User login
Which behavioral health screening tool should you use—and when?
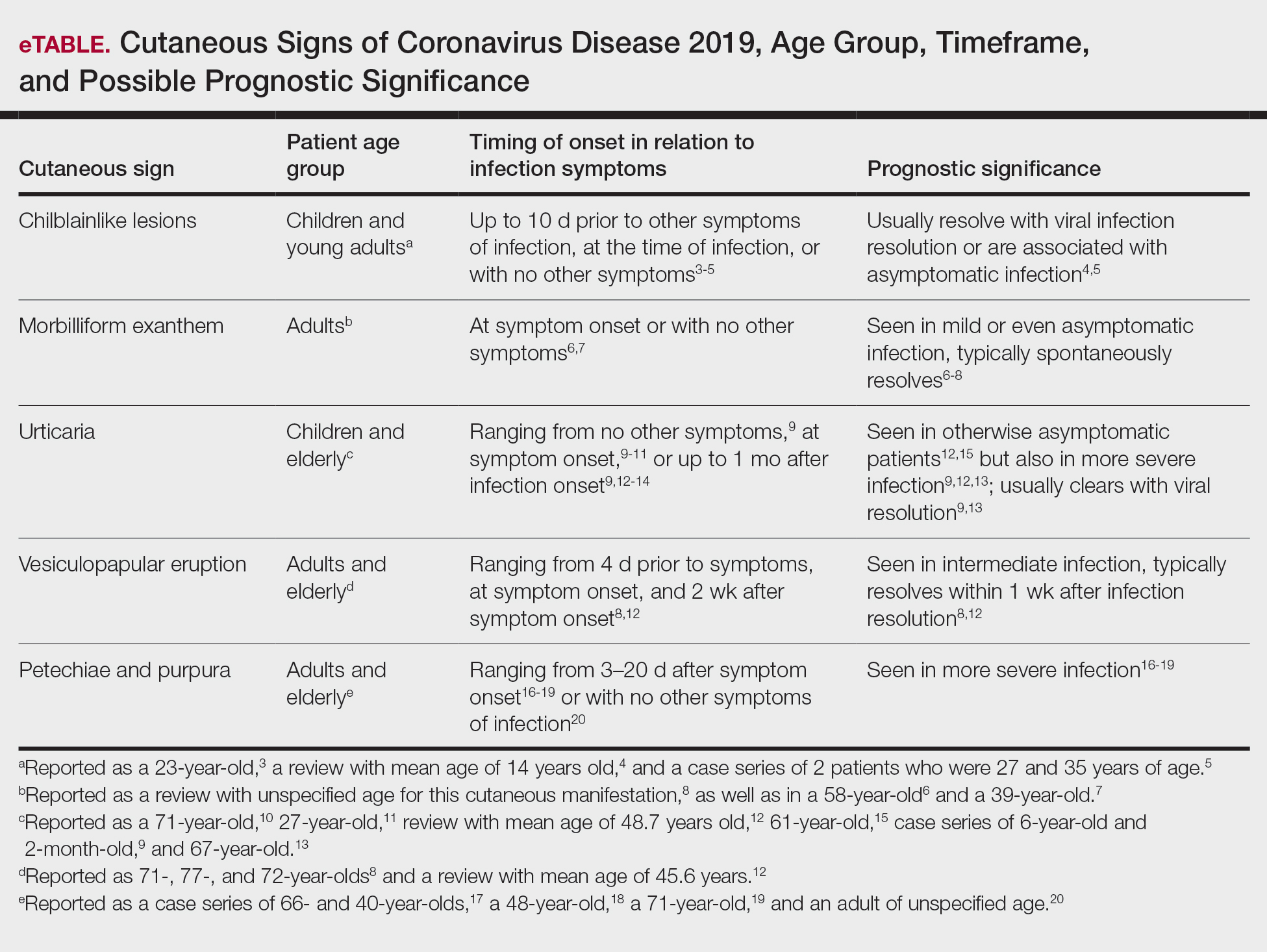
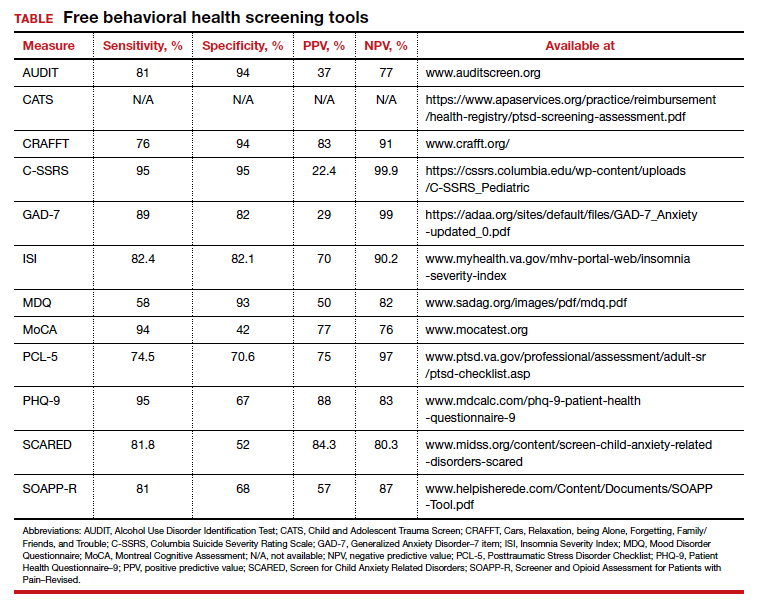
Many screening tools are available in the public domain to assess a variety of symptoms related to impaired mental health. These tools can be used to quickly evaluate for mood, suicidal ideation or behavior, anxiety, sleep, substance use, pain, trauma, memory, and cognition (TABLE). Individuals with poor mental health incur high health care costs. Those suffering from anxiety and posttraumatic stress have more outpatient and emergency department visits and hospitalizations than patients without these disorders,1,2 although use of mental health care services has been related to a decrease in the overutilization of health care services in general.3
Here we review several screening tools that can help you to identify symptoms of mental illnesses and thus, provide prompt early intervention, including referrals to psychological and psychiatric services.
Mood disorders
Most patients with mood disorders are treated in primary care settings.4 Quickly measuring patients’ mood symptoms can expedite treatment for those who need it. Many primary care clinics use the 9-item Patient Health Questionnaire (PHQ-9) to screen for depression.5 The US Preventive Services Task Force (USPSTF) has recommended screening for depression with adequate systems to ensure accurate diagnoses, effective treatment, and follow-up. Although the USPSTF did not specially endorse screening for bipolar disorder, it followed that recommendation with the qualifying statement, “positive screening results [for depression] should lead to additional assessment that considers severity of depression and comorbid psychological problems, alternate diagnoses, and medical conditions.”6 Thus, following a positive screen result for depression, consider using a screening tool for mood disorders to provide diagnostic clarification.
The Mood Disorder Questionnaire (MDQ) is a validated 15-item, self-administered questionnaire that takes only 5 minutes to use in screening adult patients for bipolar I disorder.7 The MDQ assesses specific behaviors related to bipolar disorder, symptom co-occurrence, and functional impairment. The MDQ has low sensitivity (58%) but good specificity (93%) in a primary care setting.8 However, the MDQ is not a diagnostic instrument. A positive screen result should prompt a more thorough clinical evaluation, if necessary, by a professional trained in psychiatric disorders.
We recommend completing the MDQ prior to prescribing antidepressants. You can also monitor a patient’s response to treatment with serial MDQ testing. The MDQ is useful, too, when a patient has unclear mood symptoms that may have features overlapping with bipolar disorder. Furthermore, we recommend screening for bipolar disorder with every patient who reports symptoms of depression, given that some pharmacologic treatments (predominately selective serotonin reuptake inhibitors) can induce mania in patients who actually have unrecognized bipolar disorder.9
Continue to: Suicide...
Suicide
Suicide is the 10th leading cause of death among the general population. All demographic groups are impacted by suicide; however, the most vulnerable are men ages 45 to 64 years.10 Given the imminent risk to individuals who experience suicidal ideation, properly assessing and targeting suicidal risk is paramount.
The Columbia Suicide Severity Rating Scale (C-SSRS) can be completed in an interview format or as a patient self-report. Versions of the C-SSRS are available for children, adolescents, and adults. It can be used in practice with any patient who may be at risk for suicide. Specifically, consider using the C-SSRS when a patient scores 1 or greater on the PHQ-9 or when risk is revealed with another brief screening tool that includes suicidal ideation.
The C-SSRS covers 10 categories related to suicidal ideation and behavior that the clinician explores with questions requiring only Yes/No responses. The C-SSRS demonstrates moderate-to-strong internal consistency and reliability, and it has shown a high degree of sensitivity (95%) and specificity (95%) for suicidal ideation.11
Anxiety and physiologic arousal
Generalized anxiety disorder (GAD) is one of the most common anxiety disorders, with an estimated prevalence of 2.8% to 8.5% among primary care patients.12 Brief, validated screening tools such as the Generalized Anxiety Disorder–7 item (GAD-7) scale can be effective in identifying anxiety and other related disorders in primary care settings.
The GAD-7 comprises 7 items inquiring about symptoms experienced in the past 2 weeks. Scores range from 0 to 21, with cutoffs of 5, 10, and 15 indicating mild, moderate, and severe anxiety, respectively. This questionnaire is appropriate for use with adults and has strong specificity, internal consistency, and test-retest reliability.12 Specificity and sensitivity of the GAD-7 are maximized at a cutoff score of 10 or greater, both exceeding 80%.12 The GAD-7 can be used when patients report symptoms of anxiety or when one needs to screen for anxiety with new patients or more clearly understand symptoms among patients who have complex mental health concerns.
The Screen for Child Anxiety Related Disorders (SCARED) is a 41-item self-report measure of anxiety for children ages 8 to 18. The SCARED questionnaire yields an overall anxiety score, as well as subscales for panic disorder or significant somatic symptoms, generalized anxiety disorder, separation anxiety, social anxiety disorder, and significant school avoidance.13 There is also a 5-item version of the SCARED, which can be useful for brief screening in fast-paced settings when no anxiety disorder is suspected, or for children who may have anxiety but exhibit reduced verbal capacity. The SCARED has been found to have moderate sensitivity (81.8%) and specificity (52%) for diagnosing anxiety disorders in a community sample, with an optimal cutoff point of 22 on the total scale.14
Sleep
Sleep concerns are common, with the prevalence of insomnia among adults in the United States estimated to be 19.2%.15 The importance of assessing these concerns cannot be overstated, and primary care providers are the ones patients consult most often.16 The gold standard in assessing sleep disorders is a structured clinical interview, polysomnography, sleep diary, and actigraphy (home-based monitoring of movement through a device, often worn on the wrist).17,18 However, this work-up is expensive, time-intensive, and impractical in integrated care settings; thus the need for a brief, self-report screening tool to guide further assessment and intervention.
The Insomnia Severity Index (ISI) assesses patients’ perceptions of their insomnia. The ISI was developed to aid both in the clinical evaluation of patients with insomnia and to measure treatment outcomes. Administration of the ISI takes approximately 5 minutes, and scoring takes less than 1 minute.
The ISI is composed of 7 items that measure the severity of sleep onset and sleep maintenance difficulties, satisfaction with current sleep, impact on daily functioning, impairment observable to others, and degree of distress caused by the sleep problems. Each item is scored on a 0 to 4 Likert-type scale, and the individual items are summed for a total score of 0 to 28, with higher scores suggesting more severe insomnia. Evidence-based guidelines recommend cognitive behavioral therapy for insomnia (CBT-I) as the first-line treatment for adults with primary insomnia.19
Several validation studies have found the ISI to be a reliable measure of perceived insomnia severity, and one that is sensitive to changes in patients’ perceptions of treatment outcomes.20,21 An additional validation study confirmed that in primary care settings, a cutoff score of 14 should be used to indicate the likely presence of clinical insomnia22 and to guide further assessment and intervention.
The percentage of insomniac patients correctly identified with the ISI was 82.2%, with moderate sensitivity (82.4%) and specificity (82.1%).22 A positive predictive value of 70% was found, meaning that an insomnia disorder is probable when the ISI total score is 14 or higher; conversely, the negative predictive value was 90.2%.
Continue to: Substance use and pain...
Substance use and pain
The evaluation of alcohol and drug use is an integral part of assessing risky health behaviors. The 10-item Alcohol Use Disorder Identification Test (AUDIT) is a self-report tool developed by the World Health Organization.23,24 Validated in medical settings, scores of 8 or higher suggest problematic drinking.25,26 The AUDIT has demonstrated high specificity (94%) and moderate sensitivity (81%) in primary care settings.27 The AUDIT-C (items 1, 2, and 3 of the AUDIT) has also demonstrated comparable sensitivity, although slightly lower specificity, than the full AUDIT, suggesting that this 3-question screen can also be used in primary care settings.27
Opioid medications, frequently prescribed for chronic pain, present serious risks for many patients. The Screener and Opioid Assessment for Patients with Pain–Revised (SOAPP-R) is a 24-item self-reporting scale that can be completed in approximately 10 minutes.28 A score of 18 or higher has identified 81% of patients at high risk for opioid misuse in a clinical setting, with moderate specificity (68%). Although other factors should be considered when assessing risk of opioid misuse, the SOAPP-R is a helpful and quick addition to an opioid risk assessment.
The CRAFFT Screening Tool for Adolescent Substance Use is administered by the clinician for youths ages 14 to 21. The first 3 questions ask about use of alcohol, marijuana, or other substances during the past 12 months. What follows are questions related to the young person’s specific experiences with substances in relation to Cars, Relaxation, being Alone, Forgetting, Family/Friends, and Trouble (CRAFFT). The CRAFFT has shown moderate sensitivity (76%) and good specificity (94%) for identifying any problem with substance use.29 These measures may be administered to clarify or confirm substance use patterns (ie, duration, frequency), or to determine the severity of problems related to substance use (ie, social or legal problems).
Trauma and PTSD
Approximately 7.7 million adults per year will experience posttraumatic stress disorder (PTSD) symptoms, although PTSD can affect individuals of any age.30 Given the impact that trauma can have, assess for PTSD in patients who have a history of trauma or who otherwise seem to be at risk. The Post-traumatic Stress Disorder Checklist (PCL-5) is a 20-item self-report questionnaire that screens for symptoms directly from the Diagnostic and Statistical Manual of Mental Disorders, 5th Edition (DSM-5) criteria for PTSD. One limitation is that the questionnaire is only validated for adults ages 18 years or older. Completion of the PCL-5 takes 5 to 10 minutes. The PCL-5 has strong internal consistency reliability (94%) and test-retest reliability (82%).31 With a cutoff score of 33 or higher, the sensitivity and specificity have been shown to be moderately high (74.5% and 70.6%, respectively).32
The Child and Adolescent Trauma Screen (CATS) is used to assess for potentially traumatic events and PTSD symptoms in children and adolescents. These symptoms are based on the DSM-5, and therefore the CATS can act as a useful diagnostic aid. The CATS is also available in Spanish, with both caregiver-report (for children ages 3-6 years or 7-17 years) and self-report (for ages 7-17 years) versions. Practical use of the PCL-5 and the CATS involves screening for PTSD symptoms, supporting a provisional diagnosis of PTSD, and monitoring PTSD symptom changes during and after treatment.
Memory and cognition
Cognitive screening is a first step in evaluating possible dementia and other neuropsychological disorders. The importance of brief cognitive screening in primary care cannot be understated, especially for an aging patient population. Although the Mini Mental Status Exam (MMSE) has been widely used among health care providers and researchers, we recommend the Montreal Cognitive Assessment (MoCA).
The MoCA is a simple, standalone cognitive screening tool validated for adults ages 55 to 85 years.33 The MoCA addresses many important cognitive domains, fits on one page, and can be administered by a trained provider in 10 minutes. Research also suggests that it has strong test-retest reliability and positive and negative predictive values for mild cognitive impairment and Alzheimer dementia, and it has been found to be more sensitive than the MMSE.34 We additionally recommend the MoCA as it measures several cognitive skills that are not addressed on the MMSE, including verbal fluency and abstraction.34 Scores below 25 are suggestive of cognitive impairment and should lead to a referral for neuropsychological testing.
The MoCA’s sensitivity for detecting cognitive impairment is high (94%), and specificity is low (42%).35 To ensure consistency and accuracy in administering the MoCA, certification is now required via an online training program through www.mocatest.org.
Adapting these screening tools to practice
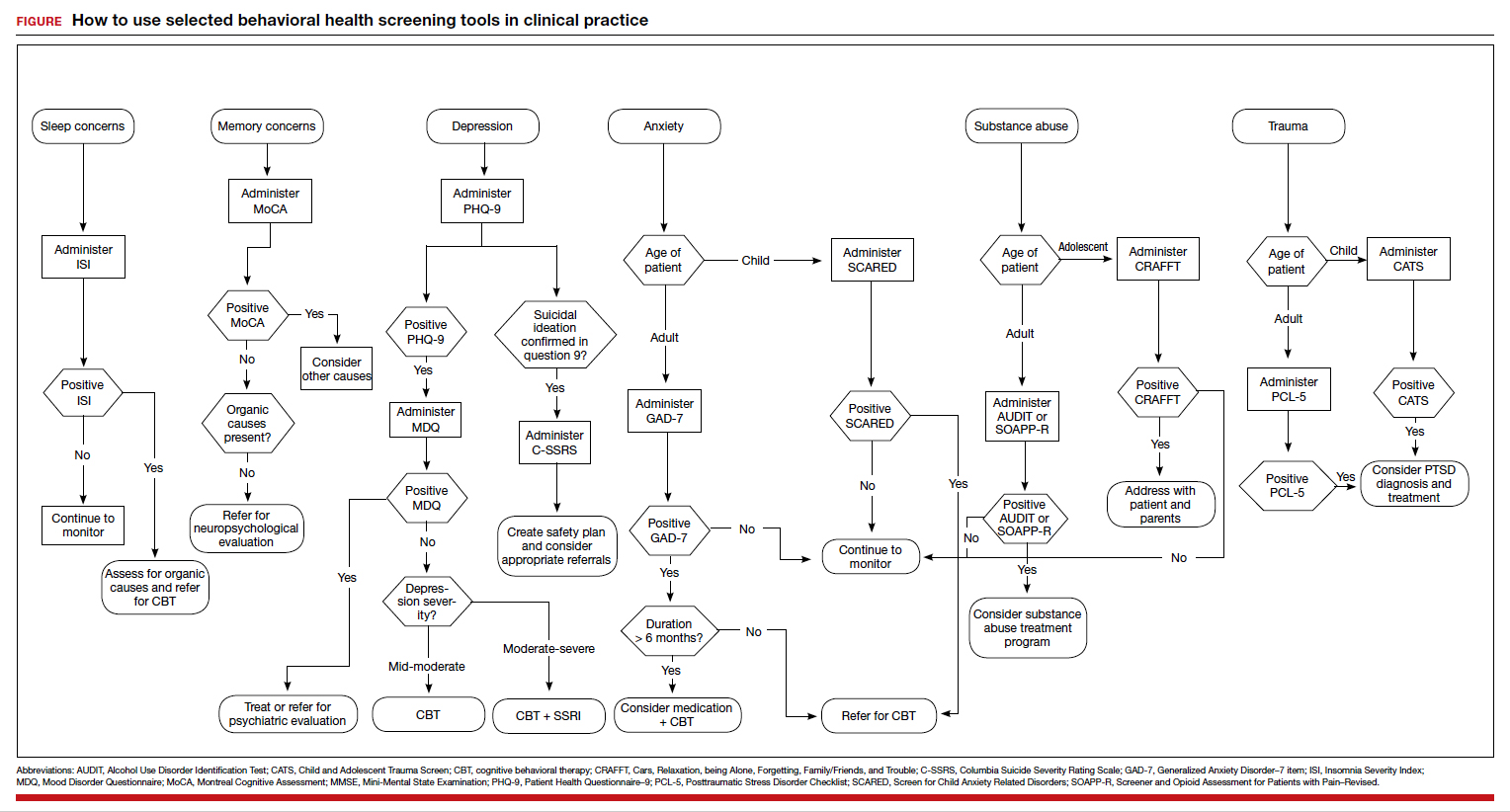
These tools are not meant to be used at every appointment. Every practice is different, and each clinic or physician can tailor the use of these screening tools to the needs of the patient population, as concerns arise, or in collaboration with other providers. Additionally, these screening tools can be used in both integrated care and in private practice, to prompt a more thorough assessment or to aid in—and inform—treatment. Although some physicians choose to administer certain screening tools at each clinic visit, knowing about the availability of other tools can be useful in assessing various issues. The FIGURE can be used to aid in the clinical decision-making process.
- Robinson RL, Grabner M, Palli SR, et al. Covariates of depression and high utilizers of healthcare: impact on resource use and costs. J Psychosom Res. 2016,85:35-43.
- Fogarty CT, Sharma S, Chetty VK, et al. Mental health conditions are associated with increased health care utilization among urban family medicine patients. J Am Board Fam Med. 2008,21:398-407.
- Weissman JD, Russell D, Beasley J, et al. Relationships between adult emotional states and indicators of health care utilization: findings from the National Health Interview Survey 2006–2014. J Psychosom Res. 2016,91:75-81.
- Haddad M, Walters P. Mood disorders in primary care. Psychiatry. 2009,8:71-75.
- Mitchell AJ, Yadegarfar M, Gill J, et al. Case finding and screening clinical utility of the Patient Health Questionnaire (PHQ-9 and PHQ-2) for depression in primary care: a diagnostic metaanalysis of 40 studies. BJPsych Open. 2016,2:127-138.
- Siu AL and US Preventive Services Task Force. Screening for depression in adults. JAMA. 2016;315:380-387.
- Hirschfeld RM, Williams JB, Spitzer RL, et al. Development and validation of a screening instrument for bipolar spectrum disorder: the Mood Disorder Questionnaire. Am J Psychiatry. 2000;157:1873-1875.
- Hirschfeld RM, Cass AR, Holt DC, et al. Screening for bipolar disorder in patients treated for depression in a family medicine clinic. J Am Board Fam Med. 2005;18:233-239.
- Das AK, Olfson M, Gameroff MJ, et al. Screening for bipolar disorder in a primary care practice. JAMA. 2005;293:956-963.
- CDC. Suicide mortality in the United States, 1999-2017. www.cdc.gov/nchs/products/databriefs/db330.htm. Accessed October 23, 2020.
- Viguera AC, Milano N, Ralston L, et al. Comparison of electronic screening for suicidal risk with Patient Health Questionnaire Item 9 and the Columbia Suicide Severity Rating Scale in an outpatient psychiatric clinic. Psychosomatics. 2015;56:460-469.
- Spitzer RL, Kroenke K, Williams JBW, et al. A brief measure for assessing generalized anxiety disorder: the GAD-7. Arch Intern Med. 2006;166:1092-1097.
- Birmaher B, Khetarpal S, Brent D, et al. The Screen for Child Anxiety Related Emotional Disorders (SCARED): scale construction and psychometric characteristics. J Am Acad Chil Adolesc Psychiatry. 1997;36:545-553.
- DeSousa DA, Salum GA, Isolan LR, et al. Sensitivity and specificity of the Screen for Child Anxiety Related Emotional Disorders (SCARED): a community-based study. Child Psychiatry Hum Dev. 2013;44:391-399.
- Ford ES, Cunningham TJ, Giles WH, et al. Trends in insomnia and excessive daytime sleepiness among U.S. adults from 2002 to 2012. Sleep Med. 2015;16:372-378.
- Morin CM, LeBlanc M, Daley M, et al. Epidemiology of insomnia: prevalence, self-help treatments, consultations, and determinants of help-seeking behaviors. Sleep Med. 2006;7:123-130.
- Buysse DJ, Ancoli-Israel S, Edinger JD, et al. Recommendations for a standard research assessment of insomnia. Sleep. 2006;29:1155-1173.
- Martin JL, Hakim AD. Wrist actigraphy. Chest. 2011;139:1514-1527.
- Riemann D, Baglioni C, Bassetti C, et al. European guideline for the diagnosis and treatment of insomnia. J Sleep Res. 2017;26:675-700.
- Bastien CH, Vallières A, Morin CM. Validation of the Insomnia Severity Index as an outcome measure for insomnia research. Sleep Med. 2001;2:297-307.
- Wong ML, Lau KNT, Espie CA, et al. Psychometric properties of the Sleep Condition Indicator and Insomnia Severity Index in the evaluation of insomnia disorder. Sleep Med. 2017;33:76-81.
- Gagnon C, Bélanger L, Ivers H, et al. Validation of the Insomnia Severity Index in primary care. J Am Board Fam Med. 2013;26:701-710.
- Saunders JB, Aasland OG, Babor TF, et al. Development of the Alcohol Use Disorders Identification Test (AUDIT): WHO Collaborative Project on Early Detection of Persons with Harmful Alcohol Consumption. Addiction. 1993;88:791-804.
- Selin KH. Test-retest reliability of the Alcohol Use Disorder Identification Test in a general population sample. Alcohol Clin Exp Res. 2003;27:1428-1435.
- Bohn MJ, Babor TF, Kranzler HR. The Alcohol Use Disorders Identification Test (AUDIT): validation of a screening instrument for use in medical settings. J Stud Alcohol. 1995;56:423-432.
- Conigrave KM, Hall WD, Saunders JB. The AUDIT questionnaire: choosing a cut-off score. Addiction. 1995;90:1349-1356.
- Gomez A, Conde A, Santana JM, et al. Diagnostic usefulness of brief versions of Alcohol Use Identification Test (AUDIT) for detecting hazardous drinkers in primary care settings. J Stud Alcohol. 2005;66:305-308.
- Butler SF, Fernandez K, Benoit C, et al. Validation of the revised Screener and Opioid Assessment for Patients with Pain (SOAPPR). J Pain. 2008;9:360-372.
- Knight JR, Sherritt L, Shrier LA, et al. Validity of the CRAFFT substance abuse screening test among adolescent clinic patients. Arch Pediatr Adolesc Med. 2002;156:607-614.
- DHHS. Post-traumatic stress disorder (PTSD). https://archives.nih.gov/asites/report/09-09-2019/report.nih.gov/nihfactsheets/ViewFactSheetfdf8.html?csid=58&key=P#P. Accessed October 23,2020.
- Blevins CA, Weathers FW, Davis MT, et al. The Posttraumatic Stress Disorder Checklist for DSM-5 (PCL-5): development and initial psychometric evaluation. J Trauma Stress. 2015;28:489-498.
- Verhey R, Chilbanda D, Gibson L, et al. Validation of the Posttraumatic Stress Disorder Checklist- 5 (PCL-5) in a primary care population with high HIV prevalence in Zimbabwe. BMC Psychiatry. 2018;18:109.
- Nasreddine ZS, Phillips NA, Bédirian V, et al. The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc. 2005;53:695-699.
- Stewart S, O’Riley A, Edelstein B, et al. A preliminary comparison of three cognitive screening instruments in long term care: the MMSE, SLUMS, and MoCA. Clin Gerontol. 2012;35:57-75.
- Godefroy O, Fickl A, Roussel M, et al. Is the Montreal Cognitive Assessment superior to the Mini-Mental State Examination to detect poststroke cognitive impairment? A study with neuropsychological evaluation. Stroke. 2011;42:1712-1716.
Many screening tools are available in the public domain to assess a variety of symptoms related to impaired mental health. These tools can be used to quickly evaluate for mood, suicidal ideation or behavior, anxiety, sleep, substance use, pain, trauma, memory, and cognition (TABLE). Individuals with poor mental health incur high health care costs. Those suffering from anxiety and posttraumatic stress have more outpatient and emergency department visits and hospitalizations than patients without these disorders,1,2 although use of mental health care services has been related to a decrease in the overutilization of health care services in general.3

Here we review several screening tools that can help you to identify symptoms of mental illnesses and thus, provide prompt early intervention, including referrals to psychological and psychiatric services.
Mood disorders
Most patients with mood disorders are treated in primary care settings.4 Quickly measuring patients’ mood symptoms can expedite treatment for those who need it. Many primary care clinics use the 9-item Patient Health Questionnaire (PHQ-9) to screen for depression.5 The US Preventive Services Task Force (USPSTF) has recommended screening for depression with adequate systems to ensure accurate diagnoses, effective treatment, and follow-up. Although the USPSTF did not specially endorse screening for bipolar disorder, it followed that recommendation with the qualifying statement, “positive screening results [for depression] should lead to additional assessment that considers severity of depression and comorbid psychological problems, alternate diagnoses, and medical conditions.”6 Thus, following a positive screen result for depression, consider using a screening tool for mood disorders to provide diagnostic clarification.
The Mood Disorder Questionnaire (MDQ) is a validated 15-item, self-administered questionnaire that takes only 5 minutes to use in screening adult patients for bipolar I disorder.7 The MDQ assesses specific behaviors related to bipolar disorder, symptom co-occurrence, and functional impairment. The MDQ has low sensitivity (58%) but good specificity (93%) in a primary care setting.8 However, the MDQ is not a diagnostic instrument. A positive screen result should prompt a more thorough clinical evaluation, if necessary, by a professional trained in psychiatric disorders.
We recommend completing the MDQ prior to prescribing antidepressants. You can also monitor a patient’s response to treatment with serial MDQ testing. The MDQ is useful, too, when a patient has unclear mood symptoms that may have features overlapping with bipolar disorder. Furthermore, we recommend screening for bipolar disorder with every patient who reports symptoms of depression, given that some pharmacologic treatments (predominately selective serotonin reuptake inhibitors) can induce mania in patients who actually have unrecognized bipolar disorder.9
Continue to: Suicide...
Suicide
Suicide is the 10th leading cause of death among the general population. All demographic groups are impacted by suicide; however, the most vulnerable are men ages 45 to 64 years.10 Given the imminent risk to individuals who experience suicidal ideation, properly assessing and targeting suicidal risk is paramount.
The Columbia Suicide Severity Rating Scale (C-SSRS) can be completed in an interview format or as a patient self-report. Versions of the C-SSRS are available for children, adolescents, and adults. It can be used in practice with any patient who may be at risk for suicide. Specifically, consider using the C-SSRS when a patient scores 1 or greater on the PHQ-9 or when risk is revealed with another brief screening tool that includes suicidal ideation.
The C-SSRS covers 10 categories related to suicidal ideation and behavior that the clinician explores with questions requiring only Yes/No responses. The C-SSRS demonstrates moderate-to-strong internal consistency and reliability, and it has shown a high degree of sensitivity (95%) and specificity (95%) for suicidal ideation.11
Anxiety and physiologic arousal
Generalized anxiety disorder (GAD) is one of the most common anxiety disorders, with an estimated prevalence of 2.8% to 8.5% among primary care patients.12 Brief, validated screening tools such as the Generalized Anxiety Disorder–7 item (GAD-7) scale can be effective in identifying anxiety and other related disorders in primary care settings.
The GAD-7 comprises 7 items inquiring about symptoms experienced in the past 2 weeks. Scores range from 0 to 21, with cutoffs of 5, 10, and 15 indicating mild, moderate, and severe anxiety, respectively. This questionnaire is appropriate for use with adults and has strong specificity, internal consistency, and test-retest reliability.12 Specificity and sensitivity of the GAD-7 are maximized at a cutoff score of 10 or greater, both exceeding 80%.12 The GAD-7 can be used when patients report symptoms of anxiety or when one needs to screen for anxiety with new patients or more clearly understand symptoms among patients who have complex mental health concerns.
The Screen for Child Anxiety Related Disorders (SCARED) is a 41-item self-report measure of anxiety for children ages 8 to 18. The SCARED questionnaire yields an overall anxiety score, as well as subscales for panic disorder or significant somatic symptoms, generalized anxiety disorder, separation anxiety, social anxiety disorder, and significant school avoidance.13 There is also a 5-item version of the SCARED, which can be useful for brief screening in fast-paced settings when no anxiety disorder is suspected, or for children who may have anxiety but exhibit reduced verbal capacity. The SCARED has been found to have moderate sensitivity (81.8%) and specificity (52%) for diagnosing anxiety disorders in a community sample, with an optimal cutoff point of 22 on the total scale.14
Sleep
Sleep concerns are common, with the prevalence of insomnia among adults in the United States estimated to be 19.2%.15 The importance of assessing these concerns cannot be overstated, and primary care providers are the ones patients consult most often.16 The gold standard in assessing sleep disorders is a structured clinical interview, polysomnography, sleep diary, and actigraphy (home-based monitoring of movement through a device, often worn on the wrist).17,18 However, this work-up is expensive, time-intensive, and impractical in integrated care settings; thus the need for a brief, self-report screening tool to guide further assessment and intervention.
The Insomnia Severity Index (ISI) assesses patients’ perceptions of their insomnia. The ISI was developed to aid both in the clinical evaluation of patients with insomnia and to measure treatment outcomes. Administration of the ISI takes approximately 5 minutes, and scoring takes less than 1 minute.
The ISI is composed of 7 items that measure the severity of sleep onset and sleep maintenance difficulties, satisfaction with current sleep, impact on daily functioning, impairment observable to others, and degree of distress caused by the sleep problems. Each item is scored on a 0 to 4 Likert-type scale, and the individual items are summed for a total score of 0 to 28, with higher scores suggesting more severe insomnia. Evidence-based guidelines recommend cognitive behavioral therapy for insomnia (CBT-I) as the first-line treatment for adults with primary insomnia.19
Several validation studies have found the ISI to be a reliable measure of perceived insomnia severity, and one that is sensitive to changes in patients’ perceptions of treatment outcomes.20,21 An additional validation study confirmed that in primary care settings, a cutoff score of 14 should be used to indicate the likely presence of clinical insomnia22 and to guide further assessment and intervention.
The percentage of insomniac patients correctly identified with the ISI was 82.2%, with moderate sensitivity (82.4%) and specificity (82.1%).22 A positive predictive value of 70% was found, meaning that an insomnia disorder is probable when the ISI total score is 14 or higher; conversely, the negative predictive value was 90.2%.
Continue to: Substance use and pain...
Substance use and pain
The evaluation of alcohol and drug use is an integral part of assessing risky health behaviors. The 10-item Alcohol Use Disorder Identification Test (AUDIT) is a self-report tool developed by the World Health Organization.23,24 Validated in medical settings, scores of 8 or higher suggest problematic drinking.25,26 The AUDIT has demonstrated high specificity (94%) and moderate sensitivity (81%) in primary care settings.27 The AUDIT-C (items 1, 2, and 3 of the AUDIT) has also demonstrated comparable sensitivity, although slightly lower specificity, than the full AUDIT, suggesting that this 3-question screen can also be used in primary care settings.27
Opioid medications, frequently prescribed for chronic pain, present serious risks for many patients. The Screener and Opioid Assessment for Patients with Pain–Revised (SOAPP-R) is a 24-item self-reporting scale that can be completed in approximately 10 minutes.28 A score of 18 or higher has identified 81% of patients at high risk for opioid misuse in a clinical setting, with moderate specificity (68%). Although other factors should be considered when assessing risk of opioid misuse, the SOAPP-R is a helpful and quick addition to an opioid risk assessment.
The CRAFFT Screening Tool for Adolescent Substance Use is administered by the clinician for youths ages 14 to 21. The first 3 questions ask about use of alcohol, marijuana, or other substances during the past 12 months. What follows are questions related to the young person’s specific experiences with substances in relation to Cars, Relaxation, being Alone, Forgetting, Family/Friends, and Trouble (CRAFFT). The CRAFFT has shown moderate sensitivity (76%) and good specificity (94%) for identifying any problem with substance use.29 These measures may be administered to clarify or confirm substance use patterns (ie, duration, frequency), or to determine the severity of problems related to substance use (ie, social or legal problems).
Trauma and PTSD
Approximately 7.7 million adults per year will experience posttraumatic stress disorder (PTSD) symptoms, although PTSD can affect individuals of any age.30 Given the impact that trauma can have, assess for PTSD in patients who have a history of trauma or who otherwise seem to be at risk. The Post-traumatic Stress Disorder Checklist (PCL-5) is a 20-item self-report questionnaire that screens for symptoms directly from the Diagnostic and Statistical Manual of Mental Disorders, 5th Edition (DSM-5) criteria for PTSD. One limitation is that the questionnaire is only validated for adults ages 18 years or older. Completion of the PCL-5 takes 5 to 10 minutes. The PCL-5 has strong internal consistency reliability (94%) and test-retest reliability (82%).31 With a cutoff score of 33 or higher, the sensitivity and specificity have been shown to be moderately high (74.5% and 70.6%, respectively).32
The Child and Adolescent Trauma Screen (CATS) is used to assess for potentially traumatic events and PTSD symptoms in children and adolescents. These symptoms are based on the DSM-5, and therefore the CATS can act as a useful diagnostic aid. The CATS is also available in Spanish, with both caregiver-report (for children ages 3-6 years or 7-17 years) and self-report (for ages 7-17 years) versions. Practical use of the PCL-5 and the CATS involves screening for PTSD symptoms, supporting a provisional diagnosis of PTSD, and monitoring PTSD symptom changes during and after treatment.
Memory and cognition
Cognitive screening is a first step in evaluating possible dementia and other neuropsychological disorders. The importance of brief cognitive screening in primary care cannot be understated, especially for an aging patient population. Although the Mini Mental Status Exam (MMSE) has been widely used among health care providers and researchers, we recommend the Montreal Cognitive Assessment (MoCA).
The MoCA is a simple, standalone cognitive screening tool validated for adults ages 55 to 85 years.33 The MoCA addresses many important cognitive domains, fits on one page, and can be administered by a trained provider in 10 minutes. Research also suggests that it has strong test-retest reliability and positive and negative predictive values for mild cognitive impairment and Alzheimer dementia, and it has been found to be more sensitive than the MMSE.34 We additionally recommend the MoCA as it measures several cognitive skills that are not addressed on the MMSE, including verbal fluency and abstraction.34 Scores below 25 are suggestive of cognitive impairment and should lead to a referral for neuropsychological testing.
The MoCA’s sensitivity for detecting cognitive impairment is high (94%), and specificity is low (42%).35 To ensure consistency and accuracy in administering the MoCA, certification is now required via an online training program through www.mocatest.org.
Adapting these screening tools to practice
These tools are not meant to be used at every appointment. Every practice is different, and each clinic or physician can tailor the use of these screening tools to the needs of the patient population, as concerns arise, or in collaboration with other providers. Additionally, these screening tools can be used in both integrated care and in private practice, to prompt a more thorough assessment or to aid in—and inform—treatment. Although some physicians choose to administer certain screening tools at each clinic visit, knowing about the availability of other tools can be useful in assessing various issues. The FIGURE can be used to aid in the clinical decision-making process.

Many screening tools are available in the public domain to assess a variety of symptoms related to impaired mental health. These tools can be used to quickly evaluate for mood, suicidal ideation or behavior, anxiety, sleep, substance use, pain, trauma, memory, and cognition (TABLE). Individuals with poor mental health incur high health care costs. Those suffering from anxiety and posttraumatic stress have more outpatient and emergency department visits and hospitalizations than patients without these disorders,1,2 although use of mental health care services has been related to a decrease in the overutilization of health care services in general.3

Here we review several screening tools that can help you to identify symptoms of mental illnesses and thus, provide prompt early intervention, including referrals to psychological and psychiatric services.
Mood disorders
Most patients with mood disorders are treated in primary care settings.4 Quickly measuring patients’ mood symptoms can expedite treatment for those who need it. Many primary care clinics use the 9-item Patient Health Questionnaire (PHQ-9) to screen for depression.5 The US Preventive Services Task Force (USPSTF) has recommended screening for depression with adequate systems to ensure accurate diagnoses, effective treatment, and follow-up. Although the USPSTF did not specially endorse screening for bipolar disorder, it followed that recommendation with the qualifying statement, “positive screening results [for depression] should lead to additional assessment that considers severity of depression and comorbid psychological problems, alternate diagnoses, and medical conditions.”6 Thus, following a positive screen result for depression, consider using a screening tool for mood disorders to provide diagnostic clarification.
The Mood Disorder Questionnaire (MDQ) is a validated 15-item, self-administered questionnaire that takes only 5 minutes to use in screening adult patients for bipolar I disorder.7 The MDQ assesses specific behaviors related to bipolar disorder, symptom co-occurrence, and functional impairment. The MDQ has low sensitivity (58%) but good specificity (93%) in a primary care setting.8 However, the MDQ is not a diagnostic instrument. A positive screen result should prompt a more thorough clinical evaluation, if necessary, by a professional trained in psychiatric disorders.
We recommend completing the MDQ prior to prescribing antidepressants. You can also monitor a patient’s response to treatment with serial MDQ testing. The MDQ is useful, too, when a patient has unclear mood symptoms that may have features overlapping with bipolar disorder. Furthermore, we recommend screening for bipolar disorder with every patient who reports symptoms of depression, given that some pharmacologic treatments (predominately selective serotonin reuptake inhibitors) can induce mania in patients who actually have unrecognized bipolar disorder.9
Continue to: Suicide...
Suicide
Suicide is the 10th leading cause of death among the general population. All demographic groups are impacted by suicide; however, the most vulnerable are men ages 45 to 64 years.10 Given the imminent risk to individuals who experience suicidal ideation, properly assessing and targeting suicidal risk is paramount.
The Columbia Suicide Severity Rating Scale (C-SSRS) can be completed in an interview format or as a patient self-report. Versions of the C-SSRS are available for children, adolescents, and adults. It can be used in practice with any patient who may be at risk for suicide. Specifically, consider using the C-SSRS when a patient scores 1 or greater on the PHQ-9 or when risk is revealed with another brief screening tool that includes suicidal ideation.
The C-SSRS covers 10 categories related to suicidal ideation and behavior that the clinician explores with questions requiring only Yes/No responses. The C-SSRS demonstrates moderate-to-strong internal consistency and reliability, and it has shown a high degree of sensitivity (95%) and specificity (95%) for suicidal ideation.11
Anxiety and physiologic arousal
Generalized anxiety disorder (GAD) is one of the most common anxiety disorders, with an estimated prevalence of 2.8% to 8.5% among primary care patients.12 Brief, validated screening tools such as the Generalized Anxiety Disorder–7 item (GAD-7) scale can be effective in identifying anxiety and other related disorders in primary care settings.
The GAD-7 comprises 7 items inquiring about symptoms experienced in the past 2 weeks. Scores range from 0 to 21, with cutoffs of 5, 10, and 15 indicating mild, moderate, and severe anxiety, respectively. This questionnaire is appropriate for use with adults and has strong specificity, internal consistency, and test-retest reliability.12 Specificity and sensitivity of the GAD-7 are maximized at a cutoff score of 10 or greater, both exceeding 80%.12 The GAD-7 can be used when patients report symptoms of anxiety or when one needs to screen for anxiety with new patients or more clearly understand symptoms among patients who have complex mental health concerns.
The Screen for Child Anxiety Related Disorders (SCARED) is a 41-item self-report measure of anxiety for children ages 8 to 18. The SCARED questionnaire yields an overall anxiety score, as well as subscales for panic disorder or significant somatic symptoms, generalized anxiety disorder, separation anxiety, social anxiety disorder, and significant school avoidance.13 There is also a 5-item version of the SCARED, which can be useful for brief screening in fast-paced settings when no anxiety disorder is suspected, or for children who may have anxiety but exhibit reduced verbal capacity. The SCARED has been found to have moderate sensitivity (81.8%) and specificity (52%) for diagnosing anxiety disorders in a community sample, with an optimal cutoff point of 22 on the total scale.14
Sleep
Sleep concerns are common, with the prevalence of insomnia among adults in the United States estimated to be 19.2%.15 The importance of assessing these concerns cannot be overstated, and primary care providers are the ones patients consult most often.16 The gold standard in assessing sleep disorders is a structured clinical interview, polysomnography, sleep diary, and actigraphy (home-based monitoring of movement through a device, often worn on the wrist).17,18 However, this work-up is expensive, time-intensive, and impractical in integrated care settings; thus the need for a brief, self-report screening tool to guide further assessment and intervention.
The Insomnia Severity Index (ISI) assesses patients’ perceptions of their insomnia. The ISI was developed to aid both in the clinical evaluation of patients with insomnia and to measure treatment outcomes. Administration of the ISI takes approximately 5 minutes, and scoring takes less than 1 minute.
The ISI is composed of 7 items that measure the severity of sleep onset and sleep maintenance difficulties, satisfaction with current sleep, impact on daily functioning, impairment observable to others, and degree of distress caused by the sleep problems. Each item is scored on a 0 to 4 Likert-type scale, and the individual items are summed for a total score of 0 to 28, with higher scores suggesting more severe insomnia. Evidence-based guidelines recommend cognitive behavioral therapy for insomnia (CBT-I) as the first-line treatment for adults with primary insomnia.19
Several validation studies have found the ISI to be a reliable measure of perceived insomnia severity, and one that is sensitive to changes in patients’ perceptions of treatment outcomes.20,21 An additional validation study confirmed that in primary care settings, a cutoff score of 14 should be used to indicate the likely presence of clinical insomnia22 and to guide further assessment and intervention.
The percentage of insomniac patients correctly identified with the ISI was 82.2%, with moderate sensitivity (82.4%) and specificity (82.1%).22 A positive predictive value of 70% was found, meaning that an insomnia disorder is probable when the ISI total score is 14 or higher; conversely, the negative predictive value was 90.2%.
Continue to: Substance use and pain...
Substance use and pain
The evaluation of alcohol and drug use is an integral part of assessing risky health behaviors. The 10-item Alcohol Use Disorder Identification Test (AUDIT) is a self-report tool developed by the World Health Organization.23,24 Validated in medical settings, scores of 8 or higher suggest problematic drinking.25,26 The AUDIT has demonstrated high specificity (94%) and moderate sensitivity (81%) in primary care settings.27 The AUDIT-C (items 1, 2, and 3 of the AUDIT) has also demonstrated comparable sensitivity, although slightly lower specificity, than the full AUDIT, suggesting that this 3-question screen can also be used in primary care settings.27
Opioid medications, frequently prescribed for chronic pain, present serious risks for many patients. The Screener and Opioid Assessment for Patients with Pain–Revised (SOAPP-R) is a 24-item self-reporting scale that can be completed in approximately 10 minutes.28 A score of 18 or higher has identified 81% of patients at high risk for opioid misuse in a clinical setting, with moderate specificity (68%). Although other factors should be considered when assessing risk of opioid misuse, the SOAPP-R is a helpful and quick addition to an opioid risk assessment.
The CRAFFT Screening Tool for Adolescent Substance Use is administered by the clinician for youths ages 14 to 21. The first 3 questions ask about use of alcohol, marijuana, or other substances during the past 12 months. What follows are questions related to the young person’s specific experiences with substances in relation to Cars, Relaxation, being Alone, Forgetting, Family/Friends, and Trouble (CRAFFT). The CRAFFT has shown moderate sensitivity (76%) and good specificity (94%) for identifying any problem with substance use.29 These measures may be administered to clarify or confirm substance use patterns (ie, duration, frequency), or to determine the severity of problems related to substance use (ie, social or legal problems).
Trauma and PTSD
Approximately 7.7 million adults per year will experience posttraumatic stress disorder (PTSD) symptoms, although PTSD can affect individuals of any age.30 Given the impact that trauma can have, assess for PTSD in patients who have a history of trauma or who otherwise seem to be at risk. The Post-traumatic Stress Disorder Checklist (PCL-5) is a 20-item self-report questionnaire that screens for symptoms directly from the Diagnostic and Statistical Manual of Mental Disorders, 5th Edition (DSM-5) criteria for PTSD. One limitation is that the questionnaire is only validated for adults ages 18 years or older. Completion of the PCL-5 takes 5 to 10 minutes. The PCL-5 has strong internal consistency reliability (94%) and test-retest reliability (82%).31 With a cutoff score of 33 or higher, the sensitivity and specificity have been shown to be moderately high (74.5% and 70.6%, respectively).32
The Child and Adolescent Trauma Screen (CATS) is used to assess for potentially traumatic events and PTSD symptoms in children and adolescents. These symptoms are based on the DSM-5, and therefore the CATS can act as a useful diagnostic aid. The CATS is also available in Spanish, with both caregiver-report (for children ages 3-6 years or 7-17 years) and self-report (for ages 7-17 years) versions. Practical use of the PCL-5 and the CATS involves screening for PTSD symptoms, supporting a provisional diagnosis of PTSD, and monitoring PTSD symptom changes during and after treatment.
Memory and cognition
Cognitive screening is a first step in evaluating possible dementia and other neuropsychological disorders. The importance of brief cognitive screening in primary care cannot be understated, especially for an aging patient population. Although the Mini Mental Status Exam (MMSE) has been widely used among health care providers and researchers, we recommend the Montreal Cognitive Assessment (MoCA).
The MoCA is a simple, standalone cognitive screening tool validated for adults ages 55 to 85 years.33 The MoCA addresses many important cognitive domains, fits on one page, and can be administered by a trained provider in 10 minutes. Research also suggests that it has strong test-retest reliability and positive and negative predictive values for mild cognitive impairment and Alzheimer dementia, and it has been found to be more sensitive than the MMSE.34 We additionally recommend the MoCA as it measures several cognitive skills that are not addressed on the MMSE, including verbal fluency and abstraction.34 Scores below 25 are suggestive of cognitive impairment and should lead to a referral for neuropsychological testing.
The MoCA’s sensitivity for detecting cognitive impairment is high (94%), and specificity is low (42%).35 To ensure consistency and accuracy in administering the MoCA, certification is now required via an online training program through www.mocatest.org.
Adapting these screening tools to practice
These tools are not meant to be used at every appointment. Every practice is different, and each clinic or physician can tailor the use of these screening tools to the needs of the patient population, as concerns arise, or in collaboration with other providers. Additionally, these screening tools can be used in both integrated care and in private practice, to prompt a more thorough assessment or to aid in—and inform—treatment. Although some physicians choose to administer certain screening tools at each clinic visit, knowing about the availability of other tools can be useful in assessing various issues. The FIGURE can be used to aid in the clinical decision-making process.

