User login
Pediatric Procedural Dermatology
Performing dermatologic procedures in infants, children, and teenagers presents many unique challenges. There may be unique diagnoses, different instruments, differences in skin biology, or different approaches to pain management and anesthesia; the inclusion of a third party (caregivers) in decision processes; or a need to assess maturity level or to optimize outcomes over the patient’s lifetime. The field of pediatric procedural dermatology is broad. This article reviews some of the more common procedures performed by pediatric dermatologists and some of the more common ethical and quality-of-life (QOL) considerations one might face in procedural pediatric dermatology. (The textbook Procedural Pediatric Dermatology1 offers a thorough discussion of this topic.)
Quality of Life
More often than not, procedures are performed in pediatric dermatology to improve QOL rather than to prevent morbidity or mortality. In the case of many self-limited conditions, such as ingrown nails or pyogenic granulomas, it is clear that intervention will improve the patient’s QOL. In the case of warts and molluscum contagiosum, emotional, social, and cultural considerations play a large role in determining whether an intervention will improve QOL. Finally, some conditions, such as genodermatoses, giant congenital melanocytic nevi, and large vascular malformations, may be associated with additional systemic symptoms and may not have good treatment options for cure. In these cases, procedural interventions will result in a mixture of positive and negative QOL outcomes that can occur at the same time.
Bemmels et al2 published a qualitative study that provides a good foundation for understanding the positive and negative effects of procedural interventions on children and teenagers. In their study, children and teenagers who underwent reconstructive surgery for craniofacial differences noted improved self-esteem and reduced stigmatization. However, they also experienced negative outcomes, including an addiction to attaining a perfect surgical face, missing school for treatments, difficulty adjusting to an evolving appearance, anxiety related to not knowing when treatments will end, and experiencing stigma related to undergoing surgery.2 Thus, a comprehensive plan for the management of children who need ongoing procedures should include some level of psychosocial support. Two good references on supporting young patients with visible differences include CBT for Appearance Anxiety: Psychosocial Interventions for Anxiety Due to Visible Difference3 and Reaching Teens: Strength-Based, Trauma-Sensitive, Resilience-Building Communication Strategies Rooted in Positive Youth Development.4
Ethics
Ethical decisions in pediatric procedural dermatology differ from adult dermatology in 3 major ways: (1) the involvement of a third party (ie, parents or legal guardians), (2) the need to assess the maturity of the patient, and (3) the need to know local laws in the jurisdiction in which care is being provided. Ethical dilemmas occur when the desires of the child, parents/guardians, and dermatologist are not in alignment. In these cases, it is important to be prepared with a moral or ethical framework to guide decision-making when conflicts occur. Two great resources are the best interest standard5 and the publication entitled, “Informed Consent in Decision-making in Pediatric Practice,” from the American Academy of Pediatrics.6
In pediatrics, it often is better to conceptualize medical decision-making as a combination of informed permission and assent of the patient rather than informed consent. Informed permission describes how a parent or surrogate makes decisions for the child or adolescent and is similar to informed consent. A parent’s informed permission may be in conflict with a child’s wishes, but it is assumed that the parent is acting in the best interest of the child. Assent of the patient is the process of obtaining a minor’s agreement to undergo an intervention even though he/she may lack legal authority or decision-making capacity to provide standard informed consent. It is important to respect the child’s right to assent to interventions to the extent that their maturity level permits to develop trust with the dermatologist and medical encounters in general.
These differences emphasize an active process in which the patient, caregiver, and physician are all involved in the health care process and allow for increasing inclusion of the child as is developmentally appropriate. In the end, however, parents have the legal authority to give or withhold permission for a procedure.7 When this conflicts with a child’s dissent, the dermatologist will need to objectively explore the reasons for the conflict and decide if a procedure is not in the child’s best interests. If a mutual understanding cannot be reached between the dermatologist and parents, obtaining a second opinion is a good option.8
Common Diagnoses
The most common diagnoses unique to procedural pediatric dermatology include congenital melanocytic nevi, vascular anomalies, midline lesions, epidermal nevi, and pilomatricomas. Prior to intervening on these lesions, it is important to consider evaluating for associated diseases.
Congenital Melanocytic Nevi
Nevus Outreach has published best practices for the management of congenital melanocytic nevi.9 In newborns with a congenital melanocytic nevus greater than 3 cm in diameter or more than 20 satellite lesions, it is recommended that magnetic resonance imaging (MRI) of the brain and spine with and without gadolinium contrast be obtained before 6 months of age. Within the first 6 months of life, these children also should see ophthalmologists, neurologists, pediatric dermatologists, and plastic surgeons. These early referrals will help to establish a baseline for the patient and plan for possible interventions, if needed. Additionally, before 3 years of age, every child should be referred to psychology, even if he/she is asymptomatic.10
Vascular Anomalies
Prior to intervening on a vascular anomaly, it is important to accurately classify the lesion. Once the lesion is classified, an evaluation and treatment plan can be developed. The International Society for the Study of Vascular Anomalies has published a detailed classification guide that is a useful starting point in the management of vascular anomalies.11 Once a diagnosis is confirmed, further evaluation may include imaging, specialty referrals, genetic testing, biopsy, or blood tests, and a pediatric dermatologist usually helps to coordinate the care of patients with complex vascular anomalies.
Midline Lesions
Certain lesions in the midline may have a higher risk for neural tube dysraphism, and imaging should be performed prior to any procedural intervention.12 Midline cutaneous findings that are highly likely to be associated with dysraphism are lipomas, acrochordons, pseudotails, true tails, aplasia cutis congenita, congenital scars, dermoid cysts, dermoid sinuses, and infantile hemangiomas that are greater than 2.5 cm in diameter. An MRI should be performed for all high-risk lesions. Intermediate-risk lesions are atypical dimples (>5 mm in diameter or >2.5 cm from the anal verge), hemangiomas less than 2.5 cm in diameter, and hypertrichosis. An ultrasound can screen for spinal dysraphism in these cases as long as imaging is performed prior to 6 months of age. If the child is older than 6 months, an MRI should be performed. Low-risk lesions that do not require imaging are simple dimples, hyperpigmentation, hypopigmentation, melanocytic nevi, port-wine stains, and telangiectases.
Epidermal Nevi
Children with epidermal nevi should have a complete physical examination, focusing on the skeletal system, central nervous system, and eyes. There are no specifically recommended imaging studies or referrals; however, several diagnostic clues can aid in the diagnosis of an epidermal nevus syndrome13:
• Schimmelpenning syndrome: extensive nevus sebaceous and bowing or pain in the legs after 2 years of age
• Phacomatosis pigmentokeratotica: nevus sebaceous and nevus spilus
• Nevus comedonicus syndrome: ipsilateral cataract
• Angora hair nevus syndrome: soft white hair within the nevus
• Becker nevus syndrome: breast hypoplasia
• Proteus syndrome: cerebriform plantar changes
• PIK3CA-related overgrowth spectrum: lipomas, macrodactyly, and/or vascular malformations
• Congenital hemidysplasia with ichthyosiform erythroderma and limb defects: inflammatory epidermal nevi, lateralization, ptychotropism, and ipsilateral limb defects
• Conradi-Hünermann-Happle syndrome: scaly red epidermal nevi without hair follicles and asymmetric limb shortening
Pilomatricomas
In addition to the tent sign—an angulated shape can be appreciated by stretching the skin overlying pilomatricomas—diagnosis of pilomatricoma can be confirmed by transillumination with an otoscope. In this case, a dark shadow typically is cast distal to where the otoscope touches the skin.14 In the case of multiple lesions, the patient should be evaluated for signs of myotonic dystrophy, Turner syndrome, and Gardner syndrome.15
Common Procedures
Pulsed Dye Laser
The pulsed dye laser is the most common laser used for red-colored lesions such as port-wine stains, facial telangiectases, and superficial hemangiomas. It also can be used to treat erythematous scars, verrucae, and psoriasis. In large vascular lesions, it typically is employed at 0.45 to 10 milliseconds every 4 to 6 weeks for 10 or more treatments. Port-wine stains preferably are treated within the first few months of life to provide the most fading without the need for general anesthesia.16 On the other hand, systemic therapy with propranolol is preferred over lasers for infantile hemangiomas.17
Long-Pulsed Alexandrite Laser (755 nm)
The alexandrite laser often is used to treat deeper vascular lesions such as venous lakes and hypertrophic port-wine stains. The operator needs to be cautious, as this laser has a higher incidence of scarring at the settings used to treat vascular lesions (typically fluences around 60–85 J/cm2).18 It also may be used for hair reduction in disorders with hypertrichosis or hidradenitis suppurativa.19
Long-Pulsed Nd:YAG Laser
The long-pulsed Nd:YAG laser also can be used to treat deep vascular lesions and remove unwanted hair. Because of its low window of safety in the treatment of vascular lesions, the alexandrite laser usually is preferred. However, it is the preferred laser for treatment of unwanted hair and hidradenitis suppurativa in darker skin types. It often provides a 50% reduction in hair density after 9 treatments.20
Quality-Switched Lasers
Pigment granules in melanosomes and tattoo particles are targeted with quality-switched (QS) lasers. Typically, a device will contain a combination of QS 532-nm potassium-titanyl-phosphate (KTP) lasers, QS 1064-nm Nd:YAG lasers, and QS 755-nm alexandrite lasers in 1 machine. In general, shorter wavelengths are used to treat epidermal lesions such as ephelides, lentigines, and café-au-lait macules. Longer wavelengths are used to treat deeper lesions such as nevus of Ota. A 2017 review suggested that café-au-lait macules with ragged borders (so-called coast of Maine borders) may respond well to QS lasers.21
Ablative Lasers
The 10,600-nm
Fractionated Lasers
Fractionated lasers can be nonablative (several devices are available in the 1410- to 1927-nm range) or ablative (CO2 or erbium:YAG). In pediatrics, they are usually used to treat burn scars, traumatic scars, and mild to moderate acne scarring.22 The most common side effects from fractionated lasers are prolonged erythema or hyperpigmentation. In addition, it typically takes at least 3 treatments to notice improvements.
Excisions
Pediatric procedural dermatologists remove a variety of unique lesions through excision. A few tips are provided for some of the more common lesions that may be excised in children.
Accessory Tragi
Prior to excising an accessory tragus, the surgeon should consider documenting a facial nerve examination, as accessory tragi can be associated with complete or partial facial nerve dysfunction. Additionally, there usually is an underlying cartilage structure present within the tragus. The cartilage stalk also should be addressed during the excision to avoid a continued palpable deformity after excision.
Dermoid Cysts
Dermoid cysts are the most commonly diagnosed benign orbital lesion in children.23 Exophytic periorbital lesions, which extend outside the orbital rim, can be removed through an infrabrow incision. Endophytic periorbital lesions, which are inside the orbital rim, should be removed through a crease incision. Midline lesions may have an intracranial extension and should be imaged through MRI and/or a computed tomography.24 Because dermoid cysts usually are located below the orbicularis oculi muscle, the muscle should be fixed with a suture prior to closing with skin sutures.
Pilomatricomas
Typically, a linear incision is made overlying the lesion, and then the underlying tumor is removed with sharp or blunt dissection. However, if the overlying skin has been stretched thin, a lenticular excision that includes the thinned skin may improve cosmesis.
Congenital Nevi
Large congenital nevi typically are removed through staged excisions. Lower extremity lesions are best removed before 10 months of age or before walking begins to minimize wound tension. However, if the procedure is not performed in infancy, it is best to wait until walking becomes stable.25 In older children, it is advisable to splint the affected lower extremity for 2 weeks to prevent dehiscence. The interval between excisions typically is 4 to 6 weeks for small lesions and 3 months for larger nevi.
Conclusion
Procedural pediatric dermatology is a broad and emerging field. As this article highlights, children are not small versions of adults and have unique biology, diseases, therapies, social situations, and ethical challenges from adults. This article provides a superficial overview of some of the more common issues faced by pediatric dermatologists and providers who perform procedures on infants, children, and teenagers. Readers who are interested in obtaining a more in-depth understanding of procedural pediatric dermatology should look at Procedural Pediatric Dermatology,1 the first textbook to provide expert opinion and evidence-based information on procedural management of pediatric skin conditions.
- Krakowski AC. Procedural Pediatric Dermatology. Phialdelphia, PA: Wolters Kluwer; 2011.
- Bemmels H, Biesecker B, Schmidt J, et al. Psychological and social factors in undergoing reconstructive surgery among individuals with craniofacial conditions: an exploratory study. Cleft Palate Craniofac J. 2013;50:158-167.
- Clarke A, Thompson AR, Jenkinson E, et al. CBT for Appearance Anxiety: Psychosocial Interventions for Anxiety Due to Visible Difference. Chichester, West Sussex: Wiley-Blackwell; 2013.
- Ginsburg KR, Ramirez McClain ZB, eds. Reaching Teens: Strength-Based, Trauma-Sensitive, Resilience-Building Communication Strategies Rooted in Positive Youth Development. 2nd ed. Itasca, IL: American Academy of Pediatrics; 2020.
- Kopelman LM. The best interests standard for incompetent or incapacitated patients of all ages. J Law Med Ethics. 2007;35:187-196.
- Katz AL, Webb SA; Committee on Bioethics. Informed consent in decision-making in pediatric practice. Pediatrics. 2016;138:e20161485. doi:10.1542/peds.2016-1485.
- Michon K. Emancipation of minors. NOLO website. https://www.nolo.com/legal-encyclopedia/emancipation-of-minors-32237.html. Accessed October 14, 2020.
- Cobb C, Bercovitch L. Ethical dilemmas. In: Krakowski AC, ed. Procedural Pediatric Dermatology. Philadelphia, PA: Wolters Kluwer; 2021:7-10.
- Nevus Outreach, Inc., releases best practice guidelines [news release]. Bartlesville, OK: Nevus Outreach Inc; July 7, 2018. https://www.nevus.org/matrices/page_file_download.php?id=239. Accessed October 14, 2020.
- 10. Masnari O, Neuhaus K, Aegerter T, et al. Predictors of health-related quality of life and psychological adjustment in children and adolescents with congenital melanocytic nevi: analysis of parent reports. J Pediatr Psychol. 2019;44:714-725.
- ISSVA classification for vascular anomalies. International Society for the Study of Vascular Anomalies website. https://www.issva.org/UserFiles/file/ISSVA-Classification-2018.pdf. Approved April 2014. Revised May 2018. Accessed October 14, 2020.
- Sewell MJ, Chiu YE, Drolet BA. Neural tube dysraphism: review of cutaneous markers and imaging. Pediatr Dermatol. 2015;32:161-170.
- Happle R. The group of epidermal nevus syndromes part I. well defined phenotypes. J Am Acad Dermatol. 2010;63:1-22.
- Berreto-Chang OL, Gorell ES, Yamaguma MA, et al. Diagnosis of pilomatricoma using an otoscope. Pediatr Dermatol. 2010;27:554-557.
- Danielson-Cohen A, Lin SJ, Hughes CA, et al. Head and neck pilomatrixoma in children. Arch Otolaryngol Head Neck Surg. 2001;127:1481-1483.
- Jeon H, Bernstein LJ, Belkin DA, et al. Pulsed dye laser treatment of port-wine stains in infancy without the need for general anesthesia. JAMA Dermatol. 2019;155:435-441.
- Krowchuk DP, Frieden IJ, Mancini AJ, et al. Clinical practice guideline for the management of infantile hemangiomas. Pediatrics. 2019;143:e20183475. doi:10.1542/peds.2018-3475.
- Tierney EP, Hanke CW. Alexandrite laser for the treatment of port wine stains refractory to pulsed dye laser. Dermatol Surg. 2011;37:1268-1278.
- Alikhan A, Sayed C, Alavi A, et al. North American clinical management guidelines for hidradenitis suppurativa: a publication from the United States and Canadian Hidradenitis Suppurativa Foundations. J Am Acad Dermatol. 2019;81:76-90.
- Rao K, Sankar TK. Long-pulsed Nd:YAG laser-assisted hair removal in Fitzpatrick skin types IV-VI. Lasers Med Sci. 2011;26:623-626.
- Belkin DA, Neckman JP, Jeon H, et al. Response to laser treatment of café au lait macules based on morphologic features. JAMA Dermatol. 2017;153:1158-1161.
- Kelly K, Lehmer L. Laser surgery. In: Krakowski AC, ed. Procedural Pediatric Dermatology. Philadelphia, PA: Wolters Kluwer; 2021:92-106.
- Eldesouky MA, Elbakary MA. Orbital dermoid cyst: classification and its impact on surgical management. Semin Ophthalmol. 2018;33:170-174.
- Pryor SG, Lewis JE, Weaver AL, et al. Pediatric dermoid cysts of the head and neck. Otolaryngol Head Neck Surg. 2005;132:938-942.
- Metz BJ. Procedural pediatric dermatology. Dermatol Clin. 2013;31:337-346.
Performing dermatologic procedures in infants, children, and teenagers presents many unique challenges. There may be unique diagnoses, different instruments, differences in skin biology, or different approaches to pain management and anesthesia; the inclusion of a third party (caregivers) in decision processes; or a need to assess maturity level or to optimize outcomes over the patient’s lifetime. The field of pediatric procedural dermatology is broad. This article reviews some of the more common procedures performed by pediatric dermatologists and some of the more common ethical and quality-of-life (QOL) considerations one might face in procedural pediatric dermatology. (The textbook Procedural Pediatric Dermatology1 offers a thorough discussion of this topic.)
Quality of Life
More often than not, procedures are performed in pediatric dermatology to improve QOL rather than to prevent morbidity or mortality. In the case of many self-limited conditions, such as ingrown nails or pyogenic granulomas, it is clear that intervention will improve the patient’s QOL. In the case of warts and molluscum contagiosum, emotional, social, and cultural considerations play a large role in determining whether an intervention will improve QOL. Finally, some conditions, such as genodermatoses, giant congenital melanocytic nevi, and large vascular malformations, may be associated with additional systemic symptoms and may not have good treatment options for cure. In these cases, procedural interventions will result in a mixture of positive and negative QOL outcomes that can occur at the same time.
Bemmels et al2 published a qualitative study that provides a good foundation for understanding the positive and negative effects of procedural interventions on children and teenagers. In their study, children and teenagers who underwent reconstructive surgery for craniofacial differences noted improved self-esteem and reduced stigmatization. However, they also experienced negative outcomes, including an addiction to attaining a perfect surgical face, missing school for treatments, difficulty adjusting to an evolving appearance, anxiety related to not knowing when treatments will end, and experiencing stigma related to undergoing surgery.2 Thus, a comprehensive plan for the management of children who need ongoing procedures should include some level of psychosocial support. Two good references on supporting young patients with visible differences include CBT for Appearance Anxiety: Psychosocial Interventions for Anxiety Due to Visible Difference3 and Reaching Teens: Strength-Based, Trauma-Sensitive, Resilience-Building Communication Strategies Rooted in Positive Youth Development.4
Ethics
Ethical decisions in pediatric procedural dermatology differ from adult dermatology in 3 major ways: (1) the involvement of a third party (ie, parents or legal guardians), (2) the need to assess the maturity of the patient, and (3) the need to know local laws in the jurisdiction in which care is being provided. Ethical dilemmas occur when the desires of the child, parents/guardians, and dermatologist are not in alignment. In these cases, it is important to be prepared with a moral or ethical framework to guide decision-making when conflicts occur. Two great resources are the best interest standard5 and the publication entitled, “Informed Consent in Decision-making in Pediatric Practice,” from the American Academy of Pediatrics.6
In pediatrics, it often is better to conceptualize medical decision-making as a combination of informed permission and assent of the patient rather than informed consent. Informed permission describes how a parent or surrogate makes decisions for the child or adolescent and is similar to informed consent. A parent’s informed permission may be in conflict with a child’s wishes, but it is assumed that the parent is acting in the best interest of the child. Assent of the patient is the process of obtaining a minor’s agreement to undergo an intervention even though he/she may lack legal authority or decision-making capacity to provide standard informed consent. It is important to respect the child’s right to assent to interventions to the extent that their maturity level permits to develop trust with the dermatologist and medical encounters in general.
These differences emphasize an active process in which the patient, caregiver, and physician are all involved in the health care process and allow for increasing inclusion of the child as is developmentally appropriate. In the end, however, parents have the legal authority to give or withhold permission for a procedure.7 When this conflicts with a child’s dissent, the dermatologist will need to objectively explore the reasons for the conflict and decide if a procedure is not in the child’s best interests. If a mutual understanding cannot be reached between the dermatologist and parents, obtaining a second opinion is a good option.8
Common Diagnoses
The most common diagnoses unique to procedural pediatric dermatology include congenital melanocytic nevi, vascular anomalies, midline lesions, epidermal nevi, and pilomatricomas. Prior to intervening on these lesions, it is important to consider evaluating for associated diseases.
Congenital Melanocytic Nevi
Nevus Outreach has published best practices for the management of congenital melanocytic nevi.9 In newborns with a congenital melanocytic nevus greater than 3 cm in diameter or more than 20 satellite lesions, it is recommended that magnetic resonance imaging (MRI) of the brain and spine with and without gadolinium contrast be obtained before 6 months of age. Within the first 6 months of life, these children also should see ophthalmologists, neurologists, pediatric dermatologists, and plastic surgeons. These early referrals will help to establish a baseline for the patient and plan for possible interventions, if needed. Additionally, before 3 years of age, every child should be referred to psychology, even if he/she is asymptomatic.10
Vascular Anomalies
Prior to intervening on a vascular anomaly, it is important to accurately classify the lesion. Once the lesion is classified, an evaluation and treatment plan can be developed. The International Society for the Study of Vascular Anomalies has published a detailed classification guide that is a useful starting point in the management of vascular anomalies.11 Once a diagnosis is confirmed, further evaluation may include imaging, specialty referrals, genetic testing, biopsy, or blood tests, and a pediatric dermatologist usually helps to coordinate the care of patients with complex vascular anomalies.
Midline Lesions
Certain lesions in the midline may have a higher risk for neural tube dysraphism, and imaging should be performed prior to any procedural intervention.12 Midline cutaneous findings that are highly likely to be associated with dysraphism are lipomas, acrochordons, pseudotails, true tails, aplasia cutis congenita, congenital scars, dermoid cysts, dermoid sinuses, and infantile hemangiomas that are greater than 2.5 cm in diameter. An MRI should be performed for all high-risk lesions. Intermediate-risk lesions are atypical dimples (>5 mm in diameter or >2.5 cm from the anal verge), hemangiomas less than 2.5 cm in diameter, and hypertrichosis. An ultrasound can screen for spinal dysraphism in these cases as long as imaging is performed prior to 6 months of age. If the child is older than 6 months, an MRI should be performed. Low-risk lesions that do not require imaging are simple dimples, hyperpigmentation, hypopigmentation, melanocytic nevi, port-wine stains, and telangiectases.
Epidermal Nevi
Children with epidermal nevi should have a complete physical examination, focusing on the skeletal system, central nervous system, and eyes. There are no specifically recommended imaging studies or referrals; however, several diagnostic clues can aid in the diagnosis of an epidermal nevus syndrome13:
• Schimmelpenning syndrome: extensive nevus sebaceous and bowing or pain in the legs after 2 years of age
• Phacomatosis pigmentokeratotica: nevus sebaceous and nevus spilus
• Nevus comedonicus syndrome: ipsilateral cataract
• Angora hair nevus syndrome: soft white hair within the nevus
• Becker nevus syndrome: breast hypoplasia
• Proteus syndrome: cerebriform plantar changes
• PIK3CA-related overgrowth spectrum: lipomas, macrodactyly, and/or vascular malformations
• Congenital hemidysplasia with ichthyosiform erythroderma and limb defects: inflammatory epidermal nevi, lateralization, ptychotropism, and ipsilateral limb defects
• Conradi-Hünermann-Happle syndrome: scaly red epidermal nevi without hair follicles and asymmetric limb shortening
Pilomatricomas
In addition to the tent sign—an angulated shape can be appreciated by stretching the skin overlying pilomatricomas—diagnosis of pilomatricoma can be confirmed by transillumination with an otoscope. In this case, a dark shadow typically is cast distal to where the otoscope touches the skin.14 In the case of multiple lesions, the patient should be evaluated for signs of myotonic dystrophy, Turner syndrome, and Gardner syndrome.15
Common Procedures
Pulsed Dye Laser
The pulsed dye laser is the most common laser used for red-colored lesions such as port-wine stains, facial telangiectases, and superficial hemangiomas. It also can be used to treat erythematous scars, verrucae, and psoriasis. In large vascular lesions, it typically is employed at 0.45 to 10 milliseconds every 4 to 6 weeks for 10 or more treatments. Port-wine stains preferably are treated within the first few months of life to provide the most fading without the need for general anesthesia.16 On the other hand, systemic therapy with propranolol is preferred over lasers for infantile hemangiomas.17
Long-Pulsed Alexandrite Laser (755 nm)
The alexandrite laser often is used to treat deeper vascular lesions such as venous lakes and hypertrophic port-wine stains. The operator needs to be cautious, as this laser has a higher incidence of scarring at the settings used to treat vascular lesions (typically fluences around 60–85 J/cm2).18 It also may be used for hair reduction in disorders with hypertrichosis or hidradenitis suppurativa.19
Long-Pulsed Nd:YAG Laser
The long-pulsed Nd:YAG laser also can be used to treat deep vascular lesions and remove unwanted hair. Because of its low window of safety in the treatment of vascular lesions, the alexandrite laser usually is preferred. However, it is the preferred laser for treatment of unwanted hair and hidradenitis suppurativa in darker skin types. It often provides a 50% reduction in hair density after 9 treatments.20
Quality-Switched Lasers
Pigment granules in melanosomes and tattoo particles are targeted with quality-switched (QS) lasers. Typically, a device will contain a combination of QS 532-nm potassium-titanyl-phosphate (KTP) lasers, QS 1064-nm Nd:YAG lasers, and QS 755-nm alexandrite lasers in 1 machine. In general, shorter wavelengths are used to treat epidermal lesions such as ephelides, lentigines, and café-au-lait macules. Longer wavelengths are used to treat deeper lesions such as nevus of Ota. A 2017 review suggested that café-au-lait macules with ragged borders (so-called coast of Maine borders) may respond well to QS lasers.21
Ablative Lasers
The 10,600-nm
Fractionated Lasers
Fractionated lasers can be nonablative (several devices are available in the 1410- to 1927-nm range) or ablative (CO2 or erbium:YAG). In pediatrics, they are usually used to treat burn scars, traumatic scars, and mild to moderate acne scarring.22 The most common side effects from fractionated lasers are prolonged erythema or hyperpigmentation. In addition, it typically takes at least 3 treatments to notice improvements.
Excisions
Pediatric procedural dermatologists remove a variety of unique lesions through excision. A few tips are provided for some of the more common lesions that may be excised in children.
Accessory Tragi
Prior to excising an accessory tragus, the surgeon should consider documenting a facial nerve examination, as accessory tragi can be associated with complete or partial facial nerve dysfunction. Additionally, there usually is an underlying cartilage structure present within the tragus. The cartilage stalk also should be addressed during the excision to avoid a continued palpable deformity after excision.
Dermoid Cysts
Dermoid cysts are the most commonly diagnosed benign orbital lesion in children.23 Exophytic periorbital lesions, which extend outside the orbital rim, can be removed through an infrabrow incision. Endophytic periorbital lesions, which are inside the orbital rim, should be removed through a crease incision. Midline lesions may have an intracranial extension and should be imaged through MRI and/or a computed tomography.24 Because dermoid cysts usually are located below the orbicularis oculi muscle, the muscle should be fixed with a suture prior to closing with skin sutures.
Pilomatricomas
Typically, a linear incision is made overlying the lesion, and then the underlying tumor is removed with sharp or blunt dissection. However, if the overlying skin has been stretched thin, a lenticular excision that includes the thinned skin may improve cosmesis.
Congenital Nevi
Large congenital nevi typically are removed through staged excisions. Lower extremity lesions are best removed before 10 months of age or before walking begins to minimize wound tension. However, if the procedure is not performed in infancy, it is best to wait until walking becomes stable.25 In older children, it is advisable to splint the affected lower extremity for 2 weeks to prevent dehiscence. The interval between excisions typically is 4 to 6 weeks for small lesions and 3 months for larger nevi.
Conclusion
Procedural pediatric dermatology is a broad and emerging field. As this article highlights, children are not small versions of adults and have unique biology, diseases, therapies, social situations, and ethical challenges from adults. This article provides a superficial overview of some of the more common issues faced by pediatric dermatologists and providers who perform procedures on infants, children, and teenagers. Readers who are interested in obtaining a more in-depth understanding of procedural pediatric dermatology should look at Procedural Pediatric Dermatology,1 the first textbook to provide expert opinion and evidence-based information on procedural management of pediatric skin conditions.
Performing dermatologic procedures in infants, children, and teenagers presents many unique challenges. There may be unique diagnoses, different instruments, differences in skin biology, or different approaches to pain management and anesthesia; the inclusion of a third party (caregivers) in decision processes; or a need to assess maturity level or to optimize outcomes over the patient’s lifetime. The field of pediatric procedural dermatology is broad. This article reviews some of the more common procedures performed by pediatric dermatologists and some of the more common ethical and quality-of-life (QOL) considerations one might face in procedural pediatric dermatology. (The textbook Procedural Pediatric Dermatology1 offers a thorough discussion of this topic.)
Quality of Life
More often than not, procedures are performed in pediatric dermatology to improve QOL rather than to prevent morbidity or mortality. In the case of many self-limited conditions, such as ingrown nails or pyogenic granulomas, it is clear that intervention will improve the patient’s QOL. In the case of warts and molluscum contagiosum, emotional, social, and cultural considerations play a large role in determining whether an intervention will improve QOL. Finally, some conditions, such as genodermatoses, giant congenital melanocytic nevi, and large vascular malformations, may be associated with additional systemic symptoms and may not have good treatment options for cure. In these cases, procedural interventions will result in a mixture of positive and negative QOL outcomes that can occur at the same time.
Bemmels et al2 published a qualitative study that provides a good foundation for understanding the positive and negative effects of procedural interventions on children and teenagers. In their study, children and teenagers who underwent reconstructive surgery for craniofacial differences noted improved self-esteem and reduced stigmatization. However, they also experienced negative outcomes, including an addiction to attaining a perfect surgical face, missing school for treatments, difficulty adjusting to an evolving appearance, anxiety related to not knowing when treatments will end, and experiencing stigma related to undergoing surgery.2 Thus, a comprehensive plan for the management of children who need ongoing procedures should include some level of psychosocial support. Two good references on supporting young patients with visible differences include CBT for Appearance Anxiety: Psychosocial Interventions for Anxiety Due to Visible Difference3 and Reaching Teens: Strength-Based, Trauma-Sensitive, Resilience-Building Communication Strategies Rooted in Positive Youth Development.4
Ethics
Ethical decisions in pediatric procedural dermatology differ from adult dermatology in 3 major ways: (1) the involvement of a third party (ie, parents or legal guardians), (2) the need to assess the maturity of the patient, and (3) the need to know local laws in the jurisdiction in which care is being provided. Ethical dilemmas occur when the desires of the child, parents/guardians, and dermatologist are not in alignment. In these cases, it is important to be prepared with a moral or ethical framework to guide decision-making when conflicts occur. Two great resources are the best interest standard5 and the publication entitled, “Informed Consent in Decision-making in Pediatric Practice,” from the American Academy of Pediatrics.6
In pediatrics, it often is better to conceptualize medical decision-making as a combination of informed permission and assent of the patient rather than informed consent. Informed permission describes how a parent or surrogate makes decisions for the child or adolescent and is similar to informed consent. A parent’s informed permission may be in conflict with a child’s wishes, but it is assumed that the parent is acting in the best interest of the child. Assent of the patient is the process of obtaining a minor’s agreement to undergo an intervention even though he/she may lack legal authority or decision-making capacity to provide standard informed consent. It is important to respect the child’s right to assent to interventions to the extent that their maturity level permits to develop trust with the dermatologist and medical encounters in general.
These differences emphasize an active process in which the patient, caregiver, and physician are all involved in the health care process and allow for increasing inclusion of the child as is developmentally appropriate. In the end, however, parents have the legal authority to give or withhold permission for a procedure.7 When this conflicts with a child’s dissent, the dermatologist will need to objectively explore the reasons for the conflict and decide if a procedure is not in the child’s best interests. If a mutual understanding cannot be reached between the dermatologist and parents, obtaining a second opinion is a good option.8
Common Diagnoses
The most common diagnoses unique to procedural pediatric dermatology include congenital melanocytic nevi, vascular anomalies, midline lesions, epidermal nevi, and pilomatricomas. Prior to intervening on these lesions, it is important to consider evaluating for associated diseases.
Congenital Melanocytic Nevi
Nevus Outreach has published best practices for the management of congenital melanocytic nevi.9 In newborns with a congenital melanocytic nevus greater than 3 cm in diameter or more than 20 satellite lesions, it is recommended that magnetic resonance imaging (MRI) of the brain and spine with and without gadolinium contrast be obtained before 6 months of age. Within the first 6 months of life, these children also should see ophthalmologists, neurologists, pediatric dermatologists, and plastic surgeons. These early referrals will help to establish a baseline for the patient and plan for possible interventions, if needed. Additionally, before 3 years of age, every child should be referred to psychology, even if he/she is asymptomatic.10
Vascular Anomalies
Prior to intervening on a vascular anomaly, it is important to accurately classify the lesion. Once the lesion is classified, an evaluation and treatment plan can be developed. The International Society for the Study of Vascular Anomalies has published a detailed classification guide that is a useful starting point in the management of vascular anomalies.11 Once a diagnosis is confirmed, further evaluation may include imaging, specialty referrals, genetic testing, biopsy, or blood tests, and a pediatric dermatologist usually helps to coordinate the care of patients with complex vascular anomalies.
Midline Lesions
Certain lesions in the midline may have a higher risk for neural tube dysraphism, and imaging should be performed prior to any procedural intervention.12 Midline cutaneous findings that are highly likely to be associated with dysraphism are lipomas, acrochordons, pseudotails, true tails, aplasia cutis congenita, congenital scars, dermoid cysts, dermoid sinuses, and infantile hemangiomas that are greater than 2.5 cm in diameter. An MRI should be performed for all high-risk lesions. Intermediate-risk lesions are atypical dimples (>5 mm in diameter or >2.5 cm from the anal verge), hemangiomas less than 2.5 cm in diameter, and hypertrichosis. An ultrasound can screen for spinal dysraphism in these cases as long as imaging is performed prior to 6 months of age. If the child is older than 6 months, an MRI should be performed. Low-risk lesions that do not require imaging are simple dimples, hyperpigmentation, hypopigmentation, melanocytic nevi, port-wine stains, and telangiectases.
Epidermal Nevi
Children with epidermal nevi should have a complete physical examination, focusing on the skeletal system, central nervous system, and eyes. There are no specifically recommended imaging studies or referrals; however, several diagnostic clues can aid in the diagnosis of an epidermal nevus syndrome13:
• Schimmelpenning syndrome: extensive nevus sebaceous and bowing or pain in the legs after 2 years of age
• Phacomatosis pigmentokeratotica: nevus sebaceous and nevus spilus
• Nevus comedonicus syndrome: ipsilateral cataract
• Angora hair nevus syndrome: soft white hair within the nevus
• Becker nevus syndrome: breast hypoplasia
• Proteus syndrome: cerebriform plantar changes
• PIK3CA-related overgrowth spectrum: lipomas, macrodactyly, and/or vascular malformations
• Congenital hemidysplasia with ichthyosiform erythroderma and limb defects: inflammatory epidermal nevi, lateralization, ptychotropism, and ipsilateral limb defects
• Conradi-Hünermann-Happle syndrome: scaly red epidermal nevi without hair follicles and asymmetric limb shortening
Pilomatricomas
In addition to the tent sign—an angulated shape can be appreciated by stretching the skin overlying pilomatricomas—diagnosis of pilomatricoma can be confirmed by transillumination with an otoscope. In this case, a dark shadow typically is cast distal to where the otoscope touches the skin.14 In the case of multiple lesions, the patient should be evaluated for signs of myotonic dystrophy, Turner syndrome, and Gardner syndrome.15
Common Procedures
Pulsed Dye Laser
The pulsed dye laser is the most common laser used for red-colored lesions such as port-wine stains, facial telangiectases, and superficial hemangiomas. It also can be used to treat erythematous scars, verrucae, and psoriasis. In large vascular lesions, it typically is employed at 0.45 to 10 milliseconds every 4 to 6 weeks for 10 or more treatments. Port-wine stains preferably are treated within the first few months of life to provide the most fading without the need for general anesthesia.16 On the other hand, systemic therapy with propranolol is preferred over lasers for infantile hemangiomas.17
Long-Pulsed Alexandrite Laser (755 nm)
The alexandrite laser often is used to treat deeper vascular lesions such as venous lakes and hypertrophic port-wine stains. The operator needs to be cautious, as this laser has a higher incidence of scarring at the settings used to treat vascular lesions (typically fluences around 60–85 J/cm2).18 It also may be used for hair reduction in disorders with hypertrichosis or hidradenitis suppurativa.19
Long-Pulsed Nd:YAG Laser
The long-pulsed Nd:YAG laser also can be used to treat deep vascular lesions and remove unwanted hair. Because of its low window of safety in the treatment of vascular lesions, the alexandrite laser usually is preferred. However, it is the preferred laser for treatment of unwanted hair and hidradenitis suppurativa in darker skin types. It often provides a 50% reduction in hair density after 9 treatments.20
Quality-Switched Lasers
Pigment granules in melanosomes and tattoo particles are targeted with quality-switched (QS) lasers. Typically, a device will contain a combination of QS 532-nm potassium-titanyl-phosphate (KTP) lasers, QS 1064-nm Nd:YAG lasers, and QS 755-nm alexandrite lasers in 1 machine. In general, shorter wavelengths are used to treat epidermal lesions such as ephelides, lentigines, and café-au-lait macules. Longer wavelengths are used to treat deeper lesions such as nevus of Ota. A 2017 review suggested that café-au-lait macules with ragged borders (so-called coast of Maine borders) may respond well to QS lasers.21
Ablative Lasers
The 10,600-nm
Fractionated Lasers
Fractionated lasers can be nonablative (several devices are available in the 1410- to 1927-nm range) or ablative (CO2 or erbium:YAG). In pediatrics, they are usually used to treat burn scars, traumatic scars, and mild to moderate acne scarring.22 The most common side effects from fractionated lasers are prolonged erythema or hyperpigmentation. In addition, it typically takes at least 3 treatments to notice improvements.
Excisions
Pediatric procedural dermatologists remove a variety of unique lesions through excision. A few tips are provided for some of the more common lesions that may be excised in children.
Accessory Tragi
Prior to excising an accessory tragus, the surgeon should consider documenting a facial nerve examination, as accessory tragi can be associated with complete or partial facial nerve dysfunction. Additionally, there usually is an underlying cartilage structure present within the tragus. The cartilage stalk also should be addressed during the excision to avoid a continued palpable deformity after excision.
Dermoid Cysts
Dermoid cysts are the most commonly diagnosed benign orbital lesion in children.23 Exophytic periorbital lesions, which extend outside the orbital rim, can be removed through an infrabrow incision. Endophytic periorbital lesions, which are inside the orbital rim, should be removed through a crease incision. Midline lesions may have an intracranial extension and should be imaged through MRI and/or a computed tomography.24 Because dermoid cysts usually are located below the orbicularis oculi muscle, the muscle should be fixed with a suture prior to closing with skin sutures.
Pilomatricomas
Typically, a linear incision is made overlying the lesion, and then the underlying tumor is removed with sharp or blunt dissection. However, if the overlying skin has been stretched thin, a lenticular excision that includes the thinned skin may improve cosmesis.
Congenital Nevi
Large congenital nevi typically are removed through staged excisions. Lower extremity lesions are best removed before 10 months of age or before walking begins to minimize wound tension. However, if the procedure is not performed in infancy, it is best to wait until walking becomes stable.25 In older children, it is advisable to splint the affected lower extremity for 2 weeks to prevent dehiscence. The interval between excisions typically is 4 to 6 weeks for small lesions and 3 months for larger nevi.
Conclusion
Procedural pediatric dermatology is a broad and emerging field. As this article highlights, children are not small versions of adults and have unique biology, diseases, therapies, social situations, and ethical challenges from adults. This article provides a superficial overview of some of the more common issues faced by pediatric dermatologists and providers who perform procedures on infants, children, and teenagers. Readers who are interested in obtaining a more in-depth understanding of procedural pediatric dermatology should look at Procedural Pediatric Dermatology,1 the first textbook to provide expert opinion and evidence-based information on procedural management of pediatric skin conditions.
- Krakowski AC. Procedural Pediatric Dermatology. Phialdelphia, PA: Wolters Kluwer; 2011.
- Bemmels H, Biesecker B, Schmidt J, et al. Psychological and social factors in undergoing reconstructive surgery among individuals with craniofacial conditions: an exploratory study. Cleft Palate Craniofac J. 2013;50:158-167.
- Clarke A, Thompson AR, Jenkinson E, et al. CBT for Appearance Anxiety: Psychosocial Interventions for Anxiety Due to Visible Difference. Chichester, West Sussex: Wiley-Blackwell; 2013.
- Ginsburg KR, Ramirez McClain ZB, eds. Reaching Teens: Strength-Based, Trauma-Sensitive, Resilience-Building Communication Strategies Rooted in Positive Youth Development. 2nd ed. Itasca, IL: American Academy of Pediatrics; 2020.
- Kopelman LM. The best interests standard for incompetent or incapacitated patients of all ages. J Law Med Ethics. 2007;35:187-196.
- Katz AL, Webb SA; Committee on Bioethics. Informed consent in decision-making in pediatric practice. Pediatrics. 2016;138:e20161485. doi:10.1542/peds.2016-1485.
- Michon K. Emancipation of minors. NOLO website. https://www.nolo.com/legal-encyclopedia/emancipation-of-minors-32237.html. Accessed October 14, 2020.
- Cobb C, Bercovitch L. Ethical dilemmas. In: Krakowski AC, ed. Procedural Pediatric Dermatology. Philadelphia, PA: Wolters Kluwer; 2021:7-10.
- Nevus Outreach, Inc., releases best practice guidelines [news release]. Bartlesville, OK: Nevus Outreach Inc; July 7, 2018. https://www.nevus.org/matrices/page_file_download.php?id=239. Accessed October 14, 2020.
- 10. Masnari O, Neuhaus K, Aegerter T, et al. Predictors of health-related quality of life and psychological adjustment in children and adolescents with congenital melanocytic nevi: analysis of parent reports. J Pediatr Psychol. 2019;44:714-725.
- ISSVA classification for vascular anomalies. International Society for the Study of Vascular Anomalies website. https://www.issva.org/UserFiles/file/ISSVA-Classification-2018.pdf. Approved April 2014. Revised May 2018. Accessed October 14, 2020.
- Sewell MJ, Chiu YE, Drolet BA. Neural tube dysraphism: review of cutaneous markers and imaging. Pediatr Dermatol. 2015;32:161-170.
- Happle R. The group of epidermal nevus syndromes part I. well defined phenotypes. J Am Acad Dermatol. 2010;63:1-22.
- Berreto-Chang OL, Gorell ES, Yamaguma MA, et al. Diagnosis of pilomatricoma using an otoscope. Pediatr Dermatol. 2010;27:554-557.
- Danielson-Cohen A, Lin SJ, Hughes CA, et al. Head and neck pilomatrixoma in children. Arch Otolaryngol Head Neck Surg. 2001;127:1481-1483.
- Jeon H, Bernstein LJ, Belkin DA, et al. Pulsed dye laser treatment of port-wine stains in infancy without the need for general anesthesia. JAMA Dermatol. 2019;155:435-441.
- Krowchuk DP, Frieden IJ, Mancini AJ, et al. Clinical practice guideline for the management of infantile hemangiomas. Pediatrics. 2019;143:e20183475. doi:10.1542/peds.2018-3475.
- Tierney EP, Hanke CW. Alexandrite laser for the treatment of port wine stains refractory to pulsed dye laser. Dermatol Surg. 2011;37:1268-1278.
- Alikhan A, Sayed C, Alavi A, et al. North American clinical management guidelines for hidradenitis suppurativa: a publication from the United States and Canadian Hidradenitis Suppurativa Foundations. J Am Acad Dermatol. 2019;81:76-90.
- Rao K, Sankar TK. Long-pulsed Nd:YAG laser-assisted hair removal in Fitzpatrick skin types IV-VI. Lasers Med Sci. 2011;26:623-626.
- Belkin DA, Neckman JP, Jeon H, et al. Response to laser treatment of café au lait macules based on morphologic features. JAMA Dermatol. 2017;153:1158-1161.
- Kelly K, Lehmer L. Laser surgery. In: Krakowski AC, ed. Procedural Pediatric Dermatology. Philadelphia, PA: Wolters Kluwer; 2021:92-106.
- Eldesouky MA, Elbakary MA. Orbital dermoid cyst: classification and its impact on surgical management. Semin Ophthalmol. 2018;33:170-174.
- Pryor SG, Lewis JE, Weaver AL, et al. Pediatric dermoid cysts of the head and neck. Otolaryngol Head Neck Surg. 2005;132:938-942.
- Metz BJ. Procedural pediatric dermatology. Dermatol Clin. 2013;31:337-346.
- Krakowski AC. Procedural Pediatric Dermatology. Phialdelphia, PA: Wolters Kluwer; 2011.
- Bemmels H, Biesecker B, Schmidt J, et al. Psychological and social factors in undergoing reconstructive surgery among individuals with craniofacial conditions: an exploratory study. Cleft Palate Craniofac J. 2013;50:158-167.
- Clarke A, Thompson AR, Jenkinson E, et al. CBT for Appearance Anxiety: Psychosocial Interventions for Anxiety Due to Visible Difference. Chichester, West Sussex: Wiley-Blackwell; 2013.
- Ginsburg KR, Ramirez McClain ZB, eds. Reaching Teens: Strength-Based, Trauma-Sensitive, Resilience-Building Communication Strategies Rooted in Positive Youth Development. 2nd ed. Itasca, IL: American Academy of Pediatrics; 2020.
- Kopelman LM. The best interests standard for incompetent or incapacitated patients of all ages. J Law Med Ethics. 2007;35:187-196.
- Katz AL, Webb SA; Committee on Bioethics. Informed consent in decision-making in pediatric practice. Pediatrics. 2016;138:e20161485. doi:10.1542/peds.2016-1485.
- Michon K. Emancipation of minors. NOLO website. https://www.nolo.com/legal-encyclopedia/emancipation-of-minors-32237.html. Accessed October 14, 2020.
- Cobb C, Bercovitch L. Ethical dilemmas. In: Krakowski AC, ed. Procedural Pediatric Dermatology. Philadelphia, PA: Wolters Kluwer; 2021:7-10.
- Nevus Outreach, Inc., releases best practice guidelines [news release]. Bartlesville, OK: Nevus Outreach Inc; July 7, 2018. https://www.nevus.org/matrices/page_file_download.php?id=239. Accessed October 14, 2020.
- 10. Masnari O, Neuhaus K, Aegerter T, et al. Predictors of health-related quality of life and psychological adjustment in children and adolescents with congenital melanocytic nevi: analysis of parent reports. J Pediatr Psychol. 2019;44:714-725.
- ISSVA classification for vascular anomalies. International Society for the Study of Vascular Anomalies website. https://www.issva.org/UserFiles/file/ISSVA-Classification-2018.pdf. Approved April 2014. Revised May 2018. Accessed October 14, 2020.
- Sewell MJ, Chiu YE, Drolet BA. Neural tube dysraphism: review of cutaneous markers and imaging. Pediatr Dermatol. 2015;32:161-170.
- Happle R. The group of epidermal nevus syndromes part I. well defined phenotypes. J Am Acad Dermatol. 2010;63:1-22.
- Berreto-Chang OL, Gorell ES, Yamaguma MA, et al. Diagnosis of pilomatricoma using an otoscope. Pediatr Dermatol. 2010;27:554-557.
- Danielson-Cohen A, Lin SJ, Hughes CA, et al. Head and neck pilomatrixoma in children. Arch Otolaryngol Head Neck Surg. 2001;127:1481-1483.
- Jeon H, Bernstein LJ, Belkin DA, et al. Pulsed dye laser treatment of port-wine stains in infancy without the need for general anesthesia. JAMA Dermatol. 2019;155:435-441.
- Krowchuk DP, Frieden IJ, Mancini AJ, et al. Clinical practice guideline for the management of infantile hemangiomas. Pediatrics. 2019;143:e20183475. doi:10.1542/peds.2018-3475.
- Tierney EP, Hanke CW. Alexandrite laser for the treatment of port wine stains refractory to pulsed dye laser. Dermatol Surg. 2011;37:1268-1278.
- Alikhan A, Sayed C, Alavi A, et al. North American clinical management guidelines for hidradenitis suppurativa: a publication from the United States and Canadian Hidradenitis Suppurativa Foundations. J Am Acad Dermatol. 2019;81:76-90.
- Rao K, Sankar TK. Long-pulsed Nd:YAG laser-assisted hair removal in Fitzpatrick skin types IV-VI. Lasers Med Sci. 2011;26:623-626.
- Belkin DA, Neckman JP, Jeon H, et al. Response to laser treatment of café au lait macules based on morphologic features. JAMA Dermatol. 2017;153:1158-1161.
- Kelly K, Lehmer L. Laser surgery. In: Krakowski AC, ed. Procedural Pediatric Dermatology. Philadelphia, PA: Wolters Kluwer; 2021:92-106.
- Eldesouky MA, Elbakary MA. Orbital dermoid cyst: classification and its impact on surgical management. Semin Ophthalmol. 2018;33:170-174.
- Pryor SG, Lewis JE, Weaver AL, et al. Pediatric dermoid cysts of the head and neck. Otolaryngol Head Neck Surg. 2005;132:938-942.
- Metz BJ. Procedural pediatric dermatology. Dermatol Clin. 2013;31:337-346.
Practice Points
- Children who require repetitive laser or surgical procedures over time benefit from regular monitoring of psychosocial needs.
- The informed consent process for children differs from adult procedural dermatology and should be adjusted to the maturity level of the patient.
- Common diagnoses unique to procedural pediatric dermatology that may require additional investigation include congenital melanocytic nevi, vascular anomalies, epidermal nevi, and midline lesions.
- Specific measures can be performed to improve outcomes when removing accessory tragi, dermoid cysts, pilomatricomas, and congenital nevi.
Translating the 2019 AAD-NPF Guidelines of Care for the Management of Psoriasis in Pediatric Patients
In November 2019, the American Academy of Dermatology (AAD) and the National Psoriasis Foundation (NPF) released their first set of recommendations for the management of pediatric psoriasis.1 The pediatric guidelines discuss methods of quantifying disease severity in children, triggers and comorbidities, and the efficacy and safety of various therapeutic agents. This review aims to discuss, in a condensed form, special considerations unique to the management of children with psoriasis as presented in the guidelines as well as grade A– and grade B–level treatment recommendations (Table).
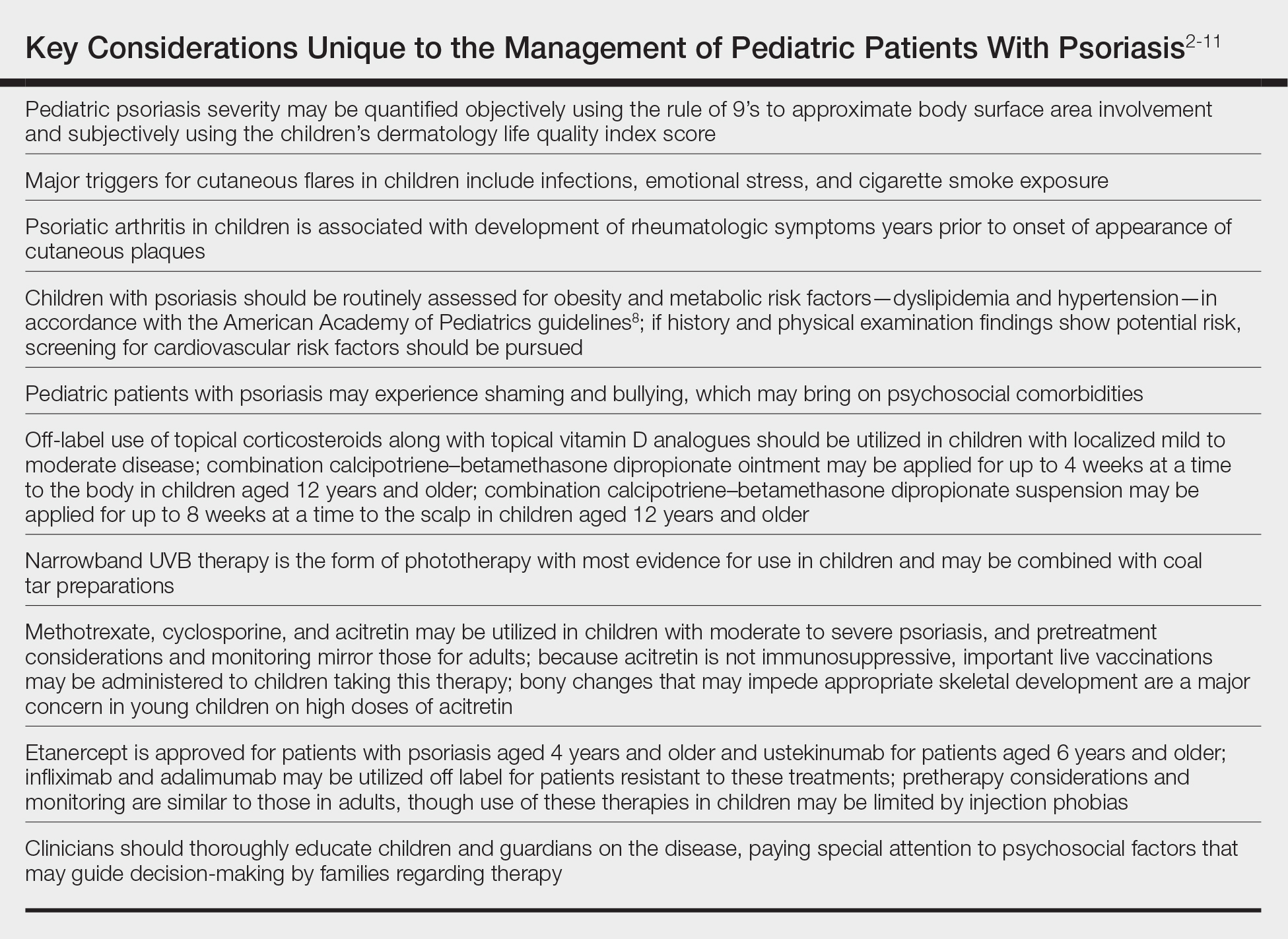
Quantifying Psoriasis Severity in Children
Percentage body surface area (BSA) involvement is the most common mode of grading psoriasis severity, with less than 3% BSA involvement being considered mild, 3% to 10% BSA moderate, and more than 10% severe disease. In children, the standard method of measuring BSA is the rule of 9’s: the head and each arm make up 9% of the total BSA, each leg and the front and back of the torso respectively each make up 18%, and the genitalia make up 1%. It also is important to consider impact on quality of life, which may be remarkable in spite of limited BSA involvement. The children’s dermatology life quality index score may be utilized in combination with affected BSA to determine the burden of psoriasis in context of impact on daily life. This metric is available in both written and cartoon form, and it consists of 10 questions that include variables such as severity of itch, impact on social life, and effects on sleep. Most notably, this tool incorporates pruritus,2 which generally is addressed inadequately in pediatric psoriasis.
Triggers and Comorbidities in Pediatric Patients
In children, it is important to identify and eliminate modifiable factors that may prompt psoriasis flares. Infections, particularly group A beta-hemolytic streptococcal infections, are a major trigger in neonates and infants. Other exacerbating factors in children include emotional stress, secondhand cigarette smoke, Kawasaki disease, and withdrawal from systemic corticosteroids.
Psoriatic arthritis (PsA) is a burdensome comorbidity affecting children with psoriasis. The prevalence of joint disease is 15-times greater in children with psoriasis vs those without,3 and 80% of children with PsA develop rheumatologic symptoms, which typically include oligoarticular disease and dactylitis in infants and girls and enthesitis and axial joint involvement in boys and older children, years prior to the onset of cutaneous disease.4 Uveitis often occurs in children with psoriasis and PsA but not in those with isolated cutaneous disease.
Compared to unaffected children, pediatric patients with psoriasis have greater prevalence of metabolic and cardiovascular risk factors during childhood, including central obesity, hypertension, hypertriglyceridemia, hypercholesterolemia, insulin resistance, atherosclerosis, arrythmia, and valvular heart disease. Family history of obesity increases the risk for early-onset development of cutaneous lesions,5,6 and weight reduction may alleviate severity of psoriasis lesions.7 In the United States, many of the metabolic associations observed are particularly robust in Black and Hispanic children vs those of other races. Furthermore, the prevalence of inflammatory bowel disease is 3- to 4-times higher in children with psoriasis compared to those without.
As with other cutaneous diseases, it is important to be aware of social and mental health concerns in children with psoriasis. The majority of pediatric patients with psoriasis experience name-calling, shaming, or bullying, and many have concerns from skin shedding and malodor. Independent risk for depression after the onset of psoriasis is high. Affected older children and adolescents are at increased risk for alcohol and drug abuse as well as eating disorders.
Despite these identified comorbidities, there are no unique screening recommendations for arthritis, ophthalmologic disease, metabolic disease, cardiovascular disease, gastrointestinal tract disease, or mental health issues in children with psoriasis. Rather, these patients should be monitored according to the American Academy of Pediatrics or American Diabetes Association guidelines for all pediatric patients.8,9 Nonetheless, educating patients and guardians about these potential issues may be warranted.
Topical Therapies
For children with mild to moderate psoriasis, topical therapies are first line. Despite being off label, topical corticosteroids are the mainstay of therapy for localized psoriatic plaques in children. Topical vitamin D analogues—calcitriol and calcipotriol/calcipotriene—are highly effective and well tolerated, and they frequently are used in combination with topical corticosteroids. Topical calcineurin inhibitors, namely tacrolimus, also are used off label but are considered first line for sensitive regions of the skin in children, including the face, genitalia, and body folds. There currently is limited evidence for supporting the use of the topical vitamin A analogue tazarotene in children with psoriasis, though some consider its off-label use effective for pediatric nail psoriasis. It also may be used as an adjunct to topical corticosteroids to minimize irritation.
Although there is no gold standard topical regimen, combination therapy with a high-potency topical steroid and topical vitamin D analogue commonly is used to minimize steroid-induced side effects. For the first 2 weeks of treatment, they each may be applied once daily or mixed together and applied twice daily. For subsequent maintenance, topical calcipotriene may be applied on weekdays and topical steroids only on weekends. Combination calcipotriol–betamethasone dipropionate also is available as cream, ointment, foam, and suspension vehicles for use on the body and scalp in children aged 12 years and older. Tacrolimus ointment 0.1% may be applied in a thin layer up to twice daily. Concurrent emollient use also is recommended with these therapies.
Health care providers should educate patients and guardians about the potential side effects of topical therapies. They also should provide explicit instructions for amount, site, frequency, and duration of application. Topical corticosteroids commonly result in burning on application and may potentially cause skin thinning and striae with overuse. Topical vitamin D analogues may result in local irritation that may be improved by concurrent emollient use, and they generally should be avoided on sensitive sites. Topical calcineurin inhibitors are associated with burning, stinging, and pruritus, and the US Food and Drug Administration has issued a black-box warning related to risk for lymphoma with their chronic intermittent use. However, it was based on rare reports of lymphoma in transplant patients taking oral calcineurin inhibitors; no clinical trials to date in humans have demonstrated an increased risk for malignancy with topical calcineurin inhibitors.10 Tazarotene should be used cautiously in females of childbearing age given its teratogenic potential.
Children younger than 7 years are especially prone to suppression of the hypothalamic-pituitary-adrenal axis from topical corticosteroid therapy and theoretically hypercalcemia and hypervitaminosis D from topical vitamin D analogues, as their high BSA-to-volume ratio increases potential for systemic absorption. Children should avoid occlusive application of topical vitamin D analogues to large areas of the skin. Monitoring of vitamin D metabolites in the serum may be considered if calcipotriene or calcipotriol application to a large BSA is warranted.
Light-Based Therapy
In children with widespread psoriasis or those refractory to topical therapy, phototherapy may be considered. Narrowband UVB (311- to 313-nm wavelength) therapy is considered a first-line form of phototherapy in pediatric psoriasis. Mineral oil or emollient pretreatment to affected areas may augment the efficacy of UV-based treatments.11 Excimer laser and UVA also may be efficacious, though evidence is limited in children. Treatment is recommended to start at 3 days a week, and once improvement is seen, the frequency can be decreased to 2 days a week. Once desired clearance is achieved, maintenance therapy can be continued at even longer intervals. Adjunctive use of tar preparations may potentiate the efficacy of phototherapy, though there is a theoretical increased risk for carcinogenicity with prolonged use of coal tar. Side effects of phototherapy include erythema, blistering hyperpigmentation, and pruritus. Psoralen is contraindicated in children younger than 12 years. All forms of phototherapy are contraindicated in children with generalized erythroderma and cutaneous cancer syndromes. Other important pediatric-specific considerations include anxiety that may be provoked by UV light machines and inconvenience of frequent appointments.
Nonbiologic Systemic Therapies
Systemic therapies may be considered in children with recalcitrant, widespread, or rapidly progressing psoriasis, particularly if the disease is accompanied by severe emotional and psychological burden. These drugs, which include methotrexate, cyclosporine, and acitretin (see eTable for recommended dosing), are advantageous in that they may be combined with other therapies; however, they have potential for dangerous toxicities.
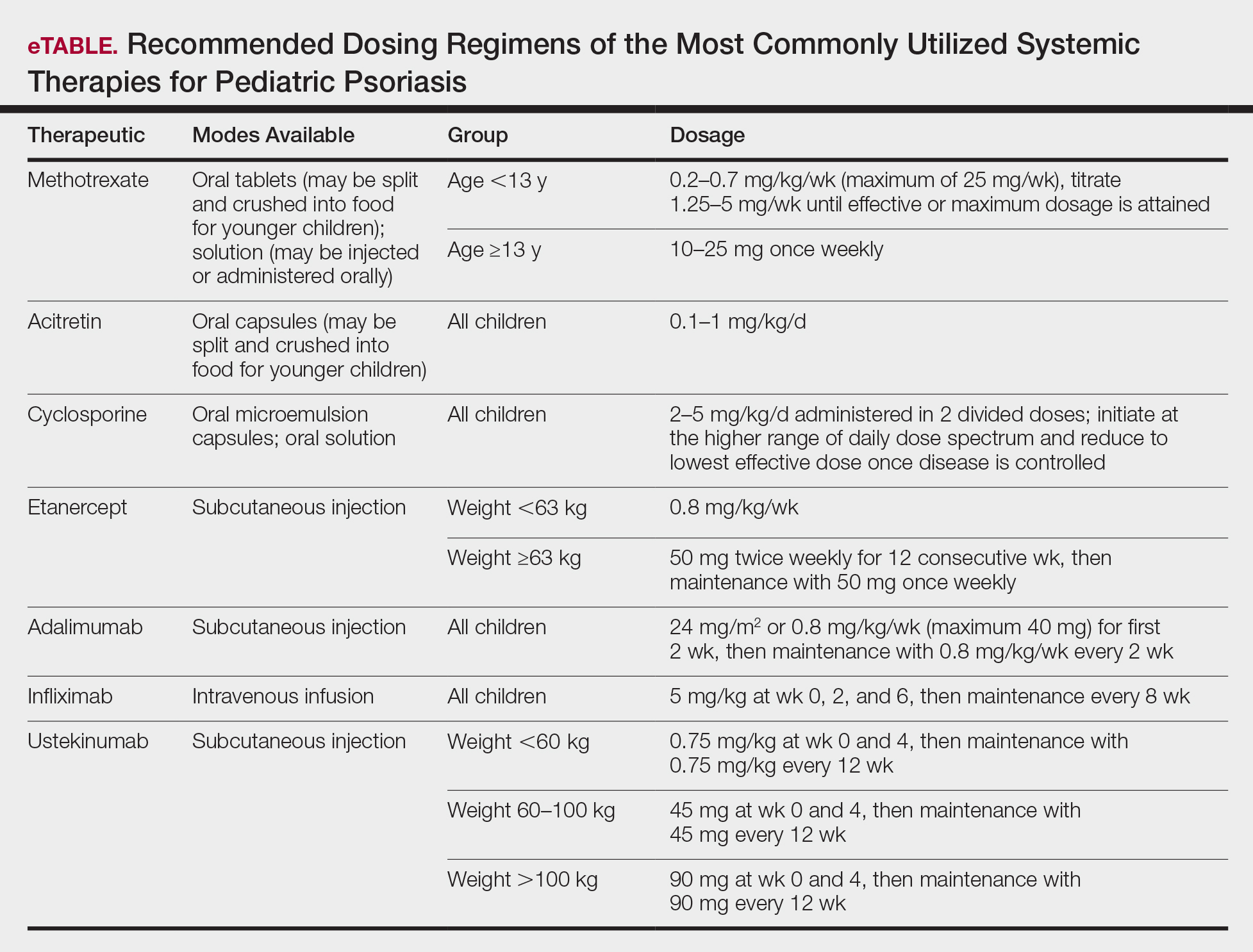
Methotrexate is the most frequently utilized systemic therapy for psoriasis worldwide in children because of its low cost, once-weekly dosing, and the substantial amount of long-term efficacy and safety data available in the pediatric population. It is slow acting initially but has excellent long-term efficacy for nearly every subtype of psoriasis. The most common side effect of methotrexate is gastrointestinal tract intolerance. Nonetheless, adverse events are rare in children without prior history, with 1 large study (N=289) reporting no adverse events in more than 90% of patients aged 9 to 14 years treated with methotrexate.12 Current guidelines recommend monitoring for bone marrow suppression and elevated transaminase levels 4 to 6 days after initiating treatment.1 The absolute contraindications for methotrexate are pregnancy and liver disease, and caution should be taken in children with metabolic risk factors. Adolescents must be counseled regarding the elevated risk for hepatotoxicity associated with alcohol ingestion. Methotrexate therapy also requires 1 mg folic acid supplementation 6 to 7 days a week, which decreases the risk for developing folic acid deficiency and may decrease gastrointestinal tract intolerance and hepatic side effects that may result from therapy.
Cyclosporine is an effective and well-tolerated option for rapid control of severe psoriasis in children. It is useful for various types of psoriasis but generally is reserved for more severe subtypes, such as generalized pustular psoriasis, erythrodermic psoriasis, and uncontrolled plaque psoriasis. Long-term use of cyclosporine may result in renal toxicity and hypertension, and this therapy is absolutely contraindicated in children with kidney disease or hypertension at baseline. It is strongly recommended to evaluate blood pressure every week for the first month of therapy and at every subsequent follow-up visit, which may occur at variable intervals based on the judgement of the provider. Evaluation before and during treatment with cyclosporine also should include a complete blood cell count, complete metabolic panel, and lipid panel.
Systemic retinoids have a unique advantage over methotrexate and cyclosporine in that they are not immunosuppressive and therefore are not contraindicated in children who are very young or immunosuppressed. Children receiving systemic retinoids also can receive routine live vaccines—measles-mumps-rubella, varicella zoster, and rotavirus—that are contraindicated with other systemic therapies. Acitretin is particularly effective in pediatric patients with diffuse guttate psoriasis, pustular psoriasis, and palmoplantar psoriasis. Narrowband UVB therapy has been shown to augment the effectiveness of acitretin in children, which may allow for reduced acitretin dosing. Pustular psoriasis may respond as quickly as 3 weeks after initiation, whereas it may take 2 to 3 months before improvement is noticed in plaque psoriasis. Side effects of retinoids include skin dryness, hyperlipidemia, and gastrointestinal tract upset. The most severe long-term concern is skeletal toxicity, including premature epiphyseal closure, hyperostosis, periosteal bone formation, and decreased bone mineral density.1 Vitamin A derivatives also are known teratogens and should be avoided in females of childbearing potential. Lipids and transaminases should be monitored routinely, and screening for depression and psychiatric symptoms should be performed frequently.1
When utilizing systemic therapies, the objective should be to control the disease, maintain stability, and ultimately taper to the lowest effective dose or transition to a topical therapy, if feasible. Although no particular systemic therapy is recommended as first line for children with psoriasis, it is important to consider comorbidities, contraindications, monitoring frequency, mode of administration (injectable therapies elicit more psychological trauma in children than oral therapies), and expense when determining the best choice.
Biologics
Biologic agents are associated with very high to total psoriatic plaque clearance rates and require infrequent dosing and monitoring. However, their use may be limited by cost and injection phobias in children as well as limited evidence for their efficacy and safety in pediatric psoriasis. Several studies have established the safety and effectiveness of biologics in children with plaque psoriasis (see eTable for recommended dosing), whereas the evidence supporting their use in treating pustular and erythrodermic variants are limited to case reports and case series. The tumor necrosis factor α (TNF-α) inhibitor etanercept has been approved for use in children aged 4 years and older, and the IL-12/IL-23 inhibitor ustekinumab is approved in children aged 6 years and older. Other TNF-α inhibitors, namely infliximab and adalimumab, commonly are utilized off label for pediatric psoriasis. The most common side effect of biologic therapies in pediatric patients is injection-site reactions.1 Prior to initiating therapy, children must undergo tuberculosis screening either by purified protein derivative testing or IFN-γ release assay. Testing should be repeated annually in individuals taking TNF-α inhibitors, though the utility of repeat testing when taking biologics in other classes is not clear. High-risk patients also should be screened for human immunodeficiency virus and hepatitis. Follow-up frequency may range from every 3 months to annually, based on judgement of the provider. In children who develop loss of response to biologics, methotrexate can be added to the regimen to attenuate formation of efficacy-reducing antidrug antibodies.
Final Thoughts
When managing children with psoriasis, it is important for dermatologists to appropriately educate guardians and children on the disease course, as well as consider the psychological, emotional, social, and financial factors that may direct decision-making regarding optimal therapeutics. Dermatologists should consider collaboration with the child’s primary care physician and other specialists to ensure that all needs are met.
These guidelines provide a framework agreed upon by numerous experts in pediatric psoriasis, but they are limited by gaps in the research. There still is much to be learned regarding the pathophysiology of psoriasis; the risk for developing comorbidities during adulthood; and the efficacy and safety of certain therapeutics, particularly biologics, in pediatric patients with psoriasis.
- Menter A, Cordoro KM, Davis DMR, et al. Joint American Academy of Dermatology–National Psoriasis Foundation guidelines of care for the management and treatment of psoriasis in pediatric patients [published online November 5, 2019]. J Am Acad Dermatol. 2020;82:161-201.
- Lewis-Jones MS, Finlay AY. The Children’s Dermatology Life Quality Index (CDLQI): initial validation and practical use. Br J Dermatol. 1995;132:942-949.
- Augustin M, Radtke MA, Glaeske G, et al. Epidemiology and comorbidity in children with psoriasis and atopic eczema. Dermatology. 2015;231:35-40.
- Osier E, Wang AS, Tollefson MM, et al. Pediatric psoriasis comorbidity screening guidelines. JAMA Dermatol. 2017;153:698-704.
- Boccardi D, Menni S, La Vecchia C, et al. Overweight and childhood psoriasis. Br J Dermatol. 2009;161:484-486.
- Becker L, Tom WL, Eshagh K, et al. Excess adiposity preceding pediatric psoriasis. JAMA Dermatol. 2014;150:573-574.
- Alotaibi HA. Effects of weight loss on psoriasis: a review of clinical trials. Cureus. 2018;10:E3491.
- Guidelines summaries—American Academy of Pediatrics. Guideline Central
website. https://www.guidelinecentral.com/summaries/organizations/american-academy-of-pediatrics/2019. Accessed October 27, 2020. - Standards of Medical Care in Diabetes. American Diabetes Association website. https://care.diabetesjournals.org/content/43/Supplement_1. Published January 1, 2020. Accessed May 8, 2020.
- Siegfried EC, Jaworski JC, Hebert AA. Topical calcineurin inhibitors and lymphoma risk: evidence update with implications for daily practice. Am J Clin Dermatol. 2013;14:163-178.
- Jain VK, Bansal A, Aggarwal K, et al. Enhanced response of childhood psoriasis to narrow-band UV-B phototherapy with preirradiation use of mineral oil. Pediatr Dermatol. 2008;25:559-564.
- Ergun T, Seckin Gencosmanoglu D, Alpsoy E, et al. Efficacy, safety and drug survival of conventional agents in pediatric psoriasis: a multicenter, cohort study. J Dermatol. 2017;44:630-634.
In November 2019, the American Academy of Dermatology (AAD) and the National Psoriasis Foundation (NPF) released their first set of recommendations for the management of pediatric psoriasis.1 The pediatric guidelines discuss methods of quantifying disease severity in children, triggers and comorbidities, and the efficacy and safety of various therapeutic agents. This review aims to discuss, in a condensed form, special considerations unique to the management of children with psoriasis as presented in the guidelines as well as grade A– and grade B–level treatment recommendations (Table).

