User login
Home pregnancy tests—Is ectopic always on your mind?
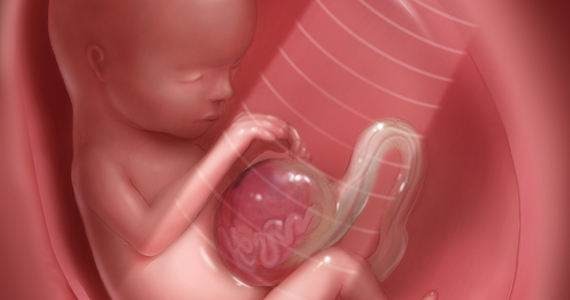
CASE Unidentified ectopic pregnancy leads to rupture*
A 33-year-old woman (G1 P0010) with 2 positive home pregnancy tests presents to the emergency department (ED) reporting intermittent vaginal bleeding for 3 days. Her last menstrual period was 10 weeks ago, but she reports that her menses are always irregular. She has a history of asymptomatic chlamydia, as well as spontaneous abortion 2 years prior. At present, she denies abdominal pain or vaginal discharge.
Upon examination her vital signs are: temperature, 98.3 °F; pulse, 112 bpm, with a resting rate of 16 bpm; blood pressure (BP), 142/91 mm Hg; pulse O2, 99%; height, 4’ 3”; weight, 115 lb. Her labs are: hemoglobin, 12.1 g/dL; hematocrit, 38%; serum human chorionic gonadotropin (hCG) 236 mIU/mL. Upon pelvic examination, no active bleeding is noted. She agrees to be followed up by her gynecologist and is given a prescription for serum hCG in 2 days. She is instructed to return to the ED should she have pain or increased vaginal bleeding.
Three days later, the patient follows up with her gynecologist reporting mild cramping. She notes having had an episode of heavy vaginal bleeding and a “weakly positive” home pregnancy test. Transvaginal ultrasonography notes endometrial thickness 0.59 mm and unremarkable adnexa. A urine pregnancy test performed in the office is positive; urinalysis is positive for nitrites. With the bleeding slowed, the gynecologist’s overall impression is that the patient has undergone complete spontaneous abortion. She prescribes Macrobid for the urinary tract infection. She does not obtain the ED-prescribed serum HCG levels, as she feels, since complete spontaneous abortion has occurred there is no need to obtain a follow-up serum HCG.
Five days later, the patient returns to the ED reporting abdominal pain after eating. Fever and productive cough of 2 days are noted. The patient states that she had a recent miscarriage. The overall impression of the patient’s condition is bronchitis, and it is noted on the patient’s record, “unlikely ectopic pregnancy and pregnancy test may be false positive,” hence a pregnancy test is not ordered. Examination reveals mild suprapubic tenderness with no rebound; no pelvic exam is performed. The patient is instructed to follow up with a health care clinic within a week, and to return to the ED with severe abdominal pain, higher fever, or any new concerning symptoms. A Zithromax Z-pak is prescribed.
Four days later, the patient is brought by ambulance to the ED of the local major medical center with severe abdominal pain involving the right lower quadrant. She states that she had a miscarriage 3 weeks prior and was recently treated for bronchitis. She has dizziness when standing. Her vital signs are: temperature, 97.8 °F; heart rate, 95 bpm; BP, 72/48 mm Hg; pulse O2, 100%. She reports her abdominal pain to be 6/10.
The patient is given a Lactated Ringer’s bolus of 1,000 mL for a hypotensive episode. Computed tomography is obtained and notes, “low attenuation in the left adnexa with a dilated fallopian tube.” A large heterogeneous collection of fluid in the pelvis is noted with active extravasation, consistent with an “acute bleed.”
The patient is brought to the operating room with a diagnosis of probable ruptured ectopic pregnancy. Intraoperatively she is noted to have a right ruptured ectopic and left tubo-ovarian abscess. The surgeon proceeds with right salpingectomy and left salpingo-oophorectomy. Three liters of hemoperitoneum is found.
She is followed postoperatively with serum hCG until levels are negative. Her postoperative course is uneventful. Her only future option for pregnancy is through assisted reproductive technology (ART) with in vitro fertilization (IVF). The patient sues the gynecologist and second ED physician for presumed inappropriate assessment for ectopic pregnancy.
*The “facts” of this case are a composite, drawn from several cases to illustrate medical and legal issues. The statement of facts should be considered hypothetical.
Continue to: WHAT’S THE VERDICT?...
WHAT’S THE VERDICT?
A defense verdict is returned.
Medical considerations
The incidence of ectopic pregnancy is 2% of all pregnancies, with a higher incidence (about 4%) among infertility patients.1 Up to 10% of ectopic pregnancies have no symptoms.2
Clinical presentations. Classic signs of ectopic pregnancy include:
- abdominal pain
- vaginal bleeding
- late menses (often noted).
A recent case of ectopic pregnancy presenting with chest pain was reported.3 Clinicians must never lose site of the fact that ectopic pregnancy is the most common cause of maternal mortality in the first trimester, with an incidence of 1% to 10% of all first-trimester deaths.4
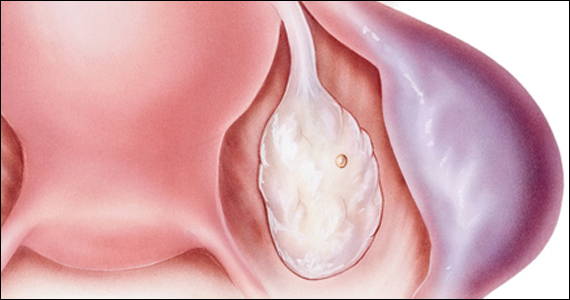
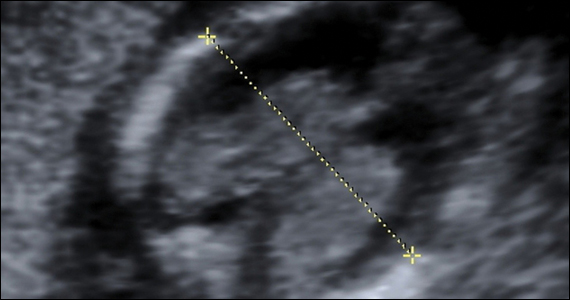
Risk factors include pelvic inflammatory disease, as demonstrated in the opening case. “The silent epidemic of chlamydia” comes to mind, and tobacco smoking can adversely affect tubal cilia, as can pelvic adhesions and/or prior tubal surgery. All of these factors can predispose a patient to ectopic pregnancy; in addition, intrauterine devices, endometriosis, tubal ligation (or ligation reversal), all can set the stage for an ectopic pregnancy.5 Appropriate serum hCG monitoring during early pregnancy can assist in sorting out pregnancies of unknown location (PUL; FIGURE). First trimester ultrasonography, at 5 weeks gestation, usually identifies early intrauterine gestation.

Imaging. With regard to pelvic sonography, the earliest sign of an intrauterine pregnancy (IUP) is a sac eccentrically located in the decidua.6 As the IUP progresses, it becomes equated with a “double decidual sign,” with double rings of tissue around the sac.6 If the pregnancy is located in an adnexal mass, it is frequently inhomogeneous or noncystic in appearance (ie, “the blob” sign); the positive predictive value (PPV) is 96%.2 The PPV of transvaginal ultrasound is 80%, as paratubal, paraovarian, ovarian cyst, and hydrosalpinx can affect the interpretation.7
Heterotopic pregnancy includes an intrauterine gestation and an ectopic pregnancy. This presentation includes the presence of a “pseudosac” in the endometrial cavity plus an extrauterine gestation. Heterotopic pregnancies have become somewhat more common as ART/IVF has unfolded, especially prior to the predominance of single embryo transfer.
Managing ectopic pregnancy
For cases of early pregnancy complicated by intermittent bleeding and/or pain, monitoring with serum hCG levels at 48-hour intervals to distinguish a viable IUP from an abnormal IUP or an ectopic is appropriate. The “discriminatory zone” collates serum hCG levels with findings on ultrasonography. Specific lower limits of serum hCG levels are not clear cut, with recommendations of 3,500 mIU/mL to provide sonographic evidence of an intrauterine gestation “to avoid misdiagnosis and possible interruption of intrauterine pregnancy,” as conveyed in the American College of Obstetricians and Gynecologists 2018 practice bulletin.8 Serum progesterone levels also have been suggested to complement hCG levels; a progesterone level of <20 nmol/L is consistent with an abnormal pregnancy, whereas levels >25 nmol/L are suggestive of a viable pregnancy.2 Inhibin A levels also have been suggested to be helpful, but they are not an ideal monitoring tool.
While most ectopic pregnancies are located in the fallopian tube, other locations also can be abdominal or ovarian. In addition, cesarean scar ectopic pregnancy can occur and often is associated with delay in diagnosis and greater morbidity due to such delay.9 With regard to ovarian ectopic, Spiegelberg criteria are established for diagnosis (TABLE 1).10
Appropriate management of an ectopic pregnancy is dependent upon the gestational age, serum hCG levels, and imaging findings, as well as the patient’s symptoms and exam findings. Treatment is established in large part on a case-by-case basis and includes, for early pregnancy, expectant management and use of methotrexate (TABLE 2).11 Dilation and curettage may be used to identify the pregnancy’s location when the serum hCG level is below 2,000 mIU/mL and there is no evidence of an IUP on ultrasound. Surgical treatment can include minimally invasive salpingostomy or salpingectomy and, depending on circumstance, laparotomy may be indicated.

Fertility following ectopic pregnancy varies and is affected by location, treatment, predisposing factors, total number of ectopic pregnancies, and other factors. Ectopic pregnancy, although rare, also can occur with use of IVF. Humans are not unique with regard to ectopic pregnancies, as they also occur in sheep.12
Continue to: Legal perspective...
Legal perspective
Lawsuits related to ectopic pregnancy are not a new phenomenon. In fact, in 1897, a physician in Ohio who misdiagnosed an “extrauterine pregnancy” as appendicitis was the center of a malpractice lawsuit.13 Unrecognized or mishandled ectopic pregnancy can result in serious injuries—in the range of 1% to 10% (see above) of maternal deaths are related to ectopic pregnancy.14 Ectopic pregnancy cases, therefore, have been the subject of substantial litigation over the years. An informal, noncomprehensive review of malpractice lawsuits brought from 2000 to 2019, found more than 300 ectopic pregnancy cases. Given the large number of malpractice claims against ObGyns,15 ectopic pregnancy cases are only a small portion of all ObGyn malpractice cases.16
A common claim: negligent diagnosis or treatment
The most common basis for lawsuits in cases of ectopic pregnancy is the clinician’s negligent failure to properly diagnose the ectopic nature of the pregnancy. There are also a number of cases claiming negligent treatment of an identified ectopic pregnancy. Not every missed diagnosis, or unsuccessful treatment, leads to liability, of course. It is only when a diagnosis or treatment fails to meet the standard of care within the profession that there should be liability. That standard of care is generally defined by what a reasonably prudent physician would do under the circumstances. Expert witnesses, who are familiar with the standard of practice within the specialty, are usually necessary to establish what that practice is. Both the plaintiff and the defense obtain experts, the former to prove what the standard of care is and that the standard was not met in the case at hand. The defense experts are usually arguing that the standard of care was met.17 Inadequate diagnosis of ectopic pregnancy or other condition may arise from a failure to take a sufficient history, conduct an appropriately thorough physical examination, recognize any of the symptoms that would suggest it is present, use and conduct ultrasound correctly, or follow-up appropriately with additional testing.18
A malpractice claim of negligent treatment can involve any the following circumstances19:
- failure to establish an appropriate treatment plan
- prescribing inappropriate medications for the patient (eg, methotrexate, when it is contraindicated)
- delivering the wrong medication or the wrong amount of the right medication
- performing a procedure badly
- undertaking a new treatment without adequate instruction and preparation.
Given the nature and risks of ectopic pregnancy, ongoing, frequent contact with the patient is essential from the point at which the condition is suspected. The greater the risk of harm (probability or consequence), the more careful any professional ought to be. Because ectopic pregnancy is not an uncommon occurrence, and because it can have devastating effects, including death, a reasonably prudent practitioner would be especially aware of the clinical presentations discussed above.20 In the opening case, the treatment plan was not well documented.
Negligence must lead to patient harm. In addition to negligence (proving that the physician did not act in accordance with the standard of care), to prevail in a malpractice case, the plaintiff-patient must prove that the negligence caused the injury, or worsened it. If the failure to make a diagnosis would not have made any difference in a harm the patient suffered, there are no damages and no liability. Suppose, for example, that a physician negligently failed to diagnose ectopic pregnancy, but performed surgery expecting to find the misdiagnosed condition. In the course of the surgery, however, the surgeon discovered and appropriately treated the ectopic pregnancy. (A version of this happened in the old 19th century case mentioned above.) The negligence of the physician did not cause harm, so there are no damages and no liability.
Continue to: Informed consent is vital...
Informed consent is vital
A part of malpractice is informed consent (or the absence of it)—issues that can arise in any medical care.21 It is wise to pay particular attention in cases where the nature of the illness is unknown, and where there are significant uncertainties and the nature of testing and treatment may change substantially over a period of a few days or few weeks. As always, informed consent should include a discussion of what process or procedure is proposed, its risks and benefits, alternative approaches that might be available, and the risk of doing nothing. Frequently, the uncertainty of ectopic pregnancy complicates the informed consent process.22
Because communication with the patient is an essential function of informed consent, the consent process should productively be used in PUL and similar cases to inform the patient about the uncertainty, and the testing and (nonsurgical) treatment that will occur. This is an opportunity to reinforce the message that the patient must maintain ongoing communication with the physician’s office about changes in her condition, and appear for each appointment scheduled. If more invasive procedures—notably surgery—become required, a separate consent process should be completed, because the risks and considerations are now meaningfully different than when treatment began. As a general matter, any possible treatment that may result in infertility or reduced reproductive capacity should specifically be included in the consent process.
In the hypothetical case, the gynecologist failed to obtain a follow-up serum hCG level. In addition, the record did not reflect ectopic pregnancy in the differential diagnosis. As noted above, the patient had predisposing factors for an ectopic pregnancy. The physician should have acknowledged the history of sexually transmitted disease predisposing her to an ectopic pregnancy. Monitoring of serum hCG levels until they are negative is appropriate with ectopic, or presumed ectopic, pregnancy management. Appropriate monitoring did not occur in this case. Each of these errors (following up on serum hCG levels and the inadequacy of notations about the possibility of ectopic pregnancy) seem inconsistent with the usual standard of care. Furthermore, as a result of the outcome, the only future option for the patient to pursue pregnancy was IVF.
Other legal issues
There are a number of other legal issues that are associated with the topic of ectopic pregnancy. There is evidence, for example, that Catholic and non-Catholic hospitals treat ectopic pregnancies differently,23 which may reflect different views on taking a life or the use of methotrexate and its association with abortion.24 In addition, the possibility of an increase in future ectopic pregnancies is one of the “risks” of abortion that pro-life organizations have pushed to see included in abortion informed consent.25 This has led some commentators to conclude that some Catholic hospitals violate federal law in managing ectopic pregnancy. There is also evidence of “overwhelming rates of medical misinformation on pregnancy center websites, including a link between abortion and ectopic pregnancy.”26
The fact that cesarean deliveries are related to an increased risk for ectopic pregnancy (because of the risk of cesarean scar ectopic pregnancy) also has been cited as information that should play a role in the consent process for cesarean delivery.27 In terms of liability, failed tubal ligation leads to a 33% risk of ectopic pregnancy.28 The risk of ectopic pregnancy is also commonly included in surrogacy contracts.29
Why the outcome was for the defense
The opening hypothetical case illustrates some of the uncertainties of medical malpractice cases. As noted, there appeared a deviation from the usual standard of care, particularly the failure to follow up on the serum hCG level. The weakness in the medical record, failing to note the possibility of ectopic pregnancy, also was probably an error but, apparently, the court felt that this did not result in any harm to the patient.
The question arises of how there would be a defense verdict in light of the failure to track consecutive serum hCG levels. A speculative explanation is that there are many uncertainties in most lawsuits. Procedural problems may result in a case being limited, expert witnesses are essential to both the plaintiff and defense, with the quality of their review and testimony possibly uneven. Judges and juries may rely on one expert witness rather than another, juries vary, and the quality of advocacy differs. Any of these situations can contribute to the unpredictability of the outcome of a case. In the case above, the liability was somewhat uncertain, and the various other factors tipped in favor of a defense verdict. ●
- Centers for Disease Control and Prevention. Ectopic pregnancy—United States, 1990‒1992. MMWR Morb Mortal Wkly Rep. 1995;44:46-48.
- Kirk E, Bottomley C, Bourne T. Diagnosing ectopic pregnancy and current concepts in the management of pregnancy of unknown location. Hum Reprod Update. 2012;20:250-261.
- Dichter E, Espinosa J, Baird J, Lucerna A. An unusual emergency department case: ruptured ectopic pregnancy presenting as chest pain. World J Emerg Med. 2017;8:71-73.
- Cecchino GN, Araujo E, Elito J. Methotrexate for ectopic pregnancy: when and how. Arch Gynecol Obstet. 2014;290:417- 423.
- Barnhart KT, Sammel MD, Cracia CR, et al. Risk factors for ectopic pregnancy in women with symptomatic firsttrimester pregnancies. Fertil Steril. 2006;86:36-43.
- Carusi D. Pregnancy of unknown location: evaluation and management. Semin Perinatol. 2019;43:95-100.
- Barnhart KT, Fay CA, Suescum M, et al. Clinical factors affecting the accuracy of ultrasonography in symptomatic first-trimester pregnancy. Obstet Gynecol. 2011;117:299-306.
- American College of Obstetricians and Gynecologists Practice Bulletin No. 193: tubal ectopic pregnancy. Obstet Gynecol. 2018;131:e91-e103.
- Bouyer J, Coste J, Fernandez H, et al. Sites of ectopic pregnancy: a 10-year population-based study of 1800 cases. Hum Reprod. 2002;17:3224-3230.
- Spiegelberg O. Zur casuistic der ovarial schwangerschaft. Arch Gynecol. 1978;13:73.
- OB Hospitalist Group. Methotrexate use for ectopic pregnancies guidelines. https://www.obhg.com/wp-content /uploads/2020/01/Methotrexate-Use-for-EctopicPregnancies_2016-updates.pdf. Accessed December 10, 2020.
- Brozos C, Kargiannis I, Kiossis E, et al. Ectopic pregnancy through a caesarean scar in a ewe. N Z Vet J. 2013;61:373-375.
- Tucker v. Gillette, 12 Ohio Cir. Dec. 401 (Cir. Ct. 1901).
- Creanga AA, Syverson C, Seed K, et al. Pregnancy-related mortality in the United States, 2011–2013. Obstet Gynecol. 2017;130:366-373.
- Matthews LR, Alvi FA, Milad MP. Reproductive surgery malpractice patterns. Fertil Steril. 2016;106:e42-e43.
- Kim B. The impact of malpractice risk on the use of obstetrics procedures. J Legal Studies. 2006;36:S79-S120.
- Abinader R, Warsof S. Complications involving obstetrical ultrasound. In: Warsof S, Shwayder JM, eds. Legal Concepts and Best Practices in Obstetrics: The Nuts and Bolts Guide to Mitigating Risk. 2019;45-48.
- Creanga AA, Shapiro-Mendoza CK, Bish CL, et al. Trends in ectopic pregnancy mortality in the United States: 1980-2007. Obstet Gynecol. 2011;117:837-843.
- Shwayder JM. IUP diagnosed and treated as ectopic: How bad can it get? Contemporary OB/GYN. 2019;64:49-46.
- Kaplan AI. Should this ectopic pregnancy have been diagnosed earlier? Contemporary OB/GYN. 2017;62:53.
- American College of Obstetricians and Gynecologists Committee on Ethics. Committee opinion 439: informed consent. Reaffirmed 2015. https://www.acog.org/clinical /clinical-guidance/committee-opinion/articles/2009/08 /informed-consent. Accessed December 9, 2020.
- Shwayder JM. Liability in ob/gyn ultrasound. Contemporary OB/GYN. 2017;62:32-49.
- Fisher LN. Institutional religious exemptions: a balancing approach. BYU Law Review. 2014;415-444.
- Makdisi J. Aquinas’s prohibition of killing reconsidered. J Catholic Legal Stud. 2019:57:67-128.
- Franzonello A. Remarks of Anna Franzonello. Alb Law J Sci Tech. 2012;23:519-530.
- Malcolm HE. Pregnancy centers and the limits of mandated disclosure. Columbia Law Rev. 2019;119:1133-1168.
- Kukura E. Contested care: the limitations of evidencebased maternity care reform. Berkeley J Gender Law Justice. 2016;31:241-298.
- Donley G. Contraceptive equity: curing the sex discrimination in the ACA’s mandate. Alabama Law Rev. 2019;71:499-560.
- Berk H. Savvy surrogates and rock star parents: compensation provisions, contracting practices, and the value of womb work. Law Social Inquiry. 2020;45:398-431.
CASE Unidentified ectopic pregnancy leads to rupture*
A 33-year-old woman (G1 P0010) with 2 positive home pregnancy tests presents to the emergency department (ED) reporting intermittent vaginal bleeding for 3 days. Her last menstrual period was 10 weeks ago, but she reports that her menses are always irregular. She has a history of asymptomatic chlamydia, as well as spontaneous abortion 2 years prior. At present, she denies abdominal pain or vaginal discharge.
Upon examination her vital signs are: temperature, 98.3 °F; pulse, 112 bpm, with a resting rate of 16 bpm; blood pressure (BP), 142/91 mm Hg; pulse O2, 99%; height, 4’ 3”; weight, 115 lb. Her labs are: hemoglobin, 12.1 g/dL; hematocrit, 38%; serum human chorionic gonadotropin (hCG) 236 mIU/mL. Upon pelvic examination, no active bleeding is noted. She agrees to be followed up by her gynecologist and is given a prescription for serum hCG in 2 days. She is instructed to return to the ED should she have pain or increased vaginal bleeding.
Three days later, the patient follows up with her gynecologist reporting mild cramping. She notes having had an episode of heavy vaginal bleeding and a “weakly positive” home pregnancy test. Transvaginal ultrasonography notes endometrial thickness 0.59 mm and unremarkable adnexa. A urine pregnancy test performed in the office is positive; urinalysis is positive for nitrites. With the bleeding slowed, the gynecologist’s overall impression is that the patient has undergone complete spontaneous abortion. She prescribes Macrobid for the urinary tract infection. She does not obtain the ED-prescribed serum HCG levels, as she feels, since complete spontaneous abortion has occurred there is no need to obtain a follow-up serum HCG.
Five days later, the patient returns to the ED reporting abdominal pain after eating. Fever and productive cough of 2 days are noted. The patient states that she had a recent miscarriage. The overall impression of the patient’s condition is bronchitis, and it is noted on the patient’s record, “unlikely ectopic pregnancy and pregnancy test may be false positive,” hence a pregnancy test is not ordered. Examination reveals mild suprapubic tenderness with no rebound; no pelvic exam is performed. The patient is instructed to follow up with a health care clinic within a week, and to return to the ED with severe abdominal pain, higher fever, or any new concerning symptoms. A Zithromax Z-pak is prescribed.
Four days later, the patient is brought by ambulance to the ED of the local major medical center with severe abdominal pain involving the right lower quadrant. She states that she had a miscarriage 3 weeks prior and was recently treated for bronchitis. She has dizziness when standing. Her vital signs are: temperature, 97.8 °F; heart rate, 95 bpm; BP, 72/48 mm Hg; pulse O2, 100%. She reports her abdominal pain to be 6/10.
The patient is given a Lactated Ringer’s bolus of 1,000 mL for a hypotensive episode. Computed tomography is obtained and notes, “low attenuation in the left adnexa with a dilated fallopian tube.” A large heterogeneous collection of fluid in the pelvis is noted with active extravasation, consistent with an “acute bleed.”
The patient is brought to the operating room with a diagnosis of probable ruptured ectopic pregnancy. Intraoperatively she is noted to have a right ruptured ectopic and left tubo-ovarian abscess. The surgeon proceeds with right salpingectomy and left salpingo-oophorectomy. Three liters of hemoperitoneum is found.
She is followed postoperatively with serum hCG until levels are negative. Her postoperative course is uneventful. Her only future option for pregnancy is through assisted reproductive technology (ART) with in vitro fertilization (IVF). The patient sues the gynecologist and second ED physician for presumed inappropriate assessment for ectopic pregnancy.
*The “facts” of this case are a composite, drawn from several cases to illustrate medical and legal issues. The statement of facts should be considered hypothetical.
Continue to: WHAT’S THE VERDICT?...
WHAT’S THE VERDICT?
A defense verdict is returned.
Medical considerations
The incidence of ectopic pregnancy is 2% of all pregnancies, with a higher incidence (about 4%) among infertility patients.1 Up to 10% of ectopic pregnancies have no symptoms.2
Clinical presentations. Classic signs of ectopic pregnancy include:
- abdominal pain
- vaginal bleeding
- late menses (often noted).
A recent case of ectopic pregnancy presenting with chest pain was reported.3 Clinicians must never lose site of the fact that ectopic pregnancy is the most common cause of maternal mortality in the first trimester, with an incidence of 1% to 10% of all first-trimester deaths.4
Risk factors include pelvic inflammatory disease, as demonstrated in the opening case. “The silent epidemic of chlamydia” comes to mind, and tobacco smoking can adversely affect tubal cilia, as can pelvic adhesions and/or prior tubal surgery. All of these factors can predispose a patient to ectopic pregnancy; in addition, intrauterine devices, endometriosis, tubal ligation (or ligation reversal), all can set the stage for an ectopic pregnancy.5 Appropriate serum hCG monitoring during early pregnancy can assist in sorting out pregnancies of unknown location (PUL; FIGURE). First trimester ultrasonography, at 5 weeks gestation, usually identifies early intrauterine gestation.

Imaging. With regard to pelvic sonography, the earliest sign of an intrauterine pregnancy (IUP) is a sac eccentrically located in the decidua.6 As the IUP progresses, it becomes equated with a “double decidual sign,” with double rings of tissue around the sac.6 If the pregnancy is located in an adnexal mass, it is frequently inhomogeneous or noncystic in appearance (ie, “the blob” sign); the positive predictive value (PPV) is 96%.2 The PPV of transvaginal ultrasound is 80%, as paratubal, paraovarian, ovarian cyst, and hydrosalpinx can affect the interpretation.7
Heterotopic pregnancy includes an intrauterine gestation and an ectopic pregnancy. This presentation includes the presence of a “pseudosac” in the endometrial cavity plus an extrauterine gestation. Heterotopic pregnancies have become somewhat more common as ART/IVF has unfolded, especially prior to the predominance of single embryo transfer.
Managing ectopic pregnancy
For cases of early pregnancy complicated by intermittent bleeding and/or pain, monitoring with serum hCG levels at 48-hour intervals to distinguish a viable IUP from an abnormal IUP or an ectopic is appropriate. The “discriminatory zone” collates serum hCG levels with findings on ultrasonography. Specific lower limits of serum hCG levels are not clear cut, with recommendations of 3,500 mIU/mL to provide sonographic evidence of an intrauterine gestation “to avoid misdiagnosis and possible interruption of intrauterine pregnancy,” as conveyed in the American College of Obstetricians and Gynecologists 2018 practice bulletin.8 Serum progesterone levels also have been suggested to complement hCG levels; a progesterone level of <20 nmol/L is consistent with an abnormal pregnancy, whereas levels >25 nmol/L are suggestive of a viable pregnancy.2 Inhibin A levels also have been suggested to be helpful, but they are not an ideal monitoring tool.
While most ectopic pregnancies are located in the fallopian tube, other locations also can be abdominal or ovarian. In addition, cesarean scar ectopic pregnancy can occur and often is associated with delay in diagnosis and greater morbidity due to such delay.9 With regard to ovarian ectopic, Spiegelberg criteria are established for diagnosis (TABLE 1).10
Appropriate management of an ectopic pregnancy is dependent upon the gestational age, serum hCG levels, and imaging findings, as well as the patient’s symptoms and exam findings. Treatment is established in large part on a case-by-case basis and includes, for early pregnancy, expectant management and use of methotrexate (TABLE 2).11 Dilation and curettage may be used to identify the pregnancy’s location when the serum hCG level is below 2,000 mIU/mL and there is no evidence of an IUP on ultrasound. Surgical treatment can include minimally invasive salpingostomy or salpingectomy and, depending on circumstance, laparotomy may be indicated.

Fertility following ectopic pregnancy varies and is affected by location, treatment, predisposing factors, total number of ectopic pregnancies, and other factors. Ectopic pregnancy, although rare, also can occur with use of IVF. Humans are not unique with regard to ectopic pregnancies, as they also occur in sheep.12
Continue to: Legal perspective...
Legal perspective
Lawsuits related to ectopic pregnancy are not a new phenomenon. In fact, in 1897, a physician in Ohio who misdiagnosed an “extrauterine pregnancy” as appendicitis was the center of a malpractice lawsuit.13 Unrecognized or mishandled ectopic pregnancy can result in serious injuries—in the range of 1% to 10% (see above) of maternal deaths are related to ectopic pregnancy.14 Ectopic pregnancy cases, therefore, have been the subject of substantial litigation over the years. An informal, noncomprehensive review of malpractice lawsuits brought from 2000 to 2019, found more than 300 ectopic pregnancy cases. Given the large number of malpractice claims against ObGyns,15 ectopic pregnancy cases are only a small portion of all ObGyn malpractice cases.16
A common claim: negligent diagnosis or treatment
The most common basis for lawsuits in cases of ectopic pregnancy is the clinician’s negligent failure to properly diagnose the ectopic nature of the pregnancy. There are also a number of cases claiming negligent treatment of an identified ectopic pregnancy. Not every missed diagnosis, or unsuccessful treatment, leads to liability, of course. It is only when a diagnosis or treatment fails to meet the standard of care within the profession that there should be liability. That standard of care is generally defined by what a reasonably prudent physician would do under the circumstances. Expert witnesses, who are familiar with the standard of practice within the specialty, are usually necessary to establish what that practice is. Both the plaintiff and the defense obtain experts, the former to prove what the standard of care is and that the standard was not met in the case at hand. The defense experts are usually arguing that the standard of care was met.17 Inadequate diagnosis of ectopic pregnancy or other condition may arise from a failure to take a sufficient history, conduct an appropriately thorough physical examination, recognize any of the symptoms that would suggest it is present, use and conduct ultrasound correctly, or follow-up appropriately with additional testing.18
A malpractice claim of negligent treatment can involve any the following circumstances19:
- failure to establish an appropriate treatment plan
- prescribing inappropriate medications for the patient (eg, methotrexate, when it is contraindicated)
- delivering the wrong medication or the wrong amount of the right medication
- performing a procedure badly
- undertaking a new treatment without adequate instruction and preparation.
Given the nature and risks of ectopic pregnancy, ongoing, frequent contact with the patient is essential from the point at which the condition is suspected. The greater the risk of harm (probability or consequence), the more careful any professional ought to be. Because ectopic pregnancy is not an uncommon occurrence, and because it can have devastating effects, including death, a reasonably prudent practitioner would be especially aware of the clinical presentations discussed above.20 In the opening case, the treatment plan was not well documented.
Negligence must lead to patient harm. In addition to negligence (proving that the physician did not act in accordance with the standard of care), to prevail in a malpractice case, the plaintiff-patient must prove that the negligence caused the injury, or worsened it. If the failure to make a diagnosis would not have made any difference in a harm the patient suffered, there are no damages and no liability. Suppose, for example, that a physician negligently failed to diagnose ectopic pregnancy, but performed surgery expecting to find the misdiagnosed condition. In the course of the surgery, however, the surgeon discovered and appropriately treated the ectopic pregnancy. (A version of this happened in the old 19th century case mentioned above.) The negligence of the physician did not cause harm, so there are no damages and no liability.
Continue to: Informed consent is vital...
Informed consent is vital
A part of malpractice is informed consent (or the absence of it)—issues that can arise in any medical care.21 It is wise to pay particular attention in cases where the nature of the illness is unknown, and where there are significant uncertainties and the nature of testing and treatment may change substantially over a period of a few days or few weeks. As always, informed consent should include a discussion of what process or procedure is proposed, its risks and benefits, alternative approaches that might be available, and the risk of doing nothing. Frequently, the uncertainty of ectopic pregnancy complicates the informed consent process.22
Because communication with the patient is an essential function of informed consent, the consent process should productively be used in PUL and similar cases to inform the patient about the uncertainty, and the testing and (nonsurgical) treatment that will occur. This is an opportunity to reinforce the message that the patient must maintain ongoing communication with the physician’s office about changes in her condition, and appear for each appointment scheduled. If more invasive procedures—notably surgery—become required, a separate consent process should be completed, because the risks and considerations are now meaningfully different than when treatment began. As a general matter, any possible treatment that may result in infertility or reduced reproductive capacity should specifically be included in the consent process.
In the hypothetical case, the gynecologist failed to obtain a follow-up serum hCG level. In addition, the record did not reflect ectopic pregnancy in the differential diagnosis. As noted above, the patient had predisposing factors for an ectopic pregnancy. The physician should have acknowledged the history of sexually transmitted disease predisposing her to an ectopic pregnancy. Monitoring of serum hCG levels until they are negative is appropriate with ectopic, or presumed ectopic, pregnancy management. Appropriate monitoring did not occur in this case. Each of these errors (following up on serum hCG levels and the inadequacy of notations about the possibility of ectopic pregnancy) seem inconsistent with the usual standard of care. Furthermore, as a result of the outcome, the only future option for the patient to pursue pregnancy was IVF.
Other legal issues
There are a number of other legal issues that are associated with the topic of ectopic pregnancy. There is evidence, for example, that Catholic and non-Catholic hospitals treat ectopic pregnancies differently,23 which may reflect different views on taking a life or the use of methotrexate and its association with abortion.24 In addition, the possibility of an increase in future ectopic pregnancies is one of the “risks” of abortion that pro-life organizations have pushed to see included in abortion informed consent.25 This has led some commentators to conclude that some Catholic hospitals violate federal law in managing ectopic pregnancy. There is also evidence of “overwhelming rates of medical misinformation on pregnancy center websites, including a link between abortion and ectopic pregnancy.”26
The fact that cesarean deliveries are related to an increased risk for ectopic pregnancy (because of the risk of cesarean scar ectopic pregnancy) also has been cited as information that should play a role in the consent process for cesarean delivery.27 In terms of liability, failed tubal ligation leads to a 33% risk of ectopic pregnancy.28 The risk of ectopic pregnancy is also commonly included in surrogacy contracts.29
Why the outcome was for the defense
The opening hypothetical case illustrates some of the uncertainties of medical malpractice cases. As noted, there appeared a deviation from the usual standard of care, particularly the failure to follow up on the serum hCG level. The weakness in the medical record, failing to note the possibility of ectopic pregnancy, also was probably an error but, apparently, the court felt that this did not result in any harm to the patient.
The question arises of how there would be a defense verdict in light of the failure to track consecutive serum hCG levels. A speculative explanation is that there are many uncertainties in most lawsuits. Procedural problems may result in a case being limited, expert witnesses are essential to both the plaintiff and defense, with the quality of their review and testimony possibly uneven. Judges and juries may rely on one expert witness rather than another, juries vary, and the quality of advocacy differs. Any of these situations can contribute to the unpredictability of the outcome of a case. In the case above, the liability was somewhat uncertain, and the various other factors tipped in favor of a defense verdict. ●
CASE Unidentified ectopic pregnancy leads to rupture*
A 33-year-old woman (G1 P0010) with 2 positive home pregnancy tests presents to the emergency department (ED) reporting intermittent vaginal bleeding for 3 days. Her last menstrual period was 10 weeks ago, but she reports that her menses are always irregular. She has a history of asymptomatic chlamydia, as well as spontaneous abortion 2 years prior. At present, she denies abdominal pain or vaginal discharge.
Upon examination her vital signs are: temperature, 98.3 °F; pulse, 112 bpm, with a resting rate of 16 bpm; blood pressure (BP), 142/91 mm Hg; pulse O2, 99%; height, 4’ 3”; weight, 115 lb. Her labs are: hemoglobin, 12.1 g/dL; hematocrit, 38%; serum human chorionic gonadotropin (hCG) 236 mIU/mL. Upon pelvic examination, no active bleeding is noted. She agrees to be followed up by her gynecologist and is given a prescription for serum hCG in 2 days. She is instructed to return to the ED should she have pain or increased vaginal bleeding.
Three days later, the patient follows up with her gynecologist reporting mild cramping. She notes having had an episode of heavy vaginal bleeding and a “weakly positive” home pregnancy test. Transvaginal ultrasonography notes endometrial thickness 0.59 mm and unremarkable adnexa. A urine pregnancy test performed in the office is positive; urinalysis is positive for nitrites. With the bleeding slowed, the gynecologist’s overall impression is that the patient has undergone complete spontaneous abortion. She prescribes Macrobid for the urinary tract infection. She does not obtain the ED-prescribed serum HCG levels, as she feels, since complete spontaneous abortion has occurred there is no need to obtain a follow-up serum HCG.
Five days later, the patient returns to the ED reporting abdominal pain after eating. Fever and productive cough of 2 days are noted. The patient states that she had a recent miscarriage. The overall impression of the patient’s condition is bronchitis, and it is noted on the patient’s record, “unlikely ectopic pregnancy and pregnancy test may be false positive,” hence a pregnancy test is not ordered. Examination reveals mild suprapubic tenderness with no rebound; no pelvic exam is performed. The patient is instructed to follow up with a health care clinic within a week, and to return to the ED with severe abdominal pain, higher fever, or any new concerning symptoms. A Zithromax Z-pak is prescribed.
Four days later, the patient is brought by ambulance to the ED of the local major medical center with severe abdominal pain involving the right lower quadrant. She states that she had a miscarriage 3 weeks prior and was recently treated for bronchitis. She has dizziness when standing. Her vital signs are: temperature, 97.8 °F; heart rate, 95 bpm; BP, 72/48 mm Hg; pulse O2, 100%. She reports her abdominal pain to be 6/10.
The patient is given a Lactated Ringer’s bolus of 1,000 mL for a hypotensive episode. Computed tomography is obtained and notes, “low attenuation in the left adnexa with a dilated fallopian tube.” A large heterogeneous collection of fluid in the pelvis is noted with active extravasation, consistent with an “acute bleed.”
The patient is brought to the operating room with a diagnosis of probable ruptured ectopic pregnancy. Intraoperatively she is noted to have a right ruptured ectopic and left tubo-ovarian abscess. The surgeon proceeds with right salpingectomy and left salpingo-oophorectomy. Three liters of hemoperitoneum is found.
She is followed postoperatively with serum hCG until levels are negative. Her postoperative course is uneventful. Her only future option for pregnancy is through assisted reproductive technology (ART) with in vitro fertilization (IVF). The patient sues the gynecologist and second ED physician for presumed inappropriate assessment for ectopic pregnancy.
*The “facts” of this case are a composite, drawn from several cases to illustrate medical and legal issues. The statement of facts should be considered hypothetical.
Continue to: WHAT’S THE VERDICT?...
WHAT’S THE VERDICT?
A defense verdict is returned.
Medical considerations
The incidence of ectopic pregnancy is 2% of all pregnancies, with a higher incidence (about 4%) among infertility patients.1 Up to 10% of ectopic pregnancies have no symptoms.2
Clinical presentations. Classic signs of ectopic pregnancy include:
- abdominal pain
- vaginal bleeding
- late menses (often noted).
A recent case of ectopic pregnancy presenting with chest pain was reported.3 Clinicians must never lose site of the fact that ectopic pregnancy is the most common cause of maternal mortality in the first trimester, with an incidence of 1% to 10% of all first-trimester deaths.4
Risk factors include pelvic inflammatory disease, as demonstrated in the opening case. “The silent epidemic of chlamydia” comes to mind, and tobacco smoking can adversely affect tubal cilia, as can pelvic adhesions and/or prior tubal surgery. All of these factors can predispose a patient to ectopic pregnancy; in addition, intrauterine devices, endometriosis, tubal ligation (or ligation reversal), all can set the stage for an ectopic pregnancy.5 Appropriate serum hCG monitoring during early pregnancy can assist in sorting out pregnancies of unknown location (PUL; FIGURE). First trimester ultrasonography, at 5 weeks gestation, usually identifies early intrauterine gestation.

Imaging. With regard to pelvic sonography, the earliest sign of an intrauterine pregnancy (IUP) is a sac eccentrically located in the decidua.6 As the IUP progresses, it becomes equated with a “double decidual sign,” with double rings of tissue around the sac.6 If the pregnancy is located in an adnexal mass, it is frequently inhomogeneous or noncystic in appearance (ie, “the blob” sign); the positive predictive value (PPV) is 96%.2 The PPV of transvaginal ultrasound is 80%, as paratubal, paraovarian, ovarian cyst, and hydrosalpinx can affect the interpretation.7
Heterotopic pregnancy includes an intrauterine gestation and an ectopic pregnancy. This presentation includes the presence of a “pseudosac” in the endometrial cavity plus an extrauterine gestation. Heterotopic pregnancies have become somewhat more common as ART/IVF has unfolded, especially prior to the predominance of single embryo transfer.
Managing ectopic pregnancy
For cases of early pregnancy complicated by intermittent bleeding and/or pain, monitoring with serum hCG levels at 48-hour intervals to distinguish a viable IUP from an abnormal IUP or an ectopic is appropriate. The “discriminatory zone” collates serum hCG levels with findings on ultrasonography. Specific lower limits of serum hCG levels are not clear cut, with recommendations of 3,500 mIU/mL to provide sonographic evidence of an intrauterine gestation “to avoid misdiagnosis and possible interruption of intrauterine pregnancy,” as conveyed in the American College of Obstetricians and Gynecologists 2018 practice bulletin.8 Serum progesterone levels also have been suggested to complement hCG levels; a progesterone level of <20 nmol/L is consistent with an abnormal pregnancy, whereas levels >25 nmol/L are suggestive of a viable pregnancy.2 Inhibin A levels also have been suggested to be helpful, but they are not an ideal monitoring tool.
While most ectopic pregnancies are located in the fallopian tube, other locations also can be abdominal or ovarian. In addition, cesarean scar ectopic pregnancy can occur and often is associated with delay in diagnosis and greater morbidity due to such delay.9 With regard to ovarian ectopic, Spiegelberg criteria are established for diagnosis (TABLE 1).10
Appropriate management of an ectopic pregnancy is dependent upon the gestational age, serum hCG levels, and imaging findings, as well as the patient’s symptoms and exam findings. Treatment is established in large part on a case-by-case basis and includes, for early pregnancy, expectant management and use of methotrexate (TABLE 2).11 Dilation and curettage may be used to identify the pregnancy’s location when the serum hCG level is below 2,000 mIU/mL and there is no evidence of an IUP on ultrasound. Surgical treatment can include minimally invasive salpingostomy or salpingectomy and, depending on circumstance, laparotomy may be indicated.

