User login
Comparing Transurethral Alprostadil with Intracavernosal Injections in a VA Impotence Clinic
Infantile Idiopathic Scoliosis
Orthopedic Trauma in Pregnancy
Management of Pelvic Fractures During Pregnancy
The legacy of WHI? Confusion and apprehension, possibly
Why? And is this state of confusion permanent? Most of all, how are your colleagues dealing with that lack of clarity in their practice?
Questions put to your peers
In early September, the Hormone Foundation, public education affiliate of the Endocrine Society, released the results of a national survey of doctors involved in menopause care.2 The survey was designed to gauge the effects of the WHI on clinical practice and was conducted on behalf of the Hormone Foundation with financial support from Novogyne Pharmaceuticals. Among the findings:
- Only 15% of the physicians believe their patients’ perceptions of the risks of hormone replacement are accurate
- Only 18% of physicians—this includes ObGyns—report that they themselves have “no confusion at all” about the findings of the WHI
- 83% of physicians believe their patients are as confused now as when the WHI findings were released in 2002—or more so
- 81% of physicians believe the media are as, or more, confused as when the findings were released.2
“There’s a lot of noise,” says Nanette Santoro, MD, director of reproductive endocrinology at Albert Einstein College of Medicine, Bronx, New York, and a member of the Hormone Foundation’s Women’s Health Task Force. “And there have been a lot of arguments back and forth.”
What can a physician do to achieve a little clarity?
Staying up-to-date on the clinical practice guidelines is the best way to combat confusion, Dr. Santoro says. A good starting point, she notes, is the Hormone Foundation Web site (Hormone. org), which links to the American College of Obstetricians and Gynecologists, the American Society for Reproductive Medicine (ASRM), and the North American Menopause Society (NAMS), all of which publish reliable guidelines.
“I think that’s probably the best way of keeping abreast of what’s happening now if [physicians] are not really deeply into menopause care,” she says. “But getting filtered information, or getting information from pundits or from the media is, I think, more hazardous because the quality of that information can be variable. And the days of getting your information from pharmaceutical representatives are long gone in this area because, again, it is not sufficiently reliable.”
During the spring of this year, 404 physicians responded to a survey about menopause management in the 5 years since the first Women’s Health Initiative (WHI) findings were published.1 The physicians represented the following primary care specialties: endocrinology, obstetrics and gynecology, internal medicine, and family and general practice. To qualify for the survey, each clinician had to devote at least 70% of his or her working day to clinical practice and see at least two women each month with menopausal symptoms.
The survey was conducted by Richard Day Research of Evanston, Illinois, for the Hormone Foundation. To review the full survey, visit www.hormone.org/pdf/meno_survey_qa.pdf.
Here are highlights:
| Primary medical specialty | |
| Family or general practice | 29% |
| Internal medicine | 27 |
| Obstetrics and gynecology | 40 |
| Endocrinology | 4 |
| Percentage of patients with menopausal symptoms currently taking HT | 37%* |
| Percentage reluctant to start HT | 42%* |
| Percentage that specifically asks to be put on HT | 19%* |
| Percentage that specifically asks not to be put on HT | 29%* |
| For moderate or severe menopausal symptoms, do you think of HT as a: | |
| first-line treatment? | 74% |
| second-line treatment (or third, fourth, etc)? | 26% |
| Which of the following are very important to you when deciding whether to prescribe HT for your patients? | |
| Severity of symptoms | 81% |
| Patient’s personal medical history | 77 |
| Risks of HT | 61 |
| Range and specific types of symptoms | 50 |
| Patient request | 44 |
| Age of patient | 33 |
| Prevention of osteoporosis | 24 |
| Which risks concern you about prescribing estrogen–progestin therapy for menopausal symptoms? | |
| Blood clots | 88% |
| Breast cancer | 87 |
| Coronary heart disease | 74 |
| Stroke | 73 |
| Dementia | 14 |
| What do you see as valuable about estrogen–progestin therapy for menopausal symptoms? | |
| Relieves hot flashes | 100% |
| Relieves vaginal dryness and painful intercourse | 92 |
| Improves sleep problems | 88 |
| Prevents bone loss | 84 |
| Reduces depression and mood changes | 68 |
| Reduces risk of colorectal Ca | 37 |
| Prevents cardiovascular disease | 16 |
| Which risks concern you about prescribing estrogen-only therapy for menopausal symptoms? | |
| Blood clots | 86% |
| Breast cancer | 71 |
| Stroke | 68 |
| Coronary heart disease | 51 |
| Dementia | 8 |
| Uterine cancer | 4 |
| What do you see as valuable about estrogen-only therapy for menopausal symptoms? | |
| Relieves hot flashes | 99% |
| Relieves vaginal dryness and painful intercourse | 94 |
| Improves sleep problems | 84 |
| Prevents bone loss | 81 |
| Reduces depression and mood changes | 71 |
| Reduces risk of colorectal Ca | 35 |
| Prevents cardiovascular disease | 19 |
| In your view, are the risks of HT understated, overstated, or accurately perceived by the following groups? | |
| Means, based on the following: | |
| 1 = understated | |
| 2 = accurately perceived | |
| 3 = overstated | |
| Media | 2.9 (Mean) |
| Patients | 2.7 |
| Family or general practitioners | 2.4 |
| Internists | 2.4 |
| ObGyns | 2.0 |
| Endocrinologists | 2.1 |
| As of today, how much confusion do you feel there is about the WHI findings? | |
| Means, based on the following: | |
| 1 = not confused at all | |
| 2 = not very much confusion | |
| 3 = some confusion | |
| 4 = great deal of confusion | |
| For you personally | 2.3 (Mean) |
| Media | 3.7 |
| Patients | 3.7 |
| Family or general practitioners | 3.1 |
| Internists | 3.0 |
| ObGyns | 2.5 |
| Endocrinologists | 2.5 |
| *Mean | |
“Afraid of hormones”
In the years since early WHI findings were published, Anita L. Nelson, MD, has not noticed confusion so much as fear among her patients. Dr. Nelson is professor of obstetrics and gynecology at the David Geffen School of Medicine at UCLA in Los Angeles.
“I think the things that are concerning to patients by and large are breast cancer and, in women who have done more reading on it, some of them are concerned about dementia,” Dr. Nelson says. “But by and large, other than those focused issues, it is hormones that patients are afraid of, and they sort of wave their hands in this global aura of ‘badness’ that they’re afraid of.”
One reason is the WHI. “Obviously that contributed to it,” she says. But a bigger cause of fear among her patients, a large percentage of whom are referred, is the fact that “their physicians have been taking them off of therapy. They’re not offering it,” she says, “or they are putting up a sort of barrier by saying, ‘You have to go see Dr. Nelson before you can start taking those medications.’”
The problem doesn’t end there, she adds. “The sad thing is that they are by and large not offering them alternative medications while they’re waiting for the transition—or if they are, sometimes they are actually giving them hazardous drugs. One of my favorite things is when patients who have high blood pressure are denied estrogen but are given Bellergal [ergotamine, belladonna alkaloids, and phenobarbital], which has a vasoconstrictive medication in it.”
“We do want folks to review the data,” she says, noting that ObGyns are “true believers” and unlikely to quit prescribing hormone therapy (HT). It is the internists and the family medicine physicians “who still have significant misgivings about the safety of these therapies in recently menopausal women.”
Joanna Shulman, MD, agrees. She is associate professor and director of the medical student clerkship in obstetrics and gynecology at Mount Sinai School of Medicine in New York City.
“The internists I work with or that my patients see tend to be terrified of hormone therapy. So I think they tend to discourage their patients.”
Mea culpa, anyone?
Confusion over the WHI is an issue for another prominent ObGyn—Wulf H. Utian, MD, PhD, editor-in-chief of Menopause Management and executive director of NAMS. In an editorial in the September/October issue of Menopause Management, Dr. Utian faults the National Heart, Lung and Blood Institute of the National Institutes of Health (NIH) for starting a “firestorm in women’s health” by publicizing the abrupt termination of the estrogen–progestin arm of the WHI study.4
Utian notes that he pointed out his dismay over the WHI way back in 2002, when he wrote, again in Menopause Management: “The manner in which the study was terminated was poorly planned, abrupt, and inhumane. Predictably, the media response was enormous, ranging from thoughtful to sensational. Panic was caused, numerous women discontinued therapy, and women and their health providers alike have been thrown into a state of confusion, distrust, and quandary of what to do next.”4
Bruce Wineman, DO, concurs. Although he retired from practice as a reproductive endocrinologist at the Marshfield Clinic in Marshfield, Wisconsin, shortly before the WHI findings were first published, he maintains his license and stays active in the ASRM. “The worst part of the WHI is that they got so much press with it,” he says, “and that the group of women that they chose was exactly the group of women that was going to have the maximum amount of negative effect.”
Utian believes a mea culpa is in order. “There are reams of important and pertinent data coming out of all the substudies of the WHI,” he writes. “For these to be accepted with confidence, it is well time for the NIH to bring all their WHI investigators together to develop a transparent and comprehensive summary of their results. It is also time for the WHI investigators to cease their stubborn defense and misrepresentation of their 2002 data, and to return to scientific integrity.”3
Same view in the trenches
Mohamed Mitwally, MD, spends 90% of his day in clinical practice at the Reproductive Medicine and Fertility Center in Colorado Springs, Colorado. He estimates that roughly half of his perimenopausal and menopausal patients troubled by vasomotor and other symptoms are currently on HT. Since the WHI’s initial findings were published, Dr. Mitwally has “absolutely” had to spend considerably more time educating his patients—“and educating physicians,” he says. His patients are reluctant to take HT because of press attention to the WHI. And other physicians are reluctant to give HT because they understand that the WHI is a randomized trial “and so don’t question it.”
Dr. Mitwally blames two entities for this state of affairs. “The credit goes to the WHI,” he says. “They did a wonderful job of screwing people up” with a “very poorly designed study.” There is also “a lot of misinformation,” thanks to the media. “They just want to get any bad news and magnify it.”
In the wake of the WHI, Dr. Mitwally recalls, “it was like chaos” for 3 or 4 years—and there is still a lot of confusion.
Nevertheless, when a patient complains of moderate or severe vasomotor symptoms, Dr. Mitwally usually turns to HT as a first-line therapy. “It is excellent for these patients,” he says, although he emphasizes that “every patient should be managed separately.”
“I think the most important thing in the whole issue of HT is that physicians should leave these patients to subspecialists,” he says, by which he means reproductive endocrinologists and ObGyns with expertise in menopause care.
Plethora of products
One of the more surprising impacts of the WHI is the array of estrogen products now available. Because the WHI was expected to confirm observational data that suggested that estrogen reduced the risk of cardiovascular disease, the number of products in development skyrocketed.
“I think something like 35 compounds got approved while the study was under way, so there is more stuff than ever,” says Dr. Santoro. “But that actually was attractive to some people in the survey and has been found to be attractive to patients because it does give more choices the way things are going, which is toward more of a customized approach to giving hormones.”
Raksha Joshi, MD, chief medical officer and medical director of Monmouth Family Health Center in Long Branch, New Jersey, a federally funded qualified health migrant center (FQHC), says the broader array of estrogen products adds to the time she spends educating patients.
“We do tell them about the other forms of estrogen and their bioeffectiveness and what they would achieve for this particular woman,” she says.
For patients who report moderate to severe menopausal symptoms, Dr. Joshi considers estrogen a first-line therapy, but recommends concurrent lifestyle changes.
“Of course, the WHI has not disappeared,” she says, so concerns about risks remain. “But in the transition, when the symptoms are paramount, I would tailor the treatment to what the woman wants to get out of it. But I think it is important for the woman to understand that this is not a panacea and that it will not cure all her symptoms. Therefore, lifestyle changes and getting hormone replacement therapy should go concurrently.”
As for alternative therapies, women are increasingly likely to ask for or about them.
“We talk about that,” says Dr. Shulman. “If they’re miserable and they don’t think they’re appropriate candidates for estrogen, we talk about other things. Or some people will come in and say, ‘I don’t want to take estrogen. Is there anything else?’”
In these cases, Dr. Shulman recommends a number of options. “Effexor has shown some benefit, apparently, in the literature,” she says. “And I mention black cohosh, which is in a lot of popular over-the-counter type remedies and which, apparently, recently was shown to have possibly some benefit.” Of course, “there’s a tremendous placebo effect with all of these,” she observes.
“And then I suggest things like getting plenty of exercise and eating sensibly, and I take my other patients’ recommendations. One patient told me that she takes a cool shower every night before going to bed and finds it beneficial, so I don’t know—it’s one of those ‘can’t hurt, might help’ things.”
Estrogen got a “bad name”
When she looks back over the past 5 years, Dr. Shulman thinks the WHI’s effects have been destructive in many ways.
“I think the most important thing is that [HT] got an undeservedly bad name when the Women’s Health Initiative was published,” she says. The WHI “really did a disservice for women who could benefit from [HT] enormously and weren’t really at risk—not just for vasomotor symptoms but also emotional lability, depression, increased anxiety, things like that.”
“I have many women for whom I did prescribe estrogen, and they’re still on it and will probably never get off because they think that I saved their lives. So for women to be scared unfairly by the Women’s Health Initiative and to have to suffer with vasomotor and emotional problems is really a disservice.”
Dr. Wineman agrees, and points out that even some professional organizations are beginning to reconsider the initial WHI findings. “They’re beginning to say, ‘I really believe that there are certain women who would probably benefit a great deal more than we once thought, and perhaps we jumped to some wrong conclusions.’”
1. WHI Investigators. Risks and benefits of estrogen plus progestin in healthy postmenopausal women. Principal results from the Women’s Health Initiative Randomized Controlled Trial. JAMA. 2002;288:321-333.
2. Hormone Foundation. Physician survey on menopause management. April 16–May 23, 2007. Available at: www.hormone.org/pdf/meno_survey_qa.pdf. Accessed September 26, 2007.
3. Utian WH. If only WHI had kept to its premise—but now it’s time for their mea culpa. Menopause Management. 2007;16(5):8-12.
4. Utian WH. Managing menopause after HERS II and WHI: coping with the aftermath. Menopause Management. 2002;11:6-7.
Why? And is this state of confusion permanent? Most of all, how are your colleagues dealing with that lack of clarity in their practice?
Questions put to your peers
In early September, the Hormone Foundation, public education affiliate of the Endocrine Society, released the results of a national survey of doctors involved in menopause care.2 The survey was designed to gauge the effects of the WHI on clinical practice and was conducted on behalf of the Hormone Foundation with financial support from Novogyne Pharmaceuticals. Among the findings:
- Only 15% of the physicians believe their patients’ perceptions of the risks of hormone replacement are accurate
- Only 18% of physicians—this includes ObGyns—report that they themselves have “no confusion at all” about the findings of the WHI
- 83% of physicians believe their patients are as confused now as when the WHI findings were released in 2002—or more so
- 81% of physicians believe the media are as, or more, confused as when the findings were released.2
“There’s a lot of noise,” says Nanette Santoro, MD, director of reproductive endocrinology at Albert Einstein College of Medicine, Bronx, New York, and a member of the Hormone Foundation’s Women’s Health Task Force. “And there have been a lot of arguments back and forth.”
What can a physician do to achieve a little clarity?
Staying up-to-date on the clinical practice guidelines is the best way to combat confusion, Dr. Santoro says. A good starting point, she notes, is the Hormone Foundation Web site (Hormone. org), which links to the American College of Obstetricians and Gynecologists, the American Society for Reproductive Medicine (ASRM), and the North American Menopause Society (NAMS), all of which publish reliable guidelines.
“I think that’s probably the best way of keeping abreast of what’s happening now if [physicians] are not really deeply into menopause care,” she says. “But getting filtered information, or getting information from pundits or from the media is, I think, more hazardous because the quality of that information can be variable. And the days of getting your information from pharmaceutical representatives are long gone in this area because, again, it is not sufficiently reliable.”
During the spring of this year, 404 physicians responded to a survey about menopause management in the 5 years since the first Women’s Health Initiative (WHI) findings were published.1 The physicians represented the following primary care specialties: endocrinology, obstetrics and gynecology, internal medicine, and family and general practice. To qualify for the survey, each clinician had to devote at least 70% of his or her working day to clinical practice and see at least two women each month with menopausal symptoms.
The survey was conducted by Richard Day Research of Evanston, Illinois, for the Hormone Foundation. To review the full survey, visit www.hormone.org/pdf/meno_survey_qa.pdf.
Here are highlights:
| Primary medical specialty | |
| Family or general practice | 29% |
| Internal medicine | 27 |
| Obstetrics and gynecology | 40 |
| Endocrinology | 4 |
| Percentage of patients with menopausal symptoms currently taking HT | 37%* |
| Percentage reluctant to start HT | 42%* |
| Percentage that specifically asks to be put on HT | 19%* |
| Percentage that specifically asks not to be put on HT | 29%* |
| For moderate or severe menopausal symptoms, do you think of HT as a: | |
| first-line treatment? | 74% |
| second-line treatment (or third, fourth, etc)? | 26% |
| Which of the following are very important to you when deciding whether to prescribe HT for your patients? | |
| Severity of symptoms | 81% |
| Patient’s personal medical history | 77 |
| Risks of HT | 61 |
| Range and specific types of symptoms | 50 |
| Patient request | 44 |
| Age of patient | 33 |
| Prevention of osteoporosis | 24 |
| Which risks concern you about prescribing estrogen–progestin therapy for menopausal symptoms? | |
| Blood clots | 88% |
| Breast cancer | 87 |
| Coronary heart disease | 74 |
| Stroke | 73 |
| Dementia | 14 |
| What do you see as valuable about estrogen–progestin therapy for menopausal symptoms? | |
| Relieves hot flashes | 100% |
| Relieves vaginal dryness and painful intercourse | 92 |
| Improves sleep problems | 88 |
| Prevents bone loss | 84 |
| Reduces depression and mood changes | 68 |
| Reduces risk of colorectal Ca | 37 |
| Prevents cardiovascular disease | 16 |
| Which risks concern you about prescribing estrogen-only therapy for menopausal symptoms? | |
| Blood clots | 86% |
| Breast cancer | 71 |
| Stroke | 68 |
| Coronary heart disease | 51 |
| Dementia | 8 |
| Uterine cancer | 4 |
| What do you see as valuable about estrogen-only therapy for menopausal symptoms? | |
| Relieves hot flashes | 99% |
| Relieves vaginal dryness and painful intercourse | 94 |
| Improves sleep problems | 84 |
| Prevents bone loss | 81 |
| Reduces depression and mood changes | 71 |
| Reduces risk of colorectal Ca | 35 |
| Prevents cardiovascular disease | 19 |
| In your view, are the risks of HT understated, overstated, or accurately perceived by the following groups? | |
| Means, based on the following: | |
| 1 = understated | |
| 2 = accurately perceived | |
| 3 = overstated | |
| Media | 2.9 (Mean) |
| Patients | 2.7 |
| Family or general practitioners | 2.4 |
| Internists | 2.4 |
| ObGyns | 2.0 |
| Endocrinologists | 2.1 |
| As of today, how much confusion do you feel there is about the WHI findings? | |
| Means, based on the following: | |
| 1 = not confused at all | |
| 2 = not very much confusion | |
| 3 = some confusion | |
| 4 = great deal of confusion | |
| For you personally | 2.3 (Mean) |
| Media | 3.7 |
| Patients | 3.7 |
| Family or general practitioners | 3.1 |
| Internists | 3.0 |
| ObGyns | 2.5 |
| Endocrinologists | 2.5 |
| *Mean | |
“Afraid of hormones”
In the years since early WHI findings were published, Anita L. Nelson, MD, has not noticed confusion so much as fear among her patients. Dr. Nelson is professor of obstetrics and gynecology at the David Geffen School of Medicine at UCLA in Los Angeles.
“I think the things that are concerning to patients by and large are breast cancer and, in women who have done more reading on it, some of them are concerned about dementia,” Dr. Nelson says. “But by and large, other than those focused issues, it is hormones that patients are afraid of, and they sort of wave their hands in this global aura of ‘badness’ that they’re afraid of.”
One reason is the WHI. “Obviously that contributed to it,” she says. But a bigger cause of fear among her patients, a large percentage of whom are referred, is the fact that “their physicians have been taking them off of therapy. They’re not offering it,” she says, “or they are putting up a sort of barrier by saying, ‘You have to go see Dr. Nelson before you can start taking those medications.’”
The problem doesn’t end there, she adds. “The sad thing is that they are by and large not offering them alternative medications while they’re waiting for the transition—or if they are, sometimes they are actually giving them hazardous drugs. One of my favorite things is when patients who have high blood pressure are denied estrogen but are given Bellergal [ergotamine, belladonna alkaloids, and phenobarbital], which has a vasoconstrictive medication in it.”
“We do want folks to review the data,” she says, noting that ObGyns are “true believers” and unlikely to quit prescribing hormone therapy (HT). It is the internists and the family medicine physicians “who still have significant misgivings about the safety of these therapies in recently menopausal women.”
Joanna Shulman, MD, agrees. She is associate professor and director of the medical student clerkship in obstetrics and gynecology at Mount Sinai School of Medicine in New York City.
“The internists I work with or that my patients see tend to be terrified of hormone therapy. So I think they tend to discourage their patients.”
Mea culpa, anyone?
Confusion over the WHI is an issue for another prominent ObGyn—Wulf H. Utian, MD, PhD, editor-in-chief of Menopause Management and executive director of NAMS. In an editorial in the September/October issue of Menopause Management, Dr. Utian faults the National Heart, Lung and Blood Institute of the National Institutes of Health (NIH) for starting a “firestorm in women’s health” by publicizing the abrupt termination of the estrogen–progestin arm of the WHI study.4
Utian notes that he pointed out his dismay over the WHI way back in 2002, when he wrote, again in Menopause Management: “The manner in which the study was terminated was poorly planned, abrupt, and inhumane. Predictably, the media response was enormous, ranging from thoughtful to sensational. Panic was caused, numerous women discontinued therapy, and women and their health providers alike have been thrown into a state of confusion, distrust, and quandary of what to do next.”4
Bruce Wineman, DO, concurs. Although he retired from practice as a reproductive endocrinologist at the Marshfield Clinic in Marshfield, Wisconsin, shortly before the WHI findings were first published, he maintains his license and stays active in the ASRM. “The worst part of the WHI is that they got so much press with it,” he says, “and that the group of women that they chose was exactly the group of women that was going to have the maximum amount of negative effect.”
Utian believes a mea culpa is in order. “There are reams of important and pertinent data coming out of all the substudies of the WHI,” he writes. “For these to be accepted with confidence, it is well time for the NIH to bring all their WHI investigators together to develop a transparent and comprehensive summary of their results. It is also time for the WHI investigators to cease their stubborn defense and misrepresentation of their 2002 data, and to return to scientific integrity.”3
Same view in the trenches
Mohamed Mitwally, MD, spends 90% of his day in clinical practice at the Reproductive Medicine and Fertility Center in Colorado Springs, Colorado. He estimates that roughly half of his perimenopausal and menopausal patients troubled by vasomotor and other symptoms are currently on HT. Since the WHI’s initial findings were published, Dr. Mitwally has “absolutely” had to spend considerably more time educating his patients—“and educating physicians,” he says. His patients are reluctant to take HT because of press attention to the WHI. And other physicians are reluctant to give HT because they understand that the WHI is a randomized trial “and so don’t question it.”
Dr. Mitwally blames two entities for this state of affairs. “The credit goes to the WHI,” he says. “They did a wonderful job of screwing people up” with a “very poorly designed study.” There is also “a lot of misinformation,” thanks to the media. “They just want to get any bad news and magnify it.”
In the wake of the WHI, Dr. Mitwally recalls, “it was like chaos” for 3 or 4 years—and there is still a lot of confusion.
Nevertheless, when a patient complains of moderate or severe vasomotor symptoms, Dr. Mitwally usually turns to HT as a first-line therapy. “It is excellent for these patients,” he says, although he emphasizes that “every patient should be managed separately.”
“I think the most important thing in the whole issue of HT is that physicians should leave these patients to subspecialists,” he says, by which he means reproductive endocrinologists and ObGyns with expertise in menopause care.
Plethora of products
One of the more surprising impacts of the WHI is the array of estrogen products now available. Because the WHI was expected to confirm observational data that suggested that estrogen reduced the risk of cardiovascular disease, the number of products in development skyrocketed.
“I think something like 35 compounds got approved while the study was under way, so there is more stuff than ever,” says Dr. Santoro. “But that actually was attractive to some people in the survey and has been found to be attractive to patients because it does give more choices the way things are going, which is toward more of a customized approach to giving hormones.”
Raksha Joshi, MD, chief medical officer and medical director of Monmouth Family Health Center in Long Branch, New Jersey, a federally funded qualified health migrant center (FQHC), says the broader array of estrogen products adds to the time she spends educating patients.
“We do tell them about the other forms of estrogen and their bioeffectiveness and what they would achieve for this particular woman,” she says.
For patients who report moderate to severe menopausal symptoms, Dr. Joshi considers estrogen a first-line therapy, but recommends concurrent lifestyle changes.
“Of course, the WHI has not disappeared,” she says, so concerns about risks remain. “But in the transition, when the symptoms are paramount, I would tailor the treatment to what the woman wants to get out of it. But I think it is important for the woman to understand that this is not a panacea and that it will not cure all her symptoms. Therefore, lifestyle changes and getting hormone replacement therapy should go concurrently.”
As for alternative therapies, women are increasingly likely to ask for or about them.
“We talk about that,” says Dr. Shulman. “If they’re miserable and they don’t think they’re appropriate candidates for estrogen, we talk about other things. Or some people will come in and say, ‘I don’t want to take estrogen. Is there anything else?’”
In these cases, Dr. Shulman recommends a number of options. “Effexor has shown some benefit, apparently, in the literature,” she says. “And I mention black cohosh, which is in a lot of popular over-the-counter type remedies and which, apparently, recently was shown to have possibly some benefit.” Of course, “there’s a tremendous placebo effect with all of these,” she observes.
“And then I suggest things like getting plenty of exercise and eating sensibly, and I take my other patients’ recommendations. One patient told me that she takes a cool shower every night before going to bed and finds it beneficial, so I don’t know—it’s one of those ‘can’t hurt, might help’ things.”
Estrogen got a “bad name”
When she looks back over the past 5 years, Dr. Shulman thinks the WHI’s effects have been destructive in many ways.
“I think the most important thing is that [HT] got an undeservedly bad name when the Women’s Health Initiative was published,” she says. The WHI “really did a disservice for women who could benefit from [HT] enormously and weren’t really at risk—not just for vasomotor symptoms but also emotional lability, depression, increased anxiety, things like that.”
“I have many women for whom I did prescribe estrogen, and they’re still on it and will probably never get off because they think that I saved their lives. So for women to be scared unfairly by the Women’s Health Initiative and to have to suffer with vasomotor and emotional problems is really a disservice.”
Dr. Wineman agrees, and points out that even some professional organizations are beginning to reconsider the initial WHI findings. “They’re beginning to say, ‘I really believe that there are certain women who would probably benefit a great deal more than we once thought, and perhaps we jumped to some wrong conclusions.’”
Why? And is this state of confusion permanent? Most of all, how are your colleagues dealing with that lack of clarity in their practice?
Questions put to your peers
In early September, the Hormone Foundation, public education affiliate of the Endocrine Society, released the results of a national survey of doctors involved in menopause care.2 The survey was designed to gauge the effects of the WHI on clinical practice and was conducted on behalf of the Hormone Foundation with financial support from Novogyne Pharmaceuticals. Among the findings:
- Only 15% of the physicians believe their patients’ perceptions of the risks of hormone replacement are accurate
- Only 18% of physicians—this includes ObGyns—report that they themselves have “no confusion at all” about the findings of the WHI
- 83% of physicians believe their patients are as confused now as when the WHI findings were released in 2002—or more so
- 81% of physicians believe the media are as, or more, confused as when the findings were released.2
“There’s a lot of noise,” says Nanette Santoro, MD, director of reproductive endocrinology at Albert Einstein College of Medicine, Bronx, New York, and a member of the Hormone Foundation’s Women’s Health Task Force. “And there have been a lot of arguments back and forth.”
What can a physician do to achieve a little clarity?
Staying up-to-date on the clinical practice guidelines is the best way to combat confusion, Dr. Santoro says. A good starting point, she notes, is the Hormone Foundation Web site (Hormone. org), which links to the American College of Obstetricians and Gynecologists, the American Society for Reproductive Medicine (ASRM), and the North American Menopause Society (NAMS), all of which publish reliable guidelines.
“I think that’s probably the best way of keeping abreast of what’s happening now if [physicians] are not really deeply into menopause care,” she says. “But getting filtered information, or getting information from pundits or from the media is, I think, more hazardous because the quality of that information can be variable. And the days of getting your information from pharmaceutical representatives are long gone in this area because, again, it is not sufficiently reliable.”
During the spring of this year, 404 physicians responded to a survey about menopause management in the 5 years since the first Women’s Health Initiative (WHI) findings were published.1 The physicians represented the following primary care specialties: endocrinology, obstetrics and gynecology, internal medicine, and family and general practice. To qualify for the survey, each clinician had to devote at least 70% of his or her working day to clinical practice and see at least two women each month with menopausal symptoms.
The survey was conducted by Richard Day Research of Evanston, Illinois, for the Hormone Foundation. To review the full survey, visit www.hormone.org/pdf/meno_survey_qa.pdf.
Here are highlights:
| Primary medical specialty | |
| Family or general practice | 29% |
| Internal medicine | 27 |
| Obstetrics and gynecology | 40 |
| Endocrinology | 4 |
| Percentage of patients with menopausal symptoms currently taking HT | 37%* |
| Percentage reluctant to start HT | 42%* |
| Percentage that specifically asks to be put on HT | 19%* |
| Percentage that specifically asks not to be put on HT | 29%* |
| For moderate or severe menopausal symptoms, do you think of HT as a: | |
| first-line treatment? | 74% |
| second-line treatment (or third, fourth, etc)? | 26% |
| Which of the following are very important to you when deciding whether to prescribe HT for your patients? | |
| Severity of symptoms | 81% |
| Patient’s personal medical history | 77 |
| Risks of HT | 61 |
| Range and specific types of symptoms | 50 |
| Patient request | 44 |
| Age of patient | 33 |
| Prevention of osteoporosis | 24 |
| Which risks concern you about prescribing estrogen–progestin therapy for menopausal symptoms? | |
| Blood clots | 88% |
| Breast cancer | 87 |
| Coronary heart disease | 74 |
| Stroke | 73 |
| Dementia | 14 |
| What do you see as valuable about estrogen–progestin therapy for menopausal symptoms? | |
| Relieves hot flashes | 100% |
| Relieves vaginal dryness and painful intercourse | 92 |
| Improves sleep problems | 88 |
| Prevents bone loss | 84 |
| Reduces depression and mood changes | 68 |
| Reduces risk of colorectal Ca | 37 |
| Prevents cardiovascular disease | 16 |
| Which risks concern you about prescribing estrogen-only therapy for menopausal symptoms? | |
| Blood clots | 86% |
| Breast cancer | 71 |
| Stroke | 68 |
| Coronary heart disease | 51 |
| Dementia | 8 |
| Uterine cancer | 4 |
| What do you see as valuable about estrogen-only therapy for menopausal symptoms? | |
| Relieves hot flashes | 99% |
| Relieves vaginal dryness and painful intercourse | 94 |
| Improves sleep problems | 84 |
| Prevents bone loss | 81 |
| Reduces depression and mood changes | 71 |
| Reduces risk of colorectal Ca | 35 |
| Prevents cardiovascular disease | 19 |
| In your view, are the risks of HT understated, overstated, or accurately perceived by the following groups? | |
| Means, based on the following: | |
| 1 = understated | |
| 2 = accurately perceived | |
| 3 = overstated | |
| Media | 2.9 (Mean) |
| Patients | 2.7 |
| Family or general practitioners | 2.4 |
| Internists | 2.4 |
| ObGyns | 2.0 |
| Endocrinologists | 2.1 |
| As of today, how much confusion do you feel there is about the WHI findings? | |
| Means, based on the following: | |
| 1 = not confused at all | |
| 2 = not very much confusion | |
| 3 = some confusion | |
| 4 = great deal of confusion | |
| For you personally | 2.3 (Mean) |
| Media | 3.7 |
| Patients | 3.7 |
| Family or general practitioners | 3.1 |
| Internists | 3.0 |
| ObGyns | 2.5 |
| Endocrinologists | 2.5 |
| *Mean | |
“Afraid of hormones”
In the years since early WHI findings were published, Anita L. Nelson, MD, has not noticed confusion so much as fear among her patients. Dr. Nelson is professor of obstetrics and gynecology at the David Geffen School of Medicine at UCLA in Los Angeles.
“I think the things that are concerning to patients by and large are breast cancer and, in women who have done more reading on it, some of them are concerned about dementia,” Dr. Nelson says. “But by and large, other than those focused issues, it is hormones that patients are afraid of, and they sort of wave their hands in this global aura of ‘badness’ that they’re afraid of.”
One reason is the WHI. “Obviously that contributed to it,” she says. But a bigger cause of fear among her patients, a large percentage of whom are referred, is the fact that “their physicians have been taking them off of therapy. They’re not offering it,” she says, “or they are putting up a sort of barrier by saying, ‘You have to go see Dr. Nelson before you can start taking those medications.’”
The problem doesn’t end there, she adds. “The sad thing is that they are by and large not offering them alternative medications while they’re waiting for the transition—or if they are, sometimes they are actually giving them hazardous drugs. One of my favorite things is when patients who have high blood pressure are denied estrogen but are given Bellergal [ergotamine, belladonna alkaloids, and phenobarbital], which has a vasoconstrictive medication in it.”
“We do want folks to review the data,” she says, noting that ObGyns are “true believers” and unlikely to quit prescribing hormone therapy (HT). It is the internists and the family medicine physicians “who still have significant misgivings about the safety of these therapies in recently menopausal women.”
Joanna Shulman, MD, agrees. She is associate professor and director of the medical student clerkship in obstetrics and gynecology at Mount Sinai School of Medicine in New York City.
“The internists I work with or that my patients see tend to be terrified of hormone therapy. So I think they tend to discourage their patients.”
Mea culpa, anyone?
Confusion over the WHI is an issue for another prominent ObGyn—Wulf H. Utian, MD, PhD, editor-in-chief of Menopause Management and executive director of NAMS. In an editorial in the September/October issue of Menopause Management, Dr. Utian faults the National Heart, Lung and Blood Institute of the National Institutes of Health (NIH) for starting a “firestorm in women’s health” by publicizing the abrupt termination of the estrogen–progestin arm of the WHI study.4
Utian notes that he pointed out his dismay over the WHI way back in 2002, when he wrote, again in Menopause Management: “The manner in which the study was terminated was poorly planned, abrupt, and inhumane. Predictably, the media response was enormous, ranging from thoughtful to sensational. Panic was caused, numerous women discontinued therapy, and women and their health providers alike have been thrown into a state of confusion, distrust, and quandary of what to do next.”4
Bruce Wineman, DO, concurs. Although he retired from practice as a reproductive endocrinologist at the Marshfield Clinic in Marshfield, Wisconsin, shortly before the WHI findings were first published, he maintains his license and stays active in the ASRM. “The worst part of the WHI is that they got so much press with it,” he says, “and that the group of women that they chose was exactly the group of women that was going to have the maximum amount of negative effect.”
