User login
Bipolar disorder: How to avoid overdiagnosis
Over the past decade, bipolar disorder (BD) has gained widespread recognition in mainstream culture and in the media,1 and awareness of this condition has increased substantially. As a result, patients commonly present with preconceived ideas about bipolarity that may or may not actually correspond with this diagnosis. In anticipation of seeing such patients, I offer 4 recommendations to help clinicians more accurately diagnose BD.
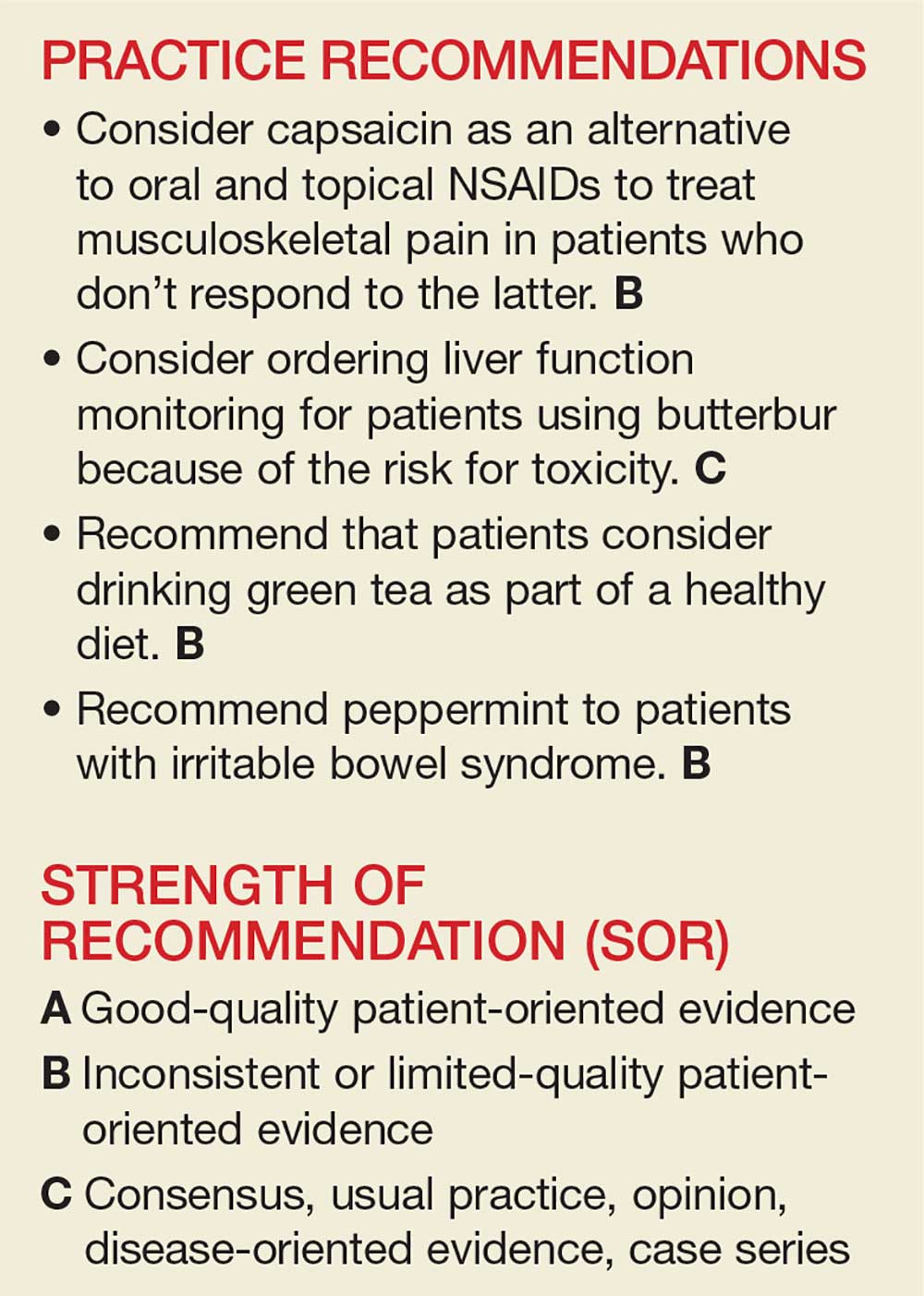
1. Screen for periods of manic or hypomanic mood. Effective screening questions include:
- “Have you ever had periods when you felt too happy, too angry, or on top of the world for several days in a row?”
- “Have you had periods when you would go several days without much sleep and still feel fine during the day?”
If the patient reports irritability rather than euphoria, try to better understand the phenomenology of his or her irritable mood. Among patients who experience mania, irritability often results from impatience, which in turn seems to be secondary to grandiosity, increased energy, and accelerated thought processes.2
2. Avoid using terms with low specificity, such as “mood swings” and “racing thoughts,” when you screen for manic symptoms. If the patient mentions these phrases, do not take them at face value; ask him or her to characterize them in detail. Differentiate chronic, quick fluctuations in affect—which are usually triggered by environmental factors and typically are reported by patients with personality disorders—from more persistent periods of mood polarization. Similarly, anxious patients commonly report having “racing thoughts.”
3. Distinguish patients who have a chronic, ongoing preoccupation with shopping from those who exhibit intermittent periods of excessive shopping and prodigality, which usually are associated with other manic symptoms.3 Spending money in excess is often cited as a classic symptom of mania or hypomania, but it may be an indicator of other conditions, such as compulsive buying.
4. Ask about any increases in goal-directed activity. This is a good way to identify true manic or hypomanic periods. Patients with anxiety or agitated depression may report an increase in psychomotor activity, but this is usually characterized more by restlessness and wandering, and not by a true increase in activity.
Consider a temporary diagnosis
When in doubt, it may be advisable to establish a temporary diagnosis of unspecified mood disorder, until you can learn more about the patient, obtain collateral information from family or friends, and request past medical records.
1. Ghouse AA, Sanches M, Zunta-Soares G, et al. Overdiagnosis of bipolar disorder: a critical analysis of the literature. Scientific World Journal. 2013;2013:297087. doi: 10.1155/2013/297087.
2. Carlat DJ. My favorite tips for sorting out diagnostic quandaries with bipolar disorder and adult attention-deficit hyperactivity disorder. Psychiatr Clin North Am. 2007;30(2):233-238.
3. Black DW. A review of compulsive buying disorder. World Psychiatry. 2007;6(1):14-18.
Over the past decade, bipolar disorder (BD) has gained widespread recognition in mainstream culture and in the media,1 and awareness of this condition has increased substantially. As a result, patients commonly present with preconceived ideas about bipolarity that may or may not actually correspond with this diagnosis. In anticipation of seeing such patients, I offer 4 recommendations to help clinicians more accurately diagnose BD.
1. Screen for periods of manic or hypomanic mood. Effective screening questions include:
- “Have you ever had periods when you felt too happy, too angry, or on top of the world for several days in a row?”
- “Have you had periods when you would go several days without much sleep and still feel fine during the day?”
If the patient reports irritability rather than euphoria, try to better understand the phenomenology of his or her irritable mood. Among patients who experience mania, irritability often results from impatience, which in turn seems to be secondary to grandiosity, increased energy, and accelerated thought processes.2
2. Avoid using terms with low specificity, such as “mood swings” and “racing thoughts,” when you screen for manic symptoms. If the patient mentions these phrases, do not take them at face value; ask him or her to characterize them in detail. Differentiate chronic, quick fluctuations in affect—which are usually triggered by environmental factors and typically are reported by patients with personality disorders—from more persistent periods of mood polarization. Similarly, anxious patients commonly report having “racing thoughts.”
3. Distinguish patients who have a chronic, ongoing preoccupation with shopping from those who exhibit intermittent periods of excessive shopping and prodigality, which usually are associated with other manic symptoms.3 Spending money in excess is often cited as a classic symptom of mania or hypomania, but it may be an indicator of other conditions, such as compulsive buying.
4. Ask about any increases in goal-directed activity. This is a good way to identify true manic or hypomanic periods. Patients with anxiety or agitated depression may report an increase in psychomotor activity, but this is usually characterized more by restlessness and wandering, and not by a true increase in activity.
Consider a temporary diagnosis
When in doubt, it may be advisable to establish a temporary diagnosis of unspecified mood disorder, until you can learn more about the patient, obtain collateral information from family or friends, and request past medical records.
Over the past decade, bipolar disorder (BD) has gained widespread recognition in mainstream culture and in the media,1 and awareness of this condition has increased substantially. As a result, patients commonly present with preconceived ideas about bipolarity that may or may not actually correspond with this diagnosis. In anticipation of seeing such patients, I offer 4 recommendations to help clinicians more accurately diagnose BD.
1. Screen for periods of manic or hypomanic mood. Effective screening questions include:
- “Have you ever had periods when you felt too happy, too angry, or on top of the world for several days in a row?”
- “Have you had periods when you would go several days without much sleep and still feel fine during the day?”
If the patient reports irritability rather than euphoria, try to better understand the phenomenology of his or her irritable mood. Among patients who experience mania, irritability often results from impatience, which in turn seems to be secondary to grandiosity, increased energy, and accelerated thought processes.2
2. Avoid using terms with low specificity, such as “mood swings” and “racing thoughts,” when you screen for manic symptoms. If the patient mentions these phrases, do not take them at face value; ask him or her to characterize them in detail. Differentiate chronic, quick fluctuations in affect—which are usually triggered by environmental factors and typically are reported by patients with personality disorders—from more persistent periods of mood polarization. Similarly, anxious patients commonly report having “racing thoughts.”
3. Distinguish patients who have a chronic, ongoing preoccupation with shopping from those who exhibit intermittent periods of excessive shopping and prodigality, which usually are associated with other manic symptoms.3 Spending money in excess is often cited as a classic symptom of mania or hypomania, but it may be an indicator of other conditions, such as compulsive buying.
4. Ask about any increases in goal-directed activity. This is a good way to identify true manic or hypomanic periods. Patients with anxiety or agitated depression may report an increase in psychomotor activity, but this is usually characterized more by restlessness and wandering, and not by a true increase in activity.
Consider a temporary diagnosis
When in doubt, it may be advisable to establish a temporary diagnosis of unspecified mood disorder, until you can learn more about the patient, obtain collateral information from family or friends, and request past medical records.
1. Ghouse AA, Sanches M, Zunta-Soares G, et al. Overdiagnosis of bipolar disorder: a critical analysis of the literature. Scientific World Journal. 2013;2013:297087. doi: 10.1155/2013/297087.
2. Carlat DJ. My favorite tips for sorting out diagnostic quandaries with bipolar disorder and adult attention-deficit hyperactivity disorder. Psychiatr Clin North Am. 2007;30(2):233-238.
3. Black DW. A review of compulsive buying disorder. World Psychiatry. 2007;6(1):14-18.
1. Ghouse AA, Sanches M, Zunta-Soares G, et al. Overdiagnosis of bipolar disorder: a critical analysis of the literature. Scientific World Journal. 2013;2013:297087. doi: 10.1155/2013/297087.
2. Carlat DJ. My favorite tips for sorting out diagnostic quandaries with bipolar disorder and adult attention-deficit hyperactivity disorder. Psychiatr Clin North Am. 2007;30(2):233-238.
3. Black DW. A review of compulsive buying disorder. World Psychiatry. 2007;6(1):14-18.
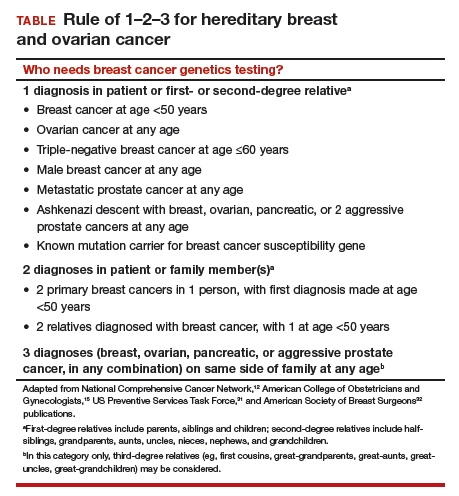
Who needs breast cancer genetics testing?
Advances in cancer genetics are rapidly changing how clinicians assess an individual’s risk for breast cancer. ObGyns counsel many women with a personal or family history of the disease, many of whom can benefit from genetics counseling and testing. As patients with a hereditary predisposition to breast cancer are at higher risk and are younger at diagnosis, it is imperative to identify them early so they can benefit from enhanced surveillance, chemoprevention, and discussions regarding risk-reducing surgeries. ObGyns are uniquely poised to identify young women at risk for hereditary cancer syndromes, and they play a crucial role in screening and prevention over the life span.
CASE Patient with breast cancer history asks about screening for her daughters
A 52-year-old woman presents for her annual examination. She underwent breast cancer treatment 10 years earlier and has done well since then. When asked about family history of breast cancer and ethnicity, she reports her mother had breast cancer later in life, and her mother’s father was of Ashkenazi Jewish ancestry.In addition, a maternal uncle had metastatic prostate cancer. You recall that breast cancer diagnosed before age 50 years and Ashkenazi ancestry are “red flags” for a hereditary cancer syndrome. The patient wonders how her daughters should be screened. What do you do next?
Having a risk assessment plan is crucial
Given increasing demands, limited time, and the abundance of information to be discussed with patients, primary care physicians may find it challenging to assess breast cancer risk, consider genetics testing for appropriate individuals, and counsel patients about risk management options. The process has become even more complex since the expansion in genetics knowledge and the advent of multigene panel testing. Not only is risk assessment crucial for this woman and her daughters, and for other patients, but a delay in diagnosing and treating breast cancer in patients with hereditary and familial cancer risks may represent a worrisome new trend in medical litigation.1,2 Clinicians must have a process in place for assessing risk in all patients and treating them appropriately.
The American Cancer Society (ACS) estimated that 252,710 cases of breast cancer would be diagnosed in 2017, leading to 40,610 deaths.3 Twelve percent to 14% of breast cancers are thought to be related to hereditary cancer predisposition syndromes.4–8 This means that, every year, almost 35,000 cases of breast cancer are attributable to hereditary risk. These cases can be detected early with enhanced surveillance, which carries the highest chance for cure, or prevented with risk-reducing surgery in identified genetic mutation carriers. Each child of a person with a genetic mutation predisposing to breast cancer has a 50% chance of inheriting the mutation and having a very high risk of cancer.
In this patient’s case, basic information is collected about her cancer-related personal and family history.
Asking a few key questions can help in stratifying risk:
- Have you or anyone in your family had cancer? What type, and at what age?
- If breast cancer, did it involve both breasts, or was it triple-negative?
- Is there a family history of ovarian cancer?
- Is there a family history of male breast cancer?
- Is there a family history of metastatic prostate cancer?
- Are you of Ashkenazi Jewish ethnicity?
- Have you or anyone in your family ever had genetics testing for cancer?
The hallmarks of hereditary cancer are multiple cancers in an individual or family; young age at diagnosis; and ovarian, pancreatic, or another rare cancer. Metastatic prostate cancer was added as a red flag for hereditary risk after a recent large series found that 11.8% of men with metastatic prostate cancer harbor germline mutations.9
CASE Continued
On further questioning, the patient reports she had triple-negative (estrogen receptor–, progesterone receptor–, and human epidermal growth factor receptor 2 [HER2]–negative) breast cancer, a feature of patients with germline BRCA1 (breast cancer susceptibility gene 1) mutations.10 In addition, her Ashkenazi ancestry is concerning, as there is a 1-in-40 chance of carrying 1 of the 3 Ashkenazi founder BRCA mutations.11 Is a genetics consultation needed?
Read about guidelines for referral and testing.
Guidelines for genetics referral and testing
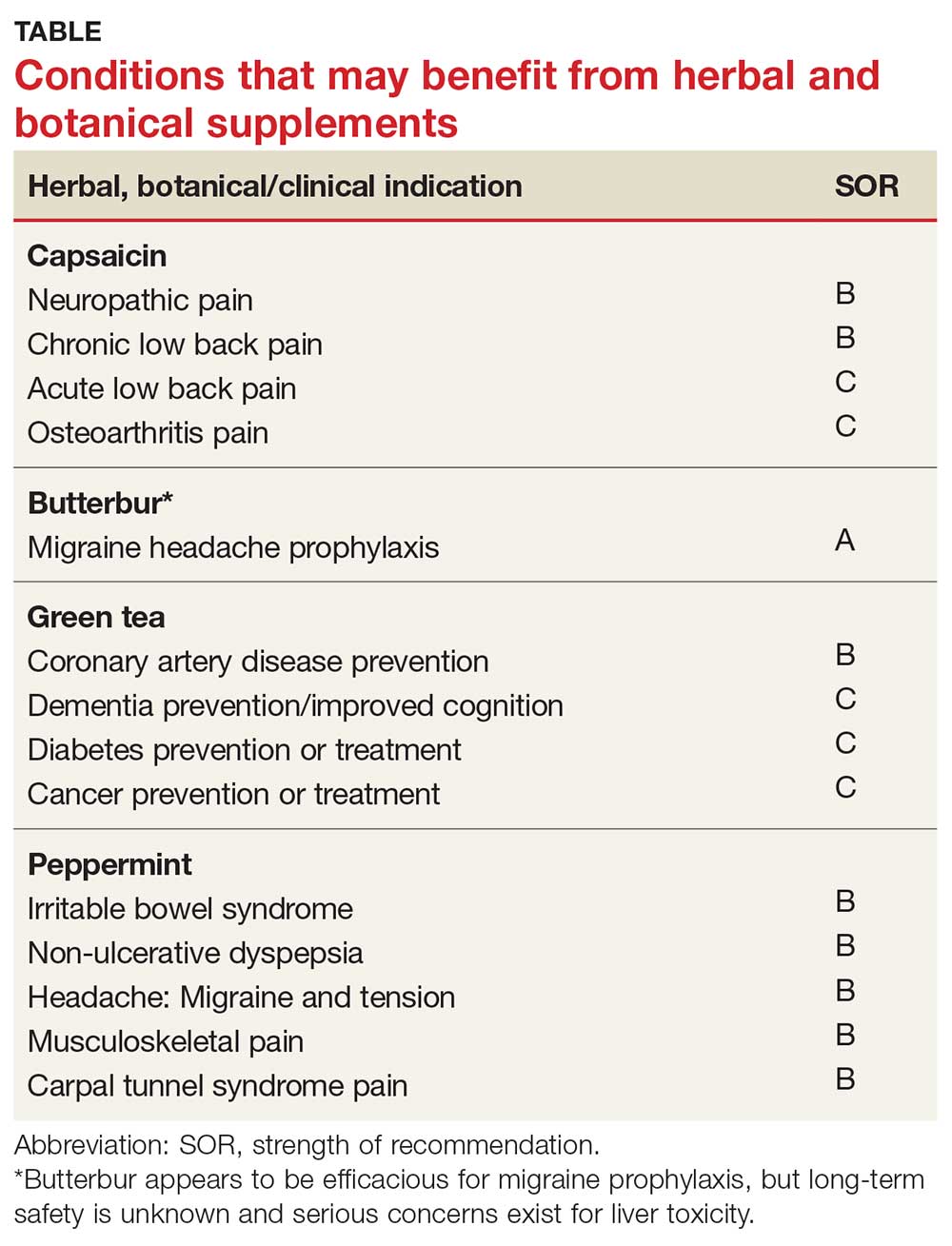
According to the TABLE, which summarizes national guidelines for genetics referral, maternal and paternal family histories are equally important. Our patient was under age 50 at diagnosis, has a history of triple-negative breast cancer, is of Ashkenazi ancestry, and has a family history of metastatic prostate cancer. She meets the criteria for genetics testing, and screening for her daughters most certainly will depend on the findings of that testing. If she carries a BRCA1 mutation, as might be anticipated, each daughter would have a 50% chance of having inherited the mutation. If they carry the mutation as well, they would begin breast magnetic resonance imaging (MRI) screening at age 25.12 If they decide against genetics testing, they could still undergo MRI screening as untested first-degree relatives of a BRCA carrier, per ACS recommendations.13
Integrating evidence and experience
Over the past 10 to 20 years, other breast cancer susceptibility genes (eg, BRCA2, PALB2, CHEK2) have been identified. More recently, next-generation sequencing has become commercially available. Laboratories can use this newer method to sequence multiple genes rapidly and in parallel, and its cost is similar to that of single-syndrome testing.14 When more than 1 gene can explain an inherited cancer syndrome, multigene panel testing may be more efficient and cost-effective. Use of multigene panel testing is supported in guidelines issued by the National Comprehensive Cancer Network,12 the American College of Obstetricians and Gynecologists,15 and other medical societies.
For our patient, the most logical strategy would be to test for the 3 mutations most common in the Ashkenazi population and then, if no mutation is found, perform multigene panel testing.
Formal genetics counseling can be very helpful for a patient, particularly in the era of multigene panel testing.16,17 A detailed pedigree (family tree) is elicited, and a genetics specialist determines whether testing is indicated and which test is best for the patient. Possible test findings are explained. The patient may be found to have a pathogenic variant with associated increased cancer risk, a negative test result (informative or uninformative), or a variant of uncertain significance (VUS). VUS is a gene mutation identified with an unknown effect on protein function and an unclear association with cancer risk. A finding of VUS may make the patient anxious,18 create uncertainty in the treating physician,19 and lead to harmful overtreatment, excessive surveillance, or unnecessary use of a preventive measure.19–21 Genetics counseling allows the patient, even the patient with VUS, to make appropriate decisions.22 Counseling may also help a patient or family process emotional responses, such as fear and guilt. In addition, counselors are familiar with relevant laws and regulations, such as the Genetic Information Nondiscrimination Act of 2008 (GINA), which protects patients from insurance and employment discrimination. Many professional guidelines recommend providing genetics counseling in conjunction with genetics testing,12,23 and some insurance companies and some states require counseling for coverage of testing.
Cost of genetics counseling. If patients are concerned about the cost of genetics testing, they can be reassured with the following information24–26:
- The Patient Protection and Affordable Care Act (ACA) identifies BRCA testing as a preventive service
- Medicare provides coverage for affected patients with a qualifying personal history
- 97% of commercial insurers and most state Medicaid programs provide coverage for hereditary cancer testing
- Most commercial laboratories have affordability programs that may provide additional support.
If a BRCA mutation is found: Many patients question the value of knowing whether they have a BRCA mutation. What our patient, her daughters, and others may not realize is that, if a BRCA mutation is found, breast MRI screening can begin at age 25. Although contrast-enhanced MRI screening is highly sensitive in detecting breast cancer,27–29 it lacks specificity and commonly yields false positives.
Some patients also worry about overdiagnosis with this highly sensitive test. Many do not realize that preventively prescribed oral contraceptives can reduce the risk of ovarian cancer by 50%, and cosmetically acceptable risk-reducing breast surgeries can reduce the risk by 90%.
Many are unaware of the associated risks with ovarian, prostate, pancreatic, and other cancers; of risk management options; and of assisted reproduction options, such as preimplantation genetics diagnosis, which can prevent the passing of a genetic mutation to future generations. The guidelines on risk management options are increasingly clear and helpful,12,30–32 and women often turn to their ObGyns for advice about health and prevention.
ObGyns are often the first-line providers for women with a personal or family history of breast cancer. Identification of at-risk patients begins with taking a careful family history and becoming familiar with the rapidly evolving guidelines in this important field. Identification of appropriate candidates for breast cancer genetics testing is a key step toward prevention, value-based care, and avoidance of legal liability.
CASE Resolved
In this case, testing for the 3 common Ashkenazi BRCA founder mutations was negative, and multigene panel testing was also negative. Her husband is not of Ashkenazi Jewish descent and there is no significant family history of cancer on his side. The daughters are advised to begin high-risk screening at the age of 32, 10 years earlier than their mother was diagnosed, but no genetic testing is indicated for them.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- Phillips RL Jr, Bartholomew LA, Dovey SM, Fryer GE Jr, Miyoshi TJ, Green LA. Learning from malpractice claims about negligent, adverse events in primary care in the United States. Qual Saf Health Care. 2004;13(2):121–126.
- Saber Tehrani AS, Lee H, Mathews SC, et al. 25-year summary of US malpractice claims for diagnostic errors 1986–2010: an analysis from the National Practitioner Data Bank. BMJ Qual Saf. 2013;22(8):672–680.
- American Cancer Society. Breast Cancer Facts & Figures 2017-2018. https://www.cancer.org/content/dam/cancer-org/research/cancer-facts-and-statistics/breast-cancer-factsand-figures/breast-cancer-facts-and-figures-2017-2018.pdf. Published 2017. Accessed December 28, 2017.
- Tung N, Battelli C, Allen B, et al. Frequency of mutations in individuals with breast cancer referred for BRCA1 and BRCA2 testing using next-generation sequencing with a 25-gene panel. Cancer. 2015;121(1):25–33.
- Tung N, Lin NU, Kidd J, et al. Frequency of germline mutations in 25 cancer susceptibility genes in a sequential series of patients with breast cancer. J Clin Oncol. 2016;34(13):1460–1468.
- Kurian AW, Hare EE, Mills MA, et al. Clinical evaluation of a multiple-gene sequencing panel for hereditary cancer risk assessment. J Clin Oncol. 2014;32(19):2001–2009.
- Easton DF, Pharoah PD, Antoniou AC, et al. Gene-panel sequencing and the prediction of breast-cancer risk. N Engl J Med. 2015;372(23):2243–2257.
- Yurgelun MB, Allen B, Kaldate RR, et al. Identification of a variety of mutations in cancer predisposition genes in patients with suspected Lynch syndrome. Gastroenterology. 2015;149(3):604–613.e20.
- Pritchard CC, Mateo J, Walsh MF, et al. Inherited DNA-repair gene mutations in men with metastatic prostate cancer. N Engl J Med. 2016;375(5):443–453.
- Mavaddat N, Barrowdale D, Andrulis IL, et al; Consortium of Investigators of Modifiers of BRCA1/2. Pathology of breast and ovarian cancers among BRCA1 and BRCA2 mutation carriers: results from the Consortium of Investigators of Modifiers of BRCA1/2 (CIMBA). Cancer Epidemiol Biomarkers Prev. 2012;21(1):134–147.
- Struewing JP, Hartge P, Wacholder S, et al. The risk of cancer associated with specific mutations of BRCA1 and BRCA2 among Ashkenazi Jews. N Engl J Med. 1997;336(20):1401–1408.
- National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines): Genetic/Familial High-Risk Assessment: Breast and Ovarian. Version 1.2018. https://www.nccn.org. Accessed December 28, 2017.
- Saslow D, Boetes C, Burke W, et al; American Cancer Society Breast Cancer Advisory Group. American Cancer Society guidelines for breast screening with MRI as an adjunct to mammography. CA Cancer J Clin. 2007;57(2):75–89.
- Heather JM, Chain B. The sequence of sequencers: the history of sequencing DNA. Genomics. 2016;107(1):1–8.
- American College of Obstetricians and Gynecologists Committee on Practice Bulletins-Gynecology. ACOG Practice Bulletin No. 182: Hereditary breast and ovarian cancer syndrome. Obstet Gynecol. 2017;130(3):e110–e126.
- Mester JL, Schreiber AH, Moran RT. Genetic counselors: your partners in clinical practice. Cleve Clin J Med. 2012;79(8):560–568.
- Smith M, Mester J, Eng C. How to spot heritable breast cancer: a primary care physician’s guide. Cleve Clin J Med. 2014;81(1):31–40.
- Welsh JL, Hoskin TL, Day CN, et al. Clinical decision-making in patients with variant of uncertain significance in BRCA1 or BRCA2 genes. Ann Surg Oncol. 2017;24(10):3067–3072.
- Kurian AW, Li Y, Hamilton AS, et al. Gaps in incorporating germline genetic testing into treatment decision-making for early-stage breast cancer. J Clin Oncol. 2017;35(20):2232–2239.
- Tung N, Domchek SM, Stadler Z, et al. Counselling framework for moderate-penetrance cancer-susceptibility mutations. Nat Rev Clin Oncol. 2016;13(9):581–588.
- Yu PP, Vose JM, Hayes DF. Genetic cancer susceptibility testing: increased technology, increased complexity. J Clin Oncol. 2015;33(31):3533–3534.
- Pederson HJ, Gopalakrishnan D, Noss R, Yanda C, Eng C, Grobmyer SR. Impact of multigene panel testing on surgical decision making in breast cancer patients. J Am Coll Surg. 2018;226(4):560–565.
- Robson ME, Bradbury AR, Arun B, et al. American Society of Clinical Oncology policy statement update: genetic and genomic testing for cancer susceptibility. J Clin Oncol. 2015;33(31):3660–3667.
- Preventive care benefits for women: What Marketplace health insurance plans cover. HealthCare.gov. https://www.healthcare.gov/coverage/what-marketplace-plans-cover/. Accessed May 15, 2018.
- Centers for Medicare & Medicaid Services. The Center for Consumer Information & Insurance Oversight: Affordable Care Act Implementation FAQs – Set 12. https://www.cms.gov/CCIIO/Resources/Fact-Sheets-and-FAQs/aca_implementation_faqs12.html. Accessed May 15, 2018.
- US Preventive Services Task Force. Final Recommendation Statement: BRCA-Related Cancer: Risk Assessment, Genetic Counseling, and Genetic Testing. https://www.uspreventiveservicestaskforce.org/Page/Document/RecommendationStatementFinal/brca-related-cancer-risk-assessment-genetic-counseling-and-genetic-testing. Published December 2013. Accessed May 15, 2018.
- Kuhl CK, Schrading S, Leutner CC, et al. Mammography, breast ultrasound, and magnetic resonance imaging for surveillance of women at high familial risk for breast cancer. J Clin Oncol. 2005;23(33):8469–8476.
- Lehman CD, Blume JD, Weatherall P, et al; International Breast MRI Consortium Working Group. Screening women at high risk for breast cancer with mammography and magnetic resonance imaging. Cancer. 2005;103(9):1898–1905.
- Kriege M, Brekelmans CT, Boetes C, et al; Magnetic Resonance Imaging Screening Study Group. Efficacy of MRI and mammography for breast-cancer screening in women with a familial or genetic predisposition. N Engl J Med. 2004;351(5):427–437.
- Pederson HJ, Padia SA, May M, Grobmyer S. Managing patients at genetic risk of breast cancer. Cleve Clin J Med. 2016;83(3):199–206.
- Moyer VA; US Preventive Services Task Force. Risk assessment, genetic counseling, and genetic testing for BRCA-related cancer in women: US Preventive Services Task Force recommendation statement. Ann Intern Med. 2014;160(4):271–281.
- American Society of Breast Surgeons. Consensus Guideline on Hereditary Genetic Testing for Patients With and Without Breast Cancer. Columbia, MD: American Society of Breast Surgeons. https://www.breastsurgeons.org/new_layout/about/statements/PDF_Statements/BRCA_Testing.pdf. Published March 14, 2017. Accessed December 28, 2017.
Advances in cancer genetics are rapidly changing how clinicians assess an individual’s risk for breast cancer. ObGyns counsel many women with a personal or family history of the disease, many of whom can benefit from genetics counseling and testing. As patients with a hereditary predisposition to breast cancer are at higher risk and are younger at diagnosis, it is imperative to identify them early so they can benefit from enhanced surveillance, chemoprevention, and discussions regarding risk-reducing surgeries. ObGyns are uniquely poised to identify young women at risk for hereditary cancer syndromes, and they play a crucial role in screening and prevention over the life span.
CASE Patient with breast cancer history asks about screening for her daughters
A 52-year-old woman presents for her annual examination. She underwent breast cancer treatment 10 years earlier and has done well since then. When asked about family history of breast cancer and ethnicity, she reports her mother had breast cancer later in life, and her mother’s father was of Ashkenazi Jewish ancestry.In addition, a maternal uncle had metastatic prostate cancer. You recall that breast cancer diagnosed before age 50 years and Ashkenazi ancestry are “red flags” for a hereditary cancer syndrome. The patient wonders how her daughters should be screened. What do you do next?
Having a risk assessment plan is crucial
Given increasing demands, limited time, and the abundance of information to be discussed with patients, primary care physicians may find it challenging to assess breast cancer risk, consider genetics testing for appropriate individuals, and counsel patients about risk management options. The process has become even more complex since the expansion in genetics knowledge and the advent of multigene panel testing. Not only is risk assessment crucial for this woman and her daughters, and for other patients, but a delay in diagnosing and treating breast cancer in patients with hereditary and familial cancer risks may represent a worrisome new trend in medical litigation.1,2 Clinicians must have a process in place for assessing risk in all patients and treating them appropriately.
The American Cancer Society (ACS) estimated that 252,710 cases of breast cancer would be diagnosed in 2017, leading to 40,610 deaths.3 Twelve percent to 14% of breast cancers are thought to be related to hereditary cancer predisposition syndromes.4–8 This means that, every year, almost 35,000 cases of breast cancer are attributable to hereditary risk. These cases can be detected early with enhanced surveillance, which carries the highest chance for cure, or prevented with risk-reducing surgery in identified genetic mutation carriers. Each child of a person with a genetic mutation predisposing to breast cancer has a 50% chance of inheriting the mutation and having a very high risk of cancer.
In this patient’s case, basic information is collected about her cancer-related personal and family history.
Asking a few key questions can help in stratifying risk:
- Have you or anyone in your family had cancer? What type, and at what age?
- If breast cancer, did it involve both breasts, or was it triple-negative?
- Is there a family history of ovarian cancer?
- Is there a family history of male breast cancer?
- Is there a family history of metastatic prostate cancer?
- Are you of Ashkenazi Jewish ethnicity?
- Have you or anyone in your family ever had genetics testing for cancer?
The hallmarks of hereditary cancer are multiple cancers in an individual or family; young age at diagnosis; and ovarian, pancreatic, or another rare cancer. Metastatic prostate cancer was added as a red flag for hereditary risk after a recent large series found that 11.8% of men with metastatic prostate cancer harbor germline mutations.9
CASE Continued
On further questioning, the patient reports she had triple-negative (estrogen receptor–, progesterone receptor–, and human epidermal growth factor receptor 2 [HER2]–negative) breast cancer, a feature of patients with germline BRCA1 (breast cancer susceptibility gene 1) mutations.10 In addition, her Ashkenazi ancestry is concerning, as there is a 1-in-40 chance of carrying 1 of the 3 Ashkenazi founder BRCA mutations.11 Is a genetics consultation needed?
Read about guidelines for referral and testing.
Guidelines for genetics referral and testing
According to the TABLE, which summarizes national guidelines for genetics referral, maternal and paternal family histories are equally important. Our patient was under age 50 at diagnosis, has a history of triple-negative breast cancer, is of Ashkenazi ancestry, and has a family history of metastatic prostate cancer. She meets the criteria for genetics testing, and screening for her daughters most certainly will depend on the findings of that testing. If she carries a BRCA1 mutation, as might be anticipated, each daughter would have a 50% chance of having inherited the mutation. If they carry the mutation as well, they would begin breast magnetic resonance imaging (MRI) screening at age 25.12 If they decide against genetics testing, they could still undergo MRI screening as untested first-degree relatives of a BRCA carrier, per ACS recommendations.13
Integrating evidence and experience
Over the past 10 to 20 years, other breast cancer susceptibility genes (eg, BRCA2, PALB2, CHEK2) have been identified. More recently, next-generation sequencing has become commercially available. Laboratories can use this newer method to sequence multiple genes rapidly and in parallel, and its cost is similar to that of single-syndrome testing.14 When more than 1 gene can explain an inherited cancer syndrome, multigene panel testing may be more efficient and cost-effective. Use of multigene panel testing is supported in guidelines issued by the National Comprehensive Cancer Network,12 the American College of Obstetricians and Gynecologists,15 and other medical societies.
For our patient, the most logical strategy would be to test for the 3 mutations most common in the Ashkenazi population and then, if no mutation is found, perform multigene panel testing.
Formal genetics counseling can be very helpful for a patient, particularly in the era of multigene panel testing.16,17 A detailed pedigree (family tree) is elicited, and a genetics specialist determines whether testing is indicated and which test is best for the patient. Possible test findings are explained. The patient may be found to have a pathogenic variant with associated increased cancer risk, a negative test result (informative or uninformative), or a variant of uncertain significance (VUS). VUS is a gene mutation identified with an unknown effect on protein function and an unclear association with cancer risk. A finding of VUS may make the patient anxious,18 create uncertainty in the treating physician,19 and lead to harmful overtreatment, excessive surveillance, or unnecessary use of a preventive measure.19–21 Genetics counseling allows the patient, even the patient with VUS, to make appropriate decisions.22 Counseling may also help a patient or family process emotional responses, such as fear and guilt. In addition, counselors are familiar with relevant laws and regulations, such as the Genetic Information Nondiscrimination Act of 2008 (GINA), which protects patients from insurance and employment discrimination. Many professional guidelines recommend providing genetics counseling in conjunction with genetics testing,12,23 and some insurance companies and some states require counseling for coverage of testing.
Cost of genetics counseling. If patients are concerned about the cost of genetics testing, they can be reassured with the following information24–26:
- The Patient Protection and Affordable Care Act (ACA) identifies BRCA testing as a preventive service
- Medicare provides coverage for affected patients with a qualifying personal history
- 97% of commercial insurers and most state Medicaid programs provide coverage for hereditary cancer testing
- Most commercial laboratories have affordability programs that may provide additional support.
If a BRCA mutation is found: Many patients question the value of knowing whether they have a BRCA mutation. What our patient, her daughters, and others may not realize is that, if a BRCA mutation is found, breast MRI screening can begin at age 25. Although contrast-enhanced MRI screening is highly sensitive in detecting breast cancer,27–29 it lacks specificity and commonly yields false positives.
Some patients also worry about overdiagnosis with this highly sensitive test. Many do not realize that preventively prescribed oral contraceptives can reduce the risk of ovarian cancer by 50%, and cosmetically acceptable risk-reducing breast surgeries can reduce the risk by 90%.
Many are unaware of the associated risks with ovarian, prostate, pancreatic, and other cancers; of risk management options; and of assisted reproduction options, such as preimplantation genetics diagnosis, which can prevent the passing of a genetic mutation to future generations. The guidelines on risk management options are increasingly clear and helpful,12,30–32 and women often turn to their ObGyns for advice about health and prevention.
ObGyns are often the first-line providers for women with a personal or family history of breast cancer. Identification of at-risk patients begins with taking a careful family history and becoming familiar with the rapidly evolving guidelines in this important field. Identification of appropriate candidates for breast cancer genetics testing is a key step toward prevention, value-based care, and avoidance of legal liability.
CASE Resolved
In this case, testing for the 3 common Ashkenazi BRCA founder mutations was negative, and multigene panel testing was also negative. Her husband is not of Ashkenazi Jewish descent and there is no significant family history of cancer on his side. The daughters are advised to begin high-risk screening at the age of 32, 10 years earlier than their mother was diagnosed, but no genetic testing is indicated for them.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
Advances in cancer genetics are rapidly changing how clinicians assess an individual’s risk for breast cancer. ObGyns counsel many women with a personal or family history of the disease, many of whom can benefit from genetics counseling and testing. As patients with a hereditary predisposition to breast cancer are at higher risk and are younger at diagnosis, it is imperative to identify them early so they can benefit from enhanced surveillance, chemoprevention, and discussions regarding risk-reducing surgeries. ObGyns are uniquely poised to identify young women at risk for hereditary cancer syndromes, and they play a crucial role in screening and prevention over the life span.
CASE Patient with breast cancer history asks about screening for her daughters
A 52-year-old woman presents for her annual examination. She underwent breast cancer treatment 10 years earlier and has done well since then. When asked about family history of breast cancer and ethnicity, she reports her mother had breast cancer later in life, and her mother’s father was of Ashkenazi Jewish ancestry.In addition, a maternal uncle had metastatic prostate cancer. You recall that breast cancer diagnosed before age 50 years and Ashkenazi ancestry are “red flags” for a hereditary cancer syndrome. The patient wonders how her daughters should be screened. What do you do next?
Having a risk assessment plan is crucial
Given increasing demands, limited time, and the abundance of information to be discussed with patients, primary care physicians may find it challenging to assess breast cancer risk, consider genetics testing for appropriate individuals, and counsel patients about risk management options. The process has become even more complex since the expansion in genetics knowledge and the advent of multigene panel testing. Not only is risk assessment crucial for this woman and her daughters, and for other patients, but a delay in diagnosing and treating breast cancer in patients with hereditary and familial cancer risks may represent a worrisome new trend in medical litigation.1,2 Clinicians must have a process in place for assessing risk in all patients and treating them appropriately.
The American Cancer Society (ACS) estimated that 252,710 cases of breast cancer would be diagnosed in 2017, leading to 40,610 deaths.3 Twelve percent to 14% of breast cancers are thought to be related to hereditary cancer predisposition syndromes.4–8 This means that, every year, almost 35,000 cases of breast cancer are attributable to hereditary risk. These cases can be detected early with enhanced surveillance, which carries the highest chance for cure, or prevented with risk-reducing surgery in identified genetic mutation carriers. Each child of a person with a genetic mutation predisposing to breast cancer has a 50% chance of inheriting the mutation and having a very high risk of cancer.
In this patient’s case, basic information is collected about her cancer-related personal and family history.
Asking a few key questions can help in stratifying risk:
- Have you or anyone in your family had cancer? What type, and at what age?
- If breast cancer, did it involve both breasts, or was it triple-negative?
- Is there a family history of ovarian cancer?
- Is there a family history of male breast cancer?
- Is there a family history of metastatic prostate cancer?
- Are you of Ashkenazi Jewish ethnicity?
- Have you or anyone in your family ever had genetics testing for cancer?
The hallmarks of hereditary cancer are multiple cancers in an individual or family; young age at diagnosis; and ovarian, pancreatic, or another rare cancer. Metastatic prostate cancer was added as a red flag for hereditary risk after a recent large series found that 11.8% of men with metastatic prostate cancer harbor germline mutations.9
CASE Continued
On further questioning, the patient reports she had triple-negative (estrogen receptor–, progesterone receptor–, and human epidermal growth factor receptor 2 [HER2]–negative) breast cancer, a feature of patients with germline BRCA1 (breast cancer susceptibility gene 1) mutations.10 In addition, her Ashkenazi ancestry is concerning, as there is a 1-in-40 chance of carrying 1 of the 3 Ashkenazi founder BRCA mutations.11 Is a genetics consultation needed?
Read about guidelines for referral and testing.
Guidelines for genetics referral and testing
According to the TABLE, which summarizes national guidelines for genetics referral, maternal and paternal family histories are equally important. Our patient was under age 50 at diagnosis, has a history of triple-negative breast cancer, is of Ashkenazi ancestry, and has a family history of metastatic prostate cancer. She meets the criteria for genetics testing, and screening for her daughters most certainly will depend on the findings of that testing. If she carries a BRCA1 mutation, as might be anticipated, each daughter would have a 50% chance of having inherited the mutation. If they carry the mutation as well, they would begin breast magnetic resonance imaging (MRI) screening at age 25.12 If they decide against genetics testing, they could still undergo MRI screening as untested first-degree relatives of a BRCA carrier, per ACS recommendations.13
Integrating evidence and experience
Over the past 10 to 20 years, other breast cancer susceptibility genes (eg, BRCA2, PALB2, CHEK2) have been identified. More recently, next-generation sequencing has become commercially available. Laboratories can use this newer method to sequence multiple genes rapidly and in parallel, and its cost is similar to that of single-syndrome testing.14 When more than 1 gene can explain an inherited cancer syndrome, multigene panel testing may be more efficient and cost-effective. Use of multigene panel testing is supported in guidelines issued by the National Comprehensive Cancer Network,12 the American College of Obstetricians and Gynecologists,15 and other medical societies.
For our patient, the most logical strategy would be to test for the 3 mutations most common in the Ashkenazi population and then, if no mutation is found, perform multigene panel testing.
Formal genetics counseling can be very helpful for a patient, particularly in the era of multigene panel testing.16,17 A detailed pedigree (family tree) is elicited, and a genetics specialist determines whether testing is indicated and which test is best for the patient. Possible test findings are explained. The patient may be found to have a pathogenic variant with associated increased cancer risk, a negative test result (informative or uninformative), or a variant of uncertain significance (VUS). VUS is a gene mutation identified with an unknown effect on protein function and an unclear association with cancer risk. A finding of VUS may make the patient anxious,18 create uncertainty in the treating physician,19 and lead to harmful overtreatment, excessive surveillance, or unnecessary use of a preventive measure.19–21 Genetics counseling allows the patient, even the patient with VUS, to make appropriate decisions.22 Counseling may also help a patient or family process emotional responses, such as fear and guilt. In addition, counselors are familiar with relevant laws and regulations, such as the Genetic Information Nondiscrimination Act of 2008 (GINA), which protects patients from insurance and employment discrimination. Many professional guidelines recommend providing genetics counseling in conjunction with genetics testing,12,23 and some insurance companies and some states require counseling for coverage of testing.
Cost of genetics counseling. If patients are concerned about the cost of genetics testing, they can be reassured with the following information24–26:
- The Patient Protection and Affordable Care Act (ACA) identifies BRCA testing as a preventive service
- Medicare provides coverage for affected patients with a qualifying personal history
- 97% of commercial insurers and most state Medicaid programs provide coverage for hereditary cancer testing
- Most commercial laboratories have affordability programs that may provide additional support.
If a BRCA mutation is found: Many patients question the value of knowing whether they have a BRCA mutation. What our patient, her daughters, and others may not realize is that, if a BRCA mutation is found, breast MRI screening can begin at age 25. Although contrast-enhanced MRI screening is highly sensitive in detecting breast cancer,27–29 it lacks specificity and commonly yields false positives.
Some patients also worry about overdiagnosis with this highly sensitive test. Many do not realize that preventively prescribed oral contraceptives can reduce the risk of ovarian cancer by 50%, and cosmetically acceptable risk-reducing breast surgeries can reduce the risk by 90%.
Many are unaware of the associated risks with ovarian, prostate, pancreatic, and other cancers; of risk management options; and of assisted reproduction options, such as preimplantation genetics diagnosis, which can prevent the passing of a genetic mutation to future generations. The guidelines on risk management options are increasingly clear and helpful,12,30–32 and women often turn to their ObGyns for advice about health and prevention.
ObGyns are often the first-line providers for women with a personal or family history of breast cancer. Identification of at-risk patients begins with taking a careful family history and becoming familiar with the rapidly evolving guidelines in this important field. Identification of appropriate candidates for breast cancer genetics testing is a key step toward prevention, value-based care, and avoidance of legal liability.
CASE Resolved
In this case, testing for the 3 common Ashkenazi BRCA founder mutations was negative, and multigene panel testing was also negative. Her husband is not of Ashkenazi Jewish descent and there is no significant family history of cancer on his side. The daughters are advised to begin high-risk screening at the age of 32, 10 years earlier than their mother was diagnosed, but no genetic testing is indicated for them.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- Phillips RL Jr, Bartholomew LA, Dovey SM, Fryer GE Jr, Miyoshi TJ, Green LA. Learning from malpractice claims about negligent, adverse events in primary care in the United States. Qual Saf Health Care. 2004;13(2):121–126.
- Saber Tehrani AS, Lee H, Mathews SC, et al. 25-year summary of US malpractice claims for diagnostic errors 1986–2010: an analysis from the National Practitioner Data Bank. BMJ Qual Saf. 2013;22(8):672–680.
- American Cancer Society. Breast Cancer Facts & Figures 2017-2018. https://www.cancer.org/content/dam/cancer-org/research/cancer-facts-and-statistics/breast-cancer-factsand-figures/breast-cancer-facts-and-figures-2017-2018.pdf. Published 2017. Accessed December 28, 2017.
- Tung N, Battelli C, Allen B, et al. Frequency of mutations in individuals with breast cancer referred for BRCA1 and BRCA2 testing using next-generation sequencing with a 25-gene panel. Cancer. 2015;121(1):25–33.
- Tung N, Lin NU, Kidd J, et al. Frequency of germline mutations in 25 cancer susceptibility genes in a sequential series of patients with breast cancer. J Clin Oncol. 2016;34(13):1460–1468.
- Kurian AW, Hare EE, Mills MA, et al. Clinical evaluation of a multiple-gene sequencing panel for hereditary cancer risk assessment. J Clin Oncol. 2014;32(19):2001–2009.
- Easton DF, Pharoah PD, Antoniou AC, et al. Gene-panel sequencing and the prediction of breast-cancer risk. N Engl J Med. 2015;372(23):2243–2257.
- Yurgelun MB, Allen B, Kaldate RR, et al. Identification of a variety of mutations in cancer predisposition genes in patients with suspected Lynch syndrome. Gastroenterology. 2015;149(3):604–613.e20.
- Pritchard CC, Mateo J, Walsh MF, et al. Inherited DNA-repair gene mutations in men with metastatic prostate cancer. N Engl J Med. 2016;375(5):443–453.
- Mavaddat N, Barrowdale D, Andrulis IL, et al; Consortium of Investigators of Modifiers of BRCA1/2. Pathology of breast and ovarian cancers among BRCA1 and BRCA2 mutation carriers: results from the Consortium of Investigators of Modifiers of BRCA1/2 (CIMBA). Cancer Epidemiol Biomarkers Prev. 2012;21(1):134–147.
- Struewing JP, Hartge P, Wacholder S, et al. The risk of cancer associated with specific mutations of BRCA1 and BRCA2 among Ashkenazi Jews. N Engl J Med. 1997;336(20):1401–1408.
- National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines): Genetic/Familial High-Risk Assessment: Breast and Ovarian. Version 1.2018. https://www.nccn.org. Accessed December 28, 2017.
- Saslow D, Boetes C, Burke W, et al; American Cancer Society Breast Cancer Advisory Group. American Cancer Society guidelines for breast screening with MRI as an adjunct to mammography. CA Cancer J Clin. 2007;57(2):75–89.
- Heather JM, Chain B. The sequence of sequencers: the history of sequencing DNA. Genomics. 2016;107(1):1–8.
- American College of Obstetricians and Gynecologists Committee on Practice Bulletins-Gynecology. ACOG Practice Bulletin No. 182: Hereditary breast and ovarian cancer syndrome. Obstet Gynecol. 2017;130(3):e110–e126.
- Mester JL, Schreiber AH, Moran RT. Genetic counselors: your partners in clinical practice. Cleve Clin J Med. 2012;79(8):560–568.
- Smith M, Mester J, Eng C. How to spot heritable breast cancer: a primary care physician’s guide. Cleve Clin J Med. 2014;81(1):31–40.
- Welsh JL, Hoskin TL, Day CN, et al. Clinical decision-making in patients with variant of uncertain significance in BRCA1 or BRCA2 genes. Ann Surg Oncol. 2017;24(10):3067–3072.
- Kurian AW, Li Y, Hamilton AS, et al. Gaps in incorporating germline genetic testing into treatment decision-making for early-stage breast cancer. J Clin Oncol. 2017;35(20):2232–2239.
- Tung N, Domchek SM, Stadler Z, et al. Counselling framework for moderate-penetrance cancer-susceptibility mutations. Nat Rev Clin Oncol. 2016;13(9):581–588.
- Yu PP, Vose JM, Hayes DF. Genetic cancer susceptibility testing: increased technology, increased complexity. J Clin Oncol. 2015;33(31):3533–3534.
- Pederson HJ, Gopalakrishnan D, Noss R, Yanda C, Eng C, Grobmyer SR. Impact of multigene panel testing on surgical decision making in breast cancer patients. J Am Coll Surg. 2018;226(4):560–565.
- Robson ME, Bradbury AR, Arun B, et al. American Society of Clinical Oncology policy statement update: genetic and genomic testing for cancer susceptibility. J Clin Oncol. 2015;33(31):3660–3667.
- Preventive care benefits for women: What Marketplace health insurance plans cover. HealthCare.gov. https://www.healthcare.gov/coverage/what-marketplace-plans-cover/. Accessed May 15, 2018.
- Centers for Medicare & Medicaid Services. The Center for Consumer Information & Insurance Oversight: Affordable Care Act Implementation FAQs – Set 12. https://www.cms.gov/CCIIO/Resources/Fact-Sheets-and-FAQs/aca_implementation_faqs12.html. Accessed May 15, 2018.
- US Preventive Services Task Force. Final Recommendation Statement: BRCA-Related Cancer: Risk Assessment, Genetic Counseling, and Genetic Testing. https://www.uspreventiveservicestaskforce.org/Page/Document/RecommendationStatementFinal/brca-related-cancer-risk-assessment-genetic-counseling-and-genetic-testing. Published December 2013. Accessed May 15, 2018.
- Kuhl CK, Schrading S, Leutner CC, et al. Mammography, breast ultrasound, and magnetic resonance imaging for surveillance of women at high familial risk for breast cancer. J Clin Oncol. 2005;23(33):8469–8476.
- Lehman CD, Blume JD, Weatherall P, et al; International Breast MRI Consortium Working Group. Screening women at high risk for breast cancer with mammography and magnetic resonance imaging. Cancer. 2005;103(9):1898–1905.
- Kriege M, Brekelmans CT, Boetes C, et al; Magnetic Resonance Imaging Screening Study Group. Efficacy of MRI and mammography for breast-cancer screening in women with a familial or genetic predisposition. N Engl J Med. 2004;351(5):427–437.
- Pederson HJ, Padia SA, May M, Grobmyer S. Managing patients at genetic risk of breast cancer. Cleve Clin J Med. 2016;83(3):199–206.
- Moyer VA; US Preventive Services Task Force. Risk assessment, genetic counseling, and genetic testing for BRCA-related cancer in women: US Preventive Services Task Force recommendation statement. Ann Intern Med. 2014;160(4):271–281.
- American Society of Breast Surgeons. Consensus Guideline on Hereditary Genetic Testing for Patients With and Without Breast Cancer. Columbia, MD: American Society of Breast Surgeons. https://www.breastsurgeons.org/new_layout/about/statements/PDF_Statements/BRCA_Testing.pdf. Published March 14, 2017. Accessed December 28, 2017.
- Phillips RL Jr, Bartholomew LA, Dovey SM, Fryer GE Jr, Miyoshi TJ, Green LA. Learning from malpractice claims about negligent, adverse events in primary care in the United States. Qual Saf Health Care. 2004;13(2):121–126.
- Saber Tehrani AS, Lee H, Mathews SC, et al. 25-year summary of US malpractice claims for diagnostic errors 1986–2010: an analysis from the National Practitioner Data Bank. BMJ Qual Saf. 2013;22(8):672–680.
- American Cancer Society. Breast Cancer Facts & Figures 2017-2018. https://www.cancer.org/content/dam/cancer-org/research/cancer-facts-and-statistics/breast-cancer-factsand-figures/breast-cancer-facts-and-figures-2017-2018.pdf. Published 2017. Accessed December 28, 2017.
- Tung N, Battelli C, Allen B, et al. Frequency of mutations in individuals with breast cancer referred for BRCA1 and BRCA2 testing using next-generation sequencing with a 25-gene panel. Cancer. 2015;121(1):25–33.
- Tung N, Lin NU, Kidd J, et al. Frequency of germline mutations in 25 cancer susceptibility genes in a sequential series of patients with breast cancer. J Clin Oncol. 2016;34(13):1460–1468.
- Kurian AW, Hare EE, Mills MA, et al. Clinical evaluation of a multiple-gene sequencing panel for hereditary cancer risk assessment. J Clin Oncol. 2014;32(19):2001–2009.
- Easton DF, Pharoah PD, Antoniou AC, et al. Gene-panel sequencing and the prediction of breast-cancer risk. N Engl J Med. 2015;372(23):2243–2257.
- Yurgelun MB, Allen B, Kaldate RR, et al. Identification of a variety of mutations in cancer predisposition genes in patients with suspected Lynch syndrome. Gastroenterology. 2015;149(3):604–613.e20.
- Pritchard CC, Mateo J, Walsh MF, et al. Inherited DNA-repair gene mutations in men with metastatic prostate cancer. N Engl J Med. 2016;375(5):443–453.
- Mavaddat N, Barrowdale D, Andrulis IL, et al; Consortium of Investigators of Modifiers of BRCA1/2. Pathology of breast and ovarian cancers among BRCA1 and BRCA2 mutation carriers: results from the Consortium of Investigators of Modifiers of BRCA1/2 (CIMBA). Cancer Epidemiol Biomarkers Prev. 2012;21(1):134–147.
- Struewing JP, Hartge P, Wacholder S, et al. The risk of cancer associated with specific mutations of BRCA1 and BRCA2 among Ashkenazi Jews. N Engl J Med. 1997;336(20):1401–1408.
- National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines): Genetic/Familial High-Risk Assessment: Breast and Ovarian. Version 1.2018. https://www.nccn.org. Accessed December 28, 2017.
- Saslow D, Boetes C, Burke W, et al; American Cancer Society Breast Cancer Advisory Group. American Cancer Society guidelines for breast screening with MRI as an adjunct to mammography. CA Cancer J Clin. 2007;57(2):75–89.
- Heather JM, Chain B. The sequence of sequencers: the history of sequencing DNA. Genomics. 2016;107(1):1–8.
- American College of Obstetricians and Gynecologists Committee on Practice Bulletins-Gynecology. ACOG Practice Bulletin No. 182: Hereditary breast and ovarian cancer syndrome. Obstet Gynecol. 2017;130(3):e110–e126.
- Mester JL, Schreiber AH, Moran RT. Genetic counselors: your partners in clinical practice. Cleve Clin J Med. 2012;79(8):560–568.
- Smith M, Mester J, Eng C. How to spot heritable breast cancer: a primary care physician’s guide. Cleve Clin J Med. 2014;81(1):31–40.
- Welsh JL, Hoskin TL, Day CN, et al. Clinical decision-making in patients with variant of uncertain significance in BRCA1 or BRCA2 genes. Ann Surg Oncol. 2017;24(10):3067–3072.
- Kurian AW, Li Y, Hamilton AS, et al. Gaps in incorporating germline genetic testing into treatment decision-making for early-stage breast cancer. J Clin Oncol. 2017;35(20):2232–2239.
- Tung N, Domchek SM, Stadler Z, et al. Counselling framework for moderate-penetrance cancer-susceptibility mutations. Nat Rev Clin Oncol. 2016;13(9):581–588.
- Yu PP, Vose JM, Hayes DF. Genetic cancer susceptibility testing: increased technology, increased complexity. J Clin Oncol. 2015;33(31):3533–3534.
- Pederson HJ, Gopalakrishnan D, Noss R, Yanda C, Eng C, Grobmyer SR. Impact of multigene panel testing on surgical decision making in breast cancer patients. J Am Coll Surg. 2018;226(4):560–565.
- Robson ME, Bradbury AR, Arun B, et al. American Society of Clinical Oncology policy statement update: genetic and genomic testing for cancer susceptibility. J Clin Oncol. 2015;33(31):3660–3667.
- Preventive care benefits for women: What Marketplace health insurance plans cover. HealthCare.gov. https://www.healthcare.gov/coverage/what-marketplace-plans-cover/. Accessed May 15, 2018.
- Centers for Medicare & Medicaid Services. The Center for Consumer Information & Insurance Oversight: Affordable Care Act Implementation FAQs – Set 12. https://www.cms.gov/CCIIO/Resources/Fact-Sheets-and-FAQs/aca_implementation_faqs12.html. Accessed May 15, 2018.
- US Preventive Services Task Force. Final Recommendation Statement: BRCA-Related Cancer: Risk Assessment, Genetic Counseling, and Genetic Testing. https://www.uspreventiveservicestaskforce.org/Page/Document/RecommendationStatementFinal/brca-related-cancer-risk-assessment-genetic-counseling-and-genetic-testing. Published December 2013. Accessed May 15, 2018.
- Kuhl CK, Schrading S, Leutner CC, et al. Mammography, breast ultrasound, and magnetic resonance imaging for surveillance of women at high familial risk for breast cancer. J Clin Oncol. 2005;23(33):8469–8476.
- Lehman CD, Blume JD, Weatherall P, et al; International Breast MRI Consortium Working Group. Screening women at high risk for breast cancer with mammography and magnetic resonance imaging. Cancer. 2005;103(9):1898–1905.
- Kriege M, Brekelmans CT, Boetes C, et al; Magnetic Resonance Imaging Screening Study Group. Efficacy of MRI and mammography for breast-cancer screening in women with a familial or genetic predisposition. N Engl J Med. 2004;351(5):427–437.
- Pederson HJ, Padia SA, May M, Grobmyer S. Managing patients at genetic risk of breast cancer. Cleve Clin J Med. 2016;83(3):199–206.
- Moyer VA; US Preventive Services Task Force. Risk assessment, genetic counseling, and genetic testing for BRCA-related cancer in women: US Preventive Services Task Force recommendation statement. Ann Intern Med. 2014;160(4):271–281.
- American Society of Breast Surgeons. Consensus Guideline on Hereditary Genetic Testing for Patients With and Without Breast Cancer. Columbia, MD: American Society of Breast Surgeons. https://www.breastsurgeons.org/new_layout/about/statements/PDF_Statements/BRCA_Testing.pdf. Published March 14, 2017. Accessed December 28, 2017.
Take-home points
- The best genetics test is a good family history, updated annually
- Each year, 35,000 breast cancers are attributable to hereditary risk
- It is crucial to identify families at risk for hereditary breast cancer early, as cancers may begin in a woman's 30s; screening begins at age 25
- Multigene panel testing is efficient and cost-effective
- For patients who have highly penetrant pathogenic variants and are of childbearing age, preimplantation genetics diagnosis is an option
Ovarian masses: Surgery or surveillance?
A meaningful evolution has occurred over the past 30 years in the evaluation of ovarian tumors. In the 1980s, any palpable ovarian tumor was recommended for surgical removal.1 In the early 2000s, studies showed that unilocular cysts were at very low risk for malignancy, and surveillance was recommended.2 In the following decade, septate cysts were added to the list of ovarian tumors unlikely to be malignant, and nonsurgical therapy was suggested.3 It is estimated that 10% of women will undergo surgery for an adnexal mass in their lifetime, despite the fact that only 1 in 6 (13%–21%) of these masses is found to be malignant.4,5
A comprehensive, morphology-based pelvic ultrasonography is the first and most important step in evaluating an ovarian tumor’s risk of malignancy to determine whether surgery or surveillance is required.
Ovarian cancer continues to be the leading cause of gynecologic cancer death. Despite achieving superior surgical and cancer outcomes, a gynecologic oncologist performs only 40% of the initial ovarian cancer surgeries.6 Premenopausal and menopausal ovarian tumors are different in cause and consequence. Only 15% of premenopausal tumors are malignant, most commonly germ cell tumors, borderline ovarian tumors, and epithelial ovarian cancers. Tumors in menopausal women are less common but are more likely to be malignant. In actuality, up to 50% of tumors in this population are malignant. The most common of these malignancies are epithelial ovarian cancers, cancers metastatic to the ovary, and malignant stromal tumors.
Effective and evidence-based preoperative evaluations are available to help the clinician estimate a tumor’s risk of malignancy and determine which tumors are appropriate for referral to a specialist for surgery.
The actual incidence and prevalence of ovarian tumors are not known. From a review of almost 40,000 ultrasonography scans performed in the University of Kentucky Ovarian Cancer Screening Program, the estimated incidence and prevalence of ovarian abnormalities are 8.2 per 100 women annuallyand 17%, respectively.7 Seventy percent of these abnormalities have a unilocular or simple septate morphology and are at low risk for malignancy.7 The remaining 30% of abnormalities are high risk, although this represents only 9% of the total population evaluated. Since the vast majority of these abnormalities are expected to be asymptomatic, most will go unrecognized in the general population. For women who have an ovarian abnormality on ultrasonography, the majority will be at low risk for malignancy and will not require surgery.
Ovarian ultrasonography plus morphologic scoring comprise a comprehensive approach
The recently published recommendations of the First International Consensus Conference report on adnexal masses are summarized in TABLE 1.8 The expert panel reviewed the evidence and concluded that effective ultrasonography strategies exist and are well validated, and that low-risk asymptomatic ovarian cysts do not require surgical removal.
While no single ultrasonographic findingcan differentiate a benign from a malignant mass, morphologic scoring systems improve our ability to estimate a tumor’s malignant potential. In the United States, most practitioners in women’s health have ready access to gynecologic ultrasonography, but individual training and proficiency vary. Since not everyone is an expert sonographer, it is useful to employ an objective strategy when evaluating an ovarian tumor. The focus of a comprehensive ovarian ultrasonography is to recognize morphologic patterns that reflect a tumor’s malignant potential. While tumor volume is useful, tumor morphology is the most prognostic feature.
International Ovarian Tumor Analysis group
The International Ovarian Tumor Analysis (IOTA) group has published extensively on sonographic definitions and patterns that categorize tumors based on appearance.9 Simple rules and the ADNEX risk model are 2 of the group’s approaches (FIGURE 1).10,11 Both methods have been validated as effective for differentiating benign from malignant ovarian tumors, but neither has been used to study serial changes in ovarian morphology.
Regardless of the strategy employed, 25% of ovarian ultrasonography evaluations will be interpreted as “indeterminate” or “risk unknown.”10 The IOTA strategies have been successfully used in Europe for years, but they have not yet been studied or adopted in the United States.
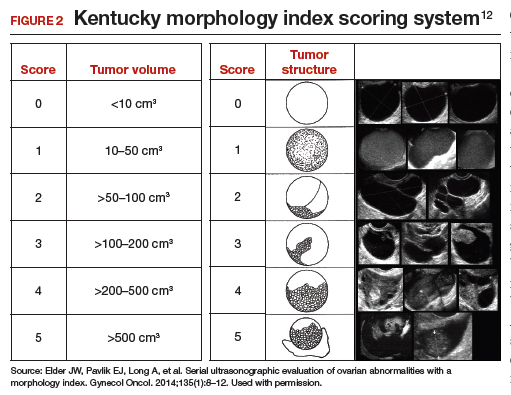
Kentucky morphology index
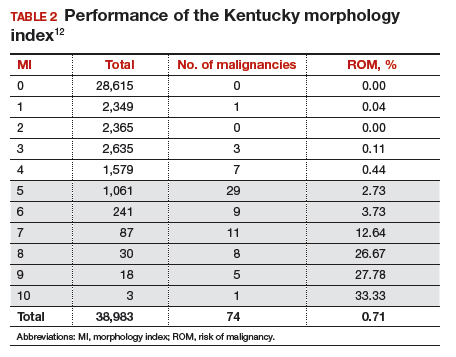
The morphology index (MI) from the University of Kentucky is an ultrasonography-based scoring system that combines tumor volume and tumor structure into a simple and effective index with a score ranging from 0 to 10 (FIGURE 2).12 A rising Kentucky MI score has a linear and predictable increase in the risk of ovarian malignancy. In a review of almost 40,000 sonograms, 85% of the malignancies had an MI score of 5 or greater (TABLE 2).12 Using this as a cutoff, the sensitivity and specificity for predicting malignancy was 86% and 98%, respectively.12
When comparing the ADNEX risk model with the Kentucky MI, investigators reviewed 45,000 ultrasound results and found that the majority of cancers were categorized by the ADNEX model in the lowest 4 of the 10 risk-of-malignancy groups, compared with only 15% for the MI.13 This clustering or skew is potentially problematic, since we expect higher scores to be more predictive of cancer than lower scores. It also infers that the ADNEX model may not be useful in serial surveillance strategies. Moreover, the ADNEX model identified only 30% of early stage cancers compared with identification of 80% with use of the MI.13
Serial ultrasonography
Serial ultrasonography is a concept similar to any longitudinal biomarker evaluation. In the United Kingdom Collaborative Trial of Ovarian Cancer Screening (UKCTOCS) program, the Risk of Ovarian Cancer Algorithm (ROCA) employs serial measurements of cancer antigen 125 (CA 125) to improve cancer detection. Serial ultrasonography similarly can be applied to better characterize a tumor’s physiology as well as its morphology. Over time, malignant ovarian tumors grow naturally in volume and complexity, and they do so at a rate faster than nonmalignant tumors. If this physical change can be measured objectively with ultrasonography, then serial sonography becomes a valuable diagnostic aid.
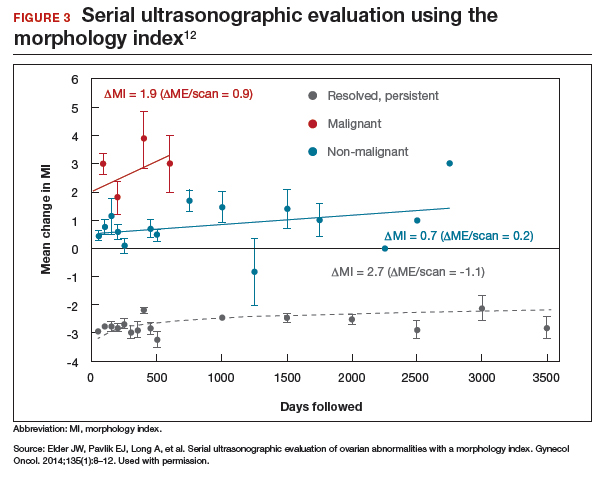
In comparing serial MI scores with clinical outcomes, studies have shown that malignant tumors exhibit a rapid increase, nonmalignant tumors have a stable or gradual rise, and resolving cysts show a decrease in MI score over time (FIGURE 3).12 An increase in the MI score of 1 or more per month (≥1 per month) is concerning for malignancy, and surgical removal should be considered. If the MI score of an asymptomatic ovarian tumor does not increase by 1 per month, it can be surveilled with intermittent ultrasonography.
Read about evaluating with serum biomarkers and sonography.
Serum biomarkers useful for determining risk, need for referral
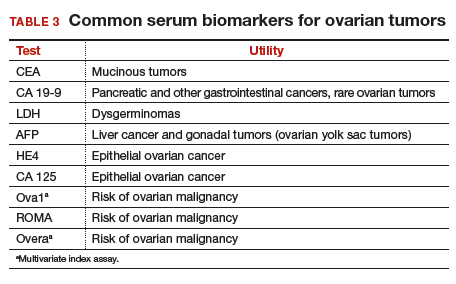
Serum biomarkers can be used to complement an ultrasonographic evaluation. They are particularly useful when surgery is recommended but the sonographic evaluation is indeterminate for malignancy risk. Many serum biomarkers are commonly used for the preoperative evaluation of an ovarian tumor or for surveillance of a malignancy following diagnosis (TABLE 3).
CA 125 is the most commonly ordered serum biomarker test for ovarian cancer. It is estimated that three‐quarters of CA 125 tests are ordered for preoperative use, which is not the US Food and Drug Administration (FDA) approved indication. Despite our clinical reliance on CA 125 as a diagnostic test prior to surgery, its utility is limited because of a low sensitivity for predicting cancer in premenopausal women and early stage disease.14,15 CA 125 specificity also varies widely, depending on patient age and other clinical factors, ranging from as low as 26% in premenopausal women to as high as 100% in postmenopausal women.16 Because CA 125 often is negative when early stage cancer is present, or positive when cancer is not, it is not recommended for preoperative use for determining whether an ovarian tumor is malignant or whether surgery is indicated. CA 125 should be used to monitor patients with a known ovarian malignancy.
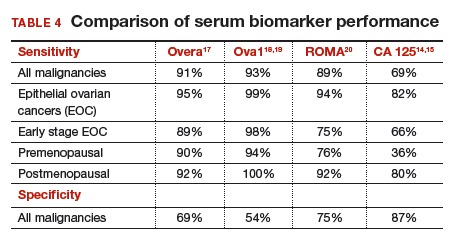
The new triage serum biomarkers, Overa, Ova1, and ROMA (Risk of Ovarian Malignancy Algorithm), are FDA cleared for preoperative use to help determine whether a woman needing surgery for an ovarian mass should be referred to a gynecologic oncologist.17–20 These tests should not be used to decide if surgery is indicated, but rather should be considered when the decision for surgery has already been made but the malignancy risk is unknown. A woman with a “high risk” result should be referred to a gynecologic oncologist, while one with a “low risk” score is very unlikely to have a malignancy and referral to a specialist is not necessary. TABLE 4 lists a comparison of the relative performance of these serum biomarkers.14,15,17–20 There are no published data on the use of serial triage biomarkers.
How to evaluate an ovarian tumor
Approximately 65% of the time, ovarian cystic tumors can be identified accurately as low risk based on the initial sonographic evaluation (TABLE 5). In this scenario, the risk of malignancy is very low (<1%), no secondary testing is needed, and no surgery is recommended.1,3,21
About 10% of tumors are expected to have a high-risk morphology on ultrasonography, where the risk of malignancy exceeds 25% and referral to a gynecologic oncologist is required.
The remaining 25% of tumors cannot be accurately classified with a single ultrasonographic evaluation and are considered indeterminate.22 Indeterminate tumors require secondary testing to ascertain whether surgery is indicated. Secondary testing may consist of serial ultrasonography, magnetic resonance imaging (MRI), or serum triage biomarker testing if the decision for surgery has been made.
A 2-step process is recommended for evaluating an ovarian tumor.
Step 1. Perform a detailed ultrasonography study using a morphology-based system. Classify the tumor as:
- low risk (65%): unilocular, simple septate, no flow on color Doppler
- simple rules: benign
- MI score 0–3
- no secondary testing; no referral is recommended
- high risk (10%): irregular, mostly solid, papillary projections, very strong flow on color Doppler
- simple rules: malignant
- MI score ≥5
- no secondary testing; refer to a gynecologic oncologist
- indeterminate (25%): partly solid, small wall abnormalities, minimal or moderate flow on color Doppler
- simple rules: both M and B rules apply or no rule applies
- MI score usually 4–6
- perform secondary testing (step 2).
Step 2. Perform secondary testing as follows:
- serum triage biomarkers if surgery is planned (Ova1, ROMA, Overa), or
- MRI, or
- serial sonography.
The 3 case scenarios that follow illustrate how the ovarian tumor evaluation process may be applied in clinical practice, with referral to a gynecologic oncologist as appropriate.
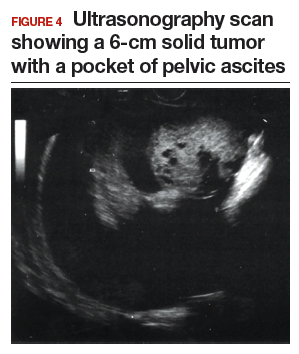
CASE 1 Postmenopausal woman with urinary symptoms and pelvic pressure
A 61-year-old woman is referred with a newly identified ovarian tumor. She has had 1 month of urinary urgency, frequency, and pelvic pressure, but she denies vaginal bleeding or fever. She has no family history of cancer. The referring physician included results of a serum CA 125 (48 U/mL; normal, ≤35 U/mL). A pelvic examination reveals a palpable, irregular mass in the anterior pelvis with limited mobility.
What would be your next step in the evaluation of this patient?
Start with ultrasonography
Step 1. Perform pelvic ultrasonography. In this patient, transvaginal sonography revealed a 6-cm (volume, 89 mL) mostly solid tumor (FIGURE 4). The maximum solid diameter of the tumor was 4.0 cm. There was a 20-mL pocket of pelvic ascites.
Results of morphology-based classification were as follows:
- simple rules: M1 and M5 positive; B rules: negative (malignant; high risk)
- ADNEX: 51.6% risk of malignancy (high risk)
- MI: 7 (high risk).
Step 2. Consider secondary testing. In this case, no secondary testing was recommended. Treatment plan. The patient was referred to a gynecologic oncologist for surgery and was found to have a stage IIA serous ovarian carcinoma.
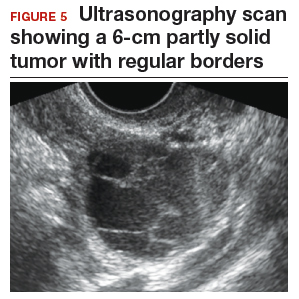
CASE 2 Woman with history of pelvic symptoms and worsening pain
A 46-year-old woman presents with worsening pelvic pain over the last month. She has a long-standing history of pelvic pain, dysmenorrhea, and dyspareunia from suspected endometriosis. She has no family history of cancer. The referring physician included the following serum biomarker results: CA 125, 48 U/mL (normal, ≤35 U/mL), and HE4, 60 pM (normal, ≤150 pM). On pelvic examination, there is a palpable mass with limited mobility in the posterior cul-de-sac.
Based on the patient’s available history, physical examination, and biomarker information, how would you proceed?
Follow the 2-step process
Step 1. Perform pelvic ultrasonography. Transvaginal sonography revealed a 6-cm (volume, 89 mL) partly solid tumor with regular internal borders (FIGURE 5). The maximum solid diameter of the tumor was 4.5 cm. There was no pelvic ascites.
Morphology classification was as follows:
- simple rules: M5 equivocal; B4 positive (indeterminate risk)
- ADNEX: 42.7% risk of malignancy (high risk)
- MI: 6 (indeterminate risk).
Step 2. Secondary testing was recommended for this patient. Test results were:
- repeat ultrasonography in 4 weeks with MI of 7 (volume score increase from 2 to 3, structure score unchanged at 4). Change in MI score +1 per month (high risk)
- Overa: 5.2 (high risk)
- ROMA: 11.8% (low risk).
Treatment plan. The patient was referred to a gynecologic oncologist because of an increasing MI score on serial sonography. Surgery revealed a stage IA grade 2 endometrioid adenocarcinoma of the ovary with surrounding endometriosis.
Read about treating a woman with postmenstrual bleeding.
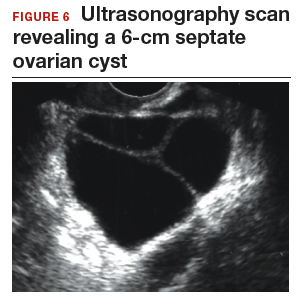
CASE 3 Woman with postmenopausal bleeding seeks medical care
A 62-year-old woman is referred with new-onset postmenopausal spotting for 1 month. She was recently prescribed antibiotics for diverticulitis. She has no family history of cancer. The referring physician included the results of a serum CA 125, which was 48 U/mL (normal, ≤35 U/mL). On pelvic examination, a mobile cystic mass is noted in the posterior cul-de-sac.
Use the stepwise protocol to sort out findings
Step 1. Pelvic ultrasonography. Transvaginal sonography suggested the presence of an endometrial polyp and revealed a 6-cm (volume, 89 mL) septate ovarian cyst (FIGURE 6).
Based on morphology classification, risk was categorized as:
- simple rules: M rules negative; B2, B4, B5 positive (benign; low risk)
- ADNEX: 2.9% risk of malignancy (low risk)
- MI: 2 (low risk).
Step 2. No secondary testing was recommended in this case.
Treatment plan. The patient’s gynecologist performed a hysteroscopic polypectomy that revealed no cancer. Serial monitoring was recommended for the low-risk ovarian cyst. The next ultrasonography scan, at 6 months, was unchanged; a subsequent scan was ordered for 12 months later, and at that time the cyst had resolved.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- Barber HR, Graber EA. The PMPO syndrome (postmenopausal palpable ovary syndrome). Obstet Gynecol. 1971;38(6):921–923.
- Modesitt SC, Pavlik EJ, Ueland FR, DePriest PD, Kryscio RJ, van Nagell JR Jr. Risk of malignancy in unilocular ovarian cystic tumors less than 10 centimeters in diameter. Obstet Gynecol. 2003;102(3):594–599.
- Saunders BA, Podzielinski I, Ware RA, et al. Risk of malignancy in sonographically confirmed septated cystic ovarian tumors. Gynecol Oncol. 2010;118(3):278–282.
- Moore RG, McMeekin DS, Brown AK, et al. A novel multiple marker bioassay utilizing HE4 and CA125 for the prediction of ovarian cancer in patients with a pelvic mass. Gynecol Oncol. 2009;112(1):40–46.
- Jordan SM, Bristow RE. Ovarian cancer biomarkers as diagnostic triage tests. Current Biomarker Findings. 2013;3:35–42.
- Giede KC, Kieser K, Dodge J, Rosen B. Who should operate on patients with ovarian cancer? An evidence-based review. Gynecol Oncol. 2005;99(2):447–461.
- Pavlik EJ, Ueland FR, Miller RW, et al. Frequency and disposition of ovarian abnormalities followed with serial transvaginal ultrasonography. Obstet Gynecol. 2013;122(2 pt 1):210–217.
- Glanc P, Benacerraf B, Bourne T, et al. First International Consensus Report on adnexal masses: management recommendations. J Ultrasound Med. 2017;36(5):849–863.
- Timmerman D, Valentin L, Bourne TGH, Collins WP, Verrelst H, Vergote I; International Ovarian Tumor Analysis (IOTA) Group. Terms, definitions and measurements to describe the sonographic features of adnexal tumors: a consensus opinion from the International Ovarian Tumor Analysis (IOTA) group. Ultrasound Obstet Gynecol. 2000;6(5):500–505.
- Timmerman D, Testa AC, Bourne T, et al. Simple ultrasound-based rules for the diagnosis of ovarian cancer. Ultrasound Obstet Gynecol. 2008;31(6):681–690.
