User login
Energy-Based Devices for Actinic Keratosis Field Therapy
In cutaneous field cancerization, focal treatments such as cryotherapy are impractical, thus necessitating the use of field-directed therapies over the lesion and the surrounding skin field. Although evidence-based guidelines do not exist, field-directed therapy has been proposed in cases of 3 or more actinic keratoses (AKs) in a 25-cm2 area or larger.1 It can be further speculated that patients who are vulnerable to aggressive phenotypes of cutaneous malignancies, such as those with a genodermatosis or who are immunocompromised, necessitate a higher index of suspicion for field effect with even 1 or 2 AKs.
Current field-directed therapies include topical agents (imiquimod, fluorouracil, ingenol mebutate, and diclo-fenac), photodynamic therapy (PDT), and resurfacing procedures (lasers, chemical peels, dermabrasion). Although topical agents and PDT currently are gold standards in field treatment, the use of energy-based devices (ie, ablative and nonablative lasers) are attractive options as monotherapy or as part of a combination therapy. These devices are attractive options for field-directed therapy because they offer defined, customizable control of settings, allowing for optimal cosmesis and precision of therapy.
Principally, lasers function by damaging skin tissue to induce resurfacing, neocollagenesis, and vascular restructuring. Fractional versions of ablative and nonablative systems are available to target a fraction of the treatment area in evenly spaced microthermal zones and to minimize overall thermal damage.2
Given recent advances in laser systems and numerous investigations reported in the literature, a review of ablative and nonablative lasers that have been studied as treatment options for cutaneous field cancerization is provided, with a focus on treatment efficacy.
Ablative Lasers
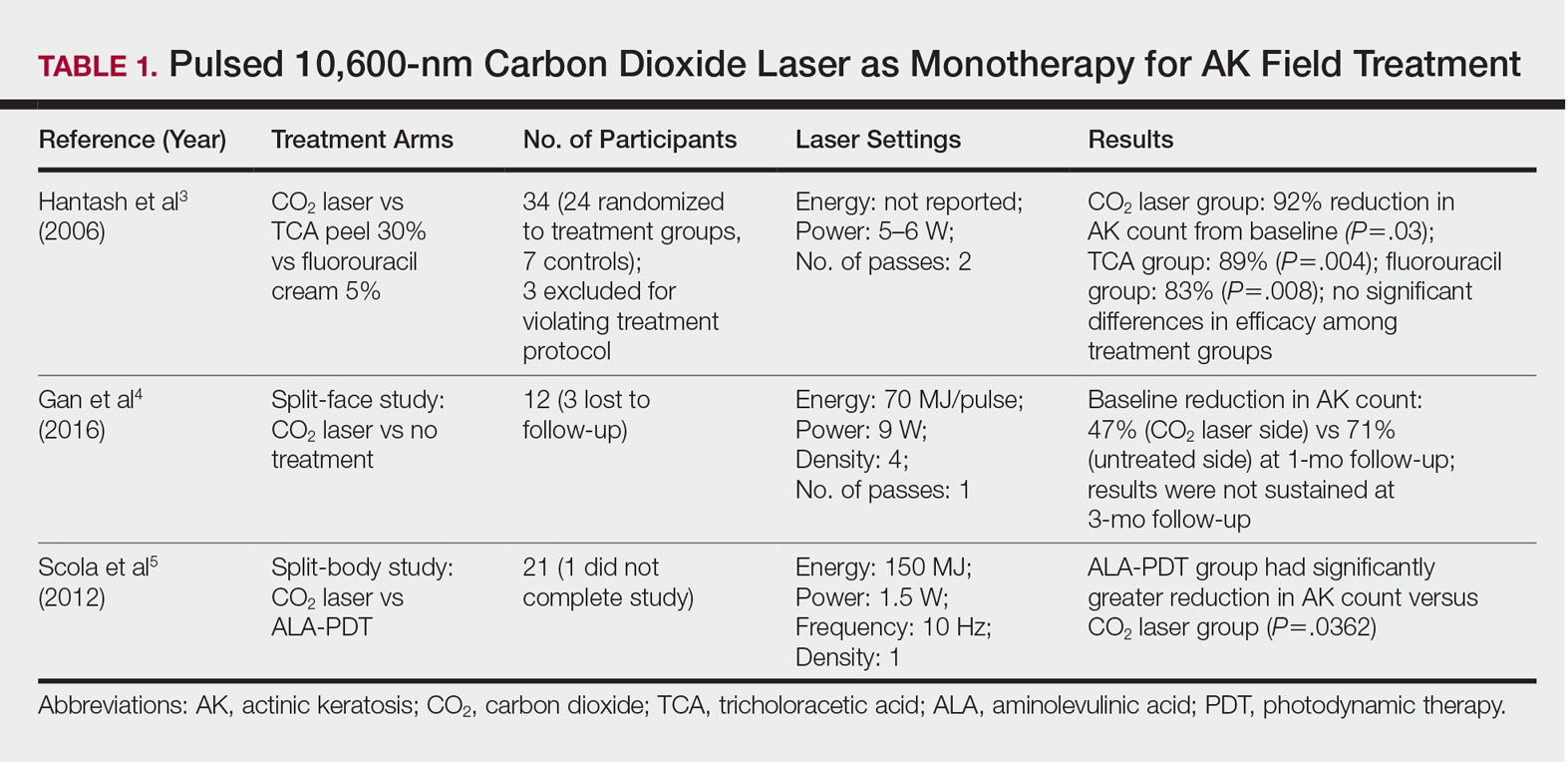
Ablative lasers operate at higher wavelengths than nonablative lasers to destroy epidermal and dermal tissue. The 10,600-nm carbon dioxide (CO2) and 2940-nm Er:YAG lasers have been heavily investigated for field therapy for multiple AKs, both as monotherapies (Table 1) and in combination with PDT (Table 2).
Monotherapy
One randomized trial with 5-year follow-up compared the efficacy of full-face pulsed CO2 laser therapy, full-face trichloroacetic acid (TCA) peel 30%, and fluorouracil cream 5% (twice daily for 3 weeks) on AKs on the face and head.3 Thirty-one participants were randomized to the 3 treatment arms and a negative control arm. The mean AK counts at baseline for the CO2, TCA, and fluorouracil treatment groups were 78.0, 83.7, and 61.8, respectively. At 3-month follow-up, all treatment groups had significant reductions in the mean AK count from baseline (CO2 group, 92% [P=.03]; TCA group, 89% [P=.004]; fluorouracil group, 83% [P=.008]). No significant differences in efficacy among the treatment groups were noted. All 3 treatment groups had a demonstrably lower incidence of nonmelanoma skin cancer over 5-year follow-up compared to the control group (P<.001).3
In contrast to these promising results, the pulsed CO2 laser showed only short-term efficacy in a split-face study of 12 participants with at least 5 facial or scalp AKs on each of 2 symmetric facial sides who were randomized to 1 treatment side.4 At 1-month follow-up, the treatment side exhibited significantly fewer AKs compared to the control side (47% vs 71% at baseline; P=.01), but the improvement was not sustained at 3-month follow-up (49% vs 57%; P=.47).4
In another study, the CO2 laser was found to be inferior to 5-aminolevulinic acid PDT.5 Twenty-one participants who had at least 4 AKs in each symmetric half of a body region (head, hands, forearms) were randomized to PDT on 1 side and CO2 laser therapy on the other. Median baseline AK counts for the PDT and CO2 laser groups were 6 and 8, respectively. Both treatment groups exhibited significant median AK reduction from baseline 4 weeks posttreatment (PDT group, 82.1% [P<.05], CO2 laser group, 100% [P<.05]); however. at 3 months posttreatment the PDT group had significantly higher absolute (P=.0155) and relative (P=.0362) reductions in AK count compared to the CO2 laser group. One participant received a topical antibiotic for superficial infection on the PDT treatment side.5
Many questions remain regarding the practical application of laser ablation monotherapy for multiple AKs. More studies are needed to determine the practicality and long-term clinical efficacy of these devices.
PDT Combination Therapy
Laser ablation may be combined with PDT to increase efficacy and prolong remission rates. In fact, laser ablation may be thought of as a physical drug-delivery system to boost uptake of topical agents—in this case, aminolevulinic acid and methyl aminolevulinate (MAL)—given that it disrupts the skin barrier.
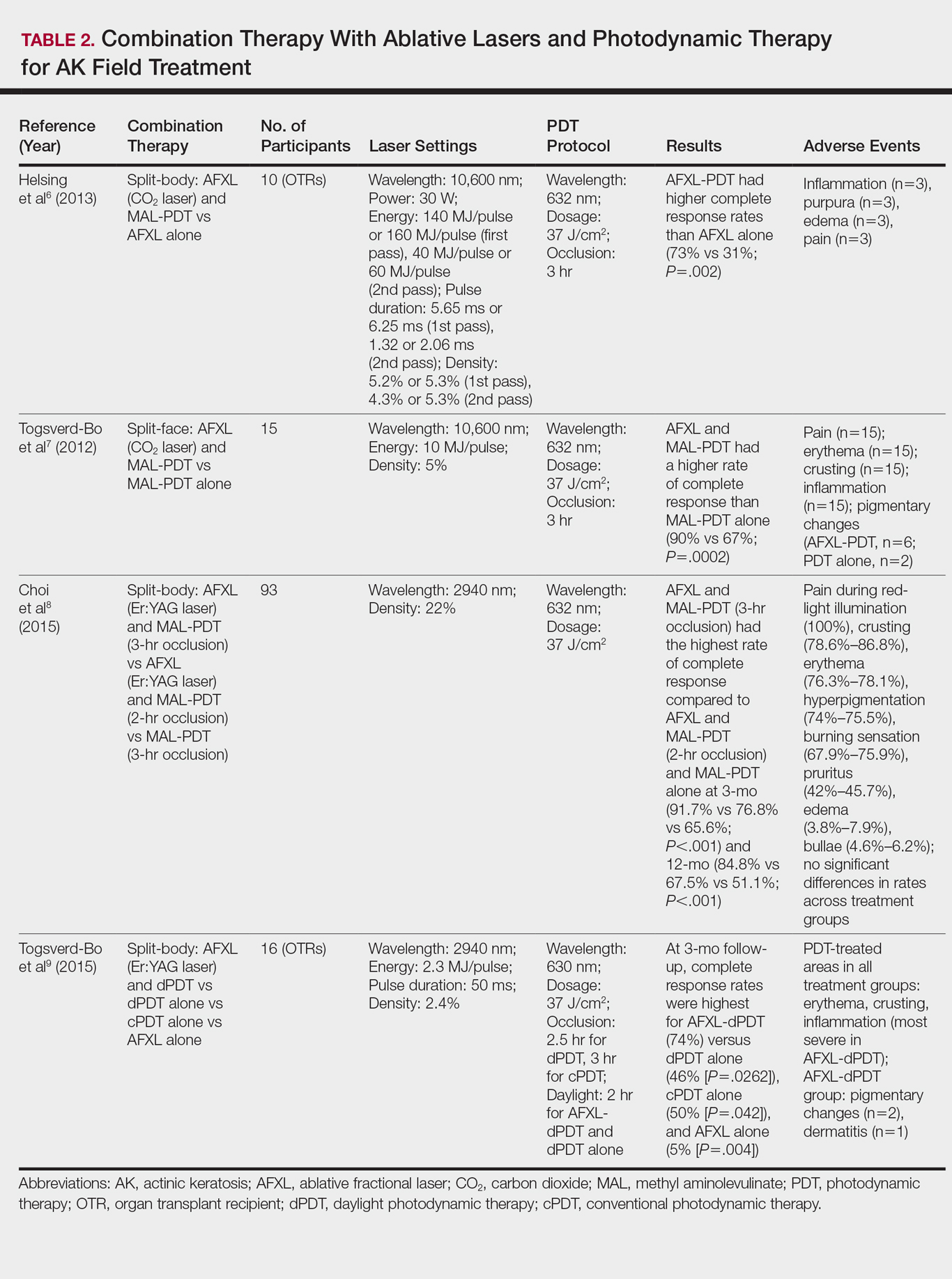
In a comparative study of ablative fractional laser (AFXL)–assisted PDT and AFXL alone in 10 organ transplant recipients on immunosuppression with at least 5 AKs on each dorsal hand, participants were randomized to AFXL-PDT on one treatment side and PDT on the other side.6 Participants received AFXL in an initial lesion-directed pass and then a second field-directed pass of a fractional CO2 laser. After AFXL exposure, methyl aminolevulinate was applied to the AFXL-PDT treatment side, with 3-hour occlusion. A total of 680 AKs were treated (335 in the AFXL-PDT group, 345 in the PDT group); results were stratified by the clinical grade of the lesion (1, slightly palpable; 2, moderately thick; 3, very thick or obvious). At 4-month follow-up, the AFXL-PDT group had a significantly higher median complete response rate of 73% compared to 31% in the AFXL group (P=.002). Interestingly, AFXL-PDT was also significantly more efficacious compared to AFXL for grades 1 (80% vs 37%; P=.02) and 2 (53% vs 7%, P=.009) AKs but not grade 3 AKs (4% vs 0%, P=.17).6
The combination of fractional CO2 laser and PDT also demonstrated superiority to PDT.7 In a split-face investigation, 15 participants with bilateral symmetric areas of 2 to 10 AKs on the face or scalp were randomized to receive fractional CO2 laser and MAL-PDT combination therapy on 1 treatment side and conventional MAL-PDT on the other side.7 The AFXL-PDT treatment side received laser ablation with immediate subsequent application of MAL to both treatment sides under 3-hour occlusion. At baseline, 103 AKs were treated by AFXL-PDT and 109 AKs were treated with conventional PDT. At 3-month follow-up, the AFXL-PDT treatment group exhibited a significantly higher rate of complete response (90%) compared to the conventional PDT group (67%)(P=.0002).7
Like the CO2 laser, the Er:YAG laser has demonstrated superior results when used in combination with PDT to treat field cancerization compared to either treatment alone. In a comparison study, 93 patients with 2 to 10 AK lesions on the face or scalp were randomized to treatment with AFXL (Er:YAG laser) and MAL-PDT with 3-hour occlusion, AFXL (Er:YAG laser) and MAL-PDT with 2-hour occlusion, and MAL-PDT with 3-hour occlusion.8 A total of 440 baseline AK lesions on the face or scalp were treated. At 3-month follow-up, the AFXL-PDT (3-hour occlusion) group had the highest rate of complete response (91.7%), compared to 76.8% (P=.001) in the AFXL-PDT (2-hour occlusion) and 65.6% (P=.001) in the PDT groups, regardless of the grade of AK lesion. The AFXL-PDT (2-hour occlusion) treatment was also superior to PDT alone (P=.038). These findings were sustained at 12-month follow-up (84.8% in the AFXL-PDT [3-hour occlusion] group [P<.001, compared to others]; 67.5% in the AFXL-PDT [2-hour occlusion] group [P<.001, compared to 3-hour PDT]; 51.1% in the PDT group). Importantly, the AK lesion recurrence rate was also lowest in the AFL-PDT (3-hour occlusion) group (7.5% vs 12.1% and 22.1% in the AFXL-PDT [2-hour occlusion] and PDT groups, respectively; P=.007).8
Combination therapy with AFXL and daylight PDT (dPDT) may improve the tolerability of PDT and the efficacy rate of field therapy in organ transplant recipients. One study demonstrated the superiority of this combination therapy in a population of 16 organ transplant recipients on immunosuppressants with at least 2 moderate to severely thick AKs in each of 4 comparable areas in the same anatomic region.9 The 4 areas were randomized to a single session of AFXL-dPDT, dPDT alone, conventional PDT, or AFXL alone. Ablation was performed with a fractional Er:YAG laser. The AFXL-dPDT and dPDT alone groups received MAL for 2.5 hours without occlusion, and the conventional PDT group received MAL for 3 hours with occlusion. Daylight exposure in dPDT groups was initiated 30 minutes after MAL application for 2 hours total. A baseline total of 542 AKs were treated. At 3-month follow-up, the complete response rate was highest for the AFXL-dPDT group (74%) compared to dPDT alone (46%; P=.0262), conventional PDT (50%; P=.042), and AFXL alone (5%; P=.004). Pain scores for AFXL–dPDT and dPDT alone were significantly lower than for conventional PDT and AFXL alone (P<.001).9
Nonablative Lasers
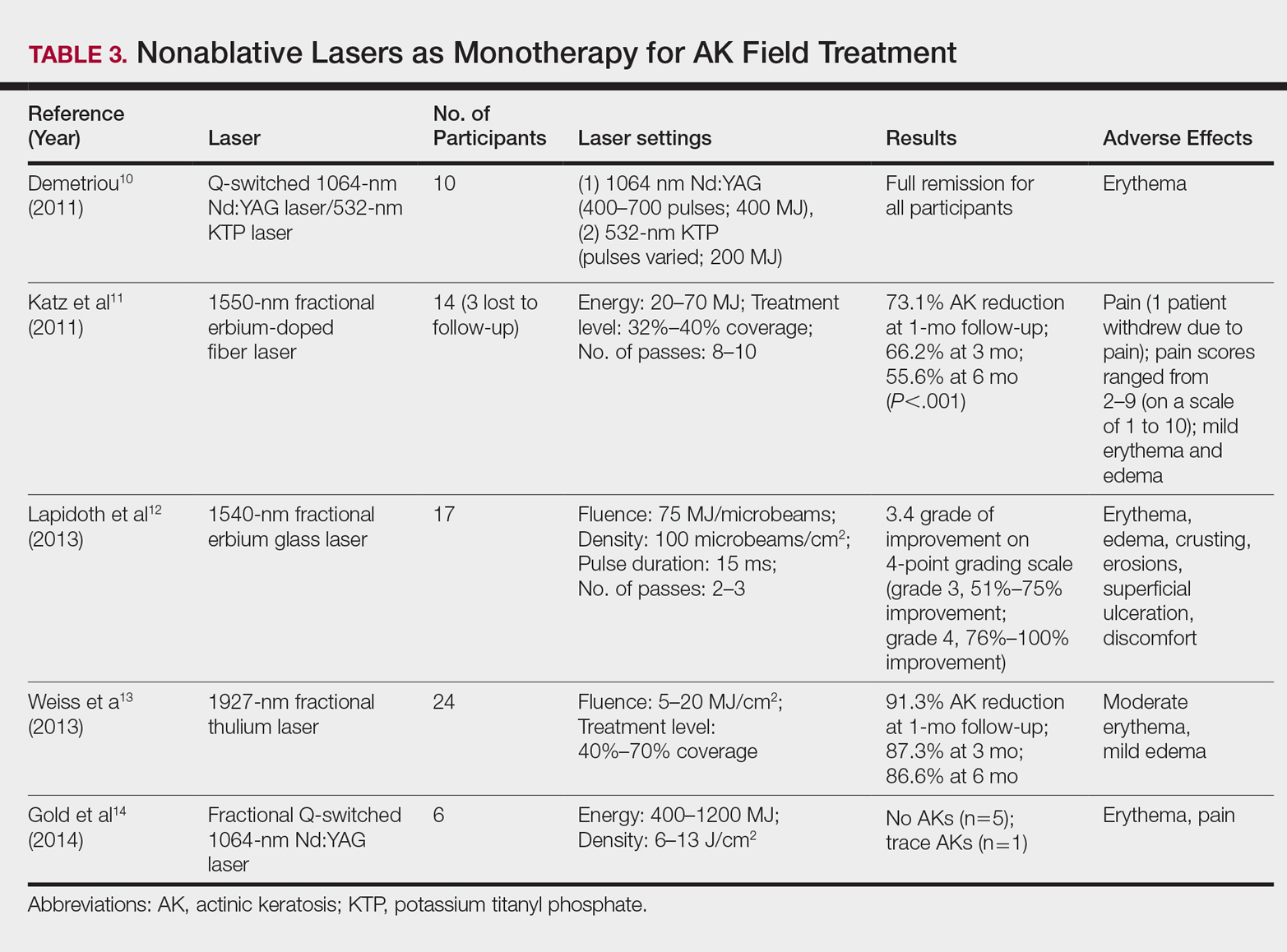
By heating the dermis to induce neogenesis without destruction, nonablative lasers offer superior healing times compared to their ablative counterparts. Multiple treatments with nonablative lasers may be necessary for maximal effect. Four nonablative laser devices have demonstrated efficacy in the treatment of multiple AKs10-14: (1) the Q-switched 1064-nm Nd:YAG laser, with or without a 532-nm potassium titanyl phosphate (KTP) laser; (2) the 1540-nm fractional erbium glass laser; (3) the 1550-nm fractional erbium-doped fiber laser; and (4) the 1927-nm fractional thulium laser (Table 3).
In a proof-of-concept study of the Q-switched Nd:YAG laser with the 532-nm KTP laser, 1 treatment session induced full remission of AKs in 10 patients at follow-up day 20, although the investigator did not grade improvement on a numerical scale.10 In a study of the fractional Q-switched 1064-nm Nd:YAG laser alone, 6 patients with trace or mild AKs received 4 treatment sessions at approximately 2-week intervals.14 All but 1 patient (who had trace AKs) had no AKs at 3-month follow-up.
The efficacy of the 1540-nm fractional erbium glass laser was examined in 17 participants with investigator-rated moderate-to-severe AK involvement of the scalp and face.12 Participants were given 2 or 3 treatment sessions at 3- to 4-week intervals and were graded by blinded dermatologists on a quartile scale of 0 (no improvement), 1 (1%–25% improvement), 2 (26%–50% improvement), 3 (51%–75% improvement), or 4 (76%–100% improvement). At 3 months posttreatment, the average grade of improvement was 3.4.12
The 1550-nm fractional erbium-doped fiber laser was tested in 14 men with multiple facial AKs (range, 9–44 AKs [mean, 22.1 AKs]).11 Participants received 5 treatment sessions at 2- to 4-week intervals, with majority energies used at 70 MJ and treatment level 11. The mean AK count was reduced significantly by 73.1%, 66.2%, and 55.6% at 1-, 3-, and 6-month follow-up, respectively (P<.001).11
The 1927-nm fractional thulium laser showed promising results in 24 participants with facial AKs.13 Participants received up to 4 treatment sessions at intervals from 2 to 6 weeks at the investigators’ discretion. At baseline, patients had an average of 14.04 facial AKs. At 1-, 3-, and 6-month follow-up, participants exhibited 91.3%, 87.3%, and 86.6% reduction in AK counts, respectively. The mean AK count at 3-month follow-up was 1.88.13
Due to limited sample sizes and/or lack of quantifiable results and controls in these studies, more studies are needed to fully elucidate the role of nonablative lasers in the treatment of AK.
Future Directions
Iontophoresis involves the noninvasive induction of an electrical current to facilitate ion movement through the skin and may be a novel method to boost the efficacy of current field therapies. In the first known study of its kisnd, iontophoresis-assisted AFXL-PDT was found to be noninferior to conventional AFXL-PDT15; however, additional studies demonstrating its superiority are needed before more widespread clinical use is considered.
Pretreatment with AFXL prior to topical field-directed therapies also has been proposed.16 In a case series of 13 patients, combination therapy with AFXL and ingenol mebutate was shown to be superior to ingenol mebutate alone (AK clearance rate, 89.2% vs 72.1%, respectively; P<.001).16 Randomized studies with longer follow-up time are needed.
Conclusion
Ablative and nonablative laser systems have yielded limited data about their potential as monotherapies for treatment of multiple AKs and are unlikely to replace topical agents and PDT as a first-line modality in field-directed treatment at this time. More studies with a larger number of participants and long-term follow-up are needed for further clarification of efficacy, safety, and clinical feasibility. Nevertheless, fractional ablative lasers in combination with PDT have shown robust efficacy and a favorable safety profile for treatment of multiple AKs.6-9 Further, this combination therapy exhibited a superior clearance rate and lower lesion recurrence in organ transplant recipients—a demographic that classically is difficult to treat.6-9
With continued rapid evolution of laser systems and more widespread use in dermatology, monotherapy and combination therapy may offer a dynamic new option in field cancerization that can decrease disease burden and treatment frequency.
- Peris K, Calzavara-Pinton PG, Neri L, et al. Italian expert consensus for the management of actinic keratosis in immunocompetent patients. J Eur Acad Dermatol Venereol. 2016;30:1077-1084.
- Alexiades-Armenakas MR, Dover JS, Arndt KA. The spectrum of laser skin resurfacing: nonablative, fractional, and ablative laser resurfacing. J Am Acad Dermatol. 2008;58:719-737; quiz 738-740.
- Hantash BM, Stewart DB, Cooper ZA, et al. Facial resurfacing for nonmelanoma skin cancer prophylaxis. Arch Dermatol. 2006;142:976-982.
- Gan SD, Hsu SH, Chuang G, et al. Ablative fractional laser therapy for the treatment of actinic keratosis: a split-face study. J Am Acad Dermatol. 2016;74:387-389.
- Scola N, Terras S, Georgas D, et al. A randomized, half-side comparative study of aminolaevulinate photodynamic therapy vs. CO(2) laser ablation in immunocompetent patients with multiple actinic keratoses. Br J Dermatol. 2012;167:1366-1373.
- Helsing P, Togsverd-Bo K, Veierod MB, et al. Intensified fractional CO2 laser-assisted photodynamic therapy vs. laser alone for organ transplant recipients with multiple actinic keratoses and wart-like lesions: a randomized half-side comparative trial on dorsal hands. Br J Dermatol. 2013;169:1087-1092.
- Togsverd-Bo K, Haak CS, Thaysen-Petersen D, et al. Intensified photodynamic therapy of actinic keratoses with fractional CO2 laser: a randomized clinical trial. Br J Dermatol. 2012;166:1262-1269.
- Choi SH, Kim KH, Song KH. Efficacy of ablative fractional laser-assisted photodynamic therapy with short-incubation time for the treatment of facial and scalp actinic keratosis: 12-month follow-up results of a randomized, prospective, comparative trial. J Eur Acad Dermatol Venereol. 2015;29:1598-1605.
- Togsverd-Bo K, Lei U, Erlendsson AM, et al. Combination of ablative fractional laser and daylight-mediated photodynamic therapy for actinic keratosis in organ transplant recipients—a randomized controlled trial. Br J Dermatol. 2015;172:467-474.
- Demetriou C. Reversing precancerous actinic damage by mixing wavelengths (1064 nm, 532 nm). J Cosmet Laser Ther. 2011;13:113-119.
- Katz TM, Goldberg LH, Marquez D, et al. Nonablative fractional photothermolysis for facial actinic keratoses: 6-month follow-up with histologic evaluation. J Am Acad Dermatol. 2011;65:349-356.
- Lapidoth M, Adatto M, Halachmi S. Treatment of actinic keratoses and photodamage with non-contact fractional 1540-nm laser quasi-ablation: an ex vivo and clinical evaluation. Lasers Med Sci. 2013;28:537-542.
- Weiss ET, Brauer JA, Anolik R, et al. 1927-nm fractional resurfacing of facial actinic keratoses: a promising new therapeutic option. J Am Acad Dermatol. 2013;68:98-102.
- Gold MH, Sensing W, Biron J. Fractional Q-switched 1,064-nm laser for the treatment of photoaged-photodamaged skin. J Cosmet Laser Ther. 2014;16:69-76.
- Choi SH, Kim TH, Song KH. Efficacy of iontophoresis-assisted ablative fractional laser photodynamic therapy with short incubation time for the treatment of actinic keratosis: 12-month follow-up results of a prospective, randomised, comparative trial. Photodiagnosis Photodyn Ther. 2017;18:105-110.
- Nisticò S, Sannino M, Del Duca E, et al. Ablative fractional laser improves treatment of actinic keratoses with ingenol mebutate. Eur J Inflamm. 2016;14:200-205.
In cutaneous field cancerization, focal treatments such as cryotherapy are impractical, thus necessitating the use of field-directed therapies over the lesion and the surrounding skin field. Although evidence-based guidelines do not exist, field-directed therapy has been proposed in cases of 3 or more actinic keratoses (AKs) in a 25-cm2 area or larger.1 It can be further speculated that patients who are vulnerable to aggressive phenotypes of cutaneous malignancies, such as those with a genodermatosis or who are immunocompromised, necessitate a higher index of suspicion for field effect with even 1 or 2 AKs.
Current field-directed therapies include topical agents (imiquimod, fluorouracil, ingenol mebutate, and diclo-fenac), photodynamic therapy (PDT), and resurfacing procedures (lasers, chemical peels, dermabrasion). Although topical agents and PDT currently are gold standards in field treatment, the use of energy-based devices (ie, ablative and nonablative lasers) are attractive options as monotherapy or as part of a combination therapy. These devices are attractive options for field-directed therapy because they offer defined, customizable control of settings, allowing for optimal cosmesis and precision of therapy.
Principally, lasers function by damaging skin tissue to induce resurfacing, neocollagenesis, and vascular restructuring. Fractional versions of ablative and nonablative systems are available to target a fraction of the treatment area in evenly spaced microthermal zones and to minimize overall thermal damage.2
Given recent advances in laser systems and numerous investigations reported in the literature, a review of ablative and nonablative lasers that have been studied as treatment options for cutaneous field cancerization is provided, with a focus on treatment efficacy.
Ablative Lasers
Ablative lasers operate at higher wavelengths than nonablative lasers to destroy epidermal and dermal tissue. The 10,600-nm carbon dioxide (CO2) and 2940-nm Er:YAG lasers have been heavily investigated for field therapy for multiple AKs, both as monotherapies (Table 1) and in combination with PDT (Table 2).
Monotherapy
One randomized trial with 5-year follow-up compared the efficacy of full-face pulsed CO2 laser therapy, full-face trichloroacetic acid (TCA) peel 30%, and fluorouracil cream 5% (twice daily for 3 weeks) on AKs on the face and head.3 Thirty-one participants were randomized to the 3 treatment arms and a negative control arm. The mean AK counts at baseline for the CO2, TCA, and fluorouracil treatment groups were 78.0, 83.7, and 61.8, respectively. At 3-month follow-up, all treatment groups had significant reductions in the mean AK count from baseline (CO2 group, 92% [P=.03]; TCA group, 89% [P=.004]; fluorouracil group, 83% [P=.008]). No significant differences in efficacy among the treatment groups were noted. All 3 treatment groups had a demonstrably lower incidence of nonmelanoma skin cancer over 5-year follow-up compared to the control group (P<.001).3
In contrast to these promising results, the pulsed CO2 laser showed only short-term efficacy in a split-face study of 12 participants with at least 5 facial or scalp AKs on each of 2 symmetric facial sides who were randomized to 1 treatment side.4 At 1-month follow-up, the treatment side exhibited significantly fewer AKs compared to the control side (47% vs 71% at baseline; P=.01), but the improvement was not sustained at 3-month follow-up (49% vs 57%; P=.47).4
In another study, the CO2 laser was found to be inferior to 5-aminolevulinic acid PDT.5 Twenty-one participants who had at least 4 AKs in each symmetric half of a body region (head, hands, forearms) were randomized to PDT on 1 side and CO2 laser therapy on the other. Median baseline AK counts for the PDT and CO2 laser groups were 6 and 8, respectively. Both treatment groups exhibited significant median AK reduction from baseline 4 weeks posttreatment (PDT group, 82.1% [P<.05], CO2 laser group, 100% [P<.05]); however. at 3 months posttreatment the PDT group had significantly higher absolute (P=.0155) and relative (P=.0362) reductions in AK count compared to the CO2 laser group. One participant received a topical antibiotic for superficial infection on the PDT treatment side.5
Many questions remain regarding the practical application of laser ablation monotherapy for multiple AKs. More studies are needed to determine the practicality and long-term clinical efficacy of these devices.
PDT Combination Therapy
Laser ablation may be combined with PDT to increase efficacy and prolong remission rates. In fact, laser ablation may be thought of as a physical drug-delivery system to boost uptake of topical agents—in this case, aminolevulinic acid and methyl aminolevulinate (MAL)—given that it disrupts the skin barrier.
In a comparative study of ablative fractional laser (AFXL)–assisted PDT and AFXL alone in 10 organ transplant recipients on immunosuppression with at least 5 AKs on each dorsal hand, participants were randomized to AFXL-PDT on one treatment side and PDT on the other side.6 Participants received AFXL in an initial lesion-directed pass and then a second field-directed pass of a fractional CO2 laser. After AFXL exposure, methyl aminolevulinate was applied to the AFXL-PDT treatment side, with 3-hour occlusion. A total of 680 AKs were treated (335 in the AFXL-PDT group, 345 in the PDT group); results were stratified by the clinical grade of the lesion (1, slightly palpable; 2, moderately thick; 3, very thick or obvious). At 4-month follow-up, the AFXL-PDT group had a significantly higher median complete response rate of 73% compared to 31% in the AFXL group (P=.002). Interestingly, AFXL-PDT was also significantly more efficacious compared to AFXL for grades 1 (80% vs 37%; P=.02) and 2 (53% vs 7%, P=.009) AKs but not grade 3 AKs (4% vs 0%, P=.17).6
The combination of fractional CO2 laser and PDT also demonstrated superiority to PDT.7 In a split-face investigation, 15 participants with bilateral symmetric areas of 2 to 10 AKs on the face or scalp were randomized to receive fractional CO2 laser and MAL-PDT combination therapy on 1 treatment side and conventional MAL-PDT on the other side.7 The AFXL-PDT treatment side received laser ablation with immediate subsequent application of MAL to both treatment sides under 3-hour occlusion. At baseline, 103 AKs were treated by AFXL-PDT and 109 AKs were treated with conventional PDT. At 3-month follow-up, the AFXL-PDT treatment group exhibited a significantly higher rate of complete response (90%) compared to the conventional PDT group (67%)(P=.0002).7
Like the CO2 laser, the Er:YAG laser has demonstrated superior results when used in combination with PDT to treat field cancerization compared to either treatment alone. In a comparison study, 93 patients with 2 to 10 AK lesions on the face or scalp were randomized to treatment with AFXL (Er:YAG laser) and MAL-PDT with 3-hour occlusion, AFXL (Er:YAG laser) and MAL-PDT with 2-hour occlusion, and MAL-PDT with 3-hour occlusion.8 A total of 440 baseline AK lesions on the face or scalp were treated. At 3-month follow-up, the AFXL-PDT (3-hour occlusion) group had the highest rate of complete response (91.7%), compared to 76.8% (P=.001) in the AFXL-PDT (2-hour occlusion) and 65.6% (P=.001) in the PDT groups, regardless of the grade of AK lesion. The AFXL-PDT (2-hour occlusion) treatment was also superior to PDT alone (P=.038). These findings were sustained at 12-month follow-up (84.8% in the AFXL-PDT [3-hour occlusion] group [P<.001, compared to others]; 67.5% in the AFXL-PDT [2-hour occlusion] group [P<.001, compared to 3-hour PDT]; 51.1% in the PDT group). Importantly, the AK lesion recurrence rate was also lowest in the AFL-PDT (3-hour occlusion) group (7.5% vs 12.1% and 22.1% in the AFXL-PDT [2-hour occlusion] and PDT groups, respectively; P=.007).8
Combination therapy with AFXL and daylight PDT (dPDT) may improve the tolerability of PDT and the efficacy rate of field therapy in organ transplant recipients. One study demonstrated the superiority of this combination therapy in a population of 16 organ transplant recipients on immunosuppressants with at least 2 moderate to severely thick AKs in each of 4 comparable areas in the same anatomic region.9 The 4 areas were randomized to a single session of AFXL-dPDT, dPDT alone, conventional PDT, or AFXL alone. Ablation was performed with a fractional Er:YAG laser. The AFXL-dPDT and dPDT alone groups received MAL for 2.5 hours without occlusion, and the conventional PDT group received MAL for 3 hours with occlusion. Daylight exposure in dPDT groups was initiated 30 minutes after MAL application for 2 hours total. A baseline total of 542 AKs were treated. At 3-month follow-up, the complete response rate was highest for the AFXL-dPDT group (74%) compared to dPDT alone (46%; P=.0262), conventional PDT (50%; P=.042), and AFXL alone (5%; P=.004). Pain scores for AFXL–dPDT and dPDT alone were significantly lower than for conventional PDT and AFXL alone (P<.001).9
Nonablative Lasers
By heating the dermis to induce neogenesis without destruction, nonablative lasers offer superior healing times compared to their ablative counterparts. Multiple treatments with nonablative lasers may be necessary for maximal effect. Four nonablative laser devices have demonstrated efficacy in the treatment of multiple AKs10-14: (1) the Q-switched 1064-nm Nd:YAG laser, with or without a 532-nm potassium titanyl phosphate (KTP) laser; (2) the 1540-nm fractional erbium glass laser; (3) the 1550-nm fractional erbium-doped fiber laser; and (4) the 1927-nm fractional thulium laser (Table 3).
In a proof-of-concept study of the Q-switched Nd:YAG laser with the 532-nm KTP laser, 1 treatment session induced full remission of AKs in 10 patients at follow-up day 20, although the investigator did not grade improvement on a numerical scale.10 In a study of the fractional Q-switched 1064-nm Nd:YAG laser alone, 6 patients with trace or mild AKs received 4 treatment sessions at approximately 2-week intervals.14 All but 1 patient (who had trace AKs) had no AKs at 3-month follow-up.
The efficacy of the 1540-nm fractional erbium glass laser was examined in 17 participants with investigator-rated moderate-to-severe AK involvement of the scalp and face.12 Participants were given 2 or 3 treatment sessions at 3- to 4-week intervals and were graded by blinded dermatologists on a quartile scale of 0 (no improvement), 1 (1%–25% improvement), 2 (26%–50% improvement), 3 (51%–75% improvement), or 4 (76%–100% improvement). At 3 months posttreatment, the average grade of improvement was 3.4.12
The 1550-nm fractional erbium-doped fiber laser was tested in 14 men with multiple facial AKs (range, 9–44 AKs [mean, 22.1 AKs]).11 Participants received 5 treatment sessions at 2- to 4-week intervals, with majority energies used at 70 MJ and treatment level 11. The mean AK count was reduced significantly by 73.1%, 66.2%, and 55.6% at 1-, 3-, and 6-month follow-up, respectively (P<.001).11
The 1927-nm fractional thulium laser showed promising results in 24 participants with facial AKs.13 Participants received up to 4 treatment sessions at intervals from 2 to 6 weeks at the investigators’ discretion. At baseline, patients had an average of 14.04 facial AKs. At 1-, 3-, and 6-month follow-up, participants exhibited 91.3%, 87.3%, and 86.6% reduction in AK counts, respectively. The mean AK count at 3-month follow-up was 1.88.13
Due to limited sample sizes and/or lack of quantifiable results and controls in these studies, more studies are needed to fully elucidate the role of nonablative lasers in the treatment of AK.
Future Directions
Iontophoresis involves the noninvasive induction of an electrical current to facilitate ion movement through the skin and may be a novel method to boost the efficacy of current field therapies. In the first known study of its kisnd, iontophoresis-assisted AFXL-PDT was found to be noninferior to conventional AFXL-PDT15; however, additional studies demonstrating its superiority are needed before more widespread clinical use is considered.
Pretreatment with AFXL prior to topical field-directed therapies also has been proposed.16 In a case series of 13 patients, combination therapy with AFXL and ingenol mebutate was shown to be superior to ingenol mebutate alone (AK clearance rate, 89.2% vs 72.1%, respectively; P<.001).16 Randomized studies with longer follow-up time are needed.
Conclusion
Ablative and nonablative laser systems have yielded limited data about their potential as monotherapies for treatment of multiple AKs and are unlikely to replace topical agents and PDT as a first-line modality in field-directed treatment at this time. More studies with a larger number of participants and long-term follow-up are needed for further clarification of efficacy, safety, and clinical feasibility. Nevertheless, fractional ablative lasers in combination with PDT have shown robust efficacy and a favorable safety profile for treatment of multiple AKs.6-9 Further, this combination therapy exhibited a superior clearance rate and lower lesion recurrence in organ transplant recipients—a demographic that classically is difficult to treat.6-9
With continued rapid evolution of laser systems and more widespread use in dermatology, monotherapy and combination therapy may offer a dynamic new option in field cancerization that can decrease disease burden and treatment frequency.
In cutaneous field cancerization, focal treatments such as cryotherapy are impractical, thus necessitating the use of field-directed therapies over the lesion and the surrounding skin field. Although evidence-based guidelines do not exist, field-directed therapy has been proposed in cases of 3 or more actinic keratoses (AKs) in a 25-cm2 area or larger.1 It can be further speculated that patients who are vulnerable to aggressive phenotypes of cutaneous malignancies, such as those with a genodermatosis or who are immunocompromised, necessitate a higher index of suspicion for field effect with even 1 or 2 AKs.
Current field-directed therapies include topical agents (imiquimod, fluorouracil, ingenol mebutate, and diclo-fenac), photodynamic therapy (PDT), and resurfacing procedures (lasers, chemical peels, dermabrasion). Although topical agents and PDT currently are gold standards in field treatment, the use of energy-based devices (ie, ablative and nonablative lasers) are attractive options as monotherapy or as part of a combination therapy. These devices are attractive options for field-directed therapy because they offer defined, customizable control of settings, allowing for optimal cosmesis and precision of therapy.
Principally, lasers function by damaging skin tissue to induce resurfacing, neocollagenesis, and vascular restructuring. Fractional versions of ablative and nonablative systems are available to target a fraction of the treatment area in evenly spaced microthermal zones and to minimize overall thermal damage.2
Given recent advances in laser systems and numerous investigations reported in the literature, a review of ablative and nonablative lasers that have been studied as treatment options for cutaneous field cancerization is provided, with a focus on treatment efficacy.
Ablative Lasers
Ablative lasers operate at higher wavelengths than nonablative lasers to destroy epidermal and dermal tissue. The 10,600-nm carbon dioxide (CO2) and 2940-nm Er:YAG lasers have been heavily investigated for field therapy for multiple AKs, both as monotherapies (Table 1) and in combination with PDT (Table 2).
Monotherapy
One randomized trial with 5-year follow-up compared the efficacy of full-face pulsed CO2 laser therapy, full-face trichloroacetic acid (TCA) peel 30%, and fluorouracil cream 5% (twice daily for 3 weeks) on AKs on the face and head.3 Thirty-one participants were randomized to the 3 treatment arms and a negative control arm. The mean AK counts at baseline for the CO2, TCA, and fluorouracil treatment groups were 78.0, 83.7, and 61.8, respectively. At 3-month follow-up, all treatment groups had significant reductions in the mean AK count from baseline (CO2 group, 92% [P=.03]; TCA group, 89% [P=.004]; fluorouracil group, 83% [P=.008]). No significant differences in efficacy among the treatment groups were noted. All 3 treatment groups had a demonstrably lower incidence of nonmelanoma skin cancer over 5-year follow-up compared to the control group (P<.001).3
In contrast to these promising results, the pulsed CO2 laser showed only short-term efficacy in a split-face study of 12 participants with at least 5 facial or scalp AKs on each of 2 symmetric facial sides who were randomized to 1 treatment side.4 At 1-month follow-up, the treatment side exhibited significantly fewer AKs compared to the control side (47% vs 71% at baseline; P=.01), but the improvement was not sustained at 3-month follow-up (49% vs 57%; P=.47).4
In another study, the CO2 laser was found to be inferior to 5-aminolevulinic acid PDT.5 Twenty-one participants who had at least 4 AKs in each symmetric half of a body region (head, hands, forearms) were randomized to PDT on 1 side and CO2 laser therapy on the other. Median baseline AK counts for the PDT and CO2 laser groups were 6 and 8, respectively. Both treatment groups exhibited significant median AK reduction from baseline 4 weeks posttreatment (PDT group, 82.1% [P<.05], CO2 laser group, 100% [P<.05]); however. at 3 months posttreatment the PDT group had significantly higher absolute (P=.0155) and relative (P=.0362) reductions in AK count compared to the CO2 laser group. One participant received a topical antibiotic for superficial infection on the PDT treatment side.5
Many questions remain regarding the practical application of laser ablation monotherapy for multiple AKs. More studies are needed to determine the practicality and long-term clinical efficacy of these devices.
PDT Combination Therapy
Laser ablation may be combined with PDT to increase efficacy and prolong remission rates. In fact, laser ablation may be thought of as a physical drug-delivery system to boost uptake of topical agents—in this case, aminolevulinic acid and methyl aminolevulinate (MAL)—given that it disrupts the skin barrier.
In a comparative study of ablative fractional laser (AFXL)–assisted PDT and AFXL alone in 10 organ transplant recipients on immunosuppression with at least 5 AKs on each dorsal hand, participants were randomized to AFXL-PDT on one treatment side and PDT on the other side.6 Participants received AFXL in an initial lesion-directed pass and then a second field-directed pass of a fractional CO2 laser. After AFXL exposure, methyl aminolevulinate was applied to the AFXL-PDT treatment side, with 3-hour occlusion. A total of 680 AKs were treated (335 in the AFXL-PDT group, 345 in the PDT group); results were stratified by the clinical grade of the lesion (1, slightly palpable; 2, moderately thick; 3, very thick or obvious). At 4-month follow-up, the AFXL-PDT group had a significantly higher median complete response rate of 73% compared to 31% in the AFXL group (P=.002). Interestingly, AFXL-PDT was also significantly more efficacious compared to AFXL for grades 1 (80% vs 37%; P=.02) and 2 (53% vs 7%, P=.009) AKs but not grade 3 AKs (4% vs 0%, P=.17).6
The combination of fractional CO2 laser and PDT also demonstrated superiority to PDT.7 In a split-face investigation, 15 participants with bilateral symmetric areas of 2 to 10 AKs on the face or scalp were randomized to receive fractional CO2 laser and MAL-PDT combination therapy on 1 treatment side and conventional MAL-PDT on the other side.7 The AFXL-PDT treatment side received laser ablation with immediate subsequent application of MAL to both treatment sides under 3-hour occlusion. At baseline, 103 AKs were treated by AFXL-PDT and 109 AKs were treated with conventional PDT. At 3-month follow-up, the AFXL-PDT treatment group exhibited a significantly higher rate of complete response (90%) compared to the conventional PDT group (67%)(P=.0002).7
Like the CO2 laser, the Er:YAG laser has demonstrated superior results when used in combination with PDT to treat field cancerization compared to either treatment alone. In a comparison study, 93 patients with 2 to 10 AK lesions on the face or scalp were randomized to treatment with AFXL (Er:YAG laser) and MAL-PDT with 3-hour occlusion, AFXL (Er:YAG laser) and MAL-PDT with 2-hour occlusion, and MAL-PDT with 3-hour occlusion.8 A total of 440 baseline AK lesions on the face or scalp were treated. At 3-month follow-up, the AFXL-PDT (3-hour occlusion) group had the highest rate of complete response (91.7%), compared to 76.8% (P=.001) in the AFXL-PDT (2-hour occlusion) and 65.6% (P=.001) in the PDT groups, regardless of the grade of AK lesion. The AFXL-PDT (2-hour occlusion) treatment was also superior to PDT alone (P=.038). These findings were sustained at 12-month follow-up (84.8% in the AFXL-PDT [3-hour occlusion] group [P<.001, compared to others]; 67.5% in the AFXL-PDT [2-hour occlusion] group [P<.001, compared to 3-hour PDT]; 51.1% in the PDT group). Importantly, the AK lesion recurrence rate was also lowest in the AFL-PDT (3-hour occlusion) group (7.5% vs 12.1% and 22.1% in the AFXL-PDT [2-hour occlusion] and PDT groups, respectively; P=.007).8
Combination therapy with AFXL and daylight PDT (dPDT) may improve the tolerability of PDT and the efficacy rate of field therapy in organ transplant recipients. One study demonstrated the superiority of this combination therapy in a population of 16 organ transplant recipients on immunosuppressants with at least 2 moderate to severely thick AKs in each of 4 comparable areas in the same anatomic region.9 The 4 areas were randomized to a single session of AFXL-dPDT, dPDT alone, conventional PDT, or AFXL alone. Ablation was performed with a fractional Er:YAG laser. The AFXL-dPDT and dPDT alone groups received MAL for 2.5 hours without occlusion, and the conventional PDT group received MAL for 3 hours with occlusion. Daylight exposure in dPDT groups was initiated 30 minutes after MAL application for 2 hours total. A baseline total of 542 AKs were treated. At 3-month follow-up, the complete response rate was highest for the AFXL-dPDT group (74%) compared to dPDT alone (46%; P=.0262), conventional PDT (50%; P=.042), and AFXL alone (5%; P=.004). Pain scores for AFXL–dPDT and dPDT alone were significantly lower than for conventional PDT and AFXL alone (P<.001).9
Nonablative Lasers
By heating the dermis to induce neogenesis without destruction, nonablative lasers offer superior healing times compared to their ablative counterparts. Multiple treatments with nonablative lasers may be necessary for maximal effect. Four nonablative laser devices have demonstrated efficacy in the treatment of multiple AKs10-14: (1) the Q-switched 1064-nm Nd:YAG laser, with or without a 532-nm potassium titanyl phosphate (KTP) laser; (2) the 1540-nm fractional erbium glass laser; (3) the 1550-nm fractional erbium-doped fiber laser; and (4) the 1927-nm fractional thulium laser (Table 3).
In a proof-of-concept study of the Q-switched Nd:YAG laser with the 532-nm KTP laser, 1 treatment session induced full remission of AKs in 10 patients at follow-up day 20, although the investigator did not grade improvement on a numerical scale.10 In a study of the fractional Q-switched 1064-nm Nd:YAG laser alone, 6 patients with trace or mild AKs received 4 treatment sessions at approximately 2-week intervals.14 All but 1 patient (who had trace AKs) had no AKs at 3-month follow-up.
The efficacy of the 1540-nm fractional erbium glass laser was examined in 17 participants with investigator-rated moderate-to-severe AK involvement of the scalp and face.12 Participants were given 2 or 3 treatment sessions at 3- to 4-week intervals and were graded by blinded dermatologists on a quartile scale of 0 (no improvement), 1 (1%–25% improvement), 2 (26%–50% improvement), 3 (51%–75% improvement), or 4 (76%–100% improvement). At 3 months posttreatment, the average grade of improvement was 3.4.12
The 1550-nm fractional erbium-doped fiber laser was tested in 14 men with multiple facial AKs (range, 9–44 AKs [mean, 22.1 AKs]).11 Participants received 5 treatment sessions at 2- to 4-week intervals, with majority energies used at 70 MJ and treatment level 11. The mean AK count was reduced significantly by 73.1%, 66.2%, and 55.6% at 1-, 3-, and 6-month follow-up, respectively (P<.001).11
The 1927-nm fractional thulium laser showed promising results in 24 participants with facial AKs.13 Participants received up to 4 treatment sessions at intervals from 2 to 6 weeks at the investigators’ discretion. At baseline, patients had an average of 14.04 facial AKs. At 1-, 3-, and 6-month follow-up, participants exhibited 91.3%, 87.3%, and 86.6% reduction in AK counts, respectively. The mean AK count at 3-month follow-up was 1.88.13
Due to limited sample sizes and/or lack of quantifiable results and controls in these studies, more studies are needed to fully elucidate the role of nonablative lasers in the treatment of AK.
Future Directions
Iontophoresis involves the noninvasive induction of an electrical current to facilitate ion movement through the skin and may be a novel method to boost the efficacy of current field therapies. In the first known study of its kisnd, iontophoresis-assisted AFXL-PDT was found to be noninferior to conventional AFXL-PDT15; however, additional studies demonstrating its superiority are needed before more widespread clinical use is considered.
Pretreatment with AFXL prior to topical field-directed therapies also has been proposed.16 In a case series of 13 patients, combination therapy with AFXL and ingenol mebutate was shown to be superior to ingenol mebutate alone (AK clearance rate, 89.2% vs 72.1%, respectively; P<.001).16 Randomized studies with longer follow-up time are needed.
Conclusion
Ablative and nonablative laser systems have yielded limited data about their potential as monotherapies for treatment of multiple AKs and are unlikely to replace topical agents and PDT as a first-line modality in field-directed treatment at this time. More studies with a larger number of participants and long-term follow-up are needed for further clarification of efficacy, safety, and clinical feasibility. Nevertheless, fractional ablative lasers in combination with PDT have shown robust efficacy and a favorable safety profile for treatment of multiple AKs.6-9 Further, this combination therapy exhibited a superior clearance rate and lower lesion recurrence in organ transplant recipients—a demographic that classically is difficult to treat.6-9
With continued rapid evolution of laser systems and more widespread use in dermatology, monotherapy and combination therapy may offer a dynamic new option in field cancerization that can decrease disease burden and treatment frequency.
- Peris K, Calzavara-Pinton PG, Neri L, et al. Italian expert consensus for the management of actinic keratosis in immunocompetent patients. J Eur Acad Dermatol Venereol. 2016;30:1077-1084.
- Alexiades-Armenakas MR, Dover JS, Arndt KA. The spectrum of laser skin resurfacing: nonablative, fractional, and ablative laser resurfacing. J Am Acad Dermatol. 2008;58:719-737; quiz 738-740.
- Hantash BM, Stewart DB, Cooper ZA, et al. Facial resurfacing for nonmelanoma skin cancer prophylaxis. Arch Dermatol. 2006;142:976-982.
- Gan SD, Hsu SH, Chuang G, et al. Ablative fractional laser therapy for the treatment of actinic keratosis: a split-face study. J Am Acad Dermatol. 2016;74:387-389.
- Scola N, Terras S, Georgas D, et al. A randomized, half-side comparative study of aminolaevulinate photodynamic therapy vs. CO(2) laser ablation in immunocompetent patients with multiple actinic keratoses. Br J Dermatol. 2012;167:1366-1373.
- Helsing P, Togsverd-Bo K, Veierod MB, et al. Intensified fractional CO2 laser-assisted photodynamic therapy vs. laser alone for organ transplant recipients with multiple actinic keratoses and wart-like lesions: a randomized half-side comparative trial on dorsal hands. Br J Dermatol. 2013;169:1087-1092.
- Togsverd-Bo K, Haak CS, Thaysen-Petersen D, et al. Intensified photodynamic therapy of actinic keratoses with fractional CO2 laser: a randomized clinical trial. Br J Dermatol. 2012;166:1262-1269.
- Choi SH, Kim KH, Song KH. Efficacy of ablative fractional laser-assisted photodynamic therapy with short-incubation time for the treatment of facial and scalp actinic keratosis: 12-month follow-up results of a randomized, prospective, comparative trial. J Eur Acad Dermatol Venereol. 2015;29:1598-1605.
- Togsverd-Bo K, Lei U, Erlendsson AM, et al. Combination of ablative fractional laser and daylight-mediated photodynamic therapy for actinic keratosis in organ transplant recipients—a randomized controlled trial. Br J Dermatol. 2015;172:467-474.
- Demetriou C. Reversing precancerous actinic damage by mixing wavelengths (1064 nm, 532 nm). J Cosmet Laser Ther. 2011;13:113-119.
- Katz TM, Goldberg LH, Marquez D, et al. Nonablative fractional photothermolysis for facial actinic keratoses: 6-month follow-up with histologic evaluation. J Am Acad Dermatol. 2011;65:349-356.
- Lapidoth M, Adatto M, Halachmi S. Treatment of actinic keratoses and photodamage with non-contact fractional 1540-nm laser quasi-ablation: an ex vivo and clinical evaluation. Lasers Med Sci. 2013;28:537-542.
- Weiss ET, Brauer JA, Anolik R, et al. 1927-nm fractional resurfacing of facial actinic keratoses: a promising new therapeutic option. J Am Acad Dermatol. 2013;68:98-102.
- Gold MH, Sensing W, Biron J. Fractional Q-switched 1,064-nm laser for the treatment of photoaged-photodamaged skin. J Cosmet Laser Ther. 2014;16:69-76.
- Choi SH, Kim TH, Song KH. Efficacy of iontophoresis-assisted ablative fractional laser photodynamic therapy with short incubation time for the treatment of actinic keratosis: 12-month follow-up results of a prospective, randomised, comparative trial. Photodiagnosis Photodyn Ther. 2017;18:105-110.
- Nisticò S, Sannino M, Del Duca E, et al. Ablative fractional laser improves treatment of actinic keratoses with ingenol mebutate. Eur J Inflamm. 2016;14:200-205.
- Peris K, Calzavara-Pinton PG, Neri L, et al. Italian expert consensus for the management of actinic keratosis in immunocompetent patients. J Eur Acad Dermatol Venereol. 2016;30:1077-1084.
- Alexiades-Armenakas MR, Dover JS, Arndt KA. The spectrum of laser skin resurfacing: nonablative, fractional, and ablative laser resurfacing. J Am Acad Dermatol. 2008;58:719-737; quiz 738-740.
- Hantash BM, Stewart DB, Cooper ZA, et al. Facial resurfacing for nonmelanoma skin cancer prophylaxis. Arch Dermatol. 2006;142:976-982.
- Gan SD, Hsu SH, Chuang G, et al. Ablative fractional laser therapy for the treatment of actinic keratosis: a split-face study. J Am Acad Dermatol. 2016;74:387-389.
- Scola N, Terras S, Georgas D, et al. A randomized, half-side comparative study of aminolaevulinate photodynamic therapy vs. CO(2) laser ablation in immunocompetent patients with multiple actinic keratoses. Br J Dermatol. 2012;167:1366-1373.
- Helsing P, Togsverd-Bo K, Veierod MB, et al. Intensified fractional CO2 laser-assisted photodynamic therapy vs. laser alone for organ transplant recipients with multiple actinic keratoses and wart-like lesions: a randomized half-side comparative trial on dorsal hands. Br J Dermatol. 2013;169:1087-1092.
- Togsverd-Bo K, Haak CS, Thaysen-Petersen D, et al. Intensified photodynamic therapy of actinic keratoses with fractional CO2 laser: a randomized clinical trial. Br J Dermatol. 2012;166:1262-1269.
- Choi SH, Kim KH, Song KH. Efficacy of ablative fractional laser-assisted photodynamic therapy with short-incubation time for the treatment of facial and scalp actinic keratosis: 12-month follow-up results of a randomized, prospective, comparative trial. J Eur Acad Dermatol Venereol. 2015;29:1598-1605.
- Togsverd-Bo K, Lei U, Erlendsson AM, et al. Combination of ablative fractional laser and daylight-mediated photodynamic therapy for actinic keratosis in organ transplant recipients—a randomized controlled trial. Br J Dermatol. 2015;172:467-474.
- Demetriou C. Reversing precancerous actinic damage by mixing wavelengths (1064 nm, 532 nm). J Cosmet Laser Ther. 2011;13:113-119.
- Katz TM, Goldberg LH, Marquez D, et al. Nonablative fractional photothermolysis for facial actinic keratoses: 6-month follow-up with histologic evaluation. J Am Acad Dermatol. 2011;65:349-356.
- Lapidoth M, Adatto M, Halachmi S. Treatment of actinic keratoses and photodamage with non-contact fractional 1540-nm laser quasi-ablation: an ex vivo and clinical evaluation. Lasers Med Sci. 2013;28:537-542.
- Weiss ET, Brauer JA, Anolik R, et al. 1927-nm fractional resurfacing of facial actinic keratoses: a promising new therapeutic option. J Am Acad Dermatol. 2013;68:98-102.
- Gold MH, Sensing W, Biron J. Fractional Q-switched 1,064-nm laser for the treatment of photoaged-photodamaged skin. J Cosmet Laser Ther. 2014;16:69-76.
- Choi SH, Kim TH, Song KH. Efficacy of iontophoresis-assisted ablative fractional laser photodynamic therapy with short incubation time for the treatment of actinic keratosis: 12-month follow-up results of a prospective, randomised, comparative trial. Photodiagnosis Photodyn Ther. 2017;18:105-110.
- Nisticò S, Sannino M, Del Duca E, et al. Ablative fractional laser improves treatment of actinic keratoses with ingenol mebutate. Eur J Inflamm. 2016;14:200-205.
Practice Points
- Ablative fractional laser therapy in combination with photodynamic therapy has demonstrated increased efficacy in treating field actinic keratoses (AKs) for up to 12 months of follow-up over either modality alone.
- Ablative and nonablative lasers as monotherapy in treating field AKs require further studies with larger sample sizes to determine efficacy and safety.
Inhaled nitrous oxide for labor analgesia: Pearls from clinical experience
Nitrous oxide, a colorless, odorless gas, has long been used for labor analgesia in many countries, including the United Kingdom, Canada, throughout Europe, Australia, and New Zealand. Recently, interest in its use in the United States has increased, since the US Food and Drug Administration (FDA) approval in 2012 of simple devices for administration of nitrous oxide in a variety of locations. Being able to offer an alternative technique, other than parenteral opioids, for women who may not wish to or who cannot have regional analgesia, and for women who have delivered and need analgesia for postdelivery repair, conveys significant benefits. Risks to its use are very low, although the quality of pain relief is inferior to that offered by regional analgesic techniques. Our experience with its use since 2014 at Brigham and Women’s Hospital in Boston, Massachusetts, corroborates that reported in the literature and leads us to continue offering inhaled nitrous oxide and advocating that others do as well.1–7 When using nitrous oxide in your labor and delivery unit, or if considering its use, keep the following points in mind.
A successful inhaled nitrous oxide program requires proper patient selection
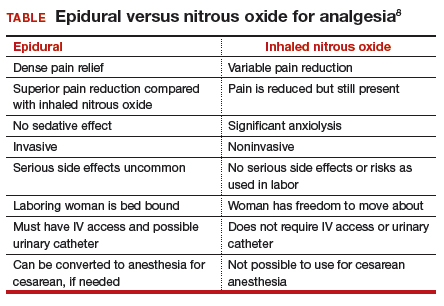
Inhaled nitrous oxide is not an epidural (TABLE).8 The pain relief is clearly inferior to that of an epidural. Inhaled nitrous oxide will not replace epidurals or even have any effect on the epidural rate at a particular institution.6 However, the use of inhaled nitrous oxide for labor analgesia has a long track record of safety (albeit with moderate efficacy for selected patients) for many years in many countries around the world. Inhaled nitrous oxide is a valuable addition to the options we can offer patients:
- who are poor responders to opioid medication or who have high opioid tolerance
- with certain disorders of coagulation
- with chronic pain or anxiety
- who for other reasons need to consider alternatives or adjuncts to neuraxial analgesia.
Although it is important to be realistic regarding the expectations of analgesia quality offered by this agent,7 compared with other agents we have tried, it has less adverse effects, is economically reasonable, and has no proven impact on neonatal outcomes.
No significant complications with inhaled nitrous oxide have been reported
Systematic reviews did not report any significant complications to either mother or newborn.1,2 Our personal experiences corroborate this, as no complications have been associated with its frequent use at Brigham and Women’s Hospital. Reported adverse effects are mild. The incidence of nausea is 13%, dizziness is 3% to 5%, and drowsiness is 4%; these rates are hard to detect over the baseline rates of those side effects associated with labor and delivery alone.1 Many other centers have now adopted the use of this agent, with several hundred locations now offering inhaled nitrous oxide for labor analgesia in the United States.
Practical use of inhaled nitrous oxide is relatively simple
Several vendors offer portable, user-friendly, cost-effective equipment that is appropriate for labor and delivery use. All devices are structured in demand-valve modality, meaning that the patient must initiate a breath in order to open a valve that allows gas to flow. Cessation of the inspiratory effort closes the valve, thus preventing the free flow of gas into the ambient atmosphere of the room. The devices generally include a tank with nitrous oxide as well as a source of oxygen. Most devices designed for labor and delivery provide a fixed mixture of 50% nitrous oxide and 50% oxygen, with fail-safe mechanisms to allow increased oxygen delivery in the event of failure or depletion of the nitrous supply. All modern, FDA–approved devices include effective scavenging systems, such that expired gases are vented outside (generally via room suction), which prevents occupational exposure to low levels of nitrous oxide.
Inhaled nitrous oxide for labor pain must be patient controlled
An essential feature of the use of inhaled nitrous oxide for labor analgesia is that it must be considered a patient-controlled system. Patients have an option to use either a mask or a mouthpiece, according to their preferences and comfort. The patient must hold the mask or mouthpiece herself; it is neither appropriate nor safe for anyone else, such as a nurse, family member, or labor support personnel, to assist with this task.
Some coordination with the nurse is essential for optimal timing of administration. Onset of a therapeutic level of pain relief is generally 30 to 60 seconds after inhalation has begun, with rapid resolution after cessation of the inhalation. The patient should thus initiate the inspiration of the gas at the earliest signs of onset of a contraction, so as to achieve maximal analgesia at the peak of the contraction. Waiting until the peak of the contraction to initiate inhalation of the nitrous oxide will not provide effective analgesia, yet will result in sedation after the contraction has ended.
Read about patient satisfaction with inhaled nitrous oxide.
No oversight by an anesthesiologist is required
The Centers for Medicare and Medicaid Services (CMS) produced a clarification statement for definitions of “anesthesia services” (42 CFR 482.52)9 that may be offered by a hospital, based on American Society of Anesthesiologists (ASA) definitions. CMS, consistent with ASA guidelines, does not define moderate or conscious sedation as “anesthesia,” thus direct oversight by an anesthesiologist is not required. Furthermore, the definition of “minimal sedation,” which is where 50% concentration delivery of inhaled nitrous oxide would be categorized, also does not meet this requirement by CMS.
Women who use inhaled nitrous oxide for labor pain typically are satisfied with its use
The use of analog pain scale measurements may not be appropriate in a setting where dissociation from pain might be the primary beneficial effect. Measurements of maternal satisfaction with their analgesic experience support this. The experiences at Vanderbilt University and Brigham and Women’s Hospital show that, while pain relief is limited, like reported in systematic reviews, maternal satisfaction scores for labor analgesia are not different among women who receive inhaled nitrous oxide analgesia, neuraxial analgesia, and those who transition from nitrous to neuraxial analgesia. In fact, published evidence supports extraordinarily high satisfaction in women who plan to use inhaled nitrous oxide, and actually successfully do so, despite only limited degrees of pain relief.10,11 Work to identify the characteristics of women who report success with inhaled nitrous oxide use needs to be performed so that patients can be better selected and informed when making analgesic choices.
Animal research on inhaled nitrous oxide may not translate well to human neonates
A very recent task force convened by the European Society of Anaesthesiology (ESA) addressed some of the potential concerns about inhaled nitrous oxide analgesia.12 Per their report:
“the potential teratogenic effect of N2O observed in experimental models cannot be extrapolated to humans. There is a lack of evidence for an association between N2O and reproductive toxicity. The incidence of health hazards and abortion was not shown to be higher in women exposed to, or spouses of men exposed to N2O than those who were not so exposed. Moreover, the incidence of congenital malformations was not higher among women who received N2O for anaesthesia during the first trimester of pregnancy nor during anaesthesia management for cervical cerclage, nor for surgery in the first two trimesters of pregnancy.”
There is a theoretical concern of an increase in neuronal apoptosis in neonates, demonstrated in laboratory animals in anesthetic concentrations, but the human relevance of this is not clear, since the data on animal developmental neurotoxicity is generally combined with data wherein potent inhalational anesthetic agents were also used, not nitrous oxide alone.13 The analgesic doses and time of exposure of inhaled nitrous oxide administered for labor analgesia are well below those required for these changes, as subanesthetic doses are associated with minimal changes, if any, in laboratory animals.
No labor analgesic is without the potential for fetal effects, and alternative labor analgesics such as systemic opioids in higher doses also may have potential adverse effects on the fetus, such as fetal heart rate effects or early tone, alertness, and breastfeeding difficulties. The low solubility and short half-life of inhaled nitrous oxide contribute to low absorption by tissues, thus contributing to the safety of this agent. Nitrous oxide via inhalation for sedation during elective cesarean has been reported to show no adverse effects on neonatal Apgar scores.14
Modern equipment keeps occupational exposure to nitrous oxide safe
One retrospective review of women exposed to high concentrations of inhaled nitrous oxide reported reduced fertility.15 However, the only effects on fertility were seen when nitrous was used without scavenging equipment, and in high concentrations. Moreover, that study examined dental offices, where nitrous was free flowing during procedures—quite a different setting than the intermittent inhalation, demand-valve modality as is used during labor—and when using appropriate modern, FDA-approved equipment, and scavenging devices. Per the recent ESA task force12:
“Members of the task force agreed that, despite theoretical concerns and laboratory data, there is no evidence indicating that the use of N2O in a clinically relevant setting would increase health risk in patients or providers exposed to this drug. With the ubiquitous availability of scavenging systems in the modern operating room, the health concern for medical staff has decreased dramatically. Properly operating scavenging systems reduce N2O concentrations by more than 70%, thereby efficiently keeping ambient N2O levels well below official limits.”
The ESA task force concludes: “An extensive amount of clinical evidence indicates that N2O can be used safely for procedural pain management, for labour pain, and for anxiolysis and sedation in dentistry.”12
Two important reminders
Inhaled nitrous oxide has been a central component of the labor pain relief menu in most of the rest of the world for decades, and the safety record is impeccable. This agent has now had extensive and growing experience in American maternity units. Remember 2 critical points: 1) patient selection is key, 2) analgesia is not like that provided by regional anesthetic techniques such as an epidural.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- Likis FE, Andrews JC, Collins MR, et al. Nitrous oxide for the management of labor pain: a systematic review. Anesth Analg. 2014;118(1):153-167.
- Rosen MA. Nitrous oxide for relief of labor pain: a systematic review. Am J Obstet Gynecol. 2002;186(5 suppl nature):S110-S126.
- Angle P, Landy CK, Charles C. Phase 1 development of an index to measure the quality of neuraxial labour analgesia: exploring the perspectives of childbearing women. Can J Anaesth. 2010;57(5):468-478.
- Migliaccio L, Lawton R, Leeman L, Holbrook A. Initiating intrapartum nitrous oxide in an academic hospital: considerations and challenges. J Midwifery Womens Health. 2017;62(3):358-362.
- Markley JC, Rollins MD. Non-neuraxial labor analgesia: options. Clin Obstet Gynecol. 2017;60(2);350-364.
- Bobb LE, Farber MK, McGovern C, Camann W. Does nitrous oxide labor analgesia influence the pattern of neuraxial analgesia usage? An impact study at an academic medical center. J Clin Anesth. 2016;35:54-57.