Quantifying Psoriasis Severity in Children
Percentage body surface area (BSA) involvement is the most common mode of grading psoriasis severity, with less than 3% BSA involvement being considered mild, 3% to 10% BSA moderate, and more than 10% severe disease. In children, the standard method of measuring BSA is the rule of 9’s: the head and each arm make up 9% of the total BSA, each leg and the front and back of the torso respectively each make up 18%, and the genitalia make up 1%. It also is important to consider impact on quality of life, which may be remarkable in spite of limited BSA involvement. The children’s dermatology life quality index score may be utilized in combination with affected BSA to determine the burden of psoriasis in context of impact on daily life. This metric is available in both written and cartoon form, and it consists of 10 questions that include variables such as severity of itch, impact on social life, and effects on sleep. Most notably, this tool incorporates pruritus,2 which generally is addressed inadequately in pediatric psoriasis.
Triggers and Comorbidities in Pediatric Patients
In children, it is important to identify and eliminate modifiable factors that may prompt psoriasis flares. Infections, particularly group A beta-hemolytic streptococcal infections, are a major trigger in neonates and infants. Other exacerbating factors in children include emotional stress, secondhand cigarette smoke, Kawasaki disease, and withdrawal from systemic corticosteroids.
Psoriatic arthritis (PsA) is a burdensome comorbidity affecting children with psoriasis. The prevalence of joint disease is 15-times greater in children with psoriasis vs those without,3 and 80% of children with PsA develop rheumatologic symptoms, which typically include oligoarticular disease and dactylitis in infants and girls and enthesitis and axial joint involvement in boys and older children, years prior to the onset of cutaneous disease.4 Uveitis often occurs in children with psoriasis and PsA but not in those with isolated cutaneous disease.
Compared to unaffected children, pediatric patients with psoriasis have greater prevalence of metabolic and cardiovascular risk factors during childhood, including central obesity, hypertension, hypertriglyceridemia, hypercholesterolemia, insulin resistance, atherosclerosis, arrythmia, and valvular heart disease. Family history of obesity increases the risk for early-onset development of cutaneous lesions,5,6 and weight reduction may alleviate severity of psoriasis lesions.7 In the United States, many of the metabolic associations observed are particularly robust in Black and Hispanic children vs those of other races. Furthermore, the prevalence of inflammatory bowel disease is 3- to 4-times higher in children with psoriasis compared to those without.
As with other cutaneous diseases, it is important to be aware of social and mental health concerns in children with psoriasis. The majority of pediatric patients with psoriasis experience name-calling, shaming, or bullying, and many have concerns from skin shedding and malodor. Independent risk for depression after the onset of psoriasis is high. Affected older children and adolescents are at increased risk for alcohol and drug abuse as well as eating disorders.
Despite these identified comorbidities, there are no unique screening recommendations for arthritis, ophthalmologic disease, metabolic disease, cardiovascular disease, gastrointestinal tract disease, or mental health issues in children with psoriasis. Rather, these patients should be monitored according to the American Academy of Pediatrics or American Diabetes Association guidelines for all pediatric patients.8,9 Nonetheless, educating patients and guardians about these potential issues may be warranted.
Topical Therapies
For children with mild to moderate psoriasis, topical therapies are first line. Despite being off label, topical corticosteroids are the mainstay of therapy for localized psoriatic plaques in children. Topical vitamin D analogues—calcitriol and calcipotriol/calcipotriene—are highly effective and well tolerated, and they frequently are used in combination with topical corticosteroids. Topical calcineurin inhibitors, namely tacrolimus, also are used off label but are considered first line for sensitive regions of the skin in children, including the face, genitalia, and body folds. There currently is limited evidence for supporting the use of the topical vitamin A analogue tazarotene in children with psoriasis, though some consider its off-label use effective for pediatric nail psoriasis. It also may be used as an adjunct to topical corticosteroids to minimize irritation.
Although there is no gold standard topical regimen, combination therapy with a high-potency topical steroid and topical vitamin D analogue commonly is used to minimize steroid-induced side effects. For the first 2 weeks of treatment, they each may be applied once daily or mixed together and applied twice daily. For subsequent maintenance, topical calcipotriene may be applied on weekdays and topical steroids only on weekends. Combination calcipotriol–betamethasone dipropionate also is available as cream, ointment, foam, and suspension vehicles for use on the body and scalp in children aged 12 years and older. Tacrolimus ointment 0.1% may be applied in a thin layer up to twice daily. Concurrent emollient use also is recommended with these therapies.
Health care providers should educate patients and guardians about the potential side effects of topical therapies. They also should provide explicit instructions for amount, site, frequency, and duration of application. Topical corticosteroids commonly result in burning on application and may potentially cause skin thinning and striae with overuse. Topical vitamin D analogues may result in local irritation that may be improved by concurrent emollient use, and they generally should be avoided on sensitive sites. Topical calcineurin inhibitors are associated with burning, stinging, and pruritus, and the US Food and Drug Administration has issued a black-box warning related to risk for lymphoma with their chronic intermittent use. However, it was based on rare reports of lymphoma in transplant patients taking oral calcineurin inhibitors; no clinical trials to date in humans have demonstrated an increased risk for malignancy with topical calcineurin inhibitors.10 Tazarotene should be used cautiously in females of childbearing age given its teratogenic potential.
Children younger than 7 years are especially prone to suppression of the hypothalamic-pituitary-adrenal axis from topical corticosteroid therapy and theoretically hypercalcemia and hypervitaminosis D from topical vitamin D analogues, as their high BSA-to-volume ratio increases potential for systemic absorption. Children should avoid occlusive application of topical vitamin D analogues to large areas of the skin. Monitoring of vitamin D metabolites in the serum may be considered if calcipotriene or calcipotriol application to a large BSA is warranted.
Light-Based Therapy
In children with widespread psoriasis or those refractory to topical therapy, phototherapy may be considered. Narrowband UVB (311- to 313-nm wavelength) therapy is considered a first-line form of phototherapy in pediatric psoriasis. Mineral oil or emollient pretreatment to affected areas may augment the efficacy of UV-based treatments.11 Excimer laser and UVA also may be efficacious, though evidence is limited in children. Treatment is recommended to start at 3 days a week, and once improvement is seen, the frequency can be decreased to 2 days a week. Once desired clearance is achieved, maintenance therapy can be continued at even longer intervals. Adjunctive use of tar preparations may potentiate the efficacy of phototherapy, though there is a theoretical increased risk for carcinogenicity with prolonged use of coal tar. Side effects of phototherapy include erythema, blistering hyperpigmentation, and pruritus. Psoralen is contraindicated in children younger than 12 years. All forms of phototherapy are contraindicated in children with generalized erythroderma and cutaneous cancer syndromes. Other important pediatric-specific considerations include anxiety that may be provoked by UV light machines and inconvenience of frequent appointments.
Nonbiologic Systemic Therapies
Systemic therapies may be considered in children with recalcitrant, widespread, or rapidly progressing psoriasis, particularly if the disease is accompanied by severe emotional and psychological burden. These drugs, which include methotrexate, cyclosporine, and acitretin (see eTable for recommended dosing), are advantageous in that they may be combined with other therapies; however, they have potential for dangerous toxicities.

Methotrexate is the most frequently utilized systemic therapy for psoriasis worldwide in children because of its low cost, once-weekly dosing, and the substantial amount of long-term efficacy and safety data available in the pediatric population. It is slow acting initially but has excellent long-term efficacy for nearly every subtype of psoriasis. The most common side effect of methotrexate is gastrointestinal tract intolerance. Nonetheless, adverse events are rare in children without prior history, with 1 large study (N=289) reporting no adverse events in more than 90% of patients aged 9 to 14 years treated with methotrexate.12 Current guidelines recommend monitoring for bone marrow suppression and elevated transaminase levels 4 to 6 days after initiating treatment.1 The absolute contraindications for methotrexate are pregnancy and liver disease, and caution should be taken in children with metabolic risk factors. Adolescents must be counseled regarding the elevated risk for hepatotoxicity associated with alcohol ingestion. Methotrexate therapy also requires 1 mg folic acid supplementation 6 to 7 days a week, which decreases the risk for developing folic acid deficiency and may decrease gastrointestinal tract intolerance and hepatic side effects that may result from therapy.
Cyclosporine is an effective and well-tolerated option for rapid control of severe psoriasis in children. It is useful for various types of psoriasis but generally is reserved for more severe subtypes, such as generalized pustular psoriasis, erythrodermic psoriasis, and uncontrolled plaque psoriasis. Long-term use of cyclosporine may result in renal toxicity and hypertension, and this therapy is absolutely contraindicated in children with kidney disease or hypertension at baseline. It is strongly recommended to evaluate blood pressure every week for the first month of therapy and at every subsequent follow-up visit, which may occur at variable intervals based on the judgement of the provider. Evaluation before and during treatment with cyclosporine also should include a complete blood cell count, complete metabolic panel, and lipid panel.
Systemic retinoids have a unique advantage over methotrexate and cyclosporine in that they are not immunosuppressive and therefore are not contraindicated in children who are very young or immunosuppressed. Children receiving systemic retinoids also can receive routine live vaccines—measles-mumps-rubella, varicella zoster, and rotavirus—that are contraindicated with other systemic therapies. Acitretin is particularly effective in pediatric patients with diffuse guttate psoriasis, pustular psoriasis, and palmoplantar psoriasis. Narrowband UVB therapy has been shown to augment the effectiveness of acitretin in children, which may allow for reduced acitretin dosing. Pustular psoriasis may respond as quickly as 3 weeks after initiation, whereas it may take 2 to 3 months before improvement is noticed in plaque psoriasis. Side effects of retinoids include skin dryness, hyperlipidemia, and gastrointestinal tract upset. The most severe long-term concern is skeletal toxicity, including premature epiphyseal closure, hyperostosis, periosteal bone formation, and decreased bone mineral density.1 Vitamin A derivatives also are known teratogens and should be avoided in females of childbearing potential. Lipids and transaminases should be monitored routinely, and screening for depression and psychiatric symptoms should be performed frequently.1
When utilizing systemic therapies, the objective should be to control the disease, maintain stability, and ultimately taper to the lowest effective dose or transition to a topical therapy, if feasible. Although no particular systemic therapy is recommended as first line for children with psoriasis, it is important to consider comorbidities, contraindications, monitoring frequency, mode of administration (injectable therapies elicit more psychological trauma in children than oral therapies), and expense when determining the best choice.
Biologics
Biologic agents are associated with very high to total psoriatic plaque clearance rates and require infrequent dosing and monitoring. However, their use may be limited by cost and injection phobias in children as well as limited evidence for their efficacy and safety in pediatric psoriasis. Several studies have established the safety and effectiveness of biologics in children with plaque psoriasis (see eTable for recommended dosing), whereas the evidence supporting their use in treating pustular and erythrodermic variants are limited to case reports and case series. The tumor necrosis factor α (TNF-α) inhibitor etanercept has been approved for use in children aged 4 years and older, and the IL-12/IL-23 inhibitor ustekinumab is approved in children aged 6 years and older. Other TNF-α inhibitors, namely infliximab and adalimumab, commonly are utilized off label for pediatric psoriasis. The most common side effect of biologic therapies in pediatric patients is injection-site reactions.1 Prior to initiating therapy, children must undergo tuberculosis screening either by purified protein derivative testing or IFN-γ release assay. Testing should be repeated annually in individuals taking TNF-α inhibitors, though the utility of repeat testing when taking biologics in other classes is not clear. High-risk patients also should be screened for human immunodeficiency virus and hepatitis. Follow-up frequency may range from every 3 months to annually, based on judgement of the provider. In children who develop loss of response to biologics, methotrexate can be added to the regimen to attenuate formation of efficacy-reducing antidrug antibodies.
Final Thoughts
When managing children with psoriasis, it is important for dermatologists to appropriately educate guardians and children on the disease course, as well as consider the psychological, emotional, social, and financial factors that may direct decision-making regarding optimal therapeutics. Dermatologists should consider collaboration with the child’s primary care physician and other specialists to ensure that all needs are met.
These guidelines provide a framework agreed upon by numerous experts in pediatric psoriasis, but they are limited by gaps in the research. There still is much to be learned regarding the pathophysiology of psoriasis; the risk for developing comorbidities during adulthood; and the efficacy and safety of certain therapeutics, particularly biologics, in pediatric patients with psoriasis.
In November 2019, the American Academy of Dermatology (AAD) and the National Psoriasis Foundation (NPF) released their first set of recommendations for the management of pediatric psoriasis.1 The pediatric guidelines discuss methods of quantifying disease severity in children, triggers and comorbidities, and the efficacy and safety of various therapeutic agents. This review aims to discuss, in a condensed form, special considerations unique to the management of children with psoriasis as presented in the guidelines as well as grade A– and grade B–level treatment recommendations (Table).

Quantifying Psoriasis Severity in Children
Percentage body surface area (BSA) involvement is the most common mode of grading psoriasis severity, with less than 3% BSA involvement being considered mild, 3% to 10% BSA moderate, and more than 10% severe disease. In children, the standard method of measuring BSA is the rule of 9’s: the head and each arm make up 9% of the total BSA, each leg and the front and back of the torso respectively each make up 18%, and the genitalia make up 1%. It also is important to consider impact on quality of life, which may be remarkable in spite of limited BSA involvement. The children’s dermatology life quality index score may be utilized in combination with affected BSA to determine the burden of psoriasis in context of impact on daily life. This metric is available in both written and cartoon form, and it consists of 10 questions that include variables such as severity of itch, impact on social life, and effects on sleep. Most notably, this tool incorporates pruritus,2 which generally is addressed inadequately in pediatric psoriasis.
Triggers and Comorbidities in Pediatric Patients
In children, it is important to identify and eliminate modifiable factors that may prompt psoriasis flares. Infections, particularly group A beta-hemolytic streptococcal infections, are a major trigger in neonates and infants. Other exacerbating factors in children include emotional stress, secondhand cigarette smoke, Kawasaki disease, and withdrawal from systemic corticosteroids.
Psoriatic arthritis (PsA) is a burdensome comorbidity affecting children with psoriasis. The prevalence of joint disease is 15-times greater in children with psoriasis vs those without,3 and 80% of children with PsA develop rheumatologic symptoms, which typically include oligoarticular disease and dactylitis in infants and girls and enthesitis and axial joint involvement in boys and older children, years prior to the onset of cutaneous disease.4 Uveitis often occurs in children with psoriasis and PsA but not in those with isolated cutaneous disease.
Compared to unaffected children, pediatric patients with psoriasis have greater prevalence of metabolic and cardiovascular risk factors during childhood, including central obesity, hypertension, hypertriglyceridemia, hypercholesterolemia, insulin resistance, atherosclerosis, arrythmia, and valvular heart disease. Family history of obesity increases the risk for early-onset development of cutaneous lesions,5,6 and weight reduction may alleviate severity of psoriasis lesions.7 In the United States, many of the metabolic associations observed are particularly robust in Black and Hispanic children vs those of other races. Furthermore, the prevalence of inflammatory bowel disease is 3- to 4-times higher in children with psoriasis compared to those without.
As with other cutaneous diseases, it is important to be aware of social and mental health concerns in children with psoriasis. The majority of pediatric patients with psoriasis experience name-calling, shaming, or bullying, and many have concerns from skin shedding and malodor. Independent risk for depression after the onset of psoriasis is high. Affected older children and adolescents are at increased risk for alcohol and drug abuse as well as eating disorders.
Despite these identified comorbidities, there are no unique screening recommendations for arthritis, ophthalmologic disease, metabolic disease, cardiovascular disease, gastrointestinal tract disease, or mental health issues in children with psoriasis. Rather, these patients should be monitored according to the American Academy of Pediatrics or American Diabetes Association guidelines for all pediatric patients.8,9 Nonetheless, educating patients and guardians about these potential issues may be warranted.
Topical Therapies
For children with mild to moderate psoriasis, topical therapies are first line. Despite being off label, topical corticosteroids are the mainstay of therapy for localized psoriatic plaques in children. Topical vitamin D analogues—calcitriol and calcipotriol/calcipotriene—are highly effective and well tolerated, and they frequently are used in combination with topical corticosteroids. Topical calcineurin inhibitors, namely tacrolimus, also are used off label but are considered first line for sensitive regions of the skin in children, including the face, genitalia, and body folds. There currently is limited evidence for supporting the use of the topical vitamin A analogue tazarotene in children with psoriasis, though some consider its off-label use effective for pediatric nail psoriasis. It also may be used as an adjunct to topical corticosteroids to minimize irritation.
Although there is no gold standard topical regimen, combination therapy with a high-potency topical steroid and topical vitamin D analogue commonly is used to minimize steroid-induced side effects. For the first 2 weeks of treatment, they each may be applied once daily or mixed together and applied twice daily. For subsequent maintenance, topical calcipotriene may be applied on weekdays and topical steroids only on weekends. Combination calcipotriol–betamethasone dipropionate also is available as cream, ointment, foam, and suspension vehicles for use on the body and scalp in children aged 12 years and older. Tacrolimus ointment 0.1% may be applied in a thin layer up to twice daily. Concurrent emollient use also is recommended with these therapies.
Health care providers should educate patients and guardians about the potential side effects of topical therapies. They also should provide explicit instructions for amount, site, frequency, and duration of application. Topical corticosteroids commonly result in burning on application and may potentially cause skin thinning and striae with overuse. Topical vitamin D analogues may result in local irritation that may be improved by concurrent emollient use, and they generally should be avoided on sensitive sites. Topical calcineurin inhibitors are associated with burning, stinging, and pruritus, and the US Food and Drug Administration has issued a black-box warning related to risk for lymphoma with their chronic intermittent use. However, it was based on rare reports of lymphoma in transplant patients taking oral calcineurin inhibitors; no clinical trials to date in humans have demonstrated an increased risk for malignancy with topical calcineurin inhibitors.10 Tazarotene should be used cautiously in females of childbearing age given its teratogenic potential.
Children younger than 7 years are especially prone to suppression of the hypothalamic-pituitary-adrenal axis from topical corticosteroid therapy and theoretically hypercalcemia and hypervitaminosis D from topical vitamin D analogues, as their high BSA-to-volume ratio increases potential for systemic absorption. Children should avoid occlusive application of topical vitamin D analogues to large areas of the skin. Monitoring of vitamin D metabolites in the serum may be considered if calcipotriene or calcipotriol application to a large BSA is warranted.
Light-Based Therapy
In children with widespread psoriasis or those refractory to topical therapy, phototherapy may be considered. Narrowband UVB (311- to 313-nm wavelength) therapy is considered a first-line form of phototherapy in pediatric psoriasis. Mineral oil or emollient pretreatment to affected areas may augment the efficacy of UV-based treatments.11 Excimer laser and UVA also may be efficacious, though evidence is limited in children. Treatment is recommended to start at 3 days a week, and once improvement is seen, the frequency can be decreased to 2 days a week. Once desired clearance is achieved, maintenance therapy can be continued at even longer intervals. Adjunctive use of tar preparations may potentiate the efficacy of phototherapy, though there is a theoretical increased risk for carcinogenicity with prolonged use of coal tar. Side effects of phototherapy include erythema, blistering hyperpigmentation, and pruritus. Psoralen is contraindicated in children younger than 12 years. All forms of phototherapy are contraindicated in children with generalized erythroderma and cutaneous cancer syndromes. Other important pediatric-specific considerations include anxiety that may be provoked by UV light machines and inconvenience of frequent appointments.
Nonbiologic Systemic Therapies
Systemic therapies may be considered in children with recalcitrant, widespread, or rapidly progressing psoriasis, particularly if the disease is accompanied by severe emotional and psychological burden. These drugs, which include methotrexate, cyclosporine, and acitretin (see eTable for recommended dosing), are advantageous in that they may be combined with other therapies; however, they have potential for dangerous toxicities.

Methotrexate is the most frequently utilized systemic therapy for psoriasis worldwide in children because of its low cost, once-weekly dosing, and the substantial amount of long-term efficacy and safety data available in the pediatric population. It is slow acting initially but has excellent long-term efficacy for nearly every subtype of psoriasis. The most common side effect of methotrexate is gastrointestinal tract intolerance. Nonetheless, adverse events are rare in children without prior history, with 1 large study (N=289) reporting no adverse events in more than 90% of patients aged 9 to 14 years treated with methotrexate.12 Current guidelines recommend monitoring for bone marrow suppression and elevated transaminase levels 4 to 6 days after initiating treatment.1 The absolute contraindications for methotrexate are pregnancy and liver disease, and caution should be taken in children with metabolic risk factors. Adolescents must be counseled regarding the elevated risk for hepatotoxicity associated with alcohol ingestion. Methotrexate therapy also requires 1 mg folic acid supplementation 6 to 7 days a week, which decreases the risk for developing folic acid deficiency and may decrease gastrointestinal tract intolerance and hepatic side effects that may result from therapy.
Cyclosporine is an effective and well-tolerated option for rapid control of severe psoriasis in children. It is useful for various types of psoriasis but generally is reserved for more severe subtypes, such as generalized pustular psoriasis, erythrodermic psoriasis, and uncontrolled plaque psoriasis. Long-term use of cyclosporine may result in renal toxicity and hypertension, and this therapy is absolutely contraindicated in children with kidney disease or hypertension at baseline. It is strongly recommended to evaluate blood pressure every week for the first month of therapy and at every subsequent follow-up visit, which may occur at variable intervals based on the judgement of the provider. Evaluation before and during treatment with cyclosporine also should include a complete blood cell count, complete metabolic panel, and lipid panel.
Systemic retinoids have a unique advantage over methotrexate and cyclosporine in that they are not immunosuppressive and therefore are not contraindicated in children who are very young or immunosuppressed. Children receiving systemic retinoids also can receive routine live vaccines—measles-mumps-rubella, varicella zoster, and rotavirus—that are contraindicated with other systemic therapies. Acitretin is particularly effective in pediatric patients with diffuse guttate psoriasis, pustular psoriasis, and palmoplantar psoriasis. Narrowband UVB therapy has been shown to augment the effectiveness of acitretin in children, which may allow for reduced acitretin dosing. Pustular psoriasis may respond as quickly as 3 weeks after initiation, whereas it may take 2 to 3 months before improvement is noticed in plaque psoriasis. Side effects of retinoids include skin dryness, hyperlipidemia, and gastrointestinal tract upset. The most severe long-term concern is skeletal toxicity, including premature epiphyseal closure, hyperostosis, periosteal bone formation, and decreased bone mineral density.1 Vitamin A derivatives also are known teratogens and should be avoided in females of childbearing potential. Lipids and transaminases should be monitored routinely, and screening for depression and psychiatric symptoms should be performed frequently.1
When utilizing systemic therapies, the objective should be to control the disease, maintain stability, and ultimately taper to the lowest effective dose or transition to a topical therapy, if feasible. Although no particular systemic therapy is recommended as first line for children with psoriasis, it is important to consider comorbidities, contraindications, monitoring frequency, mode of administration (injectable therapies elicit more psychological trauma in children than oral therapies), and expense when determining the best choice.
Biologics
Biologic agents are associated with very high to total psoriatic plaque clearance rates and require infrequent dosing and monitoring. However, their use may be limited by cost and injection phobias in children as well as limited evidence for their efficacy and safety in pediatric psoriasis. Several studies have established the safety and effectiveness of biologics in children with plaque psoriasis (see eTable for recommended dosing), whereas the evidence supporting their use in treating pustular and erythrodermic variants are limited to case reports and case series. The tumor necrosis factor α (TNF-α) inhibitor etanercept has been approved for use in children aged 4 years and older, and the IL-12/IL-23 inhibitor ustekinumab is approved in children aged 6 years and older. Other TNF-α inhibitors, namely infliximab and adalimumab, commonly are utilized off label for pediatric psoriasis. The most common side effect of biologic therapies in pediatric patients is injection-site reactions.1 Prior to initiating therapy, children must undergo tuberculosis screening either by purified protein derivative testing or IFN-γ release assay. Testing should be repeated annually in individuals taking TNF-α inhibitors, though the utility of repeat testing when taking biologics in other classes is not clear. High-risk patients also should be screened for human immunodeficiency virus and hepatitis. Follow-up frequency may range from every 3 months to annually, based on judgement of the provider. In children who develop loss of response to biologics, methotrexate can be added to the regimen to attenuate formation of efficacy-reducing antidrug antibodies.
Final Thoughts
When managing children with psoriasis, it is important for dermatologists to appropriately educate guardians and children on the disease course, as well as consider the psychological, emotional, social, and financial factors that may direct decision-making regarding optimal therapeutics. Dermatologists should consider collaboration with the child’s primary care physician and other specialists to ensure that all needs are met.
These guidelines provide a framework agreed upon by numerous experts in pediatric psoriasis, but they are limited by gaps in the research. There still is much to be learned regarding the pathophysiology of psoriasis; the risk for developing comorbidities during adulthood; and the efficacy and safety of certain therapeutics, particularly biologics, in pediatric patients with psoriasis.
- Menter A, Cordoro KM, Davis DMR, et al. Joint American Academy of Dermatology–National Psoriasis Foundation guidelines of care for the management and treatment of psoriasis in pediatric patients [published online November 5, 2019]. J Am Acad Dermatol. 2020;82:161-201.
- Lewis-Jones MS, Finlay AY. The Children’s Dermatology Life Quality Index (CDLQI): initial validation and practical use. Br J Dermatol. 1995;132:942-949.
- Augustin M, Radtke MA, Glaeske G, et al. Epidemiology and comorbidity in children with psoriasis and atopic eczema. Dermatology. 2015;231:35-40.
- Osier E, Wang AS, Tollefson MM, et al. Pediatric psoriasis comorbidity screening guidelines. JAMA Dermatol. 2017;153:698-704.
- Boccardi D, Menni S, La Vecchia C, et al. Overweight and childhood psoriasis. Br J Dermatol. 2009;161:484-486.
- Becker L, Tom WL, Eshagh K, et al. Excess adiposity preceding pediatric psoriasis. JAMA Dermatol. 2014;150:573-574.
- Alotaibi HA. Effects of weight loss on psoriasis: a review of clinical trials. Cureus. 2018;10:E3491.
- Guidelines summaries—American Academy of Pediatrics. Guideline Central
website. https://www.guidelinecentral.com/summaries/organizations/american-academy-of-pediatrics/2019. Accessed October 27, 2020. - Standards of Medical Care in Diabetes. American Diabetes Association website. https://care.diabetesjournals.org/content/43/Supplement_1. Published January 1, 2020. Accessed May 8, 2020.
- Siegfried EC, Jaworski JC, Hebert AA. Topical calcineurin inhibitors and lymphoma risk: evidence update with implications for daily practice. Am J Clin Dermatol. 2013;14:163-178.
- Jain VK, Bansal A, Aggarwal K, et al. Enhanced response of childhood psoriasis to narrow-band UV-B phototherapy with preirradiation use of mineral oil. Pediatr Dermatol. 2008;25:559-564.
- Ergun T, Seckin Gencosmanoglu D, Alpsoy E, et al. Efficacy, safety and drug survival of conventional agents in pediatric psoriasis: a multicenter, cohort study. J Dermatol. 2017;44:630-634.
- Menter A, Cordoro KM, Davis DMR, et al. Joint American Academy of Dermatology–National Psoriasis Foundation guidelines of care for the management and treatment of psoriasis in pediatric patients [published online November 5, 2019]. J Am Acad Dermatol. 2020;82:161-201.
- Lewis-Jones MS, Finlay AY. The Children’s Dermatology Life Quality Index (CDLQI): initial validation and practical use. Br J Dermatol. 1995;132:942-949.
- Augustin M, Radtke MA, Glaeske G, et al. Epidemiology and comorbidity in children with psoriasis and atopic eczema. Dermatology. 2015;231:35-40.
- Osier E, Wang AS, Tollefson MM, et al. Pediatric psoriasis comorbidity screening guidelines. JAMA Dermatol. 2017;153:698-704.
- Boccardi D, Menni S, La Vecchia C, et al. Overweight and childhood psoriasis. Br J Dermatol. 2009;161:484-486.
- Becker L, Tom WL, Eshagh K, et al. Excess adiposity preceding pediatric psoriasis. JAMA Dermatol. 2014;150:573-574.
- Alotaibi HA. Effects of weight loss on psoriasis: a review of clinical trials. Cureus. 2018;10:E3491.
- Guidelines summaries—American Academy of Pediatrics. Guideline Central
website. https://www.guidelinecentral.com/summaries/organizations/american-academy-of-pediatrics/2019. Accessed October 27, 2020. - Standards of Medical Care in Diabetes. American Diabetes Association website. https://care.diabetesjournals.org/content/43/Supplement_1. Published January 1, 2020. Accessed May 8, 2020.
- Siegfried EC, Jaworski JC, Hebert AA. Topical calcineurin inhibitors and lymphoma risk: evidence update with implications for daily practice. Am J Clin Dermatol. 2013;14:163-178.
- Jain VK, Bansal A, Aggarwal K, et al. Enhanced response of childhood psoriasis to narrow-band UV-B phototherapy with preirradiation use of mineral oil. Pediatr Dermatol. 2008;25:559-564.
- Ergun T, Seckin Gencosmanoglu D, Alpsoy E, et al. Efficacy, safety and drug survival of conventional agents in pediatric psoriasis: a multicenter, cohort study. J Dermatol. 2017;44:630-634.
Practice Points
- For children, several environmental factors may prompt psoriasis flares, and it is critical to identify and eliminate these triggers.
- Although the use of biologics may be limited by cost and injection phobias in children, they may be an appropriate option for children with moderate to severe psoriasis when other therapies have failed. A growing body of literature is establishing the safety and effectiveness of biologics in children.
- Clinicians should thoroughly educate parents/ guardians on the course of psoriasis and treatment options as well as pay special attention to treatment goals and psychosocial factors that may guide decision-making regarding therapy.
Syphilis: Cutting risk through primary prevention and prenatal screening
CASE Pregnant woman with positive Treponema pallidum antibody test
A 30-year-old primigravida at 10 weeks and 4 days of gestation by her last menstrual period presents to your office for her initial prenatal visit. She expresses no concerns. You order the standard set of laboratory tests, including a sexually transmitted infection (STI) screening panel. Consistent with your institution’s use of the reverse algorithm for syphilis screening, you obtain a Treponema pallidum antibody test, which reflexes to the rapid plasma reagin (RPR) test. Three days later, you receive a notification that this patient’s T pallidum antibody result was positive, followed by negative RPR test results. The follow-up T pallidum particle agglutination (TP-PA) test also was negative. Given these findings, you consider:
- What is the correct interpretation of the patient’s sequence of test results?
- Is she infected, and does she require treatment?
Meet our perpetrator
Syphilis has plagued society since the late 15th century, although its causative agent, the spirochete T pallidum, was not recognized until 1905.1,2T pallidum bacteria are transmitted via sexual contact, as well as through vertical transmission during pregnancy or delivery. Infection with syphilis is reported in 50% to 60% of sexual partners after a single exposure to an infected individual with early syphilis, and the mean incubation period is 21 days.3T pallidum can cross the placenta and infect a fetus as early as the sixth week of gestation.3 Congenital syphilis infections occur in the neonates of 50% to 80% of women with untreated primary, secondary, or early latent syphilis infections; maternal syphilis is associated with a 21% increased risk of stillbirth, a 6% increased risk of preterm delivery, and a 9% increased risk of neonatal death.4,5 Additionally, syphilis infection is associated with a high risk of HIV infection, as well as coinfection with other STIs.1
Given the highly infective nature of T pallidum, as well as the severity of the potential consequences of infection for both mothers and babies, primary prevention, education of at-risk populations, and early recognition of clinical features of syphilis infection are of utmost importance in preventing morbidity and mortality. In this article, we review the epidemiology and extensive clinical manifestations of syphilis, as well as current screening recommendations and treatment for pregnant women.
The extent of the problem today
Although US rates of syphilis have ebbed and flowed for the past several decades, the current incidence has grown exponentially in recent years, with the number of cases reported to the Centers for Disease Control and Prevention (CDC) increasing by 71% from 2014 to 2018.6 During this time period, reported cases of primary and secondary syphilis in women more than doubled (172.7% and 165.4%, respectively) according to CDC data, accompanied by a parallel rise in reported cases of congenital syphilis in both live and stillborn infants.6 In 2018, the CDC reported a national rate of congenital syphilis of 33.1 cases per 100,000 live births, a 39.7% rise compared with data from 2017.6
Those most at risk. Risk factors for syphilis infection include age younger than 30 years, low socioeconomic status, substance abuse, HIV infection, concurrent STIs, and high-risk sexual activity (sex with multiple high-risk partners).3 Additionally, reported rates of primary and secondary syphilis infections, as well as congenital syphilis infections, are more elevated among women who identify as Black, American Indian/Alaska Native, and/or Hispanic.6 Congenital infections in the United States are correlated with a lack of prenatal care, which has been similarly linked with racial and socioeconomic disparities, as well as with untreated mental health and substance use disorders and recent immigration to the United States.5,7
Continue to: The many phases of syphilis...
The many phases of syphilis
The characteristic lesion of primary syphilis is a chancre, which is a painless, ulcerative lesion with raised borders and a clean, indurated base appearing at the site of spirochete entry (FIGURE 1). Chancres most commonly appear in the genital area, with the most frequent sites in females being within the vaginal canal or on the cervix. Primary chancres tend to heal spontaneously within 3 to 6 weeks, even without treatment, and frequently are accompanied by painless inguinal lymphadenopathy. Given that the most common chancre sites are not immediately apparent, primary infections in women often go undetected.3 In fact, it is essential for clinicians to recognize that, in our routine practice, most patients with syphilis will not be symptomatic at all, and the diagnosis will only be made by serologic screening.