- Van Calser B, Van Hoorde K, Valentin L, et al. Evaluating the risk of ovarian cancer before surgery using the ADNEX model to differentiate between benign, borderline, early and advanced stage invasive, and secondary metastatic tumours: prospective multicentre diagnostic study. BMJ. 2014;349:g5920.
- Elder JW, Pavlik EJ, Long A, et al. Serial ultrasonographic evaluation of ovarian abnormalities with a morphology index. Gynecol Oncol. 2014;135(1):8–12.
- Lefringhouse J, Ueland F, Ore R, et al. Comparing 2 sonographic scoring systems for distinguishing benign from malignant ovarian tumors [abstract]. Gynecol Oncol. 2016;141(suppl 1):57.
- Bast RC Jr, Klug TL, St John E, et al. A radioimmunoassay using a monoclonal antibody to monitor the course of epithelial ovarian cancer. N Engl J Med. 1983;309(15):883–887.
- Jacobs I, Bast RC Jr. The CA 125 tumour-associated antigen: a review of the literature. Hum Reprod. 1989;4(1):1–12.
- Myers ER, Bastian LA, Havrilesky LJ, et al. Management of adnexal mass. Agency for Healthcare Research and Quality. https://archive.ahrq.gov/downloads/pub/evidence/pdf/adnexal/adnexal.pdf. Published February 2006. Accessed May 15, 2018.
- Coleman RL, Herzog TJ, Chan DW, et al. Validation of a second-generation multivariate index assay for malignancy risk of adnexal masses. Am J Obstet Gynecol. 2016;215(1):82.e1–e11.
- Ueland FR, Desimone CP, Seamon LG, et al. Effectiveness of a multivariate index assay in the preoperative assessment of ovarian tumors. Obstet Gynecol. 2011;117(6):1289–1297.
- Bristow RE, Smith A, Zhang Z, et al. Ovarian malignancy risk stratification of the adnexal mass using a multivariate index assay. Gynecol Oncol. 2013;128(2):252–259.
- Moore RG, McMeekin DS, Brown AK, et al. A novel multiple marker bioassay utilizing HE4 and CA125 for the prediction of ovarian cancer in patients with a pelvic mass. Gynecol Oncol. 2009;112(1):40–46.
- Valentin L, Ameye L, Franchi D, et al. Risk of malignancy in unilocular cysts: a study of 1148 adnexal masses classified as unilocular cysts on transvaginal ultrasound and review of the literature. Ultrasound Obstet Gynecol. 2013;41(1):80–89.
- Timmerman D, Ameye L, Fischerova D, et al. Simple ultrasound rules to distinguish between benign and malignant adnexal masses before surgery: prospective validation by IOTA group. BMJ. 2010;341:c6839.
A meaningful evolution has occurred over the past 30 years in the evaluation of ovarian tumors. In the 1980s, any palpable ovarian tumor was recommended for surgical removal.1 In the early 2000s, studies showed that unilocular cysts were at very low risk for malignancy, and surveillance was recommended.2 In the following decade, septate cysts were added to the list of ovarian tumors unlikely to be malignant, and nonsurgical therapy was suggested.3 It is estimated that 10% of women will undergo surgery for an adnexal mass in their lifetime, despite the fact that only 1 in 6 (13%–21%) of these masses is found to be malignant.4,5
A comprehensive, morphology-based pelvic ultrasonography is the first and most important step in evaluating an ovarian tumor’s risk of malignancy to determine whether surgery or surveillance is required.
Ovarian cancer continues to be the leading cause of gynecologic cancer death. Despite achieving superior surgical and cancer outcomes, a gynecologic oncologist performs only 40% of the initial ovarian cancer surgeries.6 Premenopausal and menopausal ovarian tumors are different in cause and consequence. Only 15% of premenopausal tumors are malignant, most commonly germ cell tumors, borderline ovarian tumors, and epithelial ovarian cancers. Tumors in menopausal women are less common but are more likely to be malignant. In actuality, up to 50% of tumors in this population are malignant. The most common of these malignancies are epithelial ovarian cancers, cancers metastatic to the ovary, and malignant stromal tumors.
Effective and evidence-based preoperative evaluations are available to help the clinician estimate a tumor’s risk of malignancy and determine which tumors are appropriate for referral to a specialist for surgery.
The actual incidence and prevalence of ovarian tumors are not known. From a review of almost 40,000 ultrasonography scans performed in the University of Kentucky Ovarian Cancer Screening Program, the estimated incidence and prevalence of ovarian abnormalities are 8.2 per 100 women annuallyand 17%, respectively.7 Seventy percent of these abnormalities have a unilocular or simple septate morphology and are at low risk for malignancy.7 The remaining 30% of abnormalities are high risk, although this represents only 9% of the total population evaluated. Since the vast majority of these abnormalities are expected to be asymptomatic, most will go unrecognized in the general population. For women who have an ovarian abnormality on ultrasonography, the majority will be at low risk for malignancy and will not require surgery.
Ovarian ultrasonography plus morphologic scoring comprise a comprehensive approach
The recently published recommendations of the First International Consensus Conference report on adnexal masses are summarized in TABLE 1.8 The expert panel reviewed the evidence and concluded that effective ultrasonography strategies exist and are well validated, and that low-risk asymptomatic ovarian cysts do not require surgical removal.
While no single ultrasonographic findingcan differentiate a benign from a malignant mass, morphologic scoring systems improve our ability to estimate a tumor’s malignant potential. In the United States, most practitioners in women’s health have ready access to gynecologic ultrasonography, but individual training and proficiency vary. Since not everyone is an expert sonographer, it is useful to employ an objective strategy when evaluating an ovarian tumor. The focus of a comprehensive ovarian ultrasonography is to recognize morphologic patterns that reflect a tumor’s malignant potential. While tumor volume is useful, tumor morphology is the most prognostic feature.
International Ovarian Tumor Analysis group
The International Ovarian Tumor Analysis (IOTA) group has published extensively on sonographic definitions and patterns that categorize tumors based on appearance.9 Simple rules and the ADNEX risk model are 2 of the group’s approaches (FIGURE 1).10,11 Both methods have been validated as effective for differentiating benign from malignant ovarian tumors, but neither has been used to study serial changes in ovarian morphology.
Regardless of the strategy employed, 25% of ovarian ultrasonography evaluations will be interpreted as “indeterminate” or “risk unknown.”10 The IOTA strategies have been successfully used in Europe for years, but they have not yet been studied or adopted in the United States.
Kentucky morphology index
The morphology index (MI) from the University of Kentucky is an ultrasonography-based scoring system that combines tumor volume and tumor structure into a simple and effective index with a score ranging from 0 to 10 (FIGURE 2).12 A rising Kentucky MI score has a linear and predictable increase in the risk of ovarian malignancy. In a review of almost 40,000 sonograms, 85% of the malignancies had an MI score of 5 or greater (TABLE 2).12 Using this as a cutoff, the sensitivity and specificity for predicting malignancy was 86% and 98%, respectively.12
When comparing the ADNEX risk model with the Kentucky MI, investigators reviewed 45,000 ultrasound results and found that the majority of cancers were categorized by the ADNEX model in the lowest 4 of the 10 risk-of-malignancy groups, compared with only 15% for the MI.13 This clustering or skew is potentially problematic, since we expect higher scores to be more predictive of cancer than lower scores. It also infers that the ADNEX model may not be useful in serial surveillance strategies. Moreover, the ADNEX model identified only 30% of early stage cancers compared with identification of 80% with use of the MI.13
Serial ultrasonography
Serial ultrasonography is a concept similar to any longitudinal biomarker evaluation. In the United Kingdom Collaborative Trial of Ovarian Cancer Screening (UKCTOCS) program, the Risk of Ovarian Cancer Algorithm (ROCA) employs serial measurements of cancer antigen 125 (CA 125) to improve cancer detection. Serial ultrasonography similarly can be applied to better characterize a tumor’s physiology as well as its morphology. Over time, malignant ovarian tumors grow naturally in volume and complexity, and they do so at a rate faster than nonmalignant tumors. If this physical change can be measured objectively with ultrasonography, then serial sonography becomes a valuable diagnostic aid.
In comparing serial MI scores with clinical outcomes, studies have shown that malignant tumors exhibit a rapid increase, nonmalignant tumors have a stable or gradual rise, and resolving cysts show a decrease in MI score over time (FIGURE 3).12 An increase in the MI score of 1 or more per month (≥1 per month) is concerning for malignancy, and surgical removal should be considered. If the MI score of an asymptomatic ovarian tumor does not increase by 1 per month, it can be surveilled with intermittent ultrasonography.
Read about evaluating with serum biomarkers and sonography.
Serum biomarkers useful for determining risk, need for referral
Serum biomarkers can be used to complement an ultrasonographic evaluation. They are particularly useful when surgery is recommended but the sonographic evaluation is indeterminate for malignancy risk. Many serum biomarkers are commonly used for the preoperative evaluation of an ovarian tumor or for surveillance of a malignancy following diagnosis (TABLE 3).
CA 125 is the most commonly ordered serum biomarker test for ovarian cancer. It is estimated that three‐quarters of CA 125 tests are ordered for preoperative use, which is not the US Food and Drug Administration (FDA) approved indication. Despite our clinical reliance on CA 125 as a diagnostic test prior to surgery, its utility is limited because of a low sensitivity for predicting cancer in premenopausal women and early stage disease.14,15 CA 125 specificity also varies widely, depending on patient age and other clinical factors, ranging from as low as 26% in premenopausal women to as high as 100% in postmenopausal women.16 Because CA 125 often is negative when early stage cancer is present, or positive when cancer is not, it is not recommended for preoperative use for determining whether an ovarian tumor is malignant or whether surgery is indicated. CA 125 should be used to monitor patients with a known ovarian malignancy.
The new triage serum biomarkers, Overa, Ova1, and ROMA (Risk of Ovarian Malignancy Algorithm), are FDA cleared for preoperative use to help determine whether a woman needing surgery for an ovarian mass should be referred to a gynecologic oncologist.17–20 These tests should not be used to decide if surgery is indicated, but rather should be considered when the decision for surgery has already been made but the malignancy risk is unknown. A woman with a “high risk” result should be referred to a gynecologic oncologist, while one with a “low risk” score is very unlikely to have a malignancy and referral to a specialist is not necessary. TABLE 4 lists a comparison of the relative performance of these serum biomarkers.14,15,17–20 There are no published data on the use of serial triage biomarkers.
How to evaluate an ovarian tumor
Approximately 65% of the time, ovarian cystic tumors can be identified accurately as low risk based on the initial sonographic evaluation (TABLE 5). In this scenario, the risk of malignancy is very low (<1%), no secondary testing is needed, and no surgery is recommended.1,3,21
About 10% of tumors are expected to have a high-risk morphology on ultrasonography, where the risk of malignancy exceeds 25% and referral to a gynecologic oncologist is required.
The remaining 25% of tumors cannot be accurately classified with a single ultrasonographic evaluation and are considered indeterminate.22 Indeterminate tumors require secondary testing to ascertain whether surgery is indicated. Secondary testing may consist of serial ultrasonography, magnetic resonance imaging (MRI), or serum triage biomarker testing if the decision for surgery has been made.
A 2-step process is recommended for evaluating an ovarian tumor.
Step 1. Perform a detailed ultrasonography study using a morphology-based system. Classify the tumor as:
- low risk (65%): unilocular, simple septate, no flow on color Doppler
- simple rules: benign
- MI score 0–3
- no secondary testing; no referral is recommended
- high risk (10%): irregular, mostly solid, papillary projections, very strong flow on color Doppler
- simple rules: malignant
- MI score ≥5
- no secondary testing; refer to a gynecologic oncologist
- indeterminate (25%): partly solid, small wall abnormalities, minimal or moderate flow on color Doppler
- simple rules: both M and B rules apply or no rule applies
- MI score usually 4–6
- perform secondary testing (step 2).
Step 2. Perform secondary testing as follows:
- serum triage biomarkers if surgery is planned (Ova1, ROMA, Overa), or
- MRI, or
- serial sonography.
The 3 case scenarios that follow illustrate how the ovarian tumor evaluation process may be applied in clinical practice, with referral to a gynecologic oncologist as appropriate.
CASE 1 Postmenopausal woman with urinary symptoms and pelvic pressure
A 61-year-old woman is referred with a newly identified ovarian tumor. She has had 1 month of urinary urgency, frequency, and pelvic pressure, but she denies vaginal bleeding or fever. She has no family history of cancer. The referring physician included results of a serum CA 125 (48 U/mL; normal, ≤35 U/mL). A pelvic examination reveals a palpable, irregular mass in the anterior pelvis with limited mobility.
What would be your next step in the evaluation of this patient?
Start with ultrasonography
Step 1. Perform pelvic ultrasonography. In this patient, transvaginal sonography revealed a 6-cm (volume, 89 mL) mostly solid tumor (FIGURE 4). The maximum solid diameter of the tumor was 4.0 cm. There was a 20-mL pocket of pelvic ascites.
Results of morphology-based classification were as follows:
- simple rules: M1 and M5 positive; B rules: negative (malignant; high risk)
- ADNEX: 51.6% risk of malignancy (high risk)
- MI: 7 (high risk).
Step 2. Consider secondary testing. In this case, no secondary testing was recommended. Treatment plan. The patient was referred to a gynecologic oncologist for surgery and was found to have a stage IIA serous ovarian carcinoma.
CASE 2 Woman with history of pelvic symptoms and worsening pain
A 46-year-old woman presents with worsening pelvic pain over the last month. She has a long-standing history of pelvic pain, dysmenorrhea, and dyspareunia from suspected endometriosis. She has no family history of cancer. The referring physician included the following serum biomarker results: CA 125, 48 U/mL (normal, ≤35 U/mL), and HE4, 60 pM (normal, ≤150 pM). On pelvic examination, there is a palpable mass with limited mobility in the posterior cul-de-sac.
Based on the patient’s available history, physical examination, and biomarker information, how would you proceed?
Follow the 2-step process
Step 1. Perform pelvic ultrasonography. Transvaginal sonography revealed a 6-cm (volume, 89 mL) partly solid tumor with regular internal borders (FIGURE 5). The maximum solid diameter of the tumor was 4.5 cm. There was no pelvic ascites.
Morphology classification was as follows:
- simple rules: M5 equivocal; B4 positive (indeterminate risk)
- ADNEX: 42.7% risk of malignancy (high risk)
- MI: 6 (indeterminate risk).
Step 2. Secondary testing was recommended for this patient. Test results were:
- repeat ultrasonography in 4 weeks with MI of 7 (volume score increase from 2 to 3, structure score unchanged at 4). Change in MI score +1 per month (high risk)
- Overa: 5.2 (high risk)
- ROMA: 11.8% (low risk).
Treatment plan. The patient was referred to a gynecologic oncologist because of an increasing MI score on serial sonography. Surgery revealed a stage IA grade 2 endometrioid adenocarcinoma of the ovary with surrounding endometriosis.
Read about treating a woman with postmenstrual bleeding.
CASE 3 Woman with postmenopausal bleeding seeks medical care
A 62-year-old woman is referred with new-onset postmenopausal spotting for 1 month. She was recently prescribed antibiotics for diverticulitis. She has no family history of cancer. The referring physician included the results of a serum CA 125, which was 48 U/mL (normal, ≤35 U/mL). On pelvic examination, a mobile cystic mass is noted in the posterior cul-de-sac.
Use the stepwise protocol to sort out findings
Step 1. Pelvic ultrasonography. Transvaginal sonography suggested the presence of an endometrial polyp and revealed a 6-cm (volume, 89 mL) septate ovarian cyst (FIGURE 6).
Based on morphology classification, risk was categorized as:
- simple rules: M rules negative; B2, B4, B5 positive (benign; low risk)
- ADNEX: 2.9% risk of malignancy (low risk)
- MI: 2 (low risk).
Step 2. No secondary testing was recommended in this case.
Treatment plan. The patient’s gynecologist performed a hysteroscopic polypectomy that revealed no cancer. Serial monitoring was recommended for the low-risk ovarian cyst. The next ultrasonography scan, at 6 months, was unchanged; a subsequent scan was ordered for 12 months later, and at that time the cyst had resolved.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
A meaningful evolution has occurred over the past 30 years in the evaluation of ovarian tumors. In the 1980s, any palpable ovarian tumor was recommended for surgical removal.1 In the early 2000s, studies showed that unilocular cysts were at very low risk for malignancy, and surveillance was recommended.2 In the following decade, septate cysts were added to the list of ovarian tumors unlikely to be malignant, and nonsurgical therapy was suggested.3 It is estimated that 10% of women will undergo surgery for an adnexal mass in their lifetime, despite the fact that only 1 in 6 (13%–21%) of these masses is found to be malignant.4,5
A comprehensive, morphology-based pelvic ultrasonography is the first and most important step in evaluating an ovarian tumor’s risk of malignancy to determine whether surgery or surveillance is required.
Ovarian cancer continues to be the leading cause of gynecologic cancer death. Despite achieving superior surgical and cancer outcomes, a gynecologic oncologist performs only 40% of the initial ovarian cancer surgeries.6 Premenopausal and menopausal ovarian tumors are different in cause and consequence. Only 15% of premenopausal tumors are malignant, most commonly germ cell tumors, borderline ovarian tumors, and epithelial ovarian cancers. Tumors in menopausal women are less common but are more likely to be malignant. In actuality, up to 50% of tumors in this population are malignant. The most common of these malignancies are epithelial ovarian cancers, cancers metastatic to the ovary, and malignant stromal tumors.
Effective and evidence-based preoperative evaluations are available to help the clinician estimate a tumor’s risk of malignancy and determine which tumors are appropriate for referral to a specialist for surgery.
The actual incidence and prevalence of ovarian tumors are not known. From a review of almost 40,000 ultrasonography scans performed in the University of Kentucky Ovarian Cancer Screening Program, the estimated incidence and prevalence of ovarian abnormalities are 8.2 per 100 women annuallyand 17%, respectively.7 Seventy percent of these abnormalities have a unilocular or simple septate morphology and are at low risk for malignancy.7 The remaining 30% of abnormalities are high risk, although this represents only 9% of the total population evaluated. Since the vast majority of these abnormalities are expected to be asymptomatic, most will go unrecognized in the general population. For women who have an ovarian abnormality on ultrasonography, the majority will be at low risk for malignancy and will not require surgery.
Ovarian ultrasonography plus morphologic scoring comprise a comprehensive approach
The recently published recommendations of the First International Consensus Conference report on adnexal masses are summarized in TABLE 1.8 The expert panel reviewed the evidence and concluded that effective ultrasonography strategies exist and are well validated, and that low-risk asymptomatic ovarian cysts do not require surgical removal.
While no single ultrasonographic findingcan differentiate a benign from a malignant mass, morphologic scoring systems improve our ability to estimate a tumor’s malignant potential. In the United States, most practitioners in women’s health have ready access to gynecologic ultrasonography, but individual training and proficiency vary. Since not everyone is an expert sonographer, it is useful to employ an objective strategy when evaluating an ovarian tumor. The focus of a comprehensive ovarian ultrasonography is to recognize morphologic patterns that reflect a tumor’s malignant potential. While tumor volume is useful, tumor morphology is the most prognostic feature.
International Ovarian Tumor Analysis group
The International Ovarian Tumor Analysis (IOTA) group has published extensively on sonographic definitions and patterns that categorize tumors based on appearance.9 Simple rules and the ADNEX risk model are 2 of the group’s approaches (FIGURE 1).10,11 Both methods have been validated as effective for differentiating benign from malignant ovarian tumors, but neither has been used to study serial changes in ovarian morphology.
Regardless of the strategy employed, 25% of ovarian ultrasonography evaluations will be interpreted as “indeterminate” or “risk unknown.”10 The IOTA strategies have been successfully used in Europe for years, but they have not yet been studied or adopted in the United States.
Kentucky morphology index
The morphology index (MI) from the University of Kentucky is an ultrasonography-based scoring system that combines tumor volume and tumor structure into a simple and effective index with a score ranging from 0 to 10 (FIGURE 2).12 A rising Kentucky MI score has a linear and predictable increase in the risk of ovarian malignancy. In a review of almost 40,000 sonograms, 85% of the malignancies had an MI score of 5 or greater (TABLE 2).12 Using this as a cutoff, the sensitivity and specificity for predicting malignancy was 86% and 98%, respectively.12
When comparing the ADNEX risk model with the Kentucky MI, investigators reviewed 45,000 ultrasound results and found that the majority of cancers were categorized by the ADNEX model in the lowest 4 of the 10 risk-of-malignancy groups, compared with only 15% for the MI.13 This clustering or skew is potentially problematic, since we expect higher scores to be more predictive of cancer than lower scores. It also infers that the ADNEX model may not be useful in serial surveillance strategies. Moreover, the ADNEX model identified only 30% of early stage cancers compared with identification of 80% with use of the MI.13
Serial ultrasonography
Serial ultrasonography is a concept similar to any longitudinal biomarker evaluation. In the United Kingdom Collaborative Trial of Ovarian Cancer Screening (UKCTOCS) program, the Risk of Ovarian Cancer Algorithm (ROCA) employs serial measurements of cancer antigen 125 (CA 125) to improve cancer detection. Serial ultrasonography similarly can be applied to better characterize a tumor’s physiology as well as its morphology. Over time, malignant ovarian tumors grow naturally in volume and complexity, and they do so at a rate faster than nonmalignant tumors. If this physical change can be measured objectively with ultrasonography, then serial sonography becomes a valuable diagnostic aid.
In comparing serial MI scores with clinical outcomes, studies have shown that malignant tumors exhibit a rapid increase, nonmalignant tumors have a stable or gradual rise, and resolving cysts show a decrease in MI score over time (FIGURE 3).12 An increase in the MI score of 1 or more per month (≥1 per month) is concerning for malignancy, and surgical removal should be considered. If the MI score of an asymptomatic ovarian tumor does not increase by 1 per month, it can be surveilled with intermittent ultrasonography.
Read about evaluating with serum biomarkers and sonography.
Serum biomarkers useful for determining risk, need for referral
Serum biomarkers can be used to complement an ultrasonographic evaluation. They are particularly useful when surgery is recommended but the sonographic evaluation is indeterminate for malignancy risk. Many serum biomarkers are commonly used for the preoperative evaluation of an ovarian tumor or for surveillance of a malignancy following diagnosis (TABLE 3).
CA 125 is the most commonly ordered serum biomarker test for ovarian cancer. It is estimated that three‐quarters of CA 125 tests are ordered for preoperative use, which is not the US Food and Drug Administration (FDA) approved indication. Despite our clinical reliance on CA 125 as a diagnostic test prior to surgery, its utility is limited because of a low sensitivity for predicting cancer in premenopausal women and early stage disease.14,15 CA 125 specificity also varies widely, depending on patient age and other clinical factors, ranging from as low as 26% in premenopausal women to as high as 100% in postmenopausal women.16 Because CA 125 often is negative when early stage cancer is present, or positive when cancer is not, it is not recommended for preoperative use for determining whether an ovarian tumor is malignant or whether surgery is indicated. CA 125 should be used to monitor patients with a known ovarian malignancy.
The new triage serum biomarkers, Overa, Ova1, and ROMA (Risk of Ovarian Malignancy Algorithm), are FDA cleared for preoperative use to help determine whether a woman needing surgery for an ovarian mass should be referred to a gynecologic oncologist.17–20 These tests should not be used to decide if surgery is indicated, but rather should be considered when the decision for surgery has already been made but the malignancy risk is unknown. A woman with a “high risk” result should be referred to a gynecologic oncologist, while one with a “low risk” score is very unlikely to have a malignancy and referral to a specialist is not necessary. TABLE 4 lists a comparison of the relative performance of these serum biomarkers.14,15,17–20 There are no published data on the use of serial triage biomarkers.
How to evaluate an ovarian tumor
Approximately 65% of the time, ovarian cystic tumors can be identified accurately as low risk based on the initial sonographic evaluation (TABLE 5). In this scenario, the risk of malignancy is very low (<1%), no secondary testing is needed, and no surgery is recommended.1,3,21
About 10% of tumors are expected to have a high-risk morphology on ultrasonography, where the risk of malignancy exceeds 25% and referral to a gynecologic oncologist is required.
The remaining 25% of tumors cannot be accurately classified with a single ultrasonographic evaluation and are considered indeterminate.22 Indeterminate tumors require secondary testing to ascertain whether surgery is indicated. Secondary testing may consist of serial ultrasonography, magnetic resonance imaging (MRI), or serum triage biomarker testing if the decision for surgery has been made.
A 2-step process is recommended for evaluating an ovarian tumor.
Step 1. Perform a detailed ultrasonography study using a morphology-based system. Classify the tumor as:
- low risk (65%): unilocular, simple septate, no flow on color Doppler
- simple rules: benign
- MI score 0–3
- no secondary testing; no referral is recommended
- high risk (10%): irregular, mostly solid, papillary projections, very strong flow on color Doppler
- simple rules: malignant
- MI score ≥5
- no secondary testing; refer to a gynecologic oncologist
- indeterminate (25%): partly solid, small wall abnormalities, minimal or moderate flow on color Doppler
- simple rules: both M and B rules apply or no rule applies
- MI score usually 4–6
- perform secondary testing (step 2).
Step 2. Perform secondary testing as follows:
- serum triage biomarkers if surgery is planned (Ova1, ROMA, Overa), or
- MRI, or
- serial sonography.
The 3 case scenarios that follow illustrate how the ovarian tumor evaluation process may be applied in clinical practice, with referral to a gynecologic oncologist as appropriate.
CASE 1 Postmenopausal woman with urinary symptoms and pelvic pressure
A 61-year-old woman is referred with a newly identified ovarian tumor. She has had 1 month of urinary urgency, frequency, and pelvic pressure, but she denies vaginal bleeding or fever. She has no family history of cancer. The referring physician included results of a serum CA 125 (48 U/mL; normal, ≤35 U/mL). A pelvic examination reveals a palpable, irregular mass in the anterior pelvis with limited mobility.
What would be your next step in the evaluation of this patient?
Start with ultrasonography
Step 1. Perform pelvic ultrasonography. In this patient, transvaginal sonography revealed a 6-cm (volume, 89 mL) mostly solid tumor (FIGURE 4). The maximum solid diameter of the tumor was 4.0 cm. There was a 20-mL pocket of pelvic ascites.
Results of morphology-based classification were as follows:
- simple rules: M1 and M5 positive; B rules: negative (malignant; high risk)
- ADNEX: 51.6% risk of malignancy (high risk)
- MI: 7 (high risk).
Step 2. Consider secondary testing. In this case, no secondary testing was recommended. Treatment plan. The patient was referred to a gynecologic oncologist for surgery and was found to have a stage IIA serous ovarian carcinoma.
CASE 2 Woman with history of pelvic symptoms and worsening pain
A 46-year-old woman presents with worsening pelvic pain over the last month. She has a long-standing history of pelvic pain, dysmenorrhea, and dyspareunia from suspected endometriosis. She has no family history of cancer. The referring physician included the following serum biomarker results: CA 125, 48 U/mL (normal, ≤35 U/mL), and HE4, 60 pM (normal, ≤150 pM). On pelvic examination, there is a palpable mass with limited mobility in the posterior cul-de-sac.
Based on the patient’s available history, physical examination, and biomarker information, how would you proceed?
Follow the 2-step process
Step 1. Perform pelvic ultrasonography. Transvaginal sonography revealed a 6-cm (volume, 89 mL) partly solid tumor with regular internal borders (FIGURE 5). The maximum solid diameter of the tumor was 4.5 cm. There was no pelvic ascites.
Morphology classification was as follows:
- simple rules: M5 equivocal; B4 positive (indeterminate risk)
- ADNEX: 42.7% risk of malignancy (high risk)
- MI: 6 (indeterminate risk).
Step 2. Secondary testing was recommended for this patient. Test results were:
- repeat ultrasonography in 4 weeks with MI of 7 (volume score increase from 2 to 3, structure score unchanged at 4). Change in MI score +1 per month (high risk)
- Overa: 5.2 (high risk)
- ROMA: 11.8% (low risk).
Treatment plan. The patient was referred to a gynecologic oncologist because of an increasing MI score on serial sonography. Surgery revealed a stage IA grade 2 endometrioid adenocarcinoma of the ovary with surrounding endometriosis.
Read about treating a woman with postmenstrual bleeding.
CASE 3 Woman with postmenopausal bleeding seeks medical care
A 62-year-old woman is referred with new-onset postmenopausal spotting for 1 month. She was recently prescribed antibiotics for diverticulitis. She has no family history of cancer. The referring physician included the results of a serum CA 125, which was 48 U/mL (normal, ≤35 U/mL). On pelvic examination, a mobile cystic mass is noted in the posterior cul-de-sac.
Use the stepwise protocol to sort out findings
Step 1. Pelvic ultrasonography. Transvaginal sonography suggested the presence of an endometrial polyp and revealed a 6-cm (volume, 89 mL) septate ovarian cyst (FIGURE 6).
Based on morphology classification, risk was categorized as:
- simple rules: M rules negative; B2, B4, B5 positive (benign; low risk)
- ADNEX: 2.9% risk of malignancy (low risk)
- MI: 2 (low risk).
Step 2. No secondary testing was recommended in this case.
Treatment plan. The patient’s gynecologist performed a hysteroscopic polypectomy that revealed no cancer. Serial monitoring was recommended for the low-risk ovarian cyst. The next ultrasonography scan, at 6 months, was unchanged; a subsequent scan was ordered for 12 months later, and at that time the cyst had resolved.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- Barber HR, Graber EA. The PMPO syndrome (postmenopausal palpable ovary syndrome). Obstet Gynecol. 1971;38(6):921–923.
- Modesitt SC, Pavlik EJ, Ueland FR, DePriest PD, Kryscio RJ, van Nagell JR Jr. Risk of malignancy in unilocular ovarian cystic tumors less than 10 centimeters in diameter. Obstet Gynecol. 2003;102(3):594–599.
- Saunders BA, Podzielinski I, Ware RA, et al. Risk of malignancy in sonographically confirmed septated cystic ovarian tumors. Gynecol Oncol. 2010;118(3):278–282.
- Moore RG, McMeekin DS, Brown AK, et al. A novel multiple marker bioassay utilizing HE4 and CA125 for the prediction of ovarian cancer in patients with a pelvic mass. Gynecol Oncol. 2009;112(1):40–46.
- Jordan SM, Bristow RE. Ovarian cancer biomarkers as diagnostic triage tests. Current Biomarker Findings. 2013;3:35–42.
- Giede KC, Kieser K, Dodge J, Rosen B. Who should operate on patients with ovarian cancer? An evidence-based review. Gynecol Oncol. 2005;99(2):447–461.
- Pavlik EJ, Ueland FR, Miller RW, et al. Frequency and disposition of ovarian abnormalities followed with serial transvaginal ultrasonography. Obstet Gynecol. 2013;122(2 pt 1):210–217.
- Glanc P, Benacerraf B, Bourne T, et al. First International Consensus Report on adnexal masses: management recommendations. J Ultrasound Med. 2017;36(5):849–863.
- Timmerman D, Valentin L, Bourne TGH, Collins WP, Verrelst H, Vergote I; International Ovarian Tumor Analysis (IOTA) Group. Terms, definitions and measurements to describe the sonographic features of adnexal tumors: a consensus opinion from the International Ovarian Tumor Analysis (IOTA) group. Ultrasound Obstet Gynecol. 2000;6(5):500–505.
- Timmerman D, Testa AC, Bourne T, et al. Simple ultrasound-based rules for the diagnosis of ovarian cancer. Ultrasound Obstet Gynecol. 2008;31(6):681–690.
- Van Calser B, Van Hoorde K, Valentin L, et al. Evaluating the risk of ovarian cancer before surgery using the ADNEX model to differentiate between benign, borderline, early and advanced stage invasive, and secondary metastatic tumours: prospective multicentre diagnostic study. BMJ. 2014;349:g5920.
- Elder JW, Pavlik EJ, Long A, et al. Serial ultrasonographic evaluation of ovarian abnormalities with a morphology index. Gynecol Oncol. 2014;135(1):8–12.
- Lefringhouse J, Ueland F, Ore R, et al. Comparing 2 sonographic scoring systems for distinguishing benign from malignant ovarian tumors [abstract]. Gynecol Oncol. 2016;141(suppl 1):57.
- Bast RC Jr, Klug TL, St John E, et al. A radioimmunoassay using a monoclonal antibody to monitor the course of epithelial ovarian cancer. N Engl J Med. 1983;309(15):883–887.
- Jacobs I, Bast RC Jr. The CA 125 tumour-associated antigen: a review of the literature. Hum Reprod. 1989;4(1):1–12.
- Myers ER, Bastian LA, Havrilesky LJ, et al. Management of adnexal mass. Agency for Healthcare Research and Quality. https://archive.ahrq.gov/downloads/pub/evidence/pdf/adnexal/adnexal.pdf. Published February 2006. Accessed May 15, 2018.
- Coleman RL, Herzog TJ, Chan DW, et al. Validation of a second-generation multivariate index assay for malignancy risk of adnexal masses. Am J Obstet Gynecol. 2016;215(1):82.e1–e11.
- Ueland FR, Desimone CP, Seamon LG, et al. Effectiveness of a multivariate index assay in the preoperative assessment of ovarian tumors. Obstet Gynecol. 2011;117(6):1289–1297.
- Bristow RE, Smith A, Zhang Z, et al. Ovarian malignancy risk stratification of the adnexal mass using a multivariate index assay. Gynecol Oncol. 2013;128(2):252–259.
- Moore RG, McMeekin DS, Brown AK, et al. A novel multiple marker bioassay utilizing HE4 and CA125 for the prediction of ovarian cancer in patients with a pelvic mass. Gynecol Oncol. 2009;112(1):40–46.
- Valentin L, Ameye L, Franchi D, et al. Risk of malignancy in unilocular cysts: a study of 1148 adnexal masses classified as unilocular cysts on transvaginal ultrasound and review of the literature. Ultrasound Obstet Gynecol. 2013;41(1):80–89.
- Timmerman D, Ameye L, Fischerova D, et al. Simple ultrasound rules to distinguish between benign and malignant adnexal masses before surgery: prospective validation by IOTA group. BMJ. 2010;341:c6839.
- Barber HR, Graber EA. The PMPO syndrome (postmenopausal palpable ovary syndrome). Obstet Gynecol. 1971;38(6):921–923.
- Modesitt SC, Pavlik EJ, Ueland FR, DePriest PD, Kryscio RJ, van Nagell JR Jr. Risk of malignancy in unilocular ovarian cystic tumors less than 10 centimeters in diameter. Obstet Gynecol. 2003;102(3):594–599.
- Saunders BA, Podzielinski I, Ware RA, et al. Risk of malignancy in sonographically confirmed septated cystic ovarian tumors. Gynecol Oncol. 2010;118(3):278–282.
- Moore RG, McMeekin DS, Brown AK, et al. A novel multiple marker bioassay utilizing HE4 and CA125 for the prediction of ovarian cancer in patients with a pelvic mass. Gynecol Oncol. 2009;112(1):40–46.
- Jordan SM, Bristow RE. Ovarian cancer biomarkers as diagnostic triage tests. Current Biomarker Findings. 2013;3:35–42.
- Giede KC, Kieser K, Dodge J, Rosen B. Who should operate on patients with ovarian cancer? An evidence-based review. Gynecol Oncol. 2005;99(2):447–461.
- Pavlik EJ, Ueland FR, Miller RW, et al. Frequency and disposition of ovarian abnormalities followed with serial transvaginal ultrasonography. Obstet Gynecol. 2013;122(2 pt 1):210–217.
- Glanc P, Benacerraf B, Bourne T, et al. First International Consensus Report on adnexal masses: management recommendations. J Ultrasound Med. 2017;36(5):849–863.
- Timmerman D, Valentin L, Bourne TGH, Collins WP, Verrelst H, Vergote I; International Ovarian Tumor Analysis (IOTA) Group. Terms, definitions and measurements to describe the sonographic features of adnexal tumors: a consensus opinion from the International Ovarian Tumor Analysis (IOTA) group. Ultrasound Obstet Gynecol. 2000;6(5):500–505.
- Timmerman D, Testa AC, Bourne T, et al. Simple ultrasound-based rules for the diagnosis of ovarian cancer. Ultrasound Obstet Gynecol. 2008;31(6):681–690.
- Van Calser B, Van Hoorde K, Valentin L, et al. Evaluating the risk of ovarian cancer before surgery using the ADNEX model to differentiate between benign, borderline, early and advanced stage invasive, and secondary metastatic tumours: prospective multicentre diagnostic study. BMJ. 2014;349:g5920.
- Elder JW, Pavlik EJ, Long A, et al. Serial ultrasonographic evaluation of ovarian abnormalities with a morphology index. Gynecol Oncol. 2014;135(1):8–12.
- Lefringhouse J, Ueland F, Ore R, et al. Comparing 2 sonographic scoring systems for distinguishing benign from malignant ovarian tumors [abstract]. Gynecol Oncol. 2016;141(suppl 1):57.
- Bast RC Jr, Klug TL, St John E, et al. A radioimmunoassay using a monoclonal antibody to monitor the course of epithelial ovarian cancer. N Engl J Med. 1983;309(15):883–887.
- Jacobs I, Bast RC Jr. The CA 125 tumour-associated antigen: a review of the literature. Hum Reprod. 1989;4(1):1–12.
- Myers ER, Bastian LA, Havrilesky LJ, et al. Management of adnexal mass. Agency for Healthcare Research and Quality. https://archive.ahrq.gov/downloads/pub/evidence/pdf/adnexal/adnexal.pdf. Published February 2006. Accessed May 15, 2018.
- Coleman RL, Herzog TJ, Chan DW, et al. Validation of a second-generation multivariate index assay for malignancy risk of adnexal masses. Am J Obstet Gynecol. 2016;215(1):82.e1–e11.
- Ueland FR, Desimone CP, Seamon LG, et al. Effectiveness of a multivariate index assay in the preoperative assessment of ovarian tumors. Obstet Gynecol. 2011;117(6):1289–1297.
- Bristow RE, Smith A, Zhang Z, et al. Ovarian malignancy risk stratification of the adnexal mass using a multivariate index assay. Gynecol Oncol. 2013;128(2):252–259.
- Moore RG, McMeekin DS, Brown AK, et al. A novel multiple marker bioassay utilizing HE4 and CA125 for the prediction of ovarian cancer in patients with a pelvic mass. Gynecol Oncol. 2009;112(1):40–46.
- Valentin L, Ameye L, Franchi D, et al. Risk of malignancy in unilocular cysts: a study of 1148 adnexal masses classified as unilocular cysts on transvaginal ultrasound and review of the literature. Ultrasound Obstet Gynecol. 2013;41(1):80–89.
- Timmerman D, Ameye L, Fischerova D, et al. Simple ultrasound rules to distinguish between benign and malignant adnexal masses before surgery: prospective validation by IOTA group. BMJ. 2010;341:c6839.
2018 Update on menopause
Our knowledge regarding the benefits and risks of systemic menopausal hormone therapy (HT) has continued to evolve since the 2002 publication of the initial findings of the Women’s Health Initiative (WHI). In late 2017, the US Preventive Services Task Force (USPSTF) issued its recommendation against the use of menopausal HT for the prevention of chronic conditions. In this Menopause Update, Dr. JoAnn Manson, Dr. JoAnn Pinkerton, and I detail why we do not support the Task Force’s recommendation. In a sidebar discussion, Dr. Manson also reviews the results of 2 WHI HT trials, published in September 2017, that analyzed mortality in trial participants over an 18-year follow-up.
In addition, I summarize an observational study that assessed the association of HT and Alzheimer disease (AD) as well as a clinical trial that compared the impact of oral versus transdermal estrogen on sexuality in recently menopausal women.
What's the impact of long-term use of systemic HT on Alzheimer disease risk?
Imtiaz B, Tuppurainen M, Rikkonen T, et al. Postmenopausal hormone therapy and Alzheimer disease: a prospective cohort study. Neurology. 2017;88(11):1062-1068.
Data from the WHI HT randomized trials have clarified that initiation of oral HT among women aged 65 and older increases the risk of cognitive decline. By contrast, an analysis of younger WHI participants found that oral HT had no impact on cognitive function. Recently, Imtiaz and colleagues conducted a prospective cohort study of postmenopausal HT and AD in women residing in a Finnish county, with 25 years of follow-up. A diagnosis of AD was based on administrative health records and use of medications prescribed specifically to treat dementia. Use of systemic HT was identified via self-report. Overall, among more than 8,000 women followed, 227 cases of AD (mean age, 72 years) were identified.
In an analysis that controlled for factors including age, body mass index, alcohol use, smoking, physical activity, occupation status, and parity, up to 5 years of HT use was not associated with a risk of being diagnosed with AD. Five to 10 years of HT use was associated with a hazard ratio (HR) of 0.89, an 11% risk reduction that did not achieve statistical significance. By contrast, more than 10 years' use of systemic HT was associated with an HR of 0.53, a statistically significant 47% reduction in risk of AD.1
Other studies found conflicting results
Three large randomized trials found that HT initiated early in menopause and continued for less than 7 years had no impact on cognitive function.2-4 The Cache County (Utah) long-term prospective cohort study, however, found that HT started early in menopause and continued for 10 years or longer was associated with a significant reduction in risk of AD.5
Of note are results from the 2017 report of 18-year cumulative mortality among WHI participants (see the box on page 30). In that study, mortality from AD and other dementia was lower among participants who were randomly assigned to treatment with estrogen alone versus placebo (HR, 0.74; 95% confidence interval [CI], 0.59-0.94). With estrogen-progestin therapy, the HR was 0.93 (95% CI, 0.77-1.11), and the pooled HR for the 2 trials was 0.85 (95% CI, 0.74-0.98).6
NAMS guidance
The North American Menopause Society (NAMS) HT position statement recommends that prevention of dementia should not be considered an indication for HT use since definitive data are not available.7 The statement indicates also that estrogen therapy may have positive cognitive benefits when initiated immediately after early surgical menopause and taken until the average age of menopause to prevent health risks seen with early loss of hormones.
Definitive data from long-term randomized clinical trials are not likely to become available. Observational trials continue to have methodologic issues, such as "healthy user bias," but the studies are reassuring that initiating HT close to menopause does not increase the risk of dementia. The long-term Finnish study by Imtiaz and colleagues and the Cache County study provide tentative observational data support for a "critical window" hypothesis, leaving open the possibility that initiating systemic HT soon after menopause onset and continuing it long term may reduce the risk of AD. Discussion is needed on individual patient characteristics, potential benefits and risks, and ongoing assessment over time.
Read Dr. Manson’s discussion of 18 years of follow-up data on menopause.
JoAnn E. Manson, MD, DrPH, NCMP
A new analysis from the Women's Health Initiative (WHI) randomized trials examined all-cause and cause-specific mortality during the intervention and postintervention follow-up periods.1 We followed more than 27,000 postmenopausal women aged 50 to 79 (mean age, 63) who were recruited to 2 randomized WHI trials of HT between 1993 and 1998. The trials continued until 2002 for the estrogen-progestin trial and to 2004 for the estrogen-alone trial. The trials ran for 5 to 7 years' duration, with post-stopping follow-up for an additional 10 to 12 years (total cumulative follow-up of 18 years).
The participants were randomly assigned to receive active treatment or placebo. The interventions were conjugated equine estrogens (CEE) plus medroxyprogesterone acetate (MPA) versus placebo for women with an intact uterus and CEE alone versus placebo for women who had a hysterectomy.
All-cause mortality did not increase with HT use
The primary outcome measure was all-cause mortality in the 2 pooled trials and in each trial individually. We found that there was no link between HT and all-cause mortality in the overall study population (ages 50-79) in either trial. However, there was a trend toward lower all-cause mortality among the younger women in both trials. In women aged 50 to 59, there was a statistically significant 31% lower risk of mortality in the pooled trials among women taking active HT compared with those taking placebo, but no reduction in mortality with HT among older women (P for trend by age = .01).
Notably, all-cause mortality provides a critically important summary measure for interventions such as HT that have a complex matrix of benefits and risks. We know that HT has a number of benefits in menopausal women. It reduces hot flashes and other menopausal symptoms. It lowers the risk of hip fracture, other types of bone fractures, and type 2 diabetes. However, HT increases the risk of venous thrombosis, stroke, and some forms of cancer.
A summary measure that assesses the net effect of a medication on serious and life-threatening health outcomes is very important. As such, all-cause mortality is the ultimate bottom line for the balance of benefits and risks. This speaks to why we conducted the mortality analysis--WHI is the largest randomized trial of HT with long-term follow-up, allowing detailed analyses by age group. Although there have been previous reports on individual health outcomes in the WHI trials, no previous report had specifically focused on all-cause and cause-specific mortality with HT, stratified by age group, over long-term follow-up.
Hopefully the results of this study will alleviate some of the anxiety associated with HT because, as mentioned, there was no increase in overall total mortality or specific major causes of death. In addition, the younger women had a trend toward benefit for all-cause mortality.
We think that these findings support the recommendations from The North American Menopause Society and other professional societies that endorse the use of HT for managing bothersome menopausal symptoms, especially when started in early menopause. These results should be reassuring that there is no increase in mortality with HT use. Although these findings do not support prescribing HT for the express purpose of trying to prevent cardiovascular disease, dementia, or other chronic diseases (due to some potential risks), they do support an important role of HT for management of bothersome hot flashes, especially in early menopause.
Cause-specific mortality
Regarding cause-specific mortality and HT use, we looked in detail at deaths from cardiovascular causes, cancer, dementia, and other major illness. Overall, we observed no increase or decrease in cardiovascular or cancer deaths. In the estrogen-alone trial, there was a surprising finding of a 26% reduction in dementia deaths. In the estrogen-progestin trial, the results were neutral for dementia deaths.
Overall, the cause-specific mortality results were neutral. This is surprising because even for total cancer deaths there was no increase or decrease, despite a great deal of anxiety about cancer risk with HT. It appears that for cancer, HT has complex effects: it increases some types of cancer, such as breast cancer, and decreases others, such as endometrial cancer (in the estrogen-progestin group), and possibly colorectal cancer. Moreover, CEE alone was associated with a reduction in breast cancer mortality, but it remains unclear if this applies to other formulations. HT's net effect on total cancer mortality was neutral in both trials, that is, no increase or decrease.
Cautions and takeaways
We need to keep in mind that in current clinical practice, lower doses and different formulations and routes of administration of HT are now often used, including transdermal estradiol patches, gels, sprays, and micronized progesterone. These formulations, and the lower doses, may have an even more favorable benefit-risk profile. We need additional research on the long-term benefits and risks of these newer formulations and lower dosages.
Generally, these findings from the WHI trials indicate that for women who have significant hot flashes, night sweats, or other bothersome menopausal symptoms, it's important to discuss their symptoms with their health care provider and understand that hormone therapy may be an option for them. If it's not an option, many other treatments are available, including nonhormonal prescription medications, nonprescription medications, and behavioral approaches.
These findings should alleviate some fear about HT use, especially in younger women who have an overall favorable trend in terms of all-cause mortality with treatment, plus a much lower absolute risk of adverse events than older women. In a woman in early menopause who has bothersome hot flashes or other symptoms that disrupt her sleep or impair her quality of life, it's likely that the benefits of HT will outweigh the risks.
Reference
- Manson JE, Aragaki AK, Rossouw JE, et al; for the WHI Investigators. Menopausal hormone therapy and long-term all-cause and cause-specific mortality: the Women's Health Initiative randomized trials. JAMA. 2017;318(10):927-938.
Read how the route of HT may affect sexuality outcomes.
Oral vs transdermal estrogen therapy: Is one preferable regarding sexuality?
Taylor HS, Tal A, Pal L, et al. Effects of oral vs transdermal estrogen therapy on sexual function in early post menopause: ancillary study of the Kronos Early Estrogen Prevention Study (KEEPS). JAMA Intern Med. 2017;177(10):1471-1479.
If route of administration of systemic HT influences sexuality outcomes in menopausal women, this would inform how we counsel our patients regarding HT.
Recently, Taylor and colleagues conducted a randomized clinical trial to examine the effects of HT's route of administration on sexual function.8 The 4-year Kronos Early Estrogen Prevention Study (KEEPS) ancillary sexual study randomly assigned 670 recently menopausal women to 0.45 mg of oral conjugated equine estrogens (CEE), an 0.05-mg estradiol transdermal patch, or placebo (with oral micronized progesterone for those on active treatment). The participants were aged 42 to 58 years and were within 36 months from their last menstrual period.
Participants were evaluated using the Female Sexual Function Inventory (FSFI) questionnaire, which assessed desire, arousal, lubrication, orgasm, satisfaction, and pain. The FSFI is scored using a point range of 0 to 36. A higher FSFI score indicates better sexual function. An FSFI score less than 26.55 depicts low sexual function (LSF).
Transdermal estrogen improved sexual function scores
Treatment with oral CEE was associated with no significant change in FSFI score compared with placebo, although benefits were seen for lubrication. By contrast, estrogen patch use improved the FSFI score (mean improvement, 2.6). Although improvement in FSFI score with transdermal estrogen was limited to participants with baseline LSF, most participants in fact had LSF at baseline.
Oral estrogen increases the liver's production of sex hormone-binding globulin, resulting in lower free (bioavailable) testosterone. Transdermal estrogen does not produce this effect. Accordingly, sexuality concerns may represent a reason to prefer the use of transdermal as opposed to oral estrogen.
Read about the authors’ concern over new USPSTF guidance.
The USPSTF recommendation against menopausal HT use for prevention of chronic conditions: Guidance that may confuse--and mislead
US Preventive Services Task Force; Grossman DC, CurrySJ, Owens DK, et al. Hormone therapy for the primary prevention of chronic conditions in postmenopausal women: US Preventive Services Task Force recommendation statement. JAMA. 2017;318(22):2224-2233.
In late 2017, the USPSTF issued its recommendation against the use of menopausal HT for prevention of chronic conditions.9 We are concerned that this recommendation will be misconstrued as suggesting that the use of HT is not appropriate for any indication, including treatment of bothersome menopausal symptoms.
Although the Task Force's report briefly indicated that the guidance does not refer to HT use for treatment of symptoms, this important disclaimer likely will be overlooked or ignored by many readers. The result may be increased uncertainty and anxiety in decision making regarding HT use. Thus, we might see a further decline in the proportion of menopausal women who are prescribed appropriate treatment for symptoms that impair quality of life.
HT use improves menopausal symptoms
According to the 2017 NAMS Position Statement, for symptomatic women in early menopause (that is, younger than age 60 or within 10 years of menopause onset) and free of contraindications to treatment, use of systemic HT is appropriate.7 Currently, clinicians are reluctant to prescribe HT, and women are apprehensive regarding its use.10 Unfortunately, the USPSTF guidance may further discourage appropriate treatment of menopausal symptoms.
Findings from randomized clinical trials, as well as preclinical, clinical, and epidemiologic studies, clarify the favorable benefit-risk profile for HT use by recently menopausal women with bothersome vasomotor and related menopausal symptoms.7,10-12
Notably, the USPSTF guidance does not address women with premature or early menopause, those with persistent (long-duration) vasomotor symptoms, or women at increased risk for osteoporosis and related fractures. Furthermore, the prevalent and undertreated condition, genitourinary syndrome of menopause, deserves but does not receive attention.
In recent decades, our understanding regarding HT's benefits and risks has advanced substantially. Guidance for clinicians and women should reflect this evolution and underscore the individualization and shared decision making that facilitates appropriate decisions regarding the use of HT.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- Imtiaz B, Tuppurainen M, Rikkonen T, et al. Postmenopausal hormone therapy and Alzheimer disease: a prospective cohort study. Neurology. 2017;88(11):1062–1068.
- Espeland MA, Shumaker SA, Leng I, et al; WHIMSY Study Group. Long-term effects on cognitive function of postmenopausal hormone therapy prescribed to women aged 50 to 55 years. JAMA Intern Med. 2013;173(15):1429–1436.
- Gleason CE, Dowling NM, Wharton W, et al. Effects of hormone therapy on cognition and mood in recently postmenopausal women: findings from the randomized, controlled KEEPS-Cognitive and Affective Study. PLoS Med. 2015;12(6):e1001833;discussion e1001833.
- Henderson VW, St John JA, Hodis HN, et al. Cognitive effects of estradiol after menopause: a randomized trial of the timing hypothesis. Neurology. 2016;87(7):699–708.
- Shao H, Breitner JC, Whitmer RA, et al; Cache County Investigators. Hormone therapy and Alzheimer disease dementia: new findings from the Cache County Study. Neurology. 2012;79(18):1846–1852.
- Manson JE, Aragaki AK, Rossouw JE, et al; the WHI Investigators. Menopausal hormone therapy and long-term all-cause and cause-specific mortality: the Women’s Health Initiative randomized trials. JAMA. 2017;318(10):927–938.
- NAMS 2017 Hormone Therapy Position Statement Advisory Panel. The 2017 hormone therapy position statement of The North American Menopause Society. Menopause. 2017;24(7):728–753.
- Taylor HS, Tal A, Pal L, et al. Effects of oral vs transdermal estrogen therapy on sexual function in early post menopause: ancillary study of the Kronos Early Estrogen Prevention Study (KEEPS). JAMA Intern Med. 2017;177(10):1471–1479.
- US Preventive Services Task Force; Grossman DC, Curry SJ, Owens DK, et al. Hormone therapy for the primary prevention of chronic conditions in postmenopausal women: US Preventive Services Task Force recommendation statement. JAMA. 2017;318(22):2224–2233.
- Manson JE, Kaunitz AM. Menopause management—getting clinical care back on track. N Engl J Med. 2016;374(9):803–806.
- Kaunitz AM, Manson JE. Management of menopausal symptoms. Obstet Gynecol. 2015; 126(4):859–876.
- Manson JE, Chlebowski RT, Stefanick ML, et al. Menopausal hormone therapy and health outcomes during the intervention and extended poststopping phases of the Women’s Health Initiative randomized trials. JAMA. 2013;310(13):1353–1368.
Our knowledge regarding the benefits and risks of systemic menopausal hormone therapy (HT) has continued to evolve since the 2002 publication of the initial findings of the Women’s Health Initiative (WHI). In late 2017, the US Preventive Services Task Force (USPSTF) issued its recommendation against the use of menopausal HT for the prevention of chronic conditions. In this Menopause Update, Dr. JoAnn Manson, Dr. JoAnn Pinkerton, and I detail why we do not support the Task Force’s recommendation. In a sidebar discussion, Dr. Manson also reviews the results of 2 WHI HT trials, published in September 2017, that analyzed mortality in trial participants over an 18-year follow-up.
In addition, I summarize an observational study that assessed the association of HT and Alzheimer disease (AD) as well as a clinical trial that compared the impact of oral versus transdermal estrogen on sexuality in recently menopausal women.
What's the impact of long-term use of systemic HT on Alzheimer disease risk?
Imtiaz B, Tuppurainen M, Rikkonen T, et al. Postmenopausal hormone therapy and Alzheimer disease: a prospective cohort study. Neurology. 2017;88(11):1062-1068.
Data from the WHI HT randomized trials have clarified that initiation of oral HT among women aged 65 and older increases the risk of cognitive decline. By contrast, an analysis of younger WHI participants found that oral HT had no impact on cognitive function. Recently, Imtiaz and colleagues conducted a prospective cohort study of postmenopausal HT and AD in women residing in a Finnish county, with 25 years of follow-up. A diagnosis of AD was based on administrative health records and use of medications prescribed specifically to treat dementia. Use of systemic HT was identified via self-report. Overall, among more than 8,000 women followed, 227 cases of AD (mean age, 72 years) were identified.
In an analysis that controlled for factors including age, body mass index, alcohol use, smoking, physical activity, occupation status, and parity, up to 5 years of HT use was not associated with a risk of being diagnosed with AD. Five to 10 years of HT use was associated with a hazard ratio (HR) of 0.89, an 11% risk reduction that did not achieve statistical significance. By contrast, more than 10 years' use of systemic HT was associated with an HR of 0.53, a statistically significant 47% reduction in risk of AD.1
Other studies found conflicting results
Three large randomized trials found that HT initiated early in menopause and continued for less than 7 years had no impact on cognitive function.2-4 The Cache County (Utah) long-term prospective cohort study, however, found that HT started early in menopause and continued for 10 years or longer was associated with a significant reduction in risk of AD.5
Of note are results from the 2017 report of 18-year cumulative mortality among WHI participants (see the box on page 30). In that study, mortality from AD and other dementia was lower among participants who were randomly assigned to treatment with estrogen alone versus placebo (HR, 0.74; 95% confidence interval [CI], 0.59-0.94). With estrogen-progestin therapy, the HR was 0.93 (95% CI, 0.77-1.11), and the pooled HR for the 2 trials was 0.85 (95% CI, 0.74-0.98).6
NAMS guidance
The North American Menopause Society (NAMS) HT position statement recommends that prevention of dementia should not be considered an indication for HT use since definitive data are not available.7 The statement indicates also that estrogen therapy may have positive cognitive benefits when initiated immediately after early surgical menopause and taken until the average age of menopause to prevent health risks seen with early loss of hormones.
Definitive data from long-term randomized clinical trials are not likely to become available. Observational trials continue to have methodologic issues, such as "healthy user bias," but the studies are reassuring that initiating HT close to menopause does not increase the risk of dementia. The long-term Finnish study by Imtiaz and colleagues and the Cache County study provide tentative observational data support for a "critical window" hypothesis, leaving open the possibility that initiating systemic HT soon after menopause onset and continuing it long term may reduce the risk of AD. Discussion is needed on individual patient characteristics, potential benefits and risks, and ongoing assessment over time.
Read Dr. Manson’s discussion of 18 years of follow-up data on menopause.
JoAnn E. Manson, MD, DrPH, NCMP
A new analysis from the Women's Health Initiative (WHI) randomized trials examined all-cause and cause-specific mortality during the intervention and postintervention follow-up periods.1 We followed more than 27,000 postmenopausal women aged 50 to 79 (mean age, 63) who were recruited to 2 randomized WHI trials of HT between 1993 and 1998. The trials continued until 2002 for the estrogen-progestin trial and to 2004 for the estrogen-alone trial. The trials ran for 5 to 7 years' duration, with post-stopping follow-up for an additional 10 to 12 years (total cumulative follow-up of 18 years).
The participants were randomly assigned to receive active treatment or placebo. The interventions were conjugated equine estrogens (CEE) plus medroxyprogesterone acetate (MPA) versus placebo for women with an intact uterus and CEE alone versus placebo for women who had a hysterectomy.
All-cause mortality did not increase with HT use
The primary outcome measure was all-cause mortality in the 2 pooled trials and in each trial individually. We found that there was no link between HT and all-cause mortality in the overall study population (ages 50-79) in either trial. However, there was a trend toward lower all-cause mortality among the younger women in both trials. In women aged 50 to 59, there was a statistically significant 31% lower risk of mortality in the pooled trials among women taking active HT compared with those taking placebo, but no reduction in mortality with HT among older women (P for trend by age = .01).
Notably, all-cause mortality provides a critically important summary measure for interventions such as HT that have a complex matrix of benefits and risks. We know that HT has a number of benefits in menopausal women. It reduces hot flashes and other menopausal symptoms. It lowers the risk of hip fracture, other types of bone fractures, and type 2 diabetes. However, HT increases the risk of venous thrombosis, stroke, and some forms of cancer.
A summary measure that assesses the net effect of a medication on serious and life-threatening health outcomes is very important. As such, all-cause mortality is the ultimate bottom line for the balance of benefits and risks. This speaks to why we conducted the mortality analysis--WHI is the largest randomized trial of HT with long-term follow-up, allowing detailed analyses by age group. Although there have been previous reports on individual health outcomes in the WHI trials, no previous report had specifically focused on all-cause and cause-specific mortality with HT, stratified by age group, over long-term follow-up.
Hopefully the results of this study will alleviate some of the anxiety associated with HT because, as mentioned, there was no increase in overall total mortality or specific major causes of death. In addition, the younger women had a trend toward benefit for all-cause mortality.
We think that these findings support the recommendations from The North American Menopause Society and other professional societies that endorse the use of HT for managing bothersome menopausal symptoms, especially when started in early menopause. These results should be reassuring that there is no increase in mortality with HT use. Although these findings do not support prescribing HT for the express purpose of trying to prevent cardiovascular disease, dementia, or other chronic diseases (due to some potential risks), they do support an important role of HT for management of bothersome hot flashes, especially in early menopause.
Cause-specific mortality
Regarding cause-specific mortality and HT use, we looked in detail at deaths from cardiovascular causes, cancer, dementia, and other major illness. Overall, we observed no increase or decrease in cardiovascular or cancer deaths. In the estrogen-alone trial, there was a surprising finding of a 26% reduction in dementia deaths. In the estrogen-progestin trial, the results were neutral for dementia deaths.
Overall, the cause-specific mortality results were neutral. This is surprising because even for total cancer deaths there was no increase or decrease, despite a great deal of anxiety about cancer risk with HT. It appears that for cancer, HT has complex effects: it increases some types of cancer, such as breast cancer, and decreases others, such as endometrial cancer (in the estrogen-progestin group), and possibly colorectal cancer. Moreover, CEE alone was associated with a reduction in breast cancer mortality, but it remains unclear if this applies to other formulations. HT's net effect on total cancer mortality was neutral in both trials, that is, no increase or decrease.
Cautions and takeaways
We need to keep in mind that in current clinical practice, lower doses and different formulations and routes of administration of HT are now often used, including transdermal estradiol patches, gels, sprays, and micronized progesterone. These formulations, and the lower doses, may have an even more favorable benefit-risk profile. We need additional research on the long-term benefits and risks of these newer formulations and lower dosages.
Generally, these findings from the WHI trials indicate that for women who have significant hot flashes, night sweats, or other bothersome menopausal symptoms, it's important to discuss their symptoms with their health care provider and understand that hormone therapy may be an option for them. If it's not an option, many other treatments are available, including nonhormonal prescription medications, nonprescription medications, and behavioral approaches.
These findings should alleviate some fear about HT use, especially in younger women who have an overall favorable trend in terms of all-cause mortality with treatment, plus a much lower absolute risk of adverse events than older women. In a woman in early menopause who has bothersome hot flashes or other symptoms that disrupt her sleep or impair her quality of life, it's likely that the benefits of HT will outweigh the risks.
Reference
- Manson JE, Aragaki AK, Rossouw JE, et al; for the WHI Investigators. Menopausal hormone therapy and long-term all-cause and cause-specific mortality: the Women's Health Initiative randomized trials. JAMA. 2017;318(10):927-938.
Read how the route of HT may affect sexuality outcomes.
Oral vs transdermal estrogen therapy: Is one preferable regarding sexuality?
Taylor HS, Tal A, Pal L, et al. Effects of oral vs transdermal estrogen therapy on sexual function in early post menopause: ancillary study of the Kronos Early Estrogen Prevention Study (KEEPS). JAMA Intern Med. 2017;177(10):1471-1479.
If route of administration of systemic HT influences sexuality outcomes in menopausal women, this would inform how we counsel our patients regarding HT.
Recently, Taylor and colleagues conducted a randomized clinical trial to examine the effects of HT's route of administration on sexual function.8 The 4-year Kronos Early Estrogen Prevention Study (KEEPS) ancillary sexual study randomly assigned 670 recently menopausal women to 0.45 mg of oral conjugated equine estrogens (CEE), an 0.05-mg estradiol transdermal patch, or placebo (with oral micronized progesterone for those on active treatment). The participants were aged 42 to 58 years and were within 36 months from their last menstrual period.
Participants were evaluated using the Female Sexual Function Inventory (FSFI) questionnaire, which assessed desire, arousal, lubrication, orgasm, satisfaction, and pain. The FSFI is scored using a point range of 0 to 36. A higher FSFI score indicates better sexual function. An FSFI score less than 26.55 depicts low sexual function (LSF).
Transdermal estrogen improved sexual function scores
Treatment with oral CEE was associated with no significant change in FSFI score compared with placebo, although benefits were seen for lubrication. By contrast, estrogen patch use improved the FSFI score (mean improvement, 2.6). Although improvement in FSFI score with transdermal estrogen was limited to participants with baseline LSF, most participants in fact had LSF at baseline.
Oral estrogen increases the liver's production of sex hormone-binding globulin, resulting in lower free (bioavailable) testosterone. Transdermal estrogen does not produce this effect. Accordingly, sexuality concerns may represent a reason to prefer the use of transdermal as opposed to oral estrogen.
Read about the authors’ concern over new USPSTF guidance.
The USPSTF recommendation against menopausal HT use for prevention of chronic conditions: Guidance that may confuse--and mislead
US Preventive Services Task Force; Grossman DC, CurrySJ, Owens DK, et al. Hormone therapy for the primary prevention of chronic conditions in postmenopausal women: US Preventive Services Task Force recommendation statement. JAMA. 2017;318(22):2224-2233.
In late 2017, the USPSTF issued its recommendation against the use of menopausal HT for prevention of chronic conditions.9 We are concerned that this recommendation will be misconstrued as suggesting that the use of HT is not appropriate for any indication, including treatment of bothersome menopausal symptoms.
Although the Task Force's report briefly indicated that the guidance does not refer to HT use for treatment of symptoms, this important disclaimer likely will be overlooked or ignored by many readers. The result may be increased uncertainty and anxiety in decision making regarding HT use. Thus, we might see a further decline in the proportion of menopausal women who are prescribed appropriate treatment for symptoms that impair quality of life.
HT use improves menopausal symptoms
According to the 2017 NAMS Position Statement, for symptomatic women in early menopause (that is, younger than age 60 or within 10 years of menopause onset) and free of contraindications to treatment, use of systemic HT is appropriate.7 Currently, clinicians are reluctant to prescribe HT, and women are apprehensive regarding its use.10 Unfortunately, the USPSTF guidance may further discourage appropriate treatment of menopausal symptoms.
Findings from randomized clinical trials, as well as preclinical, clinical, and epidemiologic studies, clarify the favorable benefit-risk profile for HT use by recently menopausal women with bothersome vasomotor and related menopausal symptoms.7,10-12
Notably, the USPSTF guidance does not address women with premature or early menopause, those with persistent (long-duration) vasomotor symptoms, or women at increased risk for osteoporosis and related fractures. Furthermore, the prevalent and undertreated condition, genitourinary syndrome of menopause, deserves but does not receive attention.
In recent decades, our understanding regarding HT's benefits and risks has advanced substantially. Guidance for clinicians and women should reflect this evolution and underscore the individualization and shared decision making that facilitates appropriate decisions regarding the use of HT.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
Our knowledge regarding the benefits and risks of systemic menopausal hormone therapy (HT) has continued to evolve since the 2002 publication of the initial findings of the Women’s Health Initiative (WHI). In late 2017, the US Preventive Services Task Force (USPSTF) issued its recommendation against the use of menopausal HT for the prevention of chronic conditions. In this Menopause Update, Dr. JoAnn Manson, Dr. JoAnn Pinkerton, and I detail why we do not support the Task Force’s recommendation. In a sidebar discussion, Dr. Manson also reviews the results of 2 WHI HT trials, published in September 2017, that analyzed mortality in trial participants over an 18-year follow-up.
In addition, I summarize an observational study that assessed the association of HT and Alzheimer disease (AD) as well as a clinical trial that compared the impact of oral versus transdermal estrogen on sexuality in recently menopausal women.
What's the impact of long-term use of systemic HT on Alzheimer disease risk?
Imtiaz B, Tuppurainen M, Rikkonen T, et al. Postmenopausal hormone therapy and Alzheimer disease: a prospective cohort study. Neurology. 2017;88(11):1062-1068.
Data from the WHI HT randomized trials have clarified that initiation of oral HT among women aged 65 and older increases the risk of cognitive decline. By contrast, an analysis of younger WHI participants found that oral HT had no impact on cognitive function. Recently, Imtiaz and colleagues conducted a prospective cohort study of postmenopausal HT and AD in women residing in a Finnish county, with 25 years of follow-up. A diagnosis of AD was based on administrative health records and use of medications prescribed specifically to treat dementia. Use of systemic HT was identified via self-report. Overall, among more than 8,000 women followed, 227 cases of AD (mean age, 72 years) were identified.
In an analysis that controlled for factors including age, body mass index, alcohol use, smoking, physical activity, occupation status, and parity, up to 5 years of HT use was not associated with a risk of being diagnosed with AD. Five to 10 years of HT use was associated with a hazard ratio (HR) of 0.89, an 11% risk reduction that did not achieve statistical significance. By contrast, more than 10 years' use of systemic HT was associated with an HR of 0.53, a statistically significant 47% reduction in risk of AD.1
Other studies found conflicting results
Three large randomized trials found that HT initiated early in menopause and continued for less than 7 years had no impact on cognitive function.2-4 The Cache County (Utah) long-term prospective cohort study, however, found that HT started early in menopause and continued for 10 years or longer was associated with a significant reduction in risk of AD.5
Of note are results from the 2017 report of 18-year cumulative mortality among WHI participants (see the box on page 30). In that study, mortality from AD and other dementia was lower among participants who were randomly assigned to treatment with estrogen alone versus placebo (HR, 0.74; 95% confidence interval [CI], 0.59-0.94). With estrogen-progestin therapy, the HR was 0.93 (95% CI, 0.77-1.11), and the pooled HR for the 2 trials was 0.85 (95% CI, 0.74-0.98).6
NAMS guidance
The North American Menopause Society (NAMS) HT position statement recommends that prevention of dementia should not be considered an indication for HT use since definitive data are not available.7 The statement indicates also that estrogen therapy may have positive cognitive benefits when initiated immediately after early surgical menopause and taken until the average age of menopause to prevent health risks seen with early loss of hormones.
Definitive data from long-term randomized clinical trials are not likely to become available. Observational trials continue to have methodologic issues, such as "healthy user bias," but the studies are reassuring that initiating HT close to menopause does not increase the risk of dementia. The long-term Finnish study by Imtiaz and colleagues and the Cache County study provide tentative observational data support for a "critical window" hypothesis, leaving open the possibility that initiating systemic HT soon after menopause onset and continuing it long term may reduce the risk of AD. Discussion is needed on individual patient characteristics, potential benefits and risks, and ongoing assessment over time.
Read Dr. Manson’s discussion of 18 years of follow-up data on menopause.
JoAnn E. Manson, MD, DrPH, NCMP
A new analysis from the Women's Health Initiative (WHI) randomized trials examined all-cause and cause-specific mortality during the intervention and postintervention follow-up periods.1 We followed more than 27,000 postmenopausal women aged 50 to 79 (mean age, 63) who were recruited to 2 randomized WHI trials of HT between 1993 and 1998. The trials continued until 2002 for the estrogen-progestin trial and to 2004 for the estrogen-alone trial. The trials ran for 5 to 7 years' duration, with post-stopping follow-up for an additional 10 to 12 years (total cumulative follow-up of 18 years).
The participants were randomly assigned to receive active treatment or placebo. The interventions were conjugated equine estrogens (CEE) plus medroxyprogesterone acetate (MPA) versus placebo for women with an intact uterus and CEE alone versus placebo for women who had a hysterectomy.
All-cause mortality did not increase with HT use
The primary outcome measure was all-cause mortality in the 2 pooled trials and in each trial individually. We found that there was no link between HT and all-cause mortality in the overall study population (ages 50-79) in either trial. However, there was a trend toward lower all-cause mortality among the younger women in both trials. In women aged 50 to 59, there was a statistically significant 31% lower risk of mortality in the pooled trials among women taking active HT compared with those taking placebo, but no reduction in mortality with HT among older women (P for trend by age = .01).
Notably, all-cause mortality provides a critically important summary measure for interventions such as HT that have a complex matrix of benefits and risks. We know that HT has a number of benefits in menopausal women. It reduces hot flashes and other menopausal symptoms. It lowers the risk of hip fracture, other types of bone fractures, and type 2 diabetes. However, HT increases the risk of venous thrombosis, stroke, and some forms of cancer.
A summary measure that assesses the net effect of a medication on serious and life-threatening health outcomes is very important. As such, all-cause mortality is the ultimate bottom line for the balance of benefits and risks. This speaks to why we conducted the mortality analysis--WHI is the largest randomized trial of HT with long-term follow-up, allowing detailed analyses by age group. Although there have been previous reports on individual health outcomes in the WHI trials, no previous report had specifically focused on all-cause and cause-specific mortality with HT, stratified by age group, over long-term follow-up.
Hopefully the results of this study will alleviate some of the anxiety associated with HT because, as mentioned, there was no increase in overall total mortality or specific major causes of death. In addition, the younger women had a trend toward benefit for all-cause mortality.
We think that these findings support the recommendations from The North American Menopause Society and other professional societies that endorse the use of HT for managing bothersome menopausal symptoms, especially when started in early menopause. These results should be reassuring that there is no increase in mortality with HT use. Although these findings do not support prescribing HT for the express purpose of trying to prevent cardiovascular disease, dementia, or other chronic diseases (due to some potential risks), they do support an important role of HT for management of bothersome hot flashes, especially in early menopause.
Cause-specific mortality
Regarding cause-specific mortality and HT use, we looked in detail at deaths from cardiovascular causes, cancer, dementia, and other major illness. Overall, we observed no increase or decrease in cardiovascular or cancer deaths. In the estrogen-alone trial, there was a surprising finding of a 26% reduction in dementia deaths. In the estrogen-progestin trial, the results were neutral for dementia deaths.
Overall, the cause-specific mortality results were neutral. This is surprising because even for total cancer deaths there was no increase or decrease, despite a great deal of anxiety about cancer risk with HT. It appears that for cancer, HT has complex effects: it increases some types of cancer, such as breast cancer, and decreases others, such as endometrial cancer (in the estrogen-progestin group), and possibly colorectal cancer. Moreover, CEE alone was associated with a reduction in breast cancer mortality, but it remains unclear if this applies to other formulations. HT's net effect on total cancer mortality was neutral in both trials, that is, no increase or decrease.
Cautions and takeaways
We need to keep in mind that in current clinical practice, lower doses and different formulations and routes of administration of HT are now often used, including transdermal estradiol patches, gels, sprays, and micronized progesterone. These formulations, and the lower doses, may have an even more favorable benefit-risk profile. We need additional research on the long-term benefits and risks of these newer formulations and lower dosages.
Generally, these findings from the WHI trials indicate that for women who have significant hot flashes, night sweats, or other bothersome menopausal symptoms, it's important to discuss their symptoms with their health care provider and understand that hormone therapy may be an option for them. If it's not an option, many other treatments are available, including nonhormonal prescription medications, nonprescription medications, and behavioral approaches.
These findings should alleviate some fear about HT use, especially in younger women who have an overall favorable trend in terms of all-cause mortality with treatment, plus a much lower absolute risk of adverse events than older women. In a woman in early menopause who has bothersome hot flashes or other symptoms that disrupt her sleep or impair her quality of life, it's likely that the benefits of HT will outweigh the risks.
Reference
- Manson JE, Aragaki AK, Rossouw JE, et al; for the WHI Investigators. Menopausal hormone therapy and long-term all-cause and cause-specific mortality: the Women's Health Initiative randomized trials. JAMA. 2017;318(10):927-938.
Read how the route of HT may affect sexuality outcomes.
Oral vs transdermal estrogen therapy: Is one preferable regarding sexuality?
Taylor HS, Tal A, Pal L, et al. Effects of oral vs transdermal estrogen therapy on sexual function in early post menopause: ancillary study of the Kronos Early Estrogen Prevention Study (KEEPS). JAMA Intern Med. 2017;177(10):1471-1479.
If route of administration of systemic HT influences sexuality outcomes in menopausal women, this would inform how we counsel our patients regarding HT.
Recently, Taylor and colleagues conducted a randomized clinical trial to examine the effects of HT's route of administration on sexual function.8 The 4-year Kronos Early Estrogen Prevention Study (KEEPS) ancillary sexual study randomly assigned 670 recently menopausal women to 0.45 mg of oral conjugated equine estrogens (CEE), an 0.05-mg estradiol transdermal patch, or placebo (with oral micronized progesterone for those on active treatment). The participants were aged 42 to 58 years and were within 36 months from their last menstrual period.
Participants were evaluated using the Female Sexual Function Inventory (FSFI) questionnaire, which assessed desire, arousal, lubrication, orgasm, satisfaction, and pain. The FSFI is scored using a point range of 0 to 36. A higher FSFI score indicates better sexual function. An FSFI score less than 26.55 depicts low sexual function (LSF).
Transdermal estrogen improved sexual function scores
Treatment with oral CEE was associated with no significant change in FSFI score compared with placebo, although benefits were seen for lubrication. By contrast, estrogen patch use improved the FSFI score (mean improvement, 2.6). Although improvement in FSFI score with transdermal estrogen was limited to participants with baseline LSF, most participants in fact had LSF at baseline.
Oral estrogen increases the liver's production of sex hormone-binding globulin, resulting in lower free (bioavailable) testosterone. Transdermal estrogen does not produce this effect. Accordingly, sexuality concerns may represent a reason to prefer the use of transdermal as opposed to oral estrogen.
Read about the authors’ concern over new USPSTF guidance.
The USPSTF recommendation against menopausal HT use for prevention of chronic conditions: Guidance that may confuse--and mislead
US Preventive Services Task Force; Grossman DC, CurrySJ, Owens DK, et al. Hormone therapy for the primary prevention of chronic conditions in postmenopausal women: US Preventive Services Task Force recommendation statement. JAMA. 2017;318(22):2224-2233.
In late 2017, the USPSTF issued its recommendation against the use of menopausal HT for prevention of chronic conditions.9 We are concerned that this recommendation will be misconstrued as suggesting that the use of HT is not appropriate for any indication, including treatment of bothersome menopausal symptoms.
Although the Task Force's report briefly indicated that the guidance does not refer to HT use for treatment of symptoms, this important disclaimer likely will be overlooked or ignored by many readers. The result may be increased uncertainty and anxiety in decision making regarding HT use. Thus, we might see a further decline in the proportion of menopausal women who are prescribed appropriate treatment for symptoms that impair quality of life.
HT use improves menopausal symptoms
According to the 2017 NAMS Position Statement, for symptomatic women in early menopause (that is, younger than age 60 or within 10 years of menopause onset) and free of contraindications to treatment, use of systemic HT is appropriate.7 Currently, clinicians are reluctant to prescribe HT, and women are apprehensive regarding its use.10 Unfortunately, the USPSTF guidance may further discourage appropriate treatment of menopausal symptoms.
Findings from randomized clinical trials, as well as preclinical, clinical, and epidemiologic studies, clarify the favorable benefit-risk profile for HT use by recently menopausal women with bothersome vasomotor and related menopausal symptoms.7,10-12
Notably, the USPSTF guidance does not address women with premature or early menopause, those with persistent (long-duration) vasomotor symptoms, or women at increased risk for osteoporosis and related fractures. Furthermore, the prevalent and undertreated condition, genitourinary syndrome of menopause, deserves but does not receive attention.
In recent decades, our understanding regarding HT's benefits and risks has advanced substantially. Guidance for clinicians and women should reflect this evolution and underscore the individualization and shared decision making that facilitates appropriate decisions regarding the use of HT.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- Imtiaz B, Tuppurainen M, Rikkonen T, et al. Postmenopausal hormone therapy and Alzheimer disease: a prospective cohort study. Neurology. 2017;88(11):1062–1068.
- Espeland MA, Shumaker SA, Leng I, et al; WHIMSY Study Group. Long-term effects on cognitive function of postmenopausal hormone therapy prescribed to women aged 50 to 55 years. JAMA Intern Med. 2013;173(15):1429–1436.
- Gleason CE, Dowling NM, Wharton W, et al. Effects of hormone therapy on cognition and mood in recently postmenopausal women: findings from the randomized, controlled KEEPS-Cognitive and Affective Study. PLoS Med. 2015;12(6):e1001833;discussion e1001833.
- Henderson VW, St John JA, Hodis HN, et al. Cognitive effects of estradiol after menopause: a randomized trial of the timing hypothesis. Neurology. 2016;87(7):699–708.
- Shao H, Breitner JC, Whitmer RA, et al; Cache County Investigators. Hormone therapy and Alzheimer disease dementia: new findings from the Cache County Study. Neurology. 2012;79(18):1846–1852.
- Manson JE, Aragaki AK, Rossouw JE, et al; the WHI Investigators. Menopausal hormone therapy and long-term all-cause and cause-specific mortality: the Women’s Health Initiative randomized trials. JAMA. 2017;318(10):927–938.
- NAMS 2017 Hormone Therapy Position Statement Advisory Panel. The 2017 hormone therapy position statement of The North American Menopause Society. Menopause. 2017;24(7):728–753.
- Taylor HS, Tal A, Pal L, et al. Effects of oral vs transdermal estrogen therapy on sexual function in early post menopause: ancillary study of the Kronos Early Estrogen Prevention Study (KEEPS). JAMA Intern Med. 2017;177(10):1471–1479.
- US Preventive Services Task Force; Grossman DC, Curry SJ, Owens DK, et al. Hormone therapy for the primary prevention of chronic conditions in postmenopausal women: US Preventive Services Task Force recommendation statement. JAMA. 2017;318(22):2224–2233.