- Sutton CD, Butwick AJ, Riley ET, Carvalho B. Nitrous oxide for labor analgesia: utilization and predictors of conversion to neuraxial analgesia. J Clin Anesth. 2017;40:40-45.
- Collins MR, Starr SA, Bishop JT, Baysinger CL. Nitrous oxide for labor analgesia: expanding analgesic options for women in the United States. Rev Obstet Gynecol. 2012;5(3-4):e126-e131.
- 42 CFR 482.52 - Condition of participation: Anesthesia services. US Government Publishing Office website. https://www.gpo.gov/fdsys/granule/CFR-2011-title42-vol5/CFR-2011-title42-vol5-sec482-52. Accessed April 16, 2018.
- Richardson MG, Lopez BM, Baysinger CL, Shotwell MS, Chestnut DH. Nitrous oxide during labor: maternal satisfaction does not depend exclusively on analgesic effectiveness. Anesth Analg. 2017;124(2):548-553.
- Camann W. Pain, pain relief, satisfaction, and excellence in obstetric anesthesia: a surprisingly complex relationship. Anesth Analg. 2017;124(2):383-385.
- European Society of Anaesthesiology Task Force on Use of Nitrous Oxide in Clinical Anaesthetic Practice. The current place of nitrous oxide in clinical practice: an expert opinion-based task force consensus statement of the European Society of Anaesthesiology. Eur J Anaesthesiol. 2015;32(8):517-520.
- Rappaport B, Mellon RD, Simone A, Woodcock J. Defining safe use of anesthesia in children. N Engl J Med. 2011;364(15):1387-1390.
- Vallejo MC, Phelps AL, Shepherd CJ, Kaul B, Mandell GL, Ramanathan S. Nitrous oxide anxiolysis for elective cesarean section. J Clin Anesth. 2005;17(7):543-548.
- Rowland AS, Baird DD, Weinberg CR, et al. Reduced fertility among women employed as dental assistants exposed to high levels of nitrous oxide. N Engl J Med. 1992;327(14):993-997.
Nitrous oxide, a colorless, odorless gas, has long been used for labor analgesia in many countries, including the United Kingdom, Canada, throughout Europe, Australia, and New Zealand. Recently, interest in its use in the United States has increased, since the US Food and Drug Administration (FDA) approval in 2012 of simple devices for administration of nitrous oxide in a variety of locations. Being able to offer an alternative technique, other than parenteral opioids, for women who may not wish to or who cannot have regional analgesia, and for women who have delivered and need analgesia for postdelivery repair, conveys significant benefits. Risks to its use are very low, although the quality of pain relief is inferior to that offered by regional analgesic techniques. Our experience with its use since 2014 at Brigham and Women’s Hospital in Boston, Massachusetts, corroborates that reported in the literature and leads us to continue offering inhaled nitrous oxide and advocating that others do as well.1–7 When using nitrous oxide in your labor and delivery unit, or if considering its use, keep the following points in mind.
A successful inhaled nitrous oxide program requires proper patient selection
Inhaled nitrous oxide is not an epidural (TABLE).8 The pain relief is clearly inferior to that of an epidural. Inhaled nitrous oxide will not replace epidurals or even have any effect on the epidural rate at a particular institution.6 However, the use of inhaled nitrous oxide for labor analgesia has a long track record of safety (albeit with moderate efficacy for selected patients) for many years in many countries around the world. Inhaled nitrous oxide is a valuable addition to the options we can offer patients:
- who are poor responders to opioid medication or who have high opioid tolerance
- with certain disorders of coagulation
- with chronic pain or anxiety
- who for other reasons need to consider alternatives or adjuncts to neuraxial analgesia.
Although it is important to be realistic regarding the expectations of analgesia quality offered by this agent,7 compared with other agents we have tried, it has less adverse effects, is economically reasonable, and has no proven impact on neonatal outcomes.
No significant complications with inhaled nitrous oxide have been reported
Systematic reviews did not report any significant complications to either mother or newborn.1,2 Our personal experiences corroborate this, as no complications have been associated with its frequent use at Brigham and Women’s Hospital. Reported adverse effects are mild. The incidence of nausea is 13%, dizziness is 3% to 5%, and drowsiness is 4%; these rates are hard to detect over the baseline rates of those side effects associated with labor and delivery alone.1 Many other centers have now adopted the use of this agent, with several hundred locations now offering inhaled nitrous oxide for labor analgesia in the United States.
Practical use of inhaled nitrous oxide is relatively simple
Several vendors offer portable, user-friendly, cost-effective equipment that is appropriate for labor and delivery use. All devices are structured in demand-valve modality, meaning that the patient must initiate a breath in order to open a valve that allows gas to flow. Cessation of the inspiratory effort closes the valve, thus preventing the free flow of gas into the ambient atmosphere of the room. The devices generally include a tank with nitrous oxide as well as a source of oxygen. Most devices designed for labor and delivery provide a fixed mixture of 50% nitrous oxide and 50% oxygen, with fail-safe mechanisms to allow increased oxygen delivery in the event of failure or depletion of the nitrous supply. All modern, FDA–approved devices include effective scavenging systems, such that expired gases are vented outside (generally via room suction), which prevents occupational exposure to low levels of nitrous oxide.
Inhaled nitrous oxide for labor pain must be patient controlled
An essential feature of the use of inhaled nitrous oxide for labor analgesia is that it must be considered a patient-controlled system. Patients have an option to use either a mask or a mouthpiece, according to their preferences and comfort. The patient must hold the mask or mouthpiece herself; it is neither appropriate nor safe for anyone else, such as a nurse, family member, or labor support personnel, to assist with this task.
Some coordination with the nurse is essential for optimal timing of administration. Onset of a therapeutic level of pain relief is generally 30 to 60 seconds after inhalation has begun, with rapid resolution after cessation of the inhalation. The patient should thus initiate the inspiration of the gas at the earliest signs of onset of a contraction, so as to achieve maximal analgesia at the peak of the contraction. Waiting until the peak of the contraction to initiate inhalation of the nitrous oxide will not provide effective analgesia, yet will result in sedation after the contraction has ended.
Read about patient satisfaction with inhaled nitrous oxide.
No oversight by an anesthesiologist is required
The Centers for Medicare and Medicaid Services (CMS) produced a clarification statement for definitions of “anesthesia services” (42 CFR 482.52)9 that may be offered by a hospital, based on American Society of Anesthesiologists (ASA) definitions. CMS, consistent with ASA guidelines, does not define moderate or conscious sedation as “anesthesia,” thus direct oversight by an anesthesiologist is not required. Furthermore, the definition of “minimal sedation,” which is where 50% concentration delivery of inhaled nitrous oxide would be categorized, also does not meet this requirement by CMS.
Women who use inhaled nitrous oxide for labor pain typically are satisfied with its use
The use of analog pain scale measurements may not be appropriate in a setting where dissociation from pain might be the primary beneficial effect. Measurements of maternal satisfaction with their analgesic experience support this. The experiences at Vanderbilt University and Brigham and Women’s Hospital show that, while pain relief is limited, like reported in systematic reviews, maternal satisfaction scores for labor analgesia are not different among women who receive inhaled nitrous oxide analgesia, neuraxial analgesia, and those who transition from nitrous to neuraxial analgesia. In fact, published evidence supports extraordinarily high satisfaction in women who plan to use inhaled nitrous oxide, and actually successfully do so, despite only limited degrees of pain relief.10,11 Work to identify the characteristics of women who report success with inhaled nitrous oxide use needs to be performed so that patients can be better selected and informed when making analgesic choices.
Animal research on inhaled nitrous oxide may not translate well to human neonates
A very recent task force convened by the European Society of Anaesthesiology (ESA) addressed some of the potential concerns about inhaled nitrous oxide analgesia.12 Per their report:
“the potential teratogenic effect of N2O observed in experimental models cannot be extrapolated to humans. There is a lack of evidence for an association between N2O and reproductive toxicity. The incidence of health hazards and abortion was not shown to be higher in women exposed to, or spouses of men exposed to N2O than those who were not so exposed. Moreover, the incidence of congenital malformations was not higher among women who received N2O for anaesthesia during the first trimester of pregnancy nor during anaesthesia management for cervical cerclage, nor for surgery in the first two trimesters of pregnancy.”
There is a theoretical concern of an increase in neuronal apoptosis in neonates, demonstrated in laboratory animals in anesthetic concentrations, but the human relevance of this is not clear, since the data on animal developmental neurotoxicity is generally combined with data wherein potent inhalational anesthetic agents were also used, not nitrous oxide alone.13 The analgesic doses and time of exposure of inhaled nitrous oxide administered for labor analgesia are well below those required for these changes, as subanesthetic doses are associated with minimal changes, if any, in laboratory animals.
No labor analgesic is without the potential for fetal effects, and alternative labor analgesics such as systemic opioids in higher doses also may have potential adverse effects on the fetus, such as fetal heart rate effects or early tone, alertness, and breastfeeding difficulties. The low solubility and short half-life of inhaled nitrous oxide contribute to low absorption by tissues, thus contributing to the safety of this agent. Nitrous oxide via inhalation for sedation during elective cesarean has been reported to show no adverse effects on neonatal Apgar scores.14
Modern equipment keeps occupational exposure to nitrous oxide safe
One retrospective review of women exposed to high concentrations of inhaled nitrous oxide reported reduced fertility.15 However, the only effects on fertility were seen when nitrous was used without scavenging equipment, and in high concentrations. Moreover, that study examined dental offices, where nitrous was free flowing during procedures—quite a different setting than the intermittent inhalation, demand-valve modality as is used during labor—and when using appropriate modern, FDA-approved equipment, and scavenging devices. Per the recent ESA task force12:
“Members of the task force agreed that, despite theoretical concerns and laboratory data, there is no evidence indicating that the use of N2O in a clinically relevant setting would increase health risk in patients or providers exposed to this drug. With the ubiquitous availability of scavenging systems in the modern operating room, the health concern for medical staff has decreased dramatically. Properly operating scavenging systems reduce N2O concentrations by more than 70%, thereby efficiently keeping ambient N2O levels well below official limits.”
The ESA task force concludes: “An extensive amount of clinical evidence indicates that N2O can be used safely for procedural pain management, for labour pain, and for anxiolysis and sedation in dentistry.”12
Two important reminders
Inhaled nitrous oxide has been a central component of the labor pain relief menu in most of the rest of the world for decades, and the safety record is impeccable. This agent has now had extensive and growing experience in American maternity units. Remember 2 critical points: 1) patient selection is key, 2) analgesia is not like that provided by regional anesthetic techniques such as an epidural.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
Nitrous oxide, a colorless, odorless gas, has long been used for labor analgesia in many countries, including the United Kingdom, Canada, throughout Europe, Australia, and New Zealand. Recently, interest in its use in the United States has increased, since the US Food and Drug Administration (FDA) approval in 2012 of simple devices for administration of nitrous oxide in a variety of locations. Being able to offer an alternative technique, other than parenteral opioids, for women who may not wish to or who cannot have regional analgesia, and for women who have delivered and need analgesia for postdelivery repair, conveys significant benefits. Risks to its use are very low, although the quality of pain relief is inferior to that offered by regional analgesic techniques. Our experience with its use since 2014 at Brigham and Women’s Hospital in Boston, Massachusetts, corroborates that reported in the literature and leads us to continue offering inhaled nitrous oxide and advocating that others do as well.1–7 When using nitrous oxide in your labor and delivery unit, or if considering its use, keep the following points in mind.
A successful inhaled nitrous oxide program requires proper patient selection
Inhaled nitrous oxide is not an epidural (TABLE).8 The pain relief is clearly inferior to that of an epidural. Inhaled nitrous oxide will not replace epidurals or even have any effect on the epidural rate at a particular institution.6 However, the use of inhaled nitrous oxide for labor analgesia has a long track record of safety (albeit with moderate efficacy for selected patients) for many years in many countries around the world. Inhaled nitrous oxide is a valuable addition to the options we can offer patients:
- who are poor responders to opioid medication or who have high opioid tolerance
- with certain disorders of coagulation
- with chronic pain or anxiety
- who for other reasons need to consider alternatives or adjuncts to neuraxial analgesia.
Although it is important to be realistic regarding the expectations of analgesia quality offered by this agent,7 compared with other agents we have tried, it has less adverse effects, is economically reasonable, and has no proven impact on neonatal outcomes.
No significant complications with inhaled nitrous oxide have been reported
Systematic reviews did not report any significant complications to either mother or newborn.1,2 Our personal experiences corroborate this, as no complications have been associated with its frequent use at Brigham and Women’s Hospital. Reported adverse effects are mild. The incidence of nausea is 13%, dizziness is 3% to 5%, and drowsiness is 4%; these rates are hard to detect over the baseline rates of those side effects associated with labor and delivery alone.1 Many other centers have now adopted the use of this agent, with several hundred locations now offering inhaled nitrous oxide for labor analgesia in the United States.
Practical use of inhaled nitrous oxide is relatively simple
Several vendors offer portable, user-friendly, cost-effective equipment that is appropriate for labor and delivery use. All devices are structured in demand-valve modality, meaning that the patient must initiate a breath in order to open a valve that allows gas to flow. Cessation of the inspiratory effort closes the valve, thus preventing the free flow of gas into the ambient atmosphere of the room. The devices generally include a tank with nitrous oxide as well as a source of oxygen. Most devices designed for labor and delivery provide a fixed mixture of 50% nitrous oxide and 50% oxygen, with fail-safe mechanisms to allow increased oxygen delivery in the event of failure or depletion of the nitrous supply. All modern, FDA–approved devices include effective scavenging systems, such that expired gases are vented outside (generally via room suction), which prevents occupational exposure to low levels of nitrous oxide.
Inhaled nitrous oxide for labor pain must be patient controlled
An essential feature of the use of inhaled nitrous oxide for labor analgesia is that it must be considered a patient-controlled system. Patients have an option to use either a mask or a mouthpiece, according to their preferences and comfort. The patient must hold the mask or mouthpiece herself; it is neither appropriate nor safe for anyone else, such as a nurse, family member, or labor support personnel, to assist with this task.
Some coordination with the nurse is essential for optimal timing of administration. Onset of a therapeutic level of pain relief is generally 30 to 60 seconds after inhalation has begun, with rapid resolution after cessation of the inhalation. The patient should thus initiate the inspiration of the gas at the earliest signs of onset of a contraction, so as to achieve maximal analgesia at the peak of the contraction. Waiting until the peak of the contraction to initiate inhalation of the nitrous oxide will not provide effective analgesia, yet will result in sedation after the contraction has ended.
Read about patient satisfaction with inhaled nitrous oxide.
No oversight by an anesthesiologist is required
The Centers for Medicare and Medicaid Services (CMS) produced a clarification statement for definitions of “anesthesia services” (42 CFR 482.52)9 that may be offered by a hospital, based on American Society of Anesthesiologists (ASA) definitions. CMS, consistent with ASA guidelines, does not define moderate or conscious sedation as “anesthesia,” thus direct oversight by an anesthesiologist is not required. Furthermore, the definition of “minimal sedation,” which is where 50% concentration delivery of inhaled nitrous oxide would be categorized, also does not meet this requirement by CMS.
Women who use inhaled nitrous oxide for labor pain typically are satisfied with its use
The use of analog pain scale measurements may not be appropriate in a setting where dissociation from pain might be the primary beneficial effect. Measurements of maternal satisfaction with their analgesic experience support this. The experiences at Vanderbilt University and Brigham and Women’s Hospital show that, while pain relief is limited, like reported in systematic reviews, maternal satisfaction scores for labor analgesia are not different among women who receive inhaled nitrous oxide analgesia, neuraxial analgesia, and those who transition from nitrous to neuraxial analgesia. In fact, published evidence supports extraordinarily high satisfaction in women who plan to use inhaled nitrous oxide, and actually successfully do so, despite only limited degrees of pain relief.10,11 Work to identify the characteristics of women who report success with inhaled nitrous oxide use needs to be performed so that patients can be better selected and informed when making analgesic choices.
Animal research on inhaled nitrous oxide may not translate well to human neonates
A very recent task force convened by the European Society of Anaesthesiology (ESA) addressed some of the potential concerns about inhaled nitrous oxide analgesia.12 Per their report:
“the potential teratogenic effect of N2O observed in experimental models cannot be extrapolated to humans. There is a lack of evidence for an association between N2O and reproductive toxicity. The incidence of health hazards and abortion was not shown to be higher in women exposed to, or spouses of men exposed to N2O than those who were not so exposed. Moreover, the incidence of congenital malformations was not higher among women who received N2O for anaesthesia during the first trimester of pregnancy nor during anaesthesia management for cervical cerclage, nor for surgery in the first two trimesters of pregnancy.”
There is a theoretical concern of an increase in neuronal apoptosis in neonates, demonstrated in laboratory animals in anesthetic concentrations, but the human relevance of this is not clear, since the data on animal developmental neurotoxicity is generally combined with data wherein potent inhalational anesthetic agents were also used, not nitrous oxide alone.13 The analgesic doses and time of exposure of inhaled nitrous oxide administered for labor analgesia are well below those required for these changes, as subanesthetic doses are associated with minimal changes, if any, in laboratory animals.
No labor analgesic is without the potential for fetal effects, and alternative labor analgesics such as systemic opioids in higher doses also may have potential adverse effects on the fetus, such as fetal heart rate effects or early tone, alertness, and breastfeeding difficulties. The low solubility and short half-life of inhaled nitrous oxide contribute to low absorption by tissues, thus contributing to the safety of this agent. Nitrous oxide via inhalation for sedation during elective cesarean has been reported to show no adverse effects on neonatal Apgar scores.14
Modern equipment keeps occupational exposure to nitrous oxide safe
One retrospective review of women exposed to high concentrations of inhaled nitrous oxide reported reduced fertility.15 However, the only effects on fertility were seen when nitrous was used without scavenging equipment, and in high concentrations. Moreover, that study examined dental offices, where nitrous was free flowing during procedures—quite a different setting than the intermittent inhalation, demand-valve modality as is used during labor—and when using appropriate modern, FDA-approved equipment, and scavenging devices. Per the recent ESA task force12:
“Members of the task force agreed that, despite theoretical concerns and laboratory data, there is no evidence indicating that the use of N2O in a clinically relevant setting would increase health risk in patients or providers exposed to this drug. With the ubiquitous availability of scavenging systems in the modern operating room, the health concern for medical staff has decreased dramatically. Properly operating scavenging systems reduce N2O concentrations by more than 70%, thereby efficiently keeping ambient N2O levels well below official limits.”
The ESA task force concludes: “An extensive amount of clinical evidence indicates that N2O can be used safely for procedural pain management, for labour pain, and for anxiolysis and sedation in dentistry.”12
Two important reminders
Inhaled nitrous oxide has been a central component of the labor pain relief menu in most of the rest of the world for decades, and the safety record is impeccable. This agent has now had extensive and growing experience in American maternity units. Remember 2 critical points: 1) patient selection is key, 2) analgesia is not like that provided by regional anesthetic techniques such as an epidural.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- Likis FE, Andrews JC, Collins MR, et al. Nitrous oxide for the management of labor pain: a systematic review. Anesth Analg. 2014;118(1):153-167.
- Rosen MA. Nitrous oxide for relief of labor pain: a systematic review. Am J Obstet Gynecol. 2002;186(5 suppl nature):S110-S126.
- Angle P, Landy CK, Charles C. Phase 1 development of an index to measure the quality of neuraxial labour analgesia: exploring the perspectives of childbearing women. Can J Anaesth. 2010;57(5):468-478.
- Migliaccio L, Lawton R, Leeman L, Holbrook A. Initiating intrapartum nitrous oxide in an academic hospital: considerations and challenges. J Midwifery Womens Health. 2017;62(3):358-362.
- Markley JC, Rollins MD. Non-neuraxial labor analgesia: options. Clin Obstet Gynecol. 2017;60(2);350-364.
- Bobb LE, Farber MK, McGovern C, Camann W. Does nitrous oxide labor analgesia influence the pattern of neuraxial analgesia usage? An impact study at an academic medical center. J Clin Anesth. 2016;35:54-57.
- Sutton CD, Butwick AJ, Riley ET, Carvalho B. Nitrous oxide for labor analgesia: utilization and predictors of conversion to neuraxial analgesia. J Clin Anesth. 2017;40:40-45.
- Collins MR, Starr SA, Bishop JT, Baysinger CL. Nitrous oxide for labor analgesia: expanding analgesic options for women in the United States. Rev Obstet Gynecol. 2012;5(3-4):e126-e131.
- 42 CFR 482.52 - Condition of participation: Anesthesia services. US Government Publishing Office website. https://www.gpo.gov/fdsys/granule/CFR-2011-title42-vol5/CFR-2011-title42-vol5-sec482-52. Accessed April 16, 2018.
- Richardson MG, Lopez BM, Baysinger CL, Shotwell MS, Chestnut DH. Nitrous oxide during labor: maternal satisfaction does not depend exclusively on analgesic effectiveness. Anesth Analg. 2017;124(2):548-553.
- Camann W. Pain, pain relief, satisfaction, and excellence in obstetric anesthesia: a surprisingly complex relationship. Anesth Analg. 2017;124(2):383-385.
- European Society of Anaesthesiology Task Force on Use of Nitrous Oxide in Clinical Anaesthetic Practice. The current place of nitrous oxide in clinical practice: an expert opinion-based task force consensus statement of the European Society of Anaesthesiology. Eur J Anaesthesiol. 2015;32(8):517-520.
- Rappaport B, Mellon RD, Simone A, Woodcock J. Defining safe use of anesthesia in children. N Engl J Med. 2011;364(15):1387-1390.
- Vallejo MC, Phelps AL, Shepherd CJ, Kaul B, Mandell GL, Ramanathan S. Nitrous oxide anxiolysis for elective cesarean section. J Clin Anesth. 2005;17(7):543-548.
- Rowland AS, Baird DD, Weinberg CR, et al. Reduced fertility among women employed as dental assistants exposed to high levels of nitrous oxide. N Engl J Med. 1992;327(14):993-997.
- Likis FE, Andrews JC, Collins MR, et al. Nitrous oxide for the management of labor pain: a systematic review. Anesth Analg. 2014;118(1):153-167.
- Rosen MA. Nitrous oxide for relief of labor pain: a systematic review. Am J Obstet Gynecol. 2002;186(5 suppl nature):S110-S126.
- Angle P, Landy CK, Charles C. Phase 1 development of an index to measure the quality of neuraxial labour analgesia: exploring the perspectives of childbearing women. Can J Anaesth. 2010;57(5):468-478.
- Migliaccio L, Lawton R, Leeman L, Holbrook A. Initiating intrapartum nitrous oxide in an academic hospital: considerations and challenges. J Midwifery Womens Health. 2017;62(3):358-362.
- Markley JC, Rollins MD. Non-neuraxial labor analgesia: options. Clin Obstet Gynecol. 2017;60(2);350-364.
- Bobb LE, Farber MK, McGovern C, Camann W. Does nitrous oxide labor analgesia influence the pattern of neuraxial analgesia usage? An impact study at an academic medical center. J Clin Anesth. 2016;35:54-57.
- Sutton CD, Butwick AJ, Riley ET, Carvalho B. Nitrous oxide for labor analgesia: utilization and predictors of conversion to neuraxial analgesia. J Clin Anesth. 2017;40:40-45.
- Collins MR, Starr SA, Bishop JT, Baysinger CL. Nitrous oxide for labor analgesia: expanding analgesic options for women in the United States. Rev Obstet Gynecol. 2012;5(3-4):e126-e131.
- 42 CFR 482.52 - Condition of participation: Anesthesia services. US Government Publishing Office website. https://www.gpo.gov/fdsys/granule/CFR-2011-title42-vol5/CFR-2011-title42-vol5-sec482-52. Accessed April 16, 2018.
- Richardson MG, Lopez BM, Baysinger CL, Shotwell MS, Chestnut DH. Nitrous oxide during labor: maternal satisfaction does not depend exclusively on analgesic effectiveness. Anesth Analg. 2017;124(2):548-553.
- Camann W. Pain, pain relief, satisfaction, and excellence in obstetric anesthesia: a surprisingly complex relationship. Anesth Analg. 2017;124(2):383-385.
- European Society of Anaesthesiology Task Force on Use of Nitrous Oxide in Clinical Anaesthetic Practice. The current place of nitrous oxide in clinical practice: an expert opinion-based task force consensus statement of the European Society of Anaesthesiology. Eur J Anaesthesiol. 2015;32(8):517-520.
- Rappaport B, Mellon RD, Simone A, Woodcock J. Defining safe use of anesthesia in children. N Engl J Med. 2011;364(15):1387-1390.
- Vallejo MC, Phelps AL, Shepherd CJ, Kaul B, Mandell GL, Ramanathan S. Nitrous oxide anxiolysis for elective cesarean section. J Clin Anesth. 2005;17(7):543-548.
- Rowland AS, Baird DD, Weinberg CR, et al. Reduced fertility among women employed as dental assistants exposed to high levels of nitrous oxide. N Engl J Med. 1992;327(14):993-997.
Association of Dioxin and Dioxin-like Congeners With Hypertension
Persistent organic pollutants (POPs), endocrine-disrupting, lipophilic chemicals that concentrate in adipose tissue, increasingly are being studied for a wide range of health effects.1 Persistent organic pollutants include bisphenol A, phthalates, dioxins, hexachlorobenzene, dichlorodiphenyltrichloroethane (DDT), polybrominated diphenyl ethers, and polychlorinated biphenyls (PCBs). Chlorinated dibenzo-p-dioxins are known as polychlorinated dibenzodioxins (PCDDs), or simply dioxins. Categorization of this group of chemicals is based on the structural chlorinated constituents. Of the 75 congener molecules, 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) is the most toxic, and this dioxin, given its more serious health implications, has been studied the most.1,2
Because it was a contaminant in the herbicide Agent Orange, the main defoliant used by the US military in southern Vietnam during the Vietnam War, TCDD is of primary interest. Agent Orange consists of 2,4-dichlorophenoxyacetic acid (2,4-D) and 2,4,5-trichlorophenoxyacetic acid (2,4,5-T) in equal parts. Like other dioxins, TCDD is lipophilic and retained in adipose tissue.1,3 Contemporaneous sources include occupational and residential exposure from pulp and paper mills, metallurgy, incinerators, industrial waste, fossil fuel combustion, and industrial accidents and poisonings.1-4
Another main class of POPs, polychlorinated benzenes, includes 209 synthetic PCB congener chemicals, a subset of which is referred to as dioxin-like PCBs.1 Organochlorine (OC) pesticides and PCBs were once manufactured as lubricants and coolants for electronics but are now banned; nevertheless, they remain concentrated in fish and mammals and persist in the food chain.3,5,6 These chemicals of interest (COIs) are graded for toxicity based on toxic equivalency factors relative to TCDDs in a 2005 World Health Organization assessment.3
Polychlorinated dibenzofurans (PCDFs), TCDD, PCBs, PCDDs, and other environmental toxins are being studied as possible contributing factors in the development of hypertension. The authors review the results of several recent studies on COI exposure and hypertension.
In 2017, the American College of Cardiology and the American Heart Association lowered the threshold for hypertension to systolic blood pressure (SBP) > 130 mm Hg and diastolic blood pressure (DBP) > 80 mm Hg.7 This new guideline would categorize 46% of the US population as having hypertension, compared with 32% under the former cutoff of 140/90 mm Hg.7 Modifiable factors (eg, diet, body mass index [BMI], smoking, alcohol, physical activity) and nonmodifiable factors (eg, age, family history, sex, race/ethnicity) have a role in the pathophysiology of hypertension. Between 90% and 95% of hypertension is considered primary. Hypertension increases the risk of developing ischemic heart disease, atherosclerosis, aortic dissection, small blood vessel disease, congestive heart failure, and renal failure, and thus results in considerable morbidity and mortality each year.8
Contaminant Exposure and Hypertension Risk
Vietnam-Era Army Chemical Corps
The US Army Chemical Corps (ACC) Vietnam-Era Veterans Health Study (2012-2013) recorded the long-term health burdens imposed by Agent Orange exposure and Vietnam War service.9,10 This cross-sectional study reexamined a subset of 5,609 Vietnam-era ACC veterans for an association of self-reported, physician-diagnosed hypertension (≥ 140/90 mm Hg) and herbicide spraying history confirmed with serum TCDD levels. The 22 Army units that made up the ACC were in charge of spreading Agent Orange and other defoliants on opposition camps between 1965 and 1973. The herbicide was dispersed aerially and on the ground. The ACC was also responsible for dispensing napalm, tear gas, and other chemicals.
A previous phone survey found an association of self-reported hypertension and herbicide spraying in ACC veterans with associated Vietnam service and herbicide spraying history, verified with serum TCDD levels (odds ratio [OR], 1.26; 95% confidence interval [CI], 1.00-1.58).9 Median age of ACC veterans with Vietnam War service at the time of the survey was 53 years.
The 2012-2013 study assessed respondents with a record of their serum TCDD measurements from the time of the survey.10 Most of the respondents were aged in their 60s. The stated purpose of the health study was to examine the association of Vietnam veterans’ occupational herbicide exposure and hypertension risk, rather than isolate a certain responsible chemical, though serum TCDD levels were used to confirm spraying history. After adjustments for age, tobacco use, alcohol use, race, and BMI but not salt intake, family history of hypertension, psychiatric health, or diabetes mellitus (DM) comorbidity, the OR of self-reported, physician-diagnosed hypertension was 1.74 (95% CI, 1.44-2.11) for sprayers and 1.26 (95% CI, 1.05-1.53) for Vietnam veterans.10
Vietnam War Veterans From Korea
Soldiers of the Republic of Korea (ROK) who fought in the Vietnam War also were exposed to Agent Orange and other defoliants and herbicides. In 2013, Yi and colleagues contacted 187,897 ROK Vietnam veterans to analyze their Agent Orange exposure and self-reported diseases decades after the war.11 By mail, the researchers administered a questionnaire of perceived Agent Orange exposure (eg, spraying, handling spray equipment, having contact with COIs). The Korean veterans were classified by military assignment and by their proximity to areas sprayed with Agent Orange, according to the military records of 3 US combat units: Capital Division, 9th Division, and Marine Second Brigade. The ROK veterans in those units presumably would have similar levels of Agent Orange exposure.
The questionnaire response rate was 69%. The 114,562 respondents were divided into groups based on self-perceived exposure (no, low, moderate, high) and qualitative exposure level, derived from service history (battalion/company, division/brigade). After adjusting for BMI, smoking, alcohol use, physical activity, use of nonoccupational herbicides, education, income, and military rank, Yi and colleagues found a statistically significant association of hypertension and self-reported perceived Agent Orange exposure (P < .001) and a statistically significant association of hypertension and exposure in the division/brigade group with the highest exposure level (P < .001).11 The highest ORs were found for high- vs low-exposure and moderate- vs low-exposure subsets in self-reported perceived Agent Orange levels: 1.60 (95% CI, 1.56-1.65) and 1.70 (95% CI, 1.64-1.77), respectively. However, adjusted ORs in proximity-based exposure for all groups were > 1.03.
Inuits in Canada and Greenland
To study total PCBs, non-dioxin-like PCBs, OC pesticides, and their metabolites in plasma, public health researchers Valera and colleagues focused on the Inuit town of Nunavik (in Canada), where there is contamination from foods like fish, a mainstay of the Inuit diet.5 A health survey was sent to 400 households randomly selected from 1,378 households in 14 villages. Data were collected between September and December 1992. In total, 518 people between ages 18 and 74 years agreed to undergo a physical examination, and 492 agreed to have blood drawn. Laboratories measured serum PCB congeners and 13 chlorinated pesticides or their metabolites. Blood pressure (BP) was measured 3 times, and the last 2 measurements averaged. Hypertension was defined as SBP ≥ 140 mm Hg and/or DBP ≥ 90 mm Hg.
Of the 518 participants, 315 (134 men, 181 women) had complete BP, serum POP levels, and confounding variables recorded, and these were subsequently analyzed. Mean age was 32.7 years. Polychlorinated biphenyls congeners 105 and 118 were higher in women than in men; no other congeners were quantitatively different. Associations between POP levels and hypertension were analyzed with multiple logistic regression modeling, with adjustments for age, sex, fasting blood glucose, waist circumference, smoking, alcohol use, and physical activity, as well as the common contaminants lead, mercury, and omega-3 polyunsaturated fatty acids (n-3 PUFA).The researchers adjusted for n-3 PUFA because of the posited BP-lowering effects. Inuits consume large amounts of the polyunsaturated fatty acids DHA (docosahexaenoic acid) and EPA (eicosapentaenoic acid).5
Using congeners found in at least 70% of the total samples, the study authors found a statistically significant association between certain PCBs, both dioxin-like (DL-PCBs) and non-dioxin-like (NDL-PCBs), and increased risk of hypertension. Congeners 101, 105 (DL-PCB), 138, and 187, as well as p,p'-DDE, were also significantly associated with a higher risk of hypertension. Congener 99 was associated with increased SBP, and congener 118 (DL-PCB) was associated with increased SBP and DBP. Some congeners, such as the OC pesticides, p,p'-DDT, β-hexachlorocyclohexane, and oxychlordane, were inversely associated with hypertension.
In 2012, Valera and colleagues conducted a similar study of Greenland Inuits who also consume marine mammals and fish and present with high POP levels.6 Despite correcting for n-3 PUFA, they found no significant association involving DL-PCBs, NDL-PCBs, or OC pesticides.
Japanese Background Exposures
Nakamoto and colleagues conducted a cross-sectional study of 2,266 Japanese women and men who had been exposed to background (vs occupational or wartime) levels of dioxins, including PCDDs, PCDFs, and DL-PCBs.12 The dioxins likely originated from combustion of chlorinated materials and older manufactured electronics components. The study participants had lived in urban areas or in farming or fishing villages for at least 10 consecutive years and had no concomitant occupational exposure to dioxins. Mean (SD) age was 43.5 (13.6) years for the men and 45.3 (14.0) years for the women. Participants volunteered their disease histories, which included physician-diagnosed hypertension (SBP ≥ 140 mm Hg or DBP ≥ 90 mm Hg). Logistic regression analyses were adjusted for BMI, sex, age, regional residential area, smoking, alcohol use, and survey year. In fishing regions, PCDDs, PCDFs, and DL-PCBs were significantly higher than in the other regions. Of the 2,266 participants, 647 reported physician-diagnosed hypertension. Dividing the toxic equivalents of serum COI levels into quartiles of concentration, Nakamoto and colleagues found a statistically significant association of hypertension and increased toxic equivalent levels of PCDDs, PCDFs, DL-PCBs, and total dioxins.
Italian Male Steelworkers
In a 2016 retrospective cohort study, Cappelletti and colleagues assessed the health burden of workers at a steel recycling plant in Trento, Italy. The plant, which had been using an electric arc furnace without a coke oven, had been exposing workers to dust containing PCBs, PCDDs, PCDFs, and other metals.13 Each hour, roughly 2 to 5 kg of dust was being released inside the plant (diffuse emissions), and exposure extended to a 2-km radius around the plant. A cohort of 331 plant workers, identified and assessed through company records, had been exposed to diffuse emissions for at least 1 year between 1979 and 2009. This group was compared with a control group of 32 office workers from that company, as identified by company records. The authors found a risk ratio (RR) of 2.23 in cases of noncomplicated hypertension and an RR of 2.01 in cases of complicated hypertension, defined as hypertension with organ damage.
Elderly in Sweden
In a study of 1,016 Swedish men and women who were aged 70 years or older and were living in Uppsala, Sweden, Lind and colleagues calculated average supine BP from 3 sphygmomanometer measurements after 30 minutes of rest.14 The researchers used high-resolution gas chromatography/high-resolution mass spectrometry (HRGC/HRMS) to measure the serum levels of a set of 23 POPs—16 PCB congeners, 5 OC pesticides, 1 brominated biphenyl ether congener, and octachloro-p-dibenzodioxin—and lipid-normalized the values. They used logistic regression to assess POP levels and prevalent hypertension (BP ≥ 140/90 mm Hg or use of antihypertensives), adjusting for sex, BMI, smoking status, exercise, and education. Among the COIs with the highest circulating lipid-normalized POP levels were PCB congeners 180, 138, and 170 and DDE. There was no clear relationship between toxic equivalents and hypertension; after multivariate adjustments, only DDE showed a statistically significant OR: 1.25 (95% CI, 1.07-1.47).
Organic Pollutants and Hypertension
Using National Health and Nutrition Examination Survey (NHANES) data from 1999 to 2002, Ha and colleagues conducted a cross-sectional study of a 524-adult subset of patients who were exposed to background levels of POPs and had newly diagnosed hypertension (≥ 140/90 mm Hg).15 In the NHANES study, the CDC collected standardized patient history information, physical examination findings, and venous blood sample results. Recorded BP data points were the averages of 3 separate SBP and DBP readings from a sphygmomanometer, as recommended by the AHA. The NHANES study recorded POPs with HRGC/HRMS.
Ha and colleagues selected 12 POPs, and standardized the COI concentrations against lipid concentration. The lipid-standardized POP concentrations used were at a higher level of detection and found in at least 60% of the study patients. The researchers used a logistic regression model to calculate multivariate-adjusted OR separately in men and women, adjusting for race/ethnicity, smoking/alcohol use, physical activity, BMI, cotinine level, and income level. Among the 56 men and 67 women with newly diagnosed prevalent hypertension, PCDD levels in women were positively associated with hypertension but not correlated with higher or lower toxic equivalency factors. Dioxin and NDL-PCBs were positively associated with hypertension in men but negatively in women. Ha and colleagues postulated that this approach of studying a US population subset of patients with background exposure to POPs, instead of groups with high concentrations of exposure (eg, Vietnam War veterans or those exposed occupationally or in industrial accidents), provides an alternative observable effect of long-term, low-dose exposure of a blend of POPs.15
Discussion
In vivo and in vitro studies have found that dioxins induce a subset of 35 genes, including microsomal P450 enzymes, kinases and phosphates, and DNA repair proteins. A microarray profile of cardiovascular murine tissue and cultured vascular smooth muscle cells exposed to TCDD found known dioxin-inducible genes Cyp1b1, a phase 1 drug metabolism enzyme, and Aldh3A1, another drug metabolism gene up-regulated, among lectin-related natural killer cell receptor, insulin-like growth factor binding protein, and cyclin G2.16
Dioxins bind avidly to the aryl hydrocarbon receptor (AhR), a cytosolic transcription factor that also interacts with other xenobiotic compounds with varying affinities. TCDD is one of the most potent ligands for AhR, and other DL compounds have a lower binding affinity. AhR dimerizes in the nucleus with the AhR nuclear translocator and then binds genomic dioxin response elements and induces the expression of cytochrome P450 genes, such as CYP1A1.17
The AhRs are highly expressed in the vascular endothelium.17 Agbor and colleagues found that mice with endothelial AhR knockouts showed decreased baseline SBP and DBP.18 When challenged with angiotensin II, a potent vasoconstrictor, AhR-/- mice failed to show an increase in DBP. AhR-/- exhibited reduced ex vivo aortic contraction in the presence of angiotensin II in aortas with perivascular adipose tissue. Notably, compared with wild-type mice, AhR-/- mice had reduced renin-angiotensinsystem gene expression in the visceral adipose, linking the AhR receptor with the endogenous renin-angiotensin-aldosterone system (RAAS).
Early studies have shown that mice lacking AhR do not demonstrate TCDD toxicity.20 More recently, Kopf and colleagues found that TCDD exposure in mice led to increased BP and cardiac hypertrophy, possibly linked to increased superoxide production in the vasculature.21 When exposed to TCDD, mice showed enhanced CYP1A1 mRNA expression in the left ventricle, kidney, and aorta by day 35 and increased CYP1B1 mRNA expression in the left ventricle after 60 days. Within the first week of TCDD exposure, the mean arterial pressure for the exposure group was statistically significantly increased, showing a trend of peaks and plateaus. Mice exposed to TCDD also showed left ventricular concentric hypertrophy, which is typical of systemic hypertension.8,21 Kerley-Hamilton and colleagues found that AhR ligand activation increased atherosclerosis.22
Most hypertension is idiopathic. Research into the downstream effects of AhR suggests it induces vascular oxidative stress and increases atherosclerosis.22 It is unclear whether this is an initiating or synergistic factor in the development of hypertension. The study results described here indicate that dioxins initiate BP changes through the endothelial AhR receptor, but this mechanism has been proved only in an animal model. Ongoing studies are needed to examine the molecular changes in humans. Clinicians can be advised that dioxin exposure, rather than being an initiating factor, would at most contribute to an accumulating series of assaults, including genetics, lifestyle, and environmental factors, and that these assaults progress to hypertension only after passing a threshold.23 Moreover, many of the studies described here categorized hypertension under the guideline of 140/90 mm Hg. Future studies may use the newer guideline, which will affect their results.
Conclusion
Studies have shown an association between dioxins and endocrine disruption, reproductive and developmental problems, and certain cancers.3,24 The Seveso Women’s Health Study of an industrial accident in Italy linked dioxins to an incidence of DM, obesity, or metabolic syndrome.25 By contrast, evidence of a link between dioxins and hypertension has been limited and inconsistent. Seven of the 8 studies reviewed in this study found moderate evidence of association in patients with at least 1 chemical congener and a certain subset of the study population (Table).
The Vietnam-Era Veterans Health Study found a higher OR of developing hypertension in herbicide sprayers than in its control group. Korean Vietnam War veterans stratified by either self-reported risk or military assignment also had significant associations. For male steelworkers in Italy, occupational exposure had a moderately higher RR in the exposure cohort. In the NHANES study, background levels of POPs were positively associated, but only in men. A nonoccupational study in urban and rural areas of Japan found a significant association between dioxins and hypertension. A nonoccupational study of elderly Swedes found a significant association with only 1 chemical congener. A study of Inuits in Greenland found no significant associations, but a study of Inuits in Canada did yield an association.Recent studies maintain the 2012 veterans update regarding a limited but suggestive association of dioxin and hypertension.4 Despite having high power because of the number of exposed patients, these observational studies can posit only an associative relationship, not a causal one. These studies also are limited by their categorization of dioxin exposure levels—ranging from perceived exposure to proximity and direct serum dioxin measurement. Moreover, chemical levels are measured an inconsistent number of years after exposure, and therefore, as dioxins are primarily metabolized by CYP genes, different metabolic rates could account for different susceptibility to health effects.2
In vivo animal studies could better characterize the effect of time point of exposure and effects on hypertension. Studies could also examine the synergistic effects of dioxins and other toxins, or smoking or alcohol use, on hypertension. New clinical guidelines for hypertension will have an impact on studies. Overall, clinicians who treat patients with known exposure to dioxins can suggest with moderate confidence that it is likely not a primary reason for the development of hypertension. At most, dioxin exposure is a contributing factor in the development of hypertension, with lifestyle, smoking, diet, and genetics playing more compelling roles.
1. Van den Berg M, Birnbaum L, Bosveld AT, et al. Toxic equivalency factors (TEFs) for PCBs, PCDDs, PCDFs for humans and wildlife. Environ Health Perspect. 1998;106(12):775-792.
2. US Department of Health and Human Services, Public Health Service, Agency for Toxic Substances and Disease Registry, Koplan JP. Toxicological profile for chlorinated dibenzo-p-dioxins. https://www.atsdr.cdc .gov/toxprofiles/tp104.pdf. Published December 1998. Accessed April 3, 2018.
3. Van den Berg M, Birnbaum LS, Denison M, et al. The 2005 World Health Organization reevaluation of human and mammalian toxic equivalency factors for dioxins and dioxin-like compounds. Toxicol Sci. 2006;93(2):223-241.
4. Committee to Review the Health Effects in Vietnam Veterans of Exposure to Herbicides (Ninth Biennial Update); Board of the Health of Select Populations, Institute of Medicine. Veterans and Agent Orange: Update 2012. Washington, DC: National Academies Press; 2014.
5. Valera B, Ayotte P, Poirier P, Dewailly E. Associations between plasma persistent organic pollutant levels and blood pressure in Inuit adults from Nunavik. Environ Int. 2013;59:282-289.
6. Valera B, Jørgensen ME, Jeppesen C, Bjerregaard P. Exposure to persistent organic pollutants and risk of hypertension among Inuit from Greenland. Environ Res. 2013;122:65-73.
7. Whelton PK, Carey RM, Aronow WS, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Hypertension. 2017;Nov 13:pii:HYP.0000000000000066. [Epub ahead of print.]
8. Kumar V, Abbas A, Aster J. Robbins and Cotran Pathologic Basis of Disease. Philadelphia, PA: Elsevier; 2014.
9. Kang HK, Dalager NA, Needham LL, et al. Health status of Army Chemical Corps Vietnam veterans who sprayed defoliant in Vietnam. Am J Ind Med. 2006;49(11):875-884.
10. Cypel YS, Kress AM, Eber SM, Schneiderman AI, Davey VJ. Herbicide exposure, Vietnam service, and hypertension risk in Army Chemical Corps veterans. J Occup Environ Med. 2016;58(11):1127-1136.
11. Yi SW, Ohrr H, Hong JS, Yi JJ. Agent Orange exposure and prevalence of self-reported diseases in Korean Vietnam veterans. J Prev Med Public Health. 2013;46(5):213-225.
12. Nakamoto M, Arisawa K, Uemura H, et al. Association between blood levels of PCDDs/PCDFs/dioxin-like PCBs and history of allergic and other diseases in the Japanese population. Int Arch Occup Environ Health. 2013;86(8):849-859.
13. Cappelletti R, Ceppi M, Claudatus J, Gennaro V. Health status of male steel workers at an electric arc furnace (EAF) in Trentino, Italy. J Occup Med Toxicol. 2016;11:7.
14. Lind PM, Penell J, Salihovic S, van Bavel B, Lind L. Circulating levels of p,p’-DDE are related to prevalent hypertension in the elderly. Environ Res. 2014;129:27-31.
15. Ha MH, Lee DH, Son HK, Park SK, Jacobs DR Jr. Association between serum concentrations of persistent organic pollutants and prevalence of newly diagnosed hypertension: results from the National Health and Nutrition Examination Survey 1999–2002. J Hum Hypertens. 2009;23(4):274-286.
16. Puga A, Sartor MA, Huang M, et al. Gene expression profiles of mouse aorta and cultured vascular smooth muscle cells differ widely, yet show common responses to dioxin exposure. Cardiovasc Toxicol. 2004;4(4):385-404.
17. Swanson HI, Bradfield CA. The AH-receptor: genetics, structure and function. Pharmacogenetics. 1993;3(5):213-230.
18. Agbor LN, Elased KM, Walker MK. Endothelial cell-specific aryl hydrocarbon receptor knockout mice exhibit hypotension mediated, in part, by an attenuated angiotensin II responsiveness. Biochem Pharmacol. 2011;82(5):514-523.
19. Fujii-Kuriyama Y, Mimura J. Molecular mechanisms of AhR functions in the regulation of cytochrome P450 genes. Biochem Biophys Res Commun. 2005;338(1):311-317.
20. Fernandez-Salguero PM, Hilbert DM, Rudikoff S, Ward JM, Gonzalez FJ. Aryl-hydrocarbon receptor-deficient mice are resistant to 2,3,7,8-tetrachlorodibenzo-p-dioxin-induced toxicity. Toxicol Appl Pharmacol. 1996;140(1):173-179.
21. Kopf PG, Scott JA, Agbor LN, et al. Cytochrome P4501A1 is required for vascular dysfunction and hypertension induced by 2,3,7,8-tetrachlorodibenzo-p-dioxin. Toxicol Sci. 2010;117(2):537-546.
22. Kerley-Hamilton JS, Trask HW, Ridley CJ, et al. Inherent and benzo[a]pyrene-induced differential aryl hydrocarbon receptor signaling greatly affects life span, atherosclerosis, cardiac gene expression, and body and heart growth in mice. Toxicol Sci. 2012;126(2):391-404.
23. Narkiewicz K, Kjeldsen SE, Hedner T. Is smoking a causative factor of hypertension? Blood Pressure. 2005;14(2):69-71.
24. Kogevinas M. Human health effects of dioxins: cancer, reproductive and endocrine system effects. Hum Reprod Update. 2001;7(3):331-339.
25. Warner M, Mocarelli P, Brambilla P, et al. Diabetes, metabolic syndrome, and obesity in relation to serum dioxin concentrations: the Seveso Women’s Health Study. Environ Health Perspect. 2013;121(8):906-911.
Persistent organic pollutants (POPs), endocrine-disrupting, lipophilic chemicals that concentrate in adipose tissue, increasingly are being studied for a wide range of health effects.1 Persistent organic pollutants include bisphenol A, phthalates, dioxins, hexachlorobenzene, dichlorodiphenyltrichloroethane (DDT), polybrominated diphenyl ethers, and polychlorinated biphenyls (PCBs). Chlorinated dibenzo-p-dioxins are known as polychlorinated dibenzodioxins (PCDDs), or simply dioxins. Categorization of this group of chemicals is based on the structural chlorinated constituents. Of the 75 congener molecules, 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) is the most toxic, and this dioxin, given its more serious health implications, has been studied the most.1,2
Because it was a contaminant in the herbicide Agent Orange, the main defoliant used by the US military in southern Vietnam during the Vietnam War, TCDD is of primary interest. Agent Orange consists of 2,4-dichlorophenoxyacetic acid (2,4-D) and 2,4,5-trichlorophenoxyacetic acid (2,4,5-T) in equal parts. Like other dioxins, TCDD is lipophilic and retained in adipose tissue.1,3 Contemporaneous sources include occupational and residential exposure from pulp and paper mills, metallurgy, incinerators, industrial waste, fossil fuel combustion, and industrial accidents and poisonings.1-4
Another main class of POPs, polychlorinated benzenes, includes 209 synthetic PCB congener chemicals, a subset of which is referred to as dioxin-like PCBs.1 Organochlorine (OC) pesticides and PCBs were once manufactured as lubricants and coolants for electronics but are now banned; nevertheless, they remain concentrated in fish and mammals and persist in the food chain.3,5,6 These chemicals of interest (COIs) are graded for toxicity based on toxic equivalency factors relative to TCDDs in a 2005 World Health Organization assessment.3
Polychlorinated dibenzofurans (PCDFs), TCDD, PCBs, PCDDs, and other environmental toxins are being studied as possible contributing factors in the development of hypertension. The authors review the results of several recent studies on COI exposure and hypertension.
In 2017, the American College of Cardiology and the American Heart Association lowered the threshold for hypertension to systolic blood pressure (SBP) > 130 mm Hg and diastolic blood pressure (DBP) > 80 mm Hg.7 This new guideline would categorize 46% of the US population as having hypertension, compared with 32% under the former cutoff of 140/90 mm Hg.7 Modifiable factors (eg, diet, body mass index [BMI], smoking, alcohol, physical activity) and nonmodifiable factors (eg, age, family history, sex, race/ethnicity) have a role in the pathophysiology of hypertension. Between 90% and 95% of hypertension is considered primary. Hypertension increases the risk of developing ischemic heart disease, atherosclerosis, aortic dissection, small blood vessel disease, congestive heart failure, and renal failure, and thus results in considerable morbidity and mortality each year.8
Contaminant Exposure and Hypertension Risk
Vietnam-Era Army Chemical Corps
The US Army Chemical Corps (ACC) Vietnam-Era Veterans Health Study (2012-2013) recorded the long-term health burdens imposed by Agent Orange exposure and Vietnam War service.9,10 This cross-sectional study reexamined a subset of 5,609 Vietnam-era ACC veterans for an association of self-reported, physician-diagnosed hypertension (≥ 140/90 mm Hg) and herbicide spraying history confirmed with serum TCDD levels. The 22 Army units that made up the ACC were in charge of spreading Agent Orange and other defoliants on opposition camps between 1965 and 1973. The herbicide was dispersed aerially and on the ground. The ACC was also responsible for dispensing napalm, tear gas, and other chemicals.
A previous phone survey found an association of self-reported hypertension and herbicide spraying in ACC veterans with associated Vietnam service and herbicide spraying history, verified with serum TCDD levels (odds ratio [OR], 1.26; 95% confidence interval [CI], 1.00-1.58).9 Median age of ACC veterans with Vietnam War service at the time of the survey was 53 years.
The 2012-2013 study assessed respondents with a record of their serum TCDD measurements from the time of the survey.10 Most of the respondents were aged in their 60s. The stated purpose of the health study was to examine the association of Vietnam veterans’ occupational herbicide exposure and hypertension risk, rather than isolate a certain responsible chemical, though serum TCDD levels were used to confirm spraying history. After adjustments for age, tobacco use, alcohol use, race, and BMI but not salt intake, family history of hypertension, psychiatric health, or diabetes mellitus (DM) comorbidity, the OR of self-reported, physician-diagnosed hypertension was 1.74 (95% CI, 1.44-2.11) for sprayers and 1.26 (95% CI, 1.05-1.53) for Vietnam veterans.10
Vietnam War Veterans From Korea
Soldiers of the Republic of Korea (ROK) who fought in the Vietnam War also were exposed to Agent Orange and other defoliants and herbicides. In 2013, Yi and colleagues contacted 187,897 ROK Vietnam veterans to analyze their Agent Orange exposure and self-reported diseases decades after the war.11 By mail, the researchers administered a questionnaire of perceived Agent Orange exposure (eg, spraying, handling spray equipment, having contact with COIs). The Korean veterans were classified by military assignment and by their proximity to areas sprayed with Agent Orange, according to the military records of 3 US combat units: Capital Division, 9th Division, and Marine Second Brigade. The ROK veterans in those units presumably would have similar levels of Agent Orange exposure.
The questionnaire response rate was 69%. The 114,562 respondents were divided into groups based on self-perceived exposure (no, low, moderate, high) and qualitative exposure level, derived from service history (battalion/company, division/brigade). After adjusting for BMI, smoking, alcohol use, physical activity, use of nonoccupational herbicides, education, income, and military rank, Yi and colleagues found a statistically significant association of hypertension and self-reported perceived Agent Orange exposure (P < .001) and a statistically significant association of hypertension and exposure in the division/brigade group with the highest exposure level (P < .001).11 The highest ORs were found for high- vs low-exposure and moderate- vs low-exposure subsets in self-reported perceived Agent Orange levels: 1.60 (95% CI, 1.56-1.65) and 1.70 (95% CI, 1.64-1.77), respectively. However, adjusted ORs in proximity-based exposure for all groups were > 1.03.
Inuits in Canada and Greenland
To study total PCBs, non-dioxin-like PCBs, OC pesticides, and their metabolites in plasma, public health researchers Valera and colleagues focused on the Inuit town of Nunavik (in Canada), where there is contamination from foods like fish, a mainstay of the Inuit diet.5 A health survey was sent to 400 households randomly selected from 1,378 households in 14 villages. Data were collected between September and December 1992. In total, 518 people between ages 18 and 74 years agreed to undergo a physical examination, and 492 agreed to have blood drawn. Laboratories measured serum PCB congeners and 13 chlorinated pesticides or their metabolites. Blood pressure (BP) was measured 3 times, and the last 2 measurements averaged. Hypertension was defined as SBP ≥ 140 mm Hg and/or DBP ≥ 90 mm Hg.
Of the 518 participants, 315 (134 men, 181 women) had complete BP, serum POP levels, and confounding variables recorded, and these were subsequently analyzed. Mean age was 32.7 years. Polychlorinated biphenyls congeners 105 and 118 were higher in women than in men; no other congeners were quantitatively different. Associations between POP levels and hypertension were analyzed with multiple logistic regression modeling, with adjustments for age, sex, fasting blood glucose, waist circumference, smoking, alcohol use, and physical activity, as well as the common contaminants lead, mercury, and omega-3 polyunsaturated fatty acids (n-3 PUFA).The researchers adjusted for n-3 PUFA because of the posited BP-lowering effects. Inuits consume large amounts of the polyunsaturated fatty acids DHA (docosahexaenoic acid) and EPA (eicosapentaenoic acid).5
Using congeners found in at least 70% of the total samples, the study authors found a statistically significant association between certain PCBs, both dioxin-like (DL-PCBs) and non-dioxin-like (NDL-PCBs), and increased risk of hypertension. Congeners 101, 105 (DL-PCB), 138, and 187, as well as p,p'-DDE, were also significantly associated with a higher risk of hypertension. Congener 99 was associated with increased SBP, and congener 118 (DL-PCB) was associated with increased SBP and DBP. Some congeners, such as the OC pesticides, p,p'-DDT, β-hexachlorocyclohexane, and oxychlordane, were inversely associated with hypertension.
In 2012, Valera and colleagues conducted a similar study of Greenland Inuits who also consume marine mammals and fish and present with high POP levels.6 Despite correcting for n-3 PUFA, they found no significant association involving DL-PCBs, NDL-PCBs, or OC pesticides.
Japanese Background Exposures
Nakamoto and colleagues conducted a cross-sectional study of 2,266 Japanese women and men who had been exposed to background (vs occupational or wartime) levels of dioxins, including PCDDs, PCDFs, and DL-PCBs.12 The dioxins likely originated from combustion of chlorinated materials and older manufactured electronics components. The study participants had lived in urban areas or in farming or fishing villages for at least 10 consecutive years and had no concomitant occupational exposure to dioxins. Mean (SD) age was 43.5 (13.6) years for the men and 45.3 (14.0) years for the women. Participants volunteered their disease histories, which included physician-diagnosed hypertension (SBP ≥ 140 mm Hg or DBP ≥ 90 mm Hg). Logistic regression analyses were adjusted for BMI, sex, age, regional residential area, smoking, alcohol use, and survey year. In fishing regions, PCDDs, PCDFs, and DL-PCBs were significantly higher than in the other regions. Of the 2,266 participants, 647 reported physician-diagnosed hypertension. Dividing the toxic equivalents of serum COI levels into quartiles of concentration, Nakamoto and colleagues found a statistically significant association of hypertension and increased toxic equivalent levels of PCDDs, PCDFs, DL-PCBs, and total dioxins.
Italian Male Steelworkers
In a 2016 retrospective cohort study, Cappelletti and colleagues assessed the health burden of workers at a steel recycling plant in Trento, Italy. The plant, which had been using an electric arc furnace without a coke oven, had been exposing workers to dust containing PCBs, PCDDs, PCDFs, and other metals.13 Each hour, roughly 2 to 5 kg of dust was being released inside the plant (diffuse emissions), and exposure extended to a 2-km radius around the plant. A cohort of 331 plant workers, identified and assessed through company records, had been exposed to diffuse emissions for at least 1 year between 1979 and 2009. This group was compared with a control group of 32 office workers from that company, as identified by company records. The authors found a risk ratio (RR) of 2.23 in cases of noncomplicated hypertension and an RR of 2.01 in cases of complicated hypertension, defined as hypertension with organ damage.
Elderly in Sweden
In a study of 1,016 Swedish men and women who were aged 70 years or older and were living in Uppsala, Sweden, Lind and colleagues calculated average supine BP from 3 sphygmomanometer measurements after 30 minutes of rest.14 The researchers used high-resolution gas chromatography/high-resolution mass spectrometry (HRGC/HRMS) to measure the serum levels of a set of 23 POPs—16 PCB congeners, 5 OC pesticides, 1 brominated biphenyl ether congener, and octachloro-p-dibenzodioxin—and lipid-normalized the values. They used logistic regression to assess POP levels and prevalent hypertension (BP ≥ 140/90 mm Hg or use of antihypertensives), adjusting for sex, BMI, smoking status, exercise, and education. Among the COIs with the highest circulating lipid-normalized POP levels were PCB congeners 180, 138, and 170 and DDE. There was no clear relationship between toxic equivalents and hypertension; after multivariate adjustments, only DDE showed a statistically significant OR: 1.25 (95% CI, 1.07-1.47).
Organic Pollutants and Hypertension
Using National Health and Nutrition Examination Survey (NHANES) data from 1999 to 2002, Ha and colleagues conducted a cross-sectional study of a 524-adult subset of patients who were exposed to background levels of POPs and had newly diagnosed hypertension (≥ 140/90 mm Hg).15 In the NHANES study, the CDC collected standardized patient history information, physical examination findings, and venous blood sample results. Recorded BP data points were the averages of 3 separate SBP and DBP readings from a sphygmomanometer, as recommended by the AHA. The NHANES study recorded POPs with HRGC/HRMS.
Ha and colleagues selected 12 POPs, and standardized the COI concentrations against lipid concentration. The lipid-standardized POP concentrations used were at a higher level of detection and found in at least 60% of the study patients. The researchers used a logistic regression model to calculate multivariate-adjusted OR separately in men and women, adjusting for race/ethnicity, smoking/alcohol use, physical activity, BMI, cotinine level, and income level. Among the 56 men and 67 women with newly diagnosed prevalent hypertension, PCDD levels in women were positively associated with hypertension but not correlated with higher or lower toxic equivalency factors. Dioxin and NDL-PCBs were positively associated with hypertension in men but negatively in women. Ha and colleagues postulated that this approach of studying a US population subset of patients with background exposure to POPs, instead of groups with high concentrations of exposure (eg, Vietnam War veterans or those exposed occupationally or in industrial accidents), provides an alternative observable effect of long-term, low-dose exposure of a blend of POPs.15
Discussion
In vivo and in vitro studies have found that dioxins induce a subset of 35 genes, including microsomal P450 enzymes, kinases and phosphates, and DNA repair proteins. A microarray profile of cardiovascular murine tissue and cultured vascular smooth muscle cells exposed to TCDD found known dioxin-inducible genes Cyp1b1, a phase 1 drug metabolism enzyme, and Aldh3A1, another drug metabolism gene up-regulated, among lectin-related natural killer cell receptor, insulin-like growth factor binding protein, and cyclin G2.16
Dioxins bind avidly to the aryl hydrocarbon receptor (AhR), a cytosolic transcription factor that also interacts with other xenobiotic compounds with varying affinities. TCDD is one of the most potent ligands for AhR, and other DL compounds have a lower binding affinity. AhR dimerizes in the nucleus with the AhR nuclear translocator and then binds genomic dioxin response elements and induces the expression of cytochrome P450 genes, such as CYP1A1.17
The AhRs are highly expressed in the vascular endothelium.17 Agbor and colleagues found that mice with endothelial AhR knockouts showed decreased baseline SBP and DBP.18 When challenged with angiotensin II, a potent vasoconstrictor, AhR-/- mice failed to show an increase in DBP. AhR-/- exhibited reduced ex vivo aortic contraction in the presence of angiotensin II in aortas with perivascular adipose tissue. Notably, compared with wild-type mice, AhR-/- mice had reduced renin-angiotensinsystem gene expression in the visceral adipose, linking the AhR receptor with the endogenous renin-angiotensin-aldosterone system (RAAS).
Early studies have shown that mice lacking AhR do not demonstrate TCDD toxicity.20 More recently, Kopf and colleagues found that TCDD exposure in mice led to increased BP and cardiac hypertrophy, possibly linked to increased superoxide production in the vasculature.21 When exposed to TCDD, mice showed enhanced CYP1A1 mRNA expression in the left ventricle, kidney, and aorta by day 35 and increased CYP1B1 mRNA expression in the left ventricle after 60 days. Within the first week of TCDD exposure, the mean arterial pressure for the exposure group was statistically significantly increased, showing a trend of peaks and plateaus. Mice exposed to TCDD also showed left ventricular concentric hypertrophy, which is typical of systemic hypertension.8,21 Kerley-Hamilton and colleagues found that AhR ligand activation increased atherosclerosis.22
Most hypertension is idiopathic. Research into the downstream effects of AhR suggests it induces vascular oxidative stress and increases atherosclerosis.22 It is unclear whether this is an initiating or synergistic factor in the development of hypertension. The study results described here indicate that dioxins initiate BP changes through the endothelial AhR receptor, but this mechanism has been proved only in an animal model. Ongoing studies are needed to examine the molecular changes in humans. Clinicians can be advised that dioxin exposure, rather than being an initiating factor, would at most contribute to an accumulating series of assaults, including genetics, lifestyle, and environmental factors, and that these assaults progress to hypertension only after passing a threshold.23 Moreover, many of the studies described here categorized hypertension under the guideline of 140/90 mm Hg. Future studies may use the newer guideline, which will affect their results.
Conclusion
Studies have shown an association between dioxins and endocrine disruption, reproductive and developmental problems, and certain cancers.3,24 The Seveso Women’s Health Study of an industrial accident in Italy linked dioxins to an incidence of DM, obesity, or metabolic syndrome.25 By contrast, evidence of a link between dioxins and hypertension has been limited and inconsistent. Seven of the 8 studies reviewed in this study found moderate evidence of association in patients with at least 1 chemical congener and a certain subset of the study population (Table).
The Vietnam-Era Veterans Health Study found a higher OR of developing hypertension in herbicide sprayers than in its control group. Korean Vietnam War veterans stratified by either self-reported risk or military assignment also had significant associations. For male steelworkers in Italy, occupational exposure had a moderately higher RR in the exposure cohort. In the NHANES study, background levels of POPs were positively associated, but only in men. A nonoccupational study in urban and rural areas of Japan found a significant association between dioxins and hypertension. A nonoccupational study of elderly Swedes found a significant association with only 1 chemical congener. A study of Inuits in Greenland found no significant associations, but a study of Inuits in Canada did yield an association.Recent studies maintain the 2012 veterans update regarding a limited but suggestive association of dioxin and hypertension.4 Despite having high power because of the number of exposed patients, these observational studies can posit only an associative relationship, not a causal one. These studies also are limited by their categorization of dioxin exposure levels—ranging from perceived exposure to proximity and direct serum dioxin measurement. Moreover, chemical levels are measured an inconsistent number of years after exposure, and therefore, as dioxins are primarily metabolized by CYP genes, different metabolic rates could account for different susceptibility to health effects.2
In vivo animal studies could better characterize the effect of time point of exposure and effects on hypertension. Studies could also examine the synergistic effects of dioxins and other toxins, or smoking or alcohol use, on hypertension. New clinical guidelines for hypertension will have an impact on studies. Overall, clinicians who treat patients with known exposure to dioxins can suggest with moderate confidence that it is likely not a primary reason for the development of hypertension. At most, dioxin exposure is a contributing factor in the development of hypertension, with lifestyle, smoking, diet, and genetics playing more compelling roles.
Persistent organic pollutants (POPs), endocrine-disrupting, lipophilic chemicals that concentrate in adipose tissue, increasingly are being studied for a wide range of health effects.1 Persistent organic pollutants include bisphenol A, phthalates, dioxins, hexachlorobenzene, dichlorodiphenyltrichloroethane (DDT), polybrominated diphenyl ethers, and polychlorinated biphenyls (PCBs). Chlorinated dibenzo-p-dioxins are known as polychlorinated dibenzodioxins (PCDDs), or simply dioxins. Categorization of this group of chemicals is based on the structural chlorinated constituents. Of the 75 congener molecules, 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) is the most toxic, and this dioxin, given its more serious health implications, has been studied the most.1,2
Because it was a contaminant in the herbicide Agent Orange, the main defoliant used by the US military in southern Vietnam during the Vietnam War, TCDD is of primary interest. Agent Orange consists of 2,4-dichlorophenoxyacetic acid (2,4-D) and 2,4,5-trichlorophenoxyacetic acid (2,4,5-T) in equal parts. Like other dioxins, TCDD is lipophilic and retained in adipose tissue.1,3 Contemporaneous sources include occupational and residential exposure from pulp and paper mills, metallurgy, incinerators, industrial waste, fossil fuel combustion, and industrial accidents and poisonings.1-4
Another main class of POPs, polychlorinated benzenes, includes 209 synthetic PCB congener chemicals, a subset of which is referred to as dioxin-like PCBs.1 Organochlorine (OC) pesticides and PCBs were once manufactured as lubricants and coolants for electronics but are now banned; nevertheless, they remain concentrated in fish and mammals and persist in the food chain.3,5,6 These chemicals of interest (COIs) are graded for toxicity based on toxic equivalency factors relative to TCDDs in a 2005 World Health Organization assessment.3
Polychlorinated dibenzofurans (PCDFs), TCDD, PCBs, PCDDs, and other environmental toxins are being studied as possible contributing factors in the development of hypertension. The authors review the results of several recent studies on COI exposure and hypertension.
In 2017, the American College of Cardiology and the American Heart Association lowered the threshold for hypertension to systolic blood pressure (SBP) > 130 mm Hg and diastolic blood pressure (DBP) > 80 mm Hg.7 This new guideline would categorize 46% of the US population as having hypertension, compared with 32% under the former cutoff of 140/90 mm Hg.7 Modifiable factors (eg, diet, body mass index [BMI], smoking, alcohol, physical activity) and nonmodifiable factors (eg, age, family history, sex, race/ethnicity) have a role in the pathophysiology of hypertension. Between 90% and 95% of hypertension is considered primary. Hypertension increases the risk of developing ischemic heart disease, atherosclerosis, aortic dissection, small blood vessel disease, congestive heart failure, and renal failure, and thus results in considerable morbidity and mortality each year.8
Contaminant Exposure and Hypertension Risk
Vietnam-Era Army Chemical Corps
The US Army Chemical Corps (ACC) Vietnam-Era Veterans Health Study (2012-2013) recorded the long-term health burdens imposed by Agent Orange exposure and Vietnam War service.9,10 This cross-sectional study reexamined a subset of 5,609 Vietnam-era ACC veterans for an association of self-reported, physician-diagnosed hypertension (≥ 140/90 mm Hg) and herbicide spraying history confirmed with serum TCDD levels. The 22 Army units that made up the ACC were in charge of spreading Agent Orange and other defoliants on opposition camps between 1965 and 1973. The herbicide was dispersed aerially and on the ground. The ACC was also responsible for dispensing napalm, tear gas, and other chemicals.
A previous phone survey found an association of self-reported hypertension and herbicide spraying in ACC veterans with associated Vietnam service and herbicide spraying history, verified with serum TCDD levels (odds ratio [OR], 1.26; 95% confidence interval [CI], 1.00-1.58).9 Median age of ACC veterans with Vietnam War service at the time of the survey was 53 years.