Fertility following ectopic pregnancy varies and is affected by location, treatment, predisposing factors, total number of ectopic pregnancies, and other factors. Ectopic pregnancy, although rare, also can occur with use of IVF. Humans are not unique with regard to ectopic pregnancies, as they also occur in sheep.12
Continue to: Legal perspective...
Legal perspective
Lawsuits related to ectopic pregnancy are not a new phenomenon. In fact, in 1897, a physician in Ohio who misdiagnosed an “extrauterine pregnancy” as appendicitis was the center of a malpractice lawsuit.13 Unrecognized or mishandled ectopic pregnancy can result in serious injuries—in the range of 1% to 10% (see above) of maternal deaths are related to ectopic pregnancy.14 Ectopic pregnancy cases, therefore, have been the subject of substantial litigation over the years. An informal, noncomprehensive review of malpractice lawsuits brought from 2000 to 2019, found more than 300 ectopic pregnancy cases. Given the large number of malpractice claims against ObGyns,15 ectopic pregnancy cases are only a small portion of all ObGyn malpractice cases.16
A common claim: negligent diagnosis or treatment
The most common basis for lawsuits in cases of ectopic pregnancy is the clinician’s negligent failure to properly diagnose the ectopic nature of the pregnancy. There are also a number of cases claiming negligent treatment of an identified ectopic pregnancy. Not every missed diagnosis, or unsuccessful treatment, leads to liability, of course. It is only when a diagnosis or treatment fails to meet the standard of care within the profession that there should be liability. That standard of care is generally defined by what a reasonably prudent physician would do under the circumstances. Expert witnesses, who are familiar with the standard of practice within the specialty, are usually necessary to establish what that practice is. Both the plaintiff and the defense obtain experts, the former to prove what the standard of care is and that the standard was not met in the case at hand. The defense experts are usually arguing that the standard of care was met.17 Inadequate diagnosis of ectopic pregnancy or other condition may arise from a failure to take a sufficient history, conduct an appropriately thorough physical examination, recognize any of the symptoms that would suggest it is present, use and conduct ultrasound correctly, or follow-up appropriately with additional testing.18
A malpractice claim of negligent treatment can involve any the following circumstances19:
- failure to establish an appropriate treatment plan
- prescribing inappropriate medications for the patient (eg, methotrexate, when it is contraindicated)
- delivering the wrong medication or the wrong amount of the right medication
- performing a procedure badly
- undertaking a new treatment without adequate instruction and preparation.
Given the nature and risks of ectopic pregnancy, ongoing, frequent contact with the patient is essential from the point at which the condition is suspected. The greater the risk of harm (probability or consequence), the more careful any professional ought to be. Because ectopic pregnancy is not an uncommon occurrence, and because it can have devastating effects, including death, a reasonably prudent practitioner would be especially aware of the clinical presentations discussed above.20 In the opening case, the treatment plan was not well documented.
Negligence must lead to patient harm. In addition to negligence (proving that the physician did not act in accordance with the standard of care), to prevail in a malpractice case, the plaintiff-patient must prove that the negligence caused the injury, or worsened it. If the failure to make a diagnosis would not have made any difference in a harm the patient suffered, there are no damages and no liability. Suppose, for example, that a physician negligently failed to diagnose ectopic pregnancy, but performed surgery expecting to find the misdiagnosed condition. In the course of the surgery, however, the surgeon discovered and appropriately treated the ectopic pregnancy. (A version of this happened in the old 19th century case mentioned above.) The negligence of the physician did not cause harm, so there are no damages and no liability.
Continue to: Informed consent is vital...
Informed consent is vital
A part of malpractice is informed consent (or the absence of it)—issues that can arise in any medical care.21 It is wise to pay particular attention in cases where the nature of the illness is unknown, and where there are significant uncertainties and the nature of testing and treatment may change substantially over a period of a few days or few weeks. As always, informed consent should include a discussion of what process or procedure is proposed, its risks and benefits, alternative approaches that might be available, and the risk of doing nothing. Frequently, the uncertainty of ectopic pregnancy complicates the informed consent process.22
Because communication with the patient is an essential function of informed consent, the consent process should productively be used in PUL and similar cases to inform the patient about the uncertainty, and the testing and (nonsurgical) treatment that will occur. This is an opportunity to reinforce the message that the patient must maintain ongoing communication with the physician’s office about changes in her condition, and appear for each appointment scheduled. If more invasive procedures—notably surgery—become required, a separate consent process should be completed, because the risks and considerations are now meaningfully different than when treatment began. As a general matter, any possible treatment that may result in infertility or reduced reproductive capacity should specifically be included in the consent process.
In the hypothetical case, the gynecologist failed to obtain a follow-up serum hCG level. In addition, the record did not reflect ectopic pregnancy in the differential diagnosis. As noted above, the patient had predisposing factors for an ectopic pregnancy. The physician should have acknowledged the history of sexually transmitted disease predisposing her to an ectopic pregnancy. Monitoring of serum hCG levels until they are negative is appropriate with ectopic, or presumed ectopic, pregnancy management. Appropriate monitoring did not occur in this case. Each of these errors (following up on serum hCG levels and the inadequacy of notations about the possibility of ectopic pregnancy) seem inconsistent with the usual standard of care. Furthermore, as a result of the outcome, the only future option for the patient to pursue pregnancy was IVF.
Other legal issues
There are a number of other legal issues that are associated with the topic of ectopic pregnancy. There is evidence, for example, that Catholic and non-Catholic hospitals treat ectopic pregnancies differently,23 which may reflect different views on taking a life or the use of methotrexate and its association with abortion.24 In addition, the possibility of an increase in future ectopic pregnancies is one of the “risks” of abortion that pro-life organizations have pushed to see included in abortion informed consent.25 This has led some commentators to conclude that some Catholic hospitals violate federal law in managing ectopic pregnancy. There is also evidence of “overwhelming rates of medical misinformation on pregnancy center websites, including a link between abortion and ectopic pregnancy.”26
The fact that cesarean deliveries are related to an increased risk for ectopic pregnancy (because of the risk of cesarean scar ectopic pregnancy) also has been cited as information that should play a role in the consent process for cesarean delivery.27 In terms of liability, failed tubal ligation leads to a 33% risk of ectopic pregnancy.28 The risk of ectopic pregnancy is also commonly included in surrogacy contracts.29
Why the outcome was for the defense
The opening hypothetical case illustrates some of the uncertainties of medical malpractice cases. As noted, there appeared a deviation from the usual standard of care, particularly the failure to follow up on the serum hCG level. The weakness in the medical record, failing to note the possibility of ectopic pregnancy, also was probably an error but, apparently, the court felt that this did not result in any harm to the patient.
The question arises of how there would be a defense verdict in light of the failure to track consecutive serum hCG levels. A speculative explanation is that there are many uncertainties in most lawsuits. Procedural problems may result in a case being limited, expert witnesses are essential to both the plaintiff and defense, with the quality of their review and testimony possibly uneven. Judges and juries may rely on one expert witness rather than another, juries vary, and the quality of advocacy differs. Any of these situations can contribute to the unpredictability of the outcome of a case. In the case above, the liability was somewhat uncertain, and the various other factors tipped in favor of a defense verdict. ●
- Centers for Disease Control and Prevention. Ectopic pregnancy—United States, 1990‒1992. MMWR Morb Mortal Wkly Rep. 1995;44:46-48.
- Kirk E, Bottomley C, Bourne T. Diagnosing ectopic pregnancy and current concepts in the management of pregnancy of unknown location. Hum Reprod Update. 2012;20:250-261.
- Dichter E, Espinosa J, Baird J, Lucerna A. An unusual emergency department case: ruptured ectopic pregnancy presenting as chest pain. World J Emerg Med. 2017;8:71-73.
- Cecchino GN, Araujo E, Elito J. Methotrexate for ectopic pregnancy: when and how. Arch Gynecol Obstet. 2014;290:417- 423.
- Barnhart KT, Sammel MD, Cracia CR, et al. Risk factors for ectopic pregnancy in women with symptomatic firsttrimester pregnancies. Fertil Steril. 2006;86:36-43.
- Carusi D. Pregnancy of unknown location: evaluation and management. Semin Perinatol. 2019;43:95-100.
- Barnhart KT, Fay CA, Suescum M, et al. Clinical factors affecting the accuracy of ultrasonography in symptomatic first-trimester pregnancy. Obstet Gynecol. 2011;117:299-306.
- American College of Obstetricians and Gynecologists Practice Bulletin No. 193: tubal ectopic pregnancy. Obstet Gynecol. 2018;131:e91-e103.
- Bouyer J, Coste J, Fernandez H, et al. Sites of ectopic pregnancy: a 10-year population-based study of 1800 cases. Hum Reprod. 2002;17:3224-3230.
- Spiegelberg O. Zur casuistic der ovarial schwangerschaft. Arch Gynecol. 1978;13:73.
- OB Hospitalist Group. Methotrexate use for ectopic pregnancies guidelines. https://www.obhg.com/wp-content /uploads/2020/01/Methotrexate-Use-for-EctopicPregnancies_2016-updates.pdf. Accessed December 10, 2020.
- Brozos C, Kargiannis I, Kiossis E, et al. Ectopic pregnancy through a caesarean scar in a ewe. N Z Vet J. 2013;61:373-375.
- Tucker v. Gillette, 12 Ohio Cir. Dec. 401 (Cir. Ct. 1901).
- Creanga AA, Syverson C, Seed K, et al. Pregnancy-related mortality in the United States, 2011–2013. Obstet Gynecol. 2017;130:366-373.
- Matthews LR, Alvi FA, Milad MP. Reproductive surgery malpractice patterns. Fertil Steril. 2016;106:e42-e43.
- Kim B. The impact of malpractice risk on the use of obstetrics procedures. J Legal Studies. 2006;36:S79-S120.
- Abinader R, Warsof S. Complications involving obstetrical ultrasound. In: Warsof S, Shwayder JM, eds. Legal Concepts and Best Practices in Obstetrics: The Nuts and Bolts Guide to Mitigating Risk. 2019;45-48.
- Creanga AA, Shapiro-Mendoza CK, Bish CL, et al. Trends in ectopic pregnancy mortality in the United States: 1980-2007. Obstet Gynecol. 2011;117:837-843.
- Shwayder JM. IUP diagnosed and treated as ectopic: How bad can it get? Contemporary OB/GYN. 2019;64:49-46.
- Kaplan AI. Should this ectopic pregnancy have been diagnosed earlier? Contemporary OB/GYN. 2017;62:53.
- American College of Obstetricians and Gynecologists Committee on Ethics. Committee opinion 439: informed consent. Reaffirmed 2015. https://www.acog.org/clinical /clinical-guidance/committee-opinion/articles/2009/08 /informed-consent. Accessed December 9, 2020.
- Shwayder JM. Liability in ob/gyn ultrasound. Contemporary OB/GYN. 2017;62:32-49.
- Fisher LN. Institutional religious exemptions: a balancing approach. BYU Law Review. 2014;415-444.
- Makdisi J. Aquinas’s prohibition of killing reconsidered. J Catholic Legal Stud. 2019:57:67-128.
- Franzonello A. Remarks of Anna Franzonello. Alb Law J Sci Tech. 2012;23:519-530.
- Malcolm HE. Pregnancy centers and the limits of mandated disclosure. Columbia Law Rev. 2019;119:1133-1168.
- Kukura E. Contested care: the limitations of evidencebased maternity care reform. Berkeley J Gender Law Justice. 2016;31:241-298.
- Donley G. Contraceptive equity: curing the sex discrimination in the ACA’s mandate. Alabama Law Rev. 2019;71:499-560.
- Berk H. Savvy surrogates and rock star parents: compensation provisions, contracting practices, and the value of womb work. Law Social Inquiry. 2020;45:398-431.
- Centers for Disease Control and Prevention. Ectopic pregnancy—United States, 1990‒1992. MMWR Morb Mortal Wkly Rep. 1995;44:46-48.
- Kirk E, Bottomley C, Bourne T. Diagnosing ectopic pregnancy and current concepts in the management of pregnancy of unknown location. Hum Reprod Update. 2012;20:250-261.
- Dichter E, Espinosa J, Baird J, Lucerna A. An unusual emergency department case: ruptured ectopic pregnancy presenting as chest pain. World J Emerg Med. 2017;8:71-73.
- Cecchino GN, Araujo E, Elito J. Methotrexate for ectopic pregnancy: when and how. Arch Gynecol Obstet. 2014;290:417- 423.
- Barnhart KT, Sammel MD, Cracia CR, et al. Risk factors for ectopic pregnancy in women with symptomatic firsttrimester pregnancies. Fertil Steril. 2006;86:36-43.
- Carusi D. Pregnancy of unknown location: evaluation and management. Semin Perinatol. 2019;43:95-100.
- Barnhart KT, Fay CA, Suescum M, et al. Clinical factors affecting the accuracy of ultrasonography in symptomatic first-trimester pregnancy. Obstet Gynecol. 2011;117:299-306.
- American College of Obstetricians and Gynecologists Practice Bulletin No. 193: tubal ectopic pregnancy. Obstet Gynecol. 2018;131:e91-e103.
- Bouyer J, Coste J, Fernandez H, et al. Sites of ectopic pregnancy: a 10-year population-based study of 1800 cases. Hum Reprod. 2002;17:3224-3230.
- Spiegelberg O. Zur casuistic der ovarial schwangerschaft. Arch Gynecol. 1978;13:73.
- OB Hospitalist Group. Methotrexate use for ectopic pregnancies guidelines. https://www.obhg.com/wp-content /uploads/2020/01/Methotrexate-Use-for-EctopicPregnancies_2016-updates.pdf. Accessed December 10, 2020.
- Brozos C, Kargiannis I, Kiossis E, et al. Ectopic pregnancy through a caesarean scar in a ewe. N Z Vet J. 2013;61:373-375.
- Tucker v. Gillette, 12 Ohio Cir. Dec. 401 (Cir. Ct. 1901).
- Creanga AA, Syverson C, Seed K, et al. Pregnancy-related mortality in the United States, 2011–2013. Obstet Gynecol. 2017;130:366-373.
- Matthews LR, Alvi FA, Milad MP. Reproductive surgery malpractice patterns. Fertil Steril. 2016;106:e42-e43.
- Kim B. The impact of malpractice risk on the use of obstetrics procedures. J Legal Studies. 2006;36:S79-S120.
- Abinader R, Warsof S. Complications involving obstetrical ultrasound. In: Warsof S, Shwayder JM, eds. Legal Concepts and Best Practices in Obstetrics: The Nuts and Bolts Guide to Mitigating Risk. 2019;45-48.
- Creanga AA, Shapiro-Mendoza CK, Bish CL, et al. Trends in ectopic pregnancy mortality in the United States: 1980-2007. Obstet Gynecol. 2011;117:837-843.
- Shwayder JM. IUP diagnosed and treated as ectopic: How bad can it get? Contemporary OB/GYN. 2019;64:49-46.
- Kaplan AI. Should this ectopic pregnancy have been diagnosed earlier? Contemporary OB/GYN. 2017;62:53.
- American College of Obstetricians and Gynecologists Committee on Ethics. Committee opinion 439: informed consent. Reaffirmed 2015. https://www.acog.org/clinical /clinical-guidance/committee-opinion/articles/2009/08 /informed-consent. Accessed December 9, 2020.
- Shwayder JM. Liability in ob/gyn ultrasound. Contemporary OB/GYN. 2017;62:32-49.
- Fisher LN. Institutional religious exemptions: a balancing approach. BYU Law Review. 2014;415-444.
- Makdisi J. Aquinas’s prohibition of killing reconsidered. J Catholic Legal Stud. 2019:57:67-128.
- Franzonello A. Remarks of Anna Franzonello. Alb Law J Sci Tech. 2012;23:519-530.
- Malcolm HE. Pregnancy centers and the limits of mandated disclosure. Columbia Law Rev. 2019;119:1133-1168.
- Kukura E. Contested care: the limitations of evidencebased maternity care reform. Berkeley J Gender Law Justice. 2016;31:241-298.
- Donley G. Contraceptive equity: curing the sex discrimination in the ACA’s mandate. Alabama Law Rev. 2019;71:499-560.
- Berk H. Savvy surrogates and rock star parents: compensation provisions, contracting practices, and the value of womb work. Law Social Inquiry. 2020;45:398-431.
2021 Update on obstetrics
While 2020 was a challenge to say the least, obstetrician-gynecologists remained on the frontline caring for women through it all. Life continued despite the COVID-19 pandemic: prenatal care was delivered, albeit at times in different ways; babies were born; and our role in improving outcomes for women and their children became even more important. This year’s Update focuses on clinical guidelines centered on safety and optimal outcomes for women and children.
ACOG and SMFM update guidance on FGR management
American College of Obstetricians and Gynecologists. Practice advisory: Updated guidance regarding fetal growth restriction. September 2020. https://www.acog.org/clinical/clinical-guidance/practice-advisory/articles/2020/09/updated-guidance-regarding-fetal-growth-restriction. Accessed December 18, 2020.
Fetal growth restriction (FGR) affects up to 10% of pregnancies and is a leading cause of infant morbidity and mortality. Suboptimal fetal growth can have lasting negative effects on development into early childhood and, some hypothesize, even into adulthood.1,2 Antenatal detection of fetuses with FGR is critical so that antenatal testing can be implemented in an attempt to deliver improved clinical outcomes. FGR is defined by several different diagnostic criteria, and many studies have been conducted to determine how best to diagnose this condition.
In September 2020, the American College of Obstetricians and Gynecologists (ACOG) released a Practice Advisory regarding guidance on FGR in an effort to align the ACOG Practice Bulletin No. 204, ACOG Committee Opinion No. 764, and SMFM (Society for Maternal-Fetal Medicine) Consult Series No. 52.3-5 This guidance updates and replaces prior guidelines, with an emphasis on 3 notable changes.
FGR definition, workup have changed
While the original definition of FGR was an estimated fetal weight (EFW) of less than the 10th percentile for gestational age, a similar level of accuracy in prediction of subsequent small for gestational age (SGA) at birth has been shown when this or an abdominal circumference (AC) of less than the 10th percentile is used. Based on these findings, SMFM now recommends that FGR be defined as an EFW or AC of less than the 10th percentile for gestational age.
Recent studies have done head-to-head comparisons of different methods of estimating fetal weight to determine the best detection and pregnancy outcome improvement in FGR. In all instances, the Hadlock formula has continued to more accurately estimate fetal weight, prediction of SGA, and composite neonatal morbidity. As such, new guidelines recommend that population-based fetal growth references (that is, the Hadlock formula) should be used to determine ultrasonography-derived fetal weight percentiles.
The new guidance also suggests classification of FGR based on gestational age at onset, with early FGR at less than 32 weeks and late FGR at 32 or more weeks. The definition of severe FGR is reserved for fetuses with an EFW of less than the 3rd percentile. A diagnosis of FGR should prompt the recommendation for a detailed obstetric ultrasonography. Diagnostic genetic testing should be offered in cases of early-onset FGR, concomitant sonographic abnormalities, and/or polyhydramnios. Routine serum screening for toxoplasmosis, rubella, herpes, or cytomegalovirus (CMV) should not be done unless there are risk factors for infection. If amniocentesis is performed for genetic diagnostic testing, consideration can be made for polymerase chain reaction for CMV in the amniotic fluid.
Continue to: Timing of delivery in isolated FGR...
Timing of delivery in isolated FGR
A complicating factor in diagnosing FGR is distinguishing between the pathologically growth-restricted fetus and the constitutionally small fetus. Antenatal testing and serial umbilical artery Doppler assessment should be done following diagnosis of FGR to monitor for evidence of fetal compromise until delivery is planned.
The current ACOG Practice Bulletin No. 204 and Committee Opinion No. 764 recommend delivery between 38 0/7 and 39 6/7 weeks in the setting of isolated FGR with reassuring fetal testing and umbilical artery Doppler assessment.To further refine this, the new recommendations use the growth percentiles. In cases of isolated FGR with EFW between the 3rd and 10th percentile in the setting of normal umbilical artery Doppler, delivery is recommended between 38 and 39 weeks’ gestation. In cases of isolated FGR with EFW of less than the 3rd percentile (severe FGR) in the setting of normal umbilical artery Doppler, delivery is recommended at 37 weeks.
Timing of delivery in complicated FGR
A normal umbilical artery Doppler reflects the low impedance that is necessary for continuous forward flow of blood to the fetus. Abnormal umbilical artery Doppler signifies aberrations of this low-pressure system that affect the amount of continuous forward flow during diastole of the cardiac cycle. With continued compromise, there is progression to absent end-diastolic velocity (AEDV) and, most concerning, reversed end-diastolic velocity (REDV).
Serial umbilical artery Doppler assessment should be done following diagnosis of FGR to monitor for progression that is associated with perinatal mortality, since intervention can be initiated in the form of delivery. Delivery at 37 weeks is recommended for FGR with elevated umbilical artery Doppler of greater than the 95th percentile for gestational age. For FGR with AEDV, delivery is recommended between 33 and 34 weeks of gestation and for FGR with REDV between 30 and 32 weeks, as the neonatal morbidity and mortality associated with continuing the pregnancy outweighs the risks of prematurity in this setting. Because of the abnormal placental-fetal circulation in FGR complicated by AEDV/REDV, there may be a higher likelihood of fetal intolerance of labor and cesarean delivery (CD) may be considered.
- Fetal growth restriction is now defined as EFW of less than the 10th percentile or AC of less than the 10th percentile.
- Evaluation of FGR includes detailed anatomic survey and consideration of genetic evaluation, but infection screening should be done only if the patient is at risk for infection.
- With reassuring antenatal testing and normal umbilical artery Doppler studies, delivery is recommended at 38 to 39 weeks for isolated FGR with EFW in the 3rd to 10th percentile and at 37 weeks for FGR with EFW of less than the 3rd percentile.
- Umbilical artery Doppler studies are used to decrease the risk of perinatal mortality and further guide timing of delivery
Continue to: New recommendations for PROM management...
New recommendations for PROM management
American College of Obstetricians and Gynecologists Committee on Practice Bulletins–Obstetrics. ACOG practice bulletin no. 217: Prelabor rupture of membranes. Obstet Gynecol. 2020;135:e80-e97.
Rupture of membranes prior to the onset of labor occurs at term in 8% of pregnancies and in the preterm period in 2% to 3% of pregnancies.6 Accurate diagnosis, gestational age, evidence of infection, and discussion of the risks and benefits to the mother and fetus/neonate are necessary to optimize outcomes. In the absence of other indications for delivery, a gestational age of 34 or more weeks traditionally has been the cutoff to proceed with delivery, although this has not been globally agreed on and/or practiced.
ACOG has published a comprehensive update that incorporates the results of the PPROMT trial and other recommendations for the diagnosis and management of both term and preterm prelabor rupture of membranes (PROM).6,7
Making the diagnosis
Diagnosis of PROM usually can be made clinically via history and the classic triad of physical exam findings—pooling of fluid, basic pH, and ferning; some institutions also use commercially available tests that detect placental-derived proteins. Both ACOG and the US Food and Drug Administration caution against using these tests alone without clinical evaluation due to concern for false-positives and false-negatives that lead to adverse maternal and fetal/neonatal outcomes. For equivocal cases, ultrasonography for amniotic fluid evaluation and ultrasonography-guided dye tests can be used to assist in accurate diagnosis, especially in the preterm period in which there are significant implications for pregnancy management.
PROM management depends on gestational age
All management recommendations require reassuring fetal testing, evaluation for infection, and no other contraindications to expectant management. Once these are established, the most important determinant of PROM management then becomes gestational age.
Previable PROM
Previable PROM (usually defined as less than 23–24 weeks) has high risks of both maternal and fetal/neonatal morbidity and mortality from infection, hemorrhage, pulmonary hypoplasia, and extreme prematurity. These very difficult cases benefit from a multidisciplinary approach to patient counseling regarding expectant management versus immediate delivery.
If expectant management is chosen, outpatient management with close monitoring for signs of maternal infection may be done until an agreed on gestational age of viability. Then inpatient management with fetal monitoring, corticosteroids, tocolysis, magnesium for neuroprotection, and group B streptococcus (GBS) prophylaxis may be considered as appropriate.
Preterm PROM at less than 34 weeks
If the mother and fetus are otherwise stable, PROM at less than 34 weeks warrants inpatient expectant management with close maternal and fetal monitoring for signs of infection and labor. Management includes latency antibiotics, antenatal corticosteroids, magnesium for neuroprotection if less than 32 weeks’ gestation and at risk for imminent delivery, and GBS prophylaxis. While tocolysis may increase latency and help with steroid course completion, it should be used cautiously and avoided in cases of abruption or chorioamnionitis. Although there is no definitive recommendation published, a rescue course of steroids may be considered as appropriate but should not delay an indicated delivery.
Continue to: Late preterm PROM...
Late preterm PROM
The biggest change to clinical management in this ACOG Practice Bulletin is for late preterm (34–36 6/7 weeks) PROM, with the recommendation for either immediate delivery or expectant management up to 37 weeks stemming from the PPROMPT study by Morris and colleagues.7
From the neonatal perspective, no difference has been demonstrated between immediate delivery and expectant management for neonatal sepsis or a composite neonatal morbidity and mortality. Expectant management may be preferred from the neonatal point of view as immediate delivery was associated with an increased rate of neonatal respiratory distress, mechanical ventilation, and length of stay in the neonatal intensive care unit. The potential for long-term neurodevelopmental outcomes of delivery at 34 versus 37 weeks also should be considered.
From the maternal perspective, expectant management has an increased risk of antepartum and postpartum hemorrhage, fever, antibiotic use, and maternal length of stay, but a decreased risk of CD.
A late preterm steroid course can be considered if delivery is planned in no less than 24 hours and likely to occur in the next 7 days and if the patient has not already received a course of steroids. A rescue course of steroids is not indicated if the patient received a steroid course prior in the pregnancy. While appropriate GBS prophylaxis is recommended, latency antibiotics and tocolysis are not, and delivery should not be delayed if chorioamnionitis is diagnosed.
Ultimately, preterm PROM management with a stable mother and fetus at or beyond 34 weeks requires comprehensive counseling of the risks and benefits for both mother and fetus/neonate. A multidisciplinary team that together counsels the patient also may help with this shared decision making.
Term PROM
For patients with term PROM, delivery is recommended. Although a short period of expectant management for 12 to 24 hours is reported as “reasonable,” the risk of infection increases with the length of rupture of membranes. Therefore, induction of labor or CD soon after rupture of membranes is recommended for patients who are GBS positive and is preferred for all others.
- Accurate diagnosis is necessary for appropriate counseling and management of PROM.
- Delivery is recommended for term PROM, chorioamnionitis, and for patients with previable PROM who do not desire expectant management.
- If the mother and fetus are otherwise stable, expectant management of preterm PROM until 34 to 37 weeks is recommended.
- The decision of when to deliver between 34 and 37 weeks is best made with multidisciplinary counseling and shared decision making with the patient.
VTE prophylaxis in pregnancy: Regimen adjustments, CD strategies, and COVID-19 considerations
Birsner ML, Turrentine M, Pettker CM, et al. ACOG practice advisory: Options for peripartum anticoagulation in areas affected by shortage of unfractionated heparin. March 2020. https://www.acog.org/clinical/clinical-guidance/practice-advisory/articles/2020/03/options-for-peripartum-anticoagulation-in-areas-affected-by-shortage-of-unfractionated-heparin. Accessed December 8, 2020.
Pacheco LD, Saade G, Metz TD. Society for Maternal-Fetal Medicine Consult Series No. 51: Thromboembolism prophylaxis for cesarean delivery. Am J Obstet Gynecol. 2020;223:B11-B17
Venous thromboembolism (VTE) prophylaxis is a timely topic for a number of reasons. First, a shortage of unfractionated heparin prompted an ACOG Practice Advisory, endorsed by SMFM and the Society for Obstetric Anesthesia and Perinatology, regarding use of low molecular weight heparin (LMWH) in the peripartum period.8 In addition, SMFM released updated recommendations for VTE prophylaxis for CD as part of the SMFM Consult Series.9 Finally, there is evidence that COVID-19 infection may increase the risk of coagulopathy, leading to consideration of additional VTE prophylaxis for pregnant and postpartum women with COVID-19.
Candidates for prophylaxis
As recommended by the ACOG Practice Bulletin on thromboembolism in pregnancy, women who may require VTE prophylaxis during pregnancy and/or the postpartum period include those with10:
- VTE diagnosed during pregnancy
- a history of VTE, including during pregnancy or with use of hormonal contraception
- a history of thrombophilia with or without a personal or family history of VTE.
For these patients, LMWH has many advantages over unfractionated heparin, including ease of use and reliability of dosing. It generally is preferred in pregnancy and postpartum (for both prophylactic and therapeutic anticoagulation) by patients and providers.
The Practice Bulletin references a strategy that describes converting LMWH to unfractionated heparin at around 36 weeks’ gestation in preparation for delivery because unfractionated heparin has the advantage of a shorter half-life and the option for anticoagulation reversal with protamine sulfate. In the Practice Advisory, a global shortage of unfractionated heparin and an argument that the above conversion was less about concern for maternal hemorrhage and more about avoiding spinal and epidural hematomas led to the following recommendations for continued use of LMWH through delivery:
- LMWH heparin can be discontinued in a planned fashion prior to scheduled induction of labor or CD (generally 12 hours for prophylactic dosing and 24 hours for intermediate dosing).
- Patients in spontaneous labor may receive neuraxial anesthesia 12 hours after the last prophylactic dose and 24 hours after the last intermediate dose of LMWH.
- Patients who require anticoagulation during pregnancy should be counseled that if they have vaginal bleeding, leakage of fluid, or regular contractions they should be evaluated prior to taking their next dose of anticoagulant.
- In the absence of other complications, delivery should not be before 39 weeks for the indication of anticoagulation requirement alone.
Continue to: Managing VTE risk in CD...
Managing VTE risk in CD
Recognizing that VTE is a major cause of maternal morbidity and mortality, as well as the variety of the published guidelines for VTE prophylaxis after CD, the SMFM Consult Series provides recommendations to assist clinicians caring for postpartum women after CD. As reviewed in the ACOG Practice Bulletin, there are good data to support pharmacologic prophylaxis during pregnancy and the postpartum period for women with a history of VTE or a thrombophilia. Solid evidence is lacking, however, for what to do for women who have a CD without this history but may have other potential risk factors for VTE, such as obesity, preeclampsia, and transfusion requirement. Universal pharmacologic prophylaxis also is not yet supported by evidence. SMFM supports LMWH as the preferred medication in pregnancy and postpartum and provides these additional recommendations:
- All women who have a CD should have sequential compression devices (SCDs) placed prior to surgery and continued until they are ambulatory.
- Women with a history of VTE or thrombophilia without history of VTE should have SCDs and pharmacologic VTE prophylaxis for 6 weeks postpartum.
- Intermediate dosing of LMWH is recommended for patients with class III obesity.
- Institutions should develop patient safety bundles for VTE prophylaxis to identify additional risk factors that may warrant pharmacologic prophylaxis after CD in select patients.
Our approach to patients with COVID-19 infection
At our institution, we recently incorporated a VTE prophylaxis protocol into our electronic medical record that provides risk stratification for each patient. In addition to the above recommendations, our patients may qualify for short-term in-house or longer postpartum prophylaxis depending on risk factors.
A new risk factor in recent months is COVID-19 infection, which appears to increase the risk of coagulopathy, especially in patients with disease severe enough to warrant hospitalization. Given the potential for additive risk in pregnancy, in consult with our medicine colleagues, we have placed some of our more ill hospitalized pregnant patients on a course of prophylactic LMWH both in the hospital and after discharge independent of delivery status or mode of delivery. ●
- Pregnant patients with a history of VTE or a thrombophilia may be candidates for pharmacologic anticoagulation during pregnancy and/or postpartum.
- LMWH is the preferred method of pharmacologic VTE prophylaxis during pregnancy and postpartum.
- For most patients, CD and neuraxial anesthesia safely can be performed 12 to 24 hours after the last dose of prophylactic or intermediate LMWH, respectively.
- All patients undergoing CD should have at least mechanical VTE prophylaxis with SCDs.
- All women who have a CD should be evaluated via institutional patient safety bundles for VTE prophylaxis for additional risk factors that potentially warrant postpartum pharmacologic VTE prophylaxis.
- More data are needed to determine recommendations for universal/ near universal pharmacologic VTE prophylaxis in the postpartum period.
- Pregnant or postpartum patients with moderate to severe COVID-19 infection may be at increased risk for VTE, warranting consideration of additional pharmacologic prophylaxis.
- Baschat AA, Gembruch U, Harman CR. The sequence of changes in Doppler and biophysical parameters as severe fetal growth restriction worsens. Ultrasound Obstet Gynecol. 2001;18:571-577.
- Almond D, Currie J. Killing me softly: the fetal origins hypothesis. J Econ Perspect. 2011;25:153-172.
- American College of Obstetricians and Gynecologists Committee on Practice Bulletins–Obstetrics and Society for Maternal-Fetal Medicine. ACOG practice bulletin no. 204: Fetal growth restriction. Obstet Gynecol. 2019;133: e97-e109.
- American College of Obstetricians and Gynecologists Committee on Obstetric Practice and Society for Maternal-Fetal Medicine. ACOG committee opinion no. 764: Medically indicated late-preterm and early-term deliveries. Obstet Gynecol. 2019;133:e151-e155.
- Society for Maternal-Fetal Medicine; Martins JG, Biggio FR, Abuhamad A. SMFM consult series no. 52: diagnosis and management of fetal growth restriction. Am J Obstet Gynecol. 2020;223:B2-B17.
- American College of Obstetricians and Gynecologists Committee on Practice Bulletins–Obstetrics. ACOG practice bulletin no. 217: Prelabor rupture of membranes. Obstet Gynecol. 2020;135:e80-e97.
- Morris JM, Roberts CL, Bowen JR, et al; PPROMT Collaboration. Immediate delivery compared with expectant management after preterm pre-labour rupture of the membranes close to term (PPROMT trial): a randomised controlled trial. Lancet. 2016;387:444-452.
- Birsner ML, Turrentine M, Pettker CM, et al. ACOG practice advisory: Options for peripartum anticoagulation in areas affected by shortage of unfractionated heparin. March 2020. https://www.acog.org/clinical/clinical-guidance/practice-advisory/articles/2020/03/options-for-peripartum-anticoagulation-in-areas-affected-by-shortage-of-unfractionated-heparin. Accessed December 8, 2020.
- Pacheco LD, Saade G, Metz TD. Society for MaternalFetal Medicine consult series no. 51: Thromboembolism prophylaxis for cesarean delivery. Am J Obstet Gynecol. 2020;223:B11-B17.
- American College of Obstetricians and Gynecologists Committee on Practice Bulletins–Obstetrics. ACOG practice bulletin no. 196: Thromboembolism in pregnancy. Obstet Gynecol. 2018;132:e1-e17.
While 2020 was a challenge to say the least, obstetrician-gynecologists remained on the frontline caring for women through it all. Life continued despite the COVID-19 pandemic: prenatal care was delivered, albeit at times in different ways; babies were born; and our role in improving outcomes for women and their children became even more important. This year’s Update focuses on clinical guidelines centered on safety and optimal outcomes for women and children.
ACOG and SMFM update guidance on FGR management
American College of Obstetricians and Gynecologists. Practice advisory: Updated guidance regarding fetal growth restriction. September 2020. https://www.acog.org/clinical/clinical-guidance/practice-advisory/articles/2020/09/updated-guidance-regarding-fetal-growth-restriction. Accessed December 18, 2020.
Fetal growth restriction (FGR) affects up to 10% of pregnancies and is a leading cause of infant morbidity and mortality. Suboptimal fetal growth can have lasting negative effects on development into early childhood and, some hypothesize, even into adulthood.1,2 Antenatal detection of fetuses with FGR is critical so that antenatal testing can be implemented in an attempt to deliver improved clinical outcomes. FGR is defined by several different diagnostic criteria, and many studies have been conducted to determine how best to diagnose this condition.
In September 2020, the American College of Obstetricians and Gynecologists (ACOG) released a Practice Advisory regarding guidance on FGR in an effort to align the ACOG Practice Bulletin No. 204, ACOG Committee Opinion No. 764, and SMFM (Society for Maternal-Fetal Medicine) Consult Series No. 52.3-5 This guidance updates and replaces prior guidelines, with an emphasis on 3 notable changes.
FGR definition, workup have changed
While the original definition of FGR was an estimated fetal weight (EFW) of less than the 10th percentile for gestational age, a similar level of accuracy in prediction of subsequent small for gestational age (SGA) at birth has been shown when this or an abdominal circumference (AC) of less than the 10th percentile is used. Based on these findings, SMFM now recommends that FGR be defined as an EFW or AC of less than the 10th percentile for gestational age.
Recent studies have done head-to-head comparisons of different methods of estimating fetal weight to determine the best detection and pregnancy outcome improvement in FGR. In all instances, the Hadlock formula has continued to more accurately estimate fetal weight, prediction of SGA, and composite neonatal morbidity. As such, new guidelines recommend that population-based fetal growth references (that is, the Hadlock formula) should be used to determine ultrasonography-derived fetal weight percentiles.
The new guidance also suggests classification of FGR based on gestational age at onset, with early FGR at less than 32 weeks and late FGR at 32 or more weeks. The definition of severe FGR is reserved for fetuses with an EFW of less than the 3rd percentile. A diagnosis of FGR should prompt the recommendation for a detailed obstetric ultrasonography. Diagnostic genetic testing should be offered in cases of early-onset FGR, concomitant sonographic abnormalities, and/or polyhydramnios. Routine serum screening for toxoplasmosis, rubella, herpes, or cytomegalovirus (CMV) should not be done unless there are risk factors for infection. If amniocentesis is performed for genetic diagnostic testing, consideration can be made for polymerase chain reaction for CMV in the amniotic fluid.
Continue to: Timing of delivery in isolated FGR...
Timing of delivery in isolated FGR
A complicating factor in diagnosing FGR is distinguishing between the pathologically growth-restricted fetus and the constitutionally small fetus. Antenatal testing and serial umbilical artery Doppler assessment should be done following diagnosis of FGR to monitor for evidence of fetal compromise until delivery is planned.
The current ACOG Practice Bulletin No. 204 and Committee Opinion No. 764 recommend delivery between 38 0/7 and 39 6/7 weeks in the setting of isolated FGR with reassuring fetal testing and umbilical artery Doppler assessment.To further refine this, the new recommendations use the growth percentiles. In cases of isolated FGR with EFW between the 3rd and 10th percentile in the setting of normal umbilical artery Doppler, delivery is recommended between 38 and 39 weeks’ gestation. In cases of isolated FGR with EFW of less than the 3rd percentile (severe FGR) in the setting of normal umbilical artery Doppler, delivery is recommended at 37 weeks.
Timing of delivery in complicated FGR
A normal umbilical artery Doppler reflects the low impedance that is necessary for continuous forward flow of blood to the fetus. Abnormal umbilical artery Doppler signifies aberrations of this low-pressure system that affect the amount of continuous forward flow during diastole of the cardiac cycle. With continued compromise, there is progression to absent end-diastolic velocity (AEDV) and, most concerning, reversed end-diastolic velocity (REDV).
Serial umbilical artery Doppler assessment should be done following diagnosis of FGR to monitor for progression that is associated with perinatal mortality, since intervention can be initiated in the form of delivery. Delivery at 37 weeks is recommended for FGR with elevated umbilical artery Doppler of greater than the 95th percentile for gestational age. For FGR with AEDV, delivery is recommended between 33 and 34 weeks of gestation and for FGR with REDV between 30 and 32 weeks, as the neonatal morbidity and mortality associated with continuing the pregnancy outweighs the risks of prematurity in this setting. Because of the abnormal placental-fetal circulation in FGR complicated by AEDV/REDV, there may be a higher likelihood of fetal intolerance of labor and cesarean delivery (CD) may be considered.
- Fetal growth restriction is now defined as EFW of less than the 10th percentile or AC of less than the 10th percentile.
- Evaluation of FGR includes detailed anatomic survey and consideration of genetic evaluation, but infection screening should be done only if the patient is at risk for infection.
- With reassuring antenatal testing and normal umbilical artery Doppler studies, delivery is recommended at 38 to 39 weeks for isolated FGR with EFW in the 3rd to 10th percentile and at 37 weeks for FGR with EFW of less than the 3rd percentile.
- Umbilical artery Doppler studies are used to decrease the risk of perinatal mortality and further guide timing of delivery
Continue to: New recommendations for PROM management...
New recommendations for PROM management
American College of Obstetricians and Gynecologists Committee on Practice Bulletins–Obstetrics. ACOG practice bulletin no. 217: Prelabor rupture of membranes. Obstet Gynecol. 2020;135:e80-e97.
Rupture of membranes prior to the onset of labor occurs at term in 8% of pregnancies and in the preterm period in 2% to 3% of pregnancies.6 Accurate diagnosis, gestational age, evidence of infection, and discussion of the risks and benefits to the mother and fetus/neonate are necessary to optimize outcomes. In the absence of other indications for delivery, a gestational age of 34 or more weeks traditionally has been the cutoff to proceed with delivery, although this has not been globally agreed on and/or practiced.
ACOG has published a comprehensive update that incorporates the results of the PPROMT trial and other recommendations for the diagnosis and management of both term and preterm prelabor rupture of membranes (PROM).6,7
Making the diagnosis
Diagnosis of PROM usually can be made clinically via history and the classic triad of physical exam findings—pooling of fluid, basic pH, and ferning; some institutions also use commercially available tests that detect placental-derived proteins. Both ACOG and the US Food and Drug Administration caution against using these tests alone without clinical evaluation due to concern for false-positives and false-negatives that lead to adverse maternal and fetal/neonatal outcomes. For equivocal cases, ultrasonography for amniotic fluid evaluation and ultrasonography-guided dye tests can be used to assist in accurate diagnosis, especially in the preterm period in which there are significant implications for pregnancy management.
PROM management depends on gestational age
All management recommendations require reassuring fetal testing, evaluation for infection, and no other contraindications to expectant management. Once these are established, the most important determinant of PROM management then becomes gestational age.
Previable PROM
Previable PROM (usually defined as less than 23–24 weeks) has high risks of both maternal and fetal/neonatal morbidity and mortality from infection, hemorrhage, pulmonary hypoplasia, and extreme prematurity. These very difficult cases benefit from a multidisciplinary approach to patient counseling regarding expectant management versus immediate delivery.
If expectant management is chosen, outpatient management with close monitoring for signs of maternal infection may be done until an agreed on gestational age of viability. Then inpatient management with fetal monitoring, corticosteroids, tocolysis, magnesium for neuroprotection, and group B streptococcus (GBS) prophylaxis may be considered as appropriate.
Preterm PROM at less than 34 weeks
If the mother and fetus are otherwise stable, PROM at less than 34 weeks warrants inpatient expectant management with close maternal and fetal monitoring for signs of infection and labor. Management includes latency antibiotics, antenatal corticosteroids, magnesium for neuroprotection if less than 32 weeks’ gestation and at risk for imminent delivery, and GBS prophylaxis. While tocolysis may increase latency and help with steroid course completion, it should be used cautiously and avoided in cases of abruption or chorioamnionitis. Although there is no definitive recommendation published, a rescue course of steroids may be considered as appropriate but should not delay an indicated delivery.
Continue to: Late preterm PROM...
Late preterm PROM
The biggest change to clinical management in this ACOG Practice Bulletin is for late preterm (34–36 6/7 weeks) PROM, with the recommendation for either immediate delivery or expectant management up to 37 weeks stemming from the PPROMPT study by Morris and colleagues.7
From the neonatal perspective, no difference has been demonstrated between immediate delivery and expectant management for neonatal sepsis or a composite neonatal morbidity and mortality. Expectant management may be preferred from the neonatal point of view as immediate delivery was associated with an increased rate of neonatal respiratory distress, mechanical ventilation, and length of stay in the neonatal intensive care unit. The potential for long-term neurodevelopmental outcomes of delivery at 34 versus 37 weeks also should be considered.
From the maternal perspective, expectant management has an increased risk of antepartum and postpartum hemorrhage, fever, antibiotic use, and maternal length of stay, but a decreased risk of CD.
A late preterm steroid course can be considered if delivery is planned in no less than 24 hours and likely to occur in the next 7 days and if the patient has not already received a course of steroids. A rescue course of steroids is not indicated if the patient received a steroid course prior in the pregnancy. While appropriate GBS prophylaxis is recommended, latency antibiotics and tocolysis are not, and delivery should not be delayed if chorioamnionitis is diagnosed.
Ultimately, preterm PROM management with a stable mother and fetus at or beyond 34 weeks requires comprehensive counseling of the risks and benefits for both mother and fetus/neonate. A multidisciplinary team that together counsels the patient also may help with this shared decision making.
Term PROM
For patients with term PROM, delivery is recommended. Although a short period of expectant management for 12 to 24 hours is reported as “reasonable,” the risk of infection increases with the length of rupture of membranes. Therefore, induction of labor or CD soon after rupture of membranes is recommended for patients who are GBS positive and is preferred for all others.
- Accurate diagnosis is necessary for appropriate counseling and management of PROM.
- Delivery is recommended for term PROM, chorioamnionitis, and for patients with previable PROM who do not desire expectant management.
- If the mother and fetus are otherwise stable, expectant management of preterm PROM until 34 to 37 weeks is recommended.
- The decision of when to deliver between 34 and 37 weeks is best made with multidisciplinary counseling and shared decision making with the patient.
VTE prophylaxis in pregnancy: Regimen adjustments, CD strategies, and COVID-19 considerations
Birsner ML, Turrentine M, Pettker CM, et al. ACOG practice advisory: Options for peripartum anticoagulation in areas affected by shortage of unfractionated heparin. March 2020. https://www.acog.org/clinical/clinical-guidance/practice-advisory/articles/2020/03/options-for-peripartum-anticoagulation-in-areas-affected-by-shortage-of-unfractionated-heparin. Accessed December 8, 2020.
Pacheco LD, Saade G, Metz TD. Society for Maternal-Fetal Medicine Consult Series No. 51: Thromboembolism prophylaxis for cesarean delivery. Am J Obstet Gynecol. 2020;223:B11-B17
Venous thromboembolism (VTE) prophylaxis is a timely topic for a number of reasons. First, a shortage of unfractionated heparin prompted an ACOG Practice Advisory, endorsed by SMFM and the Society for Obstetric Anesthesia and Perinatology, regarding use of low molecular weight heparin (LMWH) in the peripartum period.8 In addition, SMFM released updated recommendations for VTE prophylaxis for CD as part of the SMFM Consult Series.9 Finally, there is evidence that COVID-19 infection may increase the risk of coagulopathy, leading to consideration of additional VTE prophylaxis for pregnant and postpartum women with COVID-19.
Candidates for prophylaxis
As recommended by the ACOG Practice Bulletin on thromboembolism in pregnancy, women who may require VTE prophylaxis during pregnancy and/or the postpartum period include those with10:
- VTE diagnosed during pregnancy
- a history of VTE, including during pregnancy or with use of hormonal contraception
- a history of thrombophilia with or without a personal or family history of VTE.
For these patients, LMWH has many advantages over unfractionated heparin, including ease of use and reliability of dosing. It generally is preferred in pregnancy and postpartum (for both prophylactic and therapeutic anticoagulation) by patients and providers.
The Practice Bulletin references a strategy that describes converting LMWH to unfractionated heparin at around 36 weeks’ gestation in preparation for delivery because unfractionated heparin has the advantage of a shorter half-life and the option for anticoagulation reversal with protamine sulfate. In the Practice Advisory, a global shortage of unfractionated heparin and an argument that the above conversion was less about concern for maternal hemorrhage and more about avoiding spinal and epidural hematomas led to the following recommendations for continued use of LMWH through delivery:
- LMWH heparin can be discontinued in a planned fashion prior to scheduled induction of labor or CD (generally 12 hours for prophylactic dosing and 24 hours for intermediate dosing).
- Patients in spontaneous labor may receive neuraxial anesthesia 12 hours after the last prophylactic dose and 24 hours after the last intermediate dose of LMWH.
- Patients who require anticoagulation during pregnancy should be counseled that if they have vaginal bleeding, leakage of fluid, or regular contractions they should be evaluated prior to taking their next dose of anticoagulant.
- In the absence of other complications, delivery should not be before 39 weeks for the indication of anticoagulation requirement alone.
Continue to: Managing VTE risk in CD...
Managing VTE risk in CD
Recognizing that VTE is a major cause of maternal morbidity and mortality, as well as the variety of the published guidelines for VTE prophylaxis after CD, the SMFM Consult Series provides recommendations to assist clinicians caring for postpartum women after CD. As reviewed in the ACOG Practice Bulletin, there are good data to support pharmacologic prophylaxis during pregnancy and the postpartum period for women with a history of VTE or a thrombophilia. Solid evidence is lacking, however, for what to do for women who have a CD without this history but may have other potential risk factors for VTE, such as obesity, preeclampsia, and transfusion requirement. Universal pharmacologic prophylaxis also is not yet supported by evidence. SMFM supports LMWH as the preferred medication in pregnancy and postpartum and provides these additional recommendations:
- All women who have a CD should have sequential compression devices (SCDs) placed prior to surgery and continued until they are ambulatory.
- Women with a history of VTE or thrombophilia without history of VTE should have SCDs and pharmacologic VTE prophylaxis for 6 weeks postpartum.
- Intermediate dosing of LMWH is recommended for patients with class III obesity.
- Institutions should develop patient safety bundles for VTE prophylaxis to identify additional risk factors that may warrant pharmacologic prophylaxis after CD in select patients.
Our approach to patients with COVID-19 infection
At our institution, we recently incorporated a VTE prophylaxis protocol into our electronic medical record that provides risk stratification for each patient. In addition to the above recommendations, our patients may qualify for short-term in-house or longer postpartum prophylaxis depending on risk factors.
A new risk factor in recent months is COVID-19 infection, which appears to increase the risk of coagulopathy, especially in patients with disease severe enough to warrant hospitalization. Given the potential for additive risk in pregnancy, in consult with our medicine colleagues, we have placed some of our more ill hospitalized pregnant patients on a course of prophylactic LMWH both in the hospital and after discharge independent of delivery status or mode of delivery. ●
- Pregnant patients with a history of VTE or a thrombophilia may be candidates for pharmacologic anticoagulation during pregnancy and/or postpartum.
- LMWH is the preferred method of pharmacologic VTE prophylaxis during pregnancy and postpartum.
- For most patients, CD and neuraxial anesthesia safely can be performed 12 to 24 hours after the last dose of prophylactic or intermediate LMWH, respectively.
- All patients undergoing CD should have at least mechanical VTE prophylaxis with SCDs.
- All women who have a CD should be evaluated via institutional patient safety bundles for VTE prophylaxis for additional risk factors that potentially warrant postpartum pharmacologic VTE prophylaxis.
- More data are needed to determine recommendations for universal/ near universal pharmacologic VTE prophylaxis in the postpartum period.
- Pregnant or postpartum patients with moderate to severe COVID-19 infection may be at increased risk for VTE, warranting consideration of additional pharmacologic prophylaxis.
While 2020 was a challenge to say the least, obstetrician-gynecologists remained on the frontline caring for women through it all. Life continued despite the COVID-19 pandemic: prenatal care was delivered, albeit at times in different ways; babies were born; and our role in improving outcomes for women and their children became even more important. This year’s Update focuses on clinical guidelines centered on safety and optimal outcomes for women and children.
ACOG and SMFM update guidance on FGR management
American College of Obstetricians and Gynecologists. Practice advisory: Updated guidance regarding fetal growth restriction. September 2020. https://www.acog.org/clinical/clinical-guidance/practice-advisory/articles/2020/09/updated-guidance-regarding-fetal-growth-restriction. Accessed December 18, 2020.
Fetal growth restriction (FGR) affects up to 10% of pregnancies and is a leading cause of infant morbidity and mortality. Suboptimal fetal growth can have lasting negative effects on development into early childhood and, some hypothesize, even into adulthood.1,2 Antenatal detection of fetuses with FGR is critical so that antenatal testing can be implemented in an attempt to deliver improved clinical outcomes. FGR is defined by several different diagnostic criteria, and many studies have been conducted to determine how best to diagnose this condition.
In September 2020, the American College of Obstetricians and Gynecologists (ACOG) released a Practice Advisory regarding guidance on FGR in an effort to align the ACOG Practice Bulletin No. 204, ACOG Committee Opinion No. 764, and SMFM (Society for Maternal-Fetal Medicine) Consult Series No. 52.3-5 This guidance updates and replaces prior guidelines, with an emphasis on 3 notable changes.
FGR definition, workup have changed
While the original definition of FGR was an estimated fetal weight (EFW) of less than the 10th percentile for gestational age, a similar level of accuracy in prediction of subsequent small for gestational age (SGA) at birth has been shown when this or an abdominal circumference (AC) of less than the 10th percentile is used. Based on these findings, SMFM now recommends that FGR be defined as an EFW or AC of less than the 10th percentile for gestational age.
Recent studies have done head-to-head comparisons of different methods of estimating fetal weight to determine the best detection and pregnancy outcome improvement in FGR. In all instances, the Hadlock formula has continued to more accurately estimate fetal weight, prediction of SGA, and composite neonatal morbidity. As such, new guidelines recommend that population-based fetal growth references (that is, the Hadlock formula) should be used to determine ultrasonography-derived fetal weight percentiles.
The new guidance also suggests classification of FGR based on gestational age at onset, with early FGR at less than 32 weeks and late FGR at 32 or more weeks. The definition of severe FGR is reserved for fetuses with an EFW of less than the 3rd percentile. A diagnosis of FGR should prompt the recommendation for a detailed obstetric ultrasonography. Diagnostic genetic testing should be offered in cases of early-onset FGR, concomitant sonographic abnormalities, and/or polyhydramnios. Routine serum screening for toxoplasmosis, rubella, herpes, or cytomegalovirus (CMV) should not be done unless there are risk factors for infection. If amniocentesis is performed for genetic diagnostic testing, consideration can be made for polymerase chain reaction for CMV in the amniotic fluid.
Continue to: Timing of delivery in isolated FGR...
Timing of delivery in isolated FGR
A complicating factor in diagnosing FGR is distinguishing between the pathologically growth-restricted fetus and the constitutionally small fetus. Antenatal testing and serial umbilical artery Doppler assessment should be done following diagnosis of FGR to monitor for evidence of fetal compromise until delivery is planned.
The current ACOG Practice Bulletin No. 204 and Committee Opinion No. 764 recommend delivery between 38 0/7 and 39 6/7 weeks in the setting of isolated FGR with reassuring fetal testing and umbilical artery Doppler assessment.To further refine this, the new recommendations use the growth percentiles. In cases of isolated FGR with EFW between the 3rd and 10th percentile in the setting of normal umbilical artery Doppler, delivery is recommended between 38 and 39 weeks’ gestation. In cases of isolated FGR with EFW of less than the 3rd percentile (severe FGR) in the setting of normal umbilical artery Doppler, delivery is recommended at 37 weeks.
Timing of delivery in complicated FGR
A normal umbilical artery Doppler reflects the low impedance that is necessary for continuous forward flow of blood to the fetus. Abnormal umbilical artery Doppler signifies aberrations of this low-pressure system that affect the amount of continuous forward flow during diastole of the cardiac cycle. With continued compromise, there is progression to absent end-diastolic velocity (AEDV) and, most concerning, reversed end-diastolic velocity (REDV).
Serial umbilical artery Doppler assessment should be done following diagnosis of FGR to monitor for progression that is associated with perinatal mortality, since intervention can be initiated in the form of delivery. Delivery at 37 weeks is recommended for FGR with elevated umbilical artery Doppler of greater than the 95th percentile for gestational age. For FGR with AEDV, delivery is recommended between 33 and 34 weeks of gestation and for FGR with REDV between 30 and 32 weeks, as the neonatal morbidity and mortality associated with continuing the pregnancy outweighs the risks of prematurity in this setting. Because of the abnormal placental-fetal circulation in FGR complicated by AEDV/REDV, there may be a higher likelihood of fetal intolerance of labor and cesarean delivery (CD) may be considered.
- Fetal growth restriction is now defined as EFW of less than the 10th percentile or AC of less than the 10th percentile.
- Evaluation of FGR includes detailed anatomic survey and consideration of genetic evaluation, but infection screening should be done only if the patient is at risk for infection.
- With reassuring antenatal testing and normal umbilical artery Doppler studies, delivery is recommended at 38 to 39 weeks for isolated FGR with EFW in the 3rd to 10th percentile and at 37 weeks for FGR with EFW of less than the 3rd percentile.
- Umbilical artery Doppler studies are used to decrease the risk of perinatal mortality and further guide timing of delivery
Continue to: New recommendations for PROM management...
New recommendations for PROM management
American College of Obstetricians and Gynecologists Committee on Practice Bulletins–Obstetrics. ACOG practice bulletin no. 217: Prelabor rupture of membranes. Obstet Gynecol. 2020;135:e80-e97.
Rupture of membranes prior to the onset of labor occurs at term in 8% of pregnancies and in the preterm period in 2% to 3% of pregnancies.6 Accurate diagnosis, gestational age, evidence of infection, and discussion of the risks and benefits to the mother and fetus/neonate are necessary to optimize outcomes. In the absence of other indications for delivery, a gestational age of 34 or more weeks traditionally has been the cutoff to proceed with delivery, although this has not been globally agreed on and/or practiced.
ACOG has published a comprehensive update that incorporates the results of the PPROMT trial and other recommendations for the diagnosis and management of both term and preterm prelabor rupture of membranes (PROM).6,7
Making the diagnosis
Diagnosis of PROM usually can be made clinically via history and the classic triad of physical exam findings—pooling of fluid, basic pH, and ferning; some institutions also use commercially available tests that detect placental-derived proteins. Both ACOG and the US Food and Drug Administration caution against using these tests alone without clinical evaluation due to concern for false-positives and false-negatives that lead to adverse maternal and fetal/neonatal outcomes. For equivocal cases, ultrasonography for amniotic fluid evaluation and ultrasonography-guided dye tests can be used to assist in accurate diagnosis, especially in the preterm period in which there are significant implications for pregnancy management.
PROM management depends on gestational age
All management recommendations require reassuring fetal testing, evaluation for infection, and no other contraindications to expectant management. Once these are established, the most important determinant of PROM management then becomes gestational age.
Previable PROM
Previable PROM (usually defined as less than 23–24 weeks) has high risks of both maternal and fetal/neonatal morbidity and mortality from infection, hemorrhage, pulmonary hypoplasia, and extreme prematurity. These very difficult cases benefit from a multidisciplinary approach to patient counseling regarding expectant management versus immediate delivery.
If expectant management is chosen, outpatient management with close monitoring for signs of maternal infection may be done until an agreed on gestational age of viability. Then inpatient management with fetal monitoring, corticosteroids, tocolysis, magnesium for neuroprotection, and group B streptococcus (GBS) prophylaxis may be considered as appropriate.
Preterm PROM at less than 34 weeks
If the mother and fetus are otherwise stable, PROM at less than 34 weeks warrants inpatient expectant management with close maternal and fetal monitoring for signs of infection and labor. Management includes latency antibiotics, antenatal corticosteroids, magnesium for neuroprotection if less than 32 weeks’ gestation and at risk for imminent delivery, and GBS prophylaxis. While tocolysis may increase latency and help with steroid course completion, it should be used cautiously and avoided in cases of abruption or chorioamnionitis. Although there is no definitive recommendation published, a rescue course of steroids may be considered as appropriate but should not delay an indicated delivery.
Continue to: Late preterm PROM...
Late preterm PROM
The biggest change to clinical management in this ACOG Practice Bulletin is for late preterm (34–36 6/7 weeks) PROM, with the recommendation for either immediate delivery or expectant management up to 37 weeks stemming from the PPROMPT study by Morris and colleagues.7
From the neonatal perspective, no difference has been demonstrated between immediate delivery and expectant management for neonatal sepsis or a composite neonatal morbidity and mortality. Expectant management may be preferred from the neonatal point of view as immediate delivery was associated with an increased rate of neonatal respiratory distress, mechanical ventilation, and length of stay in the neonatal intensive care unit. The potential for long-term neurodevelopmental outcomes of delivery at 34 versus 37 weeks also should be considered.
From the maternal perspective, expectant management has an increased risk of antepartum and postpartum hemorrhage, fever, antibiotic use, and maternal length of stay, but a decreased risk of CD.
A late preterm steroid course can be considered if delivery is planned in no less than 24 hours and likely to occur in the next 7 days and if the patient has not already received a course of steroids. A rescue course of steroids is not indicated if the patient received a steroid course prior in the pregnancy. While appropriate GBS prophylaxis is recommended, latency antibiotics and tocolysis are not, and delivery should not be delayed if chorioamnionitis is diagnosed.
Ultimately, preterm PROM management with a stable mother and fetus at or beyond 34 weeks requires comprehensive counseling of the risks and benefits for both mother and fetus/neonate. A multidisciplinary team that together counsels the patient also may help with this shared decision making.
Term PROM
For patients with term PROM, delivery is recommended. Although a short period of expectant management for 12 to 24 hours is reported as “reasonable,” the risk of infection increases with the length of rupture of membranes. Therefore, induction of labor or CD soon after rupture of membranes is recommended for patients who are GBS positive and is preferred for all others.
- Accurate diagnosis is necessary for appropriate counseling and management of PROM.
- Delivery is recommended for term PROM, chorioamnionitis, and for patients with previable PROM who do not desire expectant management.
- If the mother and fetus are otherwise stable, expectant management of preterm PROM until 34 to 37 weeks is recommended.
- The decision of when to deliver between 34 and 37 weeks is best made with multidisciplinary counseling and shared decision making with the patient.
VTE prophylaxis in pregnancy: Regimen adjustments, CD strategies, and COVID-19 considerations
Birsner ML, Turrentine M, Pettker CM, et al. ACOG practice advisory: Options for peripartum anticoagulation in areas affected by shortage of unfractionated heparin. March 2020. https://www.acog.org/clinical/clinical-guidance/practice-advisory/articles/2020/03/options-for-peripartum-anticoagulation-in-areas-affected-by-shortage-of-unfractionated-heparin. Accessed December 8, 2020.
Pacheco LD, Saade G, Metz TD. Society for Maternal-Fetal Medicine Consult Series No. 51: Thromboembolism prophylaxis for cesarean delivery. Am J Obstet Gynecol. 2020;223:B11-B17
Venous thromboembolism (VTE) prophylaxis is a timely topic for a number of reasons. First, a shortage of unfractionated heparin prompted an ACOG Practice Advisory, endorsed by SMFM and the Society for Obstetric Anesthesia and Perinatology, regarding use of low molecular weight heparin (LMWH) in the peripartum period.8 In addition, SMFM released updated recommendations for VTE prophylaxis for CD as part of the SMFM Consult Series.9 Finally, there is evidence that COVID-19 infection may increase the risk of coagulopathy, leading to consideration of additional VTE prophylaxis for pregnant and postpartum women with COVID-19.
Candidates for prophylaxis
As recommended by the ACOG Practice Bulletin on thromboembolism in pregnancy, women who may require VTE prophylaxis during pregnancy and/or the postpartum period include those with10:
- VTE diagnosed during pregnancy
- a history of VTE, including during pregnancy or with use of hormonal contraception
- a history of thrombophilia with or without a personal or family history of VTE.
For these patients, LMWH has many advantages over unfractionated heparin, including ease of use and reliability of dosing. It generally is preferred in pregnancy and postpartum (for both prophylactic and therapeutic anticoagulation) by patients and providers.
The Practice Bulletin references a strategy that describes converting LMWH to unfractionated heparin at around 36 weeks’ gestation in preparation for delivery because unfractionated heparin has the advantage of a shorter half-life and the option for anticoagulation reversal with protamine sulfate. In the Practice Advisory, a global shortage of unfractionated heparin and an argument that the above conversion was less about concern for maternal hemorrhage and more about avoiding spinal and epidural hematomas led to the following recommendations for continued use of LMWH through delivery:
- LMWH heparin can be discontinued in a planned fashion prior to scheduled induction of labor or CD (generally 12 hours for prophylactic dosing and 24 hours for intermediate dosing).
- Patients in spontaneous labor may receive neuraxial anesthesia 12 hours after the last prophylactic dose and 24 hours after the last intermediate dose of LMWH.
- Patients who require anticoagulation during pregnancy should be counseled that if they have vaginal bleeding, leakage of fluid, or regular contractions they should be evaluated prior to taking their next dose of anticoagulant.
- In the absence of other complications, delivery should not be before 39 weeks for the indication of anticoagulation requirement alone.
Continue to: Managing VTE risk in CD...
Managing VTE risk in CD
Recognizing that VTE is a major cause of maternal morbidity and mortality, as well as the variety of the published guidelines for VTE prophylaxis after CD, the SMFM Consult Series provides recommendations to assist clinicians caring for postpartum women after CD. As reviewed in the ACOG Practice Bulletin, there are good data to support pharmacologic prophylaxis during pregnancy and the postpartum period for women with a history of VTE or a thrombophilia. Solid evidence is lacking, however, for what to do for women who have a CD without this history but may have other potential risk factors for VTE, such as obesity, preeclampsia, and transfusion requirement. Universal pharmacologic prophylaxis also is not yet supported by evidence. SMFM supports LMWH as the preferred medication in pregnancy and postpartum and provides these additional recommendations:
- All women who have a CD should have sequential compression devices (SCDs) placed prior to surgery and continued until they are ambulatory.
- Women with a history of VTE or thrombophilia without history of VTE should have SCDs and pharmacologic VTE prophylaxis for 6 weeks postpartum.
- Intermediate dosing of LMWH is recommended for patients with class III obesity.
- Institutions should develop patient safety bundles for VTE prophylaxis to identify additional risk factors that may warrant pharmacologic prophylaxis after CD in select patients.
Our approach to patients with COVID-19 infection
At our institution, we recently incorporated a VTE prophylaxis protocol into our electronic medical record that provides risk stratification for each patient. In addition to the above recommendations, our patients may qualify for short-term in-house or longer postpartum prophylaxis depending on risk factors.
A new risk factor in recent months is COVID-19 infection, which appears to increase the risk of coagulopathy, especially in patients with disease severe enough to warrant hospitalization. Given the potential for additive risk in pregnancy, in consult with our medicine colleagues, we have placed some of our more ill hospitalized pregnant patients on a course of prophylactic LMWH both in the hospital and after discharge independent of delivery status or mode of delivery. ●
- Pregnant patients with a history of VTE or a thrombophilia may be candidates for pharmacologic anticoagulation during pregnancy and/or postpartum.
- LMWH is the preferred method of pharmacologic VTE prophylaxis during pregnancy and postpartum.
- For most patients, CD and neuraxial anesthesia safely can be performed 12 to 24 hours after the last dose of prophylactic or intermediate LMWH, respectively.
- All patients undergoing CD should have at least mechanical VTE prophylaxis with SCDs.
- All women who have a CD should be evaluated via institutional patient safety bundles for VTE prophylaxis for additional risk factors that potentially warrant postpartum pharmacologic VTE prophylaxis.
- More data are needed to determine recommendations for universal/ near universal pharmacologic VTE prophylaxis in the postpartum period.
- Pregnant or postpartum patients with moderate to severe COVID-19 infection may be at increased risk for VTE, warranting consideration of additional pharmacologic prophylaxis.
- Baschat AA, Gembruch U, Harman CR. The sequence of changes in Doppler and biophysical parameters as severe fetal growth restriction worsens. Ultrasound Obstet Gynecol. 2001;18:571-577.
- Almond D, Currie J. Killing me softly: the fetal origins hypothesis. J Econ Perspect. 2011;25:153-172.
- American College of Obstetricians and Gynecologists Committee on Practice Bulletins–Obstetrics and Society for Maternal-Fetal Medicine. ACOG practice bulletin no. 204: Fetal growth restriction. Obstet Gynecol. 2019;133: e97-e109.
- American College of Obstetricians and Gynecologists Committee on Obstetric Practice and Society for Maternal-Fetal Medicine. ACOG committee opinion no. 764: Medically indicated late-preterm and early-term deliveries. Obstet Gynecol. 2019;133:e151-e155.
- Society for Maternal-Fetal Medicine; Martins JG, Biggio FR, Abuhamad A. SMFM consult series no. 52: diagnosis and management of fetal growth restriction. Am J Obstet Gynecol. 2020;223:B2-B17.
- American College of Obstetricians and Gynecologists Committee on Practice Bulletins–Obstetrics. ACOG practice bulletin no. 217: Prelabor rupture of membranes. Obstet Gynecol. 2020;135:e80-e97.
- Morris JM, Roberts CL, Bowen JR, et al; PPROMT Collaboration. Immediate delivery compared with expectant management after preterm pre-labour rupture of the membranes close to term (PPROMT trial): a randomised controlled trial. Lancet. 2016;387:444-452.
- Birsner ML, Turrentine M, Pettker CM, et al. ACOG practice advisory: Options for peripartum anticoagulation in areas affected by shortage of unfractionated heparin. March 2020. https://www.acog.org/clinical/clinical-guidance/practice-advisory/articles/2020/03/options-for-peripartum-anticoagulation-in-areas-affected-by-shortage-of-unfractionated-heparin. Accessed December 8, 2020.
- Pacheco LD, Saade G, Metz TD. Society for MaternalFetal Medicine consult series no. 51: Thromboembolism prophylaxis for cesarean delivery. Am J Obstet Gynecol. 2020;223:B11-B17.
- American College of Obstetricians and Gynecologists Committee on Practice Bulletins–Obstetrics. ACOG practice bulletin no. 196: Thromboembolism in pregnancy. Obstet Gynecol. 2018;132:e1-e17.
- Baschat AA, Gembruch U, Harman CR. The sequence of changes in Doppler and biophysical parameters as severe fetal growth restriction worsens. Ultrasound Obstet Gynecol. 2001;18:571-577.
- Almond D, Currie J. Killing me softly: the fetal origins hypothesis. J Econ Perspect. 2011;25:153-172.
- American College of Obstetricians and Gynecologists Committee on Practice Bulletins–Obstetrics and Society for Maternal-Fetal Medicine. ACOG practice bulletin no. 204: Fetal growth restriction. Obstet Gynecol. 2019;133: e97-e109.
- American College of Obstetricians and Gynecologists Committee on Obstetric Practice and Society for Maternal-Fetal Medicine. ACOG committee opinion no. 764: Medically indicated late-preterm and early-term deliveries. Obstet Gynecol. 2019;133:e151-e155.
- Society for Maternal-Fetal Medicine; Martins JG, Biggio FR, Abuhamad A. SMFM consult series no. 52: diagnosis and management of fetal growth restriction. Am J Obstet Gynecol. 2020;223:B2-B17.
- American College of Obstetricians and Gynecologists Committee on Practice Bulletins–Obstetrics. ACOG practice bulletin no. 217: Prelabor rupture of membranes. Obstet Gynecol. 2020;135:e80-e97.
- Morris JM, Roberts CL, Bowen JR, et al; PPROMT Collaboration. Immediate delivery compared with expectant management after preterm pre-labour rupture of the membranes close to term (PPROMT trial): a randomised controlled trial. Lancet. 2016;387:444-452.
- Birsner ML, Turrentine M, Pettker CM, et al. ACOG practice advisory: Options for peripartum anticoagulation in areas affected by shortage of unfractionated heparin. March 2020. https://www.acog.org/clinical/clinical-guidance/practice-advisory/articles/2020/03/options-for-peripartum-anticoagulation-in-areas-affected-by-shortage-of-unfractionated-heparin. Accessed December 8, 2020.
- Pacheco LD, Saade G, Metz TD. Society for MaternalFetal Medicine consult series no. 51: Thromboembolism prophylaxis for cesarean delivery. Am J Obstet Gynecol. 2020;223:B11-B17.
- American College of Obstetricians and Gynecologists Committee on Practice Bulletins–Obstetrics. ACOG practice bulletin no. 196: Thromboembolism in pregnancy. Obstet Gynecol. 2018;132:e1-e17.
When ultrasonography reveals a fetal abdominal wall defect
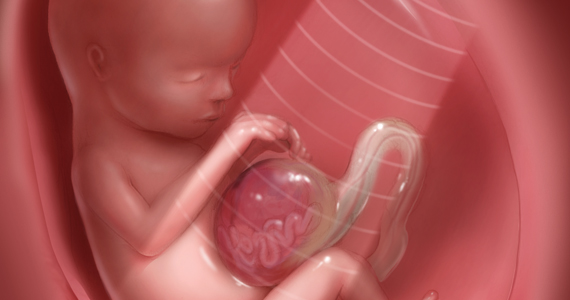
CASE Fetal anomalies detected on ultrasonography
A 34-year-old woman (G2P1) at 19 weeks’ gestation presented for fetal anatomy ultrasonography evaluation. Ultrasonography demonstrated fetal demise with fetal size less than dates, oligohydramnios, and what appeared to be a full-thickness herniation of the thoracic and abdominal contents. Due to the positioning of the fetus and the oligohydramnios, the fetus appeared to have ectopia cordis and herniated liver and bowel; the bladder was not visualized. The patient was counseled regarding the findings and the suspected diagnosis of pentalogy of Cantrell. After counseling, the patient expressed desire to bury the fetus intact according to her religious custom. She underwent a successful uterine evacuation with misoprostol administration and delivered a nonviable fetus that had a closed thoracic cage without ectopia cordis. Key findings were a very short 2-vessel umbilical cord without coiling that was tethered to the intra-abdominal organs, “pulling” the internal organs out of the abdomen, and lack of an anterior abdominal wall (FIGURE 1). Given these findings, a final diagnosis of body-stalk anomaly was made.
Fetal abdominal wall defects (AWDs) encompass a wide array of congenital defects, although they all involve herniation of 1 or more intra-abdominal content through a ventral abdominal defect.1 Overall, the estimated incidence of AWDs is approximately 6 per 10,000 births.1 Gastroschisis and omphalocele are the most common of these defect types.2
The majority of AWDs can be diagnosed during the first trimester of pregnancy via ultrasonography; however, during the first trimester the physiologic midgut herniation resolves by 12 weeks of gestation. It is therefore important to repeat imaging at a later gestational age to confirm the suspicion. Furthermore, the differential diagnosis should include the relatively benign condition of umbilical hernia.
While many AWDs share similarities, they differ significantly in prognosis and management. Early detection is therefore crucial for fetal surveillance, prenatal testing, perinatal planning, and patient counseling (TABLE). In this article, we outline antenatal surveillance and management of AWDs based on recommendations from the American College of Obstetricians and Gynecologists and the Society for Maternal-Fetal Medicine as well as on our experience and practice.
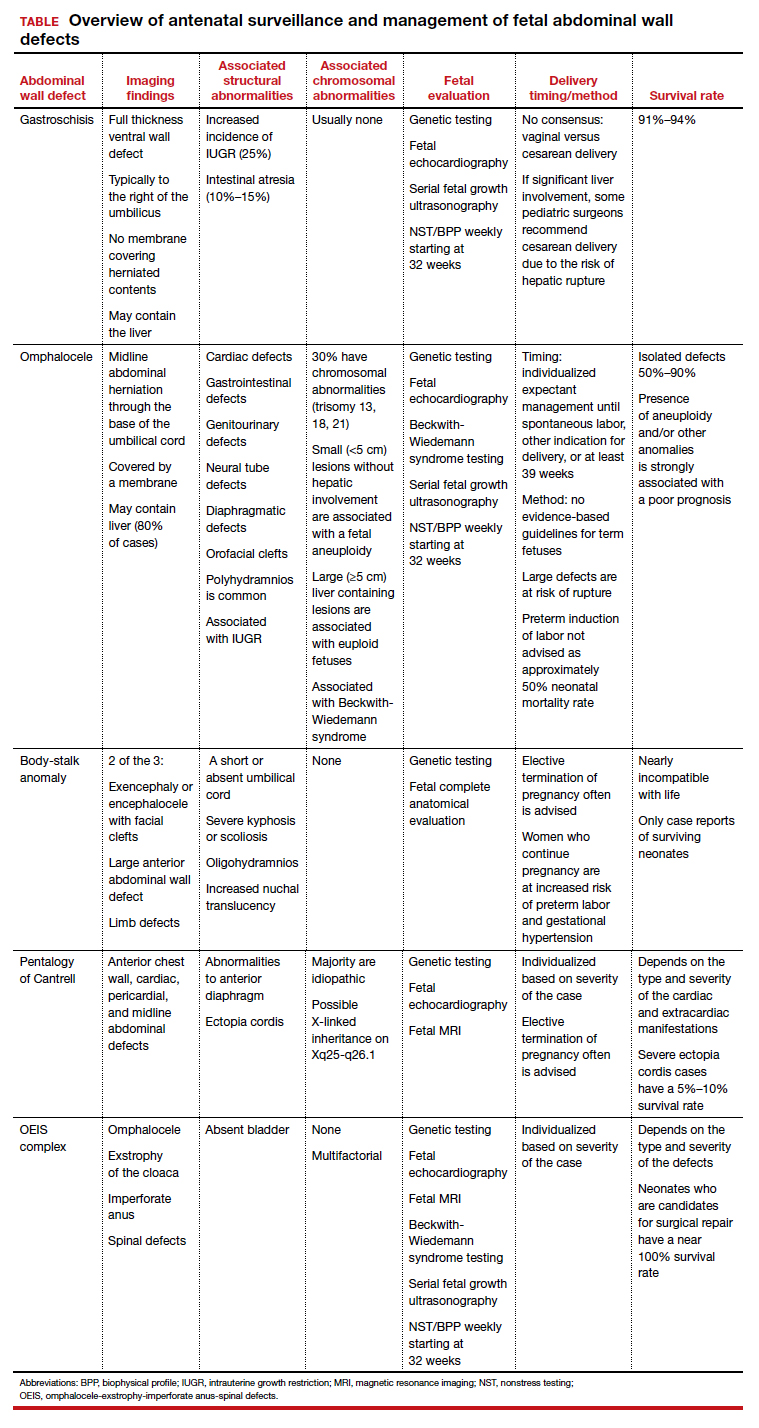
Gastroschisis is an increasingly prevalent AWD
Gastroschisis is a full-thickness, ventral wall defect that results in bowel evisceration; it typically occurs to the right of the umbilical cord insertion.3 It is one of the most common AWDs and its prevalence has increased in the past few decades, from 2 to 3 cases per 10,000 live births in 1995 to as high as 6 cases per 10,000 live births in 2011.2,4,5
The cause of gastroschisis remains unclear. The main theory is that there is an ischemic disruption of the closure of the abdominal wall at or near the omphalomesenteric artery or the right umbilical vein.6,7 In addition, investigators have reported an increased incidence of gastroschisis in mothers exposed to cigarette smoking and certain medications, such as pseudoephedrine, salicylates, ibuprofen, and acetaminophen.8,9
Continue to: Making the diagnosis...
Making the diagnosis
Prenatal diagnosis using ultrasonography is possible at around 10 weeks of gestation. As previously mentioned, however, physiologic herniation of the midgut must be excluded by performing follow-up imaging at a later gestational age. In our practice, we typically do this at around 16 weeks of gestation.
Ultrasonographic features of gastroschisis include loops of bowel herniating through a small paraumbilical wall defect (usually 2–3 cm) floating in amniotic fluid without a covering membrane4 (FIGURE 2). Direct exposure to amniotic fluid causes small bowel inflammation and fibrin deposition, leading to a thickened, echogenic appearance. Polyhydramnios and intra-abdominal bowel dilation have been associated with the presence of intestinal atresia.10
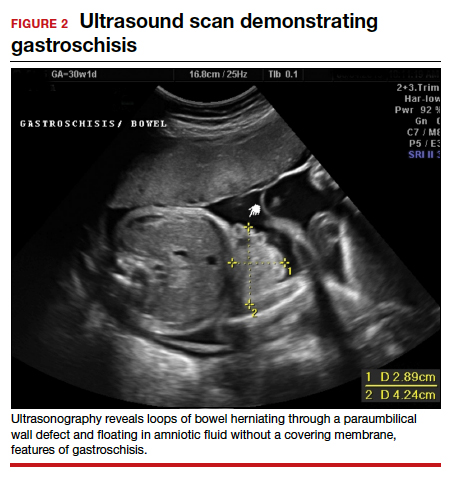
Management
There is no expert consensus regarding optimal prenatal management of gastroschisis.11-17 Prenatal care, patient counseling, and delivery planning should be individualized based on the defect and should be determined in a multidisciplinary discussion with specialists in maternal-fetal medicine, neonatology, and pediatric surgery, as necessary. In our practice, if the gastroschisis is isolated and uncomplicated, our generalist obstetricians manage the patient with maternal-fetal medicine consultation, increased fetal surveillance as described below, and delivery at our tertiary care institution.
Our standard practice is to use the initial ultrasonography imaging to evaluate the size and contents of the defect, measure the nuchal translucency, and evaluate for additional abnormalities. Serial ultrasonography monitoring of the fetus is required to assess the size and quality of the herniated intestine, amount of amniotic fluid, and fetal growth.10
As gastroschisis is a full-thickness defect of the anterior abdominal wall, the abdominal contents are exposed to amniotic fluid. This exposure causes progressive intestinal damage, which can be identified on ultrasonography as bowel thickening and dilation.12-14 Currently, intestinal thickening and dilation is not considered an indication for delivery as it is assumed that the intestinal damage has already occurred. It is debatable whether delivery around 37 weeks compared with delayed delivery beyond 37 weeks improves outcomes and decreases the stillbirth rate.11,13 Studies show that neonates delivered prior to 37 weeks have worse outcomes compared with those delivered after 37 weeks.14,15
Fetal surveillance. As standard practice, we evaluate the fetus at around 16 weeks and then again at around 20 weeks. In the absence of fetal growth restriction, which is associated with 25% of cases,16,17 our standard practice includes performing serial growth ultrasonography every 3 to 4 weeks starting at 28 weeks and biophysical profiles and nonstress testing weekly starting at 32 weeks. Fetal echocardiography can be offered. However, unlike with omphalocele, which has a high incidence of associated cardiac structural anomalies, gastroschisis has a low incidence of congenital cardiac anomalies, estimated to be between 2.5% and 4%.18,19
Delivery considerations. Little agreement exists regarding when and how to deliver pregnancies complicated by fetal gastroschisis. While some advocate for induction of labor at 36 to 38 weeks, most infants with gastroschisis can be delivered safely at term via either vaginal or cesarean delivery.14,15
Delivery timing should consider the clinical picture and incorporate performance on antenatal testing, fetal growth, the size and contents of the gastroschisis, and consultation with maternal-fetal medicine. Fetuses with gastroschisis often have non-reassuring antenatal testing. This can necessitate early delivery, although cesarean delivery should be reserved for obstetric indications, with the caveat that if there is large liver involvement, some pediatric surgeons recommend cesarean delivery due to the risk of hepatic rupture.
Neonate management. The survival rate of gastroschisis is reported to be as high as 91% to 94%.2 Morbidity is related to intestinal complications, such as strictures, adhesions, and volvulus.
In the case of simple gastroschisis, when the bowel is in good condition, the treatment method of choice is primary reduction.20 If performed in the operating room, an immediate sutured closure of the defect can be done. The benefits of primary repair include decreased length of stay, fewer intensive care bed days, and less time to achieve full feeds.20,21 Primary reduction has a reported success rate of 50% to 83%.22 A reduction with a delayed spontaneous closure also can be performed at bedside in the neonatal intensive care unit.22
For complex gastroschisis, characterized by bowel complications such as inflammation, perforation, ischemia, atresia, necrosis, or volvulus, primary closure may not be possible and reduction may need to be achieved through silo application.22-25 Additionally, further bowel surgery, such as stoma formation and bowel resection, may be required.25
Continue to: Omphalocele often is associated with abnormal karyotype...
Omphalocele often is associated with abnormal karyotype
Also known as exomphalos, omphalocele is a relatively common defect, with an estimated prevalence of 2 to 3 cases per 10,000 live births.2 In this condition, there is a midline defect in which intra-abdominal contents herniate through the base of the umbilical cord. Omphaloceles are covered by amniotic membranes, making them distinguishable from gastroschisis, which has no covering, and congenital umbilical hernias, which are covered by intact skin and subcutaneous tissue.26-33
Additionally, in omphalocele the umbilical cord insertion site varies, whereas in gastroschisis the umbilical cord insertion is usually to the right of midline. An omphalocele is often categorized based on whether or not it contains the liver (extracorporeal liver) or only the bowel (intracorporeal liver).
Genetic studies
Approximately 67% to 88% of all pregnancies with omphalocele have an abnormal karyotype and/or associated malformations, including Beckwith-Wiedemann syndrome.31 Of the aneuploidies, trisomy 18 is the one most commonly associated with omphalocele, accounting for approximately 62% to 75%, while trisomy 13 accounts for approximately 11% to 24%.32,33 The presence of other anomalies is strongly associated with poor prognosis, and increased defect size is an independent predictor of neonatal morbidity and mortality, as neonates with large omphaloceles with extracorporeal livers can develop respiratory insufficiency and require more complex surgical repairs. It is interesting, however, that the absence of an extracorporeal liver is associated with a higher risk of aneuploidy than are cases with an intracorporeal liver.33
We offer chorionic villus sampling or amniocentesis to all patients with omphalocele. If the patient undergoes invasive diagnostic testing, the sample then undergoes karyotyping, chromosomal microarray, and testing for Beckwith-Wiedemann syndrome. If the patient declines diagnostic sampling, we perform a cell-free DNA screening to rule out aneuploidy.
Continue to: Making the diagnosis...
Making the diagnosis
Omphaloceles can be diagnosed via prenatal ultrasonography as early as 11 to 14 weeks’ gestation.26 They are classified based on size, location, and contents of the sac.26,27 A small omphalocele is defined as a defect less than 5 cm with a sac that may contain a few loops of intestines (FIGURE 3).27 A giant omphalocele is a defect with more than 75% of the liver contained in the sac.29
Location can be epigastric, umbilical, or hypogastric, and both small and giant omphaloceles may have ruptured membranes that will result in exposure of the contained viscera.27 Omphaloceles are associated with such structural anomalies as cardiac, gastrointestinal, genitourinary, diaphragmatic, and neural tube defects. We do not routinely perform magnetic resonance imaging (MRI) for evaluation of omphaloceles, but MRI may be used to help predict postnatal outcomes in the case of giant omphaloceles.26
Management
Our standard practice is to use the initial ultrasonography imaging to evaluate the size and contents of defect, measure the nuchal translucency, and evaluate for additional abnormalities. As in cases of gastroschisis, serial ultrasonography monitoring of the fetus is required to assess the size and quality of the herniated intestine, amount of amniotic fluid, and fetal growth. We typically evaluate the fetus at around 16 weeks and then again at around 20 weeks. In the absence of fetal growth restriction, we recommend serial growth ultrasonography every 3 to 4 weeks starting at 28 weeks and biophysical profiles and nonstress testing weekly starting at 32 weeks. Additionally, we routinely obtain a fetal echocardiogram to rule out cardiac structural abnormalities.
Delivery considerations. Fetuses that do not undergo spontaneous abortion or medical termination of pregnancy often are born at term.26 We recommend expectant management until spontaneous labor, another indication for delivery arises, or at least 39 weeks’ estimated gestational age. There are no evidence-based guidelines for the optimal mode of delivery in fetuses with omphalocele, although we recommend cesarean delivery for fetuses with large defects to avoid postnatal sac rupture and liver damage. Preterm induction of labor is not indicated as infants born preterm have about a 50% mortality rate.26,27
Children born with isolated omphalocele typically have a good prognosis, with an estimated survival rate of 50% to 90%.32,33 However, compared to gastroschisis, omphaloceles are often associated with other anomalies.32,33
Management of omphaloceles depends on the size of the defect. In our institution, our generalist obstetricians manage the standard prenatal care with the addition of increased fetal surveillance and testing, interdisciplinary patient counseling with maternal-fetal medicine, pediatric surgeons, and neonatologists for delivery planning, and delivery is performed at our tertiary care center.
Neonate management. Small omphaloceles are amenable to primary early fascial closure.26-30 However, attempted primary closure of giant omphaloceles carries significant risks, including abdominal compartment syndrome and postoperative herniation.29,30 Instead, several options exist for staged surgical closure, in which there are multiple operations prior to final fascial closure, as well as nonoperative delayed closure for management of giant omphaloceles.29,30
Conservative management of giant omphaloceles has certain benefits, such as earlier first feeds, decreased risk of abdominal compartment syndrome, and lower risk of infection.30 Ruptured omphaloceles can be repaired through primary repair, employment of a synthetic or biologic mesh fascial bridge, or silo placement with delayed closure.28
Body-stalk anomaly: Multiple defects and poor prognosis
Also known as limb body wall complex, body-stalk anomaly is a rare malformation that has a reported prevalence of approximately 0.12 cases per 10,000 births (both live and stillbirths).34 Body-stalk anomaly is characterized by multiple defects, including severe kyphosis or scoliosis, a short or absent umbilical cord, and a large anterior abdominal wall defect.34-36 This malformation is almost entirely incompatible with life, resulting in abortion or stillbirth.35 Survival is extremely rare and limited to case reports.
While the exact etiology of body-stalk anomaly is unknown, 3 possible causes have been hypothesized: early amnion rupture, vascular compromise, and embryonic dysgenesis.37-40
Continue to: Making the diagnosis...
Making the diagnosis
Body-stalk anomaly typically can be diagnosed by 10 to 14 weeks’ gestation via ultrasonography.34-41 We currently follow the diagnostic criteria proposed by Van Allen and colleagues, which requires 2 of the following 3 anomalies34:
- exencephaly/encephalocele with facial clefts
- thoraco- and/or abdominoschisis (midline defect)
- limb defect.
Additional ultrasonographic findings can include the identification of evisceration of the abdominal contents, a short umbilical cord, and increased nuchal thickness.36,42 During the second and third trimesters, oligohydramnios may be seen.2
Management
Body-stalk anomaly is considered a fatal condition without specific therapeutic interventions. Maternal risks include an increased risk of preterm labor and gestational hypertension.35 Research on body-stalk anomaly has not shown any correlation with patients’ age, fetal sex, or abnormal karyotype, and the reported risk of recurrence for this anomaly is very low.42,43 Early diagnosis therefore is essential to provide families with information and counseling. Given the poor fetal prognosis, increased maternal risk, and low recurrence rates, mothers can be advised toward elective termination of pregnancy.
Should a patient desire expectant management, care can be provided by generalist obstetricians or care can be transferred to maternal-fetal medicine, with the addition of increased fetal surveillance and testing, interdisciplinary patient counseling with maternal-fetal medicine, pediatric surgeons, and neonatologists for delivery planning; delivery should be performed at a tertiary care center.
Pentalogy of Cantrell: Very rare, with variable prognosis
Pentalogy of Cantrell is characterized by a collection of defects in the midline abdominal wall, lower sternum, anterior diaphragm, diaphragmatic pericardium, and some manifestation of intra-cardiac defect.44 It is thought to arise early in gestation due to abnormal differentiation, migration, and fusion of the embryonic mesoderm.44 The condition is rare, with an incidence of about 1 in 5.5 million live births.45
Making the diagnosis
The diagnosis of pentalogy of Cantrell can be made via prenatal ultrasonography as early as the first trimester, although it is diagnosed more commonly in the second trimester.46 Three-dimensional ultrasonography and fetal MRI have been used to confirm the diagnosis.47
Management
Typically, corrective operations are performed during the neonatal period, and cases of successful staged and one-stage operations have been reported.48 Surgical treatment is determined based on the complexity of the condition and the presence of coexistent heart defects.49,50 However, very few patients survive surgical repair; mortality rates are estimated at around 50% to 60%, with high postsurgical morbidity risks for those who do survive.45
Prognosis varies depending on the type and severity of the associated malformations and intracardiac anomalies.46 Patients with partial ectopia cordis and incomplete presentation may have more favorable outcomes, but for patients with severe ectopia cordis, the survival rate is only 5% to 10%.47
Depending on the severity of the defects, mothers can be advised toward elective termination of pregnancy. In our institution, prenatal care usually is transferred to the maternal-fetal medicine service, and delivery is planned at our tertiary care institution.
OEIS complex comprises abdominal, pelvic, and spinal defects
Omphalocele-exstrophy-imperforate anus-spinal defects (OEIS) complex is a congenital malformation syndrome characterized by the combination of midline abdominal and pelvic defects (including omphalocele, exstrophy of the cloaca, and imperforate anus) and spinal defects.51 The condition’s etiology is unknown but is thought to be multifactorial.51-53 It is a rare condition, with an incidence of around 1 in 200,000 to 400,000 pregnancies.52
Making the diagnosis
Prenatal diagnosis of OEIS complex can be made as early as the first trimester via ultrasonographic identification of an infraumbilical abdominal wall defect with protruding mass, absent bladder, and spinal defects.52 When OEIS complex is suspected, fetal MRI can play a critical role in the diagnosis.
Management
As OEIS complex is rare, there are no evidence-based guidelines for optimal mode and timing of delivery. Cases are individualized based on their specific pathology, and we recommend cesarean delivery for fetuses with large defects to avoid postnatal sac rupture and liver damage.
The prognosis for infants with OEIS complex depends on the spectrum and severity of the structural defects.52,53 The many surgeries involved in the repair of OEIS have potential complications, such as urogenital and gastrointestinal dysfunction.52,53 Advances in medical and surgical treatment have resulted in improved survival and quality of life, and survival rates for OEIS complex are now close to 100%.53 While many OEIS patients live with a permanent colostomy, improvements in management mean that more patients are now candidates for gastrointestinal pull-through procedures, which allow for natural bowel control and a higher degree of bowel cleanliness.53
Prenatal care, patient counseling, and delivery planning should be individualized based on the defects present and determined in a multidisciplinary discussion with maternal-fetal medicine, neonatology, and pediatric surgery as necessary. In our institution, prenatal care usually is transferred to the maternal-fetal medicine service, and delivery is planned at our tertiary care institution.
Multidisciplinary team strategy is essential
Based on our experience, when faced with an anterior AWD in utero, prenatal imaging, genetic testing, increased fetal surveillance, and a multidisciplinary team approach improves outcomes. We must emphasize that careful patient counseling is paramount in our practice. ●
Acknowledgement: The authors would like to thank Ashley Tran, BS, for her assistance in the literature review and drafting of this article.
- Patients with fetuses with anterior wall defects should be referred to a maternal-fetal medicine specialist for co-management and advanced fetal imaging.
- The American College of Obstetricians and Gynecologists recommends microarray for all major fetal structural abnormalities, with the qualifier that karyotype can be offered if a specific aneuploidy is suspected based on the abnormality or prior genetic screening tests.
- If confirmatory testing is performed (amniocentesis or chorionic villus sampling), the sample should undergo karyotyping, chromosomal microarray, and if indicated, testing for Beckwith-Wiedemann syndrome. If the patient declines confirmatory sampling, performing cell-free DNA screening to rule out aneuploidy is recommended.
- Fetal echocardiography is recommended.
- Fetal magnetic resonance imaging should be considered in complex cases.
- Management should be individualized based on the type and severity of defect(s).
- Delivery timing and method should be individualized based on the defect(s) and determined in a multidisciplinary discussion with maternal-fetal medicine, neonatology, pediatric surgery, and pediatric cardiology, as necessary.
- The most common fetal abdominal wall defect is omphalocele, followed by gastroschisis.
- Maternal serum α-fetoprotein is usually elevated in all of the disorders.
- Victoria T, Andronikou S, Bowen D, et al. Fetal anterior abdominal wall defects: prenatal imaging by magnetic resonance imaging. Pediatr Radiol. 2018;48:499-512.
- Pakdaman R, Woodward PJ, Kennedy A. Complex abdominal wall defects: appearances at prenatal imaging. Radiographics. 2015;35:636-649.
- Oakes MC, Porto M, Chung JH. Advances in prenatal and perinatal diagnosis and management of gastroschisis. Semin Pediatr Surg. 2018;27:289-299.
- Mastroiacovo P, Lisi A, Castilla EE. The incidence of gastroschisis: research urgently needs resources. BMJ. 2006;332:423-424.
- Boyd PA, Haeusler M, Barisic I. EUROCAT report 9: surveillance of congenital anomalies in Europe 1980-2008. Birth Defects Res A Clin Mol Teratol. 2011;91(suppl 1):S1.
- Gamba P, Midrio P. Abdominal wall defects: prenatal diagnosis, newborn management, and long-term outcomes. Semin Pediatr Surg. 2014;23:283-290.
- Beaudoin S. Insights into the etiology and embryology of gastroschisis. Semin Pediatr Surg. 2018;27:283-288.
- Yazdy MM, Mitchell AA, Werler MM. Maternal genitourinary infections and the risk of gastroschisis. Am J Epidemiol. 2014;180:518-525.
- Werler MM, Sheehan JE, Mitchell AA. Maternal medication use and risks of gastroschisis and small intestinal atresia. Am J Epidemiol. 2002;155:26-31.
- D’Antonio F, Virgone C, Rizzo G, et al. Prenatal risk factors and outcomes in gastroschisis: a meta-analysis. Pediatrics. 2015;136:e159-e169.
- Baud D, Lausman A, Alfaraj MA, et al. Expectant management compared with elective delivery at 37 weeks for gastroschisis. Obstet Gynecol. 2013;121:990-998.
- Goetzinger KR, Tuuli MG, Longman RE, et al. Sonographic predictors of postnatal bowel atresia in fetal gastroschisis. Ultrasound Obstet Gynecol. 2014;43:420-425.
- Overton TG, Pierce MR, Gao H, et al. Antenatal management and outcomes of gastroschisis in the UK. Prenat Diagn. 2012;32:1256-1262.
- Ergün O, Barksdale E, Ergün FS, et al. The timing of delivery of infants with gastroschisis influences outcome. J Pediatr Surg. 2005;40:424-428.
- Overcash RT, DeUgarte DA, Stephenson ML, et al; University of California Fetal Consortium. Factors associated with gastroschisis outcomes. Obstet Gynecol. 2014;124:551-557.
- Wissanji H, Puligandla PS. Risk stratification and outcome determinants in gastroschisis. Semin Pediatr Surg. 2018;27: 300-303.
- Raynor BD, Richards D. Growth retardation in fetuses with gastroschisis. J Ultrasound Med. 1997;16:13-16.
- Mastroiacovo P, Lisi A, Castilla EE, et al. Gastroschisis and associated defects: an international study. Am J Med Genet A. 2007;143A:660-671.
- Kunz LH, Gilbert WM, Towner DR. Increased incidence of cardiac anomalies in pregnancies complicated by gastroschisis. Am J Obstet Gynecol. 2005;193(3 pt 2): 1248-1252.
- Lakshminarayanan B, Lakhoo K. Abdominal wall defects. Early Hum Dev. 2014;90:917-920.
- Prefumo F, Izzi C. Fetal abdominal wall defects. Best Pract Res Clin Obstet Gynaecol. 2014;28:391-402.
- Petrosyan M, Sandler AD. Closure methods in gastroschisis. Semin Pediatr Surg. 2018;27:304-308.
- Skarsgard ED. Management of gastroschisis. Curr Opin Pediatr. 2016;28:363-369.
- Bergholz R, Boettcher M, Reinshagen K, et al. Complex gastroschisis is a different entity to simple gastroschisis affecting morbidity and mortality—a systematic review and meta-analysis. J Pediatr Surg. 2014;49:1527-1532.
- Emil S. Surgical strategies in complex gastroschisis. Semin Pediatr Surg. 2018;27:309-315.
- Verla MA, Style CC, Olutoye OO. Prenatal diagnosis and management of omphalocele. Semin Pediatr Surg. 2019;28:84-88.
- Gonzalez KW, Chandler NM. Ruptured omphalocele: diagnosis and management. Semin Pediatr Surg. 2019;28:101-105.
- Sugandhi N, Saha M, Bhatnagar V, et al. Repair of ruptured omphalocele sac in the neonatal period and beyond. J Indian Assoc Pediatr Surg. 2020;25:46-48.
- Bauman B, Stephens D, Gershone H, et al. Management of giant omphaloceles: a systematic review of methods of staged surgical vs nonoperative delayed closure. J Pediatr Surg. 2016;51:1725-1730.
- Kogut KA, Fiore NF. Nonoperative management of giant omphalocele leading to early fascial closure. J Pediatr Surg. 2018;53:2404-2408.
- Conner P, Vejde JH, Burgos CM. Accuracy and impact of prenatal diagnosis in infants with omphalocele. Pediatr Surg Int. 2018;34:629-633.
- Iacovella C, Contro E, Ghi T, et al. The effect of the contents of exomphalos and nuchal translucency at 11-14 weeks on the likelihood of associated chromosomal abnormality. Prenat Diagn. 2012;32:1066-1070.
- Getachew MM, Goldstein RB, Edge V, et al. Correlation between omphalocele contents and karyotypic abnormalities: sonographic study in 37 cases. AJR Am J Roentgenol. 1992;158:133-136.
- Singh A, Singh J, Gupta K. Body stalk anomaly: antenatal sonographic diagnosis of this rare entity with review of literature. J Ultrason. 2017;17:133-135.
- Lazaroni TL, Cruzeiro PC, Piçarro C, et al. Body stalk anomaly: Three months of survival. Case report and literature review. J Pediatr Surg Case Rep. 2016;14:22-25.
- Gajzer DC, Hirzel AC, Saigal G, et al. Possible genetic origin of limb-body wall complex. Fetal Pediatr Pathol. 2015;34: 257–270.
- Maruyama H, Inagaki T, Nakata Y, et al. Minimally conjoined omphalopagus twins with a body stalk anomaly. AJP Rep. 2015;5:e124-e128.
- Bhat A, Ilyas M, Dev G. Prenatal sonographic diagnosis of limb-body wall complex: case series of a rare congenital anomaly. Radiol Case Rep. 2016;11:116-120.
- Quijano FE, Rey MM, Echeverry M, et al. Body stalk anomaly in a 9-week pregnancy. Case Rep Obstet Gynecol. 2014;2014:357285.
- Kocherla K, Kumari V, Kocherla PR. Prenatal diagnosis of body stalk complex: a rare entity and review of literature. Indian J Radiol Imaging. 2015;25:67-70.
- Panaitescu AM, Ushakov F, Kalaskar A, et al. Ultrasound features and management of body stalk anomaly. Fetal Diagn Ther. 2016;40:285-290.
- Routhu M, Thakkallapelli S, Mohan P, et al. Role of ultrasound in body stalk anomaly and amniotic band syndrome. Int J Reprod Med. 2016;2016:3974139.
- Costa ML, Couto E, Furlan E, et al. Body stalk anomaly: adverse maternal outcomes in a series of 21 cases. Prenat Diagn. 2012;32:264-267.
- Hubbard R, Hayes S, Gillis H, et al. Management challenges in an infant with pentalogy of Cantrell, giant anterior encephalocele, and craniofacial anomalies: a case report. A A Pract. 2018;11:238-240.
- Jnah AJ, Newberry DM, England A. Pentalogy of Cantrell: case report with review of the literature. Adv Neonatal Care. 2015;15:261-268.
- Williams AP, Marayati R, Beierle EA. Pentalogy of Cantrell. Semin Pediatr Surg. 2019;28:106-110.
- Restrepo MS, Cerqua A, Turek JW. Pentalogy of Cantrell with ectopia cordis totalis, total anomalous pulmonary venous connection, and tetralogy of Fallot: a case report and review of the literature. Congenit Heart Dis. 2014;9:E129–E134.
- Zhang X, Xing Q, Sun J, et al. Surgical treatment and outcomes of pentalogy of Cantrell in eight patients. J Pediatr Surg. 2014;49:1335-1340.
- Harring G, Weil J, Thiel C, et al. Management of pentalogy of Cantrell with complete ectopia cordis and double outlet right ventricle. Congenit Anom (Kyoto). 2015;55:121- 123.
- Mallula KK, Sosnowski C, Awad S. Spectrum of Cantrell’s pentalogy: case series from a single tertiary care center and review of the literature. Pediatr Cardiol. 2013;34:1703- 1710.
- Allam ES, Shetty VS, Farmakis SG. Fetal and neonatal presentation of OEIS complex. J Pediatr Surg. 2015;50:2155-2158.
- Neel N, Tarabay MS. Omphalocele, exstrophy of cloaca, imperforate anus, and spinal defect complex, multiple major reconstructive surgeries needed. Urol Ann. 2018;10:118-121.
- Sawaya D, Gearhart JP. Gastrointestinal reconstruction and outcomes for patients with the OEIS complex. Semin Pediatr Surg. 2011;20:123-125.