- Robinson RL, Grabner M, Palli SR, et al. Covariates of depression and high utilizers of healthcare: impact on resource use and costs. J Psychosom Res. 2016,85:35-43.
- Fogarty CT, Sharma S, Chetty VK, et al. Mental health conditions are associated with increased health care utilization among urban family medicine patients. J Am Board Fam Med. 2008,21:398-407.
- Weissman JD, Russell D, Beasley J, et al. Relationships between adult emotional states and indicators of health care utilization: findings from the National Health Interview Survey 2006–2014. J Psychosom Res. 2016,91:75-81.
- Haddad M, Walters P. Mood disorders in primary care. Psychiatry. 2009,8:71-75.
- Mitchell AJ, Yadegarfar M, Gill J, et al. Case finding and screening clinical utility of the Patient Health Questionnaire (PHQ-9 and PHQ-2) for depression in primary care: a diagnostic metaanalysis of 40 studies. BJPsych Open. 2016,2:127-138.
- Siu AL and US Preventive Services Task Force. Screening for depression in adults. JAMA. 2016;315:380-387.
- Hirschfeld RM, Williams JB, Spitzer RL, et al. Development and validation of a screening instrument for bipolar spectrum disorder: the Mood Disorder Questionnaire. Am J Psychiatry. 2000;157:1873-1875.
- Hirschfeld RM, Cass AR, Holt DC, et al. Screening for bipolar disorder in patients treated for depression in a family medicine clinic. J Am Board Fam Med. 2005;18:233-239.
- Das AK, Olfson M, Gameroff MJ, et al. Screening for bipolar disorder in a primary care practice. JAMA. 2005;293:956-963.
- CDC. Suicide mortality in the United States, 1999-2017. www.cdc.gov/nchs/products/databriefs/db330.htm. Accessed October 23, 2020.
- Viguera AC, Milano N, Ralston L, et al. Comparison of electronic screening for suicidal risk with Patient Health Questionnaire Item 9 and the Columbia Suicide Severity Rating Scale in an outpatient psychiatric clinic. Psychosomatics. 2015;56:460-469.
- Spitzer RL, Kroenke K, Williams JBW, et al. A brief measure for assessing generalized anxiety disorder: the GAD-7. Arch Intern Med. 2006;166:1092-1097.
- Birmaher B, Khetarpal S, Brent D, et al. The Screen for Child Anxiety Related Emotional Disorders (SCARED): scale construction and psychometric characteristics. J Am Acad Chil Adolesc Psychiatry. 1997;36:545-553.
- DeSousa DA, Salum GA, Isolan LR, et al. Sensitivity and specificity of the Screen for Child Anxiety Related Emotional Disorders (SCARED): a community-based study. Child Psychiatry Hum Dev. 2013;44:391-399.
- Ford ES, Cunningham TJ, Giles WH, et al. Trends in insomnia and excessive daytime sleepiness among U.S. adults from 2002 to 2012. Sleep Med. 2015;16:372-378.
- Morin CM, LeBlanc M, Daley M, et al. Epidemiology of insomnia: prevalence, self-help treatments, consultations, and determinants of help-seeking behaviors. Sleep Med. 2006;7:123-130.
- Buysse DJ, Ancoli-Israel S, Edinger JD, et al. Recommendations for a standard research assessment of insomnia. Sleep. 2006;29:1155-1173.
- Martin JL, Hakim AD. Wrist actigraphy. Chest. 2011;139:1514-1527.
- Riemann D, Baglioni C, Bassetti C, et al. European guideline for the diagnosis and treatment of insomnia. J Sleep Res. 2017;26:675-700.
- Bastien CH, Vallières A, Morin CM. Validation of the Insomnia Severity Index as an outcome measure for insomnia research. Sleep Med. 2001;2:297-307.
- Wong ML, Lau KNT, Espie CA, et al. Psychometric properties of the Sleep Condition Indicator and Insomnia Severity Index in the evaluation of insomnia disorder. Sleep Med. 2017;33:76-81.
- Gagnon C, Bélanger L, Ivers H, et al. Validation of the Insomnia Severity Index in primary care. J Am Board Fam Med. 2013;26:701-710.
- Saunders JB, Aasland OG, Babor TF, et al. Development of the Alcohol Use Disorders Identification Test (AUDIT): WHO Collaborative Project on Early Detection of Persons with Harmful Alcohol Consumption. Addiction. 1993;88:791-804.
- Selin KH. Test-retest reliability of the Alcohol Use Disorder Identification Test in a general population sample. Alcohol Clin Exp Res. 2003;27:1428-1435.
- Bohn MJ, Babor TF, Kranzler HR. The Alcohol Use Disorders Identification Test (AUDIT): validation of a screening instrument for use in medical settings. J Stud Alcohol. 1995;56:423-432.
- Conigrave KM, Hall WD, Saunders JB. The AUDIT questionnaire: choosing a cut-off score. Addiction. 1995;90:1349-1356.
- Gomez A, Conde A, Santana JM, et al. Diagnostic usefulness of brief versions of Alcohol Use Identification Test (AUDIT) for detecting hazardous drinkers in primary care settings. J Stud Alcohol. 2005;66:305-308.
- Butler SF, Fernandez K, Benoit C, et al. Validation of the revised Screener and Opioid Assessment for Patients with Pain (SOAPPR). J Pain. 2008;9:360-372.
- Knight JR, Sherritt L, Shrier LA, et al. Validity of the CRAFFT substance abuse screening test among adolescent clinic patients. Arch Pediatr Adolesc Med. 2002;156:607-614.
- DHHS. Post-traumatic stress disorder (PTSD). https://archives.nih.gov/asites/report/09-09-2019/report.nih.gov/nihfactsheets/ViewFactSheetfdf8.html?csid=58&key=P#P. Accessed October 23,2020.
- Blevins CA, Weathers FW, Davis MT, et al. The Posttraumatic Stress Disorder Checklist for DSM-5 (PCL-5): development and initial psychometric evaluation. J Trauma Stress. 2015;28:489-498.
- Verhey R, Chilbanda D, Gibson L, et al. Validation of the Posttraumatic Stress Disorder Checklist- 5 (PCL-5) in a primary care population with high HIV prevalence in Zimbabwe. BMC Psychiatry. 2018;18:109.
- Nasreddine ZS, Phillips NA, Bédirian V, et al. The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc. 2005;53:695-699.
- Stewart S, O’Riley A, Edelstein B, et al. A preliminary comparison of three cognitive screening instruments in long term care: the MMSE, SLUMS, and MoCA. Clin Gerontol. 2012;35:57-75.
- Godefroy O, Fickl A, Roussel M, et al. Is the Montreal Cognitive Assessment superior to the Mini-Mental State Examination to detect poststroke cognitive impairment? A study with neuropsychological evaluation. Stroke. 2011;42:1712-1716.
- Robinson RL, Grabner M, Palli SR, et al. Covariates of depression and high utilizers of healthcare: impact on resource use and costs. J Psychosom Res. 2016,85:35-43.
- Fogarty CT, Sharma S, Chetty VK, et al. Mental health conditions are associated with increased health care utilization among urban family medicine patients. J Am Board Fam Med. 2008,21:398-407.
- Weissman JD, Russell D, Beasley J, et al. Relationships between adult emotional states and indicators of health care utilization: findings from the National Health Interview Survey 2006–2014. J Psychosom Res. 2016,91:75-81.
- Haddad M, Walters P. Mood disorders in primary care. Psychiatry. 2009,8:71-75.
- Mitchell AJ, Yadegarfar M, Gill J, et al. Case finding and screening clinical utility of the Patient Health Questionnaire (PHQ-9 and PHQ-2) for depression in primary care: a diagnostic metaanalysis of 40 studies. BJPsych Open. 2016,2:127-138.
- Siu AL and US Preventive Services Task Force. Screening for depression in adults. JAMA. 2016;315:380-387.
- Hirschfeld RM, Williams JB, Spitzer RL, et al. Development and validation of a screening instrument for bipolar spectrum disorder: the Mood Disorder Questionnaire. Am J Psychiatry. 2000;157:1873-1875.
- Hirschfeld RM, Cass AR, Holt DC, et al. Screening for bipolar disorder in patients treated for depression in a family medicine clinic. J Am Board Fam Med. 2005;18:233-239.
- Das AK, Olfson M, Gameroff MJ, et al. Screening for bipolar disorder in a primary care practice. JAMA. 2005;293:956-963.
- CDC. Suicide mortality in the United States, 1999-2017. www.cdc.gov/nchs/products/databriefs/db330.htm. Accessed October 23, 2020.
- Viguera AC, Milano N, Ralston L, et al. Comparison of electronic screening for suicidal risk with Patient Health Questionnaire Item 9 and the Columbia Suicide Severity Rating Scale in an outpatient psychiatric clinic. Psychosomatics. 2015;56:460-469.
- Spitzer RL, Kroenke K, Williams JBW, et al. A brief measure for assessing generalized anxiety disorder: the GAD-7. Arch Intern Med. 2006;166:1092-1097.
- Birmaher B, Khetarpal S, Brent D, et al. The Screen for Child Anxiety Related Emotional Disorders (SCARED): scale construction and psychometric characteristics. J Am Acad Chil Adolesc Psychiatry. 1997;36:545-553.
- DeSousa DA, Salum GA, Isolan LR, et al. Sensitivity and specificity of the Screen for Child Anxiety Related Emotional Disorders (SCARED): a community-based study. Child Psychiatry Hum Dev. 2013;44:391-399.
- Ford ES, Cunningham TJ, Giles WH, et al. Trends in insomnia and excessive daytime sleepiness among U.S. adults from 2002 to 2012. Sleep Med. 2015;16:372-378.
- Morin CM, LeBlanc M, Daley M, et al. Epidemiology of insomnia: prevalence, self-help treatments, consultations, and determinants of help-seeking behaviors. Sleep Med. 2006;7:123-130.
- Buysse DJ, Ancoli-Israel S, Edinger JD, et al. Recommendations for a standard research assessment of insomnia. Sleep. 2006;29:1155-1173.
- Martin JL, Hakim AD. Wrist actigraphy. Chest. 2011;139:1514-1527.
- Riemann D, Baglioni C, Bassetti C, et al. European guideline for the diagnosis and treatment of insomnia. J Sleep Res. 2017;26:675-700.
- Bastien CH, Vallières A, Morin CM. Validation of the Insomnia Severity Index as an outcome measure for insomnia research. Sleep Med. 2001;2:297-307.
- Wong ML, Lau KNT, Espie CA, et al. Psychometric properties of the Sleep Condition Indicator and Insomnia Severity Index in the evaluation of insomnia disorder. Sleep Med. 2017;33:76-81.
- Gagnon C, Bélanger L, Ivers H, et al. Validation of the Insomnia Severity Index in primary care. J Am Board Fam Med. 2013;26:701-710.
- Saunders JB, Aasland OG, Babor TF, et al. Development of the Alcohol Use Disorders Identification Test (AUDIT): WHO Collaborative Project on Early Detection of Persons with Harmful Alcohol Consumption. Addiction. 1993;88:791-804.
- Selin KH. Test-retest reliability of the Alcohol Use Disorder Identification Test in a general population sample. Alcohol Clin Exp Res. 2003;27:1428-1435.
- Bohn MJ, Babor TF, Kranzler HR. The Alcohol Use Disorders Identification Test (AUDIT): validation of a screening instrument for use in medical settings. J Stud Alcohol. 1995;56:423-432.
- Conigrave KM, Hall WD, Saunders JB. The AUDIT questionnaire: choosing a cut-off score. Addiction. 1995;90:1349-1356.
- Gomez A, Conde A, Santana JM, et al. Diagnostic usefulness of brief versions of Alcohol Use Identification Test (AUDIT) for detecting hazardous drinkers in primary care settings. J Stud Alcohol. 2005;66:305-308.
- Butler SF, Fernandez K, Benoit C, et al. Validation of the revised Screener and Opioid Assessment for Patients with Pain (SOAPPR). J Pain. 2008;9:360-372.
- Knight JR, Sherritt L, Shrier LA, et al. Validity of the CRAFFT substance abuse screening test among adolescent clinic patients. Arch Pediatr Adolesc Med. 2002;156:607-614.
- DHHS. Post-traumatic stress disorder (PTSD). https://archives.nih.gov/asites/report/09-09-2019/report.nih.gov/nihfactsheets/ViewFactSheetfdf8.html?csid=58&key=P#P. Accessed October 23,2020.
- Blevins CA, Weathers FW, Davis MT, et al. The Posttraumatic Stress Disorder Checklist for DSM-5 (PCL-5): development and initial psychometric evaluation. J Trauma Stress. 2015;28:489-498.
- Verhey R, Chilbanda D, Gibson L, et al. Validation of the Posttraumatic Stress Disorder Checklist- 5 (PCL-5) in a primary care population with high HIV prevalence in Zimbabwe. BMC Psychiatry. 2018;18:109.
- Nasreddine ZS, Phillips NA, Bédirian V, et al. The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc. 2005;53:695-699.
- Stewart S, O’Riley A, Edelstein B, et al. A preliminary comparison of three cognitive screening instruments in long term care: the MMSE, SLUMS, and MoCA. Clin Gerontol. 2012;35:57-75.
- Godefroy O, Fickl A, Roussel M, et al. Is the Montreal Cognitive Assessment superior to the Mini-Mental State Examination to detect poststroke cognitive impairment? A study with neuropsychological evaluation. Stroke. 2011;42:1712-1716.
A case of BV during pregnancy: Best management approach
CASE Pregnant woman with abnormal vaginal discharge
A 26-year-old woman (G2P1001) at 24 weeks of gestation requests evaluation for increased frothy, whitish-gray vaginal discharge with a fishy odor. She notes that her underclothes constantly feel damp. The vaginal pH is 4.5, and the amine test is positive.
- What is the most likely diagnosis?
- What obstetrical complications may be associated with this condition?
- How should her condition be treated?
Meet our perpetrator
Bacterial vaginosis (BV) is one of the most common conditions associated with vaginal discharge among women of reproductive age. It is characterized by a polymicrobial alteration of the vaginal microbiome, and most distinctly, a relative absence of vaginal lactobacilli. This review discusses the microbiology, epidemiology, specific obstetric and gynecologic complications, clinical manifestations, diagnosis, and treatment of BV.
The role of vaginal flora
Estrogen has a fundamental role in regulating the normal state of the vagina. In a woman’s reproductive years, estrogen increases glycogen in the vaginal epithelial cells, and the increased glycogen concentration promotes colonization by lactobacilli. The lack of estrogen in pre- and postmenopausal women inhibits the growth of the vaginal lactobacilli, leading to a high vaginal pH, which facilitates the growth of bacteria, particularly anaerobes, that can cause BV.
The vaginal microbiome is polymicrobial and has been classified into at least 5 community state types (CSTs). Four CSTs are dominated by lactobacilli. A fifth CST is characterized by the absence of lactobacilli and high concentrations of obligate or facultative anaerobes.1 The hydrogen peroxide–producing lactobacilli predominate in normal vaginal flora and make up 70% to 90% of the total microbiome. These hydrogen peroxide–producing lactobacilli are associated with reduced vaginal proinflammatory cytokines and a highly acidic vaginal pH. Both factors defend against sexually transmitted infections (STIs).2
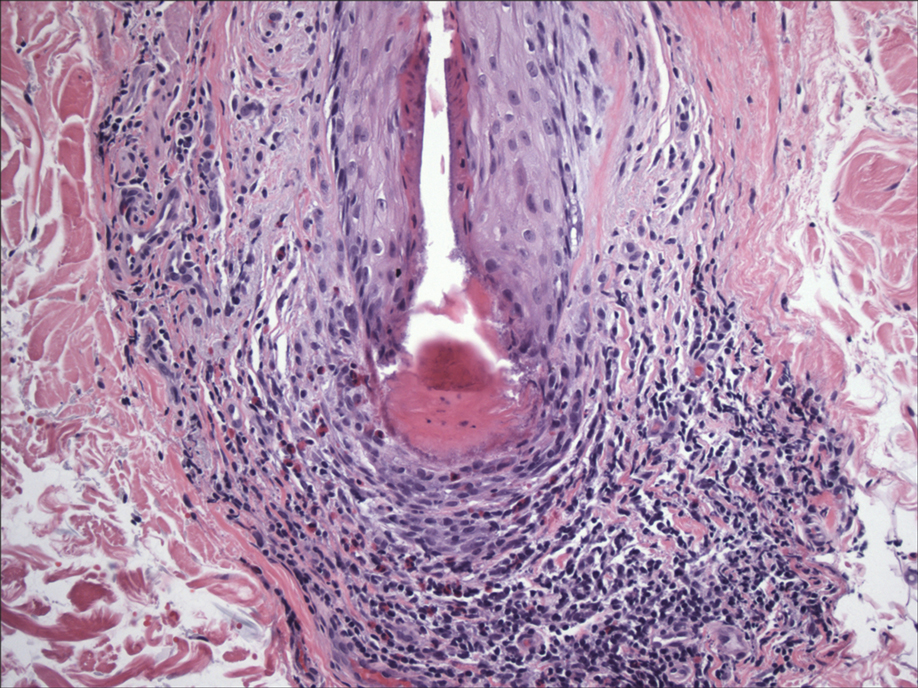
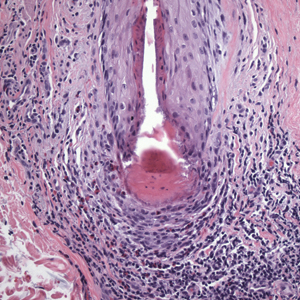
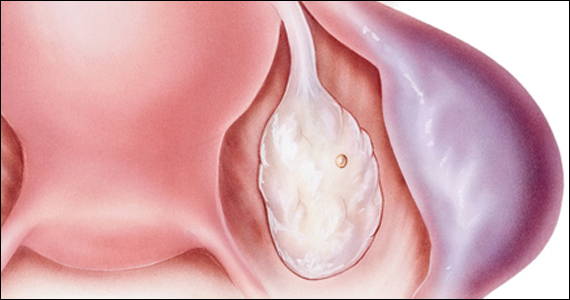
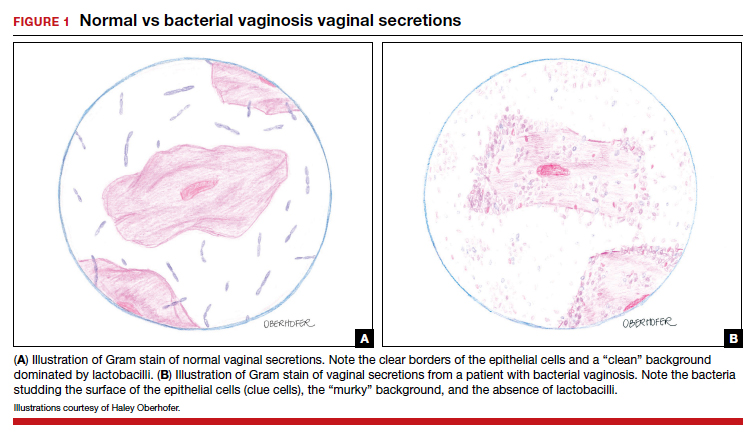
BV is a polymicrobial disorder marked by the significant reduction in the number of vaginal lactobacilli (FIGURE 1). A recent study showed that BV is associated first with a decrease in Lactobacillus crispatus, followed by increase in Prevotella bivia, Gardnerella vaginalis, Atopobium vaginae, and Megasphaera type 1.3 The polymicrobial load is increased by a factor of up to 1,000, compared with normal vaginal flora.4 BV should be considered a biofilm infection caused by adherence of G vaginalis to the vaginal epithelium.5 This biofilm creates a favorable environment for the overgrowth of obligate anaerobic bacteria.
BMI factors into epidemiology
BV is the leading cause of vaginal discharge in reproductive-age women. In the United States, the National Health and Nutrition Examination Survey estimated a prevalence of 29% in the general population and 50% in Black women aged 14 to 49 years.6 In 2013, Kenyon and colleagues performed a systematic review to assess the worldwide epidemiology of BV, and the prevalence varied by country. Within the US population, rates were highest among non-Hispanic, Black women.7 Brookheart and colleagues demonstrated that, even after controlling for race, overweight and obese women had a higher frequency of BV compared with leaner women. In this investigation, the overall prevalence of BV was 28.1%. When categorized by body mass index (BMI), the prevalence was 21.3% in lean women, 30.4% in overweight women, and 34.5% in obese women (P<.001). The authors also found that Black women had a higher prevalence, independent of BMI, compared with White women.8
Complications may occur. BV is notable for having several serious sequelae in both pregnant and nonpregnant women. For obstetric patients, these sequelae include an increased risk of preterm birth; first trimester spontaneous abortion, particularly in the setting of in vitro fertilization; intra-amniotic infection; and endometritis.9,10 The risk of preterm birth increases by a factor of 2 in infected women; however, most women with BV do not deliver preterm.4 The risk of endometritis is increased 6-fold in women with BV.11 Nonpregnant women with BV are at increased risk for pelvic inflammatory disease, postoperative infections, and an increased susceptibility to STIs such as chlamydia, gonorrhea, herpes simplex virus, and HIV.12-15 The risk for vaginal-cuff cellulitis and abscess after hysterectomy is increased 6-fold in the setting of BV.16
Continue to: Clinical manifestations...
Clinical manifestations
BV is characterized by a milky, homogenous, and malodorous vaginal discharge accompanied by vulvovaginal discomfort and vulvar irritation. Vaginal inflammation typically is absent. The associated odor is fishy, and this odor is accentuated when potassium hydroxide (KOH) is added to the vaginal discharge (amine or “whiff” test) or after the patient has coitus. The distinctive odor is due to the release of organic acids and polyamines that are byproducts of anaerobic bacterial metabolism of putrescine and cadaverine. This release is enhanced by exposure of vaginal secretions to alkaline substances such as KOH or semen.
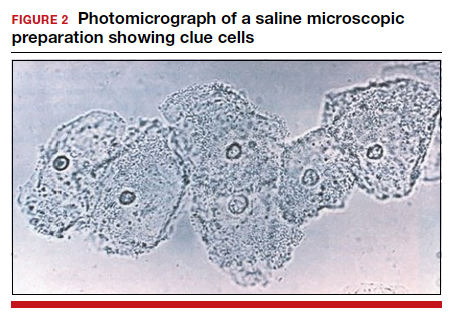
Diagnostic tests and criteria. The diagnosis of BV is made using Amsel criteria or Gram stain with Nugent scoring; bacterial culture is not recommended. Amsel criteria include:
- homogenous, thin, white-gray discharge
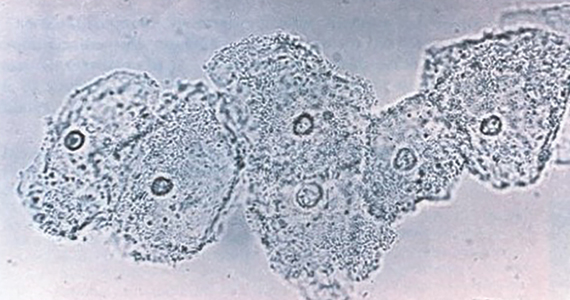
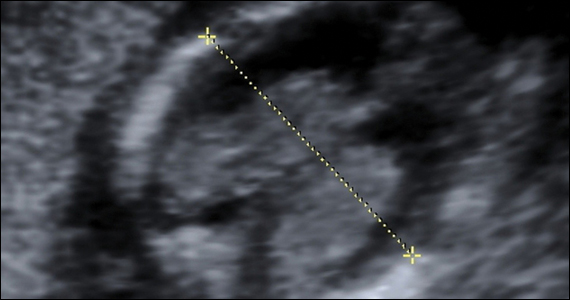
- >20% clue cells on saline microscopy (FIGURE 2)
- a pH >4.5 of vaginal fluid
- positive KOH whiff test.
For diagnosis, 3 of the 4 Amsel criteria must be present.17 Gram stain with Nugent score typically is used for research purposes. Nugent scoring assigns a value to different bacterial morphotypes on Gram stain of vaginal secretions. A score of 7 to 10 is consistent with BV.18
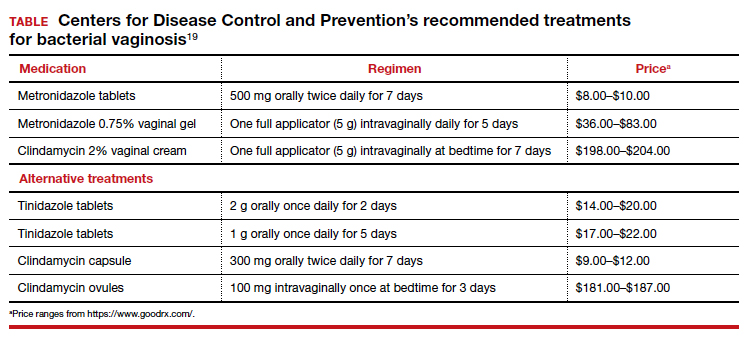
Oral and topical treatments
Treatment is recommended for symptomatic patients. Treatment may reduce the risk of transmission and acquisition of other STIs. The TABLE summarizes Centers for Disease Control and Prevention (CDC) guidelines for BV treatment,19 with options including both oral and topical regimens. Oral and topical metronidazole and oral and topical clindamycin are equally effective at eradicating the local source of infection20; however, only oral metronidazole and oral clindamycin are effective in preventing the systemic complications of BV. Oral metronidazole has more adverse effects than oral clindamycin—including nausea, vomiting, diarrhea, and a disulfiram-like reaction (characterized by flushing, dizziness, throbbing headache, chest and abdominal discomfort, and a distinct hangover effect in addition to nausea and vomiting). However, oral clindamycin can cause antibiotic-associated colitis and is more expensive than metronidazole.
Currently, there are no single-dose regimens for the treatment of BV readily available in the United States. Secnidazole, a 5-nitroimidazole with a longer half-life than metronidazole, (17 vs 8 hours) has been used as therapy in Europe and Asia but is not yet available commercially in the United States.21 Hiller and colleagues found that 1 g and 2 g secnidazole oral granules were superior to placebo in treating BV.22 A larger randomized trial comparing this regimen to standard treatment is necessary before this therapy is adopted as the standard of care.
Continue to: Managing recurrent disease...
Managing recurrent disease, a common problem. Bradshaw and colleagues noted that, although the initial treatment of BV is effective in approximately 80% of women, up to 50% have a recurrence within 12 months.23 Data are limited regarding optimal treatment for recurrent infections; however, most regimens consist of some form of suppressive therapy. One regimen includes one full applicator of metronidazole vaginal gel 0.75% twice weekly for 6 months.24 A second regimen consists of vaginal boric acid capsules 600 mg once daily at bedtime for 21 days. Upon completion of boric acid therapy, metronidazole vaginal gel 0.75% should be administered twice weekly for 6 months.25 A third option is oral metronidazole 2 g and fluconazole 250 mg once every month.26 Of note, boric acid can be fatal if consumed orally and is not recommended during pregnancy.
Most recently, a randomized trial evaluated the ability of L crispatus to prevent BV recurrence. After completion of standard treatment therapy with metronidazole, women were randomly assigned to receive vaginally administered L crispatus (152 patients) or placebo (76 patients) for 11 weeks. In the intention-to-treat population, recurrent BV occurred in 30% of patients in the L crispatus group and 45% of patients in the placebo group. The use of L crispatus significantly reduced recurrence of BV by one-third (P = .01; 95% confidence interval [CI], 0.44–0.87).27 These findings are encouraging; however, confirmatory studies are needed before adopting this as standard of care.
Should sexual partners be treated as well? BV has not traditionally been considered an STI, and the CDC does not currently recommend treatment of partners of women who have BV. However, in women who have sex with women, the rate of BV concordance is high, and in women who have sex with men, coitus can clearly influence disease activity. Therefore, in patients with refractory BV, we recommend treatment of the sexual partner(s) with metronidazole 500 mg orally twice daily for 7 days. For women having sex with men, we also recommend consistent use of condoms, at least until the patient’s infection is better controlled.28
CASE Resolved
The patient’s clinical findings are indicative of BV. This condition is associated with an increased risk of preterm delivery and intrapartum and postpartum infection. To reduce the risk of these systemic complications, she was treated with oral metronidazole 500 mg twice daily for 7 days. Within 1 week of completing treatment, she noted complete resolution of the malodorous discharge. ●
- Smith SB, Ravel J. The vaginal microbiota, host defence and reproductive physiology. J Physiol. 2017;595:451-463.
- Mitchell C, Fredricks D, Agnew K, et al. Hydrogen peroxide-producing lactobacilli are associated with lower levels of vaginal interleukin-1β, independent of bacterial vaginosis. Sex Transm Infect. 2015;42:358-363.
- Munzy CA, Blanchard E, Taylor CM, et al. Identification of key bacteria involved in the induction of incident bacterial vaginosis: a prospective study. J Infect. 2018;218:966-978.
- Paavonen J, Brunham RC. Bacterial vaginosis and desquamative inflammatory vaginitis. N Engl J Med. 2018; 379:2246-2254.
- Hardy L, Jespers V, Dahchour N, et al. Unravelling the bacterial vaginosis-associated biofilm: a multiplex Gardnerella vaginalis and Atopobium vaginae fluorescence in situ hybridization assay using peptide nucleic acid probes. PloS One. 2015;10:E0136658.
- Allswoth JE, Peipert JF. Prevalence of bacterial vaginosis: 2001-2004 national health and nutrition examination survey data. Obstet Gynecol. 2007;109:114-120.
- Kenyon C, Colebunders R, Crucitti T. The global epidemiology of bacterial vaginosis: a systematic review. Am J Obstet Gynecol. 2013;209:505-523.
- Brookheart RT, Lewis WG, Peipert JF, et al. Association between obesity and bacterial vaginosis as assessed by Nugent score. Am J Obstet Gynecol. 2019;220:476.e1-476.e11.
- Onderdonk AB, Delaney ML, Fichorova RN. The human microbiome during bacterial vaginosis. Clin Microbiol Rev. 2016;29:223-238.
- Brown RG, Marchesi JR, Lee YS, et al. Vaginal dysbiosis increases risk of preterm fetal membrane rupture, neonatal sepsis and is exacerbated by erythromycin. BMC Med. 2018;16:9.
- Watts DH, Eschenbach DA, Kenny GE. Early postpartum endometritis: the role of bacteria, genital mycoplasmas, and chlamydia trachomatis. Obstet Gynecol. 1989;73:52-60.
- Balkus JE, Richardson BA, Rabe LK, et al. Bacterial vaginosis and the risk of Trichomonas vaginalis acquisition among HIV1-negative women. Sex Transm Dis. 2014;41:123-128.
- Cherpes TL, Meyn LA, Krohn MA, et al. Association between acquisition of herpes simplex virus type 2 in women and bacterial vaginosis. Clin Infect Dis. 2003;37:319-325.
- Wiesenfeld HC, Hillier SL, Krohn MA, et al. Bacterial vaginosis is a strong predictor of Neisseria gonorrhoeae and Chlamydia trachomatis infection. Clin Infect Dis. 2003;36:663-668.
- Myer L, Denny L, Telerant R, et al. Bacterial vaginosis and susceptibility to HIV infection in South African women: a nested case-control study. J Infect. 2005;192:1372-1380.
- Soper DE, Bump RC, Hurt WG. Bacterial vaginosis and trichomoniasis vaginitis are risk factors for cuff cellulitis after abdominal hysterectomy. Am J Obstet Gynecol. 1990;163:1061-1121.
- Amsel R, Totten PA, Spiegel CA, et al. Nonspecific vaginitis. diagnostic criteria and microbial and epidemiologic associations. Am J Med. 1983;74:14-22.
- Nugent RP, Krohn MA, Hillier SL. Reliability of diagnosing bacterial vaginosis is improved by a standardized method of gram stain interpretation. J Clin Microbiol. 1991;29:297-301.
- Bacterial vaginosis. Centers for Disease Control and Prevention website. Updated June 4, 2015. Accessed December 9, 2020. https://www.cdc.gov/std/tg2015/bv.htm.
- Oduyebo OO, Anorlu RI, Ogunsola FT. The effects of antimicrobial therapy on bacterial vaginosis in non-pregnant women. Cochrane Database Syst Rev. 2009:CD006055.
- Videau D, Niel G, Siboulet A, et al. Secnidazole. a 5-nitroimidazole derivative with a long half-life. Br J Vener Dis. 1978;54:77-80.
- Hillier SL, Nyirjesy P, Waldbaum AS, et al. Secnidazole treatment of bacterial vaginosis: a randomized controlled trial. Obstet Gynecol. 2017;130:379-386.
- Bradshaw CS, Morton AN, Hocking J, et al. High recurrence rates of bacterial vaginosis over the course of 12 months after oral metronidazole therapy and factors associated with recurrence. J Infect. 2006;193:1478-1486.
- Sobel JD, Ferris D, Schwebke J, et al. Suppressive antibacterial therapy with 0.75% metronidazole vaginal gel to prevent recurrent bacterial vaginosis. Am J Obstet Gynecol. 2006;194:1283-1289.
- Reichman O, Akins R, Sobel JD. Boric acid addition to suppressive antimicrobial therapy for recurrent bacterial vaginosis. Sex Transm Dis. 2009;36:732-734.
- McClelland RS, Richardson BA, Hassan WM, et al. Improvement of vaginal health for Kenyan women at risk for acquisition of human immunodeficiency virus type 1: results of a randomized trial. J Infect. 2008;197:1361-1368.
- Cohen CR, Wierzbicki MR, French AL, et al. Randomized trial of lactin-v to prevent recurrence of bacterial vaginosis. N Engl J Med. 2020;382:906-915.
- Barbieri RL. Effective treatment of recurrent bacterial vaginosis. OBG Manag. 2017;29:7-12.
CASE Pregnant woman with abnormal vaginal discharge
A 26-year-old woman (G2P1001) at 24 weeks of gestation requests evaluation for increased frothy, whitish-gray vaginal discharge with a fishy odor. She notes that her underclothes constantly feel damp. The vaginal pH is 4.5, and the amine test is positive.
- What is the most likely diagnosis?
- What obstetrical complications may be associated with this condition?
- How should her condition be treated?
Meet our perpetrator
Bacterial vaginosis (BV) is one of the most common conditions associated with vaginal discharge among women of reproductive age. It is characterized by a polymicrobial alteration of the vaginal microbiome, and most distinctly, a relative absence of vaginal lactobacilli. This review discusses the microbiology, epidemiology, specific obstetric and gynecologic complications, clinical manifestations, diagnosis, and treatment of BV.
The role of vaginal flora
Estrogen has a fundamental role in regulating the normal state of the vagina. In a woman’s reproductive years, estrogen increases glycogen in the vaginal epithelial cells, and the increased glycogen concentration promotes colonization by lactobacilli. The lack of estrogen in pre- and postmenopausal women inhibits the growth of the vaginal lactobacilli, leading to a high vaginal pH, which facilitates the growth of bacteria, particularly anaerobes, that can cause BV.
The vaginal microbiome is polymicrobial and has been classified into at least 5 community state types (CSTs). Four CSTs are dominated by lactobacilli. A fifth CST is characterized by the absence of lactobacilli and high concentrations of obligate or facultative anaerobes.1 The hydrogen peroxide–producing lactobacilli predominate in normal vaginal flora and make up 70% to 90% of the total microbiome. These hydrogen peroxide–producing lactobacilli are associated with reduced vaginal proinflammatory cytokines and a highly acidic vaginal pH. Both factors defend against sexually transmitted infections (STIs).2
BV is a polymicrobial disorder marked by the significant reduction in the number of vaginal lactobacilli (FIGURE 1). A recent study showed that BV is associated first with a decrease in Lactobacillus crispatus, followed by increase in Prevotella bivia, Gardnerella vaginalis, Atopobium vaginae, and Megasphaera type 1.3 The polymicrobial load is increased by a factor of up to 1,000, compared with normal vaginal flora.4 BV should be considered a biofilm infection caused by adherence of G vaginalis to the vaginal epithelium.5 This biofilm creates a favorable environment for the overgrowth of obligate anaerobic bacteria.

BMI factors into epidemiology
BV is the leading cause of vaginal discharge in reproductive-age women. In the United States, the National Health and Nutrition Examination Survey estimated a prevalence of 29% in the general population and 50% in Black women aged 14 to 49 years.6 In 2013, Kenyon and colleagues performed a systematic review to assess the worldwide epidemiology of BV, and the prevalence varied by country. Within the US population, rates were highest among non-Hispanic, Black women.7 Brookheart and colleagues demonstrated that, even after controlling for race, overweight and obese women had a higher frequency of BV compared with leaner women. In this investigation, the overall prevalence of BV was 28.1%. When categorized by body mass index (BMI), the prevalence was 21.3% in lean women, 30.4% in overweight women, and 34.5% in obese women (P<.001). The authors also found that Black women had a higher prevalence, independent of BMI, compared with White women.8
Complications may occur. BV is notable for having several serious sequelae in both pregnant and nonpregnant women. For obstetric patients, these sequelae include an increased risk of preterm birth; first trimester spontaneous abortion, particularly in the setting of in vitro fertilization; intra-amniotic infection; and endometritis.9,10 The risk of preterm birth increases by a factor of 2 in infected women; however, most women with BV do not deliver preterm.4 The risk of endometritis is increased 6-fold in women with BV.11 Nonpregnant women with BV are at increased risk for pelvic inflammatory disease, postoperative infections, and an increased susceptibility to STIs such as chlamydia, gonorrhea, herpes simplex virus, and HIV.12-15 The risk for vaginal-cuff cellulitis and abscess after hysterectomy is increased 6-fold in the setting of BV.16
Continue to: Clinical manifestations...
Clinical manifestations
BV is characterized by a milky, homogenous, and malodorous vaginal discharge accompanied by vulvovaginal discomfort and vulvar irritation. Vaginal inflammation typically is absent. The associated odor is fishy, and this odor is accentuated when potassium hydroxide (KOH) is added to the vaginal discharge (amine or “whiff” test) or after the patient has coitus. The distinctive odor is due to the release of organic acids and polyamines that are byproducts of anaerobic bacterial metabolism of putrescine and cadaverine. This release is enhanced by exposure of vaginal secretions to alkaline substances such as KOH or semen.
Diagnostic tests and criteria. The diagnosis of BV is made using Amsel criteria or Gram stain with Nugent scoring; bacterial culture is not recommended. Amsel criteria include:
- homogenous, thin, white-gray discharge
- >20% clue cells on saline microscopy (FIGURE 2)
- a pH >4.5 of vaginal fluid
- positive KOH whiff test.

For diagnosis, 3 of the 4 Amsel criteria must be present.17 Gram stain with Nugent score typically is used for research purposes. Nugent scoring assigns a value to different bacterial morphotypes on Gram stain of vaginal secretions. A score of 7 to 10 is consistent with BV.18
Oral and topical treatments
Treatment is recommended for symptomatic patients. Treatment may reduce the risk of transmission and acquisition of other STIs. The TABLE summarizes Centers for Disease Control and Prevention (CDC) guidelines for BV treatment,19 with options including both oral and topical regimens. Oral and topical metronidazole and oral and topical clindamycin are equally effective at eradicating the local source of infection20; however, only oral metronidazole and oral clindamycin are effective in preventing the systemic complications of BV. Oral metronidazole has more adverse effects than oral clindamycin—including nausea, vomiting, diarrhea, and a disulfiram-like reaction (characterized by flushing, dizziness, throbbing headache, chest and abdominal discomfort, and a distinct hangover effect in addition to nausea and vomiting). However, oral clindamycin can cause antibiotic-associated colitis and is more expensive than metronidazole.

Currently, there are no single-dose regimens for the treatment of BV readily available in the United States. Secnidazole, a 5-nitroimidazole with a longer half-life than metronidazole, (17 vs 8 hours) has been used as therapy in Europe and Asia but is not yet available commercially in the United States.21 Hiller and colleagues found that 1 g and 2 g secnidazole oral granules were superior to placebo in treating BV.22 A larger randomized trial comparing this regimen to standard treatment is necessary before this therapy is adopted as the standard of care.
Continue to: Managing recurrent disease...
Managing recurrent disease, a common problem. Bradshaw and colleagues noted that, although the initial treatment of BV is effective in approximately 80% of women, up to 50% have a recurrence within 12 months.23 Data are limited regarding optimal treatment for recurrent infections; however, most regimens consist of some form of suppressive therapy. One regimen includes one full applicator of metronidazole vaginal gel 0.75% twice weekly for 6 months.24 A second regimen consists of vaginal boric acid capsules 600 mg once daily at bedtime for 21 days. Upon completion of boric acid therapy, metronidazole vaginal gel 0.75% should be administered twice weekly for 6 months.25 A third option is oral metronidazole 2 g and fluconazole 250 mg once every month.26 Of note, boric acid can be fatal if consumed orally and is not recommended during pregnancy.
Most recently, a randomized trial evaluated the ability of L crispatus to prevent BV recurrence. After completion of standard treatment therapy with metronidazole, women were randomly assigned to receive vaginally administered L crispatus (152 patients) or placebo (76 patients) for 11 weeks. In the intention-to-treat population, recurrent BV occurred in 30% of patients in the L crispatus group and 45% of patients in the placebo group. The use of L crispatus significantly reduced recurrence of BV by one-third (P = .01; 95% confidence interval [CI], 0.44–0.87).27 These findings are encouraging; however, confirmatory studies are needed before adopting this as standard of care.
Should sexual partners be treated as well? BV has not traditionally been considered an STI, and the CDC does not currently recommend treatment of partners of women who have BV. However, in women who have sex with women, the rate of BV concordance is high, and in women who have sex with men, coitus can clearly influence disease activity. Therefore, in patients with refractory BV, we recommend treatment of the sexual partner(s) with metronidazole 500 mg orally twice daily for 7 days. For women having sex with men, we also recommend consistent use of condoms, at least until the patient’s infection is better controlled.28
CASE Resolved
The patient’s clinical findings are indicative of BV. This condition is associated with an increased risk of preterm delivery and intrapartum and postpartum infection. To reduce the risk of these systemic complications, she was treated with oral metronidazole 500 mg twice daily for 7 days. Within 1 week of completing treatment, she noted complete resolution of the malodorous discharge. ●
CASE Pregnant woman with abnormal vaginal discharge
A 26-year-old woman (G2P1001) at 24 weeks of gestation requests evaluation for increased frothy, whitish-gray vaginal discharge with a fishy odor. She notes that her underclothes constantly feel damp. The vaginal pH is 4.5, and the amine test is positive.
- What is the most likely diagnosis?
- What obstetrical complications may be associated with this condition?
- How should her condition be treated?
Meet our perpetrator
Bacterial vaginosis (BV) is one of the most common conditions associated with vaginal discharge among women of reproductive age. It is characterized by a polymicrobial alteration of the vaginal microbiome, and most distinctly, a relative absence of vaginal lactobacilli. This review discusses the microbiology, epidemiology, specific obstetric and gynecologic complications, clinical manifestations, diagnosis, and treatment of BV.
The role of vaginal flora
Estrogen has a fundamental role in regulating the normal state of the vagina. In a woman’s reproductive years, estrogen increases glycogen in the vaginal epithelial cells, and the increased glycogen concentration promotes colonization by lactobacilli. The lack of estrogen in pre- and postmenopausal women inhibits the growth of the vaginal lactobacilli, leading to a high vaginal pH, which facilitates the growth of bacteria, particularly anaerobes, that can cause BV.
The vaginal microbiome is polymicrobial and has been classified into at least 5 community state types (CSTs). Four CSTs are dominated by lactobacilli. A fifth CST is characterized by the absence of lactobacilli and high concentrations of obligate or facultative anaerobes.1 The hydrogen peroxide–producing lactobacilli predominate in normal vaginal flora and make up 70% to 90% of the total microbiome. These hydrogen peroxide–producing lactobacilli are associated with reduced vaginal proinflammatory cytokines and a highly acidic vaginal pH. Both factors defend against sexually transmitted infections (STIs).2
BV is a polymicrobial disorder marked by the significant reduction in the number of vaginal lactobacilli (FIGURE 1). A recent study showed that BV is associated first with a decrease in Lactobacillus crispatus, followed by increase in Prevotella bivia, Gardnerella vaginalis, Atopobium vaginae, and Megasphaera type 1.3 The polymicrobial load is increased by a factor of up to 1,000, compared with normal vaginal flora.4 BV should be considered a biofilm infection caused by adherence of G vaginalis to the vaginal epithelium.5 This biofilm creates a favorable environment for the overgrowth of obligate anaerobic bacteria.

BMI factors into epidemiology
BV is the leading cause of vaginal discharge in reproductive-age women. In the United States, the National Health and Nutrition Examination Survey estimated a prevalence of 29% in the general population and 50% in Black women aged 14 to 49 years.6 In 2013, Kenyon and colleagues performed a systematic review to assess the worldwide epidemiology of BV, and the prevalence varied by country. Within the US population, rates were highest among non-Hispanic, Black women.7 Brookheart and colleagues demonstrated that, even after controlling for race, overweight and obese women had a higher frequency of BV compared with leaner women. In this investigation, the overall prevalence of BV was 28.1%. When categorized by body mass index (BMI), the prevalence was 21.3% in lean women, 30.4% in overweight women, and 34.5% in obese women (P<.001). The authors also found that Black women had a higher prevalence, independent of BMI, compared with White women.8
Complications may occur. BV is notable for having several serious sequelae in both pregnant and nonpregnant women. For obstetric patients, these sequelae include an increased risk of preterm birth; first trimester spontaneous abortion, particularly in the setting of in vitro fertilization; intra-amniotic infection; and endometritis.9,10 The risk of preterm birth increases by a factor of 2 in infected women; however, most women with BV do not deliver preterm.4 The risk of endometritis is increased 6-fold in women with BV.11 Nonpregnant women with BV are at increased risk for pelvic inflammatory disease, postoperative infections, and an increased susceptibility to STIs such as chlamydia, gonorrhea, herpes simplex virus, and HIV.12-15 The risk for vaginal-cuff cellulitis and abscess after hysterectomy is increased 6-fold in the setting of BV.16
Continue to: Clinical manifestations...
Clinical manifestations
BV is characterized by a milky, homogenous, and malodorous vaginal discharge accompanied by vulvovaginal discomfort and vulvar irritation. Vaginal inflammation typically is absent. The associated odor is fishy, and this odor is accentuated when potassium hydroxide (KOH) is added to the vaginal discharge (amine or “whiff” test) or after the patient has coitus. The distinctive odor is due to the release of organic acids and polyamines that are byproducts of anaerobic bacterial metabolism of putrescine and cadaverine. This release is enhanced by exposure of vaginal secretions to alkaline substances such as KOH or semen.
Diagnostic tests and criteria. The diagnosis of BV is made using Amsel criteria or Gram stain with Nugent scoring; bacterial culture is not recommended. Amsel criteria include:
- homogenous, thin, white-gray discharge
- >20% clue cells on saline microscopy (FIGURE 2)
- a pH >4.5 of vaginal fluid
- positive KOH whiff test.

For diagnosis, 3 of the 4 Amsel criteria must be present.17 Gram stain with Nugent score typically is used for research purposes. Nugent scoring assigns a value to different bacterial morphotypes on Gram stain of vaginal secretions. A score of 7 to 10 is consistent with BV.18
Oral and topical treatments
Treatment is recommended for symptomatic patients. Treatment may reduce the risk of transmission and acquisition of other STIs. The TABLE summarizes Centers for Disease Control and Prevention (CDC) guidelines for BV treatment,19 with options including both oral and topical regimens. Oral and topical metronidazole and oral and topical clindamycin are equally effective at eradicating the local source of infection20; however, only oral metronidazole and oral clindamycin are effective in preventing the systemic complications of BV. Oral metronidazole has more adverse effects than oral clindamycin—including nausea, vomiting, diarrhea, and a disulfiram-like reaction (characterized by flushing, dizziness, throbbing headache, chest and abdominal discomfort, and a distinct hangover effect in addition to nausea and vomiting). However, oral clindamycin can cause antibiotic-associated colitis and is more expensive than metronidazole.