The 2012-2013 study assessed respondents with a record of their serum TCDD measurements from the time of the survey.10 Most of the respondents were aged in their 60s. The stated purpose of the health study was to examine the association of Vietnam veterans’ occupational herbicide exposure and hypertension risk, rather than isolate a certain responsible chemical, though serum TCDD levels were used to confirm spraying history. After adjustments for age, tobacco use, alcohol use, race, and BMI but not salt intake, family history of hypertension, psychiatric health, or diabetes mellitus (DM) comorbidity, the OR of self-reported, physician-diagnosed hypertension was 1.74 (95% CI, 1.44-2.11) for sprayers and 1.26 (95% CI, 1.05-1.53) for Vietnam veterans.10
Vietnam War Veterans From Korea
Soldiers of the Republic of Korea (ROK) who fought in the Vietnam War also were exposed to Agent Orange and other defoliants and herbicides. In 2013, Yi and colleagues contacted 187,897 ROK Vietnam veterans to analyze their Agent Orange exposure and self-reported diseases decades after the war.11 By mail, the researchers administered a questionnaire of perceived Agent Orange exposure (eg, spraying, handling spray equipment, having contact with COIs). The Korean veterans were classified by military assignment and by their proximity to areas sprayed with Agent Orange, according to the military records of 3 US combat units: Capital Division, 9th Division, and Marine Second Brigade. The ROK veterans in those units presumably would have similar levels of Agent Orange exposure.
The questionnaire response rate was 69%. The 114,562 respondents were divided into groups based on self-perceived exposure (no, low, moderate, high) and qualitative exposure level, derived from service history (battalion/company, division/brigade). After adjusting for BMI, smoking, alcohol use, physical activity, use of nonoccupational herbicides, education, income, and military rank, Yi and colleagues found a statistically significant association of hypertension and self-reported perceived Agent Orange exposure (P < .001) and a statistically significant association of hypertension and exposure in the division/brigade group with the highest exposure level (P < .001).11 The highest ORs were found for high- vs low-exposure and moderate- vs low-exposure subsets in self-reported perceived Agent Orange levels: 1.60 (95% CI, 1.56-1.65) and 1.70 (95% CI, 1.64-1.77), respectively. However, adjusted ORs in proximity-based exposure for all groups were > 1.03.
Inuits in Canada and Greenland
To study total PCBs, non-dioxin-like PCBs, OC pesticides, and their metabolites in plasma, public health researchers Valera and colleagues focused on the Inuit town of Nunavik (in Canada), where there is contamination from foods like fish, a mainstay of the Inuit diet.5 A health survey was sent to 400 households randomly selected from 1,378 households in 14 villages. Data were collected between September and December 1992. In total, 518 people between ages 18 and 74 years agreed to undergo a physical examination, and 492 agreed to have blood drawn. Laboratories measured serum PCB congeners and 13 chlorinated pesticides or their metabolites. Blood pressure (BP) was measured 3 times, and the last 2 measurements averaged. Hypertension was defined as SBP ≥ 140 mm Hg and/or DBP ≥ 90 mm Hg.
Of the 518 participants, 315 (134 men, 181 women) had complete BP, serum POP levels, and confounding variables recorded, and these were subsequently analyzed. Mean age was 32.7 years. Polychlorinated biphenyls congeners 105 and 118 were higher in women than in men; no other congeners were quantitatively different. Associations between POP levels and hypertension were analyzed with multiple logistic regression modeling, with adjustments for age, sex, fasting blood glucose, waist circumference, smoking, alcohol use, and physical activity, as well as the common contaminants lead, mercury, and omega-3 polyunsaturated fatty acids (n-3 PUFA).The researchers adjusted for n-3 PUFA because of the posited BP-lowering effects. Inuits consume large amounts of the polyunsaturated fatty acids DHA (docosahexaenoic acid) and EPA (eicosapentaenoic acid).5
Using congeners found in at least 70% of the total samples, the study authors found a statistically significant association between certain PCBs, both dioxin-like (DL-PCBs) and non-dioxin-like (NDL-PCBs), and increased risk of hypertension. Congeners 101, 105 (DL-PCB), 138, and 187, as well as p,p'-DDE, were also significantly associated with a higher risk of hypertension. Congener 99 was associated with increased SBP, and congener 118 (DL-PCB) was associated with increased SBP and DBP. Some congeners, such as the OC pesticides, p,p'-DDT, β-hexachlorocyclohexane, and oxychlordane, were inversely associated with hypertension.
In 2012, Valera and colleagues conducted a similar study of Greenland Inuits who also consume marine mammals and fish and present with high POP levels.6 Despite correcting for n-3 PUFA, they found no significant association involving DL-PCBs, NDL-PCBs, or OC pesticides.
Japanese Background Exposures
Nakamoto and colleagues conducted a cross-sectional study of 2,266 Japanese women and men who had been exposed to background (vs occupational or wartime) levels of dioxins, including PCDDs, PCDFs, and DL-PCBs.12 The dioxins likely originated from combustion of chlorinated materials and older manufactured electronics components. The study participants had lived in urban areas or in farming or fishing villages for at least 10 consecutive years and had no concomitant occupational exposure to dioxins. Mean (SD) age was 43.5 (13.6) years for the men and 45.3 (14.0) years for the women. Participants volunteered their disease histories, which included physician-diagnosed hypertension (SBP ≥ 140 mm Hg or DBP ≥ 90 mm Hg). Logistic regression analyses were adjusted for BMI, sex, age, regional residential area, smoking, alcohol use, and survey year. In fishing regions, PCDDs, PCDFs, and DL-PCBs were significantly higher than in the other regions. Of the 2,266 participants, 647 reported physician-diagnosed hypertension. Dividing the toxic equivalents of serum COI levels into quartiles of concentration, Nakamoto and colleagues found a statistically significant association of hypertension and increased toxic equivalent levels of PCDDs, PCDFs, DL-PCBs, and total dioxins.
Italian Male Steelworkers
In a 2016 retrospective cohort study, Cappelletti and colleagues assessed the health burden of workers at a steel recycling plant in Trento, Italy. The plant, which had been using an electric arc furnace without a coke oven, had been exposing workers to dust containing PCBs, PCDDs, PCDFs, and other metals.13 Each hour, roughly 2 to 5 kg of dust was being released inside the plant (diffuse emissions), and exposure extended to a 2-km radius around the plant. A cohort of 331 plant workers, identified and assessed through company records, had been exposed to diffuse emissions for at least 1 year between 1979 and 2009. This group was compared with a control group of 32 office workers from that company, as identified by company records. The authors found a risk ratio (RR) of 2.23 in cases of noncomplicated hypertension and an RR of 2.01 in cases of complicated hypertension, defined as hypertension with organ damage.
Elderly in Sweden
In a study of 1,016 Swedish men and women who were aged 70 years or older and were living in Uppsala, Sweden, Lind and colleagues calculated average supine BP from 3 sphygmomanometer measurements after 30 minutes of rest.14 The researchers used high-resolution gas chromatography/high-resolution mass spectrometry (HRGC/HRMS) to measure the serum levels of a set of 23 POPs—16 PCB congeners, 5 OC pesticides, 1 brominated biphenyl ether congener, and octachloro-p-dibenzodioxin—and lipid-normalized the values. They used logistic regression to assess POP levels and prevalent hypertension (BP ≥ 140/90 mm Hg or use of antihypertensives), adjusting for sex, BMI, smoking status, exercise, and education. Among the COIs with the highest circulating lipid-normalized POP levels were PCB congeners 180, 138, and 170 and DDE. There was no clear relationship between toxic equivalents and hypertension; after multivariate adjustments, only DDE showed a statistically significant OR: 1.25 (95% CI, 1.07-1.47).
Organic Pollutants and Hypertension
Using National Health and Nutrition Examination Survey (NHANES) data from 1999 to 2002, Ha and colleagues conducted a cross-sectional study of a 524-adult subset of patients who were exposed to background levels of POPs and had newly diagnosed hypertension (≥ 140/90 mm Hg).15 In the NHANES study, the CDC collected standardized patient history information, physical examination findings, and venous blood sample results. Recorded BP data points were the averages of 3 separate SBP and DBP readings from a sphygmomanometer, as recommended by the AHA. The NHANES study recorded POPs with HRGC/HRMS.
Ha and colleagues selected 12 POPs, and standardized the COI concentrations against lipid concentration. The lipid-standardized POP concentrations used were at a higher level of detection and found in at least 60% of the study patients. The researchers used a logistic regression model to calculate multivariate-adjusted OR separately in men and women, adjusting for race/ethnicity, smoking/alcohol use, physical activity, BMI, cotinine level, and income level. Among the 56 men and 67 women with newly diagnosed prevalent hypertension, PCDD levels in women were positively associated with hypertension but not correlated with higher or lower toxic equivalency factors. Dioxin and NDL-PCBs were positively associated with hypertension in men but negatively in women. Ha and colleagues postulated that this approach of studying a US population subset of patients with background exposure to POPs, instead of groups with high concentrations of exposure (eg, Vietnam War veterans or those exposed occupationally or in industrial accidents), provides an alternative observable effect of long-term, low-dose exposure of a blend of POPs.15
Discussion
In vivo and in vitro studies have found that dioxins induce a subset of 35 genes, including microsomal P450 enzymes, kinases and phosphates, and DNA repair proteins. A microarray profile of cardiovascular murine tissue and cultured vascular smooth muscle cells exposed to TCDD found known dioxin-inducible genes Cyp1b1, a phase 1 drug metabolism enzyme, and Aldh3A1, another drug metabolism gene up-regulated, among lectin-related natural killer cell receptor, insulin-like growth factor binding protein, and cyclin G2.16
Dioxins bind avidly to the aryl hydrocarbon receptor (AhR), a cytosolic transcription factor that also interacts with other xenobiotic compounds with varying affinities. TCDD is one of the most potent ligands for AhR, and other DL compounds have a lower binding affinity. AhR dimerizes in the nucleus with the AhR nuclear translocator and then binds genomic dioxin response elements and induces the expression of cytochrome P450 genes, such as CYP1A1.17
The AhRs are highly expressed in the vascular endothelium.17 Agbor and colleagues found that mice with endothelial AhR knockouts showed decreased baseline SBP and DBP.18 When challenged with angiotensin II, a potent vasoconstrictor, AhR-/- mice failed to show an increase in DBP. AhR-/- exhibited reduced ex vivo aortic contraction in the presence of angiotensin II in aortas with perivascular adipose tissue. Notably, compared with wild-type mice, AhR-/- mice had reduced renin-angiotensinsystem gene expression in the visceral adipose, linking the AhR receptor with the endogenous renin-angiotensin-aldosterone system (RAAS).
Early studies have shown that mice lacking AhR do not demonstrate TCDD toxicity.20 More recently, Kopf and colleagues found that TCDD exposure in mice led to increased BP and cardiac hypertrophy, possibly linked to increased superoxide production in the vasculature.21 When exposed to TCDD, mice showed enhanced CYP1A1 mRNA expression in the left ventricle, kidney, and aorta by day 35 and increased CYP1B1 mRNA expression in the left ventricle after 60 days. Within the first week of TCDD exposure, the mean arterial pressure for the exposure group was statistically significantly increased, showing a trend of peaks and plateaus. Mice exposed to TCDD also showed left ventricular concentric hypertrophy, which is typical of systemic hypertension.8,21 Kerley-Hamilton and colleagues found that AhR ligand activation increased atherosclerosis.22
Most hypertension is idiopathic. Research into the downstream effects of AhR suggests it induces vascular oxidative stress and increases atherosclerosis.22 It is unclear whether this is an initiating or synergistic factor in the development of hypertension. The study results described here indicate that dioxins initiate BP changes through the endothelial AhR receptor, but this mechanism has been proved only in an animal model. Ongoing studies are needed to examine the molecular changes in humans. Clinicians can be advised that dioxin exposure, rather than being an initiating factor, would at most contribute to an accumulating series of assaults, including genetics, lifestyle, and environmental factors, and that these assaults progress to hypertension only after passing a threshold.23 Moreover, many of the studies described here categorized hypertension under the guideline of 140/90 mm Hg. Future studies may use the newer guideline, which will affect their results.
Conclusion
Studies have shown an association between dioxins and endocrine disruption, reproductive and developmental problems, and certain cancers.3,24 The Seveso Women’s Health Study of an industrial accident in Italy linked dioxins to an incidence of DM, obesity, or metabolic syndrome.25 By contrast, evidence of a link between dioxins and hypertension has been limited and inconsistent. Seven of the 8 studies reviewed in this study found moderate evidence of association in patients with at least 1 chemical congener and a certain subset of the study population (Table).
The Vietnam-Era Veterans Health Study found a higher OR of developing hypertension in herbicide sprayers than in its control group. Korean Vietnam War veterans stratified by either self-reported risk or military assignment also had significant associations. For male steelworkers in Italy, occupational exposure had a moderately higher RR in the exposure cohort. In the NHANES study, background levels of POPs were positively associated, but only in men. A nonoccupational study in urban and rural areas of Japan found a significant association between dioxins and hypertension. A nonoccupational study of elderly Swedes found a significant association with only 1 chemical congener. A study of Inuits in Greenland found no significant associations, but a study of Inuits in Canada did yield an association.Recent studies maintain the 2012 veterans update regarding a limited but suggestive association of dioxin and hypertension.4 Despite having high power because of the number of exposed patients, these observational studies can posit only an associative relationship, not a causal one. These studies also are limited by their categorization of dioxin exposure levels—ranging from perceived exposure to proximity and direct serum dioxin measurement. Moreover, chemical levels are measured an inconsistent number of years after exposure, and therefore, as dioxins are primarily metabolized by CYP genes, different metabolic rates could account for different susceptibility to health effects.2
In vivo animal studies could better characterize the effect of time point of exposure and effects on hypertension. Studies could also examine the synergistic effects of dioxins and other toxins, or smoking or alcohol use, on hypertension. New clinical guidelines for hypertension will have an impact on studies. Overall, clinicians who treat patients with known exposure to dioxins can suggest with moderate confidence that it is likely not a primary reason for the development of hypertension. At most, dioxin exposure is a contributing factor in the development of hypertension, with lifestyle, smoking, diet, and genetics playing more compelling roles.
1. Van den Berg M, Birnbaum L, Bosveld AT, et al. Toxic equivalency factors (TEFs) for PCBs, PCDDs, PCDFs for humans and wildlife. Environ Health Perspect. 1998;106(12):775-792.
2. US Department of Health and Human Services, Public Health Service, Agency for Toxic Substances and Disease Registry, Koplan JP. Toxicological profile for chlorinated dibenzo-p-dioxins. https://www.atsdr.cdc .gov/toxprofiles/tp104.pdf. Published December 1998. Accessed April 3, 2018.
3. Van den Berg M, Birnbaum LS, Denison M, et al. The 2005 World Health Organization reevaluation of human and mammalian toxic equivalency factors for dioxins and dioxin-like compounds. Toxicol Sci. 2006;93(2):223-241.
4. Committee to Review the Health Effects in Vietnam Veterans of Exposure to Herbicides (Ninth Biennial Update); Board of the Health of Select Populations, Institute of Medicine. Veterans and Agent Orange: Update 2012. Washington, DC: National Academies Press; 2014.
5. Valera B, Ayotte P, Poirier P, Dewailly E. Associations between plasma persistent organic pollutant levels and blood pressure in Inuit adults from Nunavik. Environ Int. 2013;59:282-289.
6. Valera B, Jørgensen ME, Jeppesen C, Bjerregaard P. Exposure to persistent organic pollutants and risk of hypertension among Inuit from Greenland. Environ Res. 2013;122:65-73.
7. Whelton PK, Carey RM, Aronow WS, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Hypertension. 2017;Nov 13:pii:HYP.0000000000000066. [Epub ahead of print.]
8. Kumar V, Abbas A, Aster J. Robbins and Cotran Pathologic Basis of Disease. Philadelphia, PA: Elsevier; 2014.
9. Kang HK, Dalager NA, Needham LL, et al. Health status of Army Chemical Corps Vietnam veterans who sprayed defoliant in Vietnam. Am J Ind Med. 2006;49(11):875-884.
10. Cypel YS, Kress AM, Eber SM, Schneiderman AI, Davey VJ. Herbicide exposure, Vietnam service, and hypertension risk in Army Chemical Corps veterans. J Occup Environ Med. 2016;58(11):1127-1136.
11. Yi SW, Ohrr H, Hong JS, Yi JJ. Agent Orange exposure and prevalence of self-reported diseases in Korean Vietnam veterans. J Prev Med Public Health. 2013;46(5):213-225.
12. Nakamoto M, Arisawa K, Uemura H, et al. Association between blood levels of PCDDs/PCDFs/dioxin-like PCBs and history of allergic and other diseases in the Japanese population. Int Arch Occup Environ Health. 2013;86(8):849-859.
13. Cappelletti R, Ceppi M, Claudatus J, Gennaro V. Health status of male steel workers at an electric arc furnace (EAF) in Trentino, Italy. J Occup Med Toxicol. 2016;11:7.
14. Lind PM, Penell J, Salihovic S, van Bavel B, Lind L. Circulating levels of p,p’-DDE are related to prevalent hypertension in the elderly. Environ Res. 2014;129:27-31.
15. Ha MH, Lee DH, Son HK, Park SK, Jacobs DR Jr. Association between serum concentrations of persistent organic pollutants and prevalence of newly diagnosed hypertension: results from the National Health and Nutrition Examination Survey 1999–2002. J Hum Hypertens. 2009;23(4):274-286.
16. Puga A, Sartor MA, Huang M, et al. Gene expression profiles of mouse aorta and cultured vascular smooth muscle cells differ widely, yet show common responses to dioxin exposure. Cardiovasc Toxicol. 2004;4(4):385-404.
17. Swanson HI, Bradfield CA. The AH-receptor: genetics, structure and function. Pharmacogenetics. 1993;3(5):213-230.
18. Agbor LN, Elased KM, Walker MK. Endothelial cell-specific aryl hydrocarbon receptor knockout mice exhibit hypotension mediated, in part, by an attenuated angiotensin II responsiveness. Biochem Pharmacol. 2011;82(5):514-523.
19. Fujii-Kuriyama Y, Mimura J. Molecular mechanisms of AhR functions in the regulation of cytochrome P450 genes. Biochem Biophys Res Commun. 2005;338(1):311-317.
20. Fernandez-Salguero PM, Hilbert DM, Rudikoff S, Ward JM, Gonzalez FJ. Aryl-hydrocarbon receptor-deficient mice are resistant to 2,3,7,8-tetrachlorodibenzo-p-dioxin-induced toxicity. Toxicol Appl Pharmacol. 1996;140(1):173-179.
21. Kopf PG, Scott JA, Agbor LN, et al. Cytochrome P4501A1 is required for vascular dysfunction and hypertension induced by 2,3,7,8-tetrachlorodibenzo-p-dioxin. Toxicol Sci. 2010;117(2):537-546.
22. Kerley-Hamilton JS, Trask HW, Ridley CJ, et al. Inherent and benzo[a]pyrene-induced differential aryl hydrocarbon receptor signaling greatly affects life span, atherosclerosis, cardiac gene expression, and body and heart growth in mice. Toxicol Sci. 2012;126(2):391-404.
23. Narkiewicz K, Kjeldsen SE, Hedner T. Is smoking a causative factor of hypertension? Blood Pressure. 2005;14(2):69-71.
24. Kogevinas M. Human health effects of dioxins: cancer, reproductive and endocrine system effects. Hum Reprod Update. 2001;7(3):331-339.
25. Warner M, Mocarelli P, Brambilla P, et al. Diabetes, metabolic syndrome, and obesity in relation to serum dioxin concentrations: the Seveso Women’s Health Study. Environ Health Perspect. 2013;121(8):906-911.
1. Van den Berg M, Birnbaum L, Bosveld AT, et al. Toxic equivalency factors (TEFs) for PCBs, PCDDs, PCDFs for humans and wildlife. Environ Health Perspect. 1998;106(12):775-792.
2. US Department of Health and Human Services, Public Health Service, Agency for Toxic Substances and Disease Registry, Koplan JP. Toxicological profile for chlorinated dibenzo-p-dioxins. https://www.atsdr.cdc .gov/toxprofiles/tp104.pdf. Published December 1998. Accessed April 3, 2018.
3. Van den Berg M, Birnbaum LS, Denison M, et al. The 2005 World Health Organization reevaluation of human and mammalian toxic equivalency factors for dioxins and dioxin-like compounds. Toxicol Sci. 2006;93(2):223-241.
4. Committee to Review the Health Effects in Vietnam Veterans of Exposure to Herbicides (Ninth Biennial Update); Board of the Health of Select Populations, Institute of Medicine. Veterans and Agent Orange: Update 2012. Washington, DC: National Academies Press; 2014.
5. Valera B, Ayotte P, Poirier P, Dewailly E. Associations between plasma persistent organic pollutant levels and blood pressure in Inuit adults from Nunavik. Environ Int. 2013;59:282-289.
6. Valera B, Jørgensen ME, Jeppesen C, Bjerregaard P. Exposure to persistent organic pollutants and risk of hypertension among Inuit from Greenland. Environ Res. 2013;122:65-73.
7. Whelton PK, Carey RM, Aronow WS, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Hypertension. 2017;Nov 13:pii:HYP.0000000000000066. [Epub ahead of print.]
8. Kumar V, Abbas A, Aster J. Robbins and Cotran Pathologic Basis of Disease. Philadelphia, PA: Elsevier; 2014.
9. Kang HK, Dalager NA, Needham LL, et al. Health status of Army Chemical Corps Vietnam veterans who sprayed defoliant in Vietnam. Am J Ind Med. 2006;49(11):875-884.
10. Cypel YS, Kress AM, Eber SM, Schneiderman AI, Davey VJ. Herbicide exposure, Vietnam service, and hypertension risk in Army Chemical Corps veterans. J Occup Environ Med. 2016;58(11):1127-1136.
11. Yi SW, Ohrr H, Hong JS, Yi JJ. Agent Orange exposure and prevalence of self-reported diseases in Korean Vietnam veterans. J Prev Med Public Health. 2013;46(5):213-225.
12. Nakamoto M, Arisawa K, Uemura H, et al. Association between blood levels of PCDDs/PCDFs/dioxin-like PCBs and history of allergic and other diseases in the Japanese population. Int Arch Occup Environ Health. 2013;86(8):849-859.
13. Cappelletti R, Ceppi M, Claudatus J, Gennaro V. Health status of male steel workers at an electric arc furnace (EAF) in Trentino, Italy. J Occup Med Toxicol. 2016;11:7.
14. Lind PM, Penell J, Salihovic S, van Bavel B, Lind L. Circulating levels of p,p’-DDE are related to prevalent hypertension in the elderly. Environ Res. 2014;129:27-31.
15. Ha MH, Lee DH, Son HK, Park SK, Jacobs DR Jr. Association between serum concentrations of persistent organic pollutants and prevalence of newly diagnosed hypertension: results from the National Health and Nutrition Examination Survey 1999–2002. J Hum Hypertens. 2009;23(4):274-286.
16. Puga A, Sartor MA, Huang M, et al. Gene expression profiles of mouse aorta and cultured vascular smooth muscle cells differ widely, yet show common responses to dioxin exposure. Cardiovasc Toxicol. 2004;4(4):385-404.
17. Swanson HI, Bradfield CA. The AH-receptor: genetics, structure and function. Pharmacogenetics. 1993;3(5):213-230.
18. Agbor LN, Elased KM, Walker MK. Endothelial cell-specific aryl hydrocarbon receptor knockout mice exhibit hypotension mediated, in part, by an attenuated angiotensin II responsiveness. Biochem Pharmacol. 2011;82(5):514-523.
19. Fujii-Kuriyama Y, Mimura J. Molecular mechanisms of AhR functions in the regulation of cytochrome P450 genes. Biochem Biophys Res Commun. 2005;338(1):311-317.
20. Fernandez-Salguero PM, Hilbert DM, Rudikoff S, Ward JM, Gonzalez FJ. Aryl-hydrocarbon receptor-deficient mice are resistant to 2,3,7,8-tetrachlorodibenzo-p-dioxin-induced toxicity. Toxicol Appl Pharmacol. 1996;140(1):173-179.
21. Kopf PG, Scott JA, Agbor LN, et al. Cytochrome P4501A1 is required for vascular dysfunction and hypertension induced by 2,3,7,8-tetrachlorodibenzo-p-dioxin. Toxicol Sci. 2010;117(2):537-546.
22. Kerley-Hamilton JS, Trask HW, Ridley CJ, et al. Inherent and benzo[a]pyrene-induced differential aryl hydrocarbon receptor signaling greatly affects life span, atherosclerosis, cardiac gene expression, and body and heart growth in mice. Toxicol Sci. 2012;126(2):391-404.
23. Narkiewicz K, Kjeldsen SE, Hedner T. Is smoking a causative factor of hypertension? Blood Pressure. 2005;14(2):69-71.
24. Kogevinas M. Human health effects of dioxins: cancer, reproductive and endocrine system effects. Hum Reprod Update. 2001;7(3):331-339.
25. Warner M, Mocarelli P, Brambilla P, et al. Diabetes, metabolic syndrome, and obesity in relation to serum dioxin concentrations: the Seveso Women’s Health Study. Environ Health Perspect. 2013;121(8):906-911.
2018 Update on cervical disease
In this Update, I outline important findings from several studies published in the past year. First and foremost, what are best practices for performing colposcopy in the United States? The American Society for Colposcopy and Cervical Pathology (ASCCP) released guidelines addressing such practices. Second, what are the implications of repeated negative screening and patients’ acceptance of extended screening intervals? A recent observational cohort study and a large study of Kaiser Permanente’s practices since 2003 shed light on these questions. Last, where do we stand with HPV vaccination? Two studies shed light on the efficacy of vaccination against human papillomavirus (HPV), and subsequent cervical intraepithelial neoplasia (CIN) and cervical cancer.
ASCCP releases updated quality guidelines for performing colposcopy
Khan MJ, Werner CL, Darragh TM, et al. ASCCP colposcopy standards: Role of colposcopy, benefits, potential harms, and terminology for colposcopic practice. J Low Genit Tract Dis. 2017;21(4):223-229.
Waxman AG, Conageski C, Silver MI, et al. ASCCP colposcopy standards: How do we perform colposcopy? Implications for establishing standards. J Low Genit Tract Dis. 2017;21(4):235-241.
Wentzensen N, Schiffman M, Silver MI, et al. ASCCP colposcopy standards: Risk-based colposcopy practice. J Low Genit Tract Dis. 2017;21(4):230-234.
In October 2017, the ASCCP released a set of standards on the role and performance of colposcopy that represents best practices in women's health care in the United States. The work of these groups comprised a literature search, a national survey of ASCCP members, public comment, and expert consensus, and addressed:
- establishment of a common understanding of 1) the benefits of colposcopy in health maintenance and risk prevention, 2) risks presented by the procedure, and 3) terminology and criteria for reporting results that reduce subjectivity in reporting
- the rationale for, approach to, and recommendations regarding assessment of cervical precancer at colposcopy
- both minimum and comprehensive guidelines for the colposcopic examination, from preprocedure evaluation to follow-up.
Each Working Group performed the analysis and produced its own report and recommendations, published sequentially in a 2017 issue of the Journal of Lower Urinary Tract Disease. The findings and standards that they produced 1) offer essential insight for high- and low-volume coloposcopists and 2) are intended to improve the quality of colposcopy, reduce subjectivity in reporting findings, and improve the sensitivity of the procedure. Aware of the concerns and objectives of payers and hospital credentialing committees, the ASCCP found it important to establish what would be considered US-based minimum quality standards and to present goals that providers and systems could strive to achieve.
Selected details of the 3 guideline reports
The past 6 years have brought us through a great deal of transition in the prevention of cervical precancer, with regard to screening intervals and types of screening (for example, see "HPV−cytology co-testing every 3 years lowers population rates of cervical precancer and cancer," in the 2017 "Cervical Disease Update," OBG Management, May 2017). The most significant change was in 2012, when American Cancer Society/ASCCP guidelines were revised to abandon screening with annual Pap testing on most patients--an effort to strike a balance between the lifesaving value of identifying precancer and the potential harm of excessive colposcopy.
If, as the US Preventive Services Task Force (USPSTF) has declared, excessive colposcopy is a harm of screening, then we should be adapting our practices, especially in terms of the frequency of screening, to 1) reduce the risk of unnecessarily screening and potentially triaging patients to colposcopy and 2) bring the highest standards of performance and reporting to colposcopic practice (see "Why aren't you doing a Pap on me?"). In other words, "This is the way I've always done it" shouldn't characterize efforts to detect disease, when the data are clear that doing less might be more beneficial for our patients. Adherence to extended screening intervals is not yet good enough to balance benefit and risk of harm, as Rendle and colleagues showed in an article this year in Preventive Medicine (discussed in the next section of this "Update"). We need to do better.
Adherence to extended screening intervals means fewer colposcopies and less exposure to risk of attendant harm. But adherence is not purely mechanical: It can be intertwined with how patients feel about the care we provide and about their safety. When a patient moves from years of annual Pap testing to less frequent screening, she might express her concern by challenging your expertise.
In my practice, I have a simple, 1-minute conversation with the patient that is important to wedge into our discussion of her care. I explain that increasing the frequency of screening is only going to increase the chance that I will perform a colposcopy but not increase the chance that I will identify cancer. I conclude by reassuring her that I do not want to harm her, or to cause her anxiety, pain, cramping, or bleeding--or require her to spend time away from work or show her family that she is suffering. Patients are reassured and happy after that, I find. This is a patient-centered discussion that providers need to have if they hope to establish and maintain adherence to recommended screening intervals.
-- Mark H. Einstein, MD, MS
Here is a limited encapsulation of the 3 wide-ranging reports on the ASCCP colposcopy recommendations:
Role of colposcopy; benefits, potential harms, terminology (Khan et al; Working Group 1). The authors provide reinforcement: The strategic benefit of colposcopy is clear--a "drastic" reduction in excisional procedures by limiting them to patients in whom cervical cancer precursors have been confirmed or who present a high risk of occult invasive cervical cancer. Furthermore, the rate of adverse events for colposcopy−including significant bleeding and infection−is low.
Nevertheless, the potential for harm exists when an unskilled provider performs colposcopy; the Working Group emphasizes that proficiency comes with training and experience. Even in skilled hands, however, anxiety and the discomfort of a speculum examination and from acetic acid, as well as cramping and pain, might dissuade some women from receiving regular cervical screening subsequently. The authors cite research showing that educational interventions can help soothe anxiety about colposcopy and potential findings,1,2 although consensus is lacking on the value of such interventions.
The Working Group 1) developed recommended terminology for reporting findings in colposcopy practice in the United States and 2) defined the comprehensive documentation of the procedure as comprising cervix and squamocolumnar junction visibility; acetowhitening; presence of a lesion; lesion visibility, size and location of lesion(s); vascular changes; other features; and colposcopic impression (TABLE 1).3 Minimum criteria for reporting colposcopy results were also proposed, extracted from the comprehensive standards.
Risk-based colposcopy practice (Wentzensen et al). Women referred to colposcopy present with a range of underlying risk of precancer. Assessing that risk at the colposcopy visit allows the provider to modify and individualize the procedure. Risk can be estimated by referral screening tests (eg, cytology, HPV testing) performed in conjunction with the colposcopic impression. As opposed to a lack of standards for a minimum number of biopsies, the Working Group recommends that, as a standard, multiple targeted biopsies (≥2, as many as 4) are needed to improve detection of prevalent precancers. Colposcopic impression alone is not enough to diagnose precancerous cells. Let's face it: Our eyes with a colposcopic magnification of 15X do not make a microscope.
Implementing the Working Group's recommendations is expected to lead to improved detection of cervical precancers at colposcopy and to provide stronger reassurance of negative colposcopy results. Regarding biopsy of lesions, ASCCP did not find added benefit to taking random (nondirected) biopsies for women at low risk for precancer. The sensitivity of biopsy is increased by taking multiple biopsies of suspicious lesions, based on a risk-based approach detailed in the ASCCP guidelines. So, depending on underlying risk (estimated from screening and triage tests), colposcopy practice can be adapted in a useful manner to account for differences in risk:
- When risk of precancer is very high, for example, immediate treatment might reduce cost and prevent the patient from being lost to follow-up. When risk is very low, consider expectant management (serial cytology and HPV testing) with limited need for biopsy. In a setting of intermediate risk, the Working Group proposes, "multiple biopsies of acetowhite lesions lead to increased detection of precancer."
- Perform multiple biopsies that target all areas characterized by 1) acetowhitening, 2) metaplasia, and 3) higher abnormalities.
- Do not perform nontargeted biopsies on patients at the lowest end of risk who have been referred to colposcopy−ie, those with cytology that is less than HSIL; no evidence of HPV types 16/18; and a normal colposcopic impression (ie, no acetowhitening or metaplasia, or other visible abnormality).
- Immediate excision without biopsy confirmation or colposcopy with multiple targeted biopsies is acceptable in nonpregnant women 25 years and older whose risk of precancer is very high (≥2 of the following: HSIL cytology, HPV 16- or HPV 18-positive(or both), and high-grade colposcopy impression). Endocervical sampling should be conducted according to ASCCP's 2012 management guidelines. If biopsies do not show precancer, manage the patient using ASCCP's 2012 management guidelines, the Working Group recommends.
How do we perform colposcopy? Implications for establishing standards (Waxman et al; Working Group 3). To serve as a guide to standardizing colposcopy across the United States, the authors defined and delineated 6 major components (and their constituent parts) of a comprehensive colposcopy:
- precolposcopy evaluation
- the examination
- use of colposcopy adjuncts
- documentation
- biopsy sampling
- postcolposcopy procedures.
The constituent parts of these components are laid out in TABLE 2.4 A set of components for a minimum colposcopy procedure is drawn mostly from the comprehensive protocol.
The Working Group acknowledges that, in the United States, "the accuracy and reproducibility of colposcopy with biopsy as a diagnostic tool are limited." Why? Three contributing factors, the authors write, might be the absence of practice recommendations for colposcopy-biopsy procedures; of measures of quality assurance; and of standardized terminology.
Standards arrive for practice
Minimum quality standards are becoming part of almost everything US health care providers do−whether it is documentation, billing practices, or good care. Our work in gynecology, including colposcopy, is now being assessed as it is in much of the world, where minimum standards are already in place and guidelines must be followed. (In some countries standards require performing a minimum number of colposcopies per year to be identified as a "certified" colposcopist.)
What should be considered "minimum standards" for colposcopy in the United States? These ASCCP reports ask, and deliver answers to that question, bringing a broad range of concerns about high-quality practice into focus. Physicians and advanced-practice clinicians in this country who perform colposcopies have been trained to do so, but they have never had minimum standards by which to model and assess their performance. A procedure that has the potential to lead to additional testing for either cervical cancer, or to surveillance, should have minimum standards by which it is performed and documented in the United States as it is for much of the world that has widespread cervical cancer screening.
Guidance and recommendations developed by ASCCP offer women's health care providers a set of comprehensive and, alternatively, minimum quality standards that should be incorporated into practice across all aspects of the colposcopic exam, including precolposcopy evaluation, how to perform the procedure, how to document and report findings (TABLE 2), biopsy practice, establish quality control and assurance, as well as postprocedure follow-up. In taking the initiative to draw up these standards, ASCCP encourages providers to exceed the minimum requirements.
Read about adherence to cervical cancer screening.
Cervical screening adherence is relatively low, but safe. Extended intervals are very safe.
Castle PE, Kinney WK, Xue X, et al. Effect of several negative rounds of human papillomavirus and cytology co-testing on safety against cervical cancer: an observational cohort study. Ann Intern Med. 2018;168(1):20-29.
Rendle KA, Schiffman M, Cheung LC, et al. Adherence patterns to extended cervical screening intervals in women undergoing human papillomavirus (HPV) and cytology cotesting. Prev Med. 2018;109:44-50.
Patients who have been screened for cervical cancer for a long time--decades, even--have a diminishing likelihood that cancer will ever be detected. Furthermore, highest-risk patients already have been triaged into further testing or procedures, such as a loop excision electrosurgical procedure or hysterectomy. Two recent studies examined the implications of repeated negative screening and patients' acceptance of extended screening intervals.
Details of the studies
Several negative rounds of cotesting (HPV and cytology) might justify changes to the screening interval. To determine the rate of detection of CIN3, adenocarcinoma in situ, and cervical cancer (≥CIN3) in routine practice after successive negative screening at 3-year intervals, Castle and colleagues looked at records of more than 990,000 women in an integrated health care system who underwent cotesting (HPV and cytology) between 2003 and 2014. They determined that the risk of invasive cervical cancer and ≥CIN3 declined with each round of cotesting; the absolute risk fell more between first and second rounds than between second and third rounds.
At any given round of cotesting, Castle found that the ability to reassure a patient about cancer and cancer risk was similar when looking at an HPV result alone, whatever the cytology or HPV-cytology cotest result was. The investigators concluded that similar patterns of risk would have been seen had stand-alone HPV testing been used, instead of co-testing, (HPV testing alone might have missed a few cases of CIN3 and adenocarcinoma in situ leading to cancer). A single negative cotest was so effective at ruling out ≥CIN3 and cervical cancer that, after a second round of cotesting, they found that no interval cancer cases were detected among women who had a negative HPV result.
Women aged 50 years or older had a 5- to 6-fold lower risk after their third consecutive negative cotest than women aged 30 to 39 years had after their first negative cotest. These data support the ideas, Castle noted, that 1) assigning screening intervals based on both age and number of previous negative screens and 2) extending the screening interval even further than 3 years after 2--perhaps even after 1--negative cotests or HPV tests are worth entertaining. Screening women of this age becomes inefficient and cost-ineffective, even at 5-year intervals.
Is patients' adherence to an extended interval of cotesting reliable enough to change practice? Rendle and colleagues examined the records of more than 491,000 women (in the same integrated health care system that Castle studied) who had undergone routine cervical cancer screening between 2003 and 2015. Their goal was to determine how high adherence had become to the system's recommendation of an every-3-year screening interval--an interval that mirrors long-standing guidelines elsewhere.
In short, researchers observed increasing and relatively rapid clinical adoption of every-3-year cotesting for routine cervical screening over time; between 2003 and 2009, the cohort grew significantly less likely overall to come in early for screening. In this setting, adoption of an extended screeninginterval appears to run counter to earlier understanding that patients are likely to resist such extension.
Women aged 60 to 64 were most likely to screen early across 2 consecutive intervals. What Rendle termed a "modest" decrease in the percentage of late screeners (but still within a 5-year interval) was also noted during adoption of the 3-year interval.
What next?
Molecular-based testing. Research, mostly outside of the United States, is taking us in the direction of molecular-based technologies as at least a component of cervical cancer screening. Today, we rely mostly on Pap tests and colposcopy, but these are subjective screens, with a human operator. With molecular testing (mostly of components of HPV), results are objective--a "Yes" or "No" finding based on clinically validated thresholds. Methods such as genotyping, P16INK4a/Ki-67 gene product dual-stain cytology, and testing for E6 and E7 HPV mRNA transcripts are in development, and hold promise to allow us to screen safely using almost completely molecular testing, thus eliminating human error and subjectivity and enriching the population that needs further management with very sensitive and potentially specific testing.
We are being presented with the possibility that almost all aspects of screening can be done without a provider, until the patient needs treatment.
Access to screening. Research is also looking at improving access, such as self-sampling for primary screening. That includes home cervical and vaginal sampling, with specimens mailed to the laboratory, from where results and follow-up instructions as communicated to patients. The Netherlands and the United Kingdom are moving to self-sampling primary screens; the United States is not--yet. But that is the direction research is taking us.
Modified guidelines. Eyes are on the work of the USPSTF. Last year, the Task Force issued draft recommendations (https://www.uspre ventiveservicestaskforce.org/Page/Document/draft-recommendation-statement/cervical-cancer-screening2#clinical), followed by a comment period (now closed), for updating 2012 cervical cancer screening guidelines in a way that would trigger a major change in clinical practice. Those draft recommendations and public comments are under review; final recommendations are possible within this calendar year.
Continue to follow current screening guidelines; they are safe and effective for preventing cervical cancer. This assumes adherence to intervals, which is both the provider's and the patient's responsibility: First, less is more; too much screening ("I've always done it this way") can be harmful. Second, screening at intervals set by the guidelines is extremely safe, despite earlier reports or provider concerns that suggest otherwise.
Patients who have undergone several rounds of negative screening have a markedly diminished risk of cervical cancer. Serve them best by performing this underutilized gyn procedure: Sit on your hands.
Be aware that winds of change are blowing: What constitutes appropriate screening intervals is up for discussion this year, and molecular-based testing technologies that are under investigation have the potential to someday be a vast improvement over current good, but subjective, interpretations of results.
Last, promote primary prevention of cervical cancer with HPV vaccination in your practice to increase the percentage of protected patients. Doing so will contribute not only to their long-term health but also, at a societal level, to a herd immunity effect.5 Any positive HPV infection in a future of a well-vaccinated population will be significant, and HPV-targeted technologies to identify the highest risk women will be the most efficient screening.
Read about the safety and efficacy of HPV vaccination.
Primary prevention of cervical cancer with vaccination is critical in any cancer prevention program
Benard VB, Castle PE, Jenison SA, et al; New Mexico HPV Pap Registry Steering Committee. Population-based incidence rates of cervical intraepithelial neoplasia in the human papillomavirus vaccine era. JAMA Oncol. 2017;3(6):833-837.
Luostarinen T, Apter D, Dillner J, et al. Vaccination protects against invasive HPV-associated cancers. Int J Cancer. 2018;142(10):2186-2187.
The success story of HPV vaccination, after more than a decade of use, continued to unfold in important ways over the past year.
Safety. With tens of millions of doses delivered, we know that the vaccine is safe, and we have retreated on some of the precautions that we once took: For example, we no longer perform a routine pregnancy test before vaccination on reproductive-age women.
Efficacy. We have learned, based on what we see in Australia and Western Europe, that vaccination is highly effective. We are also starting to see evidence of efficacy in areas of the United States, even though the vaccine is voluntary and there are no school-based recommendations. And we know that herd vaccination is very effective. The 2 studies described here add to our understanding of how vaccination is having an impact on endpoints.
Findings of the 2 studies
HPV vaccination has a direct impact on the precursor of cancer, CIN. Benard and colleagues examined data from the New Mexico HPV Pap Registry, a mandatory statewide surveillance system of cervical cancer screening that captured estimates of both screening prevalence and CIN since the time HPV vaccination was introduced in 2007 to 2014. The investigators examined registry data to gauge trends in the rate of CIN and to estimate the effect of HPV vaccination on that rate when adjusted for changes in screening for cervical cancer.
The incidence of CIN declined significantly across all grades in 2 groups between 2007 and 2015: females aged 15 to 19 years and females aged 20 to 24 years (but not in females 25 to 29 years of age). During those years, mean uptake of HPV vaccination among females 13 to 17 years of age reached as high as 40% (in 2014).
Although a reduction in CIN2 and CIN3 precancers "are early benchmarks for achieving this aim [of reducing the rate of cancer]," the investigators note, a reduction in CIN1 is "a direct measure of reductions in HPV infections requisite to the development of almost all invasive cervical cancer."
Benard moves on to conclude that a reduction in clinical outcomes of CIN among groups who are partially vaccinated for HPV is going to change clinical practice and reduce the cost-effectiveness of clinical care that supports prevention of cervical cancer. Of greatest importance, modalities and strategies for screening, and management algorithms, are going to need to evolve as HPV vaccination and cervical screening are integrated in a rational manner. Furthermore, it might be feasible to factor in population-level decreases in CIN among cohorts who are partially vaccinated for HPV when reassessing clinical practice guidelines for cervical cancer screening.
What does this mean? As we start to eliminate HPV from the population, any positive screening result will be that much more meaningful because the outcome--cervical cancer--will be much rarer. The onus will be on providers and public health officials to re-strategize how to screen what is going to be a widely-vaccinated population; more and more, we will be looking for needles in a haystack.
How are we going to someday screen women in their 20s who were vaccinated at 11 or 12 years of age? Likely, screening will start at a later age, and screening will be conducted at longer intervals. Any finding of HPV or disease is going to be highly significant, and likely, far less frequent.
HPV vaccination protects against invasive HPV-associated cancer. Luostarinen and colleagues report proof of highly efficacious protection offered by a population-based HPV vaccination program in Finland, in the form of a decrease in the key endpoint: cases of invasive HPV-associated cancer. Examining vaccinated (3,331 females) and unvaccinated (15,665 females) cohorts in the nationwide Finnish Cancer Registry, the investigators identified 10 cases of HPV-caused cancer (8 cervical, 1 oropharyngeal, 1 vulvar) in the unvaccinated females and 0 cases in vaccinated females--a statistically significant difference.
From the evidence gathered in this first intention-to-treat trial, the investigators conclude that vaccination protects against invasive HPV-associated cancer--what they call "an awaited, pivotal corollary" to high vaccine efficacy against HPV infection.
Summing up
This success story continues to unfold, despite well-organized, antivaccine campaigns. The HPV vaccine has been an easy target: It is novel, it involves a sexually transmitted infection, and the endpoint of protecting against invasive HPV-associated cancer is years--decades--away. But antivaccine groups can no longer argue the point that studies have not been designed to yield evidence of the impact of the vaccine on decisive endpoints, including cervical cancer.
The exciting news that the sought-out endpoint of HPV vaccination -- prevention of invasive HPV-associated cervical cancer -- is being realized. This should all the more energize you to:
- urge vaccination for your patients in whom it is indicated
- emphasize vaccine coverage in the young -- especially for the routinely recommended age group of 11 - and 12-year-olds
- strenuously reject and counter arguments made by segments of the public that HPV vaccination is neither safe nor necessary
- prepare for potential changes down the road in practice guidelines regarding screening (eg, raising the age at which screening begins) as the impact of vaccination on the health of women is felt.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- Walsh JC, Curtis R, Mylotte M. Anxiety levels in women attending a colposcopy clinic: a randomised trial of an educational intervention using video colposcopy. Patient Educ Couns. 2004;55(2):247–251.
- Tomaino-Brunner C, Freda MC, Damus K, Runowicz CD. Can precolposcopy education increase knowledge and decrease anxiety? J Obstet Gynecol Neonatal Nurs. 1998;27(6):636–645.
- Khan MJ, Werner CL, Darragh TM, et al. ASCCP colposcopy standards: Role of colposcopy, benefits, potential harms, and terminology for colposcopic practice. J Low Genit Tract Dis. 2017;21(4):223–229.
- Waxman AG, Conageski C, Silver MI, et al. ASCCP colposcopy standards: How do we perform colposcopy? Implications for establishing standards. J Low Genit Tract Dis. 2017;21(4):235–241.
- Wentzensen N, Schiffman M. Accelerating cervical cancer control and prevention. Lancet Public Health. 2018;3(1):e6–e7.
In this Update, I outline important findings from several studies published in the past year. First and foremost, what are best practices for performing colposcopy in the United States? The American Society for Colposcopy and Cervical Pathology (ASCCP) released guidelines addressing such practices. Second, what are the implications of repeated negative screening and patients’ acceptance of extended screening intervals? A recent observational cohort study and a large study of Kaiser Permanente’s practices since 2003 shed light on these questions. Last, where do we stand with HPV vaccination? Two studies shed light on the efficacy of vaccination against human papillomavirus (HPV), and subsequent cervical intraepithelial neoplasia (CIN) and cervical cancer.
ASCCP releases updated quality guidelines for performing colposcopy
Khan MJ, Werner CL, Darragh TM, et al. ASCCP colposcopy standards: Role of colposcopy, benefits, potential harms, and terminology for colposcopic practice. J Low Genit Tract Dis. 2017;21(4):223-229.
Waxman AG, Conageski C, Silver MI, et al. ASCCP colposcopy standards: How do we perform colposcopy? Implications for establishing standards. J Low Genit Tract Dis. 2017;21(4):235-241.
Wentzensen N, Schiffman M, Silver MI, et al. ASCCP colposcopy standards: Risk-based colposcopy practice. J Low Genit Tract Dis. 2017;21(4):230-234.
In October 2017, the ASCCP released a set of standards on the role and performance of colposcopy that represents best practices in women's health care in the United States. The work of these groups comprised a literature search, a national survey of ASCCP members, public comment, and expert consensus, and addressed:
- establishment of a common understanding of 1) the benefits of colposcopy in health maintenance and risk prevention, 2) risks presented by the procedure, and 3) terminology and criteria for reporting results that reduce subjectivity in reporting
- the rationale for, approach to, and recommendations regarding assessment of cervical precancer at colposcopy
- both minimum and comprehensive guidelines for the colposcopic examination, from preprocedure evaluation to follow-up.
Each Working Group performed the analysis and produced its own report and recommendations, published sequentially in a 2017 issue of the Journal of Lower Urinary Tract Disease. The findings and standards that they produced 1) offer essential insight for high- and low-volume coloposcopists and 2) are intended to improve the quality of colposcopy, reduce subjectivity in reporting findings, and improve the sensitivity of the procedure. Aware of the concerns and objectives of payers and hospital credentialing committees, the ASCCP found it important to establish what would be considered US-based minimum quality standards and to present goals that providers and systems could strive to achieve.
Selected details of the 3 guideline reports
The past 6 years have brought us through a great deal of transition in the prevention of cervical precancer, with regard to screening intervals and types of screening (for example, see "HPV−cytology co-testing every 3 years lowers population rates of cervical precancer and cancer," in the 2017 "Cervical Disease Update," OBG Management, May 2017). The most significant change was in 2012, when American Cancer Society/ASCCP guidelines were revised to abandon screening with annual Pap testing on most patients--an effort to strike a balance between the lifesaving value of identifying precancer and the potential harm of excessive colposcopy.
If, as the US Preventive Services Task Force (USPSTF) has declared, excessive colposcopy is a harm of screening, then we should be adapting our practices, especially in terms of the frequency of screening, to 1) reduce the risk of unnecessarily screening and potentially triaging patients to colposcopy and 2) bring the highest standards of performance and reporting to colposcopic practice (see "Why aren't you doing a Pap on me?"). In other words, "This is the way I've always done it" shouldn't characterize efforts to detect disease, when the data are clear that doing less might be more beneficial for our patients. Adherence to extended screening intervals is not yet good enough to balance benefit and risk of harm, as Rendle and colleagues showed in an article this year in Preventive Medicine (discussed in the next section of this "Update"). We need to do better.
Adherence to extended screening intervals means fewer colposcopies and less exposure to risk of attendant harm. But adherence is not purely mechanical: It can be intertwined with how patients feel about the care we provide and about their safety. When a patient moves from years of annual Pap testing to less frequent screening, she might express her concern by challenging your expertise.
In my practice, I have a simple, 1-minute conversation with the patient that is important to wedge into our discussion of her care. I explain that increasing the frequency of screening is only going to increase the chance that I will perform a colposcopy but not increase the chance that I will identify cancer. I conclude by reassuring her that I do not want to harm her, or to cause her anxiety, pain, cramping, or bleeding--or require her to spend time away from work or show her family that she is suffering. Patients are reassured and happy after that, I find. This is a patient-centered discussion that providers need to have if they hope to establish and maintain adherence to recommended screening intervals.
-- Mark H. Einstein, MD, MS
Here is a limited encapsulation of the 3 wide-ranging reports on the ASCCP colposcopy recommendations:
Role of colposcopy; benefits, potential harms, terminology (Khan et al; Working Group 1). The authors provide reinforcement: The strategic benefit of colposcopy is clear--a "drastic" reduction in excisional procedures by limiting them to patients in whom cervical cancer precursors have been confirmed or who present a high risk of occult invasive cervical cancer. Furthermore, the rate of adverse events for colposcopy−including significant bleeding and infection−is low.
Nevertheless, the potential for harm exists when an unskilled provider performs colposcopy; the Working Group emphasizes that proficiency comes with training and experience. Even in skilled hands, however, anxiety and the discomfort of a speculum examination and from acetic acid, as well as cramping and pain, might dissuade some women from receiving regular cervical screening subsequently. The authors cite research showing that educational interventions can help soothe anxiety about colposcopy and potential findings,1,2 although consensus is lacking on the value of such interventions.
The Working Group 1) developed recommended terminology for reporting findings in colposcopy practice in the United States and 2) defined the comprehensive documentation of the procedure as comprising cervix and squamocolumnar junction visibility; acetowhitening; presence of a lesion; lesion visibility, size and location of lesion(s); vascular changes; other features; and colposcopic impression (TABLE 1).3 Minimum criteria for reporting colposcopy results were also proposed, extracted from the comprehensive standards.
Risk-based colposcopy practice (Wentzensen et al). Women referred to colposcopy present with a range of underlying risk of precancer. Assessing that risk at the colposcopy visit allows the provider to modify and individualize the procedure. Risk can be estimated by referral screening tests (eg, cytology, HPV testing) performed in conjunction with the colposcopic impression. As opposed to a lack of standards for a minimum number of biopsies, the Working Group recommends that, as a standard, multiple targeted biopsies (≥2, as many as 4) are needed to improve detection of prevalent precancers. Colposcopic impression alone is not enough to diagnose precancerous cells. Let's face it: Our eyes with a colposcopic magnification of 15X do not make a microscope.
Implementing the Working Group's recommendations is expected to lead to improved detection of cervical precancers at colposcopy and to provide stronger reassurance of negative colposcopy results. Regarding biopsy of lesions, ASCCP did not find added benefit to taking random (nondirected) biopsies for women at low risk for precancer. The sensitivity of biopsy is increased by taking multiple biopsies of suspicious lesions, based on a risk-based approach detailed in the ASCCP guidelines. So, depending on underlying risk (estimated from screening and triage tests), colposcopy practice can be adapted in a useful manner to account for differences in risk:
- When risk of precancer is very high, for example, immediate treatment might reduce cost and prevent the patient from being lost to follow-up. When risk is very low, consider expectant management (serial cytology and HPV testing) with limited need for biopsy. In a setting of intermediate risk, the Working Group proposes, "multiple biopsies of acetowhite lesions lead to increased detection of precancer."
- Perform multiple biopsies that target all areas characterized by 1) acetowhitening, 2) metaplasia, and 3) higher abnormalities.
- Do not perform nontargeted biopsies on patients at the lowest end of risk who have been referred to colposcopy−ie, those with cytology that is less than HSIL; no evidence of HPV types 16/18; and a normal colposcopic impression (ie, no acetowhitening or metaplasia, or other visible abnormality).
- Immediate excision without biopsy confirmation or colposcopy with multiple targeted biopsies is acceptable in nonpregnant women 25 years and older whose risk of precancer is very high (≥2 of the following: HSIL cytology, HPV 16- or HPV 18-positive(or both), and high-grade colposcopy impression). Endocervical sampling should be conducted according to ASCCP's 2012 management guidelines. If biopsies do not show precancer, manage the patient using ASCCP's 2012 management guidelines, the Working Group recommends.
How do we perform colposcopy? Implications for establishing standards (Waxman et al; Working Group 3). To serve as a guide to standardizing colposcopy across the United States, the authors defined and delineated 6 major components (and their constituent parts) of a comprehensive colposcopy:
- precolposcopy evaluation
- the examination
- use of colposcopy adjuncts
- documentation
- biopsy sampling
- postcolposcopy procedures.
The constituent parts of these components are laid out in TABLE 2.4 A set of components for a minimum colposcopy procedure is drawn mostly from the comprehensive protocol.
The Working Group acknowledges that, in the United States, "the accuracy and reproducibility of colposcopy with biopsy as a diagnostic tool are limited." Why? Three contributing factors, the authors write, might be the absence of practice recommendations for colposcopy-biopsy procedures; of measures of quality assurance; and of standardized terminology.
Standards arrive for practice
Minimum quality standards are becoming part of almost everything US health care providers do−whether it is documentation, billing practices, or good care. Our work in gynecology, including colposcopy, is now being assessed as it is in much of the world, where minimum standards are already in place and guidelines must be followed. (In some countries standards require performing a minimum number of colposcopies per year to be identified as a "certified" colposcopist.)
What should be considered "minimum standards" for colposcopy in the United States? These ASCCP reports ask, and deliver answers to that question, bringing a broad range of concerns about high-quality practice into focus. Physicians and advanced-practice clinicians in this country who perform colposcopies have been trained to do so, but they have never had minimum standards by which to model and assess their performance. A procedure that has the potential to lead to additional testing for either cervical cancer, or to surveillance, should have minimum standards by which it is performed and documented in the United States as it is for much of the world that has widespread cervical cancer screening.
Guidance and recommendations developed by ASCCP offer women's health care providers a set of comprehensive and, alternatively, minimum quality standards that should be incorporated into practice across all aspects of the colposcopic exam, including precolposcopy evaluation, how to perform the procedure, how to document and report findings (TABLE 2), biopsy practice, establish quality control and assurance, as well as postprocedure follow-up. In taking the initiative to draw up these standards, ASCCP encourages providers to exceed the minimum requirements.
Read about adherence to cervical cancer screening.
Cervical screening adherence is relatively low, but safe. Extended intervals are very safe.
Castle PE, Kinney WK, Xue X, et al. Effect of several negative rounds of human papillomavirus and cytology co-testing on safety against cervical cancer: an observational cohort study. Ann Intern Med. 2018;168(1):20-29.
Rendle KA, Schiffman M, Cheung LC, et al. Adherence patterns to extended cervical screening intervals in women undergoing human papillomavirus (HPV) and cytology cotesting. Prev Med. 2018;109:44-50.
Patients who have been screened for cervical cancer for a long time--decades, even--have a diminishing likelihood that cancer will ever be detected. Furthermore, highest-risk patients already have been triaged into further testing or procedures, such as a loop excision electrosurgical procedure or hysterectomy. Two recent studies examined the implications of repeated negative screening and patients' acceptance of extended screening intervals.
Details of the studies
Several negative rounds of cotesting (HPV and cytology) might justify changes to the screening interval. To determine the rate of detection of CIN3, adenocarcinoma in situ, and cervical cancer (≥CIN3) in routine practice after successive negative screening at 3-year intervals, Castle and colleagues looked at records of more than 990,000 women in an integrated health care system who underwent cotesting (HPV and cytology) between 2003 and 2014. They determined that the risk of invasive cervical cancer and ≥CIN3 declined with each round of cotesting; the absolute risk fell more between first and second rounds than between second and third rounds.
At any given round of cotesting, Castle found that the ability to reassure a patient about cancer and cancer risk was similar when looking at an HPV result alone, whatever the cytology or HPV-cytology cotest result was. The investigators concluded that similar patterns of risk would have been seen had stand-alone HPV testing been used, instead of co-testing, (HPV testing alone might have missed a few cases of CIN3 and adenocarcinoma in situ leading to cancer). A single negative cotest was so effective at ruling out ≥CIN3 and cervical cancer that, after a second round of cotesting, they found that no interval cancer cases were detected among women who had a negative HPV result.
Women aged 50 years or older had a 5- to 6-fold lower risk after their third consecutive negative cotest than women aged 30 to 39 years had after their first negative cotest. These data support the ideas, Castle noted, that 1) assigning screening intervals based on both age and number of previous negative screens and 2) extending the screening interval even further than 3 years after 2--perhaps even after 1--negative cotests or HPV tests are worth entertaining. Screening women of this age becomes inefficient and cost-ineffective, even at 5-year intervals.
Is patients' adherence to an extended interval of cotesting reliable enough to change practice? Rendle and colleagues examined the records of more than 491,000 women (in the same integrated health care system that Castle studied) who had undergone routine cervical cancer screening between 2003 and 2015. Their goal was to determine how high adherence had become to the system's recommendation of an every-3-year screening interval--an interval that mirrors long-standing guidelines elsewhere.
In short, researchers observed increasing and relatively rapid clinical adoption of every-3-year cotesting for routine cervical screening over time; between 2003 and 2009, the cohort grew significantly less likely overall to come in early for screening. In this setting, adoption of an extended screeninginterval appears to run counter to earlier understanding that patients are likely to resist such extension.
Women aged 60 to 64 were most likely to screen early across 2 consecutive intervals. What Rendle termed a "modest" decrease in the percentage of late screeners (but still within a 5-year interval) was also noted during adoption of the 3-year interval.
What next?
Molecular-based testing. Research, mostly outside of the United States, is taking us in the direction of molecular-based technologies as at least a component of cervical cancer screening. Today, we rely mostly on Pap tests and colposcopy, but these are subjective screens, with a human operator. With molecular testing (mostly of components of HPV), results are objective--a "Yes" or "No" finding based on clinically validated thresholds. Methods such as genotyping, P16INK4a/Ki-67 gene product dual-stain cytology, and testing for E6 and E7 HPV mRNA transcripts are in development, and hold promise to allow us to screen safely using almost completely molecular testing, thus eliminating human error and subjectivity and enriching the population that needs further management with very sensitive and potentially specific testing.
We are being presented with the possibility that almost all aspects of screening can be done without a provider, until the patient needs treatment.
Access to screening. Research is also looking at improving access, such as self-sampling for primary screening. That includes home cervical and vaginal sampling, with specimens mailed to the laboratory, from where results and follow-up instructions as communicated to patients. The Netherlands and the United Kingdom are moving to self-sampling primary screens; the United States is not--yet. But that is the direction research is taking us.
Modified guidelines. Eyes are on the work of the USPSTF. Last year, the Task Force issued draft recommendations (https://www.uspre ventiveservicestaskforce.org/Page/Document/draft-recommendation-statement/cervical-cancer-screening2#clinical), followed by a comment period (now closed), for updating 2012 cervical cancer screening guidelines in a way that would trigger a major change in clinical practice. Those draft recommendations and public comments are under review; final recommendations are possible within this calendar year.
Continue to follow current screening guidelines; they are safe and effective for preventing cervical cancer. This assumes adherence to intervals, which is both the provider's and the patient's responsibility: First, less is more; too much screening ("I've always done it this way") can be harmful. Second, screening at intervals set by the guidelines is extremely safe, despite earlier reports or provider concerns that suggest otherwise.
Patients who have undergone several rounds of negative screening have a markedly diminished risk of cervical cancer. Serve them best by performing this underutilized gyn procedure: Sit on your hands.
Be aware that winds of change are blowing: What constitutes appropriate screening intervals is up for discussion this year, and molecular-based testing technologies that are under investigation have the potential to someday be a vast improvement over current good, but subjective, interpretations of results.
Last, promote primary prevention of cervical cancer with HPV vaccination in your practice to increase the percentage of protected patients. Doing so will contribute not only to their long-term health but also, at a societal level, to a herd immunity effect.5 Any positive HPV infection in a future of a well-vaccinated population will be significant, and HPV-targeted technologies to identify the highest risk women will be the most efficient screening.
Read about the safety and efficacy of HPV vaccination.
Primary prevention of cervical cancer with vaccination is critical in any cancer prevention program
Benard VB, Castle PE, Jenison SA, et al; New Mexico HPV Pap Registry Steering Committee. Population-based incidence rates of cervical intraepithelial neoplasia in the human papillomavirus vaccine era. JAMA Oncol. 2017;3(6):833-837.
Luostarinen T, Apter D, Dillner J, et al. Vaccination protects against invasive HPV-associated cancers. Int J Cancer. 2018;142(10):2186-2187.
The success story of HPV vaccination, after more than a decade of use, continued to unfold in important ways over the past year.
Safety. With tens of millions of doses delivered, we know that the vaccine is safe, and we have retreated on some of the precautions that we once took: For example, we no longer perform a routine pregnancy test before vaccination on reproductive-age women.
Efficacy. We have learned, based on what we see in Australia and Western Europe, that vaccination is highly effective. We are also starting to see evidence of efficacy in areas of the United States, even though the vaccine is voluntary and there are no school-based recommendations. And we know that herd vaccination is very effective. The 2 studies described here add to our understanding of how vaccination is having an impact on endpoints.
Findings of the 2 studies
HPV vaccination has a direct impact on the precursor of cancer, CIN. Benard and colleagues examined data from the New Mexico HPV Pap Registry, a mandatory statewide surveillance system of cervical cancer screening that captured estimates of both screening prevalence and CIN since the time HPV vaccination was introduced in 2007 to 2014. The investigators examined registry data to gauge trends in the rate of CIN and to estimate the effect of HPV vaccination on that rate when adjusted for changes in screening for cervical cancer.
The incidence of CIN declined significantly across all grades in 2 groups between 2007 and 2015: females aged 15 to 19 years and females aged 20 to 24 years (but not in females 25 to 29 years of age). During those years, mean uptake of HPV vaccination among females 13 to 17 years of age reached as high as 40% (in 2014).
Although a reduction in CIN2 and CIN3 precancers "are early benchmarks for achieving this aim [of reducing the rate of cancer]," the investigators note, a reduction in CIN1 is "a direct measure of reductions in HPV infections requisite to the development of almost all invasive cervical cancer."
Benard moves on to conclude that a reduction in clinical outcomes of CIN among groups who are partially vaccinated for HPV is going to change clinical practice and reduce the cost-effectiveness of clinical care that supports prevention of cervical cancer. Of greatest importance, modalities and strategies for screening, and management algorithms, are going to need to evolve as HPV vaccination and cervical screening are integrated in a rational manner. Furthermore, it might be feasible to factor in population-level decreases in CIN among cohorts who are partially vaccinated for HPV when reassessing clinical practice guidelines for cervical cancer screening.
What does this mean? As we start to eliminate HPV from the population, any positive screening result will be that much more meaningful because the outcome--cervical cancer--will be much rarer. The onus will be on providers and public health officials to re-strategize how to screen what is going to be a widely-vaccinated population; more and more, we will be looking for needles in a haystack.
How are we going to someday screen women in their 20s who were vaccinated at 11 or 12 years of age? Likely, screening will start at a later age, and screening will be conducted at longer intervals. Any finding of HPV or disease is going to be highly significant, and likely, far less frequent.
HPV vaccination protects against invasive HPV-associated cancer. Luostarinen and colleagues report proof of highly efficacious protection offered by a population-based HPV vaccination program in Finland, in the form of a decrease in the key endpoint: cases of invasive HPV-associated cancer. Examining vaccinated (3,331 females) and unvaccinated (15,665 females) cohorts in the nationwide Finnish Cancer Registry, the investigators identified 10 cases of HPV-caused cancer (8 cervical, 1 oropharyngeal, 1 vulvar) in the unvaccinated females and 0 cases in vaccinated females--a statistically significant difference.
From the evidence gathered in this first intention-to-treat trial, the investigators conclude that vaccination protects against invasive HPV-associated cancer--what they call "an awaited, pivotal corollary" to high vaccine efficacy against HPV infection.
Summing up
This success story continues to unfold, despite well-organized, antivaccine campaigns. The HPV vaccine has been an easy target: It is novel, it involves a sexually transmitted infection, and the endpoint of protecting against invasive HPV-associated cancer is years--decades--away. But antivaccine groups can no longer argue the point that studies have not been designed to yield evidence of the impact of the vaccine on decisive endpoints, including cervical cancer.
The exciting news that the sought-out endpoint of HPV vaccination -- prevention of invasive HPV-associated cervical cancer -- is being realized. This should all the more energize you to:
- urge vaccination for your patients in whom it is indicated
- emphasize vaccine coverage in the young -- especially for the routinely recommended age group of 11 - and 12-year-olds
- strenuously reject and counter arguments made by segments of the public that HPV vaccination is neither safe nor necessary
- prepare for potential changes down the road in practice guidelines regarding screening (eg, raising the age at which screening begins) as the impact of vaccination on the health of women is felt.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
In this Update, I outline important findings from several studies published in the past year. First and foremost, what are best practices for performing colposcopy in the United States? The American Society for Colposcopy and Cervical Pathology (ASCCP) released guidelines addressing such practices. Second, what are the implications of repeated negative screening and patients’ acceptance of extended screening intervals? A recent observational cohort study and a large study of Kaiser Permanente’s practices since 2003 shed light on these questions. Last, where do we stand with HPV vaccination? Two studies shed light on the efficacy of vaccination against human papillomavirus (HPV), and subsequent cervical intraepithelial neoplasia (CIN) and cervical cancer.
ASCCP releases updated quality guidelines for performing colposcopy
Khan MJ, Werner CL, Darragh TM, et al. ASCCP colposcopy standards: Role of colposcopy, benefits, potential harms, and terminology for colposcopic practice. J Low Genit Tract Dis. 2017;21(4):223-229.
Waxman AG, Conageski C, Silver MI, et al. ASCCP colposcopy standards: How do we perform colposcopy? Implications for establishing standards. J Low Genit Tract Dis. 2017;21(4):235-241.
Wentzensen N, Schiffman M, Silver MI, et al. ASCCP colposcopy standards: Risk-based colposcopy practice. J Low Genit Tract Dis. 2017;21(4):230-234.
In October 2017, the ASCCP released a set of standards on the role and performance of colposcopy that represents best practices in women's health care in the United States. The work of these groups comprised a literature search, a national survey of ASCCP members, public comment, and expert consensus, and addressed:
- establishment of a common understanding of 1) the benefits of colposcopy in health maintenance and risk prevention, 2) risks presented by the procedure, and 3) terminology and criteria for reporting results that reduce subjectivity in reporting
- the rationale for, approach to, and recommendations regarding assessment of cervical precancer at colposcopy
- both minimum and comprehensive guidelines for the colposcopic examination, from preprocedure evaluation to follow-up.
Each Working Group performed the analysis and produced its own report and recommendations, published sequentially in a 2017 issue of the Journal of Lower Urinary Tract Disease. The findings and standards that they produced 1) offer essential insight for high- and low-volume coloposcopists and 2) are intended to improve the quality of colposcopy, reduce subjectivity in reporting findings, and improve the sensitivity of the procedure. Aware of the concerns and objectives of payers and hospital credentialing committees, the ASCCP found it important to establish what would be considered US-based minimum quality standards and to present goals that providers and systems could strive to achieve.
Selected details of the 3 guideline reports
The past 6 years have brought us through a great deal of transition in the prevention of cervical precancer, with regard to screening intervals and types of screening (for example, see "HPV−cytology co-testing every 3 years lowers population rates of cervical precancer and cancer," in the 2017 "Cervical Disease Update," OBG Management, May 2017). The most significant change was in 2012, when American Cancer Society/ASCCP guidelines were revised to abandon screening with annual Pap testing on most patients--an effort to strike a balance between the lifesaving value of identifying precancer and the potential harm of excessive colposcopy.
