberKnife, or the linear accelerator,29,40,41 are considered appropriate for patients with smaller tumors and those who are not candidates for conventional surgery.1 Trigeminal neuropathy, injury to the facial nerves, and hydrocephaly are reported complications of Gamma Knife radiosurgery, but improvements in these technologies are ongoing.1,2,40
Patient Outcomes
The outcome in a patient with vestibular schwannoma depends on the treatment administered, but prolonged follow-up is typically necessary. For patients being managed through observation, annual brain scans are recommended for 10 years, with subsequent scans every three to five years if no tumor growth is seen. For patients who have had surgery, annual brain scans are advised for the successive eight to 10 years, with decreasing frequency if no tumor remains. In patients who undergo radiation, annual scans are recommended for 10 years, then every two years if no tumor growth is detected.36
Psychosocial experiences vary widely among patients who have undergone treatment for vestibular schwannomas. Some are unable to perform necessary or recreational activities, and others must retire early from work.42 Others, however, have minimal disruption in their lives and enjoy a good quality of life. The most difficult consequence of vestibular schwannoma and its treatment, according to patients, is the associated hearing loss.8,19
THE CASE PATIENT
The 22-year-old patient in this case had an atypical presentation of vestibular schwannoma. Although she did present with vertigo, she also complained of headache, nausea, and photophobia—which are rarely reported in investigations of these tumors. She was also younger than the typical patient and did not report tinnitus.
The case patient reportedly underwent surgery and subsequent radiation to treat the remaining portion of her tumor. She suspended her attendance at the college and, as of this writing, has not re-enrolled. She was lost to follow-up.
CONCLUSION
For the primary care provider, diagnostic challenges require great clinical acumen. Vertigo, headache, hearing loss, and tinnitus are all symptoms seen in the primary care setting; when they occur together, the clinician should be alerted to investigate further. A high level of suspicion is appropriate when a patient complains of longstanding auditory symptoms, with or without headache. Unilateral hearing loss is a common symptom in patients with vestibular schwannomas, although some may present with facial weakness or pain, imbalance, and/or vertigo.
In addition to the history and physical exam, experts recommend that audiometry and MRI be considered, particularly if hearing loss is unilateral. Genetic screening for NF2 should be performed if vestibular schwannoma is found on MRI. Referral to a neurologist, a neurosurgeon, or an otolaryngologist is appropriate.
REFERENCES
1. Arthurs BJ, Lamoreaux WT, Giddings NA, et al. Gamma Knife radiosurgery for vestibular schwannoma: case report and review of the literature. World J Surg Oncol. 2009 Dec 18;7:100.
2. Mohammed TA, Ahuja MS, Ju SS, Thomas J. Normal pressure hydrocephalus after Gamma Knife radiosurgery for vestibular schwannoma. J Postgrad Med. 2010;56(3):213-215.
3. Gal TJ, Shinn J, Huang B. Current epidemiology and management trends in acoustic neuroma. Otolaryngol Head Neck Surg. 2010;142(5):677-681.
4. Evans DG, Moran A, King A, et al. Incidence of vestibular schwannoma and neurofibromatosis 2 in the North West of England over a 10-year period: higher incidence than previously thought. Otol Neurotol. 2005;26(1):93-97.
5. Haynes D. Acoustic neuroma diagnosis and treatment options. Hearing Health. 2009;25(3):32. www.drf.org/magazine/36/Summer+2009+Issue/article/272. Accessed May 16, 2011.
6. Sobel RA. Vestibular (acoustic) schwannomas: histologic features in neurofibromatosis 2 and in unilateral cases. J Neuropathol Exp Neurol. 1993;52(2):106-113.
7. Evans DG, Huson SM, Donnai D, et al. A clinical study of type 2 neurofibromatosis. Q J Med. 1992;84(304):603-618.
8. Plotkin SR, Stemmer-Rachamimov AO, Barker FG 2nd, et al. Hearing improvement after bevacizumab in patients with neurofibromatosis type 2. N Engl J Med. 2009;361(4):358-367.
9. Evans DGR, Sainio M, Baser E. Neurofibromatosis type 2. J Med Genet. 2000:37(11):897-904.
10. Gusella JF, Ramesh V, MacCollin M, Jacoby LB. Neurofibromatosis 2: loss of Merlin’s protective spell. Curr Opin Genet Dev. 1996;6(1):87-92.
11. Sagar SM, Israel MA. Ch 374. Primary and metastatic tumors of the nervous system. In: Kasper DL, Braunwald E, Fauci AS, et al. Harrison’s Principles of Internal Medicine. 17th ed. New York, NY: McGraw-Hill Companies, Inc; 2008:2601-2610.
12. Evans DGR. Neurofibromatosis 2 [bilateral acoustic neurofibromatosis, central neurofibromatosis, NF2, neurofibromatosis type II]. Genet Med. 2009;11(9):599-610.
13. Arya R, Sahu JK, Kabra M. Neurofibromatosis type II (Wishart type). J Pediatr Neurol. 2009;7(3): 333-335.
14. Fortnum H, O’Neill C, Taylor R, et al. The role of magnetic resonance imaging in the identification of suspected acoustic neuroma: a systematic review of clinical and cost effectiveness and natural history. Health Technol Assess. 2009;13(18):iii-iv, ix-xi, 1-154.
15. Forton GE, Cremers CW, Offeciers EE. Acoustic neuroma ingrowth in the cochlear nerve: does it influence the clinical presentation? Ann Otol Rhinol Laryngol. 2004;113(7):582-586.
16. Nikolopoulos TP, Fortnum H, O’Donoghue G, Baguley D. Acoustic neuroma growth: a systematic review of the evidence. Otol Neurotol. 2010;31(3):478-485.
17. Yates CW, Weinberg M, Packer MJ, Jacob A. Fatal case of tumor-associated hemorrhage in a large vestibular schwannoma. Ann Otol Rhinol Laryngol. 2010;119(6):402-405.
18. Baser ME, Mautner VF, Parry DM, Evans DGR. Methodological issues in longitudinal studies; vestibular schwannoma growth rates in neurofibromatosis 2. J Med Genet. 2005;42(12):903-906.
19. Brooker J, Burney S, Fletcher J, Dally M. A qualitative exploration of quality of life among individuals diagnosed with an acoustic neuroma. Br J Health Psychol. 2009;14(pt 3):563-578.
20. Strupp M, Brandt T. Diagnosis and treatment of vertigo and dizziness. Dtsch Arzetbl Int. 2008;105(10):173-180.
21. Kerber KA. Dizziness and vertigo. In: Andreoli TE, Griggs RC, Benjamin I , Wing EJ, eds. Andreoli and Carpenter’s Cecil Essentials of Medicine. 8th ed. Philadelphia, PA: Elsevier Inc; 2010:1104-1105.