Currently, there are no single-dose regimens for the treatment of BV readily available in the United States. Secnidazole, a 5-nitroimidazole with a longer half-life than metronidazole, (17 vs 8 hours) has been used as therapy in Europe and Asia but is not yet available commercially in the United States.21 Hiller and colleagues found that 1 g and 2 g secnidazole oral granules were superior to placebo in treating BV.22 A larger randomized trial comparing this regimen to standard treatment is necessary before this therapy is adopted as the standard of care.
Continue to: Managing recurrent disease...
Managing recurrent disease, a common problem. Bradshaw and colleagues noted that, although the initial treatment of BV is effective in approximately 80% of women, up to 50% have a recurrence within 12 months.23 Data are limited regarding optimal treatment for recurrent infections; however, most regimens consist of some form of suppressive therapy. One regimen includes one full applicator of metronidazole vaginal gel 0.75% twice weekly for 6 months.24 A second regimen consists of vaginal boric acid capsules 600 mg once daily at bedtime for 21 days. Upon completion of boric acid therapy, metronidazole vaginal gel 0.75% should be administered twice weekly for 6 months.25 A third option is oral metronidazole 2 g and fluconazole 250 mg once every month.26 Of note, boric acid can be fatal if consumed orally and is not recommended during pregnancy.
Most recently, a randomized trial evaluated the ability of L crispatus to prevent BV recurrence. After completion of standard treatment therapy with metronidazole, women were randomly assigned to receive vaginally administered L crispatus (152 patients) or placebo (76 patients) for 11 weeks. In the intention-to-treat population, recurrent BV occurred in 30% of patients in the L crispatus group and 45% of patients in the placebo group. The use of L crispatus significantly reduced recurrence of BV by one-third (P = .01; 95% confidence interval [CI], 0.44–0.87).27 These findings are encouraging; however, confirmatory studies are needed before adopting this as standard of care.
Should sexual partners be treated as well? BV has not traditionally been considered an STI, and the CDC does not currently recommend treatment of partners of women who have BV. However, in women who have sex with women, the rate of BV concordance is high, and in women who have sex with men, coitus can clearly influence disease activity. Therefore, in patients with refractory BV, we recommend treatment of the sexual partner(s) with metronidazole 500 mg orally twice daily for 7 days. For women having sex with men, we also recommend consistent use of condoms, at least until the patient’s infection is better controlled.28
CASE Resolved
The patient’s clinical findings are indicative of BV. This condition is associated with an increased risk of preterm delivery and intrapartum and postpartum infection. To reduce the risk of these systemic complications, she was treated with oral metronidazole 500 mg twice daily for 7 days. Within 1 week of completing treatment, she noted complete resolution of the malodorous discharge. ●
- Smith SB, Ravel J. The vaginal microbiota, host defence and reproductive physiology. J Physiol. 2017;595:451-463.
- Mitchell C, Fredricks D, Agnew K, et al. Hydrogen peroxide-producing lactobacilli are associated with lower levels of vaginal interleukin-1β, independent of bacterial vaginosis. Sex Transm Infect. 2015;42:358-363.
- Munzy CA, Blanchard E, Taylor CM, et al. Identification of key bacteria involved in the induction of incident bacterial vaginosis: a prospective study. J Infect. 2018;218:966-978.
- Paavonen J, Brunham RC. Bacterial vaginosis and desquamative inflammatory vaginitis. N Engl J Med. 2018; 379:2246-2254.
- Hardy L, Jespers V, Dahchour N, et al. Unravelling the bacterial vaginosis-associated biofilm: a multiplex Gardnerella vaginalis and Atopobium vaginae fluorescence in situ hybridization assay using peptide nucleic acid probes. PloS One. 2015;10:E0136658.
- Allswoth JE, Peipert JF. Prevalence of bacterial vaginosis: 2001-2004 national health and nutrition examination survey data. Obstet Gynecol. 2007;109:114-120.
- Kenyon C, Colebunders R, Crucitti T. The global epidemiology of bacterial vaginosis: a systematic review. Am J Obstet Gynecol. 2013;209:505-523.
- Brookheart RT, Lewis WG, Peipert JF, et al. Association between obesity and bacterial vaginosis as assessed by Nugent score. Am J Obstet Gynecol. 2019;220:476.e1-476.e11.
- Onderdonk AB, Delaney ML, Fichorova RN. The human microbiome during bacterial vaginosis. Clin Microbiol Rev. 2016;29:223-238.
- Brown RG, Marchesi JR, Lee YS, et al. Vaginal dysbiosis increases risk of preterm fetal membrane rupture, neonatal sepsis and is exacerbated by erythromycin. BMC Med. 2018;16:9.
- Watts DH, Eschenbach DA, Kenny GE. Early postpartum endometritis: the role of bacteria, genital mycoplasmas, and chlamydia trachomatis. Obstet Gynecol. 1989;73:52-60.
- Balkus JE, Richardson BA, Rabe LK, et al. Bacterial vaginosis and the risk of Trichomonas vaginalis acquisition among HIV1-negative women. Sex Transm Dis. 2014;41:123-128.
- Cherpes TL, Meyn LA, Krohn MA, et al. Association between acquisition of herpes simplex virus type 2 in women and bacterial vaginosis. Clin Infect Dis. 2003;37:319-325.
- Wiesenfeld HC, Hillier SL, Krohn MA, et al. Bacterial vaginosis is a strong predictor of Neisseria gonorrhoeae and Chlamydia trachomatis infection. Clin Infect Dis. 2003;36:663-668.
- Myer L, Denny L, Telerant R, et al. Bacterial vaginosis and susceptibility to HIV infection in South African women: a nested case-control study. J Infect. 2005;192:1372-1380.
- Soper DE, Bump RC, Hurt WG. Bacterial vaginosis and trichomoniasis vaginitis are risk factors for cuff cellulitis after abdominal hysterectomy. Am J Obstet Gynecol. 1990;163:1061-1121.
- Amsel R, Totten PA, Spiegel CA, et al. Nonspecific vaginitis. diagnostic criteria and microbial and epidemiologic associations. Am J Med. 1983;74:14-22.
- Nugent RP, Krohn MA, Hillier SL. Reliability of diagnosing bacterial vaginosis is improved by a standardized method of gram stain interpretation. J Clin Microbiol. 1991;29:297-301.
- Bacterial vaginosis. Centers for Disease Control and Prevention website. Updated June 4, 2015. Accessed December 9, 2020. https://www.cdc.gov/std/tg2015/bv.htm.
- Oduyebo OO, Anorlu RI, Ogunsola FT. The effects of antimicrobial therapy on bacterial vaginosis in non-pregnant women. Cochrane Database Syst Rev. 2009:CD006055.
- Videau D, Niel G, Siboulet A, et al. Secnidazole. a 5-nitroimidazole derivative with a long half-life. Br J Vener Dis. 1978;54:77-80.
- Hillier SL, Nyirjesy P, Waldbaum AS, et al. Secnidazole treatment of bacterial vaginosis: a randomized controlled trial. Obstet Gynecol. 2017;130:379-386.
- Bradshaw CS, Morton AN, Hocking J, et al. High recurrence rates of bacterial vaginosis over the course of 12 months after oral metronidazole therapy and factors associated with recurrence. J Infect. 2006;193:1478-1486.
- Sobel JD, Ferris D, Schwebke J, et al. Suppressive antibacterial therapy with 0.75% metronidazole vaginal gel to prevent recurrent bacterial vaginosis. Am J Obstet Gynecol. 2006;194:1283-1289.
- Reichman O, Akins R, Sobel JD. Boric acid addition to suppressive antimicrobial therapy for recurrent bacterial vaginosis. Sex Transm Dis. 2009;36:732-734.
- McClelland RS, Richardson BA, Hassan WM, et al. Improvement of vaginal health for Kenyan women at risk for acquisition of human immunodeficiency virus type 1: results of a randomized trial. J Infect. 2008;197:1361-1368.
- Cohen CR, Wierzbicki MR, French AL, et al. Randomized trial of lactin-v to prevent recurrence of bacterial vaginosis. N Engl J Med. 2020;382:906-915.
- Barbieri RL. Effective treatment of recurrent bacterial vaginosis. OBG Manag. 2017;29:7-12.
- Smith SB, Ravel J. The vaginal microbiota, host defence and reproductive physiology. J Physiol. 2017;595:451-463.
- Mitchell C, Fredricks D, Agnew K, et al. Hydrogen peroxide-producing lactobacilli are associated with lower levels of vaginal interleukin-1β, independent of bacterial vaginosis. Sex Transm Infect. 2015;42:358-363.
- Munzy CA, Blanchard E, Taylor CM, et al. Identification of key bacteria involved in the induction of incident bacterial vaginosis: a prospective study. J Infect. 2018;218:966-978.
- Paavonen J, Brunham RC. Bacterial vaginosis and desquamative inflammatory vaginitis. N Engl J Med. 2018; 379:2246-2254.
- Hardy L, Jespers V, Dahchour N, et al. Unravelling the bacterial vaginosis-associated biofilm: a multiplex Gardnerella vaginalis and Atopobium vaginae fluorescence in situ hybridization assay using peptide nucleic acid probes. PloS One. 2015;10:E0136658.
- Allswoth JE, Peipert JF. Prevalence of bacterial vaginosis: 2001-2004 national health and nutrition examination survey data. Obstet Gynecol. 2007;109:114-120.
- Kenyon C, Colebunders R, Crucitti T. The global epidemiology of bacterial vaginosis: a systematic review. Am J Obstet Gynecol. 2013;209:505-523.
- Brookheart RT, Lewis WG, Peipert JF, et al. Association between obesity and bacterial vaginosis as assessed by Nugent score. Am J Obstet Gynecol. 2019;220:476.e1-476.e11.
- Onderdonk AB, Delaney ML, Fichorova RN. The human microbiome during bacterial vaginosis. Clin Microbiol Rev. 2016;29:223-238.
- Brown RG, Marchesi JR, Lee YS, et al. Vaginal dysbiosis increases risk of preterm fetal membrane rupture, neonatal sepsis and is exacerbated by erythromycin. BMC Med. 2018;16:9.
- Watts DH, Eschenbach DA, Kenny GE. Early postpartum endometritis: the role of bacteria, genital mycoplasmas, and chlamydia trachomatis. Obstet Gynecol. 1989;73:52-60.
- Balkus JE, Richardson BA, Rabe LK, et al. Bacterial vaginosis and the risk of Trichomonas vaginalis acquisition among HIV1-negative women. Sex Transm Dis. 2014;41:123-128.
- Cherpes TL, Meyn LA, Krohn MA, et al. Association between acquisition of herpes simplex virus type 2 in women and bacterial vaginosis. Clin Infect Dis. 2003;37:319-325.
- Wiesenfeld HC, Hillier SL, Krohn MA, et al. Bacterial vaginosis is a strong predictor of Neisseria gonorrhoeae and Chlamydia trachomatis infection. Clin Infect Dis. 2003;36:663-668.
- Myer L, Denny L, Telerant R, et al. Bacterial vaginosis and susceptibility to HIV infection in South African women: a nested case-control study. J Infect. 2005;192:1372-1380.
- Soper DE, Bump RC, Hurt WG. Bacterial vaginosis and trichomoniasis vaginitis are risk factors for cuff cellulitis after abdominal hysterectomy. Am J Obstet Gynecol. 1990;163:1061-1121.
- Amsel R, Totten PA, Spiegel CA, et al. Nonspecific vaginitis. diagnostic criteria and microbial and epidemiologic associations. Am J Med. 1983;74:14-22.
- Nugent RP, Krohn MA, Hillier SL. Reliability of diagnosing bacterial vaginosis is improved by a standardized method of gram stain interpretation. J Clin Microbiol. 1991;29:297-301.
- Bacterial vaginosis. Centers for Disease Control and Prevention website. Updated June 4, 2015. Accessed December 9, 2020. https://www.cdc.gov/std/tg2015/bv.htm.
- Oduyebo OO, Anorlu RI, Ogunsola FT. The effects of antimicrobial therapy on bacterial vaginosis in non-pregnant women. Cochrane Database Syst Rev. 2009:CD006055.
- Videau D, Niel G, Siboulet A, et al. Secnidazole. a 5-nitroimidazole derivative with a long half-life. Br J Vener Dis. 1978;54:77-80.
- Hillier SL, Nyirjesy P, Waldbaum AS, et al. Secnidazole treatment of bacterial vaginosis: a randomized controlled trial. Obstet Gynecol. 2017;130:379-386.
- Bradshaw CS, Morton AN, Hocking J, et al. High recurrence rates of bacterial vaginosis over the course of 12 months after oral metronidazole therapy and factors associated with recurrence. J Infect. 2006;193:1478-1486.
- Sobel JD, Ferris D, Schwebke J, et al. Suppressive antibacterial therapy with 0.75% metronidazole vaginal gel to prevent recurrent bacterial vaginosis. Am J Obstet Gynecol. 2006;194:1283-1289.
- Reichman O, Akins R, Sobel JD. Boric acid addition to suppressive antimicrobial therapy for recurrent bacterial vaginosis. Sex Transm Dis. 2009;36:732-734.
- McClelland RS, Richardson BA, Hassan WM, et al. Improvement of vaginal health for Kenyan women at risk for acquisition of human immunodeficiency virus type 1: results of a randomized trial. J Infect. 2008;197:1361-1368.
- Cohen CR, Wierzbicki MR, French AL, et al. Randomized trial of lactin-v to prevent recurrence of bacterial vaginosis. N Engl J Med. 2020;382:906-915.
- Barbieri RL. Effective treatment of recurrent bacterial vaginosis. OBG Manag. 2017;29:7-12.
2021 Update on fertility
In this Update, we discuss several aspects of infertility and emerging technologic advances in treatment. We review an important infertility fact sheet recently issued by the World Health Organization (WHO) that provides a succinct overview of infertility causes, the rights of infertility patients, treatment challenges, and advocacy efforts. In addition, we discuss what the infertility literature reveals about reducing multiple birth rates and the technologic, financial, and social factors involved. Finally, we look at the molecular progress made in germline-editing technology and the myriad complications involved in its potential future translation to clinical phenotyping.
WHO recognizes the burden of infertility and addresses fertility care needs
World Health Organization (WHO). Infertility fact sheet. September 14, 2020. https://www.who.int/news-room/fact-sheets/detail/infertility. Accessed January 24, 2021.
The WHO published its first comprehensive infertility fact sheet in September 2020. This document is important because it validates infertility as a high-burden disease and disability that diminishes quality of life for up to 186 million individuals globally. The infertility fact sheet is a comprehensive yet focused quick read that addresses the causes of infertility, why infertility is important, challenges, and the WHO response.
Factors in infertility
Infertility is caused by different factors in women and men, yet sometimes it is unexplained, and its relative importance can vary from country to country. For women, tubal disorders (for example, postinfectious), uterine problems (fibroids, congenital), endometriosis, ovarian disorders (polycystic ovary syndrome, ovulation disorders), and endocrine imbalances are the most common factors.
For men, causes of infertility include obstruction of the reproductive tract (as after injuries or infection); hormonal disorders in the hypothalamus, pituitary, and/or testicles (for example, low testosterone); testicular failure to produce sperm (such as after cancer treatment); and abnormal sperm function and quality (low count, motility, or morphology).
Environmental and lifestyle factors— including smoking, obesity, alcohol, or toxins—can affect fertility.
Continue to: Recognizing all individuals’ fertility rights...
Recognizing all individuals’ fertility rights
The WHO infertility fact sheet makes strong statements, recognizing that individuals and couples have the right to decide the number, timing, and spacing of their children. Addressing infertility is therefore an important part of realizing the right of individuals and couples to found a family. This includes heterosexual couples, same-sex partners, older persons, individuals not in sexual relationships who might require infertility management and fertility care services, and notably marginalized populations.
Addressing infertility also can help mitigate gender inequality, which has significant negative social impacts on the lives of infertile individuals, especially women. Fertility education is important to reduce the fear of infertility and contraception use in those wanting pregnancy in the future.
In most countries the biggest challenges are availability, access, and quality of interventions to address infertility. This includes the United States, where only 1 in 4 individuals receive the fertility care they need. Lack of prioritization, ineffective public health strategies, inadequate funding, and costs are barriers. Health policies need to recognize that infertility is a disease that often can be prevented, thereby reducing future costs. Comprehensive awareness and education programs, laws and policies that regulate and ensure access and the human rights of all involved, are essential.
Advocacy efforts
To address infertility and fertility care, the WHO is committed to:
- collaborate with partners on epidemiologic and etiologic research
- facilitate policy dialogue globally to frame infertility within a legal and policy framework
- support generation of data on the burden of infertility
- develop guidelines
- produce other documents of standards
- collaborate with all stakeholders to strengthen political commitment and health system capacity, and
- provide country-level technical support to develop or strengthen policies and services.
For your practice, this means that infertility is recognized as a disease that should receive its appropriate share of health care resources. Infertility and fertility care are the right of every individual according to their desires to found a family. Besides providing the best care you can to all your patients, including referring them when necessary, all health care clinicians should advocate on behalf of their patients to payors, policy makers, and the public the need to provide equitable laws, resources, and funding for infertility and fertility care.
Every person has the right to infertility and fertility care as endorsed by the recent WHO infertility fact sheet. To address this high-burden disease, all women’s health care clinicians should be aware of, equitably diagnose and treat, refer as necessary, and advocate for infertile individuals.
Continue to: Lessons learned in reducing multiple pregnancy rates in infertility treatment...
Lessons learned in reducing multiple pregnancy rates in infertility treatment
Views and reviews section. Fertil Steril. 2020;114:671- 672; 673-679; 680-689; 690-714; 715-721.
In the October 2020 issue of Fertility and Sterility, the Views and Reviews section included 5 articles on avoiding multiple live birth rates (LBRs) in assisted reproductive technologies (ART).1-5 International experts provided a comprehensive review of global multiple LBRs and their associated negative impact on maternal and perinatal outcomes, reasons for global variability, strategies to reduce multiples, single embryo transfer, and implications of funding and reporting. These international comparisons and recommendations are helpful and applicable to infertility care in the United States.3
The rise of multiple birth rates
During the first decade of in vitro fertilization (IVF), live birth rates were low, increasing to 14% in 1990. Multiple embryos needed to be transferred so that even these LBRs could be obtained. In the 1990s, however, laboratory technology improved rapidly, with increased implantation rates and subsequent rapid increases in LBR, but also with increased multiple birth rates (MBRs).
In the United States, clinic-specific reporting helped create competition among clinics for the best LBRs, and this led to MBRs of 30% and higher. Numerous studies documented the associated significantly increased morbidity and mortality of both mothers and babies. Similar situations occurred in many other countries while some, especially Nordic nations, Australia, New Zealand, and Japan, had twin rates of less than 10% or even 5% since the early 2000s. So why the difference?
The higher MBR is due largely to the transfer of more than one embryo. The immediate solution is therefore always to perform elective single embryo transfer (eSET). However, numerous factors affect the decision to perform eSET or not, and this ideal is far from being achieved. Older women, those with longer duration of infertility and/or failed treatment, often feel a time pressure and want to transfer more embryos. Of course, biologically this is reasonable because the number and quality of their embryos is lower. While attempts have been made to assess embryo quality with preimplantation genetic testing for aneuploidy, evidence that this increases the LBR is controversial except possibly in women aged 35 to 38 years. This is especially true when the cumulative LBR, that is, the number of live births after transfer of all embryos from an egg retrieval cycle, is the measured outcome.
The major factor that determines the frequency of eSET is financial. Affordability is the out-of-pocket cost (after insurance or other subsidy) as a percentage of disposable income, and it is the most important factor that determines whether eSET is performed. Less affordable treatment creates a financial incentive to transfer more than one embryo to maximize the pregnancy rates in fewer cycles.5 Other factors include whether the effectiveness of treatment, that is, LBR, is emphasized over safety, that is, MBR. In the United States, the Society for Assisted Reproductive Technology now reports cumulative LBR, singleton and multiple LBR, and preterm births as outcomes, thereby increasing the emphasis on eSET.
Sociologic, cultural, and religious factors also can affect the frequency of eSET. Even within the United States, great variation exists in values and beliefs regarding infertility treatment. It can be challenging to determine who makes decisions: the patient alone, the physician, the payor, professional guidelines, or laws. In many countries, including the United States, it is an amalgam of these.
Setting new goals
If the goal is to reduce the MBR, what should that rate be? In the past few years, the MBR in the United States has been reduced to approximately 10%. It is reasonable now to set a goal of 5% in the next several years. To do this, we can learn from countries that have been successful. The United States already has very high-quality clinical and laboratory services, knowledgeable physicians, and a reasonable regulatory environment. Improved technology, specifically embryo selection for transfer, and focus on adherence to established embryo transfer guidelines could help.
Many would argue that eSET essentially should be performed always in women younger than age 40 and in all women of any age with a known euploid embryo. The major problem that drives multiples is the lack of affordability, which can be addressed by increased subsidies from payors. Increased subsidies can result from legislative mandates or societal pressures on employers, either of which could be associated with requirements for eSET and/or reduced MBRs.
In your practice, you can now reassure your infertility patients that cumulative LBRs are excellent in the United States and that the risk of multiple pregnancy has been reduced dramatically. This should encourage more patients to accept and take advantage of this successful technology that has resulted in the birth of millions of babies globally. Further reduction of the MBR to 5% should be possible within a few years through education and advocacy by women’s health care clinicians that results in increased subsidies and more affordable IVF.
The multiple birth rate in ART has been reduced to 10% in the United States through an increased understanding of the complex factors that affect embryo transfer practices globally. Further progress will depend primarily on increased subsidies that make ART more affordable.
Continue to: Genetics and ART...
Genetics and ART: Selection versus correction
Adashi EY, Cohen IG. The case for remedial germline editing—the long-term view. JAMA. 2020;323:1762-1763.
Rosenbaum L. The future of gene editing—toward scientific and social consensus. N Engl J Med. 2019;380:971-975.
Cyranoski D. The CRISPR-baby scandal: what’s next for human gene-editing. Nature. 2019;566:440-442.
de Wert G, Pennings G, Clarke A, et al; European Society of Human Genetics and European Society of Human Reproduction and Embryology. Human germline gene editing: recommendations of ESHG and ESHRE. Hum Reprod Open. 2018;hox025.
Following the completion of the Human Genome Project in 2003 and major technologic advancements in the subsequent years, the field of human genetics became the focal point of convergence for several distinct but interrelated disciplines: bioinformatics, computational biology, and sequencing technologies. As the result, individual human genomes can now be sequenced at a single base pair level, and with higher fidelity, at a fraction of the original cost and at a much faster speed.
This molecular progress, however, has not been accompanied by an equivalent clinical progress, because in a significant number of cases a defined and predictable clinical phenotype cannot be attributed to a detected molecular genotype. This has resulted in an overabundance of variants of uncertain significance. Variable expressivity, incomplete penetrance, epigenetics, mosaicism, and the polygenic nature of many human traits further complicate reliable interpretation and prognostication of the colossal amount of molecular genetic data that are being generated by the above-mentioned technologic advances.
Considering these limitations, at this juncture it is crucial to acknowledge that any attempts to prematurely commercialize these preclinical and research studies (such as polygenic risk scores for embryos) are perilous and have the potential to cause significant harm in terms of unnecessary stress and anxiety for intended parents as well as the potential for yet-unmapped societal and legal implications.
However, it is just a matter of time until more accurate clinical phenotyping catches up with molecular genotyping. As we get closer to this next historic milestone, precision medicine in the postnatal life (with regard to both diagnostics and therapeutics) and preimplantation genetic testing (PGT) at the prenatal stage for a much wider spectrum of conditions—including both monogenic and polygenic traits—may indeed become a reality.
The potential of germline editing
Specifically regarding PGT (which requires IVF), it is important to recognize that due to the limited and nonrenewable endowment of human oocytes (ovarian reserve), combined with the detrimental impact of advancing age on the quality of the remaining cohort as manifested by a higher risk of aneuploidy, the current clinical practice of trying to “select” a nonaffected embryo can be very inefficient. As a result, the intended parents pursuing such treatments may need to undergo multiple cycles of ovarian stimulation and oocyte retrieval.
A potential solution for genes associated with known diseases is the prospect of remedial germline editing by CRISPR–Cas9 technology or its future descendants. This would take advantage of the existing embryos to try to “correct” the defective gene instead of trying to “select” a normal embryo. These technologies are still in the early stages of development and are remotely distant from clinical applications. On the other hand, although germline gene editing, if actualized, would be a monumental breakthrough in the history of genetics and medicine, we must be cognizant of its serious legal, societal, and ethical ramifications, which are currently unknown. Furthermore, even at the biologic and technical level, the technology still is not advanced enough to reliably rule out off-target modifications, and the unintended clinical consequences of the on-target corrections have not been studied either.
Regulation of genetic modifications
Due to these myriad concerns and the lack of an existing appropriate regulatory framework and oversight for such interventions, current US law (since December 2015, through provisions in annual federal appropriations laws passed by Congress and renewed annually thereafter) bars the US Food and Drug Administration from considering any clinical trial application “in which a human embryo is intentionally created or modified to include a heritable genetic modification.” Notably, this moratorium also prohibits mitochondrial replacement technology (MRT), which is a less controversial and relatively better-studied innovation.
Mitochondrial genetic disorders caused by the mutations in mitochondrial DNA (versus nuclear DNA) are amenable to a specific treatment strategy aimed at substituting the defective maternal mitochondrial genome with the mitochondrial genome of an unaffected donor oocyte. This can be achieved via either pronuclear transfer, which involves isolation and transfer of the male and female pronuclei from an affected embryo to an enucleated normal donor embryo, or maternal spindle transfer, which involves isolation and transfer of the metaphase II spindle complex of an affected oocyte to an enucleated disease-free donor egg. It is noteworthy that in 2015 in the United Kingdom, Parliament expanded the definition of “permitted eggs and embryos” to include those “where unhealthy mitochondrial DNA is replaced by healthy mitochondrial DNA from a donor.” This thereby allows the UK Human Fertilisation and Embryology Authority to formally direct and oversee clinical trials in MRT.
Summing up
Although the future of assisted human reproduction cannot be clearly outlined at this time, it is likely to be radically different from the current state given these emerging applications at the intersection of ART and diagnostic and therapeutic genetics. To ensure that exploring this uncharted territory will ultimately be in the interest of humankind and civilization, proper regulatory oversight—after careful consideration of all ethical, societal, and legal implications—needs to be developed for all preclinical and clinical research in this field. Participatory public engagement must be an integrated part of this process. ●
The field of human genetics has already transformed medicine. However, the convergence of the interrelated disciplines of bioinformatics, computational biology, sequencing technologies, and CRISPR–Cas9 technology is creating incredible new advances that will bring great benefits but also major societal challenges.
- Farquhar C. Avoiding multiple pregnancies in assisted reproductive technologies: transferring one embryo at a time should be the norm. Fertil Steril. 2020;114:671-672.
- Bergh C, Kamath MS, Wang R, et al. Strategies to reduce multiple pregnancies during medically assisted reproduction. Fertil Steril. 2020;114:673-679.
- Adamson GD, Norman RJ. Why are multiple pregnancy rates and single embryo transfer rates so different globally, and what do we do about it? Fertil Steril. 2020;114:680-689.
- Eapen A, Ryan GL, Ten Eyck P, et al. Current evidence supporting a goal of singletons: a review of maternal and neonatal outcomes associated with twin versus singleton pregnancies after in vitro fertilization and intracytoplasmic sperm injection. Fertil Steril. 2020;114: 690-714.
- Chambers GM, Keller E, Choi S, et al. Funding and public reporting strategies for reducing multiple pregnancy from fertility treatments. Fertil Steril. 2020;114:715-721.
In this Update, we discuss several aspects of infertility and emerging technologic advances in treatment. We review an important infertility fact sheet recently issued by the World Health Organization (WHO) that provides a succinct overview of infertility causes, the rights of infertility patients, treatment challenges, and advocacy efforts. In addition, we discuss what the infertility literature reveals about reducing multiple birth rates and the technologic, financial, and social factors involved. Finally, we look at the molecular progress made in germline-editing technology and the myriad complications involved in its potential future translation to clinical phenotyping.
WHO recognizes the burden of infertility and addresses fertility care needs
World Health Organization (WHO). Infertility fact sheet. September 14, 2020. https://www.who.int/news-room/fact-sheets/detail/infertility. Accessed January 24, 2021.
The WHO published its first comprehensive infertility fact sheet in September 2020. This document is important because it validates infertility as a high-burden disease and disability that diminishes quality of life for up to 186 million individuals globally. The infertility fact sheet is a comprehensive yet focused quick read that addresses the causes of infertility, why infertility is important, challenges, and the WHO response.
Factors in infertility
Infertility is caused by different factors in women and men, yet sometimes it is unexplained, and its relative importance can vary from country to country. For women, tubal disorders (for example, postinfectious), uterine problems (fibroids, congenital), endometriosis, ovarian disorders (polycystic ovary syndrome, ovulation disorders), and endocrine imbalances are the most common factors.
For men, causes of infertility include obstruction of the reproductive tract (as after injuries or infection); hormonal disorders in the hypothalamus, pituitary, and/or testicles (for example, low testosterone); testicular failure to produce sperm (such as after cancer treatment); and abnormal sperm function and quality (low count, motility, or morphology).
Environmental and lifestyle factors— including smoking, obesity, alcohol, or toxins—can affect fertility.
Continue to: Recognizing all individuals’ fertility rights...
Recognizing all individuals’ fertility rights
The WHO infertility fact sheet makes strong statements, recognizing that individuals and couples have the right to decide the number, timing, and spacing of their children. Addressing infertility is therefore an important part of realizing the right of individuals and couples to found a family. This includes heterosexual couples, same-sex partners, older persons, individuals not in sexual relationships who might require infertility management and fertility care services, and notably marginalized populations.
Addressing infertility also can help mitigate gender inequality, which has significant negative social impacts on the lives of infertile individuals, especially women. Fertility education is important to reduce the fear of infertility and contraception use in those wanting pregnancy in the future.
In most countries the biggest challenges are availability, access, and quality of interventions to address infertility. This includes the United States, where only 1 in 4 individuals receive the fertility care they need. Lack of prioritization, ineffective public health strategies, inadequate funding, and costs are barriers. Health policies need to recognize that infertility is a disease that often can be prevented, thereby reducing future costs. Comprehensive awareness and education programs, laws and policies that regulate and ensure access and the human rights of all involved, are essential.
Advocacy efforts
To address infertility and fertility care, the WHO is committed to:
- collaborate with partners on epidemiologic and etiologic research
- facilitate policy dialogue globally to frame infertility within a legal and policy framework
- support generation of data on the burden of infertility
- develop guidelines
- produce other documents of standards
- collaborate with all stakeholders to strengthen political commitment and health system capacity, and
- provide country-level technical support to develop or strengthen policies and services.
For your practice, this means that infertility is recognized as a disease that should receive its appropriate share of health care resources. Infertility and fertility care are the right of every individual according to their desires to found a family. Besides providing the best care you can to all your patients, including referring them when necessary, all health care clinicians should advocate on behalf of their patients to payors, policy makers, and the public the need to provide equitable laws, resources, and funding for infertility and fertility care.
Every person has the right to infertility and fertility care as endorsed by the recent WHO infertility fact sheet. To address this high-burden disease, all women’s health care clinicians should be aware of, equitably diagnose and treat, refer as necessary, and advocate for infertile individuals.
Continue to: Lessons learned in reducing multiple pregnancy rates in infertility treatment...
Lessons learned in reducing multiple pregnancy rates in infertility treatment
Views and reviews section. Fertil Steril. 2020;114:671- 672; 673-679; 680-689; 690-714; 715-721.
In the October 2020 issue of Fertility and Sterility, the Views and Reviews section included 5 articles on avoiding multiple live birth rates (LBRs) in assisted reproductive technologies (ART).1-5 International experts provided a comprehensive review of global multiple LBRs and their associated negative impact on maternal and perinatal outcomes, reasons for global variability, strategies to reduce multiples, single embryo transfer, and implications of funding and reporting. These international comparisons and recommendations are helpful and applicable to infertility care in the United States.3
The rise of multiple birth rates
During the first decade of in vitro fertilization (IVF), live birth rates were low, increasing to 14% in 1990. Multiple embryos needed to be transferred so that even these LBRs could be obtained. In the 1990s, however, laboratory technology improved rapidly, with increased implantation rates and subsequent rapid increases in LBR, but also with increased multiple birth rates (MBRs).
In the United States, clinic-specific reporting helped create competition among clinics for the best LBRs, and this led to MBRs of 30% and higher. Numerous studies documented the associated significantly increased morbidity and mortality of both mothers and babies. Similar situations occurred in many other countries while some, especially Nordic nations, Australia, New Zealand, and Japan, had twin rates of less than 10% or even 5% since the early 2000s. So why the difference?
The higher MBR is due largely to the transfer of more than one embryo. The immediate solution is therefore always to perform elective single embryo transfer (eSET). However, numerous factors affect the decision to perform eSET or not, and this ideal is far from being achieved. Older women, those with longer duration of infertility and/or failed treatment, often feel a time pressure and want to transfer more embryos. Of course, biologically this is reasonable because the number and quality of their embryos is lower. While attempts have been made to assess embryo quality with preimplantation genetic testing for aneuploidy, evidence that this increases the LBR is controversial except possibly in women aged 35 to 38 years. This is especially true when the cumulative LBR, that is, the number of live births after transfer of all embryos from an egg retrieval cycle, is the measured outcome.
The major factor that determines the frequency of eSET is financial. Affordability is the out-of-pocket cost (after insurance or other subsidy) as a percentage of disposable income, and it is the most important factor that determines whether eSET is performed. Less affordable treatment creates a financial incentive to transfer more than one embryo to maximize the pregnancy rates in fewer cycles.5 Other factors include whether the effectiveness of treatment, that is, LBR, is emphasized over safety, that is, MBR. In the United States, the Society for Assisted Reproductive Technology now reports cumulative LBR, singleton and multiple LBR, and preterm births as outcomes, thereby increasing the emphasis on eSET.
Sociologic, cultural, and religious factors also can affect the frequency of eSET. Even within the United States, great variation exists in values and beliefs regarding infertility treatment. It can be challenging to determine who makes decisions: the patient alone, the physician, the payor, professional guidelines, or laws. In many countries, including the United States, it is an amalgam of these.
Setting new goals
If the goal is to reduce the MBR, what should that rate be? In the past few years, the MBR in the United States has been reduced to approximately 10%. It is reasonable now to set a goal of 5% in the next several years. To do this, we can learn from countries that have been successful. The United States already has very high-quality clinical and laboratory services, knowledgeable physicians, and a reasonable regulatory environment. Improved technology, specifically embryo selection for transfer, and focus on adherence to established embryo transfer guidelines could help.
Many would argue that eSET essentially should be performed always in women younger than age 40 and in all women of any age with a known euploid embryo. The major problem that drives multiples is the lack of affordability, which can be addressed by increased subsidies from payors. Increased subsidies can result from legislative mandates or societal pressures on employers, either of which could be associated with requirements for eSET and/or reduced MBRs.
In your practice, you can now reassure your infertility patients that cumulative LBRs are excellent in the United States and that the risk of multiple pregnancy has been reduced dramatically. This should encourage more patients to accept and take advantage of this successful technology that has resulted in the birth of millions of babies globally. Further reduction of the MBR to 5% should be possible within a few years through education and advocacy by women’s health care clinicians that results in increased subsidies and more affordable IVF.
The multiple birth rate in ART has been reduced to 10% in the United States through an increased understanding of the complex factors that affect embryo transfer practices globally. Further progress will depend primarily on increased subsidies that make ART more affordable.
Continue to: Genetics and ART...
Genetics and ART: Selection versus correction
Adashi EY, Cohen IG. The case for remedial germline editing—the long-term view. JAMA. 2020;323:1762-1763.
Rosenbaum L. The future of gene editing—toward scientific and social consensus. N Engl J Med. 2019;380:971-975.
Cyranoski D. The CRISPR-baby scandal: what’s next for human gene-editing. Nature. 2019;566:440-442.
de Wert G, Pennings G, Clarke A, et al; European Society of Human Genetics and European Society of Human Reproduction and Embryology. Human germline gene editing: recommendations of ESHG and ESHRE. Hum Reprod Open. 2018;hox025.
Following the completion of the Human Genome Project in 2003 and major technologic advancements in the subsequent years, the field of human genetics became the focal point of convergence for several distinct but interrelated disciplines: bioinformatics, computational biology, and sequencing technologies. As the result, individual human genomes can now be sequenced at a single base pair level, and with higher fidelity, at a fraction of the original cost and at a much faster speed.
This molecular progress, however, has not been accompanied by an equivalent clinical progress, because in a significant number of cases a defined and predictable clinical phenotype cannot be attributed to a detected molecular genotype. This has resulted in an overabundance of variants of uncertain significance. Variable expressivity, incomplete penetrance, epigenetics, mosaicism, and the polygenic nature of many human traits further complicate reliable interpretation and prognostication of the colossal amount of molecular genetic data that are being generated by the above-mentioned technologic advances.
Considering these limitations, at this juncture it is crucial to acknowledge that any attempts to prematurely commercialize these preclinical and research studies (such as polygenic risk scores for embryos) are perilous and have the potential to cause significant harm in terms of unnecessary stress and anxiety for intended parents as well as the potential for yet-unmapped societal and legal implications.
However, it is just a matter of time until more accurate clinical phenotyping catches up with molecular genotyping. As we get closer to this next historic milestone, precision medicine in the postnatal life (with regard to both diagnostics and therapeutics) and preimplantation genetic testing (PGT) at the prenatal stage for a much wider spectrum of conditions—including both monogenic and polygenic traits—may indeed become a reality.
The potential of germline editing
Specifically regarding PGT (which requires IVF), it is important to recognize that due to the limited and nonrenewable endowment of human oocytes (ovarian reserve), combined with the detrimental impact of advancing age on the quality of the remaining cohort as manifested by a higher risk of aneuploidy, the current clinical practice of trying to “select” a nonaffected embryo can be very inefficient. As a result, the intended parents pursuing such treatments may need to undergo multiple cycles of ovarian stimulation and oocyte retrieval.
A potential solution for genes associated with known diseases is the prospect of remedial germline editing by CRISPR–Cas9 technology or its future descendants. This would take advantage of the existing embryos to try to “correct” the defective gene instead of trying to “select” a normal embryo. These technologies are still in the early stages of development and are remotely distant from clinical applications. On the other hand, although germline gene editing, if actualized, would be a monumental breakthrough in the history of genetics and medicine, we must be cognizant of its serious legal, societal, and ethical ramifications, which are currently unknown. Furthermore, even at the biologic and technical level, the technology still is not advanced enough to reliably rule out off-target modifications, and the unintended clinical consequences of the on-target corrections have not been studied either.
Regulation of genetic modifications
Due to these myriad concerns and the lack of an existing appropriate regulatory framework and oversight for such interventions, current US law (since December 2015, through provisions in annual federal appropriations laws passed by Congress and renewed annually thereafter) bars the US Food and Drug Administration from considering any clinical trial application “in which a human embryo is intentionally created or modified to include a heritable genetic modification.” Notably, this moratorium also prohibits mitochondrial replacement technology (MRT), which is a less controversial and relatively better-studied innovation.
Mitochondrial genetic disorders caused by the mutations in mitochondrial DNA (versus nuclear DNA) are amenable to a specific treatment strategy aimed at substituting the defective maternal mitochondrial genome with the mitochondrial genome of an unaffected donor oocyte. This can be achieved via either pronuclear transfer, which involves isolation and transfer of the male and female pronuclei from an affected embryo to an enucleated normal donor embryo, or maternal spindle transfer, which involves isolation and transfer of the metaphase II spindle complex of an affected oocyte to an enucleated disease-free donor egg. It is noteworthy that in 2015 in the United Kingdom, Parliament expanded the definition of “permitted eggs and embryos” to include those “where unhealthy mitochondrial DNA is replaced by healthy mitochondrial DNA from a donor.” This thereby allows the UK Human Fertilisation and Embryology Authority to formally direct and oversee clinical trials in MRT.
Summing up
Although the future of assisted human reproduction cannot be clearly outlined at this time, it is likely to be radically different from the current state given these emerging applications at the intersection of ART and diagnostic and therapeutic genetics. To ensure that exploring this uncharted territory will ultimately be in the interest of humankind and civilization, proper regulatory oversight—after careful consideration of all ethical, societal, and legal implications—needs to be developed for all preclinical and clinical research in this field. Participatory public engagement must be an integrated part of this process. ●
The field of human genetics has already transformed medicine. However, the convergence of the interrelated disciplines of bioinformatics, computational biology, sequencing technologies, and CRISPR–Cas9 technology is creating incredible new advances that will bring great benefits but also major societal challenges.
In this Update, we discuss several aspects of infertility and emerging technologic advances in treatment. We review an important infertility fact sheet recently issued by the World Health Organization (WHO) that provides a succinct overview of infertility causes, the rights of infertility patients, treatment challenges, and advocacy efforts. In addition, we discuss what the infertility literature reveals about reducing multiple birth rates and the technologic, financial, and social factors involved. Finally, we look at the molecular progress made in germline-editing technology and the myriad complications involved in its potential future translation to clinical phenotyping.
WHO recognizes the burden of infertility and addresses fertility care needs
World Health Organization (WHO). Infertility fact sheet. September 14, 2020. https://www.who.int/news-room/fact-sheets/detail/infertility. Accessed January 24, 2021.
The WHO published its first comprehensive infertility fact sheet in September 2020. This document is important because it validates infertility as a high-burden disease and disability that diminishes quality of life for up to 186 million individuals globally. The infertility fact sheet is a comprehensive yet focused quick read that addresses the causes of infertility, why infertility is important, challenges, and the WHO response.
Factors in infertility
Infertility is caused by different factors in women and men, yet sometimes it is unexplained, and its relative importance can vary from country to country. For women, tubal disorders (for example, postinfectious), uterine problems (fibroids, congenital), endometriosis, ovarian disorders (polycystic ovary syndrome, ovulation disorders), and endocrine imbalances are the most common factors.
For men, causes of infertility include obstruction of the reproductive tract (as after injuries or infection); hormonal disorders in the hypothalamus, pituitary, and/or testicles (for example, low testosterone); testicular failure to produce sperm (such as after cancer treatment); and abnormal sperm function and quality (low count, motility, or morphology).
Environmental and lifestyle factors— including smoking, obesity, alcohol, or toxins—can affect fertility.
Continue to: Recognizing all individuals’ fertility rights...
Recognizing all individuals’ fertility rights
The WHO infertility fact sheet makes strong statements, recognizing that individuals and couples have the right to decide the number, timing, and spacing of their children. Addressing infertility is therefore an important part of realizing the right of individuals and couples to found a family. This includes heterosexual couples, same-sex partners, older persons, individuals not in sexual relationships who might require infertility management and fertility care services, and notably marginalized populations.
Addressing infertility also can help mitigate gender inequality, which has significant negative social impacts on the lives of infertile individuals, especially women. Fertility education is important to reduce the fear of infertility and contraception use in those wanting pregnancy in the future.
In most countries the biggest challenges are availability, access, and quality of interventions to address infertility. This includes the United States, where only 1 in 4 individuals receive the fertility care they need. Lack of prioritization, ineffective public health strategies, inadequate funding, and costs are barriers. Health policies need to recognize that infertility is a disease that often can be prevented, thereby reducing future costs. Comprehensive awareness and education programs, laws and policies that regulate and ensure access and the human rights of all involved, are essential.
Advocacy efforts
To address infertility and fertility care, the WHO is committed to:
- collaborate with partners on epidemiologic and etiologic research
- facilitate policy dialogue globally to frame infertility within a legal and policy framework
- support generation of data on the burden of infertility
- develop guidelines
- produce other documents of standards
- collaborate with all stakeholders to strengthen political commitment and health system capacity, and
- provide country-level technical support to develop or strengthen policies and services.
For your practice, this means that infertility is recognized as a disease that should receive its appropriate share of health care resources. Infertility and fertility care are the right of every individual according to their desires to found a family. Besides providing the best care you can to all your patients, including referring them when necessary, all health care clinicians should advocate on behalf of their patients to payors, policy makers, and the public the need to provide equitable laws, resources, and funding for infertility and fertility care.
Every person has the right to infertility and fertility care as endorsed by the recent WHO infertility fact sheet. To address this high-burden disease, all women’s health care clinicians should be aware of, equitably diagnose and treat, refer as necessary, and advocate for infertile individuals.
Continue to: Lessons learned in reducing multiple pregnancy rates in infertility treatment...
Lessons learned in reducing multiple pregnancy rates in infertility treatment
Views and reviews section. Fertil Steril. 2020;114:671- 672; 673-679; 680-689; 690-714; 715-721.
In the October 2020 issue of Fertility and Sterility, the Views and Reviews section included 5 articles on avoiding multiple live birth rates (LBRs) in assisted reproductive technologies (ART).1-5 International experts provided a comprehensive review of global multiple LBRs and their associated negative impact on maternal and perinatal outcomes, reasons for global variability, strategies to reduce multiples, single embryo transfer, and implications of funding and reporting. These international comparisons and recommendations are helpful and applicable to infertility care in the United States.3
The rise of multiple birth rates
During the first decade of in vitro fertilization (IVF), live birth rates were low, increasing to 14% in 1990. Multiple embryos needed to be transferred so that even these LBRs could be obtained. In the 1990s, however, laboratory technology improved rapidly, with increased implantation rates and subsequent rapid increases in LBR, but also with increased multiple birth rates (MBRs).
In the United States, clinic-specific reporting helped create competition among clinics for the best LBRs, and this led to MBRs of 30% and higher. Numerous studies documented the associated significantly increased morbidity and mortality of both mothers and babies. Similar situations occurred in many other countries while some, especially Nordic nations, Australia, New Zealand, and Japan, had twin rates of less than 10% or even 5% since the early 2000s. So why the difference?
The higher MBR is due largely to the transfer of more than one embryo. The immediate solution is therefore always to perform elective single embryo transfer (eSET). However, numerous factors affect the decision to perform eSET or not, and this ideal is far from being achieved. Older women, those with longer duration of infertility and/or failed treatment, often feel a time pressure and want to transfer more embryos. Of course, biologically this is reasonable because the number and quality of their embryos is lower. While attempts have been made to assess embryo quality with preimplantation genetic testing for aneuploidy, evidence that this increases the LBR is controversial except possibly in women aged 35 to 38 years. This is especially true when the cumulative LBR, that is, the number of live births after transfer of all embryos from an egg retrieval cycle, is the measured outcome.
The major factor that determines the frequency of eSET is financial. Affordability is the out-of-pocket cost (after insurance or other subsidy) as a percentage of disposable income, and it is the most important factor that determines whether eSET is performed. Less affordable treatment creates a financial incentive to transfer more than one embryo to maximize the pregnancy rates in fewer cycles.5 Other factors include whether the effectiveness of treatment, that is, LBR, is emphasized over safety, that is, MBR. In the United States, the Society for Assisted Reproductive Technology now reports cumulative LBR, singleton and multiple LBR, and preterm births as outcomes, thereby increasing the emphasis on eSET.
Sociologic, cultural, and religious factors also can affect the frequency of eSET. Even within the United States, great variation exists in values and beliefs regarding infertility treatment. It can be challenging to determine who makes decisions: the patient alone, the physician, the payor, professional guidelines, or laws. In many countries, including the United States, it is an amalgam of these.
Setting new goals
If the goal is to reduce the MBR, what should that rate be? In the past few years, the MBR in the United States has been reduced to approximately 10%. It is reasonable now to set a goal of 5% in the next several years. To do this, we can learn from countries that have been successful. The United States already has very high-quality clinical and laboratory services, knowledgeable physicians, and a reasonable regulatory environment. Improved technology, specifically embryo selection for transfer, and focus on adherence to established embryo transfer guidelines could help.
Many would argue that eSET essentially should be performed always in women younger than age 40 and in all women of any age with a known euploid embryo. The major problem that drives multiples is the lack of affordability, which can be addressed by increased subsidies from payors. Increased subsidies can result from legislative mandates or societal pressures on employers, either of which could be associated with requirements for eSET and/or reduced MBRs.
In your practice, you can now reassure your infertility patients that cumulative LBRs are excellent in the United States and that the risk of multiple pregnancy has been reduced dramatically. This should encourage more patients to accept and take advantage of this successful technology that has resulted in the birth of millions of babies globally. Further reduction of the MBR to 5% should be possible within a few years through education and advocacy by women’s health care clinicians that results in increased subsidies and more affordable IVF.
The multiple birth rate in ART has been reduced to 10% in the United States through an increased understanding of the complex factors that affect embryo transfer practices globally. Further progress will depend primarily on increased subsidies that make ART more affordable.
Continue to: Genetics and ART...
Genetics and ART: Selection versus correction
Adashi EY, Cohen IG. The case for remedial germline editing—the long-term view. JAMA. 2020;323:1762-1763.
Rosenbaum L. The future of gene editing—toward scientific and social consensus. N Engl J Med. 2019;380:971-975.
Cyranoski D. The CRISPR-baby scandal: what’s next for human gene-editing. Nature. 2019;566:440-442.
de Wert G, Pennings G, Clarke A, et al; European Society of Human Genetics and European Society of Human Reproduction and Embryology. Human germline gene editing: recommendations of ESHG and ESHRE. Hum Reprod Open. 2018;hox025.
Following the completion of the Human Genome Project in 2003 and major technologic advancements in the subsequent years, the field of human genetics became the focal point of convergence for several distinct but interrelated disciplines: bioinformatics, computational biology, and sequencing technologies. As the result, individual human genomes can now be sequenced at a single base pair level, and with higher fidelity, at a fraction of the original cost and at a much faster speed.
This molecular progress, however, has not been accompanied by an equivalent clinical progress, because in a significant number of cases a defined and predictable clinical phenotype cannot be attributed to a detected molecular genotype. This has resulted in an overabundance of variants of uncertain significance. Variable expressivity, incomplete penetrance, epigenetics, mosaicism, and the polygenic nature of many human traits further complicate reliable interpretation and prognostication of the colossal amount of molecular genetic data that are being generated by the above-mentioned technologic advances.
Considering these limitations, at this juncture it is crucial to acknowledge that any attempts to prematurely commercialize these preclinical and research studies (such as polygenic risk scores for embryos) are perilous and have the potential to cause significant harm in terms of unnecessary stress and anxiety for intended parents as well as the potential for yet-unmapped societal and legal implications.
However, it is just a matter of time until more accurate clinical phenotyping catches up with molecular genotyping. As we get closer to this next historic milestone, precision medicine in the postnatal life (with regard to both diagnostics and therapeutics) and preimplantation genetic testing (PGT) at the prenatal stage for a much wider spectrum of conditions—including both monogenic and polygenic traits—may indeed become a reality.
The potential of germline editing
Specifically regarding PGT (which requires IVF), it is important to recognize that due to the limited and nonrenewable endowment of human oocytes (ovarian reserve), combined with the detrimental impact of advancing age on the quality of the remaining cohort as manifested by a higher risk of aneuploidy, the current clinical practice of trying to “select” a nonaffected embryo can be very inefficient. As a result, the intended parents pursuing such treatments may need to undergo multiple cycles of ovarian stimulation and oocyte retrieval.
A potential solution for genes associated with known diseases is the prospect of remedial germline editing by CRISPR–Cas9 technology or its future descendants. This would take advantage of the existing embryos to try to “correct” the defective gene instead of trying to “select” a normal embryo. These technologies are still in the early stages of development and are remotely distant from clinical applications. On the other hand, although germline gene editing, if actualized, would be a monumental breakthrough in the history of genetics and medicine, we must be cognizant of its serious legal, societal, and ethical ramifications, which are currently unknown. Furthermore, even at the biologic and technical level, the technology still is not advanced enough to reliably rule out off-target modifications, and the unintended clinical consequences of the on-target corrections have not been studied either.
Regulation of genetic modifications
Due to these myriad concerns and the lack of an existing appropriate regulatory framework and oversight for such interventions, current US law (since December 2015, through provisions in annual federal appropriations laws passed by Congress and renewed annually thereafter) bars the US Food and Drug Administration from considering any clinical trial application “in which a human embryo is intentionally created or modified to include a heritable genetic modification.” Notably, this moratorium also prohibits mitochondrial replacement technology (MRT), which is a less controversial and relatively better-studied innovation.
Mitochondrial genetic disorders caused by the mutations in mitochondrial DNA (versus nuclear DNA) are amenable to a specific treatment strategy aimed at substituting the defective maternal mitochondrial genome with the mitochondrial genome of an unaffected donor oocyte. This can be achieved via either pronuclear transfer, which involves isolation and transfer of the male and female pronuclei from an affected embryo to an enucleated normal donor embryo, or maternal spindle transfer, which involves isolation and transfer of the metaphase II spindle complex of an affected oocyte to an enucleated disease-free donor egg. It is noteworthy that in 2015 in the United Kingdom, Parliament expanded the definition of “permitted eggs and embryos” to include those “where unhealthy mitochondrial DNA is replaced by healthy mitochondrial DNA from a donor.” This thereby allows the UK Human Fertilisation and Embryology Authority to formally direct and oversee clinical trials in MRT.
Summing up
Although the future of assisted human reproduction cannot be clearly outlined at this time, it is likely to be radically different from the current state given these emerging applications at the intersection of ART and diagnostic and therapeutic genetics. To ensure that exploring this uncharted territory will ultimately be in the interest of humankind and civilization, proper regulatory oversight—after careful consideration of all ethical, societal, and legal implications—needs to be developed for all preclinical and clinical research in this field. Participatory public engagement must be an integrated part of this process. ●
The field of human genetics has already transformed medicine. However, the convergence of the interrelated disciplines of bioinformatics, computational biology, sequencing technologies, and CRISPR–Cas9 technology is creating incredible new advances that will bring great benefits but also major societal challenges.
- Farquhar C. Avoiding multiple pregnancies in assisted reproductive technologies: transferring one embryo at a time should be the norm. Fertil Steril. 2020;114:671-672.
- Bergh C, Kamath MS, Wang R, et al. Strategies to reduce multiple pregnancies during medically assisted reproduction. Fertil Steril. 2020;114:673-679.
- Adamson GD, Norman RJ. Why are multiple pregnancy rates and single embryo transfer rates so different globally, and what do we do about it? Fertil Steril. 2020;114:680-689.
- Eapen A, Ryan GL, Ten Eyck P, et al. Current evidence supporting a goal of singletons: a review of maternal and neonatal outcomes associated with twin versus singleton pregnancies after in vitro fertilization and intracytoplasmic sperm injection. Fertil Steril. 2020;114: 690-714.
- Chambers GM, Keller E, Choi S, et al. Funding and public reporting strategies for reducing multiple pregnancy from fertility treatments. Fertil Steril. 2020;114:715-721.
- Farquhar C. Avoiding multiple pregnancies in assisted reproductive technologies: transferring one embryo at a time should be the norm. Fertil Steril. 2020;114:671-672.
- Bergh C, Kamath MS, Wang R, et al. Strategies to reduce multiple pregnancies during medically assisted reproduction. Fertil Steril. 2020;114:673-679.
- Adamson GD, Norman RJ. Why are multiple pregnancy rates and single embryo transfer rates so different globally, and what do we do about it? Fertil Steril. 2020;114:680-689.
- Eapen A, Ryan GL, Ten Eyck P, et al. Current evidence supporting a goal of singletons: a review of maternal and neonatal outcomes associated with twin versus singleton pregnancies after in vitro fertilization and intracytoplasmic sperm injection. Fertil Steril. 2020;114: 690-714.
- Chambers GM, Keller E, Choi S, et al. Funding and public reporting strategies for reducing multiple pregnancy from fertility treatments. Fertil Steril. 2020;114:715-721.
Hair Follicle Bulb Region: A Potential Nidus for the Formation of Osteoma Cutis
The term osteoma cutis (OC) is defined as the ossification or bone formation either in the dermis or hypodermis. 1 It is heterotopic in nature, referring to extraneous bone formation in soft tissue. Osteoma cutis was first described in 1858 2,3 ; in 1868, the multiple miliary form on the face was described. 4 Cutaneous ossification can take many forms, ranging from occurrence in a nevus (nevus of Nanta) to its association with rare genetic disorders, such as fibrodysplasia ossificans progressiva and Albright hereditary osteodystrophy.
Some of these ossifications are classified as primary; others are secondary, depending on the presence of a preexisting lesion (eg, pilomatricoma, basal cell carcinoma). However, certain conditions, such as multiple miliary osteoma of the face, can be difficult to classify due to the presence or absence of a history of acne or dermabrasion, or both. The secondary forms more commonly are encountered due to their incidental association with an excised lesion, such as pilomatricoma.
A precursor of OC has been neglected in the literature despite its common occurrence. It may have been peripherally alluded to in the literature in reference to the miliary form of OC.5,6 The cases reported here demonstrate small round nodules of calcification or ossification, or both, in punch biopsies and excision specimens from hair-bearing areas of skin, especially from the head and neck. These lesions are mainly observed in the peripilar location or more specifically in the approximate location of the hair bulb.
This article reviews a possible mechanism of formation of these osteocalcific micronodules. These often-encountered micronodules are small osteocalcific lesions without typical bone or well-formed OC, such as trabeculae formation or fatty marrow, and may represent earliest stages in the formation of OC.
Clinical Observations
During routine dermatopathologic practice, I observed incidental small osteocalcific micronodules in close proximity to the lower part of the hair follicle in multiple cases. These nodules were not related to the main lesion in the specimen and were not the reason for the biopsy or excision. Most of the time, these micronodules were noted in excision or re-excision specimens or in a punch biopsy.
In my review of multiple unrelated cases over time, incidental osteocalcific micronodules were observed occasionally in punch biopsies and excision specimens during routine practice. These micronodules were mainly located in the vicinity of a hair bulb (Figure 1). If the hair bulb was not present in the sections, these micronodules were noted near or within the fibrous tract (Figure 2) or beneath a sebaceous lobule (Figure 3). In an exceptional case, a small round deposit of osteoid was seen forming just above the dermal papilla of the hair bulb (Figure 4).
Multiple osteocalcific micronodules were identified in a case of cicatricial alopecia. These micronodules were observed in sections taken at the levels of hair bulbs, and more or less corresponded to the size of the bulb (Figure 5A). Fortuitously, the patient was dark-skinned; the remnants of melanin within the micronodules provided evidence that the micronodules were formed within hair bulbs. Melanin staining confirmed the presence of melanin within some of the micronodules (Figure 5B).
Comment
Skeletogenesis in humans takes place by 2 methods: endochondral ossification and intramembranous ossification. In contrast to endochondral ossification, intramembranous ossification does not require a preexisting cartilaginous template. Instead, there is condensation of mesenchymal cells, which differentiate into osteoblasts and lay down osteoid, thus forming an ossification center. Little is known about the mechanism of formation of OC or the nidus of formation of the primary form.
Incidental micronodules of calcification and ossification are routinely encountered during histopathologic review of specimens from hair-bearing areas of the skin in dermatopathology practice. A review of the literature, however, does not reveal any specific dermatopathologic term ascribed to this phenomenon. These lesions might be similar to those described by Hopkins5 in 1928 in the setting of miliary OC of the face secondary to acne. Rossman and Freeman6 also described the same lesions when referring to facial OC as a “stage of pre-osseous calcification.”
When these osteocalcific micronodules are encountered, it usually is in close proximity to a hair follicle bulb. When a hair bulb is not seen in the sections, the micronodules are noted near fibrous tracts, arrector pili muscles, or sebaceous lobules, suggesting a close peripilar or peribulbar location. The micronodules are approximately 0.5 mm in diameter—roughly the size of a hair bulb. Due to the close anatomic association of micronodules and the hair bulb, these lesions can be called pilar osteocalcific nodules (PONs).
The role of bone morphogenetic protein (BMP) signaling in the maintenance of the hair cycle is well established. Bone morphogenetic proteins are extracellular cytokines that belong to the transforming growth factor β family. The hair bulb microenvironment is rich in BMPs
As the name implies, BMPs were discovered in relation to their important role in osteogenesis and tissue homeostasis. More than 20 BMPs have been identified, many of which promote bone formation and repair of bone fracture. Osteoinductive BMPs include BMP-2 and BMP-4 through BMP-10; BMP-2 and BMP-4 are expressed in the hair matrix and BMP-4 and BMP-6 are expressed in the FDP.8,9 All bone-inducing BMPs can cause mesenchymal stem cells to differentiate into osteoblasts in vitro.10
Overactive BMP signaling has been shown to cause heterotopic ossification in patients with fibrodysplasia ossificans progressiva.8 Immunohistochemical expression of BMP-2 has been demonstrated in shadow cells of pilomatricoma.11 Calcification and ossification are seen in as many as 20% of pilomatricomas. Both BMP-2 and BMP-4 have been shown to induce osteogenic differentiation of mouse skin−derived fibroblasts and FDP cells.12
Myllylä et al13 described 4 cases of multiple miliary osteoma cutis (MMOC). They also found 47 reported cases of MMOC, in which there was a history of acne in 55% (26/47). Only 15% (7/47) of these cases were extrafacial on the neck, chest, back, and arms. Osteomas in these cases were not associated with folliculosebaceous units or other adnexal structures, which may have been due to replacement by acne scarring, as all 4 patients had a history of acne vulgaris. The authors postulated a role for the GNAS gene mutation in the morphogenesis of MMOC; however, no supporting evidence was found for this claim. They also postulated a role for BMPs in the formation of MMOC.13
Some disturbance or imbalance in hair bulb homeostasis leads to overactivity of BMP signaling, causing osteoinduction in the hair bulb region and formation of PONs. The cause of the disturbance could be a traumatic or inflammatory injury to the hair follicle, as in the case of the secondary form of MMOC in association with chronic acne. In the primary form of osteoma cutis, the trigger could be more subtle or subclinical.
Trauma and inflammation are the main initiating factors involved in ossification in patients with fibrodysplasia ossificans progressiva due to ectopic activity of BMPs.9 The primary form of ossification appears to be similar to the mechanism by which intramembranous ossification is laid down (ie, by differentiation of mesenchymal cells into osteoblasts). In the proposed scenario, the cells of FDP, under the influence of BMPs, differentiate into osteoblasts and lay down osteoid, forming a limited-capacity “ossification center” or pilar osteocalcific nodule.
It is difficult to know the exact relationship of PONs or OC to the hair bulb due to the 2-dimensional nature of histologic sections. However, considering the finding of a rare case of osteoid forming within the bulb and in another the presence of melanin within the osteocalcific nodule, it is likely that these lesions are formed within the hair bulb or in situations in which the conditions replicate the biochemical characteristics of the hair bulb (eg, pilomatricoma).
The formation of PONs might act as a terminal phase in the hair cycle that is rarely induced to provide an exit for damaged hair follicles from cyclical perpetuity. An unspecified event or injury might render a hair follicle unable to continue its cyclical growth and cause BMPs to induce premature calcification in or around the hair bulb, which would probably be the only known quasiphysiological mechanism for a damaged hair follicle to exit the hair cycle.
Another interesting aspect of osteoma formation in human skin is the similarity to osteoderms or the integumentary skeleton of vertebrates.14 Early in evolution, the dermal skeleton was the predominant skeletal system in some lineages. Phylogenetically, osteoderms are not uniformly distributed, and show a latent ability to manifest in some groups or lay dormant or disappear in others. The occurrence of primary osteomas in the human integument might be a vestigial manifestation of deep homology,15 a latent ability to form structures that have been lost. The embryologic formation of osteoderms in the dermis of vertebrates is thought to depend on the interaction or cross-talk between ectomesenchymal cells of neural crest origin and cells of the stratum basalis of epidermis, which is somewhat similar to the formation of the hair follicles.
Conclusion
Under certain conditions, the bulb region of a hair follicle might provide a nidus for the formation of OC. The hair bulb region contains both the precursor cellular element (mesenchymal cells of FDP) and the trigger cytokine (BMP) for the induction of osteogenic metaplasia.
- Burgdorf W, Nasemann T. Cutaneous osteomas: a clinical and histopathologic review. Arch Dermatol Res. 1977;260:121-135.
- Essing M. Osteoma cutis of the forehead. HNO. 1985;33:548-550.
- Bouraoui S, Mlika M, Kort R, et al. Miliary osteoma cutis of the face. J Dermatol Case Rep. 2011;5:77-81.
- Virchow R. Die krankhaften Geschwülste. Vol 2. Hirschwald; 1864.
- Hopkins JG. Multiple miliary osteomas of the skin: report of a case. Arch Derm Syphilol. 1928;18:706-715.
- Rossman RE, Freeman RG. Osteoma cutis, a stage of preosseous calcification. Arch Dermatol. 1964;89:68-73.
- Guha U, Mecklenburg L, Cowin P, et al. Bone morphogenetic protein signaling regulates postnatal hair follicle differentiation and cycling. Am J Pathol. 2004;165:729-740.
- Rendl M, Polak L, Fuchs E. BMP signaling in dermal papilla cells is required for their hair follicle-inductive properties. Genes Dev. 2008;22:543-557.
- Shi S, de Gorter DJJ, Hoogaars WMH, et al. Overactive bone morphogenetic protein signaling in heterotopic ossification and Duchenne muscular dystrophy. Cell Mol Life Sci. 2013;70:407-423.
- Miyazono K, Kamiya Y, Morikawa M. Bone morphogenetic protein receptors and signal transduction. J Biochem. 2010;147:35-51.
- Kurokawa I, Kusumoto K, Bessho K. Immunohistochemical expression of bone morphogenetic protein-2 in pilomatricoma. Br J Dermatol. 2000;143:754-758.
- Myllylä RM, Haapasaari K-M, Lehenkari P, et al. Bone morphogenetic proteins 4 and 2/7 induce osteogenic differentiation of mouse skin derived fibroblast and dermal papilla cells. Cell Tissue Res. 2014;355:463-470.
- Myllylä RM, Haapasaari KM, Palatsi R, et al. Multiple miliary osteoma cutis is a distinct disease entity: four case reports and review of the literature. Br J Dermatol. 2011;164:544-552.
- Vickaryous MK, Sire J-Y. The integumentary skeleton of tetrapods: origin, evolution, and development. J Anat. 2009;214:441-464.
- Vickaryous MK, Hall BK. Development of the dermal skeleton in Alligator mississippiensis (Archosauria, Crocodylia) with comments on the homology of osteoderms. J Morphol. 2008;269:398-422.
The term osteoma cutis (OC) is defined as the ossification or bone formation either in the dermis or hypodermis. 1 It is heterotopic in nature, referring to extraneous bone formation in soft tissue. Osteoma cutis was first described in 1858 2,3 ; in 1868, the multiple miliary form on the face was described. 4 Cutaneous ossification can take many forms, ranging from occurrence in a nevus (nevus of Nanta) to its association with rare genetic disorders, such as fibrodysplasia ossificans progressiva and Albright hereditary osteodystrophy.
Some of these ossifications are classified as primary; others are secondary, depending on the presence of a preexisting lesion (eg, pilomatricoma, basal cell carcinoma). However, certain conditions, such as multiple miliary osteoma of the face, can be difficult to classify due to the presence or absence of a history of acne or dermabrasion, or both. The secondary forms more commonly are encountered due to their incidental association with an excised lesion, such as pilomatricoma.
A precursor of OC has been neglected in the literature despite its common occurrence. It may have been peripherally alluded to in the literature in reference to the miliary form of OC.5,6 The cases reported here demonstrate small round nodules of calcification or ossification, or both, in punch biopsies and excision specimens from hair-bearing areas of skin, especially from the head and neck. These lesions are mainly observed in the peripilar location or more specifically in the approximate location of the hair bulb.
This article reviews a possible mechanism of formation of these osteocalcific micronodules. These often-encountered micronodules are small osteocalcific lesions without typical bone or well-formed OC, such as trabeculae formation or fatty marrow, and may represent earliest stages in the formation of OC.
Clinical Observations
During routine dermatopathologic practice, I observed incidental small osteocalcific micronodules in close proximity to the lower part of the hair follicle in multiple cases. These nodules were not related to the main lesion in the specimen and were not the reason for the biopsy or excision. Most of the time, these micronodules were noted in excision or re-excision specimens or in a punch biopsy.
In my review of multiple unrelated cases over time, incidental osteocalcific micronodules were observed occasionally in punch biopsies and excision specimens during routine practice. These micronodules were mainly located in the vicinity of a hair bulb (Figure 1). If the hair bulb was not present in the sections, these micronodules were noted near or within the fibrous tract (Figure 2) or beneath a sebaceous lobule (Figure 3). In an exceptional case, a small round deposit of osteoid was seen forming just above the dermal papilla of the hair bulb (Figure 4).
Multiple osteocalcific micronodules were identified in a case of cicatricial alopecia. These micronodules were observed in sections taken at the levels of hair bulbs, and more or less corresponded to the size of the bulb (Figure 5A). Fortuitously, the patient was dark-skinned; the remnants of melanin within the micronodules provided evidence that the micronodules were formed within hair bulbs. Melanin staining confirmed the presence of melanin within some of the micronodules (Figure 5B).
Comment
Skeletogenesis in humans takes place by 2 methods: endochondral ossification and intramembranous ossification. In contrast to endochondral ossification, intramembranous ossification does not require a preexisting cartilaginous template. Instead, there is condensation of mesenchymal cells, which differentiate into osteoblasts and lay down osteoid, thus forming an ossification center. Little is known about the mechanism of formation of OC or the nidus of formation of the primary form.
Incidental micronodules of calcification and ossification are routinely encountered during histopathologic review of specimens from hair-bearing areas of the skin in dermatopathology practice. A review of the literature, however, does not reveal any specific dermatopathologic term ascribed to this phenomenon. These lesions might be similar to those described by Hopkins5 in 1928 in the setting of miliary OC of the face secondary to acne. Rossman and Freeman6 also described the same lesions when referring to facial OC as a “stage of pre-osseous calcification.”
When these osteocalcific micronodules are encountered, it usually is in close proximity to a hair follicle bulb. When a hair bulb is not seen in the sections, the micronodules are noted near fibrous tracts, arrector pili muscles, or sebaceous lobules, suggesting a close peripilar or peribulbar location. The micronodules are approximately 0.5 mm in diameter—roughly the size of a hair bulb. Due to the close anatomic association of micronodules and the hair bulb, these lesions can be called pilar osteocalcific nodules (PONs).
The role of bone morphogenetic protein (BMP) signaling in the maintenance of the hair cycle is well established. Bone morphogenetic proteins are extracellular cytokines that belong to the transforming growth factor β family. The hair bulb microenvironment is rich in BMPs
As the name implies, BMPs were discovered in relation to their important role in osteogenesis and tissue homeostasis. More than 20 BMPs have been identified, many of which promote bone formation and repair of bone fracture. Osteoinductive BMPs include BMP-2 and BMP-4 through BMP-10; BMP-2 and BMP-4 are expressed in the hair matrix and BMP-4 and BMP-6 are expressed in the FDP.8,9 All bone-inducing BMPs can cause mesenchymal stem cells to differentiate into osteoblasts in vitro.10
Overactive BMP signaling has been shown to cause heterotopic ossification in patients with fibrodysplasia ossificans progressiva.8 Immunohistochemical expression of BMP-2 has been demonstrated in shadow cells of pilomatricoma.11 Calcification and ossification are seen in as many as 20% of pilomatricomas. Both BMP-2 and BMP-4 have been shown to induce osteogenic differentiation of mouse skin−derived fibroblasts and FDP cells.12
Myllylä et al13 described 4 cases of multiple miliary osteoma cutis (MMOC). They also found 47 reported cases of MMOC, in which there was a history of acne in 55% (26/47). Only 15% (7/47) of these cases were extrafacial on the neck, chest, back, and arms. Osteomas in these cases were not associated with folliculosebaceous units or other adnexal structures, which may have been due to replacement by acne scarring, as all 4 patients had a history of acne vulgaris. The authors postulated a role for the GNAS gene mutation in the morphogenesis of MMOC; however, no supporting evidence was found for this claim. They also postulated a role for BMPs in the formation of MMOC.13
Some disturbance or imbalance in hair bulb homeostasis leads to overactivity of BMP signaling, causing osteoinduction in the hair bulb region and formation of PONs. The cause of the disturbance could be a traumatic or inflammatory injury to the hair follicle, as in the case of the secondary form of MMOC in association with chronic acne. In the primary form of osteoma cutis, the trigger could be more subtle or subclinical.
Trauma and inflammation are the main initiating factors involved in ossification in patients with fibrodysplasia ossificans progressiva due to ectopic activity of BMPs.9 The primary form of ossification appears to be similar to the mechanism by which intramembranous ossification is laid down (ie, by differentiation of mesenchymal cells into osteoblasts). In the proposed scenario, the cells of FDP, under the influence of BMPs, differentiate into osteoblasts and lay down osteoid, forming a limited-capacity “ossification center” or pilar osteocalcific nodule.
It is difficult to know the exact relationship of PONs or OC to the hair bulb due to the 2-dimensional nature of histologic sections. However, considering the finding of a rare case of osteoid forming within the bulb and in another the presence of melanin within the osteocalcific nodule, it is likely that these lesions are formed within the hair bulb or in situations in which the conditions replicate the biochemical characteristics of the hair bulb (eg, pilomatricoma).
The formation of PONs might act as a terminal phase in the hair cycle that is rarely induced to provide an exit for damaged hair follicles from cyclical perpetuity. An unspecified event or injury might render a hair follicle unable to continue its cyclical growth and cause BMPs to induce premature calcification in or around the hair bulb, which would probably be the only known quasiphysiological mechanism for a damaged hair follicle to exit the hair cycle.
Another interesting aspect of osteoma formation in human skin is the similarity to osteoderms or the integumentary skeleton of vertebrates.14 Early in evolution, the dermal skeleton was the predominant skeletal system in some lineages. Phylogenetically, osteoderms are not uniformly distributed, and show a latent ability to manifest in some groups or lay dormant or disappear in others. The occurrence of primary osteomas in the human integument might be a vestigial manifestation of deep homology,15 a latent ability to form structures that have been lost. The embryologic formation of osteoderms in the dermis of vertebrates is thought to depend on the interaction or cross-talk between ectomesenchymal cells of neural crest origin and cells of the stratum basalis of epidermis, which is somewhat similar to the formation of the hair follicles.
Conclusion
Under certain conditions, the bulb region of a hair follicle might provide a nidus for the formation of OC. The hair bulb region contains both the precursor cellular element (mesenchymal cells of FDP) and the trigger cytokine (BMP) for the induction of osteogenic metaplasia.
The term osteoma cutis (OC) is defined as the ossification or bone formation either in the dermis or hypodermis. 1 It is heterotopic in nature, referring to extraneous bone formation in soft tissue. Osteoma cutis was first described in 1858 2,3 ; in 1868, the multiple miliary form on the face was described. 4 Cutaneous ossification can take many forms, ranging from occurrence in a nevus (nevus of Nanta) to its association with rare genetic disorders, such as fibrodysplasia ossificans progressiva and Albright hereditary osteodystrophy.
Some of these ossifications are classified as primary; others are secondary, depending on the presence of a preexisting lesion (eg, pilomatricoma, basal cell carcinoma). However, certain conditions, such as multiple miliary osteoma of the face, can be difficult to classify due to the presence or absence of a history of acne or dermabrasion, or both. The secondary forms more commonly are encountered due to their incidental association with an excised lesion, such as pilomatricoma.
A precursor of OC has been neglected in the literature despite its common occurrence. It may have been peripherally alluded to in the literature in reference to the miliary form of OC.5,6 The cases reported here demonstrate small round nodules of calcification or ossification, or both, in punch biopsies and excision specimens from hair-bearing areas of skin, especially from the head and neck. These lesions are mainly observed in the peripilar location or more specifically in the approximate location of the hair bulb.
This article reviews a possible mechanism of formation of these osteocalcific micronodules. These often-encountered micronodules are small osteocalcific lesions without typical bone or well-formed OC, such as trabeculae formation or fatty marrow, and may represent earliest stages in the formation of OC.
Clinical Observations
During routine dermatopathologic practice, I observed incidental small osteocalcific micronodules in close proximity to the lower part of the hair follicle in multiple cases. These nodules were not related to the main lesion in the specimen and were not the reason for the biopsy or excision. Most of the time, these micronodules were noted in excision or re-excision specimens or in a punch biopsy.
In my review of multiple unrelated cases over time, incidental osteocalcific micronodules were observed occasionally in punch biopsies and excision specimens during routine practice. These micronodules were mainly located in the vicinity of a hair bulb (Figure 1). If the hair bulb was not present in the sections, these micronodules were noted near or within the fibrous tract (Figure 2) or beneath a sebaceous lobule (Figure 3). In an exceptional case, a small round deposit of osteoid was seen forming just above the dermal papilla of the hair bulb (Figure 4).
Multiple osteocalcific micronodules were identified in a case of cicatricial alopecia. These micronodules were observed in sections taken at the levels of hair bulbs, and more or less corresponded to the size of the bulb (Figure 5A). Fortuitously, the patient was dark-skinned; the remnants of melanin within the micronodules provided evidence that the micronodules were formed within hair bulbs. Melanin staining confirmed the presence of melanin within some of the micronodules (Figure 5B).
Comment
Skeletogenesis in humans takes place by 2 methods: endochondral ossification and intramembranous ossification. In contrast to endochondral ossification, intramembranous ossification does not require a preexisting cartilaginous template. Instead, there is condensation of mesenchymal cells, which differentiate into osteoblasts and lay down osteoid, thus forming an ossification center. Little is known about the mechanism of formation of OC or the nidus of formation of the primary form.
Incidental micronodules of calcification and ossification are routinely encountered during histopathologic review of specimens from hair-bearing areas of the skin in dermatopathology practice. A review of the literature, however, does not reveal any specific dermatopathologic term ascribed to this phenomenon. These lesions might be similar to those described by Hopkins5 in 1928 in the setting of miliary OC of the face secondary to acne. Rossman and Freeman6 also described the same lesions when referring to facial OC as a “stage of pre-osseous calcification.”
When these osteocalcific micronodules are encountered, it usually is in close proximity to a hair follicle bulb. When a hair bulb is not seen in the sections, the micronodules are noted near fibrous tracts, arrector pili muscles, or sebaceous lobules, suggesting a close peripilar or peribulbar location. The micronodules are approximately 0.5 mm in diameter—roughly the size of a hair bulb. Due to the close anatomic association of micronodules and the hair bulb, these lesions can be called pilar osteocalcific nodules (PONs).
The role of bone morphogenetic protein (BMP) signaling in the maintenance of the hair cycle is well established. Bone morphogenetic proteins are extracellular cytokines that belong to the transforming growth factor β family. The hair bulb microenvironment is rich in BMPs
As the name implies, BMPs were discovered in relation to their important role in osteogenesis and tissue homeostasis. More than 20 BMPs have been identified, many of which promote bone formation and repair of bone fracture. Osteoinductive BMPs include BMP-2 and BMP-4 through BMP-10; BMP-2 and BMP-4 are expressed in the hair matrix and BMP-4 and BMP-6 are expressed in the FDP.8,9 All bone-inducing BMPs can cause mesenchymal stem cells to differentiate into osteoblasts in vitro.10
Overactive BMP signaling has been shown to cause heterotopic ossification in patients with fibrodysplasia ossificans progressiva.8 Immunohistochemical expression of BMP-2 has been demonstrated in shadow cells of pilomatricoma.11 Calcification and ossification are seen in as many as 20% of pilomatricomas. Both BMP-2 and BMP-4 have been shown to induce osteogenic differentiation of mouse skin−derived fibroblasts and FDP cells.12
Myllylä et al13 described 4 cases of multiple miliary osteoma cutis (MMOC). They also found 47 reported cases of MMOC, in which there was a history of acne in 55% (26/47). Only 15% (7/47) of these cases were extrafacial on the neck, chest, back, and arms. Osteomas in these cases were not associated with folliculosebaceous units or other adnexal structures, which may have been due to replacement by acne scarring, as all 4 patients had a history of acne vulgaris. The authors postulated a role for the GNAS gene mutation in the morphogenesis of MMOC; however, no supporting evidence was found for this claim. They also postulated a role for BMPs in the formation of MMOC.13
Some disturbance or imbalance in hair bulb homeostasis leads to overactivity of BMP signaling, causing osteoinduction in the hair bulb region and formation of PONs. The cause of the disturbance could be a traumatic or inflammatory injury to the hair follicle, as in the case of the secondary form of MMOC in association with chronic acne. In the primary form of osteoma cutis, the trigger could be more subtle or subclinical.
Trauma and inflammation are the main initiating factors involved in ossification in patients with fibrodysplasia ossificans progressiva due to ectopic activity of BMPs.9 The primary form of ossification appears to be similar to the mechanism by which intramembranous ossification is laid down (ie, by differentiation of mesenchymal cells into osteoblasts). In the proposed scenario, the cells of FDP, under the influence of BMPs, differentiate into osteoblasts and lay down osteoid, forming a limited-capacity “ossification center” or pilar osteocalcific nodule.
It is difficult to know the exact relationship of PONs or OC to the hair bulb due to the 2-dimensional nature of histologic sections. However, considering the finding of a rare case of osteoid forming within the bulb and in another the presence of melanin within the osteocalcific nodule, it is likely that these lesions are formed within the hair bulb or in situations in which the conditions replicate the biochemical characteristics of the hair bulb (eg, pilomatricoma).
The formation of PONs might act as a terminal phase in the hair cycle that is rarely induced to provide an exit for damaged hair follicles from cyclical perpetuity. An unspecified event or injury might render a hair follicle unable to continue its cyclical growth and cause BMPs to induce premature calcification in or around the hair bulb, which would probably be the only known quasiphysiological mechanism for a damaged hair follicle to exit the hair cycle.
Another interesting aspect of osteoma formation in human skin is the similarity to osteoderms or the integumentary skeleton of vertebrates.14 Early in evolution, the dermal skeleton was the predominant skeletal system in some lineages. Phylogenetically, osteoderms are not uniformly distributed, and show a latent ability to manifest in some groups or lay dormant or disappear in others. The occurrence of primary osteomas in the human integument might be a vestigial manifestation of deep homology,15 a latent ability to form structures that have been lost. The embryologic formation of osteoderms in the dermis of vertebrates is thought to depend on the interaction or cross-talk between ectomesenchymal cells of neural crest origin and cells of the stratum basalis of epidermis, which is somewhat similar to the formation of the hair follicles.
Conclusion
Under certain conditions, the bulb region of a hair follicle might provide a nidus for the formation of OC. The hair bulb region contains both the precursor cellular element (mesenchymal cells of FDP) and the trigger cytokine (BMP) for the induction of osteogenic metaplasia.
- Burgdorf W, Nasemann T. Cutaneous osteomas: a clinical and histopathologic review. Arch Dermatol Res. 1977;260:121-135.
- Essing M. Osteoma cutis of the forehead. HNO. 1985;33:548-550.
- Bouraoui S, Mlika M, Kort R, et al. Miliary osteoma cutis of the face. J Dermatol Case Rep. 2011;5:77-81.
- Virchow R. Die krankhaften Geschwülste. Vol 2. Hirschwald; 1864.
- Hopkins JG. Multiple miliary osteomas of the skin: report of a case. Arch Derm Syphilol. 1928;18:706-715.
- Rossman RE, Freeman RG. Osteoma cutis, a stage of preosseous calcification. Arch Dermatol. 1964;89:68-73.
- Guha U, Mecklenburg L, Cowin P, et al. Bone morphogenetic protein signaling regulates postnatal hair follicle differentiation and cycling. Am J Pathol. 2004;165:729-740.
- Rendl M, Polak L, Fuchs E. BMP signaling in dermal papilla cells is required for their hair follicle-inductive properties. Genes Dev. 2008;22:543-557.
- Shi S, de Gorter DJJ, Hoogaars WMH, et al. Overactive bone morphogenetic protein signaling in heterotopic ossification and Duchenne muscular dystrophy. Cell Mol Life Sci. 2013;70:407-423.
- Miyazono K, Kamiya Y, Morikawa M. Bone morphogenetic protein receptors and signal transduction. J Biochem. 2010;147:35-51.
- Kurokawa I, Kusumoto K, Bessho K. Immunohistochemical expression of bone morphogenetic protein-2 in pilomatricoma. Br J Dermatol. 2000;143:754-758.
- Myllylä RM, Haapasaari K-M, Lehenkari P, et al. Bone morphogenetic proteins 4 and 2/7 induce osteogenic differentiation of mouse skin derived fibroblast and dermal papilla cells. Cell Tissue Res. 2014;355:463-470.
- Myllylä RM, Haapasaari KM, Palatsi R, et al. Multiple miliary osteoma cutis is a distinct disease entity: four case reports and review of the literature. Br J Dermatol. 2011;164:544-552.
- Vickaryous MK, Sire J-Y. The integumentary skeleton of tetrapods: origin, evolution, and development. J Anat. 2009;214:441-464.
- Vickaryous MK, Hall BK. Development of the dermal skeleton in Alligator mississippiensis (Archosauria, Crocodylia) with comments on the homology of osteoderms. J Morphol. 2008;269:398-422.
- Burgdorf W, Nasemann T. Cutaneous osteomas: a clinical and histopathologic review. Arch Dermatol Res. 1977;260:121-135.
- Essing M. Osteoma cutis of the forehead. HNO. 1985;33:548-550.
- Bouraoui S, Mlika M, Kort R, et al. Miliary osteoma cutis of the face. J Dermatol Case Rep. 2011;5:77-81.
- Virchow R. Die krankhaften Geschwülste. Vol 2. Hirschwald; 1864.
- Hopkins JG. Multiple miliary osteomas of the skin: report of a case. Arch Derm Syphilol. 1928;18:706-715.
- Rossman RE, Freeman RG. Osteoma cutis, a stage of preosseous calcification. Arch Dermatol. 1964;89:68-73.
- Guha U, Mecklenburg L, Cowin P, et al. Bone morphogenetic protein signaling regulates postnatal hair follicle differentiation and cycling. Am J Pathol. 2004;165:729-740.
- Rendl M, Polak L, Fuchs E. BMP signaling in dermal papilla cells is required for their hair follicle-inductive properties. Genes Dev. 2008;22:543-557.
- Shi S, de Gorter DJJ, Hoogaars WMH, et al. Overactive bone morphogenetic protein signaling in heterotopic ossification and Duchenne muscular dystrophy. Cell Mol Life Sci. 2013;70:407-423.
- Miyazono K, Kamiya Y, Morikawa M. Bone morphogenetic protein receptors and signal transduction. J Biochem. 2010;147:35-51.
- Kurokawa I, Kusumoto K, Bessho K. Immunohistochemical expression of bone morphogenetic protein-2 in pilomatricoma. Br J Dermatol. 2000;143:754-758.
- Myllylä RM, Haapasaari K-M, Lehenkari P, et al. Bone morphogenetic proteins 4 and 2/7 induce osteogenic differentiation of mouse skin derived fibroblast and dermal papilla cells. Cell Tissue Res. 2014;355:463-470.
- Myllylä RM, Haapasaari KM, Palatsi R, et al. Multiple miliary osteoma cutis is a distinct disease entity: four case reports and review of the literature. Br J Dermatol. 2011;164:544-552.
- Vickaryous MK, Sire J-Y. The integumentary skeleton of tetrapods: origin, evolution, and development. J Anat. 2009;214:441-464.
- Vickaryous MK, Hall BK. Development of the dermal skeleton in Alligator mississippiensis (Archosauria, Crocodylia) with comments on the homology of osteoderms. J Morphol. 2008;269:398-422.
Practice Points
- Understanding the pathogenesis of osteoma cutis (OC) can help physicians devise management of these disfiguring lesions.
- Small osteocalcific nodules in close proximity to the lower aspect of the hair bulb may be an important precursor to OC.
Cutaneous Manifestations of COVID-19
The pathogenesis of coronavirus disease 2019 (COVID-19), the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), is not yet completely understood. Thus far, it is known to affect multiple organ systems, including gastrointestinal, neurological, and cardiovascular, with typical clinical symptoms of COVID-19 including fever, cough, myalgia, headache, anosmia, and diarrhea.1 This multiorgan attack may be secondary to an exaggerated inflammatory reaction with vasculopathy and possibly a hypercoagulable state. Skin manifestations also are prevalent in COVID-19, and they often result in polymorphous presentations.2 This article aims to summarize cutaneous clinical signs of COVID-19 so that dermatologists can promptly identify and manage COVID-19 and prevent its spread.
Methods
A PubMed search of articles indexed for MEDLINE was conducted on June 30, 2020. The literature included observational studies, case reports, and literature reviews from January 1, 2020, to June 30, 2020. Search terms included COVID-19, SARS-CoV-2, and coronavirus used in combination with cutaneous, skin, and dermatology. All of the resulting articles were then reviewed for relevance to the cutaneous manifestations of COVID-19. Only confirmed cases of COVID-19 infection were included in this review; suspected unconfirmed cases were excluded. Further exclusion criteria included articles that discussed dermatology in the time of COVID-19 that did not explicitly address its cutaneous manifestations. The remaining literature was evaluated to provide dermatologists and patients with a concise resource for the cutaneous signs and symptoms of COVID-19. Data extracted from the literature included geographic region, number of patients with skin findings, status of COVID-19 infection and timeline, and cutaneous signs. If a cutaneous sign was not given a clear diagnosis in the literature, the senior authors (A.L. and J.J.) assigned it to its most similar classification to aid in ease of understanding and clarity for the readers.
Results
A search of the key terms resulted in 75 articles published in the specified date range. After excluding overtly irrelevant articles and dermatologic conditions in the time of COVID-19 without confirmed SARS-CoV-2 infection, 25 articles ultimately met inclusion criteria. Relevant references from the articles also were explored for cutaneous dermatologic manifestations of COVID-19. Cutaneous manifestations that were repeatedly reported included chilblainlike lesions; acrocyanosis; urticaria; pityriasis rosea–like cutaneous eruption; erythema multiforme–like, vesiculopapular, and morbilliform eruptions; petechiae; livedo reticularis; and purpuric livedo reticularis (dermatologists may label this stellate purpura). Fewer but nonetheless notable cases of androgenic alopecia, periorbital dyschromia, and herpes zoster exacerbations also were documented. The Table summarizes the reported integumentary findings. The eTable groups the common findings and describes patient age, time to onset of cutaneous sign, and any prognostic significance as seen in the literature.
Chilblainlike Lesions and Acrocyanosis
Chilblainlike lesions are edematous eruptions of the fingers and toes. They usually do not scar and are described as erythematous to violaceous papules and macules with possible bullae on the digits. Skin biopsies demonstrate a histopathologic pattern of vacuolar interface dermatitis with necrotic keratinocytes and a thickened basement membrane. Lymphocytic infiltrate presents in a perieccrine distribution, occasionally with plasma cells. The dermatopathologic findings mimic those of chilblain lupus but lack dermal edema.3
These eruptions have been reported in cases of COVID-19 that more frequently affect children and young adults. They usually resolve over the course of viral infection, averaging within 14 days. Chilblainlike eruptions often are associated with pruritus or pain. They commonly are asymmetrical and appear more often on the toes than the fingers.4 In cases of COVID-19 that lack systemic symptoms, chilblainlike lesions have been seen on the dorsal fingers as the first presenting sign of infection.5
Acral erythema and chilblainlike lesions frequently have been associated with milder infection. Another positive prognostic indicator is the manifestation of these signs in younger individuals.3
Morbilliform Exanthem
The morbilliform exanthem associated with COVID-19 also typically presents in patients with milder disease. It often affects the buttocks, lower abdomen, and thighs, but spares the palms, soles, and mucosae.4 This skin sign, which may start out as a generalized morbilliform exanthem, has been seen to morph into macular hemorrhagic purpura on the legs. These cutaneous lesions typically spontaneously resolve.8
In a case report by Najarian,6 a morbilliform exanthem was seen on the legs, arms, and trunk of a patient who was otherwise asymptomatic but tested positive for COVID-19. The morbilliform exanthem then became confluent on the trunk. Notably, the patient reported pain of the hands and feet.6
Another case report described a patient with edematous annular plaques on the palms, neck, and upper extremities who presented solely with fever.7 The biopsy specimen was nonspecific but indicated a viral exanthem. Histopathology showed perivascular lymphocytic infiltrate, dermal edema and vacuoles, spongiosis, dyskeratotic basilar keratinocytes, and few neutrophils without eosinophils.7
Eczematous Eruption
A confluent eczematous eruption in the flexural areas, the antecubital fossae, and axillary folds has been found in COVID-19 patients.21,22 An elderly patient with severe COVID-19 developed a squamous erythematous periumbilical patch 1 day after hospital admission. The cutaneous eruption rapidly progressed to digitate scaly plaques on the trunk, thighs, and flank. A biopsy specimen showed epidermal spongiosis, vesicles containing lymphocytes, and Langerhans cells. The upper dermis demonstrated a lymphohistiocytic infiltrate.23
Pityriasis Rosea–Like Eruption
In Iran, a COVID-19–infected patient developed an erythematous papulosquamous eruption with a herald patch and trailing scales 3 days after viral symptoms, resembling that of pityriasis rosea.24 Nests of Langerhans cells within the epidermis are seen in many viral exanthems, including cases of COVID-19 and pityriasis rosea.25
Urticaria
According to a number of case reports, urticarial lesions have been the first presenting sign of COVID-19 infection, most resolving with antihistamines.10,11 Some patients with more severe symptoms have had widespread urticaria. An urticarial exanthem appearing on the bilateral thighs and buttocks may be the initial sign of infection.12,15 Pruritic erythematous plaques over the face and acral areas is another initial sign. Interestingly, pediatric patients have reported nonpruritic urticaria.9
Urticaria also has been seen as a late dermatologic sign of viral infection. After battling relentless viral infection for 1 month, a pruritic, confluent, ill-defined eruption appeared along a patient’s trunk, back, and proximal extremities. Histopathologic examination concluded a perivascular lymphocytic infiltrate and dilated vessels in the dermis. The urticaria resolved a week later, and the patient’s nasopharyngeal swab finally came back negative.13
Vesiculopapular Eruption
Vesicles mimicking those of chickenpox have been reported. A study of 375 confirmed cases of COVID-19 by Galván Casas et al12 showed a 9% incidence of this vesicular eruption. A study by Sachdeva et al8 revealed vesicular eruptions in 25 of 72 patients. Pruritic papules and vesicles may resemble Grover disease. This cutaneous sign may be seen in the submammary folds, on the hips, or diffusely over the body.
Erythema Multiforme–Like Eruption
Targetoid lesions similar to those of erythema multiforme erupted in 2 of 27 patients with mild COVID-19 infection in a review by Wollina et al.4 In a study of 4 patients with erythema multiforme–like eruptions after COVID-19 symptoms resolved, 3 had palatal petechiae. Two of 4 patients had pseudovesicles in the center of the erythematous targetoid patches.26 Targetoid lesions on the extremities have been reported in pediatric patients with COVID-19 infections. These patients often present without any typical viral symptoms but rather just a febrile exanthem or exanthem alone. Thus, to minimize spread of the virus, it is vital to recognize COVID-19 infection early in patients with a viral exanthem during the time of high COVID-19 incidence.4
Livedo Reticularis
In the United States, a case series reported 2 patients with transient livedo reticularis throughout the course of COVID-19 infection. The cutaneous eruption resembled erythema ab igne, but there was no history of exposure to heat.16
Stellate Purpura
In severe COVID-19 infection, a reticulated nonblanching purpura on the buttocks has been reported to demonstrate pauci-inflammatory vascular thrombosis, complement membrane attack complex deposition, and endothelial injury on dermatopathology. Stellate purpura on palmoplantar surfaces also has shown arterial thrombosis in the deep dermis due to complement deposition.17
Petechiae and Purpura
A morbilliform exanthem may develop into significant petechiae in the popliteal fossae, buttocks, and thighs. A punch biopsy specimen demonstrates a perivascular lymphocytic infiltrate with erythrocyte extravasation and papillary dermal edema with dyskeratotic cells.18 Purpura of the lower extremities may develop, with histopathology showing fibrinoid necrosis of small vessel walls, neutrophilic infiltrate with karyorrhexis, and granular complement deposition.19
In Thailand, a patient was misdiagnosed with dengue after presenting with petechiae and low platelet count.20 Further progression of the viral illness resulted in respiratory symptoms. Subsequently, the patient tested positive for COVID-19. This case demonstrates that cutaneous signs of many sorts may be the first presenting signs of COVID-19, even prior to febrile symptoms.20
Androgenic Alopecia
Studies have shown that androgens are related in the pathogenesis of COVID-19. Coronavirus disease 2019 uses a cellular co-receptor, TMPRSS2, which is androgen regulated.27 In a study of 41 males with COVID-19, 29 had androgenic alopecia. However, this is only a correlation, and causation cannot be concluded here. It cannot be determined from this study whether androgenic alopecia is a risk factor, result of COVID-19, or confounder.28
Exaggerated Herpes Zoster
Shors29 reported a herpes zoster eruption in a patient who had symptoms of COVID-19 for 1 week. Further testing confirmed COVID-19 infection, and despite prompt treatment with valacyclovir, the eruption was slow to resolve. The patient then experienced severe postherpetic neuralgia for more than 4 weeks, even with treatment with gabapentin and lidocaine. It is hypothesized that because of the major inflammatory response caused by COVID-19, an exaggerated inflammation occurred in the dorsal root ganglion, resulting in relentless herpes zoster infection.29
Mottled Skin
Born at term, a 15-day-old neonate presented with sepsis and mottling of the skin. The patient did not have any typical COVID-19 symptoms, such as diarrhea or cough, but tested positive for COVID-19.30
Periorbital Dyschromia
Kalner and Vergilis31 reported 2 cases of periorbital dyschromia prior to any other COVID-19 infection symptoms. The discoloration improved with resolution of ensuing viral symptoms.31
Comment
Many dermatologic signs of COVID-19 have been identified. Their individual frequency and association with viral severity will become more apparent as more cases are reported. So far during this pandemic, common dermatologic manifestations have been polymorphic in clinical presentation.
Onset of Skin Manifestations
The timeline of skin signs and COVID-19 symptoms varies from the first reported sign to weeks after symptom resolution. In the Region of Murcia, Spain, Pérez-Suárez et al14 collected data on cutaneous signs of patients with COVID-19. Of the patients studied, 9 had tests confirming COVID-19 infection. Truncal urticaria, sacral ulcers, acrocyanosis, and erythema multiforme were all reported in patients more than 2 weeks after symptom onset. One case of tinea infection also was reported 4 days after fever and respiratory symptoms began.14
Presentation
Coronavirus disease 2019 has affected the skin of both the central thorax and peripheral locations. In a study of 72 patients with cutaneous signs of COVID-19 by Sachdeva et al,8 a truncal distribution was most common, but 14 patients reported acral site involvement. Sachdeva et al8 reported urticarial reactions in 7 of 72 patients with cutaneous signs. A painful acral cyanosis was seen in 11 of 72 patients. Livedo reticularis presented in 2 patients, and only 1 patient had petechiae. Cutaneous signs were the first indicators of viral infection in 9 of 72 patients; 52 patients presented with respiratory symptoms first. All of the reported cutaneous signs spontaneously resolved within 10 days.8
Recalcati32 reviewed 88 patients with COVID-19, and 18 had cutaneous signs at initial onset of viral infection or during hospitalization. The most common integumentary sign reported in this study was erythema, followed by diffuse urticaria, and then a vesicular eruption resembling varicella infection.32
Some less common phenomena have been identified in patients with COVID-19, including androgenic alopecia, exaggerated herpes zoster and postherpetic neuralgia, mottled skin, and periorbital dyschromia. Being aware of these complications may help in early treatment, diagnosis, and even prevention of viral spread.
Pathogenesis of Skin Manifestations
Few breakthroughs have been made in understanding the pathogenesis of skin manifestations of SARS-CoV-2. Acral ischemia may be a manifestation of COVID-19’s association with hypercoagulation. Increasing fibrinogen and prothrombin times lead to disseminated intravascular coagulation and microthrombi. These tiny blood clots then lodge in blood vessels and cause acral cyanosis and subsequent gangrene.2 The proposed mechanism behind this clinical manifestation in younger populations is the hypercoagulable state that COVID-19 creates. Conversely, acral erythema and chilblainlike lesions in older patients are thought to be from acral ischemia as a response to insufficient type 1 interferons. This pathophysiologic mechanism is indicative of a worse prognosis due to the large role that type 1 interferons play in antiviral responses. Coronavirus disease 2019 similarly triggers type 1 interferons; thus, their efficacy positively correlates with good disease prognosis.3
Similarly, the pathogenesis for livedo reticularis in patients with COVID-19 can only be hypothesized. Infected patients are in a hypercoagulable state, and in these cases, it was uncertain whether this was due to a disseminated intravascular coagulation, cold agglutinins, cryofibrinogens, or lupus anticoagulant.16
Nonetheless, it can be difficult to separate the primary event between vasculopathy or vasculitis in larger vessel pathology specimens. Some of the studies’ pathology reports discuss a granulocytic infiltrate and red blood cell extravasation, which represent small vessel vasculitis. However, the gangrene and necrosing livedo represent vasculopathy events. A final conclusion about the pathogenesis cannot be made without further clinical and histopathologic evaluation.
Histopathology
Biopsy specimens of reported morbilliform eruptions have demonstrated thrombosed vessels with evidence of necrosis and granulocytic infiltrate.25 Another biopsy specimen of a widespread erythematous exanthem demonstrated extravasated red blood cells and vessel wall damage similar to thrombophilic arteritis. Other reports of histopathology showed necrotic keratinocytes and lymphocytic satellitosis at the dermoepidermal junction, resembling Grover disease. These cases demonstrating necrosis suggest a strong cytokine reaction from the virus.25 A concern with these biopsy findings is that morbilliform eruptions generally show dilated vessels with lymphocytes, and these biopsy findings are consistent with a cutaneous small vessel vasculitis. Additionally, histopathologic evaluation of purpuric eruptions has shown erythrocyte extravasation and granulocytic infiltrate indicative of a cutaneous small vessel vasculitis.
Although most reported cases of cutaneous signs of COVID-19 do not have histopathologic reports, Yao et al33 conducted a dermatopathologic study that investigated the tissue in deceased patients who had COVID-19. This pathology showed hyaline thrombi within the small vessels of the skin, likely leading to the painful acral ischemia. Similarly, Yao et al33 reported autopsies finding hyaline thrombi within the small vessels of the lungs. More research should be done to explore this pathogenesis as part of prognostic factors and virulence.
Conclusion
Cutaneous signs may be the first reported symptom of COVID-19 infection, and dermatologists should be prepared to identify them. This review may be used as a guide for physicians to quickly identify potential infection as well as further understand the pathogenesis related to COVID-19. Future research is necessary to determine the dermatologic pathogenesis, infectivity, and prevalence of cutaneous manifestations of COVID-19. It also will be important to explore if vasculopathic lesions predict more severe multisystem disease.
- Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497-506.
- Criado PR, Abdalla BMZ, de Assis IC, et al. Are the cutaneous manifestations during or due to SARS-CoV-2 infection/COVID-19 frequent or not? revision of possible pathophysiologic mechanisms. Inflamm Res. 2020;69:745-756.
- Kolivras A, Dehavay F, Delplace D, et al. Coronavirus (COVID‐19) infection–induced chilblains: a case report with histopathological findings. JAAD Case Rep. 2020;6:489-492.
- Wollina U, Karadag˘ AS, Rowland-Payne C, et al. Cutaneous signs in COVID-19 patients: a review [published online May 10, 2020]. Dermatol Ther. 2020;33:E13549.
- Alramthan A, Aldaraji W. Two cases of COVID-19 presenting with a clinical picture resembling chilblains: first report from the Middle East. Clin Exp Dermatol. 2020;45:746-748.
- Najarian DJ. Morbilliform exanthem associated with COVID‐19. JAAD Case Rep. 2020;6:493-494.
- Amatore F, Macagno N, Mailhe M, et al. SARS-CoV-2 infection presenting as a febrile rash. J Eur Acad Dermatol Venereol. 2020;34:E304-E306.
- Sachdeva M, Gianotti R, Shah M, et al. Cutaneous manifestations of COVID-19: report of three cases and a review of literature. J Dermatol Sci. 2020;98:75-81.
- Morey-Olivé M, Espiau M, Mercadal-Hally M, et al. Cutaneous manifestations in the current pandemic of coronavirus infection disease (COVID 2019). An Pediatr (Engl Ed). 2020;92:374-375.
- van Damme C, Berlingin E, Saussez S, et al. Acute urticaria with pyrexia as the first manifestations of a COVID‐19 infection. J Eur Acad Dermatol Venereol. 2020;34:E300-E301.
- Henry D, Ackerman M, Sancelme E, et al. Urticarial eruption in COVID‐19 infection. J Eur Acad Dermatol Venereol. 2020;34:E244-E245.
- Galván Casas C, Català A, Carretero Hernández G, et al. Classification of the cutaneous manifestations of COVID-19: a rapid prospective nationwide consensus study in Spain with 375 cases. Br J Dermatol. 2020;183:71-77.
- Zengarini C, Orioni G, Cascavilla A, et al. Histological pattern in Covid-19-induced viral rash [published online May 2, 2020]. J Eur Acad Dermatol Venereol. doi:10.1111/jdv.16569.
- Pérez-Suárez B, Martínez-Menchón T, Cutillas-Marco E. Skin findings in the COVID-19 pandemic in the Region of Murcia [published online June 12, 2020]. Med Clin (Engl Ed). 2020;155:41-42.
- Quintana-Castanedo L, Feito-Rodríguez M, Valero-López I, et al. Urticarial exanthem as early diagnostic clue for COVID-19 infection [published online April 29, 2020]. JAAD Case Rep. 2020;6:498-499.
- Manalo IF, Smith MK, Cheeley J, et al. Reply to: “reply: a dermatologic manifestation of COVID-19: transient livedo reticularis” [published online May 7, 2020]. J Am Acad Dermatol. 2020;83:E157.
- Magro C, Mulvey JJ, Berlin D, et al. Complement associated microvascular injury and thrombosis in the pathogenesis of severe COVID-19 infection: a report of five cases. Transl Res. 2020;220:1-13.
- Diaz-Guimaraens B, Dominguez-Santas M, Suarez-Valle A, et al. Petechial skin rash associated with severe acute respiratory syndrome coronavirus 2 infection. JAMA Dermatol. 2020;156:820-822.
- Dominguez-Santas M, Diaz-Guimaraens B, Garcia Abellas P, et al. Cutaneous small-vessel vasculitis associated with novel 2019 coronavirus SARS-CoV-2 infection (COVID-19) [published online July 2, 2020]. J Eur Acad Dermatol Venereol. 2020;34:E536-E537.
- Joob B, Wiwanitkit V. COVID-19 can present with a rash and be mistaken for dengue [published online March 22, 2020]. J Am Acad Dermatol. 2020;82:E177.
- Avellana Moreno R, Estella Villa LM, Avellana Moreno V, et al. Cutaneous manifestation of COVID‐19 in images: a case report [published online May 19, 2020]. J Eur Acad Dermatol Venereol. 2020;34:E307-E309.
- Mahé A, Birckel E, Krieger S, et al. A distinctive skin rash associated with coronavirus disease 2019 [published online June 8, 2020]? J Eur Acad Dermatol Venereol. 2020;34:E246-E247.
- Sanchez A, Sohier P, Benghanem S, et al. Digitate papulosquamous eruption associated with severe acute respiratory syndrome coronavirus 2 infection. JAMA Dermatol. 2020;156:819-820.
- Ehsani AH, Nasimi M, Bigdelo Z. Pityriasis rosea as a cutaneous manifestation of COVID‐19 infection [published online May 2, 2020]. J Eur Acad Dermatol Venereol. doi:10.1111/jdv.16579.
- Gianotti R, Veraldi S, Recalcati S, et al. Cutaneous clinico-pathological findings in three COVID-19-positive patients observed in the metropolitan area of Milan, Italy. Acta Derm Venereol. 2020;100:adv00124.
- Jimenez-Cauhe J, Ortega-Quijano D, Carretero-Barrio I, et al. Erythema multiforme-like eruption in patients with COVID-19 infection: clinical and histological findings [published online May 9, 2020]. Clin Exp Dermatol. doi:10.1111/ced.14281
- Hoffmann M, Kleine‐Weber H, Schroeder S, et al. SARS‐CoV‐2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor [published online March 5, 2020]. Cell. 2020;181:271‐280.e8.
- Goren A, Vaño‐Galván S, Wambier CG, et al. A preliminary observation: male pattern hair loss among hospitalized COVID‐19 patients in Spain—a potential clue to the role of androgens in COVID‐19 severity [published online April 23, 2020]. J Cosmet Dermatol. 2020;19:1545-1547.
- Shors AR. Herpes zoster and severe acute herpetic neuralgia as a complication of COVID-19 infection. JAAD Case Rep. 2020;6:656-657.
- Kamali Aghdam M, Jafari N, Eftekhari K. Novel coronavirus in a 15‐day‐old neonate with clinical signs of sepsis, a case report. Infect Dis (London). 2020;52:427‐429.
- Kalner S, Vergilis IJ. Periorbital erythema as a presenting sign of covid-19 [published online May 11, 2020]. JAAD Case Rep. 2020;6:996-998.
- Recalcati S. Cutaneous manifestations in COVID‐19: a first perspective. J Eur Acad Dermatol Venereol. 2020;34:E212-E213.
- Yao XH, Li TY, He ZC, et al. A pathological report of three COVID‐19 cases by minimally invasive autopsies [in Chinese]. Zhonghua Bing Li Xue Za Zhi. 2020;49:411-417.
The pathogenesis of coronavirus disease 2019 (COVID-19), the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), is not yet completely understood. Thus far, it is known to affect multiple organ systems, including gastrointestinal, neurological, and cardiovascular, with typical clinical symptoms of COVID-19 including fever, cough, myalgia, headache, anosmia, and diarrhea.1 This multiorgan attack may be secondary to an exaggerated inflammatory reaction with vasculopathy and possibly a hypercoagulable state. Skin manifestations also are prevalent in COVID-19, and they often result in polymorphous presentations.2 This article aims to summarize cutaneous clinical signs of COVID-19 so that dermatologists can promptly identify and manage COVID-19 and prevent its spread.
Methods
A PubMed search of articles indexed for MEDLINE was conducted on June 30, 2020. The literature included observational studies, case reports, and literature reviews from January 1, 2020, to June 30, 2020. Search terms included COVID-19, SARS-CoV-2, and coronavirus used in combination with cutaneous, skin, and dermatology. All of the resulting articles were then reviewed for relevance to the cutaneous manifestations of COVID-19. Only confirmed cases of COVID-19 infection were included in this review; suspected unconfirmed cases were excluded. Further exclusion criteria included articles that discussed dermatology in the time of COVID-19 that did not explicitly address its cutaneous manifestations. The remaining literature was evaluated to provide dermatologists and patients with a concise resource for the cutaneous signs and symptoms of COVID-19. Data extracted from the literature included geographic region, number of patients with skin findings, status of COVID-19 infection and timeline, and cutaneous signs. If a cutaneous sign was not given a clear diagnosis in the literature, the senior authors (A.L. and J.J.) assigned it to its most similar classification to aid in ease of understanding and clarity for the readers.
Results
A search of the key terms resulted in 75 articles published in the specified date range. After excluding overtly irrelevant articles and dermatologic conditions in the time of COVID-19 without confirmed SARS-CoV-2 infection, 25 articles ultimately met inclusion criteria. Relevant references from the articles also were explored for cutaneous dermatologic manifestations of COVID-19. Cutaneous manifestations that were repeatedly reported included chilblainlike lesions; acrocyanosis; urticaria; pityriasis rosea–like cutaneous eruption; erythema multiforme–like, vesiculopapular, and morbilliform eruptions; petechiae; livedo reticularis; and purpuric livedo reticularis (dermatologists may label this stellate purpura). Fewer but nonetheless notable cases of androgenic alopecia, periorbital dyschromia, and herpes zoster exacerbations also were documented. The Table summarizes the reported integumentary findings. The eTable groups the common findings and describes patient age, time to onset of cutaneous sign, and any prognostic significance as seen in the literature.
Chilblainlike Lesions and Acrocyanosis
Chilblainlike lesions are edematous eruptions of the fingers and toes. They usually do not scar and are described as erythematous to violaceous papules and macules with possible bullae on the digits. Skin biopsies demonstrate a histopathologic pattern of vacuolar interface dermatitis with necrotic keratinocytes and a thickened basement membrane. Lymphocytic infiltrate presents in a perieccrine distribution, occasionally with plasma cells. The dermatopathologic findings mimic those of chilblain lupus but lack dermal edema.3
These eruptions have been reported in cases of COVID-19 that more frequently affect children and young adults. They usually resolve over the course of viral infection, averaging within 14 days. Chilblainlike eruptions often are associated with pruritus or pain. They commonly are asymmetrical and appear more often on the toes than the fingers.4 In cases of COVID-19 that lack systemic symptoms, chilblainlike lesions have been seen on the dorsal fingers as the first presenting sign of infection.5
Acral erythema and chilblainlike lesions frequently have been associated with milder infection. Another positive prognostic indicator is the manifestation of these signs in younger individuals.3
Morbilliform Exanthem
The morbilliform exanthem associated with COVID-19 also typically presents in patients with milder disease. It often affects the buttocks, lower abdomen, and thighs, but spares the palms, soles, and mucosae.4 This skin sign, which may start out as a generalized morbilliform exanthem, has been seen to morph into macular hemorrhagic purpura on the legs. These cutaneous lesions typically spontaneously resolve.8
In a case report by Najarian,6 a morbilliform exanthem was seen on the legs, arms, and trunk of a patient who was otherwise asymptomatic but tested positive for COVID-19. The morbilliform exanthem then became confluent on the trunk. Notably, the patient reported pain of the hands and feet.6
Another case report described a patient with edematous annular plaques on the palms, neck, and upper extremities who presented solely with fever.7 The biopsy specimen was nonspecific but indicated a viral exanthem. Histopathology showed perivascular lymphocytic infiltrate, dermal edema and vacuoles, spongiosis, dyskeratotic basilar keratinocytes, and few neutrophils without eosinophils.7
Eczematous Eruption
A confluent eczematous eruption in the flexural areas, the antecubital fossae, and axillary folds has been found in COVID-19 patients.21,22 An elderly patient with severe COVID-19 developed a squamous erythematous periumbilical patch 1 day after hospital admission. The cutaneous eruption rapidly progressed to digitate scaly plaques on the trunk, thighs, and flank. A biopsy specimen showed epidermal spongiosis, vesicles containing lymphocytes, and Langerhans cells. The upper dermis demonstrated a lymphohistiocytic infiltrate.23
Pityriasis Rosea–Like Eruption
In Iran, a COVID-19–infected patient developed an erythematous papulosquamous eruption with a herald patch and trailing scales 3 days after viral symptoms, resembling that of pityriasis rosea.24 Nests of Langerhans cells within the epidermis are seen in many viral exanthems, including cases of COVID-19 and pityriasis rosea.25
Urticaria
According to a number of case reports, urticarial lesions have been the first presenting sign of COVID-19 infection, most resolving with antihistamines.10,11 Some patients with more severe symptoms have had widespread urticaria. An urticarial exanthem appearing on the bilateral thighs and buttocks may be the initial sign of infection.12,15 Pruritic erythematous plaques over the face and acral areas is another initial sign. Interestingly, pediatric patients have reported nonpruritic urticaria.9
Urticaria also has been seen as a late dermatologic sign of viral infection. After battling relentless viral infection for 1 month, a pruritic, confluent, ill-defined eruption appeared along a patient’s trunk, back, and proximal extremities. Histopathologic examination concluded a perivascular lymphocytic infiltrate and dilated vessels in the dermis. The urticaria resolved a week later, and the patient’s nasopharyngeal swab finally came back negative.13
Vesiculopapular Eruption
Vesicles mimicking those of chickenpox have been reported. A study of 375 confirmed cases of COVID-19 by Galván Casas et al12 showed a 9% incidence of this vesicular eruption. A study by Sachdeva et al8 revealed vesicular eruptions in 25 of 72 patients. Pruritic papules and vesicles may resemble Grover disease. This cutaneous sign may be seen in the submammary folds, on the hips, or diffusely over the body.
Erythema Multiforme–Like Eruption
Targetoid lesions similar to those of erythema multiforme erupted in 2 of 27 patients with mild COVID-19 infection in a review by Wollina et al.4 In a study of 4 patients with erythema multiforme–like eruptions after COVID-19 symptoms resolved, 3 had palatal petechiae. Two of 4 patients had pseudovesicles in the center of the erythematous targetoid patches.26 Targetoid lesions on the extremities have been reported in pediatric patients with COVID-19 infections. These patients often present without any typical viral symptoms but rather just a febrile exanthem or exanthem alone. Thus, to minimize spread of the virus, it is vital to recognize COVID-19 infection early in patients with a viral exanthem during the time of high COVID-19 incidence.4
Livedo Reticularis
In the United States, a case series reported 2 patients with transient livedo reticularis throughout the course of COVID-19 infection. The cutaneous eruption resembled erythema ab igne, but there was no history of exposure to heat.16
Stellate Purpura
In severe COVID-19 infection, a reticulated nonblanching purpura on the buttocks has been reported to demonstrate pauci-inflammatory vascular thrombosis, complement membrane attack complex deposition, and endothelial injury on dermatopathology. Stellate purpura on palmoplantar surfaces also has shown arterial thrombosis in the deep dermis due to complement deposition.17
Petechiae and Purpura
A morbilliform exanthem may develop into significant petechiae in the popliteal fossae, buttocks, and thighs. A punch biopsy specimen demonstrates a perivascular lymphocytic infiltrate with erythrocyte extravasation and papillary dermal edema with dyskeratotic cells.18 Purpura of the lower extremities may develop, with histopathology showing fibrinoid necrosis of small vessel walls, neutrophilic infiltrate with karyorrhexis, and granular complement deposition.19
In Thailand, a patient was misdiagnosed with dengue after presenting with petechiae and low platelet count.20 Further progression of the viral illness resulted in respiratory symptoms. Subsequently, the patient tested positive for COVID-19. This case demonstrates that cutaneous signs of many sorts may be the first presenting signs of COVID-19, even prior to febrile symptoms.20
Androgenic Alopecia
Studies have shown that androgens are related in the pathogenesis of COVID-19. Coronavirus disease 2019 uses a cellular co-receptor, TMPRSS2, which is androgen regulated.27 In a study of 41 males with COVID-19, 29 had androgenic alopecia. However, this is only a correlation, and causation cannot be concluded here. It cannot be determined from this study whether androgenic alopecia is a risk factor, result of COVID-19, or confounder.28
Exaggerated Herpes Zoster
Shors29 reported a herpes zoster eruption in a patient who had symptoms of COVID-19 for 1 week. Further testing confirmed COVID-19 infection, and despite prompt treatment with valacyclovir, the eruption was slow to resolve. The patient then experienced severe postherpetic neuralgia for more than 4 weeks, even with treatment with gabapentin and lidocaine. It is hypothesized that because of the major inflammatory response caused by COVID-19, an exaggerated inflammation occurred in the dorsal root ganglion, resulting in relentless herpes zoster infection.29
Mottled Skin
Born at term, a 15-day-old neonate presented with sepsis and mottling of the skin. The patient did not have any typical COVID-19 symptoms, such as diarrhea or cough, but tested positive for COVID-19.30
Periorbital Dyschromia
Kalner and Vergilis31 reported 2 cases of periorbital dyschromia prior to any other COVID-19 infection symptoms. The discoloration improved with resolution of ensuing viral symptoms.31
Comment
Many dermatologic signs of COVID-19 have been identified. Their individual frequency and association with viral severity will become more apparent as more cases are reported. So far during this pandemic, common dermatologic manifestations have been polymorphic in clinical presentation.
Onset of Skin Manifestations
The timeline of skin signs and COVID-19 symptoms varies from the first reported sign to weeks after symptom resolution. In the Region of Murcia, Spain, Pérez-Suárez et al14 collected data on cutaneous signs of patients with COVID-19. Of the patients studied, 9 had tests confirming COVID-19 infection. Truncal urticaria, sacral ulcers, acrocyanosis, and erythema multiforme were all reported in patients more than 2 weeks after symptom onset. One case of tinea infection also was reported 4 days after fever and respiratory symptoms began.14
Presentation
Coronavirus disease 2019 has affected the skin of both the central thorax and peripheral locations. In a study of 72 patients with cutaneous signs of COVID-19 by Sachdeva et al,8 a truncal distribution was most common, but 14 patients reported acral site involvement. Sachdeva et al8 reported urticarial reactions in 7 of 72 patients with cutaneous signs. A painful acral cyanosis was seen in 11 of 72 patients. Livedo reticularis presented in 2 patients, and only 1 patient had petechiae. Cutaneous signs were the first indicators of viral infection in 9 of 72 patients; 52 patients presented with respiratory symptoms first. All of the reported cutaneous signs spontaneously resolved within 10 days.8
Recalcati32 reviewed 88 patients with COVID-19, and 18 had cutaneous signs at initial onset of viral infection or during hospitalization. The most common integumentary sign reported in this study was erythema, followed by diffuse urticaria, and then a vesicular eruption resembling varicella infection.32
Some less common phenomena have been identified in patients with COVID-19, including androgenic alopecia, exaggerated herpes zoster and postherpetic neuralgia, mottled skin, and periorbital dyschromia. Being aware of these complications may help in early treatment, diagnosis, and even prevention of viral spread.
Pathogenesis of Skin Manifestations
Few breakthroughs have been made in understanding the pathogenesis of skin manifestations of SARS-CoV-2. Acral ischemia may be a manifestation of COVID-19’s association with hypercoagulation. Increasing fibrinogen and prothrombin times lead to disseminated intravascular coagulation and microthrombi. These tiny blood clots then lodge in blood vessels and cause acral cyanosis and subsequent gangrene.2 The proposed mechanism behind this clinical manifestation in younger populations is the hypercoagulable state that COVID-19 creates. Conversely, acral erythema and chilblainlike lesions in older patients are thought to be from acral ischemia as a response to insufficient type 1 interferons. This pathophysiologic mechanism is indicative of a worse prognosis due to the large role that type 1 interferons play in antiviral responses. Coronavirus disease 2019 similarly triggers type 1 interferons; thus, their efficacy positively correlates with good disease prognosis.3
Similarly, the pathogenesis for livedo reticularis in patients with COVID-19 can only be hypothesized. Infected patients are in a hypercoagulable state, and in these cases, it was uncertain whether this was due to a disseminated intravascular coagulation, cold agglutinins, cryofibrinogens, or lupus anticoagulant.16
Nonetheless, it can be difficult to separate the primary event between vasculopathy or vasculitis in larger vessel pathology specimens. Some of the studies’ pathology reports discuss a granulocytic infiltrate and red blood cell extravasation, which represent small vessel vasculitis. However, the gangrene and necrosing livedo represent vasculopathy events. A final conclusion about the pathogenesis cannot be made without further clinical and histopathologic evaluation.
Histopathology
Biopsy specimens of reported morbilliform eruptions have demonstrated thrombosed vessels with evidence of necrosis and granulocytic infiltrate.25 Another biopsy specimen of a widespread erythematous exanthem demonstrated extravasated red blood cells and vessel wall damage similar to thrombophilic arteritis. Other reports of histopathology showed necrotic keratinocytes and lymphocytic satellitosis at the dermoepidermal junction, resembling Grover disease. These cases demonstrating necrosis suggest a strong cytokine reaction from the virus.25 A concern with these biopsy findings is that morbilliform eruptions generally show dilated vessels with lymphocytes, and these biopsy findings are consistent with a cutaneous small vessel vasculitis. Additionally, histopathologic evaluation of purpuric eruptions has shown erythrocyte extravasation and granulocytic infiltrate indicative of a cutaneous small vessel vasculitis.
Although most reported cases of cutaneous signs of COVID-19 do not have histopathologic reports, Yao et al33 conducted a dermatopathologic study that investigated the tissue in deceased patients who had COVID-19. This pathology showed hyaline thrombi within the small vessels of the skin, likely leading to the painful acral ischemia. Similarly, Yao et al33 reported autopsies finding hyaline thrombi within the small vessels of the lungs. More research should be done to explore this pathogenesis as part of prognostic factors and virulence.
Conclusion
Cutaneous signs may be the first reported symptom of COVID-19 infection, and dermatologists should be prepared to identify them. This review may be used as a guide for physicians to quickly identify potential infection as well as further understand the pathogenesis related to COVID-19. Future research is necessary to determine the dermatologic pathogenesis, infectivity, and prevalence of cutaneous manifestations of COVID-19. It also will be important to explore if vasculopathic lesions predict more severe multisystem disease.
The pathogenesis of coronavirus disease 2019 (COVID-19), the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), is not yet completely understood. Thus far, it is known to affect multiple organ systems, including gastrointestinal, neurological, and cardiovascular, with typical clinical symptoms of COVID-19 including fever, cough, myalgia, headache, anosmia, and diarrhea.1 This multiorgan attack may be secondary to an exaggerated inflammatory reaction with vasculopathy and possibly a hypercoagulable state. Skin manifestations also are prevalent in COVID-19, and they often result in polymorphous presentations.2 This article aims to summarize cutaneous clinical signs of COVID-19 so that dermatologists can promptly identify and manage COVID-19 and prevent its spread.
Methods
A PubMed search of articles indexed for MEDLINE was conducted on June 30, 2020. The literature included observational studies, case reports, and literature reviews from January 1, 2020, to June 30, 2020. Search terms included COVID-19, SARS-CoV-2, and coronavirus used in combination with cutaneous, skin, and dermatology. All of the resulting articles were then reviewed for relevance to the cutaneous manifestations of COVID-19. Only confirmed cases of COVID-19 infection were included in this review; suspected unconfirmed cases were excluded. Further exclusion criteria included articles that discussed dermatology in the time of COVID-19 that did not explicitly address its cutaneous manifestations. The remaining literature was evaluated to provide dermatologists and patients with a concise resource for the cutaneous signs and symptoms of COVID-19. Data extracted from the literature included geographic region, number of patients with skin findings, status of COVID-19 infection and timeline, and cutaneous signs. If a cutaneous sign was not given a clear diagnosis in the literature, the senior authors (A.L. and J.J.) assigned it to its most similar classification to aid in ease of understanding and clarity for the readers.
Results
A search of the key terms resulted in 75 articles published in the specified date range. After excluding overtly irrelevant articles and dermatologic conditions in the time of COVID-19 without confirmed SARS-CoV-2 infection, 25 articles ultimately met inclusion criteria. Relevant references from the articles also were explored for cutaneous dermatologic manifestations of COVID-19. Cutaneous manifestations that were repeatedly reported included chilblainlike lesions; acrocyanosis; urticaria; pityriasis rosea–like cutaneous eruption; erythema multiforme–like, vesiculopapular, and morbilliform eruptions; petechiae; livedo reticularis; and purpuric livedo reticularis (dermatologists may label this stellate purpura). Fewer but nonetheless notable cases of androgenic alopecia, periorbital dyschromia, and herpes zoster exacerbations also were documented. The Table summarizes the reported integumentary findings. The eTable groups the common findings and describes patient age, time to onset of cutaneous sign, and any prognostic significance as seen in the literature.
Chilblainlike Lesions and Acrocyanosis
Chilblainlike lesions are edematous eruptions of the fingers and toes. They usually do not scar and are described as erythematous to violaceous papules and macules with possible bullae on the digits. Skin biopsies demonstrate a histopathologic pattern of vacuolar interface dermatitis with necrotic keratinocytes and a thickened basement membrane. Lymphocytic infiltrate presents in a perieccrine distribution, occasionally with plasma cells. The dermatopathologic findings mimic those of chilblain lupus but lack dermal edema.3
These eruptions have been reported in cases of COVID-19 that more frequently affect children and young adults. They usually resolve over the course of viral infection, averaging within 14 days. Chilblainlike eruptions often are associated with pruritus or pain. They commonly are asymmetrical and appear more often on the toes than the fingers.4 In cases of COVID-19 that lack systemic symptoms, chilblainlike lesions have been seen on the dorsal fingers as the first presenting sign of infection.5
Acral erythema and chilblainlike lesions frequently have been associated with milder infection. Another positive prognostic indicator is the manifestation of these signs in younger individuals.3
Morbilliform Exanthem
The morbilliform exanthem associated with COVID-19 also typically presents in patients with milder disease. It often affects the buttocks, lower abdomen, and thighs, but spares the palms, soles, and mucosae.4 This skin sign, which may start out as a generalized morbilliform exanthem, has been seen to morph into macular hemorrhagic purpura on the legs. These cutaneous lesions typically spontaneously resolve.8
In a case report by Najarian,6 a morbilliform exanthem was seen on the legs, arms, and trunk of a patient who was otherwise asymptomatic but tested positive for COVID-19. The morbilliform exanthem then became confluent on the trunk. Notably, the patient reported pain of the hands and feet.6
Another case report described a patient with edematous annular plaques on the palms, neck, and upper extremities who presented solely with fever.7 The biopsy specimen was nonspecific but indicated a viral exanthem. Histopathology showed perivascular lymphocytic infiltrate, dermal edema and vacuoles, spongiosis, dyskeratotic basilar keratinocytes, and few neutrophils without eosinophils.7
Eczematous Eruption
A confluent eczematous eruption in the flexural areas, the antecubital fossae, and axillary folds has been found in COVID-19 patients.21,22 An elderly patient with severe COVID-19 developed a squamous erythematous periumbilical patch 1 day after hospital admission. The cutaneous eruption rapidly progressed to digitate scaly plaques on the trunk, thighs, and flank. A biopsy specimen showed epidermal spongiosis, vesicles containing lymphocytes, and Langerhans cells. The upper dermis demonstrated a lymphohistiocytic infiltrate.23
Pityriasis Rosea–Like Eruption
In Iran, a COVID-19–infected patient developed an erythematous papulosquamous eruption with a herald patch and trailing scales 3 days after viral symptoms, resembling that of pityriasis rosea.24 Nests of Langerhans cells within the epidermis are seen in many viral exanthems, including cases of COVID-19 and pityriasis rosea.25
Urticaria
According to a number of case reports, urticarial lesions have been the first presenting sign of COVID-19 infection, most resolving with antihistamines.10,11 Some patients with more severe symptoms have had widespread urticaria. An urticarial exanthem appearing on the bilateral thighs and buttocks may be the initial sign of infection.12,15 Pruritic erythematous plaques over the face and acral areas is another initial sign. Interestingly, pediatric patients have reported nonpruritic urticaria.9
Urticaria also has been seen as a late dermatologic sign of viral infection. After battling relentless viral infection for 1 month, a pruritic, confluent, ill-defined eruption appeared along a patient’s trunk, back, and proximal extremities. Histopathologic examination concluded a perivascular lymphocytic infiltrate and dilated vessels in the dermis. The urticaria resolved a week later, and the patient’s nasopharyngeal swab finally came back negative.13
Vesiculopapular Eruption
Vesicles mimicking those of chickenpox have been reported. A study of 375 confirmed cases of COVID-19 by Galván Casas et al12 showed a 9% incidence of this vesicular eruption. A study by Sachdeva et al8 revealed vesicular eruptions in 25 of 72 patients. Pruritic papules and vesicles may resemble Grover disease. This cutaneous sign may be seen in the submammary folds, on the hips, or diffusely over the body.
Erythema Multiforme–Like Eruption
Targetoid lesions similar to those of erythema multiforme erupted in 2 of 27 patients with mild COVID-19 infection in a review by Wollina et al.4 In a study of 4 patients with erythema multiforme–like eruptions after COVID-19 symptoms resolved, 3 had palatal petechiae. Two of 4 patients had pseudovesicles in the center of the erythematous targetoid patches.26 Targetoid lesions on the extremities have been reported in pediatric patients with COVID-19 infections. These patients often present without any typical viral symptoms but rather just a febrile exanthem or exanthem alone. Thus, to minimize spread of the virus, it is vital to recognize COVID-19 infection early in patients with a viral exanthem during the time of high COVID-19 incidence.4
Livedo Reticularis
In the United States, a case series reported 2 patients with transient livedo reticularis throughout the course of COVID-19 infection. The cutaneous eruption resembled erythema ab igne, but there was no history of exposure to heat.16
Stellate Purpura
In severe COVID-19 infection, a reticulated nonblanching purpura on the buttocks has been reported to demonstrate pauci-inflammatory vascular thrombosis, complement membrane attack complex deposition, and endothelial injury on dermatopathology. Stellate purpura on palmoplantar surfaces also has shown arterial thrombosis in the deep dermis due to complement deposition.17
Petechiae and Purpura
A morbilliform exanthem may develop into significant petechiae in the popliteal fossae, buttocks, and thighs. A punch biopsy specimen demonstrates a perivascular lymphocytic infiltrate with erythrocyte extravasation and papillary dermal edema with dyskeratotic cells.18 Purpura of the lower extremities may develop, with histopathology showing fibrinoid necrosis of small vessel walls, neutrophilic infiltrate with karyorrhexis, and granular complement deposition.19
In Thailand, a patient was misdiagnosed with dengue after presenting with petechiae and low platelet count.20 Further progression of the viral illness resulted in respiratory symptoms. Subsequently, the patient tested positive for COVID-19. This case demonstrates that cutaneous signs of many sorts may be the first presenting signs of COVID-19, even prior to febrile symptoms.20
Androgenic Alopecia
Studies have shown that androgens are related in the pathogenesis of COVID-19. Coronavirus disease 2019 uses a cellular co-receptor, TMPRSS2, which is androgen regulated.27 In a study of 41 males with COVID-19, 29 had androgenic alopecia. However, this is only a correlation, and causation cannot be concluded here. It cannot be determined from this study whether androgenic alopecia is a risk factor, result of COVID-19, or confounder.28
Exaggerated Herpes Zoster
Shors29 reported a herpes zoster eruption in a patient who had symptoms of COVID-19 for 1 week. Further testing confirmed COVID-19 infection, and despite prompt treatment with valacyclovir, the eruption was slow to resolve. The patient then experienced severe postherpetic neuralgia for more than 4 weeks, even with treatment with gabapentin and lidocaine. It is hypothesized that because of the major inflammatory response caused by COVID-19, an exaggerated inflammation occurred in the dorsal root ganglion, resulting in relentless herpes zoster infection.29
Mottled Skin
Born at term, a 15-day-old neonate presented with sepsis and mottling of the skin. The patient did not have any typical COVID-19 symptoms, such as diarrhea or cough, but tested positive for COVID-19.30
Periorbital Dyschromia
Kalner and Vergilis31 reported 2 cases of periorbital dyschromia prior to any other COVID-19 infection symptoms. The discoloration improved with resolution of ensuing viral symptoms.31
Comment
Many dermatologic signs of COVID-19 have been identified. Their individual frequency and association with viral severity will become more apparent as more cases are reported. So far during this pandemic, common dermatologic manifestations have been polymorphic in clinical presentation.
Onset of Skin Manifestations
The timeline of skin signs and COVID-19 symptoms varies from the first reported sign to weeks after symptom resolution. In the Region of Murcia, Spain, Pérez-Suárez et al14 collected data on cutaneous signs of patients with COVID-19. Of the patients studied, 9 had tests confirming COVID-19 infection. Truncal urticaria, sacral ulcers, acrocyanosis, and erythema multiforme were all reported in patients more than 2 weeks after symptom onset. One case of tinea infection also was reported 4 days after fever and respiratory symptoms began.14
Presentation
Coronavirus disease 2019 has affected the skin of both the central thorax and peripheral locations. In a study of 72 patients with cutaneous signs of COVID-19 by Sachdeva et al,8 a truncal distribution was most common, but 14 patients reported acral site involvement. Sachdeva et al8 reported urticarial reactions in 7 of 72 patients with cutaneous signs. A painful acral cyanosis was seen in 11 of 72 patients. Livedo reticularis presented in 2 patients, and only 1 patient had petechiae. Cutaneous signs were the first indicators of viral infection in 9 of 72 patients; 52 patients presented with respiratory symptoms first. All of the reported cutaneous signs spontaneously resolved within 10 days.8
Recalcati32 reviewed 88 patients with COVID-19, and 18 had cutaneous signs at initial onset of viral infection or during hospitalization. The most common integumentary sign reported in this study was erythema, followed by diffuse urticaria, and then a vesicular eruption resembling varicella infection.32
Some less common phenomena have been identified in patients with COVID-19, including androgenic alopecia, exaggerated herpes zoster and postherpetic neuralgia, mottled skin, and periorbital dyschromia. Being aware of these complications may help in early treatment, diagnosis, and even prevention of viral spread.
Pathogenesis of Skin Manifestations
Few breakthroughs have been made in understanding the pathogenesis of skin manifestations of SARS-CoV-2. Acral ischemia may be a manifestation of COVID-19’s association with hypercoagulation. Increasing fibrinogen and prothrombin times lead to disseminated intravascular coagulation and microthrombi. These tiny blood clots then lodge in blood vessels and cause acral cyanosis and subsequent gangrene.2 The proposed mechanism behind this clinical manifestation in younger populations is the hypercoagulable state that COVID-19 creates. Conversely, acral erythema and chilblainlike lesions in older patients are thought to be from acral ischemia as a response to insufficient type 1 interferons. This pathophysiologic mechanism is indicative of a worse prognosis due to the large role that type 1 interferons play in antiviral responses. Coronavirus disease 2019 similarly triggers type 1 interferons; thus, their efficacy positively correlates with good disease prognosis.3
Similarly, the pathogenesis for livedo reticularis in patients with COVID-19 can only be hypothesized. Infected patients are in a hypercoagulable state, and in these cases, it was uncertain whether this was due to a disseminated intravascular coagulation, cold agglutinins, cryofibrinogens, or lupus anticoagulant.16
Nonetheless, it can be difficult to separate the primary event between vasculopathy or vasculitis in larger vessel pathology specimens. Some of the studies’ pathology reports discuss a granulocytic infiltrate and red blood cell extravasation, which represent small vessel vasculitis. However, the gangrene and necrosing livedo represent vasculopathy events. A final conclusion about the pathogenesis cannot be made without further clinical and histopathologic evaluation.
Histopathology
Biopsy specimens of reported morbilliform eruptions have demonstrated thrombosed vessels with evidence of necrosis and granulocytic infiltrate.25 Another biopsy specimen of a widespread erythematous exanthem demonstrated extravasated red blood cells and vessel wall damage similar to thrombophilic arteritis. Other reports of histopathology showed necrotic keratinocytes and lymphocytic satellitosis at the dermoepidermal junction, resembling Grover disease. These cases demonstrating necrosis suggest a strong cytokine reaction from the virus.25 A concern with these biopsy findings is that morbilliform eruptions generally show dilated vessels with lymphocytes, and these biopsy findings are consistent with a cutaneous small vessel vasculitis. Additionally, histopathologic evaluation of purpuric eruptions has shown erythrocyte extravasation and granulocytic infiltrate indicative of a cutaneous small vessel vasculitis.
Although most reported cases of cutaneous signs of COVID-19 do not have histopathologic reports, Yao et al33 conducted a dermatopathologic study that investigated the tissue in deceased patients who had COVID-19. This pathology showed hyaline thrombi within the small vessels of the skin, likely leading to the painful acral ischemia. Similarly, Yao et al33 reported autopsies finding hyaline thrombi within the small vessels of the lungs. More research should be done to explore this pathogenesis as part of prognostic factors and virulence.
Conclusion
Cutaneous signs may be the first reported symptom of COVID-19 infection, and dermatologists should be prepared to identify them. This review may be used as a guide for physicians to quickly identify potential infection as well as further understand the pathogenesis related to COVID-19. Future research is necessary to determine the dermatologic pathogenesis, infectivity, and prevalence of cutaneous manifestations of COVID-19. It also will be important to explore if vasculopathic lesions predict more severe multisystem disease.
- Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497-506.
- Criado PR, Abdalla BMZ, de Assis IC, et al. Are the cutaneous manifestations during or due to SARS-CoV-2 infection/COVID-19 frequent or not? revision of possible pathophysiologic mechanisms. Inflamm Res. 2020;69:745-756.
- Kolivras A, Dehavay F, Delplace D, et al. Coronavirus (COVID‐19) infection–induced chilblains: a case report with histopathological findings. JAAD Case Rep. 2020;6:489-492.
- Wollina U, Karadag˘ AS, Rowland-Payne C, et al. Cutaneous signs in COVID-19 patients: a review [published online May 10, 2020]. Dermatol Ther. 2020;33:E13549.
- Alramthan A, Aldaraji W. Two cases of COVID-19 presenting with a clinical picture resembling chilblains: first report from the Middle East. Clin Exp Dermatol. 2020;45:746-748.
- Najarian DJ. Morbilliform exanthem associated with COVID‐19. JAAD Case Rep. 2020;6:493-494.
- Amatore F, Macagno N, Mailhe M, et al. SARS-CoV-2 infection presenting as a febrile rash. J Eur Acad Dermatol Venereol. 2020;34:E304-E306.
- Sachdeva M, Gianotti R, Shah M, et al. Cutaneous manifestations of COVID-19: report of three cases and a review of literature. J Dermatol Sci. 2020;98:75-81.
- Morey-Olivé M, Espiau M, Mercadal-Hally M, et al. Cutaneous manifestations in the current pandemic of coronavirus infection disease (COVID 2019). An Pediatr (Engl Ed). 2020;92:374-375.
- van Damme C, Berlingin E, Saussez S, et al. Acute urticaria with pyrexia as the first manifestations of a COVID‐19 infection. J Eur Acad Dermatol Venereol. 2020;34:E300-E301.
- Henry D, Ackerman M, Sancelme E, et al. Urticarial eruption in COVID‐19 infection. J Eur Acad Dermatol Venereol. 2020;34:E244-E245.
- Galván Casas C, Català A, Carretero Hernández G, et al. Classification of the cutaneous manifestations of COVID-19: a rapid prospective nationwide consensus study in Spain with 375 cases. Br J Dermatol. 2020;183:71-77.
- Zengarini C, Orioni G, Cascavilla A, et al. Histological pattern in Covid-19-induced viral rash [published online May 2, 2020]. J Eur Acad Dermatol Venereol. doi:10.1111/jdv.16569.
- Pérez-Suárez B, Martínez-Menchón T, Cutillas-Marco E. Skin findings in the COVID-19 pandemic in the Region of Murcia [published online June 12, 2020]. Med Clin (Engl Ed). 2020;155:41-42.
- Quintana-Castanedo L, Feito-Rodríguez M, Valero-López I, et al. Urticarial exanthem as early diagnostic clue for COVID-19 infection [published online April 29, 2020]. JAAD Case Rep. 2020;6:498-499.
- Manalo IF, Smith MK, Cheeley J, et al. Reply to: “reply: a dermatologic manifestation of COVID-19: transient livedo reticularis” [published online May 7, 2020]. J Am Acad Dermatol. 2020;83:E157.
- Magro C, Mulvey JJ, Berlin D, et al. Complement associated microvascular injury and thrombosis in the pathogenesis of severe COVID-19 infection: a report of five cases. Transl Res. 2020;220:1-13.
- Diaz-Guimaraens B, Dominguez-Santas M, Suarez-Valle A, et al. Petechial skin rash associated with severe acute respiratory syndrome coronavirus 2 infection. JAMA Dermatol. 2020;156:820-822.
- Dominguez-Santas M, Diaz-Guimaraens B, Garcia Abellas P, et al. Cutaneous small-vessel vasculitis associated with novel 2019 coronavirus SARS-CoV-2 infection (COVID-19) [published online July 2, 2020]. J Eur Acad Dermatol Venereol. 2020;34:E536-E537.
- Joob B, Wiwanitkit V. COVID-19 can present with a rash and be mistaken for dengue [published online March 22, 2020]. J Am Acad Dermatol. 2020;82:E177.
- Avellana Moreno R, Estella Villa LM, Avellana Moreno V, et al. Cutaneous manifestation of COVID‐19 in images: a case report [published online May 19, 2020]. J Eur Acad Dermatol Venereol. 2020;34:E307-E309.
- Mahé A, Birckel E, Krieger S, et al. A distinctive skin rash associated with coronavirus disease 2019 [published online June 8, 2020]? J Eur Acad Dermatol Venereol. 2020;34:E246-E247.
- Sanchez A, Sohier P, Benghanem S, et al. Digitate papulosquamous eruption associated with severe acute respiratory syndrome coronavirus 2 infection. JAMA Dermatol. 2020;156:819-820.
- Ehsani AH, Nasimi M, Bigdelo Z. Pityriasis rosea as a cutaneous manifestation of COVID‐19 infection [published online May 2, 2020]. J Eur Acad Dermatol Venereol. doi:10.1111/jdv.16579.
- Gianotti R, Veraldi S, Recalcati S, et al. Cutaneous clinico-pathological findings in three COVID-19-positive patients observed in the metropolitan area of Milan, Italy. Acta Derm Venereol. 2020;100:adv00124.
- Jimenez-Cauhe J, Ortega-Quijano D, Carretero-Barrio I, et al. Erythema multiforme-like eruption in patients with COVID-19 infection: clinical and histological findings [published online May 9, 2020]. Clin Exp Dermatol. doi:10.1111/ced.14281
- Hoffmann M, Kleine‐Weber H, Schroeder S, et al. SARS‐CoV‐2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor [published online March 5, 2020]. Cell. 2020;181:271‐280.e8.
- Goren A, Vaño‐Galván S, Wambier CG, et al. A preliminary observation: male pattern hair loss among hospitalized COVID‐19 patients in Spain—a potential clue to the role of androgens in COVID‐19 severity [published online April 23, 2020]. J Cosmet Dermatol. 2020;19:1545-1547.
- Shors AR. Herpes zoster and severe acute herpetic neuralgia as a complication of COVID-19 infection. JAAD Case Rep. 2020;6:656-657.
- Kamali Aghdam M, Jafari N, Eftekhari K. Novel coronavirus in a 15‐day‐old neonate with clinical signs of sepsis, a case report. Infect Dis (London). 2020;52:427‐429.
- Kalner S, Vergilis IJ. Periorbital erythema as a presenting sign of covid-19 [published online May 11, 2020]. JAAD Case Rep. 2020;6:996-998.
- Recalcati S. Cutaneous manifestations in COVID‐19: a first perspective. J Eur Acad Dermatol Venereol. 2020;34:E212-E213.
- Yao XH, Li TY, He ZC, et al. A pathological report of three COVID‐19 cases by minimally invasive autopsies [in Chinese]. Zhonghua Bing Li Xue Za Zhi. 2020;49:411-417.
- Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497-506.
- Criado PR, Abdalla BMZ, de Assis IC, et al. Are the cutaneous manifestations during or due to SARS-CoV-2 infection/COVID-19 frequent or not? revision of possible pathophysiologic mechanisms. Inflamm Res. 2020;69:745-756.
- Kolivras A, Dehavay F, Delplace D, et al. Coronavirus (COVID‐19) infection–induced chilblains: a case report with histopathological findings. JAAD Case Rep. 2020;6:489-492.
- Wollina U, Karadag˘ AS, Rowland-Payne C, et al. Cutaneous signs in COVID-19 patients: a review [published online May 10, 2020]. Dermatol Ther. 2020;33:E13549.
- Alramthan A, Aldaraji W. Two cases of COVID-19 presenting with a clinical picture resembling chilblains: first report from the Middle East. Clin Exp Dermatol. 2020;45:746-748.
- Najarian DJ. Morbilliform exanthem associated with COVID‐19. JAAD Case Rep. 2020;6:493-494.
- Amatore F, Macagno N, Mailhe M, et al. SARS-CoV-2 infection presenting as a febrile rash. J Eur Acad Dermatol Venereol. 2020;34:E304-E306.
- Sachdeva M, Gianotti R, Shah M, et al. Cutaneous manifestations of COVID-19: report of three cases and a review of literature. J Dermatol Sci. 2020;98:75-81.
- Morey-Olivé M, Espiau M, Mercadal-Hally M, et al. Cutaneous manifestations in the current pandemic of coronavirus infection disease (COVID 2019). An Pediatr (Engl Ed). 2020;92:374-375.
- van Damme C, Berlingin E, Saussez S, et al. Acute urticaria with pyrexia as the first manifestations of a COVID‐19 infection. J Eur Acad Dermatol Venereol. 2020;34:E300-E301.
- Henry D, Ackerman M, Sancelme E, et al. Urticarial eruption in COVID‐19 infection. J Eur Acad Dermatol Venereol. 2020;34:E244-E245.
- Galván Casas C, Català A, Carretero Hernández G, et al. Classification of the cutaneous manifestations of COVID-19: a rapid prospective nationwide consensus study in Spain with 375 cases. Br J Dermatol. 2020;183:71-77.
- Zengarini C, Orioni G, Cascavilla A, et al. Histological pattern in Covid-19-induced viral rash [published online May 2, 2020]. J Eur Acad Dermatol Venereol. doi:10.1111/jdv.16569.
- Pérez-Suárez B, Martínez-Menchón T, Cutillas-Marco E. Skin findings in the COVID-19 pandemic in the Region of Murcia [published online June 12, 2020]. Med Clin (Engl Ed). 2020;155:41-42.
- Quintana-Castanedo L, Feito-Rodríguez M, Valero-López I, et al. Urticarial exanthem as early diagnostic clue for COVID-19 infection [published online April 29, 2020]. JAAD Case Rep. 2020;6:498-499.
- Manalo IF, Smith MK, Cheeley J, et al. Reply to: “reply: a dermatologic manifestation of COVID-19: transient livedo reticularis” [published online May 7, 2020]. J Am Acad Dermatol. 2020;83:E157.
- Magro C, Mulvey JJ, Berlin D, et al. Complement associated microvascular injury and thrombosis in the pathogenesis of severe COVID-19 infection: a report of five cases. Transl Res. 2020;220:1-13.
- Diaz-Guimaraens B, Dominguez-Santas M, Suarez-Valle A, et al. Petechial skin rash associated with severe acute respiratory syndrome coronavirus 2 infection. JAMA Dermatol. 2020;156:820-822.
- Dominguez-Santas M, Diaz-Guimaraens B, Garcia Abellas P, et al. Cutaneous small-vessel vasculitis associated with novel 2019 coronavirus SARS-CoV-2 infection (COVID-19) [published online July 2, 2020]. J Eur Acad Dermatol Venereol. 2020;34:E536-E537.
- Joob B, Wiwanitkit V. COVID-19 can present with a rash and be mistaken for dengue [published online March 22, 2020]. J Am Acad Dermatol. 2020;82:E177.
- Avellana Moreno R, Estella Villa LM, Avellana Moreno V, et al. Cutaneous manifestation of COVID‐19 in images: a case report [published online May 19, 2020]. J Eur Acad Dermatol Venereol. 2020;34:E307-E309.
- Mahé A, Birckel E, Krieger S, et al. A distinctive skin rash associated with coronavirus disease 2019 [published online June 8, 2020]? J Eur Acad Dermatol Venereol. 2020;34:E246-E247.
- Sanchez A, Sohier P, Benghanem S, et al. Digitate papulosquamous eruption associated with severe acute respiratory syndrome coronavirus 2 infection. JAMA Dermatol. 2020;156:819-820.
- Ehsani AH, Nasimi M, Bigdelo Z. Pityriasis rosea as a cutaneous manifestation of COVID‐19 infection [published online May 2, 2020]. J Eur Acad Dermatol Venereol. doi:10.1111/jdv.16579.
- Gianotti R, Veraldi S, Recalcati S, et al. Cutaneous clinico-pathological findings in three COVID-19-positive patients observed in the metropolitan area of Milan, Italy. Acta Derm Venereol. 2020;100:adv00124.
- Jimenez-Cauhe J, Ortega-Quijano D, Carretero-Barrio I, et al. Erythema multiforme-like eruption in patients with COVID-19 infection: clinical and histological findings [published online May 9, 2020]. Clin Exp Dermatol. doi:10.1111/ced.14281
- Hoffmann M, Kleine‐Weber H, Schroeder S, et al. SARS‐CoV‐2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor [published online March 5, 2020]. Cell. 2020;181:271‐280.e8.
- Goren A, Vaño‐Galván S, Wambier CG, et al. A preliminary observation: male pattern hair loss among hospitalized COVID‐19 patients in Spain—a potential clue to the role of androgens in COVID‐19 severity [published online April 23, 2020]. J Cosmet Dermatol. 2020;19:1545-1547.
- Shors AR. Herpes zoster and severe acute herpetic neuralgia as a complication of COVID-19 infection. JAAD Case Rep. 2020;6:656-657.
- Kamali Aghdam M, Jafari N, Eftekhari K. Novel coronavirus in a 15‐day‐old neonate with clinical signs of sepsis, a case report. Infect Dis (London). 2020;52:427‐429.
- Kalner S, Vergilis IJ. Periorbital erythema as a presenting sign of covid-19 [published online May 11, 2020]. JAAD Case Rep. 2020;6:996-998.
- Recalcati S. Cutaneous manifestations in COVID‐19: a first perspective. J Eur Acad Dermatol Venereol. 2020;34:E212-E213.
- Yao XH, Li TY, He ZC, et al. A pathological report of three COVID‐19 cases by minimally invasive autopsies [in Chinese]. Zhonghua Bing Li Xue Za Zhi. 2020;49:411-417.
Practice Points
- Coronavirus disease 2019 (COVID-19) is a worldwide pandemic that affects multiple organ systems via a pathogenesis that is still being elucidated.
- Understanding the various cutaneous manifestations of COVID-19 will aid in early detection and proper treatment, thus increasing patient satisfaction and outcomes.
Translating the 2020 AAD-NPF Guidelines of Care for the Management of Psoriasis With Systemic Nonbiologics to Clinical Practice
Psoriasis is a chronic relapsing skin condition characterized by keratinocyte hyperproliferation and a chronic inflammatory cascade. Therefore, controlling inflammatory responses with systemic medications is beneficial in managing psoriatic lesions and their accompanying symptoms, especially in disease inadequately controlled by topicals. Ease of drug administration and treatment availability are benefits that systemic nonbiologic therapies may have over biologic therapies.
In 2020, the American Academy of Dermatology (AAD) and the National Psoriasis Foundation (NPF) published guidelines for managing psoriasis in adults with systemic nonbiologic therapies.1 Dosing, efficacy, toxicity, drug-related interactions, and contraindications are addressed alongside evidence-based treatment recommendations. This review addresses current recommendations for systemic nonbiologics in psoriasis with a focus on the treatments approved by the US Food and Drug Administration (FDA): acitretin, apremilast, cyclosporine, and methotrexate (eTable). Fumaric acid esters and tofacitinib are FDA approved for psoriatic arthritis but not for plaque psoriasis. Additional long-term safety analyses of tofacitinib for plaque psoriasis were requested by the FDA. Dimethyl fumarate is approved by the European Medicines Agency for treatment of psoriasis and is among the first-line systemic treatments used in Germany.2
Selecting a Systemic Nonbiologic Agent
Methotrexate and apremilast have a strength level A recommendation for treating moderate to severe psoriasis in adults. However, methotrexate is less effective than biologic agents, including adalimumab and infliximab, for cutaneous psoriasis. Methotrexate is believed to improve psoriasis because of its direct immunosuppressive effect and inhibition of lymphoid cell proliferation. It typically is administered orally but can be administered subcutaneously for decreased gastrointestinal (GI) adverse effects. Compliance with close laboratory monitoring and lifestyle modifications, such as contraceptive use (because of teratogenicity) and alcohol cessation (because of the risk of liver damage) are essential in patients using methotrexate.
Apremilast, the most recently FDA-approved oral systemic medication for psoriasis, inhibits phosphodiesterase 4, subsequently decreasing inflammatory responses involving helper T cells TH1 and TH17 as well as type 1 interferon pathways. Apremilast is particularly effective in treating psoriasis with scalp and palmoplantar involvement.3 Additionally, it has an encouraging safety profile and is favorable in patients with multiple comorbidities.
Among the 4 oral agents, cyclosporine has the quickest onset of effect and has a strength level A recommendation for treating severe and recalcitrant psoriasis. Because of its high-risk profile, it is recommended for short periods of time, acute flares, or during transitions to safer long-term treatment. Patients with multiple comorbidities should avoid cyclosporine as a treatment option.
Acitretin, an FDA-approved oral retinoid, is an optimal treatment option for immunosuppressed patients or patients with HIV on antiretroviral therapy because it is not immunosuppressive.4 Unlike cyclosporine, acitretin is less helpful for acute flares because it takes 3 to 6 months to reach peak therapeutic response for treating plaque psoriasis. Similar to cyclosporine, acitretin can be recommended for severe psoriatic variants of erythrodermic, generalized pustular, and palmoplantar psoriasis. Acitretin has been reported to be more effective and have a more rapid onset of action in erythrodermic and pustular psoriasis than in plaque psoriasis.5
Patient Comorbidities
Psoriatic arthritis (PsA) is a common comorbidity that affects treatment choice. Patients with coexisting PsA could be treated with apremilast, as it is approved for both psoriasis and PsA. In a phase 3 randomized, controlled trial, American College of Rheumatology (ACR) 20 response at weeks 16 and 52 was achieved by significantly more patients on apremilast at 20 mg twice daily (BID)(P=.0166) or 30 mg BID (P=.0001) than placebo.6 Although not FDA approved for PsA, methotrexate has been shown to improve concomitant PsA of the peripheral joints in patients with psoriasis. Furthermore, a trial of methotrexate has shown considerable improvements in PsA symptoms in patients with psoriasis—a 62.7% decrease in proportion of patients with dactylitis, 25.7% decrease in enthesitis, and improvements in ACR outcomes (ACR20 in 40.8%, ACR50 in 18.8%, and ACR70 in 8.6%, with 22.4% achieving minimal disease activity).7
Prior to starting a systemic medication for psoriasis, it is necessary to discuss effects on pregnancy and fertility. Pregnancy is an absolute contraindication for methotrexate and acitretin use because of the drugs’ teratogenicity. Fetal death and fetal abnormalities have been reported with methotrexate use in pregnant women.8 Bone, central nervous system, auditory, ocular, and cardiovascular fetal abnormalities have been reported with maternal acitretin use.9 Breastfeeding also is an absolute contraindication for methotrexate use, as methotrexate passes into breastmilk in small quantities. Patients taking acitretin also are strongly discouraged from nursing because of the long half-life (168 days) of etretinate, a reverse metabolism product of acitretin that is increased in the presence of alcohol. Women should wait 3 months after discontinuing methotrexate for complete drug clearance before conceiving compared to 3 years in women who have discontinued acitretin.8,10 Men also are recommended to wait 3 months after discontinuing methotrexate before attempting to conceive, as its effect on male spermatogenesis and teratogenicity is unclear. Acitretin has no documented teratogenic effect in men. For women planning to become pregnant, apremilast and cyclosporine can be continued throughout pregnancy on an individual basis. The benefit of apremilast should be weighed against its potential risk to the fetus. There is no evidence of teratogenicity of apremilast at doses of 20 mg/kg daily.11 Current research regarding cyclosporine use in pregnancy only exists in transplant patients and has revealed higher rates of prematurity and lower birth weight without teratogenic effects.10,12 The risks and benefits of continuing cyclosporine while nursing should be evaluated, as cyclosporine (and ethanol-methanol components used in some formulations) is detectable in breast milk.
Drug Contraindications
Hypersensitivity to a specific systemic nonbiologic medication is a contraindication to its use and is an absolute contraindication for methotrexate. Other absolute contraindications to methotrexate are pregnancy and nursing, alcoholism, alcoholic liver disease, chronic liver disease, immunodeficiency, and cytopenia. Contraindications to acitretin include pregnancy, severely impaired liver and kidney function, and chronic abnormally elevated lipid levels. There are no additional contraindications for apremilast, but patients must be informed of the risk for depression before initiating therapy. Cyclosporine is contraindicated in patients with prior psoralen plus UVA (PUVA) treatment or radiation therapy, abnormal renal function, uncontrolled hypertension, uncontrolled and active infections, and a history of systemic malignancy. Live vaccines should be avoided in patients on cyclosporine, and caution is advised when cyclosporine is prescribed for patients with poorly controlled diabetes.
Pretreatment Screening
Because of drug interactions, a detailed medication history is essential prior to starting any systemic medication for psoriasis. Apremilast and cyclosporine are metabolized by cytochrome P450 and therefore are more susceptible to drug-related interactions. Cyclosporine use can affect levels of other medications that are metabolized by cytochrome P450, such as statins, calcium channel blockers, and warfarin. Similarly, acitretin’s metabolism is affected by drugs that interfere with cytochrome P450. Additionally, screening laboratory tests are needed before initiating systemic nonbiologic agents for psoriasis, with the exception of apremilast.
Prior to initiating methotrexate treatment, patients may require tuberculosis (TB), hepatitis B, and hepatitis C screening tests, depending on their risk factors. A baseline liver fibrosis assessment is recommended because of the potential of hepatotoxicity in patients receiving methotrexate. Noninvasive serology tests utilized to evaluate the presence of pre-existing liver disease include Fibrosis-4, FibroMeter, FibroSure, and Hepascore. Patients with impaired renal function have an increased predisposition to methotrexate-induced hematologic toxicity. Thus, it is necessary to administer a test dose of methotrexate in these patients followed by a complete blood cell count (CBC) 5 to 7 days later. An unremarkable CBC after the test dose suggests the absence of myelosuppression, and methotrexate dosage can be increased weekly. Patients on methotrexate also must receive folate supplementation to reduce the risk for adverse effects during treatment.
Patients considering cyclosporine must undergo screening for family and personal history of renal disease. Prior to initiating treatment, patients require 2 blood pressure measurements, hepatitis screening, TB screening, urinalysis, serum creatinine (Cr), blood urea nitrogen (BUN), CBC, potassium and magnesium levels, uric acid levels, lipid profile, bilirubin, and liver function tests (LFTs). A pregnancy test also is warranted for women of childbearing potential (WOCP).
Patients receiving acitretin should receive screening laboratory tests consisting of fasting cholesterol and triglycerides, CBC, renal function tests, LFTs, and a pregnancy test, if applicable.
After baseline evaluations, the selected oral systemic can be initiated using specific dosing regimens to ensure optimal drug efficacy and reduce incidence of adverse effects (eTable).
Monitoring During Active Treatment
Physicians need to counsel patients on potential adverse effects of their medications. Because of its relatively safe profile among the systemic nonbiologic agents, apremilast requires the least monitoring during treatment. There is no required routine laboratory monitoring for patients using apremilast, though testing may be pursued at the clinician’s discretion. However, weight should be regularly measured in patients on apremilast. In a phase 3 clinical trial of patients with psoriasis, 12% of patients on apremilast experienced a 5% to 10% weight loss compared to 5% of patients on placebo.11,13 Thus, it is recommended that physicians consider discontinuing apremilast in patients with a weight loss of more than 5% from baseline, especially if it may lead to other unfavorable health effects. Because depression is reported among 1% of patients on apremilast, close monitoring for new or worsening symptoms of depression should be performed during treatment.11,13 To avoid common GI side effects, apremilast is initiated at 10 mg/d and is increased by 10 mg/d over the first 5 days to a final dose of 30 mg BID. Elderly patients in particular should be cautioned about the risk of dehydration associated with GI side effects. Patients with severe renal impairment (Cr clearance, <30 mL/min) should use apremilast at a dosage of 30 mg once daily.
For patients on methotrexate, laboratory monitoring is essential after each dose increase. It also is important for physicians to obtain regular blood work to assess for hematologic abnormalities and hepatoxicity. Patients with risk factors such as renal insufficiency, increased age, hypoalbuminemia, alcohol abuse and alcoholic liver disease, and methotrexate dosing errors, as well as those prone to drug-related interactions, must be monitored closely for pancytopenia.14,15 The protocol for screening for methotrexate-induced hepatotoxicity during treatment depends on patient risk factors. Risk factors for hepatoxicity include history of or current alcohol abuse, abnormal LFTs, personal or family history of liver disease, diabetes, obesity, use of other hepatotoxic drugs, and hyperlipidemia.16 In patients without blood work abnormalities, CBC and LFTs can be performed every 3 to 6 months. Patients with abnormally elevated LFTs require repeat blood work every 2 to 4 weeks. Persistent elevations in LFTs require further evaluation by a GI specialist. After a cumulative dose of 3.5 to 4 g, patients should receive a GI referral and further studies (such as vibration-controlled transient elastography or liver biopsy) to assess for liver fibrosis. Patients with signs of stage 3 liver fibrosis are recommended to discontinue methotrexate and switch to another medication for psoriasis. For patients with impaired renal function, periodic BUN and Cr monitoring are needed. Common adverse effects of methotrexate include diarrhea, nausea, and anorexia, which can be mitigated by taking methotrexate with food or lowering the dosage.8 Patients on methotrexate should be monitored for rare but potential risks of infection and reactivation of latent TB, hepatitis, and lymphoma. To reduce the incidence of methotrexate toxicity from drug interactions, a review of current medications at each follow-up visit is recommended.
Nephrotoxicity and hypertension are the most common adverse effects of cyclosporine. It is important to monitor BUN and Cr biweekly for the initial 3 months, then at monthly intervals if there are no persistent abnormalities. Patients also must receive monthly CBC, potassium and magnesium levels, uric acid levels, lipid panel, serum bilirubin, and LFTs to monitor for adverse effects.17 Physicians should obtain regular pregnancy tests in WOCP. Weekly monitoring of early-morning blood pressure is recommended for patients on cyclosporine to detect early cyclosporine-induced nephrotoxicity. Hypertension on 2 separate occasions warrants a reduction in cyclosporine dosage or an addition of a calcium channel blocker for blood pressure control. Dose reduction also should be performed in patients with an increase in Cr above baseline greater than 25%.17 If Cr level is persistently elevated or if blood pressure does not normalize to lower than 140/90 after dose reduction, cyclosporine should be immediately discontinued. Patients on cyclosporine for more than a year warrant an annual estimation of glomerular filtration rate because of irreversible kidney damage associated with long-term use. A systematic review of patients treated with cyclosporine for more than 2 years found that at least 50% of patients experienced a 30% increase in Cr above baseline.18
Patients taking acitretin should be monitored for hyperlipidemia, the most common laboratory abnormality seen in 25% to 50% of patients.19 Fasting lipid panel and LFTs should be performed monthly for the initial 3 months on acitretin, then at 3-month intervals. Lifestyle changes should be encouraged to reduce hyperlipidemia, and fibrates may be given to treat elevated triglyceride levels, the most common type of hyperlipidemia seen with acitretin. Acitretin-induced toxic hepatitis is a rare occurrence that warrants immediate discontinuation of the medication.20 Monthly pregnancy tests must be performed in WOCP.
Combination Therapy
For apremilast, there is anecdotal evidence supporting its use in conjunction with phototherapy or biologics in some cases, but no high-quality data.21 On the other hand, using combination therapy with other systemic therapies can reduce adverse effects and decrease the amount of medication needed to achieve psoriasis clearance. Methotrexate used with etanercept, for example, has been more effective than methotrexate monotherapy in treating psoriasis, which has been attributed to a methotrexate-mediated reduction in the production of antidrug antibodies.22,23
Methotrexate, cyclosporine, and acitretin have synergistic effects when used with phototherapy. Narrowband UVB (NB-UVB) phototherapy combined with methotrexate is more effective in clearing psoriasis than methotrexate or NB-UVB phototherapy alone. Similarly, acitretin and PUVA combination therapy is more effective than acitretin or PUVA phototherapy alone. Combination regimens of acitretin and broadband UVB phototherapy, acitretin and NB-UVB phototherapy, and acitretin and PUVA phototherapy also have been more effective than individual modalities alone. Combination therapy reduces the cumulative doses of both therapies and reduces the frequency and duration of phototherapy needed for psoriatic clearance.24 In acitretin combination therapy with UVB phototherapy, the recommended regimen is 2 weeks of acitretin monotherapy followed by UVB phototherapy. For patients with an inadequate response to UVB phototherapy, the UVB dose can be reduced by 30% to 50%, and acitretin 25 mg/d can be added to phototherapy treatment. Acitretin-UVB combination therapy has been shown to reduce the risk of UVB-induced erythema seen in UVB monotherapy. Similarly, the risk of squamous cell carcinoma is reduced in acitretin-PUVA combination therapy compared to PUVA monotherapy.25
The timing of phototherapy in combination with systemic nonbiologic agents is critical. Phototherapy used simultaneously with cyclosporine is contraindicated owing to increased risk of photocarcinogenesis, whereas phototherapy used in sequence with cyclosporine is well tolerated and effective. Furthermore, cyclosporine 3 mg/kg/d for 4 weeks followed by a rapid cyclosporine taper and initiation of NB-UVB phototherapy demonstrated resolution of psoriasis with fewer NB-UVB treatments and less UVB exposure than NB-UVB therapy alone.26
Final Thoughts
The FDA-approved systemic nonbiologic agents are accessible and effective treatment options for adults with widespread or inadequately controlled psoriasis. Selecting the ideal therapy requires careful consideration of medication toxicity, contraindications, monitoring requirements, and patient comorbidities. The AAD-NPF guidelines guide dermatologists in prescribing systemic nonbiologic treatments in adults with psoriasis. Utilizing these recommendations in combination with clinician judgment will help patients achieve safe and optimal psoriasis clearance.
- Menter A, Gelfand JM, Connor C, et al. Joint American Academy of Dermatology-National Psoriasis Foundation guidelines of care for the management of psoriasis with systemic nonbiologic therapies. J Am Acad Dermatol. 2020;82:1445-1486.
- Mrowietz U, Barker J, Boehncke WH, et al. Clinical use of dimethyl fumarate in moderate-to-severe plaque-type psoriasis: a European expert consensus. J Eur Acad Dermatol Venereol. 2018;32(suppl 3):3-14.
- Van Voorhees AS, Gold LS, Lebwohl M, et al. Efficacy and safety of apremilast in patients with moderate to severe plaque psoriasis of the scalp: results of a phase 3b, multicenter, randomized, placebo-controlled, double-blind study. J Am Acad Dermatol. 2020;83:96-103.
- Buccheri L, Katchen BR, Karter AJ, et al. Acitretin therapy is effective for psoriasis associated with human immunodeficiency virus infection. Arch Dermatol. 1997;133:711-715.
- Ormerod AD, Campalani E, Goodfield MJD. British Association of Dermatologists guidelines on the efficacy and use of acitretin in dermatology. Br J Dermatol. 2010;162:952-963.
- Kavanaugh A, Mease PJ, Gomez-Reino JJ, et al. Longterm (52-week) results of a phase III randomized, controlled trial of apremilast in patients with psoriatic arthritis. J Rheumatol. 2015;42:479-488.
- Coates LC, Aslam T, Al Balushi F, et al. Comparison of three screening tools to detect psoriatic arthritis in patients with psoriasis (CONTEST study). Br J Dermatol. 2013;168:802-807.
- Antares Pharma, Inc. Otrexup PFS (methotrexate) [package insert]. US Food and Drug Administration website. Revised June 2019. Accessed February 28, 2020. https://www.accessdata.fda.gov/drugsatfda_docs/label/2019/204824s009lbl.pdf
- David M, Hodak E, Lowe NJ. Adverse effects of retinoids. Med Toxicol Adverse Drug Exp. 1988;3:273-288.
- Stiefel Laboratories, Inc. Soriatane (acitretin) [package insert]. US Food and Drug Administration website. Revised September 2017. Accessed February 28, 2020. https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/019821s028lbl.pdf
- Celgene Corporation. Otezla (apremilast) [package insert]. US Food and Drug Administration website. Revised March 2014. Accessed February 28, 2020. https://www.accessdata.fda.gov/drugsatfda_docs/label/2014/205437s000lbl.pdf
- Ghanem ME, El-Baghdadi LA, Badawy AM, et al. Pregnancy outcome after renal allograft transplantation: 15 years experience. Eur J Obstet Gynecol Reprod Biol. 2005;121:178-181.
- Zerilli T, Ocheretyaner E. Apremilast (Otezla): A new oral treatment for adults with psoriasis and psoriatic arthritis. P T. 2015;40:495-500.
- Kivity S, Zafrir Y, Loebstein R, et al. Clinical characteristics and risk factors for low dose methotrexate toxicity: a cohort of 28 patients. Autoimmun Rev. 2014;13:1109-1113.
- Boffa MJ, Chalmers RJ. Methotrexate for psoriasis. Clin Exp Dermatol. 1996;21:399-408.
- Rosenberg P, Urwitz H, Johannesson A, et al. Psoriasis patients with diabetes type 2 are at high risk of developing liver fibrosis during methotrexate treatment. J Hepatol. 2007;46:1111-1118.
- Novartis Pharmaceuticals Corporation. Sandimmune (cyclosporine) [package insert]. US Food and Drug Administration website. Published 2015. Accessed February 28, 2020. https://www.accessdata.fda.gov/drugsatfda_docs/label/2015/050573s041,050574s051,050625s055lbl.pdf
- Maza A, Montaudie H, Sbidian E, et al. Oral cyclosporin in psoriasis: a systematic review on treatment modalities, risk of kidney toxicity and evidence for use in non-plaque psoriasis. J Eur Acad Dermatol Venereol. 2011;25(suppl 2):19-27.
- Yamauchi PS, Rizk D, Kormilli T, et al. Systemic retinoids. In: Weinstein GD, Gottlieb AB, eds. Therapy of Moderate-to-Severe Psoriasis. Marcel Dekker; 2003:137-150.
- van Ditzhuijsen TJ, van Haelst UJ, van Dooren-Greebe RJ, et al. Severe hepatotoxic reaction with progression to cirrhosis after use of a novel retinoid (acitretin). J Hepatol. 1990;11:185-188.
- AbuHilal M, Walsh S, Shear N. Use of apremilast in combination with other therapies for treatment of chronic plaque psoriasis: a retrospective study. J Cutan Med Surg. 2016;20:313-316.
- Gottlieb AB, Langley RG, Strober BE, et al. A randomized, double-blind, placebo-controlled study to evaluate the addition of methotrexate to etanercept in patients with moderate to severe plaque psoriasis. Br J Dermatol. 2012;167:649-657.
- Cronstein BN. Methotrexate BAFFles anti-drug antibodies. Nat Rev Rheumatol. 2018;14:505-506.
- Lebwohl M, Drake L, Menter A, et al. Consensus conference: acitretin in combination with UVB or PUVA in the treatment of psoriasis. J Am Acad Dermatol. 2001;45:544-553.
- Nijsten TE, Stern RS. Oral retinoid use reduces cutaneous squamous cell carcinoma risk in patients with psoriasis treated with psoralen-UVA: a nested cohort study. J Am Acad Dermatol. 2003;49:644-650.
- Calzavara-Pinton P, Leone G, Venturini M, et al. A comparative non randomized study of narrow-band (NB) (312 +/- 2 nm) UVB phototherapy versus sequential therapy with oral administration of low-dose cyclosporin A and NB-UVB phototherapy in patients with severe psoriasis vulgaris. Eur J Dermatol. 2005;15:470-473.
Psoriasis is a chronic relapsing skin condition characterized by keratinocyte hyperproliferation and a chronic inflammatory cascade. Therefore, controlling inflammatory responses with systemic medications is beneficial in managing psoriatic lesions and their accompanying symptoms, especially in disease inadequately controlled by topicals. Ease of drug administration and treatment availability are benefits that systemic nonbiologic therapies may have over biologic therapies.
In 2020, the American Academy of Dermatology (AAD) and the National Psoriasis Foundation (NPF) published guidelines for managing psoriasis in adults with systemic nonbiologic therapies.1 Dosing, efficacy, toxicity, drug-related interactions, and contraindications are addressed alongside evidence-based treatment recommendations. This review addresses current recommendations for systemic nonbiologics in psoriasis with a focus on the treatments approved by the US Food and Drug Administration (FDA): acitretin, apremilast, cyclosporine, and methotrexate (eTable). Fumaric acid esters and tofacitinib are FDA approved for psoriatic arthritis but not for plaque psoriasis. Additional long-term safety analyses of tofacitinib for plaque psoriasis were requested by the FDA. Dimethyl fumarate is approved by the European Medicines Agency for treatment of psoriasis and is among the first-line systemic treatments used in Germany.2
Selecting a Systemic Nonbiologic Agent
Methotrexate and apremilast have a strength level A recommendation for treating moderate to severe psoriasis in adults. However, methotrexate is less effective than biologic agents, including adalimumab and infliximab, for cutaneous psoriasis. Methotrexate is believed to improve psoriasis because of its direct immunosuppressive effect and inhibition of lymphoid cell proliferation. It typically is administered orally but can be administered subcutaneously for decreased gastrointestinal (GI) adverse effects. Compliance with close laboratory monitoring and lifestyle modifications, such as contraceptive use (because of teratogenicity) and alcohol cessation (because of the risk of liver damage) are essential in patients using methotrexate.
Apremilast, the most recently FDA-approved oral systemic medication for psoriasis, inhibits phosphodiesterase 4, subsequently decreasing inflammatory responses involving helper T cells TH1 and TH17 as well as type 1 interferon pathways. Apremilast is particularly effective in treating psoriasis with scalp and palmoplantar involvement.3 Additionally, it has an encouraging safety profile and is favorable in patients with multiple comorbidities.
Among the 4 oral agents, cyclosporine has the quickest onset of effect and has a strength level A recommendation for treating severe and recalcitrant psoriasis. Because of its high-risk profile, it is recommended for short periods of time, acute flares, or during transitions to safer long-term treatment. Patients with multiple comorbidities should avoid cyclosporine as a treatment option.
Acitretin, an FDA-approved oral retinoid, is an optimal treatment option for immunosuppressed patients or patients with HIV on antiretroviral therapy because it is not immunosuppressive.4 Unlike cyclosporine, acitretin is less helpful for acute flares because it takes 3 to 6 months to reach peak therapeutic response for treating plaque psoriasis. Similar to cyclosporine, acitretin can be recommended for severe psoriatic variants of erythrodermic, generalized pustular, and palmoplantar psoriasis. Acitretin has been reported to be more effective and have a more rapid onset of action in erythrodermic and pustular psoriasis than in plaque psoriasis.5
Patient Comorbidities
Psoriatic arthritis (PsA) is a common comorbidity that affects treatment choice. Patients with coexisting PsA could be treated with apremilast, as it is approved for both psoriasis and PsA. In a phase 3 randomized, controlled trial, American College of Rheumatology (ACR) 20 response at weeks 16 and 52 was achieved by significantly more patients on apremilast at 20 mg twice daily (BID)(P=.0166) or 30 mg BID (P=.0001) than placebo.6 Although not FDA approved for PsA, methotrexate has been shown to improve concomitant PsA of the peripheral joints in patients with psoriasis. Furthermore, a trial of methotrexate has shown considerable improvements in PsA symptoms in patients with psoriasis—a 62.7% decrease in proportion of patients with dactylitis, 25.7% decrease in enthesitis, and improvements in ACR outcomes (ACR20 in 40.8%, ACR50 in 18.8%, and ACR70 in 8.6%, with 22.4% achieving minimal disease activity).7
Prior to starting a systemic medication for psoriasis, it is necessary to discuss effects on pregnancy and fertility. Pregnancy is an absolute contraindication for methotrexate and acitretin use because of the drugs’ teratogenicity. Fetal death and fetal abnormalities have been reported with methotrexate use in pregnant women.8 Bone, central nervous system, auditory, ocular, and cardiovascular fetal abnormalities have been reported with maternal acitretin use.9 Breastfeeding also is an absolute contraindication for methotrexate use, as methotrexate passes into breastmilk in small quantities. Patients taking acitretin also are strongly discouraged from nursing because of the long half-life (168 days) of etretinate, a reverse metabolism product of acitretin that is increased in the presence of alcohol. Women should wait 3 months after discontinuing methotrexate for complete drug clearance before conceiving compared to 3 years in women who have discontinued acitretin.8,10 Men also are recommended to wait 3 months after discontinuing methotrexate before attempting to conceive, as its effect on male spermatogenesis and teratogenicity is unclear. Acitretin has no documented teratogenic effect in men. For women planning to become pregnant, apremilast and cyclosporine can be continued throughout pregnancy on an individual basis. The benefit of apremilast should be weighed against its potential risk to the fetus. There is no evidence of teratogenicity of apremilast at doses of 20 mg/kg daily.11 Current research regarding cyclosporine use in pregnancy only exists in transplant patients and has revealed higher rates of prematurity and lower birth weight without teratogenic effects.10,12 The risks and benefits of continuing cyclosporine while nursing should be evaluated, as cyclosporine (and ethanol-methanol components used in some formulations) is detectable in breast milk.
Drug Contraindications
Hypersensitivity to a specific systemic nonbiologic medication is a contraindication to its use and is an absolute contraindication for methotrexate. Other absolute contraindications to methotrexate are pregnancy and nursing, alcoholism, alcoholic liver disease, chronic liver disease, immunodeficiency, and cytopenia. Contraindications to acitretin include pregnancy, severely impaired liver and kidney function, and chronic abnormally elevated lipid levels. There are no additional contraindications for apremilast, but patients must be informed of the risk for depression before initiating therapy. Cyclosporine is contraindicated in patients with prior psoralen plus UVA (PUVA) treatment or radiation therapy, abnormal renal function, uncontrolled hypertension, uncontrolled and active infections, and a history of systemic malignancy. Live vaccines should be avoided in patients on cyclosporine, and caution is advised when cyclosporine is prescribed for patients with poorly controlled diabetes.
Pretreatment Screening
Because of drug interactions, a detailed medication history is essential prior to starting any systemic medication for psoriasis. Apremilast and cyclosporine are metabolized by cytochrome P450 and therefore are more susceptible to drug-related interactions. Cyclosporine use can affect levels of other medications that are metabolized by cytochrome P450, such as statins, calcium channel blockers, and warfarin. Similarly, acitretin’s metabolism is affected by drugs that interfere with cytochrome P450. Additionally, screening laboratory tests are needed before initiating systemic nonbiologic agents for psoriasis, with the exception of apremilast.
Prior to initiating methotrexate treatment, patients may require tuberculosis (TB), hepatitis B, and hepatitis C screening tests, depending on their risk factors. A baseline liver fibrosis assessment is recommended because of the potential of hepatotoxicity in patients receiving methotrexate. Noninvasive serology tests utilized to evaluate the presence of pre-existing liver disease include Fibrosis-4, FibroMeter, FibroSure, and Hepascore. Patients with impaired renal function have an increased predisposition to methotrexate-induced hematologic toxicity. Thus, it is necessary to administer a test dose of methotrexate in these patients followed by a complete blood cell count (CBC) 5 to 7 days later. An unremarkable CBC after the test dose suggests the absence of myelosuppression, and methotrexate dosage can be increased weekly. Patients on methotrexate also must receive folate supplementation to reduce the risk for adverse effects during treatment.
Patients considering cyclosporine must undergo screening for family and personal history of renal disease. Prior to initiating treatment, patients require 2 blood pressure measurements, hepatitis screening, TB screening, urinalysis, serum creatinine (Cr), blood urea nitrogen (BUN), CBC, potassium and magnesium levels, uric acid levels, lipid profile, bilirubin, and liver function tests (LFTs). A pregnancy test also is warranted for women of childbearing potential (WOCP).
Patients receiving acitretin should receive screening laboratory tests consisting of fasting cholesterol and triglycerides, CBC, renal function tests, LFTs, and a pregnancy test, if applicable.
After baseline evaluations, the selected oral systemic can be initiated using specific dosing regimens to ensure optimal drug efficacy and reduce incidence of adverse effects (eTable).
Monitoring During Active Treatment
Physicians need to counsel patients on potential adverse effects of their medications. Because of its relatively safe profile among the systemic nonbiologic agents, apremilast requires the least monitoring during treatment. There is no required routine laboratory monitoring for patients using apremilast, though testing may be pursued at the clinician’s discretion. However, weight should be regularly measured in patients on apremilast. In a phase 3 clinical trial of patients with psoriasis, 12% of patients on apremilast experienced a 5% to 10% weight loss compared to 5% of patients on placebo.11,13 Thus, it is recommended that physicians consider discontinuing apremilast in patients with a weight loss of more than 5% from baseline, especially if it may lead to other unfavorable health effects. Because depression is reported among 1% of patients on apremilast, close monitoring for new or worsening symptoms of depression should be performed during treatment.11,13 To avoid common GI side effects, apremilast is initiated at 10 mg/d and is increased by 10 mg/d over the first 5 days to a final dose of 30 mg BID. Elderly patients in particular should be cautioned about the risk of dehydration associated with GI side effects. Patients with severe renal impairment (Cr clearance, <30 mL/min) should use apremilast at a dosage of 30 mg once daily.
For patients on methotrexate, laboratory monitoring is essential after each dose increase. It also is important for physicians to obtain regular blood work to assess for hematologic abnormalities and hepatoxicity. Patients with risk factors such as renal insufficiency, increased age, hypoalbuminemia, alcohol abuse and alcoholic liver disease, and methotrexate dosing errors, as well as those prone to drug-related interactions, must be monitored closely for pancytopenia.14,15 The protocol for screening for methotrexate-induced hepatotoxicity during treatment depends on patient risk factors. Risk factors for hepatoxicity include history of or current alcohol abuse, abnormal LFTs, personal or family history of liver disease, diabetes, obesity, use of other hepatotoxic drugs, and hyperlipidemia.16 In patients without blood work abnormalities, CBC and LFTs can be performed every 3 to 6 months. Patients with abnormally elevated LFTs require repeat blood work every 2 to 4 weeks. Persistent elevations in LFTs require further evaluation by a GI specialist. After a cumulative dose of 3.5 to 4 g, patients should receive a GI referral and further studies (such as vibration-controlled transient elastography or liver biopsy) to assess for liver fibrosis. Patients with signs of stage 3 liver fibrosis are recommended to discontinue methotrexate and switch to another medication for psoriasis. For patients with impaired renal function, periodic BUN and Cr monitoring are needed. Common adverse effects of methotrexate include diarrhea, nausea, and anorexia, which can be mitigated by taking methotrexate with food or lowering the dosage.8 Patients on methotrexate should be monitored for rare but potential risks of infection and reactivation of latent TB, hepatitis, and lymphoma. To reduce the incidence of methotrexate toxicity from drug interactions, a review of current medications at each follow-up visit is recommended.
Nephrotoxicity and hypertension are the most common adverse effects of cyclosporine. It is important to monitor BUN and Cr biweekly for the initial 3 months, then at monthly intervals if there are no persistent abnormalities. Patients also must receive monthly CBC, potassium and magnesium levels, uric acid levels, lipid panel, serum bilirubin, and LFTs to monitor for adverse effects.17 Physicians should obtain regular pregnancy tests in WOCP. Weekly monitoring of early-morning blood pressure is recommended for patients on cyclosporine to detect early cyclosporine-induced nephrotoxicity. Hypertension on 2 separate occasions warrants a reduction in cyclosporine dosage or an addition of a calcium channel blocker for blood pressure control. Dose reduction also should be performed in patients with an increase in Cr above baseline greater than 25%.17 If Cr level is persistently elevated or if blood pressure does not normalize to lower than 140/90 after dose reduction, cyclosporine should be immediately discontinued. Patients on cyclosporine for more than a year warrant an annual estimation of glomerular filtration rate because of irreversible kidney damage associated with long-term use. A systematic review of patients treated with cyclosporine for more than 2 years found that at least 50% of patients experienced a 30% increase in Cr above baseline.18
Patients taking acitretin should be monitored for hyperlipidemia, the most common laboratory abnormality seen in 25% to 50% of patients.19 Fasting lipid panel and LFTs should be performed monthly for the initial 3 months on acitretin, then at 3-month intervals. Lifestyle changes should be encouraged to reduce hyperlipidemia, and fibrates may be given to treat elevated triglyceride levels, the most common type of hyperlipidemia seen with acitretin. Acitretin-induced toxic hepatitis is a rare occurrence that warrants immediate discontinuation of the medication.20 Monthly pregnancy tests must be performed in WOCP.
Combination Therapy
For apremilast, there is anecdotal evidence supporting its use in conjunction with phototherapy or biologics in some cases, but no high-quality data.21 On the other hand, using combination therapy with other systemic therapies can reduce adverse effects and decrease the amount of medication needed to achieve psoriasis clearance. Methotrexate used with etanercept, for example, has been more effective than methotrexate monotherapy in treating psoriasis, which has been attributed to a methotrexate-mediated reduction in the production of antidrug antibodies.22,23
Methotrexate, cyclosporine, and acitretin have synergistic effects when used with phototherapy. Narrowband UVB (NB-UVB) phototherapy combined with methotrexate is more effective in clearing psoriasis than methotrexate or NB-UVB phototherapy alone. Similarly, acitretin and PUVA combination therapy is more effective than acitretin or PUVA phototherapy alone. Combination regimens of acitretin and broadband UVB phototherapy, acitretin and NB-UVB phototherapy, and acitretin and PUVA phototherapy also have been more effective than individual modalities alone. Combination therapy reduces the cumulative doses of both therapies and reduces the frequency and duration of phototherapy needed for psoriatic clearance.24 In acitretin combination therapy with UVB phototherapy, the recommended regimen is 2 weeks of acitretin monotherapy followed by UVB phototherapy. For patients with an inadequate response to UVB phototherapy, the UVB dose can be reduced by 30% to 50%, and acitretin 25 mg/d can be added to phototherapy treatment. Acitretin-UVB combination therapy has been shown to reduce the risk of UVB-induced erythema seen in UVB monotherapy. Similarly, the risk of squamous cell carcinoma is reduced in acitretin-PUVA combination therapy compared to PUVA monotherapy.25
The timing of phototherapy in combination with systemic nonbiologic agents is critical. Phototherapy used simultaneously with cyclosporine is contraindicated owing to increased risk of photocarcinogenesis, whereas phototherapy used in sequence with cyclosporine is well tolerated and effective. Furthermore, cyclosporine 3 mg/kg/d for 4 weeks followed by a rapid cyclosporine taper and initiation of NB-UVB phototherapy demonstrated resolution of psoriasis with fewer NB-UVB treatments and less UVB exposure than NB-UVB therapy alone.26
Final Thoughts
The FDA-approved systemic nonbiologic agents are accessible and effective treatment options for adults with widespread or inadequately controlled psoriasis. Selecting the ideal therapy requires careful consideration of medication toxicity, contraindications, monitoring requirements, and patient comorbidities. The AAD-NPF guidelines guide dermatologists in prescribing systemic nonbiologic treatments in adults with psoriasis. Utilizing these recommendations in combination with clinician judgment will help patients achieve safe and optimal psoriasis clearance.
Psoriasis is a chronic relapsing skin condition characterized by keratinocyte hyperproliferation and a chronic inflammatory cascade. Therefore, controlling inflammatory responses with systemic medications is beneficial in managing psoriatic lesions and their accompanying symptoms, especially in disease inadequately controlled by topicals. Ease of drug administration and treatment availability are benefits that systemic nonbiologic therapies may have over biologic therapies.
In 2020, the American Academy of Dermatology (AAD) and the National Psoriasis Foundation (NPF) published guidelines for managing psoriasis in adults with systemic nonbiologic therapies.1 Dosing, efficacy, toxicity, drug-related interactions, and contraindications are addressed alongside evidence-based treatment recommendations. This review addresses current recommendations for systemic nonbiologics in psoriasis with a focus on the treatments approved by the US Food and Drug Administration (FDA): acitretin, apremilast, cyclosporine, and methotrexate (eTable). Fumaric acid esters and tofacitinib are FDA approved for psoriatic arthritis but not for plaque psoriasis. Additional long-term safety analyses of tofacitinib for plaque psoriasis were requested by the FDA. Dimethyl fumarate is approved by the European Medicines Agency for treatment of psoriasis and is among the first-line systemic treatments used in Germany.2
Selecting a Systemic Nonbiologic Agent
Methotrexate and apremilast have a strength level A recommendation for treating moderate to severe psoriasis in adults. However, methotrexate is less effective than biologic agents, including adalimumab and infliximab, for cutaneous psoriasis. Methotrexate is believed to improve psoriasis because of its direct immunosuppressive effect and inhibition of lymphoid cell proliferation. It typically is administered orally but can be administered subcutaneously for decreased gastrointestinal (GI) adverse effects. Compliance with close laboratory monitoring and lifestyle modifications, such as contraceptive use (because of teratogenicity) and alcohol cessation (because of the risk of liver damage) are essential in patients using methotrexate.
Apremilast, the most recently FDA-approved oral systemic medication for psoriasis, inhibits phosphodiesterase 4, subsequently decreasing inflammatory responses involving helper T cells TH1 and TH17 as well as type 1 interferon pathways. Apremilast is particularly effective in treating psoriasis with scalp and palmoplantar involvement.3 Additionally, it has an encouraging safety profile and is favorable in patients with multiple comorbidities.
Among the 4 oral agents, cyclosporine has the quickest onset of effect and has a strength level A recommendation for treating severe and recalcitrant psoriasis. Because of its high-risk profile, it is recommended for short periods of time, acute flares, or during transitions to safer long-term treatment. Patients with multiple comorbidities should avoid cyclosporine as a treatment option.
Acitretin, an FDA-approved oral retinoid, is an optimal treatment option for immunosuppressed patients or patients with HIV on antiretroviral therapy because it is not immunosuppressive.4 Unlike cyclosporine, acitretin is less helpful for acute flares because it takes 3 to 6 months to reach peak therapeutic response for treating plaque psoriasis. Similar to cyclosporine, acitretin can be recommended for severe psoriatic variants of erythrodermic, generalized pustular, and palmoplantar psoriasis. Acitretin has been reported to be more effective and have a more rapid onset of action in erythrodermic and pustular psoriasis than in plaque psoriasis.5
Patient Comorbidities
Psoriatic arthritis (PsA) is a common comorbidity that affects treatment choice. Patients with coexisting PsA could be treated with apremilast, as it is approved for both psoriasis and PsA. In a phase 3 randomized, controlled trial, American College of Rheumatology (ACR) 20 response at weeks 16 and 52 was achieved by significantly more patients on apremilast at 20 mg twice daily (BID)(P=.0166) or 30 mg BID (P=.0001) than placebo.6 Although not FDA approved for PsA, methotrexate has been shown to improve concomitant PsA of the peripheral joints in patients with psoriasis. Furthermore, a trial of methotrexate has shown considerable improvements in PsA symptoms in patients with psoriasis—a 62.7% decrease in proportion of patients with dactylitis, 25.7% decrease in enthesitis, and improvements in ACR outcomes (ACR20 in 40.8%, ACR50 in 18.8%, and ACR70 in 8.6%, with 22.4% achieving minimal disease activity).7
Prior to starting a systemic medication for psoriasis, it is necessary to discuss effects on pregnancy and fertility. Pregnancy is an absolute contraindication for methotrexate and acitretin use because of the drugs’ teratogenicity. Fetal death and fetal abnormalities have been reported with methotrexate use in pregnant women.8 Bone, central nervous system, auditory, ocular, and cardiovascular fetal abnormalities have been reported with maternal acitretin use.9 Breastfeeding also is an absolute contraindication for methotrexate use, as methotrexate passes into breastmilk in small quantities. Patients taking acitretin also are strongly discouraged from nursing because of the long half-life (168 days) of etretinate, a reverse metabolism product of acitretin that is increased in the presence of alcohol. Women should wait 3 months after discontinuing methotrexate for complete drug clearance before conceiving compared to 3 years in women who have discontinued acitretin.8,10 Men also are recommended to wait 3 months after discontinuing methotrexate before attempting to conceive, as its effect on male spermatogenesis and teratogenicity is unclear. Acitretin has no documented teratogenic effect in men. For women planning to become pregnant, apremilast and cyclosporine can be continued throughout pregnancy on an individual basis. The benefit of apremilast should be weighed against its potential risk to the fetus. There is no evidence of teratogenicity of apremilast at doses of 20 mg/kg daily.11 Current research regarding cyclosporine use in pregnancy only exists in transplant patients and has revealed higher rates of prematurity and lower birth weight without teratogenic effects.10,12 The risks and benefits of continuing cyclosporine while nursing should be evaluated, as cyclosporine (and ethanol-methanol components used in some formulations) is detectable in breast milk.
Drug Contraindications
Hypersensitivity to a specific systemic nonbiologic medication is a contraindication to its use and is an absolute contraindication for methotrexate. Other absolute contraindications to methotrexate are pregnancy and nursing, alcoholism, alcoholic liver disease, chronic liver disease, immunodeficiency, and cytopenia. Contraindications to acitretin include pregnancy, severely impaired liver and kidney function, and chronic abnormally elevated lipid levels. There are no additional contraindications for apremilast, but patients must be informed of the risk for depression before initiating therapy. Cyclosporine is contraindicated in patients with prior psoralen plus UVA (PUVA) treatment or radiation therapy, abnormal renal function, uncontrolled hypertension, uncontrolled and active infections, and a history of systemic malignancy. Live vaccines should be avoided in patients on cyclosporine, and caution is advised when cyclosporine is prescribed for patients with poorly controlled diabetes.
Pretreatment Screening
Because of drug interactions, a detailed medication history is essential prior to starting any systemic medication for psoriasis. Apremilast and cyclosporine are metabolized by cytochrome P450 and therefore are more susceptible to drug-related interactions. Cyclosporine use can affect levels of other medications that are metabolized by cytochrome P450, such as statins, calcium channel blockers, and warfarin. Similarly, acitretin’s metabolism is affected by drugs that interfere with cytochrome P450. Additionally, screening laboratory tests are needed before initiating systemic nonbiologic agents for psoriasis, with the exception of apremilast.
Prior to initiating methotrexate treatment, patients may require tuberculosis (TB), hepatitis B, and hepatitis C screening tests, depending on their risk factors. A baseline liver fibrosis assessment is recommended because of the potential of hepatotoxicity in patients receiving methotrexate. Noninvasive serology tests utilized to evaluate the presence of pre-existing liver disease include Fibrosis-4, FibroMeter, FibroSure, and Hepascore. Patients with impaired renal function have an increased predisposition to methotrexate-induced hematologic toxicity. Thus, it is necessary to administer a test dose of methotrexate in these patients followed by a complete blood cell count (CBC) 5 to 7 days later. An unremarkable CBC after the test dose suggests the absence of myelosuppression, and methotrexate dosage can be increased weekly. Patients on methotrexate also must receive folate supplementation to reduce the risk for adverse effects during treatment.
Patients considering cyclosporine must undergo screening for family and personal history of renal disease. Prior to initiating treatment, patients require 2 blood pressure measurements, hepatitis screening, TB screening, urinalysis, serum creatinine (Cr), blood urea nitrogen (BUN), CBC, potassium and magnesium levels, uric acid levels, lipid profile, bilirubin, and liver function tests (LFTs). A pregnancy test also is warranted for women of childbearing potential (WOCP).
Patients receiving acitretin should receive screening laboratory tests consisting of fasting cholesterol and triglycerides, CBC, renal function tests, LFTs, and a pregnancy test, if applicable.
After baseline evaluations, the selected oral systemic can be initiated using specific dosing regimens to ensure optimal drug efficacy and reduce incidence of adverse effects (eTable).
Monitoring During Active Treatment
Physicians need to counsel patients on potential adverse effects of their medications. Because of its relatively safe profile among the systemic nonbiologic agents, apremilast requires the least monitoring during treatment. There is no required routine laboratory monitoring for patients using apremilast, though testing may be pursued at the clinician’s discretion. However, weight should be regularly measured in patients on apremilast. In a phase 3 clinical trial of patients with psoriasis, 12% of patients on apremilast experienced a 5% to 10% weight loss compared to 5% of patients on placebo.11,13 Thus, it is recommended that physicians consider discontinuing apremilast in patients with a weight loss of more than 5% from baseline, especially if it may lead to other unfavorable health effects. Because depression is reported among 1% of patients on apremilast, close monitoring for new or worsening symptoms of depression should be performed during treatment.11,13 To avoid common GI side effects, apremilast is initiated at 10 mg/d and is increased by 10 mg/d over the first 5 days to a final dose of 30 mg BID. Elderly patients in particular should be cautioned about the risk of dehydration associated with GI side effects. Patients with severe renal impairment (Cr clearance, <30 mL/min) should use apremilast at a dosage of 30 mg once daily.
For patients on methotrexate, laboratory monitoring is essential after each dose increase. It also is important for physicians to obtain regular blood work to assess for hematologic abnormalities and hepatoxicity. Patients with risk factors such as renal insufficiency, increased age, hypoalbuminemia, alcohol abuse and alcoholic liver disease, and methotrexate dosing errors, as well as those prone to drug-related interactions, must be monitored closely for pancytopenia.14,15 The protocol for screening for methotrexate-induced hepatotoxicity during treatment depends on patient risk factors. Risk factors for hepatoxicity include history of or current alcohol abuse, abnormal LFTs, personal or family history of liver disease, diabetes, obesity, use of other hepatotoxic drugs, and hyperlipidemia.16 In patients without blood work abnormalities, CBC and LFTs can be performed every 3 to 6 months. Patients with abnormally elevated LFTs require repeat blood work every 2 to 4 weeks. Persistent elevations in LFTs require further evaluation by a GI specialist. After a cumulative dose of 3.5 to 4 g, patients should receive a GI referral and further studies (such as vibration-controlled transient elastography or liver biopsy) to assess for liver fibrosis. Patients with signs of stage 3 liver fibrosis are recommended to discontinue methotrexate and switch to another medication for psoriasis. For patients with impaired renal function, periodic BUN and Cr monitoring are needed. Common adverse effects of methotrexate include diarrhea, nausea, and anorexia, which can be mitigated by taking methotrexate with food or lowering the dosage.8 Patients on methotrexate should be monitored for rare but potential risks of infection and reactivation of latent TB, hepatitis, and lymphoma. To reduce the incidence of methotrexate toxicity from drug interactions, a review of current medications at each follow-up visit is recommended.
Nephrotoxicity and hypertension are the most common adverse effects of cyclosporine. It is important to monitor BUN and Cr biweekly for the initial 3 months, then at monthly intervals if there are no persistent abnormalities. Patients also must receive monthly CBC, potassium and magnesium levels, uric acid levels, lipid panel, serum bilirubin, and LFTs to monitor for adverse effects.17 Physicians should obtain regular pregnancy tests in WOCP. Weekly monitoring of early-morning blood pressure is recommended for patients on cyclosporine to detect early cyclosporine-induced nephrotoxicity. Hypertension on 2 separate occasions warrants a reduction in cyclosporine dosage or an addition of a calcium channel blocker for blood pressure control. Dose reduction also should be performed in patients with an increase in Cr above baseline greater than 25%.17 If Cr level is persistently elevated or if blood pressure does not normalize to lower than 140/90 after dose reduction, cyclosporine should be immediately discontinued. Patients on cyclosporine for more than a year warrant an annual estimation of glomerular filtration rate because of irreversible kidney damage associated with long-term use. A systematic review of patients treated with cyclosporine for more than 2 years found that at least 50% of patients experienced a 30% increase in Cr above baseline.18
Patients taking acitretin should be monitored for hyperlipidemia, the most common laboratory abnormality seen in 25% to 50% of patients.19 Fasting lipid panel and LFTs should be performed monthly for the initial 3 months on acitretin, then at 3-month intervals. Lifestyle changes should be encouraged to reduce hyperlipidemia, and fibrates may be given to treat elevated triglyceride levels, the most common type of hyperlipidemia seen with acitretin. Acitretin-induced toxic hepatitis is a rare occurrence that warrants immediate discontinuation of the medication.20 Monthly pregnancy tests must be performed in WOCP.
Combination Therapy
For apremilast, there is anecdotal evidence supporting its use in conjunction with phototherapy or biologics in some cases, but no high-quality data.21 On the other hand, using combination therapy with other systemic therapies can reduce adverse effects and decrease the amount of medication needed to achieve psoriasis clearance. Methotrexate used with etanercept, for example, has been more effective than methotrexate monotherapy in treating psoriasis, which has been attributed to a methotrexate-mediated reduction in the production of antidrug antibodies.22,23
Methotrexate, cyclosporine, and acitretin have synergistic effects when used with phototherapy. Narrowband UVB (NB-UVB) phototherapy combined with methotrexate is more effective in clearing psoriasis than methotrexate or NB-UVB phototherapy alone. Similarly, acitretin and PUVA combination therapy is more effective than acitretin or PUVA phototherapy alone. Combination regimens of acitretin and broadband UVB phototherapy, acitretin and NB-UVB phototherapy, and acitretin and PUVA phototherapy also have been more effective than individual modalities alone. Combination therapy reduces the cumulative doses of both therapies and reduces the frequency and duration of phototherapy needed for psoriatic clearance.24 In acitretin combination therapy with UVB phototherapy, the recommended regimen is 2 weeks of acitretin monotherapy followed by UVB phototherapy. For patients with an inadequate response to UVB phototherapy, the UVB dose can be reduced by 30% to 50%, and acitretin 25 mg/d can be added to phototherapy treatment. Acitretin-UVB combination therapy has been shown to reduce the risk of UVB-induced erythema seen in UVB monotherapy. Similarly, the risk of squamous cell carcinoma is reduced in acitretin-PUVA combination therapy compared to PUVA monotherapy.25
The timing of phototherapy in combination with systemic nonbiologic agents is critical. Phototherapy used simultaneously with cyclosporine is contraindicated owing to increased risk of photocarcinogenesis, whereas phototherapy used in sequence with cyclosporine is well tolerated and effective. Furthermore, cyclosporine 3 mg/kg/d for 4 weeks followed by a rapid cyclosporine taper and initiation of NB-UVB phototherapy demonstrated resolution of psoriasis with fewer NB-UVB treatments and less UVB exposure than NB-UVB therapy alone.26
Final Thoughts
The FDA-approved systemic nonbiologic agents are accessible and effective treatment options for adults with widespread or inadequately controlled psoriasis. Selecting the ideal therapy requires careful consideration of medication toxicity, contraindications, monitoring requirements, and patient comorbidities. The AAD-NPF guidelines guide dermatologists in prescribing systemic nonbiologic treatments in adults with psoriasis. Utilizing these recommendations in combination with clinician judgment will help patients achieve safe and optimal psoriasis clearance.
- Menter A, Gelfand JM, Connor C, et al. Joint American Academy of Dermatology-National Psoriasis Foundation guidelines of care for the management of psoriasis with systemic nonbiologic therapies. J Am Acad Dermatol. 2020;82:1445-1486.
- Mrowietz U, Barker J, Boehncke WH, et al. Clinical use of dimethyl fumarate in moderate-to-severe plaque-type psoriasis: a European expert consensus. J Eur Acad Dermatol Venereol. 2018;32(suppl 3):3-14.
- Van Voorhees AS, Gold LS, Lebwohl M, et al. Efficacy and safety of apremilast in patients with moderate to severe plaque psoriasis of the scalp: results of a phase 3b, multicenter, randomized, placebo-controlled, double-blind study. J Am Acad Dermatol. 2020;83:96-103.
- Buccheri L, Katchen BR, Karter AJ, et al. Acitretin therapy is effective for psoriasis associated with human immunodeficiency virus infection. Arch Dermatol. 1997;133:711-715.
- Ormerod AD, Campalani E, Goodfield MJD. British Association of Dermatologists guidelines on the efficacy and use of acitretin in dermatology. Br J Dermatol. 2010;162:952-963.
- Kavanaugh A, Mease PJ, Gomez-Reino JJ, et al. Longterm (52-week) results of a phase III randomized, controlled trial of apremilast in patients with psoriatic arthritis. J Rheumatol. 2015;42:479-488.
- Coates LC, Aslam T, Al Balushi F, et al. Comparison of three screening tools to detect psoriatic arthritis in patients with psoriasis (CONTEST study). Br J Dermatol. 2013;168:802-807.
- Antares Pharma, Inc. Otrexup PFS (methotrexate) [package insert]. US Food and Drug Administration website. Revised June 2019. Accessed February 28, 2020. https://www.accessdata.fda.gov/drugsatfda_docs/label/2019/204824s009lbl.pdf
- David M, Hodak E, Lowe NJ. Adverse effects of retinoids. Med Toxicol Adverse Drug Exp. 1988;3:273-288.
- Stiefel Laboratories, Inc. Soriatane (acitretin) [package insert]. US Food and Drug Administration website. Revised September 2017. Accessed February 28, 2020. https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/019821s028lbl.pdf
- Celgene Corporation. Otezla (apremilast) [package insert]. US Food and Drug Administration website. Revised March 2014. Accessed February 28, 2020. https://www.accessdata.fda.gov/drugsatfda_docs/label/2014/205437s000lbl.pdf
- Ghanem ME, El-Baghdadi LA, Badawy AM, et al. Pregnancy outcome after renal allograft transplantation: 15 years experience. Eur J Obstet Gynecol Reprod Biol. 2005;121:178-181.
- Zerilli T, Ocheretyaner E. Apremilast (Otezla): A new oral treatment for adults with psoriasis and psoriatic arthritis. P T. 2015;40:495-500.
- Kivity S, Zafrir Y, Loebstein R, et al. Clinical characteristics and risk factors for low dose methotrexate toxicity: a cohort of 28 patients. Autoimmun Rev. 2014;13:1109-1113.
- Boffa MJ, Chalmers RJ. Methotrexate for psoriasis. Clin Exp Dermatol. 1996;21:399-408.
- Rosenberg P, Urwitz H, Johannesson A, et al. Psoriasis patients with diabetes type 2 are at high risk of developing liver fibrosis during methotrexate treatment. J Hepatol. 2007;46:1111-1118.
- Novartis Pharmaceuticals Corporation. Sandimmune (cyclosporine) [package insert]. US Food and Drug Administration website. Published 2015. Accessed February 28, 2020. https://www.accessdata.fda.gov/drugsatfda_docs/label/2015/050573s041,050574s051,050625s055lbl.pdf
- Maza A, Montaudie H, Sbidian E, et al. Oral cyclosporin in psoriasis: a systematic review on treatment modalities, risk of kidney toxicity and evidence for use in non-plaque psoriasis. J Eur Acad Dermatol Venereol. 2011;25(suppl 2):19-27.
- Yamauchi PS, Rizk D, Kormilli T, et al. Systemic retinoids. In: Weinstein GD, Gottlieb AB, eds. Therapy of Moderate-to-Severe Psoriasis. Marcel Dekker; 2003:137-150.
- van Ditzhuijsen TJ, van Haelst UJ, van Dooren-Greebe RJ, et al. Severe hepatotoxic reaction with progression to cirrhosis after use of a novel retinoid (acitretin). J Hepatol. 1990;11:185-188.
- AbuHilal M, Walsh S, Shear N. Use of apremilast in combination with other therapies for treatment of chronic plaque psoriasis: a retrospective study. J Cutan Med Surg. 2016;20:313-316.
- Gottlieb AB, Langley RG, Strober BE, et al. A randomized, double-blind, placebo-controlled study to evaluate the addition of methotrexate to etanercept in patients with moderate to severe plaque psoriasis. Br J Dermatol. 2012;167:649-657.
- Cronstein BN. Methotrexate BAFFles anti-drug antibodies. Nat Rev Rheumatol. 2018;14:505-506.
- Lebwohl M, Drake L, Menter A, et al. Consensus conference: acitretin in combination with UVB or PUVA in the treatment of psoriasis. J Am Acad Dermatol. 2001;45:544-553.
- Nijsten TE, Stern RS. Oral retinoid use reduces cutaneous squamous cell carcinoma risk in patients with psoriasis treated with psoralen-UVA: a nested cohort study. J Am Acad Dermatol. 2003;49:644-650.
- Calzavara-Pinton P, Leone G, Venturini M, et al. A comparative non randomized study of narrow-band (NB) (312 +/- 2 nm) UVB phototherapy versus sequential therapy with oral administration of low-dose cyclosporin A and NB-UVB phototherapy in patients with severe psoriasis vulgaris. Eur J Dermatol. 2005;15:470-473.
- Menter A, Gelfand JM, Connor C, et al. Joint American Academy of Dermatology-National Psoriasis Foundation guidelines of care for the management of psoriasis with systemic nonbiologic therapies. J Am Acad Dermatol. 2020;82:1445-1486.
- Mrowietz U, Barker J, Boehncke WH, et al. Clinical use of dimethyl fumarate in moderate-to-severe plaque-type psoriasis: a European expert consensus. J Eur Acad Dermatol Venereol. 2018;32(suppl 3):3-14.
- Van Voorhees AS, Gold LS, Lebwohl M, et al. Efficacy and safety of apremilast in patients with moderate to severe plaque psoriasis of the scalp: results of a phase 3b, multicenter, randomized, placebo-controlled, double-blind study. J Am Acad Dermatol. 2020;83:96-103.
- Buccheri L, Katchen BR, Karter AJ, et al. Acitretin therapy is effective for psoriasis associated with human immunodeficiency virus infection. Arch Dermatol. 1997;133:711-715.
- Ormerod AD, Campalani E, Goodfield MJD. British Association of Dermatologists guidelines on the efficacy and use of acitretin in dermatology. Br J Dermatol. 2010;162:952-963.
- Kavanaugh A, Mease PJ, Gomez-Reino JJ, et al. Longterm (52-week) results of a phase III randomized, controlled trial of apremilast in patients with psoriatic arthritis. J Rheumatol. 2015;42:479-488.
- Coates LC, Aslam T, Al Balushi F, et al. Comparison of three screening tools to detect psoriatic arthritis in patients with psoriasis (CONTEST study). Br J Dermatol. 2013;168:802-807.
- Antares Pharma, Inc. Otrexup PFS (methotrexate) [package insert]. US Food and Drug Administration website. Revised June 2019. Accessed February 28, 2020. https://www.accessdata.fda.gov/drugsatfda_docs/label/2019/204824s009lbl.pdf
- David M, Hodak E, Lowe NJ. Adverse effects of retinoids. Med Toxicol Adverse Drug Exp. 1988;3:273-288.
- Stiefel Laboratories, Inc. Soriatane (acitretin) [package insert]. US Food and Drug Administration website. Revised September 2017. Accessed February 28, 2020. https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/019821s028lbl.pdf
- Celgene Corporation. Otezla (apremilast) [package insert]. US Food and Drug Administration website. Revised March 2014. Accessed February 28, 2020. https://www.accessdata.fda.gov/drugsatfda_docs/label/2014/205437s000lbl.pdf
- Ghanem ME, El-Baghdadi LA, Badawy AM, et al. Pregnancy outcome after renal allograft transplantation: 15 years experience. Eur J Obstet Gynecol Reprod Biol. 2005;121:178-181.
- Zerilli T, Ocheretyaner E. Apremilast (Otezla): A new oral treatment for adults with psoriasis and psoriatic arthritis. P T. 2015;40:495-500.
- Kivity S, Zafrir Y, Loebstein R, et al. Clinical characteristics and risk factors for low dose methotrexate toxicity: a cohort of 28 patients. Autoimmun Rev. 2014;13:1109-1113.
- Boffa MJ, Chalmers RJ. Methotrexate for psoriasis. Clin Exp Dermatol. 1996;21:399-408.
- Rosenberg P, Urwitz H, Johannesson A, et al. Psoriasis patients with diabetes type 2 are at high risk of developing liver fibrosis during methotrexate treatment. J Hepatol. 2007;46:1111-1118.
- Novartis Pharmaceuticals Corporation. Sandimmune (cyclosporine) [package insert]. US Food and Drug Administration website. Published 2015. Accessed February 28, 2020. https://www.accessdata.fda.gov/drugsatfda_docs/label/2015/050573s041,050574s051,050625s055lbl.pdf
- Maza A, Montaudie H, Sbidian E, et al. Oral cyclosporin in psoriasis: a systematic review on treatment modalities, risk of kidney toxicity and evidence for use in non-plaque psoriasis. J Eur Acad Dermatol Venereol. 2011;25(suppl 2):19-27.
- Yamauchi PS, Rizk D, Kormilli T, et al. Systemic retinoids. In: Weinstein GD, Gottlieb AB, eds. Therapy of Moderate-to-Severe Psoriasis. Marcel Dekker; 2003:137-150.
- van Ditzhuijsen TJ, van Haelst UJ, van Dooren-Greebe RJ, et al. Severe hepatotoxic reaction with progression to cirrhosis after use of a novel retinoid (acitretin). J Hepatol. 1990;11:185-188.
- AbuHilal M, Walsh S, Shear N. Use of apremilast in combination with other therapies for treatment of chronic plaque psoriasis: a retrospective study. J Cutan Med Surg. 2016;20:313-316.
- Gottlieb AB, Langley RG, Strober BE, et al. A randomized, double-blind, placebo-controlled study to evaluate the addition of methotrexate to etanercept in patients with moderate to severe plaque psoriasis. Br J Dermatol. 2012;167:649-657.
- Cronstein BN. Methotrexate BAFFles anti-drug antibodies. Nat Rev Rheumatol. 2018;14:505-506.
- Lebwohl M, Drake L, Menter A, et al. Consensus conference: acitretin in combination with UVB or PUVA in the treatment of psoriasis. J Am Acad Dermatol. 2001;45:544-553.
- Nijsten TE, Stern RS. Oral retinoid use reduces cutaneous squamous cell carcinoma risk in patients with psoriasis treated with psoralen-UVA: a nested cohort study. J Am Acad Dermatol. 2003;49:644-650.
- Calzavara-Pinton P, Leone G, Venturini M, et al. A comparative non randomized study of narrow-band (NB) (312 +/- 2 nm) UVB phototherapy versus sequential therapy with oral administration of low-dose cyclosporin A and NB-UVB phototherapy in patients with severe psoriasis vulgaris. Eur J Dermatol. 2005;15:470-473.
Practice Points
- Systemic nonbiologic therapies are effective treatments for adults with psoriasis. The benefits of these treatments include ease of administration and the ability to control widespread disease.
- When selecting a therapy, a thorough evaluation of patient characteristics and commitment to lifestyle adjustments is necessary, including careful consideration in women of childbearing potential and those with plans of starting a family.
- Regular drug monitoring and patient follow-up is crucial to ensure safe dosing adjustments and to mitigate potential adverse effects.
Pessaries for POP and SUI: Their fitting, care, and effectiveness in various disorders
In Part 1 of this article in the December 2020 issue of OBG Management, I discussed the reasons that pessaries are an effective treatment option for many women with pelvic organ prolapse (POP) and stress urinary incontinence (SUI) and provided details on the types of pessaries available.
In this article, I highlight the steps in fitting a pessary, pessary aftercare, and potential complications associated with pessary use. In addition, I discuss the effectiveness of pessary treatment for POP and SUI as well as for preterm labor prevention and defecatory disorders.
The pessary fitting process
For a given patient, the best size pessary is the smallest one that will not fall out. The only “rule” for fitting a pessary is that a woman’s internal vaginal caliber should be wider than her introitus.
When fitting a pessary, goals include that the selected pessary:
- should be comfortable for the patient to wear
- is not easily expelled
- does not interfere with urination or defecation
- does not cause vaginal irritation.
The presence or absence of a cervix or uterus does not affect pessary choice.
Most experts agree that the process for fitting the right size pessary is one of trial and error. As with fitting a contraceptive diaphragm, the clinician should perform a manual examination to estimate the integrity and width of the perineum and the depth of the vagina to roughly approximate the pessary size that might best fit. Using a set of “fitting pessaries,” a pessary of the estimated size should be placed into the vagina and the fit evaluated as to whether the device is too big, too small, or appropriate. If the pessary is easily expelled, larger sizes should be tried until the pessary remains in place or the patient is uncomfortable. Once the pessary is in place, the clinician should be able to run his or her finger around the entire pessary; if this is not possible, the pessary is too tight. In addition, the pessary should remain more than one finger breadth above the introitus when the patient is standing or bearing down.
Since many patients who require a pessary are elderly, their perineal skin and vaginal mucosa may be atrophic and fragile. Inserting a pessary can be uncomfortable and can cause abrasions or tears. Successfully fitting a pessary may require extra care under these circumstances. The following steps may help alleviate these difficulties:
- Explain the fitting process to the patient in detail.
- Employ lubrication liberally.
- Enlarge the introitus by applying gentle digital pressure on the posterior fourchette.
- Apply 2% lidocaine ointment several minutes prior to pessary fitting to help decrease patient discomfort.
- Treat the patient for several weeks with vaginal estrogen cream before attempting to fit a pessary if severe vulvovaginal atrophy is present.
Once the type and size of the pessary are selected and a pessary is inserted, evaluate the patient with the pessary in place. Assess for the following:
Discomfort. Ask the patient if she feels discomfort with the pessary in position. A patient with a properly fitting pessary should not feel that it is in place. If she does feel discomfort initially, the discomfort will only increase with time and the issue should be addressed at that time.
Expulsion. Test to make certain that the pessary is not easily expelled from the vagina. Have the patient walk, cough, squat, and even jump if possible.
Urination. Have the patient urinate with the pessary in place. This tests for her ability to void while wearing the pessary and shows whether the contraction of pelvic muscles during voiding results in expulsion of the pessary. (Experience shows that it is best to do this with a plastic “hat” over the toilet so that if the pessary is expelled, it does not drop into the bowl.)
Re-examination. After these provocative tests, examine the patient again to ensure that the pessary has not slid out of place.
Depending on whether or not your office stocks pessaries, at this point the patient is either given the correct type and size of pessary or it is ordered for her. If the former, the patient should try placing it herself; if she is unable to, the clinician should place it for her. In either event, its position should be checked. If the pessary has to be ordered, the patient must schedule an appointment to return for pessary insertion.
Whether the pessary is supplied by the office or ordered, instruct the patient on how to insert and remove the pessary, how frequently to remove it for cleansing (see below), and signs to watch for, such as vaginal bleeding, inability to void or defecate, or pelvic pain.
It is advisable to schedule a subsequent visit for 2 to 3 weeks after initial pessary placement to assess how the patient is doing and to address any issues that have developed.
Continue to: Special circumstances...
Special circumstances
It is safe for a patient with a pessary in place to undergo magnetic resonance imaging.1 Patients should be informed, however, that full body scans, such as at airports, will detect pessaries. Patients may need to obtain a physician’s note to document that the pessary is a medical device.
Finally, several factors may prevent successful pessary fitting. These include prior pelvic surgery, obesity, short vaginal length (less than 6–7 cm), and a vaginal introitus width of greater than 4 finger breadths.
Necessary pessary aftercare
Once a pessary is in place and the patient is comfortable with it, the only maintenance necessary is the pessary’s intermittent removal for cleansing and for evaluation of the vaginal mucosa for erosion and ulcerations. How frequently this should be done varies based on the type of pessary, the amount of discharge that a woman produces, whether or not an odor develops after prolonged wearing of the pessary, and whether or not the patient’s vaginal mucosa has been abraded.
The question of timing for pessary cleaning
Although there are many opinions about how often pessaries should be removed and cleaned, no data in the literature support any specific interval. Pessaries that are easily removed by women themselves can be cleaned as frequently as desired, often on a weekly basis. The patient simply removes the pessary, washes it with soap and water, and reinserts it. For pessaries that are difficult to remove (such as the Gellhorn, cube, or donut) or for women who are physically unable to remove their own ring pessary, the clinician should remove and clean the pessary in the office every 3 to 6 months. It has been shown that there is no difference in complications from pessary use with either of these intervals.2
Prior to any vaginal surgical procedure, patients must be instructed to remove their pessary 10 to 14 days beforehand so that the surgeon can see the full extent of prolapse when making decisions about reconstruction and so that any vaginal mucosal erosions or abrasions have time to heal.
Office visits for follow-up care
The pessary “cleaning visit” has several goals, including to:
- see if the pessary is meeting the patient’s needs in terms of resolving symptoms of prolapse and/or restoring urinary continence
- discuss with the patient any problems she may be having, such as pelvic discomfort or pressure, difficulty voiding or defecating, excessive vaginal discharge, or vaginal odor
- check for vaginal mucosal erosion or ulceration; such vaginal lesions often can be prevented by the prophylactic use of either estrogen vaginal cream twice weekly or the continuous use of an estradiol vaginal ring in addition to the pessary
- evaluate the condition of the pessary itself and clean it with soap and water.
Continue to: Potential complications of pessary use...
Potential complications of pessary use
The most common complications experienced by pessary users are:
Odor or excessive discharge. Bacterial vaginosis (BV) occurs more frequently in women who use pessaries. The symptoms of BV can be minimized—but unfortunately not totally eliminated—by the prophylactic use of antiseptic vaginal creams or gels, such as metronidazole, clindamycin, Trimo-San (oxyquinoline sulfate and sodium lauryl sulfate), and others. Inserting the gel vaginally once a week can significantly reduce discharge and odor.3
Vaginal mucosal erosion and ulceration. These are treated by removing the pessary for 2 weeks during which time estrogen cream is applied daily or an estradiol vaginal ring is put in place. If no resolution occurs after 2 weeks, the nonhealing vaginal mucosa should be biopsied.
Pressure on the rectum or bladder. If the pessary causes significant discomfort or interferes with voiding function, then either a different size or a different type pessary should be tried
Patients may discontinue pessary use for a variety of reasons. Among these are:
- discomfort
- inadequate improvement of POP or incontinence symptoms
- expulsion of the pessary during daily activities
- the patient’s desire for surgery instead
- worsening of urine leakage
- difficulty inserting or removing the pessary
- damage to the vaginal mucosa
- pain during removal of the pessary in the office.
Pessary effectiveness for POP and SUI symptoms
As might be expected with a device that is available in so many forms and is used to treat varied types of POP and SUI, the data concerning the success rates of pessary use vary considerably. These rates depend on the definition of success, that is, complete or partial control of prolapse and/or incontinence; which devices are being evaluated; and the nature and severity of the POP and/or SUI being treated.
That being said, a review of the literature reveals that the rates of prolapse symptom relief vary from 48% to 92% (TABLE 1).4-13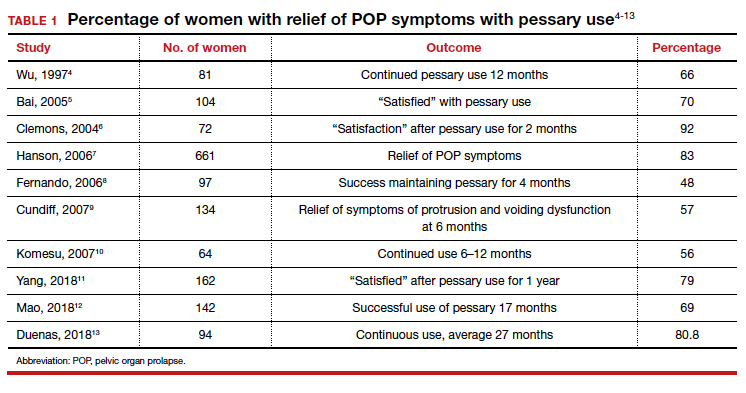
As for success in relieving symptoms of incontinence, studies show improvements in from 40% to 77% of patients (TABLE 2).6,8,14-17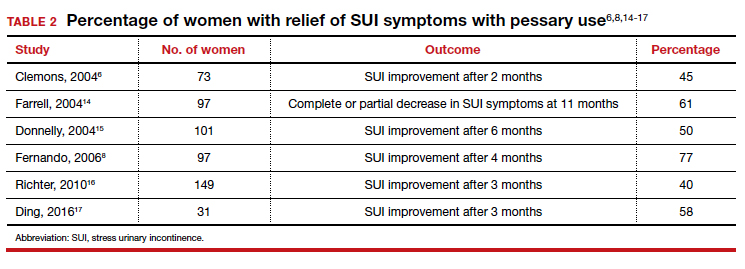
In addition, some studies show a 50% improvement in bowel symptoms (urgency, obstruction, and anal incontinence) with the use of a pessary.9,18
How pessaries compare with surgery
While surgery has the advantage of being a one-time fix with a very high rate of initial success in correcting both POP and incontinence, surgery also has potential drawbacks:
- It is an invasive procedure with the discomfort and risk of complications any surgery entails.
- There is a relatively high rate of prolapse recurrence.
- It exposes the patient to the possibility of mesh erosion if mesh is employed either for POP support or incontinence treatment.
Pessaries, on the other hand, are inexpensive, nonsurgical, removable, and allow for immediate correction of symptoms. Moreover, if the pessary is tried and is found to be unsatisfactory, surgery always can be performed subsequently.
Drawbacks of pessary treatment compared with surgery include the:
- ongoing need to wear an artificial internal device
- need for intermittent pessary removal and cleansing
- inability to have sexual intercourse with certain kinds of pessaries in place
- possible accumulation of vaginal discharge and odor.
Sexual activity and pessaries
Studies by Fernando, Meriwether, and Kuhn concur that for a substantial number of pessary users who are sexually active, both frequency and satisfaction with sexual intercourse are increased.8,19,20 Kuhn further showed that desire, orgasm, and lubrication improved with the use of pessaries.20 While some types of pessaries do require removal for intercourse, Clemons reported that issues involving sexual activity are not associated with pessary discontinuation.21
Using a pessary to predict a surgical outcome
Because a pessary elevates the pelvic organs, supports the vaginal walls, and lifts the bladder and urethra into a position that simulates the results of surgical repair, trial placement of a pessary can be used as a fairly accurate predictive tool to model what pelvic support and continence status will be after a proposed surgical procedure.22,23 This is especially important because a significant number of patients with POP will have their occult stress incontinence unmasked following a reparative procedure.24 A brief pessary trial prior to surgery, therefore, can be a useful tool for both patient and surgeon.
Continue to: Pessaries for prevention of preterm labor...
Pessaries for prevention of preterm labor
Almost 1 in 10 births in the United States occurs before 37 completed weeks of gestation.25 Obstetricians have long thought that in women at risk for preterm delivery, the use of a pessary might help reduce the pressure of the growing uterus on the cervix and thus help prevent premature cervical dilation. It also has been thought that use of a pessary would be a safer and less invasive alternative to cervical cerclage. Many studies have evaluated the use of pessaries for the prevention of preterm labor with a mixture of positive (TABLE 3)26-29 and negative results (TABLE 4).30-33
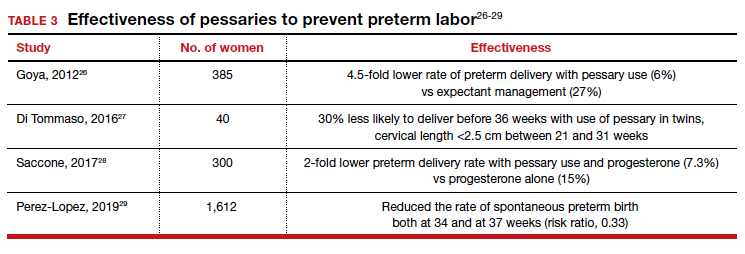
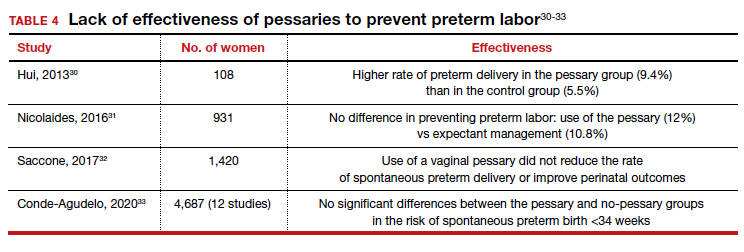
From these data, it is reasonable to conclude that:
- The final answer concerning the effectiveness or lack thereof of pessary use in preventing preterm delivery is not yet in.
- Any advantage there might be to using pessaries to prevent preterm delivery cannot be too significant if multiple studies show as many negative outcomes as positive ones.
Pessary effectiveness in defecatory disorders
Vaginal birth has the potential to create multiple anatomic injuries in the anus, lower pelvis, and perineum that can affect defecation and bowel control. Tears of the anal sphincter, whether obvious or occult, may heal incompletely or be repaired inadequately.34 Nerve innervation of the perianal and perineal areas can be interrupted or damaged by stretching, tearing, or prolonged compression. Of healthy parous adult women, 7% to 16% admit incontinence of gas or feces.35,36
In addition, when a rectocele is present, stool in the lower rectum may cause bulging of the anterior rectal wall into the vagina, preventing stool from passing out of the anus. This sometimes requires women to digitally press their posterior vaginal walls during defecation to evacuate stool successfully. The question thus arises as to whether or not pessary placement and subsequent relief of rectoceles might facilitate bowel movements and decrease or eliminate defecatory dysfunction.
As with the issue of pessary use for prevention of preterm delivery, the answer is mixed. For instance, while Brazell18 showed that there was an overall improvement in bowel symptoms in pessary users, a study by Komesu10 did not demonstrate improvement.
There is, however, a relatively new device specifically designed to control defecatory problems: the vaginal bowel control system (Eclipse; Pelvalon). The silicon device is placed intravaginally as one does a pessary. After insertion, it is inflated via a valve and syringe. It works by putting pressure on and reversibly closing the lower rectum, thus blocking the uncontrolled passage of stool and gas. It can be worn continuously or intermittently, but it does need to be deflated for normal bowel movements. One trial of this device demonstrated a 50% reduction in incontinence episodes with a patient satisfaction rate of 84% at 3 months.37 This device may well prove to be a valuable nonsurgical approach to the treatment of fecal incontinence. Unfortunately, the device is relatively expensive and usually is not covered by insurance as third-party payers do not consider it to be a pessary (which generally is covered).
Practice management particulars
Useful information on Current Procedural Terminology codes for pessaries, diagnostic codes, and the cost of various pessaries is provided in TABLE 5,38TABLE 6,39 and TABLE 7.40-42
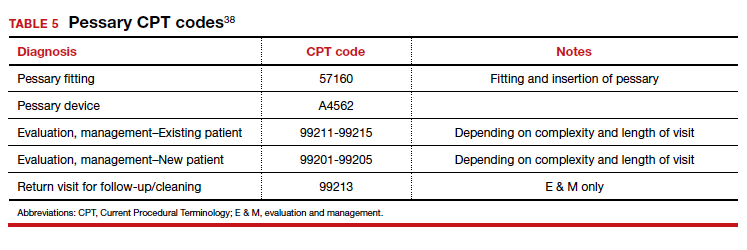
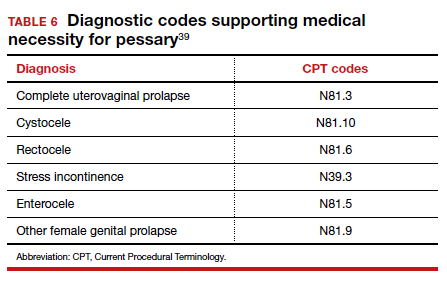
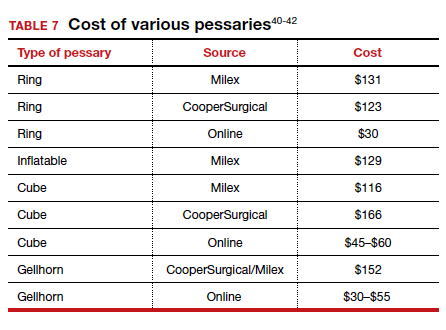
A contemporary device used since antiquity
Pessaries, considered “old-fashioned” by many gynecologists, are actually a very cost-effective and useful tool for the correction of POP and SUI. It behooves all who provide medical care to women to be familiar with them, to know when they might be useful, and to know how to fit and prescribe them. ●
- O’Dell K, Atnip S. Pessary care: follow up and management of complications. Urol Nurs. 2012;32:126-136, 145.
- Gorti M, Hudelist G, Simons A. Evaluation of vaginal pessary management: a UK-based survey. J Obstet Gynaecol. 2009;29:129-131.
- Meriwether KV, Rogers RG, Craig E, et al. The effect of hydroxyquinoline-based gel on pessary-associated bacterial vaginosis: a multicenter randomized controlled trial. Am J Obstet Gynecol. 2015;213:729.e1-9.
- Wu V, Farrell SA, Baskett TF, et al. A simplified protocol for pessary management. Obstet Gynecol. 1997;90:990-994.
- Bai SW, Yoon BS, Kwon JY, et al. Survey of the characteristics and satisfaction degree of the patients using a pessary. Int Urogynecol J Pelvic Floor Dysfunct. 2005;16:182-186.
- Clemons JL, Aguilar VC, Tillinghast TA, et al. Patient satisfaction and changes in prolapse and urinary symptoms in women who were fitted successfully with a pessary for pelvic organ prolapse. Am J Obstet Gynecol. 2004;190:1025-1029.
- Hanson LM, Schulz JA, Flood CG, et al. Vaginal pessaries in managing women with pelvic organ prolapse and urinary incontinence: patient characteristics and factors contributing to success. Int Urogynecol J Pelvic Floor Dysfunct. 2006;17: 155-159.
- Fernando RJ, Thakar R, Sultan AH, et al. Effect of vaginal pessaries on symptoms associated with pelvic organ prolapse. Obstet Gynecol. 2006;108:93-99.
- Cundiff GW, Amundsen CL, Bent AE, et al. The PESSRI study: symptom relief outcomes of a randomized crossover trial of the ring and Gellhorn pessaries. Am J Obstet Gynecol. 2007;196:405.e1-405e.8.
- Komesu YM Rogers RG, Rode MA, et al. Pelvic floor symptom changes in pessary users. Am J Obstet Gynecol. 2007;197: 620.e1-6.
- Yang J, Han J, Zhu F, et al. Ring and Gellhorn pessaries used inpatients with pelvic organ prolapse: a retrospective study of 8 years. Arch Gynecol Obstet. 2018;298:623-629.
- Mao M, Ai F, Zhang Y, et al. Changes in the symptoms and quality of life of women with symptomatic pelvic organ prolapse fitted with a ring with support pessary. Maturitas. 2018;117:51-56.
- Duenas JL, Miceli A. Effectiveness of a continuous-use ringshaped vaginal pessary without support for advanced pelvic organ prolapse in postmenopausal women. Int Urogynecol J. 2018;29:1629-1636.
- Farrell S, Singh B, Aldakhil L. Continence pessaries in the management of urinary incontinence in women. J Obstet Gynaecol Canada. 2004;26:113-117.
- Donnelly MJ, Powell-Morgan SP, Olsen AL, et al. Vaginal pessaries for the management of stress and mixed urinary incontinence. Int Urogynecol J Pelvic Floor Dysfunct. 2004;15:302-307.
- Richter HE, Burgio KL, Brubaker L, et al; Pelvic Floor Disorders Network. Continence pessary compared with behavioral therapy or combined therapy for stress incontinence: a randomized controlled trial. Obstet Gynecol. 2010;115:609-617.
- Ding J, Chen C, Song XC, et al. Changes in prolapse and urinary symptoms after successful fitting of a ring pessary with support in women with advanced pelvic organ prolapse: a prospective study. Urology. 2016;87:70-75.
- Brazell HD, Patel M, O’Sullivan DM, et al. The impact of pessary use on bowel symptoms: one-year outcomes. Female Pelvic Med Reconstr Surg. 2014;20:95-98.
- Meriwether KV, Komesu YM, Craig C, et al. Sexual function and pessary management among women using a pessary for pelvic floor disorders. J Sex Med. 2015;12:2339-2349.
- Kuhn A, Bapst D, Stadlmayr W, et al. Sexual and organ function in patients with symptomatic prolapse: are pessaries helpful? Fertil Steril. 2009;91:1914-1918.
- Clemons JL, Aguilar VC, Sokol ER, et al. Patient characteristics that are associated with continued pessary use versus surgery after 1 year. Am J Obstet Gynecol. 2004;191:159-164.
- Liang CC, Chang YL, Chang SD, et al. Pessary test to predict postoperative urinary incontinence in women undergoing hysterectomy for prolapse. Obstet Gynecol. 2004;104:795-800.
- Liapis A, Bakas P, Georgantopoulou C, et al. The use of the pessary test in preoperative assessment of women with severe genital prolapse. Eur J Obstet Gynecol Reprod Biol. 2011; 155:110-113.
- Wei JT, Nygaard I, Richter HE, et al; Pelvic Floor Disorders Network. A midurethral sling to reduce incontinence after vaginal prolapse repair. N Engl J Med. 2012;366:2358-2367.
- March of Dimes. Quick facts: preterm birth. https://www .marchofdimes.org/Peristats/ViewTopic.aspx?reg=99 &top=3&lev=0&slev=1&gclid=EAIaIQobChMI4r. Accessed December 10, 2020.
- Goya M, Pratcorona L, Merced C, et al; PECEP Trial Group. Cervical pessary in pregnant women with a short cervix (PECEP): an open-label randomized controlled trial. Lancet. 2012;379:1800-1806.
- Di Tommaso M, Seravalli V, Arduino S, et al. Arabin cervical pessary to prevent preterm birth in twin pregnancies with short cervix. J Obstet Gynaecol. 2016;36:715-718.
- Saccone G, Maruotti GM, Giudicepietro A, et al; Italian Preterm Birth Prevention (IPP) Working Group. Effect of cervical pessary on spontaneous preterm birth in women with singleton pregnancies and short cervical length: a randomized clinical trial. JAMA. 2017;318:2317-2324.
- Perez-Lopez FR, Chedraui P, Perez-Roncero GR, et al; Health Outcomes and Systematic Analyses (HOUSSAY) Project. Effectiveness of the cervical pessary for the prevention of preterm birth in singleton pregnancies with a short cervix: a meta-analysis of randomized trials. Arch Gynecol Obstet. 2019;299:1215-1231.
- Hui SYA, Chor CM, Lau TK, et al. Cerclage pessary for preventing preterm birth in women with a singleton pregnancy and a short cervix at 20 to 24 weeks: a randomized controlled trial. Am J Perinatol. 2013;30:283-288.
- Nicolaides KH, Syngelaki A, Poon LC, et al. A randomized trial of a cervical pessary to prevent preterm singleton birth. N Engl J Med. 2016;374:1044-1052.
- Saccone G, Ciardulli A, Xodo S, et al. Cervical pessary for preventing preterm birth in singleton pregnancies with short cervical length: a systematic review and meta-analyses. J Ultrasound Med. 2017;36:1535-1543.
- Conde-Agudelo A, Romero R, Nicolaides KH. Cervical pessary to prevent preterm birth in asymptomatic high-risk women: a systematic review and meta-analysis. Am J Obstet Gynecol. 2020;223:42-65.e2.
- Sultan AH, Kamm MA, Hudson CN, et al. Anal-sphincter disruption during vaginal delivery. N Engl J Med. 1993;329: 1905-1911.
- Talley NJ, O’Keefe EA, Zinsmeister AR, et al. Prevalence of gastrointestinal symptoms in the elderly: a population-based study. Gastroenterology. 1992;102:895-901.
- Denis P, Bercoff E, Bizien MF, et al. Prevalence of anal incontinence in adults [in French]. Gastroenterol Clin Biol. 1992;16:344-350.
- Richter HE, Matthew CA, Muir T, et al. A vaginal bowel-control system for the treatment of fecal incontinence. Obstet Gynecol. 2015;125:540-547.
- 2019 Current Procedural Coding Expert. Optum360; 2018.
- ICD-10-CM Expert for Physicians. Optum360; 2019.
- MDS Medical Department Store website. http://www .medicaldepartmentstore.com/Pessary-Vaginal -Pessaries-/3788.htm?gclid=CjwKCAiAlNf-BRB _EiwA2osbxdqln8fQg-AxOUEMphM9aYlTIft Skwy0xXLT0PrcpIZnb5gBhiLc1RoCsbMQAvD_BwE. Accessed December 15, 2020.
- Monarch Medical Products website. https://www .monarchmedicalproducts.com/index.php?route=product /category&path=99_67. Accessed December 15, 2020.
- CooperSurgical Medical Devices website. https://www .coopersurgical.com/our-brands/milex/. Accessed December 15, 2020.