If, as the US Preventive Services Task Force (USPSTF) has declared, excessive colposcopy is a harm of screening, then we should be adapting our practices, especially in terms of the frequency of screening, to 1) reduce the risk of unnecessarily screening and potentially triaging patients to colposcopy and 2) bring the highest standards of performance and reporting to colposcopic practice (see "Why aren't you doing a Pap on me?"). In other words, "This is the way I've always done it" shouldn't characterize efforts to detect disease, when the data are clear that doing less might be more beneficial for our patients. Adherence to extended screening intervals is not yet good enough to balance benefit and risk of harm, as Rendle and colleagues showed in an article this year in Preventive Medicine (discussed in the next section of this "Update"). We need to do better.
Adherence to extended screening intervals means fewer colposcopies and less exposure to risk of attendant harm. But adherence is not purely mechanical: It can be intertwined with how patients feel about the care we provide and about their safety. When a patient moves from years of annual Pap testing to less frequent screening, she might express her concern by challenging your expertise.
In my practice, I have a simple, 1-minute conversation with the patient that is important to wedge into our discussion of her care. I explain that increasing the frequency of screening is only going to increase the chance that I will perform a colposcopy but not increase the chance that I will identify cancer. I conclude by reassuring her that I do not want to harm her, or to cause her anxiety, pain, cramping, or bleeding--or require her to spend time away from work or show her family that she is suffering. Patients are reassured and happy after that, I find. This is a patient-centered discussion that providers need to have if they hope to establish and maintain adherence to recommended screening intervals.
-- Mark H. Einstein, MD, MS
Here is a limited encapsulation of the 3 wide-ranging reports on the ASCCP colposcopy recommendations:
Role of colposcopy; benefits, potential harms, terminology (Khan et al; Working Group 1). The authors provide reinforcement: The strategic benefit of colposcopy is clear--a "drastic" reduction in excisional procedures by limiting them to patients in whom cervical cancer precursors have been confirmed or who present a high risk of occult invasive cervical cancer. Furthermore, the rate of adverse events for colposcopy−including significant bleeding and infection−is low.
Nevertheless, the potential for harm exists when an unskilled provider performs colposcopy; the Working Group emphasizes that proficiency comes with training and experience. Even in skilled hands, however, anxiety and the discomfort of a speculum examination and from acetic acid, as well as cramping and pain, might dissuade some women from receiving regular cervical screening subsequently. The authors cite research showing that educational interventions can help soothe anxiety about colposcopy and potential findings,1,2 although consensus is lacking on the value of such interventions.
The Working Group 1) developed recommended terminology for reporting findings in colposcopy practice in the United States and 2) defined the comprehensive documentation of the procedure as comprising cervix and squamocolumnar junction visibility; acetowhitening; presence of a lesion; lesion visibility, size and location of lesion(s); vascular changes; other features; and colposcopic impression (TABLE 1).3 Minimum criteria for reporting colposcopy results were also proposed, extracted from the comprehensive standards.
Risk-based colposcopy practice (Wentzensen et al). Women referred to colposcopy present with a range of underlying risk of precancer. Assessing that risk at the colposcopy visit allows the provider to modify and individualize the procedure. Risk can be estimated by referral screening tests (eg, cytology, HPV testing) performed in conjunction with the colposcopic impression. As opposed to a lack of standards for a minimum number of biopsies, the Working Group recommends that, as a standard, multiple targeted biopsies (≥2, as many as 4) are needed to improve detection of prevalent precancers. Colposcopic impression alone is not enough to diagnose precancerous cells. Let's face it: Our eyes with a colposcopic magnification of 15X do not make a microscope.
Implementing the Working Group's recommendations is expected to lead to improved detection of cervical precancers at colposcopy and to provide stronger reassurance of negative colposcopy results. Regarding biopsy of lesions, ASCCP did not find added benefit to taking random (nondirected) biopsies for women at low risk for precancer. The sensitivity of biopsy is increased by taking multiple biopsies of suspicious lesions, based on a risk-based approach detailed in the ASCCP guidelines. So, depending on underlying risk (estimated from screening and triage tests), colposcopy practice can be adapted in a useful manner to account for differences in risk:
- When risk of precancer is very high, for example, immediate treatment might reduce cost and prevent the patient from being lost to follow-up. When risk is very low, consider expectant management (serial cytology and HPV testing) with limited need for biopsy. In a setting of intermediate risk, the Working Group proposes, "multiple biopsies of acetowhite lesions lead to increased detection of precancer."
- Perform multiple biopsies that target all areas characterized by 1) acetowhitening, 2) metaplasia, and 3) higher abnormalities.
- Do not perform nontargeted biopsies on patients at the lowest end of risk who have been referred to colposcopy−ie, those with cytology that is less than HSIL; no evidence of HPV types 16/18; and a normal colposcopic impression (ie, no acetowhitening or metaplasia, or other visible abnormality).
- Immediate excision without biopsy confirmation or colposcopy with multiple targeted biopsies is acceptable in nonpregnant women 25 years and older whose risk of precancer is very high (≥2 of the following: HSIL cytology, HPV 16- or HPV 18-positive(or both), and high-grade colposcopy impression). Endocervical sampling should be conducted according to ASCCP's 2012 management guidelines. If biopsies do not show precancer, manage the patient using ASCCP's 2012 management guidelines, the Working Group recommends.
How do we perform colposcopy? Implications for establishing standards (Waxman et al; Working Group 3). To serve as a guide to standardizing colposcopy across the United States, the authors defined and delineated 6 major components (and their constituent parts) of a comprehensive colposcopy:
- precolposcopy evaluation
- the examination
- use of colposcopy adjuncts
- documentation
- biopsy sampling
- postcolposcopy procedures.
The constituent parts of these components are laid out in TABLE 2.4 A set of components for a minimum colposcopy procedure is drawn mostly from the comprehensive protocol.
The Working Group acknowledges that, in the United States, "the accuracy and reproducibility of colposcopy with biopsy as a diagnostic tool are limited." Why? Three contributing factors, the authors write, might be the absence of practice recommendations for colposcopy-biopsy procedures; of measures of quality assurance; and of standardized terminology.
Standards arrive for practice
Minimum quality standards are becoming part of almost everything US health care providers do−whether it is documentation, billing practices, or good care. Our work in gynecology, including colposcopy, is now being assessed as it is in much of the world, where minimum standards are already in place and guidelines must be followed. (In some countries standards require performing a minimum number of colposcopies per year to be identified as a "certified" colposcopist.)
What should be considered "minimum standards" for colposcopy in the United States? These ASCCP reports ask, and deliver answers to that question, bringing a broad range of concerns about high-quality practice into focus. Physicians and advanced-practice clinicians in this country who perform colposcopies have been trained to do so, but they have never had minimum standards by which to model and assess their performance. A procedure that has the potential to lead to additional testing for either cervical cancer, or to surveillance, should have minimum standards by which it is performed and documented in the United States as it is for much of the world that has widespread cervical cancer screening.
Guidance and recommendations developed by ASCCP offer women's health care providers a set of comprehensive and, alternatively, minimum quality standards that should be incorporated into practice across all aspects of the colposcopic exam, including precolposcopy evaluation, how to perform the procedure, how to document and report findings (TABLE 2), biopsy practice, establish quality control and assurance, as well as postprocedure follow-up. In taking the initiative to draw up these standards, ASCCP encourages providers to exceed the minimum requirements.
Read about adherence to cervical cancer screening.
Cervical screening adherence is relatively low, but safe. Extended intervals are very safe.
Castle PE, Kinney WK, Xue X, et al. Effect of several negative rounds of human papillomavirus and cytology co-testing on safety against cervical cancer: an observational cohort study. Ann Intern Med. 2018;168(1):20-29.
Rendle KA, Schiffman M, Cheung LC, et al. Adherence patterns to extended cervical screening intervals in women undergoing human papillomavirus (HPV) and cytology cotesting. Prev Med. 2018;109:44-50.
Patients who have been screened for cervical cancer for a long time--decades, even--have a diminishing likelihood that cancer will ever be detected. Furthermore, highest-risk patients already have been triaged into further testing or procedures, such as a loop excision electrosurgical procedure or hysterectomy. Two recent studies examined the implications of repeated negative screening and patients' acceptance of extended screening intervals.
Details of the studies
Several negative rounds of cotesting (HPV and cytology) might justify changes to the screening interval. To determine the rate of detection of CIN3, adenocarcinoma in situ, and cervical cancer (≥CIN3) in routine practice after successive negative screening at 3-year intervals, Castle and colleagues looked at records of more than 990,000 women in an integrated health care system who underwent cotesting (HPV and cytology) between 2003 and 2014. They determined that the risk of invasive cervical cancer and ≥CIN3 declined with each round of cotesting; the absolute risk fell more between first and second rounds than between second and third rounds.
At any given round of cotesting, Castle found that the ability to reassure a patient about cancer and cancer risk was similar when looking at an HPV result alone, whatever the cytology or HPV-cytology cotest result was. The investigators concluded that similar patterns of risk would have been seen had stand-alone HPV testing been used, instead of co-testing, (HPV testing alone might have missed a few cases of CIN3 and adenocarcinoma in situ leading to cancer). A single negative cotest was so effective at ruling out ≥CIN3 and cervical cancer that, after a second round of cotesting, they found that no interval cancer cases were detected among women who had a negative HPV result.
Women aged 50 years or older had a 5- to 6-fold lower risk after their third consecutive negative cotest than women aged 30 to 39 years had after their first negative cotest. These data support the ideas, Castle noted, that 1) assigning screening intervals based on both age and number of previous negative screens and 2) extending the screening interval even further than 3 years after 2--perhaps even after 1--negative cotests or HPV tests are worth entertaining. Screening women of this age becomes inefficient and cost-ineffective, even at 5-year intervals.
Is patients' adherence to an extended interval of cotesting reliable enough to change practice? Rendle and colleagues examined the records of more than 491,000 women (in the same integrated health care system that Castle studied) who had undergone routine cervical cancer screening between 2003 and 2015. Their goal was to determine how high adherence had become to the system's recommendation of an every-3-year screening interval--an interval that mirrors long-standing guidelines elsewhere.
In short, researchers observed increasing and relatively rapid clinical adoption of every-3-year cotesting for routine cervical screening over time; between 2003 and 2009, the cohort grew significantly less likely overall to come in early for screening. In this setting, adoption of an extended screeninginterval appears to run counter to earlier understanding that patients are likely to resist such extension.
Women aged 60 to 64 were most likely to screen early across 2 consecutive intervals. What Rendle termed a "modest" decrease in the percentage of late screeners (but still within a 5-year interval) was also noted during adoption of the 3-year interval.
What next?
Molecular-based testing. Research, mostly outside of the United States, is taking us in the direction of molecular-based technologies as at least a component of cervical cancer screening. Today, we rely mostly on Pap tests and colposcopy, but these are subjective screens, with a human operator. With molecular testing (mostly of components of HPV), results are objective--a "Yes" or "No" finding based on clinically validated thresholds. Methods such as genotyping, P16INK4a/Ki-67 gene product dual-stain cytology, and testing for E6 and E7 HPV mRNA transcripts are in development, and hold promise to allow us to screen safely using almost completely molecular testing, thus eliminating human error and subjectivity and enriching the population that needs further management with very sensitive and potentially specific testing.
We are being presented with the possibility that almost all aspects of screening can be done without a provider, until the patient needs treatment.
Access to screening. Research is also looking at improving access, such as self-sampling for primary screening. That includes home cervical and vaginal sampling, with specimens mailed to the laboratory, from where results and follow-up instructions as communicated to patients. The Netherlands and the United Kingdom are moving to self-sampling primary screens; the United States is not--yet. But that is the direction research is taking us.
Modified guidelines. Eyes are on the work of the USPSTF. Last year, the Task Force issued draft recommendations (https://www.uspre ventiveservicestaskforce.org/Page/Document/draft-recommendation-statement/cervical-cancer-screening2#clinical), followed by a comment period (now closed), for updating 2012 cervical cancer screening guidelines in a way that would trigger a major change in clinical practice. Those draft recommendations and public comments are under review; final recommendations are possible within this calendar year.
Continue to follow current screening guidelines; they are safe and effective for preventing cervical cancer. This assumes adherence to intervals, which is both the provider's and the patient's responsibility: First, less is more; too much screening ("I've always done it this way") can be harmful. Second, screening at intervals set by the guidelines is extremely safe, despite earlier reports or provider concerns that suggest otherwise.
Patients who have undergone several rounds of negative screening have a markedly diminished risk of cervical cancer. Serve them best by performing this underutilized gyn procedure: Sit on your hands.
Be aware that winds of change are blowing: What constitutes appropriate screening intervals is up for discussion this year, and molecular-based testing technologies that are under investigation have the potential to someday be a vast improvement over current good, but subjective, interpretations of results.
Last, promote primary prevention of cervical cancer with HPV vaccination in your practice to increase the percentage of protected patients. Doing so will contribute not only to their long-term health but also, at a societal level, to a herd immunity effect.5 Any positive HPV infection in a future of a well-vaccinated population will be significant, and HPV-targeted technologies to identify the highest risk women will be the most efficient screening.
Read about the safety and efficacy of HPV vaccination.
Primary prevention of cervical cancer with vaccination is critical in any cancer prevention program
Benard VB, Castle PE, Jenison SA, et al; New Mexico HPV Pap Registry Steering Committee. Population-based incidence rates of cervical intraepithelial neoplasia in the human papillomavirus vaccine era. JAMA Oncol. 2017;3(6):833-837.
Luostarinen T, Apter D, Dillner J, et al. Vaccination protects against invasive HPV-associated cancers. Int J Cancer. 2018;142(10):2186-2187.
The success story of HPV vaccination, after more than a decade of use, continued to unfold in important ways over the past year.
Safety. With tens of millions of doses delivered, we know that the vaccine is safe, and we have retreated on some of the precautions that we once took: For example, we no longer perform a routine pregnancy test before vaccination on reproductive-age women.
Efficacy. We have learned, based on what we see in Australia and Western Europe, that vaccination is highly effective. We are also starting to see evidence of efficacy in areas of the United States, even though the vaccine is voluntary and there are no school-based recommendations. And we know that herd vaccination is very effective. The 2 studies described here add to our understanding of how vaccination is having an impact on endpoints.
Findings of the 2 studies
HPV vaccination has a direct impact on the precursor of cancer, CIN. Benard and colleagues examined data from the New Mexico HPV Pap Registry, a mandatory statewide surveillance system of cervical cancer screening that captured estimates of both screening prevalence and CIN since the time HPV vaccination was introduced in 2007 to 2014. The investigators examined registry data to gauge trends in the rate of CIN and to estimate the effect of HPV vaccination on that rate when adjusted for changes in screening for cervical cancer.
The incidence of CIN declined significantly across all grades in 2 groups between 2007 and 2015: females aged 15 to 19 years and females aged 20 to 24 years (but not in females 25 to 29 years of age). During those years, mean uptake of HPV vaccination among females 13 to 17 years of age reached as high as 40% (in 2014).
Although a reduction in CIN2 and CIN3 precancers "are early benchmarks for achieving this aim [of reducing the rate of cancer]," the investigators note, a reduction in CIN1 is "a direct measure of reductions in HPV infections requisite to the development of almost all invasive cervical cancer."
Benard moves on to conclude that a reduction in clinical outcomes of CIN among groups who are partially vaccinated for HPV is going to change clinical practice and reduce the cost-effectiveness of clinical care that supports prevention of cervical cancer. Of greatest importance, modalities and strategies for screening, and management algorithms, are going to need to evolve as HPV vaccination and cervical screening are integrated in a rational manner. Furthermore, it might be feasible to factor in population-level decreases in CIN among cohorts who are partially vaccinated for HPV when reassessing clinical practice guidelines for cervical cancer screening.
What does this mean? As we start to eliminate HPV from the population, any positive screening result will be that much more meaningful because the outcome--cervical cancer--will be much rarer. The onus will be on providers and public health officials to re-strategize how to screen what is going to be a widely-vaccinated population; more and more, we will be looking for needles in a haystack.
How are we going to someday screen women in their 20s who were vaccinated at 11 or 12 years of age? Likely, screening will start at a later age, and screening will be conducted at longer intervals. Any finding of HPV or disease is going to be highly significant, and likely, far less frequent.
HPV vaccination protects against invasive HPV-associated cancer. Luostarinen and colleagues report proof of highly efficacious protection offered by a population-based HPV vaccination program in Finland, in the form of a decrease in the key endpoint: cases of invasive HPV-associated cancer. Examining vaccinated (3,331 females) and unvaccinated (15,665 females) cohorts in the nationwide Finnish Cancer Registry, the investigators identified 10 cases of HPV-caused cancer (8 cervical, 1 oropharyngeal, 1 vulvar) in the unvaccinated females and 0 cases in vaccinated females--a statistically significant difference.
From the evidence gathered in this first intention-to-treat trial, the investigators conclude that vaccination protects against invasive HPV-associated cancer--what they call "an awaited, pivotal corollary" to high vaccine efficacy against HPV infection.
Summing up
This success story continues to unfold, despite well-organized, antivaccine campaigns. The HPV vaccine has been an easy target: It is novel, it involves a sexually transmitted infection, and the endpoint of protecting against invasive HPV-associated cancer is years--decades--away. But antivaccine groups can no longer argue the point that studies have not been designed to yield evidence of the impact of the vaccine on decisive endpoints, including cervical cancer.
The exciting news that the sought-out endpoint of HPV vaccination -- prevention of invasive HPV-associated cervical cancer -- is being realized. This should all the more energize you to:
- urge vaccination for your patients in whom it is indicated
- emphasize vaccine coverage in the young -- especially for the routinely recommended age group of 11 - and 12-year-olds
- strenuously reject and counter arguments made by segments of the public that HPV vaccination is neither safe nor necessary
- prepare for potential changes down the road in practice guidelines regarding screening (eg, raising the age at which screening begins) as the impact of vaccination on the health of women is felt.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- Walsh JC, Curtis R, Mylotte M. Anxiety levels in women attending a colposcopy clinic: a randomised trial of an educational intervention using video colposcopy. Patient Educ Couns. 2004;55(2):247–251.
- Tomaino-Brunner C, Freda MC, Damus K, Runowicz CD. Can precolposcopy education increase knowledge and decrease anxiety? J Obstet Gynecol Neonatal Nurs. 1998;27(6):636–645.
- Khan MJ, Werner CL, Darragh TM, et al. ASCCP colposcopy standards: Role of colposcopy, benefits, potential harms, and terminology for colposcopic practice. J Low Genit Tract Dis. 2017;21(4):223–229.
- Waxman AG, Conageski C, Silver MI, et al. ASCCP colposcopy standards: How do we perform colposcopy? Implications for establishing standards. J Low Genit Tract Dis. 2017;21(4):235–241.
- Wentzensen N, Schiffman M. Accelerating cervical cancer control and prevention. Lancet Public Health. 2018;3(1):e6–e7.
- Walsh JC, Curtis R, Mylotte M. Anxiety levels in women attending a colposcopy clinic: a randomised trial of an educational intervention using video colposcopy. Patient Educ Couns. 2004;55(2):247–251.
- Tomaino-Brunner C, Freda MC, Damus K, Runowicz CD. Can precolposcopy education increase knowledge and decrease anxiety? J Obstet Gynecol Neonatal Nurs. 1998;27(6):636–645.
- Khan MJ, Werner CL, Darragh TM, et al. ASCCP colposcopy standards: Role of colposcopy, benefits, potential harms, and terminology for colposcopic practice. J Low Genit Tract Dis. 2017;21(4):223–229.
- Waxman AG, Conageski C, Silver MI, et al. ASCCP colposcopy standards: How do we perform colposcopy? Implications for establishing standards. J Low Genit Tract Dis. 2017;21(4):235–241.
- Wentzensen N, Schiffman M. Accelerating cervical cancer control and prevention. Lancet Public Health. 2018;3(1):e6–e7.
Pharmacologic Treatments for Idiopathic Pulmonary Fibrosis
IN THIS ARTICLE
- Confirming the diagnosis
- Pirfenidone treatment
- Nintedanib treatment
A 64-year-old man has a one-year history of dyspnea on exertion and a nonproductive cough. His symptoms are gradually worsening and increasingly bothersome to him.
His medical history includes mild seasonal allergies and GERD, which is well-controlled by oral antihistamines and proton pump inhibitors. He has spent the past 30 years working a desk job as an accountant. He denies a history of smoking, exposure to secondhand smoke, and initiation of new medication.
He admits to increased fatigue, but denies fever, chills, lymphadenopathy, weight change, chest pain, wheezing, abdominal pain, diarrhea, vomiting, claudication, and swelling in the extremities. The rest of the review of systems is negative.
Lab results—complete blood count, comprehensive metabolic panel, TSH, antinuclear antibodies, erythrocyte sedimentation rate, and C-reactive protein—are within normal limits. Spirometry shows very mild restriction. A chest x-ray is abnormal but nonspecific, showing peripheral opacities. An ECG shows normal sinus rhythm.
The patient is given a trial of an inhaled steroid, which yields no improvement. Six months later, the patient is seen by a pulmonologist. Idiopathic pulmonary fibrosis (IPF) is diagnosed based on high-resolution CT (HRCT) and lung biopsy results.
IPF is a chronic, progressive, fibrosing interstitial disease that is limited to lung tissue. It most commonly manifests in older adults with vague symptoms of dyspnea on exertion and nonproductive cough, but symptoms can also include fatigue, muscle and joint aches, clubbing of the fingernails, and weight loss.1 The average life expectancy following diagnosis of IPF is two to five years, and the mortality rate is estimated at 64.3 per million men and 58.4 per million women per year.2,3
Continue to: DIAGNOSIS
DIAGNOSIS
IPF belongs in the general class of idiopathic interstitial pneumonias (IIPs), which are characterized by varying degrees of inflammation and fibrosis of lung interstitium.4 All subtypes of IIPs cause dyspnea and diffuse abnormalities on HRCT, and all vary from each other histologically. Table 1 outlines the key features of each.5-8
Because of its vague symptomology and the extensive workup needed to rule out other diseases, patients with IPF often have symptoms for one to two years before a diagnosis is made.1 Physical exam may reveal fine inspiratory rales in both lung bases and digital clubbing; eventual signs of pulmonary hypertension and right-sided heart failure may be appreciated.1,9
There are no specific diagnostic laboratory tests to confirm IPF; however, baseline labwork (as outlined in the case presentation) is typically ordered to rule out infection, thyroid disease, or connective tissue disease.10 Many patients are referred to a cardiologist before being seen by a pulmonologist; cardiac stress testing may be done, and an echocardiogram may be performed to rule out heart failure.
Diagnostic testing may include pulmonary function testing, HRCT of the chest, and lung biopsy.10 Tissue samples from patients with IPF reveal different stages of disease, including dense fibrosis with honeycombing, subpleural or paraseptal distribution, fibroblast foci, and normal tissue.11 Pulmonary function test results will show a restrictive pattern. Both forced expiratory volume in one second (FEV1) and forced vital capacity (FVC) will be reduced, and the FEV1/FVC ratio preserved. Due to decreased functional lung volume, diffusing capacity of the lung for carbon monoxide (DLCO) will also be reduced.4,12
The differential is broad and includes allergic asthma, bronchitis, COPD, lung cancer, hypersensitivity pneumonitis, asbestosis, or pulmonary embolism.
Continue to: TREATMENT HISTORY
TREATMENT HISTORY
IPF has a long history of tried and failed treatment options. The American Thoracic Society (ATS), in concert with other professional organizations, has published comprehensive guidelines and recommendations pertaining to the use of pharmacologic medications to control disease progression. Warfarin and other anticoagulants have been studied, based on the observation that a procoagulant state promotes fibrotic changes in the lung tissue.13 However, anticoagulant use is not recommended in patients with IPF due to lack of efficacy and high potential for harm.13
Immunosuppressants have also been in the spotlight as possible treatment for IPF, but a clinical study investigating the efficacy of a three-drug regimen including prednisone, azathioprine, and N-acetylcysteine was stopped early due to increased risk for harm. Endothelin antagonists and potent tyrosine kinase inhibitors are also not recommended in the most recent edition of IPF guidelines, as they lack benefit.13
In fact, prior to the 2015 edition of the guidelines, no single medication was routinely recommended for patients with IPF. But this is now changing, following the 2014 FDA approval of two new drugs, nintedanib and pirfenidone, designed specifically to treat IPF.14 These drugs have shown promise in clinical trials (results of which are summarized in Table 2).
Continue to: NEW PHARMACOLOGIC OPTIONS
NEW PHARMACOLOGIC OPTIONS
Pirfenidone
In 2008, a study was conducted in Japan to determine the mechanism of action of pirfenidone.15 Through in vitro studies of healthy adult lung fibroblasts with added pro-fibrotic factor and transforming growth factor (TGF-ß 1), the researchers found that pirfenidone was effective at decreasing the production of a collagen-binding protein called HSP47. This protein is ubiquitous in fibrotic tissue. The study also showed that pirfenidone decreased the production of collagen type 1, which, when uninhibited, increases fibrosis.15
CAPACITY trials. In the CAPACITY trials, two phase 3 multinational studies conducted from 2006 to 2008, patients were given either pirfenidone or placebo.16 In the first study arm, patients were assigned to pirfenidone 2,403 mg/d (n = 174), pirfenidone 1,197 mg/d (n = 87), or placebo (n = 174). In the second study arm, 171 patients received pirfenidone 2,403 mg/d and 173 patients received placebo. Endpoints were measured at baseline and up to week 72.
The first study arm found that the mean rate of decline of FVC—the primary endpoint—was 4.4% less in the treatment group than in the placebo group (p = 0.001), and there was a 36% decrease in risk for death or disease progression in the treatment group (HR, 0.64; p, 0.023). (Endpoints were defined as: time to confirmed > 10% decline in percentage predicted FVC, > 15% decline in percentage predicted DLCO, or death.) The researchers found no clinically significant change in the six-minute walk test—a secondary endpoint of the study.16
The second study arm, however, found no statistically significant change in FVC between the treatment and placebo groups (with a 0.6% smaller decrease in FVC in the pirfenidone group), nor did they see a difference in progression-free survival. However, there was a significant change in the six-minute walk test between the treatment and placebo groups (p = 0.0009). Throughout the study, the most common adverse effects included nausea (36%), rash (32%), and dyspepsia (19%).16
ASCEND trial. The 2014 Assessment of Pirfenidone to Confirm Efficacy and Safety in Idiopathic Pulmonary Fibrosis (ASCEND) trial was a phase 3, multinational, randomized, double-blind, placebo-controlled study of the use of pirfenidone 2,403 mg/d.17 The study was conducted from 2012 to 2013. Of the total number of patients (N = 522), half received pirfenidone and half received placebo. After 52 weeks of treatment (the end of the study), the researchers found a smaller decline in FVC—the primary endpoint—in the treatment group compared to placebo (mean decline, 235 mL vs 428 mL, respectively [p < 0.001]). Regarding the six-minute walk test, the investigators found that 25.9% of the treatment group exhibited a decrease of ≥ 50 meters, compared to 35.7% of the placebo group (p = 0.04). (Progression-free survival was defined as a confirmed ≥ 10% decrease in predicted FVC, a confirmed decrease of 50 meters in the six-minute walk test, or death.)
The pirfenidone group in the ASCEND trial showed a 43% reduced risk for death or disease progression (HR, 0.57; p, < 0.001).16,17 All-cause mortality was lower in the pirfenidone group (4%) than in the placebo group (7.2%), but this was not statistically significant. Deaths from IPF in the pirfenidone group totaled three patients (1.1%) versus seven patients (2.5%) in the placebo group; this was also not statistically significant. The most common adverse effects seen during the study were nausea (36%), rash (28.1%), and headache (25.9%).17
Recommendations for use. Liver function testing should be performed at baseline, monthly for six months, and every three months afterward, as elevations in liver enzymes have been observed.18 Pirfenidone is a CYP1A2 substrate; moderate-to-strong CYP1A2 inhibitors should therefore be discontinued prior to initiation, as they are likely to decrease exposure and efficacy of pirfenidone. There are currently no black box warnings.18
Continue to: Nintedanib
Nintedanib
Hostettler et al studied lung samples from patients with IPF to determine the mechanism of action of nintedanib.19 Evaluation of fibroblasts derived from IPF samples revealed that they contained higher levels of platelet-derived growth factor (PDGF) than did nonfibrotic control cells. They also found that nintedanib, a tyrosine kinase inhibitor, significantly inhibited the phosphorylation of fibrotic-inducing growth factors—PDGF as well as vascular endothelial growth factor (VEGF).
INPULSIS trials. A phase 3 replicate of randomized, double-blind, multinational studies, the INPULSIS trials were performed between 2011 and 2012.20 Two study arms were used to evaluate a total of 638 patients who received nintedanib 150 mg bid for 52 weeks. The primary endpoint was annual rate of decline of FVC.
The researchers also evaluated efficacy through two other endpoints: patient-reported quality of life and symptoms via the St. George’s Respiratory Questionnaire (SGRQ) and evaluation of time to acute exacerbation. The latter was defined as worsening or new dyspnea, new diffuse pulmonary infiltrates visualized on chest radiography and/or HRCT, or the development of parenchymal abnormalities with no pneumothorax or pleural effusion since the preceding visit; and exclusion of any known causes of acute worsening, including infection, heart failure, pulmonary embolism, and any identifiable cause of acute lung injury.20
INPULSIS 1 (first arm) included 309 patients in the treatment group. Results showed an adjusted annual rate of decline in FVC of 114.7 mL/year, versus 239.9 mL/year in the placebo group (p < 0.001). In the treatment group, 52.8% exhibited ≤ 5% decline in FVC, compared to 38.2% in the placebo group (p = 0.001). No significant between-group differences were found in SGRQ score or time to acute exacerbation.20
INPULSIS 2 had 329 patients receiving nintedanib. An annual rate of decline in FVC of 113.6 mL/year from baseline was observed in the treatment group, compared to 207.3 mL/year in the placebo group (p < 0.001). In the treatment group, 53.2% showed ≤ 5% decline in FVC, versus 39.3% in the placebo group (p = 0.001). There was also a significantly smaller increase in total SGRQ score (meaning, less deterioration in quality of life) in the nintedanib group versus the placebo group (p = 0.02). A statistically significant increase in time to first acute exacerbation was observed in the nintedanib group (p = 0.005).20
There was no significant difference between groups in death from any cause, death from respiratory causation, or death that occurred between randomization and 28 days post treatment. The most common adverse effects seen throughout the two trials included diarrhea (trial 1, 61.5%; trial 2, 63.2%), nausea (trial 1, 22.7%; trial 2, 26.1%), and nasopharyngitis (trial 1, 12.6%; trial 2, 14.6%).20
Recommendations for use. Liver function testing should be performed at baseline, at regular intervals during the first three months, then periodically thereafter; patients in the treatment group of both INPULSIS trials had elevated liver enzymes, and cases of drug-induced liver injury have been observed with use of nintedanib.21 This medication may increase risk for bleeding due to its mechanism of action (VEGFR inhibition). Coadministration with CYP3A4 inhibitors may increase concentration of nintedanib; therefore, close monitoring is recommended. Avoid coadministration with CYP3A4 inducers, as this may decrease concentration of nintedanib by 50%. There are currently no black box warnings.21
Continue to: Patient monitoring
Patient monitoring
The ATS recommends measuring FVC and DLCO every three to six months, or sooner if clinically indicated.13 Pulse oximetry should be measured at rest and on exertion in all patients, regardless of symptoms, to assure proper saturation and identify the need for supplemental oxygen; this should also be done every three to six months.
The ATS recommends prompt detection and treatment of comorbidities such as pulmonary hypertension, emphysema, airflow obstruction, GERD, sleep apnea, and coronary artery disease.13 These recommendations are based on the organization’s 2015 guidelines.
OUTCOME FOR THE CASE PATIENT
The patient was started on pirfenidone (2,403 mg/d). He is continuing treatment and showing improvements in quality of life and slowed deterioration of lung function.
CONCLUSION
IPF causes progressive fibrosis of lung interstitium. The etiology is unknown, the symptoms and signs are vague, and mean life expectancy following diagnosis is two to five years. The most recent IPF guidelines recommend avoiding use of anticoagulants and immunosuppressants (eg, steroids, azathioprine, and N-acetylcysteine), due to their proven ineffectiveness and harm to patients with IPF.
Since the FDA’s approval of pirfenidone and nintedanib, the ATS has made recommendations for their use in patients with IPF. Despite mixed results in clinical trials, both drugs have demonstrated the ability to slow the decline in FVC over time, with relatively benign adverse effects. It is difficult to compare pirfenidone and nintedanib, or to recommend use of one drug over the other. However, it is promising that patients with this routinely fatal disease now have treatment options that can potentially modulate their disease progression.
1. Kim DS, Collard HR, King TE Jr. Classification and natural history of the idiopathic interstitial pneumonias. Proc Am Thorac Soc. 2006;3(4):285-292.
2. Frankel SK, Schwarz MI. Update in idiopathic pulmonary fibrosis. Curr Opin Pulm Med. 2009;15(5):463-469.
3. Olson AL, Swigris JJ, Lezotte DC, et al. Mortality from pulmonary fibrosis increased in the United States from 1992 to 2003. Am J Respir Crit Care Med. 2007;176(3):277-284.
4. Chapman JT. Interstitial lung disease. Cleveland Clinic. August 2010. www.clevelandclinicmeded.com/medical pubs/diseasemanagement/pulmonary/interstitial-lung-disease. Accessed March 12, 2018.
5. Cleveland Clinic. Nonspecific interstitial pneumonia. January 16, 2015. https://my.clevelandclinic.org/health/articles/nonspecific-interstitial-pneumonia. Accessed March 12, 2018.
6. Skandhan AKP, Weerakkody Y. Non-specific interstitial pneumonia. Radiopaedia. https://radiopaedia.org/articles/non-specific-interstitial-pneumonia-1. Accessed March 12, 2018.
7. Tatco V, Weerakkody Y. Lymphocytic interstitial pneumonitis. Radiopaedia. https://radiopaedia.org/articles/lymphocytic-interstitial-pneumonitis-1. Accessed March 12, 2018.
8. King TE Jr, Flaherty KR, Hollingsworth H. Cryptogenic organizing pneumonia. UpToDate. www.uptodate.com/contents/cryptogenic-organizing-pneumonia#H12. Accessed March 12, 2018.
9. Patel NM, Lederer DJ, Borczuk AC, Kawut SM. Pulmonary hypertension in idiopathic pulmonary fibrosis. Chest. 2007; 132(3):998-1006.
10. Lee J. Overview of idiopathic interstitial pneumonias. April 2016. www.merckmanuals.com/professional/pulmonary-disorders/interstitial-lung-diseases/overview-of-idiopathic-interstitial-pneumonias. Accessed March 12, 2018.
11. Lynch DA, Sverzellati N, Travis WD, et al. Diagnostic criteria for idiopathic pulmonary fibrosis: a Fleischner Society White Paper. Lancet Respir Med. 2018;6(2):138-153.
12. Martinez FJ, Flaherty K. Pulmonary function testing in idiopathic interstitial pneumonias. Proc Am Thorac Soc. 2006; 3(4):315-321.
13. Raghu G, Rochwerg B, Zhang Y, et al; American Thoracic Society; European Respiratory Society; Japanese Respiratory Society; Latin American Thoracic Association. An official ATS/ERS/JRS/ALAT clinical practice guideline: treatment of idiopathic pulmonary fibrosis. An update of the 2011 Clinical Practice Guideline. Am J Respir Crit Care Med. 2015; 192(2):e3-e19.
14. Chowdhury BA; FDA. Two FDA drug approvals for idiopathic pulmonary fibrosis (IPF). October 15, 2014. https://blogs.fda.gov/fdavoice/index.php/2014/10/two-fda-drug-approvals-for-idiopathic-pulmonary-fibrosis-ipf/. Accessed March 12, 2018.
15. Nakayama S, Mukae H, Sakamoto N, et al. Pirfenidone inhibits the expression of HSP47 in TGF-beta1-stimulated human lung fibroblasts. Life Sci. 2008; 82(3-4):210-217.
16. Noble PW, Albera C, Bradford WZ, et al; CAPACITY Study Group. Pirfenidone in patients with idiopathic pulmonary fibrosis (CAPACITY): two randomized trials. Lancet. 2011;377: 1760-1769.
17. King TE Jr, Bradford WZ, Castro-Bernardini S, et al; ASCEND Study Group. A phase 3 trial of pirfenidone in patients with idiopathic pulmonary fibrosis. N Engl J Med. 2014;370(22): 2083-2092.
18. Esbriet [package insert]. South San Francisco, CA: Genentech, Inc; 2016.
19. Hostettler KE, Zhong J, Papakonstantinou E, et al. Anti-fibrotic effects of nintedanib in lung fibroblasts derived from patients with idiopathic pulmonary fibrosis. Respir Res. 2014;15(1):157.
20. Richeldi L, du Bois RM, Raghu G, et al; INPULSIS Trial Investigators. Efficacy and safety of nintedanib in idiopathic pulmonary fibrosis. N Engl J Med. 2014;370(22):2071-2082.
21. OFEV [package insert]. Ridgefield, CT: Boehringer Ingelheim Pharmaceuticals, Inc; 2018.
IN THIS ARTICLE
- Confirming the diagnosis
- Pirfenidone treatment
- Nintedanib treatment
A 64-year-old man has a one-year history of dyspnea on exertion and a nonproductive cough. His symptoms are gradually worsening and increasingly bothersome to him.
His medical history includes mild seasonal allergies and GERD, which is well-controlled by oral antihistamines and proton pump inhibitors. He has spent the past 30 years working a desk job as an accountant. He denies a history of smoking, exposure to secondhand smoke, and initiation of new medication.
He admits to increased fatigue, but denies fever, chills, lymphadenopathy, weight change, chest pain, wheezing, abdominal pain, diarrhea, vomiting, claudication, and swelling in the extremities. The rest of the review of systems is negative.
Lab results—complete blood count, comprehensive metabolic panel, TSH, antinuclear antibodies, erythrocyte sedimentation rate, and C-reactive protein—are within normal limits. Spirometry shows very mild restriction. A chest x-ray is abnormal but nonspecific, showing peripheral opacities. An ECG shows normal sinus rhythm.
The patient is given a trial of an inhaled steroid, which yields no improvement. Six months later, the patient is seen by a pulmonologist. Idiopathic pulmonary fibrosis (IPF) is diagnosed based on high-resolution CT (HRCT) and lung biopsy results.
IPF is a chronic, progressive, fibrosing interstitial disease that is limited to lung tissue. It most commonly manifests in older adults with vague symptoms of dyspnea on exertion and nonproductive cough, but symptoms can also include fatigue, muscle and joint aches, clubbing of the fingernails, and weight loss.1 The average life expectancy following diagnosis of IPF is two to five years, and the mortality rate is estimated at 64.3 per million men and 58.4 per million women per year.2,3
Continue to: DIAGNOSIS
DIAGNOSIS
IPF belongs in the general class of idiopathic interstitial pneumonias (IIPs), which are characterized by varying degrees of inflammation and fibrosis of lung interstitium.4 All subtypes of IIPs cause dyspnea and diffuse abnormalities on HRCT, and all vary from each other histologically. Table 1 outlines the key features of each.5-8
Because of its vague symptomology and the extensive workup needed to rule out other diseases, patients with IPF often have symptoms for one to two years before a diagnosis is made.1 Physical exam may reveal fine inspiratory rales in both lung bases and digital clubbing; eventual signs of pulmonary hypertension and right-sided heart failure may be appreciated.1,9
There are no specific diagnostic laboratory tests to confirm IPF; however, baseline labwork (as outlined in the case presentation) is typically ordered to rule out infection, thyroid disease, or connective tissue disease.10 Many patients are referred to a cardiologist before being seen by a pulmonologist; cardiac stress testing may be done, and an echocardiogram may be performed to rule out heart failure.
Diagnostic testing may include pulmonary function testing, HRCT of the chest, and lung biopsy.10 Tissue samples from patients with IPF reveal different stages of disease, including dense fibrosis with honeycombing, subpleural or paraseptal distribution, fibroblast foci, and normal tissue.11 Pulmonary function test results will show a restrictive pattern. Both forced expiratory volume in one second (FEV1) and forced vital capacity (FVC) will be reduced, and the FEV1/FVC ratio preserved. Due to decreased functional lung volume, diffusing capacity of the lung for carbon monoxide (DLCO) will also be reduced.4,12
The differential is broad and includes allergic asthma, bronchitis, COPD, lung cancer, hypersensitivity pneumonitis, asbestosis, or pulmonary embolism.
Continue to: TREATMENT HISTORY
TREATMENT HISTORY
IPF has a long history of tried and failed treatment options. The American Thoracic Society (ATS), in concert with other professional organizations, has published comprehensive guidelines and recommendations pertaining to the use of pharmacologic medications to control disease progression. Warfarin and other anticoagulants have been studied, based on the observation that a procoagulant state promotes fibrotic changes in the lung tissue.13 However, anticoagulant use is not recommended in patients with IPF due to lack of efficacy and high potential for harm.13
Immunosuppressants have also been in the spotlight as possible treatment for IPF, but a clinical study investigating the efficacy of a three-drug regimen including prednisone, azathioprine, and N-acetylcysteine was stopped early due to increased risk for harm. Endothelin antagonists and potent tyrosine kinase inhibitors are also not recommended in the most recent edition of IPF guidelines, as they lack benefit.13
In fact, prior to the 2015 edition of the guidelines, no single medication was routinely recommended for patients with IPF. But this is now changing, following the 2014 FDA approval of two new drugs, nintedanib and pirfenidone, designed specifically to treat IPF.14 These drugs have shown promise in clinical trials (results of which are summarized in Table 2).
Continue to: NEW PHARMACOLOGIC OPTIONS
NEW PHARMACOLOGIC OPTIONS
Pirfenidone
In 2008, a study was conducted in Japan to determine the mechanism of action of pirfenidone.15 Through in vitro studies of healthy adult lung fibroblasts with added pro-fibrotic factor and transforming growth factor (TGF-ß 1), the researchers found that pirfenidone was effective at decreasing the production of a collagen-binding protein called HSP47. This protein is ubiquitous in fibrotic tissue. The study also showed that pirfenidone decreased the production of collagen type 1, which, when uninhibited, increases fibrosis.15
CAPACITY trials. In the CAPACITY trials, two phase 3 multinational studies conducted from 2006 to 2008, patients were given either pirfenidone or placebo.16 In the first study arm, patients were assigned to pirfenidone 2,403 mg/d (n = 174), pirfenidone 1,197 mg/d (n = 87), or placebo (n = 174). In the second study arm, 171 patients received pirfenidone 2,403 mg/d and 173 patients received placebo. Endpoints were measured at baseline and up to week 72.
The first study arm found that the mean rate of decline of FVC—the primary endpoint—was 4.4% less in the treatment group than in the placebo group (p = 0.001), and there was a 36% decrease in risk for death or disease progression in the treatment group (HR, 0.64; p, 0.023). (Endpoints were defined as: time to confirmed > 10% decline in percentage predicted FVC, > 15% decline in percentage predicted DLCO, or death.) The researchers found no clinically significant change in the six-minute walk test—a secondary endpoint of the study.16
The second study arm, however, found no statistically significant change in FVC between the treatment and placebo groups (with a 0.6% smaller decrease in FVC in the pirfenidone group), nor did they see a difference in progression-free survival. However, there was a significant change in the six-minute walk test between the treatment and placebo groups (p = 0.0009). Throughout the study, the most common adverse effects included nausea (36%), rash (32%), and dyspepsia (19%).16
ASCEND trial. The 2014 Assessment of Pirfenidone to Confirm Efficacy and Safety in Idiopathic Pulmonary Fibrosis (ASCEND) trial was a phase 3, multinational, randomized, double-blind, placebo-controlled study of the use of pirfenidone 2,403 mg/d.17 The study was conducted from 2012 to 2013. Of the total number of patients (N = 522), half received pirfenidone and half received placebo. After 52 weeks of treatment (the end of the study), the researchers found a smaller decline in FVC—the primary endpoint—in the treatment group compared to placebo (mean decline, 235 mL vs 428 mL, respectively [p < 0.001]). Regarding the six-minute walk test, the investigators found that 25.9% of the treatment group exhibited a decrease of ≥ 50 meters, compared to 35.7% of the placebo group (p = 0.04). (Progression-free survival was defined as a confirmed ≥ 10% decrease in predicted FVC, a confirmed decrease of 50 meters in the six-minute walk test, or death.)
The pirfenidone group in the ASCEND trial showed a 43% reduced risk for death or disease progression (HR, 0.57; p, < 0.001).16,17 All-cause mortality was lower in the pirfenidone group (4%) than in the placebo group (7.2%), but this was not statistically significant. Deaths from IPF in the pirfenidone group totaled three patients (1.1%) versus seven patients (2.5%) in the placebo group; this was also not statistically significant. The most common adverse effects seen during the study were nausea (36%), rash (28.1%), and headache (25.9%).17
Recommendations for use. Liver function testing should be performed at baseline, monthly for six months, and every three months afterward, as elevations in liver enzymes have been observed.18 Pirfenidone is a CYP1A2 substrate; moderate-to-strong CYP1A2 inhibitors should therefore be discontinued prior to initiation, as they are likely to decrease exposure and efficacy of pirfenidone. There are currently no black box warnings.18
Continue to: Nintedanib
Nintedanib
Hostettler et al studied lung samples from patients with IPF to determine the mechanism of action of nintedanib.19 Evaluation of fibroblasts derived from IPF samples revealed that they contained higher levels of platelet-derived growth factor (PDGF) than did nonfibrotic control cells. They also found that nintedanib, a tyrosine kinase inhibitor, significantly inhibited the phosphorylation of fibrotic-inducing growth factors—PDGF as well as vascular endothelial growth factor (VEGF).
INPULSIS trials. A phase 3 replicate of randomized, double-blind, multinational studies, the INPULSIS trials were performed between 2011 and 2012.20 Two study arms were used to evaluate a total of 638 patients who received nintedanib 150 mg bid for 52 weeks. The primary endpoint was annual rate of decline of FVC.
The researchers also evaluated efficacy through two other endpoints: patient-reported quality of life and symptoms via the St. George’s Respiratory Questionnaire (SGRQ) and evaluation of time to acute exacerbation. The latter was defined as worsening or new dyspnea, new diffuse pulmonary infiltrates visualized on chest radiography and/or HRCT, or the development of parenchymal abnormalities with no pneumothorax or pleural effusion since the preceding visit; and exclusion of any known causes of acute worsening, including infection, heart failure, pulmonary embolism, and any identifiable cause of acute lung injury.20
INPULSIS 1 (first arm) included 309 patients in the treatment group. Results showed an adjusted annual rate of decline in FVC of 114.7 mL/year, versus 239.9 mL/year in the placebo group (p < 0.001). In the treatment group, 52.8% exhibited ≤ 5% decline in FVC, compared to 38.2% in the placebo group (p = 0.001). No significant between-group differences were found in SGRQ score or time to acute exacerbation.20
INPULSIS 2 had 329 patients receiving nintedanib. An annual rate of decline in FVC of 113.6 mL/year from baseline was observed in the treatment group, compared to 207.3 mL/year in the placebo group (p < 0.001). In the treatment group, 53.2% showed ≤ 5% decline in FVC, versus 39.3% in the placebo group (p = 0.001). There was also a significantly smaller increase in total SGRQ score (meaning, less deterioration in quality of life) in the nintedanib group versus the placebo group (p = 0.02). A statistically significant increase in time to first acute exacerbation was observed in the nintedanib group (p = 0.005).20
There was no significant difference between groups in death from any cause, death from respiratory causation, or death that occurred between randomization and 28 days post treatment. The most common adverse effects seen throughout the two trials included diarrhea (trial 1, 61.5%; trial 2, 63.2%), nausea (trial 1, 22.7%; trial 2, 26.1%), and nasopharyngitis (trial 1, 12.6%; trial 2, 14.6%).20
Recommendations for use. Liver function testing should be performed at baseline, at regular intervals during the first three months, then periodically thereafter; patients in the treatment group of both INPULSIS trials had elevated liver enzymes, and cases of drug-induced liver injury have been observed with use of nintedanib.21 This medication may increase risk for bleeding due to its mechanism of action (VEGFR inhibition). Coadministration with CYP3A4 inhibitors may increase concentration of nintedanib; therefore, close monitoring is recommended. Avoid coadministration with CYP3A4 inducers, as this may decrease concentration of nintedanib by 50%. There are currently no black box warnings.21
Continue to: Patient monitoring
Patient monitoring
The ATS recommends measuring FVC and DLCO every three to six months, or sooner if clinically indicated.13 Pulse oximetry should be measured at rest and on exertion in all patients, regardless of symptoms, to assure proper saturation and identify the need for supplemental oxygen; this should also be done every three to six months.
The ATS recommends prompt detection and treatment of comorbidities such as pulmonary hypertension, emphysema, airflow obstruction, GERD, sleep apnea, and coronary artery disease.13 These recommendations are based on the organization’s 2015 guidelines.
OUTCOME FOR THE CASE PATIENT
The patient was started on pirfenidone (2,403 mg/d). He is continuing treatment and showing improvements in quality of life and slowed deterioration of lung function.
CONCLUSION
IPF causes progressive fibrosis of lung interstitium. The etiology is unknown, the symptoms and signs are vague, and mean life expectancy following diagnosis is two to five years. The most recent IPF guidelines recommend avoiding use of anticoagulants and immunosuppressants (eg, steroids, azathioprine, and N-acetylcysteine), due to their proven ineffectiveness and harm to patients with IPF.
Since the FDA’s approval of pirfenidone and nintedanib, the ATS has made recommendations for their use in patients with IPF. Despite mixed results in clinical trials, both drugs have demonstrated the ability to slow the decline in FVC over time, with relatively benign adverse effects. It is difficult to compare pirfenidone and nintedanib, or to recommend use of one drug over the other. However, it is promising that patients with this routinely fatal disease now have treatment options that can potentially modulate their disease progression.
IN THIS ARTICLE
- Confirming the diagnosis
- Pirfenidone treatment
- Nintedanib treatment
A 64-year-old man has a one-year history of dyspnea on exertion and a nonproductive cough. His symptoms are gradually worsening and increasingly bothersome to him.
His medical history includes mild seasonal allergies and GERD, which is well-controlled by oral antihistamines and proton pump inhibitors. He has spent the past 30 years working a desk job as an accountant. He denies a history of smoking, exposure to secondhand smoke, and initiation of new medication.
He admits to increased fatigue, but denies fever, chills, lymphadenopathy, weight change, chest pain, wheezing, abdominal pain, diarrhea, vomiting, claudication, and swelling in the extremities. The rest of the review of systems is negative.
Lab results—complete blood count, comprehensive metabolic panel, TSH, antinuclear antibodies, erythrocyte sedimentation rate, and C-reactive protein—are within normal limits. Spirometry shows very mild restriction. A chest x-ray is abnormal but nonspecific, showing peripheral opacities. An ECG shows normal sinus rhythm.
The patient is given a trial of an inhaled steroid, which yields no improvement. Six months later, the patient is seen by a pulmonologist. Idiopathic pulmonary fibrosis (IPF) is diagnosed based on high-resolution CT (HRCT) and lung biopsy results.
IPF is a chronic, progressive, fibrosing interstitial disease that is limited to lung tissue. It most commonly manifests in older adults with vague symptoms of dyspnea on exertion and nonproductive cough, but symptoms can also include fatigue, muscle and joint aches, clubbing of the fingernails, and weight loss.1 The average life expectancy following diagnosis of IPF is two to five years, and the mortality rate is estimated at 64.3 per million men and 58.4 per million women per year.2,3
Continue to: DIAGNOSIS
DIAGNOSIS
IPF belongs in the general class of idiopathic interstitial pneumonias (IIPs), which are characterized by varying degrees of inflammation and fibrosis of lung interstitium.4 All subtypes of IIPs cause dyspnea and diffuse abnormalities on HRCT, and all vary from each other histologically. Table 1 outlines the key features of each.5-8
Because of its vague symptomology and the extensive workup needed to rule out other diseases, patients with IPF often have symptoms for one to two years before a diagnosis is made.1 Physical exam may reveal fine inspiratory rales in both lung bases and digital clubbing; eventual signs of pulmonary hypertension and right-sided heart failure may be appreciated.1,9
There are no specific diagnostic laboratory tests to confirm IPF; however, baseline labwork (as outlined in the case presentation) is typically ordered to rule out infection, thyroid disease, or connective tissue disease.10 Many patients are referred to a cardiologist before being seen by a pulmonologist; cardiac stress testing may be done, and an echocardiogram may be performed to rule out heart failure.
Diagnostic testing may include pulmonary function testing, HRCT of the chest, and lung biopsy.10 Tissue samples from patients with IPF reveal different stages of disease, including dense fibrosis with honeycombing, subpleural or paraseptal distribution, fibroblast foci, and normal tissue.11 Pulmonary function test results will show a restrictive pattern. Both forced expiratory volume in one second (FEV1) and forced vital capacity (FVC) will be reduced, and the FEV1/FVC ratio preserved. Due to decreased functional lung volume, diffusing capacity of the lung for carbon monoxide (DLCO) will also be reduced.4,12
The differential is broad and includes allergic asthma, bronchitis, COPD, lung cancer, hypersensitivity pneumonitis, asbestosis, or pulmonary embolism.
Continue to: TREATMENT HISTORY
TREATMENT HISTORY
IPF has a long history of tried and failed treatment options. The American Thoracic Society (ATS), in concert with other professional organizations, has published comprehensive guidelines and recommendations pertaining to the use of pharmacologic medications to control disease progression. Warfarin and other anticoagulants have been studied, based on the observation that a procoagulant state promotes fibrotic changes in the lung tissue.13 However, anticoagulant use is not recommended in patients with IPF due to lack of efficacy and high potential for harm.13
Immunosuppressants have also been in the spotlight as possible treatment for IPF, but a clinical study investigating the efficacy of a three-drug regimen including prednisone, azathioprine, and N-acetylcysteine was stopped early due to increased risk for harm. Endothelin antagonists and potent tyrosine kinase inhibitors are also not recommended in the most recent edition of IPF guidelines, as they lack benefit.13
In fact, prior to the 2015 edition of the guidelines, no single medication was routinely recommended for patients with IPF. But this is now changing, following the 2014 FDA approval of two new drugs, nintedanib and pirfenidone, designed specifically to treat IPF.14 These drugs have shown promise in clinical trials (results of which are summarized in Table 2).
Continue to: NEW PHARMACOLOGIC OPTIONS
NEW PHARMACOLOGIC OPTIONS
Pirfenidone
In 2008, a study was conducted in Japan to determine the mechanism of action of pirfenidone.15 Through in vitro studies of healthy adult lung fibroblasts with added pro-fibrotic factor and transforming growth factor (TGF-ß 1), the researchers found that pirfenidone was effective at decreasing the production of a collagen-binding protein called HSP47. This protein is ubiquitous in fibrotic tissue. The study also showed that pirfenidone decreased the production of collagen type 1, which, when uninhibited, increases fibrosis.15
CAPACITY trials. In the CAPACITY trials, two phase 3 multinational studies conducted from 2006 to 2008, patients were given either pirfenidone or placebo.16 In the first study arm, patients were assigned to pirfenidone 2,403 mg/d (n = 174), pirfenidone 1,197 mg/d (n = 87), or placebo (n = 174). In the second study arm, 171 patients received pirfenidone 2,403 mg/d and 173 patients received placebo. Endpoints were measured at baseline and up to week 72.
The first study arm found that the mean rate of decline of FVC—the primary endpoint—was 4.4% less in the treatment group than in the placebo group (p = 0.001), and there was a 36% decrease in risk for death or disease progression in the treatment group (HR, 0.64; p, 0.023). (Endpoints were defined as: time to confirmed > 10% decline in percentage predicted FVC, > 15% decline in percentage predicted DLCO, or death.) The researchers found no clinically significant change in the six-minute walk test—a secondary endpoint of the study.16
The second study arm, however, found no statistically significant change in FVC between the treatment and placebo groups (with a 0.6% smaller decrease in FVC in the pirfenidone group), nor did they see a difference in progression-free survival. However, there was a significant change in the six-minute walk test between the treatment and placebo groups (p = 0.0009). Throughout the study, the most common adverse effects included nausea (36%), rash (32%), and dyspepsia (19%).16
ASCEND trial. The 2014 Assessment of Pirfenidone to Confirm Efficacy and Safety in Idiopathic Pulmonary Fibrosis (ASCEND) trial was a phase 3, multinational, randomized, double-blind, placebo-controlled study of the use of pirfenidone 2,403 mg/d.17 The study was conducted from 2012 to 2013. Of the total number of patients (N = 522), half received pirfenidone and half received placebo. After 52 weeks of treatment (the end of the study), the researchers found a smaller decline in FVC—the primary endpoint—in the treatment group compared to placebo (mean decline, 235 mL vs 428 mL, respectively [p < 0.001]). Regarding the six-minute walk test, the investigators found that 25.9% of the treatment group exhibited a decrease of ≥ 50 meters, compared to 35.7% of the placebo group (p = 0.04). (Progression-free survival was defined as a confirmed ≥ 10% decrease in predicted FVC, a confirmed decrease of 50 meters in the six-minute walk test, or death.)
The pirfenidone group in the ASCEND trial showed a 43% reduced risk for death or disease progression (HR, 0.57; p, < 0.001).16,17 All-cause mortality was lower in the pirfenidone group (4%) than in the placebo group (7.2%), but this was not statistically significant. Deaths from IPF in the pirfenidone group totaled three patients (1.1%) versus seven patients (2.5%) in the placebo group; this was also not statistically significant. The most common adverse effects seen during the study were nausea (36%), rash (28.1%), and headache (25.9%).17
Recommendations for use. Liver function testing should be performed at baseline, monthly for six months, and every three months afterward, as elevations in liver enzymes have been observed.18 Pirfenidone is a CYP1A2 substrate; moderate-to-strong CYP1A2 inhibitors should therefore be discontinued prior to initiation, as they are likely to decrease exposure and efficacy of pirfenidone. There are currently no black box warnings.18
Continue to: Nintedanib
Nintedanib
Hostettler et al studied lung samples from patients with IPF to determine the mechanism of action of nintedanib.19 Evaluation of fibroblasts derived from IPF samples revealed that they contained higher levels of platelet-derived growth factor (PDGF) than did nonfibrotic control cells. They also found that nintedanib, a tyrosine kinase inhibitor, significantly inhibited the phosphorylation of fibrotic-inducing growth factors—PDGF as well as vascular endothelial growth factor (VEGF).
INPULSIS trials. A phase 3 replicate of randomized, double-blind, multinational studies, the INPULSIS trials were performed between 2011 and 2012.20 Two study arms were used to evaluate a total of 638 patients who received nintedanib 150 mg bid for 52 weeks. The primary endpoint was annual rate of decline of FVC.
The researchers also evaluated efficacy through two other endpoints: patient-reported quality of life and symptoms via the St. George’s Respiratory Questionnaire (SGRQ) and evaluation of time to acute exacerbation. The latter was defined as worsening or new dyspnea, new diffuse pulmonary infiltrates visualized on chest radiography and/or HRCT, or the development of parenchymal abnormalities with no pneumothorax or pleural effusion since the preceding visit; and exclusion of any known causes of acute worsening, including infection, heart failure, pulmonary embolism, and any identifiable cause of acute lung injury.20
INPULSIS 1 (first arm) included 309 patients in the treatment group. Results showed an adjusted annual rate of decline in FVC of 114.7 mL/year, versus 239.9 mL/year in the placebo group (p < 0.001). In the treatment group, 52.8% exhibited ≤ 5% decline in FVC, compared to 38.2% in the placebo group (p = 0.001). No significant between-group differences were found in SGRQ score or time to acute exacerbation.20
INPULSIS 2 had 329 patients receiving nintedanib. An annual rate of decline in FVC of 113.6 mL/year from baseline was observed in the treatment group, compared to 207.3 mL/year in the placebo group (p < 0.001). In the treatment group, 53.2% showed ≤ 5% decline in FVC, versus 39.3% in the placebo group (p = 0.001). There was also a significantly smaller increase in total SGRQ score (meaning, less deterioration in quality of life) in the nintedanib group versus the placebo group (p = 0.02). A statistically significant increase in time to first acute exacerbation was observed in the nintedanib group (p = 0.005).20
There was no significant difference between groups in death from any cause, death from respiratory causation, or death that occurred between randomization and 28 days post treatment. The most common adverse effects seen throughout the two trials included diarrhea (trial 1, 61.5%; trial 2, 63.2%), nausea (trial 1, 22.7%; trial 2, 26.1%), and nasopharyngitis (trial 1, 12.6%; trial 2, 14.6%).20
Recommendations for use. Liver function testing should be performed at baseline, at regular intervals during the first three months, then periodically thereafter; patients in the treatment group of both INPULSIS trials had elevated liver enzymes, and cases of drug-induced liver injury have been observed with use of nintedanib.21 This medication may increase risk for bleeding due to its mechanism of action (VEGFR inhibition). Coadministration with CYP3A4 inhibitors may increase concentration of nintedanib; therefore, close monitoring is recommended. Avoid coadministration with CYP3A4 inducers, as this may decrease concentration of nintedanib by 50%. There are currently no black box warnings.21
Continue to: Patient monitoring
Patient monitoring
The ATS recommends measuring FVC and DLCO every three to six months, or sooner if clinically indicated.13 Pulse oximetry should be measured at rest and on exertion in all patients, regardless of symptoms, to assure proper saturation and identify the need for supplemental oxygen; this should also be done every three to six months.
The ATS recommends prompt detection and treatment of comorbidities such as pulmonary hypertension, emphysema, airflow obstruction, GERD, sleep apnea, and coronary artery disease.13 These recommendations are based on the organization’s 2015 guidelines.
OUTCOME FOR THE CASE PATIENT
The patient was started on pirfenidone (2,403 mg/d). He is continuing treatment and showing improvements in quality of life and slowed deterioration of lung function.
CONCLUSION
IPF causes progressive fibrosis of lung interstitium. The etiology is unknown, the symptoms and signs are vague, and mean life expectancy following diagnosis is two to five years. The most recent IPF guidelines recommend avoiding use of anticoagulants and immunosuppressants (eg, steroids, azathioprine, and N-acetylcysteine), due to their proven ineffectiveness and harm to patients with IPF.
Since the FDA’s approval of pirfenidone and nintedanib, the ATS has made recommendations for their use in patients with IPF. Despite mixed results in clinical trials, both drugs have demonstrated the ability to slow the decline in FVC over time, with relatively benign adverse effects. It is difficult to compare pirfenidone and nintedanib, or to recommend use of one drug over the other. However, it is promising that patients with this routinely fatal disease now have treatment options that can potentially modulate their disease progression.
1. Kim DS, Collard HR, King TE Jr. Classification and natural history of the idiopathic interstitial pneumonias. Proc Am Thorac Soc. 2006;3(4):285-292.
2. Frankel SK, Schwarz MI. Update in idiopathic pulmonary fibrosis. Curr Opin Pulm Med. 2009;15(5):463-469.
3. Olson AL, Swigris JJ, Lezotte DC, et al. Mortality from pulmonary fibrosis increased in the United States from 1992 to 2003. Am J Respir Crit Care Med. 2007;176(3):277-284.
4. Chapman JT. Interstitial lung disease. Cleveland Clinic. August 2010. www.clevelandclinicmeded.com/medical pubs/diseasemanagement/pulmonary/interstitial-lung-disease. Accessed March 12, 2018.
5. Cleveland Clinic. Nonspecific interstitial pneumonia. January 16, 2015. https://my.clevelandclinic.org/health/articles/nonspecific-interstitial-pneumonia. Accessed March 12, 2018.
6. Skandhan AKP, Weerakkody Y. Non-specific interstitial pneumonia. Radiopaedia. https://radiopaedia.org/articles/non-specific-interstitial-pneumonia-1. Accessed March 12, 2018.
7. Tatco V, Weerakkody Y. Lymphocytic interstitial pneumonitis. Radiopaedia. https://radiopaedia.org/articles/lymphocytic-interstitial-pneumonitis-1. Accessed March 12, 2018.
8. King TE Jr, Flaherty KR, Hollingsworth H. Cryptogenic organizing pneumonia. UpToDate. www.uptodate.com/contents/cryptogenic-organizing-pneumonia#H12. Accessed March 12, 2018.
9. Patel NM, Lederer DJ, Borczuk AC, Kawut SM. Pulmonary hypertension in idiopathic pulmonary fibrosis. Chest. 2007; 132(3):998-1006.
10. Lee J. Overview of idiopathic interstitial pneumonias. April 2016. www.merckmanuals.com/professional/pulmonary-disorders/interstitial-lung-diseases/overview-of-idiopathic-interstitial-pneumonias. Accessed March 12, 2018.
11. Lynch DA, Sverzellati N, Travis WD, et al. Diagnostic criteria for idiopathic pulmonary fibrosis: a Fleischner Society White Paper. Lancet Respir Med. 2018;6(2):138-153.
12. Martinez FJ, Flaherty K. Pulmonary function testing in idiopathic interstitial pneumonias. Proc Am Thorac Soc. 2006; 3(4):315-321.
13. Raghu G, Rochwerg B, Zhang Y, et al; American Thoracic Society; European Respiratory Society; Japanese Respiratory Society; Latin American Thoracic Association. An official ATS/ERS/JRS/ALAT clinical practice guideline: treatment of idiopathic pulmonary fibrosis. An update of the 2011 Clinical Practice Guideline. Am J Respir Crit Care Med. 2015; 192(2):e3-e19.
14. Chowdhury BA; FDA. Two FDA drug approvals for idiopathic pulmonary fibrosis (IPF). October 15, 2014. https://blogs.fda.gov/fdavoice/index.php/2014/10/two-fda-drug-approvals-for-idiopathic-pulmonary-fibrosis-ipf/. Accessed March 12, 2018.
15. Nakayama S, Mukae H, Sakamoto N, et al. Pirfenidone inhibits the expression of HSP47 in TGF-beta1-stimulated human lung fibroblasts. Life Sci. 2008; 82(3-4):210-217.
16. Noble PW, Albera C, Bradford WZ, et al; CAPACITY Study Group. Pirfenidone in patients with idiopathic pulmonary fibrosis (CAPACITY): two randomized trials. Lancet. 2011;377: 1760-1769.
17. King TE Jr, Bradford WZ, Castro-Bernardini S, et al; ASCEND Study Group. A phase 3 trial of pirfenidone in patients with idiopathic pulmonary fibrosis. N Engl J Med. 2014;370(22): 2083-2092.
18. Esbriet [package insert]. South San Francisco, CA: Genentech, Inc; 2016.
19. Hostettler KE, Zhong J, Papakonstantinou E, et al. Anti-fibrotic effects of nintedanib in lung fibroblasts derived from patients with idiopathic pulmonary fibrosis. Respir Res. 2014;15(1):157.
20. Richeldi L, du Bois RM, Raghu G, et al; INPULSIS Trial Investigators. Efficacy and safety of nintedanib in idiopathic pulmonary fibrosis. N Engl J Med. 2014;370(22):2071-2082.
21. OFEV [package insert]. Ridgefield, CT: Boehringer Ingelheim Pharmaceuticals, Inc; 2018.
1. Kim DS, Collard HR, King TE Jr. Classification and natural history of the idiopathic interstitial pneumonias. Proc Am Thorac Soc. 2006;3(4):285-292.
2. Frankel SK, Schwarz MI. Update in idiopathic pulmonary fibrosis. Curr Opin Pulm Med. 2009;15(5):463-469.
3. Olson AL, Swigris JJ, Lezotte DC, et al. Mortality from pulmonary fibrosis increased in the United States from 1992 to 2003. Am J Respir Crit Care Med. 2007;176(3):277-284.
4. Chapman JT. Interstitial lung disease. Cleveland Clinic. August 2010. www.clevelandclinicmeded.com/medical pubs/diseasemanagement/pulmonary/interstitial-lung-disease. Accessed March 12, 2018.
5. Cleveland Clinic. Nonspecific interstitial pneumonia. January 16, 2015. https://my.clevelandclinic.org/health/articles/nonspecific-interstitial-pneumonia. Accessed March 12, 2018.
6. Skandhan AKP, Weerakkody Y. Non-specific interstitial pneumonia. Radiopaedia. https://radiopaedia.org/articles/non-specific-interstitial-pneumonia-1. Accessed March 12, 2018.
7. Tatco V, Weerakkody Y. Lymphocytic interstitial pneumonitis. Radiopaedia. https://radiopaedia.org/articles/lymphocytic-interstitial-pneumonitis-1. Accessed March 12, 2018.
8. King TE Jr, Flaherty KR, Hollingsworth H. Cryptogenic organizing pneumonia. UpToDate. www.uptodate.com/contents/cryptogenic-organizing-pneumonia#H12. Accessed March 12, 2018.
9. Patel NM, Lederer DJ, Borczuk AC, Kawut SM. Pulmonary hypertension in idiopathic pulmonary fibrosis. Chest. 2007; 132(3):998-1006.
10. Lee J. Overview of idiopathic interstitial pneumonias. April 2016. www.merckmanuals.com/professional/pulmonary-disorders/interstitial-lung-diseases/overview-of-idiopathic-interstitial-pneumonias. Accessed March 12, 2018.
11. Lynch DA, Sverzellati N, Travis WD, et al. Diagnostic criteria for idiopathic pulmonary fibrosis: a Fleischner Society White Paper. Lancet Respir Med. 2018;6(2):138-153.
12. Martinez FJ, Flaherty K. Pulmonary function testing in idiopathic interstitial pneumonias. Proc Am Thorac Soc. 2006; 3(4):315-321.
13. Raghu G, Rochwerg B, Zhang Y, et al; American Thoracic Society; European Respiratory Society; Japanese Respiratory Society; Latin American Thoracic Association. An official ATS/ERS/JRS/ALAT clinical practice guideline: treatment of idiopathic pulmonary fibrosis. An update of the 2011 Clinical Practice Guideline. Am J Respir Crit Care Med. 2015; 192(2):e3-e19.