22. Gimsing S. Vestibular schwannoma: when to look for it? J Laryngol Otol. 2010;124(3):258-264.
23. Agrawal Y, Clark JH, Limb CJ, et al. Predictors of vestibular schwannoma growth and clinical implications. Otol Neurotol. 2010;31(5):807-812.
24. Cheung SW, Aranda D, Driscoll CLW, Parsa AT. Mapping clinical outcomes expectations to treatment decisions: an application to vestibular schwannoma management. Otol Neurotol. 2010;31(2):284-293.
25. Myrseth E, Pedersen PH, Møller P, Lund-Johansen M. Treatment of vestibular schwannomas: why, when and how? Acta Neurochir (Wien). 2007;149(7):647-660.
26. Sidney Kimmel Comprehensive Cancer Center, Massachusetts General Hospital, National Cancer Institute. Bevacizumab for symptomatic vestibular schwannoma in neurofibromatosis type 2 (NF2). http://clinicaltrials.gov/ct2/show/NCT01207687. Accessed May 16, 2011.
27. Mautner VF, Nguyen R, Kutta H, et al. Bevacizumab induces regression of vestibular schwannomas in patients with neurofibromatosis type 2. Neuro Oncol. 2010;12(1):14-18.
28. Plotkin SR, Halpin C, McKenna MJ, et al. Erlotinib for progressive vestibular schwannoma in neurofibromatosis 2 patients. Otol Neurotol. 2010;31(7):1135-1143.
29. Arthurs BJ, Fairbanks RK, Demakas JJ, et al. A review of treatment modalities for vestibular schwannoma. Neurosurg Rev. 2011 Feb 9; [Epub ahead of print].
30. Andrews DW, Werner-Wasik M, Den RB, et al. Toward dose optimization for fractionated stereotactic radiotherapy for acoustic neuromas: comparison of two dose cohorts. Int J Radiat Oncol Biol Phys. 2009;74(2):419-426.
31. Thomas C, Di Maio S, Ma R, et al. Hearing preservation following fractionated stereotactic radiotherapy for vestibular schwannomas: prognostic implications of cochlear dose. J Neurosurg. 2007;107(5):917-926.
32. Samii M, Gerganov V, Samii A. Improved preservation of hearing and facial nerve function in vestibular schwannoma surgery via the retrosigmoid approach in a series of 200 patients. J Neurosurg. 2006;105(4):527-535.
33. Shiobara R, Ohira T, Inoue Y, et al. Extended middle cranial fossa approach for vestibular schwannoma: technical note and surgical results of 896 operations. Prog Neurol Surg. 2008;21:65-72.
34. Schmerber S, Palombi O, Boubagra K, et al. Long-term control of vestibular schwannoma after a translabyrinthine complete removal. Neurosurgery. 2005;57(4):693-698.
35. Phillips DJ, Kobylarz EJ, De Peralta ET, et al. Predictive factors of hearing preservation after surgical resection of small vestibular schwannomas. Otol Neurotol. 2010;31(9):1463-1468.
36. Park JK, Black MP, Vernick DM, Ramakrishna N. Vestibular schwannoma (acoustic neuroma) (2010). www.uptodate.com/contents/vestibular-schwannoma-acoustic-neuroma. Accessed May 16, 2011.
37. Schankin CJ, Gall C, Straube A. Headache syndromes after acoustic neuroma surgery and their implications for quality of life. Cephalalgia. 2009;29(7):760-761.
38. Ryzenman JM, Pensak ML, Tew JM Jr. Headache: a quality of life analysis in a cohort of 1,657 patients undergoing acoustic neuroma surgery: results from the Acoustic Neuroma Association. Laryngoscope. 2005;115(4):703-711.
39. Sriskandan N, Connor SE. The role of radiology in the diagnosis and management of vestibular schwannoma. Clin Radiol. 2010;66(4):357-365.
40. Yang I, Sughrue ME, Han SJ, et al. Facial nerve preservation after vestibular schwannoma Gamma Knife surgery. J Neurooncol. 2009;93(1): 41-48.
41. Unger F, Dominikus K, Haselsberger K. Stereotactic radiosurgery and fractionated stereotactic radiotherapy of acoustic neuromas [in German]. HNO. 2011;59(1):31-37.
42. Tos T, Caye-Thomasen P, Stangerup SE, et al. Long-term socio-economic impact of vestibular schwannoma for patients under observation and after surgery. J Laryngol Otol. 2003;117(12):955-964.
A 22-year-old student was brought in to a college student health center in a wheelchair by campus safety personnel. She appeared drowsy and was crying softly. She complained of a severe headache and said she was “tired of going through this all the time.” The woman said she had seen spots and become dizzy, then had gotten “the worst headache of my life” while sitting in class. She rated the headache pain at 8 on a 10-point scale and also complained of nausea and photophobia.
The history revealed dizziness that made her “feel as if I’m tipping over” and similar headaches during the previous year. The patient said she had seen “a few doctors” for her symptoms, but that they “could never find anything.” The headaches usually occurred on the left side of her head, lasted hours to days, and were only partially relieved with acetaminophen. The patient could not remember whether she had eaten breakfast and was unsure of what day it was. She described herself as frustrated and began to weep again.
She was currently under the care of a psychologist but seemed uncertain why; she said that she was sexually active and used condoms. She had undergone an appendectomy at age 12. She denied taking any medications besides acetaminophen. She denied smoking or drug use, history of migraine headaches, vision or hearing changes, facial weakness, depression, or anxiety. Her family history included a grandfather with diabetes and hypertension and an uncle with heart disease. The family history was negative for migraine or psychiatric illness.
Because of the patient’s weakness, she was assisted onto the examination table by a nurse. Physical exam revealed a pale, slightly sweaty, overweight, tearful young woman who was slow to respond. Her blood pressure was measured at 134/104 mm Hg; pulse, 100 beats/min; respirations, 14 breaths/min; and temperature, 97.0ºF. Point-of-care testing of blood glucose was 91 mg/dL, and hemoglobin was measured at 12.3 g/dL. The ophthalmologic exam was positive for photophobia and revealed slightly disconjugate gaze with horizontal nystagmus during testing of cranial nerves (CN) III, IV, and VI. The otoscopic exam revealed a slightly injected right tympanic membrane, and there were no apparent hearing deficits.