Utian believes a mea culpa is in order. “There are reams of important and pertinent data coming out of all the substudies of the WHI,” he writes. “For these to be accepted with confidence, it is well time for the NIH to bring all their WHI investigators together to develop a transparent and comprehensive summary of their results. It is also time for the WHI investigators to cease their stubborn defense and misrepresentation of their 2002 data, and to return to scientific integrity.”3
Same view in the trenches
Mohamed Mitwally, MD, spends 90% of his day in clinical practice at the Reproductive Medicine and Fertility Center in Colorado Springs, Colorado. He estimates that roughly half of his perimenopausal and menopausal patients troubled by vasomotor and other symptoms are currently on HT. Since the WHI’s initial findings were published, Dr. Mitwally has “absolutely” had to spend considerably more time educating his patients—“and educating physicians,” he says. His patients are reluctant to take HT because of press attention to the WHI. And other physicians are reluctant to give HT because they understand that the WHI is a randomized trial “and so don’t question it.”
Dr. Mitwally blames two entities for this state of affairs. “The credit goes to the WHI,” he says. “They did a wonderful job of screwing people up” with a “very poorly designed study.” There is also “a lot of misinformation,” thanks to the media. “They just want to get any bad news and magnify it.”
In the wake of the WHI, Dr. Mitwally recalls, “it was like chaos” for 3 or 4 years—and there is still a lot of confusion.
Nevertheless, when a patient complains of moderate or severe vasomotor symptoms, Dr. Mitwally usually turns to HT as a first-line therapy. “It is excellent for these patients,” he says, although he emphasizes that “every patient should be managed separately.”
“I think the most important thing in the whole issue of HT is that physicians should leave these patients to subspecialists,” he says, by which he means reproductive endocrinologists and ObGyns with expertise in menopause care.
Plethora of products
One of the more surprising impacts of the WHI is the array of estrogen products now available. Because the WHI was expected to confirm observational data that suggested that estrogen reduced the risk of cardiovascular disease, the number of products in development skyrocketed.
“I think something like 35 compounds got approved while the study was under way, so there is more stuff than ever,” says Dr. Santoro. “But that actually was attractive to some people in the survey and has been found to be attractive to patients because it does give more choices the way things are going, which is toward more of a customized approach to giving hormones.”
Raksha Joshi, MD, chief medical officer and medical director of Monmouth Family Health Center in Long Branch, New Jersey, a federally funded qualified health migrant center (FQHC), says the broader array of estrogen products adds to the time she spends educating patients.
“We do tell them about the other forms of estrogen and their bioeffectiveness and what they would achieve for this particular woman,” she says.
For patients who report moderate to severe menopausal symptoms, Dr. Joshi considers estrogen a first-line therapy, but recommends concurrent lifestyle changes.
“Of course, the WHI has not disappeared,” she says, so concerns about risks remain. “But in the transition, when the symptoms are paramount, I would tailor the treatment to what the woman wants to get out of it. But I think it is important for the woman to understand that this is not a panacea and that it will not cure all her symptoms. Therefore, lifestyle changes and getting hormone replacement therapy should go concurrently.”
As for alternative therapies, women are increasingly likely to ask for or about them.
“We talk about that,” says Dr. Shulman. “If they’re miserable and they don’t think they’re appropriate candidates for estrogen, we talk about other things. Or some people will come in and say, ‘I don’t want to take estrogen. Is there anything else?’”
In these cases, Dr. Shulman recommends a number of options. “Effexor has shown some benefit, apparently, in the literature,” she says. “And I mention black cohosh, which is in a lot of popular over-the-counter type remedies and which, apparently, recently was shown to have possibly some benefit.” Of course, “there’s a tremendous placebo effect with all of these,” she observes.
“And then I suggest things like getting plenty of exercise and eating sensibly, and I take my other patients’ recommendations. One patient told me that she takes a cool shower every night before going to bed and finds it beneficial, so I don’t know—it’s one of those ‘can’t hurt, might help’ things.”
Estrogen got a “bad name”
When she looks back over the past 5 years, Dr. Shulman thinks the WHI’s effects have been destructive in many ways.
“I think the most important thing is that [HT] got an undeservedly bad name when the Women’s Health Initiative was published,” she says. The WHI “really did a disservice for women who could benefit from [HT] enormously and weren’t really at risk—not just for vasomotor symptoms but also emotional lability, depression, increased anxiety, things like that.”
“I have many women for whom I did prescribe estrogen, and they’re still on it and will probably never get off because they think that I saved their lives. So for women to be scared unfairly by the Women’s Health Initiative and to have to suffer with vasomotor and emotional problems is really a disservice.”
Dr. Wineman agrees, and points out that even some professional organizations are beginning to reconsider the initial WHI findings. “They’re beginning to say, ‘I really believe that there are certain women who would probably benefit a great deal more than we once thought, and perhaps we jumped to some wrong conclusions.’”
1. WHI Investigators. Risks and benefits of estrogen plus progestin in healthy postmenopausal women. Principal results from the Women’s Health Initiative Randomized Controlled Trial. JAMA. 2002;288:321-333.
2. Hormone Foundation. Physician survey on menopause management. April 16–May 23, 2007. Available at: www.hormone.org/pdf/meno_survey_qa.pdf. Accessed September 26, 2007.
3. Utian WH. If only WHI had kept to its premise—but now it’s time for their mea culpa. Menopause Management. 2007;16(5):8-12.
4. Utian WH. Managing menopause after HERS II and WHI: coping with the aftermath. Menopause Management. 2002;11:6-7.
1. WHI Investigators. Risks and benefits of estrogen plus progestin in healthy postmenopausal women. Principal results from the Women’s Health Initiative Randomized Controlled Trial. JAMA. 2002;288:321-333.
2. Hormone Foundation. Physician survey on menopause management. April 16–May 23, 2007. Available at: www.hormone.org/pdf/meno_survey_qa.pdf. Accessed September 26, 2007.
3. Utian WH. If only WHI had kept to its premise—but now it’s time for their mea culpa. Menopause Management. 2007;16(5):8-12.
4. Utian WH. Managing menopause after HERS II and WHI: coping with the aftermath. Menopause Management. 2002;11:6-7.
IN THIS ARTICLE
OSTEOPOROSIS
As 2007 draws to a close, we are still awaiting the World Health Organization’s fracture risk-assessment tool. The much-anticipated instrument will calculate 5- and 10-year fracture risks using an individual’s femoral neck T-score, age, history of low-trauma fracture, body mass index, steroid exposure, family history of hip fracture, smoking status, and alcohol intake. Once it is implemented, the tool will eliminate much of the confusion that arises when the T-score is the only variable used to determine the need for pharmacotherapy.
Why is the ability to stratify risk important? Although the incidence of fragility fractures is highest in osteoporotic women (as defined by the T-score), the absolute number of fractures is greater in those who have osteopenia. All clinicians should realize that the current definitions of normal bone density, osteopenia, and osteoporosis apply to the postmenopausal population only:
- normal – T-score above -1.0
- osteopenia – T-score below -1.0 but above -2.5
- osteoporosis – T-score below -2.5.
Indications added for raloxifene
The year did bring new indications for raloxifene, based on data from the RUTH and STAR trials,1,2 which were mentioned in this Update 1 year ago. On September 14, the Food and Drug Administration approved two new indications:
- reduction of risk of invasive breast cancer in postmenopausal women with osteoporosis
- reduction of risk of invasive breast cancer in postmenopausal women at high risk of breast cancer.
These new indications are very important for clinicians who prescribe agents to prevent fragility fractures. Raloxifene should be considered for breast cancer risk reduction when deciding which agent to prescribe.
REAL study finds real advantage with risedronate
Silverman SL, Watts NB, Delmas PD, et al. Effectiveness of bisphosphonates on nonvertebral and hip fractures in the first year of therapy: the risedronate and alendronate (REAL) cohort study. Osteoporos Int. 2007;18(1):25–34.
Patients who take risedronate have lower rates of hip and nonvertebral fracture during their first year of therapy than do those who take alendronate. That is the finding of the RisedronatE and ALendronate (REAL) cohort study, a retrospective observation of the records of health-care utilization among women in the United States. Silverman and colleagues analyzed data sets for women older than age 65 who had ever used once-weekly dosing of risedronate or alendronate. The risedronate cohort included 12,215 women who were followed for a mean of 226 days of therapy. The alendronate cohort included 21,615 women followed for a mean of 238 days of therapy.
Risedronate group had more risk factors for fracture
At baseline, women taking risedronate had a statistically greater incidence of:
- advanced age
- use of concomitant medications
- glucocorticoid use
- rheumatoid arthritis.
Each of these characteristics might have been expected to increase the risk of fragility fracture. However, through 1 year of therapy, women using risedronate had an incidence of nonvertebral fractures 18% lower than those using alendronate (2.0% versus 2.3%; 95% confidence interval [CI], 0.02–0.32). They also had an incidence of hip fracture 43% lower than those using alendronate (0.4% versus 0.6%; 95% CI, 0.13–0.63). Overall, there were 507 nonvertebral fractures and 109 hip fractures.
Footnote: Large database analyses complement randomized trials
Randomized clinical trials (RCTs) are, of course, the gold standard for determining drug safety and efficacy and the key requisite for regulatory approval of new drugs. By design, RCTs have strict inclusion and exclusion criteria to meet the regulatory standard for evaluating drug efficacy and safety and to exclude internal bias. Often, the majority of patients are deemed ineligible for entry into an RCT because of comorbidity, concomitant medication use, age, or severity of disease. Therefore, database analyses are often more “real world” than RCTs.
As clinical practice data accumulate over time, observational, or outcomes, studies can be conducted to complement the data that have been generated by RCTs. For example, over the past decade, large databases of health-care utilization claims have become available in the United States and are being tapped to conduct effectiveness studies. These observational studies can supplement the efficacy measures obtained from the carefully controlled environment of a placebo-controlled RCT. They also provide a measure of effectiveness over a wide range of patients and health-care practices. Data generated from the REAL study are just another piece of the huge puzzle we must grapple with as we seek good information about agents to treat osteoporosis and prevent fragility fractures.
Annual infusion of zoledronic acid reduces risk of fracture
Black DM, Delmas PD, Eastell R, et al, for the HORIZON Pivotal Fracture Trial. Once-yearly zoledronic acid for treatment of postmenopausal osteoporosis. N Engl J Med. 2007;356:1809–1822.
Lyles KW, Colon-Emeris C, Magaziner JS, et al. Zoledronic acid and clinical fractures and mortality after hip fracture. N Engl J Med. 2007;357. DOI: 10.1056/NEJMoa074941.
Zoledronic acid (Zometa) is indicated for the treatment of high levels of serum calcium associated with Paget’s disease and various malignancies (multiple myeloma, breast, prostate, and lung). These two studies explore use of this agent to prevent fracture in postmenopausal women with osteoporosis—a use for which it proved effective. Other benefits may include improved compliance and ease of administration in some women.
Black and colleagues conducted their randomized, double-blind, placebo-controlled trial to assess the effect of annual infusion of zoledronic acid on the risk of fracture over a 3-year period. A total of 3,889 postmenopausal women with osteoporosis (mean age, 73 years; range, 65–89 years) were assigned to receive a single 15-minute, 5-mg infusion of the drug at baseline, 12 months, and 24 months, and a total of 3,876 women received placebo. Approximately half the women were from Europe, and the other half were from North and South America and Asia. All women received oral daily calcium (1,000–1,500 mg) and vitamin D (400–1,200 U), and all were monitored for 36 months.
The risk of vertebral fracture was reduced in the treatment group by 70% over 3 years, compared with the placebo group (3.3% or 92 women in the treatment group versus 10.9% or 310 women receiving placebo). The risk of hip fracture was reduced by 41% in the treatment group (1.4% or 52 women receiving zoledronic acid versus 2.5% or 88 women in the placebo group). (For all comparisons, P<.001.)
The most common postdose symptoms, seen within 3 days of infusion, included fever, flu-like symptoms, myalgia, headache, and arthralgias. There were more serious adverse events related to atrial fibrillation in the women receiving zoledronic acid (50 women receiving zoledronic acid versus 20 in the placebo group; P<.001).
Treatment is a valuable option for carefully selected populations
The trial by Black and colleagues holds great promise for some patients, especially those who have (or appear to have) upper gastrointestinal intolerance of oral bisphosphonates or who may have difficulty adhering to the positional requirements (i.e., remaining upright) of oral therapy. This may be especially true of patients in nursing homes. Once-yearly intravenous (IV) infusion may also make compliance easier for these patients.
One important detail of this trial: Of almost 8,000 women studied, the youngest was age 65. The implication: Don’t automatically assume this regimen is an appropriate alternative for younger osteopenic women who perceive themselves to have acid reflux.
Women at extremely high risk of fracture also benefit
Fracture is most likely to occur in women who have already experienced it (FIGURE 1). Lyles and colleagues chose this population for their study of once-yearly infusion of zoledronic acid. The study involved 2,127 patients within 90 days after repair of low-trauma hip fracture. Subjects were randomized to receive 5-mg IV zoledronic acid annually or placebo in a blinded fashion. All patients also received vitamin D and calcium supplements. Mean age was 74.5 years, as might be expected in a hip-fracture cohort, and median follow-up was 1.9 years.
New clinical fractures occurred in 8.6% of women in the zoledronic acid group and 13.9% of the placebo group—a 35% risk reduction with IV zoledronic acid (P=.001). Deaths occurred in 101 of 1,054 patients (9.6%) in the zoledronic acid group and 141 of 1,057 patients (13.3%) in the placebo group. This was a reduction of 28% in death from any cause in the zoledronic acid group (P=.01).
The most frequent adverse events in patients receiving zoledronic acid were pyrexia, myalgia, and bone and musculoskeletal pain. No cases of osteonecrosis of the jaw were reported. Rates of atrial fibrillation and stroke were similar in both the zoledronic acid and placebo groups.
This study provides further evidence that, for extremely high-risk women (those who have already suffered hip fracture), yearly zoledronic acid may be an extremely useful tool, especially for elderly women, in whom compliance with any medication—weekly or monthly—may be difficult.
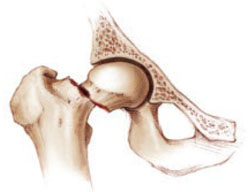
FIGURE 1 Once a fracture occurs, another is likely
Although the risk of new fractures is heightened in women who have already experienced one, Lyles and colleagues found a 35% risk reduction with zoledronic acid.
Parathyroid hormone reduces vertebral fractures in osteoporotic women
Greenspan SL, Bone HG, Ettinger MP, et al, for the Treatment of Osteoporosis with Parathyroid Hormone Study Group. Effect of recombinant human parathyroid hormone (1–84) on vertebral fracture and bone mineral density in postmenopausal women with osteoporosis: a randomized trial. Ann Intern Med. 2007;146:326–339.
Teriparatide (Forteo) is a synthetic portion (1-34) of the parathyroid hormone (PTH) molecule that is identical in sequence to the biologically active segment of the 84-amino acid human PTH.
It has been shown to prevent fracture in women with low bone mineral density (BMD). Teriparatide is the only anabolic bone agent approved for clinical use; all other pharmacotherapies are antiresorptive.
The study by Greenspan and associates—the Treatment of Osteoporosis with Parathyroid Hormone, or TOP trial—involved the full-length PTH molecule (1–84) and provides evidence that it, too, can prevent vertebral fracture in women who have low BMD (FIGURE 2). This agent is used in Europe but not yet available in the United States.
TOP was an 18-month, randomized, double-blind, placebo-controlled, parallel-group study of 2,532 women with low BMD at the hip or lumbar spine. It was conducted at 168 centers in nine countries.
The primary outcome measure was new or worsened vertebral fracture; secondary outcomes were changes in BMD and safety. The trial investigated the safety of recombinant PTH and its effect on the incidence of vertebral fractures in postmenopausal women with osteoporosis.
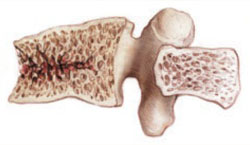
FIGURE 2 Fracture-prone bone responds to PTH
PTH prevented vertebral fracture in women with low BMD, existing fractures, or both.
Women had very low BMD, or low BMD and existing fractures
Participants were postmenopausal women aged 45 to 54 years. They had a BMD 3 standard deviations or more (T-score ≤ -3.0) below the mean peak bone mass of young adult women at the lumbar spine, femoral neck, or total hip, with no vertebral fractures, or they had a BMD T-score of -2.5 and 1 to 4 vertebral fractures. Postmenopausal women aged 55 years or older were included if their BMD T-score was -2.5 and they had no vertebral fractures, or if their BMD T-score was -2.0 and they had 1 to 4 vertebral fractures before enrollment. The women were given 100 μg of recombinant human PTH or placebo daily, as well as calcium (700 mg/day) and vitamin D3 (400 U/day).
PTH reduced the risk of new fractures or prevented worsening of existing fractures. The reduction in relative risk (RR) for vertebral fracture was 0.42 (95% CI, 0.24–0.72; P=.001). Women who received PTH had an increase in mean BMD of 6.9% at the spine (95% CI, 6.4–7.4) and 2.1% at the hip (95% CI, 1.7–2.5). Adverse events included a higher incidence of hypercalciuria, hypercalcemia, and nausea.
Although it is unlikely that many gynecologists will be ordering or monitoring injectable PTH therapy, we should be aware of the data. All too often such therapy is not even considered for women with severe osteoporosis (T-score < -2.5 and preexisting fracture), who may be excellent candidates.
Long-term alendronate users can sometimes take a “drug holiday”
Black DM, Schwartz AV, Ensrud KE, et al, for the FLEX Research Group. Effects of continuing or stopping alendronate after 5 years of treatment. The Fracture Intervention Trial Long-term Extension (FLEX): a randomized trial. JAMA. 2006;296:2927–2938.
As early as 2002, Greenspan and colleagues3 demonstrated that women on alendronate for 21 months maintained femoral-neck BMD through 15 months of crossover to placebo. This study by Black and associates, known as the FLEX trial, is a long-term extension of the Fracture Intervention Trial (FIT). A total of 1,099 women who had participated in FIT and taken alendronate for 5 years were then randomized to one of two doses of alendronate or placebo for an additional 5 years. To qualify for the FLEX trial, all women had to have low bone mass at the beginning of FIT. The average age of women in the FLEX trial was 73 years. The primary outcome was total hip BMD; secondary outcomes were BMD at other sites and biochemical markers.
Women who remained on alendronate maintained a higher BMD of the hip and spine than women on placebo, but all patients’ levels remained at or above pretreatment levels of 10 years earlier. The same was true for markers of bone remodeling. The cumulative risk of nonvertebral fractures did not differ between the two groups (19% for placebo, 18.9% for alendronate; RR, 1.0; 95% CI, 0.76–1.32). The risk of clinically recognized vertebral fractures was lower in the women who continued alendronate (5.3% for placebo and 2.4% for alendronate; RR, 0.45; 95% CI, 0.24–0.85), but there was no significant reduction in morphometric vertebral fractures.
Data can aid in determining duration of therapy
These data are extremely helpful, especially for clinicians who are trying to determine how long to continue bisphosphonate therapy and which patients may be candidates for a “drug holiday.” We have all had women whose response to a bisphosphonate has been so robust that follow-up BMD measurements have climbed to a range in which therapy would not have been initiated. This study clearly shows that the cumulative effect of 5 years of alendronate followed by 5 years of placebo is positive, compared with the bone loss one would expect in untreated women.
The answer isn’t clear, but women with a previous fracture, and those at high risk for spine fracture, are likely to benefit from continued treatment with alendronate. Patients with a lower risk of fracture are better candidates for the holiday.
As for when the drug holiday should end, that isn’t clear, either. Continued close monitoring of these lower-risk women using bone-density measurements may help identify that minority of patients who do not maintain bone mass off the medication.
1. Barrett-Connor E, Mosca L, Collins P, et al. Effects of raloxifene on cardiovascular events and breast cancer in postmenopausal women. N Engl J Med. 2006;355:125-137.
2. Vogel VG, Costantino JP, Wickerham DL, et al. Effects of tamoxifen vs raloxifene on the risk of developing invasive breast cancer and other disease outcomes. The NSABP Study of Tamoxifen and Raloxifene (STAR) P-2 Trial. JAMA. 2006;295:2727-2741.
3. Greenspan SL, Emkey RD, Bone HG, et al. Significant differential effects of alendronate, estrogen, or combination therapy on the rate of bone loss after discontinuation of treatment of postmenopausal osteoporosis. A randomized, double-blind, placebo-controlled trial. Ann Intern Med. 2002;137:875-883.
As 2007 draws to a close, we are still awaiting the World Health Organization’s fracture risk-assessment tool. The much-anticipated instrument will calculate 5- and 10-year fracture risks using an individual’s femoral neck T-score, age, history of low-trauma fracture, body mass index, steroid exposure, family history of hip fracture, smoking status, and alcohol intake. Once it is implemented, the tool will eliminate much of the confusion that arises when the T-score is the only variable used to determine the need for pharmacotherapy.
Why is the ability to stratify risk important? Although the incidence of fragility fractures is highest in osteoporotic women (as defined by the T-score), the absolute number of fractures is greater in those who have osteopenia. All clinicians should realize that the current definitions of normal bone density, osteopenia, and osteoporosis apply to the postmenopausal population only:
- normal – T-score above -1.0
- osteopenia – T-score below -1.0 but above -2.5
- osteoporosis – T-score below -2.5.
Indications added for raloxifene
The year did bring new indications for raloxifene, based on data from the RUTH and STAR trials,1,2 which were mentioned in this Update 1 year ago. On September 14, the Food and Drug Administration approved two new indications:
- reduction of risk of invasive breast cancer in postmenopausal women with osteoporosis
- reduction of risk of invasive breast cancer in postmenopausal women at high risk of breast cancer.
These new indications are very important for clinicians who prescribe agents to prevent fragility fractures. Raloxifene should be considered for breast cancer risk reduction when deciding which agent to prescribe.
REAL study finds real advantage with risedronate
Silverman SL, Watts NB, Delmas PD, et al. Effectiveness of bisphosphonates on nonvertebral and hip fractures in the first year of therapy: the risedronate and alendronate (REAL) cohort study. Osteoporos Int. 2007;18(1):25–34.
Patients who take risedronate have lower rates of hip and nonvertebral fracture during their first year of therapy than do those who take alendronate. That is the finding of the RisedronatE and ALendronate (REAL) cohort study, a retrospective observation of the records of health-care utilization among women in the United States. Silverman and colleagues analyzed data sets for women older than age 65 who had ever used once-weekly dosing of risedronate or alendronate. The risedronate cohort included 12,215 women who were followed for a mean of 226 days of therapy. The alendronate cohort included 21,615 women followed for a mean of 238 days of therapy.
Risedronate group had more risk factors for fracture
At baseline, women taking risedronate had a statistically greater incidence of:
- advanced age
- use of concomitant medications
- glucocorticoid use
- rheumatoid arthritis.
Each of these characteristics might have been expected to increase the risk of fragility fracture. However, through 1 year of therapy, women using risedronate had an incidence of nonvertebral fractures 18% lower than those using alendronate (2.0% versus 2.3%; 95% confidence interval [CI], 0.02–0.32). They also had an incidence of hip fracture 43% lower than those using alendronate (0.4% versus 0.6%; 95% CI, 0.13–0.63). Overall, there were 507 nonvertebral fractures and 109 hip fractures.
Footnote: Large database analyses complement randomized trials
Randomized clinical trials (RCTs) are, of course, the gold standard for determining drug safety and efficacy and the key requisite for regulatory approval of new drugs. By design, RCTs have strict inclusion and exclusion criteria to meet the regulatory standard for evaluating drug efficacy and safety and to exclude internal bias. Often, the majority of patients are deemed ineligible for entry into an RCT because of comorbidity, concomitant medication use, age, or severity of disease. Therefore, database analyses are often more “real world” than RCTs.
As clinical practice data accumulate over time, observational, or outcomes, studies can be conducted to complement the data that have been generated by RCTs. For example, over the past decade, large databases of health-care utilization claims have become available in the United States and are being tapped to conduct effectiveness studies. These observational studies can supplement the efficacy measures obtained from the carefully controlled environment of a placebo-controlled RCT. They also provide a measure of effectiveness over a wide range of patients and health-care practices. Data generated from the REAL study are just another piece of the huge puzzle we must grapple with as we seek good information about agents to treat osteoporosis and prevent fragility fractures.
Annual infusion of zoledronic acid reduces risk of fracture
Black DM, Delmas PD, Eastell R, et al, for the HORIZON Pivotal Fracture Trial. Once-yearly zoledronic acid for treatment of postmenopausal osteoporosis. N Engl J Med. 2007;356:1809–1822.
Lyles KW, Colon-Emeris C, Magaziner JS, et al. Zoledronic acid and clinical fractures and mortality after hip fracture. N Engl J Med. 2007;357. DOI: 10.1056/NEJMoa074941.
Zoledronic acid (Zometa) is indicated for the treatment of high levels of serum calcium associated with Paget’s disease and various malignancies (multiple myeloma, breast, prostate, and lung). These two studies explore use of this agent to prevent fracture in postmenopausal women with osteoporosis—a use for which it proved effective. Other benefits may include improved compliance and ease of administration in some women.
Black and colleagues conducted their randomized, double-blind, placebo-controlled trial to assess the effect of annual infusion of zoledronic acid on the risk of fracture over a 3-year period. A total of 3,889 postmenopausal women with osteoporosis (mean age, 73 years; range, 65–89 years) were assigned to receive a single 15-minute, 5-mg infusion of the drug at baseline, 12 months, and 24 months, and a total of 3,876 women received placebo. Approximately half the women were from Europe, and the other half were from North and South America and Asia. All women received oral daily calcium (1,000–1,500 mg) and vitamin D (400–1,200 U), and all were monitored for 36 months.
The risk of vertebral fracture was reduced in the treatment group by 70% over 3 years, compared with the placebo group (3.3% or 92 women in the treatment group versus 10.9% or 310 women receiving placebo). The risk of hip fracture was reduced by 41% in the treatment group (1.4% or 52 women receiving zoledronic acid versus 2.5% or 88 women in the placebo group). (For all comparisons, P<.001.)
The most common postdose symptoms, seen within 3 days of infusion, included fever, flu-like symptoms, myalgia, headache, and arthralgias. There were more serious adverse events related to atrial fibrillation in the women receiving zoledronic acid (50 women receiving zoledronic acid versus 20 in the placebo group; P<.001).
Treatment is a valuable option for carefully selected populations
The trial by Black and colleagues holds great promise for some patients, especially those who have (or appear to have) upper gastrointestinal intolerance of oral bisphosphonates or who may have difficulty adhering to the positional requirements (i.e., remaining upright) of oral therapy. This may be especially true of patients in nursing homes. Once-yearly intravenous (IV) infusion may also make compliance easier for these patients.
One important detail of this trial: Of almost 8,000 women studied, the youngest was age 65. The implication: Don’t automatically assume this regimen is an appropriate alternative for younger osteopenic women who perceive themselves to have acid reflux.
Women at extremely high risk of fracture also benefit
Fracture is most likely to occur in women who have already experienced it (FIGURE 1). Lyles and colleagues chose this population for their study of once-yearly infusion of zoledronic acid. The study involved 2,127 patients within 90 days after repair of low-trauma hip fracture. Subjects were randomized to receive 5-mg IV zoledronic acid annually or placebo in a blinded fashion. All patients also received vitamin D and calcium supplements. Mean age was 74.5 years, as might be expected in a hip-fracture cohort, and median follow-up was 1.9 years.
New clinical fractures occurred in 8.6% of women in the zoledronic acid group and 13.9% of the placebo group—a 35% risk reduction with IV zoledronic acid (P=.001). Deaths occurred in 101 of 1,054 patients (9.6%) in the zoledronic acid group and 141 of 1,057 patients (13.3%) in the placebo group. This was a reduction of 28% in death from any cause in the zoledronic acid group (P=.01).
The most frequent adverse events in patients receiving zoledronic acid were pyrexia, myalgia, and bone and musculoskeletal pain. No cases of osteonecrosis of the jaw were reported. Rates of atrial fibrillation and stroke were similar in both the zoledronic acid and placebo groups.
This study provides further evidence that, for extremely high-risk women (those who have already suffered hip fracture), yearly zoledronic acid may be an extremely useful tool, especially for elderly women, in whom compliance with any medication—weekly or monthly—may be difficult.

FIGURE 1 Once a fracture occurs, another is likely
Although the risk of new fractures is heightened in women who have already experienced one, Lyles and colleagues found a 35% risk reduction with zoledronic acid.
Parathyroid hormone reduces vertebral fractures in osteoporotic women
Greenspan SL, Bone HG, Ettinger MP, et al, for the Treatment of Osteoporosis with Parathyroid Hormone Study Group. Effect of recombinant human parathyroid hormone (1–84) on vertebral fracture and bone mineral density in postmenopausal women with osteoporosis: a randomized trial. Ann Intern Med. 2007;146:326–339.
Teriparatide (Forteo) is a synthetic portion (1-34) of the parathyroid hormone (PTH) molecule that is identical in sequence to the biologically active segment of the 84-amino acid human PTH.
It has been shown to prevent fracture in women with low bone mineral density (BMD). Teriparatide is the only anabolic bone agent approved for clinical use; all other pharmacotherapies are antiresorptive.
The study by Greenspan and associates—the Treatment of Osteoporosis with Parathyroid Hormone, or TOP trial—involved the full-length PTH molecule (1–84) and provides evidence that it, too, can prevent vertebral fracture in women who have low BMD (FIGURE 2). This agent is used in Europe but not yet available in the United States.
TOP was an 18-month, randomized, double-blind, placebo-controlled, parallel-group study of 2,532 women with low BMD at the hip or lumbar spine. It was conducted at 168 centers in nine countries.
The primary outcome measure was new or worsened vertebral fracture; secondary outcomes were changes in BMD and safety. The trial investigated the safety of recombinant PTH and its effect on the incidence of vertebral fractures in postmenopausal women with osteoporosis.

FIGURE 2 Fracture-prone bone responds to PTH
PTH prevented vertebral fracture in women with low BMD, existing fractures, or both.
Women had very low BMD, or low BMD and existing fractures
Participants were postmenopausal women aged 45 to 54 years. They had a BMD 3 standard deviations or more (T-score ≤ -3.0) below the mean peak bone mass of young adult women at the lumbar spine, femoral neck, or total hip, with no vertebral fractures, or they had a BMD T-score of -2.5 and 1 to 4 vertebral fractures. Postmenopausal women aged 55 years or older were included if their BMD T-score was -2.5 and they had no vertebral fractures, or if their BMD T-score was -2.0 and they had 1 to 4 vertebral fractures before enrollment. The women were given 100 μg of recombinant human PTH or placebo daily, as well as calcium (700 mg/day) and vitamin D3 (400 U/day).
PTH reduced the risk of new fractures or prevented worsening of existing fractures. The reduction in relative risk (RR) for vertebral fracture was 0.42 (95% CI, 0.24–0.72; P=.001). Women who received PTH had an increase in mean BMD of 6.9% at the spine (95% CI, 6.4–7.4) and 2.1% at the hip (95% CI, 1.7–2.5). Adverse events included a higher incidence of hypercalciuria, hypercalcemia, and nausea.
Although it is unlikely that many gynecologists will be ordering or monitoring injectable PTH therapy, we should be aware of the data. All too often such therapy is not even considered for women with severe osteoporosis (T-score < -2.5 and preexisting fracture), who may be excellent candidates.
Long-term alendronate users can sometimes take a “drug holiday”
Black DM, Schwartz AV, Ensrud KE, et al, for the FLEX Research Group. Effects of continuing or stopping alendronate after 5 years of treatment. The Fracture Intervention Trial Long-term Extension (FLEX): a randomized trial. JAMA. 2006;296:2927–2938.
As early as 2002, Greenspan and colleagues3 demonstrated that women on alendronate for 21 months maintained femoral-neck BMD through 15 months of crossover to placebo. This study by Black and associates, known as the FLEX trial, is a long-term extension of the Fracture Intervention Trial (FIT). A total of 1,099 women who had participated in FIT and taken alendronate for 5 years were then randomized to one of two doses of alendronate or placebo for an additional 5 years. To qualify for the FLEX trial, all women had to have low bone mass at the beginning of FIT. The average age of women in the FLEX trial was 73 years. The primary outcome was total hip BMD; secondary outcomes were BMD at other sites and biochemical markers.
Women who remained on alendronate maintained a higher BMD of the hip and spine than women on placebo, but all patients’ levels remained at or above pretreatment levels of 10 years earlier. The same was true for markers of bone remodeling. The cumulative risk of nonvertebral fractures did not differ between the two groups (19% for placebo, 18.9% for alendronate; RR, 1.0; 95% CI, 0.76–1.32). The risk of clinically recognized vertebral fractures was lower in the women who continued alendronate (5.3% for placebo and 2.4% for alendronate; RR, 0.45; 95% CI, 0.24–0.85), but there was no significant reduction in morphometric vertebral fractures.
Data can aid in determining duration of therapy
These data are extremely helpful, especially for clinicians who are trying to determine how long to continue bisphosphonate therapy and which patients may be candidates for a “drug holiday.” We have all had women whose response to a bisphosphonate has been so robust that follow-up BMD measurements have climbed to a range in which therapy would not have been initiated. This study clearly shows that the cumulative effect of 5 years of alendronate followed by 5 years of placebo is positive, compared with the bone loss one would expect in untreated women.
The answer isn’t clear, but women with a previous fracture, and those at high risk for spine fracture, are likely to benefit from continued treatment with alendronate. Patients with a lower risk of fracture are better candidates for the holiday.
As for when the drug holiday should end, that isn’t clear, either. Continued close monitoring of these lower-risk women using bone-density measurements may help identify that minority of patients who do not maintain bone mass off the medication.
As 2007 draws to a close, we are still awaiting the World Health Organization’s fracture risk-assessment tool. The much-anticipated instrument will calculate 5- and 10-year fracture risks using an individual’s femoral neck T-score, age, history of low-trauma fracture, body mass index, steroid exposure, family history of hip fracture, smoking status, and alcohol intake. Once it is implemented, the tool will eliminate much of the confusion that arises when the T-score is the only variable used to determine the need for pharmacotherapy.
Why is the ability to stratify risk important? Although the incidence of fragility fractures is highest in osteoporotic women (as defined by the T-score), the absolute number of fractures is greater in those who have osteopenia. All clinicians should realize that the current definitions of normal bone density, osteopenia, and osteoporosis apply to the postmenopausal population only:
- normal – T-score above -1.0
- osteopenia – T-score below -1.0 but above -2.5
- osteoporosis – T-score below -2.5.
Indications added for raloxifene
The year did bring new indications for raloxifene, based on data from the RUTH and STAR trials,1,2 which were mentioned in this Update 1 year ago. On September 14, the Food and Drug Administration approved two new indications:
- reduction of risk of invasive breast cancer in postmenopausal women with osteoporosis
- reduction of risk of invasive breast cancer in postmenopausal women at high risk of breast cancer.
These new indications are very important for clinicians who prescribe agents to prevent fragility fractures. Raloxifene should be considered for breast cancer risk reduction when deciding which agent to prescribe.
REAL study finds real advantage with risedronate
Silverman SL, Watts NB, Delmas PD, et al. Effectiveness of bisphosphonates on nonvertebral and hip fractures in the first year of therapy: the risedronate and alendronate (REAL) cohort study. Osteoporos Int. 2007;18(1):25–34.