Following resolution of the primary phase, the patient may enter the secondary stage of T pallidum infection. During this stage, spirochetes may disseminate throughout the bloodstream to infect all major organ systems. The principal manifestations of secondary syphilis include a diffuse maculopapular rash that begins on the trunk and proximal extremities and spreads to include the palms and soles (FIGURE 2); mucosal lesions, such as mucous patches and condyloma lata (FIGURE 3); nonscarring alopecia; periostitis; generalized lymphadenopathy; and, in some cases, hepatitis or nephritis.1,3

Secondary syphilis usually clears within 2 to 6 weeks, with the patient then entering the early latent stage of syphilis. During this period, up to 25% of patients are subject to flares of secondary syphilitic lesions but otherwise are asymptomatic.1,3,4 These recurrences tend to occur within 1 year, hence the distinction between early and late latent stages. Once a year has passed, patients are not contagious by sexual transmission and are unlikely to suffer a relapse of secondary symptoms.1,3 However, late latent syphilis is characterized by periods of intermittent bacteremia that allow for seeding of the placenta and infection in about 10% of fetuses.5
Untreated, about 40% of patients will progress to the tertiary stage of syphilis, which is characterized by gummas affecting the skin and mucous membranes (FIGURE 4) and cardiovascular manifestations including arterial aneurysms and aortic insufficiency.3
Neurologic manifestations of syphilis may arise during any of the above stages, though the most characteristic manifestations tend to appear decades after the primary infection. Early neurosyphilis may present as meningitis, with or without concomitant ocular syphilis (uveitis, retinitis) and/or as otic syphilis (hearing loss, persistent tinnitus).1,5 Patients with late (tertiary) neurosyphilis tend to exhibit meningovascular symptoms similar to stroke (aphasia, hemiplegia, seizures) and/or parenchymal effects such as general paresis. Tabes dorsalis (manifestations of which include urinary and rectal incontinence, lightning pains, and ataxia) is a late-onset manifestation.1,3
Congenital syphilis can be subdivided into an early and late stage. The first stage, in which clinical findings occur within the first 2 years of life, commonly features a desquamating rash, hepatomegaly, and rhinitis. Anemia, thrombocytopenia, periostitis, and osteomyelitis also have been documented.5 Of note, two-thirds of infants are asymptomatic at birth and may not develop such clinical manifestations for 3 to 8 weeks.3 If untreated, early congenital infection may progress to late manifestations, such as Hutchinson teeth, mulberry molars, interstitial keratitis, deafness, saddle nose, saber shins, and such neurologic abnormalities as developmental delay and general paresis.3
Continue to: Prenatal screening and diagnosis...
Prenatal screening and diagnosis
Current recommendations issued by the CDC and the American College of Obstetricians and Gynecologists state that all pregnant women should be screened for syphilis infection at their first presentation to care, with repeat screening between 28 and 32 weeks of gestation and at birth, for women living in areas with a high prevalence of syphilis and/or with any of the aforementioned risk factors.3,5 Given that providers may be unfamiliar with the prevalence of syphilis in their area, and that patients may acquire or develop an infection later on in their pregnancy, researchers have begun to investigate the feasibility of universal third-trimester screening. While the cost-effectiveness of such a protocol is disputed, recent studies suggest that it may result in a substantial decrease in adverse maternal and fetal outcomes.8,9
Diagnostic tests
The traditional algorithm for the diagnosis of syphilis infection begins with a nontreponemal screening test, such as the RPR or the Venereal Disease Research Laboratory test. If positive, these screening tests are followed by a confirmatory treponemal test, such as the

The “reverse” screening algorithm begins with the FTA and, if positive, reflexes to the RPR. A reactive RPR indicates an active infection, and the patient should be treated. A negative RPR should be followed by the TP-PA to rule out a false-positive immunoglobulin G test. If the TP-PA test result is positive, the diagnosis of syphilis is confirmed (FIGURE 6). It is crucial to understand, however, that treponemal antibodies will remain positive for a patient’s lifetime, and someone who may have been treated for syphilis in the past also will screen positive. Once 2 treponemal tests are positive, physicians should take a careful history to assess prior infection risk and treatment status. A negative TP-PA excludes a diagnosis of syphilis.

Advantages of the reverse screening algorithm. Nontreponemal tests are inexpensive and easy to perform, and titers allow for identification of a baseline to evaluate response to treatment.11 However, given the fluctuation of RPR sensitivity (depending on stage of disease and a decreased ability to detect primary and latent stages of syphilis), there has been a resurgence of interest in the reverse algorithm.11 While reverse screening has been found to incur higher costs, and may result in overtreatment and increased stress due to false-positive results,12 there is evidence to suggest that this algorithm is more sensitive for primary and latent infections.8,11,13-15
Given the rise in prevalence of syphilis infections in the United States over the past decade, and therefore a higher pretest probability of syphilis in the population, we favor the reverse screening algorithm in obstetrics, particularly given the risks of adverse maternal and fetal outcomes.
Treating syphilis in pregnancy
Parenteral benzathine penicillin G is the only currently recommended medication for the treatment of syphilis in pregnancy. This drug is effective in treating maternal infection and in preventing fetal infections, as well as in treating established fetal infections.3,5 Regimens differ depending on the stage of syphilis infection (TABLE). Treatment for presumed early syphilis is recommended for women who have had sexual contact with a partner diagnosed with primary, secondary, or early latent syphilis within 3 months of their current pregnancy.5 Any patient with diagnosed syphilis who demonstrates clinical signs of neurologic involvement should undergo lumbar puncture to assess for evidence of neurosyphilis.3 CDC guidelines recommend that patients who report an allergy to penicillin undergo desensitization therapy in a controlled setting, as other antibiotics that have been investigated in the treatment of syphilis are either not appropriate due to teratogenicity or due to suboptimal fetal treatment.3,5

Syphilotherapy may lead to the Jarisch-Herxheimer reaction, which is an acute systemic reaction to inflammatory cytokines produced in response to lipopolysaccharide released by dying spirochetes.5 This reaction is characterized by fever, chills, myalgia, headache, hypotension, and worsening of cutaneous lesions. Preterm labor and delivery and fetal heart rate tracing abnormalities also have been documented in pregnant women experiencing this reaction, particularly during the second half of pregnancy.16 Prior to the start of treatment, a detailed sonographic assessment should be performed to assess the fetus for signs of early syphilis, including hepatomegaly, elevated peak systolic velocity of the middle cerebral artery (indicative of fetal anemia), polyhydramnios, placentomegaly, or hydrops.5,7
CASE Resolved
The combination of the patient’s test results—positive FTA, negative RPR, and negative TP-PA—suggest a false-positive treponemal assay. This sequence of tests excludes a diagnosis of syphilis; therefore, no treatment is necessary. Depending on the prevalence of syphilis in the patient’s geographic location, as well as her sexual history, rescreening between 28 and 32 weeks may be warranted. ●
- Ghanem KG, Ram S, Rice PA. The modern epidemic of syphilis. N Engl J Med. 2020;382:845-854.
- Barnett R. Syphilis. Lancet. 2018;391:1471.
- Duff P. Maternal and fetal infections. In: Resnik R, Lockwood CJ, Moore T, et al. Creasy and Resnik's Maternal-Fetal Medicine: Principles and Practice. 8th ed. Philadelphia, PA: Elsevier; 2018:862-919.
- Gomez GB, Kamb ML, Newman LM, et al. Untreated maternal syphilis and adverse outcomes of pregnancy: a systematic review and meta-analysis. Bull World Health Organ. 2013;91:217-226.
- Adhikari EH. Syphilis in pregnancy. Obstet Gynecol. 2020;135:1121-1135.
- Syphilis. CDC website. https://www.cdc.gov/std/stats18/syphilis.htm. Published October 1, 2019. Accessed October 6, 2020.
- Rac MF, Revell PA, Eppes CS. Syphilis during pregnancy: a preventable threat to maternal-fetal health. Am J Obstet Gynecol. 2017;4:352-363.
- Dunseth CD, Ford BA, Krasowski MD. Traditional versus reverse syphilis algorithms: a comparison at a large academic medical center. Pract Lab Med. 2017;8:52-59.
- Hersh AR, Megli CJ, Caughey AB. Repeat screening for syphilis in the third trimester of pregnancy: a cost-effectiveness analysis. Obstet Gynecol. 2018;132:699-706.
- Albright CM, Emerson JB, Werner EF, et al. Third trimester prenatal syphilis screening: a cost-effectiveness analysis. Obstet Gynecol. 2015;126:479-485.
- Seña AC, White BL, Sparling PF. Novel Treponema pallidum serologic tests: a paradigm shift in syphilis screening for the 21st century. Clin Infect Dis. 2010;51:700-708.
- Owusu-Edusei K Jr, Peterman TA, Ballard RC. Serologic testing for syphilis in the United States: a cost-effectiveness analysis of two screening algorithms. Sex Transm Dis. 2011;38:1-7.
- Huh HJ, Chung JW, Park SY, et al. Comparison of automated treponemal and nontreponemal test algorithms as first-line syphilis screening assays. Ann Lab Med. 2016;36:23-27.
- Centers for Disease Control and Prevention. Syphilis testing algorithms using treponemal test for initial screening-four laboratories. New York City, 2005-2006. MMWR Morb Mortal Wkly Rep. 2008;57:872-875.
- Mishra S, Boily MC, Ng V, et al. The laboratory impact of changing syphilis screening from the rapid-plasma reagin to a treponemal enzyme immunoassay: a case-study from the greater Toronto area. Sex Transm Dis. 2011;38:190-196.
- Klein VR, Cox SM, Mitchell MD, et al. The Jarisch-Herzheimer reaction complicating syphilotherapy in pregnancy. Obstet Gynecol. 1990;75:375-380.
CASE Pregnant woman with positive Treponema pallidum antibody test
A 30-year-old primigravida at 10 weeks and 4 days of gestation by her last menstrual period presents to your office for her initial prenatal visit. She expresses no concerns. You order the standard set of laboratory tests, including a sexually transmitted infection (STI) screening panel. Consistent with your institution’s use of the reverse algorithm for syphilis screening, you obtain a Treponema pallidum antibody test, which reflexes to the rapid plasma reagin (RPR) test. Three days later, you receive a notification that this patient’s T pallidum antibody result was positive, followed by negative RPR test results. The follow-up T pallidum particle agglutination (TP-PA) test also was negative. Given these findings, you consider:
- What is the correct interpretation of the patient’s sequence of test results?
- Is she infected, and does she require treatment?
Meet our perpetrator
Syphilis has plagued society since the late 15th century, although its causative agent, the spirochete T pallidum, was not recognized until 1905.1,2T pallidum bacteria are transmitted via sexual contact, as well as through vertical transmission during pregnancy or delivery. Infection with syphilis is reported in 50% to 60% of sexual partners after a single exposure to an infected individual with early syphilis, and the mean incubation period is 21 days.3T pallidum can cross the placenta and infect a fetus as early as the sixth week of gestation.3 Congenital syphilis infections occur in the neonates of 50% to 80% of women with untreated primary, secondary, or early latent syphilis infections; maternal syphilis is associated with a 21% increased risk of stillbirth, a 6% increased risk of preterm delivery, and a 9% increased risk of neonatal death.4,5 Additionally, syphilis infection is associated with a high risk of HIV infection, as well as coinfection with other STIs.1
Given the highly infective nature of T pallidum, as well as the severity of the potential consequences of infection for both mothers and babies, primary prevention, education of at-risk populations, and early recognition of clinical features of syphilis infection are of utmost importance in preventing morbidity and mortality. In this article, we review the epidemiology and extensive clinical manifestations of syphilis, as well as current screening recommendations and treatment for pregnant women.
The extent of the problem today
Although US rates of syphilis have ebbed and flowed for the past several decades, the current incidence has grown exponentially in recent years, with the number of cases reported to the Centers for Disease Control and Prevention (CDC) increasing by 71% from 2014 to 2018.6 During this time period, reported cases of primary and secondary syphilis in women more than doubled (172.7% and 165.4%, respectively) according to CDC data, accompanied by a parallel rise in reported cases of congenital syphilis in both live and stillborn infants.6 In 2018, the CDC reported a national rate of congenital syphilis of 33.1 cases per 100,000 live births, a 39.7% rise compared with data from 2017.6
Those most at risk. Risk factors for syphilis infection include age younger than 30 years, low socioeconomic status, substance abuse, HIV infection, concurrent STIs, and high-risk sexual activity (sex with multiple high-risk partners).3 Additionally, reported rates of primary and secondary syphilis infections, as well as congenital syphilis infections, are more elevated among women who identify as Black, American Indian/Alaska Native, and/or Hispanic.6 Congenital infections in the United States are correlated with a lack of prenatal care, which has been similarly linked with racial and socioeconomic disparities, as well as with untreated mental health and substance use disorders and recent immigration to the United States.5,7
Continue to: The many phases of syphilis...
The many phases of syphilis
The characteristic lesion of primary syphilis is a chancre, which is a painless, ulcerative lesion with raised borders and a clean, indurated base appearing at the site of spirochete entry (FIGURE 1). Chancres most commonly appear in the genital area, with the most frequent sites in females being within the vaginal canal or on the cervix. Primary chancres tend to heal spontaneously within 3 to 6 weeks, even without treatment, and frequently are accompanied by painless inguinal lymphadenopathy. Given that the most common chancre sites are not immediately apparent, primary infections in women often go undetected.3 In fact, it is essential for clinicians to recognize that, in our routine practice, most patients with syphilis will not be symptomatic at all, and the diagnosis will only be made by serologic screening.

Following resolution of the primary phase, the patient may enter the secondary stage of T pallidum infection. During this stage, spirochetes may disseminate throughout the bloodstream to infect all major organ systems. The principal manifestations of secondary syphilis include a diffuse maculopapular rash that begins on the trunk and proximal extremities and spreads to include the palms and soles (FIGURE 2); mucosal lesions, such as mucous patches and condyloma lata (FIGURE 3); nonscarring alopecia; periostitis; generalized lymphadenopathy; and, in some cases, hepatitis or nephritis.1,3

Secondary syphilis usually clears within 2 to 6 weeks, with the patient then entering the early latent stage of syphilis. During this period, up to 25% of patients are subject to flares of secondary syphilitic lesions but otherwise are asymptomatic.1,3,4 These recurrences tend to occur within 1 year, hence the distinction between early and late latent stages. Once a year has passed, patients are not contagious by sexual transmission and are unlikely to suffer a relapse of secondary symptoms.1,3 However, late latent syphilis is characterized by periods of intermittent bacteremia that allow for seeding of the placenta and infection in about 10% of fetuses.5
Untreated, about 40% of patients will progress to the tertiary stage of syphilis, which is characterized by gummas affecting the skin and mucous membranes (FIGURE 4) and cardiovascular manifestations including arterial aneurysms and aortic insufficiency.3
Neurologic manifestations of syphilis may arise during any of the above stages, though the most characteristic manifestations tend to appear decades after the primary infection. Early neurosyphilis may present as meningitis, with or without concomitant ocular syphilis (uveitis, retinitis) and/or as otic syphilis (hearing loss, persistent tinnitus).1,5 Patients with late (tertiary) neurosyphilis tend to exhibit meningovascular symptoms similar to stroke (aphasia, hemiplegia, seizures) and/or parenchymal effects such as general paresis. Tabes dorsalis (manifestations of which include urinary and rectal incontinence, lightning pains, and ataxia) is a late-onset manifestation.1,3
Congenital syphilis can be subdivided into an early and late stage. The first stage, in which clinical findings occur within the first 2 years of life, commonly features a desquamating rash, hepatomegaly, and rhinitis. Anemia, thrombocytopenia, periostitis, and osteomyelitis also have been documented.5 Of note, two-thirds of infants are asymptomatic at birth and may not develop such clinical manifestations for 3 to 8 weeks.3 If untreated, early congenital infection may progress to late manifestations, such as Hutchinson teeth, mulberry molars, interstitial keratitis, deafness, saddle nose, saber shins, and such neurologic abnormalities as developmental delay and general paresis.3
Continue to: Prenatal screening and diagnosis...
Prenatal screening and diagnosis
Current recommendations issued by the CDC and the American College of Obstetricians and Gynecologists state that all pregnant women should be screened for syphilis infection at their first presentation to care, with repeat screening between 28 and 32 weeks of gestation and at birth, for women living in areas with a high prevalence of syphilis and/or with any of the aforementioned risk factors.3,5 Given that providers may be unfamiliar with the prevalence of syphilis in their area, and that patients may acquire or develop an infection later on in their pregnancy, researchers have begun to investigate the feasibility of universal third-trimester screening. While the cost-effectiveness of such a protocol is disputed, recent studies suggest that it may result in a substantial decrease in adverse maternal and fetal outcomes.8,9
Diagnostic tests
The traditional algorithm for the diagnosis of syphilis infection begins with a nontreponemal screening test, such as the RPR or the Venereal Disease Research Laboratory test. If positive, these screening tests are followed by a confirmatory treponemal test, such as the

The “reverse” screening algorithm begins with the FTA and, if positive, reflexes to the RPR. A reactive RPR indicates an active infection, and the patient should be treated. A negative RPR should be followed by the TP-PA to rule out a false-positive immunoglobulin G test. If the TP-PA test result is positive, the diagnosis of syphilis is confirmed (FIGURE 6). It is crucial to understand, however, that treponemal antibodies will remain positive for a patient’s lifetime, and someone who may have been treated for syphilis in the past also will screen positive. Once 2 treponemal tests are positive, physicians should take a careful history to assess prior infection risk and treatment status. A negative TP-PA excludes a diagnosis of syphilis.

Advantages of the reverse screening algorithm. Nontreponemal tests are inexpensive and easy to perform, and titers allow for identification of a baseline to evaluate response to treatment.11 However, given the fluctuation of RPR sensitivity (depending on stage of disease and a decreased ability to detect primary and latent stages of syphilis), there has been a resurgence of interest in the reverse algorithm.11 While reverse screening has been found to incur higher costs, and may result in overtreatment and increased stress due to false-positive results,12 there is evidence to suggest that this algorithm is more sensitive for primary and latent infections.8,11,13-15
Given the rise in prevalence of syphilis infections in the United States over the past decade, and therefore a higher pretest probability of syphilis in the population, we favor the reverse screening algorithm in obstetrics, particularly given the risks of adverse maternal and fetal outcomes.
Treating syphilis in pregnancy
Parenteral benzathine penicillin G is the only currently recommended medication for the treatment of syphilis in pregnancy. This drug is effective in treating maternal infection and in preventing fetal infections, as well as in treating established fetal infections.3,5 Regimens differ depending on the stage of syphilis infection (TABLE). Treatment for presumed early syphilis is recommended for women who have had sexual contact with a partner diagnosed with primary, secondary, or early latent syphilis within 3 months of their current pregnancy.5 Any patient with diagnosed syphilis who demonstrates clinical signs of neurologic involvement should undergo lumbar puncture to assess for evidence of neurosyphilis.3 CDC guidelines recommend that patients who report an allergy to penicillin undergo desensitization therapy in a controlled setting, as other antibiotics that have been investigated in the treatment of syphilis are either not appropriate due to teratogenicity or due to suboptimal fetal treatment.3,5

Syphilotherapy may lead to the Jarisch-Herxheimer reaction, which is an acute systemic reaction to inflammatory cytokines produced in response to lipopolysaccharide released by dying spirochetes.5 This reaction is characterized by fever, chills, myalgia, headache, hypotension, and worsening of cutaneous lesions. Preterm labor and delivery and fetal heart rate tracing abnormalities also have been documented in pregnant women experiencing this reaction, particularly during the second half of pregnancy.16 Prior to the start of treatment, a detailed sonographic assessment should be performed to assess the fetus for signs of early syphilis, including hepatomegaly, elevated peak systolic velocity of the middle cerebral artery (indicative of fetal anemia), polyhydramnios, placentomegaly, or hydrops.5,7
CASE Resolved
The combination of the patient’s test results—positive FTA, negative RPR, and negative TP-PA—suggest a false-positive treponemal assay. This sequence of tests excludes a diagnosis of syphilis; therefore, no treatment is necessary. Depending on the prevalence of syphilis in the patient’s geographic location, as well as her sexual history, rescreening between 28 and 32 weeks may be warranted. ●
CASE Pregnant woman with positive Treponema pallidum antibody test
A 30-year-old primigravida at 10 weeks and 4 days of gestation by her last menstrual period presents to your office for her initial prenatal visit. She expresses no concerns. You order the standard set of laboratory tests, including a sexually transmitted infection (STI) screening panel. Consistent with your institution’s use of the reverse algorithm for syphilis screening, you obtain a Treponema pallidum antibody test, which reflexes to the rapid plasma reagin (RPR) test. Three days later, you receive a notification that this patient’s T pallidum antibody result was positive, followed by negative RPR test results. The follow-up T pallidum particle agglutination (TP-PA) test also was negative. Given these findings, you consider:
- What is the correct interpretation of the patient’s sequence of test results?
- Is she infected, and does she require treatment?
Meet our perpetrator
Syphilis has plagued society since the late 15th century, although its causative agent, the spirochete T pallidum, was not recognized until 1905.1,2T pallidum bacteria are transmitted via sexual contact, as well as through vertical transmission during pregnancy or delivery. Infection with syphilis is reported in 50% to 60% of sexual partners after a single exposure to an infected individual with early syphilis, and the mean incubation period is 21 days.3T pallidum can cross the placenta and infect a fetus as early as the sixth week of gestation.3 Congenital syphilis infections occur in the neonates of 50% to 80% of women with untreated primary, secondary, or early latent syphilis infections; maternal syphilis is associated with a 21% increased risk of stillbirth, a 6% increased risk of preterm delivery, and a 9% increased risk of neonatal death.4,5 Additionally, syphilis infection is associated with a high risk of HIV infection, as well as coinfection with other STIs.1
Given the highly infective nature of T pallidum, as well as the severity of the potential consequences of infection for both mothers and babies, primary prevention, education of at-risk populations, and early recognition of clinical features of syphilis infection are of utmost importance in preventing morbidity and mortality. In this article, we review the epidemiology and extensive clinical manifestations of syphilis, as well as current screening recommendations and treatment for pregnant women.
The extent of the problem today
Although US rates of syphilis have ebbed and flowed for the past several decades, the current incidence has grown exponentially in recent years, with the number of cases reported to the Centers for Disease Control and Prevention (CDC) increasing by 71% from 2014 to 2018.6 During this time period, reported cases of primary and secondary syphilis in women more than doubled (172.7% and 165.4%, respectively) according to CDC data, accompanied by a parallel rise in reported cases of congenital syphilis in both live and stillborn infants.6 In 2018, the CDC reported a national rate of congenital syphilis of 33.1 cases per 100,000 live births, a 39.7% rise compared with data from 2017.6
Those most at risk. Risk factors for syphilis infection include age younger than 30 years, low socioeconomic status, substance abuse, HIV infection, concurrent STIs, and high-risk sexual activity (sex with multiple high-risk partners).3 Additionally, reported rates of primary and secondary syphilis infections, as well as congenital syphilis infections, are more elevated among women who identify as Black, American Indian/Alaska Native, and/or Hispanic.6 Congenital infections in the United States are correlated with a lack of prenatal care, which has been similarly linked with racial and socioeconomic disparities, as well as with untreated mental health and substance use disorders and recent immigration to the United States.5,7
Continue to: The many phases of syphilis...
The many phases of syphilis
The characteristic lesion of primary syphilis is a chancre, which is a painless, ulcerative lesion with raised borders and a clean, indurated base appearing at the site of spirochete entry (FIGURE 1). Chancres most commonly appear in the genital area, with the most frequent sites in females being within the vaginal canal or on the cervix. Primary chancres tend to heal spontaneously within 3 to 6 weeks, even without treatment, and frequently are accompanied by painless inguinal lymphadenopathy. Given that the most common chancre sites are not immediately apparent, primary infections in women often go undetected.3 In fact, it is essential for clinicians to recognize that, in our routine practice, most patients with syphilis will not be symptomatic at all, and the diagnosis will only be made by serologic screening.

Following resolution of the primary phase, the patient may enter the secondary stage of T pallidum infection. During this stage, spirochetes may disseminate throughout the bloodstream to infect all major organ systems. The principal manifestations of secondary syphilis include a diffuse maculopapular rash that begins on the trunk and proximal extremities and spreads to include the palms and soles (FIGURE 2); mucosal lesions, such as mucous patches and condyloma lata (FIGURE 3); nonscarring alopecia; periostitis; generalized lymphadenopathy; and, in some cases, hepatitis or nephritis.1,3

Secondary syphilis usually clears within 2 to 6 weeks, with the patient then entering the early latent stage of syphilis. During this period, up to 25% of patients are subject to flares of secondary syphilitic lesions but otherwise are asymptomatic.1,3,4 These recurrences tend to occur within 1 year, hence the distinction between early and late latent stages. Once a year has passed, patients are not contagious by sexual transmission and are unlikely to suffer a relapse of secondary symptoms.1,3 However, late latent syphilis is characterized by periods of intermittent bacteremia that allow for seeding of the placenta and infection in about 10% of fetuses.5
Untreated, about 40% of patients will progress to the tertiary stage of syphilis, which is characterized by gummas affecting the skin and mucous membranes (FIGURE 4) and cardiovascular manifestations including arterial aneurysms and aortic insufficiency.3
Neurologic manifestations of syphilis may arise during any of the above stages, though the most characteristic manifestations tend to appear decades after the primary infection. Early neurosyphilis may present as meningitis, with or without concomitant ocular syphilis (uveitis, retinitis) and/or as otic syphilis (hearing loss, persistent tinnitus).1,5 Patients with late (tertiary) neurosyphilis tend to exhibit meningovascular symptoms similar to stroke (aphasia, hemiplegia, seizures) and/or parenchymal effects such as general paresis. Tabes dorsalis (manifestations of which include urinary and rectal incontinence, lightning pains, and ataxia) is a late-onset manifestation.1,3
Congenital syphilis can be subdivided into an early and late stage. The first stage, in which clinical findings occur within the first 2 years of life, commonly features a desquamating rash, hepatomegaly, and rhinitis. Anemia, thrombocytopenia, periostitis, and osteomyelitis also have been documented.5 Of note, two-thirds of infants are asymptomatic at birth and may not develop such clinical manifestations for 3 to 8 weeks.3 If untreated, early congenital infection may progress to late manifestations, such as Hutchinson teeth, mulberry molars, interstitial keratitis, deafness, saddle nose, saber shins, and such neurologic abnormalities as developmental delay and general paresis.3
Continue to: Prenatal screening and diagnosis...
Prenatal screening and diagnosis
Current recommendations issued by the CDC and the American College of Obstetricians and Gynecologists state that all pregnant women should be screened for syphilis infection at their first presentation to care, with repeat screening between 28 and 32 weeks of gestation and at birth, for women living in areas with a high prevalence of syphilis and/or with any of the aforementioned risk factors.3,5 Given that providers may be unfamiliar with the prevalence of syphilis in their area, and that patients may acquire or develop an infection later on in their pregnancy, researchers have begun to investigate the feasibility of universal third-trimester screening. While the cost-effectiveness of such a protocol is disputed, recent studies suggest that it may result in a substantial decrease in adverse maternal and fetal outcomes.8,9
Diagnostic tests
The traditional algorithm for the diagnosis of syphilis infection begins with a nontreponemal screening test, such as the RPR or the Venereal Disease Research Laboratory test. If positive, these screening tests are followed by a confirmatory treponemal test, such as the

The “reverse” screening algorithm begins with the FTA and, if positive, reflexes to the RPR. A reactive RPR indicates an active infection, and the patient should be treated. A negative RPR should be followed by the TP-PA to rule out a false-positive immunoglobulin G test. If the TP-PA test result is positive, the diagnosis of syphilis is confirmed (FIGURE 6). It is crucial to understand, however, that treponemal antibodies will remain positive for a patient’s lifetime, and someone who may have been treated for syphilis in the past also will screen positive. Once 2 treponemal tests are positive, physicians should take a careful history to assess prior infection risk and treatment status. A negative TP-PA excludes a diagnosis of syphilis.

Advantages of the reverse screening algorithm. Nontreponemal tests are inexpensive and easy to perform, and titers allow for identification of a baseline to evaluate response to treatment.11 However, given the fluctuation of RPR sensitivity (depending on stage of disease and a decreased ability to detect primary and latent stages of syphilis), there has been a resurgence of interest in the reverse algorithm.11 While reverse screening has been found to incur higher costs, and may result in overtreatment and increased stress due to false-positive results,12 there is evidence to suggest that this algorithm is more sensitive for primary and latent infections.8,11,13-15
Given the rise in prevalence of syphilis infections in the United States over the past decade, and therefore a higher pretest probability of syphilis in the population, we favor the reverse screening algorithm in obstetrics, particularly given the risks of adverse maternal and fetal outcomes.
Treating syphilis in pregnancy
Parenteral benzathine penicillin G is the only currently recommended medication for the treatment of syphilis in pregnancy. This drug is effective in treating maternal infection and in preventing fetal infections, as well as in treating established fetal infections.3,5 Regimens differ depending on the stage of syphilis infection (TABLE). Treatment for presumed early syphilis is recommended for women who have had sexual contact with a partner diagnosed with primary, secondary, or early latent syphilis within 3 months of their current pregnancy.5 Any patient with diagnosed syphilis who demonstrates clinical signs of neurologic involvement should undergo lumbar puncture to assess for evidence of neurosyphilis.3 CDC guidelines recommend that patients who report an allergy to penicillin undergo desensitization therapy in a controlled setting, as other antibiotics that have been investigated in the treatment of syphilis are either not appropriate due to teratogenicity or due to suboptimal fetal treatment.3,5

Syphilotherapy may lead to the Jarisch-Herxheimer reaction, which is an acute systemic reaction to inflammatory cytokines produced in response to lipopolysaccharide released by dying spirochetes.5 This reaction is characterized by fever, chills, myalgia, headache, hypotension, and worsening of cutaneous lesions. Preterm labor and delivery and fetal heart rate tracing abnormalities also have been documented in pregnant women experiencing this reaction, particularly during the second half of pregnancy.16 Prior to the start of treatment, a detailed sonographic assessment should be performed to assess the fetus for signs of early syphilis, including hepatomegaly, elevated peak systolic velocity of the middle cerebral artery (indicative of fetal anemia), polyhydramnios, placentomegaly, or hydrops.5,7
CASE Resolved
The combination of the patient’s test results—positive FTA, negative RPR, and negative TP-PA—suggest a false-positive treponemal assay. This sequence of tests excludes a diagnosis of syphilis; therefore, no treatment is necessary. Depending on the prevalence of syphilis in the patient’s geographic location, as well as her sexual history, rescreening between 28 and 32 weeks may be warranted. ●
- Ghanem KG, Ram S, Rice PA. The modern epidemic of syphilis. N Engl J Med. 2020;382:845-854.
- Barnett R. Syphilis. Lancet. 2018;391:1471.
- Duff P. Maternal and fetal infections. In: Resnik R, Lockwood CJ, Moore T, et al. Creasy and Resnik's Maternal-Fetal Medicine: Principles and Practice. 8th ed. Philadelphia, PA: Elsevier; 2018:862-919.
- Gomez GB, Kamb ML, Newman LM, et al. Untreated maternal syphilis and adverse outcomes of pregnancy: a systematic review and meta-analysis. Bull World Health Organ. 2013;91:217-226.
- Adhikari EH. Syphilis in pregnancy. Obstet Gynecol. 2020;135:1121-1135.
- Syphilis. CDC website. https://www.cdc.gov/std/stats18/syphilis.htm. Published October 1, 2019. Accessed October 6, 2020.
- Rac MF, Revell PA, Eppes CS. Syphilis during pregnancy: a preventable threat to maternal-fetal health. Am J Obstet Gynecol. 2017;4:352-363.
- Dunseth CD, Ford BA, Krasowski MD. Traditional versus reverse syphilis algorithms: a comparison at a large academic medical center. Pract Lab Med. 2017;8:52-59.
- Hersh AR, Megli CJ, Caughey AB. Repeat screening for syphilis in the third trimester of pregnancy: a cost-effectiveness analysis. Obstet Gynecol. 2018;132:699-706.
- Albright CM, Emerson JB, Werner EF, et al. Third trimester prenatal syphilis screening: a cost-effectiveness analysis. Obstet Gynecol. 2015;126:479-485.
- Seña AC, White BL, Sparling PF. Novel Treponema pallidum serologic tests: a paradigm shift in syphilis screening for the 21st century. Clin Infect Dis. 2010;51:700-708.
- Owusu-Edusei K Jr, Peterman TA, Ballard RC. Serologic testing for syphilis in the United States: a cost-effectiveness analysis of two screening algorithms. Sex Transm Dis. 2011;38:1-7.
- Huh HJ, Chung JW, Park SY, et al. Comparison of automated treponemal and nontreponemal test algorithms as first-line syphilis screening assays. Ann Lab Med. 2016;36:23-27.
- Centers for Disease Control and Prevention. Syphilis testing algorithms using treponemal test for initial screening-four laboratories. New York City, 2005-2006. MMWR Morb Mortal Wkly Rep. 2008;57:872-875.
- Mishra S, Boily MC, Ng V, et al. The laboratory impact of changing syphilis screening from the rapid-plasma reagin to a treponemal enzyme immunoassay: a case-study from the greater Toronto area. Sex Transm Dis. 2011;38:190-196.
- Klein VR, Cox SM, Mitchell MD, et al. The Jarisch-Herzheimer reaction complicating syphilotherapy in pregnancy. Obstet Gynecol. 1990;75:375-380.
- Ghanem KG, Ram S, Rice PA. The modern epidemic of syphilis. N Engl J Med. 2020;382:845-854.
- Barnett R. Syphilis. Lancet. 2018;391:1471.
- Duff P. Maternal and fetal infections. In: Resnik R, Lockwood CJ, Moore T, et al. Creasy and Resnik's Maternal-Fetal Medicine: Principles and Practice. 8th ed. Philadelphia, PA: Elsevier; 2018:862-919.
- Gomez GB, Kamb ML, Newman LM, et al. Untreated maternal syphilis and adverse outcomes of pregnancy: a systematic review and meta-analysis. Bull World Health Organ. 2013;91:217-226.
- Adhikari EH. Syphilis in pregnancy. Obstet Gynecol. 2020;135:1121-1135.
- Syphilis. CDC website. https://www.cdc.gov/std/stats18/syphilis.htm. Published October 1, 2019. Accessed October 6, 2020.
- Rac MF, Revell PA, Eppes CS. Syphilis during pregnancy: a preventable threat to maternal-fetal health. Am J Obstet Gynecol. 2017;4:352-363.
- Dunseth CD, Ford BA, Krasowski MD. Traditional versus reverse syphilis algorithms: a comparison at a large academic medical center. Pract Lab Med. 2017;8:52-59.
- Hersh AR, Megli CJ, Caughey AB. Repeat screening for syphilis in the third trimester of pregnancy: a cost-effectiveness analysis. Obstet Gynecol. 2018;132:699-706.
- Albright CM, Emerson JB, Werner EF, et al. Third trimester prenatal syphilis screening: a cost-effectiveness analysis. Obstet Gynecol. 2015;126:479-485.
- Seña AC, White BL, Sparling PF. Novel Treponema pallidum serologic tests: a paradigm shift in syphilis screening for the 21st century. Clin Infect Dis. 2010;51:700-708.
- Owusu-Edusei K Jr, Peterman TA, Ballard RC. Serologic testing for syphilis in the United States: a cost-effectiveness analysis of two screening algorithms. Sex Transm Dis. 2011;38:1-7.
- Huh HJ, Chung JW, Park SY, et al. Comparison of automated treponemal and nontreponemal test algorithms as first-line syphilis screening assays. Ann Lab Med. 2016;36:23-27.
- Centers for Disease Control and Prevention. Syphilis testing algorithms using treponemal test for initial screening-four laboratories. New York City, 2005-2006. MMWR Morb Mortal Wkly Rep. 2008;57:872-875.
- Mishra S, Boily MC, Ng V, et al. The laboratory impact of changing syphilis screening from the rapid-plasma reagin to a treponemal enzyme immunoassay: a case-study from the greater Toronto area. Sex Transm Dis. 2011;38:190-196.
- Klein VR, Cox SM, Mitchell MD, et al. The Jarisch-Herzheimer reaction complicating syphilotherapy in pregnancy. Obstet Gynecol. 1990;75:375-380.
Laparoscopic specimen retrieval bags in gyn surgery: Expert guidance on selection
The use of minimally invasive gynecologic surgery (MIGS) has grown rapidly over the past 20 years. MIGS, which includes vaginal hysterectomy and laparoscopic hysterectomy, is safe and has fewer complications and a more rapid recovery period than open abdominal surgery.1,2 In 2005, the role of MIGS was expanded further when the US Food and Drug Administration (FDA) approved robot-assisted surgery for the performance of gynecologic procedures.3 As knowledge and experience in the safe performance of MIGS progresses, the rates for MIGS procedures have skyrocketed and continue to grow. Between 2007 and 2010, laparoscopic hysterectomy rates rose from 23.5% to 30.5%, while robot-assisted laparoscopic hysterectomy rates increased from 0.5% to 9.5%, representing 40% of all hysterectomies.4 Due to the benefits of minimally invasive surgery over open abdominal surgery, patient and physician preference for minimally invasive procedures has grown significantly in popularity.1,5
Because incisions are small in minimally invasive surgery, surgeons have been challenged with removing large specimens through incisions that are much smaller than the presenting pathology. One approach is to use a specimen retrieval bag for specimen extraction. Once the dissection is completed, the specimen is placed within the retrieval bag for removal, thus minimizing exposure of the specimen and its contents to the abdominopelvic cavity and incision.
The use of specimen retrieval devices has been advocated to prevent infection, avoid spillage into the peritoneal cavity, and minimize the risk of port-site metastases in cases of potentially cancerous specimens. Devices include affordable and readily available products, such as nonpowdered gloves, and commercially produced bags.6
While the use of specimen containment systems for tissue extraction has been well described in gynecology, the available systems vary widely in construction, size, durability, and shape, potentially leading to confusion and suboptimal bag selection during surgery.7 In this article, we review the most common laparoscopic bags available in the United States, provide an overview of bag characteristics, offer practice guidelines for bag selection, and review bag terminology to highlight important concepts for bag selection.
Controversy spurs change
In April 2014, the FDA warned against the use of power morcellation for specimen removal during minimally invasive surgery, citing a prevalence of 1 in 352 unsuspected uterine sarcomas and 1 in 498 unsuspected uterine leiomyosarcomas among women undergoing hysterectomy or myomectomy for presumed benign leiomyoma.8 Since then, the risk of occult uterine sarcomas, including leiomyosarcoma, in women undergoing surgery for benign gynecologic indications has been determined to be much lower.
Nonetheless, the clinical importance of contained specimen removal was clearly highlighted and the role of specimen retrieval bags soared to the forefront. Open power morcellation is no longer commonly practiced, and national societies such as the American Association of Gynecologic Laparoscopists (AAGL), the Society of Gynecologic Oncology (SGO), and the American College of Obstetricians and Gynecologists (ACOG) recommend that containment systems be used for safer specimen retrieval during gynecologic surgery.9-11 After the specimen is placed inside the containment system (typically a specimen bag), the surgeon may deliver the bag through a vaginal colpotomy or through a slightly extended laparoscopic incision to remove bulky specimens using cold-cutting extraction techniques.12-15
Continue to: Know the pathology’s characteristics...
Know the pathology’s characteristics
In most cases, based on imaging studies and physical examination, surgeons have a good idea of what to expect before proceeding with surgery. The 2 most common characteristics used for surgical planning are the specimen size (dimensions) and the tissue type (solid, cystic, soft tissue, or mixed). The mass size can range from less than 1 cm to larger than a 20-week sized fibroid uterus. Assessing the specimen in 3 dimensions is important. Tissue type also is a consideration, as soft and squishy masses, such as ovarian cysts, are easier to deflate and manipulate within the bag compared with solid or calcified tumors, such as a large fibroid uterus or a large dermoid with solid components.
Specimen shape also is a critical determinant for bag selection. Most specimen retrieval bags are tapered to varying degrees, and some have an irregular shape. Long tubular structures, such as fallopian tubes that are composed of soft tissue, fit easily into most bags regardless of bag shape or extent of bag taper, whereas the round shape of a bulky myoma may render certain bags ineffective even if the bag’s entrance accommodates the greatest diameter of the myoma. Often, a round mass will not fully fit into a bag because there is a poor fit between the mass’s shape and the bag’s shape and taper. (We discuss the concept of a poor “fit” below.) Knowing the pathology before starting a procedure can help optimize bag selection, streamline operative flow, and reduce waste.
Overview of laparoscopic bag characteristics and clinical applications
The TABLE lists the most common laparoscopic bags available for purchase in the United States. Details include the trocar size, manufacturer, product name, mouth diameter, volume, bag shape, construction material, and best clinical application.
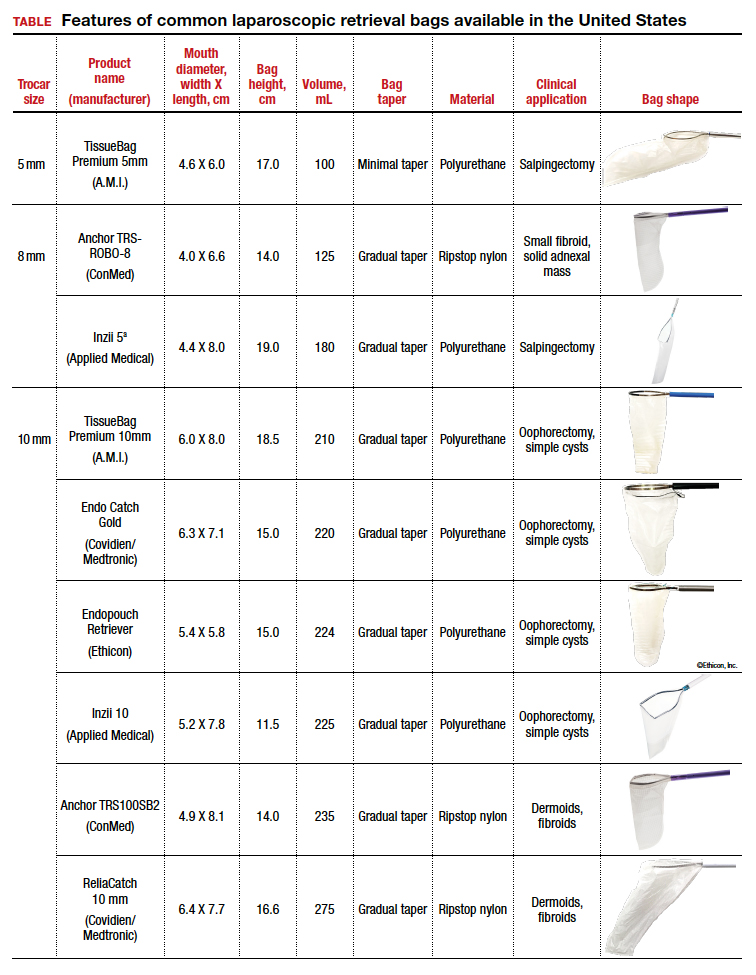
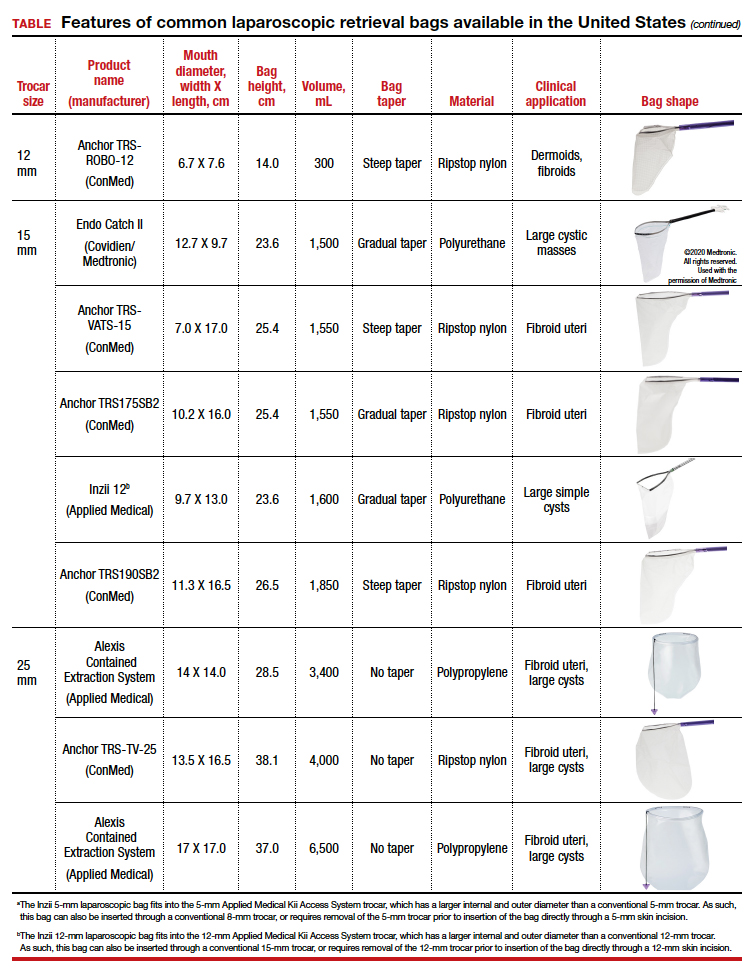
The following are terms used to refer to the components of a laparoscopic retrieval bag:
- Mouth diameter: diameter at the entrance of a fully opened bag (FIGURE 1)
- Bag volume: the total volume a bag can accommodate when completely full
- Bag rim: characteristics of the rim of the bag when opened (that is, rigid vs soft rim, complete vs partial rim mechanism to hold the bag open) (FIGURE 2)
- Bag shape: the shape of the bag when it is fully opened (square shaped vs cone shaped vs curved bag shape) (FIGURE 2)
- Bag taper (severity and type): extent the bag is tapered from the rim of the bag’s entrance to the base of the bag; categorized by taper severity (minimal, gradual, or steep taper) and type (continuous taper or curved taper) (FIGURE 3)
- Ball fit: the maximum spherical specimen size that completely fits into a bag and allows it to cinch closed (FIGURE 4)
- Bag strength: durability of a bag when placed on tension during specimen extraction (weak, moderate, or extremely durable).
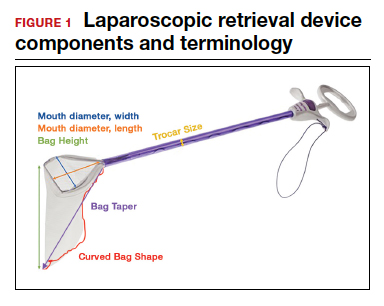