The neurologic exam showed patellar and brachial deep tendon reflexes equal, grips weak and equal, and the pupillary response intact. The patient was able to stand without assistance, although her gait was slightly unsteady. Because the patient was of college age, the clinician ruled out meningitis by negative Kernig’s and Brudzinski’s signs and absence of fever. Subarachnoid hemorrhage was also a concern when the patient mentioned the “worst headache of my life,” indicating the need for emergent imaging.
The patient’s presentation, it was felt, warranted a 911 call. The emergency medical team arrived, and its members began to question the patient. Discrepancies in the patient’s history during the paramedics’ reexamination led them to question whether an emergency department (ED) visit was necessary, but at the clinician’s insistence, they agreed to transport the student to the ED.
The following day, the student health center clinician was contacted by a member of the hospital ED staff with an update on the patient’s status. Shortly after her arrival at the hospital, she underwent MRI and was diagnosed with a vestibular schwannoma. She had surgery that same evening, during which the surgeon removed most of the tumor. Although the ED staff was not at liberty to provide more complete information, they did inform the clinician that the patient would require radiation for the remainder of the tumor.
DISCUSSION
Vestibular schwannoma is also known as acoustic schwannoma, acoustic neuroma, acoustic neurinoma, or vestibular neurilemmoma. These tumors arise from perineural elements of Schwann cells, which commonly form and lead to myelination in the vestibular area of CN VIII1 (see figure). They occur with equal frequency on the superior and inferior branches of the vestibular nerve and originate only rarely at the cochlear portion of the eighth cranial nerve. Vestibular schwannomas represent approximately 8% to 10% of brain tumors and 80% to 90% of tumors in the cerebellopontine angle in adults.2 Tumors are distributed evenly across genders, but the majority of diagnosed patients are white.3
Most likely because of improvements in diagnostic technology, the incidence of vestibular schwannoma has increased over the past 30 years. One British research team predicts that one in 1,000 persons will receive a diagnosis of vestibular schwannoma in their lifetime.4 These tumors are most commonly diagnosed in people ages 30 to 60, with a median age of 55.5
A relationship has been demonstrated between neurofibromatosis type 2 (NF2), an autosomal-dominant disease, and the development of vestibular schwannomas.6,7 NF2 has a birth prevalence of one in about 25,000 persons,4,8 and those who inherit the responsible gene inevitably develop vestibular schwannomas.9 Patients with a confirmed diagnosis of vestibular schwannoma should be screened by a geneticist for the NF2 gene; although the tumors are benign, they can cause compression of the vestibular nerve, leading to deafness and balance disorders.10 Schwannomas of the spinal nerves can also occur in persons with NF2.11 Compression of the spinal nerves in these patients can lead to significant morbidity and a shortened average life span.10
NF2 is diagnosed using the following criteria:
1) Bilateral vestibular schwannomas
2) Diagnosis of a family member with either NF2 or unilateral vestibular schwannoma, and
3) Juvenile posterior subscapular lens opacities.9,12,13
Because schwannomas grow slowly, the vestibular system can adapt to the slow destruction of CN VIII. For this reason, patients typically present with unilateral deafness or hearing impairment rather than dizziness.11 Many patients also present with tinnitus and/or vertigo.14,15
Some vestibular tumors remain stable or even regress; others progress, in some cases causing life-threatening complications.16 An extremely rare complication of a vestibular schwannoma was reported in one patient: an intratumoral hemorrhage that led to acute neurologic deterioration and death.17
Since the case patient underwent immediate surgical intervention, it appears she was experiencing significant involvement and it was likely anticipated that without surgical intervention, clinical progression would occur. Her young age could be considered a risk factor for a faster-growing neuroma.18
Clinical Presentation and Diagnosis
Primary care clinicians commonly see patients with complaints of dizziness, lightheadedness, faintness, or a sensation of spinning or tilting. Vestibular schwannoma should be considered in the differential diagnosis of the patient who presents with these complaints, as well as tinnitus or hearing loss.9 The patient with vestibular schwannoma may also have a history of headache, unsteady gait, facial pain, and numbness.19 A partial differential diagnosis is listed in the table20,21). The astute clinician will systematically rule out many of these conditions, since certain other features that may be present (eg, rapid onset, vomiting, fever) do not typically occur in the patient with vestibular schwannoma.
Because the symptoms typically associated with vestibular schwannoma are likely to occur bilaterally in patients with other conditions, unilateral symptoms should alert the clinician to investigate further. The patterns and growth rates of vestibular schwannomas are highly variable and currently unpredictable18 (according to Fortnum et al,14 at least 50% of tumors do not grow within several years after diagnosis); thus, no clear predictors of tumor growth have been identified to assist in the evaluation of an affected patient,16 although faster tumor growth rates have been reported in young patients, and Baser et al18 have called for additional research involving younger persons with vestibular schwannomas.