Patients who take risedronate have lower rates of hip and nonvertebral fracture during their first year of therapy than do those who take alendronate. That is the finding of the RisedronatE and ALendronate (REAL) cohort study, a retrospective observation of the records of health-care utilization among women in the United States. Silverman and colleagues analyzed data sets for women older than age 65 who had ever used once-weekly dosing of risedronate or alendronate. The risedronate cohort included 12,215 women who were followed for a mean of 226 days of therapy. The alendronate cohort included 21,615 women followed for a mean of 238 days of therapy.
Risedronate group had more risk factors for fracture
At baseline, women taking risedronate had a statistically greater incidence of:
- advanced age
- use of concomitant medications
- glucocorticoid use
- rheumatoid arthritis.
Each of these characteristics might have been expected to increase the risk of fragility fracture. However, through 1 year of therapy, women using risedronate had an incidence of nonvertebral fractures 18% lower than those using alendronate (2.0% versus 2.3%; 95% confidence interval [CI], 0.02–0.32). They also had an incidence of hip fracture 43% lower than those using alendronate (0.4% versus 0.6%; 95% CI, 0.13–0.63). Overall, there were 507 nonvertebral fractures and 109 hip fractures.
Footnote: Large database analyses complement randomized trials
Randomized clinical trials (RCTs) are, of course, the gold standard for determining drug safety and efficacy and the key requisite for regulatory approval of new drugs. By design, RCTs have strict inclusion and exclusion criteria to meet the regulatory standard for evaluating drug efficacy and safety and to exclude internal bias. Often, the majority of patients are deemed ineligible for entry into an RCT because of comorbidity, concomitant medication use, age, or severity of disease. Therefore, database analyses are often more “real world” than RCTs.
As clinical practice data accumulate over time, observational, or outcomes, studies can be conducted to complement the data that have been generated by RCTs. For example, over the past decade, large databases of health-care utilization claims have become available in the United States and are being tapped to conduct effectiveness studies. These observational studies can supplement the efficacy measures obtained from the carefully controlled environment of a placebo-controlled RCT. They also provide a measure of effectiveness over a wide range of patients and health-care practices. Data generated from the REAL study are just another piece of the huge puzzle we must grapple with as we seek good information about agents to treat osteoporosis and prevent fragility fractures.
Annual infusion of zoledronic acid reduces risk of fracture
Black DM, Delmas PD, Eastell R, et al, for the HORIZON Pivotal Fracture Trial. Once-yearly zoledronic acid for treatment of postmenopausal osteoporosis. N Engl J Med. 2007;356:1809–1822.
Lyles KW, Colon-Emeris C, Magaziner JS, et al. Zoledronic acid and clinical fractures and mortality after hip fracture. N Engl J Med. 2007;357. DOI: 10.1056/NEJMoa074941.
Zoledronic acid (Zometa) is indicated for the treatment of high levels of serum calcium associated with Paget’s disease and various malignancies (multiple myeloma, breast, prostate, and lung). These two studies explore use of this agent to prevent fracture in postmenopausal women with osteoporosis—a use for which it proved effective. Other benefits may include improved compliance and ease of administration in some women.
Black and colleagues conducted their randomized, double-blind, placebo-controlled trial to assess the effect of annual infusion of zoledronic acid on the risk of fracture over a 3-year period. A total of 3,889 postmenopausal women with osteoporosis (mean age, 73 years; range, 65–89 years) were assigned to receive a single 15-minute, 5-mg infusion of the drug at baseline, 12 months, and 24 months, and a total of 3,876 women received placebo. Approximately half the women were from Europe, and the other half were from North and South America and Asia. All women received oral daily calcium (1,000–1,500 mg) and vitamin D (400–1,200 U), and all were monitored for 36 months.
The risk of vertebral fracture was reduced in the treatment group by 70% over 3 years, compared with the placebo group (3.3% or 92 women in the treatment group versus 10.9% or 310 women receiving placebo). The risk of hip fracture was reduced by 41% in the treatment group (1.4% or 52 women receiving zoledronic acid versus 2.5% or 88 women in the placebo group). (For all comparisons, P<.001.)
The most common postdose symptoms, seen within 3 days of infusion, included fever, flu-like symptoms, myalgia, headache, and arthralgias. There were more serious adverse events related to atrial fibrillation in the women receiving zoledronic acid (50 women receiving zoledronic acid versus 20 in the placebo group; P<.001).
Treatment is a valuable option for carefully selected populations
The trial by Black and colleagues holds great promise for some patients, especially those who have (or appear to have) upper gastrointestinal intolerance of oral bisphosphonates or who may have difficulty adhering to the positional requirements (i.e., remaining upright) of oral therapy. This may be especially true of patients in nursing homes. Once-yearly intravenous (IV) infusion may also make compliance easier for these patients.
One important detail of this trial: Of almost 8,000 women studied, the youngest was age 65. The implication: Don’t automatically assume this regimen is an appropriate alternative for younger osteopenic women who perceive themselves to have acid reflux.
Women at extremely high risk of fracture also benefit
Fracture is most likely to occur in women who have already experienced it (FIGURE 1). Lyles and colleagues chose this population for their study of once-yearly infusion of zoledronic acid. The study involved 2,127 patients within 90 days after repair of low-trauma hip fracture. Subjects were randomized to receive 5-mg IV zoledronic acid annually or placebo in a blinded fashion. All patients also received vitamin D and calcium supplements. Mean age was 74.5 years, as might be expected in a hip-fracture cohort, and median follow-up was 1.9 years.
New clinical fractures occurred in 8.6% of women in the zoledronic acid group and 13.9% of the placebo group—a 35% risk reduction with IV zoledronic acid (P=.001). Deaths occurred in 101 of 1,054 patients (9.6%) in the zoledronic acid group and 141 of 1,057 patients (13.3%) in the placebo group. This was a reduction of 28% in death from any cause in the zoledronic acid group (P=.01).
The most frequent adverse events in patients receiving zoledronic acid were pyrexia, myalgia, and bone and musculoskeletal pain. No cases of osteonecrosis of the jaw were reported. Rates of atrial fibrillation and stroke were similar in both the zoledronic acid and placebo groups.
This study provides further evidence that, for extremely high-risk women (those who have already suffered hip fracture), yearly zoledronic acid may be an extremely useful tool, especially for elderly women, in whom compliance with any medication—weekly or monthly—may be difficult.

FIGURE 1 Once a fracture occurs, another is likely
Although the risk of new fractures is heightened in women who have already experienced one, Lyles and colleagues found a 35% risk reduction with zoledronic acid.
Parathyroid hormone reduces vertebral fractures in osteoporotic women
Greenspan SL, Bone HG, Ettinger MP, et al, for the Treatment of Osteoporosis with Parathyroid Hormone Study Group. Effect of recombinant human parathyroid hormone (1–84) on vertebral fracture and bone mineral density in postmenopausal women with osteoporosis: a randomized trial. Ann Intern Med. 2007;146:326–339.
Teriparatide (Forteo) is a synthetic portion (1-34) of the parathyroid hormone (PTH) molecule that is identical in sequence to the biologically active segment of the 84-amino acid human PTH.
It has been shown to prevent fracture in women with low bone mineral density (BMD). Teriparatide is the only anabolic bone agent approved for clinical use; all other pharmacotherapies are antiresorptive.
The study by Greenspan and associates—the Treatment of Osteoporosis with Parathyroid Hormone, or TOP trial—involved the full-length PTH molecule (1–84) and provides evidence that it, too, can prevent vertebral fracture in women who have low BMD (FIGURE 2). This agent is used in Europe but not yet available in the United States.
TOP was an 18-month, randomized, double-blind, placebo-controlled, parallel-group study of 2,532 women with low BMD at the hip or lumbar spine. It was conducted at 168 centers in nine countries.
The primary outcome measure was new or worsened vertebral fracture; secondary outcomes were changes in BMD and safety. The trial investigated the safety of recombinant PTH and its effect on the incidence of vertebral fractures in postmenopausal women with osteoporosis.

FIGURE 2 Fracture-prone bone responds to PTH
PTH prevented vertebral fracture in women with low BMD, existing fractures, or both.
Women had very low BMD, or low BMD and existing fractures
Participants were postmenopausal women aged 45 to 54 years. They had a BMD 3 standard deviations or more (T-score ≤ -3.0) below the mean peak bone mass of young adult women at the lumbar spine, femoral neck, or total hip, with no vertebral fractures, or they had a BMD T-score of -2.5 and 1 to 4 vertebral fractures. Postmenopausal women aged 55 years or older were included if their BMD T-score was -2.5 and they had no vertebral fractures, or if their BMD T-score was -2.0 and they had 1 to 4 vertebral fractures before enrollment. The women were given 100 μg of recombinant human PTH or placebo daily, as well as calcium (700 mg/day) and vitamin D3 (400 U/day).
PTH reduced the risk of new fractures or prevented worsening of existing fractures. The reduction in relative risk (RR) for vertebral fracture was 0.42 (95% CI, 0.24–0.72; P=.001). Women who received PTH had an increase in mean BMD of 6.9% at the spine (95% CI, 6.4–7.4) and 2.1% at the hip (95% CI, 1.7–2.5). Adverse events included a higher incidence of hypercalciuria, hypercalcemia, and nausea.
Although it is unlikely that many gynecologists will be ordering or monitoring injectable PTH therapy, we should be aware of the data. All too often such therapy is not even considered for women with severe osteoporosis (T-score < -2.5 and preexisting fracture), who may be excellent candidates.
Long-term alendronate users can sometimes take a “drug holiday”
Black DM, Schwartz AV, Ensrud KE, et al, for the FLEX Research Group. Effects of continuing or stopping alendronate after 5 years of treatment. The Fracture Intervention Trial Long-term Extension (FLEX): a randomized trial. JAMA. 2006;296:2927–2938.
As early as 2002, Greenspan and colleagues3 demonstrated that women on alendronate for 21 months maintained femoral-neck BMD through 15 months of crossover to placebo. This study by Black and associates, known as the FLEX trial, is a long-term extension of the Fracture Intervention Trial (FIT). A total of 1,099 women who had participated in FIT and taken alendronate for 5 years were then randomized to one of two doses of alendronate or placebo for an additional 5 years. To qualify for the FLEX trial, all women had to have low bone mass at the beginning of FIT. The average age of women in the FLEX trial was 73 years. The primary outcome was total hip BMD; secondary outcomes were BMD at other sites and biochemical markers.
Women who remained on alendronate maintained a higher BMD of the hip and spine than women on placebo, but all patients’ levels remained at or above pretreatment levels of 10 years earlier. The same was true for markers of bone remodeling. The cumulative risk of nonvertebral fractures did not differ between the two groups (19% for placebo, 18.9% for alendronate; RR, 1.0; 95% CI, 0.76–1.32). The risk of clinically recognized vertebral fractures was lower in the women who continued alendronate (5.3% for placebo and 2.4% for alendronate; RR, 0.45; 95% CI, 0.24–0.85), but there was no significant reduction in morphometric vertebral fractures.
Data can aid in determining duration of therapy
These data are extremely helpful, especially for clinicians who are trying to determine how long to continue bisphosphonate therapy and which patients may be candidates for a “drug holiday.” We have all had women whose response to a bisphosphonate has been so robust that follow-up BMD measurements have climbed to a range in which therapy would not have been initiated. This study clearly shows that the cumulative effect of 5 years of alendronate followed by 5 years of placebo is positive, compared with the bone loss one would expect in untreated women.
The answer isn’t clear, but women with a previous fracture, and those at high risk for spine fracture, are likely to benefit from continued treatment with alendronate. Patients with a lower risk of fracture are better candidates for the holiday.
As for when the drug holiday should end, that isn’t clear, either. Continued close monitoring of these lower-risk women using bone-density measurements may help identify that minority of patients who do not maintain bone mass off the medication.
1. Barrett-Connor E, Mosca L, Collins P, et al. Effects of raloxifene on cardiovascular events and breast cancer in postmenopausal women. N Engl J Med. 2006;355:125-137.
2. Vogel VG, Costantino JP, Wickerham DL, et al. Effects of tamoxifen vs raloxifene on the risk of developing invasive breast cancer and other disease outcomes. The NSABP Study of Tamoxifen and Raloxifene (STAR) P-2 Trial. JAMA. 2006;295:2727-2741.
3. Greenspan SL, Emkey RD, Bone HG, et al. Significant differential effects of alendronate, estrogen, or combination therapy on the rate of bone loss after discontinuation of treatment of postmenopausal osteoporosis. A randomized, double-blind, placebo-controlled trial. Ann Intern Med. 2002;137:875-883.
1. Barrett-Connor E, Mosca L, Collins P, et al. Effects of raloxifene on cardiovascular events and breast cancer in postmenopausal women. N Engl J Med. 2006;355:125-137.
2. Vogel VG, Costantino JP, Wickerham DL, et al. Effects of tamoxifen vs raloxifene on the risk of developing invasive breast cancer and other disease outcomes. The NSABP Study of Tamoxifen and Raloxifene (STAR) P-2 Trial. JAMA. 2006;295:2727-2741.
3. Greenspan SL, Emkey RD, Bone HG, et al. Significant differential effects of alendronate, estrogen, or combination therapy on the rate of bone loss after discontinuation of treatment of postmenopausal osteoporosis. A randomized, double-blind, placebo-controlled trial. Ann Intern Med. 2002;137:875-883.
How to overcome a resistant cervix for hysteroscopy and endometrial biopsy
CASE: Difficulty inserting a catheter suggests an unyielding cervix
A.W. is a 38-year-old nulliparous woman who seeks treatment for persistent irregular vaginal bleeding. Her physician attempts an endometrial biopsy in the office but is unable to pass the catheter through the internal cervical os. She schedules office hysteroscopy as follow-up.
What steps can the ObGyn take to reduce the difficulty of the procedure, particularly insertion of the hysteroscope through the cervical canal?
Successful hysteroscopy requires a cervical canal sufficiently dilated to allow passage of the hysteroscope. And because of inevitable variation in anatomy—and even in models of hysteroscopes, which range in diameter from 2.7 to 10 mm—passage is not always easily accomplished. Many of the complications related to hysteroscopy, including cervical tears, creation of a false passage, uterine perforation, vasovagal reaction, pain, and inability to complete the procedure, are caused by inadequate cervical dilation and an inability to insert the hysteroscope.1-6 One study noted that almost half of complications were related to cervical entry.6
In this article, I describe ways to overcome the challenging cervix for hysteroscopic procedures and endometrial biopsy (TABLES 1 and 2).
TABLE 1
10 actions that can ease entry to the cervix for hysteroscopy
| ACTION | COMMENTS |
|---|---|
| Take a careful history and perform a rigorous physical exam | Identify risk factors for cervical stenosis and assess cervical/uterine position |
| Administer an oral nonsteroidal anti-inflammatory drug 60 minutes before the procedure | Helps to reduce discomfort, especially postprocedure pain |
| Provide an anxiolytic or conscious sedation, or both | Consider this option for women who are very anxious or unlikely to tolerate pain, especially for operative procedures |
| Use a tenaculum | Consider if the uterus is not in the axial position |
| Use Hagar dilators or a lacrimal duct probe | May be helpful if mechanical dilation is necessary |
| Proceed under ultrasonographic guidance | Consider transabdominal imaging to help guide cervical dilation in difficult cases, e.g., when the patient has a history of uterine perforation |
| Opt for a smaller hysteroscope | A smaller scope will require less cervical dilation |
| Administer a paracervical block | Consider this option if cervical dilation is expected to be difficult, especially in women at risk of significant pain. Be alert for complications such as bleeding, discomfort at the time of injection, and intravascular injection leading to bradycardia and hypotension |
| Administer a topical cervical anesthetic | May be appropriate when a tenaculum is used |
| Give misoprostol to prime the cervix | Consider giving 400 μg of intravaginal misoprostol 9 to 12 hours preoperatively in premenopausal women, particularly nulliparous women and those undergoing operative hysteroscopy |
TABLE 2
6 ways to prepare the cervix for endometrial biopsy
| ACTION | COMMENTS |
|---|---|
| Take a careful history and perform a thorough physical examination | Identify risk factors for cervical stenosis and assess uterine position |
| Administer an oral nonsteroidal anti-inflammatory drug 60 minutes prior to biopsy | Helps to reduce discomfort, especially postprocedure pain |
| Use a tenaculum | May be helpful if the uterus/cervix is not in the axial position |
| Apply a topical cervical anesthetic | May help alleviate discomfort associated with use of a tenaculum |
| Use Hagar dilators or lacrimal duct probes | Provide mechanical dilation |
| Use the smallest biopsy catheter possible | Reduces degree of cervical dilation necessary |
Hysteroscopy failure rate: 3.4% to 4.2%
Hysteroscopy is, of course, common in gynecologic practice, its indications extending across a range of investigations and treatments—for menstrual disorders, postmenopausal bleeding, infertility, and recurrent pregnancy loss.1,7 Flexible hysteroscopes range in diameter from 2.7 to 5 mm; rigid hysteroscopes, from 1 to 5 mm; and operative hysteroscopes can be as large as 8 to 10 mm.2,7
A systematic review of diagnostic hysteroscopy in more than 26,000 women reported a failure rate of 4.2% for ambulatory hysteroscopy and 3.4% for inpatient procedures.4 Failed ambulatory procedures were mainly attributed to technical problems, including:
- cervical stenosis
- anatomic and structural abnormalities
- pain and intolerance.4
Ideally, hysteroscopy is performed with minimal or no cervical dilation,7 but this may not always be possible.
Things to consider before embarking
Close attention to cervical and uterine anatomy is critical because insertion of the hysteroscope can be the most difficult aspect of the procedure. A bimanual examination is imperative to assess uterine size and position. It also is useful to sound the uterus to determine its depth.
An accurate medical, gynecologic, and obstetric history is essential, including information on pregnancies, dilation and curettage, cervical procedures such as cryotherapy, and any other procedures that may increase the risk of cervical stenosis, or difficulty dilating the cervix.
Is stenosis present? Stenosis is most common in nulliparous and postmenopausal women and in those who have undergone cervical procedures such as cryotherapy. Stenosis increases the risk of laceration and uterine perforation.
Consider a mechanical dilator. When cervical dilation is difficult, a series of small Hagar or lacrimal duct dilators may be helpful (FIGURE).
FIGURE Mechanical dilation is one antidote to cervical stenosis
In challenging cases, such as cervical stenosis, mechanical dilation with a series of Hagar or lacrimal duct dilators may facilitate entry into the cervix.
Pain can be mild—or it can thwart your work
Although many women tolerate placement of a small hysteroscope without analgesia or anesthesia, pain and vasovagal reaction sometimes occur. Indeed, the level of pain experienced by the patient is a major determinant of the overall success of the procedure.3,8-10 Pain can occur when a tenaculum is used to grasp the anterior cervix, as well as during cervical dilation, injection of local anesthetic, or insertion of the hysteroscope. In some cases, a smaller scope may be all that is needed to solve the problem.11
Analgesia may not always be necessary
Some researchers have studied office hysteroscopy without analgesia or anesthesia, finding a high level of acceptance.12,13 Others have found a significant percentage of women requesting anesthesia or analgesia (16.5%)10 or requiring local anesthesia (28.8%).8
Preoperative NSAIDs may suffice. Use of oral nonsteroidal anti-inflammatory drugs (NSAIDs) 1 hour before office hysteroscopy may reduce intraoperative and postoperative pain.7 Nagele and colleagues8 compared use of mefenamic acid 1 hour before the procedure with placebo in 95 women undergoing outpatient diagnostic hysteroscopy. Mefenamic acid reduced pain at 30 and 60 minutes after—but not during—the procedure. Other studies have found that pain is reduced when an oral NSAID is taken 1 to 2 hours before insertion of an intrauterine device and before suction curettage.14,15
Other perioperative medications may help reduce discomfort and patient anxiety, including anxiolytics, such as lorazepam, analgesics, and conscious sedation.3
Paracervical block may be appropriate when pain is very likely
A number of investigators have evaluated use of paracervical anesthesia during out-patient hysteroscopy.9,13,16,17 They injected lignocaine or mepivacaine using a 21- or 22-gauge needle at 3, 5, 7, and 9 o’clock or 4 and 8 o’clock paracervically.13 One study found paracervical block to be effective in reducing the pain of tenaculum placement and insertion of the hysteroscope.17 However, some studies suggested a reduction of pain in postmenopausal women only.9 These women may be more likely to have cervical stenosis.
Paracervical block does pose a risk of complications. Studies have reported bleeding in some women16 and pain with injection of the paracervical block, as well as bradycardia and hypotension possibly secondary to intravascular injection.17
Other methods are inconsistent
Intracervical injection. Some researchers have recommended injection of local anesthetic into the cervix.13 One study found no benefit—in fact, the injection appeared to be the most painful part of the procedure.18 A case series suggested that injection of local anesthetic may be effective, but the series lacked a placebo or control arm.13
Topical intrauterine anesthetic has been investigated after administration through the channel of the hysteroscope or by a catheter passed through the cervix into the uterine cavity.13 Findings have been mixed, with some researchers demonstrating reduced pain19,20 and others showing no relief.21
Topical cervical anesthesia. Some hysteroscopists have recommended application of anesthetic cream, gel, or spray directly to the cervix immediately before the procedure.13,22 The results have been mixed, with some studies noting decreased pain overall,13 one finding decreased pain only during tenaculum placement,22 and others finding no significant reduction in pain any time during the procedure.13,23,24 A review concluded that topical cervical lignocaine spray may reduce the discomfort of tenaculum placement.13
Topical anesthesia may minimize vasovagal reaction
In one study, 1.1% of women undergoing office hysteroscopy experienced a vasovagal reaction, caused by stimulation of the parasympathetic nervous system with cervical manipulation and passage of the scope through the internal os of the cervix.25 The reaction led to hypotension and bradycardia. Several studies have suggested that a local anesthetic can reduce this complication.19,20
Cicinelli and associates found that topical local anesthesia reduced the incidence of vasovagal reaction from 32.5% in the control arm to 5%.20 They suggest that a local anesthetic be considered in selected women, such as postmenopausal patients, who are at increased risk of vasovagal attack.
In contrast, Lau and associates17 found an increased rate of bradycardia and hypotension with paracervical lignocaine (31% versus 10%), but it may have been caused by inadvertent intravascular injection.17
Researchers have also suggested that the use of smaller hysteroscopes may reduce the incidence of vasovagal reactions.26
How to prime the cervix for hysteroscopy
The use of vaginal misoprostol, a prostaglandin E1 analogue, 9 to 12 hours before hysteroscopy may help increase preprocedural cervical dilation in premenopausal women, especially in nulliparas and women undergoing operative hysteroscopy. Misoprostol, used to prevent and treat NSAID-induced gastric ulcers, is gaining favor as a cervical ripening agent. We performed a meta-analysis to assess its effectiveness in dilating the cervix and reducing the need for mechanical dilation.5
We identified 10 studies that met inclusion criteria; five of them included premenopausal women, four included postmenopausal women or women receiving a gonadotropin-releasing hormone (GnRH) agonist, and one study included both groups.5 A variety of dosing protocols were used, with dosages ranging from 100 μg to 1,000 μg of intravaginal or oral misoprostol 4 to 24 hours preoperatively (most studies evaluated the vaginal route).
We found that misoprostol significantly reduced the need for further cervical dilation, and was associated with a lower rate of cervical laceration. However, this was true only for the premenopausal group: 42.6% of premenopausal women given misoprostol needed further dilation, compared with 71.7% in the control group, and 2% of premenopausal women given misoprostol suffered cervical laceration, compared with 11% in the control group. Among postmenopausal women and those receiving a GnRH agonist, misoprostol lacked clear benefit and was associated with side effects such as nausea, diarrhea, abdominal cramping, and fever.
For every premenopausal woman who received misoprostol before hysteroscopy, one woman avoided the need for further cervical dilation. For every 12 premenopausal women receiving misoprostol, one cervical laceration was avoided.
The ideal dosing regimen could not be determined because of variations in protocols. Nor was it clear whether misoprostol had any benefit among postmenopausal women or those receiving a GnRH agonist.
Most studies of misoprostol for cervical ripening have involved intravaginal administration, with dosages of 200 μg to 400 μg given 9 to 12 hours before hysteroscopy showing the greatest benefit.
Ultrasonography may help guide dilation
Transabdominal ultrasonography has been used to guide dilation in difficult dilation and curettage procedures, and is especially useful in women with a history of uterine perforation.27 It may be helpful in cases involving difficult cervical dilation during hysteroscopy or endometrial biopsy.
Steady the cervix. A tenaculum is not always required, but its use on the anterior lip of the cervix may help steady the cervix and provide countertraction during insertion of the hysteroscope through the cervical canal, especially if the cervix is not in an axial position.7
CASE Resolved!
Because she is nulliparous and may benefit from cervical priming, the patient is given 400 μg of intravaginal misoprostol 12 hours before hysteroscopy, as well as an oral NSAID 1 hour before the procedure. A bimanual examination reveals a sharply anteverted uterus, so a topical cervical anesthetic spray is applied to the anterior cervix, and a tenaculum is placed to help straighten the uterine position. The hysteroscope passes easily through the cervical canal, making further dilation unnecessary. The procedure is completed without difficulty and is well tolerated by the patient.
Difficult entry can also hamper endometrial biopsy
Every ObGyn has used endometrial biopsy to assess abnormal uterine bleeding, postmenopausal bleeding, infertility, or recurrent pregnancy loss, or to monitor women on hormone replacement therapy28,29 —so its advantages over dilation and curettage should come as no surprise. They include the ability to perform it in an office setting, usually with minimal cervical dilation, often without anesthesia, and at less expense.28 Complications include cramping and pain,29-32 vasovagal reaction,29 bleeding,29 and inability to pass the biopsy catheter through the cervix into the uterine cavity. Another rare complication is uterine perforation.29
As with hysteroscopy, many of these complications are related to difficulty entering the uterine cavity through the cervix.
Prerequisites include thorough assessment of the uterus
As with hysteroscopy, an accurate and detailed history is necessary to identify risk factors for a difficult procedure. Assess uterine size and position with a bimanual examination. Although a tenaculum is often unnecessary, its placement on the anterior lip of the cervix may help steady the cervix and allow the catheter to pass through the cervical canal into the uterine cavity, especially if the uterus is not in the axial position.28,29 Again, it is useful to sound the uterine cavity to ascertain its depth. This may be done with the biopsy catheter.
Cervical dilation may be necessary
Even when women with cervical stenosis were excluded in one study, it was difficult to pass the Pipelle endometrial biopsy through the cervix in 41.7% of women.30
If the sampling device does not pass easily through the cervix, use a tenaculum and a lacrimal duct probe or small Hagar dilators to dilate the cervix.28
Pain may again be an issue
Almost 50% of women experience moderate or severe pain during endometrial biopsy.32 Many clinicians recommend giving an oral NSAID 60 minutes before the procedure to decrease discomfort. One study found that the use of naproxen sodium before Vabra curettage reduced the severity of pain at 30 and 60 minutes after the procedure, but did not alleviate discomfort arising during the biopsy itself.14 Another study suggested the combination of naproxen sodium and intrauterine lidocaine (5 mL of 2% lidocaine) to reduce discomfort associated with the procedure.30
Use of anesthesia is controversial
A study by Lau and colleagues17 found paracervical lignocaine to be ineffective at reducing pain during hysteroscopy and endometrial biopsy, but the drug did increase the risk of bradycardia and hypotension. Another study demonstrated a decrease in procedure-related discomfort in postmenopausal women who were given 2 mL of 2% intrauterine mepivacaine.20 These findings are similar to those of Zupi and associates.19
Consider the tool
Discomfort may be related to the size of the biopsy catheter. Pain scores appear to be significantly lower with the Pipelle biopsy catheter than with the larger Novak biopsy curette.32
Vasovagal reaction usually resolves after the procedure
As with hysteroscopy, women may occasionally experience a vasovagal reaction during endometrial biopsy. This complication usually resolves quickly once the procedure is completed.29 Some clinicians suggest that the patient be allowed to eat and drink before the procedure and be given an analgesic before it begins.28
Cervical priming is not a proven strategy
Misoprostol has been considered as a preprocedure adjunct to endometrial biopsy. Only one small randomized, controlled trial involving 42 women has evaluated the drug for this indication. It found no benefit when 400 μg of misoprostol was given orally 3 hours before the procedure, as well as cramping and increased pain during the biopsy.33 This study had several shortcomings, including its small sample size and the inclusion of both pre- and postmenopausal women. Further research is needed—separately in premenopausal and postmenopausal women and with adequately large samples—to assess the use of misoprostol.
The author reports no financial relationships relevant to this article.
1. Bradley LD. Complications in hysteroscopy: prevention, treatment and legal risk. Curr Opin Obstet Gynecol. 2002;14:409-415.
2. American College of Obstetricians and Gynecologists. ACOG technology assessment in obstetrics and gynecology, number 4, August 2005: hysteroscopy. Obstet Gynecol. 2005;106:439-442.
3. Vilos GA, Abu-Rafea B. New developments in ambulatory hysteroscopic surgery. Best Pract Res Clin Obstet Gynaecol. 2005;19:727-742.
4. Clark TJ, Voit D, Gupta JK, Hyde C, Song F, Khan KS. Accuracy of hysteroscopy in the diagnosis of endometrial cancer and hyperplasia: a systematic quantitative review. JAMA. 2002;288:1610-1621.
5. Crane JM, Healey S. Use of misoprostol before hysteroscopy: a systematic review. J Obstet Gynaecol Can. 2006;28:373-379.
6. Jansen FW, Vredevoogd CB, van Ulzen K, Hermans J, Trimbos JB, Trimbos-Kemper TC. Complications of hysteroscopy: a prospective, multicenter study. Obstet Gynecol. 2000;96:266-270.
7. Guido R, Stovall D. Hysteroscopy Version 14.3. UpToDate [cited February 15, 2007]; Available from: www.uptodate.com.
8. Nagele F, Lockwood G, Magos AL. Randomised placebo controlled trial of mefenamic acid for premedication at outpatient hysteroscopy: a pilot study. Br J Obstet Gynaecol. 1997;104:842-844.
9. Cicinelli E, Didonna T, Schonauer LM, Stragapede S, Falco N, Pansini N. Paracervical anesthesia for hysteroscopy and endometrial biopsy in postmenopausal women. A randomized, double-blind, placebo-controlled study. J Reprod Med. 1998;43:1014-1018.
10. De Iaco P, Marabini A, Stefanetti M, Del Vecchio C, Bovicelli L. Acceptability and pain of outpatient hysteroscopy. J Am Assoc Gynecol Laparosc. 2000;7:71-75.
11. Marsh F, Jackson T, Duffy S. A case controlled study comparing 3.6 mm and 3.1 mm flexible hysteroscopes. Gynaecol Endosc. 2002;11:393-396.
12. Lau WC, Ho RY, Tsang MK, Yuen PM. Patient’s acceptance of outpatient hysteroscopy. Gynecol Obstet Invest. 1999;47:191-193.
13. Hassan L, Gannon MJ. Anaesthesia and analgesia for ambulatory hysteroscopic surgery. Best Pract Res Clin Obstet Gynaecol. 2005;19:681-691.
14. Siddle NC, Young O, Sledmere CM, Reading AE, Whitehead MI. A controlled trial of naproxen sodium for relief of pain associated with Vabra suction curettage. Br J Obstet Gynaecol. 1983;90:864-869.
15. Edgren RA, Morton CJ. Naproxen sodium for Ob/Gyn use, with special reference to pain states: a review. Int J Fertil. 1986;31:135-142.
16. Giorda G, Scarabelli C, Franceschi S, Campagnutta E. Feasibility and pain control in outpatient hysteroscopy in postmenopausal women: a randomized trial. Acta Obstet Gynecol Scand. 2000;79:593-597.
17. Lau WC, Lo WK, Tam WH, Yuen PM. Paracervical anaesthesia in outpatient hysteroscopy: a randomised double-blind placebo-controlled trial. Br J Obstet Gynaecol. 1999;106:356-359.
18. Broadbent JA, Hill NC, Molnar BG, Rolfe KJ, Magos AL. Randomized placebo controlled trial to assess the role of intracervical lignocaine in outpatient hysteroscopy. Br J Obstet Gynaecol. 1992;99:777-779.
19. Zupi E, Luciano AA, Valli E, Marconi D, Maneschi F, Romanini C. The use of topical anesthesia in diagnostic hysteroscopy and endometrial biopsy. Fertil Steril. 1995;63:414-416.
20. Cicinelli E, Didonna T, Ambrosi G, Schonauer LM, Fiore G, Matteo MG. Topical anaesthesia for diagnostic hysteroscopy and endometrial biopsy in postmenopausal women: a randomised placebo-controlled double-blind study. Br J Obstet Gynaecol. 1997;104:316-319.
21. Lau WC, Tam WH, Lo WK, Yuen PM. A randomised double-blind placebo-controlled trial of transcervical intrauterine local anaesthesia in outpatient hysteroscopy. BJOG. 2000;107:610-613.
22. Davies A, Richardson RE, O’Connor H, Baskett TF, Nagele F, Magos AL. Lignocaine aerosol spray in outpatient hysteroscopy: a randomized double-blind placebo-controlled trial. Fertil Steril. 1997;67:1019-1023.
23. Clark S, Vonau B, Macdonald R. Topical anaesthesia in out-patient hysteroscopy. Gynaecol Endosc. 1996;5:141-144.
24. Wong AY, Wong K, Tang LC. Stepwise pain score analysis of the effect of local lignocaine on outpatient hysteroscopy: a randomized, double-blind, placebo-controlled trial. Fertil Steril. 2000;73:1234-1237.
25. Bellingham FR. Outpatient hysteroscopy—problems. Aust N Z J Obstet Gynaecol. 1997;37:202-205.
26. Cicinelli E, Schonauer LM, Barba B, Tartagni M, Luisi D, Di Naro E. Tolerability and cardiovascular complications of outpatient diagnostic minihysteroscopy compared with conventional hysteroscopy. J Am Assoc Gynecol Laparosc. 2003;10:399-402.
27. Hunter RE, Reuter K, Kopin E. Use of ultrasonography in the difficult postmenopausal dilation and curettage. Obstet Gynecol. 1989;73:813-816.
28. Guido R, Stovall D. Endometrial sampling procedures Version 14.3. UpToDate [cited February 15, 2007]; Available from: www.uptodate.com.
29. Cooper JM, Erickson ML. Endometrial sampling techniques in the diagnosis of abnormal uterine bleeding. Obstet Gynecol Clin North Am. 2000;27:235-244.
30. Dogan E, Celiloglu M, Sarihan E, Demir A. Anesthetic effect of intrauterine lidocaine plus naproxen sodium in endometrial biopsy. Obstet Gynecol. 2004;103:347-351.
31. Trolice MP, Fishburne C, Jr, McGrady S. Anesthetic efficacy of intrauterine lidocaine for endometrial biopsy: a randomized double-masked trial. Obstet Gynecol. 2000;95:345-347.
32. Silver MM, Miles P, Rosa C. Comparison of Novak and Pipelle endometrial biopsy instruments. Obstet Gynecol. 1991;78:828-830.
33. Perrone JF, Caldito G, Mailhes JB, Tucker AN, Ford WR, London SN. Oral misoprostol before office endometrial biopsy. Obstet Gynecol. 2002;99:439-444.