- Manson JE, Kaunitz AM. Menopause management—getting clinical care back on track. N Engl J Med. 2016;374(9):803–806.
- Kaunitz AM, Manson JE. Management of menopausal symptoms. Obstet Gynecol. 2015; 126(4):859–876.
- Manson JE, Chlebowski RT, Stefanick ML, et al. Menopausal hormone therapy and health outcomes during the intervention and extended poststopping phases of the Women’s Health Initiative randomized trials. JAMA. 2013;310(13):1353–1368.
- Imtiaz B, Tuppurainen M, Rikkonen T, et al. Postmenopausal hormone therapy and Alzheimer disease: a prospective cohort study. Neurology. 2017;88(11):1062–1068.
- Espeland MA, Shumaker SA, Leng I, et al; WHIMSY Study Group. Long-term effects on cognitive function of postmenopausal hormone therapy prescribed to women aged 50 to 55 years. JAMA Intern Med. 2013;173(15):1429–1436.
- Gleason CE, Dowling NM, Wharton W, et al. Effects of hormone therapy on cognition and mood in recently postmenopausal women: findings from the randomized, controlled KEEPS-Cognitive and Affective Study. PLoS Med. 2015;12(6):e1001833;discussion e1001833.
- Henderson VW, St John JA, Hodis HN, et al. Cognitive effects of estradiol after menopause: a randomized trial of the timing hypothesis. Neurology. 2016;87(7):699–708.
- Shao H, Breitner JC, Whitmer RA, et al; Cache County Investigators. Hormone therapy and Alzheimer disease dementia: new findings from the Cache County Study. Neurology. 2012;79(18):1846–1852.
- Manson JE, Aragaki AK, Rossouw JE, et al; the WHI Investigators. Menopausal hormone therapy and long-term all-cause and cause-specific mortality: the Women’s Health Initiative randomized trials. JAMA. 2017;318(10):927–938.
- NAMS 2017 Hormone Therapy Position Statement Advisory Panel. The 2017 hormone therapy position statement of The North American Menopause Society. Menopause. 2017;24(7):728–753.
- Taylor HS, Tal A, Pal L, et al. Effects of oral vs transdermal estrogen therapy on sexual function in early post menopause: ancillary study of the Kronos Early Estrogen Prevention Study (KEEPS). JAMA Intern Med. 2017;177(10):1471–1479.
- US Preventive Services Task Force; Grossman DC, Curry SJ, Owens DK, et al. Hormone therapy for the primary prevention of chronic conditions in postmenopausal women: US Preventive Services Task Force recommendation statement. JAMA. 2017;318(22):2224–2233.
- Manson JE, Kaunitz AM. Menopause management—getting clinical care back on track. N Engl J Med. 2016;374(9):803–806.
- Kaunitz AM, Manson JE. Management of menopausal symptoms. Obstet Gynecol. 2015; 126(4):859–876.
- Manson JE, Chlebowski RT, Stefanick ML, et al. Menopausal hormone therapy and health outcomes during the intervention and extended poststopping phases of the Women’s Health Initiative randomized trials. JAMA. 2013;310(13):1353–1368.
The Potential Value of Dual-Energy X-Ray Absorptiometry in Orthopedics
ABSTRACT
Dual-energy X-ray absorptiometry (DXA) is a well-established technology with an important and well-known role in measuring bone mineral density (BMD) for the purpose of determining fracture risk, diagnosing osteoporosis, and monitoring treatment efficacy. However, aside from the assessment of bone status, DXA is likely underutilized in the field of orthopedics, and most orthopedists may not be aware of the full capabilities of DXA, particularly with regard to total body scans and body composition assessment. For example, DXA would be a valuable tool for monitoring body composition after surgery where compensatory changes in the affected limb may lead to right-left asymmetry (eg, tracking lean mass change after knee surgery), rehabilitation regimens for athletes, congenital and metabolic disorders that affect the musculoskeletal system, or monitoring sarcopenia and frailty in the elderly. Furthermore, preoperative and postoperative regional scans can track BMD changes during healing or alert surgeons to impending problems such as loss of periprosthetic bone, which could lead to implant failure. This article discusses the capabilities of DXA and how this technology could be better used to the advantage of the attending orthopedist.
Dual-energy X-ray absorptiometry, abbreviated as “DXA,” (although usually abbreviated in older literature as “DEXA”) was first introduced in 1987 (Hologic QDR-1000 system, Hologic, Inc) and immediately made all previous forms of radiation-based bone mineral density (BMD) measurement systems obsolete.1 Since then, there have been many generations of the technology, with the main US manufacturers in 2017 being Hologic, Inc. and GE Lunar. There are 2 forms of DXA, peripheral systems (which usually measure BMD only in the radius, finger bones, or calcaneus) and central systems (which measure the radius, proximal femur [“hip”], lumbar spine, total body, and custom sites). The general principle of how DXA works is based on the differential attenuation of photons by bone, fat, and lean mass.2 The DXA technique uses a low- and high-energy X-ray beam produced by an X-ray tube. With the low-energy beam, attenuation by bone is greater than attenuation by soft tissue. With the high-energy beam, attenuation by bone and soft tissues are similar. The dual X-ray beams are passed through the body regions being scanned (usually posterioanteriorly), and the differential attenuation by bone and soft tissue is analyzed to produce BMD estimates. In addition, a high-quality image is produced to enable the operator of the DXA system to verify that the appropriate body region was scanned. It is important to realize that DXA is 2-dimensional (which is sometimes cited as a weakness of DXA), and the units of BMD are grams of mineral per centimeter squared (g/cm2).
Continue to: When assessing bone status...
When assessing bone status for the purpose of determining if a patient is normal, osteopenic, or osteoporotic, the skeletal sites (called regions of interest [ROI]) typically scanned are the proximal femur, lumbar spine, and radius. The BMD of the patient is then compared to a manufacturer-provided normative database of young adults (the logic being that the BMD in the young adult normative population represents maximal peak bone mass). Total body BMD and body composition can also be quantified (grams of lean and fat mass), and custom scans can be designed for other skeletal sites. Specifically, a patient’s BMD is compared to a database of sex- and age-adjusted normal values, and the deviation from normal is expressed as a T-score (the number of standard deviations the patient's BMD is above or below the average BMD of the young adult reference population) and Z-scores (the number of standard deviations a patient's BMD is above or below the average BMD of a sex- and age-matched reference population).3 The International Society for Clinical Densitometry (ISCD) has developed and published well-accepted guidelines used to assist in acquiring high-quality DXA scans and for the diagnosis of osteoporosis using BMD. The accuracy and, especially, the precision of DXA scans can be remarkable when they are performed by trained technologists, and thus, serial scans can be performed to monitor BMD and body composition changes with aging or in response to treatment.
Because of the nature of the scan mechanics and speed, the effective radiation dose with DXA is very low, expressed in microSieverts.4,5 Generally, the radiation exposure from a series of the lumbar spine, proximal femur, and distal radius is about the same as daily background radiation. Even total body scans present very low exposure due to the scan speed at which any 1 body part is exposed for only a fraction of a second.
BENEFITS OF USING DXA FOR THE ORTHEOPEDIST
At the time of this writing in 2018, the presumption could be made that most physicians in the specialties of internal medicine, rheumatology, endocrinology, radiology, and orthopedics were familiar with the capabilities of DXA to assess BMD for the purpose of diagnosing osteoporosis. However, DXA is likely underused for other purposes, as orthopedists may be unaware of the full capabilities of DXA. Printouts after a scan contain more information than simply BMD, and there are more features and applications of DXA that can potentially be useful to orthopedists.
BONE SIZE
Data from a DXA scan are expressed not only as g/cm2 (BMD) but also as total grams in the ROI (known as bone mineral content, abbreviated as BMC), and cm2 (area of the ROI). These data may appear on a separate page, being considered ancillary results. The latter 2 variables are rarely included on a report sent to a referring physician; therefore, awareness of their value is probably limited. However, there are instances where such information could be valuable when interpreting results, especially bone size.6,7 For example, on occasion, patients present with osteopenic lumbar vertebrate but larger than normal vertebral size (area). Many studies have shown that bone size is directly related to bone strength and thus fracture risk.8,9 Although an understudied phenomenon, large vertebral body size could be protective, counteracting a lower than optimal BMD. Further, because the area of the ROI is measured, it is possible to calculate the bone width (or measure directly with a ruler tool in the software if available) for the area measured. This is especially feasible for tubular bones such as the midshaft of the radius, or more specifically, the classic DXA ROI being the area approximately one third the length of the radius from the distal end, the radius 33% region (actually based on ulna length). Consequently, it is possible to use the width of the radius 33% ROI in addition to BMD and T-score when assessing fracture risk.
CASE STUDY
A 60-year-old man had a DXA series of the lumbar spine, proximal femur, and whole body. His total body T-score was 0.6 (normal), and his total proximal femur T-score was −0.8 (normal), but his lumbar spine vertebrae 2 to 4 T-score was −1.9. As the patient was osteopenic based on the lumbar spine T-score, some physicians may have initiated antiresorptive therapy, especially if other risk factors for fracture were present. Further examination of the ancillary results of the DXA scan revealed that the vertebral body height T-score was a remarkable 1.11 and 1.53 after adjustment for stature (automatic software calculation). These results suggested that the patient had vertebral bodies of above average size, which theoretically would be protective against fracture even though the BMD T-score was below normal. For this patient, this finding mitigated immediate concern about the lumbar spine T-score of −1.9. Although vertebral body size is not typically used in assessing fracture risk, it is useful information that could be factored into the decision to start treatment or watch for further change with aging.
Continue to: Case Series: Distal Radius Fractures...
CASE SERIES: DISTAL RADIUS FRACTURES
Table 1 summarizes the data comparing radius 33% ROI T-scores and ROI width in patients who fractured the contralateral radius and normal nonfractured controls.10
Table 1. Comparison of Radius Width at the 33% Region of Interest (ROI) and Bone Mineral Density T-Scores in Premenopausal Women With and Without Fractures
| 33% ROI T-score | Width of ROI, cm |
White women with distal radius fractures |
|
|
Premenopausal (<49 years), n = 36 | -0.2 + 0.9 | 1.22 + 0.11a |
Controls matched for race, age, BMIb |
|
|
Premenopausal (<49 years), n = 65 | -0.1 + 0.8 | 1.45 + 0.25 |
For premenopausal women with distal radius fractures, the width of the radius at the radius 33% ROI was significantly smaller than that in controls. However, there was no difference in T-scores between premenopausal women with distal radius fractures and controls. Thus, bone width more accurately identified women with fractures than T-scores based on BMD, and the orthopedist could use bone size in addition to BMD to predict fracture risk in a patient.
PREPARATION FOR SURGERY
For some procedures, there is potential benefit of assessing bone status prior to surgery. That is, determination of low BMD could potentially influence the type of hardware or fixation techniques used in surgery. Various studies have shown that poor bone quality and low BMD can impair purchase with various types of fixation.11-13 Low preoperative BMD has been shown to be related to high implant migration.14 Knowledge of BMD could influence the choice of screw type used or the type of implant metal (titanium vs cobalt chrome). Another example is predicting the risk of spine curvature progression in adolescent idiopathic scoliosis.15-17 It has been reported that low BMD is a risk factor for progression.15 Knowledge of BMD could potentially help with patient management strategies. For example, a patient with low BMD and vitamin D deficiency could be treated (vitamin D supplementation) prior to planning surgery in an effort to improve the low BMD.
PERIOPROSTHETIC BMD
It is possible to monitor changes in BMD around implants using the periprosthetic software application (this usually needs to be purchased separately from standard software that is installed with a system set-up). Dramatic loss of bone due to stress shielding after total hip arthroplasty (THA) can be a risk factor for implant migration or potentially outright failure of fixation or breakthrough. If bone loss occurs and is observed in the early stages, then antiresorptive treatment can be initiated to limit further loss.18,19 (Figure 1) shows the image from a periprosthetic scan.
Continue to: A 60-year-old, 215-lb man...
CASE REPORT
A 60-year-old, 215-lb man had a total hip replacement using a newly introduced cemented collared cobalt-chromium alloy femoral stem. A baseline periprosthetic DXA scan was performed 6 weeks postoperatively. Compared to baseline, the change in BMD in the Gruen zone 5 was −8.2%, +6.5%, +4.9%, and +9.46% at 3, 6, 12, and 24 months, respectively. In contrast, dramatic BMD loss was seen in Gruen zone 7 (calcar region): −33.2%, −40.8%, −37.1%, and −34.1% at 3, 6, 12, and 24 months, respectively. Similar findings in other patients led to discontinuation of use of this stem in favor of a collarless stem in which less BMD loss was seen in Gruen zone 7. Although additional technologist training is required and scans may not be reimbursable, for research purposes or for evaluating new component prototypes, the periprosthetic DXA scan capability can be useful.
Various other custom scans can be used to detect and quantify vertebral fractures (vertebral fracture assessment application), monitor healing of fractures by scanning through radiolucent cast materials, or for research purposes to assess BMD at unusual locations.21-23 Other new innovations, such as the ability to perform full-length scans of the femoral shaft and to quantify focal thickening of the lateral cortex to identify beaking, an abnormality associated with atypical femur fracture after long-term bisphosphonate use, continue to expand the utility of DXA. Using standard software, cadaver bones can be scanned prior to biomechanical testing for a variety of purposes, such as ensuring proper matching specimens in test groups. It has been reported that the common practice of using contralateral bone specimens can lead to bias, as the BMD can be significantly different in right and left bones from the same individual.9,24
TOTAL BODY BMD AND BODY COMPOSITION SCANS
Perhaps the least understood capability of DXA from our experience working with orthopedists is the ability to perform total body scans and to obtain not only total body and regional BMD but also body composition data, namely grams of lean and fat mass.25 Soft tissue (no bone pixels) is partitioned into fat and lean body mass by a calibration procedure (lean mass = total soft tissue –fat mass). DXA has become the standard for body composition assessment given the ease of data acquisition (a total body scan takes only a few minutes), accuracy, and precision of measurements. Compared with other methods (eg, skinfold thickness, bioelectrical impedance, and underwater weighing), it is the only method that gives regional values for fat mass, lean mass, and BMC (this allows the ability to compare left vs right sides).25-27 The ability to perform regional measurements cannot be overstated, as stable body weight belies potential changes with age and disease that relate to redistribution of fat and lean mass. It is not possible to identify, let alone track, such changes by measuring gross body weight on a scale or with BMI calculations. However, redistribution of fat and lean mass can be monitored in great detail using DXA. Figures 2 and 3 show the typical output from a DXA total body/body composition scan.
Total body scans with body composition analyses have many applications. For example, monitoring growth and development or treatment in patients with congenital deformity, metabolic bone disease, osteoporosis, and frailty; patients undergoing rehabilitation; and patients having surgery that could affect the use of a contralateral limb with potential hypertrophy or atrophy. Accurate assessment of percent body fat and fat distribution may help surgeons to improve risk stratification and surgical outcome.28-30 Fracture risk has been associated with muscle area.28 Simple measurements of quadriceps size underestimates atrophy, and total body composition can quantitate lean mass.30
In sports medicine, body composition assessments could be useful to monitor postoperative recovery and effectiveness of rehabilitation protocols after injury, effectiveness of conditioning and training programs, developmental changes due to sports participation, and for obtaining baseline assessment at the time of preseason physicals.27,31-34 In athletes, baseline status and morphological adaptations to training have traditionally been measured by anthropometry (eg, skinfold thickness, BMI, limb circumference, etc.), but DXA total body scanning allows for much more detailed assessments with the possibility of subregional quantitation. There is evidence for sports-specific body composition profiles and characteristic adaptations.27,31-34 Using DXA, adaptive changes as a result of training as well as changes and recovery after surgery or injury can be monitored. For example, quadriceps atrophy usually occurs to some extent after ACL repair, and bone mineral loss and muscle atrophy occur after a limb has been immobilized with a cast. DXA body composition assessment could be used to monitor leg lean mass after surgery for comparison with presurgery values or those of the contralateral noninjured side, or to track recovery of bone mineral and muscle after a cast is removed. Some technical sports, such as tennis and baseball pitching, are known to result in limb asymmetry; DXA body composition could be used to monitor development of right-left arm asymmetry in tennis players or baseball pitchers, and then measures could be taken to balance the asymmetry. Wrestlers and elite dancers are expected to maintain strict weight requirements, but diets are often poor, and as such, DXA body composition could be used to track the effects of dieting and training by comparing serial measurements to baseline to ensure that weight changes include preservation or gain of muscle mass.31
Continue to: For older patients...
For older patients being followed after orthopedic care, there is a growing concern about age-related loss of muscle mass, or sarcopenia, which can lead to functional impairment (eg, balance, gait, etc.), and physical disability leading to falling and increased risk of fracture.35-40 Even obese patients can be sarcopenic (a concept known as sarcopenic obesity), and their large body mass can mask the relative deficiency of lean mass.40 DXA total body scans can be used to monitor patients at risk for sarcopenia.
Finally, DXA total body composition scans are underused in the pediatric population. Given the low radiation burden, DXA can be used safely in children of all ages. In addition to the same uses as in adults for presurgical assessment, monitoring bone and soft-tissue changes after treatment and rehabilitation, scans can be used to monitor growth and development.41
CASE STUDY: MONITORING DEVELOPMENT AND TREATMENT
A 12-year-old boy with polyostotic fibrous dysplasia (McCune Albright Syndrome) was started on treatment with cyclic pamidronate to mitigate bone pain and reduce fracture risk. Use of DXA was planned to provide evidence of treatment efficacy by documenting increasing BMD. However, the severe skeletal deformity prevented standard site-specific DXA scans, and consequently, total body scans were effectively used to acquire the BMD data needed to monitor treatment (Figure 4).
CASE STUDY: AGE-RELATED SARCOPENIA
Figure 5 shows images of a 64-year-old woman who was followed after a distal radius fracture. A total body scan and body composition assessment was performed in 2002. At follow-up in 2004, total body weight seemed stable with only a seemingly benign 5.1-lb loss of weight, and the patient’s overall physical appearance was unchanged (Table 2).
Table 2. Age-Related Changes Potentially Leading to Sarcopenia
| Baseline, 2002 | Follow-up, 2004 | Change, % |
Body weight, kg | 57.9 (127.6 lb) | 55.6 (122.5 lb) | 4 |
BMI | 20.6 | 19.8 |
|
Total body fat, g | 13,619 | 13,390 | −1.7 |
Total body percent fat | 23.5 | 24.1 |
|
Total body lean, g | 42,038 | 39,949 | −5.0 |
Dual-energy X-ray absorptiometry scans were performed using a GE Lunar Prodigy system.
However, body composition assessment revealed a disproportionate loss of lean mass, with a resultant total percent body fat increase. This imbalance between the change in fat and lean mass could lead to clinical sarcopenia unless appropriate dietary and exercise measures are taken. Such subtle developing imbalances in body composition could only be quantitated using DXA total body scans.
Continue to: It is not uncommon...
CASE STUDY: WEIGHT CHANGE IN A RECREATIONAL ATHLETE
It is not uncommon to encounter patients who have substantial weight changes as a result of lifestyle changes, such as dieting. It is also possible that body weight remains stable, but variable changes occur in the amount and distribution of fat and lean mass. Combining exercise with dieting is more likely to be associated with preservation or gain of lean mass. Such a case is presented. After a knee injury, a club tennis player reported gaining 30 lb in the subsequent 12 months. She enrolled in a DXA study, and serial body composition assessments were performed as she started a diet program and exercised on a treadmill and stationary bike. Table 3 shows body composition changes from baseline.
Table 3. Body Composition Changes After Dieting and Exercise
|
|
| Total Body | ||
| Weight, lb | Body Mass Index | Bone Mineral Density, g/cm2 | Fat, g | Lean, g |
Baseline | 160 | 27.5 | 1.245 | 29,023 | 39,610 |
12-month follow-up | 148 | 25.4 | 1.230 | 22,581 | 41,979 |
Dual-energy X-ray absorptiometry scans were performed using a GE Lunar Prodigy system.
Although gross weight using a scale clearly showed progress in losing weight, it did not provide information about redistribution of fat and lean mass. The DXA body composition assessment showed that at follow up, there was a 22% decrease in total grams of fat and a 6% increase in lean mass (changes were uniform over different body regions). Her BMI still categorized her as being overweight; however, her body composition changes demonstrated that diet and exercise were producing positive results.
CONCLUSION
There are many ways in which DXA technology could provide orthopedists with valuable baseline and postoperative and post-treatment information about their patients. This technology could be used more effectively by orthopedists in both general clinical practice and research.
1. Miller PD. The history of bone densitometry. Bone. 2017;104:4-6 [Epub ahead of print].
2. Blake GM, Fogelman I. Technical principles of dual energy X ray absorptiometry. Semin Nucl Med. 1997;27(3):210-228.
3. Faulkner KG. The tale of the T-score: review and perspective. Osteoporo Int. 2005;16(4):347-352. doi:10.1007/s00198-004-1779-y.
4. Solomou G, Damilakis J. Radiation exposure in bone densitometry. Semin Musculoskelet Radiol. 2016;20(4):392-398. doi:10.1055/s-0036-1592430.
5. Adams J. Bone densitometry in children. Semin Musculoskelet Radiol. 2016;20(3):254-268. doi:10.1055/s-0036-1592369.
6. Duan Y, Parfitt AM, Seeman E. Vertebral bone mass, size, and volumetric density in women with spinal fractures. J Bone Miner Res. 1999;14(10):1796-1802. doi:10.1359/jbmr.1999.14.10.1796.
7. Szaulc P, Munoz F, Duboeuf F, Delmas PD. Low width of tubular bones is associated with increased risk of fragility fracture in elderly men–the MINOS study. Bone 2006;38(4):595-602. doi:10.1016/j.bone.2005.09.004.
8. Mi J, Li K, Zhao X, Zhao CQ, Li H, Zhao J. Vertebral body compressive strength evaluated by dual-energy x-ray absorptiometry and Hounsfield units in vitro. J Clin Densitom. 2018;21(1):148-153. doi:10.1016/j.jocd.2016.08.011.
9. Ambrose CG, Kiebzak GM, Sabonghy EP, et al. Biomechanical testing of cadaveric specimens: importance of bone mineral density assessment. Foot Ankle Int. 2002;23(9):850-855. doi:10.1177/107110070202300913.
10. Kiebzak G, Sassard WR. Smaller radius width in women with distal radius fractures compared to women without fractures. Cureus. 2017;9(12):e1950. doi:10.775/cureus.1950.
11. Krappinger D, Bizzotto N, Riedmann S, Kammerlander C, Hengg C, Kralinger FS. Predicting failure after surgical fixation of proximal humerus fractures. Injury 2011;42(11):1283-1288. doi:10.1016/j.injury.2011.01.017.
12. Suhm N, Hengg C, Schwyn R, Windolf M, Quarz V, Hänni M. Mechanical torque measurement predicts load to implant cut-out: a biomechanical study investigating DHS anchorage in femoral heads. Arch Orthop Trauma Surg. 2007;127(6):469-474. doi:10.1007/s00402-006-0265-8.
13. Persiani P, Ranaldi FM, Graci J, et al. Isolated olecranon fractures in children affected by osteogenesis imperfecta type I treated with single screw or tension band wiring system: outcomes and pitfalls in relation to bone mineral density. Medicine (Baltimore). 2017;96(20):e6766. doi:10.1097/MD.0000000000006766.
14. Andersen MR, Winther NS, Lind T, Schrøder HM, Flivik G, Petersen MM. Low preoperative BMD is related to high migration of tibia components in uncemented TKA–92 patients in a combined DEXA and RSA study with 2-year follow-up. J Arthroplasty. 2017;32(7):2141-2146. doi:10.1016/j.arth.2017.02.032.
15. Yip BH, Yu FW, Wang Z, et al. Prognostic value of bone mineral density on curve progression: A longitudinal cohort study of 513 girls with adolescent idiopathic scoliosis. Sci Rep. 2016;6:39220. doi:10.1038/srep39220.
16. Pourabbas Tahvildari B, Erfani MA, Nouraei H, Sadeghian M. Evaluation of bone mineral status in adolescent idiopathic scoliosis. Clin Orthop Surg. 2014;6(2):180-184. doi:10.4055/cios.2014.6.2.180.
17. Li XF, Li H, Liu ZD, Dai LY. Low bone mineral status in adolescent idiopathic scoliosis. Eur Spine J. 2008;17(11):1431-1440. doi:10.1007/s00586-008-0757-z.
18. Venesmaa PK, Kröger HP, Miettinen HJ, Jurvelin JS, Suomalainen OT, Alhava EM. Monitoring of periprosthetic BMD after uncemented total hip arthroplasty with dual-energy X-ray absorptiometry--a 3-year follow-up study. J Bone Miner Res. 2001;16(6):1056-1061. doi:10.1359/jbmr.2001.16.6.1056.
19. Arabmotlagh M, Pilz M, Warzecha J, Rauschmann M. Changes of femoral periprosthetic bone mineral density 6 years after treatment with alendronate following total hip arthroplasty J Orthop Res. 2009;27(2):183-188. doi:10.1002/jor.20748.
20. Gruen TA, McNeice GM, Amstutz HC. Modes of failure of cemented stem-type femoral components: a radiographic analysis of loosening. Clin Orthop Relat Res. 1979;(141):17-27.
21. Zeytinoglu M, Jain RK, Vokes TJ. Vertebral fracture assessment: Enhancing the diagnosis, prevention, and treatment of osteoporosis. Bone. 2017;104:54-65. doi:10.1016/j.bone.2017.03.004.
22. Kiebzak GM. Radiolucent casting tape allows for accurate measurement of forearm bone mineral density using dual-energy X-ray absorptiometry. J Clin Densitom. 1998;1(4):369-374.
23. Sung KH, Chung CY, Lee KM, et al. Correlation between central and peripheral bone mineral density around the elbow measured by dual-energy x-ray absorptiometry in healthy children and adolescents. J Clin Densitom. 2017;20(1):114-119. doi:10.1016/j.jocd.2016.04.007.
24. Hamdy R, Kiebzak GM, Seier E, Watts NB. The prevalence of significant left-right differences in hip bone mineral density. Osteoporos Int. 2006;17(12):1772-1780. doi:10.1007/s00198-006-0192-0.
25. Kelly TL, Berger N, Richardson TL. DXA body composition: Theory and practice. Appl Radiat Isot. 1998;49(5-6):511-513.
26. Kiebzak GM, Leamy LJ, Pierson LM, Nord RH, Zhang ZY. Measurement precision of body composition variables using the lunar DPX-L densitometer. J Clin Densitom. 2000;3(1):35-41.
27. Bilborough JC, Greenway k, Par D, Coutts AJ. The accuracy and precision of DXA for assessing body composition in team sport athletes. J Sports Sci. 2014;32(19):1821-1828. doi:10.1080/02640414.2014.926380.
28. Malkov S, Cawthon PM, Peters KW, et al. Health ABC Study. Hip fractures risk in older men and women associated with DXA-derived measures of thigh subcutaneous fat thickness, cross-sectional muscle area, and muscle density. J Bone Miner Res. 2015;30(8):1414-1421. doi:10.1002/jbmr.2469.
29. Arangio GA, Chen C, Klady M, Reed JF. Thigh muscle size and strength after anterior cruciate ligament reconstruction and rehabilitation. J Orthop Sports Phys Ther. 1997;26(5):238-245. doi:10.2519/jospt.1997.26.5.238.
30. Ledford CK, Millikan PD, Nickel BT, et al. Percent body fat Is more predictive of function after total joint arthroplasty than body mass index. J Bone Joint Surg. 2016;98(10):849-857. doi:10.2106/JBJS.15.00509.
31. Berlet G, Kiebzak GM, Dandar A, et al. Prospective analysis of body composition and SF36 profiles in professional dancers over a 7-month season: is there a correlation to injury? J Dance Med Sci. 2002;6(2):54-61.
32. Grant JA, Bedi A, Kurz J, Bancroft R, Gagnier JJ, Miller BS. Ability of preseason body composition and physical fitness to predict the risk of injury in male collegiate hockey players. Sports Health. 2015;7(1):45-51. doi:10.1177/1941738114540445.
33. Stewart AD, Hannan J. Subregional tissue morphometry in male athletes and controls using DXA. Int J Sport Nutr Exerc Metab. 2000;10(2):157-169. doi:10.1123/ijsnem.10.2.157.
34. Sannicandro I, Cofano G, Rosa RA, Piccinno A. Balance training exercises decrease lower-limb strength asymmetry in young tennis players. J Sports Sci Med. 2014;13(2):397-402.
35. Guglielmi G, Ponti F, Agostini M, Amadori M, Battista G, Bazzocchi A. The role of DXA in sarcopenia. Aging Clin Exp Res. 2016;28(6):1047-1060. doi:10.1007/s40520-016-0589-3.
36. Janssen I, Baumgartner RN, Ross R, Rosenberg IH, Roubenoff R. Skeletal muscle cutpoints associated with elevated physical disability risk in older men and women. Am J Epidemiol. 2004;159(4):413-421.
37. Landi F, Calvani R, Ortolani E, et al. The association between sarcopenia and functional outcomes among older patients with hip fracture undergoing in-hospital rehabilitation. Osteoporos Int. 2017;28(5):1569-1576. doi:10.1007/s00198-017-3929-z.
38. Roh YH, Noh JH, Gong HS, Baek GH. Effect of low appendicular lean mass, grip strength, and gait speed on the functional outcome after surgery for distal radius fractures. Arch Osteoporos. 2017;12(1):41. doi:10.1007/s11657-017-0335-2.
39. Miller MS, Callahan DM, Toth MJ. Skeletal muscle myofilament adaptations to aging, disease, and disuse and their effects on whole muscle performance in older adult humans. Front Physiol. 2014;5:369. doi:10.3389/fphys.2014.00369.
40. Waters DJ, Baumgartner RN. Sarcopenia and obesity. Clin Geriatr Med. 2011;27(3):401-421. doi:10.1016/j.cger.2011.03.007.
41. Bachrach LK, Gordon CM. Bone densitometry in children and adolescents. Pediatrics. 2016;138(4):e20162398. doi:10.1542/peds.2016-2398.
ABSTRACT
Dual-energy X-ray absorptiometry (DXA) is a well-established technology with an important and well-known role in measuring bone mineral density (BMD) for the purpose of determining fracture risk, diagnosing osteoporosis, and monitoring treatment efficacy. However, aside from the assessment of bone status, DXA is likely underutilized in the field of orthopedics, and most orthopedists may not be aware of the full capabilities of DXA, particularly with regard to total body scans and body composition assessment. For example, DXA would be a valuable tool for monitoring body composition after surgery where compensatory changes in the affected limb may lead to right-left asymmetry (eg, tracking lean mass change after knee surgery), rehabilitation regimens for athletes, congenital and metabolic disorders that affect the musculoskeletal system, or monitoring sarcopenia and frailty in the elderly. Furthermore, preoperative and postoperative regional scans can track BMD changes during healing or alert surgeons to impending problems such as loss of periprosthetic bone, which could lead to implant failure. This article discusses the capabilities of DXA and how this technology could be better used to the advantage of the attending orthopedist.
Dual-energy X-ray absorptiometry, abbreviated as “DXA,” (although usually abbreviated in older literature as “DEXA”) was first introduced in 1987 (Hologic QDR-1000 system, Hologic, Inc) and immediately made all previous forms of radiation-based bone mineral density (BMD) measurement systems obsolete.1 Since then, there have been many generations of the technology, with the main US manufacturers in 2017 being Hologic, Inc. and GE Lunar. There are 2 forms of DXA, peripheral systems (which usually measure BMD only in the radius, finger bones, or calcaneus) and central systems (which measure the radius, proximal femur [“hip”], lumbar spine, total body, and custom sites). The general principle of how DXA works is based on the differential attenuation of photons by bone, fat, and lean mass.2 The DXA technique uses a low- and high-energy X-ray beam produced by an X-ray tube. With the low-energy beam, attenuation by bone is greater than attenuation by soft tissue. With the high-energy beam, attenuation by bone and soft tissues are similar. The dual X-ray beams are passed through the body regions being scanned (usually posterioanteriorly), and the differential attenuation by bone and soft tissue is analyzed to produce BMD estimates. In addition, a high-quality image is produced to enable the operator of the DXA system to verify that the appropriate body region was scanned. It is important to realize that DXA is 2-dimensional (which is sometimes cited as a weakness of DXA), and the units of BMD are grams of mineral per centimeter squared (g/cm2).
Continue to: When assessing bone status...
When assessing bone status for the purpose of determining if a patient is normal, osteopenic, or osteoporotic, the skeletal sites (called regions of interest [ROI]) typically scanned are the proximal femur, lumbar spine, and radius. The BMD of the patient is then compared to a manufacturer-provided normative database of young adults (the logic being that the BMD in the young adult normative population represents maximal peak bone mass). Total body BMD and body composition can also be quantified (grams of lean and fat mass), and custom scans can be designed for other skeletal sites. Specifically, a patient’s BMD is compared to a database of sex- and age-adjusted normal values, and the deviation from normal is expressed as a T-score (the number of standard deviations the patient's BMD is above or below the average BMD of the young adult reference population) and Z-scores (the number of standard deviations a patient's BMD is above or below the average BMD of a sex- and age-matched reference population).3 The International Society for Clinical Densitometry (ISCD) has developed and published well-accepted guidelines used to assist in acquiring high-quality DXA scans and for the diagnosis of osteoporosis using BMD. The accuracy and, especially, the precision of DXA scans can be remarkable when they are performed by trained technologists, and thus, serial scans can be performed to monitor BMD and body composition changes with aging or in response to treatment.
Because of the nature of the scan mechanics and speed, the effective radiation dose with DXA is very low, expressed in microSieverts.4,5 Generally, the radiation exposure from a series of the lumbar spine, proximal femur, and distal radius is about the same as daily background radiation. Even total body scans present very low exposure due to the scan speed at which any 1 body part is exposed for only a fraction of a second.
BENEFITS OF USING DXA FOR THE ORTHEOPEDIST
At the time of this writing in 2018, the presumption could be made that most physicians in the specialties of internal medicine, rheumatology, endocrinology, radiology, and orthopedics were familiar with the capabilities of DXA to assess BMD for the purpose of diagnosing osteoporosis. However, DXA is likely underused for other purposes, as orthopedists may be unaware of the full capabilities of DXA. Printouts after a scan contain more information than simply BMD, and there are more features and applications of DXA that can potentially be useful to orthopedists.
BONE SIZE
Data from a DXA scan are expressed not only as g/cm2 (BMD) but also as total grams in the ROI (known as bone mineral content, abbreviated as BMC), and cm2 (area of the ROI). These data may appear on a separate page, being considered ancillary results. The latter 2 variables are rarely included on a report sent to a referring physician; therefore, awareness of their value is probably limited. However, there are instances where such information could be valuable when interpreting results, especially bone size.6,7 For example, on occasion, patients present with osteopenic lumbar vertebrate but larger than normal vertebral size (area). Many studies have shown that bone size is directly related to bone strength and thus fracture risk.8,9 Although an understudied phenomenon, large vertebral body size could be protective, counteracting a lower than optimal BMD. Further, because the area of the ROI is measured, it is possible to calculate the bone width (or measure directly with a ruler tool in the software if available) for the area measured. This is especially feasible for tubular bones such as the midshaft of the radius, or more specifically, the classic DXA ROI being the area approximately one third the length of the radius from the distal end, the radius 33% region (actually based on ulna length). Consequently, it is possible to use the width of the radius 33% ROI in addition to BMD and T-score when assessing fracture risk.
CASE STUDY
A 60-year-old man had a DXA series of the lumbar spine, proximal femur, and whole body. His total body T-score was 0.6 (normal), and his total proximal femur T-score was −0.8 (normal), but his lumbar spine vertebrae 2 to 4 T-score was −1.9. As the patient was osteopenic based on the lumbar spine T-score, some physicians may have initiated antiresorptive therapy, especially if other risk factors for fracture were present. Further examination of the ancillary results of the DXA scan revealed that the vertebral body height T-score was a remarkable 1.11 and 1.53 after adjustment for stature (automatic software calculation). These results suggested that the patient had vertebral bodies of above average size, which theoretically would be protective against fracture even though the BMD T-score was below normal. For this patient, this finding mitigated immediate concern about the lumbar spine T-score of −1.9. Although vertebral body size is not typically used in assessing fracture risk, it is useful information that could be factored into the decision to start treatment or watch for further change with aging.
Continue to: Case Series: Distal Radius Fractures...
CASE SERIES: DISTAL RADIUS FRACTURES
Table 1 summarizes the data comparing radius 33% ROI T-scores and ROI width in patients who fractured the contralateral radius and normal nonfractured controls.10
Table 1. Comparison of Radius Width at the 33% Region of Interest (ROI) and Bone Mineral Density T-Scores in Premenopausal Women With and Without Fractures
| 33% ROI T-score | Width of ROI, cm |
White women with distal radius fractures |
|
|
Premenopausal (<49 years), n = 36 | -0.2 + 0.9 | 1.22 + 0.11a |
Controls matched for race, age, BMIb |
|
|
Premenopausal (<49 years), n = 65 | -0.1 + 0.8 | 1.45 + 0.25 |
For premenopausal women with distal radius fractures, the width of the radius at the radius 33% ROI was significantly smaller than that in controls. However, there was no difference in T-scores between premenopausal women with distal radius fractures and controls. Thus, bone width more accurately identified women with fractures than T-scores based on BMD, and the orthopedist could use bone size in addition to BMD to predict fracture risk in a patient.
PREPARATION FOR SURGERY
For some procedures, there is potential benefit of assessing bone status prior to surgery. That is, determination of low BMD could potentially influence the type of hardware or fixation techniques used in surgery. Various studies have shown that poor bone quality and low BMD can impair purchase with various types of fixation.11-13 Low preoperative BMD has been shown to be related to high implant migration.14 Knowledge of BMD could influence the choice of screw type used or the type of implant metal (titanium vs cobalt chrome). Another example is predicting the risk of spine curvature progression in adolescent idiopathic scoliosis.15-17 It has been reported that low BMD is a risk factor for progression.15 Knowledge of BMD could potentially help with patient management strategies. For example, a patient with low BMD and vitamin D deficiency could be treated (vitamin D supplementation) prior to planning surgery in an effort to improve the low BMD.
PERIOPROSTHETIC BMD
It is possible to monitor changes in BMD around implants using the periprosthetic software application (this usually needs to be purchased separately from standard software that is installed with a system set-up). Dramatic loss of bone due to stress shielding after total hip arthroplasty (THA) can be a risk factor for implant migration or potentially outright failure of fixation or breakthrough. If bone loss occurs and is observed in the early stages, then antiresorptive treatment can be initiated to limit further loss.18,19 (Figure 1) shows the image from a periprosthetic scan.
Continue to: A 60-year-old, 215-lb man...
CASE REPORT
A 60-year-old, 215-lb man had a total hip replacement using a newly introduced cemented collared cobalt-chromium alloy femoral stem. A baseline periprosthetic DXA scan was performed 6 weeks postoperatively. Compared to baseline, the change in BMD in the Gruen zone 5 was −8.2%, +6.5%, +4.9%, and +9.46% at 3, 6, 12, and 24 months, respectively. In contrast, dramatic BMD loss was seen in Gruen zone 7 (calcar region): −33.2%, −40.8%, −37.1%, and −34.1% at 3, 6, 12, and 24 months, respectively. Similar findings in other patients led to discontinuation of use of this stem in favor of a collarless stem in which less BMD loss was seen in Gruen zone 7. Although additional technologist training is required and scans may not be reimbursable, for research purposes or for evaluating new component prototypes, the periprosthetic DXA scan capability can be useful.
Various other custom scans can be used to detect and quantify vertebral fractures (vertebral fracture assessment application), monitor healing of fractures by scanning through radiolucent cast materials, or for research purposes to assess BMD at unusual locations.21-23 Other new innovations, such as the ability to perform full-length scans of the femoral shaft and to quantify focal thickening of the lateral cortex to identify beaking, an abnormality associated with atypical femur fracture after long-term bisphosphonate use, continue to expand the utility of DXA. Using standard software, cadaver bones can be scanned prior to biomechanical testing for a variety of purposes, such as ensuring proper matching specimens in test groups. It has been reported that the common practice of using contralateral bone specimens can lead to bias, as the BMD can be significantly different in right and left bones from the same individual.9,24
TOTAL BODY BMD AND BODY COMPOSITION SCANS
Perhaps the least understood capability of DXA from our experience working with orthopedists is the ability to perform total body scans and to obtain not only total body and regional BMD but also body composition data, namely grams of lean and fat mass.25 Soft tissue (no bone pixels) is partitioned into fat and lean body mass by a calibration procedure (lean mass = total soft tissue –fat mass). DXA has become the standard for body composition assessment given the ease of data acquisition (a total body scan takes only a few minutes), accuracy, and precision of measurements. Compared with other methods (eg, skinfold thickness, bioelectrical impedance, and underwater weighing), it is the only method that gives regional values for fat mass, lean mass, and BMC (this allows the ability to compare left vs right sides).25-27 The ability to perform regional measurements cannot be overstated, as stable body weight belies potential changes with age and disease that relate to redistribution of fat and lean mass. It is not possible to identify, let alone track, such changes by measuring gross body weight on a scale or with BMI calculations. However, redistribution of fat and lean mass can be monitored in great detail using DXA. Figures 2 and 3 show the typical output from a DXA total body/body composition scan.
Total body scans with body composition analyses have many applications. For example, monitoring growth and development or treatment in patients with congenital deformity, metabolic bone disease, osteoporosis, and frailty; patients undergoing rehabilitation; and patients having surgery that could affect the use of a contralateral limb with potential hypertrophy or atrophy. Accurate assessment of percent body fat and fat distribution may help surgeons to improve risk stratification and surgical outcome.28-30 Fracture risk has been associated with muscle area.28 Simple measurements of quadriceps size underestimates atrophy, and total body composition can quantitate lean mass.30
In sports medicine, body composition assessments could be useful to monitor postoperative recovery and effectiveness of rehabilitation protocols after injury, effectiveness of conditioning and training programs, developmental changes due to sports participation, and for obtaining baseline assessment at the time of preseason physicals.27,31-34 In athletes, baseline status and morphological adaptations to training have traditionally been measured by anthropometry (eg, skinfold thickness, BMI, limb circumference, etc.), but DXA total body scanning allows for much more detailed assessments with the possibility of subregional quantitation. There is evidence for sports-specific body composition profiles and characteristic adaptations.27,31-34 Using DXA, adaptive changes as a result of training as well as changes and recovery after surgery or injury can be monitored. For example, quadriceps atrophy usually occurs to some extent after ACL repair, and bone mineral loss and muscle atrophy occur after a limb has been immobilized with a cast. DXA body composition assessment could be used to monitor leg lean mass after surgery for comparison with presurgery values or those of the contralateral noninjured side, or to track recovery of bone mineral and muscle after a cast is removed. Some technical sports, such as tennis and baseball pitching, are known to result in limb asymmetry; DXA body composition could be used to monitor development of right-left arm asymmetry in tennis players or baseball pitchers, and then measures could be taken to balance the asymmetry. Wrestlers and elite dancers are expected to maintain strict weight requirements, but diets are often poor, and as such, DXA body composition could be used to track the effects of dieting and training by comparing serial measurements to baseline to ensure that weight changes include preservation or gain of muscle mass.31
Continue to: For older patients...
For older patients being followed after orthopedic care, there is a growing concern about age-related loss of muscle mass, or sarcopenia, which can lead to functional impairment (eg, balance, gait, etc.), and physical disability leading to falling and increased risk of fracture.35-40 Even obese patients can be sarcopenic (a concept known as sarcopenic obesity), and their large body mass can mask the relative deficiency of lean mass.40 DXA total body scans can be used to monitor patients at risk for sarcopenia.
Finally, DXA total body composition scans are underused in the pediatric population. Given the low radiation burden, DXA can be used safely in children of all ages. In addition to the same uses as in adults for presurgical assessment, monitoring bone and soft-tissue changes after treatment and rehabilitation, scans can be used to monitor growth and development.41
CASE STUDY: MONITORING DEVELOPMENT AND TREATMENT
A 12-year-old boy with polyostotic fibrous dysplasia (McCune Albright Syndrome) was started on treatment with cyclic pamidronate to mitigate bone pain and reduce fracture risk. Use of DXA was planned to provide evidence of treatment efficacy by documenting increasing BMD. However, the severe skeletal deformity prevented standard site-specific DXA scans, and consequently, total body scans were effectively used to acquire the BMD data needed to monitor treatment (Figure 4).
CASE STUDY: AGE-RELATED SARCOPENIA
Figure 5 shows images of a 64-year-old woman who was followed after a distal radius fracture. A total body scan and body composition assessment was performed in 2002. At follow-up in 2004, total body weight seemed stable with only a seemingly benign 5.1-lb loss of weight, and the patient’s overall physical appearance was unchanged (Table 2).
Table 2. Age-Related Changes Potentially Leading to Sarcopenia
| Baseline, 2002 | Follow-up, 2004 | Change, % |
Body weight, kg | 57.9 (127.6 lb) | 55.6 (122.5 lb) | 4 |
BMI | 20.6 | 19.8 |
|
Total body fat, g | 13,619 | 13,390 | −1.7 |
Total body percent fat | 23.5 | 24.1 |
|
Total body lean, g | 42,038 | 39,949 | −5.0 |
Dual-energy X-ray absorptiometry scans were performed using a GE Lunar Prodigy system.
However, body composition assessment revealed a disproportionate loss of lean mass, with a resultant total percent body fat increase. This imbalance between the change in fat and lean mass could lead to clinical sarcopenia unless appropriate dietary and exercise measures are taken. Such subtle developing imbalances in body composition could only be quantitated using DXA total body scans.
Continue to: It is not uncommon...
CASE STUDY: WEIGHT CHANGE IN A RECREATIONAL ATHLETE
It is not uncommon to encounter patients who have substantial weight changes as a result of lifestyle changes, such as dieting. It is also possible that body weight remains stable, but variable changes occur in the amount and distribution of fat and lean mass. Combining exercise with dieting is more likely to be associated with preservation or gain of lean mass. Such a case is presented. After a knee injury, a club tennis player reported gaining 30 lb in the subsequent 12 months. She enrolled in a DXA study, and serial body composition assessments were performed as she started a diet program and exercised on a treadmill and stationary bike. Table 3 shows body composition changes from baseline.
Table 3. Body Composition Changes After Dieting and Exercise
|
|
| Total Body | ||
| Weight, lb | Body Mass Index | Bone Mineral Density, g/cm2 | Fat, g | Lean, g |
Baseline | 160 | 27.5 | 1.245 | 29,023 | 39,610 |
12-month follow-up | 148 | 25.4 | 1.230 | 22,581 | 41,979 |
Dual-energy X-ray absorptiometry scans were performed using a GE Lunar Prodigy system.
Although gross weight using a scale clearly showed progress in losing weight, it did not provide information about redistribution of fat and lean mass. The DXA body composition assessment showed that at follow up, there was a 22% decrease in total grams of fat and a 6% increase in lean mass (changes were uniform over different body regions). Her BMI still categorized her as being overweight; however, her body composition changes demonstrated that diet and exercise were producing positive results.
CONCLUSION
There are many ways in which DXA technology could provide orthopedists with valuable baseline and postoperative and post-treatment information about their patients. This technology could be used more effectively by orthopedists in both general clinical practice and research.
ABSTRACT
Dual-energy X-ray absorptiometry (DXA) is a well-established technology with an important and well-known role in measuring bone mineral density (BMD) for the purpose of determining fracture risk, diagnosing osteoporosis, and monitoring treatment efficacy. However, aside from the assessment of bone status, DXA is likely underutilized in the field of orthopedics, and most orthopedists may not be aware of the full capabilities of DXA, particularly with regard to total body scans and body composition assessment. For example, DXA would be a valuable tool for monitoring body composition after surgery where compensatory changes in the affected limb may lead to right-left asymmetry (eg, tracking lean mass change after knee surgery), rehabilitation regimens for athletes, congenital and metabolic disorders that affect the musculoskeletal system, or monitoring sarcopenia and frailty in the elderly. Furthermore, preoperative and postoperative regional scans can track BMD changes during healing or alert surgeons to impending problems such as loss of periprosthetic bone, which could lead to implant failure. This article discusses the capabilities of DXA and how this technology could be better used to the advantage of the attending orthopedist.
Dual-energy X-ray absorptiometry, abbreviated as “DXA,” (although usually abbreviated in older literature as “DEXA”) was first introduced in 1987 (Hologic QDR-1000 system, Hologic, Inc) and immediately made all previous forms of radiation-based bone mineral density (BMD) measurement systems obsolete.1 Since then, there have been many generations of the technology, with the main US manufacturers in 2017 being Hologic, Inc. and GE Lunar. There are 2 forms of DXA, peripheral systems (which usually measure BMD only in the radius, finger bones, or calcaneus) and central systems (which measure the radius, proximal femur [“hip”], lumbar spine, total body, and custom sites). The general principle of how DXA works is based on the differential attenuation of photons by bone, fat, and lean mass.2 The DXA technique uses a low- and high-energy X-ray beam produced by an X-ray tube. With the low-energy beam, attenuation by bone is greater than attenuation by soft tissue. With the high-energy beam, attenuation by bone and soft tissues are similar. The dual X-ray beams are passed through the body regions being scanned (usually posterioanteriorly), and the differential attenuation by bone and soft tissue is analyzed to produce BMD estimates. In addition, a high-quality image is produced to enable the operator of the DXA system to verify that the appropriate body region was scanned. It is important to realize that DXA is 2-dimensional (which is sometimes cited as a weakness of DXA), and the units of BMD are grams of mineral per centimeter squared (g/cm2).
Continue to: When assessing bone status...
When assessing bone status for the purpose of determining if a patient is normal, osteopenic, or osteoporotic, the skeletal sites (called regions of interest [ROI]) typically scanned are the proximal femur, lumbar spine, and radius. The BMD of the patient is then compared to a manufacturer-provided normative database of young adults (the logic being that the BMD in the young adult normative population represents maximal peak bone mass). Total body BMD and body composition can also be quantified (grams of lean and fat mass), and custom scans can be designed for other skeletal sites. Specifically, a patient’s BMD is compared to a database of sex- and age-adjusted normal values, and the deviation from normal is expressed as a T-score (the number of standard deviations the patient's BMD is above or below the average BMD of the young adult reference population) and Z-scores (the number of standard deviations a patient's BMD is above or below the average BMD of a sex- and age-matched reference population).3 The International Society for Clinical Densitometry (ISCD) has developed and published well-accepted guidelines used to assist in acquiring high-quality DXA scans and for the diagnosis of osteoporosis using BMD. The accuracy and, especially, the precision of DXA scans can be remarkable when they are performed by trained technologists, and thus, serial scans can be performed to monitor BMD and body composition changes with aging or in response to treatment.
Because of the nature of the scan mechanics and speed, the effective radiation dose with DXA is very low, expressed in microSieverts.4,5 Generally, the radiation exposure from a series of the lumbar spine, proximal femur, and distal radius is about the same as daily background radiation. Even total body scans present very low exposure due to the scan speed at which any 1 body part is exposed for only a fraction of a second.
BENEFITS OF USING DXA FOR THE ORTHEOPEDIST
At the time of this writing in 2018, the presumption could be made that most physicians in the specialties of internal medicine, rheumatology, endocrinology, radiology, and orthopedics were familiar with the capabilities of DXA to assess BMD for the purpose of diagnosing osteoporosis. However, DXA is likely underused for other purposes, as orthopedists may be unaware of the full capabilities of DXA. Printouts after a scan contain more information than simply BMD, and there are more features and applications of DXA that can potentially be useful to orthopedists.
BONE SIZE
Data from a DXA scan are expressed not only as g/cm2 (BMD) but also as total grams in the ROI (known as bone mineral content, abbreviated as BMC), and cm2 (area of the ROI). These data may appear on a separate page, being considered ancillary results. The latter 2 variables are rarely included on a report sent to a referring physician; therefore, awareness of their value is probably limited. However, there are instances where such information could be valuable when interpreting results, especially bone size.6,7 For example, on occasion, patients present with osteopenic lumbar vertebrate but larger than normal vertebral size (area). Many studies have shown that bone size is directly related to bone strength and thus fracture risk.8,9 Although an understudied phenomenon, large vertebral body size could be protective, counteracting a lower than optimal BMD. Further, because the area of the ROI is measured, it is possible to calculate the bone width (or measure directly with a ruler tool in the software if available) for the area measured. This is especially feasible for tubular bones such as the midshaft of the radius, or more specifically, the classic DXA ROI being the area approximately one third the length of the radius from the distal end, the radius 33% region (actually based on ulna length). Consequently, it is possible to use the width of the radius 33% ROI in addition to BMD and T-score when assessing fracture risk.
CASE STUDY
A 60-year-old man had a DXA series of the lumbar spine, proximal femur, and whole body. His total body T-score was 0.6 (normal), and his total proximal femur T-score was −0.8 (normal), but his lumbar spine vertebrae 2 to 4 T-score was −1.9. As the patient was osteopenic based on the lumbar spine T-score, some physicians may have initiated antiresorptive therapy, especially if other risk factors for fracture were present. Further examination of the ancillary results of the DXA scan revealed that the vertebral body height T-score was a remarkable 1.11 and 1.53 after adjustment for stature (automatic software calculation). These results suggested that the patient had vertebral bodies of above average size, which theoretically would be protective against fracture even though the BMD T-score was below normal. For this patient, this finding mitigated immediate concern about the lumbar spine T-score of −1.9. Although vertebral body size is not typically used in assessing fracture risk, it is useful information that could be factored into the decision to start treatment or watch for further change with aging.
Continue to: Case Series: Distal Radius Fractures...
CASE SERIES: DISTAL RADIUS FRACTURES
Table 1 summarizes the data comparing radius 33% ROI T-scores and ROI width in patients who fractured the contralateral radius and normal nonfractured controls.10
Table 1. Comparison of Radius Width at the 33% Region of Interest (ROI) and Bone Mineral Density T-Scores in Premenopausal Women With and Without Fractures
| 33% ROI T-score | Width of ROI, cm |
White women with distal radius fractures |
|
|
Premenopausal (<49 years), n = 36 | -0.2 + 0.9 | 1.22 + 0.11a |
Controls matched for race, age, BMIb |
|
|
Premenopausal (<49 years), n = 65 | -0.1 + 0.8 | 1.45 + 0.25 |
For premenopausal women with distal radius fractures, the width of the radius at the radius 33% ROI was significantly smaller than that in controls. However, there was no difference in T-scores between premenopausal women with distal radius fractures and controls. Thus, bone width more accurately identified women with fractures than T-scores based on BMD, and the orthopedist could use bone size in addition to BMD to predict fracture risk in a patient.
PREPARATION FOR SURGERY
For some procedures, there is potential benefit of assessing bone status prior to surgery. That is, determination of low BMD could potentially influence the type of hardware or fixation techniques used in surgery. Various studies have shown that poor bone quality and low BMD can impair purchase with various types of fixation.11-13 Low preoperative BMD has been shown to be related to high implant migration.14 Knowledge of BMD could influence the choice of screw type used or the type of implant metal (titanium vs cobalt chrome). Another example is predicting the risk of spine curvature progression in adolescent idiopathic scoliosis.15-17 It has been reported that low BMD is a risk factor for progression.15 Knowledge of BMD could potentially help with patient management strategies. For example, a patient with low BMD and vitamin D deficiency could be treated (vitamin D supplementation) prior to planning surgery in an effort to improve the low BMD.
PERIOPROSTHETIC BMD
It is possible to monitor changes in BMD around implants using the periprosthetic software application (this usually needs to be purchased separately from standard software that is installed with a system set-up). Dramatic loss of bone due to stress shielding after total hip arthroplasty (THA) can be a risk factor for implant migration or potentially outright failure of fixation or breakthrough. If bone loss occurs and is observed in the early stages, then antiresorptive treatment can be initiated to limit further loss.18,19 (Figure 1) shows the image from a periprosthetic scan.
Continue to: A 60-year-old, 215-lb man...
CASE REPORT
A 60-year-old, 215-lb man had a total hip replacement using a newly introduced cemented collared cobalt-chromium alloy femoral stem. A baseline periprosthetic DXA scan was performed 6 weeks postoperatively. Compared to baseline, the change in BMD in the Gruen zone 5 was −8.2%, +6.5%, +4.9%, and +9.46% at 3, 6, 12, and 24 months, respectively. In contrast, dramatic BMD loss was seen in Gruen zone 7 (calcar region): −33.2%, −40.8%, −37.1%, and −34.1% at 3, 6, 12, and 24 months, respectively. Similar findings in other patients led to discontinuation of use of this stem in favor of a collarless stem in which less BMD loss was seen in Gruen zone 7. Although additional technologist training is required and scans may not be reimbursable, for research purposes or for evaluating new component prototypes, the periprosthetic DXA scan capability can be useful.
Various other custom scans can be used to detect and quantify vertebral fractures (vertebral fracture assessment application), monitor healing of fractures by scanning through radiolucent cast materials, or for research purposes to assess BMD at unusual locations.21-23 Other new innovations, such as the ability to perform full-length scans of the femoral shaft and to quantify focal thickening of the lateral cortex to identify beaking, an abnormality associated with atypical femur fracture after long-term bisphosphonate use, continue to expand the utility of DXA. Using standard software, cadaver bones can be scanned prior to biomechanical testing for a variety of purposes, such as ensuring proper matching specimens in test groups. It has been reported that the common practice of using contralateral bone specimens can lead to bias, as the BMD can be significantly different in right and left bones from the same individual.9,24
TOTAL BODY BMD AND BODY COMPOSITION SCANS
Perhaps the least understood capability of DXA from our experience working with orthopedists is the ability to perform total body scans and to obtain not only total body and regional BMD but also body composition data, namely grams of lean and fat mass.25 Soft tissue (no bone pixels) is partitioned into fat and lean body mass by a calibration procedure (lean mass = total soft tissue –fat mass). DXA has become the standard for body composition assessment given the ease of data acquisition (a total body scan takes only a few minutes), accuracy, and precision of measurements. Compared with other methods (eg, skinfold thickness, bioelectrical impedance, and underwater weighing), it is the only method that gives regional values for fat mass, lean mass, and BMC (this allows the ability to compare left vs right sides).25-27 The ability to perform regional measurements cannot be overstated, as stable body weight belies potential changes with age and disease that relate to redistribution of fat and lean mass. It is not possible to identify, let alone track, such changes by measuring gross body weight on a scale or with BMI calculations. However, redistribution of fat and lean mass can be monitored in great detail using DXA. Figures 2 and 3 show the typical output from a DXA total body/body composition scan.
Total body scans with body composition analyses have many applications. For example, monitoring growth and development or treatment in patients with congenital deformity, metabolic bone disease, osteoporosis, and frailty; patients undergoing rehabilitation; and patients having surgery that could affect the use of a contralateral limb with potential hypertrophy or atrophy. Accurate assessment of percent body fat and fat distribution may help surgeons to improve risk stratification and surgical outcome.28-30 Fracture risk has been associated with muscle area.28 Simple measurements of quadriceps size underestimates atrophy, and total body composition can quantitate lean mass.30
In sports medicine, body composition assessments could be useful to monitor postoperative recovery and effectiveness of rehabilitation protocols after injury, effectiveness of conditioning and training programs, developmental changes due to sports participation, and for obtaining baseline assessment at the time of preseason physicals.27,31-34 In athletes, baseline status and morphological adaptations to training have traditionally been measured by anthropometry (eg, skinfold thickness, BMI, limb circumference, etc.), but DXA total body scanning allows for much more detailed assessments with the possibility of subregional quantitation. There is evidence for sports-specific body composition profiles and characteristic adaptations.27,31-34 Using DXA, adaptive changes as a result of training as well as changes and recovery after surgery or injury can be monitored. For example, quadriceps atrophy usually occurs to some extent after ACL repair, and bone mineral loss and muscle atrophy occur after a limb has been immobilized with a cast. DXA body composition assessment could be used to monitor leg lean mass after surgery for comparison with presurgery values or those of the contralateral noninjured side, or to track recovery of bone mineral and muscle after a cast is removed. Some technical sports, such as tennis and baseball pitching, are known to result in limb asymmetry; DXA body composition could be used to monitor development of right-left arm asymmetry in tennis players or baseball pitchers, and then measures could be taken to balance the asymmetry. Wrestlers and elite dancers are expected to maintain strict weight requirements, but diets are often poor, and as such, DXA body composition could be used to track the effects of dieting and training by comparing serial measurements to baseline to ensure that weight changes include preservation or gain of muscle mass.31
Continue to: For older patients...
For older patients being followed after orthopedic care, there is a growing concern about age-related loss of muscle mass, or sarcopenia, which can lead to functional impairment (eg, balance, gait, etc.), and physical disability leading to falling and increased risk of fracture.35-40 Even obese patients can be sarcopenic (a concept known as sarcopenic obesity), and their large body mass can mask the relative deficiency of lean mass.40 DXA total body scans can be used to monitor patients at risk for sarcopenia.
Finally, DXA total body composition scans are underused in the pediatric population. Given the low radiation burden, DXA can be used safely in children of all ages. In addition to the same uses as in adults for presurgical assessment, monitoring bone and soft-tissue changes after treatment and rehabilitation, scans can be used to monitor growth and development.41
CASE STUDY: MONITORING DEVELOPMENT AND TREATMENT
A 12-year-old boy with polyostotic fibrous dysplasia (McCune Albright Syndrome) was started on treatment with cyclic pamidronate to mitigate bone pain and reduce fracture risk. Use of DXA was planned to provide evidence of treatment efficacy by documenting increasing BMD. However, the severe skeletal deformity prevented standard site-specific DXA scans, and consequently, total body scans were effectively used to acquire the BMD data needed to monitor treatment (Figure 4).
CASE STUDY: AGE-RELATED SARCOPENIA
Figure 5 shows images of a 64-year-old woman who was followed after a distal radius fracture. A total body scan and body composition assessment was performed in 2002. At follow-up in 2004, total body weight seemed stable with only a seemingly benign 5.1-lb loss of weight, and the patient’s overall physical appearance was unchanged (Table 2).
Table 2. Age-Related Changes Potentially Leading to Sarcopenia
| Baseline, 2002 | Follow-up, 2004 | Change, % |
Body weight, kg | 57.9 (127.6 lb) | 55.6 (122.5 lb) | 4 |
BMI | 20.6 | 19.8 |
|
Total body fat, g | 13,619 | 13,390 | −1.7 |
Total body percent fat | 23.5 | 24.1 |
|
Total body lean, g | 42,038 | 39,949 | −5.0 |
Dual-energy X-ray absorptiometry scans were performed using a GE Lunar Prodigy system.
However, body composition assessment revealed a disproportionate loss of lean mass, with a resultant total percent body fat increase. This imbalance between the change in fat and lean mass could lead to clinical sarcopenia unless appropriate dietary and exercise measures are taken. Such subtle developing imbalances in body composition could only be quantitated using DXA total body scans.
Continue to: It is not uncommon...
CASE STUDY: WEIGHT CHANGE IN A RECREATIONAL ATHLETE
It is not uncommon to encounter patients who have substantial weight changes as a result of lifestyle changes, such as dieting. It is also possible that body weight remains stable, but variable changes occur in the amount and distribution of fat and lean mass. Combining exercise with dieting is more likely to be associated with preservation or gain of lean mass. Such a case is presented. After a knee injury, a club tennis player reported gaining 30 lb in the subsequent 12 months. She enrolled in a DXA study, and serial body composition assessments were performed as she started a diet program and exercised on a treadmill and stationary bike. Table 3 shows body composition changes from baseline.
Table 3. Body Composition Changes After Dieting and Exercise
|
|
| Total Body | ||
| Weight, lb | Body Mass Index | Bone Mineral Density, g/cm2 | Fat, g | Lean, g |
Baseline | 160 | 27.5 | 1.245 | 29,023 | 39,610 |
12-month follow-up | 148 | 25.4 | 1.230 | 22,581 | 41,979 |
Dual-energy X-ray absorptiometry scans were performed using a GE Lunar Prodigy system.
Although gross weight using a scale clearly showed progress in losing weight, it did not provide information about redistribution of fat and lean mass. The DXA body composition assessment showed that at follow up, there was a 22% decrease in total grams of fat and a 6% increase in lean mass (changes were uniform over different body regions). Her BMI still categorized her as being overweight; however, her body composition changes demonstrated that diet and exercise were producing positive results.
CONCLUSION
There are many ways in which DXA technology could provide orthopedists with valuable baseline and postoperative and post-treatment information about their patients. This technology could be used more effectively by orthopedists in both general clinical practice and research.
1. Miller PD. The history of bone densitometry. Bone. 2017;104:4-6 [Epub ahead of print].
2. Blake GM, Fogelman I. Technical principles of dual energy X ray absorptiometry. Semin Nucl Med. 1997;27(3):210-228.
3. Faulkner KG. The tale of the T-score: review and perspective. Osteoporo Int. 2005;16(4):347-352. doi:10.1007/s00198-004-1779-y.
4. Solomou G, Damilakis J. Radiation exposure in bone densitometry. Semin Musculoskelet Radiol. 2016;20(4):392-398. doi:10.1055/s-0036-1592430.
5. Adams J. Bone densitometry in children. Semin Musculoskelet Radiol. 2016;20(3):254-268. doi:10.1055/s-0036-1592369.
6. Duan Y, Parfitt AM, Seeman E. Vertebral bone mass, size, and volumetric density in women with spinal fractures. J Bone Miner Res. 1999;14(10):1796-1802. doi:10.1359/jbmr.1999.14.10.1796.
7. Szaulc P, Munoz F, Duboeuf F, Delmas PD. Low width of tubular bones is associated with increased risk of fragility fracture in elderly men–the MINOS study. Bone 2006;38(4):595-602. doi:10.1016/j.bone.2005.09.004.
8. Mi J, Li K, Zhao X, Zhao CQ, Li H, Zhao J. Vertebral body compressive strength evaluated by dual-energy x-ray absorptiometry and Hounsfield units in vitro. J Clin Densitom. 2018;21(1):148-153. doi:10.1016/j.jocd.2016.08.011.
9. Ambrose CG, Kiebzak GM, Sabonghy EP, et al. Biomechanical testing of cadaveric specimens: importance of bone mineral density assessment. Foot Ankle Int. 2002;23(9):850-855. doi:10.1177/107110070202300913.
10. Kiebzak G, Sassard WR. Smaller radius width in women with distal radius fractures compared to women without fractures. Cureus. 2017;9(12):e1950. doi:10.775/cureus.1950.
11. Krappinger D, Bizzotto N, Riedmann S, Kammerlander C, Hengg C, Kralinger FS. Predicting failure after surgical fixation of proximal humerus fractures. Injury 2011;42(11):1283-1288. doi:10.1016/j.injury.2011.01.017.
12. Suhm N, Hengg C, Schwyn R, Windolf M, Quarz V, Hänni M. Mechanical torque measurement predicts load to implant cut-out: a biomechanical study investigating DHS anchorage in femoral heads. Arch Orthop Trauma Surg. 2007;127(6):469-474. doi:10.1007/s00402-006-0265-8.
13. Persiani P, Ranaldi FM, Graci J, et al. Isolated olecranon fractures in children affected by osteogenesis imperfecta type I treated with single screw or tension band wiring system: outcomes and pitfalls in relation to bone mineral density. Medicine (Baltimore). 2017;96(20):e6766. doi:10.1097/MD.0000000000006766.
14. Andersen MR, Winther NS, Lind T, Schrøder HM, Flivik G, Petersen MM. Low preoperative BMD is related to high migration of tibia components in uncemented TKA–92 patients in a combined DEXA and RSA study with 2-year follow-up. J Arthroplasty. 2017;32(7):2141-2146. doi:10.1016/j.arth.2017.02.032.
15. Yip BH, Yu FW, Wang Z, et al. Prognostic value of bone mineral density on curve progression: A longitudinal cohort study of 513 girls with adolescent idiopathic scoliosis. Sci Rep. 2016;6:39220. doi:10.1038/srep39220.
16. Pourabbas Tahvildari B, Erfani MA, Nouraei H, Sadeghian M. Evaluation of bone mineral status in adolescent idiopathic scoliosis. Clin Orthop Surg. 2014;6(2):180-184. doi:10.4055/cios.2014.6.2.180.
17. Li XF, Li H, Liu ZD, Dai LY. Low bone mineral status in adolescent idiopathic scoliosis. Eur Spine J. 2008;17(11):1431-1440. doi:10.1007/s00586-008-0757-z.
18. Venesmaa PK, Kröger HP, Miettinen HJ, Jurvelin JS, Suomalainen OT, Alhava EM. Monitoring of periprosthetic BMD after uncemented total hip arthroplasty with dual-energy X-ray absorptiometry--a 3-year follow-up study. J Bone Miner Res. 2001;16(6):1056-1061. doi:10.1359/jbmr.2001.16.6.1056.
19. Arabmotlagh M, Pilz M, Warzecha J, Rauschmann M. Changes of femoral periprosthetic bone mineral density 6 years after treatment with alendronate following total hip arthroplasty J Orthop Res. 2009;27(2):183-188. doi:10.1002/jor.20748.
20. Gruen TA, McNeice GM, Amstutz HC. Modes of failure of cemented stem-type femoral components: a radiographic analysis of loosening. Clin Orthop Relat Res. 1979;(141):17-27.
21. Zeytinoglu M, Jain RK, Vokes TJ. Vertebral fracture assessment: Enhancing the diagnosis, prevention, and treatment of osteoporosis. Bone. 2017;104:54-65. doi:10.1016/j.bone.2017.03.004.
22. Kiebzak GM. Radiolucent casting tape allows for accurate measurement of forearm bone mineral density using dual-energy X-ray absorptiometry. J Clin Densitom. 1998;1(4):369-374.
23. Sung KH, Chung CY, Lee KM, et al. Correlation between central and peripheral bone mineral density around the elbow measured by dual-energy x-ray absorptiometry in healthy children and adolescents. J Clin Densitom. 2017;20(1):114-119. doi:10.1016/j.jocd.2016.04.007.
24. Hamdy R, Kiebzak GM, Seier E, Watts NB. The prevalence of significant left-right differences in hip bone mineral density. Osteoporos Int. 2006;17(12):1772-1780. doi:10.1007/s00198-006-0192-0.
25. Kelly TL, Berger N, Richardson TL. DXA body composition: Theory and practice. Appl Radiat Isot. 1998;49(5-6):511-513.
26. Kiebzak GM, Leamy LJ, Pierson LM, Nord RH, Zhang ZY. Measurement precision of body composition variables using the lunar DPX-L densitometer. J Clin Densitom. 2000;3(1):35-41.
27. Bilborough JC, Greenway k, Par D, Coutts AJ. The accuracy and precision of DXA for assessing body composition in team sport athletes. J Sports Sci. 2014;32(19):1821-1828. doi:10.1080/02640414.2014.926380.
28. Malkov S, Cawthon PM, Peters KW, et al. Health ABC Study. Hip fractures risk in older men and women associated with DXA-derived measures of thigh subcutaneous fat thickness, cross-sectional muscle area, and muscle density. J Bone Miner Res. 2015;30(8):1414-1421. doi:10.1002/jbmr.2469.
29. Arangio GA, Chen C, Klady M, Reed JF. Thigh muscle size and strength after anterior cruciate ligament reconstruction and rehabilitation. J Orthop Sports Phys Ther. 1997;26(5):238-245. doi:10.2519/jospt.1997.26.5.238.
30. Ledford CK, Millikan PD, Nickel BT, et al. Percent body fat Is more predictive of function after total joint arthroplasty than body mass index. J Bone Joint Surg. 2016;98(10):849-857. doi:10.2106/JBJS.15.00509.
31. Berlet G, Kiebzak GM, Dandar A, et al. Prospective analysis of body composition and SF36 profiles in professional dancers over a 7-month season: is there a correlation to injury? J Dance Med Sci. 2002;6(2):54-61.
32. Grant JA, Bedi A, Kurz J, Bancroft R, Gagnier JJ, Miller BS. Ability of preseason body composition and physical fitness to predict the risk of injury in male collegiate hockey players. Sports Health. 2015;7(1):45-51. doi:10.1177/1941738114540445.
33. Stewart AD, Hannan J. Subregional tissue morphometry in male athletes and controls using DXA. Int J Sport Nutr Exerc Metab. 2000;10(2):157-169. doi:10.1123/ijsnem.10.2.157.
34. Sannicandro I, Cofano G, Rosa RA, Piccinno A. Balance training exercises decrease lower-limb strength asymmetry in young tennis players. J Sports Sci Med. 2014;13(2):397-402.
35. Guglielmi G, Ponti F, Agostini M, Amadori M, Battista G, Bazzocchi A. The role of DXA in sarcopenia. Aging Clin Exp Res. 2016;28(6):1047-1060. doi:10.1007/s40520-016-0589-3.
36. Janssen I, Baumgartner RN, Ross R, Rosenberg IH, Roubenoff R. Skeletal muscle cutpoints associated with elevated physical disability risk in older men and women. Am J Epidemiol. 2004;159(4):413-421.
37. Landi F, Calvani R, Ortolani E, et al. The association between sarcopenia and functional outcomes among older patients with hip fracture undergoing in-hospital rehabilitation. Osteoporos Int. 2017;28(5):1569-1576. doi:10.1007/s00198-017-3929-z.
38. Roh YH, Noh JH, Gong HS, Baek GH. Effect of low appendicular lean mass, grip strength, and gait speed on the functional outcome after surgery for distal radius fractures. Arch Osteoporos. 2017;12(1):41. doi:10.1007/s11657-017-0335-2.
39. Miller MS, Callahan DM, Toth MJ. Skeletal muscle myofilament adaptations to aging, disease, and disuse and their effects on whole muscle performance in older adult humans. Front Physiol. 2014;5:369. doi:10.3389/fphys.2014.00369.
40. Waters DJ, Baumgartner RN. Sarcopenia and obesity. Clin Geriatr Med. 2011;27(3):401-421. doi:10.1016/j.cger.2011.03.007.
41. Bachrach LK, Gordon CM. Bone densitometry in children and adolescents. Pediatrics. 2016;138(4):e20162398. doi:10.1542/peds.2016-2398.
1. Miller PD. The history of bone densitometry. Bone. 2017;104:4-6 [Epub ahead of print].
2. Blake GM, Fogelman I. Technical principles of dual energy X ray absorptiometry. Semin Nucl Med. 1997;27(3):210-228.
3. Faulkner KG. The tale of the T-score: review and perspective. Osteoporo Int. 2005;16(4):347-352. doi:10.1007/s00198-004-1779-y.
4. Solomou G, Damilakis J. Radiation exposure in bone densitometry. Semin Musculoskelet Radiol. 2016;20(4):392-398. doi:10.1055/s-0036-1592430.
5. Adams J. Bone densitometry in children. Semin Musculoskelet Radiol. 2016;20(3):254-268. doi:10.1055/s-0036-1592369.
6. Duan Y, Parfitt AM, Seeman E. Vertebral bone mass, size, and volumetric density in women with spinal fractures. J Bone Miner Res. 1999;14(10):1796-1802. doi:10.1359/jbmr.1999.14.10.1796.
7. Szaulc P, Munoz F, Duboeuf F, Delmas PD. Low width of tubular bones is associated with increased risk of fragility fracture in elderly men–the MINOS study. Bone 2006;38(4):595-602. doi:10.1016/j.bone.2005.09.004.
8. Mi J, Li K, Zhao X, Zhao CQ, Li H, Zhao J. Vertebral body compressive strength evaluated by dual-energy x-ray absorptiometry and Hounsfield units in vitro. J Clin Densitom. 2018;21(1):148-153. doi:10.1016/j.jocd.2016.08.011.
9. Ambrose CG, Kiebzak GM, Sabonghy EP, et al. Biomechanical testing of cadaveric specimens: importance of bone mineral density assessment. Foot Ankle Int. 2002;23(9):850-855. doi:10.1177/107110070202300913.
10. Kiebzak G, Sassard WR. Smaller radius width in women with distal radius fractures compared to women without fractures. Cureus. 2017;9(12):e1950. doi:10.775/cureus.1950.
11. Krappinger D, Bizzotto N, Riedmann S, Kammerlander C, Hengg C, Kralinger FS. Predicting failure after surgical fixation of proximal humerus fractures. Injury 2011;42(11):1283-1288. doi:10.1016/j.injury.2011.01.017.
12. Suhm N, Hengg C, Schwyn R, Windolf M, Quarz V, Hänni M. Mechanical torque measurement predicts load to implant cut-out: a biomechanical study investigating DHS anchorage in femoral heads. Arch Orthop Trauma Surg. 2007;127(6):469-474. doi:10.1007/s00402-006-0265-8.
13. Persiani P, Ranaldi FM, Graci J, et al. Isolated olecranon fractures in children affected by osteogenesis imperfecta type I treated with single screw or tension band wiring system: outcomes and pitfalls in relation to bone mineral density. Medicine (Baltimore). 2017;96(20):e6766. doi:10.1097/MD.0000000000006766.
14. Andersen MR, Winther NS, Lind T, Schrøder HM, Flivik G, Petersen MM. Low preoperative BMD is related to high migration of tibia components in uncemented TKA–92 patients in a combined DEXA and RSA study with 2-year follow-up. J Arthroplasty. 2017;32(7):2141-2146. doi:10.1016/j.arth.2017.02.032.
15. Yip BH, Yu FW, Wang Z, et al. Prognostic value of bone mineral density on curve progression: A longitudinal cohort study of 513 girls with adolescent idiopathic scoliosis. Sci Rep. 2016;6:39220. doi:10.1038/srep39220.
16. Pourabbas Tahvildari B, Erfani MA, Nouraei H, Sadeghian M. Evaluation of bone mineral status in adolescent idiopathic scoliosis. Clin Orthop Surg. 2014;6(2):180-184. doi:10.4055/cios.2014.6.2.180.
17. Li XF, Li H, Liu ZD, Dai LY. Low bone mineral status in adolescent idiopathic scoliosis. Eur Spine J. 2008;17(11):1431-1440. doi:10.1007/s00586-008-0757-z.
18. Venesmaa PK, Kröger HP, Miettinen HJ, Jurvelin JS, Suomalainen OT, Alhava EM. Monitoring of periprosthetic BMD after uncemented total hip arthroplasty with dual-energy X-ray absorptiometry--a 3-year follow-up study. J Bone Miner Res. 2001;16(6):1056-1061. doi:10.1359/jbmr.2001.16.6.1056.
19. Arabmotlagh M, Pilz M, Warzecha J, Rauschmann M. Changes of femoral periprosthetic bone mineral density 6 years after treatment with alendronate following total hip arthroplasty J Orthop Res. 2009;27(2):183-188. doi:10.1002/jor.20748.
20. Gruen TA, McNeice GM, Amstutz HC. Modes of failure of cemented stem-type femoral components: a radiographic analysis of loosening. Clin Orthop Relat Res. 1979;(141):17-27.
21. Zeytinoglu M, Jain RK, Vokes TJ. Vertebral fracture assessment: Enhancing the diagnosis, prevention, and treatment of osteoporosis. Bone. 2017;104:54-65. doi:10.1016/j.bone.2017.03.004.
22. Kiebzak GM. Radiolucent casting tape allows for accurate measurement of forearm bone mineral density using dual-energy X-ray absorptiometry. J Clin Densitom. 1998;1(4):369-374.
23. Sung KH, Chung CY, Lee KM, et al. Correlation between central and peripheral bone mineral density around the elbow measured by dual-energy x-ray absorptiometry in healthy children and adolescents. J Clin Densitom. 2017;20(1):114-119. doi:10.1016/j.jocd.2016.04.007.
24. Hamdy R, Kiebzak GM, Seier E, Watts NB. The prevalence of significant left-right differences in hip bone mineral density. Osteoporos Int. 2006;17(12):1772-1780. doi:10.1007/s00198-006-0192-0.
25. Kelly TL, Berger N, Richardson TL. DXA body composition: Theory and practice. Appl Radiat Isot. 1998;49(5-6):511-513.
26. Kiebzak GM, Leamy LJ, Pierson LM, Nord RH, Zhang ZY. Measurement precision of body composition variables using the lunar DPX-L densitometer. J Clin Densitom. 2000;3(1):35-41.
27. Bilborough JC, Greenway k, Par D, Coutts AJ. The accuracy and precision of DXA for assessing body composition in team sport athletes. J Sports Sci. 2014;32(19):1821-1828. doi:10.1080/02640414.2014.926380.
28. Malkov S, Cawthon PM, Peters KW, et al. Health ABC Study. Hip fractures risk in older men and women associated with DXA-derived measures of thigh subcutaneous fat thickness, cross-sectional muscle area, and muscle density. J Bone Miner Res. 2015;30(8):1414-1421. doi:10.1002/jbmr.2469.