Standard testing is audiometry followed by MRI, which is considered the most effective means to confirm a diagnosis of vestibular schwannoma.5,14,22
Treatment for Vestibular Schwannoma
Treatment, whether with surgery or radiation, is associated with significant morbidity and possibly decreased quality of life.16 Therefore, distinguishing patients whose tumors will grow and pose a threat to them from those whose tumors are likely to remain stable is central to appropriate management.23
Treatment modalities are considered based on tumor size, growth, presence or absence of tinnitus, and the patient’s preferences and life expectancy.23 In most cases, decision making is complex and should be customized to meet the patient’s individual circumstances. Patients with similar clinical scenarios have been reported to opt for different treatment choices.24
Four treatment options are currently available for patients with vestibular schwannoma:
Serial observation with periodic MRI studies. Since vestibular schwannomas are benign and slow-growing, conservative management can be a reasonable option, particularly if the patient is elderly, the tumor is small, and/or little hearing loss has taken place. However, use of observation is associated with a risk for progressive and permanent hearing loss.2 Between 15% and 50% of patients who opt for serial observation will undergo subsequent surgical intervention, particularly in cases involving worsening tinnitus, balance problems, or hearing loss.23-25
Chemotherapy. Agents including bevacizumab (a humanized monoclonal antibody against vascular endothelial growth factor)8,26,27 and erlotinib (an epidermal growth factor receptor inhibitor) may delay progression or even facilitate regression of vestibular schwannomas.28 Hearing improvement has also been reported in patients with NF2 who were treated with bevacizumab8; research is ongoing.26
Fractionated radiotherapy. Hearing may be preserved in 60% to 95% of patients, depending on levels of dosing to the cochlea, but 3% to 7% of patients will need further treatment.29-31 Radiation treatment is a likely choice in patients with tumors measuring 2.0 cm or less. Larger tumors are considered a surgical disease, and directed radiotherapy may be administered postoperatively (as in the case patient) for residual portions of the tumor.16
Microsurgery. Compared with other treatment modalities, the emphasis of microsurgery is on removing tumors (particularly larger tumors) rather than controlling their growth.29 The three common approaches are retrosigmoid, middle fossa, or translabyrinthine.32-34 Preservation of hearing is reportedly better following retrosigmoid or middle fossa microsurgery, compared with a translabyrinthine procedure (because in the latter, the tumor cannot be exposed without damage to the inner ear).32,35
With any such surgery, risks include cranial nerve damage, leakage of cerebrospinal fluid, and infection.29,32 Postsurgically, about half of patients report frequent headaches, which are persistent in about half of these cases.36-38 Another concern is preservation of the facial nerves, with a risk for temporary facial weakness or dysfunction.3,24,39 Less than 2% of patients who undergo microsurgery require additional treatment.29
Stereotactic radiosurgery. These procedures, which are performed using the Gamma Knife,® the CyberKnife, or the linear accelerator,29,40,41 are considered appropriate for patients with smaller tumors and those who are not candidates for conventional surgery.1 Trigeminal neuropathy, injury to the facial nerves, and hydrocephaly are reported complications of Gamma Knife radiosurgery, but improvements in these technologies are ongoing.1,2,40
Patient Outcomes
The outcome in a patient with vestibular schwannoma depends on the treatment administered, but prolonged follow-up is typically necessary. For patients being managed through observation, annual brain scans are recommended for 10 years, with subsequent scans every three to five years if no tumor growth is seen. For patients who have had surgery, annual brain scans are advised for the successive eight to 10 years, with decreasing frequency if no tumor remains. In patients who undergo radiation, annual scans are recommended for 10 years, then every two years if no tumor growth is detected.36
Psychosocial experiences vary widely among patients who have undergone treatment for vestibular schwannomas. Some are unable to perform necessary or recreational activities, and others must retire early from work.42 Others, however, have minimal disruption in their lives and enjoy a good quality of life. The most difficult consequence of vestibular schwannoma and its treatment, according to patients, is the associated hearing loss.8,19
THE CASE PATIENT
The 22-year-old patient in this case had an atypical presentation of vestibular schwannoma. Although she did present with vertigo, she also complained of headache, nausea, and photophobia—which are rarely reported in investigations of these tumors. She was also younger than the typical patient and did not report tinnitus.
The case patient reportedly underwent surgery and subsequent radiation to treat the remaining portion of her tumor. She suspended her attendance at the college and, as of this writing, has not re-enrolled. She was lost to follow-up.
CONCLUSION
For the primary care provider, diagnostic challenges require great clinical acumen. Vertigo, headache, hearing loss, and tinnitus are all symptoms seen in the primary care setting; when they occur together, the clinician should be alerted to investigate further. A high level of suspicion is appropriate when a patient complains of longstanding auditory symptoms, with or without headache. Unilateral hearing loss is a common symptom in patients with vestibular schwannomas, although some may present with facial weakness or pain, imbalance, and/or vertigo.
In addition to the history and physical exam, experts recommend that audiometry and MRI be considered, particularly if hearing loss is unilateral. Genetic screening for NF2 should be performed if vestibular schwannoma is found on MRI. Referral to a neurologist, a neurosurgeon, or an otolaryngologist is appropriate.
REFERENCES
1. Arthurs BJ, Lamoreaux WT, Giddings NA, et al. Gamma Knife radiosurgery for vestibular schwannoma: case report and review of the literature. World J Surg Oncol. 2009 Dec 18;7:100.
2. Mohammed TA, Ahuja MS, Ju SS, Thomas J. Normal pressure hydrocephalus after Gamma Knife radiosurgery for vestibular schwannoma. J Postgrad Med. 2010;56(3):213-215.
3. Gal TJ, Shinn J, Huang B. Current epidemiology and management trends in acoustic neuroma. Otolaryngol Head Neck Surg. 2010;142(5):677-681.
4. Evans DG, Moran A, King A, et al. Incidence of vestibular schwannoma and neurofibromatosis 2 in the North West of England over a 10-year period: higher incidence than previously thought. Otol Neurotol. 2005;26(1):93-97.
5. Haynes D. Acoustic neuroma diagnosis and treatment options. Hearing Health. 2009;25(3):32. www.drf.org/magazine/36/Summer+2009+Issue/article/272. Accessed May 16, 2011.
6. Sobel RA. Vestibular (acoustic) schwannomas: histologic features in neurofibromatosis 2 and in unilateral cases. J Neuropathol Exp Neurol. 1993;52(2):106-113.
7. Evans DG, Huson SM, Donnai D, et al. A clinical study of type 2 neurofibromatosis. Q J Med. 1992;84(304):603-618.
8. Plotkin SR, Stemmer-Rachamimov AO, Barker FG 2nd, et al. Hearing improvement after bevacizumab in patients with neurofibromatosis type 2. N Engl J Med. 2009;361(4):358-367.
9. Evans DGR, Sainio M, Baser E. Neurofibromatosis type 2. J Med Genet. 2000:37(11):897-904.
10. Gusella JF, Ramesh V, MacCollin M, Jacoby LB. Neurofibromatosis 2: loss of Merlin’s protective spell. Curr Opin Genet Dev. 1996;6(1):87-92.
11. Sagar SM, Israel MA. Ch 374. Primary and metastatic tumors of the nervous system. In: Kasper DL, Braunwald E, Fauci AS, et al. Harrison’s Principles of Internal Medicine. 17th ed. New York, NY: McGraw-Hill Companies, Inc; 2008:2601-2610.
12. Evans DGR. Neurofibromatosis 2 [bilateral acoustic neurofibromatosis, central neurofibromatosis, NF2, neurofibromatosis type II]. Genet Med. 2009;11(9):599-610.
13. Arya R, Sahu JK, Kabra M. Neurofibromatosis type II (Wishart type). J Pediatr Neurol. 2009;7(3): 333-335.
14. Fortnum H, O’Neill C, Taylor R, et al. The role of magnetic resonance imaging in the identification of suspected acoustic neuroma: a systematic review of clinical and cost effectiveness and natural history. Health Technol Assess. 2009;13(18):iii-iv, ix-xi, 1-154.
15. Forton GE, Cremers CW, Offeciers EE. Acoustic neuroma ingrowth in the cochlear nerve: does it influence the clinical presentation? Ann Otol Rhinol Laryngol. 2004;113(7):582-586.
16. Nikolopoulos TP, Fortnum H, O’Donoghue G, Baguley D. Acoustic neuroma growth: a systematic review of the evidence. Otol Neurotol. 2010;31(3):478-485.