CASE: Difficulty inserting a catheter suggests an unyielding cervix
A.W. is a 38-year-old nulliparous woman who seeks treatment for persistent irregular vaginal bleeding. Her physician attempts an endometrial biopsy in the office but is unable to pass the catheter through the internal cervical os. She schedules office hysteroscopy as follow-up.
What steps can the ObGyn take to reduce the difficulty of the procedure, particularly insertion of the hysteroscope through the cervical canal?
Successful hysteroscopy requires a cervical canal sufficiently dilated to allow passage of the hysteroscope. And because of inevitable variation in anatomy—and even in models of hysteroscopes, which range in diameter from 2.7 to 10 mm—passage is not always easily accomplished. Many of the complications related to hysteroscopy, including cervical tears, creation of a false passage, uterine perforation, vasovagal reaction, pain, and inability to complete the procedure, are caused by inadequate cervical dilation and an inability to insert the hysteroscope.1-6 One study noted that almost half of complications were related to cervical entry.6
In this article, I describe ways to overcome the challenging cervix for hysteroscopic procedures and endometrial biopsy (TABLES 1 and 2).
TABLE 1
10 actions that can ease entry to the cervix for hysteroscopy
| ACTION | COMMENTS |
|---|---|
| Take a careful history and perform a rigorous physical exam | Identify risk factors for cervical stenosis and assess cervical/uterine position |
| Administer an oral nonsteroidal anti-inflammatory drug 60 minutes before the procedure | Helps to reduce discomfort, especially postprocedure pain |
| Provide an anxiolytic or conscious sedation, or both | Consider this option for women who are very anxious or unlikely to tolerate pain, especially for operative procedures |
| Use a tenaculum | Consider if the uterus is not in the axial position |
| Use Hagar dilators or a lacrimal duct probe | May be helpful if mechanical dilation is necessary |
| Proceed under ultrasonographic guidance | Consider transabdominal imaging to help guide cervical dilation in difficult cases, e.g., when the patient has a history of uterine perforation |
| Opt for a smaller hysteroscope | A smaller scope will require less cervical dilation |
| Administer a paracervical block | Consider this option if cervical dilation is expected to be difficult, especially in women at risk of significant pain. Be alert for complications such as bleeding, discomfort at the time of injection, and intravascular injection leading to bradycardia and hypotension |
| Administer a topical cervical anesthetic | May be appropriate when a tenaculum is used |
| Give misoprostol to prime the cervix | Consider giving 400 μg of intravaginal misoprostol 9 to 12 hours preoperatively in premenopausal women, particularly nulliparous women and those undergoing operative hysteroscopy |
TABLE 2
6 ways to prepare the cervix for endometrial biopsy
| ACTION | COMMENTS |
|---|---|
| Take a careful history and perform a thorough physical examination | Identify risk factors for cervical stenosis and assess uterine position |
| Administer an oral nonsteroidal anti-inflammatory drug 60 minutes prior to biopsy | Helps to reduce discomfort, especially postprocedure pain |
| Use a tenaculum | May be helpful if the uterus/cervix is not in the axial position |
| Apply a topical cervical anesthetic | May help alleviate discomfort associated with use of a tenaculum |
| Use Hagar dilators or lacrimal duct probes | Provide mechanical dilation |
| Use the smallest biopsy catheter possible | Reduces degree of cervical dilation necessary |
Hysteroscopy failure rate: 3.4% to 4.2%
Hysteroscopy is, of course, common in gynecologic practice, its indications extending across a range of investigations and treatments—for menstrual disorders, postmenopausal bleeding, infertility, and recurrent pregnancy loss.1,7 Flexible hysteroscopes range in diameter from 2.7 to 5 mm; rigid hysteroscopes, from 1 to 5 mm; and operative hysteroscopes can be as large as 8 to 10 mm.2,7
A systematic review of diagnostic hysteroscopy in more than 26,000 women reported a failure rate of 4.2% for ambulatory hysteroscopy and 3.4% for inpatient procedures.4 Failed ambulatory procedures were mainly attributed to technical problems, including:
- cervical stenosis
- anatomic and structural abnormalities
- pain and intolerance.4
Ideally, hysteroscopy is performed with minimal or no cervical dilation,7 but this may not always be possible.
Things to consider before embarking
Close attention to cervical and uterine anatomy is critical because insertion of the hysteroscope can be the most difficult aspect of the procedure. A bimanual examination is imperative to assess uterine size and position. It also is useful to sound the uterus to determine its depth.
An accurate medical, gynecologic, and obstetric history is essential, including information on pregnancies, dilation and curettage, cervical procedures such as cryotherapy, and any other procedures that may increase the risk of cervical stenosis, or difficulty dilating the cervix.
Is stenosis present? Stenosis is most common in nulliparous and postmenopausal women and in those who have undergone cervical procedures such as cryotherapy. Stenosis increases the risk of laceration and uterine perforation.
Consider a mechanical dilator. When cervical dilation is difficult, a series of small Hagar or lacrimal duct dilators may be helpful (FIGURE).
FIGURE Mechanical dilation is one antidote to cervical stenosis
In challenging cases, such as cervical stenosis, mechanical dilation with a series of Hagar or lacrimal duct dilators may facilitate entry into the cervix.
Pain can be mild—or it can thwart your work
Although many women tolerate placement of a small hysteroscope without analgesia or anesthesia, pain and vasovagal reaction sometimes occur. Indeed, the level of pain experienced by the patient is a major determinant of the overall success of the procedure.3,8-10 Pain can occur when a tenaculum is used to grasp the anterior cervix, as well as during cervical dilation, injection of local anesthetic, or insertion of the hysteroscope. In some cases, a smaller scope may be all that is needed to solve the problem.11
Analgesia may not always be necessary
Some researchers have studied office hysteroscopy without analgesia or anesthesia, finding a high level of acceptance.12,13 Others have found a significant percentage of women requesting anesthesia or analgesia (16.5%)10 or requiring local anesthesia (28.8%).8
Preoperative NSAIDs may suffice. Use of oral nonsteroidal anti-inflammatory drugs (NSAIDs) 1 hour before office hysteroscopy may reduce intraoperative and postoperative pain.7 Nagele and colleagues8 compared use of mefenamic acid 1 hour before the procedure with placebo in 95 women undergoing outpatient diagnostic hysteroscopy. Mefenamic acid reduced pain at 30 and 60 minutes after—but not during—the procedure. Other studies have found that pain is reduced when an oral NSAID is taken 1 to 2 hours before insertion of an intrauterine device and before suction curettage.14,15
Other perioperative medications may help reduce discomfort and patient anxiety, including anxiolytics, such as lorazepam, analgesics, and conscious sedation.3
Paracervical block may be appropriate when pain is very likely
A number of investigators have evaluated use of paracervical anesthesia during out-patient hysteroscopy.9,13,16,17 They injected lignocaine or mepivacaine using a 21- or 22-gauge needle at 3, 5, 7, and 9 o’clock or 4 and 8 o’clock paracervically.13 One study found paracervical block to be effective in reducing the pain of tenaculum placement and insertion of the hysteroscope.17 However, some studies suggested a reduction of pain in postmenopausal women only.9 These women may be more likely to have cervical stenosis.
Paracervical block does pose a risk of complications. Studies have reported bleeding in some women16 and pain with injection of the paracervical block, as well as bradycardia and hypotension possibly secondary to intravascular injection.17
Other methods are inconsistent
Intracervical injection. Some researchers have recommended injection of local anesthetic into the cervix.13 One study found no benefit—in fact, the injection appeared to be the most painful part of the procedure.18 A case series suggested that injection of local anesthetic may be effective, but the series lacked a placebo or control arm.13
Topical intrauterine anesthetic has been investigated after administration through the channel of the hysteroscope or by a catheter passed through the cervix into the uterine cavity.13 Findings have been mixed, with some researchers demonstrating reduced pain19,20 and others showing no relief.21
Topical cervical anesthesia. Some hysteroscopists have recommended application of anesthetic cream, gel, or spray directly to the cervix immediately before the procedure.13,22 The results have been mixed, with some studies noting decreased pain overall,13 one finding decreased pain only during tenaculum placement,22 and others finding no significant reduction in pain any time during the procedure.13,23,24 A review concluded that topical cervical lignocaine spray may reduce the discomfort of tenaculum placement.13
Topical anesthesia may minimize vasovagal reaction
In one study, 1.1% of women undergoing office hysteroscopy experienced a vasovagal reaction, caused by stimulation of the parasympathetic nervous system with cervical manipulation and passage of the scope through the internal os of the cervix.25 The reaction led to hypotension and bradycardia. Several studies have suggested that a local anesthetic can reduce this complication.19,20
Cicinelli and associates found that topical local anesthesia reduced the incidence of vasovagal reaction from 32.5% in the control arm to 5%.20 They suggest that a local anesthetic be considered in selected women, such as postmenopausal patients, who are at increased risk of vasovagal attack.
In contrast, Lau and associates17 found an increased rate of bradycardia and hypotension with paracervical lignocaine (31% versus 10%), but it may have been caused by inadvertent intravascular injection.17
Researchers have also suggested that the use of smaller hysteroscopes may reduce the incidence of vasovagal reactions.26
How to prime the cervix for hysteroscopy
The use of vaginal misoprostol, a prostaglandin E1 analogue, 9 to 12 hours before hysteroscopy may help increase preprocedural cervical dilation in premenopausal women, especially in nulliparas and women undergoing operative hysteroscopy. Misoprostol, used to prevent and treat NSAID-induced gastric ulcers, is gaining favor as a cervical ripening agent. We performed a meta-analysis to assess its effectiveness in dilating the cervix and reducing the need for mechanical dilation.5
We identified 10 studies that met inclusion criteria; five of them included premenopausal women, four included postmenopausal women or women receiving a gonadotropin-releasing hormone (GnRH) agonist, and one study included both groups.5 A variety of dosing protocols were used, with dosages ranging from 100 μg to 1,000 μg of intravaginal or oral misoprostol 4 to 24 hours preoperatively (most studies evaluated the vaginal route).
We found that misoprostol significantly reduced the need for further cervical dilation, and was associated with a lower rate of cervical laceration. However, this was true only for the premenopausal group: 42.6% of premenopausal women given misoprostol needed further dilation, compared with 71.7% in the control group, and 2% of premenopausal women given misoprostol suffered cervical laceration, compared with 11% in the control group. Among postmenopausal women and those receiving a GnRH agonist, misoprostol lacked clear benefit and was associated with side effects such as nausea, diarrhea, abdominal cramping, and fever.
For every premenopausal woman who received misoprostol before hysteroscopy, one woman avoided the need for further cervical dilation. For every 12 premenopausal women receiving misoprostol, one cervical laceration was avoided.
The ideal dosing regimen could not be determined because of variations in protocols. Nor was it clear whether misoprostol had any benefit among postmenopausal women or those receiving a GnRH agonist.
Most studies of misoprostol for cervical ripening have involved intravaginal administration, with dosages of 200 μg to 400 μg given 9 to 12 hours before hysteroscopy showing the greatest benefit.
Ultrasonography may help guide dilation
Transabdominal ultrasonography has been used to guide dilation in difficult dilation and curettage procedures, and is especially useful in women with a history of uterine perforation.27 It may be helpful in cases involving difficult cervical dilation during hysteroscopy or endometrial biopsy.
Steady the cervix. A tenaculum is not always required, but its use on the anterior lip of the cervix may help steady the cervix and provide countertraction during insertion of the hysteroscope through the cervical canal, especially if the cervix is not in an axial position.7
CASE Resolved!
Because she is nulliparous and may benefit from cervical priming, the patient is given 400 μg of intravaginal misoprostol 12 hours before hysteroscopy, as well as an oral NSAID 1 hour before the procedure. A bimanual examination reveals a sharply anteverted uterus, so a topical cervical anesthetic spray is applied to the anterior cervix, and a tenaculum is placed to help straighten the uterine position. The hysteroscope passes easily through the cervical canal, making further dilation unnecessary. The procedure is completed without difficulty and is well tolerated by the patient.
Difficult entry can also hamper endometrial biopsy
Every ObGyn has used endometrial biopsy to assess abnormal uterine bleeding, postmenopausal bleeding, infertility, or recurrent pregnancy loss, or to monitor women on hormone replacement therapy28,29 —so its advantages over dilation and curettage should come as no surprise. They include the ability to perform it in an office setting, usually with minimal cervical dilation, often without anesthesia, and at less expense.28 Complications include cramping and pain,29-32 vasovagal reaction,29 bleeding,29 and inability to pass the biopsy catheter through the cervix into the uterine cavity. Another rare complication is uterine perforation.29
As with hysteroscopy, many of these complications are related to difficulty entering the uterine cavity through the cervix.
Prerequisites include thorough assessment of the uterus
As with hysteroscopy, an accurate and detailed history is necessary to identify risk factors for a difficult procedure. Assess uterine size and position with a bimanual examination. Although a tenaculum is often unnecessary, its placement on the anterior lip of the cervix may help steady the cervix and allow the catheter to pass through the cervical canal into the uterine cavity, especially if the uterus is not in the axial position.28,29 Again, it is useful to sound the uterine cavity to ascertain its depth. This may be done with the biopsy catheter.
Cervical dilation may be necessary
Even when women with cervical stenosis were excluded in one study, it was difficult to pass the Pipelle endometrial biopsy through the cervix in 41.7% of women.30
If the sampling device does not pass easily through the cervix, use a tenaculum and a lacrimal duct probe or small Hagar dilators to dilate the cervix.28
Pain may again be an issue
Almost 50% of women experience moderate or severe pain during endometrial biopsy.32 Many clinicians recommend giving an oral NSAID 60 minutes before the procedure to decrease discomfort. One study found that the use of naproxen sodium before Vabra curettage reduced the severity of pain at 30 and 60 minutes after the procedure, but did not alleviate discomfort arising during the biopsy itself.14 Another study suggested the combination of naproxen sodium and intrauterine lidocaine (5 mL of 2% lidocaine) to reduce discomfort associated with the procedure.30
Use of anesthesia is controversial
A study by Lau and colleagues17 found paracervical lignocaine to be ineffective at reducing pain during hysteroscopy and endometrial biopsy, but the drug did increase the risk of bradycardia and hypotension. Another study demonstrated a decrease in procedure-related discomfort in postmenopausal women who were given 2 mL of 2% intrauterine mepivacaine.20 These findings are similar to those of Zupi and associates.19
Consider the tool
Discomfort may be related to the size of the biopsy catheter. Pain scores appear to be significantly lower with the Pipelle biopsy catheter than with the larger Novak biopsy curette.32
Vasovagal reaction usually resolves after the procedure
As with hysteroscopy, women may occasionally experience a vasovagal reaction during endometrial biopsy. This complication usually resolves quickly once the procedure is completed.29 Some clinicians suggest that the patient be allowed to eat and drink before the procedure and be given an analgesic before it begins.28
Cervical priming is not a proven strategy
Misoprostol has been considered as a preprocedure adjunct to endometrial biopsy. Only one small randomized, controlled trial involving 42 women has evaluated the drug for this indication. It found no benefit when 400 μg of misoprostol was given orally 3 hours before the procedure, as well as cramping and increased pain during the biopsy.33 This study had several shortcomings, including its small sample size and the inclusion of both pre- and postmenopausal women. Further research is needed—separately in premenopausal and postmenopausal women and with adequately large samples—to assess the use of misoprostol.
The author reports no financial relationships relevant to this article.
CASE: Difficulty inserting a catheter suggests an unyielding cervix
A.W. is a 38-year-old nulliparous woman who seeks treatment for persistent irregular vaginal bleeding. Her physician attempts an endometrial biopsy in the office but is unable to pass the catheter through the internal cervical os. She schedules office hysteroscopy as follow-up.
What steps can the ObGyn take to reduce the difficulty of the procedure, particularly insertion of the hysteroscope through the cervical canal?
Successful hysteroscopy requires a cervical canal sufficiently dilated to allow passage of the hysteroscope. And because of inevitable variation in anatomy—and even in models of hysteroscopes, which range in diameter from 2.7 to 10 mm—passage is not always easily accomplished. Many of the complications related to hysteroscopy, including cervical tears, creation of a false passage, uterine perforation, vasovagal reaction, pain, and inability to complete the procedure, are caused by inadequate cervical dilation and an inability to insert the hysteroscope.1-6 One study noted that almost half of complications were related to cervical entry.6
In this article, I describe ways to overcome the challenging cervix for hysteroscopic procedures and endometrial biopsy (TABLES 1 and 2).
TABLE 1
10 actions that can ease entry to the cervix for hysteroscopy
| ACTION | COMMENTS |
|---|---|
| Take a careful history and perform a rigorous physical exam | Identify risk factors for cervical stenosis and assess cervical/uterine position |
| Administer an oral nonsteroidal anti-inflammatory drug 60 minutes before the procedure | Helps to reduce discomfort, especially postprocedure pain |
| Provide an anxiolytic or conscious sedation, or both | Consider this option for women who are very anxious or unlikely to tolerate pain, especially for operative procedures |
| Use a tenaculum | Consider if the uterus is not in the axial position |
| Use Hagar dilators or a lacrimal duct probe | May be helpful if mechanical dilation is necessary |
| Proceed under ultrasonographic guidance | Consider transabdominal imaging to help guide cervical dilation in difficult cases, e.g., when the patient has a history of uterine perforation |
| Opt for a smaller hysteroscope | A smaller scope will require less cervical dilation |
| Administer a paracervical block | Consider this option if cervical dilation is expected to be difficult, especially in women at risk of significant pain. Be alert for complications such as bleeding, discomfort at the time of injection, and intravascular injection leading to bradycardia and hypotension |
| Administer a topical cervical anesthetic | May be appropriate when a tenaculum is used |
| Give misoprostol to prime the cervix | Consider giving 400 μg of intravaginal misoprostol 9 to 12 hours preoperatively in premenopausal women, particularly nulliparous women and those undergoing operative hysteroscopy |
TABLE 2
6 ways to prepare the cervix for endometrial biopsy
| ACTION | COMMENTS |
|---|---|
| Take a careful history and perform a thorough physical examination | Identify risk factors for cervical stenosis and assess uterine position |
| Administer an oral nonsteroidal anti-inflammatory drug 60 minutes prior to biopsy | Helps to reduce discomfort, especially postprocedure pain |
| Use a tenaculum | May be helpful if the uterus/cervix is not in the axial position |
| Apply a topical cervical anesthetic | May help alleviate discomfort associated with use of a tenaculum |
| Use Hagar dilators or lacrimal duct probes | Provide mechanical dilation |
| Use the smallest biopsy catheter possible | Reduces degree of cervical dilation necessary |
Hysteroscopy failure rate: 3.4% to 4.2%
Hysteroscopy is, of course, common in gynecologic practice, its indications extending across a range of investigations and treatments—for menstrual disorders, postmenopausal bleeding, infertility, and recurrent pregnancy loss.1,7 Flexible hysteroscopes range in diameter from 2.7 to 5 mm; rigid hysteroscopes, from 1 to 5 mm; and operative hysteroscopes can be as large as 8 to 10 mm.2,7
A systematic review of diagnostic hysteroscopy in more than 26,000 women reported a failure rate of 4.2% for ambulatory hysteroscopy and 3.4% for inpatient procedures.4 Failed ambulatory procedures were mainly attributed to technical problems, including:
- cervical stenosis
- anatomic and structural abnormalities
- pain and intolerance.4
Ideally, hysteroscopy is performed with minimal or no cervical dilation,7 but this may not always be possible.
Things to consider before embarking
Close attention to cervical and uterine anatomy is critical because insertion of the hysteroscope can be the most difficult aspect of the procedure. A bimanual examination is imperative to assess uterine size and position. It also is useful to sound the uterus to determine its depth.
An accurate medical, gynecologic, and obstetric history is essential, including information on pregnancies, dilation and curettage, cervical procedures such as cryotherapy, and any other procedures that may increase the risk of cervical stenosis, or difficulty dilating the cervix.
Is stenosis present? Stenosis is most common in nulliparous and postmenopausal women and in those who have undergone cervical procedures such as cryotherapy. Stenosis increases the risk of laceration and uterine perforation.
Consider a mechanical dilator. When cervical dilation is difficult, a series of small Hagar or lacrimal duct dilators may be helpful (FIGURE).
FIGURE Mechanical dilation is one antidote to cervical stenosis
In challenging cases, such as cervical stenosis, mechanical dilation with a series of Hagar or lacrimal duct dilators may facilitate entry into the cervix.
Pain can be mild—or it can thwart your work
Although many women tolerate placement of a small hysteroscope without analgesia or anesthesia, pain and vasovagal reaction sometimes occur. Indeed, the level of pain experienced by the patient is a major determinant of the overall success of the procedure.3,8-10 Pain can occur when a tenaculum is used to grasp the anterior cervix, as well as during cervical dilation, injection of local anesthetic, or insertion of the hysteroscope. In some cases, a smaller scope may be all that is needed to solve the problem.11
Analgesia may not always be necessary
Some researchers have studied office hysteroscopy without analgesia or anesthesia, finding a high level of acceptance.12,13 Others have found a significant percentage of women requesting anesthesia or analgesia (16.5%)10 or requiring local anesthesia (28.8%).8
Preoperative NSAIDs may suffice. Use of oral nonsteroidal anti-inflammatory drugs (NSAIDs) 1 hour before office hysteroscopy may reduce intraoperative and postoperative pain.7 Nagele and colleagues8 compared use of mefenamic acid 1 hour before the procedure with placebo in 95 women undergoing outpatient diagnostic hysteroscopy. Mefenamic acid reduced pain at 30 and 60 minutes after—but not during—the procedure. Other studies have found that pain is reduced when an oral NSAID is taken 1 to 2 hours before insertion of an intrauterine device and before suction curettage.14,15
Other perioperative medications may help reduce discomfort and patient anxiety, including anxiolytics, such as lorazepam, analgesics, and conscious sedation.3
Paracervical block may be appropriate when pain is very likely
A number of investigators have evaluated use of paracervical anesthesia during out-patient hysteroscopy.9,13,16,17 They injected lignocaine or mepivacaine using a 21- or 22-gauge needle at 3, 5, 7, and 9 o’clock or 4 and 8 o’clock paracervically.13 One study found paracervical block to be effective in reducing the pain of tenaculum placement and insertion of the hysteroscope.17 However, some studies suggested a reduction of pain in postmenopausal women only.9 These women may be more likely to have cervical stenosis.
Paracervical block does pose a risk of complications. Studies have reported bleeding in some women16 and pain with injection of the paracervical block, as well as bradycardia and hypotension possibly secondary to intravascular injection.17
Other methods are inconsistent
Intracervical injection. Some researchers have recommended injection of local anesthetic into the cervix.13 One study found no benefit—in fact, the injection appeared to be the most painful part of the procedure.18 A case series suggested that injection of local anesthetic may be effective, but the series lacked a placebo or control arm.13
Topical intrauterine anesthetic has been investigated after administration through the channel of the hysteroscope or by a catheter passed through the cervix into the uterine cavity.13 Findings have been mixed, with some researchers demonstrating reduced pain19,20 and others showing no relief.21
Topical cervical anesthesia. Some hysteroscopists have recommended application of anesthetic cream, gel, or spray directly to the cervix immediately before the procedure.13,22 The results have been mixed, with some studies noting decreased pain overall,13 one finding decreased pain only during tenaculum placement,22 and others finding no significant reduction in pain any time during the procedure.13,23,24 A review concluded that topical cervical lignocaine spray may reduce the discomfort of tenaculum placement.13
Topical anesthesia may minimize vasovagal reaction
In one study, 1.1% of women undergoing office hysteroscopy experienced a vasovagal reaction, caused by stimulation of the parasympathetic nervous system with cervical manipulation and passage of the scope through the internal os of the cervix.25 The reaction led to hypotension and bradycardia. Several studies have suggested that a local anesthetic can reduce this complication.19,20
Cicinelli and associates found that topical local anesthesia reduced the incidence of vasovagal reaction from 32.5% in the control arm to 5%.20 They suggest that a local anesthetic be considered in selected women, such as postmenopausal patients, who are at increased risk of vasovagal attack.
In contrast, Lau and associates17 found an increased rate of bradycardia and hypotension with paracervical lignocaine (31% versus 10%), but it may have been caused by inadvertent intravascular injection.17
Researchers have also suggested that the use of smaller hysteroscopes may reduce the incidence of vasovagal reactions.26
How to prime the cervix for hysteroscopy
The use of vaginal misoprostol, a prostaglandin E1 analogue, 9 to 12 hours before hysteroscopy may help increase preprocedural cervical dilation in premenopausal women, especially in nulliparas and women undergoing operative hysteroscopy. Misoprostol, used to prevent and treat NSAID-induced gastric ulcers, is gaining favor as a cervical ripening agent. We performed a meta-analysis to assess its effectiveness in dilating the cervix and reducing the need for mechanical dilation.5
We identified 10 studies that met inclusion criteria; five of them included premenopausal women, four included postmenopausal women or women receiving a gonadotropin-releasing hormone (GnRH) agonist, and one study included both groups.5 A variety of dosing protocols were used, with dosages ranging from 100 μg to 1,000 μg of intravaginal or oral misoprostol 4 to 24 hours preoperatively (most studies evaluated the vaginal route).
We found that misoprostol significantly reduced the need for further cervical dilation, and was associated with a lower rate of cervical laceration. However, this was true only for the premenopausal group: 42.6% of premenopausal women given misoprostol needed further dilation, compared with 71.7% in the control group, and 2% of premenopausal women given misoprostol suffered cervical laceration, compared with 11% in the control group. Among postmenopausal women and those receiving a GnRH agonist, misoprostol lacked clear benefit and was associated with side effects such as nausea, diarrhea, abdominal cramping, and fever.
For every premenopausal woman who received misoprostol before hysteroscopy, one woman avoided the need for further cervical dilation. For every 12 premenopausal women receiving misoprostol, one cervical laceration was avoided.
The ideal dosing regimen could not be determined because of variations in protocols. Nor was it clear whether misoprostol had any benefit among postmenopausal women or those receiving a GnRH agonist.
Most studies of misoprostol for cervical ripening have involved intravaginal administration, with dosages of 200 μg to 400 μg given 9 to 12 hours before hysteroscopy showing the greatest benefit.
Ultrasonography may help guide dilation
Transabdominal ultrasonography has been used to guide dilation in difficult dilation and curettage procedures, and is especially useful in women with a history of uterine perforation.27 It may be helpful in cases involving difficult cervical dilation during hysteroscopy or endometrial biopsy.
Steady the cervix. A tenaculum is not always required, but its use on the anterior lip of the cervix may help steady the cervix and provide countertraction during insertion of the hysteroscope through the cervical canal, especially if the cervix is not in an axial position.7
CASE Resolved!
Because she is nulliparous and may benefit from cervical priming, the patient is given 400 μg of intravaginal misoprostol 12 hours before hysteroscopy, as well as an oral NSAID 1 hour before the procedure. A bimanual examination reveals a sharply anteverted uterus, so a topical cervical anesthetic spray is applied to the anterior cervix, and a tenaculum is placed to help straighten the uterine position. The hysteroscope passes easily through the cervical canal, making further dilation unnecessary. The procedure is completed without difficulty and is well tolerated by the patient.
Difficult entry can also hamper endometrial biopsy
Every ObGyn has used endometrial biopsy to assess abnormal uterine bleeding, postmenopausal bleeding, infertility, or recurrent pregnancy loss, or to monitor women on hormone replacement therapy28,29 —so its advantages over dilation and curettage should come as no surprise. They include the ability to perform it in an office setting, usually with minimal cervical dilation, often without anesthesia, and at less expense.28 Complications include cramping and pain,29-32 vasovagal reaction,29 bleeding,29 and inability to pass the biopsy catheter through the cervix into the uterine cavity. Another rare complication is uterine perforation.29
As with hysteroscopy, many of these complications are related to difficulty entering the uterine cavity through the cervix.
Prerequisites include thorough assessment of the uterus
As with hysteroscopy, an accurate and detailed history is necessary to identify risk factors for a difficult procedure. Assess uterine size and position with a bimanual examination. Although a tenaculum is often unnecessary, its placement on the anterior lip of the cervix may help steady the cervix and allow the catheter to pass through the cervical canal into the uterine cavity, especially if the uterus is not in the axial position.28,29 Again, it is useful to sound the uterine cavity to ascertain its depth. This may be done with the biopsy catheter.
Cervical dilation may be necessary
Even when women with cervical stenosis were excluded in one study, it was difficult to pass the Pipelle endometrial biopsy through the cervix in 41.7% of women.30
If the sampling device does not pass easily through the cervix, use a tenaculum and a lacrimal duct probe or small Hagar dilators to dilate the cervix.28
Pain may again be an issue
Almost 50% of women experience moderate or severe pain during endometrial biopsy.32 Many clinicians recommend giving an oral NSAID 60 minutes before the procedure to decrease discomfort. One study found that the use of naproxen sodium before Vabra curettage reduced the severity of pain at 30 and 60 minutes after the procedure, but did not alleviate discomfort arising during the biopsy itself.14 Another study suggested the combination of naproxen sodium and intrauterine lidocaine (5 mL of 2% lidocaine) to reduce discomfort associated with the procedure.30
Use of anesthesia is controversial
A study by Lau and colleagues17 found paracervical lignocaine to be ineffective at reducing pain during hysteroscopy and endometrial biopsy, but the drug did increase the risk of bradycardia and hypotension. Another study demonstrated a decrease in procedure-related discomfort in postmenopausal women who were given 2 mL of 2% intrauterine mepivacaine.20 These findings are similar to those of Zupi and associates.19
Consider the tool
Discomfort may be related to the size of the biopsy catheter. Pain scores appear to be significantly lower with the Pipelle biopsy catheter than with the larger Novak biopsy curette.32
Vasovagal reaction usually resolves after the procedure
As with hysteroscopy, women may occasionally experience a vasovagal reaction during endometrial biopsy. This complication usually resolves quickly once the procedure is completed.29 Some clinicians suggest that the patient be allowed to eat and drink before the procedure and be given an analgesic before it begins.28
Cervical priming is not a proven strategy
Misoprostol has been considered as a preprocedure adjunct to endometrial biopsy. Only one small randomized, controlled trial involving 42 women has evaluated the drug for this indication. It found no benefit when 400 μg of misoprostol was given orally 3 hours before the procedure, as well as cramping and increased pain during the biopsy.33 This study had several shortcomings, including its small sample size and the inclusion of both pre- and postmenopausal women. Further research is needed—separately in premenopausal and postmenopausal women and with adequately large samples—to assess the use of misoprostol.
The author reports no financial relationships relevant to this article.
1. Bradley LD. Complications in hysteroscopy: prevention, treatment and legal risk. Curr Opin Obstet Gynecol. 2002;14:409-415.
2. American College of Obstetricians and Gynecologists. ACOG technology assessment in obstetrics and gynecology, number 4, August 2005: hysteroscopy. Obstet Gynecol. 2005;106:439-442.
3. Vilos GA, Abu-Rafea B. New developments in ambulatory hysteroscopic surgery. Best Pract Res Clin Obstet Gynaecol. 2005;19:727-742.
4. Clark TJ, Voit D, Gupta JK, Hyde C, Song F, Khan KS. Accuracy of hysteroscopy in the diagnosis of endometrial cancer and hyperplasia: a systematic quantitative review. JAMA. 2002;288:1610-1621.
5. Crane JM, Healey S. Use of misoprostol before hysteroscopy: a systematic review. J Obstet Gynaecol Can. 2006;28:373-379.
6. Jansen FW, Vredevoogd CB, van Ulzen K, Hermans J, Trimbos JB, Trimbos-Kemper TC. Complications of hysteroscopy: a prospective, multicenter study. Obstet Gynecol. 2000;96:266-270.
7. Guido R, Stovall D. Hysteroscopy Version 14.3. UpToDate [cited February 15, 2007]; Available from: www.uptodate.com.
8. Nagele F, Lockwood G, Magos AL. Randomised placebo controlled trial of mefenamic acid for premedication at outpatient hysteroscopy: a pilot study. Br J Obstet Gynaecol. 1997;104:842-844.
9. Cicinelli E, Didonna T, Schonauer LM, Stragapede S, Falco N, Pansini N. Paracervical anesthesia for hysteroscopy and endometrial biopsy in postmenopausal women. A randomized, double-blind, placebo-controlled study. J Reprod Med. 1998;43:1014-1018.
10. De Iaco P, Marabini A, Stefanetti M, Del Vecchio C, Bovicelli L. Acceptability and pain of outpatient hysteroscopy. J Am Assoc Gynecol Laparosc. 2000;7:71-75.
11. Marsh F, Jackson T, Duffy S. A case controlled study comparing 3.6 mm and 3.1 mm flexible hysteroscopes. Gynaecol Endosc. 2002;11:393-396.
12. Lau WC, Ho RY, Tsang MK, Yuen PM. Patient’s acceptance of outpatient hysteroscopy. Gynecol Obstet Invest. 1999;47:191-193.
13. Hassan L, Gannon MJ. Anaesthesia and analgesia for ambulatory hysteroscopic surgery. Best Pract Res Clin Obstet Gynaecol. 2005;19:681-691.
14. Siddle NC, Young O, Sledmere CM, Reading AE, Whitehead MI. A controlled trial of naproxen sodium for relief of pain associated with Vabra suction curettage. Br J Obstet Gynaecol. 1983;90:864-869.
15. Edgren RA, Morton CJ. Naproxen sodium for Ob/Gyn use, with special reference to pain states: a review. Int J Fertil. 1986;31:135-142.
16. Giorda G, Scarabelli C, Franceschi S, Campagnutta E. Feasibility and pain control in outpatient hysteroscopy in postmenopausal women: a randomized trial. Acta Obstet Gynecol Scand. 2000;79:593-597.
17. Lau WC, Lo WK, Tam WH, Yuen PM. Paracervical anaesthesia in outpatient hysteroscopy: a randomised double-blind placebo-controlled trial. Br J Obstet Gynaecol. 1999;106:356-359.
18. Broadbent JA, Hill NC, Molnar BG, Rolfe KJ, Magos AL. Randomized placebo controlled trial to assess the role of intracervical lignocaine in outpatient hysteroscopy. Br J Obstet Gynaecol. 1992;99:777-779.
19. Zupi E, Luciano AA, Valli E, Marconi D, Maneschi F, Romanini C. The use of topical anesthesia in diagnostic hysteroscopy and endometrial biopsy. Fertil Steril. 1995;63:414-416.
20. Cicinelli E, Didonna T, Ambrosi G, Schonauer LM, Fiore G, Matteo MG. Topical anaesthesia for diagnostic hysteroscopy and endometrial biopsy in postmenopausal women: a randomised placebo-controlled double-blind study. Br J Obstet Gynaecol. 1997;104:316-319.
21. Lau WC, Tam WH, Lo WK, Yuen PM. A randomised double-blind placebo-controlled trial of transcervical intrauterine local anaesthesia in outpatient hysteroscopy. BJOG. 2000;107:610-613.
22. Davies A, Richardson RE, O’Connor H, Baskett TF, Nagele F, Magos AL. Lignocaine aerosol spray in outpatient hysteroscopy: a randomized double-blind placebo-controlled trial. Fertil Steril. 1997;67:1019-1023.
23. Clark S, Vonau B, Macdonald R. Topical anaesthesia in out-patient hysteroscopy. Gynaecol Endosc. 1996;5:141-144.
24. Wong AY, Wong K, Tang LC. Stepwise pain score analysis of the effect of local lignocaine on outpatient hysteroscopy: a randomized, double-blind, placebo-controlled trial. Fertil Steril. 2000;73:1234-1237.