CASE Fetal anomalies detected on ultrasonography
A 34-year-old woman (G2P1) at 19 weeks’ gestation presented for fetal anatomy ultrasonography evaluation. Ultrasonography demonstrated fetal demise with fetal size less than dates, oligohydramnios, and what appeared to be a full-thickness herniation of the thoracic and abdominal contents. Due to the positioning of the fetus and the oligohydramnios, the fetus appeared to have ectopia cordis and herniated liver and bowel; the bladder was not visualized. The patient was counseled regarding the findings and the suspected diagnosis of pentalogy of Cantrell. After counseling, the patient expressed desire to bury the fetus intact according to her religious custom. She underwent a successful uterine evacuation with misoprostol administration and delivered a nonviable fetus that had a closed thoracic cage without ectopia cordis. Key findings were a very short 2-vessel umbilical cord without coiling that was tethered to the intra-abdominal organs, “pulling” the internal organs out of the abdomen, and lack of an anterior abdominal wall (FIGURE 1). Given these findings, a final diagnosis of body-stalk anomaly was made.

Fetal abdominal wall defects (AWDs) encompass a wide array of congenital defects, although they all involve herniation of 1 or more intra-abdominal content through a ventral abdominal defect.1 Overall, the estimated incidence of AWDs is approximately 6 per 10,000 births.1 Gastroschisis and omphalocele are the most common of these defect types.2
The majority of AWDs can be diagnosed during the first trimester of pregnancy via ultrasonography; however, during the first trimester the physiologic midgut herniation resolves by 12 weeks of gestation. It is therefore important to repeat imaging at a later gestational age to confirm the suspicion. Furthermore, the differential diagnosis should include the relatively benign condition of umbilical hernia.
While many AWDs share similarities, they differ significantly in prognosis and management. Early detection is therefore crucial for fetal surveillance, prenatal testing, perinatal planning, and patient counseling (TABLE). In this article, we outline antenatal surveillance and management of AWDs based on recommendations from the American College of Obstetricians and Gynecologists and the Society for Maternal-Fetal Medicine as well as on our experience and practice.

Gastroschisis is an increasingly prevalent AWD
Gastroschisis is a full-thickness, ventral wall defect that results in bowel evisceration; it typically occurs to the right of the umbilical cord insertion.3 It is one of the most common AWDs and its prevalence has increased in the past few decades, from 2 to 3 cases per 10,000 live births in 1995 to as high as 6 cases per 10,000 live births in 2011.2,4,5
The cause of gastroschisis remains unclear. The main theory is that there is an ischemic disruption of the closure of the abdominal wall at or near the omphalomesenteric artery or the right umbilical vein.6,7 In addition, investigators have reported an increased incidence of gastroschisis in mothers exposed to cigarette smoking and certain medications, such as pseudoephedrine, salicylates, ibuprofen, and acetaminophen.8,9
Continue to: Making the diagnosis...
Making the diagnosis
Prenatal diagnosis using ultrasonography is possible at around 10 weeks of gestation. As previously mentioned, however, physiologic herniation of the midgut must be excluded by performing follow-up imaging at a later gestational age. In our practice, we typically do this at around 16 weeks of gestation.
Ultrasonographic features of gastroschisis include loops of bowel herniating through a small paraumbilical wall defect (usually 2–3 cm) floating in amniotic fluid without a covering membrane4 (FIGURE 2). Direct exposure to amniotic fluid causes small bowel inflammation and fibrin deposition, leading to a thickened, echogenic appearance. Polyhydramnios and intra-abdominal bowel dilation have been associated with the presence of intestinal atresia.10

Management
There is no expert consensus regarding optimal prenatal management of gastroschisis.11-17 Prenatal care, patient counseling, and delivery planning should be individualized based on the defect and should be determined in a multidisciplinary discussion with specialists in maternal-fetal medicine, neonatology, and pediatric surgery, as necessary. In our practice, if the gastroschisis is isolated and uncomplicated, our generalist obstetricians manage the patient with maternal-fetal medicine consultation, increased fetal surveillance as described below, and delivery at our tertiary care institution.
Our standard practice is to use the initial ultrasonography imaging to evaluate the size and contents of the defect, measure the nuchal translucency, and evaluate for additional abnormalities. Serial ultrasonography monitoring of the fetus is required to assess the size and quality of the herniated intestine, amount of amniotic fluid, and fetal growth.10
As gastroschisis is a full-thickness defect of the anterior abdominal wall, the abdominal contents are exposed to amniotic fluid. This exposure causes progressive intestinal damage, which can be identified on ultrasonography as bowel thickening and dilation.12-14 Currently, intestinal thickening and dilation is not considered an indication for delivery as it is assumed that the intestinal damage has already occurred. It is debatable whether delivery around 37 weeks compared with delayed delivery beyond 37 weeks improves outcomes and decreases the stillbirth rate.11,13 Studies show that neonates delivered prior to 37 weeks have worse outcomes compared with those delivered after 37 weeks.14,15
Fetal surveillance. As standard practice, we evaluate the fetus at around 16 weeks and then again at around 20 weeks. In the absence of fetal growth restriction, which is associated with 25% of cases,16,17 our standard practice includes performing serial growth ultrasonography every 3 to 4 weeks starting at 28 weeks and biophysical profiles and nonstress testing weekly starting at 32 weeks. Fetal echocardiography can be offered. However, unlike with omphalocele, which has a high incidence of associated cardiac structural anomalies, gastroschisis has a low incidence of congenital cardiac anomalies, estimated to be between 2.5% and 4%.18,19
Delivery considerations. Little agreement exists regarding when and how to deliver pregnancies complicated by fetal gastroschisis. While some advocate for induction of labor at 36 to 38 weeks, most infants with gastroschisis can be delivered safely at term via either vaginal or cesarean delivery.14,15
Delivery timing should consider the clinical picture and incorporate performance on antenatal testing, fetal growth, the size and contents of the gastroschisis, and consultation with maternal-fetal medicine. Fetuses with gastroschisis often have non-reassuring antenatal testing. This can necessitate early delivery, although cesarean delivery should be reserved for obstetric indications, with the caveat that if there is large liver involvement, some pediatric surgeons recommend cesarean delivery due to the risk of hepatic rupture.
Neonate management. The survival rate of gastroschisis is reported to be as high as 91% to 94%.2 Morbidity is related to intestinal complications, such as strictures, adhesions, and volvulus.
In the case of simple gastroschisis, when the bowel is in good condition, the treatment method of choice is primary reduction.20 If performed in the operating room, an immediate sutured closure of the defect can be done. The benefits of primary repair include decreased length of stay, fewer intensive care bed days, and less time to achieve full feeds.20,21 Primary reduction has a reported success rate of 50% to 83%.22 A reduction with a delayed spontaneous closure also can be performed at bedside in the neonatal intensive care unit.22
For complex gastroschisis, characterized by bowel complications such as inflammation, perforation, ischemia, atresia, necrosis, or volvulus, primary closure may not be possible and reduction may need to be achieved through silo application.22-25 Additionally, further bowel surgery, such as stoma formation and bowel resection, may be required.25
Continue to: Omphalocele often is associated with abnormal karyotype...
Omphalocele often is associated with abnormal karyotype
Also known as exomphalos, omphalocele is a relatively common defect, with an estimated prevalence of 2 to 3 cases per 10,000 live births.2 In this condition, there is a midline defect in which intra-abdominal contents herniate through the base of the umbilical cord. Omphaloceles are covered by amniotic membranes, making them distinguishable from gastroschisis, which has no covering, and congenital umbilical hernias, which are covered by intact skin and subcutaneous tissue.26-33
Additionally, in omphalocele the umbilical cord insertion site varies, whereas in gastroschisis the umbilical cord insertion is usually to the right of midline. An omphalocele is often categorized based on whether or not it contains the liver (extracorporeal liver) or only the bowel (intracorporeal liver).
Genetic studies
Approximately 67% to 88% of all pregnancies with omphalocele have an abnormal karyotype and/or associated malformations, including Beckwith-Wiedemann syndrome.31 Of the aneuploidies, trisomy 18 is the one most commonly associated with omphalocele, accounting for approximately 62% to 75%, while trisomy 13 accounts for approximately 11% to 24%.32,33 The presence of other anomalies is strongly associated with poor prognosis, and increased defect size is an independent predictor of neonatal morbidity and mortality, as neonates with large omphaloceles with extracorporeal livers can develop respiratory insufficiency and require more complex surgical repairs. It is interesting, however, that the absence of an extracorporeal liver is associated with a higher risk of aneuploidy than are cases with an intracorporeal liver.33
We offer chorionic villus sampling or amniocentesis to all patients with omphalocele. If the patient undergoes invasive diagnostic testing, the sample then undergoes karyotyping, chromosomal microarray, and testing for Beckwith-Wiedemann syndrome. If the patient declines diagnostic sampling, we perform a cell-free DNA screening to rule out aneuploidy.
Continue to: Making the diagnosis...
Making the diagnosis
Omphaloceles can be diagnosed via prenatal ultrasonography as early as 11 to 14 weeks’ gestation.26 They are classified based on size, location, and contents of the sac.26,27 A small omphalocele is defined as a defect less than 5 cm with a sac that may contain a few loops of intestines (FIGURE 3).27 A giant omphalocele is a defect with more than 75% of the liver contained in the sac.29

Location can be epigastric, umbilical, or hypogastric, and both small and giant omphaloceles may have ruptured membranes that will result in exposure of the contained viscera.27 Omphaloceles are associated with such structural anomalies as cardiac, gastrointestinal, genitourinary, diaphragmatic, and neural tube defects. We do not routinely perform magnetic resonance imaging (MRI) for evaluation of omphaloceles, but MRI may be used to help predict postnatal outcomes in the case of giant omphaloceles.26
Management
Our standard practice is to use the initial ultrasonography imaging to evaluate the size and contents of defect, measure the nuchal translucency, and evaluate for additional abnormalities. As in cases of gastroschisis, serial ultrasonography monitoring of the fetus is required to assess the size and quality of the herniated intestine, amount of amniotic fluid, and fetal growth. We typically evaluate the fetus at around 16 weeks and then again at around 20 weeks. In the absence of fetal growth restriction, we recommend serial growth ultrasonography every 3 to 4 weeks starting at 28 weeks and biophysical profiles and nonstress testing weekly starting at 32 weeks. Additionally, we routinely obtain a fetal echocardiogram to rule out cardiac structural abnormalities.
Delivery considerations. Fetuses that do not undergo spontaneous abortion or medical termination of pregnancy often are born at term.26 We recommend expectant management until spontaneous labor, another indication for delivery arises, or at least 39 weeks’ estimated gestational age. There are no evidence-based guidelines for the optimal mode of delivery in fetuses with omphalocele, although we recommend cesarean delivery for fetuses with large defects to avoid postnatal sac rupture and liver damage. Preterm induction of labor is not indicated as infants born preterm have about a 50% mortality rate.26,27
Children born with isolated omphalocele typically have a good prognosis, with an estimated survival rate of 50% to 90%.32,33 However, compared to gastroschisis, omphaloceles are often associated with other anomalies.32,33
Management of omphaloceles depends on the size of the defect. In our institution, our generalist obstetricians manage the standard prenatal care with the addition of increased fetal surveillance and testing, interdisciplinary patient counseling with maternal-fetal medicine, pediatric surgeons, and neonatologists for delivery planning, and delivery is performed at our tertiary care center.
Neonate management. Small omphaloceles are amenable to primary early fascial closure.26-30 However, attempted primary closure of giant omphaloceles carries significant risks, including abdominal compartment syndrome and postoperative herniation.29,30 Instead, several options exist for staged surgical closure, in which there are multiple operations prior to final fascial closure, as well as nonoperative delayed closure for management of giant omphaloceles.29,30
Conservative management of giant omphaloceles has certain benefits, such as earlier first feeds, decreased risk of abdominal compartment syndrome, and lower risk of infection.30 Ruptured omphaloceles can be repaired through primary repair, employment of a synthetic or biologic mesh fascial bridge, or silo placement with delayed closure.28
Body-stalk anomaly: Multiple defects and poor prognosis
Also known as limb body wall complex, body-stalk anomaly is a rare malformation that has a reported prevalence of approximately 0.12 cases per 10,000 births (both live and stillbirths).34 Body-stalk anomaly is characterized by multiple defects, including severe kyphosis or scoliosis, a short or absent umbilical cord, and a large anterior abdominal wall defect.34-36 This malformation is almost entirely incompatible with life, resulting in abortion or stillbirth.35 Survival is extremely rare and limited to case reports.
While the exact etiology of body-stalk anomaly is unknown, 3 possible causes have been hypothesized: early amnion rupture, vascular compromise, and embryonic dysgenesis.37-40
Continue to: Making the diagnosis...
Making the diagnosis
Body-stalk anomaly typically can be diagnosed by 10 to 14 weeks’ gestation via ultrasonography.34-41 We currently follow the diagnostic criteria proposed by Van Allen and colleagues, which requires 2 of the following 3 anomalies34:
- exencephaly/encephalocele with facial clefts
- thoraco- and/or abdominoschisis (midline defect)
- limb defect.
Additional ultrasonographic findings can include the identification of evisceration of the abdominal contents, a short umbilical cord, and increased nuchal thickness.36,42 During the second and third trimesters, oligohydramnios may be seen.2
Management
Body-stalk anomaly is considered a fatal condition without specific therapeutic interventions. Maternal risks include an increased risk of preterm labor and gestational hypertension.35 Research on body-stalk anomaly has not shown any correlation with patients’ age, fetal sex, or abnormal karyotype, and the reported risk of recurrence for this anomaly is very low.42,43 Early diagnosis therefore is essential to provide families with information and counseling. Given the poor fetal prognosis, increased maternal risk, and low recurrence rates, mothers can be advised toward elective termination of pregnancy.
Should a patient desire expectant management, care can be provided by generalist obstetricians or care can be transferred to maternal-fetal medicine, with the addition of increased fetal surveillance and testing, interdisciplinary patient counseling with maternal-fetal medicine, pediatric surgeons, and neonatologists for delivery planning; delivery should be performed at a tertiary care center.
Pentalogy of Cantrell: Very rare, with variable prognosis
Pentalogy of Cantrell is characterized by a collection of defects in the midline abdominal wall, lower sternum, anterior diaphragm, diaphragmatic pericardium, and some manifestation of intra-cardiac defect.44 It is thought to arise early in gestation due to abnormal differentiation, migration, and fusion of the embryonic mesoderm.44 The condition is rare, with an incidence of about 1 in 5.5 million live births.45
Making the diagnosis
The diagnosis of pentalogy of Cantrell can be made via prenatal ultrasonography as early as the first trimester, although it is diagnosed more commonly in the second trimester.46 Three-dimensional ultrasonography and fetal MRI have been used to confirm the diagnosis.47
Management
Typically, corrective operations are performed during the neonatal period, and cases of successful staged and one-stage operations have been reported.48 Surgical treatment is determined based on the complexity of the condition and the presence of coexistent heart defects.49,50 However, very few patients survive surgical repair; mortality rates are estimated at around 50% to 60%, with high postsurgical morbidity risks for those who do survive.45
Prognosis varies depending on the type and severity of the associated malformations and intracardiac anomalies.46 Patients with partial ectopia cordis and incomplete presentation may have more favorable outcomes, but for patients with severe ectopia cordis, the survival rate is only 5% to 10%.47
Depending on the severity of the defects, mothers can be advised toward elective termination of pregnancy. In our institution, prenatal care usually is transferred to the maternal-fetal medicine service, and delivery is planned at our tertiary care institution.
OEIS complex comprises abdominal, pelvic, and spinal defects
Omphalocele-exstrophy-imperforate anus-spinal defects (OEIS) complex is a congenital malformation syndrome characterized by the combination of midline abdominal and pelvic defects (including omphalocele, exstrophy of the cloaca, and imperforate anus) and spinal defects.51 The condition’s etiology is unknown but is thought to be multifactorial.51-53 It is a rare condition, with an incidence of around 1 in 200,000 to 400,000 pregnancies.52
Making the diagnosis
Prenatal diagnosis of OEIS complex can be made as early as the first trimester via ultrasonographic identification of an infraumbilical abdominal wall defect with protruding mass, absent bladder, and spinal defects.52 When OEIS complex is suspected, fetal MRI can play a critical role in the diagnosis.
Management
As OEIS complex is rare, there are no evidence-based guidelines for optimal mode and timing of delivery. Cases are individualized based on their specific pathology, and we recommend cesarean delivery for fetuses with large defects to avoid postnatal sac rupture and liver damage.
The prognosis for infants with OEIS complex depends on the spectrum and severity of the structural defects.52,53 The many surgeries involved in the repair of OEIS have potential complications, such as urogenital and gastrointestinal dysfunction.52,53 Advances in medical and surgical treatment have resulted in improved survival and quality of life, and survival rates for OEIS complex are now close to 100%.53 While many OEIS patients live with a permanent colostomy, improvements in management mean that more patients are now candidates for gastrointestinal pull-through procedures, which allow for natural bowel control and a higher degree of bowel cleanliness.53
Prenatal care, patient counseling, and delivery planning should be individualized based on the defects present and determined in a multidisciplinary discussion with maternal-fetal medicine, neonatology, and pediatric surgery as necessary. In our institution, prenatal care usually is transferred to the maternal-fetal medicine service, and delivery is planned at our tertiary care institution.
Multidisciplinary team strategy is essential
Based on our experience, when faced with an anterior AWD in utero, prenatal imaging, genetic testing, increased fetal surveillance, and a multidisciplinary team approach improves outcomes. We must emphasize that careful patient counseling is paramount in our practice. ●
Acknowledgement: The authors would like to thank Ashley Tran, BS, for her assistance in the literature review and drafting of this article.
- Patients with fetuses with anterior wall defects should be referred to a maternal-fetal medicine specialist for co-management and advanced fetal imaging.
- The American College of Obstetricians and Gynecologists recommends microarray for all major fetal structural abnormalities, with the qualifier that karyotype can be offered if a specific aneuploidy is suspected based on the abnormality or prior genetic screening tests.
- If confirmatory testing is performed (amniocentesis or chorionic villus sampling), the sample should undergo karyotyping, chromosomal microarray, and if indicated, testing for Beckwith-Wiedemann syndrome. If the patient declines confirmatory sampling, performing cell-free DNA screening to rule out aneuploidy is recommended.
- Fetal echocardiography is recommended.
- Fetal magnetic resonance imaging should be considered in complex cases.
- Management should be individualized based on the type and severity of defect(s).
- Delivery timing and method should be individualized based on the defect(s) and determined in a multidisciplinary discussion with maternal-fetal medicine, neonatology, pediatric surgery, and pediatric cardiology, as necessary.
- The most common fetal abdominal wall defect is omphalocele, followed by gastroschisis.
- Maternal serum α-fetoprotein is usually elevated in all of the disorders.

CASE Fetal anomalies detected on ultrasonography
A 34-year-old woman (G2P1) at 19 weeks’ gestation presented for fetal anatomy ultrasonography evaluation. Ultrasonography demonstrated fetal demise with fetal size less than dates, oligohydramnios, and what appeared to be a full-thickness herniation of the thoracic and abdominal contents. Due to the positioning of the fetus and the oligohydramnios, the fetus appeared to have ectopia cordis and herniated liver and bowel; the bladder was not visualized. The patient was counseled regarding the findings and the suspected diagnosis of pentalogy of Cantrell. After counseling, the patient expressed desire to bury the fetus intact according to her religious custom. She underwent a successful uterine evacuation with misoprostol administration and delivered a nonviable fetus that had a closed thoracic cage without ectopia cordis. Key findings were a very short 2-vessel umbilical cord without coiling that was tethered to the intra-abdominal organs, “pulling” the internal organs out of the abdomen, and lack of an anterior abdominal wall (FIGURE 1). Given these findings, a final diagnosis of body-stalk anomaly was made.

Fetal abdominal wall defects (AWDs) encompass a wide array of congenital defects, although they all involve herniation of 1 or more intra-abdominal content through a ventral abdominal defect.1 Overall, the estimated incidence of AWDs is approximately 6 per 10,000 births.1 Gastroschisis and omphalocele are the most common of these defect types.2
The majority of AWDs can be diagnosed during the first trimester of pregnancy via ultrasonography; however, during the first trimester the physiologic midgut herniation resolves by 12 weeks of gestation. It is therefore important to repeat imaging at a later gestational age to confirm the suspicion. Furthermore, the differential diagnosis should include the relatively benign condition of umbilical hernia.
While many AWDs share similarities, they differ significantly in prognosis and management. Early detection is therefore crucial for fetal surveillance, prenatal testing, perinatal planning, and patient counseling (TABLE). In this article, we outline antenatal surveillance and management of AWDs based on recommendations from the American College of Obstetricians and Gynecologists and the Society for Maternal-Fetal Medicine as well as on our experience and practice.

Gastroschisis is an increasingly prevalent AWD
Gastroschisis is a full-thickness, ventral wall defect that results in bowel evisceration; it typically occurs to the right of the umbilical cord insertion.3 It is one of the most common AWDs and its prevalence has increased in the past few decades, from 2 to 3 cases per 10,000 live births in 1995 to as high as 6 cases per 10,000 live births in 2011.2,4,5
The cause of gastroschisis remains unclear. The main theory is that there is an ischemic disruption of the closure of the abdominal wall at or near the omphalomesenteric artery or the right umbilical vein.6,7 In addition, investigators have reported an increased incidence of gastroschisis in mothers exposed to cigarette smoking and certain medications, such as pseudoephedrine, salicylates, ibuprofen, and acetaminophen.8,9
Continue to: Making the diagnosis...
Making the diagnosis
Prenatal diagnosis using ultrasonography is possible at around 10 weeks of gestation. As previously mentioned, however, physiologic herniation of the midgut must be excluded by performing follow-up imaging at a later gestational age. In our practice, we typically do this at around 16 weeks of gestation.
Ultrasonographic features of gastroschisis include loops of bowel herniating through a small paraumbilical wall defect (usually 2–3 cm) floating in amniotic fluid without a covering membrane4 (FIGURE 2). Direct exposure to amniotic fluid causes small bowel inflammation and fibrin deposition, leading to a thickened, echogenic appearance. Polyhydramnios and intra-abdominal bowel dilation have been associated with the presence of intestinal atresia.10

Management
There is no expert consensus regarding optimal prenatal management of gastroschisis.11-17 Prenatal care, patient counseling, and delivery planning should be individualized based on the defect and should be determined in a multidisciplinary discussion with specialists in maternal-fetal medicine, neonatology, and pediatric surgery, as necessary. In our practice, if the gastroschisis is isolated and uncomplicated, our generalist obstetricians manage the patient with maternal-fetal medicine consultation, increased fetal surveillance as described below, and delivery at our tertiary care institution.
Our standard practice is to use the initial ultrasonography imaging to evaluate the size and contents of the defect, measure the nuchal translucency, and evaluate for additional abnormalities. Serial ultrasonography monitoring of the fetus is required to assess the size and quality of the herniated intestine, amount of amniotic fluid, and fetal growth.10
As gastroschisis is a full-thickness defect of the anterior abdominal wall, the abdominal contents are exposed to amniotic fluid. This exposure causes progressive intestinal damage, which can be identified on ultrasonography as bowel thickening and dilation.12-14 Currently, intestinal thickening and dilation is not considered an indication for delivery as it is assumed that the intestinal damage has already occurred. It is debatable whether delivery around 37 weeks compared with delayed delivery beyond 37 weeks improves outcomes and decreases the stillbirth rate.11,13 Studies show that neonates delivered prior to 37 weeks have worse outcomes compared with those delivered after 37 weeks.14,15
Fetal surveillance. As standard practice, we evaluate the fetus at around 16 weeks and then again at around 20 weeks. In the absence of fetal growth restriction, which is associated with 25% of cases,16,17 our standard practice includes performing serial growth ultrasonography every 3 to 4 weeks starting at 28 weeks and biophysical profiles and nonstress testing weekly starting at 32 weeks. Fetal echocardiography can be offered. However, unlike with omphalocele, which has a high incidence of associated cardiac structural anomalies, gastroschisis has a low incidence of congenital cardiac anomalies, estimated to be between 2.5% and 4%.18,19
Delivery considerations. Little agreement exists regarding when and how to deliver pregnancies complicated by fetal gastroschisis. While some advocate for induction of labor at 36 to 38 weeks, most infants with gastroschisis can be delivered safely at term via either vaginal or cesarean delivery.14,15
Delivery timing should consider the clinical picture and incorporate performance on antenatal testing, fetal growth, the size and contents of the gastroschisis, and consultation with maternal-fetal medicine. Fetuses with gastroschisis often have non-reassuring antenatal testing. This can necessitate early delivery, although cesarean delivery should be reserved for obstetric indications, with the caveat that if there is large liver involvement, some pediatric surgeons recommend cesarean delivery due to the risk of hepatic rupture.
Neonate management. The survival rate of gastroschisis is reported to be as high as 91% to 94%.2 Morbidity is related to intestinal complications, such as strictures, adhesions, and volvulus.
In the case of simple gastroschisis, when the bowel is in good condition, the treatment method of choice is primary reduction.20 If performed in the operating room, an immediate sutured closure of the defect can be done. The benefits of primary repair include decreased length of stay, fewer intensive care bed days, and less time to achieve full feeds.20,21 Primary reduction has a reported success rate of 50% to 83%.22 A reduction with a delayed spontaneous closure also can be performed at bedside in the neonatal intensive care unit.22
For complex gastroschisis, characterized by bowel complications such as inflammation, perforation, ischemia, atresia, necrosis, or volvulus, primary closure may not be possible and reduction may need to be achieved through silo application.22-25 Additionally, further bowel surgery, such as stoma formation and bowel resection, may be required.25
Continue to: Omphalocele often is associated with abnormal karyotype...
Omphalocele often is associated with abnormal karyotype
Also known as exomphalos, omphalocele is a relatively common defect, with an estimated prevalence of 2 to 3 cases per 10,000 live births.2 In this condition, there is a midline defect in which intra-abdominal contents herniate through the base of the umbilical cord. Omphaloceles are covered by amniotic membranes, making them distinguishable from gastroschisis, which has no covering, and congenital umbilical hernias, which are covered by intact skin and subcutaneous tissue.26-33
Additionally, in omphalocele the umbilical cord insertion site varies, whereas in gastroschisis the umbilical cord insertion is usually to the right of midline. An omphalocele is often categorized based on whether or not it contains the liver (extracorporeal liver) or only the bowel (intracorporeal liver).
Genetic studies
Approximately 67% to 88% of all pregnancies with omphalocele have an abnormal karyotype and/or associated malformations, including Beckwith-Wiedemann syndrome.31 Of the aneuploidies, trisomy 18 is the one most commonly associated with omphalocele, accounting for approximately 62% to 75%, while trisomy 13 accounts for approximately 11% to 24%.32,33 The presence of other anomalies is strongly associated with poor prognosis, and increased defect size is an independent predictor of neonatal morbidity and mortality, as neonates with large omphaloceles with extracorporeal livers can develop respiratory insufficiency and require more complex surgical repairs. It is interesting, however, that the absence of an extracorporeal liver is associated with a higher risk of aneuploidy than are cases with an intracorporeal liver.33
We offer chorionic villus sampling or amniocentesis to all patients with omphalocele. If the patient undergoes invasive diagnostic testing, the sample then undergoes karyotyping, chromosomal microarray, and testing for Beckwith-Wiedemann syndrome. If the patient declines diagnostic sampling, we perform a cell-free DNA screening to rule out aneuploidy.
Continue to: Making the diagnosis...
Making the diagnosis
Omphaloceles can be diagnosed via prenatal ultrasonography as early as 11 to 14 weeks’ gestation.26 They are classified based on size, location, and contents of the sac.26,27 A small omphalocele is defined as a defect less than 5 cm with a sac that may contain a few loops of intestines (FIGURE 3).27 A giant omphalocele is a defect with more than 75% of the liver contained in the sac.29

Location can be epigastric, umbilical, or hypogastric, and both small and giant omphaloceles may have ruptured membranes that will result in exposure of the contained viscera.27 Omphaloceles are associated with such structural anomalies as cardiac, gastrointestinal, genitourinary, diaphragmatic, and neural tube defects. We do not routinely perform magnetic resonance imaging (MRI) for evaluation of omphaloceles, but MRI may be used to help predict postnatal outcomes in the case of giant omphaloceles.26
Management
Our standard practice is to use the initial ultrasonography imaging to evaluate the size and contents of defect, measure the nuchal translucency, and evaluate for additional abnormalities. As in cases of gastroschisis, serial ultrasonography monitoring of the fetus is required to assess the size and quality of the herniated intestine, amount of amniotic fluid, and fetal growth. We typically evaluate the fetus at around 16 weeks and then again at around 20 weeks. In the absence of fetal growth restriction, we recommend serial growth ultrasonography every 3 to 4 weeks starting at 28 weeks and biophysical profiles and nonstress testing weekly starting at 32 weeks. Additionally, we routinely obtain a fetal echocardiogram to rule out cardiac structural abnormalities.
Delivery considerations. Fetuses that do not undergo spontaneous abortion or medical termination of pregnancy often are born at term.26 We recommend expectant management until spontaneous labor, another indication for delivery arises, or at least 39 weeks’ estimated gestational age. There are no evidence-based guidelines for the optimal mode of delivery in fetuses with omphalocele, although we recommend cesarean delivery for fetuses with large defects to avoid postnatal sac rupture and liver damage. Preterm induction of labor is not indicated as infants born preterm have about a 50% mortality rate.26,27
Children born with isolated omphalocele typically have a good prognosis, with an estimated survival rate of 50% to 90%.32,33 However, compared to gastroschisis, omphaloceles are often associated with other anomalies.32,33
Management of omphaloceles depends on the size of the defect. In our institution, our generalist obstetricians manage the standard prenatal care with the addition of increased fetal surveillance and testing, interdisciplinary patient counseling with maternal-fetal medicine, pediatric surgeons, and neonatologists for delivery planning, and delivery is performed at our tertiary care center.
Neonate management. Small omphaloceles are amenable to primary early fascial closure.26-30 However, attempted primary closure of giant omphaloceles carries significant risks, including abdominal compartment syndrome and postoperative herniation.29,30 Instead, several options exist for staged surgical closure, in which there are multiple operations prior to final fascial closure, as well as nonoperative delayed closure for management of giant omphaloceles.29,30
Conservative management of giant omphaloceles has certain benefits, such as earlier first feeds, decreased risk of abdominal compartment syndrome, and lower risk of infection.30 Ruptured omphaloceles can be repaired through primary repair, employment of a synthetic or biologic mesh fascial bridge, or silo placement with delayed closure.28
Body-stalk anomaly: Multiple defects and poor prognosis
Also known as limb body wall complex, body-stalk anomaly is a rare malformation that has a reported prevalence of approximately 0.12 cases per 10,000 births (both live and stillbirths).34 Body-stalk anomaly is characterized by multiple defects, including severe kyphosis or scoliosis, a short or absent umbilical cord, and a large anterior abdominal wall defect.34-36 This malformation is almost entirely incompatible with life, resulting in abortion or stillbirth.35 Survival is extremely rare and limited to case reports.
While the exact etiology of body-stalk anomaly is unknown, 3 possible causes have been hypothesized: early amnion rupture, vascular compromise, and embryonic dysgenesis.37-40
Continue to: Making the diagnosis...
Making the diagnosis
Body-stalk anomaly typically can be diagnosed by 10 to 14 weeks’ gestation via ultrasonography.34-41 We currently follow the diagnostic criteria proposed by Van Allen and colleagues, which requires 2 of the following 3 anomalies34:
- exencephaly/encephalocele with facial clefts
- thoraco- and/or abdominoschisis (midline defect)
- limb defect.
Additional ultrasonographic findings can include the identification of evisceration of the abdominal contents, a short umbilical cord, and increased nuchal thickness.36,42 During the second and third trimesters, oligohydramnios may be seen.2
Management
Body-stalk anomaly is considered a fatal condition without specific therapeutic interventions. Maternal risks include an increased risk of preterm labor and gestational hypertension.35 Research on body-stalk anomaly has not shown any correlation with patients’ age, fetal sex, or abnormal karyotype, and the reported risk of recurrence for this anomaly is very low.42,43 Early diagnosis therefore is essential to provide families with information and counseling. Given the poor fetal prognosis, increased maternal risk, and low recurrence rates, mothers can be advised toward elective termination of pregnancy.
Should a patient desire expectant management, care can be provided by generalist obstetricians or care can be transferred to maternal-fetal medicine, with the addition of increased fetal surveillance and testing, interdisciplinary patient counseling with maternal-fetal medicine, pediatric surgeons, and neonatologists for delivery planning; delivery should be performed at a tertiary care center.
Pentalogy of Cantrell: Very rare, with variable prognosis
Pentalogy of Cantrell is characterized by a collection of defects in the midline abdominal wall, lower sternum, anterior diaphragm, diaphragmatic pericardium, and some manifestation of intra-cardiac defect.44 It is thought to arise early in gestation due to abnormal differentiation, migration, and fusion of the embryonic mesoderm.44 The condition is rare, with an incidence of about 1 in 5.5 million live births.45
Making the diagnosis
The diagnosis of pentalogy of Cantrell can be made via prenatal ultrasonography as early as the first trimester, although it is diagnosed more commonly in the second trimester.46 Three-dimensional ultrasonography and fetal MRI have been used to confirm the diagnosis.47
Management
Typically, corrective operations are performed during the neonatal period, and cases of successful staged and one-stage operations have been reported.48 Surgical treatment is determined based on the complexity of the condition and the presence of coexistent heart defects.49,50 However, very few patients survive surgical repair; mortality rates are estimated at around 50% to 60%, with high postsurgical morbidity risks for those who do survive.45
Prognosis varies depending on the type and severity of the associated malformations and intracardiac anomalies.46 Patients with partial ectopia cordis and incomplete presentation may have more favorable outcomes, but for patients with severe ectopia cordis, the survival rate is only 5% to 10%.47
Depending on the severity of the defects, mothers can be advised toward elective termination of pregnancy. In our institution, prenatal care usually is transferred to the maternal-fetal medicine service, and delivery is planned at our tertiary care institution.
OEIS complex comprises abdominal, pelvic, and spinal defects
Omphalocele-exstrophy-imperforate anus-spinal defects (OEIS) complex is a congenital malformation syndrome characterized by the combination of midline abdominal and pelvic defects (including omphalocele, exstrophy of the cloaca, and imperforate anus) and spinal defects.51 The condition’s etiology is unknown but is thought to be multifactorial.51-53 It is a rare condition, with an incidence of around 1 in 200,000 to 400,000 pregnancies.52
Making the diagnosis
Prenatal diagnosis of OEIS complex can be made as early as the first trimester via ultrasonographic identification of an infraumbilical abdominal wall defect with protruding mass, absent bladder, and spinal defects.52 When OEIS complex is suspected, fetal MRI can play a critical role in the diagnosis.
Management
As OEIS complex is rare, there are no evidence-based guidelines for optimal mode and timing of delivery. Cases are individualized based on their specific pathology, and we recommend cesarean delivery for fetuses with large defects to avoid postnatal sac rupture and liver damage.
The prognosis for infants with OEIS complex depends on the spectrum and severity of the structural defects.52,53 The many surgeries involved in the repair of OEIS have potential complications, such as urogenital and gastrointestinal dysfunction.52,53 Advances in medical and surgical treatment have resulted in improved survival and quality of life, and survival rates for OEIS complex are now close to 100%.53 While many OEIS patients live with a permanent colostomy, improvements in management mean that more patients are now candidates for gastrointestinal pull-through procedures, which allow for natural bowel control and a higher degree of bowel cleanliness.53
Prenatal care, patient counseling, and delivery planning should be individualized based on the defects present and determined in a multidisciplinary discussion with maternal-fetal medicine, neonatology, and pediatric surgery as necessary. In our institution, prenatal care usually is transferred to the maternal-fetal medicine service, and delivery is planned at our tertiary care institution.
Multidisciplinary team strategy is essential
Based on our experience, when faced with an anterior AWD in utero, prenatal imaging, genetic testing, increased fetal surveillance, and a multidisciplinary team approach improves outcomes. We must emphasize that careful patient counseling is paramount in our practice. ●
Acknowledgement: The authors would like to thank Ashley Tran, BS, for her assistance in the literature review and drafting of this article.
- Patients with fetuses with anterior wall defects should be referred to a maternal-fetal medicine specialist for co-management and advanced fetal imaging.
- The American College of Obstetricians and Gynecologists recommends microarray for all major fetal structural abnormalities, with the qualifier that karyotype can be offered if a specific aneuploidy is suspected based on the abnormality or prior genetic screening tests.
- If confirmatory testing is performed (amniocentesis or chorionic villus sampling), the sample should undergo karyotyping, chromosomal microarray, and if indicated, testing for Beckwith-Wiedemann syndrome. If the patient declines confirmatory sampling, performing cell-free DNA screening to rule out aneuploidy is recommended.
- Fetal echocardiography is recommended.
- Fetal magnetic resonance imaging should be considered in complex cases.
- Management should be individualized based on the type and severity of defect(s).
- Delivery timing and method should be individualized based on the defect(s) and determined in a multidisciplinary discussion with maternal-fetal medicine, neonatology, pediatric surgery, and pediatric cardiology, as necessary.
- The most common fetal abdominal wall defect is omphalocele, followed by gastroschisis.
- Maternal serum α-fetoprotein is usually elevated in all of the disorders.
- Victoria T, Andronikou S, Bowen D, et al. Fetal anterior abdominal wall defects: prenatal imaging by magnetic resonance imaging. Pediatr Radiol. 2018;48:499-512.
- Pakdaman R, Woodward PJ, Kennedy A. Complex abdominal wall defects: appearances at prenatal imaging. Radiographics. 2015;35:636-649.
- Oakes MC, Porto M, Chung JH. Advances in prenatal and perinatal diagnosis and management of gastroschisis. Semin Pediatr Surg. 2018;27:289-299.
- Mastroiacovo P, Lisi A, Castilla EE. The incidence of gastroschisis: research urgently needs resources. BMJ. 2006;332:423-424.
- Boyd PA, Haeusler M, Barisic I. EUROCAT report 9: surveillance of congenital anomalies in Europe 1980-2008. Birth Defects Res A Clin Mol Teratol. 2011;91(suppl 1):S1.
- Gamba P, Midrio P. Abdominal wall defects: prenatal diagnosis, newborn management, and long-term outcomes. Semin Pediatr Surg. 2014;23:283-290.
- Beaudoin S. Insights into the etiology and embryology of gastroschisis. Semin Pediatr Surg. 2018;27:283-288.
- Yazdy MM, Mitchell AA, Werler MM. Maternal genitourinary infections and the risk of gastroschisis. Am J Epidemiol. 2014;180:518-525.
- Werler MM, Sheehan JE, Mitchell AA. Maternal medication use and risks of gastroschisis and small intestinal atresia. Am J Epidemiol. 2002;155:26-31.
- D’Antonio F, Virgone C, Rizzo G, et al. Prenatal risk factors and outcomes in gastroschisis: a meta-analysis. Pediatrics. 2015;136:e159-e169.
- Baud D, Lausman A, Alfaraj MA, et al. Expectant management compared with elective delivery at 37 weeks for gastroschisis. Obstet Gynecol. 2013;121:990-998.
- Goetzinger KR, Tuuli MG, Longman RE, et al. Sonographic predictors of postnatal bowel atresia in fetal gastroschisis. Ultrasound Obstet Gynecol. 2014;43:420-425.
- Overton TG, Pierce MR, Gao H, et al. Antenatal management and outcomes of gastroschisis in the UK. Prenat Diagn. 2012;32:1256-1262.
- Ergün O, Barksdale E, Ergün FS, et al. The timing of delivery of infants with gastroschisis influences outcome. J Pediatr Surg. 2005;40:424-428.
- Overcash RT, DeUgarte DA, Stephenson ML, et al; University of California Fetal Consortium. Factors associated with gastroschisis outcomes. Obstet Gynecol. 2014;124:551-557.
- Wissanji H, Puligandla PS. Risk stratification and outcome determinants in gastroschisis. Semin Pediatr Surg. 2018;27: 300-303.
- Raynor BD, Richards D. Growth retardation in fetuses with gastroschisis. J Ultrasound Med. 1997;16:13-16.
- Mastroiacovo P, Lisi A, Castilla EE, et al. Gastroschisis and associated defects: an international study. Am J Med Genet A. 2007;143A:660-671.
- Kunz LH, Gilbert WM, Towner DR. Increased incidence of cardiac anomalies in pregnancies complicated by gastroschisis. Am J Obstet Gynecol. 2005;193(3 pt 2): 1248-1252.
- Lakshminarayanan B, Lakhoo K. Abdominal wall defects. Early Hum Dev. 2014;90:917-920.
- Prefumo F, Izzi C. Fetal abdominal wall defects. Best Pract Res Clin Obstet Gynaecol. 2014;28:391-402.
- Petrosyan M, Sandler AD. Closure methods in gastroschisis. Semin Pediatr Surg. 2018;27:304-308.
- Skarsgard ED. Management of gastroschisis. Curr Opin Pediatr. 2016;28:363-369.
- Bergholz R, Boettcher M, Reinshagen K, et al. Complex gastroschisis is a different entity to simple gastroschisis affecting morbidity and mortality—a systematic review and meta-analysis. J Pediatr Surg. 2014;49:1527-1532.
- Emil S. Surgical strategies in complex gastroschisis. Semin Pediatr Surg. 2018;27:309-315.
- Verla MA, Style CC, Olutoye OO. Prenatal diagnosis and management of omphalocele. Semin Pediatr Surg. 2019;28:84-88.
- Gonzalez KW, Chandler NM. Ruptured omphalocele: diagnosis and management. Semin Pediatr Surg. 2019;28:101-105.
- Sugandhi N, Saha M, Bhatnagar V, et al. Repair of ruptured omphalocele sac in the neonatal period and beyond. J Indian Assoc Pediatr Surg. 2020;25:46-48.
- Bauman B, Stephens D, Gershone H, et al. Management of giant omphaloceles: a systematic review of methods of staged surgical vs nonoperative delayed closure. J Pediatr Surg. 2016;51:1725-1730.
- Kogut KA, Fiore NF. Nonoperative management of giant omphalocele leading to early fascial closure. J Pediatr Surg. 2018;53:2404-2408.
- Conner P, Vejde JH, Burgos CM. Accuracy and impact of prenatal diagnosis in infants with omphalocele. Pediatr Surg Int. 2018;34:629-633.
- Iacovella C, Contro E, Ghi T, et al. The effect of the contents of exomphalos and nuchal translucency at 11-14 weeks on the likelihood of associated chromosomal abnormality. Prenat Diagn. 2012;32:1066-1070.
- Getachew MM, Goldstein RB, Edge V, et al. Correlation between omphalocele contents and karyotypic abnormalities: sonographic study in 37 cases. AJR Am J Roentgenol. 1992;158:133-136.
- Singh A, Singh J, Gupta K. Body stalk anomaly: antenatal sonographic diagnosis of this rare entity with review of literature. J Ultrason. 2017;17:133-135.
- Lazaroni TL, Cruzeiro PC, Piçarro C, et al. Body stalk anomaly: Three months of survival. Case report and literature review. J Pediatr Surg Case Rep. 2016;14:22-25.
- Gajzer DC, Hirzel AC, Saigal G, et al. Possible genetic origin of limb-body wall complex. Fetal Pediatr Pathol. 2015;34: 257–270.
- Maruyama H, Inagaki T, Nakata Y, et al. Minimally conjoined omphalopagus twins with a body stalk anomaly. AJP Rep. 2015;5:e124-e128.
- Bhat A, Ilyas M, Dev G. Prenatal sonographic diagnosis of limb-body wall complex: case series of a rare congenital anomaly. Radiol Case Rep. 2016;11:116-120.
- Quijano FE, Rey MM, Echeverry M, et al. Body stalk anomaly in a 9-week pregnancy. Case Rep Obstet Gynecol. 2014;2014:357285.
- Kocherla K, Kumari V, Kocherla PR. Prenatal diagnosis of body stalk complex: a rare entity and review of literature. Indian J Radiol Imaging. 2015;25:67-70.
- Panaitescu AM, Ushakov F, Kalaskar A, et al. Ultrasound features and management of body stalk anomaly. Fetal Diagn Ther. 2016;40:285-290.
- Routhu M, Thakkallapelli S, Mohan P, et al. Role of ultrasound in body stalk anomaly and amniotic band syndrome. Int J Reprod Med. 2016;2016:3974139.
- Costa ML, Couto E, Furlan E, et al. Body stalk anomaly: adverse maternal outcomes in a series of 21 cases. Prenat Diagn. 2012;32:264-267.
- Hubbard R, Hayes S, Gillis H, et al. Management challenges in an infant with pentalogy of Cantrell, giant anterior encephalocele, and craniofacial anomalies: a case report. A A Pract. 2018;11:238-240.
- Jnah AJ, Newberry DM, England A. Pentalogy of Cantrell: case report with review of the literature. Adv Neonatal Care. 2015;15:261-268.
- Williams AP, Marayati R, Beierle EA. Pentalogy of Cantrell. Semin Pediatr Surg. 2019;28:106-110.
- Restrepo MS, Cerqua A, Turek JW. Pentalogy of Cantrell with ectopia cordis totalis, total anomalous pulmonary venous connection, and tetralogy of Fallot: a case report and review of the literature. Congenit Heart Dis. 2014;9:E129–E134.
- Zhang X, Xing Q, Sun J, et al. Surgical treatment and outcomes of pentalogy of Cantrell in eight patients. J Pediatr Surg. 2014;49:1335-1340.
- Harring G, Weil J, Thiel C, et al. Management of pentalogy of Cantrell with complete ectopia cordis and double outlet right ventricle. Congenit Anom (Kyoto). 2015;55:121- 123.
- Mallula KK, Sosnowski C, Awad S. Spectrum of Cantrell’s pentalogy: case series from a single tertiary care center and review of the literature. Pediatr Cardiol. 2013;34:1703- 1710.
- Allam ES, Shetty VS, Farmakis SG. Fetal and neonatal presentation of OEIS complex. J Pediatr Surg. 2015;50:2155-2158.
- Neel N, Tarabay MS. Omphalocele, exstrophy of cloaca, imperforate anus, and spinal defect complex, multiple major reconstructive surgeries needed. Urol Ann. 2018;10:118-121.
- Sawaya D, Gearhart JP. Gastrointestinal reconstruction and outcomes for patients with the OEIS complex. Semin Pediatr Surg. 2011;20:123-125.
- Victoria T, Andronikou S, Bowen D, et al. Fetal anterior abdominal wall defects: prenatal imaging by magnetic resonance imaging. Pediatr Radiol. 2018;48:499-512.
- Pakdaman R, Woodward PJ, Kennedy A. Complex abdominal wall defects: appearances at prenatal imaging. Radiographics. 2015;35:636-649.
- Oakes MC, Porto M, Chung JH. Advances in prenatal and perinatal diagnosis and management of gastroschisis. Semin Pediatr Surg. 2018;27:289-299.
- Mastroiacovo P, Lisi A, Castilla EE. The incidence of gastroschisis: research urgently needs resources. BMJ. 2006;332:423-424.
- Boyd PA, Haeusler M, Barisic I. EUROCAT report 9: surveillance of congenital anomalies in Europe 1980-2008. Birth Defects Res A Clin Mol Teratol. 2011;91(suppl 1):S1.
- Gamba P, Midrio P. Abdominal wall defects: prenatal diagnosis, newborn management, and long-term outcomes. Semin Pediatr Surg. 2014;23:283-290.
- Beaudoin S. Insights into the etiology and embryology of gastroschisis. Semin Pediatr Surg. 2018;27:283-288.
- Yazdy MM, Mitchell AA, Werler MM. Maternal genitourinary infections and the risk of gastroschisis. Am J Epidemiol. 2014;180:518-525.
- Werler MM, Sheehan JE, Mitchell AA. Maternal medication use and risks of gastroschisis and small intestinal atresia. Am J Epidemiol. 2002;155:26-31.
- D’Antonio F, Virgone C, Rizzo G, et al. Prenatal risk factors and outcomes in gastroschisis: a meta-analysis. Pediatrics. 2015;136:e159-e169.
- Baud D, Lausman A, Alfaraj MA, et al. Expectant management compared with elective delivery at 37 weeks for gastroschisis. Obstet Gynecol. 2013;121:990-998.
- Goetzinger KR, Tuuli MG, Longman RE, et al. Sonographic predictors of postnatal bowel atresia in fetal gastroschisis. Ultrasound Obstet Gynecol. 2014;43:420-425.
- Overton TG, Pierce MR, Gao H, et al. Antenatal management and outcomes of gastroschisis in the UK. Prenat Diagn. 2012;32:1256-1262.
- Ergün O, Barksdale E, Ergün FS, et al. The timing of delivery of infants with gastroschisis influences outcome. J Pediatr Surg. 2005;40:424-428.
- Overcash RT, DeUgarte DA, Stephenson ML, et al; University of California Fetal Consortium. Factors associated with gastroschisis outcomes. Obstet Gynecol. 2014;124:551-557.
- Wissanji H, Puligandla PS. Risk stratification and outcome determinants in gastroschisis. Semin Pediatr Surg. 2018;27: 300-303.
- Raynor BD, Richards D. Growth retardation in fetuses with gastroschisis. J Ultrasound Med. 1997;16:13-16.
- Mastroiacovo P, Lisi A, Castilla EE, et al. Gastroschisis and associated defects: an international study. Am J Med Genet A. 2007;143A:660-671.
- Kunz LH, Gilbert WM, Towner DR. Increased incidence of cardiac anomalies in pregnancies complicated by gastroschisis. Am J Obstet Gynecol. 2005;193(3 pt 2): 1248-1252.
- Lakshminarayanan B, Lakhoo K. Abdominal wall defects. Early Hum Dev. 2014;90:917-920.
- Prefumo F, Izzi C. Fetal abdominal wall defects. Best Pract Res Clin Obstet Gynaecol. 2014;28:391-402.
- Petrosyan M, Sandler AD. Closure methods in gastroschisis. Semin Pediatr Surg. 2018;27:304-308.
- Skarsgard ED. Management of gastroschisis. Curr Opin Pediatr. 2016;28:363-369.
- Bergholz R, Boettcher M, Reinshagen K, et al. Complex gastroschisis is a different entity to simple gastroschisis affecting morbidity and mortality—a systematic review and meta-analysis. J Pediatr Surg. 2014;49:1527-1532.
- Emil S. Surgical strategies in complex gastroschisis. Semin Pediatr Surg. 2018;27:309-315.
- Verla MA, Style CC, Olutoye OO. Prenatal diagnosis and management of omphalocele. Semin Pediatr Surg. 2019;28:84-88.
- Gonzalez KW, Chandler NM. Ruptured omphalocele: diagnosis and management. Semin Pediatr Surg. 2019;28:101-105.
- Sugandhi N, Saha M, Bhatnagar V, et al. Repair of ruptured omphalocele sac in the neonatal period and beyond. J Indian Assoc Pediatr Surg. 2020;25:46-48.
- Bauman B, Stephens D, Gershone H, et al. Management of giant omphaloceles: a systematic review of methods of staged surgical vs nonoperative delayed closure. J Pediatr Surg. 2016;51:1725-1730.
- Kogut KA, Fiore NF. Nonoperative management of giant omphalocele leading to early fascial closure. J Pediatr Surg. 2018;53:2404-2408.
- Conner P, Vejde JH, Burgos CM. Accuracy and impact of prenatal diagnosis in infants with omphalocele. Pediatr Surg Int. 2018;34:629-633.
- Iacovella C, Contro E, Ghi T, et al. The effect of the contents of exomphalos and nuchal translucency at 11-14 weeks on the likelihood of associated chromosomal abnormality. Prenat Diagn. 2012;32:1066-1070.
- Getachew MM, Goldstein RB, Edge V, et al. Correlation between omphalocele contents and karyotypic abnormalities: sonographic study in 37 cases. AJR Am J Roentgenol. 1992;158:133-136.
- Singh A, Singh J, Gupta K. Body stalk anomaly: antenatal sonographic diagnosis of this rare entity with review of literature. J Ultrason. 2017;17:133-135.
- Lazaroni TL, Cruzeiro PC, Piçarro C, et al. Body stalk anomaly: Three months of survival. Case report and literature review. J Pediatr Surg Case Rep. 2016;14:22-25.
- Gajzer DC, Hirzel AC, Saigal G, et al. Possible genetic origin of limb-body wall complex. Fetal Pediatr Pathol. 2015;34: 257–270.
- Maruyama H, Inagaki T, Nakata Y, et al. Minimally conjoined omphalopagus twins with a body stalk anomaly. AJP Rep. 2015;5:e124-e128.
- Bhat A, Ilyas M, Dev G. Prenatal sonographic diagnosis of limb-body wall complex: case series of a rare congenital anomaly. Radiol Case Rep. 2016;11:116-120.
- Quijano FE, Rey MM, Echeverry M, et al. Body stalk anomaly in a 9-week pregnancy. Case Rep Obstet Gynecol. 2014;2014:357285.
- Kocherla K, Kumari V, Kocherla PR. Prenatal diagnosis of body stalk complex: a rare entity and review of literature. Indian J Radiol Imaging. 2015;25:67-70.
- Panaitescu AM, Ushakov F, Kalaskar A, et al. Ultrasound features and management of body stalk anomaly. Fetal Diagn Ther. 2016;40:285-290.
- Routhu M, Thakkallapelli S, Mohan P, et al. Role of ultrasound in body stalk anomaly and amniotic band syndrome. Int J Reprod Med. 2016;2016:3974139.
- Costa ML, Couto E, Furlan E, et al. Body stalk anomaly: adverse maternal outcomes in a series of 21 cases. Prenat Diagn. 2012;32:264-267.
- Hubbard R, Hayes S, Gillis H, et al. Management challenges in an infant with pentalogy of Cantrell, giant anterior encephalocele, and craniofacial anomalies: a case report. A A Pract. 2018;11:238-240.
- Jnah AJ, Newberry DM, England A. Pentalogy of Cantrell: case report with review of the literature. Adv Neonatal Care. 2015;15:261-268.
- Williams AP, Marayati R, Beierle EA. Pentalogy of Cantrell. Semin Pediatr Surg. 2019;28:106-110.
- Restrepo MS, Cerqua A, Turek JW. Pentalogy of Cantrell with ectopia cordis totalis, total anomalous pulmonary venous connection, and tetralogy of Fallot: a case report and review of the literature. Congenit Heart Dis. 2014;9:E129–E134.
- Zhang X, Xing Q, Sun J, et al. Surgical treatment and outcomes of pentalogy of Cantrell in eight patients. J Pediatr Surg. 2014;49:1335-1340.
- Harring G, Weil J, Thiel C, et al. Management of pentalogy of Cantrell with complete ectopia cordis and double outlet right ventricle. Congenit Anom (Kyoto). 2015;55:121- 123.
- Mallula KK, Sosnowski C, Awad S. Spectrum of Cantrell’s pentalogy: case series from a single tertiary care center and review of the literature. Pediatr Cardiol. 2013;34:1703- 1710.
- Allam ES, Shetty VS, Farmakis SG. Fetal and neonatal presentation of OEIS complex. J Pediatr Surg. 2015;50:2155-2158.
- Neel N, Tarabay MS. Omphalocele, exstrophy of cloaca, imperforate anus, and spinal defect complex, multiple major reconstructive surgeries needed. Urol Ann. 2018;10:118-121.
- Sawaya D, Gearhart JP. Gastrointestinal reconstruction and outcomes for patients with the OEIS complex. Semin Pediatr Surg. 2011;20:123-125.
Doctor in a Bottle: Examining the Increase in Essential Oil Use
What Are Essential Oils?
Essential oils are aromatic volatile oils produced by medicinal plants that give them their distinct flavors and aromas. They are extracted using a variety of different techniques, such as microwave-assisted extraction, headspace extraction, and the most commonly employed hydrodistillation.1 Different parts of the plant are used for the specific oils; the shoots and leaves of Origanum vulgare are used for oregano oil, whereas the skins of Citrus limonum are used for lemon oil.2 Historically, essential oils have been used for cooking, food preservation, perfume, and medicine.3,4
Historical Uses for Essential Oils
Essential oils and their intact medicinal plants were among the first medicines widely available to the ancient world. The Ancient Greeks used topical and oral oregano as a cure-all for ailments including wounds, sore muscles, and diarrhea. Because of its use as a cure-all medicine, it remains a popular folk remedy in parts of Europe today.3 Lavender also has a long history of being a cure-all plant and oil. Some of the many claims behind this flower include treatment of burns, insect bites, parasites, muscle spasms, nausea, and anxiety/depression.5 With an extensive list of historical uses, many essential oils are being researched to determine if their acclaimed qualities have quantifiable properties.
Science Behind the Belief
In vitro experiments with oregano (O vulgare) have demonstrated notable antifungal and antimicrobial effects.6 Gas chromatographic analysis of the oil shows much of it is composed of phenolic monoterpenes, such as thymol and carvacrol. They exhibit strong antifungal effects with a slightly stronger effect on the dermatophyte Trichophyton rubrum over other yeast species such as Candida.7,8 The full effect of the monoterpenes on fungi is not completely understood, but early data show it has a strong affinity for the ergosterol used in the cell-wall synthesis. Other effects demonstrated in in vitro studies include the ability to block drug efflux pumps, biofilm formation, cellular communication among bacteria, and mycotoxin production.9
A double-blind, randomized trial by Akhondzadeh et al10 demonstrated lavender (Lavandula officinalis) to have a mild antidepressant quality but a noticeably more potent effect when combined with imipramine. The effects of the lavender with imipramine were stronger and provided earlier improvement than imipramine alone for treatment of mild to moderate depression. The team concluded that lavender may be an effective adjunct therapy in treating depression.10
In a study by Mori et al,11 full-thickness circular wounds were made in rats and treated with either lavender oil (L officinalis), nothing, or a control oil. With the lavender oil being at only 1% solution, the wounds treated with lavender oil demonstrated earlier closure than the other 2 groups of wounds, where no major difference was noted. On cellular analysis, it was seen that the lavender had increased the rate of granulation as well as expression of types I and III collagen. The most striking result was the large expression of transforming growth factor β seen in the lavender group compared to the others. The final thoughts on this experiment were that lavender may provide new approaches to wound care in the future.11
Potential Problems With Purity
One major concern raised about essential oils is their purity and the fidelity of their chemical composition. The specific aromatic chemicals in each essential oil are maintained for each species, but the proportions of each change even with the time of year.12 Gas chromatograph analysis of the same oil distilled with different techniques showed that the proportions of aromatic chemicals varied with technique. However, the major constituents of the oil remained present in large quantities, just at different percentages.1 Even using the same distillation technique for different time periods can greatly affect the yield and composition of the oil. Although the percentage of each aromatic compound can be affected by distillation times, the antioxidant and antimicrobial effects of the oil remain constant regardless of these variables.2 There is clearly a lack in standardization in essential oil production, which may not be an issue for its use in complementary medicine if its properties are maintained regardless.
Safety Concerns and Regulations
With essential oils being a natural cure for everyday ailments, some people are turning first to oils for every cut and bruise. The danger in these natural cures is that essential oils can cause several types of dermatitis and allergic reactions. The development of allergies to essential oils is at an even higher risk, considering people frequently put them on wounds and rashes where the skin barrier is already weakened. Many essential oils fall into the fragrance category in patch tests, negating the widely circulating blogger and online reports that essential oils cannot cause allergies.
Some of the oils, although regarded safe by the US Food and Drug Administration for consumption, can cause dermatitis from simple contact or with sun exposure.13 Members of the citrus family are notorious for the phytophotodermatitis reaction, which can leave hyperpigmented scarring after exposure of the oils to sunlight.14 Most companies that sell essential oils are aware of this reaction and include it in the warning labels.
The legal problem with selling and classifying essential oils is that the US Food and Drug Administration requires products intended for treatment to be labeled as drugs, which hinders their sales on the open market.13 It all boils down to intended use, so some companies sell the oils under a food or fragrance classification with vague instructions on how to use said oil for medicinal purposes, which leads to lack of supervision, anecdotal cures, and false health claims. One company claims in their safety guide for topical applications of their oils that “[i]f a rash occurs, this may be a sign of detoxification.”15 If essential oils had only minimal absorption topically, their safety would be less concerning, but this does not appear to be the case.
Absorption and Systemics
The effects of essential oils on the skin is one aspect of their use to be studied; another is the more systemic effects from absorption through the skin. Most essential oils used in small quantities for fragrance in over-the-counter lotions prove only to be an issue for allergens in sensitive patient groups. However, topical applications of essential oils in their pure concentrated form get absorbed into the skin faster than if used with a carrier oil, emulsion, or solvent.16 For most minor uses of essential oils, the body can detoxify absorbed chemicals the same way it does when a person eats the plants the oils came from (eg, basil essential oils leaching from the leaves into a tomato sauce). A possible danger of the oils’ systemic properties lies in the pregnant patient population who use essential oils thinking that natural is safe.
Many essential oils, such as lavender (L officinalis), exhibit hormonal mimicry with phytoestrogens and can produce emmenagogue (increasing menstrual flow) effects in women. Other oils, such as those of nutmeg (Myristica fragrans) and myrrh (Commiphora myrrha), can have abortifacient effects. These natural essential oils can lead to unintended health risks for mother and baby.17 With implications this serious, many essential oil companies put pregnancy warnings on most if not all of their products, but pregnant patients may not always note the risk.
Conclusion
Essential oils are not the newest medical fad. They outdate every drug on the market and were used by some of the first physicians in history. It is important to continue research into the antimicrobial effects of essential oils, as they may hold the secret to treatment options with the continued rise of multidrug-resistant organisms. The danger of these oils lies not in their hidden potential but in the belief that natural things are safe. A few animal studies have been performed, but little is known about the full effects of essential oils in humans. Patients need to be educated that these are not panaceas with freedom from side effects and that treatment options backed by the scientific method should be their first choice under the supervision of trained physicians. The Table outlines the uses and side effects of the essential oils discussed here.
- Fan S, Chang J, Zong Y, et al. GC-MS analysis of the composition of the essential oil from Dendranthema indicum var. aromaticum using three extraction methods and two columns. Molecules. 2018;23:576.
- Zheljazkov VD, Astatkie T, Schlegel V. Distillation time changes oregano essential oil yields and composition but not the antioxidant or antimicrobial activities. HortScience. 2012;47:777-784.
- Singletary K. Oregano: overview of the literature on health benefits. Nutr Today. 2010;45:129-138.
- Cortés-Rojas DF, de Souza CRF, Oliveira WP. Clove (Syzygium aromaticum): a precious spice. Asian Pac J Trop Biomed. 2014;4:90-96.
- Koulivand PH, Khaleghi Ghadiri M, Gorji A. Lavender and the nervous system. Evid Based Complement Alternat Med. 2013;2013:681304.
- Cleff MB, Meinerz AR, Xavier M, et al. In vitro activity of Origanum vulgare essential oil against Candida species. Brazilian J Microbiol. 2010;41:116-123.
- Adam K, Sivropoulou A, Kokkini S, et al. Antifungal activities of Origanum vulgare subsp. hirtum, Mentha spicata, Lavandula angustifolia, and Salvia fruticosa essential oils against human pathogenic fungi. J Agric Food Chem. 1998;46:1739-1745.
- Miron D, Battisti F, Silva FK, et al. Antifungal activity and mechanism of action of monoterpenes against dermatophytes and yeasts. Brazil J Pharmacognosy. 2014;24:660-667.
- Nazzaro F, Fratianni F, Coppola R, et al. Essential oils and antifungal activity. Pharmaceuticals (Basel). 2017;10:86.
- Akhondzadeh S, Kashani L, Fotouhi A, et al. Comparison of Lavandula angustifolia Mill. tincture and imipramine in the treatment of mild to moderate depression: a double-blind, randomized trial. Prog Neuropsychopharmacol Biol Psychiatry. 2003;27:123-127.
- Mori H-M, Kawanami H, Kawahata H, et al. Wound healing potential of lavender oil by acceleration of granulation and wound contraction through induction of TGF-β in a rat model. BMC Complement Altern Med. 2016;16:144.
- Vekiari SA, Protopapadakis EE, Papadopoulou P, et al. Composition and seasonal variation of the essential oil from leaves and peel of a cretan lemon variety. J Agric Food Chem. 2002;50:147-153.
- Aromatherapy. US Food & Drug Administration website. https://www.fda.gov/cosmetics/productsingredients/products/ucm127054.htm. Accessed October 14, 2020.
- Hankinson A, Lloyd B, Alweis R. Lime-induced phytophotodermatitis. J Community Hosp Intern Med Perspect. 2014;4. doi:10.3402/jchimp.v4.25090.
- Essential Oil Safety Guide. Young Living Essential Oils website. https://www.youngliving.com/en_US/discover/essential-oil-safety. Accessed October 14, 2020.
- Cal K. Skin penetration of terpenes from essential oils and topical vehicles. Planta Medica. 2006;72:311-316.
- Ernst E. Herbal medicinal products during pregnancy: are they safe? BJOG. 2002;109:227-235.
- Hsouna AB, Halima NB, Smaoui S, et al. Citrus lemon essential oil: chemical composition, antioxidant and antimicrobial activities with its preservative effect against Listeria monocytogenes inoculated in minced beef meat. Lipids Health Dis. 2017;16:146.
- Chen Y, Zhou C, Ge Z, et al. Composition and potential anticancer activities of essential oils obtained from myrrh and frankincense. Oncol Lett. 2013;6:1140-1146.
- Zhang WK, Tao S-S, Li T-T, et al. Nutmeg oil alleviates chronic inflammatory pain through inhibition of COX-2 expression and substance P release in vivo. Food Nutr Res. 2016;60:30849.
- Glodde N, Jakobs M, Bald T, et al. Differential role of cannabinoids in the pathogenesis of skin cancer. Life Sci. 2015;138:35-40.
What Are Essential Oils?
Essential oils are aromatic volatile oils produced by medicinal plants that give them their distinct flavors and aromas. They are extracted using a variety of different techniques, such as microwave-assisted extraction, headspace extraction, and the most commonly employed hydrodistillation.1 Different parts of the plant are used for the specific oils; the shoots and leaves of Origanum vulgare are used for oregano oil, whereas the skins of Citrus limonum are used for lemon oil.2 Historically, essential oils have been used for cooking, food preservation, perfume, and medicine.3,4
Historical Uses for Essential Oils
Essential oils and their intact medicinal plants were among the first medicines widely available to the ancient world. The Ancient Greeks used topical and oral oregano as a cure-all for ailments including wounds, sore muscles, and diarrhea. Because of its use as a cure-all medicine, it remains a popular folk remedy in parts of Europe today.3 Lavender also has a long history of being a cure-all plant and oil. Some of the many claims behind this flower include treatment of burns, insect bites, parasites, muscle spasms, nausea, and anxiety/depression.5 With an extensive list of historical uses, many essential oils are being researched to determine if their acclaimed qualities have quantifiable properties.
Science Behind the Belief
In vitro experiments with oregano (O vulgare) have demonstrated notable antifungal and antimicrobial effects.6 Gas chromatographic analysis of the oil shows much of it is composed of phenolic monoterpenes, such as thymol and carvacrol. They exhibit strong antifungal effects with a slightly stronger effect on the dermatophyte Trichophyton rubrum over other yeast species such as Candida.7,8 The full effect of the monoterpenes on fungi is not completely understood, but early data show it has a strong affinity for the ergosterol used in the cell-wall synthesis. Other effects demonstrated in in vitro studies include the ability to block drug efflux pumps, biofilm formation, cellular communication among bacteria, and mycotoxin production.9
A double-blind, randomized trial by Akhondzadeh et al10 demonstrated lavender (Lavandula officinalis) to have a mild antidepressant quality but a noticeably more potent effect when combined with imipramine. The effects of the lavender with imipramine were stronger and provided earlier improvement than imipramine alone for treatment of mild to moderate depression. The team concluded that lavender may be an effective adjunct therapy in treating depression.10
In a study by Mori et al,11 full-thickness circular wounds were made in rats and treated with either lavender oil (L officinalis), nothing, or a control oil. With the lavender oil being at only 1% solution, the wounds treated with lavender oil demonstrated earlier closure than the other 2 groups of wounds, where no major difference was noted. On cellular analysis, it was seen that the lavender had increased the rate of granulation as well as expression of types I and III collagen. The most striking result was the large expression of transforming growth factor β seen in the lavender group compared to the others. The final thoughts on this experiment were that lavender may provide new approaches to wound care in the future.11
Potential Problems With Purity
One major concern raised about essential oils is their purity and the fidelity of their chemical composition. The specific aromatic chemicals in each essential oil are maintained for each species, but the proportions of each change even with the time of year.12 Gas chromatograph analysis of the same oil distilled with different techniques showed that the proportions of aromatic chemicals varied with technique. However, the major constituents of the oil remained present in large quantities, just at different percentages.1 Even using the same distillation technique for different time periods can greatly affect the yield and composition of the oil. Although the percentage of each aromatic compound can be affected by distillation times, the antioxidant and antimicrobial effects of the oil remain constant regardless of these variables.2 There is clearly a lack in standardization in essential oil production, which may not be an issue for its use in complementary medicine if its properties are maintained regardless.
Safety Concerns and Regulations
With essential oils being a natural cure for everyday ailments, some people are turning first to oils for every cut and bruise. The danger in these natural cures is that essential oils can cause several types of dermatitis and allergic reactions. The development of allergies to essential oils is at an even higher risk, considering people frequently put them on wounds and rashes where the skin barrier is already weakened. Many essential oils fall into the fragrance category in patch tests, negating the widely circulating blogger and online reports that essential oils cannot cause allergies.
Some of the oils, although regarded safe by the US Food and Drug Administration for consumption, can cause dermatitis from simple contact or with sun exposure.13 Members of the citrus family are notorious for the phytophotodermatitis reaction, which can leave hyperpigmented scarring after exposure of the oils to sunlight.14 Most companies that sell essential oils are aware of this reaction and include it in the warning labels.
The legal problem with selling and classifying essential oils is that the US Food and Drug Administration requires products intended for treatment to be labeled as drugs, which hinders their sales on the open market.13 It all boils down to intended use, so some companies sell the oils under a food or fragrance classification with vague instructions on how to use said oil for medicinal purposes, which leads to lack of supervision, anecdotal cures, and false health claims. One company claims in their safety guide for topical applications of their oils that “[i]f a rash occurs, this may be a sign of detoxification.”15 If essential oils had only minimal absorption topically, their safety would be less concerning, but this does not appear to be the case.
Absorption and Systemics
The effects of essential oils on the skin is one aspect of their use to be studied; another is the more systemic effects from absorption through the skin. Most essential oils used in small quantities for fragrance in over-the-counter lotions prove only to be an issue for allergens in sensitive patient groups. However, topical applications of essential oils in their pure concentrated form get absorbed into the skin faster than if used with a carrier oil, emulsion, or solvent.16 For most minor uses of essential oils, the body can detoxify absorbed chemicals the same way it does when a person eats the plants the oils came from (eg, basil essential oils leaching from the leaves into a tomato sauce). A possible danger of the oils’ systemic properties lies in the pregnant patient population who use essential oils thinking that natural is safe.
Many essential oils, such as lavender (L officinalis), exhibit hormonal mimicry with phytoestrogens and can produce emmenagogue (increasing menstrual flow) effects in women. Other oils, such as those of nutmeg (Myristica fragrans) and myrrh (Commiphora myrrha), can have abortifacient effects. These natural essential oils can lead to unintended health risks for mother and baby.17 With implications this serious, many essential oil companies put pregnancy warnings on most if not all of their products, but pregnant patients may not always note the risk.
Conclusion
Essential oils are not the newest medical fad. They outdate every drug on the market and were used by some of the first physicians in history. It is important to continue research into the antimicrobial effects of essential oils, as they may hold the secret to treatment options with the continued rise of multidrug-resistant organisms. The danger of these oils lies not in their hidden potential but in the belief that natural things are safe. A few animal studies have been performed, but little is known about the full effects of essential oils in humans. Patients need to be educated that these are not panaceas with freedom from side effects and that treatment options backed by the scientific method should be their first choice under the supervision of trained physicians. The Table outlines the uses and side effects of the essential oils discussed here.