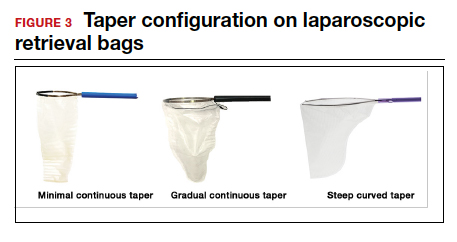
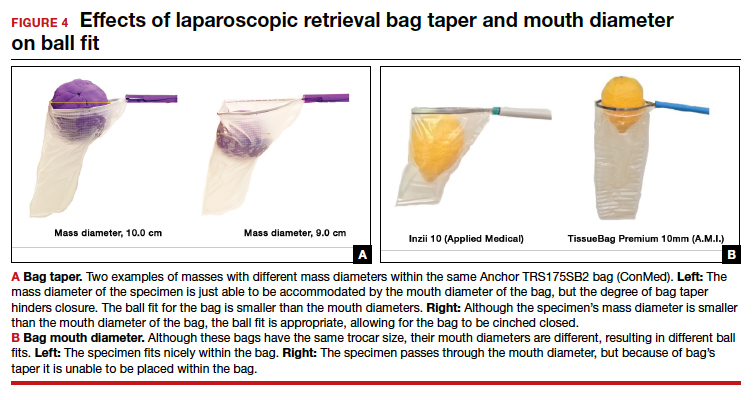
Continue to: Mouth diameter...
Mouth diameter
Bag manufacturers often differentiate bag sizes by indicating “volume” in milliliters. Bag volume, however, offers little clinical value to surgeons, as pelvic mass dimensions are usually measured in centimeters on imaging. Rather, an important characteristic for bag selection is the diameter of the rim of the bag when it is fully opened—the so-called bag mouth diameter. For a specimen to fit, the 2 dimensions of the specimen must be smaller than the dimensions of the bag entrance.
Notably, the number often linked to the specimen bag—as, for example, in the 10-mm Endo Catch bag (Covidien/Medtronic)— describes the width of the shaft of the bag before it is opened rather than the mouth diameter of the opened bag. The number actually correlates with the trocar size necessary for bag insertion rather than with the specimen size that can fit into the bag. Therefore, a 10-mm Endo Catch bag cannot fit a 10-cm mass, but rather requires a trocar size of 10 mm or greater for insertion of the bag. Fully opened, the mouth diameters of the 10-mm Endo Catch bag are roughly 6 cm x 7 cm, which allows for delivery of a 6-cm mass.
Because 2 bags that use the same trocar size for insertion may have vastly differing bag dimensions, the surgeon must know the bag mouth diameters when selecting a bag to remove the presenting pathology. For example, the Inzii 12 (Applied Medical) laparoscopic bag has mouth diameters of 9.7 cm × 13.0 cm, whereas the Anchor TRSROBO-12 (ConMed) has mouth diameters of 6.7 cm × 7.6 cm (TABLE). Although both bags can be inserted through a 12-mm trocar, both bags cannot fit the same size mass for removal.
Shape and taper
Laparoscopic bags come in various shapes (curved, cone, or square shaped), with varying levels of bag taper (steep, gradual, or no taper) (FIGURES 2 and 3). While taper has little impact on long and skinny specimens, taper may hinder successful bagging of bulky or spherical specimens.
Each bag has different grades of taper regardless of mouth diameter or trocar size. For round masses, the steeper the taper, the smaller the mass that can comfortably fit within the bag. This concept is connected to the idea of “ball fit,” explained below.
In addition, bag shape may affect what mass size can fit into the bag. An irregularly shaped curved bag or a bag with a steep taper may be well suited for removal of multiple specimens of varying sizes or soft masses that are malleable enough to conform to the bag’s shape (such as a ruptured ovarian cyst). Alternatively, a square-shaped bag or a bag with minimal taper would better accommodate a round mass.
Ball fit
When thinking about large circular masses, such as myomas or ovarian cysts, one must consider the ball fit. This refers to the maximum spherical size of the specimen that fits completely within a bag while allowing the bag to cinch closed. Generally, this is an estimation that factors in the bag shape, extent of the bag taper, bag mouth diameter, and specimen shape and tissue type. At times, although a mass can fit through the bag’s mouth diameter, a steep taper may prevent the mass from being fully bagged and limit closure of the bag (FIGURE 4).
Curved bags like the Anchor TRSVATS-15 (ConMed), which have a very narrow bottom, are prone to a limited ball fit, and thus the bag mouth diameter will not correlate with the largest mass size that can be fitted within the bag. Therefore, if using a steeply tapered bag for removal of large round masses, do not rely on the bag’s mouth diameter for bag selection. The surgeon must visualize the ball fit within the bag, taking into account the specimen size and shape, bag shape, and bag taper. In these scenarios, using the diameter of the midportion of the opened bag may better reflect the mass size that can fit into that bag.
Bag strength
Bag strength depends on the material used for bag construction. Most laparoscopic bags in the United States are made of 3 different materials: polyurethane, polypropylene, and ripstop nylon.
Polyurethane and polypropylene are synthetic plastic polymers; in bag form they are stretchy and, under extreme force, may tear. They are best used for bagging fluid-filled cysts or soft pliable masses that will not require extensive bag or tissue handling, such as extraction of large leiomyomas. Polyurethane and polypropylene bags are more susceptible to puncture with sharp laparoscopic instruments or scalpels, and care must be taken to avoid accidentally cutting the bag during tissue extraction.
Alternatively, bags made of ripstop nylon are favored for their bag strength. Ripstop nylon is a synthetic fabric that is woven together in a crosshatch pattern that makes it resistant to tearing and ripping. It was developed originally during World War II as a replacement for silk parachutes. Modern applications include its use in sails, kites, and high-quality camping equipment. This material has a favorable strength-to-weight ratio, and, in case of a tear, it is less prone to extension of the tear. For surgical applications, these bags are best used for bagging specimens that will require a lot of bag manipulation and tissue extraction. However, the ripstop fabric takes up more space in the incision than polyurethane or polypropylene, leaving the surgeon with less space for tissue extraction. Thus, as a tradeoff for bag strength, the surgeon may need to extend the incision a little, and a small self-retracting wound retractor may be necessary to allow visibility for safe tissue extraction when using a ripstop nylon bag compared with others.
Continue to: Trocar selection is important...
Trocar selection is important
While considering bag selection, the surgeon also must consider trocar selection to allow for laparoscopic insertion of the bag. Trocar size for bag selection refers to the minimum trocar diameter needed to insert the laparoscopic bag. Most bags are designed to fit into a laparoscopic trocar or into the skin incision that previously housed the trocar. Trocar size does not directly correlate with bag mouth diameter; for example, a 10-mm laparoscopic bag that can be inserted through a 10- or 12-mm trocar size cannot fit a 10-cm mass (see the mouth diameter section above).
A tip to maximize operating room (OR) efficiency is to start off with a larger trocar, such as a 12-mm trocar, if it is known that a laparoscopic bag with a 12-mm trocar size will be used, rather than starting with a 5-mm trocar and upsizing the port site incision. This saves time and offers intraoperative flexibility, allowing for the use of larger instruments and quicker insufflation.
Furthermore, if the specimen has a solid component and tissue extraction is anticipated, consider starting off with a large trocar, one that is larger than the bag’s trocar size since the incision likely will be extended. For example, even if a myoma will fit within a 10-mm laparoscopic bag made of ripstop nylon, using a 15-mm trocar rather than a 10-mm trocar may be considered since the skin and fascial incisions will need to be extended to allow for cold-cut tissue extraction. Starting with the larger 15-mm trocar may offer surgical advantages, such as direct needle delivery of larger needles for myometrial closure after myomectomy or direct removal of smaller myomas through the trocar to avoid bagging multiple specimens.
Putting it all together
To optimize efficiency in the OR for specimen removal, we recommend streamlining OR flow and reducing waste by first considering the specimen size, tissue type, bag shape, and trocar selection. Choose a bag by taking into account the bag mouth diameter and the amount of taper you will need to obtain an appropriate ball fit. If the tissue type is soft and pliable, consider a polyurethane or polypropylene bag and the smallest bag size possible, even if it has a narrow bag shape and taper.
However, if the tissue type is solid, the shape is round, and the mass is large (requiring extensive tissue extraction for removal), consider a bag made of ripstop nylon and factor in the bag shape as well as the bag taper. Using a bag without a steep taper may allow a better fit.
After choosing a laparoscopic bag, select the appropriate trocars necessary for completion of the surgery. Consider starting off with a larger trocar rather than spending the time to upsize a trocar if you plan to use a large bag or intend to extend the trocar incision for a contained tissue extraction. These tips will help optimize efficiency, reduce equipment wastage, and prevent intra-abdominal spillage.
Keep in mind that all procedures, including specimen removal using containment systems, have inherent risks. For example, visualization of the mass within the bag and visualization of vital structures may be hindered by bulkiness of the bag or specimen. There is also a risk of bag compromise and leakage, whether through manipulation of the bag or puncture during specimen extraction. Lastly, even though removing a specimen within a containment system minimizes spillage and reports of in-bag cold-knife tissue extraction in women with histologically proven endometrial cancer have suggested that it is safe, laparoscopic bags have not been proven to prevent the dissemination of malignant tissue fragments.16,17
Overall, the inherent risks of specimen extraction during minimally invasive surgery are far outweighed by the well-established advantages of laparoscopic surgery, which carries lower risks of surgical complications such as bleeding and infection, shorter hospital stay, and quicker recovery time compared to laparotomy. There is no doubt minimally invasive surgery offers many benefits.
In summary, for best bag selection, it is equally important to know the characteristics of the pathology as it is to know the features of the specimen retrieval systems available at your institution. Understanding both the pathology and the equipment available will allow the surgeon to make the best surgical decisions for the case. ●
- Desai VB, Wright JD, Lin H, et al. Laparoscopic hysterectomy route, resource use, and outcomes: change after power morcellation warning. Obstet Gynecol. 2019;134:227-238.
- American College of Obstetricians and Gynecologists. ACOG committee opinion No. 444: choosing the route of hysterectomy for benign disease. Obstet Gynecol. 2009;114:1156-1158.
- Liu H, Lu D, Wang L, et al. Robotic surgery for benign gynecological disease. Cochrane Database Syst Rev. 2012;2:CD008978.
- Wright JD, Herzog TJ, Tsui J, et al. Nationwide trends in the performance of inpatient hysterectomy in the United States. Obstet Gynecol. 2013;122(2 pt 1):233-241.
- Turner LC, Shepherd JP, Wang L, et al. Hysterectomy surgery trends: a more accurate depiction of the last decade? Am J Obstet Gynecol. 2013;208:277.e1-7.
- Holme JB, Mortensen FV. A powder-free surgical glove bag for retraction of the gallbladder during laparoscopic cholecystectomy. Surg Laparosc Endosc Percutan Tech. 2005;15:209-211.
- Siedhoff MT, Cohen SL. Tissue extraction techniques for leiomyomas and uteri during minimally invasive surgery. Obstet Gynecol. 2017;130:1251-1260.
- US Food and Drug Administration. Laparoscopic uterine power morcellation in hysterectomy and myomectomy: FDA safety communication. April 17, 2014. https://wayback .archive-it.org/7993/20170722215731/https:/www.fda.gov /MedicalDevices/Safety/AlertsandNotices/ucm393576.htm. Accessed September 22, 2020.
- AAGL. AAGL practice report: morcellation during uterine tissue extraction. J Minim Invasive Gynecol. 2014;21:517-530.
- American College of Obstetricians and Gynecologists. ACOG committee opinion No. 770: uterine morcellation for presumed leiomyomas. Obstet Gynecol. 2019;133:e238-e248.
- Society of Gynecologic Oncology website. SGO position statement: morcellation. December 1, 2013. https://www .sgo.org/newsroom/position-statements-2/morcellation/. Accessed September 22, 2020.
- Advincula AP, Truong MD. ExCITE: minimally invasive tissue extraction made simple with simulation. OBG Manag. 2015;27(12):40-45.
- Solima E, Scagnelli G, Austoni V, et al. Vaginal uterine morcellation within a specimen containment system: a study of bag integrity. J Minim Invasive Gynecol. 2015;22:1244-1246.
- Ghezzi F, Casarin J, De Francesco G, et al. Transvaginal contained tissue extraction after laparoscopic myomectomy: a cohort study. BJOG. 2018;125:367-373.
- Dotson S, Landa A, Ehrisman J, et al. Safety and feasibility of contained uterine morcellation in women undergoing laparoscopic hysterectomy. Gynecol Oncol Res Pract. 2018;5:8.
- Favero G, Miglino G, Köhler C, et al. Vaginal morcellation inside protective pouch: a safe strategy for uterine extration in cases of bulky endometrial cancers: operative and oncological safety of the method. J Minim Invasive Gynecol. 2015;22:938-943.
- Montella F, Riboni F, Cosma S, et al. A safe method of vaginal longitudinal morcellation of bulky uterus with endometrial cancer in a bag at laparoscopy. Surg Endosc. 2014;28:1949-1953.
The use of minimally invasive gynecologic surgery (MIGS) has grown rapidly over the past 20 years. MIGS, which includes vaginal hysterectomy and laparoscopic hysterectomy, is safe and has fewer complications and a more rapid recovery period than open abdominal surgery.1,2 In 2005, the role of MIGS was expanded further when the US Food and Drug Administration (FDA) approved robot-assisted surgery for the performance of gynecologic procedures.3 As knowledge and experience in the safe performance of MIGS progresses, the rates for MIGS procedures have skyrocketed and continue to grow. Between 2007 and 2010, laparoscopic hysterectomy rates rose from 23.5% to 30.5%, while robot-assisted laparoscopic hysterectomy rates increased from 0.5% to 9.5%, representing 40% of all hysterectomies.4 Due to the benefits of minimally invasive surgery over open abdominal surgery, patient and physician preference for minimally invasive procedures has grown significantly in popularity.1,5
Because incisions are small in minimally invasive surgery, surgeons have been challenged with removing large specimens through incisions that are much smaller than the presenting pathology. One approach is to use a specimen retrieval bag for specimen extraction. Once the dissection is completed, the specimen is placed within the retrieval bag for removal, thus minimizing exposure of the specimen and its contents to the abdominopelvic cavity and incision.
The use of specimen retrieval devices has been advocated to prevent infection, avoid spillage into the peritoneal cavity, and minimize the risk of port-site metastases in cases of potentially cancerous specimens. Devices include affordable and readily available products, such as nonpowdered gloves, and commercially produced bags.6
While the use of specimen containment systems for tissue extraction has been well described in gynecology, the available systems vary widely in construction, size, durability, and shape, potentially leading to confusion and suboptimal bag selection during surgery.7 In this article, we review the most common laparoscopic bags available in the United States, provide an overview of bag characteristics, offer practice guidelines for bag selection, and review bag terminology to highlight important concepts for bag selection.

Controversy spurs change
In April 2014, the FDA warned against the use of power morcellation for specimen removal during minimally invasive surgery, citing a prevalence of 1 in 352 unsuspected uterine sarcomas and 1 in 498 unsuspected uterine leiomyosarcomas among women undergoing hysterectomy or myomectomy for presumed benign leiomyoma.8 Since then, the risk of occult uterine sarcomas, including leiomyosarcoma, in women undergoing surgery for benign gynecologic indications has been determined to be much lower.
Nonetheless, the clinical importance of contained specimen removal was clearly highlighted and the role of specimen retrieval bags soared to the forefront. Open power morcellation is no longer commonly practiced, and national societies such as the American Association of Gynecologic Laparoscopists (AAGL), the Society of Gynecologic Oncology (SGO), and the American College of Obstetricians and Gynecologists (ACOG) recommend that containment systems be used for safer specimen retrieval during gynecologic surgery.9-11 After the specimen is placed inside the containment system (typically a specimen bag), the surgeon may deliver the bag through a vaginal colpotomy or through a slightly extended laparoscopic incision to remove bulky specimens using cold-cutting extraction techniques.12-15
Continue to: Know the pathology’s characteristics...
Know the pathology’s characteristics
In most cases, based on imaging studies and physical examination, surgeons have a good idea of what to expect before proceeding with surgery. The 2 most common characteristics used for surgical planning are the specimen size (dimensions) and the tissue type (solid, cystic, soft tissue, or mixed). The mass size can range from less than 1 cm to larger than a 20-week sized fibroid uterus. Assessing the specimen in 3 dimensions is important. Tissue type also is a consideration, as soft and squishy masses, such as ovarian cysts, are easier to deflate and manipulate within the bag compared with solid or calcified tumors, such as a large fibroid uterus or a large dermoid with solid components.
Specimen shape also is a critical determinant for bag selection. Most specimen retrieval bags are tapered to varying degrees, and some have an irregular shape. Long tubular structures, such as fallopian tubes that are composed of soft tissue, fit easily into most bags regardless of bag shape or extent of bag taper, whereas the round shape of a bulky myoma may render certain bags ineffective even if the bag’s entrance accommodates the greatest diameter of the myoma. Often, a round mass will not fully fit into a bag because there is a poor fit between the mass’s shape and the bag’s shape and taper. (We discuss the concept of a poor “fit” below.) Knowing the pathology before starting a procedure can help optimize bag selection, streamline operative flow, and reduce waste.
Overview of laparoscopic bag characteristics and clinical applications
The TABLE lists the most common laparoscopic bags available for purchase in the United States. Details include the trocar size, manufacturer, product name, mouth diameter, volume, bag shape, construction material, and best clinical application.


The following are terms used to refer to the components of a laparoscopic retrieval bag:
- Mouth diameter: diameter at the entrance of a fully opened bag (FIGURE 1)
- Bag volume: the total volume a bag can accommodate when completely full
- Bag rim: characteristics of the rim of the bag when opened (that is, rigid vs soft rim, complete vs partial rim mechanism to hold the bag open) (FIGURE 2)
- Bag shape: the shape of the bag when it is fully opened (square shaped vs cone shaped vs curved bag shape) (FIGURE 2)
- Bag taper (severity and type): extent the bag is tapered from the rim of the bag’s entrance to the base of the bag; categorized by taper severity (minimal, gradual, or steep taper) and type (continuous taper or curved taper) (FIGURE 3)
- Ball fit: the maximum spherical specimen size that completely fits into a bag and allows it to cinch closed (FIGURE 4)
- Bag strength: durability of a bag when placed on tension during specimen extraction (weak, moderate, or extremely durable).




Continue to: Mouth diameter...
Mouth diameter
Bag manufacturers often differentiate bag sizes by indicating “volume” in milliliters. Bag volume, however, offers little clinical value to surgeons, as pelvic mass dimensions are usually measured in centimeters on imaging. Rather, an important characteristic for bag selection is the diameter of the rim of the bag when it is fully opened—the so-called bag mouth diameter. For a specimen to fit, the 2 dimensions of the specimen must be smaller than the dimensions of the bag entrance.
Notably, the number often linked to the specimen bag—as, for example, in the 10-mm Endo Catch bag (Covidien/Medtronic)— describes the width of the shaft of the bag before it is opened rather than the mouth diameter of the opened bag. The number actually correlates with the trocar size necessary for bag insertion rather than with the specimen size that can fit into the bag. Therefore, a 10-mm Endo Catch bag cannot fit a 10-cm mass, but rather requires a trocar size of 10 mm or greater for insertion of the bag. Fully opened, the mouth diameters of the 10-mm Endo Catch bag are roughly 6 cm x 7 cm, which allows for delivery of a 6-cm mass.
Because 2 bags that use the same trocar size for insertion may have vastly differing bag dimensions, the surgeon must know the bag mouth diameters when selecting a bag to remove the presenting pathology. For example, the Inzii 12 (Applied Medical) laparoscopic bag has mouth diameters of 9.7 cm × 13.0 cm, whereas the Anchor TRSROBO-12 (ConMed) has mouth diameters of 6.7 cm × 7.6 cm (TABLE). Although both bags can be inserted through a 12-mm trocar, both bags cannot fit the same size mass for removal.
Shape and taper
Laparoscopic bags come in various shapes (curved, cone, or square shaped), with varying levels of bag taper (steep, gradual, or no taper) (FIGURES 2 and 3). While taper has little impact on long and skinny specimens, taper may hinder successful bagging of bulky or spherical specimens.
Each bag has different grades of taper regardless of mouth diameter or trocar size. For round masses, the steeper the taper, the smaller the mass that can comfortably fit within the bag. This concept is connected to the idea of “ball fit,” explained below.
In addition, bag shape may affect what mass size can fit into the bag. An irregularly shaped curved bag or a bag with a steep taper may be well suited for removal of multiple specimens of varying sizes or soft masses that are malleable enough to conform to the bag’s shape (such as a ruptured ovarian cyst). Alternatively, a square-shaped bag or a bag with minimal taper would better accommodate a round mass.
Ball fit
When thinking about large circular masses, such as myomas or ovarian cysts, one must consider the ball fit. This refers to the maximum spherical size of the specimen that fits completely within a bag while allowing the bag to cinch closed. Generally, this is an estimation that factors in the bag shape, extent of the bag taper, bag mouth diameter, and specimen shape and tissue type. At times, although a mass can fit through the bag’s mouth diameter, a steep taper may prevent the mass from being fully bagged and limit closure of the bag (FIGURE 4).
Curved bags like the Anchor TRSVATS-15 (ConMed), which have a very narrow bottom, are prone to a limited ball fit, and thus the bag mouth diameter will not correlate with the largest mass size that can be fitted within the bag. Therefore, if using a steeply tapered bag for removal of large round masses, do not rely on the bag’s mouth diameter for bag selection. The surgeon must visualize the ball fit within the bag, taking into account the specimen size and shape, bag shape, and bag taper. In these scenarios, using the diameter of the midportion of the opened bag may better reflect the mass size that can fit into that bag.
Bag strength
Bag strength depends on the material used for bag construction. Most laparoscopic bags in the United States are made of 3 different materials: polyurethane, polypropylene, and ripstop nylon.
Polyurethane and polypropylene are synthetic plastic polymers; in bag form they are stretchy and, under extreme force, may tear. They are best used for bagging fluid-filled cysts or soft pliable masses that will not require extensive bag or tissue handling, such as extraction of large leiomyomas. Polyurethane and polypropylene bags are more susceptible to puncture with sharp laparoscopic instruments or scalpels, and care must be taken to avoid accidentally cutting the bag during tissue extraction.
Alternatively, bags made of ripstop nylon are favored for their bag strength. Ripstop nylon is a synthetic fabric that is woven together in a crosshatch pattern that makes it resistant to tearing and ripping. It was developed originally during World War II as a replacement for silk parachutes. Modern applications include its use in sails, kites, and high-quality camping equipment. This material has a favorable strength-to-weight ratio, and, in case of a tear, it is less prone to extension of the tear. For surgical applications, these bags are best used for bagging specimens that will require a lot of bag manipulation and tissue extraction. However, the ripstop fabric takes up more space in the incision than polyurethane or polypropylene, leaving the surgeon with less space for tissue extraction. Thus, as a tradeoff for bag strength, the surgeon may need to extend the incision a little, and a small self-retracting wound retractor may be necessary to allow visibility for safe tissue extraction when using a ripstop nylon bag compared with others.
Continue to: Trocar selection is important...
Trocar selection is important
While considering bag selection, the surgeon also must consider trocar selection to allow for laparoscopic insertion of the bag. Trocar size for bag selection refers to the minimum trocar diameter needed to insert the laparoscopic bag. Most bags are designed to fit into a laparoscopic trocar or into the skin incision that previously housed the trocar. Trocar size does not directly correlate with bag mouth diameter; for example, a 10-mm laparoscopic bag that can be inserted through a 10- or 12-mm trocar size cannot fit a 10-cm mass (see the mouth diameter section above).
A tip to maximize operating room (OR) efficiency is to start off with a larger trocar, such as a 12-mm trocar, if it is known that a laparoscopic bag with a 12-mm trocar size will be used, rather than starting with a 5-mm trocar and upsizing the port site incision. This saves time and offers intraoperative flexibility, allowing for the use of larger instruments and quicker insufflation.
Furthermore, if the specimen has a solid component and tissue extraction is anticipated, consider starting off with a large trocar, one that is larger than the bag’s trocar size since the incision likely will be extended. For example, even if a myoma will fit within a 10-mm laparoscopic bag made of ripstop nylon, using a 15-mm trocar rather than a 10-mm trocar may be considered since the skin and fascial incisions will need to be extended to allow for cold-cut tissue extraction. Starting with the larger 15-mm trocar may offer surgical advantages, such as direct needle delivery of larger needles for myometrial closure after myomectomy or direct removal of smaller myomas through the trocar to avoid bagging multiple specimens.
Putting it all together
To optimize efficiency in the OR for specimen removal, we recommend streamlining OR flow and reducing waste by first considering the specimen size, tissue type, bag shape, and trocar selection. Choose a bag by taking into account the bag mouth diameter and the amount of taper you will need to obtain an appropriate ball fit. If the tissue type is soft and pliable, consider a polyurethane or polypropylene bag and the smallest bag size possible, even if it has a narrow bag shape and taper.
However, if the tissue type is solid, the shape is round, and the mass is large (requiring extensive tissue extraction for removal), consider a bag made of ripstop nylon and factor in the bag shape as well as the bag taper. Using a bag without a steep taper may allow a better fit.
After choosing a laparoscopic bag, select the appropriate trocars necessary for completion of the surgery. Consider starting off with a larger trocar rather than spending the time to upsize a trocar if you plan to use a large bag or intend to extend the trocar incision for a contained tissue extraction. These tips will help optimize efficiency, reduce equipment wastage, and prevent intra-abdominal spillage.
Keep in mind that all procedures, including specimen removal using containment systems, have inherent risks. For example, visualization of the mass within the bag and visualization of vital structures may be hindered by bulkiness of the bag or specimen. There is also a risk of bag compromise and leakage, whether through manipulation of the bag or puncture during specimen extraction. Lastly, even though removing a specimen within a containment system minimizes spillage and reports of in-bag cold-knife tissue extraction in women with histologically proven endometrial cancer have suggested that it is safe, laparoscopic bags have not been proven to prevent the dissemination of malignant tissue fragments.16,17
Overall, the inherent risks of specimen extraction during minimally invasive surgery are far outweighed by the well-established advantages of laparoscopic surgery, which carries lower risks of surgical complications such as bleeding and infection, shorter hospital stay, and quicker recovery time compared to laparotomy. There is no doubt minimally invasive surgery offers many benefits.
In summary, for best bag selection, it is equally important to know the characteristics of the pathology as it is to know the features of the specimen retrieval systems available at your institution. Understanding both the pathology and the equipment available will allow the surgeon to make the best surgical decisions for the case. ●
The use of minimally invasive gynecologic surgery (MIGS) has grown rapidly over the past 20 years. MIGS, which includes vaginal hysterectomy and laparoscopic hysterectomy, is safe and has fewer complications and a more rapid recovery period than open abdominal surgery.1,2 In 2005, the role of MIGS was expanded further when the US Food and Drug Administration (FDA) approved robot-assisted surgery for the performance of gynecologic procedures.3 As knowledge and experience in the safe performance of MIGS progresses, the rates for MIGS procedures have skyrocketed and continue to grow. Between 2007 and 2010, laparoscopic hysterectomy rates rose from 23.5% to 30.5%, while robot-assisted laparoscopic hysterectomy rates increased from 0.5% to 9.5%, representing 40% of all hysterectomies.4 Due to the benefits of minimally invasive surgery over open abdominal surgery, patient and physician preference for minimally invasive procedures has grown significantly in popularity.1,5
Because incisions are small in minimally invasive surgery, surgeons have been challenged with removing large specimens through incisions that are much smaller than the presenting pathology. One approach is to use a specimen retrieval bag for specimen extraction. Once the dissection is completed, the specimen is placed within the retrieval bag for removal, thus minimizing exposure of the specimen and its contents to the abdominopelvic cavity and incision.
The use of specimen retrieval devices has been advocated to prevent infection, avoid spillage into the peritoneal cavity, and minimize the risk of port-site metastases in cases of potentially cancerous specimens. Devices include affordable and readily available products, such as nonpowdered gloves, and commercially produced bags.6
While the use of specimen containment systems for tissue extraction has been well described in gynecology, the available systems vary widely in construction, size, durability, and shape, potentially leading to confusion and suboptimal bag selection during surgery.7 In this article, we review the most common laparoscopic bags available in the United States, provide an overview of bag characteristics, offer practice guidelines for bag selection, and review bag terminology to highlight important concepts for bag selection.

Controversy spurs change
In April 2014, the FDA warned against the use of power morcellation for specimen removal during minimally invasive surgery, citing a prevalence of 1 in 352 unsuspected uterine sarcomas and 1 in 498 unsuspected uterine leiomyosarcomas among women undergoing hysterectomy or myomectomy for presumed benign leiomyoma.8 Since then, the risk of occult uterine sarcomas, including leiomyosarcoma, in women undergoing surgery for benign gynecologic indications has been determined to be much lower.
Nonetheless, the clinical importance of contained specimen removal was clearly highlighted and the role of specimen retrieval bags soared to the forefront. Open power morcellation is no longer commonly practiced, and national societies such as the American Association of Gynecologic Laparoscopists (AAGL), the Society of Gynecologic Oncology (SGO), and the American College of Obstetricians and Gynecologists (ACOG) recommend that containment systems be used for safer specimen retrieval during gynecologic surgery.9-11 After the specimen is placed inside the containment system (typically a specimen bag), the surgeon may deliver the bag through a vaginal colpotomy or through a slightly extended laparoscopic incision to remove bulky specimens using cold-cutting extraction techniques.12-15
Continue to: Know the pathology’s characteristics...
Know the pathology’s characteristics
In most cases, based on imaging studies and physical examination, surgeons have a good idea of what to expect before proceeding with surgery. The 2 most common characteristics used for surgical planning are the specimen size (dimensions) and the tissue type (solid, cystic, soft tissue, or mixed). The mass size can range from less than 1 cm to larger than a 20-week sized fibroid uterus. Assessing the specimen in 3 dimensions is important. Tissue type also is a consideration, as soft and squishy masses, such as ovarian cysts, are easier to deflate and manipulate within the bag compared with solid or calcified tumors, such as a large fibroid uterus or a large dermoid with solid components.
Specimen shape also is a critical determinant for bag selection. Most specimen retrieval bags are tapered to varying degrees, and some have an irregular shape. Long tubular structures, such as fallopian tubes that are composed of soft tissue, fit easily into most bags regardless of bag shape or extent of bag taper, whereas the round shape of a bulky myoma may render certain bags ineffective even if the bag’s entrance accommodates the greatest diameter of the myoma. Often, a round mass will not fully fit into a bag because there is a poor fit between the mass’s shape and the bag’s shape and taper. (We discuss the concept of a poor “fit” below.) Knowing the pathology before starting a procedure can help optimize bag selection, streamline operative flow, and reduce waste.
Overview of laparoscopic bag characteristics and clinical applications
The TABLE lists the most common laparoscopic bags available for purchase in the United States. Details include the trocar size, manufacturer, product name, mouth diameter, volume, bag shape, construction material, and best clinical application.


The following are terms used to refer to the components of a laparoscopic retrieval bag:
- Mouth diameter: diameter at the entrance of a fully opened bag (FIGURE 1)
- Bag volume: the total volume a bag can accommodate when completely full
- Bag rim: characteristics of the rim of the bag when opened (that is, rigid vs soft rim, complete vs partial rim mechanism to hold the bag open) (FIGURE 2)
- Bag shape: the shape of the bag when it is fully opened (square shaped vs cone shaped vs curved bag shape) (FIGURE 2)
- Bag taper (severity and type): extent the bag is tapered from the rim of the bag’s entrance to the base of the bag; categorized by taper severity (minimal, gradual, or steep taper) and type (continuous taper or curved taper) (FIGURE 3)
- Ball fit: the maximum spherical specimen size that completely fits into a bag and allows it to cinch closed (FIGURE 4)
- Bag strength: durability of a bag when placed on tension during specimen extraction (weak, moderate, or extremely durable).




Continue to: Mouth diameter...
Mouth diameter
Bag manufacturers often differentiate bag sizes by indicating “volume” in milliliters. Bag volume, however, offers little clinical value to surgeons, as pelvic mass dimensions are usually measured in centimeters on imaging. Rather, an important characteristic for bag selection is the diameter of the rim of the bag when it is fully opened—the so-called bag mouth diameter. For a specimen to fit, the 2 dimensions of the specimen must be smaller than the dimensions of the bag entrance.
Notably, the number often linked to the specimen bag—as, for example, in the 10-mm Endo Catch bag (Covidien/Medtronic)— describes the width of the shaft of the bag before it is opened rather than the mouth diameter of the opened bag. The number actually correlates with the trocar size necessary for bag insertion rather than with the specimen size that can fit into the bag. Therefore, a 10-mm Endo Catch bag cannot fit a 10-cm mass, but rather requires a trocar size of 10 mm or greater for insertion of the bag. Fully opened, the mouth diameters of the 10-mm Endo Catch bag are roughly 6 cm x 7 cm, which allows for delivery of a 6-cm mass.
Because 2 bags that use the same trocar size for insertion may have vastly differing bag dimensions, the surgeon must know the bag mouth diameters when selecting a bag to remove the presenting pathology. For example, the Inzii 12 (Applied Medical) laparoscopic bag has mouth diameters of 9.7 cm × 13.0 cm, whereas the Anchor TRSROBO-12 (ConMed) has mouth diameters of 6.7 cm × 7.6 cm (TABLE). Although both bags can be inserted through a 12-mm trocar, both bags cannot fit the same size mass for removal.
Shape and taper
Laparoscopic bags come in various shapes (curved, cone, or square shaped), with varying levels of bag taper (steep, gradual, or no taper) (FIGURES 2 and 3). While taper has little impact on long and skinny specimens, taper may hinder successful bagging of bulky or spherical specimens.
Each bag has different grades of taper regardless of mouth diameter or trocar size. For round masses, the steeper the taper, the smaller the mass that can comfortably fit within the bag. This concept is connected to the idea of “ball fit,” explained below.
In addition, bag shape may affect what mass size can fit into the bag. An irregularly shaped curved bag or a bag with a steep taper may be well suited for removal of multiple specimens of varying sizes or soft masses that are malleable enough to conform to the bag’s shape (such as a ruptured ovarian cyst). Alternatively, a square-shaped bag or a bag with minimal taper would better accommodate a round mass.
Ball fit
When thinking about large circular masses, such as myomas or ovarian cysts, one must consider the ball fit. This refers to the maximum spherical size of the specimen that fits completely within a bag while allowing the bag to cinch closed. Generally, this is an estimation that factors in the bag shape, extent of the bag taper, bag mouth diameter, and specimen shape and tissue type. At times, although a mass can fit through the bag’s mouth diameter, a steep taper may prevent the mass from being fully bagged and limit closure of the bag (FIGURE 4).
Curved bags like the Anchor TRSVATS-15 (ConMed), which have a very narrow bottom, are prone to a limited ball fit, and thus the bag mouth diameter will not correlate with the largest mass size that can be fitted within the bag. Therefore, if using a steeply tapered bag for removal of large round masses, do not rely on the bag’s mouth diameter for bag selection. The surgeon must visualize the ball fit within the bag, taking into account the specimen size and shape, bag shape, and bag taper. In these scenarios, using the diameter of the midportion of the opened bag may better reflect the mass size that can fit into that bag.
Bag strength
Bag strength depends on the material used for bag construction. Most laparoscopic bags in the United States are made of 3 different materials: polyurethane, polypropylene, and ripstop nylon.
Polyurethane and polypropylene are synthetic plastic polymers; in bag form they are stretchy and, under extreme force, may tear. They are best used for bagging fluid-filled cysts or soft pliable masses that will not require extensive bag or tissue handling, such as extraction of large leiomyomas. Polyurethane and polypropylene bags are more susceptible to puncture with sharp laparoscopic instruments or scalpels, and care must be taken to avoid accidentally cutting the bag during tissue extraction.
Alternatively, bags made of ripstop nylon are favored for their bag strength. Ripstop nylon is a synthetic fabric that is woven together in a crosshatch pattern that makes it resistant to tearing and ripping. It was developed originally during World War II as a replacement for silk parachutes. Modern applications include its use in sails, kites, and high-quality camping equipment. This material has a favorable strength-to-weight ratio, and, in case of a tear, it is less prone to extension of the tear. For surgical applications, these bags are best used for bagging specimens that will require a lot of bag manipulation and tissue extraction. However, the ripstop fabric takes up more space in the incision than polyurethane or polypropylene, leaving the surgeon with less space for tissue extraction. Thus, as a tradeoff for bag strength, the surgeon may need to extend the incision a little, and a small self-retracting wound retractor may be necessary to allow visibility for safe tissue extraction when using a ripstop nylon bag compared with others.
Continue to: Trocar selection is important...
Trocar selection is important
While considering bag selection, the surgeon also must consider trocar selection to allow for laparoscopic insertion of the bag. Trocar size for bag selection refers to the minimum trocar diameter needed to insert the laparoscopic bag. Most bags are designed to fit into a laparoscopic trocar or into the skin incision that previously housed the trocar. Trocar size does not directly correlate with bag mouth diameter; for example, a 10-mm laparoscopic bag that can be inserted through a 10- or 12-mm trocar size cannot fit a 10-cm mass (see the mouth diameter section above).
A tip to maximize operating room (OR) efficiency is to start off with a larger trocar, such as a 12-mm trocar, if it is known that a laparoscopic bag with a 12-mm trocar size will be used, rather than starting with a 5-mm trocar and upsizing the port site incision. This saves time and offers intraoperative flexibility, allowing for the use of larger instruments and quicker insufflation.
Furthermore, if the specimen has a solid component and tissue extraction is anticipated, consider starting off with a large trocar, one that is larger than the bag’s trocar size since the incision likely will be extended. For example, even if a myoma will fit within a 10-mm laparoscopic bag made of ripstop nylon, using a 15-mm trocar rather than a 10-mm trocar may be considered since the skin and fascial incisions will need to be extended to allow for cold-cut tissue extraction. Starting with the larger 15-mm trocar may offer surgical advantages, such as direct needle delivery of larger needles for myometrial closure after myomectomy or direct removal of smaller myomas through the trocar to avoid bagging multiple specimens.
Putting it all together
To optimize efficiency in the OR for specimen removal, we recommend streamlining OR flow and reducing waste by first considering the specimen size, tissue type, bag shape, and trocar selection. Choose a bag by taking into account the bag mouth diameter and the amount of taper you will need to obtain an appropriate ball fit. If the tissue type is soft and pliable, consider a polyurethane or polypropylene bag and the smallest bag size possible, even if it has a narrow bag shape and taper.
However, if the tissue type is solid, the shape is round, and the mass is large (requiring extensive tissue extraction for removal), consider a bag made of ripstop nylon and factor in the bag shape as well as the bag taper. Using a bag without a steep taper may allow a better fit.
After choosing a laparoscopic bag, select the appropriate trocars necessary for completion of the surgery. Consider starting off with a larger trocar rather than spending the time to upsize a trocar if you plan to use a large bag or intend to extend the trocar incision for a contained tissue extraction. These tips will help optimize efficiency, reduce equipment wastage, and prevent intra-abdominal spillage.
Keep in mind that all procedures, including specimen removal using containment systems, have inherent risks. For example, visualization of the mass within the bag and visualization of vital structures may be hindered by bulkiness of the bag or specimen. There is also a risk of bag compromise and leakage, whether through manipulation of the bag or puncture during specimen extraction. Lastly, even though removing a specimen within a containment system minimizes spillage and reports of in-bag cold-knife tissue extraction in women with histologically proven endometrial cancer have suggested that it is safe, laparoscopic bags have not been proven to prevent the dissemination of malignant tissue fragments.16,17
Overall, the inherent risks of specimen extraction during minimally invasive surgery are far outweighed by the well-established advantages of laparoscopic surgery, which carries lower risks of surgical complications such as bleeding and infection, shorter hospital stay, and quicker recovery time compared to laparotomy. There is no doubt minimally invasive surgery offers many benefits.
In summary, for best bag selection, it is equally important to know the characteristics of the pathology as it is to know the features of the specimen retrieval systems available at your institution. Understanding both the pathology and the equipment available will allow the surgeon to make the best surgical decisions for the case. ●
- Desai VB, Wright JD, Lin H, et al. Laparoscopic hysterectomy route, resource use, and outcomes: change after power morcellation warning. Obstet Gynecol. 2019;134:227-238.
- American College of Obstetricians and Gynecologists. ACOG committee opinion No. 444: choosing the route of hysterectomy for benign disease. Obstet Gynecol. 2009;114:1156-1158.
- Liu H, Lu D, Wang L, et al. Robotic surgery for benign gynecological disease. Cochrane Database Syst Rev. 2012;2:CD008978.
- Wright JD, Herzog TJ, Tsui J, et al. Nationwide trends in the performance of inpatient hysterectomy in the United States. Obstet Gynecol. 2013;122(2 pt 1):233-241.
- Turner LC, Shepherd JP, Wang L, et al. Hysterectomy surgery trends: a more accurate depiction of the last decade? Am J Obstet Gynecol. 2013;208:277.e1-7.
- Holme JB, Mortensen FV. A powder-free surgical glove bag for retraction of the gallbladder during laparoscopic cholecystectomy. Surg Laparosc Endosc Percutan Tech. 2005;15:209-211.
- Siedhoff MT, Cohen SL. Tissue extraction techniques for leiomyomas and uteri during minimally invasive surgery. Obstet Gynecol. 2017;130:1251-1260.
- US Food and Drug Administration. Laparoscopic uterine power morcellation in hysterectomy and myomectomy: FDA safety communication. April 17, 2014. https://wayback .archive-it.org/7993/20170722215731/https:/www.fda.gov /MedicalDevices/Safety/AlertsandNotices/ucm393576.htm. Accessed September 22, 2020.
- AAGL. AAGL practice report: morcellation during uterine tissue extraction. J Minim Invasive Gynecol. 2014;21:517-530.
- American College of Obstetricians and Gynecologists. ACOG committee opinion No. 770: uterine morcellation for presumed leiomyomas. Obstet Gynecol. 2019;133:e238-e248.
- Society of Gynecologic Oncology website. SGO position statement: morcellation. December 1, 2013. https://www .sgo.org/newsroom/position-statements-2/morcellation/. Accessed September 22, 2020.
- Advincula AP, Truong MD. ExCITE: minimally invasive tissue extraction made simple with simulation. OBG Manag. 2015;27(12):40-45.
- Solima E, Scagnelli G, Austoni V, et al. Vaginal uterine morcellation within a specimen containment system: a study of bag integrity. J Minim Invasive Gynecol. 2015;22:1244-1246.
- Ghezzi F, Casarin J, De Francesco G, et al. Transvaginal contained tissue extraction after laparoscopic myomectomy: a cohort study. BJOG. 2018;125:367-373.
- Dotson S, Landa A, Ehrisman J, et al. Safety and feasibility of contained uterine morcellation in women undergoing laparoscopic hysterectomy. Gynecol Oncol Res Pract. 2018;5:8.
- Favero G, Miglino G, Köhler C, et al. Vaginal morcellation inside protective pouch: a safe strategy for uterine extration in cases of bulky endometrial cancers: operative and oncological safety of the method. J Minim Invasive Gynecol. 2015;22:938-943.
- Montella F, Riboni F, Cosma S, et al. A safe method of vaginal longitudinal morcellation of bulky uterus with endometrial cancer in a bag at laparoscopy. Surg Endosc. 2014;28:1949-1953.
- Desai VB, Wright JD, Lin H, et al. Laparoscopic hysterectomy route, resource use, and outcomes: change after power morcellation warning. Obstet Gynecol. 2019;134:227-238.
- American College of Obstetricians and Gynecologists. ACOG committee opinion No. 444: choosing the route of hysterectomy for benign disease. Obstet Gynecol. 2009;114:1156-1158.
- Liu H, Lu D, Wang L, et al. Robotic surgery for benign gynecological disease. Cochrane Database Syst Rev. 2012;2:CD008978.
- Wright JD, Herzog TJ, Tsui J, et al. Nationwide trends in the performance of inpatient hysterectomy in the United States. Obstet Gynecol. 2013;122(2 pt 1):233-241.
- Turner LC, Shepherd JP, Wang L, et al. Hysterectomy surgery trends: a more accurate depiction of the last decade? Am J Obstet Gynecol. 2013;208:277.e1-7.
- Holme JB, Mortensen FV. A powder-free surgical glove bag for retraction of the gallbladder during laparoscopic cholecystectomy. Surg Laparosc Endosc Percutan Tech. 2005;15:209-211.
- Siedhoff MT, Cohen SL. Tissue extraction techniques for leiomyomas and uteri during minimally invasive surgery. Obstet Gynecol. 2017;130:1251-1260.
- US Food and Drug Administration. Laparoscopic uterine power morcellation in hysterectomy and myomectomy: FDA safety communication. April 17, 2014. https://wayback .archive-it.org/7993/20170722215731/https:/www.fda.gov /MedicalDevices/Safety/AlertsandNotices/ucm393576.htm. Accessed September 22, 2020.
- AAGL. AAGL practice report: morcellation during uterine tissue extraction. J Minim Invasive Gynecol. 2014;21:517-530.
- American College of Obstetricians and Gynecologists. ACOG committee opinion No. 770: uterine morcellation for presumed leiomyomas. Obstet Gynecol. 2019;133:e238-e248.
- Society of Gynecologic Oncology website. SGO position statement: morcellation. December 1, 2013. https://www .sgo.org/newsroom/position-statements-2/morcellation/. Accessed September 22, 2020.
- Advincula AP, Truong MD. ExCITE: minimally invasive tissue extraction made simple with simulation. OBG Manag. 2015;27(12):40-45.
- Solima E, Scagnelli G, Austoni V, et al. Vaginal uterine morcellation within a specimen containment system: a study of bag integrity. J Minim Invasive Gynecol. 2015;22:1244-1246.
- Ghezzi F, Casarin J, De Francesco G, et al. Transvaginal contained tissue extraction after laparoscopic myomectomy: a cohort study. BJOG. 2018;125:367-373.
- Dotson S, Landa A, Ehrisman J, et al. Safety and feasibility of contained uterine morcellation in women undergoing laparoscopic hysterectomy. Gynecol Oncol Res Pract. 2018;5:8.
- Favero G, Miglino G, Köhler C, et al. Vaginal morcellation inside protective pouch: a safe strategy for uterine extration in cases of bulky endometrial cancers: operative and oncological safety of the method. J Minim Invasive Gynecol. 2015;22:938-943.
- Montella F, Riboni F, Cosma S, et al. A safe method of vaginal longitudinal morcellation of bulky uterus with endometrial cancer in a bag at laparoscopy. Surg Endosc. 2014;28:1949-1953.
2020 Update on contraception
A vaginal ring that can be reused for up to 1 year and a progestin-only pill (POP) with a wider window for missed pills are 2 of the novel contraceptive products introduced to the market this year. In addition, an ongoing study of the levonorgestrel 52-mg intrauterine system (IUS) continues to provide evidence on its extended duration of use, now approved through 6 years.
The segesterone acetate (SA) and ethinyl estradiol (EE) vaginal ring (Annovera) is new among contraceptive options. Segesterone acetate is a novel progestin that can be used only via nonoral routes; it binds specifically to progesterone receptors without estrogenic or antiandrogen effects.1 Unlike the etonogestrel and ethinyl estradiol ring (NuvaRing; for which generic products became available this past year), which is used for 1 cycle and then thrown away, the SA/EE ring is effective for 13 consecutive cycles. It does not require refrigeration when not in use.2 Because a single ring can be used for 13 cycles, users in locations without laws that mandate a 12-month supply of pills, patches, and rings need less frequent visits to the pharmacy or clinic.
Progestin-only contraceptive pills are an important option for patients who desire hormonal contraception and have contraindications to estrogen, such as migraines with aura, cardiovascular risk factors, and being in the early postpartum period.3 In the United States, current POPs contain norethindrone, which has a 3-hour window for missed pills4; a desogestrel-only pill available outside the United States has a 12-hour window.5 Both are provided as a 28-day pill pack for continuous use, and both result in undesirable bleeding patterns in some users.
The prolonged half-life of drospirenone, another progestin, gives it the potential to increase reliability in the setting of missed or delayed pills and improve bleeding patterns. A new POP contraceptive contains drospirenone (Slynd) and is available in a 28-day pack with a 24-day supply of hormone and a 4-day supply of placebo; it provides a window for missed pill use similar to that for combined hormonal contraception (CHC) as well as a placebo period for a timed withdrawal bleed.6,7
Liletta is a well-known levonorgestrel 52-mg IUS that was first approved by the US Food and Drug Administration (FDA) in 2015. An ongoing clinical trial has been the basis for approval of this IUS for use in increasing durations, from 3 years initially to 4 and then 5 years. The newest data indicate efficacy up to 6 years.8
Continue to: Combined hormonal vaginal system provides a year's contraception with an acceptable safety profile...
Combined hormonal vaginal system provides a year's contraception with an acceptable safety profile
Archer DF, Merkatz RB, Bahamondes L, et al. Efficacy of the 1-year (13-cycle) segesterone acetate and ethinylestradiol contraceptive vaginal system: results of two multicentre, open-label, single-arm, phase 3 trials. Lancet Glob Health. 2019;7:e1054-e1064.
Gemzell-Danielsson K, Sitruk-Ware R, Creinin MD, et al. Segesterone acetate/ethinyl estradiol 12-month contraceptive vaginal system safety evaluation. Contraception. 2019;99:323-328.
Archer and colleagues reported the results of 2 pivotal multicenter, open-label, phase 3 trials, which included 2,265 users, conducted to evaluate efficacy and return to menses or pregnancy after use of the 1-year (13 cycles) SA/EE contraceptive vaginal system (CVS).
Details of the efficacy study
The study included 1,130 women in a US-only study and 1,135 women in an international study with sites in the United States, Australia, Brazil, Chile, Dominican Republic, Finland, Hungary, and Sweden. Participants used the CVS for 21 days followed by a 7-day use-free interval for up to 13 consecutive cycles; they were instructed not to remove the CVS for more than 2 hours during the 21 days of use.
Primary and secondary efficacy outcomes were calculated using the Pearl Index and an intention-to-treat Kaplan-Meier life table, respectively. At the end of the study, users who desired not to continue hormonal contraception or to become pregnant were followed up for 6 months to evaluate return to menses or pregnancy.
Year-long effectiveness
The investigators reported an overall Pearl index of 2.98 (95% confidence interval [CI], 2.13-4.06) and a Kaplan-Meier life table cumulative efficacy rate of 97.5% (FIGURE 1), consistent with other recently approved CHC methods. Women from non-European sites, who primarily were US participants, had a Pearl Index of 3.25 (95% CI, 2.35-4.37), and participants from the European sites had a Pearl Index of 0.47 (95% CI, 0.03-2.07). Importantly, CVS removal had a significant impact on efficacy, with a Pearl Index of 5.98 (95% CI, 2.46-9.27) in users reporting CVS removals for longer than 2 hours, suggesting escape ovulation with improper use. The Pearl Index was highest in users aged 18 to 19 years and was not affected by body mass index (BMI), although 91% of users had a BMI of 29.0 kg/m2 or lower.
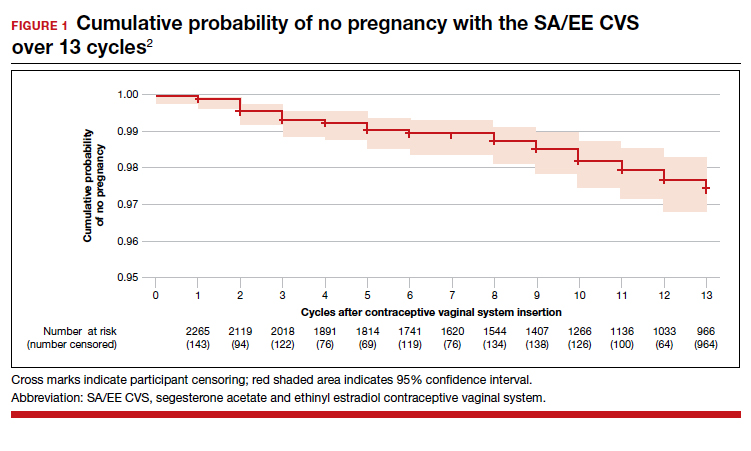
There was no trend for a change in pregnancy risk across 13 cycles, providing evidence of CVS efficacy throughout a full year's use. The follow-up portion of the study included 290 users who were not continuing hormonal contraception at study end; all follow-up participants reported return to menses after method discontinuation.
Clinical safety data
To evaluate safety outcomes from clinical studies on the CVS containing SA/EE, Gemzell-Danielsson and colleagues analyzed 9 studies. Most of the data were derived from 2 phase 3, multicenter trials (as discussed above), with supporting evidence from 7 other studies.
Adverse events reported
Among 2,308 CVS users in the phase 3 trials, 87% reported at least 1 adverse effect, with most of mild or moderate severity. These included headache, 26%; nausea, 18%; vaginal discharge, 10%; and metrorrhagia, 7%. Overall, 12% of CVS users discontinued use due to an adverse effect. Two percent of users experienced severe adverse effects, including venous thromboembolism (VTE), allergic reaction, gallbladder disease, and spontaneous abortion.
In the US-only phase 3 trial, 2 VTE events occurred in the first 6 months in women with baseline BMI greater than 29.0 kg/m2; therefore, enrollment of patients with a BMI greater than 29.0 kg/m2 was halted and current users meeting that criteria were discontinued. Notably, no cases of VTE occurred in studies with a segesterone acetate-only CVS; this suggests that risk can be attributed to the estrogen component. Overall, 4 nonfatal VTEs occurred, all among the 1,536 women enrolled in the phase 3 trials (4 of 1,536 [0.3%]); at least 3 of these cases occurred in users with VTE risk factors (TABLE 1). The estimated VTE rate in CVS users with a BMI greater than 29.0 kg/m2 is 10.8/10,000 women-years (95% CI, 8.9-13.1).
Complete expulsion of the CVS occurred in 7% of cycles and partial expulsion in 19.5% of cycles; users reported expulsion more frequently in the first cycle, most (about 70%) of which were partial expulsions. Of the laboratory values and vital signs studied, including weight, users had no clinically relevant changes from baseline.
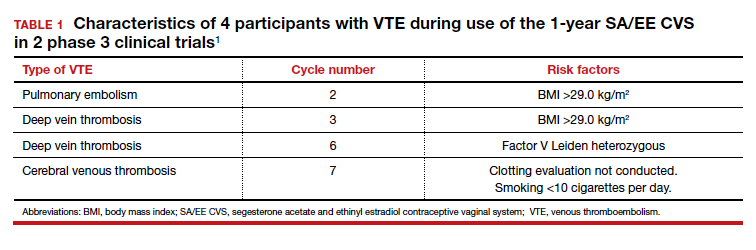
The 13-cycle efficacy and general adverse events rates of the new SA/EE CVS are consistent with those of other CHCs. However, the efficacy and safety findings are not necessarily generalizable to all patients. Because users with a BMI greater than 29.0 kg/m2 were excluded following 2 early VTE events in women with a BMI of 29.1 and 30.8 kg/m2 , only 9% of the phase 3 study population had a BMI greater than 29.0 kg/m2 . Clinicians may question whether the 1-year SA/EE CVS is an acceptable method for obese users. We know that EE causes similar changes in hemostatic factors regardless of oral or vaginal route,9 but these studies as well as pharmacokinetic studies typically include relatively few participants. While studies demonstrate that the SA/EE CVS delivers EE 13 µg daily,1 individual hormone absorption can vary. It is possible that the amount of EE in the CVS (17.4 mg) could, in a person predisposed to higher absorption, increase VTE risk. We do not know if this potential or actual risk is different for nonobese and obese users. To be fair, most of the EE-containing combined hormonal contraceptives were approved with study data that did not include obese women; the FDA first discussed the importance of including obese women in contraceptive approval studies in 2007.10 Thus, we do not know if this CVS has a significantly higher VTE risk in obese users than other methods.
All available information is based on cyclic CVS use (28-day cycles with a 7-day use-free interval). No data are available on drug levels, safety, or efficacy over extended periods of continuous use with the same CVS. During counseling, special emphasis should be placed on the increased pregnancy risk for patients who remove the ring for more than 2 hours.
Continue to: New drospirenone pill is an effective POP option...
New drospirenone pill is an effective POP option
Kimble T, Burke AE, Barnhart KT, et al. A 1-year prospective, open-label, single-arm, multicenter, phase 3 trial of the contraceptive efficacy and safety of the oral progestin-only pill drospirenone 4 mg using a 24/4-day regimen. Contracept X. 2020;2:100020.
Palacios S, Colli E, Regidor PA. Multicenter, phase III trials on the contraceptive efficacy, tolerability and safety of a new drospirenone-only pill. Acta Obstet Gynecol Scand. 2019;98:1549-1557.
In a prospective, single-arm, multicenter phase 3 trial in the United States, Kimble and colleagues evaluated the efficacy and safety of an oral drospirenone POP in a cyclic 24-day hormone/4-day placebo regimen. The trial included 1,006 users. No BMI cutoff was used, and about one-third of study participants were obese (BMI >30.0 kg/m2). Women were instructed to take a missed tablet as soon as remembered if within 24 hours or with the next scheduled dose if more than 24 hours late.
Contraceptive effectiveness
The Pearl Index for nonbreastfeeding users aged 35 years or younger with pregnancies confirmed by a quantitative serum ß-human chorionic gonadotropin test (915 users) was 2.9 (95% CI, 1.5-5.1). Of note, 2 out of 15 on-treatment pregnancies were excluded from this calculation because of protocol site violations, as were 3 pregnancies that were unconfirmed. In the modified full analysis set of 915 users, 36% were obese (BMI≥30 kg/m2), and the Pearl Index was noted to be unaffected by BMI (TABLE 2).