25. Bellingham FR. Outpatient hysteroscopy—problems. Aust N Z J Obstet Gynaecol. 1997;37:202-205.
26. Cicinelli E, Schonauer LM, Barba B, Tartagni M, Luisi D, Di Naro E. Tolerability and cardiovascular complications of outpatient diagnostic minihysteroscopy compared with conventional hysteroscopy. J Am Assoc Gynecol Laparosc. 2003;10:399-402.
27. Hunter RE, Reuter K, Kopin E. Use of ultrasonography in the difficult postmenopausal dilation and curettage. Obstet Gynecol. 1989;73:813-816.
28. Guido R, Stovall D. Endometrial sampling procedures Version 14.3. UpToDate [cited February 15, 2007]; Available from: www.uptodate.com.
29. Cooper JM, Erickson ML. Endometrial sampling techniques in the diagnosis of abnormal uterine bleeding. Obstet Gynecol Clin North Am. 2000;27:235-244.
30. Dogan E, Celiloglu M, Sarihan E, Demir A. Anesthetic effect of intrauterine lidocaine plus naproxen sodium in endometrial biopsy. Obstet Gynecol. 2004;103:347-351.
31. Trolice MP, Fishburne C, Jr, McGrady S. Anesthetic efficacy of intrauterine lidocaine for endometrial biopsy: a randomized double-masked trial. Obstet Gynecol. 2000;95:345-347.
32. Silver MM, Miles P, Rosa C. Comparison of Novak and Pipelle endometrial biopsy instruments. Obstet Gynecol. 1991;78:828-830.
33. Perrone JF, Caldito G, Mailhes JB, Tucker AN, Ford WR, London SN. Oral misoprostol before office endometrial biopsy. Obstet Gynecol. 2002;99:439-444.
1. Bradley LD. Complications in hysteroscopy: prevention, treatment and legal risk. Curr Opin Obstet Gynecol. 2002;14:409-415.
2. American College of Obstetricians and Gynecologists. ACOG technology assessment in obstetrics and gynecology, number 4, August 2005: hysteroscopy. Obstet Gynecol. 2005;106:439-442.
3. Vilos GA, Abu-Rafea B. New developments in ambulatory hysteroscopic surgery. Best Pract Res Clin Obstet Gynaecol. 2005;19:727-742.
4. Clark TJ, Voit D, Gupta JK, Hyde C, Song F, Khan KS. Accuracy of hysteroscopy in the diagnosis of endometrial cancer and hyperplasia: a systematic quantitative review. JAMA. 2002;288:1610-1621.
5. Crane JM, Healey S. Use of misoprostol before hysteroscopy: a systematic review. J Obstet Gynaecol Can. 2006;28:373-379.
6. Jansen FW, Vredevoogd CB, van Ulzen K, Hermans J, Trimbos JB, Trimbos-Kemper TC. Complications of hysteroscopy: a prospective, multicenter study. Obstet Gynecol. 2000;96:266-270.
7. Guido R, Stovall D. Hysteroscopy Version 14.3. UpToDate [cited February 15, 2007]; Available from: www.uptodate.com.
8. Nagele F, Lockwood G, Magos AL. Randomised placebo controlled trial of mefenamic acid for premedication at outpatient hysteroscopy: a pilot study. Br J Obstet Gynaecol. 1997;104:842-844.
9. Cicinelli E, Didonna T, Schonauer LM, Stragapede S, Falco N, Pansini N. Paracervical anesthesia for hysteroscopy and endometrial biopsy in postmenopausal women. A randomized, double-blind, placebo-controlled study. J Reprod Med. 1998;43:1014-1018.
10. De Iaco P, Marabini A, Stefanetti M, Del Vecchio C, Bovicelli L. Acceptability and pain of outpatient hysteroscopy. J Am Assoc Gynecol Laparosc. 2000;7:71-75.
11. Marsh F, Jackson T, Duffy S. A case controlled study comparing 3.6 mm and 3.1 mm flexible hysteroscopes. Gynaecol Endosc. 2002;11:393-396.
12. Lau WC, Ho RY, Tsang MK, Yuen PM. Patient’s acceptance of outpatient hysteroscopy. Gynecol Obstet Invest. 1999;47:191-193.
13. Hassan L, Gannon MJ. Anaesthesia and analgesia for ambulatory hysteroscopic surgery. Best Pract Res Clin Obstet Gynaecol. 2005;19:681-691.
14. Siddle NC, Young O, Sledmere CM, Reading AE, Whitehead MI. A controlled trial of naproxen sodium for relief of pain associated with Vabra suction curettage. Br J Obstet Gynaecol. 1983;90:864-869.
15. Edgren RA, Morton CJ. Naproxen sodium for Ob/Gyn use, with special reference to pain states: a review. Int J Fertil. 1986;31:135-142.
16. Giorda G, Scarabelli C, Franceschi S, Campagnutta E. Feasibility and pain control in outpatient hysteroscopy in postmenopausal women: a randomized trial. Acta Obstet Gynecol Scand. 2000;79:593-597.
17. Lau WC, Lo WK, Tam WH, Yuen PM. Paracervical anaesthesia in outpatient hysteroscopy: a randomised double-blind placebo-controlled trial. Br J Obstet Gynaecol. 1999;106:356-359.
18. Broadbent JA, Hill NC, Molnar BG, Rolfe KJ, Magos AL. Randomized placebo controlled trial to assess the role of intracervical lignocaine in outpatient hysteroscopy. Br J Obstet Gynaecol. 1992;99:777-779.
19. Zupi E, Luciano AA, Valli E, Marconi D, Maneschi F, Romanini C. The use of topical anesthesia in diagnostic hysteroscopy and endometrial biopsy. Fertil Steril. 1995;63:414-416.
20. Cicinelli E, Didonna T, Ambrosi G, Schonauer LM, Fiore G, Matteo MG. Topical anaesthesia for diagnostic hysteroscopy and endometrial biopsy in postmenopausal women: a randomised placebo-controlled double-blind study. Br J Obstet Gynaecol. 1997;104:316-319.
21. Lau WC, Tam WH, Lo WK, Yuen PM. A randomised double-blind placebo-controlled trial of transcervical intrauterine local anaesthesia in outpatient hysteroscopy. BJOG. 2000;107:610-613.
22. Davies A, Richardson RE, O’Connor H, Baskett TF, Nagele F, Magos AL. Lignocaine aerosol spray in outpatient hysteroscopy: a randomized double-blind placebo-controlled trial. Fertil Steril. 1997;67:1019-1023.
23. Clark S, Vonau B, Macdonald R. Topical anaesthesia in out-patient hysteroscopy. Gynaecol Endosc. 1996;5:141-144.
24. Wong AY, Wong K, Tang LC. Stepwise pain score analysis of the effect of local lignocaine on outpatient hysteroscopy: a randomized, double-blind, placebo-controlled trial. Fertil Steril. 2000;73:1234-1237.
25. Bellingham FR. Outpatient hysteroscopy—problems. Aust N Z J Obstet Gynaecol. 1997;37:202-205.
26. Cicinelli E, Schonauer LM, Barba B, Tartagni M, Luisi D, Di Naro E. Tolerability and cardiovascular complications of outpatient diagnostic minihysteroscopy compared with conventional hysteroscopy. J Am Assoc Gynecol Laparosc. 2003;10:399-402.
27. Hunter RE, Reuter K, Kopin E. Use of ultrasonography in the difficult postmenopausal dilation and curettage. Obstet Gynecol. 1989;73:813-816.
28. Guido R, Stovall D. Endometrial sampling procedures Version 14.3. UpToDate [cited February 15, 2007]; Available from: www.uptodate.com.
29. Cooper JM, Erickson ML. Endometrial sampling techniques in the diagnosis of abnormal uterine bleeding. Obstet Gynecol Clin North Am. 2000;27:235-244.
30. Dogan E, Celiloglu M, Sarihan E, Demir A. Anesthetic effect of intrauterine lidocaine plus naproxen sodium in endometrial biopsy. Obstet Gynecol. 2004;103:347-351.
31. Trolice MP, Fishburne C, Jr, McGrady S. Anesthetic efficacy of intrauterine lidocaine for endometrial biopsy: a randomized double-masked trial. Obstet Gynecol. 2000;95:345-347.
32. Silver MM, Miles P, Rosa C. Comparison of Novak and Pipelle endometrial biopsy instruments. Obstet Gynecol. 1991;78:828-830.
33. Perrone JF, Caldito G, Mailhes JB, Tucker AN, Ford WR, London SN. Oral misoprostol before office endometrial biopsy. Obstet Gynecol. 2002;99:439-444.
What you need to know about medication safety in pregnancy
The author reports no financial relationships relevant to this article.
Fifty years ago, the thalidomide experience—a high incidence of major birth defects following prenatal use of the drug—made clear the devastating potential of drug exposure during pregnancy. Since that disaster, healthcare providers and patients have adopted a conservative approach to medication use during pregnancy, especially during the first trimester and lactation. That is a wise strategy, although very few medications are associated with abnormal fetal development.
In this article, I’ll guide you through some of the issues that must be considered when assessing a drug’s teratogenicity, help you find information on a host of medications, and familiarize you with some of the challenges involved in counseling the patient. I also present a table listing the adverse effects known to be associated with selected drugs during the first, second, and third trimesters and lactation (TABLE). We are fortunate that a large body of information about medication use during pregnancy and lactation is readily available on the Web and in books and medical journals. This information is far from definitive, however, because much of the evidence concerning prescribed drugs is anecdotal or presented with insufficient warning about their use during pregnancy and lactation.
A discussion of these issues with the patient will help set the risks and benefits of a particular drug into proper perspective, alleviate fears, and improve compliance. Nonprescription medications should also be discussed, and the patient should be advised that we have very little data concerning their use during pregnancy.
Assignment of risk is an uncertain science
Major structural defects are apparent at birth in about 3% of all pregnancies and in about 4.5% of all children by the age of 5 years.1 A cause or proposed mechanism for the defects can be determined in fewer than 50% of these cases. Nor can we count on expert consensus about the safety of many medications during pregnancy because it rarely occurs and, in some cases, may be impossible to achieve.
Animal studies are the means of assessing the teratogenicity of most drugs. Animals commonly used to study fetal effects include rodents (fertility, birth defects, birth weight, behavior), rabbits (birth defects), baboons (uterine blood flow), and sheep (uterine blood flow, cardiovascular effects, fetal hypoxia, and acidosis). Dosages are often much higher (in relation to body weight or surface area) to “test the systems” for any possible reproductive harm. Although these studies may be helpful, they do not reliably predict the human response.
Even when humans are the subject of study, conclusions must be viewed with caution. To determine the risk of teratogenesis, it is necessary to know the stage of development during which the exposure occurred, as well as the identity and dose of the medication and the genetic susceptibility of the mother and fetus.
Three critical stages. In utero exposure to a drug occurs in one of three periods of fetal development:
- ovum – from fertilization to implantation
- embryo – from the second through the eighth week of gestation
- fetus – from the eighth completed week until delivery.
An “all-or-none” effect (i.e., spontaneous abortion or not) is believed to arise from exposure during the first period, but the embryo stage is the most critical time because it involves organogenesis. Detrimental effects may occur even beyond this period as cells continue to divide in the hematologic, reproductive, and central nervous systems.
Many fine points of exposure are difficult to clarify
Retrospective and uncontrolled studies, as well as individual case reports or small series, may overestimate the risk to the fetus of exposure to a specific drug or combination of medications. Case reports do not establish causation.
It can also be difficult to differentiate between the risks of a specific drug and the hazards of maternal illness to explain an unfavorable outcome. For example, is a particular case of stillbirth the result of fetal exposure to enoxaparin or maternal thrombophilia, or both? Can fetal growth restriction be attributed to use of azathioprine during pregnancy or to the mother’s underlying illness? And so on.
In addition, it is necessary to distinguish between a defect’s natural prevalence—i.e., the rate at which it occurs in a population—and the additional risk posed by exposure to a particular drug. Studies in large populations are needed—but usually unattainable—to determine the relative risk from specific potential teratogens.
Finally, it is very difficult to assess neurobehavioral effects of in utero exposure to centrally acting drugs beyond the immediate neonatal period. The dose, offspring’s age and gender, and behavioral test system must all be considered.
Few drugs are implicated in restricting fetal growth or reducing organ size. We also lack consistent information about long-term effects such as learning or behavioral problems (i.e., functional teratogenesis) that may result from chronic prenatal exposure to a certain medication.
In 1979, the Food and Drug Administration created five pregnancy risk categories to be used by manufacturers to rate their products in the drug formulary for use during pregnancy: categories A, B, C, D, and X, which range from no evidence of damage to the fetus (category A) to clear teratogenicity (D and X).
The D rating is generally reserved for drugs with no safer alternatives, such as secobarbital, doxycycline, and lorazepam. The X rating means there is absolutely no reason to risk using the drug in pregnancy, as in the case of oral contraceptives, benzodiazepines, and misoprostol.
Approximately 2% of drugs fall into category A, 50% in category B, 38% in category C, 3–5% in category D, and 1–5% in category X.3 These categories do not often accurately reflect the available information on risk to the fetus. A major initiative is under way to declare these categories obsolete and provide more informative drug labeling. Pregnancy labels of the future will likely address three important areas:
- clinical considerations–issues relevant to prescription of a particular drug in pregnancy, including the risk of disease versus no treatment. Also included will be information of use when counseling a patient whose fetus was inadvertently exposed to a medication in early gestation
- summary risk assessment–a narrative text that describes, as comprehensively as possible, the risk of exposure based on animal and human data
- data to support the assessment.
All drugs cross the placenta
Most medications are easily absorbed during pregnancy, and serum concentrations of albumin for drug binding are lower than in the nonpregnant state. Pharmacokinetic changes during pregnancy include:
- higher volume of distribution
- lower maximum plasma concentration
- lower steady-state serum concentration
- shorter plasma half-life
- higher clearance rate.1
The small spatial configuration and high lipid solubility of most medications permit easy transfer of an unbound drug or its metabolite across the placenta or into breast milk. Virtually all drugs and their end products cross the placenta, with unbound concentrations of the drug in the fetal serum similar to the level in maternal serum—sometimes even higher (FIGURE).
A few drugs with high molecular weight do not cross the placenta in significant amounts (e.g., glyburide, interferon, thyroid supplements, insulin).
FIGURE An elaborate nutrient (and drug) delivery system
The placenta and umbilical cord deliver the nutrients and oxygen the fetus needs for normal growth—as well as most medications used by the mother.
Medication use tends to increase as pregnancy progresses
The drugs most commonly taken during pregnancy include vitamins, iron preparations, calcium, analgesics, antibiotics, and antacids. Excluding vitamins and mineral supplements, an average of one to two medications are taken during gestation. Over-the-counter formulations account for about half of these drugs, with acetaminophen being the single most commonly used medication during pregnancy. Antibiotics are the most widely prescribed drugs.
Although caffeine, tobacco, alcohol, and illicit substance use tends to diminish as pregnancy progresses, medications are usually taken at the same frequency or more often during gestation.
My colleagues and I found a significantly higher mean number of medications (3.3 and 4.1, respectively) used during the second and third trimesters of gestation than were taken before pregnancy (2.6).2
How to counsel the patient
Counseling a woman before or during pregnancy about the continuation or initiation of a medication should take place in an open, supportive, and informative manner. Most inquiries relate to exposures involving very low levels of relative and absolute risk.
A detailed fetal ultrasonographic examination is often used to accurately date the pregnancy and, if possible, screen for any structural defects. The patient should be advised that first-trimester screening, chorionic villus sampling, maternal serum quadruple screening, amniocentesis, and fetal blood sampling are not very predictive of a drug’s fetal effects. Exceptions may be the observation of open neural tube defects (approximate 1% risk associated with valproic acid and carbamazepine) by maternal serum quadruple screening and facial clefting by targeted ultrasonography.
When a patient inquires about a particular drug, it is important to gather the following information:
- When did she take the medication?
- Why did she take it?
- For how long did she take the medication?
- Did she take other medications, or any substances of abuse, at the same time?
A number of sources of information about potential teratogens are available.3-5 These include national computerized databases that are accessible on the Web:
- National Library of Medicine (http://sis.nlm.nih.gov/enviro.html)
- pregnancy exposure registries (www.fda.gov/womens/registries/default.htm)
- Reproductive Toxicology Center (http://reprotox.org) (access requires a paid subscription)
- LactMed, National Library of Medicine guide to drug safety in lactation (http://toxnet.nlm.nih.gov/cgi-bin/sis/htmlgen?LACT)
- Organization of Teratology Information Specialists (OTIS) (www.otispregnancy.org).
The last source (OTIS) consolidates teratology information nationwide and reports it by state or region. It also publishes a host of fact sheets on various drugs that may be useful to dispense to the patient during counseling. In addition, many teratogen information services or poison control centers (often at children’s hospitals) are available throughout the United States to serve specific geographic areas. And teratogen registries at pharmaceutical companies may provide limited information about newer medications.
The Physician’s Desk Reference (PDR) is a common source of information about the use of prescription drugs in pregnancy.3 But be aware that, to avoid liability, pharmaceutical manufacturers do not encourage use of their drugs during pregnancy unless the benefit clearly outweighs the risk. It would be unrealistic for them to market a medication for specific use during pregnancy because it would require considerable time and cost, and raise ethical objections, to conduct research in a vulnerable population that is limited in number.
Effects of agents used more than 40 years ago were reported by the Collaborative Perinatal Project or the Boston Collaborative Drug Surveillance Program.6 Those findings are often inconclusive, reflect bias in study designs, and do not help a clinician evaluate current medications or those less commonly prescribed during pregnancy.
The risks and experience associated with new drugs are usually not well explored in regard to pregnancy. As a result, older medications are more likely to be prescribed as maintenance therapy during gestation for the simple reason that they have a larger body of information regarding their effects. These older drugs may no longer be preferred once the patient delivers.
Most drugs are not teratogens
The TABLE lists adverse effects in the human fetus known to arise from exposure to specific drugs. The information comes largely from the Reprotox database, which was reviewed as recently as 2006, describes human data only, and is reported by first trimester (anomalies, abortion) and the second and third trimesters (fetal growth restriction, stillbirth, low birth weight, preterm delivery, immediate neonatal problems).7 Typical dosages of most drugs are not anticipated to increase the risk of congenital anomalies.
Most human data come from small series or case reports. Although these types of studies are helpful, they tend to be biased or reflect the pregnancy’s background risk of birth defects rather than the risk posed by a specific drug. In addition, case reports of malformation after prenatal exposure to a certain drug may involve exposures to other agents and a lack of uniformity of abnormalities, making the association between adverse effects and a single agent unlikely. Dissimilarities in the dosage and route of delivery also limit interpretation. for example, short-term intravenous or sublingual administration of a drug may pose a different risk than taking that medication orally or vaginally, in a lower dose, for a longer period, or at a different period of gestation.
Randomized controlled trials of drugs are rare during pregnancy, as are prospective cohort investigations. Because a control population is often impossible to identify, it becomes difficult to separate any heightened risk identified during use of the medication from the underlying disease. When the gravida has significant medical problems, it is important to assess the potential risk of a drug—or its omission—in her as well as her fetus. The lowest effective dose is preferred, but keep in mind that inadequate treatment may lead to minimal benefit and potentially greater risk to the pregnancy.
When reviewing or planning maintenance drug therapy, follow the same principles as you would in a nonpregnant patient. Be familiar with more than one medication for each disorder. Also be aware that some drugs may need to be prescribed at a higher dose or greater frequency to attain a therapeutic concentration in the expanded intravascular volume of pregnancy. In addition, side effects such as nausea, fatigue, and gastrointestinal disturbance may mimic symptoms arising from physiologic changes of pregnancy.
Assessing the risks associated with over-the-counter medications and natural food products is even harder. The PDR for Nonprescription Drugs, Dietary Supplements, and Herbs8 contains little or no information about the reproductive hazards of most of these products. Many agents contain multiple ingredients, both active and inactive, thereby complicating counseling about their risks during pregnancy. Although the recommended dosage is usually low, many product labels do not specify what it should be.
Most drugs enter breast milk
The amount of drug that an infant consumes from breast milk depends on the medication’s chemical properties as well as the dosage, frequency, and duration of exposure.2 Contraindications and cautions are usually either theoretical or based on findings from case reports that often conflict or confuse. In theory, it is safer for the mother to take the medication just after infant feeding or just before the infant’s longest sleep period.
The TABLE also lists the effects of drugs in the breastfed human infant. Again, the information comes from the Reprotox database, access to which requires a subscription. For additional information, try the free LactMed site at http://toxnet.nlm.nih.gov/cgi-bin/sis/htmlgen?LACT.
Nearly all drugs are excreted in breast milk, usually in small amounts (often less than 5% of the weight-adjusted maternal daily dose). The amount of drug or metabolite in an infant’s serum also is determined by the volume of breast milk, age of the infant, and other exposures.
Avoid prescribing multiple medications, if possible, and choose “safe” drugs from among the options in categories that include a number of teratogenic medications, such as anticonvulsants.
Determine the best method to monitor therapy. For example, use a peak flow meter for asthma, a portable blood pressure monitor for hypertension, and so on.
Focus on keeping the patient healthy. The healthiest mother is most likely to deliver the healthiest infant.
Keep the underlying disorder in mind, as well as the drug, when choosing a drug.
Know which drugs are clearly linked to birth defects. These include phenytoin, warfarin, alcohol, methotrexate, diethylstilbestrol, cis-retinoic acid, valproic acid, and carbamazepine.
Pay special attention to the first trimester. Too little is known about the first-trimester effects of the vast majority of drugs for them to be considered safe.
Suspect a drug-related effect
A medication may be the cause in any newborn manifesting signs of anemia, hepatitis, hepatotoxicity, hepatorenal dysfunction, and hyperbilirubinemia. This includes breastfed infants. An adverse drug-related effect should also be suspected when an infant exhibits signs of jaundice, floppiness, jitteriness, poor suck, diarrhea, or growth restriction.
Reference
1. American College of Obstetricians and Gynecologists. Teratology. ACOG Educational Bulletin #236. Washington, DC: ACOG; 1997.
2. Splinter M, Nightingale B, Sawgraves R, Rayburn W. Medication use during pregnancy by women delivering at a tertiary university hospital. South Med J. 1997;90:498-502.
3. Physician’s Desk Reference. 61st ed. Montvale, NJ: Medical Economics; 2007.
4. Briggs GG, Freeman RK, Yaffee FJ. Drugs in Pregnancy and Lactation: Reference Guide to Fetal and Neonatal Risk. 7th ed. Baltimore: Williams & Wilkins; 2005.
5. Briggs GG, Freeman RK, Yaffee FJ. ReproTox Database. Vol. 13. No. 1. Bethesda, Md: Reproductive Toxicology Center; 2000.
6. Heinonen OP, Sloan ED, Shapiro S. Birth Defects and Drugs in Pregnancy. Boston: John Wright PSG; 1973.
7. Reproductive Toxicology Center. Bethesda, Md. Available at http://reprotox.org. Accessed Oct. 5, 2007.
8. PDR for Nonprescription Drugs, Dietary Supplements, and Herbs. 28th ed. Montvale, NJ: Medical Economics; 2007.
<b>How selected drugs affect the human fetus and breastfed infant</b><cs cn="1" a="l"> <cs cn="2" a="l"> <cs cn="3" a="l"> <cs cn="4" a="l"> </cs></cs></cs></cs><row><entry v="t">DRUG</entry> <entry v="t">FIRST-TRIMESTER EFFECTS</entry> <entry v="t">EFFECTS DURING SECOND AND THIRD TRIMESTER</entry> <entry v="t">SAFETY DURING BREASTFEEDING</entry></row><row><entry cs="4">ANALGESICS</entry></row><row><entry v="t">Acetaminophen</entry> <entry v="t">None known</entry> <entry v="t">Hepatotoxicity/nephrotoxicity</entry> <entry v="t">Safe</entry></row><row><entry v="t">Ibuprofen</entry> <entry v="t">Gastroschisis (?)</entry> <entry v="t">Closure of ductus</entry> <entry v="t">Small amount passed; no other information</entry></row><row><entry v="t">Narcotics</entry> <entry v="t">None known</entry> <entry v="t">Depression, withdrawal</entry> <entry v="t">Not recommended if dosing is repetitive</entry></row><row><entry v="t">Salicylates</entry> <entry v="t">None known</entry> <entry v="t">Prolonged pregnancy and labor, hemorrhage, altered hemostasis, intracranial hemorrhage</entry> <entry v="t">Use with caution; may have adverse effects in newborn</entry></row><row><entry cs="4">ANESTHETICS</entry></row><row><entry v="t">General</entry> <entry v="t">Anomalies (?), abortion (?)</entry> <entry v="t">Depression</entry> <entry v="t"> </entry></row><row><entry v="t">Local</entry> <entry v="t">None known</entry> <entry v="t">Bradycardia, seizures</entry> <entry v="t"> </entry></row><row><entry cs="4">ANTI-ASTHMATICS</entry></row><row><entry v="t">Metaproterenol, salmeterol, albuterol</entry> <entry v="t">None known</entry> <entry v="t">None known</entry> <entry v="t">No information available</entry></row><row><entry v="t">Terbutaline</entry> <entry v="t">None known</entry> <entry v="t">Tachycardia, hypothermia, hypocalcemia, hypoglycemia, and hyperglycemia</entry> <entry v="t">Compatible</entry></row><row><entry v="t">Theophylline</entry> <entry v="t">None known</entry> <entry v="t">Jitteriness, tachycardia</entry> <entry v="t">May produce jitteriness, poor feeding, vomiting, cardiac arrhythmias</entry></row><row><entry cs="4">ANTICOAGULANTS</entry></row><row><entry v="t">Warfarin</entry> <entry v="t">Nasal hypoplasia, ophthalmic abnormalities, epiphyseal stippling</entry> <entry v="t">Hemorrhage, stillbirth</entry> <entry v="t">Safe</entry></row><row><entry v="t">Heparin, low molecular weight</entry> <entry v="t">None known</entry> <entry v="t">Hemorrhage (?), stillbirth (?)</entry> <entry v="t">Safe</entry></row><row><entry cs="4">ANTICONVULSANTS</entry></row><row><entry v="t">Barbiturates</entry> <entry v="t">Malformations (?)</entry> <entry v="t">Bleeding, withdrawal</entry> <entry v="t">Not recommended</entry></row><row><entry v="t">Carbamazepine, oxcarbazepine</entry> <entry v="t">Craniofacial, neural tube (?)</entry> <entry v="t">Bleeding, withdrawal, growth restriction</entry> <entry v="t">Probably safe</entry></row><row><entry v="t">Clonazepam</entry> <entry v="t">None known</entry> <entry v="t">Withdrawal, depression</entry> <entry v="t">Not recommended (potential for apnea, cyanosis, or hypotonia); serum levels should be monitored</entry></row><row><entry v="t">Ethosuximide</entry> <entry v="t">None known</entry> <entry v="t">None known</entry> <entry v="t">Compatible</entry></row><row><entry v="t">Gabapentin</entry> <entry v="t">Unknown</entry> <entry v="t">None known</entry> <entry v="t">Unknown</entry></row><row><entry v="t">Phenytoin*</entry> <entry v="t">Craniofacial abnormalities, mental retardation, hypoplasia of phalanges</entry> <entry v="t">Hemorrhage, depletion of vitamin K-dependent clotting factors</entry> <entry v="t">Probably safe</entry></row><row><entry v="t">Primidone</entry> <entry v="t">Orofacial clefts</entry> <entry v="t">Hemorrhage, depletion of vitamin K-dependent clotting factors, intrauterine growth restriction</entry> <entry v="t">May produce significant adverse effects in infants; use with caution</entry></row><row><entry v="t">Trimethadione*</entry> <entry v="t">Mental retardation, facial dysmorphogenesis, cardiovascular effects</entry> <entry v="t">Hemorrhage, depletion of vitamin K-dependent clotting factors, intrauterine growth restriction</entry> <entry v="t">No information available</entry></row><row><entry v="t">Valproic acid*</entry> <entry v="t">Spina bifida, facial dysmorphogenesis</entry> <entry v="t">Perinatal distress, behavioral abnormalities</entry> <entry v="t">Safe</entry></row><row><entry cs="4">ANTI-EMETICS</entry></row><row><entry v="t">Diphenhydramine</entry> <entry v="t">None known, clefting unlikely</entry> <entry v="t">None known</entry> <entry v="t">Safe, but may cause drowsiness</entry></row><row><entry v="t">Doxylamine (with pyridoxine)</entry> <entry v="t">None known</entry> <entry v="t">None known</entry> <entry v="t">Unknown; probably sedating</entry></row><row><entry v="t">Meclizine</entry> <entry v="t">None known</entry> <entry v="t">Retrolental fibrosis in premature infant</entry> <entry v="t">Unknown</entry></row><row><entry v="t">Metoclopramide</entry> <entry v="t">None known</entry> <entry v="t">None known</entry> <entry v="t">Potential central nervous system effects</entry></row><row><entry v="t">Ondansetron</entry> <entry v="t">Unknown</entry> <entry v="t">Unknown</entry> <entry v="t">Unknown</entry></row><row><entry v="t">Promethazine</entry> <entry v="t">None known</entry> <entry v="t">None known</entry> <entry v="t">Compatible</entry></row><row><entry v="t">Scopolamine</entry> <entry v="t">None known</entry> <entry v="t">Fetal heart rate changes</entry> <entry v="t">Compatible</entry></row><row><entry cs="4">ANTIBACTERIALS</entry></row><row><entry v="t">Aminoglycosides</entry> <entry v="t">None known</entry> <entry v="t">Nephrotoxic (?), ototoxic (?)</entry> <entry v="t">Depends on level of exposure and renal function of infant</entry></row><row><entry v="t">Azithromycin</entry> <entry v="t">None known</entry> <entry v="t">None known</entry> <entry v="t">Compatible</entry></row><row><entry v="t">Cephalosporins</entry> <entry v="t">None known</entry> <entry v="t">None known</entry> <entry v="t">Probably Compatible</entry></row><row><entry v="t">Chloramphenicol</entry> <entry v="t">None known</entry> <entry v="t">Vascular collapse</entry> <entry v="t">Contraindicated</entry></row><row><entry v="t">Ciprofloxacin</entry> <entry v="t">Toxic to developing cartilage (?)</entry> <entry v="t">Toxic to developing cartilage (?)</entry> <entry v="t">Compatible</entry></row><row><entry v="t">Clindamycin</entry> <entry v="t">Unknown</entry> <entry v="t">Unknown</entry> <entry v="t">Compatible, but potential modification of bowel flora, interference with culture interpretation after fever work-up in infants</entry></row><row><entry v="t">Erythromycin</entry> <entry v="t">None known</entry> <entry v="t">None known</entry> <entry v="t">Compatible</entry></row><row><entry v="t">Isoniazid</entry> <entry v="t">Malformations (?)</entry> <entry v="t">Behavioral abnormality</entry> <entry v="t">Compatible</entry></row><row><entry v="t">Metronidazole</entry> <entry v="t">None known</entry> <entry v="t">None known</entry> <entry v="t">Use with caution because of mutagenic and carcinogenic effects in some species; abstain from breastfeeding for 12–24 hours after single dose</entry></row><row><entry v="t">Nitrofurantoin</entry> <entry v="t">None known</entry> <entry v="t">Hemolysis (?)</entry> <entry v="t">Compatible</entry></row><row><entry v="t">Penicillins</entry> <entry v="t">None known</entry> <entry v="t">None known</entry> <entry v="t">Compatible</entry></row><row><entry v="t">Rifampin</entry> <entry v="t">Risk of malformation not greater than in general population</entry> <entry v="t">None known</entry> <entry v="t">Compatible</entry></row><row><entry v="t">Sulfonamides</entry> <entry v="t">None known</entry> <entry v="t">None known</entry> <entry v="t">Generally Compatible, but avoid in infants with hyperbilirubinemia, premature infants, and infants with G6PD deficiency</entry></row><row><entry v="t">Tetracyclines</entry> <entry v="t">None known</entry> <entry v="t">Stained deciduous teeth (enamel hypoplasia)</entry> <entry v="t">Compatible</entry></row><row><entry v="t">Trimethoprim</entry> <entry v="t">Cleft palate, micrognathia, limb shortening</entry> <entry v="t">Unknown</entry> <entry v="t">Compatible</entry></row><row><entry cs="4">ANTIFUNGALS</entry></row><row><entry v="t">Amphotericin-B</entry> <entry v="t">Unknown</entry> <entry v="t">Unknown</entry> <entry v="t">Unknown</entry></row><row><entry v="t">Fluconazole</entry> <entry v="t">None known</entry> <entry v="t">None known</entry> <entry v="t">Compatible</entry></row><row><entry cs="4">ANTIRETROVIRALS</entry></row><row><entry v="t">Class of drugs in general</entry> <entry v="t">None known</entry> <entry v="t">None known</entry> <entry v="t">Contraindicated (HIV)</entry></row><row><entry cs="4">ANTIVIRALS</entry></row><row><entry v="t">Acyclovir</entry> <entry v="t">None known</entry> <entry v="t">None known</entry> <entry v="t">Compatible</entry></row><row><entry v="t">Interferon</entry> <entry v="t">None known</entry> <entry v="t">Intrauterine growth restriction (?)</entry> <entry v="t">Likely safe</entry></row><row><entry cs="4">CANCER CHEMOTHERAPY</entry></row><row><entry v="t">Alkylating agents</entry> <entry v="t">Abortion, anomalies</entry> <entry v="t">Hypoplastic gonads, growth restriction and delay</entry> <entry v="t">Contraindicated</entry></row><row><entry v="t">Antimetabolites <list type="bullet"> <item><para>Folic acid analogues (methotrexate)</para></item> <item><para>Purine analogues</para></item> <item><para>Pyrimidine analogues (cytosine arabinoside, 5-fluorouracil)</para></item> </list></entry> <entry v="t"><list type="bullet"> <item><para>Abortion, intrauterine growth restriction, cranial anomalies</para></item> <item><para>Same as above</para></item> <item><para>Same as above</para></item></list></entry> <entry v="t"><list type="bullet"> <item><para>Hypoplastic gonads, growth restriction and delay</para></item> <item><para>Same as above, plus transient anemia</para></item> <item><para>Same as above</para></item></list></entry> <entry v="t"><list type="bullet"> <item><para>Contraindicated</para></item> <item><para>Contraindicated</para></item> <item><para>Contraindicated</para></item></list></entry></row><row><entry v="t">Antibiotics <list type="bullet"> <item><para>Actinomycin</para></item> <item><para>Vinca alkaloids (vincristine)</para></item></list></entry> <entry v="t"><list type="bullet"> <item><para>Abortion, intrauterine growth restriction, cranial anomalies</para></item> <item><para>Same as above</para></item></list></entry> <entry v="t"><list type="bullet"> <item><para>Hypoplastic gonads, growth restriction and delay</para></item> <item><para>Same as above</para></item></list></entry> <entry v="t"><list type="bullet"> <item><para>Contraindicated</para></item> <item><para>Contraindicated</para></item></list></entry></row><row><entry cs="4">CARDIOVASCULAR DRUGS</entry></row><row><entry v="t">ACE inhibitors</entry> <entry v="t">None known</entry> <entry v="t">Oliguria, skull defects, death</entry> <entry v="t">Compatible</entry></row><row><entry v="t">Adenosine</entry> <entry v="t">None known</entry> <entry v="t">No effects on fetal heart rate</entry> <entry v="t">Unknown</entry></row><row><entry v="t">Beta-sympathomimetics</entry> <entry v="t">None known</entry> <entry v="t">Tachycardia, hypothermia, hypocalcemia, hypoglycemia, and hyperglycemia</entry> <entry v="t">Compatible</entry></row><row><entry v="t">Calcium channel blockers</entry> <entry v="t">Unknown</entry> <entry v="t">None known</entry> <entry v="t">Compatible</entry></row><row><entry v="t">Digitalis</entry> <entry v="t">None known</entry> <entry v="t">Lower heart rate</entry> <entry v="t">Compatible</entry></row><row><entry v="t">Hydralazine</entry> <entry v="t">Skeletal defects (?)</entry> <entry v="t">Tachycardia, thrombocytopenia, fetal distress</entry> <entry v="t">Compatible</entry></row><row><entry v="t">Methyldopa</entry> <entry v="t">None known</entry> <entry v="t">Hemolytic anemia, tremor, hypotension</entry> <entry v="t">Compatible</entry></row><row><entry v="t">Propranolol, labetalol</entry> <entry v="t">Unknown</entry> <entry v="t">Lower heart rate, intrauterine growth restriction (?), hypoglycemia, respiratory distress</entry> <entry v="t">Compatible; hypoglycemia (?)