What Are Essential Oils?
Essential oils are aromatic volatile oils produced by medicinal plants that give them their distinct flavors and aromas. They are extracted using a variety of different techniques, such as microwave-assisted extraction, headspace extraction, and the most commonly employed hydrodistillation.1 Different parts of the plant are used for the specific oils; the shoots and leaves of Origanum vulgare are used for oregano oil, whereas the skins of Citrus limonum are used for lemon oil.2 Historically, essential oils have been used for cooking, food preservation, perfume, and medicine.3,4
Historical Uses for Essential Oils
Essential oils and their intact medicinal plants were among the first medicines widely available to the ancient world. The Ancient Greeks used topical and oral oregano as a cure-all for ailments including wounds, sore muscles, and diarrhea. Because of its use as a cure-all medicine, it remains a popular folk remedy in parts of Europe today.3 Lavender also has a long history of being a cure-all plant and oil. Some of the many claims behind this flower include treatment of burns, insect bites, parasites, muscle spasms, nausea, and anxiety/depression.5 With an extensive list of historical uses, many essential oils are being researched to determine if their acclaimed qualities have quantifiable properties.
Science Behind the Belief
In vitro experiments with oregano (O vulgare) have demonstrated notable antifungal and antimicrobial effects.6 Gas chromatographic analysis of the oil shows much of it is composed of phenolic monoterpenes, such as thymol and carvacrol. They exhibit strong antifungal effects with a slightly stronger effect on the dermatophyte Trichophyton rubrum over other yeast species such as Candida.7,8 The full effect of the monoterpenes on fungi is not completely understood, but early data show it has a strong affinity for the ergosterol used in the cell-wall synthesis. Other effects demonstrated in in vitro studies include the ability to block drug efflux pumps, biofilm formation, cellular communication among bacteria, and mycotoxin production.9
A double-blind, randomized trial by Akhondzadeh et al10 demonstrated lavender (Lavandula officinalis) to have a mild antidepressant quality but a noticeably more potent effect when combined with imipramine. The effects of the lavender with imipramine were stronger and provided earlier improvement than imipramine alone for treatment of mild to moderate depression. The team concluded that lavender may be an effective adjunct therapy in treating depression.10
In a study by Mori et al,11 full-thickness circular wounds were made in rats and treated with either lavender oil (L officinalis), nothing, or a control oil. With the lavender oil being at only 1% solution, the wounds treated with lavender oil demonstrated earlier closure than the other 2 groups of wounds, where no major difference was noted. On cellular analysis, it was seen that the lavender had increased the rate of granulation as well as expression of types I and III collagen. The most striking result was the large expression of transforming growth factor β seen in the lavender group compared to the others. The final thoughts on this experiment were that lavender may provide new approaches to wound care in the future.11
Potential Problems With Purity
One major concern raised about essential oils is their purity and the fidelity of their chemical composition. The specific aromatic chemicals in each essential oil are maintained for each species, but the proportions of each change even with the time of year.12 Gas chromatograph analysis of the same oil distilled with different techniques showed that the proportions of aromatic chemicals varied with technique. However, the major constituents of the oil remained present in large quantities, just at different percentages.1 Even using the same distillation technique for different time periods can greatly affect the yield and composition of the oil. Although the percentage of each aromatic compound can be affected by distillation times, the antioxidant and antimicrobial effects of the oil remain constant regardless of these variables.2 There is clearly a lack in standardization in essential oil production, which may not be an issue for its use in complementary medicine if its properties are maintained regardless.
Safety Concerns and Regulations
With essential oils being a natural cure for everyday ailments, some people are turning first to oils for every cut and bruise. The danger in these natural cures is that essential oils can cause several types of dermatitis and allergic reactions. The development of allergies to essential oils is at an even higher risk, considering people frequently put them on wounds and rashes where the skin barrier is already weakened. Many essential oils fall into the fragrance category in patch tests, negating the widely circulating blogger and online reports that essential oils cannot cause allergies.
Some of the oils, although regarded safe by the US Food and Drug Administration for consumption, can cause dermatitis from simple contact or with sun exposure.13 Members of the citrus family are notorious for the phytophotodermatitis reaction, which can leave hyperpigmented scarring after exposure of the oils to sunlight.14 Most companies that sell essential oils are aware of this reaction and include it in the warning labels.
The legal problem with selling and classifying essential oils is that the US Food and Drug Administration requires products intended for treatment to be labeled as drugs, which hinders their sales on the open market.13 It all boils down to intended use, so some companies sell the oils under a food or fragrance classification with vague instructions on how to use said oil for medicinal purposes, which leads to lack of supervision, anecdotal cures, and false health claims. One company claims in their safety guide for topical applications of their oils that “[i]f a rash occurs, this may be a sign of detoxification.”15 If essential oils had only minimal absorption topically, their safety would be less concerning, but this does not appear to be the case.
Absorption and Systemics
The effects of essential oils on the skin is one aspect of their use to be studied; another is the more systemic effects from absorption through the skin. Most essential oils used in small quantities for fragrance in over-the-counter lotions prove only to be an issue for allergens in sensitive patient groups. However, topical applications of essential oils in their pure concentrated form get absorbed into the skin faster than if used with a carrier oil, emulsion, or solvent.16 For most minor uses of essential oils, the body can detoxify absorbed chemicals the same way it does when a person eats the plants the oils came from (eg, basil essential oils leaching from the leaves into a tomato sauce). A possible danger of the oils’ systemic properties lies in the pregnant patient population who use essential oils thinking that natural is safe.
Many essential oils, such as lavender (L officinalis), exhibit hormonal mimicry with phytoestrogens and can produce emmenagogue (increasing menstrual flow) effects in women. Other oils, such as those of nutmeg (Myristica fragrans) and myrrh (Commiphora myrrha), can have abortifacient effects. These natural essential oils can lead to unintended health risks for mother and baby.17 With implications this serious, many essential oil companies put pregnancy warnings on most if not all of their products, but pregnant patients may not always note the risk.
Conclusion
Essential oils are not the newest medical fad. They outdate every drug on the market and were used by some of the first physicians in history. It is important to continue research into the antimicrobial effects of essential oils, as they may hold the secret to treatment options with the continued rise of multidrug-resistant organisms. The danger of these oils lies not in their hidden potential but in the belief that natural things are safe. A few animal studies have been performed, but little is known about the full effects of essential oils in humans. Patients need to be educated that these are not panaceas with freedom from side effects and that treatment options backed by the scientific method should be their first choice under the supervision of trained physicians. The Table outlines the uses and side effects of the essential oils discussed here.