29. Arangio GA, Chen C, Klady M, Reed JF. Thigh muscle size and strength after anterior cruciate ligament reconstruction and rehabilitation. J Orthop Sports Phys Ther. 1997;26(5):238-245. doi:10.2519/jospt.1997.26.5.238.
30. Ledford CK, Millikan PD, Nickel BT, et al. Percent body fat Is more predictive of function after total joint arthroplasty than body mass index. J Bone Joint Surg. 2016;98(10):849-857. doi:10.2106/JBJS.15.00509.
31. Berlet G, Kiebzak GM, Dandar A, et al. Prospective analysis of body composition and SF36 profiles in professional dancers over a 7-month season: is there a correlation to injury? J Dance Med Sci. 2002;6(2):54-61.
32. Grant JA, Bedi A, Kurz J, Bancroft R, Gagnier JJ, Miller BS. Ability of preseason body composition and physical fitness to predict the risk of injury in male collegiate hockey players. Sports Health. 2015;7(1):45-51. doi:10.1177/1941738114540445.
33. Stewart AD, Hannan J. Subregional tissue morphometry in male athletes and controls using DXA. Int J Sport Nutr Exerc Metab. 2000;10(2):157-169. doi:10.1123/ijsnem.10.2.157.
34. Sannicandro I, Cofano G, Rosa RA, Piccinno A. Balance training exercises decrease lower-limb strength asymmetry in young tennis players. J Sports Sci Med. 2014;13(2):397-402.
35. Guglielmi G, Ponti F, Agostini M, Amadori M, Battista G, Bazzocchi A. The role of DXA in sarcopenia. Aging Clin Exp Res. 2016;28(6):1047-1060. doi:10.1007/s40520-016-0589-3.
36. Janssen I, Baumgartner RN, Ross R, Rosenberg IH, Roubenoff R. Skeletal muscle cutpoints associated with elevated physical disability risk in older men and women. Am J Epidemiol. 2004;159(4):413-421.
37. Landi F, Calvani R, Ortolani E, et al. The association between sarcopenia and functional outcomes among older patients with hip fracture undergoing in-hospital rehabilitation. Osteoporos Int. 2017;28(5):1569-1576. doi:10.1007/s00198-017-3929-z.
38. Roh YH, Noh JH, Gong HS, Baek GH. Effect of low appendicular lean mass, grip strength, and gait speed on the functional outcome after surgery for distal radius fractures. Arch Osteoporos. 2017;12(1):41. doi:10.1007/s11657-017-0335-2.
39. Miller MS, Callahan DM, Toth MJ. Skeletal muscle myofilament adaptations to aging, disease, and disuse and their effects on whole muscle performance in older adult humans. Front Physiol. 2014;5:369. doi:10.3389/fphys.2014.00369.
40. Waters DJ, Baumgartner RN. Sarcopenia and obesity. Clin Geriatr Med. 2011;27(3):401-421. doi:10.1016/j.cger.2011.03.007.
41. Bachrach LK, Gordon CM. Bone densitometry in children and adolescents. Pediatrics. 2016;138(4):e20162398. doi:10.1542/peds.2016-2398.
TAKE-HOME POINTS
- DXA is underutilized technology in orthopedics.
- More data ("ancillary data") are often available from a DXA scan then typically included in a standard report from a referral center.
- Most orthopedists are likely unaware of the detailed body composition data available with a total body scan.
- Preoperative DXA scans and knowledge of BMD may be informative when planning the type of fixation and implant metal to used.
- Serial follow-up body composition scans can be useful in monitoring the course of bone healing (mineralization) and soft tissue changes (fat and lean mass).
Open Clinical Trials for Patients With Colorectal Cancer (FULL)
Providing access to clinical trials for veteran and active-duty military patients can be a challenge, but a significant number of trials are now recruiting patients from those patient populations. Many trials explicitly recruit patients from the VA, the military, and IHS. The VA Office of Research and Development alone sponsors > 300 research initiatives, and many more are sponsored by Walter Reed National Medical Center and other major defense and VA facilities. The clinical trials listed below are all open as of April 1, 2017; have at least 1 VA, DoD, or IHS location recruiting patients; and are focused on treatment for colorectal cancer. For additional information and full inclusion/exclusion criteria, please consult clinicaltrials.gov.
Impact of Family History and Decision Support on High-Risk Cancer Screening
There is no standardized system for collecting and updating family health history, using this information to determine a patient’s disease risk level, and providing screening recommendations to patients and providers. Patients will enter their family health history into a program that will produce screening recommendations tailored to the patient’s family health history. The investigators will examine whether this process increases physician referrals for, and patient uptake of, guideline-recommended screening for colorectal cancer.
ID: NCT02247336
Sponsor: VA Office of Research and Development
Location (contact): Durham VAMC, North Carolina (Jamiyla Bolton, Susan B. Armstrong); William S. Middleton Memorial Veterans Hospital, Madison, Wisconsin (Corrine Voils)
Colonoscopy Versus Fecal Immunochemical Test in Reducing Mortality From Colorectal Cancer
The investigators propose to perform a large, simple, multicenter, randomized, parallel-group trial directly comparing screening colonoscopy with annual fecal immunochemical test screening in average risk individuals. The hypothesis is that colonoscopy will be superior to fecal immunochemical testing in the prevention of colorectal cancer (CRC) mortality measured over 10 years. The primary study endpoint will be CRC mortality within 10 years of enrollment.
ID: NCT01239082
Sponsor: VA Office of Research and Development
Locations: 48 current locations
S0820, Adenoma and Second Primary Prevention Trial (PACES)
The investigators hypothesize that the combination of eflornithine and sulindac will be effective in reducing a 3-year event rate of adenomas and second primary colorectal cancers in patients previously treated for Stages 0 through III colon cancer.
ID: NCT01349881
Sponsor: Southwest Oncology Group
Location (contact): VA Connecticut Healthcare System-West Haven Campus (Michal Rose); Edward Hines Jr. VA Hospital, Hines, Illinois (Abdul Choudhury); Kansas City VAMC, Missouri (Joaquina Baranda); White River Junction VAMC, Vermont (Nancy Kuemmerle); Eisenhower Army Medical Center, Fort Gordon, Georgia (Andrew Delmas); Tripler Army Medical Center, Honolulu, Hawaii (Jeffrey Berenberg); Brooke Army Medical Center, Fort Sam Houston, Texas (John Renshaw)
Irinotecan Hydrochloride and Cetuximab With or Without Ramucirumab in Treating Patients With Advanced Colorectal Cancer With Progressive Disease After Treatment With Bevacizumab
This randomized phase II trial is studying the adverse effects and how well giving cetuximab and irinotecan hydrochloride with or without ramucirumab work in treating patients with advanced colorectal cancer with progressive disease after treatment with bevacizumab-containing chemotherapy.
ID: NCT01079780
Sponsor: Eastern Cooperative Oncology Group
Location (contact): Atlanta VAMC, Decatur, Georgia (Samuel Chan); VA New Jersey Health Care System East Orange Campus (Basil Kasimis)
Cancer Associated Thrombosis and Isoquercetin
This research study is evaluating a drug called isoquercetin to prevent venous thrombosis (blood clots) in participants who have pancreas, non-small cell lung cancer or colorectal cancer.
ID: NCT02195232
Sponsor: Dana-Farber Cancer Institute
Location (contact): Washington DC VAMC (Anita Aggarwal); Boston VA Healthcare System, Massachusetts (Kenneth Bauer); White River Junction VAMC, Vermont (Nancy Kuemmerle)
Studying Lymph Nodes in Patients With Stage II Colon Cancer
Diagnostic procedures that look for micrometastases in lymph nodes removed during surgery for colon cancer may help doctors learn the extent of disease. This phase I trial is studying lymph nodes in patients with stage II colon cancer.
ID: NCT00949312
Sponsor: John Wayne Cancer Institute
Location: Walter Reed Army Medical Center, Washington, DC
Click here to read the digital edition.
Providing access to clinical trials for veteran and active-duty military patients can be a challenge, but a significant number of trials are now recruiting patients from those patient populations. Many trials explicitly recruit patients from the VA, the military, and IHS. The VA Office of Research and Development alone sponsors > 300 research initiatives, and many more are sponsored by Walter Reed National Medical Center and other major defense and VA facilities. The clinical trials listed below are all open as of April 1, 2017; have at least 1 VA, DoD, or IHS location recruiting patients; and are focused on treatment for colorectal cancer. For additional information and full inclusion/exclusion criteria, please consult clinicaltrials.gov.
Impact of Family History and Decision Support on High-Risk Cancer Screening
There is no standardized system for collecting and updating family health history, using this information to determine a patient’s disease risk level, and providing screening recommendations to patients and providers. Patients will enter their family health history into a program that will produce screening recommendations tailored to the patient’s family health history. The investigators will examine whether this process increases physician referrals for, and patient uptake of, guideline-recommended screening for colorectal cancer.
ID: NCT02247336
Sponsor: VA Office of Research and Development
Location (contact): Durham VAMC, North Carolina (Jamiyla Bolton, Susan B. Armstrong); William S. Middleton Memorial Veterans Hospital, Madison, Wisconsin (Corrine Voils)
Colonoscopy Versus Fecal Immunochemical Test in Reducing Mortality From Colorectal Cancer
The investigators propose to perform a large, simple, multicenter, randomized, parallel-group trial directly comparing screening colonoscopy with annual fecal immunochemical test screening in average risk individuals. The hypothesis is that colonoscopy will be superior to fecal immunochemical testing in the prevention of colorectal cancer (CRC) mortality measured over 10 years. The primary study endpoint will be CRC mortality within 10 years of enrollment.
ID: NCT01239082
Sponsor: VA Office of Research and Development
Locations: 48 current locations
S0820, Adenoma and Second Primary Prevention Trial (PACES)
The investigators hypothesize that the combination of eflornithine and sulindac will be effective in reducing a 3-year event rate of adenomas and second primary colorectal cancers in patients previously treated for Stages 0 through III colon cancer.
ID: NCT01349881
Sponsor: Southwest Oncology Group
Location (contact): VA Connecticut Healthcare System-West Haven Campus (Michal Rose); Edward Hines Jr. VA Hospital, Hines, Illinois (Abdul Choudhury); Kansas City VAMC, Missouri (Joaquina Baranda); White River Junction VAMC, Vermont (Nancy Kuemmerle); Eisenhower Army Medical Center, Fort Gordon, Georgia (Andrew Delmas); Tripler Army Medical Center, Honolulu, Hawaii (Jeffrey Berenberg); Brooke Army Medical Center, Fort Sam Houston, Texas (John Renshaw)
Irinotecan Hydrochloride and Cetuximab With or Without Ramucirumab in Treating Patients With Advanced Colorectal Cancer With Progressive Disease After Treatment With Bevacizumab
This randomized phase II trial is studying the adverse effects and how well giving cetuximab and irinotecan hydrochloride with or without ramucirumab work in treating patients with advanced colorectal cancer with progressive disease after treatment with bevacizumab-containing chemotherapy.
ID: NCT01079780
Sponsor: Eastern Cooperative Oncology Group
Location (contact): Atlanta VAMC, Decatur, Georgia (Samuel Chan); VA New Jersey Health Care System East Orange Campus (Basil Kasimis)
Cancer Associated Thrombosis and Isoquercetin
This research study is evaluating a drug called isoquercetin to prevent venous thrombosis (blood clots) in participants who have pancreas, non-small cell lung cancer or colorectal cancer.
ID: NCT02195232
Sponsor: Dana-Farber Cancer Institute
Location (contact): Washington DC VAMC (Anita Aggarwal); Boston VA Healthcare System, Massachusetts (Kenneth Bauer); White River Junction VAMC, Vermont (Nancy Kuemmerle)
Studying Lymph Nodes in Patients With Stage II Colon Cancer
Diagnostic procedures that look for micrometastases in lymph nodes removed during surgery for colon cancer may help doctors learn the extent of disease. This phase I trial is studying lymph nodes in patients with stage II colon cancer.
ID: NCT00949312
Sponsor: John Wayne Cancer Institute
Location: Walter Reed Army Medical Center, Washington, DC
Click here to read the digital edition.
Providing access to clinical trials for veteran and active-duty military patients can be a challenge, but a significant number of trials are now recruiting patients from those patient populations. Many trials explicitly recruit patients from the VA, the military, and IHS. The VA Office of Research and Development alone sponsors > 300 research initiatives, and many more are sponsored by Walter Reed National Medical Center and other major defense and VA facilities. The clinical trials listed below are all open as of April 1, 2017; have at least 1 VA, DoD, or IHS location recruiting patients; and are focused on treatment for colorectal cancer. For additional information and full inclusion/exclusion criteria, please consult clinicaltrials.gov.
Impact of Family History and Decision Support on High-Risk Cancer Screening
There is no standardized system for collecting and updating family health history, using this information to determine a patient’s disease risk level, and providing screening recommendations to patients and providers. Patients will enter their family health history into a program that will produce screening recommendations tailored to the patient’s family health history. The investigators will examine whether this process increases physician referrals for, and patient uptake of, guideline-recommended screening for colorectal cancer.
ID: NCT02247336
Sponsor: VA Office of Research and Development
Location (contact): Durham VAMC, North Carolina (Jamiyla Bolton, Susan B. Armstrong); William S. Middleton Memorial Veterans Hospital, Madison, Wisconsin (Corrine Voils)
Colonoscopy Versus Fecal Immunochemical Test in Reducing Mortality From Colorectal Cancer
The investigators propose to perform a large, simple, multicenter, randomized, parallel-group trial directly comparing screening colonoscopy with annual fecal immunochemical test screening in average risk individuals. The hypothesis is that colonoscopy will be superior to fecal immunochemical testing in the prevention of colorectal cancer (CRC) mortality measured over 10 years. The primary study endpoint will be CRC mortality within 10 years of enrollment.
ID: NCT01239082
Sponsor: VA Office of Research and Development
Locations: 48 current locations
S0820, Adenoma and Second Primary Prevention Trial (PACES)
The investigators hypothesize that the combination of eflornithine and sulindac will be effective in reducing a 3-year event rate of adenomas and second primary colorectal cancers in patients previously treated for Stages 0 through III colon cancer.
ID: NCT01349881
Sponsor: Southwest Oncology Group
Location (contact): VA Connecticut Healthcare System-West Haven Campus (Michal Rose); Edward Hines Jr. VA Hospital, Hines, Illinois (Abdul Choudhury); Kansas City VAMC, Missouri (Joaquina Baranda); White River Junction VAMC, Vermont (Nancy Kuemmerle); Eisenhower Army Medical Center, Fort Gordon, Georgia (Andrew Delmas); Tripler Army Medical Center, Honolulu, Hawaii (Jeffrey Berenberg); Brooke Army Medical Center, Fort Sam Houston, Texas (John Renshaw)
Irinotecan Hydrochloride and Cetuximab With or Without Ramucirumab in Treating Patients With Advanced Colorectal Cancer With Progressive Disease After Treatment With Bevacizumab
This randomized phase II trial is studying the adverse effects and how well giving cetuximab and irinotecan hydrochloride with or without ramucirumab work in treating patients with advanced colorectal cancer with progressive disease after treatment with bevacizumab-containing chemotherapy.
ID: NCT01079780
Sponsor: Eastern Cooperative Oncology Group
Location (contact): Atlanta VAMC, Decatur, Georgia (Samuel Chan); VA New Jersey Health Care System East Orange Campus (Basil Kasimis)
Cancer Associated Thrombosis and Isoquercetin
This research study is evaluating a drug called isoquercetin to prevent venous thrombosis (blood clots) in participants who have pancreas, non-small cell lung cancer or colorectal cancer.
ID: NCT02195232
Sponsor: Dana-Farber Cancer Institute
Location (contact): Washington DC VAMC (Anita Aggarwal); Boston VA Healthcare System, Massachusetts (Kenneth Bauer); White River Junction VAMC, Vermont (Nancy Kuemmerle)
Studying Lymph Nodes in Patients With Stage II Colon Cancer
Diagnostic procedures that look for micrometastases in lymph nodes removed during surgery for colon cancer may help doctors learn the extent of disease. This phase I trial is studying lymph nodes in patients with stage II colon cancer.
ID: NCT00949312
Sponsor: John Wayne Cancer Institute
Location: Walter Reed Army Medical Center, Washington, DC
Click here to read the digital edition.
A Practical Approach to Management of the Patient with Inflammatory Bowel Disease Following Tumor Necrosis Factor Antagonist Failure
From the Division of Gastroenterology University of Washington, Seattle, WA (Dr. Tiderington), and the Division of Gastroenterology, Hepatology and Nutrition, The Ohio State University Wexner Medical Center and The Ohio State University Inflammatory Bowel Disease Center, Columbus, OH (Dr. Afzali).
Abstract
- Objective: To provide a practical approach to the management of patients with inflammatory bowel disease (IBD) following tumor necrosis factor (TNF) alpha antagonist failure.
- Methods: Review of the literature.
- Results: TNF alpha antagonists play a central role in the treatment of IBD. Unfortunately, some patients will not respond to therapy with TNF antagonists, and others will lose response during treatment. When patients present with persistent or recurrent symptoms suggesting active IBD while on anti-TNF therapy it can present a dilemma for the clinician. In this paper we review the mechanisms of drug failure, the use of reactive therapeutic drug monitoring to guide clinical decision making, and propose an evidence-based method for managing this common clinical scenario.
- Conclusion: Despite the improved clinical outcomes seen since the introduction of TNF antagonists for the management of IBD, there remains a significant need for additional medical therapies. Fortunately, the armamentarium is expected to expand dramatically over the next decade.
Key words: TNF antagonists; therapeutic drug monitoring; biologic failure; Crohn’s disease treatment; ulcerative colitis treatment.
Ulcerative colitis and Crohn’s disease are the two types of inflammatory bowel disease (IBD), and they are characterized by chronic, immunologically mediated inflammation involving the gastrointestinal tract [1]. Guided by an understanding of the role of tumor necrosis factor (TNF) alpha in the pathogenesis of IBD, TNF antagonists have played a central role in modern treatment algorithms [2]. Unfortunately, roughly one third of patients will not have a clinical response when given induction dosing of the currently available anti-TNF agents, and of those who do respond to treatment, up to one half will lose response to treatment within the first year [3]. When patients present with persistent or recurrent symptoms suggesting active IBD while on anti-TNF therapy it can present a dilemma for the clinician. Once the clinician has confirmed that active IBD is present based on endoscopic, cross-sectional imaging and/or biochemical markers of inflammation, the next step is to identify the cause of the treatment failure, as this guides management. Here we review the body of literature that guides our understanding of treatment failure as well as therapeutic drug monitoring and propose an evidence-based algorithm for managing this common clinical scenario.
Defining Treatment Failure
Patients who receive anti-TNF therapy but demonstrate active IBD should be classified as having either primary nonresponse or secondary loss of response. Primary nonresponse is defined as having either no response, or only partial response, to induction with anti-TNF therapy [4]. Data from pivotal trials and meta-analyses suggest that about one third of patients will not show any clinical response to induction with anti-TNF therapies, with response typically being defined using composite endpoints favoring clinical symptoms and only sometimes incorporating endoscopic findings [5]. An additional one third of patients will have only a partial response, without remission. Patients with ulcerative colitis are at a slightly increased risk of primary nonresponse compared to patients with Crohn’s disease. Though the time frame for defining primary nonresponse is different for each agent because each agent has a slightly different induction schedule, in general the maximal response to therapy is typically seen by week 12, and it is reasonable to use this as a time cutoff [6].
Secondary loss of response is likewise defined as recrudescence of clinically active disease after an initial response. In general, the presence of secondary loss of response should not be invoked until week 12 of therapy. In most pivotal trials, secondary loss of response was seen in roughly half of patients at 1 year. In clinical practice, however, particularly as therapeutic drug monitoring has become more common, the observed rates of secondary loss of response have been lower [6].
Applying these definitions appropriately is important because it dictates the next steps in management. When a patient presents with symptoms suggesting active IBD while on anti-TNF therapy, either during induction when primary nonresponse is possible, or in maintenance when secondary loss of response would be invoked, the first step is to determine if active IBD is the etiology for the presenting symptoms. The initial evaluation should rule out common infectious causes of symptoms mimicking IBD. In particular, Clostridium difficile infection should be ruled out with stool testing. In certain circumstances, ruling out cytomegalovirus (CMV) colitis is important, so an endoscopic evaluation with colonic biopsies should be considered. In the absence of infectious colitis, the presence of active inflammation can often be identified endoscopically, or can be inferred from noninvasive markers with a fair degree of certainty. Fecal calprotectin is an ideal choice for this purpose. In ulcerative colitis it is estimated to have a sensitivity of 0.88 and a specificity of 0.79 for the prediction of endoscopically active disease. The estimated sensitivity for detecting endoscopically active Crohn’s disease is essentially the same (0.87), and the specificity is only slightly lower (0.67). C-reactive protein demonstrates a better specificity (0.92), but has a marginal sensitivity (0.49) [7]. Other etiologies for the patient’s symptoms should also be considered, including medication side effects including use of nonsteroidal anti-inflammatory medications, bile acid malabsorption, small intestinal bacterial overgrowth (SIBO), irritable bowel syndrome (IBS), diverticular disease, ischemic colitis, fibrostenotic strictures, and cancer, depending on comorbidities and the history of present illness.
Once it has been determined that active IBD is the etiology for the patient’s symptoms, the patient should be classified as having either primary nonresponse or secondary loss of response as described above. For the clinician, the next question is how to alter or optimize therapy.
The decision of how to optimize therapy will largely depend on which anti-TNF therapy the patient is currently receiving, and whether they are receiving it as monotherapy or as combination therapy with an immunomodulator. Optimizing therapy will involve either increasing the dose or frequency of administration of the anti-TNF therapy, increasing the dose of azathioprine if indicated, adding an immunomodulator if the patient is on anti-TNF monotherapy, changing to a different anti-TNF agent, or changing to a different class of medication with a different mechanism of action. The recently released American Gastroenterological Association (AGA) guidelines on therapeutic drug monitoring in IBD provide a framework for making these decisions [8]. In general, the clinical choice will be dictated by the etiology of the drug failure.
Types of TNF Antagonist Drug Failure
Our understanding of the causes of biologic treatment failure are evolving but are typically classified as due to mechanistic failure, non-immune-mediated pharmacokinetic failure, or immune-mediated pharmacokinetic failure [9]. Differentiating between these classes of treatment failure requires therapeutic drug monitoring (TDM), which will be discussed in more detail below.
Mechanistic failure is encountered when the underlying biology does not favor a response to a particular therapy. Studies indicate a strong association between particular genetic phenotypes and the probability of a response to induction with anti-TNF agents [10]. This suggests that some individuals have IBD driven by a biochemical inflammatory cascade in which TNF features prominently, while others have alternative mechanistic drivers of inflammation without significantly elevated TNF levels. Mechanistic failure will typically present as primary nonresponse, but can also be seen in patients with secondary loss of response. Mechanistic failure can be elucidated clinically by the use of TDM. In the case of mechanistic failure, active disease is seen in the presence of adequate drug level, without the presence of anti-drug antibodies. The AGA recommends considering switching to a biologic with a different mechanism of action when mechanistic failure is identified [8].
Non-immune-mediated pharmacokinetic failure is encountered when a patient who would otherwise respond to a drug at adequate drug levels experiences suboptimal drug levels because of pharmacokinetic factors. In the case of anti-TNF therapy, this can be conceptualized as either an increased clearance of anti-TNF from the body (eg, in patients with significant hypoalbuminemia or severe colitis), a reduction in the average serum anti-TNF level because of the redistribution of drug in patients with a large body mass index, or inadequate saturation of the total body burden of TNF-alpha in subjects with a high baseline level of inflammation [11]. Non-immune-mediated pharmacokinetic failure can also be identified clinically through TDM. In this case, active disease is seen in the presence of a suboptimal drug level, without the presence of anti-drug antibodies. The AGA recommends considering dose-escalation of the current TNF antagonist when non-immune-mediated pharmacokinetic failure is identified [8], as this can improve clinical response in an estimated 82% of patients [9].
Finally, immune-mediated pharmacokinetic failure is encountered when a patient who would otherwise respond to the current biologic therapy when at adequate drug concentration levels experiences suboptimal drug levels because of increased drug clearance mediated by anti-drug antibodies [9]. Because anti-TNF agents are monoclonal antibodies, they are inherently immunogenic, and it is well established that episodic dosing and lower serum drug concentrations are strong risk factors for the development of anti-drug antibodies [12]. When anti-drug antibodies are present, and are associated with both a decreased serum drug concentration and active inflammatory bowel disease, immune-mediate pharmacokinetic failure can be invoked. When anti-drug antibodies are present, but at a low level, the AGA recommends dose escalation of current TNF antagonist. When anti-drug antibodies are present at a high level, the AGA recommends considering either the addition of an immunomodulator (if not already being used), or changing to a different class of biologic therapy [8]. This recommendation is based in part on data showing that the proportion of patients with sustained anti-drug antibodies during the first year of therapy with an TNF antagonist is likely between 14% and 20% for those on monotherapy, but between 1% and 5% for those on concomitant immunomodulatory therapy [13,14].
Therapeutic Drug Monitoring of Anti-TNF Agents
As described above, TDM, which is the process of testing the patient’s serum for both the concentration of the TNF antagonist and for the presence and concentration of anti-drug antibodies, can help differentiate between mechanistic failure, non-immune-mediated pharmacokinetic failure, and immune-mediated pharmacokinetic failure (Table 1).
Therapeutic drug monitoring can be classified as either proactive or reactive. Proactive TDM is performed during induction or maintenance therapy when the patient does not have signs or symptoms of active disease to suggest a loss of response. Theoretically, this would allow dose modification and optimization, including dose de-escalation in certain circumstances, and could thus provide cost savings with minimal impact on clinical outcomes. The TAXIT trial provides the most robust evaluation of proactive TDM in TNF antagonist therapy. In this study, patients with Crohn’s disease or ulcerative colitis who had a stable clinical response while on maintenance infliximab were first dose optimized proactively to a target trough concentration of 3–7 μg/mL, then randomized to having dose modifications made based on clinical factors alone, defined as reactive monitoring, or dose modifications based on proactive monitoring, performed by checking the drug concentration and antibody levels before each infusion. At 1 year there was no statistically significant difference in the proportion of patients in remission. In addition, some patients in the proactive TDM group were able to have a dose reduction without a subsequent flare of disease, thus providing cost savings [15]. This study suggests that proactive TDM may have a role in drug optimization, particularly with respect to cost-effectiveness, but provides only indirect evidence of a clinical benefit, since all subjects enrolled in the study were proactively dose optimized prior to randomization. This study had a limited follow-up time of 1 year so was not able to assess for longer-term benefits and risks associated with proactive TDM.
More recently, a large, multicenter, retrospective cohort study provided additional evidence that proactive TDM may provide a clinical benefit in addition to cost savings. This study retrospectively evaluated consecutive patients receiving maintenance infliximab for Crohn’s disease between 2006 and 2015, with a median follow-up time of 2.4 years. They were classified as having had either proactive TDM or reactive TDM. Proactive TDM was associated with statistically significant reductions in the risk of treatment failure (hazard ratio [HR] 0.16, 95% confidence interval [CI] 0.09–0.27), the need for surgery (HR 0.30, 95% CI 0.11–0.80), hospitalization (HR 0.16, 95% CI 0.07–0.33), and anti-drug antibody formation (HR 0.25, 95% CI 0.07–0.84) [16].
To date, however, no randomized controlled trials have been published comparing proactive TDM to reactive TDM in treatment-naive patients. Because of the paucity of prospective studies, the AGA currently makes no recommendation regarding the use of proactive TDM in clinical practice. However, the current AGA guidelines do recommend reactive TDM in the setting of secondary loss of response based on the results of one randomized controlled trial (RCT) and several observational studies. The RCT was small (n = 69), and enrolled patients with Crohn’s disease on maintenance therapy with infliximab. Similar to the TAXIT trial, the study did not show a statistically significant difference in rates of clinical remission when subjects were randomized to either empiric dose escalation (to 5 mg/kg every 4 weeks) based on symptoms, or to dose escalations based on the results of reactive TDM. Also similar to the TAXIT trial, it showed an estimated cost savings of about 34% based on local prices in Denmark for reactive TDM over empiric dose escalation [17].
Meanwhile, the observational studies for reactive TDM provided additional support to the clinical benefit of reactive TDM, but also to the underlying hypotheses that drive reactive TDM, namely that subjects with mechanistic failure benefit from a change in drug class, those with non-immune-mediated pharmacokinetic failure benefit from dose escalation, and that those with immune-mediated pharmacokinetic failure may benefit from either dose escalation or a change in mechanism of action, depending on antibody titers. Specifically, on pooled analysis of 2 of these studies, 82% of subjects who were found to have non-immune-mediated pharmacokinetic failure responded to empiric dose escalation, whereas only 8% of subjects who were found to have immune-mediated pharmacokinetic failure with high anti-drug antibody titers responded to dose escalation [9]. Likewise, in a retrospective study involving subjects who were being treated with infliximab and then had reactive TDM performed, when non-immune-mediated pharmacokinetic failure was identified, a clinical response was seen in 86% of subjects who underwent dose escalation, and only 33% among those who were switched to a different anti-TNF (P < 0.016). Conversely, dose escalation resulted in a clinical response only 17% of the time when anti-drug antibodies were detectable, compared to a 92% response rate when the subject was switched to a different anti-TNF (P < 0.004) [18].
Interpreting the Results of Reactive Therapeutic Drug Monitoring
The implementation of reactive TDM involves obtaining a serum drug and antibody level and then interpreting what those results suggest about the mechanism of drug failure, in order to decide on a course of action. The serum drug level should be a trough concentration, so it should be obtained just prior to a timed dose, while on a stable treatment regimen. Exactly what serum drug concentration we should be targeting in reactive therapeutic drug monitoring remains an area of investigation. No RCTs have been published. There is ample observational, cross-sectional data from cohorts of patients on maintenance therapy, though heterogeneity in study design and study populations, as well as use of different assays, limit interpretation of the data. In a secondary analysis of data from 6 observational studies of patients on infliximab maintenance therapy, there was a highly statistically significant concentration-dependent trend in rates of clinical remission depending on the measured infliximab trough concentration, with 96% of those with infliximab > 7 μg/mL in remission, 92% of those with infliximab > 5 μg/mL in remission, and 75% of those with infliximab > 1 μg/mL in remission. Likewise, data from 4 studies of patients receiving adalimumab showed a statistically significant concentration-dependent trend in clinical remission, with 90% of those with adalimumab trough concentrations > 7.5 μg/mL being in clinical remission, compared with only 83% of those with concentrations > 5 μg/mL. Similarly, data from 9 studies suggested that a certolizumab trough concentration > 20 μg/mL was associated with a 75% probability of being in clinical remission, compared to a 60% probability when the trough concentration was > 10 μg/mL [9]. Based on these analyses, the AGA suggests target trough concentrations for reactive therapeutic drug monitoring of anti-TNF agents of ≥ 5 μg/mL for infliximab, ≥ 7.5 μg/mL for adalimumamb, and ≥ 20 μg/mL for certolizumab. They did not suggest a target trough concentration for golimumab because of insufficient evidence [8].
When interpreting TDM test results, it is important to know if the test you have used is drug-sensitive or drug-tolerant (Table 2). Drug-sensitive tests will be less likely to reveal the presence of anti-drug antibodies when the drug level is above a certain threshold. A post-hoc analysis of the TAXIT trial recently suggested that subjects who have antibodies detected on a drug-tolerant test which were not detected on a drug-sensitive test are more likely to respond to higher doses of infliximab [19]. It follows that there should be a threshold anti-drug antibody titer below which someone who has immune-mediated pharmacokinetic failure will still respond to TNF antagonist dose escalation, but above which they will fail to respond to dose escalation. To be sure, our understanding of the clinical implications of a drug-tolerant test demonstrating an adequate drug level while also detectable anti-drug antibodies is evolving. Complicating the issue further is the fact that anti-drug antibody concentrations cannot be compared between assays because of assay-specific characteristics. As such, though the presence of low antibody titers and high antibody titers seems to be clinically important, recommendations cannot yet be made on how to interpret specific thresholds. Furthermore, development of transient versus sustained antibodies requires further clinical investigation to determine impact and treatment algorithms.
Optimizing Therapy
Once you have determined the most likely cause of drug failure, the next step is to make a change in medical therapy.
When switching within class (to another anti-TNF agent), the choice of which agent to use next will largely depend on patient preference (route of administration, infusion versus injection), insurance, and costs of treatment. When making the decision to switch within class, it should be kept in mind that the probability of achieving remission is modestly reduced compared to the probability seen in anti-TNF-naive patients [20], and even more so when the patient is switching to their third anti-TNF agent [21]. Thus, for the patient who has already previously switched from one TNF antagonist to a second TNF antagonist, it may be better to switch to a different class of biologic rather than attempting to capture a clinical remission with a third TNF antagonist.
When adding an immunomodulator (azathioprine or methotrexate), the expectation is that the therapy will increase the serum concentration of the anti-TNF agent [14] and/or reduce the ongoing risk of anti-drug antibody formation [22]. There could also be a direct treatment effect on the bowel disease by the immunomodulator.
When switching to an alternate mechanism of action, the currently FDA-approved options include the biologic agents vedolizumab (for both moderate-to-severe ulcerative colitis and moderate-to-severe Crohn’s disease) and ustekinumab (for moderate-to-severe Crohn’s disease), as well as the recently FDA-approved oral, small-molecule JAK1 and JAK3 inhibitor tofacitinib (for moderate-to-severe ulcerative colitis). Prospective comparative effectiveness studies for these agents are lacking and are unlikely to be performed in part due to the cost and time required to accomplish these studies. A recent post-hoc analysis of clinical trials data suggests that there are no significant differences in the rates of clinical response, clinical remission, or in adverse outcomes to vedolizumab or ustekinumab when they are used in patients who have failed anti-TNF therapy [23]. Thus, one cannot be recommended over the other, and the decision of which to use is usually guided by patient preference and insurance coverage.
Meanwhile, the role of tofacitinib in the treatment algorithm of patients who have failed anti-TNF therapy remains unclear. The phase III clinical trials OCTAVE 1, OCTAVE 2, and OCTAVE Sustain showed efficacy for both the induction and maintenance of remission in patients with moderate-to-severe ulcerative colitis who had previously failed anti-TNF agents. However, there remain concerns about the safety profile of tofacitinib compared to vedolizumab and ustekinumab, particularly regarding herpes zoster infection, dyslipidemia, and adverse cardiovascular events. Notable findings from the tofacitinib induction trials include robust rates of clinical remission (18.5% vs 8.2% for placebo in Octave 1, and 16.6% vs 3.6% in Octave 2, P < 0.001 for both comparisons) and mucosal healing (31.3% vs 15.6% for placebo in Octave 1, and 28.4% and 11.6% in Octave 2, P < 0.001 for both comparisons) after 8 weeks of induction therapy [24]. These results suggest that tofacitinib, or other JAK inhibitors that become approved in the future, may be excellent oral agents for the induction of remission in moderate-to-severe ulcerative colitis, and may demonstrate a better side effect profile than steroids. Whether cost factors (compared to steroid therapy) will limit the role of JAK-inhibitor therapy in induction, and whether safety concerns will limit their use in maintenance therapy, remains to be seen.
Off-Label Rescue Therapy and Surgery
Though the armamentarium of IBD therapies has expanded greatly over the past 2 decades, and will continue to do so for the foreseeable future, there are still a limited selection of therapies available to patients. Patients who have failed anti-TNF therapy, and subsequently fail vedolizumab and/or ustekinumab, have limited options. These options include clinical trials, off-label small molecule rescue therapy, and surgery. Patients who are felt to require any of these options should be referred to a tertiary center for evaluation by a gastroenterologist specializing in the treatment of IBD and/or a colorectal surgeon specializing in the surgical management of IBD.
Tacrolimus
Tacrolimus, a macrolide calcineurin inhibitor, has been studied as a small molecule therapy for IBD, though not in randomized controlled trials. There is biological plausibility for its use as a disease modifying agent. Mucosal T cells in subjects with active Crohn’s disease have been found to express increased levels of mRNA encoding IL-2, and tacrolimus acts primarily by reducing IL-2 production [25]. The largest observational cohort evaluating the use of tacrolimus, published by Thin et al, included patients with both ulcerative colitis (n = 24) and Crohn’s disease (n = 11) who had moderate to severely active IBD. All patients had failed dose-optimized thiopurine therapy, 89% had primary nonresponse or secondary loss of response to at least one anti-TNF agent, and 74% were either steroid-refractory or steroid-dependent at the time tacrolimus was started. With close monitoring, they targeted a tacrolimus trough of 8–12 ng/mL. At 30 days, 66% had a clinical response, and 40% were in clinical remission. At 90 days, 60% had a clinical response, and 37% were in clinical remission. At 1 year, 31% had a clinical response, and 23% were in clinical remission. Of those in clinical remission at 1 year, 88% were either off of steroids or on less than 5 mg of prednisone per day. Renal impairment was seen in 25% of patients, including severe renal impairment in 11%, requiring drug cessation. Infectious complications were seen in 9% of patients. Headaches, tremor, and pancreatitis were also observed, though less commonly. The majority of patients ultimately had a surgical intervention, particularly if they were steroid-refractory at baseline, but the time to surgery was delayed in those who achieved a response or remission in the first 90 days of tacrolimus therapy. The authors suggested that while tacrolimus may lack clear long-term benefit in patients with medically refractory IBD, a therapeutic trial should be considered in select patients with the goal of medical and nutritional optimization before surgical intervention [26].
Cyclosporine
Cyclosporine, which also exerts its effect by inhibiting IL-2 production, has an established role in the management of severe ulcerative colitis. Data from randomized, placebo-controlled trials, along with numerous open label observational studies, have shown that intravenous cyclosporine can induce remission and potentially obviate the need for urgent/emergent colectomy in steroid-refractory patients who are hospitalized with severe ulcerative colitis [27,28]. Its use in maintenance therapy remains controversial, however. Older observational data suggest that even among those who have an initial clinical response to cyclosporine induction, 33% will undergo colectomy by 1 year, and 88% will undergo colectomy by 7 years [27). Though the concomitant administration of a thiopurine may delay the need for colectomy [29,30], cyclosporine seems to be, at best, a temporizing therapy for patients with severe ulcerative colitis. Studies on the use of cyclosporine for the induction of remission in Crohn’s disease have been less robust, as have studies on the use of cyclosporine for the maintenance of remission in Crohn’s disease [31]. Dose-dependent toxicity also remains a concern, particularly when being considered as maintenance therapy. Though some observational data suggest that the absolute risks of serious side effects from maintenance cyclosporine are small, cyclosporine is still generally avoided as a maintenance therapy [30].
Mycophenolate Mofetil
Mycophenolate mofetil (MMF), which inhibits both B and T cell proliferation by inhibiting de novo purine synthesis, has been studied in both Crohn’s disease and ulcerative colitis. Studies have been small, observational, and heterogeneous. On the whole, they suggest that MMF does have some efficacy, but it is not necessarily more effective than azathioprine and may have a slightly increased risk of side effects [32]. Given that the side effects of MMF include diarrhea, and an IBD-like enterocolitis (MMF-induced colitis) when given to subjects without an established diagnosis of IBD, it is likely best to avoid using the drug in patients with IBD [33].
Surgery
Finally, when medical management has failed, or when fibrostenotic and/or penetrating complications of inflammatory bowel disease are present, surgery should be considered. Surgery can provide a cure in patients with ulcerative colitis, and can induce remission in patients with Crohn’s disease. Managing IBD medications around the time of surgery is always challenging. Multiple large, retrospective cohort studies have suggested that the risk for postoperative infectious complications, anastomotic leaks, and thrombotic complications do not differ between those who receive anti-TNF therapy within several months of surgery and those who do not. Nevertheless, some surgeons may prefer to time elective surgery halfway between doses of anti-TNF therapy. Additionally, there is some data to suggest that patients who are on both thiopurines and anti-TNF agents have an increased risk of postoperative complications compared to those who are on anti-TNF agents alone [34].
After a surgical evaluation, a plan of action should be formulated in a multidisciplinary fashion to determine how medical management will proceed. For those with an established diagnosis of ulcerative colitis, medical therapy can often be stopped postoperatively and the patient can be monitored prospectively for pouch complications including possible new-onset Crohn’s disease. For those who undergo surgery for the management of Crohn’s disease, though a resection completed with negative margins does induce remission, nearly 90% can be expected to have histologic, endoscopic, or clinical recurrence by 1 year. A randomized controlled trial showed that postoperative anti-TNF therapy can reduce this risk to 9% [35]. Unfortunately, a subsequently conducted large, multicenter, randomized controlled trial comparing postoperative infliximab to placebo was terminated early because of a lack of a statistically significant difference in clinical recurrence between the 2 groups at week 74. However, this lack of demonstrated efficacy may have been obscured by the relatively mild phenotype of the enrolled participants, who had a median CDAI score of 105.5 at baseline [36]. Based on available data, the AGA does conditionally recommend postoperative anti-TNF and/or thiopurine therapy for those patients with Crohn’s disease who are in a surgically induced remission [37]. The patients who are most likely to benefit from postoperative medical therapy are those who have the highest risk of recurrence, namely those who were young at the time of diagnosis, had a short disease duration prior to surgery, have multiple sites of disease, and who use tobacco products [34].
Emerging and Future Options
Despite the improved clinical outcomes seen since the introduction of TNF antagonists for the management of IBD, there remains a significant need for additional medical therapies. Fortunately, the armamentarium is expected to expand dramatically over the next decade.
Based on our improved, and evolving understanding of the pathogenesis of IBD, several new biochemical targets have emerged, offering novel ways to modulate the cytokine cascade which drives IBD [38]. Well over a dozen phase II and phase III trials for IBD therapeutic agents are ongoing, including biologic agents targeting interleukin-23, β7-Integrin, and MAdCAM-1, as well as small molecule agents targeting the JAK/STAT pathway and the sphingosine-1-phosphate receptor modulators [39]. As new agents are approved, it may be possible to develop a more patient-centered approach to care by targeting therapies to the particular pathogenesis of each patient’s disease. Nevertheless, integrating these therapies into practice algorithms will remain a challenge in the absence of meaningful comparative effectiveness trials [40].
Conclusion
When evaluating a patient who seems to have failed anti-TNF therapy for IBD, the first step is to confirm that active inflammatory disease is present. This process includes ruling out other potential causes of the patient’s symptoms, including infectious colitis, and ideally includes obtaining objective evidence of inflammation, whether through non-invasive biomarkers, an endoscopic evaluation and/or cross-sectional imaging. Once active IBD is confirmed, reactive therapeutic drug monitoring can help elucidate the likely mechanism of drug failure, which in turn can guide medical decision making.
Corresponding author: Anita Afzali MD, MPH, The Ohio State University Wexner Medical Center, 395 West 12th Ave, Room 280, Columbus, OH 43210, [email protected].
Financial disclosures: Dr. Afzali has served as a speaker/consultant for Abbvie, UCB, Takeda, Pfizer, Janssen; on the advisory board of Abbvie, UCB; received grant support from UCB; and is a board member of IBD Horizons.
1. Abraham C, Cho JH. Inflammatory bowel disease. N Engl J Med 2009;361:2066–78.
2. Danese S. New therapies for inflammatory bowel disease: from the bench to the bedside. Gut 2012;61:918–32.
3. Mitrev N, Leong RW. Therapeutic drug monitoring of anti-tumour necrosis factor-α agents in inflammatory bowel disease. Expert Opin Drug Saf 2017;16:303–17.
4. Papamichael K, Gils A, Rutgeerts P, et al. Role for therapeutic drug monitoring during induction therapy with TNF antagonists in IBD: evolution in the definition and management of primary nonresponse. Inflamm Bowel Dis 2015;21:182–97.
5. Levesque BG, Sandborn WJ, Ruel J, et al. Converging goals of treatment of inflammatory bowel disease from clinical trials and practice. Gastroenterology 2015;148:37–51.
6. Allez M, Karmiris K, Louis E, et al. Report of the ECCO pathogenesis workshop on anti-TNF therapy failures in inflammatory bowel diseases: definitions, frequency and pharmacological aspects. J Crohns Colitis 2010;4:355–66.
7. Mosli MH, Zou G, Garg SK, et al. C-Reactive protein, fecal calprotectin, and stool lactoferrin for detection of endoscopic activity in symptomatic inflammatory bowel disease patients: a systematic review and meta-analysis. Am J Gastroenterol 2015;110:802–19.
8. Feuerstein JD, Nguyen GC, Kupfer SS, et al. American Gastroenterological Association Institute guideline on therapeutic drug monitoring in inflammatory bowel disease. Gastroenterology 2017;153:827–34.
9. Vande Casteele N, Herfarth H, Katz J, et al. American Gastroenterological Association Institute technical review on the role of therapeutic drug monitoring in the management of inflammatory bowel diseases. Gastroenterology 2017;153:835–57.
10. López-Hernández R, Valdés M, Campillo JA, et al. Genetic polymorphisms of tumour necrosis factor alpha (TNF-α) promoter gene and response to TNF-α inhibitors in Spanish patients with inflammatory bowel disease. Int J Immunogenet 2014;41:63–8.
11. Ordás I, Mould DR, Feagan BG, Sandborn WJ. Anti-TNF monoclonal antibodies in inflammatory bowel disease: pharmacokinetics-based dosing paradigms. Clin Pharmacol Ther 2012;91:635–46.
12. Hindryckx P, Novak G, Vande Casteele N, et al. Incidence, prevention and management of anti-drug antibodies against therapeutic antibodies in inflammatory bowel disease: a practical overview. Drugs 2017;77:363–77.
13. Colombel JF, Sandborn WJ, Reinisch W, et al. Infliximab, azathioprine, or combination therapy for Crohn’s disease. N Engl J Med 2010;362:1383–95.
14. Lichtenstein GR, Diamond RH, Wagner CL, et al. Clinical trial: benefits and risks of immunomodulators and maintenance infliximab for IBD-subgroup analyses across four randomized trials. Aliment Pharmacol Ther 2009;30:210–26.
15. Vande Casteele N, Ferrante M, Van Assche G, et al. Trough concentrations of infliximab guide dosing for patients with inflammatory bowel disease. Gastroenterology 2015;148:1320–9.
16. Papamichael K, Chachu KA, Vajravelu RK, et al. Improved long-term outcomes of patients with inflammatory bowel disease receiving proactive compared with reactive monitoring of serum concentrations of infliximab. Clin Gastroenterol Hepatol 2017;15:1580–8.
17. Steenholdt C, Brynskov J, Thomsen OØ, et al. Individualised therapy is more cost-effective than dose intensification in patients with Crohn’s disease who lose response to anti-TNF treatment: a randomised, controlled trial. Gut 2014;63:919–27.
18. Afif W, Loftus EV Jr, Faubion WA, et al. Clinical utility of measuring infliximab and human anti-chimeric antibody concentrations in patients with inflammatory bowel disease. Am J Gastroenterol 2010;105:1133–9.
19. Van Stappen T, Vande Casteele N, Van Assche G, et al. Clinical relevance of detecting anti-infliximab antibodies with a drug-tolerant assay: post hoc analysis of the TAXIT trial. Gut 2017.
20. Gisbert JP, Marín AC, McNicholl AG, Chaparro M. Systematic review with meta-analysis: the efficacy of a second anti-TNF in patients with inflammatory bowel disease whose previous anti-TNF treatment has failed. Aliment Pharmacol Ther 2015;41:613–23.
21. Gisbert JP, Chaparro M. Use of a third anti-TNF after failure of two previous anti-TNFs in patients with inflammatory bowel disease: is it worth it? Scand J Gastroenterol 2015;50:379–86.
22. Ordás I, Feagan BG, Sandborn WJ. Therapeutic drug monitoring of tumor necrosis factor antagonists in inflammatory bowel disease. Clin Gastroenterol Hepatol 2012;10:1079–87.
23. Kawalec P, Moćko P. An indirect comparison of ustekinumab and vedolizumab in the therapy of TNF-failure Crohn’s disease patients. J Comp Eff Res 2017;7:101–11.
24. Sandborn WJ, Su C, Panes J, et al. Tofacitinib as induction and maintenance therapy for ulcerative colitis. N Engl J Med 2017;377:1723–36.
25. Yarkoni S, Sagiv Y, Kaminitz A, Askenasy N. Interleukin 2 targeted therapy in inflammatory bowel disease. Gut 2009;58:1705–6.
26. Thin LW, Murray K, Lawrance IC. Oral tacrolimus for the treatment of refractory inflammatory bowel disease in the biologic era. Inflamm Bowel Dis 2013;19:1490–8.
27. Moskovitz DN, Van Assche G, Maenhout B, et al. Incidence of colectomy during long-term follow-up after cyclosporine-induced remission of severe ulcerative colitis. Clin Gastroenterol Hepatol 2006;4:760–5.
28. Arts J, D’Haens G, Zeegers M, et al. Long-term outcome of treatment with intravenous cyclosporin in patients with severe ulcerative colitis. Inflamm Bowel Dis 2004;10:73–8.
29. Cohen RD, Stein R, Hanauer SB. Intravenous cyclosporin in ulcerative colitis: a five-year experience. Am J Gastroenterol 1999;94:1587–92.
30. Cheifetz AS, Stern J, Garud S, et al. Cyclosporine is safe and effective in patients with severe ulcerative colitis. J Clin Gastroenterol 2011;45:107–12.
31. Lazarev M, Present DH, Lichtiger S, et al. The effect of intravenous cyclosporine on rates of colonic surgery in hospitalized patients with severe Crohn’s colitis. J Clin Gastroenterol 2012;46:764–7.
32. Renna S, Cottone M, Orlando A. Optimization of the treatment with immunosuppressants and biologics in inflammatory bowel disease. World J Gastroenterol 2014;20:9675–90.
33. Izower MA, Rahman M, Molmenti EP, et al. Correlation of abnormal histology with endoscopic findings among mycophenolate mofetil treated patients. World J Gastrointest Endosc 2017;9:405–10.
34. Ferrari L, Krane MK, Fichera A. Inflammatory bowel disease surgery in the biologic era. World J Gastrointest Surg 2016;8:363–70.
35. Regueiro M, Schraut W, Baidoo L, et al. Infliximab prevents Crohn’s disease recurrence after ileal resection. Gastroenterology 2009;136:441–50.
36. Regueiro M, Feagan BG, Zou B, et al. Infliximab reduces endoscopic, but not clinical, recurrence of Crohn’s disease after ileocolonic resection. Gastroenterology 2016;150:1568–78.
37. Regueiro M, Velayos F, Greer JB, et al. American Gastroenterological Association Institute technical review on the management of Crohn’s disease after surgical resection. Gastroenterology 2017;152:277–95.
38. Abraham C, Dulai PS, Vermeire S, Sandborn WJ. Lessons learned from trials targeting cytokine pathways in patients with inflammatory bowel diseases. Gastroenterology 2017;152:374–88.
39. Coskun M, Vermeire S, Nielsen OH. Novel targeted therapies for inflammatory bowel disease. Trends Pharmacol Sci 2017;38:127–42.
40. Khanna R, Feagan BG. Emerging therapies for inflammatory bowel diseases. Dig Dis 2016;34 Suppl 1:67–
From the Division of Gastroenterology University of Washington, Seattle, WA (Dr. Tiderington), and the Division of Gastroenterology, Hepatology and Nutrition, The Ohio State University Wexner Medical Center and The Ohio State University Inflammatory Bowel Disease Center, Columbus, OH (Dr. Afzali).
Abstract
- Objective: To provide a practical approach to the management of patients with inflammatory bowel disease (IBD) following tumor necrosis factor (TNF) alpha antagonist failure.
- Methods: Review of the literature.
- Results: TNF alpha antagonists play a central role in the treatment of IBD. Unfortunately, some patients will not respond to therapy with TNF antagonists, and others will lose response during treatment. When patients present with persistent or recurrent symptoms suggesting active IBD while on anti-TNF therapy it can present a dilemma for the clinician. In this paper we review the mechanisms of drug failure, the use of reactive therapeutic drug monitoring to guide clinical decision making, and propose an evidence-based method for managing this common clinical scenario.
- Conclusion: Despite the improved clinical outcomes seen since the introduction of TNF antagonists for the management of IBD, there remains a significant need for additional medical therapies. Fortunately, the armamentarium is expected to expand dramatically over the next decade.
Key words: TNF antagonists; therapeutic drug monitoring; biologic failure; Crohn’s disease treatment; ulcerative colitis treatment.
Ulcerative colitis and Crohn’s disease are the two types of inflammatory bowel disease (IBD), and they are characterized by chronic, immunologically mediated inflammation involving the gastrointestinal tract [1]. Guided by an understanding of the role of tumor necrosis factor (TNF) alpha in the pathogenesis of IBD, TNF antagonists have played a central role in modern treatment algorithms [2]. Unfortunately, roughly one third of patients will not have a clinical response when given induction dosing of the currently available anti-TNF agents, and of those who do respond to treatment, up to one half will lose response to treatment within the first year [3]. When patients present with persistent or recurrent symptoms suggesting active IBD while on anti-TNF therapy it can present a dilemma for the clinician. Once the clinician has confirmed that active IBD is present based on endoscopic, cross-sectional imaging and/or biochemical markers of inflammation, the next step is to identify the cause of the treatment failure, as this guides management. Here we review the body of literature that guides our understanding of treatment failure as well as therapeutic drug monitoring and propose an evidence-based algorithm for managing this common clinical scenario.
Defining Treatment Failure
Patients who receive anti-TNF therapy but demonstrate active IBD should be classified as having either primary nonresponse or secondary loss of response. Primary nonresponse is defined as having either no response, or only partial response, to induction with anti-TNF therapy [4]. Data from pivotal trials and meta-analyses suggest that about one third of patients will not show any clinical response to induction with anti-TNF therapies, with response typically being defined using composite endpoints favoring clinical symptoms and only sometimes incorporating endoscopic findings [5]. An additional one third of patients will have only a partial response, without remission. Patients with ulcerative colitis are at a slightly increased risk of primary nonresponse compared to patients with Crohn’s disease. Though the time frame for defining primary nonresponse is different for each agent because each agent has a slightly different induction schedule, in general the maximal response to therapy is typically seen by week 12, and it is reasonable to use this as a time cutoff [6].
Secondary loss of response is likewise defined as recrudescence of clinically active disease after an initial response. In general, the presence of secondary loss of response should not be invoked until week 12 of therapy. In most pivotal trials, secondary loss of response was seen in roughly half of patients at 1 year. In clinical practice, however, particularly as therapeutic drug monitoring has become more common, the observed rates of secondary loss of response have been lower [6].
Applying these definitions appropriately is important because it dictates the next steps in management. When a patient presents with symptoms suggesting active IBD while on anti-TNF therapy, either during induction when primary nonresponse is possible, or in maintenance when secondary loss of response would be invoked, the first step is to determine if active IBD is the etiology for the presenting symptoms. The initial evaluation should rule out common infectious causes of symptoms mimicking IBD. In particular, Clostridium difficile infection should be ruled out with stool testing. In certain circumstances, ruling out cytomegalovirus (CMV) colitis is important, so an endoscopic evaluation with colonic biopsies should be considered. In the absence of infectious colitis, the presence of active inflammation can often be identified endoscopically, or can be inferred from noninvasive markers with a fair degree of certainty. Fecal calprotectin is an ideal choice for this purpose. In ulcerative colitis it is estimated to have a sensitivity of 0.88 and a specificity of 0.79 for the prediction of endoscopically active disease. The estimated sensitivity for detecting endoscopically active Crohn’s disease is essentially the same (0.87), and the specificity is only slightly lower (0.67). C-reactive protein demonstrates a better specificity (0.92), but has a marginal sensitivity (0.49) [7]. Other etiologies for the patient’s symptoms should also be considered, including medication side effects including use of nonsteroidal anti-inflammatory medications, bile acid malabsorption, small intestinal bacterial overgrowth (SIBO), irritable bowel syndrome (IBS), diverticular disease, ischemic colitis, fibrostenotic strictures, and cancer, depending on comorbidities and the history of present illness.
Once it has been determined that active IBD is the etiology for the patient’s symptoms, the patient should be classified as having either primary nonresponse or secondary loss of response as described above. For the clinician, the next question is how to alter or optimize therapy.
The decision of how to optimize therapy will largely depend on which anti-TNF therapy the patient is currently receiving, and whether they are receiving it as monotherapy or as combination therapy with an immunomodulator. Optimizing therapy will involve either increasing the dose or frequency of administration of the anti-TNF therapy, increasing the dose of azathioprine if indicated, adding an immunomodulator if the patient is on anti-TNF monotherapy, changing to a different anti-TNF agent, or changing to a different class of medication with a different mechanism of action. The recently released American Gastroenterological Association (AGA) guidelines on therapeutic drug monitoring in IBD provide a framework for making these decisions [8]. In general, the clinical choice will be dictated by the etiology of the drug failure.
Types of TNF Antagonist Drug Failure
Our understanding of the causes of biologic treatment failure are evolving but are typically classified as due to mechanistic failure, non-immune-mediated pharmacokinetic failure, or immune-mediated pharmacokinetic failure [9]. Differentiating between these classes of treatment failure requires therapeutic drug monitoring (TDM), which will be discussed in more detail below.
Mechanistic failure is encountered when the underlying biology does not favor a response to a particular therapy. Studies indicate a strong association between particular genetic phenotypes and the probability of a response to induction with anti-TNF agents [10]. This suggests that some individuals have IBD driven by a biochemical inflammatory cascade in which TNF features prominently, while others have alternative mechanistic drivers of inflammation without significantly elevated TNF levels. Mechanistic failure will typically present as primary nonresponse, but can also be seen in patients with secondary loss of response. Mechanistic failure can be elucidated clinically by the use of TDM. In the case of mechanistic failure, active disease is seen in the presence of adequate drug level, without the presence of anti-drug antibodies. The AGA recommends considering switching to a biologic with a different mechanism of action when mechanistic failure is identified [8].
Non-immune-mediated pharmacokinetic failure is encountered when a patient who would otherwise respond to a drug at adequate drug levels experiences suboptimal drug levels because of pharmacokinetic factors. In the case of anti-TNF therapy, this can be conceptualized as either an increased clearance of anti-TNF from the body (eg, in patients with significant hypoalbuminemia or severe colitis), a reduction in the average serum anti-TNF level because of the redistribution of drug in patients with a large body mass index, or inadequate saturation of the total body burden of TNF-alpha in subjects with a high baseline level of inflammation [11]. Non-immune-mediated pharmacokinetic failure can also be identified clinically through TDM. In this case, active disease is seen in the presence of a suboptimal drug level, without the presence of anti-drug antibodies. The AGA recommends considering dose-escalation of the current TNF antagonist when non-immune-mediated pharmacokinetic failure is identified [8], as this can improve clinical response in an estimated 82% of patients [9].
Finally, immune-mediated pharmacokinetic failure is encountered when a patient who would otherwise respond to the current biologic therapy when at adequate drug concentration levels experiences suboptimal drug levels because of increased drug clearance mediated by anti-drug antibodies [9]. Because anti-TNF agents are monoclonal antibodies, they are inherently immunogenic, and it is well established that episodic dosing and lower serum drug concentrations are strong risk factors for the development of anti-drug antibodies [12]. When anti-drug antibodies are present, and are associated with both a decreased serum drug concentration and active inflammatory bowel disease, immune-mediate pharmacokinetic failure can be invoked. When anti-drug antibodies are present, but at a low level, the AGA recommends dose escalation of current TNF antagonist. When anti-drug antibodies are present at a high level, the AGA recommends considering either the addition of an immunomodulator (if not already being used), or changing to a different class of biologic therapy [8]. This recommendation is based in part on data showing that the proportion of patients with sustained anti-drug antibodies during the first year of therapy with an TNF antagonist is likely between 14% and 20% for those on monotherapy, but between 1% and 5% for those on concomitant immunomodulatory therapy [13,14].
Therapeutic Drug Monitoring of Anti-TNF Agents
As described above, TDM, which is the process of testing the patient’s serum for both the concentration of the TNF antagonist and for the presence and concentration of anti-drug antibodies, can help differentiate between mechanistic failure, non-immune-mediated pharmacokinetic failure, and immune-mediated pharmacokinetic failure (Table 1).
Therapeutic drug monitoring can be classified as either proactive or reactive. Proactive TDM is performed during induction or maintenance therapy when the patient does not have signs or symptoms of active disease to suggest a loss of response. Theoretically, this would allow dose modification and optimization, including dose de-escalation in certain circumstances, and could thus provide cost savings with minimal impact on clinical outcomes. The TAXIT trial provides the most robust evaluation of proactive TDM in TNF antagonist therapy. In this study, patients with Crohn’s disease or ulcerative colitis who had a stable clinical response while on maintenance infliximab were first dose optimized proactively to a target trough concentration of 3–7 μg/mL, then randomized to having dose modifications made based on clinical factors alone, defined as reactive monitoring, or dose modifications based on proactive monitoring, performed by checking the drug concentration and antibody levels before each infusion. At 1 year there was no statistically significant difference in the proportion of patients in remission. In addition, some patients in the proactive TDM group were able to have a dose reduction without a subsequent flare of disease, thus providing cost savings [15]. This study suggests that proactive TDM may have a role in drug optimization, particularly with respect to cost-effectiveness, but provides only indirect evidence of a clinical benefit, since all subjects enrolled in the study were proactively dose optimized prior to randomization. This study had a limited follow-up time of 1 year so was not able to assess for longer-term benefits and risks associated with proactive TDM.
More recently, a large, multicenter, retrospective cohort study provided additional evidence that proactive TDM may provide a clinical benefit in addition to cost savings. This study retrospectively evaluated consecutive patients receiving maintenance infliximab for Crohn’s disease between 2006 and 2015, with a median follow-up time of 2.4 years. They were classified as having had either proactive TDM or reactive TDM. Proactive TDM was associated with statistically significant reductions in the risk of treatment failure (hazard ratio [HR] 0.16, 95% confidence interval [CI] 0.09–0.27), the need for surgery (HR 0.30, 95% CI 0.11–0.80), hospitalization (HR 0.16, 95% CI 0.07–0.33), and anti-drug antibody formation (HR 0.25, 95% CI 0.07–0.84) [16].
To date, however, no randomized controlled trials have been published comparing proactive TDM to reactive TDM in treatment-naive patients. Because of the paucity of prospective studies, the AGA currently makes no recommendation regarding the use of proactive TDM in clinical practice. However, the current AGA guidelines do recommend reactive TDM in the setting of secondary loss of response based on the results of one randomized controlled trial (RCT) and several observational studies. The RCT was small (n = 69), and enrolled patients with Crohn’s disease on maintenance therapy with infliximab. Similar to the TAXIT trial, the study did not show a statistically significant difference in rates of clinical remission when subjects were randomized to either empiric dose escalation (to 5 mg/kg every 4 weeks) based on symptoms, or to dose escalations based on the results of reactive TDM. Also similar to the TAXIT trial, it showed an estimated cost savings of about 34% based on local prices in Denmark for reactive TDM over empiric dose escalation [17].
Meanwhile, the observational studies for reactive TDM provided additional support to the clinical benefit of reactive TDM, but also to the underlying hypotheses that drive reactive TDM, namely that subjects with mechanistic failure benefit from a change in drug class, those with non-immune-mediated pharmacokinetic failure benefit from dose escalation, and that those with immune-mediated pharmacokinetic failure may benefit from either dose escalation or a change in mechanism of action, depending on antibody titers. Specifically, on pooled analysis of 2 of these studies, 82% of subjects who were found to have non-immune-mediated pharmacokinetic failure responded to empiric dose escalation, whereas only 8% of subjects who were found to have immune-mediated pharmacokinetic failure with high anti-drug antibody titers responded to dose escalation [9]. Likewise, in a retrospective study involving subjects who were being treated with infliximab and then had reactive TDM performed, when non-immune-mediated pharmacokinetic failure was identified, a clinical response was seen in 86% of subjects who underwent dose escalation, and only 33% among those who were switched to a different anti-TNF (P < 0.016). Conversely, dose escalation resulted in a clinical response only 17% of the time when anti-drug antibodies were detectable, compared to a 92% response rate when the subject was switched to a different anti-TNF (P < 0.004) [18].
Interpreting the Results of Reactive Therapeutic Drug Monitoring
The implementation of reactive TDM involves obtaining a serum drug and antibody level and then interpreting what those results suggest about the mechanism of drug failure, in order to decide on a course of action. The serum drug level should be a trough concentration, so it should be obtained just prior to a timed dose, while on a stable treatment regimen. Exactly what serum drug concentration we should be targeting in reactive therapeutic drug monitoring remains an area of investigation. No RCTs have been published. There is ample observational, cross-sectional data from cohorts of patients on maintenance therapy, though heterogeneity in study design and study populations, as well as use of different assays, limit interpretation of the data. In a secondary analysis of data from 6 observational studies of patients on infliximab maintenance therapy, there was a highly statistically significant concentration-dependent trend in rates of clinical remission depending on the measured infliximab trough concentration, with 96% of those with infliximab > 7 μg/mL in remission, 92% of those with infliximab > 5 μg/mL in remission, and 75% of those with infliximab > 1 μg/mL in remission. Likewise, data from 4 studies of patients receiving adalimumab showed a statistically significant concentration-dependent trend in clinical remission, with 90% of those with adalimumab trough concentrations > 7.5 μg/mL being in clinical remission, compared with only 83% of those with concentrations > 5 μg/mL. Similarly, data from 9 studies suggested that a certolizumab trough concentration > 20 μg/mL was associated with a 75% probability of being in clinical remission, compared to a 60% probability when the trough concentration was > 10 μg/mL [9]. Based on these analyses, the AGA suggests target trough concentrations for reactive therapeutic drug monitoring of anti-TNF agents of ≥ 5 μg/mL for infliximab, ≥ 7.5 μg/mL for adalimumamb, and ≥ 20 μg/mL for certolizumab. They did not suggest a target trough concentration for golimumab because of insufficient evidence [8].
When interpreting TDM test results, it is important to know if the test you have used is drug-sensitive or drug-tolerant (Table 2). Drug-sensitive tests will be less likely to reveal the presence of anti-drug antibodies when the drug level is above a certain threshold. A post-hoc analysis of the TAXIT trial recently suggested that subjects who have antibodies detected on a drug-tolerant test which were not detected on a drug-sensitive test are more likely to respond to higher doses of infliximab [19]. It follows that there should be a threshold anti-drug antibody titer below which someone who has immune-mediated pharmacokinetic failure will still respond to TNF antagonist dose escalation, but above which they will fail to respond to dose escalation. To be sure, our understanding of the clinical implications of a drug-tolerant test demonstrating an adequate drug level while also detectable anti-drug antibodies is evolving. Complicating the issue further is the fact that anti-drug antibody concentrations cannot be compared between assays because of assay-specific characteristics. As such, though the presence of low antibody titers and high antibody titers seems to be clinically important, recommendations cannot yet be made on how to interpret specific thresholds. Furthermore, development of transient versus sustained antibodies requires further clinical investigation to determine impact and treatment algorithms.
Optimizing Therapy
Once you have determined the most likely cause of drug failure, the next step is to make a change in medical therapy.
When switching within class (to another anti-TNF agent), the choice of which agent to use next will largely depend on patient preference (route of administration, infusion versus injection), insurance, and costs of treatment. When making the decision to switch within class, it should be kept in mind that the probability of achieving remission is modestly reduced compared to the probability seen in anti-TNF-naive patients [20], and even more so when the patient is switching to their third anti-TNF agent [21]. Thus, for the patient who has already previously switched from one TNF antagonist to a second TNF antagonist, it may be better to switch to a different class of biologic rather than attempting to capture a clinical remission with a third TNF antagonist.
When adding an immunomodulator (azathioprine or methotrexate), the expectation is that the therapy will increase the serum concentration of the anti-TNF agent [14] and/or reduce the ongoing risk of anti-drug antibody formation [22]. There could also be a direct treatment effect on the bowel disease by the immunomodulator.
When switching to an alternate mechanism of action, the currently FDA-approved options include the biologic agents vedolizumab (for both moderate-to-severe ulcerative colitis and moderate-to-severe Crohn’s disease) and ustekinumab (for moderate-to-severe Crohn’s disease), as well as the recently FDA-approved oral, small-molecule JAK1 and JAK3 inhibitor tofacitinib (for moderate-to-severe ulcerative colitis). Prospective comparative effectiveness studies for these agents are lacking and are unlikely to be performed in part due to the cost and time required to accomplish these studies. A recent post-hoc analysis of clinical trials data suggests that there are no significant differences in the rates of clinical response, clinical remission, or in adverse outcomes to vedolizumab or ustekinumab when they are used in patients who have failed anti-TNF therapy [23]. Thus, one cannot be recommended over the other, and the decision of which to use is usually guided by patient preference and insurance coverage.
Meanwhile, the role of tofacitinib in the treatment algorithm of patients who have failed anti-TNF therapy remains unclear. The phase III clinical trials OCTAVE 1, OCTAVE 2, and OCTAVE Sustain showed efficacy for both the induction and maintenance of remission in patients with moderate-to-severe ulcerative colitis who had previously failed anti-TNF agents. However, there remain concerns about the safety profile of tofacitinib compared to vedolizumab and ustekinumab, particularly regarding herpes zoster infection, dyslipidemia, and adverse cardiovascular events. Notable findings from the tofacitinib induction trials include robust rates of clinical remission (18.5% vs 8.2% for placebo in Octave 1, and 16.6% vs 3.6% in Octave 2, P < 0.001 for both comparisons) and mucosal healing (31.3% vs 15.6% for placebo in Octave 1, and 28.4% and 11.6% in Octave 2, P < 0.001 for both comparisons) after 8 weeks of induction therapy [24]. These results suggest that tofacitinib, or other JAK inhibitors that become approved in the future, may be excellent oral agents for the induction of remission in moderate-to-severe ulcerative colitis, and may demonstrate a better side effect profile than steroids. Whether cost factors (compared to steroid therapy) will limit the role of JAK-inhibitor therapy in induction, and whether safety concerns will limit their use in maintenance therapy, remains to be seen.
Off-Label Rescue Therapy and Surgery
Though the armamentarium of IBD therapies has expanded greatly over the past 2 decades, and will continue to do so for the foreseeable future, there are still a limited selection of therapies available to patients. Patients who have failed anti-TNF therapy, and subsequently fail vedolizumab and/or ustekinumab, have limited options. These options include clinical trials, off-label small molecule rescue therapy, and surgery. Patients who are felt to require any of these options should be referred to a tertiary center for evaluation by a gastroenterologist specializing in the treatment of IBD and/or a colorectal surgeon specializing in the surgical management of IBD.
Tacrolimus
Tacrolimus, a macrolide calcineurin inhibitor, has been studied as a small molecule therapy for IBD, though not in randomized controlled trials. There is biological plausibility for its use as a disease modifying agent. Mucosal T cells in subjects with active Crohn’s disease have been found to express increased levels of mRNA encoding IL-2, and tacrolimus acts primarily by reducing IL-2 production [25]. The largest observational cohort evaluating the use of tacrolimus, published by Thin et al, included patients with both ulcerative colitis (n = 24) and Crohn’s disease (n = 11) who had moderate to severely active IBD. All patients had failed dose-optimized thiopurine therapy, 89% had primary nonresponse or secondary loss of response to at least one anti-TNF agent, and 74% were either steroid-refractory or steroid-dependent at the time tacrolimus was started. With close monitoring, they targeted a tacrolimus trough of 8–12 ng/mL. At 30 days, 66% had a clinical response, and 40% were in clinical remission. At 90 days, 60% had a clinical response, and 37% were in clinical remission. At 1 year, 31% had a clinical response, and 23% were in clinical remission. Of those in clinical remission at 1 year, 88% were either off of steroids or on less than 5 mg of prednisone per day. Renal impairment was seen in 25% of patients, including severe renal impairment in 11%, requiring drug cessation. Infectious complications were seen in 9% of patients. Headaches, tremor, and pancreatitis were also observed, though less commonly. The majority of patients ultimately had a surgical intervention, particularly if they were steroid-refractory at baseline, but the time to surgery was delayed in those who achieved a response or remission in the first 90 days of tacrolimus therapy. The authors suggested that while tacrolimus may lack clear long-term benefit in patients with medically refractory IBD, a therapeutic trial should be considered in select patients with the goal of medical and nutritional optimization before surgical intervention [26].
Cyclosporine
Cyclosporine, which also exerts its effect by inhibiting IL-2 production, has an established role in the management of severe ulcerative colitis. Data from randomized, placebo-controlled trials, along with numerous open label observational studies, have shown that intravenous cyclosporine can induce remission and potentially obviate the need for urgent/emergent colectomy in steroid-refractory patients who are hospitalized with severe ulcerative colitis [27,28]. Its use in maintenance therapy remains controversial, however. Older observational data suggest that even among those who have an initial clinical response to cyclosporine induction, 33% will undergo colectomy by 1 year, and 88% will undergo colectomy by 7 years [27). Though the concomitant administration of a thiopurine may delay the need for colectomy [29,30], cyclosporine seems to be, at best, a temporizing therapy for patients with severe ulcerative colitis. Studies on the use of cyclosporine for the induction of remission in Crohn’s disease have been less robust, as have studies on the use of cyclosporine for the maintenance of remission in Crohn’s disease [31]. Dose-dependent toxicity also remains a concern, particularly when being considered as maintenance therapy. Though some observational data suggest that the absolute risks of serious side effects from maintenance cyclosporine are small, cyclosporine is still generally avoided as a maintenance therapy [30].
Mycophenolate Mofetil
Mycophenolate mofetil (MMF), which inhibits both B and T cell proliferation by inhibiting de novo purine synthesis, has been studied in both Crohn’s disease and ulcerative colitis. Studies have been small, observational, and heterogeneous. On the whole, they suggest that MMF does have some efficacy, but it is not necessarily more effective than azathioprine and may have a slightly increased risk of side effects [32]. Given that the side effects of MMF include diarrhea, and an IBD-like enterocolitis (MMF-induced colitis) when given to subjects without an established diagnosis of IBD, it is likely best to avoid using the drug in patients with IBD [33].
Surgery
Finally, when medical management has failed, or when fibrostenotic and/or penetrating complications of inflammatory bowel disease are present, surgery should be considered. Surgery can provide a cure in patients with ulcerative colitis, and can induce remission in patients with Crohn’s disease. Managing IBD medications around the time of surgery is always challenging. Multiple large, retrospective cohort studies have suggested that the risk for postoperative infectious complications, anastomotic leaks, and thrombotic complications do not differ between those who receive anti-TNF therapy within several months of surgery and those who do not. Nevertheless, some surgeons may prefer to time elective surgery halfway between doses of anti-TNF therapy. Additionally, there is some data to suggest that patients who are on both thiopurines and anti-TNF agents have an increased risk of postoperative complications compared to those who are on anti-TNF agents alone [34].
After a surgical evaluation, a plan of action should be formulated in a multidisciplinary fashion to determine how medical management will proceed. For those with an established diagnosis of ulcerative colitis, medical therapy can often be stopped postoperatively and the patient can be monitored prospectively for pouch complications including possible new-onset Crohn’s disease. For those who undergo surgery for the management of Crohn’s disease, though a resection completed with negative margins does induce remission, nearly 90% can be expected to have histologic, endoscopic, or clinical recurrence by 1 year. A randomized controlled trial showed that postoperative anti-TNF therapy can reduce this risk to 9% [35]. Unfortunately, a subsequently conducted large, multicenter, randomized controlled trial comparing postoperative infliximab to placebo was terminated early because of a lack of a statistically significant difference in clinical recurrence between the 2 groups at week 74. However, this lack of demonstrated efficacy may have been obscured by the relatively mild phenotype of the enrolled participants, who had a median CDAI score of 105.5 at baseline [36]. Based on available data, the AGA does conditionally recommend postoperative anti-TNF and/or thiopurine therapy for those patients with Crohn’s disease who are in a surgically induced remission [37]. The patients who are most likely to benefit from postoperative medical therapy are those who have the highest risk of recurrence, namely those who were young at the time of diagnosis, had a short disease duration prior to surgery, have multiple sites of disease, and who use tobacco products [34].
Emerging and Future Options
Despite the improved clinical outcomes seen since the introduction of TNF antagonists for the management of IBD, there remains a significant need for additional medical therapies. Fortunately, the armamentarium is expected to expand dramatically over the next decade.
Based on our improved, and evolving understanding of the pathogenesis of IBD, several new biochemical targets have emerged, offering novel ways to modulate the cytokine cascade which drives IBD [38]. Well over a dozen phase II and phase III trials for IBD therapeutic agents are ongoing, including biologic agents targeting interleukin-23, β7-Integrin, and MAdCAM-1, as well as small molecule agents targeting the JAK/STAT pathway and the sphingosine-1-phosphate receptor modulators [39]. As new agents are approved, it may be possible to develop a more patient-centered approach to care by targeting therapies to the particular pathogenesis of each patient’s disease. Nevertheless, integrating these therapies into practice algorithms will remain a challenge in the absence of meaningful comparative effectiveness trials [40].
Conclusion
When evaluating a patient who seems to have failed anti-TNF therapy for IBD, the first step is to confirm that active inflammatory disease is present. This process includes ruling out other potential causes of the patient’s symptoms, including infectious colitis, and ideally includes obtaining objective evidence of inflammation, whether through non-invasive biomarkers, an endoscopic evaluation and/or cross-sectional imaging. Once active IBD is confirmed, reactive therapeutic drug monitoring can help elucidate the likely mechanism of drug failure, which in turn can guide medical decision making.
Corresponding author: Anita Afzali MD, MPH, The Ohio State University Wexner Medical Center, 395 West 12th Ave, Room 280, Columbus, OH 43210, [email protected].
Financial disclosures: Dr. Afzali has served as a speaker/consultant for Abbvie, UCB, Takeda, Pfizer, Janssen; on the advisory board of Abbvie, UCB; received grant support from UCB; and is a board member of IBD Horizons.
From the Division of Gastroenterology University of Washington, Seattle, WA (Dr. Tiderington), and the Division of Gastroenterology, Hepatology and Nutrition, The Ohio State University Wexner Medical Center and The Ohio State University Inflammatory Bowel Disease Center, Columbus, OH (Dr. Afzali).
Abstract
- Objective: To provide a practical approach to the management of patients with inflammatory bowel disease (IBD) following tumor necrosis factor (TNF) alpha antagonist failure.
- Methods: Review of the literature.
- Results: TNF alpha antagonists play a central role in the treatment of IBD. Unfortunately, some patients will not respond to therapy with TNF antagonists, and others will lose response during treatment. When patients present with persistent or recurrent symptoms suggesting active IBD while on anti-TNF therapy it can present a dilemma for the clinician. In this paper we review the mechanisms of drug failure, the use of reactive therapeutic drug monitoring to guide clinical decision making, and propose an evidence-based method for managing this common clinical scenario.
- Conclusion: Despite the improved clinical outcomes seen since the introduction of TNF antagonists for the management of IBD, there remains a significant need for additional medical therapies. Fortunately, the armamentarium is expected to expand dramatically over the next decade.
Key words: TNF antagonists; therapeutic drug monitoring; biologic failure; Crohn’s disease treatment; ulcerative colitis treatment.
Ulcerative colitis and Crohn’s disease are the two types of inflammatory bowel disease (IBD), and they are characterized by chronic, immunologically mediated inflammation involving the gastrointestinal tract [1]. Guided by an understanding of the role of tumor necrosis factor (TNF) alpha in the pathogenesis of IBD, TNF antagonists have played a central role in modern treatment algorithms [2]. Unfortunately, roughly one third of patients will not have a clinical response when given induction dosing of the currently available anti-TNF agents, and of those who do respond to treatment, up to one half will lose response to treatment within the first year [3]. When patients present with persistent or recurrent symptoms suggesting active IBD while on anti-TNF therapy it can present a dilemma for the clinician. Once the clinician has confirmed that active IBD is present based on endoscopic, cross-sectional imaging and/or biochemical markers of inflammation, the next step is to identify the cause of the treatment failure, as this guides management. Here we review the body of literature that guides our understanding of treatment failure as well as therapeutic drug monitoring and propose an evidence-based algorithm for managing this common clinical scenario.
Defining Treatment Failure
Patients who receive anti-TNF therapy but demonstrate active IBD should be classified as having either primary nonresponse or secondary loss of response. Primary nonresponse is defined as having either no response, or only partial response, to induction with anti-TNF therapy [4]. Data from pivotal trials and meta-analyses suggest that about one third of patients will not show any clinical response to induction with anti-TNF therapies, with response typically being defined using composite endpoints favoring clinical symptoms and only sometimes incorporating endoscopic findings [5]. An additional one third of patients will have only a partial response, without remission. Patients with ulcerative colitis are at a slightly increased risk of primary nonresponse compared to patients with Crohn’s disease. Though the time frame for defining primary nonresponse is different for each agent because each agent has a slightly different induction schedule, in general the maximal response to therapy is typically seen by week 12, and it is reasonable to use this as a time cutoff [6].