</entry></row><row><entry v="t">Reserpine</entry> <entry v="t">None known</entry> <entry v="t">Lethargy, respiratory distress</entry> <entry v="t">Unknown</entry></row><row><entry cs="4">COLD AND COUGH PREPARATIONS</entry></row><row><entry v="t">Antihistamines</entry> <entry v="t">None known</entry> <entry v="t">None known</entry> <entry v="t">Reduced milk (?); drowsiness</entry></row><row><entry v="t">Cough suppressants</entry> <entry v="t">None known</entry> <entry v="t">None known</entry> <entry v="t">Compatible</entry></row><row><entry v="t">Decongestants</entry> <entry v="t">None known</entry> <entry v="t">None known</entry> <entry v="t">Compatible</entry></row><row><entry v="t">Dextromethorphan</entry> <entry v="t">None known</entry> <entry v="t">None known</entry> <entry v="t">No information</entry></row><row><entry v="t">Expectorants</entry> <entry v="t">Fetal goiter (?)</entry> <entry v="t">None known</entry> <entry v="t">Compatible</entry></row><row><entry v="t">Loratadine</entry> <entry v="t">Likely none</entry> <entry v="t">Likely none</entry> <entry v="t">Compatible</entry></row><row><entry cs="4">DIURETICS</entry></row><row><entry v="t">Furosemide</entry> <entry v="t">Unknown</entry> <entry v="t">Death from sudden hypoperfusion, electrolyte imbalance</entry> <entry v="t">Found to suppress lactation</entry></row><row><entry v="t">Thiazides</entry> <entry v="t">None known</entry> <entry v="t">Thrombocytopenia, hypokalemia, hyperbilirubinemia, hyponatremia</entry> <entry v="t">Compatible</entry></row><row><entry cs="4">FERTILITY DRUGS</entry></row><row><entry v="t">Clomiphene</entry> <entry v="t">Meiotic nondisjunction (?), neural tube defects (?)</entry> <entry v="t">Unknown</entry> <entry v="t">No data available</entry></row><row><entry cs="4">GASTROINTESTINAL AGENTS</entry></row><row><entry v="t">Bisacodyl</entry> <entry v="t">Unknown</entry> <entry v="t">Unknown</entry> <entry v="t">No reports of adverse effects</entry></row><row><entry v="t">Cholestyramine</entry> <entry v="t">None known</entry> <entry v="t">None known, but fat-soluble vitamins are depleted</entry> <entry v="t">Unknown</entry></row><row><entry v="t">Colestipol</entry> <entry v="t">Unknown</entry> <entry v="t">Unknown, but minimal absorption</entry> <entry v="t">No data available</entry></row><row><entry v="t">Docusate</entry> <entry v="t">None known</entry> <entry v="t">None known</entry> <entry v="t">Compatible</entry></row><row><entry v="t">H2-histamine receptor blockers</entry> <entry v="t">None known</entry> <entry v="t">Anti-androgen effect (cimetidine)</entry> <entry v="t">Compatible</entry></row><row><entry v="t">Magnesium hydroxide (Milk of Magnesia)</entry> <entry v="t">None known</entry> <entry v="t">None known</entry> <entry v="t">Compatible</entry></row><row><entry v="t">Mineral oil</entry> <entry v="t">Decreased maternal vitamin absorption</entry> <entry v="t">Decreased maternal vitamin absorption</entry> <entry v="t">Compatible</entry></row><row><entry v="t">Proton pump inhibitors</entry> <entry v="t">None known</entry> <entry v="t">None known</entry> <entry v="t">Unknown</entry></row><row><entry v="t">Sulfasalazine</entry> <entry v="t">None known</entry> <entry v="t">None known</entry> <entry v="t">Caution with ill infants</entry></row><row><entry cs="4">HORMONES</entry></row><row><entry v="t">Androgens*</entry> <entry v="t">Virilization of female fetus</entry> <entry v="t">Adrenal suppression</entry> <entry v="t">No adverse effects reported</entry></row><row><entry v="t">Corticosteroids</entry> <entry v="t">Orofacial cleft in animals, not in humans</entry> <entry v="t">No adverse effects in humans</entry> <entry v="t">No data available</entry></row><row><entry v="t">Danazol</entry> <entry v="t">Virilization of female fetus (?)</entry> <entry v="t">None known</entry> <entry v="t">No information available</entry></row><row><entry v="t">Estrogens</entry> <entry v="t">Cardiovascular anomalies (?)</entry> <entry v="t">None known</entry> <entry v="t">No reported adverse effects</entry></row><row><entry v="t">Progestins</entry> <entry v="t">Limb and cardiovascular anomalies (?), VACTERL syndrome (?), masculinization of female fetus (?)</entry> <entry v="t">None known</entry> <entry v="t">No reported adverse effects</entry></row><row><entry cs="4">DIABETES CARE</entry></row><row><entry v="t">Glucagon</entry> <entry v="t">None known</entry> <entry v="t">None known</entry> <entry v="t">Compatible</entry></row><row><entry v="t">Glyburide</entry> <entry v="t">None known</entry> <entry v="t">Not thought to cross the placenta in significant amounts; no neonatal hypoglycemia</entry> <entry v="t">Compatible</entry></row><row><entry v="t">Insulin</entry> <entry v="t">None known</entry> <entry v="t">None known</entry> <entry v="t">Safe</entry></row><row><entry v="t">Metformin</entry> <entry v="t">None known</entry> <entry v="t">Neonatal hypoglycemia</entry> <entry v="t">Unknown</entry></row><row><entry v="t">Sulfonylureas</entry> <entry v="t">Anomalies (?)</entry> <entry v="t">Suppressed insulin secretion</entry> <entry v="t">Compatible</entry></row><row><entry cs="4">MIGRAINE REMEDIES</entry></row><row><entry v="t">Ergotamine</entry> <entry v="t">None known</entry> <entry v="t">May stimulate contractions</entry> <entry v="t">Use with caution</entry></row><row><entry v="t">Sumatriptan</entry> <entry v="t">None known</entry> <entry v="t">None known</entry> <entry v="t">Compatible</entry></row><row><entry cs="4">PSYCHOACTIVE DRUGS, ANTIDEPRESSANTS</entry></row><row><entry v="t">Amphetamine</entry> <entry v="t">Inconsistent; likely none</entry> <entry v="t">Reduced weight</entry> <entry v="t">Contraindicated</entry></row><row><entry v="t">Benzodiazepines</entry> <entry v="t">Facial dysmorphism (?)</entry> <entry v="t">Depression, floppy infant, hypothermia, withdrawal</entry> <entry v="t">Some concern about central nervous system toxicity with long-term use</entry></row><row><entry v="t">Fluoxetine</entry> <entry v="t">None known</entry> <entry v="t">None known</entry> <entry v="t">Symptoms of colic</entry></row><row><entry v="t">Hydroxyzine</entry> <entry v="t">None known</entry> <entry v="t">None known</entry> <entry v="t">No information</entry></row><row><entry v="t">Lithium</entry> <entry v="t">Facial clefts; cardiovascular anomaly</entry> <entry v="t">Lithium toxicity (neurologic and hepatic dysfunction)</entry> <entry v="t">Contraindicated</entry></row><row><entry v="t">Meprobamate</entry> <entry v="t">Cardiac anomalies (?), major malformations (?)</entry> <entry v="t">None known</entry> <entry v="t">Unknown</entry></row><row><entry v="t">Phenothiazines</entry> <entry v="t">None known</entry> <entry v="t">Muscle rigidity, hypothermia, tremor</entry> <entry v="t">Unknown</entry></row><row><entry v="t">Sedatives</entry> <entry v="t">None known</entry> <entry v="t">Depression, slow learning</entry> <entry v="t">Not recommended</entry></row><row><entry v="t">Thalidomide*</entry> <entry v="t">Phocomelia in 20% of cases</entry> <entry v="t">None known</entry> <entry v="t">No information</entry></row><row><entry v="t">Tricyclics</entry> <entry v="t">None known</entry> <entry v="t">None known</entry> <entry v="t">Unknown/caution</entry></row><row><entry v="t">Zolpiden</entry> <entry v="t">Unknown</entry> <entry v="t">Withdrawal or floppy infant (?)</entry> <entry v="t">Compatible</entry></row><row><entry cs="4">RADIOLABELED DIAGNOSTICS</entry></row><row><entry v="t">Albumin</entry> <entry v="t">None known</entry> <entry v="t">None known</entry> <entry v="t">No information available</entry></row><row><entry v="t">I131 (diagnostic)</entry> <entry v="t">None known</entry> <entry v="t">None known</entry> <entry v="t">Not recommended during exposure; may continue 24 hours after exposure</entry></row><row><entry v="t">Technetium</entry> <entry v="t">None known</entry> <entry v="t">None known</entry> <entry v="t">Not recommended during exposure; may continue 24 hours after exposure</entry></row><row><entry cs="4">SMOKING CESSATION</entry></row><row><entry v="t">Bupropion</entry> <entry v="t">Likely none</entry> <entry v="t">None known</entry> <entry v="t">Compatible</entry></row><row><entry v="t">Nicotine</entry> <entry v="t">Spontaneous abortion (?)</entry> <entry v="t">Impaired growth (?)</entry> <entry v="t">Consistent with passive smoking</entry></row><row><entry cs="4">THYROID MEDICATION</entry></row><row><entry v="t">I131 (therapeutic)</entry> <entry v="t">Goiter, abortion, anomalies</entry> <entry v="t">Goiter, airway obstruction, hyperthyroid, mental retardation</entry> <entry v="t">Contraindicated</entry></row><row><entry v="t">Methimazole</entry> <entry v="t">Aplasia cutis (?), goiter</entry> <entry v="t">Goiter, airway obstruction, hyperthyroid, mental retardation, aplasia cutis (?)</entry> <entry v="t">Compatible, but monitor fetal thyroid function</entry></row><row><entry v="t">Propylthiouracil</entry> <entry v="t">Goiter</entry> <entry v="t">Same as above</entry> <entry v="t">Safe, but monitor baby’s thyroid status</entry></row><row><entry v="t">Thyroid USP</entry> <entry v="t">None known</entry> <entry v="t">None known</entry> <entry v="t">Compatible</entry></row><row><entry v="t">Thyroxine</entry> <entry v="t">None known</entry> <entry v="t">None known</entry> <entry v="t">Compatible</entry></row><row><entry cs="4">TOCOLYTICS</entry></row><row><entry v="t">Beta-sympathomimetics</entry> <entry v="t">None known</entry> <entry v="t">Tachycardia, hypothermia, hypocalcemia, hypoglycemia, hyperglycemia</entry> <entry v="t">—</entry></row><row><entry v="t">Indomethacin</entry> <entry v="t">None known</entry> <entry v="t">Oligohydramnios (>48 hours of use)</entry> <entry v="t">—</entry></row><row><entry v="t">Magnesium sulfate</entry> <entry v="t">None known</entry> <entry v="t">Hypermagnesemia, respiratory depression</entry> <entry v="t">—</entry></row><row><entry v="t">Nifedipine</entry> <entry v="t">Unknown</entry> <entry v="t">None known</entry> <entry v="t">—</entry></row><row><entry cs="4">VACCINATIONS</entry></row><row><entry v="t">Influenza</entry> <entry v="t">None known</entry> <entry v="t">Passive immunization</entry> <entry v="t">Compatible</entry></row><row><entry v="t">Pneumovaccine</entry> <entry v="t">None known</entry> <entry v="t">Passive immunization</entry> <entry v="t">Compatible</entry></row><row><entry v="t">Tetanus toxoid</entry> <entry v="t">None known</entry> <entry v="t">Passive immunization</entry> <entry v="t">Compatible</entry></row><row><entry cs="4">VAGINAL PREPARATIONS</entry></row><row><entry v="t">Antifungal agents</entry> <entry v="t">None known</entry> <entry v="t">None known</entry> <entry v="t">Compatible</entry></row><row><entry v="t">Podophyllin</entry> <entry v="t">Mutagenesis (?)</entry> <entry v="t">Central nervous system effects (?)</entry> <entry v="t">Contraindicated</entry></row><row><entry cs="4">VITAMINS (high dose)</entry></row><row><entry v="t">A</entry> <entry v="t">Urogenital and craniofacial anomalies (?)</entry> <entry v="t">None known</entry> <entry v="t">No data available</entry></row><row><entry v="t">C</entry> <entry v="t">None known</entry> <entry v="t">Scurvy after delivery</entry> <entry v="t">Compatible</entry></row><row><entry v="t">D</entry> <entry v="t">Supravalvular aortic stenosis (?)</entry> <entry v="t">None known</entry> <entry v="t">Compatible</entry></row><row><entry v="t">E</entry> <entry v="t">Unknown</entry> <entry v="t">None known</entry> <entry v="t">Compatible</entry></row><row><entry v="t">K</entry> <entry v="t">Unknown</entry> <entry v="t">Hemorrhage, if deficiency</entry> <entry v="t">Compatible</entry></row><row><entry cs="4">“STREET” DRUGS</entry></row><row><entry v="t">Cocaine</entry> <entry v="t">Placental abruption, vascular disruption, urinary tract anomalies</entry> <entry v="t">Withdrawal, placental abruption, vascular disruption, growth restriction</entry> <entry v="t">Contraindicated</entry></row><row><entry v="t">Heroin</entry> <entry v="t">None known</entry> <entry v="t">Depression, withdrawal, growth restriction</entry> <entry v="t">Contraindicated</entry></row><row><entry v="t">LSD</entry> <entry v="t">None known</entry> <entry v="t">Withdrawal, behavioral effects</entry> <entry v="t">Contraindicated</entry></row><row><entry v="t">Marijuana</entry> <entry v="t">None known</entry> <entry v="t">Behavioral effects, growth restriction</entry> <entry v="t">Contraindicated</entry></row><row><entry v="t">Methadone</entry> <entry v="t">None known</entry> <entry v="t">Withdrawal, growth restriction</entry> <entry v="t">Contraindicated</entry></row><row><entry v="t">Methamphetamine</entry> <entry v="t">None known</entry> <entry v="t">Withdrawal, growth restriction</entry> <entry v="t">Contraindicated</entry></row><row><entry v="t">Pentazocine</entry> <entry v="t">None known</entry> <entry v="t">Withdrawal, growth restriction</entry> <entry v="t">Contraindicated</entry></row><row><entry v="t">Phencyclidine</entry> <entry v="t">None known</entry> <entry v="t">Withdrawal, neurobehavioral effects, growth restriction</entry> <entry v="t">Contraindicated</entry></row><row><entry cs="4">OTHER DRUGS</entry></row><row><entry v="t">Azathioprine</entry> <entry v="t">Abortion</entry> <entry v="t">Anemia, thrombocytopenia, lymphopenia, growth retardation</entry> <entry v="t">Not recommended</entry></row><row><entry v="t">Bromocriptine</entry> <entry v="t">None known</entry> <entry v="t">None known</entry> <entry v="t">Compatible</entry></row><row><entry v="t">Caffeine</entry> <entry v="t">Anomalies (?) in high doses, abortion (?)</entry> <entry v="t">Jitteriness</entry> <entry v="t">Not recommended</entry></row><row><entry v="t">Immune gamma globulin</entry> <entry v="t">None known</entry> <entry v="t">None known</entry> <entry v="t">Compatible</entry></row><row><entry v="t">Isotretinoin*</entry> <entry v="t">Central nervous system, cardiac, facial anomalies</entry> <entry v="t">Stillbirth, mental retardation (?)</entry> <entry v="t">Contraindicated</entry></row><row><entry v="t">Misoprostol</entry> <entry v="t">Abortion; variety of anomalies (cranium, limb, oral cleft); Mobius sequence</entry> <entry v="t">None with low dose for cervical ripening; placental abruption</entry> <entry v="t">Contraindicated, especially if diarrhea occurs</entry></row><row><entry v="t">Spermicides</entry> <entry v="t">None known</entry> <entry v="t">None known</entry> <entry v="t">No information</entry></row><row><entry cs="4">* Proven teratogen.</entry></row><row><entry cs="4">Unknown=no studies to investigate fetal effects; none known=no malformations reported in human studies or no consistent malformations in animal studies; (?)=conflicting information</entry></row><row><entry cs="4">Source: Reprotox data from humans, last reviewed in 2006.</entry></row>
The author reports no financial relationships relevant to this article.
Fifty years ago, the thalidomide experience—a high incidence of major birth defects following prenatal use of the drug—made clear the devastating potential of drug exposure during pregnancy. Since that disaster, healthcare providers and patients have adopted a conservative approach to medication use during pregnancy, especially during the first trimester and lactation. That is a wise strategy, although very few medications are associated with abnormal fetal development.
In this article, I’ll guide you through some of the issues that must be considered when assessing a drug’s teratogenicity, help you find information on a host of medications, and familiarize you with some of the challenges involved in counseling the patient. I also present a table listing the adverse effects known to be associated with selected drugs during the first, second, and third trimesters and lactation (TABLE). We are fortunate that a large body of information about medication use during pregnancy and lactation is readily available on the Web and in books and medical journals. This information is far from definitive, however, because much of the evidence concerning prescribed drugs is anecdotal or presented with insufficient warning about their use during pregnancy and lactation.
A discussion of these issues with the patient will help set the risks and benefits of a particular drug into proper perspective, alleviate fears, and improve compliance. Nonprescription medications should also be discussed, and the patient should be advised that we have very little data concerning their use during pregnancy.
Assignment of risk is an uncertain science
Major structural defects are apparent at birth in about 3% of all pregnancies and in about 4.5% of all children by the age of 5 years.1 A cause or proposed mechanism for the defects can be determined in fewer than 50% of these cases. Nor can we count on expert consensus about the safety of many medications during pregnancy because it rarely occurs and, in some cases, may be impossible to achieve.
Animal studies are the means of assessing the teratogenicity of most drugs. Animals commonly used to study fetal effects include rodents (fertility, birth defects, birth weight, behavior), rabbits (birth defects), baboons (uterine blood flow), and sheep (uterine blood flow, cardiovascular effects, fetal hypoxia, and acidosis). Dosages are often much higher (in relation to body weight or surface area) to “test the systems” for any possible reproductive harm. Although these studies may be helpful, they do not reliably predict the human response.
Even when humans are the subject of study, conclusions must be viewed with caution. To determine the risk of teratogenesis, it is necessary to know the stage of development during which the exposure occurred, as well as the identity and dose of the medication and the genetic susceptibility of the mother and fetus.
Three critical stages. In utero exposure to a drug occurs in one of three periods of fetal development:
- ovum – from fertilization to implantation
- embryo – from the second through the eighth week of gestation
- fetus – from the eighth completed week until delivery.
An “all-or-none” effect (i.e., spontaneous abortion or not) is believed to arise from exposure during the first period, but the embryo stage is the most critical time because it involves organogenesis. Detrimental effects may occur even beyond this period as cells continue to divide in the hematologic, reproductive, and central nervous systems.
Many fine points of exposure are difficult to clarify
Retrospective and uncontrolled studies, as well as individual case reports or small series, may overestimate the risk to the fetus of exposure to a specific drug or combination of medications. Case reports do not establish causation.
It can also be difficult to differentiate between the risks of a specific drug and the hazards of maternal illness to explain an unfavorable outcome. For example, is a particular case of stillbirth the result of fetal exposure to enoxaparin or maternal thrombophilia, or both? Can fetal growth restriction be attributed to use of azathioprine during pregnancy or to the mother’s underlying illness? And so on.
In addition, it is necessary to distinguish between a defect’s natural prevalence—i.e., the rate at which it occurs in a population—and the additional risk posed by exposure to a particular drug. Studies in large populations are needed—but usually unattainable—to determine the relative risk from specific potential teratogens.
Finally, it is very difficult to assess neurobehavioral effects of in utero exposure to centrally acting drugs beyond the immediate neonatal period. The dose, offspring’s age and gender, and behavioral test system must all be considered.
Few drugs are implicated in restricting fetal growth or reducing organ size. We also lack consistent information about long-term effects such as learning or behavioral problems (i.e., functional teratogenesis) that may result from chronic prenatal exposure to a certain medication.
In 1979, the Food and Drug Administration created five pregnancy risk categories to be used by manufacturers to rate their products in the drug formulary for use during pregnancy: categories A, B, C, D, and X, which range from no evidence of damage to the fetus (category A) to clear teratogenicity (D and X).
The D rating is generally reserved for drugs with no safer alternatives, such as secobarbital, doxycycline, and lorazepam. The X rating means there is absolutely no reason to risk using the drug in pregnancy, as in the case of oral contraceptives, benzodiazepines, and misoprostol.
Approximately 2% of drugs fall into category A, 50% in category B, 38% in category C, 3–5% in category D, and 1–5% in category X.3 These categories do not often accurately reflect the available information on risk to the fetus. A major initiative is under way to declare these categories obsolete and provide more informative drug labeling. Pregnancy labels of the future will likely address three important areas:
- clinical considerations–issues relevant to prescription of a particular drug in pregnancy, including the risk of disease versus no treatment. Also included will be information of use when counseling a patient whose fetus was inadvertently exposed to a medication in early gestation
- summary risk assessment–a narrative text that describes, as comprehensively as possible, the risk of exposure based on animal and human data
- data to support the assessment.
All drugs cross the placenta
Most medications are easily absorbed during pregnancy, and serum concentrations of albumin for drug binding are lower than in the nonpregnant state. Pharmacokinetic changes during pregnancy include:
- higher volume of distribution
- lower maximum plasma concentration
- lower steady-state serum concentration
- shorter plasma half-life
- higher clearance rate.1
The small spatial configuration and high lipid solubility of most medications permit easy transfer of an unbound drug or its metabolite across the placenta or into breast milk. Virtually all drugs and their end products cross the placenta, with unbound concentrations of the drug in the fetal serum similar to the level in maternal serum—sometimes even higher (FIGURE).
A few drugs with high molecular weight do not cross the placenta in significant amounts (e.g., glyburide, interferon, thyroid supplements, insulin).
FIGURE An elaborate nutrient (and drug) delivery system
The placenta and umbilical cord deliver the nutrients and oxygen the fetus needs for normal growth—as well as most medications used by the mother.
Medication use tends to increase as pregnancy progresses
The drugs most commonly taken during pregnancy include vitamins, iron preparations, calcium, analgesics, antibiotics, and antacids. Excluding vitamins and mineral supplements, an average of one to two medications are taken during gestation. Over-the-counter formulations account for about half of these drugs, with acetaminophen being the single most commonly used medication during pregnancy. Antibiotics are the most widely prescribed drugs.
Although caffeine, tobacco, alcohol, and illicit substance use tends to diminish as pregnancy progresses, medications are usually taken at the same frequency or more often during gestation.
My colleagues and I found a significantly higher mean number of medications (3.3 and 4.1, respectively) used during the second and third trimesters of gestation than were taken before pregnancy (2.6).2
How to counsel the patient
Counseling a woman before or during pregnancy about the continuation or initiation of a medication should take place in an open, supportive, and informative manner. Most inquiries relate to exposures involving very low levels of relative and absolute risk.
A detailed fetal ultrasonographic examination is often used to accurately date the pregnancy and, if possible, screen for any structural defects. The patient should be advised that first-trimester screening, chorionic villus sampling, maternal serum quadruple screening, amniocentesis, and fetal blood sampling are not very predictive of a drug’s fetal effects. Exceptions may be the observation of open neural tube defects (approximate 1% risk associated with valproic acid and carbamazepine) by maternal serum quadruple screening and facial clefting by targeted ultrasonography.
When a patient inquires about a particular drug, it is important to gather the following information:
- When did she take the medication?
- Why did she take it?
- For how long did she take the medication?
- Did she take other medications, or any substances of abuse, at the same time?
A number of sources of information about potential teratogens are available.3-5 These include national computerized databases that are accessible on the Web:
- National Library of Medicine (http://sis.nlm.nih.gov/enviro.html)
- pregnancy exposure registries (www.fda.gov/womens/registries/default.htm)
- Reproductive Toxicology Center (http://reprotox.org) (access requires a paid subscription)
- LactMed, National Library of Medicine guide to drug safety in lactation (http://toxnet.nlm.nih.gov/cgi-bin/sis/htmlgen?LACT)
- Organization of Teratology Information Specialists (OTIS) (www.otispregnancy.org).
The last source (OTIS) consolidates teratology information nationwide and reports it by state or region. It also publishes a host of fact sheets on various drugs that may be useful to dispense to the patient during counseling. In addition, many teratogen information services or poison control centers (often at children’s hospitals) are available throughout the United States to serve specific geographic areas. And teratogen registries at pharmaceutical companies may provide limited information about newer medications.
The Physician’s Desk Reference (PDR) is a common source of information about the use of prescription drugs in pregnancy.3 But be aware that, to avoid liability, pharmaceutical manufacturers do not encourage use of their drugs during pregnancy unless the benefit clearly outweighs the risk. It would be unrealistic for them to market a medication for specific use during pregnancy because it would require considerable time and cost, and raise ethical objections, to conduct research in a vulnerable population that is limited in number.
Effects of agents used more than 40 years ago were reported by the Collaborative Perinatal Project or the Boston Collaborative Drug Surveillance Program.6 Those findings are often inconclusive, reflect bias in study designs, and do not help a clinician evaluate current medications or those less commonly prescribed during pregnancy.
The risks and experience associated with new drugs are usually not well explored in regard to pregnancy. As a result, older medications are more likely to be prescribed as maintenance therapy during gestation for the simple reason that they have a larger body of information regarding their effects. These older drugs may no longer be preferred once the patient delivers.
Most drugs are not teratogens
The TABLE lists adverse effects in the human fetus known to arise from exposure to specific drugs. The information comes largely from the Reprotox database, which was reviewed as recently as 2006, describes human data only, and is reported by first trimester (anomalies, abortion) and the second and third trimesters (fetal growth restriction, stillbirth, low birth weight, preterm delivery, immediate neonatal problems).7 Typical dosages of most drugs are not anticipated to increase the risk of congenital anomalies.
Most human data come from small series or case reports. Although these types of studies are helpful, they tend to be biased or reflect the pregnancy’s background risk of birth defects rather than the risk posed by a specific drug. In addition, case reports of malformation after prenatal exposure to a certain drug may involve exposures to other agents and a lack of uniformity of abnormalities, making the association between adverse effects and a single agent unlikely. Dissimilarities in the dosage and route of delivery also limit interpretation. for example, short-term intravenous or sublingual administration of a drug may pose a different risk than taking that medication orally or vaginally, in a lower dose, for a longer period, or at a different period of gestation.
Randomized controlled trials of drugs are rare during pregnancy, as are prospective cohort investigations. Because a control population is often impossible to identify, it becomes difficult to separate any heightened risk identified during use of the medication from the underlying disease. When the gravida has significant medical problems, it is important to assess the potential risk of a drug—or its omission—in her as well as her fetus. The lowest effective dose is preferred, but keep in mind that inadequate treatment may lead to minimal benefit and potentially greater risk to the pregnancy.
When reviewing or planning maintenance drug therapy, follow the same principles as you would in a nonpregnant patient. Be familiar with more than one medication for each disorder. Also be aware that some drugs may need to be prescribed at a higher dose or greater frequency to attain a therapeutic concentration in the expanded intravascular volume of pregnancy. In addition, side effects such as nausea, fatigue, and gastrointestinal disturbance may mimic symptoms arising from physiologic changes of pregnancy.
Assessing the risks associated with over-the-counter medications and natural food products is even harder. The PDR for Nonprescription Drugs, Dietary Supplements, and Herbs8 contains little or no information about the reproductive hazards of most of these products. Many agents contain multiple ingredients, both active and inactive, thereby complicating counseling about their risks during pregnancy. Although the recommended dosage is usually low, many product labels do not specify what it should be.
Most drugs enter breast milk
The amount of drug that an infant consumes from breast milk depends on the medication’s chemical properties as well as the dosage, frequency, and duration of exposure.2 Contraindications and cautions are usually either theoretical or based on findings from case reports that often conflict or confuse. In theory, it is safer for the mother to take the medication just after infant feeding or just before the infant’s longest sleep period.
The TABLE also lists the effects of drugs in the breastfed human infant. Again, the information comes from the Reprotox database, access to which requires a subscription. For additional information, try the free LactMed site at http://toxnet.nlm.nih.gov/cgi-bin/sis/htmlgen?LACT.
Nearly all drugs are excreted in breast milk, usually in small amounts (often less than 5% of the weight-adjusted maternal daily dose). The amount of drug or metabolite in an infant’s serum also is determined by the volume of breast milk, age of the infant, and other exposures.
Avoid prescribing multiple medications, if possible, and choose “safe” drugs from among the options in categories that include a number of teratogenic medications, such as anticonvulsants.
Determine the best method to monitor therapy. For example, use a peak flow meter for asthma, a portable blood pressure monitor for hypertension, and so on.
Focus on keeping the patient healthy. The healthiest mother is most likely to deliver the healthiest infant.
Keep the underlying disorder in mind, as well as the drug, when choosing a drug.
Know which drugs are clearly linked to birth defects. These include phenytoin, warfarin, alcohol, methotrexate, diethylstilbestrol, cis-retinoic acid, valproic acid, and carbamazepine.
Pay special attention to the first trimester. Too little is known about the first-trimester effects of the vast majority of drugs for them to be considered safe.
Suspect a drug-related effect
A medication may be the cause in any newborn manifesting signs of anemia, hepatitis, hepatotoxicity, hepatorenal dysfunction, and hyperbilirubinemia. This includes breastfed infants. An adverse drug-related effect should also be suspected when an infant exhibits signs of jaundice, floppiness, jitteriness, poor suck, diarrhea, or growth restriction.
The author reports no financial relationships relevant to this article.
Fifty years ago, the thalidomide experience—a high incidence of major birth defects following prenatal use of the drug—made clear the devastating potential of drug exposure during pregnancy. Since that disaster, healthcare providers and patients have adopted a conservative approach to medication use during pregnancy, especially during the first trimester and lactation. That is a wise strategy, although very few medications are associated with abnormal fetal development.
In this article, I’ll guide you through some of the issues that must be considered when assessing a drug’s teratogenicity, help you find information on a host of medications, and familiarize you with some of the challenges involved in counseling the patient. I also present a table listing the adverse effects known to be associated with selected drugs during the first, second, and third trimesters and lactation (TABLE). We are fortunate that a large body of information about medication use during pregnancy and lactation is readily available on the Web and in books and medical journals. This information is far from definitive, however, because much of the evidence concerning prescribed drugs is anecdotal or presented with insufficient warning about their use during pregnancy and lactation.
A discussion of these issues with the patient will help set the risks and benefits of a particular drug into proper perspective, alleviate fears, and improve compliance. Nonprescription medications should also be discussed, and the patient should be advised that we have very little data concerning their use during pregnancy.
Assignment of risk is an uncertain science
Major structural defects are apparent at birth in about 3% of all pregnancies and in about 4.5% of all children by the age of 5 years.1 A cause or proposed mechanism for the defects can be determined in fewer than 50% of these cases. Nor can we count on expert consensus about the safety of many medications during pregnancy because it rarely occurs and, in some cases, may be impossible to achieve.
Animal studies are the means of assessing the teratogenicity of most drugs. Animals commonly used to study fetal effects include rodents (fertility, birth defects, birth weight, behavior), rabbits (birth defects), baboons (uterine blood flow), and sheep (uterine blood flow, cardiovascular effects, fetal hypoxia, and acidosis). Dosages are often much higher (in relation to body weight or surface area) to “test the systems” for any possible reproductive harm. Although these studies may be helpful, they do not reliably predict the human response.
Even when humans are the subject of study, conclusions must be viewed with caution. To determine the risk of teratogenesis, it is necessary to know the stage of development during which the exposure occurred, as well as the identity and dose of the medication and the genetic susceptibility of the mother and fetus.
Three critical stages. In utero exposure to a drug occurs in one of three periods of fetal development:
- ovum – from fertilization to implantation
- embryo – from the second through the eighth week of gestation
- fetus – from the eighth completed week until delivery.
An “all-or-none” effect (i.e., spontaneous abortion or not) is believed to arise from exposure during the first period, but the embryo stage is the most critical time because it involves organogenesis. Detrimental effects may occur even beyond this period as cells continue to divide in the hematologic, reproductive, and central nervous systems.
Many fine points of exposure are difficult to clarify
Retrospective and uncontrolled studies, as well as individual case reports or small series, may overestimate the risk to the fetus of exposure to a specific drug or combination of medications. Case reports do not establish causation.
It can also be difficult to differentiate between the risks of a specific drug and the hazards of maternal illness to explain an unfavorable outcome. For example, is a particular case of stillbirth the result of fetal exposure to enoxaparin or maternal thrombophilia, or both? Can fetal growth restriction be attributed to use of azathioprine during pregnancy or to the mother’s underlying illness? And so on.
In addition, it is necessary to distinguish between a defect’s natural prevalence—i.e., the rate at which it occurs in a population—and the additional risk posed by exposure to a particular drug. Studies in large populations are needed—but usually unattainable—to determine the relative risk from specific potential teratogens.
Finally, it is very difficult to assess neurobehavioral effects of in utero exposure to centrally acting drugs beyond the immediate neonatal period. The dose, offspring’s age and gender, and behavioral test system must all be considered.
Few drugs are implicated in restricting fetal growth or reducing organ size. We also lack consistent information about long-term effects such as learning or behavioral problems (i.e., functional teratogenesis) that may result from chronic prenatal exposure to a certain medication.
In 1979, the Food and Drug Administration created five pregnancy risk categories to be used by manufacturers to rate their products in the drug formulary for use during pregnancy: categories A, B, C, D, and X, which range from no evidence of damage to the fetus (category A) to clear teratogenicity (D and X).
The D rating is generally reserved for drugs with no safer alternatives, such as secobarbital, doxycycline, and lorazepam. The X rating means there is absolutely no reason to risk using the drug in pregnancy, as in the case of oral contraceptives, benzodiazepines, and misoprostol.