While 61% of women reported adverse effects, more than 95% of these were mild or moderate in intensity, including headache, nausea, dysmenorrhea, metrorrhagia, and breast pain. No VTE occurred. The frequency of hyperkalemia was 0.5%, and there was no evidence of hypotension, which is significant due to the antimineralocorticoid activity of drospirenone. All cases of hyperkalemia were considered mild, and all women were asymptomatic. There were no clinically relevant changes in body weight, gynecologic exam, or other laboratory values.
With increased cycles of use, the number of days with bleeding or spotting generally decreased and amenorrhea increased. However, in cycles 11 to 13, 41.6% of users still had unscheduled bleeding (reduced from 57.0% at cycles 2-4), and 29.0% had scheduled bleeding (decreased from 44% at cycles 2-4) (FIGURE 2). With these bleeding patterns, 86.2% of users agreed or strongly agreed that they were satisfied with the product.
European multicenter study of drospironene
In a European investigation, Palacios and colleagues pooled and analyzed data from 2 phase 3 multicenter trials to assess the efficacy, tolerability, and safety of the same drospirenone-only pill (24 days of drospironene 4 mg and 4 days of placebo) in 1,571 users. No BMI cutoff was used, but overall only 71 participants (4.6%) were obese. One study included desogestrel 0.075 mg (in a regimen of 28 active pills) as a comparator for safety.
The overall Pearl Index for users 35 years or younger (1,251 users) was 1.0 (95% CI, 0.4-2.0). The "method failure Pearl Index" in users 35 years or younger, which included all pregnancies during "perfect medication cycles," was 1.3 (95% CI, 0.5-2.5).
The most common adverse effects were acne (6.6% in study 1 and 4.4% in study 2), headache (4.5% in study 1), and irregular bleeding (4.4% in study 2). No cases of VTE occurred; there was 1 case of asymptomatic hyperkalemia. Additional laboratory values and vital signs showed no significant changes. The trend in bleeding was similar to that in the US studies, but it is interesting to note that there were significantly lower rates of unscheduled bleeding or spotting in drospirenone users than in desogestrel users (67.9% vs 86.5%, respectively; P<.001).
In the US study, the higher Pearl Index compared with that found in the European study (2.9 vs 1.0) likely reflects an increased proportion of study participants with a BMI of 30 kg/m2 or higher, a younger average age of participants, and a historical tendency toward better contraceptive efficacy in European than in US study participants. Kimble and colleagues’ finding of a Pearl Index of 2.9 is similar to that seen with other CHCs and POPs, and the data from the US study are potentially more generalizable.
Among the 2,257 participants in 3 studies, 423 (19%) were obese. No VTE events occurred with drospirenone use, as compared with 4 events in the SA/EE CVS study with 2,308 participants in the phase 3 studies.
Historically, POPs were associated with more days of bleeding than CHCs and require stricter adherence to daily use within a narrow window for missed pills. The new drospirenone-only pill may provide women with more flexibility since it maintains contraceptive efficacy even with 24-hour delayed or missed-pill errors. Although intermenstrual bleeding rates are high, participants still had a very favorable assessment, and the profile may be more tolerable compared with other POPs. Clinicians prescribing this new POP should counsel patients that the cyclic regimen does not always result in regular bleeding patterns.
Continue to: Evidence supports 6 years' use of a levonorgestrel 52-mg IUS...
Evidence supports 6 years' use of a levonorgestrel 52-mg IUS
Westhoff CL, Keder LM, Gangestad A, et al. Six-year contraceptive efficacy and continued safety of a levonorgestrel 52-mg intrauterine system. Contraception. 2020;101:159-161.
Two levonorgestrel 52-mg IUS products are on the market, both of which were approved for 5 years of use. The ACCESS IUS study (A Comprehensive Contraceptive Efficacy and Safety Study of an IUS) is an ongoing phase 3 trial to assess the safety and efficacy of a levonorgestrel 52-mg IUS (Liletta) for up to 10 years of use in US women. Westhoff and colleagues presented the data used for this IUS to gain approval for 6 years of use as of October 2019. The report included safety information for all users, with use exceeding 8 years in 122 participants.
In year 6 of the ongoing trial, there were no on-treatment pregnancies with a 6-year life table pregnancy rate of 0.87 (95% CI, 0.44-1.70). Forty percent of users reported amenorrhea in the 90 days preceding the end of year 6, consistent with prior data after 3 years of use (FIGURE 3). The most common adverse effects over 6 or more years of use were bacterial vulvovaginal infections and urinary tract infections.
Long-term IUS effectiveness
Overall, in users aged 16 to 35 years, 72% discontinued study participation, most frequently due to an adverse event (19.2%) or to seeking pregnancy (15.5%). Through 6 or more years of use, overall discontinuation rates for expulsion (4.0%) and bleeding symptoms (2.3%) were very low, with 2 expulsions occurring in year 6 and only 1 participant discontinuing in year 6 for a bleeding symptom. These findings are consistent with those found at 5 years of IUS use and are representative of continued efficacy as well as overall low frequency of new significant events with extended use.11
Clinicians and patients should be aware of data that support the continued use of levonorgestrel 52-mg IUS products for 6 years, and likely even longer. A low incidence of new significant events and a steady state of amenorrhea are also indications that users who like using a hormonal IUS will likely continue to do so for an extended time, if recommended. This extension, as well as continued study up to 10 years, will allow users who desire reversible long-acting hormonal contraception to have fewer removals and reinsertions; this in turn will decrease the risks and pain associated with IUS insertion and removal as well as health care costs.
- Gemzell-Danielsson K, Sitruk-Ware R, Creinin MD, et al. Segesterone acetate/ethinyl estradiol 12-month contraceptive vaginal system safety evaluation. Contraception. 2019;99:323-328.
- Archer DF, Merkatz RB, Bahamondes L, et al. Efficacy of the 1-year (13-cycle) segesterone acetate and ethinylestradiol contraceptive vaginal system: results of two multicentre, open-label, single-arm, phase 3 trials. Lancet Glob Health. 2019;7:e1054-e1064.
- American College of Obstetricians and Gynecologists. ACOG practice bulletin No. 206. Use of hormonal contraception in women with coexisiting medical conditions. Obstet Gynecol. 2019;133:e128-e150.
- Ortho Micronor [package insert]. Raritan, NJ: Ortho-McNeil Pharmaceutical Inc; 2008.
- Cerazette [package insert]. Oss, Netherlands: Merck Sharp & Dohme Limited; 2019.
- Kimble T, Burke AE, Barnhart KT, et al. A 1-year prospective, open-label, single-arm, multicenter, phase 3 trial of the contraceptive efficacy and safety of the oral progestin-only pill drospirenone 4 mg using a 24/4-day regimen. Contracept X. 2020;2:100020.
- Palacios S, Colli E, Regidor PA. Multicenter, phase III trials on the contraceptive efficacy, tolerability and safety of a new drospirenone-only pill. Acta Obstet Gynecol Scand. 2019;98:1549-1557.
- Westhoff CL, Keder LM, Gangestad A, et al. Six-year contraceptive efficacy and continued safety of a levonorgestrel 52-mg intrauterine system. Contraception. 2020;101:159-161.
- Sitruk-Ware R, Plu-Bureau G, Menard J, et al. Effects of oral and transvaginal ethinyl estradiol on hemostatic factors and hepatic proteins in a randomized, crossover study. J Clin Endocrinol Metab. 2007;92:2074-2079.
- Food and Drug Administration Advisory Committee for Reproductive Health Drugs meeting. Final summary minutes, January 23-24, 2007. https://wayback.archive-it.org/7993/20170404050830/https://www.fda.gov/ohrms/dockets/ac/07/minutes/2007-4274m1.pdf. Accessed July 28, 2020.
- Teal SB, Turok DK, Chen BA, et al. Five-year contraceptive efficacy and safety of a levonorgestrel 52-mg intrauterine system. Obstet Gynecol. 2019;133:63-70.
A vaginal ring that can be reused for up to 1 year and a progestin-only pill (POP) with a wider window for missed pills are 2 of the novel contraceptive products introduced to the market this year. In addition, an ongoing study of the levonorgestrel 52-mg intrauterine system (IUS) continues to provide evidence on its extended duration of use, now approved through 6 years.
The segesterone acetate (SA) and ethinyl estradiol (EE) vaginal ring (Annovera) is new among contraceptive options. Segesterone acetate is a novel progestin that can be used only via nonoral routes; it binds specifically to progesterone receptors without estrogenic or antiandrogen effects.1 Unlike the etonogestrel and ethinyl estradiol ring (NuvaRing; for which generic products became available this past year), which is used for 1 cycle and then thrown away, the SA/EE ring is effective for 13 consecutive cycles. It does not require refrigeration when not in use.2 Because a single ring can be used for 13 cycles, users in locations without laws that mandate a 12-month supply of pills, patches, and rings need less frequent visits to the pharmacy or clinic.
Progestin-only contraceptive pills are an important option for patients who desire hormonal contraception and have contraindications to estrogen, such as migraines with aura, cardiovascular risk factors, and being in the early postpartum period.3 In the United States, current POPs contain norethindrone, which has a 3-hour window for missed pills4; a desogestrel-only pill available outside the United States has a 12-hour window.5 Both are provided as a 28-day pill pack for continuous use, and both result in undesirable bleeding patterns in some users.
The prolonged half-life of drospirenone, another progestin, gives it the potential to increase reliability in the setting of missed or delayed pills and improve bleeding patterns. A new POP contraceptive contains drospirenone (Slynd) and is available in a 28-day pack with a 24-day supply of hormone and a 4-day supply of placebo; it provides a window for missed pill use similar to that for combined hormonal contraception (CHC) as well as a placebo period for a timed withdrawal bleed.6,7
Liletta is a well-known levonorgestrel 52-mg IUS that was first approved by the US Food and Drug Administration (FDA) in 2015. An ongoing clinical trial has been the basis for approval of this IUS for use in increasing durations, from 3 years initially to 4 and then 5 years. The newest data indicate efficacy up to 6 years.8
Continue to: Combined hormonal vaginal system provides a year's contraception with an acceptable safety profile...
Combined hormonal vaginal system provides a year's contraception with an acceptable safety profile
Archer DF, Merkatz RB, Bahamondes L, et al. Efficacy of the 1-year (13-cycle) segesterone acetate and ethinylestradiol contraceptive vaginal system: results of two multicentre, open-label, single-arm, phase 3 trials. Lancet Glob Health. 2019;7:e1054-e1064.
Gemzell-Danielsson K, Sitruk-Ware R, Creinin MD, et al. Segesterone acetate/ethinyl estradiol 12-month contraceptive vaginal system safety evaluation. Contraception. 2019;99:323-328.
Archer and colleagues reported the results of 2 pivotal multicenter, open-label, phase 3 trials, which included 2,265 users, conducted to evaluate efficacy and return to menses or pregnancy after use of the 1-year (13 cycles) SA/EE contraceptive vaginal system (CVS).
Details of the efficacy study
The study included 1,130 women in a US-only study and 1,135 women in an international study with sites in the United States, Australia, Brazil, Chile, Dominican Republic, Finland, Hungary, and Sweden. Participants used the CVS for 21 days followed by a 7-day use-free interval for up to 13 consecutive cycles; they were instructed not to remove the CVS for more than 2 hours during the 21 days of use.
Primary and secondary efficacy outcomes were calculated using the Pearl Index and an intention-to-treat Kaplan-Meier life table, respectively. At the end of the study, users who desired not to continue hormonal contraception or to become pregnant were followed up for 6 months to evaluate return to menses or pregnancy.
Year-long effectiveness
The investigators reported an overall Pearl index of 2.98 (95% confidence interval [CI], 2.13-4.06) and a Kaplan-Meier life table cumulative efficacy rate of 97.5% (FIGURE 1), consistent with other recently approved CHC methods. Women from non-European sites, who primarily were US participants, had a Pearl Index of 3.25 (95% CI, 2.35-4.37), and participants from the European sites had a Pearl Index of 0.47 (95% CI, 0.03-2.07). Importantly, CVS removal had a significant impact on efficacy, with a Pearl Index of 5.98 (95% CI, 2.46-9.27) in users reporting CVS removals for longer than 2 hours, suggesting escape ovulation with improper use. The Pearl Index was highest in users aged 18 to 19 years and was not affected by body mass index (BMI), although 91% of users had a BMI of 29.0 kg/m2 or lower.

There was no trend for a change in pregnancy risk across 13 cycles, providing evidence of CVS efficacy throughout a full year's use. The follow-up portion of the study included 290 users who were not continuing hormonal contraception at study end; all follow-up participants reported return to menses after method discontinuation.
Clinical safety data
To evaluate safety outcomes from clinical studies on the CVS containing SA/EE, Gemzell-Danielsson and colleagues analyzed 9 studies. Most of the data were derived from 2 phase 3, multicenter trials (as discussed above), with supporting evidence from 7 other studies.
Adverse events reported
Among 2,308 CVS users in the phase 3 trials, 87% reported at least 1 adverse effect, with most of mild or moderate severity. These included headache, 26%; nausea, 18%; vaginal discharge, 10%; and metrorrhagia, 7%. Overall, 12% of CVS users discontinued use due to an adverse effect. Two percent of users experienced severe adverse effects, including venous thromboembolism (VTE), allergic reaction, gallbladder disease, and spontaneous abortion.
In the US-only phase 3 trial, 2 VTE events occurred in the first 6 months in women with baseline BMI greater than 29.0 kg/m2; therefore, enrollment of patients with a BMI greater than 29.0 kg/m2 was halted and current users meeting that criteria were discontinued. Notably, no cases of VTE occurred in studies with a segesterone acetate-only CVS; this suggests that risk can be attributed to the estrogen component. Overall, 4 nonfatal VTEs occurred, all among the 1,536 women enrolled in the phase 3 trials (4 of 1,536 [0.3%]); at least 3 of these cases occurred in users with VTE risk factors (TABLE 1). The estimated VTE rate in CVS users with a BMI greater than 29.0 kg/m2 is 10.8/10,000 women-years (95% CI, 8.9-13.1).
Complete expulsion of the CVS occurred in 7% of cycles and partial expulsion in 19.5% of cycles; users reported expulsion more frequently in the first cycle, most (about 70%) of which were partial expulsions. Of the laboratory values and vital signs studied, including weight, users had no clinically relevant changes from baseline.

The 13-cycle efficacy and general adverse events rates of the new SA/EE CVS are consistent with those of other CHCs. However, the efficacy and safety findings are not necessarily generalizable to all patients. Because users with a BMI greater than 29.0 kg/m2 were excluded following 2 early VTE events in women with a BMI of 29.1 and 30.8 kg/m2 , only 9% of the phase 3 study population had a BMI greater than 29.0 kg/m2 . Clinicians may question whether the 1-year SA/EE CVS is an acceptable method for obese users. We know that EE causes similar changes in hemostatic factors regardless of oral or vaginal route,9 but these studies as well as pharmacokinetic studies typically include relatively few participants. While studies demonstrate that the SA/EE CVS delivers EE 13 µg daily,1 individual hormone absorption can vary. It is possible that the amount of EE in the CVS (17.4 mg) could, in a person predisposed to higher absorption, increase VTE risk. We do not know if this potential or actual risk is different for nonobese and obese users. To be fair, most of the EE-containing combined hormonal contraceptives were approved with study data that did not include obese women; the FDA first discussed the importance of including obese women in contraceptive approval studies in 2007.10 Thus, we do not know if this CVS has a significantly higher VTE risk in obese users than other methods.
All available information is based on cyclic CVS use (28-day cycles with a 7-day use-free interval). No data are available on drug levels, safety, or efficacy over extended periods of continuous use with the same CVS. During counseling, special emphasis should be placed on the increased pregnancy risk for patients who remove the ring for more than 2 hours.
Continue to: New drospirenone pill is an effective POP option...
New drospirenone pill is an effective POP option
Kimble T, Burke AE, Barnhart KT, et al. A 1-year prospective, open-label, single-arm, multicenter, phase 3 trial of the contraceptive efficacy and safety of the oral progestin-only pill drospirenone 4 mg using a 24/4-day regimen. Contracept X. 2020;2:100020.
Palacios S, Colli E, Regidor PA. Multicenter, phase III trials on the contraceptive efficacy, tolerability and safety of a new drospirenone-only pill. Acta Obstet Gynecol Scand. 2019;98:1549-1557.
In a prospective, single-arm, multicenter phase 3 trial in the United States, Kimble and colleagues evaluated the efficacy and safety of an oral drospirenone POP in a cyclic 24-day hormone/4-day placebo regimen. The trial included 1,006 users. No BMI cutoff was used, and about one-third of study participants were obese (BMI >30.0 kg/m2). Women were instructed to take a missed tablet as soon as remembered if within 24 hours or with the next scheduled dose if more than 24 hours late.
Contraceptive effectiveness
The Pearl Index for nonbreastfeeding users aged 35 years or younger with pregnancies confirmed by a quantitative serum ß-human chorionic gonadotropin test (915 users) was 2.9 (95% CI, 1.5-5.1). Of note, 2 out of 15 on-treatment pregnancies were excluded from this calculation because of protocol site violations, as were 3 pregnancies that were unconfirmed. In the modified full analysis set of 915 users, 36% were obese (BMI≥30 kg/m2), and the Pearl Index was noted to be unaffected by BMI (TABLE 2).

While 61% of women reported adverse effects, more than 95% of these were mild or moderate in intensity, including headache, nausea, dysmenorrhea, metrorrhagia, and breast pain. No VTE occurred. The frequency of hyperkalemia was 0.5%, and there was no evidence of hypotension, which is significant due to the antimineralocorticoid activity of drospirenone. All cases of hyperkalemia were considered mild, and all women were asymptomatic. There were no clinically relevant changes in body weight, gynecologic exam, or other laboratory values.
With increased cycles of use, the number of days with bleeding or spotting generally decreased and amenorrhea increased. However, in cycles 11 to 13, 41.6% of users still had unscheduled bleeding (reduced from 57.0% at cycles 2-4), and 29.0% had scheduled bleeding (decreased from 44% at cycles 2-4) (FIGURE 2). With these bleeding patterns, 86.2% of users agreed or strongly agreed that they were satisfied with the product.

European multicenter study of drospironene
In a European investigation, Palacios and colleagues pooled and analyzed data from 2 phase 3 multicenter trials to assess the efficacy, tolerability, and safety of the same drospirenone-only pill (24 days of drospironene 4 mg and 4 days of placebo) in 1,571 users. No BMI cutoff was used, but overall only 71 participants (4.6%) were obese. One study included desogestrel 0.075 mg (in a regimen of 28 active pills) as a comparator for safety.
The overall Pearl Index for users 35 years or younger (1,251 users) was 1.0 (95% CI, 0.4-2.0). The "method failure Pearl Index" in users 35 years or younger, which included all pregnancies during "perfect medication cycles," was 1.3 (95% CI, 0.5-2.5).
The most common adverse effects were acne (6.6% in study 1 and 4.4% in study 2), headache (4.5% in study 1), and irregular bleeding (4.4% in study 2). No cases of VTE occurred; there was 1 case of asymptomatic hyperkalemia. Additional laboratory values and vital signs showed no significant changes. The trend in bleeding was similar to that in the US studies, but it is interesting to note that there were significantly lower rates of unscheduled bleeding or spotting in drospirenone users than in desogestrel users (67.9% vs 86.5%, respectively; P<.001).
In the US study, the higher Pearl Index compared with that found in the European study (2.9 vs 1.0) likely reflects an increased proportion of study participants with a BMI of 30 kg/m2 or higher, a younger average age of participants, and a historical tendency toward better contraceptive efficacy in European than in US study participants. Kimble and colleagues’ finding of a Pearl Index of 2.9 is similar to that seen with other CHCs and POPs, and the data from the US study are potentially more generalizable.
Among the 2,257 participants in 3 studies, 423 (19%) were obese. No VTE events occurred with drospirenone use, as compared with 4 events in the SA/EE CVS study with 2,308 participants in the phase 3 studies.
Historically, POPs were associated with more days of bleeding than CHCs and require stricter adherence to daily use within a narrow window for missed pills. The new drospirenone-only pill may provide women with more flexibility since it maintains contraceptive efficacy even with 24-hour delayed or missed-pill errors. Although intermenstrual bleeding rates are high, participants still had a very favorable assessment, and the profile may be more tolerable compared with other POPs. Clinicians prescribing this new POP should counsel patients that the cyclic regimen does not always result in regular bleeding patterns.
Continue to: Evidence supports 6 years' use of a levonorgestrel 52-mg IUS...
Evidence supports 6 years' use of a levonorgestrel 52-mg IUS
Westhoff CL, Keder LM, Gangestad A, et al. Six-year contraceptive efficacy and continued safety of a levonorgestrel 52-mg intrauterine system. Contraception. 2020;101:159-161.
Two levonorgestrel 52-mg IUS products are on the market, both of which were approved for 5 years of use. The ACCESS IUS study (A Comprehensive Contraceptive Efficacy and Safety Study of an IUS) is an ongoing phase 3 trial to assess the safety and efficacy of a levonorgestrel 52-mg IUS (Liletta) for up to 10 years of use in US women. Westhoff and colleagues presented the data used for this IUS to gain approval for 6 years of use as of October 2019. The report included safety information for all users, with use exceeding 8 years in 122 participants.
In year 6 of the ongoing trial, there were no on-treatment pregnancies with a 6-year life table pregnancy rate of 0.87 (95% CI, 0.44-1.70). Forty percent of users reported amenorrhea in the 90 days preceding the end of year 6, consistent with prior data after 3 years of use (FIGURE 3). The most common adverse effects over 6 or more years of use were bacterial vulvovaginal infections and urinary tract infections.

Long-term IUS effectiveness
Overall, in users aged 16 to 35 years, 72% discontinued study participation, most frequently due to an adverse event (19.2%) or to seeking pregnancy (15.5%). Through 6 or more years of use, overall discontinuation rates for expulsion (4.0%) and bleeding symptoms (2.3%) were very low, with 2 expulsions occurring in year 6 and only 1 participant discontinuing in year 6 for a bleeding symptom. These findings are consistent with those found at 5 years of IUS use and are representative of continued efficacy as well as overall low frequency of new significant events with extended use.11
Clinicians and patients should be aware of data that support the continued use of levonorgestrel 52-mg IUS products for 6 years, and likely even longer. A low incidence of new significant events and a steady state of amenorrhea are also indications that users who like using a hormonal IUS will likely continue to do so for an extended time, if recommended. This extension, as well as continued study up to 10 years, will allow users who desire reversible long-acting hormonal contraception to have fewer removals and reinsertions; this in turn will decrease the risks and pain associated with IUS insertion and removal as well as health care costs.
A vaginal ring that can be reused for up to 1 year and a progestin-only pill (POP) with a wider window for missed pills are 2 of the novel contraceptive products introduced to the market this year. In addition, an ongoing study of the levonorgestrel 52-mg intrauterine system (IUS) continues to provide evidence on its extended duration of use, now approved through 6 years.
The segesterone acetate (SA) and ethinyl estradiol (EE) vaginal ring (Annovera) is new among contraceptive options. Segesterone acetate is a novel progestin that can be used only via nonoral routes; it binds specifically to progesterone receptors without estrogenic or antiandrogen effects.1 Unlike the etonogestrel and ethinyl estradiol ring (NuvaRing; for which generic products became available this past year), which is used for 1 cycle and then thrown away, the SA/EE ring is effective for 13 consecutive cycles. It does not require refrigeration when not in use.2 Because a single ring can be used for 13 cycles, users in locations without laws that mandate a 12-month supply of pills, patches, and rings need less frequent visits to the pharmacy or clinic.
Progestin-only contraceptive pills are an important option for patients who desire hormonal contraception and have contraindications to estrogen, such as migraines with aura, cardiovascular risk factors, and being in the early postpartum period.3 In the United States, current POPs contain norethindrone, which has a 3-hour window for missed pills4; a desogestrel-only pill available outside the United States has a 12-hour window.5 Both are provided as a 28-day pill pack for continuous use, and both result in undesirable bleeding patterns in some users.
The prolonged half-life of drospirenone, another progestin, gives it the potential to increase reliability in the setting of missed or delayed pills and improve bleeding patterns. A new POP contraceptive contains drospirenone (Slynd) and is available in a 28-day pack with a 24-day supply of hormone and a 4-day supply of placebo; it provides a window for missed pill use similar to that for combined hormonal contraception (CHC) as well as a placebo period for a timed withdrawal bleed.6,7
Liletta is a well-known levonorgestrel 52-mg IUS that was first approved by the US Food and Drug Administration (FDA) in 2015. An ongoing clinical trial has been the basis for approval of this IUS for use in increasing durations, from 3 years initially to 4 and then 5 years. The newest data indicate efficacy up to 6 years.8
Continue to: Combined hormonal vaginal system provides a year's contraception with an acceptable safety profile...
Combined hormonal vaginal system provides a year's contraception with an acceptable safety profile
Archer DF, Merkatz RB, Bahamondes L, et al. Efficacy of the 1-year (13-cycle) segesterone acetate and ethinylestradiol contraceptive vaginal system: results of two multicentre, open-label, single-arm, phase 3 trials. Lancet Glob Health. 2019;7:e1054-e1064.
Gemzell-Danielsson K, Sitruk-Ware R, Creinin MD, et al. Segesterone acetate/ethinyl estradiol 12-month contraceptive vaginal system safety evaluation. Contraception. 2019;99:323-328.
Archer and colleagues reported the results of 2 pivotal multicenter, open-label, phase 3 trials, which included 2,265 users, conducted to evaluate efficacy and return to menses or pregnancy after use of the 1-year (13 cycles) SA/EE contraceptive vaginal system (CVS).
Details of the efficacy study
The study included 1,130 women in a US-only study and 1,135 women in an international study with sites in the United States, Australia, Brazil, Chile, Dominican Republic, Finland, Hungary, and Sweden. Participants used the CVS for 21 days followed by a 7-day use-free interval for up to 13 consecutive cycles; they were instructed not to remove the CVS for more than 2 hours during the 21 days of use.
Primary and secondary efficacy outcomes were calculated using the Pearl Index and an intention-to-treat Kaplan-Meier life table, respectively. At the end of the study, users who desired not to continue hormonal contraception or to become pregnant were followed up for 6 months to evaluate return to menses or pregnancy.
Year-long effectiveness
The investigators reported an overall Pearl index of 2.98 (95% confidence interval [CI], 2.13-4.06) and a Kaplan-Meier life table cumulative efficacy rate of 97.5% (FIGURE 1), consistent with other recently approved CHC methods. Women from non-European sites, who primarily were US participants, had a Pearl Index of 3.25 (95% CI, 2.35-4.37), and participants from the European sites had a Pearl Index of 0.47 (95% CI, 0.03-2.07). Importantly, CVS removal had a significant impact on efficacy, with a Pearl Index of 5.98 (95% CI, 2.46-9.27) in users reporting CVS removals for longer than 2 hours, suggesting escape ovulation with improper use. The Pearl Index was highest in users aged 18 to 19 years and was not affected by body mass index (BMI), although 91% of users had a BMI of 29.0 kg/m2 or lower.

There was no trend for a change in pregnancy risk across 13 cycles, providing evidence of CVS efficacy throughout a full year's use. The follow-up portion of the study included 290 users who were not continuing hormonal contraception at study end; all follow-up participants reported return to menses after method discontinuation.
Clinical safety data
To evaluate safety outcomes from clinical studies on the CVS containing SA/EE, Gemzell-Danielsson and colleagues analyzed 9 studies. Most of the data were derived from 2 phase 3, multicenter trials (as discussed above), with supporting evidence from 7 other studies.
Adverse events reported
Among 2,308 CVS users in the phase 3 trials, 87% reported at least 1 adverse effect, with most of mild or moderate severity. These included headache, 26%; nausea, 18%; vaginal discharge, 10%; and metrorrhagia, 7%. Overall, 12% of CVS users discontinued use due to an adverse effect. Two percent of users experienced severe adverse effects, including venous thromboembolism (VTE), allergic reaction, gallbladder disease, and spontaneous abortion.
In the US-only phase 3 trial, 2 VTE events occurred in the first 6 months in women with baseline BMI greater than 29.0 kg/m2; therefore, enrollment of patients with a BMI greater than 29.0 kg/m2 was halted and current users meeting that criteria were discontinued. Notably, no cases of VTE occurred in studies with a segesterone acetate-only CVS; this suggests that risk can be attributed to the estrogen component. Overall, 4 nonfatal VTEs occurred, all among the 1,536 women enrolled in the phase 3 trials (4 of 1,536 [0.3%]); at least 3 of these cases occurred in users with VTE risk factors (TABLE 1). The estimated VTE rate in CVS users with a BMI greater than 29.0 kg/m2 is 10.8/10,000 women-years (95% CI, 8.9-13.1).
Complete expulsion of the CVS occurred in 7% of cycles and partial expulsion in 19.5% of cycles; users reported expulsion more frequently in the first cycle, most (about 70%) of which were partial expulsions. Of the laboratory values and vital signs studied, including weight, users had no clinically relevant changes from baseline.

The 13-cycle efficacy and general adverse events rates of the new SA/EE CVS are consistent with those of other CHCs. However, the efficacy and safety findings are not necessarily generalizable to all patients. Because users with a BMI greater than 29.0 kg/m2 were excluded following 2 early VTE events in women with a BMI of 29.1 and 30.8 kg/m2 , only 9% of the phase 3 study population had a BMI greater than 29.0 kg/m2 . Clinicians may question whether the 1-year SA/EE CVS is an acceptable method for obese users. We know that EE causes similar changes in hemostatic factors regardless of oral or vaginal route,9 but these studies as well as pharmacokinetic studies typically include relatively few participants. While studies demonstrate that the SA/EE CVS delivers EE 13 µg daily,1 individual hormone absorption can vary. It is possible that the amount of EE in the CVS (17.4 mg) could, in a person predisposed to higher absorption, increase VTE risk. We do not know if this potential or actual risk is different for nonobese and obese users. To be fair, most of the EE-containing combined hormonal contraceptives were approved with study data that did not include obese women; the FDA first discussed the importance of including obese women in contraceptive approval studies in 2007.10 Thus, we do not know if this CVS has a significantly higher VTE risk in obese users than other methods.
All available information is based on cyclic CVS use (28-day cycles with a 7-day use-free interval). No data are available on drug levels, safety, or efficacy over extended periods of continuous use with the same CVS. During counseling, special emphasis should be placed on the increased pregnancy risk for patients who remove the ring for more than 2 hours.
Continue to: New drospirenone pill is an effective POP option...
New drospirenone pill is an effective POP option
Kimble T, Burke AE, Barnhart KT, et al. A 1-year prospective, open-label, single-arm, multicenter, phase 3 trial of the contraceptive efficacy and safety of the oral progestin-only pill drospirenone 4 mg using a 24/4-day regimen. Contracept X. 2020;2:100020.
Palacios S, Colli E, Regidor PA. Multicenter, phase III trials on the contraceptive efficacy, tolerability and safety of a new drospirenone-only pill. Acta Obstet Gynecol Scand. 2019;98:1549-1557.
In a prospective, single-arm, multicenter phase 3 trial in the United States, Kimble and colleagues evaluated the efficacy and safety of an oral drospirenone POP in a cyclic 24-day hormone/4-day placebo regimen. The trial included 1,006 users. No BMI cutoff was used, and about one-third of study participants were obese (BMI >30.0 kg/m2). Women were instructed to take a missed tablet as soon as remembered if within 24 hours or with the next scheduled dose if more than 24 hours late.
Contraceptive effectiveness
The Pearl Index for nonbreastfeeding users aged 35 years or younger with pregnancies confirmed by a quantitative serum ß-human chorionic gonadotropin test (915 users) was 2.9 (95% CI, 1.5-5.1). Of note, 2 out of 15 on-treatment pregnancies were excluded from this calculation because of protocol site violations, as were 3 pregnancies that were unconfirmed. In the modified full analysis set of 915 users, 36% were obese (BMI≥30 kg/m2), and the Pearl Index was noted to be unaffected by BMI (TABLE 2).

While 61% of women reported adverse effects, more than 95% of these were mild or moderate in intensity, including headache, nausea, dysmenorrhea, metrorrhagia, and breast pain. No VTE occurred. The frequency of hyperkalemia was 0.5%, and there was no evidence of hypotension, which is significant due to the antimineralocorticoid activity of drospirenone. All cases of hyperkalemia were considered mild, and all women were asymptomatic. There were no clinically relevant changes in body weight, gynecologic exam, or other laboratory values.
With increased cycles of use, the number of days with bleeding or spotting generally decreased and amenorrhea increased. However, in cycles 11 to 13, 41.6% of users still had unscheduled bleeding (reduced from 57.0% at cycles 2-4), and 29.0% had scheduled bleeding (decreased from 44% at cycles 2-4) (FIGURE 2). With these bleeding patterns, 86.2% of users agreed or strongly agreed that they were satisfied with the product.

European multicenter study of drospironene
In a European investigation, Palacios and colleagues pooled and analyzed data from 2 phase 3 multicenter trials to assess the efficacy, tolerability, and safety of the same drospirenone-only pill (24 days of drospironene 4 mg and 4 days of placebo) in 1,571 users. No BMI cutoff was used, but overall only 71 participants (4.6%) were obese. One study included desogestrel 0.075 mg (in a regimen of 28 active pills) as a comparator for safety.
The overall Pearl Index for users 35 years or younger (1,251 users) was 1.0 (95% CI, 0.4-2.0). The "method failure Pearl Index" in users 35 years or younger, which included all pregnancies during "perfect medication cycles," was 1.3 (95% CI, 0.5-2.5).
The most common adverse effects were acne (6.6% in study 1 and 4.4% in study 2), headache (4.5% in study 1), and irregular bleeding (4.4% in study 2). No cases of VTE occurred; there was 1 case of asymptomatic hyperkalemia. Additional laboratory values and vital signs showed no significant changes. The trend in bleeding was similar to that in the US studies, but it is interesting to note that there were significantly lower rates of unscheduled bleeding or spotting in drospirenone users than in desogestrel users (67.9% vs 86.5%, respectively; P<.001).
In the US study, the higher Pearl Index compared with that found in the European study (2.9 vs 1.0) likely reflects an increased proportion of study participants with a BMI of 30 kg/m2 or higher, a younger average age of participants, and a historical tendency toward better contraceptive efficacy in European than in US study participants. Kimble and colleagues’ finding of a Pearl Index of 2.9 is similar to that seen with other CHCs and POPs, and the data from the US study are potentially more generalizable.
Among the 2,257 participants in 3 studies, 423 (19%) were obese. No VTE events occurred with drospirenone use, as compared with 4 events in the SA/EE CVS study with 2,308 participants in the phase 3 studies.
Historically, POPs were associated with more days of bleeding than CHCs and require stricter adherence to daily use within a narrow window for missed pills. The new drospirenone-only pill may provide women with more flexibility since it maintains contraceptive efficacy even with 24-hour delayed or missed-pill errors. Although intermenstrual bleeding rates are high, participants still had a very favorable assessment, and the profile may be more tolerable compared with other POPs. Clinicians prescribing this new POP should counsel patients that the cyclic regimen does not always result in regular bleeding patterns.
Continue to: Evidence supports 6 years' use of a levonorgestrel 52-mg IUS...
Evidence supports 6 years' use of a levonorgestrel 52-mg IUS
Westhoff CL, Keder LM, Gangestad A, et al. Six-year contraceptive efficacy and continued safety of a levonorgestrel 52-mg intrauterine system. Contraception. 2020;101:159-161.
Two levonorgestrel 52-mg IUS products are on the market, both of which were approved for 5 years of use. The ACCESS IUS study (A Comprehensive Contraceptive Efficacy and Safety Study of an IUS) is an ongoing phase 3 trial to assess the safety and efficacy of a levonorgestrel 52-mg IUS (Liletta) for up to 10 years of use in US women. Westhoff and colleagues presented the data used for this IUS to gain approval for 6 years of use as of October 2019. The report included safety information for all users, with use exceeding 8 years in 122 participants.
In year 6 of the ongoing trial, there were no on-treatment pregnancies with a 6-year life table pregnancy rate of 0.87 (95% CI, 0.44-1.70). Forty percent of users reported amenorrhea in the 90 days preceding the end of year 6, consistent with prior data after 3 years of use (FIGURE 3). The most common adverse effects over 6 or more years of use were bacterial vulvovaginal infections and urinary tract infections.