14. Chowdhury BA; FDA. Two FDA drug approvals for idiopathic pulmonary fibrosis (IPF). October 15, 2014. https://blogs.fda.gov/fdavoice/index.php/2014/10/two-fda-drug-approvals-for-idiopathic-pulmonary-fibrosis-ipf/. Accessed March 12, 2018.
15. Nakayama S, Mukae H, Sakamoto N, et al. Pirfenidone inhibits the expression of HSP47 in TGF-beta1-stimulated human lung fibroblasts. Life Sci. 2008; 82(3-4):210-217.
16. Noble PW, Albera C, Bradford WZ, et al; CAPACITY Study Group. Pirfenidone in patients with idiopathic pulmonary fibrosis (CAPACITY): two randomized trials. Lancet. 2011;377: 1760-1769.
17. King TE Jr, Bradford WZ, Castro-Bernardini S, et al; ASCEND Study Group. A phase 3 trial of pirfenidone in patients with idiopathic pulmonary fibrosis. N Engl J Med. 2014;370(22): 2083-2092.
18. Esbriet [package insert]. South San Francisco, CA: Genentech, Inc; 2016.
19. Hostettler KE, Zhong J, Papakonstantinou E, et al. Anti-fibrotic effects of nintedanib in lung fibroblasts derived from patients with idiopathic pulmonary fibrosis. Respir Res. 2014;15(1):157.
20. Richeldi L, du Bois RM, Raghu G, et al; INPULSIS Trial Investigators. Efficacy and safety of nintedanib in idiopathic pulmonary fibrosis. N Engl J Med. 2014;370(22):2071-2082.
21. OFEV [package insert]. Ridgefield, CT: Boehringer Ingelheim Pharmaceuticals, Inc; 2018.
Secondary Prevention of Low-Trauma Fractures: In Search of an Effective Solution
From the Department of Internal Medicine, University of Iowa Carver College of Medicine, Iowa City, IA.
Abstract
- Objective: To review and summarize the literature regarding current approaches to secondary prevention of low-trauma osteoporotic fractures.
- Methods: PubMed search and summary of existing literature related to complications and secondary prevention of osteoporotic fractures was performed.
- Results: Fragility fractures are associated with high rates of short and long term morbidities and carry a high risk of mortality and fracture recurrence. Several of the currently available anti-osteoporosis medications have been shown to decrease the risk of fracture recurrence in patients with prevalent osteoporotic fractures and some may even decrease mortality. However, only a minority of patients with fragility fractures are adequately evaluated and treated for osteoporosis. Fracture liaison services that ensure identification and risk stratification of patients with fragility fractures and proper evaluation and treatment of osteoporosis have proven effective at enhancing osteoporosis care in these patients, decreasing fracture recurrence and possibly even decreasing long-term mortality, while providing long-term cost savings. Unfortunately, however, this model of care has not been widely adopted and implemented.
- Conclusion: Fragility fractures represent a major health care problem for aging populations. Unfortunately, most patients with low-trauma fractures still receive suboptimal osteoporosis care.
Key words: osteoporosis; fracture; fragility; low-trauma; bone density.
Low-trauma fractures are fractures that occur from a trauma equivalent to a fall from standing height or less [1,2]. They can involve any skeletal site, but the most significant are vertebral, pelvic, wrist and hip fractures, which together represent close to 90% of all low-trauma fractures [3,4]. The overall burden of low-trauma fractures is quite high worldwide and is projected to increase over time [3–6]. In 2010, 3.5 million new low-trauma fractures were reported in the European Union [3]. In the United States, there were more than 2 million fractures in 2005, and it is estimated that more than 3 million fractures will occur in year 2025 [4].
Low-trauma fractures are generally indicative of compromised bone strength—especially when they involve the hip—and are thus often referred to as fragility fractures. While the traditional definition of osteoporosis is a bone mineral density (BMD) T-score of -2.5 or lower, low-trauma fractures of the hip are also diagnostic of osteoporosis, regardless of bone mineral density [2,7–9]. In addition, low-trauma fractures of the vertebrae, the proximal humerus, and the pelvis are considered diagnostic of osteoporosis when combined with T-scores between -1 and -2.5 [2,7]. Bone biopsies and high-resolution peripheral quantitative computed tomography (HR-pQCT) in patients with low-trauma fractures and normal BMD suggest microarchitectural alterations and abnormalities of collagen orientation and crosslinking within the bone matrix [10-12], leading to decreased bone strength.
This review will address the individual and societal costs of low-trauma fractures and issues related to secondary prevention of fractures, with specific emphasis on pharmacotherapy and fracture liaison services.
Impact of Low-Trauma Fractures
Acute and Long-Term Complications
Of all fragility fractures, hip fractures are the ones most likely to result in serious acute complications. The most common acute complications are delirium in up to 50% of patients and malnutrition in up to 60%, both of which predict slower and less complete recovery [13–16]. Other complications include urinary tract infections in up to 60% of patients in certain reports [17], thromboembolic disease with deep venous thrombosis in around 27% of patients and pulmonary embolism in up to 7% [16], and acute kidney injury in about 15% [18].
In addition, it is not uncommon for patients to suffer from significant long-term functional limitations following fragility fractures. While vertebral fractures do not frequently lead to hospitalization or institutionalization, they often lead to significant physical limitations and chronic pain [19,20] and to negative effects on self-esteem, mood, and body image [21,22]. However, the most remarkable functional decline and limitations are seen after hip fractures [23–25]. In a study of 2800 women and men with hip fracture, Beringer et al found that more than 30% were still institutionalized, and only 40% were able to walk outdoors independently 1 year later. Predictors of poor outcome included male sex, advanced age, cognitive impairment, and presence of comorbidities [23].
It is not surprising then that a fracture is often associated with an overall decline in the individual’s quality of life and this has been demonstrated in several studies [26–28]. In the largest study of this type, Tarride et al examined over 23,000 patients with fragility fractures and found a sharp decline in health-related quality of life (HRQOL) immediately after the fracture, which remained below baseline for up to 3 years [26]. The decline was worse in patients with hip and spine fractures compared to other fractures [27].
Mortality Following Fragility Fractures
Perhaps the most concerning complication, however, is the excess mortality seen after fractures. Several studies have demonstrated excess mortality after vertebral fractures, especially in the year following the fracture [29–33], but the highest increase in mortality was observed following hip fractures. In fact, the 30-day mortality after a hip fracture approximates 7% [23] and the excess 1-year mortality is estimated at 8% to 36% [34,35]. While the highest risk of mortality is seen in the first year following the fracture, the increased risk persists for at least 5 to 6 years [36]. Malnutrition, decreased mobility, male sex, and the number of coexisting medical comorbidities further increase the risk of mortality [29,32,34,36,37].
Risk of Fracture Recurrence
In both men and women, a fragility fracture at any site increases the risk of subsequent fractures [38–41], and the risk increases with the number of prevalent fractures [42]. Gehlbach et al estimated an 80% increase in the risk of fracture recurrence after 1 fracture, a threefold increase after 2 fractures, and an almost fivefold increase after 3 fractures [42]. The increase in risk is even more pronounced following vertebral fractures specifically, doubling after the first fracture an increasing by up to ninefold after 3 fractures [42, 43]. This increase in risk is highest in the first year following the fracture but may persist for up to 10 years [39,43].
Fracture Impact on Society
Fractures are associated with a high financial burden to society, in terms of direct acute care costs and long-term rehabilitation [3,4,44–48]. In 2010, the direct cost from fractures in the EU was estimated at €24.6 billion [3]. In the US, this cost was around $14.0 billion in 2002 and $16.9 billion in 2005 [4,48], and in Canada it was $1.5 billion in 2011 [47]. These numbers increase substantially when costs associated with long-term post-fracture rehabilitation are included, with an additional estimated yearly cost of €10.7 billion in the EU and $1.03 billion in Canada [3,47].
While hip fractures account for only about 18% of all low-trauma fractures, they are associated with the highest cost burden, accounting for about 50% to 70% of the total fracture-associated expenditures [3,4,44]. This is likely due to the fact almost all hip fractures require hospitalization, most require surgical repair and rehabilitation, and because they lead to the highest rates of morbidity and mortality.
Can Fracture Recurrence After a Low-Trauma Fracture Be Prevented?
Many approaches to secondary fracture prevention have been proposed, including but not limited to fall prevention, exercise therapy, nutrition therapy, prevention and treatment of sarcopenia, vitamin D and calcium supplementation, and osteoporosis pharmacotherapy [49–53]. Of those, osteoporosis pharmacotherapy has the strongest and most compelling efficacy data and will be reviewed in the following sections.
Effect of Antiresorptive Therapy After a Fracture
In the Fracture Intervention Trial (FIT), alendronate decreased the risk of new vertebral fractures by about 47% and of hip fractures by about 50% in women with preexisting vertebral fractures [54,55]. Similar fracture protection benefits were demonstrated in the Hip Intervention Program (HIP), where risedronate decreased the risk of hip fractures by 60% in women with prior history of vertebral fractures [56].
The best data regarding secondary prevention of hip fractures however comes from the Health Outcomes and Reduced Incidence with Zoledronic Acid Once Yearly (HORIZON) trial, where patients were randomized to zoledronic acid or placebo within 90 days of a hip fracture. Over a median duration of therapy of about 2 years, zoledronic acid decreased the risk of any new clinical fracture by 35%, of new vertebral fractures by 46%, and of recurrent hip fractures by 30% [57].
Effect of Anabolic Therapy After a Fracture
The Fracture Prevention Trial (FPT) compared the effect of teriparatide to placebo in women with at least 1 moderate or 2 mild atraumatic vertebral fractures and showed a 65% reduction in the risk of new vertebral fractures and a 53% reduction in the risk of new non-vertebral fractures [58]. Likewise, the Abaloparatide Comparator Trial In Vertebral Endpoints (ACTIVE) enrolled women with at least 2 mild vertebral fractures, 1 moderate vertebral fracture or history of a low trauma fracture of the forearm, humerus, sacrum, pelvis, hip, femur, or tibia. In this trial, abaloparatide decreased the risk of new vertebral fractures by 85% and of new non-vertebral fractures by 43% compared to placebo [59].
Will Anti-Osteoporosis Therapy After a Low-Trauma Fracture Impact Fracture Healing?
One major question regarding the use of anti-osteoporosis drugs in patients with a recent fracture is the effect that treatment might have on bone healing after fracture or fracture-repair surgery. With antiresorptive agents in particular, the main concern is whether suppression of bone turnover may lead to delayed bone healing, since healing requires callus remodeling. A small prospective study evaluated fracture healing in 196 patients treated for a distal radius fracture, 153 of whom were on a bisphosphonate at the time of the fracture. While bisphosphonate use was associated with a longer time to radiographic union, the time to union was only 6 days longer in the bisphosphonate group (55 days versus 49 days to union in the bisphosphonate and control groups, respectively), and has generally not been felt to be clinically significant [60]. The most reassuring data regarding this question however, comes from the HORIZON trial where 2127 men and women were randomized to zoledronic acid or placebo within 90 days of a hip fracture. No difference in healing between the 2 groups was seen, regardless of the time of initiation of zoledronic acid (within 2 weeks of fracture, between 2 and 4 weeks, between 4 and 6 weeks or after 6 weeks) [61].
The stimulation of bone turnover that occurs with anabolic agents is generally thought to accelerate bone healing. In animal studies, teriparatide has been found to enhance callus formation and mechanical strength [62–64], but there is no definitive data in humans to prove this effect [65].
In summary, there is strong evidence demonstrating the effectiveness of bisphosphonates and anabolic agents at decreasing the risk of fracture recurrence in patients with preexisting vertebral fractures. Zoledronic acid has also been shown to decrease the risk of fracture recurrence after a hip fracture. Anti-osteoporosis therapy after a fracture has no clinically significant effect on fracture healing.
The Gap Between Science and Practice
Practice Guidelines Versus Actual Practice
Based on the data presented above, multiple professional societies and expert groups have developed guidelines emphasizing the importance of evaluation and treatment for osteoporosis following a low-trauma fracture, especially those of the hip and spine [8,9,66–69]. In a 2009 multidisciplinary workshop of the International Society of Fracture Repair, an in-depth review of existing data showed no evidence for a negative effect of anti-osteoporosis drugs on fracture healing. As a result, it was recommended not to withhold osteoporosis therapy until fracture healing has occurred, and to initiate treatment before patient discharge from the fracture ward in order to improve follow-up [70].
However, despite these expert guidelines and the availability of several effective agents to decrease the risk of fracture and fracture recurrence, evaluation and treatment of patients for osteoporosis after a low-trauma fracture are very low. Several large-scale studies involving older patients with fractures in North America, Europe, Asia, and Australia have shown that the rates of BMD measurement or drug therapy for osteoporosis after a fragility fracture do not exceed 25% to 30% [71–80]. While treatment trends over time may have shown some improvement, they remain overall disappointing. For example, in a study of over 150,000 patients who sustained a fracture between 1997 and 2004, Roerholt et al found that around 20% of women were started on therapy after a vertebral fracture in 1997, while 40% received therapy in 2004. Among women with hip fracture, 3% received treatment in 1997 and 9% in 2004 [71]. Furthermore, when osteoporosis treatment rates are examined more closely, most of the patients who receive treatment after a fracture are those who were being treated prior to the fracture, so treatment is simply continued in them. New osteoporosis therapy is initiated in only 5% to 15% of patients who are not already on osteoporosis therapy at the time of fracture [72,73,77,81,82].
Analyses of prescription patterns suggest that patients with vertebral fractures are more likely to receive treatment compared to those with hip fractures [71,82], and that women are much more likely to receive therapy than men [71,74,77,83–88]. Other factors that decrease the chance of receiving therapy include black race [84], low income [74], older age, presence of multiple comorbidities, and polypharmacy [83].
Barriers to Care: Where Are We Failing?
The large discrepancy between science and practice when it comes to secondary prevention of fractures is quite puzzling and has been the subject of several investigations. A major barrier to proper care seems to be the lack of ownership of the problem by the orthopedic surgeons and medical providers, and the less than ideal collaboration between the 2 services in coordinating and providing secondary prevention [89–94]. The orthopedic surgeons are one of the first points of contact with health care for a patient with a low-trauma hip fracture. They are mainly charged with providing acute fracture care and often cannot provide long-term osteoporosis care, which would be more suitable for a medical specialist. However, while the acute care surgical team is not best suited to treat osteoporosis, it is still very important that they initiate patient referral to a provider who can provide long-term osteoporosis care. This transition of care–of lack of it–seems to be one of the major missing links, leading to patient loss [88] and suboptimal secondary prevention.
However, patient referral may not be a sufficient solution and interestingly, a medical consultation during an acute admission for hip fracture does not seem to increase the frequency of osteoporosis diagnosis [95]. This points to a deficiency in knowledge, and as a matter of fact, studies do suggest a problem with under-recognition of the connection between low-trauma fractures and underlying osteoporosis among medical and surgical providers alike [92,93,96]. In a survey of orthopedic surgeons and consultant physicians involved in the care of patients with low-trauma hip fractures, only 24% of respondents felt that osteoporosis therapy was indicated. The majority of providers thought that treatment with a bisphosphonate was indicated only if low BMD was present, rather than in all patients with low-trauma hip fractures [92]. This is further illustrated by the fact that only a minority of patients with a low-trauma fracture are formally given the diagnosis of osteoporosis [75,80,97] or are told that they have osteoporosis [79].
Fracture Liaison Services—A Potential Solution to Enhance Secondary Fracture Prevention
What is a Fracture Liaison Service?
Several solutions have been proposed to remedy the main barriers that interfere with proper secondary treatment of osteoporosis, namely patient education, provider education, and the initiation of programs to enhance coordination and continuity of care between treating teams. Taken together, these interventions have been modestly effective at increasing the odds of BMD measurement and initiation of osteoporosis therapy [98, 99]. Interventions that focused mainly on provider and/or patient education were the least effective, especially when they did not rely on direct in-person interactions, and programs intended to enhance transitions of care were more effective [96,99,100].
These programs are commonly referred to as fracture liaison services (FLS). They aim to identify patients with low-trauma fractures, provide risk assessment and education to the patient, and in some cases provide the patient with post-fracture osteoporosis care. These services typically require a dedicated case manager, who is often a clinical nurse specialist, ideally supported by a medical practitioner with expertise in the treatment of osteoporosis. The FLS case manager uses predetermined protocols that facilitate patient identification, risk assessment and management [101]. Some programs are hospital-based, identifying and evaluating patients while still hospitalized for their hip fracture, and others are based in clinics, aiming to provide services after discharge from the initial acute hospitalization [96,99–101].
How Effective Are Fracture Liaison Services?
Several FLS models have been proposed and tested, with some limited to patient identification and risk stratification, and others more intensive, involving initiation of BMD testing or BMD testing and osteoporosis treatment. In a meta-analysis of FLS programs, Ganda et al grouped programs into 3 categories: Type A programs involved patient identification, assessment and treatment, type B programs involved patient identification and assessment only without treatment, and type C programs involved patient identification combined with alerting of the patients and providers to the need to assess and treat. The effectiveness of the programs in terms of BMD testing and initiation of therapy increased with intensity. Type A programs were the most effective with BMD testing and treatment initiation rates of 79.4% and 46.4% respectively, followed by type B programs which had BMD testing and treatment initiation rates of 59.5% and 40.6% respectively, then type C programs which had BMD testing and treatment initiation rates of 43.4% and 23.4% respectively [100].
The most intensive programs have also been shown to significantly decrease the risk of fracture recurrence, with a reduction in the rate of re-fracture from 19.7% to 4.1% within 4 weeks [102], and a 37.2 % reduction within 3 years [103,104]. Additionally, intensive FLS programs involving pharmacotherapy with a bisphosphonate may be associated with a reduction in mortality after a hip fracture. Beaupre et al evaluated the mortality benefit associated with oral bisphosphonate therapy in the setting of a FLS and demonstrated an 8% decline in mortality per month of oral bisphosphonate use, and an approximate 60% reduction per year of use in comparison to patients who did not receive treatment [105]. This finding was consistent with the reduction in mortality seen with zoledronic acid in the HORIZON trial, which was in part attributable to decreased re-fracture rates, but primarily due to reduction in the occurrence of pneumonia and arrhythmias in patients receiving the drug [57,106].
While fracture liaison services may be associated with increased immediate costs—such as the costs of hiring a case manager, BMD testing and pharmacotherapy, and in some cases a data management system—several cost-effectiveness analyses have shown associated long-term cost savings [107–109]. This is not surprising given that they decrease re-fracture rates, leading to a decline in the very costly immediate and long-term fracture care costs.
Summary
In summary, fragility fractures present a major health care problem for aging populations, leading to significant costs and high morbidity and mortality. Assessment and treatment of osteoporosis following a fragility fracture can decrease the risk of fracture recurrence, long-term costs, morbidities, and possibly mortality. In the last decade, several national and international initiatives have been created to promote and encourage secondary prevention of fragility fractures [110–113]. However, these programs have all been voluntary and there are currently no reliable mechanisms to ensure broad implementation of secondary fracture prevention interventions. As a result, and while several isolated secondary prevention programs have shown great success, most patients with low-trauma fractures still receive suboptimal osteoporosis care.
Corresponding author: Amal Shibli-Rahhal, MD, MS, Dept. of Internal Medicine, University of Iowa Hospitals and Clinics, Iowa City, IA 52242.
Financial disclosures:
1. Bergström U, Björnstig U, Stenlund H, Jonsson H, Svensson O. Fracture mechanisms and fracture pattern in men and women aged 50 years and older: a study of a 12-year population-based injury register, Umeå, Sweden. Osteoporos Int 2008;19:1267–73.
2. Siris ES, Adler R, Bilezikian J, et al. The clinical diagnosis of osteoporosis: a position statement from the National Bone Health Alliance Working Group. Osteoporos Int 2014;25:1439–43.
3. Kanis JA, Borgström F, Compston J, et al. SCOPE: a scorecard for osteoporosis in Europe. Arch Osteoporos 2013;8:144.
4. Burge R, Dawson-Hughes B, Solomon DH, Wong JB, King A, Tosteson A. Incidence and economic burden of osteoporosis-related fractures in the United States, 2005-2025. J Bone Miner Res 2007;22:465–75.
5. Rosengren BE, Karlsson MK. The annual number of hip fractures in Sweden will double from year 2002 to 2050: projections based on local and nationwide data. Acta Orthop 2014;85:234–7.
6. Chen IJ, Chiang CY, Li YH, et al. Nationwide cohort study of hip fractures: time trends in the incidence rates and projections up to 2035. Osteoporos Int 2015;26:681–8.
7. Siris ES, Boonen S, Mitchell PJ, Bilezikian J, Silverman S. What’s in a name? What constitutes the clinical diagnosis of osteoporosis? Osteoporos Int 2012;23:2093–7.
8. Cosman F, de Beur SJ, LeBoff MS, et al. Clinician’s guide to prevention and treatment of osteoporosis. Osteoporos Int 2014;25:2359–81.
9. Papaioannou A, Morin S, Cheung AM, et al; Scientific Advisory Council of Osteoporosis Canada. 2010 Clinical practice guidelines for the diagnosis and management of osteoporosis in Canada: summary. CMAJ 2010;182:1864–73.
10. Malluche HH, Porter DS, Mawad H, Monier-Faugere MC, Pienkowski D. Low-energy fractures without low T-scores characteristic of osteoporosis: a possible bone matrix disorder. J Bone Joint Surg Am 2013;95:e1391–6.
11. Ascenzi MG, Chin J, Lappe J, Recker R. Non-osteoporotic women with low-trauma fracture present altered birefringence in cortical bone. Bone 2016;84:104–12.
12. Nishiyama KK, Macdonald HM, Hanley DA, Boyd SK. Women with previous fragility fractures can be classified based on bone microarchitecture and finite element analysis measured with HR-pQCT. Osteoporos Int 2013;24:1733–40.
13. Dolan MM, Hawkes WG, Zimmerman SI, Morrison RS, Gruber-Baldini AL, Hebel JR, Magaziner J. Delirium on hospital admission in aged hip fracture patients: prediction of mortality and 2-year functional outcomes. J Gerontol A Biol Sci Med Sci 2000;55:M527–34.
14. Bruce AJ, Ritchie CW, Blizard R, Lai R, Raven P. The incidence of delirium associated with orthopedic surgery: a meta-analytic review. Int Psychogeriatr 2007;19:197–214.
15. Patterson BM, Cornell CN, Carbone B, Levine B, Chapman D. Protein depletion and metabolic stress in elderly patients who have a fracture of the hip. J Bone Joint Surg Am 1992;74:251–60.
16. Beaupre LA, Jones CA, Saunders LD, Johnston DW, Buckingham J, Majumdar SR. Best practices for elderly hip fracture patients. A systematic overview of the evidence. J Gen Intern Med 2005;20:1019–25.
17. Johansson I, Athlin E, Frykholm L, Bolinder H, Larsson G. Intermittent versus indwelling catheters for older patients with hip fractures. J Clin Nurs 2002;11:651–6.
18. Ulucay C, Eren Z, Kaspar EC, et al. Risk factors for acute kidney injury after hip fracture surgery in the elderly individuals. Geriatr Orthop Surg Rehabil 2012;3:150–6.
19. Greendale GA, Barrett-Connor E, Ingles S, Haile R. Late physical and functional effects of osteoporotic fracture in women: the Rancho Bernardo Study. J Am Geriatr Soc 1995;43:955–61.
20. Nevitt MC, Thompson DE, Black DM, et al. Effect of alendronate on limited-activity days and bed-disability days caused by back pain in postmenopausal women with existing vertebral fractures. Fracture Intervention Trial Research Group. Arch Intern Med 2000;160:77–85.
21. Gold DT, Lyles KW, Shipp KM, Drezner MK. Osteoporosis and its nonskeletal consequences: their impact on treatment decisions. In: Marcus R, Feldman D, Kelsey J, eds. Osteoporosis. 2nd ed. San Diego, CA: Academic Press; 2001:479–84.
22. Sale JEM, Frankel L, Thielke S, Funnell L. Pain and fracture-related limitations persist 6 months after a fragility fracture. Rheumatol Int 2017;37:1317–22.
23. Beringer TR, Clarke J, Elliott JR, Marsh DR, Heyburn G, Steele IC. Outcome following proximal femoral fracture in Northern Ireland. Ulster Med J 2006;75:200–6.
24. Córcoles-Jiménez MP, Villada-Munera A, Del Egido-Fernández MÁ, et al. Recovery of activities of daily living among older people one year after hip fracture. Clin Nurs Res 2015;24:604–23.
25. Kammerlander C, Gosch M, Kammerlander-Knauer U, Luger TJ, Blauth M, Roth T. Long-term functional outcome in geriatric hip fracture patients. Arch Orthop Trauma Surg 2011;131:1435–44.
26. Tarride JE, Burke N, Leslie WD, et al. Loss of health related quality of life following low-trauma fractures in the elderly. BMC Geriatr 2016;16:84.
27. Abimanyi-Ochom J, Watts JJ, Borgström F, et al. Changes in quality of life associated with fragility fractures: Australian arm of the International Cost and Utility Related to Osteoporotic Fractures Study (AusICUROS). Osteoporos Int 2015;26:1781–90.
28. Adachi JD, Loannidis G, Berger C, et al; Canadian Multicentre Osteoporosis Study (CaMos) Research Group. The influence of osteoporotic fractures on health-related quality of life in community-dwelling men and women across Canada. Osteoporos Int 2001;12:903–8.
29. Center JR, Nguyen TV, Schneider D, Sambrook PN, Eisman JA. Mortality after all major types of osteoporotic fracture in men and women: an observational study. Lancet 1999;353:878–82.
30. Kanis JA, Oden A, Johnell O, De Laet C, Jonsson B. Excess mortality after hospitalisation for vertebral fracture. Osteoporos Int 2004;15:108–12.
31. Johnell O, Kanis JA, Odén A, et al. Mortality after osteoporotic fractures. Osteoporos Int 2004;15:38–42.
32. Gosch M, Druml T, Nicholas JA, et al. Fragility non-hip fracture patients are at risk. Arch Orthop Trauma Surg 2015;135:69–77.
33. Ensrud KE, Thompson DE, Cauley JA, et al. Prevalent vertebral deformities predict mortality and hospitalization in older women with low bone mass. Fracture Intervention Trial Research Group. J Am Geriatr Soc 2000;48:241–9.
34. Abrahamsen B, van Staa T, Ariely R, Olson M, Cooper C. Excess mortality following hip fracture: a systematic epidemiological review. Osteoporos Int 2009;20:1633–50.
35. Kanis JA, Oden A, Johnell O, De Laet C, Jonsson B, Oglesby AK. The components of excess mortality after hip fracture. Bone 2003;32:468–73.
36. Forsén L, Sogaard AJ, Meyer HE, Edna T, Kopjar B. Survival after hip fracture: short- and long-term excess mortality according to age and gender. Osteoporos Int 1999;10:73–8.
37. Trombetti A, Herrmann F, Hoffmeyer P, Schurch MA, Bonjour JP, Rizzoli R. Survival and potential years of life lost after hip fracture in men and age-matched women. Osteoporos Int 2002;13:731–7.
38. Kanis JA, Johnell O, De Laet C, et al. A meta-analysis of previous fracture and subsequent fracture risk. Bone 2004;35:375–82.
39. Center JR, Bliuc D, Nguyen TV, Eisman JA. Risk of subsequent fracture after low-trauma fracture in men and women. JAMA 2007;297:387–94.
40. Hasserius R, Karlsson MK, Nilsson BE, Redlund-Johnell I, Johnell O; European Vertebral Osteoporosis Study. Prevalent vertebral deformities predict increased mortality and increased fracture rate in both men and women: a 10-year population-based study of 598 individuals from the Swedish cohort in the European Vertebral Osteoporosis Study. Osteoporos Int 2003;14:61–8.
41. Edwards BJ, Bunta AD, Simonelli C, Bolander M, Fitzpatrick LA. Prior fractures are common in patients with subsequent hip fractures. Clin Orthop Relat Res 2007;461:226–30.
42. Gehlbach S, Saag KG, Adachi JD, et al. Previous fractures at multiple sites increase the risk for subsequent fractures: the Global Longitudinal Study of Osteoporosis in Women. J Bone Miner Res 2012;27:645–53.
43. Lindsay R, Silverman SL, Cooper C, et al. Risk of new vertebral fracture in the year following a fracture. JAMA 2001;285:320–3.
44. Hansen L, Mathiesen AS, Vestergaard P, Ehlers LH, Petersen KD. A health economic analysis of osteoporotic fractures: who carries the burden? Arch Osteoporos 2013;8:126.
45. Lippuner K, Grifone S, Schwenkglenks M, et al. Comparative trends in hospitalizations for osteoporotic fractures and other frequent diseases between 2000 and 2008. Osteoporos Int 2012;23:829–39.
46. Lange A, Zeidler J, Braun S. One-year disease-related health care costs of incident vertebral fractures in osteoporotic patients. Osteoporos Int 2014;25:2435–43.
47. Hopkins RB, Burke N, Von Keyserlingk C, Leslie WD, Morin SN, Adachi JD, Papaioannou A, Bessette L, Brown JP, Pericleous L, Tarride J. The current economic burden of illness of osteoporosis in Canada. Osteoporos Int 2016;27:3023–32.
48. Blume SW, Curtis JR. Medical costs of osteoporosis in the elderly Medicare population. Osteoporos Int 2011;22:1835–44.
49. Avenell A, Mak JC, O’Connell D. Vitamin D and vitamin D analogues for preventing fractures in post-menopausal women and older men. Cochrane Database Syst Rev 2014: CD000227.
50. Santesso N, Carrasco-Labra A, Brignardello-Petersen R. Hip protectors for preventing hip fractures in older people. Cochrane Database Syst Rev 2014: CD001255
51. Avenell A, Smith TO, Curtain J, Mak JC, Myint PK. Nutritional supplementation for hip fracture aftercare in older people. Cochrane Database Syst Rev 2016;11: CD001880
52. Giangregorio LM, MacIntyre NJ, Thabane L, Skidmore CJ, Papaioannou A. Exercise for improving outcomes after osteoporotic vertebral fracture. Cochrane Database Syst Rev 2013: CD008618
53. Gillespie LD, Robertson M, Gillespie WJ, et al. Interventions for preventing falls in older people living in the community. Cochrane Database Syst Rev 2012:CD007146.
54. Ensrud KE, Black DM, Palermo L, et al. Treatment with alendronate prevents fractures in women at highest risk: results from the Fracture Intervention Trial. Arch Intern Med 1997;157:2617–24.
55. Black DM, Cummings SR, Karpf DB, et al. Randomised trial of effect of alendronate on risk of fracture in women with existing vertebral fractures. Fracture Intervention Trial Research Group. Lancet 1996;348:1535–41.
56. McClung MR, Geusens P, Miller PD, Zippel H, Bensen WG, Roux C, Adami S, Fogelman I, Diamond T, Eastell R, Meunier PJ, Reginster JY; Hip Intervention Program Study Group. Effect of risedronate on the risk of hip fracture in elderly women. Hip Intervention Program Study Group. N Engl J Med. 2001;344:333–40.
57. Lyles KW, Colón-Emeric CS, Magaziner JS, et al; HORIZON Recurrent Fracture Trial. Zoledronic acid and clinical fractures and mortality after hip fracture. N Engl J Med 2007;357:1799–809.
58. Neer RM, Arnaud CD, Zanchetta JR, et al. Effect of parathyroid hormone (1-34) on fractures and bone mineral density in postmenopausal women with osteoporosis. N Engl J Med 2001;344:1434–41.
59. Miller PD, Hattersley G, Riis BJ, et al; ACTIVE Study Investigators. Effect of abaloparatide vs placebo on new vertebral fractures in postmenopausal women with osteoporosis: a randomized clinical trial. JAMA. 2016;316:722–33.
60. Rozental TD, Vazquez MA, Chacko AT, Ayogu N, Bouxsein ML. Comparison of radiographic fracture healing in the distal radius for patients on and off bisphosphonate therapy. J Hand Surg Am 2009;34:595–602.
61. Colón-Emeric C, Nordsletten L, Olson S, et al; HORIZON Recurrent Fracture Trial. Association between timing of zoledronic acid infusion and hip fracture healing. Osteoporos Int 2011;22:2329–36.
62. Nakajima A, Shimoji N, Shiomi K, et al. Mechanisms for the enhancement of fracture healing in rats treated with intermittent low-dose human parathyroid hormone (1-34). J Bone Miner Res 2002;17:2038–47.
63. Alkhiary YM, Gerstenfeld LC, Krall E, et al. Enhancement of experimental fracture-healing by systemic administration of recombinant human parathyroid hormone (PTH 1-34). J Bone Joint Surg Am 2005;87:731–41.
64. Andreassen TT, Ejersted C, Oxlund H. Intermittent parathyroid hormone (1-34) treatment increases callus formation and mechanical strength of healing rat fractures. J Bone Miner Res 1999;14:960–8.
65. Bhandari M, Jin L, See K, et al. Does teriparatide improve femoral neck fracture healing: results from a randomized placebo-controlled trial. Clin Orthop Relat Res 2016;474:1234–44.
66. Lems WF, Dreinhöfer KE, Bischoff-Ferrari H, et al. EULAR/EFORT recommendations for management of patients older than 50 years with a fragility fracture and prevention of subsequent fractures. Ann Rheum Dis 2017;76:802–810.
67. American Academy of Orthopaedics Surgeons. Management of Hip Fractures in the Elderly. Evidence-Based Clinical Practice Guideline. Available at https://www.aaos.org/research/guidelines/hipfxguideline.pdf. Accessed March 2, 2018
68. The International Society For Clinical Densitometry. Official Positions 2015 ISCD Combined. Available at https://iscd.app.box.com/v/OP-ISCD-2015-Adult. Accessed March 2, 2018.
69. International Osteoporosis Foundation. National and Regional Osteoporosis Guidelines. https://www.iofbonehealth.org/national-regional-osteoporosis-guidelines. Accessed March 2, 2018.
70. Goldhahn J, Little D, Mitchell P, et al; ISFR working group drugs and fracture repair. Evidence for anti-osteoporosis therapy in acute fracture situations—recommendations of a multidisciplinary workshop of the International Society for Fracture Repair. Bone 2010;46:267–71.
71. Roerholt C, Eiken P, Abrahamsen B. Initiation of anti-osteoporotic therapy in patients with recent fractures: a nationwide analysis of prescription rates and persistence. Osteoporos Int 2009;20:299–307.
72. Panneman MJ, Lips P, Sen SS, Herings RM. Undertreatment with anti-osteoporotic drugs after hospitalization for fracture. Osteoporos Int 2004;15:120–4.
73. Wilk A, Sajjan S, Modi A, Fan CPS, Mavros P. Post-fracture pharmacotherapy for women with osteoporotic fractures: analysis of a managed care population in the USA. Osteoporos Int 2014; 25:2777–86.
74. Leslie WD, Giangregorio LM, Yogendran M, et al. A population-based analysis of the post-fracture care gap 1996-2008: the situation is not improving. Osteoporos Int 2012;23:1623–9.
75. Kung AW, Fan T, Xu L, et al. Factors influencing diagnosis and treatment of osteoporosis after a fragility fracture among postmenopausal women in Asian countries: a retrospective study. BMC Womens Health 2013;13:7.
76. Wang O, Hu Y, Gong S, et al. A survey of outcomes and management of patients post fragility fractures in China. Osteoporos Int 2015;26:2631–40.
77. Yusuf AA, Matlon TJ, Grauer A, Barron R, Chandler D, Peng Y. Utilization of osteoporosis medication after a fragility fracture among elderly Medicare beneficiaries. Arch Osteoporos 2016;11:31
78. Munson JC, Bynum JP, Bell JE, et al. Patterns of prescription drug use before and after fragility fracture. JAMA Intern Med 2016;176:1531–8.
79. Eisman J, Clapham S, Kehoe L; Australian BoneCare Study. Osteoporosis prevalence and levels of treatment in primary care: the Australian BoneCare Study. J Bone Miner Res 2004;19:1969–75.
80. Duncan R, Francis RM, Jagger C, et al. Magnitude of fragility fracture risk in the very old—are we meeting their needs? The Newcastle 85+ Study. Osteoporos Int 2015;26:123–30.
81. Singh S, Foster R, Khan KM. Accident or osteoporosis?: Survey of community follow-up after low-trauma fracture. Can Fam Physician. 2011;57:e128–33.
82. Andrade SE, Majumdar SR, Chan KA, et al. Low frequency of treatment of osteoporosis among postmenopausal women following a fracture. Arch Intern Med 2003;163:2052–7.
83. Blecher R, Wasrbrout Z, Arama Y, Kardosh R, Agar G, Mirovsky Y. Who is at risk of receiving inadequate care for osteoporosis following fragility fractures? A retrospective study. Isr Med Assoc J 2013;15:634–8.
84. Shibli-Rahhal A, Vaughan-Sarrazin MS, Richardson K, Cram P. Testing and treatment for osteoporosis following hip fracture in an integrated U.S. healthcare delivery system. Osteoporos Int 2011;22:2973–80.
85. Freedman BA, Potter BK, Nesti LJ, Giuliani JR, Hampton C, Kuklo TR. Osteoporosis and vertebral compression fractures-continued missed opportunities. Spine J 2008;8:756–62.
86. Kiebzak GM, Beinart GA, Perser K, Ambrose CG, Siff SJ, Heggeness MH. Undertreatment of osteoporosis in men with hip fracture. Arch Intern Med 2002;162:2217–22.
87. Kamel HK, Bida A, Montagnini M. Secondary prevention of hip fractures in veterans: can we do better? J Am Geriatr Soc 2004;52:647–8.
88. Skorupski N, Alexander IM. Multidisciplinary osteoporosis management of post low-energy trauma hip-fracture patients. J Am Assoc Nurse Pract 2013;25:3–10.
89. Simonelli C, Killeen K, Mehle S, Swanson L. Barriers to osteoporosis identification and treatment among primary care physicians and orthopedic surgeons. Mayo Clin Proc 2002;77:334–8.
90. Abraham A. Undertreatment of osteoporosis in men who have had a hip fracture. Arch Intern Med 2003;163:1236.
91. Sheehan J, Mohamed F, Reilly M, Perry IJ. Secondary prevention following fractured neck of femur: a survey of orthopaedic surgeons practice. Ir Med J. 2000;93:105–7.
92. Levinson MR, Clay FJ. Barriers to the implementation of evidence in osteoporosis treatment in hip fracture. Intern Med J 2009;39:199–202.
93. Kaufman JD, Bolander ME, Bunta AD, Edwards BJ, Fitzpatrick LA, Simonelli C. Barriers and solutions to osteoporosis care in patients with a hip fracture. J Bone Joint Surg Am 2003;85-A:1837–43.
94. Sorbi R, Aghamirsalim M. Osteoporotic Fracture Program management: who should be in charge? A comparative survey of knowledge in orthopaedic surgeons and internists. Orthop Traumatol Surg Res 2013;99:723–30.
95. Kamel HK, Hussain MS, Tariq S, Perry HM, Morley JE. Failure to diagnose and treat osteoporosis in elderly patients hospitalized with hip fracture. Am J Med 2000;109:326–8.
96. Eisman JA, Bogoch ER, Dell R, et al; ASBMR Task Force on Secondary Fracture Prevention. Making the first fracture the last fracture: ASBMR task force report on secondary fracture prevention. J Bone Miner Res 2012;27:2039–46.
97. Riley RL, Carnes ML, Gudmundsson A, Elliott ME. Outcomes and secondary prevention strategies for male hip fractures. Ann Pharmacother 2002;36:17–23.
98. Little EA, Eccles MP. A systematic review of the effectiveness of interventions to improve post-fracture investigation and management of patients at risk of osteoporosis. Implement Sci 2010;5:80.
99. Sale JE, Beaton D, Posen J, Elliot-Gibson V, Bogoch E. Systematic review on interventions to improve osteoporosis investigation and treatment in fragility fracture patients. Osteoporos Int 2011;22:2067–82.
100. Ganda K, Puech M, Chen JS, et al. Models of care for the secondary prevention of osteoporotic fractures: a systematic review and meta-analysis. Osteoporos Int 2013;24:393–406.
101. Akesson K, Marsh D, Mitchell PJ, et al; IOF Fracture Working Group. Capture the fracture: a best practice framework and global campaign to break the fragility fracture cycle. Osteoporos Int 2013;24:2135–52.
102. Lih A, Nandapalan H, Kim M, et al. Targeted intervention reduces refracture rates in patients with incident non-vertebral osteoporotic fractures: a 4-year prospective controlled study. Osteoporos Int 2011;22:849–58.
103. Dell R, Greene D, Schelkun SR, Williams K. Osteoporosis disease management: the role of the orthopaedic surgeon. J Bone Joint Surg Am 2008;90:188–94.
104. Dell R. Fracture prevention in Kaiser Permanente Southern California. Osteoporos Int 2011;22:457–60.
105. Beaupre LA, Morrish DW, Hanley DA, et al. Oral bisphosphonates are associated with reduced mortality after hip fracture. Osteoporos Int 2011;22:983–91.
106. Colón-Emeric CS, Mesenbrink P, Lyles KW, et al. Potential mediators of the mortality reduction with zoledronic acid after hip fracture. J Bone Miner Res 2010;25:91–7.
107. Cooper MS, Palmer AJ, Seibel MJ. Cost-effectiveness of the Concord Minimal Trauma Fracture Liaison service, a prospective, controlled fracture prevention study. Osteoporos Int 2012;23:97–107.
108. McLellan AR, Wolowacz SE, Zimovetz EA, et al. Fracture liaison services for the evaluation and management of patients with osteoporotic fracture: a cost-effectiveness evaluation based on data collected over 8 years of service provision. Osteoporos Int 2011;22:2083–98.
109. Solomon DH, Patrick AR, Schousboe J, Losina E. The potential economic benefits of improved postfracture care: a cost-effectiveness analysis of a fracture liaison service in the US health-care system. J Bone Miner Res 2014;29:1667–74.
110. Fragility Fracture Network of the Bone and Joint Decade. National bone health alliance: http://fragilityfracturenetwork.org/other-leading-organisations/national/united-states-of-america/national-bone-health-alliance-nbha/. Accessed March 3, 2018.
111. International Osteoporosis Foundation. Capture the fracture. https://www.iofbonehealth.org/capture-fracture. Accessed March 3, 2018.
112. Osteoporosis Canada. Towards a fracture free future: postoperative management of fragility fractures-a focus on osteoporosis care. Available at http://www.osteoporosis.ca/multimedia/pdf/COA_Bulletin_Winter_2012.pdf . Accessed March 3, 2018.
113. The American Orthopaedic Association. Own the bone. http://www.ownthebone.org/ . Accessed March 3, 2018.
From the Department of Internal Medicine, University of Iowa Carver College of Medicine, Iowa City, IA.
Abstract
- Objective: To review and summarize the literature regarding current approaches to secondary prevention of low-trauma osteoporotic fractures.
- Methods: PubMed search and summary of existing literature related to complications and secondary prevention of osteoporotic fractures was performed.
- Results: Fragility fractures are associated with high rates of short and long term morbidities and carry a high risk of mortality and fracture recurrence. Several of the currently available anti-osteoporosis medications have been shown to decrease the risk of fracture recurrence in patients with prevalent osteoporotic fractures and some may even decrease mortality. However, only a minority of patients with fragility fractures are adequately evaluated and treated for osteoporosis. Fracture liaison services that ensure identification and risk stratification of patients with fragility fractures and proper evaluation and treatment of osteoporosis have proven effective at enhancing osteoporosis care in these patients, decreasing fracture recurrence and possibly even decreasing long-term mortality, while providing long-term cost savings. Unfortunately, however, this model of care has not been widely adopted and implemented.
- Conclusion: Fragility fractures represent a major health care problem for aging populations. Unfortunately, most patients with low-trauma fractures still receive suboptimal osteoporosis care.
Key words: osteoporosis; fracture; fragility; low-trauma; bone density.
Low-trauma fractures are fractures that occur from a trauma equivalent to a fall from standing height or less [1,2]. They can involve any skeletal site, but the most significant are vertebral, pelvic, wrist and hip fractures, which together represent close to 90% of all low-trauma fractures [3,4]. The overall burden of low-trauma fractures is quite high worldwide and is projected to increase over time [3–6]. In 2010, 3.5 million new low-trauma fractures were reported in the European Union [3]. In the United States, there were more than 2 million fractures in 2005, and it is estimated that more than 3 million fractures will occur in year 2025 [4].
Low-trauma fractures are generally indicative of compromised bone strength—especially when they involve the hip—and are thus often referred to as fragility fractures. While the traditional definition of osteoporosis is a bone mineral density (BMD) T-score of -2.5 or lower, low-trauma fractures of the hip are also diagnostic of osteoporosis, regardless of bone mineral density [2,7–9]. In addition, low-trauma fractures of the vertebrae, the proximal humerus, and the pelvis are considered diagnostic of osteoporosis when combined with T-scores between -1 and -2.5 [2,7]. Bone biopsies and high-resolution peripheral quantitative computed tomography (HR-pQCT) in patients with low-trauma fractures and normal BMD suggest microarchitectural alterations and abnormalities of collagen orientation and crosslinking within the bone matrix [10-12], leading to decreased bone strength.
This review will address the individual and societal costs of low-trauma fractures and issues related to secondary prevention of fractures, with specific emphasis on pharmacotherapy and fracture liaison services.
Impact of Low-Trauma Fractures
Acute and Long-Term Complications
Of all fragility fractures, hip fractures are the ones most likely to result in serious acute complications. The most common acute complications are delirium in up to 50% of patients and malnutrition in up to 60%, both of which predict slower and less complete recovery [13–16]. Other complications include urinary tract infections in up to 60% of patients in certain reports [17], thromboembolic disease with deep venous thrombosis in around 27% of patients and pulmonary embolism in up to 7% [16], and acute kidney injury in about 15% [18].
In addition, it is not uncommon for patients to suffer from significant long-term functional limitations following fragility fractures. While vertebral fractures do not frequently lead to hospitalization or institutionalization, they often lead to significant physical limitations and chronic pain [19,20] and to negative effects on self-esteem, mood, and body image [21,22]. However, the most remarkable functional decline and limitations are seen after hip fractures [23–25]. In a study of 2800 women and men with hip fracture, Beringer et al found that more than 30% were still institutionalized, and only 40% were able to walk outdoors independently 1 year later. Predictors of poor outcome included male sex, advanced age, cognitive impairment, and presence of comorbidities [23].
It is not surprising then that a fracture is often associated with an overall decline in the individual’s quality of life and this has been demonstrated in several studies [26–28]. In the largest study of this type, Tarride et al examined over 23,000 patients with fragility fractures and found a sharp decline in health-related quality of life (HRQOL) immediately after the fracture, which remained below baseline for up to 3 years [26]. The decline was worse in patients with hip and spine fractures compared to other fractures [27].
Mortality Following Fragility Fractures
Perhaps the most concerning complication, however, is the excess mortality seen after fractures. Several studies have demonstrated excess mortality after vertebral fractures, especially in the year following the fracture [29–33], but the highest increase in mortality was observed following hip fractures. In fact, the 30-day mortality after a hip fracture approximates 7% [23] and the excess 1-year mortality is estimated at 8% to 36% [34,35]. While the highest risk of mortality is seen in the first year following the fracture, the increased risk persists for at least 5 to 6 years [36]. Malnutrition, decreased mobility, male sex, and the number of coexisting medical comorbidities further increase the risk of mortality [29,32,34,36,37].
Risk of Fracture Recurrence
In both men and women, a fragility fracture at any site increases the risk of subsequent fractures [38–41], and the risk increases with the number of prevalent fractures [42]. Gehlbach et al estimated an 80% increase in the risk of fracture recurrence after 1 fracture, a threefold increase after 2 fractures, and an almost fivefold increase after 3 fractures [42]. The increase in risk is even more pronounced following vertebral fractures specifically, doubling after the first fracture an increasing by up to ninefold after 3 fractures [42, 43]. This increase in risk is highest in the first year following the fracture but may persist for up to 10 years [39,43].
Fracture Impact on Society
Fractures are associated with a high financial burden to society, in terms of direct acute care costs and long-term rehabilitation [3,4,44–48]. In 2010, the direct cost from fractures in the EU was estimated at €24.6 billion [3]. In the US, this cost was around $14.0 billion in 2002 and $16.9 billion in 2005 [4,48], and in Canada it was $1.5 billion in 2011 [47]. These numbers increase substantially when costs associated with long-term post-fracture rehabilitation are included, with an additional estimated yearly cost of €10.7 billion in the EU and $1.03 billion in Canada [3,47].
While hip fractures account for only about 18% of all low-trauma fractures, they are associated with the highest cost burden, accounting for about 50% to 70% of the total fracture-associated expenditures [3,4,44]. This is likely due to the fact almost all hip fractures require hospitalization, most require surgical repair and rehabilitation, and because they lead to the highest rates of morbidity and mortality.
Can Fracture Recurrence After a Low-Trauma Fracture Be Prevented?
Many approaches to secondary fracture prevention have been proposed, including but not limited to fall prevention, exercise therapy, nutrition therapy, prevention and treatment of sarcopenia, vitamin D and calcium supplementation, and osteoporosis pharmacotherapy [49–53]. Of those, osteoporosis pharmacotherapy has the strongest and most compelling efficacy data and will be reviewed in the following sections.
Effect of Antiresorptive Therapy After a Fracture
In the Fracture Intervention Trial (FIT), alendronate decreased the risk of new vertebral fractures by about 47% and of hip fractures by about 50% in women with preexisting vertebral fractures [54,55]. Similar fracture protection benefits were demonstrated in the Hip Intervention Program (HIP), where risedronate decreased the risk of hip fractures by 60% in women with prior history of vertebral fractures [56].
The best data regarding secondary prevention of hip fractures however comes from the Health Outcomes and Reduced Incidence with Zoledronic Acid Once Yearly (HORIZON) trial, where patients were randomized to zoledronic acid or placebo within 90 days of a hip fracture. Over a median duration of therapy of about 2 years, zoledronic acid decreased the risk of any new clinical fracture by 35%, of new vertebral fractures by 46%, and of recurrent hip fractures by 30% [57].
Effect of Anabolic Therapy After a Fracture
The Fracture Prevention Trial (FPT) compared the effect of teriparatide to placebo in women with at least 1 moderate or 2 mild atraumatic vertebral fractures and showed a 65% reduction in the risk of new vertebral fractures and a 53% reduction in the risk of new non-vertebral fractures [58]. Likewise, the Abaloparatide Comparator Trial In Vertebral Endpoints (ACTIVE) enrolled women with at least 2 mild vertebral fractures, 1 moderate vertebral fracture or history of a low trauma fracture of the forearm, humerus, sacrum, pelvis, hip, femur, or tibia. In this trial, abaloparatide decreased the risk of new vertebral fractures by 85% and of new non-vertebral fractures by 43% compared to placebo [59].
Will Anti-Osteoporosis Therapy After a Low-Trauma Fracture Impact Fracture Healing?
One major question regarding the use of anti-osteoporosis drugs in patients with a recent fracture is the effect that treatment might have on bone healing after fracture or fracture-repair surgery. With antiresorptive agents in particular, the main concern is whether suppression of bone turnover may lead to delayed bone healing, since healing requires callus remodeling. A small prospective study evaluated fracture healing in 196 patients treated for a distal radius fracture, 153 of whom were on a bisphosphonate at the time of the fracture. While bisphosphonate use was associated with a longer time to radiographic union, the time to union was only 6 days longer in the bisphosphonate group (55 days versus 49 days to union in the bisphosphonate and control groups, respectively), and has generally not been felt to be clinically significant [60]. The most reassuring data regarding this question however, comes from the HORIZON trial where 2127 men and women were randomized to zoledronic acid or placebo within 90 days of a hip fracture. No difference in healing between the 2 groups was seen, regardless of the time of initiation of zoledronic acid (within 2 weeks of fracture, between 2 and 4 weeks, between 4 and 6 weeks or after 6 weeks) [61].
The stimulation of bone turnover that occurs with anabolic agents is generally thought to accelerate bone healing. In animal studies, teriparatide has been found to enhance callus formation and mechanical strength [62–64], but there is no definitive data in humans to prove this effect [65].
In summary, there is strong evidence demonstrating the effectiveness of bisphosphonates and anabolic agents at decreasing the risk of fracture recurrence in patients with preexisting vertebral fractures. Zoledronic acid has also been shown to decrease the risk of fracture recurrence after a hip fracture. Anti-osteoporosis therapy after a fracture has no clinically significant effect on fracture healing.
The Gap Between Science and Practice
Practice Guidelines Versus Actual Practice
Based on the data presented above, multiple professional societies and expert groups have developed guidelines emphasizing the importance of evaluation and treatment for osteoporosis following a low-trauma fracture, especially those of the hip and spine [8,9,66–69]. In a 2009 multidisciplinary workshop of the International Society of Fracture Repair, an in-depth review of existing data showed no evidence for a negative effect of anti-osteoporosis drugs on fracture healing. As a result, it was recommended not to withhold osteoporosis therapy until fracture healing has occurred, and to initiate treatment before patient discharge from the fracture ward in order to improve follow-up [70].
However, despite these expert guidelines and the availability of several effective agents to decrease the risk of fracture and fracture recurrence, evaluation and treatment of patients for osteoporosis after a low-trauma fracture are very low. Several large-scale studies involving older patients with fractures in North America, Europe, Asia, and Australia have shown that the rates of BMD measurement or drug therapy for osteoporosis after a fragility fracture do not exceed 25% to 30% [71–80]. While treatment trends over time may have shown some improvement, they remain overall disappointing. For example, in a study of over 150,000 patients who sustained a fracture between 1997 and 2004, Roerholt et al found that around 20% of women were started on therapy after a vertebral fracture in 1997, while 40% received therapy in 2004. Among women with hip fracture, 3% received treatment in 1997 and 9% in 2004 [71]. Furthermore, when osteoporosis treatment rates are examined more closely, most of the patients who receive treatment after a fracture are those who were being treated prior to the fracture, so treatment is simply continued in them. New osteoporosis therapy is initiated in only 5% to 15% of patients who are not already on osteoporosis therapy at the time of fracture [72,73,77,81,82].
Analyses of prescription patterns suggest that patients with vertebral fractures are more likely to receive treatment compared to those with hip fractures [71,82], and that women are much more likely to receive therapy than men [71,74,77,83–88]. Other factors that decrease the chance of receiving therapy include black race [84], low income [74], older age, presence of multiple comorbidities, and polypharmacy [83].
Barriers to Care: Where Are We Failing?
The large discrepancy between science and practice when it comes to secondary prevention of fractures is quite puzzling and has been the subject of several investigations. A major barrier to proper care seems to be the lack of ownership of the problem by the orthopedic surgeons and medical providers, and the less than ideal collaboration between the 2 services in coordinating and providing secondary prevention [89–94]. The orthopedic surgeons are one of the first points of contact with health care for a patient with a low-trauma hip fracture. They are mainly charged with providing acute fracture care and often cannot provide long-term osteoporosis care, which would be more suitable for a medical specialist. However, while the acute care surgical team is not best suited to treat osteoporosis, it is still very important that they initiate patient referral to a provider who can provide long-term osteoporosis care. This transition of care–of lack of it–seems to be one of the major missing links, leading to patient loss [88] and suboptimal secondary prevention.
However, patient referral may not be a sufficient solution and interestingly, a medical consultation during an acute admission for hip fracture does not seem to increase the frequency of osteoporosis diagnosis [95]. This points to a deficiency in knowledge, and as a matter of fact, studies do suggest a problem with under-recognition of the connection between low-trauma fractures and underlying osteoporosis among medical and surgical providers alike [92,93,96]. In a survey of orthopedic surgeons and consultant physicians involved in the care of patients with low-trauma hip fractures, only 24% of respondents felt that osteoporosis therapy was indicated. The majority of providers thought that treatment with a bisphosphonate was indicated only if low BMD was present, rather than in all patients with low-trauma hip fractures [92]. This is further illustrated by the fact that only a minority of patients with a low-trauma fracture are formally given the diagnosis of osteoporosis [75,80,97] or are told that they have osteoporosis [79].
Fracture Liaison Services—A Potential Solution to Enhance Secondary Fracture Prevention
What is a Fracture Liaison Service?
Several solutions have been proposed to remedy the main barriers that interfere with proper secondary treatment of osteoporosis, namely patient education, provider education, and the initiation of programs to enhance coordination and continuity of care between treating teams. Taken together, these interventions have been modestly effective at increasing the odds of BMD measurement and initiation of osteoporosis therapy [98, 99]. Interventions that focused mainly on provider and/or patient education were the least effective, especially when they did not rely on direct in-person interactions, and programs intended to enhance transitions of care were more effective [96,99,100].
These programs are commonly referred to as fracture liaison services (FLS). They aim to identify patients with low-trauma fractures, provide risk assessment and education to the patient, and in some cases provide the patient with post-fracture osteoporosis care. These services typically require a dedicated case manager, who is often a clinical nurse specialist, ideally supported by a medical practitioner with expertise in the treatment of osteoporosis. The FLS case manager uses predetermined protocols that facilitate patient identification, risk assessment and management [101]. Some programs are hospital-based, identifying and evaluating patients while still hospitalized for their hip fracture, and others are based in clinics, aiming to provide services after discharge from the initial acute hospitalization [96,99–101].
How Effective Are Fracture Liaison Services?
Several FLS models have been proposed and tested, with some limited to patient identification and risk stratification, and others more intensive, involving initiation of BMD testing or BMD testing and osteoporosis treatment. In a meta-analysis of FLS programs, Ganda et al grouped programs into 3 categories: Type A programs involved patient identification, assessment and treatment, type B programs involved patient identification and assessment only without treatment, and type C programs involved patient identification combined with alerting of the patients and providers to the need to assess and treat. The effectiveness of the programs in terms of BMD testing and initiation of therapy increased with intensity. Type A programs were the most effective with BMD testing and treatment initiation rates of 79.4% and 46.4% respectively, followed by type B programs which had BMD testing and treatment initiation rates of 59.5% and 40.6% respectively, then type C programs which had BMD testing and treatment initiation rates of 43.4% and 23.4% respectively [100].
The most intensive programs have also been shown to significantly decrease the risk of fracture recurrence, with a reduction in the rate of re-fracture from 19.7% to 4.1% within 4 weeks [102], and a 37.2 % reduction within 3 years [103,104]. Additionally, intensive FLS programs involving pharmacotherapy with a bisphosphonate may be associated with a reduction in mortality after a hip fracture. Beaupre et al evaluated the mortality benefit associated with oral bisphosphonate therapy in the setting of a FLS and demonstrated an 8% decline in mortality per month of oral bisphosphonate use, and an approximate 60% reduction per year of use in comparison to patients who did not receive treatment [105]. This finding was consistent with the reduction in mortality seen with zoledronic acid in the HORIZON trial, which was in part attributable to decreased re-fracture rates, but primarily due to reduction in the occurrence of pneumonia and arrhythmias in patients receiving the drug [57,106].
While fracture liaison services may be associated with increased immediate costs—such as the costs of hiring a case manager, BMD testing and pharmacotherapy, and in some cases a data management system—several cost-effectiveness analyses have shown associated long-term cost savings [107–109]. This is not surprising given that they decrease re-fracture rates, leading to a decline in the very costly immediate and long-term fracture care costs.
Summary
In summary, fragility fractures present a major health care problem for aging populations, leading to significant costs and high morbidity and mortality. Assessment and treatment of osteoporosis following a fragility fracture can decrease the risk of fracture recurrence, long-term costs, morbidities, and possibly mortality. In the last decade, several national and international initiatives have been created to promote and encourage secondary prevention of fragility fractures [110–113]. However, these programs have all been voluntary and there are currently no reliable mechanisms to ensure broad implementation of secondary fracture prevention interventions. As a result, and while several isolated secondary prevention programs have shown great success, most patients with low-trauma fractures still receive suboptimal osteoporosis care.
Corresponding author: Amal Shibli-Rahhal, MD, MS, Dept. of Internal Medicine, University of Iowa Hospitals and Clinics, Iowa City, IA 52242.
Financial disclosures:
From the Department of Internal Medicine, University of Iowa Carver College of Medicine, Iowa City, IA.
Abstract
- Objective: To review and summarize the literature regarding current approaches to secondary prevention of low-trauma osteoporotic fractures.
- Methods: PubMed search and summary of existing literature related to complications and secondary prevention of osteoporotic fractures was performed.
- Results: Fragility fractures are associated with high rates of short and long term morbidities and carry a high risk of mortality and fracture recurrence. Several of the currently available anti-osteoporosis medications have been shown to decrease the risk of fracture recurrence in patients with prevalent osteoporotic fractures and some may even decrease mortality. However, only a minority of patients with fragility fractures are adequately evaluated and treated for osteoporosis. Fracture liaison services that ensure identification and risk stratification of patients with fragility fractures and proper evaluation and treatment of osteoporosis have proven effective at enhancing osteoporosis care in these patients, decreasing fracture recurrence and possibly even decreasing long-term mortality, while providing long-term cost savings. Unfortunately, however, this model of care has not been widely adopted and implemented.
- Conclusion: Fragility fractures represent a major health care problem for aging populations. Unfortunately, most patients with low-trauma fractures still receive suboptimal osteoporosis care.
Key words: osteoporosis; fracture; fragility; low-trauma; bone density.
Low-trauma fractures are fractures that occur from a trauma equivalent to a fall from standing height or less [1,2]. They can involve any skeletal site, but the most significant are vertebral, pelvic, wrist and hip fractures, which together represent close to 90% of all low-trauma fractures [3,4]. The overall burden of low-trauma fractures is quite high worldwide and is projected to increase over time [3–6]. In 2010, 3.5 million new low-trauma fractures were reported in the European Union [3]. In the United States, there were more than 2 million fractures in 2005, and it is estimated that more than 3 million fractures will occur in year 2025 [4].
Low-trauma fractures are generally indicative of compromised bone strength—especially when they involve the hip—and are thus often referred to as fragility fractures. While the traditional definition of osteoporosis is a bone mineral density (BMD) T-score of -2.5 or lower, low-trauma fractures of the hip are also diagnostic of osteoporosis, regardless of bone mineral density [2,7–9]. In addition, low-trauma fractures of the vertebrae, the proximal humerus, and the pelvis are considered diagnostic of osteoporosis when combined with T-scores between -1 and -2.5 [2,7]. Bone biopsies and high-resolution peripheral quantitative computed tomography (HR-pQCT) in patients with low-trauma fractures and normal BMD suggest microarchitectural alterations and abnormalities of collagen orientation and crosslinking within the bone matrix [10-12], leading to decreased bone strength.
This review will address the individual and societal costs of low-trauma fractures and issues related to secondary prevention of fractures, with specific emphasis on pharmacotherapy and fracture liaison services.
Impact of Low-Trauma Fractures
Acute and Long-Term Complications
Of all fragility fractures, hip fractures are the ones most likely to result in serious acute complications. The most common acute complications are delirium in up to 50% of patients and malnutrition in up to 60%, both of which predict slower and less complete recovery [13–16]. Other complications include urinary tract infections in up to 60% of patients in certain reports [17], thromboembolic disease with deep venous thrombosis in around 27% of patients and pulmonary embolism in up to 7% [16], and acute kidney injury in about 15% [18].
In addition, it is not uncommon for patients to suffer from significant long-term functional limitations following fragility fractures. While vertebral fractures do not frequently lead to hospitalization or institutionalization, they often lead to significant physical limitations and chronic pain [19,20] and to negative effects on self-esteem, mood, and body image [21,22]. However, the most remarkable functional decline and limitations are seen after hip fractures [23–25]. In a study of 2800 women and men with hip fracture, Beringer et al found that more than 30% were still institutionalized, and only 40% were able to walk outdoors independently 1 year later. Predictors of poor outcome included male sex, advanced age, cognitive impairment, and presence of comorbidities [23].
It is not surprising then that a fracture is often associated with an overall decline in the individual’s quality of life and this has been demonstrated in several studies [26–28]. In the largest study of this type, Tarride et al examined over 23,000 patients with fragility fractures and found a sharp decline in health-related quality of life (HRQOL) immediately after the fracture, which remained below baseline for up to 3 years [26]. The decline was worse in patients with hip and spine fractures compared to other fractures [27].
Mortality Following Fragility Fractures
Perhaps the most concerning complication, however, is the excess mortality seen after fractures. Several studies have demonstrated excess mortality after vertebral fractures, especially in the year following the fracture [29–33], but the highest increase in mortality was observed following hip fractures. In fact, the 30-day mortality after a hip fracture approximates 7% [23] and the excess 1-year mortality is estimated at 8% to 36% [34,35]. While the highest risk of mortality is seen in the first year following the fracture, the increased risk persists for at least 5 to 6 years [36]. Malnutrition, decreased mobility, male sex, and the number of coexisting medical comorbidities further increase the risk of mortality [29,32,34,36,37].
Risk of Fracture Recurrence
In both men and women, a fragility fracture at any site increases the risk of subsequent fractures [38–41], and the risk increases with the number of prevalent fractures [42]. Gehlbach et al estimated an 80% increase in the risk of fracture recurrence after 1 fracture, a threefold increase after 2 fractures, and an almost fivefold increase after 3 fractures [42]. The increase in risk is even more pronounced following vertebral fractures specifically, doubling after the first fracture an increasing by up to ninefold after 3 fractures [42, 43]. This increase in risk is highest in the first year following the fracture but may persist for up to 10 years [39,43].
Fracture Impact on Society
Fractures are associated with a high financial burden to society, in terms of direct acute care costs and long-term rehabilitation [3,4,44–48]. In 2010, the direct cost from fractures in the EU was estimated at €24.6 billion [3]. In the US, this cost was around $14.0 billion in 2002 and $16.9 billion in 2005 [4,48], and in Canada it was $1.5 billion in 2011 [47]. These numbers increase substantially when costs associated with long-term post-fracture rehabilitation are included, with an additional estimated yearly cost of €10.7 billion in the EU and $1.03 billion in Canada [3,47].
While hip fractures account for only about 18% of all low-trauma fractures, they are associated with the highest cost burden, accounting for about 50% to 70% of the total fracture-associated expenditures [3,4,44]. This is likely due to the fact almost all hip fractures require hospitalization, most require surgical repair and rehabilitation, and because they lead to the highest rates of morbidity and mortality.
Can Fracture Recurrence After a Low-Trauma Fracture Be Prevented?
Many approaches to secondary fracture prevention have been proposed, including but not limited to fall prevention, exercise therapy, nutrition therapy, prevention and treatment of sarcopenia, vitamin D and calcium supplementation, and osteoporosis pharmacotherapy [49–53]. Of those, osteoporosis pharmacotherapy has the strongest and most compelling efficacy data and will be reviewed in the following sections.
Effect of Antiresorptive Therapy After a Fracture
In the Fracture Intervention Trial (FIT), alendronate decreased the risk of new vertebral fractures by about 47% and of hip fractures by about 50% in women with preexisting vertebral fractures [54,55]. Similar fracture protection benefits were demonstrated in the Hip Intervention Program (HIP), where risedronate decreased the risk of hip fractures by 60% in women with prior history of vertebral fractures [56].
The best data regarding secondary prevention of hip fractures however comes from the Health Outcomes and Reduced Incidence with Zoledronic Acid Once Yearly (HORIZON) trial, where patients were randomized to zoledronic acid or placebo within 90 days of a hip fracture. Over a median duration of therapy of about 2 years, zoledronic acid decreased the risk of any new clinical fracture by 35%, of new vertebral fractures by 46%, and of recurrent hip fractures by 30% [57].
Effect of Anabolic Therapy After a Fracture
The Fracture Prevention Trial (FPT) compared the effect of teriparatide to placebo in women with at least 1 moderate or 2 mild atraumatic vertebral fractures and showed a 65% reduction in the risk of new vertebral fractures and a 53% reduction in the risk of new non-vertebral fractures [58]. Likewise, the Abaloparatide Comparator Trial In Vertebral Endpoints (ACTIVE) enrolled women with at least 2 mild vertebral fractures, 1 moderate vertebral fracture or history of a low trauma fracture of the forearm, humerus, sacrum, pelvis, hip, femur, or tibia. In this trial, abaloparatide decreased the risk of new vertebral fractures by 85% and of new non-vertebral fractures by 43% compared to placebo [59].
Will Anti-Osteoporosis Therapy After a Low-Trauma Fracture Impact Fracture Healing?
One major question regarding the use of anti-osteoporosis drugs in patients with a recent fracture is the effect that treatment might have on bone healing after fracture or fracture-repair surgery. With antiresorptive agents in particular, the main concern is whether suppression of bone turnover may lead to delayed bone healing, since healing requires callus remodeling. A small prospective study evaluated fracture healing in 196 patients treated for a distal radius fracture, 153 of whom were on a bisphosphonate at the time of the fracture. While bisphosphonate use was associated with a longer time to radiographic union, the time to union was only 6 days longer in the bisphosphonate group (55 days versus 49 days to union in the bisphosphonate and control groups, respectively), and has generally not been felt to be clinically significant [60]. The most reassuring data regarding this question however, comes from the HORIZON trial where 2127 men and women were randomized to zoledronic acid or placebo within 90 days of a hip fracture. No difference in healing between the 2 groups was seen, regardless of the time of initiation of zoledronic acid (within 2 weeks of fracture, between 2 and 4 weeks, between 4 and 6 weeks or after 6 weeks) [61].
The stimulation of bone turnover that occurs with anabolic agents is generally thought to accelerate bone healing. In animal studies, teriparatide has been found to enhance callus formation and mechanical strength [62–64], but there is no definitive data in humans to prove this effect [65].
In summary, there is strong evidence demonstrating the effectiveness of bisphosphonates and anabolic agents at decreasing the risk of fracture recurrence in patients with preexisting vertebral fractures. Zoledronic acid has also been shown to decrease the risk of fracture recurrence after a hip fracture. Anti-osteoporosis therapy after a fracture has no clinically significant effect on fracture healing.