Secondary loss of response is likewise defined as recrudescence of clinically active disease after an initial response. In general, the presence of secondary loss of response should not be invoked until week 12 of therapy. In most pivotal trials, secondary loss of response was seen in roughly half of patients at 1 year. In clinical practice, however, particularly as therapeutic drug monitoring has become more common, the observed rates of secondary loss of response have been lower [6].
Applying these definitions appropriately is important because it dictates the next steps in management. When a patient presents with symptoms suggesting active IBD while on anti-TNF therapy, either during induction when primary nonresponse is possible, or in maintenance when secondary loss of response would be invoked, the first step is to determine if active IBD is the etiology for the presenting symptoms. The initial evaluation should rule out common infectious causes of symptoms mimicking IBD. In particular, Clostridium difficile infection should be ruled out with stool testing. In certain circumstances, ruling out cytomegalovirus (CMV) colitis is important, so an endoscopic evaluation with colonic biopsies should be considered. In the absence of infectious colitis, the presence of active inflammation can often be identified endoscopically, or can be inferred from noninvasive markers with a fair degree of certainty. Fecal calprotectin is an ideal choice for this purpose. In ulcerative colitis it is estimated to have a sensitivity of 0.88 and a specificity of 0.79 for the prediction of endoscopically active disease. The estimated sensitivity for detecting endoscopically active Crohn’s disease is essentially the same (0.87), and the specificity is only slightly lower (0.67). C-reactive protein demonstrates a better specificity (0.92), but has a marginal sensitivity (0.49) [7]. Other etiologies for the patient’s symptoms should also be considered, including medication side effects including use of nonsteroidal anti-inflammatory medications, bile acid malabsorption, small intestinal bacterial overgrowth (SIBO), irritable bowel syndrome (IBS), diverticular disease, ischemic colitis, fibrostenotic strictures, and cancer, depending on comorbidities and the history of present illness.
Once it has been determined that active IBD is the etiology for the patient’s symptoms, the patient should be classified as having either primary nonresponse or secondary loss of response as described above. For the clinician, the next question is how to alter or optimize therapy.
The decision of how to optimize therapy will largely depend on which anti-TNF therapy the patient is currently receiving, and whether they are receiving it as monotherapy or as combination therapy with an immunomodulator. Optimizing therapy will involve either increasing the dose or frequency of administration of the anti-TNF therapy, increasing the dose of azathioprine if indicated, adding an immunomodulator if the patient is on anti-TNF monotherapy, changing to a different anti-TNF agent, or changing to a different class of medication with a different mechanism of action. The recently released American Gastroenterological Association (AGA) guidelines on therapeutic drug monitoring in IBD provide a framework for making these decisions [8]. In general, the clinical choice will be dictated by the etiology of the drug failure.
Types of TNF Antagonist Drug Failure
Our understanding of the causes of biologic treatment failure are evolving but are typically classified as due to mechanistic failure, non-immune-mediated pharmacokinetic failure, or immune-mediated pharmacokinetic failure [9]. Differentiating between these classes of treatment failure requires therapeutic drug monitoring (TDM), which will be discussed in more detail below.
Mechanistic failure is encountered when the underlying biology does not favor a response to a particular therapy. Studies indicate a strong association between particular genetic phenotypes and the probability of a response to induction with anti-TNF agents [10]. This suggests that some individuals have IBD driven by a biochemical inflammatory cascade in which TNF features prominently, while others have alternative mechanistic drivers of inflammation without significantly elevated TNF levels. Mechanistic failure will typically present as primary nonresponse, but can also be seen in patients with secondary loss of response. Mechanistic failure can be elucidated clinically by the use of TDM. In the case of mechanistic failure, active disease is seen in the presence of adequate drug level, without the presence of anti-drug antibodies. The AGA recommends considering switching to a biologic with a different mechanism of action when mechanistic failure is identified [8].
Non-immune-mediated pharmacokinetic failure is encountered when a patient who would otherwise respond to a drug at adequate drug levels experiences suboptimal drug levels because of pharmacokinetic factors. In the case of anti-TNF therapy, this can be conceptualized as either an increased clearance of anti-TNF from the body (eg, in patients with significant hypoalbuminemia or severe colitis), a reduction in the average serum anti-TNF level because of the redistribution of drug in patients with a large body mass index, or inadequate saturation of the total body burden of TNF-alpha in subjects with a high baseline level of inflammation [11]. Non-immune-mediated pharmacokinetic failure can also be identified clinically through TDM. In this case, active disease is seen in the presence of a suboptimal drug level, without the presence of anti-drug antibodies. The AGA recommends considering dose-escalation of the current TNF antagonist when non-immune-mediated pharmacokinetic failure is identified [8], as this can improve clinical response in an estimated 82% of patients [9].
Finally, immune-mediated pharmacokinetic failure is encountered when a patient who would otherwise respond to the current biologic therapy when at adequate drug concentration levels experiences suboptimal drug levels because of increased drug clearance mediated by anti-drug antibodies [9]. Because anti-TNF agents are monoclonal antibodies, they are inherently immunogenic, and it is well established that episodic dosing and lower serum drug concentrations are strong risk factors for the development of anti-drug antibodies [12]. When anti-drug antibodies are present, and are associated with both a decreased serum drug concentration and active inflammatory bowel disease, immune-mediate pharmacokinetic failure can be invoked. When anti-drug antibodies are present, but at a low level, the AGA recommends dose escalation of current TNF antagonist. When anti-drug antibodies are present at a high level, the AGA recommends considering either the addition of an immunomodulator (if not already being used), or changing to a different class of biologic therapy [8]. This recommendation is based in part on data showing that the proportion of patients with sustained anti-drug antibodies during the first year of therapy with an TNF antagonist is likely between 14% and 20% for those on monotherapy, but between 1% and 5% for those on concomitant immunomodulatory therapy [13,14].
Therapeutic Drug Monitoring of Anti-TNF Agents
As described above, TDM, which is the process of testing the patient’s serum for both the concentration of the TNF antagonist and for the presence and concentration of anti-drug antibodies, can help differentiate between mechanistic failure, non-immune-mediated pharmacokinetic failure, and immune-mediated pharmacokinetic failure (Table 1).
Therapeutic drug monitoring can be classified as either proactive or reactive. Proactive TDM is performed during induction or maintenance therapy when the patient does not have signs or symptoms of active disease to suggest a loss of response. Theoretically, this would allow dose modification and optimization, including dose de-escalation in certain circumstances, and could thus provide cost savings with minimal impact on clinical outcomes. The TAXIT trial provides the most robust evaluation of proactive TDM in TNF antagonist therapy. In this study, patients with Crohn’s disease or ulcerative colitis who had a stable clinical response while on maintenance infliximab were first dose optimized proactively to a target trough concentration of 3–7 μg/mL, then randomized to having dose modifications made based on clinical factors alone, defined as reactive monitoring, or dose modifications based on proactive monitoring, performed by checking the drug concentration and antibody levels before each infusion. At 1 year there was no statistically significant difference in the proportion of patients in remission. In addition, some patients in the proactive TDM group were able to have a dose reduction without a subsequent flare of disease, thus providing cost savings [15]. This study suggests that proactive TDM may have a role in drug optimization, particularly with respect to cost-effectiveness, but provides only indirect evidence of a clinical benefit, since all subjects enrolled in the study were proactively dose optimized prior to randomization. This study had a limited follow-up time of 1 year so was not able to assess for longer-term benefits and risks associated with proactive TDM.
More recently, a large, multicenter, retrospective cohort study provided additional evidence that proactive TDM may provide a clinical benefit in addition to cost savings. This study retrospectively evaluated consecutive patients receiving maintenance infliximab for Crohn’s disease between 2006 and 2015, with a median follow-up time of 2.4 years. They were classified as having had either proactive TDM or reactive TDM. Proactive TDM was associated with statistically significant reductions in the risk of treatment failure (hazard ratio [HR] 0.16, 95% confidence interval [CI] 0.09–0.27), the need for surgery (HR 0.30, 95% CI 0.11–0.80), hospitalization (HR 0.16, 95% CI 0.07–0.33), and anti-drug antibody formation (HR 0.25, 95% CI 0.07–0.84) [16].
To date, however, no randomized controlled trials have been published comparing proactive TDM to reactive TDM in treatment-naive patients. Because of the paucity of prospective studies, the AGA currently makes no recommendation regarding the use of proactive TDM in clinical practice. However, the current AGA guidelines do recommend reactive TDM in the setting of secondary loss of response based on the results of one randomized controlled trial (RCT) and several observational studies. The RCT was small (n = 69), and enrolled patients with Crohn’s disease on maintenance therapy with infliximab. Similar to the TAXIT trial, the study did not show a statistically significant difference in rates of clinical remission when subjects were randomized to either empiric dose escalation (to 5 mg/kg every 4 weeks) based on symptoms, or to dose escalations based on the results of reactive TDM. Also similar to the TAXIT trial, it showed an estimated cost savings of about 34% based on local prices in Denmark for reactive TDM over empiric dose escalation [17].
Meanwhile, the observational studies for reactive TDM provided additional support to the clinical benefit of reactive TDM, but also to the underlying hypotheses that drive reactive TDM, namely that subjects with mechanistic failure benefit from a change in drug class, those with non-immune-mediated pharmacokinetic failure benefit from dose escalation, and that those with immune-mediated pharmacokinetic failure may benefit from either dose escalation or a change in mechanism of action, depending on antibody titers. Specifically, on pooled analysis of 2 of these studies, 82% of subjects who were found to have non-immune-mediated pharmacokinetic failure responded to empiric dose escalation, whereas only 8% of subjects who were found to have immune-mediated pharmacokinetic failure with high anti-drug antibody titers responded to dose escalation [9]. Likewise, in a retrospective study involving subjects who were being treated with infliximab and then had reactive TDM performed, when non-immune-mediated pharmacokinetic failure was identified, a clinical response was seen in 86% of subjects who underwent dose escalation, and only 33% among those who were switched to a different anti-TNF (P < 0.016). Conversely, dose escalation resulted in a clinical response only 17% of the time when anti-drug antibodies were detectable, compared to a 92% response rate when the subject was switched to a different anti-TNF (P < 0.004) [18].
Interpreting the Results of Reactive Therapeutic Drug Monitoring
The implementation of reactive TDM involves obtaining a serum drug and antibody level and then interpreting what those results suggest about the mechanism of drug failure, in order to decide on a course of action. The serum drug level should be a trough concentration, so it should be obtained just prior to a timed dose, while on a stable treatment regimen. Exactly what serum drug concentration we should be targeting in reactive therapeutic drug monitoring remains an area of investigation. No RCTs have been published. There is ample observational, cross-sectional data from cohorts of patients on maintenance therapy, though heterogeneity in study design and study populations, as well as use of different assays, limit interpretation of the data. In a secondary analysis of data from 6 observational studies of patients on infliximab maintenance therapy, there was a highly statistically significant concentration-dependent trend in rates of clinical remission depending on the measured infliximab trough concentration, with 96% of those with infliximab > 7 μg/mL in remission, 92% of those with infliximab > 5 μg/mL in remission, and 75% of those with infliximab > 1 μg/mL in remission. Likewise, data from 4 studies of patients receiving adalimumab showed a statistically significant concentration-dependent trend in clinical remission, with 90% of those with adalimumab trough concentrations > 7.5 μg/mL being in clinical remission, compared with only 83% of those with concentrations > 5 μg/mL. Similarly, data from 9 studies suggested that a certolizumab trough concentration > 20 μg/mL was associated with a 75% probability of being in clinical remission, compared to a 60% probability when the trough concentration was > 10 μg/mL [9]. Based on these analyses, the AGA suggests target trough concentrations for reactive therapeutic drug monitoring of anti-TNF agents of ≥ 5 μg/mL for infliximab, ≥ 7.5 μg/mL for adalimumamb, and ≥ 20 μg/mL for certolizumab. They did not suggest a target trough concentration for golimumab because of insufficient evidence [8].
When interpreting TDM test results, it is important to know if the test you have used is drug-sensitive or drug-tolerant (Table 2). Drug-sensitive tests will be less likely to reveal the presence of anti-drug antibodies when the drug level is above a certain threshold. A post-hoc analysis of the TAXIT trial recently suggested that subjects who have antibodies detected on a drug-tolerant test which were not detected on a drug-sensitive test are more likely to respond to higher doses of infliximab [19]. It follows that there should be a threshold anti-drug antibody titer below which someone who has immune-mediated pharmacokinetic failure will still respond to TNF antagonist dose escalation, but above which they will fail to respond to dose escalation. To be sure, our understanding of the clinical implications of a drug-tolerant test demonstrating an adequate drug level while also detectable anti-drug antibodies is evolving. Complicating the issue further is the fact that anti-drug antibody concentrations cannot be compared between assays because of assay-specific characteristics. As such, though the presence of low antibody titers and high antibody titers seems to be clinically important, recommendations cannot yet be made on how to interpret specific thresholds. Furthermore, development of transient versus sustained antibodies requires further clinical investigation to determine impact and treatment algorithms.
Optimizing Therapy
Once you have determined the most likely cause of drug failure, the next step is to make a change in medical therapy.
When switching within class (to another anti-TNF agent), the choice of which agent to use next will largely depend on patient preference (route of administration, infusion versus injection), insurance, and costs of treatment. When making the decision to switch within class, it should be kept in mind that the probability of achieving remission is modestly reduced compared to the probability seen in anti-TNF-naive patients [20], and even more so when the patient is switching to their third anti-TNF agent [21]. Thus, for the patient who has already previously switched from one TNF antagonist to a second TNF antagonist, it may be better to switch to a different class of biologic rather than attempting to capture a clinical remission with a third TNF antagonist.
When adding an immunomodulator (azathioprine or methotrexate), the expectation is that the therapy will increase the serum concentration of the anti-TNF agent [14] and/or reduce the ongoing risk of anti-drug antibody formation [22]. There could also be a direct treatment effect on the bowel disease by the immunomodulator.
When switching to an alternate mechanism of action, the currently FDA-approved options include the biologic agents vedolizumab (for both moderate-to-severe ulcerative colitis and moderate-to-severe Crohn’s disease) and ustekinumab (for moderate-to-severe Crohn’s disease), as well as the recently FDA-approved oral, small-molecule JAK1 and JAK3 inhibitor tofacitinib (for moderate-to-severe ulcerative colitis). Prospective comparative effectiveness studies for these agents are lacking and are unlikely to be performed in part due to the cost and time required to accomplish these studies. A recent post-hoc analysis of clinical trials data suggests that there are no significant differences in the rates of clinical response, clinical remission, or in adverse outcomes to vedolizumab or ustekinumab when they are used in patients who have failed anti-TNF therapy [23]. Thus, one cannot be recommended over the other, and the decision of which to use is usually guided by patient preference and insurance coverage.
Meanwhile, the role of tofacitinib in the treatment algorithm of patients who have failed anti-TNF therapy remains unclear. The phase III clinical trials OCTAVE 1, OCTAVE 2, and OCTAVE Sustain showed efficacy for both the induction and maintenance of remission in patients with moderate-to-severe ulcerative colitis who had previously failed anti-TNF agents. However, there remain concerns about the safety profile of tofacitinib compared to vedolizumab and ustekinumab, particularly regarding herpes zoster infection, dyslipidemia, and adverse cardiovascular events. Notable findings from the tofacitinib induction trials include robust rates of clinical remission (18.5% vs 8.2% for placebo in Octave 1, and 16.6% vs 3.6% in Octave 2, P < 0.001 for both comparisons) and mucosal healing (31.3% vs 15.6% for placebo in Octave 1, and 28.4% and 11.6% in Octave 2, P < 0.001 for both comparisons) after 8 weeks of induction therapy [24]. These results suggest that tofacitinib, or other JAK inhibitors that become approved in the future, may be excellent oral agents for the induction of remission in moderate-to-severe ulcerative colitis, and may demonstrate a better side effect profile than steroids. Whether cost factors (compared to steroid therapy) will limit the role of JAK-inhibitor therapy in induction, and whether safety concerns will limit their use in maintenance therapy, remains to be seen.
Off-Label Rescue Therapy and Surgery
Though the armamentarium of IBD therapies has expanded greatly over the past 2 decades, and will continue to do so for the foreseeable future, there are still a limited selection of therapies available to patients. Patients who have failed anti-TNF therapy, and subsequently fail vedolizumab and/or ustekinumab, have limited options. These options include clinical trials, off-label small molecule rescue therapy, and surgery. Patients who are felt to require any of these options should be referred to a tertiary center for evaluation by a gastroenterologist specializing in the treatment of IBD and/or a colorectal surgeon specializing in the surgical management of IBD.
Tacrolimus
Tacrolimus, a macrolide calcineurin inhibitor, has been studied as a small molecule therapy for IBD, though not in randomized controlled trials. There is biological plausibility for its use as a disease modifying agent. Mucosal T cells in subjects with active Crohn’s disease have been found to express increased levels of mRNA encoding IL-2, and tacrolimus acts primarily by reducing IL-2 production [25]. The largest observational cohort evaluating the use of tacrolimus, published by Thin et al, included patients with both ulcerative colitis (n = 24) and Crohn’s disease (n = 11) who had moderate to severely active IBD. All patients had failed dose-optimized thiopurine therapy, 89% had primary nonresponse or secondary loss of response to at least one anti-TNF agent, and 74% were either steroid-refractory or steroid-dependent at the time tacrolimus was started. With close monitoring, they targeted a tacrolimus trough of 8–12 ng/mL. At 30 days, 66% had a clinical response, and 40% were in clinical remission. At 90 days, 60% had a clinical response, and 37% were in clinical remission. At 1 year, 31% had a clinical response, and 23% were in clinical remission. Of those in clinical remission at 1 year, 88% were either off of steroids or on less than 5 mg of prednisone per day. Renal impairment was seen in 25% of patients, including severe renal impairment in 11%, requiring drug cessation. Infectious complications were seen in 9% of patients. Headaches, tremor, and pancreatitis were also observed, though less commonly. The majority of patients ultimately had a surgical intervention, particularly if they were steroid-refractory at baseline, but the time to surgery was delayed in those who achieved a response or remission in the first 90 days of tacrolimus therapy. The authors suggested that while tacrolimus may lack clear long-term benefit in patients with medically refractory IBD, a therapeutic trial should be considered in select patients with the goal of medical and nutritional optimization before surgical intervention [26].
Cyclosporine
Cyclosporine, which also exerts its effect by inhibiting IL-2 production, has an established role in the management of severe ulcerative colitis. Data from randomized, placebo-controlled trials, along with numerous open label observational studies, have shown that intravenous cyclosporine can induce remission and potentially obviate the need for urgent/emergent colectomy in steroid-refractory patients who are hospitalized with severe ulcerative colitis [27,28]. Its use in maintenance therapy remains controversial, however. Older observational data suggest that even among those who have an initial clinical response to cyclosporine induction, 33% will undergo colectomy by 1 year, and 88% will undergo colectomy by 7 years [27). Though the concomitant administration of a thiopurine may delay the need for colectomy [29,30], cyclosporine seems to be, at best, a temporizing therapy for patients with severe ulcerative colitis. Studies on the use of cyclosporine for the induction of remission in Crohn’s disease have been less robust, as have studies on the use of cyclosporine for the maintenance of remission in Crohn’s disease [31]. Dose-dependent toxicity also remains a concern, particularly when being considered as maintenance therapy. Though some observational data suggest that the absolute risks of serious side effects from maintenance cyclosporine are small, cyclosporine is still generally avoided as a maintenance therapy [30].
Mycophenolate Mofetil
Mycophenolate mofetil (MMF), which inhibits both B and T cell proliferation by inhibiting de novo purine synthesis, has been studied in both Crohn’s disease and ulcerative colitis. Studies have been small, observational, and heterogeneous. On the whole, they suggest that MMF does have some efficacy, but it is not necessarily more effective than azathioprine and may have a slightly increased risk of side effects [32]. Given that the side effects of MMF include diarrhea, and an IBD-like enterocolitis (MMF-induced colitis) when given to subjects without an established diagnosis of IBD, it is likely best to avoid using the drug in patients with IBD [33].
Surgery
Finally, when medical management has failed, or when fibrostenotic and/or penetrating complications of inflammatory bowel disease are present, surgery should be considered. Surgery can provide a cure in patients with ulcerative colitis, and can induce remission in patients with Crohn’s disease. Managing IBD medications around the time of surgery is always challenging. Multiple large, retrospective cohort studies have suggested that the risk for postoperative infectious complications, anastomotic leaks, and thrombotic complications do not differ between those who receive anti-TNF therapy within several months of surgery and those who do not. Nevertheless, some surgeons may prefer to time elective surgery halfway between doses of anti-TNF therapy. Additionally, there is some data to suggest that patients who are on both thiopurines and anti-TNF agents have an increased risk of postoperative complications compared to those who are on anti-TNF agents alone [34].
After a surgical evaluation, a plan of action should be formulated in a multidisciplinary fashion to determine how medical management will proceed. For those with an established diagnosis of ulcerative colitis, medical therapy can often be stopped postoperatively and the patient can be monitored prospectively for pouch complications including possible new-onset Crohn’s disease. For those who undergo surgery for the management of Crohn’s disease, though a resection completed with negative margins does induce remission, nearly 90% can be expected to have histologic, endoscopic, or clinical recurrence by 1 year. A randomized controlled trial showed that postoperative anti-TNF therapy can reduce this risk to 9% [35]. Unfortunately, a subsequently conducted large, multicenter, randomized controlled trial comparing postoperative infliximab to placebo was terminated early because of a lack of a statistically significant difference in clinical recurrence between the 2 groups at week 74. However, this lack of demonstrated efficacy may have been obscured by the relatively mild phenotype of the enrolled participants, who had a median CDAI score of 105.5 at baseline [36]. Based on available data, the AGA does conditionally recommend postoperative anti-TNF and/or thiopurine therapy for those patients with Crohn’s disease who are in a surgically induced remission [37]. The patients who are most likely to benefit from postoperative medical therapy are those who have the highest risk of recurrence, namely those who were young at the time of diagnosis, had a short disease duration prior to surgery, have multiple sites of disease, and who use tobacco products [34].
Emerging and Future Options
Despite the improved clinical outcomes seen since the introduction of TNF antagonists for the management of IBD, there remains a significant need for additional medical therapies. Fortunately, the armamentarium is expected to expand dramatically over the next decade.
Based on our improved, and evolving understanding of the pathogenesis of IBD, several new biochemical targets have emerged, offering novel ways to modulate the cytokine cascade which drives IBD [38]. Well over a dozen phase II and phase III trials for IBD therapeutic agents are ongoing, including biologic agents targeting interleukin-23, β7-Integrin, and MAdCAM-1, as well as small molecule agents targeting the JAK/STAT pathway and the sphingosine-1-phosphate receptor modulators [39]. As new agents are approved, it may be possible to develop a more patient-centered approach to care by targeting therapies to the particular pathogenesis of each patient’s disease. Nevertheless, integrating these therapies into practice algorithms will remain a challenge in the absence of meaningful comparative effectiveness trials [40].
Conclusion
When evaluating a patient who seems to have failed anti-TNF therapy for IBD, the first step is to confirm that active inflammatory disease is present. This process includes ruling out other potential causes of the patient’s symptoms, including infectious colitis, and ideally includes obtaining objective evidence of inflammation, whether through non-invasive biomarkers, an endoscopic evaluation and/or cross-sectional imaging. Once active IBD is confirmed, reactive therapeutic drug monitoring can help elucidate the likely mechanism of drug failure, which in turn can guide medical decision making.
Corresponding author: Anita Afzali MD, MPH, The Ohio State University Wexner Medical Center, 395 West 12th Ave, Room 280, Columbus, OH 43210, [email protected].
Financial disclosures: Dr. Afzali has served as a speaker/consultant for Abbvie, UCB, Takeda, Pfizer, Janssen; on the advisory board of Abbvie, UCB; received grant support from UCB; and is a board member of IBD Horizons.
1. Abraham C, Cho JH. Inflammatory bowel disease. N Engl J Med 2009;361:2066–78.
2. Danese S. New therapies for inflammatory bowel disease: from the bench to the bedside. Gut 2012;61:918–32.
3. Mitrev N, Leong RW. Therapeutic drug monitoring of anti-tumour necrosis factor-α agents in inflammatory bowel disease. Expert Opin Drug Saf 2017;16:303–17.
4. Papamichael K, Gils A, Rutgeerts P, et al. Role for therapeutic drug monitoring during induction therapy with TNF antagonists in IBD: evolution in the definition and management of primary nonresponse. Inflamm Bowel Dis 2015;21:182–97.
5. Levesque BG, Sandborn WJ, Ruel J, et al. Converging goals of treatment of inflammatory bowel disease from clinical trials and practice. Gastroenterology 2015;148:37–51.
6. Allez M, Karmiris K, Louis E, et al. Report of the ECCO pathogenesis workshop on anti-TNF therapy failures in inflammatory bowel diseases: definitions, frequency and pharmacological aspects. J Crohns Colitis 2010;4:355–66.
7. Mosli MH, Zou G, Garg SK, et al. C-Reactive protein, fecal calprotectin, and stool lactoferrin for detection of endoscopic activity in symptomatic inflammatory bowel disease patients: a systematic review and meta-analysis. Am J Gastroenterol 2015;110:802–19.
8. Feuerstein JD, Nguyen GC, Kupfer SS, et al. American Gastroenterological Association Institute guideline on therapeutic drug monitoring in inflammatory bowel disease. Gastroenterology 2017;153:827–34.
9. Vande Casteele N, Herfarth H, Katz J, et al. American Gastroenterological Association Institute technical review on the role of therapeutic drug monitoring in the management of inflammatory bowel diseases. Gastroenterology 2017;153:835–57.
10. López-Hernández R, Valdés M, Campillo JA, et al. Genetic polymorphisms of tumour necrosis factor alpha (TNF-α) promoter gene and response to TNF-α inhibitors in Spanish patients with inflammatory bowel disease. Int J Immunogenet 2014;41:63–8.
11. Ordás I, Mould DR, Feagan BG, Sandborn WJ. Anti-TNF monoclonal antibodies in inflammatory bowel disease: pharmacokinetics-based dosing paradigms. Clin Pharmacol Ther 2012;91:635–46.
12. Hindryckx P, Novak G, Vande Casteele N, et al. Incidence, prevention and management of anti-drug antibodies against therapeutic antibodies in inflammatory bowel disease: a practical overview. Drugs 2017;77:363–77.
13. Colombel JF, Sandborn WJ, Reinisch W, et al. Infliximab, azathioprine, or combination therapy for Crohn’s disease. N Engl J Med 2010;362:1383–95.
14. Lichtenstein GR, Diamond RH, Wagner CL, et al. Clinical trial: benefits and risks of immunomodulators and maintenance infliximab for IBD-subgroup analyses across four randomized trials. Aliment Pharmacol Ther 2009;30:210–26.
15. Vande Casteele N, Ferrante M, Van Assche G, et al. Trough concentrations of infliximab guide dosing for patients with inflammatory bowel disease. Gastroenterology 2015;148:1320–9.
16. Papamichael K, Chachu KA, Vajravelu RK, et al. Improved long-term outcomes of patients with inflammatory bowel disease receiving proactive compared with reactive monitoring of serum concentrations of infliximab. Clin Gastroenterol Hepatol 2017;15:1580–8.
17. Steenholdt C, Brynskov J, Thomsen OØ, et al. Individualised therapy is more cost-effective than dose intensification in patients with Crohn’s disease who lose response to anti-TNF treatment: a randomised, controlled trial. Gut 2014;63:919–27.
18. Afif W, Loftus EV Jr, Faubion WA, et al. Clinical utility of measuring infliximab and human anti-chimeric antibody concentrations in patients with inflammatory bowel disease. Am J Gastroenterol 2010;105:1133–9.
19. Van Stappen T, Vande Casteele N, Van Assche G, et al. Clinical relevance of detecting anti-infliximab antibodies with a drug-tolerant assay: post hoc analysis of the TAXIT trial. Gut 2017.
20. Gisbert JP, Marín AC, McNicholl AG, Chaparro M. Systematic review with meta-analysis: the efficacy of a second anti-TNF in patients with inflammatory bowel disease whose previous anti-TNF treatment has failed. Aliment Pharmacol Ther 2015;41:613–23.
21. Gisbert JP, Chaparro M. Use of a third anti-TNF after failure of two previous anti-TNFs in patients with inflammatory bowel disease: is it worth it? Scand J Gastroenterol 2015;50:379–86.
22. Ordás I, Feagan BG, Sandborn WJ. Therapeutic drug monitoring of tumor necrosis factor antagonists in inflammatory bowel disease. Clin Gastroenterol Hepatol 2012;10:1079–87.
23. Kawalec P, Moćko P. An indirect comparison of ustekinumab and vedolizumab in the therapy of TNF-failure Crohn’s disease patients. J Comp Eff Res 2017;7:101–11.
24. Sandborn WJ, Su C, Panes J, et al. Tofacitinib as induction and maintenance therapy for ulcerative colitis. N Engl J Med 2017;377:1723–36.
25. Yarkoni S, Sagiv Y, Kaminitz A, Askenasy N. Interleukin 2 targeted therapy in inflammatory bowel disease. Gut 2009;58:1705–6.
26. Thin LW, Murray K, Lawrance IC. Oral tacrolimus for the treatment of refractory inflammatory bowel disease in the biologic era. Inflamm Bowel Dis 2013;19:1490–8.
27. Moskovitz DN, Van Assche G, Maenhout B, et al. Incidence of colectomy during long-term follow-up after cyclosporine-induced remission of severe ulcerative colitis. Clin Gastroenterol Hepatol 2006;4:760–5.
28. Arts J, D’Haens G, Zeegers M, et al. Long-term outcome of treatment with intravenous cyclosporin in patients with severe ulcerative colitis. Inflamm Bowel Dis 2004;10:73–8.
29. Cohen RD, Stein R, Hanauer SB. Intravenous cyclosporin in ulcerative colitis: a five-year experience. Am J Gastroenterol 1999;94:1587–92.
30. Cheifetz AS, Stern J, Garud S, et al. Cyclosporine is safe and effective in patients with severe ulcerative colitis. J Clin Gastroenterol 2011;45:107–12.
31. Lazarev M, Present DH, Lichtiger S, et al. The effect of intravenous cyclosporine on rates of colonic surgery in hospitalized patients with severe Crohn’s colitis. J Clin Gastroenterol 2012;46:764–7.
32. Renna S, Cottone M, Orlando A. Optimization of the treatment with immunosuppressants and biologics in inflammatory bowel disease. World J Gastroenterol 2014;20:9675–90.
33. Izower MA, Rahman M, Molmenti EP, et al. Correlation of abnormal histology with endoscopic findings among mycophenolate mofetil treated patients. World J Gastrointest Endosc 2017;9:405–10.
34. Ferrari L, Krane MK, Fichera A. Inflammatory bowel disease surgery in the biologic era. World J Gastrointest Surg 2016;8:363–70.
35. Regueiro M, Schraut W, Baidoo L, et al. Infliximab prevents Crohn’s disease recurrence after ileal resection. Gastroenterology 2009;136:441–50.
36. Regueiro M, Feagan BG, Zou B, et al. Infliximab reduces endoscopic, but not clinical, recurrence of Crohn’s disease after ileocolonic resection. Gastroenterology 2016;150:1568–78.
37. Regueiro M, Velayos F, Greer JB, et al. American Gastroenterological Association Institute technical review on the management of Crohn’s disease after surgical resection. Gastroenterology 2017;152:277–95.
38. Abraham C, Dulai PS, Vermeire S, Sandborn WJ. Lessons learned from trials targeting cytokine pathways in patients with inflammatory bowel diseases. Gastroenterology 2017;152:374–88.
39. Coskun M, Vermeire S, Nielsen OH. Novel targeted therapies for inflammatory bowel disease. Trends Pharmacol Sci 2017;38:127–42.
40. Khanna R, Feagan BG. Emerging therapies for inflammatory bowel diseases. Dig Dis 2016;34 Suppl 1:67–
1. Abraham C, Cho JH. Inflammatory bowel disease. N Engl J Med 2009;361:2066–78.
2. Danese S. New therapies for inflammatory bowel disease: from the bench to the bedside. Gut 2012;61:918–32.
3. Mitrev N, Leong RW. Therapeutic drug monitoring of anti-tumour necrosis factor-α agents in inflammatory bowel disease. Expert Opin Drug Saf 2017;16:303–17.
4. Papamichael K, Gils A, Rutgeerts P, et al. Role for therapeutic drug monitoring during induction therapy with TNF antagonists in IBD: evolution in the definition and management of primary nonresponse. Inflamm Bowel Dis 2015;21:182–97.
5. Levesque BG, Sandborn WJ, Ruel J, et al. Converging goals of treatment of inflammatory bowel disease from clinical trials and practice. Gastroenterology 2015;148:37–51.
6. Allez M, Karmiris K, Louis E, et al. Report of the ECCO pathogenesis workshop on anti-TNF therapy failures in inflammatory bowel diseases: definitions, frequency and pharmacological aspects. J Crohns Colitis 2010;4:355–66.
7. Mosli MH, Zou G, Garg SK, et al. C-Reactive protein, fecal calprotectin, and stool lactoferrin for detection of endoscopic activity in symptomatic inflammatory bowel disease patients: a systematic review and meta-analysis. Am J Gastroenterol 2015;110:802–19.
8. Feuerstein JD, Nguyen GC, Kupfer SS, et al. American Gastroenterological Association Institute guideline on therapeutic drug monitoring in inflammatory bowel disease. Gastroenterology 2017;153:827–34.
9. Vande Casteele N, Herfarth H, Katz J, et al. American Gastroenterological Association Institute technical review on the role of therapeutic drug monitoring in the management of inflammatory bowel diseases. Gastroenterology 2017;153:835–57.
10. López-Hernández R, Valdés M, Campillo JA, et al. Genetic polymorphisms of tumour necrosis factor alpha (TNF-α) promoter gene and response to TNF-α inhibitors in Spanish patients with inflammatory bowel disease. Int J Immunogenet 2014;41:63–8.
11. Ordás I, Mould DR, Feagan BG, Sandborn WJ. Anti-TNF monoclonal antibodies in inflammatory bowel disease: pharmacokinetics-based dosing paradigms. Clin Pharmacol Ther 2012;91:635–46.
12. Hindryckx P, Novak G, Vande Casteele N, et al. Incidence, prevention and management of anti-drug antibodies against therapeutic antibodies in inflammatory bowel disease: a practical overview. Drugs 2017;77:363–77.
13. Colombel JF, Sandborn WJ, Reinisch W, et al. Infliximab, azathioprine, or combination therapy for Crohn’s disease. N Engl J Med 2010;362:1383–95.
14. Lichtenstein GR, Diamond RH, Wagner CL, et al. Clinical trial: benefits and risks of immunomodulators and maintenance infliximab for IBD-subgroup analyses across four randomized trials. Aliment Pharmacol Ther 2009;30:210–26.
15. Vande Casteele N, Ferrante M, Van Assche G, et al. Trough concentrations of infliximab guide dosing for patients with inflammatory bowel disease. Gastroenterology 2015;148:1320–9.
16. Papamichael K, Chachu KA, Vajravelu RK, et al. Improved long-term outcomes of patients with inflammatory bowel disease receiving proactive compared with reactive monitoring of serum concentrations of infliximab. Clin Gastroenterol Hepatol 2017;15:1580–8.
17. Steenholdt C, Brynskov J, Thomsen OØ, et al. Individualised therapy is more cost-effective than dose intensification in patients with Crohn’s disease who lose response to anti-TNF treatment: a randomised, controlled trial. Gut 2014;63:919–27.
18. Afif W, Loftus EV Jr, Faubion WA, et al. Clinical utility of measuring infliximab and human anti-chimeric antibody concentrations in patients with inflammatory bowel disease. Am J Gastroenterol 2010;105:1133–9.
19. Van Stappen T, Vande Casteele N, Van Assche G, et al. Clinical relevance of detecting anti-infliximab antibodies with a drug-tolerant assay: post hoc analysis of the TAXIT trial. Gut 2017.
20. Gisbert JP, Marín AC, McNicholl AG, Chaparro M. Systematic review with meta-analysis: the efficacy of a second anti-TNF in patients with inflammatory bowel disease whose previous anti-TNF treatment has failed. Aliment Pharmacol Ther 2015;41:613–23.
21. Gisbert JP, Chaparro M. Use of a third anti-TNF after failure of two previous anti-TNFs in patients with inflammatory bowel disease: is it worth it? Scand J Gastroenterol 2015;50:379–86.
22. Ordás I, Feagan BG, Sandborn WJ. Therapeutic drug monitoring of tumor necrosis factor antagonists in inflammatory bowel disease. Clin Gastroenterol Hepatol 2012;10:1079–87.
23. Kawalec P, Moćko P. An indirect comparison of ustekinumab and vedolizumab in the therapy of TNF-failure Crohn’s disease patients. J Comp Eff Res 2017;7:101–11.
24. Sandborn WJ, Su C, Panes J, et al. Tofacitinib as induction and maintenance therapy for ulcerative colitis. N Engl J Med 2017;377:1723–36.
25. Yarkoni S, Sagiv Y, Kaminitz A, Askenasy N. Interleukin 2 targeted therapy in inflammatory bowel disease. Gut 2009;58:1705–6.
26. Thin LW, Murray K, Lawrance IC. Oral tacrolimus for the treatment of refractory inflammatory bowel disease in the biologic era. Inflamm Bowel Dis 2013;19:1490–8.
27. Moskovitz DN, Van Assche G, Maenhout B, et al. Incidence of colectomy during long-term follow-up after cyclosporine-induced remission of severe ulcerative colitis. Clin Gastroenterol Hepatol 2006;4:760–5.
28. Arts J, D’Haens G, Zeegers M, et al. Long-term outcome of treatment with intravenous cyclosporin in patients with severe ulcerative colitis. Inflamm Bowel Dis 2004;10:73–8.
29. Cohen RD, Stein R, Hanauer SB. Intravenous cyclosporin in ulcerative colitis: a five-year experience. Am J Gastroenterol 1999;94:1587–92.
30. Cheifetz AS, Stern J, Garud S, et al. Cyclosporine is safe and effective in patients with severe ulcerative colitis. J Clin Gastroenterol 2011;45:107–12.
31. Lazarev M, Present DH, Lichtiger S, et al. The effect of intravenous cyclosporine on rates of colonic surgery in hospitalized patients with severe Crohn’s colitis. J Clin Gastroenterol 2012;46:764–7.
32. Renna S, Cottone M, Orlando A. Optimization of the treatment with immunosuppressants and biologics in inflammatory bowel disease. World J Gastroenterol 2014;20:9675–90.
33. Izower MA, Rahman M, Molmenti EP, et al. Correlation of abnormal histology with endoscopic findings among mycophenolate mofetil treated patients. World J Gastrointest Endosc 2017;9:405–10.
34. Ferrari L, Krane MK, Fichera A. Inflammatory bowel disease surgery in the biologic era. World J Gastrointest Surg 2016;8:363–70.
35. Regueiro M, Schraut W, Baidoo L, et al. Infliximab prevents Crohn’s disease recurrence after ileal resection. Gastroenterology 2009;136:441–50.
36. Regueiro M, Feagan BG, Zou B, et al. Infliximab reduces endoscopic, but not clinical, recurrence of Crohn’s disease after ileocolonic resection. Gastroenterology 2016;150:1568–78.
37. Regueiro M, Velayos F, Greer JB, et al. American Gastroenterological Association Institute technical review on the management of Crohn’s disease after surgical resection. Gastroenterology 2017;152:277–95.
38. Abraham C, Dulai PS, Vermeire S, Sandborn WJ. Lessons learned from trials targeting cytokine pathways in patients with inflammatory bowel diseases. Gastroenterology 2017;152:374–88.
39. Coskun M, Vermeire S, Nielsen OH. Novel targeted therapies for inflammatory bowel disease. Trends Pharmacol Sci 2017;38:127–42.
40. Khanna R, Feagan BG. Emerging therapies for inflammatory bowel diseases. Dig Dis 2016;34 Suppl 1:67–
The Management of Hypertension in Elderly Patients with Chronic Kidney Disease
From the Division of Nephrology, University of New Mexico Health Sciences Center, Albuquerque, NM.
Abstract
- Objective: To review the available literature regarding hypertension and chronic kidney disease (CKD) in the elderly and provide a framework for clinical management of hypertension in this subset of the elderly population.
- Methods: Review of the available literature.
- Results: Though several large, well-designed randomized trials exist examining the treatment of isolated hypertension in the elderly, these trials have uniformly excluded patients with CKD, thus reducing the generalizability of these results to this subgroup. CKD in the elderly is poorly studied overall, and whether CKD in the elderly is an expected product of senescence or a pathology from modifiable risk factors is debatable. Concern exists regarding the increased potential of acute kidney injury events and a more rapid progression of CKD with more aggressive hypertension lowering in elderly patients.
- Conclusion: Though data is limited regarding hypertension treatment in the subset of elderly patients with CKD, given the consistent benefits in cardiovascular reduction with hypertension treatment in the general elderly population, it is likewise recommended that elderly patients with hypertension and CKD receive antihypertensive therapy, though with more careful monitoring for adverse renal effects. We provide a practical approach to management for this clinical scenario.
Chronic kidney disease (CKD) is an increasingly recognized finding in elderly patients, with approximately half of all patients over the age of 70 meeting the most common currently accepted definition of CKD stage III, an estimated glomerular filtration rate (eGFR) of less than 60 mL/min/1.73 m2 [1]. Whether this finding is a result of normal physiologic aging or whether it represents a true disease process in elderly patients has been a matter of considerable debate [2–4]. Nonetheless, the decline in eGFR in elderly patients has important implications regarding drug dosing and the potential risk of acute kidney injury (AKI) in this population [5–9]. Additionally, elderly patients with reduced GFR may have an increased risk of cardiovascular events and progression to end-stage kidney disease (ESKD), though extensive studies are lacking in this population [10–13].
In contrast, isolated hypertension and its treatment in the elderly population has now been extensively evaluated in several well-designed, prospective randomized studies, with generally favorable results arguing for the treatment of hypertension in elderly individuals [14–17]. Unfortunately, however, these studies have uniformly excluded patients with CKD in their study designs. Thus, the impact of aggressive hypertension management in elderly patients with CKD is unknown. As a considerable proportion of CKD in this population has been felt secondary to vascular disease and poor overall vascular health, many have questioned whether aggressive blood pressure reduction, particularly in patients with wide pulse pressure as an indicator of vascular disease, may result in decreased overall renal perfusion and greater risk for AKI, and thus potentially accelerate renal decline in this population [18–22].
In this paper, we review the epidemiology and physiology of renal disease in the elderly, provide an analysis of the available data regarding management of hypertension in the elderly, and suggest an approach to management of hypertension in this specific patient population. Though a multitude of age cutoffs defining elderly have been proposed, for the purposes of this paper we define elderly as age greater than 65 years unless otherwise specified.
Definition of CKD
The currently accepted definition of CKD represents any composite of pathology resulting in impaired kidney function, defined as a drop of GFR < 60 mL/min/1.73 m2 for 3 months or longer, or a higher GFR but with evidence of structural or functional abnormalities, such as proteinuria [23]. However, there are key aspects and important limitations to the above diagnostic criteria to be considered in the elderly patient population. Importantly, the most commonly used GFR estimation equations use serum creatinine as the marker for impaired renal function. As serum creatinine levels are also determined by overall muscle mass, significant error in estimating GFR can occur using these equations in elderly patients, who may have widely varying degrees of musculature and thus creatinine production. Additionally, these equations were often derived using all CKD or mostly CKD patients, which may result in healthy individuals having a higher GFR at the same serum creatinine levels than CKD patients, thus incorrectly classifying many patients with normal kidney function as having CKD [24].
Serum cystatin C has been proposed as an alternative surrogate marker for impaired kidney function, particularly in the elderly, as it is not affected by muscle mass. However, cystatin C levels are affected by obesity, inflammation, and atherosclerosis, and thus equations using this marker to determine GFR also face some limitations in the elderly population [25]. Evidence comparing various GFR estimating equations in the elderly suggest that formulas that use a combination of serum creatinine and cystatin C do best at predicting GFR when compared to gold standard techniques, such as iohexol clearance, though it is important to note that yet the ideal GFR estimating equation for elderly patients has not been determined [26–28].
It has been suggested that given these limitations and potential to underestimate GFR in the elderly population, a lower GFR reference range of 45 mL/min/1.73 m2 be used in the absence of other signs of kidney damage given the multiple unique characteristics of the aging kidneys, as we will explore in this review [29]. In general, we are in agreement with this suggestion that all elderly patients with a creatinine based estimated GFR of < 45 mL/min/1.73 m2 can safely be assumed to have CKD, and it is our opinion that elderly patients with a GFR > 45 mL/min/1.73 m2 but less < 60 mL/min/1.73 m2, without other signs of structural of functional renal disease such as proteinuria, have additional evaluation for the presence of impaired renal function, including but not limited to the addition of cystatin C to estimate GFR.
Epidemiology of CKD in the Elderly
According to the Centers for Disease Control and Prevention (CDC), the number of elderly patients in the United States is expected to double in the next 25 years to 72 million patients, representing approximately 20% of the adult population by 2030 [30]. Analysis of the National Health and Nutrition Examination Surveys (NHANES) from 1999–2004 revealed an overall prevalence of CKD in the US population of 13.1%. However, when sub-grouped into patients greater than or equal to 70 years of age, the prevalence of CKD in this population increased to a staggering 47.5% [31]. Likewise, analysis of other elderly populations from Canada, China, Italy, and Spain indicated a roughly 3- to 7-fold increase in CKD prevalence in those elderly populations compared to younger patients [3]. Additionally, according to the United States Renal Data System there is evidence of a progressive rise in the number of end-stage renal disease (ESRD) patients enrolled in Medicare-funded programs over the past decades [32]. In extrapolating these estimates, it is conceivable to predict that approximately 30 million elderly patients may have CKD in the United States by year 2030, with enormous implications to treatment recommendations and healthcare associated costs.
The Aging Kidney and Expected Rate of Nephron Loss
A progressive, age-related decline in GFR has been demonstrated in many studies. In an earlier analysis of the Baltimore Longitudinal Study of Aging by Lindeman et al, a decline in measured creatinine clearance of 0.75 mL/min/year was demonstrated. It is important to note that in this analysis, patients with suspected pre-existing renal or urologic disease and those on diuretics or other antihypertensives were excluded from analysis, and a normal Gaussian distribution of creatinine clearance slopes versus time was demonstrated, suggesting the GFR loss was a process of normal aging [4].
In support of the theory of a physiologic age-related decline in renal function, a study by Rule et al analyzed potential kidney transplant donors for age-related decline in renal function and determined an approximately 6.3 mL/min/1.73 m2 decline in GFR for each decade. In this investigation, core needle biopsies were obtained at the time of donation and transplantation. The investigators found a progressive increase in the histologic prevalence of nephrosclerosis with each age group analyzed, increasing from 2.7% at ages 18 to 29 to 16% for ages 30 to 39, 28% for ages 40 to 49, 44% for ages 50 to 59, 58% for ages 60 to 69, and finally 73% in donors older than age 70. It is important to note that this study only examined live kidney donors, a group heavily screened and selected on the basis of optimal health, thus strongly arguing for progressive renal decline as a consequence of “normal” aging. Furthermore, though controlled hypertensive patients (treated with 2 or less medications) were allowed to be donors in this study, exclusion of this group had only a minimal impact on the findings of the study [33].
However, whether this age-related decline is purely a result of normal senescence or is a consequence of modifiable risk factors that could alter this outcome remains debatable. Additionally, vascular disease is clearly implicated in more accelerated renal decline. This concept was well demonstrated in an analysis of the longitudinal Age, Gene/Environment Susceptibility – Reykjavik Study, which showed that although age was associated with both reduced GFR and albuminuria, reduced GFR and albuminuria in elderly patients (mean age 80.8 yr) was strongly associated with midlife systolic and diastolic blood pressure, thus suggesting that potentially modifiable vascular pathology may play a much stronger role in CKD in the elderly than aging alone [34].
Finally, it has been hypothesized that reduced nephron mass at birth may contribute to CKD in the elderly [29]. Reduced nephron mass appears to be associated with low birth weight and prematurity, and this has been associated with an increased risk for ESRD later in life [35,36].
Hypertension in the Elderly
Pathophysiology
Age-associated hypertension is felt to arise from several mechanisms and hemodynamic changes. Systolic blood pressure has been noted to progressively rise with age, whereas diastolic blood pressure rises to the 5th or 6th decade, after which it appears to slowly decline. This pattern is felt likely secondary to increasing large vessel stiffness from collagen deposition and calcification with aging, and fracturing and degradation of elastin fibers. As large vessels become less distensible, pulse pressure and pulse wave velocity increases with this drop in diastolic BP, with less forward flow seen in diastole, leading to decreased organ perfusion. Additionally and alternatively, concentric left ventricular hypertrophy develops with aging, leading to reduced cardiac output from decreased stroke volume, which may also contribute to reduced organ perfusion [37,38]. These findings have led many to speculate that hypertension in the elderly may actually serve as a protective mechanism to maintain organ perfusion, and have led to great concern regarding excessive lowering of diastolic blood pressure and increasing of pulse pressure in this population with antihypertensive therapy. This theory was initially corroborated with a sub-analysis of the Systolic Hypertension in the Elderly Program (SHEP) where an increase in pulse pressure by 10 mm Hg was accompanied by increased risk of stroke and congestive heart failure in the treatment arm [39]. Nonetheless, the bulk of evidence continues to support a lower overall risk of cardiovascular events with treatment of hypertension in elderly patients, and general expert consensus recommends treatment with gradual reduction to normal levels of systolic blood pressure accompanied by careful monitoring for adverse effects [40,41].
In addition to these above changes, reduced GFR in elderly likewise results in impaired natriuresis, thereby fostering hypertension via volume expansion. Age-related arteriolosclerosis may result in renal artery stenosis, resulting in decreased renal perfusion and upregulation of the renin-angiotensin-aldosterone cascade. Further challenging treatment decisions is the frequent development of autonomic dysregulation in the elderly, a major risk factor for falls and cardiovascular events [40].
The result of these abnormalities is that roughly 65% of patients greater than the age of 60 have at least isolated systolic hypertension [42]. Similarly corresponding to the underlying physiology highlighted above, rising pulse pressure, rather than systolic or diastolic blood pressure, appears to be the greatest risk factor for cardiovascular events in the elderly population [43,44]. In an interesting analysis of the Framingham Heart Study by Franklin et al, the authors noted that in patients < 50 years of age, diastolic blood pressure was the strongest risk factor for events. However, at age 50 to 59, a change occurred where all 3 blood pressure indexes were comparable risk predictors, and then from age 60 years and on pulse pressure became the superior predictor, with diastolic blood pressure being negatively correlated to cardiovascular risk, highlighting the potential importance for organ perfusion during diastole in this group [45].
Likewise, in the elderly population pulse pressure also appears to be inversely related to GFR, suggesting that vascular stiffness and the reduced forward flow in diastole may contribute to microvascular damage and CKD [46]. In elderly patients with untreated isolated systolic hypertension, increasing systolic blood pressure (a reflection of rising pulse pressure) was associated with the greatest risk of renal decline when compared to diastolic blood pressure, pulse, and mean arterial pressure [47]. In the normal state, high renal blood flow and low renal arterial resistance can contribute to regular large intrarenal pressure variations. Because of vascular stiffness, these pressure variations increase with time, increasing up to 4-fold in the elderly compared with young peers, and likely contribute to renal damage seen in older patients [48].
Treatment
In comparison to the paucity of randomized trials examining CKD progression in the elderly, 4 very large, well designed randomized trials (SHEP, MRC trial, Syst-Eur trial, and HYVET) specifically examining the treatment of hypertension in the elderly have now been conducted [14–17] and confirmed earlier and smaller trials demonstrating the benefits of treatment of hypertension in the elderly [49,50]. In addition to this, several of the other large landmark hypertension trials such as ALLHAT, ACCOMPLISH, and the SPRINT trial included a considerable number of elderly patients [51–53]. Though the primary aim of those trials was not to determine the effects of hypertension treatment in the elderly per se, sub-analysis of this population in these trials has further added to our knowledge of this condition.
In the largest initial trial of hypertension in the elderly (SHEP), the researchers randomized 4376 patients over the age of 60 with an average blood pressure of 170/77 mm Hg into a treatment versus placebo arm. Such a study would be inconceivable today due to the consistent benefit derived from antihypertensive therapy now demonstrated in multiple trials. An achieved systolic blood pressure of 143 mm Hg in the treatment arm versus 155 mm Hg in the placebo arm was obtained. Stroke and nonfatal cardiac events were significantly reduced with treatment. The development of renal dysfunction occurred in 7 patients in the treatment arm and 11 patients in the placebo arm, a nonsignificant difference. As we have noted previously, however, patients with pre-existing kidney disease were excluded from the study [14]. A subsequent analysis of the SHEP trial results by Vaccarino et al, however, showed that in patients on treatment who developed an increase in pulse pressure of 10 mm Hg or more carried a 23% higher risk for developing heart failure and a 24% higher risk for stroke. This effect was not seen in the placebo arm [39].
Shortly following the publication of the SHEP results, the Medical Research Council trial of treatment of hypertension in older adults (MRC) further confirmed the initial findings by demonstrating a 25% reduction in stroke and a 17% reduction in all cardiac events in 4396 patients aged 65 to 74 with a systolic blood pressure greater than 160 mm Hg randomized to treatment of hypertension with either atenolol or a diuretic combination of amiloride and hydrochlorothiazide versus placebo. Like SHEP, however, patients with pre-existing renal disease were excluded, and no report of renal outcomes was published in the initial results [15]. Similarly, the Systolic Hypertension in Europe Trial (Syst-Eur) revealed a 42% reduction in stroke and a 26% reduction in all cardiac endpoints in 4695 patients with a systolic blood pressure of greater than 160 mm Hg randomized to receive nitrendipine with addition of enalapril and hydrochlorothiazide as required. However, CKD patients were likewise excluded in this trial [16].
Finally, the Hypertension in the Very Elderly Trial (HYVET) was unique in that it sought to enroll only patients greater than 80 years of age, a significant departure from the earlier hypertension in elderly trials. This trial randomized 3845 patients, again with a systolic blood pressure of 160 mm Hg or greater, to a placebo arm versus a treatment arm of the thiazide type diuretic indapamide, with addition of the ACE inhibitor perindopril if blood pressure was still greater than 150 mm Hg on monotherapy. Despite the older age of the participants in this trial, patients still benefited from blood pressure reduction with a 30% reduction in rate of stroke, a 21% reduction in the rate of death from any cause, and an impressive 64% reduction in the rate of heart failure [17]. These findings from HYVET, combined with the earlier SHEP, MRC and Syst-Eur trials, confirmed that treatment of hypertension in the elderly of any age should be attempted.
Recomendations for Managing Hypertension in the Elderly with CKD
Though a lack of data exists regarding the treatment of hypertension in elderly patients with the comorbidity of CKD, given the consistent and robust data that exists demonstrating a reduction in cardiovascular risk and mortality in the general elderly population without renal impairment, it is our opinion that elderly patients with CKD and hypertension should receive antihypertensive treatment. This opinion is supported by the fact that in the recently published SPRINT trial, 28.1% of patients in the standard treatment arm (targeting a blood pressure of less than 140 mm Hg), and 28.4% of patients in the intensive treatment arm (blood pressure target less than 120 mm Hg) had CKD, and similarly 28.2% of the trial participants in each group were greater than the age of 75. The percentage of patients with both CKD and age greater than 75 years was not reported in the initial trial results, though it is assumed a significant portion of these patients had both CKD and age greater than 75 years. It is nonetheless reassuring that patients with CKD in the SPRINT trial, as well as those with age > 75 years, both seemed to derive the same benefit in cardiovascular and mortality benefit in the intensive treatment arm compared to the standard treatment arm [53].
It should be noted, however, that though cardiovascular events and mortality were lower in the more intensive treatment arm of the SPRINT trial, CKD progression did not differ between the two treatment groups. Additionally, the risk of acute kidney injury was significantly greater in the intensive treatment arm when compared to the standard treatment arm, with 3.8% of patients in the intensive treatment arm suffering AKI compared to 2.3% in the standard arm [22]. Thus, it should be understood by both the clinician and the elderly patient with hypertension and CKD that the goal of more aggressively lowering blood pressure is to prevent cardiovascular events and not slow renal disease progression.
The recently published 2017 hypertension guidelines by the American College of Cardiology/American Heart Association is the most comprehensive set of hypertension treatment recommendations published to date and includes a section regarding patients with CKD as well as a section on the elderly [54]. Regarding CKD, the guidelines recommend a goal blood pressure of less than 130/80 mm Hg in patients with CKD, and that patients with macroalbuminuria (defined as a daily urine protein excretion of greater than 300 mg/dL or a urine albumin to creatinine ratio of 300 mg/g) be treated with and angiotensin-converting enzyme inhibitor (ACEI) or angiotensin receptor blocker (ARB). We feel these are reasonable recommendations for CKD targets and agree with the guideline, with the understanding that the target of 130/80 mm Hg is based largely on the SPRINT data. It is important to recognize that in the Action to Control Cardiovascular Risk in Diabetes trial (ACCORD), a more intensive blood pressure target of 120 mm Hg did not result in further improvement in cardiovascular events compared to a traditional target of 140/90 mm Hg [55]. However, given the larger and more robust sample size from SPRINT, we feel the target of 130/80 mm Hg is warranted and therefore should be the first target for elderly patients with CKD. With this goal in mind, it has been our clinical experience that some elderly patients with CKD have difficulty tolerating this goal, either from the development of worsening of GFR, acute kidney injury events, or due to orthostatic hypotension. Additionally, it should be noted that patients with orthostatic hypotension were excluded from SPRINT, though an increase in falls was not seen in the primary study. Therefore, for patients who are unable to tolerate the SPRINT goal of 130/80 mm Hg, an individualized goal of at least less than 160 mm Hg systolic and ideally less than 140 mm Hg, reflecting achieved blood pressure endpoints from earlier trials, may be a reasonable alternative [55]. The recent hypertension guidelines also recommend that for elderly adults with a high burden of comorbidities or limited life expectancy, “clinical judgement, patient preference, and at team-based approach to risk/benefit is reasonable for decisions regarding intensity of BP lowering and choice of antihypertensive drugs.” We agree that all treatment decisions must be individualized based upon each patient’s clinical scenario, and that a guideline is only a general aid for treatment decisions, not a mandate for care.
Therefore, with the acknowledgement that there is a lack of literature specifically examining blood pressure goals in elderly patients with CKD, it is our opinion based on available evidence that the following suggestions constitute a reasonable approach to this scenario: (1) a blood pressure target of less than 130/80 mm Hg should be sought as the primary blood pressure target; (2) if the patient cannot tolerate this due to rapidly declining GFR, acute kidney injury, orthostatic hypotension and or falls; or in other situations where this is not a practical a goal, individualized goal of at ideally less than 140 mm Hg, though at least less than 160 mm Hg systolic, could be considered; (3) the clinician should attempt careful and gradual reduction of blood pressure, with no more than one agent added or one escalation of medication dose attempted per visit; (4) the patient should have close follow up-after medication changes with an adjustment period of at least 4 weeks before additional medication or dose escalations are made; (5) if CKD is accompanied by albuminuria (daily urine protein excretion of greater than 300 mg/dL or a urine albumin to creatinine ratio of 300 mg/g) an ACEI or ARB should be used in management; (6) a rise in serum creatinine of up to 30% of baseline after addition of an ACEI may be acceptable; however, a rise greater than this amount should prompt discontinuation of the drug and evaluation for renal artery stenosis; (7) frequent monitoring of creatinine is required, with repeat chemistry performed after medication adjustments; (8) patients with a high pulse pressure should be monitored especially closely for symptoms or changes in renal function; and finally (9) individualized treatment and clinical judgement, with the patient being an informed participant, should take priority over all other recommendations and guidelines. We feel that further research in this growing subgroup of elderly patients is needed and will be sought, and we expect recommendations will continue to evolve as future literature becomes available.
Corresponding author: Jonathan G. Owen, MD, MSC04 2785, 1 University of New Mexico, Albuquerque, NM 87131, [email protected].
Financial disclosures: None.
Author contributions: conception and design, JGO; analysis and interpretation of data, KA, FXR, JGO; drafting of article, KA, FXR, JGO; critical revision of the article, KA, FXR, JGO; collection and assembly of data, KA, FXR, JGO.
1. Schaeffner ES, Ebert N, Delanaye P, et al. Two novel equations to estimate kidney function in persons aged 70 years or older. Ann Intern Med 2012;157:471–81.
2. Wetzels JF, Kiemeney LA, Swinkels DW, et al. Age- and gender-specific reference values of estimated GFR in Caucasians: the Nijmegen Biomedical Study. Kidney Int 2007;72:632–7.
3. Minutolo R, Borrelli S, De Nicola L. CKD in the elderly: kidney senescence or blood pressure-related nephropathy? Am J Kidney Dis 2015;66:184–6.
4. Lindeman RD, Tobin J, Shock NW. Longitudinal studies on the rate of decline in renal function with age. J Am Geriatr Soc 1985; 33:278–85.
5. Gill J, Malyk R, Djurdiev O, Levin A. Use of GFR equations to adjust drug doses in an elderly multi-ethnic group – a cautionary tale. Nephrol Dial Transplant 2007;22:2894–9.
6. Spruill WJ, Wade WE, Cobb HH 3rd. Comparison of estimated glomerular filtration rate with estimated creatinine clearance in the dosing of drugs requiring adjustments in elderly patients with declining renal function. Am J Geriatr Pharmacother 2008;6:153–60.
7. Dowling TC, Wang ES, Ferruci L, Sorkin JD. Glomerular filtration rate equations overestimate creatinine clearance in older individuals enrolled in the Baltimore Longitudinal Study on Aging: impact on renal drug dosing. Pharmacotherapy 2013;33:912–21.
8. Ballew SH, Chen Y, Daya NR, Godino JG, Windham BG, McAdams-DeMarco M, Coresh J, Selvin E, Grams ME. Frailty, kidney function, and polypharmacy: the atherosclerosis risk in communities (ARIC) study. Am J Kidney Dis 2017;69:228–36.
9. Grams ME, Sang Y, Ballew SH, et al; CKD Prognosis Consortium. A meta-analysis of the association of estimated GFR, albuminuria, race, and sex with acute kidney injury. Am J Kidney Dis 2015;66:591–601.
10. Gansevoort RT, Matsushita K, van der Velde M, et al; Chronic Kidney Disease Prognosis Consortium. Lower estimated GFR and higher albuminuria are associated with adverse kidney outcomes. A collaborative meta-analysis of general and high-risk population cohorts. Kidney Int 2011;80:93–104.
11. Masson P, Webster AC, Hong M, et al. Chronic kidney disease and the risk of stroke: a systematic review and meta-analysis. Nephrol Dial Transplant 2015;30:1162–9.
12. Tellez-Plaza M, Orozco-Beltran D, Gil-Guillen V, et al; ESCARVAL STUDY GROUP. Renal function and attributable risk of death and cardiovascular hospitalization in patients with cardiovascular risk factors from a registry-based cohort: the Estudio Cardiovascular Valencia-risk study. J Hypertens 2016;34:2266–73.
13. Smink PA, Lambers-Heerspink HJ, Gansevoort RT, et al. Albuminuria, estimated GFR, traditional risk factors, and incident cardiovascular disease: the PREVEND (Prevention of Renal and Vascular Endstage Disease) study. Am J Kidney Dis 2012;60:804–11.
14. SHEP Cooperative Research Group. Prevention of stroke by antihypertensive drug treatment in older persons with isolated systolic hypertension. Final results of the systolic hypertension in the elderly program (SHEP). JAMA 1991;265:3255–64.
15. Working Party. Medical Research Council trial of treatment of hypertension in older adults: principal results. BMJ 1992;304:405–12.
16. Staessen JA, Faggard R, Thijs L, et al. Randomised double-blind comparison of placebo and active treatment for older patients with isolated systolic hypertension. Lancet 1997;350:757–64.
17. Beckett NS, Peters R, Fletcher AE, et al; HYVET Study Group. Treatment of hypertension in patients 80 years of age or older. N Engl J Med 2008;358:1887–98.
18. Fesler P, Safar ME, du Cailar G, et al. Pulse pressure is an independent determinant of renal function decline during treatment of essential hypertension. J Hypertens 2007;25:1915–20.
19. Weir MR, Dworkin LD. Antihypertensive drugs, dietary salt, and renal protection: how low should you go and with which therapy? Am J Kidney Dis 1998;32:1–22.
20. Obi Y, Kalantar-Zadeh K, Shintani A, et al. Estimated glomerular filtration rate and the risk-benefit profile of intensive blood pressure control amongst nondiabetic patients: a post hoc analysis of a randomized clinical trial. J Intern Med 2017;283:314–27.
21. Peralta CA, McClure LA, Scherzer R, et al. Effect of intensive versus usual blood pressure control on kidney function among individuals with prior lacunar stroke: a post hoc analysis of the secondary prevention of small subcortical strokes (SPS3) randomized trial. Circulation 2016;133:584–91.
22. Rocco MV, Sink KM, Lovato LC, et al; SPRINT Research Group. Effects of intensive blood pressure treatment on acute kidney injury events in the systolic blood pressure intervention trial (SPRINT). Am J Kidney Dis 2018;71:352–61.
23. Chapter 1: Definition and classification of CKD. Kidney Int Suppl (2011) 2013;3:19–62.
24. Rule AD, Larson TS, Bergstralh EJ, et al. Using serum creatinine to estimate glomerular filtration rate: accuracy in good health and in chronic kidney disease. Ann Intern Med 2004;141:929–37.
25. Stevens LA, Schmid CH, Green T, et al. Factors other than glomerular filtration rate affect serum cystatin C levels. Kidney Int 2009;75:652–60.
26. Biork J, Grubb A, Gudnason V, et al. Comparison of glomerular filtration rate estimating equations derived from creatinine and cystatin C: validation in the age, gene/environment susceptibility-Reykjavik elderly cohort. Nephrol Dial Transplant 2017.
27. Bevc S, Hojs N, Hois R, et al. Estimation of glomerular filtration rate in elderly chronic kidney disease patients: comparison of three novel sophisticated equations and simple cystatin C equation. Therapeutic Apheresis and Dialysis 2017;21:126–32.
28. Pottel H, Delanaye P, Schaeffner E, et al. Estimating glomerular filtration rate for the full age spectrum from serum creatinine and cystatin C. Nephrol Dial Transplant 2017;32:497–507.
29. Denic A, Glassock RJ, Rule AD. Structural and functional changes with the aging kidney. Adv Chronic Kidney Dis 2016;23:19–28.
30. US Department of Health and Human Services, Centers for Disease Control and Prevention. The state of aging and health in America 2013. Centers for Disease Control and Prevention website. https://www.cdc.gov/aging/pdf/state-aging-health-in-america-2013.pdf. Published 2013. Accessed April 5, 2018.
31. Coresh J, Selvin E, Stevens LA, et al. Prevalence of chronic kidney disease in the United States. JAMA 2007;298:2038–47.
32. United Stated Renal Data System. USRD 2013 Annual Data Report: Atlas of Chronic Kidney Disease and End-Stage Renal Disease in the United States. United States Renal Data System website. https://www.usrds.org/atlas13.aspx . Published 2013. Accessed April 5, 2018.
33. Rule AD, Amer H, Cornell LD, et al. The association between age and nephroclerosis on renal biopsy among healthy adults. Ann Intern Med 2010;152:561–7.
34. Inker LA, Okparavero A, Tighiouart H, et al. Midlife blood pressure and late-life GFR and albuminuria: an elderly general population cohort. Am J Kidney Dis 2015;66:240–8.
35. Vikse BE, Irgens LM, Leivestad T, et al. Low birth weight increases risk for end-stage renal disease. J Am Soc Nephrol 2008;19:151–7.
36. Luyckx VA, Bertram JF, Brenner BM, et al. Effect of fetal and child health on kidney development and long-term risk of hypertension and kidney disease. Lancet 2013;382:273–83.
37. Franklin SS, Gustin W 4th, Wong ND, et al. Hemodynamic patterns of age-related changes in blood pressure. The Framingham Heart Study. Circulation 1997;96:308–15.
38. Messerli FH, Sundgaard-Riise K, Ventura HO, et al. Essential hypertension in the elderly: haemodynamics, intravascular volume, plasma renin activity, and circulating catecholamine levels. Lancet 1983;2:983–6.
39. Vaccarino V, Berger AK, Abramson J, et al. Pulse pressure and risk of cardiovascular events in the systolic hypertension in the elderly program. Am J Cardiol 2001;88:980–6.
40. Aronow WS, Harrington RA, Fleg JL, Pepine CJ, Artinian NT, Bakris G, Brown AS, Ferdinand KC, Forciea MA, Frishman WH, Jaigobin C, Kostis JB, Mancia G, Oparil S, Ortiz E, Reisin E, Rich MW, Schocken DD, Weber MA, Wesley DJ. ACCF/AHA 2011 expert consensus document on hypertension in the elderly: a report of the American College of Cardiology Foundation task force on clinical expert consensus documents. Circulation 2011;123:2434–506.
41. Chaudhry SI, Krumholz HM, Foody JM. Systolic hypertension in older persons. JAMA 2004;292:1074–80.
42. Burt VL, Whelton P, Roccella EJ, et al. Prevalence of hypertension in the US adult population. Results from the Third National Health and Nutrition Examination Survey, 1988-1991. Hypertension 1995;25: 305–13.
43. Blacher J, Staessen JA, Girerd X, et al. Pulse pressure not mean pressure determines cardiovascular risk in older hypertensive patients. Arch Intern Med 2000;160:1085–90.
44. Franklin SS, Lopez VA, Wong ND, et al. Single versus combined blood pressure components and risk for cardiovascular disease: the Framingham Heart Study. Circulation 2009;119:243–50.
45. Franklin SS, Larson MG, Khan SA, et al. Does the relation of blood pressure to coronary heart disease risk change with aging? The Framingham Heart Study. Circulation 2001;103:1245–9.
46. Verhave JC, Fesler P, du Cailar G, et al. Elevated pulse pressure is associated with low renal function in elderly patients with isolated systolic hypertension. Hypertension 2005;45:586–91.
47. Young JH, Klaq MJ, Muntner P, et al. Blood pressure and decline in kidney function: findings from the systolic hypertension in elderly program (SHEP). J Am Soc Nephrol 2002;13:2776–82.
48. O’Rourke MF, Safar ME. Relationship between aortic stiffening and microvascular disease in brain and kidney: cause and logic of therapy. Hypertension 2005;46:200–4.
49. Coope J, Warrender TS. Randomised trial of treatment of hypertension in elderly patients in primary care. Br Med J (Clin Res Ed) 1986;293:1145–8.
50. Dahlof B, Lindholm LH, Hansson L, et al. Morbidity and mortality in the Swedish trial in old patients with hypertension (STOP-Hypertension). Lancet 1991;338:1281–5.
51. ALLHAT Officers and Coordinators for the ALLHAT Collaborative Research Group; The Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial. Major outcomes in high-risk hypertensive patients randomized to angiotensin-converting enzyme inhibitor or calcium channel blocker vs diuretic: the antihypertensive and lipid-lowering treatment to prevent heart attack trial (ALLHAT). JAMA 2002;288:2981–97.
52. Jamerson K, Weber MA, Bakris GL, et al; ACCOMPLISH Trial Investigators. Benazapril plus amlodipine or hydrochlorothiazide for hypertension in high-risk patients. N Engl J Med 2008;359:2417–28.
53. SPRINT Research Group; Wright JT Jr, Williamson JD, Whelton PK, et al. A randomized trial of intensive versus standard blood-pressure control. N Engl J Med 2015;373: 2103–16.
54. Whelton PK, Carey RM, Aronow WS, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: a report of the American College of Cardiology/American Heart Association task force on clinical practice guidelines. Hypertension 2017.
55. ACCORD Study Group; Cushman WC, Evans GW, Byington RP, et al. Effects of intensive blood-pressure control in type 2 diabetes mellitus. N Engl J Med 2010;362:1575–85.
From the Division of Nephrology, University of New Mexico Health Sciences Center, Albuquerque, NM.
Abstract
- Objective: To review the available literature regarding hypertension and chronic kidney disease (CKD) in the elderly and provide a framework for clinical management of hypertension in this subset of the elderly population.
- Methods: Review of the available literature.
- Results: Though several large, well-designed randomized trials exist examining the treatment of isolated hypertension in the elderly, these trials have uniformly excluded patients with CKD, thus reducing the generalizability of these results to this subgroup. CKD in the elderly is poorly studied overall, and whether CKD in the elderly is an expected product of senescence or a pathology from modifiable risk factors is debatable. Concern exists regarding the increased potential of acute kidney injury events and a more rapid progression of CKD with more aggressive hypertension lowering in elderly patients.
- Conclusion: Though data is limited regarding hypertension treatment in the subset of elderly patients with CKD, given the consistent benefits in cardiovascular reduction with hypertension treatment in the general elderly population, it is likewise recommended that elderly patients with hypertension and CKD receive antihypertensive therapy, though with more careful monitoring for adverse renal effects. We provide a practical approach to management for this clinical scenario.
Chronic kidney disease (CKD) is an increasingly recognized finding in elderly patients, with approximately half of all patients over the age of 70 meeting the most common currently accepted definition of CKD stage III, an estimated glomerular filtration rate (eGFR) of less than 60 mL/min/1.73 m2 [1]. Whether this finding is a result of normal physiologic aging or whether it represents a true disease process in elderly patients has been a matter of considerable debate [2–4]. Nonetheless, the decline in eGFR in elderly patients has important implications regarding drug dosing and the potential risk of acute kidney injury (AKI) in this population [5–9]. Additionally, elderly patients with reduced GFR may have an increased risk of cardiovascular events and progression to end-stage kidney disease (ESKD), though extensive studies are lacking in this population [10–13].
In contrast, isolated hypertension and its treatment in the elderly population has now been extensively evaluated in several well-designed, prospective randomized studies, with generally favorable results arguing for the treatment of hypertension in elderly individuals [14–17]. Unfortunately, however, these studies have uniformly excluded patients with CKD in their study designs. Thus, the impact of aggressive hypertension management in elderly patients with CKD is unknown. As a considerable proportion of CKD in this population has been felt secondary to vascular disease and poor overall vascular health, many have questioned whether aggressive blood pressure reduction, particularly in patients with wide pulse pressure as an indicator of vascular disease, may result in decreased overall renal perfusion and greater risk for AKI, and thus potentially accelerate renal decline in this population [18–22].
In this paper, we review the epidemiology and physiology of renal disease in the elderly, provide an analysis of the available data regarding management of hypertension in the elderly, and suggest an approach to management of hypertension in this specific patient population. Though a multitude of age cutoffs defining elderly have been proposed, for the purposes of this paper we define elderly as age greater than 65 years unless otherwise specified.
Definition of CKD
The currently accepted definition of CKD represents any composite of pathology resulting in impaired kidney function, defined as a drop of GFR < 60 mL/min/1.73 m2 for 3 months or longer, or a higher GFR but with evidence of structural or functional abnormalities, such as proteinuria [23]. However, there are key aspects and important limitations to the above diagnostic criteria to be considered in the elderly patient population. Importantly, the most commonly used GFR estimation equations use serum creatinine as the marker for impaired renal function. As serum creatinine levels are also determined by overall muscle mass, significant error in estimating GFR can occur using these equations in elderly patients, who may have widely varying degrees of musculature and thus creatinine production. Additionally, these equations were often derived using all CKD or mostly CKD patients, which may result in healthy individuals having a higher GFR at the same serum creatinine levels than CKD patients, thus incorrectly classifying many patients with normal kidney function as having CKD [24].
Serum cystatin C has been proposed as an alternative surrogate marker for impaired kidney function, particularly in the elderly, as it is not affected by muscle mass. However, cystatin C levels are affected by obesity, inflammation, and atherosclerosis, and thus equations using this marker to determine GFR also face some limitations in the elderly population [25]. Evidence comparing various GFR estimating equations in the elderly suggest that formulas that use a combination of serum creatinine and cystatin C do best at predicting GFR when compared to gold standard techniques, such as iohexol clearance, though it is important to note that yet the ideal GFR estimating equation for elderly patients has not been determined [26–28].
It has been suggested that given these limitations and potential to underestimate GFR in the elderly population, a lower GFR reference range of 45 mL/min/1.73 m2 be used in the absence of other signs of kidney damage given the multiple unique characteristics of the aging kidneys, as we will explore in this review [29]. In general, we are in agreement with this suggestion that all elderly patients with a creatinine based estimated GFR of < 45 mL/min/1.73 m2 can safely be assumed to have CKD, and it is our opinion that elderly patients with a GFR > 45 mL/min/1.73 m2 but less < 60 mL/min/1.73 m2, without other signs of structural of functional renal disease such as proteinuria, have additional evaluation for the presence of impaired renal function, including but not limited to the addition of cystatin C to estimate GFR.
Epidemiology of CKD in the Elderly
According to the Centers for Disease Control and Prevention (CDC), the number of elderly patients in the United States is expected to double in the next 25 years to 72 million patients, representing approximately 20% of the adult population by 2030 [30]. Analysis of the National Health and Nutrition Examination Surveys (NHANES) from 1999–2004 revealed an overall prevalence of CKD in the US population of 13.1%. However, when sub-grouped into patients greater than or equal to 70 years of age, the prevalence of CKD in this population increased to a staggering 47.5% [31]. Likewise, analysis of other elderly populations from Canada, China, Italy, and Spain indicated a roughly 3- to 7-fold increase in CKD prevalence in those elderly populations compared to younger patients [3]. Additionally, according to the United States Renal Data System there is evidence of a progressive rise in the number of end-stage renal disease (ESRD) patients enrolled in Medicare-funded programs over the past decades [32]. In extrapolating these estimates, it is conceivable to predict that approximately 30 million elderly patients may have CKD in the United States by year 2030, with enormous implications to treatment recommendations and healthcare associated costs.
The Aging Kidney and Expected Rate of Nephron Loss
A progressive, age-related decline in GFR has been demonstrated in many studies. In an earlier analysis of the Baltimore Longitudinal Study of Aging by Lindeman et al, a decline in measured creatinine clearance of 0.75 mL/min/year was demonstrated. It is important to note that in this analysis, patients with suspected pre-existing renal or urologic disease and those on diuretics or other antihypertensives were excluded from analysis, and a normal Gaussian distribution of creatinine clearance slopes versus time was demonstrated, suggesting the GFR loss was a process of normal aging [4].
In support of the theory of a physiologic age-related decline in renal function, a study by Rule et al analyzed potential kidney transplant donors for age-related decline in renal function and determined an approximately 6.3 mL/min/1.73 m2 decline in GFR for each decade. In this investigation, core needle biopsies were obtained at the time of donation and transplantation. The investigators found a progressive increase in the histologic prevalence of nephrosclerosis with each age group analyzed, increasing from 2.7% at ages 18 to 29 to 16% for ages 30 to 39, 28% for ages 40 to 49, 44% for ages 50 to 59, 58% for ages 60 to 69, and finally 73% in donors older than age 70. It is important to note that this study only examined live kidney donors, a group heavily screened and selected on the basis of optimal health, thus strongly arguing for progressive renal decline as a consequence of “normal” aging. Furthermore, though controlled hypertensive patients (treated with 2 or less medications) were allowed to be donors in this study, exclusion of this group had only a minimal impact on the findings of the study [33].
However, whether this age-related decline is purely a result of normal senescence or is a consequence of modifiable risk factors that could alter this outcome remains debatable. Additionally, vascular disease is clearly implicated in more accelerated renal decline. This concept was well demonstrated in an analysis of the longitudinal Age, Gene/Environment Susceptibility – Reykjavik Study, which showed that although age was associated with both reduced GFR and albuminuria, reduced GFR and albuminuria in elderly patients (mean age 80.8 yr) was strongly associated with midlife systolic and diastolic blood pressure, thus suggesting that potentially modifiable vascular pathology may play a much stronger role in CKD in the elderly than aging alone [34].
Finally, it has been hypothesized that reduced nephron mass at birth may contribute to CKD in the elderly [29]. Reduced nephron mass appears to be associated with low birth weight and prematurity, and this has been associated with an increased risk for ESRD later in life [35,36].