- Fan S, Chang J, Zong Y, et al. GC-MS analysis of the composition of the essential oil from Dendranthema indicum var. aromaticum using three extraction methods and two columns. Molecules. 2018;23:576.
- Zheljazkov VD, Astatkie T, Schlegel V. Distillation time changes oregano essential oil yields and composition but not the antioxidant or antimicrobial activities. HortScience. 2012;47:777-784.
- Singletary K. Oregano: overview of the literature on health benefits. Nutr Today. 2010;45:129-138.
- Cortés-Rojas DF, de Souza CRF, Oliveira WP. Clove (Syzygium aromaticum): a precious spice. Asian Pac J Trop Biomed. 2014;4:90-96.
- Koulivand PH, Khaleghi Ghadiri M, Gorji A. Lavender and the nervous system. Evid Based Complement Alternat Med. 2013;2013:681304.
- Cleff MB, Meinerz AR, Xavier M, et al. In vitro activity of Origanum vulgare essential oil against Candida species. Brazilian J Microbiol. 2010;41:116-123.
- Adam K, Sivropoulou A, Kokkini S, et al. Antifungal activities of Origanum vulgare subsp. hirtum, Mentha spicata, Lavandula angustifolia, and Salvia fruticosa essential oils against human pathogenic fungi. J Agric Food Chem. 1998;46:1739-1745.
- Miron D, Battisti F, Silva FK, et al. Antifungal activity and mechanism of action of monoterpenes against dermatophytes and yeasts. Brazil J Pharmacognosy. 2014;24:660-667.
- Nazzaro F, Fratianni F, Coppola R, et al. Essential oils and antifungal activity. Pharmaceuticals (Basel). 2017;10:86.
- Akhondzadeh S, Kashani L, Fotouhi A, et al. Comparison of Lavandula angustifolia Mill. tincture and imipramine in the treatment of mild to moderate depression: a double-blind, randomized trial. Prog Neuropsychopharmacol Biol Psychiatry. 2003;27:123-127.
- Mori H-M, Kawanami H, Kawahata H, et al. Wound healing potential of lavender oil by acceleration of granulation and wound contraction through induction of TGF-β in a rat model. BMC Complement Altern Med. 2016;16:144.
- Vekiari SA, Protopapadakis EE, Papadopoulou P, et al. Composition and seasonal variation of the essential oil from leaves and peel of a cretan lemon variety. J Agric Food Chem. 2002;50:147-153.
- Aromatherapy. US Food & Drug Administration website. https://www.fda.gov/cosmetics/productsingredients/products/ucm127054.htm. Accessed October 14, 2020.
- Hankinson A, Lloyd B, Alweis R. Lime-induced phytophotodermatitis. J Community Hosp Intern Med Perspect. 2014;4. doi:10.3402/jchimp.v4.25090.
- Essential Oil Safety Guide. Young Living Essential Oils website. https://www.youngliving.com/en_US/discover/essential-oil-safety. Accessed October 14, 2020.
- Cal K. Skin penetration of terpenes from essential oils and topical vehicles. Planta Medica. 2006;72:311-316.
- Ernst E. Herbal medicinal products during pregnancy: are they safe? BJOG. 2002;109:227-235.
- Hsouna AB, Halima NB, Smaoui S, et al. Citrus lemon essential oil: chemical composition, antioxidant and antimicrobial activities with its preservative effect against Listeria monocytogenes inoculated in minced beef meat. Lipids Health Dis. 2017;16:146.
- Chen Y, Zhou C, Ge Z, et al. Composition and potential anticancer activities of essential oils obtained from myrrh and frankincense. Oncol Lett. 2013;6:1140-1146.
- Zhang WK, Tao S-S, Li T-T, et al. Nutmeg oil alleviates chronic inflammatory pain through inhibition of COX-2 expression and substance P release in vivo. Food Nutr Res. 2016;60:30849.
- Glodde N, Jakobs M, Bald T, et al. Differential role of cannabinoids in the pathogenesis of skin cancer. Life Sci. 2015;138:35-40.
- Fan S, Chang J, Zong Y, et al. GC-MS analysis of the composition of the essential oil from Dendranthema indicum var. aromaticum using three extraction methods and two columns. Molecules. 2018;23:576.
- Zheljazkov VD, Astatkie T, Schlegel V. Distillation time changes oregano essential oil yields and composition but not the antioxidant or antimicrobial activities. HortScience. 2012;47:777-784.
- Singletary K. Oregano: overview of the literature on health benefits. Nutr Today. 2010;45:129-138.
- Cortés-Rojas DF, de Souza CRF, Oliveira WP. Clove (Syzygium aromaticum): a precious spice. Asian Pac J Trop Biomed. 2014;4:90-96.
- Koulivand PH, Khaleghi Ghadiri M, Gorji A. Lavender and the nervous system. Evid Based Complement Alternat Med. 2013;2013:681304.
- Cleff MB, Meinerz AR, Xavier M, et al. In vitro activity of Origanum vulgare essential oil against Candida species. Brazilian J Microbiol. 2010;41:116-123.
- Adam K, Sivropoulou A, Kokkini S, et al. Antifungal activities of Origanum vulgare subsp. hirtum, Mentha spicata, Lavandula angustifolia, and Salvia fruticosa essential oils against human pathogenic fungi. J Agric Food Chem. 1998;46:1739-1745.
- Miron D, Battisti F, Silva FK, et al. Antifungal activity and mechanism of action of monoterpenes against dermatophytes and yeasts. Brazil J Pharmacognosy. 2014;24:660-667.
- Nazzaro F, Fratianni F, Coppola R, et al. Essential oils and antifungal activity. Pharmaceuticals (Basel). 2017;10:86.
- Akhondzadeh S, Kashani L, Fotouhi A, et al. Comparison of Lavandula angustifolia Mill. tincture and imipramine in the treatment of mild to moderate depression: a double-blind, randomized trial. Prog Neuropsychopharmacol Biol Psychiatry. 2003;27:123-127.
- Mori H-M, Kawanami H, Kawahata H, et al. Wound healing potential of lavender oil by acceleration of granulation and wound contraction through induction of TGF-β in a rat model. BMC Complement Altern Med. 2016;16:144.
- Vekiari SA, Protopapadakis EE, Papadopoulou P, et al. Composition and seasonal variation of the essential oil from leaves and peel of a cretan lemon variety. J Agric Food Chem. 2002;50:147-153.
- Aromatherapy. US Food & Drug Administration website. https://www.fda.gov/cosmetics/productsingredients/products/ucm127054.htm. Accessed October 14, 2020.
- Hankinson A, Lloyd B, Alweis R. Lime-induced phytophotodermatitis. J Community Hosp Intern Med Perspect. 2014;4. doi:10.3402/jchimp.v4.25090.
- Essential Oil Safety Guide. Young Living Essential Oils website. https://www.youngliving.com/en_US/discover/essential-oil-safety. Accessed October 14, 2020.
- Cal K. Skin penetration of terpenes from essential oils and topical vehicles. Planta Medica. 2006;72:311-316.
- Ernst E. Herbal medicinal products during pregnancy: are they safe? BJOG. 2002;109:227-235.
- Hsouna AB, Halima NB, Smaoui S, et al. Citrus lemon essential oil: chemical composition, antioxidant and antimicrobial activities with its preservative effect against Listeria monocytogenes inoculated in minced beef meat. Lipids Health Dis. 2017;16:146.
- Chen Y, Zhou C, Ge Z, et al. Composition and potential anticancer activities of essential oils obtained from myrrh and frankincense. Oncol Lett. 2013;6:1140-1146.
- Zhang WK, Tao S-S, Li T-T, et al. Nutmeg oil alleviates chronic inflammatory pain through inhibition of COX-2 expression and substance P release in vivo. Food Nutr Res. 2016;60:30849.
- Glodde N, Jakobs M, Bald T, et al. Differential role of cannabinoids in the pathogenesis of skin cancer. Life Sci. 2015;138:35-40.
Practice Points
- Essential oils are a rising trend of nonprescribed topical supplements used by patients to self-treat.
- Research into historically medicinal essential oils may unlock treatment opportunities in the near future.
- Keeping an open-minded line of communication is critical for divulgence of potential home remedies that could be causing patients harm.
- Understanding the mindset of the essential oil–using community is key to building trust and treating these patients who are often distrusting of Western medicine.
2020 Update on bone health
Increasingly, bone health and fragility fracture prevention is one of the most important aspects of healthy aging that we, as women’s health care providers (HCPs), must be sure is part of our thought process in caring for women at midlife and beyond. Virtually all ObGyn HCPs are aware of breast health, both in terms of the clinical breast exam and imaging surveillance. The 5-year relative survival rate for “localized breast cancer” is 99%.1 Most recent data on hip fracture, however, indicate that it is associated with a mortality in the first year of 21%!2 We need to be sure that our patients understand this.
Previously, this column provided an update on osteoporosis. In 2016, I asked to change the focus to “Update on bone health” to highlight that simply relying on dual energy x-ray absorptiometry (DXA) testing of bone mass with arbitrary cutoffs for osteoporosis, osteopenia, and normal bone mass is not adequate for improving overall bone health. The addition of the FRAX fracture risk assessment tool, now widely employed, as well as the trabecular bone score (TBS), not widely employed, helps to refine the assessment of patients’ risk status. Further, issues such as sarcopenia, adequate dietary calcium and vitamin D supplementation, and fall prevention (improving balance, use of nonskid rugs in the bathroom, avoiding black ice when present, having nothing to slip on between the bed and the bathroom in the middle of the night, and so on) also are essential elements of “bone health.”
Finally, I cannot stress enough the importance of developing a good relationship with whatever facility one uses for DXA testing in order to maximize use of the reports and potential limitations. In addition, we should identify a metabolic bone specialist for referral of unusual cases or patients who require medications unlikely to be prescribed by us as ObGyns, and develop some familiarity with therapies that may be utilized.
Osteosarcopenia greatly enhances fall and fracture risk
Sepúlveda-Loyola W, Phu S, Bani Hassan E, et al. The joint occurrence of osteoporosis and sarcopenia (osteosarcopenia): definitions and characteristics. J Am Med Dir Assoc. 2020;21:220-225.
Tokeshi S, Eguchi Y, Suzuki M, et al. Relationship between skeletal muscle mass, bone mineral density, and trabecular bone score in osteoporotic vertebral compression fractures. Asian Spine J. 2020 Sep 3. doi: 10.31616/asj.2020.0045.
Kirk B, Zanker J, Duque G. Osteosarcopenia: epidemiology, diagnosis, and treatment—facts and numbers. J Cachexia Sarcopenia Muscle. 2020;11:609-618.
The topic of sarcopenia as defined by the concurrent presence of low muscle mass, physical performance, and strength has been discussed previously in this Update series.3 Now, osteosarcopenia, defined as the concomitant presence of osteoporosis or osteopenia combined with sarcopenia, seems to be an extremely important gauge of fracture risk, especially now as the population’s longevity has increased dramatically. This new syndrome is associated with higher disability and rates of fracture and falls in older people compared with either entity (the bone component or the sarcopenia component) alone.4,5 In fact, in the 2016 ICD-10-CM, sarcopenia was finally recognized as a disease entity.
Severe sarcopenia is known to increase the risk for falls.6 Furthermore, evidence is increasing of cross talk between muscle and bone.4 The diagnostic criteria of osteopenia and osteoporosis are well established; however, absolute criteria for sarcopenia lack an international consensus.
Continue to: Assess for osteopenia/osteoporosis plus sarcopenia to determine those at greatest fracture risk...
Assess for osteopenia/osteoporosis plus sarcopenia to determine those at greatest fracture risk
Sepúlveda-Loyola and colleagues performed a cross-sectional analysis of 253 participants, of which 77% were women, average age 78, who presented for a “falls and fractures” risk assessment. T-scores were measured by DXA. In addition, the investigators measured components of sarcopenia, including physical performance (evaluated by hand grip strength, gait speed, timed up and go test, and 5-time sit to stand test) and dynamic and static balance. Falls in the previous year were self-reported, with 42% of participants having fallen once and 54%, more than once.
Results. Participants with osteosarcopenia had a statistically significant increased rate of falls of approximately threefold and an increased rate of fractures that was approximately fourfold when compared with osteopenia or osteoporosis alone.
Another important finding was that, despite the links between osteoporosis, fracture, and poor clinical outcomes, the investigators did not find differences in fracture rates in the osteopenic compared with the osteoporotic classifications. Their findings corroborated those of other studies that reported discrepancies in fractures and bone mineral density (BMD), with osteopenic older adults experiencing fracture rates similar to and in some cases greater than those diagnosed with osteoporosis.7
Thus, it appears that the use of T-scores that combine osteopenic and osteoporotic criteria into the osteosarcopenic category may be sufficient to capture individuals at the greatest risk of fracture.
Skeletal muscle mass plays a role in vertebral compression fractures
Tokeshi and colleagues conducted retrospective observational study to investigate the relationships between skeletal muscle mass, BMD, and TBS in individuals with osteoporotic vertebral compression fractures.
They evaluated 142 patients with an average age of 75; of these, 30% had radiographically diagnosed vertebral compression fractures (average age, 79) and 70% had no vertebral compression fractures (average age, 70). Body composition was measured using whole-body DXA; appendicular skeletal muscle mass index was determined as the sum of upper and lower extremities’ lean mass (kg/height in m2 ). TBS was measured using the patented algorithm software on DXA scans for the lumbar vertebrae.
Results. The investigators found that the vertebral compression fracture group was statistically significantly older, had lower femur BMD, and had decreased leg muscle mass. The TBS was not identified as a risk factor.
Certain lifestyle factors add to risk of osteosarcopenia
In an editorial, Kirk and colleagues summarized the epidemiology, diagnosis, and treatment of osteosarcopenia. They concluded that this syndrome can be expected to grow in age-related and disease-related states as a consequence of immunosenescence coinciding with an increase in sedentary lifestyle, obesity, and fat infiltration of muscle and bone.
Increasingly, clinicians should screen for osteosarcopenia via imaging methods (DXA) to quantitate bone mass (as is currently done) and, increasingly, quantify muscle mass. In addition, assessment of muscle strength, easily done by testing grip strength, as well as functional capacity (gait speed), will become increasingly important.
Finally, the authors call for a more comprehensive geriatric assessment that includes medical history and risk factors as well as treatment (including osteoporosis drugs, where indicated), and progressive resistance and balance exercises. Nutritional recommendations, in terms of protein, vitamin D, and calcium, also are necessary. They anticipate that diagnosis and treatment of osteosarcopenia will become part of routine health care in the future.
In the past, our assessment of risk for fragility fracture was based mostly on bone mass measurement by DXA. Scoring systems like the FRAX tool have included other risk factors, such as age, body mass index, previous fracture, family history of hip fracture, smoking, any history of rheumatoid arthritis, use of glucocorticoids, and alcohol consumption. However, sarcopenia is a condition characterized by loss of skeletal muscle mass, strength, and function. While it is a natural part of the aging process, when it is severe and coupled with osteopenia or osteoporosis, it significantly increases the risks of falls as well as fracture. Women’s HCPs should increasingly think about the presence of sarcopenia in their patients, especially those with low bone mass (osteopenia or osteoporosis), particularly when making decisions about initiating pharmaceutical intervention. In addition, recommendations for resistive and balance exercises virtually should be universal.
Continue to: The denosumab discontinuation dilemma...
The denosumab discontinuation dilemma
Lyu H, Yoshida K, Zhao SS, et al. Delayed denosumab injections and fracture risk among patients with osteoporosis: a population-based cohort study. Ann Intern Med. 2020;173:516-526.
Tripto-Shkolnik L, Fund N, Rouach V, et al. Fracture incidence after denosumab discontinuation: real-world data from a large healthcare provider. Bone. 2020;130:115150.
Denosumab, marketed under the brand name Prolia, is a human monoclonal antibody that blocks the binding of RANK ligand and inhibits development and activity of osteoclast, thus decreasing bone resorption and increasing BMD. In the original pivotal clinical trial of denosumab, almost 7,900 women between the ages of 60 and 90 (average age, 73) with osteoporotic T-scores were enrolled.8 The women were randomly assigned to receive 60 mg of denosumab subcutaneously every 6 months or placebo for a total of 3 years. In that trial, the denosumabtreated group, relative to the placebo group, showed a statistically significant decrease in radiographic vertebral fracture, hip fracture, and nonvertebral fracture.
An open-label extension study looked at denosumab use for a total of 10 years.9 That study found that denosumab treatment for up to 10 years was associated with low rates of adverse events, low fracture incidence compared with that observed during the original trial, and continued increases in BMD without plateau. Thus, denosumab appeared to be an extremely safe and effective agent for treating postmenopausal women with osteoporosis.
Denosumab cessation leads to rebound vertebral fractures
As opposed to bisphosphonates, denosumab does not incorporate into bone matrix, and bone turnover is not suppressed after cessation of its use. Reports have implied that denosumab discontinuation may lead to an increased risk of multiple vertebral fractures.10 One theory is that unlike atypical femoral fractures that seem to emerge from failure of microdamage repair in cortical bone with long-term antiresorptive treatment, denosumab rebound–associated vertebral fractures seem to originate from the synergy of rapid bone resorption and accelerated microdamage accumulation in trabecular bone triggered by the discontinuation of this highly potent reversible agent.11
Post hoc analysis of the denosumab placebo-controlled trial and its extension reported that the vertebral fracture rate increased after denosumab discontinuation to the level observed in untreated patients.12 Further, a majority of participants who did sustain vertebral fracture after discontinuing denosumab had multiple vertebral fractures, with the risk being greatest in participants who had a prior vertebral facture. This caused those authors to suggest that patients who discontinued denosumab should rapidly transition to an alternative antiresorptive treatment.
Effect of dose delays, discontinuation on vertebral fracture rate
Lyu and colleagues recently described their population-based cohort study of the United Kingdom’s Health Improvement Network primary care database between 2010 and 2019. They found that delayed administration of a subsequent denosumab dose by more than 16 weeks was associated with an increased risk for vertebral fracture compared with on-time dosing. They noted, however, that the evidence was insufficient to conclude that fracture risk at any other anatomic sites is increased with such a delay.
In a similar study, Tripto-Shkolnik and colleagues examined an Israeli database of 2.3 million members in a state-mandated health organization. They identified osteoporotic patients with at least 2 denosumab prescription dispenses and defined treatment discontinuation as a refill gap of 3 months or more. Fractures were identified by an osteoporosis registry, including fractures that occurred within 1 year from discontinuation in denosumab discontinuers as well as from the second year of treatment forward for persistent users. They identified 1,500 denosumab discontinuers (average age, 72) and 1,610 persistent users (average age also 72). At baseline, the groups were comparable in fracture history, smoking, and bone density.
In the discontinuation group, 0.8% had multiple vertebral fractures versus 0.1% in the persistent users (P = .006); the overall rate of fractures per 100 patient-years of follow-up was 3 times higher in the discontinuation group than in the persistent user group, and the rate of vertebral fractures was almost 5 times higher in the discontinuation group.
Denosumab is an extremely safe and effective treatment for postmenopausal osteoporosis. Discontinuation or even delay in dosing seems to result in a “rebound” effect of increased vertebral fractures and even multiple vertebral fractures, especially in those with history of a previous vertebral fracture. This is extremely important in this era of COVID-19, in which patients—especially elderly patients who are perceived to be at the greatest risk—often delay management of chronic disease to limit their potential exposure to the virus. Further, even in normal, nonpandemic times, clinicians need to make patients receiving denosumab aware of the importance of timely administration of doses as scheduled. If such dosing is not possible, then clinicians and patients need to be aware of the potential need for instituting other antiresorptive therapies. In addition, the need to ostensibly continue denosumab therapy for long periods of time and indefinitely may make it a less desirable choice for younger patients.
Continue to: Atypical femur fracture risk and bisphosphonate use...
Atypical femur fracture risk and bisphosphonate use
Black DM, Geiger EJ, Eastell R, et al. Atypical femur fracture risk versus fragility fracture prevention with bisphosphonates. N Engl J Med. 2020;383:743-753.
Since their introduction in the 1990s, bisphosphonates have been the mainstay of osteoporosis treatment. This category of medications inhibits osteoclast-mediated resorption and remodeling of bone. Various large, randomized, controlled trials have established the efficacy of bisphosphonates to increase BMD and decrease the risk of hip and vertebral fracture by as much as 40% to 70%.13
However, case reports of unusual fragility fractures in the subtrochanteric region and along the femoral diaphysis in patients treated with bisphosphonates started to appear approximately 15 years ago.14 Since then, concerns and publicity about these atypical fractures have led to substantial declines in bisphosphonate use clinically.
Bisphosphonate preventive benefits versus atypical fracture risk
Black and colleagues reviewed data on women 50 years and older who were enrolled in the Kaiser Permanente health care system in California. The total cohort included slightly more than 1 million women, of which almost 200,000 (17.9%) used bisphosphonates at any point from 2007–2017.
A total of 277 atypical femur fractures occurred. Among bisphosphonate users, there were 1.74 fractures per 10,000 patient-years. Overall, there were almost 59 fractures per 10,000 person-years. The incidence of atypical fractures was highest in women between the ages of 75 and 84 years, and the incidence diminished after age 85. Rates of atypical fractures increased as the duration of bisphosphonate use increased. In addition, rates of atypical fractures decreased with time since bisphosphonate discontinuation.
The rate of atypical fractures in women who had never received bisphosphonate therapy was 0.1 per 10,000 person-years. The number of fractures prevented for each fracture type far outweighed bisphosphonate-associated atypical fractures at all time points along the 10 years of study. In White women, for instance, at 3 years there were 541 clinical fractures prevented and 149 hip fractures prevented, while 2 bisphosphonate-associated atypical fractures occurred, all per 10,000 women.
Interestingly, in the Asian population at the same time point, 330 clinical fractures were prevented and 91 hip fractures were prevented, but 8 atypical fractures of the femur occurred, per 10,000 women. The authors further referenced an earlier Kaiser study that showed that 49% of 142 atypical femur fractures occurred in Asian patients who comprised only 10% of the study population.15
The authors concluded that the risk of atypical femur fracture increases with longer duration of bisphosphate use and rapidly decreases after bisphosphate discontinuation. Asian women have a higher risk than White women. With bisphosphonate treatment, the absolute risk of atypical femur fracture is very low compared with the reduction in the risk of hip and other fractures.
Many patients and even clinicians have moved away from the use of bisphosphonates to reduce fragility fracture risk because of fears of atypical femur fractures. With bisphosphonate use, the reduction in hip fracture as well as other fractures far overshadows the small but real complication of atypical femur fracture. The Asian population seems to have 4 to 6 times the risk for these atypical femur fractures. Thus, bisphosphonate therapy, especially now that it is available in generic formulations, should remain an important option for appropriate patients.
Continue to: Romosozumab increases BMD gains and improves T-scores...
Romosozumab increases BMD gains and improves T-scores
Cosman F, Lewiecki EM, Ebeling PR, et al. T-score as an indicator of fracture risk during treatment with romosozumab or alendronate in the ARCH trial. J Bone Miner Res. 2020;35:1333-1342
Romosozumab (Evenity) is a monoclonal antibody that binds and inhibits sclerostin, thus having the dual effect of increasing bone formation and decreasing bone resorption.16 It is administered for 1 year as monthly doses of 210 mg subcutaneously. Previous studies have shown that romosozumab produces large increases in lumbar spine and total hip BMD,17 reduces the risk of new vertebral and clinical fractures compared with placebo,16 and reduces the risk of vertebral, clinical, nonvertebral, and hip fractures compared with alendronate over a median treatment period of 33 months (the ARCH study).18
According to the package insert, romosozumab is indicated “for the treatment of osteoporosis in postmenopausal women at high risk for fracture, defined as a history of osteoporotic fracture, or multiple risk factors for fracture; or patients who have failed or are intolerant to other available osteoporosis therapy.”
Should T-score be a therapeutic target?
Cosman and colleagues performed a post hoc analysis of the ARCH trial specifically to evaluate mean BMD and corresponding mean T-score changes (and the relationships between T-scores) after 1 year of romosozumab or alendronate therapy and subsequent fracture incidence. The study is quite detailed with much numerical data and statistical analysis.
Basically, the ARCH trial randomly assigned patients with osteoporosis to receive either monthly subcutaneous romosozumab 210 mg or weekly oral alendronate 70 mg for 12 months. After the double-blind portion of the trial, all patients received open label weekly oral alendronate 70 mg through the end of study (24 months), although they were still blinded to the initial treatment assignment. In addition, patients received daily calcium and vitamin D supplements.
The data analysis found that 1 year of romosozumab led to larger BMD gains than alendronate therapy. Also, the T-score achieved with either therapy was directly related to subsequent fracture risk. The authors thus proposed that these data support the use of the T-score as a therapeutic target for patients with osteoporosis.
It is important to note that in the original ARCH study, the participants’ average age was 71 years and approximately one-third were older than 75. The average T-score was -2.7 at both the lumbar spine and femoral neck. Approximately 20% of patients had a pre-existing vertebral fracture, and approximately 20% had a previous nonvertebral fracture.
The authors of the current study, furthermore, found that mean BMD gains after 1 year of romosozumab treatment were more than twice those seen with alendronate at the total hip, femoral neck, and lumbar spine. These BMD changes resulted in a larger proportion of patients who achieved T-scores above the osteoporosis level at each of the skeletal sites after 1 year of therapy. Fewer fractures occurred during the second year and the entire open label period among patients who had received romosozumab first compared with those who received alendronate.●
Women’s HCPs need to be aware of romosozumab even if they are not the ones primarily to prescribe it. Perhaps familiarity with the drug will allow some clinicians to begin to implement this treatment into their care for elderly patients with osteoporosis, especially those with pre-existing fractures. It may be useful to monitor patients’ total hip T-score while on treatment if osteoporosis treatment goals have been achieved to minimize future fracture risk.
- American Cancer Society. Cancer Facts & Figures 2020. Atlanta, Ga: American Cancer Society; 2020. https://www .cancer.org/content/dam/cancer-org/research/cancer-facts -and-statistics/annual-cancer-facts-and-figures/2020/cancer -facts-and-figures-2020.pdf. Accessed November 17, 2020.
- DowneyC, Kelly M, Quinlan JF. Changing trends in the mortality rate at 1-year post hip fracture—a systematic review. World J Orthop. 2019;10:166-175.
- Goldstein SR. 2019 Update on bone health. OBG Manag. 2019;31(12):16-21.
- Hassan EB, Duque G. Osteosarcopenia: a new geriatric syndrome. Aust Fam Physician. 2017;46:849-853.
- Drey M, Sieber CC, Bertsch T, et al; FiAT Intervention Group. Osteosarcopenia is more than sarcopenia and osteopenia alone. Aging Clin Exp Res. 2016;28:895-899.
- Landi F, Liperoti R, Russo A, et al. Sarcopenia as a risk factor for falls in elderly individuals: results from the ilSIRENTE study. Clin Nutr. 2012;31:652-658.
- Kopperdahl DL, Aspelund T, Hoffmann PF, et al. Assessment of incident spine and hip fractures in women and men using finite element analysis of CT scans. J Bone Miner Res. 2014;29:570-580.
- Cummings SR, San Martin J, McClung MR, et al; FREEDOM Trial. Denosumab for prevention of fractures in postmenopausal women with osteoporosis. N Engl J Med. 2009;361: 756-765.
- Bone HG, Wagman RB, Brandi ML, et al. 10 years of denosumab treatment in postmenopausal women with osteoporosis: results from the phase 3 randomised FREEDOM trial and open-label extension. Lancet Diabetes Endocrinol. 2017;5:513-523.
- Tsourdi E, Langdahl B, Cohen-Solal M, et al. Discontinuation of denosumab therapy for osteoporosis: a systematic review and position statement by ECTS. Bone. 2017;105:11-17.
- Popp AW, Zysset PK, Lippuner K. Rebound-associated vertebral fractures after discontinuation of denosumab—from clinic and biomechanics. Osteoporos Int. 2016;27:1917-1921.
- Cummings SR, Ferrari S, Eastell R, et al. Vertebral fractures after discontinuation of denosumab: a post hoc analysis of the randomized placebo-controlled FREEDOM Trial and its extension. J Bone Miner Res. 2018;33:190-198.
- Eastell R, Rosen CJ, Black DM, et al. Pharmacological management of osteoporosis in postmenopausal women: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2019;104:1595-1622.
- Goh SK, Yang KY, Koh JS, et al. Subtrochanteric insufficiency fractures in patients on alendronate therapy: a caution. J Bone Joint Surg Br. 2007;89:349-353.
- Dell RM, Adams AL, Greene DF, et al. Incidence of atypical nontraumatic diaphyseal fractures of the femur. J Bone Miner Res. 2012;27:2544-2550.
- Cosman F, Crittenden DB, Adachi JD, et al. Romosozumab treatment in postmenopausal women with osteoporosis. N Engl J Med. 2016;375:1532-1543.
- McClung MR, Grauer A, Boonen S, et al. Romosozumab in postmenopausal women with low bone mineral density. N Engl J Med. 2014;370:412-420.
- Saag KG, Petersen J, Brandi ML, et al. Romosozumab or alendronate for fracture prevention in women with osteoporosis. N Engl J Med. 2017;377:1417-1427.
Increasingly, bone health and fragility fracture prevention is one of the most important aspects of healthy aging that we, as women’s health care providers (HCPs), must be sure is part of our thought process in caring for women at midlife and beyond. Virtually all ObGyn HCPs are aware of breast health, both in terms of the clinical breast exam and imaging surveillance. The 5-year relative survival rate for “localized breast cancer” is 99%.1 Most recent data on hip fracture, however, indicate that it is associated with a mortality in the first year of 21%!2 We need to be sure that our patients understand this.
Previously, this column provided an update on osteoporosis. In 2016, I asked to change the focus to “Update on bone health” to highlight that simply relying on dual energy x-ray absorptiometry (DXA) testing of bone mass with arbitrary cutoffs for osteoporosis, osteopenia, and normal bone mass is not adequate for improving overall bone health. The addition of the FRAX fracture risk assessment tool, now widely employed, as well as the trabecular bone score (TBS), not widely employed, helps to refine the assessment of patients’ risk status. Further, issues such as sarcopenia, adequate dietary calcium and vitamin D supplementation, and fall prevention (improving balance, use of nonskid rugs in the bathroom, avoiding black ice when present, having nothing to slip on between the bed and the bathroom in the middle of the night, and so on) also are essential elements of “bone health.”
Finally, I cannot stress enough the importance of developing a good relationship with whatever facility one uses for DXA testing in order to maximize use of the reports and potential limitations. In addition, we should identify a metabolic bone specialist for referral of unusual cases or patients who require medications unlikely to be prescribed by us as ObGyns, and develop some familiarity with therapies that may be utilized.
Osteosarcopenia greatly enhances fall and fracture risk
Sepúlveda-Loyola W, Phu S, Bani Hassan E, et al. The joint occurrence of osteoporosis and sarcopenia (osteosarcopenia): definitions and characteristics. J Am Med Dir Assoc. 2020;21:220-225.
Tokeshi S, Eguchi Y, Suzuki M, et al. Relationship between skeletal muscle mass, bone mineral density, and trabecular bone score in osteoporotic vertebral compression fractures. Asian Spine J. 2020 Sep 3. doi: 10.31616/asj.2020.0045.
Kirk B, Zanker J, Duque G. Osteosarcopenia: epidemiology, diagnosis, and treatment—facts and numbers. J Cachexia Sarcopenia Muscle. 2020;11:609-618.
The topic of sarcopenia as defined by the concurrent presence of low muscle mass, physical performance, and strength has been discussed previously in this Update series.3 Now, osteosarcopenia, defined as the concomitant presence of osteoporosis or osteopenia combined with sarcopenia, seems to be an extremely important gauge of fracture risk, especially now as the population’s longevity has increased dramatically. This new syndrome is associated with higher disability and rates of fracture and falls in older people compared with either entity (the bone component or the sarcopenia component) alone.4,5 In fact, in the 2016 ICD-10-CM, sarcopenia was finally recognized as a disease entity.
Severe sarcopenia is known to increase the risk for falls.6 Furthermore, evidence is increasing of cross talk between muscle and bone.4 The diagnostic criteria of osteopenia and osteoporosis are well established; however, absolute criteria for sarcopenia lack an international consensus.
Continue to: Assess for osteopenia/osteoporosis plus sarcopenia to determine those at greatest fracture risk...
Assess for osteopenia/osteoporosis plus sarcopenia to determine those at greatest fracture risk
Sepúlveda-Loyola and colleagues performed a cross-sectional analysis of 253 participants, of which 77% were women, average age 78, who presented for a “falls and fractures” risk assessment. T-scores were measured by DXA. In addition, the investigators measured components of sarcopenia, including physical performance (evaluated by hand grip strength, gait speed, timed up and go test, and 5-time sit to stand test) and dynamic and static balance. Falls in the previous year were self-reported, with 42% of participants having fallen once and 54%, more than once.
Results. Participants with osteosarcopenia had a statistically significant increased rate of falls of approximately threefold and an increased rate of fractures that was approximately fourfold when compared with osteopenia or osteoporosis alone.
Another important finding was that, despite the links between osteoporosis, fracture, and poor clinical outcomes, the investigators did not find differences in fracture rates in the osteopenic compared with the osteoporotic classifications. Their findings corroborated those of other studies that reported discrepancies in fractures and bone mineral density (BMD), with osteopenic older adults experiencing fracture rates similar to and in some cases greater than those diagnosed with osteoporosis.7
Thus, it appears that the use of T-scores that combine osteopenic and osteoporotic criteria into the osteosarcopenic category may be sufficient to capture individuals at the greatest risk of fracture.
Skeletal muscle mass plays a role in vertebral compression fractures
Tokeshi and colleagues conducted retrospective observational study to investigate the relationships between skeletal muscle mass, BMD, and TBS in individuals with osteoporotic vertebral compression fractures.
They evaluated 142 patients with an average age of 75; of these, 30% had radiographically diagnosed vertebral compression fractures (average age, 79) and 70% had no vertebral compression fractures (average age, 70). Body composition was measured using whole-body DXA; appendicular skeletal muscle mass index was determined as the sum of upper and lower extremities’ lean mass (kg/height in m2 ). TBS was measured using the patented algorithm software on DXA scans for the lumbar vertebrae.
Results. The investigators found that the vertebral compression fracture group was statistically significantly older, had lower femur BMD, and had decreased leg muscle mass. The TBS was not identified as a risk factor.
Certain lifestyle factors add to risk of osteosarcopenia
In an editorial, Kirk and colleagues summarized the epidemiology, diagnosis, and treatment of osteosarcopenia. They concluded that this syndrome can be expected to grow in age-related and disease-related states as a consequence of immunosenescence coinciding with an increase in sedentary lifestyle, obesity, and fat infiltration of muscle and bone.
Increasingly, clinicians should screen for osteosarcopenia via imaging methods (DXA) to quantitate bone mass (as is currently done) and, increasingly, quantify muscle mass. In addition, assessment of muscle strength, easily done by testing grip strength, as well as functional capacity (gait speed), will become increasingly important.
Finally, the authors call for a more comprehensive geriatric assessment that includes medical history and risk factors as well as treatment (including osteoporosis drugs, where indicated), and progressive resistance and balance exercises. Nutritional recommendations, in terms of protein, vitamin D, and calcium, also are necessary. They anticipate that diagnosis and treatment of osteosarcopenia will become part of routine health care in the future.
In the past, our assessment of risk for fragility fracture was based mostly on bone mass measurement by DXA. Scoring systems like the FRAX tool have included other risk factors, such as age, body mass index, previous fracture, family history of hip fracture, smoking, any history of rheumatoid arthritis, use of glucocorticoids, and alcohol consumption. However, sarcopenia is a condition characterized by loss of skeletal muscle mass, strength, and function. While it is a natural part of the aging process, when it is severe and coupled with osteopenia or osteoporosis, it significantly increases the risks of falls as well as fracture. Women’s HCPs should increasingly think about the presence of sarcopenia in their patients, especially those with low bone mass (osteopenia or osteoporosis), particularly when making decisions about initiating pharmaceutical intervention. In addition, recommendations for resistive and balance exercises virtually should be universal.
Continue to: The denosumab discontinuation dilemma...
The denosumab discontinuation dilemma
Lyu H, Yoshida K, Zhao SS, et al. Delayed denosumab injections and fracture risk among patients with osteoporosis: a population-based cohort study. Ann Intern Med. 2020;173:516-526.
Tripto-Shkolnik L, Fund N, Rouach V, et al. Fracture incidence after denosumab discontinuation: real-world data from a large healthcare provider. Bone. 2020;130:115150.
Denosumab, marketed under the brand name Prolia, is a human monoclonal antibody that blocks the binding of RANK ligand and inhibits development and activity of osteoclast, thus decreasing bone resorption and increasing BMD. In the original pivotal clinical trial of denosumab, almost 7,900 women between the ages of 60 and 90 (average age, 73) with osteoporotic T-scores were enrolled.8 The women were randomly assigned to receive 60 mg of denosumab subcutaneously every 6 months or placebo for a total of 3 years. In that trial, the denosumabtreated group, relative to the placebo group, showed a statistically significant decrease in radiographic vertebral fracture, hip fracture, and nonvertebral fracture.
An open-label extension study looked at denosumab use for a total of 10 years.9 That study found that denosumab treatment for up to 10 years was associated with low rates of adverse events, low fracture incidence compared with that observed during the original trial, and continued increases in BMD without plateau. Thus, denosumab appeared to be an extremely safe and effective agent for treating postmenopausal women with osteoporosis.
Denosumab cessation leads to rebound vertebral fractures
As opposed to bisphosphonates, denosumab does not incorporate into bone matrix, and bone turnover is not suppressed after cessation of its use. Reports have implied that denosumab discontinuation may lead to an increased risk of multiple vertebral fractures.10 One theory is that unlike atypical femoral fractures that seem to emerge from failure of microdamage repair in cortical bone with long-term antiresorptive treatment, denosumab rebound–associated vertebral fractures seem to originate from the synergy of rapid bone resorption and accelerated microdamage accumulation in trabecular bone triggered by the discontinuation of this highly potent reversible agent.11
Post hoc analysis of the denosumab placebo-controlled trial and its extension reported that the vertebral fracture rate increased after denosumab discontinuation to the level observed in untreated patients.12 Further, a majority of participants who did sustain vertebral fracture after discontinuing denosumab had multiple vertebral fractures, with the risk being greatest in participants who had a prior vertebral facture. This caused those authors to suggest that patients who discontinued denosumab should rapidly transition to an alternative antiresorptive treatment.
Effect of dose delays, discontinuation on vertebral fracture rate
Lyu and colleagues recently described their population-based cohort study of the United Kingdom’s Health Improvement Network primary care database between 2010 and 2019. They found that delayed administration of a subsequent denosumab dose by more than 16 weeks was associated with an increased risk for vertebral fracture compared with on-time dosing. They noted, however, that the evidence was insufficient to conclude that fracture risk at any other anatomic sites is increased with such a delay.
In a similar study, Tripto-Shkolnik and colleagues examined an Israeli database of 2.3 million members in a state-mandated health organization. They identified osteoporotic patients with at least 2 denosumab prescription dispenses and defined treatment discontinuation as a refill gap of 3 months or more. Fractures were identified by an osteoporosis registry, including fractures that occurred within 1 year from discontinuation in denosumab discontinuers as well as from the second year of treatment forward for persistent users. They identified 1,500 denosumab discontinuers (average age, 72) and 1,610 persistent users (average age also 72). At baseline, the groups were comparable in fracture history, smoking, and bone density.
In the discontinuation group, 0.8% had multiple vertebral fractures versus 0.1% in the persistent users (P = .006); the overall rate of fractures per 100 patient-years of follow-up was 3 times higher in the discontinuation group than in the persistent user group, and the rate of vertebral fractures was almost 5 times higher in the discontinuation group.
Denosumab is an extremely safe and effective treatment for postmenopausal osteoporosis. Discontinuation or even delay in dosing seems to result in a “rebound” effect of increased vertebral fractures and even multiple vertebral fractures, especially in those with history of a previous vertebral fracture. This is extremely important in this era of COVID-19, in which patients—especially elderly patients who are perceived to be at the greatest risk—often delay management of chronic disease to limit their potential exposure to the virus. Further, even in normal, nonpandemic times, clinicians need to make patients receiving denosumab aware of the importance of timely administration of doses as scheduled. If such dosing is not possible, then clinicians and patients need to be aware of the potential need for instituting other antiresorptive therapies. In addition, the need to ostensibly continue denosumab therapy for long periods of time and indefinitely may make it a less desirable choice for younger patients.
Continue to: Atypical femur fracture risk and bisphosphonate use...
Atypical femur fracture risk and bisphosphonate use
Black DM, Geiger EJ, Eastell R, et al. Atypical femur fracture risk versus fragility fracture prevention with bisphosphonates. N Engl J Med. 2020;383:743-753.
Since their introduction in the 1990s, bisphosphonates have been the mainstay of osteoporosis treatment. This category of medications inhibits osteoclast-mediated resorption and remodeling of bone. Various large, randomized, controlled trials have established the efficacy of bisphosphonates to increase BMD and decrease the risk of hip and vertebral fracture by as much as 40% to 70%.13
However, case reports of unusual fragility fractures in the subtrochanteric region and along the femoral diaphysis in patients treated with bisphosphonates started to appear approximately 15 years ago.14 Since then, concerns and publicity about these atypical fractures have led to substantial declines in bisphosphonate use clinically.
Bisphosphonate preventive benefits versus atypical fracture risk
Black and colleagues reviewed data on women 50 years and older who were enrolled in the Kaiser Permanente health care system in California. The total cohort included slightly more than 1 million women, of which almost 200,000 (17.9%) used bisphosphonates at any point from 2007–2017.
A total of 277 atypical femur fractures occurred. Among bisphosphonate users, there were 1.74 fractures per 10,000 patient-years. Overall, there were almost 59 fractures per 10,000 person-years. The incidence of atypical fractures was highest in women between the ages of 75 and 84 years, and the incidence diminished after age 85. Rates of atypical fractures increased as the duration of bisphosphonate use increased. In addition, rates of atypical fractures decreased with time since bisphosphonate discontinuation.
The rate of atypical fractures in women who had never received bisphosphonate therapy was 0.1 per 10,000 person-years. The number of fractures prevented for each fracture type far outweighed bisphosphonate-associated atypical fractures at all time points along the 10 years of study. In White women, for instance, at 3 years there were 541 clinical fractures prevented and 149 hip fractures prevented, while 2 bisphosphonate-associated atypical fractures occurred, all per 10,000 women.
Interestingly, in the Asian population at the same time point, 330 clinical fractures were prevented and 91 hip fractures were prevented, but 8 atypical fractures of the femur occurred, per 10,000 women. The authors further referenced an earlier Kaiser study that showed that 49% of 142 atypical femur fractures occurred in Asian patients who comprised only 10% of the study population.15
The authors concluded that the risk of atypical femur fracture increases with longer duration of bisphosphate use and rapidly decreases after bisphosphate discontinuation. Asian women have a higher risk than White women. With bisphosphonate treatment, the absolute risk of atypical femur fracture is very low compared with the reduction in the risk of hip and other fractures.
Many patients and even clinicians have moved away from the use of bisphosphonates to reduce fragility fracture risk because of fears of atypical femur fractures. With bisphosphonate use, the reduction in hip fracture as well as other fractures far overshadows the small but real complication of atypical femur fracture. The Asian population seems to have 4 to 6 times the risk for these atypical femur fractures. Thus, bisphosphonate therapy, especially now that it is available in generic formulations, should remain an important option for appropriate patients.
Continue to: Romosozumab increases BMD gains and improves T-scores...
Romosozumab increases BMD gains and improves T-scores
Cosman F, Lewiecki EM, Ebeling PR, et al. T-score as an indicator of fracture risk during treatment with romosozumab or alendronate in the ARCH trial. J Bone Miner Res. 2020;35:1333-1342
Romosozumab (Evenity) is a monoclonal antibody that binds and inhibits sclerostin, thus having the dual effect of increasing bone formation and decreasing bone resorption.16 It is administered for 1 year as monthly doses of 210 mg subcutaneously. Previous studies have shown that romosozumab produces large increases in lumbar spine and total hip BMD,17 reduces the risk of new vertebral and clinical fractures compared with placebo,16 and reduces the risk of vertebral, clinical, nonvertebral, and hip fractures compared with alendronate over a median treatment period of 33 months (the ARCH study).18
According to the package insert, romosozumab is indicated “for the treatment of osteoporosis in postmenopausal women at high risk for fracture, defined as a history of osteoporotic fracture, or multiple risk factors for fracture; or patients who have failed or are intolerant to other available osteoporosis therapy.”
Should T-score be a therapeutic target?
Cosman and colleagues performed a post hoc analysis of the ARCH trial specifically to evaluate mean BMD and corresponding mean T-score changes (and the relationships between T-scores) after 1 year of romosozumab or alendronate therapy and subsequent fracture incidence. The study is quite detailed with much numerical data and statistical analysis.
Basically, the ARCH trial randomly assigned patients with osteoporosis to receive either monthly subcutaneous romosozumab 210 mg or weekly oral alendronate 70 mg for 12 months. After the double-blind portion of the trial, all patients received open label weekly oral alendronate 70 mg through the end of study (24 months), although they were still blinded to the initial treatment assignment. In addition, patients received daily calcium and vitamin D supplements.
The data analysis found that 1 year of romosozumab led to larger BMD gains than alendronate therapy. Also, the T-score achieved with either therapy was directly related to subsequent fracture risk. The authors thus proposed that these data support the use of the T-score as a therapeutic target for patients with osteoporosis.
It is important to note that in the original ARCH study, the participants’ average age was 71 years and approximately one-third were older than 75. The average T-score was -2.7 at both the lumbar spine and femoral neck. Approximately 20% of patients had a pre-existing vertebral fracture, and approximately 20% had a previous nonvertebral fracture.
The authors of the current study, furthermore, found that mean BMD gains after 1 year of romosozumab treatment were more than twice those seen with alendronate at the total hip, femoral neck, and lumbar spine. These BMD changes resulted in a larger proportion of patients who achieved T-scores above the osteoporosis level at each of the skeletal sites after 1 year of therapy. Fewer fractures occurred during the second year and the entire open label period among patients who had received romosozumab first compared with those who received alendronate.●
Women’s HCPs need to be aware of romosozumab even if they are not the ones primarily to prescribe it. Perhaps familiarity with the drug will allow some clinicians to begin to implement this treatment into their care for elderly patients with osteoporosis, especially those with pre-existing fractures. It may be useful to monitor patients’ total hip T-score while on treatment if osteoporosis treatment goals have been achieved to minimize future fracture risk.
Increasingly, bone health and fragility fracture prevention is one of the most important aspects of healthy aging that we, as women’s health care providers (HCPs), must be sure is part of our thought process in caring for women at midlife and beyond. Virtually all ObGyn HCPs are aware of breast health, both in terms of the clinical breast exam and imaging surveillance. The 5-year relative survival rate for “localized breast cancer” is 99%.1 Most recent data on hip fracture, however, indicate that it is associated with a mortality in the first year of 21%!2 We need to be sure that our patients understand this.
Previously, this column provided an update on osteoporosis. In 2016, I asked to change the focus to “Update on bone health” to highlight that simply relying on dual energy x-ray absorptiometry (DXA) testing of bone mass with arbitrary cutoffs for osteoporosis, osteopenia, and normal bone mass is not adequate for improving overall bone health. The addition of the FRAX fracture risk assessment tool, now widely employed, as well as the trabecular bone score (TBS), not widely employed, helps to refine the assessment of patients’ risk status. Further, issues such as sarcopenia, adequate dietary calcium and vitamin D supplementation, and fall prevention (improving balance, use of nonskid rugs in the bathroom, avoiding black ice when present, having nothing to slip on between the bed and the bathroom in the middle of the night, and so on) also are essential elements of “bone health.”
Finally, I cannot stress enough the importance of developing a good relationship with whatever facility one uses for DXA testing in order to maximize use of the reports and potential limitations. In addition, we should identify a metabolic bone specialist for referral of unusual cases or patients who require medications unlikely to be prescribed by us as ObGyns, and develop some familiarity with therapies that may be utilized.
Osteosarcopenia greatly enhances fall and fracture risk
Sepúlveda-Loyola W, Phu S, Bani Hassan E, et al. The joint occurrence of osteoporosis and sarcopenia (osteosarcopenia): definitions and characteristics. J Am Med Dir Assoc. 2020;21:220-225.
Tokeshi S, Eguchi Y, Suzuki M, et al. Relationship between skeletal muscle mass, bone mineral density, and trabecular bone score in osteoporotic vertebral compression fractures. Asian Spine J. 2020 Sep 3. doi: 10.31616/asj.2020.0045.
Kirk B, Zanker J, Duque G. Osteosarcopenia: epidemiology, diagnosis, and treatment—facts and numbers. J Cachexia Sarcopenia Muscle. 2020;11:609-618.
The topic of sarcopenia as defined by the concurrent presence of low muscle mass, physical performance, and strength has been discussed previously in this Update series.3 Now, osteosarcopenia, defined as the concomitant presence of osteoporosis or osteopenia combined with sarcopenia, seems to be an extremely important gauge of fracture risk, especially now as the population’s longevity has increased dramatically. This new syndrome is associated with higher disability and rates of fracture and falls in older people compared with either entity (the bone component or the sarcopenia component) alone.4,5 In fact, in the 2016 ICD-10-CM, sarcopenia was finally recognized as a disease entity.
Severe sarcopenia is known to increase the risk for falls.6 Furthermore, evidence is increasing of cross talk between muscle and bone.4 The diagnostic criteria of osteopenia and osteoporosis are well established; however, absolute criteria for sarcopenia lack an international consensus.
Continue to: Assess for osteopenia/osteoporosis plus sarcopenia to determine those at greatest fracture risk...
Assess for osteopenia/osteoporosis plus sarcopenia to determine those at greatest fracture risk
Sepúlveda-Loyola and colleagues performed a cross-sectional analysis of 253 participants, of which 77% were women, average age 78, who presented for a “falls and fractures” risk assessment. T-scores were measured by DXA. In addition, the investigators measured components of sarcopenia, including physical performance (evaluated by hand grip strength, gait speed, timed up and go test, and 5-time sit to stand test) and dynamic and static balance. Falls in the previous year were self-reported, with 42% of participants having fallen once and 54%, more than once.
Results. Participants with osteosarcopenia had a statistically significant increased rate of falls of approximately threefold and an increased rate of fractures that was approximately fourfold when compared with osteopenia or osteoporosis alone.
Another important finding was that, despite the links between osteoporosis, fracture, and poor clinical outcomes, the investigators did not find differences in fracture rates in the osteopenic compared with the osteoporotic classifications. Their findings corroborated those of other studies that reported discrepancies in fractures and bone mineral density (BMD), with osteopenic older adults experiencing fracture rates similar to and in some cases greater than those diagnosed with osteoporosis.7
Thus, it appears that the use of T-scores that combine osteopenic and osteoporotic criteria into the osteosarcopenic category may be sufficient to capture individuals at the greatest risk of fracture.
Skeletal muscle mass plays a role in vertebral compression fractures
Tokeshi and colleagues conducted retrospective observational study to investigate the relationships between skeletal muscle mass, BMD, and TBS in individuals with osteoporotic vertebral compression fractures.
They evaluated 142 patients with an average age of 75; of these, 30% had radiographically diagnosed vertebral compression fractures (average age, 79) and 70% had no vertebral compression fractures (average age, 70). Body composition was measured using whole-body DXA; appendicular skeletal muscle mass index was determined as the sum of upper and lower extremities’ lean mass (kg/height in m2 ). TBS was measured using the patented algorithm software on DXA scans for the lumbar vertebrae.
Results. The investigators found that the vertebral compression fracture group was statistically significantly older, had lower femur BMD, and had decreased leg muscle mass. The TBS was not identified as a risk factor.
Certain lifestyle factors add to risk of osteosarcopenia
In an editorial, Kirk and colleagues summarized the epidemiology, diagnosis, and treatment of osteosarcopenia. They concluded that this syndrome can be expected to grow in age-related and disease-related states as a consequence of immunosenescence coinciding with an increase in sedentary lifestyle, obesity, and fat infiltration of muscle and bone.
Increasingly, clinicians should screen for osteosarcopenia via imaging methods (DXA) to quantitate bone mass (as is currently done) and, increasingly, quantify muscle mass. In addition, assessment of muscle strength, easily done by testing grip strength, as well as functional capacity (gait speed), will become increasingly important.
Finally, the authors call for a more comprehensive geriatric assessment that includes medical history and risk factors as well as treatment (including osteoporosis drugs, where indicated), and progressive resistance and balance exercises. Nutritional recommendations, in terms of protein, vitamin D, and calcium, also are necessary. They anticipate that diagnosis and treatment of osteosarcopenia will become part of routine health care in the future.
In the past, our assessment of risk for fragility fracture was based mostly on bone mass measurement by DXA. Scoring systems like the FRAX tool have included other risk factors, such as age, body mass index, previous fracture, family history of hip fracture, smoking, any history of rheumatoid arthritis, use of glucocorticoids, and alcohol consumption. However, sarcopenia is a condition characterized by loss of skeletal muscle mass, strength, and function. While it is a natural part of the aging process, when it is severe and coupled with osteopenia or osteoporosis, it significantly increases the risks of falls as well as fracture. Women’s HCPs should increasingly think about the presence of sarcopenia in their patients, especially those with low bone mass (osteopenia or osteoporosis), particularly when making decisions about initiating pharmaceutical intervention. In addition, recommendations for resistive and balance exercises virtually should be universal.
Continue to: The denosumab discontinuation dilemma...
The denosumab discontinuation dilemma
Lyu H, Yoshida K, Zhao SS, et al. Delayed denosumab injections and fracture risk among patients with osteoporosis: a population-based cohort study. Ann Intern Med. 2020;173:516-526.
Tripto-Shkolnik L, Fund N, Rouach V, et al. Fracture incidence after denosumab discontinuation: real-world data from a large healthcare provider. Bone. 2020;130:115150.
Denosumab, marketed under the brand name Prolia, is a human monoclonal antibody that blocks the binding of RANK ligand and inhibits development and activity of osteoclast, thus decreasing bone resorption and increasing BMD. In the original pivotal clinical trial of denosumab, almost 7,900 women between the ages of 60 and 90 (average age, 73) with osteoporotic T-scores were enrolled.8 The women were randomly assigned to receive 60 mg of denosumab subcutaneously every 6 months or placebo for a total of 3 years. In that trial, the denosumabtreated group, relative to the placebo group, showed a statistically significant decrease in radiographic vertebral fracture, hip fracture, and nonvertebral fracture.
An open-label extension study looked at denosumab use for a total of 10 years.9 That study found that denosumab treatment for up to 10 years was associated with low rates of adverse events, low fracture incidence compared with that observed during the original trial, and continued increases in BMD without plateau. Thus, denosumab appeared to be an extremely safe and effective agent for treating postmenopausal women with osteoporosis.
Denosumab cessation leads to rebound vertebral fractures
As opposed to bisphosphonates, denosumab does not incorporate into bone matrix, and bone turnover is not suppressed after cessation of its use. Reports have implied that denosumab discontinuation may lead to an increased risk of multiple vertebral fractures.10 One theory is that unlike atypical femoral fractures that seem to emerge from failure of microdamage repair in cortical bone with long-term antiresorptive treatment, denosumab rebound–associated vertebral fractures seem to originate from the synergy of rapid bone resorption and accelerated microdamage accumulation in trabecular bone triggered by the discontinuation of this highly potent reversible agent.11
Post hoc analysis of the denosumab placebo-controlled trial and its extension reported that the vertebral fracture rate increased after denosumab discontinuation to the level observed in untreated patients.12 Further, a majority of participants who did sustain vertebral fracture after discontinuing denosumab had multiple vertebral fractures, with the risk being greatest in participants who had a prior vertebral facture. This caused those authors to suggest that patients who discontinued denosumab should rapidly transition to an alternative antiresorptive treatment.
Effect of dose delays, discontinuation on vertebral fracture rate
Lyu and colleagues recently described their population-based cohort study of the United Kingdom’s Health Improvement Network primary care database between 2010 and 2019. They found that delayed administration of a subsequent denosumab dose by more than 16 weeks was associated with an increased risk for vertebral fracture compared with on-time dosing. They noted, however, that the evidence was insufficient to conclude that fracture risk at any other anatomic sites is increased with such a delay.
In a similar study, Tripto-Shkolnik and colleagues examined an Israeli database of 2.3 million members in a state-mandated health organization. They identified osteoporotic patients with at least 2 denosumab prescription dispenses and defined treatment discontinuation as a refill gap of 3 months or more. Fractures were identified by an osteoporosis registry, including fractures that occurred within 1 year from discontinuation in denosumab discontinuers as well as from the second year of treatment forward for persistent users. They identified 1,500 denosumab discontinuers (average age, 72) and 1,610 persistent users (average age also 72). At baseline, the groups were comparable in fracture history, smoking, and bone density.
In the discontinuation group, 0.8% had multiple vertebral fractures versus 0.1% in the persistent users (P = .006); the overall rate of fractures per 100 patient-years of follow-up was 3 times higher in the discontinuation group than in the persistent user group, and the rate of vertebral fractures was almost 5 times higher in the discontinuation group.
Denosumab is an extremely safe and effective treatment for postmenopausal osteoporosis. Discontinuation or even delay in dosing seems to result in a “rebound” effect of increased vertebral fractures and even multiple vertebral fractures, especially in those with history of a previous vertebral fracture. This is extremely important in this era of COVID-19, in which patients—especially elderly patients who are perceived to be at the greatest risk—often delay management of chronic disease to limit their potential exposure to the virus. Further, even in normal, nonpandemic times, clinicians need to make patients receiving denosumab aware of the importance of timely administration of doses as scheduled. If such dosing is not possible, then clinicians and patients need to be aware of the potential need for instituting other antiresorptive therapies. In addition, the need to ostensibly continue denosumab therapy for long periods of time and indefinitely may make it a less desirable choice for younger patients.
Continue to: Atypical femur fracture risk and bisphosphonate use...
Atypical femur fracture risk and bisphosphonate use
Black DM, Geiger EJ, Eastell R, et al. Atypical femur fracture risk versus fragility fracture prevention with bisphosphonates. N Engl J Med. 2020;383:743-753.
Since their introduction in the 1990s, bisphosphonates have been the mainstay of osteoporosis treatment. This category of medications inhibits osteoclast-mediated resorption and remodeling of bone. Various large, randomized, controlled trials have established the efficacy of bisphosphonates to increase BMD and decrease the risk of hip and vertebral fracture by as much as 40% to 70%.13
However, case reports of unusual fragility fractures in the subtrochanteric region and along the femoral diaphysis in patients treated with bisphosphonates started to appear approximately 15 years ago.14 Since then, concerns and publicity about these atypical fractures have led to substantial declines in bisphosphonate use clinically.
Bisphosphonate preventive benefits versus atypical fracture risk
Black and colleagues reviewed data on women 50 years and older who were enrolled in the Kaiser Permanente health care system in California. The total cohort included slightly more than 1 million women, of which almost 200,000 (17.9%) used bisphosphonates at any point from 2007–2017.
A total of 277 atypical femur fractures occurred. Among bisphosphonate users, there were 1.74 fractures per 10,000 patient-years. Overall, there were almost 59 fractures per 10,000 person-years. The incidence of atypical fractures was highest in women between the ages of 75 and 84 years, and the incidence diminished after age 85. Rates of atypical fractures increased as the duration of bisphosphonate use increased. In addition, rates of atypical fractures decreased with time since bisphosphonate discontinuation.
The rate of atypical fractures in women who had never received bisphosphonate therapy was 0.1 per 10,000 person-years. The number of fractures prevented for each fracture type far outweighed bisphosphonate-associated atypical fractures at all time points along the 10 years of study. In White women, for instance, at 3 years there were 541 clinical fractures prevented and 149 hip fractures prevented, while 2 bisphosphonate-associated atypical fractures occurred, all per 10,000 women.
Interestingly, in the Asian population at the same time point, 330 clinical fractures were prevented and 91 hip fractures were prevented, but 8 atypical fractures of the femur occurred, per 10,000 women. The authors further referenced an earlier Kaiser study that showed that 49% of 142 atypical femur fractures occurred in Asian patients who comprised only 10% of the study population.15
The authors concluded that the risk of atypical femur fracture increases with longer duration of bisphosphate use and rapidly decreases after bisphosphate discontinuation. Asian women have a higher risk than White women. With bisphosphonate treatment, the absolute risk of atypical femur fracture is very low compared with the reduction in the risk of hip and other fractures.
Many patients and even clinicians have moved away from the use of bisphosphonates to reduce fragility fracture risk because of fears of atypical femur fractures. With bisphosphonate use, the reduction in hip fracture as well as other fractures far overshadows the small but real complication of atypical femur fracture. The Asian population seems to have 4 to 6 times the risk for these atypical femur fractures. Thus, bisphosphonate therapy, especially now that it is available in generic formulations, should remain an important option for appropriate patients.
Continue to: Romosozumab increases BMD gains and improves T-scores...
Romosozumab increases BMD gains and improves T-scores
Cosman F, Lewiecki EM, Ebeling PR, et al. T-score as an indicator of fracture risk during treatment with romosozumab or alendronate in the ARCH trial. J Bone Miner Res. 2020;35:1333-1342
Romosozumab (Evenity) is a monoclonal antibody that binds and inhibits sclerostin, thus having the dual effect of increasing bone formation and decreasing bone resorption.16 It is administered for 1 year as monthly doses of 210 mg subcutaneously. Previous studies have shown that romosozumab produces large increases in lumbar spine and total hip BMD,17 reduces the risk of new vertebral and clinical fractures compared with placebo,16 and reduces the risk of vertebral, clinical, nonvertebral, and hip fractures compared with alendronate over a median treatment period of 33 months (the ARCH study).18
According to the package insert, romosozumab is indicated “for the treatment of osteoporosis in postmenopausal women at high risk for fracture, defined as a history of osteoporotic fracture, or multiple risk factors for fracture; or patients who have failed or are intolerant to other available osteoporosis therapy.”
Should T-score be a therapeutic target?
Cosman and colleagues performed a post hoc analysis of the ARCH trial specifically to evaluate mean BMD and corresponding mean T-score changes (and the relationships between T-scores) after 1 year of romosozumab or alendronate therapy and subsequent fracture incidence. The study is quite detailed with much numerical data and statistical analysis.
Basically, the ARCH trial randomly assigned patients with osteoporosis to receive either monthly subcutaneous romosozumab 210 mg or weekly oral alendronate 70 mg for 12 months. After the double-blind portion of the trial, all patients received open label weekly oral alendronate 70 mg through the end of study (24 months), although they were still blinded to the initial treatment assignment. In addition, patients received daily calcium and vitamin D supplements.
The data analysis found that 1 year of romosozumab led to larger BMD gains than alendronate therapy. Also, the T-score achieved with either therapy was directly related to subsequent fracture risk. The authors thus proposed that these data support the use of the T-score as a therapeutic target for patients with osteoporosis.
It is important to note that in the original ARCH study, the participants’ average age was 71 years and approximately one-third were older than 75. The average T-score was -2.7 at both the lumbar spine and femoral neck. Approximately 20% of patients had a pre-existing vertebral fracture, and approximately 20% had a previous nonvertebral fracture.
The authors of the current study, furthermore, found that mean BMD gains after 1 year of romosozumab treatment were more than twice those seen with alendronate at the total hip, femoral neck, and lumbar spine. These BMD changes resulted in a larger proportion of patients who achieved T-scores above the osteoporosis level at each of the skeletal sites after 1 year of therapy. Fewer fractures occurred during the second year and the entire open label period among patients who had received romosozumab first compared with those who received alendronate.●
Women’s HCPs need to be aware of romosozumab even if they are not the ones primarily to prescribe it. Perhaps familiarity with the drug will allow some clinicians to begin to implement this treatment into their care for elderly patients with osteoporosis, especially those with pre-existing fractures. It may be useful to monitor patients’ total hip T-score while on treatment if osteoporosis treatment goals have been achieved to minimize future fracture risk.
- American Cancer Society. Cancer Facts & Figures 2020. Atlanta, Ga: American Cancer Society; 2020. https://www .cancer.org/content/dam/cancer-org/research/cancer-facts -and-statistics/annual-cancer-facts-and-figures/2020/cancer -facts-and-figures-2020.pdf. Accessed November 17, 2020.
- DowneyC, Kelly M, Quinlan JF. Changing trends in the mortality rate at 1-year post hip fracture—a systematic review. World J Orthop. 2019;10:166-175.
- Goldstein SR. 2019 Update on bone health. OBG Manag. 2019;31(12):16-21.
- Hassan EB, Duque G. Osteosarcopenia: a new geriatric syndrome. Aust Fam Physician. 2017;46:849-853.
- Drey M, Sieber CC, Bertsch T, et al; FiAT Intervention Group. Osteosarcopenia is more than sarcopenia and osteopenia alone. Aging Clin Exp Res. 2016;28:895-899.
- Landi F, Liperoti R, Russo A, et al. Sarcopenia as a risk factor for falls in elderly individuals: results from the ilSIRENTE study. Clin Nutr. 2012;31:652-658.
- Kopperdahl DL, Aspelund T, Hoffmann PF, et al. Assessment of incident spine and hip fractures in women and men using finite element analysis of CT scans. J Bone Miner Res. 2014;29:570-580.
- Cummings SR, San Martin J, McClung MR, et al; FREEDOM Trial. Denosumab for prevention of fractures in postmenopausal women with osteoporosis. N Engl J Med. 2009;361: 756-765.
- Bone HG, Wagman RB, Brandi ML, et al. 10 years of denosumab treatment in postmenopausal women with osteoporosis: results from the phase 3 randomised FREEDOM trial and open-label extension. Lancet Diabetes Endocrinol. 2017;5:513-523.
- Tsourdi E, Langdahl B, Cohen-Solal M, et al. Discontinuation of denosumab therapy for osteoporosis: a systematic review and position statement by ECTS. Bone. 2017;105:11-17.
- Popp AW, Zysset PK, Lippuner K. Rebound-associated vertebral fractures after discontinuation of denosumab—from clinic and biomechanics. Osteoporos Int. 2016;27:1917-1921.
- Cummings SR, Ferrari S, Eastell R, et al. Vertebral fractures after discontinuation of denosumab: a post hoc analysis of the randomized placebo-controlled FREEDOM Trial and its extension. J Bone Miner Res. 2018;33:190-198.
- Eastell R, Rosen CJ, Black DM, et al. Pharmacological management of osteoporosis in postmenopausal women: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2019;104:1595-1622.
- Goh SK, Yang KY, Koh JS, et al. Subtrochanteric insufficiency fractures in patients on alendronate therapy: a caution. J Bone Joint Surg Br. 2007;89:349-353.
- Dell RM, Adams AL, Greene DF, et al. Incidence of atypical nontraumatic diaphyseal fractures of the femur. J Bone Miner Res. 2012;27:2544-2550.
- Cosman F, Crittenden DB, Adachi JD, et al. Romosozumab treatment in postmenopausal women with osteoporosis. N Engl J Med. 2016;375:1532-1543.
- McClung MR, Grauer A, Boonen S, et al. Romosozumab in postmenopausal women with low bone mineral density. N Engl J Med. 2014;370:412-420.
- Saag KG, Petersen J, Brandi ML, et al. Romosozumab or alendronate for fracture prevention in women with osteoporosis. N Engl J Med. 2017;377:1417-1427.
- American Cancer Society. Cancer Facts & Figures 2020. Atlanta, Ga: American Cancer Society; 2020. https://www .cancer.org/content/dam/cancer-org/research/cancer-facts -and-statistics/annual-cancer-facts-and-figures/2020/cancer -facts-and-figures-2020.pdf. Accessed November 17, 2020.
- DowneyC, Kelly M, Quinlan JF. Changing trends in the mortality rate at 1-year post hip fracture—a systematic review. World J Orthop. 2019;10:166-175.
- Goldstein SR. 2019 Update on bone health. OBG Manag. 2019;31(12):16-21.
- Hassan EB, Duque G. Osteosarcopenia: a new geriatric syndrome. Aust Fam Physician. 2017;46:849-853.
- Drey M, Sieber CC, Bertsch T, et al; FiAT Intervention Group. Osteosarcopenia is more than sarcopenia and osteopenia alone. Aging Clin Exp Res. 2016;28:895-899.
- Landi F, Liperoti R, Russo A, et al. Sarcopenia as a risk factor for falls in elderly individuals: results from the ilSIRENTE study. Clin Nutr. 2012;31:652-658.
- Kopperdahl DL, Aspelund T, Hoffmann PF, et al. Assessment of incident spine and hip fractures in women and men using finite element analysis of CT scans. J Bone Miner Res. 2014;29:570-580.
- Cummings SR, San Martin J, McClung MR, et al; FREEDOM Trial. Denosumab for prevention of fractures in postmenopausal women with osteoporosis. N Engl J Med. 2009;361: 756-765.
- Bone HG, Wagman RB, Brandi ML, et al. 10 years of denosumab treatment in postmenopausal women with osteoporosis: results from the phase 3 randomised FREEDOM trial and open-label extension. Lancet Diabetes Endocrinol. 2017;5:513-523.
- Tsourdi E, Langdahl B, Cohen-Solal M, et al. Discontinuation of denosumab therapy for osteoporosis: a systematic review and position statement by ECTS. Bone. 2017;105:11-17.
- Popp AW, Zysset PK, Lippuner K. Rebound-associated vertebral fractures after discontinuation of denosumab—from clinic and biomechanics. Osteoporos Int. 2016;27:1917-1921.
- Cummings SR, Ferrari S, Eastell R, et al. Vertebral fractures after discontinuation of denosumab: a post hoc analysis of the randomized placebo-controlled FREEDOM Trial and its extension. J Bone Miner Res. 2018;33:190-198.
- Eastell R, Rosen CJ, Black DM, et al. Pharmacological management of osteoporosis in postmenopausal women: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2019;104:1595-1622.
- Goh SK, Yang KY, Koh JS, et al. Subtrochanteric insufficiency fractures in patients on alendronate therapy: a caution. J Bone Joint Surg Br. 2007;89:349-353.
- Dell RM, Adams AL, Greene DF, et al. Incidence of atypical nontraumatic diaphyseal fractures of the femur. J Bone Miner Res. 2012;27:2544-2550.
- Cosman F, Crittenden DB, Adachi JD, et al. Romosozumab treatment in postmenopausal women with osteoporosis. N Engl J Med. 2016;375:1532-1543.
- McClung MR, Grauer A, Boonen S, et al. Romosozumab in postmenopausal women with low bone mineral density. N Engl J Med. 2014;370:412-420.
- Saag KG, Petersen J, Brandi ML, et al. Romosozumab or alendronate for fracture prevention in women with osteoporosis. N Engl J Med. 2017;377:1417-1427.
Facing systemic racism in health care: Inequities in medical education
Finding inspiration among life’s challenges
Barbara Levy, MD: I am fortunate to have met Pierre serendipitously at a training that we were both attending and was impressed by Dr. Johnson’s life story, his passion and commitment, and his dedication—not only to his personal career but also to raising up other young men of color by trying to break down barriers that face them. His life story highlights those areas of systemic and structural problems that all of us together need to address if we are going to make any progress.
Pierre Johnson, MD: Thank you, Barbara. A little about myself: I am a board-certified ObGyn, and I specialize in minimally invasive surgery. I was born on the South side of Chicago, experiencing gang violence, drugs, and substandard, underserved schools. Long story short, I had a very rough upbringing. I had a single mom and several different issues at home. I am the oldest of 5 siblings, and life was tough.
But I knew that I wanted to do something different with my life. I saw that there was a need in my community as far as health care was concerned, in particular women’s health and childbirth. I knew early on that I wanted to be an ObGyn, and the reason had a lot to do with The Cosby Show. It was the only example of a positive, successful Black man that I saw. No one graduated from college in my family. There weren’t any models of young Black excellence around me. Saying that I wanted to be a doctor planted a seed. I was 9 when my mom became pregnant with my first sibling, and it was fascinating to me. The physiology of pregnancy, and eventually childbirth, was extremely fascinating to me; it set me off on my journey to be an ObGyn.
As I got older, things didn’t get any easier. I went to high school in one of the toughest areas on the South side of Chicago. Gang violence, and violence in and of itself, were all around me, but I was able to stay focused. I went on to Xavier University in Louisiana.
Dr. Levy: There are some important things that I learned from your book and from talking to you at our first meeting. Your mom’s ObGyn, when she was pregnant with your next youngest sibling, was also a Black ObGyn. He took some time to take you under wing?
Dr. Johnson: He did. My mom’s ObGyn was a Black man. Other than The Cosby Show, that’s the only time I saw something like that. When I spoke to him, he really took the time to answer my questions and show me that he was like me; he wasn’t just a far-off mythical person, or something that I could not obtain.
Continue to: Seeing is believing when it comes to success...
Seeing is believing when it comes to success
Dr. Levy: Do you think it was important to have a role model who wasn’t a sports star?
Dr. Johnson: If you can’t see it, you can’t achieve it. He took his time to really talk to me, and it’s the little things for kids that go a long way in their life experience. I still have a relationship with him to this day. How he handled me as a kid made me realize that this is something that I could do. That was extremely important for me.
Dr. Levy: One of the structural things I think we need to point out is that the ability to see yourself as someone successful is critical. When we see 1,000 images a day and they are all White, and they are all so different from where we are that it gets incorporated into our sense of being. I think that’s really difficult for those of us of with privilege to understand what that privilege is.
Dr. Johnson: Absolutely, and I’ll even go further. In residency, 2 White females were my classmates, and both of their parents were doctors. They had grandparents who were doctors. My mom was addicted to drugs; my father was not around. They had been talking medicine since they were 5. You have to make things equitable, but in medicine it’s really not equitable. In medicine, what we don’t realize is that there is an importance for all aspects of someone’s upbringing and environment, and it’s not just what they can regurgitate on a standardized test. If a patient can’t relate to you and tell you what is wrong with them, how can you adequately treat them?
Dr. Levy: Even if they are trying to tell me, but I can’t hear it because I don’t have the language and I don’t have the background. There are really good data to show, in fact, that Black male physicians do a better job at engaging Black men to manage their hypertension.1 When we look at the inequities in birth outcomes for women of color, indigenous women and Black women, there’s evidence that providers who come from a similar background do a better job.
Dr. Johnson: There was the study of Black infants that just came out about them dying at a 3-time higher rate in non-Black physicians’ hands.2 These things need to be recognized. They need to be discussed, and they need to be identified as issues and then, realistically, we need to start talking about solutions, not get offended by what actual statistics are saying.
Foundational inequities in education
Dr. Levy: To address some of the barriers that you faced: I know that you went to a high school that was not geared toward pushing students into professional careers. Your colleagues, however, had educations that prepared them for the standardized tests and other things that they would face academically.
Dr. Johnson: People think I am kidding when I say it, but when I went into college, I didn’t know what a periodic table was. I saw it, but I had no idea what these things meant. I didn’t have any sciences or any AP classes in high school. I did well, but grades are smoke and mirrors. The true test of medicine comes with testing. From the MCATs to the boards, every step of the way there is a standardized test.
Knowledge is something that you can obtain, but test taking is a cultivated skill that happens from a very early age. Trying to teach an adult or someone in their late teens a skill that they should have learned as a kid is difficult. For me, I did not have that, so I had to program myself. I had to learn how to fundamentally take tests as an adult, where most people understand how to do that going into college and professional school.
Dr. Levy: I was impressed with your resilience. I think all of us as human beings, if we fail a test, we take it personally and think it’s about our lack of knowledge. One of the insights that you came to was that failure on those things was not that you didn’t study hard enough. In fact, you probably studied 4 times harder than most other people. You had the knowledge. Being able to get that knowledge into a standardized structured test score was the huge challenge for you.
Dr. Johnson: That’s it. I can remember taking the MCAT, and if you looked at the step 1 book, I could regurgitate to you everything on that page. However, it’s not a test about do you know it or not. It’s an understanding of the English language and how to break things down to make things fit into particular scenarios.
Continue to: A college experience focused on growth and exposure...
A college experience focused on growth and exposure
Dr. Levy: I was impressed by the distinction between your experience at Xavier University where there was a lot of support and guidance and help in your premed program, and what happened to you when you hit medical school.
Dr. Johnson: Xavier University in Louisiana is the number 1 institution in the country for getting minorities into professional school. They understand that they have kids that are brilliant but underprepared, and just have not had the background to actually tackle some of these tough curriculums. I always had good grades in school. But by not being challenged, I didn’t know what I didn’t really know. So now that I was seeing biology, chemistry for the first time, and trying to tackle it; there’s a failure point. I didn’t know how to take tests, and I didn’t know how to study properly. The harder I tried, the worse things got for me.
Xavier has seen that story a multitude of times. If I went to a bigger or predominantly White university, a counselor would have told me, “Well, medicine’s maybe not for you. You can’t handle a premed curriculum.” Instead, I said, “Listen, I’m studying. I’m doing all of these things, and I’m not hacking it.” And they broke it down: “Let’s get you into study groups with kids that have had these type of AP classes before. We’ll have you watch how they study,” and everything started to click. That facilitation of how to adjust to this curriculum was a godsend. It’s the only reason I’m here. I am a prime example of being brilliant enough to be able to do it, but needing the infrastructure and a system set up.
Dr. Levy: There’s a great book by Carol Dweck called Mindset that talks about education of young kids and putting them into silos so early in life; the brilliant kids go into the AP courses and the rest are labeled as inadequate. It’s assumed in a fixed mindset based on their heredity and IQ, and not based on the fact that they have not been exposed to the right things.
Xavier was growing you into the man who could, in fact, do all of those things. I think that is one of the systemic and structural issues that we have—that fixed mindset that frames a kid who is not succeeding as therefore unable to succeed, as opposed to framing that child as not having the correct tools.
New tribulations of medical school
Dr. Johnson: Absolutely. I think what Xavier did for me is to at least let me understand what I needed to do, how to comprehend and retain information, which I never had been exposed to before. Those years were very important to establishing a foundation. When going to medical school, it was like, “There’s no more excuses. What could be the problem now?” Well, now let’s talk about taking tests—a whole different skill. Xavier focused on getting me to understand how to structure my thought process and knowledge base. In medical school I had to apply those skills (because if you can’t apply them, there’s no fit).
My second through fourth year of medical school, I was the only African-American kid in my class. I was spending 20-hour days sometimes just studying, trying to overcompensate by knowing as much as I possibly could and thinking that would propel me from the test-taking standpoint. Even though I didn’t have a lot of classmates in medical school that looked like me, I did have mentors that looked similarly, who really saw potential in me. Dr. Frederick Horvath, a nephrologist in Peoria said, “What are you doing? I want you to get out of these books, and let’s go out to lunch.”
He ended up buying me some instrumental books, really talked to me, listening to my background and understanding how driven I was as a person. He took me under his wing for the rest of medical school and said, “This is how you navigate through these spaces. Yes, you need to have a fund of knowledge to be able to take these tests, but you need to start understanding how to apply it to these questions.” I’m forever grateful to Dr. Horvath for doing that because it was a point in time where I was lost and struggling.
Continue to: Hitting a stride but facing racism head-on...
Hitting a stride but facing racism head-on
Dr. Levy: You talk about the systemic and pervasive racism that was on the wards when you hit them in fourth year. If you don’t mind sharing just a little bit of that, it would help people reading this to have a better understanding of the kinds of barriers that are out there.
Dr. Johnson: Even when I talk about it today, it bothers me.
I went to medical school in Peoria, Illinois, not far from the home of the Ku Klux Klan. At that time, once you got out of Chicago it was a very brutal place, with systemic racism throughout. I was a young Black kid going through a process that not many young Black kids from the South side of Chicago go through, and you had people who had never seen anyone like me. When I was going through my clinical rotations, I knew what I was up against. I was dressed “to the T” every day, arriving early, leaving late, trying to answer questions. I would look at the evaluations, and they would be disparaging. I would look at my counterparts, how their evaluations were, and how people would respond to them, and it would be completely different.
Surgery was the part of ObGyn that I really grew to love more than anything, even more than obstetrics. When general surgery came, I wanted to take it very seriously and learn as much as I possibly could. From the beginning, I knew there was a problem because the chief resident, an older White man, wouldn’t look me in the eye or talk to me. He would make disparaging remarks. The thing that stuck out in my mind the most was when I was in the operating room transporting patients, just like a medical student did, and he came up behind me and said, “You know, Pierre, this is where a small mind and a strong back come into play.” For me, it took me to a place where I had to corral my emotions and thoughts because I just wanted to lash out and just tell him how racist and horrible that was for him to say that to me. I explained this to the powers that be, the director of the department, and they basically blew it off to the side.
When it came down to the end of the evaluation period, I passed with flying colors. But they gave me an incomplete because of that chief resident and his remarks on my evaluations. He had 3 pages of report about me as a person and as a student. He said that he had difficulty in expressing his opinions about me because of possible cultural biases that he may have had. He put “cultural biases” in an evaluation, and they looked at that and said that was enough for me to have to remediate my time. I was required to do an extra month in Pontiac, Illinois, which is even more rural than Peoria, because of a racist person that did not give me a fair opportunity because I was Black.
Like everything else in life, it was a learning experience. It’s why I fight so hard today. It’s why I’m so passionate about equity, not only in medicine but also in all aspects of society. It shows why we have police brutality and Black men dying in the streets. It shows how this happens because there are cultural and implicit biases that play out in every part of life, and we are not honest about it. Until we are honest about it and until we say that this is happening and there is something that needs to be done to address it, it’s going to continue to happen. That is my fight.
Exposing the unspoken power struggle
Dr. Levy: I couldn’t agree more. Attributing things like that to the individual, where you talk about a White man in power and a power structure that didn’t literally physically beat you but did beat you into submission. You talk about how to succeed in medical school, and how you had to suck it up and submit to something that was incredibly unfair. You understood, you were old enough, mature enough, to understand that if you fought back, you were going to lose. The only opportunity you had was to submit to that inequity and push forward.
Dr. Johnson: When I did try to fight, the chair of the department told me that either I accept the consequences or I would not graduate from medical school and be forced to do another year. That struck a chord with me. I think that happens a lot in our society, and it needs to be exposed.
Past experiences reflected in today’s society
Dr. Levy: Can you talk about what you faced in your ObGyn residency in terms of the systemic pushback, people not taking your orders, people questioning you. I know that I have heard that a great deal, and I experienced that myself as a woman.
Dr. Johnson: We look at the things that are happening now, everything from George Floyd’s murder to Colin Kaepernick taking a knee. These things are 10 years past when I first started residency. The year before I started residency, there was a noose hanging on the capitol lawn of Springfield, Illinois’ capital city. There’s systemic racism and hatred there. When I first started on the wards of my first year of ObGyn, again, I was the very first Black resident of my program’s history. Nobody could relate to me.
I went from a year-long general surgery internship at Washington Hospital Center in Washington, DC, to ObGyn residency. In the first 2 months, there were complaints of, “He’s not answering his pages. He’s not being prompt.” I went to my program director and said, “Listen, I have never had one complaint like this. There’s a problem here. And there’s a problem when I’m on the floor: When trying to give orders to nurses, they’re not taking them. I had to tell a couple of nurses, ‘I’m Dr. Johnson. Don’t call me by my first name, especially not in front of patients.’”
My director was just not hearing me, because the entire scenario was something they had never been exposed to. Systemic racism is real, and unless you experience it, it’s very difficult to accept that it is happening. But biases happen when you are not cognizant. People are used to things a certain way. Things play out in the media that make your mind think a certain way, and you don’t even realize it. You may not even want to be that way.
Continue to: Unconscious bias is a barrier to ensuring equity...
Unconscious bias is a barrier to ensuring equity
Dr. Levy: One very important point you just made is that we as the system need to be able to recognize those unconscious things, the language that we use, the disparaging remarks, the things that put people down, as well as the things that keep people out of promotion.
There are some interesting data about both race and gender and the language that we use when we write recommendations for people, that we do things unconsciously. The big message to all of us at the end is to open our minds to where those things can occur. For myself, professionally, I keep a list of words that I use when I write recommendations. I measure myself to ensure that I am using the same language for men and women, for Black and White. I think we need to overcome the system and the structure to create real equity—not equality but equity.
It begins with being real about the issues
Dr. Johnson: It’s a bigger problem than the existence of bias and racism. I think these are systemic issues that have been cultivated over centuries that have never been addressed. The true issue is that we deny that these are problems and refuse to talk about it because it makes us uncomfortable. To truly make things more equitable, we have to push our levels of comfort to be able to talk about things in a healthy manner, be open and transparent, and to start to understand how we are thinking about certain things. When you can see it, you can start to implement changes and start to change mentalities and thought processes.
For me, people say, “You don’t look like a doctor.” I get that all the time—because I have tattoos and earrings. I wear my hair in a mohawk. The image of what success looks like has been manifested through our media and culture, and it has imprinted on our minds as to how things are supposed to be. If someone doesn’t fit those molds, we start to shun them out, or we start to exhibit biases against those things. What I am trying to do is change that thought process of what a successful or a professional person looks like. It doesn’t have a look. It is not a White or Black thing. It’s an intellect, a mindset, a way of living. You have to treat every person as an individual and take all the biases out of it and understand where they are coming from and what they have to offer to the profession.
Dr. Levy: I personally was so impressed by you when I met you. I was impressed by the tattoos and the earrings, and my initial response to them was exactly that biased, “Oh, who is this person?” I checked that at the door, listened to you, and was really impressed at your surgical skill, your knowledge, your background. I am really grateful that you have been willing to spend the time to share that with everyone.
Dr. Johnson: Thank you for this discussion.
To watch the full interview between Drs. Levy and Johnson, visit: https://www.mdedge.com/obgyn/article/228507/facing-systemic-racism-health-care-inequities-medical-education. ●
- The Pulse of Perseverance:
Three Black Doctors on Their Journey to Success Pierre Johnson, MD; Maxime Madhere, MD; and Joseph Semien Jr, MD - Mindset:
The New Psychology of Success
Carol S. Dweck
- Benkert R, Peters R, Tate N, et al. Trust of nurse practitioners and physicians among African Americans with hypertension. J Am Acad Nurse Pract. 2008;20:273-280.
- Greenwood BN, Hardeman RR, Huang L, et al. Physician– patient racial concordance and disparities in birthing mortality for newborns. Proc Natl Acad Sci U S A. 2020; 117:21194-21200.
Finding inspiration among life’s challenges
Barbara Levy, MD: I am fortunate to have met Pierre serendipitously at a training that we were both attending and was impressed by Dr. Johnson’s life story, his passion and commitment, and his dedication—not only to his personal career but also to raising up other young men of color by trying to break down barriers that face them. His life story highlights those areas of systemic and structural problems that all of us together need to address if we are going to make any progress.
Pierre Johnson, MD: Thank you, Barbara. A little about myself: I am a board-certified ObGyn, and I specialize in minimally invasive surgery. I was born on the South side of Chicago, experiencing gang violence, drugs, and substandard, underserved schools. Long story short, I had a very rough upbringing. I had a single mom and several different issues at home. I am the oldest of 5 siblings, and life was tough.
But I knew that I wanted to do something different with my life. I saw that there was a need in my community as far as health care was concerned, in particular women’s health and childbirth. I knew early on that I wanted to be an ObGyn, and the reason had a lot to do with The Cosby Show. It was the only example of a positive, successful Black man that I saw. No one graduated from college in my family. There weren’t any models of young Black excellence around me. Saying that I wanted to be a doctor planted a seed. I was 9 when my mom became pregnant with my first sibling, and it was fascinating to me. The physiology of pregnancy, and eventually childbirth, was extremely fascinating to me; it set me off on my journey to be an ObGyn.
As I got older, things didn’t get any easier. I went to high school in one of the toughest areas on the South side of Chicago. Gang violence, and violence in and of itself, were all around me, but I was able to stay focused. I went on to Xavier University in Louisiana.
Dr. Levy: There are some important things that I learned from your book and from talking to you at our first meeting. Your mom’s ObGyn, when she was pregnant with your next youngest sibling, was also a Black ObGyn. He took some time to take you under wing?
Dr. Johnson: He did. My mom’s ObGyn was a Black man. Other than The Cosby Show, that’s the only time I saw something like that. When I spoke to him, he really took the time to answer my questions and show me that he was like me; he wasn’t just a far-off mythical person, or something that I could not obtain.
Continue to: Seeing is believing when it comes to success...
Seeing is believing when it comes to success
Dr. Levy: Do you think it was important to have a role model who wasn’t a sports star?
Dr. Johnson: If you can’t see it, you can’t achieve it. He took his time to really talk to me, and it’s the little things for kids that go a long way in their life experience. I still have a relationship with him to this day. How he handled me as a kid made me realize that this is something that I could do. That was extremely important for me.
Dr. Levy: One of the structural things I think we need to point out is that the ability to see yourself as someone successful is critical. When we see 1,000 images a day and they are all White, and they are all so different from where we are that it gets incorporated into our sense of being. I think that’s really difficult for those of us of with privilege to understand what that privilege is.
Dr. Johnson: Absolutely, and I’ll even go further. In residency, 2 White females were my classmates, and both of their parents were doctors. They had grandparents who were doctors. My mom was addicted to drugs; my father was not around. They had been talking medicine since they were 5. You have to make things equitable, but in medicine it’s really not equitable. In medicine, what we don’t realize is that there is an importance for all aspects of someone’s upbringing and environment, and it’s not just what they can regurgitate on a standardized test. If a patient can’t relate to you and tell you what is wrong with them, how can you adequately treat them?
Dr. Levy: Even if they are trying to tell me, but I can’t hear it because I don’t have the language and I don’t have the background. There are really good data to show, in fact, that Black male physicians do a better job at engaging Black men to manage their hypertension.1 When we look at the inequities in birth outcomes for women of color, indigenous women and Black women, there’s evidence that providers who come from a similar background do a better job.
Dr. Johnson: There was the study of Black infants that just came out about them dying at a 3-time higher rate in non-Black physicians’ hands.2 These things need to be recognized. They need to be discussed, and they need to be identified as issues and then, realistically, we need to start talking about solutions, not get offended by what actual statistics are saying.
Foundational inequities in education
Dr. Levy: To address some of the barriers that you faced: I know that you went to a high school that was not geared toward pushing students into professional careers. Your colleagues, however, had educations that prepared them for the standardized tests and other things that they would face academically.
Dr. Johnson: People think I am kidding when I say it, but when I went into college, I didn’t know what a periodic table was. I saw it, but I had no idea what these things meant. I didn’t have any sciences or any AP classes in high school. I did well, but grades are smoke and mirrors. The true test of medicine comes with testing. From the MCATs to the boards, every step of the way there is a standardized test.
Knowledge is something that you can obtain, but test taking is a cultivated skill that happens from a very early age. Trying to teach an adult or someone in their late teens a skill that they should have learned as a kid is difficult. For me, I did not have that, so I had to program myself. I had to learn how to fundamentally take tests as an adult, where most people understand how to do that going into college and professional school.
Dr. Levy: I was impressed with your resilience. I think all of us as human beings, if we fail a test, we take it personally and think it’s about our lack of knowledge. One of the insights that you came to was that failure on those things was not that you didn’t study hard enough. In fact, you probably studied 4 times harder than most other people. You had the knowledge. Being able to get that knowledge into a standardized structured test score was the huge challenge for you.
Dr. Johnson: That’s it. I can remember taking the MCAT, and if you looked at the step 1 book, I could regurgitate to you everything on that page. However, it’s not a test about do you know it or not. It’s an understanding of the English language and how to break things down to make things fit into particular scenarios.
Continue to: A college experience focused on growth and exposure...
A college experience focused on growth and exposure
Dr. Levy: I was impressed by the distinction between your experience at Xavier University where there was a lot of support and guidance and help in your premed program, and what happened to you when you hit medical school.
Dr. Johnson: Xavier University in Louisiana is the number 1 institution in the country for getting minorities into professional school. They understand that they have kids that are brilliant but underprepared, and just have not had the background to actually tackle some of these tough curriculums. I always had good grades in school. But by not being challenged, I didn’t know what I didn’t really know. So now that I was seeing biology, chemistry for the first time, and trying to tackle it; there’s a failure point. I didn’t know how to take tests, and I didn’t know how to study properly. The harder I tried, the worse things got for me.
Xavier has seen that story a multitude of times. If I went to a bigger or predominantly White university, a counselor would have told me, “Well, medicine’s maybe not for you. You can’t handle a premed curriculum.” Instead, I said, “Listen, I’m studying. I’m doing all of these things, and I’m not hacking it.” And they broke it down: “Let’s get you into study groups with kids that have had these type of AP classes before. We’ll have you watch how they study,” and everything started to click. That facilitation of how to adjust to this curriculum was a godsend. It’s the only reason I’m here. I am a prime example of being brilliant enough to be able to do it, but needing the infrastructure and a system set up.
Dr. Levy: There’s a great book by Carol Dweck called Mindset that talks about education of young kids and putting them into silos so early in life; the brilliant kids go into the AP courses and the rest are labeled as inadequate. It’s assumed in a fixed mindset based on their heredity and IQ, and not based on the fact that they have not been exposed to the right things.
Xavier was growing you into the man who could, in fact, do all of those things. I think that is one of the systemic and structural issues that we have—that fixed mindset that frames a kid who is not succeeding as therefore unable to succeed, as opposed to framing that child as not having the correct tools.
New tribulations of medical school
Dr. Johnson: Absolutely. I think what Xavier did for me is to at least let me understand what I needed to do, how to comprehend and retain information, which I never had been exposed to before. Those years were very important to establishing a foundation. When going to medical school, it was like, “There’s no more excuses. What could be the problem now?” Well, now let’s talk about taking tests—a whole different skill. Xavier focused on getting me to understand how to structure my thought process and knowledge base. In medical school I had to apply those skills (because if you can’t apply them, there’s no fit).
My second through fourth year of medical school, I was the only African-American kid in my class. I was spending 20-hour days sometimes just studying, trying to overcompensate by knowing as much as I possibly could and thinking that would propel me from the test-taking standpoint. Even though I didn’t have a lot of classmates in medical school that looked like me, I did have mentors that looked similarly, who really saw potential in me. Dr. Frederick Horvath, a nephrologist in Peoria said, “What are you doing? I want you to get out of these books, and let’s go out to lunch.”
He ended up buying me some instrumental books, really talked to me, listening to my background and understanding how driven I was as a person. He took me under his wing for the rest of medical school and said, “This is how you navigate through these spaces. Yes, you need to have a fund of knowledge to be able to take these tests, but you need to start understanding how to apply it to these questions.” I’m forever grateful to Dr. Horvath for doing that because it was a point in time where I was lost and struggling.
Continue to: Hitting a stride but facing racism head-on...
Hitting a stride but facing racism head-on
Dr. Levy: You talk about the systemic and pervasive racism that was on the wards when you hit them in fourth year. If you don’t mind sharing just a little bit of that, it would help people reading this to have a better understanding of the kinds of barriers that are out there.
Dr. Johnson: Even when I talk about it today, it bothers me.
I went to medical school in Peoria, Illinois, not far from the home of the Ku Klux Klan. At that time, once you got out of Chicago it was a very brutal place, with systemic racism throughout. I was a young Black kid going through a process that not many young Black kids from the South side of Chicago go through, and you had people who had never seen anyone like me. When I was going through my clinical rotations, I knew what I was up against. I was dressed “to the T” every day, arriving early, leaving late, trying to answer questions. I would look at the evaluations, and they would be disparaging. I would look at my counterparts, how their evaluations were, and how people would respond to them, and it would be completely different.
Surgery was the part of ObGyn that I really grew to love more than anything, even more than obstetrics. When general surgery came, I wanted to take it very seriously and learn as much as I possibly could. From the beginning, I knew there was a problem because the chief resident, an older White man, wouldn’t look me in the eye or talk to me. He would make disparaging remarks. The thing that stuck out in my mind the most was when I was in the operating room transporting patients, just like a medical student did, and he came up behind me and said, “You know, Pierre, this is where a small mind and a strong back come into play.” For me, it took me to a place where I had to corral my emotions and thoughts because I just wanted to lash out and just tell him how racist and horrible that was for him to say that to me. I explained this to the powers that be, the director of the department, and they basically blew it off to the side.
When it came down to the end of the evaluation period, I passed with flying colors. But they gave me an incomplete because of that chief resident and his remarks on my evaluations. He had 3 pages of report about me as a person and as a student. He said that he had difficulty in expressing his opinions about me because of possible cultural biases that he may have had. He put “cultural biases” in an evaluation, and they looked at that and said that was enough for me to have to remediate my time. I was required to do an extra month in Pontiac, Illinois, which is even more rural than Peoria, because of a racist person that did not give me a fair opportunity because I was Black.
Like everything else in life, it was a learning experience. It’s why I fight so hard today. It’s why I’m so passionate about equity, not only in medicine but also in all aspects of society. It shows why we have police brutality and Black men dying in the streets. It shows how this happens because there are cultural and implicit biases that play out in every part of life, and we are not honest about it. Until we are honest about it and until we say that this is happening and there is something that needs to be done to address it, it’s going to continue to happen. That is my fight.
Exposing the unspoken power struggle
Dr. Levy: I couldn’t agree more. Attributing things like that to the individual, where you talk about a White man in power and a power structure that didn’t literally physically beat you but did beat you into submission. You talk about how to succeed in medical school, and how you had to suck it up and submit to something that was incredibly unfair. You understood, you were old enough, mature enough, to understand that if you fought back, you were going to lose. The only opportunity you had was to submit to that inequity and push forward.
Dr. Johnson: When I did try to fight, the chair of the department told me that either I accept the consequences or I would not graduate from medical school and be forced to do another year. That struck a chord with me. I think that happens a lot in our society, and it needs to be exposed.
Past experiences reflected in today’s society
Dr. Levy: Can you talk about what you faced in your ObGyn residency in terms of the systemic pushback, people not taking your orders, people questioning you. I know that I have heard that a great deal, and I experienced that myself as a woman.
Dr. Johnson: We look at the things that are happening now, everything from George Floyd’s murder to Colin Kaepernick taking a knee. These things are 10 years past when I first started residency. The year before I started residency, there was a noose hanging on the capitol lawn of Springfield, Illinois’ capital city. There’s systemic racism and hatred there. When I first started on the wards of my first year of ObGyn, again, I was the very first Black resident of my program’s history. Nobody could relate to me.
I went from a year-long general surgery internship at Washington Hospital Center in Washington, DC, to ObGyn residency. In the first 2 months, there were complaints of, “He’s not answering his pages. He’s not being prompt.” I went to my program director and said, “Listen, I have never had one complaint like this. There’s a problem here. And there’s a problem when I’m on the floor: When trying to give orders to nurses, they’re not taking them. I had to tell a couple of nurses, ‘I’m Dr. Johnson. Don’t call me by my first name, especially not in front of patients.’”
My director was just not hearing me, because the entire scenario was something they had never been exposed to. Systemic racism is real, and unless you experience it, it’s very difficult to accept that it is happening. But biases happen when you are not cognizant. People are used to things a certain way. Things play out in the media that make your mind think a certain way, and you don’t even realize it. You may not even want to be that way.
Continue to: Unconscious bias is a barrier to ensuring equity...
Unconscious bias is a barrier to ensuring equity
Dr. Levy: One very important point you just made is that we as the system need to be able to recognize those unconscious things, the language that we use, the disparaging remarks, the things that put people down, as well as the things that keep people out of promotion.
There are some interesting data about both race and gender and the language that we use when we write recommendations for people, that we do things unconsciously. The big message to all of us at the end is to open our minds to where those things can occur. For myself, professionally, I keep a list of words that I use when I write recommendations. I measure myself to ensure that I am using the same language for men and women, for Black and White. I think we need to overcome the system and the structure to create real equity—not equality but equity.
It begins with being real about the issues
Dr. Johnson: It’s a bigger problem than the existence of bias and racism. I think these are systemic issues that have been cultivated over centuries that have never been addressed. The true issue is that we deny that these are problems and refuse to talk about it because it makes us uncomfortable. To truly make things more equitable, we have to push our levels of comfort to be able to talk about things in a healthy manner, be open and transparent, and to start to understand how we are thinking about certain things. When you can see it, you can start to implement changes and start to change mentalities and thought processes.
For me, people say, “You don’t look like a doctor.” I get that all the time—because I have tattoos and earrings. I wear my hair in a mohawk. The image of what success looks like has been manifested through our media and culture, and it has imprinted on our minds as to how things are supposed to be. If someone doesn’t fit those molds, we start to shun them out, or we start to exhibit biases against those things. What I am trying to do is change that thought process of what a successful or a professional person looks like. It doesn’t have a look. It is not a White or Black thing. It’s an intellect, a mindset, a way of living. You have to treat every person as an individual and take all the biases out of it and understand where they are coming from and what they have to offer to the profession.
Dr. Levy: I personally was so impressed by you when I met you. I was impressed by the tattoos and the earrings, and my initial response to them was exactly that biased, “Oh, who is this person?” I checked that at the door, listened to you, and was really impressed at your surgical skill, your knowledge, your background. I am really grateful that you have been willing to spend the time to share that with everyone.
Dr. Johnson: Thank you for this discussion.
To watch the full interview between Drs. Levy and Johnson, visit: https://www.mdedge.com/obgyn/article/228507/facing-systemic-racism-health-care-inequities-medical-education. ●
- The Pulse of Perseverance:
Three Black Doctors on Their Journey to Success Pierre Johnson, MD; Maxime Madhere, MD; and Joseph Semien Jr, MD - Mindset:
The New Psychology of Success
Carol S. Dweck
Finding inspiration among life’s challenges
Barbara Levy, MD: I am fortunate to have met Pierre serendipitously at a training that we were both attending and was impressed by Dr. Johnson’s life story, his passion and commitment, and his dedication—not only to his personal career but also to raising up other young men of color by trying to break down barriers that face them. His life story highlights those areas of systemic and structural problems that all of us together need to address if we are going to make any progress.
Pierre Johnson, MD: Thank you, Barbara. A little about myself: I am a board-certified ObGyn, and I specialize in minimally invasive surgery. I was born on the South side of Chicago, experiencing gang violence, drugs, and substandard, underserved schools. Long story short, I had a very rough upbringing. I had a single mom and several different issues at home. I am the oldest of 5 siblings, and life was tough.
But I knew that I wanted to do something different with my life. I saw that there was a need in my community as far as health care was concerned, in particular women’s health and childbirth. I knew early on that I wanted to be an ObGyn, and the reason had a lot to do with The Cosby Show. It was the only example of a positive, successful Black man that I saw. No one graduated from college in my family. There weren’t any models of young Black excellence around me. Saying that I wanted to be a doctor planted a seed. I was 9 when my mom became pregnant with my first sibling, and it was fascinating to me. The physiology of pregnancy, and eventually childbirth, was extremely fascinating to me; it set me off on my journey to be an ObGyn.
As I got older, things didn’t get any easier. I went to high school in one of the toughest areas on the South side of Chicago. Gang violence, and violence in and of itself, were all around me, but I was able to stay focused. I went on to Xavier University in Louisiana.
Dr. Levy: There are some important things that I learned from your book and from talking to you at our first meeting. Your mom’s ObGyn, when she was pregnant with your next youngest sibling, was also a Black ObGyn. He took some time to take you under wing?
Dr. Johnson: He did. My mom’s ObGyn was a Black man. Other than The Cosby Show, that’s the only time I saw something like that. When I spoke to him, he really took the time to answer my questions and show me that he was like me; he wasn’t just a far-off mythical person, or something that I could not obtain.
Continue to: Seeing is believing when it comes to success...
Seeing is believing when it comes to success
Dr. Levy: Do you think it was important to have a role model who wasn’t a sports star?
Dr. Johnson: If you can’t see it, you can’t achieve it. He took his time to really talk to me, and it’s the little things for kids that go a long way in their life experience. I still have a relationship with him to this day. How he handled me as a kid made me realize that this is something that I could do. That was extremely important for me.
Dr. Levy: One of the structural things I think we need to point out is that the ability to see yourself as someone successful is critical. When we see 1,000 images a day and they are all White, and they are all so different from where we are that it gets incorporated into our sense of being. I think that’s really difficult for those of us of with privilege to understand what that privilege is.
Dr. Johnson: Absolutely, and I’ll even go further. In residency, 2 White females were my classmates, and both of their parents were doctors. They had grandparents who were doctors. My mom was addicted to drugs; my father was not around. They had been talking medicine since they were 5. You have to make things equitable, but in medicine it’s really not equitable. In medicine, what we don’t realize is that there is an importance for all aspects of someone’s upbringing and environment, and it’s not just what they can regurgitate on a standardized test. If a patient can’t relate to you and tell you what is wrong with them, how can you adequately treat them?
Dr. Levy: Even if they are trying to tell me, but I can’t hear it because I don’t have the language and I don’t have the background. There are really good data to show, in fact, that Black male physicians do a better job at engaging Black men to manage their hypertension.1 When we look at the inequities in birth outcomes for women of color, indigenous women and Black women, there’s evidence that providers who come from a similar background do a better job.
Dr. Johnson: There was the study of Black infants that just came out about them dying at a 3-time higher rate in non-Black physicians’ hands.2 These things need to be recognized. They need to be discussed, and they need to be identified as issues and then, realistically, we need to start talking about solutions, not get offended by what actual statistics are saying.
Foundational inequities in education
Dr. Levy: To address some of the barriers that you faced: I know that you went to a high school that was not geared toward pushing students into professional careers. Your colleagues, however, had educations that prepared them for the standardized tests and other things that they would face academically.
Dr. Johnson: People think I am kidding when I say it, but when I went into college, I didn’t know what a periodic table was. I saw it, but I had no idea what these things meant. I didn’t have any sciences or any AP classes in high school. I did well, but grades are smoke and mirrors. The true test of medicine comes with testing. From the MCATs to the boards, every step of the way there is a standardized test.
Knowledge is something that you can obtain, but test taking is a cultivated skill that happens from a very early age. Trying to teach an adult or someone in their late teens a skill that they should have learned as a kid is difficult. For me, I did not have that, so I had to program myself. I had to learn how to fundamentally take tests as an adult, where most people understand how to do that going into college and professional school.
Dr. Levy: I was impressed with your resilience. I think all of us as human beings, if we fail a test, we take it personally and think it’s about our lack of knowledge. One of the insights that you came to was that failure on those things was not that you didn’t study hard enough. In fact, you probably studied 4 times harder than most other people. You had the knowledge. Being able to get that knowledge into a standardized structured test score was the huge challenge for you.
Dr. Johnson: That’s it. I can remember taking the MCAT, and if you looked at the step 1 book, I could regurgitate to you everything on that page. However, it’s not a test about do you know it or not. It’s an understanding of the English language and how to break things down to make things fit into particular scenarios.
Continue to: A college experience focused on growth and exposure...
A college experience focused on growth and exposure
Dr. Levy: I was impressed by the distinction between your experience at Xavier University where there was a lot of support and guidance and help in your premed program, and what happened to you when you hit medical school.
Dr. Johnson: Xavier University in Louisiana is the number 1 institution in the country for getting minorities into professional school. They understand that they have kids that are brilliant but underprepared, and just have not had the background to actually tackle some of these tough curriculums. I always had good grades in school. But by not being challenged, I didn’t know what I didn’t really know. So now that I was seeing biology, chemistry for the first time, and trying to tackle it; there’s a failure point. I didn’t know how to take tests, and I didn’t know how to study properly. The harder I tried, the worse things got for me.
Xavier has seen that story a multitude of times. If I went to a bigger or predominantly White university, a counselor would have told me, “Well, medicine’s maybe not for you. You can’t handle a premed curriculum.” Instead, I said, “Listen, I’m studying. I’m doing all of these things, and I’m not hacking it.” And they broke it down: “Let’s get you into study groups with kids that have had these type of AP classes before. We’ll have you watch how they study,” and everything started to click. That facilitation of how to adjust to this curriculum was a godsend. It’s the only reason I’m here. I am a prime example of being brilliant enough to be able to do it, but needing the infrastructure and a system set up.
Dr. Levy: There’s a great book by Carol Dweck called Mindset that talks about education of young kids and putting them into silos so early in life; the brilliant kids go into the AP courses and the rest are labeled as inadequate. It’s assumed in a fixed mindset based on their heredity and IQ, and not based on the fact that they have not been exposed to the right things.
Xavier was growing you into the man who could, in fact, do all of those things. I think that is one of the systemic and structural issues that we have—that fixed mindset that frames a kid who is not succeeding as therefore unable to succeed, as opposed to framing that child as not having the correct tools.
New tribulations of medical school
Dr. Johnson: Absolutely. I think what Xavier did for me is to at least let me understand what I needed to do, how to comprehend and retain information, which I never had been exposed to before. Those years were very important to establishing a foundation. When going to medical school, it was like, “There’s no more excuses. What could be the problem now?” Well, now let’s talk about taking tests—a whole different skill. Xavier focused on getting me to understand how to structure my thought process and knowledge base. In medical school I had to apply those skills (because if you can’t apply them, there’s no fit).
My second through fourth year of medical school, I was the only African-American kid in my class. I was spending 20-hour days sometimes just studying, trying to overcompensate by knowing as much as I possibly could and thinking that would propel me from the test-taking standpoint. Even though I didn’t have a lot of classmates in medical school that looked like me, I did have mentors that looked similarly, who really saw potential in me. Dr. Frederick Horvath, a nephrologist in Peoria said, “What are you doing? I want you to get out of these books, and let’s go out to lunch.”
He ended up buying me some instrumental books, really talked to me, listening to my background and understanding how driven I was as a person. He took me under his wing for the rest of medical school and said, “This is how you navigate through these spaces. Yes, you need to have a fund of knowledge to be able to take these tests, but you need to start understanding how to apply it to these questions.” I’m forever grateful to Dr. Horvath for doing that because it was a point in time where I was lost and struggling.
Continue to: Hitting a stride but facing racism head-on...
Hitting a stride but facing racism head-on
Dr. Levy: You talk about the systemic and pervasive racism that was on the wards when you hit them in fourth year. If you don’t mind sharing just a little bit of that, it would help people reading this to have a better understanding of the kinds of barriers that are out there.
Dr. Johnson: Even when I talk about it today, it bothers me.
I went to medical school in Peoria, Illinois, not far from the home of the Ku Klux Klan. At that time, once you got out of Chicago it was a very brutal place, with systemic racism throughout. I was a young Black kid going through a process that not many young Black kids from the South side of Chicago go through, and you had people who had never seen anyone like me. When I was going through my clinical rotations, I knew what I was up against. I was dressed “to the T” every day, arriving early, leaving late, trying to answer questions. I would look at the evaluations, and they would be disparaging. I would look at my counterparts, how their evaluations were, and how people would respond to them, and it would be completely different.
Surgery was the part of ObGyn that I really grew to love more than anything, even more than obstetrics. When general surgery came, I wanted to take it very seriously and learn as much as I possibly could. From the beginning, I knew there was a problem because the chief resident, an older White man, wouldn’t look me in the eye or talk to me. He would make disparaging remarks. The thing that stuck out in my mind the most was when I was in the operating room transporting patients, just like a medical student did, and he came up behind me and said, “You know, Pierre, this is where a small mind and a strong back come into play.” For me, it took me to a place where I had to corral my emotions and thoughts because I just wanted to lash out and just tell him how racist and horrible that was for him to say that to me. I explained this to the powers that be, the director of the department, and they basically blew it off to the side.
When it came down to the end of the evaluation period, I passed with flying colors. But they gave me an incomplete because of that chief resident and his remarks on my evaluations. He had 3 pages of report about me as a person and as a student. He said that he had difficulty in expressing his opinions about me because of possible cultural biases that he may have had. He put “cultural biases” in an evaluation, and they looked at that and said that was enough for me to have to remediate my time. I was required to do an extra month in Pontiac, Illinois, which is even more rural than Peoria, because of a racist person that did not give me a fair opportunity because I was Black.
Like everything else in life, it was a learning experience. It’s why I fight so hard today. It’s why I’m so passionate about equity, not only in medicine but also in all aspects of society. It shows why we have police brutality and Black men dying in the streets. It shows how this happens because there are cultural and implicit biases that play out in every part of life, and we are not honest about it. Until we are honest about it and until we say that this is happening and there is something that needs to be done to address it, it’s going to continue to happen. That is my fight.
Exposing the unspoken power struggle
Dr. Levy: I couldn’t agree more. Attributing things like that to the individual, where you talk about a White man in power and a power structure that didn’t literally physically beat you but did beat you into submission. You talk about how to succeed in medical school, and how you had to suck it up and submit to something that was incredibly unfair. You understood, you were old enough, mature enough, to understand that if you fought back, you were going to lose. The only opportunity you had was to submit to that inequity and push forward.
Dr. Johnson: When I did try to fight, the chair of the department told me that either I accept the consequences or I would not graduate from medical school and be forced to do another year. That struck a chord with me. I think that happens a lot in our society, and it needs to be exposed.
Past experiences reflected in today’s society
Dr. Levy: Can you talk about what you faced in your ObGyn residency in terms of the systemic pushback, people not taking your orders, people questioning you. I know that I have heard that a great deal, and I experienced that myself as a woman.
Dr. Johnson: We look at the things that are happening now, everything from George Floyd’s murder to Colin Kaepernick taking a knee. These things are 10 years past when I first started residency. The year before I started residency, there was a noose hanging on the capitol lawn of Springfield, Illinois’ capital city. There’s systemic racism and hatred there. When I first started on the wards of my first year of ObGyn, again, I was the very first Black resident of my program’s history. Nobody could relate to me.
I went from a year-long general surgery internship at Washington Hospital Center in Washington, DC, to ObGyn residency. In the first 2 months, there were complaints of, “He’s not answering his pages. He’s not being prompt.” I went to my program director and said, “Listen, I have never had one complaint like this. There’s a problem here. And there’s a problem when I’m on the floor: When trying to give orders to nurses, they’re not taking them. I had to tell a couple of nurses, ‘I’m Dr. Johnson. Don’t call me by my first name, especially not in front of patients.’”
My director was just not hearing me, because the entire scenario was something they had never been exposed to. Systemic racism is real, and unless you experience it, it’s very difficult to accept that it is happening. But biases happen when you are not cognizant. People are used to things a certain way. Things play out in the media that make your mind think a certain way, and you don’t even realize it. You may not even want to be that way.
Continue to: Unconscious bias is a barrier to ensuring equity...
Unconscious bias is a barrier to ensuring equity
Dr. Levy: One very important point you just made is that we as the system need to be able to recognize those unconscious things, the language that we use, the disparaging remarks, the things that put people down, as well as the things that keep people out of promotion.
There are some interesting data about both race and gender and the language that we use when we write recommendations for people, that we do things unconsciously. The big message to all of us at the end is to open our minds to where those things can occur. For myself, professionally, I keep a list of words that I use when I write recommendations. I measure myself to ensure that I am using the same language for men and women, for Black and White. I think we need to overcome the system and the structure to create real equity—not equality but equity.
It begins with being real about the issues
Dr. Johnson: It’s a bigger problem than the existence of bias and racism. I think these are systemic issues that have been cultivated over centuries that have never been addressed. The true issue is that we deny that these are problems and refuse to talk about it because it makes us uncomfortable. To truly make things more equitable, we have to push our levels of comfort to be able to talk about things in a healthy manner, be open and transparent, and to start to understand how we are thinking about certain things. When you can see it, you can start to implement changes and start to change mentalities and thought processes.
For me, people say, “You don’t look like a doctor.” I get that all the time—because I have tattoos and earrings. I wear my hair in a mohawk. The image of what success looks like has been manifested through our media and culture, and it has imprinted on our minds as to how things are supposed to be. If someone doesn’t fit those molds, we start to shun them out, or we start to exhibit biases against those things. What I am trying to do is change that thought process of what a successful or a professional person looks like. It doesn’t have a look. It is not a White or Black thing. It’s an intellect, a mindset, a way of living. You have to treat every person as an individual and take all the biases out of it and understand where they are coming from and what they have to offer to the profession.
Dr. Levy: I personally was so impressed by you when I met you. I was impressed by the tattoos and the earrings, and my initial response to them was exactly that biased, “Oh, who is this person?” I checked that at the door, listened to you, and was really impressed at your surgical skill, your knowledge, your background. I am really grateful that you have been willing to spend the time to share that with everyone.
Dr. Johnson: Thank you for this discussion.
To watch the full interview between Drs. Levy and Johnson, visit: https://www.mdedge.com/obgyn/article/228507/facing-systemic-racism-health-care-inequities-medical-education. ●
- The Pulse of Perseverance:
Three Black Doctors on Their Journey to Success Pierre Johnson, MD; Maxime Madhere, MD; and Joseph Semien Jr, MD - Mindset:
The New Psychology of Success
Carol S. Dweck
- Benkert R, Peters R, Tate N, et al. Trust of nurse practitioners and physicians among African Americans with hypertension. J Am Acad Nurse Pract. 2008;20:273-280.
- Greenwood BN, Hardeman RR, Huang L, et al. Physician– patient racial concordance and disparities in birthing mortality for newborns. Proc Natl Acad Sci U S A. 2020; 117:21194-21200.
- Benkert R, Peters R, Tate N, et al. Trust of nurse practitioners and physicians among African Americans with hypertension. J Am Acad Nurse Pract. 2008;20:273-280.
- Greenwood BN, Hardeman RR, Huang L, et al. Physician– patient racial concordance and disparities in birthing mortality for newborns. Proc Natl Acad Sci U S A. 2020; 117:21194-21200.
Pessaries for POP and SUI: Your options and guidance on use
Over the last 30 years, surgical correction of the common condition pelvic organ prolapse (POP) and stress urinary incontinence (SUI) has become so routine and straightforward that many gynecologists and urogynecologists choose surgery as their first choice for treating these conditions, withholding it only from the riskiest patients or from those who, for a variety of reasons, do not choose surgery. Moreover, as generalist gynecologists increasingly refer patients with POP or incontinence to their urogynecologist colleagues, they increasingly lack the skills, or have not been trained, to use conservative treatment strategies for these disorders. Thus, pessaries—devices constructed of inert plastic, silicone, or latex and placed inside the vagina to support prolapsed pelvic structures—frequently are not part of the general gynecologist’s armamentarium.
When properly selected, however, pessaries used for indicated purposes and correctly fitted are an excellent, inexpensive, low-risk, and noninvasive tool that can provide immediate relief not only of POP but also of SUI and defecatory difficulties. As an alternative to surgery, pessaries are especially valuable, because the other major nonsurgical modality for treatment of POP and incontinence—pelvic floor muscle training—often is not covered by insurance (making it expensive for patients), takes many weekly sessions to complete (which can make access challenging), and frequently is not readily available.1
POP is very common. An estimated 15% to 30% of women in North America have some degree of prolapse, and more than 500,000 surgeries for this condition are performed in the United States each year.2 Risk factors for POP include:
- vaginal childbirth, especially higher parity
- advancing age
- high body mass index (BMI)
- prior hysterectomy
- raised intra-abdominal pressure, such as from obesity, chronic cough, or heavy lifting.
In addition to the discomfort caused by the herniation of pelvic and vaginal structures, POP also is associated with urinary incontinence (73%), urinary urgency and frequency (86%), and fecal incontinence (31%).3
Moreover, according to the US Census Bureau, the number of American women aged 65 or older will double to more than 40 million by 2030.4 This will greatly increase the population of women at risk for POP who may be candidates for pessary use. It therefore behooves gynecologists to become familiar with the correct usage, fitting, and maintenance of this effective, nonsurgical mode of treatment for POP.
In this article, I discuss why pessaries are a good option for many patients with POP, review the types of pessaries available, and offer guidance on how to choose the right pessary for an individual patient’s needs. In addition, the box at the end of this article provides an interesting timeline of pessary history dating back to antiquity.
Next month in Part 2 of this article, I cover how to fit a pessary; device aftercare; potential complications of use; and effectiveness of pessaries for POP, SUI, preterm labor prevention, and defecatory disorders.
Continue to: Potential candidates for pessary use...
Potential candidates for pessary use
Almost all women with POP—and in many cases accompanying SUI—are potential candidates for a pessary. In fact, many urogynecologists believe that a trial of pessary usage should be the first treatment modality offered for POP.5 Women who cannot use a pessary include those with an extremely short vagina (<6 cm) and those who have severely eroded vaginal mucosa. In the latter situation, the mucosa can be treated with estrogen cream for several weeks and, once the tissue has healed, a pessary can be fitted.
Given that surgical repair is generally a straightforward, one-time procedure that obviates the need for long-term use of an artificial device worn internally, why might a patient or her physician opt for a pessary instead?
Some of the many reasons include:
- Many patients prefer to avoid surgery.
- Many patients are not appropriate candidates for surgery because they have significant comorbid risk factors or high BMI.
- Patients may have recurrent prolapse or incontinence and wish to avoid repeat surgery.
- Patients with SUI may have heard of the occurrence of mesh erosion and wish to avoid that possibility.
- Women who live in low-resource environments or countries where elective surgical care is relatively unavailable may not have the option of surgery.
A clinician might also recommend pessary use:
- as a diagnostic tool to attempt to assess the potential results of vaginal repair surgery
- to estimate the potential effectiveness of a midurethral sling procedure; several investigators have found this to be approximately as accurate as urodynamic testing6,7
- as prophylaxis for pregnant women with either a history of preterm cervical dilation or a short cervix detected on ultrasonography
- for pregnant women with POP that is worsening and becoming increasingly uncomfortable
- for women with POP who wish to have more children
- for short-term use while a patient is delaying or awaiting POP surgery or to allow time for other medical issues to resolve
- for patients who wish only intermittent, temporary support while exercising or engaging in sports.
Patient acceptance may be contingent on counseling
Numerous studies show that women who choose pessaries to treat POP are generally older than women who elect surgery. Still, patient acceptance of a trial of pessary use depends much on the counseling and information she receives. Properly informed, many patients with POP will opt for a trial of pessary placement. One study showed that, of women with untreated POP, 36% preferred pessary placement to surgery.8 Other investigators reported that when women with symptomatic POP had the benefits of a pessary versus surgery explained to them, nearly two-thirds opted for a pessary as their mode of treatment.9
Exceptions to pessary use
Fortunately, there are relatively few contraindications to pessary use. These are vaginal or pelvic infection and an exposed foreign body in the vagina, such as eroded vaginal mesh. In addition, patients at risk for nonadherence with follow-up care are poor candidates, as it could lead to missing such problems as mucosal erosion, ulceration, or even (extremely rarely) fistula formation. Pessaries may be inappropriate for sexually active women who on their own are unable to remove and reinsert pessary types that do not allow for intercourse while in place (see below).
Continue to: Types of pessaries...
Types of pessaries
The numerous kinds of pessaries available fall into 3 general categories: support, space filling, and lever, and devices within each group have modifications and variations. As with most areas of prescribing and treatment in medicine, it is best to become very familiar with just a few kinds of pessaries, know their indications, and use them when appropriate.
Most pessaries are constructed of inert silicone which, unlike earlier rubber pessaries, does not absorb odor or discharge. They are easy to clean, long lasting, and are autoclavable and hypoallergenic.
Support pessaries
Support pessaries look like contraceptive diaphragms. They are easy to place and remove, are comfortable, and do an excellent job correcting moderate POP. They also can control or eliminate symptoms of SUI by the pressure they exert on the urethra and their alteration of the urethrovesicular angle.
Ring pessaries. The most commonly used type of pessary, the ring pessary,10 comes in 4 variations:
- a simple open ring
- a ring with a web of material, called a “support shield,” that fills the ring
- an open ring with a firm 2-cm “incontinence knob” attached that is positioned over the urethra
- a ring with support shield and incontinence knob.
When in position, the deepest edge of a ring pessary fits behind the cervix (or in the vaginal apex for women who have had a hysterectomy) while the front of the ring slips into place behind the pubic symphysis, just like a diaphragm. When a ring with an incontinence knob is used, the ring is rotated until the knob is directly over the urethra.
Sexual intercourse is possible with any of the ring pessaries in place. Of the various types of pessaries, the ring pessary is the easiest to insert and remove. Some women tie a piece of dental floss to the edge of the ring to make its removal even easier.
The ring pessary is available in sizes 0 (44.5 mm) to 13 (127 mm). For most women a size 3, 4, or 5 ring pessary fits well.
The Marland pessary is similar to the ring pessary with the addition of a wedge-shaped piece of material approximately 3 cm in height that arises from half of the ring. It rarely is used in the United States because most American gynecologists are unfamiliar with it, and there is little evidence that it is more effective than the ring pessary.11
The Shaatz pessary is a rigid round pessary, smaller in diameter than the standard ring pessary, and similar to the Gellhorn pessary (discussed below) but without a stem. It is placed the same way one places a ring pessary but with its concave surface up against the cervix or, if there is no cervix, against the upper anterior vaginal wall. Its main benefit is that it provides firmer support than the ring pessary. This pessary is not widely used in the United States.
The Gehrung pessary looks like a flat strip of material that has been bent into the shape of a “U.” It is designed to correct severe cystoceles and rectoceles. For insertion, the edges at the open end of the pessary are squeezed together and the pessary is inserted with the closed part of the “U” facing the anterior vaginal wall. The upper edge is advanced until it rests in the anterior fornix of the vagina (or in the vaginal apex in women who have had a hysterectomy). Although it is more efficacious than some other pessaries for control of vaginal wall prolapse, its unfamiliarity to clinicians and its unusual shape result in it being used rarely.
Continue to: Space-filling pessaries...
Space-filling pessaries
Space-filling pessaries are used when more severe degrees of prolapse are present than can be managed by the ring or other support pessaries. This is especially the case when the vagina is so capacious or the introitus so lax that a standard ring pessary cannot be kept in place, resulting in frequent expulsions.
Space-filling pessaries are 3 dimensional and work by filling the vagina with a relatively large object that prevents the cervix/vaginal apex from dropping down and the vaginal walls from prolapsing. They have a special role for women who:
- are posthysterectomy and have an enterocele and/or vaginal apex prolapse
- have significant rectoceles for which support pessaries are not effective
- have a wide vaginal hiatus and thus are prone to expel support pessaries.
Space-filling pessaries do have some drawbacks compared with support pessaries. For example, they do not help in controlling SUI, and they are difficult for patients to remove on their own for cleaning. In addition, sexual intercourse is impossible with a space-filling pessary in place.
The Gellhorn pessary is the most common of the space-filling pessaries, and it is the one gynecologists and urogynecologists most often use for severe prolapse. It has a concave disc that fits up against the cervix or vaginal apex and a solid stem that points down the vagina. The stem itself is supported by the perineal body. It offers excellent support for severe uterine and vaginal wall prolapse, as long as the perineal body is intact. The stem stabilizes the disc portion of the pessary and prevents pessary expulsion. Gellhorn pessaries are available with long or short stems.
The Gellhorn is inserted into the vagina by folding the stem 90 degrees until it is in the same plane as the disc. With lubricated fingers, the patient’s perineal body is depressed and the disc of the pessary is folded and slid in. The disc is then placed up against the cervix or vaginal apex with the stem pointing down the vagina and tucked just inside the posterior edge of the introitus.
Removing the Gellhorn pessary can be problematic and is difficult for patients to do on their own. Clinicians often must use a ring forceps to grasp the stem of the pessary in order to bring it into the lower vagina, where the stem is folded up against the disc and the entire pessary removed. As with all space-filling pessaries, the Gellhorn must be taken out prior to intercourse.
The Gellhorn pessary is available in sizes that range from a disc diameter of 1.5 to 3.75 inches. Those measuring 2.5, 2.75, or 3 inches are used most commonly.
The cube pessary is a soft, dice-shaped piece of silicone with an indentation in each of its 6 sides. It is used for severe prolapse.
Squeezing the cube allows for easier insertion into the vagina; once it is at the top of the vagina, the cube expands back to its normal shape. The indentations on each side of the cube attach to the vaginal walls with moderate suction, which helps to keep the pessary in place. Because of the suction, the cube pessary can be used in cases of severe prolapse when other pessaries will not stay in place; a drawback is that the suction created by the indented sides can cause vaginal mucosal erosion.10 Ideally, the cube pessary should be removed every night for cleansing as discharge and accompanying odor can accumulate. The string attached to the cube pessary aids in its removal.
The cube pessary is available in sizes 0 to 7, with edge lengths that range from 1 to 2.25 inches.
The donut pessary, as its name suggests, has the form of a large donut. It can be compressed slightly to help with insertion. Because it occupies a large space within the vagina, it is used (like the cube pessary) for treatment of severe prolapse. The size and shape of the donut pessary, however, can make it difficult for patients to insert and take out on their own.
The donut pessary is available in sizes 0 (51 mm) to 8 (95 mm).
The inflatable pessary has the same basic shape as the donut pessary and serves the same purpose: It acts as a large semisoft object that fills the vagina to support the vaginal walls and cervix (or vaginal apex) in cases of severe prolapse. The inflatable pessary differs in that it has a valve on a stem through which air can be inserted and removed. This allows the uninflated pessary to be placed relatively easily into the vagina and then pumped full of air to the dimensions necessary to prevent vaginal, cervical, uterine, or apex prolapse. Air likewise can be removed to facilitate pessary removal.
One drawback of the inflatable pessary is that it is made of latex and thus cannot be used by anyone with a latex allergy. Also, as latex retains discharge and odors, this pessary should be removed and washed daily.
The inflatable pessary is available in sizes that range from 2 to 2.75 inches in 0.25-inch increments.
Continue to: Space-filling pessaries...
Lever pessaries
In addition to the more commonly used support and space-filling pessaries, there is a third kind that is rarely used in current practice: the lever pessaries. These pessaries—the Hodge, the Smith, and the Risser—are rectangles made of inert plastic that are folded into 3 planes to facilitate positioning in the vagina. The narrower of the 2 shorter ends of the folded rectangle is placed behind the cervix or at the vaginal apex while the other short end is placed behind the symphysis pubis.
Although sometimes used to correct POP in nonpregnant women, the lever pessary’s main purpose is to antivert a retroflexed uterus and to support the cervix and uterus in cases of prolapse during pregnancy or impending cervical incompetence.
The 3 lever pessaries differ in terms of whether the narrow ends of the pessary are straight or curved and wider or narrower.
How to choose the right pessary for your patient
If a patient’s POP or urinary incontinence symptoms would best be treated with a pessary, the next step is to select the pessary type and size best suited for that patient’s needs and the size that should be prescribed. While there is controversy among experts as to whether or not certain pessaries are better than others for different indications,12 most gynecologists and urogynecologists who use pessaries on a regular basis agree on the following:
1. Support pessaries will meet the needs of most women with moderate POP and/or SUI. These include the ring pessary with or without the support shield and with or without an incontinence knob. A support pessary is the go-to pessary in most cases. Most women find it comfortable to wear, it is easy to put in and take out, and sexual intercourse is possible with the pessary in place.
2. The specific degree of a patient’s prolapse and/or incontinence dictates whether or not to prescribe the support shield feature or the incontinence knob with a ring pessary. The shield helps support a prolapsed cervix and uterus when they are present.5,13 The knob is a useful feature if incontinence is a prominent symptom.
3. The Gellhorn pessary is usually the first choice for more severe prolapse. As long as there is some degree of posterior perineal support, this pessary does an excellent job of correcting even severe prolapse whether of a cervix and uterus or of vaginal walls and apex. It does require the patient to have some practice and dexterity for inserting and removing it on her own; individuals not comfortable or physically able to do so will need to have the pessary removed and cleaned by a clinician on a regular basis in the office. (Part 2 of this article will discuss pessary cleansing intervals).
4. Space-filling pessaries (such as the cube and donut) are useful when there is a severe degree of prolapse and insufficient perineal support to maintain a Gellhorn pessary. In practice, they are generally used less frequently—which is unfortunate, as they are a potentially useful solution for older women with severe prolapse who might not be candidates for surgical repair. As mentioned, both the cube and donut pessaries require more frequent removal for cleaning.
5. In unusual cases, the use of 2 pessaries simultaneously may resolve a difficult problem, such as when a pessary is the only option for treatment, the prolapse is severe, or it is impossible to find a pessary that resists being expelled from the vagina.14 A space-filling pessary in the most cephalad aspect of the vagina used in conjunction with a ring pessary with support shield below it can sometimes resolve even the worst cases of prolapse.
Continue to: Stay tuned...
Stay tuned
Part 2 of this article next month will provide more information on pessaries, including fitting, aftercare, potential complications, and effectiveness in various disorders. ●
Pessaries have been used in one form or another to help resolve pelvic organ prolapse (POP) in women for at least 2,500 years. They have come in many shapes and have been made of many materials. Here is a brief sketch of the history of the pessary.
Antiquity
Kahun papyrus (ancient Egypt, c. 2000 BCE)
Women with POP were made to stand over a fire in which different ingredients were burned. It was thought that the disagreeable odors emitted would cause the uterus to “rebel” and thus revert back into place.1
Hippocrates (c. 460–375 BCE)
Used several techniques to resolve uterine prolapse:
- Tipping the woman upside down and shaking her, using gravity as an aid to return the prolapsed organs into the pelvis2
- Cupping of the buttocks and the lower abdomen in hopes of “sucking” the prolapsed uterus back into place3
The Greek physician Polybus (c. 400 BCE)
Placed half a pomegranate in the vagina to hold prolapsed structures in place2
Cleopatra (c. 70–30 BCE)
Treated prolapse with the vaginal application of an astringent liquid2
Celsus (c. 25 BCE–50 CE)
Used cone-shaped pessaries made of bronze with a perforated circular plate on the lower edge through which bands were attached. The bands were then tied around the body to keep the device in place4
The Greek physician Soranus (c. 98–138)
Utilized linen tampons soaked with vinegar—along with a piece of beef—to treat prolapse. These were then held in place by bands passed around the loins2
Galen (c. 130–210)
Used fumigation to “encourage” the uterus to return to the pelvis2
Middle Ages
Paulus of Aegina (c. 625–690) and Abbas (c. 949–982)
Both wrote about the use of pessaries made of wax3
Myrepsus (late 13th century)
Described the preparation of 45 types of pessaries consisting of different solid materials treated with perfumes, wax, honey, and herbs5
16th century
Caspar Stromayr (Practica Copiosa, 1559)
Used as pessaries tightly rolled sponges bound with string, dipped in wax, and covered with oil or butter6
Ambroise Paré (c. 1510–1590)
Developed the first ring-type pessary in the late 16th century. He used hammered brass and waxed cork in the shape of an oval to treat uterine prolapse. He also made ring-shaped devices of gold, silver, or brass which were kept in place by a belt around the waist.7
17th century de Castro (1546–1627)
Urged “attacking” uterine prolapse with application of a red-hot iron thus “frightening it” into receding back into the vagina8
Hendrik van Roonhuyse (1625–1672)
In his gynecology textbook, discussed the etiology and treatment of prolapse. He utilized a cork with a hole in it (to allow for passage of discharge) as prolapse treatment. He also wrote of removing an obstructed wax pessary that had blocked discharge of a patient’s vaginal secretions for many years4
18th century Thomas Simson (1696-1764)
Invented a metal spring device that kept a pessary made of cork in place9
John Leake (1729-1792)
Recommended the use of sponges as pessaries to avoid vaginal prolapse10
Juville (1783)
Was the first to use rubber pessaries, resembling today’s contraceptive cup, to avoid injuring the vaginal mucosa. The center of the cup was perforated with a gold tip which allowed for the discharge of vaginal secretions10
19th century
Scanzoni (1821-1891)
Recommended massage and the application of leeches to reduce local congestion and swelling of prolapsed pelvic organs before manual replacement11
Hugh Lenox Hodge (1796-1873)
In his 1860 textbook Diseases Peculiar to Women, Hodge discussed at length the use of pessaries for uterine displacement. He suggested that metals, alloys, glass, and porcelain be used for pessaries rather than cork, wax, and sponges12
20th century
1950s—
Pessaries made of rubber, which absorb discharge and odor, are replaced by polystyrene pessaries. Currently, pessaries are made of silicone, plastic, and latex.
References
- Stevens JM. Gynecology from ancient Egypt: the papyrus Kahun, a translation of the oldest treatise on gynecology that has survived from the ancient world. Med J Austr. 1975;2:949-952.
- Emge LA, Durfee RB. Pelvic organ prolapse: four thousand years of treatment. Clin Obstet Gynecol. 1966;9:997-1032.
- Van Dongen L. The anatomy of genital prolapse. South Afr Med J. 1981;60:357-359.
- Cianfrani T. Short History of Obstetrics and Gynecology. Springfield, IL: Charles C Thomas; 1960.
- Leonardo RA. History of Gynecology. New York, NY: Froben Press; 1944.
- Tizzano AP, Muffly TM. Historical milestones in female pelvic surgery, gynecology, and female urology. In: Walters M, Karram M. Urogynecology and Reconstructive Pelvic Surgery, 4th ed. Philadelphia, PA: Elsevier Saunders; 2015
- Farrell SA. Pessaries in Clinical Practice. Switzerland: Springer-Verlag; 2006.
- Tam T, Davies MF, eds. Vaginal Pessaries. Boca Raton, FL: CRC Press; 2019.
- Ricci JV. Genealogy of Gynaecology. Philadelphia, PA: Blakiston; 1950.
- Miller DS. Contemporary use of the pessary. In Sciarra JJ, ed. Gynecology and Obstetrics. Philadelphia, PA: JB Lippincott Company; 1995.
- Thomas TG. A Practical Treatise on the Disorders of Women. Philadelphia, PA: Lea Brothers and Co; 1891.
- Hodge HL. Diseases Peculiar to Women, Including Displacements of the Uterus. Philadelphia, PA: Blanchard and Lea; 1860.
- Zoorob D, Higgins M, Swan K, et al. Barriers to pelvic floor physical therapy regarding treatment of high-tone pelvic floor dysfunction. Female Pelvic Med Reconstr Surg. 2017;23:444-448.
- Kirby AC, Luber KM, Menefee SA. An update on the current and future demand for care of pelvic floor disorders in the United States. Am J Obstet Gynecol. 2013;209:584.e1-584.e5.
- Ellerkmann RM, Cundiff GW, Melick CF, et al. Correlation of symptoms with location and severity of pelvic organ prolapse. Am J Obstet Gynecol. 2001;185:1332-1337.
- US Census Bureau. United States population projections: 2000 to 2050. https://www.census.gov/library/workingpapers/2009/demo/us-pop-proj-2000-2050.html. Accessed November 13, 2020.
- Pott-Grinstein E, Newcomer JR. Gynecologists’ patterns of prescribing pessaries. J Reprod Med. 2001;46:205-208.
- Chaikin DC, Groutz A, Blaivas JG. Predicting the need for anti-incontinence surgery in continent women undergoing repair of severe urogenital prolapse. J Urol. 2000;163:531-534.
- Reena C, Kekre AN, Kekre N. Occult stress incontinence in women with pelvic organ prolapse. Int J Gynaecol Obstet. 2007;97:31-34.
- Thys SD, Roovers JP, Geomini PM, et al. Do patients prefer a pessary or surgery as primary treatment for pelvic organ prolapse. Gynecol Obstet Invest. 2012;74:6-12.
- Kapoor DS, Thakar R, Sultan AH, et al. Conservative versus surgical management of prolapse: what dictates patient choice? Int Urogynecol J Pelvic Floor Dysfunct. 2009;20: 1157-1161.
- Wu V, Farrel SA, Baskett TF, et al. A simplified protocol for pessary management. Obstet Gynecol. 1997;90:990-994.
- Culligan PJ. Nonsurgical management of pelvic organ prolapse. Obstet Gynecol. 2012;119:852-860.
- Cundiff GW, Amundsen CL, Bent AE, et al. The PESSRI study: symptom relief outcomes of a randomized crossover trial of the ring and Gellhorn pessaries. Am J Obstet Gynecol. 2007;196:405.e1-404.e8.
- Cundiff GW, Weidner AC, Visco AG, et al. A survey of pessary use by members of the American Urogynecologic Society. Obstet Gynecol. 2000;95(6 pt 1):931-935.
- Singh K, Reid W. Nonsurgical treatment of uterovaginal prolapse using double vaginal rings. Br J Obstet Gynecol. 2001;108:112-113.