In Part 1 of this article in the December 2020 issue of OBG Management, I discussed the reasons that pessaries are an effective treatment option for many women with pelvic organ prolapse (POP) and stress urinary incontinence (SUI) and provided details on the types of pessaries available.
In this article, I highlight the steps in fitting a pessary, pessary aftercare, and potential complications associated with pessary use. In addition, I discuss the effectiveness of pessary treatment for POP and SUI as well as for preterm labor prevention and defecatory disorders.
The pessary fitting process
For a given patient, the best size pessary is the smallest one that will not fall out. The only “rule” for fitting a pessary is that a woman’s internal vaginal caliber should be wider than her introitus.
When fitting a pessary, goals include that the selected pessary:
- should be comfortable for the patient to wear
- is not easily expelled
- does not interfere with urination or defecation
- does not cause vaginal irritation.
The presence or absence of a cervix or uterus does not affect pessary choice.
Most experts agree that the process for fitting the right size pessary is one of trial and error. As with fitting a contraceptive diaphragm, the clinician should perform a manual examination to estimate the integrity and width of the perineum and the depth of the vagina to roughly approximate the pessary size that might best fit. Using a set of “fitting pessaries,” a pessary of the estimated size should be placed into the vagina and the fit evaluated as to whether the device is too big, too small, or appropriate. If the pessary is easily expelled, larger sizes should be tried until the pessary remains in place or the patient is uncomfortable. Once the pessary is in place, the clinician should be able to run his or her finger around the entire pessary; if this is not possible, the pessary is too tight. In addition, the pessary should remain more than one finger breadth above the introitus when the patient is standing or bearing down.
Since many patients who require a pessary are elderly, their perineal skin and vaginal mucosa may be atrophic and fragile. Inserting a pessary can be uncomfortable and can cause abrasions or tears. Successfully fitting a pessary may require extra care under these circumstances. The following steps may help alleviate these difficulties:
- Explain the fitting process to the patient in detail.
- Employ lubrication liberally.
- Enlarge the introitus by applying gentle digital pressure on the posterior fourchette.
- Apply 2% lidocaine ointment several minutes prior to pessary fitting to help decrease patient discomfort.
- Treat the patient for several weeks with vaginal estrogen cream before attempting to fit a pessary if severe vulvovaginal atrophy is present.
Once the type and size of the pessary are selected and a pessary is inserted, evaluate the patient with the pessary in place. Assess for the following:
Discomfort. Ask the patient if she feels discomfort with the pessary in position. A patient with a properly fitting pessary should not feel that it is in place. If she does feel discomfort initially, the discomfort will only increase with time and the issue should be addressed at that time.
Expulsion. Test to make certain that the pessary is not easily expelled from the vagina. Have the patient walk, cough, squat, and even jump if possible.
Urination. Have the patient urinate with the pessary in place. This tests for her ability to void while wearing the pessary and shows whether the contraction of pelvic muscles during voiding results in expulsion of the pessary. (Experience shows that it is best to do this with a plastic “hat” over the toilet so that if the pessary is expelled, it does not drop into the bowl.)
Re-examination. After these provocative tests, examine the patient again to ensure that the pessary has not slid out of place.
Depending on whether or not your office stocks pessaries, at this point the patient is either given the correct type and size of pessary or it is ordered for her. If the former, the patient should try placing it herself; if she is unable to, the clinician should place it for her. In either event, its position should be checked. If the pessary has to be ordered, the patient must schedule an appointment to return for pessary insertion.
Whether the pessary is supplied by the office or ordered, instruct the patient on how to insert and remove the pessary, how frequently to remove it for cleansing (see below), and signs to watch for, such as vaginal bleeding, inability to void or defecate, or pelvic pain.
It is advisable to schedule a subsequent visit for 2 to 3 weeks after initial pessary placement to assess how the patient is doing and to address any issues that have developed.
Continue to: Special circumstances...
Special circumstances
It is safe for a patient with a pessary in place to undergo magnetic resonance imaging.1 Patients should be informed, however, that full body scans, such as at airports, will detect pessaries. Patients may need to obtain a physician’s note to document that the pessary is a medical device.
Finally, several factors may prevent successful pessary fitting. These include prior pelvic surgery, obesity, short vaginal length (less than 6–7 cm), and a vaginal introitus width of greater than 4 finger breadths.
Necessary pessary aftercare
Once a pessary is in place and the patient is comfortable with it, the only maintenance necessary is the pessary’s intermittent removal for cleansing and for evaluation of the vaginal mucosa for erosion and ulcerations. How frequently this should be done varies based on the type of pessary, the amount of discharge that a woman produces, whether or not an odor develops after prolonged wearing of the pessary, and whether or not the patient’s vaginal mucosa has been abraded.
The question of timing for pessary cleaning
Although there are many opinions about how often pessaries should be removed and cleaned, no data in the literature support any specific interval. Pessaries that are easily removed by women themselves can be cleaned as frequently as desired, often on a weekly basis. The patient simply removes the pessary, washes it with soap and water, and reinserts it. For pessaries that are difficult to remove (such as the Gellhorn, cube, or donut) or for women who are physically unable to remove their own ring pessary, the clinician should remove and clean the pessary in the office every 3 to 6 months. It has been shown that there is no difference in complications from pessary use with either of these intervals.2
Prior to any vaginal surgical procedure, patients must be instructed to remove their pessary 10 to 14 days beforehand so that the surgeon can see the full extent of prolapse when making decisions about reconstruction and so that any vaginal mucosal erosions or abrasions have time to heal.
Office visits for follow-up care
The pessary “cleaning visit” has several goals, including to:
- see if the pessary is meeting the patient’s needs in terms of resolving symptoms of prolapse and/or restoring urinary continence
- discuss with the patient any problems she may be having, such as pelvic discomfort or pressure, difficulty voiding or defecating, excessive vaginal discharge, or vaginal odor
- check for vaginal mucosal erosion or ulceration; such vaginal lesions often can be prevented by the prophylactic use of either estrogen vaginal cream twice weekly or the continuous use of an estradiol vaginal ring in addition to the pessary
- evaluate the condition of the pessary itself and clean it with soap and water.
Continue to: Potential complications of pessary use...
Potential complications of pessary use
The most common complications experienced by pessary users are:
Odor or excessive discharge. Bacterial vaginosis (BV) occurs more frequently in women who use pessaries. The symptoms of BV can be minimized—but unfortunately not totally eliminated—by the prophylactic use of antiseptic vaginal creams or gels, such as metronidazole, clindamycin, Trimo-San (oxyquinoline sulfate and sodium lauryl sulfate), and others. Inserting the gel vaginally once a week can significantly reduce discharge and odor.3
Vaginal mucosal erosion and ulceration. These are treated by removing the pessary for 2 weeks during which time estrogen cream is applied daily or an estradiol vaginal ring is put in place. If no resolution occurs after 2 weeks, the nonhealing vaginal mucosa should be biopsied.
Pressure on the rectum or bladder. If the pessary causes significant discomfort or interferes with voiding function, then either a different size or a different type pessary should be tried
Patients may discontinue pessary use for a variety of reasons. Among these are:
- discomfort
- inadequate improvement of POP or incontinence symptoms
- expulsion of the pessary during daily activities
- the patient’s desire for surgery instead
- worsening of urine leakage
- difficulty inserting or removing the pessary
- damage to the vaginal mucosa
- pain during removal of the pessary in the office.
Pessary effectiveness for POP and SUI symptoms
As might be expected with a device that is available in so many forms and is used to treat varied types of POP and SUI, the data concerning the success rates of pessary use vary considerably. These rates depend on the definition of success, that is, complete or partial control of prolapse and/or incontinence; which devices are being evaluated; and the nature and severity of the POP and/or SUI being treated.
That being said, a review of the literature reveals that the rates of prolapse symptom relief vary from 48% to 92% (TABLE 1).4-13
As for success in relieving symptoms of incontinence, studies show improvements in from 40% to 77% of patients (TABLE 2).6,8,14-17
In addition, some studies show a 50% improvement in bowel symptoms (urgency, obstruction, and anal incontinence) with the use of a pessary.9,18
How pessaries compare with surgery
While surgery has the advantage of being a one-time fix with a very high rate of initial success in correcting both POP and incontinence, surgery also has potential drawbacks:
- It is an invasive procedure with the discomfort and risk of complications any surgery entails.
- There is a relatively high rate of prolapse recurrence.
- It exposes the patient to the possibility of mesh erosion if mesh is employed either for POP support or incontinence treatment.
Pessaries, on the other hand, are inexpensive, nonsurgical, removable, and allow for immediate correction of symptoms. Moreover, if the pessary is tried and is found to be unsatisfactory, surgery always can be performed subsequently.
Drawbacks of pessary treatment compared with surgery include the:
- ongoing need to wear an artificial internal device
- need for intermittent pessary removal and cleansing
- inability to have sexual intercourse with certain kinds of pessaries in place
- possible accumulation of vaginal discharge and odor.
Sexual activity and pessaries
Studies by Fernando, Meriwether, and Kuhn concur that for a substantial number of pessary users who are sexually active, both frequency and satisfaction with sexual intercourse are increased.8,19,20 Kuhn further showed that desire, orgasm, and lubrication improved with the use of pessaries.20 While some types of pessaries do require removal for intercourse, Clemons reported that issues involving sexual activity are not associated with pessary discontinuation.21
Using a pessary to predict a surgical outcome
Because a pessary elevates the pelvic organs, supports the vaginal walls, and lifts the bladder and urethra into a position that simulates the results of surgical repair, trial placement of a pessary can be used as a fairly accurate predictive tool to model what pelvic support and continence status will be after a proposed surgical procedure.22,23 This is especially important because a significant number of patients with POP will have their occult stress incontinence unmasked following a reparative procedure.24 A brief pessary trial prior to surgery, therefore, can be a useful tool for both patient and surgeon.
Continue to: Pessaries for prevention of preterm labor...
Pessaries for prevention of preterm labor
Almost 1 in 10 births in the United States occurs before 37 completed weeks of gestation.25 Obstetricians have long thought that in women at risk for preterm delivery, the use of a pessary might help reduce the pressure of the growing uterus on the cervix and thus help prevent premature cervical dilation. It also has been thought that use of a pessary would be a safer and less invasive alternative to cervical cerclage. Many studies have evaluated the use of pessaries for the prevention of preterm labor with a mixture of positive (TABLE 3)26-29 and negative results (TABLE 4).30-33