17. Yates CW, Weinberg M, Packer MJ, Jacob A. Fatal case of tumor-associated hemorrhage in a large vestibular schwannoma. Ann Otol Rhinol Laryngol. 2010;119(6):402-405.
18. Baser ME, Mautner VF, Parry DM, Evans DGR. Methodological issues in longitudinal studies; vestibular schwannoma growth rates in neurofibromatosis 2. J Med Genet. 2005;42(12):903-906.
19. Brooker J, Burney S, Fletcher J, Dally M. A qualitative exploration of quality of life among individuals diagnosed with an acoustic neuroma. Br J Health Psychol. 2009;14(pt 3):563-578.
20. Strupp M, Brandt T. Diagnosis and treatment of vertigo and dizziness. Dtsch Arzetbl Int. 2008;105(10):173-180.
21. Kerber KA. Dizziness and vertigo. In: Andreoli TE, Griggs RC, Benjamin I , Wing EJ, eds. Andreoli and Carpenter’s Cecil Essentials of Medicine. 8th ed. Philadelphia, PA: Elsevier Inc; 2010:1104-1105.
22. Gimsing S. Vestibular schwannoma: when to look for it? J Laryngol Otol. 2010;124(3):258-264.
23. Agrawal Y, Clark JH, Limb CJ, et al. Predictors of vestibular schwannoma growth and clinical implications. Otol Neurotol. 2010;31(5):807-812.
24. Cheung SW, Aranda D, Driscoll CLW, Parsa AT. Mapping clinical outcomes expectations to treatment decisions: an application to vestibular schwannoma management. Otol Neurotol. 2010;31(2):284-293.
25. Myrseth E, Pedersen PH, Møller P, Lund-Johansen M. Treatment of vestibular schwannomas: why, when and how? Acta Neurochir (Wien). 2007;149(7):647-660.
26. Sidney Kimmel Comprehensive Cancer Center, Massachusetts General Hospital, National Cancer Institute. Bevacizumab for symptomatic vestibular schwannoma in neurofibromatosis type 2 (NF2). http://clinicaltrials.gov/ct2/show/NCT01207687. Accessed May 16, 2011.
27. Mautner VF, Nguyen R, Kutta H, et al. Bevacizumab induces regression of vestibular schwannomas in patients with neurofibromatosis type 2. Neuro Oncol. 2010;12(1):14-18.
28. Plotkin SR, Halpin C, McKenna MJ, et al. Erlotinib for progressive vestibular schwannoma in neurofibromatosis 2 patients. Otol Neurotol. 2010;31(7):1135-1143.
29. Arthurs BJ, Fairbanks RK, Demakas JJ, et al. A review of treatment modalities for vestibular schwannoma. Neurosurg Rev. 2011 Feb 9; [Epub ahead of print].
30. Andrews DW, Werner-Wasik M, Den RB, et al. Toward dose optimization for fractionated stereotactic radiotherapy for acoustic neuromas: comparison of two dose cohorts. Int J Radiat Oncol Biol Phys. 2009;74(2):419-426.
31. Thomas C, Di Maio S, Ma R, et al. Hearing preservation following fractionated stereotactic radiotherapy for vestibular schwannomas: prognostic implications of cochlear dose. J Neurosurg. 2007;107(5):917-926.
32. Samii M, Gerganov V, Samii A. Improved preservation of hearing and facial nerve function in vestibular schwannoma surgery via the retrosigmoid approach in a series of 200 patients. J Neurosurg. 2006;105(4):527-535.
33. Shiobara R, Ohira T, Inoue Y, et al. Extended middle cranial fossa approach for vestibular schwannoma: technical note and surgical results of 896 operations. Prog Neurol Surg. 2008;21:65-72.
34. Schmerber S, Palombi O, Boubagra K, et al. Long-term control of vestibular schwannoma after a translabyrinthine complete removal. Neurosurgery. 2005;57(4):693-698.
35. Phillips DJ, Kobylarz EJ, De Peralta ET, et al. Predictive factors of hearing preservation after surgical resection of small vestibular schwannomas. Otol Neurotol. 2010;31(9):1463-1468.
36. Park JK, Black MP, Vernick DM, Ramakrishna N. Vestibular schwannoma (acoustic neuroma) (2010). www.uptodate.com/contents/vestibular-schwannoma-acoustic-neuroma. Accessed May 16, 2011.
37. Schankin CJ, Gall C, Straube A. Headache syndromes after acoustic neuroma surgery and their implications for quality of life. Cephalalgia. 2009;29(7):760-761.
38. Ryzenman JM, Pensak ML, Tew JM Jr. Headache: a quality of life analysis in a cohort of 1,657 patients undergoing acoustic neuroma surgery: results from the Acoustic Neuroma Association. Laryngoscope. 2005;115(4):703-711.
39. Sriskandan N, Connor SE. The role of radiology in the diagnosis and management of vestibular schwannoma. Clin Radiol. 2010;66(4):357-365.
40. Yang I, Sughrue ME, Han SJ, et al. Facial nerve preservation after vestibular schwannoma Gamma Knife surgery. J Neurooncol. 2009;93(1): 41-48.
41. Unger F, Dominikus K, Haselsberger K. Stereotactic radiosurgery and fractionated stereotactic radiotherapy of acoustic neuromas [in German]. HNO. 2011;59(1):31-37.
42. Tos T, Caye-Thomasen P, Stangerup SE, et al. Long-term socio-economic impact of vestibular schwannoma for patients under observation and after surgery. J Laryngol Otol. 2003;117(12):955-964.
A 22-year-old student was brought in to a college student health center in a wheelchair by campus safety personnel. She appeared drowsy and was crying softly. She complained of a severe headache and said she was “tired of going through this all the time.” The woman said she had seen spots and become dizzy, then had gotten “the worst headache of my life” while sitting in class. She rated the headache pain at 8 on a 10-point scale and also complained of nausea and photophobia.
The history revealed dizziness that made her “feel as if I’m tipping over” and similar headaches during the previous year. The patient said she had seen “a few doctors” for her symptoms, but that they “could never find anything.” The headaches usually occurred on the left side of her head, lasted hours to days, and were only partially relieved with acetaminophen. The patient could not remember whether she had eaten breakfast and was unsure of what day it was. She described herself as frustrated and began to weep again.
She was currently under the care of a psychologist but seemed uncertain why; she said that she was sexually active and used condoms. She had undergone an appendectomy at age 12. She denied taking any medications besides acetaminophen. She denied smoking or drug use, history of migraine headaches, vision or hearing changes, facial weakness, depression, or anxiety. Her family history included a grandfather with diabetes and hypertension and an uncle with heart disease. The family history was negative for migraine or psychiatric illness.