Hypertension in the Elderly
Pathophysiology
Age-associated hypertension is felt to arise from several mechanisms and hemodynamic changes. Systolic blood pressure has been noted to progressively rise with age, whereas diastolic blood pressure rises to the 5th or 6th decade, after which it appears to slowly decline. This pattern is felt likely secondary to increasing large vessel stiffness from collagen deposition and calcification with aging, and fracturing and degradation of elastin fibers. As large vessels become less distensible, pulse pressure and pulse wave velocity increases with this drop in diastolic BP, with less forward flow seen in diastole, leading to decreased organ perfusion. Additionally and alternatively, concentric left ventricular hypertrophy develops with aging, leading to reduced cardiac output from decreased stroke volume, which may also contribute to reduced organ perfusion [37,38]. These findings have led many to speculate that hypertension in the elderly may actually serve as a protective mechanism to maintain organ perfusion, and have led to great concern regarding excessive lowering of diastolic blood pressure and increasing of pulse pressure in this population with antihypertensive therapy. This theory was initially corroborated with a sub-analysis of the Systolic Hypertension in the Elderly Program (SHEP) where an increase in pulse pressure by 10 mm Hg was accompanied by increased risk of stroke and congestive heart failure in the treatment arm [39]. Nonetheless, the bulk of evidence continues to support a lower overall risk of cardiovascular events with treatment of hypertension in elderly patients, and general expert consensus recommends treatment with gradual reduction to normal levels of systolic blood pressure accompanied by careful monitoring for adverse effects [40,41].
In addition to these above changes, reduced GFR in elderly likewise results in impaired natriuresis, thereby fostering hypertension via volume expansion. Age-related arteriolosclerosis may result in renal artery stenosis, resulting in decreased renal perfusion and upregulation of the renin-angiotensin-aldosterone cascade. Further challenging treatment decisions is the frequent development of autonomic dysregulation in the elderly, a major risk factor for falls and cardiovascular events [40].
The result of these abnormalities is that roughly 65% of patients greater than the age of 60 have at least isolated systolic hypertension [42]. Similarly corresponding to the underlying physiology highlighted above, rising pulse pressure, rather than systolic or diastolic blood pressure, appears to be the greatest risk factor for cardiovascular events in the elderly population [43,44]. In an interesting analysis of the Framingham Heart Study by Franklin et al, the authors noted that in patients < 50 years of age, diastolic blood pressure was the strongest risk factor for events. However, at age 50 to 59, a change occurred where all 3 blood pressure indexes were comparable risk predictors, and then from age 60 years and on pulse pressure became the superior predictor, with diastolic blood pressure being negatively correlated to cardiovascular risk, highlighting the potential importance for organ perfusion during diastole in this group [45].
Likewise, in the elderly population pulse pressure also appears to be inversely related to GFR, suggesting that vascular stiffness and the reduced forward flow in diastole may contribute to microvascular damage and CKD [46]. In elderly patients with untreated isolated systolic hypertension, increasing systolic blood pressure (a reflection of rising pulse pressure) was associated with the greatest risk of renal decline when compared to diastolic blood pressure, pulse, and mean arterial pressure [47]. In the normal state, high renal blood flow and low renal arterial resistance can contribute to regular large intrarenal pressure variations. Because of vascular stiffness, these pressure variations increase with time, increasing up to 4-fold in the elderly compared with young peers, and likely contribute to renal damage seen in older patients [48].
Treatment
In comparison to the paucity of randomized trials examining CKD progression in the elderly, 4 very large, well designed randomized trials (SHEP, MRC trial, Syst-Eur trial, and HYVET) specifically examining the treatment of hypertension in the elderly have now been conducted [14–17] and confirmed earlier and smaller trials demonstrating the benefits of treatment of hypertension in the elderly [49,50]. In addition to this, several of the other large landmark hypertension trials such as ALLHAT, ACCOMPLISH, and the SPRINT trial included a considerable number of elderly patients [51–53]. Though the primary aim of those trials was not to determine the effects of hypertension treatment in the elderly per se, sub-analysis of this population in these trials has further added to our knowledge of this condition.
In the largest initial trial of hypertension in the elderly (SHEP), the researchers randomized 4376 patients over the age of 60 with an average blood pressure of 170/77 mm Hg into a treatment versus placebo arm. Such a study would be inconceivable today due to the consistent benefit derived from antihypertensive therapy now demonstrated in multiple trials. An achieved systolic blood pressure of 143 mm Hg in the treatment arm versus 155 mm Hg in the placebo arm was obtained. Stroke and nonfatal cardiac events were significantly reduced with treatment. The development of renal dysfunction occurred in 7 patients in the treatment arm and 11 patients in the placebo arm, a nonsignificant difference. As we have noted previously, however, patients with pre-existing kidney disease were excluded from the study [14]. A subsequent analysis of the SHEP trial results by Vaccarino et al, however, showed that in patients on treatment who developed an increase in pulse pressure of 10 mm Hg or more carried a 23% higher risk for developing heart failure and a 24% higher risk for stroke. This effect was not seen in the placebo arm [39].
Shortly following the publication of the SHEP results, the Medical Research Council trial of treatment of hypertension in older adults (MRC) further confirmed the initial findings by demonstrating a 25% reduction in stroke and a 17% reduction in all cardiac events in 4396 patients aged 65 to 74 with a systolic blood pressure greater than 160 mm Hg randomized to treatment of hypertension with either atenolol or a diuretic combination of amiloride and hydrochlorothiazide versus placebo. Like SHEP, however, patients with pre-existing renal disease were excluded, and no report of renal outcomes was published in the initial results [15]. Similarly, the Systolic Hypertension in Europe Trial (Syst-Eur) revealed a 42% reduction in stroke and a 26% reduction in all cardiac endpoints in 4695 patients with a systolic blood pressure of greater than 160 mm Hg randomized to receive nitrendipine with addition of enalapril and hydrochlorothiazide as required. However, CKD patients were likewise excluded in this trial [16].
Finally, the Hypertension in the Very Elderly Trial (HYVET) was unique in that it sought to enroll only patients greater than 80 years of age, a significant departure from the earlier hypertension in elderly trials. This trial randomized 3845 patients, again with a systolic blood pressure of 160 mm Hg or greater, to a placebo arm versus a treatment arm of the thiazide type diuretic indapamide, with addition of the ACE inhibitor perindopril if blood pressure was still greater than 150 mm Hg on monotherapy. Despite the older age of the participants in this trial, patients still benefited from blood pressure reduction with a 30% reduction in rate of stroke, a 21% reduction in the rate of death from any cause, and an impressive 64% reduction in the rate of heart failure [17]. These findings from HYVET, combined with the earlier SHEP, MRC and Syst-Eur trials, confirmed that treatment of hypertension in the elderly of any age should be attempted.
Recomendations for Managing Hypertension in the Elderly with CKD
Though a lack of data exists regarding the treatment of hypertension in elderly patients with the comorbidity of CKD, given the consistent and robust data that exists demonstrating a reduction in cardiovascular risk and mortality in the general elderly population without renal impairment, it is our opinion that elderly patients with CKD and hypertension should receive antihypertensive treatment. This opinion is supported by the fact that in the recently published SPRINT trial, 28.1% of patients in the standard treatment arm (targeting a blood pressure of less than 140 mm Hg), and 28.4% of patients in the intensive treatment arm (blood pressure target less than 120 mm Hg) had CKD, and similarly 28.2% of the trial participants in each group were greater than the age of 75. The percentage of patients with both CKD and age greater than 75 years was not reported in the initial trial results, though it is assumed a significant portion of these patients had both CKD and age greater than 75 years. It is nonetheless reassuring that patients with CKD in the SPRINT trial, as well as those with age > 75 years, both seemed to derive the same benefit in cardiovascular and mortality benefit in the intensive treatment arm compared to the standard treatment arm [53].
It should be noted, however, that though cardiovascular events and mortality were lower in the more intensive treatment arm of the SPRINT trial, CKD progression did not differ between the two treatment groups. Additionally, the risk of acute kidney injury was significantly greater in the intensive treatment arm when compared to the standard treatment arm, with 3.8% of patients in the intensive treatment arm suffering AKI compared to 2.3% in the standard arm [22]. Thus, it should be understood by both the clinician and the elderly patient with hypertension and CKD that the goal of more aggressively lowering blood pressure is to prevent cardiovascular events and not slow renal disease progression.
The recently published 2017 hypertension guidelines by the American College of Cardiology/American Heart Association is the most comprehensive set of hypertension treatment recommendations published to date and includes a section regarding patients with CKD as well as a section on the elderly [54]. Regarding CKD, the guidelines recommend a goal blood pressure of less than 130/80 mm Hg in patients with CKD, and that patients with macroalbuminuria (defined as a daily urine protein excretion of greater than 300 mg/dL or a urine albumin to creatinine ratio of 300 mg/g) be treated with and angiotensin-converting enzyme inhibitor (ACEI) or angiotensin receptor blocker (ARB). We feel these are reasonable recommendations for CKD targets and agree with the guideline, with the understanding that the target of 130/80 mm Hg is based largely on the SPRINT data. It is important to recognize that in the Action to Control Cardiovascular Risk in Diabetes trial (ACCORD), a more intensive blood pressure target of 120 mm Hg did not result in further improvement in cardiovascular events compared to a traditional target of 140/90 mm Hg [55]. However, given the larger and more robust sample size from SPRINT, we feel the target of 130/80 mm Hg is warranted and therefore should be the first target for elderly patients with CKD. With this goal in mind, it has been our clinical experience that some elderly patients with CKD have difficulty tolerating this goal, either from the development of worsening of GFR, acute kidney injury events, or due to orthostatic hypotension. Additionally, it should be noted that patients with orthostatic hypotension were excluded from SPRINT, though an increase in falls was not seen in the primary study. Therefore, for patients who are unable to tolerate the SPRINT goal of 130/80 mm Hg, an individualized goal of at least less than 160 mm Hg systolic and ideally less than 140 mm Hg, reflecting achieved blood pressure endpoints from earlier trials, may be a reasonable alternative [55]. The recent hypertension guidelines also recommend that for elderly adults with a high burden of comorbidities or limited life expectancy, “clinical judgement, patient preference, and at team-based approach to risk/benefit is reasonable for decisions regarding intensity of BP lowering and choice of antihypertensive drugs.” We agree that all treatment decisions must be individualized based upon each patient’s clinical scenario, and that a guideline is only a general aid for treatment decisions, not a mandate for care.
Therefore, with the acknowledgement that there is a lack of literature specifically examining blood pressure goals in elderly patients with CKD, it is our opinion based on available evidence that the following suggestions constitute a reasonable approach to this scenario: (1) a blood pressure target of less than 130/80 mm Hg should be sought as the primary blood pressure target; (2) if the patient cannot tolerate this due to rapidly declining GFR, acute kidney injury, orthostatic hypotension and or falls; or in other situations where this is not a practical a goal, individualized goal of at ideally less than 140 mm Hg, though at least less than 160 mm Hg systolic, could be considered; (3) the clinician should attempt careful and gradual reduction of blood pressure, with no more than one agent added or one escalation of medication dose attempted per visit; (4) the patient should have close follow up-after medication changes with an adjustment period of at least 4 weeks before additional medication or dose escalations are made; (5) if CKD is accompanied by albuminuria (daily urine protein excretion of greater than 300 mg/dL or a urine albumin to creatinine ratio of 300 mg/g) an ACEI or ARB should be used in management; (6) a rise in serum creatinine of up to 30% of baseline after addition of an ACEI may be acceptable; however, a rise greater than this amount should prompt discontinuation of the drug and evaluation for renal artery stenosis; (7) frequent monitoring of creatinine is required, with repeat chemistry performed after medication adjustments; (8) patients with a high pulse pressure should be monitored especially closely for symptoms or changes in renal function; and finally (9) individualized treatment and clinical judgement, with the patient being an informed participant, should take priority over all other recommendations and guidelines. We feel that further research in this growing subgroup of elderly patients is needed and will be sought, and we expect recommendations will continue to evolve as future literature becomes available.
Corresponding author: Jonathan G. Owen, MD, MSC04 2785, 1 University of New Mexico, Albuquerque, NM 87131, [email protected].
Financial disclosures: None.
Author contributions: conception and design, JGO; analysis and interpretation of data, KA, FXR, JGO; drafting of article, KA, FXR, JGO; critical revision of the article, KA, FXR, JGO; collection and assembly of data, KA, FXR, JGO.
From the Division of Nephrology, University of New Mexico Health Sciences Center, Albuquerque, NM.
Abstract
- Objective: To review the available literature regarding hypertension and chronic kidney disease (CKD) in the elderly and provide a framework for clinical management of hypertension in this subset of the elderly population.
- Methods: Review of the available literature.
- Results: Though several large, well-designed randomized trials exist examining the treatment of isolated hypertension in the elderly, these trials have uniformly excluded patients with CKD, thus reducing the generalizability of these results to this subgroup. CKD in the elderly is poorly studied overall, and whether CKD in the elderly is an expected product of senescence or a pathology from modifiable risk factors is debatable. Concern exists regarding the increased potential of acute kidney injury events and a more rapid progression of CKD with more aggressive hypertension lowering in elderly patients.
- Conclusion: Though data is limited regarding hypertension treatment in the subset of elderly patients with CKD, given the consistent benefits in cardiovascular reduction with hypertension treatment in the general elderly population, it is likewise recommended that elderly patients with hypertension and CKD receive antihypertensive therapy, though with more careful monitoring for adverse renal effects. We provide a practical approach to management for this clinical scenario.
Chronic kidney disease (CKD) is an increasingly recognized finding in elderly patients, with approximately half of all patients over the age of 70 meeting the most common currently accepted definition of CKD stage III, an estimated glomerular filtration rate (eGFR) of less than 60 mL/min/1.73 m2 [1]. Whether this finding is a result of normal physiologic aging or whether it represents a true disease process in elderly patients has been a matter of considerable debate [2–4]. Nonetheless, the decline in eGFR in elderly patients has important implications regarding drug dosing and the potential risk of acute kidney injury (AKI) in this population [5–9]. Additionally, elderly patients with reduced GFR may have an increased risk of cardiovascular events and progression to end-stage kidney disease (ESKD), though extensive studies are lacking in this population [10–13].
In contrast, isolated hypertension and its treatment in the elderly population has now been extensively evaluated in several well-designed, prospective randomized studies, with generally favorable results arguing for the treatment of hypertension in elderly individuals [14–17]. Unfortunately, however, these studies have uniformly excluded patients with CKD in their study designs. Thus, the impact of aggressive hypertension management in elderly patients with CKD is unknown. As a considerable proportion of CKD in this population has been felt secondary to vascular disease and poor overall vascular health, many have questioned whether aggressive blood pressure reduction, particularly in patients with wide pulse pressure as an indicator of vascular disease, may result in decreased overall renal perfusion and greater risk for AKI, and thus potentially accelerate renal decline in this population [18–22].
In this paper, we review the epidemiology and physiology of renal disease in the elderly, provide an analysis of the available data regarding management of hypertension in the elderly, and suggest an approach to management of hypertension in this specific patient population. Though a multitude of age cutoffs defining elderly have been proposed, for the purposes of this paper we define elderly as age greater than 65 years unless otherwise specified.
Definition of CKD
The currently accepted definition of CKD represents any composite of pathology resulting in impaired kidney function, defined as a drop of GFR < 60 mL/min/1.73 m2 for 3 months or longer, or a higher GFR but with evidence of structural or functional abnormalities, such as proteinuria [23]. However, there are key aspects and important limitations to the above diagnostic criteria to be considered in the elderly patient population. Importantly, the most commonly used GFR estimation equations use serum creatinine as the marker for impaired renal function. As serum creatinine levels are also determined by overall muscle mass, significant error in estimating GFR can occur using these equations in elderly patients, who may have widely varying degrees of musculature and thus creatinine production. Additionally, these equations were often derived using all CKD or mostly CKD patients, which may result in healthy individuals having a higher GFR at the same serum creatinine levels than CKD patients, thus incorrectly classifying many patients with normal kidney function as having CKD [24].
Serum cystatin C has been proposed as an alternative surrogate marker for impaired kidney function, particularly in the elderly, as it is not affected by muscle mass. However, cystatin C levels are affected by obesity, inflammation, and atherosclerosis, and thus equations using this marker to determine GFR also face some limitations in the elderly population [25]. Evidence comparing various GFR estimating equations in the elderly suggest that formulas that use a combination of serum creatinine and cystatin C do best at predicting GFR when compared to gold standard techniques, such as iohexol clearance, though it is important to note that yet the ideal GFR estimating equation for elderly patients has not been determined [26–28].
It has been suggested that given these limitations and potential to underestimate GFR in the elderly population, a lower GFR reference range of 45 mL/min/1.73 m2 be used in the absence of other signs of kidney damage given the multiple unique characteristics of the aging kidneys, as we will explore in this review [29]. In general, we are in agreement with this suggestion that all elderly patients with a creatinine based estimated GFR of < 45 mL/min/1.73 m2 can safely be assumed to have CKD, and it is our opinion that elderly patients with a GFR > 45 mL/min/1.73 m2 but less < 60 mL/min/1.73 m2, without other signs of structural of functional renal disease such as proteinuria, have additional evaluation for the presence of impaired renal function, including but not limited to the addition of cystatin C to estimate GFR.
Epidemiology of CKD in the Elderly
According to the Centers for Disease Control and Prevention (CDC), the number of elderly patients in the United States is expected to double in the next 25 years to 72 million patients, representing approximately 20% of the adult population by 2030 [30]. Analysis of the National Health and Nutrition Examination Surveys (NHANES) from 1999–2004 revealed an overall prevalence of CKD in the US population of 13.1%. However, when sub-grouped into patients greater than or equal to 70 years of age, the prevalence of CKD in this population increased to a staggering 47.5% [31]. Likewise, analysis of other elderly populations from Canada, China, Italy, and Spain indicated a roughly 3- to 7-fold increase in CKD prevalence in those elderly populations compared to younger patients [3]. Additionally, according to the United States Renal Data System there is evidence of a progressive rise in the number of end-stage renal disease (ESRD) patients enrolled in Medicare-funded programs over the past decades [32]. In extrapolating these estimates, it is conceivable to predict that approximately 30 million elderly patients may have CKD in the United States by year 2030, with enormous implications to treatment recommendations and healthcare associated costs.
The Aging Kidney and Expected Rate of Nephron Loss
A progressive, age-related decline in GFR has been demonstrated in many studies. In an earlier analysis of the Baltimore Longitudinal Study of Aging by Lindeman et al, a decline in measured creatinine clearance of 0.75 mL/min/year was demonstrated. It is important to note that in this analysis, patients with suspected pre-existing renal or urologic disease and those on diuretics or other antihypertensives were excluded from analysis, and a normal Gaussian distribution of creatinine clearance slopes versus time was demonstrated, suggesting the GFR loss was a process of normal aging [4].
In support of the theory of a physiologic age-related decline in renal function, a study by Rule et al analyzed potential kidney transplant donors for age-related decline in renal function and determined an approximately 6.3 mL/min/1.73 m2 decline in GFR for each decade. In this investigation, core needle biopsies were obtained at the time of donation and transplantation. The investigators found a progressive increase in the histologic prevalence of nephrosclerosis with each age group analyzed, increasing from 2.7% at ages 18 to 29 to 16% for ages 30 to 39, 28% for ages 40 to 49, 44% for ages 50 to 59, 58% for ages 60 to 69, and finally 73% in donors older than age 70. It is important to note that this study only examined live kidney donors, a group heavily screened and selected on the basis of optimal health, thus strongly arguing for progressive renal decline as a consequence of “normal” aging. Furthermore, though controlled hypertensive patients (treated with 2 or less medications) were allowed to be donors in this study, exclusion of this group had only a minimal impact on the findings of the study [33].
However, whether this age-related decline is purely a result of normal senescence or is a consequence of modifiable risk factors that could alter this outcome remains debatable. Additionally, vascular disease is clearly implicated in more accelerated renal decline. This concept was well demonstrated in an analysis of the longitudinal Age, Gene/Environment Susceptibility – Reykjavik Study, which showed that although age was associated with both reduced GFR and albuminuria, reduced GFR and albuminuria in elderly patients (mean age 80.8 yr) was strongly associated with midlife systolic and diastolic blood pressure, thus suggesting that potentially modifiable vascular pathology may play a much stronger role in CKD in the elderly than aging alone [34].
Finally, it has been hypothesized that reduced nephron mass at birth may contribute to CKD in the elderly [29]. Reduced nephron mass appears to be associated with low birth weight and prematurity, and this has been associated with an increased risk for ESRD later in life [35,36].
Hypertension in the Elderly
Pathophysiology
Age-associated hypertension is felt to arise from several mechanisms and hemodynamic changes. Systolic blood pressure has been noted to progressively rise with age, whereas diastolic blood pressure rises to the 5th or 6th decade, after which it appears to slowly decline. This pattern is felt likely secondary to increasing large vessel stiffness from collagen deposition and calcification with aging, and fracturing and degradation of elastin fibers. As large vessels become less distensible, pulse pressure and pulse wave velocity increases with this drop in diastolic BP, with less forward flow seen in diastole, leading to decreased organ perfusion. Additionally and alternatively, concentric left ventricular hypertrophy develops with aging, leading to reduced cardiac output from decreased stroke volume, which may also contribute to reduced organ perfusion [37,38]. These findings have led many to speculate that hypertension in the elderly may actually serve as a protective mechanism to maintain organ perfusion, and have led to great concern regarding excessive lowering of diastolic blood pressure and increasing of pulse pressure in this population with antihypertensive therapy. This theory was initially corroborated with a sub-analysis of the Systolic Hypertension in the Elderly Program (SHEP) where an increase in pulse pressure by 10 mm Hg was accompanied by increased risk of stroke and congestive heart failure in the treatment arm [39]. Nonetheless, the bulk of evidence continues to support a lower overall risk of cardiovascular events with treatment of hypertension in elderly patients, and general expert consensus recommends treatment with gradual reduction to normal levels of systolic blood pressure accompanied by careful monitoring for adverse effects [40,41].
In addition to these above changes, reduced GFR in elderly likewise results in impaired natriuresis, thereby fostering hypertension via volume expansion. Age-related arteriolosclerosis may result in renal artery stenosis, resulting in decreased renal perfusion and upregulation of the renin-angiotensin-aldosterone cascade. Further challenging treatment decisions is the frequent development of autonomic dysregulation in the elderly, a major risk factor for falls and cardiovascular events [40].
The result of these abnormalities is that roughly 65% of patients greater than the age of 60 have at least isolated systolic hypertension [42]. Similarly corresponding to the underlying physiology highlighted above, rising pulse pressure, rather than systolic or diastolic blood pressure, appears to be the greatest risk factor for cardiovascular events in the elderly population [43,44]. In an interesting analysis of the Framingham Heart Study by Franklin et al, the authors noted that in patients < 50 years of age, diastolic blood pressure was the strongest risk factor for events. However, at age 50 to 59, a change occurred where all 3 blood pressure indexes were comparable risk predictors, and then from age 60 years and on pulse pressure became the superior predictor, with diastolic blood pressure being negatively correlated to cardiovascular risk, highlighting the potential importance for organ perfusion during diastole in this group [45].
Likewise, in the elderly population pulse pressure also appears to be inversely related to GFR, suggesting that vascular stiffness and the reduced forward flow in diastole may contribute to microvascular damage and CKD [46]. In elderly patients with untreated isolated systolic hypertension, increasing systolic blood pressure (a reflection of rising pulse pressure) was associated with the greatest risk of renal decline when compared to diastolic blood pressure, pulse, and mean arterial pressure [47]. In the normal state, high renal blood flow and low renal arterial resistance can contribute to regular large intrarenal pressure variations. Because of vascular stiffness, these pressure variations increase with time, increasing up to 4-fold in the elderly compared with young peers, and likely contribute to renal damage seen in older patients [48].
Treatment
In comparison to the paucity of randomized trials examining CKD progression in the elderly, 4 very large, well designed randomized trials (SHEP, MRC trial, Syst-Eur trial, and HYVET) specifically examining the treatment of hypertension in the elderly have now been conducted [14–17] and confirmed earlier and smaller trials demonstrating the benefits of treatment of hypertension in the elderly [49,50]. In addition to this, several of the other large landmark hypertension trials such as ALLHAT, ACCOMPLISH, and the SPRINT trial included a considerable number of elderly patients [51–53]. Though the primary aim of those trials was not to determine the effects of hypertension treatment in the elderly per se, sub-analysis of this population in these trials has further added to our knowledge of this condition.
In the largest initial trial of hypertension in the elderly (SHEP), the researchers randomized 4376 patients over the age of 60 with an average blood pressure of 170/77 mm Hg into a treatment versus placebo arm. Such a study would be inconceivable today due to the consistent benefit derived from antihypertensive therapy now demonstrated in multiple trials. An achieved systolic blood pressure of 143 mm Hg in the treatment arm versus 155 mm Hg in the placebo arm was obtained. Stroke and nonfatal cardiac events were significantly reduced with treatment. The development of renal dysfunction occurred in 7 patients in the treatment arm and 11 patients in the placebo arm, a nonsignificant difference. As we have noted previously, however, patients with pre-existing kidney disease were excluded from the study [14]. A subsequent analysis of the SHEP trial results by Vaccarino et al, however, showed that in patients on treatment who developed an increase in pulse pressure of 10 mm Hg or more carried a 23% higher risk for developing heart failure and a 24% higher risk for stroke. This effect was not seen in the placebo arm [39].
Shortly following the publication of the SHEP results, the Medical Research Council trial of treatment of hypertension in older adults (MRC) further confirmed the initial findings by demonstrating a 25% reduction in stroke and a 17% reduction in all cardiac events in 4396 patients aged 65 to 74 with a systolic blood pressure greater than 160 mm Hg randomized to treatment of hypertension with either atenolol or a diuretic combination of amiloride and hydrochlorothiazide versus placebo. Like SHEP, however, patients with pre-existing renal disease were excluded, and no report of renal outcomes was published in the initial results [15]. Similarly, the Systolic Hypertension in Europe Trial (Syst-Eur) revealed a 42% reduction in stroke and a 26% reduction in all cardiac endpoints in 4695 patients with a systolic blood pressure of greater than 160 mm Hg randomized to receive nitrendipine with addition of enalapril and hydrochlorothiazide as required. However, CKD patients were likewise excluded in this trial [16].
Finally, the Hypertension in the Very Elderly Trial (HYVET) was unique in that it sought to enroll only patients greater than 80 years of age, a significant departure from the earlier hypertension in elderly trials. This trial randomized 3845 patients, again with a systolic blood pressure of 160 mm Hg or greater, to a placebo arm versus a treatment arm of the thiazide type diuretic indapamide, with addition of the ACE inhibitor perindopril if blood pressure was still greater than 150 mm Hg on monotherapy. Despite the older age of the participants in this trial, patients still benefited from blood pressure reduction with a 30% reduction in rate of stroke, a 21% reduction in the rate of death from any cause, and an impressive 64% reduction in the rate of heart failure [17]. These findings from HYVET, combined with the earlier SHEP, MRC and Syst-Eur trials, confirmed that treatment of hypertension in the elderly of any age should be attempted.
Recomendations for Managing Hypertension in the Elderly with CKD
Though a lack of data exists regarding the treatment of hypertension in elderly patients with the comorbidity of CKD, given the consistent and robust data that exists demonstrating a reduction in cardiovascular risk and mortality in the general elderly population without renal impairment, it is our opinion that elderly patients with CKD and hypertension should receive antihypertensive treatment. This opinion is supported by the fact that in the recently published SPRINT trial, 28.1% of patients in the standard treatment arm (targeting a blood pressure of less than 140 mm Hg), and 28.4% of patients in the intensive treatment arm (blood pressure target less than 120 mm Hg) had CKD, and similarly 28.2% of the trial participants in each group were greater than the age of 75. The percentage of patients with both CKD and age greater than 75 years was not reported in the initial trial results, though it is assumed a significant portion of these patients had both CKD and age greater than 75 years. It is nonetheless reassuring that patients with CKD in the SPRINT trial, as well as those with age > 75 years, both seemed to derive the same benefit in cardiovascular and mortality benefit in the intensive treatment arm compared to the standard treatment arm [53].
It should be noted, however, that though cardiovascular events and mortality were lower in the more intensive treatment arm of the SPRINT trial, CKD progression did not differ between the two treatment groups. Additionally, the risk of acute kidney injury was significantly greater in the intensive treatment arm when compared to the standard treatment arm, with 3.8% of patients in the intensive treatment arm suffering AKI compared to 2.3% in the standard arm [22]. Thus, it should be understood by both the clinician and the elderly patient with hypertension and CKD that the goal of more aggressively lowering blood pressure is to prevent cardiovascular events and not slow renal disease progression.
The recently published 2017 hypertension guidelines by the American College of Cardiology/American Heart Association is the most comprehensive set of hypertension treatment recommendations published to date and includes a section regarding patients with CKD as well as a section on the elderly [54]. Regarding CKD, the guidelines recommend a goal blood pressure of less than 130/80 mm Hg in patients with CKD, and that patients with macroalbuminuria (defined as a daily urine protein excretion of greater than 300 mg/dL or a urine albumin to creatinine ratio of 300 mg/g) be treated with and angiotensin-converting enzyme inhibitor (ACEI) or angiotensin receptor blocker (ARB). We feel these are reasonable recommendations for CKD targets and agree with the guideline, with the understanding that the target of 130/80 mm Hg is based largely on the SPRINT data. It is important to recognize that in the Action to Control Cardiovascular Risk in Diabetes trial (ACCORD), a more intensive blood pressure target of 120 mm Hg did not result in further improvement in cardiovascular events compared to a traditional target of 140/90 mm Hg [55]. However, given the larger and more robust sample size from SPRINT, we feel the target of 130/80 mm Hg is warranted and therefore should be the first target for elderly patients with CKD. With this goal in mind, it has been our clinical experience that some elderly patients with CKD have difficulty tolerating this goal, either from the development of worsening of GFR, acute kidney injury events, or due to orthostatic hypotension. Additionally, it should be noted that patients with orthostatic hypotension were excluded from SPRINT, though an increase in falls was not seen in the primary study. Therefore, for patients who are unable to tolerate the SPRINT goal of 130/80 mm Hg, an individualized goal of at least less than 160 mm Hg systolic and ideally less than 140 mm Hg, reflecting achieved blood pressure endpoints from earlier trials, may be a reasonable alternative [55]. The recent hypertension guidelines also recommend that for elderly adults with a high burden of comorbidities or limited life expectancy, “clinical judgement, patient preference, and at team-based approach to risk/benefit is reasonable for decisions regarding intensity of BP lowering and choice of antihypertensive drugs.” We agree that all treatment decisions must be individualized based upon each patient’s clinical scenario, and that a guideline is only a general aid for treatment decisions, not a mandate for care.
Therefore, with the acknowledgement that there is a lack of literature specifically examining blood pressure goals in elderly patients with CKD, it is our opinion based on available evidence that the following suggestions constitute a reasonable approach to this scenario: (1) a blood pressure target of less than 130/80 mm Hg should be sought as the primary blood pressure target; (2) if the patient cannot tolerate this due to rapidly declining GFR, acute kidney injury, orthostatic hypotension and or falls; or in other situations where this is not a practical a goal, individualized goal of at ideally less than 140 mm Hg, though at least less than 160 mm Hg systolic, could be considered; (3) the clinician should attempt careful and gradual reduction of blood pressure, with no more than one agent added or one escalation of medication dose attempted per visit; (4) the patient should have close follow up-after medication changes with an adjustment period of at least 4 weeks before additional medication or dose escalations are made; (5) if CKD is accompanied by albuminuria (daily urine protein excretion of greater than 300 mg/dL or a urine albumin to creatinine ratio of 300 mg/g) an ACEI or ARB should be used in management; (6) a rise in serum creatinine of up to 30% of baseline after addition of an ACEI may be acceptable; however, a rise greater than this amount should prompt discontinuation of the drug and evaluation for renal artery stenosis; (7) frequent monitoring of creatinine is required, with repeat chemistry performed after medication adjustments; (8) patients with a high pulse pressure should be monitored especially closely for symptoms or changes in renal function; and finally (9) individualized treatment and clinical judgement, with the patient being an informed participant, should take priority over all other recommendations and guidelines. We feel that further research in this growing subgroup of elderly patients is needed and will be sought, and we expect recommendations will continue to evolve as future literature becomes available.
Corresponding author: Jonathan G. Owen, MD, MSC04 2785, 1 University of New Mexico, Albuquerque, NM 87131, [email protected].
Financial disclosures: None.
Author contributions: conception and design, JGO; analysis and interpretation of data, KA, FXR, JGO; drafting of article, KA, FXR, JGO; critical revision of the article, KA, FXR, JGO; collection and assembly of data, KA, FXR, JGO.
1. Schaeffner ES, Ebert N, Delanaye P, et al. Two novel equations to estimate kidney function in persons aged 70 years or older. Ann Intern Med 2012;157:471–81.
2. Wetzels JF, Kiemeney LA, Swinkels DW, et al. Age- and gender-specific reference values of estimated GFR in Caucasians: the Nijmegen Biomedical Study. Kidney Int 2007;72:632–7.
3. Minutolo R, Borrelli S, De Nicola L. CKD in the elderly: kidney senescence or blood pressure-related nephropathy? Am J Kidney Dis 2015;66:184–6.
4. Lindeman RD, Tobin J, Shock NW. Longitudinal studies on the rate of decline in renal function with age. J Am Geriatr Soc 1985; 33:278–85.
5. Gill J, Malyk R, Djurdiev O, Levin A. Use of GFR equations to adjust drug doses in an elderly multi-ethnic group – a cautionary tale. Nephrol Dial Transplant 2007;22:2894–9.
6. Spruill WJ, Wade WE, Cobb HH 3rd. Comparison of estimated glomerular filtration rate with estimated creatinine clearance in the dosing of drugs requiring adjustments in elderly patients with declining renal function. Am J Geriatr Pharmacother 2008;6:153–60.
7. Dowling TC, Wang ES, Ferruci L, Sorkin JD. Glomerular filtration rate equations overestimate creatinine clearance in older individuals enrolled in the Baltimore Longitudinal Study on Aging: impact on renal drug dosing. Pharmacotherapy 2013;33:912–21.
8. Ballew SH, Chen Y, Daya NR, Godino JG, Windham BG, McAdams-DeMarco M, Coresh J, Selvin E, Grams ME. Frailty, kidney function, and polypharmacy: the atherosclerosis risk in communities (ARIC) study. Am J Kidney Dis 2017;69:228–36.
9. Grams ME, Sang Y, Ballew SH, et al; CKD Prognosis Consortium. A meta-analysis of the association of estimated GFR, albuminuria, race, and sex with acute kidney injury. Am J Kidney Dis 2015;66:591–601.
10. Gansevoort RT, Matsushita K, van der Velde M, et al; Chronic Kidney Disease Prognosis Consortium. Lower estimated GFR and higher albuminuria are associated with adverse kidney outcomes. A collaborative meta-analysis of general and high-risk population cohorts. Kidney Int 2011;80:93–104.
11. Masson P, Webster AC, Hong M, et al. Chronic kidney disease and the risk of stroke: a systematic review and meta-analysis. Nephrol Dial Transplant 2015;30:1162–9.
12. Tellez-Plaza M, Orozco-Beltran D, Gil-Guillen V, et al; ESCARVAL STUDY GROUP. Renal function and attributable risk of death and cardiovascular hospitalization in patients with cardiovascular risk factors from a registry-based cohort: the Estudio Cardiovascular Valencia-risk study. J Hypertens 2016;34:2266–73.
13. Smink PA, Lambers-Heerspink HJ, Gansevoort RT, et al. Albuminuria, estimated GFR, traditional risk factors, and incident cardiovascular disease: the PREVEND (Prevention of Renal and Vascular Endstage Disease) study. Am J Kidney Dis 2012;60:804–11.
14. SHEP Cooperative Research Group. Prevention of stroke by antihypertensive drug treatment in older persons with isolated systolic hypertension. Final results of the systolic hypertension in the elderly program (SHEP). JAMA 1991;265:3255–64.
15. Working Party. Medical Research Council trial of treatment of hypertension in older adults: principal results. BMJ 1992;304:405–12.
16. Staessen JA, Faggard R, Thijs L, et al. Randomised double-blind comparison of placebo and active treatment for older patients with isolated systolic hypertension. Lancet 1997;350:757–64.
17. Beckett NS, Peters R, Fletcher AE, et al; HYVET Study Group. Treatment of hypertension in patients 80 years of age or older. N Engl J Med 2008;358:1887–98.
18. Fesler P, Safar ME, du Cailar G, et al. Pulse pressure is an independent determinant of renal function decline during treatment of essential hypertension. J Hypertens 2007;25:1915–20.
19. Weir MR, Dworkin LD. Antihypertensive drugs, dietary salt, and renal protection: how low should you go and with which therapy? Am J Kidney Dis 1998;32:1–22.
20. Obi Y, Kalantar-Zadeh K, Shintani A, et al. Estimated glomerular filtration rate and the risk-benefit profile of intensive blood pressure control amongst nondiabetic patients: a post hoc analysis of a randomized clinical trial. J Intern Med 2017;283:314–27.
21. Peralta CA, McClure LA, Scherzer R, et al. Effect of intensive versus usual blood pressure control on kidney function among individuals with prior lacunar stroke: a post hoc analysis of the secondary prevention of small subcortical strokes (SPS3) randomized trial. Circulation 2016;133:584–91.
22. Rocco MV, Sink KM, Lovato LC, et al; SPRINT Research Group. Effects of intensive blood pressure treatment on acute kidney injury events in the systolic blood pressure intervention trial (SPRINT). Am J Kidney Dis 2018;71:352–61.
23. Chapter 1: Definition and classification of CKD. Kidney Int Suppl (2011) 2013;3:19–62.
24. Rule AD, Larson TS, Bergstralh EJ, et al. Using serum creatinine to estimate glomerular filtration rate: accuracy in good health and in chronic kidney disease. Ann Intern Med 2004;141:929–37.
25. Stevens LA, Schmid CH, Green T, et al. Factors other than glomerular filtration rate affect serum cystatin C levels. Kidney Int 2009;75:652–60.
26. Biork J, Grubb A, Gudnason V, et al. Comparison of glomerular filtration rate estimating equations derived from creatinine and cystatin C: validation in the age, gene/environment susceptibility-Reykjavik elderly cohort. Nephrol Dial Transplant 2017.
27. Bevc S, Hojs N, Hois R, et al. Estimation of glomerular filtration rate in elderly chronic kidney disease patients: comparison of three novel sophisticated equations and simple cystatin C equation. Therapeutic Apheresis and Dialysis 2017;21:126–32.
28. Pottel H, Delanaye P, Schaeffner E, et al. Estimating glomerular filtration rate for the full age spectrum from serum creatinine and cystatin C. Nephrol Dial Transplant 2017;32:497–507.
29. Denic A, Glassock RJ, Rule AD. Structural and functional changes with the aging kidney. Adv Chronic Kidney Dis 2016;23:19–28.
30. US Department of Health and Human Services, Centers for Disease Control and Prevention. The state of aging and health in America 2013. Centers for Disease Control and Prevention website. https://www.cdc.gov/aging/pdf/state-aging-health-in-america-2013.pdf. Published 2013. Accessed April 5, 2018.
31. Coresh J, Selvin E, Stevens LA, et al. Prevalence of chronic kidney disease in the United States. JAMA 2007;298:2038–47.
32. United Stated Renal Data System. USRD 2013 Annual Data Report: Atlas of Chronic Kidney Disease and End-Stage Renal Disease in the United States. United States Renal Data System website. https://www.usrds.org/atlas13.aspx . Published 2013. Accessed April 5, 2018.
33. Rule AD, Amer H, Cornell LD, et al. The association between age and nephroclerosis on renal biopsy among healthy adults. Ann Intern Med 2010;152:561–7.
34. Inker LA, Okparavero A, Tighiouart H, et al. Midlife blood pressure and late-life GFR and albuminuria: an elderly general population cohort. Am J Kidney Dis 2015;66:240–8.
35. Vikse BE, Irgens LM, Leivestad T, et al. Low birth weight increases risk for end-stage renal disease. J Am Soc Nephrol 2008;19:151–7.
36. Luyckx VA, Bertram JF, Brenner BM, et al. Effect of fetal and child health on kidney development and long-term risk of hypertension and kidney disease. Lancet 2013;382:273–83.
37. Franklin SS, Gustin W 4th, Wong ND, et al. Hemodynamic patterns of age-related changes in blood pressure. The Framingham Heart Study. Circulation 1997;96:308–15.
38. Messerli FH, Sundgaard-Riise K, Ventura HO, et al. Essential hypertension in the elderly: haemodynamics, intravascular volume, plasma renin activity, and circulating catecholamine levels. Lancet 1983;2:983–6.
39. Vaccarino V, Berger AK, Abramson J, et al. Pulse pressure and risk of cardiovascular events in the systolic hypertension in the elderly program. Am J Cardiol 2001;88:980–6.
40. Aronow WS, Harrington RA, Fleg JL, Pepine CJ, Artinian NT, Bakris G, Brown AS, Ferdinand KC, Forciea MA, Frishman WH, Jaigobin C, Kostis JB, Mancia G, Oparil S, Ortiz E, Reisin E, Rich MW, Schocken DD, Weber MA, Wesley DJ. ACCF/AHA 2011 expert consensus document on hypertension in the elderly: a report of the American College of Cardiology Foundation task force on clinical expert consensus documents. Circulation 2011;123:2434–506.
41. Chaudhry SI, Krumholz HM, Foody JM. Systolic hypertension in older persons. JAMA 2004;292:1074–80.
42. Burt VL, Whelton P, Roccella EJ, et al. Prevalence of hypertension in the US adult population. Results from the Third National Health and Nutrition Examination Survey, 1988-1991. Hypertension 1995;25: 305–13.
43. Blacher J, Staessen JA, Girerd X, et al. Pulse pressure not mean pressure determines cardiovascular risk in older hypertensive patients. Arch Intern Med 2000;160:1085–90.
44. Franklin SS, Lopez VA, Wong ND, et al. Single versus combined blood pressure components and risk for cardiovascular disease: the Framingham Heart Study. Circulation 2009;119:243–50.
45. Franklin SS, Larson MG, Khan SA, et al. Does the relation of blood pressure to coronary heart disease risk change with aging? The Framingham Heart Study. Circulation 2001;103:1245–9.
46. Verhave JC, Fesler P, du Cailar G, et al. Elevated pulse pressure is associated with low renal function in elderly patients with isolated systolic hypertension. Hypertension 2005;45:586–91.
47. Young JH, Klaq MJ, Muntner P, et al. Blood pressure and decline in kidney function: findings from the systolic hypertension in elderly program (SHEP). J Am Soc Nephrol 2002;13:2776–82.
48. O’Rourke MF, Safar ME. Relationship between aortic stiffening and microvascular disease in brain and kidney: cause and logic of therapy. Hypertension 2005;46:200–4.
49. Coope J, Warrender TS. Randomised trial of treatment of hypertension in elderly patients in primary care. Br Med J (Clin Res Ed) 1986;293:1145–8.
50. Dahlof B, Lindholm LH, Hansson L, et al. Morbidity and mortality in the Swedish trial in old patients with hypertension (STOP-Hypertension). Lancet 1991;338:1281–5.
51. ALLHAT Officers and Coordinators for the ALLHAT Collaborative Research Group; The Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial. Major outcomes in high-risk hypertensive patients randomized to angiotensin-converting enzyme inhibitor or calcium channel blocker vs diuretic: the antihypertensive and lipid-lowering treatment to prevent heart attack trial (ALLHAT). JAMA 2002;288:2981–97.
52. Jamerson K, Weber MA, Bakris GL, et al; ACCOMPLISH Trial Investigators. Benazapril plus amlodipine or hydrochlorothiazide for hypertension in high-risk patients. N Engl J Med 2008;359:2417–28.
53. SPRINT Research Group; Wright JT Jr, Williamson JD, Whelton PK, et al. A randomized trial of intensive versus standard blood-pressure control. N Engl J Med 2015;373: 2103–16.
54. Whelton PK, Carey RM, Aronow WS, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: a report of the American College of Cardiology/American Heart Association task force on clinical practice guidelines. Hypertension 2017.
55. ACCORD Study Group; Cushman WC, Evans GW, Byington RP, et al. Effects of intensive blood-pressure control in type 2 diabetes mellitus. N Engl J Med 2010;362:1575–85.
1. Schaeffner ES, Ebert N, Delanaye P, et al. Two novel equations to estimate kidney function in persons aged 70 years or older. Ann Intern Med 2012;157:471–81.
2. Wetzels JF, Kiemeney LA, Swinkels DW, et al. Age- and gender-specific reference values of estimated GFR in Caucasians: the Nijmegen Biomedical Study. Kidney Int 2007;72:632–7.
3. Minutolo R, Borrelli S, De Nicola L. CKD in the elderly: kidney senescence or blood pressure-related nephropathy? Am J Kidney Dis 2015;66:184–6.
4. Lindeman RD, Tobin J, Shock NW. Longitudinal studies on the rate of decline in renal function with age. J Am Geriatr Soc 1985; 33:278–85.
5. Gill J, Malyk R, Djurdiev O, Levin A. Use of GFR equations to adjust drug doses in an elderly multi-ethnic group – a cautionary tale. Nephrol Dial Transplant 2007;22:2894–9.
6. Spruill WJ, Wade WE, Cobb HH 3rd. Comparison of estimated glomerular filtration rate with estimated creatinine clearance in the dosing of drugs requiring adjustments in elderly patients with declining renal function. Am J Geriatr Pharmacother 2008;6:153–60.
7. Dowling TC, Wang ES, Ferruci L, Sorkin JD. Glomerular filtration rate equations overestimate creatinine clearance in older individuals enrolled in the Baltimore Longitudinal Study on Aging: impact on renal drug dosing. Pharmacotherapy 2013;33:912–21.
8. Ballew SH, Chen Y, Daya NR, Godino JG, Windham BG, McAdams-DeMarco M, Coresh J, Selvin E, Grams ME. Frailty, kidney function, and polypharmacy: the atherosclerosis risk in communities (ARIC) study. Am J Kidney Dis 2017;69:228–36.
9. Grams ME, Sang Y, Ballew SH, et al; CKD Prognosis Consortium. A meta-analysis of the association of estimated GFR, albuminuria, race, and sex with acute kidney injury. Am J Kidney Dis 2015;66:591–601.
10. Gansevoort RT, Matsushita K, van der Velde M, et al; Chronic Kidney Disease Prognosis Consortium. Lower estimated GFR and higher albuminuria are associated with adverse kidney outcomes. A collaborative meta-analysis of general and high-risk population cohorts. Kidney Int 2011;80:93–104.
11. Masson P, Webster AC, Hong M, et al. Chronic kidney disease and the risk of stroke: a systematic review and meta-analysis. Nephrol Dial Transplant 2015;30:1162–9.
12. Tellez-Plaza M, Orozco-Beltran D, Gil-Guillen V, et al; ESCARVAL STUDY GROUP. Renal function and attributable risk of death and cardiovascular hospitalization in patients with cardiovascular risk factors from a registry-based cohort: the Estudio Cardiovascular Valencia-risk study. J Hypertens 2016;34:2266–73.
13. Smink PA, Lambers-Heerspink HJ, Gansevoort RT, et al. Albuminuria, estimated GFR, traditional risk factors, and incident cardiovascular disease: the PREVEND (Prevention of Renal and Vascular Endstage Disease) study. Am J Kidney Dis 2012;60:804–11.
14. SHEP Cooperative Research Group. Prevention of stroke by antihypertensive drug treatment in older persons with isolated systolic hypertension. Final results of the systolic hypertension in the elderly program (SHEP). JAMA 1991;265:3255–64.
15. Working Party. Medical Research Council trial of treatment of hypertension in older adults: principal results. BMJ 1992;304:405–12.
16. Staessen JA, Faggard R, Thijs L, et al. Randomised double-blind comparison of placebo and active treatment for older patients with isolated systolic hypertension. Lancet 1997;350:757–64.
17. Beckett NS, Peters R, Fletcher AE, et al; HYVET Study Group. Treatment of hypertension in patients 80 years of age or older. N Engl J Med 2008;358:1887–98.
18. Fesler P, Safar ME, du Cailar G, et al. Pulse pressure is an independent determinant of renal function decline during treatment of essential hypertension. J Hypertens 2007;25:1915–20.
19. Weir MR, Dworkin LD. Antihypertensive drugs, dietary salt, and renal protection: how low should you go and with which therapy? Am J Kidney Dis 1998;32:1–22.
20. Obi Y, Kalantar-Zadeh K, Shintani A, et al. Estimated glomerular filtration rate and the risk-benefit profile of intensive blood pressure control amongst nondiabetic patients: a post hoc analysis of a randomized clinical trial. J Intern Med 2017;283:314–27.
21. Peralta CA, McClure LA, Scherzer R, et al. Effect of intensive versus usual blood pressure control on kidney function among individuals with prior lacunar stroke: a post hoc analysis of the secondary prevention of small subcortical strokes (SPS3) randomized trial. Circulation 2016;133:584–91.
22. Rocco MV, Sink KM, Lovato LC, et al; SPRINT Research Group. Effects of intensive blood pressure treatment on acute kidney injury events in the systolic blood pressure intervention trial (SPRINT). Am J Kidney Dis 2018;71:352–61.
23. Chapter 1: Definition and classification of CKD. Kidney Int Suppl (2011) 2013;3:19–62.
24. Rule AD, Larson TS, Bergstralh EJ, et al. Using serum creatinine to estimate glomerular filtration rate: accuracy in good health and in chronic kidney disease. Ann Intern Med 2004;141:929–37.
25. Stevens LA, Schmid CH, Green T, et al. Factors other than glomerular filtration rate affect serum cystatin C levels. Kidney Int 2009;75:652–60.
26. Biork J, Grubb A, Gudnason V, et al. Comparison of glomerular filtration rate estimating equations derived from creatinine and cystatin C: validation in the age, gene/environment susceptibility-Reykjavik elderly cohort. Nephrol Dial Transplant 2017.
27. Bevc S, Hojs N, Hois R, et al. Estimation of glomerular filtration rate in elderly chronic kidney disease patients: comparison of three novel sophisticated equations and simple cystatin C equation. Therapeutic Apheresis and Dialysis 2017;21:126–32.
28. Pottel H, Delanaye P, Schaeffner E, et al. Estimating glomerular filtration rate for the full age spectrum from serum creatinine and cystatin C. Nephrol Dial Transplant 2017;32:497–507.
29. Denic A, Glassock RJ, Rule AD. Structural and functional changes with the aging kidney. Adv Chronic Kidney Dis 2016;23:19–28.
30. US Department of Health and Human Services, Centers for Disease Control and Prevention. The state of aging and health in America 2013. Centers for Disease Control and Prevention website. https://www.cdc.gov/aging/pdf/state-aging-health-in-america-2013.pdf. Published 2013. Accessed April 5, 2018.
31. Coresh J, Selvin E, Stevens LA, et al. Prevalence of chronic kidney disease in the United States. JAMA 2007;298:2038–47.
32. United Stated Renal Data System. USRD 2013 Annual Data Report: Atlas of Chronic Kidney Disease and End-Stage Renal Disease in the United States. United States Renal Data System website. https://www.usrds.org/atlas13.aspx . Published 2013. Accessed April 5, 2018.
33. Rule AD, Amer H, Cornell LD, et al. The association between age and nephroclerosis on renal biopsy among healthy adults. Ann Intern Med 2010;152:561–7.
34. Inker LA, Okparavero A, Tighiouart H, et al. Midlife blood pressure and late-life GFR and albuminuria: an elderly general population cohort. Am J Kidney Dis 2015;66:240–8.
35. Vikse BE, Irgens LM, Leivestad T, et al. Low birth weight increases risk for end-stage renal disease. J Am Soc Nephrol 2008;19:151–7.
36. Luyckx VA, Bertram JF, Brenner BM, et al. Effect of fetal and child health on kidney development and long-term risk of hypertension and kidney disease. Lancet 2013;382:273–83.
37. Franklin SS, Gustin W 4th, Wong ND, et al. Hemodynamic patterns of age-related changes in blood pressure. The Framingham Heart Study. Circulation 1997;96:308–15.
38. Messerli FH, Sundgaard-Riise K, Ventura HO, et al. Essential hypertension in the elderly: haemodynamics, intravascular volume, plasma renin activity, and circulating catecholamine levels. Lancet 1983;2:983–6.
39. Vaccarino V, Berger AK, Abramson J, et al. Pulse pressure and risk of cardiovascular events in the systolic hypertension in the elderly program. Am J Cardiol 2001;88:980–6.
40. Aronow WS, Harrington RA, Fleg JL, Pepine CJ, Artinian NT, Bakris G, Brown AS, Ferdinand KC, Forciea MA, Frishman WH, Jaigobin C, Kostis JB, Mancia G, Oparil S, Ortiz E, Reisin E, Rich MW, Schocken DD, Weber MA, Wesley DJ. ACCF/AHA 2011 expert consensus document on hypertension in the elderly: a report of the American College of Cardiology Foundation task force on clinical expert consensus documents. Circulation 2011;123:2434–506.
41. Chaudhry SI, Krumholz HM, Foody JM. Systolic hypertension in older persons. JAMA 2004;292:1074–80.
42. Burt VL, Whelton P, Roccella EJ, et al. Prevalence of hypertension in the US adult population. Results from the Third National Health and Nutrition Examination Survey, 1988-1991. Hypertension 1995;25: 305–13.
43. Blacher J, Staessen JA, Girerd X, et al. Pulse pressure not mean pressure determines cardiovascular risk in older hypertensive patients. Arch Intern Med 2000;160:1085–90.
44. Franklin SS, Lopez VA, Wong ND, et al. Single versus combined blood pressure components and risk for cardiovascular disease: the Framingham Heart Study. Circulation 2009;119:243–50.
45. Franklin SS, Larson MG, Khan SA, et al. Does the relation of blood pressure to coronary heart disease risk change with aging? The Framingham Heart Study. Circulation 2001;103:1245–9.
46. Verhave JC, Fesler P, du Cailar G, et al. Elevated pulse pressure is associated with low renal function in elderly patients with isolated systolic hypertension. Hypertension 2005;45:586–91.
47. Young JH, Klaq MJ, Muntner P, et al. Blood pressure and decline in kidney function: findings from the systolic hypertension in elderly program (SHEP). J Am Soc Nephrol 2002;13:2776–82.
48. O’Rourke MF, Safar ME. Relationship between aortic stiffening and microvascular disease in brain and kidney: cause and logic of therapy. Hypertension 2005;46:200–4.
49. Coope J, Warrender TS. Randomised trial of treatment of hypertension in elderly patients in primary care. Br Med J (Clin Res Ed) 1986;293:1145–8.
50. Dahlof B, Lindholm LH, Hansson L, et al. Morbidity and mortality in the Swedish trial in old patients with hypertension (STOP-Hypertension). Lancet 1991;338:1281–5.
51. ALLHAT Officers and Coordinators for the ALLHAT Collaborative Research Group; The Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial. Major outcomes in high-risk hypertensive patients randomized to angiotensin-converting enzyme inhibitor or calcium channel blocker vs diuretic: the antihypertensive and lipid-lowering treatment to prevent heart attack trial (ALLHAT). JAMA 2002;288:2981–97.
52. Jamerson K, Weber MA, Bakris GL, et al; ACCOMPLISH Trial Investigators. Benazapril plus amlodipine or hydrochlorothiazide for hypertension in high-risk patients. N Engl J Med 2008;359:2417–28.
53. SPRINT Research Group; Wright JT Jr, Williamson JD, Whelton PK, et al. A randomized trial of intensive versus standard blood-pressure control. N Engl J Med 2015;373: 2103–16.
54. Whelton PK, Carey RM, Aronow WS, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: a report of the American College of Cardiology/American Heart Association task force on clinical practice guidelines. Hypertension 2017.
55. ACCORD Study Group; Cushman WC, Evans GW, Byington RP, et al. Effects of intensive blood-pressure control in type 2 diabetes mellitus. N Engl J Med 2010;362:1575–85.
Current Concepts in Clinical Research: Anterior Cruciate Ligament Outcome Instruments
ABSTRACT
Outcome instruments have become an essential part of the evaluation of functional recovery after anterior cruciate ligament (ACL) reconstruction. Although the clinical examination provides important objective information to assess graft integrity, stability, range of motion, and strength, these measurements do not take the patient’s perception into account. There are many knee outcome instruments, and it is challenging for surgeons to understand how to interpret clinical research and utilize these measures in a practical way. The purpose of this review is to provide an overview of the most commonly used outcome measures in patients undergoing ACL reconstruction and to examine and compare the psychometric performance (validity, reliability, responsiveness) of these measurement tools.
Anterior cruciate ligament (ACL) reconstruction is one of the most common elective orthopedic procedures.1 Despite advances in surgical techniques, ACL reconstruction is associated with a lengthy recovery time, decreased performance, and increased rate of reinjury.2 Patients undergoing ACL reconstruction are often active individuals who participate in demanding activities, and accurate assessment of their recovery helps to guide recovery counseling. In addition to objective clinical outcomes measured through physical examination, patient-reported outcome (PRO) instruments add the patient’s perspective, information critical in determining a successful outcome. A variety of outcome instruments have been used and validated for patients with ACL tears. It is important for orthopedic surgeons to know the advantages and disadvantages of each outcome tool in order to interpret clinical studies and assess postoperative patients.
Over the last 10 years, there has been an increase in the number of knee instruments and rating scales designed to measure PROs, with >54 scores designed for the ACL-deficient knee.3 No standardized instrument is currently universally accepted as superior following ACL reconstruction across the spectrum of patient populations. Clinicians and researchers must carefully consider an outcome instrument’s utility based on specific patient populations in which it has been evaluated. Appropriate selection of outcome measures is of fundamental importance for adequate demonstration of the efficacy and value of treatment interventions, especially in an era of healthcare reform with a focus on providing high-quality and cost-effective care.
The purpose of this review is to highlight current tools used to measure outcomes after ACL reconstruction. Current outcome measures vary widely in regards to their validity, reliability, minimal clinically important difference, and applicability to specific patient populations. We have thus identified the measures most commonly used today in studies and clinical follow-up after ACL reconstruction and their various advantages and limitations. This information may enhance the orthopedic surgeon’s understanding of what outcome measures may be utilized in clinical studies.
Continue to: Patient-Reported Outcome Instruments...
PATIENT-REPORTED OUTCOME INSTRUMENTS
Recently, there has been a transition to increased use of PRO instruments rather than clinician-based postoperative assessment, largely due to the increasing emphasis on patient satisfaction in determining the value of an orthopedic intervention.4 PRO instruments are widely used to capture the patient’s perception of general health, quality of life (QOL), daily function, and pain. PRO instruments offer the benefit of allowing patients to subjectively assess their knee function during daily living and sports activities, conveying to the provider the impact of ACL reconstruction on physical, psychological, and social aspects of everyday activities. Furthermore, patient satisfaction has been shown to closely follow outcome scores related to symptoms and function.5 A multitude of specific knee-related PRO instruments have been developed and validated to measure outcomes after ACL reconstruction for both research and clinical purposes (Table).
Table. ACL Outcome Measures
|
|
|
|
| ||
Outcome Measure | Condition/Intervention | Measures | Internal Consistency (Cronbach’s a) | Test-Retest Reliability | Minimal Clinically Important Difference | Ref |
AAOS Sports Knee Scale | Many Knee | Stiffness, swelling, pain/function, locking/catching, giving way, limitation of activity, pain with activity | 0.86-0.95 | 0.68-0.96 | Unknown | 59, 60 |
ACL-QOL | Chronic ACL deficiency | Physical complaints, work, recreation and sports competition, lifestyle, social and emotional functioning | 0.93-0.98 | 6% average error | Unknown | 35, 36 |
Cincinnati Knee Rating System | ACL | Symptoms, daily and sports activities, physical examination, stability, radiographs, functional testing |
| 0.80-0.97 | 14 points (6 months), 26 points (12 months) | 39, 40, 47, 52 |
IKDC (Subjective Knee Form) | ACL | Symptoms, function, sports activity | 0.92 | 0.91-0.93 | 11.5 points; 6.3 at 6 months, 16.7 at 12 months | 48, 52, 54 |
KOOS | ACL | Pain, symptoms, activities of daily living, sport/recreation, knee-related quality of life | 0.71-0.95 | 0.75-0.93 | 8-10 points | 17 |
Lysholm | ACL | Pain, instability, locking, squatting, limp, support, swelling, stair-climbing | 0.72 | 0.94 | 8.9 | 46, 47, 55 |
Marx | Healthy patients | Activity level | 0.87 | 0.97 | Unknown | 42, 56, 57 |
Tegner | ACL | Activity level | 0.81 | 0.82 | 1 | 55, 56 |
PROMIS | Many lower extremity orthopedic conditions | Lower extremity function, central body function, activities of daily living | 0.98 | 0.96-0.99 |
| 30, 31 |
WOMAC | Hip/knee OA | Physical function, pain, stiffness | 0.81-0.95 | 0.80-0.92 | 12% baseline score or 6% max score; 9-12 points | 13, 14 |
Abbreviations: AAOS, American Academy of Orthopaedic Surgeons; ACL, anterior cruciate ligament; ACL-QOL, anterior cruciate ligament quality of life score; CAT, computer-adapting testing; IKDC, International Knee Documentation Committee; KOOS, Knee Injury and Osteoarthritis Outcome Score; OA, osteoarthritis; PF, physical function; PROMIS, Patient-Reported Outcome Measurement Information System; Ref, references; WOMAC, Western Ontario and McMaster Universities Osteoarthritis Index.
MEASUREMENT PROPERTIES
In general, clinicians and investigators should use health-related outcome measures with established reliability, validity, patient relevance, and responsiveness for assessing the specific condition.6
Reliability refers to the degree to which a measurement score is free from random error, reflecting how consistent or reproducible the instrument is when administered under the same testing conditions. Internal consistency, test-retest reliability, and measurement error are measures of reliability. Internal consistency is tested after a single administration and assesses how well items within a scale measure a single underlying dimension, represented using item-total correlation coefficients and Cronbach’s alpha. A Cronbach’s alpha of 0.70 to 0.95 is generally defined as good.7 Test-retest reliability is designed to appraise variation over time in stable patients and is represented using the intraclass correlation coefficient (ICC).8 An ICC >0.7 is considered acceptable; >0.8, good; and >0.9, excellent.9 An aspect of accuracy is whether the scoring system measures the full range of the disease or complaints. The incidence of minimum (floor) and maximum (ceiling) scores can be calculated for outcome scores. An instrument with low floor and ceiling effects, below 10% to 15%, is more inconclusive and can be more reliably used to measure patients at the high and low end of the scoring system.10
Validity is the ability of an outcome instrument to measure what it is intended to measure. Establishing validity is complex and requires evaluation of several facets, including content validity, construct validity, and criterion validity. Content validity is a relatively subjective judgment explaining the ability of an instrument to assess the critical features of the problem. Construct validity evaluates whether the questionnaire measures what it intends to measure, and is often assessed by correlating scores form one instrument to those from other proven instruments that are already accepted as valid. Finally, criterion validity assesses the correlation between the score and a previously established “gold standard” instrument.
Responsiveness is the ability of the instrument to detect a change or identify improvement or worsening of a clinical condition over time. Most frequently, the effect size (observed change/standard deviation of baseline scores) and standardized response mean (observed change/standard deviation of change) are used as measures of responsiveness. The minimal clinically important difference of an outcome measure is the smallest change in an outcome score that corresponds to a change in patient condition.
Continue to: ACL Outcome Instruments...
ACL OUTCOME INSTRUMENTS
WESTERN ONTARIO AND MCMASTER UNIVERSITIES OSTEOARTHRITIS INDEX (WOMAC LK 3.0)
The Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC LK 3.0) was developed in 1982 and is a widely used, disease-specific instrument recommended for the evaluation of treatment effects in patients with hip and knee osteoarthritis.11 Available in more than 80 languages, it is a self-administered, generic health status questionnaire developed to assess pain, function, and stiffness in daily living, taking respondents between 3 to 7.5 minutes for completion.12 Using visual analog scales, the 24 items probe the 3 subscales: pain (5 items), stiffness (2 items), and functional difficulty (17 items). Scores are calculated for each dimension, and the total score is normalized to a 100-point scale, with 0 indicating severe symptoms and 100 indicating no symptoms and higher function. The WOMAC score can also be calculated from the Knee Injury and Osteoarthritis Outcome Score (KOOS). The WOMAC questionnaire is well recognized for its good validity, reliability, and responsiveness, and is the most commonly used outcome measure for osteoarthritis.13-15 Considering its focus on older patients with osteoarthritis, it may not be appropriate for use in a young and active population.
KNEE INJURY AND OSTEOARTHRITIS OUTCOME SCORE (KOOS)
The KOOS is a knee-specific questionnaire developed as an extension of the WOMAC to evaluate the functional status and QOL of patients with any type of knee injury who are at an increased risk of developing osteoarthritis.16 The patient-based questionnaire is available in over 30 languages and covers both the short- and long-term consequences of an injury of the knee causing traumatic damage to cartilage, ligaments, and menisci. The KOOS is 42 items graded on a 5-point Likert scale, covering 5 subscales: pain (9 items), symptoms (7 items), function in activities of daily living (17 items), function in sports/recreation (5 items), and knee-related QOL (4 items). The questionnaire is self-administered and takes about 10 minutes to complete. Scores are calculated for each dimension, and the total score is transformed to a 0 to 100 scale, with 0 representing severe knee problems and 100 representing no knee problems and better outcome. An advantage of the KOOS is that it evaluates both knee injuries and osteoarthritis; therefore, it is arguably more suitable for evaluating patients over the long-term. The KOOS has been validated for several orthopedic interventions, including ACL reconstruction and rehabilitation16,17 as well as meniscectomy18 and total knee replacement.19 Population-based reference data for the adult population according to age and gender have also been established.20 The KOOS is increasingly utilized in clinical studies on ACL reconstruction.21-25 The questions of the WOMAC were retained so that a WOMAC score might be calculated separately and compared with the KOOS score.26
PATIENT-REPORTED OUTCOMES INFORMATION SYSTEM (PROMIS)
Since 2004, The National Institutes of Health (NIH) has funded the development of the Patient-Reported Outcome Measurement Information System (PROMIS), a set of flexible tools that reliably and validly measure PROs. The PROMIS consists of a library of question banks that has been developed and operated by a network of National Institutes of Health-funded research sites and coordinating centers and covers many different health domains including pain, fatigue, anxiety, depression, social functioning, physical functioning, and sleep. PROMIS items are developed using Item Response Theory (IRT), wherein the answer to any individual item has a known mathematical probability of predicting the test taker’s overall measurement of the specific trait being tested. This is commonly administered using computer-adaptive testing (CAT), which presents to the test taker an initial item, scores the response to that item, and from the response then presents the most informative second item, and so forth until a predefined level of precision is reached. Because the items are individually validated, they can be used alone or in any combination, a feature that distinguishes the PROMIS from traditional fixed-length PRO instruments that require the completion of an instrument in its entirety to be valid.27 In recent years, orthopedic research has been published with PROMIS physical function (PF) scores as primary outcome measures.28-30 The PF item bank includes 124 items measuring upper extremity, lower extremity, central and instrumental activities of daily living. PF can be completed as a short form (SF) with a set number of questions or utilizing CAT and evaluates self-reported function and physical activity. An advantage is its ease of use and potential to minimize test burden with very few questions, often as little as 4 items, as compared to other traditional PROMs.31
Previously published work has demonstrated that, in patients undergoing meniscal surgery, the PROMIS PF CAT maintains construct validity and correlates well with currently used knee outcome instruments, including KOOS.28 Work by the same group looking at the performance of the PROMIS PF CAT in patients indicated for ACL reconstruction shows that the PROMIS PF CAT correlates well with other PRO instruments for patients with ACL injuries, (SF-36 PF [r = 0.82, P < 0.01], KOOS Sport [r = 0.70, P < 0.01], KOOS ADL [r = 0.74, P < 0.01]), does not have floor or ceiling effects in this relatively young and healthy population, and has a low test burden.32,33 Papuga and colleagues33 also compared the International Knee Documentation Committee (IKDC) and PROMIS PF CAT on 106 subjects after ACL reconstruction and found good correlation.
Continue to: Quality of Life Outcome Measure...
QUALITY OF LIFE OUTCOME MEASURE FOR ACL DEFICIENCY (ACL-QOL)
The ACL-QOL Score was developed in 1998 as a disease-specific measure for patients with chronic ACL deficiency.34 This scale consists of 32 separate items in 31 visual analog questions regarding symptoms and physical complaints, work-related concerns, recreational activities and sport participation or competition, lifestyle, and social and emotional health status relating to the knee. The raw score is transformed into a 0- to 100-point scale, with higher scores indicating a better outcome. The scale is valid, reliable, and responsive for patients with ACL insufficiency,35,36 and is not applicable to other disorders of the knee. We recommend the ACL-QOL questionnaire be used in conjunction with other currently available objective and functional outcome measures.
CINCINNATI KNEE RATING SYSTEM
The Cincinnati Knee Rating System (CKRS) was first described in 1983 and was modified to include occupational activities, athletic activities, symptoms, and functional limitations.37,38 There are 11 components, measuring symptoms and disability in sports activity, activities of daily living function, occupational rating, as well as sections that measure physical examination, laxity of the knee, and radiographic evidence of degenerative joint disease.39 The measure is scored on a 100-point scale, with higher scores indicating better outcomes. Scores have been shown to be lower as compared with other outcome measures assessing the same clinical condition.40,41 Barber-Westin and colleagues39 confirmed the reliability, validity, and responsiveness of the CKRS by testing 350 subjects with and without knee ligament injuries. In 2001, Marx42 tested the CKRS subjective form for reliability, validity, and responsiveness and found it to be acceptable for clinical research.
LYSHOLM KNEE SCORE
The Lysholm Knee Score was published in 1982 and modified in 1985, consisting of an 8-question survey that evaluates outcomes after knee ligament surgery. Items include pain, instability, locking, squatting, limping, support usage, swelling, and stair-climbing ability, with pain and instability carrying the highest weight.43 It is scored on a scale of 0 to 100, with high scores indicating higher functioning and fewer symptoms. It has been validated in patients with ACL injuries and meniscal injuries.44 Although it is widely used to measure outcomes after ACL reconstruction,45 it has received criticism in the evaluation of patients with other knee conditions.46 The main advantage of the Lysholm Knee Score is its ability to note changes in activity in the same patient across different time periods (responsiveness). A limitation of the Lysholm Knee Score is that it does not measure the domains of functioning in daily activities, sports, and recreational activities. The Lysholm scoring system’s test-retest reliability and construct validity have been evaluated,42,43,46 although there has been some concern regarding a ceiling effect and its validity, sensitivity, and reliability has been questioned.47 Therefore, it is advised that this score be used in conjunction with other PRO scores.
INTERNATIONAL KNEE DOCUMENTATION COMMITTEE (IKDC) SUBJECTIVE KNEE FORM
In 1987, members of the European Society for Knee Surgery and Arthroscopy and the American Orthopaedic Society for Sports Medicine formed the IKDC to develop a standardized method for evaluating knee injuries and treatment. The IKDC Subjective Knee Evaluation Form was initially published in 1993, and in 2001 the form was revised by the American Orthopaedic Society for Sports Medicine to become a knee-specific assessment tool rather than a disease or condition-specific tool.48 The IKDC subjective form is an 18-question, knee-specific survey designed to detect improvement or deterioration in symptoms, function, and ability to participate in sports activities experienced by patients following knee surgery or other interventions. The individual items are summed and transformed into a 0- to 100-point scale, with high scores representing higher levels of function and minimal symptoms. The IKDC is utilized to assess a variety of knee conditions including ligament, meniscus, articular cartilage, osteoarthritis, and patellofemoral pain.48,49 Thus, this form can be used to assess any condition involving the knee and allow comparison between groups with different diagnoses. The IKDC has been validated for an ACL reconstruction population,47 has been used to assess outcomes in recent clinical studies on ACL reconstruction,50,51 and is one of the most frequently used measures for patients with ACL deficiency.3 The validity, responsiveness, and reliability of the IKDC subjective form has been confirmed for both adult and adolescent populations.48,49,52-54
TEGNER ACTIVITY SCORES
The Tegner activity score was developed in 1985 and was designed to provide an objective value for a patient’s activity level.44 This scale was developed to complement the Lysholm score. It consists of 1 sport-specific activity level question on a 0 to 10 scale that evaluates an individual’s ability to compete in a sporting activity. Scores between 1 and 5 represent work or recreational sports. Scores >5 represent higher-level recreational and competitive sports. The Tegner activity score is one of the most widely used activity scoring systems for patients with knee disorders,55,56 commonly utilized with the Lysholm Knee Score.44 One disadvantage of the Tegner activity score is that it relates to specific sports rather than functional activities, which limits its generalizability. We are not aware of any studies documenting the reliability or validity of this instrument.
Continue on: Marx Activity Rating Scale...
MARX ACTIVITY RATING SCALE
The Marx activity rating scale was developed to be utilized with other knee rating scales and outcome measures as an activity assessment.57 In contrast to the Tegner activity score, the Marx activity rating scale measures function rather than sport-specific activity. The scale is a short, patient-based activity assessment that consists of a 4-question survey evaluating patients’ knee health by recording the frequency and intensity of participation in a sporting activity. Questions are scored from 0 to 4 on the basis of how often the activity is performed. The 4 sections of the Marx scale that are rated include running, cutting, decelerating, and pivoting. This scale has been validated in patients with ACL injuries, chondromalacia patellae, and meniscal lesions.42,56-58 Acceptable ceiling effects of 3% and floor effects of 8% were noted in the study of ACL-injured patients.57
AMERICAN ACADEMY OF ORTHOPAEDIC SURGEONS (AAOS) SPORTS KNEE SCALE
The American Academy of Orthopaedic Surgeons (AAOS) Sports Knee Rating Scale consists of 5 parts and 23 items, including a section addressing stiffness, swelling, pain and function (7 questions), locking/catching (4 questions), giving way (4 questions), limitations of activity (4 questions), and pain with activity (4 questions).59,60 Items may be dropped if patients select particular responses, which can lead to difficulties when using the survey. This scoring system has been found to be satisfactory when all subscales were combined and the mean was calculated.42
DISCUSSION
PRO measures play an increasingly important role in the measurement of success and impact of health care services. Specifically, for ACL reconstruction, patient satisfaction is key for demonstrating the value of operative or other interventions. Selecting a suitable outcome measurement tool can be daunting, as it can be difficult to ascertain which outcome measures are appropriate for the patient or disorder in question. As there is currently no instrument that is universally superior in the evaluation of ACL outcomes, clinicians must consider the specific patient population in which the outcome instrument has been evaluated. Investigators should also use instruments with reported minimal clinically important differences so that variation in scores can be interpreted as either clinically significant or not. When choosing which outcome instrument to use, there is rarely a single appropriate rating system that is entirely comprehensive. In most cases, a general health outcome measure should be used in combination with a condition-specific rating scale. Activity rating scales, such as Marx or Tegner, should be included, especially when evaluating patients with low-activity lifestyles.
CONCLUSION
There are a number of reliable, valid, and responsive outcome measures that can be utilized to evaluate outcomes following ACL reconstruction in an array of patient populations. Outcome measures should be relevant to patients, easy to use, reliable, valid, and responsive to change. By increasing familiarity with these outcome measures, orthopedic surgeons and investigators can develop better studies, interpret data, and implement findings in practice with sound and informed judgment. Future research should focus on identifying the most relevant outcome metrics for assessing function following ACL reconstruction.
This paper will be judged for the Resident Writer’s Award.
1. Mall NA, Chalmers PN, Moric M, et al. Incidence and trends of anterior cruciate ligament reconstruction in the United States. Am J Sports Med. 2014;42(10):2363-2370. doi:10.1177/0363546514542796.
2. Brophy RH, Schmitz L, Wright RW, et al. Return to play and future ACL injury risk after ACL reconstruction in soccer athletes from the Multicenter Orthopaedic Outcomes Network (MOON) group. Am J Sports Med. 2012;40(11):2517-2522. doi:10.1177/0363546512459476.
3. Johnson DS, Smith RB. Outcome measurement in the ACL deficient knee- what’s the score? Knee. 2001;8(1):51-57. doi:10.1016/S0968-0160(01)00068-0.
4. Graham B, Green A, James M, Katz J, Swiontkowski M. Measuring patient satisfaction in orthopaedic surgery. J Bone Joint Surg Am. 2015;97(1):80-84. doi:10.2106/JBJS.N.00811.
5. Kocher MS, Steadman JR, Briggs K, Zurakowski D, Sterett WI, Hawkins RJ. Determinants of patient satisfaction with outcome after anterior cruciate ligament reconstruction. J Bone Joint Surg Am. 2002;84(9):1560-1572. doi:10.2106/00004623-200209000-00008.
6. Streiner DL, Norman GR. Health Measurement Scales: A Practical Guide to their Development and Use. Oxford: Oxford University Press; 1989.
7. Terwee CB, Bot SD, de Boer MR, et al. Quality criteria were proposed for measurement properties of health status questionnaires. J Clin Epidemiol. 2007;60(1):34-42. doi:10.1016/j.jclinepi.2006.03.012.
8. Bartko JJ. The intraclass correlation coefficient as a measure of reliability. Psychol Rep. 1966;19(1):3-11. doi:10.2466/pr0.1966.19.1.3.
9. Scholtes VA, Terwee CB, Poolman RW. What makes a measurement instrument valid and reliable? Injury. 2011;42(3):236-240. doi:10.1016/j.injury.2010.11.042.
10. Fries J, Rose M, Krishnan E. The PROMIS of better outcome assessment: responsiveness, floor and ceiling effects, and internet administration. J Rheumatol. 2011;38(8):1759-1764. doi:10.3899/jrheum.110402.
11. Bellamy N, Buchanan WW, Goldsmith CH, Campbell J, Stitt L. Validation study of WOMAC: a health status instrument for measuring clinically important patient relevant outcomes to antirheumatic drug therapy in patients with osteoarthritis of the hip or knee. J Rheumatol. 1988;15(12):1833-1840.
12. Gandek B. Measurement properties of the Western Ontario and McMaster Universities Osteoarthritis Index: a systematic review. Arthritis Care Res (Hoboken). 2015;67(2):216-229. doi:10.1002/acr.22415.
13. Angst F, Aeschlimann A, Stucki G. Smallest detectable and minimal clinically important differences of rehabilitation intervention with their implications for required sample sizes using WOMAC and SF-36 quality of life measurement instruments in patients with osteoarthritis of the lower extremities. Arthritis Rheum. 2001;45(4):384-391. doi:10.1002/1529-0131(200108)45:4<384::AID-ART352>3.0.CO;2-0.
14. Ryser L, Wright BD, Aeschlimann A, Mariacher-Gehler S, Stuckl G. A new look at the Western Ontario and McMaster Universities Osteoarthritis Index using Rasch analysis. Arthritis Care Res. 1999;12(5):331-335.
15. Wolfe F, Kong SX. Rasch analysis of the Western Ontario MacMaster questionnaire (WOMAC) in 2205 patients with osteoarthritis, rheumatoid arthritis, and fibromyalgia. Ann Rheum Dis. 1999;58(9):563-568. doi:10.1136/ard.58.9.563.
16. Roos EM, Roos HP, Lohmander LS, Ekdahl C, Beynnon BD. Knee Injury and Osteoarthritis Outcome Score (KOOS)—development of a self-administered outcome measure. J Orthop Sports Phys Ther. 1998;28(2):88-96. doi:10.2519/jospt.1998.28.2.88.
17. Salavati M, Akhbari B, Mohammadi F, Mazaheri M, Khorrami M. Knee injury and Osteoarthritis Outcome Score (KOOS): reliability and validity in competitive athletes after anterior cruciate ligament reconstruction. Osteoarthritis Cartilage. 2011;19(4):406-410. doi:10.1016/j.joca.2011.01.010.
18. Roos EM, Roos HP, Lohmander LS. WOMAC Osteoarthritis Index—additional dimensions for use in subjects with post-traumatic osteoarthritis of the knee. Western Ontario and MacMaster Universities. Osteoarthritis Cartilage. 1999;7(2):216-221. doi:10.1053/joca.1998.0153.
19. Roos EM, Toksvig-Larsen S. Knee injury and osteoarthritis outcome score (KOOS)—validation and comparison to the WOMAC in total knee replacement. Health Qual Life Outcomes. 2003;1(1):17. doi:10.1186/1477-7525-1-17.
20. Paradowski PT, Bergman S, Sunden-Lundius A, Lohmander LS, Roos EM. Knee complaints vary with age and gender in the adult population: population-based reference data for the Knee injury and Osteoarthritis Outcome Score (KOOS). BMC Musculoskeletal Disord. 2006;7(1):38. doi:10.1186/1471-2474-7-38.
21. MARS Group. Effect of graft choice on the outcome of revision anterior cruciate ligament reconstruction in the Multicenter ACL Revision Study (MARS) Cohort. Am J Sports Med. 2014;42(10):2301-2310. doi:10.1177/0363546514549005.
22. Ventura A, Legnani C, Terzaghi C, Borgo E, Albisetti W. Revision surgery after failed ACL reconstruction with artificial ligaments: clinical, histologic and radiographic evaluation. Eur J Orthop Surg Traumatol. 2014;21(1):93-98. doi:10.1007/s00590-012-1136-3.