From these data, it is reasonable to conclude that:
- The final answer concerning the effectiveness or lack thereof of pessary use in preventing preterm delivery is not yet in.
- Any advantage there might be to using pessaries to prevent preterm delivery cannot be too significant if multiple studies show as many negative outcomes as positive ones.
Pessary effectiveness in defecatory disorders
Vaginal birth has the potential to create multiple anatomic injuries in the anus, lower pelvis, and perineum that can affect defecation and bowel control. Tears of the anal sphincter, whether obvious or occult, may heal incompletely or be repaired inadequately.34 Nerve innervation of the perianal and perineal areas can be interrupted or damaged by stretching, tearing, or prolonged compression. Of healthy parous adult women, 7% to 16% admit incontinence of gas or feces.35,36
In addition, when a rectocele is present, stool in the lower rectum may cause bulging of the anterior rectal wall into the vagina, preventing stool from passing out of the anus. This sometimes requires women to digitally press their posterior vaginal walls during defecation to evacuate stool successfully. The question thus arises as to whether or not pessary placement and subsequent relief of rectoceles might facilitate bowel movements and decrease or eliminate defecatory dysfunction.
As with the issue of pessary use for prevention of preterm delivery, the answer is mixed. For instance, while Brazell18 showed that there was an overall improvement in bowel symptoms in pessary users, a study by Komesu10 did not demonstrate improvement.
There is, however, a relatively new device specifically designed to control defecatory problems: the vaginal bowel control system (Eclipse; Pelvalon). The silicon device is placed intravaginally as one does a pessary. After insertion, it is inflated via a valve and syringe. It works by putting pressure on and reversibly closing the lower rectum, thus blocking the uncontrolled passage of stool and gas. It can be worn continuously or intermittently, but it does need to be deflated for normal bowel movements. One trial of this device demonstrated a 50% reduction in incontinence episodes with a patient satisfaction rate of 84% at 3 months.37 This device may well prove to be a valuable nonsurgical approach to the treatment of fecal incontinence. Unfortunately, the device is relatively expensive and usually is not covered by insurance as third-party payers do not consider it to be a pessary (which generally is covered).
Practice management particulars
Useful information on Current Procedural Terminology codes for pessaries, diagnostic codes, and the cost of various pessaries is provided in TABLE 5,38TABLE 6,39 and TABLE 7.40-42