The Gap Between Science and Practice
Practice Guidelines Versus Actual Practice
Based on the data presented above, multiple professional societies and expert groups have developed guidelines emphasizing the importance of evaluation and treatment for osteoporosis following a low-trauma fracture, especially those of the hip and spine [8,9,66–69]. In a 2009 multidisciplinary workshop of the International Society of Fracture Repair, an in-depth review of existing data showed no evidence for a negative effect of anti-osteoporosis drugs on fracture healing. As a result, it was recommended not to withhold osteoporosis therapy until fracture healing has occurred, and to initiate treatment before patient discharge from the fracture ward in order to improve follow-up [70].
However, despite these expert guidelines and the availability of several effective agents to decrease the risk of fracture and fracture recurrence, evaluation and treatment of patients for osteoporosis after a low-trauma fracture are very low. Several large-scale studies involving older patients with fractures in North America, Europe, Asia, and Australia have shown that the rates of BMD measurement or drug therapy for osteoporosis after a fragility fracture do not exceed 25% to 30% [71–80]. While treatment trends over time may have shown some improvement, they remain overall disappointing. For example, in a study of over 150,000 patients who sustained a fracture between 1997 and 2004, Roerholt et al found that around 20% of women were started on therapy after a vertebral fracture in 1997, while 40% received therapy in 2004. Among women with hip fracture, 3% received treatment in 1997 and 9% in 2004 [71]. Furthermore, when osteoporosis treatment rates are examined more closely, most of the patients who receive treatment after a fracture are those who were being treated prior to the fracture, so treatment is simply continued in them. New osteoporosis therapy is initiated in only 5% to 15% of patients who are not already on osteoporosis therapy at the time of fracture [72,73,77,81,82].
Analyses of prescription patterns suggest that patients with vertebral fractures are more likely to receive treatment compared to those with hip fractures [71,82], and that women are much more likely to receive therapy than men [71,74,77,83–88]. Other factors that decrease the chance of receiving therapy include black race [84], low income [74], older age, presence of multiple comorbidities, and polypharmacy [83].
Barriers to Care: Where Are We Failing?
The large discrepancy between science and practice when it comes to secondary prevention of fractures is quite puzzling and has been the subject of several investigations. A major barrier to proper care seems to be the lack of ownership of the problem by the orthopedic surgeons and medical providers, and the less than ideal collaboration between the 2 services in coordinating and providing secondary prevention [89–94]. The orthopedic surgeons are one of the first points of contact with health care for a patient with a low-trauma hip fracture. They are mainly charged with providing acute fracture care and often cannot provide long-term osteoporosis care, which would be more suitable for a medical specialist. However, while the acute care surgical team is not best suited to treat osteoporosis, it is still very important that they initiate patient referral to a provider who can provide long-term osteoporosis care. This transition of care–of lack of it–seems to be one of the major missing links, leading to patient loss [88] and suboptimal secondary prevention.
However, patient referral may not be a sufficient solution and interestingly, a medical consultation during an acute admission for hip fracture does not seem to increase the frequency of osteoporosis diagnosis [95]. This points to a deficiency in knowledge, and as a matter of fact, studies do suggest a problem with under-recognition of the connection between low-trauma fractures and underlying osteoporosis among medical and surgical providers alike [92,93,96]. In a survey of orthopedic surgeons and consultant physicians involved in the care of patients with low-trauma hip fractures, only 24% of respondents felt that osteoporosis therapy was indicated. The majority of providers thought that treatment with a bisphosphonate was indicated only if low BMD was present, rather than in all patients with low-trauma hip fractures [92]. This is further illustrated by the fact that only a minority of patients with a low-trauma fracture are formally given the diagnosis of osteoporosis [75,80,97] or are told that they have osteoporosis [79].
Fracture Liaison Services—A Potential Solution to Enhance Secondary Fracture Prevention
What is a Fracture Liaison Service?
Several solutions have been proposed to remedy the main barriers that interfere with proper secondary treatment of osteoporosis, namely patient education, provider education, and the initiation of programs to enhance coordination and continuity of care between treating teams. Taken together, these interventions have been modestly effective at increasing the odds of BMD measurement and initiation of osteoporosis therapy [98, 99]. Interventions that focused mainly on provider and/or patient education were the least effective, especially when they did not rely on direct in-person interactions, and programs intended to enhance transitions of care were more effective [96,99,100].
These programs are commonly referred to as fracture liaison services (FLS). They aim to identify patients with low-trauma fractures, provide risk assessment and education to the patient, and in some cases provide the patient with post-fracture osteoporosis care. These services typically require a dedicated case manager, who is often a clinical nurse specialist, ideally supported by a medical practitioner with expertise in the treatment of osteoporosis. The FLS case manager uses predetermined protocols that facilitate patient identification, risk assessment and management [101]. Some programs are hospital-based, identifying and evaluating patients while still hospitalized for their hip fracture, and others are based in clinics, aiming to provide services after discharge from the initial acute hospitalization [96,99–101].
How Effective Are Fracture Liaison Services?
Several FLS models have been proposed and tested, with some limited to patient identification and risk stratification, and others more intensive, involving initiation of BMD testing or BMD testing and osteoporosis treatment. In a meta-analysis of FLS programs, Ganda et al grouped programs into 3 categories: Type A programs involved patient identification, assessment and treatment, type B programs involved patient identification and assessment only without treatment, and type C programs involved patient identification combined with alerting of the patients and providers to the need to assess and treat. The effectiveness of the programs in terms of BMD testing and initiation of therapy increased with intensity. Type A programs were the most effective with BMD testing and treatment initiation rates of 79.4% and 46.4% respectively, followed by type B programs which had BMD testing and treatment initiation rates of 59.5% and 40.6% respectively, then type C programs which had BMD testing and treatment initiation rates of 43.4% and 23.4% respectively [100].
The most intensive programs have also been shown to significantly decrease the risk of fracture recurrence, with a reduction in the rate of re-fracture from 19.7% to 4.1% within 4 weeks [102], and a 37.2 % reduction within 3 years [103,104]. Additionally, intensive FLS programs involving pharmacotherapy with a bisphosphonate may be associated with a reduction in mortality after a hip fracture. Beaupre et al evaluated the mortality benefit associated with oral bisphosphonate therapy in the setting of a FLS and demonstrated an 8% decline in mortality per month of oral bisphosphonate use, and an approximate 60% reduction per year of use in comparison to patients who did not receive treatment [105]. This finding was consistent with the reduction in mortality seen with zoledronic acid in the HORIZON trial, which was in part attributable to decreased re-fracture rates, but primarily due to reduction in the occurrence of pneumonia and arrhythmias in patients receiving the drug [57,106].
While fracture liaison services may be associated with increased immediate costs—such as the costs of hiring a case manager, BMD testing and pharmacotherapy, and in some cases a data management system—several cost-effectiveness analyses have shown associated long-term cost savings [107–109]. This is not surprising given that they decrease re-fracture rates, leading to a decline in the very costly immediate and long-term fracture care costs.
Summary
In summary, fragility fractures present a major health care problem for aging populations, leading to significant costs and high morbidity and mortality. Assessment and treatment of osteoporosis following a fragility fracture can decrease the risk of fracture recurrence, long-term costs, morbidities, and possibly mortality. In the last decade, several national and international initiatives have been created to promote and encourage secondary prevention of fragility fractures [110–113]. However, these programs have all been voluntary and there are currently no reliable mechanisms to ensure broad implementation of secondary fracture prevention interventions. As a result, and while several isolated secondary prevention programs have shown great success, most patients with low-trauma fractures still receive suboptimal osteoporosis care.
Corresponding author: Amal Shibli-Rahhal, MD, MS, Dept. of Internal Medicine, University of Iowa Hospitals and Clinics, Iowa City, IA 52242.
Financial disclosures:
1. Bergström U, Björnstig U, Stenlund H, Jonsson H, Svensson O. Fracture mechanisms and fracture pattern in men and women aged 50 years and older: a study of a 12-year population-based injury register, Umeå, Sweden. Osteoporos Int 2008;19:1267–73.
2. Siris ES, Adler R, Bilezikian J, et al. The clinical diagnosis of osteoporosis: a position statement from the National Bone Health Alliance Working Group. Osteoporos Int 2014;25:1439–43.
3. Kanis JA, Borgström F, Compston J, et al. SCOPE: a scorecard for osteoporosis in Europe. Arch Osteoporos 2013;8:144.
4. Burge R, Dawson-Hughes B, Solomon DH, Wong JB, King A, Tosteson A. Incidence and economic burden of osteoporosis-related fractures in the United States, 2005-2025. J Bone Miner Res 2007;22:465–75.
5. Rosengren BE, Karlsson MK. The annual number of hip fractures in Sweden will double from year 2002 to 2050: projections based on local and nationwide data. Acta Orthop 2014;85:234–7.
6. Chen IJ, Chiang CY, Li YH, et al. Nationwide cohort study of hip fractures: time trends in the incidence rates and projections up to 2035. Osteoporos Int 2015;26:681–8.
7. Siris ES, Boonen S, Mitchell PJ, Bilezikian J, Silverman S. What’s in a name? What constitutes the clinical diagnosis of osteoporosis? Osteoporos Int 2012;23:2093–7.
8. Cosman F, de Beur SJ, LeBoff MS, et al. Clinician’s guide to prevention and treatment of osteoporosis. Osteoporos Int 2014;25:2359–81.
9. Papaioannou A, Morin S, Cheung AM, et al; Scientific Advisory Council of Osteoporosis Canada. 2010 Clinical practice guidelines for the diagnosis and management of osteoporosis in Canada: summary. CMAJ 2010;182:1864–73.
10. Malluche HH, Porter DS, Mawad H, Monier-Faugere MC, Pienkowski D. Low-energy fractures without low T-scores characteristic of osteoporosis: a possible bone matrix disorder. J Bone Joint Surg Am 2013;95:e1391–6.
11. Ascenzi MG, Chin J, Lappe J, Recker R. Non-osteoporotic women with low-trauma fracture present altered birefringence in cortical bone. Bone 2016;84:104–12.
12. Nishiyama KK, Macdonald HM, Hanley DA, Boyd SK. Women with previous fragility fractures can be classified based on bone microarchitecture and finite element analysis measured with HR-pQCT. Osteoporos Int 2013;24:1733–40.
13. Dolan MM, Hawkes WG, Zimmerman SI, Morrison RS, Gruber-Baldini AL, Hebel JR, Magaziner J. Delirium on hospital admission in aged hip fracture patients: prediction of mortality and 2-year functional outcomes. J Gerontol A Biol Sci Med Sci 2000;55:M527–34.
14. Bruce AJ, Ritchie CW, Blizard R, Lai R, Raven P. The incidence of delirium associated with orthopedic surgery: a meta-analytic review. Int Psychogeriatr 2007;19:197–214.
15. Patterson BM, Cornell CN, Carbone B, Levine B, Chapman D. Protein depletion and metabolic stress in elderly patients who have a fracture of the hip. J Bone Joint Surg Am 1992;74:251–60.
16. Beaupre LA, Jones CA, Saunders LD, Johnston DW, Buckingham J, Majumdar SR. Best practices for elderly hip fracture patients. A systematic overview of the evidence. J Gen Intern Med 2005;20:1019–25.
17. Johansson I, Athlin E, Frykholm L, Bolinder H, Larsson G. Intermittent versus indwelling catheters for older patients with hip fractures. J Clin Nurs 2002;11:651–6.
18. Ulucay C, Eren Z, Kaspar EC, et al. Risk factors for acute kidney injury after hip fracture surgery in the elderly individuals. Geriatr Orthop Surg Rehabil 2012;3:150–6.
19. Greendale GA, Barrett-Connor E, Ingles S, Haile R. Late physical and functional effects of osteoporotic fracture in women: the Rancho Bernardo Study. J Am Geriatr Soc 1995;43:955–61.
20. Nevitt MC, Thompson DE, Black DM, et al. Effect of alendronate on limited-activity days and bed-disability days caused by back pain in postmenopausal women with existing vertebral fractures. Fracture Intervention Trial Research Group. Arch Intern Med 2000;160:77–85.
21. Gold DT, Lyles KW, Shipp KM, Drezner MK. Osteoporosis and its nonskeletal consequences: their impact on treatment decisions. In: Marcus R, Feldman D, Kelsey J, eds. Osteoporosis. 2nd ed. San Diego, CA: Academic Press; 2001:479–84.
22. Sale JEM, Frankel L, Thielke S, Funnell L. Pain and fracture-related limitations persist 6 months after a fragility fracture. Rheumatol Int 2017;37:1317–22.
23. Beringer TR, Clarke J, Elliott JR, Marsh DR, Heyburn G, Steele IC. Outcome following proximal femoral fracture in Northern Ireland. Ulster Med J 2006;75:200–6.
24. Córcoles-Jiménez MP, Villada-Munera A, Del Egido-Fernández MÁ, et al. Recovery of activities of daily living among older people one year after hip fracture. Clin Nurs Res 2015;24:604–23.
25. Kammerlander C, Gosch M, Kammerlander-Knauer U, Luger TJ, Blauth M, Roth T. Long-term functional outcome in geriatric hip fracture patients. Arch Orthop Trauma Surg 2011;131:1435–44.
26. Tarride JE, Burke N, Leslie WD, et al. Loss of health related quality of life following low-trauma fractures in the elderly. BMC Geriatr 2016;16:84.
27. Abimanyi-Ochom J, Watts JJ, Borgström F, et al. Changes in quality of life associated with fragility fractures: Australian arm of the International Cost and Utility Related to Osteoporotic Fractures Study (AusICUROS). Osteoporos Int 2015;26:1781–90.
28. Adachi JD, Loannidis G, Berger C, et al; Canadian Multicentre Osteoporosis Study (CaMos) Research Group. The influence of osteoporotic fractures on health-related quality of life in community-dwelling men and women across Canada. Osteoporos Int 2001;12:903–8.
29. Center JR, Nguyen TV, Schneider D, Sambrook PN, Eisman JA. Mortality after all major types of osteoporotic fracture in men and women: an observational study. Lancet 1999;353:878–82.
30. Kanis JA, Oden A, Johnell O, De Laet C, Jonsson B. Excess mortality after hospitalisation for vertebral fracture. Osteoporos Int 2004;15:108–12.
31. Johnell O, Kanis JA, Odén A, et al. Mortality after osteoporotic fractures. Osteoporos Int 2004;15:38–42.
32. Gosch M, Druml T, Nicholas JA, et al. Fragility non-hip fracture patients are at risk. Arch Orthop Trauma Surg 2015;135:69–77.
33. Ensrud KE, Thompson DE, Cauley JA, et al. Prevalent vertebral deformities predict mortality and hospitalization in older women with low bone mass. Fracture Intervention Trial Research Group. J Am Geriatr Soc 2000;48:241–9.
34. Abrahamsen B, van Staa T, Ariely R, Olson M, Cooper C. Excess mortality following hip fracture: a systematic epidemiological review. Osteoporos Int 2009;20:1633–50.
35. Kanis JA, Oden A, Johnell O, De Laet C, Jonsson B, Oglesby AK. The components of excess mortality after hip fracture. Bone 2003;32:468–73.
36. Forsén L, Sogaard AJ, Meyer HE, Edna T, Kopjar B. Survival after hip fracture: short- and long-term excess mortality according to age and gender. Osteoporos Int 1999;10:73–8.
37. Trombetti A, Herrmann F, Hoffmeyer P, Schurch MA, Bonjour JP, Rizzoli R. Survival and potential years of life lost after hip fracture in men and age-matched women. Osteoporos Int 2002;13:731–7.
38. Kanis JA, Johnell O, De Laet C, et al. A meta-analysis of previous fracture and subsequent fracture risk. Bone 2004;35:375–82.
39. Center JR, Bliuc D, Nguyen TV, Eisman JA. Risk of subsequent fracture after low-trauma fracture in men and women. JAMA 2007;297:387–94.
40. Hasserius R, Karlsson MK, Nilsson BE, Redlund-Johnell I, Johnell O; European Vertebral Osteoporosis Study. Prevalent vertebral deformities predict increased mortality and increased fracture rate in both men and women: a 10-year population-based study of 598 individuals from the Swedish cohort in the European Vertebral Osteoporosis Study. Osteoporos Int 2003;14:61–8.
41. Edwards BJ, Bunta AD, Simonelli C, Bolander M, Fitzpatrick LA. Prior fractures are common in patients with subsequent hip fractures. Clin Orthop Relat Res 2007;461:226–30.
42. Gehlbach S, Saag KG, Adachi JD, et al. Previous fractures at multiple sites increase the risk for subsequent fractures: the Global Longitudinal Study of Osteoporosis in Women. J Bone Miner Res 2012;27:645–53.
43. Lindsay R, Silverman SL, Cooper C, et al. Risk of new vertebral fracture in the year following a fracture. JAMA 2001;285:320–3.
44. Hansen L, Mathiesen AS, Vestergaard P, Ehlers LH, Petersen KD. A health economic analysis of osteoporotic fractures: who carries the burden? Arch Osteoporos 2013;8:126.
45. Lippuner K, Grifone S, Schwenkglenks M, et al. Comparative trends in hospitalizations for osteoporotic fractures and other frequent diseases between 2000 and 2008. Osteoporos Int 2012;23:829–39.
46. Lange A, Zeidler J, Braun S. One-year disease-related health care costs of incident vertebral fractures in osteoporotic patients. Osteoporos Int 2014;25:2435–43.
47. Hopkins RB, Burke N, Von Keyserlingk C, Leslie WD, Morin SN, Adachi JD, Papaioannou A, Bessette L, Brown JP, Pericleous L, Tarride J. The current economic burden of illness of osteoporosis in Canada. Osteoporos Int 2016;27:3023–32.
48. Blume SW, Curtis JR. Medical costs of osteoporosis in the elderly Medicare population. Osteoporos Int 2011;22:1835–44.
49. Avenell A, Mak JC, O’Connell D. Vitamin D and vitamin D analogues for preventing fractures in post-menopausal women and older men. Cochrane Database Syst Rev 2014: CD000227.
50. Santesso N, Carrasco-Labra A, Brignardello-Petersen R. Hip protectors for preventing hip fractures in older people. Cochrane Database Syst Rev 2014: CD001255
51. Avenell A, Smith TO, Curtain J, Mak JC, Myint PK. Nutritional supplementation for hip fracture aftercare in older people. Cochrane Database Syst Rev 2016;11: CD001880
52. Giangregorio LM, MacIntyre NJ, Thabane L, Skidmore CJ, Papaioannou A. Exercise for improving outcomes after osteoporotic vertebral fracture. Cochrane Database Syst Rev 2013: CD008618
53. Gillespie LD, Robertson M, Gillespie WJ, et al. Interventions for preventing falls in older people living in the community. Cochrane Database Syst Rev 2012:CD007146.
54. Ensrud KE, Black DM, Palermo L, et al. Treatment with alendronate prevents fractures in women at highest risk: results from the Fracture Intervention Trial. Arch Intern Med 1997;157:2617–24.
55. Black DM, Cummings SR, Karpf DB, et al. Randomised trial of effect of alendronate on risk of fracture in women with existing vertebral fractures. Fracture Intervention Trial Research Group. Lancet 1996;348:1535–41.
56. McClung MR, Geusens P, Miller PD, Zippel H, Bensen WG, Roux C, Adami S, Fogelman I, Diamond T, Eastell R, Meunier PJ, Reginster JY; Hip Intervention Program Study Group. Effect of risedronate on the risk of hip fracture in elderly women. Hip Intervention Program Study Group. N Engl J Med. 2001;344:333–40.
57. Lyles KW, Colón-Emeric CS, Magaziner JS, et al; HORIZON Recurrent Fracture Trial. Zoledronic acid and clinical fractures and mortality after hip fracture. N Engl J Med 2007;357:1799–809.
58. Neer RM, Arnaud CD, Zanchetta JR, et al. Effect of parathyroid hormone (1-34) on fractures and bone mineral density in postmenopausal women with osteoporosis. N Engl J Med 2001;344:1434–41.
59. Miller PD, Hattersley G, Riis BJ, et al; ACTIVE Study Investigators. Effect of abaloparatide vs placebo on new vertebral fractures in postmenopausal women with osteoporosis: a randomized clinical trial. JAMA. 2016;316:722–33.
60. Rozental TD, Vazquez MA, Chacko AT, Ayogu N, Bouxsein ML. Comparison of radiographic fracture healing in the distal radius for patients on and off bisphosphonate therapy. J Hand Surg Am 2009;34:595–602.
61. Colón-Emeric C, Nordsletten L, Olson S, et al; HORIZON Recurrent Fracture Trial. Association between timing of zoledronic acid infusion and hip fracture healing. Osteoporos Int 2011;22:2329–36.
62. Nakajima A, Shimoji N, Shiomi K, et al. Mechanisms for the enhancement of fracture healing in rats treated with intermittent low-dose human parathyroid hormone (1-34). J Bone Miner Res 2002;17:2038–47.
63. Alkhiary YM, Gerstenfeld LC, Krall E, et al. Enhancement of experimental fracture-healing by systemic administration of recombinant human parathyroid hormone (PTH 1-34). J Bone Joint Surg Am 2005;87:731–41.
64. Andreassen TT, Ejersted C, Oxlund H. Intermittent parathyroid hormone (1-34) treatment increases callus formation and mechanical strength of healing rat fractures. J Bone Miner Res 1999;14:960–8.
65. Bhandari M, Jin L, See K, et al. Does teriparatide improve femoral neck fracture healing: results from a randomized placebo-controlled trial. Clin Orthop Relat Res 2016;474:1234–44.
66. Lems WF, Dreinhöfer KE, Bischoff-Ferrari H, et al. EULAR/EFORT recommendations for management of patients older than 50 years with a fragility fracture and prevention of subsequent fractures. Ann Rheum Dis 2017;76:802–810.
67. American Academy of Orthopaedics Surgeons. Management of Hip Fractures in the Elderly. Evidence-Based Clinical Practice Guideline. Available at https://www.aaos.org/research/guidelines/hipfxguideline.pdf. Accessed March 2, 2018
68. The International Society For Clinical Densitometry. Official Positions 2015 ISCD Combined. Available at https://iscd.app.box.com/v/OP-ISCD-2015-Adult. Accessed March 2, 2018.
69. International Osteoporosis Foundation. National and Regional Osteoporosis Guidelines. https://www.iofbonehealth.org/national-regional-osteoporosis-guidelines. Accessed March 2, 2018.
70. Goldhahn J, Little D, Mitchell P, et al; ISFR working group drugs and fracture repair. Evidence for anti-osteoporosis therapy in acute fracture situations—recommendations of a multidisciplinary workshop of the International Society for Fracture Repair. Bone 2010;46:267–71.
71. Roerholt C, Eiken P, Abrahamsen B. Initiation of anti-osteoporotic therapy in patients with recent fractures: a nationwide analysis of prescription rates and persistence. Osteoporos Int 2009;20:299–307.
72. Panneman MJ, Lips P, Sen SS, Herings RM. Undertreatment with anti-osteoporotic drugs after hospitalization for fracture. Osteoporos Int 2004;15:120–4.
73. Wilk A, Sajjan S, Modi A, Fan CPS, Mavros P. Post-fracture pharmacotherapy for women with osteoporotic fractures: analysis of a managed care population in the USA. Osteoporos Int 2014; 25:2777–86.
74. Leslie WD, Giangregorio LM, Yogendran M, et al. A population-based analysis of the post-fracture care gap 1996-2008: the situation is not improving. Osteoporos Int 2012;23:1623–9.
75. Kung AW, Fan T, Xu L, et al. Factors influencing diagnosis and treatment of osteoporosis after a fragility fracture among postmenopausal women in Asian countries: a retrospective study. BMC Womens Health 2013;13:7.
76. Wang O, Hu Y, Gong S, et al. A survey of outcomes and management of patients post fragility fractures in China. Osteoporos Int 2015;26:2631–40.
77. Yusuf AA, Matlon TJ, Grauer A, Barron R, Chandler D, Peng Y. Utilization of osteoporosis medication after a fragility fracture among elderly Medicare beneficiaries. Arch Osteoporos 2016;11:31
78. Munson JC, Bynum JP, Bell JE, et al. Patterns of prescription drug use before and after fragility fracture. JAMA Intern Med 2016;176:1531–8.
79. Eisman J, Clapham S, Kehoe L; Australian BoneCare Study. Osteoporosis prevalence and levels of treatment in primary care: the Australian BoneCare Study. J Bone Miner Res 2004;19:1969–75.
80. Duncan R, Francis RM, Jagger C, et al. Magnitude of fragility fracture risk in the very old—are we meeting their needs? The Newcastle 85+ Study. Osteoporos Int 2015;26:123–30.
81. Singh S, Foster R, Khan KM. Accident or osteoporosis?: Survey of community follow-up after low-trauma fracture. Can Fam Physician. 2011;57:e128–33.
82. Andrade SE, Majumdar SR, Chan KA, et al. Low frequency of treatment of osteoporosis among postmenopausal women following a fracture. Arch Intern Med 2003;163:2052–7.
83. Blecher R, Wasrbrout Z, Arama Y, Kardosh R, Agar G, Mirovsky Y. Who is at risk of receiving inadequate care for osteoporosis following fragility fractures? A retrospective study. Isr Med Assoc J 2013;15:634–8.
84. Shibli-Rahhal A, Vaughan-Sarrazin MS, Richardson K, Cram P. Testing and treatment for osteoporosis following hip fracture in an integrated U.S. healthcare delivery system. Osteoporos Int 2011;22:2973–80.
85. Freedman BA, Potter BK, Nesti LJ, Giuliani JR, Hampton C, Kuklo TR. Osteoporosis and vertebral compression fractures-continued missed opportunities. Spine J 2008;8:756–62.
86. Kiebzak GM, Beinart GA, Perser K, Ambrose CG, Siff SJ, Heggeness MH. Undertreatment of osteoporosis in men with hip fracture. Arch Intern Med 2002;162:2217–22.
87. Kamel HK, Bida A, Montagnini M. Secondary prevention of hip fractures in veterans: can we do better? J Am Geriatr Soc 2004;52:647–8.
88. Skorupski N, Alexander IM. Multidisciplinary osteoporosis management of post low-energy trauma hip-fracture patients. J Am Assoc Nurse Pract 2013;25:3–10.
89. Simonelli C, Killeen K, Mehle S, Swanson L. Barriers to osteoporosis identification and treatment among primary care physicians and orthopedic surgeons. Mayo Clin Proc 2002;77:334–8.
90. Abraham A. Undertreatment of osteoporosis in men who have had a hip fracture. Arch Intern Med 2003;163:1236.
91. Sheehan J, Mohamed F, Reilly M, Perry IJ. Secondary prevention following fractured neck of femur: a survey of orthopaedic surgeons practice. Ir Med J. 2000;93:105–7.
92. Levinson MR, Clay FJ. Barriers to the implementation of evidence in osteoporosis treatment in hip fracture. Intern Med J 2009;39:199–202.
93. Kaufman JD, Bolander ME, Bunta AD, Edwards BJ, Fitzpatrick LA, Simonelli C. Barriers and solutions to osteoporosis care in patients with a hip fracture. J Bone Joint Surg Am 2003;85-A:1837–43.
94. Sorbi R, Aghamirsalim M. Osteoporotic Fracture Program management: who should be in charge? A comparative survey of knowledge in orthopaedic surgeons and internists. Orthop Traumatol Surg Res 2013;99:723–30.
95. Kamel HK, Hussain MS, Tariq S, Perry HM, Morley JE. Failure to diagnose and treat osteoporosis in elderly patients hospitalized with hip fracture. Am J Med 2000;109:326–8.
96. Eisman JA, Bogoch ER, Dell R, et al; ASBMR Task Force on Secondary Fracture Prevention. Making the first fracture the last fracture: ASBMR task force report on secondary fracture prevention. J Bone Miner Res 2012;27:2039–46.
97. Riley RL, Carnes ML, Gudmundsson A, Elliott ME. Outcomes and secondary prevention strategies for male hip fractures. Ann Pharmacother 2002;36:17–23.
98. Little EA, Eccles MP. A systematic review of the effectiveness of interventions to improve post-fracture investigation and management of patients at risk of osteoporosis. Implement Sci 2010;5:80.
99. Sale JE, Beaton D, Posen J, Elliot-Gibson V, Bogoch E. Systematic review on interventions to improve osteoporosis investigation and treatment in fragility fracture patients. Osteoporos Int 2011;22:2067–82.
100. Ganda K, Puech M, Chen JS, et al. Models of care for the secondary prevention of osteoporotic fractures: a systematic review and meta-analysis. Osteoporos Int 2013;24:393–406.
101. Akesson K, Marsh D, Mitchell PJ, et al; IOF Fracture Working Group. Capture the fracture: a best practice framework and global campaign to break the fragility fracture cycle. Osteoporos Int 2013;24:2135–52.
102. Lih A, Nandapalan H, Kim M, et al. Targeted intervention reduces refracture rates in patients with incident non-vertebral osteoporotic fractures: a 4-year prospective controlled study. Osteoporos Int 2011;22:849–58.
103. Dell R, Greene D, Schelkun SR, Williams K. Osteoporosis disease management: the role of the orthopaedic surgeon. J Bone Joint Surg Am 2008;90:188–94.
104. Dell R. Fracture prevention in Kaiser Permanente Southern California. Osteoporos Int 2011;22:457–60.
105. Beaupre LA, Morrish DW, Hanley DA, et al. Oral bisphosphonates are associated with reduced mortality after hip fracture. Osteoporos Int 2011;22:983–91.
106. Colón-Emeric CS, Mesenbrink P, Lyles KW, et al. Potential mediators of the mortality reduction with zoledronic acid after hip fracture. J Bone Miner Res 2010;25:91–7.
107. Cooper MS, Palmer AJ, Seibel MJ. Cost-effectiveness of the Concord Minimal Trauma Fracture Liaison service, a prospective, controlled fracture prevention study. Osteoporos Int 2012;23:97–107.
108. McLellan AR, Wolowacz SE, Zimovetz EA, et al. Fracture liaison services for the evaluation and management of patients with osteoporotic fracture: a cost-effectiveness evaluation based on data collected over 8 years of service provision. Osteoporos Int 2011;22:2083–98.
109. Solomon DH, Patrick AR, Schousboe J, Losina E. The potential economic benefits of improved postfracture care: a cost-effectiveness analysis of a fracture liaison service in the US health-care system. J Bone Miner Res 2014;29:1667–74.
110. Fragility Fracture Network of the Bone and Joint Decade. National bone health alliance: http://fragilityfracturenetwork.org/other-leading-organisations/national/united-states-of-america/national-bone-health-alliance-nbha/. Accessed March 3, 2018.
111. International Osteoporosis Foundation. Capture the fracture. https://www.iofbonehealth.org/capture-fracture. Accessed March 3, 2018.
112. Osteoporosis Canada. Towards a fracture free future: postoperative management of fragility fractures-a focus on osteoporosis care. Available at http://www.osteoporosis.ca/multimedia/pdf/COA_Bulletin_Winter_2012.pdf . Accessed March 3, 2018.
113. The American Orthopaedic Association. Own the bone. http://www.ownthebone.org/ . Accessed March 3, 2018.
1. Bergström U, Björnstig U, Stenlund H, Jonsson H, Svensson O. Fracture mechanisms and fracture pattern in men and women aged 50 years and older: a study of a 12-year population-based injury register, Umeå, Sweden. Osteoporos Int 2008;19:1267–73.
2. Siris ES, Adler R, Bilezikian J, et al. The clinical diagnosis of osteoporosis: a position statement from the National Bone Health Alliance Working Group. Osteoporos Int 2014;25:1439–43.
3. Kanis JA, Borgström F, Compston J, et al. SCOPE: a scorecard for osteoporosis in Europe. Arch Osteoporos 2013;8:144.
4. Burge R, Dawson-Hughes B, Solomon DH, Wong JB, King A, Tosteson A. Incidence and economic burden of osteoporosis-related fractures in the United States, 2005-2025. J Bone Miner Res 2007;22:465–75.
5. Rosengren BE, Karlsson MK. The annual number of hip fractures in Sweden will double from year 2002 to 2050: projections based on local and nationwide data. Acta Orthop 2014;85:234–7.
6. Chen IJ, Chiang CY, Li YH, et al. Nationwide cohort study of hip fractures: time trends in the incidence rates and projections up to 2035. Osteoporos Int 2015;26:681–8.
7. Siris ES, Boonen S, Mitchell PJ, Bilezikian J, Silverman S. What’s in a name? What constitutes the clinical diagnosis of osteoporosis? Osteoporos Int 2012;23:2093–7.
8. Cosman F, de Beur SJ, LeBoff MS, et al. Clinician’s guide to prevention and treatment of osteoporosis. Osteoporos Int 2014;25:2359–81.
9. Papaioannou A, Morin S, Cheung AM, et al; Scientific Advisory Council of Osteoporosis Canada. 2010 Clinical practice guidelines for the diagnosis and management of osteoporosis in Canada: summary. CMAJ 2010;182:1864–73.
10. Malluche HH, Porter DS, Mawad H, Monier-Faugere MC, Pienkowski D. Low-energy fractures without low T-scores characteristic of osteoporosis: a possible bone matrix disorder. J Bone Joint Surg Am 2013;95:e1391–6.
11. Ascenzi MG, Chin J, Lappe J, Recker R. Non-osteoporotic women with low-trauma fracture present altered birefringence in cortical bone. Bone 2016;84:104–12.
12. Nishiyama KK, Macdonald HM, Hanley DA, Boyd SK. Women with previous fragility fractures can be classified based on bone microarchitecture and finite element analysis measured with HR-pQCT. Osteoporos Int 2013;24:1733–40.
13. Dolan MM, Hawkes WG, Zimmerman SI, Morrison RS, Gruber-Baldini AL, Hebel JR, Magaziner J. Delirium on hospital admission in aged hip fracture patients: prediction of mortality and 2-year functional outcomes. J Gerontol A Biol Sci Med Sci 2000;55:M527–34.
14. Bruce AJ, Ritchie CW, Blizard R, Lai R, Raven P. The incidence of delirium associated with orthopedic surgery: a meta-analytic review. Int Psychogeriatr 2007;19:197–214.
15. Patterson BM, Cornell CN, Carbone B, Levine B, Chapman D. Protein depletion and metabolic stress in elderly patients who have a fracture of the hip. J Bone Joint Surg Am 1992;74:251–60.
16. Beaupre LA, Jones CA, Saunders LD, Johnston DW, Buckingham J, Majumdar SR. Best practices for elderly hip fracture patients. A systematic overview of the evidence. J Gen Intern Med 2005;20:1019–25.
17. Johansson I, Athlin E, Frykholm L, Bolinder H, Larsson G. Intermittent versus indwelling catheters for older patients with hip fractures. J Clin Nurs 2002;11:651–6.
18. Ulucay C, Eren Z, Kaspar EC, et al. Risk factors for acute kidney injury after hip fracture surgery in the elderly individuals. Geriatr Orthop Surg Rehabil 2012;3:150–6.
19. Greendale GA, Barrett-Connor E, Ingles S, Haile R. Late physical and functional effects of osteoporotic fracture in women: the Rancho Bernardo Study. J Am Geriatr Soc 1995;43:955–61.
20. Nevitt MC, Thompson DE, Black DM, et al. Effect of alendronate on limited-activity days and bed-disability days caused by back pain in postmenopausal women with existing vertebral fractures. Fracture Intervention Trial Research Group. Arch Intern Med 2000;160:77–85.
21. Gold DT, Lyles KW, Shipp KM, Drezner MK. Osteoporosis and its nonskeletal consequences: their impact on treatment decisions. In: Marcus R, Feldman D, Kelsey J, eds. Osteoporosis. 2nd ed. San Diego, CA: Academic Press; 2001:479–84.
22. Sale JEM, Frankel L, Thielke S, Funnell L. Pain and fracture-related limitations persist 6 months after a fragility fracture. Rheumatol Int 2017;37:1317–22.
23. Beringer TR, Clarke J, Elliott JR, Marsh DR, Heyburn G, Steele IC. Outcome following proximal femoral fracture in Northern Ireland. Ulster Med J 2006;75:200–6.
24. Córcoles-Jiménez MP, Villada-Munera A, Del Egido-Fernández MÁ, et al. Recovery of activities of daily living among older people one year after hip fracture. Clin Nurs Res 2015;24:604–23.
25. Kammerlander C, Gosch M, Kammerlander-Knauer U, Luger TJ, Blauth M, Roth T. Long-term functional outcome in geriatric hip fracture patients. Arch Orthop Trauma Surg 2011;131:1435–44.
26. Tarride JE, Burke N, Leslie WD, et al. Loss of health related quality of life following low-trauma fractures in the elderly. BMC Geriatr 2016;16:84.
27. Abimanyi-Ochom J, Watts JJ, Borgström F, et al. Changes in quality of life associated with fragility fractures: Australian arm of the International Cost and Utility Related to Osteoporotic Fractures Study (AusICUROS). Osteoporos Int 2015;26:1781–90.
28. Adachi JD, Loannidis G, Berger C, et al; Canadian Multicentre Osteoporosis Study (CaMos) Research Group. The influence of osteoporotic fractures on health-related quality of life in community-dwelling men and women across Canada. Osteoporos Int 2001;12:903–8.
29. Center JR, Nguyen TV, Schneider D, Sambrook PN, Eisman JA. Mortality after all major types of osteoporotic fracture in men and women: an observational study. Lancet 1999;353:878–82.
30. Kanis JA, Oden A, Johnell O, De Laet C, Jonsson B. Excess mortality after hospitalisation for vertebral fracture. Osteoporos Int 2004;15:108–12.
31. Johnell O, Kanis JA, Odén A, et al. Mortality after osteoporotic fractures. Osteoporos Int 2004;15:38–42.
32. Gosch M, Druml T, Nicholas JA, et al. Fragility non-hip fracture patients are at risk. Arch Orthop Trauma Surg 2015;135:69–77.
33. Ensrud KE, Thompson DE, Cauley JA, et al. Prevalent vertebral deformities predict mortality and hospitalization in older women with low bone mass. Fracture Intervention Trial Research Group. J Am Geriatr Soc 2000;48:241–9.
34. Abrahamsen B, van Staa T, Ariely R, Olson M, Cooper C. Excess mortality following hip fracture: a systematic epidemiological review. Osteoporos Int 2009;20:1633–50.
35. Kanis JA, Oden A, Johnell O, De Laet C, Jonsson B, Oglesby AK. The components of excess mortality after hip fracture. Bone 2003;32:468–73.
36. Forsén L, Sogaard AJ, Meyer HE, Edna T, Kopjar B. Survival after hip fracture: short- and long-term excess mortality according to age and gender. Osteoporos Int 1999;10:73–8.
37. Trombetti A, Herrmann F, Hoffmeyer P, Schurch MA, Bonjour JP, Rizzoli R. Survival and potential years of life lost after hip fracture in men and age-matched women. Osteoporos Int 2002;13:731–7.
38. Kanis JA, Johnell O, De Laet C, et al. A meta-analysis of previous fracture and subsequent fracture risk. Bone 2004;35:375–82.
39. Center JR, Bliuc D, Nguyen TV, Eisman JA. Risk of subsequent fracture after low-trauma fracture in men and women. JAMA 2007;297:387–94.
40. Hasserius R, Karlsson MK, Nilsson BE, Redlund-Johnell I, Johnell O; European Vertebral Osteoporosis Study. Prevalent vertebral deformities predict increased mortality and increased fracture rate in both men and women: a 10-year population-based study of 598 individuals from the Swedish cohort in the European Vertebral Osteoporosis Study. Osteoporos Int 2003;14:61–8.
41. Edwards BJ, Bunta AD, Simonelli C, Bolander M, Fitzpatrick LA. Prior fractures are common in patients with subsequent hip fractures. Clin Orthop Relat Res 2007;461:226–30.
42. Gehlbach S, Saag KG, Adachi JD, et al. Previous fractures at multiple sites increase the risk for subsequent fractures: the Global Longitudinal Study of Osteoporosis in Women. J Bone Miner Res 2012;27:645–53.
43. Lindsay R, Silverman SL, Cooper C, et al. Risk of new vertebral fracture in the year following a fracture. JAMA 2001;285:320–3.
44. Hansen L, Mathiesen AS, Vestergaard P, Ehlers LH, Petersen KD. A health economic analysis of osteoporotic fractures: who carries the burden? Arch Osteoporos 2013;8:126.
45. Lippuner K, Grifone S, Schwenkglenks M, et al. Comparative trends in hospitalizations for osteoporotic fractures and other frequent diseases between 2000 and 2008. Osteoporos Int 2012;23:829–39.
46. Lange A, Zeidler J, Braun S. One-year disease-related health care costs of incident vertebral fractures in osteoporotic patients. Osteoporos Int 2014;25:2435–43.
47. Hopkins RB, Burke N, Von Keyserlingk C, Leslie WD, Morin SN, Adachi JD, Papaioannou A, Bessette L, Brown JP, Pericleous L, Tarride J. The current economic burden of illness of osteoporosis in Canada. Osteoporos Int 2016;27:3023–32.
48. Blume SW, Curtis JR. Medical costs of osteoporosis in the elderly Medicare population. Osteoporos Int 2011;22:1835–44.
49. Avenell A, Mak JC, O’Connell D. Vitamin D and vitamin D analogues for preventing fractures in post-menopausal women and older men. Cochrane Database Syst Rev 2014: CD000227.
50. Santesso N, Carrasco-Labra A, Brignardello-Petersen R. Hip protectors for preventing hip fractures in older people. Cochrane Database Syst Rev 2014: CD001255
51. Avenell A, Smith TO, Curtain J, Mak JC, Myint PK. Nutritional supplementation for hip fracture aftercare in older people. Cochrane Database Syst Rev 2016;11: CD001880
52. Giangregorio LM, MacIntyre NJ, Thabane L, Skidmore CJ, Papaioannou A. Exercise for improving outcomes after osteoporotic vertebral fracture. Cochrane Database Syst Rev 2013: CD008618
53. Gillespie LD, Robertson M, Gillespie WJ, et al. Interventions for preventing falls in older people living in the community. Cochrane Database Syst Rev 2012:CD007146.
54. Ensrud KE, Black DM, Palermo L, et al. Treatment with alendronate prevents fractures in women at highest risk: results from the Fracture Intervention Trial. Arch Intern Med 1997;157:2617–24.
55. Black DM, Cummings SR, Karpf DB, et al. Randomised trial of effect of alendronate on risk of fracture in women with existing vertebral fractures. Fracture Intervention Trial Research Group. Lancet 1996;348:1535–41.
56. McClung MR, Geusens P, Miller PD, Zippel H, Bensen WG, Roux C, Adami S, Fogelman I, Diamond T, Eastell R, Meunier PJ, Reginster JY; Hip Intervention Program Study Group. Effect of risedronate on the risk of hip fracture in elderly women. Hip Intervention Program Study Group. N Engl J Med. 2001;344:333–40.
57. Lyles KW, Colón-Emeric CS, Magaziner JS, et al; HORIZON Recurrent Fracture Trial. Zoledronic acid and clinical fractures and mortality after hip fracture. N Engl J Med 2007;357:1799–809.
58. Neer RM, Arnaud CD, Zanchetta JR, et al. Effect of parathyroid hormone (1-34) on fractures and bone mineral density in postmenopausal women with osteoporosis. N Engl J Med 2001;344:1434–41.
59. Miller PD, Hattersley G, Riis BJ, et al; ACTIVE Study Investigators. Effect of abaloparatide vs placebo on new vertebral fractures in postmenopausal women with osteoporosis: a randomized clinical trial. JAMA. 2016;316:722–33.
60. Rozental TD, Vazquez MA, Chacko AT, Ayogu N, Bouxsein ML. Comparison of radiographic fracture healing in the distal radius for patients on and off bisphosphonate therapy. J Hand Surg Am 2009;34:595–602.
61. Colón-Emeric C, Nordsletten L, Olson S, et al; HORIZON Recurrent Fracture Trial. Association between timing of zoledronic acid infusion and hip fracture healing. Osteoporos Int 2011;22:2329–36.
62. Nakajima A, Shimoji N, Shiomi K, et al. Mechanisms for the enhancement of fracture healing in rats treated with intermittent low-dose human parathyroid hormone (1-34). J Bone Miner Res 2002;17:2038–47.
63. Alkhiary YM, Gerstenfeld LC, Krall E, et al. Enhancement of experimental fracture-healing by systemic administration of recombinant human parathyroid hormone (PTH 1-34). J Bone Joint Surg Am 2005;87:731–41.
64. Andreassen TT, Ejersted C, Oxlund H. Intermittent parathyroid hormone (1-34) treatment increases callus formation and mechanical strength of healing rat fractures. J Bone Miner Res 1999;14:960–8.
65. Bhandari M, Jin L, See K, et al. Does teriparatide improve femoral neck fracture healing: results from a randomized placebo-controlled trial. Clin Orthop Relat Res 2016;474:1234–44.
66. Lems WF, Dreinhöfer KE, Bischoff-Ferrari H, et al. EULAR/EFORT recommendations for management of patients older than 50 years with a fragility fracture and prevention of subsequent fractures. Ann Rheum Dis 2017;76:802–810.
67. American Academy of Orthopaedics Surgeons. Management of Hip Fractures in the Elderly. Evidence-Based Clinical Practice Guideline. Available at https://www.aaos.org/research/guidelines/hipfxguideline.pdf. Accessed March 2, 2018
68. The International Society For Clinical Densitometry. Official Positions 2015 ISCD Combined. Available at https://iscd.app.box.com/v/OP-ISCD-2015-Adult. Accessed March 2, 2018.
69. International Osteoporosis Foundation. National and Regional Osteoporosis Guidelines. https://www.iofbonehealth.org/national-regional-osteoporosis-guidelines. Accessed March 2, 2018.
70. Goldhahn J, Little D, Mitchell P, et al; ISFR working group drugs and fracture repair. Evidence for anti-osteoporosis therapy in acute fracture situations—recommendations of a multidisciplinary workshop of the International Society for Fracture Repair. Bone 2010;46:267–71.
71. Roerholt C, Eiken P, Abrahamsen B. Initiation of anti-osteoporotic therapy in patients with recent fractures: a nationwide analysis of prescription rates and persistence. Osteoporos Int 2009;20:299–307.
72. Panneman MJ, Lips P, Sen SS, Herings RM. Undertreatment with anti-osteoporotic drugs after hospitalization for fracture. Osteoporos Int 2004;15:120–4.
73. Wilk A, Sajjan S, Modi A, Fan CPS, Mavros P. Post-fracture pharmacotherapy for women with osteoporotic fractures: analysis of a managed care population in the USA. Osteoporos Int 2014; 25:2777–86.
74. Leslie WD, Giangregorio LM, Yogendran M, et al. A population-based analysis of the post-fracture care gap 1996-2008: the situation is not improving. Osteoporos Int 2012;23:1623–9.
75. Kung AW, Fan T, Xu L, et al. Factors influencing diagnosis and treatment of osteoporosis after a fragility fracture among postmenopausal women in Asian countries: a retrospective study. BMC Womens Health 2013;13:7.
76. Wang O, Hu Y, Gong S, et al. A survey of outcomes and management of patients post fragility fractures in China. Osteoporos Int 2015;26:2631–40.
77. Yusuf AA, Matlon TJ, Grauer A, Barron R, Chandler D, Peng Y. Utilization of osteoporosis medication after a fragility fracture among elderly Medicare beneficiaries. Arch Osteoporos 2016;11:31
78. Munson JC, Bynum JP, Bell JE, et al. Patterns of prescription drug use before and after fragility fracture. JAMA Intern Med 2016;176:1531–8.
79. Eisman J, Clapham S, Kehoe L; Australian BoneCare Study. Osteoporosis prevalence and levels of treatment in primary care: the Australian BoneCare Study. J Bone Miner Res 2004;19:1969–75.
80. Duncan R, Francis RM, Jagger C, et al. Magnitude of fragility fracture risk in the very old—are we meeting their needs? The Newcastle 85+ Study. Osteoporos Int 2015;26:123–30.
81. Singh S, Foster R, Khan KM. Accident or osteoporosis?: Survey of community follow-up after low-trauma fracture. Can Fam Physician. 2011;57:e128–33.
82. Andrade SE, Majumdar SR, Chan KA, et al. Low frequency of treatment of osteoporosis among postmenopausal women following a fracture. Arch Intern Med 2003;163:2052–7.
83. Blecher R, Wasrbrout Z, Arama Y, Kardosh R, Agar G, Mirovsky Y. Who is at risk of receiving inadequate care for osteoporosis following fragility fractures? A retrospective study. Isr Med Assoc J 2013;15:634–8.
84. Shibli-Rahhal A, Vaughan-Sarrazin MS, Richardson K, Cram P. Testing and treatment for osteoporosis following hip fracture in an integrated U.S. healthcare delivery system. Osteoporos Int 2011;22:2973–80.
85. Freedman BA, Potter BK, Nesti LJ, Giuliani JR, Hampton C, Kuklo TR. Osteoporosis and vertebral compression fractures-continued missed opportunities. Spine J 2008;8:756–62.
86. Kiebzak GM, Beinart GA, Perser K, Ambrose CG, Siff SJ, Heggeness MH. Undertreatment of osteoporosis in men with hip fracture. Arch Intern Med 2002;162:2217–22.
87. Kamel HK, Bida A, Montagnini M. Secondary prevention of hip fractures in veterans: can we do better? J Am Geriatr Soc 2004;52:647–8.
88. Skorupski N, Alexander IM. Multidisciplinary osteoporosis management of post low-energy trauma hip-fracture patients. J Am Assoc Nurse Pract 2013;25:3–10.
89. Simonelli C, Killeen K, Mehle S, Swanson L. Barriers to osteoporosis identification and treatment among primary care physicians and orthopedic surgeons. Mayo Clin Proc 2002;77:334–8.
90. Abraham A. Undertreatment of osteoporosis in men who have had a hip fracture. Arch Intern Med 2003;163:1236.
91. Sheehan J, Mohamed F, Reilly M, Perry IJ. Secondary prevention following fractured neck of femur: a survey of orthopaedic surgeons practice. Ir Med J. 2000;93:105–7.
92. Levinson MR, Clay FJ. Barriers to the implementation of evidence in osteoporosis treatment in hip fracture. Intern Med J 2009;39:199–202.
93. Kaufman JD, Bolander ME, Bunta AD, Edwards BJ, Fitzpatrick LA, Simonelli C. Barriers and solutions to osteoporosis care in patients with a hip fracture. J Bone Joint Surg Am 2003;85-A:1837–43.
94. Sorbi R, Aghamirsalim M. Osteoporotic Fracture Program management: who should be in charge? A comparative survey of knowledge in orthopaedic surgeons and internists. Orthop Traumatol Surg Res 2013;99:723–30.
95. Kamel HK, Hussain MS, Tariq S, Perry HM, Morley JE. Failure to diagnose and treat osteoporosis in elderly patients hospitalized with hip fracture. Am J Med 2000;109:326–8.
96. Eisman JA, Bogoch ER, Dell R, et al; ASBMR Task Force on Secondary Fracture Prevention. Making the first fracture the last fracture: ASBMR task force report on secondary fracture prevention. J Bone Miner Res 2012;27:2039–46.
97. Riley RL, Carnes ML, Gudmundsson A, Elliott ME. Outcomes and secondary prevention strategies for male hip fractures. Ann Pharmacother 2002;36:17–23.
98. Little EA, Eccles MP. A systematic review of the effectiveness of interventions to improve post-fracture investigation and management of patients at risk of osteoporosis. Implement Sci 2010;5:80.
99. Sale JE, Beaton D, Posen J, Elliot-Gibson V, Bogoch E. Systematic review on interventions to improve osteoporosis investigation and treatment in fragility fracture patients. Osteoporos Int 2011;22:2067–82.
100. Ganda K, Puech M, Chen JS, et al. Models of care for the secondary prevention of osteoporotic fractures: a systematic review and meta-analysis. Osteoporos Int 2013;24:393–406.
101. Akesson K, Marsh D, Mitchell PJ, et al; IOF Fracture Working Group. Capture the fracture: a best practice framework and global campaign to break the fragility fracture cycle. Osteoporos Int 2013;24:2135–52.
102. Lih A, Nandapalan H, Kim M, et al. Targeted intervention reduces refracture rates in patients with incident non-vertebral osteoporotic fractures: a 4-year prospective controlled study. Osteoporos Int 2011;22:849–58.
103. Dell R, Greene D, Schelkun SR, Williams K. Osteoporosis disease management: the role of the orthopaedic surgeon. J Bone Joint Surg Am 2008;90:188–94.
104. Dell R. Fracture prevention in Kaiser Permanente Southern California. Osteoporos Int 2011;22:457–60.
105. Beaupre LA, Morrish DW, Hanley DA, et al. Oral bisphosphonates are associated with reduced mortality after hip fracture. Osteoporos Int 2011;22:983–91.
106. Colón-Emeric CS, Mesenbrink P, Lyles KW, et al. Potential mediators of the mortality reduction with zoledronic acid after hip fracture. J Bone Miner Res 2010;25:91–7.
107. Cooper MS, Palmer AJ, Seibel MJ. Cost-effectiveness of the Concord Minimal Trauma Fracture Liaison service, a prospective, controlled fracture prevention study. Osteoporos Int 2012;23:97–107.
108. McLellan AR, Wolowacz SE, Zimovetz EA, et al. Fracture liaison services for the evaluation and management of patients with osteoporotic fracture: a cost-effectiveness evaluation based on data collected over 8 years of service provision. Osteoporos Int 2011;22:2083–98.
109. Solomon DH, Patrick AR, Schousboe J, Losina E. The potential economic benefits of improved postfracture care: a cost-effectiveness analysis of a fracture liaison service in the US health-care system. J Bone Miner Res 2014;29:1667–74.
110. Fragility Fracture Network of the Bone and Joint Decade. National bone health alliance: http://fragilityfracturenetwork.org/other-leading-organisations/national/united-states-of-america/national-bone-health-alliance-nbha/. Accessed March 3, 2018.
111. International Osteoporosis Foundation. Capture the fracture. https://www.iofbonehealth.org/capture-fracture. Accessed March 3, 2018.
112. Osteoporosis Canada. Towards a fracture free future: postoperative management of fragility fractures-a focus on osteoporosis care. Available at http://www.osteoporosis.ca/multimedia/pdf/COA_Bulletin_Winter_2012.pdf . Accessed March 3, 2018.
113. The American Orthopaedic Association. Own the bone. http://www.ownthebone.org/ . Accessed March 3, 2018.
Evaluation and Management of Female Sexual Dysfunction
IN THIS ARTICLE
- Causes of pain
- Screening
- Multimodal treatment
Care of women with sexual disorders has made great strides since Masters and Johnson began their study in 1957. In 2000, the Sexual Function Health Council of the American Foundation for Urologic Disease devised the classification system for female sexual dysfunction, which was officially defined in the Diagnostic and Statistical Manual of Mental Disorders-IV-TR.1 There are now definitions for sexual desire disorders, sexual arousal disorders, orgasmic disorder, and sexual pain disorders.
Female sexual dysfunction (FSD) has complex physiologic and psychologic components that require a detailed screening, history, and physical examination. Our goal in this review is to provide primary care providers with insights and practical advice to help screen, diagnose, and treat FSD, which can have a profound impact on patients’ most intimate relationships.
UNDERSTANDING THE TYPES OF FSD
Most women consider sexual health an important part of their overall health.2 Factors that can disrupt normal sexual function include aging, socioeconomics, and other medical comorbidities. FSD is common in women throughout their lives and refers to various sexual dysfunctions including diminished arousal, problems achieving orgasm, dyspareunia, and low desire. Its prevalence is reported to be as high as 20% to 43%.3,4
The World Health Organization and the US Surgeon General have released statements encouraging health care providers to address sexual health during a patient’s annual visits.5 Unfortunately, despite this call to action, many patients and providers are initially hesitant to discuss these problems.6
The Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM-5) provides the definition and diagnostic guidelines for the different components of FSD. Its classification of sexual disorders was simplified and published in May 2013.7 There are now only three female dysfunctions (as opposed to five in DSM-IV):
- Female hypoactive desire dysfunction and female arousal dysfunction were merged into a single syndrome labeled female sexual interest/arousal disorder.
- The formerly separate dyspareunia (painful intercourse) and vaginismus are now called genitopelvic pain/penetration disorder.
- Female orgasmic disorder remains as a category and is unchanged.
To qualify as a dysfunction, the problem must be present more than 75% of the time, for more than six months, causing significant distress, and must not be explained by a nonsexual mental disorder, relationship distress, substance abuse, or a medical condition.
Substance- or medication-induced sexual dysfunction falls under “Other Dysfunctions” and is defined as a clinically significant disturbance in sexual function that is predominant in the clinical picture. The criteria for substance- and medication-induced sexual dysfunction are unchanged and include neither the 75% nor the six-month requirement. The diagnosis of sexual dysfunction due to a general medical condition and sexual aversion disorder are absent from the DSM-5.7
Continue to: A common symptom
A common symptom. Female sexual disorders can be caused by several complex physiologic and psychologic factors. A common symptom among many women is dyspareunia. It is seen more often in postmenopausal women, and its prevalence ranges from 8% to 22%.8 Pain on vaginal entry usually indicates vaginal atrophy, vaginal dermatitis, or provoked vestibulodynia. Pain on deep penetration could be caused by endometriosis, interstitial cystitis, or uterine leiomyomas.9
The physical examination will reproduce the pain when the vulva or vagina is touched with a cotton swab or when you insert a finger into the vagina. The differential diagnosis is listed in the Table.9-11
EVALUATING THE PATIENT
Initially, many patients and providers may hesitate to discuss sexual dysfunction, but the annual exam is a good opportunity to broach the topic of sexual health.
Screening and history
Clinicians can screen all patients, regardless of age, with the help of a validated sex questionnaire or during a routine review of systems. There are many validated screening tools available. A simple, integrated screening tool to use is the Brief Sexual Symptom Checklist for Women (BSSC-W), created by the International Consultation on Sexual Medicine.12 Although recommended by the American Congress of Obstetricians and Gynecologists, the BSSC-W is not validated.9 The four items in the questionnaire ascertain personal information regarding an individual’s overall sexual function satisfaction, the problem causing dysfunction, how bothersome the symptoms are, and whether the patient is interested in discussing it with her provider.12
It’s important to obtain a detailed obstetric and gynecologic history that includes any sexually transmitted diseases, sexual abuse, urinary and bowel complaints, or surgeries. In addition, you’ll want to differentiate between various types of dysfunctions. A thorough physical examination, including an external and internal pelvic exam, can help to rule out other causes of sexual dysfunction.
Continue to: General exam: What to look for
General exam: What to look for
The external pelvic examination begins with visual inspection of the vulva, labia majora, and labia minora. Often, this is best accomplished gently with a gloved hand and a cotton swab. This inspection may reveal changes in pubic hair distribution, vulvar skin disorders, lesions, masses, cracks, or fissures. Inspection may also reveal redness and pain typical of vestibulitis, a flattening and pallor of the labia that suggests estrogen deficiency, or pelvic organ prolapse.
The internal pelvic examination begins with a manual evaluation of the muscles of the pelvic floor, uterus, bladder, urethra, anus, and adnexa. Make careful note of any unusual tenderness or pelvic masses. Pelvic floor muscles (PFMs) should voluntarily contract and relax and are not normally tender to palpation. Pelvic organ prolapse and/or hypermobility of the bladder may indicate a weakening of the endopelvic fascia and may cause sexual pain. The size and flexion of the uterus, tenderness in the vaginal fornix possibly indicating endometriosis, and adnexal fullness and/or masses should be identified and evaluated.
Neurologic exam of the pelvis will involve evaluation of sensory and motor function of both lower extremities and include a screening lumbosacral neurologic examination. Lumbosacral examination includes assessment of PFM strength, anal sphincter resting tone, voluntary anal contraction, and perineal sensation. If abnormalities are noted in the screening assessment, a complete comprehensive neurologic examination should be performed.
It’s important to assess pelvic floor muscle strength
Sexual function is associated with normal PFM function.13,14 The PFMs, particularly the pubococcygeus and iliococcygeus, are responsible for involuntary contractions during orgasm.13 Orgasm has been considered a reflex, which is preceded by increased blood flow to the genital organs, tumescence of the vulva and vagina, increased secretions during sexual arousal, and increased tension and contractions of the PFMs.15
Lowenstein et al found that women with strong or moderate PFM contractions scored significantly higher on both orgasm and arousal domains of the Female Sexual Function Index (FSFI), compared with women with weak PFM contractions.16 Orgasm and arousal functions may be associated with PFM strength, with a positive association between pelvic floor strength and sexual activity and function.17,18
The function and dysfunction of the PFMs have been characterized as normal, overactive (high tone), underactive (low tone), and nonfunctioning.
Continue to: Normal PFMs
Normal PFMs are those that can voluntarily and involuntarily contract and relax.19,20
Overactive (high-tone) muscles are those that do not relax and possibly contract during times of relaxation for micturition or defecation. This type of dysfunction can lead to voiding dysfunction, defecatory dysfunction, and dyspareunia.19
Underactive, or low-tone, PFMs cannot contract voluntarily. This can be associated with urinary and anal incontinence and pelvic organ prolapse.
Nonfunctioning muscles are completely inactive.19
How to assess. There are several ways to assess PFM tone and strength.20 The first is intravaginal or intrarectal digital palpation, which can be performed when the patient is in a supine or standing position. This examination evaluates PFM tone, squeeze pressure during contraction, symmetry, and relaxation. However, there is no validated scale to quantify PFM strength. Contractions can be further divided into voluntary and involuntary.19
During the exam, ask the patient to contract as much as she can to evaluate the maximum strength and sustained contraction for endurance. This measurement can be done with digital palpation or with pressure manometry or dynamometry.
Examination can be focused on the levator ani, piriformis, and internal obturator muscles bilaterally and rated by the patient’s reactions. Pelvic muscle tenderness, which can be highly prevalent in women with chronic pelvic pain, is associated with higher degrees of dyspareunia.21 Digital evaluation of the pelvic floor musculature varies in scale, number of fingers used, and parameters evaluated.
Lukban et al have described a 0 to 4 numbered scale that evaluates tenderness in the pelvic floor.22 The scale denotes “1” as comfortable pressure associated with the exam, “2” as uncomfortable pressure associated with the exam, “3” as moderate pain associated with the exam that intensifies with contraction, and “4” indicating severe pain with the exam and inability to perform the contraction maneuver due to pain.
Continue to: EFFECTIVE TREATMENT INCLUDES MULTIPLE OPTIONS
EFFECTIVE TREATMENT INCLUDES MULTIPLE OPTIONS
Lifestyle modifications can help
Lifestyle changes may help improve sexual function. These modifications include physical activity, healthy diet, nutrition counseling, and adequate sleep.23,24
Identifying medical conditions such as depression and anxiety will help delineate differential diagnoses of sexual dysfunction. Cardiovascular diseases may contribute to arousal disorder as a result of atherosclerosis of the vessels supplying the vagina and clitoris. Neurologic diseases such as multiple sclerosis and diabetes can affect sexual dysfunction by impairing arousal and orgasm.
Identification of concurrent comorbidities and implementation of lifestyle changes will help improve overall health and may improve sexual function.25
In addition, Herati et al found food sensitivities to grapefruit juice, spicy foods, alcohol, and caffeine were more prevalent in patients with interstitial cystitis and chronic pelvic pain.26 Avoiding irritants such as soap and other detergents in the perineal region may help decrease dysfunction.27 Finally, foods high in oxalate and other acidic items may cause bladder pain and worsening symptoms of vulvodynia.28
Topical therapies worth considering
Lubricants and moisturizers may help women with dyspareunia or symptoms of vaginal atrophy. For instance
Zestra, which contains a patented blend of botanical oils and extracts and is applied to the vulva prior to sexual activity, has been proven more effective than placebo for improving desire and arousal.29
Neogyn, a nonhormonal cream containing cutaneous lysate, has been shown to improve vulvar pain in women with vulvodynia. A double-blind placebo-controlled randomized crossover trial followed 30 patients for three months and found a significant reduction in pain during sexual activity and a significant reduction in erythema.30
Alprostadil, a prostaglandin E1 analogue that increases genital vasodilation when applied topically, is currently undergoing investigational trials.31,32
Patients can also choose from many OTC lubricants that contain water-based, oil-based, or silicone-based ingredients.
Continue to: Don't overlook physical therapy
Don’t overlook physical therapy
Manual therapies, including the transvaginal technique, are used for FSD that results from a variety of causes, including high-tone pelvic floor dysfunction. The transvaginal technique can identify myofascial pain; treatment involves internal release of the PFMs and external trigger-point identification and alleviation.
One pilot study examined use of transvaginal Thiele massage twice a week for five weeks in 21 symptomatic women with interstitial cystitis and high-tone pelvic floor dysfunction. The researchers found it decreased hypertonicity of the pelvic floor and generated statistically significant improvement in the Symptom and Problem Indexes of the O’Leary-Sant Questionnaire, Likert Visual Analogue Scales for urgency and pain, and the Physical and Mental Component Summary from the SF-12 Quality-of-Life Scale.33 Transvaginal physical therapy is also an effective treatment for myofascial pelvic pain.34
Biofeedback, which can be used in combination with pelvic floor physical therapy, teaches the patient to control the PFMs by visualizing the activity to achieve conscious control over contraction of the pelvic floor and ceasing the cycle of spasm.35 Ger et al investigated patients with levator spasm and found biofeedback decreased pain; relief was rated as good or excellent at 15-month follow-up in six of 14 patients (43%).36
Home devices such as Eros Therapy, an FDA-approved, nonpharmacologic battery-operated device, provide vacuum suction to the clitoris with vibratory sensation. Eros Therapy has been shown to increase blood flow to the clitoris, vagina, and pelvic floor and increase sensation, orgasm, lubrication, and satisfaction.37
Vaginal dilators allow increasing lengths and girths designed to treat vaginal and pelvic floor pain.38 In our practice, we encourage pelvic muscle strengthening tools in the form of Kegel trainers and other insertion devices that may improve PFM coordination and strength.
Continue to: Pharmacotherapy has its place
Pharmacotherapy has its place
The treatment of FSD may require a multimodal systematic approach targeting genitopelvic pain. But before the best options can be found, it is important to first establish the cause of the pain. Several drug formulations have been effectively used, including hormonal and nonhormonal options.
Conjugated estrogens are FDA approved for the treatment of dyspareunia, which can contribute to decreased desire. Systemic estrogen in oral form, transdermal preparations, and topical formulations may increase sexual desire and arousal and decrease dyspareunia.39 Even synthetic steroid compounds such as tibolone may improve sexual function, although it is not FDA approved for that purpose.40
Ospemifene is a selective estrogen receptor modulator that acts as an estrogen agonist in select tissues, including vaginal epithelium. It is FDA approved for dyspareunia in postmenopausal women.41,42 A daily dose of 60 mg is effective and safe, with minimal adverse effects.42 Studies suggest that testosterone, although not FDA approved in the United States for this purpose, improves sexual desire, pleasure, orgasm, and arousal satisfaction.39 The hormone has not gained FDA approval because of concerns about long-term safety and efficacy.42
Nonhormonal drugs including flibanserin, a well-tolerated serotonin receptor 1A agonist, 2A antagonist shown to improve sexual desire, increase the number of satisfying sexual events and reduce distress associated with low sexual desire when compared with placebo.43 The FDA has approved flibanserin as the first treatment targeted for women with hypoactive sexual desire disorder (HSDD). It can, however, cause severe hypotension and syncope, is not well tolerated with alcohol, and is contraindicated in patients who take strong CYP3A4 inhibitors, such as fluconazole, verapamil, and erythromycin, or who have liver impairment.
Bupropion, a mild dopamine and norepinephrine reuptake inhibitor and acetylcholine receptor antagonist, has been shown to improve desire in women with and without depression. Although it is FDA approved for major depressive disorder, it is not approved for female sexual dysfunction and is still under investigation.
Tricyclic antidepressants, such as nortriptyline and amitriptyline, may be effective in treating neuropathic pain. Starting doses of both amitriptyline and nortriptyline are 10 mg/d and can be increased to a maximum of 100 mg/d.44 Tricyclic antidepressants are still under investigation for the treatment of FSD.
Muscle relaxants in oral and topical compounded form are used to treat increased pelvic floor tension and spasticity. Cyclobenzaprine and tizanidine are FDA-approved muscle relaxants indicated for muscle spasticity.
Cyclobenzaprine, at a starting dose of 10 mg, can be taken up to three times a day for pelvic floor tension. Tizanidine is a centrally active alpha 2 agonist that’s superior to placebo in treating high-tone pelvic floor dysfunction.44
Other medications include benzodiazepines, such as oral clonazepam and intravaginal diazepam, although they are not FDA approved for high-tone pelvic floor dysfunction. Rogalski et al evaluated data for 26 patients who received vaginal diazepam for bladder pain, sexual pain, and levator hypertonus.45 They found subjective and sexual pain improvement assessed on FSFI and the visual analog pain scale. PFM tone significantly improved during resting, squeezing, and relaxation phases. Multimodal therapy can be used for muscle spasticity and high-tone pelvic floor dysfunction.
Continue to: Trigger point and Botox injections
Trigger point and Botox injections
Although drug therapy has its place in the management of sexual dysfunction, other modalities that involve trigger-point injections or botulinum toxin injections to the PFMs may prove helpful for patients with high-tone pelvic floor dysfunction.
A prospective study investigated the role of trigger-point injections in 18 women with levator ani muscle spasm using a mixture of 0.25% bupivacaine in 10 mL, 2% lidocaine in 10 mL, and 40 mg of triamcinolone in 1 mL combined and used for injection of 5 mL per trigger point.46 Three months after injections, 13 of the 18 women showed improvement, resulting in a success rate of 72%. Trigger point injections can be applied externally or transvaginally.