23. Wasserstein D, Huston LJ, Nwosu S, et al. KOOS pain as a marker for significant knee pain two and six years after primary ACL reconstruction: a Multicenter Orthopaedic Outcomes Network (MOON) prospective longitudinal cohort study. Osteoarthritis Cartilage. 2015;23(10):1674-1684. doi:10.1016/j.joca.2015.05.025.
24. Zaffagnini S, Grassi A, Muccioli GM, et al. Return to sport after anterior cruciate ligament reconstruction in professional soccer players. Knee. 2014;21(3):731-735. doi:10.1016/j.knee.2014.02.005.
25. Duffee A, Magnussen RA, Pedroza AD, Flanigan DC; MOON Group, Kaeding CC. Transtibial ACL femoral tunnel preparation increases odds of repeat ipsilateral knee surgery. J Bone Joint Surg Am. 2013;95(22):2035-2042. doi:10.2106/JBJS.M.00187.
26. Bellamy N, Buchanan WW. A preliminary evaluation of the dimensionality and clinical importance of pain and disability in osteoarthritis of the hip and knee. Clin Rheumatol. 1986;5(2):231-241. doi:10.1007/BF02032362.
27. Fries J, Rose M, Krishnan E. The PROMIS of better outcome assessment: responsiveness, floor and ceiling effects, and Internet administration. J Rheumatol. 2011;38(8):1759-1764. doi:10.3899/jrheum.110402.
28. Hancock KJ, Glass NA, Anthony CA, et al. Performance of PROMIS for healthy patients undergoing meniscal surgery. J Bone Joint Surg Am. 2017;99(11):954-958. doi:10.2106/JBJS.16.00848.
29. Hung M, Clegg Do, Greene T, et al. Evaluation of the PROMIS physical function item bank in orthopedic patients. J Orthop Res. 2011;29(6):947-953. doi:10.1002/jor.21308.
30. Hung M, Baumhauer JF, Brodsky JW, et al; Orthopaedic Foot & Ankle Outcomes Research (OFAR) of the American Orthopaedic Foot & Ankle Society (AOFAS). Psychometric comparison of the PROMIS physical function CAT with the FAAM and FFI for measuring patient-reported outcomes. Foot Ankle Int. 2014;35(6):592-599. doi:10.1177/1071100714528492.
31. Hung M, Stuart AR, Higgins TF, Saltzman CL, Kubiak EN. Computerized adaptive testing using the PROMIS physical function item bank reduces test burden with less ceiling effects compared with the short musculoskeletal function assessment in orthopaedic trauma patients. J Orthop Trauma. 2014;28(8):439-443. doi:10.1097/BOT.0000000000000059.
32. Hancock, et al. PROMIS: A valid and efficient outcomes instrument for patients with ACL tears. KSSTA. In press.
33. Scott, et al. Performance of PROMIS physical function compared with KOOS, SF-36, Eq5D, and Marx activity scale in patients who undergo ACL reconstruction. In press.
34. Papuga MO, Beck CA, Kates SL, Schwarz EM, Maloney MD. Validation of GAITRite and PROMIS as high-throughput physical function outcome measures following ACL reconstruction. J Orthop Res. 2014;32(6):793-801. doi:10.1002/jor.22591.
35. Mohtadi N. Development and validation of the quality of life outcome measure (questionnaire) for chronic anterior cruciate ligament deficiency. Am J Sports Med. 1998;26(3):350-359. doi:10.1177/03635465980260030201.
36. Lafave MR, Hiemstra L, Kerslake S, Heard M, Buchko G. Validity, reliability, and responsiveness of the anterior cruciate ligament quality of life measure: a continuation of its overall validation. Clin J Sport Med. 2017;27(1):57-63. doi:10.1097/JSM.0000000000000292.
37. Noyes FR, McGinniss GH, Mooar LA. Functional disability in the anterior cruciate insufficient knee syndrome: Review of knee rating systems and projected risk factors in determining treatment. Sports Med. 1984;1(4):278-302. doi:10.2165/00007256-198401040-00004.
38. Noyes FR, Matthews DS, Mooar PA, Grood ES. The symptomatic anterior cruciate-deficient knee: Part II. The results of rehabilitation, activity modification, and counseling on functional disability. J Bone Joint Surg Am. 1983;65(2):163-174. doi:10.2106/00004623-198365020-00004.
39. Barber-Westin SD, Noyes FR, McCloskey JW. Rigorous statistical reliability, validity, and responsiveness testing of the Cincinnati knee rating system in 350 subjects with uninjured, injured, or anterior cruciate ligament-reconstructed knees. Am J Sports Med. 1999;27(4):402-416. doi:10.1177/03635465990270040201.
40. Bollen S, Seedhorn BB. A comparison of the Lysholm and Cincinnati knee scoring questionnaires. Am J Sports Med. 1991;19(2):189-190. doi:10.1177/036354659101900215.
41. Sgaglione NA, Del Pizzo W, Fox JM, Friedman MJ. Critical analysis of knee ligament rating systems. Am J Sports Med. 1995;23(6):660-667. doi:10.1177/036354659502300604.
42. Marx RG, Jones EC, Allen AA, et al. Reliability, validity, and responsiveness of four knee outcome scales for athletic patients. J Bone Joint Surg Am. 2001;83(10):1459-1469. doi:10.2106/00004623-200110000-00001.
43. Lysholm J, Gillquist J. Evaluation of knee ligament surgery results with special emphasis on use of a scoring scale. Am J Sports Med. 1982;10(3):150-154. doi:10.1177/036354658201000306.
44. Tegner Y, Lysholm J. Rating systems in the evaluation of knee ligament injuries. Clin Orthop Relat Res. 1985;198:43-49. doi:10.1097/00003086-198509000-00007.
45. Lukianov AV, Gillquist J, Grana WA, DeHaven KE. An anterior cruciate ligament (ACL) evaluation format for assessment of artificial or autologous anterior cruciate reconstruction results. Clin Orthop Relat Res. 1987;218:167-180. doi:10.1097/00003086-198705000-00024.
46. Bengtsson J, Mollborg J, Werner S. A study for testing the sensitivity and reliability of the Lysholm knee scoring scale. Knee Surg Sports Traumatol Arthrosc. 1996;4(1):27-31. doi:10.1007/BF01565994.
47. Risberg MA, Holm I, Steen J, Beynnon BD. Sensitivity to changes over time for the IKDC form, the Lysholm score, and the Cincinnati knee score. A prospective study of 120 ACL reconstructed patients with a 2-year follow-up. Knee Surg Sports Traumatol Arthrosc. 1999;7(3):152-159. doi:10.1007/s001670050140.
48. Irrgang JJ, Anderson AF, Boland AL, et al. Development and validation of the international knee documentation committee subjective knee form. Am J Sports Med. 2001;29(5):600-613. doi:10.1177/03635465010290051301.
49. Irrgang JJ, Anderson AF, Boland AL, et al. Responsiveness of the International Knee Documentation Committee Subjective Knee Form. Am J Sports Med. 2006;34(10):1567-1573. doi:10.1177/0363546506288855.
50. Logerstedt D, Di Stasi S, Grindem H, et al. Self-reported knee function can identify athletes who fail return-to-activity criteria up to 1 year after anterior cruciate ligament reconstruction: a Delaware-Oslo ACL cohort study. J Orthop Sports Phys Ther. 2014;44(2):914-923. doi:10.2519/jospt.2014.4852.
51. Lentz TA, Zeppieri G Jr, George SZ, et al. Comparison of physical impairment, functional and psychosocial measures based on fear of reinjury/lack of confidence and return-to-sport status after ACL reconstruction. Am J Sports Med. 2015;43(2):345-353. doi:10.1177/0363546514559707.
52. Greco NJ, Anderson AF, Mann BJ, et al. Responsiveness of the International Knee Documentation Committee Subjective Knee Form in comparison to the Western Ontario and McMaster Universities Osteoarthritis Index, modified Cincinnati Knee Rating System, and Short Form 36 in patients with focal articular cartilage defects. Am J Sports Med. 2010;38(5):891-902. doi:10.1177/0363546509354163.
53. Hefti F, Muller W, Jakob RP, Staubli HU. Evaluation of knee ligament injuries with the IKDC form. Knee Surg Sports Traumatol Arthrosc. 1993;1(3-4):226-234. doi:10.1007/BF01560215.
54. Schmitt LC, Paterno MV, Huang S. Validity and internal consistency of the International Knee Documentation Committee Subjective Knee Evaluation Form in children and adolescents. Am J Sports Med. 2010;38(12):2443-2447. doi:10.1177/0363546510374873.
55. Briggs KK, Lysholm J, Tegner Y, Rodkey WG, Kocher MS, Steadman JR. The reliability, validity, and responsiveness of the Lysholm and Tegner activity scale for anterior cruciate ligament injuries of the knee: 25 years later. Am J Sports Med. 2009;37(5):890-897. doi:10.1177/0363546508330143.
56. Negahban H, Mostafaee N, Sohani SM, et al. Reliability and validity of the Tegner and Marx activity rating scales in Iranian patients with anterior cruciate ligament injury. Disabil Rehabil. 2011;33(23-24):2305-2310. doi:10.3109/09638288.2011.570409.
57. Marx RG, Stump TJ, Jones EC, Wickiewicz TL, Warren RF. Development and evaluation of an activity rating scale for disorders of the knee. Am J Sports Med. 2001;29(2):213-218. doi:10.1177/03635465010290021601.
58. Garratt AM, Brealey S, Gillespie WJ, in collaboration with the DAM-ASK Trial Team. Patient-assessed health instruments for the knee: a structured review. Rheumatology. 2004;43(11):1414-1423. doi:10.1093/rheumatology/keh362.
59. American Academy of Orthopaedic Surgeons. Scoring algorithms for the lower limb: Outcomes data collection instrument. Rosemon, IL: American Academy of Orthopaedic Surgeons; 1998.
60. Johanson NA, Liang MH, Daltroy L, Rudicel S, Richmond J. American Academy of Orthopaedic Surgeons lower limb outcomes assessment instruments. Reliability, validity, and sensitivity to change. J Bone Joint Surg Am. 2004;86-A(5):902-909.
ABSTRACT
Outcome instruments have become an essential part of the evaluation of functional recovery after anterior cruciate ligament (ACL) reconstruction. Although the clinical examination provides important objective information to assess graft integrity, stability, range of motion, and strength, these measurements do not take the patient’s perception into account. There are many knee outcome instruments, and it is challenging for surgeons to understand how to interpret clinical research and utilize these measures in a practical way. The purpose of this review is to provide an overview of the most commonly used outcome measures in patients undergoing ACL reconstruction and to examine and compare the psychometric performance (validity, reliability, responsiveness) of these measurement tools.
Anterior cruciate ligament (ACL) reconstruction is one of the most common elective orthopedic procedures.1 Despite advances in surgical techniques, ACL reconstruction is associated with a lengthy recovery time, decreased performance, and increased rate of reinjury.2 Patients undergoing ACL reconstruction are often active individuals who participate in demanding activities, and accurate assessment of their recovery helps to guide recovery counseling. In addition to objective clinical outcomes measured through physical examination, patient-reported outcome (PRO) instruments add the patient’s perspective, information critical in determining a successful outcome. A variety of outcome instruments have been used and validated for patients with ACL tears. It is important for orthopedic surgeons to know the advantages and disadvantages of each outcome tool in order to interpret clinical studies and assess postoperative patients.
Over the last 10 years, there has been an increase in the number of knee instruments and rating scales designed to measure PROs, with >54 scores designed for the ACL-deficient knee.3 No standardized instrument is currently universally accepted as superior following ACL reconstruction across the spectrum of patient populations. Clinicians and researchers must carefully consider an outcome instrument’s utility based on specific patient populations in which it has been evaluated. Appropriate selection of outcome measures is of fundamental importance for adequate demonstration of the efficacy and value of treatment interventions, especially in an era of healthcare reform with a focus on providing high-quality and cost-effective care.
The purpose of this review is to highlight current tools used to measure outcomes after ACL reconstruction. Current outcome measures vary widely in regards to their validity, reliability, minimal clinically important difference, and applicability to specific patient populations. We have thus identified the measures most commonly used today in studies and clinical follow-up after ACL reconstruction and their various advantages and limitations. This information may enhance the orthopedic surgeon’s understanding of what outcome measures may be utilized in clinical studies.
Continue to: Patient-Reported Outcome Instruments...
PATIENT-REPORTED OUTCOME INSTRUMENTS
Recently, there has been a transition to increased use of PRO instruments rather than clinician-based postoperative assessment, largely due to the increasing emphasis on patient satisfaction in determining the value of an orthopedic intervention.4 PRO instruments are widely used to capture the patient’s perception of general health, quality of life (QOL), daily function, and pain. PRO instruments offer the benefit of allowing patients to subjectively assess their knee function during daily living and sports activities, conveying to the provider the impact of ACL reconstruction on physical, psychological, and social aspects of everyday activities. Furthermore, patient satisfaction has been shown to closely follow outcome scores related to symptoms and function.5 A multitude of specific knee-related PRO instruments have been developed and validated to measure outcomes after ACL reconstruction for both research and clinical purposes (Table).
Table. ACL Outcome Measures
|
|
|
|
| ||
Outcome Measure | Condition/Intervention | Measures | Internal Consistency (Cronbach’s a) | Test-Retest Reliability | Minimal Clinically Important Difference | Ref |
AAOS Sports Knee Scale | Many Knee | Stiffness, swelling, pain/function, locking/catching, giving way, limitation of activity, pain with activity | 0.86-0.95 | 0.68-0.96 | Unknown | 59, 60 |
ACL-QOL | Chronic ACL deficiency | Physical complaints, work, recreation and sports competition, lifestyle, social and emotional functioning | 0.93-0.98 | 6% average error | Unknown | 35, 36 |
Cincinnati Knee Rating System | ACL | Symptoms, daily and sports activities, physical examination, stability, radiographs, functional testing |
| 0.80-0.97 | 14 points (6 months), 26 points (12 months) | 39, 40, 47, 52 |
IKDC (Subjective Knee Form) | ACL | Symptoms, function, sports activity | 0.92 | 0.91-0.93 | 11.5 points; 6.3 at 6 months, 16.7 at 12 months | 48, 52, 54 |
KOOS | ACL | Pain, symptoms, activities of daily living, sport/recreation, knee-related quality of life | 0.71-0.95 | 0.75-0.93 | 8-10 points | 17 |
Lysholm | ACL | Pain, instability, locking, squatting, limp, support, swelling, stair-climbing | 0.72 | 0.94 | 8.9 | 46, 47, 55 |
Marx | Healthy patients | Activity level | 0.87 | 0.97 | Unknown | 42, 56, 57 |
Tegner | ACL | Activity level | 0.81 | 0.82 | 1 | 55, 56 |
PROMIS | Many lower extremity orthopedic conditions | Lower extremity function, central body function, activities of daily living | 0.98 | 0.96-0.99 |
| 30, 31 |
WOMAC | Hip/knee OA | Physical function, pain, stiffness | 0.81-0.95 | 0.80-0.92 | 12% baseline score or 6% max score; 9-12 points | 13, 14 |
Abbreviations: AAOS, American Academy of Orthopaedic Surgeons; ACL, anterior cruciate ligament; ACL-QOL, anterior cruciate ligament quality of life score; CAT, computer-adapting testing; IKDC, International Knee Documentation Committee; KOOS, Knee Injury and Osteoarthritis Outcome Score; OA, osteoarthritis; PF, physical function; PROMIS, Patient-Reported Outcome Measurement Information System; Ref, references; WOMAC, Western Ontario and McMaster Universities Osteoarthritis Index.
MEASUREMENT PROPERTIES
In general, clinicians and investigators should use health-related outcome measures with established reliability, validity, patient relevance, and responsiveness for assessing the specific condition.6
Reliability refers to the degree to which a measurement score is free from random error, reflecting how consistent or reproducible the instrument is when administered under the same testing conditions. Internal consistency, test-retest reliability, and measurement error are measures of reliability. Internal consistency is tested after a single administration and assesses how well items within a scale measure a single underlying dimension, represented using item-total correlation coefficients and Cronbach’s alpha. A Cronbach’s alpha of 0.70 to 0.95 is generally defined as good.7 Test-retest reliability is designed to appraise variation over time in stable patients and is represented using the intraclass correlation coefficient (ICC).8 An ICC >0.7 is considered acceptable; >0.8, good; and >0.9, excellent.9 An aspect of accuracy is whether the scoring system measures the full range of the disease or complaints. The incidence of minimum (floor) and maximum (ceiling) scores can be calculated for outcome scores. An instrument with low floor and ceiling effects, below 10% to 15%, is more inconclusive and can be more reliably used to measure patients at the high and low end of the scoring system.10
Validity is the ability of an outcome instrument to measure what it is intended to measure. Establishing validity is complex and requires evaluation of several facets, including content validity, construct validity, and criterion validity. Content validity is a relatively subjective judgment explaining the ability of an instrument to assess the critical features of the problem. Construct validity evaluates whether the questionnaire measures what it intends to measure, and is often assessed by correlating scores form one instrument to those from other proven instruments that are already accepted as valid. Finally, criterion validity assesses the correlation between the score and a previously established “gold standard” instrument.
Responsiveness is the ability of the instrument to detect a change or identify improvement or worsening of a clinical condition over time. Most frequently, the effect size (observed change/standard deviation of baseline scores) and standardized response mean (observed change/standard deviation of change) are used as measures of responsiveness. The minimal clinically important difference of an outcome measure is the smallest change in an outcome score that corresponds to a change in patient condition.
Continue to: ACL Outcome Instruments...
ACL OUTCOME INSTRUMENTS
WESTERN ONTARIO AND MCMASTER UNIVERSITIES OSTEOARTHRITIS INDEX (WOMAC LK 3.0)
The Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC LK 3.0) was developed in 1982 and is a widely used, disease-specific instrument recommended for the evaluation of treatment effects in patients with hip and knee osteoarthritis.11 Available in more than 80 languages, it is a self-administered, generic health status questionnaire developed to assess pain, function, and stiffness in daily living, taking respondents between 3 to 7.5 minutes for completion.12 Using visual analog scales, the 24 items probe the 3 subscales: pain (5 items), stiffness (2 items), and functional difficulty (17 items). Scores are calculated for each dimension, and the total score is normalized to a 100-point scale, with 0 indicating severe symptoms and 100 indicating no symptoms and higher function. The WOMAC score can also be calculated from the Knee Injury and Osteoarthritis Outcome Score (KOOS). The WOMAC questionnaire is well recognized for its good validity, reliability, and responsiveness, and is the most commonly used outcome measure for osteoarthritis.13-15 Considering its focus on older patients with osteoarthritis, it may not be appropriate for use in a young and active population.
KNEE INJURY AND OSTEOARTHRITIS OUTCOME SCORE (KOOS)
The KOOS is a knee-specific questionnaire developed as an extension of the WOMAC to evaluate the functional status and QOL of patients with any type of knee injury who are at an increased risk of developing osteoarthritis.16 The patient-based questionnaire is available in over 30 languages and covers both the short- and long-term consequences of an injury of the knee causing traumatic damage to cartilage, ligaments, and menisci. The KOOS is 42 items graded on a 5-point Likert scale, covering 5 subscales: pain (9 items), symptoms (7 items), function in activities of daily living (17 items), function in sports/recreation (5 items), and knee-related QOL (4 items). The questionnaire is self-administered and takes about 10 minutes to complete. Scores are calculated for each dimension, and the total score is transformed to a 0 to 100 scale, with 0 representing severe knee problems and 100 representing no knee problems and better outcome. An advantage of the KOOS is that it evaluates both knee injuries and osteoarthritis; therefore, it is arguably more suitable for evaluating patients over the long-term. The KOOS has been validated for several orthopedic interventions, including ACL reconstruction and rehabilitation16,17 as well as meniscectomy18 and total knee replacement.19 Population-based reference data for the adult population according to age and gender have also been established.20 The KOOS is increasingly utilized in clinical studies on ACL reconstruction.21-25 The questions of the WOMAC were retained so that a WOMAC score might be calculated separately and compared with the KOOS score.26
PATIENT-REPORTED OUTCOMES INFORMATION SYSTEM (PROMIS)
Since 2004, The National Institutes of Health (NIH) has funded the development of the Patient-Reported Outcome Measurement Information System (PROMIS), a set of flexible tools that reliably and validly measure PROs. The PROMIS consists of a library of question banks that has been developed and operated by a network of National Institutes of Health-funded research sites and coordinating centers and covers many different health domains including pain, fatigue, anxiety, depression, social functioning, physical functioning, and sleep. PROMIS items are developed using Item Response Theory (IRT), wherein the answer to any individual item has a known mathematical probability of predicting the test taker’s overall measurement of the specific trait being tested. This is commonly administered using computer-adaptive testing (CAT), which presents to the test taker an initial item, scores the response to that item, and from the response then presents the most informative second item, and so forth until a predefined level of precision is reached. Because the items are individually validated, they can be used alone or in any combination, a feature that distinguishes the PROMIS from traditional fixed-length PRO instruments that require the completion of an instrument in its entirety to be valid.27 In recent years, orthopedic research has been published with PROMIS physical function (PF) scores as primary outcome measures.28-30 The PF item bank includes 124 items measuring upper extremity, lower extremity, central and instrumental activities of daily living. PF can be completed as a short form (SF) with a set number of questions or utilizing CAT and evaluates self-reported function and physical activity. An advantage is its ease of use and potential to minimize test burden with very few questions, often as little as 4 items, as compared to other traditional PROMs.31
Previously published work has demonstrated that, in patients undergoing meniscal surgery, the PROMIS PF CAT maintains construct validity and correlates well with currently used knee outcome instruments, including KOOS.28 Work by the same group looking at the performance of the PROMIS PF CAT in patients indicated for ACL reconstruction shows that the PROMIS PF CAT correlates well with other PRO instruments for patients with ACL injuries, (SF-36 PF [r = 0.82, P < 0.01], KOOS Sport [r = 0.70, P < 0.01], KOOS ADL [r = 0.74, P < 0.01]), does not have floor or ceiling effects in this relatively young and healthy population, and has a low test burden.32,33 Papuga and colleagues33 also compared the International Knee Documentation Committee (IKDC) and PROMIS PF CAT on 106 subjects after ACL reconstruction and found good correlation.
Continue to: Quality of Life Outcome Measure...
QUALITY OF LIFE OUTCOME MEASURE FOR ACL DEFICIENCY (ACL-QOL)
The ACL-QOL Score was developed in 1998 as a disease-specific measure for patients with chronic ACL deficiency.34 This scale consists of 32 separate items in 31 visual analog questions regarding symptoms and physical complaints, work-related concerns, recreational activities and sport participation or competition, lifestyle, and social and emotional health status relating to the knee. The raw score is transformed into a 0- to 100-point scale, with higher scores indicating a better outcome. The scale is valid, reliable, and responsive for patients with ACL insufficiency,35,36 and is not applicable to other disorders of the knee. We recommend the ACL-QOL questionnaire be used in conjunction with other currently available objective and functional outcome measures.
CINCINNATI KNEE RATING SYSTEM
The Cincinnati Knee Rating System (CKRS) was first described in 1983 and was modified to include occupational activities, athletic activities, symptoms, and functional limitations.37,38 There are 11 components, measuring symptoms and disability in sports activity, activities of daily living function, occupational rating, as well as sections that measure physical examination, laxity of the knee, and radiographic evidence of degenerative joint disease.39 The measure is scored on a 100-point scale, with higher scores indicating better outcomes. Scores have been shown to be lower as compared with other outcome measures assessing the same clinical condition.40,41 Barber-Westin and colleagues39 confirmed the reliability, validity, and responsiveness of the CKRS by testing 350 subjects with and without knee ligament injuries. In 2001, Marx42 tested the CKRS subjective form for reliability, validity, and responsiveness and found it to be acceptable for clinical research.
LYSHOLM KNEE SCORE
The Lysholm Knee Score was published in 1982 and modified in 1985, consisting of an 8-question survey that evaluates outcomes after knee ligament surgery. Items include pain, instability, locking, squatting, limping, support usage, swelling, and stair-climbing ability, with pain and instability carrying the highest weight.43 It is scored on a scale of 0 to 100, with high scores indicating higher functioning and fewer symptoms. It has been validated in patients with ACL injuries and meniscal injuries.44 Although it is widely used to measure outcomes after ACL reconstruction,45 it has received criticism in the evaluation of patients with other knee conditions.46 The main advantage of the Lysholm Knee Score is its ability to note changes in activity in the same patient across different time periods (responsiveness). A limitation of the Lysholm Knee Score is that it does not measure the domains of functioning in daily activities, sports, and recreational activities. The Lysholm scoring system’s test-retest reliability and construct validity have been evaluated,42,43,46 although there has been some concern regarding a ceiling effect and its validity, sensitivity, and reliability has been questioned.47 Therefore, it is advised that this score be used in conjunction with other PRO scores.
INTERNATIONAL KNEE DOCUMENTATION COMMITTEE (IKDC) SUBJECTIVE KNEE FORM
In 1987, members of the European Society for Knee Surgery and Arthroscopy and the American Orthopaedic Society for Sports Medicine formed the IKDC to develop a standardized method for evaluating knee injuries and treatment. The IKDC Subjective Knee Evaluation Form was initially published in 1993, and in 2001 the form was revised by the American Orthopaedic Society for Sports Medicine to become a knee-specific assessment tool rather than a disease or condition-specific tool.48 The IKDC subjective form is an 18-question, knee-specific survey designed to detect improvement or deterioration in symptoms, function, and ability to participate in sports activities experienced by patients following knee surgery or other interventions. The individual items are summed and transformed into a 0- to 100-point scale, with high scores representing higher levels of function and minimal symptoms. The IKDC is utilized to assess a variety of knee conditions including ligament, meniscus, articular cartilage, osteoarthritis, and patellofemoral pain.48,49 Thus, this form can be used to assess any condition involving the knee and allow comparison between groups with different diagnoses. The IKDC has been validated for an ACL reconstruction population,47 has been used to assess outcomes in recent clinical studies on ACL reconstruction,50,51 and is one of the most frequently used measures for patients with ACL deficiency.3 The validity, responsiveness, and reliability of the IKDC subjective form has been confirmed for both adult and adolescent populations.48,49,52-54
TEGNER ACTIVITY SCORES
The Tegner activity score was developed in 1985 and was designed to provide an objective value for a patient’s activity level.44 This scale was developed to complement the Lysholm score. It consists of 1 sport-specific activity level question on a 0 to 10 scale that evaluates an individual’s ability to compete in a sporting activity. Scores between 1 and 5 represent work or recreational sports. Scores >5 represent higher-level recreational and competitive sports. The Tegner activity score is one of the most widely used activity scoring systems for patients with knee disorders,55,56 commonly utilized with the Lysholm Knee Score.44 One disadvantage of the Tegner activity score is that it relates to specific sports rather than functional activities, which limits its generalizability. We are not aware of any studies documenting the reliability or validity of this instrument.
Continue on: Marx Activity Rating Scale...
MARX ACTIVITY RATING SCALE
The Marx activity rating scale was developed to be utilized with other knee rating scales and outcome measures as an activity assessment.57 In contrast to the Tegner activity score, the Marx activity rating scale measures function rather than sport-specific activity. The scale is a short, patient-based activity assessment that consists of a 4-question survey evaluating patients’ knee health by recording the frequency and intensity of participation in a sporting activity. Questions are scored from 0 to 4 on the basis of how often the activity is performed. The 4 sections of the Marx scale that are rated include running, cutting, decelerating, and pivoting. This scale has been validated in patients with ACL injuries, chondromalacia patellae, and meniscal lesions.42,56-58 Acceptable ceiling effects of 3% and floor effects of 8% were noted in the study of ACL-injured patients.57
AMERICAN ACADEMY OF ORTHOPAEDIC SURGEONS (AAOS) SPORTS KNEE SCALE
The American Academy of Orthopaedic Surgeons (AAOS) Sports Knee Rating Scale consists of 5 parts and 23 items, including a section addressing stiffness, swelling, pain and function (7 questions), locking/catching (4 questions), giving way (4 questions), limitations of activity (4 questions), and pain with activity (4 questions).59,60 Items may be dropped if patients select particular responses, which can lead to difficulties when using the survey. This scoring system has been found to be satisfactory when all subscales were combined and the mean was calculated.42
DISCUSSION
PRO measures play an increasingly important role in the measurement of success and impact of health care services. Specifically, for ACL reconstruction, patient satisfaction is key for demonstrating the value of operative or other interventions. Selecting a suitable outcome measurement tool can be daunting, as it can be difficult to ascertain which outcome measures are appropriate for the patient or disorder in question. As there is currently no instrument that is universally superior in the evaluation of ACL outcomes, clinicians must consider the specific patient population in which the outcome instrument has been evaluated. Investigators should also use instruments with reported minimal clinically important differences so that variation in scores can be interpreted as either clinically significant or not. When choosing which outcome instrument to use, there is rarely a single appropriate rating system that is entirely comprehensive. In most cases, a general health outcome measure should be used in combination with a condition-specific rating scale. Activity rating scales, such as Marx or Tegner, should be included, especially when evaluating patients with low-activity lifestyles.
CONCLUSION
There are a number of reliable, valid, and responsive outcome measures that can be utilized to evaluate outcomes following ACL reconstruction in an array of patient populations. Outcome measures should be relevant to patients, easy to use, reliable, valid, and responsive to change. By increasing familiarity with these outcome measures, orthopedic surgeons and investigators can develop better studies, interpret data, and implement findings in practice with sound and informed judgment. Future research should focus on identifying the most relevant outcome metrics for assessing function following ACL reconstruction.
This paper will be judged for the Resident Writer’s Award.
ABSTRACT
Outcome instruments have become an essential part of the evaluation of functional recovery after anterior cruciate ligament (ACL) reconstruction. Although the clinical examination provides important objective information to assess graft integrity, stability, range of motion, and strength, these measurements do not take the patient’s perception into account. There are many knee outcome instruments, and it is challenging for surgeons to understand how to interpret clinical research and utilize these measures in a practical way. The purpose of this review is to provide an overview of the most commonly used outcome measures in patients undergoing ACL reconstruction and to examine and compare the psychometric performance (validity, reliability, responsiveness) of these measurement tools.
Anterior cruciate ligament (ACL) reconstruction is one of the most common elective orthopedic procedures.1 Despite advances in surgical techniques, ACL reconstruction is associated with a lengthy recovery time, decreased performance, and increased rate of reinjury.2 Patients undergoing ACL reconstruction are often active individuals who participate in demanding activities, and accurate assessment of their recovery helps to guide recovery counseling. In addition to objective clinical outcomes measured through physical examination, patient-reported outcome (PRO) instruments add the patient’s perspective, information critical in determining a successful outcome. A variety of outcome instruments have been used and validated for patients with ACL tears. It is important for orthopedic surgeons to know the advantages and disadvantages of each outcome tool in order to interpret clinical studies and assess postoperative patients.
Over the last 10 years, there has been an increase in the number of knee instruments and rating scales designed to measure PROs, with >54 scores designed for the ACL-deficient knee.3 No standardized instrument is currently universally accepted as superior following ACL reconstruction across the spectrum of patient populations. Clinicians and researchers must carefully consider an outcome instrument’s utility based on specific patient populations in which it has been evaluated. Appropriate selection of outcome measures is of fundamental importance for adequate demonstration of the efficacy and value of treatment interventions, especially in an era of healthcare reform with a focus on providing high-quality and cost-effective care.
The purpose of this review is to highlight current tools used to measure outcomes after ACL reconstruction. Current outcome measures vary widely in regards to their validity, reliability, minimal clinically important difference, and applicability to specific patient populations. We have thus identified the measures most commonly used today in studies and clinical follow-up after ACL reconstruction and their various advantages and limitations. This information may enhance the orthopedic surgeon’s understanding of what outcome measures may be utilized in clinical studies.
Continue to: Patient-Reported Outcome Instruments...
PATIENT-REPORTED OUTCOME INSTRUMENTS
Recently, there has been a transition to increased use of PRO instruments rather than clinician-based postoperative assessment, largely due to the increasing emphasis on patient satisfaction in determining the value of an orthopedic intervention.4 PRO instruments are widely used to capture the patient’s perception of general health, quality of life (QOL), daily function, and pain. PRO instruments offer the benefit of allowing patients to subjectively assess their knee function during daily living and sports activities, conveying to the provider the impact of ACL reconstruction on physical, psychological, and social aspects of everyday activities. Furthermore, patient satisfaction has been shown to closely follow outcome scores related to symptoms and function.5 A multitude of specific knee-related PRO instruments have been developed and validated to measure outcomes after ACL reconstruction for both research and clinical purposes (Table).
Table. ACL Outcome Measures
|
|
|
|
| ||
Outcome Measure | Condition/Intervention | Measures | Internal Consistency (Cronbach’s a) | Test-Retest Reliability | Minimal Clinically Important Difference | Ref |
AAOS Sports Knee Scale | Many Knee | Stiffness, swelling, pain/function, locking/catching, giving way, limitation of activity, pain with activity | 0.86-0.95 | 0.68-0.96 | Unknown | 59, 60 |
ACL-QOL | Chronic ACL deficiency | Physical complaints, work, recreation and sports competition, lifestyle, social and emotional functioning | 0.93-0.98 | 6% average error | Unknown | 35, 36 |
Cincinnati Knee Rating System | ACL | Symptoms, daily and sports activities, physical examination, stability, radiographs, functional testing |
| 0.80-0.97 | 14 points (6 months), 26 points (12 months) | 39, 40, 47, 52 |
IKDC (Subjective Knee Form) | ACL | Symptoms, function, sports activity | 0.92 | 0.91-0.93 | 11.5 points; 6.3 at 6 months, 16.7 at 12 months | 48, 52, 54 |
KOOS | ACL | Pain, symptoms, activities of daily living, sport/recreation, knee-related quality of life | 0.71-0.95 | 0.75-0.93 | 8-10 points | 17 |
Lysholm | ACL | Pain, instability, locking, squatting, limp, support, swelling, stair-climbing | 0.72 | 0.94 | 8.9 | 46, 47, 55 |
Marx | Healthy patients | Activity level | 0.87 | 0.97 | Unknown | 42, 56, 57 |
Tegner | ACL | Activity level | 0.81 | 0.82 | 1 | 55, 56 |
PROMIS | Many lower extremity orthopedic conditions | Lower extremity function, central body function, activities of daily living | 0.98 | 0.96-0.99 |
| 30, 31 |
WOMAC | Hip/knee OA | Physical function, pain, stiffness | 0.81-0.95 | 0.80-0.92 | 12% baseline score or 6% max score; 9-12 points | 13, 14 |
Abbreviations: AAOS, American Academy of Orthopaedic Surgeons; ACL, anterior cruciate ligament; ACL-QOL, anterior cruciate ligament quality of life score; CAT, computer-adapting testing; IKDC, International Knee Documentation Committee; KOOS, Knee Injury and Osteoarthritis Outcome Score; OA, osteoarthritis; PF, physical function; PROMIS, Patient-Reported Outcome Measurement Information System; Ref, references; WOMAC, Western Ontario and McMaster Universities Osteoarthritis Index.
MEASUREMENT PROPERTIES
In general, clinicians and investigators should use health-related outcome measures with established reliability, validity, patient relevance, and responsiveness for assessing the specific condition.6
Reliability refers to the degree to which a measurement score is free from random error, reflecting how consistent or reproducible the instrument is when administered under the same testing conditions. Internal consistency, test-retest reliability, and measurement error are measures of reliability. Internal consistency is tested after a single administration and assesses how well items within a scale measure a single underlying dimension, represented using item-total correlation coefficients and Cronbach’s alpha. A Cronbach’s alpha of 0.70 to 0.95 is generally defined as good.7 Test-retest reliability is designed to appraise variation over time in stable patients and is represented using the intraclass correlation coefficient (ICC).8 An ICC >0.7 is considered acceptable; >0.8, good; and >0.9, excellent.9 An aspect of accuracy is whether the scoring system measures the full range of the disease or complaints. The incidence of minimum (floor) and maximum (ceiling) scores can be calculated for outcome scores. An instrument with low floor and ceiling effects, below 10% to 15%, is more inconclusive and can be more reliably used to measure patients at the high and low end of the scoring system.10
Validity is the ability of an outcome instrument to measure what it is intended to measure. Establishing validity is complex and requires evaluation of several facets, including content validity, construct validity, and criterion validity. Content validity is a relatively subjective judgment explaining the ability of an instrument to assess the critical features of the problem. Construct validity evaluates whether the questionnaire measures what it intends to measure, and is often assessed by correlating scores form one instrument to those from other proven instruments that are already accepted as valid. Finally, criterion validity assesses the correlation between the score and a previously established “gold standard” instrument.
Responsiveness is the ability of the instrument to detect a change or identify improvement or worsening of a clinical condition over time. Most frequently, the effect size (observed change/standard deviation of baseline scores) and standardized response mean (observed change/standard deviation of change) are used as measures of responsiveness. The minimal clinically important difference of an outcome measure is the smallest change in an outcome score that corresponds to a change in patient condition.
Continue to: ACL Outcome Instruments...
ACL OUTCOME INSTRUMENTS
WESTERN ONTARIO AND MCMASTER UNIVERSITIES OSTEOARTHRITIS INDEX (WOMAC LK 3.0)
The Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC LK 3.0) was developed in 1982 and is a widely used, disease-specific instrument recommended for the evaluation of treatment effects in patients with hip and knee osteoarthritis.11 Available in more than 80 languages, it is a self-administered, generic health status questionnaire developed to assess pain, function, and stiffness in daily living, taking respondents between 3 to 7.5 minutes for completion.12 Using visual analog scales, the 24 items probe the 3 subscales: pain (5 items), stiffness (2 items), and functional difficulty (17 items). Scores are calculated for each dimension, and the total score is normalized to a 100-point scale, with 0 indicating severe symptoms and 100 indicating no symptoms and higher function. The WOMAC score can also be calculated from the Knee Injury and Osteoarthritis Outcome Score (KOOS). The WOMAC questionnaire is well recognized for its good validity, reliability, and responsiveness, and is the most commonly used outcome measure for osteoarthritis.13-15 Considering its focus on older patients with osteoarthritis, it may not be appropriate for use in a young and active population.
KNEE INJURY AND OSTEOARTHRITIS OUTCOME SCORE (KOOS)
The KOOS is a knee-specific questionnaire developed as an extension of the WOMAC to evaluate the functional status and QOL of patients with any type of knee injury who are at an increased risk of developing osteoarthritis.16 The patient-based questionnaire is available in over 30 languages and covers both the short- and long-term consequences of an injury of the knee causing traumatic damage to cartilage, ligaments, and menisci. The KOOS is 42 items graded on a 5-point Likert scale, covering 5 subscales: pain (9 items), symptoms (7 items), function in activities of daily living (17 items), function in sports/recreation (5 items), and knee-related QOL (4 items). The questionnaire is self-administered and takes about 10 minutes to complete. Scores are calculated for each dimension, and the total score is transformed to a 0 to 100 scale, with 0 representing severe knee problems and 100 representing no knee problems and better outcome. An advantage of the KOOS is that it evaluates both knee injuries and osteoarthritis; therefore, it is arguably more suitable for evaluating patients over the long-term. The KOOS has been validated for several orthopedic interventions, including ACL reconstruction and rehabilitation16,17 as well as meniscectomy18 and total knee replacement.19 Population-based reference data for the adult population according to age and gender have also been established.20 The KOOS is increasingly utilized in clinical studies on ACL reconstruction.21-25 The questions of the WOMAC were retained so that a WOMAC score might be calculated separately and compared with the KOOS score.26
PATIENT-REPORTED OUTCOMES INFORMATION SYSTEM (PROMIS)
Since 2004, The National Institutes of Health (NIH) has funded the development of the Patient-Reported Outcome Measurement Information System (PROMIS), a set of flexible tools that reliably and validly measure PROs. The PROMIS consists of a library of question banks that has been developed and operated by a network of National Institutes of Health-funded research sites and coordinating centers and covers many different health domains including pain, fatigue, anxiety, depression, social functioning, physical functioning, and sleep. PROMIS items are developed using Item Response Theory (IRT), wherein the answer to any individual item has a known mathematical probability of predicting the test taker’s overall measurement of the specific trait being tested. This is commonly administered using computer-adaptive testing (CAT), which presents to the test taker an initial item, scores the response to that item, and from the response then presents the most informative second item, and so forth until a predefined level of precision is reached. Because the items are individually validated, they can be used alone or in any combination, a feature that distinguishes the PROMIS from traditional fixed-length PRO instruments that require the completion of an instrument in its entirety to be valid.27 In recent years, orthopedic research has been published with PROMIS physical function (PF) scores as primary outcome measures.28-30 The PF item bank includes 124 items measuring upper extremity, lower extremity, central and instrumental activities of daily living. PF can be completed as a short form (SF) with a set number of questions or utilizing CAT and evaluates self-reported function and physical activity. An advantage is its ease of use and potential to minimize test burden with very few questions, often as little as 4 items, as compared to other traditional PROMs.31
Previously published work has demonstrated that, in patients undergoing meniscal surgery, the PROMIS PF CAT maintains construct validity and correlates well with currently used knee outcome instruments, including KOOS.28 Work by the same group looking at the performance of the PROMIS PF CAT in patients indicated for ACL reconstruction shows that the PROMIS PF CAT correlates well with other PRO instruments for patients with ACL injuries, (SF-36 PF [r = 0.82, P < 0.01], KOOS Sport [r = 0.70, P < 0.01], KOOS ADL [r = 0.74, P < 0.01]), does not have floor or ceiling effects in this relatively young and healthy population, and has a low test burden.32,33 Papuga and colleagues33 also compared the International Knee Documentation Committee (IKDC) and PROMIS PF CAT on 106 subjects after ACL reconstruction and found good correlation.
Continue to: Quality of Life Outcome Measure...
QUALITY OF LIFE OUTCOME MEASURE FOR ACL DEFICIENCY (ACL-QOL)
The ACL-QOL Score was developed in 1998 as a disease-specific measure for patients with chronic ACL deficiency.34 This scale consists of 32 separate items in 31 visual analog questions regarding symptoms and physical complaints, work-related concerns, recreational activities and sport participation or competition, lifestyle, and social and emotional health status relating to the knee. The raw score is transformed into a 0- to 100-point scale, with higher scores indicating a better outcome. The scale is valid, reliable, and responsive for patients with ACL insufficiency,35,36 and is not applicable to other disorders of the knee. We recommend the ACL-QOL questionnaire be used in conjunction with other currently available objective and functional outcome measures.
CINCINNATI KNEE RATING SYSTEM
The Cincinnati Knee Rating System (CKRS) was first described in 1983 and was modified to include occupational activities, athletic activities, symptoms, and functional limitations.37,38 There are 11 components, measuring symptoms and disability in sports activity, activities of daily living function, occupational rating, as well as sections that measure physical examination, laxity of the knee, and radiographic evidence of degenerative joint disease.39 The measure is scored on a 100-point scale, with higher scores indicating better outcomes. Scores have been shown to be lower as compared with other outcome measures assessing the same clinical condition.40,41 Barber-Westin and colleagues39 confirmed the reliability, validity, and responsiveness of the CKRS by testing 350 subjects with and without knee ligament injuries. In 2001, Marx42 tested the CKRS subjective form for reliability, validity, and responsiveness and found it to be acceptable for clinical research.
LYSHOLM KNEE SCORE
The Lysholm Knee Score was published in 1982 and modified in 1985, consisting of an 8-question survey that evaluates outcomes after knee ligament surgery. Items include pain, instability, locking, squatting, limping, support usage, swelling, and stair-climbing ability, with pain and instability carrying the highest weight.43 It is scored on a scale of 0 to 100, with high scores indicating higher functioning and fewer symptoms. It has been validated in patients with ACL injuries and meniscal injuries.44 Although it is widely used to measure outcomes after ACL reconstruction,45 it has received criticism in the evaluation of patients with other knee conditions.46 The main advantage of the Lysholm Knee Score is its ability to note changes in activity in the same patient across different time periods (responsiveness). A limitation of the Lysholm Knee Score is that it does not measure the domains of functioning in daily activities, sports, and recreational activities. The Lysholm scoring system’s test-retest reliability and construct validity have been evaluated,42,43,46 although there has been some concern regarding a ceiling effect and its validity, sensitivity, and reliability has been questioned.47 Therefore, it is advised that this score be used in conjunction with other PRO scores.
INTERNATIONAL KNEE DOCUMENTATION COMMITTEE (IKDC) SUBJECTIVE KNEE FORM
In 1987, members of the European Society for Knee Surgery and Arthroscopy and the American Orthopaedic Society for Sports Medicine formed the IKDC to develop a standardized method for evaluating knee injuries and treatment. The IKDC Subjective Knee Evaluation Form was initially published in 1993, and in 2001 the form was revised by the American Orthopaedic Society for Sports Medicine to become a knee-specific assessment tool rather than a disease or condition-specific tool.48 The IKDC subjective form is an 18-question, knee-specific survey designed to detect improvement or deterioration in symptoms, function, and ability to participate in sports activities experienced by patients following knee surgery or other interventions. The individual items are summed and transformed into a 0- to 100-point scale, with high scores representing higher levels of function and minimal symptoms. The IKDC is utilized to assess a variety of knee conditions including ligament, meniscus, articular cartilage, osteoarthritis, and patellofemoral pain.48,49 Thus, this form can be used to assess any condition involving the knee and allow comparison between groups with different diagnoses. The IKDC has been validated for an ACL reconstruction population,47 has been used to assess outcomes in recent clinical studies on ACL reconstruction,50,51 and is one of the most frequently used measures for patients with ACL deficiency.3 The validity, responsiveness, and reliability of the IKDC subjective form has been confirmed for both adult and adolescent populations.48,49,52-54
TEGNER ACTIVITY SCORES
The Tegner activity score was developed in 1985 and was designed to provide an objective value for a patient’s activity level.44 This scale was developed to complement the Lysholm score. It consists of 1 sport-specific activity level question on a 0 to 10 scale that evaluates an individual’s ability to compete in a sporting activity. Scores between 1 and 5 represent work or recreational sports. Scores >5 represent higher-level recreational and competitive sports. The Tegner activity score is one of the most widely used activity scoring systems for patients with knee disorders,55,56 commonly utilized with the Lysholm Knee Score.44 One disadvantage of the Tegner activity score is that it relates to specific sports rather than functional activities, which limits its generalizability. We are not aware of any studies documenting the reliability or validity of this instrument.
Continue on: Marx Activity Rating Scale...
MARX ACTIVITY RATING SCALE
The Marx activity rating scale was developed to be utilized with other knee rating scales and outcome measures as an activity assessment.57 In contrast to the Tegner activity score, the Marx activity rating scale measures function rather than sport-specific activity. The scale is a short, patient-based activity assessment that consists of a 4-question survey evaluating patients’ knee health by recording the frequency and intensity of participation in a sporting activity. Questions are scored from 0 to 4 on the basis of how often the activity is performed. The 4 sections of the Marx scale that are rated include running, cutting, decelerating, and pivoting. This scale has been validated in patients with ACL injuries, chondromalacia patellae, and meniscal lesions.42,56-58 Acceptable ceiling effects of 3% and floor effects of 8% were noted in the study of ACL-injured patients.57
AMERICAN ACADEMY OF ORTHOPAEDIC SURGEONS (AAOS) SPORTS KNEE SCALE
The American Academy of Orthopaedic Surgeons (AAOS) Sports Knee Rating Scale consists of 5 parts and 23 items, including a section addressing stiffness, swelling, pain and function (7 questions), locking/catching (4 questions), giving way (4 questions), limitations of activity (4 questions), and pain with activity (4 questions).59,60 Items may be dropped if patients select particular responses, which can lead to difficulties when using the survey. This scoring system has been found to be satisfactory when all subscales were combined and the mean was calculated.42
DISCUSSION
PRO measures play an increasingly important role in the measurement of success and impact of health care services. Specifically, for ACL reconstruction, patient satisfaction is key for demonstrating the value of operative or other interventions. Selecting a suitable outcome measurement tool can be daunting, as it can be difficult to ascertain which outcome measures are appropriate for the patient or disorder in question. As there is currently no instrument that is universally superior in the evaluation of ACL outcomes, clinicians must consider the specific patient population in which the outcome instrument has been evaluated. Investigators should also use instruments with reported minimal clinically important differences so that variation in scores can be interpreted as either clinically significant or not. When choosing which outcome instrument to use, there is rarely a single appropriate rating system that is entirely comprehensive. In most cases, a general health outcome measure should be used in combination with a condition-specific rating scale. Activity rating scales, such as Marx or Tegner, should be included, especially when evaluating patients with low-activity lifestyles.
CONCLUSION
There are a number of reliable, valid, and responsive outcome measures that can be utilized to evaluate outcomes following ACL reconstruction in an array of patient populations. Outcome measures should be relevant to patients, easy to use, reliable, valid, and responsive to change. By increasing familiarity with these outcome measures, orthopedic surgeons and investigators can develop better studies, interpret data, and implement findings in practice with sound and informed judgment. Future research should focus on identifying the most relevant outcome metrics for assessing function following ACL reconstruction.
This paper will be judged for the Resident Writer’s Award.
1. Mall NA, Chalmers PN, Moric M, et al. Incidence and trends of anterior cruciate ligament reconstruction in the United States. Am J Sports Med. 2014;42(10):2363-2370. doi:10.1177/0363546514542796.
2. Brophy RH, Schmitz L, Wright RW, et al. Return to play and future ACL injury risk after ACL reconstruction in soccer athletes from the Multicenter Orthopaedic Outcomes Network (MOON) group. Am J Sports Med. 2012;40(11):2517-2522. doi:10.1177/0363546512459476.
3. Johnson DS, Smith RB. Outcome measurement in the ACL deficient knee- what’s the score? Knee. 2001;8(1):51-57. doi:10.1016/S0968-0160(01)00068-0.
4. Graham B, Green A, James M, Katz J, Swiontkowski M. Measuring patient satisfaction in orthopaedic surgery. J Bone Joint Surg Am. 2015;97(1):80-84. doi:10.2106/JBJS.N.00811.
5. Kocher MS, Steadman JR, Briggs K, Zurakowski D, Sterett WI, Hawkins RJ. Determinants of patient satisfaction with outcome after anterior cruciate ligament reconstruction. J Bone Joint Surg Am. 2002;84(9):1560-1572. doi:10.2106/00004623-200209000-00008.
6. Streiner DL, Norman GR. Health Measurement Scales: A Practical Guide to their Development and Use. Oxford: Oxford University Press; 1989.
7. Terwee CB, Bot SD, de Boer MR, et al. Quality criteria were proposed for measurement properties of health status questionnaires. J Clin Epidemiol. 2007;60(1):34-42. doi:10.1016/j.jclinepi.2006.03.012.
8. Bartko JJ. The intraclass correlation coefficient as a measure of reliability. Psychol Rep. 1966;19(1):3-11. doi:10.2466/pr0.1966.19.1.3.
9. Scholtes VA, Terwee CB, Poolman RW. What makes a measurement instrument valid and reliable? Injury. 2011;42(3):236-240. doi:10.1016/j.injury.2010.11.042.
10. Fries J, Rose M, Krishnan E. The PROMIS of better outcome assessment: responsiveness, floor and ceiling effects, and internet administration. J Rheumatol. 2011;38(8):1759-1764. doi:10.3899/jrheum.110402.
11. Bellamy N, Buchanan WW, Goldsmith CH, Campbell J, Stitt L. Validation study of WOMAC: a health status instrument for measuring clinically important patient relevant outcomes to antirheumatic drug therapy in patients with osteoarthritis of the hip or knee. J Rheumatol. 1988;15(12):1833-1840.
12. Gandek B. Measurement properties of the Western Ontario and McMaster Universities Osteoarthritis Index: a systematic review. Arthritis Care Res (Hoboken). 2015;67(2):216-229. doi:10.1002/acr.22415.
13. Angst F, Aeschlimann A, Stucki G. Smallest detectable and minimal clinically important differences of rehabilitation intervention with their implications for required sample sizes using WOMAC and SF-36 quality of life measurement instruments in patients with osteoarthritis of the lower extremities. Arthritis Rheum. 2001;45(4):384-391. doi:10.1002/1529-0131(200108)45:4<384::AID-ART352>3.0.CO;2-0.
14. Ryser L, Wright BD, Aeschlimann A, Mariacher-Gehler S, Stuckl G. A new look at the Western Ontario and McMaster Universities Osteoarthritis Index using Rasch analysis. Arthritis Care Res. 1999;12(5):331-335.
15. Wolfe F, Kong SX. Rasch analysis of the Western Ontario MacMaster questionnaire (WOMAC) in 2205 patients with osteoarthritis, rheumatoid arthritis, and fibromyalgia. Ann Rheum Dis. 1999;58(9):563-568. doi:10.1136/ard.58.9.563.
16. Roos EM, Roos HP, Lohmander LS, Ekdahl C, Beynnon BD. Knee Injury and Osteoarthritis Outcome Score (KOOS)—development of a self-administered outcome measure. J Orthop Sports Phys Ther. 1998;28(2):88-96. doi:10.2519/jospt.1998.28.2.88.
17. Salavati M, Akhbari B, Mohammadi F, Mazaheri M, Khorrami M. Knee injury and Osteoarthritis Outcome Score (KOOS): reliability and validity in competitive athletes after anterior cruciate ligament reconstruction. Osteoarthritis Cartilage. 2011;19(4):406-410. doi:10.1016/j.joca.2011.01.010.
18. Roos EM, Roos HP, Lohmander LS. WOMAC Osteoarthritis Index—additional dimensions for use in subjects with post-traumatic osteoarthritis of the knee. Western Ontario and MacMaster Universities. Osteoarthritis Cartilage. 1999;7(2):216-221. doi:10.1053/joca.1998.0153.
19. Roos EM, Toksvig-Larsen S. Knee injury and osteoarthritis outcome score (KOOS)—validation and comparison to the WOMAC in total knee replacement. Health Qual Life Outcomes. 2003;1(1):17. doi:10.1186/1477-7525-1-17.
20. Paradowski PT, Bergman S, Sunden-Lundius A, Lohmander LS, Roos EM. Knee complaints vary with age and gender in the adult population: population-based reference data for the Knee injury and Osteoarthritis Outcome Score (KOOS). BMC Musculoskeletal Disord. 2006;7(1):38. doi:10.1186/1471-2474-7-38.
21. MARS Group. Effect of graft choice on the outcome of revision anterior cruciate ligament reconstruction in the Multicenter ACL Revision Study (MARS) Cohort. Am J Sports Med. 2014;42(10):2301-2310. doi:10.1177/0363546514549005.
22. Ventura A, Legnani C, Terzaghi C, Borgo E, Albisetti W. Revision surgery after failed ACL reconstruction with artificial ligaments: clinical, histologic and radiographic evaluation. Eur J Orthop Surg Traumatol. 2014;21(1):93-98. doi:10.1007/s00590-012-1136-3.
23. Wasserstein D, Huston LJ, Nwosu S, et al. KOOS pain as a marker for significant knee pain two and six years after primary ACL reconstruction: a Multicenter Orthopaedic Outcomes Network (MOON) prospective longitudinal cohort study. Osteoarthritis Cartilage. 2015;23(10):1674-1684. doi:10.1016/j.joca.2015.05.025.
24. Zaffagnini S, Grassi A, Muccioli GM, et al. Return to sport after anterior cruciate ligament reconstruction in professional soccer players. Knee. 2014;21(3):731-735. doi:10.1016/j.knee.2014.02.005.
25. Duffee A, Magnussen RA, Pedroza AD, Flanigan DC; MOON Group, Kaeding CC. Transtibial ACL femoral tunnel preparation increases odds of repeat ipsilateral knee surgery. J Bone Joint Surg Am. 2013;95(22):2035-2042. doi:10.2106/JBJS.M.00187.
26. Bellamy N, Buchanan WW. A preliminary evaluation of the dimensionality and clinical importance of pain and disability in osteoarthritis of the hip and knee. Clin Rheumatol. 1986;5(2):231-241. doi:10.1007/BF02032362.
27. Fries J, Rose M, Krishnan E. The PROMIS of better outcome assessment: responsiveness, floor and ceiling effects, and Internet administration. J Rheumatol. 2011;38(8):1759-1764. doi:10.3899/jrheum.110402.
28. Hancock KJ, Glass NA, Anthony CA, et al. Performance of PROMIS for healthy patients undergoing meniscal surgery. J Bone Joint Surg Am. 2017;99(11):954-958. doi:10.2106/JBJS.16.00848.
29. Hung M, Clegg Do, Greene T, et al. Evaluation of the PROMIS physical function item bank in orthopedic patients. J Orthop Res. 2011;29(6):947-953. doi:10.1002/jor.21308.
30. Hung M, Baumhauer JF, Brodsky JW, et al; Orthopaedic Foot & Ankle Outcomes Research (OFAR) of the American Orthopaedic Foot & Ankle Society (AOFAS). Psychometric comparison of the PROMIS physical function CAT with the FAAM and FFI for measuring patient-reported outcomes. Foot Ankle Int. 2014;35(6):592-599. doi:10.1177/1071100714528492.
31. Hung M, Stuart AR, Higgins TF, Saltzman CL, Kubiak EN. Computerized adaptive testing using the PROMIS physical function item bank reduces test burden with less ceiling effects compared with the short musculoskeletal function assessment in orthopaedic trauma patients. J Orthop Trauma. 2014;28(8):439-443. doi:10.1097/BOT.0000000000000059.
32. Hancock, et al. PROMIS: A valid and efficient outcomes instrument for patients with ACL tears. KSSTA. In press.
33. Scott, et al. Performance of PROMIS physical function compared with KOOS, SF-36, Eq5D, and Marx activity scale in patients who undergo ACL reconstruction. In press.
34. Papuga MO, Beck CA, Kates SL, Schwarz EM, Maloney MD. Validation of GAITRite and PROMIS as high-throughput physical function outcome measures following ACL reconstruction. J Orthop Res. 2014;32(6):793-801. doi:10.1002/jor.22591.
35. Mohtadi N. Development and validation of the quality of life outcome measure (questionnaire) for chronic anterior cruciate ligament deficiency. Am J Sports Med. 1998;26(3):350-359. doi:10.1177/03635465980260030201.
36. Lafave MR, Hiemstra L, Kerslake S, Heard M, Buchko G. Validity, reliability, and responsiveness of the anterior cruciate ligament quality of life measure: a continuation of its overall validation. Clin J Sport Med. 2017;27(1):57-63. doi:10.1097/JSM.0000000000000292.
37. Noyes FR, McGinniss GH, Mooar LA. Functional disability in the anterior cruciate insufficient knee syndrome: Review of knee rating systems and projected risk factors in determining treatment. Sports Med. 1984;1(4):278-302. doi:10.2165/00007256-198401040-00004.
38. Noyes FR, Matthews DS, Mooar PA, Grood ES. The symptomatic anterior cruciate-deficient knee: Part II. The results of rehabilitation, activity modification, and counseling on functional disability. J Bone Joint Surg Am. 1983;65(2):163-174. doi:10.2106/00004623-198365020-00004.
39. Barber-Westin SD, Noyes FR, McCloskey JW. Rigorous statistical reliability, validity, and responsiveness testing of the Cincinnati knee rating system in 350 subjects with uninjured, injured, or anterior cruciate ligament-reconstructed knees. Am J Sports Med. 1999;27(4):402-416. doi:10.1177/03635465990270040201.
40. Bollen S, Seedhorn BB. A comparison of the Lysholm and Cincinnati knee scoring questionnaires. Am J Sports Med. 1991;19(2):189-190. doi:10.1177/036354659101900215.
41. Sgaglione NA, Del Pizzo W, Fox JM, Friedman MJ. Critical analysis of knee ligament rating systems. Am J Sports Med. 1995;23(6):660-667. doi:10.1177/036354659502300604.
42. Marx RG, Jones EC, Allen AA, et al. Reliability, validity, and responsiveness of four knee outcome scales for athletic patients. J Bone Joint Surg Am. 2001;83(10):1459-1469. doi:10.2106/00004623-200110000-00001.
43. Lysholm J, Gillquist J. Evaluation of knee ligament surgery results with special emphasis on use of a scoring scale. Am J Sports Med. 1982;10(3):150-154. doi:10.1177/036354658201000306.
44. Tegner Y, Lysholm J. Rating systems in the evaluation of knee ligament injuries. Clin Orthop Relat Res. 1985;198:43-49. doi:10.1097/00003086-198509000-00007.
45. Lukianov AV, Gillquist J, Grana WA, DeHaven KE. An anterior cruciate ligament (ACL) evaluation format for assessment of artificial or autologous anterior cruciate reconstruction results. Clin Orthop Relat Res. 1987;218:167-180. doi:10.1097/00003086-198705000-00024.
46. Bengtsson J, Mollborg J, Werner S. A study for testing the sensitivity and reliability of the Lysholm knee scoring scale. Knee Surg Sports Traumatol Arthrosc. 1996;4(1):27-31. doi:10.1007/BF01565994.
47. Risberg MA, Holm I, Steen J, Beynnon BD. Sensitivity to changes over time for the IKDC form, the Lysholm score, and the Cincinnati knee score. A prospective study of 120 ACL reconstructed patients with a 2-year follow-up. Knee Surg Sports Traumatol Arthrosc. 1999;7(3):152-159. doi:10.1007/s001670050140.
48. Irrgang JJ, Anderson AF, Boland AL, et al. Development and validation of the international knee documentation committee subjective knee form. Am J Sports Med. 2001;29(5):600-613. doi:10.1177/03635465010290051301.
49. Irrgang JJ, Anderson AF, Boland AL, et al. Responsiveness of the International Knee Documentation Committee Subjective Knee Form. Am J Sports Med. 2006;34(10):1567-1573. doi:10.1177/0363546506288855.
50. Logerstedt D, Di Stasi S, Grindem H, et al. Self-reported knee function can identify athletes who fail return-to-activity criteria up to 1 year after anterior cruciate ligament reconstruction: a Delaware-Oslo ACL cohort study. J Orthop Sports Phys Ther. 2014;44(2):914-923. doi:10.2519/jospt.2014.4852.
51. Lentz TA, Zeppieri G Jr, George SZ, et al. Comparison of physical impairment, functional and psychosocial measures based on fear of reinjury/lack of confidence and return-to-sport status after ACL reconstruction. Am J Sports Med. 2015;43(2):345-353. doi:10.1177/0363546514559707.
52. Greco NJ, Anderson AF, Mann BJ, et al. Responsiveness of the International Knee Documentation Committee Subjective Knee Form in comparison to the Western Ontario and McMaster Universities Osteoarthritis Index, modified Cincinnati Knee Rating System, and Short Form 36 in patients with focal articular cartilage defects. Am J Sports Med. 2010;38(5):891-902. doi:10.1177/0363546509354163.
53. Hefti F, Muller W, Jakob RP, Staubli HU. Evaluation of knee ligament injuries with the IKDC form. Knee Surg Sports Traumatol Arthrosc. 1993;1(3-4):226-234. doi:10.1007/BF01560215.
54. Schmitt LC, Paterno MV, Huang S. Validity and internal consistency of the International Knee Documentation Committee Subjective Knee Evaluation Form in children and adolescents. Am J Sports Med. 2010;38(12):2443-2447. doi:10.1177/0363546510374873.
55. Briggs KK, Lysholm J, Tegner Y, Rodkey WG, Kocher MS, Steadman JR. The reliability, validity, and responsiveness of the Lysholm and Tegner activity scale for anterior cruciate ligament injuries of the knee: 25 years later. Am J Sports Med. 2009;37(5):890-897. doi:10.1177/0363546508330143.
56. Negahban H, Mostafaee N, Sohani SM, et al. Reliability and validity of the Tegner and Marx activity rating scales in Iranian patients with anterior cruciate ligament injury. Disabil Rehabil. 2011;33(23-24):2305-2310. doi:10.3109/09638288.2011.570409.
57. Marx RG, Stump TJ, Jones EC, Wickiewicz TL, Warren RF. Development and evaluation of an activity rating scale for disorders of the knee. Am J Sports Med. 2001;29(2):213-218. doi:10.1177/03635465010290021601.
58. Garratt AM, Brealey S, Gillespie WJ, in collaboration with the DAM-ASK Trial Team. Patient-assessed health instruments for the knee: a structured review. Rheumatology. 2004;43(11):1414-1423. doi:10.1093/rheumatology/keh362.
59. American Academy of Orthopaedic Surgeons. Scoring algorithms for the lower limb: Outcomes data collection instrument. Rosemon, IL: American Academy of Orthopaedic Surgeons; 1998.
60. Johanson NA, Liang MH, Daltroy L, Rudicel S, Richmond J. American Academy of Orthopaedic Surgeons lower limb outcomes assessment instruments. Reliability, validity, and sensitivity to change. J Bone Joint Surg Am. 2004;86-A(5):902-909.
1. Mall NA, Chalmers PN, Moric M, et al. Incidence and trends of anterior cruciate ligament reconstruction in the United States. Am J Sports Med. 2014;42(10):2363-2370. doi:10.1177/0363546514542796.
2. Brophy RH, Schmitz L, Wright RW, et al. Return to play and future ACL injury risk after ACL reconstruction in soccer athletes from the Multicenter Orthopaedic Outcomes Network (MOON) group. Am J Sports Med. 2012;40(11):2517-2522. doi:10.1177/0363546512459476.
3. Johnson DS, Smith RB. Outcome measurement in the ACL deficient knee- what’s the score? Knee. 2001;8(1):51-57. doi:10.1016/S0968-0160(01)00068-0.
4. Graham B, Green A, James M, Katz J, Swiontkowski M. Measuring patient satisfaction in orthopaedic surgery. J Bone Joint Surg Am. 2015;97(1):80-84. doi:10.2106/JBJS.N.00811.
5. Kocher MS, Steadman JR, Briggs K, Zurakowski D, Sterett WI, Hawkins RJ. Determinants of patient satisfaction with outcome after anterior cruciate ligament reconstruction. J Bone Joint Surg Am. 2002;84(9):1560-1572. doi:10.2106/00004623-200209000-00008.
6. Streiner DL, Norman GR. Health Measurement Scales: A Practical Guide to their Development and Use. Oxford: Oxford University Press; 1989.
7. Terwee CB, Bot SD, de Boer MR, et al. Quality criteria were proposed for measurement properties of health status questionnaires. J Clin Epidemiol. 2007;60(1):34-42. doi:10.1016/j.jclinepi.2006.03.012.
8. Bartko JJ. The intraclass correlation coefficient as a measure of reliability. Psychol Rep. 1966;19(1):3-11. doi:10.2466/pr0.1966.19.1.3.
9. Scholtes VA, Terwee CB, Poolman RW. What makes a measurement instrument valid and reliable? Injury. 2011;42(3):236-240. doi:10.1016/j.injury.2010.11.042.
10. Fries J, Rose M, Krishnan E. The PROMIS of better outcome assessment: responsiveness, floor and ceiling effects, and internet administration. J Rheumatol. 2011;38(8):1759-1764. doi:10.3899/jrheum.110402.
11. Bellamy N, Buchanan WW, Goldsmith CH, Campbell J, Stitt L. Validation study of WOMAC: a health status instrument for measuring clinically important patient relevant outcomes to antirheumatic drug therapy in patients with osteoarthritis of the hip or knee. J Rheumatol. 1988;15(12):1833-1840.
12. Gandek B. Measurement properties of the Western Ontario and McMaster Universities Osteoarthritis Index: a systematic review. Arthritis Care Res (Hoboken). 2015;67(2):216-229. doi:10.1002/acr.22415.
13. Angst F, Aeschlimann A, Stucki G. Smallest detectable and minimal clinically important differences of rehabilitation intervention with their implications for required sample sizes using WOMAC and SF-36 quality of life measurement instruments in patients with osteoarthritis of the lower extremities. Arthritis Rheum. 2001;45(4):384-391. doi:10.1002/1529-0131(200108)45:4<384::AID-ART352>3.0.CO;2-0.
14. Ryser L, Wright BD, Aeschlimann A, Mariacher-Gehler S, Stuckl G. A new look at the Western Ontario and McMaster Universities Osteoarthritis Index using Rasch analysis. Arthritis Care Res. 1999;12(5):331-335.
15. Wolfe F, Kong SX. Rasch analysis of the Western Ontario MacMaster questionnaire (WOMAC) in 2205 patients with osteoarthritis, rheumatoid arthritis, and fibromyalgia. Ann Rheum Dis. 1999;58(9):563-568. doi:10.1136/ard.58.9.563.
16. Roos EM, Roos HP, Lohmander LS, Ekdahl C, Beynnon BD. Knee Injury and Osteoarthritis Outcome Score (KOOS)—development of a self-administered outcome measure. J Orthop Sports Phys Ther. 1998;28(2):88-96. doi:10.2519/jospt.1998.28.2.88.
17. Salavati M, Akhbari B, Mohammadi F, Mazaheri M, Khorrami M. Knee injury and Osteoarthritis Outcome Score (KOOS): reliability and validity in competitive athletes after anterior cruciate ligament reconstruction. Osteoarthritis Cartilage. 2011;19(4):406-410. doi:10.1016/j.joca.2011.01.010.
18. Roos EM, Roos HP, Lohmander LS. WOMAC Osteoarthritis Index—additional dimensions for use in subjects with post-traumatic osteoarthritis of the knee. Western Ontario and MacMaster Universities. Osteoarthritis Cartilage. 1999;7(2):216-221. doi:10.1053/joca.1998.0153.
19. Roos EM, Toksvig-Larsen S. Knee injury and osteoarthritis outcome score (KOOS)—validation and comparison to the WOMAC in total knee replacement. Health Qual Life Outcomes. 2003;1(1):17. doi:10.1186/1477-7525-1-17.
20. Paradowski PT, Bergman S, Sunden-Lundius A, Lohmander LS, Roos EM. Knee complaints vary with age and gender in the adult population: population-based reference data for the Knee injury and Osteoarthritis Outcome Score (KOOS). BMC Musculoskeletal Disord. 2006;7(1):38. doi:10.1186/1471-2474-7-38.
21. MARS Group. Effect of graft choice on the outcome of revision anterior cruciate ligament reconstruction in the Multicenter ACL Revision Study (MARS) Cohort. Am J Sports Med. 2014;42(10):2301-2310. doi:10.1177/0363546514549005.
22. Ventura A, Legnani C, Terzaghi C, Borgo E, Albisetti W. Revision surgery after failed ACL reconstruction with artificial ligaments: clinical, histologic and radiographic evaluation. Eur J Orthop Surg Traumatol. 2014;21(1):93-98. doi:10.1007/s00590-012-1136-3.
23. Wasserstein D, Huston LJ, Nwosu S, et al. KOOS pain as a marker for significant knee pain two and six years after primary ACL reconstruction: a Multicenter Orthopaedic Outcomes Network (MOON) prospective longitudinal cohort study. Osteoarthritis Cartilage. 2015;23(10):1674-1684. doi:10.1016/j.joca.2015.05.025.
24. Zaffagnini S, Grassi A, Muccioli GM, et al. Return to sport after anterior cruciate ligament reconstruction in professional soccer players. Knee. 2014;21(3):731-735. doi:10.1016/j.knee.2014.02.005.
25. Duffee A, Magnussen RA, Pedroza AD, Flanigan DC; MOON Group, Kaeding CC. Transtibial ACL femoral tunnel preparation increases odds of repeat ipsilateral knee surgery. J Bone Joint Surg Am. 2013;95(22):2035-2042. doi:10.2106/JBJS.M.00187.
26. Bellamy N, Buchanan WW. A preliminary evaluation of the dimensionality and clinical importance of pain and disability in osteoarthritis of the hip and knee. Clin Rheumatol. 1986;5(2):231-241. doi:10.1007/BF02032362.
27. Fries J, Rose M, Krishnan E. The PROMIS of better outcome assessment: responsiveness, floor and ceiling effects, and Internet administration. J Rheumatol. 2011;38(8):1759-1764. doi:10.3899/jrheum.110402.
28. Hancock KJ, Glass NA, Anthony CA, et al. Performance of PROMIS for healthy patients undergoing meniscal surgery. J Bone Joint Surg Am. 2017;99(11):954-958. doi:10.2106/JBJS.16.00848.
29. Hung M, Clegg Do, Greene T, et al. Evaluation of the PROMIS physical function item bank in orthopedic patients. J Orthop Res. 2011;29(6):947-953. doi:10.1002/jor.21308.
30. Hung M, Baumhauer JF, Brodsky JW, et al; Orthopaedic Foot & Ankle Outcomes Research (OFAR) of the American Orthopaedic Foot & Ankle Society (AOFAS). Psychometric comparison of the PROMIS physical function CAT with the FAAM and FFI for measuring patient-reported outcomes. Foot Ankle Int. 2014;35(6):592-599. doi:10.1177/1071100714528492.
31. Hung M, Stuart AR, Higgins TF, Saltzman CL, Kubiak EN. Computerized adaptive testing using the PROMIS physical function item bank reduces test burden with less ceiling effects compared with the short musculoskeletal function assessment in orthopaedic trauma patients. J Orthop Trauma. 2014;28(8):439-443. doi:10.1097/BOT.0000000000000059.
32. Hancock, et al. PROMIS: A valid and efficient outcomes instrument for patients with ACL tears. KSSTA. In press.
33. Scott, et al. Performance of PROMIS physical function compared with KOOS, SF-36, Eq5D, and Marx activity scale in patients who undergo ACL reconstruction. In press.
34. Papuga MO, Beck CA, Kates SL, Schwarz EM, Maloney MD. Validation of GAITRite and PROMIS as high-throughput physical function outcome measures following ACL reconstruction. J Orthop Res. 2014;32(6):793-801. doi:10.1002/jor.22591.
35. Mohtadi N. Development and validation of the quality of life outcome measure (questionnaire) for chronic anterior cruciate ligament deficiency. Am J Sports Med. 1998;26(3):350-359. doi:10.1177/03635465980260030201.
36. Lafave MR, Hiemstra L, Kerslake S, Heard M, Buchko G. Validity, reliability, and responsiveness of the anterior cruciate ligament quality of life measure: a continuation of its overall validation. Clin J Sport Med. 2017;27(1):57-63. doi:10.1097/JSM.0000000000000292.
37. Noyes FR, McGinniss GH, Mooar LA. Functional disability in the anterior cruciate insufficient knee syndrome: Review of knee rating systems and projected risk factors in determining treatment. Sports Med. 1984;1(4):278-302. doi:10.2165/00007256-198401040-00004.
38. Noyes FR, Matthews DS, Mooar PA, Grood ES. The symptomatic anterior cruciate-deficient knee: Part II. The results of rehabilitation, activity modification, and counseling on functional disability. J Bone Joint Surg Am. 1983;65(2):163-174. doi:10.2106/00004623-198365020-00004.
39. Barber-Westin SD, Noyes FR, McCloskey JW. Rigorous statistical reliability, validity, and responsiveness testing of the Cincinnati knee rating system in 350 subjects with uninjured, injured, or anterior cruciate ligament-reconstructed knees. Am J Sports Med. 1999;27(4):402-416. doi:10.1177/03635465990270040201.
40. Bollen S, Seedhorn BB. A comparison of the Lysholm and Cincinnati knee scoring questionnaires. Am J Sports Med. 1991;19(2):189-190. doi:10.1177/036354659101900215.
41. Sgaglione NA, Del Pizzo W, Fox JM, Friedman MJ. Critical analysis of knee ligament rating systems. Am J Sports Med. 1995;23(6):660-667. doi:10.1177/036354659502300604.
42. Marx RG, Jones EC, Allen AA, et al. Reliability, validity, and responsiveness of four knee outcome scales for athletic patients. J Bone Joint Surg Am. 2001;83(10):1459-1469. doi:10.2106/00004623-200110000-00001.
43. Lysholm J, Gillquist J. Evaluation of knee ligament surgery results with special emphasis on use of a scoring scale. Am J Sports Med. 1982;10(3):150-154. doi:10.1177/036354658201000306.
44. Tegner Y, Lysholm J. Rating systems in the evaluation of knee ligament injuries. Clin Orthop Relat Res. 1985;198:43-49. doi:10.1097/00003086-198509000-00007.
45. Lukianov AV, Gillquist J, Grana WA, DeHaven KE. An anterior cruciate ligament (ACL) evaluation format for assessment of artificial or autologous anterior cruciate reconstruction results. Clin Orthop Relat Res. 1987;218:167-180. doi:10.1097/00003086-198705000-00024.
46. Bengtsson J, Mollborg J, Werner S. A study for testing the sensitivity and reliability of the Lysholm knee scoring scale. Knee Surg Sports Traumatol Arthrosc. 1996;4(1):27-31. doi:10.1007/BF01565994.
47. Risberg MA, Holm I, Steen J, Beynnon BD. Sensitivity to changes over time for the IKDC form, the Lysholm score, and the Cincinnati knee score. A prospective study of 120 ACL reconstructed patients with a 2-year follow-up. Knee Surg Sports Traumatol Arthrosc. 1999;7(3):152-159. doi:10.1007/s001670050140.
48. Irrgang JJ, Anderson AF, Boland AL, et al. Development and validation of the international knee documentation committee subjective knee form. Am J Sports Med. 2001;29(5):600-613. doi:10.1177/03635465010290051301.
49. Irrgang JJ, Anderson AF, Boland AL, et al. Responsiveness of the International Knee Documentation Committee Subjective Knee Form. Am J Sports Med. 2006;34(10):1567-1573. doi:10.1177/0363546506288855.
50. Logerstedt D, Di Stasi S, Grindem H, et al. Self-reported knee function can identify athletes who fail return-to-activity criteria up to 1 year after anterior cruciate ligament reconstruction: a Delaware-Oslo ACL cohort study. J Orthop Sports Phys Ther. 2014;44(2):914-923. doi:10.2519/jospt.2014.4852.
51. Lentz TA, Zeppieri G Jr, George SZ, et al. Comparison of physical impairment, functional and psychosocial measures based on fear of reinjury/lack of confidence and return-to-sport status after ACL reconstruction. Am J Sports Med. 2015;43(2):345-353. doi:10.1177/0363546514559707.
52. Greco NJ, Anderson AF, Mann BJ, et al. Responsiveness of the International Knee Documentation Committee Subjective Knee Form in comparison to the Western Ontario and McMaster Universities Osteoarthritis Index, modified Cincinnati Knee Rating System, and Short Form 36 in patients with focal articular cartilage defects. Am J Sports Med. 2010;38(5):891-902. doi:10.1177/0363546509354163.
53. Hefti F, Muller W, Jakob RP, Staubli HU. Evaluation of knee ligament injuries with the IKDC form. Knee Surg Sports Traumatol Arthrosc. 1993;1(3-4):226-234. doi:10.1007/BF01560215.
54. Schmitt LC, Paterno MV, Huang S. Validity and internal consistency of the International Knee Documentation Committee Subjective Knee Evaluation Form in children and adolescents. Am J Sports Med. 2010;38(12):2443-2447. doi:10.1177/0363546510374873.
55. Briggs KK, Lysholm J, Tegner Y, Rodkey WG, Kocher MS, Steadman JR. The reliability, validity, and responsiveness of the Lysholm and Tegner activity scale for anterior cruciate ligament injuries of the knee: 25 years later. Am J Sports Med. 2009;37(5):890-897. doi:10.1177/0363546508330143.
56. Negahban H, Mostafaee N, Sohani SM, et al. Reliability and validity of the Tegner and Marx activity rating scales in Iranian patients with anterior cruciate ligament injury. Disabil Rehabil. 2011;33(23-24):2305-2310. doi:10.3109/09638288.2011.570409.
57. Marx RG, Stump TJ, Jones EC, Wickiewicz TL, Warren RF. Development and evaluation of an activity rating scale for disorders of the knee. Am J Sports Med. 2001;29(2):213-218. doi:10.1177/03635465010290021601.
58. Garratt AM, Brealey S, Gillespie WJ, in collaboration with the DAM-ASK Trial Team. Patient-assessed health instruments for the knee: a structured review. Rheumatology. 2004;43(11):1414-1423. doi:10.1093/rheumatology/keh362.
59. American Academy of Orthopaedic Surgeons. Scoring algorithms for the lower limb: Outcomes data collection instrument. Rosemon, IL: American Academy of Orthopaedic Surgeons; 1998.
60. Johanson NA, Liang MH, Daltroy L, Rudicel S, Richmond J. American Academy of Orthopaedic Surgeons lower limb outcomes assessment instruments. Reliability, validity, and sensitivity to change. J Bone Joint Surg Am. 2004;86-A(5):902-909.
TAKE-HOME POINTS
- PRO instruments are widely used to capture patient perception of general health, QOL, daily function, and pain, and are an essential part of evaluation after ACL reconstruction.
- ACL outcome measures vary widely in regards to their validity, reliability, minimal clinically important difference, and applicability to specific patient populations.
- There is currently no standardized instrument universally accepted as superior following ACL reconstruction.