A contemporary device used since antiquity
Pessaries, considered “old-fashioned” by many gynecologists, are actually a very cost-effective and useful tool for the correction of POP and SUI. It behooves all who provide medical care to women to be familiar with them, to know when they might be useful, and to know how to fit and prescribe them. ●

In Part 1 of this article in the December 2020 issue of OBG Management, I discussed the reasons that pessaries are an effective treatment option for many women with pelvic organ prolapse (POP) and stress urinary incontinence (SUI) and provided details on the types of pessaries available.
In this article, I highlight the steps in fitting a pessary, pessary aftercare, and potential complications associated with pessary use. In addition, I discuss the effectiveness of pessary treatment for POP and SUI as well as for preterm labor prevention and defecatory disorders.
The pessary fitting process
For a given patient, the best size pessary is the smallest one that will not fall out. The only “rule” for fitting a pessary is that a woman’s internal vaginal caliber should be wider than her introitus.
When fitting a pessary, goals include that the selected pessary:
- should be comfortable for the patient to wear
- is not easily expelled
- does not interfere with urination or defecation
- does not cause vaginal irritation.
The presence or absence of a cervix or uterus does not affect pessary choice.
Most experts agree that the process for fitting the right size pessary is one of trial and error. As with fitting a contraceptive diaphragm, the clinician should perform a manual examination to estimate the integrity and width of the perineum and the depth of the vagina to roughly approximate the pessary size that might best fit. Using a set of “fitting pessaries,” a pessary of the estimated size should be placed into the vagina and the fit evaluated as to whether the device is too big, too small, or appropriate. If the pessary is easily expelled, larger sizes should be tried until the pessary remains in place or the patient is uncomfortable. Once the pessary is in place, the clinician should be able to run his or her finger around the entire pessary; if this is not possible, the pessary is too tight. In addition, the pessary should remain more than one finger breadth above the introitus when the patient is standing or bearing down.
Since many patients who require a pessary are elderly, their perineal skin and vaginal mucosa may be atrophic and fragile. Inserting a pessary can be uncomfortable and can cause abrasions or tears. Successfully fitting a pessary may require extra care under these circumstances. The following steps may help alleviate these difficulties:
- Explain the fitting process to the patient in detail.
- Employ lubrication liberally.
- Enlarge the introitus by applying gentle digital pressure on the posterior fourchette.
- Apply 2% lidocaine ointment several minutes prior to pessary fitting to help decrease patient discomfort.
- Treat the patient for several weeks with vaginal estrogen cream before attempting to fit a pessary if severe vulvovaginal atrophy is present.
Once the type and size of the pessary are selected and a pessary is inserted, evaluate the patient with the pessary in place. Assess for the following:
Discomfort. Ask the patient if she feels discomfort with the pessary in position. A patient with a properly fitting pessary should not feel that it is in place. If she does feel discomfort initially, the discomfort will only increase with time and the issue should be addressed at that time.
Expulsion. Test to make certain that the pessary is not easily expelled from the vagina. Have the patient walk, cough, squat, and even jump if possible.
Urination. Have the patient urinate with the pessary in place. This tests for her ability to void while wearing the pessary and shows whether the contraction of pelvic muscles during voiding results in expulsion of the pessary. (Experience shows that it is best to do this with a plastic “hat” over the toilet so that if the pessary is expelled, it does not drop into the bowl.)
Re-examination. After these provocative tests, examine the patient again to ensure that the pessary has not slid out of place.
Depending on whether or not your office stocks pessaries, at this point the patient is either given the correct type and size of pessary or it is ordered for her. If the former, the patient should try placing it herself; if she is unable to, the clinician should place it for her. In either event, its position should be checked. If the pessary has to be ordered, the patient must schedule an appointment to return for pessary insertion.
Whether the pessary is supplied by the office or ordered, instruct the patient on how to insert and remove the pessary, how frequently to remove it for cleansing (see below), and signs to watch for, such as vaginal bleeding, inability to void or defecate, or pelvic pain.
It is advisable to schedule a subsequent visit for 2 to 3 weeks after initial pessary placement to assess how the patient is doing and to address any issues that have developed.
Continue to: Special circumstances...
Special circumstances
It is safe for a patient with a pessary in place to undergo magnetic resonance imaging.1 Patients should be informed, however, that full body scans, such as at airports, will detect pessaries. Patients may need to obtain a physician’s note to document that the pessary is a medical device.
Finally, several factors may prevent successful pessary fitting. These include prior pelvic surgery, obesity, short vaginal length (less than 6–7 cm), and a vaginal introitus width of greater than 4 finger breadths.
Necessary pessary aftercare
Once a pessary is in place and the patient is comfortable with it, the only maintenance necessary is the pessary’s intermittent removal for cleansing and for evaluation of the vaginal mucosa for erosion and ulcerations. How frequently this should be done varies based on the type of pessary, the amount of discharge that a woman produces, whether or not an odor develops after prolonged wearing of the pessary, and whether or not the patient’s vaginal mucosa has been abraded.
The question of timing for pessary cleaning
Although there are many opinions about how often pessaries should be removed and cleaned, no data in the literature support any specific interval. Pessaries that are easily removed by women themselves can be cleaned as frequently as desired, often on a weekly basis. The patient simply removes the pessary, washes it with soap and water, and reinserts it. For pessaries that are difficult to remove (such as the Gellhorn, cube, or donut) or for women who are physically unable to remove their own ring pessary, the clinician should remove and clean the pessary in the office every 3 to 6 months. It has been shown that there is no difference in complications from pessary use with either of these intervals.2
Prior to any vaginal surgical procedure, patients must be instructed to remove their pessary 10 to 14 days beforehand so that the surgeon can see the full extent of prolapse when making decisions about reconstruction and so that any vaginal mucosal erosions or abrasions have time to heal.
Office visits for follow-up care
The pessary “cleaning visit” has several goals, including to:
- see if the pessary is meeting the patient’s needs in terms of resolving symptoms of prolapse and/or restoring urinary continence
- discuss with the patient any problems she may be having, such as pelvic discomfort or pressure, difficulty voiding or defecating, excessive vaginal discharge, or vaginal odor
- check for vaginal mucosal erosion or ulceration; such vaginal lesions often can be prevented by the prophylactic use of either estrogen vaginal cream twice weekly or the continuous use of an estradiol vaginal ring in addition to the pessary
- evaluate the condition of the pessary itself and clean it with soap and water.
Continue to: Potential complications of pessary use...
Potential complications of pessary use
The most common complications experienced by pessary users are:
Odor or excessive discharge. Bacterial vaginosis (BV) occurs more frequently in women who use pessaries. The symptoms of BV can be minimized—but unfortunately not totally eliminated—by the prophylactic use of antiseptic vaginal creams or gels, such as metronidazole, clindamycin, Trimo-San (oxyquinoline sulfate and sodium lauryl sulfate), and others. Inserting the gel vaginally once a week can significantly reduce discharge and odor.3
Vaginal mucosal erosion and ulceration. These are treated by removing the pessary for 2 weeks during which time estrogen cream is applied daily or an estradiol vaginal ring is put in place. If no resolution occurs after 2 weeks, the nonhealing vaginal mucosa should be biopsied.
Pressure on the rectum or bladder. If the pessary causes significant discomfort or interferes with voiding function, then either a different size or a different type pessary should be tried
Patients may discontinue pessary use for a variety of reasons. Among these are:
- discomfort
- inadequate improvement of POP or incontinence symptoms
- expulsion of the pessary during daily activities
- the patient’s desire for surgery instead
- worsening of urine leakage
- difficulty inserting or removing the pessary
- damage to the vaginal mucosa
- pain during removal of the pessary in the office.
Pessary effectiveness for POP and SUI symptoms
As might be expected with a device that is available in so many forms and is used to treat varied types of POP and SUI, the data concerning the success rates of pessary use vary considerably. These rates depend on the definition of success, that is, complete or partial control of prolapse and/or incontinence; which devices are being evaluated; and the nature and severity of the POP and/or SUI being treated.
That being said, a review of the literature reveals that the rates of prolapse symptom relief vary from 48% to 92% (TABLE 1).4-13
As for success in relieving symptoms of incontinence, studies show improvements in from 40% to 77% of patients (TABLE 2).6,8,14-17
In addition, some studies show a 50% improvement in bowel symptoms (urgency, obstruction, and anal incontinence) with the use of a pessary.9,18
How pessaries compare with surgery
While surgery has the advantage of being a one-time fix with a very high rate of initial success in correcting both POP and incontinence, surgery also has potential drawbacks:
- It is an invasive procedure with the discomfort and risk of complications any surgery entails.
- There is a relatively high rate of prolapse recurrence.
- It exposes the patient to the possibility of mesh erosion if mesh is employed either for POP support or incontinence treatment.
Pessaries, on the other hand, are inexpensive, nonsurgical, removable, and allow for immediate correction of symptoms. Moreover, if the pessary is tried and is found to be unsatisfactory, surgery always can be performed subsequently.
Drawbacks of pessary treatment compared with surgery include the:
- ongoing need to wear an artificial internal device
- need for intermittent pessary removal and cleansing
- inability to have sexual intercourse with certain kinds of pessaries in place
- possible accumulation of vaginal discharge and odor.
Sexual activity and pessaries
Studies by Fernando, Meriwether, and Kuhn concur that for a substantial number of pessary users who are sexually active, both frequency and satisfaction with sexual intercourse are increased.8,19,20 Kuhn further showed that desire, orgasm, and lubrication improved with the use of pessaries.20 While some types of pessaries do require removal for intercourse, Clemons reported that issues involving sexual activity are not associated with pessary discontinuation.21
Using a pessary to predict a surgical outcome
Because a pessary elevates the pelvic organs, supports the vaginal walls, and lifts the bladder and urethra into a position that simulates the results of surgical repair, trial placement of a pessary can be used as a fairly accurate predictive tool to model what pelvic support and continence status will be after a proposed surgical procedure.22,23 This is especially important because a significant number of patients with POP will have their occult stress incontinence unmasked following a reparative procedure.24 A brief pessary trial prior to surgery, therefore, can be a useful tool for both patient and surgeon.
Continue to: Pessaries for prevention of preterm labor...
Pessaries for prevention of preterm labor
Almost 1 in 10 births in the United States occurs before 37 completed weeks of gestation.25 Obstetricians have long thought that in women at risk for preterm delivery, the use of a pessary might help reduce the pressure of the growing uterus on the cervix and thus help prevent premature cervical dilation. It also has been thought that use of a pessary would be a safer and less invasive alternative to cervical cerclage. Many studies have evaluated the use of pessaries for the prevention of preterm labor with a mixture of positive (TABLE 3)26-29 and negative results (TABLE 4).30-33


From these data, it is reasonable to conclude that:
- The final answer concerning the effectiveness or lack thereof of pessary use in preventing preterm delivery is not yet in.
- Any advantage there might be to using pessaries to prevent preterm delivery cannot be too significant if multiple studies show as many negative outcomes as positive ones.
Pessary effectiveness in defecatory disorders
Vaginal birth has the potential to create multiple anatomic injuries in the anus, lower pelvis, and perineum that can affect defecation and bowel control. Tears of the anal sphincter, whether obvious or occult, may heal incompletely or be repaired inadequately.34 Nerve innervation of the perianal and perineal areas can be interrupted or damaged by stretching, tearing, or prolonged compression. Of healthy parous adult women, 7% to 16% admit incontinence of gas or feces.35,36
In addition, when a rectocele is present, stool in the lower rectum may cause bulging of the anterior rectal wall into the vagina, preventing stool from passing out of the anus. This sometimes requires women to digitally press their posterior vaginal walls during defecation to evacuate stool successfully. The question thus arises as to whether or not pessary placement and subsequent relief of rectoceles might facilitate bowel movements and decrease or eliminate defecatory dysfunction.
As with the issue of pessary use for prevention of preterm delivery, the answer is mixed. For instance, while Brazell18 showed that there was an overall improvement in bowel symptoms in pessary users, a study by Komesu10 did not demonstrate improvement.
There is, however, a relatively new device specifically designed to control defecatory problems: the vaginal bowel control system (Eclipse; Pelvalon). The silicon device is placed intravaginally as one does a pessary. After insertion, it is inflated via a valve and syringe. It works by putting pressure on and reversibly closing the lower rectum, thus blocking the uncontrolled passage of stool and gas. It can be worn continuously or intermittently, but it does need to be deflated for normal bowel movements. One trial of this device demonstrated a 50% reduction in incontinence episodes with a patient satisfaction rate of 84% at 3 months.37 This device may well prove to be a valuable nonsurgical approach to the treatment of fecal incontinence. Unfortunately, the device is relatively expensive and usually is not covered by insurance as third-party payers do not consider it to be a pessary (which generally is covered).
Practice management particulars
Useful information on Current Procedural Terminology codes for pessaries, diagnostic codes, and the cost of various pessaries is provided in TABLE 5,38TABLE 6,39 and TABLE 7.40-42