Because of the patient’s weakness, she was assisted onto the examination table by a nurse. Physical exam revealed a pale, slightly sweaty, overweight, tearful young woman who was slow to respond. Her blood pressure was measured at 134/104 mm Hg; pulse, 100 beats/min; respirations, 14 breaths/min; and temperature, 97.0ºF. Point-of-care testing of blood glucose was 91 mg/dL, and hemoglobin was measured at 12.3 g/dL. The ophthalmologic exam was positive for photophobia and revealed slightly disconjugate gaze with horizontal nystagmus during testing of cranial nerves (CN) III, IV, and VI. The otoscopic exam revealed a slightly injected right tympanic membrane, and there were no apparent hearing deficits.
The neurologic exam showed patellar and brachial deep tendon reflexes equal, grips weak and equal, and the pupillary response intact. The patient was able to stand without assistance, although her gait was slightly unsteady. Because the patient was of college age, the clinician ruled out meningitis by negative Kernig’s and Brudzinski’s signs and absence of fever. Subarachnoid hemorrhage was also a concern when the patient mentioned the “worst headache of my life,” indicating the need for emergent imaging.
The patient’s presentation, it was felt, warranted a 911 call. The emergency medical team arrived, and its members began to question the patient. Discrepancies in the patient’s history during the paramedics’ reexamination led them to question whether an emergency department (ED) visit was necessary, but at the clinician’s insistence, they agreed to transport the student to the ED.
The following day, the student health center clinician was contacted by a member of the hospital ED staff with an update on the patient’s status. Shortly after her arrival at the hospital, she underwent MRI and was diagnosed with a vestibular schwannoma. She had surgery that same evening, during which the surgeon removed most of the tumor. Although the ED staff was not at liberty to provide more complete information, they did inform the clinician that the patient would require radiation for the remainder of the tumor.
DISCUSSION
Vestibular schwannoma is also known as acoustic schwannoma, acoustic neuroma, acoustic neurinoma, or vestibular neurilemmoma. These tumors arise from perineural elements of Schwann cells, which commonly form and lead to myelination in the vestibular area of CN VIII1 (see figure). They occur with equal frequency on the superior and inferior branches of the vestibular nerve and originate only rarely at the cochlear portion of the eighth cranial nerve. Vestibular schwannomas represent approximately 8% to 10% of brain tumors and 80% to 90% of tumors in the cerebellopontine angle in adults.2 Tumors are distributed evenly across genders, but the majority of diagnosed patients are white.3
Most likely because of improvements in diagnostic technology, the incidence of vestibular schwannoma has increased over the past 30 years. One British research team predicts that one in 1,000 persons will receive a diagnosis of vestibular schwannoma in their lifetime.4 These tumors are most commonly diagnosed in people ages 30 to 60, with a median age of 55.5
A relationship has been demonstrated between neurofibromatosis type 2 (NF2), an autosomal-dominant disease, and the development of vestibular schwannomas.6,7 NF2 has a birth prevalence of one in about 25,000 persons,4,8 and those who inherit the responsible gene inevitably develop vestibular schwannomas.9 Patients with a confirmed diagnosis of vestibular schwannoma should be screened by a geneticist for the NF2 gene; although the tumors are benign, they can cause compression of the vestibular nerve, leading to deafness and balance disorders.10 Schwannomas of the spinal nerves can also occur in persons with NF2.11 Compression of the spinal nerves in these patients can lead to significant morbidity and a shortened average life span.10
NF2 is diagnosed using the following criteria:
1) Bilateral vestibular schwannomas
2) Diagnosis of a family member with either NF2 or unilateral vestibular schwannoma, and
3) Juvenile posterior subscapular lens opacities.9,12,13
Because schwannomas grow slowly, the vestibular system can adapt to the slow destruction of CN VIII. For this reason, patients typically present with unilateral deafness or hearing impairment rather than dizziness.11 Many patients also present with tinnitus and/or vertigo.14,15
Some vestibular tumors remain stable or even regress; others progress, in some cases causing life-threatening complications.16 An extremely rare complication of a vestibular schwannoma was reported in one patient: an intratumoral hemorrhage that led to acute neurologic deterioration and death.17
Since the case patient underwent immediate surgical intervention, it appears she was experiencing significant involvement and it was likely anticipated that without surgical intervention, clinical progression would occur. Her young age could be considered a risk factor for a faster-growing neuroma.18
Clinical Presentation and Diagnosis
Primary care clinicians commonly see patients with complaints of dizziness, lightheadedness, faintness, or a sensation of spinning or tilting. Vestibular schwannoma should be considered in the differential diagnosis of the patient who presents with these complaints, as well as tinnitus or hearing loss.9 The patient with vestibular schwannoma may also have a history of headache, unsteady gait, facial pain, and numbness.19 A partial differential diagnosis is listed in the table20,21). The astute clinician will systematically rule out many of these conditions, since certain other features that may be present (eg, rapid onset, vomiting, fever) do not typically occur in the patient with vestibular schwannoma.
Because the symptoms typically associated with vestibular schwannoma are likely to occur bilaterally in patients with other conditions, unilateral symptoms should alert the clinician to investigate further. The patterns and growth rates of vestibular schwannomas are highly variable and currently unpredictable18 (according to Fortnum et al,14 at least 50% of tumors do not grow within several years after diagnosis); thus, no clear predictors of tumor growth have been identified to assist in the evaluation of an affected patient,16 although faster tumor growth rates have been reported in young patients, and Baser et al18 have called for additional research involving younger persons with vestibular schwannomas.