- In most cases, a general health outcome measure should be used in combination with a condition-specific rating scale.
- Activity rating scales, such as Marx or Tegner, should be included when evaluating patients with low-activity lifestyles.
The Evidence for Herbal and Botanical Remedies
The National Center for Complementary and Integrative Health, a division of the National Institutes of Medicine, estimates that 38% of American adults use complementary and alternative medicine (including 17.7% who say they use “natural products”).1 Despite the popularity of these products, many providers remain skeptical—and for good reason. Enthusiasts may offer dramatic anecdotes to “prove” their supplements’ worth, but little scientific support is available for most herbal remedies. There are, however, exceptions—capsaicin, butterbur, green tea, and peppermint—as this review of the medical literature reveals.
Worth noting as you consider this—or any—review of herbals is that while there is limited scientific evidence to establish the safety and efficacy of most herbal products, they are nonetheless freely sold without FDA approval because, under current regulations, they are considered dietary supplements. That legal designation means companies can manufacture, sell, and market herbs without first demonstrating safety and efficacy, as is required for pharmaceutical drugs. Because herbal medications do not require the same testing through the large randomized controlled trials (RCTs) required for pharmaceuticals, evidence is often based on smaller RCTs and other studies of lower overall quality. Despite these limitations, we believe it’s worth keeping an open mind about the value of evidence-based herbal and botanical treatments.
CAPSAICIN
Capsaicin, an active compound in chili peppers, provokes a burning sensation but also has a long history of use in pain treatment.2 Qutenza, an FDA-approved, chemically synthesized 8% capsaicin patch, is identical to the naturally occurring molecule.2 Topical capsaicin exerts its therapeutic effect by rapidly depleting substance P, thus reducing the transmission of pain from C fibers to higher neurologic centers in the area of administration.3
Meta-analyses and systematic reviews have shown capsaicin is effective for various painful conditions, including peripheral diabetic neuropathy, osteoarthritis (OA), low back pain (LBP), and postherpetic neuralgia (PHN).
Peripheral neuropathy. A Cochrane review of six randomized, double-blind, placebo-controlled studies of at least six weeks’ duration using topical 8% capsaicin to treat PHN and HIV-associated neuropathy concluded that high-concentration topical capsaicin provided more relief in patients with high pain levels than control patients who received a subtherapeutic (0.04%) capsaicin cream. Number-needed-to-treat values were between 8 and 12. Local adverse events were common, but not consistently reported enough to calculate a number needed to harm.4
OA. In randomized trials, capsaicin provided mild-to-moderate efficacy for patients with hand and knee OA, when compared with placebo.5-7 A systematic review of capsaicin for all osteoarthritic conditions noted that there was consistent evidence that capsaicin gel was effective for OA.8 However, a 2013 Cochrane review of only knee OA noted that capsicum extract did not provide significant clinical improvement for pain or function and resulted in a significant number of adverse events.9
LBP. Based on a 2014 Cochrane review of three trials (755 subjects) of moderate quality, capsicum frutescens cream or plaster appeared more efficacious than placebo in people with chronic LBP.10 Based on current (low-quality) evidence in one trial, however, it’s not clear whether topical capsicum cream is more beneficial for acute LBP than placebo.10
PHN. Topical capsaicin is an FDA-approved treatment for PHN. A review and cost-effectiveness analysis demonstrated that 8% capsaicin had significantly higher effectiveness rates than the oral agents (tricyclic antidepressants, duloxetine, gabapentin, pregabalin) used to treat PHN.11 The cost of the capsaicin patch was similar to a topical lidocaine patch and oral products for PHN.11 A meta-analysis of seven RCTs indicated that 8% topical capsaicin was superior to the low-dose capsaicin patch for relieving pain associated with PHN.12
Continue to: Adverse effects
Adverse effects
Very few toxic effects have been reported during a half-century of capsaicin use. Those that have been reported are mainly limited to mild local reactions.2 The most common adverse effect of topical capsaicin is local irritation (burning, stinging, and erythema), which was reported in approximately 40% of patients.6 Nevertheless, more than 90% of the subjects in clinical studies were able to complete the studies, and pain rapidly resolved after patch removal.2 Washing with soap and water may help prevent the compound from spreading to other parts of the body unintentionally.
The safety of the patch has been demonstrated with repeated dosing every three months for up to one year. However, the long-term risks of chronic capsaicin use and its effect on epidermal innervation are uncertain.5
The bottom line
Capsaicin appears to be an effective treatment for neuropathy and chronic LBP. It is FDA approved for the treatment of PHN. It may also benefit patients with OA and acute LBP. Serious adverse effects are uncommon with topical use. Common adverse effects include burning pain and irritation in the area of application, which can be intense and cause discontinuation.2
Continue to: BUTTERBUR
BUTTERBUR
Petasites hybridus, also known as butterbur, is a member of the daisy family, Asteraceae, and is a perennial plant found throughout Europe and Asia.13 It was used as a remedy for ulcers, wounds, and inflammation in ancient Greece. Its calcium channel–blocking effects may counteract vasoconstriction and play a role in preventing hyperexcitation of neurons.14 Sesquiterpenes, the pharmacologically active compounds in butterbur, have strong anti-inflammatory and vasodilatory effects through lipoxygenase and leukotriene inhibition.14
Migraine headache. Butterbur appears to be effective in migraine prophylaxis. Several studies have shown butterbur to significantly reduce the number of migraine attacks per month when compared with placebo. In a small, randomized, placebo-controlled, parallel-group study on the efficacy and tolerability of a special butterbur root extract (Petadolex) for the prevention of migraine, response rate was 45% in the butterbur group vs 15% in the placebo group. Butterbur was well tolerated.15 Similar results were found in another RCT in which butterbur 75 mg bid significantly reduced migraine frequency by 48%, compared with 26% for the placebo group.16 Butterbur was well tolerated in this study, too, and no serious adverse events occurred. Findings suggest that 75 mg bid may be a good option for migraine prevention, given the agent’s safety profile.
Petadolex may also be a good option in pediatric migraine. A 2005 study in children and adolescents found that 77% of patients experienced a reduction in attacks by at least 50% with butterbur. Patients were treated with 50 mg to 150 mg over four months.17
In their 2012 guidelines for migraine prevention, the American Academy of Neurology (AAN) and American Headache Society gave butterbur a Level A recommendation, concluding that butterbur should be offered to patients with migraine to reduce the frequency and severity of migraine attacks.18 However, the AAN changed its position in 2015, redacting the recommendation due to serious safety concerns.19
Allergic rhinitis. Although the data are not convincing, some studies have shown that butterbur may be beneficial for the treatment of allergic rhinitis.20,21
Continue to: Adverse effects
Adverse effects
While the butterbur plant itself contains pyrrolizidine alkaloids (PA), which are hepatotoxic and carcinogenic, extracts of butterbur root that are almost completely free from these alkaloids are available. Patients who choose to use butterbur should be advised to use only products that are certified and labeled PA free.
Petadolex, the medication used in migraine studies, was initially approved by the German health regulatory authority, but approval was later withdrawn due to concerns about liver toxicity.22 In 2012, the United Kingdom’s Medicines and Health Care Products Regulatory Agency withdrew all butterbur products from the market due to associated cases of liver toxicity.22 Butterbur products are still available in the US market, and the risks and benefits should be discussed with all patients considering this treatment. Liver function monitoring is recommended for all patients using butterbur.22
The herb can also cause dyspepsia, headache, itchy eyes, gastrointestinal symptoms, asthma, fatigue, and drowsiness. Additionally, people who are allergic to ragweed and daisies may have allergic reactions to butterbur. Eructation (belching) occurred in 7% of patients in a pediatric study.17
The bottom line
Butterbur appears to be efficacious for migraine prophylaxis, but long-term safety is unknown and serious concerns exist for liver toxicity.
Continue to: GREEN TEA
GREEN TEA
Most tea leaves come from the Camellia sinensis bush, but green and black tea are processed differently to produce different end products.23 It is estimated that green tea accounts for approximately a quarter of all tea consumption and is most commonly consumed in Asian countries.23 The health-promoting effects of green tea are mainly attributed to its polyphenol content.24 Of the many types of tea, green tea has the highest concentration of polyphenols, including catechins, which are powerful antioxidants.23,24 Green tea has been used in traditional Chinese and Indian medicine to control bleeding, improve digestion, and promote overall health.23
Dementia. Green tea polyphenols may enhance cognition and may protect against the development of dementia. In-vitro studies have shown that green tea reduces hydrogen peroxide and ß-amyloid peptides, which are significant in the development of Alzheimer’s disease.25 A 12-subject double-blind study found green tea increased working memory and had an impact on frontoparietal brain connections.26 Furthermore, a cohort study with 13,645 Japanese participants over a five-year period found that frequent green tea consumption (> 5 cups per day) was associated with a lower risk for dementia.27 Additional studies are needed, but green tea may be useful in the treatment or prevention of dementia in the future.
Coronary artery disease. In one study, green tea plasma and urinary concentrations were associated with plasma biomarkers of cardiovascular disease and diabetes.28 In one review, the consumption of green tea was associated with a statistically significant reduction in LDL cholesterol.29 Furthermore, a 2015 systematic review and meta-analysis of prospective observational studies concluded that increased tea consumption (of any type) is associated with a reduced risk for coronary heart disease, cardiac death, stroke, and total mortality.30
Cancer. Many studies have shown that green tea may reduce the risk for cancer, although epidemiologic evidence is inconsistent. Studies have shown that cancer rates tend to be lower in those who consume higher levels of green tea.31,32 Whether this can be attributed solely to green tea remains debatable. Several other studies have shown that polyphenols in green tea can inhibit the growth of cancer cells, but the exact mechanism by which tea interacts with cancerous cells is unknown.23
Several population-based studies have been performed, mostly in Japan, which showed green tea consumption reduced the risk for cancer. Fewer prostate cancer cases have been reported in men who consume green tea.33 While studies have been performed to determine whether green tea has effects on pancreatic, esophageal, ovarian, breast, bladder, and colorectal cancer, the evidence remains inadequate.32
Diabetes. Green tea has been shown in several studies to have a beneficial effect on diabetes. A retrospective Japanese cohort study showed that those who consumed green tea were one-third less likely to develop type 2 diabetes.34 A 10-year study from Taiwan found lower body fat and smaller waist circumference in those who consumed green tea regularly.35 A 2014 meta-analysis and systematic review of tea (any type) consumption and the risk for diabetes concluded that three or more cups of tea per day was associated with a lower risk for diabetes.36 Another meta-analysis of 17 RCTs focused on green tea concluded that green tea improves glucose control and A1C values.37
Continue to: Adverse effects
Adverse effects
There have been concerns about potential hepatotoxicity induced by green tea intake.38 However, a systematic review of 34 RCTs on liver-related adverse events from green tea showed only a slight elevation in liver function tests; no serious liver-related adverse events were reported.38 This review suggested that liver-related adverse events after intake of green tea extracts are rare.38
Consuming green tea in the diet may lower the risk for adverse effects since the concentration consumed is generally much lower than that found in extracts.
Contraindications to drinking green tea are few. Individuals with caffeine sensitivities could experience insomnia, anxiety, irritability, or upset stomach. Additionally, patients who are taking anticoagulation drugs, such as warfarin, should avoid green tea due to its vitamin K content, which can counter the effects of warfarin. Pregnant or breastfeeding women, those with heart problems or high blood pressure, kidney or liver problems, stomach ulcers, or anxiety disorders should use caution with green tea consumption.
The bottom line
Green tea consumption in the diet appears to be safe and may have beneficial effects on weight, dementia, and risk for diabetes, cancer, and cardiovascular disease. Patients may want to consider drinking green tea as part of a healthy diet, in combination with exercise.
Continue to: PEPPERMINT
PEPPERMINT
Mentha piperita, also known as peppermint, is a hybrid between water mint and spearmint. It is found throughout Europe and North America and is commonly used in tea and toothpaste and as a flavoring for gum. Menthol and methyl salicylate are the main active ingredients in peppermint, and peppermint has calcium channel–blocker effects.39 Menthol has been shown to help regulate cold and pain sensation through the TRPM8 receptor.40 The peppermint herb is used both orally and topically, and has been studied in the treatment of multiple conditions.
Irritable bowel syndrome (IBS). It appears that peppermint inhibits spontaneous peristaltic activity, which reduces gastric emptying, decreases basal tone in the gastrointestinal tract, and slows down peristalsis in the gut.39
The American College of Gastroenterology guidelines currently note that there is moderate-quality evidence for peppermint oil in the treatment of IBS.41 A Cochrane review concluded that peppermint appears to be beneficial for IBS-related symptoms and pain.42 In a systematic review of nine studies from 2014, peppermint oil was found to be more effective than placebo for IBS symptoms such as pain, bloating, gas, and diarrhea.43 The review also indicated that peppermint oil is safe, with heartburn being the most common complaint.43 A 2016 study also found that triple-coated microspheres containing peppermint oil reduced the frequency and intensity of IBS symptoms.44
Non-ulcer dyspepsia. In combination with caraway oil, peppermint oil can be used to reduce symptoms of non-ulcer dyspepsia.45,46 A multicenter, randomized, placebo-controlled, double-blind study found that 43.3% of subjects improved with a peppermint-caraway oil combination after eight weeks, compared with 3.5% receiving placebo.46
Barium enema–related colonic spasm. Peppermint can relax the lower esophageal sphincter, and it has been shown to be useful as an antispasmodic agent for barium enema–related colonic spasm.47,48
Itching/skin irritation. Peppermint, when applied topically, has been used to calm pruritus and relieve irritation and inflammation. It has a soothing and cooling effect on the skin. At least one study found it to be effective in the treatment of pruritus gravidarum, although the study population consisted of only 96 subjects.49
Migraine headache. Initial small trials suggest that menthol is likely beneficial for migraine headaches. A pilot trial of 25 patients treated with topical menthol 6% gel for an acute migraine attack showed a significant improvement in headache intensity two hours after gel application.50 In a randomized, triple-blind, placebo-controlled, crossover study of 35 patients, a menthol 10% solution was shown to be more efficacious as abortive treatment of migraine headaches than placebo.51
Tension headache. In a randomized, placebo-controlled, double-blind crossover study, topical peppermint oil showed a significant clinical reduction in tension headache pain.52 Another small, randomized, double-blind trial showed that tiger balm (containing menthol as the main ingredient) also produced statistically significant improvement in tension headache discomfort compared with placebo.53
Continue to: Musculoskeletal pain
Musculoskeletal pain. A small study comparing topical menthol to ice for muscle soreness noted decreased perceived discomfort with menthol.54 Menthol has also been shown to reduce pain in patients with knee OA.55
Carpal tunnel syndrome (CTS). A triple-blind RCT concluded that topical menthol acutely reduced pain intensity in slaughterhouse workers with CTS, and it should be considered as an effective nonsystemic alternative to regular analgesics in the workplace management of chronic and neuropathic pain.56
Adverse effects
Peppermint appears to be safe for most adults when used in small doses, and serious adverse effects are rare.43,57 While peppermint tea appears to be safe in moderate-to-large amounts, people allergic to plants in the peppermint family (eg, mint, thyme, sage, rosemary, marjoram, basil, lavender) may experience allergic reactions with swelling, wheals, or erythema. Peppermint may also cause heartburn due to relaxation of the cardiac sphincter.
Other symptoms may include nausea, vomiting, flushing, and headache.58 The herb may also be both hepatotoxic and nephrotoxic at extremely high doses.59 Other considerations for women are that it can trigger menstruation and should be avoided during pregnancy. Due to uncertain efficacy in this population, peppermint oil should not be used on the face of infants, young children, or pregnant women.58,59
The bottom line
Peppermint appears to be safe and well tolerated. It is useful in alleviating IBS symptoms and may be effective in the treatment of non-ulcerative dyspepsia, musculoskeletal pain, headache, and CTS.54,55
1. National Center for Complementary and Integrative Health. The Use of Complementary and Alternative Medicine in the United States. https://nccih.nih.gov/research/statistics/2007/camsurvey_fs1.htm. Accessed April 19, 2018.
2. Wallace M, Pappagallo M. Qutenza: a capsaicin 8% patch for the management of postherpetic neuralgia. Expert Rev Neurother. 2011;11:15-27.
3. Rains C, Bryson HM. Topical capsaicin. A review of its pharmacological properties and therapeutic potential in post-herpetic neuralgia, diabetic neuropathy and osteoarthritis. Drugs Aging. 1995;7:317-328.
4. Derry S, Sven-Rice A, Cole P, et al. Topical capsaicin (high concentration) for chronic neuropathic pain in adults. Cochrane Database Syst Rev. 2013;(2):CD007393.
5. Mason L, Moore RA, Derry S, et al. Systematic review of topical capsaicin for the treatment of chronic pain. BMJ. 2004;328:991.
6. Deal CL, Schnitzer TJ, Lipstein E, et al. Treatment of arthritis with topical capsaicin: a double-blind trial. Clin Ther. 1991; 13:383.
7. McCarthy GM, McCarty DJ. Effect of topical capsaicin in the therapy of painful osteoarthritis of the hands. J Rheumatol. 1992;19:604.
8. De Silva V, El-Metwally A, Ernst E, et al; Arthritis Research UK Working Group on Complementary and Alternative Medicines. Evidence for the efficacy of complementary and alternative medicines in the management of osteoarthritis: a systematic review. Rheumatology (Oxford). 2011;50:911-920.
9. Cameron M, Chrubasik S. Topical herbal therapies for treating osteoarthritis. Cochrane Database Syst Rev. 2013;(5): CD010538.
10. Oltean H, Robbins C, van Tulder MW, et al. Herbal medicine for low-back pain. Cochrane Database Syst Rev. 2014;(12): CD004504.
11. Armstrong EP, Malone DC, McCarberg B, et al. Cost-effectiveness analysis of a new 8% capsaicin patch compared to existing therapies for postherpetic neuralgia. Curr Med Res Opin. 2011;27:939-950.
12. Mou J, Paillard F, Turnbull B, et al. Efficacy of Qutenza (capsaicin) 8% patch for neuropathic pain: a meta-analysis of the Qutenza Clinical Trials Database. Pain. 2013;154:1632-1639.
13. Sun-Edelstein C, Mauskop A. Alternative headache treatments: nutraceuticals, behavioral and physical treatments. Headache. 2011;51:469-483.
14. D’Andrea G, Cevoli S, Cologno D. Herbal therapy in migraine. Neurol Sci. 2014;35(suppl 1):135-140.
15. Diener HC, Rahlfs VW, Danesch U. The first placebo-controlled trial of a special butterbur root extract for the prevention of migraine: reanalysis of efficacy criteria. Eur Neurol. 2004;51:89-97.
16. Lipton RB, Göbel H, Einhäupl KM, et al. Petasites hybridus root (butterbur) is an effective preventive treatment for migraine. Neurology. 2004;63:2240-2244.
17. Pothmann R, Danesch U. Migraine prevention in children and adolescents: results of an open study with a special butterbur root extract. Headache. 2005;45:196-203.
18. Holland S, Silberstein SD, Freitag F, et al; Quality Standards Subcommittee of the American Academy of Neurology and the American Headache Society. Evidence-based guideline update: NSAIDs and other complementary treatments for episodic migraine prevention in adults: report of the Quality Standards Subcommittee of the American Academy of Neurology and the American Headache Society. Neurology. 2012;78:1346-1353.
19. American Academy of Neurology. Evidence-based guideline update: NSAIDs and other complementary treatments for episodic migraine prevention in adults: [RETIRED]. http://n.neurology.org/content/78/17/1346. Accessed April 29, 2018.
20. Man LX. Complementary and alternative medicine for allergic rhinitis. Curr Opin Otolaryngol Head Neck Surg. 2009;17:226-231.
21. Guo R, Pittler MH, Ernst E. Herbal medicines for the treatment of allergic rhinitis: a systematic review. Ann Allergy Asthma Immunol. 2007;99:483-495.
22. Daniel O, Mauskop A. Nutraceuticals in acute and prophylactic treatment of migraine. Curr Treat Options Neurol. 2016; 18:14.
23. Chacko SM, Thambi PT, Kuttan R, et al. Beneficial effects of green tea: a literature review. Chin Med. 2010;6:13.
24. Naghma K, Hasan M. Tea polyphenols for health promotion. Life Sci. 2007;81:519-533.
25. Okello EJ, McDougall GJ, Kumar S, et al. In vitro protective effects of colon-available extract of Camellia sinensis (tea) against hydrogen peroxide and beta-amyloid (Aβ((1-42))) induced cytotoxicity in differentiated PC12 cells. Phytomedicine. 2011;18:691-696.
26. Schmidt A, Hammann F, Wölnerhanssen B, et al. Green tea extract enhances parieto-frontal connectivity during working memory processing. Psychopharmacology (Berl). 2014;231: 3879-3888.
27. Tomata Y, Sugiyama K, Kaiho Y, et al. Green tea consumption and the risk of incident dementia in elderly Japanese: The Ohsaki Cohort 2006 Study. Am J Geriatr Psychiatry. 2016;24: 881-889.
28. Takechi R, Alfonso H, Hiramatsu N, et al. Elevated plasma and urinary concentrations of green tea catechins associated with improved plasma lipid profile in healthy Japanese women. Nutr Res. 2016;36:220-226.
29. Kim A, Chiu A, Barone MK, et al. Green tea catechins decrease total and low-density lipoprotein cholesterol: a systematic review and meta-analysis. J Am Diet Assoc. 2011; 111:1720-1729.
30. Zhang C, Qin YY, Wei X, et al. Tea consumption and risk of cardiovascular outcomes and total mortality: a systematic review and meta-analysis of prospective observational studies. Eur J Epidemiol. 2015;30:103-113.
31. Imai K, Suga K, Nakachi K. Cancer-preventive effects of drinking green tea among a Japanese population. Prev Med. 1997;26:769-775.
32. Yuan JM. Cancer prevention by green tea: evidence from epidemiologic studies. Am J Clin Nutr. 2013;98(6 suppl): 1676S-1681S.
33. Kurahashi N, Sasazuki S, Iwasaki M, et al. Green tea consumption and prostate cancer risk in Japanese men: a prospective study. Am J Epidemiol. 2008;167:71-77.
34. Iso H, Date C, Wakai K, et al. The relationship between green tea and total caffeine intake and risk for self-reported type 2 diabetes among Japanese adults. Ann Intern Med. 2006; 144:554-562.
35. Kim HM, Kim J. The effects of green tea on obesity and type 2 diabetes. Diab Metab J. 2013;37:173-175.
36. Yang J, Mao Q, Xu H, et al. Tea consumption and risk of type 2 diabetes mellitus: a systematic review and meta-analysis update. BMJ Open. 2014;4:e005632.
37. Liu K, Zhou R, Wang B, et al. Effect of green tea on glucose control and insulin sensitivity: a meta-analysis of 17 randomized controlled trials. Am J Clin Nutr. 2013;98:340-348.
38. Isomura T, Suzuki S, Origasa H, et al. Liver-related safety assessment of green tea extracts in humans: a systematic review of randomized controlled trials. Eur J Clin Nutr. 2016;70:1340.
39. Tillisch K. Complementary and alternative medicine for gastrointestinal disorders. Clin Med (Lond). 2007;7:224-227.
40. Knowlton WM, McKemy DD. TRPM8: from cold to cancer, peppermint to pain. Curr Pharm Biotechnol. 2011;12:68-77.
41. Ford AC, Moayyedi P, Lacy BE, et al. Task Force on the Management of Functional Bowel Disorders. American College of Gastroenterology monograph on the management of irritable bowel syndrome and chronic idiopathic constipation. Am J Gastroenterol. 2014;109(suppl 1):S2-S26.
42. Ruepert L, Quartero AO, de Wit NJ, et al. Bulking agents, antispasmodics and antidepressants for the treatment of irritable bowel syndrome. Cochrane Database Syst Rev. 2011;(8):CD003460.
43. Khanna R, MacDonald JK, Levesque BG. Peppermint oil for the treatment of irritable bowel syndrome: a systematic review and meta-analysis. J Clin Gastroenterol. 2014;48:505-512.
44. Cash BD, Epstein MS, Shah SM. A novel delivery system of peppermint oil is an effective therapy for irritable bowel syndrome symptoms. Dig Dis Sci. 2016;61:560-571.
45. Holtmann G, Haag S, Adam B, et al. Effects of a fixed combination of peppermint oil and caraway oil on symptoms and quality of life in patients suffering from functional dyspepsia. Phytomedicine. 2003;10(suppl 4):56-57.
46. Madisch A, Heydenreich CJ, Wieland V, et al. Treatment of functional dyspepsia with a fixed peppermint oil and caraway oil combination preparation as compared to cisapride. A multicenter, reference-controlled double-blind equivalence study. Arzneimittelforschung. 1999;49:925-932.
47. Asao T, Kuwano H, Ide M, et al. Spasmolytic effect of peppermint oil in barium during double-contrast barium enema compared with Buscopan. Clin Radiol. 2003;58:301-305.
48. Sparks MJ, O’Sullivan P, Herrington AA, et al. Does peppermint oil relieve spasm during barium enema? Br J Radiol. 1995;68:841-843.
49. Akhavan Amjadi M, Mojab F, Kamranpour SB. The effect of peppermint oil on symptomatic treatment of pruritus in pregnant women. Iran J Pharm Res. 2012;11:1073-1077.
50. St Cyr A, Chen A, Bradley KC, et al. Efficacy and tolerability of STOPAIN for a migraine attack. Front Neurol. 2015;6:11.
51. Borhani Haghighi A, Motazedian S, Rezaii R, et al. Cutaneous application of menthol 10% solution as an abortive treatment of migraine without aura: a randomised, double-blind, placebo-controlled, crossed-over study. Int J Clin Pract. 2010; 64:451-456.
52. Gobel H, Fresenius J, Heinze A, et al. Effectiveness of oleum menthae piperitae and paracetamol in therapy of headache of the tension type [German]. Nervenarzt. 1996;67:672-681.
53. Schattner P, Randerson D. Tiger Balm as a treatment of tension headache. A clinical trial in general practice. Aust Fam Physician. 1996;25:216-220.
54. Johar P, Grover V, Topp R, et al. A comparison of topical menthol to ice on pain, evoked tetanic and voluntary force during delayed onset muscle soreness. Int J Sports Phys Ther. 2012;7:314-322.
55. Topp R, Brosky JA Jr, Pieschel D. The effect of either topical menthol or a placebo on functioning and knee pain among patients with knee OA. J Geriatr Phys Ther. 2013;36:92-99.
56. Sundstrup E, Jakobsen MD, Brandt M, et al. Acute effect of topical menthol on chronic pain in slaughterhouse workers with carpal tunnel syndrome: triple-blind, randomized placebo-controlled trial. Rehabil Res Pract. 2014;2014:310913.
57. Nair B. Final report on the safety assessment of mentha piperita (peppermint) oil, mentha piperita (peppermint) leaf extract, mentha piperita (peppermint) leaf, and mentha piperita (peppermint) leaf water. Int J Toxicol. 2001;20(suppl 3):61-73.
58. Klingler B, Chadhary S. Peppermint oil. Am Fam Physician. 2007;75:1027-1030.
59. Nath SS, Pandey C, Roy D. A near fatal case of high dose peppermint oil ingestion—lessons learnt. Indian J Anaesth. 2012;56:582-584.
The National Center for Complementary and Integrative Health, a division of the National Institutes of Medicine, estimates that 38% of American adults use complementary and alternative medicine (including 17.7% who say they use “natural products”).1 Despite the popularity of these products, many providers remain skeptical—and for good reason. Enthusiasts may offer dramatic anecdotes to “prove” their supplements’ worth, but little scientific support is available for most herbal remedies. There are, however, exceptions—capsaicin, butterbur, green tea, and peppermint—as this review of the medical literature reveals.
Worth noting as you consider this—or any—review of herbals is that while there is limited scientific evidence to establish the safety and efficacy of most herbal products, they are nonetheless freely sold without FDA approval because, under current regulations, they are considered dietary supplements. That legal designation means companies can manufacture, sell, and market herbs without first demonstrating safety and efficacy, as is required for pharmaceutical drugs. Because herbal medications do not require the same testing through the large randomized controlled trials (RCTs) required for pharmaceuticals, evidence is often based on smaller RCTs and other studies of lower overall quality. Despite these limitations, we believe it’s worth keeping an open mind about the value of evidence-based herbal and botanical treatments.
CAPSAICIN
Capsaicin, an active compound in chili peppers, provokes a burning sensation but also has a long history of use in pain treatment.2 Qutenza, an FDA-approved, chemically synthesized 8% capsaicin patch, is identical to the naturally occurring molecule.2 Topical capsaicin exerts its therapeutic effect by rapidly depleting substance P, thus reducing the transmission of pain from C fibers to higher neurologic centers in the area of administration.3
Meta-analyses and systematic reviews have shown capsaicin is effective for various painful conditions, including peripheral diabetic neuropathy, osteoarthritis (OA), low back pain (LBP), and postherpetic neuralgia (PHN).
Peripheral neuropathy. A Cochrane review of six randomized, double-blind, placebo-controlled studies of at least six weeks’ duration using topical 8% capsaicin to treat PHN and HIV-associated neuropathy concluded that high-concentration topical capsaicin provided more relief in patients with high pain levels than control patients who received a subtherapeutic (0.04%) capsaicin cream. Number-needed-to-treat values were between 8 and 12. Local adverse events were common, but not consistently reported enough to calculate a number needed to harm.4
OA. In randomized trials, capsaicin provided mild-to-moderate efficacy for patients with hand and knee OA, when compared with placebo.5-7 A systematic review of capsaicin for all osteoarthritic conditions noted that there was consistent evidence that capsaicin gel was effective for OA.8 However, a 2013 Cochrane review of only knee OA noted that capsicum extract did not provide significant clinical improvement for pain or function and resulted in a significant number of adverse events.9
LBP. Based on a 2014 Cochrane review of three trials (755 subjects) of moderate quality, capsicum frutescens cream or plaster appeared more efficacious than placebo in people with chronic LBP.10 Based on current (low-quality) evidence in one trial, however, it’s not clear whether topical capsicum cream is more beneficial for acute LBP than placebo.10
PHN. Topical capsaicin is an FDA-approved treatment for PHN. A review and cost-effectiveness analysis demonstrated that 8% capsaicin had significantly higher effectiveness rates than the oral agents (tricyclic antidepressants, duloxetine, gabapentin, pregabalin) used to treat PHN.11 The cost of the capsaicin patch was similar to a topical lidocaine patch and oral products for PHN.11 A meta-analysis of seven RCTs indicated that 8% topical capsaicin was superior to the low-dose capsaicin patch for relieving pain associated with PHN.12
Continue to: Adverse effects
Adverse effects
Very few toxic effects have been reported during a half-century of capsaicin use. Those that have been reported are mainly limited to mild local reactions.2 The most common adverse effect of topical capsaicin is local irritation (burning, stinging, and erythema), which was reported in approximately 40% of patients.6 Nevertheless, more than 90% of the subjects in clinical studies were able to complete the studies, and pain rapidly resolved after patch removal.2 Washing with soap and water may help prevent the compound from spreading to other parts of the body unintentionally.
The safety of the patch has been demonstrated with repeated dosing every three months for up to one year. However, the long-term risks of chronic capsaicin use and its effect on epidermal innervation are uncertain.5
The bottom line
Capsaicin appears to be an effective treatment for neuropathy and chronic LBP. It is FDA approved for the treatment of PHN. It may also benefit patients with OA and acute LBP. Serious adverse effects are uncommon with topical use. Common adverse effects include burning pain and irritation in the area of application, which can be intense and cause discontinuation.2
Continue to: BUTTERBUR
BUTTERBUR
Petasites hybridus, also known as butterbur, is a member of the daisy family, Asteraceae, and is a perennial plant found throughout Europe and Asia.13 It was used as a remedy for ulcers, wounds, and inflammation in ancient Greece. Its calcium channel–blocking effects may counteract vasoconstriction and play a role in preventing hyperexcitation of neurons.14 Sesquiterpenes, the pharmacologically active compounds in butterbur, have strong anti-inflammatory and vasodilatory effects through lipoxygenase and leukotriene inhibition.14
Migraine headache. Butterbur appears to be effective in migraine prophylaxis. Several studies have shown butterbur to significantly reduce the number of migraine attacks per month when compared with placebo. In a small, randomized, placebo-controlled, parallel-group study on the efficacy and tolerability of a special butterbur root extract (Petadolex) for the prevention of migraine, response rate was 45% in the butterbur group vs 15% in the placebo group. Butterbur was well tolerated.15 Similar results were found in another RCT in which butterbur 75 mg bid significantly reduced migraine frequency by 48%, compared with 26% for the placebo group.16 Butterbur was well tolerated in this study, too, and no serious adverse events occurred. Findings suggest that 75 mg bid may be a good option for migraine prevention, given the agent’s safety profile.
Petadolex may also be a good option in pediatric migraine. A 2005 study in children and adolescents found that 77% of patients experienced a reduction in attacks by at least 50% with butterbur. Patients were treated with 50 mg to 150 mg over four months.17
In their 2012 guidelines for migraine prevention, the American Academy of Neurology (AAN) and American Headache Society gave butterbur a Level A recommendation, concluding that butterbur should be offered to patients with migraine to reduce the frequency and severity of migraine attacks.18 However, the AAN changed its position in 2015, redacting the recommendation due to serious safety concerns.19
Allergic rhinitis. Although the data are not convincing, some studies have shown that butterbur may be beneficial for the treatment of allergic rhinitis.20,21
Continue to: Adverse effects
Adverse effects
While the butterbur plant itself contains pyrrolizidine alkaloids (PA), which are hepatotoxic and carcinogenic, extracts of butterbur root that are almost completely free from these alkaloids are available. Patients who choose to use butterbur should be advised to use only products that are certified and labeled PA free.
Petadolex, the medication used in migraine studies, was initially approved by the German health regulatory authority, but approval was later withdrawn due to concerns about liver toxicity.22 In 2012, the United Kingdom’s Medicines and Health Care Products Regulatory Agency withdrew all butterbur products from the market due to associated cases of liver toxicity.22 Butterbur products are still available in the US market, and the risks and benefits should be discussed with all patients considering this treatment. Liver function monitoring is recommended for all patients using butterbur.22
The herb can also cause dyspepsia, headache, itchy eyes, gastrointestinal symptoms, asthma, fatigue, and drowsiness. Additionally, people who are allergic to ragweed and daisies may have allergic reactions to butterbur. Eructation (belching) occurred in 7% of patients in a pediatric study.17
The bottom line
Butterbur appears to be efficacious for migraine prophylaxis, but long-term safety is unknown and serious concerns exist for liver toxicity.
Continue to: GREEN TEA
GREEN TEA
Most tea leaves come from the Camellia sinensis bush, but green and black tea are processed differently to produce different end products.23 It is estimated that green tea accounts for approximately a quarter of all tea consumption and is most commonly consumed in Asian countries.23 The health-promoting effects of green tea are mainly attributed to its polyphenol content.24 Of the many types of tea, green tea has the highest concentration of polyphenols, including catechins, which are powerful antioxidants.23,24 Green tea has been used in traditional Chinese and Indian medicine to control bleeding, improve digestion, and promote overall health.23
Dementia. Green tea polyphenols may enhance cognition and may protect against the development of dementia. In-vitro studies have shown that green tea reduces hydrogen peroxide and ß-amyloid peptides, which are significant in the development of Alzheimer’s disease.25 A 12-subject double-blind study found green tea increased working memory and had an impact on frontoparietal brain connections.26 Furthermore, a cohort study with 13,645 Japanese participants over a five-year period found that frequent green tea consumption (> 5 cups per day) was associated with a lower risk for dementia.27 Additional studies are needed, but green tea may be useful in the treatment or prevention of dementia in the future.
Coronary artery disease. In one study, green tea plasma and urinary concentrations were associated with plasma biomarkers of cardiovascular disease and diabetes.28 In one review, the consumption of green tea was associated with a statistically significant reduction in LDL cholesterol.29 Furthermore, a 2015 systematic review and meta-analysis of prospective observational studies concluded that increased tea consumption (of any type) is associated with a reduced risk for coronary heart disease, cardiac death, stroke, and total mortality.30
Cancer. Many studies have shown that green tea may reduce the risk for cancer, although epidemiologic evidence is inconsistent. Studies have shown that cancer rates tend to be lower in those who consume higher levels of green tea.31,32 Whether this can be attributed solely to green tea remains debatable. Several other studies have shown that polyphenols in green tea can inhibit the growth of cancer cells, but the exact mechanism by which tea interacts with cancerous cells is unknown.23
Several population-based studies have been performed, mostly in Japan, which showed green tea consumption reduced the risk for cancer. Fewer prostate cancer cases have been reported in men who consume green tea.33 While studies have been performed to determine whether green tea has effects on pancreatic, esophageal, ovarian, breast, bladder, and colorectal cancer, the evidence remains inadequate.32
Diabetes. Green tea has been shown in several studies to have a beneficial effect on diabetes. A retrospective Japanese cohort study showed that those who consumed green tea were one-third less likely to develop type 2 diabetes.34 A 10-year study from Taiwan found lower body fat and smaller waist circumference in those who consumed green tea regularly.35 A 2014 meta-analysis and systematic review of tea (any type) consumption and the risk for diabetes concluded that three or more cups of tea per day was associated with a lower risk for diabetes.36 Another meta-analysis of 17 RCTs focused on green tea concluded that green tea improves glucose control and A1C values.37
Continue to: Adverse effects
Adverse effects
There have been concerns about potential hepatotoxicity induced by green tea intake.38 However, a systematic review of 34 RCTs on liver-related adverse events from green tea showed only a slight elevation in liver function tests; no serious liver-related adverse events were reported.38 This review suggested that liver-related adverse events after intake of green tea extracts are rare.38
Consuming green tea in the diet may lower the risk for adverse effects since the concentration consumed is generally much lower than that found in extracts.
Contraindications to drinking green tea are few. Individuals with caffeine sensitivities could experience insomnia, anxiety, irritability, or upset stomach. Additionally, patients who are taking anticoagulation drugs, such as warfarin, should avoid green tea due to its vitamin K content, which can counter the effects of warfarin. Pregnant or breastfeeding women, those with heart problems or high blood pressure, kidney or liver problems, stomach ulcers, or anxiety disorders should use caution with green tea consumption.
The bottom line
Green tea consumption in the diet appears to be safe and may have beneficial effects on weight, dementia, and risk for diabetes, cancer, and cardiovascular disease. Patients may want to consider drinking green tea as part of a healthy diet, in combination with exercise.
Continue to: PEPPERMINT
PEPPERMINT
Mentha piperita, also known as peppermint, is a hybrid between water mint and spearmint. It is found throughout Europe and North America and is commonly used in tea and toothpaste and as a flavoring for gum. Menthol and methyl salicylate are the main active ingredients in peppermint, and peppermint has calcium channel–blocker effects.39 Menthol has been shown to help regulate cold and pain sensation through the TRPM8 receptor.40 The peppermint herb is used both orally and topically, and has been studied in the treatment of multiple conditions.
Irritable bowel syndrome (IBS). It appears that peppermint inhibits spontaneous peristaltic activity, which reduces gastric emptying, decreases basal tone in the gastrointestinal tract, and slows down peristalsis in the gut.39
The American College of Gastroenterology guidelines currently note that there is moderate-quality evidence for peppermint oil in the treatment of IBS.41 A Cochrane review concluded that peppermint appears to be beneficial for IBS-related symptoms and pain.42 In a systematic review of nine studies from 2014, peppermint oil was found to be more effective than placebo for IBS symptoms such as pain, bloating, gas, and diarrhea.43 The review also indicated that peppermint oil is safe, with heartburn being the most common complaint.43 A 2016 study also found that triple-coated microspheres containing peppermint oil reduced the frequency and intensity of IBS symptoms.44
Non-ulcer dyspepsia. In combination with caraway oil, peppermint oil can be used to reduce symptoms of non-ulcer dyspepsia.45,46 A multicenter, randomized, placebo-controlled, double-blind study found that 43.3% of subjects improved with a peppermint-caraway oil combination after eight weeks, compared with 3.5% receiving placebo.46
Barium enema–related colonic spasm. Peppermint can relax the lower esophageal sphincter, and it has been shown to be useful as an antispasmodic agent for barium enema–related colonic spasm.47,48
Itching/skin irritation. Peppermint, when applied topically, has been used to calm pruritus and relieve irritation and inflammation. It has a soothing and cooling effect on the skin. At least one study found it to be effective in the treatment of pruritus gravidarum, although the study population consisted of only 96 subjects.49
Migraine headache. Initial small trials suggest that menthol is likely beneficial for migraine headaches. A pilot trial of 25 patients treated with topical menthol 6% gel for an acute migraine attack showed a significant improvement in headache intensity two hours after gel application.50 In a randomized, triple-blind, placebo-controlled, crossover study of 35 patients, a menthol 10% solution was shown to be more efficacious as abortive treatment of migraine headaches than placebo.51
Tension headache. In a randomized, placebo-controlled, double-blind crossover study, topical peppermint oil showed a significant clinical reduction in tension headache pain.52 Another small, randomized, double-blind trial showed that tiger balm (containing menthol as the main ingredient) also produced statistically significant improvement in tension headache discomfort compared with placebo.53
Continue to: Musculoskeletal pain
Musculoskeletal pain. A small study comparing topical menthol to ice for muscle soreness noted decreased perceived discomfort with menthol.54 Menthol has also been shown to reduce pain in patients with knee OA.55
Carpal tunnel syndrome (CTS). A triple-blind RCT concluded that topical menthol acutely reduced pain intensity in slaughterhouse workers with CTS, and it should be considered as an effective nonsystemic alternative to regular analgesics in the workplace management of chronic and neuropathic pain.56
Adverse effects
Peppermint appears to be safe for most adults when used in small doses, and serious adverse effects are rare.43,57 While peppermint tea appears to be safe in moderate-to-large amounts, people allergic to plants in the peppermint family (eg, mint, thyme, sage, rosemary, marjoram, basil, lavender) may experience allergic reactions with swelling, wheals, or erythema. Peppermint may also cause heartburn due to relaxation of the cardiac sphincter.
Other symptoms may include nausea, vomiting, flushing, and headache.58 The herb may also be both hepatotoxic and nephrotoxic at extremely high doses.59 Other considerations for women are that it can trigger menstruation and should be avoided during pregnancy. Due to uncertain efficacy in this population, peppermint oil should not be used on the face of infants, young children, or pregnant women.58,59
The bottom line
Peppermint appears to be safe and well tolerated. It is useful in alleviating IBS symptoms and may be effective in the treatment of non-ulcerative dyspepsia, musculoskeletal pain, headache, and CTS.54,55
The National Center for Complementary and Integrative Health, a division of the National Institutes of Medicine, estimates that 38% of American adults use complementary and alternative medicine (including 17.7% who say they use “natural products”).1 Despite the popularity of these products, many providers remain skeptical—and for good reason. Enthusiasts may offer dramatic anecdotes to “prove” their supplements’ worth, but little scientific support is available for most herbal remedies. There are, however, exceptions—capsaicin, butterbur, green tea, and peppermint—as this review of the medical literature reveals.
Worth noting as you consider this—or any—review of herbals is that while there is limited scientific evidence to establish the safety and efficacy of most herbal products, they are nonetheless freely sold without FDA approval because, under current regulations, they are considered dietary supplements. That legal designation means companies can manufacture, sell, and market herbs without first demonstrating safety and efficacy, as is required for pharmaceutical drugs. Because herbal medications do not require the same testing through the large randomized controlled trials (RCTs) required for pharmaceuticals, evidence is often based on smaller RCTs and other studies of lower overall quality. Despite these limitations, we believe it’s worth keeping an open mind about the value of evidence-based herbal and botanical treatments.
CAPSAICIN
Capsaicin, an active compound in chili peppers, provokes a burning sensation but also has a long history of use in pain treatment.2 Qutenza, an FDA-approved, chemically synthesized 8% capsaicin patch, is identical to the naturally occurring molecule.2 Topical capsaicin exerts its therapeutic effect by rapidly depleting substance P, thus reducing the transmission of pain from C fibers to higher neurologic centers in the area of administration.3
Meta-analyses and systematic reviews have shown capsaicin is effective for various painful conditions, including peripheral diabetic neuropathy, osteoarthritis (OA), low back pain (LBP), and postherpetic neuralgia (PHN).
Peripheral neuropathy. A Cochrane review of six randomized, double-blind, placebo-controlled studies of at least six weeks’ duration using topical 8% capsaicin to treat PHN and HIV-associated neuropathy concluded that high-concentration topical capsaicin provided more relief in patients with high pain levels than control patients who received a subtherapeutic (0.04%) capsaicin cream. Number-needed-to-treat values were between 8 and 12. Local adverse events were common, but not consistently reported enough to calculate a number needed to harm.4
OA. In randomized trials, capsaicin provided mild-to-moderate efficacy for patients with hand and knee OA, when compared with placebo.5-7 A systematic review of capsaicin for all osteoarthritic conditions noted that there was consistent evidence that capsaicin gel was effective for OA.8 However, a 2013 Cochrane review of only knee OA noted that capsicum extract did not provide significant clinical improvement for pain or function and resulted in a significant number of adverse events.9
LBP. Based on a 2014 Cochrane review of three trials (755 subjects) of moderate quality, capsicum frutescens cream or plaster appeared more efficacious than placebo in people with chronic LBP.10 Based on current (low-quality) evidence in one trial, however, it’s not clear whether topical capsicum cream is more beneficial for acute LBP than placebo.10
PHN. Topical capsaicin is an FDA-approved treatment for PHN. A review and cost-effectiveness analysis demonstrated that 8% capsaicin had significantly higher effectiveness rates than the oral agents (tricyclic antidepressants, duloxetine, gabapentin, pregabalin) used to treat PHN.11 The cost of the capsaicin patch was similar to a topical lidocaine patch and oral products for PHN.11 A meta-analysis of seven RCTs indicated that 8% topical capsaicin was superior to the low-dose capsaicin patch for relieving pain associated with PHN.12
Continue to: Adverse effects
Adverse effects
Very few toxic effects have been reported during a half-century of capsaicin use. Those that have been reported are mainly limited to mild local reactions.2 The most common adverse effect of topical capsaicin is local irritation (burning, stinging, and erythema), which was reported in approximately 40% of patients.6 Nevertheless, more than 90% of the subjects in clinical studies were able to complete the studies, and pain rapidly resolved after patch removal.2 Washing with soap and water may help prevent the compound from spreading to other parts of the body unintentionally.
The safety of the patch has been demonstrated with repeated dosing every three months for up to one year. However, the long-term risks of chronic capsaicin use and its effect on epidermal innervation are uncertain.5
The bottom line
Capsaicin appears to be an effective treatment for neuropathy and chronic LBP. It is FDA approved for the treatment of PHN. It may also benefit patients with OA and acute LBP. Serious adverse effects are uncommon with topical use. Common adverse effects include burning pain and irritation in the area of application, which can be intense and cause discontinuation.2
Continue to: BUTTERBUR
BUTTERBUR
Petasites hybridus, also known as butterbur, is a member of the daisy family, Asteraceae, and is a perennial plant found throughout Europe and Asia.13 It was used as a remedy for ulcers, wounds, and inflammation in ancient Greece. Its calcium channel–blocking effects may counteract vasoconstriction and play a role in preventing hyperexcitation of neurons.14 Sesquiterpenes, the pharmacologically active compounds in butterbur, have strong anti-inflammatory and vasodilatory effects through lipoxygenase and leukotriene inhibition.14
Migraine headache. Butterbur appears to be effective in migraine prophylaxis. Several studies have shown butterbur to significantly reduce the number of migraine attacks per month when compared with placebo. In a small, randomized, placebo-controlled, parallel-group study on the efficacy and tolerability of a special butterbur root extract (Petadolex) for the prevention of migraine, response rate was 45% in the butterbur group vs 15% in the placebo group. Butterbur was well tolerated.15 Similar results were found in another RCT in which butterbur 75 mg bid significantly reduced migraine frequency by 48%, compared with 26% for the placebo group.16 Butterbur was well tolerated in this study, too, and no serious adverse events occurred. Findings suggest that 75 mg bid may be a good option for migraine prevention, given the agent’s safety profile.
Petadolex may also be a good option in pediatric migraine. A 2005 study in children and adolescents found that 77% of patients experienced a reduction in attacks by at least 50% with butterbur. Patients were treated with 50 mg to 150 mg over four months.17
In their 2012 guidelines for migraine prevention, the American Academy of Neurology (AAN) and American Headache Society gave butterbur a Level A recommendation, concluding that butterbur should be offered to patients with migraine to reduce the frequency and severity of migraine attacks.18 However, the AAN changed its position in 2015, redacting the recommendation due to serious safety concerns.19
Allergic rhinitis. Although the data are not convincing, some studies have shown that butterbur may be beneficial for the treatment of allergic rhinitis.20,21
Continue to: Adverse effects
Adverse effects
While the butterbur plant itself contains pyrrolizidine alkaloids (PA), which are hepatotoxic and carcinogenic, extracts of butterbur root that are almost completely free from these alkaloids are available. Patients who choose to use butterbur should be advised to use only products that are certified and labeled PA free.
Petadolex, the medication used in migraine studies, was initially approved by the German health regulatory authority, but approval was later withdrawn due to concerns about liver toxicity.22 In 2012, the United Kingdom’s Medicines and Health Care Products Regulatory Agency withdrew all butterbur products from the market due to associated cases of liver toxicity.22 Butterbur products are still available in the US market, and the risks and benefits should be discussed with all patients considering this treatment. Liver function monitoring is recommended for all patients using butterbur.22
The herb can also cause dyspepsia, headache, itchy eyes, gastrointestinal symptoms, asthma, fatigue, and drowsiness. Additionally, people who are allergic to ragweed and daisies may have allergic reactions to butterbur. Eructation (belching) occurred in 7% of patients in a pediatric study.17
The bottom line
Butterbur appears to be efficacious for migraine prophylaxis, but long-term safety is unknown and serious concerns exist for liver toxicity.
Continue to: GREEN TEA
GREEN TEA
Most tea leaves come from the Camellia sinensis bush, but green and black tea are processed differently to produce different end products.23 It is estimated that green tea accounts for approximately a quarter of all tea consumption and is most commonly consumed in Asian countries.23 The health-promoting effects of green tea are mainly attributed to its polyphenol content.24 Of the many types of tea, green tea has the highest concentration of polyphenols, including catechins, which are powerful antioxidants.23,24 Green tea has been used in traditional Chinese and Indian medicine to control bleeding, improve digestion, and promote overall health.23
Dementia. Green tea polyphenols may enhance cognition and may protect against the development of dementia. In-vitro studies have shown that green tea reduces hydrogen peroxide and ß-amyloid peptides, which are significant in the development of Alzheimer’s disease.25 A 12-subject double-blind study found green tea increased working memory and had an impact on frontoparietal brain connections.26 Furthermore, a cohort study with 13,645 Japanese participants over a five-year period found that frequent green tea consumption (> 5 cups per day) was associated with a lower risk for dementia.27 Additional studies are needed, but green tea may be useful in the treatment or prevention of dementia in the future.
Coronary artery disease. In one study, green tea plasma and urinary concentrations were associated with plasma biomarkers of cardiovascular disease and diabetes.28 In one review, the consumption of green tea was associated with a statistically significant reduction in LDL cholesterol.29 Furthermore, a 2015 systematic review and meta-analysis of prospective observational studies concluded that increased tea consumption (of any type) is associated with a reduced risk for coronary heart disease, cardiac death, stroke, and total mortality.30
Cancer. Many studies have shown that green tea may reduce the risk for cancer, although epidemiologic evidence is inconsistent. Studies have shown that cancer rates tend to be lower in those who consume higher levels of green tea.31,32 Whether this can be attributed solely to green tea remains debatable. Several other studies have shown that polyphenols in green tea can inhibit the growth of cancer cells, but the exact mechanism by which tea interacts with cancerous cells is unknown.23
Several population-based studies have been performed, mostly in Japan, which showed green tea consumption reduced the risk for cancer. Fewer prostate cancer cases have been reported in men who consume green tea.33 While studies have been performed to determine whether green tea has effects on pancreatic, esophageal, ovarian, breast, bladder, and colorectal cancer, the evidence remains inadequate.32
Diabetes. Green tea has been shown in several studies to have a beneficial effect on diabetes. A retrospective Japanese cohort study showed that those who consumed green tea were one-third less likely to develop type 2 diabetes.34 A 10-year study from Taiwan found lower body fat and smaller waist circumference in those who consumed green tea regularly.35 A 2014 meta-analysis and systematic review of tea (any type) consumption and the risk for diabetes concluded that three or more cups of tea per day was associated with a lower risk for diabetes.36 Another meta-analysis of 17 RCTs focused on green tea concluded that green tea improves glucose control and A1C values.37
Continue to: Adverse effects
Adverse effects
There have been concerns about potential hepatotoxicity induced by green tea intake.38 However, a systematic review of 34 RCTs on liver-related adverse events from green tea showed only a slight elevation in liver function tests; no serious liver-related adverse events were reported.38 This review suggested that liver-related adverse events after intake of green tea extracts are rare.38
Consuming green tea in the diet may lower the risk for adverse effects since the concentration consumed is generally much lower than that found in extracts.
Contraindications to drinking green tea are few. Individuals with caffeine sensitivities could experience insomnia, anxiety, irritability, or upset stomach. Additionally, patients who are taking anticoagulation drugs, such as warfarin, should avoid green tea due to its vitamin K content, which can counter the effects of warfarin. Pregnant or breastfeeding women, those with heart problems or high blood pressure, kidney or liver problems, stomach ulcers, or anxiety disorders should use caution with green tea consumption.
The bottom line
Green tea consumption in the diet appears to be safe and may have beneficial effects on weight, dementia, and risk for diabetes, cancer, and cardiovascular disease. Patients may want to consider drinking green tea as part of a healthy diet, in combination with exercise.
Continue to: PEPPERMINT
PEPPERMINT
Mentha piperita, also known as peppermint, is a hybrid between water mint and spearmint. It is found throughout Europe and North America and is commonly used in tea and toothpaste and as a flavoring for gum. Menthol and methyl salicylate are the main active ingredients in peppermint, and peppermint has calcium channel–blocker effects.39 Menthol has been shown to help regulate cold and pain sensation through the TRPM8 receptor.40 The peppermint herb is used both orally and topically, and has been studied in the treatment of multiple conditions.
Irritable bowel syndrome (IBS). It appears that peppermint inhibits spontaneous peristaltic activity, which reduces gastric emptying, decreases basal tone in the gastrointestinal tract, and slows down peristalsis in the gut.39
The American College of Gastroenterology guidelines currently note that there is moderate-quality evidence for peppermint oil in the treatment of IBS.41 A Cochrane review concluded that peppermint appears to be beneficial for IBS-related symptoms and pain.42 In a systematic review of nine studies from 2014, peppermint oil was found to be more effective than placebo for IBS symptoms such as pain, bloating, gas, and diarrhea.43 The review also indicated that peppermint oil is safe, with heartburn being the most common complaint.43 A 2016 study also found that triple-coated microspheres containing peppermint oil reduced the frequency and intensity of IBS symptoms.44
Non-ulcer dyspepsia. In combination with caraway oil, peppermint oil can be used to reduce symptoms of non-ulcer dyspepsia.45,46 A multicenter, randomized, placebo-controlled, double-blind study found that 43.3% of subjects improved with a peppermint-caraway oil combination after eight weeks, compared with 3.5% receiving placebo.46
Barium enema–related colonic spasm. Peppermint can relax the lower esophageal sphincter, and it has been shown to be useful as an antispasmodic agent for barium enema–related colonic spasm.47,48
Itching/skin irritation. Peppermint, when applied topically, has been used to calm pruritus and relieve irritation and inflammation. It has a soothing and cooling effect on the skin. At least one study found it to be effective in the treatment of pruritus gravidarum, although the study population consisted of only 96 subjects.49
Migraine headache. Initial small trials suggest that menthol is likely beneficial for migraine headaches. A pilot trial of 25 patients treated with topical menthol 6% gel for an acute migraine attack showed a significant improvement in headache intensity two hours after gel application.50 In a randomized, triple-blind, placebo-controlled, crossover study of 35 patients, a menthol 10% solution was shown to be more efficacious as abortive treatment of migraine headaches than placebo.51
Tension headache. In a randomized, placebo-controlled, double-blind crossover study, topical peppermint oil showed a significant clinical reduction in tension headache pain.52 Another small, randomized, double-blind trial showed that tiger balm (containing menthol as the main ingredient) also produced statistically significant improvement in tension headache discomfort compared with placebo.53
Continue to: Musculoskeletal pain
Musculoskeletal pain. A small study comparing topical menthol to ice for muscle soreness noted decreased perceived discomfort with menthol.54 Menthol has also been shown to reduce pain in patients with knee OA.55
Carpal tunnel syndrome (CTS). A triple-blind RCT concluded that topical menthol acutely reduced pain intensity in slaughterhouse workers with CTS, and it should be considered as an effective nonsystemic alternative to regular analgesics in the workplace management of chronic and neuropathic pain.56
Adverse effects
Peppermint appears to be safe for most adults when used in small doses, and serious adverse effects are rare.43,57 While peppermint tea appears to be safe in moderate-to-large amounts, people allergic to plants in the peppermint family (eg, mint, thyme, sage, rosemary, marjoram, basil, lavender) may experience allergic reactions with swelling, wheals, or erythema. Peppermint may also cause heartburn due to relaxation of the cardiac sphincter.
Other symptoms may include nausea, vomiting, flushing, and headache.58 The herb may also be both hepatotoxic and nephrotoxic at extremely high doses.59 Other considerations for women are that it can trigger menstruation and should be avoided during pregnancy. Due to uncertain efficacy in this population, peppermint oil should not be used on the face of infants, young children, or pregnant women.58,59
The bottom line
Peppermint appears to be safe and well tolerated. It is useful in alleviating IBS symptoms and may be effective in the treatment of non-ulcerative dyspepsia, musculoskeletal pain, headache, and CTS.54,55
1. National Center for Complementary and Integrative Health. The Use of Complementary and Alternative Medicine in the United States. https://nccih.nih.gov/research/statistics/2007/camsurvey_fs1.htm. Accessed April 19, 2018.
2. Wallace M, Pappagallo M. Qutenza: a capsaicin 8% patch for the management of postherpetic neuralgia. Expert Rev Neurother. 2011;11:15-27.
3. Rains C, Bryson HM. Topical capsaicin. A review of its pharmacological properties and therapeutic potential in post-herpetic neuralgia, diabetic neuropathy and osteoarthritis. Drugs Aging. 1995;7:317-328.
4. Derry S, Sven-Rice A, Cole P, et al. Topical capsaicin (high concentration) for chronic neuropathic pain in adults. Cochrane Database Syst Rev. 2013;(2):CD007393.
5. Mason L, Moore RA, Derry S, et al. Systematic review of topical capsaicin for the treatment of chronic pain. BMJ. 2004;328:991.
6. Deal CL, Schnitzer TJ, Lipstein E, et al. Treatment of arthritis with topical capsaicin: a double-blind trial. Clin Ther. 1991; 13:383.
7. McCarthy GM, McCarty DJ. Effect of topical capsaicin in the therapy of painful osteoarthritis of the hands. J Rheumatol. 1992;19:604.
8. De Silva V, El-Metwally A, Ernst E, et al; Arthritis Research UK Working Group on Complementary and Alternative Medicines. Evidence for the efficacy of complementary and alternative medicines in the management of osteoarthritis: a systematic review. Rheumatology (Oxford). 2011;50:911-920.
9. Cameron M, Chrubasik S. Topical herbal therapies for treating osteoarthritis. Cochrane Database Syst Rev. 2013;(5): CD010538.
10. Oltean H, Robbins C, van Tulder MW, et al. Herbal medicine for low-back pain. Cochrane Database Syst Rev. 2014;(12): CD004504.
11. Armstrong EP, Malone DC, McCarberg B, et al. Cost-effectiveness analysis of a new 8% capsaicin patch compared to existing therapies for postherpetic neuralgia. Curr Med Res Opin. 2011;27:939-950.
12. Mou J, Paillard F, Turnbull B, et al. Efficacy of Qutenza (capsaicin) 8% patch for neuropathic pain: a meta-analysis of the Qutenza Clinical Trials Database. Pain. 2013;154:1632-1639.
13. Sun-Edelstein C, Mauskop A. Alternative headache treatments: nutraceuticals, behavioral and physical treatments. Headache. 2011;51:469-483.
14. D’Andrea G, Cevoli S, Cologno D. Herbal therapy in migraine. Neurol Sci. 2014;35(suppl 1):135-140.
15. Diener HC, Rahlfs VW, Danesch U. The first placebo-controlled trial of a special butterbur root extract for the prevention of migraine: reanalysis of efficacy criteria. Eur Neurol. 2004;51:89-97.
16. Lipton RB, Göbel H, Einhäupl KM, et al. Petasites hybridus root (butterbur) is an effective preventive treatment for migraine. Neurology. 2004;63:2240-2244.
17. Pothmann R, Danesch U. Migraine prevention in children and adolescents: results of an open study with a special butterbur root extract. Headache. 2005;45:196-203.
18. Holland S, Silberstein SD, Freitag F, et al; Quality Standards Subcommittee of the American Academy of Neurology and the American Headache Society. Evidence-based guideline update: NSAIDs and other complementary treatments for episodic migraine prevention in adults: report of the Quality Standards Subcommittee of the American Academy of Neurology and the American Headache Society. Neurology. 2012;78:1346-1353.
19. American Academy of Neurology. Evidence-based guideline update: NSAIDs and other complementary treatments for episodic migraine prevention in adults: [RETIRED]. http://n.neurology.org/content/78/17/1346. Accessed April 29, 2018.
20. Man LX. Complementary and alternative medicine for allergic rhinitis. Curr Opin Otolaryngol Head Neck Surg. 2009;17:226-231.
21. Guo R, Pittler MH, Ernst E. Herbal medicines for the treatment of allergic rhinitis: a systematic review. Ann Allergy Asthma Immunol. 2007;99:483-495.
22. Daniel O, Mauskop A. Nutraceuticals in acute and prophylactic treatment of migraine. Curr Treat Options Neurol. 2016; 18:14.
23. Chacko SM, Thambi PT, Kuttan R, et al. Beneficial effects of green tea: a literature review. Chin Med. 2010;6:13.
24. Naghma K, Hasan M. Tea polyphenols for health promotion. Life Sci. 2007;81:519-533.
25. Okello EJ, McDougall GJ, Kumar S, et al. In vitro protective effects of colon-available extract of Camellia sinensis (tea) against hydrogen peroxide and beta-amyloid (Aβ((1-42))) induced cytotoxicity in differentiated PC12 cells. Phytomedicine. 2011;18:691-696.
26. Schmidt A, Hammann F, Wölnerhanssen B, et al. Green tea extract enhances parieto-frontal connectivity during working memory processing. Psychopharmacology (Berl). 2014;231: 3879-3888.
27. Tomata Y, Sugiyama K, Kaiho Y, et al. Green tea consumption and the risk of incident dementia in elderly Japanese: The Ohsaki Cohort 2006 Study. Am J Geriatr Psychiatry. 2016;24: 881-889.
28. Takechi R, Alfonso H, Hiramatsu N, et al. Elevated plasma and urinary concentrations of green tea catechins associated with improved plasma lipid profile in healthy Japanese women. Nutr Res. 2016;36:220-226.
29. Kim A, Chiu A, Barone MK, et al. Green tea catechins decrease total and low-density lipoprotein cholesterol: a systematic review and meta-analysis. J Am Diet Assoc. 2011; 111:1720-1729.
30. Zhang C, Qin YY, Wei X, et al. Tea consumption and risk of cardiovascular outcomes and total mortality: a systematic review and meta-analysis of prospective observational studies. Eur J Epidemiol. 2015;30:103-113.
31. Imai K, Suga K, Nakachi K. Cancer-preventive effects of drinking green tea among a Japanese population. Prev Med. 1997;26:769-775.
32. Yuan JM. Cancer prevention by green tea: evidence from epidemiologic studies. Am J Clin Nutr. 2013;98(6 suppl): 1676S-1681S.
33. Kurahashi N, Sasazuki S, Iwasaki M, et al. Green tea consumption and prostate cancer risk in Japanese men: a prospective study. Am J Epidemiol. 2008;167:71-77.
34. Iso H, Date C, Wakai K, et al. The relationship between green tea and total caffeine intake and risk for self-reported type 2 diabetes among Japanese adults. Ann Intern Med. 2006; 144:554-562.
35. Kim HM, Kim J. The effects of green tea on obesity and type 2 diabetes. Diab Metab J. 2013;37:173-175.
36. Yang J, Mao Q, Xu H, et al. Tea consumption and risk of type 2 diabetes mellitus: a systematic review and meta-analysis update. BMJ Open. 2014;4:e005632.
37. Liu K, Zhou R, Wang B, et al. Effect of green tea on glucose control and insulin sensitivity: a meta-analysis of 17 randomized controlled trials. Am J Clin Nutr. 2013;98:340-348.
38. Isomura T, Suzuki S, Origasa H, et al. Liver-related safety assessment of green tea extracts in humans: a systematic review of randomized controlled trials. Eur J Clin Nutr. 2016;70:1340.
39. Tillisch K. Complementary and alternative medicine for gastrointestinal disorders. Clin Med (Lond). 2007;7:224-227.
40. Knowlton WM, McKemy DD. TRPM8: from cold to cancer, peppermint to pain. Curr Pharm Biotechnol. 2011;12:68-77.
41. Ford AC, Moayyedi P, Lacy BE, et al. Task Force on the Management of Functional Bowel Disorders. American College of Gastroenterology monograph on the management of irritable bowel syndrome and chronic idiopathic constipation. Am J Gastroenterol. 2014;109(suppl 1):S2-S26.
42. Ruepert L, Quartero AO, de Wit NJ, et al. Bulking agents, antispasmodics and antidepressants for the treatment of irritable bowel syndrome. Cochrane Database Syst Rev. 2011;(8):CD003460.
43. Khanna R, MacDonald JK, Levesque BG. Peppermint oil for the treatment of irritable bowel syndrome: a systematic review and meta-analysis. J Clin Gastroenterol. 2014;48:505-512.
44. Cash BD, Epstein MS, Shah SM. A novel delivery system of peppermint oil is an effective therapy for irritable bowel syndrome symptoms. Dig Dis Sci. 2016;61:560-571.
45. Holtmann G, Haag S, Adam B, et al. Effects of a fixed combination of peppermint oil and caraway oil on symptoms and quality of life in patients suffering from functional dyspepsia. Phytomedicine. 2003;10(suppl 4):56-57.
46. Madisch A, Heydenreich CJ, Wieland V, et al. Treatment of functional dyspepsia with a fixed peppermint oil and caraway oil combination preparation as compared to cisapride. A multicenter, reference-controlled double-blind equivalence study. Arzneimittelforschung. 1999;49:925-932.
47. Asao T, Kuwano H, Ide M, et al. Spasmolytic effect of peppermint oil in barium during double-contrast barium enema compared with Buscopan. Clin Radiol. 2003;58:301-305.
48. Sparks MJ, O’Sullivan P, Herrington AA, et al. Does peppermint oil relieve spasm during barium enema? Br J Radiol. 1995;68:841-843.
49. Akhavan Amjadi M, Mojab F, Kamranpour SB. The effect of peppermint oil on symptomatic treatment of pruritus in pregnant women. Iran J Pharm Res. 2012;11:1073-1077.
50. St Cyr A, Chen A, Bradley KC, et al. Efficacy and tolerability of STOPAIN for a migraine attack. Front Neurol. 2015;6:11.
51. Borhani Haghighi A, Motazedian S, Rezaii R, et al. Cutaneous application of menthol 10% solution as an abortive treatment of migraine without aura: a randomised, double-blind, placebo-controlled, crossed-over study. Int J Clin Pract. 2010; 64:451-456.
52. Gobel H, Fresenius J, Heinze A, et al. Effectiveness of oleum menthae piperitae and paracetamol in therapy of headache of the tension type [German]. Nervenarzt. 1996;67:672-681.
53. Schattner P, Randerson D. Tiger Balm as a treatment of tension headache. A clinical trial in general practice. Aust Fam Physician. 1996;25:216-220.
54. Johar P, Grover V, Topp R, et al. A comparison of topical menthol to ice on pain, evoked tetanic and voluntary force during delayed onset muscle soreness. Int J Sports Phys Ther. 2012;7:314-322.
55. Topp R, Brosky JA Jr, Pieschel D. The effect of either topical menthol or a placebo on functioning and knee pain among patients with knee OA. J Geriatr Phys Ther. 2013;36:92-99.
56. Sundstrup E, Jakobsen MD, Brandt M, et al. Acute effect of topical menthol on chronic pain in slaughterhouse workers with carpal tunnel syndrome: triple-blind, randomized placebo-controlled trial. Rehabil Res Pract. 2014;2014:310913.
57. Nair B. Final report on the safety assessment of mentha piperita (peppermint) oil, mentha piperita (peppermint) leaf extract, mentha piperita (peppermint) leaf, and mentha piperita (peppermint) leaf water. Int J Toxicol. 2001;20(suppl 3):61-73.
58. Klingler B, Chadhary S. Peppermint oil. Am Fam Physician. 2007;75:1027-1030.
59. Nath SS, Pandey C, Roy D. A near fatal case of high dose peppermint oil ingestion—lessons learnt. Indian J Anaesth. 2012;56:582-584.
1. National Center for Complementary and Integrative Health. The Use of Complementary and Alternative Medicine in the United States. https://nccih.nih.gov/research/statistics/2007/camsurvey_fs1.htm. Accessed April 19, 2018.
2. Wallace M, Pappagallo M. Qutenza: a capsaicin 8% patch for the management of postherpetic neuralgia. Expert Rev Neurother. 2011;11:15-27.
3. Rains C, Bryson HM. Topical capsaicin. A review of its pharmacological properties and therapeutic potential in post-herpetic neuralgia, diabetic neuropathy and osteoarthritis. Drugs Aging. 1995;7:317-328.
4. Derry S, Sven-Rice A, Cole P, et al. Topical capsaicin (high concentration) for chronic neuropathic pain in adults. Cochrane Database Syst Rev. 2013;(2):CD007393.
5. Mason L, Moore RA, Derry S, et al. Systematic review of topical capsaicin for the treatment of chronic pain. BMJ. 2004;328:991.
6. Deal CL, Schnitzer TJ, Lipstein E, et al. Treatment of arthritis with topical capsaicin: a double-blind trial. Clin Ther. 1991; 13:383.
7. McCarthy GM, McCarty DJ. Effect of topical capsaicin in the therapy of painful osteoarthritis of the hands. J Rheumatol. 1992;19:604.
8. De Silva V, El-Metwally A, Ernst E, et al; Arthritis Research UK Working Group on Complementary and Alternative Medicines. Evidence for the efficacy of complementary and alternative medicines in the management of osteoarthritis: a systematic review. Rheumatology (Oxford). 2011;50:911-920.
9. Cameron M, Chrubasik S. Topical herbal therapies for treating osteoarthritis. Cochrane Database Syst Rev. 2013;(5): CD010538.
10. Oltean H, Robbins C, van Tulder MW, et al. Herbal medicine for low-back pain. Cochrane Database Syst Rev. 2014;(12): CD004504.
11. Armstrong EP, Malone DC, McCarberg B, et al. Cost-effectiveness analysis of a new 8% capsaicin patch compared to existing therapies for postherpetic neuralgia. Curr Med Res Opin. 2011;27:939-950.
12. Mou J, Paillard F, Turnbull B, et al. Efficacy of Qutenza (capsaicin) 8% patch for neuropathic pain: a meta-analysis of the Qutenza Clinical Trials Database. Pain. 2013;154:1632-1639.
13. Sun-Edelstein C, Mauskop A. Alternative headache treatments: nutraceuticals, behavioral and physical treatments. Headache. 2011;51:469-483.
14. D’Andrea G, Cevoli S, Cologno D. Herbal therapy in migraine. Neurol Sci. 2014;35(suppl 1):135-140.
15. Diener HC, Rahlfs VW, Danesch U. The first placebo-controlled trial of a special butterbur root extract for the prevention of migraine: reanalysis of efficacy criteria. Eur Neurol. 2004;51:89-97.
16. Lipton RB, Göbel H, Einhäupl KM, et al. Petasites hybridus root (butterbur) is an effective preventive treatment for migraine. Neurology. 2004;63:2240-2244.
17. Pothmann R, Danesch U. Migraine prevention in children and adolescents: results of an open study with a special butterbur root extract. Headache. 2005;45:196-203.
18. Holland S, Silberstein SD, Freitag F, et al; Quality Standards Subcommittee of the American Academy of Neurology and the American Headache Society. Evidence-based guideline update: NSAIDs and other complementary treatments for episodic migraine prevention in adults: report of the Quality Standards Subcommittee of the American Academy of Neurology and the American Headache Society. Neurology. 2012;78:1346-1353.
19. American Academy of Neurology. Evidence-based guideline update: NSAIDs and other complementary treatments for episodic migraine prevention in adults: [RETIRED]. http://n.neurology.org/content/78/17/1346. Accessed April 29, 2018.
20. Man LX. Complementary and alternative medicine for allergic rhinitis. Curr Opin Otolaryngol Head Neck Surg. 2009;17:226-231.
21. Guo R, Pittler MH, Ernst E. Herbal medicines for the treatment of allergic rhinitis: a systematic review. Ann Allergy Asthma Immunol. 2007;99:483-495.
22. Daniel O, Mauskop A. Nutraceuticals in acute and prophylactic treatment of migraine. Curr Treat Options Neurol. 2016; 18:14.
23. Chacko SM, Thambi PT, Kuttan R, et al. Beneficial effects of green tea: a literature review. Chin Med. 2010;6:13.
24. Naghma K, Hasan M. Tea polyphenols for health promotion. Life Sci. 2007;81:519-533.
25. Okello EJ, McDougall GJ, Kumar S, et al. In vitro protective effects of colon-available extract of Camellia sinensis (tea) against hydrogen peroxide and beta-amyloid (Aβ((1-42))) induced cytotoxicity in differentiated PC12 cells. Phytomedicine. 2011;18:691-696.
26. Schmidt A, Hammann F, Wölnerhanssen B, et al. Green tea extract enhances parieto-frontal connectivity during working memory processing. Psychopharmacology (Berl). 2014;231: 3879-3888.
27. Tomata Y, Sugiyama K, Kaiho Y, et al. Green tea consumption and the risk of incident dementia in elderly Japanese: The Ohsaki Cohort 2006 Study. Am J Geriatr Psychiatry. 2016;24: 881-889.
28. Takechi R, Alfonso H, Hiramatsu N, et al. Elevated plasma and urinary concentrations of green tea catechins associated with improved plasma lipid profile in healthy Japanese women. Nutr Res. 2016;36:220-226.
29. Kim A, Chiu A, Barone MK, et al. Green tea catechins decrease total and low-density lipoprotein cholesterol: a systematic review and meta-analysis. J Am Diet Assoc. 2011; 111:1720-1729.
30. Zhang C, Qin YY, Wei X, et al. Tea consumption and risk of cardiovascular outcomes and total mortality: a systematic review and meta-analysis of prospective observational studies. Eur J Epidemiol. 2015;30:103-113.
31. Imai K, Suga K, Nakachi K. Cancer-preventive effects of drinking green tea among a Japanese population. Prev Med. 1997;26:769-775.
32. Yuan JM. Cancer prevention by green tea: evidence from epidemiologic studies. Am J Clin Nutr. 2013;98(6 suppl): 1676S-1681S.
33. Kurahashi N, Sasazuki S, Iwasaki M, et al. Green tea consumption and prostate cancer risk in Japanese men: a prospective study. Am J Epidemiol. 2008;167:71-77.
34. Iso H, Date C, Wakai K, et al. The relationship between green tea and total caffeine intake and risk for self-reported type 2 diabetes among Japanese adults. Ann Intern Med. 2006; 144:554-562.
35. Kim HM, Kim J. The effects of green tea on obesity and type 2 diabetes. Diab Metab J. 2013;37:173-175.
36. Yang J, Mao Q, Xu H, et al. Tea consumption and risk of type 2 diabetes mellitus: a systematic review and meta-analysis update. BMJ Open. 2014;4:e005632.
37. Liu K, Zhou R, Wang B, et al. Effect of green tea on glucose control and insulin sensitivity: a meta-analysis of 17 randomized controlled trials. Am J Clin Nutr. 2013;98:340-348.
38. Isomura T, Suzuki S, Origasa H, et al. Liver-related safety assessment of green tea extracts in humans: a systematic review of randomized controlled trials. Eur J Clin Nutr. 2016;70:1340.
39. Tillisch K. Complementary and alternative medicine for gastrointestinal disorders. Clin Med (Lond). 2007;7:224-227.
40. Knowlton WM, McKemy DD. TRPM8: from cold to cancer, peppermint to pain. Curr Pharm Biotechnol. 2011;12:68-77.
41. Ford AC, Moayyedi P, Lacy BE, et al. Task Force on the Management of Functional Bowel Disorders. American College of Gastroenterology monograph on the management of irritable bowel syndrome and chronic idiopathic constipation. Am J Gastroenterol. 2014;109(suppl 1):S2-S26.
42. Ruepert L, Quartero AO, de Wit NJ, et al. Bulking agents, antispasmodics and antidepressants for the treatment of irritable bowel syndrome. Cochrane Database Syst Rev. 2011;(8):CD003460.
43. Khanna R, MacDonald JK, Levesque BG. Peppermint oil for the treatment of irritable bowel syndrome: a systematic review and meta-analysis. J Clin Gastroenterol. 2014;48:505-512.
44. Cash BD, Epstein MS, Shah SM. A novel delivery system of peppermint oil is an effective therapy for irritable bowel syndrome symptoms. Dig Dis Sci. 2016;61:560-571.
45. Holtmann G, Haag S, Adam B, et al. Effects of a fixed combination of peppermint oil and caraway oil on symptoms and quality of life in patients suffering from functional dyspepsia. Phytomedicine. 2003;10(suppl 4):56-57.
46. Madisch A, Heydenreich CJ, Wieland V, et al. Treatment of functional dyspepsia with a fixed peppermint oil and caraway oil combination preparation as compared to cisapride. A multicenter, reference-controlled double-blind equivalence study. Arzneimittelforschung. 1999;49:925-932.
47. Asao T, Kuwano H, Ide M, et al. Spasmolytic effect of peppermint oil in barium during double-contrast barium enema compared with Buscopan. Clin Radiol. 2003;58:301-305.
48. Sparks MJ, O’Sullivan P, Herrington AA, et al. Does peppermint oil relieve spasm during barium enema? Br J Radiol. 1995;68:841-843.
49. Akhavan Amjadi M, Mojab F, Kamranpour SB. The effect of peppermint oil on symptomatic treatment of pruritus in pregnant women. Iran J Pharm Res. 2012;11:1073-1077.
50. St Cyr A, Chen A, Bradley KC, et al. Efficacy and tolerability of STOPAIN for a migraine attack. Front Neurol. 2015;6:11.
51. Borhani Haghighi A, Motazedian S, Rezaii R, et al. Cutaneous application of menthol 10% solution as an abortive treatment of migraine without aura: a randomised, double-blind, placebo-controlled, crossed-over study. Int J Clin Pract. 2010; 64:451-456.
52. Gobel H, Fresenius J, Heinze A, et al. Effectiveness of oleum menthae piperitae and paracetamol in therapy of headache of the tension type [German]. Nervenarzt. 1996;67:672-681.
53. Schattner P, Randerson D. Tiger Balm as a treatment of tension headache. A clinical trial in general practice. Aust Fam Physician. 1996;25:216-220.
54. Johar P, Grover V, Topp R, et al. A comparison of topical menthol to ice on pain, evoked tetanic and voluntary force during delayed onset muscle soreness. Int J Sports Phys Ther. 2012;7:314-322.
55. Topp R, Brosky JA Jr, Pieschel D. The effect of either topical menthol or a placebo on functioning and knee pain among patients with knee OA. J Geriatr Phys Ther. 2013;36:92-99.
56. Sundstrup E, Jakobsen MD, Brandt M, et al. Acute effect of topical menthol on chronic pain in slaughterhouse workers with carpal tunnel syndrome: triple-blind, randomized placebo-controlled trial. Rehabil Res Pract. 2014;2014:310913.
57. Nair B. Final report on the safety assessment of mentha piperita (peppermint) oil, mentha piperita (peppermint) leaf extract, mentha piperita (peppermint) leaf, and mentha piperita (peppermint) leaf water. Int J Toxicol. 2001;20(suppl 3):61-73.
58. Klingler B, Chadhary S. Peppermint oil. Am Fam Physician. 2007;75:1027-1030.
59. Nath SS, Pandey C, Roy D. A near fatal case of high dose peppermint oil ingestion—lessons learnt. Indian J Anaesth. 2012;56:582-584.