OnabotulinumtoxinA (Botox) has also been tested for relief of levator ani muscle spasm. Botox is FDA approved for upper and lower limb spasticity but is not approved for pelvic floor spasticity or tension. It may reduce pressure in the PFMs and may be useful in women with high-tone pelvic floor dysfunction.47
In a prospective six-month pilot study, 28 patients with pelvic pain for whom conservative treatment did not work received up to 300 U Botox into the pelvic floor.11 The study, which used needle electromyography guidance and a transperineal approach, found that the dyspareunia visual analog scale improved significantly at weeks 12 and 24. Keep in mind, however, that onabotulinumtoxinA should be reserved for patients for whom conventional treatments fail.47,48
Addressing psychologic issues
Sex therapy is a traditional approach that aims to improve individual or couples’ sexual experiences and help reduce anxiety related to sex.42 Cognitive behavioral sex therapy includes traditional sex therapy components but puts greater emphasis on modifying thought patterns that interfere with intimacy and sex.42
Mindfulness-based cognitive behavioral treatments have shown promise for sexual desire problems. It is an ancient eastern practice with Buddhist roots. This therapy is a nonjudgmental, present-moment awareness comprised of self-regulation of attention and accepting orientation to the present.49 Although there is little evidence from prospective studies, it may benefit women with sexual dysfunction after intervention with sex therapy and cognitive behavioral therapy.
CONCLUSION
Female sexual dysfunction is common and affects women of all ages. It can negatively impact a woman’s quality of life and overall well-being. The etiology of FSD is complex, and treatments are based on the causes of the dysfunction. Difficult cases warrant referral to a specialist in sexual health and female pelvic medicine. Future prospective trials, randomized controlled trials, the use of validated questionnaires, and meta-analyses will continue to move us forward as we find better ways to understand, identify, and treat female sexual dysfunction.
1. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders (4th ed, text revision). Washington, DC; 1994.
2. Shifren JL, Monz BU, Russo PA, et al. Sexual problems and distress in United States women: prevalence and correlates. Obstet Gynecol. 2008;112:970-978.
3. Lewis RW, Fugl-Meyer KS, Bosch R, et al. Epidemiology/risk factors of sexual dysfunction. J Sex Med. 2004;1:35-39.
4. Laumann E, Paik A, Rosen RC. Sexual dysfunction in the United States: prevalence and predictors. JAMA. 1999;281:537-544.
5. Office of the Surgeon General. The Surgeon General’s Call to Action to Promote Sexual Health and Responsible Sexual Behavior. Rockville, MD; 2001.
6. Pauls RN, Kleeman SD, Segal JL, et al. Practice patterns of physician members of the American Urogynecologic Society regarding female sexual dysfunction: results of a national survey. Int Urogynecol J Pelvic Floor Dysfunct. 2005;16:460-467.
7. American Psychiatric Association. Sexual dysfunction. In: Diagnostic and Statistical Manual of Mental Disorders (5th ed). Washington, DC; 2013.
8. Steege JF, Zolnoun DA. Evaluation and treatment of dyspareunia. Obstet Gynecol. 2009;113:1124-1136.
9. American College of Obstetricians and Gynecologists. ACOG Practice Bulletin No. 119: Female sexual dysfunction. Obstet Gynecol. 2011;117:996-1007.
10. Clayton AH, Hamilton DV. Female sexual dysfunction. Psychiatr Clin North Am. 2017;40:267-284.
11. Morrissey D, El-Khawand D, Ginzburg N, et al. Botulinum Toxin A injections into pelvic floor muscles under electromyographic guidance for women with refractory high-tone pelvic floor dysfunction: a 6-month prospective pilot study. Female Pelvic Med Reconstr Surg. 2015;21:277-282.
12. Hatzichristou D, Rosen RC, Derogatis LR, et al. Recommendations for the clinical evaluation of men and women with sexual dysfunction. J Sex Med. 2010;7(1 pt 2):337-348.
13. Kegel A. Sexual functions of the pubococcygeus muscle. West J Surg Obstet Gynecol. 1952;60:521-524.
14. Shafik A. The role of the levator ani muscle in evacuation, sexual performance and pelvic floor disorders. Int Urogynecol J. 2000;11:361-376.
15. Kinsey A, Pomeroy WB, Martin CE, et al. Sexual Behavior in the Human Female. Philadelphia, PA: WB Saunders; 1998.
16. Lowenstein L, Gruenwald I, Gartman I, et al. Can stronger pelvic muscle floor improve sexual function? Int Urogynecol J. 2010;21:553-556.
17. Kanter G, Rogers RG, Pauls RN, et al. A strong pelvic floor is associated with higher rates of sexual activity in women with pelvic floor disorders. Int Urogynecol J. 2015;26:991-996.
18. Wehbe SA, Kellogg-Spadt S, Whitmore K. Urogenital complaints and female sexual dysfunction. Part 2. J Sex Med. 2010;7:2304-2317.
19. Messelink B, Benson T, Berghmans B, et al. Standardization of terminology of pelvic floor muscle function and dysfunction: report from the Pelvic Floor Clinical Assessment Group of the International Continence Society. Neurourol Urodyn. 2005;24:374-380.
20. Haylen BT, de Ridder D, Freeman RM, et al. An International Urogynecological Association (IUGA)/International Continence Society (ICS) joint report on the terminology for female pelvic floor dysfunction. Neurourol Urodyn. 2010;29:4-20.
21. Montenegro ML, Mateus-Vasconcelos EC, Rosa e Silva JC, et al. Importance of pelvic muscle tenderness evaluation in women with chronic pelvic pain. Pain Med. 2010;11:224-228.
22. Lukban JC, Whitmore KE. Pelvic floor muscle re-education treatment of the overactive bladder and painful bladder syndrome. Clin Obstet Gynecol. 2002;45:273-285.
23. Kalmbach DA, Arnedt JT, Pillai V, et al. The impact of sleep on female sexual response and behavior: a pilot study. J Sex Med. 2015;12:1221-1232.
24. Aversa A, Bruzziches R, Francomano D, et al. Weight loss by multidisciplinary intervention improves endothelial and sexual function in obese fertile women. J Sex Med. 2013;10:1024-1033.
25. Pauls RN, Kleeman SD, Karram MM. Female sexual dysfunction: principles of diagnosis and therapy. Obstet Gynecol Surv. 2005;60:196-205.
26. Herati AS, Shorter B, Tai J, et al. Differences in food sensitivities between female interstitial cystitis/painful bladder syndrome (IC/PBS) and chronic prostatitis/chronic pelvic pain syndrome (CP/CPPS) patients. J Urol. 2009;181(4)(suppl):22.
27. Farrell J, Cacchioni T. The medicalization of women’s sexual pain. J Sex Res. 2012;49:328-336.
28. De Andres J, Sanchis-Lopez NM, Asensio-Samper JM, et al. Vulvodynia—an evidence-based literature review and proposed treatment algorithm. Pain Pract. 2016;16:204-236.
29. Herbenick D, Reece M, Schick V, et al. Women’s use and perceptions of commercial lubricants: prevalence and characteristics in a nationally representative sample of American adults. J Sex Med. 2014;11:642-652.
30. Donders GG, Bellen G. Cream with cutaneous fibroblast lysate for the treatment of provoked vestibulodynia: a double-blind randomized placebo-controlled crossover study. J Low Genit Tract Dis. 2012;16:427-436.
31. Belkin ZR, Krapf JM, Goldstein AT. Drugs in early clinical development for the treatment of female sexual dysfunction. Expert Opin Investig Drugs. 2015;24:159-167.
32. Islam A, Mitchel J, Rosen R, et al. Topical alprostadil in the treatment of female sexual arousal disorder: a pilot study. J Sex Marital Ther. 2001;27:531-540.
33. Oyama IA, Rejba A, Lukban JC, et al. Modified Thiele massage as therapeutic intervention for female patients with interstitial cystitis and high-tone pelvic floor dysfunction. Urology. 2004;64:862-865.
34. Bedaiwy MA, Patterson B, Mahajan S. Prevalence of myofascial chronic pelvic pain and the effectiveness of pelvic floor physical therapy. J Reprod Med. 2013;58:504-510.
35. Wehbe SA, Fariello JY, Whitmore K. Minimally invasive therapies for chronic pelvic pain syndrome. Curr Urol Rep. 2010;11:276-285.
36. Ger GC, Wexner SD, Jorge JM, et al. Evaluation and treatment of chronic intractable rectal pain—a frustrating endeavor. Dis Colon Rectum. 1993;36:139-145.
37. Billups KL, Berman L, Berman J, et al. A new non-pharmacological vacuum therapy for female sexual dysfunction. J Sex Marital Ther. 2001;27:435-441.
38. Miles T, Johnson N. Vaginal dilator therapy for women receiving pelvic radiotherapy. Cochrane Database Syst Rev. 2014;9:CD007291.
39. Goldstein I. Current management strategies of the postmenopausal patient with sexual health problems. J Sex Med. 2007;4(suppl 3):235-253.
40. Modelska K, Cummings S. Female sexual dysfunction in postmenopausal women: systematic review of placebo-controlled trials. Am J Obstet Gynecol. 2003;188:286-293.
41. Constantine G, Graham S, Portman DJ, et al. Female sexual function improved with ospemifene in postmenopausal women with vulvar and vaginal atrophy: results of a randomized, placebo-controlled trial. Climacteric. 2015;18:226-232.
42. Kingsberg SA, Woodard T. Female sexual dysfunction: focus on low desire. Obstet Gynecol. 2015;125:477-486.
43. Simon JA, Kingsberg SA, Shumel B, et al. Efficacy and safety of flibanserin in postmenopausal women with hypoactive sexual desire disorder: results of the SNOWDROP trial. Menopause. 2014;21:633-640.
44. Curtis Nickel J, Baranowski AP, Pontari M, et al. Management of men diagnosed with chronic prostatitis/chronic pelvic pain syndrome who have failed traditional management. Rev Urol. 2007;9:63-72.
45. Rogalski MJ, Kellogg-Spadt S, Hoffmann AR, et al. Retrospective chart review of vaginal diazepam suppository use in high-tone pelvic floor dysfunction. Int Urogynecol J. 2010;21:895-899.
46. Langford CF, Udvari Nagy S, Ghoniem GM. Levator ani trigger point injections: an underutilized treatment for chronic pelvic pain. Neurourol Urodyn. 2007;26:59-62.
47. Abbott JA, Jarvis SK, Lyons SD, et al. Botulinum toxin type A for chronic pain and pelvic floor spasm in women: a randomized controlled trial. Obstet Gynecol. 2006;108:915-923.
48. Kamanli A, Kaya A, Ardicoglu O, et al. Comparison of lidocaine injection, botulinum toxin injection, and dry needling to trigger points in myofascial pain syndrome. Rheumatol Int. 2005;25:604-611.
49. Brotto LA, Erskine Y, Carey M, et al. A brief mindfulness-based cognitive behavioral intervention improves sexual functioning versus wait-list control in women treated for gynecologic cancer. Gynecol Oncol. 2012;125:320-325.
IN THIS ARTICLE
- Causes of pain
- Screening
- Multimodal treatment
Care of women with sexual disorders has made great strides since Masters and Johnson began their study in 1957. In 2000, the Sexual Function Health Council of the American Foundation for Urologic Disease devised the classification system for female sexual dysfunction, which was officially defined in the Diagnostic and Statistical Manual of Mental Disorders-IV-TR.1 There are now definitions for sexual desire disorders, sexual arousal disorders, orgasmic disorder, and sexual pain disorders.
Female sexual dysfunction (FSD) has complex physiologic and psychologic components that require a detailed screening, history, and physical examination. Our goal in this review is to provide primary care providers with insights and practical advice to help screen, diagnose, and treat FSD, which can have a profound impact on patients’ most intimate relationships.
UNDERSTANDING THE TYPES OF FSD
Most women consider sexual health an important part of their overall health.2 Factors that can disrupt normal sexual function include aging, socioeconomics, and other medical comorbidities. FSD is common in women throughout their lives and refers to various sexual dysfunctions including diminished arousal, problems achieving orgasm, dyspareunia, and low desire. Its prevalence is reported to be as high as 20% to 43%.3,4
The World Health Organization and the US Surgeon General have released statements encouraging health care providers to address sexual health during a patient’s annual visits.5 Unfortunately, despite this call to action, many patients and providers are initially hesitant to discuss these problems.6
The Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM-5) provides the definition and diagnostic guidelines for the different components of FSD. Its classification of sexual disorders was simplified and published in May 2013.7 There are now only three female dysfunctions (as opposed to five in DSM-IV):
- Female hypoactive desire dysfunction and female arousal dysfunction were merged into a single syndrome labeled female sexual interest/arousal disorder.
- The formerly separate dyspareunia (painful intercourse) and vaginismus are now called genitopelvic pain/penetration disorder.
- Female orgasmic disorder remains as a category and is unchanged.
To qualify as a dysfunction, the problem must be present more than 75% of the time, for more than six months, causing significant distress, and must not be explained by a nonsexual mental disorder, relationship distress, substance abuse, or a medical condition.
Substance- or medication-induced sexual dysfunction falls under “Other Dysfunctions” and is defined as a clinically significant disturbance in sexual function that is predominant in the clinical picture. The criteria for substance- and medication-induced sexual dysfunction are unchanged and include neither the 75% nor the six-month requirement. The diagnosis of sexual dysfunction due to a general medical condition and sexual aversion disorder are absent from the DSM-5.7
Continue to: A common symptom
A common symptom. Female sexual disorders can be caused by several complex physiologic and psychologic factors. A common symptom among many women is dyspareunia. It is seen more often in postmenopausal women, and its prevalence ranges from 8% to 22%.8 Pain on vaginal entry usually indicates vaginal atrophy, vaginal dermatitis, or provoked vestibulodynia. Pain on deep penetration could be caused by endometriosis, interstitial cystitis, or uterine leiomyomas.9
The physical examination will reproduce the pain when the vulva or vagina is touched with a cotton swab or when you insert a finger into the vagina. The differential diagnosis is listed in the Table.9-11
EVALUATING THE PATIENT
Initially, many patients and providers may hesitate to discuss sexual dysfunction, but the annual exam is a good opportunity to broach the topic of sexual health.
Screening and history
Clinicians can screen all patients, regardless of age, with the help of a validated sex questionnaire or during a routine review of systems. There are many validated screening tools available. A simple, integrated screening tool to use is the Brief Sexual Symptom Checklist for Women (BSSC-W), created by the International Consultation on Sexual Medicine.12 Although recommended by the American Congress of Obstetricians and Gynecologists, the BSSC-W is not validated.9 The four items in the questionnaire ascertain personal information regarding an individual’s overall sexual function satisfaction, the problem causing dysfunction, how bothersome the symptoms are, and whether the patient is interested in discussing it with her provider.12
It’s important to obtain a detailed obstetric and gynecologic history that includes any sexually transmitted diseases, sexual abuse, urinary and bowel complaints, or surgeries. In addition, you’ll want to differentiate between various types of dysfunctions. A thorough physical examination, including an external and internal pelvic exam, can help to rule out other causes of sexual dysfunction.
Continue to: General exam: What to look for
General exam: What to look for
The external pelvic examination begins with visual inspection of the vulva, labia majora, and labia minora. Often, this is best accomplished gently with a gloved hand and a cotton swab. This inspection may reveal changes in pubic hair distribution, vulvar skin disorders, lesions, masses, cracks, or fissures. Inspection may also reveal redness and pain typical of vestibulitis, a flattening and pallor of the labia that suggests estrogen deficiency, or pelvic organ prolapse.
The internal pelvic examination begins with a manual evaluation of the muscles of the pelvic floor, uterus, bladder, urethra, anus, and adnexa. Make careful note of any unusual tenderness or pelvic masses. Pelvic floor muscles (PFMs) should voluntarily contract and relax and are not normally tender to palpation. Pelvic organ prolapse and/or hypermobility of the bladder may indicate a weakening of the endopelvic fascia and may cause sexual pain. The size and flexion of the uterus, tenderness in the vaginal fornix possibly indicating endometriosis, and adnexal fullness and/or masses should be identified and evaluated.
Neurologic exam of the pelvis will involve evaluation of sensory and motor function of both lower extremities and include a screening lumbosacral neurologic examination. Lumbosacral examination includes assessment of PFM strength, anal sphincter resting tone, voluntary anal contraction, and perineal sensation. If abnormalities are noted in the screening assessment, a complete comprehensive neurologic examination should be performed.
It’s important to assess pelvic floor muscle strength
Sexual function is associated with normal PFM function.13,14 The PFMs, particularly the pubococcygeus and iliococcygeus, are responsible for involuntary contractions during orgasm.13 Orgasm has been considered a reflex, which is preceded by increased blood flow to the genital organs, tumescence of the vulva and vagina, increased secretions during sexual arousal, and increased tension and contractions of the PFMs.15
Lowenstein et al found that women with strong or moderate PFM contractions scored significantly higher on both orgasm and arousal domains of the Female Sexual Function Index (FSFI), compared with women with weak PFM contractions.16 Orgasm and arousal functions may be associated with PFM strength, with a positive association between pelvic floor strength and sexual activity and function.17,18
The function and dysfunction of the PFMs have been characterized as normal, overactive (high tone), underactive (low tone), and nonfunctioning.
Continue to: Normal PFMs
Normal PFMs are those that can voluntarily and involuntarily contract and relax.19,20
Overactive (high-tone) muscles are those that do not relax and possibly contract during times of relaxation for micturition or defecation. This type of dysfunction can lead to voiding dysfunction, defecatory dysfunction, and dyspareunia.19
Underactive, or low-tone, PFMs cannot contract voluntarily. This can be associated with urinary and anal incontinence and pelvic organ prolapse.
Nonfunctioning muscles are completely inactive.19
How to assess. There are several ways to assess PFM tone and strength.20 The first is intravaginal or intrarectal digital palpation, which can be performed when the patient is in a supine or standing position. This examination evaluates PFM tone, squeeze pressure during contraction, symmetry, and relaxation. However, there is no validated scale to quantify PFM strength. Contractions can be further divided into voluntary and involuntary.19
During the exam, ask the patient to contract as much as she can to evaluate the maximum strength and sustained contraction for endurance. This measurement can be done with digital palpation or with pressure manometry or dynamometry.
Examination can be focused on the levator ani, piriformis, and internal obturator muscles bilaterally and rated by the patient’s reactions. Pelvic muscle tenderness, which can be highly prevalent in women with chronic pelvic pain, is associated with higher degrees of dyspareunia.21 Digital evaluation of the pelvic floor musculature varies in scale, number of fingers used, and parameters evaluated.
Lukban et al have described a 0 to 4 numbered scale that evaluates tenderness in the pelvic floor.22 The scale denotes “1” as comfortable pressure associated with the exam, “2” as uncomfortable pressure associated with the exam, “3” as moderate pain associated with the exam that intensifies with contraction, and “4” indicating severe pain with the exam and inability to perform the contraction maneuver due to pain.
Continue to: EFFECTIVE TREATMENT INCLUDES MULTIPLE OPTIONS
EFFECTIVE TREATMENT INCLUDES MULTIPLE OPTIONS
Lifestyle modifications can help
Lifestyle changes may help improve sexual function. These modifications include physical activity, healthy diet, nutrition counseling, and adequate sleep.23,24
Identifying medical conditions such as depression and anxiety will help delineate differential diagnoses of sexual dysfunction. Cardiovascular diseases may contribute to arousal disorder as a result of atherosclerosis of the vessels supplying the vagina and clitoris. Neurologic diseases such as multiple sclerosis and diabetes can affect sexual dysfunction by impairing arousal and orgasm.
Identification of concurrent comorbidities and implementation of lifestyle changes will help improve overall health and may improve sexual function.25
In addition, Herati et al found food sensitivities to grapefruit juice, spicy foods, alcohol, and caffeine were more prevalent in patients with interstitial cystitis and chronic pelvic pain.26 Avoiding irritants such as soap and other detergents in the perineal region may help decrease dysfunction.27 Finally, foods high in oxalate and other acidic items may cause bladder pain and worsening symptoms of vulvodynia.28
Topical therapies worth considering
Lubricants and moisturizers may help women with dyspareunia or symptoms of vaginal atrophy. For instance
Zestra, which contains a patented blend of botanical oils and extracts and is applied to the vulva prior to sexual activity, has been proven more effective than placebo for improving desire and arousal.29
Neogyn, a nonhormonal cream containing cutaneous lysate, has been shown to improve vulvar pain in women with vulvodynia. A double-blind placebo-controlled randomized crossover trial followed 30 patients for three months and found a significant reduction in pain during sexual activity and a significant reduction in erythema.30
Alprostadil, a prostaglandin E1 analogue that increases genital vasodilation when applied topically, is currently undergoing investigational trials.31,32
Patients can also choose from many OTC lubricants that contain water-based, oil-based, or silicone-based ingredients.
Continue to: Don't overlook physical therapy
Don’t overlook physical therapy
Manual therapies, including the transvaginal technique, are used for FSD that results from a variety of causes, including high-tone pelvic floor dysfunction. The transvaginal technique can identify myofascial pain; treatment involves internal release of the PFMs and external trigger-point identification and alleviation.
One pilot study examined use of transvaginal Thiele massage twice a week for five weeks in 21 symptomatic women with interstitial cystitis and high-tone pelvic floor dysfunction. The researchers found it decreased hypertonicity of the pelvic floor and generated statistically significant improvement in the Symptom and Problem Indexes of the O’Leary-Sant Questionnaire, Likert Visual Analogue Scales for urgency and pain, and the Physical and Mental Component Summary from the SF-12 Quality-of-Life Scale.33 Transvaginal physical therapy is also an effective treatment for myofascial pelvic pain.34
Biofeedback, which can be used in combination with pelvic floor physical therapy, teaches the patient to control the PFMs by visualizing the activity to achieve conscious control over contraction of the pelvic floor and ceasing the cycle of spasm.35 Ger et al investigated patients with levator spasm and found biofeedback decreased pain; relief was rated as good or excellent at 15-month follow-up in six of 14 patients (43%).36
Home devices such as Eros Therapy, an FDA-approved, nonpharmacologic battery-operated device, provide vacuum suction to the clitoris with vibratory sensation. Eros Therapy has been shown to increase blood flow to the clitoris, vagina, and pelvic floor and increase sensation, orgasm, lubrication, and satisfaction.37
Vaginal dilators allow increasing lengths and girths designed to treat vaginal and pelvic floor pain.38 In our practice, we encourage pelvic muscle strengthening tools in the form of Kegel trainers and other insertion devices that may improve PFM coordination and strength.
Continue to: Pharmacotherapy has its place
Pharmacotherapy has its place
The treatment of FSD may require a multimodal systematic approach targeting genitopelvic pain. But before the best options can be found, it is important to first establish the cause of the pain. Several drug formulations have been effectively used, including hormonal and nonhormonal options.
Conjugated estrogens are FDA approved for the treatment of dyspareunia, which can contribute to decreased desire. Systemic estrogen in oral form, transdermal preparations, and topical formulations may increase sexual desire and arousal and decrease dyspareunia.39 Even synthetic steroid compounds such as tibolone may improve sexual function, although it is not FDA approved for that purpose.40
Ospemifene is a selective estrogen receptor modulator that acts as an estrogen agonist in select tissues, including vaginal epithelium. It is FDA approved for dyspareunia in postmenopausal women.41,42 A daily dose of 60 mg is effective and safe, with minimal adverse effects.42 Studies suggest that testosterone, although not FDA approved in the United States for this purpose, improves sexual desire, pleasure, orgasm, and arousal satisfaction.39 The hormone has not gained FDA approval because of concerns about long-term safety and efficacy.42
Nonhormonal drugs including flibanserin, a well-tolerated serotonin receptor 1A agonist, 2A antagonist shown to improve sexual desire, increase the number of satisfying sexual events and reduce distress associated with low sexual desire when compared with placebo.43 The FDA has approved flibanserin as the first treatment targeted for women with hypoactive sexual desire disorder (HSDD). It can, however, cause severe hypotension and syncope, is not well tolerated with alcohol, and is contraindicated in patients who take strong CYP3A4 inhibitors, such as fluconazole, verapamil, and erythromycin, or who have liver impairment.
Bupropion, a mild dopamine and norepinephrine reuptake inhibitor and acetylcholine receptor antagonist, has been shown to improve desire in women with and without depression. Although it is FDA approved for major depressive disorder, it is not approved for female sexual dysfunction and is still under investigation.
Tricyclic antidepressants, such as nortriptyline and amitriptyline, may be effective in treating neuropathic pain. Starting doses of both amitriptyline and nortriptyline are 10 mg/d and can be increased to a maximum of 100 mg/d.44 Tricyclic antidepressants are still under investigation for the treatment of FSD.
Muscle relaxants in oral and topical compounded form are used to treat increased pelvic floor tension and spasticity. Cyclobenzaprine and tizanidine are FDA-approved muscle relaxants indicated for muscle spasticity.
Cyclobenzaprine, at a starting dose of 10 mg, can be taken up to three times a day for pelvic floor tension. Tizanidine is a centrally active alpha 2 agonist that’s superior to placebo in treating high-tone pelvic floor dysfunction.44
Other medications include benzodiazepines, such as oral clonazepam and intravaginal diazepam, although they are not FDA approved for high-tone pelvic floor dysfunction. Rogalski et al evaluated data for 26 patients who received vaginal diazepam for bladder pain, sexual pain, and levator hypertonus.45 They found subjective and sexual pain improvement assessed on FSFI and the visual analog pain scale. PFM tone significantly improved during resting, squeezing, and relaxation phases. Multimodal therapy can be used for muscle spasticity and high-tone pelvic floor dysfunction.
Continue to: Trigger point and Botox injections
Trigger point and Botox injections
Although drug therapy has its place in the management of sexual dysfunction, other modalities that involve trigger-point injections or botulinum toxin injections to the PFMs may prove helpful for patients with high-tone pelvic floor dysfunction.
A prospective study investigated the role of trigger-point injections in 18 women with levator ani muscle spasm using a mixture of 0.25% bupivacaine in 10 mL, 2% lidocaine in 10 mL, and 40 mg of triamcinolone in 1 mL combined and used for injection of 5 mL per trigger point.46 Three months after injections, 13 of the 18 women showed improvement, resulting in a success rate of 72%. Trigger point injections can be applied externally or transvaginally.
OnabotulinumtoxinA (Botox) has also been tested for relief of levator ani muscle spasm. Botox is FDA approved for upper and lower limb spasticity but is not approved for pelvic floor spasticity or tension. It may reduce pressure in the PFMs and may be useful in women with high-tone pelvic floor dysfunction.47
In a prospective six-month pilot study, 28 patients with pelvic pain for whom conservative treatment did not work received up to 300 U Botox into the pelvic floor.11 The study, which used needle electromyography guidance and a transperineal approach, found that the dyspareunia visual analog scale improved significantly at weeks 12 and 24. Keep in mind, however, that onabotulinumtoxinA should be reserved for patients for whom conventional treatments fail.47,48
Addressing psychologic issues
Sex therapy is a traditional approach that aims to improve individual or couples’ sexual experiences and help reduce anxiety related to sex.42 Cognitive behavioral sex therapy includes traditional sex therapy components but puts greater emphasis on modifying thought patterns that interfere with intimacy and sex.42
Mindfulness-based cognitive behavioral treatments have shown promise for sexual desire problems. It is an ancient eastern practice with Buddhist roots. This therapy is a nonjudgmental, present-moment awareness comprised of self-regulation of attention and accepting orientation to the present.49 Although there is little evidence from prospective studies, it may benefit women with sexual dysfunction after intervention with sex therapy and cognitive behavioral therapy.
CONCLUSION
Female sexual dysfunction is common and affects women of all ages. It can negatively impact a woman’s quality of life and overall well-being. The etiology of FSD is complex, and treatments are based on the causes of the dysfunction. Difficult cases warrant referral to a specialist in sexual health and female pelvic medicine. Future prospective trials, randomized controlled trials, the use of validated questionnaires, and meta-analyses will continue to move us forward as we find better ways to understand, identify, and treat female sexual dysfunction.
IN THIS ARTICLE
- Causes of pain
- Screening
- Multimodal treatment
Care of women with sexual disorders has made great strides since Masters and Johnson began their study in 1957. In 2000, the Sexual Function Health Council of the American Foundation for Urologic Disease devised the classification system for female sexual dysfunction, which was officially defined in the Diagnostic and Statistical Manual of Mental Disorders-IV-TR.1 There are now definitions for sexual desire disorders, sexual arousal disorders, orgasmic disorder, and sexual pain disorders.
Female sexual dysfunction (FSD) has complex physiologic and psychologic components that require a detailed screening, history, and physical examination. Our goal in this review is to provide primary care providers with insights and practical advice to help screen, diagnose, and treat FSD, which can have a profound impact on patients’ most intimate relationships.
UNDERSTANDING THE TYPES OF FSD
Most women consider sexual health an important part of their overall health.2 Factors that can disrupt normal sexual function include aging, socioeconomics, and other medical comorbidities. FSD is common in women throughout their lives and refers to various sexual dysfunctions including diminished arousal, problems achieving orgasm, dyspareunia, and low desire. Its prevalence is reported to be as high as 20% to 43%.3,4
The World Health Organization and the US Surgeon General have released statements encouraging health care providers to address sexual health during a patient’s annual visits.5 Unfortunately, despite this call to action, many patients and providers are initially hesitant to discuss these problems.6
The Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM-5) provides the definition and diagnostic guidelines for the different components of FSD. Its classification of sexual disorders was simplified and published in May 2013.7 There are now only three female dysfunctions (as opposed to five in DSM-IV):
- Female hypoactive desire dysfunction and female arousal dysfunction were merged into a single syndrome labeled female sexual interest/arousal disorder.
- The formerly separate dyspareunia (painful intercourse) and vaginismus are now called genitopelvic pain/penetration disorder.
- Female orgasmic disorder remains as a category and is unchanged.
To qualify as a dysfunction, the problem must be present more than 75% of the time, for more than six months, causing significant distress, and must not be explained by a nonsexual mental disorder, relationship distress, substance abuse, or a medical condition.
Substance- or medication-induced sexual dysfunction falls under “Other Dysfunctions” and is defined as a clinically significant disturbance in sexual function that is predominant in the clinical picture. The criteria for substance- and medication-induced sexual dysfunction are unchanged and include neither the 75% nor the six-month requirement. The diagnosis of sexual dysfunction due to a general medical condition and sexual aversion disorder are absent from the DSM-5.7
Continue to: A common symptom
A common symptom. Female sexual disorders can be caused by several complex physiologic and psychologic factors. A common symptom among many women is dyspareunia. It is seen more often in postmenopausal women, and its prevalence ranges from 8% to 22%.8 Pain on vaginal entry usually indicates vaginal atrophy, vaginal dermatitis, or provoked vestibulodynia. Pain on deep penetration could be caused by endometriosis, interstitial cystitis, or uterine leiomyomas.9
The physical examination will reproduce the pain when the vulva or vagina is touched with a cotton swab or when you insert a finger into the vagina. The differential diagnosis is listed in the Table.9-11
EVALUATING THE PATIENT
Initially, many patients and providers may hesitate to discuss sexual dysfunction, but the annual exam is a good opportunity to broach the topic of sexual health.
Screening and history
Clinicians can screen all patients, regardless of age, with the help of a validated sex questionnaire or during a routine review of systems. There are many validated screening tools available. A simple, integrated screening tool to use is the Brief Sexual Symptom Checklist for Women (BSSC-W), created by the International Consultation on Sexual Medicine.12 Although recommended by the American Congress of Obstetricians and Gynecologists, the BSSC-W is not validated.9 The four items in the questionnaire ascertain personal information regarding an individual’s overall sexual function satisfaction, the problem causing dysfunction, how bothersome the symptoms are, and whether the patient is interested in discussing it with her provider.12
It’s important to obtain a detailed obstetric and gynecologic history that includes any sexually transmitted diseases, sexual abuse, urinary and bowel complaints, or surgeries. In addition, you’ll want to differentiate between various types of dysfunctions. A thorough physical examination, including an external and internal pelvic exam, can help to rule out other causes of sexual dysfunction.
Continue to: General exam: What to look for
General exam: What to look for
The external pelvic examination begins with visual inspection of the vulva, labia majora, and labia minora. Often, this is best accomplished gently with a gloved hand and a cotton swab. This inspection may reveal changes in pubic hair distribution, vulvar skin disorders, lesions, masses, cracks, or fissures. Inspection may also reveal redness and pain typical of vestibulitis, a flattening and pallor of the labia that suggests estrogen deficiency, or pelvic organ prolapse.
The internal pelvic examination begins with a manual evaluation of the muscles of the pelvic floor, uterus, bladder, urethra, anus, and adnexa. Make careful note of any unusual tenderness or pelvic masses. Pelvic floor muscles (PFMs) should voluntarily contract and relax and are not normally tender to palpation. Pelvic organ prolapse and/or hypermobility of the bladder may indicate a weakening of the endopelvic fascia and may cause sexual pain. The size and flexion of the uterus, tenderness in the vaginal fornix possibly indicating endometriosis, and adnexal fullness and/or masses should be identified and evaluated.
Neurologic exam of the pelvis will involve evaluation of sensory and motor function of both lower extremities and include a screening lumbosacral neurologic examination. Lumbosacral examination includes assessment of PFM strength, anal sphincter resting tone, voluntary anal contraction, and perineal sensation. If abnormalities are noted in the screening assessment, a complete comprehensive neurologic examination should be performed.
It’s important to assess pelvic floor muscle strength
Sexual function is associated with normal PFM function.13,14 The PFMs, particularly the pubococcygeus and iliococcygeus, are responsible for involuntary contractions during orgasm.13 Orgasm has been considered a reflex, which is preceded by increased blood flow to the genital organs, tumescence of the vulva and vagina, increased secretions during sexual arousal, and increased tension and contractions of the PFMs.15
Lowenstein et al found that women with strong or moderate PFM contractions scored significantly higher on both orgasm and arousal domains of the Female Sexual Function Index (FSFI), compared with women with weak PFM contractions.16 Orgasm and arousal functions may be associated with PFM strength, with a positive association between pelvic floor strength and sexual activity and function.17,18
The function and dysfunction of the PFMs have been characterized as normal, overactive (high tone), underactive (low tone), and nonfunctioning.
Continue to: Normal PFMs
Normal PFMs are those that can voluntarily and involuntarily contract and relax.19,20
Overactive (high-tone) muscles are those that do not relax and possibly contract during times of relaxation for micturition or defecation. This type of dysfunction can lead to voiding dysfunction, defecatory dysfunction, and dyspareunia.19
Underactive, or low-tone, PFMs cannot contract voluntarily. This can be associated with urinary and anal incontinence and pelvic organ prolapse.
Nonfunctioning muscles are completely inactive.19
How to assess. There are several ways to assess PFM tone and strength.20 The first is intravaginal or intrarectal digital palpation, which can be performed when the patient is in a supine or standing position. This examination evaluates PFM tone, squeeze pressure during contraction, symmetry, and relaxation. However, there is no validated scale to quantify PFM strength. Contractions can be further divided into voluntary and involuntary.19
During the exam, ask the patient to contract as much as she can to evaluate the maximum strength and sustained contraction for endurance. This measurement can be done with digital palpation or with pressure manometry or dynamometry.
Examination can be focused on the levator ani, piriformis, and internal obturator muscles bilaterally and rated by the patient’s reactions. Pelvic muscle tenderness, which can be highly prevalent in women with chronic pelvic pain, is associated with higher degrees of dyspareunia.21 Digital evaluation of the pelvic floor musculature varies in scale, number of fingers used, and parameters evaluated.
Lukban et al have described a 0 to 4 numbered scale that evaluates tenderness in the pelvic floor.22 The scale denotes “1” as comfortable pressure associated with the exam, “2” as uncomfortable pressure associated with the exam, “3” as moderate pain associated with the exam that intensifies with contraction, and “4” indicating severe pain with the exam and inability to perform the contraction maneuver due to pain.
Continue to: EFFECTIVE TREATMENT INCLUDES MULTIPLE OPTIONS
EFFECTIVE TREATMENT INCLUDES MULTIPLE OPTIONS
Lifestyle modifications can help
Lifestyle changes may help improve sexual function. These modifications include physical activity, healthy diet, nutrition counseling, and adequate sleep.23,24
Identifying medical conditions such as depression and anxiety will help delineate differential diagnoses of sexual dysfunction. Cardiovascular diseases may contribute to arousal disorder as a result of atherosclerosis of the vessels supplying the vagina and clitoris. Neurologic diseases such as multiple sclerosis and diabetes can affect sexual dysfunction by impairing arousal and orgasm.
Identification of concurrent comorbidities and implementation of lifestyle changes will help improve overall health and may improve sexual function.25
In addition, Herati et al found food sensitivities to grapefruit juice, spicy foods, alcohol, and caffeine were more prevalent in patients with interstitial cystitis and chronic pelvic pain.26 Avoiding irritants such as soap and other detergents in the perineal region may help decrease dysfunction.27 Finally, foods high in oxalate and other acidic items may cause bladder pain and worsening symptoms of vulvodynia.28
Topical therapies worth considering
Lubricants and moisturizers may help women with dyspareunia or symptoms of vaginal atrophy. For instance
Zestra, which contains a patented blend of botanical oils and extracts and is applied to the vulva prior to sexual activity, has been proven more effective than placebo for improving desire and arousal.29
Neogyn, a nonhormonal cream containing cutaneous lysate, has been shown to improve vulvar pain in women with vulvodynia. A double-blind placebo-controlled randomized crossover trial followed 30 patients for three months and found a significant reduction in pain during sexual activity and a significant reduction in erythema.30
Alprostadil, a prostaglandin E1 analogue that increases genital vasodilation when applied topically, is currently undergoing investigational trials.31,32
Patients can also choose from many OTC lubricants that contain water-based, oil-based, or silicone-based ingredients.
Continue to: Don't overlook physical therapy
Don’t overlook physical therapy
Manual therapies, including the transvaginal technique, are used for FSD that results from a variety of causes, including high-tone pelvic floor dysfunction. The transvaginal technique can identify myofascial pain; treatment involves internal release of the PFMs and external trigger-point identification and alleviation.
One pilot study examined use of transvaginal Thiele massage twice a week for five weeks in 21 symptomatic women with interstitial cystitis and high-tone pelvic floor dysfunction. The researchers found it decreased hypertonicity of the pelvic floor and generated statistically significant improvement in the Symptom and Problem Indexes of the O’Leary-Sant Questionnaire, Likert Visual Analogue Scales for urgency and pain, and the Physical and Mental Component Summary from the SF-12 Quality-of-Life Scale.33 Transvaginal physical therapy is also an effective treatment for myofascial pelvic pain.34
Biofeedback, which can be used in combination with pelvic floor physical therapy, teaches the patient to control the PFMs by visualizing the activity to achieve conscious control over contraction of the pelvic floor and ceasing the cycle of spasm.35 Ger et al investigated patients with levator spasm and found biofeedback decreased pain; relief was rated as good or excellent at 15-month follow-up in six of 14 patients (43%).36
Home devices such as Eros Therapy, an FDA-approved, nonpharmacologic battery-operated device, provide vacuum suction to the clitoris with vibratory sensation. Eros Therapy has been shown to increase blood flow to the clitoris, vagina, and pelvic floor and increase sensation, orgasm, lubrication, and satisfaction.37
Vaginal dilators allow increasing lengths and girths designed to treat vaginal and pelvic floor pain.38 In our practice, we encourage pelvic muscle strengthening tools in the form of Kegel trainers and other insertion devices that may improve PFM coordination and strength.
Continue to: Pharmacotherapy has its place
Pharmacotherapy has its place
The treatment of FSD may require a multimodal systematic approach targeting genitopelvic pain. But before the best options can be found, it is important to first establish the cause of the pain. Several drug formulations have been effectively used, including hormonal and nonhormonal options.
Conjugated estrogens are FDA approved for the treatment of dyspareunia, which can contribute to decreased desire. Systemic estrogen in oral form, transdermal preparations, and topical formulations may increase sexual desire and arousal and decrease dyspareunia.39 Even synthetic steroid compounds such as tibolone may improve sexual function, although it is not FDA approved for that purpose.40
Ospemifene is a selective estrogen receptor modulator that acts as an estrogen agonist in select tissues, including vaginal epithelium. It is FDA approved for dyspareunia in postmenopausal women.41,42 A daily dose of 60 mg is effective and safe, with minimal adverse effects.42 Studies suggest that testosterone, although not FDA approved in the United States for this purpose, improves sexual desire, pleasure, orgasm, and arousal satisfaction.39 The hormone has not gained FDA approval because of concerns about long-term safety and efficacy.42
Nonhormonal drugs including flibanserin, a well-tolerated serotonin receptor 1A agonist, 2A antagonist shown to improve sexual desire, increase the number of satisfying sexual events and reduce distress associated with low sexual desire when compared with placebo.43 The FDA has approved flibanserin as the first treatment targeted for women with hypoactive sexual desire disorder (HSDD). It can, however, cause severe hypotension and syncope, is not well tolerated with alcohol, and is contraindicated in patients who take strong CYP3A4 inhibitors, such as fluconazole, verapamil, and erythromycin, or who have liver impairment.
Bupropion, a mild dopamine and norepinephrine reuptake inhibitor and acetylcholine receptor antagonist, has been shown to improve desire in women with and without depression. Although it is FDA approved for major depressive disorder, it is not approved for female sexual dysfunction and is still under investigation.
Tricyclic antidepressants, such as nortriptyline and amitriptyline, may be effective in treating neuropathic pain. Starting doses of both amitriptyline and nortriptyline are 10 mg/d and can be increased to a maximum of 100 mg/d.44 Tricyclic antidepressants are still under investigation for the treatment of FSD.
Muscle relaxants in oral and topical compounded form are used to treat increased pelvic floor tension and spasticity. Cyclobenzaprine and tizanidine are FDA-approved muscle relaxants indicated for muscle spasticity.
Cyclobenzaprine, at a starting dose of 10 mg, can be taken up to three times a day for pelvic floor tension. Tizanidine is a centrally active alpha 2 agonist that’s superior to placebo in treating high-tone pelvic floor dysfunction.44
Other medications include benzodiazepines, such as oral clonazepam and intravaginal diazepam, although they are not FDA approved for high-tone pelvic floor dysfunction. Rogalski et al evaluated data for 26 patients who received vaginal diazepam for bladder pain, sexual pain, and levator hypertonus.45 They found subjective and sexual pain improvement assessed on FSFI and the visual analog pain scale. PFM tone significantly improved during resting, squeezing, and relaxation phases. Multimodal therapy can be used for muscle spasticity and high-tone pelvic floor dysfunction.
Continue to: Trigger point and Botox injections
Trigger point and Botox injections
Although drug therapy has its place in the management of sexual dysfunction, other modalities that involve trigger-point injections or botulinum toxin injections to the PFMs may prove helpful for patients with high-tone pelvic floor dysfunction.
A prospective study investigated the role of trigger-point injections in 18 women with levator ani muscle spasm using a mixture of 0.25% bupivacaine in 10 mL, 2% lidocaine in 10 mL, and 40 mg of triamcinolone in 1 mL combined and used for injection of 5 mL per trigger point.46 Three months after injections, 13 of the 18 women showed improvement, resulting in a success rate of 72%. Trigger point injections can be applied externally or transvaginally.
OnabotulinumtoxinA (Botox) has also been tested for relief of levator ani muscle spasm. Botox is FDA approved for upper and lower limb spasticity but is not approved for pelvic floor spasticity or tension. It may reduce pressure in the PFMs and may be useful in women with high-tone pelvic floor dysfunction.47
In a prospective six-month pilot study, 28 patients with pelvic pain for whom conservative treatment did not work received up to 300 U Botox into the pelvic floor.11 The study, which used needle electromyography guidance and a transperineal approach, found that the dyspareunia visual analog scale improved significantly at weeks 12 and 24. Keep in mind, however, that onabotulinumtoxinA should be reserved for patients for whom conventional treatments fail.47,48
Addressing psychologic issues
Sex therapy is a traditional approach that aims to improve individual or couples’ sexual experiences and help reduce anxiety related to sex.42 Cognitive behavioral sex therapy includes traditional sex therapy components but puts greater emphasis on modifying thought patterns that interfere with intimacy and sex.42
Mindfulness-based cognitive behavioral treatments have shown promise for sexual desire problems. It is an ancient eastern practice with Buddhist roots. This therapy is a nonjudgmental, present-moment awareness comprised of self-regulation of attention and accepting orientation to the present.49 Although there is little evidence from prospective studies, it may benefit women with sexual dysfunction after intervention with sex therapy and cognitive behavioral therapy.
CONCLUSION
Female sexual dysfunction is common and affects women of all ages. It can negatively impact a woman’s quality of life and overall well-being. The etiology of FSD is complex, and treatments are based on the causes of the dysfunction. Difficult cases warrant referral to a specialist in sexual health and female pelvic medicine. Future prospective trials, randomized controlled trials, the use of validated questionnaires, and meta-analyses will continue to move us forward as we find better ways to understand, identify, and treat female sexual dysfunction.
1. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders (4th ed, text revision). Washington, DC; 1994.
2. Shifren JL, Monz BU, Russo PA, et al. Sexual problems and distress in United States women: prevalence and correlates. Obstet Gynecol. 2008;112:970-978.
3. Lewis RW, Fugl-Meyer KS, Bosch R, et al. Epidemiology/risk factors of sexual dysfunction. J Sex Med. 2004;1:35-39.
4. Laumann E, Paik A, Rosen RC. Sexual dysfunction in the United States: prevalence and predictors. JAMA. 1999;281:537-544.
5. Office of the Surgeon General. The Surgeon General’s Call to Action to Promote Sexual Health and Responsible Sexual Behavior. Rockville, MD; 2001.
6. Pauls RN, Kleeman SD, Segal JL, et al. Practice patterns of physician members of the American Urogynecologic Society regarding female sexual dysfunction: results of a national survey. Int Urogynecol J Pelvic Floor Dysfunct. 2005;16:460-467.
7. American Psychiatric Association. Sexual dysfunction. In: Diagnostic and Statistical Manual of Mental Disorders (5th ed). Washington, DC; 2013.
8. Steege JF, Zolnoun DA. Evaluation and treatment of dyspareunia. Obstet Gynecol. 2009;113:1124-1136.
9. American College of Obstetricians and Gynecologists. ACOG Practice Bulletin No. 119: Female sexual dysfunction. Obstet Gynecol. 2011;117:996-1007.
10. Clayton AH, Hamilton DV. Female sexual dysfunction. Psychiatr Clin North Am. 2017;40:267-284.
11. Morrissey D, El-Khawand D, Ginzburg N, et al. Botulinum Toxin A injections into pelvic floor muscles under electromyographic guidance for women with refractory high-tone pelvic floor dysfunction: a 6-month prospective pilot study. Female Pelvic Med Reconstr Surg. 2015;21:277-282.
12. Hatzichristou D, Rosen RC, Derogatis LR, et al. Recommendations for the clinical evaluation of men and women with sexual dysfunction. J Sex Med. 2010;7(1 pt 2):337-348.
13. Kegel A. Sexual functions of the pubococcygeus muscle. West J Surg Obstet Gynecol. 1952;60:521-524.
14. Shafik A. The role of the levator ani muscle in evacuation, sexual performance and pelvic floor disorders. Int Urogynecol J. 2000;11:361-376.
15. Kinsey A, Pomeroy WB, Martin CE, et al. Sexual Behavior in the Human Female. Philadelphia, PA: WB Saunders; 1998.
16. Lowenstein L, Gruenwald I, Gartman I, et al. Can stronger pelvic muscle floor improve sexual function? Int Urogynecol J. 2010;21:553-556.
17. Kanter G, Rogers RG, Pauls RN, et al. A strong pelvic floor is associated with higher rates of sexual activity in women with pelvic floor disorders. Int Urogynecol J. 2015;26:991-996.
18. Wehbe SA, Kellogg-Spadt S, Whitmore K. Urogenital complaints and female sexual dysfunction. Part 2. J Sex Med. 2010;7:2304-2317.
19. Messelink B, Benson T, Berghmans B, et al. Standardization of terminology of pelvic floor muscle function and dysfunction: report from the Pelvic Floor Clinical Assessment Group of the International Continence Society. Neurourol Urodyn. 2005;24:374-380.
20. Haylen BT, de Ridder D, Freeman RM, et al. An International Urogynecological Association (IUGA)/International Continence Society (ICS) joint report on the terminology for female pelvic floor dysfunction. Neurourol Urodyn. 2010;29:4-20.
21. Montenegro ML, Mateus-Vasconcelos EC, Rosa e Silva JC, et al. Importance of pelvic muscle tenderness evaluation in women with chronic pelvic pain. Pain Med. 2010;11:224-228.
22. Lukban JC, Whitmore KE. Pelvic floor muscle re-education treatment of the overactive bladder and painful bladder syndrome. Clin Obstet Gynecol. 2002;45:273-285.
23. Kalmbach DA, Arnedt JT, Pillai V, et al. The impact of sleep on female sexual response and behavior: a pilot study. J Sex Med. 2015;12:1221-1232.
24. Aversa A, Bruzziches R, Francomano D, et al. Weight loss by multidisciplinary intervention improves endothelial and sexual function in obese fertile women. J Sex Med. 2013;10:1024-1033.
25. Pauls RN, Kleeman SD, Karram MM. Female sexual dysfunction: principles of diagnosis and therapy. Obstet Gynecol Surv. 2005;60:196-205.
26. Herati AS, Shorter B, Tai J, et al. Differences in food sensitivities between female interstitial cystitis/painful bladder syndrome (IC/PBS) and chronic prostatitis/chronic pelvic pain syndrome (CP/CPPS) patients. J Urol. 2009;181(4)(suppl):22.
27. Farrell J, Cacchioni T. The medicalization of women’s sexual pain. J Sex Res. 2012;49:328-336.
28. De Andres J, Sanchis-Lopez NM, Asensio-Samper JM, et al. Vulvodynia—an evidence-based literature review and proposed treatment algorithm. Pain Pract. 2016;16:204-236.
29. Herbenick D, Reece M, Schick V, et al. Women’s use and perceptions of commercial lubricants: prevalence and characteristics in a nationally representative sample of American adults. J Sex Med. 2014;11:642-652.
30. Donders GG, Bellen G. Cream with cutaneous fibroblast lysate for the treatment of provoked vestibulodynia: a double-blind randomized placebo-controlled crossover study. J Low Genit Tract Dis. 2012;16:427-436.
31. Belkin ZR, Krapf JM, Goldstein AT. Drugs in early clinical development for the treatment of female sexual dysfunction. Expert Opin Investig Drugs. 2015;24:159-167.
32. Islam A, Mitchel J, Rosen R, et al. Topical alprostadil in the treatment of female sexual arousal disorder: a pilot study. J Sex Marital Ther. 2001;27:531-540.
33. Oyama IA, Rejba A, Lukban JC, et al. Modified Thiele massage as therapeutic intervention for female patients with interstitial cystitis and high-tone pelvic floor dysfunction. Urology. 2004;64:862-865.
34. Bedaiwy MA, Patterson B, Mahajan S. Prevalence of myofascial chronic pelvic pain and the effectiveness of pelvic floor physical therapy. J Reprod Med. 2013;58:504-510.
35. Wehbe SA, Fariello JY, Whitmore K. Minimally invasive therapies for chronic pelvic pain syndrome. Curr Urol Rep. 2010;11:276-285.
36. Ger GC, Wexner SD, Jorge JM, et al. Evaluation and treatment of chronic intractable rectal pain—a frustrating endeavor. Dis Colon Rectum. 1993;36:139-145.
37. Billups KL, Berman L, Berman J, et al. A new non-pharmacological vacuum therapy for female sexual dysfunction. J Sex Marital Ther. 2001;27:435-441.
38. Miles T, Johnson N. Vaginal dilator therapy for women receiving pelvic radiotherapy. Cochrane Database Syst Rev. 2014;9:CD007291.
39. Goldstein I. Current management strategies of the postmenopausal patient with sexual health problems. J Sex Med. 2007;4(suppl 3):235-253.
40. Modelska K, Cummings S. Female sexual dysfunction in postmenopausal women: systematic review of placebo-controlled trials. Am J Obstet Gynecol. 2003;188:286-293.
41. Constantine G, Graham S, Portman DJ, et al. Female sexual function improved with ospemifene in postmenopausal women with vulvar and vaginal atrophy: results of a randomized, placebo-controlled trial. Climacteric. 2015;18:226-232.
42. Kingsberg SA, Woodard T. Female sexual dysfunction: focus on low desire. Obstet Gynecol. 2015;125:477-486.
43. Simon JA, Kingsberg SA, Shumel B, et al. Efficacy and safety of flibanserin in postmenopausal women with hypoactive sexual desire disorder: results of the SNOWDROP trial. Menopause. 2014;21:633-640.
44. Curtis Nickel J, Baranowski AP, Pontari M, et al. Management of men diagnosed with chronic prostatitis/chronic pelvic pain syndrome who have failed traditional management. Rev Urol. 2007;9:63-72.
45. Rogalski MJ, Kellogg-Spadt S, Hoffmann AR, et al. Retrospective chart review of vaginal diazepam suppository use in high-tone pelvic floor dysfunction. Int Urogynecol J. 2010;21:895-899.
46. Langford CF, Udvari Nagy S, Ghoniem GM. Levator ani trigger point injections: an underutilized treatment for chronic pelvic pain. Neurourol Urodyn. 2007;26:59-62.
47. Abbott JA, Jarvis SK, Lyons SD, et al. Botulinum toxin type A for chronic pain and pelvic floor spasm in women: a randomized controlled trial. Obstet Gynecol. 2006;108:915-923.
48. Kamanli A, Kaya A, Ardicoglu O, et al. Comparison of lidocaine injection, botulinum toxin injection, and dry needling to trigger points in myofascial pain syndrome. Rheumatol Int. 2005;25:604-611.
49. Brotto LA, Erskine Y, Carey M, et al. A brief mindfulness-based cognitive behavioral intervention improves sexual functioning versus wait-list control in women treated for gynecologic cancer. Gynecol Oncol. 2012;125:320-325.
1. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders (4th ed, text revision). Washington, DC; 1994.
2. Shifren JL, Monz BU, Russo PA, et al. Sexual problems and distress in United States women: prevalence and correlates. Obstet Gynecol. 2008;112:970-978.
3. Lewis RW, Fugl-Meyer KS, Bosch R, et al. Epidemiology/risk factors of sexual dysfunction. J Sex Med. 2004;1:35-39.
4. Laumann E, Paik A, Rosen RC. Sexual dysfunction in the United States: prevalence and predictors. JAMA. 1999;281:537-544.
5. Office of the Surgeon General. The Surgeon General’s Call to Action to Promote Sexual Health and Responsible Sexual Behavior. Rockville, MD; 2001.
6. Pauls RN, Kleeman SD, Segal JL, et al. Practice patterns of physician members of the American Urogynecologic Society regarding female sexual dysfunction: results of a national survey. Int Urogynecol J Pelvic Floor Dysfunct. 2005;16:460-467.
7. American Psychiatric Association. Sexual dysfunction. In: Diagnostic and Statistical Manual of Mental Disorders (5th ed). Washington, DC; 2013.
8. Steege JF, Zolnoun DA. Evaluation and treatment of dyspareunia. Obstet Gynecol. 2009;113:1124-1136.
9. American College of Obstetricians and Gynecologists. ACOG Practice Bulletin No. 119: Female sexual dysfunction. Obstet Gynecol. 2011;117:996-1007.
10. Clayton AH, Hamilton DV. Female sexual dysfunction. Psychiatr Clin North Am. 2017;40:267-284.
11. Morrissey D, El-Khawand D, Ginzburg N, et al. Botulinum Toxin A injections into pelvic floor muscles under electromyographic guidance for women with refractory high-tone pelvic floor dysfunction: a 6-month prospective pilot study. Female Pelvic Med Reconstr Surg. 2015;21:277-282.
12. Hatzichristou D, Rosen RC, Derogatis LR, et al. Recommendations for the clinical evaluation of men and women with sexual dysfunction. J Sex Med. 2010;7(1 pt 2):337-348.
13. Kegel A. Sexual functions of the pubococcygeus muscle. West J Surg Obstet Gynecol. 1952;60:521-524.
14. Shafik A. The role of the levator ani muscle in evacuation, sexual performance and pelvic floor disorders. Int Urogynecol J. 2000;11:361-376.
15. Kinsey A, Pomeroy WB, Martin CE, et al. Sexual Behavior in the Human Female. Philadelphia, PA: WB Saunders; 1998.
16. Lowenstein L, Gruenwald I, Gartman I, et al. Can stronger pelvic muscle floor improve sexual function? Int Urogynecol J. 2010;21:553-556.
17. Kanter G, Rogers RG, Pauls RN, et al. A strong pelvic floor is associated with higher rates of sexual activity in women with pelvic floor disorders. Int Urogynecol J. 2015;26:991-996.
18. Wehbe SA, Kellogg-Spadt S, Whitmore K. Urogenital complaints and female sexual dysfunction. Part 2. J Sex Med. 2010;7:2304-2317.
19. Messelink B, Benson T, Berghmans B, et al. Standardization of terminology of pelvic floor muscle function and dysfunction: report from the Pelvic Floor Clinical Assessment Group of the International Continence Society. Neurourol Urodyn. 2005;24:374-380.
20. Haylen BT, de Ridder D, Freeman RM, et al. An International Urogynecological Association (IUGA)/International Continence Society (ICS) joint report on the terminology for female pelvic floor dysfunction. Neurourol Urodyn. 2010;29:4-20.
21. Montenegro ML, Mateus-Vasconcelos EC, Rosa e Silva JC, et al. Importance of pelvic muscle tenderness evaluation in women with chronic pelvic pain. Pain Med. 2010;11:224-228.
22. Lukban JC, Whitmore KE. Pelvic floor muscle re-education treatment of the overactive bladder and painful bladder syndrome. Clin Obstet Gynecol. 2002;45:273-285.
23. Kalmbach DA, Arnedt JT, Pillai V, et al. The impact of sleep on female sexual response and behavior: a pilot study. J Sex Med. 2015;12:1221-1232.
24. Aversa A, Bruzziches R, Francomano D, et al. Weight loss by multidisciplinary intervention improves endothelial and sexual function in obese fertile women. J Sex Med. 2013;10:1024-1033.
25. Pauls RN, Kleeman SD, Karram MM. Female sexual dysfunction: principles of diagnosis and therapy. Obstet Gynecol Surv. 2005;60:196-205.
26. Herati AS, Shorter B, Tai J, et al. Differences in food sensitivities between female interstitial cystitis/painful bladder syndrome (IC/PBS) and chronic prostatitis/chronic pelvic pain syndrome (CP/CPPS) patients. J Urol. 2009;181(4)(suppl):22.
27. Farrell J, Cacchioni T. The medicalization of women’s sexual pain. J Sex Res. 2012;49:328-336.
28. De Andres J, Sanchis-Lopez NM, Asensio-Samper JM, et al. Vulvodynia—an evidence-based literature review and proposed treatment algorithm. Pain Pract. 2016;16:204-236.
29. Herbenick D, Reece M, Schick V, et al. Women’s use and perceptions of commercial lubricants: prevalence and characteristics in a nationally representative sample of American adults. J Sex Med. 2014;11:642-652.
30. Donders GG, Bellen G. Cream with cutaneous fibroblast lysate for the treatment of provoked vestibulodynia: a double-blind randomized placebo-controlled crossover study. J Low Genit Tract Dis. 2012;16:427-436.
31. Belkin ZR, Krapf JM, Goldstein AT. Drugs in early clinical development for the treatment of female sexual dysfunction. Expert Opin Investig Drugs. 2015;24:159-167.
32. Islam A, Mitchel J, Rosen R, et al. Topical alprostadil in the treatment of female sexual arousal disorder: a pilot study. J Sex Marital Ther. 2001;27:531-540.
33. Oyama IA, Rejba A, Lukban JC, et al. Modified Thiele massage as therapeutic intervention for female patients with interstitial cystitis and high-tone pelvic floor dysfunction. Urology. 2004;64:862-865.
34. Bedaiwy MA, Patterson B, Mahajan S. Prevalence of myofascial chronic pelvic pain and the effectiveness of pelvic floor physical therapy. J Reprod Med. 2013;58:504-510.
35. Wehbe SA, Fariello JY, Whitmore K. Minimally invasive therapies for chronic pelvic pain syndrome. Curr Urol Rep. 2010;11:276-285.
36. Ger GC, Wexner SD, Jorge JM, et al. Evaluation and treatment of chronic intractable rectal pain—a frustrating endeavor. Dis Colon Rectum. 1993;36:139-145.
37. Billups KL, Berman L, Berman J, et al. A new non-pharmacological vacuum therapy for female sexual dysfunction. J Sex Marital Ther. 2001;27:435-441.
38. Miles T, Johnson N. Vaginal dilator therapy for women receiving pelvic radiotherapy. Cochrane Database Syst Rev. 2014;9:CD007291.
39. Goldstein I. Current management strategies of the postmenopausal patient with sexual health problems. J Sex Med. 2007;4(suppl 3):235-253.
40. Modelska K, Cummings S. Female sexual dysfunction in postmenopausal women: systematic review of placebo-controlled trials. Am J Obstet Gynecol. 2003;188:286-293.
41. Constantine G, Graham S, Portman DJ, et al. Female sexual function improved with ospemifene in postmenopausal women with vulvar and vaginal atrophy: results of a randomized, placebo-controlled trial. Climacteric. 2015;18:226-232.
42. Kingsberg SA, Woodard T. Female sexual dysfunction: focus on low desire. Obstet Gynecol. 2015;125:477-486.
43. Simon JA, Kingsberg SA, Shumel B, et al. Efficacy and safety of flibanserin in postmenopausal women with hypoactive sexual desire disorder: results of the SNOWDROP trial. Menopause. 2014;21:633-640.
44. Curtis Nickel J, Baranowski AP, Pontari M, et al. Management of men diagnosed with chronic prostatitis/chronic pelvic pain syndrome who have failed traditional management. Rev Urol. 2007;9:63-72.
45. Rogalski MJ, Kellogg-Spadt S, Hoffmann AR, et al. Retrospective chart review of vaginal diazepam suppository use in high-tone pelvic floor dysfunction. Int Urogynecol J. 2010;21:895-899.
46. Langford CF, Udvari Nagy S, Ghoniem GM. Levator ani trigger point injections: an underutilized treatment for chronic pelvic pain. Neurourol Urodyn. 2007;26:59-62.
47. Abbott JA, Jarvis SK, Lyons SD, et al. Botulinum toxin type A for chronic pain and pelvic floor spasm in women: a randomized controlled trial. Obstet Gynecol. 2006;108:915-923.
48. Kamanli A, Kaya A, Ardicoglu O, et al. Comparison of lidocaine injection, botulinum toxin injection, and dry needling to trigger points in myofascial pain syndrome. Rheumatol Int. 2005;25:604-611.
49. Brotto LA, Erskine Y, Carey M, et al. A brief mindfulness-based cognitive behavioral intervention improves sexual functioning versus wait-list control in women treated for gynecologic cancer. Gynecol Oncol. 2012;125:320-325.
Understanding, Assessing, and Conceptualizing Suicide Risk Among Veterans With PTSD
Increased risk of suicide among veterans with posttraumatic stress disorder (PTSD) is well established. Posttraumatic stress disorder and related consequences are associated with higher rates of suicidal ideation and suicidal self-directed violence (S-SDV).1 Based on a systematic review, several explanations for this relationship have been hypothesized.1 Particular emphasis has been placed on trauma type (eg, premilitary childhood abuse, combat exposure), frequency of trauma exposure (ie, a single traumatic episode vs multiple traumatic experiences), specific PTSD symptoms (eg, avoidance, sleep disturbance, alteration in mood and cognitions, risky behaviors), and other psychosocial consequences associated with PTSD (eg, low social support, psychiatric comorbidity, substance use). However, there is limited understanding regarding how to conceptualize and assess risk for suicide when treating veterans who have PTSD.
PTSD and the Interpersonal-Psychological Theory of Suicide
Although PTSD is associated with risk for S-SDV among veterans, a diagnosis-specific approach to conceptualizing risk of suicide (ie, an explanation specific to PTSD) might not be enough because most individuals with a psychiatric diagnosis do not engage in S-SDV.2 Rather, theories that are able to conceptualize suicide risk across many different psychiatric diagnoses are likely to improve mental health providers’ ability to understand risk of suicide. Although many theories attempt to understand suicide risk, the Interpersonal-Psychological Theory of Suicide (IPTS) has robust empirical support.3
The IPTS proposes that suicidal ideation is driven by perceptions of stable and unchanging thwarted belongingness (TB), defined as an unmet psychological need to socially belong, and perceived burdensomeness (PB), defined as the perception that one is a burden on others.4 However, PB and TB are not considered sufficient for S-SDV to occur unless an individual also has acquired the capability for suicide. Capability for suicide is thought to happen when an individual loses the fear of dying by suicide and develops tolerance to physical pain, which is proposed to occur through habituation or repeated exposure to painful stimuli.3
Several studies have examined the IPTS in a number of clinical populations, including veterans and active-duty service members; yet limited research has applied the IPTS to veterans with PTSD.3 However, a recent article proposed that a number of PTSD-related factors increase risk of suicide through the lens of the IPTS.5 In particular, repeated exposure to painful and provocative events—especially those characterized by violence and aggression—might increase acquired capability for suicide by causing habituation to physical pain and discomfort and reducing fear of injury and death. This concept is especially concerning because of the frequent occurrence of both military- (eg, combat, military sexual trauma) and nonmilitary-related (eg, childhood abuse, intimate partner violence) stressful and traumatic events among veterans, especially individuals with PTSD.
Moreover, the acquired capability for suicide correlates highly with anxious, intrusive, and hyperarousal symptoms of PTSD.5-7 Over time, these PTSD symptoms are thought to increase habituation to the physically painful and frightening aspects of S-SDV, resulting in increased pain tolerance and fearlessness about death.3
In addition, PTSD-related cognitive-affective states (ie, thoughts and emotions), such as guilt, shame, and self-deprecation, might drive beliefs of PB and TB.5,8 Repeated exposure to such trauma-related thoughts and emotions could further reinforce beliefs of self-hate or inadequacy (PB).2 Trauma-related beliefs that the world or others are unsafe also might reduce the likelihood of seeking social support, thereby increasing TB.2 The PTSD symptoms of avoidance and self-blame also are likely to reinforce beliefs of PB and TB.2
Assessing Suicide Risk in the Context of PTSD
The IPTS framework is one that can be used by mental health providers to conceptualize risk of suicide across populations and psychiatric diagnoses, including veterans with PTSD. However, integrating additional risk assessment and management techniques is essential to guide appropriate risk stratification and treatment.
One such method of suicide risk assessment and management is therapeutic risk management (TRM).9 Therapeutic risk management involves a stratification process by which temporal aspects (ie, acute and chronic) and severity (ie, low, moderate, and high) of suicide risk are assessed using a combination of clinical interview and psychometrically sound self-report measures, such as the Beck Scale for Suicide Ideation, Beck Hopelessness Scale, and Reasons for Living Inventory. Appropriate clinical interventions that correspond to acute and chronic suicide risk stratification are then implemented (eg, safety planning, lethal means counseling, increasing frequency of care, hospitalization if warranted).
Therapeutic risk management emphasizes the necessity of assessing current and past suicidal ideation, intent, plan, and access to means. Moreover, additional considerations might be indicated when assessing and conceptualizing suicide risk among veterans with PTSD. Assessing lifetime trauma history, including traumas that occurred before, during, and after military service, is important for understanding whether traumatic experiences influence acute and chronic risks of suicide. As previously described, careful attention to stressful and traumatic experiences with violent and aggressive characteristics is recommended because research suggests that these experiences are associated with increased capability for suicide.5 Awareness of the diversity of traumatic experiences and the importance of contextual factors surrounding such experiences also are essential. For example, the nature of violence and proximity to violence (eg, directly involved in a firefight vs hearing a mortar explosion in the distance) are key components of military-related combat trauma that might differentially influence risk of suicide.10
Similarly, although military sexual trauma can include repeated threatening sexual harassment or sexual assault, research suggests that military sexual assault is particularly important for understanding suicidal ideation, and experiences of military sexual harassment are less important.11 Therefore, a careful and nuanced understanding of how contextual aspects of a veteran’s trauma history might relate to his or her chronic and acute risk of suicide is critical.
Also important is considering the individual and institutional reactions to trauma. For example, veterans whose behaviors during traumatic experiences violated their values and moral code (ie, moral injury) might be at increased risk for S-SDV. Similarly, veterans who believe that the military institution did not adequately protect them from or support them in the aftermath of traumatic experience(s) (ie, institutional betrayal) might be at higher risk of suicide.
During a clinical interview, mental health providers should pay attention to beliefs and behaviors the veteran is reporting. For example, endorsement of perceptions of low social support (eg, “no one likes me”) or self-esteem (eg, “I’m just not as good as I used to be”) might be indicative of TB or PB, respectively. Additionally, providers should be aware of current or lifetime exposure to painful stimuli (eg, nonsuicidal self-injury, such as cutting or burning, previous suicide attempts) because these exposures might increase the veteran’s acquired capability of future S-SDV.
Although unstructured clinical interviews are a common suicide risk assessment approach, TRM proposes that using a thorough clinical interview along with valid self-report measures could further illuminate a patient’s risk of suicide.9 Implementing brief measures allows mental health providers to quickly assess several risk factors and decrease the likelihood of missing important aspects of suicide risk assessment. Providers can use a number of measures to inform their suicide risk assessment, including augmenting a clinical interview of suicide risk with a valid self-report measure of recent suicidal ideation (eg, Beck Scale for Suicide Ideation, which assesses the severity of suicidal ideation in the past week).
Additionally for veterans with PTSD, mental health providers can include measures of PTSD symptoms (eg, PTSD checklist in the Diagnostic and Statistical Manual of Mental Disorders–5) and common PTSD comorbidities (eg, Beck Depression Inventory-II for depressive symptoms) that might contribute to current risk of suicide. Based on previous research, providers also might consider adding measures of trauma-related beliefs (eg, Posttraumatic Cognitions Inventory) and emotions, such as guilt (eg, Trauma-Related Guilt Inventory).5
These measures could aid in identifying modifiable risk factors of suicide among veterans with PTSD, such as the extent to which certain beliefs or emotions relate to an individual’s risk of suicide. In addition to asking about characteristics of traumatic events during the clinical interview, measures of moral injury (eg, Moral Injury Events Scale) and institutional betrayal (eg, Institutional Betrayal Questionnaire) might further inform understanding of contextual aspects of trauma that could help explain an individual’s risk of suicide.