Standard testing is audiometry followed by MRI, which is considered the most effective means to confirm a diagnosis of vestibular schwannoma.5,14,22
Treatment for Vestibular Schwannoma
Treatment, whether with surgery or radiation, is associated with significant morbidity and possibly decreased quality of life.16 Therefore, distinguishing patients whose tumors will grow and pose a threat to them from those whose tumors are likely to remain stable is central to appropriate management.23
Treatment modalities are considered based on tumor size, growth, presence or absence of tinnitus, and the patient’s preferences and life expectancy.23 In most cases, decision making is complex and should be customized to meet the patient’s individual circumstances. Patients with similar clinical scenarios have been reported to opt for different treatment choices.24
Four treatment options are currently available for patients with vestibular schwannoma:
Serial observation with periodic MRI studies. Since vestibular schwannomas are benign and slow-growing, conservative management can be a reasonable option, particularly if the patient is elderly, the tumor is small, and/or little hearing loss has taken place. However, use of observation is associated with a risk for progressive and permanent hearing loss.2 Between 15% and 50% of patients who opt for serial observation will undergo subsequent surgical intervention, particularly in cases involving worsening tinnitus, balance problems, or hearing loss.23-25
Chemotherapy. Agents including bevacizumab (a humanized monoclonal antibody against vascular endothelial growth factor)8,26,27 and erlotinib (an epidermal growth factor receptor inhibitor) may delay progression or even facilitate regression of vestibular schwannomas.28 Hearing improvement has also been reported in patients with NF2 who were treated with bevacizumab8; research is ongoing.26
Fractionated radiotherapy. Hearing may be preserved in 60% to 95% of patients, depending on levels of dosing to the cochlea, but 3% to 7% of patients will need further treatment.29-31 Radiation treatment is a likely choice in patients with tumors measuring 2.0 cm or less. Larger tumors are considered a surgical disease, and directed radiotherapy may be administered postoperatively (as in the case patient) for residual portions of the tumor.16
Microsurgery. Compared with other treatment modalities, the emphasis of microsurgery is on removing tumors (particularly larger tumors) rather than controlling their growth.29 The three common approaches are retrosigmoid, middle fossa, or translabyrinthine.32-34 Preservation of hearing is reportedly better following retrosigmoid or middle fossa microsurgery, compared with a translabyrinthine procedure (because in the latter, the tumor cannot be exposed without damage to the inner ear).32,35
With any such surgery, risks include cranial nerve damage, leakage of cerebrospinal fluid, and infection.29,32 Postsurgically, about half of patients report frequent headaches, which are persistent in about half of these cases.36-38 Another concern is preservation of the facial nerves, with a risk for temporary facial weakness or dysfunction.3,24,39 Less than 2% of patients who undergo microsurgery require additional treatment.29
Stereotactic radiosurgery. These procedures, which are performed using the Gamma Knife,® the CyberKnife, or the linear accelerator,29,40,41 are considered appropriate for patients with smaller tumors and those who are not candidates for conventional surgery.1 Trigeminal neuropathy, injury to the facial nerves, and hydrocephaly are reported complications of Gamma Knife radiosurgery, but improvements in these technologies are ongoing.1,2,40
Patient Outcomes
The outcome in a patient with vestibular schwannoma depends on the treatment administered, but prolonged follow-up is typically necessary. For patients being managed through observation, annual brain scans are recommended for 10 years, with subsequent scans every three to five years if no tumor growth is seen. For patients who have had surgery, annual brain scans are advised for the successive eight to 10 years, with decreasing frequency if no tumor remains. In patients who undergo radiation, annual scans are recommended for 10 years, then every two years if no tumor growth is detected.36
Psychosocial experiences vary widely among patients who have undergone treatment for vestibular schwannomas. Some are unable to perform necessary or recreational activities, and others must retire early from work.42 Others, however, have minimal disruption in their lives and enjoy a good quality of life. The most difficult consequence of vestibular schwannoma and its treatment, according to patients, is the associated hearing loss.8,19
THE CASE PATIENT
The 22-year-old patient in this case had an atypical presentation of vestibular schwannoma. Although she did present with vertigo, she also complained of headache, nausea, and photophobia—which are rarely reported in investigations of these tumors. She was also younger than the typical patient and did not report tinnitus.
The case patient reportedly underwent surgery and subsequent radiation to treat the remaining portion of her tumor. She suspended her attendance at the college and, as of this writing, has not re-enrolled. She was lost to follow-up.
CONCLUSION
For the primary care provider, diagnostic challenges require great clinical acumen. Vertigo, headache, hearing loss, and tinnitus are all symptoms seen in the primary care setting; when they occur together, the clinician should be alerted to investigate further. A high level of suspicion is appropriate when a patient complains of longstanding auditory symptoms, with or without headache. Unilateral hearing loss is a common symptom in patients with vestibular schwannomas, although some may present with facial weakness or pain, imbalance, and/or vertigo.
In addition to the history and physical exam, experts recommend that audiometry and MRI be considered, particularly if hearing loss is unilateral. Genetic screening for NF2 should be performed if vestibular schwannoma is found on MRI. Referral to a neurologist, a neurosurgeon, or an otolaryngologist is appropriate.
REFERENCES
1. Arthurs BJ, Lamoreaux WT, Giddings NA, et al. Gamma Knife radiosurgery for vestibular schwannoma: case report and review of the literature. World J Surg Oncol. 2009 Dec 18;7:100.
2. Mohammed TA, Ahuja MS, Ju SS, Thomas J. Normal pressure hydrocephalus after Gamma Knife radiosurgery for vestibular schwannoma. J Postgrad Med. 2010;56(3):213-215.
3. Gal TJ, Shinn J, Huang B. Current epidemiology and management trends in acoustic neuroma. Otolaryngol Head Neck Surg. 2010;142(5):677-681.
4. Evans DG, Moran A, King A, et al. Incidence of vestibular schwannoma and neurofibromatosis 2 in the North West of England over a 10-year period: higher incidence than previously thought. Otol Neurotol. 2005;26(1):93-97.
5. Haynes D. Acoustic neuroma diagnosis and treatment options. Hearing Health. 2009;25(3):32. www.drf.org/magazine/36/Summer+2009+Issue/article/272. Accessed May 16, 2011.
6. Sobel RA. Vestibular (acoustic) schwannomas: histologic features in neurofibromatosis 2 and in unilateral cases. J Neuropathol Exp Neurol. 1993;52(2):106-113.
7. Evans DG, Huson SM, Donnai D, et al. A clinical study of type 2 neurofibromatosis. Q J Med. 1992;84(304):603-618.
8. Plotkin SR, Stemmer-Rachamimov AO, Barker FG 2nd, et al. Hearing improvement after bevacizumab in patients with neurofibromatosis type 2. N Engl J Med. 2009;361(4):358-367.
9. Evans DGR, Sainio M, Baser E. Neurofibromatosis type 2. J Med Genet. 2000:37(11):897-904.
10. Gusella JF, Ramesh V, MacCollin M, Jacoby LB. Neurofibromatosis 2: loss of Merlin’s protective spell. Curr Opin Genet Dev. 1996;6(1):87-92.
11. Sagar SM, Israel MA. Ch 374. Primary and metastatic tumors of the nervous system. In: Kasper DL, Braunwald E, Fauci AS, et al. Harrison’s Principles of Internal Medicine. 17th ed. New York, NY: McGraw-Hill Companies, Inc; 2008:2601-2610.