Long-term IUS effectiveness
Overall, in users aged 16 to 35 years, 72% discontinued study participation, most frequently due to an adverse event (19.2%) or to seeking pregnancy (15.5%). Through 6 or more years of use, overall discontinuation rates for expulsion (4.0%) and bleeding symptoms (2.3%) were very low, with 2 expulsions occurring in year 6 and only 1 participant discontinuing in year 6 for a bleeding symptom. These findings are consistent with those found at 5 years of IUS use and are representative of continued efficacy as well as overall low frequency of new significant events with extended use.11
Clinicians and patients should be aware of data that support the continued use of levonorgestrel 52-mg IUS products for 6 years, and likely even longer. A low incidence of new significant events and a steady state of amenorrhea are also indications that users who like using a hormonal IUS will likely continue to do so for an extended time, if recommended. This extension, as well as continued study up to 10 years, will allow users who desire reversible long-acting hormonal contraception to have fewer removals and reinsertions; this in turn will decrease the risks and pain associated with IUS insertion and removal as well as health care costs.
- Gemzell-Danielsson K, Sitruk-Ware R, Creinin MD, et al. Segesterone acetate/ethinyl estradiol 12-month contraceptive vaginal system safety evaluation. Contraception. 2019;99:323-328.
- Archer DF, Merkatz RB, Bahamondes L, et al. Efficacy of the 1-year (13-cycle) segesterone acetate and ethinylestradiol contraceptive vaginal system: results of two multicentre, open-label, single-arm, phase 3 trials. Lancet Glob Health. 2019;7:e1054-e1064.
- American College of Obstetricians and Gynecologists. ACOG practice bulletin No. 206. Use of hormonal contraception in women with coexisiting medical conditions. Obstet Gynecol. 2019;133:e128-e150.
- Ortho Micronor [package insert]. Raritan, NJ: Ortho-McNeil Pharmaceutical Inc; 2008.
- Cerazette [package insert]. Oss, Netherlands: Merck Sharp & Dohme Limited; 2019.
- Kimble T, Burke AE, Barnhart KT, et al. A 1-year prospective, open-label, single-arm, multicenter, phase 3 trial of the contraceptive efficacy and safety of the oral progestin-only pill drospirenone 4 mg using a 24/4-day regimen. Contracept X. 2020;2:100020.
- Palacios S, Colli E, Regidor PA. Multicenter, phase III trials on the contraceptive efficacy, tolerability and safety of a new drospirenone-only pill. Acta Obstet Gynecol Scand. 2019;98:1549-1557.
- Westhoff CL, Keder LM, Gangestad A, et al. Six-year contraceptive efficacy and continued safety of a levonorgestrel 52-mg intrauterine system. Contraception. 2020;101:159-161.
- Sitruk-Ware R, Plu-Bureau G, Menard J, et al. Effects of oral and transvaginal ethinyl estradiol on hemostatic factors and hepatic proteins in a randomized, crossover study. J Clin Endocrinol Metab. 2007;92:2074-2079.
- Food and Drug Administration Advisory Committee for Reproductive Health Drugs meeting. Final summary minutes, January 23-24, 2007. https://wayback.archive-it.org/7993/20170404050830/https://www.fda.gov/ohrms/dockets/ac/07/minutes/2007-4274m1.pdf. Accessed July 28, 2020.
- Teal SB, Turok DK, Chen BA, et al. Five-year contraceptive efficacy and safety of a levonorgestrel 52-mg intrauterine system. Obstet Gynecol. 2019;133:63-70.
- Gemzell-Danielsson K, Sitruk-Ware R, Creinin MD, et al. Segesterone acetate/ethinyl estradiol 12-month contraceptive vaginal system safety evaluation. Contraception. 2019;99:323-328.
- Archer DF, Merkatz RB, Bahamondes L, et al. Efficacy of the 1-year (13-cycle) segesterone acetate and ethinylestradiol contraceptive vaginal system: results of two multicentre, open-label, single-arm, phase 3 trials. Lancet Glob Health. 2019;7:e1054-e1064.
- American College of Obstetricians and Gynecologists. ACOG practice bulletin No. 206. Use of hormonal contraception in women with coexisiting medical conditions. Obstet Gynecol. 2019;133:e128-e150.
- Ortho Micronor [package insert]. Raritan, NJ: Ortho-McNeil Pharmaceutical Inc; 2008.
- Cerazette [package insert]. Oss, Netherlands: Merck Sharp & Dohme Limited; 2019.
- Kimble T, Burke AE, Barnhart KT, et al. A 1-year prospective, open-label, single-arm, multicenter, phase 3 trial of the contraceptive efficacy and safety of the oral progestin-only pill drospirenone 4 mg using a 24/4-day regimen. Contracept X. 2020;2:100020.
- Palacios S, Colli E, Regidor PA. Multicenter, phase III trials on the contraceptive efficacy, tolerability and safety of a new drospirenone-only pill. Acta Obstet Gynecol Scand. 2019;98:1549-1557.
- Westhoff CL, Keder LM, Gangestad A, et al. Six-year contraceptive efficacy and continued safety of a levonorgestrel 52-mg intrauterine system. Contraception. 2020;101:159-161.
- Sitruk-Ware R, Plu-Bureau G, Menard J, et al. Effects of oral and transvaginal ethinyl estradiol on hemostatic factors and hepatic proteins in a randomized, crossover study. J Clin Endocrinol Metab. 2007;92:2074-2079.
- Food and Drug Administration Advisory Committee for Reproductive Health Drugs meeting. Final summary minutes, January 23-24, 2007. https://wayback.archive-it.org/7993/20170404050830/https://www.fda.gov/ohrms/dockets/ac/07/minutes/2007-4274m1.pdf. Accessed July 28, 2020.
- Teal SB, Turok DK, Chen BA, et al. Five-year contraceptive efficacy and safety of a levonorgestrel 52-mg intrauterine system. Obstet Gynecol. 2019;133:63-70.
Risk for Deep Fungal Infections During IL-17 and IL-23 Inhibitor Therapy for Psoriasis
Psoriasis is a common chronic, multisystem, inflammatory disease with predominantly skin and joint manifestations that affects approximately 2% of the world’s population.1 It occurs in a variety of clinical forms, from a few well-demarcated, erythematous plaques with a silvery scale to involvement of almost the entire body surface area. Beyond the debilitating physical ailments of the disease, psoriasis also may have psychosocial effects on quality of life.2 The pathogenesis of psoriasis is not fully understood but represents a complex multifactorial disease with both immune-mediated and genetic components. Characterized by hyperplasia of epidermal keratinocytes, psoriasis is shown to be mediated by infiltration of T-cell lymphocytes with an increase of various inflammatory cytokines, including
With the growing understanding of the pathophysiology of psoriasis, focused biologics have been developed to target specific cytokines implicated in the disease process and have been increasingly utilized. Tumor necrosis factor α inhibitors, including adalimumab, infliximab, and etanercept, along with the IL-12/IL-23 inhibitor ustekinumab, have been revolutionary in psoriasis treatment by providing safe and effective long-term therapy; however, there is concern of life-threatening infections with biologics because of the immunosuppressive effects and mechanisms of action.6 Specifically, there have been reported cases of deep fungal infections associated with TNF-α inhibitor use.7
Recently, the advent of IL-17 and IL-23 inhibitors has garnered notable interest in these biologics as promising treatments for psoriasis. With IL-17 and IL-23 supported to have a major role in the pathogenesis of psoriasis, targeting the cytokine is not only logical but also has proven to be effacacious.8-10 Secukinumab, ixekizumab, and brodalumab are IL-17 inhibitors that have been approved by the US Food and Drug Administration (FDA) for the treatment of psoriasis. Secukinumab and ixekizumab are anti–IL-17A monoclonal antibodies, whereas brodalumab is an anti–IL-17 receptor antibody. Risankizumab, guselkumab, and tildrakizumab are IL-23 inhibitors that also have been approved by the FDA for the treatment of psoriasis. As with older biologics, there is concern over the safety of these inhibitors because of the central role of IL-17 and IL-23 in both innate and adaptive immune responses, particularly against fungi.11 Therefore, use of biologics targeting IL-17 and IL-23 may increase susceptibility to deep fungal infections.
Safety data and discussion of the risk for deep fungal infections from IL-17, IL-12/IL-23, and IL-23 inhibitor use for psoriasis treatment currently are lacking. Given the knowledge gap, we sought to synthesize and review the current evidence on risks for deep fungal infections during biologic therapy in patients with psoriasis, with a focus on IL-17 inhibitor therapies.
METHODS
A PubMed search of articles indexed for MEDLINE from database inception to 2019 (1946-2019) was performed to find randomized controlled trials (RCTs), including extended trials and clinical trials, for IL-17, IL-12/IL-23, and IL-23 inhibitors approved by the FDA for psoriasis treatment. The following keywords were used: psoriasis or inflammatory disease and secukinumab, ixekizumab, brodalumab, ustekinumab, risankizumab, guselkumab, or tildrakizumab. Studies were restricted to the English-language literature, and those that did not provide adequate safety data on the specific types of infections that occurred were excluded.
RESULTSIL-17 Inhibitors
Our search yielded RCTs, some including extension trials, and clinical trials of IL-17 inhibitors used for psoriatic disease and other nonpsoriatic conditions (Table).

Risk for Deep Fungal Infection With Secukinumab
The queried studies included 20 RCTs or clinical trials along with extension trials of 3746 patients with psoriasis or other inflammatory conditions, with follow-up ranging from 12 to 52 weeks. In a 3-year extension study of SCULPTURE, Bissonnette et al12 reported no new safety concerns for the 340 patients with moderate to severe psoriasis treated with secukinumab. Common adverse events (AEs) included nasopharyngitis, upper respiratory tract infections, and headache, but there were no reports of deep fungal infections.12 In a subsequent 5-year analysis of 168 patients that focused on the 300-mg fixed interval treatment with secukinumab, the safety profile remained favorable, with 0 reports of invasive fungal infections.13 A study (FEATURE) of 118 patients with psoriasis treated with a prefilled syringe of 300 or 150 mg of secukinumab also described an acceptable safety profile and reported no deep fungal infections.14 JUNCTURE, another study utilizing autoinjectors, also found that treatment with 300 or 150 mg of secukinumab was well tolerated in 121 patients, with no deep fungal infections.15 Common AEs for both studies included nasopharyngitis and headache.14,15 A 24-week phase 3 study for scalp psoriasis treated with secukinumab also reported 0 deep fungal infections in 51 patients.16 In an RCT comparing secukinumab and ustekinumab for moderate to severe plaque psoriasis, Blauvelt et al17 demonstrated that the incidence of serious AEs was comparable between the 2 groups, with no reports of invasive fungal infections in the 334 patients exposed to secukinumab. The CLEAR study, which compared secukinumab and ustekinumab, also found no reported deep fungal disease in the 335 patients exposed to secukinumab.18 Secukinumab exhibited a similar safety profile to ustekinumab in both studies, with common AEs being headache and nasopharyngitis.17,18 The GESTURE study investigated the efficacy of secukinumab in 137 patients with palmoplantar psoriasis and reported a favorable profile with no reports of deep fungal disease.19 In a subanalysis of the phase 3 study ERASURE, secukinumab was shown to have a robust and sustainable efficacy in 58 Japanese patients with moderate to severe plaque psoriasis, and there were no reports of invasive fungal infections.20 Another subanalysis of 36 Taiwanese patients from the ERASURE study also had similar findings, with no dose relationship observed for AEs.21 In a phase 2 study of 103 patients with psoriasis, Papp et al22 demonstrated AE rates that were similar across different doses of secukinumab—3×150 mg, 3×75 mg, 3×25 mg, and 1×25 mg—and described no incidences of invasive fungal disease. In a phase 2 regimen-finding study of 337 patients conducted by Rich et al,23 the most commonly reported AEs included nasopharyngitis, worsening psoriasis, and upper respiratory tract infections, but there were no reported deep fungal infections.
Our search also resulted in studies specific to the treatment of psoriatic arthritis (PsA) with secukinumab. McInnes et al9 conducted a phase 2 proof-of-concept trial for patients with PsA and reported no deep fungal infections in 28 patients exposed to 10 mg/kg of secukinumab. A 2-year follow-up with the cohort from FUTURE 1, a phase 3 clinical trial, also showed no new or unexpected safety signals in 404 patients exposed to 150 or 75 mg of secukinumab, including no reports of invasive fungal disease.24 FUTURE 2, a phase 3 clinical trial, demonstrated that the most common AE was upper respiratory tract infection in the 299 patients treatedwith secukinumab, but there were no recorded invasive fungal infections.25 In FUTURE 3, 277 patients were treated with secukinumab, with 14 nonserious candida infections but no observed deep fungal infections.26 A study comparing secukinumab to fumaric acid esters reported that 6 of 105 patients treated with secukinumab also experienced superficial candidiasis, but there were no reports of deep fungal disease.27
Secukinumab also has been used in the treatment of ankylosing spondylitis in a phase 3 RCT (MEASURE 1) in which 4 cases of superficial candidiasis were reported (0.7 cases per 100 patient-years of secukinumab) that were all resolved with standard antifungal therapy.28 In MEASURE 2, a 5-year phase 3 RCT, 145 patients were treated with secukinumab for ankylosing spondylitis, with common AEs including nasopharyngitis, diarrhea, and upper respiratory tract infection, but there were no reports of any invasive fungal infections.29 MEASURE 3 also demonstrated similar results in which no invasive fungal infections were observed.30
Risk for Deep Fungal Infection With Ixekizumab
The queried studies included 7 RCTs or clinical trials of 3523 patients with psoriasis or other inflammatory conditions, with follow-up ranging from 12 to 52 weeks. In UNCOVER-A, a phase 3 RCT of the pharmacokinetics and safety of ixekizumab, 204 patients were randomized to a prefilled syringe or autoinjector; 48% of patients experienced AEs, but no invasive fungal infections were observed.31 In an analysis of 3 phase 3 trials of ixekizumab including a total 2334 patients treated with ixekizumab from UNCOVER-1, UNCOVER-2, and UNCOVER-3, oral candidiasis frequently was reported, but no candidal infections met criteria for serious invasive infection.32 In UNCOVER-J, a 52-week phase 3 open-label trial of Japanese patients, 91 patients were treated for plaque psoriasis, erythrodermic psoriasis, or generalized pustular psoriasis using ixekizumab; the most common AEs included allergic reactions and injection-site reactions. One case of oral candidiasis was reported, but there were no reported cases of invasive fungal infections.33 A comparison of ixekizumab vs ustekinumab from the IXORA-S trial demonstrated no substantial differences in AEs between the two, and no cases of deep fungal infections were reported. The most common AE between the 2 groups was nasopharyngitis.34 An open-label extension over 4 years of a phase 2 RCT treated 211 patients with either 120 or 80 mg of ixekizumab; 87% of patients had experienced at least 1 AE, and all AEs were considered mild or moderate in severity, with no invasive fungal disease.35
Our search also resulted in 1 study specific to the treatment of PsA with ixekizumab. A phase 3, 52-week study of patients treated with ixekizumab for PsA observed 2 incidences of oral candidiasis and nail candida infections, but no invasive fungal infections were reported.36
We also found 1 study of ixekizumab used in the treatment of ankylosing spondylitis. COAST-V was a phase 3 RCT of patients treated for ankylosing spondylitis in which 164 patients were treated with ixekizumab; no serious AEs were recorded, including 0 deep fungal infections. The most common AEs observed were nasopharyngitis and upper respiratory tract infections.37
Risk for Deep Fungal Infection With Brodalumab
The queried studies included 9 RCTs and 3 clinical trials along with extension trials of 1599 patients with psoriasis or other inflammatory conditions, with follow-up ranging from 12 to 120 weeks. In a phase 2 RCT of Japanese patients with moderate to severe plaque psoriasis, 113 patients were treated with 70, 140, or 210 mg of brodalumab, and the most common AEs were nasopharyngitis, diarrhea, and upper respiratory tract inflammation. There were no reported cases of fungal infections in the study.38 In an open-label extension study of Japanese patients that evaluated the long-term clinical safety of brodalumab, 145 patients were enrolled and observed similar AEs to the RCT, with 7 patients experiencing oral candidiasis and 1 patient having skin candidiasis, but there were no observed deep fungal infections.39 In AMG 827, which evaluated the efficacy and safety of brodalumab, 320 patients were treated, and only 2 serious AEs were reported, neither of which were deep fungal disease.10 A phase 3 RCT conducted by Papp et al40 (AMAGINE-1) also treated 441 patients with moderate to severe plaque psoriasis with brodalumab and observed candida infections in 9 patients that were mild to moderate and responsive to treatment, with no patients discontinuing the study. In a 120-week open-label extension study of 181 patients, Papp et al41 reported 8% of patients experienced serious AEs, with 1 case of latent tuberculosis that led to withdrawal of treatment. A study also investigated the efficacy and safety of brodalumab in 30 patients with generalized pustular psoriasis or psoriatic erythroderma and observed 2 cases of mild candida infections that resolved with treatment. There were no reports of invasive fungal disease.42
Our search also resulted in studies of brodalumab used in the treatment of PsA and nonpsoriatic diseases. In one phase 2 RCT, 113 patients with PsA were treated with 140 mg, 280 mg, or combined doses of brodalumab, with the most common AEs being nasopharyngitis, upper respiratory tract infection, and diarrhea, but there were no reports of deep fungal infection.43 In a phase 1b trial of patients with methotrexate-resistant rheumatoid arthritis treated with brodalumab, common AEs reported included headache, cough, and abdominal pain, with only 1 case of oral candidiasis that was determined not to be drug related.44 Finally, an RCT of patients with moderate to severe asthma treated 226 patients with brodalumab and reported a greater incidence of oral candidiasis in treatment groups compared with placebo (3.5% vs 0%) but saw no instances of invasive fungal infection.45
IL-12/IL-23 Inhibitor
Risk for Deep Fungal Infection With Ustekinumab
The queried studies included 4 RCTs of 954 patients with psoriasis treated with ustekinumab (eTable).46-49 Within these trials, there were no reported cases of serious infections involving deep fungal organisms during the stated follow-up period. The literature search also found long-term safety data from the ACCEPT and PHOENIX trials that included 5437 patients with psoriasis treated with ustekinumab.66,67 There also were no demonstrated incidences of invasive fungal disease in these studies, with most cases of infection being common bacterial or viral infections.
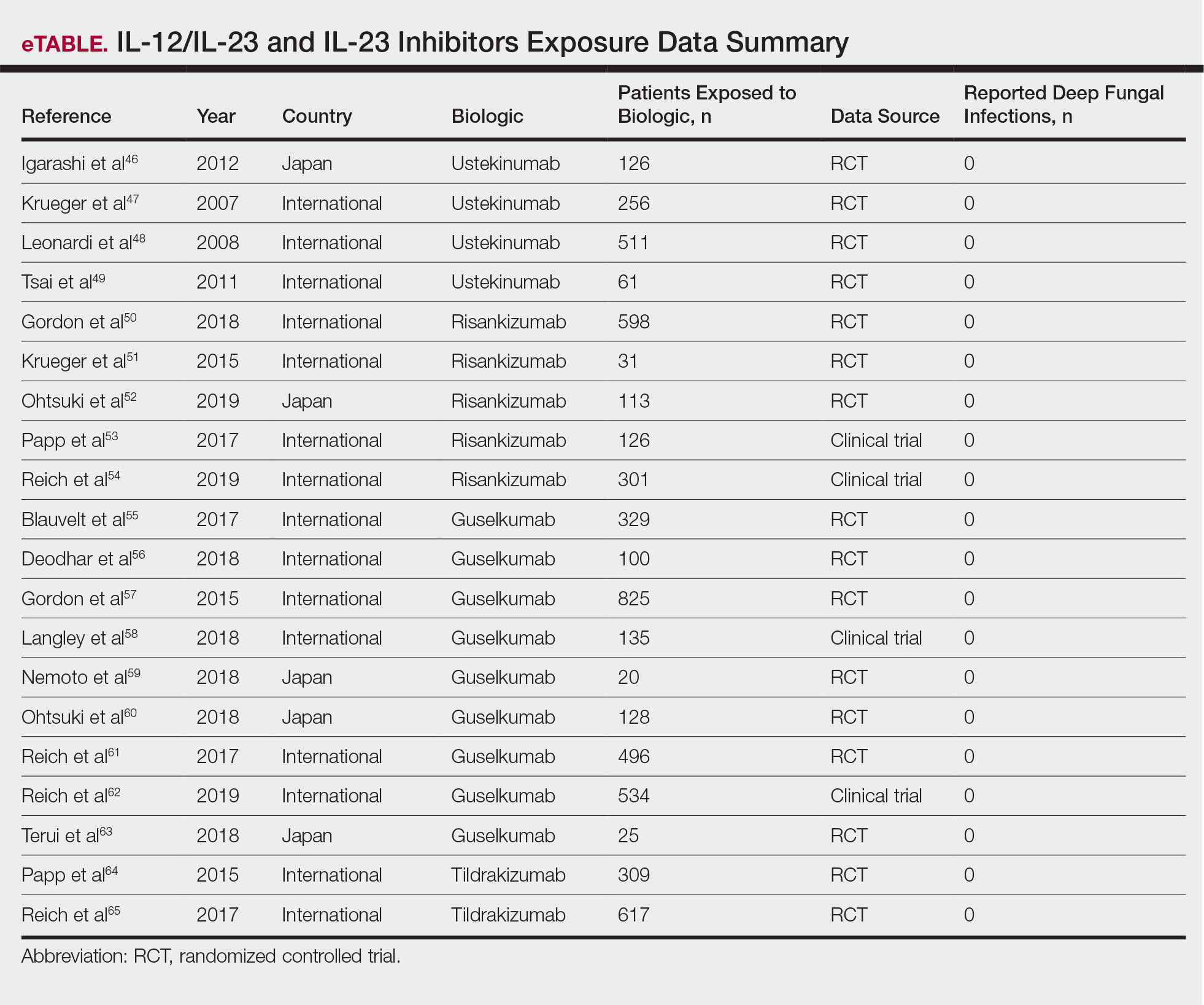
IL-23 Inhibitors
Risk for Deep Fungal Infection With Risankizumab, Guselkumab, and Tildrakizumab
The queried studies included 16 RCTs or clinical trials for psoriatic patients treated with IL-23 inhibitors, including 5 with risankizumab,50-54 9 with guselkumab,55-63 and 2 with tildrakizumab.64,65 Within these trials there were no observed cases of serious infections with deep fungal disease.
COMMENT
Our literature review has demonstrated that there does not appear to be an increased incidence of deep fungal infections for patients treated with IL-17, IL-12/IL-23, or IL-23 inhibitors for psoriatic disease. All of the reviewed studies found no cases of invasive fungal infections for patients with psoriasis treated with secukinumab, ixekizumab, brodalumab, ustekinumab, risankizumab, guselkumab, or tildrakizumab. Patients with other inflammatory conditions, such as ankylosing spondylitis, rheumatoid arthritis, and asthma, also did not appear to show an increased incidence of deep fungal disease.
Although these results show promising safety data for the use of these biologic therapies in treating inflammatory conditions, caution still is warranted, as these medications still are relatively new, with FDA approvals within the last 5 years. Safety data among different study populations also cannot be derived without further investigation, and much of the available literature is limited in long-term data. More extended trials or registry data from a large, broadly representative cohort are necessary to establish the long-term safety and risk for deep fungal infections with IL-17 and especially the newer IL-23 inhibitors.
A small percentage of patients from the reviewed literature did develop superficial candidiasis. This outcome can be expected, as the central role of IL-17 and IL-23 has been recognized in immunologic protection against infections, specifically against fungi.11 Because all of the fungal infections reported for patients on IL-17 inhibitors were superficial candidiasis, guides for practical management and treatment should be implemented to standardize future research and care. A proposed screening algorithm for patients on these biologic therapies involves safety monitoring, including inspection of the oral cavity, folds, and genitals, along with inquiring about symptoms such as burning, dysgeusia, and dysuria.68 If infection is suspected, confirmation by culture, molecular method, or optimally with esophagoscopy can be performed, and appropriate treatment may be initiated.68 Patients with candida infections of the oral cavity, folds, or genitals can be placed on topical therapy such as nystatin, amphotericin B, ciclopirox, or other azoles, while those with infections of the esophagus can be started on oral fluconazole.68
Although there were no reported cases of deep fungal infections, the theoretical risk for developing one while on IL-17 and IL-23 inhibitors may warrant further screening prior to beginning therapy. The TNF inhibitors approved for the treatment of psoriasis currently contain a black box warning for risk for disseminated and extrapulmonary histoplasmosis, coccidioidomycosis, blastomycosis, and other invasive fungal infections, which may highlight the importance of thorough evaluation and awareness of endemic areas for patients on biologics. Prior to initiating treatment with TNF inhibitors, current suggestions involve performing a thorough examination along with keeping a high index of suspicion for invasive fungal infections in patients who live in or have traveled to endemic regions.69
Screening for invasive fungal infections for patients on TNF inhibitors involves questioning about potential exposures, such as demolition of old buildings, bird roosts, or spelunking.70 Serologies or antigen testing can be used routinely, but as these tests are insensitive, empiric antifungal therapy should be initiated if there is high enough clinical suspicion.71 Currently, there are no clinical guidelines regarding fungal screening and initiation of IL-17 and IL-23 inhibitors for treatment of psoriasis and other inflammatory conditions, but careful stewardship over using these effective medications should still be practiced.
Upon review of the available safety data on the use of IL-17 and IL-23 inhibitors for the treatment of psoriasis and other inflammatory conditions, there does not appear to be an increased incidence of deep fungal infections. Physicians, however, should still be cautiously optimistic in prescribing these medications, as there is a theoretical risk for infection for all patients on biologics. A high index of suspicion for patients presenting with symptoms of fungal infections should be maintained, and appropriate diagnosis and management should be initiated if they do occur.
- Parisi R, Symmons DP, Griffiths CE, et al. Global epidemiology of psoriasis: a systematic review of incidence and prevalence. J Invest Dermatol. 2013;133:377-385.
- Koo J, Marangell LB, Nakamura M, et al. Depression and suicidality in psoriasis: review of the literature including the cytokine theory of depression. J Eur Acad Dermatol Venereol. 2017;31:1999-2009.
- Krueger JG, Bowcock A. Psoriasis pathophysiology: current concepts of pathogenesis. Ann Rheum Dis. 2005;64 (suppl 2):ii30-36.
- Lee E, Trepicchio WL, Oestreicher JL, et al. Increased expression of interleukin 23 p19 and p40 in lesional skin of patients with psoriasis vulgaris. J Exp Med. 2004;199:125-130.
- Lowes MA, Kikuchi T, Fuentes-Duculan J, et al. Psoriasis vulgaris lesions contain discrete populations of Th1 and Th17 T cells. J Invest Dermatol. 2008;128:1207-1211.
- Shear NH. Fulfilling an unmet need in psoriasis: do biologicals hold the key to improved tolerability? Drug Saf. 2006;29:49-66.
- Lee JH, Slifman NR, Gershon SK, et al. Life-threatening histoplasmosis complicating immunotherapy with tumor necrosis factor alpha antagonists infliximab and etanercept. Arthritis Rheum. 2002;46:2565-2570.
- Leonardi C, Matheson R, Zachariae C, et al. Anti-interleukin-17 monoclonal antibody ixekizumab in chronic plaque psoriasis. N Engl J Med. 2012;366:1190-1199.
- McInnes IB, Sieper J, Braun J, et al. Efficacy and safety of secukinumab, a fully human anti-interleukin-17A monoclonal antibody, in patients with moderate-to-severe psoriatic arthritis: a 24-week, randomised, double-blind, placebo-controlled, phase II proof-of-concept trial. Ann Rheum Dis. 2014;73:349-356.
- Papp KA, Leonardi C, Menter A, et al. Brodalumab, an anti-interleukin-17-receptor antibody for psoriasis. N Engl J Med. 2012;366:1181-1189.
- Isailovic N, Daigo K, Mantovani A, et al. Interleukin-17 and innate immunity in infections and chronic inflammation. J Autoimmun. 2015;60:1-11.
- Bissonnette R, Luger T, Thaci D, et al. Secukinumab sustains good efficacy and favourable safety in moderate-to-severe psoriasis after up to 3 years of treatment: results from a double-blind extension study. Br J Dermatol. 2017;177:1033-1042.
- Bissonnette R, Luger T, Thaci D, et al. Secukinumab demonstrates high sustained efficacy and a favourable safety profile in patients with moderate-to-severe psoriasis through 5 years of treatment (SCULPTURE Extension Study). J Eur Acad Dermatol Venereol. 2018;32:1507-1514.
- Blauvelt A, Prinz JC, Gottlieb AB, et al. Secukinumab administration by pre-filled syringe: efficacy, safety and usability results from a randomized controlled trial in psoriasis (FEATURE). Br J Dermatol. 2015;172:484-493.
- Paul C, Lacour JP, Tedremets L, et al. Efficacy, safety and usability of secukinumab administration by autoinjector/pen in psoriasis: a randomized, controlled trial (JUNCTURE). J Eur Acad Dermatol Venereol. 2015;29:1082-1090.
- Bagel J, Duffin KC, Moore A, et al. The effect of secukinumab on moderate-to-severe scalp psoriasis: Results of a 24-week, randomized, double-blind, placebo-controlled phase 3b study. J Am Acad Dermatol. 2017;77:667-674.
- Blauvelt A, Reich K, Tsai TF, et al. Secukinumab is superior to ustekinumab in clearing skin of subjects with moderate-to-severe plaque psoriasis up to 1 year: results from the CLEAR study. J Am Acad Dermatol. 2017;76:60.e9-69.e9.
- Thaci D, Blauvelt A, Reich K, et al. Secukinumab is superior to ustekinumab in clearing skin of subjects with moderate to severe plaque psoriasis: CLEAR, a randomized controlled trial. J Am Acad Dermatol. 2015;73:400-409.
- Gottlieb A, Sullivan J, van Doorn M, et al. Secukinumab shows significant efficacy in palmoplantar psoriasis: results from GESTURE, a randomized controlled trial. J Am Acad Dermatol. 2017;76:70-80.
- Ohtsuki M, Morita A, Abe M, et al. Secukinumab efficacy and safety in Japanese patients with moderate-to-severe plaque psoriasis: subanalysis from ERASURE, a randomized, placebo-controlled, phase 3 study. J Dermatol. 2014;41:1039-1046.
- Wu NL, Hsu CJ, Sun FJ, et al. Efficacy and safety of secukinumab in Taiwanese patients with moderate to severe plaque psoriasis: subanalysis from ERASURE phase III study. J Dermatol. 2017;44:1129-1137.
- Papp KA, Langley RG, Sigurgeirsson B, et al. Efficacy and safety of secukinumab in the treatment of moderate-to-severe plaque psoriasis: a randomized, double-blind, placebo-controlled phase II dose-ranging study. Br J Dermatol. 2013;168:412-421.
- Rich P, Sigurgeirsson B, Thaci D, et al. Secukinumab induction and maintenance therapy in moderate-to-severe plaque psoriasis: a randomized, double-blind, placebo-controlled, phase II regimen-finding study. Br J Dermatol. 2013;168:402-411.
- Kavanaugh A, Mease PJ, Reimold AM, et al. Secukinumab for long-term treatment of psoriatic arthritis: a two-year followup from a phase III, randomized, double-blind placebo-controlled study. Arthritis Care Res (Hoboken). 2017;69:347-355.
- McInnes IB, Mease PJ, Kirkham B, et al. Secukinumab, a human anti-interleukin-17A monoclonal antibody, in patients with psoriatic arthritis (FUTURE 2): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2015;386:1137-1146.
- Nash P, Mease PJ, McInnes IB, et al. Efficacy and safety of secukinumab administration by autoinjector in patients with psoriatic arthritis: results from a randomized, placebo-controlled trial (FUTURE 3). Arthritis Res Ther. 2018;20:47.
- Sticherling M, Mrowietz U, Augustin M, et al. Secukinumab is superior to fumaric acid esters in treating patients with moderate-to-severe plaque psoriasis who are naive to systemic treatments: results from the randomized controlled PRIME trial. Br J Dermatol. 2017;177:1024-1032.
- Braun J, Baraliakos X, Deodhar A, et al. Effect of secukinumab on clinical and radiographic outcomes in ankylosing spondylitis: 2-year results from the randomised phase III MEASURE 1 study. Ann Rheum Dis. 2017;76:1070-1077.
- Marzo-Ortega H, Sieper J, Kivitz A, et al. Secukinumab provides sustained improvements in the signs and symptoms of active ankylosing spondylitis with high retention rate: 3-year results from the phase III trial, MEASURE 2. RMD Open. 2017;3:e000592.
- Pavelka K, Kivitz A, Dokoupilova E, et al. Efficacy, safety, and tolerability of secukinumab in patients with active ankylosing spondylitis: a randomized, double-blind phase 3 study, MEASURE 3. Arthritis Res Ther. 2017;19:285.
- Callis Duffin K, Bagel J, Bukhalo M, et al. Phase 3, open-label, randomized study of the pharmacokinetics, efficacy and safety of ixekizumab following subcutaneous administration using a prefilled syringe or an autoinjector in patients with moderate-to-severe plaque psoriasis (UNCOVER-A). J Eur Acad Dermatol Venereol. 2017;31:107-113.
- Gordon KB, Colombel JF, Hardin DS. Phase 3 trials of ixekizumab in moderate-to-severe plaque psoriasis. N Engl J Med. 2016;375:2102.
- Saeki H, Nakagawa H, Nakajo K, et al. Efficacy and safety of ixekizumab treatment for Japanese patients with moderate to severe plaque psoriasis, erythrodermic psoriasis and generalized pustular psoriasis: results from a 52-week, open-label, phase 3 study (UNCOVER-J). J Dermatol. 2017;44:355-362.
- Reich K, Pinter A, Lacour JP, et al. Comparison of ixekizumab with ustekinumab in moderate-to-severe psoriasis: 24-week results from IXORA-S, a phase III study. Br J Dermatol. 2017;177:1014-1023.
- Zachariae C, Gordon K, Kimball AB, et al. Efficacy and safety of ixekizumab over 4 years of open-label treatment in a phase 2 study in chronic plaque psoriasis. J Am Acad Dermatol. 2018;79:294.e6-301.e6.
- van der Heijde D, Gladman DD, Kishimoto M, et al. Efficacy and safety of ixekizumab in patients with active psoriatic arthritis: 52-week results from a phase III study (SPIRIT-P1). J Rheumatol. 2018;45:367-377.
- van der Heijde D, Cheng-Chung Wei J, Dougados M, et al. Ixekizumab, an interleukin-17A antagonist in the treatment of ankylosing spondylitis or radiographic axial spondyloarthritis in patients previously untreated with biological disease-modifying anti-rheumatic drugs (COAST-V): 16 week results of a phase 3 randomised, double-blind, active-controlled and placebo-controlled trial. Lancet. 2018;392:2441-2451.
- Nakagawa H, Niiro H, Ootaki K, et al. Brodalumab, a human anti-interleukin-17-receptor antibody in the treatment of Japanese patients with moderate-to-severe plaque psoriasis: efficacy and safety results from a phase II randomized controlled study. J Dermatol Sci. 2016;81:44-52.
- Umezawa Y, Nakagawa H, Niiro H, et al. Long-term clinical safety and efficacy of brodalumab in the treatment of Japanese patients with moderate-to-severe plaque psoriasis. J Eur Acad Dermatol Venereol. 2016;30:1957-1960.
- Papp KA, Reich K, Paul C, et al. A prospective phase III, randomized, double-blind, placebo-controlled study of brodalumab in patients with moderate-to-severe plaque psoriasis. Br J Dermatol. 2016;175:273-286.
- Papp K, Leonardi C, Menter A, et al. Safety and efficacy of brodalumab for psoriasis after 120 weeks of treatment. J Am Acad Dermatol. 2014;71:1183.e3-1190.e3.
- Yamasaki K, Nakagawa H, Kubo Y, et al. Efficacy and safety of brodalumab in patients with generalized pustular psoriasis and psoriatic erythroderma: results from a 52-week, open-label study. Br J Dermatol. 2017;176:741-751.
- Mease PJ, Genovese MC, Greenwald MW, et al. Brodalumab, an anti-IL17RA monoclonal antibody, in psoriatic arthritis. N Engl J Med. 2014;370:2295-2306.
- Martin DA, Churchill M, Flores-Suarez L, et al. A phase Ib multiple ascending dose study evaluating safety, pharmacokinetics, and early clinical response of brodalumab, a human anti-IL-17R antibody, in methotrexate-resistant rheumatoid arthritis. Arthritis Res Ther. 2013;15:R164.
- Busse WW, Holgate S, Kerwin E, et al. Randomized, double-blind, placebo-controlled study of brodalumab, a human anti-IL-17 receptor monoclonal antibody, in moderate to severe asthma. Am J Respir Crit Care Med. 2013;188:1294-1302.
- Igarashi A, Kato T, Kato M, et al. Efficacy and safety of ustekinumab in Japanese patients with moderate-to-severe plaque-type psoriasis: long-term results from a phase 2/3 clinical trial. J Dermatol. 2012;39:242-252.
- Krueger GG, Langley RG, Leonardi C, et al. A human interleukin-12/23 monoclonal antibody for the treatment of psoriasis. N Engl J Med. 2007;356:580-592.
- Leonardi CL, Kimball AB, Papp KA, et al. Efficacy and safety of ustekinumab, a human interleukin-12/23 monoclonal antibody, in patients with psoriasis: 76-week results from a randomised, double-blind, placebo-controlled trial (PHOENIX 1). Lancet. 2008;371:1665-1674.
- Tsai TF, Ho JC, Song M, et al. Efficacy and safety of ustekinumab for the treatment of moderate-to-severe psoriasis: a phase III, randomized, placebo-controlled trial in Taiwanese and Korean patients (PEARL). J Dermatol Sci. 2011;63:154-163.
- Gordon KB, Strober B, Lebwohl M, et al. Efficacy and safety of risankizumab in moderate-to-severe plaque psoriasis (UltIMMa-1 and UltIMMa-2): results from two double-blind, randomised, placebo-controlled and ustekinumab-controlled phase 3 trials. Lancet. 2018;392:650-661.
- Krueger JG, Ferris LK, Menter A, et al. Anti-IL-23A mAb BI 655066 for treatment of moderate-to-severe psoriasis: safety, efficacy, pharmacokinetics, and biomarker results of a single-rising-dose, randomized, double-blind, placebo-controlled trial. J Allergy Clin Immunol. 2015;136:116.e7-124.e7.
- Ohtsuki M, Fujita H, Watanabe M, et al. Efficacy and safety of risankizumab in Japanese patients with moderate to severe plaque psoriasis: results from the SustaIMM phase 2/3 trial. J Dermatol. 2019;46:686-694.
- Papp KA, Blauvelt A, Bukhalo M, et al. Risankizumab versus ustekinumab for moderate-to-severe plaque psoriasis. N Engl J Med. 2017;376:1551-1560.
- Reich K, Gooderham M, Thaci D, et al. Risankizumab compared with adalimumab in patients with moderate-to-severe plaque psoriasis (IMMvent): a randomised, double-blind, active-comparator-controlled phase 3 trial. Lancet. 2019;394:576-586.
- Blauvelt A, Papp KA, Griffiths CE, et al. Efficacy and safety of guselkumab, an anti-interleukin-23 monoclonal antibody, compared with adalimumab for the continuous treatment of patients with moderate to severe psoriasis: results from the phase III, double-blinded, placebo- and active comparator-controlled VOYAGE 1 trial. J Am Acad Dermatol. 2017;76:405-417.
- Deodhar A, Gottlieb AB, Boehncke WH, et al. Efficacy and safety of guselkumab in patients with active psoriatic arthritis: a randomised, double-blind, placebo-controlled, phase 2 study. Lancet. 2018;391:2213-2224.
- Gordon KB, Duffin KC, Bissonnette R, et al. A phase 2 trial of guselkumab versus adalimumab for plaque psoriasis. N Engl J Med. 2015;373:136-144.
- Langley RG, Tsai TF, Flavin S, et al. Efficacy and safety of guselkumab in patients with psoriasis who have an inadequate response to ustekinumab: results of the randomized, double-blind, phase III NAVIGATE trial. Br J Dermatol. 2018;178:114-123.
- Nemoto O, Hirose K, Shibata S, et al. Safety and efficacy of guselkumab in Japanese patients with moderate-to-severe plaque psoriasis: a randomized, placebo-controlled, ascending-dose study. Br J Dermatol. 2018;178:689-696.
- Ohtsuki M, Kubo H, Morishima H, et al. Guselkumab, an anti-interleukin-23 monoclonal antibody, for the treatment of moderate to severe plaque-type psoriasis in Japanese patients: Efficacy and safety results from a phase 3, randomized, double-blind, placebo-controlled study. J Dermatol. 2018;45:1053-1062.
- Reich K, Armstrong AW, Foley P, et al. Efficacy and safety of guselkumab, an anti-interleukin-23 monoclonal antibody, compared with adalimumab for the treatment of patients with moderate to severe psoriasis with randomized withdrawal and retreatment: results from the phase III, double-blind, placebo- and active comparator-controlled VOYAGE 2 trial. J Am Acad Dermatol. 2017;76:418-431.
- Reich K, Armstrong AW, Langley RG, et al. Guselkumab versus secukinumab for the treatment of moderate-to-severe psoriasis (ECLIPSE): results from a phase 3, randomised controlled trial. Lancet. 2019;394:831-839.
- Terui T, Kobayashi S, Okubo Y, et al. Efficacy and safety of guselkumab, an anti-interleukin 23 monoclonal antibody, for palmoplantar pustulosis: a randomized clinical trial. JAMA Dermatol. 2018;154:309-316.
- Papp K, Thaci D, Reich K, et al. Tildrakizumab (MK-3222), an anti-interleukin-23p19 monoclonal antibody, improves psoriasis in a phase IIb randomized placebo-controlled trial. Br J Dermatol. 2015;173:930-939.
- Reich K, Papp KA, Blauvelt A, et al. Tildrakizumab versus placebo or etanercept for chronic plaque psoriasis (reSURFACE 1 and reSURFACE 2): results from two randomised controlled, phase 3 trials. Lancet. 2017;390:276-288.
- Gordon KB, Papp KA, Langley RG, et al. Long-term safety experience of ustekinumab in patients with moderate to severe psoriasis (part II of II): results from analyses of infections and malignancy from pooled phase II and III clinical trials. J Am Acad Dermatol. 2012;66:742-751.
- Papp KA, Griffiths CE, Gordon K, et al. Long-term safety of ustekinumab in patients with moderate-to-severe psoriasis: final results from 5 years of follow-up. Br J Dermatol. 2013;168:844-854.
- Saunte DM, Mrowietz U, Puig L, et al. Candida infections in patients with psoriasis and psoriatic arthritis treated with interleukin-17 inhibitors and their practical management. Br J Dermatol. 2017;177:47-62.
- Lis K, Kuzawinska O, Balkowiec-Iskra E. Tumor necrosis factor inhibitors—state of knowledge. Arch Med Sci. 2014;10:1175-1185.
- Hage CA, Bowyer S, Tarvin SE, et al. Recognition, diagnosis, and treatment of histoplasmosis complicating tumor necrosis factor blocker therapy. Clin Infect Dis. 2010;50:85-92
- Hage CA, Ribes JA, Wengenack NL, et al. A multicenter evaluation of tests for diagnosis of histoplasmosis. Clin Infect Dis. 2011;53:448-454.
Psoriasis is a common chronic, multisystem, inflammatory disease with predominantly skin and joint manifestations that affects approximately 2% of the world’s population.1 It occurs in a variety of clinical forms, from a few well-demarcated, erythematous plaques with a silvery scale to involvement of almost the entire body surface area. Beyond the debilitating physical ailments of the disease, psoriasis also may have psychosocial effects on quality of life.2 The pathogenesis of psoriasis is not fully understood but represents a complex multifactorial disease with both immune-mediated and genetic components. Characterized by hyperplasia of epidermal keratinocytes, psoriasis is shown to be mediated by infiltration of T-cell lymphocytes with an increase of various inflammatory cytokines, including
With the growing understanding of the pathophysiology of psoriasis, focused biologics have been developed to target specific cytokines implicated in the disease process and have been increasingly utilized. Tumor necrosis factor α inhibitors, including adalimumab, infliximab, and etanercept, along with the IL-12/IL-23 inhibitor ustekinumab, have been revolutionary in psoriasis treatment by providing safe and effective long-term therapy; however, there is concern of life-threatening infections with biologics because of the immunosuppressive effects and mechanisms of action.6 Specifically, there have been reported cases of deep fungal infections associated with TNF-α inhibitor use.7
Recently, the advent of IL-17 and IL-23 inhibitors has garnered notable interest in these biologics as promising treatments for psoriasis. With IL-17 and IL-23 supported to have a major role in the pathogenesis of psoriasis, targeting the cytokine is not only logical but also has proven to be effacacious.8-10 Secukinumab, ixekizumab, and brodalumab are IL-17 inhibitors that have been approved by the US Food and Drug Administration (FDA) for the treatment of psoriasis. Secukinumab and ixekizumab are anti–IL-17A monoclonal antibodies, whereas brodalumab is an anti–IL-17 receptor antibody. Risankizumab, guselkumab, and tildrakizumab are IL-23 inhibitors that also have been approved by the FDA for the treatment of psoriasis. As with older biologics, there is concern over the safety of these inhibitors because of the central role of IL-17 and IL-23 in both innate and adaptive immune responses, particularly against fungi.11 Therefore, use of biologics targeting IL-17 and IL-23 may increase susceptibility to deep fungal infections.
Safety data and discussion of the risk for deep fungal infections from IL-17, IL-12/IL-23, and IL-23 inhibitor use for psoriasis treatment currently are lacking. Given the knowledge gap, we sought to synthesize and review the current evidence on risks for deep fungal infections during biologic therapy in patients with psoriasis, with a focus on IL-17 inhibitor therapies.
METHODS
A PubMed search of articles indexed for MEDLINE from database inception to 2019 (1946-2019) was performed to find randomized controlled trials (RCTs), including extended trials and clinical trials, for IL-17, IL-12/IL-23, and IL-23 inhibitors approved by the FDA for psoriasis treatment. The following keywords were used: psoriasis or inflammatory disease and secukinumab, ixekizumab, brodalumab, ustekinumab, risankizumab, guselkumab, or tildrakizumab. Studies were restricted to the English-language literature, and those that did not provide adequate safety data on the specific types of infections that occurred were excluded.
RESULTSIL-17 Inhibitors
Our search yielded RCTs, some including extension trials, and clinical trials of IL-17 inhibitors used for psoriatic disease and other nonpsoriatic conditions (Table).

Risk for Deep Fungal Infection With Secukinumab
The queried studies included 20 RCTs or clinical trials along with extension trials of 3746 patients with psoriasis or other inflammatory conditions, with follow-up ranging from 12 to 52 weeks. In a 3-year extension study of SCULPTURE, Bissonnette et al12 reported no new safety concerns for the 340 patients with moderate to severe psoriasis treated with secukinumab. Common adverse events (AEs) included nasopharyngitis, upper respiratory tract infections, and headache, but there were no reports of deep fungal infections.12 In a subsequent 5-year analysis of 168 patients that focused on the 300-mg fixed interval treatment with secukinumab, the safety profile remained favorable, with 0 reports of invasive fungal infections.13 A study (FEATURE) of 118 patients with psoriasis treated with a prefilled syringe of 300 or 150 mg of secukinumab also described an acceptable safety profile and reported no deep fungal infections.14 JUNCTURE, another study utilizing autoinjectors, also found that treatment with 300 or 150 mg of secukinumab was well tolerated in 121 patients, with no deep fungal infections.15 Common AEs for both studies included nasopharyngitis and headache.14,15 A 24-week phase 3 study for scalp psoriasis treated with secukinumab also reported 0 deep fungal infections in 51 patients.16 In an RCT comparing secukinumab and ustekinumab for moderate to severe plaque psoriasis, Blauvelt et al17 demonstrated that the incidence of serious AEs was comparable between the 2 groups, with no reports of invasive fungal infections in the 334 patients exposed to secukinumab. The CLEAR study, which compared secukinumab and ustekinumab, also found no reported deep fungal disease in the 335 patients exposed to secukinumab.18 Secukinumab exhibited a similar safety profile to ustekinumab in both studies, with common AEs being headache and nasopharyngitis.17,18 The GESTURE study investigated the efficacy of secukinumab in 137 patients with palmoplantar psoriasis and reported a favorable profile with no reports of deep fungal disease.19 In a subanalysis of the phase 3 study ERASURE, secukinumab was shown to have a robust and sustainable efficacy in 58 Japanese patients with moderate to severe plaque psoriasis, and there were no reports of invasive fungal infections.20 Another subanalysis of 36 Taiwanese patients from the ERASURE study also had similar findings, with no dose relationship observed for AEs.21 In a phase 2 study of 103 patients with psoriasis, Papp et al22 demonstrated AE rates that were similar across different doses of secukinumab—3×150 mg, 3×75 mg, 3×25 mg, and 1×25 mg—and described no incidences of invasive fungal disease. In a phase 2 regimen-finding study of 337 patients conducted by Rich et al,23 the most commonly reported AEs included nasopharyngitis, worsening psoriasis, and upper respiratory tract infections, but there were no reported deep fungal infections.
Our search also resulted in studies specific to the treatment of psoriatic arthritis (PsA) with secukinumab. McInnes et al9 conducted a phase 2 proof-of-concept trial for patients with PsA and reported no deep fungal infections in 28 patients exposed to 10 mg/kg of secukinumab. A 2-year follow-up with the cohort from FUTURE 1, a phase 3 clinical trial, also showed no new or unexpected safety signals in 404 patients exposed to 150 or 75 mg of secukinumab, including no reports of invasive fungal disease.24 FUTURE 2, a phase 3 clinical trial, demonstrated that the most common AE was upper respiratory tract infection in the 299 patients treatedwith secukinumab, but there were no recorded invasive fungal infections.25 In FUTURE 3, 277 patients were treated with secukinumab, with 14 nonserious candida infections but no observed deep fungal infections.26 A study comparing secukinumab to fumaric acid esters reported that 6 of 105 patients treated with secukinumab also experienced superficial candidiasis, but there were no reports of deep fungal disease.27
Secukinumab also has been used in the treatment of ankylosing spondylitis in a phase 3 RCT (MEASURE 1) in which 4 cases of superficial candidiasis were reported (0.7 cases per 100 patient-years of secukinumab) that were all resolved with standard antifungal therapy.28 In MEASURE 2, a 5-year phase 3 RCT, 145 patients were treated with secukinumab for ankylosing spondylitis, with common AEs including nasopharyngitis, diarrhea, and upper respiratory tract infection, but there were no reports of any invasive fungal infections.29 MEASURE 3 also demonstrated similar results in which no invasive fungal infections were observed.30
Risk for Deep Fungal Infection With Ixekizumab
The queried studies included 7 RCTs or clinical trials of 3523 patients with psoriasis or other inflammatory conditions, with follow-up ranging from 12 to 52 weeks. In UNCOVER-A, a phase 3 RCT of the pharmacokinetics and safety of ixekizumab, 204 patients were randomized to a prefilled syringe or autoinjector; 48% of patients experienced AEs, but no invasive fungal infections were observed.31 In an analysis of 3 phase 3 trials of ixekizumab including a total 2334 patients treated with ixekizumab from UNCOVER-1, UNCOVER-2, and UNCOVER-3, oral candidiasis frequently was reported, but no candidal infections met criteria for serious invasive infection.32 In UNCOVER-J, a 52-week phase 3 open-label trial of Japanese patients, 91 patients were treated for plaque psoriasis, erythrodermic psoriasis, or generalized pustular psoriasis using ixekizumab; the most common AEs included allergic reactions and injection-site reactions. One case of oral candidiasis was reported, but there were no reported cases of invasive fungal infections.33 A comparison of ixekizumab vs ustekinumab from the IXORA-S trial demonstrated no substantial differences in AEs between the two, and no cases of deep fungal infections were reported. The most common AE between the 2 groups was nasopharyngitis.34 An open-label extension over 4 years of a phase 2 RCT treated 211 patients with either 120 or 80 mg of ixekizumab; 87% of patients had experienced at least 1 AE, and all AEs were considered mild or moderate in severity, with no invasive fungal disease.35
Our search also resulted in 1 study specific to the treatment of PsA with ixekizumab. A phase 3, 52-week study of patients treated with ixekizumab for PsA observed 2 incidences of oral candidiasis and nail candida infections, but no invasive fungal infections were reported.36
We also found 1 study of ixekizumab used in the treatment of ankylosing spondylitis. COAST-V was a phase 3 RCT of patients treated for ankylosing spondylitis in which 164 patients were treated with ixekizumab; no serious AEs were recorded, including 0 deep fungal infections. The most common AEs observed were nasopharyngitis and upper respiratory tract infections.37
Risk for Deep Fungal Infection With Brodalumab
The queried studies included 9 RCTs and 3 clinical trials along with extension trials of 1599 patients with psoriasis or other inflammatory conditions, with follow-up ranging from 12 to 120 weeks. In a phase 2 RCT of Japanese patients with moderate to severe plaque psoriasis, 113 patients were treated with 70, 140, or 210 mg of brodalumab, and the most common AEs were nasopharyngitis, diarrhea, and upper respiratory tract inflammation. There were no reported cases of fungal infections in the study.38 In an open-label extension study of Japanese patients that evaluated the long-term clinical safety of brodalumab, 145 patients were enrolled and observed similar AEs to the RCT, with 7 patients experiencing oral candidiasis and 1 patient having skin candidiasis, but there were no observed deep fungal infections.39 In AMG 827, which evaluated the efficacy and safety of brodalumab, 320 patients were treated, and only 2 serious AEs were reported, neither of which were deep fungal disease.10 A phase 3 RCT conducted by Papp et al40 (AMAGINE-1) also treated 441 patients with moderate to severe plaque psoriasis with brodalumab and observed candida infections in 9 patients that were mild to moderate and responsive to treatment, with no patients discontinuing the study. In a 120-week open-label extension study of 181 patients, Papp et al41 reported 8% of patients experienced serious AEs, with 1 case of latent tuberculosis that led to withdrawal of treatment. A study also investigated the efficacy and safety of brodalumab in 30 patients with generalized pustular psoriasis or psoriatic erythroderma and observed 2 cases of mild candida infections that resolved with treatment. There were no reports of invasive fungal disease.42
Our search also resulted in studies of brodalumab used in the treatment of PsA and nonpsoriatic diseases. In one phase 2 RCT, 113 patients with PsA were treated with 140 mg, 280 mg, or combined doses of brodalumab, with the most common AEs being nasopharyngitis, upper respiratory tract infection, and diarrhea, but there were no reports of deep fungal infection.43 In a phase 1b trial of patients with methotrexate-resistant rheumatoid arthritis treated with brodalumab, common AEs reported included headache, cough, and abdominal pain, with only 1 case of oral candidiasis that was determined not to be drug related.44 Finally, an RCT of patients with moderate to severe asthma treated 226 patients with brodalumab and reported a greater incidence of oral candidiasis in treatment groups compared with placebo (3.5% vs 0%) but saw no instances of invasive fungal infection.45
IL-12/IL-23 Inhibitor
Risk for Deep Fungal Infection With Ustekinumab
The queried studies included 4 RCTs of 954 patients with psoriasis treated with ustekinumab (eTable).46-49 Within these trials, there were no reported cases of serious infections involving deep fungal organisms during the stated follow-up period. The literature search also found long-term safety data from the ACCEPT and PHOENIX trials that included 5437 patients with psoriasis treated with ustekinumab.66,67 There also were no demonstrated incidences of invasive fungal disease in these studies, with most cases of infection being common bacterial or viral infections.