A contemporary device used since antiquity
Pessaries, considered “old-fashioned” by many gynecologists, are actually a very cost-effective and useful tool for the correction of POP and SUI. It behooves all who provide medical care to women to be familiar with them, to know when they might be useful, and to know how to fit and prescribe them. ●
- O’Dell K, Atnip S. Pessary care: follow up and management of complications. Urol Nurs. 2012;32:126-136, 145.
- Gorti M, Hudelist G, Simons A. Evaluation of vaginal pessary management: a UK-based survey. J Obstet Gynaecol. 2009;29:129-131.
- Meriwether KV, Rogers RG, Craig E, et al. The effect of hydroxyquinoline-based gel on pessary-associated bacterial vaginosis: a multicenter randomized controlled trial. Am J Obstet Gynecol. 2015;213:729.e1-9.
- Wu V, Farrell SA, Baskett TF, et al. A simplified protocol for pessary management. Obstet Gynecol. 1997;90:990-994.
- Bai SW, Yoon BS, Kwon JY, et al. Survey of the characteristics and satisfaction degree of the patients using a pessary. Int Urogynecol J Pelvic Floor Dysfunct. 2005;16:182-186.
- Clemons JL, Aguilar VC, Tillinghast TA, et al. Patient satisfaction and changes in prolapse and urinary symptoms in women who were fitted successfully with a pessary for pelvic organ prolapse. Am J Obstet Gynecol. 2004;190:1025-1029.
- Hanson LM, Schulz JA, Flood CG, et al. Vaginal pessaries in managing women with pelvic organ prolapse and urinary incontinence: patient characteristics and factors contributing to success. Int Urogynecol J Pelvic Floor Dysfunct. 2006;17: 155-159.
- Fernando RJ, Thakar R, Sultan AH, et al. Effect of vaginal pessaries on symptoms associated with pelvic organ prolapse. Obstet Gynecol. 2006;108:93-99.
- Cundiff GW, Amundsen CL, Bent AE, et al. The PESSRI study: symptom relief outcomes of a randomized crossover trial of the ring and Gellhorn pessaries. Am J Obstet Gynecol. 2007;196:405.e1-405e.8.
- Komesu YM Rogers RG, Rode MA, et al. Pelvic floor symptom changes in pessary users. Am J Obstet Gynecol. 2007;197: 620.e1-6.
- Yang J, Han J, Zhu F, et al. Ring and Gellhorn pessaries used inpatients with pelvic organ prolapse: a retrospective study of 8 years. Arch Gynecol Obstet. 2018;298:623-629.
- Mao M, Ai F, Zhang Y, et al. Changes in the symptoms and quality of life of women with symptomatic pelvic organ prolapse fitted with a ring with support pessary. Maturitas. 2018;117:51-56.
- Duenas JL, Miceli A. Effectiveness of a continuous-use ringshaped vaginal pessary without support for advanced pelvic organ prolapse in postmenopausal women. Int Urogynecol J. 2018;29:1629-1636.
- Farrell S, Singh B, Aldakhil L. Continence pessaries in the management of urinary incontinence in women. J Obstet Gynaecol Canada. 2004;26:113-117.
- Donnelly MJ, Powell-Morgan SP, Olsen AL, et al. Vaginal pessaries for the management of stress and mixed urinary incontinence. Int Urogynecol J Pelvic Floor Dysfunct. 2004;15:302-307.
- Richter HE, Burgio KL, Brubaker L, et al; Pelvic Floor Disorders Network. Continence pessary compared with behavioral therapy or combined therapy for stress incontinence: a randomized controlled trial. Obstet Gynecol. 2010;115:609-617.
- Ding J, Chen C, Song XC, et al. Changes in prolapse and urinary symptoms after successful fitting of a ring pessary with support in women with advanced pelvic organ prolapse: a prospective study. Urology. 2016;87:70-75.
- Brazell HD, Patel M, O’Sullivan DM, et al. The impact of pessary use on bowel symptoms: one-year outcomes. Female Pelvic Med Reconstr Surg. 2014;20:95-98.
- Meriwether KV, Komesu YM, Craig C, et al. Sexual function and pessary management among women using a pessary for pelvic floor disorders. J Sex Med. 2015;12:2339-2349.
- Kuhn A, Bapst D, Stadlmayr W, et al. Sexual and organ function in patients with symptomatic prolapse: are pessaries helpful? Fertil Steril. 2009;91:1914-1918.
- Clemons JL, Aguilar VC, Sokol ER, et al. Patient characteristics that are associated with continued pessary use versus surgery after 1 year. Am J Obstet Gynecol. 2004;191:159-164.
- Liang CC, Chang YL, Chang SD, et al. Pessary test to predict postoperative urinary incontinence in women undergoing hysterectomy for prolapse. Obstet Gynecol. 2004;104:795-800.
- Liapis A, Bakas P, Georgantopoulou C, et al. The use of the pessary test in preoperative assessment of women with severe genital prolapse. Eur J Obstet Gynecol Reprod Biol. 2011; 155:110-113.
- Wei JT, Nygaard I, Richter HE, et al; Pelvic Floor Disorders Network. A midurethral sling to reduce incontinence after vaginal prolapse repair. N Engl J Med. 2012;366:2358-2367.
- March of Dimes. Quick facts: preterm birth. https://www .marchofdimes.org/Peristats/ViewTopic.aspx?reg=99 &top=3&lev=0&slev=1&gclid=EAIaIQobChMI4r. Accessed December 10, 2020.
- Goya M, Pratcorona L, Merced C, et al; PECEP Trial Group. Cervical pessary in pregnant women with a short cervix (PECEP): an open-label randomized controlled trial. Lancet. 2012;379:1800-1806.
- Di Tommaso M, Seravalli V, Arduino S, et al. Arabin cervical pessary to prevent preterm birth in twin pregnancies with short cervix. J Obstet Gynaecol. 2016;36:715-718.
- Saccone G, Maruotti GM, Giudicepietro A, et al; Italian Preterm Birth Prevention (IPP) Working Group. Effect of cervical pessary on spontaneous preterm birth in women with singleton pregnancies and short cervical length: a randomized clinical trial. JAMA. 2017;318:2317-2324.
- Perez-Lopez FR, Chedraui P, Perez-Roncero GR, et al; Health Outcomes and Systematic Analyses (HOUSSAY) Project. Effectiveness of the cervical pessary for the prevention of preterm birth in singleton pregnancies with a short cervix: a meta-analysis of randomized trials. Arch Gynecol Obstet. 2019;299:1215-1231.
- Hui SYA, Chor CM, Lau TK, et al. Cerclage pessary for preventing preterm birth in women with a singleton pregnancy and a short cervix at 20 to 24 weeks: a randomized controlled trial. Am J Perinatol. 2013;30:283-288.
- Nicolaides KH, Syngelaki A, Poon LC, et al. A randomized trial of a cervical pessary to prevent preterm singleton birth. N Engl J Med. 2016;374:1044-1052.
- Saccone G, Ciardulli A, Xodo S, et al. Cervical pessary for preventing preterm birth in singleton pregnancies with short cervical length: a systematic review and meta-analyses. J Ultrasound Med. 2017;36:1535-1543.
- Conde-Agudelo A, Romero R, Nicolaides KH. Cervical pessary to prevent preterm birth in asymptomatic high-risk women: a systematic review and meta-analysis. Am J Obstet Gynecol. 2020;223:42-65.e2.
- Sultan AH, Kamm MA, Hudson CN, et al. Anal-sphincter disruption during vaginal delivery. N Engl J Med. 1993;329: 1905-1911.
- Talley NJ, O’Keefe EA, Zinsmeister AR, et al. Prevalence of gastrointestinal symptoms in the elderly: a population-based study. Gastroenterology. 1992;102:895-901.
- Denis P, Bercoff E, Bizien MF, et al. Prevalence of anal incontinence in adults [in French]. Gastroenterol Clin Biol. 1992;16:344-350.
- Richter HE, Matthew CA, Muir T, et al. A vaginal bowel-control system for the treatment of fecal incontinence. Obstet Gynecol. 2015;125:540-547.
- 2019 Current Procedural Coding Expert. Optum360; 2018.
- ICD-10-CM Expert for Physicians. Optum360; 2019.
- MDS Medical Department Store website. http://www .medicaldepartmentstore.com/Pessary-Vaginal -Pessaries-/3788.htm?gclid=CjwKCAiAlNf-BRB _EiwA2osbxdqln8fQg-AxOUEMphM9aYlTIft Skwy0xXLT0PrcpIZnb5gBhiLc1RoCsbMQAvD_BwE. Accessed December 15, 2020.
- Monarch Medical Products website. https://www .monarchmedicalproducts.com/index.php?route=product /category&path=99_67. Accessed December 15, 2020.
- CooperSurgical Medical Devices website. https://www .coopersurgical.com/our-brands/milex/. Accessed December 15, 2020.
- O’Dell K, Atnip S. Pessary care: follow up and management of complications. Urol Nurs. 2012;32:126-136, 145.
- Gorti M, Hudelist G, Simons A. Evaluation of vaginal pessary management: a UK-based survey. J Obstet Gynaecol. 2009;29:129-131.
- Meriwether KV, Rogers RG, Craig E, et al. The effect of hydroxyquinoline-based gel on pessary-associated bacterial vaginosis: a multicenter randomized controlled trial. Am J Obstet Gynecol. 2015;213:729.e1-9.
- Wu V, Farrell SA, Baskett TF, et al. A simplified protocol for pessary management. Obstet Gynecol. 1997;90:990-994.
- Bai SW, Yoon BS, Kwon JY, et al. Survey of the characteristics and satisfaction degree of the patients using a pessary. Int Urogynecol J Pelvic Floor Dysfunct. 2005;16:182-186.
- Clemons JL, Aguilar VC, Tillinghast TA, et al. Patient satisfaction and changes in prolapse and urinary symptoms in women who were fitted successfully with a pessary for pelvic organ prolapse. Am J Obstet Gynecol. 2004;190:1025-1029.
- Hanson LM, Schulz JA, Flood CG, et al. Vaginal pessaries in managing women with pelvic organ prolapse and urinary incontinence: patient characteristics and factors contributing to success. Int Urogynecol J Pelvic Floor Dysfunct. 2006;17: 155-159.
- Fernando RJ, Thakar R, Sultan AH, et al. Effect of vaginal pessaries on symptoms associated with pelvic organ prolapse. Obstet Gynecol. 2006;108:93-99.
- Cundiff GW, Amundsen CL, Bent AE, et al. The PESSRI study: symptom relief outcomes of a randomized crossover trial of the ring and Gellhorn pessaries. Am J Obstet Gynecol. 2007;196:405.e1-405e.8.
- Komesu YM Rogers RG, Rode MA, et al. Pelvic floor symptom changes in pessary users. Am J Obstet Gynecol. 2007;197: 620.e1-6.
- Yang J, Han J, Zhu F, et al. Ring and Gellhorn pessaries used inpatients with pelvic organ prolapse: a retrospective study of 8 years. Arch Gynecol Obstet. 2018;298:623-629.
- Mao M, Ai F, Zhang Y, et al. Changes in the symptoms and quality of life of women with symptomatic pelvic organ prolapse fitted with a ring with support pessary. Maturitas. 2018;117:51-56.
- Duenas JL, Miceli A. Effectiveness of a continuous-use ringshaped vaginal pessary without support for advanced pelvic organ prolapse in postmenopausal women. Int Urogynecol J. 2018;29:1629-1636.
- Farrell S, Singh B, Aldakhil L. Continence pessaries in the management of urinary incontinence in women. J Obstet Gynaecol Canada. 2004;26:113-117.
- Donnelly MJ, Powell-Morgan SP, Olsen AL, et al. Vaginal pessaries for the management of stress and mixed urinary incontinence. Int Urogynecol J Pelvic Floor Dysfunct. 2004;15:302-307.
- Richter HE, Burgio KL, Brubaker L, et al; Pelvic Floor Disorders Network. Continence pessary compared with behavioral therapy or combined therapy for stress incontinence: a randomized controlled trial. Obstet Gynecol. 2010;115:609-617.
- Ding J, Chen C, Song XC, et al. Changes in prolapse and urinary symptoms after successful fitting of a ring pessary with support in women with advanced pelvic organ prolapse: a prospective study. Urology. 2016;87:70-75.
- Brazell HD, Patel M, O’Sullivan DM, et al. The impact of pessary use on bowel symptoms: one-year outcomes. Female Pelvic Med Reconstr Surg. 2014;20:95-98.
- Meriwether KV, Komesu YM, Craig C, et al. Sexual function and pessary management among women using a pessary for pelvic floor disorders. J Sex Med. 2015;12:2339-2349.
- Kuhn A, Bapst D, Stadlmayr W, et al. Sexual and organ function in patients with symptomatic prolapse: are pessaries helpful? Fertil Steril. 2009;91:1914-1918.
- Clemons JL, Aguilar VC, Sokol ER, et al. Patient characteristics that are associated with continued pessary use versus surgery after 1 year. Am J Obstet Gynecol. 2004;191:159-164.
- Liang CC, Chang YL, Chang SD, et al. Pessary test to predict postoperative urinary incontinence in women undergoing hysterectomy for prolapse. Obstet Gynecol. 2004;104:795-800.
- Liapis A, Bakas P, Georgantopoulou C, et al. The use of the pessary test in preoperative assessment of women with severe genital prolapse. Eur J Obstet Gynecol Reprod Biol. 2011; 155:110-113.
- Wei JT, Nygaard I, Richter HE, et al; Pelvic Floor Disorders Network. A midurethral sling to reduce incontinence after vaginal prolapse repair. N Engl J Med. 2012;366:2358-2367.
- March of Dimes. Quick facts: preterm birth. https://www .marchofdimes.org/Peristats/ViewTopic.aspx?reg=99 &top=3&lev=0&slev=1&gclid=EAIaIQobChMI4r. Accessed December 10, 2020.
- Goya M, Pratcorona L, Merced C, et al; PECEP Trial Group. Cervical pessary in pregnant women with a short cervix (PECEP): an open-label randomized controlled trial. Lancet. 2012;379:1800-1806.
- Di Tommaso M, Seravalli V, Arduino S, et al. Arabin cervical pessary to prevent preterm birth in twin pregnancies with short cervix. J Obstet Gynaecol. 2016;36:715-718.
- Saccone G, Maruotti GM, Giudicepietro A, et al; Italian Preterm Birth Prevention (IPP) Working Group. Effect of cervical pessary on spontaneous preterm birth in women with singleton pregnancies and short cervical length: a randomized clinical trial. JAMA. 2017;318:2317-2324.
- Perez-Lopez FR, Chedraui P, Perez-Roncero GR, et al; Health Outcomes and Systematic Analyses (HOUSSAY) Project. Effectiveness of the cervical pessary for the prevention of preterm birth in singleton pregnancies with a short cervix: a meta-analysis of randomized trials. Arch Gynecol Obstet. 2019;299:1215-1231.
- Hui SYA, Chor CM, Lau TK, et al. Cerclage pessary for preventing preterm birth in women with a singleton pregnancy and a short cervix at 20 to 24 weeks: a randomized controlled trial. Am J Perinatol. 2013;30:283-288.
- Nicolaides KH, Syngelaki A, Poon LC, et al. A randomized trial of a cervical pessary to prevent preterm singleton birth. N Engl J Med. 2016;374:1044-1052.
- Saccone G, Ciardulli A, Xodo S, et al. Cervical pessary for preventing preterm birth in singleton pregnancies with short cervical length: a systematic review and meta-analyses. J Ultrasound Med. 2017;36:1535-1543.
- Conde-Agudelo A, Romero R, Nicolaides KH. Cervical pessary to prevent preterm birth in asymptomatic high-risk women: a systematic review and meta-analysis. Am J Obstet Gynecol. 2020;223:42-65.e2.
- Sultan AH, Kamm MA, Hudson CN, et al. Anal-sphincter disruption during vaginal delivery. N Engl J Med. 1993;329: 1905-1911.
- Talley NJ, O’Keefe EA, Zinsmeister AR, et al. Prevalence of gastrointestinal symptoms in the elderly: a population-based study. Gastroenterology. 1992;102:895-901.
- Denis P, Bercoff E, Bizien MF, et al. Prevalence of anal incontinence in adults [in French]. Gastroenterol Clin Biol. 1992;16:344-350.
- Richter HE, Matthew CA, Muir T, et al. A vaginal bowel-control system for the treatment of fecal incontinence. Obstet Gynecol. 2015;125:540-547.
- 2019 Current Procedural Coding Expert. Optum360; 2018.
- ICD-10-CM Expert for Physicians. Optum360; 2019.
- MDS Medical Department Store website. http://www .medicaldepartmentstore.com/Pessary-Vaginal -Pessaries-/3788.htm?gclid=CjwKCAiAlNf-BRB _EiwA2osbxdqln8fQg-AxOUEMphM9aYlTIft Skwy0xXLT0PrcpIZnb5gBhiLc1RoCsbMQAvD_BwE. Accessed December 15, 2020.
- Monarch Medical Products website. https://www .monarchmedicalproducts.com/index.php?route=product /category&path=99_67. Accessed December 15, 2020.
- CooperSurgical Medical Devices website. https://www .coopersurgical.com/our-brands/milex/. Accessed December 15, 2020.
Home pregnancy tests—Is ectopic always on your mind?
CASE Unidentified ectopic pregnancy leads to rupture*
A 33-year-old woman (G1 P0010) with 2 positive home pregnancy tests presents to the emergency department (ED) reporting intermittent vaginal bleeding for 3 days. Her last menstrual period was 10 weeks ago, but she reports that her menses are always irregular. She has a history of asymptomatic chlamydia, as well as spontaneous abortion 2 years prior. At present, she denies abdominal pain or vaginal discharge.
Upon examination her vital signs are: temperature, 98.3 °F; pulse, 112 bpm, with a resting rate of 16 bpm; blood pressure (BP), 142/91 mm Hg; pulse O2, 99%; height, 4’ 3”; weight, 115 lb. Her labs are: hemoglobin, 12.1 g/dL; hematocrit, 38%; serum human chorionic gonadotropin (hCG) 236 mIU/mL. Upon pelvic examination, no active bleeding is noted. She agrees to be followed up by her gynecologist and is given a prescription for serum hCG in 2 days. She is instructed to return to the ED should she have pain or increased vaginal bleeding.
Three days later, the patient follows up with her gynecologist reporting mild cramping. She notes having had an episode of heavy vaginal bleeding and a “weakly positive” home pregnancy test. Transvaginal ultrasonography notes endometrial thickness 0.59 mm and unremarkable adnexa. A urine pregnancy test performed in the office is positive; urinalysis is positive for nitrites. With the bleeding slowed, the gynecologist’s overall impression is that the patient has undergone complete spontaneous abortion. She prescribes Macrobid for the urinary tract infection. She does not obtain the ED-prescribed serum HCG levels, as she feels, since complete spontaneous abortion has occurred there is no need to obtain a follow-up serum HCG.
Five days later, the patient returns to the ED reporting abdominal pain after eating. Fever and productive cough of 2 days are noted. The patient states that she had a recent miscarriage. The overall impression of the patient’s condition is bronchitis, and it is noted on the patient’s record, “unlikely ectopic pregnancy and pregnancy test may be false positive,” hence a pregnancy test is not ordered. Examination reveals mild suprapubic tenderness with no rebound; no pelvic exam is performed. The patient is instructed to follow up with a health care clinic within a week, and to return to the ED with severe abdominal pain, higher fever, or any new concerning symptoms. A Zithromax Z-pak is prescribed.
Four days later, the patient is brought by ambulance to the ED of the local major medical center with severe abdominal pain involving the right lower quadrant. She states that she had a miscarriage 3 weeks prior and was recently treated for bronchitis. She has dizziness when standing. Her vital signs are: temperature, 97.8 °F; heart rate, 95 bpm; BP, 72/48 mm Hg; pulse O2, 100%. She reports her abdominal pain to be 6/10.
The patient is given a Lactated Ringer’s bolus of 1,000 mL for a hypotensive episode. Computed tomography is obtained and notes, “low attenuation in the left adnexa with a dilated fallopian tube.” A large heterogeneous collection of fluid in the pelvis is noted with active extravasation, consistent with an “acute bleed.”
The patient is brought to the operating room with a diagnosis of probable ruptured ectopic pregnancy. Intraoperatively she is noted to have a right ruptured ectopic and left tubo-ovarian abscess. The surgeon proceeds with right salpingectomy and left salpingo-oophorectomy. Three liters of hemoperitoneum is found.
She is followed postoperatively with serum hCG until levels are negative. Her postoperative course is uneventful. Her only future option for pregnancy is through assisted reproductive technology (ART) with in vitro fertilization (IVF). The patient sues the gynecologist and second ED physician for presumed inappropriate assessment for ectopic pregnancy.
*The “facts” of this case are a composite, drawn from several cases to illustrate medical and legal issues. The statement of facts should be considered hypothetical.
Continue to: WHAT’S THE VERDICT?...
WHAT’S THE VERDICT?
A defense verdict is returned.
Medical considerations
The incidence of ectopic pregnancy is 2% of all pregnancies, with a higher incidence (about 4%) among infertility patients.1 Up to 10% of ectopic pregnancies have no symptoms.2
Clinical presentations. Classic signs of ectopic pregnancy include:
- abdominal pain
- vaginal bleeding
- late menses (often noted).
A recent case of ectopic pregnancy presenting with chest pain was reported.3 Clinicians must never lose site of the fact that ectopic pregnancy is the most common cause of maternal mortality in the first trimester, with an incidence of 1% to 10% of all first-trimester deaths.4
Risk factors include pelvic inflammatory disease, as demonstrated in the opening case. “The silent epidemic of chlamydia” comes to mind, and tobacco smoking can adversely affect tubal cilia, as can pelvic adhesions and/or prior tubal surgery. All of these factors can predispose a patient to ectopic pregnancy; in addition, intrauterine devices, endometriosis, tubal ligation (or ligation reversal), all can set the stage for an ectopic pregnancy.5 Appropriate serum hCG monitoring during early pregnancy can assist in sorting out pregnancies of unknown location (PUL; FIGURE). First trimester ultrasonography, at 5 weeks gestation, usually identifies early intrauterine gestation.

Imaging. With regard to pelvic sonography, the earliest sign of an intrauterine pregnancy (IUP) is a sac eccentrically located in the decidua.6 As the IUP progresses, it becomes equated with a “double decidual sign,” with double rings of tissue around the sac.6 If the pregnancy is located in an adnexal mass, it is frequently inhomogeneous or noncystic in appearance (ie, “the blob” sign); the positive predictive value (PPV) is 96%.2 The PPV of transvaginal ultrasound is 80%, as paratubal, paraovarian, ovarian cyst, and hydrosalpinx can affect the interpretation.7
Heterotopic pregnancy includes an intrauterine gestation and an ectopic pregnancy. This presentation includes the presence of a “pseudosac” in the endometrial cavity plus an extrauterine gestation. Heterotopic pregnancies have become somewhat more common as ART/IVF has unfolded, especially prior to the predominance of single embryo transfer.
Managing ectopic pregnancy
For cases of early pregnancy complicated by intermittent bleeding and/or pain, monitoring with serum hCG levels at 48-hour intervals to distinguish a viable IUP from an abnormal IUP or an ectopic is appropriate. The “discriminatory zone” collates serum hCG levels with findings on ultrasonography. Specific lower limits of serum hCG levels are not clear cut, with recommendations of 3,500 mIU/mL to provide sonographic evidence of an intrauterine gestation “to avoid misdiagnosis and possible interruption of intrauterine pregnancy,” as conveyed in the American College of Obstetricians and Gynecologists 2018 practice bulletin.8 Serum progesterone levels also have been suggested to complement hCG levels; a progesterone level of <20 nmol/L is consistent with an abnormal pregnancy, whereas levels >25 nmol/L are suggestive of a viable pregnancy.2 Inhibin A levels also have been suggested to be helpful, but they are not an ideal monitoring tool.
While most ectopic pregnancies are located in the fallopian tube, other locations also can be abdominal or ovarian. In addition, cesarean scar ectopic pregnancy can occur and often is associated with delay in diagnosis and greater morbidity due to such delay.9 With regard to ovarian ectopic, Spiegelberg criteria are established for diagnosis (TABLE 1).10
Appropriate management of an ectopic pregnancy is dependent upon the gestational age, serum hCG levels, and imaging findings, as well as the patient’s symptoms and exam findings. Treatment is established in large part on a case-by-case basis and includes, for early pregnancy, expectant management and use of methotrexate (TABLE 2).11 Dilation and curettage may be used to identify the pregnancy’s location when the serum hCG level is below 2,000 mIU/mL and there is no evidence of an IUP on ultrasound. Surgical treatment can include minimally invasive salpingostomy or salpingectomy and, depending on circumstance, laparotomy may be indicated.

Fertility following ectopic pregnancy varies and is affected by location, treatment, predisposing factors, total number of ectopic pregnancies, and other factors. Ectopic pregnancy, although rare, also can occur with use of IVF. Humans are not unique with regard to ectopic pregnancies, as they also occur in sheep.12
Continue to: Legal perspective...
Legal perspective
Lawsuits related to ectopic pregnancy are not a new phenomenon. In fact, in 1897, a physician in Ohio who misdiagnosed an “extrauterine pregnancy” as appendicitis was the center of a malpractice lawsuit.13 Unrecognized or mishandled ectopic pregnancy can result in serious injuries—in the range of 1% to 10% (see above) of maternal deaths are related to ectopic pregnancy.14 Ectopic pregnancy cases, therefore, have been the subject of substantial litigation over the years. An informal, noncomprehensive review of malpractice lawsuits brought from 2000 to 2019, found more than 300 ectopic pregnancy cases. Given the large number of malpractice claims against ObGyns,15 ectopic pregnancy cases are only a small portion of all ObGyn malpractice cases.16
A common claim: negligent diagnosis or treatment
The most common basis for lawsuits in cases of ectopic pregnancy is the clinician’s negligent failure to properly diagnose the ectopic nature of the pregnancy. There are also a number of cases claiming negligent treatment of an identified ectopic pregnancy. Not every missed diagnosis, or unsuccessful treatment, leads to liability, of course. It is only when a diagnosis or treatment fails to meet the standard of care within the profession that there should be liability. That standard of care is generally defined by what a reasonably prudent physician would do under the circumstances. Expert witnesses, who are familiar with the standard of practice within the specialty, are usually necessary to establish what that practice is. Both the plaintiff and the defense obtain experts, the former to prove what the standard of care is and that the standard was not met in the case at hand. The defense experts are usually arguing that the standard of care was met.17 Inadequate diagnosis of ectopic pregnancy or other condition may arise from a failure to take a sufficient history, conduct an appropriately thorough physical examination, recognize any of the symptoms that would suggest it is present, use and conduct ultrasound correctly, or follow-up appropriately with additional testing.18
A malpractice claim of negligent treatment can involve any the following circumstances19:
- failure to establish an appropriate treatment plan
- prescribing inappropriate medications for the patient (eg, methotrexate, when it is contraindicated)
- delivering the wrong medication or the wrong amount of the right medication
- performing a procedure badly
- undertaking a new treatment without adequate instruction and preparation.
Given the nature and risks of ectopic pregnancy, ongoing, frequent contact with the patient is essential from the point at which the condition is suspected. The greater the risk of harm (probability or consequence), the more careful any professional ought to be. Because ectopic pregnancy is not an uncommon occurrence, and because it can have devastating effects, including death, a reasonably prudent practitioner would be especially aware of the clinical presentations discussed above.20 In the opening case, the treatment plan was not well documented.
Negligence must lead to patient harm. In addition to negligence (proving that the physician did not act in accordance with the standard of care), to prevail in a malpractice case, the plaintiff-patient must prove that the negligence caused the injury, or worsened it. If the failure to make a diagnosis would not have made any difference in a harm the patient suffered, there are no damages and no liability. Suppose, for example, that a physician negligently failed to diagnose ectopic pregnancy, but performed surgery expecting to find the misdiagnosed condition. In the course of the surgery, however, the surgeon discovered and appropriately treated the ectopic pregnancy. (A version of this happened in the old 19th century case mentioned above.) The negligence of the physician did not cause harm, so there are no damages and no liability.
Continue to: Informed consent is vital...
Informed consent is vital
A part of malpractice is informed consent (or the absence of it)—issues that can arise in any medical care.21 It is wise to pay particular attention in cases where the nature of the illness is unknown, and where there are significant uncertainties and the nature of testing and treatment may change substantially over a period of a few days or few weeks. As always, informed consent should include a discussion of what process or procedure is proposed, its risks and benefits, alternative approaches that might be available, and the risk of doing nothing. Frequently, the uncertainty of ectopic pregnancy complicates the informed consent process.22
Because communication with the patient is an essential function of informed consent, the consent process should productively be used in PUL and similar cases to inform the patient about the uncertainty, and the testing and (nonsurgical) treatment that will occur. This is an opportunity to reinforce the message that the patient must maintain ongoing communication with the physician’s office about changes in her condition, and appear for each appointment scheduled. If more invasive procedures—notably surgery—become required, a separate consent process should be completed, because the risks and considerations are now meaningfully different than when treatment began. As a general matter, any possible treatment that may result in infertility or reduced reproductive capacity should specifically be included in the consent process.
In the hypothetical case, the gynecologist failed to obtain a follow-up serum hCG level. In addition, the record did not reflect ectopic pregnancy in the differential diagnosis. As noted above, the patient had predisposing factors for an ectopic pregnancy. The physician should have acknowledged the history of sexually transmitted disease predisposing her to an ectopic pregnancy. Monitoring of serum hCG levels until they are negative is appropriate with ectopic, or presumed ectopic, pregnancy management. Appropriate monitoring did not occur in this case. Each of these errors (following up on serum hCG levels and the inadequacy of notations about the possibility of ectopic pregnancy) seem inconsistent with the usual standard of care. Furthermore, as a result of the outcome, the only future option for the patient to pursue pregnancy was IVF.
Other legal issues
There are a number of other legal issues that are associated with the topic of ectopic pregnancy. There is evidence, for example, that Catholic and non-Catholic hospitals treat ectopic pregnancies differently,23 which may reflect different views on taking a life or the use of methotrexate and its association with abortion.24 In addition, the possibility of an increase in future ectopic pregnancies is one of the “risks” of abortion that pro-life organizations have pushed to see included in abortion informed consent.25 This has led some commentators to conclude that some Catholic hospitals violate federal law in managing ectopic pregnancy. There is also evidence of “overwhelming rates of medical misinformation on pregnancy center websites, including a link between abortion and ectopic pregnancy.”26
The fact that cesarean deliveries are related to an increased risk for ectopic pregnancy (because of the risk of cesarean scar ectopic pregnancy) also has been cited as information that should play a role in the consent process for cesarean delivery.27 In terms of liability, failed tubal ligation leads to a 33% risk of ectopic pregnancy.28 The risk of ectopic pregnancy is also commonly included in surrogacy contracts.29
Why the outcome was for the defense
The opening hypothetical case illustrates some of the uncertainties of medical malpractice cases. As noted, there appeared a deviation from the usual standard of care, particularly the failure to follow up on the serum hCG level. The weakness in the medical record, failing to note the possibility of ectopic pregnancy, also was probably an error but, apparently, the court felt that this did not result in any harm to the patient.
The question arises of how there would be a defense verdict in light of the failure to track consecutive serum hCG levels. A speculative explanation is that there are many uncertainties in most lawsuits. Procedural problems may result in a case being limited, expert witnesses are essential to both the plaintiff and defense, with the quality of their review and testimony possibly uneven. Judges and juries may rely on one expert witness rather than another, juries vary, and the quality of advocacy differs. Any of these situations can contribute to the unpredictability of the outcome of a case. In the case above, the liability was somewhat uncertain, and the various other factors tipped in favor of a defense verdict. ●
- Centers for Disease Control and Prevention. Ectopic pregnancy—United States, 1990‒1992. MMWR Morb Mortal Wkly Rep. 1995;44:46-48.
- Kirk E, Bottomley C, Bourne T. Diagnosing ectopic pregnancy and current concepts in the management of pregnancy of unknown location. Hum Reprod Update. 2012;20:250-261.
- Dichter E, Espinosa J, Baird J, Lucerna A. An unusual emergency department case: ruptured ectopic pregnancy presenting as chest pain. World J Emerg Med. 2017;8:71-73.
- Cecchino GN, Araujo E, Elito J. Methotrexate for ectopic pregnancy: when and how. Arch Gynecol Obstet. 2014;290:417- 423.
- Barnhart KT, Sammel MD, Cracia CR, et al. Risk factors for ectopic pregnancy in women with symptomatic firsttrimester pregnancies. Fertil Steril. 2006;86:36-43.
- Carusi D. Pregnancy of unknown location: evaluation and management. Semin Perinatol. 2019;43:95-100.
- Barnhart KT, Fay CA, Suescum M, et al. Clinical factors affecting the accuracy of ultrasonography in symptomatic first-trimester pregnancy. Obstet Gynecol. 2011;117:299-306.
- American College of Obstetricians and Gynecologists Practice Bulletin No. 193: tubal ectopic pregnancy. Obstet Gynecol. 2018;131:e91-e103.
- Bouyer J, Coste J, Fernandez H, et al. Sites of ectopic pregnancy: a 10-year population-based study of 1800 cases. Hum Reprod. 2002;17:3224-3230.
- Spiegelberg O. Zur casuistic der ovarial schwangerschaft. Arch Gynecol. 1978;13:73.
- OB Hospitalist Group. Methotrexate use for ectopic pregnancies guidelines. https://www.obhg.com/wp-content /uploads/2020/01/Methotrexate-Use-for-EctopicPregnancies_2016-updates.pdf. Accessed December 10, 2020.
- Brozos C, Kargiannis I, Kiossis E, et al. Ectopic pregnancy through a caesarean scar in a ewe. N Z Vet J. 2013;61:373-375.
- Tucker v. Gillette, 12 Ohio Cir. Dec. 401 (Cir. Ct. 1901).
- Creanga AA, Syverson C, Seed K, et al. Pregnancy-related mortality in the United States, 2011–2013. Obstet Gynecol. 2017;130:366-373.
- Matthews LR, Alvi FA, Milad MP. Reproductive surgery malpractice patterns. Fertil Steril. 2016;106:e42-e43.
- Kim B. The impact of malpractice risk on the use of obstetrics procedures. J Legal Studies. 2006;36:S79-S120.
- Abinader R, Warsof S. Complications involving obstetrical ultrasound. In: Warsof S, Shwayder JM, eds. Legal Concepts and Best Practices in Obstetrics: The Nuts and Bolts Guide to Mitigating Risk. 2019;45-48.
- Creanga AA, Shapiro-Mendoza CK, Bish CL, et al. Trends in ectopic pregnancy mortality in the United States: 1980-2007. Obstet Gynecol. 2011;117:837-843.
- Shwayder JM. IUP diagnosed and treated as ectopic: How bad can it get? Contemporary OB/GYN. 2019;64:49-46.
- Kaplan AI. Should this ectopic pregnancy have been diagnosed earlier? Contemporary OB/GYN. 2017;62:53.
- American College of Obstetricians and Gynecologists Committee on Ethics. Committee opinion 439: informed consent. Reaffirmed 2015. https://www.acog.org/clinical /clinical-guidance/committee-opinion/articles/2009/08 /informed-consent. Accessed December 9, 2020.
- Shwayder JM. Liability in ob/gyn ultrasound. Contemporary OB/GYN. 2017;62:32-49.
- Fisher LN. Institutional religious exemptions: a balancing approach. BYU Law Review. 2014;415-444.
- Makdisi J. Aquinas’s prohibition of killing reconsidered. J Catholic Legal Stud. 2019:57:67-128.
- Franzonello A. Remarks of Anna Franzonello. Alb Law J Sci Tech. 2012;23:519-530.
- Malcolm HE. Pregnancy centers and the limits of mandated disclosure. Columbia Law Rev. 2019;119:1133-1168.
- Kukura E. Contested care: the limitations of evidencebased maternity care reform. Berkeley J Gender Law Justice. 2016;31:241-298.
- Donley G. Contraceptive equity: curing the sex discrimination in the ACA’s mandate. Alabama Law Rev. 2019;71:499-560.
- Berk H. Savvy surrogates and rock star parents: compensation provisions, contracting practices, and the value of womb work. Law Social Inquiry. 2020;45:398-431.
CASE Unidentified ectopic pregnancy leads to rupture*
A 33-year-old woman (G1 P0010) with 2 positive home pregnancy tests presents to the emergency department (ED) reporting intermittent vaginal bleeding for 3 days. Her last menstrual period was 10 weeks ago, but she reports that her menses are always irregular. She has a history of asymptomatic chlamydia, as well as spontaneous abortion 2 years prior. At present, she denies abdominal pain or vaginal discharge.
Upon examination her vital signs are: temperature, 98.3 °F; pulse, 112 bpm, with a resting rate of 16 bpm; blood pressure (BP), 142/91 mm Hg; pulse O2, 99%; height, 4’ 3”; weight, 115 lb. Her labs are: hemoglobin, 12.1 g/dL; hematocrit, 38%; serum human chorionic gonadotropin (hCG) 236 mIU/mL. Upon pelvic examination, no active bleeding is noted. She agrees to be followed up by her gynecologist and is given a prescription for serum hCG in 2 days. She is instructed to return to the ED should she have pain or increased vaginal bleeding.
Three days later, the patient follows up with her gynecologist reporting mild cramping. She notes having had an episode of heavy vaginal bleeding and a “weakly positive” home pregnancy test. Transvaginal ultrasonography notes endometrial thickness 0.59 mm and unremarkable adnexa. A urine pregnancy test performed in the office is positive; urinalysis is positive for nitrites. With the bleeding slowed, the gynecologist’s overall impression is that the patient has undergone complete spontaneous abortion. She prescribes Macrobid for the urinary tract infection. She does not obtain the ED-prescribed serum HCG levels, as she feels, since complete spontaneous abortion has occurred there is no need to obtain a follow-up serum HCG.
Five days later, the patient returns to the ED reporting abdominal pain after eating. Fever and productive cough of 2 days are noted. The patient states that she had a recent miscarriage. The overall impression of the patient’s condition is bronchitis, and it is noted on the patient’s record, “unlikely ectopic pregnancy and pregnancy test may be false positive,” hence a pregnancy test is not ordered. Examination reveals mild suprapubic tenderness with no rebound; no pelvic exam is performed. The patient is instructed to follow up with a health care clinic within a week, and to return to the ED with severe abdominal pain, higher fever, or any new concerning symptoms. A Zithromax Z-pak is prescribed.
Four days later, the patient is brought by ambulance to the ED of the local major medical center with severe abdominal pain involving the right lower quadrant. She states that she had a miscarriage 3 weeks prior and was recently treated for bronchitis. She has dizziness when standing. Her vital signs are: temperature, 97.8 °F; heart rate, 95 bpm; BP, 72/48 mm Hg; pulse O2, 100%. She reports her abdominal pain to be 6/10.
The patient is given a Lactated Ringer’s bolus of 1,000 mL for a hypotensive episode. Computed tomography is obtained and notes, “low attenuation in the left adnexa with a dilated fallopian tube.” A large heterogeneous collection of fluid in the pelvis is noted with active extravasation, consistent with an “acute bleed.”
The patient is brought to the operating room with a diagnosis of probable ruptured ectopic pregnancy. Intraoperatively she is noted to have a right ruptured ectopic and left tubo-ovarian abscess. The surgeon proceeds with right salpingectomy and left salpingo-oophorectomy. Three liters of hemoperitoneum is found.
She is followed postoperatively with serum hCG until levels are negative. Her postoperative course is uneventful. Her only future option for pregnancy is through assisted reproductive technology (ART) with in vitro fertilization (IVF). The patient sues the gynecologist and second ED physician for presumed inappropriate assessment for ectopic pregnancy.
*The “facts” of this case are a composite, drawn from several cases to illustrate medical and legal issues. The statement of facts should be considered hypothetical.
Continue to: WHAT’S THE VERDICT?...
WHAT’S THE VERDICT?
A defense verdict is returned.
Medical considerations
The incidence of ectopic pregnancy is 2% of all pregnancies, with a higher incidence (about 4%) among infertility patients.1 Up to 10% of ectopic pregnancies have no symptoms.2
Clinical presentations. Classic signs of ectopic pregnancy include:
- abdominal pain
- vaginal bleeding
- late menses (often noted).
A recent case of ectopic pregnancy presenting with chest pain was reported.3 Clinicians must never lose site of the fact that ectopic pregnancy is the most common cause of maternal mortality in the first trimester, with an incidence of 1% to 10% of all first-trimester deaths.4
Risk factors include pelvic inflammatory disease, as demonstrated in the opening case. “The silent epidemic of chlamydia” comes to mind, and tobacco smoking can adversely affect tubal cilia, as can pelvic adhesions and/or prior tubal surgery. All of these factors can predispose a patient to ectopic pregnancy; in addition, intrauterine devices, endometriosis, tubal ligation (or ligation reversal), all can set the stage for an ectopic pregnancy.5 Appropriate serum hCG monitoring during early pregnancy can assist in sorting out pregnancies of unknown location (PUL; FIGURE). First trimester ultrasonography, at 5 weeks gestation, usually identifies early intrauterine gestation.

Imaging. With regard to pelvic sonography, the earliest sign of an intrauterine pregnancy (IUP) is a sac eccentrically located in the decidua.6 As the IUP progresses, it becomes equated with a “double decidual sign,” with double rings of tissue around the sac.6 If the pregnancy is located in an adnexal mass, it is frequently inhomogeneous or noncystic in appearance (ie, “the blob” sign); the positive predictive value (PPV) is 96%.2 The PPV of transvaginal ultrasound is 80%, as paratubal, paraovarian, ovarian cyst, and hydrosalpinx can affect the interpretation.7
Heterotopic pregnancy includes an intrauterine gestation and an ectopic pregnancy. This presentation includes the presence of a “pseudosac” in the endometrial cavity plus an extrauterine gestation. Heterotopic pregnancies have become somewhat more common as ART/IVF has unfolded, especially prior to the predominance of single embryo transfer.
Managing ectopic pregnancy
For cases of early pregnancy complicated by intermittent bleeding and/or pain, monitoring with serum hCG levels at 48-hour intervals to distinguish a viable IUP from an abnormal IUP or an ectopic is appropriate. The “discriminatory zone” collates serum hCG levels with findings on ultrasonography. Specific lower limits of serum hCG levels are not clear cut, with recommendations of 3,500 mIU/mL to provide sonographic evidence of an intrauterine gestation “to avoid misdiagnosis and possible interruption of intrauterine pregnancy,” as conveyed in the American College of Obstetricians and Gynecologists 2018 practice bulletin.8 Serum progesterone levels also have been suggested to complement hCG levels; a progesterone level of <20 nmol/L is consistent with an abnormal pregnancy, whereas levels >25 nmol/L are suggestive of a viable pregnancy.2 Inhibin A levels also have been suggested to be helpful, but they are not an ideal monitoring tool.
While most ectopic pregnancies are located in the fallopian tube, other locations also can be abdominal or ovarian. In addition, cesarean scar ectopic pregnancy can occur and often is associated with delay in diagnosis and greater morbidity due to such delay.9 With regard to ovarian ectopic, Spiegelberg criteria are established for diagnosis (TABLE 1).10
Appropriate management of an ectopic pregnancy is dependent upon the gestational age, serum hCG levels, and imaging findings, as well as the patient’s symptoms and exam findings. Treatment is established in large part on a case-by-case basis and includes, for early pregnancy, expectant management and use of methotrexate (TABLE 2).11 Dilation and curettage may be used to identify the pregnancy’s location when the serum hCG level is below 2,000 mIU/mL and there is no evidence of an IUP on ultrasound. Surgical treatment can include minimally invasive salpingostomy or salpingectomy and, depending on circumstance, laparotomy may be indicated.

Fertility following ectopic pregnancy varies and is affected by location, treatment, predisposing factors, total number of ectopic pregnancies, and other factors. Ectopic pregnancy, although rare, also can occur with use of IVF. Humans are not unique with regard to ectopic pregnancies, as they also occur in sheep.12
Continue to: Legal perspective...
Legal perspective
Lawsuits related to ectopic pregnancy are not a new phenomenon. In fact, in 1897, a physician in Ohio who misdiagnosed an “extrauterine pregnancy” as appendicitis was the center of a malpractice lawsuit.13 Unrecognized or mishandled ectopic pregnancy can result in serious injuries—in the range of 1% to 10% (see above) of maternal deaths are related to ectopic pregnancy.14 Ectopic pregnancy cases, therefore, have been the subject of substantial litigation over the years. An informal, noncomprehensive review of malpractice lawsuits brought from 2000 to 2019, found more than 300 ectopic pregnancy cases. Given the large number of malpractice claims against ObGyns,15 ectopic pregnancy cases are only a small portion of all ObGyn malpractice cases.16
A common claim: negligent diagnosis or treatment
The most common basis for lawsuits in cases of ectopic pregnancy is the clinician’s negligent failure to properly diagnose the ectopic nature of the pregnancy. There are also a number of cases claiming negligent treatment of an identified ectopic pregnancy. Not every missed diagnosis, or unsuccessful treatment, leads to liability, of course. It is only when a diagnosis or treatment fails to meet the standard of care within the profession that there should be liability. That standard of care is generally defined by what a reasonably prudent physician would do under the circumstances. Expert witnesses, who are familiar with the standard of practice within the specialty, are usually necessary to establish what that practice is. Both the plaintiff and the defense obtain experts, the former to prove what the standard of care is and that the standard was not met in the case at hand. The defense experts are usually arguing that the standard of care was met.17 Inadequate diagnosis of ectopic pregnancy or other condition may arise from a failure to take a sufficient history, conduct an appropriately thorough physical examination, recognize any of the symptoms that would suggest it is present, use and conduct ultrasound correctly, or follow-up appropriately with additional testing.18
A malpractice claim of negligent treatment can involve any the following circumstances19:
- failure to establish an appropriate treatment plan
- prescribing inappropriate medications for the patient (eg, methotrexate, when it is contraindicated)
- delivering the wrong medication or the wrong amount of the right medication
- performing a procedure badly
- undertaking a new treatment without adequate instruction and preparation.
Given the nature and risks of ectopic pregnancy, ongoing, frequent contact with the patient is essential from the point at which the condition is suspected. The greater the risk of harm (probability or consequence), the more careful any professional ought to be. Because ectopic pregnancy is not an uncommon occurrence, and because it can have devastating effects, including death, a reasonably prudent practitioner would be especially aware of the clinical presentations discussed above.20 In the opening case, the treatment plan was not well documented.
Negligence must lead to patient harm. In addition to negligence (proving that the physician did not act in accordance with the standard of care), to prevail in a malpractice case, the plaintiff-patient must prove that the negligence caused the injury, or worsened it. If the failure to make a diagnosis would not have made any difference in a harm the patient suffered, there are no damages and no liability. Suppose, for example, that a physician negligently failed to diagnose ectopic pregnancy, but performed surgery expecting to find the misdiagnosed condition. In the course of the surgery, however, the surgeon discovered and appropriately treated the ectopic pregnancy. (A version of this happened in the old 19th century case mentioned above.) The negligence of the physician did not cause harm, so there are no damages and no liability.
Continue to: Informed consent is vital...
Informed consent is vital
A part of malpractice is informed consent (or the absence of it)—issues that can arise in any medical care.21 It is wise to pay particular attention in cases where the nature of the illness is unknown, and where there are significant uncertainties and the nature of testing and treatment may change substantially over a period of a few days or few weeks. As always, informed consent should include a discussion of what process or procedure is proposed, its risks and benefits, alternative approaches that might be available, and the risk of doing nothing. Frequently, the uncertainty of ectopic pregnancy complicates the informed consent process.22
Because communication with the patient is an essential function of informed consent, the consent process should productively be used in PUL and similar cases to inform the patient about the uncertainty, and the testing and (nonsurgical) treatment that will occur. This is an opportunity to reinforce the message that the patient must maintain ongoing communication with the physician’s office about changes in her condition, and appear for each appointment scheduled. If more invasive procedures—notably surgery—become required, a separate consent process should be completed, because the risks and considerations are now meaningfully different than when treatment began. As a general matter, any possible treatment that may result in infertility or reduced reproductive capacity should specifically be included in the consent process.
In the hypothetical case, the gynecologist failed to obtain a follow-up serum hCG level. In addition, the record did not reflect ectopic pregnancy in the differential diagnosis. As noted above, the patient had predisposing factors for an ectopic pregnancy. The physician should have acknowledged the history of sexually transmitted disease predisposing her to an ectopic pregnancy. Monitoring of serum hCG levels until they are negative is appropriate with ectopic, or presumed ectopic, pregnancy management. Appropriate monitoring did not occur in this case. Each of these errors (following up on serum hCG levels and the inadequacy of notations about the possibility of ectopic pregnancy) seem inconsistent with the usual standard of care. Furthermore, as a result of the outcome, the only future option for the patient to pursue pregnancy was IVF.
Other legal issues
There are a number of other legal issues that are associated with the topic of ectopic pregnancy. There is evidence, for example, that Catholic and non-Catholic hospitals treat ectopic pregnancies differently,23 which may reflect different views on taking a life or the use of methotrexate and its association with abortion.24 In addition, the possibility of an increase in future ectopic pregnancies is one of the “risks” of abortion that pro-life organizations have pushed to see included in abortion informed consent.25 This has led some commentators to conclude that some Catholic hospitals violate federal law in managing ectopic pregnancy. There is also evidence of “overwhelming rates of medical misinformation on pregnancy center websites, including a link between abortion and ectopic pregnancy.”26
The fact that cesarean deliveries are related to an increased risk for ectopic pregnancy (because of the risk of cesarean scar ectopic pregnancy) also has been cited as information that should play a role in the consent process for cesarean delivery.27 In terms of liability, failed tubal ligation leads to a 33% risk of ectopic pregnancy.28 The risk of ectopic pregnancy is also commonly included in surrogacy contracts.29
Why the outcome was for the defense
The opening hypothetical case illustrates some of the uncertainties of medical malpractice cases. As noted, there appeared a deviation from the usual standard of care, particularly the failure to follow up on the serum hCG level. The weakness in the medical record, failing to note the possibility of ectopic pregnancy, also was probably an error but, apparently, the court felt that this did not result in any harm to the patient.
The question arises of how there would be a defense verdict in light of the failure to track consecutive serum hCG levels. A speculative explanation is that there are many uncertainties in most lawsuits. Procedural problems may result in a case being limited, expert witnesses are essential to both the plaintiff and defense, with the quality of their review and testimony possibly uneven. Judges and juries may rely on one expert witness rather than another, juries vary, and the quality of advocacy differs. Any of these situations can contribute to the unpredictability of the outcome of a case. In the case above, the liability was somewhat uncertain, and the various other factors tipped in favor of a defense verdict. ●
CASE Unidentified ectopic pregnancy leads to rupture*
A 33-year-old woman (G1 P0010) with 2 positive home pregnancy tests presents to the emergency department (ED) reporting intermittent vaginal bleeding for 3 days. Her last menstrual period was 10 weeks ago, but she reports that her menses are always irregular. She has a history of asymptomatic chlamydia, as well as spontaneous abortion 2 years prior. At present, she denies abdominal pain or vaginal discharge.
Upon examination her vital signs are: temperature, 98.3 °F; pulse, 112 bpm, with a resting rate of 16 bpm; blood pressure (BP), 142/91 mm Hg; pulse O2, 99%; height, 4’ 3”; weight, 115 lb. Her labs are: hemoglobin, 12.1 g/dL; hematocrit, 38%; serum human chorionic gonadotropin (hCG) 236 mIU/mL. Upon pelvic examination, no active bleeding is noted. She agrees to be followed up by her gynecologist and is given a prescription for serum hCG in 2 days. She is instructed to return to the ED should she have pain or increased vaginal bleeding.
Three days later, the patient follows up with her gynecologist reporting mild cramping. She notes having had an episode of heavy vaginal bleeding and a “weakly positive” home pregnancy test. Transvaginal ultrasonography notes endometrial thickness 0.59 mm and unremarkable adnexa. A urine pregnancy test performed in the office is positive; urinalysis is positive for nitrites. With the bleeding slowed, the gynecologist’s overall impression is that the patient has undergone complete spontaneous abortion. She prescribes Macrobid for the urinary tract infection. She does not obtain the ED-prescribed serum HCG levels, as she feels, since complete spontaneous abortion has occurred there is no need to obtain a follow-up serum HCG.
Five days later, the patient returns to the ED reporting abdominal pain after eating. Fever and productive cough of 2 days are noted. The patient states that she had a recent miscarriage. The overall impression of the patient’s condition is bronchitis, and it is noted on the patient’s record, “unlikely ectopic pregnancy and pregnancy test may be false positive,” hence a pregnancy test is not ordered. Examination reveals mild suprapubic tenderness with no rebound; no pelvic exam is performed. The patient is instructed to follow up with a health care clinic within a week, and to return to the ED with severe abdominal pain, higher fever, or any new concerning symptoms. A Zithromax Z-pak is prescribed.
Four days later, the patient is brought by ambulance to the ED of the local major medical center with severe abdominal pain involving the right lower quadrant. She states that she had a miscarriage 3 weeks prior and was recently treated for bronchitis. She has dizziness when standing. Her vital signs are: temperature, 97.8 °F; heart rate, 95 bpm; BP, 72/48 mm Hg; pulse O2, 100%. She reports her abdominal pain to be 6/10.
The patient is given a Lactated Ringer’s bolus of 1,000 mL for a hypotensive episode. Computed tomography is obtained and notes, “low attenuation in the left adnexa with a dilated fallopian tube.” A large heterogeneous collection of fluid in the pelvis is noted with active extravasation, consistent with an “acute bleed.”
The patient is brought to the operating room with a diagnosis of probable ruptured ectopic pregnancy. Intraoperatively she is noted to have a right ruptured ectopic and left tubo-ovarian abscess. The surgeon proceeds with right salpingectomy and left salpingo-oophorectomy. Three liters of hemoperitoneum is found.
She is followed postoperatively with serum hCG until levels are negative. Her postoperative course is uneventful. Her only future option for pregnancy is through assisted reproductive technology (ART) with in vitro fertilization (IVF). The patient sues the gynecologist and second ED physician for presumed inappropriate assessment for ectopic pregnancy.
*The “facts” of this case are a composite, drawn from several cases to illustrate medical and legal issues. The statement of facts should be considered hypothetical.
Continue to: WHAT’S THE VERDICT?...
WHAT’S THE VERDICT?
A defense verdict is returned.
Medical considerations
The incidence of ectopic pregnancy is 2% of all pregnancies, with a higher incidence (about 4%) among infertility patients.1 Up to 10% of ectopic pregnancies have no symptoms.2
Clinical presentations. Classic signs of ectopic pregnancy include:
- abdominal pain
- vaginal bleeding
- late menses (often noted).
A recent case of ectopic pregnancy presenting with chest pain was reported.3 Clinicians must never lose site of the fact that ectopic pregnancy is the most common cause of maternal mortality in the first trimester, with an incidence of 1% to 10% of all first-trimester deaths.4
Risk factors include pelvic inflammatory disease, as demonstrated in the opening case. “The silent epidemic of chlamydia” comes to mind, and tobacco smoking can adversely affect tubal cilia, as can pelvic adhesions and/or prior tubal surgery. All of these factors can predispose a patient to ectopic pregnancy; in addition, intrauterine devices, endometriosis, tubal ligation (or ligation reversal), all can set the stage for an ectopic pregnancy.5 Appropriate serum hCG monitoring during early pregnancy can assist in sorting out pregnancies of unknown location (PUL; FIGURE). First trimester ultrasonography, at 5 weeks gestation, usually identifies early intrauterine gestation.

Imaging. With regard to pelvic sonography, the earliest sign of an intrauterine pregnancy (IUP) is a sac eccentrically located in the decidua.6 As the IUP progresses, it becomes equated with a “double decidual sign,” with double rings of tissue around the sac.6 If the pregnancy is located in an adnexal mass, it is frequently inhomogeneous or noncystic in appearance (ie, “the blob” sign); the positive predictive value (PPV) is 96%.2 The PPV of transvaginal ultrasound is 80%, as paratubal, paraovarian, ovarian cyst, and hydrosalpinx can affect the interpretation.7
Heterotopic pregnancy includes an intrauterine gestation and an ectopic pregnancy. This presentation includes the presence of a “pseudosac” in the endometrial cavity plus an extrauterine gestation. Heterotopic pregnancies have become somewhat more common as ART/IVF has unfolded, especially prior to the predominance of single embryo transfer.
Managing ectopic pregnancy
For cases of early pregnancy complicated by intermittent bleeding and/or pain, monitoring with serum hCG levels at 48-hour intervals to distinguish a viable IUP from an abnormal IUP or an ectopic is appropriate. The “discriminatory zone” collates serum hCG levels with findings on ultrasonography. Specific lower limits of serum hCG levels are not clear cut, with recommendations of 3,500 mIU/mL to provide sonographic evidence of an intrauterine gestation “to avoid misdiagnosis and possible interruption of intrauterine pregnancy,” as conveyed in the American College of Obstetricians and Gynecologists 2018 practice bulletin.8 Serum progesterone levels also have been suggested to complement hCG levels; a progesterone level of <20 nmol/L is consistent with an abnormal pregnancy, whereas levels >25 nmol/L are suggestive of a viable pregnancy.2 Inhibin A levels also have been suggested to be helpful, but they are not an ideal monitoring tool.
While most ectopic pregnancies are located in the fallopian tube, other locations also can be abdominal or ovarian. In addition, cesarean scar ectopic pregnancy can occur and often is associated with delay in diagnosis and greater morbidity due to such delay.9 With regard to ovarian ectopic, Spiegelberg criteria are established for diagnosis (TABLE 1).10
Appropriate management of an ectopic pregnancy is dependent upon the gestational age, serum hCG levels, and imaging findings, as well as the patient’s symptoms and exam findings. Treatment is established in large part on a case-by-case basis and includes, for early pregnancy, expectant management and use of methotrexate (TABLE 2).11 Dilation and curettage may be used to identify the pregnancy’s location when the serum hCG level is below 2,000 mIU/mL and there is no evidence of an IUP on ultrasound. Surgical treatment can include minimally invasive salpingostomy or salpingectomy and, depending on circumstance, laparotomy may be indicated.