Finally, interpersonal measures also could be helpful. For example, because avoidance and social isolation are risk factors for suicidal ideation among veterans with PTSD, measures of perceived interpersonal functioning (eg, Interpersonal Needs Questionnaire) might add further data to assist in suicide risk conceptualization. Although the selection of specific measures likely varies based on the specific needs of an individual patient, these are examples of measures that can be used with veterans with PTSD to inform suicide risk assessment and conceptualization.
By combining data from various measures across multiple domains with a thorough clinical interview, mental health providers can use a TRM approach to understand and conceptualize suicide risk among veterans with PTSD. This approach can facilitate mental health providers’ ability to provide optimal care and guide intervention(s) for veterans with PTSD. One brief intervention that has been used with veterans is safety planning. During safety planning, the provider assists the veteran in identifying warning signs, internal and external coping strategies, and individuals the veteran can reach out to for help (eg, friends and family, providers, Veterans Crisis Line), in addition to collaboratively brainstorming ways the veteran can make his or her environment safer (eg, reducing access to lethal means, identifying reminders of their reasons for living).
Specific to veterans with PTSD, symptoms such as avoidance, hyperarousal, social isolation, and beliefs that others and the world are unsafe might affect safety planning. Such symptoms could hinder identification and use of coping strategies while deterring openness to reach out to others for help. A collaborative method can be used to identify alternate means of coping that take into account PTSD-related avoidance and hyperarousal (eg, rather than going to a crowded store or isolating at home, taking a walk in a quiet park with few people). Similarly, because substance use and risky behaviors are common among veterans with PTSD and might further increase risk of suicide, exploring healthy (eg, exercise) vs unhealthy (eg, substance use; unprotected sex) coping strategies could be helpful.
Further, based on their lived experience, veterans with PTSD could experience difficulty identifying a support system or be reluctant to reach out to others during acute crisis. This might be particularly daunting in the presence of PB and TB. In these situations, it is important to validate the veteran’s difficulty with reaching out while simultaneously encouraging the veteran to examine the accuracy of such beliefs and/or helping the veteran develop skills to overcome these obstacles.
The mental health provider also can work with the individual to ensure that the veteran understands that if he or she does engage emergency resources (eg, Veterans Crisis Line), information likely will be held confidential. Providers can tell their patients that breaks in confidentiality are rare and occur only in circumstances in which it is necessary to protect the veteran. In doing so, the provider facilitates the veteran’s understanding of the role of crisis resources and clarifies any misconceptions the veteran might have (eg, calling the crisis line will always result in hospitalization or police presence).
Conclusion
Several PTSD-related factors might increase PB, TB, and the acquired capability for suicide among veterans with PTSD. Because suicide risk assessment and management can be time sensitive and anxiety provoking, mental health providers can use a TRM approach to increase their confidence in instituting optimal care and mitigating risk by having a structured, therapeutic assessment process that gathers appropriate suicide- and PTSD-related data to assist in developing suicide risk-related treatment. However, more research is needed to determine the most useful self-report measures and effective interventions when working with veterans with PTSD at risk of suicide.
1. Pompili M, Sher L, Serafini G, et al. Posttraumatic stress disorder and suicide risk among veterans: a literature review
2. Goldsmith SK, Pellmar TC, Kleinman AM, Bunney WE, eds. Reducing Suicide: A National Imperative. Washington, DC: The National Academies Press; 2002.
3. Chu C, Buchman-Schmitt JM, Stanley IH, et al. The interpersonal psychological theory of suicide: a systematic review and meta-analysis of a decade of cross-national research. Psychol Bull. 2017;143(12):1313-1345.
4. Van Orden KA, Witte TK, Cukrowicz KC, et al. The interpersonal theory of suicide. Psychol Rev. 2010;117(2):575-600.
5. Bryan CJ, Grove JL, Kimbrel NA. Theory-driven models of self-directed violence among individuals with PTSD. Curr Opin Psychol. 2017;14:12-17.
6. Bryan CJ, Anestis M. Reexperiencing symptoms and the interpersonal-psychological theory of suicidal behavior among deployed service members evaluated for traumatic brain injury. J Clin Psychol. 2011;67(9):856-865.
7. Zuromski KL, Davis MT, Witte TK, Weathers F, Blevins C. PTSD symptom clusters are differentially associated with components of the acquired capability for suicide. Suicide Life Threat Behav. 2014;44(6):682-697.
8. Davis MT, Witte TK, Weathers FW, Blevins CA. The role of posttraumatic stress disorder symptom clusters in the prediction of passive suicidal ideation. Psychol Trauma. 2014;6(suppl 1):S82-S91.
9. Wortzel HS, Matarazzo B, Homaifar B. A model for therapeutic risk management of the suicidal patient. J Psychiatr Pract. 2013;19(4):323-326.
10. Bryan CJ, Cukrowicz KC. Associations between types of combat violence and the acquired capability for suicide. Suicide Life Threat Behav. 2011;41(2):126-136.
11. Monteith LL, Menefee DS, Forster JE, Bahraini NH. A closer examination of sexual trauma during deployment: not all sexual traumas are associated with suicidal ideation. Suicide Life Threat Behav. 2016;46(1):46-54.
Increased risk of suicide among veterans with posttraumatic stress disorder (PTSD) is well established. Posttraumatic stress disorder and related consequences are associated with higher rates of suicidal ideation and suicidal self-directed violence (S-SDV).1 Based on a systematic review, several explanations for this relationship have been hypothesized.1 Particular emphasis has been placed on trauma type (eg, premilitary childhood abuse, combat exposure), frequency of trauma exposure (ie, a single traumatic episode vs multiple traumatic experiences), specific PTSD symptoms (eg, avoidance, sleep disturbance, alteration in mood and cognitions, risky behaviors), and other psychosocial consequences associated with PTSD (eg, low social support, psychiatric comorbidity, substance use). However, there is limited understanding regarding how to conceptualize and assess risk for suicide when treating veterans who have PTSD.
PTSD and the Interpersonal-Psychological Theory of Suicide
Although PTSD is associated with risk for S-SDV among veterans, a diagnosis-specific approach to conceptualizing risk of suicide (ie, an explanation specific to PTSD) might not be enough because most individuals with a psychiatric diagnosis do not engage in S-SDV.2 Rather, theories that are able to conceptualize suicide risk across many different psychiatric diagnoses are likely to improve mental health providers’ ability to understand risk of suicide. Although many theories attempt to understand suicide risk, the Interpersonal-Psychological Theory of Suicide (IPTS) has robust empirical support.3
The IPTS proposes that suicidal ideation is driven by perceptions of stable and unchanging thwarted belongingness (TB), defined as an unmet psychological need to socially belong, and perceived burdensomeness (PB), defined as the perception that one is a burden on others.4 However, PB and TB are not considered sufficient for S-SDV to occur unless an individual also has acquired the capability for suicide. Capability for suicide is thought to happen when an individual loses the fear of dying by suicide and develops tolerance to physical pain, which is proposed to occur through habituation or repeated exposure to painful stimuli.3
Several studies have examined the IPTS in a number of clinical populations, including veterans and active-duty service members; yet limited research has applied the IPTS to veterans with PTSD.3 However, a recent article proposed that a number of PTSD-related factors increase risk of suicide through the lens of the IPTS.5 In particular, repeated exposure to painful and provocative events—especially those characterized by violence and aggression—might increase acquired capability for suicide by causing habituation to physical pain and discomfort and reducing fear of injury and death. This concept is especially concerning because of the frequent occurrence of both military- (eg, combat, military sexual trauma) and nonmilitary-related (eg, childhood abuse, intimate partner violence) stressful and traumatic events among veterans, especially individuals with PTSD.
Moreover, the acquired capability for suicide correlates highly with anxious, intrusive, and hyperarousal symptoms of PTSD.5-7 Over time, these PTSD symptoms are thought to increase habituation to the physically painful and frightening aspects of S-SDV, resulting in increased pain tolerance and fearlessness about death.3
In addition, PTSD-related cognitive-affective states (ie, thoughts and emotions), such as guilt, shame, and self-deprecation, might drive beliefs of PB and TB.5,8 Repeated exposure to such trauma-related thoughts and emotions could further reinforce beliefs of self-hate or inadequacy (PB).2 Trauma-related beliefs that the world or others are unsafe also might reduce the likelihood of seeking social support, thereby increasing TB.2 The PTSD symptoms of avoidance and self-blame also are likely to reinforce beliefs of PB and TB.2
Assessing Suicide Risk in the Context of PTSD
The IPTS framework is one that can be used by mental health providers to conceptualize risk of suicide across populations and psychiatric diagnoses, including veterans with PTSD. However, integrating additional risk assessment and management techniques is essential to guide appropriate risk stratification and treatment.
One such method of suicide risk assessment and management is therapeutic risk management (TRM).9 Therapeutic risk management involves a stratification process by which temporal aspects (ie, acute and chronic) and severity (ie, low, moderate, and high) of suicide risk are assessed using a combination of clinical interview and psychometrically sound self-report measures, such as the Beck Scale for Suicide Ideation, Beck Hopelessness Scale, and Reasons for Living Inventory. Appropriate clinical interventions that correspond to acute and chronic suicide risk stratification are then implemented (eg, safety planning, lethal means counseling, increasing frequency of care, hospitalization if warranted).
Therapeutic risk management emphasizes the necessity of assessing current and past suicidal ideation, intent, plan, and access to means. Moreover, additional considerations might be indicated when assessing and conceptualizing suicide risk among veterans with PTSD. Assessing lifetime trauma history, including traumas that occurred before, during, and after military service, is important for understanding whether traumatic experiences influence acute and chronic risks of suicide. As previously described, careful attention to stressful and traumatic experiences with violent and aggressive characteristics is recommended because research suggests that these experiences are associated with increased capability for suicide.5 Awareness of the diversity of traumatic experiences and the importance of contextual factors surrounding such experiences also are essential. For example, the nature of violence and proximity to violence (eg, directly involved in a firefight vs hearing a mortar explosion in the distance) are key components of military-related combat trauma that might differentially influence risk of suicide.10
Similarly, although military sexual trauma can include repeated threatening sexual harassment or sexual assault, research suggests that military sexual assault is particularly important for understanding suicidal ideation, and experiences of military sexual harassment are less important.11 Therefore, a careful and nuanced understanding of how contextual aspects of a veteran’s trauma history might relate to his or her chronic and acute risk of suicide is critical.
Also important is considering the individual and institutional reactions to trauma. For example, veterans whose behaviors during traumatic experiences violated their values and moral code (ie, moral injury) might be at increased risk for S-SDV. Similarly, veterans who believe that the military institution did not adequately protect them from or support them in the aftermath of traumatic experience(s) (ie, institutional betrayal) might be at higher risk of suicide.
During a clinical interview, mental health providers should pay attention to beliefs and behaviors the veteran is reporting. For example, endorsement of perceptions of low social support (eg, “no one likes me”) or self-esteem (eg, “I’m just not as good as I used to be”) might be indicative of TB or PB, respectively. Additionally, providers should be aware of current or lifetime exposure to painful stimuli (eg, nonsuicidal self-injury, such as cutting or burning, previous suicide attempts) because these exposures might increase the veteran’s acquired capability of future S-SDV.
Although unstructured clinical interviews are a common suicide risk assessment approach, TRM proposes that using a thorough clinical interview along with valid self-report measures could further illuminate a patient’s risk of suicide.9 Implementing brief measures allows mental health providers to quickly assess several risk factors and decrease the likelihood of missing important aspects of suicide risk assessment. Providers can use a number of measures to inform their suicide risk assessment, including augmenting a clinical interview of suicide risk with a valid self-report measure of recent suicidal ideation (eg, Beck Scale for Suicide Ideation, which assesses the severity of suicidal ideation in the past week).
Additionally for veterans with PTSD, mental health providers can include measures of PTSD symptoms (eg, PTSD checklist in the Diagnostic and Statistical Manual of Mental Disorders–5) and common PTSD comorbidities (eg, Beck Depression Inventory-II for depressive symptoms) that might contribute to current risk of suicide. Based on previous research, providers also might consider adding measures of trauma-related beliefs (eg, Posttraumatic Cognitions Inventory) and emotions, such as guilt (eg, Trauma-Related Guilt Inventory).5
These measures could aid in identifying modifiable risk factors of suicide among veterans with PTSD, such as the extent to which certain beliefs or emotions relate to an individual’s risk of suicide. In addition to asking about characteristics of traumatic events during the clinical interview, measures of moral injury (eg, Moral Injury Events Scale) and institutional betrayal (eg, Institutional Betrayal Questionnaire) might further inform understanding of contextual aspects of trauma that could help explain an individual’s risk of suicide.
Finally, interpersonal measures also could be helpful. For example, because avoidance and social isolation are risk factors for suicidal ideation among veterans with PTSD, measures of perceived interpersonal functioning (eg, Interpersonal Needs Questionnaire) might add further data to assist in suicide risk conceptualization. Although the selection of specific measures likely varies based on the specific needs of an individual patient, these are examples of measures that can be used with veterans with PTSD to inform suicide risk assessment and conceptualization.
By combining data from various measures across multiple domains with a thorough clinical interview, mental health providers can use a TRM approach to understand and conceptualize suicide risk among veterans with PTSD. This approach can facilitate mental health providers’ ability to provide optimal care and guide intervention(s) for veterans with PTSD. One brief intervention that has been used with veterans is safety planning. During safety planning, the provider assists the veteran in identifying warning signs, internal and external coping strategies, and individuals the veteran can reach out to for help (eg, friends and family, providers, Veterans Crisis Line), in addition to collaboratively brainstorming ways the veteran can make his or her environment safer (eg, reducing access to lethal means, identifying reminders of their reasons for living).
Specific to veterans with PTSD, symptoms such as avoidance, hyperarousal, social isolation, and beliefs that others and the world are unsafe might affect safety planning. Such symptoms could hinder identification and use of coping strategies while deterring openness to reach out to others for help. A collaborative method can be used to identify alternate means of coping that take into account PTSD-related avoidance and hyperarousal (eg, rather than going to a crowded store or isolating at home, taking a walk in a quiet park with few people). Similarly, because substance use and risky behaviors are common among veterans with PTSD and might further increase risk of suicide, exploring healthy (eg, exercise) vs unhealthy (eg, substance use; unprotected sex) coping strategies could be helpful.
Further, based on their lived experience, veterans with PTSD could experience difficulty identifying a support system or be reluctant to reach out to others during acute crisis. This might be particularly daunting in the presence of PB and TB. In these situations, it is important to validate the veteran’s difficulty with reaching out while simultaneously encouraging the veteran to examine the accuracy of such beliefs and/or helping the veteran develop skills to overcome these obstacles.
The mental health provider also can work with the individual to ensure that the veteran understands that if he or she does engage emergency resources (eg, Veterans Crisis Line), information likely will be held confidential. Providers can tell their patients that breaks in confidentiality are rare and occur only in circumstances in which it is necessary to protect the veteran. In doing so, the provider facilitates the veteran’s understanding of the role of crisis resources and clarifies any misconceptions the veteran might have (eg, calling the crisis line will always result in hospitalization or police presence).
Conclusion
Several PTSD-related factors might increase PB, TB, and the acquired capability for suicide among veterans with PTSD. Because suicide risk assessment and management can be time sensitive and anxiety provoking, mental health providers can use a TRM approach to increase their confidence in instituting optimal care and mitigating risk by having a structured, therapeutic assessment process that gathers appropriate suicide- and PTSD-related data to assist in developing suicide risk-related treatment. However, more research is needed to determine the most useful self-report measures and effective interventions when working with veterans with PTSD at risk of suicide.
Increased risk of suicide among veterans with posttraumatic stress disorder (PTSD) is well established. Posttraumatic stress disorder and related consequences are associated with higher rates of suicidal ideation and suicidal self-directed violence (S-SDV).1 Based on a systematic review, several explanations for this relationship have been hypothesized.1 Particular emphasis has been placed on trauma type (eg, premilitary childhood abuse, combat exposure), frequency of trauma exposure (ie, a single traumatic episode vs multiple traumatic experiences), specific PTSD symptoms (eg, avoidance, sleep disturbance, alteration in mood and cognitions, risky behaviors), and other psychosocial consequences associated with PTSD (eg, low social support, psychiatric comorbidity, substance use). However, there is limited understanding regarding how to conceptualize and assess risk for suicide when treating veterans who have PTSD.
PTSD and the Interpersonal-Psychological Theory of Suicide
Although PTSD is associated with risk for S-SDV among veterans, a diagnosis-specific approach to conceptualizing risk of suicide (ie, an explanation specific to PTSD) might not be enough because most individuals with a psychiatric diagnosis do not engage in S-SDV.2 Rather, theories that are able to conceptualize suicide risk across many different psychiatric diagnoses are likely to improve mental health providers’ ability to understand risk of suicide. Although many theories attempt to understand suicide risk, the Interpersonal-Psychological Theory of Suicide (IPTS) has robust empirical support.3
The IPTS proposes that suicidal ideation is driven by perceptions of stable and unchanging thwarted belongingness (TB), defined as an unmet psychological need to socially belong, and perceived burdensomeness (PB), defined as the perception that one is a burden on others.4 However, PB and TB are not considered sufficient for S-SDV to occur unless an individual also has acquired the capability for suicide. Capability for suicide is thought to happen when an individual loses the fear of dying by suicide and develops tolerance to physical pain, which is proposed to occur through habituation or repeated exposure to painful stimuli.3
Several studies have examined the IPTS in a number of clinical populations, including veterans and active-duty service members; yet limited research has applied the IPTS to veterans with PTSD.3 However, a recent article proposed that a number of PTSD-related factors increase risk of suicide through the lens of the IPTS.5 In particular, repeated exposure to painful and provocative events—especially those characterized by violence and aggression—might increase acquired capability for suicide by causing habituation to physical pain and discomfort and reducing fear of injury and death. This concept is especially concerning because of the frequent occurrence of both military- (eg, combat, military sexual trauma) and nonmilitary-related (eg, childhood abuse, intimate partner violence) stressful and traumatic events among veterans, especially individuals with PTSD.
Moreover, the acquired capability for suicide correlates highly with anxious, intrusive, and hyperarousal symptoms of PTSD.5-7 Over time, these PTSD symptoms are thought to increase habituation to the physically painful and frightening aspects of S-SDV, resulting in increased pain tolerance and fearlessness about death.3
In addition, PTSD-related cognitive-affective states (ie, thoughts and emotions), such as guilt, shame, and self-deprecation, might drive beliefs of PB and TB.5,8 Repeated exposure to such trauma-related thoughts and emotions could further reinforce beliefs of self-hate or inadequacy (PB).2 Trauma-related beliefs that the world or others are unsafe also might reduce the likelihood of seeking social support, thereby increasing TB.2 The PTSD symptoms of avoidance and self-blame also are likely to reinforce beliefs of PB and TB.2
Assessing Suicide Risk in the Context of PTSD
The IPTS framework is one that can be used by mental health providers to conceptualize risk of suicide across populations and psychiatric diagnoses, including veterans with PTSD. However, integrating additional risk assessment and management techniques is essential to guide appropriate risk stratification and treatment.
One such method of suicide risk assessment and management is therapeutic risk management (TRM).9 Therapeutic risk management involves a stratification process by which temporal aspects (ie, acute and chronic) and severity (ie, low, moderate, and high) of suicide risk are assessed using a combination of clinical interview and psychometrically sound self-report measures, such as the Beck Scale for Suicide Ideation, Beck Hopelessness Scale, and Reasons for Living Inventory. Appropriate clinical interventions that correspond to acute and chronic suicide risk stratification are then implemented (eg, safety planning, lethal means counseling, increasing frequency of care, hospitalization if warranted).
Therapeutic risk management emphasizes the necessity of assessing current and past suicidal ideation, intent, plan, and access to means. Moreover, additional considerations might be indicated when assessing and conceptualizing suicide risk among veterans with PTSD. Assessing lifetime trauma history, including traumas that occurred before, during, and after military service, is important for understanding whether traumatic experiences influence acute and chronic risks of suicide. As previously described, careful attention to stressful and traumatic experiences with violent and aggressive characteristics is recommended because research suggests that these experiences are associated with increased capability for suicide.5 Awareness of the diversity of traumatic experiences and the importance of contextual factors surrounding such experiences also are essential. For example, the nature of violence and proximity to violence (eg, directly involved in a firefight vs hearing a mortar explosion in the distance) are key components of military-related combat trauma that might differentially influence risk of suicide.10
Similarly, although military sexual trauma can include repeated threatening sexual harassment or sexual assault, research suggests that military sexual assault is particularly important for understanding suicidal ideation, and experiences of military sexual harassment are less important.11 Therefore, a careful and nuanced understanding of how contextual aspects of a veteran’s trauma history might relate to his or her chronic and acute risk of suicide is critical.
Also important is considering the individual and institutional reactions to trauma. For example, veterans whose behaviors during traumatic experiences violated their values and moral code (ie, moral injury) might be at increased risk for S-SDV. Similarly, veterans who believe that the military institution did not adequately protect them from or support them in the aftermath of traumatic experience(s) (ie, institutional betrayal) might be at higher risk of suicide.
During a clinical interview, mental health providers should pay attention to beliefs and behaviors the veteran is reporting. For example, endorsement of perceptions of low social support (eg, “no one likes me”) or self-esteem (eg, “I’m just not as good as I used to be”) might be indicative of TB or PB, respectively. Additionally, providers should be aware of current or lifetime exposure to painful stimuli (eg, nonsuicidal self-injury, such as cutting or burning, previous suicide attempts) because these exposures might increase the veteran’s acquired capability of future S-SDV.
Although unstructured clinical interviews are a common suicide risk assessment approach, TRM proposes that using a thorough clinical interview along with valid self-report measures could further illuminate a patient’s risk of suicide.9 Implementing brief measures allows mental health providers to quickly assess several risk factors and decrease the likelihood of missing important aspects of suicide risk assessment. Providers can use a number of measures to inform their suicide risk assessment, including augmenting a clinical interview of suicide risk with a valid self-report measure of recent suicidal ideation (eg, Beck Scale for Suicide Ideation, which assesses the severity of suicidal ideation in the past week).
Additionally for veterans with PTSD, mental health providers can include measures of PTSD symptoms (eg, PTSD checklist in the Diagnostic and Statistical Manual of Mental Disorders–5) and common PTSD comorbidities (eg, Beck Depression Inventory-II for depressive symptoms) that might contribute to current risk of suicide. Based on previous research, providers also might consider adding measures of trauma-related beliefs (eg, Posttraumatic Cognitions Inventory) and emotions, such as guilt (eg, Trauma-Related Guilt Inventory).5
These measures could aid in identifying modifiable risk factors of suicide among veterans with PTSD, such as the extent to which certain beliefs or emotions relate to an individual’s risk of suicide. In addition to asking about characteristics of traumatic events during the clinical interview, measures of moral injury (eg, Moral Injury Events Scale) and institutional betrayal (eg, Institutional Betrayal Questionnaire) might further inform understanding of contextual aspects of trauma that could help explain an individual’s risk of suicide.
Finally, interpersonal measures also could be helpful. For example, because avoidance and social isolation are risk factors for suicidal ideation among veterans with PTSD, measures of perceived interpersonal functioning (eg, Interpersonal Needs Questionnaire) might add further data to assist in suicide risk conceptualization. Although the selection of specific measures likely varies based on the specific needs of an individual patient, these are examples of measures that can be used with veterans with PTSD to inform suicide risk assessment and conceptualization.
By combining data from various measures across multiple domains with a thorough clinical interview, mental health providers can use a TRM approach to understand and conceptualize suicide risk among veterans with PTSD. This approach can facilitate mental health providers’ ability to provide optimal care and guide intervention(s) for veterans with PTSD. One brief intervention that has been used with veterans is safety planning. During safety planning, the provider assists the veteran in identifying warning signs, internal and external coping strategies, and individuals the veteran can reach out to for help (eg, friends and family, providers, Veterans Crisis Line), in addition to collaboratively brainstorming ways the veteran can make his or her environment safer (eg, reducing access to lethal means, identifying reminders of their reasons for living).
Specific to veterans with PTSD, symptoms such as avoidance, hyperarousal, social isolation, and beliefs that others and the world are unsafe might affect safety planning. Such symptoms could hinder identification and use of coping strategies while deterring openness to reach out to others for help. A collaborative method can be used to identify alternate means of coping that take into account PTSD-related avoidance and hyperarousal (eg, rather than going to a crowded store or isolating at home, taking a walk in a quiet park with few people). Similarly, because substance use and risky behaviors are common among veterans with PTSD and might further increase risk of suicide, exploring healthy (eg, exercise) vs unhealthy (eg, substance use; unprotected sex) coping strategies could be helpful.
Further, based on their lived experience, veterans with PTSD could experience difficulty identifying a support system or be reluctant to reach out to others during acute crisis. This might be particularly daunting in the presence of PB and TB. In these situations, it is important to validate the veteran’s difficulty with reaching out while simultaneously encouraging the veteran to examine the accuracy of such beliefs and/or helping the veteran develop skills to overcome these obstacles.
The mental health provider also can work with the individual to ensure that the veteran understands that if he or she does engage emergency resources (eg, Veterans Crisis Line), information likely will be held confidential. Providers can tell their patients that breaks in confidentiality are rare and occur only in circumstances in which it is necessary to protect the veteran. In doing so, the provider facilitates the veteran’s understanding of the role of crisis resources and clarifies any misconceptions the veteran might have (eg, calling the crisis line will always result in hospitalization or police presence).
Conclusion
Several PTSD-related factors might increase PB, TB, and the acquired capability for suicide among veterans with PTSD. Because suicide risk assessment and management can be time sensitive and anxiety provoking, mental health providers can use a TRM approach to increase their confidence in instituting optimal care and mitigating risk by having a structured, therapeutic assessment process that gathers appropriate suicide- and PTSD-related data to assist in developing suicide risk-related treatment. However, more research is needed to determine the most useful self-report measures and effective interventions when working with veterans with PTSD at risk of suicide.
1. Pompili M, Sher L, Serafini G, et al. Posttraumatic stress disorder and suicide risk among veterans: a literature review
2. Goldsmith SK, Pellmar TC, Kleinman AM, Bunney WE, eds. Reducing Suicide: A National Imperative. Washington, DC: The National Academies Press; 2002.
3. Chu C, Buchman-Schmitt JM, Stanley IH, et al. The interpersonal psychological theory of suicide: a systematic review and meta-analysis of a decade of cross-national research. Psychol Bull. 2017;143(12):1313-1345.
4. Van Orden KA, Witte TK, Cukrowicz KC, et al. The interpersonal theory of suicide. Psychol Rev. 2010;117(2):575-600.
5. Bryan CJ, Grove JL, Kimbrel NA. Theory-driven models of self-directed violence among individuals with PTSD. Curr Opin Psychol. 2017;14:12-17.
6. Bryan CJ, Anestis M. Reexperiencing symptoms and the interpersonal-psychological theory of suicidal behavior among deployed service members evaluated for traumatic brain injury. J Clin Psychol. 2011;67(9):856-865.
7. Zuromski KL, Davis MT, Witte TK, Weathers F, Blevins C. PTSD symptom clusters are differentially associated with components of the acquired capability for suicide. Suicide Life Threat Behav. 2014;44(6):682-697.
8. Davis MT, Witte TK, Weathers FW, Blevins CA. The role of posttraumatic stress disorder symptom clusters in the prediction of passive suicidal ideation. Psychol Trauma. 2014;6(suppl 1):S82-S91.
9. Wortzel HS, Matarazzo B, Homaifar B. A model for therapeutic risk management of the suicidal patient. J Psychiatr Pract. 2013;19(4):323-326.
10. Bryan CJ, Cukrowicz KC. Associations between types of combat violence and the acquired capability for suicide. Suicide Life Threat Behav. 2011;41(2):126-136.
11. Monteith LL, Menefee DS, Forster JE, Bahraini NH. A closer examination of sexual trauma during deployment: not all sexual traumas are associated with suicidal ideation. Suicide Life Threat Behav. 2016;46(1):46-54.
1. Pompili M, Sher L, Serafini G, et al. Posttraumatic stress disorder and suicide risk among veterans: a literature review
2. Goldsmith SK, Pellmar TC, Kleinman AM, Bunney WE, eds. Reducing Suicide: A National Imperative. Washington, DC: The National Academies Press; 2002.
3. Chu C, Buchman-Schmitt JM, Stanley IH, et al. The interpersonal psychological theory of suicide: a systematic review and meta-analysis of a decade of cross-national research. Psychol Bull. 2017;143(12):1313-1345.
4. Van Orden KA, Witte TK, Cukrowicz KC, et al. The interpersonal theory of suicide. Psychol Rev. 2010;117(2):575-600.
5. Bryan CJ, Grove JL, Kimbrel NA. Theory-driven models of self-directed violence among individuals with PTSD. Curr Opin Psychol. 2017;14:12-17.
6. Bryan CJ, Anestis M. Reexperiencing symptoms and the interpersonal-psychological theory of suicidal behavior among deployed service members evaluated for traumatic brain injury. J Clin Psychol. 2011;67(9):856-865.
7. Zuromski KL, Davis MT, Witte TK, Weathers F, Blevins C. PTSD symptom clusters are differentially associated with components of the acquired capability for suicide. Suicide Life Threat Behav. 2014;44(6):682-697.
8. Davis MT, Witte TK, Weathers FW, Blevins CA. The role of posttraumatic stress disorder symptom clusters in the prediction of passive suicidal ideation. Psychol Trauma. 2014;6(suppl 1):S82-S91.
9. Wortzel HS, Matarazzo B, Homaifar B. A model for therapeutic risk management of the suicidal patient. J Psychiatr Pract. 2013;19(4):323-326.
10. Bryan CJ, Cukrowicz KC. Associations between types of combat violence and the acquired capability for suicide. Suicide Life Threat Behav. 2011;41(2):126-136.
11. Monteith LL, Menefee DS, Forster JE, Bahraini NH. A closer examination of sexual trauma during deployment: not all sexual traumas are associated with suicidal ideation. Suicide Life Threat Behav. 2016;46(1):46-54.
2018 Update on prenatal carrier screening
Prenatal care has long included carrier screening for genetic diseases, such as cystic fibrosis and Tay-Sachs disease. Recently, advances in genetics technologies led to the development of multiplex panels that can be used to test for hundreds of genetic disorders simultaneously, and can be used to assess carrier status for expectant couples or those planning a pregnancy. Although such screening covers many more conditions than those recommended in traditional guidelines, the benefit of expanded carrier screening (ECS) over standard gene-by-gene testing is not clear.
In this Update, I review recent ECS research that can be helpful to those who practice reproductive endocrinology and infertility medicine, maternal–fetal medicine, and general ObGyn. This research considered some of the many complexities of ECS:
- number and type of severe autosomal recessive conditions identified by an ECS panel, or by panethnic screening for 3 common conditions (cystic fibrosis, fragile X syndrome, spinal muscular atrophy)
- whether the disorders covered by ECS panels meet recommended criteria regarding severity, prevalence, and test accuracy
- women’s thoughts and perspectives on ECS
- whether the marketing materials disseminated by commercial providers of ECS are accurate and balanced.
Genetic diseases identified by expanded carrier screening
Haque IS, Lazarin GA, Kang HP, Evans EA, Goldberg JD, Wapner RJ. Modeled fetal risk of genetic diseases identified by expanded carrier screening. JAMA. 2016;316(7):734-742.
Screening during pregnancy to determine if one or both parents are carriers of genetic disorders historically has involved testing for a limited number of conditions, such as cystic fibrosis, hemoglobinopathies, and Tay-Sachs disease. Patients usually are offered testing for 1 or 2 disorders, with test choices primarily based on patient race and ethnicity. Unfortunately, ancestry-based screening may result in inequitable distribution of genetic testing and resources, as it has significant limitations in our increasingly multicultural society, which includes many people of uncertain or mixed race and ethnicity.
Advantages of expanded carrier screening
Several commercial laboratories now offer ECS. Haque and colleagues used data from one of these laboratories and modeled the predicted number of potentially affected fetuses that would be identified with traditional, ethnicity-based screening as compared with ECS. In one of their hypothetical cohorts, of Northern European couples, traditional screening would identify 55 affected fetuses per 100,000 (1 in 1,800), and ECS would identify 159 per 100,000 (almost 3 times more). The numbers identified with ECS varied with race or ethnicity and ranged from 94 per 100,000 (about 1 in 1,000) for Hispanic couples to 392 per 100,000 (about 1 in 250) for Ashkenazi Jewish couples.
In Australia, Archibald and colleagues conducted a similar study, of panethnic screening of 12,000 women for cystic fibrosis, fragile X syndrome, and spinal muscular atrophy.1 The number of affected fetuses identified was about 1 per 1,000 screened couples--not much different from the ECS number, though comparison is difficult given the likely very different racial and ethnic backgrounds of the 2 cohorts.
Although these data suggest ECS increases detection of genetic disorders, and it seems almost self-evident that more screening is better, there are concerns about ECS.2 Traditional carrier screening methods focus on conditions that significantly affect quality of life--owing to cognitive or physical disabilities or required lifelong medical therapies--and that have a fetal, neonatal, or early-childhood onset and well-defined phenotype. In ECS panels, additional conditions may vary significantly in severity or age of onset. Although some genetic variants on ECS panels have a consistent phenotype, the natural history of others is less well understood. Panels often include conditions for which carrier screening of the general population is not recommended by current guidelines--for example, hemochromatosis and factor V Leiden. Moreover, almost by definition, ECS panels include rare conditions for which the natural history may not be well understood, and the carrier frequency as well as the proportion of condition-causing variants that can be detected may be unclear, leaving the residual risk unknown.
This study provides additional information on the number and type of conditions that can be detected with ECS in different populations. Although ever larger panels can detect more conditions, the veracity of the results and the types of conditions detected are important considerations as providers and patients weigh the risks and benefits of this screening.
Read about the ideal expanded carrier screening panel.
The ideal expanded carrier screening panel
Stevens B, Krstic N, Jones M, Murphy L, Hoskovec J. Finding middle ground in constructing a clinically useful expanded carrier screening panel. Obstet Gynecol. 2017;130(2):279-284.
Both the American College of Obstetricians and Gynecologists (ACOG) and the American College of Medical Genetics and Genomics (ACMG) have proposed criteria for including specific disorders on ECS panels.3,4 These criteria consider disorder characteristics, such as carrier prevalence, which should be at least 1 in 100; severity; early-childhood onset; and complete penetrance. In addition, they consider test characteristics, such as sensitivity, which should be at least 70%.
Details of the study
Stevens and colleagues evaluated the ECS panels offered by 6 commercial laboratories in the United States. They found that only 27% of included conditions met the recommended criteria, and concluded that these panels are putting patients at risk for undue anxiety, and that time and money are being spent on follow-up testing for rare and mild conditions for which the benefits of testing are unclear or unlikely. The potential benefits of the extra screening should be weighed against the significant resulting harms.
Across the 6 ECS panels, 96 conditions met the criteria. As some laboratories allow providers to customize their panels, members of my practice, after reviewing this thought-provoking article, agreed we should create a custom panel that includes only these 96 conditions. Unfortunately, no commercial laboratory includes all 96 conditions, so it is not feasible to create an "ideal" panel at this time.
Arguments favoring ECS include its low cost and the efficiency of screening with multigene panels. In a 2013 study, however, 24% of patients were identified as carriers, and in most cases this finding led to screening for the reproductive partner as well.5 If the rate of detection of the disorder is low, the utility of screening with the same panel may be limited, and couples may require more extensive testing, such as gene sequencing, which is far more expensive. These findings and the additional testing also will increase the need for genetic counseling, and may lead to invasive prenatal diagnostic testing with further increases in costs. If counseling and prenatal testing yield improved outcomes--increased detection of important findings--the benefit will justify the higher costs. However, if the increased costs are largely generated chasing down and explaining findings that are not important to patients or providers, the costs may be incurred without benefit.
For practices that want to offer ECS, it is important to consider the type of conditions on a given laboratory's panel. Panels that include more conditions will detect at least one condition in more patients. As each positive test requires follow-up (typically partner testing), careful consideration should be given up-front to which test is used.
Read about the pregnant women’s perspectives on ECS.
Pregnant women's perspectives on expanded carrier screening
Propst L, Connor G, Hinton M, Poorvu T, Dungan J. Pregnant women's perspectives on expanded carrier screening [published online February 23, 2018]. J Genet Couns. doi:10.1007/s10897-018-0232-x.
Although several authors have discussed ECS detection rates, less has been reported on how women perceive ECS or how they elect or decline screening. Studies have found that the decision to undergo screening for cystic fibrosis is influenced by factors that include age, sex, ethnicity, socioeconomic status, lack of family history, cost, fear of a blood test, lack of knowledge about the condition, already having children, wanting to avoid having a disabled child, abortion preferences, and feeling pressured by health care providers.6,7 Propst and colleagues asked women for their perspectives on ECS, on electing or declining screening, and on any anxiety associated with their decision.
Details of the study
Women who declined ECS said they did so because they:
- had no family history
- knew there was a very small chance their partner carried the same condition
- would not change the course of their pregnancy on the basis of the test results.
Women who elected ECS said they did so because they wanted to:
- know their risk of having a child with a genetic condition
- have all available information about their genetic risks
- be able to make decisions about continuing or terminating their pregnancy.
Women also were asked what they would do if they discovered their fetus had a genetic disorder. About 42% said they were unsure what they would do, 34% said they would continue their pregnancy and prepare for the birth of an affected child, and 24% said they likely would terminate their pregnancy.
The most common reason women gave for declining ECS was that they had no family history. However, ECS is not a good option for women with a positive family history, as they need genetic counseling and specific consideration of their own risks and what testing should be done. The majority of couples who have a child with a genetic disease have no other family history of the disorder. In a study of reproductive carrier screening in Australia, 88% of carriers had no family history.1 Careful pretest counseling is needed to explain the distinction between, on one hand, genetic counseling and testing for those with a family history of genetic disease and, on the other hand, population screening performed to identify unsuspecting individuals who are healthy carriers of genetic disorders.
Another crucial point about carrier screening is the need to consider how its results will be used, and what options the carrier couple will have. For women who are pregnant when a risk is identified, options include expectant management, with diagnosis after birth, or prenatal diagnosis with termination of an affected fetus, out-adoption of an affected fetus, or expectant management with preparation for caring for an affected child. For women who are not pregnant when they have ECS, additional options include use of a gamete (ovum or sperm) donor to achieve pregnancy, or preimplantation genetic diagnosis with implantation of only unaffected embryos.
Different pregnant women may have very different preferences regarding genetic testing. Although many are unsure how they would proceed following the diagnosis of a fetal genetic disorder, it is important to carefully explain their options before any testing is done.
Read about the marketing of ECS.
Marketing of expanded carrier screening
Chokoshvili D, Borry P, Vears DF. A systematic analysis of online marketing materials used by providers of expanded carrier screening [published online December 14, 2017]. Genet Med. doi:10.1038/gim.2017.222.
Prenatal carrier screening can be helpful to women and their families, but it is also a high-volume, lucrative business, with many commercial laboratories competing for the growing ECS market. Professional medical societies recommend making all screening candidates aware of the purpose, characteristics, and limitations of the tests, and of the potential significance of their results. As becoming familiar and comfortable with the tests and explaining them to each patient can be time-consuming, and daunting, many busy clinicians have started relying on marketing materials and other information from the commercial laboratories. Therefore analysis of the accuracy of such materials is in order.
Details of the study
Chokoshvili and colleagues performed a systematic analysis of the quality and accuracy of online marketing materials for ECS. They identified 18 providers: 16 commercial laboratories and 2 medical services providers. All described ECS as a useful tool for family planning, and some were very directive in stating that this testing is "one of the most important steps in preparing for parenthood." In their materials, most of the companies cover some limitations, such as residual risk, but none of the commercial laboratories indicate that ECS can overestimate risk (many variants have incomplete penetrance, meaning that some individuals with a positive test result may in fact be asymptomatic throughout their lifetime).
In addition, whereas a large amount of the marketing materials implies the test was developed in line with professional recommendations, none in fact complies with ACOG and ACMG guidance. Finally, though some of the online information provided by laboratories can be helpful, it is important for clinicians to remember that reproductive genetic counseling should be nondirective and balanced. Carrier testing should be based on patient (not provider) values regarding reproductive autonomy.
Determining that a woman carries a genetic disorder in the preconception period allows more time to evaluate her reproductive partner. If both partners in the couple carry the same genetic disorder, there are more options available to avoid an affected pregnancy. These options include the use of an ovum or sperm donor, or use of preimplantation genetic diagnosis on embryos conceived through in vitro fertilization. While obstetric providers commonly offer carrier screening, and most women are only screened during pregnancy, such genetic testing should be part of pregnancy planning. When gyn providers see patients who are considering a pregnancy, he or she should discuss the options of expanded carrier screening, or ethnicity-based screening.
Summary
ECS increasingly is being adopted into clinical practice. According to ACOG, traditional ethnicity-based screening, panethnic screening (the same limited panel of tests for all patients), and ECS are all acceptable alternatives for prenatal carrier screening.3 For providers who offer ECS, it is important to have a good understanding of each selected test and its limitations. Providers should have a plan for following up patients who have positive test results; this plan may include having genetic counseling and prenatal genetic diagnostic testing in place. Although treatment is available for a few genetic conditions, for the large majority, prenatal screening has not been proved to lead to improved therapeutic options. Providers should try to make sure that patients do not have unrealistic expectations of the outcomes of carrier screening.
Laboratories' educational materials can be useful, but clinicians must carefully assess them before recommending them to patients. Some commercial laboratory information is helpful and balanced; other information is directive or even coercive. Nonbiased information on prenatal genetic testing, for both patients and clinicians, is available in the Genetic Education Modules offered by the Perinatal Quality Foundation (https://www.perinatalquality.org).
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- Archibald AD, Smith MJ, Burgess T, et al. Reproductive genetic carrier screening for cystic fibrosis, fragile X syndrome, and spinal muscular atrophy in Australia: outcomes of 12,000 tests [published online October 26, 2017; published correction appears in Genet Med. 2018. doi:10.1038/gim.2017.266]. Genet Med. doi:10.1038/gim.2017.134.
- Edwards JG, Feldman G, Goldberg J, et al. Expanded carrier screening in reproductive medicine—points to consider: a joint statement of the American College of Medical Genetics and Genomics, American College of Obstetricians and Gynecologists, National Society of Genetic Counselors, Perinatal Quality Foundation, and Society for Maternal-Fetal Medicine. Obstet Gynecol. 2015;125(3):653–662.
- Committee on Genetics. Committee opinion no. 690: carrier screening in the age of genomic medicine. Obstet Gynecol. 2017;129(3):e35–e40.
- Grody WW, Thompson BH, Gregg AR, et al. ACMG position statement on prenatal/preconception expanded carrier screening. Genet Med. 2013;15(6):482–483.
- Lazarin GA, Haque IS, Nazareth S, et al. An empirical estimate of carrier frequencies for 400+ causal Mendelian variants: results from an ethnically diverse clinical sample of 23,453 individuals. Genet Med. 2013;15(3):178–186.
- Chen LS, Goodson P. Factors affecting decisions to accept or decline cystic fibrosis carrier testing/screening: a theory-guided systematic review. Genet Med. 2007;9(7):442–450.
- Ioannou L, McClaren BJ, Massie J, et al. Population-based carrier screening for cystic fibrosis: a systematic review of 23 years of research. Genet Med. 2014;16(3):207-216.
Prenatal care has long included carrier screening for genetic diseases, such as cystic fibrosis and Tay-Sachs disease. Recently, advances in genetics technologies led to the development of multiplex panels that can be used to test for hundreds of genetic disorders simultaneously, and can be used to assess carrier status for expectant couples or those planning a pregnancy. Although such screening covers many more conditions than those recommended in traditional guidelines, the benefit of expanded carrier screening (ECS) over standard gene-by-gene testing is not clear.
In this Update, I review recent ECS research that can be helpful to those who practice reproductive endocrinology and infertility medicine, maternal–fetal medicine, and general ObGyn. This research considered some of the many complexities of ECS:
- number and type of severe autosomal recessive conditions identified by an ECS panel, or by panethnic screening for 3 common conditions (cystic fibrosis, fragile X syndrome, spinal muscular atrophy)
- whether the disorders covered by ECS panels meet recommended criteria regarding severity, prevalence, and test accuracy
- women’s thoughts and perspectives on ECS
- whether the marketing materials disseminated by commercial providers of ECS are accurate and balanced.
Genetic diseases identified by expanded carrier screening
Haque IS, Lazarin GA, Kang HP, Evans EA, Goldberg JD, Wapner RJ. Modeled fetal risk of genetic diseases identified by expanded carrier screening. JAMA. 2016;316(7):734-742.
Screening during pregnancy to determine if one or both parents are carriers of genetic disorders historically has involved testing for a limited number of conditions, such as cystic fibrosis, hemoglobinopathies, and Tay-Sachs disease. Patients usually are offered testing for 1 or 2 disorders, with test choices primarily based on patient race and ethnicity. Unfortunately, ancestry-based screening may result in inequitable distribution of genetic testing and resources, as it has significant limitations in our increasingly multicultural society, which includes many people of uncertain or mixed race and ethnicity.
Advantages of expanded carrier screening
Several commercial laboratories now offer ECS. Haque and colleagues used data from one of these laboratories and modeled the predicted number of potentially affected fetuses that would be identified with traditional, ethnicity-based screening as compared with ECS. In one of their hypothetical cohorts, of Northern European couples, traditional screening would identify 55 affected fetuses per 100,000 (1 in 1,800), and ECS would identify 159 per 100,000 (almost 3 times more). The numbers identified with ECS varied with race or ethnicity and ranged from 94 per 100,000 (about 1 in 1,000) for Hispanic couples to 392 per 100,000 (about 1 in 250) for Ashkenazi Jewish couples.
In Australia, Archibald and colleagues conducted a similar study, of panethnic screening of 12,000 women for cystic fibrosis, fragile X syndrome, and spinal muscular atrophy.1 The number of affected fetuses identified was about 1 per 1,000 screened couples--not much different from the ECS number, though comparison is difficult given the likely very different racial and ethnic backgrounds of the 2 cohorts.
Although these data suggest ECS increases detection of genetic disorders, and it seems almost self-evident that more screening is better, there are concerns about ECS.2 Traditional carrier screening methods focus on conditions that significantly affect quality of life--owing to cognitive or physical disabilities or required lifelong medical therapies--and that have a fetal, neonatal, or early-childhood onset and well-defined phenotype. In ECS panels, additional conditions may vary significantly in severity or age of onset. Although some genetic variants on ECS panels have a consistent phenotype, the natural history of others is less well understood. Panels often include conditions for which carrier screening of the general population is not recommended by current guidelines--for example, hemochromatosis and factor V Leiden. Moreover, almost by definition, ECS panels include rare conditions for which the natural history may not be well understood, and the carrier frequency as well as the proportion of condition-causing variants that can be detected may be unclear, leaving the residual risk unknown.
This study provides additional information on the number and type of conditions that can be detected with ECS in different populations. Although ever larger panels can detect more conditions, the veracity of the results and the types of conditions detected are important considerations as providers and patients weigh the risks and benefits of this screening.
Read about the ideal expanded carrier screening panel.
The ideal expanded carrier screening panel
Stevens B, Krstic N, Jones M, Murphy L, Hoskovec J. Finding middle ground in constructing a clinically useful expanded carrier screening panel. Obstet Gynecol. 2017;130(2):279-284.
Both the American College of Obstetricians and Gynecologists (ACOG) and the American College of Medical Genetics and Genomics (ACMG) have proposed criteria for including specific disorders on ECS panels.3,4 These criteria consider disorder characteristics, such as carrier prevalence, which should be at least 1 in 100; severity; early-childhood onset; and complete penetrance. In addition, they consider test characteristics, such as sensitivity, which should be at least 70%.
Details of the study
Stevens and colleagues evaluated the ECS panels offered by 6 commercial laboratories in the United States. They found that only 27% of included conditions met the recommended criteria, and concluded that these panels are putting patients at risk for undue anxiety, and that time and money are being spent on follow-up testing for rare and mild conditions for which the benefits of testing are unclear or unlikely. The potential benefits of the extra screening should be weighed against the significant resulting harms.
Across the 6 ECS panels, 96 conditions met the criteria. As some laboratories allow providers to customize their panels, members of my practice, after reviewing this thought-provoking article, agreed we should create a custom panel that includes only these 96 conditions. Unfortunately, no commercial laboratory includes all 96 conditions, so it is not feasible to create an "ideal" panel at this time.
Arguments favoring ECS include its low cost and the efficiency of screening with multigene panels. In a 2013 study, however, 24% of patients were identified as carriers, and in most cases this finding led to screening for the reproductive partner as well.5 If the rate of detection of the disorder is low, the utility of screening with the same panel may be limited, and couples may require more extensive testing, such as gene sequencing, which is far more expensive. These findings and the additional testing also will increase the need for genetic counseling, and may lead to invasive prenatal diagnostic testing with further increases in costs. If counseling and prenatal testing yield improved outcomes--increased detection of important findings--the benefit will justify the higher costs. However, if the increased costs are largely generated chasing down and explaining findings that are not important to patients or providers, the costs may be incurred without benefit.
For practices that want to offer ECS, it is important to consider the type of conditions on a given laboratory's panel. Panels that include more conditions will detect at least one condition in more patients. As each positive test requires follow-up (typically partner testing), careful consideration should be given up-front to which test is used.
Read about the pregnant women’s perspectives on ECS.
Pregnant women's perspectives on expanded carrier screening
Propst L, Connor G, Hinton M, Poorvu T, Dungan J. Pregnant women's perspectives on expanded carrier screening [published online February 23, 2018]. J Genet Couns. doi:10.1007/s10897-018-0232-x.
Although several authors have discussed ECS detection rates, less has been reported on how women perceive ECS or how they elect or decline screening. Studies have found that the decision to undergo screening for cystic fibrosis is influenced by factors that include age, sex, ethnicity, socioeconomic status, lack of family history, cost, fear of a blood test, lack of knowledge about the condition, already having children, wanting to avoid having a disabled child, abortion preferences, and feeling pressured by health care providers.6,7 Propst and colleagues asked women for their perspectives on ECS, on electing or declining screening, and on any anxiety associated with their decision.
Details of the study
Women who declined ECS said they did so because they:
- had no family history
- knew there was a very small chance their partner carried the same condition
- would not change the course of their pregnancy on the basis of the test results.
Women who elected ECS said they did so because they wanted to:
- know their risk of having a child with a genetic condition
- have all available information about their genetic risks
- be able to make decisions about continuing or terminating their pregnancy.
Women also were asked what they would do if they discovered their fetus had a genetic disorder. About 42% said they were unsure what they would do, 34% said they would continue their pregnancy and prepare for the birth of an affected child, and 24% said they likely would terminate their pregnancy.
The most common reason women gave for declining ECS was that they had no family history. However, ECS is not a good option for women with a positive family history, as they need genetic counseling and specific consideration of their own risks and what testing should be done. The majority of couples who have a child with a genetic disease have no other family history of the disorder. In a study of reproductive carrier screening in Australia, 88% of carriers had no family history.1 Careful pretest counseling is needed to explain the distinction between, on one hand, genetic counseling and testing for those with a family history of genetic disease and, on the other hand, population screening performed to identify unsuspecting individuals who are healthy carriers of genetic disorders.
Another crucial point about carrier screening is the need to consider how its results will be used, and what options the carrier couple will have. For women who are pregnant when a risk is identified, options include expectant management, with diagnosis after birth, or prenatal diagnosis with termination of an affected fetus, out-adoption of an affected fetus, or expectant management with preparation for caring for an affected child. For women who are not pregnant when they have ECS, additional options include use of a gamete (ovum or sperm) donor to achieve pregnancy, or preimplantation genetic diagnosis with implantation of only unaffected embryos.
Different pregnant women may have very different preferences regarding genetic testing. Although many are unsure how they would proceed following the diagnosis of a fetal genetic disorder, it is important to carefully explain their options before any testing is done.
Read about the marketing of ECS.
Marketing of expanded carrier screening
Chokoshvili D, Borry P, Vears DF. A systematic analysis of online marketing materials used by providers of expanded carrier screening [published online December 14, 2017]. Genet Med. doi:10.1038/gim.2017.222.
Prenatal carrier screening can be helpful to women and their families, but it is also a high-volume, lucrative business, with many commercial laboratories competing for the growing ECS market. Professional medical societies recommend making all screening candidates aware of the purpose, characteristics, and limitations of the tests, and of the potential significance of their results. As becoming familiar and comfortable with the tests and explaining them to each patient can be time-consuming, and daunting, many busy clinicians have started relying on marketing materials and other information from the commercial laboratories. Therefore analysis of the accuracy of such materials is in order.
Details of the study
Chokoshvili and colleagues performed a systematic analysis of the quality and accuracy of online marketing materials for ECS. They identified 18 providers: 16 commercial laboratories and 2 medical services providers. All described ECS as a useful tool for family planning, and some were very directive in stating that this testing is "one of the most important steps in preparing for parenthood." In their materials, most of the companies cover some limitations, such as residual risk, but none of the commercial laboratories indicate that ECS can overestimate risk (many variants have incomplete penetrance, meaning that some individuals with a positive test result may in fact be asymptomatic throughout their lifetime).
In addition, whereas a large amount of the marketing materials implies the test was developed in line with professional recommendations, none in fact complies with ACOG and ACMG guidance. Finally, though some of the online information provided by laboratories can be helpful, it is important for clinicians to remember that reproductive genetic counseling should be nondirective and balanced. Carrier testing should be based on patient (not provider) values regarding reproductive autonomy.
Determining that a woman carries a genetic disorder in the preconception period allows more time to evaluate her reproductive partner. If both partners in the couple carry the same genetic disorder, there are more options available to avoid an affected pregnancy. These options include the use of an ovum or sperm donor, or use of preimplantation genetic diagnosis on embryos conceived through in vitro fertilization. While obstetric providers commonly offer carrier screening, and most women are only screened during pregnancy, such genetic testing should be part of pregnancy planning. When gyn providers see patients who are considering a pregnancy, he or she should discuss the options of expanded carrier screening, or ethnicity-based screening.
Summary
ECS increasingly is being adopted into clinical practice. According to ACOG, traditional ethnicity-based screening, panethnic screening (the same limited panel of tests for all patients), and ECS are all acceptable alternatives for prenatal carrier screening.3 For providers who offer ECS, it is important to have a good understanding of each selected test and its limitations. Providers should have a plan for following up patients who have positive test results; this plan may include having genetic counseling and prenatal genetic diagnostic testing in place. Although treatment is available for a few genetic conditions, for the large majority, prenatal screening has not been proved to lead to improved therapeutic options. Providers should try to make sure that patients do not have unrealistic expectations of the outcomes of carrier screening.
Laboratories' educational materials can be useful, but clinicians must carefully assess them before recommending them to patients. Some commercial laboratory information is helpful and balanced; other information is directive or even coercive. Nonbiased information on prenatal genetic testing, for both patients and clinicians, is available in the Genetic Education Modules offered by the Perinatal Quality Foundation (https://www.perinatalquality.org).
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
Prenatal care has long included carrier screening for genetic diseases, such as cystic fibrosis and Tay-Sachs disease. Recently, advances in genetics technologies led to the development of multiplex panels that can be used to test for hundreds of genetic disorders simultaneously, and can be used to assess carrier status for expectant couples or those planning a pregnancy. Although such screening covers many more conditions than those recommended in traditional guidelines, the benefit of expanded carrier screening (ECS) over standard gene-by-gene testing is not clear.
In this Update, I review recent ECS research that can be helpful to those who practice reproductive endocrinology and infertility medicine, maternal–fetal medicine, and general ObGyn. This research considered some of the many complexities of ECS:
- number and type of severe autosomal recessive conditions identified by an ECS panel, or by panethnic screening for 3 common conditions (cystic fibrosis, fragile X syndrome, spinal muscular atrophy)
- whether the disorders covered by ECS panels meet recommended criteria regarding severity, prevalence, and test accuracy
- women’s thoughts and perspectives on ECS
- whether the marketing materials disseminated by commercial providers of ECS are accurate and balanced.
Genetic diseases identified by expanded carrier screening
Haque IS, Lazarin GA, Kang HP, Evans EA, Goldberg JD, Wapner RJ. Modeled fetal risk of genetic diseases identified by expanded carrier screening. JAMA. 2016;316(7):734-742.
Screening during pregnancy to determine if one or both parents are carriers of genetic disorders historically has involved testing for a limited number of conditions, such as cystic fibrosis, hemoglobinopathies, and Tay-Sachs disease. Patients usually are offered testing for 1 or 2 disorders, with test choices primarily based on patient race and ethnicity. Unfortunately, ancestry-based screening may result in inequitable distribution of genetic testing and resources, as it has significant limitations in our increasingly multicultural society, which includes many people of uncertain or mixed race and ethnicity.
Advantages of expanded carrier screening
Several commercial laboratories now offer ECS. Haque and colleagues used data from one of these laboratories and modeled the predicted number of potentially affected fetuses that would be identified with traditional, ethnicity-based screening as compared with ECS. In one of their hypothetical cohorts, of Northern European couples, traditional screening would identify 55 affected fetuses per 100,000 (1 in 1,800), and ECS would identify 159 per 100,000 (almost 3 times more). The numbers identified with ECS varied with race or ethnicity and ranged from 94 per 100,000 (about 1 in 1,000) for Hispanic couples to 392 per 100,000 (about 1 in 250) for Ashkenazi Jewish couples.
In Australia, Archibald and colleagues conducted a similar study, of panethnic screening of 12,000 women for cystic fibrosis, fragile X syndrome, and spinal muscular atrophy.1 The number of affected fetuses identified was about 1 per 1,000 screened couples--not much different from the ECS number, though comparison is difficult given the likely very different racial and ethnic backgrounds of the 2 cohorts.
Although these data suggest ECS increases detection of genetic disorders, and it seems almost self-evident that more screening is better, there are concerns about ECS.2 Traditional carrier screening methods focus on conditions that significantly affect quality of life--owing to cognitive or physical disabilities or required lifelong medical therapies--and that have a fetal, neonatal, or early-childhood onset and well-defined phenotype. In ECS panels, additional conditions may vary significantly in severity or age of onset. Although some genetic variants on ECS panels have a consistent phenotype, the natural history of others is less well understood. Panels often include conditions for which carrier screening of the general population is not recommended by current guidelines--for example, hemochromatosis and factor V Leiden. Moreover, almost by definition, ECS panels include rare conditions for which the natural history may not be well understood, and the carrier frequency as well as the proportion of condition-causing variants that can be detected may be unclear, leaving the residual risk unknown.
This study provides additional information on the number and type of conditions that can be detected with ECS in different populations. Although ever larger panels can detect more conditions, the veracity of the results and the types of conditions detected are important considerations as providers and patients weigh the risks and benefits of this screening.
Read about the ideal expanded carrier screening panel.
The ideal expanded carrier screening panel
Stevens B, Krstic N, Jones M, Murphy L, Hoskovec J. Finding middle ground in constructing a clinically useful expanded carrier screening panel. Obstet Gynecol. 2017;130(2):279-284.
Both the American College of Obstetricians and Gynecologists (ACOG) and the American College of Medical Genetics and Genomics (ACMG) have proposed criteria for including specific disorders on ECS panels.3,4 These criteria consider disorder characteristics, such as carrier prevalence, which should be at least 1 in 100; severity; early-childhood onset; and complete penetrance. In addition, they consider test characteristics, such as sensitivity, which should be at least 70%.
Details of the study
Stevens and colleagues evaluated the ECS panels offered by 6 commercial laboratories in the United States. They found that only 27% of included conditions met the recommended criteria, and concluded that these panels are putting patients at risk for undue anxiety, and that time and money are being spent on follow-up testing for rare and mild conditions for which the benefits of testing are unclear or unlikely. The potential benefits of the extra screening should be weighed against the significant resulting harms.
Across the 6 ECS panels, 96 conditions met the criteria. As some laboratories allow providers to customize their panels, members of my practice, after reviewing this thought-provoking article, agreed we should create a custom panel that includes only these 96 conditions. Unfortunately, no commercial laboratory includes all 96 conditions, so it is not feasible to create an "ideal" panel at this time.
Arguments favoring ECS include its low cost and the efficiency of screening with multigene panels. In a 2013 study, however, 24% of patients were identified as carriers, and in most cases this finding led to screening for the reproductive partner as well.5 If the rate of detection of the disorder is low, the utility of screening with the same panel may be limited, and couples may require more extensive testing, such as gene sequencing, which is far more expensive. These findings and the additional testing also will increase the need for genetic counseling, and may lead to invasive prenatal diagnostic testing with further increases in costs. If counseling and prenatal testing yield improved outcomes--increased detection of important findings--the benefit will justify the higher costs. However, if the increased costs are largely generated chasing down and explaining findings that are not important to patients or providers, the costs may be incurred without benefit.
For practices that want to offer ECS, it is important to consider the type of conditions on a given laboratory's panel. Panels that include more conditions will detect at least one condition in more patients. As each positive test requires follow-up (typically partner testing), careful consideration should be given up-front to which test is used.
Read about the pregnant women’s perspectives on ECS.
Pregnant women's perspectives on expanded carrier screening
Propst L, Connor G, Hinton M, Poorvu T, Dungan J. Pregnant women's perspectives on expanded carrier screening [published online February 23, 2018]. J Genet Couns. doi:10.1007/s10897-018-0232-x.
Although several authors have discussed ECS detection rates, less has been reported on how women perceive ECS or how they elect or decline screening. Studies have found that the decision to undergo screening for cystic fibrosis is influenced by factors that include age, sex, ethnicity, socioeconomic status, lack of family history, cost, fear of a blood test, lack of knowledge about the condition, already having children, wanting to avoid having a disabled child, abortion preferences, and feeling pressured by health care providers.6,7 Propst and colleagues asked women for their perspectives on ECS, on electing or declining screening, and on any anxiety associated with their decision.
Details of the study
Women who declined ECS said they did so because they:
- had no family history
- knew there was a very small chance their partner carried the same condition
- would not change the course of their pregnancy on the basis of the test results.
Women who elected ECS said they did so because they wanted to:
- know their risk of having a child with a genetic condition
- have all available information about their genetic risks
- be able to make decisions about continuing or terminating their pregnancy.
Women also were asked what they would do if they discovered their fetus had a genetic disorder. About 42% said they were unsure what they would do, 34% said they would continue their pregnancy and prepare for the birth of an affected child, and 24% said they likely would terminate their pregnancy.
The most common reason women gave for declining ECS was that they had no family history. However, ECS is not a good option for women with a positive family history, as they need genetic counseling and specific consideration of their own risks and what testing should be done. The majority of couples who have a child with a genetic disease have no other family history of the disorder. In a study of reproductive carrier screening in Australia, 88% of carriers had no family history.1 Careful pretest counseling is needed to explain the distinction between, on one hand, genetic counseling and testing for those with a family history of genetic disease and, on the other hand, population screening performed to identify unsuspecting individuals who are healthy carriers of genetic disorders.
Another crucial point about carrier screening is the need to consider how its results will be used, and what options the carrier couple will have. For women who are pregnant when a risk is identified, options include expectant management, with diagnosis after birth, or prenatal diagnosis with termination of an affected fetus, out-adoption of an affected fetus, or expectant management with preparation for caring for an affected child. For women who are not pregnant when they have ECS, additional options include use of a gamete (ovum or sperm) donor to achieve pregnancy, or preimplantation genetic diagnosis with implantation of only unaffected embryos.
Different pregnant women may have very different preferences regarding genetic testing. Although many are unsure how they would proceed following the diagnosis of a fetal genetic disorder, it is important to carefully explain their options before any testing is done.
Read about the marketing of ECS.
Marketing of expanded carrier screening
Chokoshvili D, Borry P, Vears DF. A systematic analysis of online marketing materials used by providers of expanded carrier screening [published online December 14, 2017]. Genet Med. doi:10.1038/gim.2017.222.
Prenatal carrier screening can be helpful to women and their families, but it is also a high-volume, lucrative business, with many commercial laboratories competing for the growing ECS market. Professional medical societies recommend making all screening candidates aware of the purpose, characteristics, and limitations of the tests, and of the potential significance of their results. As becoming familiar and comfortable with the tests and explaining them to each patient can be time-consuming, and daunting, many busy clinicians have started relying on marketing materials and other information from the commercial laboratories. Therefore analysis of the accuracy of such materials is in order.
Details of the study
Chokoshvili and colleagues performed a systematic analysis of the quality and accuracy of online marketing materials for ECS. They identified 18 providers: 16 commercial laboratories and 2 medical services providers. All described ECS as a useful tool for family planning, and some were very directive in stating that this testing is "one of the most important steps in preparing for parenthood." In their materials, most of the companies cover some limitations, such as residual risk, but none of the commercial laboratories indicate that ECS can overestimate risk (many variants have incomplete penetrance, meaning that some individuals with a positive test result may in fact be asymptomatic throughout their lifetime).
In addition, whereas a large amount of the marketing materials implies the test was developed in line with professional recommendations, none in fact complies with ACOG and ACMG guidance. Finally, though some of the online information provided by laboratories can be helpful, it is important for clinicians to remember that reproductive genetic counseling should be nondirective and balanced. Carrier testing should be based on patient (not provider) values regarding reproductive autonomy.
Determining that a woman carries a genetic disorder in the preconception period allows more time to evaluate her reproductive partner. If both partners in the couple carry the same genetic disorder, there are more options available to avoid an affected pregnancy. These options include the use of an ovum or sperm donor, or use of preimplantation genetic diagnosis on embryos conceived through in vitro fertilization. While obstetric providers commonly offer carrier screening, and most women are only screened during pregnancy, such genetic testing should be part of pregnancy planning. When gyn providers see patients who are considering a pregnancy, he or she should discuss the options of expanded carrier screening, or ethnicity-based screening.
Summary
ECS increasingly is being adopted into clinical practice. According to ACOG, traditional ethnicity-based screening, panethnic screening (the same limited panel of tests for all patients), and ECS are all acceptable alternatives for prenatal carrier screening.3 For providers who offer ECS, it is important to have a good understanding of each selected test and its limitations. Providers should have a plan for following up patients who have positive test results; this plan may include having genetic counseling and prenatal genetic diagnostic testing in place. Although treatment is available for a few genetic conditions, for the large majority, prenatal screening has not been proved to lead to improved therapeutic options. Providers should try to make sure that patients do not have unrealistic expectations of the outcomes of carrier screening.
Laboratories' educational materials can be useful, but clinicians must carefully assess them before recommending them to patients. Some commercial laboratory information is helpful and balanced; other information is directive or even coercive. Nonbiased information on prenatal genetic testing, for both patients and clinicians, is available in the Genetic Education Modules offered by the Perinatal Quality Foundation (https://www.perinatalquality.org).
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- Archibald AD, Smith MJ, Burgess T, et al. Reproductive genetic carrier screening for cystic fibrosis, fragile X syndrome, and spinal muscular atrophy in Australia: outcomes of 12,000 tests [published online October 26, 2017; published correction appears in Genet Med. 2018. doi:10.1038/gim.2017.266]. Genet Med. doi:10.1038/gim.2017.134.
- Edwards JG, Feldman G, Goldberg J, et al. Expanded carrier screening in reproductive medicine—points to consider: a joint statement of the American College of Medical Genetics and Genomics, American College of Obstetricians and Gynecologists, National Society of Genetic Counselors, Perinatal Quality Foundation, and Society for Maternal-Fetal Medicine. Obstet Gynecol. 2015;125(3):653–662.
- Committee on Genetics. Committee opinion no. 690: carrier screening in the age of genomic medicine. Obstet Gynecol. 2017;129(3):e35–e40.
- Grody WW, Thompson BH, Gregg AR, et al. ACMG position statement on prenatal/preconception expanded carrier screening. Genet Med. 2013;15(6):482–483.
- Lazarin GA, Haque IS, Nazareth S, et al. An empirical estimate of carrier frequencies for 400+ causal Mendelian variants: results from an ethnically diverse clinical sample of 23,453 individuals. Genet Med. 2013;15(3):178–186.
- Chen LS, Goodson P. Factors affecting decisions to accept or decline cystic fibrosis carrier testing/screening: a theory-guided systematic review. Genet Med. 2007;9(7):442–450.
- Ioannou L, McClaren BJ, Massie J, et al. Population-based carrier screening for cystic fibrosis: a systematic review of 23 years of research. Genet Med. 2014;16(3):207-216.
- Archibald AD, Smith MJ, Burgess T, et al. Reproductive genetic carrier screening for cystic fibrosis, fragile X syndrome, and spinal muscular atrophy in Australia: outcomes of 12,000 tests [published online October 26, 2017; published correction appears in Genet Med. 2018. doi:10.1038/gim.2017.266]. Genet Med. doi:10.1038/gim.2017.134.
- Edwards JG, Feldman G, Goldberg J, et al. Expanded carrier screening in reproductive medicine—points to consider: a joint statement of the American College of Medical Genetics and Genomics, American College of Obstetricians and Gynecologists, National Society of Genetic Counselors, Perinatal Quality Foundation, and Society for Maternal-Fetal Medicine. Obstet Gynecol. 2015;125(3):653–662.
- Committee on Genetics. Committee opinion no. 690: carrier screening in the age of genomic medicine. Obstet Gynecol. 2017;129(3):e35–e40.
- Grody WW, Thompson BH, Gregg AR, et al. ACMG position statement on prenatal/preconception expanded carrier screening. Genet Med. 2013;15(6):482–483.
- Lazarin GA, Haque IS, Nazareth S, et al. An empirical estimate of carrier frequencies for 400+ causal Mendelian variants: results from an ethnically diverse clinical sample of 23,453 individuals. Genet Med. 2013;15(3):178–186.
- Chen LS, Goodson P. Factors affecting decisions to accept or decline cystic fibrosis carrier testing/screening: a theory-guided systematic review. Genet Med. 2007;9(7):442–450.
- Ioannou L, McClaren BJ, Massie J, et al. Population-based carrier screening for cystic fibrosis: a systematic review of 23 years of research. Genet Med. 2014;16(3):207-216.