12. Evans DGR. Neurofibromatosis 2 [bilateral acoustic neurofibromatosis, central neurofibromatosis, NF2, neurofibromatosis type II]. Genet Med. 2009;11(9):599-610.
13. Arya R, Sahu JK, Kabra M. Neurofibromatosis type II (Wishart type). J Pediatr Neurol. 2009;7(3): 333-335.
14. Fortnum H, O’Neill C, Taylor R, et al. The role of magnetic resonance imaging in the identification of suspected acoustic neuroma: a systematic review of clinical and cost effectiveness and natural history. Health Technol Assess. 2009;13(18):iii-iv, ix-xi, 1-154.
15. Forton GE, Cremers CW, Offeciers EE. Acoustic neuroma ingrowth in the cochlear nerve: does it influence the clinical presentation? Ann Otol Rhinol Laryngol. 2004;113(7):582-586.
16. Nikolopoulos TP, Fortnum H, O’Donoghue G, Baguley D. Acoustic neuroma growth: a systematic review of the evidence. Otol Neurotol. 2010;31(3):478-485.
17. Yates CW, Weinberg M, Packer MJ, Jacob A. Fatal case of tumor-associated hemorrhage in a large vestibular schwannoma. Ann Otol Rhinol Laryngol. 2010;119(6):402-405.
18. Baser ME, Mautner VF, Parry DM, Evans DGR. Methodological issues in longitudinal studies; vestibular schwannoma growth rates in neurofibromatosis 2. J Med Genet. 2005;42(12):903-906.
19. Brooker J, Burney S, Fletcher J, Dally M. A qualitative exploration of quality of life among individuals diagnosed with an acoustic neuroma. Br J Health Psychol. 2009;14(pt 3):563-578.
20. Strupp M, Brandt T. Diagnosis and treatment of vertigo and dizziness. Dtsch Arzetbl Int. 2008;105(10):173-180.
21. Kerber KA. Dizziness and vertigo. In: Andreoli TE, Griggs RC, Benjamin I , Wing EJ, eds. Andreoli and Carpenter’s Cecil Essentials of Medicine. 8th ed. Philadelphia, PA: Elsevier Inc; 2010:1104-1105.
22. Gimsing S. Vestibular schwannoma: when to look for it? J Laryngol Otol. 2010;124(3):258-264.
23. Agrawal Y, Clark JH, Limb CJ, et al. Predictors of vestibular schwannoma growth and clinical implications. Otol Neurotol. 2010;31(5):807-812.
24. Cheung SW, Aranda D, Driscoll CLW, Parsa AT. Mapping clinical outcomes expectations to treatment decisions: an application to vestibular schwannoma management. Otol Neurotol. 2010;31(2):284-293.
25. Myrseth E, Pedersen PH, Møller P, Lund-Johansen M. Treatment of vestibular schwannomas: why, when and how? Acta Neurochir (Wien). 2007;149(7):647-660.
26. Sidney Kimmel Comprehensive Cancer Center, Massachusetts General Hospital, National Cancer Institute. Bevacizumab for symptomatic vestibular schwannoma in neurofibromatosis type 2 (NF2). http://clinicaltrials.gov/ct2/show/NCT01207687. Accessed May 16, 2011.
27. Mautner VF, Nguyen R, Kutta H, et al. Bevacizumab induces regression of vestibular schwannomas in patients with neurofibromatosis type 2. Neuro Oncol. 2010;12(1):14-18.
28. Plotkin SR, Halpin C, McKenna MJ, et al. Erlotinib for progressive vestibular schwannoma in neurofibromatosis 2 patients. Otol Neurotol. 2010;31(7):1135-1143.
29. Arthurs BJ, Fairbanks RK, Demakas JJ, et al. A review of treatment modalities for vestibular schwannoma. Neurosurg Rev. 2011 Feb 9; [Epub ahead of print].
30. Andrews DW, Werner-Wasik M, Den RB, et al. Toward dose optimization for fractionated stereotactic radiotherapy for acoustic neuromas: comparison of two dose cohorts. Int J Radiat Oncol Biol Phys. 2009;74(2):419-426.
31. Thomas C, Di Maio S, Ma R, et al. Hearing preservation following fractionated stereotactic radiotherapy for vestibular schwannomas: prognostic implications of cochlear dose. J Neurosurg. 2007;107(5):917-926.
32. Samii M, Gerganov V, Samii A. Improved preservation of hearing and facial nerve function in vestibular schwannoma surgery via the retrosigmoid approach in a series of 200 patients. J Neurosurg. 2006;105(4):527-535.
33. Shiobara R, Ohira T, Inoue Y, et al. Extended middle cranial fossa approach for vestibular schwannoma: technical note and surgical results of 896 operations. Prog Neurol Surg. 2008;21:65-72.
34. Schmerber S, Palombi O, Boubagra K, et al. Long-term control of vestibular schwannoma after a translabyrinthine complete removal. Neurosurgery. 2005;57(4):693-698.
35. Phillips DJ, Kobylarz EJ, De Peralta ET, et al. Predictive factors of hearing preservation after surgical resection of small vestibular schwannomas. Otol Neurotol. 2010;31(9):1463-1468.
36. Park JK, Black MP, Vernick DM, Ramakrishna N. Vestibular schwannoma (acoustic neuroma) (2010). www.uptodate.com/contents/vestibular-schwannoma-acoustic-neuroma. Accessed May 16, 2011.
37. Schankin CJ, Gall C, Straube A. Headache syndromes after acoustic neuroma surgery and their implications for quality of life. Cephalalgia. 2009;29(7):760-761.
38. Ryzenman JM, Pensak ML, Tew JM Jr. Headache: a quality of life analysis in a cohort of 1,657 patients undergoing acoustic neuroma surgery: results from the Acoustic Neuroma Association. Laryngoscope. 2005;115(4):703-711.
39. Sriskandan N, Connor SE. The role of radiology in the diagnosis and management of vestibular schwannoma. Clin Radiol. 2010;66(4):357-365.
40. Yang I, Sughrue ME, Han SJ, et al. Facial nerve preservation after vestibular schwannoma Gamma Knife surgery. J Neurooncol. 2009;93(1): 41-48.
41. Unger F, Dominikus K, Haselsberger K. Stereotactic radiosurgery and fractionated stereotactic radiotherapy of acoustic neuromas [in German]. HNO. 2011;59(1):31-37.
42. Tos T, Caye-Thomasen P, Stangerup SE, et al. Long-term socio-economic impact of vestibular schwannoma for patients under observation and after surgery. J Laryngol Otol. 2003;117(12):955-964.