Approximately 2% of drugs fall into category A, 50% in category B, 38% in category C, 3–5% in category D, and 1–5% in category X.3 These categories do not often accurately reflect the available information on risk to the fetus. A major initiative is under way to declare these categories obsolete and provide more informative drug labeling. Pregnancy labels of the future will likely address three important areas:
- clinical considerations–issues relevant to prescription of a particular drug in pregnancy, including the risk of disease versus no treatment. Also included will be information of use when counseling a patient whose fetus was inadvertently exposed to a medication in early gestation
- summary risk assessment–a narrative text that describes, as comprehensively as possible, the risk of exposure based on animal and human data
- data to support the assessment.
All drugs cross the placenta
Most medications are easily absorbed during pregnancy, and serum concentrations of albumin for drug binding are lower than in the nonpregnant state. Pharmacokinetic changes during pregnancy include:
- higher volume of distribution
- lower maximum plasma concentration
- lower steady-state serum concentration
- shorter plasma half-life
- higher clearance rate.1
The small spatial configuration and high lipid solubility of most medications permit easy transfer of an unbound drug or its metabolite across the placenta or into breast milk. Virtually all drugs and their end products cross the placenta, with unbound concentrations of the drug in the fetal serum similar to the level in maternal serum—sometimes even higher (FIGURE).
A few drugs with high molecular weight do not cross the placenta in significant amounts (e.g., glyburide, interferon, thyroid supplements, insulin).
FIGURE An elaborate nutrient (and drug) delivery system
The placenta and umbilical cord deliver the nutrients and oxygen the fetus needs for normal growth—as well as most medications used by the mother.
Medication use tends to increase as pregnancy progresses
The drugs most commonly taken during pregnancy include vitamins, iron preparations, calcium, analgesics, antibiotics, and antacids. Excluding vitamins and mineral supplements, an average of one to two medications are taken during gestation. Over-the-counter formulations account for about half of these drugs, with acetaminophen being the single most commonly used medication during pregnancy. Antibiotics are the most widely prescribed drugs.
Although caffeine, tobacco, alcohol, and illicit substance use tends to diminish as pregnancy progresses, medications are usually taken at the same frequency or more often during gestation.
My colleagues and I found a significantly higher mean number of medications (3.3 and 4.1, respectively) used during the second and third trimesters of gestation than were taken before pregnancy (2.6).2
How to counsel the patient
Counseling a woman before or during pregnancy about the continuation or initiation of a medication should take place in an open, supportive, and informative manner. Most inquiries relate to exposures involving very low levels of relative and absolute risk.
A detailed fetal ultrasonographic examination is often used to accurately date the pregnancy and, if possible, screen for any structural defects. The patient should be advised that first-trimester screening, chorionic villus sampling, maternal serum quadruple screening, amniocentesis, and fetal blood sampling are not very predictive of a drug’s fetal effects. Exceptions may be the observation of open neural tube defects (approximate 1% risk associated with valproic acid and carbamazepine) by maternal serum quadruple screening and facial clefting by targeted ultrasonography.
When a patient inquires about a particular drug, it is important to gather the following information:
- When did she take the medication?
- Why did she take it?
- For how long did she take the medication?
- Did she take other medications, or any substances of abuse, at the same time?
A number of sources of information about potential teratogens are available.3-5 These include national computerized databases that are accessible on the Web:
- National Library of Medicine (http://sis.nlm.nih.gov/enviro.html)
- pregnancy exposure registries (www.fda.gov/womens/registries/default.htm)
- Reproductive Toxicology Center (http://reprotox.org) (access requires a paid subscription)
- LactMed, National Library of Medicine guide to drug safety in lactation (http://toxnet.nlm.nih.gov/cgi-bin/sis/htmlgen?LACT)
- Organization of Teratology Information Specialists (OTIS) (www.otispregnancy.org).
The last source (OTIS) consolidates teratology information nationwide and reports it by state or region. It also publishes a host of fact sheets on various drugs that may be useful to dispense to the patient during counseling. In addition, many teratogen information services or poison control centers (often at children’s hospitals) are available throughout the United States to serve specific geographic areas. And teratogen registries at pharmaceutical companies may provide limited information about newer medications.
The Physician’s Desk Reference (PDR) is a common source of information about the use of prescription drugs in pregnancy.3 But be aware that, to avoid liability, pharmaceutical manufacturers do not encourage use of their drugs during pregnancy unless the benefit clearly outweighs the risk. It would be unrealistic for them to market a medication for specific use during pregnancy because it would require considerable time and cost, and raise ethical objections, to conduct research in a vulnerable population that is limited in number.
Effects of agents used more than 40 years ago were reported by the Collaborative Perinatal Project or the Boston Collaborative Drug Surveillance Program.6 Those findings are often inconclusive, reflect bias in study designs, and do not help a clinician evaluate current medications or those less commonly prescribed during pregnancy.
The risks and experience associated with new drugs are usually not well explored in regard to pregnancy. As a result, older medications are more likely to be prescribed as maintenance therapy during gestation for the simple reason that they have a larger body of information regarding their effects. These older drugs may no longer be preferred once the patient delivers.
Most drugs are not teratogens
The TABLE lists adverse effects in the human fetus known to arise from exposure to specific drugs. The information comes largely from the Reprotox database, which was reviewed as recently as 2006, describes human data only, and is reported by first trimester (anomalies, abortion) and the second and third trimesters (fetal growth restriction, stillbirth, low birth weight, preterm delivery, immediate neonatal problems).7 Typical dosages of most drugs are not anticipated to increase the risk of congenital anomalies.
Most human data come from small series or case reports. Although these types of studies are helpful, they tend to be biased or reflect the pregnancy’s background risk of birth defects rather than the risk posed by a specific drug. In addition, case reports of malformation after prenatal exposure to a certain drug may involve exposures to other agents and a lack of uniformity of abnormalities, making the association between adverse effects and a single agent unlikely. Dissimilarities in the dosage and route of delivery also limit interpretation. for example, short-term intravenous or sublingual administration of a drug may pose a different risk than taking that medication orally or vaginally, in a lower dose, for a longer period, or at a different period of gestation.
Randomized controlled trials of drugs are rare during pregnancy, as are prospective cohort investigations. Because a control population is often impossible to identify, it becomes difficult to separate any heightened risk identified during use of the medication from the underlying disease. When the gravida has significant medical problems, it is important to assess the potential risk of a drug—or its omission—in her as well as her fetus. The lowest effective dose is preferred, but keep in mind that inadequate treatment may lead to minimal benefit and potentially greater risk to the pregnancy.
When reviewing or planning maintenance drug therapy, follow the same principles as you would in a nonpregnant patient. Be familiar with more than one medication for each disorder. Also be aware that some drugs may need to be prescribed at a higher dose or greater frequency to attain a therapeutic concentration in the expanded intravascular volume of pregnancy. In addition, side effects such as nausea, fatigue, and gastrointestinal disturbance may mimic symptoms arising from physiologic changes of pregnancy.
Assessing the risks associated with over-the-counter medications and natural food products is even harder. The PDR for Nonprescription Drugs, Dietary Supplements, and Herbs8 contains little or no information about the reproductive hazards of most of these products. Many agents contain multiple ingredients, both active and inactive, thereby complicating counseling about their risks during pregnancy. Although the recommended dosage is usually low, many product labels do not specify what it should be.
Most drugs enter breast milk
The amount of drug that an infant consumes from breast milk depends on the medication’s chemical properties as well as the dosage, frequency, and duration of exposure.2 Contraindications and cautions are usually either theoretical or based on findings from case reports that often conflict or confuse. In theory, it is safer for the mother to take the medication just after infant feeding or just before the infant’s longest sleep period.
The TABLE also lists the effects of drugs in the breastfed human infant. Again, the information comes from the Reprotox database, access to which requires a subscription. For additional information, try the free LactMed site at http://toxnet.nlm.nih.gov/cgi-bin/sis/htmlgen?LACT.
Nearly all drugs are excreted in breast milk, usually in small amounts (often less than 5% of the weight-adjusted maternal daily dose). The amount of drug or metabolite in an infant’s serum also is determined by the volume of breast milk, age of the infant, and other exposures.
Avoid prescribing multiple medications, if possible, and choose “safe” drugs from among the options in categories that include a number of teratogenic medications, such as anticonvulsants.
Determine the best method to monitor therapy. For example, use a peak flow meter for asthma, a portable blood pressure monitor for hypertension, and so on.
Focus on keeping the patient healthy. The healthiest mother is most likely to deliver the healthiest infant.
Keep the underlying disorder in mind, as well as the drug, when choosing a drug.
Know which drugs are clearly linked to birth defects. These include phenytoin, warfarin, alcohol, methotrexate, diethylstilbestrol, cis-retinoic acid, valproic acid, and carbamazepine.
Pay special attention to the first trimester. Too little is known about the first-trimester effects of the vast majority of drugs for them to be considered safe.
Suspect a drug-related effect
A medication may be the cause in any newborn manifesting signs of anemia, hepatitis, hepatotoxicity, hepatorenal dysfunction, and hyperbilirubinemia. This includes breastfed infants. An adverse drug-related effect should also be suspected when an infant exhibits signs of jaundice, floppiness, jitteriness, poor suck, diarrhea, or growth restriction.
Reference
1. American College of Obstetricians and Gynecologists. Teratology. ACOG Educational Bulletin #236. Washington, DC: ACOG; 1997.
2. Splinter M, Nightingale B, Sawgraves R, Rayburn W. Medication use during pregnancy by women delivering at a tertiary university hospital. South Med J. 1997;90:498-502.
3. Physician’s Desk Reference. 61st ed. Montvale, NJ: Medical Economics; 2007.
4. Briggs GG, Freeman RK, Yaffee FJ. Drugs in Pregnancy and Lactation: Reference Guide to Fetal and Neonatal Risk. 7th ed. Baltimore: Williams & Wilkins; 2005.
5. Briggs GG, Freeman RK, Yaffee FJ. ReproTox Database. Vol. 13. No. 1. Bethesda, Md: Reproductive Toxicology Center; 2000.
6. Heinonen OP, Sloan ED, Shapiro S. Birth Defects and Drugs in Pregnancy. Boston: John Wright PSG; 1973.
7. Reproductive Toxicology Center. Bethesda, Md. Available at http://reprotox.org. Accessed Oct. 5, 2007.
8. PDR for Nonprescription Drugs, Dietary Supplements, and Herbs. 28th ed. Montvale, NJ: Medical Economics; 2007.
<b>How selected drugs affect the human fetus and breastfed infant</b><cs cn="1" a="l"> <cs cn="2" a="l"> <cs cn="3" a="l"> <cs cn="4" a="l"> </cs></cs></cs></cs><row><entry v="t">DRUG</entry> <entry v="t">FIRST-TRIMESTER EFFECTS</entry> <entry v="t">EFFECTS DURING SECOND AND THIRD TRIMESTER</entry> <entry v="t">SAFETY DURING BREASTFEEDING</entry></row><row><entry cs="4">ANALGESICS</entry></row><row><entry v="t">Acetaminophen</entry> <entry v="t">None known</entry> <entry v="t">Hepatotoxicity/nephrotoxicity</entry> <entry v="t">Safe</entry></row><row><entry v="t">Ibuprofen</entry> <entry v="t">Gastroschisis (?)</entry> <entry v="t">Closure of ductus</entry> <entry v="t">Small amount passed; no other information</entry></row><row><entry v="t">Narcotics</entry> <entry v="t">None known</entry> <entry v="t">Depression, withdrawal</entry> <entry v="t">Not recommended if dosing is repetitive</entry></row><row><entry v="t">Salicylates</entry> <entry v="t">None known</entry> <entry v="t">Prolonged pregnancy and labor, hemorrhage, altered hemostasis, intracranial hemorrhage</entry> <entry v="t">Use with caution; may have adverse effects in newborn</entry></row><row><entry cs="4">ANESTHETICS</entry></row><row><entry v="t">General</entry> <entry v="t">Anomalies (?), abortion (?)</entry> <entry v="t">Depression</entry> <entry v="t"> </entry></row><row><entry v="t">Local</entry> <entry v="t">None known</entry> <entry v="t">Bradycardia, seizures</entry> <entry v="t"> </entry></row><row><entry cs="4">ANTI-ASTHMATICS</entry></row><row><entry v="t">Metaproterenol, salmeterol, albuterol</entry> <entry v="t">None known</entry> <entry v="t">None known</entry> <entry v="t">No information available</entry></row><row><entry v="t">Terbutaline</entry> <entry v="t">None known</entry> <entry v="t">Tachycardia, hypothermia, hypocalcemia, hypoglycemia, and hyperglycemia</entry> <entry v="t">Compatible</entry></row><row><entry v="t">Theophylline</entry> <entry v="t">None known</entry> <entry v="t">Jitteriness, tachycardia</entry> <entry v="t">May produce jitteriness, poor feeding, vomiting, cardiac arrhythmias</entry></row><row><entry cs="4">ANTICOAGULANTS</entry></row><row><entry v="t">Warfarin</entry> <entry v="t">Nasal hypoplasia, ophthalmic abnormalities, epiphyseal stippling</entry> <entry v="t">Hemorrhage, stillbirth</entry> <entry v="t">Safe</entry></row><row><entry v="t">Heparin, low molecular weight</entry> <entry v="t">None known</entry> <entry v="t">Hemorrhage (?), stillbirth (?)</entry> <entry v="t">Safe</entry></row><row><entry cs="4">ANTICONVULSANTS</entry></row><row><entry v="t">Barbiturates</entry> <entry v="t">Malformations (?)</entry> <entry v="t">Bleeding, withdrawal</entry> <entry v="t">Not recommended</entry></row><row><entry v="t">Carbamazepine, oxcarbazepine</entry> <entry v="t">Craniofacial, neural tube (?)</entry> <entry v="t">Bleeding, withdrawal, growth restriction</entry> <entry v="t">Probably safe</entry></row><row><entry v="t">Clonazepam</entry> <entry v="t">None known</entry> <entry v="t">Withdrawal, depression</entry> <entry v="t">Not recommended (potential for apnea, cyanosis, or hypotonia); serum levels should be monitored</entry></row><row><entry v="t">Ethosuximide</entry> <entry v="t">None known</entry> <entry v="t">None known</entry> <entry v="t">Compatible</entry></row><row><entry v="t">Gabapentin</entry> <entry v="t">Unknown</entry> <entry v="t">None known</entry> <entry v="t">Unknown</entry></row><row><entry v="t">Phenytoin*</entry> <entry v="t">Craniofacial abnormalities, mental retardation, hypoplasia of phalanges</entry> <entry v="t">Hemorrhage, depletion of vitamin K-dependent clotting factors</entry> <entry v="t">Probably safe</entry></row><row><entry v="t">Primidone</entry> <entry v="t">Orofacial clefts</entry> <entry v="t">Hemorrhage, depletion of vitamin K-dependent clotting factors, intrauterine growth restriction</entry> <entry v="t">May produce significant adverse effects in infants; use with caution</entry></row><row><entry v="t">Trimethadione*</entry> <entry v="t">Mental retardation, facial dysmorphogenesis, cardiovascular effects</entry> <entry v="t">Hemorrhage, depletion of vitamin K-dependent clotting factors, intrauterine growth restriction</entry> <entry v="t">No information available</entry></row><row><entry v="t">Valproic acid*</entry> <entry v="t">Spina bifida, facial dysmorphogenesis</entry> <entry v="t">Perinatal distress, behavioral abnormalities</entry> <entry v="t">Safe</entry></row><row><entry cs="4">ANTI-EMETICS</entry></row><row><entry v="t">Diphenhydramine</entry> <entry v="t">None known, clefting unlikely</entry> <entry v="t">None known</entry> <entry v="t">Safe, but may cause drowsiness</entry></row><row><entry v="t">Doxylamine (with pyridoxine)</entry> <entry v="t">None known</entry> <entry v="t">None known</entry> <entry v="t">Unknown; probably sedating</entry></row><row><entry v="t">Meclizine</entry> <entry v="t">None known</entry> <entry v="t">Retrolental fibrosis in premature infant</entry> <entry v="t">Unknown</entry></row><row><entry v="t">Metoclopramide</entry> <entry v="t">None known</entry> <entry v="t">None known</entry> <entry v="t">Potential central nervous system effects</entry></row><row><entry v="t">Ondansetron</entry> <entry v="t">Unknown</entry> <entry v="t">Unknown</entry> <entry v="t">Unknown</entry></row><row><entry v="t">Promethazine</entry> <entry v="t">None known</entry> <entry v="t">None known</entry> <entry v="t">Compatible</entry></row><row><entry v="t">Scopolamine</entry> <entry v="t">None known</entry> <entry v="t">Fetal heart rate changes</entry> <entry v="t">Compatible</entry></row><row><entry cs="4">ANTIBACTERIALS</entry></row><row><entry v="t">Aminoglycosides</entry> <entry v="t">None known</entry> <entry v="t">Nephrotoxic (?), ototoxic (?)</entry> <entry v="t">Depends on level of exposure and renal function of infant</entry></row><row><entry v="t">Azithromycin</entry> <entry v="t">None known</entry> <entry v="t">None known</entry> <entry v="t">Compatible</entry></row><row><entry v="t">Cephalosporins</entry> <entry v="t">None known</entry> <entry v="t">None known</entry> <entry v="t">Probably Compatible</entry></row><row><entry v="t">Chloramphenicol</entry> <entry v="t">None known</entry> <entry v="t">Vascular collapse</entry> <entry v="t">Contraindicated</entry></row><row><entry v="t">Ciprofloxacin</entry> <entry v="t">Toxic to developing cartilage (?)</entry> <entry v="t">Toxic to developing cartilage (?)</entry> <entry v="t">Compatible</entry></row><row><entry v="t">Clindamycin</entry> <entry v="t">Unknown</entry> <entry v="t">Unknown</entry> <entry v="t">Compatible, but potential modification of bowel flora, interference with culture interpretation after fever work-up in infants</entry></row><row><entry v="t">Erythromycin</entry> <entry v="t">None known</entry> <entry v="t">None known</entry> <entry v="t">Compatible</entry></row><row><entry v="t">Isoniazid</entry> <entry v="t">Malformations (?)</entry> <entry v="t">Behavioral abnormality</entry> <entry v="t">Compatible</entry></row><row><entry v="t">Metronidazole</entry> <entry v="t">None known</entry> <entry v="t">None known</entry> <entry v="t">Use with caution because of mutagenic and carcinogenic effects in some species; abstain from breastfeeding for 12–24 hours after single dose</entry></row><row><entry v="t">Nitrofurantoin</entry> <entry v="t">None known</entry> <entry v="t">Hemolysis (?)</entry> <entry v="t">Compatible</entry></row><row><entry v="t">Penicillins</entry> <entry v="t">None known</entry> <entry v="t">None known</entry> <entry v="t">Compatible</entry></row><row><entry v="t">Rifampin</entry> <entry v="t">Risk of malformation not greater than in general population</entry> <entry v="t">None known</entry> <entry v="t">Compatible</entry></row><row><entry v="t">Sulfonamides</entry> <entry v="t">None known</entry> <entry v="t">None known</entry> <entry v="t">Generally Compatible, but avoid in infants with hyperbilirubinemia, premature infants, and infants with G6PD deficiency</entry></row><row><entry v="t">Tetracyclines</entry> <entry v="t">None known</entry> <entry v="t">Stained deciduous teeth (enamel hypoplasia)</entry> <entry v="t">Compatible</entry></row><row><entry v="t">Trimethoprim</entry> <entry v="t">Cleft palate, micrognathia, limb shortening</entry> <entry v="t">Unknown</entry> <entry v="t">Compatible</entry></row><row><entry cs="4">ANTIFUNGALS</entry></row><row><entry v="t">Amphotericin-B</entry> <entry v="t">Unknown</entry> <entry v="t">Unknown</entry> <entry v="t">Unknown</entry></row><row><entry v="t">Fluconazole</entry> <entry v="t">None known</entry> <entry v="t">None known</entry> <entry v="t">Compatible</entry></row><row><entry cs="4">ANTIRETROVIRALS</entry></row><row><entry v="t">Class of drugs in general</entry> <entry v="t">None known</entry> <entry v="t">None known</entry> <entry v="t">Contraindicated (HIV)</entry></row><row><entry cs="4">ANTIVIRALS</entry></row><row><entry v="t">Acyclovir</entry> <entry v="t">None known</entry> <entry v="t">None known</entry> <entry v="t">Compatible</entry></row><row><entry v="t">Interferon</entry> <entry v="t">None known</entry> <entry v="t">Intrauterine growth restriction (?)</entry> <entry v="t">Likely safe</entry></row><row><entry cs="4">CANCER CHEMOTHERAPY</entry></row><row><entry v="t">Alkylating agents</entry> <entry v="t">Abortion, anomalies</entry> <entry v="t">Hypoplastic gonads, growth restriction and delay</entry> <entry v="t">Contraindicated</entry></row><row><entry v="t">Antimetabolites <list type="bullet"> <item><para>Folic acid analogues (methotrexate)</para></item> <item><para>Purine analogues</para></item> <item><para>Pyrimidine analogues (cytosine arabinoside, 5-fluorouracil)</para></item> </list></entry> <entry v="t"><list type="bullet"> <item><para>Abortion, intrauterine growth restriction, cranial anomalies</para></item> <item><para>Same as above</para></item> <item><para>Same as above</para></item></list></entry> <entry v="t"><list type="bullet"> <item><para>Hypoplastic gonads, growth restriction and delay</para></item> <item><para>Same as above, plus transient anemia</para></item> <item><para>Same as above</para></item></list></entry> <entry v="t"><list type="bullet"> <item><para>Contraindicated</para></item> <item><para>Contraindicated</para></item> <item><para>Contraindicated</para></item></list></entry></row><row><entry v="t">Antibiotics <list type="bullet"> <item><para>Actinomycin</para></item> <item><para>Vinca alkaloids (vincristine)</para></item></list></entry> <entry v="t"><list type="bullet"> <item><para>Abortion, intrauterine growth restriction, cranial anomalies</para></item> <item><para>Same as above</para></item></list></entry> <entry v="t"><list type="bullet"> <item><para>Hypoplastic gonads, growth restriction and delay</para></item> <item><para>Same as above</para></item></list></entry> <entry v="t"><list type="bullet"> <item><para>Contraindicated</para></item> <item><para>Contraindicated</para></item></list></entry></row><row><entry cs="4">CARDIOVASCULAR DRUGS</entry></row><row><entry v="t">ACE inhibitors</entry> <entry v="t">None known</entry> <entry v="t">Oliguria, skull defects, death</entry> <entry v="t">Compatible</entry></row><row><entry v="t">Adenosine</entry> <entry v="t">None known</entry> <entry v="t">No effects on fetal heart rate</entry> <entry v="t">Unknown</entry></row><row><entry v="t">Beta-sympathomimetics</entry> <entry v="t">None known</entry> <entry v="t">Tachycardia, hypothermia, hypocalcemia, hypoglycemia, and hyperglycemia</entry> <entry v="t">Compatible</entry></row><row><entry v="t">Calcium channel blockers</entry> <entry v="t">Unknown</entry> <entry v="t">None known</entry> <entry v="t">Compatible</entry></row><row><entry v="t">Digitalis</entry> <entry v="t">None known</entry> <entry v="t">Lower heart rate</entry> <entry v="t">Compatible</entry></row><row><entry v="t">Hydralazine</entry> <entry v="t">Skeletal defects (?)</entry> <entry v="t">Tachycardia, thrombocytopenia, fetal distress</entry> <entry v="t">Compatible</entry></row><row><entry v="t">Methyldopa</entry> <entry v="t">None known</entry> <entry v="t">Hemolytic anemia, tremor, hypotension</entry> <entry v="t">Compatible</entry></row><row><entry v="t">Propranolol, labetalol</entry> <entry v="t">Unknown</entry> <entry v="t">Lower heart rate, intrauterine growth restriction (?), hypoglycemia, respiratory distress</entry> <entry v="t">Compatible; hypoglycemia (?)</entry></row><row><entry v="t">Reserpine</entry> <entry v="t">None known</entry> <entry v="t">Lethargy, respiratory distress</entry> <entry v="t">Unknown</entry></row><row><entry cs="4">COLD AND COUGH PREPARATIONS</entry></row><row><entry v="t">Antihistamines</entry> <entry v="t">None known</entry> <entry v="t">None known</entry> <entry v="t">Reduced milk (?); drowsiness</entry></row><row><entry v="t">Cough suppressants</entry> <entry v="t">None known</entry> <entry v="t">None known</entry> <entry v="t">Compatible</entry></row><row><entry v="t">Decongestants</entry> <entry v="t">None known</entry> <entry v="t">None known</entry> <entry v="t">Compatible</entry></row><row><entry v="t">Dextromethorphan</entry> <entry v="t">None known</entry> <entry v="t">None known</entry> <entry v="t">No information</entry></row><row><entry v="t">Expectorants</entry> <entry v="t">Fetal goiter (?)</entry> <entry v="t">None known</entry> <entry v="t">Compatible</entry></row><row><entry v="t">Loratadine</entry> <entry v="t">Likely none</entry> <entry v="t">Likely none</entry> <entry v="t">Compatible</entry></row><row><entry cs="4">DIURETICS</entry></row><row><entry v="t">Furosemide</entry> <entry v="t">Unknown</entry> <entry v="t">Death from sudden hypoperfusion, electrolyte imbalance</entry> <entry v="t">Found to suppress lactation</entry></row><row><entry v="t">Thiazides</entry> <entry v="t">None known</entry> <entry v="t">Thrombocytopenia, hypokalemia, hyperbilirubinemia, hyponatremia</entry> <entry v="t">Compatible</entry></row><row><entry cs="4">FERTILITY DRUGS</entry></row><row><entry v="t">Clomiphene</entry> <entry v="t">Meiotic nondisjunction (?), neural tube defects (?)</entry> <entry v="t">Unknown</entry> <entry v="t">No data available</entry></row><row><entry cs="4">GASTROINTESTINAL AGENTS</entry></row><row><entry v="t">Bisacodyl</entry> <entry v="t">Unknown</entry> <entry v="t">Unknown</entry> <entry v="t">No reports of adverse effects</entry></row><row><entry v="t">Cholestyramine</entry> <entry v="t">None known</entry> <entry v="t">None known, but fat-soluble vitamins are depleted</entry> <entry v="t">Unknown</entry></row><row><entry v="t">Colestipol</entry> <entry v="t">Unknown</entry> <entry v="t">Unknown, but minimal absorption</entry> <entry v="t">No data available</entry></row><row><entry v="t">Docusate</entry> <entry v="t">None known</entry> <entry v="t">None known</entry> <entry v="t">Compatible</entry></row><row><entry v="t">H2-histamine receptor blockers</entry> <entry v="t">None known</entry> <entry v="t">Anti-androgen effect (cimetidine)</entry> <entry v="t">Compatible</entry></row><row><entry v="t">Magnesium hydroxide (Milk of Magnesia)</entry> <entry v="t">None known</entry> <entry v="t">None known</entry> <entry v="t">Compatible</entry></row><row><entry v="t">Mineral oil</entry> <entry v="t">Decreased maternal vitamin absorption</entry> <entry v="t">Decreased maternal vitamin absorption</entry> <entry v="t">Compatible</entry></row><row><entry v="t">Proton pump inhibitors</entry> <entry v="t">None known</entry> <entry v="t">None known</entry> <entry v="t">Unknown</entry></row><row><entry v="t">Sulfasalazine</entry> <entry v="t">None known</entry> <entry v="t">None known</entry> <entry v="t">Caution with ill infants</entry></row><row><entry cs="4">HORMONES</entry></row><row><entry v="t">Androgens*</entry> <entry v="t">Virilization of female fetus</entry> <entry v="t">Adrenal suppression</entry> <entry v="t">No adverse effects reported</entry></row><row><entry v="t">Corticosteroids</entry> <entry v="t">Orofacial cleft in animals, not in humans</entry> <entry v="t">No adverse effects in humans</entry> <entry v="t">No data available</entry></row><row><entry v="t">Danazol</entry> <entry v="t">Virilization of female fetus (?)</entry> <entry v="t">None known</entry> <entry v="t">No information available</entry></row><row><entry v="t">Estrogens</entry> <entry v="t">Cardiovascular anomalies (?)</entry> <entry v="t">None known</entry> <entry v="t">No reported adverse effects</entry></row><row><entry v="t">Progestins</entry> <entry v="t">Limb and cardiovascular anomalies (?), VACTERL syndrome (?), masculinization of female fetus (?)</entry> <entry v="t">None known</entry> <entry v="t">No reported adverse effects</entry></row><row><entry cs="4">DIABETES CARE</entry></row><row><entry v="t">Glucagon</entry> <entry v="t">None known</entry> <entry v="t">None known</entry> <entry v="t">Compatible</entry></row><row><entry v="t">Glyburide</entry> <entry v="t">None known</entry> <entry v="t">Not thought to cross the placenta in significant amounts; no neonatal hypoglycemia</entry> <entry v="t">Compatible</entry></row><row><entry v="t">Insulin</entry> <entry v="t">None known</entry> <entry v="t">None known</entry> <entry v="t">Safe</entry></row><row><entry v="t">Metformin</entry> <entry v="t">None known</entry> <entry v="t">Neonatal hypoglycemia</entry> <entry v="t">Unknown</entry></row><row><entry v="t">Sulfonylureas</entry> <entry v="t">Anomalies (?)</entry> <entry v="t">Suppressed insulin secretion</entry> <entry v="t">Compatible</entry></row><row><entry cs="4">MIGRAINE REMEDIES</entry></row><row><entry v="t">Ergotamine</entry> <entry v="t">None known</entry> <entry v="t">May stimulate contractions</entry> <entry v="t">Use with caution</entry></row><row><entry v="t">Sumatriptan</entry> <entry v="t">None known</entry> <entry v="t">None known</entry> <entry v="t">Compatible</entry></row><row><entry cs="4">PSYCHOACTIVE DRUGS, ANTIDEPRESSANTS</entry></row><row><entry v="t">Amphetamine</entry> <entry v="t">Inconsistent; likely none</entry> <entry v="t">Reduced weight</entry> <entry v="t">Contraindicated</entry></row><row><entry v="t">Benzodiazepines</entry> <entry v="t">Facial dysmorphism (?)</entry> <entry v="t">Depression, floppy infant, hypothermia, withdrawal</entry> <entry v="t">Some concern about central nervous system toxicity with long-term use</entry></row><row><entry v="t">Fluoxetine</entry> <entry v="t">None known</entry> <entry v="t">None known</entry> <entry v="t">Symptoms of colic</entry></row><row><entry v="t">Hydroxyzine</entry> <entry v="t">None known</entry> <entry v="t">None known</entry> <entry v="t">No information</entry></row><row><entry v="t">Lithium</entry> <entry v="t">Facial clefts; cardiovascular anomaly</entry> <entry v="t">Lithium toxicity (neurologic and hepatic dysfunction)</entry> <entry v="t">Contraindicated</entry></row><row><entry v="t">Meprobamate</entry> <entry v="t">Cardiac anomalies (?), major malformations (?)</entry> <entry v="t">None known</entry> <entry v="t">Unknown</entry></row><row><entry v="t">Phenothiazines</entry> <entry v="t">None known</entry> <entry v="t">Muscle rigidity, hypothermia, tremor</entry> <entry v="t">Unknown</entry></row><row><entry v="t">Sedatives</entry> <entry v="t">None known</entry> <entry v="t">Depression, slow learning</entry> <entry v="t">Not recommended</entry></row><row><entry v="t">Thalidomide*</entry> <entry v="t">Phocomelia in 20% of cases</entry> <entry v="t">None known</entry> <entry v="t">No information</entry></row><row><entry v="t">Tricyclics</entry> <entry v="t">None known</entry> <entry v="t">None known</entry> <entry v="t">Unknown/caution</entry></row><row><entry v="t">Zolpiden</entry> <entry v="t">Unknown</entry> <entry v="t">Withdrawal or floppy infant (?)</entry> <entry v="t">Compatible</entry></row><row><entry cs="4">RADIOLABELED DIAGNOSTICS</entry></row><row><entry v="t">Albumin</entry> <entry v="t">None known</entry> <entry v="t">None known</entry> <entry v="t">No information available</entry></row><row><entry v="t">I131 (diagnostic)</entry> <entry v="t">None known</entry> <entry v="t">None known</entry> <entry v="t">Not recommended during exposure; may continue 24 hours after exposure</entry></row><row><entry v="t">Technetium</entry> <entry v="t">None known</entry> <entry v="t">None known</entry> <entry v="t">Not recommended during exposure; may continue 24 hours after exposure</entry></row><row><entry cs="4">SMOKING CESSATION</entry></row><row><entry v="t">Bupropion</entry> <entry v="t">Likely none</entry> <entry v="t">None known</entry> <entry v="t">Compatible</entry></row><row><entry v="t">Nicotine</entry> <entry v="t">Spontaneous abortion (?)</entry> <entry v="t">Impaired growth (?)</entry> <entry v="t">Consistent with passive smoking</entry></row><row><entry cs="4">THYROID MEDICATION</entry></row><row><entry v="t">I131 (therapeutic)</entry> <entry v="t">Goiter, abortion, anomalies</entry> <entry v="t">Goiter, airway obstruction, hyperthyroid, mental retardation</entry> <entry v="t">Contraindicated</entry></row><row><entry v="t">Methimazole</entry> <entry v="t">Aplasia cutis (?), goiter</entry> <entry v="t">Goiter, airway obstruction, hyperthyroid, mental retardation, aplasia cutis (?)</entry> <entry v="t">Compatible, but monitor fetal thyroid function</entry></row><row><entry v="t">Propylthiouracil</entry> <entry v="t">Goiter</entry> <entry v="t">Same as above</entry> <entry v="t">Safe, but monitor baby’s thyroid status</entry></row><row><entry v="t">Thyroid USP</entry> <entry v="t">None known</entry> <entry v="t">None known</entry> <entry v="t">Compatible</entry></row><row><entry v="t">Thyroxine</entry> <entry v="t">None known</entry> <entry v="t">None known</entry> <entry v="t">Compatible</entry></row><row><entry cs="4">TOCOLYTICS</entry></row><row><entry v="t">Beta-sympathomimetics</entry> <entry v="t">None known</entry> <entry v="t">Tachycardia, hypothermia, hypocalcemia, hypoglycemia, hyperglycemia</entry> <entry v="t">—</entry></row><row><entry v="t">Indomethacin</entry> <entry v="t">None known</entry> <entry v="t">Oligohydramnios (>48 hours of use)</entry> <entry v="t">—</entry></row><row><entry v="t">Magnesium sulfate</entry> <entry v="t">None known</entry> <entry v="t">Hypermagnesemia, respiratory depression</entry> <entry v="t">—</entry></row><row><entry v="t">Nifedipine</entry> <entry v="t">Unknown</entry> <entry v="t">None known</entry> <entry v="t">—</entry></row><row><entry cs="4">VACCINATIONS</entry></row><row><entry v="t">Influenza</entry> <entry v="t">None known</entry> <entry v="t">Passive immunization</entry> <entry v="t">Compatible</entry></row><row><entry v="t">Pneumovaccine</entry> <entry v="t">None known</entry> <entry v="t">Passive immunization</entry> <entry v="t">Compatible</entry></row><row><entry v="t">Tetanus toxoid</entry> <entry v="t">None known</entry> <entry v="t">Passive immunization</entry> <entry v="t">Compatible</entry></row><row><entry cs="4">VAGINAL PREPARATIONS</entry></row><row><entry v="t">Antifungal agents</entry> <entry v="t">None known</entry> <entry v="t">None known</entry> <entry v="t">Compatible</entry></row><row><entry v="t">Podophyllin</entry> <entry v="t">Mutagenesis (?)</entry> <entry v="t">Central nervous system effects (?)</entry> <entry v="t">Contraindicated</entry></row><row><entry cs="4">VITAMINS (high dose)</entry></row><row><entry v="t">A</entry> <entry v="t">Urogenital and craniofacial anomalies (?)</entry> <entry v="t">None known</entry> <entry v="t">No data available</entry></row><row><entry v="t">C</entry> <entry v="t">None known</entry> <entry v="t">Scurvy after delivery</entry> <entry v="t">Compatible</entry></row><row><entry v="t">D</entry> <entry v="t">Supravalvular aortic stenosis (?)</entry> <entry v="t">None known</entry> <entry v="t">Compatible</entry></row><row><entry v="t">E</entry> <entry v="t">Unknown</entry> <entry v="t">None known</entry> <entry v="t">Compatible</entry></row><row><entry v="t">K</entry> <entry v="t">Unknown</entry> <entry v="t">Hemorrhage, if deficiency</entry> <entry v="t">Compatible</entry></row><row><entry cs="4">“STREET” DRUGS</entry></row><row><entry v="t">Cocaine</entry> <entry v="t">Placental abruption, vascular disruption, urinary tract anomalies</entry> <entry v="t">Withdrawal, placental abruption, vascular disruption, growth restriction</entry> <entry v="t">Contraindicated</entry></row><row><entry v="t">Heroin</entry> <entry v="t">None known</entry> <entry v="t">Depression, withdrawal, growth restriction</entry> <entry v="t">Contraindicated</entry></row><row><entry v="t">LSD</entry> <entry v="t">None known</entry> <entry v="t">Withdrawal, behavioral effects</entry> <entry v="t">Contraindicated</entry></row><row><entry v="t">Marijuana</entry> <entry v="t">None known</entry> <entry v="t">Behavioral effects, growth restriction</entry> <entry v="t">Contraindicated</entry></row><row><entry v="t">Methadone</entry> <entry v="t">None known</entry> <entry v="t">Withdrawal, growth restriction</entry> <entry v="t">Contraindicated</entry></row><row><entry v="t">Methamphetamine</entry> <entry v="t">None known</entry> <entry v="t">Withdrawal, growth restriction</entry> <entry v="t">Contraindicated</entry></row><row><entry v="t">Pentazocine</entry> <entry v="t">None known</entry> <entry v="t">Withdrawal, growth restriction</entry> <entry v="t">Contraindicated</entry></row><row><entry v="t">Phencyclidine</entry> <entry v="t">None known</entry> <entry v="t">Withdrawal, neurobehavioral effects, growth restriction</entry> <entry v="t">Contraindicated</entry></row><row><entry cs="4">OTHER DRUGS</entry></row><row><entry v="t">Azathioprine</entry> <entry v="t">Abortion</entry> <entry v="t">Anemia, thrombocytopenia, lymphopenia, growth retardation</entry> <entry v="t">Not recommended</entry></row><row><entry v="t">Bromocriptine</entry> <entry v="t">None known</entry> <entry v="t">None known</entry> <entry v="t">Compatible</entry></row><row><entry v="t">Caffeine</entry> <entry v="t">Anomalies (?) in high doses, abortion (?)</entry> <entry v="t">Jitteriness</entry> <entry v="t">Not recommended</entry></row><row><entry v="t">Immune gamma globulin</entry> <entry v="t">None known</entry> <entry v="t">None known</entry> <entry v="t">Compatible</entry></row><row><entry v="t">Isotretinoin*</entry> <entry v="t">Central nervous system, cardiac, facial anomalies</entry> <entry v="t">Stillbirth, mental retardation (?)</entry> <entry v="t">Contraindicated</entry></row><row><entry v="t">Misoprostol</entry> <entry v="t">Abortion; variety of anomalies (cranium, limb, oral cleft); Mobius sequence</entry> <entry v="t">None with low dose for cervical ripening; placental abruption</entry> <entry v="t">Contraindicated, especially if diarrhea occurs</entry></row><row><entry v="t">Spermicides</entry> <entry v="t">None known</entry> <entry v="t">None known</entry> <entry v="t">No information</entry></row><row><entry cs="4">* Proven teratogen.</entry></row><row><entry cs="4">Unknown=no studies to investigate fetal effects; none known=no malformations reported in human studies or no consistent malformations in animal studies; (?)=conflicting information</entry></row><row><entry cs="4">Source: Reprotox data from humans, last reviewed in 2006.</entry></row>
Reference
1. American College of Obstetricians and Gynecologists. Teratology. ACOG Educational Bulletin #236. Washington, DC: ACOG; 1997.