Over the last 30 years, surgical correction of the common condition pelvic organ prolapse (POP) and stress urinary incontinence (SUI) has become so routine and straightforward that many gynecologists and urogynecologists choose surgery as their first choice for treating these conditions, withholding it only from the riskiest patients or from those who, for a variety of reasons, do not choose surgery. Moreover, as generalist gynecologists increasingly refer patients with POP or incontinence to their urogynecologist colleagues, they increasingly lack the skills, or have not been trained, to use conservative treatment strategies for these disorders. Thus, pessaries—devices constructed of inert plastic, silicone, or latex and placed inside the vagina to support prolapsed pelvic structures—frequently are not part of the general gynecologist’s armamentarium.
When properly selected, however, pessaries used for indicated purposes and correctly fitted are an excellent, inexpensive, low-risk, and noninvasive tool that can provide immediate relief not only of POP but also of SUI and defecatory difficulties. As an alternative to surgery, pessaries are especially valuable, because the other major nonsurgical modality for treatment of POP and incontinence—pelvic floor muscle training—often is not covered by insurance (making it expensive for patients), takes many weekly sessions to complete (which can make access challenging), and frequently is not readily available.1
POP is very common. An estimated 15% to 30% of women in North America have some degree of prolapse, and more than 500,000 surgeries for this condition are performed in the United States each year.2 Risk factors for POP include:
- vaginal childbirth, especially higher parity
- advancing age
- high body mass index (BMI)
- prior hysterectomy
- raised intra-abdominal pressure, such as from obesity, chronic cough, or heavy lifting.
In addition to the discomfort caused by the herniation of pelvic and vaginal structures, POP also is associated with urinary incontinence (73%), urinary urgency and frequency (86%), and fecal incontinence (31%).3
Moreover, according to the US Census Bureau, the number of American women aged 65 or older will double to more than 40 million by 2030.4 This will greatly increase the population of women at risk for POP who may be candidates for pessary use. It therefore behooves gynecologists to become familiar with the correct usage, fitting, and maintenance of this effective, nonsurgical mode of treatment for POP.
In this article, I discuss why pessaries are a good option for many patients with POP, review the types of pessaries available, and offer guidance on how to choose the right pessary for an individual patient’s needs. In addition, the box at the end of this article provides an interesting timeline of pessary history dating back to antiquity.
Next month in Part 2 of this article, I cover how to fit a pessary; device aftercare; potential complications of use; and effectiveness of pessaries for POP, SUI, preterm labor prevention, and defecatory disorders.
Continue to: Potential candidates for pessary use...
Potential candidates for pessary use
Almost all women with POP—and in many cases accompanying SUI—are potential candidates for a pessary. In fact, many urogynecologists believe that a trial of pessary usage should be the first treatment modality offered for POP.5 Women who cannot use a pessary include those with an extremely short vagina (<6 cm) and those who have severely eroded vaginal mucosa. In the latter situation, the mucosa can be treated with estrogen cream for several weeks and, once the tissue has healed, a pessary can be fitted.
Given that surgical repair is generally a straightforward, one-time procedure that obviates the need for long-term use of an artificial device worn internally, why might a patient or her physician opt for a pessary instead?
Some of the many reasons include:
- Many patients prefer to avoid surgery.
- Many patients are not appropriate candidates for surgery because they have significant comorbid risk factors or high BMI.
- Patients may have recurrent prolapse or incontinence and wish to avoid repeat surgery.
- Patients with SUI may have heard of the occurrence of mesh erosion and wish to avoid that possibility.
- Women who live in low-resource environments or countries where elective surgical care is relatively unavailable may not have the option of surgery.
A clinician might also recommend pessary use:
- as a diagnostic tool to attempt to assess the potential results of vaginal repair surgery
- to estimate the potential effectiveness of a midurethral sling procedure; several investigators have found this to be approximately as accurate as urodynamic testing6,7
- as prophylaxis for pregnant women with either a history of preterm cervical dilation or a short cervix detected on ultrasonography
- for pregnant women with POP that is worsening and becoming increasingly uncomfortable
- for women with POP who wish to have more children
- for short-term use while a patient is delaying or awaiting POP surgery or to allow time for other medical issues to resolve
- for patients who wish only intermittent, temporary support while exercising or engaging in sports.
Patient acceptance may be contingent on counseling
Numerous studies show that women who choose pessaries to treat POP are generally older than women who elect surgery. Still, patient acceptance of a trial of pessary use depends much on the counseling and information she receives. Properly informed, many patients with POP will opt for a trial of pessary placement. One study showed that, of women with untreated POP, 36% preferred pessary placement to surgery.8 Other investigators reported that when women with symptomatic POP had the benefits of a pessary versus surgery explained to them, nearly two-thirds opted for a pessary as their mode of treatment.9
Exceptions to pessary use
Fortunately, there are relatively few contraindications to pessary use. These are vaginal or pelvic infection and an exposed foreign body in the vagina, such as eroded vaginal mesh. In addition, patients at risk for nonadherence with follow-up care are poor candidates, as it could lead to missing such problems as mucosal erosion, ulceration, or even (extremely rarely) fistula formation. Pessaries may be inappropriate for sexually active women who on their own are unable to remove and reinsert pessary types that do not allow for intercourse while in place (see below).
Continue to: Types of pessaries...
Types of pessaries
The numerous kinds of pessaries available fall into 3 general categories: support, space filling, and lever, and devices within each group have modifications and variations. As with most areas of prescribing and treatment in medicine, it is best to become very familiar with just a few kinds of pessaries, know their indications, and use them when appropriate.
Most pessaries are constructed of inert silicone which, unlike earlier rubber pessaries, does not absorb odor or discharge. They are easy to clean, long lasting, and are autoclavable and hypoallergenic.
Support pessaries
Support pessaries look like contraceptive diaphragms. They are easy to place and remove, are comfortable, and do an excellent job correcting moderate POP. They also can control or eliminate symptoms of SUI by the pressure they exert on the urethra and their alteration of the urethrovesicular angle.
Ring pessaries. The most commonly used type of pessary, the ring pessary,10 comes in 4 variations:
- a simple open ring
- a ring with a web of material, called a “support shield,” that fills the ring
- an open ring with a firm 2-cm “incontinence knob” attached that is positioned over the urethra
- a ring with support shield and incontinence knob.
When in position, the deepest edge of a ring pessary fits behind the cervix (or in the vaginal apex for women who have had a hysterectomy) while the front of the ring slips into place behind the pubic symphysis, just like a diaphragm. When a ring with an incontinence knob is used, the ring is rotated until the knob is directly over the urethra.
Sexual intercourse is possible with any of the ring pessaries in place. Of the various types of pessaries, the ring pessary is the easiest to insert and remove. Some women tie a piece of dental floss to the edge of the ring to make its removal even easier.
The ring pessary is available in sizes 0 (44.5 mm) to 13 (127 mm). For most women a size 3, 4, or 5 ring pessary fits well.

The Marland pessary is similar to the ring pessary with the addition of a wedge-shaped piece of material approximately 3 cm in height that arises from half of the ring. It rarely is used in the United States because most American gynecologists are unfamiliar with it, and there is little evidence that it is more effective than the ring pessary.11

The Shaatz pessary is a rigid round pessary, smaller in diameter than the standard ring pessary, and similar to the Gellhorn pessary (discussed below) but without a stem. It is placed the same way one places a ring pessary but with its concave surface up against the cervix or, if there is no cervix, against the upper anterior vaginal wall. Its main benefit is that it provides firmer support than the ring pessary. This pessary is not widely used in the United States.

The Gehrung pessary looks like a flat strip of material that has been bent into the shape of a “U.” It is designed to correct severe cystoceles and rectoceles. For insertion, the edges at the open end of the pessary are squeezed together and the pessary is inserted with the closed part of the “U” facing the anterior vaginal wall. The upper edge is advanced until it rests in the anterior fornix of the vagina (or in the vaginal apex in women who have had a hysterectomy). Although it is more efficacious than some other pessaries for control of vaginal wall prolapse, its unfamiliarity to clinicians and its unusual shape result in it being used rarely.

Continue to: Space-filling pessaries...
Space-filling pessaries
Space-filling pessaries are used when more severe degrees of prolapse are present than can be managed by the ring or other support pessaries. This is especially the case when the vagina is so capacious or the introitus so lax that a standard ring pessary cannot be kept in place, resulting in frequent expulsions.
Space-filling pessaries are 3 dimensional and work by filling the vagina with a relatively large object that prevents the cervix/vaginal apex from dropping down and the vaginal walls from prolapsing. They have a special role for women who:
- are posthysterectomy and have an enterocele and/or vaginal apex prolapse
- have significant rectoceles for which support pessaries are not effective
- have a wide vaginal hiatus and thus are prone to expel support pessaries.
Space-filling pessaries do have some drawbacks compared with support pessaries. For example, they do not help in controlling SUI, and they are difficult for patients to remove on their own for cleaning. In addition, sexual intercourse is impossible with a space-filling pessary in place.
The Gellhorn pessary is the most common of the space-filling pessaries, and it is the one gynecologists and urogynecologists most often use for severe prolapse. It has a concave disc that fits up against the cervix or vaginal apex and a solid stem that points down the vagina. The stem itself is supported by the perineal body. It offers excellent support for severe uterine and vaginal wall prolapse, as long as the perineal body is intact. The stem stabilizes the disc portion of the pessary and prevents pessary expulsion. Gellhorn pessaries are available with long or short stems.
The Gellhorn is inserted into the vagina by folding the stem 90 degrees until it is in the same plane as the disc. With lubricated fingers, the patient’s perineal body is depressed and the disc of the pessary is folded and slid in. The disc is then placed up against the cervix or vaginal apex with the stem pointing down the vagina and tucked just inside the posterior edge of the introitus.
Removing the Gellhorn pessary can be problematic and is difficult for patients to do on their own. Clinicians often must use a ring forceps to grasp the stem of the pessary in order to bring it into the lower vagina, where the stem is folded up against the disc and the entire pessary removed. As with all space-filling pessaries, the Gellhorn must be taken out prior to intercourse.
The Gellhorn pessary is available in sizes that range from a disc diameter of 1.5 to 3.75 inches. Those measuring 2.5, 2.75, or 3 inches are used most commonly.

The cube pessary is a soft, dice-shaped piece of silicone with an indentation in each of its 6 sides. It is used for severe prolapse.
Squeezing the cube allows for easier insertion into the vagina; once it is at the top of the vagina, the cube expands back to its normal shape. The indentations on each side of the cube attach to the vaginal walls with moderate suction, which helps to keep the pessary in place. Because of the suction, the cube pessary can be used in cases of severe prolapse when other pessaries will not stay in place; a drawback is that the suction created by the indented sides can cause vaginal mucosal erosion.10 Ideally, the cube pessary should be removed every night for cleansing as discharge and accompanying odor can accumulate. The string attached to the cube pessary aids in its removal.
The cube pessary is available in sizes 0 to 7, with edge lengths that range from 1 to 2.25 inches.

The donut pessary, as its name suggests, has the form of a large donut. It can be compressed slightly to help with insertion. Because it occupies a large space within the vagina, it is used (like the cube pessary) for treatment of severe prolapse. The size and shape of the donut pessary, however, can make it difficult for patients to insert and take out on their own.
The donut pessary is available in sizes 0 (51 mm) to 8 (95 mm).

The inflatable pessary has the same basic shape as the donut pessary and serves the same purpose: It acts as a large semisoft object that fills the vagina to support the vaginal walls and cervix (or vaginal apex) in cases of severe prolapse. The inflatable pessary differs in that it has a valve on a stem through which air can be inserted and removed. This allows the uninflated pessary to be placed relatively easily into the vagina and then pumped full of air to the dimensions necessary to prevent vaginal, cervical, uterine, or apex prolapse. Air likewise can be removed to facilitate pessary removal.
One drawback of the inflatable pessary is that it is made of latex and thus cannot be used by anyone with a latex allergy. Also, as latex retains discharge and odors, this pessary should be removed and washed daily.
The inflatable pessary is available in sizes that range from 2 to 2.75 inches in 0.25-inch increments.

Continue to: Space-filling pessaries...
Lever pessaries
In addition to the more commonly used support and space-filling pessaries, there is a third kind that is rarely used in current practice: the lever pessaries. These pessaries—the Hodge, the Smith, and the Risser—are rectangles made of inert plastic that are folded into 3 planes to facilitate positioning in the vagina. The narrower of the 2 shorter ends of the folded rectangle is placed behind the cervix or at the vaginal apex while the other short end is placed behind the symphysis pubis.
Although sometimes used to correct POP in nonpregnant women, the lever pessary’s main purpose is to antivert a retroflexed uterus and to support the cervix and uterus in cases of prolapse during pregnancy or impending cervical incompetence.
The 3 lever pessaries differ in terms of whether the narrow ends of the pessary are straight or curved and wider or narrower.
How to choose the right pessary for your patient
If a patient’s POP or urinary incontinence symptoms would best be treated with a pessary, the next step is to select the pessary type and size best suited for that patient’s needs and the size that should be prescribed. While there is controversy among experts as to whether or not certain pessaries are better than others for different indications,12 most gynecologists and urogynecologists who use pessaries on a regular basis agree on the following:
1. Support pessaries will meet the needs of most women with moderate POP and/or SUI. These include the ring pessary with or without the support shield and with or without an incontinence knob. A support pessary is the go-to pessary in most cases. Most women find it comfortable to wear, it is easy to put in and take out, and sexual intercourse is possible with the pessary in place.
2. The specific degree of a patient’s prolapse and/or incontinence dictates whether or not to prescribe the support shield feature or the incontinence knob with a ring pessary. The shield helps support a prolapsed cervix and uterus when they are present.5,13 The knob is a useful feature if incontinence is a prominent symptom.
3. The Gellhorn pessary is usually the first choice for more severe prolapse. As long as there is some degree of posterior perineal support, this pessary does an excellent job of correcting even severe prolapse whether of a cervix and uterus or of vaginal walls and apex. It does require the patient to have some practice and dexterity for inserting and removing it on her own; individuals not comfortable or physically able to do so will need to have the pessary removed and cleaned by a clinician on a regular basis in the office. (Part 2 of this article will discuss pessary cleansing intervals).
4. Space-filling pessaries (such as the cube and donut) are useful when there is a severe degree of prolapse and insufficient perineal support to maintain a Gellhorn pessary. In practice, they are generally used less frequently—which is unfortunate, as they are a potentially useful solution for older women with severe prolapse who might not be candidates for surgical repair. As mentioned, both the cube and donut pessaries require more frequent removal for cleaning.
5. In unusual cases, the use of 2 pessaries simultaneously may resolve a difficult problem, such as when a pessary is the only option for treatment, the prolapse is severe, or it is impossible to find a pessary that resists being expelled from the vagina.14 A space-filling pessary in the most cephalad aspect of the vagina used in conjunction with a ring pessary with support shield below it can sometimes resolve even the worst cases of prolapse.
Continue to: Stay tuned...
Stay tuned
Part 2 of this article next month will provide more information on pessaries, including fitting, aftercare, potential complications, and effectiveness in various disorders. ●
Pessaries have been used in one form or another to help resolve pelvic organ prolapse (POP) in women for at least 2,500 years. They have come in many shapes and have been made of many materials. Here is a brief sketch of the history of the pessary.
Antiquity
Kahun papyrus (ancient Egypt, c. 2000 BCE)
Women with POP were made to stand over a fire in which different ingredients were burned. It was thought that the disagreeable odors emitted would cause the uterus to “rebel” and thus revert back into place.1
Hippocrates (c. 460–375 BCE)
Used several techniques to resolve uterine prolapse:
- Tipping the woman upside down and shaking her, using gravity as an aid to return the prolapsed organs into the pelvis2
- Cupping of the buttocks and the lower abdomen in hopes of “sucking” the prolapsed uterus back into place3
The Greek physician Polybus (c. 400 BCE)
Placed half a pomegranate in the vagina to hold prolapsed structures in place2
Cleopatra (c. 70–30 BCE)
Treated prolapse with the vaginal application of an astringent liquid2
Celsus (c. 25 BCE–50 CE)
Used cone-shaped pessaries made of bronze with a perforated circular plate on the lower edge through which bands were attached. The bands were then tied around the body to keep the device in place4
The Greek physician Soranus (c. 98–138)
Utilized linen tampons soaked with vinegar—along with a piece of beef—to treat prolapse. These were then held in place by bands passed around the loins2
Galen (c. 130–210)
Used fumigation to “encourage” the uterus to return to the pelvis2
Middle Ages
Paulus of Aegina (c. 625–690) and Abbas (c. 949–982)
Both wrote about the use of pessaries made of wax3
Myrepsus (late 13th century)
Described the preparation of 45 types of pessaries consisting of different solid materials treated with perfumes, wax, honey, and herbs5
16th century
Caspar Stromayr (Practica Copiosa, 1559)
Used as pessaries tightly rolled sponges bound with string, dipped in wax, and covered with oil or butter6
Ambroise Paré (c. 1510–1590)
Developed the first ring-type pessary in the late 16th century. He used hammered brass and waxed cork in the shape of an oval to treat uterine prolapse. He also made ring-shaped devices of gold, silver, or brass which were kept in place by a belt around the waist.7
17th century de Castro (1546–1627)
Urged “attacking” uterine prolapse with application of a red-hot iron thus “frightening it” into receding back into the vagina8
Hendrik van Roonhuyse (1625–1672)
In his gynecology textbook, discussed the etiology and treatment of prolapse. He utilized a cork with a hole in it (to allow for passage of discharge) as prolapse treatment. He also wrote of removing an obstructed wax pessary that had blocked discharge of a patient’s vaginal secretions for many years4
18th century Thomas Simson (1696-1764)
Invented a metal spring device that kept a pessary made of cork in place9
John Leake (1729-1792)
Recommended the use of sponges as pessaries to avoid vaginal prolapse10
Juville (1783)
Was the first to use rubber pessaries, resembling today’s contraceptive cup, to avoid injuring the vaginal mucosa. The center of the cup was perforated with a gold tip which allowed for the discharge of vaginal secretions10
19th century
Scanzoni (1821-1891)
Recommended massage and the application of leeches to reduce local congestion and swelling of prolapsed pelvic organs before manual replacement11
Hugh Lenox Hodge (1796-1873)
In his 1860 textbook Diseases Peculiar to Women, Hodge discussed at length the use of pessaries for uterine displacement. He suggested that metals, alloys, glass, and porcelain be used for pessaries rather than cork, wax, and sponges12
20th century
1950s—
Pessaries made of rubber, which absorb discharge and odor, are replaced by polystyrene pessaries. Currently, pessaries are made of silicone, plastic, and latex.
References
- Stevens JM. Gynecology from ancient Egypt: the papyrus Kahun, a translation of the oldest treatise on gynecology that has survived from the ancient world. Med J Austr. 1975;2:949-952.
- Emge LA, Durfee RB. Pelvic organ prolapse: four thousand years of treatment. Clin Obstet Gynecol. 1966;9:997-1032.
- Van Dongen L. The anatomy of genital prolapse. South Afr Med J. 1981;60:357-359.
- Cianfrani T. Short History of Obstetrics and Gynecology. Springfield, IL: Charles C Thomas; 1960.
- Leonardo RA. History of Gynecology. New York, NY: Froben Press; 1944.
- Tizzano AP, Muffly TM. Historical milestones in female pelvic surgery, gynecology, and female urology. In: Walters M, Karram M. Urogynecology and Reconstructive Pelvic Surgery, 4th ed. Philadelphia, PA: Elsevier Saunders; 2015
- Farrell SA. Pessaries in Clinical Practice. Switzerland: Springer-Verlag; 2006.
- Tam T, Davies MF, eds. Vaginal Pessaries. Boca Raton, FL: CRC Press; 2019.
- Ricci JV. Genealogy of Gynaecology. Philadelphia, PA: Blakiston; 1950.
- Miller DS. Contemporary use of the pessary. In Sciarra JJ, ed. Gynecology and Obstetrics. Philadelphia, PA: JB Lippincott Company; 1995.
- Thomas TG. A Practical Treatise on the Disorders of Women. Philadelphia, PA: Lea Brothers and Co; 1891.
- Hodge HL. Diseases Peculiar to Women, Including Displacements of the Uterus. Philadelphia, PA: Blanchard and Lea; 1860.

Over the last 30 years, surgical correction of the common condition pelvic organ prolapse (POP) and stress urinary incontinence (SUI) has become so routine and straightforward that many gynecologists and urogynecologists choose surgery as their first choice for treating these conditions, withholding it only from the riskiest patients or from those who, for a variety of reasons, do not choose surgery. Moreover, as generalist gynecologists increasingly refer patients with POP or incontinence to their urogynecologist colleagues, they increasingly lack the skills, or have not been trained, to use conservative treatment strategies for these disorders. Thus, pessaries—devices constructed of inert plastic, silicone, or latex and placed inside the vagina to support prolapsed pelvic structures—frequently are not part of the general gynecologist’s armamentarium.
When properly selected, however, pessaries used for indicated purposes and correctly fitted are an excellent, inexpensive, low-risk, and noninvasive tool that can provide immediate relief not only of POP but also of SUI and defecatory difficulties. As an alternative to surgery, pessaries are especially valuable, because the other major nonsurgical modality for treatment of POP and incontinence—pelvic floor muscle training—often is not covered by insurance (making it expensive for patients), takes many weekly sessions to complete (which can make access challenging), and frequently is not readily available.1
POP is very common. An estimated 15% to 30% of women in North America have some degree of prolapse, and more than 500,000 surgeries for this condition are performed in the United States each year.2 Risk factors for POP include:
- vaginal childbirth, especially higher parity
- advancing age
- high body mass index (BMI)
- prior hysterectomy
- raised intra-abdominal pressure, such as from obesity, chronic cough, or heavy lifting.
In addition to the discomfort caused by the herniation of pelvic and vaginal structures, POP also is associated with urinary incontinence (73%), urinary urgency and frequency (86%), and fecal incontinence (31%).3
Moreover, according to the US Census Bureau, the number of American women aged 65 or older will double to more than 40 million by 2030.4 This will greatly increase the population of women at risk for POP who may be candidates for pessary use. It therefore behooves gynecologists to become familiar with the correct usage, fitting, and maintenance of this effective, nonsurgical mode of treatment for POP.
In this article, I discuss why pessaries are a good option for many patients with POP, review the types of pessaries available, and offer guidance on how to choose the right pessary for an individual patient’s needs. In addition, the box at the end of this article provides an interesting timeline of pessary history dating back to antiquity.
Next month in Part 2 of this article, I cover how to fit a pessary; device aftercare; potential complications of use; and effectiveness of pessaries for POP, SUI, preterm labor prevention, and defecatory disorders.
Continue to: Potential candidates for pessary use...
Potential candidates for pessary use
Almost all women with POP—and in many cases accompanying SUI—are potential candidates for a pessary. In fact, many urogynecologists believe that a trial of pessary usage should be the first treatment modality offered for POP.5 Women who cannot use a pessary include those with an extremely short vagina (<6 cm) and those who have severely eroded vaginal mucosa. In the latter situation, the mucosa can be treated with estrogen cream for several weeks and, once the tissue has healed, a pessary can be fitted.
Given that surgical repair is generally a straightforward, one-time procedure that obviates the need for long-term use of an artificial device worn internally, why might a patient or her physician opt for a pessary instead?
Some of the many reasons include:
- Many patients prefer to avoid surgery.
- Many patients are not appropriate candidates for surgery because they have significant comorbid risk factors or high BMI.
- Patients may have recurrent prolapse or incontinence and wish to avoid repeat surgery.
- Patients with SUI may have heard of the occurrence of mesh erosion and wish to avoid that possibility.
- Women who live in low-resource environments or countries where elective surgical care is relatively unavailable may not have the option of surgery.
A clinician might also recommend pessary use:
- as a diagnostic tool to attempt to assess the potential results of vaginal repair surgery
- to estimate the potential effectiveness of a midurethral sling procedure; several investigators have found this to be approximately as accurate as urodynamic testing6,7
- as prophylaxis for pregnant women with either a history of preterm cervical dilation or a short cervix detected on ultrasonography
- for pregnant women with POP that is worsening and becoming increasingly uncomfortable
- for women with POP who wish to have more children
- for short-term use while a patient is delaying or awaiting POP surgery or to allow time for other medical issues to resolve
- for patients who wish only intermittent, temporary support while exercising or engaging in sports.
Patient acceptance may be contingent on counseling
Numerous studies show that women who choose pessaries to treat POP are generally older than women who elect surgery. Still, patient acceptance of a trial of pessary use depends much on the counseling and information she receives. Properly informed, many patients with POP will opt for a trial of pessary placement. One study showed that, of women with untreated POP, 36% preferred pessary placement to surgery.8 Other investigators reported that when women with symptomatic POP had the benefits of a pessary versus surgery explained to them, nearly two-thirds opted for a pessary as their mode of treatment.9
Exceptions to pessary use
Fortunately, there are relatively few contraindications to pessary use. These are vaginal or pelvic infection and an exposed foreign body in the vagina, such as eroded vaginal mesh. In addition, patients at risk for nonadherence with follow-up care are poor candidates, as it could lead to missing such problems as mucosal erosion, ulceration, or even (extremely rarely) fistula formation. Pessaries may be inappropriate for sexually active women who on their own are unable to remove and reinsert pessary types that do not allow for intercourse while in place (see below).
Continue to: Types of pessaries...
Types of pessaries
The numerous kinds of pessaries available fall into 3 general categories: support, space filling, and lever, and devices within each group have modifications and variations. As with most areas of prescribing and treatment in medicine, it is best to become very familiar with just a few kinds of pessaries, know their indications, and use them when appropriate.
Most pessaries are constructed of inert silicone which, unlike earlier rubber pessaries, does not absorb odor or discharge. They are easy to clean, long lasting, and are autoclavable and hypoallergenic.
Support pessaries
Support pessaries look like contraceptive diaphragms. They are easy to place and remove, are comfortable, and do an excellent job correcting moderate POP. They also can control or eliminate symptoms of SUI by the pressure they exert on the urethra and their alteration of the urethrovesicular angle.
Ring pessaries. The most commonly used type of pessary, the ring pessary,10 comes in 4 variations:
- a simple open ring
- a ring with a web of material, called a “support shield,” that fills the ring
- an open ring with a firm 2-cm “incontinence knob” attached that is positioned over the urethra
- a ring with support shield and incontinence knob.
When in position, the deepest edge of a ring pessary fits behind the cervix (or in the vaginal apex for women who have had a hysterectomy) while the front of the ring slips into place behind the pubic symphysis, just like a diaphragm. When a ring with an incontinence knob is used, the ring is rotated until the knob is directly over the urethra.
Sexual intercourse is possible with any of the ring pessaries in place. Of the various types of pessaries, the ring pessary is the easiest to insert and remove. Some women tie a piece of dental floss to the edge of the ring to make its removal even easier.
The ring pessary is available in sizes 0 (44.5 mm) to 13 (127 mm). For most women a size 3, 4, or 5 ring pessary fits well.

The Marland pessary is similar to the ring pessary with the addition of a wedge-shaped piece of material approximately 3 cm in height that arises from half of the ring. It rarely is used in the United States because most American gynecologists are unfamiliar with it, and there is little evidence that it is more effective than the ring pessary.11

The Shaatz pessary is a rigid round pessary, smaller in diameter than the standard ring pessary, and similar to the Gellhorn pessary (discussed below) but without a stem. It is placed the same way one places a ring pessary but with its concave surface up against the cervix or, if there is no cervix, against the upper anterior vaginal wall. Its main benefit is that it provides firmer support than the ring pessary. This pessary is not widely used in the United States.

The Gehrung pessary looks like a flat strip of material that has been bent into the shape of a “U.” It is designed to correct severe cystoceles and rectoceles. For insertion, the edges at the open end of the pessary are squeezed together and the pessary is inserted with the closed part of the “U” facing the anterior vaginal wall. The upper edge is advanced until it rests in the anterior fornix of the vagina (or in the vaginal apex in women who have had a hysterectomy). Although it is more efficacious than some other pessaries for control of vaginal wall prolapse, its unfamiliarity to clinicians and its unusual shape result in it being used rarely.

Continue to: Space-filling pessaries...
Space-filling pessaries
Space-filling pessaries are used when more severe degrees of prolapse are present than can be managed by the ring or other support pessaries. This is especially the case when the vagina is so capacious or the introitus so lax that a standard ring pessary cannot be kept in place, resulting in frequent expulsions.
Space-filling pessaries are 3 dimensional and work by filling the vagina with a relatively large object that prevents the cervix/vaginal apex from dropping down and the vaginal walls from prolapsing. They have a special role for women who:
- are posthysterectomy and have an enterocele and/or vaginal apex prolapse
- have significant rectoceles for which support pessaries are not effective
- have a wide vaginal hiatus and thus are prone to expel support pessaries.
Space-filling pessaries do have some drawbacks compared with support pessaries. For example, they do not help in controlling SUI, and they are difficult for patients to remove on their own for cleaning. In addition, sexual intercourse is impossible with a space-filling pessary in place.
The Gellhorn pessary is the most common of the space-filling pessaries, and it is the one gynecologists and urogynecologists most often use for severe prolapse. It has a concave disc that fits up against the cervix or vaginal apex and a solid stem that points down the vagina. The stem itself is supported by the perineal body. It offers excellent support for severe uterine and vaginal wall prolapse, as long as the perineal body is intact. The stem stabilizes the disc portion of the pessary and prevents pessary expulsion. Gellhorn pessaries are available with long or short stems.
The Gellhorn is inserted into the vagina by folding the stem 90 degrees until it is in the same plane as the disc. With lubricated fingers, the patient’s perineal body is depressed and the disc of the pessary is folded and slid in. The disc is then placed up against the cervix or vaginal apex with the stem pointing down the vagina and tucked just inside the posterior edge of the introitus.
Removing the Gellhorn pessary can be problematic and is difficult for patients to do on their own. Clinicians often must use a ring forceps to grasp the stem of the pessary in order to bring it into the lower vagina, where the stem is folded up against the disc and the entire pessary removed. As with all space-filling pessaries, the Gellhorn must be taken out prior to intercourse.
The Gellhorn pessary is available in sizes that range from a disc diameter of 1.5 to 3.75 inches. Those measuring 2.5, 2.75, or 3 inches are used most commonly.

The cube pessary is a soft, dice-shaped piece of silicone with an indentation in each of its 6 sides. It is used for severe prolapse.
Squeezing the cube allows for easier insertion into the vagina; once it is at the top of the vagina, the cube expands back to its normal shape. The indentations on each side of the cube attach to the vaginal walls with moderate suction, which helps to keep the pessary in place. Because of the suction, the cube pessary can be used in cases of severe prolapse when other pessaries will not stay in place; a drawback is that the suction created by the indented sides can cause vaginal mucosal erosion.10 Ideally, the cube pessary should be removed every night for cleansing as discharge and accompanying odor can accumulate. The string attached to the cube pessary aids in its removal.
The cube pessary is available in sizes 0 to 7, with edge lengths that range from 1 to 2.25 inches.

The donut pessary, as its name suggests, has the form of a large donut. It can be compressed slightly to help with insertion. Because it occupies a large space within the vagina, it is used (like the cube pessary) for treatment of severe prolapse. The size and shape of the donut pessary, however, can make it difficult for patients to insert and take out on their own.
The donut pessary is available in sizes 0 (51 mm) to 8 (95 mm).

The inflatable pessary has the same basic shape as the donut pessary and serves the same purpose: It acts as a large semisoft object that fills the vagina to support the vaginal walls and cervix (or vaginal apex) in cases of severe prolapse. The inflatable pessary differs in that it has a valve on a stem through which air can be inserted and removed. This allows the uninflated pessary to be placed relatively easily into the vagina and then pumped full of air to the dimensions necessary to prevent vaginal, cervical, uterine, or apex prolapse. Air likewise can be removed to facilitate pessary removal.
One drawback of the inflatable pessary is that it is made of latex and thus cannot be used by anyone with a latex allergy. Also, as latex retains discharge and odors, this pessary should be removed and washed daily.
The inflatable pessary is available in sizes that range from 2 to 2.75 inches in 0.25-inch increments.

Continue to: Space-filling pessaries...
Lever pessaries
In addition to the more commonly used support and space-filling pessaries, there is a third kind that is rarely used in current practice: the lever pessaries. These pessaries—the Hodge, the Smith, and the Risser—are rectangles made of inert plastic that are folded into 3 planes to facilitate positioning in the vagina. The narrower of the 2 shorter ends of the folded rectangle is placed behind the cervix or at the vaginal apex while the other short end is placed behind the symphysis pubis.
Although sometimes used to correct POP in nonpregnant women, the lever pessary’s main purpose is to antivert a retroflexed uterus and to support the cervix and uterus in cases of prolapse during pregnancy or impending cervical incompetence.
The 3 lever pessaries differ in terms of whether the narrow ends of the pessary are straight or curved and wider or narrower.
How to choose the right pessary for your patient
If a patient’s POP or urinary incontinence symptoms would best be treated with a pessary, the next step is to select the pessary type and size best suited for that patient’s needs and the size that should be prescribed. While there is controversy among experts as to whether or not certain pessaries are better than others for different indications,12 most gynecologists and urogynecologists who use pessaries on a regular basis agree on the following:
1. Support pessaries will meet the needs of most women with moderate POP and/or SUI. These include the ring pessary with or without the support shield and with or without an incontinence knob. A support pessary is the go-to pessary in most cases. Most women find it comfortable to wear, it is easy to put in and take out, and sexual intercourse is possible with the pessary in place.
2. The specific degree of a patient’s prolapse and/or incontinence dictates whether or not to prescribe the support shield feature or the incontinence knob with a ring pessary. The shield helps support a prolapsed cervix and uterus when they are present.5,13 The knob is a useful feature if incontinence is a prominent symptom.
3. The Gellhorn pessary is usually the first choice for more severe prolapse. As long as there is some degree of posterior perineal support, this pessary does an excellent job of correcting even severe prolapse whether of a cervix and uterus or of vaginal walls and apex. It does require the patient to have some practice and dexterity for inserting and removing it on her own; individuals not comfortable or physically able to do so will need to have the pessary removed and cleaned by a clinician on a regular basis in the office. (Part 2 of this article will discuss pessary cleansing intervals).
4. Space-filling pessaries (such as the cube and donut) are useful when there is a severe degree of prolapse and insufficient perineal support to maintain a Gellhorn pessary. In practice, they are generally used less frequently—which is unfortunate, as they are a potentially useful solution for older women with severe prolapse who might not be candidates for surgical repair. As mentioned, both the cube and donut pessaries require more frequent removal for cleaning.
5. In unusual cases, the use of 2 pessaries simultaneously may resolve a difficult problem, such as when a pessary is the only option for treatment, the prolapse is severe, or it is impossible to find a pessary that resists being expelled from the vagina.14 A space-filling pessary in the most cephalad aspect of the vagina used in conjunction with a ring pessary with support shield below it can sometimes resolve even the worst cases of prolapse.
Continue to: Stay tuned...
Stay tuned
Part 2 of this article next month will provide more information on pessaries, including fitting, aftercare, potential complications, and effectiveness in various disorders. ●
Pessaries have been used in one form or another to help resolve pelvic organ prolapse (POP) in women for at least 2,500 years. They have come in many shapes and have been made of many materials. Here is a brief sketch of the history of the pessary.
Antiquity
Kahun papyrus (ancient Egypt, c. 2000 BCE)
Women with POP were made to stand over a fire in which different ingredients were burned. It was thought that the disagreeable odors emitted would cause the uterus to “rebel” and thus revert back into place.1
Hippocrates (c. 460–375 BCE)
Used several techniques to resolve uterine prolapse:
- Tipping the woman upside down and shaking her, using gravity as an aid to return the prolapsed organs into the pelvis2
- Cupping of the buttocks and the lower abdomen in hopes of “sucking” the prolapsed uterus back into place3
The Greek physician Polybus (c. 400 BCE)
Placed half a pomegranate in the vagina to hold prolapsed structures in place2
Cleopatra (c. 70–30 BCE)
Treated prolapse with the vaginal application of an astringent liquid2
Celsus (c. 25 BCE–50 CE)
Used cone-shaped pessaries made of bronze with a perforated circular plate on the lower edge through which bands were attached. The bands were then tied around the body to keep the device in place4
The Greek physician Soranus (c. 98–138)
Utilized linen tampons soaked with vinegar—along with a piece of beef—to treat prolapse. These were then held in place by bands passed around the loins2
Galen (c. 130–210)
Used fumigation to “encourage” the uterus to return to the pelvis2
Middle Ages
Paulus of Aegina (c. 625–690) and Abbas (c. 949–982)
Both wrote about the use of pessaries made of wax3
Myrepsus (late 13th century)
Described the preparation of 45 types of pessaries consisting of different solid materials treated with perfumes, wax, honey, and herbs5
16th century
Caspar Stromayr (Practica Copiosa, 1559)
Used as pessaries tightly rolled sponges bound with string, dipped in wax, and covered with oil or butter6
Ambroise Paré (c. 1510–1590)
Developed the first ring-type pessary in the late 16th century. He used hammered brass and waxed cork in the shape of an oval to treat uterine prolapse. He also made ring-shaped devices of gold, silver, or brass which were kept in place by a belt around the waist.7
17th century de Castro (1546–1627)
Urged “attacking” uterine prolapse with application of a red-hot iron thus “frightening it” into receding back into the vagina8
Hendrik van Roonhuyse (1625–1672)
In his gynecology textbook, discussed the etiology and treatment of prolapse. He utilized a cork with a hole in it (to allow for passage of discharge) as prolapse treatment. He also wrote of removing an obstructed wax pessary that had blocked discharge of a patient’s vaginal secretions for many years4
18th century Thomas Simson (1696-1764)
Invented a metal spring device that kept a pessary made of cork in place9
John Leake (1729-1792)
Recommended the use of sponges as pessaries to avoid vaginal prolapse10
Juville (1783)
Was the first to use rubber pessaries, resembling today’s contraceptive cup, to avoid injuring the vaginal mucosa. The center of the cup was perforated with a gold tip which allowed for the discharge of vaginal secretions10
19th century
Scanzoni (1821-1891)
Recommended massage and the application of leeches to reduce local congestion and swelling of prolapsed pelvic organs before manual replacement11
Hugh Lenox Hodge (1796-1873)
In his 1860 textbook Diseases Peculiar to Women, Hodge discussed at length the use of pessaries for uterine displacement. He suggested that metals, alloys, glass, and porcelain be used for pessaries rather than cork, wax, and sponges12
20th century
1950s—
Pessaries made of rubber, which absorb discharge and odor, are replaced by polystyrene pessaries. Currently, pessaries are made of silicone, plastic, and latex.
References
- Stevens JM. Gynecology from ancient Egypt: the papyrus Kahun, a translation of the oldest treatise on gynecology that has survived from the ancient world. Med J Austr. 1975;2:949-952.
- Emge LA, Durfee RB. Pelvic organ prolapse: four thousand years of treatment. Clin Obstet Gynecol. 1966;9:997-1032.
- Van Dongen L. The anatomy of genital prolapse. South Afr Med J. 1981;60:357-359.
- Cianfrani T. Short History of Obstetrics and Gynecology. Springfield, IL: Charles C Thomas; 1960.
- Leonardo RA. History of Gynecology. New York, NY: Froben Press; 1944.
- Tizzano AP, Muffly TM. Historical milestones in female pelvic surgery, gynecology, and female urology. In: Walters M, Karram M. Urogynecology and Reconstructive Pelvic Surgery, 4th ed. Philadelphia, PA: Elsevier Saunders; 2015
- Farrell SA. Pessaries in Clinical Practice. Switzerland: Springer-Verlag; 2006.
- Tam T, Davies MF, eds. Vaginal Pessaries. Boca Raton, FL: CRC Press; 2019.
- Ricci JV. Genealogy of Gynaecology. Philadelphia, PA: Blakiston; 1950.
- Miller DS. Contemporary use of the pessary. In Sciarra JJ, ed. Gynecology and Obstetrics. Philadelphia, PA: JB Lippincott Company; 1995.
- Thomas TG. A Practical Treatise on the Disorders of Women. Philadelphia, PA: Lea Brothers and Co; 1891.
- Hodge HL. Diseases Peculiar to Women, Including Displacements of the Uterus. Philadelphia, PA: Blanchard and Lea; 1860.
- Zoorob D, Higgins M, Swan K, et al. Barriers to pelvic floor physical therapy regarding treatment of high-tone pelvic floor dysfunction. Female Pelvic Med Reconstr Surg. 2017;23:444-448.
- Kirby AC, Luber KM, Menefee SA. An update on the current and future demand for care of pelvic floor disorders in the United States. Am J Obstet Gynecol. 2013;209:584.e1-584.e5.
- Ellerkmann RM, Cundiff GW, Melick CF, et al. Correlation of symptoms with location and severity of pelvic organ prolapse. Am J Obstet Gynecol. 2001;185:1332-1337.
- US Census Bureau. United States population projections: 2000 to 2050. https://www.census.gov/library/workingpapers/2009/demo/us-pop-proj-2000-2050.html. Accessed November 13, 2020.
- Pott-Grinstein E, Newcomer JR. Gynecologists’ patterns of prescribing pessaries. J Reprod Med. 2001;46:205-208.
- Chaikin DC, Groutz A, Blaivas JG. Predicting the need for anti-incontinence surgery in continent women undergoing repair of severe urogenital prolapse. J Urol. 2000;163:531-534.
- Reena C, Kekre AN, Kekre N. Occult stress incontinence in women with pelvic organ prolapse. Int J Gynaecol Obstet. 2007;97:31-34.
- Thys SD, Roovers JP, Geomini PM, et al. Do patients prefer a pessary or surgery as primary treatment for pelvic organ prolapse. Gynecol Obstet Invest. 2012;74:6-12.
- Kapoor DS, Thakar R, Sultan AH, et al. Conservative versus surgical management of prolapse: what dictates patient choice? Int Urogynecol J Pelvic Floor Dysfunct. 2009;20: 1157-1161.
- Wu V, Farrel SA, Baskett TF, et al. A simplified protocol for pessary management. Obstet Gynecol. 1997;90:990-994.
- Culligan PJ. Nonsurgical management of pelvic organ prolapse. Obstet Gynecol. 2012;119:852-860.
- Cundiff GW, Amundsen CL, Bent AE, et al. The PESSRI study: symptom relief outcomes of a randomized crossover trial of the ring and Gellhorn pessaries. Am J Obstet Gynecol. 2007;196:405.e1-404.e8.
- Cundiff GW, Weidner AC, Visco AG, et al. A survey of pessary use by members of the American Urogynecologic Society. Obstet Gynecol. 2000;95(6 pt 1):931-935.
- Singh K, Reid W. Nonsurgical treatment of uterovaginal prolapse using double vaginal rings. Br J Obstet Gynecol. 2001;108:112-113.
- Zoorob D, Higgins M, Swan K, et al. Barriers to pelvic floor physical therapy regarding treatment of high-tone pelvic floor dysfunction. Female Pelvic Med Reconstr Surg. 2017;23:444-448.
- Kirby AC, Luber KM, Menefee SA. An update on the current and future demand for care of pelvic floor disorders in the United States. Am J Obstet Gynecol. 2013;209:584.e1-584.e5.
- Ellerkmann RM, Cundiff GW, Melick CF, et al. Correlation of symptoms with location and severity of pelvic organ prolapse. Am J Obstet Gynecol. 2001;185:1332-1337.
- US Census Bureau. United States population projections: 2000 to 2050. https://www.census.gov/library/workingpapers/2009/demo/us-pop-proj-2000-2050.html. Accessed November 13, 2020.
- Pott-Grinstein E, Newcomer JR. Gynecologists’ patterns of prescribing pessaries. J Reprod Med. 2001;46:205-208.
- Chaikin DC, Groutz A, Blaivas JG. Predicting the need for anti-incontinence surgery in continent women undergoing repair of severe urogenital prolapse. J Urol. 2000;163:531-534.
- Reena C, Kekre AN, Kekre N. Occult stress incontinence in women with pelvic organ prolapse. Int J Gynaecol Obstet. 2007;97:31-34.
- Thys SD, Roovers JP, Geomini PM, et al. Do patients prefer a pessary or surgery as primary treatment for pelvic organ prolapse. Gynecol Obstet Invest. 2012;74:6-12.
- Kapoor DS, Thakar R, Sultan AH, et al. Conservative versus surgical management of prolapse: what dictates patient choice? Int Urogynecol J Pelvic Floor Dysfunct. 2009;20: 1157-1161.
- Wu V, Farrel SA, Baskett TF, et al. A simplified protocol for pessary management. Obstet Gynecol. 1997;90:990-994.
- Culligan PJ. Nonsurgical management of pelvic organ prolapse. Obstet Gynecol. 2012;119:852-860.
- Cundiff GW, Amundsen CL, Bent AE, et al. The PESSRI study: symptom relief outcomes of a randomized crossover trial of the ring and Gellhorn pessaries. Am J Obstet Gynecol. 2007;196:405.e1-404.e8.
- Cundiff GW, Weidner AC, Visco AG, et al. A survey of pessary use by members of the American Urogynecologic Society. Obstet Gynecol. 2000;95(6 pt 1):931-935.
- Singh K, Reid W. Nonsurgical treatment of uterovaginal prolapse using double vaginal rings. Br J Obstet Gynecol. 2001;108:112-113.
The pill toolbox: How to choose a combined oral contraceptive
In the era of long-acting reversible contraceptives (LARCs), the pill can seem obsolete. However, it is still the second most commonly used birth control method in the United States, chosen by 19% of female contraceptive users as of 2015–2017.1 It also has noncontraceptive benefits, so it is important that obstetrician-gynecologists are well-versed in its uses. In this article, I will focus on combined oral contraceptives (COCs; TABLE 1), reviewing the major risks, benefits, and adverse effects of COCs before focusing on recommendations for particular formulations of COCs for various patient populations.
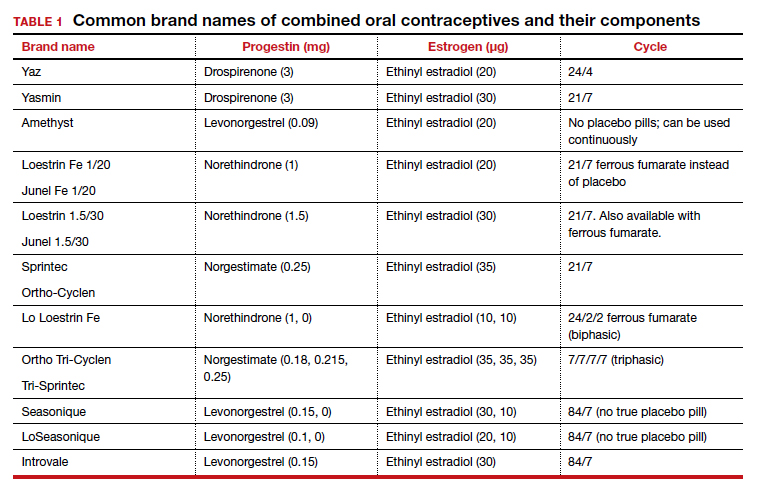
Benefits and risks
There are numerous noncontraceptive benefits of COCs, including menstrual cycle regulation; reduced risk of ovarian, endometrial, and colorectal cancer; and treatment of menorrhagia, dysmenorrhea, acne, menstrual migraine, premenstrual syndrome and premenstrual dysphoric disorder, pelvic pain due to endometriosis, and hirsutism.
Common patient concerns
In terms of adverse effects, there are more potential unwanted effects of concern to women than there are ones validated in the literature. Accepted adverse effects include nausea, breast tenderness, and decreased libido. However, one of the most common concerns voiced during contraceptive counseling is that COCs will cause weight gain. A 2014 Cochrane review identified 49 trials studying the weight gain question.2 Of those, only 4 had a placebo or nonintervention group. Of these 4, there was no significant difference in weight change between the COC-receiving group and the control group. When patients bring up their concerns, it may help to remind them that women tend to gain weight over time whether or not they are taking a COC.
Another common concern is that COCs cause mood changes. A 2016 review by Schaffir and colleagues sheds some light on this topic,3 albeit limited by the paucity of prospective studies. This review identified only 1 randomized controlled trial comparing depression incidence among women initiating a COC versus a placebo. There was no difference in the incidence of depression among the groups at 3 months. Among 4 large retrospective studies of women using COCs, the agents either had no or a beneficial effect on mood. Schaffir’s review reports that there may be greater mood adverse effects with COCs among women with underlying mood disorders.
Patients may worry that COC use will permanently impair their fertility or delay return to fertility after discontinuation. Research does indicate that return of fertility after stopping COCs often takes several months (compared with immediate fertility after discontinuing a barrier method). However, there still seem to be comparable conception rates within 12 months after discontinuing COCs as there are after discontinuing other common nonhormonal or hormonal contraceptive methods. Fertility is not impacted by the duration of COC use. In addition, return to fertility seems to be comparable after discontinuation of extended cycle or continuous COCs compared with traditional-cycle COCs.4
COC safety
Known major risks of COCs include venous thromboembolism (VTE). The risk of VTE is about double among COC users than among nonpregnant nonusers: 3–9 per 10,000 woman-years compared with 1–5.5 In a study by the US Food and Drug Administration, drospirenone-containing COCs had double the risk of VTE than other COCs. However, the position of the American College of Obstetricians and Gynecologists on this increased risk of VTE with drospirenone-containing pills is that it is “possible” and “minimal.”5 It is important to remember that an alternative to COC use is pregnancy, in which the VTE risk is about double that among COC users, at 5–20 per 10,000 woman-years. This risk increases further in the postpartum period, to 40–65 per 10,000 woman-years.5
Another known major risk of COCs is arterial embolic disease, including cerebrovascular accidents and myocardial infarctions. Women at increased risk for these complications include those with hypertension, diabetes, and/or obesity and women who are aged 35 or older and smoke. Interestingly, women with migraines with aura are at increased risk for stroke but not for myocardial infarction. These women increase their risk of stroke 2- to 4-fold if they use COCs.
Continue to: Different pills for different problems...
Different pills for different problems
With so many pills on the market, it is important for clinicians to know how to choose a particular pill for a particular patient. The following discussion assumes that the patient in question desires a COC for contraception, then offers guidance on how to choose a pill with patient-specific noncontraceptive benefits (TABLE 2).
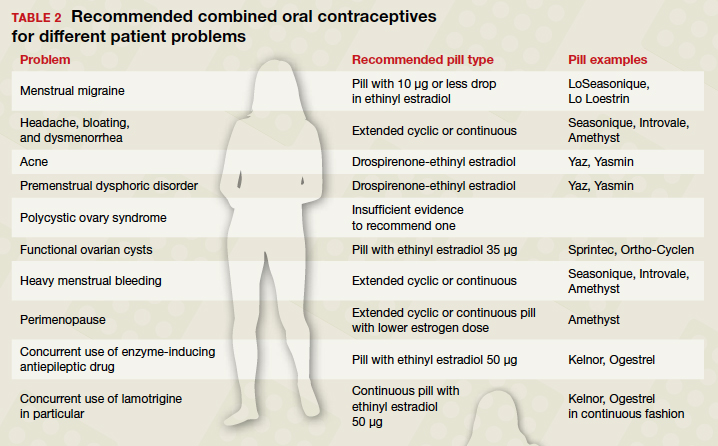
When HMB is a concern. Patients with heavy menstrual bleeding may experience fewer bleeding and/or spotting days with extended cyclic or continuous use of a COC rather than with traditional cyclic use.6 Examples of such COC options include:
- Introvale and Seasonique, both extended-cycle formulations
- Amethyst, which is formulated without placebo pills so that it can be used continuously
- any other COC prescribed with instructions for the patient to skip placebo pills.
An extrapolated benefit to extended-cycle or continuous COCs use for heavy menstrual bleeding is addressing anemia.
For premenstrual dysphoric disorder, the only randomized controlled trials showing improvement involve drospirenone-ethinyl estradiol pills (Yaz and Yasmin).7 There is also evidence that extended cyclic or continuous use of these formulations is more impactful for premenstrual dysphoric disorder than a traditional cycle.8
Keeping migraine avoidance and prevention in mind. Various studies have looked at the impact of different COC formulations on menstrual-related symptoms. There is evidence of greater improvement in headache, bloating, and dysmenorrhea with extended cyclic or continuous use compared with traditional cyclic use.6
In terms of headache, let us delve into menstrual migraine in particular. Menstrual migraines occur sometime between 2 days prior to 2 days after the first day of menses and are linked to a sharp drop in estrogen levels. COCs are contraindicated in women with menstrual migraines with aura because of the increased stroke risk. For women with menstrual migraines without aura, COCs can prevent migraines. Prevention depends on minimizing fluctuations in estrogen levels; any change in estrogen level greater than 10 µg of ethinyl estradiol may trigger an estrogen-related migraine. All currently available regimens of COCs that comprise 21 days of active pills and 7 days of placebo involve a drop of more than 10 µg. Options that involve a drop of 10 µg or less include any continuous formulation, the extended formulation LoSeasonique (levonorgestrel 0.1 mg and ethinyl estradiol 20 µg for 84 days, then ethinyl estradiol 10 µg for 7 days), and Lo Loestrin (ethinyl estradiol 10 µg and norethindrone 1 mg for 24 days, then ethinyl estradiol 10 µg for 2 days, then placebo for 2 days).9
What’s best for acne-prone patients? All COCs should improve acne by increasing levels of sex hormone binding globulin. However, some comparative studies have shown drospirenone-containing COCs to be the most effective for acne. This finding makes sense in light of studies demonstrating antiandrogenic effects of drospirenone.10
Managing PCOS symptoms. It seems logical, by extension, that drospirenone-containing COCs would be particularly beneficial for treating hirsutism associated with polycystic ovary syndrome (PCOS). Other low‒androgenic-potential progestins, such as a third-generation progestin (norgestimate or desogestrel), might similarly be hypothesized to be advantageous. However, there is currently insufficient evidence to recommend any one COC formulation over another for the indication of PCOS.11
Ovarian cysts: Can COCs be helpful? COCs are commonly prescribed by gynecologists for patients with functional ovarian cysts. It is important to note that COCs have not been found to hasten the resolution of existing cysts, so they should not be used for this purpose.12 Studies of early COCs, which had high doses of estrogen (on the order of 50 µg), showed lower rates of cysts among users. This effect seems to be attenuated with the lower-estrogen-dose pills that are currently available, but there still appears to be benefit. Therefore, for a patient prone to cysts who desires an oral contraceptive, a COC containing estrogen 35 µg is likely to be the most beneficial of COCs currently on the market.13,14
Lower-dosage COCs in perimenopause may be beneficial. COCs can ameliorate perimenopausal symptoms including abnormal uterine bleeding and vasomotor symptoms. Clinicians are often hesitant to prescribe COCs for perimenopausal women because of increased risk of VTE, stroke, myocardial infarction, and breast cancer with increasing age. However, age alone is not a contraindication to any contraceptive method. An extended cyclic or continuous regimen COC may be the best choice for a perimenopausal woman in order to avoid vasomotor symptoms that occur during hormone-free intervals. In addition, given the increasing risk of adverse effects like VTE with estrogen dose, a lower estrogen formulation is advisable.15
Patients with epilepsy who are taking antiepileptic drugs (AEDs) are a special population when it comes to COCs. Certain AEDs induce hepatic enzymes involved in the metabolism and protein binding of COCs, which can result in contraceptive failure. Strong inducers are carbamazepine, oxcarbazepine, perampanel, phenobarbital, phenytoin, and primidone. Weak inducers are clobazam, eslicarbazepine, felbamate, lamotrigine, rufinamide, and topiramate. Women taking any of the above AEDs are recommended to choose a different form of contraception than a COC. However, if they are limited to COCs for some reason, a preparation containing estrogen 50 µg is recommended. It is speculated that the efficacy and adverse effects of COCs with increased hormone doses, used in combination with enzyme-inducing AEDs, should be comparable to those with standard doses when not combined with AEDs; however, this speculation is unproven.16 There are few COCs on the market with estrogen doses of 50 µg, but a couple of examples are Kelnor and Ogestrel.
Additional factors have to be considered with concurrent COC use with the AED lamotrigine since COCs increase clearance of this agent. Therefore, patients taking lamotrigine who start COCs will need an increase in lamotrigine dose. To avoid fluctuations in lamotrigine serum levels, use of a continuous COC is recommended.17
Continue to: Pill types to minimize adverse effects or risks...
Pill types to minimize adverse effects or risks
For women who desire to use a COC for contraception but who are at risk for a particular complication or are bothered by a particular adverse effect, ObGyns can optimize the choice of pill (TABLE 3). For example, women who have adverse effects of nausea and/or breast tenderness may benefit from reducing the estrogen dose to 20 µg or lower.18