IL-23 Inhibitors
Risk for Deep Fungal Infection With Risankizumab, Guselkumab, and Tildrakizumab
The queried studies included 16 RCTs or clinical trials for psoriatic patients treated with IL-23 inhibitors, including 5 with risankizumab,50-54 9 with guselkumab,55-63 and 2 with tildrakizumab.64,65 Within these trials there were no observed cases of serious infections with deep fungal disease.
COMMENT
Our literature review has demonstrated that there does not appear to be an increased incidence of deep fungal infections for patients treated with IL-17, IL-12/IL-23, or IL-23 inhibitors for psoriatic disease. All of the reviewed studies found no cases of invasive fungal infections for patients with psoriasis treated with secukinumab, ixekizumab, brodalumab, ustekinumab, risankizumab, guselkumab, or tildrakizumab. Patients with other inflammatory conditions, such as ankylosing spondylitis, rheumatoid arthritis, and asthma, also did not appear to show an increased incidence of deep fungal disease.
Although these results show promising safety data for the use of these biologic therapies in treating inflammatory conditions, caution still is warranted, as these medications still are relatively new, with FDA approvals within the last 5 years. Safety data among different study populations also cannot be derived without further investigation, and much of the available literature is limited in long-term data. More extended trials or registry data from a large, broadly representative cohort are necessary to establish the long-term safety and risk for deep fungal infections with IL-17 and especially the newer IL-23 inhibitors.
A small percentage of patients from the reviewed literature did develop superficial candidiasis. This outcome can be expected, as the central role of IL-17 and IL-23 has been recognized in immunologic protection against infections, specifically against fungi.11 Because all of the fungal infections reported for patients on IL-17 inhibitors were superficial candidiasis, guides for practical management and treatment should be implemented to standardize future research and care. A proposed screening algorithm for patients on these biologic therapies involves safety monitoring, including inspection of the oral cavity, folds, and genitals, along with inquiring about symptoms such as burning, dysgeusia, and dysuria.68 If infection is suspected, confirmation by culture, molecular method, or optimally with esophagoscopy can be performed, and appropriate treatment may be initiated.68 Patients with candida infections of the oral cavity, folds, or genitals can be placed on topical therapy such as nystatin, amphotericin B, ciclopirox, or other azoles, while those with infections of the esophagus can be started on oral fluconazole.68
Although there were no reported cases of deep fungal infections, the theoretical risk for developing one while on IL-17 and IL-23 inhibitors may warrant further screening prior to beginning therapy. The TNF inhibitors approved for the treatment of psoriasis currently contain a black box warning for risk for disseminated and extrapulmonary histoplasmosis, coccidioidomycosis, blastomycosis, and other invasive fungal infections, which may highlight the importance of thorough evaluation and awareness of endemic areas for patients on biologics. Prior to initiating treatment with TNF inhibitors, current suggestions involve performing a thorough examination along with keeping a high index of suspicion for invasive fungal infections in patients who live in or have traveled to endemic regions.69
Screening for invasive fungal infections for patients on TNF inhibitors involves questioning about potential exposures, such as demolition of old buildings, bird roosts, or spelunking.70 Serologies or antigen testing can be used routinely, but as these tests are insensitive, empiric antifungal therapy should be initiated if there is high enough clinical suspicion.71 Currently, there are no clinical guidelines regarding fungal screening and initiation of IL-17 and IL-23 inhibitors for treatment of psoriasis and other inflammatory conditions, but careful stewardship over using these effective medications should still be practiced.
Upon review of the available safety data on the use of IL-17 and IL-23 inhibitors for the treatment of psoriasis and other inflammatory conditions, there does not appear to be an increased incidence of deep fungal infections. Physicians, however, should still be cautiously optimistic in prescribing these medications, as there is a theoretical risk for infection for all patients on biologics. A high index of suspicion for patients presenting with symptoms of fungal infections should be maintained, and appropriate diagnosis and management should be initiated if they do occur.
Psoriasis is a common chronic, multisystem, inflammatory disease with predominantly skin and joint manifestations that affects approximately 2% of the world’s population.1 It occurs in a variety of clinical forms, from a few well-demarcated, erythematous plaques with a silvery scale to involvement of almost the entire body surface area. Beyond the debilitating physical ailments of the disease, psoriasis also may have psychosocial effects on quality of life.2 The pathogenesis of psoriasis is not fully understood but represents a complex multifactorial disease with both immune-mediated and genetic components. Characterized by hyperplasia of epidermal keratinocytes, psoriasis is shown to be mediated by infiltration of T-cell lymphocytes with an increase of various inflammatory cytokines, including
With the growing understanding of the pathophysiology of psoriasis, focused biologics have been developed to target specific cytokines implicated in the disease process and have been increasingly utilized. Tumor necrosis factor α inhibitors, including adalimumab, infliximab, and etanercept, along with the IL-12/IL-23 inhibitor ustekinumab, have been revolutionary in psoriasis treatment by providing safe and effective long-term therapy; however, there is concern of life-threatening infections with biologics because of the immunosuppressive effects and mechanisms of action.6 Specifically, there have been reported cases of deep fungal infections associated with TNF-α inhibitor use.7
Recently, the advent of IL-17 and IL-23 inhibitors has garnered notable interest in these biologics as promising treatments for psoriasis. With IL-17 and IL-23 supported to have a major role in the pathogenesis of psoriasis, targeting the cytokine is not only logical but also has proven to be effacacious.8-10 Secukinumab, ixekizumab, and brodalumab are IL-17 inhibitors that have been approved by the US Food and Drug Administration (FDA) for the treatment of psoriasis. Secukinumab and ixekizumab are anti–IL-17A monoclonal antibodies, whereas brodalumab is an anti–IL-17 receptor antibody. Risankizumab, guselkumab, and tildrakizumab are IL-23 inhibitors that also have been approved by the FDA for the treatment of psoriasis. As with older biologics, there is concern over the safety of these inhibitors because of the central role of IL-17 and IL-23 in both innate and adaptive immune responses, particularly against fungi.11 Therefore, use of biologics targeting IL-17 and IL-23 may increase susceptibility to deep fungal infections.
Safety data and discussion of the risk for deep fungal infections from IL-17, IL-12/IL-23, and IL-23 inhibitor use for psoriasis treatment currently are lacking. Given the knowledge gap, we sought to synthesize and review the current evidence on risks for deep fungal infections during biologic therapy in patients with psoriasis, with a focus on IL-17 inhibitor therapies.
METHODS
A PubMed search of articles indexed for MEDLINE from database inception to 2019 (1946-2019) was performed to find randomized controlled trials (RCTs), including extended trials and clinical trials, for IL-17, IL-12/IL-23, and IL-23 inhibitors approved by the FDA for psoriasis treatment. The following keywords were used: psoriasis or inflammatory disease and secukinumab, ixekizumab, brodalumab, ustekinumab, risankizumab, guselkumab, or tildrakizumab. Studies were restricted to the English-language literature, and those that did not provide adequate safety data on the specific types of infections that occurred were excluded.
RESULTSIL-17 Inhibitors
Our search yielded RCTs, some including extension trials, and clinical trials of IL-17 inhibitors used for psoriatic disease and other nonpsoriatic conditions (Table).

Risk for Deep Fungal Infection With Secukinumab
The queried studies included 20 RCTs or clinical trials along with extension trials of 3746 patients with psoriasis or other inflammatory conditions, with follow-up ranging from 12 to 52 weeks. In a 3-year extension study of SCULPTURE, Bissonnette et al12 reported no new safety concerns for the 340 patients with moderate to severe psoriasis treated with secukinumab. Common adverse events (AEs) included nasopharyngitis, upper respiratory tract infections, and headache, but there were no reports of deep fungal infections.12 In a subsequent 5-year analysis of 168 patients that focused on the 300-mg fixed interval treatment with secukinumab, the safety profile remained favorable, with 0 reports of invasive fungal infections.13 A study (FEATURE) of 118 patients with psoriasis treated with a prefilled syringe of 300 or 150 mg of secukinumab also described an acceptable safety profile and reported no deep fungal infections.14 JUNCTURE, another study utilizing autoinjectors, also found that treatment with 300 or 150 mg of secukinumab was well tolerated in 121 patients, with no deep fungal infections.15 Common AEs for both studies included nasopharyngitis and headache.14,15 A 24-week phase 3 study for scalp psoriasis treated with secukinumab also reported 0 deep fungal infections in 51 patients.16 In an RCT comparing secukinumab and ustekinumab for moderate to severe plaque psoriasis, Blauvelt et al17 demonstrated that the incidence of serious AEs was comparable between the 2 groups, with no reports of invasive fungal infections in the 334 patients exposed to secukinumab. The CLEAR study, which compared secukinumab and ustekinumab, also found no reported deep fungal disease in the 335 patients exposed to secukinumab.18 Secukinumab exhibited a similar safety profile to ustekinumab in both studies, with common AEs being headache and nasopharyngitis.17,18 The GESTURE study investigated the efficacy of secukinumab in 137 patients with palmoplantar psoriasis and reported a favorable profile with no reports of deep fungal disease.19 In a subanalysis of the phase 3 study ERASURE, secukinumab was shown to have a robust and sustainable efficacy in 58 Japanese patients with moderate to severe plaque psoriasis, and there were no reports of invasive fungal infections.20 Another subanalysis of 36 Taiwanese patients from the ERASURE study also had similar findings, with no dose relationship observed for AEs.21 In a phase 2 study of 103 patients with psoriasis, Papp et al22 demonstrated AE rates that were similar across different doses of secukinumab—3×150 mg, 3×75 mg, 3×25 mg, and 1×25 mg—and described no incidences of invasive fungal disease. In a phase 2 regimen-finding study of 337 patients conducted by Rich et al,23 the most commonly reported AEs included nasopharyngitis, worsening psoriasis, and upper respiratory tract infections, but there were no reported deep fungal infections.
Our search also resulted in studies specific to the treatment of psoriatic arthritis (PsA) with secukinumab. McInnes et al9 conducted a phase 2 proof-of-concept trial for patients with PsA and reported no deep fungal infections in 28 patients exposed to 10 mg/kg of secukinumab. A 2-year follow-up with the cohort from FUTURE 1, a phase 3 clinical trial, also showed no new or unexpected safety signals in 404 patients exposed to 150 or 75 mg of secukinumab, including no reports of invasive fungal disease.24 FUTURE 2, a phase 3 clinical trial, demonstrated that the most common AE was upper respiratory tract infection in the 299 patients treatedwith secukinumab, but there were no recorded invasive fungal infections.25 In FUTURE 3, 277 patients were treated with secukinumab, with 14 nonserious candida infections but no observed deep fungal infections.26 A study comparing secukinumab to fumaric acid esters reported that 6 of 105 patients treated with secukinumab also experienced superficial candidiasis, but there were no reports of deep fungal disease.27
Secukinumab also has been used in the treatment of ankylosing spondylitis in a phase 3 RCT (MEASURE 1) in which 4 cases of superficial candidiasis were reported (0.7 cases per 100 patient-years of secukinumab) that were all resolved with standard antifungal therapy.28 In MEASURE 2, a 5-year phase 3 RCT, 145 patients were treated with secukinumab for ankylosing spondylitis, with common AEs including nasopharyngitis, diarrhea, and upper respiratory tract infection, but there were no reports of any invasive fungal infections.29 MEASURE 3 also demonstrated similar results in which no invasive fungal infections were observed.30
Risk for Deep Fungal Infection With Ixekizumab
The queried studies included 7 RCTs or clinical trials of 3523 patients with psoriasis or other inflammatory conditions, with follow-up ranging from 12 to 52 weeks. In UNCOVER-A, a phase 3 RCT of the pharmacokinetics and safety of ixekizumab, 204 patients were randomized to a prefilled syringe or autoinjector; 48% of patients experienced AEs, but no invasive fungal infections were observed.31 In an analysis of 3 phase 3 trials of ixekizumab including a total 2334 patients treated with ixekizumab from UNCOVER-1, UNCOVER-2, and UNCOVER-3, oral candidiasis frequently was reported, but no candidal infections met criteria for serious invasive infection.32 In UNCOVER-J, a 52-week phase 3 open-label trial of Japanese patients, 91 patients were treated for plaque psoriasis, erythrodermic psoriasis, or generalized pustular psoriasis using ixekizumab; the most common AEs included allergic reactions and injection-site reactions. One case of oral candidiasis was reported, but there were no reported cases of invasive fungal infections.33 A comparison of ixekizumab vs ustekinumab from the IXORA-S trial demonstrated no substantial differences in AEs between the two, and no cases of deep fungal infections were reported. The most common AE between the 2 groups was nasopharyngitis.34 An open-label extension over 4 years of a phase 2 RCT treated 211 patients with either 120 or 80 mg of ixekizumab; 87% of patients had experienced at least 1 AE, and all AEs were considered mild or moderate in severity, with no invasive fungal disease.35
Our search also resulted in 1 study specific to the treatment of PsA with ixekizumab. A phase 3, 52-week study of patients treated with ixekizumab for PsA observed 2 incidences of oral candidiasis and nail candida infections, but no invasive fungal infections were reported.36
We also found 1 study of ixekizumab used in the treatment of ankylosing spondylitis. COAST-V was a phase 3 RCT of patients treated for ankylosing spondylitis in which 164 patients were treated with ixekizumab; no serious AEs were recorded, including 0 deep fungal infections. The most common AEs observed were nasopharyngitis and upper respiratory tract infections.37
Risk for Deep Fungal Infection With Brodalumab
The queried studies included 9 RCTs and 3 clinical trials along with extension trials of 1599 patients with psoriasis or other inflammatory conditions, with follow-up ranging from 12 to 120 weeks. In a phase 2 RCT of Japanese patients with moderate to severe plaque psoriasis, 113 patients were treated with 70, 140, or 210 mg of brodalumab, and the most common AEs were nasopharyngitis, diarrhea, and upper respiratory tract inflammation. There were no reported cases of fungal infections in the study.38 In an open-label extension study of Japanese patients that evaluated the long-term clinical safety of brodalumab, 145 patients were enrolled and observed similar AEs to the RCT, with 7 patients experiencing oral candidiasis and 1 patient having skin candidiasis, but there were no observed deep fungal infections.39 In AMG 827, which evaluated the efficacy and safety of brodalumab, 320 patients were treated, and only 2 serious AEs were reported, neither of which were deep fungal disease.10 A phase 3 RCT conducted by Papp et al40 (AMAGINE-1) also treated 441 patients with moderate to severe plaque psoriasis with brodalumab and observed candida infections in 9 patients that were mild to moderate and responsive to treatment, with no patients discontinuing the study. In a 120-week open-label extension study of 181 patients, Papp et al41 reported 8% of patients experienced serious AEs, with 1 case of latent tuberculosis that led to withdrawal of treatment. A study also investigated the efficacy and safety of brodalumab in 30 patients with generalized pustular psoriasis or psoriatic erythroderma and observed 2 cases of mild candida infections that resolved with treatment. There were no reports of invasive fungal disease.42
Our search also resulted in studies of brodalumab used in the treatment of PsA and nonpsoriatic diseases. In one phase 2 RCT, 113 patients with PsA were treated with 140 mg, 280 mg, or combined doses of brodalumab, with the most common AEs being nasopharyngitis, upper respiratory tract infection, and diarrhea, but there were no reports of deep fungal infection.43 In a phase 1b trial of patients with methotrexate-resistant rheumatoid arthritis treated with brodalumab, common AEs reported included headache, cough, and abdominal pain, with only 1 case of oral candidiasis that was determined not to be drug related.44 Finally, an RCT of patients with moderate to severe asthma treated 226 patients with brodalumab and reported a greater incidence of oral candidiasis in treatment groups compared with placebo (3.5% vs 0%) but saw no instances of invasive fungal infection.45
IL-12/IL-23 Inhibitor
Risk for Deep Fungal Infection With Ustekinumab
The queried studies included 4 RCTs of 954 patients with psoriasis treated with ustekinumab (eTable).46-49 Within these trials, there were no reported cases of serious infections involving deep fungal organisms during the stated follow-up period. The literature search also found long-term safety data from the ACCEPT and PHOENIX trials that included 5437 patients with psoriasis treated with ustekinumab.66,67 There also were no demonstrated incidences of invasive fungal disease in these studies, with most cases of infection being common bacterial or viral infections.