2. Splinter M, Nightingale B, Sawgraves R, Rayburn W. Medication use during pregnancy by women delivering at a tertiary university hospital. South Med J. 1997;90:498-502.
3. Physician’s Desk Reference. 61st ed. Montvale, NJ: Medical Economics; 2007.
4. Briggs GG, Freeman RK, Yaffee FJ. Drugs in Pregnancy and Lactation: Reference Guide to Fetal and Neonatal Risk. 7th ed. Baltimore: Williams & Wilkins; 2005.
5. Briggs GG, Freeman RK, Yaffee FJ. ReproTox Database. Vol. 13. No. 1. Bethesda, Md: Reproductive Toxicology Center; 2000.
6. Heinonen OP, Sloan ED, Shapiro S. Birth Defects and Drugs in Pregnancy. Boston: John Wright PSG; 1973.
7. Reproductive Toxicology Center. Bethesda, Md. Available at http://reprotox.org. Accessed Oct. 5, 2007.
8. PDR for Nonprescription Drugs, Dietary Supplements, and Herbs. 28th ed. Montvale, NJ: Medical Economics; 2007.
<b>How selected drugs affect the human fetus and breastfed infant</b><cs cn="1" a="l"> <cs cn="2" a="l"> <cs cn="3" a="l"> <cs cn="4" a="l"> </cs></cs></cs></cs><row><entry v="t">DRUG</entry> <entry v="t">FIRST-TRIMESTER EFFECTS</entry> <entry v="t">EFFECTS DURING SECOND AND THIRD TRIMESTER</entry> <entry v="t">SAFETY DURING BREASTFEEDING</entry></row><row><entry cs="4">ANALGESICS</entry></row><row><entry v="t">Acetaminophen</entry> <entry v="t">None known</entry> <entry v="t">Hepatotoxicity/nephrotoxicity</entry> <entry v="t">Safe</entry></row><row><entry v="t">Ibuprofen</entry> <entry v="t">Gastroschisis (?)</entry> <entry v="t">Closure of ductus</entry> <entry v="t">Small amount passed; no other information</entry></row><row><entry v="t">Narcotics</entry> <entry v="t">None known</entry> <entry v="t">Depression, withdrawal</entry> <entry v="t">Not recommended if dosing is repetitive</entry></row><row><entry v="t">Salicylates</entry> <entry v="t">None known</entry> <entry v="t">Prolonged pregnancy and labor, hemorrhage, altered hemostasis, intracranial hemorrhage</entry> <entry v="t">Use with caution; may have adverse effects in newborn</entry></row><row><entry cs="4">ANESTHETICS</entry></row><row><entry v="t">General</entry> <entry v="t">Anomalies (?), abortion (?)</entry> <entry v="t">Depression</entry> <entry v="t"> </entry></row><row><entry v="t">Local</entry> <entry v="t">None known</entry> <entry v="t">Bradycardia, seizures</entry> <entry v="t"> </entry></row><row><entry cs="4">ANTI-ASTHMATICS</entry></row><row><entry v="t">Metaproterenol, salmeterol, albuterol</entry> <entry v="t">None known</entry> <entry v="t">None known</entry> <entry v="t">No information available</entry></row><row><entry v="t">Terbutaline</entry> <entry v="t">None known</entry> <entry v="t">Tachycardia, hypothermia, hypocalcemia, hypoglycemia, and hyperglycemia</entry> <entry v="t">Compatible</entry></row><row><entry v="t">Theophylline</entry> <entry v="t">None known</entry> <entry v="t">Jitteriness, tachycardia</entry> <entry v="t">May produce jitteriness, poor feeding, vomiting, cardiac arrhythmias</entry></row><row><entry cs="4">ANTICOAGULANTS</entry></row><row><entry v="t">Warfarin</entry> <entry v="t">Nasal hypoplasia, ophthalmic abnormalities, epiphyseal stippling</entry> <entry v="t">Hemorrhage, stillbirth</entry> <entry v="t">Safe</entry></row><row><entry v="t">Heparin, low molecular weight</entry> <entry v="t">None known</entry> <entry v="t">Hemorrhage (?), stillbirth (?)</entry> <entry v="t">Safe</entry></row><row><entry cs="4">ANTICONVULSANTS</entry></row><row><entry v="t">Barbiturates</entry> <entry v="t">Malformations (?)</entry> <entry v="t">Bleeding, withdrawal</entry> <entry v="t">Not recommended</entry></row><row><entry v="t">Carbamazepine, oxcarbazepine</entry> <entry v="t">Craniofacial, neural tube (?)</entry> <entry v="t">Bleeding, withdrawal, growth restriction</entry> <entry v="t">Probably safe</entry></row><row><entry v="t">Clonazepam</entry> <entry v="t">None known</entry> <entry v="t">Withdrawal, depression</entry> <entry v="t">Not recommended (potential for apnea, cyanosis, or hypotonia); serum levels should be monitored</entry></row><row><entry v="t">Ethosuximide</entry> <entry v="t">None known</entry> <entry v="t">None known</entry> <entry v="t">Compatible</entry></row><row><entry v="t">Gabapentin</entry> <entry v="t">Unknown</entry> <entry v="t">None known</entry> <entry v="t">Unknown</entry></row><row><entry v="t">Phenytoin*</entry> <entry v="t">Craniofacial abnormalities, mental retardation, hypoplasia of phalanges</entry> <entry v="t">Hemorrhage, depletion of vitamin K-dependent clotting factors</entry> <entry v="t">Probably safe</entry></row><row><entry v="t">Primidone</entry> <entry v="t">Orofacial clefts</entry> <entry v="t">Hemorrhage, depletion of vitamin K-dependent clotting factors, intrauterine growth restriction</entry> <entry v="t">May produce significant adverse effects in infants; use with caution</entry></row><row><entry v="t">Trimethadione*</entry> <entry v="t">Mental retardation, facial dysmorphogenesis, cardiovascular effects</entry> <entry v="t">Hemorrhage, depletion of vitamin K-dependent clotting factors, intrauterine growth restriction</entry> <entry v="t">No information available</entry></row><row><entry v="t">Valproic acid*</entry> <entry v="t">Spina bifida, facial dysmorphogenesis</entry> <entry v="t">Perinatal distress, behavioral abnormalities</entry> <entry v="t">Safe</entry></row><row><entry cs="4">ANTI-EMETICS</entry></row><row><entry v="t">Diphenhydramine</entry> <entry v="t">None known, clefting unlikely</entry> <entry v="t">None known</entry> <entry v="t">Safe, but may cause drowsiness</entry></row><row><entry v="t">Doxylamine (with pyridoxine)</entry> <entry v="t">None known</entry> <entry v="t">None known</entry> <entry v="t">Unknown; probably sedating</entry></row><row><entry v="t">Meclizine</entry> <entry v="t">None known</entry> <entry v="t">Retrolental fibrosis in premature infant</entry> <entry v="t">Unknown</entry></row><row><entry v="t">Metoclopramide</entry> <entry v="t">None known</entry> <entry v="t">None known</entry> <entry v="t">Potential central nervous system effects</entry></row><row><entry v="t">Ondansetron</entry> <entry v="t">Unknown</entry> <entry v="t">Unknown</entry> <entry v="t">Unknown</entry></row><row><entry v="t">Promethazine</entry> <entry v="t">None known</entry> <entry v="t">None known</entry> <entry v="t">Compatible</entry></row><row><entry v="t">Scopolamine</entry> <entry v="t">None known</entry> <entry v="t">Fetal heart rate changes</entry> <entry v="t">Compatible</entry></row><row><entry cs="4">ANTIBACTERIALS</entry></row><row><entry v="t">Aminoglycosides</entry> <entry v="t">None known</entry> <entry v="t">Nephrotoxic (?), ototoxic (?)</entry> <entry v="t">Depends on level of exposure and renal function of infant</entry></row><row><entry v="t">Azithromycin</entry> <entry v="t">None known</entry> <entry v="t">None known</entry> <entry v="t">Compatible</entry></row><row><entry v="t">Cephalosporins</entry> <entry v="t">None known</entry> <entry v="t">None known</entry> <entry v="t">Probably Compatible</entry></row><row><entry v="t">Chloramphenicol</entry> <entry v="t">None known</entry> <entry v="t">Vascular collapse</entry> <entry v="t">Contraindicated</entry></row><row><entry v="t">Ciprofloxacin</entry> <entry v="t">Toxic to developing cartilage (?)</entry> <entry v="t">Toxic to developing cartilage (?)</entry> <entry v="t">Compatible</entry></row><row><entry v="t">Clindamycin</entry> <entry v="t">Unknown</entry> <entry v="t">Unknown</entry> <entry v="t">Compatible, but potential modification of bowel flora, interference with culture interpretation after fever work-up in infants</entry></row><row><entry v="t">Erythromycin</entry> <entry v="t">None known</entry> <entry v="t">None known</entry> <entry v="t">Compatible</entry></row><row><entry v="t">Isoniazid</entry> <entry v="t">Malformations (?)</entry> <entry v="t">Behavioral abnormality</entry> <entry v="t">Compatible</entry></row><row><entry v="t">Metronidazole</entry> <entry v="t">None known</entry> <entry v="t">None known</entry> <entry v="t">Use with caution because of mutagenic and carcinogenic effects in some species; abstain from breastfeeding for 12–24 hours after single dose</entry></row><row><entry v="t">Nitrofurantoin</entry> <entry v="t">None known</entry> <entry v="t">Hemolysis (?)</entry> <entry v="t">Compatible</entry></row><row><entry v="t">Penicillins</entry> <entry v="t">None known</entry> <entry v="t">None known</entry> <entry v="t">Compatible</entry></row><row><entry v="t">Rifampin</entry> <entry v="t">Risk of malformation not greater than in general population</entry> <entry v="t">None known</entry> <entry v="t">Compatible</entry></row><row><entry v="t">Sulfonamides</entry> <entry v="t">None known</entry> <entry v="t">None known</entry> <entry v="t">Generally Compatible, but avoid in infants with hyperbilirubinemia, premature infants, and infants with G6PD deficiency</entry></row><row><entry v="t">Tetracyclines</entry> <entry v="t">None known</entry> <entry v="t">Stained deciduous teeth (enamel hypoplasia)</entry> <entry v="t">Compatible</entry></row><row><entry v="t">Trimethoprim</entry> <entry v="t">Cleft palate, micrognathia, limb shortening</entry> <entry v="t">Unknown</entry> <entry v="t">Compatible</entry></row><row><entry cs="4">ANTIFUNGALS</entry></row><row><entry v="t">Amphotericin-B</entry> <entry v="t">Unknown</entry> <entry v="t">Unknown</entry> <entry v="t">Unknown</entry></row><row><entry v="t">Fluconazole</entry> <entry v="t">None known</entry> <entry v="t">None known</entry> <entry v="t">Compatible</entry></row><row><entry cs="4">ANTIRETROVIRALS</entry></row><row><entry v="t">Class of drugs in general</entry> <entry v="t">None known</entry> <entry v="t">None known</entry> <entry v="t">Contraindicated (HIV)</entry></row><row><entry cs="4">ANTIVIRALS</entry></row><row><entry v="t">Acyclovir</entry> <entry v="t">None known</entry> <entry v="t">None known</entry> <entry v="t">Compatible</entry></row><row><entry v="t">Interferon</entry> <entry v="t">None known</entry> <entry v="t">Intrauterine growth restriction (?)</entry> <entry v="t">Likely safe</entry></row><row><entry cs="4">CANCER CHEMOTHERAPY</entry></row><row><entry v="t">Alkylating agents</entry> <entry v="t">Abortion, anomalies</entry> <entry v="t">Hypoplastic gonads, growth restriction and delay</entry> <entry v="t">Contraindicated</entry></row><row><entry v="t">Antimetabolites <list type="bullet"> <item><para>Folic acid analogues (methotrexate)</para></item> <item><para>Purine analogues</para></item> <item><para>Pyrimidine analogues (cytosine arabinoside, 5-fluorouracil)</para></item> </list></entry> <entry v="t"><list type="bullet"> <item><para>Abortion, intrauterine growth restriction, cranial anomalies</para></item> <item><para>Same as above</para></item> <item><para>Same as above</para></item></list></entry> <entry v="t"><list type="bullet"> <item><para>Hypoplastic gonads, growth restriction and delay</para></item> <item><para>Same as above, plus transient anemia</para></item> <item><para>Same as above</para></item></list></entry> <entry v="t"><list type="bullet"> <item><para>Contraindicated</para></item> <item><para>Contraindicated</para></item> <item><para>Contraindicated</para></item></list></entry></row><row><entry v="t">Antibiotics <list type="bullet"> <item><para>Actinomycin</para></item> <item><para>Vinca alkaloids (vincristine)</para></item></list></entry> <entry v="t"><list type="bullet"> <item><para>Abortion, intrauterine growth restriction, cranial anomalies</para></item> <item><para>Same as above</para></item></list></entry> <entry v="t"><list type="bullet"> <item><para>Hypoplastic gonads, growth restriction and delay</para></item> <item><para>Same as above</para></item></list></entry> <entry v="t"><list type="bullet"> <item><para>Contraindicated</para></item> <item><para>Contraindicated</para></item></list></entry></row><row><entry cs="4">CARDIOVASCULAR DRUGS</entry></row><row><entry v="t">ACE inhibitors</entry> <entry v="t">None known</entry> <entry v="t">Oliguria, skull defects, death</entry> <entry v="t">Compatible</entry></row><row><entry v="t">Adenosine</entry> <entry v="t">None known</entry> <entry v="t">No effects on fetal heart rate</entry> <entry v="t">Unknown</entry></row><row><entry v="t">Beta-sympathomimetics</entry> <entry v="t">None known</entry> <entry v="t">Tachycardia, hypothermia, hypocalcemia, hypoglycemia, and hyperglycemia</entry> <entry v="t">Compatible</entry></row><row><entry v="t">Calcium channel blockers</entry> <entry v="t">Unknown</entry> <entry v="t">None known</entry> <entry v="t">Compatible</entry></row><row><entry v="t">Digitalis</entry> <entry v="t">None known</entry> <entry v="t">Lower heart rate</entry> <entry v="t">Compatible</entry></row><row><entry v="t">Hydralazine</entry> <entry v="t">Skeletal defects (?)</entry> <entry v="t">Tachycardia, thrombocytopenia, fetal distress</entry> <entry v="t">Compatible</entry></row><row><entry v="t">Methyldopa</entry> <entry v="t">None known</entry> <entry v="t">Hemolytic anemia, tremor, hypotension</entry> <entry v="t">Compatible</entry></row><row><entry v="t">Propranolol, labetalol</entry> <entry v="t">Unknown</entry> <entry v="t">Lower heart rate, intrauterine growth restriction (?), hypoglycemia, respiratory distress</entry> <entry v="t">Compatible; hypoglycemia (?)</entry></row><row><entry v="t">Reserpine</entry> <entry v="t">None known</entry> <entry v="t">Lethargy, respiratory distress</entry> <entry v="t">Unknown</entry></row><row><entry cs="4">COLD AND COUGH PREPARATIONS</entry></row><row><entry v="t">Antihistamines</entry> <entry v="t">None known</entry> <entry v="t">None known</entry> <entry v="t">Reduced milk (?); drowsiness</entry></row><row><entry v="t">Cough suppressants</entry> <entry v="t">None known</entry> <entry v="t">None known</entry> <entry v="t">Compatible</entry></row><row><entry v="t">Decongestants</entry> <entry v="t">None known</entry> <entry v="t">None known</entry> <entry v="t">Compatible</entry></row><row><entry v="t">Dextromethorphan</entry> <entry v="t">None known</entry> <entry v="t">None known</entry> <entry v="t">No information</entry></row><row><entry v="t">Expectorants</entry> <entry v="t">Fetal goiter (?)</entry> <entry v="t">None known</entry> <entry v="t">Compatible</entry></row><row><entry v="t">Loratadine</entry> <entry v="t">Likely none</entry> <entry v="t">Likely none</entry> <entry v="t">Compatible</entry></row><row><entry cs="4">DIURETICS</entry></row><row><entry v="t">Furosemide</entry> <entry v="t">Unknown</entry> <entry v="t">Death from sudden hypoperfusion, electrolyte imbalance</entry> <entry v="t">Found to suppress lactation</entry></row><row><entry v="t">Thiazides</entry> <entry v="t">None known</entry> <entry v="t">Thrombocytopenia, hypokalemia, hyperbilirubinemia, hyponatremia</entry> <entry v="t">Compatible</entry></row><row><entry cs="4">FERTILITY DRUGS</entry></row><row><entry v="t">Clomiphene</entry> <entry v="t">Meiotic nondisjunction (?), neural tube defects (?)</entry> <entry v="t">Unknown</entry> <entry v="t">No data available</entry></row><row><entry cs="4">GASTROINTESTINAL AGENTS</entry></row><row><entry v="t">Bisacodyl</entry> <entry v="t">Unknown</entry> <entry v="t">Unknown</entry> <entry v="t">No reports of adverse effects</entry></row><row><entry v="t">Cholestyramine</entry> <entry v="t">None known</entry> <entry v="t">None known, but fat-soluble vitamins are depleted</entry> <entry v="t">Unknown</entry></row><row><entry v="t">Colestipol</entry> <entry v="t">Unknown</entry> <entry v="t">Unknown, but minimal absorption</entry> <entry v="t">No data available</entry></row><row><entry v="t">Docusate</entry> <entry v="t">None known</entry> <entry v="t">None known</entry> <entry v="t">Compatible</entry></row><row><entry v="t">H2-histamine receptor blockers</entry> <entry v="t">None known</entry> <entry v="t">Anti-androgen effect (cimetidine)</entry> <entry v="t">Compatible</entry></row><row><entry v="t">Magnesium hydroxide (Milk of Magnesia)</entry> <entry v="t">None known</entry> <entry v="t">None known</entry> <entry v="t">Compatible</entry></row><row><entry v="t">Mineral oil</entry> <entry v="t">Decreased maternal vitamin absorption</entry> <entry v="t">Decreased maternal vitamin absorption</entry> <entry v="t">Compatible</entry></row><row><entry v="t">Proton pump inhibitors</entry> <entry v="t">None known</entry> <entry v="t">None known</entry> <entry v="t">Unknown</entry></row><row><entry v="t">Sulfasalazine</entry> <entry v="t">None known</entry> <entry v="t">None known</entry> <entry v="t">Caution with ill infants</entry></row><row><entry cs="4">HORMONES</entry></row><row><entry v="t">Androgens*</entry> <entry v="t">Virilization of female fetus</entry> <entry v="t">Adrenal suppression</entry> <entry v="t">No adverse effects reported</entry></row><row><entry v="t">Corticosteroids</entry> <entry v="t">Orofacial cleft in animals, not in humans</entry> <entry v="t">No adverse effects in humans</entry> <entry v="t">No data available</entry></row><row><entry v="t">Danazol</entry> <entry v="t">Virilization of female fetus (?)</entry> <entry v="t">None known</entry> <entry v="t">No information available</entry></row><row><entry v="t">Estrogens</entry> <entry v="t">Cardiovascular anomalies (?)</entry> <entry v="t">None known</entry> <entry v="t">No reported adverse effects</entry></row><row><entry v="t">Progestins</entry> <entry v="t">Limb and cardiovascular anomalies (?), VACTERL syndrome (?), masculinization of female fetus (?)</entry> <entry v="t">None known</entry> <entry v="t">No reported adverse effects</entry></row><row><entry cs="4">DIABETES CARE</entry></row><row><entry v="t">Glucagon</entry> <entry v="t">None known</entry> <entry v="t">None known</entry> <entry v="t">Compatible</entry></row><row><entry v="t">Glyburide</entry> <entry v="t">None known</entry> <entry v="t">Not thought to cross the placenta in significant amounts; no neonatal hypoglycemia</entry> <entry v="t">Compatible</entry></row><row><entry v="t">Insulin</entry> <entry v="t">None known</entry> <entry v="t">None known</entry> <entry v="t">Safe</entry></row><row><entry v="t">Metformin</entry> <entry v="t">None known</entry> <entry v="t">Neonatal hypoglycemia</entry> <entry v="t">Unknown</entry></row><row><entry v="t">Sulfonylureas</entry> <entry v="t">Anomalies (?)</entry> <entry v="t">Suppressed insulin secretion</entry> <entry v="t">Compatible</entry></row><row><entry cs="4">MIGRAINE REMEDIES</entry></row><row><entry v="t">Ergotamine</entry> <entry v="t">None known</entry> <entry v="t">May stimulate contractions</entry> <entry v="t">Use with caution</entry></row><row><entry v="t">Sumatriptan</entry> <entry v="t">None known</entry> <entry v="t">None known</entry> <entry v="t">Compatible</entry></row><row><entry cs="4">PSYCHOACTIVE DRUGS, ANTIDEPRESSANTS</entry></row><row><entry v="t">Amphetamine</entry> <entry v="t">Inconsistent; likely none</entry> <entry v="t">Reduced weight</entry> <entry v="t">Contraindicated</entry></row><row><entry v="t">Benzodiazepines</entry> <entry v="t">Facial dysmorphism (?)</entry> <entry v="t">Depression, floppy infant, hypothermia, withdrawal</entry> <entry v="t">Some concern about central nervous system toxicity with long-term use</entry></row><row><entry v="t">Fluoxetine</entry> <entry v="t">None known</entry> <entry v="t">None known</entry> <entry v="t">Symptoms of colic</entry></row><row><entry v="t">Hydroxyzine</entry> <entry v="t">None known</entry> <entry v="t">None known</entry> <entry v="t">No information</entry></row><row><entry v="t">Lithium</entry> <entry v="t">Facial clefts; cardiovascular anomaly</entry> <entry v="t">Lithium toxicity (neurologic and hepatic dysfunction)</entry> <entry v="t">Contraindicated</entry></row><row><entry v="t">Meprobamate</entry> <entry v="t">Cardiac anomalies (?), major malformations (?)</entry> <entry v="t">None known</entry> <entry v="t">Unknown</entry></row><row><entry v="t">Phenothiazines</entry> <entry v="t">None known</entry> <entry v="t">Muscle rigidity, hypothermia, tremor</entry> <entry v="t">Unknown</entry></row><row><entry v="t">Sedatives</entry> <entry v="t">None known</entry> <entry v="t">Depression, slow learning</entry> <entry v="t">Not recommended</entry></row><row><entry v="t">Thalidomide*</entry> <entry v="t">Phocomelia in 20% of cases</entry> <entry v="t">None known</entry> <entry v="t">No information</entry></row><row><entry v="t">Tricyclics</entry> <entry v="t">None known</entry> <entry v="t">None known</entry> <entry v="t">Unknown/caution</entry></row><row><entry v="t">Zolpiden</entry> <entry v="t">Unknown</entry> <entry v="t">Withdrawal or floppy infant (?)</entry> <entry v="t">Compatible</entry></row><row><entry cs="4">RADIOLABELED DIAGNOSTICS</entry></row><row><entry v="t">Albumin</entry> <entry v="t">None known</entry> <entry v="t">None known</entry> <entry v="t">No information available</entry></row><row><entry v="t">I131 (diagnostic)</entry> <entry v="t">None known</entry> <entry v="t">None known</entry> <entry v="t">Not recommended during exposure; may continue 24 hours after exposure</entry></row><row><entry v="t">Technetium</entry> <entry v="t">None known</entry> <entry v="t">None known</entry> <entry v="t">Not recommended during exposure; may continue 24 hours after exposure</entry></row><row><entry cs="4">SMOKING CESSATION</entry></row><row><entry v="t">Bupropion</entry> <entry v="t">Likely none</entry> <entry v="t">None known</entry> <entry v="t">Compatible</entry></row><row><entry v="t">Nicotine</entry> <entry v="t">Spontaneous abortion (?)</entry> <entry v="t">Impaired growth (?)</entry> <entry v="t">Consistent with passive smoking</entry></row><row><entry cs="4">THYROID MEDICATION</entry></row><row><entry v="t">I131 (therapeutic)</entry> <entry v="t">Goiter, abortion, anomalies</entry> <entry v="t">Goiter, airway obstruction, hyperthyroid, mental retardation</entry> <entry v="t">Contraindicated</entry></row><row><entry v="t">Methimazole</entry> <entry v="t">Aplasia cutis (?), goiter</entry> <entry v="t">Goiter, airway obstruction, hyperthyroid, mental retardation, aplasia cutis (?)</entry> <entry v="t">Compatible, but monitor fetal thyroid function</entry></row><row><entry v="t">Propylthiouracil</entry> <entry v="t">Goiter</entry> <entry v="t">Same as above</entry> <entry v="t">Safe, but monitor baby’s thyroid status</entry></row><row><entry v="t">Thyroid USP</entry> <entry v="t">None known</entry> <entry v="t">None known</entry> <entry v="t">Compatible</entry></row><row><entry v="t">Thyroxine</entry> <entry v="t">None known</entry> <entry v="t">None known</entry> <entry v="t">Compatible</entry></row><row><entry cs="4">TOCOLYTICS</entry></row><row><entry v="t">Beta-sympathomimetics</entry> <entry v="t">None known</entry> <entry v="t">Tachycardia, hypothermia, hypocalcemia, hypoglycemia, hyperglycemia</entry> <entry v="t">—</entry></row><row><entry v="t">Indomethacin</entry> <entry v="t">None known</entry> <entry v="t">Oligohydramnios (>48 hours of use)</entry> <entry v="t">—</entry></row><row><entry v="t">Magnesium sulfate</entry> <entry v="t">None known</entry> <entry v="t">Hypermagnesemia, respiratory depression</entry> <entry v="t">—</entry></row><row><entry v="t">Nifedipine</entry> <entry v="t">Unknown</entry> <entry v="t">None known</entry> <entry v="t">—</entry></row><row><entry cs="4">VACCINATIONS</entry></row><row><entry v="t">Influenza</entry> <entry v="t">None known</entry> <entry v="t">Passive immunization</entry> <entry v="t">Compatible</entry></row><row><entry v="t">Pneumovaccine</entry> <entry v="t">None known</entry> <entry v="t">Passive immunization</entry> <entry v="t">Compatible</entry></row><row><entry v="t">Tetanus toxoid</entry> <entry v="t">None known</entry> <entry v="t">Passive immunization</entry> <entry v="t">Compatible</entry></row><row><entry cs="4">VAGINAL PREPARATIONS</entry></row><row><entry v="t">Antifungal agents</entry> <entry v="t">None known</entry> <entry v="t">None known</entry> <entry v="t">Compatible</entry></row><row><entry v="t">Podophyllin</entry> <entry v="t">Mutagenesis (?)</entry> <entry v="t">Central nervous system effects (?)</entry> <entry v="t">Contraindicated</entry></row><row><entry cs="4">VITAMINS (high dose)</entry></row><row><entry v="t">A</entry> <entry v="t">Urogenital and craniofacial anomalies (?)</entry> <entry v="t">None known</entry> <entry v="t">No data available</entry></row><row><entry v="t">C</entry> <entry v="t">None known</entry> <entry v="t">Scurvy after delivery</entry> <entry v="t">Compatible</entry></row><row><entry v="t">D</entry> <entry v="t">Supravalvular aortic stenosis (?)</entry> <entry v="t">None known</entry> <entry v="t">Compatible</entry></row><row><entry v="t">E</entry> <entry v="t">Unknown</entry> <entry v="t">None known</entry> <entry v="t">Compatible</entry></row><row><entry v="t">K</entry> <entry v="t">Unknown</entry> <entry v="t">Hemorrhage, if deficiency</entry> <entry v="t">Compatible</entry></row><row><entry cs="4">“STREET” DRUGS</entry></row><row><entry v="t">Cocaine</entry> <entry v="t">Placental abruption, vascular disruption, urinary tract anomalies</entry> <entry v="t">Withdrawal, placental abruption, vascular disruption, growth restriction</entry> <entry v="t">Contraindicated</entry></row><row><entry v="t">Heroin</entry> <entry v="t">None known</entry> <entry v="t">Depression, withdrawal, growth restriction</entry> <entry v="t">Contraindicated</entry></row><row><entry v="t">LSD</entry> <entry v="t">None known</entry> <entry v="t">Withdrawal, behavioral effects</entry> <entry v="t">Contraindicated</entry></row><row><entry v="t">Marijuana</entry> <entry v="t">None known</entry> <entry v="t">Behavioral effects, growth restriction</entry> <entry v="t">Contraindicated</entry></row><row><entry v="t">Methadone</entry> <entry v="t">None known</entry> <entry v="t">Withdrawal, growth restriction</entry> <entry v="t">Contraindicated</entry></row><row><entry v="t">Methamphetamine</entry> <entry v="t">None known</entry> <entry v="t">Withdrawal, growth restriction</entry> <entry v="t">Contraindicated</entry></row><row><entry v="t">Pentazocine</entry> <entry v="t">None known</entry> <entry v="t">Withdrawal, growth restriction</entry> <entry v="t">Contraindicated</entry></row><row><entry v="t">Phencyclidine</entry> <entry v="t">None known</entry> <entry v="t">Withdrawal, neurobehavioral effects, growth restriction</entry> <entry v="t">Contraindicated</entry></row><row><entry cs="4">OTHER DRUGS</entry></row><row><entry v="t">Azathioprine</entry> <entry v="t">Abortion</entry> <entry v="t">Anemia, thrombocytopenia, lymphopenia, growth retardation</entry> <entry v="t">Not recommended</entry></row><row><entry v="t">Bromocriptine</entry> <entry v="t">None known</entry> <entry v="t">None known</entry> <entry v="t">Compatible</entry></row><row><entry v="t">Caffeine</entry> <entry v="t">Anomalies (?) in high doses, abortion (?)</entry> <entry v="t">Jitteriness</entry> <entry v="t">Not recommended</entry></row><row><entry v="t">Immune gamma globulin</entry> <entry v="t">None known</entry> <entry v="t">None known</entry> <entry v="t">Compatible</entry></row><row><entry v="t">Isotretinoin*</entry> <entry v="t">Central nervous system, cardiac, facial anomalies</entry> <entry v="t">Stillbirth, mental retardation (?)</entry> <entry v="t">Contraindicated</entry></row><row><entry v="t">Misoprostol</entry> <entry v="t">Abortion; variety of anomalies (cranium, limb, oral cleft); Mobius sequence</entry> <entry v="t">None with low dose for cervical ripening; placental abruption</entry> <entry v="t">Contraindicated, especially if diarrhea occurs</entry></row><row><entry v="t">Spermicides</entry> <entry v="t">None known</entry> <entry v="t">None known</entry> <entry v="t">No information</entry></row><row><entry cs="4">* Proven teratogen.</entry></row><row><entry cs="4">Unknown=no studies to investigate fetal effects; none known=no malformations reported in human studies or no consistent malformations in animal studies; (?)=conflicting information</entry></row><row><entry cs="4">Source: Reprotox data from humans, last reviewed in 2006.</entry></row>