Fertility following ectopic pregnancy varies and is affected by location, treatment, predisposing factors, total number of ectopic pregnancies, and other factors. Ectopic pregnancy, although rare, also can occur with use of IVF. Humans are not unique with regard to ectopic pregnancies, as they also occur in sheep.12
Continue to: Legal perspective...
Legal perspective
Lawsuits related to ectopic pregnancy are not a new phenomenon. In fact, in 1897, a physician in Ohio who misdiagnosed an “extrauterine pregnancy” as appendicitis was the center of a malpractice lawsuit.13 Unrecognized or mishandled ectopic pregnancy can result in serious injuries—in the range of 1% to 10% (see above) of maternal deaths are related to ectopic pregnancy.14 Ectopic pregnancy cases, therefore, have been the subject of substantial litigation over the years. An informal, noncomprehensive review of malpractice lawsuits brought from 2000 to 2019, found more than 300 ectopic pregnancy cases. Given the large number of malpractice claims against ObGyns,15 ectopic pregnancy cases are only a small portion of all ObGyn malpractice cases.16
A common claim: negligent diagnosis or treatment
The most common basis for lawsuits in cases of ectopic pregnancy is the clinician’s negligent failure to properly diagnose the ectopic nature of the pregnancy. There are also a number of cases claiming negligent treatment of an identified ectopic pregnancy. Not every missed diagnosis, or unsuccessful treatment, leads to liability, of course. It is only when a diagnosis or treatment fails to meet the standard of care within the profession that there should be liability. That standard of care is generally defined by what a reasonably prudent physician would do under the circumstances. Expert witnesses, who are familiar with the standard of practice within the specialty, are usually necessary to establish what that practice is. Both the plaintiff and the defense obtain experts, the former to prove what the standard of care is and that the standard was not met in the case at hand. The defense experts are usually arguing that the standard of care was met.17 Inadequate diagnosis of ectopic pregnancy or other condition may arise from a failure to take a sufficient history, conduct an appropriately thorough physical examination, recognize any of the symptoms that would suggest it is present, use and conduct ultrasound correctly, or follow-up appropriately with additional testing.18
A malpractice claim of negligent treatment can involve any the following circumstances19:
- failure to establish an appropriate treatment plan
- prescribing inappropriate medications for the patient (eg, methotrexate, when it is contraindicated)
- delivering the wrong medication or the wrong amount of the right medication
- performing a procedure badly
- undertaking a new treatment without adequate instruction and preparation.
Given the nature and risks of ectopic pregnancy, ongoing, frequent contact with the patient is essential from the point at which the condition is suspected. The greater the risk of harm (probability or consequence), the more careful any professional ought to be. Because ectopic pregnancy is not an uncommon occurrence, and because it can have devastating effects, including death, a reasonably prudent practitioner would be especially aware of the clinical presentations discussed above.20 In the opening case, the treatment plan was not well documented.
Negligence must lead to patient harm. In addition to negligence (proving that the physician did not act in accordance with the standard of care), to prevail in a malpractice case, the plaintiff-patient must prove that the negligence caused the injury, or worsened it. If the failure to make a diagnosis would not have made any difference in a harm the patient suffered, there are no damages and no liability. Suppose, for example, that a physician negligently failed to diagnose ectopic pregnancy, but performed surgery expecting to find the misdiagnosed condition. In the course of the surgery, however, the surgeon discovered and appropriately treated the ectopic pregnancy. (A version of this happened in the old 19th century case mentioned above.) The negligence of the physician did not cause harm, so there are no damages and no liability.
Continue to: Informed consent is vital...
Informed consent is vital
A part of malpractice is informed consent (or the absence of it)—issues that can arise in any medical care.21 It is wise to pay particular attention in cases where the nature of the illness is unknown, and where there are significant uncertainties and the nature of testing and treatment may change substantially over a period of a few days or few weeks. As always, informed consent should include a discussion of what process or procedure is proposed, its risks and benefits, alternative approaches that might be available, and the risk of doing nothing. Frequently, the uncertainty of ectopic pregnancy complicates the informed consent process.22
Because communication with the patient is an essential function of informed consent, the consent process should productively be used in PUL and similar cases to inform the patient about the uncertainty, and the testing and (nonsurgical) treatment that will occur. This is an opportunity to reinforce the message that the patient must maintain ongoing communication with the physician’s office about changes in her condition, and appear for each appointment scheduled. If more invasive procedures—notably surgery—become required, a separate consent process should be completed, because the risks and considerations are now meaningfully different than when treatment began. As a general matter, any possible treatment that may result in infertility or reduced reproductive capacity should specifically be included in the consent process.
In the hypothetical case, the gynecologist failed to obtain a follow-up serum hCG level. In addition, the record did not reflect ectopic pregnancy in the differential diagnosis. As noted above, the patient had predisposing factors for an ectopic pregnancy. The physician should have acknowledged the history of sexually transmitted disease predisposing her to an ectopic pregnancy. Monitoring of serum hCG levels until they are negative is appropriate with ectopic, or presumed ectopic, pregnancy management. Appropriate monitoring did not occur in this case. Each of these errors (following up on serum hCG levels and the inadequacy of notations about the possibility of ectopic pregnancy) seem inconsistent with the usual standard of care. Furthermore, as a result of the outcome, the only future option for the patient to pursue pregnancy was IVF.
Other legal issues
There are a number of other legal issues that are associated with the topic of ectopic pregnancy. There is evidence, for example, that Catholic and non-Catholic hospitals treat ectopic pregnancies differently,23 which may reflect different views on taking a life or the use of methotrexate and its association with abortion.24 In addition, the possibility of an increase in future ectopic pregnancies is one of the “risks” of abortion that pro-life organizations have pushed to see included in abortion informed consent.25 This has led some commentators to conclude that some Catholic hospitals violate federal law in managing ectopic pregnancy. There is also evidence of “overwhelming rates of medical misinformation on pregnancy center websites, including a link between abortion and ectopic pregnancy.”26
The fact that cesarean deliveries are related to an increased risk for ectopic pregnancy (because of the risk of cesarean scar ectopic pregnancy) also has been cited as information that should play a role in the consent process for cesarean delivery.27 In terms of liability, failed tubal ligation leads to a 33% risk of ectopic pregnancy.28 The risk of ectopic pregnancy is also commonly included in surrogacy contracts.29
Why the outcome was for the defense
The opening hypothetical case illustrates some of the uncertainties of medical malpractice cases. As noted, there appeared a deviation from the usual standard of care, particularly the failure to follow up on the serum hCG level. The weakness in the medical record, failing to note the possibility of ectopic pregnancy, also was probably an error but, apparently, the court felt that this did not result in any harm to the patient.
The question arises of how there would be a defense verdict in light of the failure to track consecutive serum hCG levels. A speculative explanation is that there are many uncertainties in most lawsuits. Procedural problems may result in a case being limited, expert witnesses are essential to both the plaintiff and defense, with the quality of their review and testimony possibly uneven. Judges and juries may rely on one expert witness rather than another, juries vary, and the quality of advocacy differs. Any of these situations can contribute to the unpredictability of the outcome of a case. In the case above, the liability was somewhat uncertain, and the various other factors tipped in favor of a defense verdict. ●
- Centers for Disease Control and Prevention. Ectopic pregnancy—United States, 1990‒1992. MMWR Morb Mortal Wkly Rep. 1995;44:46-48.
- Kirk E, Bottomley C, Bourne T. Diagnosing ectopic pregnancy and current concepts in the management of pregnancy of unknown location. Hum Reprod Update. 2012;20:250-261.
- Dichter E, Espinosa J, Baird J, Lucerna A. An unusual emergency department case: ruptured ectopic pregnancy presenting as chest pain. World J Emerg Med. 2017;8:71-73.
- Cecchino GN, Araujo E, Elito J. Methotrexate for ectopic pregnancy: when and how. Arch Gynecol Obstet. 2014;290:417- 423.
- Barnhart KT, Sammel MD, Cracia CR, et al. Risk factors for ectopic pregnancy in women with symptomatic firsttrimester pregnancies. Fertil Steril. 2006;86:36-43.
- Carusi D. Pregnancy of unknown location: evaluation and management. Semin Perinatol. 2019;43:95-100.
- Barnhart KT, Fay CA, Suescum M, et al. Clinical factors affecting the accuracy of ultrasonography in symptomatic first-trimester pregnancy. Obstet Gynecol. 2011;117:299-306.
- American College of Obstetricians and Gynecologists Practice Bulletin No. 193: tubal ectopic pregnancy. Obstet Gynecol. 2018;131:e91-e103.
- Bouyer J, Coste J, Fernandez H, et al. Sites of ectopic pregnancy: a 10-year population-based study of 1800 cases. Hum Reprod. 2002;17:3224-3230.
- Spiegelberg O. Zur casuistic der ovarial schwangerschaft. Arch Gynecol. 1978;13:73.
- OB Hospitalist Group. Methotrexate use for ectopic pregnancies guidelines. https://www.obhg.com/wp-content /uploads/2020/01/Methotrexate-Use-for-EctopicPregnancies_2016-updates.pdf. Accessed December 10, 2020.
- Brozos C, Kargiannis I, Kiossis E, et al. Ectopic pregnancy through a caesarean scar in a ewe. N Z Vet J. 2013;61:373-375.
- Tucker v. Gillette, 12 Ohio Cir. Dec. 401 (Cir. Ct. 1901).
- Creanga AA, Syverson C, Seed K, et al. Pregnancy-related mortality in the United States, 2011–2013. Obstet Gynecol. 2017;130:366-373.
- Matthews LR, Alvi FA, Milad MP. Reproductive surgery malpractice patterns. Fertil Steril. 2016;106:e42-e43.
- Kim B. The impact of malpractice risk on the use of obstetrics procedures. J Legal Studies. 2006;36:S79-S120.
- Abinader R, Warsof S. Complications involving obstetrical ultrasound. In: Warsof S, Shwayder JM, eds. Legal Concepts and Best Practices in Obstetrics: The Nuts and Bolts Guide to Mitigating Risk. 2019;45-48.
- Creanga AA, Shapiro-Mendoza CK, Bish CL, et al. Trends in ectopic pregnancy mortality in the United States: 1980-2007. Obstet Gynecol. 2011;117:837-843.
- Shwayder JM. IUP diagnosed and treated as ectopic: How bad can it get? Contemporary OB/GYN. 2019;64:49-46.
- Kaplan AI. Should this ectopic pregnancy have been diagnosed earlier? Contemporary OB/GYN. 2017;62:53.
- American College of Obstetricians and Gynecologists Committee on Ethics. Committee opinion 439: informed consent. Reaffirmed 2015. https://www.acog.org/clinical /clinical-guidance/committee-opinion/articles/2009/08 /informed-consent. Accessed December 9, 2020.
- Shwayder JM. Liability in ob/gyn ultrasound. Contemporary OB/GYN. 2017;62:32-49.
- Fisher LN. Institutional religious exemptions: a balancing approach. BYU Law Review. 2014;415-444.
- Makdisi J. Aquinas’s prohibition of killing reconsidered. J Catholic Legal Stud. 2019:57:67-128.
- Franzonello A. Remarks of Anna Franzonello. Alb Law J Sci Tech. 2012;23:519-530.
- Malcolm HE. Pregnancy centers and the limits of mandated disclosure. Columbia Law Rev. 2019;119:1133-1168.
- Kukura E. Contested care: the limitations of evidencebased maternity care reform. Berkeley J Gender Law Justice. 2016;31:241-298.
- Donley G. Contraceptive equity: curing the sex discrimination in the ACA’s mandate. Alabama Law Rev. 2019;71:499-560.
- Berk H. Savvy surrogates and rock star parents: compensation provisions, contracting practices, and the value of womb work. Law Social Inquiry. 2020;45:398-431.
- Centers for Disease Control and Prevention. Ectopic pregnancy—United States, 1990‒1992. MMWR Morb Mortal Wkly Rep. 1995;44:46-48.
- Kirk E, Bottomley C, Bourne T. Diagnosing ectopic pregnancy and current concepts in the management of pregnancy of unknown location. Hum Reprod Update. 2012;20:250-261.
- Dichter E, Espinosa J, Baird J, Lucerna A. An unusual emergency department case: ruptured ectopic pregnancy presenting as chest pain. World J Emerg Med. 2017;8:71-73.
- Cecchino GN, Araujo E, Elito J. Methotrexate for ectopic pregnancy: when and how. Arch Gynecol Obstet. 2014;290:417- 423.
- Barnhart KT, Sammel MD, Cracia CR, et al. Risk factors for ectopic pregnancy in women with symptomatic firsttrimester pregnancies. Fertil Steril. 2006;86:36-43.
- Carusi D. Pregnancy of unknown location: evaluation and management. Semin Perinatol. 2019;43:95-100.
- Barnhart KT, Fay CA, Suescum M, et al. Clinical factors affecting the accuracy of ultrasonography in symptomatic first-trimester pregnancy. Obstet Gynecol. 2011;117:299-306.
- American College of Obstetricians and Gynecologists Practice Bulletin No. 193: tubal ectopic pregnancy. Obstet Gynecol. 2018;131:e91-e103.
- Bouyer J, Coste J, Fernandez H, et al. Sites of ectopic pregnancy: a 10-year population-based study of 1800 cases. Hum Reprod. 2002;17:3224-3230.
- Spiegelberg O. Zur casuistic der ovarial schwangerschaft. Arch Gynecol. 1978;13:73.
- OB Hospitalist Group. Methotrexate use for ectopic pregnancies guidelines. https://www.obhg.com/wp-content /uploads/2020/01/Methotrexate-Use-for-EctopicPregnancies_2016-updates.pdf. Accessed December 10, 2020.
- Brozos C, Kargiannis I, Kiossis E, et al. Ectopic pregnancy through a caesarean scar in a ewe. N Z Vet J. 2013;61:373-375.
- Tucker v. Gillette, 12 Ohio Cir. Dec. 401 (Cir. Ct. 1901).
- Creanga AA, Syverson C, Seed K, et al. Pregnancy-related mortality in the United States, 2011–2013. Obstet Gynecol. 2017;130:366-373.
- Matthews LR, Alvi FA, Milad MP. Reproductive surgery malpractice patterns. Fertil Steril. 2016;106:e42-e43.
- Kim B. The impact of malpractice risk on the use of obstetrics procedures. J Legal Studies. 2006;36:S79-S120.
- Abinader R, Warsof S. Complications involving obstetrical ultrasound. In: Warsof S, Shwayder JM, eds. Legal Concepts and Best Practices in Obstetrics: The Nuts and Bolts Guide to Mitigating Risk. 2019;45-48.
- Creanga AA, Shapiro-Mendoza CK, Bish CL, et al. Trends in ectopic pregnancy mortality in the United States: 1980-2007. Obstet Gynecol. 2011;117:837-843.
- Shwayder JM. IUP diagnosed and treated as ectopic: How bad can it get? Contemporary OB/GYN. 2019;64:49-46.
- Kaplan AI. Should this ectopic pregnancy have been diagnosed earlier? Contemporary OB/GYN. 2017;62:53.
- American College of Obstetricians and Gynecologists Committee on Ethics. Committee opinion 439: informed consent. Reaffirmed 2015. https://www.acog.org/clinical /clinical-guidance/committee-opinion/articles/2009/08 /informed-consent. Accessed December 9, 2020.
- Shwayder JM. Liability in ob/gyn ultrasound. Contemporary OB/GYN. 2017;62:32-49.
- Fisher LN. Institutional religious exemptions: a balancing approach. BYU Law Review. 2014;415-444.
- Makdisi J. Aquinas’s prohibition of killing reconsidered. J Catholic Legal Stud. 2019:57:67-128.
- Franzonello A. Remarks of Anna Franzonello. Alb Law J Sci Tech. 2012;23:519-530.
- Malcolm HE. Pregnancy centers and the limits of mandated disclosure. Columbia Law Rev. 2019;119:1133-1168.
- Kukura E. Contested care: the limitations of evidencebased maternity care reform. Berkeley J Gender Law Justice. 2016;31:241-298.
- Donley G. Contraceptive equity: curing the sex discrimination in the ACA’s mandate. Alabama Law Rev. 2019;71:499-560.
- Berk H. Savvy surrogates and rock star parents: compensation provisions, contracting practices, and the value of womb work. Law Social Inquiry. 2020;45:398-431.
2021 Update on obstetrics
While 2020 was a challenge to say the least, obstetrician-gynecologists remained on the frontline caring for women through it all. Life continued despite the COVID-19 pandemic: prenatal care was delivered, albeit at times in different ways; babies were born; and our role in improving outcomes for women and their children became even more important. This year’s Update focuses on clinical guidelines centered on safety and optimal outcomes for women and children.
ACOG and SMFM update guidance on FGR management
American College of Obstetricians and Gynecologists. Practice advisory: Updated guidance regarding fetal growth restriction. September 2020. https://www.acog.org/clinical/clinical-guidance/practice-advisory/articles/2020/09/updated-guidance-regarding-fetal-growth-restriction. Accessed December 18, 2020.
Fetal growth restriction (FGR) affects up to 10% of pregnancies and is a leading cause of infant morbidity and mortality. Suboptimal fetal growth can have lasting negative effects on development into early childhood and, some hypothesize, even into adulthood.1,2 Antenatal detection of fetuses with FGR is critical so that antenatal testing can be implemented in an attempt to deliver improved clinical outcomes. FGR is defined by several different diagnostic criteria, and many studies have been conducted to determine how best to diagnose this condition.
In September 2020, the American College of Obstetricians and Gynecologists (ACOG) released a Practice Advisory regarding guidance on FGR in an effort to align the ACOG Practice Bulletin No. 204, ACOG Committee Opinion No. 764, and SMFM (Society for Maternal-Fetal Medicine) Consult Series No. 52.3-5 This guidance updates and replaces prior guidelines, with an emphasis on 3 notable changes.
FGR definition, workup have changed
While the original definition of FGR was an estimated fetal weight (EFW) of less than the 10th percentile for gestational age, a similar level of accuracy in prediction of subsequent small for gestational age (SGA) at birth has been shown when this or an abdominal circumference (AC) of less than the 10th percentile is used. Based on these findings, SMFM now recommends that FGR be defined as an EFW or AC of less than the 10th percentile for gestational age.
Recent studies have done head-to-head comparisons of different methods of estimating fetal weight to determine the best detection and pregnancy outcome improvement in FGR. In all instances, the Hadlock formula has continued to more accurately estimate fetal weight, prediction of SGA, and composite neonatal morbidity. As such, new guidelines recommend that population-based fetal growth references (that is, the Hadlock formula) should be used to determine ultrasonography-derived fetal weight percentiles.
The new guidance also suggests classification of FGR based on gestational age at onset, with early FGR at less than 32 weeks and late FGR at 32 or more weeks. The definition of severe FGR is reserved for fetuses with an EFW of less than the 3rd percentile. A diagnosis of FGR should prompt the recommendation for a detailed obstetric ultrasonography. Diagnostic genetic testing should be offered in cases of early-onset FGR, concomitant sonographic abnormalities, and/or polyhydramnios. Routine serum screening for toxoplasmosis, rubella, herpes, or cytomegalovirus (CMV) should not be done unless there are risk factors for infection. If amniocentesis is performed for genetic diagnostic testing, consideration can be made for polymerase chain reaction for CMV in the amniotic fluid.
Continue to: Timing of delivery in isolated FGR...
Timing of delivery in isolated FGR
A complicating factor in diagnosing FGR is distinguishing between the pathologically growth-restricted fetus and the constitutionally small fetus. Antenatal testing and serial umbilical artery Doppler assessment should be done following diagnosis of FGR to monitor for evidence of fetal compromise until delivery is planned.
The current ACOG Practice Bulletin No. 204 and Committee Opinion No. 764 recommend delivery between 38 0/7 and 39 6/7 weeks in the setting of isolated FGR with reassuring fetal testing and umbilical artery Doppler assessment.To further refine this, the new recommendations use the growth percentiles. In cases of isolated FGR with EFW between the 3rd and 10th percentile in the setting of normal umbilical artery Doppler, delivery is recommended between 38 and 39 weeks’ gestation. In cases of isolated FGR with EFW of less than the 3rd percentile (severe FGR) in the setting of normal umbilical artery Doppler, delivery is recommended at 37 weeks.
Timing of delivery in complicated FGR
A normal umbilical artery Doppler reflects the low impedance that is necessary for continuous forward flow of blood to the fetus. Abnormal umbilical artery Doppler signifies aberrations of this low-pressure system that affect the amount of continuous forward flow during diastole of the cardiac cycle. With continued compromise, there is progression to absent end-diastolic velocity (AEDV) and, most concerning, reversed end-diastolic velocity (REDV).
Serial umbilical artery Doppler assessment should be done following diagnosis of FGR to monitor for progression that is associated with perinatal mortality, since intervention can be initiated in the form of delivery. Delivery at 37 weeks is recommended for FGR with elevated umbilical artery Doppler of greater than the 95th percentile for gestational age. For FGR with AEDV, delivery is recommended between 33 and 34 weeks of gestation and for FGR with REDV between 30 and 32 weeks, as the neonatal morbidity and mortality associated with continuing the pregnancy outweighs the risks of prematurity in this setting. Because of the abnormal placental-fetal circulation in FGR complicated by AEDV/REDV, there may be a higher likelihood of fetal intolerance of labor and cesarean delivery (CD) may be considered.
- Fetal growth restriction is now defined as EFW of less than the 10th percentile or AC of less than the 10th percentile.
- Evaluation of FGR includes detailed anatomic survey and consideration of genetic evaluation, but infection screening should be done only if the patient is at risk for infection.
- With reassuring antenatal testing and normal umbilical artery Doppler studies, delivery is recommended at 38 to 39 weeks for isolated FGR with EFW in the 3rd to 10th percentile and at 37 weeks for FGR with EFW of less than the 3rd percentile.
- Umbilical artery Doppler studies are used to decrease the risk of perinatal mortality and further guide timing of delivery
Continue to: New recommendations for PROM management...
New recommendations for PROM management
American College of Obstetricians and Gynecologists Committee on Practice Bulletins–Obstetrics. ACOG practice bulletin no. 217: Prelabor rupture of membranes. Obstet Gynecol. 2020;135:e80-e97.
Rupture of membranes prior to the onset of labor occurs at term in 8% of pregnancies and in the preterm period in 2% to 3% of pregnancies.6 Accurate diagnosis, gestational age, evidence of infection, and discussion of the risks and benefits to the mother and fetus/neonate are necessary to optimize outcomes. In the absence of other indications for delivery, a gestational age of 34 or more weeks traditionally has been the cutoff to proceed with delivery, although this has not been globally agreed on and/or practiced.
ACOG has published a comprehensive update that incorporates the results of the PPROMT trial and other recommendations for the diagnosis and management of both term and preterm prelabor rupture of membranes (PROM).6,7
Making the diagnosis
Diagnosis of PROM usually can be made clinically via history and the classic triad of physical exam findings—pooling of fluid, basic pH, and ferning; some institutions also use commercially available tests that detect placental-derived proteins. Both ACOG and the US Food and Drug Administration caution against using these tests alone without clinical evaluation due to concern for false-positives and false-negatives that lead to adverse maternal and fetal/neonatal outcomes. For equivocal cases, ultrasonography for amniotic fluid evaluation and ultrasonography-guided dye tests can be used to assist in accurate diagnosis, especially in the preterm period in which there are significant implications for pregnancy management.
PROM management depends on gestational age
All management recommendations require reassuring fetal testing, evaluation for infection, and no other contraindications to expectant management. Once these are established, the most important determinant of PROM management then becomes gestational age.
Previable PROM
Previable PROM (usually defined as less than 23–24 weeks) has high risks of both maternal and fetal/neonatal morbidity and mortality from infection, hemorrhage, pulmonary hypoplasia, and extreme prematurity. These very difficult cases benefit from a multidisciplinary approach to patient counseling regarding expectant management versus immediate delivery.
If expectant management is chosen, outpatient management with close monitoring for signs of maternal infection may be done until an agreed on gestational age of viability. Then inpatient management with fetal monitoring, corticosteroids, tocolysis, magnesium for neuroprotection, and group B streptococcus (GBS) prophylaxis may be considered as appropriate.
Preterm PROM at less than 34 weeks
If the mother and fetus are otherwise stable, PROM at less than 34 weeks warrants inpatient expectant management with close maternal and fetal monitoring for signs of infection and labor. Management includes latency antibiotics, antenatal corticosteroids, magnesium for neuroprotection if less than 32 weeks’ gestation and at risk for imminent delivery, and GBS prophylaxis. While tocolysis may increase latency and help with steroid course completion, it should be used cautiously and avoided in cases of abruption or chorioamnionitis. Although there is no definitive recommendation published, a rescue course of steroids may be considered as appropriate but should not delay an indicated delivery.
Continue to: Late preterm PROM...
Late preterm PROM
The biggest change to clinical management in this ACOG Practice Bulletin is for late preterm (34–36 6/7 weeks) PROM, with the recommendation for either immediate delivery or expectant management up to 37 weeks stemming from the PPROMPT study by Morris and colleagues.7
From the neonatal perspective, no difference has been demonstrated between immediate delivery and expectant management for neonatal sepsis or a composite neonatal morbidity and mortality. Expectant management may be preferred from the neonatal point of view as immediate delivery was associated with an increased rate of neonatal respiratory distress, mechanical ventilation, and length of stay in the neonatal intensive care unit. The potential for long-term neurodevelopmental outcomes of delivery at 34 versus 37 weeks also should be considered.
From the maternal perspective, expectant management has an increased risk of antepartum and postpartum hemorrhage, fever, antibiotic use, and maternal length of stay, but a decreased risk of CD.
A late preterm steroid course can be considered if delivery is planned in no less than 24 hours and likely to occur in the next 7 days and if the patient has not already received a course of steroids. A rescue course of steroids is not indicated if the patient received a steroid course prior in the pregnancy. While appropriate GBS prophylaxis is recommended, latency antibiotics and tocolysis are not, and delivery should not be delayed if chorioamnionitis is diagnosed.
Ultimately, preterm PROM management with a stable mother and fetus at or beyond 34 weeks requires comprehensive counseling of the risks and benefits for both mother and fetus/neonate. A multidisciplinary team that together counsels the patient also may help with this shared decision making.
Term PROM
For patients with term PROM, delivery is recommended. Although a short period of expectant management for 12 to 24 hours is reported as “reasonable,” the risk of infection increases with the length of rupture of membranes. Therefore, induction of labor or CD soon after rupture of membranes is recommended for patients who are GBS positive and is preferred for all others.
- Accurate diagnosis is necessary for appropriate counseling and management of PROM.
- Delivery is recommended for term PROM, chorioamnionitis, and for patients with previable PROM who do not desire expectant management.
- If the mother and fetus are otherwise stable, expectant management of preterm PROM until 34 to 37 weeks is recommended.
- The decision of when to deliver between 34 and 37 weeks is best made with multidisciplinary counseling and shared decision making with the patient.
VTE prophylaxis in pregnancy: Regimen adjustments, CD strategies, and COVID-19 considerations
Birsner ML, Turrentine M, Pettker CM, et al. ACOG practice advisory: Options for peripartum anticoagulation in areas affected by shortage of unfractionated heparin. March 2020. https://www.acog.org/clinical/clinical-guidance/practice-advisory/articles/2020/03/options-for-peripartum-anticoagulation-in-areas-affected-by-shortage-of-unfractionated-heparin. Accessed December 8, 2020.
Pacheco LD, Saade G, Metz TD. Society for Maternal-Fetal Medicine Consult Series No. 51: Thromboembolism prophylaxis for cesarean delivery. Am J Obstet Gynecol. 2020;223:B11-B17
Venous thromboembolism (VTE) prophylaxis is a timely topic for a number of reasons. First, a shortage of unfractionated heparin prompted an ACOG Practice Advisory, endorsed by SMFM and the Society for Obstetric Anesthesia and Perinatology, regarding use of low molecular weight heparin (LMWH) in the peripartum period.8 In addition, SMFM released updated recommendations for VTE prophylaxis for CD as part of the SMFM Consult Series.9 Finally, there is evidence that COVID-19 infection may increase the risk of coagulopathy, leading to consideration of additional VTE prophylaxis for pregnant and postpartum women with COVID-19.
Candidates for prophylaxis
As recommended by the ACOG Practice Bulletin on thromboembolism in pregnancy, women who may require VTE prophylaxis during pregnancy and/or the postpartum period include those with10:
- VTE diagnosed during pregnancy
- a history of VTE, including during pregnancy or with use of hormonal contraception
- a history of thrombophilia with or without a personal or family history of VTE.
For these patients, LMWH has many advantages over unfractionated heparin, including ease of use and reliability of dosing. It generally is preferred in pregnancy and postpartum (for both prophylactic and therapeutic anticoagulation) by patients and providers.
The Practice Bulletin references a strategy that describes converting LMWH to unfractionated heparin at around 36 weeks’ gestation in preparation for delivery because unfractionated heparin has the advantage of a shorter half-life and the option for anticoagulation reversal with protamine sulfate. In the Practice Advisory, a global shortage of unfractionated heparin and an argument that the above conversion was less about concern for maternal hemorrhage and more about avoiding spinal and epidural hematomas led to the following recommendations for continued use of LMWH through delivery:
- LMWH heparin can be discontinued in a planned fashion prior to scheduled induction of labor or CD (generally 12 hours for prophylactic dosing and 24 hours for intermediate dosing).
- Patients in spontaneous labor may receive neuraxial anesthesia 12 hours after the last prophylactic dose and 24 hours after the last intermediate dose of LMWH.
- Patients who require anticoagulation during pregnancy should be counseled that if they have vaginal bleeding, leakage of fluid, or regular contractions they should be evaluated prior to taking their next dose of anticoagulant.
- In the absence of other complications, delivery should not be before 39 weeks for the indication of anticoagulation requirement alone.
Continue to: Managing VTE risk in CD...
Managing VTE risk in CD
Recognizing that VTE is a major cause of maternal morbidity and mortality, as well as the variety of the published guidelines for VTE prophylaxis after CD, the SMFM Consult Series provides recommendations to assist clinicians caring for postpartum women after CD. As reviewed in the ACOG Practice Bulletin, there are good data to support pharmacologic prophylaxis during pregnancy and the postpartum period for women with a history of VTE or a thrombophilia. Solid evidence is lacking, however, for what to do for women who have a CD without this history but may have other potential risk factors for VTE, such as obesity, preeclampsia, and transfusion requirement. Universal pharmacologic prophylaxis also is not yet supported by evidence. SMFM supports LMWH as the preferred medication in pregnancy and postpartum and provides these additional recommendations:
- All women who have a CD should have sequential compression devices (SCDs) placed prior to surgery and continued until they are ambulatory.
- Women with a history of VTE or thrombophilia without history of VTE should have SCDs and pharmacologic VTE prophylaxis for 6 weeks postpartum.
- Intermediate dosing of LMWH is recommended for patients with class III obesity.
- Institutions should develop patient safety bundles for VTE prophylaxis to identify additional risk factors that may warrant pharmacologic prophylaxis after CD in select patients.
Our approach to patients with COVID-19 infection
At our institution, we recently incorporated a VTE prophylaxis protocol into our electronic medical record that provides risk stratification for each patient. In addition to the above recommendations, our patients may qualify for short-term in-house or longer postpartum prophylaxis depending on risk factors.
A new risk factor in recent months is COVID-19 infection, which appears to increase the risk of coagulopathy, especially in patients with disease severe enough to warrant hospitalization. Given the potential for additive risk in pregnancy, in consult with our medicine colleagues, we have placed some of our more ill hospitalized pregnant patients on a course of prophylactic LMWH both in the hospital and after discharge independent of delivery status or mode of delivery. ●
- Pregnant patients with a history of VTE or a thrombophilia may be candidates for pharmacologic anticoagulation during pregnancy and/or postpartum.
- LMWH is the preferred method of pharmacologic VTE prophylaxis during pregnancy and postpartum.
- For most patients, CD and neuraxial anesthesia safely can be performed 12 to 24 hours after the last dose of prophylactic or intermediate LMWH, respectively.
- All patients undergoing CD should have at least mechanical VTE prophylaxis with SCDs.
- All women who have a CD should be evaluated via institutional patient safety bundles for VTE prophylaxis for additional risk factors that potentially warrant postpartum pharmacologic VTE prophylaxis.
- More data are needed to determine recommendations for universal/ near universal pharmacologic VTE prophylaxis in the postpartum period.
- Pregnant or postpartum patients with moderate to severe COVID-19 infection may be at increased risk for VTE, warranting consideration of additional pharmacologic prophylaxis.
- Baschat AA, Gembruch U, Harman CR. The sequence of changes in Doppler and biophysical parameters as severe fetal growth restriction worsens. Ultrasound Obstet Gynecol. 2001;18:571-577.
- Almond D, Currie J. Killing me softly: the fetal origins hypothesis. J Econ Perspect. 2011;25:153-172.
- American College of Obstetricians and Gynecologists Committee on Practice Bulletins–Obstetrics and Society for Maternal-Fetal Medicine. ACOG practice bulletin no. 204: Fetal growth restriction. Obstet Gynecol. 2019;133: e97-e109.
- American College of Obstetricians and Gynecologists Committee on Obstetric Practice and Society for Maternal-Fetal Medicine. ACOG committee opinion no. 764: Medically indicated late-preterm and early-term deliveries. Obstet Gynecol. 2019;133:e151-e155.
- Society for Maternal-Fetal Medicine; Martins JG, Biggio FR, Abuhamad A. SMFM consult series no. 52: diagnosis and management of fetal growth restriction. Am J Obstet Gynecol. 2020;223:B2-B17.
- American College of Obstetricians and Gynecologists Committee on Practice Bulletins–Obstetrics. ACOG practice bulletin no. 217: Prelabor rupture of membranes. Obstet Gynecol. 2020;135:e80-e97.
- Morris JM, Roberts CL, Bowen JR, et al; PPROMT Collaboration. Immediate delivery compared with expectant management after preterm pre-labour rupture of the membranes close to term (PPROMT trial): a randomised controlled trial. Lancet. 2016;387:444-452.
- Birsner ML, Turrentine M, Pettker CM, et al. ACOG practice advisory: Options for peripartum anticoagulation in areas affected by shortage of unfractionated heparin. March 2020. https://www.acog.org/clinical/clinical-guidance/practice-advisory/articles/2020/03/options-for-peripartum-anticoagulation-in-areas-affected-by-shortage-of-unfractionated-heparin. Accessed December 8, 2020.
- Pacheco LD, Saade G, Metz TD. Society for MaternalFetal Medicine consult series no. 51: Thromboembolism prophylaxis for cesarean delivery. Am J Obstet Gynecol. 2020;223:B11-B17.
- American College of Obstetricians and Gynecologists Committee on Practice Bulletins–Obstetrics. ACOG practice bulletin no. 196: Thromboembolism in pregnancy. Obstet Gynecol. 2018;132:e1-e17.
While 2020 was a challenge to say the least, obstetrician-gynecologists remained on the frontline caring for women through it all. Life continued despite the COVID-19 pandemic: prenatal care was delivered, albeit at times in different ways; babies were born; and our role in improving outcomes for women and their children became even more important. This year’s Update focuses on clinical guidelines centered on safety and optimal outcomes for women and children.
ACOG and SMFM update guidance on FGR management
American College of Obstetricians and Gynecologists. Practice advisory: Updated guidance regarding fetal growth restriction. September 2020. https://www.acog.org/clinical/clinical-guidance/practice-advisory/articles/2020/09/updated-guidance-regarding-fetal-growth-restriction. Accessed December 18, 2020.
Fetal growth restriction (FGR) affects up to 10% of pregnancies and is a leading cause of infant morbidity and mortality. Suboptimal fetal growth can have lasting negative effects on development into early childhood and, some hypothesize, even into adulthood.1,2 Antenatal detection of fetuses with FGR is critical so that antenatal testing can be implemented in an attempt to deliver improved clinical outcomes. FGR is defined by several different diagnostic criteria, and many studies have been conducted to determine how best to diagnose this condition.
In September 2020, the American College of Obstetricians and Gynecologists (ACOG) released a Practice Advisory regarding guidance on FGR in an effort to align the ACOG Practice Bulletin No. 204, ACOG Committee Opinion No. 764, and SMFM (Society for Maternal-Fetal Medicine) Consult Series No. 52.3-5 This guidance updates and replaces prior guidelines, with an emphasis on 3 notable changes.
FGR definition, workup have changed
While the original definition of FGR was an estimated fetal weight (EFW) of less than the 10th percentile for gestational age, a similar level of accuracy in prediction of subsequent small for gestational age (SGA) at birth has been shown when this or an abdominal circumference (AC) of less than the 10th percentile is used. Based on these findings, SMFM now recommends that FGR be defined as an EFW or AC of less than the 10th percentile for gestational age.
Recent studies have done head-to-head comparisons of different methods of estimating fetal weight to determine the best detection and pregnancy outcome improvement in FGR. In all instances, the Hadlock formula has continued to more accurately estimate fetal weight, prediction of SGA, and composite neonatal morbidity. As such, new guidelines recommend that population-based fetal growth references (that is, the Hadlock formula) should be used to determine ultrasonography-derived fetal weight percentiles.
The new guidance also suggests classification of FGR based on gestational age at onset, with early FGR at less than 32 weeks and late FGR at 32 or more weeks. The definition of severe FGR is reserved for fetuses with an EFW of less than the 3rd percentile. A diagnosis of FGR should prompt the recommendation for a detailed obstetric ultrasonography. Diagnostic genetic testing should be offered in cases of early-onset FGR, concomitant sonographic abnormalities, and/or polyhydramnios. Routine serum screening for toxoplasmosis, rubella, herpes, or cytomegalovirus (CMV) should not be done unless there are risk factors for infection. If amniocentesis is performed for genetic diagnostic testing, consideration can be made for polymerase chain reaction for CMV in the amniotic fluid.
Continue to: Timing of delivery in isolated FGR...
Timing of delivery in isolated FGR
A complicating factor in diagnosing FGR is distinguishing between the pathologically growth-restricted fetus and the constitutionally small fetus. Antenatal testing and serial umbilical artery Doppler assessment should be done following diagnosis of FGR to monitor for evidence of fetal compromise until delivery is planned.
The current ACOG Practice Bulletin No. 204 and Committee Opinion No. 764 recommend delivery between 38 0/7 and 39 6/7 weeks in the setting of isolated FGR with reassuring fetal testing and umbilical artery Doppler assessment.To further refine this, the new recommendations use the growth percentiles. In cases of isolated FGR with EFW between the 3rd and 10th percentile in the setting of normal umbilical artery Doppler, delivery is recommended between 38 and 39 weeks’ gestation. In cases of isolated FGR with EFW of less than the 3rd percentile (severe FGR) in the setting of normal umbilical artery Doppler, delivery is recommended at 37 weeks.
Timing of delivery in complicated FGR
A normal umbilical artery Doppler reflects the low impedance that is necessary for continuous forward flow of blood to the fetus. Abnormal umbilical artery Doppler signifies aberrations of this low-pressure system that affect the amount of continuous forward flow during diastole of the cardiac cycle. With continued compromise, there is progression to absent end-diastolic velocity (AEDV) and, most concerning, reversed end-diastolic velocity (REDV).
Serial umbilical artery Doppler assessment should be done following diagnosis of FGR to monitor for progression that is associated with perinatal mortality, since intervention can be initiated in the form of delivery. Delivery at 37 weeks is recommended for FGR with elevated umbilical artery Doppler of greater than the 95th percentile for gestational age. For FGR with AEDV, delivery is recommended between 33 and 34 weeks of gestation and for FGR with REDV between 30 and 32 weeks, as the neonatal morbidity and mortality associated with continuing the pregnancy outweighs the risks of prematurity in this setting. Because of the abnormal placental-fetal circulation in FGR complicated by AEDV/REDV, there may be a higher likelihood of fetal intolerance of labor and cesarean delivery (CD) may be considered.
- Fetal growth restriction is now defined as EFW of less than the 10th percentile or AC of less than the 10th percentile.
- Evaluation of FGR includes detailed anatomic survey and consideration of genetic evaluation, but infection screening should be done only if the patient is at risk for infection.
- With reassuring antenatal testing and normal umbilical artery Doppler studies, delivery is recommended at 38 to 39 weeks for isolated FGR with EFW in the 3rd to 10th percentile and at 37 weeks for FGR with EFW of less than the 3rd percentile.
- Umbilical artery Doppler studies are used to decrease the risk of perinatal mortality and further guide timing of delivery
Continue to: New recommendations for PROM management...
New recommendations for PROM management
American College of Obstetricians and Gynecologists Committee on Practice Bulletins–Obstetrics. ACOG practice bulletin no. 217: Prelabor rupture of membranes. Obstet Gynecol. 2020;135:e80-e97.
Rupture of membranes prior to the onset of labor occurs at term in 8% of pregnancies and in the preterm period in 2% to 3% of pregnancies.6 Accurate diagnosis, gestational age, evidence of infection, and discussion of the risks and benefits to the mother and fetus/neonate are necessary to optimize outcomes. In the absence of other indications for delivery, a gestational age of 34 or more weeks traditionally has been the cutoff to proceed with delivery, although this has not been globally agreed on and/or practiced.
ACOG has published a comprehensive update that incorporates the results of the PPROMT trial and other recommendations for the diagnosis and management of both term and preterm prelabor rupture of membranes (PROM).6,7
Making the diagnosis
Diagnosis of PROM usually can be made clinically via history and the classic triad of physical exam findings—pooling of fluid, basic pH, and ferning; some institutions also use commercially available tests that detect placental-derived proteins. Both ACOG and the US Food and Drug Administration caution against using these tests alone without clinical evaluation due to concern for false-positives and false-negatives that lead to adverse maternal and fetal/neonatal outcomes. For equivocal cases, ultrasonography for amniotic fluid evaluation and ultrasonography-guided dye tests can be used to assist in accurate diagnosis, especially in the preterm period in which there are significant implications for pregnancy management.
PROM management depends on gestational age
All management recommendations require reassuring fetal testing, evaluation for infection, and no other contraindications to expectant management. Once these are established, the most important determinant of PROM management then becomes gestational age.
Previable PROM
Previable PROM (usually defined as less than 23–24 weeks) has high risks of both maternal and fetal/neonatal morbidity and mortality from infection, hemorrhage, pulmonary hypoplasia, and extreme prematurity. These very difficult cases benefit from a multidisciplinary approach to patient counseling regarding expectant management versus immediate delivery.
If expectant management is chosen, outpatient management with close monitoring for signs of maternal infection may be done until an agreed on gestational age of viability. Then inpatient management with fetal monitoring, corticosteroids, tocolysis, magnesium for neuroprotection, and group B streptococcus (GBS) prophylaxis may be considered as appropriate.
Preterm PROM at less than 34 weeks
If the mother and fetus are otherwise stable, PROM at less than 34 weeks warrants inpatient expectant management with close maternal and fetal monitoring for signs of infection and labor. Management includes latency antibiotics, antenatal corticosteroids, magnesium for neuroprotection if less than 32 weeks’ gestation and at risk for imminent delivery, and GBS prophylaxis. While tocolysis may increase latency and help with steroid course completion, it should be used cautiously and avoided in cases of abruption or chorioamnionitis. Although there is no definitive recommendation published, a rescue course of steroids may be considered as appropriate but should not delay an indicated delivery.
Continue to: Late preterm PROM...
Late preterm PROM
The biggest change to clinical management in this ACOG Practice Bulletin is for late preterm (34–36 6/7 weeks) PROM, with the recommendation for either immediate delivery or expectant management up to 37 weeks stemming from the PPROMPT study by Morris and colleagues.7
From the neonatal perspective, no difference has been demonstrated between immediate delivery and expectant management for neonatal sepsis or a composite neonatal morbidity and mortality. Expectant management may be preferred from the neonatal point of view as immediate delivery was associated with an increased rate of neonatal respiratory distress, mechanical ventilation, and length of stay in the neonatal intensive care unit. The potential for long-term neurodevelopmental outcomes of delivery at 34 versus 37 weeks also should be considered.
From the maternal perspective, expectant management has an increased risk of antepartum and postpartum hemorrhage, fever, antibiotic use, and maternal length of stay, but a decreased risk of CD.
A late preterm steroid course can be considered if delivery is planned in no less than 24 hours and likely to occur in the next 7 days and if the patient has not already received a course of steroids. A rescue course of steroids is not indicated if the patient received a steroid course prior in the pregnancy. While appropriate GBS prophylaxis is recommended, latency antibiotics and tocolysis are not, and delivery should not be delayed if chorioamnionitis is diagnosed.
Ultimately, preterm PROM management with a stable mother and fetus at or beyond 34 weeks requires comprehensive counseling of the risks and benefits for both mother and fetus/neonate. A multidisciplinary team that together counsels the patient also may help with this shared decision making.
Term PROM
For patients with term PROM, delivery is recommended. Although a short period of expectant management for 12 to 24 hours is reported as “reasonable,” the risk of infection increases with the length of rupture of membranes. Therefore, induction of labor or CD soon after rupture of membranes is recommended for patients who are GBS positive and is preferred for all others.
- Accurate diagnosis is necessary for appropriate counseling and management of PROM.
- Delivery is recommended for term PROM, chorioamnionitis, and for patients with previable PROM who do not desire expectant management.
- If the mother and fetus are otherwise stable, expectant management of preterm PROM until 34 to 37 weeks is recommended.
- The decision of when to deliver between 34 and 37 weeks is best made with multidisciplinary counseling and shared decision making with the patient.
VTE prophylaxis in pregnancy: Regimen adjustments, CD strategies, and COVID-19 considerations
Birsner ML, Turrentine M, Pettker CM, et al. ACOG practice advisory: Options for peripartum anticoagulation in areas affected by shortage of unfractionated heparin. March 2020. https://www.acog.org/clinical/clinical-guidance/practice-advisory/articles/2020/03/options-for-peripartum-anticoagulation-in-areas-affected-by-shortage-of-unfractionated-heparin. Accessed December 8, 2020.
Pacheco LD, Saade G, Metz TD. Society for Maternal-Fetal Medicine Consult Series No. 51: Thromboembolism prophylaxis for cesarean delivery. Am J Obstet Gynecol. 2020;223:B11-B17
Venous thromboembolism (VTE) prophylaxis is a timely topic for a number of reasons. First, a shortage of unfractionated heparin prompted an ACOG Practice Advisory, endorsed by SMFM and the Society for Obstetric Anesthesia and Perinatology, regarding use of low molecular weight heparin (LMWH) in the peripartum period.8 In addition, SMFM released updated recommendations for VTE prophylaxis for CD as part of the SMFM Consult Series.9 Finally, there is evidence that COVID-19 infection may increase the risk of coagulopathy, leading to consideration of additional VTE prophylaxis for pregnant and postpartum women with COVID-19.
Candidates for prophylaxis
As recommended by the ACOG Practice Bulletin on thromboembolism in pregnancy, women who may require VTE prophylaxis during pregnancy and/or the postpartum period include those with10:
- VTE diagnosed during pregnancy
- a history of VTE, including during pregnancy or with use of hormonal contraception
- a history of thrombophilia with or without a personal or family history of VTE.
For these patients, LMWH has many advantages over unfractionated heparin, including ease of use and reliability of dosing. It generally is preferred in pregnancy and postpartum (for both prophylactic and therapeutic anticoagulation) by patients and providers.
The Practice Bulletin references a strategy that describes converting LMWH to unfractionated heparin at around 36 weeks’ gestation in preparation for delivery because unfractionated heparin has the advantage of a shorter half-life and the option for anticoagulation reversal with protamine sulfate. In the Practice Advisory, a global shortage of unfractionated heparin and an argument that the above conversion was less about concern for maternal hemorrhage and more about avoiding spinal and epidural hematomas led to the following recommendations for continued use of LMWH through delivery:
- LMWH heparin can be discontinued in a planned fashion prior to scheduled induction of labor or CD (generally 12 hours for prophylactic dosing and 24 hours for intermediate dosing).
- Patients in spontaneous labor may receive neuraxial anesthesia 12 hours after the last prophylactic dose and 24 hours after the last intermediate dose of LMWH.
- Patients who require anticoagulation during pregnancy should be counseled that if they have vaginal bleeding, leakage of fluid, or regular contractions they should be evaluated prior to taking their next dose of anticoagulant.
- In the absence of other complications, delivery should not be before 39 weeks for the indication of anticoagulation requirement alone.
Continue to: Managing VTE risk in CD...
Managing VTE risk in CD
Recognizing that VTE is a major cause of maternal morbidity and mortality, as well as the variety of the published guidelines for VTE prophylaxis after CD, the SMFM Consult Series provides recommendations to assist clinicians caring for postpartum women after CD. As reviewed in the ACOG Practice Bulletin, there are good data to support pharmacologic prophylaxis during pregnancy and the postpartum period for women with a history of VTE or a thrombophilia. Solid evidence is lacking, however, for what to do for women who have a CD without this history but may have other potential risk factors for VTE, such as obesity, preeclampsia, and transfusion requirement. Universal pharmacologic prophylaxis also is not yet supported by evidence. SMFM supports LMWH as the preferred medication in pregnancy and postpartum and provides these additional recommendations:
- All women who have a CD should have sequential compression devices (SCDs) placed prior to surgery and continued until they are ambulatory.
- Women with a history of VTE or thrombophilia without history of VTE should have SCDs and pharmacologic VTE prophylaxis for 6 weeks postpartum.
- Intermediate dosing of LMWH is recommended for patients with class III obesity.
- Institutions should develop patient safety bundles for VTE prophylaxis to identify additional risk factors that may warrant pharmacologic prophylaxis after CD in select patients.
Our approach to patients with COVID-19 infection
At our institution, we recently incorporated a VTE prophylaxis protocol into our electronic medical record that provides risk stratification for each patient. In addition to the above recommendations, our patients may qualify for short-term in-house or longer postpartum prophylaxis depending on risk factors.
A new risk factor in recent months is COVID-19 infection, which appears to increase the risk of coagulopathy, especially in patients with disease severe enough to warrant hospitalization. Given the potential for additive risk in pregnancy, in consult with our medicine colleagues, we have placed some of our more ill hospitalized pregnant patients on a course of prophylactic LMWH both in the hospital and after discharge independent of delivery status or mode of delivery. ●
- Pregnant patients with a history of VTE or a thrombophilia may be candidates for pharmacologic anticoagulation during pregnancy and/or postpartum.
- LMWH is the preferred method of pharmacologic VTE prophylaxis during pregnancy and postpartum.
- For most patients, CD and neuraxial anesthesia safely can be performed 12 to 24 hours after the last dose of prophylactic or intermediate LMWH, respectively.
- All patients undergoing CD should have at least mechanical VTE prophylaxis with SCDs.
- All women who have a CD should be evaluated via institutional patient safety bundles for VTE prophylaxis for additional risk factors that potentially warrant postpartum pharmacologic VTE prophylaxis.
- More data are needed to determine recommendations for universal/ near universal pharmacologic VTE prophylaxis in the postpartum period.
- Pregnant or postpartum patients with moderate to severe COVID-19 infection may be at increased risk for VTE, warranting consideration of additional pharmacologic prophylaxis.
While 2020 was a challenge to say the least, obstetrician-gynecologists remained on the frontline caring for women through it all. Life continued despite the COVID-19 pandemic: prenatal care was delivered, albeit at times in different ways; babies were born; and our role in improving outcomes for women and their children became even more important. This year’s Update focuses on clinical guidelines centered on safety and optimal outcomes for women and children.
ACOG and SMFM update guidance on FGR management
American College of Obstetricians and Gynecologists. Practice advisory: Updated guidance regarding fetal growth restriction. September 2020. https://www.acog.org/clinical/clinical-guidance/practice-advisory/articles/2020/09/updated-guidance-regarding-fetal-growth-restriction. Accessed December 18, 2020.
Fetal growth restriction (FGR) affects up to 10% of pregnancies and is a leading cause of infant morbidity and mortality. Suboptimal fetal growth can have lasting negative effects on development into early childhood and, some hypothesize, even into adulthood.1,2 Antenatal detection of fetuses with FGR is critical so that antenatal testing can be implemented in an attempt to deliver improved clinical outcomes. FGR is defined by several different diagnostic criteria, and many studies have been conducted to determine how best to diagnose this condition.
In September 2020, the American College of Obstetricians and Gynecologists (ACOG) released a Practice Advisory regarding guidance on FGR in an effort to align the ACOG Practice Bulletin No. 204, ACOG Committee Opinion No. 764, and SMFM (Society for Maternal-Fetal Medicine) Consult Series No. 52.3-5 This guidance updates and replaces prior guidelines, with an emphasis on 3 notable changes.
FGR definition, workup have changed
While the original definition of FGR was an estimated fetal weight (EFW) of less than the 10th percentile for gestational age, a similar level of accuracy in prediction of subsequent small for gestational age (SGA) at birth has been shown when this or an abdominal circumference (AC) of less than the 10th percentile is used. Based on these findings, SMFM now recommends that FGR be defined as an EFW or AC of less than the 10th percentile for gestational age.
Recent studies have done head-to-head comparisons of different methods of estimating fetal weight to determine the best detection and pregnancy outcome improvement in FGR. In all instances, the Hadlock formula has continued to more accurately estimate fetal weight, prediction of SGA, and composite neonatal morbidity. As such, new guidelines recommend that population-based fetal growth references (that is, the Hadlock formula) should be used to determine ultrasonography-derived fetal weight percentiles.
The new guidance also suggests classification of FGR based on gestational age at onset, with early FGR at less than 32 weeks and late FGR at 32 or more weeks. The definition of severe FGR is reserved for fetuses with an EFW of less than the 3rd percentile. A diagnosis of FGR should prompt the recommendation for a detailed obstetric ultrasonography. Diagnostic genetic testing should be offered in cases of early-onset FGR, concomitant sonographic abnormalities, and/or polyhydramnios. Routine serum screening for toxoplasmosis, rubella, herpes, or cytomegalovirus (CMV) should not be done unless there are risk factors for infection. If amniocentesis is performed for genetic diagnostic testing, consideration can be made for polymerase chain reaction for CMV in the amniotic fluid.
Continue to: Timing of delivery in isolated FGR...
Timing of delivery in isolated FGR
A complicating factor in diagnosing FGR is distinguishing between the pathologically growth-restricted fetus and the constitutionally small fetus. Antenatal testing and serial umbilical artery Doppler assessment should be done following diagnosis of FGR to monitor for evidence of fetal compromise until delivery is planned.
The current ACOG Practice Bulletin No. 204 and Committee Opinion No. 764 recommend delivery between 38 0/7 and 39 6/7 weeks in the setting of isolated FGR with reassuring fetal testing and umbilical artery Doppler assessment.To further refine this, the new recommendations use the growth percentiles. In cases of isolated FGR with EFW between the 3rd and 10th percentile in the setting of normal umbilical artery Doppler, delivery is recommended between 38 and 39 weeks’ gestation. In cases of isolated FGR with EFW of less than the 3rd percentile (severe FGR) in the setting of normal umbilical artery Doppler, delivery is recommended at 37 weeks.
Timing of delivery in complicated FGR
A normal umbilical artery Doppler reflects the low impedance that is necessary for continuous forward flow of blood to the fetus. Abnormal umbilical artery Doppler signifies aberrations of this low-pressure system that affect the amount of continuous forward flow during diastole of the cardiac cycle. With continued compromise, there is progression to absent end-diastolic velocity (AEDV) and, most concerning, reversed end-diastolic velocity (REDV).
Serial umbilical artery Doppler assessment should be done following diagnosis of FGR to monitor for progression that is associated with perinatal mortality, since intervention can be initiated in the form of delivery. Delivery at 37 weeks is recommended for FGR with elevated umbilical artery Doppler of greater than the 95th percentile for gestational age. For FGR with AEDV, delivery is recommended between 33 and 34 weeks of gestation and for FGR with REDV between 30 and 32 weeks, as the neonatal morbidity and mortality associated with continuing the pregnancy outweighs the risks of prematurity in this setting. Because of the abnormal placental-fetal circulation in FGR complicated by AEDV/REDV, there may be a higher likelihood of fetal intolerance of labor and cesarean delivery (CD) may be considered.
- Fetal growth restriction is now defined as EFW of less than the 10th percentile or AC of less than the 10th percentile.
- Evaluation of FGR includes detailed anatomic survey and consideration of genetic evaluation, but infection screening should be done only if the patient is at risk for infection.
- With reassuring antenatal testing and normal umbilical artery Doppler studies, delivery is recommended at 38 to 39 weeks for isolated FGR with EFW in the 3rd to 10th percentile and at 37 weeks for FGR with EFW of less than the 3rd percentile.
- Umbilical artery Doppler studies are used to decrease the risk of perinatal mortality and further guide timing of delivery
Continue to: New recommendations for PROM management...
New recommendations for PROM management
American College of Obstetricians and Gynecologists Committee on Practice Bulletins–Obstetrics. ACOG practice bulletin no. 217: Prelabor rupture of membranes. Obstet Gynecol. 2020;135:e80-e97.
Rupture of membranes prior to the onset of labor occurs at term in 8% of pregnancies and in the preterm period in 2% to 3% of pregnancies.6 Accurate diagnosis, gestational age, evidence of infection, and discussion of the risks and benefits to the mother and fetus/neonate are necessary to optimize outcomes. In the absence of other indications for delivery, a gestational age of 34 or more weeks traditionally has been the cutoff to proceed with delivery, although this has not been globally agreed on and/or practiced.
ACOG has published a comprehensive update that incorporates the results of the PPROMT trial and other recommendations for the diagnosis and management of both term and preterm prelabor rupture of membranes (PROM).6,7
Making the diagnosis
Diagnosis of PROM usually can be made clinically via history and the classic triad of physical exam findings—pooling of fluid, basic pH, and ferning; some institutions also use commercially available tests that detect placental-derived proteins. Both ACOG and the US Food and Drug Administration caution against using these tests alone without clinical evaluation due to concern for false-positives and false-negatives that lead to adverse maternal and fetal/neonatal outcomes. For equivocal cases, ultrasonography for amniotic fluid evaluation and ultrasonography-guided dye tests can be used to assist in accurate diagnosis, especially in the preterm period in which there are significant implications for pregnancy management.
PROM management depends on gestational age
All management recommendations require reassuring fetal testing, evaluation for infection, and no other contraindications to expectant management. Once these are established, the most important determinant of PROM management then becomes gestational age.
Previable PROM
Previable PROM (usually defined as less than 23–24 weeks) has high risks of both maternal and fetal/neonatal morbidity and mortality from infection, hemorrhage, pulmonary hypoplasia, and extreme prematurity. These very difficult cases benefit from a multidisciplinary approach to patient counseling regarding expectant management versus immediate delivery.
If expectant management is chosen, outpatient management with close monitoring for signs of maternal infection may be done until an agreed on gestational age of viability. Then inpatient management with fetal monitoring, corticosteroids, tocolysis, magnesium for neuroprotection, and group B streptococcus (GBS) prophylaxis may be considered as appropriate.
Preterm PROM at less than 34 weeks
If the mother and fetus are otherwise stable, PROM at less than 34 weeks warrants inpatient expectant management with close maternal and fetal monitoring for signs of infection and labor. Management includes latency antibiotics, antenatal corticosteroids, magnesium for neuroprotection if less than 32 weeks’ gestation and at risk for imminent delivery, and GBS prophylaxis. While tocolysis may increase latency and help with steroid course completion, it should be used cautiously and avoided in cases of abruption or chorioamnionitis. Although there is no definitive recommendation published, a rescue course of steroids may be considered as appropriate but should not delay an indicated delivery.
Continue to: Late preterm PROM...
Late preterm PROM
The biggest change to clinical management in this ACOG Practice Bulletin is for late preterm (34–36 6/7 weeks) PROM, with the recommendation for either immediate delivery or expectant management up to 37 weeks stemming from the PPROMPT study by Morris and colleagues.7
From the neonatal perspective, no difference has been demonstrated between immediate delivery and expectant management for neonatal sepsis or a composite neonatal morbidity and mortality. Expectant management may be preferred from the neonatal point of view as immediate delivery was associated with an increased rate of neonatal respiratory distress, mechanical ventilation, and length of stay in the neonatal intensive care unit. The potential for long-term neurodevelopmental outcomes of delivery at 34 versus 37 weeks also should be considered.
From the maternal perspective, expectant management has an increased risk of antepartum and postpartum hemorrhage, fever, antibiotic use, and maternal length of stay, but a decreased risk of CD.
A late preterm steroid course can be considered if delivery is planned in no less than 24 hours and likely to occur in the next 7 days and if the patient has not already received a course of steroids. A rescue course of steroids is not indicated if the patient received a steroid course prior in the pregnancy. While appropriate GBS prophylaxis is recommended, latency antibiotics and tocolysis are not, and delivery should not be delayed if chorioamnionitis is diagnosed.
Ultimately, preterm PROM management with a stable mother and fetus at or beyond 34 weeks requires comprehensive counseling of the risks and benefits for both mother and fetus/neonate. A multidisciplinary team that together counsels the patient also may help with this shared decision making.
Term PROM
For patients with term PROM, delivery is recommended. Although a short period of expectant management for 12 to 24 hours is reported as “reasonable,” the risk of infection increases with the length of rupture of membranes. Therefore, induction of labor or CD soon after rupture of membranes is recommended for patients who are GBS positive and is preferred for all others.
- Accurate diagnosis is necessary for appropriate counseling and management of PROM.
- Delivery is recommended for term PROM, chorioamnionitis, and for patients with previable PROM who do not desire expectant management.
- If the mother and fetus are otherwise stable, expectant management of preterm PROM until 34 to 37 weeks is recommended.
- The decision of when to deliver between 34 and 37 weeks is best made with multidisciplinary counseling and shared decision making with the patient.
VTE prophylaxis in pregnancy: Regimen adjustments, CD strategies, and COVID-19 considerations
Birsner ML, Turrentine M, Pettker CM, et al. ACOG practice advisory: Options for peripartum anticoagulation in areas affected by shortage of unfractionated heparin. March 2020. https://www.acog.org/clinical/clinical-guidance/practice-advisory/articles/2020/03/options-for-peripartum-anticoagulation-in-areas-affected-by-shortage-of-unfractionated-heparin. Accessed December 8, 2020.
Pacheco LD, Saade G, Metz TD. Society for Maternal-Fetal Medicine Consult Series No. 51: Thromboembolism prophylaxis for cesarean delivery. Am J Obstet Gynecol. 2020;223:B11-B17
Venous thromboembolism (VTE) prophylaxis is a timely topic for a number of reasons. First, a shortage of unfractionated heparin prompted an ACOG Practice Advisory, endorsed by SMFM and the Society for Obstetric Anesthesia and Perinatology, regarding use of low molecular weight heparin (LMWH) in the peripartum period.8 In addition, SMFM released updated recommendations for VTE prophylaxis for CD as part of the SMFM Consult Series.9 Finally, there is evidence that COVID-19 infection may increase the risk of coagulopathy, leading to consideration of additional VTE prophylaxis for pregnant and postpartum women with COVID-19.
Candidates for prophylaxis
As recommended by the ACOG Practice Bulletin on thromboembolism in pregnancy, women who may require VTE prophylaxis during pregnancy and/or the postpartum period include those with10:
- VTE diagnosed during pregnancy
- a history of VTE, including during pregnancy or with use of hormonal contraception
- a history of thrombophilia with or without a personal or family history of VTE.
For these patients, LMWH has many advantages over unfractionated heparin, including ease of use and reliability of dosing. It generally is preferred in pregnancy and postpartum (for both prophylactic and therapeutic anticoagulation) by patients and providers.
The Practice Bulletin references a strategy that describes converting LMWH to unfractionated heparin at around 36 weeks’ gestation in preparation for delivery because unfractionated heparin has the advantage of a shorter half-life and the option for anticoagulation reversal with protamine sulfate. In the Practice Advisory, a global shortage of unfractionated heparin and an argument that the above conversion was less about concern for maternal hemorrhage and more about avoiding spinal and epidural hematomas led to the following recommendations for continued use of LMWH through delivery:
- LMWH heparin can be discontinued in a planned fashion prior to scheduled induction of labor or CD (generally 12 hours for prophylactic dosing and 24 hours for intermediate dosing).
- Patients in spontaneous labor may receive neuraxial anesthesia 12 hours after the last prophylactic dose and 24 hours after the last intermediate dose of LMWH.
- Patients who require anticoagulation during pregnancy should be counseled that if they have vaginal bleeding, leakage of fluid, or regular contractions they should be evaluated prior to taking their next dose of anticoagulant.
- In the absence of other complications, delivery should not be before 39 weeks for the indication of anticoagulation requirement alone.
Continue to: Managing VTE risk in CD...
Managing VTE risk in CD
Recognizing that VTE is a major cause of maternal morbidity and mortality, as well as the variety of the published guidelines for VTE prophylaxis after CD, the SMFM Consult Series provides recommendations to assist clinicians caring for postpartum women after CD. As reviewed in the ACOG Practice Bulletin, there are good data to support pharmacologic prophylaxis during pregnancy and the postpartum period for women with a history of VTE or a thrombophilia. Solid evidence is lacking, however, for what to do for women who have a CD without this history but may have other potential risk factors for VTE, such as obesity, preeclampsia, and transfusion requirement. Universal pharmacologic prophylaxis also is not yet supported by evidence. SMFM supports LMWH as the preferred medication in pregnancy and postpartum and provides these additional recommendations:
- All women who have a CD should have sequential compression devices (SCDs) placed prior to surgery and continued until they are ambulatory.
- Women with a history of VTE or thrombophilia without history of VTE should have SCDs and pharmacologic VTE prophylaxis for 6 weeks postpartum.
- Intermediate dosing of LMWH is recommended for patients with class III obesity.
- Institutions should develop patient safety bundles for VTE prophylaxis to identify additional risk factors that may warrant pharmacologic prophylaxis after CD in select patients.
Our approach to patients with COVID-19 infection
At our institution, we recently incorporated a VTE prophylaxis protocol into our electronic medical record that provides risk stratification for each patient. In addition to the above recommendations, our patients may qualify for short-term in-house or longer postpartum prophylaxis depending on risk factors.
A new risk factor in recent months is COVID-19 infection, which appears to increase the risk of coagulopathy, especially in patients with disease severe enough to warrant hospitalization. Given the potential for additive risk in pregnancy, in consult with our medicine colleagues, we have placed some of our more ill hospitalized pregnant patients on a course of prophylactic LMWH both in the hospital and after discharge independent of delivery status or mode of delivery. ●
- Pregnant patients with a history of VTE or a thrombophilia may be candidates for pharmacologic anticoagulation during pregnancy and/or postpartum.
- LMWH is the preferred method of pharmacologic VTE prophylaxis during pregnancy and postpartum.
- For most patients, CD and neuraxial anesthesia safely can be performed 12 to 24 hours after the last dose of prophylactic or intermediate LMWH, respectively.
- All patients undergoing CD should have at least mechanical VTE prophylaxis with SCDs.
- All women who have a CD should be evaluated via institutional patient safety bundles for VTE prophylaxis for additional risk factors that potentially warrant postpartum pharmacologic VTE prophylaxis.
- More data are needed to determine recommendations for universal/ near universal pharmacologic VTE prophylaxis in the postpartum period.
- Pregnant or postpartum patients with moderate to severe COVID-19 infection may be at increased risk for VTE, warranting consideration of additional pharmacologic prophylaxis.
- Baschat AA, Gembruch U, Harman CR. The sequence of changes in Doppler and biophysical parameters as severe fetal growth restriction worsens. Ultrasound Obstet Gynecol. 2001;18:571-577.
- Almond D, Currie J. Killing me softly: the fetal origins hypothesis. J Econ Perspect. 2011;25:153-172.
- American College of Obstetricians and Gynecologists Committee on Practice Bulletins–Obstetrics and Society for Maternal-Fetal Medicine. ACOG practice bulletin no. 204: Fetal growth restriction. Obstet Gynecol. 2019;133: e97-e109.
- American College of Obstetricians and Gynecologists Committee on Obstetric Practice and Society for Maternal-Fetal Medicine. ACOG committee opinion no. 764: Medically indicated late-preterm and early-term deliveries. Obstet Gynecol. 2019;133:e151-e155.
- Society for Maternal-Fetal Medicine; Martins JG, Biggio FR, Abuhamad A. SMFM consult series no. 52: diagnosis and management of fetal growth restriction. Am J Obstet Gynecol. 2020;223:B2-B17.
- American College of Obstetricians and Gynecologists Committee on Practice Bulletins–Obstetrics. ACOG practice bulletin no. 217: Prelabor rupture of membranes. Obstet Gynecol. 2020;135:e80-e97.
- Morris JM, Roberts CL, Bowen JR, et al; PPROMT Collaboration. Immediate delivery compared with expectant management after preterm pre-labour rupture of the membranes close to term (PPROMT trial): a randomised controlled trial. Lancet. 2016;387:444-452.
- Birsner ML, Turrentine M, Pettker CM, et al. ACOG practice advisory: Options for peripartum anticoagulation in areas affected by shortage of unfractionated heparin. March 2020. https://www.acog.org/clinical/clinical-guidance/practice-advisory/articles/2020/03/options-for-peripartum-anticoagulation-in-areas-affected-by-shortage-of-unfractionated-heparin. Accessed December 8, 2020.
- Pacheco LD, Saade G, Metz TD. Society for MaternalFetal Medicine consult series no. 51: Thromboembolism prophylaxis for cesarean delivery. Am J Obstet Gynecol. 2020;223:B11-B17.
- American College of Obstetricians and Gynecologists Committee on Practice Bulletins–Obstetrics. ACOG practice bulletin no. 196: Thromboembolism in pregnancy. Obstet Gynecol. 2018;132:e1-e17.
- Baschat AA, Gembruch U, Harman CR. The sequence of changes in Doppler and biophysical parameters as severe fetal growth restriction worsens. Ultrasound Obstet Gynecol. 2001;18:571-577.
- Almond D, Currie J. Killing me softly: the fetal origins hypothesis. J Econ Perspect. 2011;25:153-172.
- American College of Obstetricians and Gynecologists Committee on Practice Bulletins–Obstetrics and Society for Maternal-Fetal Medicine. ACOG practice bulletin no. 204: Fetal growth restriction. Obstet Gynecol. 2019;133: e97-e109.
- American College of Obstetricians and Gynecologists Committee on Obstetric Practice and Society for Maternal-Fetal Medicine. ACOG committee opinion no. 764: Medically indicated late-preterm and early-term deliveries. Obstet Gynecol. 2019;133:e151-e155.
- Society for Maternal-Fetal Medicine; Martins JG, Biggio FR, Abuhamad A. SMFM consult series no. 52: diagnosis and management of fetal growth restriction. Am J Obstet Gynecol. 2020;223:B2-B17.
- American College of Obstetricians and Gynecologists Committee on Practice Bulletins–Obstetrics. ACOG practice bulletin no. 217: Prelabor rupture of membranes. Obstet Gynecol. 2020;135:e80-e97.
- Morris JM, Roberts CL, Bowen JR, et al; PPROMT Collaboration. Immediate delivery compared with expectant management after preterm pre-labour rupture of the membranes close to term (PPROMT trial): a randomised controlled trial. Lancet. 2016;387:444-452.
- Birsner ML, Turrentine M, Pettker CM, et al. ACOG practice advisory: Options for peripartum anticoagulation in areas affected by shortage of unfractionated heparin. March 2020. https://www.acog.org/clinical/clinical-guidance/practice-advisory/articles/2020/03/options-for-peripartum-anticoagulation-in-areas-affected-by-shortage-of-unfractionated-heparin. Accessed December 8, 2020.
- Pacheco LD, Saade G, Metz TD. Society for MaternalFetal Medicine consult series no. 51: Thromboembolism prophylaxis for cesarean delivery. Am J Obstet Gynecol. 2020;223:B11-B17.
- American College of Obstetricians and Gynecologists Committee on Practice Bulletins–Obstetrics. ACOG practice bulletin no. 196: Thromboembolism in pregnancy. Obstet Gynecol. 2018;132:e1-e17.