IL-23 Inhibitors
Risk for Deep Fungal Infection With Risankizumab, Guselkumab, and Tildrakizumab
The queried studies included 16 RCTs or clinical trials for psoriatic patients treated with IL-23 inhibitors, including 5 with risankizumab,50-54 9 with guselkumab,55-63 and 2 with tildrakizumab.64,65 Within these trials there were no observed cases of serious infections with deep fungal disease.
COMMENT
Our literature review has demonstrated that there does not appear to be an increased incidence of deep fungal infections for patients treated with IL-17, IL-12/IL-23, or IL-23 inhibitors for psoriatic disease. All of the reviewed studies found no cases of invasive fungal infections for patients with psoriasis treated with secukinumab, ixekizumab, brodalumab, ustekinumab, risankizumab, guselkumab, or tildrakizumab. Patients with other inflammatory conditions, such as ankylosing spondylitis, rheumatoid arthritis, and asthma, also did not appear to show an increased incidence of deep fungal disease.
Although these results show promising safety data for the use of these biologic therapies in treating inflammatory conditions, caution still is warranted, as these medications still are relatively new, with FDA approvals within the last 5 years. Safety data among different study populations also cannot be derived without further investigation, and much of the available literature is limited in long-term data. More extended trials or registry data from a large, broadly representative cohort are necessary to establish the long-term safety and risk for deep fungal infections with IL-17 and especially the newer IL-23 inhibitors.
A small percentage of patients from the reviewed literature did develop superficial candidiasis. This outcome can be expected, as the central role of IL-17 and IL-23 has been recognized in immunologic protection against infections, specifically against fungi.11 Because all of the fungal infections reported for patients on IL-17 inhibitors were superficial candidiasis, guides for practical management and treatment should be implemented to standardize future research and care. A proposed screening algorithm for patients on these biologic therapies involves safety monitoring, including inspection of the oral cavity, folds, and genitals, along with inquiring about symptoms such as burning, dysgeusia, and dysuria.68 If infection is suspected, confirmation by culture, molecular method, or optimally with esophagoscopy can be performed, and appropriate treatment may be initiated.68 Patients with candida infections of the oral cavity, folds, or genitals can be placed on topical therapy such as nystatin, amphotericin B, ciclopirox, or other azoles, while those with infections of the esophagus can be started on oral fluconazole.68
Although there were no reported cases of deep fungal infections, the theoretical risk for developing one while on IL-17 and IL-23 inhibitors may warrant further screening prior to beginning therapy. The TNF inhibitors approved for the treatment of psoriasis currently contain a black box warning for risk for disseminated and extrapulmonary histoplasmosis, coccidioidomycosis, blastomycosis, and other invasive fungal infections, which may highlight the importance of thorough evaluation and awareness of endemic areas for patients on biologics. Prior to initiating treatment with TNF inhibitors, current suggestions involve performing a thorough examination along with keeping a high index of suspicion for invasive fungal infections in patients who live in or have traveled to endemic regions.69
Screening for invasive fungal infections for patients on TNF inhibitors involves questioning about potential exposures, such as demolition of old buildings, bird roosts, or spelunking.70 Serologies or antigen testing can be used routinely, but as these tests are insensitive, empiric antifungal therapy should be initiated if there is high enough clinical suspicion.71 Currently, there are no clinical guidelines regarding fungal screening and initiation of IL-17 and IL-23 inhibitors for treatment of psoriasis and other inflammatory conditions, but careful stewardship over using these effective medications should still be practiced.
Upon review of the available safety data on the use of IL-17 and IL-23 inhibitors for the treatment of psoriasis and other inflammatory conditions, there does not appear to be an increased incidence of deep fungal infections. Physicians, however, should still be cautiously optimistic in prescribing these medications, as there is a theoretical risk for infection for all patients on biologics. A high index of suspicion for patients presenting with symptoms of fungal infections should be maintained, and appropriate diagnosis and management should be initiated if they do occur.
- Parisi R, Symmons DP, Griffiths CE, et al. Global epidemiology of psoriasis: a systematic review of incidence and prevalence. J Invest Dermatol. 2013;133:377-385.
- Koo J, Marangell LB, Nakamura M, et al. Depression and suicidality in psoriasis: review of the literature including the cytokine theory of depression. J Eur Acad Dermatol Venereol. 2017;31:1999-2009.
- Krueger JG, Bowcock A. Psoriasis pathophysiology: current concepts of pathogenesis. Ann Rheum Dis. 2005;64 (suppl 2):ii30-36.
- Lee E, Trepicchio WL, Oestreicher JL, et al. Increased expression of interleukin 23 p19 and p40 in lesional skin of patients with psoriasis vulgaris. J Exp Med. 2004;199:125-130.
- Lowes MA, Kikuchi T, Fuentes-Duculan J, et al. Psoriasis vulgaris lesions contain discrete populations of Th1 and Th17 T cells. J Invest Dermatol. 2008;128:1207-1211.
- Shear NH. Fulfilling an unmet need in psoriasis: do biologicals hold the key to improved tolerability? Drug Saf. 2006;29:49-66.
- Lee JH, Slifman NR, Gershon SK, et al. Life-threatening histoplasmosis complicating immunotherapy with tumor necrosis factor alpha antagonists infliximab and etanercept. Arthritis Rheum. 2002;46:2565-2570.
- Leonardi C, Matheson R, Zachariae C, et al. Anti-interleukin-17 monoclonal antibody ixekizumab in chronic plaque psoriasis. N Engl J Med. 2012;366:1190-1199.
- McInnes IB, Sieper J, Braun J, et al. Efficacy and safety of secukinumab, a fully human anti-interleukin-17A monoclonal antibody, in patients with moderate-to-severe psoriatic arthritis: a 24-week, randomised, double-blind, placebo-controlled, phase II proof-of-concept trial. Ann Rheum Dis. 2014;73:349-356.
- Papp KA, Leonardi C, Menter A, et al. Brodalumab, an anti-interleukin-17-receptor antibody for psoriasis. N Engl J Med. 2012;366:1181-1189.
- Isailovic N, Daigo K, Mantovani A, et al. Interleukin-17 and innate immunity in infections and chronic inflammation. J Autoimmun. 2015;60:1-11.
- Bissonnette R, Luger T, Thaci D, et al. Secukinumab sustains good efficacy and favourable safety in moderate-to-severe psoriasis after up to 3 years of treatment: results from a double-blind extension study. Br J Dermatol. 2017;177:1033-1042.
- Bissonnette R, Luger T, Thaci D, et al. Secukinumab demonstrates high sustained efficacy and a favourable safety profile in patients with moderate-to-severe psoriasis through 5 years of treatment (SCULPTURE Extension Study). J Eur Acad Dermatol Venereol. 2018;32:1507-1514.
- Blauvelt A, Prinz JC, Gottlieb AB, et al. Secukinumab administration by pre-filled syringe: efficacy, safety and usability results from a randomized controlled trial in psoriasis (FEATURE). Br J Dermatol. 2015;172:484-493.
- Paul C, Lacour JP, Tedremets L, et al. Efficacy, safety and usability of secukinumab administration by autoinjector/pen in psoriasis: a randomized, controlled trial (JUNCTURE). J Eur Acad Dermatol Venereol. 2015;29:1082-1090.
- Bagel J, Duffin KC, Moore A, et al. The effect of secukinumab on moderate-to-severe scalp psoriasis: Results of a 24-week, randomized, double-blind, placebo-controlled phase 3b study. J Am Acad Dermatol. 2017;77:667-674.
- Blauvelt A, Reich K, Tsai TF, et al. Secukinumab is superior to ustekinumab in clearing skin of subjects with moderate-to-severe plaque psoriasis up to 1 year: results from the CLEAR study. J Am Acad Dermatol. 2017;76:60.e9-69.e9.
- Thaci D, Blauvelt A, Reich K, et al. Secukinumab is superior to ustekinumab in clearing skin of subjects with moderate to severe plaque psoriasis: CLEAR, a randomized controlled trial. J Am Acad Dermatol. 2015;73:400-409.
- Gottlieb A, Sullivan J, van Doorn M, et al. Secukinumab shows significant efficacy in palmoplantar psoriasis: results from GESTURE, a randomized controlled trial. J Am Acad Dermatol. 2017;76:70-80.
- Ohtsuki M, Morita A, Abe M, et al. Secukinumab efficacy and safety in Japanese patients with moderate-to-severe plaque psoriasis: subanalysis from ERASURE, a randomized, placebo-controlled, phase 3 study. J Dermatol. 2014;41:1039-1046.
- Wu NL, Hsu CJ, Sun FJ, et al. Efficacy and safety of secukinumab in Taiwanese patients with moderate to severe plaque psoriasis: subanalysis from ERASURE phase III study. J Dermatol. 2017;44:1129-1137.
- Papp KA, Langley RG, Sigurgeirsson B, et al. Efficacy and safety of secukinumab in the treatment of moderate-to-severe plaque psoriasis: a randomized, double-blind, placebo-controlled phase II dose-ranging study. Br J Dermatol. 2013;168:412-421.
- Rich P, Sigurgeirsson B, Thaci D, et al. Secukinumab induction and maintenance therapy in moderate-to-severe plaque psoriasis: a randomized, double-blind, placebo-controlled, phase II regimen-finding study. Br J Dermatol. 2013;168:402-411.
- Kavanaugh A, Mease PJ, Reimold AM, et al. Secukinumab for long-term treatment of psoriatic arthritis: a two-year followup from a phase III, randomized, double-blind placebo-controlled study. Arthritis Care Res (Hoboken). 2017;69:347-355.
- McInnes IB, Mease PJ, Kirkham B, et al. Secukinumab, a human anti-interleukin-17A monoclonal antibody, in patients with psoriatic arthritis (FUTURE 2): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2015;386:1137-1146.
- Nash P, Mease PJ, McInnes IB, et al. Efficacy and safety of secukinumab administration by autoinjector in patients with psoriatic arthritis: results from a randomized, placebo-controlled trial (FUTURE 3). Arthritis Res Ther. 2018;20:47.
- Sticherling M, Mrowietz U, Augustin M, et al. Secukinumab is superior to fumaric acid esters in treating patients with moderate-to-severe plaque psoriasis who are naive to systemic treatments: results from the randomized controlled PRIME trial. Br J Dermatol. 2017;177:1024-1032.
- Braun J, Baraliakos X, Deodhar A, et al. Effect of secukinumab on clinical and radiographic outcomes in ankylosing spondylitis: 2-year results from the randomised phase III MEASURE 1 study. Ann Rheum Dis. 2017;76:1070-1077.
- Marzo-Ortega H, Sieper J, Kivitz A, et al. Secukinumab provides sustained improvements in the signs and symptoms of active ankylosing spondylitis with high retention rate: 3-year results from the phase III trial, MEASURE 2. RMD Open. 2017;3:e000592.
- Pavelka K, Kivitz A, Dokoupilova E, et al. Efficacy, safety, and tolerability of secukinumab in patients with active ankylosing spondylitis: a randomized, double-blind phase 3 study, MEASURE 3. Arthritis Res Ther. 2017;19:285.
- Callis Duffin K, Bagel J, Bukhalo M, et al. Phase 3, open-label, randomized study of the pharmacokinetics, efficacy and safety of ixekizumab following subcutaneous administration using a prefilled syringe or an autoinjector in patients with moderate-to-severe plaque psoriasis (UNCOVER-A). J Eur Acad Dermatol Venereol. 2017;31:107-113.
- Gordon KB, Colombel JF, Hardin DS. Phase 3 trials of ixekizumab in moderate-to-severe plaque psoriasis. N Engl J Med. 2016;375:2102.
- Saeki H, Nakagawa H, Nakajo K, et al. Efficacy and safety of ixekizumab treatment for Japanese patients with moderate to severe plaque psoriasis, erythrodermic psoriasis and generalized pustular psoriasis: results from a 52-week, open-label, phase 3 study (UNCOVER-J). J Dermatol. 2017;44:355-362.
- Reich K, Pinter A, Lacour JP, et al. Comparison of ixekizumab with ustekinumab in moderate-to-severe psoriasis: 24-week results from IXORA-S, a phase III study. Br J Dermatol. 2017;177:1014-1023.
- Zachariae C, Gordon K, Kimball AB, et al. Efficacy and safety of ixekizumab over 4 years of open-label treatment in a phase 2 study in chronic plaque psoriasis. J Am Acad Dermatol. 2018;79:294.e6-301.e6.
- van der Heijde D, Gladman DD, Kishimoto M, et al. Efficacy and safety of ixekizumab in patients with active psoriatic arthritis: 52-week results from a phase III study (SPIRIT-P1). J Rheumatol. 2018;45:367-377.
- van der Heijde D, Cheng-Chung Wei J, Dougados M, et al. Ixekizumab, an interleukin-17A antagonist in the treatment of ankylosing spondylitis or radiographic axial spondyloarthritis in patients previously untreated with biological disease-modifying anti-rheumatic drugs (COAST-V): 16 week results of a phase 3 randomised, double-blind, active-controlled and placebo-controlled trial. Lancet. 2018;392:2441-2451.
- Nakagawa H, Niiro H, Ootaki K, et al. Brodalumab, a human anti-interleukin-17-receptor antibody in the treatment of Japanese patients with moderate-to-severe plaque psoriasis: efficacy and safety results from a phase II randomized controlled study. J Dermatol Sci. 2016;81:44-52.
- Umezawa Y, Nakagawa H, Niiro H, et al. Long-term clinical safety and efficacy of brodalumab in the treatment of Japanese patients with moderate-to-severe plaque psoriasis. J Eur Acad Dermatol Venereol. 2016;30:1957-1960.
- Papp KA, Reich K, Paul C, et al. A prospective phase III, randomized, double-blind, placebo-controlled study of brodalumab in patients with moderate-to-severe plaque psoriasis. Br J Dermatol. 2016;175:273-286.
- Papp K, Leonardi C, Menter A, et al. Safety and efficacy of brodalumab for psoriasis after 120 weeks of treatment. J Am Acad Dermatol. 2014;71:1183.e3-1190.e3.
- Yamasaki K, Nakagawa H, Kubo Y, et al. Efficacy and safety of brodalumab in patients with generalized pustular psoriasis and psoriatic erythroderma: results from a 52-week, open-label study. Br J Dermatol. 2017;176:741-751.
- Mease PJ, Genovese MC, Greenwald MW, et al. Brodalumab, an anti-IL17RA monoclonal antibody, in psoriatic arthritis. N Engl J Med. 2014;370:2295-2306.
- Martin DA, Churchill M, Flores-Suarez L, et al. A phase Ib multiple ascending dose study evaluating safety, pharmacokinetics, and early clinical response of brodalumab, a human anti-IL-17R antibody, in methotrexate-resistant rheumatoid arthritis. Arthritis Res Ther. 2013;15:R164.
- Busse WW, Holgate S, Kerwin E, et al. Randomized, double-blind, placebo-controlled study of brodalumab, a human anti-IL-17 receptor monoclonal antibody, in moderate to severe asthma. Am J Respir Crit Care Med. 2013;188:1294-1302.
- Igarashi A, Kato T, Kato M, et al. Efficacy and safety of ustekinumab in Japanese patients with moderate-to-severe plaque-type psoriasis: long-term results from a phase 2/3 clinical trial. J Dermatol. 2012;39:242-252.
- Krueger GG, Langley RG, Leonardi C, et al. A human interleukin-12/23 monoclonal antibody for the treatment of psoriasis. N Engl J Med. 2007;356:580-592.
- Leonardi CL, Kimball AB, Papp KA, et al. Efficacy and safety of ustekinumab, a human interleukin-12/23 monoclonal antibody, in patients with psoriasis: 76-week results from a randomised, double-blind, placebo-controlled trial (PHOENIX 1). Lancet. 2008;371:1665-1674.
- Tsai TF, Ho JC, Song M, et al. Efficacy and safety of ustekinumab for the treatment of moderate-to-severe psoriasis: a phase III, randomized, placebo-controlled trial in Taiwanese and Korean patients (PEARL). J Dermatol Sci. 2011;63:154-163.
- Gordon KB, Strober B, Lebwohl M, et al. Efficacy and safety of risankizumab in moderate-to-severe plaque psoriasis (UltIMMa-1 and UltIMMa-2): results from two double-blind, randomised, placebo-controlled and ustekinumab-controlled phase 3 trials. Lancet. 2018;392:650-661.
- Krueger JG, Ferris LK, Menter A, et al. Anti-IL-23A mAb BI 655066 for treatment of moderate-to-severe psoriasis: safety, efficacy, pharmacokinetics, and biomarker results of a single-rising-dose, randomized, double-blind, placebo-controlled trial. J Allergy Clin Immunol. 2015;136:116.e7-124.e7.
- Ohtsuki M, Fujita H, Watanabe M, et al. Efficacy and safety of risankizumab in Japanese patients with moderate to severe plaque psoriasis: results from the SustaIMM phase 2/3 trial. J Dermatol. 2019;46:686-694.
- Papp KA, Blauvelt A, Bukhalo M, et al. Risankizumab versus ustekinumab for moderate-to-severe plaque psoriasis. N Engl J Med. 2017;376:1551-1560.
- Reich K, Gooderham M, Thaci D, et al. Risankizumab compared with adalimumab in patients with moderate-to-severe plaque psoriasis (IMMvent): a randomised, double-blind, active-comparator-controlled phase 3 trial. Lancet. 2019;394:576-586.
- Blauvelt A, Papp KA, Griffiths CE, et al. Efficacy and safety of guselkumab, an anti-interleukin-23 monoclonal antibody, compared with adalimumab for the continuous treatment of patients with moderate to severe psoriasis: results from the phase III, double-blinded, placebo- and active comparator-controlled VOYAGE 1 trial. J Am Acad Dermatol. 2017;76:405-417.
- Deodhar A, Gottlieb AB, Boehncke WH, et al. Efficacy and safety of guselkumab in patients with active psoriatic arthritis: a randomised, double-blind, placebo-controlled, phase 2 study. Lancet. 2018;391:2213-2224.
- Gordon KB, Duffin KC, Bissonnette R, et al. A phase 2 trial of guselkumab versus adalimumab for plaque psoriasis. N Engl J Med. 2015;373:136-144.
- Langley RG, Tsai TF, Flavin S, et al. Efficacy and safety of guselkumab in patients with psoriasis who have an inadequate response to ustekinumab: results of the randomized, double-blind, phase III NAVIGATE trial. Br J Dermatol. 2018;178:114-123.
- Nemoto O, Hirose K, Shibata S, et al. Safety and efficacy of guselkumab in Japanese patients with moderate-to-severe plaque psoriasis: a randomized, placebo-controlled, ascending-dose study. Br J Dermatol. 2018;178:689-696.
- Ohtsuki M, Kubo H, Morishima H, et al. Guselkumab, an anti-interleukin-23 monoclonal antibody, for the treatment of moderate to severe plaque-type psoriasis in Japanese patients: Efficacy and safety results from a phase 3, randomized, double-blind, placebo-controlled study. J Dermatol. 2018;45:1053-1062.
- Reich K, Armstrong AW, Foley P, et al. Efficacy and safety of guselkumab, an anti-interleukin-23 monoclonal antibody, compared with adalimumab for the treatment of patients with moderate to severe psoriasis with randomized withdrawal and retreatment: results from the phase III, double-blind, placebo- and active comparator-controlled VOYAGE 2 trial. J Am Acad Dermatol. 2017;76:418-431.
- Reich K, Armstrong AW, Langley RG, et al. Guselkumab versus secukinumab for the treatment of moderate-to-severe psoriasis (ECLIPSE): results from a phase 3, randomised controlled trial. Lancet. 2019;394:831-839.
- Terui T, Kobayashi S, Okubo Y, et al. Efficacy and safety of guselkumab, an anti-interleukin 23 monoclonal antibody, for palmoplantar pustulosis: a randomized clinical trial. JAMA Dermatol. 2018;154:309-316.
- Papp K, Thaci D, Reich K, et al. Tildrakizumab (MK-3222), an anti-interleukin-23p19 monoclonal antibody, improves psoriasis in a phase IIb randomized placebo-controlled trial. Br J Dermatol. 2015;173:930-939.
- Reich K, Papp KA, Blauvelt A, et al. Tildrakizumab versus placebo or etanercept for chronic plaque psoriasis (reSURFACE 1 and reSURFACE 2): results from two randomised controlled, phase 3 trials. Lancet. 2017;390:276-288.
- Gordon KB, Papp KA, Langley RG, et al. Long-term safety experience of ustekinumab in patients with moderate to severe psoriasis (part II of II): results from analyses of infections and malignancy from pooled phase II and III clinical trials. J Am Acad Dermatol. 2012;66:742-751.
- Papp KA, Griffiths CE, Gordon K, et al. Long-term safety of ustekinumab in patients with moderate-to-severe psoriasis: final results from 5 years of follow-up. Br J Dermatol. 2013;168:844-854.
- Saunte DM, Mrowietz U, Puig L, et al. Candida infections in patients with psoriasis and psoriatic arthritis treated with interleukin-17 inhibitors and their practical management. Br J Dermatol. 2017;177:47-62.
- Lis K, Kuzawinska O, Balkowiec-Iskra E. Tumor necrosis factor inhibitors—state of knowledge. Arch Med Sci. 2014;10:1175-1185.
- Hage CA, Bowyer S, Tarvin SE, et al. Recognition, diagnosis, and treatment of histoplasmosis complicating tumor necrosis factor blocker therapy. Clin Infect Dis. 2010;50:85-92
- Hage CA, Ribes JA, Wengenack NL, et al. A multicenter evaluation of tests for diagnosis of histoplasmosis. Clin Infect Dis. 2011;53:448-454.
- Parisi R, Symmons DP, Griffiths CE, et al. Global epidemiology of psoriasis: a systematic review of incidence and prevalence. J Invest Dermatol. 2013;133:377-385.
- Koo J, Marangell LB, Nakamura M, et al. Depression and suicidality in psoriasis: review of the literature including the cytokine theory of depression. J Eur Acad Dermatol Venereol. 2017;31:1999-2009.
- Krueger JG, Bowcock A. Psoriasis pathophysiology: current concepts of pathogenesis. Ann Rheum Dis. 2005;64 (suppl 2):ii30-36.
- Lee E, Trepicchio WL, Oestreicher JL, et al. Increased expression of interleukin 23 p19 and p40 in lesional skin of patients with psoriasis vulgaris. J Exp Med. 2004;199:125-130.
- Lowes MA, Kikuchi T, Fuentes-Duculan J, et al. Psoriasis vulgaris lesions contain discrete populations of Th1 and Th17 T cells. J Invest Dermatol. 2008;128:1207-1211.
- Shear NH. Fulfilling an unmet need in psoriasis: do biologicals hold the key to improved tolerability? Drug Saf. 2006;29:49-66.
- Lee JH, Slifman NR, Gershon SK, et al. Life-threatening histoplasmosis complicating immunotherapy with tumor necrosis factor alpha antagonists infliximab and etanercept. Arthritis Rheum. 2002;46:2565-2570.
- Leonardi C, Matheson R, Zachariae C, et al. Anti-interleukin-17 monoclonal antibody ixekizumab in chronic plaque psoriasis. N Engl J Med. 2012;366:1190-1199.
- McInnes IB, Sieper J, Braun J, et al. Efficacy and safety of secukinumab, a fully human anti-interleukin-17A monoclonal antibody, in patients with moderate-to-severe psoriatic arthritis: a 24-week, randomised, double-blind, placebo-controlled, phase II proof-of-concept trial. Ann Rheum Dis. 2014;73:349-356.
- Papp KA, Leonardi C, Menter A, et al. Brodalumab, an anti-interleukin-17-receptor antibody for psoriasis. N Engl J Med. 2012;366:1181-1189.
- Isailovic N, Daigo K, Mantovani A, et al. Interleukin-17 and innate immunity in infections and chronic inflammation. J Autoimmun. 2015;60:1-11.
- Bissonnette R, Luger T, Thaci D, et al. Secukinumab sustains good efficacy and favourable safety in moderate-to-severe psoriasis after up to 3 years of treatment: results from a double-blind extension study. Br J Dermatol. 2017;177:1033-1042.
- Bissonnette R, Luger T, Thaci D, et al. Secukinumab demonstrates high sustained efficacy and a favourable safety profile in patients with moderate-to-severe psoriasis through 5 years of treatment (SCULPTURE Extension Study). J Eur Acad Dermatol Venereol. 2018;32:1507-1514.
- Blauvelt A, Prinz JC, Gottlieb AB, et al. Secukinumab administration by pre-filled syringe: efficacy, safety and usability results from a randomized controlled trial in psoriasis (FEATURE). Br J Dermatol. 2015;172:484-493.
- Paul C, Lacour JP, Tedremets L, et al. Efficacy, safety and usability of secukinumab administration by autoinjector/pen in psoriasis: a randomized, controlled trial (JUNCTURE). J Eur Acad Dermatol Venereol. 2015;29:1082-1090.
- Bagel J, Duffin KC, Moore A, et al. The effect of secukinumab on moderate-to-severe scalp psoriasis: Results of a 24-week, randomized, double-blind, placebo-controlled phase 3b study. J Am Acad Dermatol. 2017;77:667-674.
- Blauvelt A, Reich K, Tsai TF, et al. Secukinumab is superior to ustekinumab in clearing skin of subjects with moderate-to-severe plaque psoriasis up to 1 year: results from the CLEAR study. J Am Acad Dermatol. 2017;76:60.e9-69.e9.
- Thaci D, Blauvelt A, Reich K, et al. Secukinumab is superior to ustekinumab in clearing skin of subjects with moderate to severe plaque psoriasis: CLEAR, a randomized controlled trial. J Am Acad Dermatol. 2015;73:400-409.
- Gottlieb A, Sullivan J, van Doorn M, et al. Secukinumab shows significant efficacy in palmoplantar psoriasis: results from GESTURE, a randomized controlled trial. J Am Acad Dermatol. 2017;76:70-80.
- Ohtsuki M, Morita A, Abe M, et al. Secukinumab efficacy and safety in Japanese patients with moderate-to-severe plaque psoriasis: subanalysis from ERASURE, a randomized, placebo-controlled, phase 3 study. J Dermatol. 2014;41:1039-1046.
- Wu NL, Hsu CJ, Sun FJ, et al. Efficacy and safety of secukinumab in Taiwanese patients with moderate to severe plaque psoriasis: subanalysis from ERASURE phase III study. J Dermatol. 2017;44:1129-1137.
- Papp KA, Langley RG, Sigurgeirsson B, et al. Efficacy and safety of secukinumab in the treatment of moderate-to-severe plaque psoriasis: a randomized, double-blind, placebo-controlled phase II dose-ranging study. Br J Dermatol. 2013;168:412-421.
- Rich P, Sigurgeirsson B, Thaci D, et al. Secukinumab induction and maintenance therapy in moderate-to-severe plaque psoriasis: a randomized, double-blind, placebo-controlled, phase II regimen-finding study. Br J Dermatol. 2013;168:402-411.
- Kavanaugh A, Mease PJ, Reimold AM, et al. Secukinumab for long-term treatment of psoriatic arthritis: a two-year followup from a phase III, randomized, double-blind placebo-controlled study. Arthritis Care Res (Hoboken). 2017;69:347-355.
- McInnes IB, Mease PJ, Kirkham B, et al. Secukinumab, a human anti-interleukin-17A monoclonal antibody, in patients with psoriatic arthritis (FUTURE 2): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2015;386:1137-1146.
- Nash P, Mease PJ, McInnes IB, et al. Efficacy and safety of secukinumab administration by autoinjector in patients with psoriatic arthritis: results from a randomized, placebo-controlled trial (FUTURE 3). Arthritis Res Ther. 2018;20:47.
- Sticherling M, Mrowietz U, Augustin M, et al. Secukinumab is superior to fumaric acid esters in treating patients with moderate-to-severe plaque psoriasis who are naive to systemic treatments: results from the randomized controlled PRIME trial. Br J Dermatol. 2017;177:1024-1032.
- Braun J, Baraliakos X, Deodhar A, et al. Effect of secukinumab on clinical and radiographic outcomes in ankylosing spondylitis: 2-year results from the randomised phase III MEASURE 1 study. Ann Rheum Dis. 2017;76:1070-1077.
- Marzo-Ortega H, Sieper J, Kivitz A, et al. Secukinumab provides sustained improvements in the signs and symptoms of active ankylosing spondylitis with high retention rate: 3-year results from the phase III trial, MEASURE 2. RMD Open. 2017;3:e000592.
- Pavelka K, Kivitz A, Dokoupilova E, et al. Efficacy, safety, and tolerability of secukinumab in patients with active ankylosing spondylitis: a randomized, double-blind phase 3 study, MEASURE 3. Arthritis Res Ther. 2017;19:285.
- Callis Duffin K, Bagel J, Bukhalo M, et al. Phase 3, open-label, randomized study of the pharmacokinetics, efficacy and safety of ixekizumab following subcutaneous administration using a prefilled syringe or an autoinjector in patients with moderate-to-severe plaque psoriasis (UNCOVER-A). J Eur Acad Dermatol Venereol. 2017;31:107-113.
- Gordon KB, Colombel JF, Hardin DS. Phase 3 trials of ixekizumab in moderate-to-severe plaque psoriasis. N Engl J Med. 2016;375:2102.
- Saeki H, Nakagawa H, Nakajo K, et al. Efficacy and safety of ixekizumab treatment for Japanese patients with moderate to severe plaque psoriasis, erythrodermic psoriasis and generalized pustular psoriasis: results from a 52-week, open-label, phase 3 study (UNCOVER-J). J Dermatol. 2017;44:355-362.
- Reich K, Pinter A, Lacour JP, et al. Comparison of ixekizumab with ustekinumab in moderate-to-severe psoriasis: 24-week results from IXORA-S, a phase III study. Br J Dermatol. 2017;177:1014-1023.
- Zachariae C, Gordon K, Kimball AB, et al. Efficacy and safety of ixekizumab over 4 years of open-label treatment in a phase 2 study in chronic plaque psoriasis. J Am Acad Dermatol. 2018;79:294.e6-301.e6.
- van der Heijde D, Gladman DD, Kishimoto M, et al. Efficacy and safety of ixekizumab in patients with active psoriatic arthritis: 52-week results from a phase III study (SPIRIT-P1). J Rheumatol. 2018;45:367-377.
- van der Heijde D, Cheng-Chung Wei J, Dougados M, et al. Ixekizumab, an interleukin-17A antagonist in the treatment of ankylosing spondylitis or radiographic axial spondyloarthritis in patients previously untreated with biological disease-modifying anti-rheumatic drugs (COAST-V): 16 week results of a phase 3 randomised, double-blind, active-controlled and placebo-controlled trial. Lancet. 2018;392:2441-2451.
- Nakagawa H, Niiro H, Ootaki K, et al. Brodalumab, a human anti-interleukin-17-receptor antibody in the treatment of Japanese patients with moderate-to-severe plaque psoriasis: efficacy and safety results from a phase II randomized controlled study. J Dermatol Sci. 2016;81:44-52.
- Umezawa Y, Nakagawa H, Niiro H, et al. Long-term clinical safety and efficacy of brodalumab in the treatment of Japanese patients with moderate-to-severe plaque psoriasis. J Eur Acad Dermatol Venereol. 2016;30:1957-1960.
- Papp KA, Reich K, Paul C, et al. A prospective phase III, randomized, double-blind, placebo-controlled study of brodalumab in patients with moderate-to-severe plaque psoriasis. Br J Dermatol. 2016;175:273-286.
- Papp K, Leonardi C, Menter A, et al. Safety and efficacy of brodalumab for psoriasis after 120 weeks of treatment. J Am Acad Dermatol. 2014;71:1183.e3-1190.e3.
- Yamasaki K, Nakagawa H, Kubo Y, et al. Efficacy and safety of brodalumab in patients with generalized pustular psoriasis and psoriatic erythroderma: results from a 52-week, open-label study. Br J Dermatol. 2017;176:741-751.
- Mease PJ, Genovese MC, Greenwald MW, et al. Brodalumab, an anti-IL17RA monoclonal antibody, in psoriatic arthritis. N Engl J Med. 2014;370:2295-2306.
- Martin DA, Churchill M, Flores-Suarez L, et al. A phase Ib multiple ascending dose study evaluating safety, pharmacokinetics, and early clinical response of brodalumab, a human anti-IL-17R antibody, in methotrexate-resistant rheumatoid arthritis. Arthritis Res Ther. 2013;15:R164.
- Busse WW, Holgate S, Kerwin E, et al. Randomized, double-blind, placebo-controlled study of brodalumab, a human anti-IL-17 receptor monoclonal antibody, in moderate to severe asthma. Am J Respir Crit Care Med. 2013;188:1294-1302.
- Igarashi A, Kato T, Kato M, et al. Efficacy and safety of ustekinumab in Japanese patients with moderate-to-severe plaque-type psoriasis: long-term results from a phase 2/3 clinical trial. J Dermatol. 2012;39:242-252.
- Krueger GG, Langley RG, Leonardi C, et al. A human interleukin-12/23 monoclonal antibody for the treatment of psoriasis. N Engl J Med. 2007;356:580-592.
- Leonardi CL, Kimball AB, Papp KA, et al. Efficacy and safety of ustekinumab, a human interleukin-12/23 monoclonal antibody, in patients with psoriasis: 76-week results from a randomised, double-blind, placebo-controlled trial (PHOENIX 1). Lancet. 2008;371:1665-1674.
- Tsai TF, Ho JC, Song M, et al. Efficacy and safety of ustekinumab for the treatment of moderate-to-severe psoriasis: a phase III, randomized, placebo-controlled trial in Taiwanese and Korean patients (PEARL). J Dermatol Sci. 2011;63:154-163.
- Gordon KB, Strober B, Lebwohl M, et al. Efficacy and safety of risankizumab in moderate-to-severe plaque psoriasis (UltIMMa-1 and UltIMMa-2): results from two double-blind, randomised, placebo-controlled and ustekinumab-controlled phase 3 trials. Lancet. 2018;392:650-661.
- Krueger JG, Ferris LK, Menter A, et al. Anti-IL-23A mAb BI 655066 for treatment of moderate-to-severe psoriasis: safety, efficacy, pharmacokinetics, and biomarker results of a single-rising-dose, randomized, double-blind, placebo-controlled trial. J Allergy Clin Immunol. 2015;136:116.e7-124.e7.
- Ohtsuki M, Fujita H, Watanabe M, et al. Efficacy and safety of risankizumab in Japanese patients with moderate to severe plaque psoriasis: results from the SustaIMM phase 2/3 trial. J Dermatol. 2019;46:686-694.
- Papp KA, Blauvelt A, Bukhalo M, et al. Risankizumab versus ustekinumab for moderate-to-severe plaque psoriasis. N Engl J Med. 2017;376:1551-1560.
- Reich K, Gooderham M, Thaci D, et al. Risankizumab compared with adalimumab in patients with moderate-to-severe plaque psoriasis (IMMvent): a randomised, double-blind, active-comparator-controlled phase 3 trial. Lancet. 2019;394:576-586.
- Blauvelt A, Papp KA, Griffiths CE, et al. Efficacy and safety of guselkumab, an anti-interleukin-23 monoclonal antibody, compared with adalimumab for the continuous treatment of patients with moderate to severe psoriasis: results from the phase III, double-blinded, placebo- and active comparator-controlled VOYAGE 1 trial. J Am Acad Dermatol. 2017;76:405-417.
- Deodhar A, Gottlieb AB, Boehncke WH, et al. Efficacy and safety of guselkumab in patients with active psoriatic arthritis: a randomised, double-blind, placebo-controlled, phase 2 study. Lancet. 2018;391:2213-2224.
- Gordon KB, Duffin KC, Bissonnette R, et al. A phase 2 trial of guselkumab versus adalimumab for plaque psoriasis. N Engl J Med. 2015;373:136-144.
- Langley RG, Tsai TF, Flavin S, et al. Efficacy and safety of guselkumab in patients with psoriasis who have an inadequate response to ustekinumab: results of the randomized, double-blind, phase III NAVIGATE trial. Br J Dermatol. 2018;178:114-123.
- Nemoto O, Hirose K, Shibata S, et al. Safety and efficacy of guselkumab in Japanese patients with moderate-to-severe plaque psoriasis: a randomized, placebo-controlled, ascending-dose study. Br J Dermatol. 2018;178:689-696.
- Ohtsuki M, Kubo H, Morishima H, et al. Guselkumab, an anti-interleukin-23 monoclonal antibody, for the treatment of moderate to severe plaque-type psoriasis in Japanese patients: Efficacy and safety results from a phase 3, randomized, double-blind, placebo-controlled study. J Dermatol. 2018;45:1053-1062.
- Reich K, Armstrong AW, Foley P, et al. Efficacy and safety of guselkumab, an anti-interleukin-23 monoclonal antibody, compared with adalimumab for the treatment of patients with moderate to severe psoriasis with randomized withdrawal and retreatment: results from the phase III, double-blind, placebo- and active comparator-controlled VOYAGE 2 trial. J Am Acad Dermatol. 2017;76:418-431.
- Reich K, Armstrong AW, Langley RG, et al. Guselkumab versus secukinumab for the treatment of moderate-to-severe psoriasis (ECLIPSE): results from a phase 3, randomised controlled trial. Lancet. 2019;394:831-839.
- Terui T, Kobayashi S, Okubo Y, et al. Efficacy and safety of guselkumab, an anti-interleukin 23 monoclonal antibody, for palmoplantar pustulosis: a randomized clinical trial. JAMA Dermatol. 2018;154:309-316.
- Papp K, Thaci D, Reich K, et al. Tildrakizumab (MK-3222), an anti-interleukin-23p19 monoclonal antibody, improves psoriasis in a phase IIb randomized placebo-controlled trial. Br J Dermatol. 2015;173:930-939.
- Reich K, Papp KA, Blauvelt A, et al. Tildrakizumab versus placebo or etanercept for chronic plaque psoriasis (reSURFACE 1 and reSURFACE 2): results from two randomised controlled, phase 3 trials. Lancet. 2017;390:276-288.
- Gordon KB, Papp KA, Langley RG, et al. Long-term safety experience of ustekinumab in patients with moderate to severe psoriasis (part II of II): results from analyses of infections and malignancy from pooled phase II and III clinical trials. J Am Acad Dermatol. 2012;66:742-751.
- Papp KA, Griffiths CE, Gordon K, et al. Long-term safety of ustekinumab in patients with moderate-to-severe psoriasis: final results from 5 years of follow-up. Br J Dermatol. 2013;168:844-854.
- Saunte DM, Mrowietz U, Puig L, et al. Candida infections in patients with psoriasis and psoriatic arthritis treated with interleukin-17 inhibitors and their practical management. Br J Dermatol. 2017;177:47-62.
- Lis K, Kuzawinska O, Balkowiec-Iskra E. Tumor necrosis factor inhibitors—state of knowledge. Arch Med Sci. 2014;10:1175-1185.
- Hage CA, Bowyer S, Tarvin SE, et al. Recognition, diagnosis, and treatment of histoplasmosis complicating tumor necrosis factor blocker therapy. Clin Infect Dis. 2010;50:85-92
- Hage CA, Ribes JA, Wengenack NL, et al. A multicenter evaluation of tests for diagnosis of histoplasmosis. Clin Infect Dis. 2011;53:448-454.
Practice Points
- The use of IL-17, IL-12/IL-23, and IL-23 inhibitors for psoriasis and other inflammatory conditions does not appear to increase the risk for deep fungal infections.
- Physicians should still be cautiously optimistic in prescribing these medications, as IL-17 and IL-23 play a central role in immunologic defenses, particularly against fungi.
- A high index of suspicion should be maintained for patients from endemic areas who are being treated with biologics.
Work-life balance: How 5 surgeons manage life in and out of the operating room
Patrick J. Culligan, MD:
My impression of our younger colleagues, however, is that many of them are not attracted to the traditional ivory tower research model of academic advancement to which many in previous generations aspired. They seem more concerned with work-life balance as their measure of success rather than the classic metrics of money and prestige. Everyone still needs role models and mentors, though, and that’s where all of you come in. I asked each of you to be on this panel because I admire you for your varying approaches to work-life balance while achieving success as gynecologic surgeons. I thought others in the field might be inspired by hearing your stories.
Cultivating your passions
Kristie Greene, MD: What I have come to learn and appreciate is a really simple point: you do not have to do everything. Determining who you want to be both personally and professionally is step 1.
Granted, answering the question, “Who do I want to be?” is not as simple as it sounds. Many factors figure into the decisions we make in our personal and professional lives. Also, it is not a question we often stop and ask ourselves. From early on, we are placed on an escalator moving up through medical school, residency, fellowship, good job, better job, etc. We are so accustomed to being competitive, to winning, and to wanting to be the best that we sometimes forget to ask ourselves, “What is it exactly that I want, and why? What is my endpoint? And does it make me happy?”
Multitasking is regarded as a talent. As much as we would like to believe that we can do everything at the same time and do it all well, we actually can’t. A friend of mine made me read a book a couple of years ago, called Feeling Good, by David Burns. The book encourages you to consider the different tasks you do in a day and rate how good you are at each of them on a scale of 1 to 10. It then asks you to think about how much enjoyment you derive from each of the tasks and about why you are doing the ones that bring you little to no enjoyment.
I ultimately decided that, for me professionally, the most important thing was my interest in global health. So I decided to do whatever it took to make this happen. But you don’t get something for nothing, and everything comes with sacrifices.
Continue to: Charles Rardin, MD...
Charles Rardin, MD: How exactly did you decide that you were going to focus your career toward pursuing international health? How did you know it was more important? And how did you overcome some of those obstacles?
Dr. Greene: You have to ask the hard question again about what brings you the most joy professionally and personally. That was the easy part of it for me because global health has always been that source of happiness and fulfillment for me. The more challenging parts are the sacrifices and hard choices that come with it. With global health, it can be difficult to balance the demands of a clinical practice.
All of our jobs are a business. I am still struggling with the money part of it. For my husband and I, that meant we had to start small—do what we could afford. But then it blossomed into something that was involving residents, fellows, and med students, which requires far more funding than we had. So I reached out to family. Most of our families donate to different organizations or charities every year, so why not donate to a loved one for something they are passionate about?
At the University of South Florida (USF), we set up a fund, a foundation for global health, which helps support our work abroad as well as the costs associated with involvement of our trainees. Right now, what we have is still small potatoes to a country, but we are making it happen by starting at a small level and growing it.
Beyond the money aspect, traveling abroad means less involvement in meetings, missed opportunities to teach courses that might interest me, and time away from my family. I guess my advice on this whole thing is that you can make things happen if they are important enough to you, and if you are willing to make sacrifices in other areas because you can’t have it all.
Making time for you
Dr. Culligan: So you have found what is important to you, and you have found a way to make it happen. But you are faced with more work; you have given yourself additional work on top of your regular work. How do you make time for a personal life?
Catherine Matthews, MD: In preparing for this discussion, I decided to break down my advice into 3 buckets: The first bucket is discovering and knowing your authentic self. The second is building a community, which I’ll elaborate on. And the third, which we have discussed, is to let go of the money.
Dr. Culligan: I love the concept of the authentic self, but how does that jive with a tendency to strive for perfection? We all think we can do it all. How do we narrow down to what really matters?
Dr. Matthews: We often focus on the things that bring us happiness and what we are good at, but it’s the things that make us unhappy that tend to bring us down. It’s the presence of unhappiness, not the absence of happiness, that seems to be the undoing of many, including myself.
None of us are born with dramatic insight. It is experience that leads to insight. People who are actually present are able to gain insight through observation. A person becomes a better surgeon by observing the outcome of doing a stitch this way versus that; you learn how to do it by seeing what it looks like afterward.
Finding our authentic selves happens in much the same way. Having the presence of mind to ask the right questions, such as, “How am I feeling while I’m doing this?” leads to insights into the true self.
Continue to: It takes a village...
It takes a village
Dr. Greene: Catherine mentioned community earlier, and that is extremely important. The people who surround us can have a huge impact on the way we perceive things, including ourselves. Having a mix of people in our lives—some who practice medicine and others who don’t—helps us stay balanced and answer some of the tough questions. Catherine, for example, has helped me in various stages of my career to ask myself meaningful questions and get real answers.
Dr. Rardin: Part of finding balance is luck, and part of it is making a choice between money and everything else. In considering my first job out of training, I knew that money had the potential to distract me from what was important to me. So I chose a position that was almost entirely salaried so that the decisions I made clinically, surgically, and regarding work-life balance would be less likely to directly impact what was important to me.
Sally Huber, MD: I am still in the “getting there” phase of my life, but one thing I have found is that getting my family involved and excited about what I do has made them much more accepting of when I have longer work days or work to do on the weekend. My spouse has become quite involved with what I have been doing with transgender health in Atlanta. It has been a great bonding experience; she shares my passions, and together we are creating something about which we both can be proud.
When work invades home life
Dr. Culligan: That is great. Sally, I think when we talked, you were just learning about the necessity of mental separation and of not taking your work home with you, which is so hard for all of us with all of our devices.
Dr. Huber: Yes, this year has been about seeing what works best as far as being efficient at work and having quality time at home. At the end of every day I ask myself, “What worked well today? What didn’t work well? What else can I do to maximize time with my family?” I am slowly becoming more efficient, but it has been a challenge. During fellowship, your day is pretty set, but once you are practicing on your own, your hours and responsibilities are completely different, and you have to figure out what works best for you, your values, and your expectations of private life. It takes some time, and I am still figuring it out.
Dr. Culligan: How often would you say that you bring work home? I try hard once I am home to quit working, but sometimes on the weekends I break that rule.
Dr. Matthews: I must say that I do feel like there are certain times when I am better at that than others. Work comes in waves with pressing deadlines. If I averaged it out, probably a third of the time I have some email or some conference call or something that I have got to do at home. I do really try to limit the obligations that I have after 5:30 or 6:00 pm. I resent intrusions after that time. As far as weekends, I delegate about one weekend every 2 months to work, instead of doing a little bit every weekend.
Dr. Greene: I agree. I try hard to make 5:30 to 7:30 pm unequivocal time for a family dinner and time for my kids. During that time, I do not have my phone near me so I can’t look at email or texts. I try not to schedule conference calls. I try to be there to read books to my kids at night. Then if I need to do work, I do it later at night, which interferes with time with my spouse, and is not ideal, but that’s what happens.
Dr. Matthews: One of the things that I think is a huge part of work-life balance is work-related travel. When you are present at work on a consistent basis, the work does not pile up to the extent that it does when you are absent on a trip. When you come back, you invariably pay the price by seeing more patients and doing more surgery. Then it becomes a stressful event.
My advice to young people is to be very thoughtful about planning trips, especially distant ones. You do not want to sit on a plane all day when you could be doing something more productive. If I could have done something differently in my mid-career, I would have traveled less.
Continue to: Prioritizing “out of office” time...
Prioritizing “out of office” time
Dr. Greene: How do you all mentally separate yourself from work, so that when you are on vacation with your family you are not thinking about the office, the patients, and all of the things on your to-do list?
Dr. Rardin: I don’t have a great answer for that except that it is about being present. You have to decide that now is the time when I am home, now is the time when I am a parent, now is the time when I am a boy scout leader, etc. I guess maybe it’s a skill, or maybe it’s about making something a priority. Work will always be waiting for you when you turn your attention back to it.
Dr. Matthews: Kristie, the answer to your question goes back to community. Partners in a practice cover for each other. You have to trust them to take care of things so that you can relax during your time away.
Some people recommend not scheduling challenging cases right before going away because invariably something goes wrong, and then you are asking, “Why did I schedule 3 colpopexies before getting on a plane?”
Dr. Rardin: Yes, I completely agree with all of that. Personally, I feel fortunate that I can compartmentalize pretty well. When I am home with my kids, I allow myself to shed some of the doctor/surgeon/leadership persona; I am able to be goofy and completely non–doctor-like. It works to help me leave work behind.
Dr. Matthews: Other things you can do include setting up an out-of-office notice on your email that says when you will be back and what to do in case of urgent matters. This basically says to the world, “Don’t expect to hear from me until X date.” It removes the expectation that you will respond sooner. Otherwise, we would all be on our smartphones all the time and not enjoying our time away.
What I wish I knew then
Dr. Culligan: How would you complete the sentence, “I wish they had told me X when I was embarking on my career?”
Dr. Rardin: I keep coming back to the phrase, “Don’t do anything that you can reasonably pay someone else to do.” By that I mean, if you don’t get energy from housework, consider spending some of your money to get help with the housework. Resolve to make a relatively small expenditure to maximize the quality of the time that you give to yourself and your family. Those are the sorts of things that I think can go a long way.
Dr. Culligan: Charley, your wife is an ObGyn. How do you navigate a dual medical career household? What advice do you have for others?
Dr. Rardin: When I was going into fellowship, we had a conversation about how hard it is for both people in a relationship to have an academic fire in the belly and to be truly engaged in climbing the academic ladder. We made a decision that Jane would go into private practice. There has got to be some give and take in a dual medical relationship; a lot of sacrifices and compromises need to happen. We are fortunate in that there are complementary aspects to our jobs. We both spend about the same number of nights away from the house, but my travel is more in chunks and hers is overnight calls for labor and delivery. We have different ways of (briefly) single-parenting, and you have to come up with ways to handle the domestic chores.
Dr. Matthews: I wish someone had explained to me that the people you work with are much more important than the place. The human connection is what defines your experience, much more than any ego-driven outcome.
Dr. Greene: I wish someone had explained to me the competing aspects of academic medicine. The cards are stacked in a way that make it difficult for you to win. For example, you may love to teach and may be really good at it, but if you let your students handle too many cases, your relative value units plummet and then the hospital is on your back. There are the interests of people, and there are the interests of the business. Everything is a balance, and it’s really tricky.
Dr. Huber: Luckily, Pat counselled me as I was finishing my fellowship about the importance of negotiating a good contract, of being pushy and knowing what you want out of it and knowing what your limitations are. I joined a private practice that had 3 different physical locations. If I had to drive to all of them, as they wanted, it would have meant up to a one-and-a-half-hour commute. But I pushed to stay in one location and to put that extra hour to better use. I am glad I did, but it was terrifying at the time because I didn’t want to lose the offer. I know people that did not do that and took the first thing they got. Now, they are driving all over the place or they have these crazy hours or terrible call responsibilities that if they had just been a little firmer, they probably could have gotten out of. As they start trying to find work-life balance, they are already handicapped.
Continue to: Passions outside the office...
Passions outside the office
Dr. Culligan: One thing I would like to touch on is what is going on in each of your personal lives because all of you have interesting stories to tell outside of what you do professionally. What drives you other than medicine?
Dr. Rardin: I am the father of 3 boys. The oldest one just got his Eagle Scout rank yesterday in Boy Scouts. I would be a woodworker if I wasn’t in medicine. I am a Deacon at church. And I love to spend my downtime reading with my family in front of the fireplace.
Dr. Matthews: For me, it’s music. When my husband and I first met, he asked me if I played a musical instrument. I said I played the cello in primary school. He said, “Great, go rent a cello.” I was never at all interested in playing the cello by myself, but because he plays guitar and piano we became able to play a lot of music together. Our son, Alexander, plays drums. We now have a family band.
In addition, I do yoga. I would never have labeled myself an anxious person, but I learned through this process that I am and need to manage it. It took a lot of years to figure that out. If I don’t leave myself an hour each day to go to a yoga class, I am not a happy person and neither is anyone around me. Also, I get tremendous pleasure from reading books and magazines as opposed to watching a screen.
Dr. Greene: I have found that my passions outside of work often change depending on my stage of life. Right now, I have two young babies and so my life outside of work revolves around them. Before the babies, my dad, who lives in Buffalo, was ill. So for awhile, we were flying to Buffalo almost every weekend that I was not on call. I would say, in general what fuels me is connecting with the people I love as often as I can. A typical night involves me and my husband going for a walk with our kids and dog after dinner and talking to each other. We connect with neighbors and chat on the front porch. It doesn’t really matter what we are doing; it is about being surrounded by people who matter.
Dr. Huber: It’s similar for me. Having a child completely shifts your world view. My goal every day is to give my daughter her first feeding in the morning and to get home as soon as possible at the end of the day to do her last feeding and put her to sleep. She crawled for the first time yesterday, and I was so excited that I could be there for that.
Also, I love being outdoors. I love hiking and camping. Going on a hike and being outside with nature is my way of decompressing.
Continue to: Thinking about upcoming generations...
Thinking about upcoming generations
Dr. Matthews: One other thing I would like to propose is looking at what can we do to make the profession better for the next generation. As a group, our profession is somewhat inflexible. We tend to fall into the trap of, “since this is the way we have always done it this is how we should continue doing it.” The OR still starts at 7:00 or 7:30 am, ignoring the need for school drop-offs, etc. We are not innovative about flexibility in the work week. Honestly, it does not work well for many people, patients and physicians alike. Flexible scheduling should be something that is on the table for both men and women who are trying to balance being full-time parents and full-time surgeons. We need to create an environment in which it is okay for you to spend 10 years instead of 6 as an assistant professor because you are also a young parent, and it will not count against you when you come up for promotion.
Dr. Culligan: I agree with you, Catherine. Full “Professor” is a nice title, but it means time away from family and a lot of other things. Each of us has to decide whether it is worth it, especially since it often does not come with any extra money.
Dr. Huber: A question on a recent survey of residents asked, “Do you see yourself going into private practice or academic medicine when you’ve completed your residency?” When I was a resident, everyone wanted to go into academic medicine, but now it seems like more and more residents have their sights set on private practice because that is where they see the opportunities to create work-life balance.
In the academic world, you have to try to get a promotion in X number of years, and get X number of publications, and be a great teacher, doctor, and administrator all at the same time. I am wondering if we are going to start seeing more and more residents and fellows going into private or hospital-owned practice where there aren’t those added expectations.
Dr. Rardin: I agree, and we are back to what we said in the beginning about doing an honest assessment of what is meaningful and important. We are all trained to try to reach for that shiny brass ring, but do we really want that brass ring? Will it be an asset or a hindrance once we get it? It is okay to be honest and say, “I really don’t want that promotion. I would rather spend more time with my family.” ●
Patrick J. Culligan, MD:
My impression of our younger colleagues, however, is that many of them are not attracted to the traditional ivory tower research model of academic advancement to which many in previous generations aspired. They seem more concerned with work-life balance as their measure of success rather than the classic metrics of money and prestige. Everyone still needs role models and mentors, though, and that’s where all of you come in. I asked each of you to be on this panel because I admire you for your varying approaches to work-life balance while achieving success as gynecologic surgeons. I thought others in the field might be inspired by hearing your stories.
Cultivating your passions
Kristie Greene, MD: What I have come to learn and appreciate is a really simple point: you do not have to do everything. Determining who you want to be both personally and professionally is step 1.
Granted, answering the question, “Who do I want to be?” is not as simple as it sounds. Many factors figure into the decisions we make in our personal and professional lives. Also, it is not a question we often stop and ask ourselves. From early on, we are placed on an escalator moving up through medical school, residency, fellowship, good job, better job, etc. We are so accustomed to being competitive, to winning, and to wanting to be the best that we sometimes forget to ask ourselves, “What is it exactly that I want, and why? What is my endpoint? And does it make me happy?”
Multitasking is regarded as a talent. As much as we would like to believe that we can do everything at the same time and do it all well, we actually can’t. A friend of mine made me read a book a couple of years ago, called Feeling Good, by David Burns. The book encourages you to consider the different tasks you do in a day and rate how good you are at each of them on a scale of 1 to 10. It then asks you to think about how much enjoyment you derive from each of the tasks and about why you are doing the ones that bring you little to no enjoyment.
I ultimately decided that, for me professionally, the most important thing was my interest in global health. So I decided to do whatever it took to make this happen. But you don’t get something for nothing, and everything comes with sacrifices.
Continue to: Charles Rardin, MD...
Charles Rardin, MD: How exactly did you decide that you were going to focus your career toward pursuing international health? How did you know it was more important? And how did you overcome some of those obstacles?
Dr. Greene: You have to ask the hard question again about what brings you the most joy professionally and personally. That was the easy part of it for me because global health has always been that source of happiness and fulfillment for me. The more challenging parts are the sacrifices and hard choices that come with it. With global health, it can be difficult to balance the demands of a clinical practice.
All of our jobs are a business. I am still struggling with the money part of it. For my husband and I, that meant we had to start small—do what we could afford. But then it blossomed into something that was involving residents, fellows, and med students, which requires far more funding than we had. So I reached out to family. Most of our families donate to different organizations or charities every year, so why not donate to a loved one for something they are passionate about?
At the University of South Florida (USF), we set up a fund, a foundation for global health, which helps support our work abroad as well as the costs associated with involvement of our trainees. Right now, what we have is still small potatoes to a country, but we are making it happen by starting at a small level and growing it.
Beyond the money aspect, traveling abroad means less involvement in meetings, missed opportunities to teach courses that might interest me, and time away from my family. I guess my advice on this whole thing is that you can make things happen if they are important enough to you, and if you are willing to make sacrifices in other areas because you can’t have it all.
Making time for you
Dr. Culligan: So you have found what is important to you, and you have found a way to make it happen. But you are faced with more work; you have given yourself additional work on top of your regular work. How do you make time for a personal life?
Catherine Matthews, MD: In preparing for this discussion, I decided to break down my advice into 3 buckets: The first bucket is discovering and knowing your authentic self. The second is building a community, which I’ll elaborate on. And the third, which we have discussed, is to let go of the money.
Dr. Culligan: I love the concept of the authentic self, but how does that jive with a tendency to strive for perfection? We all think we can do it all. How do we narrow down to what really matters?
Dr. Matthews: We often focus on the things that bring us happiness and what we are good at, but it’s the things that make us unhappy that tend to bring us down. It’s the presence of unhappiness, not the absence of happiness, that seems to be the undoing of many, including myself.
None of us are born with dramatic insight. It is experience that leads to insight. People who are actually present are able to gain insight through observation. A person becomes a better surgeon by observing the outcome of doing a stitch this way versus that; you learn how to do it by seeing what it looks like afterward.
Finding our authentic selves happens in much the same way. Having the presence of mind to ask the right questions, such as, “How am I feeling while I’m doing this?” leads to insights into the true self.
Continue to: It takes a village...
It takes a village
Dr. Greene: Catherine mentioned community earlier, and that is extremely important. The people who surround us can have a huge impact on the way we perceive things, including ourselves. Having a mix of people in our lives—some who practice medicine and others who don’t—helps us stay balanced and answer some of the tough questions. Catherine, for example, has helped me in various stages of my career to ask myself meaningful questions and get real answers.
Dr. Rardin: Part of finding balance is luck, and part of it is making a choice between money and everything else. In considering my first job out of training, I knew that money had the potential to distract me from what was important to me. So I chose a position that was almost entirely salaried so that the decisions I made clinically, surgically, and regarding work-life balance would be less likely to directly impact what was important to me.
Sally Huber, MD: I am still in the “getting there” phase of my life, but one thing I have found is that getting my family involved and excited about what I do has made them much more accepting of when I have longer work days or work to do on the weekend. My spouse has become quite involved with what I have been doing with transgender health in Atlanta. It has been a great bonding experience; she shares my passions, and together we are creating something about which we both can be proud.
When work invades home life
Dr. Culligan: That is great. Sally, I think when we talked, you were just learning about the necessity of mental separation and of not taking your work home with you, which is so hard for all of us with all of our devices.
Dr. Huber: Yes, this year has been about seeing what works best as far as being efficient at work and having quality time at home. At the end of every day I ask myself, “What worked well today? What didn’t work well? What else can I do to maximize time with my family?” I am slowly becoming more efficient, but it has been a challenge. During fellowship, your day is pretty set, but once you are practicing on your own, your hours and responsibilities are completely different, and you have to figure out what works best for you, your values, and your expectations of private life. It takes some time, and I am still figuring it out.
Dr. Culligan: How often would you say that you bring work home? I try hard once I am home to quit working, but sometimes on the weekends I break that rule.
Dr. Matthews: I must say that I do feel like there are certain times when I am better at that than others. Work comes in waves with pressing deadlines. If I averaged it out, probably a third of the time I have some email or some conference call or something that I have got to do at home. I do really try to limit the obligations that I have after 5:30 or 6:00 pm. I resent intrusions after that time. As far as weekends, I delegate about one weekend every 2 months to work, instead of doing a little bit every weekend.
Dr. Greene: I agree. I try hard to make 5:30 to 7:30 pm unequivocal time for a family dinner and time for my kids. During that time, I do not have my phone near me so I can’t look at email or texts. I try not to schedule conference calls. I try to be there to read books to my kids at night. Then if I need to do work, I do it later at night, which interferes with time with my spouse, and is not ideal, but that’s what happens.
Dr. Matthews: One of the things that I think is a huge part of work-life balance is work-related travel. When you are present at work on a consistent basis, the work does not pile up to the extent that it does when you are absent on a trip. When you come back, you invariably pay the price by seeing more patients and doing more surgery. Then it becomes a stressful event.
My advice to young people is to be very thoughtful about planning trips, especially distant ones. You do not want to sit on a plane all day when you could be doing something more productive. If I could have done something differently in my mid-career, I would have traveled less.
Continue to: Prioritizing “out of office” time...
Prioritizing “out of office” time
Dr. Greene: How do you all mentally separate yourself from work, so that when you are on vacation with your family you are not thinking about the office, the patients, and all of the things on your to-do list?
Dr. Rardin: I don’t have a great answer for that except that it is about being present. You have to decide that now is the time when I am home, now is the time when I am a parent, now is the time when I am a boy scout leader, etc. I guess maybe it’s a skill, or maybe it’s about making something a priority. Work will always be waiting for you when you turn your attention back to it.
Dr. Matthews: Kristie, the answer to your question goes back to community. Partners in a practice cover for each other. You have to trust them to take care of things so that you can relax during your time away.
Some people recommend not scheduling challenging cases right before going away because invariably something goes wrong, and then you are asking, “Why did I schedule 3 colpopexies before getting on a plane?”
Dr. Rardin: Yes, I completely agree with all of that. Personally, I feel fortunate that I can compartmentalize pretty well. When I am home with my kids, I allow myself to shed some of the doctor/surgeon/leadership persona; I am able to be goofy and completely non–doctor-like. It works to help me leave work behind.
Dr. Matthews: Other things you can do include setting up an out-of-office notice on your email that says when you will be back and what to do in case of urgent matters. This basically says to the world, “Don’t expect to hear from me until X date.” It removes the expectation that you will respond sooner. Otherwise, we would all be on our smartphones all the time and not enjoying our time away.
What I wish I knew then
Dr. Culligan: How would you complete the sentence, “I wish they had told me X when I was embarking on my career?”
Dr. Rardin: I keep coming back to the phrase, “Don’t do anything that you can reasonably pay someone else to do.” By that I mean, if you don’t get energy from housework, consider spending some of your money to get help with the housework. Resolve to make a relatively small expenditure to maximize the quality of the time that you give to yourself and your family. Those are the sorts of things that I think can go a long way.
Dr. Culligan: Charley, your wife is an ObGyn. How do you navigate a dual medical career household? What advice do you have for others?
Dr. Rardin: When I was going into fellowship, we had a conversation about how hard it is for both people in a relationship to have an academic fire in the belly and to be truly engaged in climbing the academic ladder. We made a decision that Jane would go into private practice. There has got to be some give and take in a dual medical relationship; a lot of sacrifices and compromises need to happen. We are fortunate in that there are complementary aspects to our jobs. We both spend about the same number of nights away from the house, but my travel is more in chunks and hers is overnight calls for labor and delivery. We have different ways of (briefly) single-parenting, and you have to come up with ways to handle the domestic chores.
Dr. Matthews: I wish someone had explained to me that the people you work with are much more important than the place. The human connection is what defines your experience, much more than any ego-driven outcome.
Dr. Greene: I wish someone had explained to me the competing aspects of academic medicine. The cards are stacked in a way that make it difficult for you to win. For example, you may love to teach and may be really good at it, but if you let your students handle too many cases, your relative value units plummet and then the hospital is on your back. There are the interests of people, and there are the interests of the business. Everything is a balance, and it’s really tricky.
Dr. Huber: Luckily, Pat counselled me as I was finishing my fellowship about the importance of negotiating a good contract, of being pushy and knowing what you want out of it and knowing what your limitations are. I joined a private practice that had 3 different physical locations. If I had to drive to all of them, as they wanted, it would have meant up to a one-and-a-half-hour commute. But I pushed to stay in one location and to put that extra hour to better use. I am glad I did, but it was terrifying at the time because I didn’t want to lose the offer. I know people that did not do that and took the first thing they got. Now, they are driving all over the place or they have these crazy hours or terrible call responsibilities that if they had just been a little firmer, they probably could have gotten out of. As they start trying to find work-life balance, they are already handicapped.
Continue to: Passions outside the office...
Passions outside the office
Dr. Culligan: One thing I would like to touch on is what is going on in each of your personal lives because all of you have interesting stories to tell outside of what you do professionally. What drives you other than medicine?
Dr. Rardin: I am the father of 3 boys. The oldest one just got his Eagle Scout rank yesterday in Boy Scouts. I would be a woodworker if I wasn’t in medicine. I am a Deacon at church. And I love to spend my downtime reading with my family in front of the fireplace.
Dr. Matthews: For me, it’s music. When my husband and I first met, he asked me if I played a musical instrument. I said I played the cello in primary school. He said, “Great, go rent a cello.” I was never at all interested in playing the cello by myself, but because he plays guitar and piano we became able to play a lot of music together. Our son, Alexander, plays drums. We now have a family band.
In addition, I do yoga. I would never have labeled myself an anxious person, but I learned through this process that I am and need to manage it. It took a lot of years to figure that out. If I don’t leave myself an hour each day to go to a yoga class, I am not a happy person and neither is anyone around me. Also, I get tremendous pleasure from reading books and magazines as opposed to watching a screen.
Dr. Greene: I have found that my passions outside of work often change depending on my stage of life. Right now, I have two young babies and so my life outside of work revolves around them. Before the babies, my dad, who lives in Buffalo, was ill. So for awhile, we were flying to Buffalo almost every weekend that I was not on call. I would say, in general what fuels me is connecting with the people I love as often as I can. A typical night involves me and my husband going for a walk with our kids and dog after dinner and talking to each other. We connect with neighbors and chat on the front porch. It doesn’t really matter what we are doing; it is about being surrounded by people who matter.
Dr. Huber: It’s similar for me. Having a child completely shifts your world view. My goal every day is to give my daughter her first feeding in the morning and to get home as soon as possible at the end of the day to do her last feeding and put her to sleep. She crawled for the first time yesterday, and I was so excited that I could be there for that.
Also, I love being outdoors. I love hiking and camping. Going on a hike and being outside with nature is my way of decompressing.
Continue to: Thinking about upcoming generations...
Thinking about upcoming generations
Dr. Matthews: One other thing I would like to propose is looking at what can we do to make the profession better for the next generation. As a group, our profession is somewhat inflexible. We tend to fall into the trap of, “since this is the way we have always done it this is how we should continue doing it.” The OR still starts at 7:00 or 7:30 am, ignoring the need for school drop-offs, etc. We are not innovative about flexibility in the work week. Honestly, it does not work well for many people, patients and physicians alike. Flexible scheduling should be something that is on the table for both men and women who are trying to balance being full-time parents and full-time surgeons. We need to create an environment in which it is okay for you to spend 10 years instead of 6 as an assistant professor because you are also a young parent, and it will not count against you when you come up for promotion.
Dr. Culligan: I agree with you, Catherine. Full “Professor” is a nice title, but it means time away from family and a lot of other things. Each of us has to decide whether it is worth it, especially since it often does not come with any extra money.
Dr. Huber: A question on a recent survey of residents asked, “Do you see yourself going into private practice or academic medicine when you’ve completed your residency?” When I was a resident, everyone wanted to go into academic medicine, but now it seems like more and more residents have their sights set on private practice because that is where they see the opportunities to create work-life balance.
In the academic world, you have to try to get a promotion in X number of years, and get X number of publications, and be a great teacher, doctor, and administrator all at the same time. I am wondering if we are going to start seeing more and more residents and fellows going into private or hospital-owned practice where there aren’t those added expectations.
Dr. Rardin: I agree, and we are back to what we said in the beginning about doing an honest assessment of what is meaningful and important. We are all trained to try to reach for that shiny brass ring, but do we really want that brass ring? Will it be an asset or a hindrance once we get it? It is okay to be honest and say, “I really don’t want that promotion. I would rather spend more time with my family.” ●
Patrick J. Culligan, MD:
My impression of our younger colleagues, however, is that many of them are not attracted to the traditional ivory tower research model of academic advancement to which many in previous generations aspired. They seem more concerned with work-life balance as their measure of success rather than the classic metrics of money and prestige. Everyone still needs role models and mentors, though, and that’s where all of you come in. I asked each of you to be on this panel because I admire you for your varying approaches to work-life balance while achieving success as gynecologic surgeons. I thought others in the field might be inspired by hearing your stories.
Cultivating your passions
Kristie Greene, MD: What I have come to learn and appreciate is a really simple point: you do not have to do everything. Determining who you want to be both personally and professionally is step 1.
Granted, answering the question, “Who do I want to be?” is not as simple as it sounds. Many factors figure into the decisions we make in our personal and professional lives. Also, it is not a question we often stop and ask ourselves. From early on, we are placed on an escalator moving up through medical school, residency, fellowship, good job, better job, etc. We are so accustomed to being competitive, to winning, and to wanting to be the best that we sometimes forget to ask ourselves, “What is it exactly that I want, and why? What is my endpoint? And does it make me happy?”
Multitasking is regarded as a talent. As much as we would like to believe that we can do everything at the same time and do it all well, we actually can’t. A friend of mine made me read a book a couple of years ago, called Feeling Good, by David Burns. The book encourages you to consider the different tasks you do in a day and rate how good you are at each of them on a scale of 1 to 10. It then asks you to think about how much enjoyment you derive from each of the tasks and about why you are doing the ones that bring you little to no enjoyment.
I ultimately decided that, for me professionally, the most important thing was my interest in global health. So I decided to do whatever it took to make this happen. But you don’t get something for nothing, and everything comes with sacrifices.
Continue to: Charles Rardin, MD...
Charles Rardin, MD: How exactly did you decide that you were going to focus your career toward pursuing international health? How did you know it was more important? And how did you overcome some of those obstacles?
Dr. Greene: You have to ask the hard question again about what brings you the most joy professionally and personally. That was the easy part of it for me because global health has always been that source of happiness and fulfillment for me. The more challenging parts are the sacrifices and hard choices that come with it. With global health, it can be difficult to balance the demands of a clinical practice.
All of our jobs are a business. I am still struggling with the money part of it. For my husband and I, that meant we had to start small—do what we could afford. But then it blossomed into something that was involving residents, fellows, and med students, which requires far more funding than we had. So I reached out to family. Most of our families donate to different organizations or charities every year, so why not donate to a loved one for something they are passionate about?
At the University of South Florida (USF), we set up a fund, a foundation for global health, which helps support our work abroad as well as the costs associated with involvement of our trainees. Right now, what we have is still small potatoes to a country, but we are making it happen by starting at a small level and growing it.
Beyond the money aspect, traveling abroad means less involvement in meetings, missed opportunities to teach courses that might interest me, and time away from my family. I guess my advice on this whole thing is that you can make things happen if they are important enough to you, and if you are willing to make sacrifices in other areas because you can’t have it all.
Making time for you
Dr. Culligan: So you have found what is important to you, and you have found a way to make it happen. But you are faced with more work; you have given yourself additional work on top of your regular work. How do you make time for a personal life?
Catherine Matthews, MD: In preparing for this discussion, I decided to break down my advice into 3 buckets: The first bucket is discovering and knowing your authentic self. The second is building a community, which I’ll elaborate on. And the third, which we have discussed, is to let go of the money.
Dr. Culligan: I love the concept of the authentic self, but how does that jive with a tendency to strive for perfection? We all think we can do it all. How do we narrow down to what really matters?
Dr. Matthews: We often focus on the things that bring us happiness and what we are good at, but it’s the things that make us unhappy that tend to bring us down. It’s the presence of unhappiness, not the absence of happiness, that seems to be the undoing of many, including myself.
None of us are born with dramatic insight. It is experience that leads to insight. People who are actually present are able to gain insight through observation. A person becomes a better surgeon by observing the outcome of doing a stitch this way versus that; you learn how to do it by seeing what it looks like afterward.
Finding our authentic selves happens in much the same way. Having the presence of mind to ask the right questions, such as, “How am I feeling while I’m doing this?” leads to insights into the true self.
Continue to: It takes a village...
It takes a village
Dr. Greene: Catherine mentioned community earlier, and that is extremely important. The people who surround us can have a huge impact on the way we perceive things, including ourselves. Having a mix of people in our lives—some who practice medicine and others who don’t—helps us stay balanced and answer some of the tough questions. Catherine, for example, has helped me in various stages of my career to ask myself meaningful questions and get real answers.
Dr. Rardin: Part of finding balance is luck, and part of it is making a choice between money and everything else. In considering my first job out of training, I knew that money had the potential to distract me from what was important to me. So I chose a position that was almost entirely salaried so that the decisions I made clinically, surgically, and regarding work-life balance would be less likely to directly impact what was important to me.
Sally Huber, MD: I am still in the “getting there” phase of my life, but one thing I have found is that getting my family involved and excited about what I do has made them much more accepting of when I have longer work days or work to do on the weekend. My spouse has become quite involved with what I have been doing with transgender health in Atlanta. It has been a great bonding experience; she shares my passions, and together we are creating something about which we both can be proud.
When work invades home life
Dr. Culligan: That is great. Sally, I think when we talked, you were just learning about the necessity of mental separation and of not taking your work home with you, which is so hard for all of us with all of our devices.
Dr. Huber: Yes, this year has been about seeing what works best as far as being efficient at work and having quality time at home. At the end of every day I ask myself, “What worked well today? What didn’t work well? What else can I do to maximize time with my family?” I am slowly becoming more efficient, but it has been a challenge. During fellowship, your day is pretty set, but once you are practicing on your own, your hours and responsibilities are completely different, and you have to figure out what works best for you, your values, and your expectations of private life. It takes some time, and I am still figuring it out.
Dr. Culligan: How often would you say that you bring work home? I try hard once I am home to quit working, but sometimes on the weekends I break that rule.
Dr. Matthews: I must say that I do feel like there are certain times when I am better at that than others. Work comes in waves with pressing deadlines. If I averaged it out, probably a third of the time I have some email or some conference call or something that I have got to do at home. I do really try to limit the obligations that I have after 5:30 or 6:00 pm. I resent intrusions after that time. As far as weekends, I delegate about one weekend every 2 months to work, instead of doing a little bit every weekend.
Dr. Greene: I agree. I try hard to make 5:30 to 7:30 pm unequivocal time for a family dinner and time for my kids. During that time, I do not have my phone near me so I can’t look at email or texts. I try not to schedule conference calls. I try to be there to read books to my kids at night. Then if I need to do work, I do it later at night, which interferes with time with my spouse, and is not ideal, but that’s what happens.
Dr. Matthews: One of the things that I think is a huge part of work-life balance is work-related travel. When you are present at work on a consistent basis, the work does not pile up to the extent that it does when you are absent on a trip. When you come back, you invariably pay the price by seeing more patients and doing more surgery. Then it becomes a stressful event.
My advice to young people is to be very thoughtful about planning trips, especially distant ones. You do not want to sit on a plane all day when you could be doing something more productive. If I could have done something differently in my mid-career, I would have traveled less.
Continue to: Prioritizing “out of office” time...
Prioritizing “out of office” time
Dr. Greene: How do you all mentally separate yourself from work, so that when you are on vacation with your family you are not thinking about the office, the patients, and all of the things on your to-do list?
Dr. Rardin: I don’t have a great answer for that except that it is about being present. You have to decide that now is the time when I am home, now is the time when I am a parent, now is the time when I am a boy scout leader, etc. I guess maybe it’s a skill, or maybe it’s about making something a priority. Work will always be waiting for you when you turn your attention back to it.
Dr. Matthews: Kristie, the answer to your question goes back to community. Partners in a practice cover for each other. You have to trust them to take care of things so that you can relax during your time away.
Some people recommend not scheduling challenging cases right before going away because invariably something goes wrong, and then you are asking, “Why did I schedule 3 colpopexies before getting on a plane?”
Dr. Rardin: Yes, I completely agree with all of that. Personally, I feel fortunate that I can compartmentalize pretty well. When I am home with my kids, I allow myself to shed some of the doctor/surgeon/leadership persona; I am able to be goofy and completely non–doctor-like. It works to help me leave work behind.
Dr. Matthews: Other things you can do include setting up an out-of-office notice on your email that says when you will be back and what to do in case of urgent matters. This basically says to the world, “Don’t expect to hear from me until X date.” It removes the expectation that you will respond sooner. Otherwise, we would all be on our smartphones all the time and not enjoying our time away.
What I wish I knew then
Dr. Culligan: How would you complete the sentence, “I wish they had told me X when I was embarking on my career?”
Dr. Rardin: I keep coming back to the phrase, “Don’t do anything that you can reasonably pay someone else to do.” By that I mean, if you don’t get energy from housework, consider spending some of your money to get help with the housework. Resolve to make a relatively small expenditure to maximize the quality of the time that you give to yourself and your family. Those are the sorts of things that I think can go a long way.
Dr. Culligan: Charley, your wife is an ObGyn. How do you navigate a dual medical career household? What advice do you have for others?
Dr. Rardin: When I was going into fellowship, we had a conversation about how hard it is for both people in a relationship to have an academic fire in the belly and to be truly engaged in climbing the academic ladder. We made a decision that Jane would go into private practice. There has got to be some give and take in a dual medical relationship; a lot of sacrifices and compromises need to happen. We are fortunate in that there are complementary aspects to our jobs. We both spend about the same number of nights away from the house, but my travel is more in chunks and hers is overnight calls for labor and delivery. We have different ways of (briefly) single-parenting, and you have to come up with ways to handle the domestic chores.
Dr. Matthews: I wish someone had explained to me that the people you work with are much more important than the place. The human connection is what defines your experience, much more than any ego-driven outcome.
Dr. Greene: I wish someone had explained to me the competing aspects of academic medicine. The cards are stacked in a way that make it difficult for you to win. For example, you may love to teach and may be really good at it, but if you let your students handle too many cases, your relative value units plummet and then the hospital is on your back. There are the interests of people, and there are the interests of the business. Everything is a balance, and it’s really tricky.
Dr. Huber: Luckily, Pat counselled me as I was finishing my fellowship about the importance of negotiating a good contract, of being pushy and knowing what you want out of it and knowing what your limitations are. I joined a private practice that had 3 different physical locations. If I had to drive to all of them, as they wanted, it would have meant up to a one-and-a-half-hour commute. But I pushed to stay in one location and to put that extra hour to better use. I am glad I did, but it was terrifying at the time because I didn’t want to lose the offer. I know people that did not do that and took the first thing they got. Now, they are driving all over the place or they have these crazy hours or terrible call responsibilities that if they had just been a little firmer, they probably could have gotten out of. As they start trying to find work-life balance, they are already handicapped.
Continue to: Passions outside the office...
Passions outside the office
Dr. Culligan: One thing I would like to touch on is what is going on in each of your personal lives because all of you have interesting stories to tell outside of what you do professionally. What drives you other than medicine?
Dr. Rardin: I am the father of 3 boys. The oldest one just got his Eagle Scout rank yesterday in Boy Scouts. I would be a woodworker if I wasn’t in medicine. I am a Deacon at church. And I love to spend my downtime reading with my family in front of the fireplace.
Dr. Matthews: For me, it’s music. When my husband and I first met, he asked me if I played a musical instrument. I said I played the cello in primary school. He said, “Great, go rent a cello.” I was never at all interested in playing the cello by myself, but because he plays guitar and piano we became able to play a lot of music together. Our son, Alexander, plays drums. We now have a family band.
In addition, I do yoga. I would never have labeled myself an anxious person, but I learned through this process that I am and need to manage it. It took a lot of years to figure that out. If I don’t leave myself an hour each day to go to a yoga class, I am not a happy person and neither is anyone around me. Also, I get tremendous pleasure from reading books and magazines as opposed to watching a screen.
Dr. Greene: I have found that my passions outside of work often change depending on my stage of life. Right now, I have two young babies and so my life outside of work revolves around them. Before the babies, my dad, who lives in Buffalo, was ill. So for awhile, we were flying to Buffalo almost every weekend that I was not on call. I would say, in general what fuels me is connecting with the people I love as often as I can. A typical night involves me and my husband going for a walk with our kids and dog after dinner and talking to each other. We connect with neighbors and chat on the front porch. It doesn’t really matter what we are doing; it is about being surrounded by people who matter.
Dr. Huber: It’s similar for me. Having a child completely shifts your world view. My goal every day is to give my daughter her first feeding in the morning and to get home as soon as possible at the end of the day to do her last feeding and put her to sleep. She crawled for the first time yesterday, and I was so excited that I could be there for that.
Also, I love being outdoors. I love hiking and camping. Going on a hike and being outside with nature is my way of decompressing.
Continue to: Thinking about upcoming generations...
Thinking about upcoming generations
Dr. Matthews: One other thing I would like to propose is looking at what can we do to make the profession better for the next generation. As a group, our profession is somewhat inflexible. We tend to fall into the trap of, “since this is the way we have always done it this is how we should continue doing it.” The OR still starts at 7:00 or 7:30 am, ignoring the need for school drop-offs, etc. We are not innovative about flexibility in the work week. Honestly, it does not work well for many people, patients and physicians alike. Flexible scheduling should be something that is on the table for both men and women who are trying to balance being full-time parents and full-time surgeons. We need to create an environment in which it is okay for you to spend 10 years instead of 6 as an assistant professor because you are also a young parent, and it will not count against you when you come up for promotion.
Dr. Culligan: I agree with you, Catherine. Full “Professor” is a nice title, but it means time away from family and a lot of other things. Each of us has to decide whether it is worth it, especially since it often does not come with any extra money.
Dr. Huber: A question on a recent survey of residents asked, “Do you see yourself going into private practice or academic medicine when you’ve completed your residency?” When I was a resident, everyone wanted to go into academic medicine, but now it seems like more and more residents have their sights set on private practice because that is where they see the opportunities to create work-life balance.
In the academic world, you have to try to get a promotion in X number of years, and get X number of publications, and be a great teacher, doctor, and administrator all at the same time. I am wondering if we are going to start seeing more and more residents and fellows going into private or hospital-owned practice where there aren’t those added expectations.
Dr. Rardin: I agree, and we are back to what we said in the beginning about doing an honest assessment of what is meaningful and important. We are all trained to try to reach for that shiny brass ring, but do we really want that brass ring? Will it be an asset or a hindrance once we get it? It is okay to be honest and say, “I really don’t want that promotion. I would rather spend more time with my family.” ●