Considering VTE
As discussed previously, VTE is a risk with all COCs, but some pills confer greater risk than others. For one, VTE risk increases with estrogen dose. In addition, VTE risk depends on the type of progestin. Drospirenone and third-generation progestins (norgestimate, gestodene, and desogestrel) confer a higher risk of VTE than first- or second-generation progestins. For example, a pill with estradiol 30 µg and either a third-generation progestin or drospirenone has a 50% to 80% higher risk of VTE compared with a pill with estradiol 30 µg and levonorgestrel.
For patients at particularly high risk for VTE, COCs are contraindicated. For patients for whom COCs are considered medically appropriate but who are at higher risk (eg, obese women), it is wise to use a pill containing a first-generation (norethindrone) or second-generation progestin (levonorgestrel) combined with the lowest dose of estrogen that has tolerable adverse effects.19
What about hypertension concerns?
Let us turn our attention briefly to hypertension and its relation to COC use. While the American College of Cardiology and the American Heart Association redefined hypertension in 2017 using a threshold of 130/80 mm Hg, the American College of Obstetricians and Gynecologists (ACOG) considers hypertension to be 140/90 mm Hg in terms of safety of using COCs. ACOG states, “women with blood pressure below 140/90 mm Hg may use any hormonal contraceptive method.”20 In women with hypertension in the range of 140‒159 mm Hg systolic or 90‒99 mm Hg diastolic, COCs are category 3 according to the US Medical Eligibility Criteria for Contraceptive Use, meaning that the risks usually outweigh the benefits. For women with blood pressures of 160/110 mm Hg or greater, COCs are category 4 (contraindicated). If a woman with mild hypertension is started on a COC, a drospirenone-containing pill may be the best choice because of its diuretic effects. While other contemporary COCs have been associated with a mild increase in blood pressure, drospirenone-containing pills have not shown this association.21
Continue to: At issue: Break-through bleeding, mood, and weight gain...
At issue: Break-through bleeding, mood, and weight gain
For women bothered by intermenstrual bleeding, use of a COC with a third-generation progestin may be preferable to use of one with a first- or second-generation. It may be because of decreased abnormal bleeding that COCs with third-generation progestins have lower discontinuation rates.22 In addition, COCs containing estrogen 20 µg or less are associated with more intermenstrual bleeding than those with more than 20 µg estrogen.23 Keep in mind that it is common with any COC to have intermenstrual bleeding for the first several months.
For women with pre-existing mood disorders or who report mood changes with COCs, it appears that fluctuations in hormone levels are problematic. Consistently, there is evidence that monophasic pills are preferable to multiphasic and that extended cyclic or continuous use is preferable to traditional cyclic use for mitigating mood adverse effects. There is mixed evidence on whether a low dose of ethinyl estradiol is better for mood.3
Although it is discussed above that randomized controlled trials have not shown an association between COC use and weight gain, many women remain concerned. For these women, a drospirenone-containing COC may be the best choice. Drospirenone has antimineralocorticoid activity, so it may help prevent water retention.
A brief word about multiphasic COCs. While these pills were designed to mimic physiologic hormone fluctuations and minimize hormonal adverse effects, there is insufficient evidence to compare their effects to those of monophasic pills.24 Without such evidence, there is little reason to recommend a multiphasic pill to a patient over the more straightforward monophasic formulation.
Conclusion
There are more nuances to prescribing an optimal COC for a patient than may initially come to mind. It is useful to remember that any formulation of pill may be prescribed in an extended or continuous fashion, and there are benefits for such use for premenstrual dysphoric disorder, heavy menstrual bleeding, perimenopause, and menstrual symptoms. Although there are numerous brands of COCs available, a small cadre will suffice for almost all purposes. Such a “toolbox” of pills could include a pill formatted for continuous use (Seasonique), a low estrogen pill (Loestrin), a drospirenone-containing pill (Yaz), and a pill containing a third-generation progestin and a higher dose of estrogen (Sprintec). ●
- Daniels K, Abma JC. Current contraceptive status among women aged 15-49: United States, 2015-2017. NCHS Data Brief, no 327. Hyattsville, MD; 2018.
- Gallo MF, Lopez LM, Grimes DA, et al. Combination contraceptives: effects on weight. Cochrane Database Syst Rev. 2014:CD003987.
- Schaffir J, Worly BL, Gur TL. Combined hormonal contraception and its effects on mood: a critical review. Eur J Contracept Reprod Health Care. 2016;21:347-355.
- Barnhart KT, Schreiber CA. Return to fertility following discontinuation of oral contraceptives. Fertil Steril. 2009;91:659-663.
- American College of Obstetricians and Gynecologists. Committee Opinion #540: Risk of Venous Thromboembolism Among Users of Drospirenone-Containing Oral Contraceptive Pills. Obstet Gynecol. 2012;120:1239-1242.
- Edelman A, Micks E, Gallo MF, et al. Continuous or extended cycle vs. cyclic use of combined hormonal contraceptives for contraception. Cochrane Database Syst Rev. 2014:CD004695.
- American College of Obstetricians and Gynecologists. Practice Bulletin #110: Noncontraceptive Uses of Hormonal Contraceptives. Obstet Gynecol. 2010:206-218.
- Coffee AL, Kuehl TJ, Willis S, et al. Oral contraceptives and premenstrual symptoms: comparison of a 21/7 and extended regimen. Am J Obstet Gynecol. 2006;195:1311-1319.
- Calhoun AH, Batur P. Combined hormonal contraceptives and migraine: an update on the evidence. Cleve Clin J Med. 2017;84:631-638.
- Arowojolu AO, Gallo MF, Lopez LM, et al. Combined oral contraceptive pills for treatment of acne. Cochrane Database Syst Rev. 2012:CD004425.
- McCartney CR, Marshall JC. CLINICAL PRACTICE. Polycystic Ovary Syndrome. N Engl J Med. 2016;375:54-64.
- Grimes DA, Jones LB, Lopez LM, et al. Oral contraceptives for functional ovarian cysts. Cochrane Database Syst Rev. 2014:CD006134.
- Grimes DA, Godwin AJ, Rubin A, et al. Ovulation and follicular development associated with three low-dose oral contraceptives: a randomized controlled trial. Obstet Gynecol. 1994;83:29-34.
- Christensen JT, Boldsen JL, Westergaard JG. Functional ovarian cysts in premenopausal and gynecologically healthy women. Contraception. 2002;66:153-157.
- Hardman SM, Gebbie AE. Hormonal contraceptive regimens in the perimenopause. Maturitas. 2009;63:204-212.
- Zupanc ML. Antiepileptic drugs and hormonal contraceptives in adolescent women with epilepsy. Neurology. 2006;66 (6 suppl 3):S37-S45.
- Wegner I, Edelbroek PM, Bulk S, et al. Lamotrigine kinetics within the menstrual cycle, after menopause, and with oral contraceptives. Neurology. 2009;73:1388-1393.
- Stewart M, Black K. Choosing a combined oral contraceptive pill. Australian Prescriber. 2015;38:6-11.
- de Bastos M, Stegeman BH, Rosendaal FR, et al. Combined oral contraceptives: venous thrombosis. Cochrane Database Syst Rev. 2014:CD010813.
- American College of Obstetricians and Gynecologists. Practice Bulletin #206: use of hormonal contraception in women with coexisting medical conditions. Obstet Gynecol. 2019;133:e128-e150.
- de Morais TL, Giribela C, Nisenbaum MG, et al. Effects of a contraceptive containing drospirenone and ethinylestradiol on blood pressure, metabolic profile and neurohumoral axis in hypertensive women at reproductive age. Eur J Obstet Gynecol Reprod Biol. 2014;182:113-117.
- Lawrie TA, Helmerhorst FM, Maitra NK, et al. Types of progestogens in combined oral contraception: effectiveness and side-effects. Cochrane Database Syst Rev. 2011:CD004861.
- Gallo MF, Nanda K, Grimes DA, et al. 20 µg versus >20 µg estrogen combined oral contraceptives for contraception. Cochrane Database Syst Rev. 2013:CD003989.
- van Vliet HA, Grimes DA, Lopez LM, et al. Triphasic versus monophasic oral contraceptives for contraception. Cochrane Database Syst Rev. 2006:CD003553
In the era of long-acting reversible contraceptives (LARCs), the pill can seem obsolete. However, it is still the second most commonly used birth control method in the United States, chosen by 19% of female contraceptive users as of 2015–2017.1 It also has noncontraceptive benefits, so it is important that obstetrician-gynecologists are well-versed in its uses. In this article, I will focus on combined oral contraceptives (COCs; TABLE 1), reviewing the major risks, benefits, and adverse effects of COCs before focusing on recommendations for particular formulations of COCs for various patient populations.

Benefits and risks
There are numerous noncontraceptive benefits of COCs, including menstrual cycle regulation; reduced risk of ovarian, endometrial, and colorectal cancer; and treatment of menorrhagia, dysmenorrhea, acne, menstrual migraine, premenstrual syndrome and premenstrual dysphoric disorder, pelvic pain due to endometriosis, and hirsutism.
Common patient concerns
In terms of adverse effects, there are more potential unwanted effects of concern to women than there are ones validated in the literature. Accepted adverse effects include nausea, breast tenderness, and decreased libido. However, one of the most common concerns voiced during contraceptive counseling is that COCs will cause weight gain. A 2014 Cochrane review identified 49 trials studying the weight gain question.2 Of those, only 4 had a placebo or nonintervention group. Of these 4, there was no significant difference in weight change between the COC-receiving group and the control group. When patients bring up their concerns, it may help to remind them that women tend to gain weight over time whether or not they are taking a COC.
Another common concern is that COCs cause mood changes. A 2016 review by Schaffir and colleagues sheds some light on this topic,3 albeit limited by the paucity of prospective studies. This review identified only 1 randomized controlled trial comparing depression incidence among women initiating a COC versus a placebo. There was no difference in the incidence of depression among the groups at 3 months. Among 4 large retrospective studies of women using COCs, the agents either had no or a beneficial effect on mood. Schaffir’s review reports that there may be greater mood adverse effects with COCs among women with underlying mood disorders.
Patients may worry that COC use will permanently impair their fertility or delay return to fertility after discontinuation. Research does indicate that return of fertility after stopping COCs often takes several months (compared with immediate fertility after discontinuing a barrier method). However, there still seem to be comparable conception rates within 12 months after discontinuing COCs as there are after discontinuing other common nonhormonal or hormonal contraceptive methods. Fertility is not impacted by the duration of COC use. In addition, return to fertility seems to be comparable after discontinuation of extended cycle or continuous COCs compared with traditional-cycle COCs.4
COC safety
Known major risks of COCs include venous thromboembolism (VTE). The risk of VTE is about double among COC users than among nonpregnant nonusers: 3–9 per 10,000 woman-years compared with 1–5.5 In a study by the US Food and Drug Administration, drospirenone-containing COCs had double the risk of VTE than other COCs. However, the position of the American College of Obstetricians and Gynecologists on this increased risk of VTE with drospirenone-containing pills is that it is “possible” and “minimal.”5 It is important to remember that an alternative to COC use is pregnancy, in which the VTE risk is about double that among COC users, at 5–20 per 10,000 woman-years. This risk increases further in the postpartum period, to 40–65 per 10,000 woman-years.5
Another known major risk of COCs is arterial embolic disease, including cerebrovascular accidents and myocardial infarctions. Women at increased risk for these complications include those with hypertension, diabetes, and/or obesity and women who are aged 35 or older and smoke. Interestingly, women with migraines with aura are at increased risk for stroke but not for myocardial infarction. These women increase their risk of stroke 2- to 4-fold if they use COCs.
Continue to: Different pills for different problems...
Different pills for different problems
With so many pills on the market, it is important for clinicians to know how to choose a particular pill for a particular patient. The following discussion assumes that the patient in question desires a COC for contraception, then offers guidance on how to choose a pill with patient-specific noncontraceptive benefits (TABLE 2).

When HMB is a concern. Patients with heavy menstrual bleeding may experience fewer bleeding and/or spotting days with extended cyclic or continuous use of a COC rather than with traditional cyclic use.6 Examples of such COC options include:
- Introvale and Seasonique, both extended-cycle formulations
- Amethyst, which is formulated without placebo pills so that it can be used continuously
- any other COC prescribed with instructions for the patient to skip placebo pills.
An extrapolated benefit to extended-cycle or continuous COCs use for heavy menstrual bleeding is addressing anemia.
For premenstrual dysphoric disorder, the only randomized controlled trials showing improvement involve drospirenone-ethinyl estradiol pills (Yaz and Yasmin).7 There is also evidence that extended cyclic or continuous use of these formulations is more impactful for premenstrual dysphoric disorder than a traditional cycle.8
Keeping migraine avoidance and prevention in mind. Various studies have looked at the impact of different COC formulations on menstrual-related symptoms. There is evidence of greater improvement in headache, bloating, and dysmenorrhea with extended cyclic or continuous use compared with traditional cyclic use.6
In terms of headache, let us delve into menstrual migraine in particular. Menstrual migraines occur sometime between 2 days prior to 2 days after the first day of menses and are linked to a sharp drop in estrogen levels. COCs are contraindicated in women with menstrual migraines with aura because of the increased stroke risk. For women with menstrual migraines without aura, COCs can prevent migraines. Prevention depends on minimizing fluctuations in estrogen levels; any change in estrogen level greater than 10 µg of ethinyl estradiol may trigger an estrogen-related migraine. All currently available regimens of COCs that comprise 21 days of active pills and 7 days of placebo involve a drop of more than 10 µg. Options that involve a drop of 10 µg or less include any continuous formulation, the extended formulation LoSeasonique (levonorgestrel 0.1 mg and ethinyl estradiol 20 µg for 84 days, then ethinyl estradiol 10 µg for 7 days), and Lo Loestrin (ethinyl estradiol 10 µg and norethindrone 1 mg for 24 days, then ethinyl estradiol 10 µg for 2 days, then placebo for 2 days).9
What’s best for acne-prone patients? All COCs should improve acne by increasing levels of sex hormone binding globulin. However, some comparative studies have shown drospirenone-containing COCs to be the most effective for acne. This finding makes sense in light of studies demonstrating antiandrogenic effects of drospirenone.10
Managing PCOS symptoms. It seems logical, by extension, that drospirenone-containing COCs would be particularly beneficial for treating hirsutism associated with polycystic ovary syndrome (PCOS). Other low‒androgenic-potential progestins, such as a third-generation progestin (norgestimate or desogestrel), might similarly be hypothesized to be advantageous. However, there is currently insufficient evidence to recommend any one COC formulation over another for the indication of PCOS.11
Ovarian cysts: Can COCs be helpful? COCs are commonly prescribed by gynecologists for patients with functional ovarian cysts. It is important to note that COCs have not been found to hasten the resolution of existing cysts, so they should not be used for this purpose.12 Studies of early COCs, which had high doses of estrogen (on the order of 50 µg), showed lower rates of cysts among users. This effect seems to be attenuated with the lower-estrogen-dose pills that are currently available, but there still appears to be benefit. Therefore, for a patient prone to cysts who desires an oral contraceptive, a COC containing estrogen 35 µg is likely to be the most beneficial of COCs currently on the market.13,14
Lower-dosage COCs in perimenopause may be beneficial. COCs can ameliorate perimenopausal symptoms including abnormal uterine bleeding and vasomotor symptoms. Clinicians are often hesitant to prescribe COCs for perimenopausal women because of increased risk of VTE, stroke, myocardial infarction, and breast cancer with increasing age. However, age alone is not a contraindication to any contraceptive method. An extended cyclic or continuous regimen COC may be the best choice for a perimenopausal woman in order to avoid vasomotor symptoms that occur during hormone-free intervals. In addition, given the increasing risk of adverse effects like VTE with estrogen dose, a lower estrogen formulation is advisable.15
Patients with epilepsy who are taking antiepileptic drugs (AEDs) are a special population when it comes to COCs. Certain AEDs induce hepatic enzymes involved in the metabolism and protein binding of COCs, which can result in contraceptive failure. Strong inducers are carbamazepine, oxcarbazepine, perampanel, phenobarbital, phenytoin, and primidone. Weak inducers are clobazam, eslicarbazepine, felbamate, lamotrigine, rufinamide, and topiramate. Women taking any of the above AEDs are recommended to choose a different form of contraception than a COC. However, if they are limited to COCs for some reason, a preparation containing estrogen 50 µg is recommended. It is speculated that the efficacy and adverse effects of COCs with increased hormone doses, used in combination with enzyme-inducing AEDs, should be comparable to those with standard doses when not combined with AEDs; however, this speculation is unproven.16 There are few COCs on the market with estrogen doses of 50 µg, but a couple of examples are Kelnor and Ogestrel.
Additional factors have to be considered with concurrent COC use with the AED lamotrigine since COCs increase clearance of this agent. Therefore, patients taking lamotrigine who start COCs will need an increase in lamotrigine dose. To avoid fluctuations in lamotrigine serum levels, use of a continuous COC is recommended.17
Continue to: Pill types to minimize adverse effects or risks...
Pill types to minimize adverse effects or risks
For women who desire to use a COC for contraception but who are at risk for a particular complication or are bothered by a particular adverse effect, ObGyns can optimize the choice of pill (TABLE 3). For example, women who have adverse effects of nausea and/or breast tenderness may benefit from reducing the estrogen dose to 20 µg or lower.18

Considering VTE
As discussed previously, VTE is a risk with all COCs, but some pills confer greater risk than others. For one, VTE risk increases with estrogen dose. In addition, VTE risk depends on the type of progestin. Drospirenone and third-generation progestins (norgestimate, gestodene, and desogestrel) confer a higher risk of VTE than first- or second-generation progestins. For example, a pill with estradiol 30 µg and either a third-generation progestin or drospirenone has a 50% to 80% higher risk of VTE compared with a pill with estradiol 30 µg and levonorgestrel.
For patients at particularly high risk for VTE, COCs are contraindicated. For patients for whom COCs are considered medically appropriate but who are at higher risk (eg, obese women), it is wise to use a pill containing a first-generation (norethindrone) or second-generation progestin (levonorgestrel) combined with the lowest dose of estrogen that has tolerable adverse effects.19
What about hypertension concerns?
Let us turn our attention briefly to hypertension and its relation to COC use. While the American College of Cardiology and the American Heart Association redefined hypertension in 2017 using a threshold of 130/80 mm Hg, the American College of Obstetricians and Gynecologists (ACOG) considers hypertension to be 140/90 mm Hg in terms of safety of using COCs. ACOG states, “women with blood pressure below 140/90 mm Hg may use any hormonal contraceptive method.”20 In women with hypertension in the range of 140‒159 mm Hg systolic or 90‒99 mm Hg diastolic, COCs are category 3 according to the US Medical Eligibility Criteria for Contraceptive Use, meaning that the risks usually outweigh the benefits. For women with blood pressures of 160/110 mm Hg or greater, COCs are category 4 (contraindicated). If a woman with mild hypertension is started on a COC, a drospirenone-containing pill may be the best choice because of its diuretic effects. While other contemporary COCs have been associated with a mild increase in blood pressure, drospirenone-containing pills have not shown this association.21
Continue to: At issue: Break-through bleeding, mood, and weight gain...
At issue: Break-through bleeding, mood, and weight gain
For women bothered by intermenstrual bleeding, use of a COC with a third-generation progestin may be preferable to use of one with a first- or second-generation. It may be because of decreased abnormal bleeding that COCs with third-generation progestins have lower discontinuation rates.22 In addition, COCs containing estrogen 20 µg or less are associated with more intermenstrual bleeding than those with more than 20 µg estrogen.23 Keep in mind that it is common with any COC to have intermenstrual bleeding for the first several months.
For women with pre-existing mood disorders or who report mood changes with COCs, it appears that fluctuations in hormone levels are problematic. Consistently, there is evidence that monophasic pills are preferable to multiphasic and that extended cyclic or continuous use is preferable to traditional cyclic use for mitigating mood adverse effects. There is mixed evidence on whether a low dose of ethinyl estradiol is better for mood.3
Although it is discussed above that randomized controlled trials have not shown an association between COC use and weight gain, many women remain concerned. For these women, a drospirenone-containing COC may be the best choice. Drospirenone has antimineralocorticoid activity, so it may help prevent water retention.
A brief word about multiphasic COCs. While these pills were designed to mimic physiologic hormone fluctuations and minimize hormonal adverse effects, there is insufficient evidence to compare their effects to those of monophasic pills.24 Without such evidence, there is little reason to recommend a multiphasic pill to a patient over the more straightforward monophasic formulation.
Conclusion
There are more nuances to prescribing an optimal COC for a patient than may initially come to mind. It is useful to remember that any formulation of pill may be prescribed in an extended or continuous fashion, and there are benefits for such use for premenstrual dysphoric disorder, heavy menstrual bleeding, perimenopause, and menstrual symptoms. Although there are numerous brands of COCs available, a small cadre will suffice for almost all purposes. Such a “toolbox” of pills could include a pill formatted for continuous use (Seasonique), a low estrogen pill (Loestrin), a drospirenone-containing pill (Yaz), and a pill containing a third-generation progestin and a higher dose of estrogen (Sprintec). ●
In the era of long-acting reversible contraceptives (LARCs), the pill can seem obsolete. However, it is still the second most commonly used birth control method in the United States, chosen by 19% of female contraceptive users as of 2015–2017.1 It also has noncontraceptive benefits, so it is important that obstetrician-gynecologists are well-versed in its uses. In this article, I will focus on combined oral contraceptives (COCs; TABLE 1), reviewing the major risks, benefits, and adverse effects of COCs before focusing on recommendations for particular formulations of COCs for various patient populations.

Benefits and risks
There are numerous noncontraceptive benefits of COCs, including menstrual cycle regulation; reduced risk of ovarian, endometrial, and colorectal cancer; and treatment of menorrhagia, dysmenorrhea, acne, menstrual migraine, premenstrual syndrome and premenstrual dysphoric disorder, pelvic pain due to endometriosis, and hirsutism.
Common patient concerns
In terms of adverse effects, there are more potential unwanted effects of concern to women than there are ones validated in the literature. Accepted adverse effects include nausea, breast tenderness, and decreased libido. However, one of the most common concerns voiced during contraceptive counseling is that COCs will cause weight gain. A 2014 Cochrane review identified 49 trials studying the weight gain question.2 Of those, only 4 had a placebo or nonintervention group. Of these 4, there was no significant difference in weight change between the COC-receiving group and the control group. When patients bring up their concerns, it may help to remind them that women tend to gain weight over time whether or not they are taking a COC.
Another common concern is that COCs cause mood changes. A 2016 review by Schaffir and colleagues sheds some light on this topic,3 albeit limited by the paucity of prospective studies. This review identified only 1 randomized controlled trial comparing depression incidence among women initiating a COC versus a placebo. There was no difference in the incidence of depression among the groups at 3 months. Among 4 large retrospective studies of women using COCs, the agents either had no or a beneficial effect on mood. Schaffir’s review reports that there may be greater mood adverse effects with COCs among women with underlying mood disorders.
Patients may worry that COC use will permanently impair their fertility or delay return to fertility after discontinuation. Research does indicate that return of fertility after stopping COCs often takes several months (compared with immediate fertility after discontinuing a barrier method). However, there still seem to be comparable conception rates within 12 months after discontinuing COCs as there are after discontinuing other common nonhormonal or hormonal contraceptive methods. Fertility is not impacted by the duration of COC use. In addition, return to fertility seems to be comparable after discontinuation of extended cycle or continuous COCs compared with traditional-cycle COCs.4
COC safety
Known major risks of COCs include venous thromboembolism (VTE). The risk of VTE is about double among COC users than among nonpregnant nonusers: 3–9 per 10,000 woman-years compared with 1–5.5 In a study by the US Food and Drug Administration, drospirenone-containing COCs had double the risk of VTE than other COCs. However, the position of the American College of Obstetricians and Gynecologists on this increased risk of VTE with drospirenone-containing pills is that it is “possible” and “minimal.”5 It is important to remember that an alternative to COC use is pregnancy, in which the VTE risk is about double that among COC users, at 5–20 per 10,000 woman-years. This risk increases further in the postpartum period, to 40–65 per 10,000 woman-years.5
Another known major risk of COCs is arterial embolic disease, including cerebrovascular accidents and myocardial infarctions. Women at increased risk for these complications include those with hypertension, diabetes, and/or obesity and women who are aged 35 or older and smoke. Interestingly, women with migraines with aura are at increased risk for stroke but not for myocardial infarction. These women increase their risk of stroke 2- to 4-fold if they use COCs.
Continue to: Different pills for different problems...
Different pills for different problems
With so many pills on the market, it is important for clinicians to know how to choose a particular pill for a particular patient. The following discussion assumes that the patient in question desires a COC for contraception, then offers guidance on how to choose a pill with patient-specific noncontraceptive benefits (TABLE 2).

When HMB is a concern. Patients with heavy menstrual bleeding may experience fewer bleeding and/or spotting days with extended cyclic or continuous use of a COC rather than with traditional cyclic use.6 Examples of such COC options include:
- Introvale and Seasonique, both extended-cycle formulations
- Amethyst, which is formulated without placebo pills so that it can be used continuously
- any other COC prescribed with instructions for the patient to skip placebo pills.
An extrapolated benefit to extended-cycle or continuous COCs use for heavy menstrual bleeding is addressing anemia.
For premenstrual dysphoric disorder, the only randomized controlled trials showing improvement involve drospirenone-ethinyl estradiol pills (Yaz and Yasmin).7 There is also evidence that extended cyclic or continuous use of these formulations is more impactful for premenstrual dysphoric disorder than a traditional cycle.8
Keeping migraine avoidance and prevention in mind. Various studies have looked at the impact of different COC formulations on menstrual-related symptoms. There is evidence of greater improvement in headache, bloating, and dysmenorrhea with extended cyclic or continuous use compared with traditional cyclic use.6
In terms of headache, let us delve into menstrual migraine in particular. Menstrual migraines occur sometime between 2 days prior to 2 days after the first day of menses and are linked to a sharp drop in estrogen levels. COCs are contraindicated in women with menstrual migraines with aura because of the increased stroke risk. For women with menstrual migraines without aura, COCs can prevent migraines. Prevention depends on minimizing fluctuations in estrogen levels; any change in estrogen level greater than 10 µg of ethinyl estradiol may trigger an estrogen-related migraine. All currently available regimens of COCs that comprise 21 days of active pills and 7 days of placebo involve a drop of more than 10 µg. Options that involve a drop of 10 µg or less include any continuous formulation, the extended formulation LoSeasonique (levonorgestrel 0.1 mg and ethinyl estradiol 20 µg for 84 days, then ethinyl estradiol 10 µg for 7 days), and Lo Loestrin (ethinyl estradiol 10 µg and norethindrone 1 mg for 24 days, then ethinyl estradiol 10 µg for 2 days, then placebo for 2 days).9
What’s best for acne-prone patients? All COCs should improve acne by increasing levels of sex hormone binding globulin. However, some comparative studies have shown drospirenone-containing COCs to be the most effective for acne. This finding makes sense in light of studies demonstrating antiandrogenic effects of drospirenone.10
Managing PCOS symptoms. It seems logical, by extension, that drospirenone-containing COCs would be particularly beneficial for treating hirsutism associated with polycystic ovary syndrome (PCOS). Other low‒androgenic-potential progestins, such as a third-generation progestin (norgestimate or desogestrel), might similarly be hypothesized to be advantageous. However, there is currently insufficient evidence to recommend any one COC formulation over another for the indication of PCOS.11
Ovarian cysts: Can COCs be helpful? COCs are commonly prescribed by gynecologists for patients with functional ovarian cysts. It is important to note that COCs have not been found to hasten the resolution of existing cysts, so they should not be used for this purpose.12 Studies of early COCs, which had high doses of estrogen (on the order of 50 µg), showed lower rates of cysts among users. This effect seems to be attenuated with the lower-estrogen-dose pills that are currently available, but there still appears to be benefit. Therefore, for a patient prone to cysts who desires an oral contraceptive, a COC containing estrogen 35 µg is likely to be the most beneficial of COCs currently on the market.13,14
Lower-dosage COCs in perimenopause may be beneficial. COCs can ameliorate perimenopausal symptoms including abnormal uterine bleeding and vasomotor symptoms. Clinicians are often hesitant to prescribe COCs for perimenopausal women because of increased risk of VTE, stroke, myocardial infarction, and breast cancer with increasing age. However, age alone is not a contraindication to any contraceptive method. An extended cyclic or continuous regimen COC may be the best choice for a perimenopausal woman in order to avoid vasomotor symptoms that occur during hormone-free intervals. In addition, given the increasing risk of adverse effects like VTE with estrogen dose, a lower estrogen formulation is advisable.15
Patients with epilepsy who are taking antiepileptic drugs (AEDs) are a special population when it comes to COCs. Certain AEDs induce hepatic enzymes involved in the metabolism and protein binding of COCs, which can result in contraceptive failure. Strong inducers are carbamazepine, oxcarbazepine, perampanel, phenobarbital, phenytoin, and primidone. Weak inducers are clobazam, eslicarbazepine, felbamate, lamotrigine, rufinamide, and topiramate. Women taking any of the above AEDs are recommended to choose a different form of contraception than a COC. However, if they are limited to COCs for some reason, a preparation containing estrogen 50 µg is recommended. It is speculated that the efficacy and adverse effects of COCs with increased hormone doses, used in combination with enzyme-inducing AEDs, should be comparable to those with standard doses when not combined with AEDs; however, this speculation is unproven.16 There are few COCs on the market with estrogen doses of 50 µg, but a couple of examples are Kelnor and Ogestrel.
Additional factors have to be considered with concurrent COC use with the AED lamotrigine since COCs increase clearance of this agent. Therefore, patients taking lamotrigine who start COCs will need an increase in lamotrigine dose. To avoid fluctuations in lamotrigine serum levels, use of a continuous COC is recommended.17
Continue to: Pill types to minimize adverse effects or risks...
Pill types to minimize adverse effects or risks
For women who desire to use a COC for contraception but who are at risk for a particular complication or are bothered by a particular adverse effect, ObGyns can optimize the choice of pill (TABLE 3). For example, women who have adverse effects of nausea and/or breast tenderness may benefit from reducing the estrogen dose to 20 µg or lower.18

Considering VTE
As discussed previously, VTE is a risk with all COCs, but some pills confer greater risk than others. For one, VTE risk increases with estrogen dose. In addition, VTE risk depends on the type of progestin. Drospirenone and third-generation progestins (norgestimate, gestodene, and desogestrel) confer a higher risk of VTE than first- or second-generation progestins. For example, a pill with estradiol 30 µg and either a third-generation progestin or drospirenone has a 50% to 80% higher risk of VTE compared with a pill with estradiol 30 µg and levonorgestrel.
For patients at particularly high risk for VTE, COCs are contraindicated. For patients for whom COCs are considered medically appropriate but who are at higher risk (eg, obese women), it is wise to use a pill containing a first-generation (norethindrone) or second-generation progestin (levonorgestrel) combined with the lowest dose of estrogen that has tolerable adverse effects.19
What about hypertension concerns?
Let us turn our attention briefly to hypertension and its relation to COC use. While the American College of Cardiology and the American Heart Association redefined hypertension in 2017 using a threshold of 130/80 mm Hg, the American College of Obstetricians and Gynecologists (ACOG) considers hypertension to be 140/90 mm Hg in terms of safety of using COCs. ACOG states, “women with blood pressure below 140/90 mm Hg may use any hormonal contraceptive method.”20 In women with hypertension in the range of 140‒159 mm Hg systolic or 90‒99 mm Hg diastolic, COCs are category 3 according to the US Medical Eligibility Criteria for Contraceptive Use, meaning that the risks usually outweigh the benefits. For women with blood pressures of 160/110 mm Hg or greater, COCs are category 4 (contraindicated). If a woman with mild hypertension is started on a COC, a drospirenone-containing pill may be the best choice because of its diuretic effects. While other contemporary COCs have been associated with a mild increase in blood pressure, drospirenone-containing pills have not shown this association.21
Continue to: At issue: Break-through bleeding, mood, and weight gain...
At issue: Break-through bleeding, mood, and weight gain
For women bothered by intermenstrual bleeding, use of a COC with a third-generation progestin may be preferable to use of one with a first- or second-generation. It may be because of decreased abnormal bleeding that COCs with third-generation progestins have lower discontinuation rates.22 In addition, COCs containing estrogen 20 µg or less are associated with more intermenstrual bleeding than those with more than 20 µg estrogen.23 Keep in mind that it is common with any COC to have intermenstrual bleeding for the first several months.
For women with pre-existing mood disorders or who report mood changes with COCs, it appears that fluctuations in hormone levels are problematic. Consistently, there is evidence that monophasic pills are preferable to multiphasic and that extended cyclic or continuous use is preferable to traditional cyclic use for mitigating mood adverse effects. There is mixed evidence on whether a low dose of ethinyl estradiol is better for mood.3
Although it is discussed above that randomized controlled trials have not shown an association between COC use and weight gain, many women remain concerned. For these women, a drospirenone-containing COC may be the best choice. Drospirenone has antimineralocorticoid activity, so it may help prevent water retention.
A brief word about multiphasic COCs. While these pills were designed to mimic physiologic hormone fluctuations and minimize hormonal adverse effects, there is insufficient evidence to compare their effects to those of monophasic pills.24 Without such evidence, there is little reason to recommend a multiphasic pill to a patient over the more straightforward monophasic formulation.
Conclusion
There are more nuances to prescribing an optimal COC for a patient than may initially come to mind. It is useful to remember that any formulation of pill may be prescribed in an extended or continuous fashion, and there are benefits for such use for premenstrual dysphoric disorder, heavy menstrual bleeding, perimenopause, and menstrual symptoms. Although there are numerous brands of COCs available, a small cadre will suffice for almost all purposes. Such a “toolbox” of pills could include a pill formatted for continuous use (Seasonique), a low estrogen pill (Loestrin), a drospirenone-containing pill (Yaz), and a pill containing a third-generation progestin and a higher dose of estrogen (Sprintec). ●
- Daniels K, Abma JC. Current contraceptive status among women aged 15-49: United States, 2015-2017. NCHS Data Brief, no 327. Hyattsville, MD; 2018.
- Gallo MF, Lopez LM, Grimes DA, et al. Combination contraceptives: effects on weight. Cochrane Database Syst Rev. 2014:CD003987.
- Schaffir J, Worly BL, Gur TL. Combined hormonal contraception and its effects on mood: a critical review. Eur J Contracept Reprod Health Care. 2016;21:347-355.
- Barnhart KT, Schreiber CA. Return to fertility following discontinuation of oral contraceptives. Fertil Steril. 2009;91:659-663.
- American College of Obstetricians and Gynecologists. Committee Opinion #540: Risk of Venous Thromboembolism Among Users of Drospirenone-Containing Oral Contraceptive Pills. Obstet Gynecol. 2012;120:1239-1242.
- Edelman A, Micks E, Gallo MF, et al. Continuous or extended cycle vs. cyclic use of combined hormonal contraceptives for contraception. Cochrane Database Syst Rev. 2014:CD004695.
- American College of Obstetricians and Gynecologists. Practice Bulletin #110: Noncontraceptive Uses of Hormonal Contraceptives. Obstet Gynecol. 2010:206-218.
- Coffee AL, Kuehl TJ, Willis S, et al. Oral contraceptives and premenstrual symptoms: comparison of a 21/7 and extended regimen. Am J Obstet Gynecol. 2006;195:1311-1319.
- Calhoun AH, Batur P. Combined hormonal contraceptives and migraine: an update on the evidence. Cleve Clin J Med. 2017;84:631-638.
- Arowojolu AO, Gallo MF, Lopez LM, et al. Combined oral contraceptive pills for treatment of acne. Cochrane Database Syst Rev. 2012:CD004425.
- McCartney CR, Marshall JC. CLINICAL PRACTICE. Polycystic Ovary Syndrome. N Engl J Med. 2016;375:54-64.
- Grimes DA, Jones LB, Lopez LM, et al. Oral contraceptives for functional ovarian cysts. Cochrane Database Syst Rev. 2014:CD006134.
- Grimes DA, Godwin AJ, Rubin A, et al. Ovulation and follicular development associated with three low-dose oral contraceptives: a randomized controlled trial. Obstet Gynecol. 1994;83:29-34.
- Christensen JT, Boldsen JL, Westergaard JG. Functional ovarian cysts in premenopausal and gynecologically healthy women. Contraception. 2002;66:153-157.
- Hardman SM, Gebbie AE. Hormonal contraceptive regimens in the perimenopause. Maturitas. 2009;63:204-212.
- Zupanc ML. Antiepileptic drugs and hormonal contraceptives in adolescent women with epilepsy. Neurology. 2006;66 (6 suppl 3):S37-S45.
- Wegner I, Edelbroek PM, Bulk S, et al. Lamotrigine kinetics within the menstrual cycle, after menopause, and with oral contraceptives. Neurology. 2009;73:1388-1393.
- Stewart M, Black K. Choosing a combined oral contraceptive pill. Australian Prescriber. 2015;38:6-11.
- de Bastos M, Stegeman BH, Rosendaal FR, et al. Combined oral contraceptives: venous thrombosis. Cochrane Database Syst Rev. 2014:CD010813.
- American College of Obstetricians and Gynecologists. Practice Bulletin #206: use of hormonal contraception in women with coexisting medical conditions. Obstet Gynecol. 2019;133:e128-e150.
- de Morais TL, Giribela C, Nisenbaum MG, et al. Effects of a contraceptive containing drospirenone and ethinylestradiol on blood pressure, metabolic profile and neurohumoral axis in hypertensive women at reproductive age. Eur J Obstet Gynecol Reprod Biol. 2014;182:113-117.
- Lawrie TA, Helmerhorst FM, Maitra NK, et al. Types of progestogens in combined oral contraception: effectiveness and side-effects. Cochrane Database Syst Rev. 2011:CD004861.
- Gallo MF, Nanda K, Grimes DA, et al. 20 µg versus >20 µg estrogen combined oral contraceptives for contraception. Cochrane Database Syst Rev. 2013:CD003989.
- van Vliet HA, Grimes DA, Lopez LM, et al. Triphasic versus monophasic oral contraceptives for contraception. Cochrane Database Syst Rev. 2006:CD003553
- Daniels K, Abma JC. Current contraceptive status among women aged 15-49: United States, 2015-2017. NCHS Data Brief, no 327. Hyattsville, MD; 2018.
- Gallo MF, Lopez LM, Grimes DA, et al. Combination contraceptives: effects on weight. Cochrane Database Syst Rev. 2014:CD003987.
- Schaffir J, Worly BL, Gur TL. Combined hormonal contraception and its effects on mood: a critical review. Eur J Contracept Reprod Health Care. 2016;21:347-355.
- Barnhart KT, Schreiber CA. Return to fertility following discontinuation of oral contraceptives. Fertil Steril. 2009;91:659-663.
- American College of Obstetricians and Gynecologists. Committee Opinion #540: Risk of Venous Thromboembolism Among Users of Drospirenone-Containing Oral Contraceptive Pills. Obstet Gynecol. 2012;120:1239-1242.
- Edelman A, Micks E, Gallo MF, et al. Continuous or extended cycle vs. cyclic use of combined hormonal contraceptives for contraception. Cochrane Database Syst Rev. 2014:CD004695.
- American College of Obstetricians and Gynecologists. Practice Bulletin #110: Noncontraceptive Uses of Hormonal Contraceptives. Obstet Gynecol. 2010:206-218.
- Coffee AL, Kuehl TJ, Willis S, et al. Oral contraceptives and premenstrual symptoms: comparison of a 21/7 and extended regimen. Am J Obstet Gynecol. 2006;195:1311-1319.
- Calhoun AH, Batur P. Combined hormonal contraceptives and migraine: an update on the evidence. Cleve Clin J Med. 2017;84:631-638.
- Arowojolu AO, Gallo MF, Lopez LM, et al. Combined oral contraceptive pills for treatment of acne. Cochrane Database Syst Rev. 2012:CD004425.
- McCartney CR, Marshall JC. CLINICAL PRACTICE. Polycystic Ovary Syndrome. N Engl J Med. 2016;375:54-64.
- Grimes DA, Jones LB, Lopez LM, et al. Oral contraceptives for functional ovarian cysts. Cochrane Database Syst Rev. 2014:CD006134.
- Grimes DA, Godwin AJ, Rubin A, et al. Ovulation and follicular development associated with three low-dose oral contraceptives: a randomized controlled trial. Obstet Gynecol. 1994;83:29-34.
- Christensen JT, Boldsen JL, Westergaard JG. Functional ovarian cysts in premenopausal and gynecologically healthy women. Contraception. 2002;66:153-157.
- Hardman SM, Gebbie AE. Hormonal contraceptive regimens in the perimenopause. Maturitas. 2009;63:204-212.
- Zupanc ML. Antiepileptic drugs and hormonal contraceptives in adolescent women with epilepsy. Neurology. 2006;66 (6 suppl 3):S37-S45.
- Wegner I, Edelbroek PM, Bulk S, et al. Lamotrigine kinetics within the menstrual cycle, after menopause, and with oral contraceptives. Neurology. 2009;73:1388-1393.
- Stewart M, Black K. Choosing a combined oral contraceptive pill. Australian Prescriber. 2015;38:6-11.
- de Bastos M, Stegeman BH, Rosendaal FR, et al. Combined oral contraceptives: venous thrombosis. Cochrane Database Syst Rev. 2014:CD010813.
- American College of Obstetricians and Gynecologists. Practice Bulletin #206: use of hormonal contraception in women with coexisting medical conditions. Obstet Gynecol. 2019;133:e128-e150.
- de Morais TL, Giribela C, Nisenbaum MG, et al. Effects of a contraceptive containing drospirenone and ethinylestradiol on blood pressure, metabolic profile and neurohumoral axis in hypertensive women at reproductive age. Eur J Obstet Gynecol Reprod Biol. 2014;182:113-117.
- Lawrie TA, Helmerhorst FM, Maitra NK, et al. Types of progestogens in combined oral contraception: effectiveness and side-effects. Cochrane Database Syst Rev. 2011:CD004861.
- Gallo MF, Nanda K, Grimes DA, et al. 20 µg versus >20 µg estrogen combined oral contraceptives for contraception. Cochrane Database Syst Rev. 2013:CD003989.
- van Vliet HA, Grimes DA, Lopez LM, et al. Triphasic versus monophasic oral contraceptives for contraception. Cochrane Database Syst Rev. 2006:CD003553
Challenges in the Management of Peptic Ulcer Disease
From the University of Alabama at Birmingham, Birmingham, AL.
Abstract
Objective: To review current challenges in the management of peptic ulcer disease.
Methods: Review of the literature.
Results: Peptic ulcer disease affects 5% to 10% of the population worldwide, with recent decreases in lifetime prevalence in high-income countries. Helicobacter pylori infection and nonsteroidal anti-inflammatory drug (NSAID) use are the most important drivers of peptic ulcer disease. Current management strategies for peptic ulcer disease focus on ulcer healing; management of complications such as bleeding, perforation, and obstruction; and prevention of ulcer recurrence. Proton pump inhibitors (PPIs) are the cornerstone of medical therapy for peptic ulcers, and complement testing for and treatment of H. pylori infection as well as elimination of NSAID use. Although advances have been made in the medical and endoscopic treatment of peptic ulcer disease and the management of ulcer complications, such as bleeding and obstruction, challenges remain.
Conclusion: Peptic ulcer disease is a common health problem globally, with persistent challenges related to refractory ulcers, antiplatelet and anticoagulant use, and continued bleeding in the face of endoscopic therapy. These challenges should be met with PPI therapy of adequate frequency and duration, vigilant attention to and treatment of ulcer etiology, evidence-based handling of antiplatelet and anticoagulant medications, and utilization of novel endoscopic tools to obtain improved clinical outcomes.
Keywords: H. pylori; nonsteroidal anti-inflammatory drugs; NSAIDs; proton pump inhibitor; PPI; bleeding; perforation; obstruction; refractory ulcer; salvage endoscopic therapy; transcatheter angiographic embolization.
A peptic ulcer is a fibrin-covered break in the mucosa of the digestive tract extending to the submucosa that is caused by acid injury (Figure 1). Most peptic ulcers occur in the stomach or proximal duodenum, though they may also occur in the esophagus or, less frequently, in a Meckel’s diverticulum.1,2 The estimated worldwide prevalence of peptic ulcer disease is 5% to 10%, with an annual incidence of 0.1% to 0.3%1; both rates are declining.3 The annual incidence of peptic ulcer disease requiring medical or surgical treatment is also declining, and currently is estimated to be 0.1% to 0.2%.4 The lifetime prevalence of peptic ulcers has been decreasing in high-income countries since the mid-20th century due to both the widespread use of medications that suppress gastric acid secretion and the declining prevalence of Helicobacter pylori infection.1,3
Peptic ulcer disease in most individuals results from H. pylori infection, chronic use of nonsteroidal anti-inflammatory drugs (NSAIDs), including aspirin, or both. A combination of H. pylori factors and host factors lead to mucosal disruption in infected individuals who develop peptic ulcers. H. pylori–specific factors include the expression of virulence factors such as CagA and VacA, which interact with the host inflammatory response to cause mucosal injury. The mucosal inflammatory response is at least partially determined by polymorphisms in the host’s cytokine genes.1,4 NSAIDs inhibit the production of cyclooxygenase-1-derived prostaglandins, with subsequent decreases in epithelial mucous formation, bicarbonate secretion, cell proliferation, and mucosal blood flow, all of which are key elements in the maintenance of mucosal integrity.1,5 Less common causes of peptic ulcers include gastrinoma, adenocarcinoma, idiopathic ulcers, use of sympathomimetic drugs (eg, cocaine or methamphetamine), certain anticancer agents, and bariatric surgery.4,6
This article provides an overview of current management principles for peptic ulcer disease and discusses current challenges in peptic ulcer management, including proton pump inhibitor (PPI) therapy, refractory ulcers, handling of antiplatelet and anticoagulants during and after peptic ulcer bleeding, and ulcer bleeding that continues despite salvage endoscopic therapy.
Methods
We searched MEDLINE using the term peptic ulcer disease in combination with the terms current challenges, epidemiology, bleeding, anticoagulant, antiplatelet, PPI potency, etiology, treatment, management, and refractory. We selected publications from the past 35 years that we judged to be relevant.
Current Management
The goals of peptic ulcer disease management are ulcer healing and prevention of recurrence. The primary interventions used in the management of peptic ulcer disease are medical therapy and implementation of measures that address the underlying etiology of the disease.
Medical Therapy
Introduced in the late 1980s, PPIs are the cornerstone of medical therapy for peptic ulcer disease.6 These agents irreversibly inhibit the H+/K+-ATPase pump in the gastric mucosa and thereby inhibit gastric acid secretion, promoting ulcer healing. PPIs improve rates of ulcer healing compared to H2-receptor antagonists.4,7
Underlying Causes
The underlying cause of peptic ulcer disease should be addressed, in addition to initiating medical therapy. A detailed history of NSAID use should be obtained, and patients with peptic ulcers caused by NSAIDs should be counseled to avoid them, if possible. Patients with peptic ulcer disease who require long-term use of NSAIDs should be placed on long-term PPI therapy.6 Any patient with peptic ulcer disease, regardless of any history of H. pylori infection or treatment, should be tested for infection. Tests that identify active infection, such as urea breath test, stool antigen assay, or mucosal biopsy–based testing, are preferred to IgG antibody testing, although the latter is acceptable in the context of peptic ulcer disease with a high pretest probability of infection.8 Any evidence of active infection warrants appropriate treatment to allow ulcer healing and prevent recurrence.1H. pylori infection is most often treated with clarithromycin triple therapy or bismuth quadruple therapy for 14 days, with regimens selected based on the presence or absence of penicillin allergy, prior antibiotic exposure, and local clarithromycin resistance rates, when known.4,8
Managing Complications
An additional aspect of care in peptic ulcer disease is managing the complications of bleeding, perforation, and gastric outlet obstruction. Acute upper gastrointestinal bleeding (GIB) is the most common complication of peptic ulcer disease, which accounts for 40% to 60% of nonvariceal acute upper GIB.1,6 The first step in the management of acute GIB from a peptic ulcer is fluid resuscitation to ensure hemodynamic stability. If there is associated anemia with a hemoglobin level < 8 g/dL, blood transfusion should be undertaken to target a hemoglobin level > 8 g/dL. In patients with peptic ulcer disease–related acute upper GIB and comorbid cardiovascular disease, the transfusion threshold is higher, with the specific cutoff depending on clinical status, type and severity of cardiovascular disease, and degree of bleeding. Endoscopic management should generally be undertaken within 24 hours of presentation and should not be delayed in patients taking anticoagulants.9 Combination endoscopic treatment with through-the-scope clips plus thermocoagulation or sclerosant injection is recommended for acutely bleeding peptic ulcers with high-risk stigmata.
Pharmacologic management of patients with bleeding peptic ulcers with high-risk stigmata includes PPI therapy, with an 80 mg intravenous (IV) loading dose followed by continuous infusion of 8 mg/hr for 72 hours to reduce rebleeding and mortality. Following completion of IV therapy, oral PPI therapy should be continued twice daily for 14 days, followed by once-daily dosing thereafter.9Patients with peptic ulcer perforation present with sudden-onset epigastric abdominal pain and have tenderness to palpation, guarding, and rigidity on examination, often along with tachycardia and hypotension.1,4 Computed tomography (CT) of the abdomen is 98% sensitive for identifying and localizing a perforation. Most perforations occur in the duodenum or antrum.
Management of a peptic ulcer perforation requires consultation with a surgeon to determine whether a nonoperative approach may be employed (eg, a stable patient with a contained perforation), or if surgery is indicated. The surgical approach to peptic ulcer perforation has been impacted by the clinical success of gastric acid suppression with PPIs and H. pylori eradication, but a range of surgical approaches are still used to repair perforations, from omental patch repair with peritoneal drain placement, to more extensive surgeries such as wedge resection or partial gastrectomy.4 Perforation carries a high mortality risk, up to 20% to 30%, and is the leading cause of death in patients with peptic ulcer disease.1,4
Gastric outlet obstruction, a rare complication of peptic ulcer disease, results from recurrent ulcer formation and scarring. Obstruction often presents with hypovolemia and metabolic alkalosis from prolonged vomiting. CT imaging with oral contrast is often the first diagnostic test employed to demonstrate obstruction. Upper endoscopy should be performed to evaluate the appearance and degree of obstruction as well as to obtain biopsies to evaluate for a malignant etiology of the ulcer disease. Endoscopic balloon dilation has become the cornerstone of initial therapy for obstruction from peptic ulcer disease, especially in the case of ulcers due to reversible causes. Surgery is now typically reserved for cases of refractory obstruction, after repeated endoscopic balloon dilation has failed to remove the obstruction. However, because nearly all patients with gastric outlet obstruction present with malnutrition, nutritional deficiencies should be addressed prior to the patient undergoing surgical intervention. Surgical options include pyloroplasty, antrectomy, and gastrojejunostomy.4
Current Challenges
Rapid Metabolism of PPIs
High-dose PPI therapy is a key component of therapy for peptic ulcer healing. PPIs are metabolized by the cytochrome P450 system, which is comprised of multiple isoenzymes. CYP2C19, an isoenzyme involved in PPI metabolism, has 21 polymorphisms, which have variable effects leading to ultra-rapid, extensive, intermediate, or poor metabolism of PPIs.10 With rapid metabolism of PPIs, standard dosing can result in inadequate suppression of acid secretion. Despite this knowledge, routine testing of CYP2C19 phenotype is not recommended due to the cost of testing. Instead, inadequate ulcer healing should prompt consideration of increased PPI dosing to 80 mg orally twice daily, which may be sufficient to overcome rapid PPI metabolism.11
Relative Potency of PPIs
In addition to variation in PPI metabolism, the relative potency of various PPIs has been questioned. A review of all available clinical studies of the effects of PPIs on mean 24-hour intragastric pH reported a quantitative difference in the potency of 5 PPIs, with omeprazole as the reference standard. Potencies ranged from 0.23 omeprazole equivalents for pantoprazole to 1.82 omeprazole equivalents for rabeprazole.12 An additional study of data from 56 randomized clinical trials confirmed that PPIs vary in potency, which was measured as time that gastric pH is less than 4. A linear increase in intragastric pH time less than 4 was observed from 9 to 64 mg omeprazole equivalents; higher doses yielded no additional benefit. An increase in PPI dosing from once daily to twice daily also increased the duration of intragastric pH time less than 4 from 15 to 21 hours.13 Earlier modeling of the relationship between duodenal ulcer healing and antisecretory therapy showed a strong correlation of ulcer healing with the duration of acid suppression, length of therapy, and the degree of acid suppression. Additional benefit was not observed after intragastric pH rose above 3.14 Thus, as the frequency and duration of acid suppression therapy are more important than PPI potency, PPIs can be used interchangeably.13,14
Addressing Underlying Causes
Continued NSAID Use. Refractory peptic ulcers are defined as those that do not heal despite adherence to 8 to 12 weeks of standard acid-suppression therapy. A cause of refractory peptic ulcer disease that must be considered is continued NSAID use.1,15 In a study of patients with refractory peptic ulcers, 27% of patients continued NSAID use, as determined by eventual disclosure by the patients or platelet cyclooxygenase activity assay, despite extensive counseling to avoid NSAIDs at the time of the diagnosis of their refractory ulcer and at subsequent visits.16 Pain may make NSAID cessation difficult for some patients, while others do not realize that over-the-counter preparations they take contain NSAIDs.15
Another group of patients with continued NSAID exposure are those who require long-term NSAID therapy for control of arthritis or the management of cardiovascular conditions. If NSAID therapy cannot be discontinued, the risk of NSAID-related gastrointestinal injury can be assessed based on the presence of multiple risk factors, including age > 65 years, high-dose NSAID therapy, a history of peptic ulcer, and concurrent use of aspirin, corticosteroids, or anticoagulants. Individuals with 3 or more of the preceding risk factors or a history of a peptic ulcer with a complication, especially if recent, are considered to be at high risk of developing an NSAID-related ulcer and possible subsequent complications.17 In these individuals, NSAID therapy should be continued with agents that have the lowest risk for gastrointestinal toxicity and at the lowest possible dose. A meta-analysis comparing nonselective NSAIDs to placebo demonstrated naproxen to have the highest risk of gastrointestinal complications, including GIB, perforation, and obstruction (adjusted rate ratio, 4.2), while diclofenac demonstrated the lowest risk (adjusted rate ratio, 1.89). High-dose NSAID therapy demonstrated a 2-fold increase in risk of peptic ulcer formation as compared to low-dose therapy.18
In addition to selecting the NSAID with the least gastrointestinal toxicity at the lowest possible dose, additional strategies to prevent peptic ulcer disease and its complications in chronic NSAID users include co-administration of a PPI and substitution of a COX-2 selective NSAID for nonselective NSAIDs.1,9 Prior double-blind, placebo-controlled, randomized, multicenter trials with patients requiring daily NSAIDs demonstrated an up to 15% absolute reduction in the risk of developing peptic ulcers over 6 months while taking esomeprazole.19
Persistent Infection. Persistent H. pylori infection, due either to initial false-negative testing or ongoing infection despite first-line therapy, is another cause of refractory peptic ulcer disease.1,15 Because antibiotics and PPIs can reduce the number of H. pylori bacteria, use of these medications concurrent with H. pylori testing can lead to false-negative results with several testing modalities. When suspicion for H. pylori is high, 2 or more diagnostic tests may be needed to effectively rule out infection.15
When H. pylori is detected, successful eradication is becoming more difficult due to an increasing prevalence of antibiotic resistance, leading to persistent infection in many cases and maintained risk of peptic ulcer disease, despite appropriate first-line therapy.8 Options for salvage therapy for persistent H. pylori, as well as information on the role and best timing of susceptibility testing, are beyond the scope of this review, but are reviewed by Lanas and Chan1 and in the American College of Gastroenterology guideline on the treatment of H. pylori infection.8
Other Causes. In a meta-analysis of rigorously designed studies from North America, 20% of patients experienced ulcer recurrence at 6 months, despite successful H. pylori eradication and no NSAID use.20 In addition, as H. pylori prevalence is decreasing, idiopathic ulcers are increasingly being diagnosed, and such ulcers may be associated with high rates of GIB and mortality.1 In this subset of patients with non-H. pylori, non-NSAID ulcers, increased effort is required to further evaluate the differential diagnosis for rarer causes of upper GI tract ulcer disease (Table). Certain malignancies, including adenocarcinoma and lymphoma, can cause ulcer formation and should be considered in refractory cases. Repeat biopsy at follow-up endoscopy for persistent ulcers should always be obtained to further evaluate for malignancy.1,15 Infectious diseases other than H. pylori infection, such as tuberculosis, syphilis, cytomegalovirus, and herpes simplex virus, are also reported as etiologies of refractory ulcers, and require specific antimicrobial treatment over and above PPI monotherapy. Special attention in biopsy sampling and sample processing is often required when infectious etiologies are being considered, as specific histologic stains and cultures may be needed for identification.15
Systemic conditions, including sarcoidosis,21 Behçet disease,22 and polyarteritis nodosa,15,23 can also cause refractory ulcers. Approximately 15% of patients with Crohn disease have gastroduodenal involvement, which may include ulcers of variable sizes.1,15,24 The increased gastric acid production seen in Zollinger-Ellison syndrome commonly presents as refractory peptic ulcers in the duodenum beyond the bulb that do not heal with standard doses of PPIs.1,15 More rare causes of acid hypersecretion leading to refractory ulcers include idiopathic gastric acid hypersecretion and retained gastric antrum syndrome after partial gastrectomy with Billroth II anastomosis.15 Smoking is a known risk factor for impaired tissue healing throughout the body, and can contribute to impaired healing of peptic ulcers through decreased prostaglandin synthesis25 and reduced gastric mucosal blood flow.26 Smoking should always be addressed in patients with refractory peptic ulcers, and cessation should be strongly encouraged. Other less common causes of refractory upper GI tract ulcers include radiation therapy, crack cocaine use, and mesenteric ischemia.15
Managing Antiplatelet and Anticoagulant Medications
Use of antiplatelets and anticoagulants, alone or in combination, increases the risk of peptic ulcer bleeding. In patients who continue to take aspirin after a peptic ulcer bleed, recurrent bleeding occurs in up to 300 cases per 1000 person-years. The rate of GIB associated with aspirin use ranges from 1.1% to 2.5%, depending on the dose. Prior peptic ulcer disease, age greater than 70 years, and concurrent NSAID, steroid, anticoagulant, or dual antiplatelet therapy (DAPT) use increase the risk of bleeding while on aspirin. The rate of GIB while taking a thienopyridine alone is slightly less than that when taking aspirin, ranging from 0.5% to 1.6%. Studies to date have yielded mixed estimates of the effect of DAPT on the risk of GIB. Estimates of the risk of GIB with DAPT range from an odds ratio for serious GIB of 7.4 to an absolute risk increase of only 1.3% when compared to clopidogrel alone.27
Many patients are also on warfarin or a direct oral anticoagulant (DOAC). In a study from the United Kingdom, the adjusted rate ratio of GIB with warfarin alone was 1.94, and this increased to 6.48 when warfarin was used with aspirin.28 The use of warfarin and DAPT, often called triple therapy, further increases the risk of GIB, with a hazard ratio of 5.0 compared to DAPT alone, and 5.38 when compared to warfarin alone. DOACs are increasingly prescribed for the treatment and prevention of thromboembolism, and by 2014 were prescribed as often as warfarin for stroke prevention in atrial fibrillation in the United States. A meta-analysis showed the risk of major GIB did not differ between DOACs and warfarin or low-molecular-weight heparin, but among DOACs factor Xa inhibitors showed a reduced risk of GIB compared with dabigatran, a direct thrombin inhibitor.29
The use of antiplatelets and anticoagulants in the context of peptic ulcer bleeding is a current management challenge. Data to guide decision-making in patients on antiplatelet and/or anticoagulant therapy who experience peptic ulcer bleeding are scarce. Decision-making in this group of patients requires balancing the severity and risk of bleeding with the risk of thromboembolism.1,27 In patients on antiplatelet therapy for primary prophylaxis of atherothrombosis who develop bleeding from a peptic ulcer, the antiplatelet should generally be held and the indication for the medication reassessed. In patients on antiplatelet therapy for secondary prevention, the agent may be immediately resumed after endoscopy if bleeding is found to be due to an ulcer with low-risk stigmata. With bleeding resulting from an ulcer with high-risk stigmata, antiplatelet agents employed for secondary prevention may be held initially, with consideration given to early reintroduction, as early as day 3 after endoscopy.1 In patients at high risk for atherothrombotic events, including those on aspirin for secondary prophylaxis, withholding aspirin leads to a 3-fold increase in the risk of a major adverse cardiac event, with events occurring as early as 5 days after aspirin cessation in some cases.27 A randomized controlled trial of continuing low-dose aspirin versus withholding it for 8 weeks in patients on aspirin for secondary prophylaxis of cardiovascular events who experienced peptic ulcer bleeding that required endoscopic therapy demonstrated lower all-cause mortality (1.3% vs 12.9%), including death from cardiovascular or cerebrovascular events, among those who continued aspirin therapy, with a small increased risk of recurrent ulcer bleeding (10.3% vs 5.4%).30 Thus, it is recommended that antiplatelet therapy, when held, be resumed as early as possible when the risk of a cardiovascular or cerebrovascular event is considered to be higher than the risk of bleeding.27
When patients are on DAPT for a history of drug-eluting stent placement, withholding both antiplatelet medications should be avoided, even for a brief period of time, given the risk of in-stent thrombosis. When DAPT is employed for other reasons, it should be continued, if indicated, after bleeding that is found to be due to peptic ulcers with low-risk stigmata. If bleeding is due to a peptic ulcer with high-risk stigmata at endoscopy, then aspirin monotherapy should be continued and consultation should be obtained with a cardiologist to determine optimal timing to resume the second antiplatelet agent.1 In patients on anticoagulants, anticoagulation should be resumed once hemostasis is achieved when the risk of withholding anticoagulation is thought to be greater than the risk of rebleeding. For example, anticoagulation should be resumed early in a patient with a mechanical heart valve to prevent thrombosis.1,27 Following upper GIB from peptic ulcer disease, patients who will require long-term aspirin, DAPT, or anticoagulation with either warfarin or DOACs should be maintained on long-term PPI therapy to reduce the risk of recurrent bleeding.9,27
Failure of Endoscopic Therapy to Control Peptic Ulcer Bleeding
Bleeding recurs in as many as 10% to 20% of patients after initial endoscopic control of peptic ulcer bleeding.4,31 In this context, repeat upper endoscopy for hemostasis is preferred to surgery, as it leads to less morbidity while providing long-term control of bleeding in more than 70% of cases.31,32 Two potential endoscopic rescue therapies that may be employed are over-the-scope clips (OTSCs) and hemostatic powder.32,33
While through-the-scope (TTS) hemostatic clips are often used during endoscopy to control active peptic ulcer bleeding, their use may be limited in large or fibrotic ulcers due to the smaller size of the clips and method of application. OTSCs have several advantages over TTS clips; notably, their larger size allows the endoscopist to achieve deeper mucosal or submucosal clip attachment via suction of the targeted tissue into the endoscopic cap (Figure 2). In a systematic review of OTSCs, successful hemostasis was achieved in 84% of 761 lesions, including 75% of lesions due to peptic ulcer disease.34 Some have argued that OTSCs may be preferred as first-line therapy over epinephrine with TTS clips for hemostasis in bleeding from high-risk peptic ulcers (ie, those with visualized arterial bleeding or a visible vessel) given observed decreases in rebleeding events.35
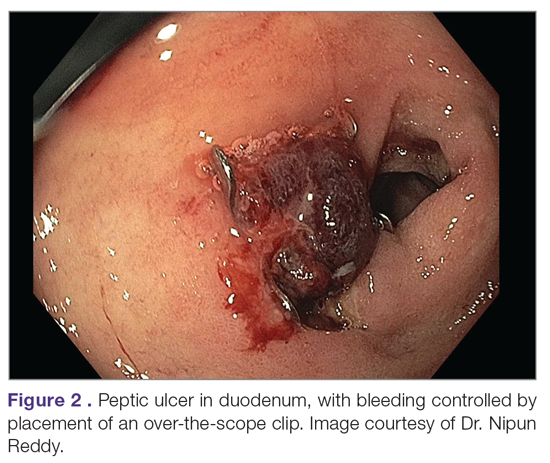
Despite the advantages of OTSCs, endoscopists should be mindful of the potential complications of OTSC use, including luminal obstruction, particularly in the duodenum, and perforation, which occurs in 0.3% to 2% of cases. Additionally, retrieval of misplaced OTSCs presents a significant challenge. Careful decision-making with consideration of the location, size, and depth of lesions is required when deciding on OTSC placement.34,36
A newer endoscopic tool developed for refractory bleeding from peptic ulcers and other causes is hemostatic powder. Hemostatic powders accelerate the coagulation cascade, leading to shortened coagulation times and enhanced clot formation.37 A recent meta-analysis showed that immediate hemostasis could be achieved in 95% of cases of bleeding, including in 96% of cases of bleeding from peptic ulcer disease.38 The primary limitation of hemostatic powders is the temporary nature of hemostasis, which requires the underlying etiology of bleeding to be addressed in order to provide long-term hemostasis. In the above meta-analysis, rebleeding occurred in 17% of cases after 30 days.38
Hypotension and ulcer diameter ≥ 2 cm are independent predictors of failure of endoscopic salvage therapy.31 When severe bleeding is not controlled with initial endoscopic therapy or bleeding recurs despite salvage endoscopic therapy, transcatheter angiographic embolization (TAE) is the treatment of choice.4 Systematic reviews and meta-analyses of studies that compared TAE to surgery have shown that the rate of rebleeding may be higher with TAE, but with less morbidity and either decreased or equivalent rates of mortality, with no increased need for additional interventions.4,32 In a case series examining 5 years of experience at a single medical center in China, massive GIB from duodenal ulcers was successfully treated with TAE in 27 of 29 cases (93% clinical success rate), with no mucosal ischemic necrosis observed.39
If repeated endoscopic therapy has not led to hemostasis of a bleeding peptic ulcer and TAE is not available, then surgery is the next best option. Bleeding gastric ulcers may be excised, wedge resected, or oversewn after an anterior gastrostomy. Bleeding duodenal ulcers may require use of a Kocher maneuver and linear incision of the anterior duodenum followed by ligation of the gastroduodenal artery. Fortunately, such surgical management is rarely necessary given the availability of TAE at most centers.4
Conclusion
Peptic ulcer disease is a common health problem globally, with persistent challenges related to refractory ulcers, antiplatelet and anticoagulant use, and continued bleeding in the face of endoscopic therapy. These challenges should be met with adequate frequency and duration of PPI therapy, vigilant attention to and treatment of ulcer etiology, evidence-based handling of antiplatelet and anticoagulant medications, and utilization of novel endoscopic tools to obtain improved clinical outcomes.
Acknowledgment: We thank Dr. Nipun Reddy from our institution for providing the endoscopic images used in this article.
Corresponding author: Adam L. Edwards, MD, MS; [email protected].
Financial disclosures: None.
1. Lanas A, Chan FKL. Peptic ulcer disease. Lancet. 2017;390:613-624.
2. Malfertheiner P, Chan FK, McColl KE. Peptic ulcer disease. Lancet. 2009;374:1449-1461.
3. Roberts-Thomson IC. Rise and fall of peptic ulceration: A disease of civilization? J Gastroenterol Hepatol. 2018;33:1321-1326.
4. Kempenich JW, Sirinek KR. Acid peptic disease. Surg Clin North Am. 2018;98:933-944.
5. Cryer B, Feldman M. Effects of very low dose daily, long-term aspirin therapy on gastric, duodenal, and rectal prostaglandin levels and on mucosal injury in healthy humans. Gastroenterology. 1999;117:17-25.
6. Kavitt RT, Lipowska AM, Anyane-Yeboa A, Gralnek IM. Diagnosis and treatment of peptic ulcer disease. Am J Med. 2019;132:447-456.
7. Walan A, Bader JP, Classen M, et al. Effect of omeprazole and ranitidine on ulcer healing and relapse rates in patients with benign gastric ulcer. New Engl J Med. 1989;320:69-75.
8. Chey WD, Leontiadis GI, Howden CW, Moss SF. ACG Clinical Guideline: Treatment of Helicobacter pylori Infection. Am J Gastroenterol. 2017;112:212-239.
9. Barkun AN, Almadi M, Kuipers EJ, et al. Management of nonvariceal upper gastrointestinal bleeding: Guideline recommendations from the International Consensus Group. Ann Intern Med. 2019;171:805-822.
10. Arevalo Galvis A, Trespalacios Rangel AA, Otero Regino W. Personalized therapy for Helicobacter pylori: CYP2C19 genotype effect on first-line triple therapy. Helicobacter. 2019;24:e12574.
11. Furuta T, Ohashi K, Kamata T, et al. Effect of genetic differences in omeprazole metabolism on cure rates for Helicobacter pylori infection and peptic ulcer. Ann Intern Med. 1998;129:1027-1030.
12. Kirchheiner J, Glatt S, Fuhr U, et al. Relative potency of proton-pump inhibitors-comparison of effects on intragastric pH. Eur J Clin Pharmacol. 2009;65:19-31.
13. Graham DY, Tansel A. Interchangeable use of proton pump inhibitors based on relative potency. Clin Gastroenterol Hepatol. 2018;16:800-808.e7.
14. Burget DW, Chiverton SG, Hunt RH. Is there an optimal degree of acid suppression for healing of duodenal ulcers? A model of the relationship between ulcer healing and acid suppression. Gastroenterology. 1990;99:345-351.
15. Kim HU. Diagnostic and treatment approaches for refractory peptic ulcers. Clin Endosc. 2015;48:285-290.
16. Lanas AI, Remacha B, Esteva F, Sainz R. Risk factors associated with refractory peptic ulcers. Gastroenterology. 1995;109:124-133.
17. Lanza FL, Chan FK, Quigley EM. Guidelines for prevention of NSAID-related ulcer complications. Am J Gastroenterol. 2009;104:728-738.
18. Richy F, Bruyere O, Ethgen O, et al. Time dependent risk of gastrointestinal complications induced by non-steroidal anti-inflammatory drug use: a consensus statement using a meta-analytic approach. Ann Rheum Dis. 2004;63:759-766.
19. Scheiman JM, Yeomans ND, Talley NJ, et al. Prevention of ulcers by esomeprazole in at-risk patients using non-selective NSAIDs and COX-2 inhibitors. Am J Gastroenterol. 2006;101:701-710.
20. Laine L, Hopkins RJ, Girardi LS. Has the impact of Helicobacter pylori therapy on ulcer recurrence in the United States been overstated? A meta-analysis of rigorously designed trials. Am J Gastroenterol. 1998;93:1409-1415.
21. Akiyama T, Endo H, Inamori M, et al. Symptomatic gastric sarcoidosis with multiple antral ulcers. Endoscopy. 2009;41 Suppl 2:E159.
22. Sonoda A, Ogawa R, Mizukami K, et al. Marked improvement in gastric involvement in Behcet’s disease with adalimumab treatment. Turk J Gastroenterol. 2017;28:405-407.
23. Saikia N, Talukdar R, Mazumder S, et al. Polyarteritis nodosa presenting as massive upper gastrointestinal hemorrhage. Gastrointest Endosc. 2006;63:868-870.
24. Annunziata ML, Caviglia R, Papparella LG, Cicala M. Upper gastrointestinal involvement of Crohn’s disease: a prospective study on the role of upper endoscopy in the diagnostic work-up. Dig Dis Sci. 2012;57:1618-1623.
25. Quimby GF, Bonnice CA, Burstein SH, Eastwood GL. Active smoking depresses prostaglandin synthesis in human gastric mucosa. Ann Intern Med. 1986;104:616-619.
26. Iwao T, Toyonaga A, Ikegami M, et al. Gastric mucosal blood flow after smoking in healthy human beings assessed by laser Doppler flowmetry. Gastrointest Endosc. 1993;39:400-403.
27. Almadi MA, Barkun A, Brophy J. Antiplatelet and anticoagulant therapy in patients with gastrointestinal bleeding: an 86-year-old woman with peptic ulcer disease. JAMA. 2011;306:2367-2374.
28. Delaney JA, Opatrny L, Brophy JM, Suissa S. Drug drug interactions between antithrombotic medications and the risk of gastrointestinal bleeding. CMAJ. 2007;177:347-351.
29. Burr N, Lummis K, Sood R, et al. Risk of gastrointestinal bleeding with direct oral anticoagulants: a systematic review and network meta-analysis. Lancet Gastroenterol Hepatol. 2017;2:85-93.
30. Sung JJ, Lau JY, Ching JY, et al. Continuation of low-dose aspirin therapy in peptic ulcer bleeding: a randomized trial. Ann Intern Med. 2010;152:1-9.
31. Lau JY, Sung JJ, Lam YH, et al. Endoscopic retreatment compared with surgery in patients with recurrent bleeding after initial endoscopic control of bleeding ulcers. N Engl J Med. 1999;340:751-756.
32. Gralnek IM, Dumonceau JM, Kuipers EJ, et al. Diagnosis and management of nonvariceal upper gastrointestinal hemorrhage: European Society of Gastrointestinal Endoscopy (ESGE) Guideline. Endoscopy. 2015;47:a1-46.
33. Skinner M, Gutierrez JP, Neumann H, et al. Over-the-scope clip placement is effective rescue therapy for severe acute upper gastrointestinal bleeding. Endosc Int Open. 2014;2:E37-40.
34. Zhong C, Tan S, Ren Y, et al. Clinical outcomes of over-the-scope-clip system for the treatment of acute upper non-variceal gastrointestinal bleeding: a systematic review and meta-analysis. BMC Gastroenterol. 2019;19:225.
35. Mangiafico S, Pigo F, Bertani H, et al. Over-the-scope clip vs epinephrine with clip for first-line hemostasis in non-variceal upper gastrointestinal bleeding: a propensity score match analysis. Endosc Int Open. 2020;8:E50-e8.
36. Wedi E, Gonzalez S, Menke D, et al. One hundred and one over-the-scope-clip applications for severe gastrointestinal bleeding, leaks and fistulas. World J Gastroenterol. 2016;22:1844-1853.
37. Holster IL, van Beusekom HM, Kuipers EJ, et al. Effects of a hemostatic powder hemospray on coagulation and clot formation. Endoscopy. 2015;47:638-645.
38. Facciorusso A, Straus Takahashi M, et al. Efficacy of hemostatic powders in upper gastrointestinal bleeding: A systematic review and meta-analysis. Dig Liver Dis. 2019;51:1633-1640.
39. Wang YL, Cheng YS, et al. Emergency transcatheter arterial embolization for patients with acute massive duodenal ulcer hemorrhage. World J Gastroenterol. 2012;18:4765-4770.
From the University of Alabama at Birmingham, Birmingham, AL.
Abstract
Objective: To review current challenges in the management of peptic ulcer disease.
Methods: Review of the literature.
Results: Peptic ulcer disease affects 5% to 10% of the population worldwide, with recent decreases in lifetime prevalence in high-income countries. Helicobacter pylori infection and nonsteroidal anti-inflammatory drug (NSAID) use are the most important drivers of peptic ulcer disease. Current management strategies for peptic ulcer disease focus on ulcer healing; management of complications such as bleeding, perforation, and obstruction; and prevention of ulcer recurrence. Proton pump inhibitors (PPIs) are the cornerstone of medical therapy for peptic ulcers, and complement testing for and treatment of H. pylori infection as well as elimination of NSAID use. Although advances have been made in the medical and endoscopic treatment of peptic ulcer disease and the management of ulcer complications, such as bleeding and obstruction, challenges remain.
Conclusion: Peptic ulcer disease is a common health problem globally, with persistent challenges related to refractory ulcers, antiplatelet and anticoagulant use, and continued bleeding in the face of endoscopic therapy. These challenges should be met with PPI therapy of adequate frequency and duration, vigilant attention to and treatment of ulcer etiology, evidence-based handling of antiplatelet and anticoagulant medications, and utilization of novel endoscopic tools to obtain improved clinical outcomes.
Keywords: H. pylori; nonsteroidal anti-inflammatory drugs; NSAIDs; proton pump inhibitor; PPI; bleeding; perforation; obstruction; refractory ulcer; salvage endoscopic therapy; transcatheter angiographic embolization.
A peptic ulcer is a fibrin-covered break in the mucosa of the digestive tract extending to the submucosa that is caused by acid injury (Figure 1). Most peptic ulcers occur in the stomach or proximal duodenum, though they may also occur in the esophagus or, less frequently, in a Meckel’s diverticulum.1,2 The estimated worldwide prevalence of peptic ulcer disease is 5% to 10%, with an annual incidence of 0.1% to 0.3%1; both rates are declining.3 The annual incidence of peptic ulcer disease requiring medical or surgical treatment is also declining, and currently is estimated to be 0.1% to 0.2%.4 The lifetime prevalence of peptic ulcers has been decreasing in high-income countries since the mid-20th century due to both the widespread use of medications that suppress gastric acid secretion and the declining prevalence of Helicobacter pylori infection.1,3

Peptic ulcer disease in most individuals results from H. pylori infection, chronic use of nonsteroidal anti-inflammatory drugs (NSAIDs), including aspirin, or both. A combination of H. pylori factors and host factors lead to mucosal disruption in infected individuals who develop peptic ulcers. H. pylori–specific factors include the expression of virulence factors such as CagA and VacA, which interact with the host inflammatory response to cause mucosal injury. The mucosal inflammatory response is at least partially determined by polymorphisms in the host’s cytokine genes.1,4 NSAIDs inhibit the production of cyclooxygenase-1-derived prostaglandins, with subsequent decreases in epithelial mucous formation, bicarbonate secretion, cell proliferation, and mucosal blood flow, all of which are key elements in the maintenance of mucosal integrity.1,5 Less common causes of peptic ulcers include gastrinoma, adenocarcinoma, idiopathic ulcers, use of sympathomimetic drugs (eg, cocaine or methamphetamine), certain anticancer agents, and bariatric surgery.4,6
This article provides an overview of current management principles for peptic ulcer disease and discusses current challenges in peptic ulcer management, including proton pump inhibitor (PPI) therapy, refractory ulcers, handling of antiplatelet and anticoagulants during and after peptic ulcer bleeding, and ulcer bleeding that continues despite salvage endoscopic therapy.
Methods
We searched MEDLINE using the term peptic ulcer disease in combination with the terms current challenges, epidemiology, bleeding, anticoagulant, antiplatelet, PPI potency, etiology, treatment, management, and refractory. We selected publications from the past 35 years that we judged to be relevant.
Current Management
The goals of peptic ulcer disease management are ulcer healing and prevention of recurrence. The primary interventions used in the management of peptic ulcer disease are medical therapy and implementation of measures that address the underlying etiology of the disease.
Medical Therapy
Introduced in the late 1980s, PPIs are the cornerstone of medical therapy for peptic ulcer disease.6 These agents irreversibly inhibit the H+/K+-ATPase pump in the gastric mucosa and thereby inhibit gastric acid secretion, promoting ulcer healing. PPIs improve rates of ulcer healing compared to H2-receptor antagonists.4,7
Underlying Causes
The underlying cause of peptic ulcer disease should be addressed, in addition to initiating medical therapy. A detailed history of NSAID use should be obtained, and patients with peptic ulcers caused by NSAIDs should be counseled to avoid them, if possible. Patients with peptic ulcer disease who require long-term use of NSAIDs should be placed on long-term PPI therapy.6 Any patient with peptic ulcer disease, regardless of any history of H. pylori infection or treatment, should be tested for infection. Tests that identify active infection, such as urea breath test, stool antigen assay, or mucosal biopsy–based testing, are preferred to IgG antibody testing, although the latter is acceptable in the context of peptic ulcer disease with a high pretest probability of infection.8 Any evidence of active infection warrants appropriate treatment to allow ulcer healing and prevent recurrence.1H. pylori infection is most often treated with clarithromycin triple therapy or bismuth quadruple therapy for 14 days, with regimens selected based on the presence or absence of penicillin allergy, prior antibiotic exposure, and local clarithromycin resistance rates, when known.4,8
Managing Complications
An additional aspect of care in peptic ulcer disease is managing the complications of bleeding, perforation, and gastric outlet obstruction. Acute upper gastrointestinal bleeding (GIB) is the most common complication of peptic ulcer disease, which accounts for 40% to 60% of nonvariceal acute upper GIB.1,6 The first step in the management of acute GIB from a peptic ulcer is fluid resuscitation to ensure hemodynamic stability. If there is associated anemia with a hemoglobin level < 8 g/dL, blood transfusion should be undertaken to target a hemoglobin level > 8 g/dL. In patients with peptic ulcer disease–related acute upper GIB and comorbid cardiovascular disease, the transfusion threshold is higher, with the specific cutoff depending on clinical status, type and severity of cardiovascular disease, and degree of bleeding. Endoscopic management should generally be undertaken within 24 hours of presentation and should not be delayed in patients taking anticoagulants.9 Combination endoscopic treatment with through-the-scope clips plus thermocoagulation or sclerosant injection is recommended for acutely bleeding peptic ulcers with high-risk stigmata.
Pharmacologic management of patients with bleeding peptic ulcers with high-risk stigmata includes PPI therapy, with an 80 mg intravenous (IV) loading dose followed by continuous infusion of 8 mg/hr for 72 hours to reduce rebleeding and mortality. Following completion of IV therapy, oral PPI therapy should be continued twice daily for 14 days, followed by once-daily dosing thereafter.9Patients with peptic ulcer perforation present with sudden-onset epigastric abdominal pain and have tenderness to palpation, guarding, and rigidity on examination, often along with tachycardia and hypotension.1,4 Computed tomography (CT) of the abdomen is 98% sensitive for identifying and localizing a perforation. Most perforations occur in the duodenum or antrum.
Management of a peptic ulcer perforation requires consultation with a surgeon to determine whether a nonoperative approach may be employed (eg, a stable patient with a contained perforation), or if surgery is indicated. The surgical approach to peptic ulcer perforation has been impacted by the clinical success of gastric acid suppression with PPIs and H. pylori eradication, but a range of surgical approaches are still used to repair perforations, from omental patch repair with peritoneal drain placement, to more extensive surgeries such as wedge resection or partial gastrectomy.4 Perforation carries a high mortality risk, up to 20% to 30%, and is the leading cause of death in patients with peptic ulcer disease.1,4
Gastric outlet obstruction, a rare complication of peptic ulcer disease, results from recurrent ulcer formation and scarring. Obstruction often presents with hypovolemia and metabolic alkalosis from prolonged vomiting. CT imaging with oral contrast is often the first diagnostic test employed to demonstrate obstruction. Upper endoscopy should be performed to evaluate the appearance and degree of obstruction as well as to obtain biopsies to evaluate for a malignant etiology of the ulcer disease. Endoscopic balloon dilation has become the cornerstone of initial therapy for obstruction from peptic ulcer disease, especially in the case of ulcers due to reversible causes. Surgery is now typically reserved for cases of refractory obstruction, after repeated endoscopic balloon dilation has failed to remove the obstruction. However, because nearly all patients with gastric outlet obstruction present with malnutrition, nutritional deficiencies should be addressed prior to the patient undergoing surgical intervention. Surgical options include pyloroplasty, antrectomy, and gastrojejunostomy.4
Current Challenges
Rapid Metabolism of PPIs
High-dose PPI therapy is a key component of therapy for peptic ulcer healing. PPIs are metabolized by the cytochrome P450 system, which is comprised of multiple isoenzymes. CYP2C19, an isoenzyme involved in PPI metabolism, has 21 polymorphisms, which have variable effects leading to ultra-rapid, extensive, intermediate, or poor metabolism of PPIs.10 With rapid metabolism of PPIs, standard dosing can result in inadequate suppression of acid secretion. Despite this knowledge, routine testing of CYP2C19 phenotype is not recommended due to the cost of testing. Instead, inadequate ulcer healing should prompt consideration of increased PPI dosing to 80 mg orally twice daily, which may be sufficient to overcome rapid PPI metabolism.11
Relative Potency of PPIs
In addition to variation in PPI metabolism, the relative potency of various PPIs has been questioned. A review of all available clinical studies of the effects of PPIs on mean 24-hour intragastric pH reported a quantitative difference in the potency of 5 PPIs, with omeprazole as the reference standard. Potencies ranged from 0.23 omeprazole equivalents for pantoprazole to 1.82 omeprazole equivalents for rabeprazole.12 An additional study of data from 56 randomized clinical trials confirmed that PPIs vary in potency, which was measured as time that gastric pH is less than 4. A linear increase in intragastric pH time less than 4 was observed from 9 to 64 mg omeprazole equivalents; higher doses yielded no additional benefit. An increase in PPI dosing from once daily to twice daily also increased the duration of intragastric pH time less than 4 from 15 to 21 hours.13 Earlier modeling of the relationship between duodenal ulcer healing and antisecretory therapy showed a strong correlation of ulcer healing with the duration of acid suppression, length of therapy, and the degree of acid suppression. Additional benefit was not observed after intragastric pH rose above 3.14 Thus, as the frequency and duration of acid suppression therapy are more important than PPI potency, PPIs can be used interchangeably.13,14
Addressing Underlying Causes
Continued NSAID Use. Refractory peptic ulcers are defined as those that do not heal despite adherence to 8 to 12 weeks of standard acid-suppression therapy. A cause of refractory peptic ulcer disease that must be considered is continued NSAID use.1,15 In a study of patients with refractory peptic ulcers, 27% of patients continued NSAID use, as determined by eventual disclosure by the patients or platelet cyclooxygenase activity assay, despite extensive counseling to avoid NSAIDs at the time of the diagnosis of their refractory ulcer and at subsequent visits.16 Pain may make NSAID cessation difficult for some patients, while others do not realize that over-the-counter preparations they take contain NSAIDs.15
Another group of patients with continued NSAID exposure are those who require long-term NSAID therapy for control of arthritis or the management of cardiovascular conditions. If NSAID therapy cannot be discontinued, the risk of NSAID-related gastrointestinal injury can be assessed based on the presence of multiple risk factors, including age > 65 years, high-dose NSAID therapy, a history of peptic ulcer, and concurrent use of aspirin, corticosteroids, or anticoagulants. Individuals with 3 or more of the preceding risk factors or a history of a peptic ulcer with a complication, especially if recent, are considered to be at high risk of developing an NSAID-related ulcer and possible subsequent complications.17 In these individuals, NSAID therapy should be continued with agents that have the lowest risk for gastrointestinal toxicity and at the lowest possible dose. A meta-analysis comparing nonselective NSAIDs to placebo demonstrated naproxen to have the highest risk of gastrointestinal complications, including GIB, perforation, and obstruction (adjusted rate ratio, 4.2), while diclofenac demonstrated the lowest risk (adjusted rate ratio, 1.89). High-dose NSAID therapy demonstrated a 2-fold increase in risk of peptic ulcer formation as compared to low-dose therapy.18
In addition to selecting the NSAID with the least gastrointestinal toxicity at the lowest possible dose, additional strategies to prevent peptic ulcer disease and its complications in chronic NSAID users include co-administration of a PPI and substitution of a COX-2 selective NSAID for nonselective NSAIDs.1,9 Prior double-blind, placebo-controlled, randomized, multicenter trials with patients requiring daily NSAIDs demonstrated an up to 15% absolute reduction in the risk of developing peptic ulcers over 6 months while taking esomeprazole.19
Persistent Infection. Persistent H. pylori infection, due either to initial false-negative testing or ongoing infection despite first-line therapy, is another cause of refractory peptic ulcer disease.1,15 Because antibiotics and PPIs can reduce the number of H. pylori bacteria, use of these medications concurrent with H. pylori testing can lead to false-negative results with several testing modalities. When suspicion for H. pylori is high, 2 or more diagnostic tests may be needed to effectively rule out infection.15
When H. pylori is detected, successful eradication is becoming more difficult due to an increasing prevalence of antibiotic resistance, leading to persistent infection in many cases and maintained risk of peptic ulcer disease, despite appropriate first-line therapy.8 Options for salvage therapy for persistent H. pylori, as well as information on the role and best timing of susceptibility testing, are beyond the scope of this review, but are reviewed by Lanas and Chan1 and in the American College of Gastroenterology guideline on the treatment of H. pylori infection.8
Other Causes. In a meta-analysis of rigorously designed studies from North America, 20% of patients experienced ulcer recurrence at 6 months, despite successful H. pylori eradication and no NSAID use.20 In addition, as H. pylori prevalence is decreasing, idiopathic ulcers are increasingly being diagnosed, and such ulcers may be associated with high rates of GIB and mortality.1 In this subset of patients with non-H. pylori, non-NSAID ulcers, increased effort is required to further evaluate the differential diagnosis for rarer causes of upper GI tract ulcer disease (Table). Certain malignancies, including adenocarcinoma and lymphoma, can cause ulcer formation and should be considered in refractory cases. Repeat biopsy at follow-up endoscopy for persistent ulcers should always be obtained to further evaluate for malignancy.1,15 Infectious diseases other than H. pylori infection, such as tuberculosis, syphilis, cytomegalovirus, and herpes simplex virus, are also reported as etiologies of refractory ulcers, and require specific antimicrobial treatment over and above PPI monotherapy. Special attention in biopsy sampling and sample processing is often required when infectious etiologies are being considered, as specific histologic stains and cultures may be needed for identification.15

Systemic conditions, including sarcoidosis,21 Behçet disease,22 and polyarteritis nodosa,15,23 can also cause refractory ulcers. Approximately 15% of patients with Crohn disease have gastroduodenal involvement, which may include ulcers of variable sizes.1,15,24 The increased gastric acid production seen in Zollinger-Ellison syndrome commonly presents as refractory peptic ulcers in the duodenum beyond the bulb that do not heal with standard doses of PPIs.1,15 More rare causes of acid hypersecretion leading to refractory ulcers include idiopathic gastric acid hypersecretion and retained gastric antrum syndrome after partial gastrectomy with Billroth II anastomosis.15 Smoking is a known risk factor for impaired tissue healing throughout the body, and can contribute to impaired healing of peptic ulcers through decreased prostaglandin synthesis25 and reduced gastric mucosal blood flow.26 Smoking should always be addressed in patients with refractory peptic ulcers, and cessation should be strongly encouraged. Other less common causes of refractory upper GI tract ulcers include radiation therapy, crack cocaine use, and mesenteric ischemia.15
Managing Antiplatelet and Anticoagulant Medications
Use of antiplatelets and anticoagulants, alone or in combination, increases the risk of peptic ulcer bleeding. In patients who continue to take aspirin after a peptic ulcer bleed, recurrent bleeding occurs in up to 300 cases per 1000 person-years. The rate of GIB associated with aspirin use ranges from 1.1% to 2.5%, depending on the dose. Prior peptic ulcer disease, age greater than 70 years, and concurrent NSAID, steroid, anticoagulant, or dual antiplatelet therapy (DAPT) use increase the risk of bleeding while on aspirin. The rate of GIB while taking a thienopyridine alone is slightly less than that when taking aspirin, ranging from 0.5% to 1.6%. Studies to date have yielded mixed estimates of the effect of DAPT on the risk of GIB. Estimates of the risk of GIB with DAPT range from an odds ratio for serious GIB of 7.4 to an absolute risk increase of only 1.3% when compared to clopidogrel alone.27
Many patients are also on warfarin or a direct oral anticoagulant (DOAC). In a study from the United Kingdom, the adjusted rate ratio of GIB with warfarin alone was 1.94, and this increased to 6.48 when warfarin was used with aspirin.28 The use of warfarin and DAPT, often called triple therapy, further increases the risk of GIB, with a hazard ratio of 5.0 compared to DAPT alone, and 5.38 when compared to warfarin alone. DOACs are increasingly prescribed for the treatment and prevention of thromboembolism, and by 2014 were prescribed as often as warfarin for stroke prevention in atrial fibrillation in the United States. A meta-analysis showed the risk of major GIB did not differ between DOACs and warfarin or low-molecular-weight heparin, but among DOACs factor Xa inhibitors showed a reduced risk of GIB compared with dabigatran, a direct thrombin inhibitor.29
The use of antiplatelets and anticoagulants in the context of peptic ulcer bleeding is a current management challenge. Data to guide decision-making in patients on antiplatelet and/or anticoagulant therapy who experience peptic ulcer bleeding are scarce. Decision-making in this group of patients requires balancing the severity and risk of bleeding with the risk of thromboembolism.1,27 In patients on antiplatelet therapy for primary prophylaxis of atherothrombosis who develop bleeding from a peptic ulcer, the antiplatelet should generally be held and the indication for the medication reassessed. In patients on antiplatelet therapy for secondary prevention, the agent may be immediately resumed after endoscopy if bleeding is found to be due to an ulcer with low-risk stigmata. With bleeding resulting from an ulcer with high-risk stigmata, antiplatelet agents employed for secondary prevention may be held initially, with consideration given to early reintroduction, as early as day 3 after endoscopy.1 In patients at high risk for atherothrombotic events, including those on aspirin for secondary prophylaxis, withholding aspirin leads to a 3-fold increase in the risk of a major adverse cardiac event, with events occurring as early as 5 days after aspirin cessation in some cases.27 A randomized controlled trial of continuing low-dose aspirin versus withholding it for 8 weeks in patients on aspirin for secondary prophylaxis of cardiovascular events who experienced peptic ulcer bleeding that required endoscopic therapy demonstrated lower all-cause mortality (1.3% vs 12.9%), including death from cardiovascular or cerebrovascular events, among those who continued aspirin therapy, with a small increased risk of recurrent ulcer bleeding (10.3% vs 5.4%).30 Thus, it is recommended that antiplatelet therapy, when held, be resumed as early as possible when the risk of a cardiovascular or cerebrovascular event is considered to be higher than the risk of bleeding.27
When patients are on DAPT for a history of drug-eluting stent placement, withholding both antiplatelet medications should be avoided, even for a brief period of time, given the risk of in-stent thrombosis. When DAPT is employed for other reasons, it should be continued, if indicated, after bleeding that is found to be due to peptic ulcers with low-risk stigmata. If bleeding is due to a peptic ulcer with high-risk stigmata at endoscopy, then aspirin monotherapy should be continued and consultation should be obtained with a cardiologist to determine optimal timing to resume the second antiplatelet agent.1 In patients on anticoagulants, anticoagulation should be resumed once hemostasis is achieved when the risk of withholding anticoagulation is thought to be greater than the risk of rebleeding. For example, anticoagulation should be resumed early in a patient with a mechanical heart valve to prevent thrombosis.1,27 Following upper GIB from peptic ulcer disease, patients who will require long-term aspirin, DAPT, or anticoagulation with either warfarin or DOACs should be maintained on long-term PPI therapy to reduce the risk of recurrent bleeding.9,27
Failure of Endoscopic Therapy to Control Peptic Ulcer Bleeding
Bleeding recurs in as many as 10% to 20% of patients after initial endoscopic control of peptic ulcer bleeding.4,31 In this context, repeat upper endoscopy for hemostasis is preferred to surgery, as it leads to less morbidity while providing long-term control of bleeding in more than 70% of cases.31,32 Two potential endoscopic rescue therapies that may be employed are over-the-scope clips (OTSCs) and hemostatic powder.32,33
While through-the-scope (TTS) hemostatic clips are often used during endoscopy to control active peptic ulcer bleeding, their use may be limited in large or fibrotic ulcers due to the smaller size of the clips and method of application. OTSCs have several advantages over TTS clips; notably, their larger size allows the endoscopist to achieve deeper mucosal or submucosal clip attachment via suction of the targeted tissue into the endoscopic cap (Figure 2). In a systematic review of OTSCs, successful hemostasis was achieved in 84% of 761 lesions, including 75% of lesions due to peptic ulcer disease.34 Some have argued that OTSCs may be preferred as first-line therapy over epinephrine with TTS clips for hemostasis in bleeding from high-risk peptic ulcers (ie, those with visualized arterial bleeding or a visible vessel) given observed decreases in rebleeding events.35

Despite the advantages of OTSCs, endoscopists should be mindful of the potential complications of OTSC use, including luminal obstruction, particularly in the duodenum, and perforation, which occurs in 0.3% to 2% of cases. Additionally, retrieval of misplaced OTSCs presents a significant challenge. Careful decision-making with consideration of the location, size, and depth of lesions is required when deciding on OTSC placement.34,36
A newer endoscopic tool developed for refractory bleeding from peptic ulcers and other causes is hemostatic powder. Hemostatic powders accelerate the coagulation cascade, leading to shortened coagulation times and enhanced clot formation.37 A recent meta-analysis showed that immediate hemostasis could be achieved in 95% of cases of bleeding, including in 96% of cases of bleeding from peptic ulcer disease.38 The primary limitation of hemostatic powders is the temporary nature of hemostasis, which requires the underlying etiology of bleeding to be addressed in order to provide long-term hemostasis. In the above meta-analysis, rebleeding occurred in 17% of cases after 30 days.38
Hypotension and ulcer diameter ≥ 2 cm are independent predictors of failure of endoscopic salvage therapy.31 When severe bleeding is not controlled with initial endoscopic therapy or bleeding recurs despite salvage endoscopic therapy, transcatheter angiographic embolization (TAE) is the treatment of choice.4 Systematic reviews and meta-analyses of studies that compared TAE to surgery have shown that the rate of rebleeding may be higher with TAE, but with less morbidity and either decreased or equivalent rates of mortality, with no increased need for additional interventions.4,32 In a case series examining 5 years of experience at a single medical center in China, massive GIB from duodenal ulcers was successfully treated with TAE in 27 of 29 cases (93% clinical success rate), with no mucosal ischemic necrosis observed.39
If repeated endoscopic therapy has not led to hemostasis of a bleeding peptic ulcer and TAE is not available, then surgery is the next best option. Bleeding gastric ulcers may be excised, wedge resected, or oversewn after an anterior gastrostomy. Bleeding duodenal ulcers may require use of a Kocher maneuver and linear incision of the anterior duodenum followed by ligation of the gastroduodenal artery. Fortunately, such surgical management is rarely necessary given the availability of TAE at most centers.4
Conclusion
Peptic ulcer disease is a common health problem globally, with persistent challenges related to refractory ulcers, antiplatelet and anticoagulant use, and continued bleeding in the face of endoscopic therapy. These challenges should be met with adequate frequency and duration of PPI therapy, vigilant attention to and treatment of ulcer etiology, evidence-based handling of antiplatelet and anticoagulant medications, and utilization of novel endoscopic tools to obtain improved clinical outcomes.
Acknowledgment: We thank Dr. Nipun Reddy from our institution for providing the endoscopic images used in this article.
Corresponding author: Adam L. Edwards, MD, MS; [email protected].
Financial disclosures: None.
From the University of Alabama at Birmingham, Birmingham, AL.
Abstract
Objective: To review current challenges in the management of peptic ulcer disease.
Methods: Review of the literature.
Results: Peptic ulcer disease affects 5% to 10% of the population worldwide, with recent decreases in lifetime prevalence in high-income countries. Helicobacter pylori infection and nonsteroidal anti-inflammatory drug (NSAID) use are the most important drivers of peptic ulcer disease. Current management strategies for peptic ulcer disease focus on ulcer healing; management of complications such as bleeding, perforation, and obstruction; and prevention of ulcer recurrence. Proton pump inhibitors (PPIs) are the cornerstone of medical therapy for peptic ulcers, and complement testing for and treatment of H. pylori infection as well as elimination of NSAID use. Although advances have been made in the medical and endoscopic treatment of peptic ulcer disease and the management of ulcer complications, such as bleeding and obstruction, challenges remain.
Conclusion: Peptic ulcer disease is a common health problem globally, with persistent challenges related to refractory ulcers, antiplatelet and anticoagulant use, and continued bleeding in the face of endoscopic therapy. These challenges should be met with PPI therapy of adequate frequency and duration, vigilant attention to and treatment of ulcer etiology, evidence-based handling of antiplatelet and anticoagulant medications, and utilization of novel endoscopic tools to obtain improved clinical outcomes.
Keywords: H. pylori; nonsteroidal anti-inflammatory drugs; NSAIDs; proton pump inhibitor; PPI; bleeding; perforation; obstruction; refractory ulcer; salvage endoscopic therapy; transcatheter angiographic embolization.
A peptic ulcer is a fibrin-covered break in the mucosa of the digestive tract extending to the submucosa that is caused by acid injury (Figure 1). Most peptic ulcers occur in the stomach or proximal duodenum, though they may also occur in the esophagus or, less frequently, in a Meckel’s diverticulum.1,2 The estimated worldwide prevalence of peptic ulcer disease is 5% to 10%, with an annual incidence of 0.1% to 0.3%1; both rates are declining.3 The annual incidence of peptic ulcer disease requiring medical or surgical treatment is also declining, and currently is estimated to be 0.1% to 0.2%.4 The lifetime prevalence of peptic ulcers has been decreasing in high-income countries since the mid-20th century due to both the widespread use of medications that suppress gastric acid secretion and the declining prevalence of Helicobacter pylori infection.1,3

Peptic ulcer disease in most individuals results from H. pylori infection, chronic use of nonsteroidal anti-inflammatory drugs (NSAIDs), including aspirin, or both. A combination of H. pylori factors and host factors lead to mucosal disruption in infected individuals who develop peptic ulcers. H. pylori–specific factors include the expression of virulence factors such as CagA and VacA, which interact with the host inflammatory response to cause mucosal injury. The mucosal inflammatory response is at least partially determined by polymorphisms in the host’s cytokine genes.1,4 NSAIDs inhibit the production of cyclooxygenase-1-derived prostaglandins, with subsequent decreases in epithelial mucous formation, bicarbonate secretion, cell proliferation, and mucosal blood flow, all of which are key elements in the maintenance of mucosal integrity.1,5 Less common causes of peptic ulcers include gastrinoma, adenocarcinoma, idiopathic ulcers, use of sympathomimetic drugs (eg, cocaine or methamphetamine), certain anticancer agents, and bariatric surgery.4,6
This article provides an overview of current management principles for peptic ulcer disease and discusses current challenges in peptic ulcer management, including proton pump inhibitor (PPI) therapy, refractory ulcers, handling of antiplatelet and anticoagulants during and after peptic ulcer bleeding, and ulcer bleeding that continues despite salvage endoscopic therapy.
Methods
We searched MEDLINE using the term peptic ulcer disease in combination with the terms current challenges, epidemiology, bleeding, anticoagulant, antiplatelet, PPI potency, etiology, treatment, management, and refractory. We selected publications from the past 35 years that we judged to be relevant.
Current Management
The goals of peptic ulcer disease management are ulcer healing and prevention of recurrence. The primary interventions used in the management of peptic ulcer disease are medical therapy and implementation of measures that address the underlying etiology of the disease.
Medical Therapy
Introduced in the late 1980s, PPIs are the cornerstone of medical therapy for peptic ulcer disease.6 These agents irreversibly inhibit the H+/K+-ATPase pump in the gastric mucosa and thereby inhibit gastric acid secretion, promoting ulcer healing. PPIs improve rates of ulcer healing compared to H2-receptor antagonists.4,7
Underlying Causes
The underlying cause of peptic ulcer disease should be addressed, in addition to initiating medical therapy. A detailed history of NSAID use should be obtained, and patients with peptic ulcers caused by NSAIDs should be counseled to avoid them, if possible. Patients with peptic ulcer disease who require long-term use of NSAIDs should be placed on long-term PPI therapy.6 Any patient with peptic ulcer disease, regardless of any history of H. pylori infection or treatment, should be tested for infection. Tests that identify active infection, such as urea breath test, stool antigen assay, or mucosal biopsy–based testing, are preferred to IgG antibody testing, although the latter is acceptable in the context of peptic ulcer disease with a high pretest probability of infection.8 Any evidence of active infection warrants appropriate treatment to allow ulcer healing and prevent recurrence.1H. pylori infection is most often treated with clarithromycin triple therapy or bismuth quadruple therapy for 14 days, with regimens selected based on the presence or absence of penicillin allergy, prior antibiotic exposure, and local clarithromycin resistance rates, when known.4,8
Managing Complications
An additional aspect of care in peptic ulcer disease is managing the complications of bleeding, perforation, and gastric outlet obstruction. Acute upper gastrointestinal bleeding (GIB) is the most common complication of peptic ulcer disease, which accounts for 40% to 60% of nonvariceal acute upper GIB.1,6 The first step in the management of acute GIB from a peptic ulcer is fluid resuscitation to ensure hemodynamic stability. If there is associated anemia with a hemoglobin level < 8 g/dL, blood transfusion should be undertaken to target a hemoglobin level > 8 g/dL. In patients with peptic ulcer disease–related acute upper GIB and comorbid cardiovascular disease, the transfusion threshold is higher, with the specific cutoff depending on clinical status, type and severity of cardiovascular disease, and degree of bleeding. Endoscopic management should generally be undertaken within 24 hours of presentation and should not be delayed in patients taking anticoagulants.9 Combination endoscopic treatment with through-the-scope clips plus thermocoagulation or sclerosant injection is recommended for acutely bleeding peptic ulcers with high-risk stigmata.
Pharmacologic management of patients with bleeding peptic ulcers with high-risk stigmata includes PPI therapy, with an 80 mg intravenous (IV) loading dose followed by continuous infusion of 8 mg/hr for 72 hours to reduce rebleeding and mortality. Following completion of IV therapy, oral PPI therapy should be continued twice daily for 14 days, followed by once-daily dosing thereafter.9Patients with peptic ulcer perforation present with sudden-onset epigastric abdominal pain and have tenderness to palpation, guarding, and rigidity on examination, often along with tachycardia and hypotension.1,4 Computed tomography (CT) of the abdomen is 98% sensitive for identifying and localizing a perforation. Most perforations occur in the duodenum or antrum.
Management of a peptic ulcer perforation requires consultation with a surgeon to determine whether a nonoperative approach may be employed (eg, a stable patient with a contained perforation), or if surgery is indicated. The surgical approach to peptic ulcer perforation has been impacted by the clinical success of gastric acid suppression with PPIs and H. pylori eradication, but a range of surgical approaches are still used to repair perforations, from omental patch repair with peritoneal drain placement, to more extensive surgeries such as wedge resection or partial gastrectomy.4 Perforation carries a high mortality risk, up to 20% to 30%, and is the leading cause of death in patients with peptic ulcer disease.1,4
Gastric outlet obstruction, a rare complication of peptic ulcer disease, results from recurrent ulcer formation and scarring. Obstruction often presents with hypovolemia and metabolic alkalosis from prolonged vomiting. CT imaging with oral contrast is often the first diagnostic test employed to demonstrate obstruction. Upper endoscopy should be performed to evaluate the appearance and degree of obstruction as well as to obtain biopsies to evaluate for a malignant etiology of the ulcer disease. Endoscopic balloon dilation has become the cornerstone of initial therapy for obstruction from peptic ulcer disease, especially in the case of ulcers due to reversible causes. Surgery is now typically reserved for cases of refractory obstruction, after repeated endoscopic balloon dilation has failed to remove the obstruction. However, because nearly all patients with gastric outlet obstruction present with malnutrition, nutritional deficiencies should be addressed prior to the patient undergoing surgical intervention. Surgical options include pyloroplasty, antrectomy, and gastrojejunostomy.4
Current Challenges
Rapid Metabolism of PPIs
High-dose PPI therapy is a key component of therapy for peptic ulcer healing. PPIs are metabolized by the cytochrome P450 system, which is comprised of multiple isoenzymes. CYP2C19, an isoenzyme involved in PPI metabolism, has 21 polymorphisms, which have variable effects leading to ultra-rapid, extensive, intermediate, or poor metabolism of PPIs.10 With rapid metabolism of PPIs, standard dosing can result in inadequate suppression of acid secretion. Despite this knowledge, routine testing of CYP2C19 phenotype is not recommended due to the cost of testing. Instead, inadequate ulcer healing should prompt consideration of increased PPI dosing to 80 mg orally twice daily, which may be sufficient to overcome rapid PPI metabolism.11
Relative Potency of PPIs
In addition to variation in PPI metabolism, the relative potency of various PPIs has been questioned. A review of all available clinical studies of the effects of PPIs on mean 24-hour intragastric pH reported a quantitative difference in the potency of 5 PPIs, with omeprazole as the reference standard. Potencies ranged from 0.23 omeprazole equivalents for pantoprazole to 1.82 omeprazole equivalents for rabeprazole.12 An additional study of data from 56 randomized clinical trials confirmed that PPIs vary in potency, which was measured as time that gastric pH is less than 4. A linear increase in intragastric pH time less than 4 was observed from 9 to 64 mg omeprazole equivalents; higher doses yielded no additional benefit. An increase in PPI dosing from once daily to twice daily also increased the duration of intragastric pH time less than 4 from 15 to 21 hours.13 Earlier modeling of the relationship between duodenal ulcer healing and antisecretory therapy showed a strong correlation of ulcer healing with the duration of acid suppression, length of therapy, and the degree of acid suppression. Additional benefit was not observed after intragastric pH rose above 3.14 Thus, as the frequency and duration of acid suppression therapy are more important than PPI potency, PPIs can be used interchangeably.13,14
Addressing Underlying Causes
Continued NSAID Use. Refractory peptic ulcers are defined as those that do not heal despite adherence to 8 to 12 weeks of standard acid-suppression therapy. A cause of refractory peptic ulcer disease that must be considered is continued NSAID use.1,15 In a study of patients with refractory peptic ulcers, 27% of patients continued NSAID use, as determined by eventual disclosure by the patients or platelet cyclooxygenase activity assay, despite extensive counseling to avoid NSAIDs at the time of the diagnosis of their refractory ulcer and at subsequent visits.16 Pain may make NSAID cessation difficult for some patients, while others do not realize that over-the-counter preparations they take contain NSAIDs.15
Another group of patients with continued NSAID exposure are those who require long-term NSAID therapy for control of arthritis or the management of cardiovascular conditions. If NSAID therapy cannot be discontinued, the risk of NSAID-related gastrointestinal injury can be assessed based on the presence of multiple risk factors, including age > 65 years, high-dose NSAID therapy, a history of peptic ulcer, and concurrent use of aspirin, corticosteroids, or anticoagulants. Individuals with 3 or more of the preceding risk factors or a history of a peptic ulcer with a complication, especially if recent, are considered to be at high risk of developing an NSAID-related ulcer and possible subsequent complications.17 In these individuals, NSAID therapy should be continued with agents that have the lowest risk for gastrointestinal toxicity and at the lowest possible dose. A meta-analysis comparing nonselective NSAIDs to placebo demonstrated naproxen to have the highest risk of gastrointestinal complications, including GIB, perforation, and obstruction (adjusted rate ratio, 4.2), while diclofenac demonstrated the lowest risk (adjusted rate ratio, 1.89). High-dose NSAID therapy demonstrated a 2-fold increase in risk of peptic ulcer formation as compared to low-dose therapy.18
In addition to selecting the NSAID with the least gastrointestinal toxicity at the lowest possible dose, additional strategies to prevent peptic ulcer disease and its complications in chronic NSAID users include co-administration of a PPI and substitution of a COX-2 selective NSAID for nonselective NSAIDs.1,9 Prior double-blind, placebo-controlled, randomized, multicenter trials with patients requiring daily NSAIDs demonstrated an up to 15% absolute reduction in the risk of developing peptic ulcers over 6 months while taking esomeprazole.19
Persistent Infection. Persistent H. pylori infection, due either to initial false-negative testing or ongoing infection despite first-line therapy, is another cause of refractory peptic ulcer disease.1,15 Because antibiotics and PPIs can reduce the number of H. pylori bacteria, use of these medications concurrent with H. pylori testing can lead to false-negative results with several testing modalities. When suspicion for H. pylori is high, 2 or more diagnostic tests may be needed to effectively rule out infection.15
When H. pylori is detected, successful eradication is becoming more difficult due to an increasing prevalence of antibiotic resistance, leading to persistent infection in many cases and maintained risk of peptic ulcer disease, despite appropriate first-line therapy.8 Options for salvage therapy for persistent H. pylori, as well as information on the role and best timing of susceptibility testing, are beyond the scope of this review, but are reviewed by Lanas and Chan1 and in the American College of Gastroenterology guideline on the treatment of H. pylori infection.8
Other Causes. In a meta-analysis of rigorously designed studies from North America, 20% of patients experienced ulcer recurrence at 6 months, despite successful H. pylori eradication and no NSAID use.20 In addition, as H. pylori prevalence is decreasing, idiopathic ulcers are increasingly being diagnosed, and such ulcers may be associated with high rates of GIB and mortality.1 In this subset of patients with non-H. pylori, non-NSAID ulcers, increased effort is required to further evaluate the differential diagnosis for rarer causes of upper GI tract ulcer disease (Table). Certain malignancies, including adenocarcinoma and lymphoma, can cause ulcer formation and should be considered in refractory cases. Repeat biopsy at follow-up endoscopy for persistent ulcers should always be obtained to further evaluate for malignancy.1,15 Infectious diseases other than H. pylori infection, such as tuberculosis, syphilis, cytomegalovirus, and herpes simplex virus, are also reported as etiologies of refractory ulcers, and require specific antimicrobial treatment over and above PPI monotherapy. Special attention in biopsy sampling and sample processing is often required when infectious etiologies are being considered, as specific histologic stains and cultures may be needed for identification.15

Systemic conditions, including sarcoidosis,21 Behçet disease,22 and polyarteritis nodosa,15,23 can also cause refractory ulcers. Approximately 15% of patients with Crohn disease have gastroduodenal involvement, which may include ulcers of variable sizes.1,15,24 The increased gastric acid production seen in Zollinger-Ellison syndrome commonly presents as refractory peptic ulcers in the duodenum beyond the bulb that do not heal with standard doses of PPIs.1,15 More rare causes of acid hypersecretion leading to refractory ulcers include idiopathic gastric acid hypersecretion and retained gastric antrum syndrome after partial gastrectomy with Billroth II anastomosis.15 Smoking is a known risk factor for impaired tissue healing throughout the body, and can contribute to impaired healing of peptic ulcers through decreased prostaglandin synthesis25 and reduced gastric mucosal blood flow.26 Smoking should always be addressed in patients with refractory peptic ulcers, and cessation should be strongly encouraged. Other less common causes of refractory upper GI tract ulcers include radiation therapy, crack cocaine use, and mesenteric ischemia.15
Managing Antiplatelet and Anticoagulant Medications
Use of antiplatelets and anticoagulants, alone or in combination, increases the risk of peptic ulcer bleeding. In patients who continue to take aspirin after a peptic ulcer bleed, recurrent bleeding occurs in up to 300 cases per 1000 person-years. The rate of GIB associated with aspirin use ranges from 1.1% to 2.5%, depending on the dose. Prior peptic ulcer disease, age greater than 70 years, and concurrent NSAID, steroid, anticoagulant, or dual antiplatelet therapy (DAPT) use increase the risk of bleeding while on aspirin. The rate of GIB while taking a thienopyridine alone is slightly less than that when taking aspirin, ranging from 0.5% to 1.6%. Studies to date have yielded mixed estimates of the effect of DAPT on the risk of GIB. Estimates of the risk of GIB with DAPT range from an odds ratio for serious GIB of 7.4 to an absolute risk increase of only 1.3% when compared to clopidogrel alone.27
Many patients are also on warfarin or a direct oral anticoagulant (DOAC). In a study from the United Kingdom, the adjusted rate ratio of GIB with warfarin alone was 1.94, and this increased to 6.48 when warfarin was used with aspirin.28 The use of warfarin and DAPT, often called triple therapy, further increases the risk of GIB, with a hazard ratio of 5.0 compared to DAPT alone, and 5.38 when compared to warfarin alone. DOACs are increasingly prescribed for the treatment and prevention of thromboembolism, and by 2014 were prescribed as often as warfarin for stroke prevention in atrial fibrillation in the United States. A meta-analysis showed the risk of major GIB did not differ between DOACs and warfarin or low-molecular-weight heparin, but among DOACs factor Xa inhibitors showed a reduced risk of GIB compared with dabigatran, a direct thrombin inhibitor.29
The use of antiplatelets and anticoagulants in the context of peptic ulcer bleeding is a current management challenge. Data to guide decision-making in patients on antiplatelet and/or anticoagulant therapy who experience peptic ulcer bleeding are scarce. Decision-making in this group of patients requires balancing the severity and risk of bleeding with the risk of thromboembolism.1,27 In patients on antiplatelet therapy for primary prophylaxis of atherothrombosis who develop bleeding from a peptic ulcer, the antiplatelet should generally be held and the indication for the medication reassessed. In patients on antiplatelet therapy for secondary prevention, the agent may be immediately resumed after endoscopy if bleeding is found to be due to an ulcer with low-risk stigmata. With bleeding resulting from an ulcer with high-risk stigmata, antiplatelet agents employed for secondary prevention may be held initially, with consideration given to early reintroduction, as early as day 3 after endoscopy.1 In patients at high risk for atherothrombotic events, including those on aspirin for secondary prophylaxis, withholding aspirin leads to a 3-fold increase in the risk of a major adverse cardiac event, with events occurring as early as 5 days after aspirin cessation in some cases.27 A randomized controlled trial of continuing low-dose aspirin versus withholding it for 8 weeks in patients on aspirin for secondary prophylaxis of cardiovascular events who experienced peptic ulcer bleeding that required endoscopic therapy demonstrated lower all-cause mortality (1.3% vs 12.9%), including death from cardiovascular or cerebrovascular events, among those who continued aspirin therapy, with a small increased risk of recurrent ulcer bleeding (10.3% vs 5.4%).30 Thus, it is recommended that antiplatelet therapy, when held, be resumed as early as possible when the risk of a cardiovascular or cerebrovascular event is considered to be higher than the risk of bleeding.27
When patients are on DAPT for a history of drug-eluting stent placement, withholding both antiplatelet medications should be avoided, even for a brief period of time, given the risk of in-stent thrombosis. When DAPT is employed for other reasons, it should be continued, if indicated, after bleeding that is found to be due to peptic ulcers with low-risk stigmata. If bleeding is due to a peptic ulcer with high-risk stigmata at endoscopy, then aspirin monotherapy should be continued and consultation should be obtained with a cardiologist to determine optimal timing to resume the second antiplatelet agent.1 In patients on anticoagulants, anticoagulation should be resumed once hemostasis is achieved when the risk of withholding anticoagulation is thought to be greater than the risk of rebleeding. For example, anticoagulation should be resumed early in a patient with a mechanical heart valve to prevent thrombosis.1,27 Following upper GIB from peptic ulcer disease, patients who will require long-term aspirin, DAPT, or anticoagulation with either warfarin or DOACs should be maintained on long-term PPI therapy to reduce the risk of recurrent bleeding.9,27
Failure of Endoscopic Therapy to Control Peptic Ulcer Bleeding
Bleeding recurs in as many as 10% to 20% of patients after initial endoscopic control of peptic ulcer bleeding.4,31 In this context, repeat upper endoscopy for hemostasis is preferred to surgery, as it leads to less morbidity while providing long-term control of bleeding in more than 70% of cases.31,32 Two potential endoscopic rescue therapies that may be employed are over-the-scope clips (OTSCs) and hemostatic powder.32,33
While through-the-scope (TTS) hemostatic clips are often used during endoscopy to control active peptic ulcer bleeding, their use may be limited in large or fibrotic ulcers due to the smaller size of the clips and method of application. OTSCs have several advantages over TTS clips; notably, their larger size allows the endoscopist to achieve deeper mucosal or submucosal clip attachment via suction of the targeted tissue into the endoscopic cap (Figure 2). In a systematic review of OTSCs, successful hemostasis was achieved in 84% of 761 lesions, including 75% of lesions due to peptic ulcer disease.34 Some have argued that OTSCs may be preferred as first-line therapy over epinephrine with TTS clips for hemostasis in bleeding from high-risk peptic ulcers (ie, those with visualized arterial bleeding or a visible vessel) given observed decreases in rebleeding events.35

Despite the advantages of OTSCs, endoscopists should be mindful of the potential complications of OTSC use, including luminal obstruction, particularly in the duodenum, and perforation, which occurs in 0.3% to 2% of cases. Additionally, retrieval of misplaced OTSCs presents a significant challenge. Careful decision-making with consideration of the location, size, and depth of lesions is required when deciding on OTSC placement.34,36
A newer endoscopic tool developed for refractory bleeding from peptic ulcers and other causes is hemostatic powder. Hemostatic powders accelerate the coagulation cascade, leading to shortened coagulation times and enhanced clot formation.37 A recent meta-analysis showed that immediate hemostasis could be achieved in 95% of cases of bleeding, including in 96% of cases of bleeding from peptic ulcer disease.38 The primary limitation of hemostatic powders is the temporary nature of hemostasis, which requires the underlying etiology of bleeding to be addressed in order to provide long-term hemostasis. In the above meta-analysis, rebleeding occurred in 17% of cases after 30 days.38
Hypotension and ulcer diameter ≥ 2 cm are independent predictors of failure of endoscopic salvage therapy.31 When severe bleeding is not controlled with initial endoscopic therapy or bleeding recurs despite salvage endoscopic therapy, transcatheter angiographic embolization (TAE) is the treatment of choice.4 Systematic reviews and meta-analyses of studies that compared TAE to surgery have shown that the rate of rebleeding may be higher with TAE, but with less morbidity and either decreased or equivalent rates of mortality, with no increased need for additional interventions.4,32 In a case series examining 5 years of experience at a single medical center in China, massive GIB from duodenal ulcers was successfully treated with TAE in 27 of 29 cases (93% clinical success rate), with no mucosal ischemic necrosis observed.39
If repeated endoscopic therapy has not led to hemostasis of a bleeding peptic ulcer and TAE is not available, then surgery is the next best option. Bleeding gastric ulcers may be excised, wedge resected, or oversewn after an anterior gastrostomy. Bleeding duodenal ulcers may require use of a Kocher maneuver and linear incision of the anterior duodenum followed by ligation of the gastroduodenal artery. Fortunately, such surgical management is rarely necessary given the availability of TAE at most centers.4
Conclusion
Peptic ulcer disease is a common health problem globally, with persistent challenges related to refractory ulcers, antiplatelet and anticoagulant use, and continued bleeding in the face of endoscopic therapy. These challenges should be met with adequate frequency and duration of PPI therapy, vigilant attention to and treatment of ulcer etiology, evidence-based handling of antiplatelet and anticoagulant medications, and utilization of novel endoscopic tools to obtain improved clinical outcomes.
Acknowledgment: We thank Dr. Nipun Reddy from our institution for providing the endoscopic images used in this article.
Corresponding author: Adam L. Edwards, MD, MS; [email protected].
Financial disclosures: None.
1. Lanas A, Chan FKL. Peptic ulcer disease. Lancet. 2017;390:613-624.
2. Malfertheiner P, Chan FK, McColl KE. Peptic ulcer disease. Lancet. 2009;374:1449-1461.
3. Roberts-Thomson IC. Rise and fall of peptic ulceration: A disease of civilization? J Gastroenterol Hepatol. 2018;33:1321-1326.
4. Kempenich JW, Sirinek KR. Acid peptic disease. Surg Clin North Am. 2018;98:933-944.
5. Cryer B, Feldman M. Effects of very low dose daily, long-term aspirin therapy on gastric, duodenal, and rectal prostaglandin levels and on mucosal injury in healthy humans. Gastroenterology. 1999;117:17-25.
6. Kavitt RT, Lipowska AM, Anyane-Yeboa A, Gralnek IM. Diagnosis and treatment of peptic ulcer disease. Am J Med. 2019;132:447-456.
7. Walan A, Bader JP, Classen M, et al. Effect of omeprazole and ranitidine on ulcer healing and relapse rates in patients with benign gastric ulcer. New Engl J Med. 1989;320:69-75.
8. Chey WD, Leontiadis GI, Howden CW, Moss SF. ACG Clinical Guideline: Treatment of Helicobacter pylori Infection. Am J Gastroenterol. 2017;112:212-239.
9. Barkun AN, Almadi M, Kuipers EJ, et al. Management of nonvariceal upper gastrointestinal bleeding: Guideline recommendations from the International Consensus Group. Ann Intern Med. 2019;171:805-822.
10. Arevalo Galvis A, Trespalacios Rangel AA, Otero Regino W. Personalized therapy for Helicobacter pylori: CYP2C19 genotype effect on first-line triple therapy. Helicobacter. 2019;24:e12574.
11. Furuta T, Ohashi K, Kamata T, et al. Effect of genetic differences in omeprazole metabolism on cure rates for Helicobacter pylori infection and peptic ulcer. Ann Intern Med. 1998;129:1027-1030.
12. Kirchheiner J, Glatt S, Fuhr U, et al. Relative potency of proton-pump inhibitors-comparison of effects on intragastric pH. Eur J Clin Pharmacol. 2009;65:19-31.
13. Graham DY, Tansel A. Interchangeable use of proton pump inhibitors based on relative potency. Clin Gastroenterol Hepatol. 2018;16:800-808.e7.
14. Burget DW, Chiverton SG, Hunt RH. Is there an optimal degree of acid suppression for healing of duodenal ulcers? A model of the relationship between ulcer healing and acid suppression. Gastroenterology. 1990;99:345-351.
15. Kim HU. Diagnostic and treatment approaches for refractory peptic ulcers. Clin Endosc. 2015;48:285-290.
16. Lanas AI, Remacha B, Esteva F, Sainz R. Risk factors associated with refractory peptic ulcers. Gastroenterology. 1995;109:124-133.
17. Lanza FL, Chan FK, Quigley EM. Guidelines for prevention of NSAID-related ulcer complications. Am J Gastroenterol. 2009;104:728-738.
18. Richy F, Bruyere O, Ethgen O, et al. Time dependent risk of gastrointestinal complications induced by non-steroidal anti-inflammatory drug use: a consensus statement using a meta-analytic approach. Ann Rheum Dis. 2004;63:759-766.
19. Scheiman JM, Yeomans ND, Talley NJ, et al. Prevention of ulcers by esomeprazole in at-risk patients using non-selective NSAIDs and COX-2 inhibitors. Am J Gastroenterol. 2006;101:701-710.
20. Laine L, Hopkins RJ, Girardi LS. Has the impact of Helicobacter pylori therapy on ulcer recurrence in the United States been overstated? A meta-analysis of rigorously designed trials. Am J Gastroenterol. 1998;93:1409-1415.
21. Akiyama T, Endo H, Inamori M, et al. Symptomatic gastric sarcoidosis with multiple antral ulcers. Endoscopy. 2009;41 Suppl 2:E159.
22. Sonoda A, Ogawa R, Mizukami K, et al. Marked improvement in gastric involvement in Behcet’s disease with adalimumab treatment. Turk J Gastroenterol. 2017;28:405-407.
23. Saikia N, Talukdar R, Mazumder S, et al. Polyarteritis nodosa presenting as massive upper gastrointestinal hemorrhage. Gastrointest Endosc. 2006;63:868-870.
24. Annunziata ML, Caviglia R, Papparella LG, Cicala M. Upper gastrointestinal involvement of Crohn’s disease: a prospective study on the role of upper endoscopy in the diagnostic work-up. Dig Dis Sci. 2012;57:1618-1623.
25. Quimby GF, Bonnice CA, Burstein SH, Eastwood GL. Active smoking depresses prostaglandin synthesis in human gastric mucosa. Ann Intern Med. 1986;104:616-619.
26. Iwao T, Toyonaga A, Ikegami M, et al. Gastric mucosal blood flow after smoking in healthy human beings assessed by laser Doppler flowmetry. Gastrointest Endosc. 1993;39:400-403.
27. Almadi MA, Barkun A, Brophy J. Antiplatelet and anticoagulant therapy in patients with gastrointestinal bleeding: an 86-year-old woman with peptic ulcer disease. JAMA. 2011;306:2367-2374.
28. Delaney JA, Opatrny L, Brophy JM, Suissa S. Drug drug interactions between antithrombotic medications and the risk of gastrointestinal bleeding. CMAJ. 2007;177:347-351.
29. Burr N, Lummis K, Sood R, et al. Risk of gastrointestinal bleeding with direct oral anticoagulants: a systematic review and network meta-analysis. Lancet Gastroenterol Hepatol. 2017;2:85-93.
30. Sung JJ, Lau JY, Ching JY, et al. Continuation of low-dose aspirin therapy in peptic ulcer bleeding: a randomized trial. Ann Intern Med. 2010;152:1-9.
31. Lau JY, Sung JJ, Lam YH, et al. Endoscopic retreatment compared with surgery in patients with recurrent bleeding after initial endoscopic control of bleeding ulcers. N Engl J Med. 1999;340:751-756.
32. Gralnek IM, Dumonceau JM, Kuipers EJ, et al. Diagnosis and management of nonvariceal upper gastrointestinal hemorrhage: European Society of Gastrointestinal Endoscopy (ESGE) Guideline. Endoscopy. 2015;47:a1-46.
33. Skinner M, Gutierrez JP, Neumann H, et al. Over-the-scope clip placement is effective rescue therapy for severe acute upper gastrointestinal bleeding. Endosc Int Open. 2014;2:E37-40.
34. Zhong C, Tan S, Ren Y, et al. Clinical outcomes of over-the-scope-clip system for the treatment of acute upper non-variceal gastrointestinal bleeding: a systematic review and meta-analysis. BMC Gastroenterol. 2019;19:225.
35. Mangiafico S, Pigo F, Bertani H, et al. Over-the-scope clip vs epinephrine with clip for first-line hemostasis in non-variceal upper gastrointestinal bleeding: a propensity score match analysis. Endosc Int Open. 2020;8:E50-e8.
36. Wedi E, Gonzalez S, Menke D, et al. One hundred and one over-the-scope-clip applications for severe gastrointestinal bleeding, leaks and fistulas. World J Gastroenterol. 2016;22:1844-1853.
37. Holster IL, van Beusekom HM, Kuipers EJ, et al. Effects of a hemostatic powder hemospray on coagulation and clot formation. Endoscopy. 2015;47:638-645.
38. Facciorusso A, Straus Takahashi M, et al. Efficacy of hemostatic powders in upper gastrointestinal bleeding: A systematic review and meta-analysis. Dig Liver Dis. 2019;51:1633-1640.
39. Wang YL, Cheng YS, et al. Emergency transcatheter arterial embolization for patients with acute massive duodenal ulcer hemorrhage. World J Gastroenterol. 2012;18:4765-4770.
1. Lanas A, Chan FKL. Peptic ulcer disease. Lancet. 2017;390:613-624.
2. Malfertheiner P, Chan FK, McColl KE. Peptic ulcer disease. Lancet. 2009;374:1449-1461.
3. Roberts-Thomson IC. Rise and fall of peptic ulceration: A disease of civilization? J Gastroenterol Hepatol. 2018;33:1321-1326.
4. Kempenich JW, Sirinek KR. Acid peptic disease. Surg Clin North Am. 2018;98:933-944.
5. Cryer B, Feldman M. Effects of very low dose daily, long-term aspirin therapy on gastric, duodenal, and rectal prostaglandin levels and on mucosal injury in healthy humans. Gastroenterology. 1999;117:17-25.
6. Kavitt RT, Lipowska AM, Anyane-Yeboa A, Gralnek IM. Diagnosis and treatment of peptic ulcer disease. Am J Med. 2019;132:447-456.
7. Walan A, Bader JP, Classen M, et al. Effect of omeprazole and ranitidine on ulcer healing and relapse rates in patients with benign gastric ulcer. New Engl J Med. 1989;320:69-75.
8. Chey WD, Leontiadis GI, Howden CW, Moss SF. ACG Clinical Guideline: Treatment of Helicobacter pylori Infection. Am J Gastroenterol. 2017;112:212-239.
9. Barkun AN, Almadi M, Kuipers EJ, et al. Management of nonvariceal upper gastrointestinal bleeding: Guideline recommendations from the International Consensus Group. Ann Intern Med. 2019;171:805-822.
10. Arevalo Galvis A, Trespalacios Rangel AA, Otero Regino W. Personalized therapy for Helicobacter pylori: CYP2C19 genotype effect on first-line triple therapy. Helicobacter. 2019;24:e12574.
11. Furuta T, Ohashi K, Kamata T, et al. Effect of genetic differences in omeprazole metabolism on cure rates for Helicobacter pylori infection and peptic ulcer. Ann Intern Med. 1998;129:1027-1030.
12. Kirchheiner J, Glatt S, Fuhr U, et al. Relative potency of proton-pump inhibitors-comparison of effects on intragastric pH. Eur J Clin Pharmacol. 2009;65:19-31.
13. Graham DY, Tansel A. Interchangeable use of proton pump inhibitors based on relative potency. Clin Gastroenterol Hepatol. 2018;16:800-808.e7.
14. Burget DW, Chiverton SG, Hunt RH. Is there an optimal degree of acid suppression for healing of duodenal ulcers? A model of the relationship between ulcer healing and acid suppression. Gastroenterology. 1990;99:345-351.
15. Kim HU. Diagnostic and treatment approaches for refractory peptic ulcers. Clin Endosc. 2015;48:285-290.
16. Lanas AI, Remacha B, Esteva F, Sainz R. Risk factors associated with refractory peptic ulcers. Gastroenterology. 1995;109:124-133.
17. Lanza FL, Chan FK, Quigley EM. Guidelines for prevention of NSAID-related ulcer complications. Am J Gastroenterol. 2009;104:728-738.
18. Richy F, Bruyere O, Ethgen O, et al. Time dependent risk of gastrointestinal complications induced by non-steroidal anti-inflammatory drug use: a consensus statement using a meta-analytic approach. Ann Rheum Dis. 2004;63:759-766.
19. Scheiman JM, Yeomans ND, Talley NJ, et al. Prevention of ulcers by esomeprazole in at-risk patients using non-selective NSAIDs and COX-2 inhibitors. Am J Gastroenterol. 2006;101:701-710.
20. Laine L, Hopkins RJ, Girardi LS. Has the impact of Helicobacter pylori therapy on ulcer recurrence in the United States been overstated? A meta-analysis of rigorously designed trials. Am J Gastroenterol. 1998;93:1409-1415.
21. Akiyama T, Endo H, Inamori M, et al. Symptomatic gastric sarcoidosis with multiple antral ulcers. Endoscopy. 2009;41 Suppl 2:E159.
22. Sonoda A, Ogawa R, Mizukami K, et al. Marked improvement in gastric involvement in Behcet’s disease with adalimumab treatment. Turk J Gastroenterol. 2017;28:405-407.
23. Saikia N, Talukdar R, Mazumder S, et al. Polyarteritis nodosa presenting as massive upper gastrointestinal hemorrhage. Gastrointest Endosc. 2006;63:868-870.
24. Annunziata ML, Caviglia R, Papparella LG, Cicala M. Upper gastrointestinal involvement of Crohn’s disease: a prospective study on the role of upper endoscopy in the diagnostic work-up. Dig Dis Sci. 2012;57:1618-1623.
25. Quimby GF, Bonnice CA, Burstein SH, Eastwood GL. Active smoking depresses prostaglandin synthesis in human gastric mucosa. Ann Intern Med. 1986;104:616-619.
26. Iwao T, Toyonaga A, Ikegami M, et al. Gastric mucosal blood flow after smoking in healthy human beings assessed by laser Doppler flowmetry. Gastrointest Endosc. 1993;39:400-403.
27. Almadi MA, Barkun A, Brophy J. Antiplatelet and anticoagulant therapy in patients with gastrointestinal bleeding: an 86-year-old woman with peptic ulcer disease. JAMA. 2011;306:2367-2374.
28. Delaney JA, Opatrny L, Brophy JM, Suissa S. Drug drug interactions between antithrombotic medications and the risk of gastrointestinal bleeding. CMAJ. 2007;177:347-351.
29. Burr N, Lummis K, Sood R, et al. Risk of gastrointestinal bleeding with direct oral anticoagulants: a systematic review and network meta-analysis. Lancet Gastroenterol Hepatol. 2017;2:85-93.
30. Sung JJ, Lau JY, Ching JY, et al. Continuation of low-dose aspirin therapy in peptic ulcer bleeding: a randomized trial. Ann Intern Med. 2010;152:1-9.
31. Lau JY, Sung JJ, Lam YH, et al. Endoscopic retreatment compared with surgery in patients with recurrent bleeding after initial endoscopic control of bleeding ulcers. N Engl J Med. 1999;340:751-756.
32. Gralnek IM, Dumonceau JM, Kuipers EJ, et al. Diagnosis and management of nonvariceal upper gastrointestinal hemorrhage: European Society of Gastrointestinal Endoscopy (ESGE) Guideline. Endoscopy. 2015;47:a1-46.
33. Skinner M, Gutierrez JP, Neumann H, et al. Over-the-scope clip placement is effective rescue therapy for severe acute upper gastrointestinal bleeding. Endosc Int Open. 2014;2:E37-40.
34. Zhong C, Tan S, Ren Y, et al. Clinical outcomes of over-the-scope-clip system for the treatment of acute upper non-variceal gastrointestinal bleeding: a systematic review and meta-analysis. BMC Gastroenterol. 2019;19:225.
35. Mangiafico S, Pigo F, Bertani H, et al. Over-the-scope clip vs epinephrine with clip for first-line hemostasis in non-variceal upper gastrointestinal bleeding: a propensity score match analysis. Endosc Int Open. 2020;8:E50-e8.
36. Wedi E, Gonzalez S, Menke D, et al. One hundred and one over-the-scope-clip applications for severe gastrointestinal bleeding, leaks and fistulas. World J Gastroenterol. 2016;22:1844-1853.
37. Holster IL, van Beusekom HM, Kuipers EJ, et al. Effects of a hemostatic powder hemospray on coagulation and clot formation. Endoscopy. 2015;47:638-645.
38. Facciorusso A, Straus Takahashi M, et al. Efficacy of hemostatic powders in upper gastrointestinal bleeding: A systematic review and meta-analysis. Dig Liver Dis. 2019;51:1633-1640.
39. Wang YL, Cheng YS, et al. Emergency transcatheter arterial embolization for patients with acute massive duodenal ulcer hemorrhage. World J Gastroenterol. 2012;18:4765-4770.